User login
Combo produces disappointing PFS, promising OS in metastatic colorectal cancer
NATIONAL HARBOR, MD. – An immunochemotherapy regimen produced mixed results in a phase 2 trial of patients with previously untreated metastatic colorectal cancer.
The regimen – avelumab and cetuximab plus oxaliplatin, leucovorin, and 5-fluorouracil (mFOLFOX6) – failed to meet the primary endpoint for progression-free survival (PFS) but was associated with “promising” yet “preliminary” overall survival, according to Joseph Tintelnot, MD, of University Medical Center Hamburg-Eppendorf (Germany).
Dr. Tintelnot presented these results from the AVETUX trial (NCT03174405) at the annual meeting of the Society for Immunotherapy of Cancer.
The trial enrolled 43 patients with previously untreated, metastatic colorectal cancer, and 39 of them had wild-type RAS and BRAF mutations. Among those 39 patients, the median age was 62 years (range, 29-82 years), 13 patients were female, and 36 patients had left-sided tumors.
A total of 30 patients had liver metastasis, 12 had lung metastasis, and 18 had lymph node metastasis. Most patients (n = 36) had microsatellite stable tumors, 2 were microsatellite instability high, and 1 was microsatellite instability low.
Patients received IV cetuximab at 250 mg/m2 over 60-90 minutes (day 1 and 8), with a first dose of 400 mg/m2; mFOLFOX6 according to local standard – IV oxaliplatin at 85 mg/m2 IV (day 1), IV leucovorin at 400 mg/m2 IV (day 1), and IV bolus 5-fluorouracil at 400 mg/m2 (day 1) and IV at 2,400 mg/m2 (days 1-3); and IV avelumab at 10 mg/kg over 60-90 minutes (day 1 from cycle 2 onward).
The median number of treatment cycles was 8 (range, 1-34) for oxaliplatin, 13 (range, 1-35) for 5-fluorouracil, 12 (range, 1-35) for cetuximab, and 16 (range, 0-34) for avelumab. The median duration of cetuximab/avelumab treatment was 5.4 months (range, 0.7-18.4 months).
The study’s primary endpoint was 12-month PFS, and the researchers expected the PFS to rise from 40% to 57%. Unfortunately, the 12-month PFS was 40%, so the primary endpoint was not met.
However, the treatment produced a “very high” overall response rate at 81% (30/37), according to Dr. Tintelnot. A total of 4 patients achieved a complete response, 26 had a partial response, 4 had stable disease, and 3 progressed.
Dr. Tintelnot also noted a “promising” but “preliminary” overall survival rate – 84% at a median follow-up of 16.2 months. He said these results suggest PFS may not be the ideal endpoint for this combination.
Dr. Tintelnot said the combination was safe, with no unexpected toxicities. The most common grade 3-4 adverse events were infection of catheter, device, urinary tract, etc. (32%); abdominal pain, diarrhea, etc. (24%); skin reaction (21%); anemia, blood disorders, and hemolytic-uremic syndrome (18%); administration, infusion-related, and allergic reactions (16%); cognitive disturbance, meningism, syncope, and psychiatric disorders (16%); and peripheral sensory polyneuropathy and paresthesia (16%).
Dr. Tintelnot and colleagues also conducted translational research evaluating programmed death-ligand 1 (PD-L1) expression and serial circulating tumor DNA in patients on this trial.
The team found no clear correlation between PFS and T-cell diversification, tumor-infiltrating lymphocytes, or tumor proportion score. Dr. Tintelnot said this suggests classical predictive factors for PD-1/PD-L1 inhibitor treatment have a limited role with this combination.
The researchers did find that circulating tumor mutations might help predict early relapse with the regimen. The team identified 26 patients with mutations detectable in their blood. There was an immediate decline of circulating tumor mutations after treatment initiation, and reemergence of mutation clones was associated with progression.
Lastly, the researchers found that avelumab, cetuximab, and mFOLFOX6 suppressed the development of epidermal growth factor receptor–resistant subclones. There were no epidermal growth factor receptor mutations detected during follow-up.
This research was sponsored by AIO-Studien-gGmbH. Dr. Tintelnot disclosed no conflicts of interest.
SOURCE: Tintelnot J et al. SITC 2019, Abstract O16.
NATIONAL HARBOR, MD. – An immunochemotherapy regimen produced mixed results in a phase 2 trial of patients with previously untreated metastatic colorectal cancer.
The regimen – avelumab and cetuximab plus oxaliplatin, leucovorin, and 5-fluorouracil (mFOLFOX6) – failed to meet the primary endpoint for progression-free survival (PFS) but was associated with “promising” yet “preliminary” overall survival, according to Joseph Tintelnot, MD, of University Medical Center Hamburg-Eppendorf (Germany).
Dr. Tintelnot presented these results from the AVETUX trial (NCT03174405) at the annual meeting of the Society for Immunotherapy of Cancer.
The trial enrolled 43 patients with previously untreated, metastatic colorectal cancer, and 39 of them had wild-type RAS and BRAF mutations. Among those 39 patients, the median age was 62 years (range, 29-82 years), 13 patients were female, and 36 patients had left-sided tumors.
A total of 30 patients had liver metastasis, 12 had lung metastasis, and 18 had lymph node metastasis. Most patients (n = 36) had microsatellite stable tumors, 2 were microsatellite instability high, and 1 was microsatellite instability low.
Patients received IV cetuximab at 250 mg/m2 over 60-90 minutes (day 1 and 8), with a first dose of 400 mg/m2; mFOLFOX6 according to local standard – IV oxaliplatin at 85 mg/m2 IV (day 1), IV leucovorin at 400 mg/m2 IV (day 1), and IV bolus 5-fluorouracil at 400 mg/m2 (day 1) and IV at 2,400 mg/m2 (days 1-3); and IV avelumab at 10 mg/kg over 60-90 minutes (day 1 from cycle 2 onward).
The median number of treatment cycles was 8 (range, 1-34) for oxaliplatin, 13 (range, 1-35) for 5-fluorouracil, 12 (range, 1-35) for cetuximab, and 16 (range, 0-34) for avelumab. The median duration of cetuximab/avelumab treatment was 5.4 months (range, 0.7-18.4 months).
The study’s primary endpoint was 12-month PFS, and the researchers expected the PFS to rise from 40% to 57%. Unfortunately, the 12-month PFS was 40%, so the primary endpoint was not met.
However, the treatment produced a “very high” overall response rate at 81% (30/37), according to Dr. Tintelnot. A total of 4 patients achieved a complete response, 26 had a partial response, 4 had stable disease, and 3 progressed.
Dr. Tintelnot also noted a “promising” but “preliminary” overall survival rate – 84% at a median follow-up of 16.2 months. He said these results suggest PFS may not be the ideal endpoint for this combination.
Dr. Tintelnot said the combination was safe, with no unexpected toxicities. The most common grade 3-4 adverse events were infection of catheter, device, urinary tract, etc. (32%); abdominal pain, diarrhea, etc. (24%); skin reaction (21%); anemia, blood disorders, and hemolytic-uremic syndrome (18%); administration, infusion-related, and allergic reactions (16%); cognitive disturbance, meningism, syncope, and psychiatric disorders (16%); and peripheral sensory polyneuropathy and paresthesia (16%).
Dr. Tintelnot and colleagues also conducted translational research evaluating programmed death-ligand 1 (PD-L1) expression and serial circulating tumor DNA in patients on this trial.
The team found no clear correlation between PFS and T-cell diversification, tumor-infiltrating lymphocytes, or tumor proportion score. Dr. Tintelnot said this suggests classical predictive factors for PD-1/PD-L1 inhibitor treatment have a limited role with this combination.
The researchers did find that circulating tumor mutations might help predict early relapse with the regimen. The team identified 26 patients with mutations detectable in their blood. There was an immediate decline of circulating tumor mutations after treatment initiation, and reemergence of mutation clones was associated with progression.
Lastly, the researchers found that avelumab, cetuximab, and mFOLFOX6 suppressed the development of epidermal growth factor receptor–resistant subclones. There were no epidermal growth factor receptor mutations detected during follow-up.
This research was sponsored by AIO-Studien-gGmbH. Dr. Tintelnot disclosed no conflicts of interest.
SOURCE: Tintelnot J et al. SITC 2019, Abstract O16.
NATIONAL HARBOR, MD. – An immunochemotherapy regimen produced mixed results in a phase 2 trial of patients with previously untreated metastatic colorectal cancer.
The regimen – avelumab and cetuximab plus oxaliplatin, leucovorin, and 5-fluorouracil (mFOLFOX6) – failed to meet the primary endpoint for progression-free survival (PFS) but was associated with “promising” yet “preliminary” overall survival, according to Joseph Tintelnot, MD, of University Medical Center Hamburg-Eppendorf (Germany).
Dr. Tintelnot presented these results from the AVETUX trial (NCT03174405) at the annual meeting of the Society for Immunotherapy of Cancer.
The trial enrolled 43 patients with previously untreated, metastatic colorectal cancer, and 39 of them had wild-type RAS and BRAF mutations. Among those 39 patients, the median age was 62 years (range, 29-82 years), 13 patients were female, and 36 patients had left-sided tumors.
A total of 30 patients had liver metastasis, 12 had lung metastasis, and 18 had lymph node metastasis. Most patients (n = 36) had microsatellite stable tumors, 2 were microsatellite instability high, and 1 was microsatellite instability low.
Patients received IV cetuximab at 250 mg/m2 over 60-90 minutes (day 1 and 8), with a first dose of 400 mg/m2; mFOLFOX6 according to local standard – IV oxaliplatin at 85 mg/m2 IV (day 1), IV leucovorin at 400 mg/m2 IV (day 1), and IV bolus 5-fluorouracil at 400 mg/m2 (day 1) and IV at 2,400 mg/m2 (days 1-3); and IV avelumab at 10 mg/kg over 60-90 minutes (day 1 from cycle 2 onward).
The median number of treatment cycles was 8 (range, 1-34) for oxaliplatin, 13 (range, 1-35) for 5-fluorouracil, 12 (range, 1-35) for cetuximab, and 16 (range, 0-34) for avelumab. The median duration of cetuximab/avelumab treatment was 5.4 months (range, 0.7-18.4 months).
The study’s primary endpoint was 12-month PFS, and the researchers expected the PFS to rise from 40% to 57%. Unfortunately, the 12-month PFS was 40%, so the primary endpoint was not met.
However, the treatment produced a “very high” overall response rate at 81% (30/37), according to Dr. Tintelnot. A total of 4 patients achieved a complete response, 26 had a partial response, 4 had stable disease, and 3 progressed.
Dr. Tintelnot also noted a “promising” but “preliminary” overall survival rate – 84% at a median follow-up of 16.2 months. He said these results suggest PFS may not be the ideal endpoint for this combination.
Dr. Tintelnot said the combination was safe, with no unexpected toxicities. The most common grade 3-4 adverse events were infection of catheter, device, urinary tract, etc. (32%); abdominal pain, diarrhea, etc. (24%); skin reaction (21%); anemia, blood disorders, and hemolytic-uremic syndrome (18%); administration, infusion-related, and allergic reactions (16%); cognitive disturbance, meningism, syncope, and psychiatric disorders (16%); and peripheral sensory polyneuropathy and paresthesia (16%).
Dr. Tintelnot and colleagues also conducted translational research evaluating programmed death-ligand 1 (PD-L1) expression and serial circulating tumor DNA in patients on this trial.
The team found no clear correlation between PFS and T-cell diversification, tumor-infiltrating lymphocytes, or tumor proportion score. Dr. Tintelnot said this suggests classical predictive factors for PD-1/PD-L1 inhibitor treatment have a limited role with this combination.
The researchers did find that circulating tumor mutations might help predict early relapse with the regimen. The team identified 26 patients with mutations detectable in their blood. There was an immediate decline of circulating tumor mutations after treatment initiation, and reemergence of mutation clones was associated with progression.
Lastly, the researchers found that avelumab, cetuximab, and mFOLFOX6 suppressed the development of epidermal growth factor receptor–resistant subclones. There were no epidermal growth factor receptor mutations detected during follow-up.
This research was sponsored by AIO-Studien-gGmbH. Dr. Tintelnot disclosed no conflicts of interest.
SOURCE: Tintelnot J et al. SITC 2019, Abstract O16.
REPORTING FROM SITC 2019
Disentangling sleep problems and bipolar disorder
COPENHAGEN – Sleep spindle density is diminished in euthymic patients with bipolar disorder, suggesting that this sleep architecture abnormality might offer potential for early differentiation of bipolar from unipolar depression, Philipp S. Ritter, MD, said at the annual congress of the European College of Neuropsychopharmacology.
“Hopefully in the future our finding, if replicated, might have clinical utility. It might be a kind of soft biomarker that could be used in early detection, or, in people having their first depressive episode, you could perhaps use this to risk-stratify. And if you see there’s a great reduction in spindle density then a patient might have a higher likelihood of a bipolar disorder, so you might not want to treat with antidepressants that have a high switch rate,” explained Dr. Ritter, a psychiatrist at Technical University of Dresden (Germany).
Sleep spindles are a specific sleep architecture formation evident on the sleep EEG. They are sudden high-amplitude bursts occurring in stage N2 sleep. They are thought to be associated with sensory gating and memory processes. Other investigators have repeatedly demonstrated that patients with schizophrenia, as well as their asymptomatic first-degree relatives, have a reduced density of fast spindles greater than 13 Hz, compared with the general population. In contrast, patients with unipolar depression do not display this polysomnographic abnormality.
These findings prompted Dr. Ritter and his coinvestigators to conduct an all-night polysomnographic study in 24 euthymic patients with bipolar disorder and 25 healthy controls. The bipolar patients demonstrated a reduced density and mean frequency of fast sleep spindles, but not slow spindles (Acta Psychiatr Scand. 2018 Aug;138[2]:163-72).
These sleep spindle findings implicate thalamic dysfunction as a potential neurobiologic mechanism in bipolar disorder, since spindles are generated in the thalamus and spun off in thalamocortical feedback loops, Dr. Ritter observed.
Which came first: the chicken (bipolar disorder) or the egg (sleep disturbance)?
Sleep problems are a prominent issue in patients with bipolar disorder, even when they are euthymic.
“Anybody who deals with bipolar patients knows that sleep is a constant issue. You are always talking to your patients about their sleep. They’re sleeping too much, or not enough, or they’re sleeping just about right but it’s unsatisfactory. They do not sleep well. And if there’s something that disrupts their sleep, it can precipitate episodes,” Dr. Ritter said.
He wondered whether sleep problems are an intrinsic part of the bipolar illness, or a byproduct of the stress of having a severe mental disorder, perhaps a medication side effect, or whether the disordered sleep actually precedes the clinical expression of the mood disorder. So he and his coinvestigators turned to a Munich-based cohort sample of 3,021 adolescents and young adults assessed via the standardized Composite International Diagnostic Interview four times during 10 years of prospective follow-up.
Among 1,943 participants in the epidemiologic study who were free of major mental disorders at entry, the presence of sleep disturbance at baseline as quantified using the Symptom Checklist-90-Revised doubled the risk of developing bipolar disorder within the next 10 years. After the researchers controlled for potential confounders, including parental mood disorder, gender, age, and a history of alcohol or cannabis dependence, poor sleep quality at baseline remained independently associated with a 1.75-fold increased chance of subsequently developing bipolar disorder (J Psychiatr Res. 2015 Sep;68:76-82).
“This is a little bit higher, actually, than the odds ratio usually found for depressive disorders,” said Dr. Ritter.
he added.
Dr. Ritter reported having no financial conflicts regarding these studies.
COPENHAGEN – Sleep spindle density is diminished in euthymic patients with bipolar disorder, suggesting that this sleep architecture abnormality might offer potential for early differentiation of bipolar from unipolar depression, Philipp S. Ritter, MD, said at the annual congress of the European College of Neuropsychopharmacology.
“Hopefully in the future our finding, if replicated, might have clinical utility. It might be a kind of soft biomarker that could be used in early detection, or, in people having their first depressive episode, you could perhaps use this to risk-stratify. And if you see there’s a great reduction in spindle density then a patient might have a higher likelihood of a bipolar disorder, so you might not want to treat with antidepressants that have a high switch rate,” explained Dr. Ritter, a psychiatrist at Technical University of Dresden (Germany).
Sleep spindles are a specific sleep architecture formation evident on the sleep EEG. They are sudden high-amplitude bursts occurring in stage N2 sleep. They are thought to be associated with sensory gating and memory processes. Other investigators have repeatedly demonstrated that patients with schizophrenia, as well as their asymptomatic first-degree relatives, have a reduced density of fast spindles greater than 13 Hz, compared with the general population. In contrast, patients with unipolar depression do not display this polysomnographic abnormality.
These findings prompted Dr. Ritter and his coinvestigators to conduct an all-night polysomnographic study in 24 euthymic patients with bipolar disorder and 25 healthy controls. The bipolar patients demonstrated a reduced density and mean frequency of fast sleep spindles, but not slow spindles (Acta Psychiatr Scand. 2018 Aug;138[2]:163-72).
These sleep spindle findings implicate thalamic dysfunction as a potential neurobiologic mechanism in bipolar disorder, since spindles are generated in the thalamus and spun off in thalamocortical feedback loops, Dr. Ritter observed.
Which came first: the chicken (bipolar disorder) or the egg (sleep disturbance)?
Sleep problems are a prominent issue in patients with bipolar disorder, even when they are euthymic.
“Anybody who deals with bipolar patients knows that sleep is a constant issue. You are always talking to your patients about their sleep. They’re sleeping too much, or not enough, or they’re sleeping just about right but it’s unsatisfactory. They do not sleep well. And if there’s something that disrupts their sleep, it can precipitate episodes,” Dr. Ritter said.
He wondered whether sleep problems are an intrinsic part of the bipolar illness, or a byproduct of the stress of having a severe mental disorder, perhaps a medication side effect, or whether the disordered sleep actually precedes the clinical expression of the mood disorder. So he and his coinvestigators turned to a Munich-based cohort sample of 3,021 adolescents and young adults assessed via the standardized Composite International Diagnostic Interview four times during 10 years of prospective follow-up.
Among 1,943 participants in the epidemiologic study who were free of major mental disorders at entry, the presence of sleep disturbance at baseline as quantified using the Symptom Checklist-90-Revised doubled the risk of developing bipolar disorder within the next 10 years. After the researchers controlled for potential confounders, including parental mood disorder, gender, age, and a history of alcohol or cannabis dependence, poor sleep quality at baseline remained independently associated with a 1.75-fold increased chance of subsequently developing bipolar disorder (J Psychiatr Res. 2015 Sep;68:76-82).
“This is a little bit higher, actually, than the odds ratio usually found for depressive disorders,” said Dr. Ritter.
he added.
Dr. Ritter reported having no financial conflicts regarding these studies.
COPENHAGEN – Sleep spindle density is diminished in euthymic patients with bipolar disorder, suggesting that this sleep architecture abnormality might offer potential for early differentiation of bipolar from unipolar depression, Philipp S. Ritter, MD, said at the annual congress of the European College of Neuropsychopharmacology.
“Hopefully in the future our finding, if replicated, might have clinical utility. It might be a kind of soft biomarker that could be used in early detection, or, in people having their first depressive episode, you could perhaps use this to risk-stratify. And if you see there’s a great reduction in spindle density then a patient might have a higher likelihood of a bipolar disorder, so you might not want to treat with antidepressants that have a high switch rate,” explained Dr. Ritter, a psychiatrist at Technical University of Dresden (Germany).
Sleep spindles are a specific sleep architecture formation evident on the sleep EEG. They are sudden high-amplitude bursts occurring in stage N2 sleep. They are thought to be associated with sensory gating and memory processes. Other investigators have repeatedly demonstrated that patients with schizophrenia, as well as their asymptomatic first-degree relatives, have a reduced density of fast spindles greater than 13 Hz, compared with the general population. In contrast, patients with unipolar depression do not display this polysomnographic abnormality.
These findings prompted Dr. Ritter and his coinvestigators to conduct an all-night polysomnographic study in 24 euthymic patients with bipolar disorder and 25 healthy controls. The bipolar patients demonstrated a reduced density and mean frequency of fast sleep spindles, but not slow spindles (Acta Psychiatr Scand. 2018 Aug;138[2]:163-72).
These sleep spindle findings implicate thalamic dysfunction as a potential neurobiologic mechanism in bipolar disorder, since spindles are generated in the thalamus and spun off in thalamocortical feedback loops, Dr. Ritter observed.
Which came first: the chicken (bipolar disorder) or the egg (sleep disturbance)?
Sleep problems are a prominent issue in patients with bipolar disorder, even when they are euthymic.
“Anybody who deals with bipolar patients knows that sleep is a constant issue. You are always talking to your patients about their sleep. They’re sleeping too much, or not enough, or they’re sleeping just about right but it’s unsatisfactory. They do not sleep well. And if there’s something that disrupts their sleep, it can precipitate episodes,” Dr. Ritter said.
He wondered whether sleep problems are an intrinsic part of the bipolar illness, or a byproduct of the stress of having a severe mental disorder, perhaps a medication side effect, or whether the disordered sleep actually precedes the clinical expression of the mood disorder. So he and his coinvestigators turned to a Munich-based cohort sample of 3,021 adolescents and young adults assessed via the standardized Composite International Diagnostic Interview four times during 10 years of prospective follow-up.
Among 1,943 participants in the epidemiologic study who were free of major mental disorders at entry, the presence of sleep disturbance at baseline as quantified using the Symptom Checklist-90-Revised doubled the risk of developing bipolar disorder within the next 10 years. After the researchers controlled for potential confounders, including parental mood disorder, gender, age, and a history of alcohol or cannabis dependence, poor sleep quality at baseline remained independently associated with a 1.75-fold increased chance of subsequently developing bipolar disorder (J Psychiatr Res. 2015 Sep;68:76-82).
“This is a little bit higher, actually, than the odds ratio usually found for depressive disorders,” said Dr. Ritter.
he added.
Dr. Ritter reported having no financial conflicts regarding these studies.
REPORTING FROM ECNP 2019
Office of Inspector General
Question: Which one of the following statements is incorrect?
A. Office of Inspector General (OIG) is a federal agency of Department of Health & Human Services (HHS) that investigates statutory violations of health care fraud and abuse.
B. The three main legal minefields for physicians are false claims, kickbacks, and self-referrals.
C. Jail terms are part of the penalties provided by law.
D. OIG is also responsible for excluding violators from participating in Medicare/Medicaid programs, as well as curtailing a physician’s license to practice.
E. A private citizen can file a qui tam lawsuit against an errant practitioner or health care entity for fraud and abuse.
Answer: D. Health care fraud, waste, and abuse consume some 10% of federal health expenditures despite well-established laws that attempt to prevent and reduce such losses. The Department of Health & Human Services (HHS) Office of Inspector General, as well as the Department of Justice and the Centers for Medicare & Medicaid Services are charged with enforcing these and other laws like the Emergency Medical Treatment and Labor Act. Their web pages, referenced throughout this article, contain a wealth of information for the practitioner.
The term “Office of Inspector General” (OIG) refers to the oversight division of a federal or state agency charged with identifying and investigating fraud, waste, abuse, and mismanagement within that department or agency. There are currently 73 separate federal offices of inspectors general, which employ armed and unarmed criminal investigators, auditors, forensic auditors called “audigators,” and a variety of other specialists. An Act of Congress in 1976 established the first OIG under HHS. Besides being the first, HHS-OIG is also the largest, with a staff of approximately 1,600. A majority of resources goes toward the oversight of Medicare and Medicaid, as well as programs under other HHS institutions such as the Centers for Disease Control and Prevention, National Institutes of Health, and the Food and Drug Administration.1
HHS-OIG has the authority to seek civil monetary penalties, assessments, and exclusion against an individual or entity based on a wide variety of prohibited conduct affecting federal health care programs. Stiff penalties are regularly assessed against violators, and jail terms are not uncommon; however, it has no direct jurisdictions over physician licensure or nonfederal programs. The government maintains a pictorial list of its most wanted health care fugitives2 and provides an excellent set of Physician Education Training Materials on its website.3
False Claims Act (FCA)
In the health care arena, violation of FCA (31 U.S.C. §§3729-3733) is the foremost infraction. False claims by physicians can include billing for noncovered services such as experimental treatments, double billing, billing the government as the primary payer, or regularly waiving deductibles and copayments, as well as quality of care issues and unnecessary services. Wrongdoing also includes knowingly using another patient’s name for purposes of, say, federal drug coverage, billing for no-shows, and misrepresenting the diagnosis to justify services, as well as other claims. In the modern doctor’s office, the EMR enables easy check-offs on a preprinted form as documentation of actual work done. However, fraud is implicated if the information is deliberately misleading such as for purposes of upcoding. Importantly, physicians are liable for the actions of their office staff, so it is prudent to oversee and supervise all such activities. Naturally, one should document all claims that are sent and know the rules for allowable and excluded services.
FCA is an old law that was first enacted in 1863. It imposes liability for submitting a payment demand to the federal government where there is actual or constructive knowledge that the claim is false. Intent to defraud is not a required element but knowing or showing reckless disregard of the truth is. However, an error that is negligently committed is insufficient to constitute a violation. Penalties include treble damages, costs and attorney fees, and fines of $11,000 per false claim, as well as possible imprisonment and criminal fines. A so-called whistle-blower may file a lawsuit on behalf of the government and is entitled to a percentage of any recoveries. Whistle-blowers may be current or ex-business partners, hospital or office staff, patients, or competitors. The fact that a claim results from a kickback or is made in violation of the Stark law (discussed below) may also render it fraudulent, thus creating additional liability under FCA.
HHS-OIG, as well as the Department of Justice, discloses named cases of statutory violations on their websites. A few random 2019 examples include a New York licensed doctor was convicted of nine counts in connection with Oxycodone and Fentanyl diversion scheme; a Newton, Mass., geriatrician agreed to pay $680,000 to resolve allegations that he violated the False Claims Act by submitting inflated claims to Medicare and the Massachusetts Medicaid program (MassHealth) for care rendered to nursing home patients; and two Clermont, Fla., ophthalmologists agreed to pay a combined total of $157,312.32 to resolve allegations that they violated FCA by knowingly billing the government for mutually exclusive eyelid repair surgeries.
Anti-Kickback Statute (AKS)
AKS (42 U.S.C. § 1320a-7b[b]) is a criminal law that prohibits the knowing and willful payment of “remuneration” to induce or reward patient referrals or the generation of business involving any item or service payable by federal health care programs. Remuneration includes anything of value and can take many forms besides cash, such as free rent, lavish travel, and excessive compensation for medical directorships or consultancies. Rewarding a referral source may be acceptable in some industries, but is a crime in federal health care programs.
Moreover, the statute covers both the payers of kickbacks (those who offer or pay remuneration) and the recipients of kickbacks. Each party’s intent is a key element of their liability under AKS. Physicians who pay or accept kickbacks face penalties of up to $50,000 per kickback plus three times the amount of the remuneration and criminal prosecution. As an example, a Tulsa, Okla., doctor earlier this year agreed to pay the government $84,666.42 for allegedly accepting illegal kickback payments from a pharmacy, and in another case, a marketer agreed to pay nearly $340,000 for receiving kickbacks in exchange for prescription referrals.
A physician is an attractive target for kickback schemes. The kickback prohibition applies to all sources, even patients. For example, where the Medicare and Medicaid programs require patients to be responsible for copays for services, the health care provider is generally required to collect that money from the patients. Advertising the forgiveness of copayments or routinely waiving these copays would violate AKS. However, one may waive a copayment when a patient cannot afford to pay one or is uninsured. AKS also imposes civil monetary penalties on physicians who offer remuneration to Medicare and Medicaid beneficiaries to influence them to use their services. Note that the government does not need to prove patient harm or financial loss to show that a violation has occurred, and physicians can be guilty even if they rendered services that are medically necessary.
There are so-called safe harbors that protect certain payment and business practices from running afoul of AKS. The rules and requirements are complex, and require full understanding and strict adherence.4
Physician Self-Referral Law
The Physician Self-Referral Law (42 U.S.C. § 1395nn), commonly referred to as the Stark law, prohibits physicians from referring patients to receive “designated health services” payable by Medicare or Medicaid from entities with which the physician or an immediate family member has a financial relationship, unless an exception applies. Financial relationships include both ownership/investment interests and compensation arrangements. A partial list of “designated health services” includes services related to clinical laboratory, physical therapy, radiology, parenteral and enteral supplies, prosthetic devices and supplies, home health care outpatient prescription drugs, and inpatient and outpatient hospital services. This list is not meant to be an exhaustive one.
Stark is a strict liability statute, which means proof of specific intent to violate the law is not required. The law prohibits the submission, or causing the submission, of claims in violation of the law’s restrictions on referrals. Penalties for physicians who violate the Stark law include fines, as well as exclusion from participation in federal health care programs. Like AKS, Stark law features its own safe harbors.
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some materials may have been discussed in earlier columns. For additional information, readers may contact the author at siang@hawaii.edu.
References
1. HHS Office of Inspector General. About OIG. https://oig.hhs.gov/about-oig/about-us/index.asp
2. HHS Office of Inspector General. OIG most wanted fugitives.
3. HHS Office of Inspector General. “Physician education training materials.” A roadmap for new physicians: Avoiding Medicare and Medicaid fraud and abuse.
4. HHS Office of Inspector General. Safe harbor regulations.
Question: Which one of the following statements is incorrect?
A. Office of Inspector General (OIG) is a federal agency of Department of Health & Human Services (HHS) that investigates statutory violations of health care fraud and abuse.
B. The three main legal minefields for physicians are false claims, kickbacks, and self-referrals.
C. Jail terms are part of the penalties provided by law.
D. OIG is also responsible for excluding violators from participating in Medicare/Medicaid programs, as well as curtailing a physician’s license to practice.
E. A private citizen can file a qui tam lawsuit against an errant practitioner or health care entity for fraud and abuse.
Answer: D. Health care fraud, waste, and abuse consume some 10% of federal health expenditures despite well-established laws that attempt to prevent and reduce such losses. The Department of Health & Human Services (HHS) Office of Inspector General, as well as the Department of Justice and the Centers for Medicare & Medicaid Services are charged with enforcing these and other laws like the Emergency Medical Treatment and Labor Act. Their web pages, referenced throughout this article, contain a wealth of information for the practitioner.
The term “Office of Inspector General” (OIG) refers to the oversight division of a federal or state agency charged with identifying and investigating fraud, waste, abuse, and mismanagement within that department or agency. There are currently 73 separate federal offices of inspectors general, which employ armed and unarmed criminal investigators, auditors, forensic auditors called “audigators,” and a variety of other specialists. An Act of Congress in 1976 established the first OIG under HHS. Besides being the first, HHS-OIG is also the largest, with a staff of approximately 1,600. A majority of resources goes toward the oversight of Medicare and Medicaid, as well as programs under other HHS institutions such as the Centers for Disease Control and Prevention, National Institutes of Health, and the Food and Drug Administration.1
HHS-OIG has the authority to seek civil monetary penalties, assessments, and exclusion against an individual or entity based on a wide variety of prohibited conduct affecting federal health care programs. Stiff penalties are regularly assessed against violators, and jail terms are not uncommon; however, it has no direct jurisdictions over physician licensure or nonfederal programs. The government maintains a pictorial list of its most wanted health care fugitives2 and provides an excellent set of Physician Education Training Materials on its website.3
False Claims Act (FCA)
In the health care arena, violation of FCA (31 U.S.C. §§3729-3733) is the foremost infraction. False claims by physicians can include billing for noncovered services such as experimental treatments, double billing, billing the government as the primary payer, or regularly waiving deductibles and copayments, as well as quality of care issues and unnecessary services. Wrongdoing also includes knowingly using another patient’s name for purposes of, say, federal drug coverage, billing for no-shows, and misrepresenting the diagnosis to justify services, as well as other claims. In the modern doctor’s office, the EMR enables easy check-offs on a preprinted form as documentation of actual work done. However, fraud is implicated if the information is deliberately misleading such as for purposes of upcoding. Importantly, physicians are liable for the actions of their office staff, so it is prudent to oversee and supervise all such activities. Naturally, one should document all claims that are sent and know the rules for allowable and excluded services.
FCA is an old law that was first enacted in 1863. It imposes liability for submitting a payment demand to the federal government where there is actual or constructive knowledge that the claim is false. Intent to defraud is not a required element but knowing or showing reckless disregard of the truth is. However, an error that is negligently committed is insufficient to constitute a violation. Penalties include treble damages, costs and attorney fees, and fines of $11,000 per false claim, as well as possible imprisonment and criminal fines. A so-called whistle-blower may file a lawsuit on behalf of the government and is entitled to a percentage of any recoveries. Whistle-blowers may be current or ex-business partners, hospital or office staff, patients, or competitors. The fact that a claim results from a kickback or is made in violation of the Stark law (discussed below) may also render it fraudulent, thus creating additional liability under FCA.
HHS-OIG, as well as the Department of Justice, discloses named cases of statutory violations on their websites. A few random 2019 examples include a New York licensed doctor was convicted of nine counts in connection with Oxycodone and Fentanyl diversion scheme; a Newton, Mass., geriatrician agreed to pay $680,000 to resolve allegations that he violated the False Claims Act by submitting inflated claims to Medicare and the Massachusetts Medicaid program (MassHealth) for care rendered to nursing home patients; and two Clermont, Fla., ophthalmologists agreed to pay a combined total of $157,312.32 to resolve allegations that they violated FCA by knowingly billing the government for mutually exclusive eyelid repair surgeries.
Anti-Kickback Statute (AKS)
AKS (42 U.S.C. § 1320a-7b[b]) is a criminal law that prohibits the knowing and willful payment of “remuneration” to induce or reward patient referrals or the generation of business involving any item or service payable by federal health care programs. Remuneration includes anything of value and can take many forms besides cash, such as free rent, lavish travel, and excessive compensation for medical directorships or consultancies. Rewarding a referral source may be acceptable in some industries, but is a crime in federal health care programs.
Moreover, the statute covers both the payers of kickbacks (those who offer or pay remuneration) and the recipients of kickbacks. Each party’s intent is a key element of their liability under AKS. Physicians who pay or accept kickbacks face penalties of up to $50,000 per kickback plus three times the amount of the remuneration and criminal prosecution. As an example, a Tulsa, Okla., doctor earlier this year agreed to pay the government $84,666.42 for allegedly accepting illegal kickback payments from a pharmacy, and in another case, a marketer agreed to pay nearly $340,000 for receiving kickbacks in exchange for prescription referrals.
A physician is an attractive target for kickback schemes. The kickback prohibition applies to all sources, even patients. For example, where the Medicare and Medicaid programs require patients to be responsible for copays for services, the health care provider is generally required to collect that money from the patients. Advertising the forgiveness of copayments or routinely waiving these copays would violate AKS. However, one may waive a copayment when a patient cannot afford to pay one or is uninsured. AKS also imposes civil monetary penalties on physicians who offer remuneration to Medicare and Medicaid beneficiaries to influence them to use their services. Note that the government does not need to prove patient harm or financial loss to show that a violation has occurred, and physicians can be guilty even if they rendered services that are medically necessary.
There are so-called safe harbors that protect certain payment and business practices from running afoul of AKS. The rules and requirements are complex, and require full understanding and strict adherence.4
Physician Self-Referral Law
The Physician Self-Referral Law (42 U.S.C. § 1395nn), commonly referred to as the Stark law, prohibits physicians from referring patients to receive “designated health services” payable by Medicare or Medicaid from entities with which the physician or an immediate family member has a financial relationship, unless an exception applies. Financial relationships include both ownership/investment interests and compensation arrangements. A partial list of “designated health services” includes services related to clinical laboratory, physical therapy, radiology, parenteral and enteral supplies, prosthetic devices and supplies, home health care outpatient prescription drugs, and inpatient and outpatient hospital services. This list is not meant to be an exhaustive one.
Stark is a strict liability statute, which means proof of specific intent to violate the law is not required. The law prohibits the submission, or causing the submission, of claims in violation of the law’s restrictions on referrals. Penalties for physicians who violate the Stark law include fines, as well as exclusion from participation in federal health care programs. Like AKS, Stark law features its own safe harbors.
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some materials may have been discussed in earlier columns. For additional information, readers may contact the author at siang@hawaii.edu.
References
1. HHS Office of Inspector General. About OIG. https://oig.hhs.gov/about-oig/about-us/index.asp
2. HHS Office of Inspector General. OIG most wanted fugitives.
3. HHS Office of Inspector General. “Physician education training materials.” A roadmap for new physicians: Avoiding Medicare and Medicaid fraud and abuse.
4. HHS Office of Inspector General. Safe harbor regulations.
Question: Which one of the following statements is incorrect?
A. Office of Inspector General (OIG) is a federal agency of Department of Health & Human Services (HHS) that investigates statutory violations of health care fraud and abuse.
B. The three main legal minefields for physicians are false claims, kickbacks, and self-referrals.
C. Jail terms are part of the penalties provided by law.
D. OIG is also responsible for excluding violators from participating in Medicare/Medicaid programs, as well as curtailing a physician’s license to practice.
E. A private citizen can file a qui tam lawsuit against an errant practitioner or health care entity for fraud and abuse.
Answer: D. Health care fraud, waste, and abuse consume some 10% of federal health expenditures despite well-established laws that attempt to prevent and reduce such losses. The Department of Health & Human Services (HHS) Office of Inspector General, as well as the Department of Justice and the Centers for Medicare & Medicaid Services are charged with enforcing these and other laws like the Emergency Medical Treatment and Labor Act. Their web pages, referenced throughout this article, contain a wealth of information for the practitioner.
The term “Office of Inspector General” (OIG) refers to the oversight division of a federal or state agency charged with identifying and investigating fraud, waste, abuse, and mismanagement within that department or agency. There are currently 73 separate federal offices of inspectors general, which employ armed and unarmed criminal investigators, auditors, forensic auditors called “audigators,” and a variety of other specialists. An Act of Congress in 1976 established the first OIG under HHS. Besides being the first, HHS-OIG is also the largest, with a staff of approximately 1,600. A majority of resources goes toward the oversight of Medicare and Medicaid, as well as programs under other HHS institutions such as the Centers for Disease Control and Prevention, National Institutes of Health, and the Food and Drug Administration.1
HHS-OIG has the authority to seek civil monetary penalties, assessments, and exclusion against an individual or entity based on a wide variety of prohibited conduct affecting federal health care programs. Stiff penalties are regularly assessed against violators, and jail terms are not uncommon; however, it has no direct jurisdictions over physician licensure or nonfederal programs. The government maintains a pictorial list of its most wanted health care fugitives2 and provides an excellent set of Physician Education Training Materials on its website.3
False Claims Act (FCA)
In the health care arena, violation of FCA (31 U.S.C. §§3729-3733) is the foremost infraction. False claims by physicians can include billing for noncovered services such as experimental treatments, double billing, billing the government as the primary payer, or regularly waiving deductibles and copayments, as well as quality of care issues and unnecessary services. Wrongdoing also includes knowingly using another patient’s name for purposes of, say, federal drug coverage, billing for no-shows, and misrepresenting the diagnosis to justify services, as well as other claims. In the modern doctor’s office, the EMR enables easy check-offs on a preprinted form as documentation of actual work done. However, fraud is implicated if the information is deliberately misleading such as for purposes of upcoding. Importantly, physicians are liable for the actions of their office staff, so it is prudent to oversee and supervise all such activities. Naturally, one should document all claims that are sent and know the rules for allowable and excluded services.
FCA is an old law that was first enacted in 1863. It imposes liability for submitting a payment demand to the federal government where there is actual or constructive knowledge that the claim is false. Intent to defraud is not a required element but knowing or showing reckless disregard of the truth is. However, an error that is negligently committed is insufficient to constitute a violation. Penalties include treble damages, costs and attorney fees, and fines of $11,000 per false claim, as well as possible imprisonment and criminal fines. A so-called whistle-blower may file a lawsuit on behalf of the government and is entitled to a percentage of any recoveries. Whistle-blowers may be current or ex-business partners, hospital or office staff, patients, or competitors. The fact that a claim results from a kickback or is made in violation of the Stark law (discussed below) may also render it fraudulent, thus creating additional liability under FCA.
HHS-OIG, as well as the Department of Justice, discloses named cases of statutory violations on their websites. A few random 2019 examples include a New York licensed doctor was convicted of nine counts in connection with Oxycodone and Fentanyl diversion scheme; a Newton, Mass., geriatrician agreed to pay $680,000 to resolve allegations that he violated the False Claims Act by submitting inflated claims to Medicare and the Massachusetts Medicaid program (MassHealth) for care rendered to nursing home patients; and two Clermont, Fla., ophthalmologists agreed to pay a combined total of $157,312.32 to resolve allegations that they violated FCA by knowingly billing the government for mutually exclusive eyelid repair surgeries.
Anti-Kickback Statute (AKS)
AKS (42 U.S.C. § 1320a-7b[b]) is a criminal law that prohibits the knowing and willful payment of “remuneration” to induce or reward patient referrals or the generation of business involving any item or service payable by federal health care programs. Remuneration includes anything of value and can take many forms besides cash, such as free rent, lavish travel, and excessive compensation for medical directorships or consultancies. Rewarding a referral source may be acceptable in some industries, but is a crime in federal health care programs.
Moreover, the statute covers both the payers of kickbacks (those who offer or pay remuneration) and the recipients of kickbacks. Each party’s intent is a key element of their liability under AKS. Physicians who pay or accept kickbacks face penalties of up to $50,000 per kickback plus three times the amount of the remuneration and criminal prosecution. As an example, a Tulsa, Okla., doctor earlier this year agreed to pay the government $84,666.42 for allegedly accepting illegal kickback payments from a pharmacy, and in another case, a marketer agreed to pay nearly $340,000 for receiving kickbacks in exchange for prescription referrals.
A physician is an attractive target for kickback schemes. The kickback prohibition applies to all sources, even patients. For example, where the Medicare and Medicaid programs require patients to be responsible for copays for services, the health care provider is generally required to collect that money from the patients. Advertising the forgiveness of copayments or routinely waiving these copays would violate AKS. However, one may waive a copayment when a patient cannot afford to pay one or is uninsured. AKS also imposes civil monetary penalties on physicians who offer remuneration to Medicare and Medicaid beneficiaries to influence them to use their services. Note that the government does not need to prove patient harm or financial loss to show that a violation has occurred, and physicians can be guilty even if they rendered services that are medically necessary.
There are so-called safe harbors that protect certain payment and business practices from running afoul of AKS. The rules and requirements are complex, and require full understanding and strict adherence.4
Physician Self-Referral Law
The Physician Self-Referral Law (42 U.S.C. § 1395nn), commonly referred to as the Stark law, prohibits physicians from referring patients to receive “designated health services” payable by Medicare or Medicaid from entities with which the physician or an immediate family member has a financial relationship, unless an exception applies. Financial relationships include both ownership/investment interests and compensation arrangements. A partial list of “designated health services” includes services related to clinical laboratory, physical therapy, radiology, parenteral and enteral supplies, prosthetic devices and supplies, home health care outpatient prescription drugs, and inpatient and outpatient hospital services. This list is not meant to be an exhaustive one.
Stark is a strict liability statute, which means proof of specific intent to violate the law is not required. The law prohibits the submission, or causing the submission, of claims in violation of the law’s restrictions on referrals. Penalties for physicians who violate the Stark law include fines, as well as exclusion from participation in federal health care programs. Like AKS, Stark law features its own safe harbors.
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some materials may have been discussed in earlier columns. For additional information, readers may contact the author at siang@hawaii.edu.
References
1. HHS Office of Inspector General. About OIG. https://oig.hhs.gov/about-oig/about-us/index.asp
2. HHS Office of Inspector General. OIG most wanted fugitives.
3. HHS Office of Inspector General. “Physician education training materials.” A roadmap for new physicians: Avoiding Medicare and Medicaid fraud and abuse.
4. HHS Office of Inspector General. Safe harbor regulations.
Antecedent Chronic Lymphocytic Leukemia May Be Associated With More Aggressive Mycosis Fungoides
To the Editor:
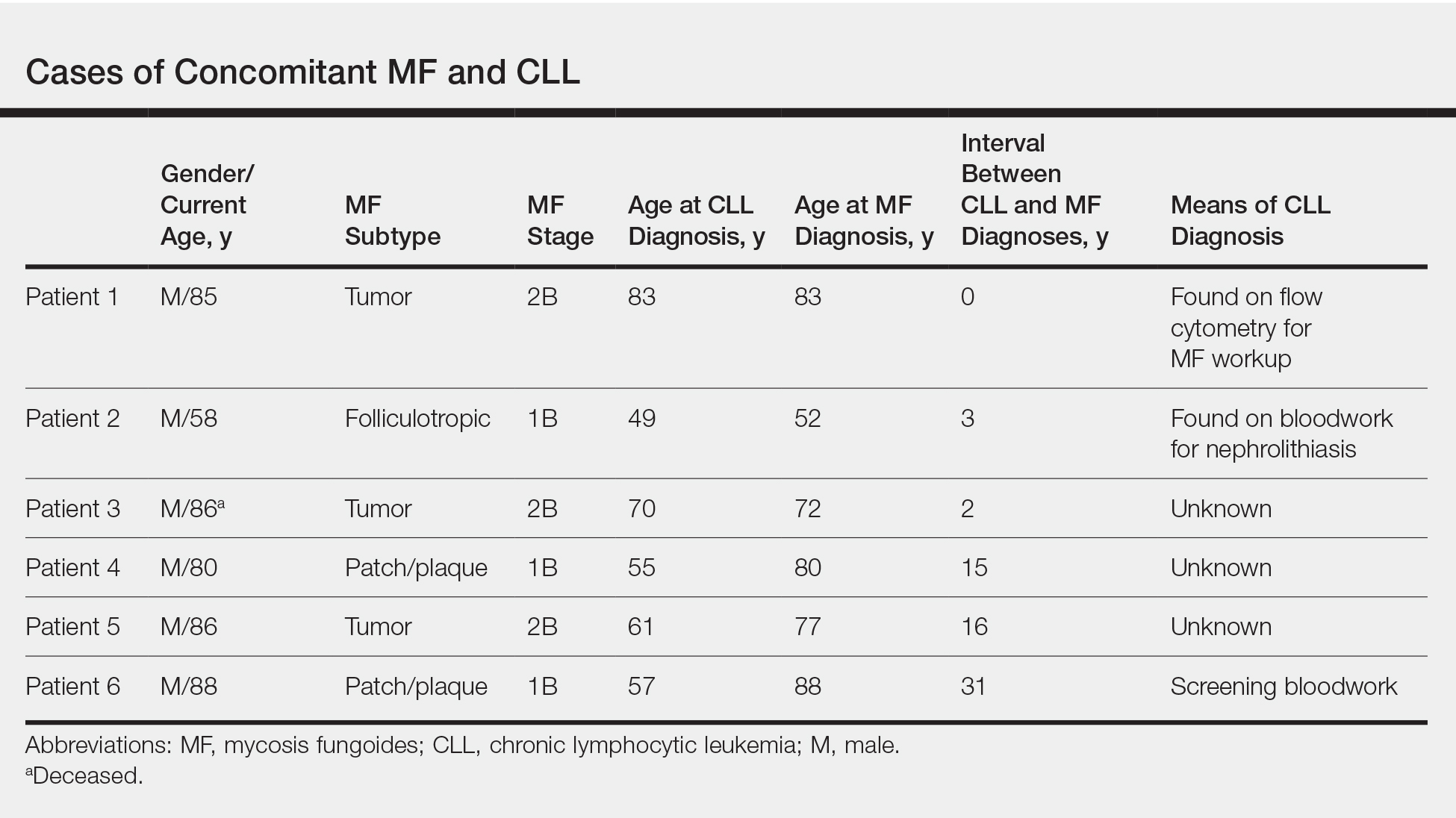
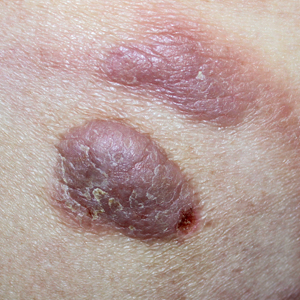
Mycosis fungoides (MF) is the most common form of primary cutaneous T-cell lymphoma. It has been associated with increased risk for other visceral and hematologic malignancies.1 Chronic lymphocytic leukemia (CLL) is one of the most common hematologic malignancies. In the United States, a patient’s lifetime risk for CLL is 0.6%. Chronic lymphocytic leukemia often is diagnosed as an incidental finding and typically is not detrimental to a patient’s health. Six cases of MF with antecedent or concomitant CLL were identified in a cohort of patients treated at the University of Minnesota (Minneapolis, Minnesota) from 2005 to 2017 (Table).
All 6 patients were male, with a mean age of 80.5 years. The mean age at CLL diagnosis was 62.5 years, while the mean age at MF diagnosis was 75.3 years. Three patients were younger than 60 years when their CLL was diagnosed: 49, 55, and 57 years. Notably, 4 patients had more aggressive types of MF: 3 with tumor-stage disease, and 1 with folliculotropic MF. Five patients were diagnosed with CLL before their MF was diagnosed (mean, 13.4 years prior; range, 3–31 years), and 1 was diagnosed as part of the initial MF workup.
Given the frequency of both MF and CLL, the co-occurrence of these diseases is not surprising, as other case reports and a larger case series have described the relationship between MF and malignancy.2 It is possible that CLL patients are more likely to be diagnosed with MF because of their regular hematology/oncology follow-up; however, none of our patients were referred from hematology/oncology to dermatology. Alternatively, patients with MF may be more likely to be diagnosed with CLL because of repeated bloodwork performed for diagnosis and screening, which occurred in only 1 of 6 cases. Most of the other patients were diagnosed with MF more than a decade after being diagnosed with CLL.
Does having CLL make patients more likely to develop MF? It is known that patients with CLL may experience immunodeficiency secondary to immune dysregulation, making them more susceptible to infection and secondary malignancies.3 Of our 6 cases, 4 had aggressive or advanced forms of MF, which is similar to the findings of Chang et al.2 In their report, of 8 patients with MF, 2 had tumor-stage disease and 2 had erythrodermic MF. They determined that these patients had worse overall survival.2 Our data corroborate the finding that patients with CLL may develop more severe MF, which leads to the conclusion that patients diagnosed with CLL before, concomitantly, or after their diagnosis of MF should be closely monitored. It is notable that patients with more advanced disease tend to be older at the time of diagnosis and that patients who are diagnosed at 57 years or older have been found to have worse disease-specific survival.4,5
This report is limited by the small sample size (6 cases), but it serves to draw attention to the phenomenon of co-occurrence of MF and CLL, and the concern that patients with CLL may develop more aggressive MF.
- Huang KP, Weinstock MA, Clarke CA, et al. Second lymphomas and other malignant neoplasms in patients with mycosis fungoides and Sézary syndrome. Arch Dermatol. 2007;143:45-50.
- Chang MB, Weaver AL, Brewer JD. Cutaneous T-cell lymphoma in patients with chronic lymphocytic leukemia: clinical characteristics, temporal relationships, and survival data in a series of 14 patients at Mayo Clinic. Int J Dermatol. 2014;53:966-970.
- Hamblin AD, Hamblin TJ. The immunodeficiency of chronic lymphocytic leukaemia. Br Med Bull. 2008;87:49-62.
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866.
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730-4739.
To the Editor:
Mycosis fungoides (MF) is the most common form of primary cutaneous T-cell lymphoma. It has been associated with increased risk for other visceral and hematologic malignancies.1 Chronic lymphocytic leukemia (CLL) is one of the most common hematologic malignancies. In the United States, a patient’s lifetime risk for CLL is 0.6%. Chronic lymphocytic leukemia often is diagnosed as an incidental finding and typically is not detrimental to a patient’s health. Six cases of MF with antecedent or concomitant CLL were identified in a cohort of patients treated at the University of Minnesota (Minneapolis, Minnesota) from 2005 to 2017 (Table).
All 6 patients were male, with a mean age of 80.5 years. The mean age at CLL diagnosis was 62.5 years, while the mean age at MF diagnosis was 75.3 years. Three patients were younger than 60 years when their CLL was diagnosed: 49, 55, and 57 years. Notably, 4 patients had more aggressive types of MF: 3 with tumor-stage disease, and 1 with folliculotropic MF. Five patients were diagnosed with CLL before their MF was diagnosed (mean, 13.4 years prior; range, 3–31 years), and 1 was diagnosed as part of the initial MF workup.
Given the frequency of both MF and CLL, the co-occurrence of these diseases is not surprising, as other case reports and a larger case series have described the relationship between MF and malignancy.2 It is possible that CLL patients are more likely to be diagnosed with MF because of their regular hematology/oncology follow-up; however, none of our patients were referred from hematology/oncology to dermatology. Alternatively, patients with MF may be more likely to be diagnosed with CLL because of repeated bloodwork performed for diagnosis and screening, which occurred in only 1 of 6 cases. Most of the other patients were diagnosed with MF more than a decade after being diagnosed with CLL.
Does having CLL make patients more likely to develop MF? It is known that patients with CLL may experience immunodeficiency secondary to immune dysregulation, making them more susceptible to infection and secondary malignancies.3 Of our 6 cases, 4 had aggressive or advanced forms of MF, which is similar to the findings of Chang et al.2 In their report, of 8 patients with MF, 2 had tumor-stage disease and 2 had erythrodermic MF. They determined that these patients had worse overall survival.2 Our data corroborate the finding that patients with CLL may develop more severe MF, which leads to the conclusion that patients diagnosed with CLL before, concomitantly, or after their diagnosis of MF should be closely monitored. It is notable that patients with more advanced disease tend to be older at the time of diagnosis and that patients who are diagnosed at 57 years or older have been found to have worse disease-specific survival.4,5
This report is limited by the small sample size (6 cases), but it serves to draw attention to the phenomenon of co-occurrence of MF and CLL, and the concern that patients with CLL may develop more aggressive MF.
To the Editor:
Mycosis fungoides (MF) is the most common form of primary cutaneous T-cell lymphoma. It has been associated with increased risk for other visceral and hematologic malignancies.1 Chronic lymphocytic leukemia (CLL) is one of the most common hematologic malignancies. In the United States, a patient’s lifetime risk for CLL is 0.6%. Chronic lymphocytic leukemia often is diagnosed as an incidental finding and typically is not detrimental to a patient’s health. Six cases of MF with antecedent or concomitant CLL were identified in a cohort of patients treated at the University of Minnesota (Minneapolis, Minnesota) from 2005 to 2017 (Table).
All 6 patients were male, with a mean age of 80.5 years. The mean age at CLL diagnosis was 62.5 years, while the mean age at MF diagnosis was 75.3 years. Three patients were younger than 60 years when their CLL was diagnosed: 49, 55, and 57 years. Notably, 4 patients had more aggressive types of MF: 3 with tumor-stage disease, and 1 with folliculotropic MF. Five patients were diagnosed with CLL before their MF was diagnosed (mean, 13.4 years prior; range, 3–31 years), and 1 was diagnosed as part of the initial MF workup.
Given the frequency of both MF and CLL, the co-occurrence of these diseases is not surprising, as other case reports and a larger case series have described the relationship between MF and malignancy.2 It is possible that CLL patients are more likely to be diagnosed with MF because of their regular hematology/oncology follow-up; however, none of our patients were referred from hematology/oncology to dermatology. Alternatively, patients with MF may be more likely to be diagnosed with CLL because of repeated bloodwork performed for diagnosis and screening, which occurred in only 1 of 6 cases. Most of the other patients were diagnosed with MF more than a decade after being diagnosed with CLL.
Does having CLL make patients more likely to develop MF? It is known that patients with CLL may experience immunodeficiency secondary to immune dysregulation, making them more susceptible to infection and secondary malignancies.3 Of our 6 cases, 4 had aggressive or advanced forms of MF, which is similar to the findings of Chang et al.2 In their report, of 8 patients with MF, 2 had tumor-stage disease and 2 had erythrodermic MF. They determined that these patients had worse overall survival.2 Our data corroborate the finding that patients with CLL may develop more severe MF, which leads to the conclusion that patients diagnosed with CLL before, concomitantly, or after their diagnosis of MF should be closely monitored. It is notable that patients with more advanced disease tend to be older at the time of diagnosis and that patients who are diagnosed at 57 years or older have been found to have worse disease-specific survival.4,5
This report is limited by the small sample size (6 cases), but it serves to draw attention to the phenomenon of co-occurrence of MF and CLL, and the concern that patients with CLL may develop more aggressive MF.
- Huang KP, Weinstock MA, Clarke CA, et al. Second lymphomas and other malignant neoplasms in patients with mycosis fungoides and Sézary syndrome. Arch Dermatol. 2007;143:45-50.
- Chang MB, Weaver AL, Brewer JD. Cutaneous T-cell lymphoma in patients with chronic lymphocytic leukemia: clinical characteristics, temporal relationships, and survival data in a series of 14 patients at Mayo Clinic. Int J Dermatol. 2014;53:966-970.
- Hamblin AD, Hamblin TJ. The immunodeficiency of chronic lymphocytic leukaemia. Br Med Bull. 2008;87:49-62.
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866.
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730-4739.
- Huang KP, Weinstock MA, Clarke CA, et al. Second lymphomas and other malignant neoplasms in patients with mycosis fungoides and Sézary syndrome. Arch Dermatol. 2007;143:45-50.
- Chang MB, Weaver AL, Brewer JD. Cutaneous T-cell lymphoma in patients with chronic lymphocytic leukemia: clinical characteristics, temporal relationships, and survival data in a series of 14 patients at Mayo Clinic. Int J Dermatol. 2014;53:966-970.
- Hamblin AD, Hamblin TJ. The immunodeficiency of chronic lymphocytic leukaemia. Br Med Bull. 2008;87:49-62.
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866.
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730-4739.
Practice Points
- Patients with mycosis fungoides (MF) are at increased risk for second hematologic malignancies, including chronic lymphocytic leukemia (CLL).
- Anecdotal information suggests that patients with CLL prior to developing MF may have more severe phenotypes of MF.
Over half of NASH patients have improvement in liver fibrosis after bariatric surgery
BOSTON – For almost half of patients with nonalcoholic steatohepatitis (NASH), bariatric surgery does not resolve severe fibrosis, even after significant weight loss and resolution of multiple metabolic comorbidities, according to investigators.
Still, bariatric surgery was highly effective at improving liver histology in patients without severe fibrosis, reported lead author Raluca Pais, MD, of Sorbonne University, Paris, and colleagues.
“There are many targeted agents for NASH at this time, but their response rate is limited to less than 30%,” Dr. Pais said in a presentation at the annual meeting of the American Association for the Study of Liver Diseases. “In very selective patients, bariatric surgery is a very attractive therapeutic option, as it promotes massive weight loss and sustained improvement in metabolic comorbidities, which are concomitant with very high rates of hepatic histological improvement in most but not all patients.” Despite the known benefits of bariatric surgery, histologic outcomes have been minimally studied, Dr. Pais said, which prompted the present trial.
The investigators began by analyzing data from 868 patients with NASH who underwent bariatric surgery with perioperative liver biopsy between 2004 and 2014. Of these patients, 181 had advanced NASH, a diagnosis that was subclassified by severe fibrosis (F3 or F4) or high activity (steatosis, activity, and fibrosis score of 3-4 with F0-2). Out of the 181 patients with advanced NASH, 65 consented to follow-up liver biopsy, which was conducted a mean of 6 years after surgery. Among these patients, 53 had undergone gastric bypass surgery, while 12 had undergone sleeve gastrectomy.
Almost one-third (29%) of the 65 patients who underwent bariatric surgery had normal livers at follow-up biopsy. Among the 35 patients who had severe fibrosis at baseline, slightly more than half (54%) had resolution of severe fibrosis. In contrast, resolution of high activity occurred in almost all affected patients (97%).
While the findings highlighted some of the benefits associated with bariatric surgery, Dr. Pais emphasized the other side of the coin; many patients did not have resolution of severe fibrosis, even years after surgery. Specifically, 45% of patients had persistent severe fibrosis at follow-up biopsy, despite improvements in comorbidities. On average, these patients lost 23% of their baseline body weight, and two-thirds of the group achieved normal ALT and resolution of NASH. Many also had improvements in insulin resistance and nonalcoholic fatty liver disease activity scores. These findings suggest that changes in severe fibrosis occur independently of many other improvements, Dr. Pais said.
Although multiple comorbidities were not correlated with changes in severe fibrosis, several other predictors were identified. Compared with patients who had resolution of severe fibrosis, nonresponders were typically older (56 vs. 49 years), more often had persistent diabetes (79% vs. 50%), and generally had shorter time between surgery and follow-up biopsy (4.2 vs. 7.5 years). Compared with nonresponders, responders were more likely to have undergone gastric bypass surgery (100% vs. 69%), suggesting that this procedure was more effective at resolving severe fibrosis than sleeve gastrectomy.
During the question-and-answer session following the presentation, multiple conference attendees suggested that the title of the study, “Persistence of severe liver fibrosis despite substantial weight loss with bariatric surgery,” was unnecessarily negative, when in fact the study offered strong support for bariatric surgery.
“This is wonderful data that shows surgery is very effective,” one attendee said.
“This is really positive data,” said another. “I mean, you’ve got reversal of advanced fibrosis in half of the population by the time you follow up for several years, so I would say this is really, very positive data.”
The investigators disclosed relationships with Allergan, Boehringer Ingelheim, Novo Nordisk, and others.
*This story was updated on November 15, 2019.
SOURCE: Pais R et al. The Liver Meeting 2019, Abstract 66.
BOSTON – For almost half of patients with nonalcoholic steatohepatitis (NASH), bariatric surgery does not resolve severe fibrosis, even after significant weight loss and resolution of multiple metabolic comorbidities, according to investigators.
Still, bariatric surgery was highly effective at improving liver histology in patients without severe fibrosis, reported lead author Raluca Pais, MD, of Sorbonne University, Paris, and colleagues.
“There are many targeted agents for NASH at this time, but their response rate is limited to less than 30%,” Dr. Pais said in a presentation at the annual meeting of the American Association for the Study of Liver Diseases. “In very selective patients, bariatric surgery is a very attractive therapeutic option, as it promotes massive weight loss and sustained improvement in metabolic comorbidities, which are concomitant with very high rates of hepatic histological improvement in most but not all patients.” Despite the known benefits of bariatric surgery, histologic outcomes have been minimally studied, Dr. Pais said, which prompted the present trial.
The investigators began by analyzing data from 868 patients with NASH who underwent bariatric surgery with perioperative liver biopsy between 2004 and 2014. Of these patients, 181 had advanced NASH, a diagnosis that was subclassified by severe fibrosis (F3 or F4) or high activity (steatosis, activity, and fibrosis score of 3-4 with F0-2). Out of the 181 patients with advanced NASH, 65 consented to follow-up liver biopsy, which was conducted a mean of 6 years after surgery. Among these patients, 53 had undergone gastric bypass surgery, while 12 had undergone sleeve gastrectomy.
Almost one-third (29%) of the 65 patients who underwent bariatric surgery had normal livers at follow-up biopsy. Among the 35 patients who had severe fibrosis at baseline, slightly more than half (54%) had resolution of severe fibrosis. In contrast, resolution of high activity occurred in almost all affected patients (97%).
While the findings highlighted some of the benefits associated with bariatric surgery, Dr. Pais emphasized the other side of the coin; many patients did not have resolution of severe fibrosis, even years after surgery. Specifically, 45% of patients had persistent severe fibrosis at follow-up biopsy, despite improvements in comorbidities. On average, these patients lost 23% of their baseline body weight, and two-thirds of the group achieved normal ALT and resolution of NASH. Many also had improvements in insulin resistance and nonalcoholic fatty liver disease activity scores. These findings suggest that changes in severe fibrosis occur independently of many other improvements, Dr. Pais said.
Although multiple comorbidities were not correlated with changes in severe fibrosis, several other predictors were identified. Compared with patients who had resolution of severe fibrosis, nonresponders were typically older (56 vs. 49 years), more often had persistent diabetes (79% vs. 50%), and generally had shorter time between surgery and follow-up biopsy (4.2 vs. 7.5 years). Compared with nonresponders, responders were more likely to have undergone gastric bypass surgery (100% vs. 69%), suggesting that this procedure was more effective at resolving severe fibrosis than sleeve gastrectomy.
During the question-and-answer session following the presentation, multiple conference attendees suggested that the title of the study, “Persistence of severe liver fibrosis despite substantial weight loss with bariatric surgery,” was unnecessarily negative, when in fact the study offered strong support for bariatric surgery.
“This is wonderful data that shows surgery is very effective,” one attendee said.
“This is really positive data,” said another. “I mean, you’ve got reversal of advanced fibrosis in half of the population by the time you follow up for several years, so I would say this is really, very positive data.”
The investigators disclosed relationships with Allergan, Boehringer Ingelheim, Novo Nordisk, and others.
*This story was updated on November 15, 2019.
SOURCE: Pais R et al. The Liver Meeting 2019, Abstract 66.
BOSTON – For almost half of patients with nonalcoholic steatohepatitis (NASH), bariatric surgery does not resolve severe fibrosis, even after significant weight loss and resolution of multiple metabolic comorbidities, according to investigators.
Still, bariatric surgery was highly effective at improving liver histology in patients without severe fibrosis, reported lead author Raluca Pais, MD, of Sorbonne University, Paris, and colleagues.
“There are many targeted agents for NASH at this time, but their response rate is limited to less than 30%,” Dr. Pais said in a presentation at the annual meeting of the American Association for the Study of Liver Diseases. “In very selective patients, bariatric surgery is a very attractive therapeutic option, as it promotes massive weight loss and sustained improvement in metabolic comorbidities, which are concomitant with very high rates of hepatic histological improvement in most but not all patients.” Despite the known benefits of bariatric surgery, histologic outcomes have been minimally studied, Dr. Pais said, which prompted the present trial.
The investigators began by analyzing data from 868 patients with NASH who underwent bariatric surgery with perioperative liver biopsy between 2004 and 2014. Of these patients, 181 had advanced NASH, a diagnosis that was subclassified by severe fibrosis (F3 or F4) or high activity (steatosis, activity, and fibrosis score of 3-4 with F0-2). Out of the 181 patients with advanced NASH, 65 consented to follow-up liver biopsy, which was conducted a mean of 6 years after surgery. Among these patients, 53 had undergone gastric bypass surgery, while 12 had undergone sleeve gastrectomy.
Almost one-third (29%) of the 65 patients who underwent bariatric surgery had normal livers at follow-up biopsy. Among the 35 patients who had severe fibrosis at baseline, slightly more than half (54%) had resolution of severe fibrosis. In contrast, resolution of high activity occurred in almost all affected patients (97%).
While the findings highlighted some of the benefits associated with bariatric surgery, Dr. Pais emphasized the other side of the coin; many patients did not have resolution of severe fibrosis, even years after surgery. Specifically, 45% of patients had persistent severe fibrosis at follow-up biopsy, despite improvements in comorbidities. On average, these patients lost 23% of their baseline body weight, and two-thirds of the group achieved normal ALT and resolution of NASH. Many also had improvements in insulin resistance and nonalcoholic fatty liver disease activity scores. These findings suggest that changes in severe fibrosis occur independently of many other improvements, Dr. Pais said.
Although multiple comorbidities were not correlated with changes in severe fibrosis, several other predictors were identified. Compared with patients who had resolution of severe fibrosis, nonresponders were typically older (56 vs. 49 years), more often had persistent diabetes (79% vs. 50%), and generally had shorter time between surgery and follow-up biopsy (4.2 vs. 7.5 years). Compared with nonresponders, responders were more likely to have undergone gastric bypass surgery (100% vs. 69%), suggesting that this procedure was more effective at resolving severe fibrosis than sleeve gastrectomy.
During the question-and-answer session following the presentation, multiple conference attendees suggested that the title of the study, “Persistence of severe liver fibrosis despite substantial weight loss with bariatric surgery,” was unnecessarily negative, when in fact the study offered strong support for bariatric surgery.
“This is wonderful data that shows surgery is very effective,” one attendee said.
“This is really positive data,” said another. “I mean, you’ve got reversal of advanced fibrosis in half of the population by the time you follow up for several years, so I would say this is really, very positive data.”
The investigators disclosed relationships with Allergan, Boehringer Ingelheim, Novo Nordisk, and others.
*This story was updated on November 15, 2019.
SOURCE: Pais R et al. The Liver Meeting 2019, Abstract 66.
REPORTING FROM THE LIVER MEETING 2019
Weekly tocilizumab provides durable benefits for giant cell arteritis patients
ATLANTA –
Of 250 patients treated in the double-blind portion of the trial, 215 entered the extension period (part 2 of the trial), and 197 (92%) completed all 3 years of the trial. A total of 38 of 81 (47%) extension participants in clinical remission (CR) at week 52 after receiving tocilizumab (Actemra) weekly maintained it throughout the extension, and 13 of 36 (36%) treated every other week maintained it, John Stone, MD, reported at the annual meeting of the American College of Rheumatology.
Notably, more of those 51 patients treated with tocilizumab who maintained CR throughout the extension were treatment free at the end of the extension, compared with those originally assigned to the placebo groups (65% vs. 45%), and their median time to first flare after stopping treatment was longer, said Dr. Stone, director of clinical rheumatology at Massachusetts General Hospital and associate professor of medicine in the division of rheumatology at Harvard Medical School, both in Boston.
For example, times to first flare were 575 and 428 days for the weekly and biweekly tocilizumab dosing groups – which each had 26-week prednisone tapering – but they were only 162 days in a placebo group with 26-week prednisone tapering and 295 days for patients in another placebo group with 52-week prednisone tapering, Dr. Stone explained. Retreatment with tocilizumab restored CR in patients who experienced flares after discontinuing treatment, he added.
Importantly, cumulative glucocorticoid doses over the 3 years were also lower in the tocilizumab groups (2,647 and 3,782 mg/day with weekly and biweekly tocilizumab and 5,248, and 5,323 mg/day in the placebo groups, respectively), Dr. Stone said.
No new safety signals were observed during the extension. Rates of serious adverse events (SAEs) and serious infections per 100 patient-years during the entire 3 years of the study were comparable among those who never received tocilizumab and those who received at least 1 dose (23.2 and 25.4 for SAEs and 4.6 and 3.5 for serious infections).
Detailed results of the extension period
GiACTA enrolled patients with GCA and randomized them to receive weekly or biweekly treatment with the interleukin-6 receptor–alpha inhibitor tocilizumab at doses of 162 mg delivered subcutaneously with a 26-week prednisone taper, or to the placebo groups with a 26- or 52-week prednisone taper. The results, reported in 2017, demonstrated the superiority of tocilizumab over placebo for providing sustained glucocorticoid-free remission and ultimately led to approval from Food and Drug Administration of the biologic agent for the treatment of GCA.
The current analysis was carried out to determine long-term safety, to explore maintenance of efficacy after discontinuation, and to “gain insight into the long-term glucocorticoid-sparing effects of tocilizumab,” Dr. Stone said.
He noted that the extension period was not randomized because of the way that patients in the “original four randomized groups had sorted themselves out into very different groups by virtue of their response in part 1 [of the trial],” which made randomization impossible because of the varying categories of patients.
Patients in the extension were also treated at investigators’ discretion to prevent “catastrophic GCA flares, vision loss, and other problems” that could potentially occur with discontinuation of the blinded injections in part 1, he said.
Nevertheless, the analysis “provides some incredibly important information about the management of GCA now,” he said, adding that the dramatic effect of the original randomization was still profound at 3 years “despite the fact that investigators could treat the patients as they wished.”
“Even at 3 years there was still a profound difference in the cumulative glucocorticoid [use], compared to those patients who were randomized to one of the placebo versus prednisone groups,” he explained.
However, the most important question from this study relates to what happened when weekly tocilizumab was stopped: Of the 81 patients in part 2 who were in CR on tocilizumab after part 1 of the study, 59 (73%) were not started on any treatment by their investigator, and of those, 25 (42%) maintained treatment-free remission for 2 full years following discontinuation of tocilizumab, he said.
What happened when prednisone was stopped?
In the absence of a direct comparison group (since the extension wasn’t a randomized, controlled part of the trial), the best comparison to that is what happens when prednisone is stopped, he noted.
“And we did that experiment in part 1” of the trial, he said. Overall, 68% of patients in the prednisone/placebo groups flared in 1-year of follow-up, and most of those did so even before they stopped prednisone. In the group that received placebo plus a 52-week prednisone taper, 49% of patients flared and “every single one of those patients flared before they got off prednisone,” he said.
“So in that context, 2 years of treatment-free remission following weekly tocilizumab discontinuation seems very good,” he added.
Of the patients who flared in part 2 of the trial, 11 whose investigator elected to treat them with only tocilizumab reentered remission in a median of 15.8 days, 13 treated with tocilizumab and glucocorticoids reentered remission in a median of 8.5 days (1 didn’t reenter remission at all), and 15 treated with only glucocorticoids reentered remission in a median of 38 days.
“There are a number of biases in these data, but they do point out the fact that some patients can be treated with tocilizumab alone,” Dr. Stone said. “I would be very careful doing that.”
The trial was not powered for the comparison of weekly versus biweekly dosing, he said. However, “multiple lines of investigation and analysis support the idea that weekly tocilizumab is more effective at controlling disease,” he added. But “if you want to be assured that you are doing everything you can to control the disease, weekly tocilizumab would be better than every-other-week tocilizumab,” he said.
GiACTA was funded by Roche. Dr. Stone reported research grants from Roche/Genentech and consulting fees from Chugal, Roche/Genentech, and Xencor.
SOURCE: Stone J et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 808.
ATLANTA –
Of 250 patients treated in the double-blind portion of the trial, 215 entered the extension period (part 2 of the trial), and 197 (92%) completed all 3 years of the trial. A total of 38 of 81 (47%) extension participants in clinical remission (CR) at week 52 after receiving tocilizumab (Actemra) weekly maintained it throughout the extension, and 13 of 36 (36%) treated every other week maintained it, John Stone, MD, reported at the annual meeting of the American College of Rheumatology.
Notably, more of those 51 patients treated with tocilizumab who maintained CR throughout the extension were treatment free at the end of the extension, compared with those originally assigned to the placebo groups (65% vs. 45%), and their median time to first flare after stopping treatment was longer, said Dr. Stone, director of clinical rheumatology at Massachusetts General Hospital and associate professor of medicine in the division of rheumatology at Harvard Medical School, both in Boston.
For example, times to first flare were 575 and 428 days for the weekly and biweekly tocilizumab dosing groups – which each had 26-week prednisone tapering – but they were only 162 days in a placebo group with 26-week prednisone tapering and 295 days for patients in another placebo group with 52-week prednisone tapering, Dr. Stone explained. Retreatment with tocilizumab restored CR in patients who experienced flares after discontinuing treatment, he added.
Importantly, cumulative glucocorticoid doses over the 3 years were also lower in the tocilizumab groups (2,647 and 3,782 mg/day with weekly and biweekly tocilizumab and 5,248, and 5,323 mg/day in the placebo groups, respectively), Dr. Stone said.
No new safety signals were observed during the extension. Rates of serious adverse events (SAEs) and serious infections per 100 patient-years during the entire 3 years of the study were comparable among those who never received tocilizumab and those who received at least 1 dose (23.2 and 25.4 for SAEs and 4.6 and 3.5 for serious infections).
Detailed results of the extension period
GiACTA enrolled patients with GCA and randomized them to receive weekly or biweekly treatment with the interleukin-6 receptor–alpha inhibitor tocilizumab at doses of 162 mg delivered subcutaneously with a 26-week prednisone taper, or to the placebo groups with a 26- or 52-week prednisone taper. The results, reported in 2017, demonstrated the superiority of tocilizumab over placebo for providing sustained glucocorticoid-free remission and ultimately led to approval from Food and Drug Administration of the biologic agent for the treatment of GCA.
The current analysis was carried out to determine long-term safety, to explore maintenance of efficacy after discontinuation, and to “gain insight into the long-term glucocorticoid-sparing effects of tocilizumab,” Dr. Stone said.
He noted that the extension period was not randomized because of the way that patients in the “original four randomized groups had sorted themselves out into very different groups by virtue of their response in part 1 [of the trial],” which made randomization impossible because of the varying categories of patients.
Patients in the extension were also treated at investigators’ discretion to prevent “catastrophic GCA flares, vision loss, and other problems” that could potentially occur with discontinuation of the blinded injections in part 1, he said.
Nevertheless, the analysis “provides some incredibly important information about the management of GCA now,” he said, adding that the dramatic effect of the original randomization was still profound at 3 years “despite the fact that investigators could treat the patients as they wished.”
“Even at 3 years there was still a profound difference in the cumulative glucocorticoid [use], compared to those patients who were randomized to one of the placebo versus prednisone groups,” he explained.
However, the most important question from this study relates to what happened when weekly tocilizumab was stopped: Of the 81 patients in part 2 who were in CR on tocilizumab after part 1 of the study, 59 (73%) were not started on any treatment by their investigator, and of those, 25 (42%) maintained treatment-free remission for 2 full years following discontinuation of tocilizumab, he said.
What happened when prednisone was stopped?
In the absence of a direct comparison group (since the extension wasn’t a randomized, controlled part of the trial), the best comparison to that is what happens when prednisone is stopped, he noted.
“And we did that experiment in part 1” of the trial, he said. Overall, 68% of patients in the prednisone/placebo groups flared in 1-year of follow-up, and most of those did so even before they stopped prednisone. In the group that received placebo plus a 52-week prednisone taper, 49% of patients flared and “every single one of those patients flared before they got off prednisone,” he said.
“So in that context, 2 years of treatment-free remission following weekly tocilizumab discontinuation seems very good,” he added.
Of the patients who flared in part 2 of the trial, 11 whose investigator elected to treat them with only tocilizumab reentered remission in a median of 15.8 days, 13 treated with tocilizumab and glucocorticoids reentered remission in a median of 8.5 days (1 didn’t reenter remission at all), and 15 treated with only glucocorticoids reentered remission in a median of 38 days.
“There are a number of biases in these data, but they do point out the fact that some patients can be treated with tocilizumab alone,” Dr. Stone said. “I would be very careful doing that.”
The trial was not powered for the comparison of weekly versus biweekly dosing, he said. However, “multiple lines of investigation and analysis support the idea that weekly tocilizumab is more effective at controlling disease,” he added. But “if you want to be assured that you are doing everything you can to control the disease, weekly tocilizumab would be better than every-other-week tocilizumab,” he said.
GiACTA was funded by Roche. Dr. Stone reported research grants from Roche/Genentech and consulting fees from Chugal, Roche/Genentech, and Xencor.
SOURCE: Stone J et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 808.
ATLANTA –
Of 250 patients treated in the double-blind portion of the trial, 215 entered the extension period (part 2 of the trial), and 197 (92%) completed all 3 years of the trial. A total of 38 of 81 (47%) extension participants in clinical remission (CR) at week 52 after receiving tocilizumab (Actemra) weekly maintained it throughout the extension, and 13 of 36 (36%) treated every other week maintained it, John Stone, MD, reported at the annual meeting of the American College of Rheumatology.
Notably, more of those 51 patients treated with tocilizumab who maintained CR throughout the extension were treatment free at the end of the extension, compared with those originally assigned to the placebo groups (65% vs. 45%), and their median time to first flare after stopping treatment was longer, said Dr. Stone, director of clinical rheumatology at Massachusetts General Hospital and associate professor of medicine in the division of rheumatology at Harvard Medical School, both in Boston.
For example, times to first flare were 575 and 428 days for the weekly and biweekly tocilizumab dosing groups – which each had 26-week prednisone tapering – but they were only 162 days in a placebo group with 26-week prednisone tapering and 295 days for patients in another placebo group with 52-week prednisone tapering, Dr. Stone explained. Retreatment with tocilizumab restored CR in patients who experienced flares after discontinuing treatment, he added.
Importantly, cumulative glucocorticoid doses over the 3 years were also lower in the tocilizumab groups (2,647 and 3,782 mg/day with weekly and biweekly tocilizumab and 5,248, and 5,323 mg/day in the placebo groups, respectively), Dr. Stone said.
No new safety signals were observed during the extension. Rates of serious adverse events (SAEs) and serious infections per 100 patient-years during the entire 3 years of the study were comparable among those who never received tocilizumab and those who received at least 1 dose (23.2 and 25.4 for SAEs and 4.6 and 3.5 for serious infections).
Detailed results of the extension period
GiACTA enrolled patients with GCA and randomized them to receive weekly or biweekly treatment with the interleukin-6 receptor–alpha inhibitor tocilizumab at doses of 162 mg delivered subcutaneously with a 26-week prednisone taper, or to the placebo groups with a 26- or 52-week prednisone taper. The results, reported in 2017, demonstrated the superiority of tocilizumab over placebo for providing sustained glucocorticoid-free remission and ultimately led to approval from Food and Drug Administration of the biologic agent for the treatment of GCA.
The current analysis was carried out to determine long-term safety, to explore maintenance of efficacy after discontinuation, and to “gain insight into the long-term glucocorticoid-sparing effects of tocilizumab,” Dr. Stone said.
He noted that the extension period was not randomized because of the way that patients in the “original four randomized groups had sorted themselves out into very different groups by virtue of their response in part 1 [of the trial],” which made randomization impossible because of the varying categories of patients.
Patients in the extension were also treated at investigators’ discretion to prevent “catastrophic GCA flares, vision loss, and other problems” that could potentially occur with discontinuation of the blinded injections in part 1, he said.
Nevertheless, the analysis “provides some incredibly important information about the management of GCA now,” he said, adding that the dramatic effect of the original randomization was still profound at 3 years “despite the fact that investigators could treat the patients as they wished.”
“Even at 3 years there was still a profound difference in the cumulative glucocorticoid [use], compared to those patients who were randomized to one of the placebo versus prednisone groups,” he explained.
However, the most important question from this study relates to what happened when weekly tocilizumab was stopped: Of the 81 patients in part 2 who were in CR on tocilizumab after part 1 of the study, 59 (73%) were not started on any treatment by their investigator, and of those, 25 (42%) maintained treatment-free remission for 2 full years following discontinuation of tocilizumab, he said.
What happened when prednisone was stopped?
In the absence of a direct comparison group (since the extension wasn’t a randomized, controlled part of the trial), the best comparison to that is what happens when prednisone is stopped, he noted.
“And we did that experiment in part 1” of the trial, he said. Overall, 68% of patients in the prednisone/placebo groups flared in 1-year of follow-up, and most of those did so even before they stopped prednisone. In the group that received placebo plus a 52-week prednisone taper, 49% of patients flared and “every single one of those patients flared before they got off prednisone,” he said.
“So in that context, 2 years of treatment-free remission following weekly tocilizumab discontinuation seems very good,” he added.
Of the patients who flared in part 2 of the trial, 11 whose investigator elected to treat them with only tocilizumab reentered remission in a median of 15.8 days, 13 treated with tocilizumab and glucocorticoids reentered remission in a median of 8.5 days (1 didn’t reenter remission at all), and 15 treated with only glucocorticoids reentered remission in a median of 38 days.
“There are a number of biases in these data, but they do point out the fact that some patients can be treated with tocilizumab alone,” Dr. Stone said. “I would be very careful doing that.”
The trial was not powered for the comparison of weekly versus biweekly dosing, he said. However, “multiple lines of investigation and analysis support the idea that weekly tocilizumab is more effective at controlling disease,” he added. But “if you want to be assured that you are doing everything you can to control the disease, weekly tocilizumab would be better than every-other-week tocilizumab,” he said.
GiACTA was funded by Roche. Dr. Stone reported research grants from Roche/Genentech and consulting fees from Chugal, Roche/Genentech, and Xencor.
SOURCE: Stone J et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 808.
REPORTING FROM ACR 2019
Amneal Pharma issues recall for ranitidine products
Amneal Pharmaceuticals has announced a voluntary recall for both ranitidine tablets and ranitidine syrup because of potential N-nitrosodimethylamine (NDMA) levels above Food and Drug Administration–allowed cutoffs, according to an FDA MedWatch alert.
NDMA, a probable human carcinogen, has been found in several different ranitidine products since an initial FDA announcement in September 2019. Ranitidine, a histamine2 receptor blocker, is indicated for multiple conditions, including treatment and prevention of ulcers of the stomach and intestines and treatment of gastroesophageal reflux disease.
The affected ranitidine products being recalled by Amneal include 60-, 100-, 180-, 500-, and 1,000-count 150-mg tablets; 30-, 100-, 250-, and 1,000-count 300-mg tablets, and a 15-mg/mL syrup. The manufacturer has not received any reports of adverse events confirmed to be directly related to the recall.
Consumers should contact their physicians or health care providers if they have experienced any problems that may be related to the use of this drug product, the FDA said. Adverse reactions or quality problems can be reported to Amneal Drug Safety by phone at 1-877-835-5472.
Amneal Pharmaceuticals has announced a voluntary recall for both ranitidine tablets and ranitidine syrup because of potential N-nitrosodimethylamine (NDMA) levels above Food and Drug Administration–allowed cutoffs, according to an FDA MedWatch alert.
NDMA, a probable human carcinogen, has been found in several different ranitidine products since an initial FDA announcement in September 2019. Ranitidine, a histamine2 receptor blocker, is indicated for multiple conditions, including treatment and prevention of ulcers of the stomach and intestines and treatment of gastroesophageal reflux disease.
The affected ranitidine products being recalled by Amneal include 60-, 100-, 180-, 500-, and 1,000-count 150-mg tablets; 30-, 100-, 250-, and 1,000-count 300-mg tablets, and a 15-mg/mL syrup. The manufacturer has not received any reports of adverse events confirmed to be directly related to the recall.
Consumers should contact their physicians or health care providers if they have experienced any problems that may be related to the use of this drug product, the FDA said. Adverse reactions or quality problems can be reported to Amneal Drug Safety by phone at 1-877-835-5472.
Amneal Pharmaceuticals has announced a voluntary recall for both ranitidine tablets and ranitidine syrup because of potential N-nitrosodimethylamine (NDMA) levels above Food and Drug Administration–allowed cutoffs, according to an FDA MedWatch alert.
NDMA, a probable human carcinogen, has been found in several different ranitidine products since an initial FDA announcement in September 2019. Ranitidine, a histamine2 receptor blocker, is indicated for multiple conditions, including treatment and prevention of ulcers of the stomach and intestines and treatment of gastroesophageal reflux disease.
The affected ranitidine products being recalled by Amneal include 60-, 100-, 180-, 500-, and 1,000-count 150-mg tablets; 30-, 100-, 250-, and 1,000-count 300-mg tablets, and a 15-mg/mL syrup. The manufacturer has not received any reports of adverse events confirmed to be directly related to the recall.
Consumers should contact their physicians or health care providers if they have experienced any problems that may be related to the use of this drug product, the FDA said. Adverse reactions or quality problems can be reported to Amneal Drug Safety by phone at 1-877-835-5472.
Lorlatinib induces deep responses in ROS1-positive NSCLC
The tyrosine kinase inhibitor (TKI) lorlatinib showed deep responses and intracranial activity in both TKI-pretreated and TKI-naive patients with advanced ROS1-positive non–small cell lung cancer (NSCLC), according to results from a phase 1-2 trial.
“We investigated the antitumour activity and safety of lorlatinib in advanced, ROS1-positive NSCLC,” wrote Alice T. Shaw, MD, PhD, of Massachusetts General Hospital Cancer Center, Boston, and colleagues. Their report is in The Lancet Oncology.
The single-arm, open-label study included 69 patients with advanced ROS1-positive disease with or without CNS involvement. The effects of lorlatinib were evaluated across 28 institutions in 12 different countries around the globe.
At baseline, the median age of study participants was 54 years (range, 44-61 years), and 57% were positive for brain metastases.
Study participants received 100 mg of oral lorlatinib once daily in repeated 21-day cycles. Drug therapy was continued until death, disease progression, unacceptable toxicity, or withdrawal of consent.
The primary outcome measured was intracranial and overall response. Activity outcomes were evaluated in subjects given a minimum of one dose of lorlatinib.
A total of 58% of patients were previously treated with crizotinib, while 30% of patients were TKI-naive. Among 40 crizotinib-pretreated patients, 14 patients (35%) had an objective response, with a median duration of response and PFS of 13.8 and 8.5 months, respectively.
Among 21 TKI-naive patients, 13 patients (62%) had an objective response, with a median duration of response and PFS of 25.3 and 21 months, respectively.
“Intracranial responses were achieved in seven (64%) of 11 TKI-naive patients and 12 (50%) of 24 previous crizotinib-only patients,” they reported.
With respect to safety, serious lorlatinib-related adverse events were observed in 7% of patients, with no therapy-related deaths reported. The most frequently seen grade 3-4 TEAEs were hypertriglyceridemia (19%) and hypercholesterolemia (14%).
The researchers noted a key limitation of the study was the small sample size; however, due to the rare nature of ROS1 rearrangements in patients with NSCLC, increasing enrollment for future studies could be challenging.
“Because crizotinib-refractory patients have few treatment options, lorlatinib could represent an important next-line targeted agent,” they concluded.
Pfizer funded the study. The authors reported financial affiliations with Ariad, Blueprint Medicines, Chugai Pharmaceutical, Daiichi Sankyo, EMD Serono, Pfizer, KSQ Therapeutics, Servier, TP Therapeutics, and other companies.
SOURCE: Shaw AT et al. Lancet Oncol. 2019 Oct 25. doi: 10.1016/S1470-2045(19)30655-2.
The tyrosine kinase inhibitor (TKI) crizotinib was recently established as an optimal first-line treatment option for patients with ROS1-positive non–small cell lung cancer (NSCLC). Despite strong efficacy seen in clinical trials, disease progression can still occur in patients on crizotinib, often through the development of resistance, which is largely the result of on-target mutations, such as Gly2032Arg.
Early results suggest the novel oral TKI candidate, lorlatinib, a potent inhibitor of the Gly2032Arg mutation, may be a treatment of choice in patients with crizotinib-resistance. Recent phase 1 data showed lorlatinib had antitumor activity in ROS1-positive patients.
Correspondingly, the deep and durable responses reported by Dr. Shaw and colleagues represents a significant milestone for lorlatinib, particularly in the setting of crizotinib resistance, where a paucity of later-line treatment options exist. In comparison to platinum-pemetrexed chemotherapy, lorlatinib is better tolerated and has demonstrated potent intracranial activity, which may prevent or delay CNS progression in the disease.
One question that remains from the current study is whether other ROS1 TKI drug candidates, such as repotrectinib and entrectinib, will show similar results to lorlatinib. Several trials are presently ongoing in an attempt to help answer this, and other remaining questions.
Michaël Duruisseaux, MD, PhD, is affiliated with the Hospices Civils de Lyon (France), Universit é Claude Bernard Lyon. Dr. Duruisseaux reported financial affiliations with Boehringer Ingelheim, Bristol-Myers Squibb, Roche, and Takeda. These comments are adapted from his editorial (Lancet Oncol. 2019 Oct 25. doi: 10.1016/S1470-2045[19]30716-8 ).
The tyrosine kinase inhibitor (TKI) crizotinib was recently established as an optimal first-line treatment option for patients with ROS1-positive non–small cell lung cancer (NSCLC). Despite strong efficacy seen in clinical trials, disease progression can still occur in patients on crizotinib, often through the development of resistance, which is largely the result of on-target mutations, such as Gly2032Arg.
Early results suggest the novel oral TKI candidate, lorlatinib, a potent inhibitor of the Gly2032Arg mutation, may be a treatment of choice in patients with crizotinib-resistance. Recent phase 1 data showed lorlatinib had antitumor activity in ROS1-positive patients.
Correspondingly, the deep and durable responses reported by Dr. Shaw and colleagues represents a significant milestone for lorlatinib, particularly in the setting of crizotinib resistance, where a paucity of later-line treatment options exist. In comparison to platinum-pemetrexed chemotherapy, lorlatinib is better tolerated and has demonstrated potent intracranial activity, which may prevent or delay CNS progression in the disease.
One question that remains from the current study is whether other ROS1 TKI drug candidates, such as repotrectinib and entrectinib, will show similar results to lorlatinib. Several trials are presently ongoing in an attempt to help answer this, and other remaining questions.
Michaël Duruisseaux, MD, PhD, is affiliated with the Hospices Civils de Lyon (France), Universit é Claude Bernard Lyon. Dr. Duruisseaux reported financial affiliations with Boehringer Ingelheim, Bristol-Myers Squibb, Roche, and Takeda. These comments are adapted from his editorial (Lancet Oncol. 2019 Oct 25. doi: 10.1016/S1470-2045[19]30716-8 ).
The tyrosine kinase inhibitor (TKI) crizotinib was recently established as an optimal first-line treatment option for patients with ROS1-positive non–small cell lung cancer (NSCLC). Despite strong efficacy seen in clinical trials, disease progression can still occur in patients on crizotinib, often through the development of resistance, which is largely the result of on-target mutations, such as Gly2032Arg.
Early results suggest the novel oral TKI candidate, lorlatinib, a potent inhibitor of the Gly2032Arg mutation, may be a treatment of choice in patients with crizotinib-resistance. Recent phase 1 data showed lorlatinib had antitumor activity in ROS1-positive patients.
Correspondingly, the deep and durable responses reported by Dr. Shaw and colleagues represents a significant milestone for lorlatinib, particularly in the setting of crizotinib resistance, where a paucity of later-line treatment options exist. In comparison to platinum-pemetrexed chemotherapy, lorlatinib is better tolerated and has demonstrated potent intracranial activity, which may prevent or delay CNS progression in the disease.
One question that remains from the current study is whether other ROS1 TKI drug candidates, such as repotrectinib and entrectinib, will show similar results to lorlatinib. Several trials are presently ongoing in an attempt to help answer this, and other remaining questions.
Michaël Duruisseaux, MD, PhD, is affiliated with the Hospices Civils de Lyon (France), Universit é Claude Bernard Lyon. Dr. Duruisseaux reported financial affiliations with Boehringer Ingelheim, Bristol-Myers Squibb, Roche, and Takeda. These comments are adapted from his editorial (Lancet Oncol. 2019 Oct 25. doi: 10.1016/S1470-2045[19]30716-8 ).
The tyrosine kinase inhibitor (TKI) lorlatinib showed deep responses and intracranial activity in both TKI-pretreated and TKI-naive patients with advanced ROS1-positive non–small cell lung cancer (NSCLC), according to results from a phase 1-2 trial.
“We investigated the antitumour activity and safety of lorlatinib in advanced, ROS1-positive NSCLC,” wrote Alice T. Shaw, MD, PhD, of Massachusetts General Hospital Cancer Center, Boston, and colleagues. Their report is in The Lancet Oncology.
The single-arm, open-label study included 69 patients with advanced ROS1-positive disease with or without CNS involvement. The effects of lorlatinib were evaluated across 28 institutions in 12 different countries around the globe.
At baseline, the median age of study participants was 54 years (range, 44-61 years), and 57% were positive for brain metastases.
Study participants received 100 mg of oral lorlatinib once daily in repeated 21-day cycles. Drug therapy was continued until death, disease progression, unacceptable toxicity, or withdrawal of consent.
The primary outcome measured was intracranial and overall response. Activity outcomes were evaluated in subjects given a minimum of one dose of lorlatinib.
A total of 58% of patients were previously treated with crizotinib, while 30% of patients were TKI-naive. Among 40 crizotinib-pretreated patients, 14 patients (35%) had an objective response, with a median duration of response and PFS of 13.8 and 8.5 months, respectively.
Among 21 TKI-naive patients, 13 patients (62%) had an objective response, with a median duration of response and PFS of 25.3 and 21 months, respectively.
“Intracranial responses were achieved in seven (64%) of 11 TKI-naive patients and 12 (50%) of 24 previous crizotinib-only patients,” they reported.
With respect to safety, serious lorlatinib-related adverse events were observed in 7% of patients, with no therapy-related deaths reported. The most frequently seen grade 3-4 TEAEs were hypertriglyceridemia (19%) and hypercholesterolemia (14%).
The researchers noted a key limitation of the study was the small sample size; however, due to the rare nature of ROS1 rearrangements in patients with NSCLC, increasing enrollment for future studies could be challenging.
“Because crizotinib-refractory patients have few treatment options, lorlatinib could represent an important next-line targeted agent,” they concluded.
Pfizer funded the study. The authors reported financial affiliations with Ariad, Blueprint Medicines, Chugai Pharmaceutical, Daiichi Sankyo, EMD Serono, Pfizer, KSQ Therapeutics, Servier, TP Therapeutics, and other companies.
SOURCE: Shaw AT et al. Lancet Oncol. 2019 Oct 25. doi: 10.1016/S1470-2045(19)30655-2.
The tyrosine kinase inhibitor (TKI) lorlatinib showed deep responses and intracranial activity in both TKI-pretreated and TKI-naive patients with advanced ROS1-positive non–small cell lung cancer (NSCLC), according to results from a phase 1-2 trial.
“We investigated the antitumour activity and safety of lorlatinib in advanced, ROS1-positive NSCLC,” wrote Alice T. Shaw, MD, PhD, of Massachusetts General Hospital Cancer Center, Boston, and colleagues. Their report is in The Lancet Oncology.
The single-arm, open-label study included 69 patients with advanced ROS1-positive disease with or without CNS involvement. The effects of lorlatinib were evaluated across 28 institutions in 12 different countries around the globe.
At baseline, the median age of study participants was 54 years (range, 44-61 years), and 57% were positive for brain metastases.
Study participants received 100 mg of oral lorlatinib once daily in repeated 21-day cycles. Drug therapy was continued until death, disease progression, unacceptable toxicity, or withdrawal of consent.
The primary outcome measured was intracranial and overall response. Activity outcomes were evaluated in subjects given a minimum of one dose of lorlatinib.
A total of 58% of patients were previously treated with crizotinib, while 30% of patients were TKI-naive. Among 40 crizotinib-pretreated patients, 14 patients (35%) had an objective response, with a median duration of response and PFS of 13.8 and 8.5 months, respectively.
Among 21 TKI-naive patients, 13 patients (62%) had an objective response, with a median duration of response and PFS of 25.3 and 21 months, respectively.
“Intracranial responses were achieved in seven (64%) of 11 TKI-naive patients and 12 (50%) of 24 previous crizotinib-only patients,” they reported.
With respect to safety, serious lorlatinib-related adverse events were observed in 7% of patients, with no therapy-related deaths reported. The most frequently seen grade 3-4 TEAEs were hypertriglyceridemia (19%) and hypercholesterolemia (14%).
The researchers noted a key limitation of the study was the small sample size; however, due to the rare nature of ROS1 rearrangements in patients with NSCLC, increasing enrollment for future studies could be challenging.
“Because crizotinib-refractory patients have few treatment options, lorlatinib could represent an important next-line targeted agent,” they concluded.
Pfizer funded the study. The authors reported financial affiliations with Ariad, Blueprint Medicines, Chugai Pharmaceutical, Daiichi Sankyo, EMD Serono, Pfizer, KSQ Therapeutics, Servier, TP Therapeutics, and other companies.
SOURCE: Shaw AT et al. Lancet Oncol. 2019 Oct 25. doi: 10.1016/S1470-2045(19)30655-2.
FROM THE LANCET ONCOLOGY
Monoclonal Antibodies in MS
A 19-year-old man was diagnosed with relapsing multiple sclerosis (MS) at age 7 and is currently being treated with an infusible monoclonal antibody (mAb) therapy. Early in the day, he receives an infusion at an outpatient clinic. That night, he begins to experience numbness and tingling in his right upper extremity, which prompts a visit to an urgent care clinic. There, the clinician administers IV fluids to the patient. After his symptoms improve, the patient is discharged home.
The next morning, he has a new onset of left-side shoulder and neck pain with a pulsating headache. The patient reports his symptoms to his primary care provider (PCP), who instructs him to visit the emergency department (ED) for evaluation and treatment of a possible infection.
EXAMINATION
The patient arrives at the ED with a 102.4°F fever, vomiting, cough, mild congestion, diaphoresis, generalized myalgias, and chills. He also reports depression and anxiety, saying that for the past 7 days, “I haven’t felt like my normal self.”
Medical history includes moderate persistent asthma that is not well controlled, status asthmaticus, and use of an electronic vaporizing device for inhaling nicotine and marijuana/tetrahydrocannabinol (THC). Besides mAb infusions, his medications include hydrocodone/acetaminophen, prochlorperazine, gabapentin, hydroxyzine, trazodone, albuterol, and montelukast.
Examination reveals vital signs within normal limits. Lab work confirms elevated white blood cell count and absolute neutrophil count. Chest x-ray shows diffuse bilateral interstitial and patchy airspace opacities. He is diagnosed with bilateral pneumonia and is admitted and started on an IV antibiotic.
Within 24 hours, a new chest x-ray shows worsening symptoms. CT of the chest with contrast reveals diffuse bilateral ground-glass and airspace opacities suggestive of acute respiratory distress syndrome; bilateral thickening of the pulmonary interstitium; trace bilateral pleural effusions; increased caliber of the main pulmonary artery; and mediastinal and right hilar lymphadenopathy.
Subsequently, the patient developed sepsis and went into acute hypoxemic respiratory failure. He is transferred to the ICU, and pulmonology is consulted. A bronchoscopy with bronchoalveolar lavage (BAL) reveals neutrophil predominance; fungal, bacterial, and viral cultures are negative. The patient is started on broad-spectrum IV antibiotics and high-dose IV steroids. After 4 days, he begins to improve and is transferred out of the ICU. He is discharged with oral steroids and antibiotics.
Continue to: DISCUSSION
DISCUSSION
Fortunately, the PCP and the ED provider identified risk factors that contributed to the patient’s pneumonia and its subsequent worsening to sepsis and acute hypoxemic respiratory failure. The immunosuppressive/immunomodulatory effect of mAb therapy increased the patient’s risk for infection and the severity of infection, which is why vigilant safety monitoring and surveillance is essential with mAb treatment.1 Bloodwork should be performed at least every 6 months and include a complete blood count, complete metabolic panel with differential, and JC virus antibody test. Additionally, urinalysis should be performed prior to every mAb infusion. All testing recommended in the package insert for the patient’s prescribed therapy should be performed.
The patient’s history of asthma and his chronic vaping predisposed him to respiratory infections. In mice studies, exposure to e-cigarette vapor has been shown to be cytotoxic to airway cells and to decrease macrophage and neutrophil antimicrobial function.2 Exposure also alters immunomodulating cytokines in the airway, increases inflammatory markers seen in BAL and serum samples, and increases the virulence of Staphylococcus aureus
TREATMENT AND PATIENT EDUCATION
The PCP’s treatment plan included patient education about the importance of infection control measures when receiving a mAb; this includes practicing good hand and environmental hygiene, maintaining vaccinations, avoiding or reducing exposure to individuals who have infections or colds, avoiding large crowds (especially during flu season), and following recommendations for nutrition and hydration. The PCP also discussed how to recognize the early signs and symptoms of an infection—and the need for vigilant safety monitoring. The PCP described available options for smoking cessation, including nicotine replacement products, prescription non-nicotine medications, behavioral therapy, and/or counseling (individual, group or telephone) and discussed the risks associated with consuming nicotine and/or marijuana/THC and using electronic vaporizing devices.
The PCP emphasized the importance of completing the entire course of the oral antibiotics prescribed at discharge. The patient and the PCP agreed to the following plan of care: appointments with a pulmonologist and a neurologist within the next 2 weeks, and follow-up visits with the
1. Celius EG. Infections in patients with multiple sclerosis: implications for disease-modifying therapy. Acta Neurol Scand. 2017;136(suppl 201):34-36.
2. Hwang JH, Lyes M, Sladewski K, et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med (Berl). 2016;94(6):667-679.
A 19-year-old man was diagnosed with relapsing multiple sclerosis (MS) at age 7 and is currently being treated with an infusible monoclonal antibody (mAb) therapy. Early in the day, he receives an infusion at an outpatient clinic. That night, he begins to experience numbness and tingling in his right upper extremity, which prompts a visit to an urgent care clinic. There, the clinician administers IV fluids to the patient. After his symptoms improve, the patient is discharged home.
The next morning, he has a new onset of left-side shoulder and neck pain with a pulsating headache. The patient reports his symptoms to his primary care provider (PCP), who instructs him to visit the emergency department (ED) for evaluation and treatment of a possible infection.
EXAMINATION
The patient arrives at the ED with a 102.4°F fever, vomiting, cough, mild congestion, diaphoresis, generalized myalgias, and chills. He also reports depression and anxiety, saying that for the past 7 days, “I haven’t felt like my normal self.”
Medical history includes moderate persistent asthma that is not well controlled, status asthmaticus, and use of an electronic vaporizing device for inhaling nicotine and marijuana/tetrahydrocannabinol (THC). Besides mAb infusions, his medications include hydrocodone/acetaminophen, prochlorperazine, gabapentin, hydroxyzine, trazodone, albuterol, and montelukast.
Examination reveals vital signs within normal limits. Lab work confirms elevated white blood cell count and absolute neutrophil count. Chest x-ray shows diffuse bilateral interstitial and patchy airspace opacities. He is diagnosed with bilateral pneumonia and is admitted and started on an IV antibiotic.
Within 24 hours, a new chest x-ray shows worsening symptoms. CT of the chest with contrast reveals diffuse bilateral ground-glass and airspace opacities suggestive of acute respiratory distress syndrome; bilateral thickening of the pulmonary interstitium; trace bilateral pleural effusions; increased caliber of the main pulmonary artery; and mediastinal and right hilar lymphadenopathy.
Subsequently, the patient developed sepsis and went into acute hypoxemic respiratory failure. He is transferred to the ICU, and pulmonology is consulted. A bronchoscopy with bronchoalveolar lavage (BAL) reveals neutrophil predominance; fungal, bacterial, and viral cultures are negative. The patient is started on broad-spectrum IV antibiotics and high-dose IV steroids. After 4 days, he begins to improve and is transferred out of the ICU. He is discharged with oral steroids and antibiotics.
Continue to: DISCUSSION
DISCUSSION
Fortunately, the PCP and the ED provider identified risk factors that contributed to the patient’s pneumonia and its subsequent worsening to sepsis and acute hypoxemic respiratory failure. The immunosuppressive/immunomodulatory effect of mAb therapy increased the patient’s risk for infection and the severity of infection, which is why vigilant safety monitoring and surveillance is essential with mAb treatment.1 Bloodwork should be performed at least every 6 months and include a complete blood count, complete metabolic panel with differential, and JC virus antibody test. Additionally, urinalysis should be performed prior to every mAb infusion. All testing recommended in the package insert for the patient’s prescribed therapy should be performed.
The patient’s history of asthma and his chronic vaping predisposed him to respiratory infections. In mice studies, exposure to e-cigarette vapor has been shown to be cytotoxic to airway cells and to decrease macrophage and neutrophil antimicrobial function.2 Exposure also alters immunomodulating cytokines in the airway, increases inflammatory markers seen in BAL and serum samples, and increases the virulence of Staphylococcus aureus
TREATMENT AND PATIENT EDUCATION
The PCP’s treatment plan included patient education about the importance of infection control measures when receiving a mAb; this includes practicing good hand and environmental hygiene, maintaining vaccinations, avoiding or reducing exposure to individuals who have infections or colds, avoiding large crowds (especially during flu season), and following recommendations for nutrition and hydration. The PCP also discussed how to recognize the early signs and symptoms of an infection—and the need for vigilant safety monitoring. The PCP described available options for smoking cessation, including nicotine replacement products, prescription non-nicotine medications, behavioral therapy, and/or counseling (individual, group or telephone) and discussed the risks associated with consuming nicotine and/or marijuana/THC and using electronic vaporizing devices.
The PCP emphasized the importance of completing the entire course of the oral antibiotics prescribed at discharge. The patient and the PCP agreed to the following plan of care: appointments with a pulmonologist and a neurologist within the next 2 weeks, and follow-up visits with the
A 19-year-old man was diagnosed with relapsing multiple sclerosis (MS) at age 7 and is currently being treated with an infusible monoclonal antibody (mAb) therapy. Early in the day, he receives an infusion at an outpatient clinic. That night, he begins to experience numbness and tingling in his right upper extremity, which prompts a visit to an urgent care clinic. There, the clinician administers IV fluids to the patient. After his symptoms improve, the patient is discharged home.
The next morning, he has a new onset of left-side shoulder and neck pain with a pulsating headache. The patient reports his symptoms to his primary care provider (PCP), who instructs him to visit the emergency department (ED) for evaluation and treatment of a possible infection.
EXAMINATION
The patient arrives at the ED with a 102.4°F fever, vomiting, cough, mild congestion, diaphoresis, generalized myalgias, and chills. He also reports depression and anxiety, saying that for the past 7 days, “I haven’t felt like my normal self.”
Medical history includes moderate persistent asthma that is not well controlled, status asthmaticus, and use of an electronic vaporizing device for inhaling nicotine and marijuana/tetrahydrocannabinol (THC). Besides mAb infusions, his medications include hydrocodone/acetaminophen, prochlorperazine, gabapentin, hydroxyzine, trazodone, albuterol, and montelukast.
Examination reveals vital signs within normal limits. Lab work confirms elevated white blood cell count and absolute neutrophil count. Chest x-ray shows diffuse bilateral interstitial and patchy airspace opacities. He is diagnosed with bilateral pneumonia and is admitted and started on an IV antibiotic.
Within 24 hours, a new chest x-ray shows worsening symptoms. CT of the chest with contrast reveals diffuse bilateral ground-glass and airspace opacities suggestive of acute respiratory distress syndrome; bilateral thickening of the pulmonary interstitium; trace bilateral pleural effusions; increased caliber of the main pulmonary artery; and mediastinal and right hilar lymphadenopathy.
Subsequently, the patient developed sepsis and went into acute hypoxemic respiratory failure. He is transferred to the ICU, and pulmonology is consulted. A bronchoscopy with bronchoalveolar lavage (BAL) reveals neutrophil predominance; fungal, bacterial, and viral cultures are negative. The patient is started on broad-spectrum IV antibiotics and high-dose IV steroids. After 4 days, he begins to improve and is transferred out of the ICU. He is discharged with oral steroids and antibiotics.
Continue to: DISCUSSION
DISCUSSION
Fortunately, the PCP and the ED provider identified risk factors that contributed to the patient’s pneumonia and its subsequent worsening to sepsis and acute hypoxemic respiratory failure. The immunosuppressive/immunomodulatory effect of mAb therapy increased the patient’s risk for infection and the severity of infection, which is why vigilant safety monitoring and surveillance is essential with mAb treatment.1 Bloodwork should be performed at least every 6 months and include a complete blood count, complete metabolic panel with differential, and JC virus antibody test. Additionally, urinalysis should be performed prior to every mAb infusion. All testing recommended in the package insert for the patient’s prescribed therapy should be performed.
The patient’s history of asthma and his chronic vaping predisposed him to respiratory infections. In mice studies, exposure to e-cigarette vapor has been shown to be cytotoxic to airway cells and to decrease macrophage and neutrophil antimicrobial function.2 Exposure also alters immunomodulating cytokines in the airway, increases inflammatory markers seen in BAL and serum samples, and increases the virulence of Staphylococcus aureus
TREATMENT AND PATIENT EDUCATION
The PCP’s treatment plan included patient education about the importance of infection control measures when receiving a mAb; this includes practicing good hand and environmental hygiene, maintaining vaccinations, avoiding or reducing exposure to individuals who have infections or colds, avoiding large crowds (especially during flu season), and following recommendations for nutrition and hydration. The PCP also discussed how to recognize the early signs and symptoms of an infection—and the need for vigilant safety monitoring. The PCP described available options for smoking cessation, including nicotine replacement products, prescription non-nicotine medications, behavioral therapy, and/or counseling (individual, group or telephone) and discussed the risks associated with consuming nicotine and/or marijuana/THC and using electronic vaporizing devices.
The PCP emphasized the importance of completing the entire course of the oral antibiotics prescribed at discharge. The patient and the PCP agreed to the following plan of care: appointments with a pulmonologist and a neurologist within the next 2 weeks, and follow-up visits with the
1. Celius EG. Infections in patients with multiple sclerosis: implications for disease-modifying therapy. Acta Neurol Scand. 2017;136(suppl 201):34-36.
2. Hwang JH, Lyes M, Sladewski K, et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med (Berl). 2016;94(6):667-679.
1. Celius EG. Infections in patients with multiple sclerosis: implications for disease-modifying therapy. Acta Neurol Scand. 2017;136(suppl 201):34-36.
2. Hwang JH, Lyes M, Sladewski K, et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med (Berl). 2016;94(6):667-679.
Letters From Maine: An albatross or your identity?
The last time I saw her she was coiled up like a garter snake resting comfortable in the old toiletries travel case that was my “black bag” for more than 40 years. Joining her in peaceful solitude were a couple of ear curettes, an insufflator, and a dead pocket flashlight. The Kermit the Frog sticker on her diaphragm was faded to a barely recognizable blur. The chest piece was frozen in the diaphragm position as it had been for several decades. I never felt comfortable using the bell side.
She was the gift from a drug company back when medical students were more interested in freebies than making a statement about conflicts of interest. I have had to change her tubing several times when cracks at the bifurcation would allow me to hear my own breath sounds better than the patient’s. The ear pieces were the originals that I modified to fit my auditory canals more comfortably.
I suspect that many of you have developed a close relationship with your stethoscope, as I did. We were always close. She was either her coiled up in my pants’ pocket or clasped around my neck where she wore through collars at a costly clip. Her chest piece was kept tucked in my shirt to keep it warm for the patients. I never hung her over my shoulders the way physicians do in publicity photos. I always found that practice pretentious and impractical.
If I decided tomorrow to leave the challenges of retirement behind and reopen my practice would it make any sense to go down to the basement and roust out my old stethoscope from her slumber? There are better ways evaluate hearts and lungs and many of them will fit in my pocket just as well as that old stethoscope. Paul Wallach, MD, an executive associate dean at the Indiana University, Indianapolis, predicts that within a decade hand-held ultrasound devices with become part of a routine part of the physical exam (Lindsey Tanner. “Is the stethoscope dying? High-tech rivals pose a challenge.” Associated Press. 2019 Oct 23). Instruction in the use of these devices has already become part of the curriculum in some medical schools.
There have been several studies demonstrating that chest auscultation is a skill that some of us have lost and many others never successfully mastered. As much as I treasure my old stethoscope, is it time to get rid of those albatrosses hanging around our necks? They do bang against desks with a deafening ring. Cute infants and toddlers yank on them while we are trying to listen to their chests. If there are better ways to auscultate chests that will fit in our pockets shouldn’t we be using them?
Well, there is the cost for one thing. But, inevitably the price will come down and portability will go up. If we allow our stethoscopes to become nothing more than nostalgic museum pieces to sit along with the head mirror, What will photographers drape over our shoulders? With very few of us in office practice wearing white coats or scrub suits, we run the risk of losing our identity.
Sadly, I fear we will have to accept the disappearance of the stethoscope as a natural consequence of the technological march. But, it also is an unfortunate reflection of the fact that the art of doing a physical exam is fading. With auscultation and palpation disappearing from our diagnostic tool kit we must be careful to preserve and improve the one skill that is indispensable to the practice of medicine.
And, that is listening to what the patient has to tell us.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
The last time I saw her she was coiled up like a garter snake resting comfortable in the old toiletries travel case that was my “black bag” for more than 40 years. Joining her in peaceful solitude were a couple of ear curettes, an insufflator, and a dead pocket flashlight. The Kermit the Frog sticker on her diaphragm was faded to a barely recognizable blur. The chest piece was frozen in the diaphragm position as it had been for several decades. I never felt comfortable using the bell side.
She was the gift from a drug company back when medical students were more interested in freebies than making a statement about conflicts of interest. I have had to change her tubing several times when cracks at the bifurcation would allow me to hear my own breath sounds better than the patient’s. The ear pieces were the originals that I modified to fit my auditory canals more comfortably.
I suspect that many of you have developed a close relationship with your stethoscope, as I did. We were always close. She was either her coiled up in my pants’ pocket or clasped around my neck where she wore through collars at a costly clip. Her chest piece was kept tucked in my shirt to keep it warm for the patients. I never hung her over my shoulders the way physicians do in publicity photos. I always found that practice pretentious and impractical.
If I decided tomorrow to leave the challenges of retirement behind and reopen my practice would it make any sense to go down to the basement and roust out my old stethoscope from her slumber? There are better ways evaluate hearts and lungs and many of them will fit in my pocket just as well as that old stethoscope. Paul Wallach, MD, an executive associate dean at the Indiana University, Indianapolis, predicts that within a decade hand-held ultrasound devices with become part of a routine part of the physical exam (Lindsey Tanner. “Is the stethoscope dying? High-tech rivals pose a challenge.” Associated Press. 2019 Oct 23). Instruction in the use of these devices has already become part of the curriculum in some medical schools.
There have been several studies demonstrating that chest auscultation is a skill that some of us have lost and many others never successfully mastered. As much as I treasure my old stethoscope, is it time to get rid of those albatrosses hanging around our necks? They do bang against desks with a deafening ring. Cute infants and toddlers yank on them while we are trying to listen to their chests. If there are better ways to auscultate chests that will fit in our pockets shouldn’t we be using them?
Well, there is the cost for one thing. But, inevitably the price will come down and portability will go up. If we allow our stethoscopes to become nothing more than nostalgic museum pieces to sit along with the head mirror, What will photographers drape over our shoulders? With very few of us in office practice wearing white coats or scrub suits, we run the risk of losing our identity.
Sadly, I fear we will have to accept the disappearance of the stethoscope as a natural consequence of the technological march. But, it also is an unfortunate reflection of the fact that the art of doing a physical exam is fading. With auscultation and palpation disappearing from our diagnostic tool kit we must be careful to preserve and improve the one skill that is indispensable to the practice of medicine.
And, that is listening to what the patient has to tell us.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
The last time I saw her she was coiled up like a garter snake resting comfortable in the old toiletries travel case that was my “black bag” for more than 40 years. Joining her in peaceful solitude were a couple of ear curettes, an insufflator, and a dead pocket flashlight. The Kermit the Frog sticker on her diaphragm was faded to a barely recognizable blur. The chest piece was frozen in the diaphragm position as it had been for several decades. I never felt comfortable using the bell side.
She was the gift from a drug company back when medical students were more interested in freebies than making a statement about conflicts of interest. I have had to change her tubing several times when cracks at the bifurcation would allow me to hear my own breath sounds better than the patient’s. The ear pieces were the originals that I modified to fit my auditory canals more comfortably.
I suspect that many of you have developed a close relationship with your stethoscope, as I did. We were always close. She was either her coiled up in my pants’ pocket or clasped around my neck where she wore through collars at a costly clip. Her chest piece was kept tucked in my shirt to keep it warm for the patients. I never hung her over my shoulders the way physicians do in publicity photos. I always found that practice pretentious and impractical.
If I decided tomorrow to leave the challenges of retirement behind and reopen my practice would it make any sense to go down to the basement and roust out my old stethoscope from her slumber? There are better ways evaluate hearts and lungs and many of them will fit in my pocket just as well as that old stethoscope. Paul Wallach, MD, an executive associate dean at the Indiana University, Indianapolis, predicts that within a decade hand-held ultrasound devices with become part of a routine part of the physical exam (Lindsey Tanner. “Is the stethoscope dying? High-tech rivals pose a challenge.” Associated Press. 2019 Oct 23). Instruction in the use of these devices has already become part of the curriculum in some medical schools.
There have been several studies demonstrating that chest auscultation is a skill that some of us have lost and many others never successfully mastered. As much as I treasure my old stethoscope, is it time to get rid of those albatrosses hanging around our necks? They do bang against desks with a deafening ring. Cute infants and toddlers yank on them while we are trying to listen to their chests. If there are better ways to auscultate chests that will fit in our pockets shouldn’t we be using them?
Well, there is the cost for one thing. But, inevitably the price will come down and portability will go up. If we allow our stethoscopes to become nothing more than nostalgic museum pieces to sit along with the head mirror, What will photographers drape over our shoulders? With very few of us in office practice wearing white coats or scrub suits, we run the risk of losing our identity.
Sadly, I fear we will have to accept the disappearance of the stethoscope as a natural consequence of the technological march. But, it also is an unfortunate reflection of the fact that the art of doing a physical exam is fading. With auscultation and palpation disappearing from our diagnostic tool kit we must be careful to preserve and improve the one skill that is indispensable to the practice of medicine.
And, that is listening to what the patient has to tell us.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.