User login
Adding mental health clinicians to your practice is full of benefits
NEW ORLEANS – The way Jay Rabinowitz, MD, MPH, sees it, providing mental and behavioral health care services in your primary care pediatrics practice is a win-win for patients, parents, and clinicians.
For one thing, children with mental and behavioral issues – especially depression and anxiety – make up a good chunk of any pediatrician’s workday. Dr. Rabinowitz, clinical professor of pediatrics at the University of Colorado, Aurora, said at the annual meeting of the American Academy of Pediatrics. “It is the most costly issue in children’s health care today.”
According to “Behavioral Health Integration in Pediatric Primary Care,” a report supported by the Milbank Memorial Fund, one in five children aged 9-17 years have a diagnosable psychiatric disorder, and up to 70% of children in the juvenile justice system have a mental health disorder. The report also found that the treatment of mental health disorders accounts for the most costly childhood medical expenditure, and that between 15% and 20% of children with psychiatric disorders receive specialty care; the rest see their primary care provider. A long-term cost analysis showed significant cost savings: $1 spent on collaborative care saves $6.50 on health care costs.
More recently, the Guidelines for Adolescent Depression in Primary Care (GLAD-PC) found that only 50% of adolescents with depression are diagnosed before reaching adulthood (Pediatrics. March 2018;141[3]:e20174081). As many as two out of three youth with depression are not identified by their pediatrician and do not receive any kind of care.
“Even when diagnosed, only half of these patients are treated appropriately,” said Dr. Rabinowitz, who also practices at Parker (Colo.) Pediatrics and Adolescents.
The guidelines also found that reliance on self-report depression checklists alone lead to substantial numbers of false-positive and false-negative cases. “Primary care providers will benefit from having access to ongoing consultation with mental health providers,” according to the guidelines.
“Integrative care was associated with significant decreases in depression scores, and improved response and remission rates at 12 months, compared with treatment as usual,” Dr. Rabinowitz said.
Providing mental health services in a primary care pediatrics setting also makes sense because there’s a shortage of psychiatrists and psychologists to see them, and it enables patients to get evaluated quicker. “It’s convenient, and it reduces stigma,” he added. “It’s a familiar setting, a familiar provider, and they’re more likely to initiate counseling. Nationwide, 50% of patients who are referred for mental health do not make their initial appointment. Think about that. If you had diabetics in your practice and only 50% would go to the endocrinologist, what would you think?”
How Dr. Rabinowitz and his partners got started
Dr. Rabinowitz and his colleagues created an integrated care model in 2008 by adding a psychologist to their practice, but before doing that, they asked parents of children with mental and behavioral health issues what type of insurance they had. Then they obtained a referral list from the family’s insurer and hoped for the best. “Sometimes I referred to someone I may not have heard of,” Dr. Rabinowitz said. “Usually I did not get follow-up reports, or even know for sure if the patient ever went.”
Today, Parker Pediatrics and Adolescents employs three doctoral-level psychologists: one full-time, one three-quarter time, and one half-time, as well as one master’s-level therapist who works half-time.
“On any given day, we have at least two counselors in our office,” said Lindsey Einhorn, PhD, a licensed clinical psychologist who joined the practice in 2011. She and her colleagues care for children and teens with ADHD, depression, anxiety, behavioral and adjustment disorders, drug counseling, behavioral addictions, social struggles such as bullying, obsessive compulsive disorder (OCD), loss, hair or eyelash pulling, mood dysregulation, and sibling conflict. They refer for educational testing, comprehensive psychological evaluations, difficult divorce cases, play therapy, complex cases requiring more than 20 sessions, and children of staff employed by the practice.
The practice features a separate waiting room for psychology patients and front office staff dedicated to managing their schedules. “For anyone who’s trying to make a psychology appointment but can’t be seen in an efficient manner or wants a different day or time, we keep an ongoing move-up list,” Dr. Einhorn said. “If a family calls to cancel an appointment, the front desk person who makes that cancellation will fill out a slip and give it to one of our psychology schedulers. That person will create a move-up list and start filling that appointment. If there’s a cancellation, it’s rare that it goes unfilled.”
Key forms for parents to complete include informed consent, a notice of privacy practices, a late cancel/no show policy, an initial intake agreement, and a summary of parent concerns.
Patient and clinician reaction
According to results from a recent survey of parents whose children were seen by a psychologist at Parker Pediatrics and Adolescents, 89% said it was important for their children to receive mental health services in the same location as their medical care, and 96% were satisfied with the services provided. In addition, 93% said that the experience benefited their child, 72% were satisfied with appointment times, and 55% expressed interest in virtual visits via telemedicine. Meanwhile, a survey of parents whose children have not been seen by a psychologist at the practice found that 65% knew a psychologist was on staff, and only 9% said that there were barriers to their child seeing a psychologist there.
Clinicians themselves benefit from having mental health specialists on site for referrals. “It enables you to be more efficient, and it saves time,” Dr. Rabinowitz said. “There’s knowledge and confidence gained, and it improves satisfaction because physicians don’t have to stay at the office later filling out referral forms. It meets the needs of your patients and their families, it attracts new patients, and you may be able to make some income on this.”
How to get started
Dr. Rabinowitz recommended that, once clinicians at a pediatric practice commit to expanding their services to include mental and behavioral health care, they should hold a corporate/partner meeting, assign responsibilities, and establish a timeline for implementation. “This is all very important,” he said. “Then you have to talk about what kind of arrangement you want to have. You could employ someone to join your practice, hire an independent contractor, establish a space share agreement, or have an out-of-office arrangement.”
For many years, clinicians at Parker Pediatrics and Adolescents had a psychologist perform ADHD evaluations on a consultative basis. “Then, as we saw a need for mental health services about a decade ago, we hired a part-time psychologist who did testing as well as counseling,” Dr. Rabinowitz said. “But that psychologist got very busy, so we hired a full-time psychologist. We continued to hire additional psychologists as need increased.”
Reimbursement issues
Numerous reimbursement barriers to providing mental health services in pediatric primary care exist, he noted, including a lack of payment if mental health codes are used, a lack of “incident to” payments in some areas of the country, existing reimbursement levels, and the fact that same-day billing of physical and mental health often is not allowed. “However, we have found that if we give flu shots during their mental health visit, [insurers] will cover the flu shot,” he said. “Reimbursement for screening is sometimes not covered very well.”
One reimbursement option is the fee-for-service/concierge model, “but that’s not an economic option for many,” he said. “You can’t see Medicaid patients in that model.” Joining a mental health networks is feasible, “but there is poor reimbursement,” he said. “It also creates another layer of administration.”
He recommends financial integration, “but you need to research your options because a lot of it is state dependent.” Other options include grants, insurance contracts, and seeking permission from Medicaid.
Mental health CPT codes that mental health clinicians at the practice commonly use include bill by time (CPT code 99214/15); psychotherapy session that lasts 16-37 minutes (CPT code 90832); psychotherapy session that lasts 38-52 minutes (CPT code 90834); and psychotherapy session that lasts more than 53 minutes (CPT code 90837). Clinicians also can bill by interactive complexity (CPT code 90785) and psychotherapy for crisis (CPT code 90839).
Dr. Rabinowitz and Dr. Einhorn reported having no financial disclosures.
NEW ORLEANS – The way Jay Rabinowitz, MD, MPH, sees it, providing mental and behavioral health care services in your primary care pediatrics practice is a win-win for patients, parents, and clinicians.
For one thing, children with mental and behavioral issues – especially depression and anxiety – make up a good chunk of any pediatrician’s workday. Dr. Rabinowitz, clinical professor of pediatrics at the University of Colorado, Aurora, said at the annual meeting of the American Academy of Pediatrics. “It is the most costly issue in children’s health care today.”
According to “Behavioral Health Integration in Pediatric Primary Care,” a report supported by the Milbank Memorial Fund, one in five children aged 9-17 years have a diagnosable psychiatric disorder, and up to 70% of children in the juvenile justice system have a mental health disorder. The report also found that the treatment of mental health disorders accounts for the most costly childhood medical expenditure, and that between 15% and 20% of children with psychiatric disorders receive specialty care; the rest see their primary care provider. A long-term cost analysis showed significant cost savings: $1 spent on collaborative care saves $6.50 on health care costs.
More recently, the Guidelines for Adolescent Depression in Primary Care (GLAD-PC) found that only 50% of adolescents with depression are diagnosed before reaching adulthood (Pediatrics. March 2018;141[3]:e20174081). As many as two out of three youth with depression are not identified by their pediatrician and do not receive any kind of care.
“Even when diagnosed, only half of these patients are treated appropriately,” said Dr. Rabinowitz, who also practices at Parker (Colo.) Pediatrics and Adolescents.
The guidelines also found that reliance on self-report depression checklists alone lead to substantial numbers of false-positive and false-negative cases. “Primary care providers will benefit from having access to ongoing consultation with mental health providers,” according to the guidelines.
“Integrative care was associated with significant decreases in depression scores, and improved response and remission rates at 12 months, compared with treatment as usual,” Dr. Rabinowitz said.
Providing mental health services in a primary care pediatrics setting also makes sense because there’s a shortage of psychiatrists and psychologists to see them, and it enables patients to get evaluated quicker. “It’s convenient, and it reduces stigma,” he added. “It’s a familiar setting, a familiar provider, and they’re more likely to initiate counseling. Nationwide, 50% of patients who are referred for mental health do not make their initial appointment. Think about that. If you had diabetics in your practice and only 50% would go to the endocrinologist, what would you think?”
How Dr. Rabinowitz and his partners got started
Dr. Rabinowitz and his colleagues created an integrated care model in 2008 by adding a psychologist to their practice, but before doing that, they asked parents of children with mental and behavioral health issues what type of insurance they had. Then they obtained a referral list from the family’s insurer and hoped for the best. “Sometimes I referred to someone I may not have heard of,” Dr. Rabinowitz said. “Usually I did not get follow-up reports, or even know for sure if the patient ever went.”
Today, Parker Pediatrics and Adolescents employs three doctoral-level psychologists: one full-time, one three-quarter time, and one half-time, as well as one master’s-level therapist who works half-time.
“On any given day, we have at least two counselors in our office,” said Lindsey Einhorn, PhD, a licensed clinical psychologist who joined the practice in 2011. She and her colleagues care for children and teens with ADHD, depression, anxiety, behavioral and adjustment disorders, drug counseling, behavioral addictions, social struggles such as bullying, obsessive compulsive disorder (OCD), loss, hair or eyelash pulling, mood dysregulation, and sibling conflict. They refer for educational testing, comprehensive psychological evaluations, difficult divorce cases, play therapy, complex cases requiring more than 20 sessions, and children of staff employed by the practice.
The practice features a separate waiting room for psychology patients and front office staff dedicated to managing their schedules. “For anyone who’s trying to make a psychology appointment but can’t be seen in an efficient manner or wants a different day or time, we keep an ongoing move-up list,” Dr. Einhorn said. “If a family calls to cancel an appointment, the front desk person who makes that cancellation will fill out a slip and give it to one of our psychology schedulers. That person will create a move-up list and start filling that appointment. If there’s a cancellation, it’s rare that it goes unfilled.”
Key forms for parents to complete include informed consent, a notice of privacy practices, a late cancel/no show policy, an initial intake agreement, and a summary of parent concerns.
Patient and clinician reaction
According to results from a recent survey of parents whose children were seen by a psychologist at Parker Pediatrics and Adolescents, 89% said it was important for their children to receive mental health services in the same location as their medical care, and 96% were satisfied with the services provided. In addition, 93% said that the experience benefited their child, 72% were satisfied with appointment times, and 55% expressed interest in virtual visits via telemedicine. Meanwhile, a survey of parents whose children have not been seen by a psychologist at the practice found that 65% knew a psychologist was on staff, and only 9% said that there were barriers to their child seeing a psychologist there.
Clinicians themselves benefit from having mental health specialists on site for referrals. “It enables you to be more efficient, and it saves time,” Dr. Rabinowitz said. “There’s knowledge and confidence gained, and it improves satisfaction because physicians don’t have to stay at the office later filling out referral forms. It meets the needs of your patients and their families, it attracts new patients, and you may be able to make some income on this.”
How to get started
Dr. Rabinowitz recommended that, once clinicians at a pediatric practice commit to expanding their services to include mental and behavioral health care, they should hold a corporate/partner meeting, assign responsibilities, and establish a timeline for implementation. “This is all very important,” he said. “Then you have to talk about what kind of arrangement you want to have. You could employ someone to join your practice, hire an independent contractor, establish a space share agreement, or have an out-of-office arrangement.”
For many years, clinicians at Parker Pediatrics and Adolescents had a psychologist perform ADHD evaluations on a consultative basis. “Then, as we saw a need for mental health services about a decade ago, we hired a part-time psychologist who did testing as well as counseling,” Dr. Rabinowitz said. “But that psychologist got very busy, so we hired a full-time psychologist. We continued to hire additional psychologists as need increased.”
Reimbursement issues
Numerous reimbursement barriers to providing mental health services in pediatric primary care exist, he noted, including a lack of payment if mental health codes are used, a lack of “incident to” payments in some areas of the country, existing reimbursement levels, and the fact that same-day billing of physical and mental health often is not allowed. “However, we have found that if we give flu shots during their mental health visit, [insurers] will cover the flu shot,” he said. “Reimbursement for screening is sometimes not covered very well.”
One reimbursement option is the fee-for-service/concierge model, “but that’s not an economic option for many,” he said. “You can’t see Medicaid patients in that model.” Joining a mental health networks is feasible, “but there is poor reimbursement,” he said. “It also creates another layer of administration.”
He recommends financial integration, “but you need to research your options because a lot of it is state dependent.” Other options include grants, insurance contracts, and seeking permission from Medicaid.
Mental health CPT codes that mental health clinicians at the practice commonly use include bill by time (CPT code 99214/15); psychotherapy session that lasts 16-37 minutes (CPT code 90832); psychotherapy session that lasts 38-52 minutes (CPT code 90834); and psychotherapy session that lasts more than 53 minutes (CPT code 90837). Clinicians also can bill by interactive complexity (CPT code 90785) and psychotherapy for crisis (CPT code 90839).
Dr. Rabinowitz and Dr. Einhorn reported having no financial disclosures.
NEW ORLEANS – The way Jay Rabinowitz, MD, MPH, sees it, providing mental and behavioral health care services in your primary care pediatrics practice is a win-win for patients, parents, and clinicians.
For one thing, children with mental and behavioral issues – especially depression and anxiety – make up a good chunk of any pediatrician’s workday. Dr. Rabinowitz, clinical professor of pediatrics at the University of Colorado, Aurora, said at the annual meeting of the American Academy of Pediatrics. “It is the most costly issue in children’s health care today.”
According to “Behavioral Health Integration in Pediatric Primary Care,” a report supported by the Milbank Memorial Fund, one in five children aged 9-17 years have a diagnosable psychiatric disorder, and up to 70% of children in the juvenile justice system have a mental health disorder. The report also found that the treatment of mental health disorders accounts for the most costly childhood medical expenditure, and that between 15% and 20% of children with psychiatric disorders receive specialty care; the rest see their primary care provider. A long-term cost analysis showed significant cost savings: $1 spent on collaborative care saves $6.50 on health care costs.
More recently, the Guidelines for Adolescent Depression in Primary Care (GLAD-PC) found that only 50% of adolescents with depression are diagnosed before reaching adulthood (Pediatrics. March 2018;141[3]:e20174081). As many as two out of three youth with depression are not identified by their pediatrician and do not receive any kind of care.
“Even when diagnosed, only half of these patients are treated appropriately,” said Dr. Rabinowitz, who also practices at Parker (Colo.) Pediatrics and Adolescents.
The guidelines also found that reliance on self-report depression checklists alone lead to substantial numbers of false-positive and false-negative cases. “Primary care providers will benefit from having access to ongoing consultation with mental health providers,” according to the guidelines.
“Integrative care was associated with significant decreases in depression scores, and improved response and remission rates at 12 months, compared with treatment as usual,” Dr. Rabinowitz said.
Providing mental health services in a primary care pediatrics setting also makes sense because there’s a shortage of psychiatrists and psychologists to see them, and it enables patients to get evaluated quicker. “It’s convenient, and it reduces stigma,” he added. “It’s a familiar setting, a familiar provider, and they’re more likely to initiate counseling. Nationwide, 50% of patients who are referred for mental health do not make their initial appointment. Think about that. If you had diabetics in your practice and only 50% would go to the endocrinologist, what would you think?”
How Dr. Rabinowitz and his partners got started
Dr. Rabinowitz and his colleagues created an integrated care model in 2008 by adding a psychologist to their practice, but before doing that, they asked parents of children with mental and behavioral health issues what type of insurance they had. Then they obtained a referral list from the family’s insurer and hoped for the best. “Sometimes I referred to someone I may not have heard of,” Dr. Rabinowitz said. “Usually I did not get follow-up reports, or even know for sure if the patient ever went.”
Today, Parker Pediatrics and Adolescents employs three doctoral-level psychologists: one full-time, one three-quarter time, and one half-time, as well as one master’s-level therapist who works half-time.
“On any given day, we have at least two counselors in our office,” said Lindsey Einhorn, PhD, a licensed clinical psychologist who joined the practice in 2011. She and her colleagues care for children and teens with ADHD, depression, anxiety, behavioral and adjustment disorders, drug counseling, behavioral addictions, social struggles such as bullying, obsessive compulsive disorder (OCD), loss, hair or eyelash pulling, mood dysregulation, and sibling conflict. They refer for educational testing, comprehensive psychological evaluations, difficult divorce cases, play therapy, complex cases requiring more than 20 sessions, and children of staff employed by the practice.
The practice features a separate waiting room for psychology patients and front office staff dedicated to managing their schedules. “For anyone who’s trying to make a psychology appointment but can’t be seen in an efficient manner or wants a different day or time, we keep an ongoing move-up list,” Dr. Einhorn said. “If a family calls to cancel an appointment, the front desk person who makes that cancellation will fill out a slip and give it to one of our psychology schedulers. That person will create a move-up list and start filling that appointment. If there’s a cancellation, it’s rare that it goes unfilled.”
Key forms for parents to complete include informed consent, a notice of privacy practices, a late cancel/no show policy, an initial intake agreement, and a summary of parent concerns.
Patient and clinician reaction
According to results from a recent survey of parents whose children were seen by a psychologist at Parker Pediatrics and Adolescents, 89% said it was important for their children to receive mental health services in the same location as their medical care, and 96% were satisfied with the services provided. In addition, 93% said that the experience benefited their child, 72% were satisfied with appointment times, and 55% expressed interest in virtual visits via telemedicine. Meanwhile, a survey of parents whose children have not been seen by a psychologist at the practice found that 65% knew a psychologist was on staff, and only 9% said that there were barriers to their child seeing a psychologist there.
Clinicians themselves benefit from having mental health specialists on site for referrals. “It enables you to be more efficient, and it saves time,” Dr. Rabinowitz said. “There’s knowledge and confidence gained, and it improves satisfaction because physicians don’t have to stay at the office later filling out referral forms. It meets the needs of your patients and their families, it attracts new patients, and you may be able to make some income on this.”
How to get started
Dr. Rabinowitz recommended that, once clinicians at a pediatric practice commit to expanding their services to include mental and behavioral health care, they should hold a corporate/partner meeting, assign responsibilities, and establish a timeline for implementation. “This is all very important,” he said. “Then you have to talk about what kind of arrangement you want to have. You could employ someone to join your practice, hire an independent contractor, establish a space share agreement, or have an out-of-office arrangement.”
For many years, clinicians at Parker Pediatrics and Adolescents had a psychologist perform ADHD evaluations on a consultative basis. “Then, as we saw a need for mental health services about a decade ago, we hired a part-time psychologist who did testing as well as counseling,” Dr. Rabinowitz said. “But that psychologist got very busy, so we hired a full-time psychologist. We continued to hire additional psychologists as need increased.”
Reimbursement issues
Numerous reimbursement barriers to providing mental health services in pediatric primary care exist, he noted, including a lack of payment if mental health codes are used, a lack of “incident to” payments in some areas of the country, existing reimbursement levels, and the fact that same-day billing of physical and mental health often is not allowed. “However, we have found that if we give flu shots during their mental health visit, [insurers] will cover the flu shot,” he said. “Reimbursement for screening is sometimes not covered very well.”
One reimbursement option is the fee-for-service/concierge model, “but that’s not an economic option for many,” he said. “You can’t see Medicaid patients in that model.” Joining a mental health networks is feasible, “but there is poor reimbursement,” he said. “It also creates another layer of administration.”
He recommends financial integration, “but you need to research your options because a lot of it is state dependent.” Other options include grants, insurance contracts, and seeking permission from Medicaid.
Mental health CPT codes that mental health clinicians at the practice commonly use include bill by time (CPT code 99214/15); psychotherapy session that lasts 16-37 minutes (CPT code 90832); psychotherapy session that lasts 38-52 minutes (CPT code 90834); and psychotherapy session that lasts more than 53 minutes (CPT code 90837). Clinicians also can bill by interactive complexity (CPT code 90785) and psychotherapy for crisis (CPT code 90839).
Dr. Rabinowitz and Dr. Einhorn reported having no financial disclosures.
EXPERT ANALYSIS FROM AAP 2019
Multidisciplinary care could address fertility preservation in transgender youth
PHILADELPHIA – A multidisciplinary approach is needed to care for gender-diverse transgender adolescents interested in fertility preservation, Leena Nahata, MD, said at the annual meeting of the American Society for Reproductive Medicine.
especially in the absence of longitudinal data, said Dr. Nahata, medical director of the fertility and reproductive health program at Nationwide Children’s Hospital, Columbus, Ohio. “We’re trying to counsel these youth and their parents about long-term outcomes of hormone therapies. However, despite the lack of data, not treating them also is not a viable option.”
Another concern among transgender individuals, Dr. Nahata said, is a high risk of mental health issues. Approximately one-third of transgender individuals experience depression, and between one-third and one-half have suicidal ideation or attempted suicide.
“It’s important to realize that these risks are not inevitable,” she said. Support from parents, peers, and social groups; engaging with the health care system; and having access to puberty suppression, gender-affirming hormones, and surgery are protective outcomes for mental health concerns. “It’s because of this that so many of us feel obligated to move on with treatments even in a setting of a lack of data.”
According to 2017 guidelines from the Endocrine Society on gender-dysphoric and gender-incongruent persons, patients can begin gonadotropin-releasing hormone (GnRH) agonists at Tanner Stage 2 of puberty (J Clin Endocrinol Metab. 2017 Nov. doi: 10.1210/jc.2017-01658). Before starting treatment, a mental health provider should confirm gender dysphoria or incongruence, and determine whether the patient has “sufficient mental capacity” to understand the long-term consequences of treatment with gender-affirming hormones such as estrogen and testosterone because the effects are partially irreversible, including a potential loss of fertility. Most pediatric patients will have this ability by 16 years old, but some programs across the country begin treatment between 13.5 years and 14 years of age, said Dr. Nahata. One consideration of beginning GnRH agonists and then moving directly to gender-affirming hormone therapy, there may not be an opportunity to explore fertility preservation.
Dr. Nahata acknowledged the data for the long-term effects of testosterone and estrogen on fertility is “murky,” but despite a lack of data, the American Society for Reproductive Medicine released an ethics statement in 2015 affirming that transgender patients “have the same interests as other persons in having children and in accessing fertility services for fertility preservation and reproduction” and pediatric providers “should offer fertility preservation options to individuals before gender transition” (Fertil Steril. 2015 Sep 9. doi: 10.1016/j.fertnstert.2015.08.021).
There also is mixed evidence that transgender individuals take advantage of fertility preservation services, whether offered or not. Two studies from Belgium that surveyed transgender individuals on parenthood preferences found 54% of adult trans men had a desire for children and that 38% of adult trans men and 51% of adult trans women would consider fertility preservation if it was an option. However, Dr. Nahata said a retrospective study from her own group of 50 adolescent trans males and 23 adolescent trans females found 99% of the cohort was counseled on fertility preservation, but only 3% (2 patients) attempted fertility preservation, and both were trans females (J Adolesc Health. 2017 Jul. doi: 10.1016/j.jadohealth.2016.12.012).
Another study examining use of fertility preservation in trans females in the Netherlands by Brik et al. found a much higher use of fertility preservation, with 38% of patients attempting cryopreservation after counseling (J Adolesc Health. 2019 May. doi: 10.1016/j.jadohealth.2018.11.008). “It’s unclear whether this is a regional difference or whether things are actually shifting over a short period of time,” said Dr. Nahata.
Attitudes about fertility preservation among gender-diverse transgender youth also impact its use in this patient population. A survey of transgender youth found less than 40% preferred adoption to biological parenthood, but said their feelings might change as time passes. However, more than half wanted more information on their family-building options. For other transgender youth aged 12-19 years, having children was their “lowest life priority,” compared with having friends, their health, and other issues in their lives, said Dr. Nahata.
In a 24-item survey Dr. Nahata and her team administered to 44 trans nonbinary adolescents, the most common reasons for not seeking fertility preservation were feelings of being too young, not wanting to be a parent or have a biological child, not wanting to delay treatment, and not being able to afford the cost of fertility preservation.
“This just speaks to the complexities of counseling in this population, and the importance of having a multidisciplinary team to see these youth and families to do more comprehensive counseling,” she said.
Dr. Nahata reported no relevant conflicts of interest.
PHILADELPHIA – A multidisciplinary approach is needed to care for gender-diverse transgender adolescents interested in fertility preservation, Leena Nahata, MD, said at the annual meeting of the American Society for Reproductive Medicine.
especially in the absence of longitudinal data, said Dr. Nahata, medical director of the fertility and reproductive health program at Nationwide Children’s Hospital, Columbus, Ohio. “We’re trying to counsel these youth and their parents about long-term outcomes of hormone therapies. However, despite the lack of data, not treating them also is not a viable option.”
Another concern among transgender individuals, Dr. Nahata said, is a high risk of mental health issues. Approximately one-third of transgender individuals experience depression, and between one-third and one-half have suicidal ideation or attempted suicide.
“It’s important to realize that these risks are not inevitable,” she said. Support from parents, peers, and social groups; engaging with the health care system; and having access to puberty suppression, gender-affirming hormones, and surgery are protective outcomes for mental health concerns. “It’s because of this that so many of us feel obligated to move on with treatments even in a setting of a lack of data.”
According to 2017 guidelines from the Endocrine Society on gender-dysphoric and gender-incongruent persons, patients can begin gonadotropin-releasing hormone (GnRH) agonists at Tanner Stage 2 of puberty (J Clin Endocrinol Metab. 2017 Nov. doi: 10.1210/jc.2017-01658). Before starting treatment, a mental health provider should confirm gender dysphoria or incongruence, and determine whether the patient has “sufficient mental capacity” to understand the long-term consequences of treatment with gender-affirming hormones such as estrogen and testosterone because the effects are partially irreversible, including a potential loss of fertility. Most pediatric patients will have this ability by 16 years old, but some programs across the country begin treatment between 13.5 years and 14 years of age, said Dr. Nahata. One consideration of beginning GnRH agonists and then moving directly to gender-affirming hormone therapy, there may not be an opportunity to explore fertility preservation.
Dr. Nahata acknowledged the data for the long-term effects of testosterone and estrogen on fertility is “murky,” but despite a lack of data, the American Society for Reproductive Medicine released an ethics statement in 2015 affirming that transgender patients “have the same interests as other persons in having children and in accessing fertility services for fertility preservation and reproduction” and pediatric providers “should offer fertility preservation options to individuals before gender transition” (Fertil Steril. 2015 Sep 9. doi: 10.1016/j.fertnstert.2015.08.021).
There also is mixed evidence that transgender individuals take advantage of fertility preservation services, whether offered or not. Two studies from Belgium that surveyed transgender individuals on parenthood preferences found 54% of adult trans men had a desire for children and that 38% of adult trans men and 51% of adult trans women would consider fertility preservation if it was an option. However, Dr. Nahata said a retrospective study from her own group of 50 adolescent trans males and 23 adolescent trans females found 99% of the cohort was counseled on fertility preservation, but only 3% (2 patients) attempted fertility preservation, and both were trans females (J Adolesc Health. 2017 Jul. doi: 10.1016/j.jadohealth.2016.12.012).
Another study examining use of fertility preservation in trans females in the Netherlands by Brik et al. found a much higher use of fertility preservation, with 38% of patients attempting cryopreservation after counseling (J Adolesc Health. 2019 May. doi: 10.1016/j.jadohealth.2018.11.008). “It’s unclear whether this is a regional difference or whether things are actually shifting over a short period of time,” said Dr. Nahata.
Attitudes about fertility preservation among gender-diverse transgender youth also impact its use in this patient population. A survey of transgender youth found less than 40% preferred adoption to biological parenthood, but said their feelings might change as time passes. However, more than half wanted more information on their family-building options. For other transgender youth aged 12-19 years, having children was their “lowest life priority,” compared with having friends, their health, and other issues in their lives, said Dr. Nahata.
In a 24-item survey Dr. Nahata and her team administered to 44 trans nonbinary adolescents, the most common reasons for not seeking fertility preservation were feelings of being too young, not wanting to be a parent or have a biological child, not wanting to delay treatment, and not being able to afford the cost of fertility preservation.
“This just speaks to the complexities of counseling in this population, and the importance of having a multidisciplinary team to see these youth and families to do more comprehensive counseling,” she said.
Dr. Nahata reported no relevant conflicts of interest.
PHILADELPHIA – A multidisciplinary approach is needed to care for gender-diverse transgender adolescents interested in fertility preservation, Leena Nahata, MD, said at the annual meeting of the American Society for Reproductive Medicine.
especially in the absence of longitudinal data, said Dr. Nahata, medical director of the fertility and reproductive health program at Nationwide Children’s Hospital, Columbus, Ohio. “We’re trying to counsel these youth and their parents about long-term outcomes of hormone therapies. However, despite the lack of data, not treating them also is not a viable option.”
Another concern among transgender individuals, Dr. Nahata said, is a high risk of mental health issues. Approximately one-third of transgender individuals experience depression, and between one-third and one-half have suicidal ideation or attempted suicide.
“It’s important to realize that these risks are not inevitable,” she said. Support from parents, peers, and social groups; engaging with the health care system; and having access to puberty suppression, gender-affirming hormones, and surgery are protective outcomes for mental health concerns. “It’s because of this that so many of us feel obligated to move on with treatments even in a setting of a lack of data.”
According to 2017 guidelines from the Endocrine Society on gender-dysphoric and gender-incongruent persons, patients can begin gonadotropin-releasing hormone (GnRH) agonists at Tanner Stage 2 of puberty (J Clin Endocrinol Metab. 2017 Nov. doi: 10.1210/jc.2017-01658). Before starting treatment, a mental health provider should confirm gender dysphoria or incongruence, and determine whether the patient has “sufficient mental capacity” to understand the long-term consequences of treatment with gender-affirming hormones such as estrogen and testosterone because the effects are partially irreversible, including a potential loss of fertility. Most pediatric patients will have this ability by 16 years old, but some programs across the country begin treatment between 13.5 years and 14 years of age, said Dr. Nahata. One consideration of beginning GnRH agonists and then moving directly to gender-affirming hormone therapy, there may not be an opportunity to explore fertility preservation.
Dr. Nahata acknowledged the data for the long-term effects of testosterone and estrogen on fertility is “murky,” but despite a lack of data, the American Society for Reproductive Medicine released an ethics statement in 2015 affirming that transgender patients “have the same interests as other persons in having children and in accessing fertility services for fertility preservation and reproduction” and pediatric providers “should offer fertility preservation options to individuals before gender transition” (Fertil Steril. 2015 Sep 9. doi: 10.1016/j.fertnstert.2015.08.021).
There also is mixed evidence that transgender individuals take advantage of fertility preservation services, whether offered or not. Two studies from Belgium that surveyed transgender individuals on parenthood preferences found 54% of adult trans men had a desire for children and that 38% of adult trans men and 51% of adult trans women would consider fertility preservation if it was an option. However, Dr. Nahata said a retrospective study from her own group of 50 adolescent trans males and 23 adolescent trans females found 99% of the cohort was counseled on fertility preservation, but only 3% (2 patients) attempted fertility preservation, and both were trans females (J Adolesc Health. 2017 Jul. doi: 10.1016/j.jadohealth.2016.12.012).
Another study examining use of fertility preservation in trans females in the Netherlands by Brik et al. found a much higher use of fertility preservation, with 38% of patients attempting cryopreservation after counseling (J Adolesc Health. 2019 May. doi: 10.1016/j.jadohealth.2018.11.008). “It’s unclear whether this is a regional difference or whether things are actually shifting over a short period of time,” said Dr. Nahata.
Attitudes about fertility preservation among gender-diverse transgender youth also impact its use in this patient population. A survey of transgender youth found less than 40% preferred adoption to biological parenthood, but said their feelings might change as time passes. However, more than half wanted more information on their family-building options. For other transgender youth aged 12-19 years, having children was their “lowest life priority,” compared with having friends, their health, and other issues in their lives, said Dr. Nahata.
In a 24-item survey Dr. Nahata and her team administered to 44 trans nonbinary adolescents, the most common reasons for not seeking fertility preservation were feelings of being too young, not wanting to be a parent or have a biological child, not wanting to delay treatment, and not being able to afford the cost of fertility preservation.
“This just speaks to the complexities of counseling in this population, and the importance of having a multidisciplinary team to see these youth and families to do more comprehensive counseling,” she said.
Dr. Nahata reported no relevant conflicts of interest.
EXPERT ANALYSIS FROM ASRM 2019
CDC identifies probable culprit in vaping lung injuries
found in lung fluid of victims.
In a telebriefing on Friday, Anne Schuchat, MD, the CDC’s principal deputy director, provided an update on recent lab findings and on case and death numbers reported so far to the CDC. The findings and more case information were published in the Mortality and Morbidity Weekly Report.
At the telebriefing, Dr. Schuchat stated that CDC has received 29 samples of bronchoalveolar lavage (BAL) fluid from EVALI patients from 10 states and that vitamin E acetate was identified in all samples. Vitamin E acetate has already been found in some vaping devices and the discovery of the chemical in the lungs of patients increases the likelihood that this toxin is at least one source of EVALI. These findings are the first to link substances found in vaping products with biological samples from patients hospitalized with EVALI.
Tetrahydrocannabinol (THC) was found in 23 of 28 samples tested and nicotine was found in 16 of 26 samples tested. Other diluents and additives of concern (such as plant oils, medium chain triglyceride oil, petroleum distillates, and diluent terpenes) were not detected in BAL fluid specimens from EVALI patients.
BAL fluid specimens were collected from hospitalized EVALI patients in the course of their treatment, although not for the specific purpose of the CDC investigation, and sent to the CDC by public health laboratories and health departments in California, Connecticut, Hawaii, Illinois, Maryland, Michigan, Minnesota, Texas, Utah, and Wisconsin for analysis.
Dr. Schuchat stated that, as of Nov. 5, there have been 2,051 cases of EVALI reported to the CDC and 39 EVALI patients have died, with other deaths still under investigation as possibly related to EVALI. She said that the trend in new EVALI cases reported appears to be decreasing, but some states continue to see new cases. She cautioned that the lab findings of vitamin E acetate in BAL fluid do not rule out other possible compounds or ingredients that may contribute to EVALI and said the investigation will continue.
E-cigarette user survey
During the telebriefing, Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist with the Illinois Department of Public Health (IDPH), gave an update on her department’s efforts to investigate vaping behaviors that might have led to EVALI in e-cigarette users and also to obtain more information on sources of vaping devices that could be linked to EVALI. The data were also reported in a MMWR.
The IDPH conducted an online public survey during September 2019 to October 2019 targeting e-cigarette, or vaping, product users in Illinois. The survey was promoted via social media on the IDPH website, local health departments, and other outlets. The survey yielded 4,631 respondents who answered questions about the frequency of vaping, sources of supply, and types of substances used. The investigators were then able to compare vaping-use habits and behaviors with similar information gleaned from EVALI patients.
Among survey respondents, 94% reported using any nicotine-containing e-cigarette, or vaping, products in the past 3 months; 21% used any THC-containing products; and 11% used both THC-containing products and nicotine-containing products. THC-containing product use was highest among survey respondents aged 18-24 years (36%) and decreased with increasing age. Compared with these survey respondents, EVALI patients were more likely to report exclusive use of THC-containing products (adjusted odds ratio, 2.0; 95% confidence interval, 1.1-3.6), frequent use (more than five times per day) of these products (aOR, 3.1; 95% CI, 1.6-6.0), and obtaining these products from informal sources, such as from a dealer, off the street, or from a friend (aOR, 9.2; 95% CI, 2.2-39.4). In addition, “the odds of using Dank Vapes, a class of largely counterfeit THC-containing products, was also higher among EVALI patients” (aOR, 8.5; 95% CI, 3.8-19.0), according to the MMWR.
Recommendations
CDC recommends that people should not buy any type of e-cigarette, or vaping, products, particularly those containing THC, off the street. They should also refrain from modifying or adding any substances to e-cigarette, or vaping, products that are not intended by the manufacturer, including products purchased through retail establishments.
Dr. Layden concluded, “we are in a better place today than we were a few weeks ago in terms of having one very strong culprit of concern based on the lung fluid testing,” but since the specific substances causing lung injury are not yet known, the only way to assure that individuals are not at risk while the investigation continues is to consider refraining from use of all vaping products.
For more information and resources visit For the Public, For Healthcare Providers, and For Health Departments pages, as well as the CDC’s Publications and Resources page.
found in lung fluid of victims.
In a telebriefing on Friday, Anne Schuchat, MD, the CDC’s principal deputy director, provided an update on recent lab findings and on case and death numbers reported so far to the CDC. The findings and more case information were published in the Mortality and Morbidity Weekly Report.
At the telebriefing, Dr. Schuchat stated that CDC has received 29 samples of bronchoalveolar lavage (BAL) fluid from EVALI patients from 10 states and that vitamin E acetate was identified in all samples. Vitamin E acetate has already been found in some vaping devices and the discovery of the chemical in the lungs of patients increases the likelihood that this toxin is at least one source of EVALI. These findings are the first to link substances found in vaping products with biological samples from patients hospitalized with EVALI.
Tetrahydrocannabinol (THC) was found in 23 of 28 samples tested and nicotine was found in 16 of 26 samples tested. Other diluents and additives of concern (such as plant oils, medium chain triglyceride oil, petroleum distillates, and diluent terpenes) were not detected in BAL fluid specimens from EVALI patients.
BAL fluid specimens were collected from hospitalized EVALI patients in the course of their treatment, although not for the specific purpose of the CDC investigation, and sent to the CDC by public health laboratories and health departments in California, Connecticut, Hawaii, Illinois, Maryland, Michigan, Minnesota, Texas, Utah, and Wisconsin for analysis.
Dr. Schuchat stated that, as of Nov. 5, there have been 2,051 cases of EVALI reported to the CDC and 39 EVALI patients have died, with other deaths still under investigation as possibly related to EVALI. She said that the trend in new EVALI cases reported appears to be decreasing, but some states continue to see new cases. She cautioned that the lab findings of vitamin E acetate in BAL fluid do not rule out other possible compounds or ingredients that may contribute to EVALI and said the investigation will continue.
E-cigarette user survey
During the telebriefing, Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist with the Illinois Department of Public Health (IDPH), gave an update on her department’s efforts to investigate vaping behaviors that might have led to EVALI in e-cigarette users and also to obtain more information on sources of vaping devices that could be linked to EVALI. The data were also reported in a MMWR.
The IDPH conducted an online public survey during September 2019 to October 2019 targeting e-cigarette, or vaping, product users in Illinois. The survey was promoted via social media on the IDPH website, local health departments, and other outlets. The survey yielded 4,631 respondents who answered questions about the frequency of vaping, sources of supply, and types of substances used. The investigators were then able to compare vaping-use habits and behaviors with similar information gleaned from EVALI patients.
Among survey respondents, 94% reported using any nicotine-containing e-cigarette, or vaping, products in the past 3 months; 21% used any THC-containing products; and 11% used both THC-containing products and nicotine-containing products. THC-containing product use was highest among survey respondents aged 18-24 years (36%) and decreased with increasing age. Compared with these survey respondents, EVALI patients were more likely to report exclusive use of THC-containing products (adjusted odds ratio, 2.0; 95% confidence interval, 1.1-3.6), frequent use (more than five times per day) of these products (aOR, 3.1; 95% CI, 1.6-6.0), and obtaining these products from informal sources, such as from a dealer, off the street, or from a friend (aOR, 9.2; 95% CI, 2.2-39.4). In addition, “the odds of using Dank Vapes, a class of largely counterfeit THC-containing products, was also higher among EVALI patients” (aOR, 8.5; 95% CI, 3.8-19.0), according to the MMWR.
Recommendations
CDC recommends that people should not buy any type of e-cigarette, or vaping, products, particularly those containing THC, off the street. They should also refrain from modifying or adding any substances to e-cigarette, or vaping, products that are not intended by the manufacturer, including products purchased through retail establishments.
Dr. Layden concluded, “we are in a better place today than we were a few weeks ago in terms of having one very strong culprit of concern based on the lung fluid testing,” but since the specific substances causing lung injury are not yet known, the only way to assure that individuals are not at risk while the investigation continues is to consider refraining from use of all vaping products.
For more information and resources visit For the Public, For Healthcare Providers, and For Health Departments pages, as well as the CDC’s Publications and Resources page.
found in lung fluid of victims.
In a telebriefing on Friday, Anne Schuchat, MD, the CDC’s principal deputy director, provided an update on recent lab findings and on case and death numbers reported so far to the CDC. The findings and more case information were published in the Mortality and Morbidity Weekly Report.
At the telebriefing, Dr. Schuchat stated that CDC has received 29 samples of bronchoalveolar lavage (BAL) fluid from EVALI patients from 10 states and that vitamin E acetate was identified in all samples. Vitamin E acetate has already been found in some vaping devices and the discovery of the chemical in the lungs of patients increases the likelihood that this toxin is at least one source of EVALI. These findings are the first to link substances found in vaping products with biological samples from patients hospitalized with EVALI.
Tetrahydrocannabinol (THC) was found in 23 of 28 samples tested and nicotine was found in 16 of 26 samples tested. Other diluents and additives of concern (such as plant oils, medium chain triglyceride oil, petroleum distillates, and diluent terpenes) were not detected in BAL fluid specimens from EVALI patients.
BAL fluid specimens were collected from hospitalized EVALI patients in the course of their treatment, although not for the specific purpose of the CDC investigation, and sent to the CDC by public health laboratories and health departments in California, Connecticut, Hawaii, Illinois, Maryland, Michigan, Minnesota, Texas, Utah, and Wisconsin for analysis.
Dr. Schuchat stated that, as of Nov. 5, there have been 2,051 cases of EVALI reported to the CDC and 39 EVALI patients have died, with other deaths still under investigation as possibly related to EVALI. She said that the trend in new EVALI cases reported appears to be decreasing, but some states continue to see new cases. She cautioned that the lab findings of vitamin E acetate in BAL fluid do not rule out other possible compounds or ingredients that may contribute to EVALI and said the investigation will continue.
E-cigarette user survey
During the telebriefing, Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist with the Illinois Department of Public Health (IDPH), gave an update on her department’s efforts to investigate vaping behaviors that might have led to EVALI in e-cigarette users and also to obtain more information on sources of vaping devices that could be linked to EVALI. The data were also reported in a MMWR.
The IDPH conducted an online public survey during September 2019 to October 2019 targeting e-cigarette, or vaping, product users in Illinois. The survey was promoted via social media on the IDPH website, local health departments, and other outlets. The survey yielded 4,631 respondents who answered questions about the frequency of vaping, sources of supply, and types of substances used. The investigators were then able to compare vaping-use habits and behaviors with similar information gleaned from EVALI patients.
Among survey respondents, 94% reported using any nicotine-containing e-cigarette, or vaping, products in the past 3 months; 21% used any THC-containing products; and 11% used both THC-containing products and nicotine-containing products. THC-containing product use was highest among survey respondents aged 18-24 years (36%) and decreased with increasing age. Compared with these survey respondents, EVALI patients were more likely to report exclusive use of THC-containing products (adjusted odds ratio, 2.0; 95% confidence interval, 1.1-3.6), frequent use (more than five times per day) of these products (aOR, 3.1; 95% CI, 1.6-6.0), and obtaining these products from informal sources, such as from a dealer, off the street, or from a friend (aOR, 9.2; 95% CI, 2.2-39.4). In addition, “the odds of using Dank Vapes, a class of largely counterfeit THC-containing products, was also higher among EVALI patients” (aOR, 8.5; 95% CI, 3.8-19.0), according to the MMWR.
Recommendations
CDC recommends that people should not buy any type of e-cigarette, or vaping, products, particularly those containing THC, off the street. They should also refrain from modifying or adding any substances to e-cigarette, or vaping, products that are not intended by the manufacturer, including products purchased through retail establishments.
Dr. Layden concluded, “we are in a better place today than we were a few weeks ago in terms of having one very strong culprit of concern based on the lung fluid testing,” but since the specific substances causing lung injury are not yet known, the only way to assure that individuals are not at risk while the investigation continues is to consider refraining from use of all vaping products.
For more information and resources visit For the Public, For Healthcare Providers, and For Health Departments pages, as well as the CDC’s Publications and Resources page.
Tide beginning to turn on vaccine hesitancy
NEW ORLEANS –
The shift began with the measles outbreak in Southern California in late 2014, he said. According to the Centers for Disease Control and Prevention, 125 measles cases with rash that occurred between Dec. 28, 2014, and Feb. 8, 2015, were confirmed in U.S. residents. Of these, 100 were California residents (MMWR. 2015 Feb 20;64[06];153-4).
“This outbreak spread ultimately to 25 states and involved 189 people,” Dr. Offit said at the annual meeting of the American Academy of Pediatrics. “It was in the news almost every day. As a consequence, there were measles outbreaks in New York, New Jersey, Florida, Oregon, and Texas, and Washington, which began to turn the public sentiment against the antivaccine movement.”
Even longstanding skeptics are changing their tune. Dr. Offit, professor of pediatrics in the division of infectious diseases at the Children’s Hospital of Philadelphia, cited a recent study from the Autism Science Foundation which found that 85% of parents of children with autism spectrum disorder don’t believe that vaccines cause the condition. “Although there will be parents who continue to believe that vaccines cause autism, most parents of children with autism don’t believe that,” he said. “Also, it’s a little hard to make your case that vaccines are dangerous and that you shouldn’t get them in the midst of outbreaks.”
Perhaps the greatest pushback against antivaccination efforts has been made in the legal arena. In 2019 alone, legislators in California banned parents from not vaccinating their kids because of personal beliefs, while lawmakers in New York repealed the religious exemption to vaccinate, those in Maine repealed the religious and philosophical exemption, those in New Jersey required detailed written explanation for religious exemption, and those in Washington State repealed the philosophical exemption for the MMR vaccine.
Pushback also is apparent on various social media platforms. For example, Dr. Offit said, Pinterest restricts vaccine search results to curb the spread of misinformation, YouTube removes ads from antivaccine channels, Amazon Prime has pulled antivaccination documentaries from its video service, and Facebook has taken steps to curb misinformation about vaccines. “With outbreaks and with children suffering, the media and public sentiment has largely turned against those who are vehemently against vaccines,” he said. “I’m talking about an angry, politically connected, lawyer-backed group of people who are conspiracy theorists, [those] who no matter what you say, they’re going to believe there’s a conspiracy theory to hurt their children and not believe you. When that group becomes big enough and you start to see outbreaks like we’ve seen, then it becomes an issue. That’s where it comes down to legislation. Is it your inalienable right as a U.S. citizen to allow your child to catch and transmit a potentially fatal infection? That’s what we’re struggling with now.”
When meeting with parents who are skeptical about vaccines or refuse their children to have them, Dr. Offit advises clinicians to “go down swinging” in favor of vaccination. He shared how his wife, Bonnie, a pediatrician who practices in suburban Philadelphia, counsels parents who raise such concerns. “The way she handled it initially was to do the best she could to eventually get people vaccinated,” he said. “She was successful about one-quarter of the time. Then she drew a line. She started saying to parents, ‘Look; don’t put me in a position where you are asking me to practice substandard care. I can’t send them out of this room knowing that there’s more measles out there, knowing that there’s mumps out there, knowing that there’s whooping cough out there, knowing that there’s pneumococcus and varicella out there. If this child leaves this office and is hurt by any of those viruses or bacteria and I knew I could have done something to prevent it, I couldn’t live with myself. If you’re going to let this child out without being vaccinated I can’t see you anymore because I’m responsible for the health of this child.’ With that [approach], she has been far more successful. Because at some level, if you continue to see that patient, you’re tacitly agreeing that it’s okay to [not vaccinate].”
In 2000, Dr. Offit and colleagues created the Vaccine Education Center at Children’s Hospital of Philadelphia, which provides complete, up-to-date, and reliable information about vaccines to parents and clinicians. It summarizes the purpose of each vaccine, and the relative risks and benefits in easy-to-read language. The CDC also maintains updated information about vaccines and immunizations on its web site. For his part, Dr. Offit tells parents that passing on an opportunity to vaccinate their child is not a risk-free choice. “If you choose not to get a vaccine you probably will get away with it, but you might not,” he said. “You are playing a game of Russian roulette. It may not be five empty chambers and one bullet, but maybe it’s 100,000 empty chambers and one bullet. There’s a bullet there.”
Dr. Offit reported having no relevant financial disclosures.
NEW ORLEANS –
The shift began with the measles outbreak in Southern California in late 2014, he said. According to the Centers for Disease Control and Prevention, 125 measles cases with rash that occurred between Dec. 28, 2014, and Feb. 8, 2015, were confirmed in U.S. residents. Of these, 100 were California residents (MMWR. 2015 Feb 20;64[06];153-4).
“This outbreak spread ultimately to 25 states and involved 189 people,” Dr. Offit said at the annual meeting of the American Academy of Pediatrics. “It was in the news almost every day. As a consequence, there were measles outbreaks in New York, New Jersey, Florida, Oregon, and Texas, and Washington, which began to turn the public sentiment against the antivaccine movement.”
Even longstanding skeptics are changing their tune. Dr. Offit, professor of pediatrics in the division of infectious diseases at the Children’s Hospital of Philadelphia, cited a recent study from the Autism Science Foundation which found that 85% of parents of children with autism spectrum disorder don’t believe that vaccines cause the condition. “Although there will be parents who continue to believe that vaccines cause autism, most parents of children with autism don’t believe that,” he said. “Also, it’s a little hard to make your case that vaccines are dangerous and that you shouldn’t get them in the midst of outbreaks.”
Perhaps the greatest pushback against antivaccination efforts has been made in the legal arena. In 2019 alone, legislators in California banned parents from not vaccinating their kids because of personal beliefs, while lawmakers in New York repealed the religious exemption to vaccinate, those in Maine repealed the religious and philosophical exemption, those in New Jersey required detailed written explanation for religious exemption, and those in Washington State repealed the philosophical exemption for the MMR vaccine.
Pushback also is apparent on various social media platforms. For example, Dr. Offit said, Pinterest restricts vaccine search results to curb the spread of misinformation, YouTube removes ads from antivaccine channels, Amazon Prime has pulled antivaccination documentaries from its video service, and Facebook has taken steps to curb misinformation about vaccines. “With outbreaks and with children suffering, the media and public sentiment has largely turned against those who are vehemently against vaccines,” he said. “I’m talking about an angry, politically connected, lawyer-backed group of people who are conspiracy theorists, [those] who no matter what you say, they’re going to believe there’s a conspiracy theory to hurt their children and not believe you. When that group becomes big enough and you start to see outbreaks like we’ve seen, then it becomes an issue. That’s where it comes down to legislation. Is it your inalienable right as a U.S. citizen to allow your child to catch and transmit a potentially fatal infection? That’s what we’re struggling with now.”
When meeting with parents who are skeptical about vaccines or refuse their children to have them, Dr. Offit advises clinicians to “go down swinging” in favor of vaccination. He shared how his wife, Bonnie, a pediatrician who practices in suburban Philadelphia, counsels parents who raise such concerns. “The way she handled it initially was to do the best she could to eventually get people vaccinated,” he said. “She was successful about one-quarter of the time. Then she drew a line. She started saying to parents, ‘Look; don’t put me in a position where you are asking me to practice substandard care. I can’t send them out of this room knowing that there’s more measles out there, knowing that there’s mumps out there, knowing that there’s whooping cough out there, knowing that there’s pneumococcus and varicella out there. If this child leaves this office and is hurt by any of those viruses or bacteria and I knew I could have done something to prevent it, I couldn’t live with myself. If you’re going to let this child out without being vaccinated I can’t see you anymore because I’m responsible for the health of this child.’ With that [approach], she has been far more successful. Because at some level, if you continue to see that patient, you’re tacitly agreeing that it’s okay to [not vaccinate].”
In 2000, Dr. Offit and colleagues created the Vaccine Education Center at Children’s Hospital of Philadelphia, which provides complete, up-to-date, and reliable information about vaccines to parents and clinicians. It summarizes the purpose of each vaccine, and the relative risks and benefits in easy-to-read language. The CDC also maintains updated information about vaccines and immunizations on its web site. For his part, Dr. Offit tells parents that passing on an opportunity to vaccinate their child is not a risk-free choice. “If you choose not to get a vaccine you probably will get away with it, but you might not,” he said. “You are playing a game of Russian roulette. It may not be five empty chambers and one bullet, but maybe it’s 100,000 empty chambers and one bullet. There’s a bullet there.”
Dr. Offit reported having no relevant financial disclosures.
NEW ORLEANS –
The shift began with the measles outbreak in Southern California in late 2014, he said. According to the Centers for Disease Control and Prevention, 125 measles cases with rash that occurred between Dec. 28, 2014, and Feb. 8, 2015, were confirmed in U.S. residents. Of these, 100 were California residents (MMWR. 2015 Feb 20;64[06];153-4).
“This outbreak spread ultimately to 25 states and involved 189 people,” Dr. Offit said at the annual meeting of the American Academy of Pediatrics. “It was in the news almost every day. As a consequence, there were measles outbreaks in New York, New Jersey, Florida, Oregon, and Texas, and Washington, which began to turn the public sentiment against the antivaccine movement.”
Even longstanding skeptics are changing their tune. Dr. Offit, professor of pediatrics in the division of infectious diseases at the Children’s Hospital of Philadelphia, cited a recent study from the Autism Science Foundation which found that 85% of parents of children with autism spectrum disorder don’t believe that vaccines cause the condition. “Although there will be parents who continue to believe that vaccines cause autism, most parents of children with autism don’t believe that,” he said. “Also, it’s a little hard to make your case that vaccines are dangerous and that you shouldn’t get them in the midst of outbreaks.”
Perhaps the greatest pushback against antivaccination efforts has been made in the legal arena. In 2019 alone, legislators in California banned parents from not vaccinating their kids because of personal beliefs, while lawmakers in New York repealed the religious exemption to vaccinate, those in Maine repealed the religious and philosophical exemption, those in New Jersey required detailed written explanation for religious exemption, and those in Washington State repealed the philosophical exemption for the MMR vaccine.
Pushback also is apparent on various social media platforms. For example, Dr. Offit said, Pinterest restricts vaccine search results to curb the spread of misinformation, YouTube removes ads from antivaccine channels, Amazon Prime has pulled antivaccination documentaries from its video service, and Facebook has taken steps to curb misinformation about vaccines. “With outbreaks and with children suffering, the media and public sentiment has largely turned against those who are vehemently against vaccines,” he said. “I’m talking about an angry, politically connected, lawyer-backed group of people who are conspiracy theorists, [those] who no matter what you say, they’re going to believe there’s a conspiracy theory to hurt their children and not believe you. When that group becomes big enough and you start to see outbreaks like we’ve seen, then it becomes an issue. That’s where it comes down to legislation. Is it your inalienable right as a U.S. citizen to allow your child to catch and transmit a potentially fatal infection? That’s what we’re struggling with now.”
When meeting with parents who are skeptical about vaccines or refuse their children to have them, Dr. Offit advises clinicians to “go down swinging” in favor of vaccination. He shared how his wife, Bonnie, a pediatrician who practices in suburban Philadelphia, counsels parents who raise such concerns. “The way she handled it initially was to do the best she could to eventually get people vaccinated,” he said. “She was successful about one-quarter of the time. Then she drew a line. She started saying to parents, ‘Look; don’t put me in a position where you are asking me to practice substandard care. I can’t send them out of this room knowing that there’s more measles out there, knowing that there’s mumps out there, knowing that there’s whooping cough out there, knowing that there’s pneumococcus and varicella out there. If this child leaves this office and is hurt by any of those viruses or bacteria and I knew I could have done something to prevent it, I couldn’t live with myself. If you’re going to let this child out without being vaccinated I can’t see you anymore because I’m responsible for the health of this child.’ With that [approach], she has been far more successful. Because at some level, if you continue to see that patient, you’re tacitly agreeing that it’s okay to [not vaccinate].”
In 2000, Dr. Offit and colleagues created the Vaccine Education Center at Children’s Hospital of Philadelphia, which provides complete, up-to-date, and reliable information about vaccines to parents and clinicians. It summarizes the purpose of each vaccine, and the relative risks and benefits in easy-to-read language. The CDC also maintains updated information about vaccines and immunizations on its web site. For his part, Dr. Offit tells parents that passing on an opportunity to vaccinate their child is not a risk-free choice. “If you choose not to get a vaccine you probably will get away with it, but you might not,” he said. “You are playing a game of Russian roulette. It may not be five empty chambers and one bullet, but maybe it’s 100,000 empty chambers and one bullet. There’s a bullet there.”
Dr. Offit reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM AAP 2019
AAD-NPF Pediatric psoriasis guideline advises on physical and mental care
Psoriasis management in children involves attention not only to treatment of the physical condition but also psychosocial wellness and quality of life, according to
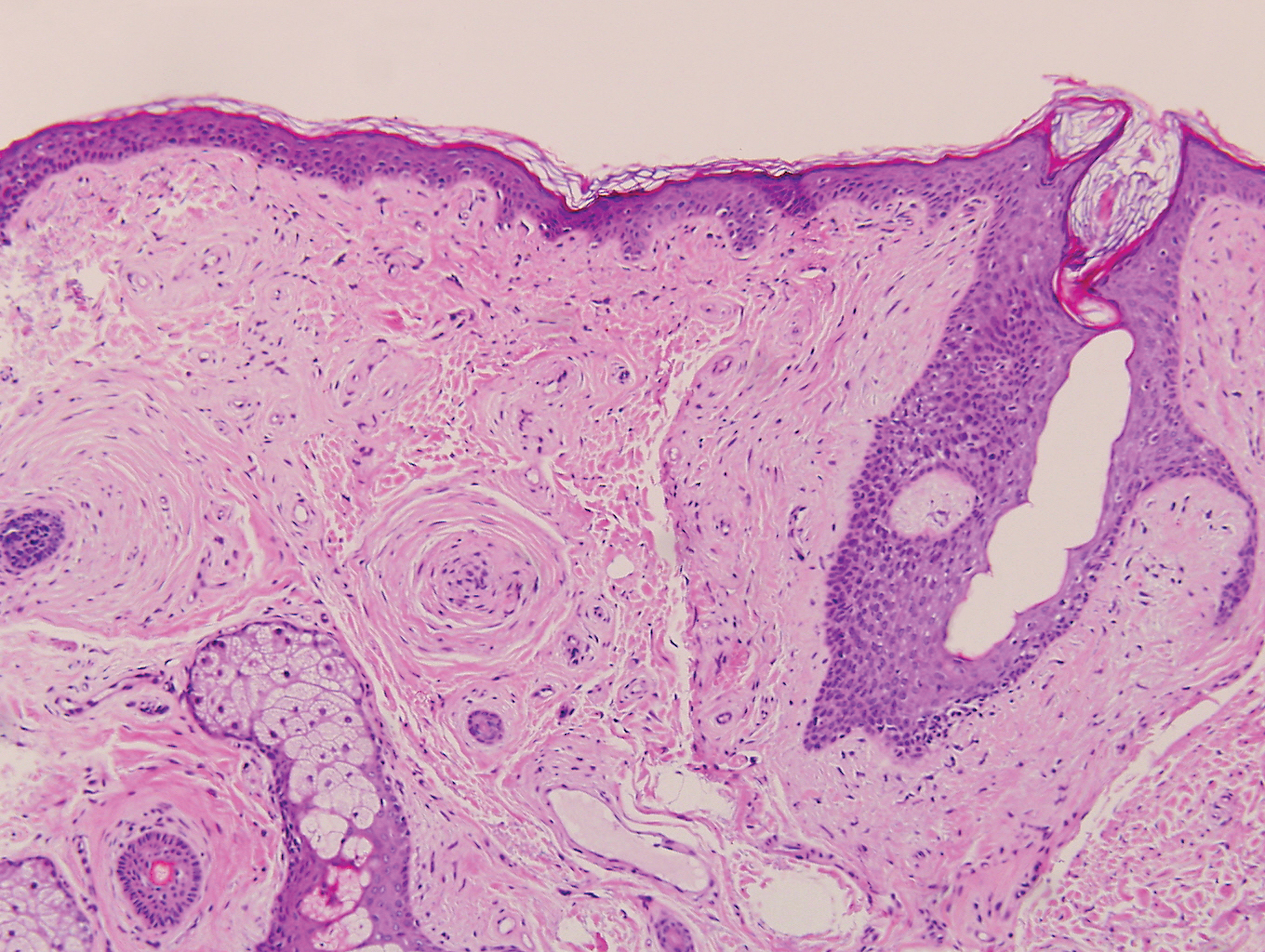
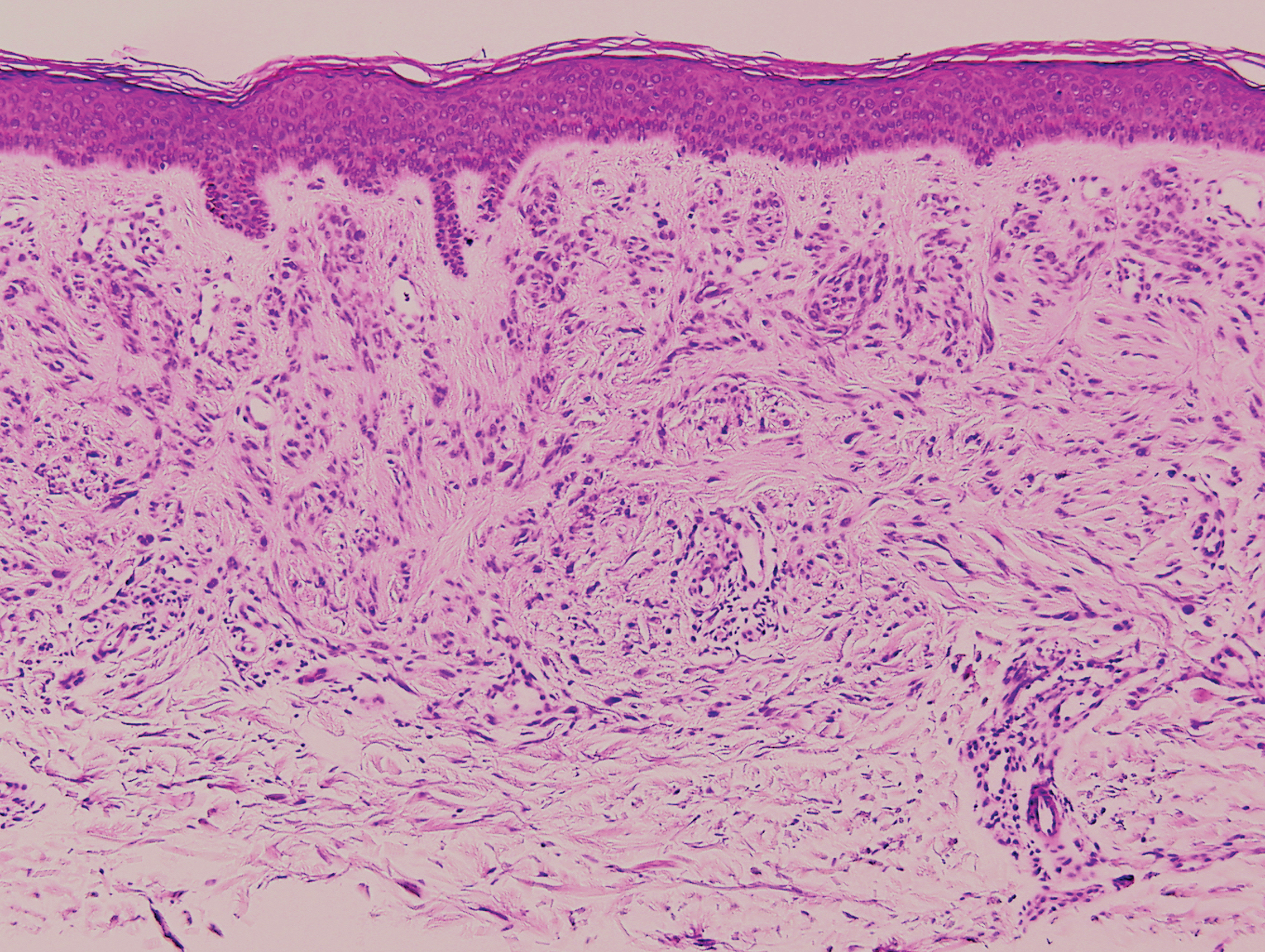
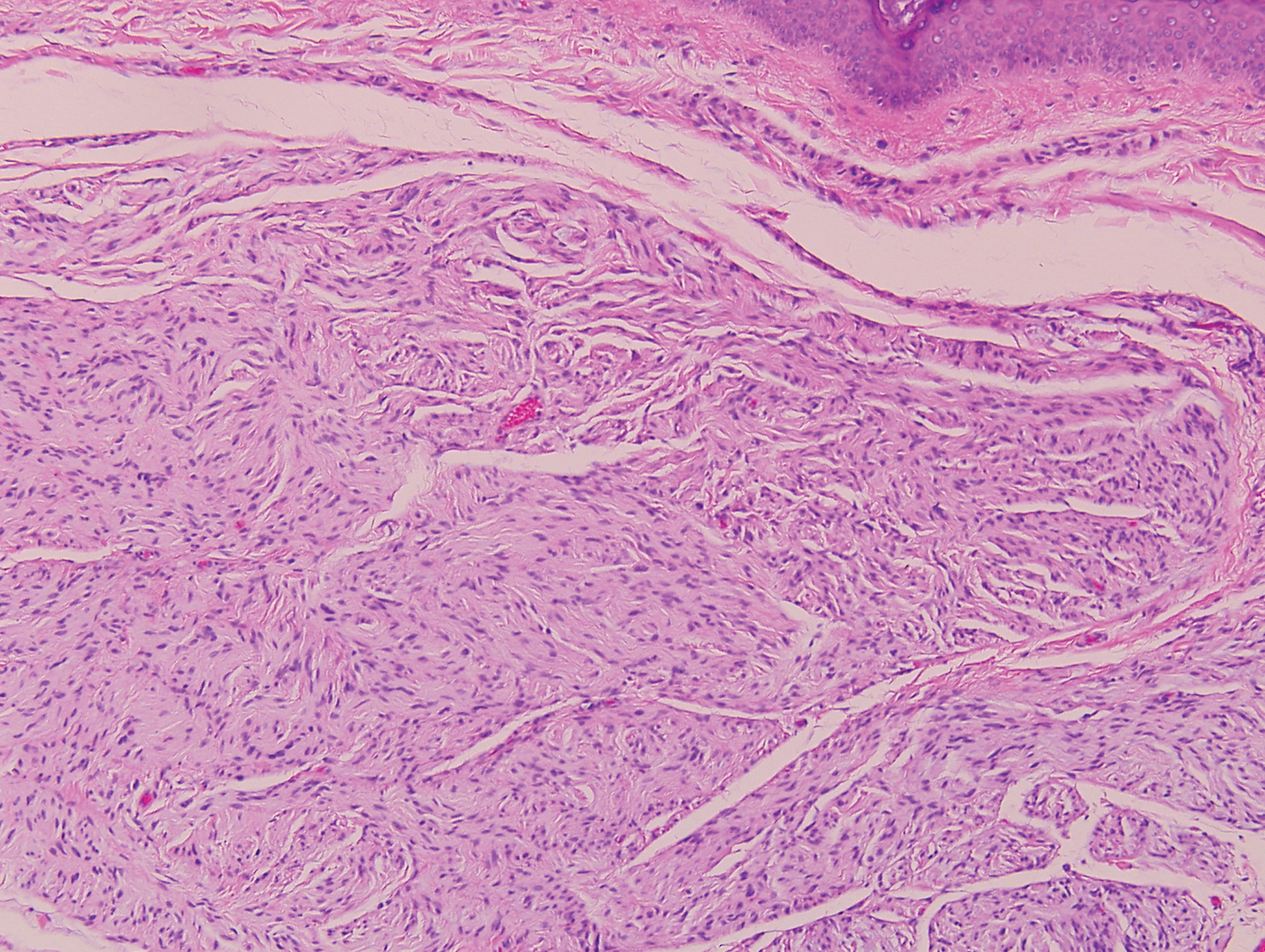
Psoriasis affects approximately 1% of children, either alone or associated with comorbid conditions such as psoriatic arthritis (PsA), wrote Alan Menter, MD, of Baylor University Medical Center, Dallas, and coauthors of the guideline.
In the guideline, published in the Journal of the American Academy of Dermatology, the multidisciplinary work group identified screening tools to measure disease severity, strategies for management of comorbidities, and the safety and effectiveness of topical, systemic, and phototherapy treatments.
To assess disease severity, the work group recommended not only the use of body surface area (BSA), similar to measurement of severity in adults, but also the use of the Children’s Dermatology Life Quality Index, a 10-question quality of life survey, as BSA alone does not account for the potential negative impact of the disease on quality of life in terms of physical, emotional, social, and psychological function.
“For example, a child with psoriasis limited to the face or the entire scalp does not have severe disease based on BSA definitions, but if this involvement causes shame, social withdrawal, or bullying, it satisfies criteria for severe disease based on impact beyond the skin,” they said.
The work group stated that a variety of conditions may trigger or exacerbate psoriasis in children, including infections, cutaneous trauma, or physiological, emotional, and environmental stressors.
The majority of children with PsA develop joint inflammation before skin disease, the work group wrote. In addition, children with psoriasis are at increased risk for rheumatoid arthritis, so clinicians may need to distinguish between a combination of psoriasis and musculoskeletal issues and cases of either psoriatic or rheumatoid arthritis in young patients.
The cardiovascular risk factors associated with metabolic syndrome are greater in children with psoriasis, compared with children without psoriasis, the work group noted. In addition, pediatric psoriasis patients have a higher prevalence of obesity than children without psoriasis, and they recommended that children with psoriasis be monitored for the development of obesity, and that obese children with psoriasis should be referred for weight management.
The work group noted that data are insufficient in children to support the link between psoriasis and cardiovascular disease that has been documented in adults with psoriasis. However, “patients with pediatric psoriasis should have American Academy of Pediatrics (AAP)–recommended age-related cardiovascular screening regardless of the presence of signs or symptoms,” they said.
The guideline also recommends screening for dyslipidemia and hypertension according to AAP guidelines and educating pediatric psoriasis patients about the risk of diabetes and regularly screening for diabetes and insulin resistance in those who are obese. Overweight children with psoriasis may be screened at the provider’s discretion, they wrote. Patients with signs of inflammatory bowel disease, which also is associated with psoriasis in adults, should be considered for referral to a gastroenterologist, they noted.
Children with psoriasis should be screened regularly for mental health conditions regardless of age, and they should be asked about substance abuse, according to the guideline, and those with concerns should be referred for additional assessment and management.
The guideline divides treatment of psoriasis in children into three categories: topical, phototherapy and photochemotherapy, and systemic treatments (nonbiologic or biologic).
For topicals, the guideline recommendations include corticosteroids as an off-label therapy, as well as ultra-high-potency topical corticosteroids as monotherapy. Overall, “selection of a therapeutic routine (potency, delivery vehicle, frequency of application) should take into account sites of involvement, type and thickness of psoriasis, age of the patient, total BSA of application, anticipated occlusion, and disease acuity, among other patient-, disease-, and drug-related factors,” the authors wrote. Other topical options included in the recommendations: calcineurin inhibitors, topical vitamin D analogues, tazarotene (off label), anthralin, and coal tar.
Phototherapy has a history of use in psoriasis treatment and remains part of the current recommendations, although data in children are limited, and data on the use of phototherapy for pustular psoriasis in children are insufficient to make specific treatment and dosing recommendations, the work group noted. The researchers also noted that in-office phototherapy may not be feasible for many patients, but that in-home ultraviolet light equipment or natural sunlight in moderation could be recommended as an alternative.
The use of systemic, nonbiologic treatments for pediatric psoriasis should be “based on baseline severity of disease, subtype of psoriasis, speed of disease progression, lack of response to more conservative therapies such as topical agents and phototherapy (when appropriate), impaired physical or psychological functioning or [quality of life] due to disease extent, and the presence of comorbidities such as PsA,” the workgroup said.
Options for systemic treatment include methotrexate, cyclosporine (notably for pustular as well as plaque and erythrodermic psoriasis), and systemic retinoids. In addition, fumaric acid esters may be an option for children with moderate to severe psoriasis, with recommended clinical and laboratory monitoring.
The increasing safety and efficacy data on biologics in pediatric psoriasis patients support their consideration among first-line systemic treatments, the work group suggested. “Etanercept and ustekinumab are now [Food and Drug Administration] approved for patients with psoriasis 4 years and older and 12 years and older, respectively,” they said, and infliximab and adalimumab have been used off label in children.
The work group concluded that research and knowledge gaps about pediatric psoriasis persist and include mechanism of disease onset, development of comorbidities, and identification of ideal dosing for various treatments.
Finally, the work group emphasized the importance of collaboration between dermatologists and primary care providers for managing psoriasis in children, as well as the importance of patient education.
“Dermatologists should be mindful of the unique aspects of the emotional development of children and the social dynamics of having a visible difference,” they wrote. “Shared decision making with the patient (if age appropriate) and the caregivers is a useful approach, particularly as related to the use of off-label medications to treat severe disease,” they said.
“This is the first time that pediatric psoriasis has been discussed as an independent topic within the guideline,” said one of the guideline authors, Dawn M.R. Davis, MD, of the Mayo Clinic, Rochester, Minn., in an interview. “Children have unique physiology and psychosocial aspects to their care relative to adults. In addition, psoriasis has some clinical manifestations that are oftentimes distinctly seen in children,” she commented. “Creation of a guideline specific to children allows us to summarize the similarities and differences of disease presentation and management. It also allows an opportunity to clarify what research data (especially therapeutics) have been studied in children and their uses, safety profiles, and dosing,” she noted.
Psoriasis can be a psychosocially debilitating disease, she emphasized. “In children, for example, isolated or prominent facial involvement is common, which can be embarrassing and impact relationships.”
The take-home message for clinicians, Dr. Davis said, is to keep in mind the multisystemic nature of psoriasis. “It is not limited to the skin,” she said. “Treating a patient with psoriasis necessitates practicing whole-person care” and considering the multiple comorbidities that impact quality of life and overall health in children, as well as adults with psoriasis, she commented. “Dermatologists can empower patients and their caregivers by educating them on the multifocal, complex nature of the disease.” She added, “We have much to learn regarding psoriasis in the pediatric population. More research into therapeutics, topical and systemic, is necessary to optimize patient care.”
The guideline was based on studies published in the PubMed and MEDLINE databases from January 2011 through December 31, 2017.
Dr. Menter and Craig A. Elmets, MD, professor of dermatology, at the University of Alabama, Birmingham, were cochairs of the work group. The pediatric guideline is the latest in a multipart series of AAD-NPF guidelines on psoriasis being published this year in the Journal of the American Academy of Dermatology.
Many of the guideline authors, including lead author Dr. Menter, disclosed relationships with multiple companies; however, a minimum 51% of workgroup members had no relevant conflicts of interest in accordance with AAD policy. There was no funding source. Dr. Davis disclosed serving as an investigator for Regeneron, with no compensation.
SOURCE: Menter et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2019.08.049.
Psoriasis management in children involves attention not only to treatment of the physical condition but also psychosocial wellness and quality of life, according to
Psoriasis affects approximately 1% of children, either alone or associated with comorbid conditions such as psoriatic arthritis (PsA), wrote Alan Menter, MD, of Baylor University Medical Center, Dallas, and coauthors of the guideline.
In the guideline, published in the Journal of the American Academy of Dermatology, the multidisciplinary work group identified screening tools to measure disease severity, strategies for management of comorbidities, and the safety and effectiveness of topical, systemic, and phototherapy treatments.
To assess disease severity, the work group recommended not only the use of body surface area (BSA), similar to measurement of severity in adults, but also the use of the Children’s Dermatology Life Quality Index, a 10-question quality of life survey, as BSA alone does not account for the potential negative impact of the disease on quality of life in terms of physical, emotional, social, and psychological function.
“For example, a child with psoriasis limited to the face or the entire scalp does not have severe disease based on BSA definitions, but if this involvement causes shame, social withdrawal, or bullying, it satisfies criteria for severe disease based on impact beyond the skin,” they said.
The work group stated that a variety of conditions may trigger or exacerbate psoriasis in children, including infections, cutaneous trauma, or physiological, emotional, and environmental stressors.
The majority of children with PsA develop joint inflammation before skin disease, the work group wrote. In addition, children with psoriasis are at increased risk for rheumatoid arthritis, so clinicians may need to distinguish between a combination of psoriasis and musculoskeletal issues and cases of either psoriatic or rheumatoid arthritis in young patients.
The cardiovascular risk factors associated with metabolic syndrome are greater in children with psoriasis, compared with children without psoriasis, the work group noted. In addition, pediatric psoriasis patients have a higher prevalence of obesity than children without psoriasis, and they recommended that children with psoriasis be monitored for the development of obesity, and that obese children with psoriasis should be referred for weight management.
The work group noted that data are insufficient in children to support the link between psoriasis and cardiovascular disease that has been documented in adults with psoriasis. However, “patients with pediatric psoriasis should have American Academy of Pediatrics (AAP)–recommended age-related cardiovascular screening regardless of the presence of signs or symptoms,” they said.
The guideline also recommends screening for dyslipidemia and hypertension according to AAP guidelines and educating pediatric psoriasis patients about the risk of diabetes and regularly screening for diabetes and insulin resistance in those who are obese. Overweight children with psoriasis may be screened at the provider’s discretion, they wrote. Patients with signs of inflammatory bowel disease, which also is associated with psoriasis in adults, should be considered for referral to a gastroenterologist, they noted.
Children with psoriasis should be screened regularly for mental health conditions regardless of age, and they should be asked about substance abuse, according to the guideline, and those with concerns should be referred for additional assessment and management.
The guideline divides treatment of psoriasis in children into three categories: topical, phototherapy and photochemotherapy, and systemic treatments (nonbiologic or biologic).
For topicals, the guideline recommendations include corticosteroids as an off-label therapy, as well as ultra-high-potency topical corticosteroids as monotherapy. Overall, “selection of a therapeutic routine (potency, delivery vehicle, frequency of application) should take into account sites of involvement, type and thickness of psoriasis, age of the patient, total BSA of application, anticipated occlusion, and disease acuity, among other patient-, disease-, and drug-related factors,” the authors wrote. Other topical options included in the recommendations: calcineurin inhibitors, topical vitamin D analogues, tazarotene (off label), anthralin, and coal tar.
Phototherapy has a history of use in psoriasis treatment and remains part of the current recommendations, although data in children are limited, and data on the use of phototherapy for pustular psoriasis in children are insufficient to make specific treatment and dosing recommendations, the work group noted. The researchers also noted that in-office phototherapy may not be feasible for many patients, but that in-home ultraviolet light equipment or natural sunlight in moderation could be recommended as an alternative.
The use of systemic, nonbiologic treatments for pediatric psoriasis should be “based on baseline severity of disease, subtype of psoriasis, speed of disease progression, lack of response to more conservative therapies such as topical agents and phototherapy (when appropriate), impaired physical or psychological functioning or [quality of life] due to disease extent, and the presence of comorbidities such as PsA,” the workgroup said.
Options for systemic treatment include methotrexate, cyclosporine (notably for pustular as well as plaque and erythrodermic psoriasis), and systemic retinoids. In addition, fumaric acid esters may be an option for children with moderate to severe psoriasis, with recommended clinical and laboratory monitoring.
The increasing safety and efficacy data on biologics in pediatric psoriasis patients support their consideration among first-line systemic treatments, the work group suggested. “Etanercept and ustekinumab are now [Food and Drug Administration] approved for patients with psoriasis 4 years and older and 12 years and older, respectively,” they said, and infliximab and adalimumab have been used off label in children.
The work group concluded that research and knowledge gaps about pediatric psoriasis persist and include mechanism of disease onset, development of comorbidities, and identification of ideal dosing for various treatments.
Finally, the work group emphasized the importance of collaboration between dermatologists and primary care providers for managing psoriasis in children, as well as the importance of patient education.
“Dermatologists should be mindful of the unique aspects of the emotional development of children and the social dynamics of having a visible difference,” they wrote. “Shared decision making with the patient (if age appropriate) and the caregivers is a useful approach, particularly as related to the use of off-label medications to treat severe disease,” they said.
“This is the first time that pediatric psoriasis has been discussed as an independent topic within the guideline,” said one of the guideline authors, Dawn M.R. Davis, MD, of the Mayo Clinic, Rochester, Minn., in an interview. “Children have unique physiology and psychosocial aspects to their care relative to adults. In addition, psoriasis has some clinical manifestations that are oftentimes distinctly seen in children,” she commented. “Creation of a guideline specific to children allows us to summarize the similarities and differences of disease presentation and management. It also allows an opportunity to clarify what research data (especially therapeutics) have been studied in children and their uses, safety profiles, and dosing,” she noted.
Psoriasis can be a psychosocially debilitating disease, she emphasized. “In children, for example, isolated or prominent facial involvement is common, which can be embarrassing and impact relationships.”
The take-home message for clinicians, Dr. Davis said, is to keep in mind the multisystemic nature of psoriasis. “It is not limited to the skin,” she said. “Treating a patient with psoriasis necessitates practicing whole-person care” and considering the multiple comorbidities that impact quality of life and overall health in children, as well as adults with psoriasis, she commented. “Dermatologists can empower patients and their caregivers by educating them on the multifocal, complex nature of the disease.” She added, “We have much to learn regarding psoriasis in the pediatric population. More research into therapeutics, topical and systemic, is necessary to optimize patient care.”
The guideline was based on studies published in the PubMed and MEDLINE databases from January 2011 through December 31, 2017.
Dr. Menter and Craig A. Elmets, MD, professor of dermatology, at the University of Alabama, Birmingham, were cochairs of the work group. The pediatric guideline is the latest in a multipart series of AAD-NPF guidelines on psoriasis being published this year in the Journal of the American Academy of Dermatology.
Many of the guideline authors, including lead author Dr. Menter, disclosed relationships with multiple companies; however, a minimum 51% of workgroup members had no relevant conflicts of interest in accordance with AAD policy. There was no funding source. Dr. Davis disclosed serving as an investigator for Regeneron, with no compensation.
SOURCE: Menter et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2019.08.049.
Psoriasis management in children involves attention not only to treatment of the physical condition but also psychosocial wellness and quality of life, according to
Psoriasis affects approximately 1% of children, either alone or associated with comorbid conditions such as psoriatic arthritis (PsA), wrote Alan Menter, MD, of Baylor University Medical Center, Dallas, and coauthors of the guideline.
In the guideline, published in the Journal of the American Academy of Dermatology, the multidisciplinary work group identified screening tools to measure disease severity, strategies for management of comorbidities, and the safety and effectiveness of topical, systemic, and phototherapy treatments.
To assess disease severity, the work group recommended not only the use of body surface area (BSA), similar to measurement of severity in adults, but also the use of the Children’s Dermatology Life Quality Index, a 10-question quality of life survey, as BSA alone does not account for the potential negative impact of the disease on quality of life in terms of physical, emotional, social, and psychological function.
“For example, a child with psoriasis limited to the face or the entire scalp does not have severe disease based on BSA definitions, but if this involvement causes shame, social withdrawal, or bullying, it satisfies criteria for severe disease based on impact beyond the skin,” they said.
The work group stated that a variety of conditions may trigger or exacerbate psoriasis in children, including infections, cutaneous trauma, or physiological, emotional, and environmental stressors.
The majority of children with PsA develop joint inflammation before skin disease, the work group wrote. In addition, children with psoriasis are at increased risk for rheumatoid arthritis, so clinicians may need to distinguish between a combination of psoriasis and musculoskeletal issues and cases of either psoriatic or rheumatoid arthritis in young patients.
The cardiovascular risk factors associated with metabolic syndrome are greater in children with psoriasis, compared with children without psoriasis, the work group noted. In addition, pediatric psoriasis patients have a higher prevalence of obesity than children without psoriasis, and they recommended that children with psoriasis be monitored for the development of obesity, and that obese children with psoriasis should be referred for weight management.
The work group noted that data are insufficient in children to support the link between psoriasis and cardiovascular disease that has been documented in adults with psoriasis. However, “patients with pediatric psoriasis should have American Academy of Pediatrics (AAP)–recommended age-related cardiovascular screening regardless of the presence of signs or symptoms,” they said.
The guideline also recommends screening for dyslipidemia and hypertension according to AAP guidelines and educating pediatric psoriasis patients about the risk of diabetes and regularly screening for diabetes and insulin resistance in those who are obese. Overweight children with psoriasis may be screened at the provider’s discretion, they wrote. Patients with signs of inflammatory bowel disease, which also is associated with psoriasis in adults, should be considered for referral to a gastroenterologist, they noted.
Children with psoriasis should be screened regularly for mental health conditions regardless of age, and they should be asked about substance abuse, according to the guideline, and those with concerns should be referred for additional assessment and management.
The guideline divides treatment of psoriasis in children into three categories: topical, phototherapy and photochemotherapy, and systemic treatments (nonbiologic or biologic).
For topicals, the guideline recommendations include corticosteroids as an off-label therapy, as well as ultra-high-potency topical corticosteroids as monotherapy. Overall, “selection of a therapeutic routine (potency, delivery vehicle, frequency of application) should take into account sites of involvement, type and thickness of psoriasis, age of the patient, total BSA of application, anticipated occlusion, and disease acuity, among other patient-, disease-, and drug-related factors,” the authors wrote. Other topical options included in the recommendations: calcineurin inhibitors, topical vitamin D analogues, tazarotene (off label), anthralin, and coal tar.
Phototherapy has a history of use in psoriasis treatment and remains part of the current recommendations, although data in children are limited, and data on the use of phototherapy for pustular psoriasis in children are insufficient to make specific treatment and dosing recommendations, the work group noted. The researchers also noted that in-office phototherapy may not be feasible for many patients, but that in-home ultraviolet light equipment or natural sunlight in moderation could be recommended as an alternative.
The use of systemic, nonbiologic treatments for pediatric psoriasis should be “based on baseline severity of disease, subtype of psoriasis, speed of disease progression, lack of response to more conservative therapies such as topical agents and phototherapy (when appropriate), impaired physical or psychological functioning or [quality of life] due to disease extent, and the presence of comorbidities such as PsA,” the workgroup said.
Options for systemic treatment include methotrexate, cyclosporine (notably for pustular as well as plaque and erythrodermic psoriasis), and systemic retinoids. In addition, fumaric acid esters may be an option for children with moderate to severe psoriasis, with recommended clinical and laboratory monitoring.
The increasing safety and efficacy data on biologics in pediatric psoriasis patients support their consideration among first-line systemic treatments, the work group suggested. “Etanercept and ustekinumab are now [Food and Drug Administration] approved for patients with psoriasis 4 years and older and 12 years and older, respectively,” they said, and infliximab and adalimumab have been used off label in children.
The work group concluded that research and knowledge gaps about pediatric psoriasis persist and include mechanism of disease onset, development of comorbidities, and identification of ideal dosing for various treatments.
Finally, the work group emphasized the importance of collaboration between dermatologists and primary care providers for managing psoriasis in children, as well as the importance of patient education.
“Dermatologists should be mindful of the unique aspects of the emotional development of children and the social dynamics of having a visible difference,” they wrote. “Shared decision making with the patient (if age appropriate) and the caregivers is a useful approach, particularly as related to the use of off-label medications to treat severe disease,” they said.
“This is the first time that pediatric psoriasis has been discussed as an independent topic within the guideline,” said one of the guideline authors, Dawn M.R. Davis, MD, of the Mayo Clinic, Rochester, Minn., in an interview. “Children have unique physiology and psychosocial aspects to their care relative to adults. In addition, psoriasis has some clinical manifestations that are oftentimes distinctly seen in children,” she commented. “Creation of a guideline specific to children allows us to summarize the similarities and differences of disease presentation and management. It also allows an opportunity to clarify what research data (especially therapeutics) have been studied in children and their uses, safety profiles, and dosing,” she noted.
Psoriasis can be a psychosocially debilitating disease, she emphasized. “In children, for example, isolated or prominent facial involvement is common, which can be embarrassing and impact relationships.”
The take-home message for clinicians, Dr. Davis said, is to keep in mind the multisystemic nature of psoriasis. “It is not limited to the skin,” she said. “Treating a patient with psoriasis necessitates practicing whole-person care” and considering the multiple comorbidities that impact quality of life and overall health in children, as well as adults with psoriasis, she commented. “Dermatologists can empower patients and their caregivers by educating them on the multifocal, complex nature of the disease.” She added, “We have much to learn regarding psoriasis in the pediatric population. More research into therapeutics, topical and systemic, is necessary to optimize patient care.”
The guideline was based on studies published in the PubMed and MEDLINE databases from January 2011 through December 31, 2017.
Dr. Menter and Craig A. Elmets, MD, professor of dermatology, at the University of Alabama, Birmingham, were cochairs of the work group. The pediatric guideline is the latest in a multipart series of AAD-NPF guidelines on psoriasis being published this year in the Journal of the American Academy of Dermatology.
Many of the guideline authors, including lead author Dr. Menter, disclosed relationships with multiple companies; however, a minimum 51% of workgroup members had no relevant conflicts of interest in accordance with AAD policy. There was no funding source. Dr. Davis disclosed serving as an investigator for Regeneron, with no compensation.
SOURCE: Menter et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2019.08.049.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Observational secukinumab data reflect clinical trial results in patients with moderate to severe psoriasis
A in a report published in the Journal of the European Academy of Dermatology and Venereology.
“The safety profile of secukinumab was similar to that reported in previous clinical trials, and no new or unexpected safety signals were observed,” according to Diamant Thaci, MD, of the Comprehensive Centre of Inflammation Medicine, University of Lübeck (Germany) and coauthors. Moreover, effectiveness in those who started treatment with secukinumab at baseline, they added, “was comparable to that observed in Phase 3 trials. High levels of effectiveness were observed also in subjects who had received previous biologic therapies, although the response rates were numerically lower, as might be expected in a difficult to treat population. In addition, lower baseline PASI [Psoriasis Area and Severity Index] in patients with prior biologic treatment could also reduce the relative decrease in PASI observed over the course of the study.”
They reported on an interim analysis of the first 1,988 patients enrolled in the PROSPECT study, an observational 24-week study conducted in Germany; 1,323 patients completed the 24 week study; total cumulative exposure to secukinumab was 746.3 patient-years. Their mean baseline PASI was 17.7, slightly lower than those in typical clinical trials, and most (91%) had received systemic therapies before.
Almost half the patients (46%) experienced an adverse event during treatment, and about 4% experienced a serious adverse event; only 1% of serious adverse events were considered related to the study drug. About 7% discontinued treatment with secukinumab because of an adverse event. The most common reasons for discontinuation were lack of benefit in 2.4%, psoriasis in 2.3%, and upper respiratory tract viral infection in 0.5%.
The most common adverse events were nasopharyngitis (8.7%), pruritus (2.9%), and headache (2.4%). Rates of neoplastic disorders and major cerebrovascular events were similar to published data, with 5 patients (0.3%) experiencing a major adverse cardiovascular event and 10 (0.5%) experiencing a malignancy. Four patients (0.2%) developed inflammatory bowel disease, 42 (2.1%) developed Candida infection, 2 (0.1%) developed hepatotoxicity, and 11 (0.6%) an injection-site reaction. There were three deaths, determined not to be related to secukinumab, the authors wrote.
Efficacy was also similar to that observed in earlier studies, they noted, with positive results regardless of concomitant medication. Overall, 44% of the cohort used concomitant medications.
Of the 829 patients using concomitant topical treatments, 73% had started before baseline. In all, 110 patients were also using conventional systemic medications and phototherapy; 77 started treatment before baseline. The most commonly employed concomitant therapies were topical steroids and phototherapy.
Overall, most patients (86%) achieved a PASI 75 by week 24, with 68.5% achieving a PASI 90, and 40% achieving a PASI 100 at that time point.
Secukinumab was most effective among the 83 patients who were naive to systemic therapies; in these patients, results at week 24 were as follows: PASI 75, 93%; PASI 90, 84%; and PASI 100, 66%. Among patients who had previously received a biologic, scores were slightly lower: PASI 75, 78%; PASI 90, 55%; and PASI 100, 29%.
“These interim data from PROSPECT confirm the effectiveness and safety of secukinumab in the routine clinical setting, in a large cohort of psoriasis patients with high disease severity,” the investigators concluded.
Initially approved in the United States in 2015, secukinumab, an interleukin-17A antagonist, is indicated for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy, adults with psoriatic arthritis, and adults with active ankylosing spondylitis.
The study was funded by Novartis, Germany; four authors are employees of the company. Dr. Thaci has served as an investigator and/or consultant for multiple pharmaceutical companies, including Novartis, AbbVie, Amgen, Arena, Biogen Idec, Boehringer Ingelheim, and Celgene. Other authors also disclosed serving as investigators, consultants, and/or speakers for Novartis and other companies.
SOURCE: J Eur Acad Dermatol Venereol. 2019 Sep 21. doi: 10.1111/jdv.15962.
A in a report published in the Journal of the European Academy of Dermatology and Venereology.
“The safety profile of secukinumab was similar to that reported in previous clinical trials, and no new or unexpected safety signals were observed,” according to Diamant Thaci, MD, of the Comprehensive Centre of Inflammation Medicine, University of Lübeck (Germany) and coauthors. Moreover, effectiveness in those who started treatment with secukinumab at baseline, they added, “was comparable to that observed in Phase 3 trials. High levels of effectiveness were observed also in subjects who had received previous biologic therapies, although the response rates were numerically lower, as might be expected in a difficult to treat population. In addition, lower baseline PASI [Psoriasis Area and Severity Index] in patients with prior biologic treatment could also reduce the relative decrease in PASI observed over the course of the study.”
They reported on an interim analysis of the first 1,988 patients enrolled in the PROSPECT study, an observational 24-week study conducted in Germany; 1,323 patients completed the 24 week study; total cumulative exposure to secukinumab was 746.3 patient-years. Their mean baseline PASI was 17.7, slightly lower than those in typical clinical trials, and most (91%) had received systemic therapies before.
Almost half the patients (46%) experienced an adverse event during treatment, and about 4% experienced a serious adverse event; only 1% of serious adverse events were considered related to the study drug. About 7% discontinued treatment with secukinumab because of an adverse event. The most common reasons for discontinuation were lack of benefit in 2.4%, psoriasis in 2.3%, and upper respiratory tract viral infection in 0.5%.
The most common adverse events were nasopharyngitis (8.7%), pruritus (2.9%), and headache (2.4%). Rates of neoplastic disorders and major cerebrovascular events were similar to published data, with 5 patients (0.3%) experiencing a major adverse cardiovascular event and 10 (0.5%) experiencing a malignancy. Four patients (0.2%) developed inflammatory bowel disease, 42 (2.1%) developed Candida infection, 2 (0.1%) developed hepatotoxicity, and 11 (0.6%) an injection-site reaction. There were three deaths, determined not to be related to secukinumab, the authors wrote.
Efficacy was also similar to that observed in earlier studies, they noted, with positive results regardless of concomitant medication. Overall, 44% of the cohort used concomitant medications.
Of the 829 patients using concomitant topical treatments, 73% had started before baseline. In all, 110 patients were also using conventional systemic medications and phototherapy; 77 started treatment before baseline. The most commonly employed concomitant therapies were topical steroids and phototherapy.
Overall, most patients (86%) achieved a PASI 75 by week 24, with 68.5% achieving a PASI 90, and 40% achieving a PASI 100 at that time point.
Secukinumab was most effective among the 83 patients who were naive to systemic therapies; in these patients, results at week 24 were as follows: PASI 75, 93%; PASI 90, 84%; and PASI 100, 66%. Among patients who had previously received a biologic, scores were slightly lower: PASI 75, 78%; PASI 90, 55%; and PASI 100, 29%.
“These interim data from PROSPECT confirm the effectiveness and safety of secukinumab in the routine clinical setting, in a large cohort of psoriasis patients with high disease severity,” the investigators concluded.
Initially approved in the United States in 2015, secukinumab, an interleukin-17A antagonist, is indicated for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy, adults with psoriatic arthritis, and adults with active ankylosing spondylitis.
The study was funded by Novartis, Germany; four authors are employees of the company. Dr. Thaci has served as an investigator and/or consultant for multiple pharmaceutical companies, including Novartis, AbbVie, Amgen, Arena, Biogen Idec, Boehringer Ingelheim, and Celgene. Other authors also disclosed serving as investigators, consultants, and/or speakers for Novartis and other companies.
SOURCE: J Eur Acad Dermatol Venereol. 2019 Sep 21. doi: 10.1111/jdv.15962.
A in a report published in the Journal of the European Academy of Dermatology and Venereology.
“The safety profile of secukinumab was similar to that reported in previous clinical trials, and no new or unexpected safety signals were observed,” according to Diamant Thaci, MD, of the Comprehensive Centre of Inflammation Medicine, University of Lübeck (Germany) and coauthors. Moreover, effectiveness in those who started treatment with secukinumab at baseline, they added, “was comparable to that observed in Phase 3 trials. High levels of effectiveness were observed also in subjects who had received previous biologic therapies, although the response rates were numerically lower, as might be expected in a difficult to treat population. In addition, lower baseline PASI [Psoriasis Area and Severity Index] in patients with prior biologic treatment could also reduce the relative decrease in PASI observed over the course of the study.”
They reported on an interim analysis of the first 1,988 patients enrolled in the PROSPECT study, an observational 24-week study conducted in Germany; 1,323 patients completed the 24 week study; total cumulative exposure to secukinumab was 746.3 patient-years. Their mean baseline PASI was 17.7, slightly lower than those in typical clinical trials, and most (91%) had received systemic therapies before.
Almost half the patients (46%) experienced an adverse event during treatment, and about 4% experienced a serious adverse event; only 1% of serious adverse events were considered related to the study drug. About 7% discontinued treatment with secukinumab because of an adverse event. The most common reasons for discontinuation were lack of benefit in 2.4%, psoriasis in 2.3%, and upper respiratory tract viral infection in 0.5%.
The most common adverse events were nasopharyngitis (8.7%), pruritus (2.9%), and headache (2.4%). Rates of neoplastic disorders and major cerebrovascular events were similar to published data, with 5 patients (0.3%) experiencing a major adverse cardiovascular event and 10 (0.5%) experiencing a malignancy. Four patients (0.2%) developed inflammatory bowel disease, 42 (2.1%) developed Candida infection, 2 (0.1%) developed hepatotoxicity, and 11 (0.6%) an injection-site reaction. There were three deaths, determined not to be related to secukinumab, the authors wrote.
Efficacy was also similar to that observed in earlier studies, they noted, with positive results regardless of concomitant medication. Overall, 44% of the cohort used concomitant medications.
Of the 829 patients using concomitant topical treatments, 73% had started before baseline. In all, 110 patients were also using conventional systemic medications and phototherapy; 77 started treatment before baseline. The most commonly employed concomitant therapies were topical steroids and phototherapy.
Overall, most patients (86%) achieved a PASI 75 by week 24, with 68.5% achieving a PASI 90, and 40% achieving a PASI 100 at that time point.
Secukinumab was most effective among the 83 patients who were naive to systemic therapies; in these patients, results at week 24 were as follows: PASI 75, 93%; PASI 90, 84%; and PASI 100, 66%. Among patients who had previously received a biologic, scores were slightly lower: PASI 75, 78%; PASI 90, 55%; and PASI 100, 29%.
“These interim data from PROSPECT confirm the effectiveness and safety of secukinumab in the routine clinical setting, in a large cohort of psoriasis patients with high disease severity,” the investigators concluded.
Initially approved in the United States in 2015, secukinumab, an interleukin-17A antagonist, is indicated for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy, adults with psoriatic arthritis, and adults with active ankylosing spondylitis.
The study was funded by Novartis, Germany; four authors are employees of the company. Dr. Thaci has served as an investigator and/or consultant for multiple pharmaceutical companies, including Novartis, AbbVie, Amgen, Arena, Biogen Idec, Boehringer Ingelheim, and Celgene. Other authors also disclosed serving as investigators, consultants, and/or speakers for Novartis and other companies.
SOURCE: J Eur Acad Dermatol Venereol. 2019 Sep 21. doi: 10.1111/jdv.15962.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Level of hepatitis B core–related antigen is risk factor for hepatocellular carcinoma
A high level of hepatitis B core–related antigen (HBcrAg) was a complementary risk factor for hepatocellular carcinoma, according to the results of a retrospective cohort study of more than 2,600 noncirrhotic adults with untreated hepatitis B virus (HBV) infection with a median of 16 years of follow-up.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
“Patients with an intermediate viral load and high levels of HBcrAg had a risk for hepatocellular carcinoma that did not differ significantly from that of patients with a high viral load. [An] HBcrAg of 10 KU/mL may serve as a novel biomarker for the management of patients with intermediate viral load in our clinical practice,” wrote Tai-Chung Tseng, MD, PhD, of National Taiwan University Hospital in Taipei and associates in Gastroenterology.
Deciding whether to start antiviral therapy is controversial for some patients with HBV infection. Typically, monitoring without treatment is recommended for patients who have both low hepatitis B surface antigen levels (less than 1,000 IU/mL) and low levels of HBV DNA (less than 2,000 IU/mL), and early antiviral therapy is recommended for patients who have high levels of HBV DNA (20,000 IU/mL or more). However, there is no clear evidence that early antiviral therapy benefits patients who have intermediate levels of HBV DNA (2,000-19,999 IU/mL) and are negative for hepatitis B e antigen. Biomarkers for risk-stratifying these patients also are lacking, the researchers noted.
Therefore, they studied a cohort of 2,666 adults who had tested positive for hepatitis B surface antigen and were followed at National Taiwan University Hospital from 1985 through 2000. No patient had cirrhosis at baseline. In all, 209 patients developed hepatocellular carcinoma, yielding an incidence rate of 4.91 cases per 1,000 person-years.
Hepatitis B core–related antigen level remained an independent risk factor for hepatocellular carcinoma after accounting for age, sex, serum alanine aminotransferase (ALT) level, FIB-4 index, hepatitis B e antigen status, hepatitis B genotype (B, C, or undetermined), and HBV DNA level. Compared with patients whose HBcrAg level was less than 10 KU, a level of 10-99 KU/mL was associated with a nearly threefold increase in risk for hepatocellular carcinoma (HR, 2.93; 95% CI, 1.67-4.80), and this risk rose even further as HBcrAg levels increased.
In the subgroup of patients who tested negative for hepatitis B e antigen, had an intermediate HBV DNA load (2,000-19,999 IU/mL), and had a normal baseline ALT level (less than 40 U/L), a high HBcrAg level (10 KU/mL or more) was tied to a nearly fivefold greater risk for hepatocellular carcinoma (HR, 4.89; 95% CI, 2.18-10.93). This approximated the risk that is observed with high viral load (20,000 IU/mL), the researchers noted. In contrast, a low HBcrAg level was associated with a risk similar to that of minimal risk carriers (annual incidence rate, 0.10%; 95% CI, 0.04%-0.24%).
“To the best of our knowledge, this is the first study to report HBcrAg level as an independent viral biomarker to stratify hepatocellular risks in a large number of patients with intermediate viral load,” the researchers commented. Among the study limitations, 412 patients received antiviral therapy during follow-up. “This is a retrospective cohort study including Asian HBV patients with genotype B or C infection,” the investigators added. “It is unclear whether this finding could be extrapolated to populations with other HBV genotype infections. Nonetheless, we had a sound cohort, as several HBsAg-related clinical findings based on our cohort have already been validated by other prospective cohort studies, implying that our data were unlikely to be biased by the study design.”
Funders included National Taiwan University Hospital, the Ministry of Science and Technology, Executive Yuan in Taiwan, and National Health Research Institutes. The researchers reported having no conflicts of interest.
SOURCE: Tseng T-C et al. Gastroenterology. 2019 Aug 27. doi: 10.1053/j.gastro.2019.08.028.
A high level of hepatitis B core–related antigen (HBcrAg) was a complementary risk factor for hepatocellular carcinoma, according to the results of a retrospective cohort study of more than 2,600 noncirrhotic adults with untreated hepatitis B virus (HBV) infection with a median of 16 years of follow-up.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
“Patients with an intermediate viral load and high levels of HBcrAg had a risk for hepatocellular carcinoma that did not differ significantly from that of patients with a high viral load. [An] HBcrAg of 10 KU/mL may serve as a novel biomarker for the management of patients with intermediate viral load in our clinical practice,” wrote Tai-Chung Tseng, MD, PhD, of National Taiwan University Hospital in Taipei and associates in Gastroenterology.
Deciding whether to start antiviral therapy is controversial for some patients with HBV infection. Typically, monitoring without treatment is recommended for patients who have both low hepatitis B surface antigen levels (less than 1,000 IU/mL) and low levels of HBV DNA (less than 2,000 IU/mL), and early antiviral therapy is recommended for patients who have high levels of HBV DNA (20,000 IU/mL or more). However, there is no clear evidence that early antiviral therapy benefits patients who have intermediate levels of HBV DNA (2,000-19,999 IU/mL) and are negative for hepatitis B e antigen. Biomarkers for risk-stratifying these patients also are lacking, the researchers noted.
Therefore, they studied a cohort of 2,666 adults who had tested positive for hepatitis B surface antigen and were followed at National Taiwan University Hospital from 1985 through 2000. No patient had cirrhosis at baseline. In all, 209 patients developed hepatocellular carcinoma, yielding an incidence rate of 4.91 cases per 1,000 person-years.
Hepatitis B core–related antigen level remained an independent risk factor for hepatocellular carcinoma after accounting for age, sex, serum alanine aminotransferase (ALT) level, FIB-4 index, hepatitis B e antigen status, hepatitis B genotype (B, C, or undetermined), and HBV DNA level. Compared with patients whose HBcrAg level was less than 10 KU, a level of 10-99 KU/mL was associated with a nearly threefold increase in risk for hepatocellular carcinoma (HR, 2.93; 95% CI, 1.67-4.80), and this risk rose even further as HBcrAg levels increased.
In the subgroup of patients who tested negative for hepatitis B e antigen, had an intermediate HBV DNA load (2,000-19,999 IU/mL), and had a normal baseline ALT level (less than 40 U/L), a high HBcrAg level (10 KU/mL or more) was tied to a nearly fivefold greater risk for hepatocellular carcinoma (HR, 4.89; 95% CI, 2.18-10.93). This approximated the risk that is observed with high viral load (20,000 IU/mL), the researchers noted. In contrast, a low HBcrAg level was associated with a risk similar to that of minimal risk carriers (annual incidence rate, 0.10%; 95% CI, 0.04%-0.24%).
“To the best of our knowledge, this is the first study to report HBcrAg level as an independent viral biomarker to stratify hepatocellular risks in a large number of patients with intermediate viral load,” the researchers commented. Among the study limitations, 412 patients received antiviral therapy during follow-up. “This is a retrospective cohort study including Asian HBV patients with genotype B or C infection,” the investigators added. “It is unclear whether this finding could be extrapolated to populations with other HBV genotype infections. Nonetheless, we had a sound cohort, as several HBsAg-related clinical findings based on our cohort have already been validated by other prospective cohort studies, implying that our data were unlikely to be biased by the study design.”
Funders included National Taiwan University Hospital, the Ministry of Science and Technology, Executive Yuan in Taiwan, and National Health Research Institutes. The researchers reported having no conflicts of interest.
SOURCE: Tseng T-C et al. Gastroenterology. 2019 Aug 27. doi: 10.1053/j.gastro.2019.08.028.
A high level of hepatitis B core–related antigen (HBcrAg) was a complementary risk factor for hepatocellular carcinoma, according to the results of a retrospective cohort study of more than 2,600 noncirrhotic adults with untreated hepatitis B virus (HBV) infection with a median of 16 years of follow-up.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
“Patients with an intermediate viral load and high levels of HBcrAg had a risk for hepatocellular carcinoma that did not differ significantly from that of patients with a high viral load. [An] HBcrAg of 10 KU/mL may serve as a novel biomarker for the management of patients with intermediate viral load in our clinical practice,” wrote Tai-Chung Tseng, MD, PhD, of National Taiwan University Hospital in Taipei and associates in Gastroenterology.
Deciding whether to start antiviral therapy is controversial for some patients with HBV infection. Typically, monitoring without treatment is recommended for patients who have both low hepatitis B surface antigen levels (less than 1,000 IU/mL) and low levels of HBV DNA (less than 2,000 IU/mL), and early antiviral therapy is recommended for patients who have high levels of HBV DNA (20,000 IU/mL or more). However, there is no clear evidence that early antiviral therapy benefits patients who have intermediate levels of HBV DNA (2,000-19,999 IU/mL) and are negative for hepatitis B e antigen. Biomarkers for risk-stratifying these patients also are lacking, the researchers noted.
Therefore, they studied a cohort of 2,666 adults who had tested positive for hepatitis B surface antigen and were followed at National Taiwan University Hospital from 1985 through 2000. No patient had cirrhosis at baseline. In all, 209 patients developed hepatocellular carcinoma, yielding an incidence rate of 4.91 cases per 1,000 person-years.
Hepatitis B core–related antigen level remained an independent risk factor for hepatocellular carcinoma after accounting for age, sex, serum alanine aminotransferase (ALT) level, FIB-4 index, hepatitis B e antigen status, hepatitis B genotype (B, C, or undetermined), and HBV DNA level. Compared with patients whose HBcrAg level was less than 10 KU, a level of 10-99 KU/mL was associated with a nearly threefold increase in risk for hepatocellular carcinoma (HR, 2.93; 95% CI, 1.67-4.80), and this risk rose even further as HBcrAg levels increased.
In the subgroup of patients who tested negative for hepatitis B e antigen, had an intermediate HBV DNA load (2,000-19,999 IU/mL), and had a normal baseline ALT level (less than 40 U/L), a high HBcrAg level (10 KU/mL or more) was tied to a nearly fivefold greater risk for hepatocellular carcinoma (HR, 4.89; 95% CI, 2.18-10.93). This approximated the risk that is observed with high viral load (20,000 IU/mL), the researchers noted. In contrast, a low HBcrAg level was associated with a risk similar to that of minimal risk carriers (annual incidence rate, 0.10%; 95% CI, 0.04%-0.24%).
“To the best of our knowledge, this is the first study to report HBcrAg level as an independent viral biomarker to stratify hepatocellular risks in a large number of patients with intermediate viral load,” the researchers commented. Among the study limitations, 412 patients received antiviral therapy during follow-up. “This is a retrospective cohort study including Asian HBV patients with genotype B or C infection,” the investigators added. “It is unclear whether this finding could be extrapolated to populations with other HBV genotype infections. Nonetheless, we had a sound cohort, as several HBsAg-related clinical findings based on our cohort have already been validated by other prospective cohort studies, implying that our data were unlikely to be biased by the study design.”
Funders included National Taiwan University Hospital, the Ministry of Science and Technology, Executive Yuan in Taiwan, and National Health Research Institutes. The researchers reported having no conflicts of interest.
SOURCE: Tseng T-C et al. Gastroenterology. 2019 Aug 27. doi: 10.1053/j.gastro.2019.08.028.
FROM GASTROENTEROLOGY
Solitary Papule on the Nose
The Diagnosis: Sclerosing Perineurioma
Sclerosing perineurioma, first described in 1997 by Fetsch and Miettinen,1 is a subtype of perineurioma with a strong predilection for the fingers and palms of young adults. Rare cases involving extra-acral sites including the forearm, elbow, axilla, back, neck, lower leg, thigh, knee, lips, nose, and mouth have been reported.2-4 Perineurioma is a relatively uncommon and benign peripheral nerve sheath tumor with exclusive perineurial differentiation.5 Perineurioma is divided into intraneural and extraneural types; the latter are further subclassified into soft tissue, sclerosing, reticular, and plexiform types. Other rare forms include the sclerosing, Pacinian corpuscle-like perineurioma, lipomatous perineurioma, perineurioma with xanthomatous areas, and perineurioma with granular cells.6,7
Clinically, sclerosing perineurioma usually presents as a solitary lesion; however, rare cases of multiple lesions have been reported.8 Our patient presented with a solitary papule on the nose. Histopathologically, sclerosing perineurioma demonstrates slender spindle cells in a whorled growth pattern (onion skin) embedded in a hyalinized, lamellar, and dense collagenous stroma with intervening cleftlike spaces. Immunohistochemically, the spindle cells of our case stained positive for epithelial membrane antigen (quiz images). Other positive immunostains for perineurioma include claudin-1 and glucose transporter 1 (GLUT1). Perineurioma lacks expression of S-100 but can express CD34.2 As a benign tumor, the prognosis of sclerosing perineurioma is excellent. Complete local excision is considered curative.1
Angiofibroma, also known as fibrous papule, is a common and benign lesion located primarily on or in close proximity to the nose.9 Angiofibromas can be associated with genodermatoses such as tuberous sclerosis, multiple endocrine neoplasia type 1, or Birt-Hogg-Dubé syndrome. When angiofibromas involve the penis, they are called pearly penile papules. Ungual angiofibroma, also known as Koenen tumor, occurs underneath the nail.10-12 Both facial angiofibromas (>3) and ungual angiofibromas (>2) are independent major criteria for tuberous sclerosis.13 Clinically, angiofibroma presents as a small, dome-shaped, pink papule arising on the lower portion of the nose or nearby area of the central face. Histopathologically, angiofibromas classically demonstrate increased dilated vessels and fibrosis in the dermis. Stellate, plump, spindle-shaped, and multinucleated cells can be seen in the collagenous stroma. The collagen fibers around hair follicles are arranged concentrically, resulting in an onion skin-like appearance. The epidermal rete ridges can be effaced (Figure 1). Increased numbers of single-unit melanocytes along the dermoepidermal junction can be seen in some cases. Immunohistochemically, a variable number of spindled and multinucleated cells in the dermis stain with factor XIIIa. There are at least 7 histologic variants of angiofibroma including hypercellular, pigmented, inflammatory, pleomorphic, clear cell, granular cell, and epithelioid.9,14
Desmoplastic nevus (DN) is a benign melanocytic neoplasm characterized by predominantly spindle-shaped nevus cells embedded within a fibrotic stroma. Although it can resemble a Spitz nevus, it is recognized as a distinct entity.15-17 Clinically, DN presents as a small and flesh-colored, erythematous or slightly pigmented papule or nodule that usually occurs on the arms and legs of young adults. Histopathologically, DN demonstrates a dermal-based proliferation of spindled melanocytes embedded in a dense collagenous stroma with sparse or absent melanin deposition. The collagen bundles often show artifactual clefts and onion skin-like accentuation around vessels. Melanocytes may be epithelioid (Figure 2).16 Immunohistochemically, DN expresses melanocytic markers such as S-100, Melan-A, and human melanoma black 45, but epithelial membrane antigen is negative. Human melanoma black 45 demonstrates maturation with stronger staining in superficial portions of the lesion and diminution of staining with increasing dermal depth.18 Many other melanocytic tumors share histologic similarities to DN including blue nevus and desmoplastic melanoma.17,19,20
Palisaded encapsulated neuroma, also referred to as solitary circumscribed neuroma, was first described by Reed et al21 in 1972. It is a benign and solitary, firm, dome-shaped, flesh-colored papule that occurs in middle-aged adults, predominately near mucocutaneous junctions of the face. Other locations include the oral mucosa, eyelid, nasal fossa, shoulder, arm, hand, foot, and glans penis.22,23 Histopathologically, palisaded encapsulated neuroma demonstrates a solitary, well-circumscribed, partially encapsulated, intradermal nodule composed of interweaving fascicles of spindle cells with prominent clefts (Figure 3). Rarely, palisaded encapsulated neuroma may have a plexiform or multinodular architecture.24 Immunohistochemically, tumor cells stain positively for S-100 protein, type IV collagen, and vimentin. The capsule, composed of perineural cells, stains positive for epithelial membrane antigen. A neurofilament stain will highlight axons within the tumor.24,25 Currently, palisaded encapsulated neuroma does not have a well-established link to known neurocutaneous or inherited syndromes. Excision is curative with a low risk of recurrence.26
Sclerotic fibromas (SFs) were first reported by Weary et al27 as multiple tumors involving the tongues of patients with Cowden syndrome. Sporadic or solitary SFs of the skin in patients without Cowden syndrome have been reported, and both multiple and solitary SFs present with similar pathologic changes.28-30 Clinically, the solitary variant manifests as a well-demarcated, flesh-colored to erythematous, waxy papule or nodule with no site or sex predilection.30,31 Histologically, SF demonstrates a well-demarcated, nonencapsulated dermal nodule composed of hypocellular and sclerotic collagen bundles with scattered spindled cells and prominent clefts resembling Vincent van Gogh's Starry Night or plywood (Figure 4). Immunohistochemically, the spindled cells strongly express CD34. Factor XIIIa and markers of melanocytic, neural, and muscular differentiation are negative. When rendering a diagnosis in a patient with multiple SFs, a comment regarding the possibility of Cowden syndrome should be mentioned.32
- Fetsch JF, Miettinen M. Sclerosing perineurioma: a clinicopathologic study of 19 cases of a distinctive soft tissue lesion with a predilection for the fingers and palms of young adults. Am J Surg Pathol. 1997;21:1433-1442.
- Fox MD, Gleason BC, Thomas AB, et al. Extra-acral cutaneous/soft tissue sclerosing perineurioma: an under-recognized entity in the differential of CD34-positive cutaneous neoplasms. J Cutan Pathol. 2010;37:1053-1056.
- Erstine EM, Ko JS, Rubin BP, et al. Broadening the anatomic landscape of sclerosing perineurioma: a series of 5 cases in nonacral sites. Am J Dermatopathol. 2017;39:679-681.
- Senghore N, Cunliffe D, Watt-Smith S, et al. Extraneural perineurioma of the face: an unusual cutaneous presentation of an uncommon tumour. Br J Oral Maxillofac Surg. 2001;39:315-319.
- Lazarus SS, Trombetta LD. Ultrastructural identification of a benign perineurial cell tumor. Cancer. 1978;41:1823-1829.
- Macarenco RS, Cury-Martins J. Extra-acral cutaneous sclerosing perineurioma with CD34 fingerprint pattern. J Cutan Pathol. 2017;44:388-392.
- Santos-Briz A, Godoy E, Canueto J, et al. Cutaneous intraneural perineurioma: a case report. Am J Dermatopathol. 2013;35:E45-E48.
- Rubin AI, Yassaee M, Johnson W, et al. Multiple cutaneous sclerosing perineuriomas: an extensive presentation with involvement of the bilateral upper extremities. J Cutan Pathol. 2009;36(suppl 1):60-65.
- Damman J, Biswas A. Fibrous papule: a histopathologic review. Am J Dermatopathol. 2018;40:551-560.
- Macri A, Tanner LS. Cutaneous angiofibroma. StatPearls. https://www.statpearls.com/kb/viewarticle/17566/. Updated January 24, 2019. Accessed October 21, 2019.
- Darling TN, Skarulis MC, Steinberg SM, et al. Multiple facial angiofibromas and collagenomas in patients with multiple endocrine neoplasia type 1. Arch Dermatol. 1997;133:853-857.
- Schaffer JV, Gohara MA, McNiff JM, et al. Multiple facial angiofibromas: a cutaneous manifestation of Birt-Hogg-Dube syndrome. J Am Acad Dermatol. 2005;53:S108-S111.
- Northrup H, Krueger DA; International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243-254.
- Bansal C, Stewart D, Li A, et al. Histologic variants of fibrous papule. J Cutan Pathol. 2005;32:424-428.
- Harris GR, Shea CR, Horenstein MG, et al. Desmoplastic (sclerotic) nevus: an underrecognized entity that resembles dermatofibroma and desmoplastic melanoma. Am J Surg Pathol. 1999;23:786-794.
- Ferrara G, Brasiello M, Annese P, et al. Desmoplastic nevus: clinicopathologic keynotes. Am J Dermatopathol. 2009;31:718-722.
- Sherrill AM, Crespo G, Prakash AV, et al. Desmoplastic nevus: an entity distinct from Spitz nevus and blue nevus. Am J Dermatopathol. 2011;33:35-39.
- Kucher C, Zhang PJ, Pasha T, et al. Expression of Melan-A and Ki-67 in desmoplastic melanoma and desmoplastic nevi. Am J Dermatopathol. 2004;26:452-457.
- Sidiropoulos M, Sholl LM, Obregon R, et al. Desmoplastic nevus of chronically sun-damaged skin: an entity to be distinguished from desmoplastic melanoma. Am J Dermatopathol. 2014;36:629-634.
- Kiuru M, Patel RM, Busam KJ. Desmoplastic melanocytic nevi with lymphocytic aggregates. J Cutan Pathol. 2012;39:940-944.
- Reed RJ, Fine RM, Meltzer HD. Palisaded, encapsulated neuromas of the skin. Arch Dermatol. 1972;106:865-870.
- Newman MD, Milgraum S. Palisaded encapsulated neuroma (PEN): an often misdiagnosed neural tumor. Dermatol Online J. 2008;14:12.
- Beutler B, Cohen PR. Palisaded encapsulated neuroma of the trunk: a case report and review of palisaded encapsulated neuroma. Cureus. 2016;8:E726.
- Jokinen CH, Ragsdale BD, Argenyi ZB. Expanding the clinicopathologic spectrum of palisaded encapsulated neuroma. J Cutan Pathol. 2010;37:43-48.
- Argenyi ZB. Immunohistochemical characterization of palisaded, encapsulated neuroma. J Cutan Pathol. 1990;17:329-335.
- Batra J, Ramesh V, Molpariya A, et al. Palisaded encapsulated neuroma: an unusual presentation. Indian Dermatol Online J. 2018;9:262-264.
- Weary PE, Gorlin RJ, Gentry WC Jr, et al. Multiple hamartoma syndrome (Cowden's disease). Arch Dermatol. 1972;106:682-690.
- Mahmood MN, Salama ME, Chaffins M, et al. Solitary sclerotic fibroma of skin: a possible link with pleomorphic fibroma with immunophenotypic expression for O13 (CD99) and CD34. J Cutan Pathol. 2003;30:631-636.
- Nakashima K, Yamada N, Adachi K, et al. Solitary sclerotic fibroma of the skin: morphological characterization of the 'plywood-like pattern'. J Cutan Pathol. 2008;35(suppl 1):74-79.
- Rapini RP, Golitz LE. Sclerotic fibromas of the skin. J Am Acad Dermatol. 1989;20:266-271.
- Abbas O, Ghosn S, Bahhady R, et al. Solitary sclerotic fibroma on the scalp of a young girl: reactive sclerosis pattern? J Dermatol. 2010;37:575-577.
- Hanft VN, Shea CR, McNutt NS, et al. Expression of CD34 in sclerotic ("plywood") fibromas. Am J Dermatopathol. 2000;22:17-21.
The Diagnosis: Sclerosing Perineurioma
Sclerosing perineurioma, first described in 1997 by Fetsch and Miettinen,1 is a subtype of perineurioma with a strong predilection for the fingers and palms of young adults. Rare cases involving extra-acral sites including the forearm, elbow, axilla, back, neck, lower leg, thigh, knee, lips, nose, and mouth have been reported.2-4 Perineurioma is a relatively uncommon and benign peripheral nerve sheath tumor with exclusive perineurial differentiation.5 Perineurioma is divided into intraneural and extraneural types; the latter are further subclassified into soft tissue, sclerosing, reticular, and plexiform types. Other rare forms include the sclerosing, Pacinian corpuscle-like perineurioma, lipomatous perineurioma, perineurioma with xanthomatous areas, and perineurioma with granular cells.6,7
Clinically, sclerosing perineurioma usually presents as a solitary lesion; however, rare cases of multiple lesions have been reported.8 Our patient presented with a solitary papule on the nose. Histopathologically, sclerosing perineurioma demonstrates slender spindle cells in a whorled growth pattern (onion skin) embedded in a hyalinized, lamellar, and dense collagenous stroma with intervening cleftlike spaces. Immunohistochemically, the spindle cells of our case stained positive for epithelial membrane antigen (quiz images). Other positive immunostains for perineurioma include claudin-1 and glucose transporter 1 (GLUT1). Perineurioma lacks expression of S-100 but can express CD34.2 As a benign tumor, the prognosis of sclerosing perineurioma is excellent. Complete local excision is considered curative.1
Angiofibroma, also known as fibrous papule, is a common and benign lesion located primarily on or in close proximity to the nose.9 Angiofibromas can be associated with genodermatoses such as tuberous sclerosis, multiple endocrine neoplasia type 1, or Birt-Hogg-Dubé syndrome. When angiofibromas involve the penis, they are called pearly penile papules. Ungual angiofibroma, also known as Koenen tumor, occurs underneath the nail.10-12 Both facial angiofibromas (>3) and ungual angiofibromas (>2) are independent major criteria for tuberous sclerosis.13 Clinically, angiofibroma presents as a small, dome-shaped, pink papule arising on the lower portion of the nose or nearby area of the central face. Histopathologically, angiofibromas classically demonstrate increased dilated vessels and fibrosis in the dermis. Stellate, plump, spindle-shaped, and multinucleated cells can be seen in the collagenous stroma. The collagen fibers around hair follicles are arranged concentrically, resulting in an onion skin-like appearance. The epidermal rete ridges can be effaced (Figure 1). Increased numbers of single-unit melanocytes along the dermoepidermal junction can be seen in some cases. Immunohistochemically, a variable number of spindled and multinucleated cells in the dermis stain with factor XIIIa. There are at least 7 histologic variants of angiofibroma including hypercellular, pigmented, inflammatory, pleomorphic, clear cell, granular cell, and epithelioid.9,14
Desmoplastic nevus (DN) is a benign melanocytic neoplasm characterized by predominantly spindle-shaped nevus cells embedded within a fibrotic stroma. Although it can resemble a Spitz nevus, it is recognized as a distinct entity.15-17 Clinically, DN presents as a small and flesh-colored, erythematous or slightly pigmented papule or nodule that usually occurs on the arms and legs of young adults. Histopathologically, DN demonstrates a dermal-based proliferation of spindled melanocytes embedded in a dense collagenous stroma with sparse or absent melanin deposition. The collagen bundles often show artifactual clefts and onion skin-like accentuation around vessels. Melanocytes may be epithelioid (Figure 2).16 Immunohistochemically, DN expresses melanocytic markers such as S-100, Melan-A, and human melanoma black 45, but epithelial membrane antigen is negative. Human melanoma black 45 demonstrates maturation with stronger staining in superficial portions of the lesion and diminution of staining with increasing dermal depth.18 Many other melanocytic tumors share histologic similarities to DN including blue nevus and desmoplastic melanoma.17,19,20
Palisaded encapsulated neuroma, also referred to as solitary circumscribed neuroma, was first described by Reed et al21 in 1972. It is a benign and solitary, firm, dome-shaped, flesh-colored papule that occurs in middle-aged adults, predominately near mucocutaneous junctions of the face. Other locations include the oral mucosa, eyelid, nasal fossa, shoulder, arm, hand, foot, and glans penis.22,23 Histopathologically, palisaded encapsulated neuroma demonstrates a solitary, well-circumscribed, partially encapsulated, intradermal nodule composed of interweaving fascicles of spindle cells with prominent clefts (Figure 3). Rarely, palisaded encapsulated neuroma may have a plexiform or multinodular architecture.24 Immunohistochemically, tumor cells stain positively for S-100 protein, type IV collagen, and vimentin. The capsule, composed of perineural cells, stains positive for epithelial membrane antigen. A neurofilament stain will highlight axons within the tumor.24,25 Currently, palisaded encapsulated neuroma does not have a well-established link to known neurocutaneous or inherited syndromes. Excision is curative with a low risk of recurrence.26
Sclerotic fibromas (SFs) were first reported by Weary et al27 as multiple tumors involving the tongues of patients with Cowden syndrome. Sporadic or solitary SFs of the skin in patients without Cowden syndrome have been reported, and both multiple and solitary SFs present with similar pathologic changes.28-30 Clinically, the solitary variant manifests as a well-demarcated, flesh-colored to erythematous, waxy papule or nodule with no site or sex predilection.30,31 Histologically, SF demonstrates a well-demarcated, nonencapsulated dermal nodule composed of hypocellular and sclerotic collagen bundles with scattered spindled cells and prominent clefts resembling Vincent van Gogh's Starry Night or plywood (Figure 4). Immunohistochemically, the spindled cells strongly express CD34. Factor XIIIa and markers of melanocytic, neural, and muscular differentiation are negative. When rendering a diagnosis in a patient with multiple SFs, a comment regarding the possibility of Cowden syndrome should be mentioned.32
The Diagnosis: Sclerosing Perineurioma
Sclerosing perineurioma, first described in 1997 by Fetsch and Miettinen,1 is a subtype of perineurioma with a strong predilection for the fingers and palms of young adults. Rare cases involving extra-acral sites including the forearm, elbow, axilla, back, neck, lower leg, thigh, knee, lips, nose, and mouth have been reported.2-4 Perineurioma is a relatively uncommon and benign peripheral nerve sheath tumor with exclusive perineurial differentiation.5 Perineurioma is divided into intraneural and extraneural types; the latter are further subclassified into soft tissue, sclerosing, reticular, and plexiform types. Other rare forms include the sclerosing, Pacinian corpuscle-like perineurioma, lipomatous perineurioma, perineurioma with xanthomatous areas, and perineurioma with granular cells.6,7
Clinically, sclerosing perineurioma usually presents as a solitary lesion; however, rare cases of multiple lesions have been reported.8 Our patient presented with a solitary papule on the nose. Histopathologically, sclerosing perineurioma demonstrates slender spindle cells in a whorled growth pattern (onion skin) embedded in a hyalinized, lamellar, and dense collagenous stroma with intervening cleftlike spaces. Immunohistochemically, the spindle cells of our case stained positive for epithelial membrane antigen (quiz images). Other positive immunostains for perineurioma include claudin-1 and glucose transporter 1 (GLUT1). Perineurioma lacks expression of S-100 but can express CD34.2 As a benign tumor, the prognosis of sclerosing perineurioma is excellent. Complete local excision is considered curative.1
Angiofibroma, also known as fibrous papule, is a common and benign lesion located primarily on or in close proximity to the nose.9 Angiofibromas can be associated with genodermatoses such as tuberous sclerosis, multiple endocrine neoplasia type 1, or Birt-Hogg-Dubé syndrome. When angiofibromas involve the penis, they are called pearly penile papules. Ungual angiofibroma, also known as Koenen tumor, occurs underneath the nail.10-12 Both facial angiofibromas (>3) and ungual angiofibromas (>2) are independent major criteria for tuberous sclerosis.13 Clinically, angiofibroma presents as a small, dome-shaped, pink papule arising on the lower portion of the nose or nearby area of the central face. Histopathologically, angiofibromas classically demonstrate increased dilated vessels and fibrosis in the dermis. Stellate, plump, spindle-shaped, and multinucleated cells can be seen in the collagenous stroma. The collagen fibers around hair follicles are arranged concentrically, resulting in an onion skin-like appearance. The epidermal rete ridges can be effaced (Figure 1). Increased numbers of single-unit melanocytes along the dermoepidermal junction can be seen in some cases. Immunohistochemically, a variable number of spindled and multinucleated cells in the dermis stain with factor XIIIa. There are at least 7 histologic variants of angiofibroma including hypercellular, pigmented, inflammatory, pleomorphic, clear cell, granular cell, and epithelioid.9,14
Desmoplastic nevus (DN) is a benign melanocytic neoplasm characterized by predominantly spindle-shaped nevus cells embedded within a fibrotic stroma. Although it can resemble a Spitz nevus, it is recognized as a distinct entity.15-17 Clinically, DN presents as a small and flesh-colored, erythematous or slightly pigmented papule or nodule that usually occurs on the arms and legs of young adults. Histopathologically, DN demonstrates a dermal-based proliferation of spindled melanocytes embedded in a dense collagenous stroma with sparse or absent melanin deposition. The collagen bundles often show artifactual clefts and onion skin-like accentuation around vessels. Melanocytes may be epithelioid (Figure 2).16 Immunohistochemically, DN expresses melanocytic markers such as S-100, Melan-A, and human melanoma black 45, but epithelial membrane antigen is negative. Human melanoma black 45 demonstrates maturation with stronger staining in superficial portions of the lesion and diminution of staining with increasing dermal depth.18 Many other melanocytic tumors share histologic similarities to DN including blue nevus and desmoplastic melanoma.17,19,20
Palisaded encapsulated neuroma, also referred to as solitary circumscribed neuroma, was first described by Reed et al21 in 1972. It is a benign and solitary, firm, dome-shaped, flesh-colored papule that occurs in middle-aged adults, predominately near mucocutaneous junctions of the face. Other locations include the oral mucosa, eyelid, nasal fossa, shoulder, arm, hand, foot, and glans penis.22,23 Histopathologically, palisaded encapsulated neuroma demonstrates a solitary, well-circumscribed, partially encapsulated, intradermal nodule composed of interweaving fascicles of spindle cells with prominent clefts (Figure 3). Rarely, palisaded encapsulated neuroma may have a plexiform or multinodular architecture.24 Immunohistochemically, tumor cells stain positively for S-100 protein, type IV collagen, and vimentin. The capsule, composed of perineural cells, stains positive for epithelial membrane antigen. A neurofilament stain will highlight axons within the tumor.24,25 Currently, palisaded encapsulated neuroma does not have a well-established link to known neurocutaneous or inherited syndromes. Excision is curative with a low risk of recurrence.26
Sclerotic fibromas (SFs) were first reported by Weary et al27 as multiple tumors involving the tongues of patients with Cowden syndrome. Sporadic or solitary SFs of the skin in patients without Cowden syndrome have been reported, and both multiple and solitary SFs present with similar pathologic changes.28-30 Clinically, the solitary variant manifests as a well-demarcated, flesh-colored to erythematous, waxy papule or nodule with no site or sex predilection.30,31 Histologically, SF demonstrates a well-demarcated, nonencapsulated dermal nodule composed of hypocellular and sclerotic collagen bundles with scattered spindled cells and prominent clefts resembling Vincent van Gogh's Starry Night or plywood (Figure 4). Immunohistochemically, the spindled cells strongly express CD34. Factor XIIIa and markers of melanocytic, neural, and muscular differentiation are negative. When rendering a diagnosis in a patient with multiple SFs, a comment regarding the possibility of Cowden syndrome should be mentioned.32
- Fetsch JF, Miettinen M. Sclerosing perineurioma: a clinicopathologic study of 19 cases of a distinctive soft tissue lesion with a predilection for the fingers and palms of young adults. Am J Surg Pathol. 1997;21:1433-1442.
- Fox MD, Gleason BC, Thomas AB, et al. Extra-acral cutaneous/soft tissue sclerosing perineurioma: an under-recognized entity in the differential of CD34-positive cutaneous neoplasms. J Cutan Pathol. 2010;37:1053-1056.
- Erstine EM, Ko JS, Rubin BP, et al. Broadening the anatomic landscape of sclerosing perineurioma: a series of 5 cases in nonacral sites. Am J Dermatopathol. 2017;39:679-681.
- Senghore N, Cunliffe D, Watt-Smith S, et al. Extraneural perineurioma of the face: an unusual cutaneous presentation of an uncommon tumour. Br J Oral Maxillofac Surg. 2001;39:315-319.
- Lazarus SS, Trombetta LD. Ultrastructural identification of a benign perineurial cell tumor. Cancer. 1978;41:1823-1829.
- Macarenco RS, Cury-Martins J. Extra-acral cutaneous sclerosing perineurioma with CD34 fingerprint pattern. J Cutan Pathol. 2017;44:388-392.
- Santos-Briz A, Godoy E, Canueto J, et al. Cutaneous intraneural perineurioma: a case report. Am J Dermatopathol. 2013;35:E45-E48.
- Rubin AI, Yassaee M, Johnson W, et al. Multiple cutaneous sclerosing perineuriomas: an extensive presentation with involvement of the bilateral upper extremities. J Cutan Pathol. 2009;36(suppl 1):60-65.
- Damman J, Biswas A. Fibrous papule: a histopathologic review. Am J Dermatopathol. 2018;40:551-560.
- Macri A, Tanner LS. Cutaneous angiofibroma. StatPearls. https://www.statpearls.com/kb/viewarticle/17566/. Updated January 24, 2019. Accessed October 21, 2019.
- Darling TN, Skarulis MC, Steinberg SM, et al. Multiple facial angiofibromas and collagenomas in patients with multiple endocrine neoplasia type 1. Arch Dermatol. 1997;133:853-857.
- Schaffer JV, Gohara MA, McNiff JM, et al. Multiple facial angiofibromas: a cutaneous manifestation of Birt-Hogg-Dube syndrome. J Am Acad Dermatol. 2005;53:S108-S111.
- Northrup H, Krueger DA; International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243-254.
- Bansal C, Stewart D, Li A, et al. Histologic variants of fibrous papule. J Cutan Pathol. 2005;32:424-428.
- Harris GR, Shea CR, Horenstein MG, et al. Desmoplastic (sclerotic) nevus: an underrecognized entity that resembles dermatofibroma and desmoplastic melanoma. Am J Surg Pathol. 1999;23:786-794.
- Ferrara G, Brasiello M, Annese P, et al. Desmoplastic nevus: clinicopathologic keynotes. Am J Dermatopathol. 2009;31:718-722.
- Sherrill AM, Crespo G, Prakash AV, et al. Desmoplastic nevus: an entity distinct from Spitz nevus and blue nevus. Am J Dermatopathol. 2011;33:35-39.
- Kucher C, Zhang PJ, Pasha T, et al. Expression of Melan-A and Ki-67 in desmoplastic melanoma and desmoplastic nevi. Am J Dermatopathol. 2004;26:452-457.
- Sidiropoulos M, Sholl LM, Obregon R, et al. Desmoplastic nevus of chronically sun-damaged skin: an entity to be distinguished from desmoplastic melanoma. Am J Dermatopathol. 2014;36:629-634.
- Kiuru M, Patel RM, Busam KJ. Desmoplastic melanocytic nevi with lymphocytic aggregates. J Cutan Pathol. 2012;39:940-944.
- Reed RJ, Fine RM, Meltzer HD. Palisaded, encapsulated neuromas of the skin. Arch Dermatol. 1972;106:865-870.
- Newman MD, Milgraum S. Palisaded encapsulated neuroma (PEN): an often misdiagnosed neural tumor. Dermatol Online J. 2008;14:12.
- Beutler B, Cohen PR. Palisaded encapsulated neuroma of the trunk: a case report and review of palisaded encapsulated neuroma. Cureus. 2016;8:E726.
- Jokinen CH, Ragsdale BD, Argenyi ZB. Expanding the clinicopathologic spectrum of palisaded encapsulated neuroma. J Cutan Pathol. 2010;37:43-48.
- Argenyi ZB. Immunohistochemical characterization of palisaded, encapsulated neuroma. J Cutan Pathol. 1990;17:329-335.
- Batra J, Ramesh V, Molpariya A, et al. Palisaded encapsulated neuroma: an unusual presentation. Indian Dermatol Online J. 2018;9:262-264.
- Weary PE, Gorlin RJ, Gentry WC Jr, et al. Multiple hamartoma syndrome (Cowden's disease). Arch Dermatol. 1972;106:682-690.
- Mahmood MN, Salama ME, Chaffins M, et al. Solitary sclerotic fibroma of skin: a possible link with pleomorphic fibroma with immunophenotypic expression for O13 (CD99) and CD34. J Cutan Pathol. 2003;30:631-636.
- Nakashima K, Yamada N, Adachi K, et al. Solitary sclerotic fibroma of the skin: morphological characterization of the 'plywood-like pattern'. J Cutan Pathol. 2008;35(suppl 1):74-79.
- Rapini RP, Golitz LE. Sclerotic fibromas of the skin. J Am Acad Dermatol. 1989;20:266-271.
- Abbas O, Ghosn S, Bahhady R, et al. Solitary sclerotic fibroma on the scalp of a young girl: reactive sclerosis pattern? J Dermatol. 2010;37:575-577.
- Hanft VN, Shea CR, McNutt NS, et al. Expression of CD34 in sclerotic ("plywood") fibromas. Am J Dermatopathol. 2000;22:17-21.
- Fetsch JF, Miettinen M. Sclerosing perineurioma: a clinicopathologic study of 19 cases of a distinctive soft tissue lesion with a predilection for the fingers and palms of young adults. Am J Surg Pathol. 1997;21:1433-1442.
- Fox MD, Gleason BC, Thomas AB, et al. Extra-acral cutaneous/soft tissue sclerosing perineurioma: an under-recognized entity in the differential of CD34-positive cutaneous neoplasms. J Cutan Pathol. 2010;37:1053-1056.
- Erstine EM, Ko JS, Rubin BP, et al. Broadening the anatomic landscape of sclerosing perineurioma: a series of 5 cases in nonacral sites. Am J Dermatopathol. 2017;39:679-681.
- Senghore N, Cunliffe D, Watt-Smith S, et al. Extraneural perineurioma of the face: an unusual cutaneous presentation of an uncommon tumour. Br J Oral Maxillofac Surg. 2001;39:315-319.
- Lazarus SS, Trombetta LD. Ultrastructural identification of a benign perineurial cell tumor. Cancer. 1978;41:1823-1829.
- Macarenco RS, Cury-Martins J. Extra-acral cutaneous sclerosing perineurioma with CD34 fingerprint pattern. J Cutan Pathol. 2017;44:388-392.
- Santos-Briz A, Godoy E, Canueto J, et al. Cutaneous intraneural perineurioma: a case report. Am J Dermatopathol. 2013;35:E45-E48.
- Rubin AI, Yassaee M, Johnson W, et al. Multiple cutaneous sclerosing perineuriomas: an extensive presentation with involvement of the bilateral upper extremities. J Cutan Pathol. 2009;36(suppl 1):60-65.
- Damman J, Biswas A. Fibrous papule: a histopathologic review. Am J Dermatopathol. 2018;40:551-560.
- Macri A, Tanner LS. Cutaneous angiofibroma. StatPearls. https://www.statpearls.com/kb/viewarticle/17566/. Updated January 24, 2019. Accessed October 21, 2019.
- Darling TN, Skarulis MC, Steinberg SM, et al. Multiple facial angiofibromas and collagenomas in patients with multiple endocrine neoplasia type 1. Arch Dermatol. 1997;133:853-857.
- Schaffer JV, Gohara MA, McNiff JM, et al. Multiple facial angiofibromas: a cutaneous manifestation of Birt-Hogg-Dube syndrome. J Am Acad Dermatol. 2005;53:S108-S111.
- Northrup H, Krueger DA; International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243-254.
- Bansal C, Stewart D, Li A, et al. Histologic variants of fibrous papule. J Cutan Pathol. 2005;32:424-428.
- Harris GR, Shea CR, Horenstein MG, et al. Desmoplastic (sclerotic) nevus: an underrecognized entity that resembles dermatofibroma and desmoplastic melanoma. Am J Surg Pathol. 1999;23:786-794.
- Ferrara G, Brasiello M, Annese P, et al. Desmoplastic nevus: clinicopathologic keynotes. Am J Dermatopathol. 2009;31:718-722.
- Sherrill AM, Crespo G, Prakash AV, et al. Desmoplastic nevus: an entity distinct from Spitz nevus and blue nevus. Am J Dermatopathol. 2011;33:35-39.
- Kucher C, Zhang PJ, Pasha T, et al. Expression of Melan-A and Ki-67 in desmoplastic melanoma and desmoplastic nevi. Am J Dermatopathol. 2004;26:452-457.
- Sidiropoulos M, Sholl LM, Obregon R, et al. Desmoplastic nevus of chronically sun-damaged skin: an entity to be distinguished from desmoplastic melanoma. Am J Dermatopathol. 2014;36:629-634.
- Kiuru M, Patel RM, Busam KJ. Desmoplastic melanocytic nevi with lymphocytic aggregates. J Cutan Pathol. 2012;39:940-944.
- Reed RJ, Fine RM, Meltzer HD. Palisaded, encapsulated neuromas of the skin. Arch Dermatol. 1972;106:865-870.
- Newman MD, Milgraum S. Palisaded encapsulated neuroma (PEN): an often misdiagnosed neural tumor. Dermatol Online J. 2008;14:12.
- Beutler B, Cohen PR. Palisaded encapsulated neuroma of the trunk: a case report and review of palisaded encapsulated neuroma. Cureus. 2016;8:E726.
- Jokinen CH, Ragsdale BD, Argenyi ZB. Expanding the clinicopathologic spectrum of palisaded encapsulated neuroma. J Cutan Pathol. 2010;37:43-48.
- Argenyi ZB. Immunohistochemical characterization of palisaded, encapsulated neuroma. J Cutan Pathol. 1990;17:329-335.
- Batra J, Ramesh V, Molpariya A, et al. Palisaded encapsulated neuroma: an unusual presentation. Indian Dermatol Online J. 2018;9:262-264.
- Weary PE, Gorlin RJ, Gentry WC Jr, et al. Multiple hamartoma syndrome (Cowden's disease). Arch Dermatol. 1972;106:682-690.
- Mahmood MN, Salama ME, Chaffins M, et al. Solitary sclerotic fibroma of skin: a possible link with pleomorphic fibroma with immunophenotypic expression for O13 (CD99) and CD34. J Cutan Pathol. 2003;30:631-636.
- Nakashima K, Yamada N, Adachi K, et al. Solitary sclerotic fibroma of the skin: morphological characterization of the 'plywood-like pattern'. J Cutan Pathol. 2008;35(suppl 1):74-79.
- Rapini RP, Golitz LE. Sclerotic fibromas of the skin. J Am Acad Dermatol. 1989;20:266-271.
- Abbas O, Ghosn S, Bahhady R, et al. Solitary sclerotic fibroma on the scalp of a young girl: reactive sclerosis pattern? J Dermatol. 2010;37:575-577.
- Hanft VN, Shea CR, McNutt NS, et al. Expression of CD34 in sclerotic ("plywood") fibromas. Am J Dermatopathol. 2000;22:17-21.
A 25-year-old man presented with a flesh-colored papule on the left side of the nose of 2 years' duration.
Medicare beneficiaries pay most for Alzheimer’s
according to the Kaiser Family Foundation.
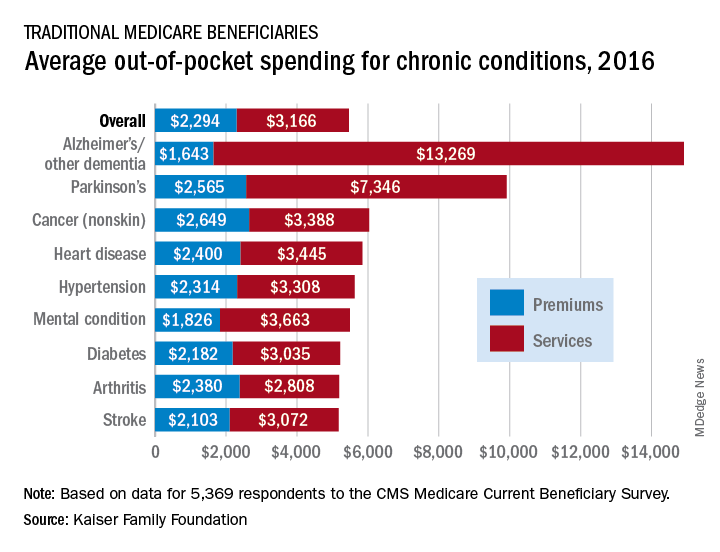
Out-of-pocket spending for Alzheimer’s disease or other dementia was higher than any other chronic condition, averaging $14,913 in 2016 (the latest year for which data are available), compared with $5,460 for all beneficiaries in traditional Medicare, Kaiser investigators said in a recent report based on data for 5,369 respondents to the Medicare Current Beneficiary Survey.
Those totals were divided between services – including long-term care facilities, medical providers and supplies, and prescription drugs – and premiums for Medicare and other types of supplemental insurance. The premium associated with Alzheimer’s, $1,643, was the lowest of any major chronic condition, but the average cost for services, $13,269, was almost twice as high as the next most expensive condition, Parkinson’s disease, and more than four times higher than the overall Medicare average, Juliette Cubanski, PhD, and associates said.
Out-of-pocket costs are higher for patients with Alzheimer’s and Parkinson’s because “these beneficiaries are more likely to reside in a long-term care facility than those with other conditions,” they said. In 2016, out-of-pocket spending on long-term care facility services averaged over $27,000 for Medicare beneficiaries with Alzheimer’s and other dementia and over $28,000 for those with Parkinson’s disease. For all traditional Medicare beneficiaries, average out-of-pocket spending on such services was $1,014.
“The fact that traditional Medicare does not have an annual out-of-pocket limit and does not cover certain services that older adults are more likely to need may undermine the financial security that Medicare provides, especially for people with significant needs and limited incomes. Addressing these gaps would help to alleviate the financial burden of health care for people with Medicare, although doing so would also increase federal spending and taxes,” Dr. Cubanski and associates wrote.
according to the Kaiser Family Foundation.

Out-of-pocket spending for Alzheimer’s disease or other dementia was higher than any other chronic condition, averaging $14,913 in 2016 (the latest year for which data are available), compared with $5,460 for all beneficiaries in traditional Medicare, Kaiser investigators said in a recent report based on data for 5,369 respondents to the Medicare Current Beneficiary Survey.
Those totals were divided between services – including long-term care facilities, medical providers and supplies, and prescription drugs – and premiums for Medicare and other types of supplemental insurance. The premium associated with Alzheimer’s, $1,643, was the lowest of any major chronic condition, but the average cost for services, $13,269, was almost twice as high as the next most expensive condition, Parkinson’s disease, and more than four times higher than the overall Medicare average, Juliette Cubanski, PhD, and associates said.
Out-of-pocket costs are higher for patients with Alzheimer’s and Parkinson’s because “these beneficiaries are more likely to reside in a long-term care facility than those with other conditions,” they said. In 2016, out-of-pocket spending on long-term care facility services averaged over $27,000 for Medicare beneficiaries with Alzheimer’s and other dementia and over $28,000 for those with Parkinson’s disease. For all traditional Medicare beneficiaries, average out-of-pocket spending on such services was $1,014.
“The fact that traditional Medicare does not have an annual out-of-pocket limit and does not cover certain services that older adults are more likely to need may undermine the financial security that Medicare provides, especially for people with significant needs and limited incomes. Addressing these gaps would help to alleviate the financial burden of health care for people with Medicare, although doing so would also increase federal spending and taxes,” Dr. Cubanski and associates wrote.
according to the Kaiser Family Foundation.

Out-of-pocket spending for Alzheimer’s disease or other dementia was higher than any other chronic condition, averaging $14,913 in 2016 (the latest year for which data are available), compared with $5,460 for all beneficiaries in traditional Medicare, Kaiser investigators said in a recent report based on data for 5,369 respondents to the Medicare Current Beneficiary Survey.
Those totals were divided between services – including long-term care facilities, medical providers and supplies, and prescription drugs – and premiums for Medicare and other types of supplemental insurance. The premium associated with Alzheimer’s, $1,643, was the lowest of any major chronic condition, but the average cost for services, $13,269, was almost twice as high as the next most expensive condition, Parkinson’s disease, and more than four times higher than the overall Medicare average, Juliette Cubanski, PhD, and associates said.
Out-of-pocket costs are higher for patients with Alzheimer’s and Parkinson’s because “these beneficiaries are more likely to reside in a long-term care facility than those with other conditions,” they said. In 2016, out-of-pocket spending on long-term care facility services averaged over $27,000 for Medicare beneficiaries with Alzheimer’s and other dementia and over $28,000 for those with Parkinson’s disease. For all traditional Medicare beneficiaries, average out-of-pocket spending on such services was $1,014.
“The fact that traditional Medicare does not have an annual out-of-pocket limit and does not cover certain services that older adults are more likely to need may undermine the financial security that Medicare provides, especially for people with significant needs and limited incomes. Addressing these gaps would help to alleviate the financial burden of health care for people with Medicare, although doing so would also increase federal spending and taxes,” Dr. Cubanski and associates wrote.
Options for acne treatment continue to advance
LAS VEGAS – according to Linda Stein Gold, MD, who reviewed the data on these two therapies, as well as cannabidiol (CBD) and an androgen receptor antagonist, which are currently in clinical trials, at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
When considering antibiotic therapy for patients with moderate to severe acne, sarecycline, approved for that indication in October 2018, has improved anti-inflammatory properties and a narrower spectrum of activity, compared with other tetracycline-class antibiotics used for the condition, according to Dr. Stein Gold, director of dermatology research at Henry Ford Health System in Detroit. In two identically designed, 12-week randomized trials, SC 1401 and SC1402, researchers evaluated the efficacy and safety of an approximate 1.5–mg/kg per day dose of sarecycline in comparison with placebo in patients with moderate to severe facial acne aged 9-45 years (J Drugs Dermatol. 2018 Sept 1;17[9]:987-96). “We started to see separation in the inflammatory lesions as early as week 3, and a nice separation versus placebo over the course of 12 weeks,” said Dr. Stein Gold, one of the study investigators. “In all, 22% of patients got clear or almost clear with monotherapy. That’s fairly good.”
She noted that there was consistency in the reductions of lesion count achieved in both studies. Improvements were seen through to 12 weeks, with statistically significant reduction seen as early as 3 weeks in both studies. Sarecycline was also statistically superior to placebo at every time point studied in both trials.
In order to be judged a successful outcome, the subject had to have a 12-week Investigator’s Global Assessment (IGA) score with a 2-point or greater decrease (improvement) from baseline score on the IGA in each location, for patients who have a baseline IGA of 2 or greater, and to be clear (0) or almost clear (1). The same IGA scale was used for the chest and back assessments as was used for facial acne; the researchers observed statistically significant improvements in IGA score for both chest and back acne across both studies at week 12.
Sarecycline also had a favorable safety profile, with no treatment-emergent vertigo or tinnitus adverse events, she noted. Treatment-emergent vestibular and phototoxic adverse events both occurred in fewer than 1% of sarecycline patients. Among females, rates of yeast infections were low. When she recommends a course of this drug for her patients, “I never overpromise,” she said. “I tell my acne patients, ‘We measure your success in weeks and months, not days.’ I always tell them, ‘Take a selfie today and take a selfie every few weeks. When you come back in, we’ll review your progress and see how things went.’ ”
Researchers have also been studying topical minocycline as a treatment option. Minocycline is a large molecule that Dr. Stein Gold characterized as being “very challenging” to deliver topically. “It’s also challenging to keep it stable in a topical formulation.” However, results from two identical phase 3 trials found 4% topical minocycline foam significantly reduced both inflammatory and noninflammatory lesions and improved IGA scores in patients with moderate to severe acne when treated daily for 12 weeks (J Am Acad Dermatol. 2019 Jan;80[1]:168-77).
“This drug has an interesting vehicle,” said Dr. Stein Gold, who was one of the study investigators. “If you take the vehicle itself and you put it next to sebum, it causes sebum to melt at lower temperatures. Why does this matter? If you’re dissolving sebum, maybe you’re creating an easier pathway for the drug to get delivered into the skin and into the hair follicles. We don’t know all the details.” In the two trials, 15%-31% of patients achieved clearance or near clearance of all lesions. “How did it do in terms of decreasing papules and pustules? It did pretty well,” she said. “We want drugs to meet their match in everything that we do.” In terms of tolerability, skin-related adverse events were reported in fewer than 1% of subjects treated with 4% topical minocycline foam. She noted that by delivering minocycline topically, “we get huge concentrations in the skin, but almost negligible amounts in the systemic circulation, which is important. We want to keep [the drug] in the skin; we don’t want it in our system.”
(The Food and Drug Administration approved minocycline foam 4% in October 2019 for treating inflammatory lesions associated with non-nodular moderate to severe acne).
Another potential treatment on the horizon is cortexolone 17a-propionate, a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. “When used around the sebaceous gland, we find that the amount of sebum produced goes down,” she said, noting that phase 3 trials of the agent have been completed.
“We also find that abnormal keratinization subsequently goes down. Just putting this on the skin significantly reduced acne as monotherapy in patients with moderate to severe acne. We were able to get them to clear or almost clear. It worked on comedones, papules, and pustules. Hopefully, it will get FDA approval. This fills the one unmet need we haven’t had topically in terms of decreasing sebum production.”
Clinical trials of CBD are also under way for acne and atopic dermatitis. “It could work for acne because there are some studies showing that might work on sebaceous glands to decrease sebum production,” she said. “CBD has been shown to have positive effects on abnormal keratinization, and it has been shown to have anti-inflammatory effects. Maybe we’ll have another mechanism of action for acne.”
Dr. Gold disclosed that she is on the speakers bureau for Almirall, Galderma, Leo Pharma, Ortho Dermatologics, Pfizer, and Sanofi/Regeneron. She is a consultant for Dermavant, Foamix, Galderma, Leo Pharma, Pfizer, Novartis, Ortho Dermatologics, and holds stock/stock options in AbbVie, Dermavant, Eli Lilly, Foamix, Galderma, Incyte, Leo Pharma, Novartis, Ortho Dermatologics, Pfizer, and Sol-Gel.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – according to Linda Stein Gold, MD, who reviewed the data on these two therapies, as well as cannabidiol (CBD) and an androgen receptor antagonist, which are currently in clinical trials, at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
When considering antibiotic therapy for patients with moderate to severe acne, sarecycline, approved for that indication in October 2018, has improved anti-inflammatory properties and a narrower spectrum of activity, compared with other tetracycline-class antibiotics used for the condition, according to Dr. Stein Gold, director of dermatology research at Henry Ford Health System in Detroit. In two identically designed, 12-week randomized trials, SC 1401 and SC1402, researchers evaluated the efficacy and safety of an approximate 1.5–mg/kg per day dose of sarecycline in comparison with placebo in patients with moderate to severe facial acne aged 9-45 years (J Drugs Dermatol. 2018 Sept 1;17[9]:987-96). “We started to see separation in the inflammatory lesions as early as week 3, and a nice separation versus placebo over the course of 12 weeks,” said Dr. Stein Gold, one of the study investigators. “In all, 22% of patients got clear or almost clear with monotherapy. That’s fairly good.”
She noted that there was consistency in the reductions of lesion count achieved in both studies. Improvements were seen through to 12 weeks, with statistically significant reduction seen as early as 3 weeks in both studies. Sarecycline was also statistically superior to placebo at every time point studied in both trials.
In order to be judged a successful outcome, the subject had to have a 12-week Investigator’s Global Assessment (IGA) score with a 2-point or greater decrease (improvement) from baseline score on the IGA in each location, for patients who have a baseline IGA of 2 or greater, and to be clear (0) or almost clear (1). The same IGA scale was used for the chest and back assessments as was used for facial acne; the researchers observed statistically significant improvements in IGA score for both chest and back acne across both studies at week 12.
Sarecycline also had a favorable safety profile, with no treatment-emergent vertigo or tinnitus adverse events, she noted. Treatment-emergent vestibular and phototoxic adverse events both occurred in fewer than 1% of sarecycline patients. Among females, rates of yeast infections were low. When she recommends a course of this drug for her patients, “I never overpromise,” she said. “I tell my acne patients, ‘We measure your success in weeks and months, not days.’ I always tell them, ‘Take a selfie today and take a selfie every few weeks. When you come back in, we’ll review your progress and see how things went.’ ”
Researchers have also been studying topical minocycline as a treatment option. Minocycline is a large molecule that Dr. Stein Gold characterized as being “very challenging” to deliver topically. “It’s also challenging to keep it stable in a topical formulation.” However, results from two identical phase 3 trials found 4% topical minocycline foam significantly reduced both inflammatory and noninflammatory lesions and improved IGA scores in patients with moderate to severe acne when treated daily for 12 weeks (J Am Acad Dermatol. 2019 Jan;80[1]:168-77).
“This drug has an interesting vehicle,” said Dr. Stein Gold, who was one of the study investigators. “If you take the vehicle itself and you put it next to sebum, it causes sebum to melt at lower temperatures. Why does this matter? If you’re dissolving sebum, maybe you’re creating an easier pathway for the drug to get delivered into the skin and into the hair follicles. We don’t know all the details.” In the two trials, 15%-31% of patients achieved clearance or near clearance of all lesions. “How did it do in terms of decreasing papules and pustules? It did pretty well,” she said. “We want drugs to meet their match in everything that we do.” In terms of tolerability, skin-related adverse events were reported in fewer than 1% of subjects treated with 4% topical minocycline foam. She noted that by delivering minocycline topically, “we get huge concentrations in the skin, but almost negligible amounts in the systemic circulation, which is important. We want to keep [the drug] in the skin; we don’t want it in our system.”
(The Food and Drug Administration approved minocycline foam 4% in October 2019 for treating inflammatory lesions associated with non-nodular moderate to severe acne).
Another potential treatment on the horizon is cortexolone 17a-propionate, a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. “When used around the sebaceous gland, we find that the amount of sebum produced goes down,” she said, noting that phase 3 trials of the agent have been completed.
“We also find that abnormal keratinization subsequently goes down. Just putting this on the skin significantly reduced acne as monotherapy in patients with moderate to severe acne. We were able to get them to clear or almost clear. It worked on comedones, papules, and pustules. Hopefully, it will get FDA approval. This fills the one unmet need we haven’t had topically in terms of decreasing sebum production.”
Clinical trials of CBD are also under way for acne and atopic dermatitis. “It could work for acne because there are some studies showing that might work on sebaceous glands to decrease sebum production,” she said. “CBD has been shown to have positive effects on abnormal keratinization, and it has been shown to have anti-inflammatory effects. Maybe we’ll have another mechanism of action for acne.”
Dr. Gold disclosed that she is on the speakers bureau for Almirall, Galderma, Leo Pharma, Ortho Dermatologics, Pfizer, and Sanofi/Regeneron. She is a consultant for Dermavant, Foamix, Galderma, Leo Pharma, Pfizer, Novartis, Ortho Dermatologics, and holds stock/stock options in AbbVie, Dermavant, Eli Lilly, Foamix, Galderma, Incyte, Leo Pharma, Novartis, Ortho Dermatologics, Pfizer, and Sol-Gel.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – according to Linda Stein Gold, MD, who reviewed the data on these two therapies, as well as cannabidiol (CBD) and an androgen receptor antagonist, which are currently in clinical trials, at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
When considering antibiotic therapy for patients with moderate to severe acne, sarecycline, approved for that indication in October 2018, has improved anti-inflammatory properties and a narrower spectrum of activity, compared with other tetracycline-class antibiotics used for the condition, according to Dr. Stein Gold, director of dermatology research at Henry Ford Health System in Detroit. In two identically designed, 12-week randomized trials, SC 1401 and SC1402, researchers evaluated the efficacy and safety of an approximate 1.5–mg/kg per day dose of sarecycline in comparison with placebo in patients with moderate to severe facial acne aged 9-45 years (J Drugs Dermatol. 2018 Sept 1;17[9]:987-96). “We started to see separation in the inflammatory lesions as early as week 3, and a nice separation versus placebo over the course of 12 weeks,” said Dr. Stein Gold, one of the study investigators. “In all, 22% of patients got clear or almost clear with monotherapy. That’s fairly good.”
She noted that there was consistency in the reductions of lesion count achieved in both studies. Improvements were seen through to 12 weeks, with statistically significant reduction seen as early as 3 weeks in both studies. Sarecycline was also statistically superior to placebo at every time point studied in both trials.
In order to be judged a successful outcome, the subject had to have a 12-week Investigator’s Global Assessment (IGA) score with a 2-point or greater decrease (improvement) from baseline score on the IGA in each location, for patients who have a baseline IGA of 2 or greater, and to be clear (0) or almost clear (1). The same IGA scale was used for the chest and back assessments as was used for facial acne; the researchers observed statistically significant improvements in IGA score for both chest and back acne across both studies at week 12.
Sarecycline also had a favorable safety profile, with no treatment-emergent vertigo or tinnitus adverse events, she noted. Treatment-emergent vestibular and phototoxic adverse events both occurred in fewer than 1% of sarecycline patients. Among females, rates of yeast infections were low. When she recommends a course of this drug for her patients, “I never overpromise,” she said. “I tell my acne patients, ‘We measure your success in weeks and months, not days.’ I always tell them, ‘Take a selfie today and take a selfie every few weeks. When you come back in, we’ll review your progress and see how things went.’ ”
Researchers have also been studying topical minocycline as a treatment option. Minocycline is a large molecule that Dr. Stein Gold characterized as being “very challenging” to deliver topically. “It’s also challenging to keep it stable in a topical formulation.” However, results from two identical phase 3 trials found 4% topical minocycline foam significantly reduced both inflammatory and noninflammatory lesions and improved IGA scores in patients with moderate to severe acne when treated daily for 12 weeks (J Am Acad Dermatol. 2019 Jan;80[1]:168-77).
“This drug has an interesting vehicle,” said Dr. Stein Gold, who was one of the study investigators. “If you take the vehicle itself and you put it next to sebum, it causes sebum to melt at lower temperatures. Why does this matter? If you’re dissolving sebum, maybe you’re creating an easier pathway for the drug to get delivered into the skin and into the hair follicles. We don’t know all the details.” In the two trials, 15%-31% of patients achieved clearance or near clearance of all lesions. “How did it do in terms of decreasing papules and pustules? It did pretty well,” she said. “We want drugs to meet their match in everything that we do.” In terms of tolerability, skin-related adverse events were reported in fewer than 1% of subjects treated with 4% topical minocycline foam. She noted that by delivering minocycline topically, “we get huge concentrations in the skin, but almost negligible amounts in the systemic circulation, which is important. We want to keep [the drug] in the skin; we don’t want it in our system.”
(The Food and Drug Administration approved minocycline foam 4% in October 2019 for treating inflammatory lesions associated with non-nodular moderate to severe acne).
Another potential treatment on the horizon is cortexolone 17a-propionate, a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. “When used around the sebaceous gland, we find that the amount of sebum produced goes down,” she said, noting that phase 3 trials of the agent have been completed.
“We also find that abnormal keratinization subsequently goes down. Just putting this on the skin significantly reduced acne as monotherapy in patients with moderate to severe acne. We were able to get them to clear or almost clear. It worked on comedones, papules, and pustules. Hopefully, it will get FDA approval. This fills the one unmet need we haven’t had topically in terms of decreasing sebum production.”
Clinical trials of CBD are also under way for acne and atopic dermatitis. “It could work for acne because there are some studies showing that might work on sebaceous glands to decrease sebum production,” she said. “CBD has been shown to have positive effects on abnormal keratinization, and it has been shown to have anti-inflammatory effects. Maybe we’ll have another mechanism of action for acne.”
Dr. Gold disclosed that she is on the speakers bureau for Almirall, Galderma, Leo Pharma, Ortho Dermatologics, Pfizer, and Sanofi/Regeneron. She is a consultant for Dermavant, Foamix, Galderma, Leo Pharma, Pfizer, Novartis, Ortho Dermatologics, and holds stock/stock options in AbbVie, Dermavant, Eli Lilly, Foamix, Galderma, Incyte, Leo Pharma, Novartis, Ortho Dermatologics, Pfizer, and Sol-Gel.
SDEF and this news organization are owned by the same parent company.
AT THE SDEF LAS VEGAS DERMATOLOGY SEMINAR