User login
Several factors may drive recent improvements in allo-HCT outcomes
A cancer center has seen improved outcomes of allogeneic transplant in recent years, despite increases in patient age and comorbidities.
Researchers compared patients who received allogeneic hematopoietic cell transplants (allo-HCTs) during two periods, 2003-2007 and 2013-2017.
Patients treated in the 2013-2017 period were older and had more HCT-specific comorbidities at baseline, but they had lower rates of mortality, relapse, and graft-versus-host disease (GVHD) post transplant. George B. McDonald, MD, an emeritus member at Fred Hutchinson Cancer Research Center in Seattle, and coauthors described these findings in Annals of Internal Medicine.
“The primary question being addressed by this study was whether the striking improvement in survival … from the 1990s to the early 2000s, that we and other transplant centers have reported, had reached a plateau or whether further improvements in survival were being seen,” Dr. McDonald said in an interview.
“We knew that older and sicker patients were now coming for transplant, compared to 10 years ago. Our transplant protocols have backed away from the highest doses of chemotherapy and irradiation used to prepare patients for transplant, toward less toxic therapies, including reduced-intensity conditioning,” he added. “Our investigators have sought to prevent and more effectively treat the myriad of complications of allogeneic transplant, based on research done at the Fred Hutchinson Cancer Research Center and at transplant centers throughout the world.”
Baseline characteristics and treatment
Dr. McDonald and his colleagues analyzed data on patients who received allo-HCTs at Seattle Cancer Care Alliance. There were 1,148 patients treated in the 2003-2007 period and 1,131 patients treated in the 2013-2017 period.
Indications for allo-HCT were similar between the time periods. Patients were diagnosed with aplastic anemia, acute and chronic leukemias, Hodgkin and non-Hodgkin lymphomas, myelodysplastic syndromes, and multiple myeloma.
Patients in the 2013-2017 group were older and had more HCT-specific comorbidities than did the patients in the 2003-2007 group. The median age was 50.0 years (range, 0.1-80.9 years) and 47.2 years (range, 0.4-78.9 years), respectively. The median score on the augmented HCT-specific comorbidity index was 4.0 and 3.0, respectively.
The 2013-2017 group was more likely to have intermediate-risk disease (73% vs. 54%) but less likely to have high-risk disease (14% vs. 31%). The 2013-2017 group was less likely to receive high-dose myeloablative conditioning (15% vs. 67%) but more likely to have an unrelated donor (70% vs. 59%) or receive a cord blood transplant (13% vs. 4%).
GVHD prophylaxis differed between the time periods, with patients in the 2013-2017 group being more likely to receive sirolimus, posttransplant cyclophosphamide, and abatacept.
Outcomes
Overall, outcomes were superior in the 2013-2017 group. The rate of nonrelapse mortality at day 200 was higher in the 2003-2007 group than in the 2013-2017 group – 16% and 11%, respectively (adjusted hazard ratio, 0.66; P = .008).
Relapse or progression was more common in the 2003-2007 group – 348 patients vs. 244 patients (aHR, 0.76; P = .011). More patients died from relapse in the 2003-2007 group – 307 patients vs. 186 patients (aHR, 0.69; P = .002). More patients died from any cause in the 2003-2007 group – 653 patients vs. 418 patients (aHR, 0.66; P less than .001). The rate of grade 2-4 acute GVHD was higher in the 2003-2007 group – 71% vs. 69% (aHR, 0.80) – and so was the rate of chronic GVHD – 44% vs. 29% (aHR, 0.40). The risk of developing gram-negative bacteremia was lower in the 2013-2017 group (aHR, 0.42), as was the risk of invasive mold infection (aHR, 0.55).
Patients in the 2013-2017 group had a higher risk of cytomegalovirus (CMV) infection (aHR = 1.15), but they were less likely to have high levels of CMV viremia (aHR, 0.78 for greater than 250 IU/mL; aHR, 0.46 for greater than 1,000 IU/mL). Having higher levels of CMV viremia was associated with an increased risk of non-relapse mortality.
Potential drivers of outcome
Dr. McDonald said this study’s design makes it difficult to determine the causes of improved outcomes in the 2013-2017 period. However, the researchers do have theories about which practice changes may have contributed to better allo-HCT outcomes.
Dr. McDonald said the decrease in GVHD over time was “likely owing to the introduction of newer preventive strategies and immune-suppressive drugs.”
The decrease in nonrelapse mortality may have been driven, in part, by a reduction in fatal infections. Dr. McDonald said these infections were less frequent in the 2013-2017 period because of “molecular methods of diagnosis (especially for herpesviruses) and newer treatments (especially for fungal infections).”
“Another reason for a lower frequency of serious infection was a change in practice for treating graft-versus-host disease,” Dr. McDonald added. “Based on a randomized trial comparing lower- versus higher-dose prednisone for less-severe GVHD … both initial doses of prednisone and total prednisone exposure were reduced.”
Another factor that may have improved allo-HCT outcomes is the center’s change in approach to conditioning therapy over time.
“The gradual shift from very-high-dose conditioning therapy to less-intense myeloablative therapy and to reduced-intensity conditioning was likely responsible for a reduction in damage to the liver, lungs, and kidneys over the last 10 years,” Dr. McDonald said. “We were able to identify patients who were at especially high risk for mortality during a screening process before transplant ... thus allowing patients at highest risk to receive less intense conditioning therapy.”
Dr. McDonald added that this study’s results are encouraging, particularly the reduction in nonrelapse mortality. However, there is still room for improvement when it comes to relapse and progression.
This research was funded by the National Institutes of Health, the American Cancer Society, and the Patient-Centered Outcomes Research Institute. Dr. McDonald reported relationships with Sangamo Therapeutics, Soligenix Therapeutics, and Lucent Medical Systems. His coauthors disclosed relationships with a range of companies.
SOURCE: McDonald GB et al. Ann Intern Med. 2020 Jan 20. doi: 10.7326/M19-2936.
A cancer center has seen improved outcomes of allogeneic transplant in recent years, despite increases in patient age and comorbidities.
Researchers compared patients who received allogeneic hematopoietic cell transplants (allo-HCTs) during two periods, 2003-2007 and 2013-2017.
Patients treated in the 2013-2017 period were older and had more HCT-specific comorbidities at baseline, but they had lower rates of mortality, relapse, and graft-versus-host disease (GVHD) post transplant. George B. McDonald, MD, an emeritus member at Fred Hutchinson Cancer Research Center in Seattle, and coauthors described these findings in Annals of Internal Medicine.
“The primary question being addressed by this study was whether the striking improvement in survival … from the 1990s to the early 2000s, that we and other transplant centers have reported, had reached a plateau or whether further improvements in survival were being seen,” Dr. McDonald said in an interview.
“We knew that older and sicker patients were now coming for transplant, compared to 10 years ago. Our transplant protocols have backed away from the highest doses of chemotherapy and irradiation used to prepare patients for transplant, toward less toxic therapies, including reduced-intensity conditioning,” he added. “Our investigators have sought to prevent and more effectively treat the myriad of complications of allogeneic transplant, based on research done at the Fred Hutchinson Cancer Research Center and at transplant centers throughout the world.”
Baseline characteristics and treatment
Dr. McDonald and his colleagues analyzed data on patients who received allo-HCTs at Seattle Cancer Care Alliance. There were 1,148 patients treated in the 2003-2007 period and 1,131 patients treated in the 2013-2017 period.
Indications for allo-HCT were similar between the time periods. Patients were diagnosed with aplastic anemia, acute and chronic leukemias, Hodgkin and non-Hodgkin lymphomas, myelodysplastic syndromes, and multiple myeloma.
Patients in the 2013-2017 group were older and had more HCT-specific comorbidities than did the patients in the 2003-2007 group. The median age was 50.0 years (range, 0.1-80.9 years) and 47.2 years (range, 0.4-78.9 years), respectively. The median score on the augmented HCT-specific comorbidity index was 4.0 and 3.0, respectively.
The 2013-2017 group was more likely to have intermediate-risk disease (73% vs. 54%) but less likely to have high-risk disease (14% vs. 31%). The 2013-2017 group was less likely to receive high-dose myeloablative conditioning (15% vs. 67%) but more likely to have an unrelated donor (70% vs. 59%) or receive a cord blood transplant (13% vs. 4%).
GVHD prophylaxis differed between the time periods, with patients in the 2013-2017 group being more likely to receive sirolimus, posttransplant cyclophosphamide, and abatacept.
Outcomes
Overall, outcomes were superior in the 2013-2017 group. The rate of nonrelapse mortality at day 200 was higher in the 2003-2007 group than in the 2013-2017 group – 16% and 11%, respectively (adjusted hazard ratio, 0.66; P = .008).
Relapse or progression was more common in the 2003-2007 group – 348 patients vs. 244 patients (aHR, 0.76; P = .011). More patients died from relapse in the 2003-2007 group – 307 patients vs. 186 patients (aHR, 0.69; P = .002). More patients died from any cause in the 2003-2007 group – 653 patients vs. 418 patients (aHR, 0.66; P less than .001). The rate of grade 2-4 acute GVHD was higher in the 2003-2007 group – 71% vs. 69% (aHR, 0.80) – and so was the rate of chronic GVHD – 44% vs. 29% (aHR, 0.40). The risk of developing gram-negative bacteremia was lower in the 2013-2017 group (aHR, 0.42), as was the risk of invasive mold infection (aHR, 0.55).
Patients in the 2013-2017 group had a higher risk of cytomegalovirus (CMV) infection (aHR = 1.15), but they were less likely to have high levels of CMV viremia (aHR, 0.78 for greater than 250 IU/mL; aHR, 0.46 for greater than 1,000 IU/mL). Having higher levels of CMV viremia was associated with an increased risk of non-relapse mortality.
Potential drivers of outcome
Dr. McDonald said this study’s design makes it difficult to determine the causes of improved outcomes in the 2013-2017 period. However, the researchers do have theories about which practice changes may have contributed to better allo-HCT outcomes.
Dr. McDonald said the decrease in GVHD over time was “likely owing to the introduction of newer preventive strategies and immune-suppressive drugs.”
The decrease in nonrelapse mortality may have been driven, in part, by a reduction in fatal infections. Dr. McDonald said these infections were less frequent in the 2013-2017 period because of “molecular methods of diagnosis (especially for herpesviruses) and newer treatments (especially for fungal infections).”
“Another reason for a lower frequency of serious infection was a change in practice for treating graft-versus-host disease,” Dr. McDonald added. “Based on a randomized trial comparing lower- versus higher-dose prednisone for less-severe GVHD … both initial doses of prednisone and total prednisone exposure were reduced.”
Another factor that may have improved allo-HCT outcomes is the center’s change in approach to conditioning therapy over time.
“The gradual shift from very-high-dose conditioning therapy to less-intense myeloablative therapy and to reduced-intensity conditioning was likely responsible for a reduction in damage to the liver, lungs, and kidneys over the last 10 years,” Dr. McDonald said. “We were able to identify patients who were at especially high risk for mortality during a screening process before transplant ... thus allowing patients at highest risk to receive less intense conditioning therapy.”
Dr. McDonald added that this study’s results are encouraging, particularly the reduction in nonrelapse mortality. However, there is still room for improvement when it comes to relapse and progression.
This research was funded by the National Institutes of Health, the American Cancer Society, and the Patient-Centered Outcomes Research Institute. Dr. McDonald reported relationships with Sangamo Therapeutics, Soligenix Therapeutics, and Lucent Medical Systems. His coauthors disclosed relationships with a range of companies.
SOURCE: McDonald GB et al. Ann Intern Med. 2020 Jan 20. doi: 10.7326/M19-2936.
A cancer center has seen improved outcomes of allogeneic transplant in recent years, despite increases in patient age and comorbidities.
Researchers compared patients who received allogeneic hematopoietic cell transplants (allo-HCTs) during two periods, 2003-2007 and 2013-2017.
Patients treated in the 2013-2017 period were older and had more HCT-specific comorbidities at baseline, but they had lower rates of mortality, relapse, and graft-versus-host disease (GVHD) post transplant. George B. McDonald, MD, an emeritus member at Fred Hutchinson Cancer Research Center in Seattle, and coauthors described these findings in Annals of Internal Medicine.
“The primary question being addressed by this study was whether the striking improvement in survival … from the 1990s to the early 2000s, that we and other transplant centers have reported, had reached a plateau or whether further improvements in survival were being seen,” Dr. McDonald said in an interview.
“We knew that older and sicker patients were now coming for transplant, compared to 10 years ago. Our transplant protocols have backed away from the highest doses of chemotherapy and irradiation used to prepare patients for transplant, toward less toxic therapies, including reduced-intensity conditioning,” he added. “Our investigators have sought to prevent and more effectively treat the myriad of complications of allogeneic transplant, based on research done at the Fred Hutchinson Cancer Research Center and at transplant centers throughout the world.”
Baseline characteristics and treatment
Dr. McDonald and his colleagues analyzed data on patients who received allo-HCTs at Seattle Cancer Care Alliance. There were 1,148 patients treated in the 2003-2007 period and 1,131 patients treated in the 2013-2017 period.
Indications for allo-HCT were similar between the time periods. Patients were diagnosed with aplastic anemia, acute and chronic leukemias, Hodgkin and non-Hodgkin lymphomas, myelodysplastic syndromes, and multiple myeloma.
Patients in the 2013-2017 group were older and had more HCT-specific comorbidities than did the patients in the 2003-2007 group. The median age was 50.0 years (range, 0.1-80.9 years) and 47.2 years (range, 0.4-78.9 years), respectively. The median score on the augmented HCT-specific comorbidity index was 4.0 and 3.0, respectively.
The 2013-2017 group was more likely to have intermediate-risk disease (73% vs. 54%) but less likely to have high-risk disease (14% vs. 31%). The 2013-2017 group was less likely to receive high-dose myeloablative conditioning (15% vs. 67%) but more likely to have an unrelated donor (70% vs. 59%) or receive a cord blood transplant (13% vs. 4%).
GVHD prophylaxis differed between the time periods, with patients in the 2013-2017 group being more likely to receive sirolimus, posttransplant cyclophosphamide, and abatacept.
Outcomes
Overall, outcomes were superior in the 2013-2017 group. The rate of nonrelapse mortality at day 200 was higher in the 2003-2007 group than in the 2013-2017 group – 16% and 11%, respectively (adjusted hazard ratio, 0.66; P = .008).
Relapse or progression was more common in the 2003-2007 group – 348 patients vs. 244 patients (aHR, 0.76; P = .011). More patients died from relapse in the 2003-2007 group – 307 patients vs. 186 patients (aHR, 0.69; P = .002). More patients died from any cause in the 2003-2007 group – 653 patients vs. 418 patients (aHR, 0.66; P less than .001). The rate of grade 2-4 acute GVHD was higher in the 2003-2007 group – 71% vs. 69% (aHR, 0.80) – and so was the rate of chronic GVHD – 44% vs. 29% (aHR, 0.40). The risk of developing gram-negative bacteremia was lower in the 2013-2017 group (aHR, 0.42), as was the risk of invasive mold infection (aHR, 0.55).
Patients in the 2013-2017 group had a higher risk of cytomegalovirus (CMV) infection (aHR = 1.15), but they were less likely to have high levels of CMV viremia (aHR, 0.78 for greater than 250 IU/mL; aHR, 0.46 for greater than 1,000 IU/mL). Having higher levels of CMV viremia was associated with an increased risk of non-relapse mortality.
Potential drivers of outcome
Dr. McDonald said this study’s design makes it difficult to determine the causes of improved outcomes in the 2013-2017 period. However, the researchers do have theories about which practice changes may have contributed to better allo-HCT outcomes.
Dr. McDonald said the decrease in GVHD over time was “likely owing to the introduction of newer preventive strategies and immune-suppressive drugs.”
The decrease in nonrelapse mortality may have been driven, in part, by a reduction in fatal infections. Dr. McDonald said these infections were less frequent in the 2013-2017 period because of “molecular methods of diagnosis (especially for herpesviruses) and newer treatments (especially for fungal infections).”
“Another reason for a lower frequency of serious infection was a change in practice for treating graft-versus-host disease,” Dr. McDonald added. “Based on a randomized trial comparing lower- versus higher-dose prednisone for less-severe GVHD … both initial doses of prednisone and total prednisone exposure were reduced.”
Another factor that may have improved allo-HCT outcomes is the center’s change in approach to conditioning therapy over time.
“The gradual shift from very-high-dose conditioning therapy to less-intense myeloablative therapy and to reduced-intensity conditioning was likely responsible for a reduction in damage to the liver, lungs, and kidneys over the last 10 years,” Dr. McDonald said. “We were able to identify patients who were at especially high risk for mortality during a screening process before transplant ... thus allowing patients at highest risk to receive less intense conditioning therapy.”
Dr. McDonald added that this study’s results are encouraging, particularly the reduction in nonrelapse mortality. However, there is still room for improvement when it comes to relapse and progression.
This research was funded by the National Institutes of Health, the American Cancer Society, and the Patient-Centered Outcomes Research Institute. Dr. McDonald reported relationships with Sangamo Therapeutics, Soligenix Therapeutics, and Lucent Medical Systems. His coauthors disclosed relationships with a range of companies.
SOURCE: McDonald GB et al. Ann Intern Med. 2020 Jan 20. doi: 10.7326/M19-2936.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: At a single center, outcomes of allogeneic hematopoietic cell transplant improved for patients treated in 2013-2017, compared with patients treated in 2003-2007.
Major finding: Rates of nonrelapse mortality at day 200 were higher in the 2003-2007 group than in the 2013-2017 group – 16% and 11%, respectively (adjusted hazard ratio, 0.66; P = .008).
Study details: A single-center study of 1,148 patients treated in the 2003-2007 period and 1,131 patients treated in the 2013-2017 period.
Disclosures: The research was funded by the National Institutes of Health, the American Cancer Society, and the Patient-Centered Outcomes Research Institute. Dr. McDonald reported relationships with Sangamo Therapeutics, Soligenix Therapeutics, and Lucent Medical Systems. His coauthors disclosed relationships with a range of companies.
Source: McDonald GB et al. Ann Intern Med. 2020 Jan 20. doi: 10.7326/M19-2936.
Cardiovascular risks associated with cannabis use
Researchers are recommending routine screening of marijuana use in cardiovascular care settings.
A review of current evidence suggests an association between marijuana use and adverse cardiovascular effects, as well as interactions between marijuana and cardiovascular medications.
Although more research is needed, the review authors suggested patients may benefit from marijuana screening and testing as well as discussions about the potential risks of marijuana use in the setting of cardiovascular disease.
Ersilia M. DeFilippis, MD, of Columbia University Irving Medical Center in New York and colleagues conducted this review, which was published in the Journal of the American College of Cardiology.
The authors noted that research on marijuana use and cardiovascular disease is limited. The different forms of cannabis and various routes of administration have made it difficult to draw concrete conclusions about marijuana products. Additionally, there have been no randomized, controlled trials of marijuana products in the United States because such trials are illegal; however, there are observational studies linking marijuana use and adverse cardiovascular effects.
Snapshot of available evidence
One study showed that smoking marijuana produces many of the same cardiotoxic chemicals produced by smoking tobacco (BMJ. 2003 May 3;326[7396]:942-3). Another study suggested marijuana smokers may have greater exposure to harmful chemicals (J Psychoactive Drugs. 1988 Jan-Mar;20[1]:43-6).
More specifically, a meta-analysis suggested that smoking marijuana was one of the top three triggers of myocardial infarction (Lancet. 2011 Feb 26;377[9767]:732-40). And in a systematic analysis, 28 of 33 studies linked marijuana use to an increased risk of acute coronary syndromes (Clin Toxicol [Phila]. 2019 Oct;57[10]:831-41).
Furthermore, a study of 2.5 million marijuana users showed that 3% experienced arrhythmias (Int J Cardiol. 2018 Aug 1;264:91-2). A population survey showed that people who smoked marijuana in the past year experienced a 3.3-fold higher rate of cerebrovascular events (Aust N Z J Public Health. 2016 Jun;40[3]:226-30).
Studies have also indicated that cannabinoids can affect cardiovascular medications, including antiarrhythmics, calcium-channel blockers, isosorbide dinitrate/mononitrate, statins, beta-blockers, warfarin, theophylline, and nonsteroidal anti-inflammatory drugs (Medicines [Basel]. 2018 Dec 23;6[1] pii: E3; Curr Top Behav Neurosci. 2017;32:249-62; Pharmacogenet Genomics. 2009 Jul;19[7]:559-62; Ann Pharmacother. 2009 Jul;43[7]:1347-53; Pharmacol Ther. 2019 Sep;201:25-38).
Reviewer recommendations
Cardiovascular specialists should be informed about regulations governing marijuana products, as well as “potential health consequences of marijuana and its derivatives,” according to Dr. DeFilippis and colleagues.
The authors recommend routinely screening patients for marijuana use, perhaps using the Daily Sessions, Frequency, Age of Onset, and Quantity of Cannabis Use Inventory (PLoS One. 2017 May 26;12[5]:e0178194) or the Cannabis Abuse Screening Test (Int J Methods Psychiatr Res. 2018 Jun;27[2]:e1597).
The authors say urine toxicology “may be reasonable” for patients with myocardial infarction or new-onset heart failure. Such testing is required for patients undergoing a heart transplant because marijuana use may affect their candidacy.
Dr. DeFilippis and colleagues say cardiovascular specialists should inform patients about the risks associated with marijuana use. The authors recommend shared decision making for patients who use marijuana for symptom management or palliative purposes.
Three review authors disclosed relationships with many different pharmaceutical companies. One author disclosed relationships with Medscape Cardiology and WebMD, which are owned by the same parent company as MDedge.
SOURCE: J Am Coll Cardiol. 2020 Jan 20. doi: 10.1016/j.jacc.2019.11.025.
Researchers are recommending routine screening of marijuana use in cardiovascular care settings.
A review of current evidence suggests an association between marijuana use and adverse cardiovascular effects, as well as interactions between marijuana and cardiovascular medications.
Although more research is needed, the review authors suggested patients may benefit from marijuana screening and testing as well as discussions about the potential risks of marijuana use in the setting of cardiovascular disease.
Ersilia M. DeFilippis, MD, of Columbia University Irving Medical Center in New York and colleagues conducted this review, which was published in the Journal of the American College of Cardiology.
The authors noted that research on marijuana use and cardiovascular disease is limited. The different forms of cannabis and various routes of administration have made it difficult to draw concrete conclusions about marijuana products. Additionally, there have been no randomized, controlled trials of marijuana products in the United States because such trials are illegal; however, there are observational studies linking marijuana use and adverse cardiovascular effects.
Snapshot of available evidence
One study showed that smoking marijuana produces many of the same cardiotoxic chemicals produced by smoking tobacco (BMJ. 2003 May 3;326[7396]:942-3). Another study suggested marijuana smokers may have greater exposure to harmful chemicals (J Psychoactive Drugs. 1988 Jan-Mar;20[1]:43-6).
More specifically, a meta-analysis suggested that smoking marijuana was one of the top three triggers of myocardial infarction (Lancet. 2011 Feb 26;377[9767]:732-40). And in a systematic analysis, 28 of 33 studies linked marijuana use to an increased risk of acute coronary syndromes (Clin Toxicol [Phila]. 2019 Oct;57[10]:831-41).
Furthermore, a study of 2.5 million marijuana users showed that 3% experienced arrhythmias (Int J Cardiol. 2018 Aug 1;264:91-2). A population survey showed that people who smoked marijuana in the past year experienced a 3.3-fold higher rate of cerebrovascular events (Aust N Z J Public Health. 2016 Jun;40[3]:226-30).
Studies have also indicated that cannabinoids can affect cardiovascular medications, including antiarrhythmics, calcium-channel blockers, isosorbide dinitrate/mononitrate, statins, beta-blockers, warfarin, theophylline, and nonsteroidal anti-inflammatory drugs (Medicines [Basel]. 2018 Dec 23;6[1] pii: E3; Curr Top Behav Neurosci. 2017;32:249-62; Pharmacogenet Genomics. 2009 Jul;19[7]:559-62; Ann Pharmacother. 2009 Jul;43[7]:1347-53; Pharmacol Ther. 2019 Sep;201:25-38).
Reviewer recommendations
Cardiovascular specialists should be informed about regulations governing marijuana products, as well as “potential health consequences of marijuana and its derivatives,” according to Dr. DeFilippis and colleagues.
The authors recommend routinely screening patients for marijuana use, perhaps using the Daily Sessions, Frequency, Age of Onset, and Quantity of Cannabis Use Inventory (PLoS One. 2017 May 26;12[5]:e0178194) or the Cannabis Abuse Screening Test (Int J Methods Psychiatr Res. 2018 Jun;27[2]:e1597).
The authors say urine toxicology “may be reasonable” for patients with myocardial infarction or new-onset heart failure. Such testing is required for patients undergoing a heart transplant because marijuana use may affect their candidacy.
Dr. DeFilippis and colleagues say cardiovascular specialists should inform patients about the risks associated with marijuana use. The authors recommend shared decision making for patients who use marijuana for symptom management or palliative purposes.
Three review authors disclosed relationships with many different pharmaceutical companies. One author disclosed relationships with Medscape Cardiology and WebMD, which are owned by the same parent company as MDedge.
SOURCE: J Am Coll Cardiol. 2020 Jan 20. doi: 10.1016/j.jacc.2019.11.025.
Researchers are recommending routine screening of marijuana use in cardiovascular care settings.
A review of current evidence suggests an association between marijuana use and adverse cardiovascular effects, as well as interactions between marijuana and cardiovascular medications.
Although more research is needed, the review authors suggested patients may benefit from marijuana screening and testing as well as discussions about the potential risks of marijuana use in the setting of cardiovascular disease.
Ersilia M. DeFilippis, MD, of Columbia University Irving Medical Center in New York and colleagues conducted this review, which was published in the Journal of the American College of Cardiology.
The authors noted that research on marijuana use and cardiovascular disease is limited. The different forms of cannabis and various routes of administration have made it difficult to draw concrete conclusions about marijuana products. Additionally, there have been no randomized, controlled trials of marijuana products in the United States because such trials are illegal; however, there are observational studies linking marijuana use and adverse cardiovascular effects.
Snapshot of available evidence
One study showed that smoking marijuana produces many of the same cardiotoxic chemicals produced by smoking tobacco (BMJ. 2003 May 3;326[7396]:942-3). Another study suggested marijuana smokers may have greater exposure to harmful chemicals (J Psychoactive Drugs. 1988 Jan-Mar;20[1]:43-6).
More specifically, a meta-analysis suggested that smoking marijuana was one of the top three triggers of myocardial infarction (Lancet. 2011 Feb 26;377[9767]:732-40). And in a systematic analysis, 28 of 33 studies linked marijuana use to an increased risk of acute coronary syndromes (Clin Toxicol [Phila]. 2019 Oct;57[10]:831-41).
Furthermore, a study of 2.5 million marijuana users showed that 3% experienced arrhythmias (Int J Cardiol. 2018 Aug 1;264:91-2). A population survey showed that people who smoked marijuana in the past year experienced a 3.3-fold higher rate of cerebrovascular events (Aust N Z J Public Health. 2016 Jun;40[3]:226-30).
Studies have also indicated that cannabinoids can affect cardiovascular medications, including antiarrhythmics, calcium-channel blockers, isosorbide dinitrate/mononitrate, statins, beta-blockers, warfarin, theophylline, and nonsteroidal anti-inflammatory drugs (Medicines [Basel]. 2018 Dec 23;6[1] pii: E3; Curr Top Behav Neurosci. 2017;32:249-62; Pharmacogenet Genomics. 2009 Jul;19[7]:559-62; Ann Pharmacother. 2009 Jul;43[7]:1347-53; Pharmacol Ther. 2019 Sep;201:25-38).
Reviewer recommendations
Cardiovascular specialists should be informed about regulations governing marijuana products, as well as “potential health consequences of marijuana and its derivatives,” according to Dr. DeFilippis and colleagues.
The authors recommend routinely screening patients for marijuana use, perhaps using the Daily Sessions, Frequency, Age of Onset, and Quantity of Cannabis Use Inventory (PLoS One. 2017 May 26;12[5]:e0178194) or the Cannabis Abuse Screening Test (Int J Methods Psychiatr Res. 2018 Jun;27[2]:e1597).
The authors say urine toxicology “may be reasonable” for patients with myocardial infarction or new-onset heart failure. Such testing is required for patients undergoing a heart transplant because marijuana use may affect their candidacy.
Dr. DeFilippis and colleagues say cardiovascular specialists should inform patients about the risks associated with marijuana use. The authors recommend shared decision making for patients who use marijuana for symptom management or palliative purposes.
Three review authors disclosed relationships with many different pharmaceutical companies. One author disclosed relationships with Medscape Cardiology and WebMD, which are owned by the same parent company as MDedge.
SOURCE: J Am Coll Cardiol. 2020 Jan 20. doi: 10.1016/j.jacc.2019.11.025.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Telehealth appears to help speed front end of liver transplant process
The incorporation of telehealth in the liver transplantation process is demonstrating the potential to expedite the evaluation of patients and get them listed on the transplant wait list.
New research shows “a ,” Binu V. John, MD, of McGuire VA Medical Center, Richmond, and colleagues wrote in a report published in Clinical Gastroenterology and Hepatology (2019 Dec 27. doi: 10.1016/j.cgh.2019.12.021).
Researchers looked at 465 patients who had evaluations for liver transplants at the Richmond Veterans Affairs Medical Center from 2005 through 2017. Nearly half (232 patients) were evaluated via telehealth, with the remaining 233 evaluated with traditional in-person evaluations.
“Patients in the telehealth group were evaluated significantly faster than patients in the usual care group (22 vs. 54 days, P less than .001),” Dr. John and colleagues wrote, adding that, after conducting a propensity-matched analysis, “telehealth was associated with an 85% reduction in time from referral to evaluation.”
Additionally, patients “who underwent the initial evaluation by telehealth were listed significantly earlier than the usual care group (95 vs. 149 days; P less than .001),” the authors stated, adding that “telehealth was associated with a 74% reduction in time to listing” after conducting a propensity-matched analysis.
However, while speeding up time to referral and listing, “the median time to transplant was not significantly different between the two groups on unadjusted (218 vs. 244 days; P = .084) or adjusted analysis (325 vs. 409 days; P = .08),” they added.
Additionally, “there was no difference in pretransplant mortality between [those] evaluated by telehealth or usual care in unadjusted analysis,” Dr. John and colleagues observed, noting that 169 of 465 patients (51 on the waiting list for a transplant and 118 who were not listed) who were referred died without receiving a liver transplant.
Researchers suggested that while evaluation times may have been shorter with the use of telehealth, they did not translate to shorter transplantation times “likely because the latter is a complex metric that is driven primarily by organ availability.”
Dr. John and colleagues cautioned that the centralized nature of the VA medical system could make the results of this study not generalizable across private care settings, particularly when care needs to cross state lines, which does not present an issue within the VA medical system.
That being said, the “ability to successfully evaluate and list patients via telehealth and obtain the same outcomes in terms of time to transplant and pretransplant mortality is significant because of the numerous advantages that telehealth offers to improve overall access to transplantation,” they stated, adding that more studies are needed, both in and out of the VA system, “to confirm that telehealth is a safe and effective way to expand access for patients undergoing evaluation for liver transplantation.”
Lead author Dr. Binu John serves on medical advisory boards for Gilead and Eisai and received research funding from a number of pharmaceutical manufacturers. No conflicts of interest were reported by the other authors.
SOURCE: John BV et al. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2019.12.021.
The incorporation of telehealth in the liver transplantation process is demonstrating the potential to expedite the evaluation of patients and get them listed on the transplant wait list.
New research shows “a ,” Binu V. John, MD, of McGuire VA Medical Center, Richmond, and colleagues wrote in a report published in Clinical Gastroenterology and Hepatology (2019 Dec 27. doi: 10.1016/j.cgh.2019.12.021).
Researchers looked at 465 patients who had evaluations for liver transplants at the Richmond Veterans Affairs Medical Center from 2005 through 2017. Nearly half (232 patients) were evaluated via telehealth, with the remaining 233 evaluated with traditional in-person evaluations.
“Patients in the telehealth group were evaluated significantly faster than patients in the usual care group (22 vs. 54 days, P less than .001),” Dr. John and colleagues wrote, adding that, after conducting a propensity-matched analysis, “telehealth was associated with an 85% reduction in time from referral to evaluation.”
Additionally, patients “who underwent the initial evaluation by telehealth were listed significantly earlier than the usual care group (95 vs. 149 days; P less than .001),” the authors stated, adding that “telehealth was associated with a 74% reduction in time to listing” after conducting a propensity-matched analysis.
However, while speeding up time to referral and listing, “the median time to transplant was not significantly different between the two groups on unadjusted (218 vs. 244 days; P = .084) or adjusted analysis (325 vs. 409 days; P = .08),” they added.
Additionally, “there was no difference in pretransplant mortality between [those] evaluated by telehealth or usual care in unadjusted analysis,” Dr. John and colleagues observed, noting that 169 of 465 patients (51 on the waiting list for a transplant and 118 who were not listed) who were referred died without receiving a liver transplant.
Researchers suggested that while evaluation times may have been shorter with the use of telehealth, they did not translate to shorter transplantation times “likely because the latter is a complex metric that is driven primarily by organ availability.”
Dr. John and colleagues cautioned that the centralized nature of the VA medical system could make the results of this study not generalizable across private care settings, particularly when care needs to cross state lines, which does not present an issue within the VA medical system.
That being said, the “ability to successfully evaluate and list patients via telehealth and obtain the same outcomes in terms of time to transplant and pretransplant mortality is significant because of the numerous advantages that telehealth offers to improve overall access to transplantation,” they stated, adding that more studies are needed, both in and out of the VA system, “to confirm that telehealth is a safe and effective way to expand access for patients undergoing evaluation for liver transplantation.”
Lead author Dr. Binu John serves on medical advisory boards for Gilead and Eisai and received research funding from a number of pharmaceutical manufacturers. No conflicts of interest were reported by the other authors.
SOURCE: John BV et al. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2019.12.021.
The incorporation of telehealth in the liver transplantation process is demonstrating the potential to expedite the evaluation of patients and get them listed on the transplant wait list.
New research shows “a ,” Binu V. John, MD, of McGuire VA Medical Center, Richmond, and colleagues wrote in a report published in Clinical Gastroenterology and Hepatology (2019 Dec 27. doi: 10.1016/j.cgh.2019.12.021).
Researchers looked at 465 patients who had evaluations for liver transplants at the Richmond Veterans Affairs Medical Center from 2005 through 2017. Nearly half (232 patients) were evaluated via telehealth, with the remaining 233 evaluated with traditional in-person evaluations.
“Patients in the telehealth group were evaluated significantly faster than patients in the usual care group (22 vs. 54 days, P less than .001),” Dr. John and colleagues wrote, adding that, after conducting a propensity-matched analysis, “telehealth was associated with an 85% reduction in time from referral to evaluation.”
Additionally, patients “who underwent the initial evaluation by telehealth were listed significantly earlier than the usual care group (95 vs. 149 days; P less than .001),” the authors stated, adding that “telehealth was associated with a 74% reduction in time to listing” after conducting a propensity-matched analysis.
However, while speeding up time to referral and listing, “the median time to transplant was not significantly different between the two groups on unadjusted (218 vs. 244 days; P = .084) or adjusted analysis (325 vs. 409 days; P = .08),” they added.
Additionally, “there was no difference in pretransplant mortality between [those] evaluated by telehealth or usual care in unadjusted analysis,” Dr. John and colleagues observed, noting that 169 of 465 patients (51 on the waiting list for a transplant and 118 who were not listed) who were referred died without receiving a liver transplant.
Researchers suggested that while evaluation times may have been shorter with the use of telehealth, they did not translate to shorter transplantation times “likely because the latter is a complex metric that is driven primarily by organ availability.”
Dr. John and colleagues cautioned that the centralized nature of the VA medical system could make the results of this study not generalizable across private care settings, particularly when care needs to cross state lines, which does not present an issue within the VA medical system.
That being said, the “ability to successfully evaluate and list patients via telehealth and obtain the same outcomes in terms of time to transplant and pretransplant mortality is significant because of the numerous advantages that telehealth offers to improve overall access to transplantation,” they stated, adding that more studies are needed, both in and out of the VA system, “to confirm that telehealth is a safe and effective way to expand access for patients undergoing evaluation for liver transplantation.”
Lead author Dr. Binu John serves on medical advisory boards for Gilead and Eisai and received research funding from a number of pharmaceutical manufacturers. No conflicts of interest were reported by the other authors.
SOURCE: John BV et al. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2019.12.021.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Travelers to three U.S. airports to be screened for novel coronavirus
according to an announcement from the Centers for Disease Control and Prevention.
Starting today, Jan. 17, 2020, people traveling from Wuhan to New York (JFK), San Francisco (SFO), and Los Angeles (LAX) airports will be screened for symptoms associated with 2019-nCoV, which include fever, cough, and difficulty breathing.
“Based on the information that CDC has today, we believe the current risk for this virus to the general public is low,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a CDC telebriefing.
To date, 45 cases of 2019-nCoV have been reported in Wuhan, according to the CDC. The Wuhan Municipal Health Commission said 15 patients have been cured and discharged, 5 severe cases are still being treated, and 2 patients have died. Both deaths occurred in older patients, one of whom was aged 69 years and one aged 61 years. One of the patients was known to have underlying health conditions.
Three cases of 2019-nCoV have been confirmed outside of Wuhan, one in Japan and two in Thailand. All three were travelers from Wuhan.
The virus is believed to have originated at Wuhan South China Seafood City, a market that sold seafood, chickens, bats, cats, marmots, and other wild animals. (The market has since been closed and disinfected.) The origin suggests animal-to-human transmission of 2019-nCoV, but it appears that human-to-human transmission can occur as well.
“While most of these infections seem to be happening from animals to people, there is some indication that limited person-to-person spread is happening,” Dr. Messonnier said.
Because of this potential risk, the CDC is working with the Department of Homeland Security’s Customs and Border Protection to screen travelers from Wuhan to the United States. The CDC is deploying about 100 additional staff to JFK, SFO, and LAX, where direct flights (JFK and SFO) or connecting flights (LAX) from Wuhan land.
The CDC could not confirm if exit screening is planned for people traveling abroad from Wuhan.
At the U.S. airports, travelers from Wuhan will be given a questionnaire asking about symptoms of 2019-nCoV (fever, cough, and difficulty breathing). People who exhibit symptoms will be assessed and questioned further. If they are believed to have 2019-nCoV, they will be sent to designated hospitals, where they will be examined, and samples will be collected.
Samples from patients with suspected 2019-nCoV will be sent to the CDC for analysis. Chinese health authorities made the full genome of 2019-nCoV publicly available, which will allow the CDC to confirm any cases that may arise in the United States. The CDC is currently working on a test to detect 2019-nCoV, which can be distributed to state health departments.
Earlier this month, the CDC issued a Level 1 Travel Health Notice for travelers to Wuhan and a Health Alert on 2019-nCoV. The latest information on 2019-nCoV can be found on the CDC’s Novel Coronavirus 2019 webpage.
according to an announcement from the Centers for Disease Control and Prevention.
Starting today, Jan. 17, 2020, people traveling from Wuhan to New York (JFK), San Francisco (SFO), and Los Angeles (LAX) airports will be screened for symptoms associated with 2019-nCoV, which include fever, cough, and difficulty breathing.
“Based on the information that CDC has today, we believe the current risk for this virus to the general public is low,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a CDC telebriefing.
To date, 45 cases of 2019-nCoV have been reported in Wuhan, according to the CDC. The Wuhan Municipal Health Commission said 15 patients have been cured and discharged, 5 severe cases are still being treated, and 2 patients have died. Both deaths occurred in older patients, one of whom was aged 69 years and one aged 61 years. One of the patients was known to have underlying health conditions.
Three cases of 2019-nCoV have been confirmed outside of Wuhan, one in Japan and two in Thailand. All three were travelers from Wuhan.
The virus is believed to have originated at Wuhan South China Seafood City, a market that sold seafood, chickens, bats, cats, marmots, and other wild animals. (The market has since been closed and disinfected.) The origin suggests animal-to-human transmission of 2019-nCoV, but it appears that human-to-human transmission can occur as well.
“While most of these infections seem to be happening from animals to people, there is some indication that limited person-to-person spread is happening,” Dr. Messonnier said.
Because of this potential risk, the CDC is working with the Department of Homeland Security’s Customs and Border Protection to screen travelers from Wuhan to the United States. The CDC is deploying about 100 additional staff to JFK, SFO, and LAX, where direct flights (JFK and SFO) or connecting flights (LAX) from Wuhan land.
The CDC could not confirm if exit screening is planned for people traveling abroad from Wuhan.
At the U.S. airports, travelers from Wuhan will be given a questionnaire asking about symptoms of 2019-nCoV (fever, cough, and difficulty breathing). People who exhibit symptoms will be assessed and questioned further. If they are believed to have 2019-nCoV, they will be sent to designated hospitals, where they will be examined, and samples will be collected.
Samples from patients with suspected 2019-nCoV will be sent to the CDC for analysis. Chinese health authorities made the full genome of 2019-nCoV publicly available, which will allow the CDC to confirm any cases that may arise in the United States. The CDC is currently working on a test to detect 2019-nCoV, which can be distributed to state health departments.
Earlier this month, the CDC issued a Level 1 Travel Health Notice for travelers to Wuhan and a Health Alert on 2019-nCoV. The latest information on 2019-nCoV can be found on the CDC’s Novel Coronavirus 2019 webpage.
according to an announcement from the Centers for Disease Control and Prevention.
Starting today, Jan. 17, 2020, people traveling from Wuhan to New York (JFK), San Francisco (SFO), and Los Angeles (LAX) airports will be screened for symptoms associated with 2019-nCoV, which include fever, cough, and difficulty breathing.
“Based on the information that CDC has today, we believe the current risk for this virus to the general public is low,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a CDC telebriefing.
To date, 45 cases of 2019-nCoV have been reported in Wuhan, according to the CDC. The Wuhan Municipal Health Commission said 15 patients have been cured and discharged, 5 severe cases are still being treated, and 2 patients have died. Both deaths occurred in older patients, one of whom was aged 69 years and one aged 61 years. One of the patients was known to have underlying health conditions.
Three cases of 2019-nCoV have been confirmed outside of Wuhan, one in Japan and two in Thailand. All three were travelers from Wuhan.
The virus is believed to have originated at Wuhan South China Seafood City, a market that sold seafood, chickens, bats, cats, marmots, and other wild animals. (The market has since been closed and disinfected.) The origin suggests animal-to-human transmission of 2019-nCoV, but it appears that human-to-human transmission can occur as well.
“While most of these infections seem to be happening from animals to people, there is some indication that limited person-to-person spread is happening,” Dr. Messonnier said.
Because of this potential risk, the CDC is working with the Department of Homeland Security’s Customs and Border Protection to screen travelers from Wuhan to the United States. The CDC is deploying about 100 additional staff to JFK, SFO, and LAX, where direct flights (JFK and SFO) or connecting flights (LAX) from Wuhan land.
The CDC could not confirm if exit screening is planned for people traveling abroad from Wuhan.
At the U.S. airports, travelers from Wuhan will be given a questionnaire asking about symptoms of 2019-nCoV (fever, cough, and difficulty breathing). People who exhibit symptoms will be assessed and questioned further. If they are believed to have 2019-nCoV, they will be sent to designated hospitals, where they will be examined, and samples will be collected.
Samples from patients with suspected 2019-nCoV will be sent to the CDC for analysis. Chinese health authorities made the full genome of 2019-nCoV publicly available, which will allow the CDC to confirm any cases that may arise in the United States. The CDC is currently working on a test to detect 2019-nCoV, which can be distributed to state health departments.
Earlier this month, the CDC issued a Level 1 Travel Health Notice for travelers to Wuhan and a Health Alert on 2019-nCoV. The latest information on 2019-nCoV can be found on the CDC’s Novel Coronavirus 2019 webpage.
European marketing of Picato suspended while skin cancer risk reviewed
As a precaution, the European Medicines Agency (EMA) has recommended that patients stop using ingenol mebutate (Picato) while the agency continues to review the safety of the topical treatment, which is indicated for the treatment of actinic keratosis in Europe and the United States.
No such action has been taken in the United States.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) is reviewing data on skin cancer in patients treated with ingenol mebutate. In a trial comparing Picato and imiquimod, skin cancer was more common in the areas treated with Picato than in areas treated with imiquimod, the statement said.
“While uncertainties remain, the EMA said in a Jan. 17 news release. “The PRAC has therefore recommended suspending the medicine’s marketing authorization as a precaution and noted that alternative treatments are available.”
FDA is looking at the situation
LEO Pharma, the company that markets Picato, announced on Jan. 9 that it was initiating voluntary withdrawal of marketing authorization and possible voluntary withdrawal of Picato in the European Union (EU) and European Economic Area (EEA). The statement says, however, that “LEO Pharma has carefully reviewed the information received from PRAC, and the company disagrees with the ongoing assessment of PRAC.” There are “no additional safety data and it is LEO Pharma’s position that there is no evidence of a causal relationship or plausible mechanism hypothesis between the use of Picato and the development of skin malignancies.” An update added to the press release on Jan. 17 restates that the company disagrees with the assessment of PRAC.
“This matter does not affect Picato in the U.S., and there are no new developments in the [United States]. Picato continues to be available to patients in the U.S. We remain in dialogue with the U.S. Food and Drug Administration about Picato in the EU/EEA,” Rhonda Sciarra, associate director of global external communications for LEO Pharma, said in an email. “We remain committed to ensuring patient safety, rigorous pharmacovigilance monitoring, and transparency,” she added.
The FDA “is gathering data and information to investigate the safety concern related to Picato,” a spokesperson for the FDA told Dermatology News. “We are committed to sharing relevant findings when we have sufficient understanding of the situation and of what actions should be taken,” he added.
Examining the data
The EMA announcement described data about the risk of skin cancer in studies of Picato. A 3-year study in 484 patients found a higher incidence of skin malignancy with ingenol mebutate than with the comparator, imiquimod. In all, 3.3% of patients developed cancer in the ingenol mebutate group, compared with 0.4% in the comparator group.
In an 8-week vehicle-controlled trial in 1,262 patients, there were more skin tumors in patients who received ingenol mebutate than in those in the vehicle arm (1.0% vs. 0.1%).
In addition, according to the EMA statement, in four trials of a related ester that included 1,234 patients, a higher incidence of skin tumors occurred with the related drug, ingenol disoxate, than with a vehicle control (7.7% vs. 2.9%). PRAC considered these data because ingenol disoxate and ingenol mebutate are closely related, the EMA said.
“Health care professionals should stop prescribing Picato and consider different treatment options while authorities review the data,” according to the European agency. “Health care professionals should advise patients to be vigilant for any skin lesions developing and to seek medical advice promptly should any occur,” the statement adds.
Picato has been authorized in the EU since 2012, and the FDA approved Picato the same year. Patients have received about 2.8 million treatment courses in that time, according to the LEO Pharma press release.
As a precaution, the European Medicines Agency (EMA) has recommended that patients stop using ingenol mebutate (Picato) while the agency continues to review the safety of the topical treatment, which is indicated for the treatment of actinic keratosis in Europe and the United States.
No such action has been taken in the United States.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) is reviewing data on skin cancer in patients treated with ingenol mebutate. In a trial comparing Picato and imiquimod, skin cancer was more common in the areas treated with Picato than in areas treated with imiquimod, the statement said.
“While uncertainties remain, the EMA said in a Jan. 17 news release. “The PRAC has therefore recommended suspending the medicine’s marketing authorization as a precaution and noted that alternative treatments are available.”
FDA is looking at the situation
LEO Pharma, the company that markets Picato, announced on Jan. 9 that it was initiating voluntary withdrawal of marketing authorization and possible voluntary withdrawal of Picato in the European Union (EU) and European Economic Area (EEA). The statement says, however, that “LEO Pharma has carefully reviewed the information received from PRAC, and the company disagrees with the ongoing assessment of PRAC.” There are “no additional safety data and it is LEO Pharma’s position that there is no evidence of a causal relationship or plausible mechanism hypothesis between the use of Picato and the development of skin malignancies.” An update added to the press release on Jan. 17 restates that the company disagrees with the assessment of PRAC.
“This matter does not affect Picato in the U.S., and there are no new developments in the [United States]. Picato continues to be available to patients in the U.S. We remain in dialogue with the U.S. Food and Drug Administration about Picato in the EU/EEA,” Rhonda Sciarra, associate director of global external communications for LEO Pharma, said in an email. “We remain committed to ensuring patient safety, rigorous pharmacovigilance monitoring, and transparency,” she added.
The FDA “is gathering data and information to investigate the safety concern related to Picato,” a spokesperson for the FDA told Dermatology News. “We are committed to sharing relevant findings when we have sufficient understanding of the situation and of what actions should be taken,” he added.
Examining the data
The EMA announcement described data about the risk of skin cancer in studies of Picato. A 3-year study in 484 patients found a higher incidence of skin malignancy with ingenol mebutate than with the comparator, imiquimod. In all, 3.3% of patients developed cancer in the ingenol mebutate group, compared with 0.4% in the comparator group.
In an 8-week vehicle-controlled trial in 1,262 patients, there were more skin tumors in patients who received ingenol mebutate than in those in the vehicle arm (1.0% vs. 0.1%).
In addition, according to the EMA statement, in four trials of a related ester that included 1,234 patients, a higher incidence of skin tumors occurred with the related drug, ingenol disoxate, than with a vehicle control (7.7% vs. 2.9%). PRAC considered these data because ingenol disoxate and ingenol mebutate are closely related, the EMA said.
“Health care professionals should stop prescribing Picato and consider different treatment options while authorities review the data,” according to the European agency. “Health care professionals should advise patients to be vigilant for any skin lesions developing and to seek medical advice promptly should any occur,” the statement adds.
Picato has been authorized in the EU since 2012, and the FDA approved Picato the same year. Patients have received about 2.8 million treatment courses in that time, according to the LEO Pharma press release.
As a precaution, the European Medicines Agency (EMA) has recommended that patients stop using ingenol mebutate (Picato) while the agency continues to review the safety of the topical treatment, which is indicated for the treatment of actinic keratosis in Europe and the United States.
No such action has been taken in the United States.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) is reviewing data on skin cancer in patients treated with ingenol mebutate. In a trial comparing Picato and imiquimod, skin cancer was more common in the areas treated with Picato than in areas treated with imiquimod, the statement said.
“While uncertainties remain, the EMA said in a Jan. 17 news release. “The PRAC has therefore recommended suspending the medicine’s marketing authorization as a precaution and noted that alternative treatments are available.”
FDA is looking at the situation
LEO Pharma, the company that markets Picato, announced on Jan. 9 that it was initiating voluntary withdrawal of marketing authorization and possible voluntary withdrawal of Picato in the European Union (EU) and European Economic Area (EEA). The statement says, however, that “LEO Pharma has carefully reviewed the information received from PRAC, and the company disagrees with the ongoing assessment of PRAC.” There are “no additional safety data and it is LEO Pharma’s position that there is no evidence of a causal relationship or plausible mechanism hypothesis between the use of Picato and the development of skin malignancies.” An update added to the press release on Jan. 17 restates that the company disagrees with the assessment of PRAC.
“This matter does not affect Picato in the U.S., and there are no new developments in the [United States]. Picato continues to be available to patients in the U.S. We remain in dialogue with the U.S. Food and Drug Administration about Picato in the EU/EEA,” Rhonda Sciarra, associate director of global external communications for LEO Pharma, said in an email. “We remain committed to ensuring patient safety, rigorous pharmacovigilance monitoring, and transparency,” she added.
The FDA “is gathering data and information to investigate the safety concern related to Picato,” a spokesperson for the FDA told Dermatology News. “We are committed to sharing relevant findings when we have sufficient understanding of the situation and of what actions should be taken,” he added.
Examining the data
The EMA announcement described data about the risk of skin cancer in studies of Picato. A 3-year study in 484 patients found a higher incidence of skin malignancy with ingenol mebutate than with the comparator, imiquimod. In all, 3.3% of patients developed cancer in the ingenol mebutate group, compared with 0.4% in the comparator group.
In an 8-week vehicle-controlled trial in 1,262 patients, there were more skin tumors in patients who received ingenol mebutate than in those in the vehicle arm (1.0% vs. 0.1%).
In addition, according to the EMA statement, in four trials of a related ester that included 1,234 patients, a higher incidence of skin tumors occurred with the related drug, ingenol disoxate, than with a vehicle control (7.7% vs. 2.9%). PRAC considered these data because ingenol disoxate and ingenol mebutate are closely related, the EMA said.
“Health care professionals should stop prescribing Picato and consider different treatment options while authorities review the data,” according to the European agency. “Health care professionals should advise patients to be vigilant for any skin lesions developing and to seek medical advice promptly should any occur,” the statement adds.
Picato has been authorized in the EU since 2012, and the FDA approved Picato the same year. Patients have received about 2.8 million treatment courses in that time, according to the LEO Pharma press release.
Flu activity declines for second straight week
Flu activity dropped nationally for a second consecutive week, but the changing predominance in type from influenza B to A suggests that “it is too early to know whether the season has peaked,” the Centers for Disease Control and Prevention said Jan. 17.
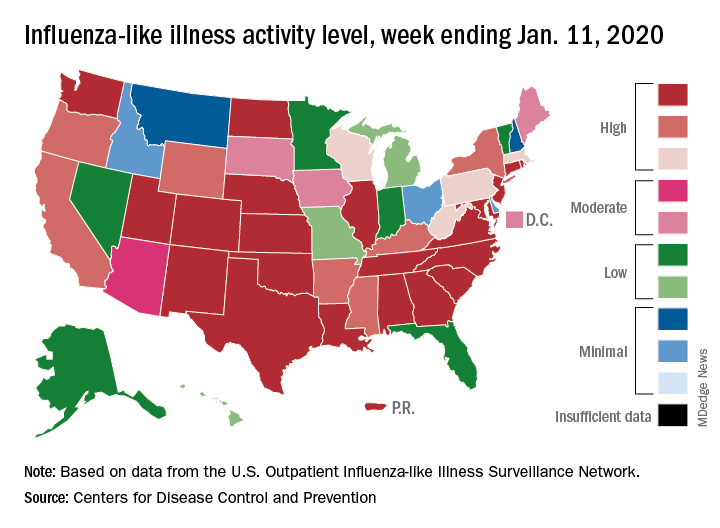
Patients with influenza-like illness (ILI) dropped from 5.7% to 4.7% of all visits to outpatient providers for the week ending Jan. 11, and the proportion of respiratory specimens positive for influenza decreased from 23.6% the week before to 22.9%, the CDC’s influenza division reported.
Despite that overall drop in positive specimens, however, “the percent positive for influenza A viruses increased and some regions are seeing increases in the proportion of influenza A(H1N1)pdm09 viruses compared to other influenza viruses,” the influenza division noted.
Outpatient activity on the state level also was down for the week. There were 23 jurisdictions – 21 states, New York City, and Puerto Rico – at level 10 on the CDC’s 1-10 scale of ILI activity for the week ending Jan. 11, compared with 33 the previous week, data from the CDC’s Outpatient Influenza-like Illness Surveillance Network show.
Indicators of ILI severity have not risen to high levels. “The percentage of deaths attributed to pneumonia and influenza increased from 6.0% to 6.9% but remains below the epidemic threshold” of 7.0% for the week, and the hospitalization rate remains at a fairly typical level for this time of year, the influenza division said.
For the week ending Jan. 11, 7 new ILI-related pediatric deaths were reported, which brings the total to 39 for the 2019-2020 season. Children aged 0-4 years are the second-most likely age group to be hospitalized with the flu (34.4/100,000 population) after adults aged 65 years and older, who have a cumulative rate of 47.6/100,000 for the season, the CDC reported.
Flu activity dropped nationally for a second consecutive week, but the changing predominance in type from influenza B to A suggests that “it is too early to know whether the season has peaked,” the Centers for Disease Control and Prevention said Jan. 17.

Patients with influenza-like illness (ILI) dropped from 5.7% to 4.7% of all visits to outpatient providers for the week ending Jan. 11, and the proportion of respiratory specimens positive for influenza decreased from 23.6% the week before to 22.9%, the CDC’s influenza division reported.
Despite that overall drop in positive specimens, however, “the percent positive for influenza A viruses increased and some regions are seeing increases in the proportion of influenza A(H1N1)pdm09 viruses compared to other influenza viruses,” the influenza division noted.
Outpatient activity on the state level also was down for the week. There were 23 jurisdictions – 21 states, New York City, and Puerto Rico – at level 10 on the CDC’s 1-10 scale of ILI activity for the week ending Jan. 11, compared with 33 the previous week, data from the CDC’s Outpatient Influenza-like Illness Surveillance Network show.
Indicators of ILI severity have not risen to high levels. “The percentage of deaths attributed to pneumonia and influenza increased from 6.0% to 6.9% but remains below the epidemic threshold” of 7.0% for the week, and the hospitalization rate remains at a fairly typical level for this time of year, the influenza division said.
For the week ending Jan. 11, 7 new ILI-related pediatric deaths were reported, which brings the total to 39 for the 2019-2020 season. Children aged 0-4 years are the second-most likely age group to be hospitalized with the flu (34.4/100,000 population) after adults aged 65 years and older, who have a cumulative rate of 47.6/100,000 for the season, the CDC reported.
Flu activity dropped nationally for a second consecutive week, but the changing predominance in type from influenza B to A suggests that “it is too early to know whether the season has peaked,” the Centers for Disease Control and Prevention said Jan. 17.

Patients with influenza-like illness (ILI) dropped from 5.7% to 4.7% of all visits to outpatient providers for the week ending Jan. 11, and the proportion of respiratory specimens positive for influenza decreased from 23.6% the week before to 22.9%, the CDC’s influenza division reported.
Despite that overall drop in positive specimens, however, “the percent positive for influenza A viruses increased and some regions are seeing increases in the proportion of influenza A(H1N1)pdm09 viruses compared to other influenza viruses,” the influenza division noted.
Outpatient activity on the state level also was down for the week. There were 23 jurisdictions – 21 states, New York City, and Puerto Rico – at level 10 on the CDC’s 1-10 scale of ILI activity for the week ending Jan. 11, compared with 33 the previous week, data from the CDC’s Outpatient Influenza-like Illness Surveillance Network show.
Indicators of ILI severity have not risen to high levels. “The percentage of deaths attributed to pneumonia and influenza increased from 6.0% to 6.9% but remains below the epidemic threshold” of 7.0% for the week, and the hospitalization rate remains at a fairly typical level for this time of year, the influenza division said.
For the week ending Jan. 11, 7 new ILI-related pediatric deaths were reported, which brings the total to 39 for the 2019-2020 season. Children aged 0-4 years are the second-most likely age group to be hospitalized with the flu (34.4/100,000 population) after adults aged 65 years and older, who have a cumulative rate of 47.6/100,000 for the season, the CDC reported.
Value analysis of JAK inhibitors for RA hampered by limited data
Adequate evidence shows that adding a Janus kinase (JAK) inhibitor to conventional disease-modifying antirheumatic drug therapy provides a net health benefit for patients with rheumatoid arthritis, compared with conventional drugs alone, according to a report by an independent research institute. But the long-term economic value of JAK inhibitors for rheumatoid arthritis is less clear, the report by the Institute for Clinical and Economic Review (ICER) indicates.
ICER on Jan. 9 released a finalized report and policy recommendations on JAK inhibitors and biosimilars for rheumatoid arthritis. The report reviews current evidence for JAK inhibitors for adults with moderately active to severely active rheumatoid arthritis.
Since the nonprofit’s 2017 review of targeted immune modulators for rheumatoid arthritis, two JAK inhibitors, baricitinib (Olumiant) and upadacitinib (Rinvoq), were approved by the Food and Drug Administration. At a December 2019 public meeting of the California Technology Assessment Forum (CTAF), one of ICER’s independent evidence appraisal committees, panelists reviewed recent evidence.
A pricey comparator
In ICER’s analysis, the JAK inhibitor upadacitinib reached common thresholds for cost-effectiveness when compared with adalimumab (Humira). Nevertheless, the 14 members of the independent evidence appraisal committee voted that upadacitinib’s long-term economic value was “low” (8 votes) or “intermediate” (6 votes). Concerns about the generalizability of phase 3 clinical trial data to patients in the real world were among the reservations noted by panelists. Furthermore, “legitimate questions remain about whether or not adalimumab, launched 17 years ago, is fairly priced to begin with,” Pamela Bradt, MD, MPH, ICER’s chief scientific officer, said in a news release.
The panel did not vote on the economic value of tofacitinib (Xeljanz) or baricitinib, the two other JAK inhibitors that are approved for rheumatoid arthritis, because head-to-head evidence against adalimumab was insufficient, ICER said.
“Rheumatoid arthritis is a progressively disabling condition, and patients are fortunate to have multiple therapy options – including biosimilars – that effectively slow disease progression,” Dr. Bradt said. “Many economists might expect medicines to become more affordable in an increasingly crowded therapeutic class; however, because the current rebate structure has erected barriers between patients and several emerging RA therapies, traditional market dynamics have been unable to drive down prices.”
Weighing efficacy and cost
Panelists found that the net health benefit provided by upadacitinib is superior to that provided by adalimumab. At the same time, they said that there is insufficient head-to-head evidence to distinguish between the net health benefit of upadacitinib and tofacitinib or to demonstrate that tofacitinib is superior to adalimumab. Evidence comparing baricitinib to adalimumab does not exist.
CTAF members unanimously agreed that adequate evidence demonstrates that the biosimilar infliximab-dyyb (Inflectra) is clinically equivalent to its reference biologic, infliximab (Remicade).
Economic modeling demonstrated that upadacitinib plus a conventional drug achieves marginally higher quality of life than adalimumab plus a conventional drug does, at similar costs. “Based on this comparison with adalimumab, ICER’s value-based price benchmark range for upadacitinib is between $44,000 and $45,000,” according to the ICER news release. “This benchmark represents a 25% discount off of upadacitinib’s annual list price of $59,860, a suggested discount that is consistent with the rebates we assume the manufacturer is currently offering.”
After the voting session, various experts, including clinicians, patient advocates, and representatives from manufacturers and insurance companies, made the following policy recommendations:
- Regulatory intervention may be needed to ensure that drug prices do not continue to increase further from reasonable alignment with added benefits for patients.
- Insurers, pharmacy benefit managers, and employers should increase transparency around the role of discounts and rebates in formulary design.
- Policymakers should aim to create a system that rewards lower-priced biosimilar treatment options.
The findings of the clinical review by the Institute for Clinical and Economic Review (ICER) are generally in line with our clinical perceptions. We have an increasing number of treatment options for our RA patients, and the results of this review support the efficacy of tofacitinib and upadacitinib, compared with currently available biologic treatments. While ICER’s voting panel did find the data supported the superiority of upadacitinib over adalimumab, the cost analysis notes a WAC (wholesale acquisition cost) for upadacitinib of $59,860. While at expected discounted rates it is felt to be cost effective when compared with adalimumab, it is difficult to know what this means since ICER found adalimumab itself not to be cost effective, compared with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), in its 2017 review.
ICER’s focus is drug pricing and cost effectiveness, so obviously our biologic drugs are in the institute’s crosshairs. This review provided context for a policy roundtable discussion that included patient, payer, and manufacturer input, as well as American College of Rheumatology (ACR) input. We are thankful that ACR had a seat at the table, and thankful ICER is attempting to bring light to the important issues and barriers that perpetuate high drug prices in our marketplace. The discussion was wide ranging but focused on step-edit policies, the role of pharmacy benefit managers (PBMs) in perpetuating high drug prices and the relatively slow uptake of biosimilars in our marketplace.
These issues are critical to every practicing rheumatologist because we each deal daily with the hassles of step-edit/fail-first policies, which hijack our otherwise thoughtful and evidence-based decision making regarding the best treatments for our patients. We know how much (unreimbursed) time it takes our staff to sort through these step edits and prior authorizations, and we have seen recent data regarding how these policies delay care and harm patients. We were thankful to see ICER validate these concerns and note that their suggested guidelines for rational step therapy somewhat mirror those in the Safe Step Act, which ACR supports on a federal legislative level. ACR continues to vigorously support the grandfathering of any patient on an effective treatment, regardless of changes in insurance or formulary; this was an issue of robust debate at their meeting, and this patient-centric position is not uniformly held among policymakers, unfortunately.
ACR agrees with ICER’s conclusion that transparency in the PBM system regarding rebates should be promoted and that opaque rebate negotiations between PBMs and manufacturers both incentivize higher prices and block access to the marketplace for cheaper biosimilar options.
Additionally, ICER and ACR agree about the critical role that biosimilar uptake will play in controlling drug costs. While we do not yet have any biosimilars that have been deemed interchangeable by the Food and Drug Administration, we agree with ICER that data regarding comparable efficacy and safety of biosimilars to their originator products is very reassuring. While the decision to switch to a biosimilar should be an individual decision between a provider and patient, and while we recognize with frustration that many FDA-approved biosimilars are not commercially available because of patent law, it is clear that the current costs of our biologic drugs are not sustainable and the uptake of biosimilars will be critical if we hope our health care economy can continue to support coverage of these life-changing drugs in years to come. We agree with ICER that it is incumbent upon prescribers to reassure our patients regarding the safety and efficacy of these drugs.
Christopher Phillips, MD , is a community rheumatologist in Paducah, Ky., who serves as chair of the insurance subcommittee of the ACR, under the guidance of the Committee on Rheumatologic Care. He attended the initial ICER rheumatoid arthritis review meeting in 2017 on behalf of ACR. In 2019, Dr. Phillips served as a reviewer and clinical expert to the ICER panel and participated in the policy roundtable discussion.
The findings of the clinical review by the Institute for Clinical and Economic Review (ICER) are generally in line with our clinical perceptions. We have an increasing number of treatment options for our RA patients, and the results of this review support the efficacy of tofacitinib and upadacitinib, compared with currently available biologic treatments. While ICER’s voting panel did find the data supported the superiority of upadacitinib over adalimumab, the cost analysis notes a WAC (wholesale acquisition cost) for upadacitinib of $59,860. While at expected discounted rates it is felt to be cost effective when compared with adalimumab, it is difficult to know what this means since ICER found adalimumab itself not to be cost effective, compared with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), in its 2017 review.
ICER’s focus is drug pricing and cost effectiveness, so obviously our biologic drugs are in the institute’s crosshairs. This review provided context for a policy roundtable discussion that included patient, payer, and manufacturer input, as well as American College of Rheumatology (ACR) input. We are thankful that ACR had a seat at the table, and thankful ICER is attempting to bring light to the important issues and barriers that perpetuate high drug prices in our marketplace. The discussion was wide ranging but focused on step-edit policies, the role of pharmacy benefit managers (PBMs) in perpetuating high drug prices and the relatively slow uptake of biosimilars in our marketplace.
These issues are critical to every practicing rheumatologist because we each deal daily with the hassles of step-edit/fail-first policies, which hijack our otherwise thoughtful and evidence-based decision making regarding the best treatments for our patients. We know how much (unreimbursed) time it takes our staff to sort through these step edits and prior authorizations, and we have seen recent data regarding how these policies delay care and harm patients. We were thankful to see ICER validate these concerns and note that their suggested guidelines for rational step therapy somewhat mirror those in the Safe Step Act, which ACR supports on a federal legislative level. ACR continues to vigorously support the grandfathering of any patient on an effective treatment, regardless of changes in insurance or formulary; this was an issue of robust debate at their meeting, and this patient-centric position is not uniformly held among policymakers, unfortunately.
ACR agrees with ICER’s conclusion that transparency in the PBM system regarding rebates should be promoted and that opaque rebate negotiations between PBMs and manufacturers both incentivize higher prices and block access to the marketplace for cheaper biosimilar options.
Additionally, ICER and ACR agree about the critical role that biosimilar uptake will play in controlling drug costs. While we do not yet have any biosimilars that have been deemed interchangeable by the Food and Drug Administration, we agree with ICER that data regarding comparable efficacy and safety of biosimilars to their originator products is very reassuring. While the decision to switch to a biosimilar should be an individual decision between a provider and patient, and while we recognize with frustration that many FDA-approved biosimilars are not commercially available because of patent law, it is clear that the current costs of our biologic drugs are not sustainable and the uptake of biosimilars will be critical if we hope our health care economy can continue to support coverage of these life-changing drugs in years to come. We agree with ICER that it is incumbent upon prescribers to reassure our patients regarding the safety and efficacy of these drugs.
Christopher Phillips, MD , is a community rheumatologist in Paducah, Ky., who serves as chair of the insurance subcommittee of the ACR, under the guidance of the Committee on Rheumatologic Care. He attended the initial ICER rheumatoid arthritis review meeting in 2017 on behalf of ACR. In 2019, Dr. Phillips served as a reviewer and clinical expert to the ICER panel and participated in the policy roundtable discussion.
The findings of the clinical review by the Institute for Clinical and Economic Review (ICER) are generally in line with our clinical perceptions. We have an increasing number of treatment options for our RA patients, and the results of this review support the efficacy of tofacitinib and upadacitinib, compared with currently available biologic treatments. While ICER’s voting panel did find the data supported the superiority of upadacitinib over adalimumab, the cost analysis notes a WAC (wholesale acquisition cost) for upadacitinib of $59,860. While at expected discounted rates it is felt to be cost effective when compared with adalimumab, it is difficult to know what this means since ICER found adalimumab itself not to be cost effective, compared with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), in its 2017 review.
ICER’s focus is drug pricing and cost effectiveness, so obviously our biologic drugs are in the institute’s crosshairs. This review provided context for a policy roundtable discussion that included patient, payer, and manufacturer input, as well as American College of Rheumatology (ACR) input. We are thankful that ACR had a seat at the table, and thankful ICER is attempting to bring light to the important issues and barriers that perpetuate high drug prices in our marketplace. The discussion was wide ranging but focused on step-edit policies, the role of pharmacy benefit managers (PBMs) in perpetuating high drug prices and the relatively slow uptake of biosimilars in our marketplace.
These issues are critical to every practicing rheumatologist because we each deal daily with the hassles of step-edit/fail-first policies, which hijack our otherwise thoughtful and evidence-based decision making regarding the best treatments for our patients. We know how much (unreimbursed) time it takes our staff to sort through these step edits and prior authorizations, and we have seen recent data regarding how these policies delay care and harm patients. We were thankful to see ICER validate these concerns and note that their suggested guidelines for rational step therapy somewhat mirror those in the Safe Step Act, which ACR supports on a federal legislative level. ACR continues to vigorously support the grandfathering of any patient on an effective treatment, regardless of changes in insurance or formulary; this was an issue of robust debate at their meeting, and this patient-centric position is not uniformly held among policymakers, unfortunately.
ACR agrees with ICER’s conclusion that transparency in the PBM system regarding rebates should be promoted and that opaque rebate negotiations between PBMs and manufacturers both incentivize higher prices and block access to the marketplace for cheaper biosimilar options.
Additionally, ICER and ACR agree about the critical role that biosimilar uptake will play in controlling drug costs. While we do not yet have any biosimilars that have been deemed interchangeable by the Food and Drug Administration, we agree with ICER that data regarding comparable efficacy and safety of biosimilars to their originator products is very reassuring. While the decision to switch to a biosimilar should be an individual decision between a provider and patient, and while we recognize with frustration that many FDA-approved biosimilars are not commercially available because of patent law, it is clear that the current costs of our biologic drugs are not sustainable and the uptake of biosimilars will be critical if we hope our health care economy can continue to support coverage of these life-changing drugs in years to come. We agree with ICER that it is incumbent upon prescribers to reassure our patients regarding the safety and efficacy of these drugs.
Christopher Phillips, MD , is a community rheumatologist in Paducah, Ky., who serves as chair of the insurance subcommittee of the ACR, under the guidance of the Committee on Rheumatologic Care. He attended the initial ICER rheumatoid arthritis review meeting in 2017 on behalf of ACR. In 2019, Dr. Phillips served as a reviewer and clinical expert to the ICER panel and participated in the policy roundtable discussion.
Adequate evidence shows that adding a Janus kinase (JAK) inhibitor to conventional disease-modifying antirheumatic drug therapy provides a net health benefit for patients with rheumatoid arthritis, compared with conventional drugs alone, according to a report by an independent research institute. But the long-term economic value of JAK inhibitors for rheumatoid arthritis is less clear, the report by the Institute for Clinical and Economic Review (ICER) indicates.
ICER on Jan. 9 released a finalized report and policy recommendations on JAK inhibitors and biosimilars for rheumatoid arthritis. The report reviews current evidence for JAK inhibitors for adults with moderately active to severely active rheumatoid arthritis.
Since the nonprofit’s 2017 review of targeted immune modulators for rheumatoid arthritis, two JAK inhibitors, baricitinib (Olumiant) and upadacitinib (Rinvoq), were approved by the Food and Drug Administration. At a December 2019 public meeting of the California Technology Assessment Forum (CTAF), one of ICER’s independent evidence appraisal committees, panelists reviewed recent evidence.
A pricey comparator
In ICER’s analysis, the JAK inhibitor upadacitinib reached common thresholds for cost-effectiveness when compared with adalimumab (Humira). Nevertheless, the 14 members of the independent evidence appraisal committee voted that upadacitinib’s long-term economic value was “low” (8 votes) or “intermediate” (6 votes). Concerns about the generalizability of phase 3 clinical trial data to patients in the real world were among the reservations noted by panelists. Furthermore, “legitimate questions remain about whether or not adalimumab, launched 17 years ago, is fairly priced to begin with,” Pamela Bradt, MD, MPH, ICER’s chief scientific officer, said in a news release.
The panel did not vote on the economic value of tofacitinib (Xeljanz) or baricitinib, the two other JAK inhibitors that are approved for rheumatoid arthritis, because head-to-head evidence against adalimumab was insufficient, ICER said.
“Rheumatoid arthritis is a progressively disabling condition, and patients are fortunate to have multiple therapy options – including biosimilars – that effectively slow disease progression,” Dr. Bradt said. “Many economists might expect medicines to become more affordable in an increasingly crowded therapeutic class; however, because the current rebate structure has erected barriers between patients and several emerging RA therapies, traditional market dynamics have been unable to drive down prices.”
Weighing efficacy and cost
Panelists found that the net health benefit provided by upadacitinib is superior to that provided by adalimumab. At the same time, they said that there is insufficient head-to-head evidence to distinguish between the net health benefit of upadacitinib and tofacitinib or to demonstrate that tofacitinib is superior to adalimumab. Evidence comparing baricitinib to adalimumab does not exist.
CTAF members unanimously agreed that adequate evidence demonstrates that the biosimilar infliximab-dyyb (Inflectra) is clinically equivalent to its reference biologic, infliximab (Remicade).
Economic modeling demonstrated that upadacitinib plus a conventional drug achieves marginally higher quality of life than adalimumab plus a conventional drug does, at similar costs. “Based on this comparison with adalimumab, ICER’s value-based price benchmark range for upadacitinib is between $44,000 and $45,000,” according to the ICER news release. “This benchmark represents a 25% discount off of upadacitinib’s annual list price of $59,860, a suggested discount that is consistent with the rebates we assume the manufacturer is currently offering.”
After the voting session, various experts, including clinicians, patient advocates, and representatives from manufacturers and insurance companies, made the following policy recommendations:
- Regulatory intervention may be needed to ensure that drug prices do not continue to increase further from reasonable alignment with added benefits for patients.
- Insurers, pharmacy benefit managers, and employers should increase transparency around the role of discounts and rebates in formulary design.
- Policymakers should aim to create a system that rewards lower-priced biosimilar treatment options.
Adequate evidence shows that adding a Janus kinase (JAK) inhibitor to conventional disease-modifying antirheumatic drug therapy provides a net health benefit for patients with rheumatoid arthritis, compared with conventional drugs alone, according to a report by an independent research institute. But the long-term economic value of JAK inhibitors for rheumatoid arthritis is less clear, the report by the Institute for Clinical and Economic Review (ICER) indicates.
ICER on Jan. 9 released a finalized report and policy recommendations on JAK inhibitors and biosimilars for rheumatoid arthritis. The report reviews current evidence for JAK inhibitors for adults with moderately active to severely active rheumatoid arthritis.
Since the nonprofit’s 2017 review of targeted immune modulators for rheumatoid arthritis, two JAK inhibitors, baricitinib (Olumiant) and upadacitinib (Rinvoq), were approved by the Food and Drug Administration. At a December 2019 public meeting of the California Technology Assessment Forum (CTAF), one of ICER’s independent evidence appraisal committees, panelists reviewed recent evidence.
A pricey comparator
In ICER’s analysis, the JAK inhibitor upadacitinib reached common thresholds for cost-effectiveness when compared with adalimumab (Humira). Nevertheless, the 14 members of the independent evidence appraisal committee voted that upadacitinib’s long-term economic value was “low” (8 votes) or “intermediate” (6 votes). Concerns about the generalizability of phase 3 clinical trial data to patients in the real world were among the reservations noted by panelists. Furthermore, “legitimate questions remain about whether or not adalimumab, launched 17 years ago, is fairly priced to begin with,” Pamela Bradt, MD, MPH, ICER’s chief scientific officer, said in a news release.
The panel did not vote on the economic value of tofacitinib (Xeljanz) or baricitinib, the two other JAK inhibitors that are approved for rheumatoid arthritis, because head-to-head evidence against adalimumab was insufficient, ICER said.
“Rheumatoid arthritis is a progressively disabling condition, and patients are fortunate to have multiple therapy options – including biosimilars – that effectively slow disease progression,” Dr. Bradt said. “Many economists might expect medicines to become more affordable in an increasingly crowded therapeutic class; however, because the current rebate structure has erected barriers between patients and several emerging RA therapies, traditional market dynamics have been unable to drive down prices.”
Weighing efficacy and cost
Panelists found that the net health benefit provided by upadacitinib is superior to that provided by adalimumab. At the same time, they said that there is insufficient head-to-head evidence to distinguish between the net health benefit of upadacitinib and tofacitinib or to demonstrate that tofacitinib is superior to adalimumab. Evidence comparing baricitinib to adalimumab does not exist.
CTAF members unanimously agreed that adequate evidence demonstrates that the biosimilar infliximab-dyyb (Inflectra) is clinically equivalent to its reference biologic, infliximab (Remicade).
Economic modeling demonstrated that upadacitinib plus a conventional drug achieves marginally higher quality of life than adalimumab plus a conventional drug does, at similar costs. “Based on this comparison with adalimumab, ICER’s value-based price benchmark range for upadacitinib is between $44,000 and $45,000,” according to the ICER news release. “This benchmark represents a 25% discount off of upadacitinib’s annual list price of $59,860, a suggested discount that is consistent with the rebates we assume the manufacturer is currently offering.”
After the voting session, various experts, including clinicians, patient advocates, and representatives from manufacturers and insurance companies, made the following policy recommendations:
- Regulatory intervention may be needed to ensure that drug prices do not continue to increase further from reasonable alignment with added benefits for patients.
- Insurers, pharmacy benefit managers, and employers should increase transparency around the role of discounts and rebates in formulary design.
- Policymakers should aim to create a system that rewards lower-priced biosimilar treatment options.
Cognitive screening of older physicians: What’s fair?
Cognitive screening of 141 clinicians 70 years or older at Yale New Haven (Conn.) Hospital identified 18 with cognitive deficits likely to impair their ability to practice medicine. Six retired and 12 agreed to limit their practice to closely proctored environments, according to a report in JAMA.
It was part of a program to screen all practitioners 70 years or older who apply for reappointment to the medical staff, and every 2 years thereafter, due to “concerns about the potentially compromised ability of older clinicians,” said the authors, Yale rheumatologist and geriatrician Leo M. Cooney Jr., MD, and Thomas Balcezak, MD, Yale New Haven’s chief medical officer.
Yale is not alone. Intermountain Healthcare, Stanford Hospitals and Clinics, Scripps Health Care, Penn Medicine, and the University of California, San Diego, are among the institutions with similar programs.
The move is being driven by the aging of the medical community. About 15% of U.S. physicians are over 65 years old, a tripling from 23,000 in 1980 to 73,000 in 2012-2016, and the number is growing, according to an editorial by Jeffrey L. Saver, MD, professor of neurology and senior associate vice president of neurology at the University of California, Los Angeles.
Given the trend, “it is not surprising that the issue of screening aging physicians for cognitive deficits has gained attention over the last decade,” Katrina Armstrong, MD, chair of the department of medicine at Massachusetts General Hospital, Boston, and Eileen E. Reynolds, MD, associate professor of medicine at Beth Israel Deaconess Medical Center, Boston, noted in a second editorial.
“Cognitive decline often accompanies aging, and the prevalence of dementia increases rapidly after age 70 years,” they said.
The data on whether older clinicians pose a risk to patients is limited and somewhat mixed. An analysis of 736,537 Medicare hospitalizations found no association between physician age and 30-day patient mortality among physicians 60 years or older with more than 201 admissions per year, but higher mortality among older physicians with lower volumes.
A meta-analysis of 62 studies showed that “older physicians have less factual knowledge, are less likely to adhere to appropriate standards of care, and may also have poorer patient outcomes.”
The new Yale data, meanwhile, suggests that “approximately 13% [18 of 141] of physicians and other clinicians older than 70 years should not be practicing independently,” Dr. Armstrong and Dr. Reynolds said in their editorial.
There is support for screening efforts. “As a profession that deals with human life, medical practitioners must obviously have the cognitive capacity to safely practice medicine. I applaud the approach taken by Yale New Haven Hospital in that cognitive abilities themselves, and not simply funds of knowledge, are assessed,” said Richard J. Caselli, MD, professor of neurology at the Mayo Clinic Arizona, Scottsdale, and a leader of the Alzheimer’s disease program there.
However, it’s not hard to imagine highly competent but older physicians taking umbrage at cognitive screening, and there’s been pushback. Stanford was considering a Yale-like approach but opted instead for peer review after opposition. Objections from the Utah Medical Association led Utah to enact a law banning age-based physician screening. In 2015, the American Medical Association issued a report calling for the development of guidelines and standards for assessing competency in aging physicians, but the AMA House of Delegates shelved it pending further study.
There are concerns about age discrimination, discounting the accumulated wisdom of long-practicing physicians, and misclassifying competent physicians, particularly those who provide quality care in rural and other underserved areas. Indeed, 8 of 14 clinicians who screened positive at Yale and underwent more extensive testing were allowed to recredential, “suggesting that the false-positive screening rate could be as high as 57%,” Dr. Armstrong and Dr. Reynolds noted.
The consensus seems to be that there probably is a need for some sort of screening, but it must be both sound and fair. Rather than a piecemeal institutional approach, perhaps there is “an important opportunity for other groups, including specialty boards and state licensing boards” to standardize the process, they said.
Among other things, assessments could focus less on test scores and more on the practice of medicine. For instance, fine motor skill/motor planning assessments for surgeons, and intermediate results could trigger a more extensive assessment of actual clinical performance, perhaps even direct observation, Dr. Saver said in his editorial.
As far as clinical performance goes, none of the 18 clinicians at Yale had previous performance problems. “Was this a failure of the system to report impaired physicians or were these physicians compensating sufficiently to avoid detection?” In either case, “cognitive testing should be a red flag that triggers other clinical assessments,” said Carl I. Cohen, MD, professor and director of the division of geriatric psychiatry at the State University of New York, Brooklyn.
The original plan at Yale was for neurologic and ophthalmologic examinations beginning at age 70, but ultimately it was decided to go with a battery of 16 tests to assess visual scanning and psychomotor efficiency, processing speed under pressure, concentration, and working memory, among other things. Testing takes about 50-90 minutes, and is graded by single neuropsychologist to ensure consistency. Results were compared with normative scores from both older and younger clinicians.
To prevent clinicians from preparing for it, Yale isn’t releasing its test battery.
Suboptimal performance triggered additional evaluations, including in-depth assessment of intellectual, memory, and executive function. Final reviews and recommendations were made by a committee that included a geriatrician, the clinician’s section or department chair, and current and past chief medical officers.
Among the 18 providers who demonstrated deficits impairing their ability to practice medicine, 5 were 70-74 years old; 4 were 75-79; and 9 were 80 years or older. Minor abnormalities were found in 34 other candidates (24.1%); they were allowed to recredential but were scheduled for rescreening at 1-year intervals, instead of every 2 years.
The mean age among the 141 screened clinicians was 74.3 years and ranged from 69 to 92 years; 86% were men. Applicants included 125 physicians (88.7%) as well as 5 advanced practice registered nurses; 4 dentists; 3 psychologists; 2 podiatrists; 1 physician associate; and 1 midwife.
The authors had no relevant disclosures.
SOURCE: Cooney L et al. JAMA. 2020 Jan 14;323(2):179-80.
Cognitive screening of 141 clinicians 70 years or older at Yale New Haven (Conn.) Hospital identified 18 with cognitive deficits likely to impair their ability to practice medicine. Six retired and 12 agreed to limit their practice to closely proctored environments, according to a report in JAMA.
It was part of a program to screen all practitioners 70 years or older who apply for reappointment to the medical staff, and every 2 years thereafter, due to “concerns about the potentially compromised ability of older clinicians,” said the authors, Yale rheumatologist and geriatrician Leo M. Cooney Jr., MD, and Thomas Balcezak, MD, Yale New Haven’s chief medical officer.
Yale is not alone. Intermountain Healthcare, Stanford Hospitals and Clinics, Scripps Health Care, Penn Medicine, and the University of California, San Diego, are among the institutions with similar programs.
The move is being driven by the aging of the medical community. About 15% of U.S. physicians are over 65 years old, a tripling from 23,000 in 1980 to 73,000 in 2012-2016, and the number is growing, according to an editorial by Jeffrey L. Saver, MD, professor of neurology and senior associate vice president of neurology at the University of California, Los Angeles.
Given the trend, “it is not surprising that the issue of screening aging physicians for cognitive deficits has gained attention over the last decade,” Katrina Armstrong, MD, chair of the department of medicine at Massachusetts General Hospital, Boston, and Eileen E. Reynolds, MD, associate professor of medicine at Beth Israel Deaconess Medical Center, Boston, noted in a second editorial.
“Cognitive decline often accompanies aging, and the prevalence of dementia increases rapidly after age 70 years,” they said.
The data on whether older clinicians pose a risk to patients is limited and somewhat mixed. An analysis of 736,537 Medicare hospitalizations found no association between physician age and 30-day patient mortality among physicians 60 years or older with more than 201 admissions per year, but higher mortality among older physicians with lower volumes.
A meta-analysis of 62 studies showed that “older physicians have less factual knowledge, are less likely to adhere to appropriate standards of care, and may also have poorer patient outcomes.”
The new Yale data, meanwhile, suggests that “approximately 13% [18 of 141] of physicians and other clinicians older than 70 years should not be practicing independently,” Dr. Armstrong and Dr. Reynolds said in their editorial.
There is support for screening efforts. “As a profession that deals with human life, medical practitioners must obviously have the cognitive capacity to safely practice medicine. I applaud the approach taken by Yale New Haven Hospital in that cognitive abilities themselves, and not simply funds of knowledge, are assessed,” said Richard J. Caselli, MD, professor of neurology at the Mayo Clinic Arizona, Scottsdale, and a leader of the Alzheimer’s disease program there.
However, it’s not hard to imagine highly competent but older physicians taking umbrage at cognitive screening, and there’s been pushback. Stanford was considering a Yale-like approach but opted instead for peer review after opposition. Objections from the Utah Medical Association led Utah to enact a law banning age-based physician screening. In 2015, the American Medical Association issued a report calling for the development of guidelines and standards for assessing competency in aging physicians, but the AMA House of Delegates shelved it pending further study.
There are concerns about age discrimination, discounting the accumulated wisdom of long-practicing physicians, and misclassifying competent physicians, particularly those who provide quality care in rural and other underserved areas. Indeed, 8 of 14 clinicians who screened positive at Yale and underwent more extensive testing were allowed to recredential, “suggesting that the false-positive screening rate could be as high as 57%,” Dr. Armstrong and Dr. Reynolds noted.
The consensus seems to be that there probably is a need for some sort of screening, but it must be both sound and fair. Rather than a piecemeal institutional approach, perhaps there is “an important opportunity for other groups, including specialty boards and state licensing boards” to standardize the process, they said.
Among other things, assessments could focus less on test scores and more on the practice of medicine. For instance, fine motor skill/motor planning assessments for surgeons, and intermediate results could trigger a more extensive assessment of actual clinical performance, perhaps even direct observation, Dr. Saver said in his editorial.
As far as clinical performance goes, none of the 18 clinicians at Yale had previous performance problems. “Was this a failure of the system to report impaired physicians or were these physicians compensating sufficiently to avoid detection?” In either case, “cognitive testing should be a red flag that triggers other clinical assessments,” said Carl I. Cohen, MD, professor and director of the division of geriatric psychiatry at the State University of New York, Brooklyn.
The original plan at Yale was for neurologic and ophthalmologic examinations beginning at age 70, but ultimately it was decided to go with a battery of 16 tests to assess visual scanning and psychomotor efficiency, processing speed under pressure, concentration, and working memory, among other things. Testing takes about 50-90 minutes, and is graded by single neuropsychologist to ensure consistency. Results were compared with normative scores from both older and younger clinicians.
To prevent clinicians from preparing for it, Yale isn’t releasing its test battery.
Suboptimal performance triggered additional evaluations, including in-depth assessment of intellectual, memory, and executive function. Final reviews and recommendations were made by a committee that included a geriatrician, the clinician’s section or department chair, and current and past chief medical officers.
Among the 18 providers who demonstrated deficits impairing their ability to practice medicine, 5 were 70-74 years old; 4 were 75-79; and 9 were 80 years or older. Minor abnormalities were found in 34 other candidates (24.1%); they were allowed to recredential but were scheduled for rescreening at 1-year intervals, instead of every 2 years.
The mean age among the 141 screened clinicians was 74.3 years and ranged from 69 to 92 years; 86% were men. Applicants included 125 physicians (88.7%) as well as 5 advanced practice registered nurses; 4 dentists; 3 psychologists; 2 podiatrists; 1 physician associate; and 1 midwife.
The authors had no relevant disclosures.
SOURCE: Cooney L et al. JAMA. 2020 Jan 14;323(2):179-80.
Cognitive screening of 141 clinicians 70 years or older at Yale New Haven (Conn.) Hospital identified 18 with cognitive deficits likely to impair their ability to practice medicine. Six retired and 12 agreed to limit their practice to closely proctored environments, according to a report in JAMA.
It was part of a program to screen all practitioners 70 years or older who apply for reappointment to the medical staff, and every 2 years thereafter, due to “concerns about the potentially compromised ability of older clinicians,” said the authors, Yale rheumatologist and geriatrician Leo M. Cooney Jr., MD, and Thomas Balcezak, MD, Yale New Haven’s chief medical officer.
Yale is not alone. Intermountain Healthcare, Stanford Hospitals and Clinics, Scripps Health Care, Penn Medicine, and the University of California, San Diego, are among the institutions with similar programs.
The move is being driven by the aging of the medical community. About 15% of U.S. physicians are over 65 years old, a tripling from 23,000 in 1980 to 73,000 in 2012-2016, and the number is growing, according to an editorial by Jeffrey L. Saver, MD, professor of neurology and senior associate vice president of neurology at the University of California, Los Angeles.
Given the trend, “it is not surprising that the issue of screening aging physicians for cognitive deficits has gained attention over the last decade,” Katrina Armstrong, MD, chair of the department of medicine at Massachusetts General Hospital, Boston, and Eileen E. Reynolds, MD, associate professor of medicine at Beth Israel Deaconess Medical Center, Boston, noted in a second editorial.
“Cognitive decline often accompanies aging, and the prevalence of dementia increases rapidly after age 70 years,” they said.
The data on whether older clinicians pose a risk to patients is limited and somewhat mixed. An analysis of 736,537 Medicare hospitalizations found no association between physician age and 30-day patient mortality among physicians 60 years or older with more than 201 admissions per year, but higher mortality among older physicians with lower volumes.
A meta-analysis of 62 studies showed that “older physicians have less factual knowledge, are less likely to adhere to appropriate standards of care, and may also have poorer patient outcomes.”
The new Yale data, meanwhile, suggests that “approximately 13% [18 of 141] of physicians and other clinicians older than 70 years should not be practicing independently,” Dr. Armstrong and Dr. Reynolds said in their editorial.
There is support for screening efforts. “As a profession that deals with human life, medical practitioners must obviously have the cognitive capacity to safely practice medicine. I applaud the approach taken by Yale New Haven Hospital in that cognitive abilities themselves, and not simply funds of knowledge, are assessed,” said Richard J. Caselli, MD, professor of neurology at the Mayo Clinic Arizona, Scottsdale, and a leader of the Alzheimer’s disease program there.
However, it’s not hard to imagine highly competent but older physicians taking umbrage at cognitive screening, and there’s been pushback. Stanford was considering a Yale-like approach but opted instead for peer review after opposition. Objections from the Utah Medical Association led Utah to enact a law banning age-based physician screening. In 2015, the American Medical Association issued a report calling for the development of guidelines and standards for assessing competency in aging physicians, but the AMA House of Delegates shelved it pending further study.
There are concerns about age discrimination, discounting the accumulated wisdom of long-practicing physicians, and misclassifying competent physicians, particularly those who provide quality care in rural and other underserved areas. Indeed, 8 of 14 clinicians who screened positive at Yale and underwent more extensive testing were allowed to recredential, “suggesting that the false-positive screening rate could be as high as 57%,” Dr. Armstrong and Dr. Reynolds noted.
The consensus seems to be that there probably is a need for some sort of screening, but it must be both sound and fair. Rather than a piecemeal institutional approach, perhaps there is “an important opportunity for other groups, including specialty boards and state licensing boards” to standardize the process, they said.
Among other things, assessments could focus less on test scores and more on the practice of medicine. For instance, fine motor skill/motor planning assessments for surgeons, and intermediate results could trigger a more extensive assessment of actual clinical performance, perhaps even direct observation, Dr. Saver said in his editorial.
As far as clinical performance goes, none of the 18 clinicians at Yale had previous performance problems. “Was this a failure of the system to report impaired physicians or were these physicians compensating sufficiently to avoid detection?” In either case, “cognitive testing should be a red flag that triggers other clinical assessments,” said Carl I. Cohen, MD, professor and director of the division of geriatric psychiatry at the State University of New York, Brooklyn.
The original plan at Yale was for neurologic and ophthalmologic examinations beginning at age 70, but ultimately it was decided to go with a battery of 16 tests to assess visual scanning and psychomotor efficiency, processing speed under pressure, concentration, and working memory, among other things. Testing takes about 50-90 minutes, and is graded by single neuropsychologist to ensure consistency. Results were compared with normative scores from both older and younger clinicians.
To prevent clinicians from preparing for it, Yale isn’t releasing its test battery.
Suboptimal performance triggered additional evaluations, including in-depth assessment of intellectual, memory, and executive function. Final reviews and recommendations were made by a committee that included a geriatrician, the clinician’s section or department chair, and current and past chief medical officers.
Among the 18 providers who demonstrated deficits impairing their ability to practice medicine, 5 were 70-74 years old; 4 were 75-79; and 9 were 80 years or older. Minor abnormalities were found in 34 other candidates (24.1%); they were allowed to recredential but were scheduled for rescreening at 1-year intervals, instead of every 2 years.
The mean age among the 141 screened clinicians was 74.3 years and ranged from 69 to 92 years; 86% were men. Applicants included 125 physicians (88.7%) as well as 5 advanced practice registered nurses; 4 dentists; 3 psychologists; 2 podiatrists; 1 physician associate; and 1 midwife.
The authors had no relevant disclosures.
SOURCE: Cooney L et al. JAMA. 2020 Jan 14;323(2):179-80.
FROM JAMA
Reducing alarm fatigue in the hospital
Noise increases patient anxiety
Researchers are exploring ways to make alarms and monitors less irritating and more informative.
“Hospitals today can be sonic hellscapes, which studies have shown regularly exceed levels set by the World Health Organization: droning IV pumps, ding-donging nurse call buttons, voices crackling on loudspeakers, ringing telephones, beeping elevators, buzzing ID scanners, clattering carts, coughing, screaming, vomiting,” according to a recent article in the New York Times.
And that’s not to mention all the alarms that blare regularly, day and night. “A single patient might trigger hundreds each day, challenging caregivers to figure out which machine is beeping, and what is wrong with the patient, if anything,” according to the article.
All this noise contributes to patient anxiety and delirium and to staff burnout too. Alarm fatigue is a serious problem, related to the high rate of false alarms, the lack of alarm standardization, and the number of medical devices that emit an alarm. Its effect is to make caregivers less responsive.
A group of researchers is developing new sounds that could replace current alarms. These new signals might mimic electronic dance music or the sounds of a heartbeat; they may combine audible alarms with visual cues such as interactive screens; they will certainly be quieter. Testing remains to be done around how quickly clinicians will be able to learn the sounds and how loud they need to be. The researchers say a new standard is likely to go into effect in 2020.
Reference
1. Rueb ES. To Reduce Hospital Noise, Researchers Create Alarms That Whistle and Sing. New York Times. July 9, 2019.
Noise increases patient anxiety
Noise increases patient anxiety
Researchers are exploring ways to make alarms and monitors less irritating and more informative.
“Hospitals today can be sonic hellscapes, which studies have shown regularly exceed levels set by the World Health Organization: droning IV pumps, ding-donging nurse call buttons, voices crackling on loudspeakers, ringing telephones, beeping elevators, buzzing ID scanners, clattering carts, coughing, screaming, vomiting,” according to a recent article in the New York Times.
And that’s not to mention all the alarms that blare regularly, day and night. “A single patient might trigger hundreds each day, challenging caregivers to figure out which machine is beeping, and what is wrong with the patient, if anything,” according to the article.
All this noise contributes to patient anxiety and delirium and to staff burnout too. Alarm fatigue is a serious problem, related to the high rate of false alarms, the lack of alarm standardization, and the number of medical devices that emit an alarm. Its effect is to make caregivers less responsive.
A group of researchers is developing new sounds that could replace current alarms. These new signals might mimic electronic dance music or the sounds of a heartbeat; they may combine audible alarms with visual cues such as interactive screens; they will certainly be quieter. Testing remains to be done around how quickly clinicians will be able to learn the sounds and how loud they need to be. The researchers say a new standard is likely to go into effect in 2020.
Reference
1. Rueb ES. To Reduce Hospital Noise, Researchers Create Alarms That Whistle and Sing. New York Times. July 9, 2019.
Researchers are exploring ways to make alarms and monitors less irritating and more informative.
“Hospitals today can be sonic hellscapes, which studies have shown regularly exceed levels set by the World Health Organization: droning IV pumps, ding-donging nurse call buttons, voices crackling on loudspeakers, ringing telephones, beeping elevators, buzzing ID scanners, clattering carts, coughing, screaming, vomiting,” according to a recent article in the New York Times.
And that’s not to mention all the alarms that blare regularly, day and night. “A single patient might trigger hundreds each day, challenging caregivers to figure out which machine is beeping, and what is wrong with the patient, if anything,” according to the article.
All this noise contributes to patient anxiety and delirium and to staff burnout too. Alarm fatigue is a serious problem, related to the high rate of false alarms, the lack of alarm standardization, and the number of medical devices that emit an alarm. Its effect is to make caregivers less responsive.
A group of researchers is developing new sounds that could replace current alarms. These new signals might mimic electronic dance music or the sounds of a heartbeat; they may combine audible alarms with visual cues such as interactive screens; they will certainly be quieter. Testing remains to be done around how quickly clinicians will be able to learn the sounds and how loud they need to be. The researchers say a new standard is likely to go into effect in 2020.
Reference
1. Rueb ES. To Reduce Hospital Noise, Researchers Create Alarms That Whistle and Sing. New York Times. July 9, 2019.
Cannabis users struggle to quit cigarettes
a large national survey has found.
“Over the past decade, there has been an increase in the use of cannabis among cigarette smokers and prevalence of cigarettes and cannabis co-use, suggesting that the negative consequences of cigarette–cannabis co-use may also become more prevalent over time,” wrote Andrea H. Weinberger, PhD, of Yeshiva University, New York, and colleagues. They noted that the prevalence of cigarette smoking is nearly three times higher among persons who use cannabis and have cannabis use disorders relative to those who do not.
The 2019 National Survey of Drug Use and Health estimated that 15.9% of Americans aged 12 years or older used cannabis in the past year. This number has been rising throughout the 2000s.
In that same report, cannabis use disorder (or marijuana use disorder) was defined as when an individual experiences clinically significant impairment caused by the recurrent use of marijuana, including health problems, persistent or increasing use, and failure to meet major responsibilities at work, school, or home. The report stated that approximately 1.6% of Americans aged 12 or older in 2018 had marijuana use disorder.
In the study published in Tobacco Control, the researchers used the National Survey on Drug Use and Health data to analyze cigarette smoking quit ratios among U.S. adults with and without cannabis use and cannabis use disorders. “Quit ratio was calculated as the proportion of former smokers among lifetime smokers and is considered a measure of total cessation in a population,” the researchers said.
In 2016, the quit ratios for adults with a history of cannabis use or cannabis use disorders were 23% and 15%, respectively, compared with 51% and 48%, respectively, in those with no cannabis use or cannabis use disorders.
Overall, quit ratios did not change significantly from 2002 to 2016 for individuals with cannabis use disorders after controlling for multiple demographic factors and other substance use disorders. However, during the same time period, quit ratios showed a nonlinear increase in cannabis users, nonusers, and individuals without cannabis use disorders.
The study findings were limited by several factors including the inability to generalize results to youth or individuals living outside the United States, the use of DSM-IV criteria to identify cannabis use disorder, the use of self-reports, and the inability to examine the timing of cannabis use as related to attempts to quit smoking, the researchers noted. However, the results highlight the need to consider offering smoking cessation treatment to individuals being treated for cannabis use disorders, and to include cannabis users in smoking cessation programs, the researchers noted.
“Based on our results, both public health and clinical efforts to improve cigarette quit outcomes may benefit from including those with any cannabis use,” they said. More research is needed to determine whether trends in the quit ratio change over time for cannabis users or those with cannabis use disorder, they added.
The study was funded by the National Institute on Drug Abuse. The researchers had no financial conflicts to disclose.
SOURCE: Weinberger AH et al. Tob Control. 2020;29(1):74-80. doi: 10.1136/tobaccocontrol-2018-054590.
a large national survey has found.
“Over the past decade, there has been an increase in the use of cannabis among cigarette smokers and prevalence of cigarettes and cannabis co-use, suggesting that the negative consequences of cigarette–cannabis co-use may also become more prevalent over time,” wrote Andrea H. Weinberger, PhD, of Yeshiva University, New York, and colleagues. They noted that the prevalence of cigarette smoking is nearly three times higher among persons who use cannabis and have cannabis use disorders relative to those who do not.
The 2019 National Survey of Drug Use and Health estimated that 15.9% of Americans aged 12 years or older used cannabis in the past year. This number has been rising throughout the 2000s.
In that same report, cannabis use disorder (or marijuana use disorder) was defined as when an individual experiences clinically significant impairment caused by the recurrent use of marijuana, including health problems, persistent or increasing use, and failure to meet major responsibilities at work, school, or home. The report stated that approximately 1.6% of Americans aged 12 or older in 2018 had marijuana use disorder.
In the study published in Tobacco Control, the researchers used the National Survey on Drug Use and Health data to analyze cigarette smoking quit ratios among U.S. adults with and without cannabis use and cannabis use disorders. “Quit ratio was calculated as the proportion of former smokers among lifetime smokers and is considered a measure of total cessation in a population,” the researchers said.
In 2016, the quit ratios for adults with a history of cannabis use or cannabis use disorders were 23% and 15%, respectively, compared with 51% and 48%, respectively, in those with no cannabis use or cannabis use disorders.
Overall, quit ratios did not change significantly from 2002 to 2016 for individuals with cannabis use disorders after controlling for multiple demographic factors and other substance use disorders. However, during the same time period, quit ratios showed a nonlinear increase in cannabis users, nonusers, and individuals without cannabis use disorders.
The study findings were limited by several factors including the inability to generalize results to youth or individuals living outside the United States, the use of DSM-IV criteria to identify cannabis use disorder, the use of self-reports, and the inability to examine the timing of cannabis use as related to attempts to quit smoking, the researchers noted. However, the results highlight the need to consider offering smoking cessation treatment to individuals being treated for cannabis use disorders, and to include cannabis users in smoking cessation programs, the researchers noted.
“Based on our results, both public health and clinical efforts to improve cigarette quit outcomes may benefit from including those with any cannabis use,” they said. More research is needed to determine whether trends in the quit ratio change over time for cannabis users or those with cannabis use disorder, they added.
The study was funded by the National Institute on Drug Abuse. The researchers had no financial conflicts to disclose.
SOURCE: Weinberger AH et al. Tob Control. 2020;29(1):74-80. doi: 10.1136/tobaccocontrol-2018-054590.
a large national survey has found.
“Over the past decade, there has been an increase in the use of cannabis among cigarette smokers and prevalence of cigarettes and cannabis co-use, suggesting that the negative consequences of cigarette–cannabis co-use may also become more prevalent over time,” wrote Andrea H. Weinberger, PhD, of Yeshiva University, New York, and colleagues. They noted that the prevalence of cigarette smoking is nearly three times higher among persons who use cannabis and have cannabis use disorders relative to those who do not.
The 2019 National Survey of Drug Use and Health estimated that 15.9% of Americans aged 12 years or older used cannabis in the past year. This number has been rising throughout the 2000s.
In that same report, cannabis use disorder (or marijuana use disorder) was defined as when an individual experiences clinically significant impairment caused by the recurrent use of marijuana, including health problems, persistent or increasing use, and failure to meet major responsibilities at work, school, or home. The report stated that approximately 1.6% of Americans aged 12 or older in 2018 had marijuana use disorder.
In the study published in Tobacco Control, the researchers used the National Survey on Drug Use and Health data to analyze cigarette smoking quit ratios among U.S. adults with and without cannabis use and cannabis use disorders. “Quit ratio was calculated as the proportion of former smokers among lifetime smokers and is considered a measure of total cessation in a population,” the researchers said.
In 2016, the quit ratios for adults with a history of cannabis use or cannabis use disorders were 23% and 15%, respectively, compared with 51% and 48%, respectively, in those with no cannabis use or cannabis use disorders.
Overall, quit ratios did not change significantly from 2002 to 2016 for individuals with cannabis use disorders after controlling for multiple demographic factors and other substance use disorders. However, during the same time period, quit ratios showed a nonlinear increase in cannabis users, nonusers, and individuals without cannabis use disorders.
The study findings were limited by several factors including the inability to generalize results to youth or individuals living outside the United States, the use of DSM-IV criteria to identify cannabis use disorder, the use of self-reports, and the inability to examine the timing of cannabis use as related to attempts to quit smoking, the researchers noted. However, the results highlight the need to consider offering smoking cessation treatment to individuals being treated for cannabis use disorders, and to include cannabis users in smoking cessation programs, the researchers noted.
“Based on our results, both public health and clinical efforts to improve cigarette quit outcomes may benefit from including those with any cannabis use,” they said. More research is needed to determine whether trends in the quit ratio change over time for cannabis users or those with cannabis use disorder, they added.
The study was funded by the National Institute on Drug Abuse. The researchers had no financial conflicts to disclose.
SOURCE: Weinberger AH et al. Tob Control. 2020;29(1):74-80. doi: 10.1136/tobaccocontrol-2018-054590.
FROM TOBACCO CONTROL