User login
Implementing Pediatric Asthma Pathways in Community Hospitals: A National Qualitative Study
Despite the widespread availability of evidence-based guidelines,1 there is inappropriate variation in the care and outcomes for children with asthma in both the emergency department (ED) and the inpatient setting.2-6 Operational versions of evidence-based guidelines known as “pathways” have been shown to improve adoption of evidence-based guidelines, quality of care, and health outcomes for children with asthma.7-14 However, little is known about how to successfully implement pathways outside of free-standing children’s hospitals.15-19
The majority of children with asthma in the United States are cared for in community hospitals, which provide services for both adults and children.20 However, prior studies of pediatric asthma pathways have largely excluded community hospitals. These studies primarily focused on determining clinical effectiveness, rather than detailing the implementation process. These approaches have left critical gaps that hinder our ability to implement pathways and improve care in community hospitals, which have unique barriers and less resources.21,22 Therefore, understanding the process of pathway implementation in community hospitals is critical to improving care for children.22 Our objective was to identify the key determinants of successful pediatric asthma pathway implementation using a national sample of community hospitals. This knowledge can guide hospital leaders and healthcare providers in efforts to improve pediatric care and outcomes in these settings.
METHODS
Study Setting, Design, and Population
In Fall 2017, the Value in Inpatient Pediatrics (VIP) network launched PIPA, Pathways to Improving Pediatric Asthma care.23 The VIP network, a part of the American Academy of Pediatrics (AAP), aims to improve the value of care delivered to any pediatric patient in a hospital bed, from rural to free-standing children’s hospitals.24 PIPA used a learning collaborative model25 and recruited local project leaders (physicians, nurses, respiratory therapists (RT), and pharmacists) from 89 hospitals around the country. PIPA provided these hospital teams with asthma pathways and several resources for implementation support, including educational meetings, quality improvement (QI) training, audit and feedback, and facilitation. Facilitation is a process of interactive problem-solving and support that occurs in the context of a supportive interpersonal relationship and a recognized need for improvement.26 A facilitator, or a “coach”, is an external expert who provides project mentorship and assists the process of making meaningful changes to improve patient care.26 Facilitation was provided by external consultants with QI expertise.
For this qualitative study, facilitators conducted semi-structured interviews with a convenience sample of project leaders from community hospitals participating in PIPA, with some interviews including multiple project leaders (eg, nursing, inpatient, and Emergency Department [ED] leaders). Verbal consent was obtained from all participants. No incentives were provided. This study was approved by the AAP institutional review board.
Data Collection
We used the constructs described in the Consolidated Framework for Implementation Research (CFIR)27 and adapted those salient to pediatric asthma pathways to develop an interview guide that was used with all participants (Appendix 1). The CFIR offers an overarching typology to understand what works where and why across five major domains that influence implementation: intervention characteristics, inner setting (hospital), outer setting (economic, political, and social context of the hospital), characteristics of the individuals involved, and the process of implementation. Data were collected across these domains to inform our analysis of the key determinants of pediatric asthma pathway implementation in community hospitals.
Interviews were conducted by phone from December 2017 to April 2018 (first four months of pathway implementation). Interviews lasted 30-60 minutes and were recorded and transcribed verbatim. Transcripts were edited for accuracy using the audio recordings. As data collection occurred concurrently with analysis, the interview guide was iteratively revised to reflect new insights and patterns that emerged from our analysis. All sites were anonymized in the data analysis. New interviews were coded until thematic saturation was reached.
Analysis
We conducted an inductive thematic analysis using the CFIR as our conceptual framework.28,29 Four investigators (CM, MJ, ES, and SK) performed the initial open coding of the data. Investigators met twice during the open coding process to develop and then finalize a codebook of standard definitions for codes. This codebook facilitated coding consistency through the remainder of the analytic process. Two investigators (CM and MJ) then independently read and coded all data to ensure intercoder reliability. During this process, CM and MJ met every two weeks to compare coding consistency, resolve discrepancies, and discuss preliminary findings. When the coding was complete, all investigators met to explore and develop themes that encompassed related common codes.
The CFIR was used at two stages of the study: (1) developing the interview guide and (2) cross-checking for any potentially important codes that were missing/needed to be explored further. Thus, the investigators maintained an inductive approach grounded in the data. To assure study rigor, we employed investigator triangulation (use of multiple investigators and participants from multiple clinical roles) and reflexivity (ongoing critique and critical reflection of the individual biases of the investigators).30 Coding was performed using Dedoose (version 7.0.23; Los Angeles, California).
RESULTS
A total of 34 community hospitals completed the PIPA project, of which the project leaders of 25 hospitals connected with the facilitators and were approached to participate; 18 (72%) hospitals’ project leaders participated in the study. We analyzed 18 interviews conducted between facilitators and project leaders, which included a total of 32 project leaders (one to five leaders per interview). The hospitals represented were diverse in geographic location and size (range 4-50 pediatric beds per hospital), and the majority of sites (78%) supported the trainees (Table 1).
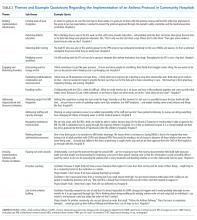
We identified four overarching themes that described the key determinants of pathway implementation in community hospitals. These themes are presented in order of their frequency of occurrence in the data. They included (1) building an implementation infrastructure, (2) engaging and motivating providers, (3) addressing organizational and resource limitations, and (4) devising implementation solutions with facilitators. Descriptions and exemplary quotations for each theme are provided in Table 2 and Appendix Figure 1.
Building an Implementation Infrastructure
“So, I’m going to sit down with the primary nursing staff and the other four physicians in the group to go over the expectations…We’re not going to have the actual EMR [changes] and we’re not going to have the nursing documentation field built right away but [we want to] make sure that people are documenting the respiratory score in their generic nursing note so that the information is easily accessible.” (Physician leader, Hospital G)
Participants also described the need to deliver education on the evidence supporting changes in practice and skills training specific to pediatric asthma care:
“Once we realized that we were going to be doing this pathway, we started training our nurses on the inpatient side on [pediatric respiratory scoring].” (Nursing leader, Hospital P)
In addition, pathway implementation required modification of clinical workflows via changes to hospital policies or guidelines, electronic medical records (EMR), and/or the physical environment (eg, placing supplies in proximity to care delivery):
“I think it can help if we could get an order set or a nursing protocol where asthmatics over a certain severity can just get steroids in triage.” (Physician leader, Hospital A)
Engaging and Motivating Providers
Another crucial step in pathway implementation was engaging and motivating providers. This included overcoming inertia to practice change, facilitating multidisciplinary collaboration, and handling conflicts regarding practice changes. Participants discussed the excitement of participating in a national collaborative as especially motivating to help drive engagement and overcome barriers to change, particularly the ability to compare local hospital performance to national peers.
“I think everyone is a little competitive. So I think that when we see how we compare to other institutions—both our group and the ER—I think it also adds a little oomph…I think for our nurses too; we’re able to say, ‘[look how we compare to] most of the other hospitals.’ I think that helps.” (Physician leader, Hospital B)
Multidisciplinary collaboration across a wide variety of frontline pediatric and nonpediatric providers was key to understanding current workflows and identifying needed modifications for pathway implementation:
“I do think clearly our biggest obstacles are the fact that we have adult ED providers. We have the opportunity on the inpatient side [with nursing and respiratory therapy], who really do awesome with pediatric changes, to take our wins where we can and make the changes with the ED. In the ED we have an RN educator. She’s very on board with doing the respiratory scoring and getting this whole thing started.” (Physician leader, Hospital L)
Intentional communication and leadership skills also played key roles in engaging hesitant providers and handling conflict:
“Just sitting and talking with our respiratory therapist about the ability to provide this type of service or support and seeing what their reservations have been, at least it’s open to conversation so that we could provide these types of therapies in the future and we’re able to see like what people’s concerns are. I think just basically increasing familiarity with not only these processes, but different types of therapy will hopefully in the future help us provide better care to our patients.” (Physician leader, Hospital Q)
Addressing Organizational and Resource Limitations
Participants recognized organizational and resource limitations, some of which may be unique to community hospitals that prioritize resources for adult care. The limitations described included EMR staff support, healthcare provider staffing/capacity, navigating IRBs, and addressing administrative processes. Competing demands for information technology staff support and lack of prioritization of pediatric-specific initiatives often hindered efforts to modify the EMR.
“Resource wise, we are hoping to implement an order set in our Epic EMR, [but] finding the availability from the Epic team may be a challenge.” (Physician Leader, Hospital A)
Participants also reported that limited staff capacity (eg, nursing, RT) hindered pathway implementation efforts. This limited capacity hindered workflow changes and limited the time available for education and training on pathways:
“[Respiratory scoring for asthma is] an added responsibility for the [nursing] staff and we don’t have patient technicians. So they’re doing everything from changing the sheets to bringing water to all of the medical patients. So, that I think may be a barrier.” (Physician leader, Hospital B)
Across sites, navigating the IRB posed various challenges. Some sites were required to obtain approval from regional IRBs, which did not have resources to devote to pediatric projects. Other sites did not have IRBs at all, but instead required separate approvals for the project from hospital leadership or other entities:
“On the IRB, I contacted the manager of the IRB and she’s said, ‘No, it’s not an IRB project,’ but she sent it to another director for review, and it took forever to be able to get a data agreement with [the local university hospital] so that we can pull the data. I just couldn’t believe it took months to get done.” (Physician Leader, Hospital K)
Finally, administrative barriers such as addressing formulary changes in the context of adult-focused settings were challenging. For example, at one hospital, metered dose inhalers (MDIs) were not used for adult patients, and the hospital administration was resistant to incorporate their use into practice for pediatric patients due to the cost of such changes.
“The [general hospital] didn’t have MDI’s anymore because of cost reasons, and when we started the pediatric work, we really made it a point to get the MDI’s for pediatric patients back in the formulary.” (Physician leader, Hospital A)
Devising Implementation Solutions with Practice Facilitators
Participants often devised pathway implementation solutions with facilitators in-the-moment during meetings. This problem-solving included figuring out work-arounds, proactive coaching by external facilitators, and just-in-time solution building. Furthermore, in meetings that included more than one project leader, leaders would often work with each other to devise solutions. Meetings provided forums that stimulated identification of implementation barriers, brainstorming, and subsequently solution building.
Physician leader: I’m wondering if we could, as an interim solution, try out an algorithm on paper, I don’t know if that’s allowed, until we get Epic approval. Do you know?
Nurse Leader: You mean having an algorithm posted in triage? Yeah, I don’t see why not. (Hospital A)
Next, problem solving was often driven by the facilitator’s experience and knowledge, drawn from their interactions with other collaborative sites or their own prior experiences with asthma, QI, or pathway implementation. The facilitators brought an outside perspective, not bound by that particular hospital’s local culture or structural intricacies. This proactive coaching spurred the identification of creative, yet practical solutions:
Project Leader: We’re still trying to get all our templates [for the EMR]…because [currently they are] all adult templates.
Facilitator: If you’re making templates right now, could you also add the three asterisks? Like smoking or exposure to second hand tobacco smoke or marijuana…then have the three asterisks there and then “Referral made?***”. That would force people to document in a certain place in the template as well.Project Leader: That’s definitely something we could add right now. (Hospital O)
Check-in meetings with facilitators offered an opportunity to trouble shoot, brainstorm work-arounds, devise in-the-moment site-specific solutions to enable successful pathway implementation, and provide ongoing support throughout implementation.
DISCUSSION
Pathways can improve the quality of care for children with asthma.31 However, there is little evidence-based guidance on how to implement pathways and improve pediatric care in community hospitals,17-20 where the majority of children are cared for nationally. This is the first study to our knowledge that details the key determinants of pediatric asthma pathway implementation in community hospital settings. We identified four key determinants of implementation that can help guide others in similar settings. These include building an implementation infrastructure, engaging and motivating multidisciplinary providers, addressing organizational and resource limitations, and using external facilitators to devise implementation solutions.
Existing frameworks such as the CFIR outline the potential determinants of implementation success but do not provide population- or setting-specific guidance.27 There have been prior studies detailing pathway implementation for pediatric populations, but these studies did not focus on community hospitals.32,33 Our findings align with these prior studies, which highlight the importance of identifying implementation champions, engaging and motivating multidisciplinary providers, establishing a QI infrastructure, and addressing organizational and resource limitations, such as EMR integration.32,33 However, our study provides unique insights into issues that are important to successful pathway implementation in community hospitals, including engagement of adult-focused healthcare providers, reprioritization of resources toward the care of children, and the potentially critical role of external facilitators.
Our findings indicate that community hospitals seeking to improve care for children may particularly benefit from using external facilitators and/or partnering with external organizations. We found that external facilitators played a significant and proactive role in community hospitals’ efforts to improve care for children. Facilitators helped devise work-arounds and engaged in just-in-time solution building with local project leaders. For instance, facilitators helped develop strategies for training healthcare providers in performing new clinical tasks, building reminders of pathway recommendations into clinical workflows, and overcoming resource barriers. Thus, community hospitals may uniquely benefit from participation in national learning collaboratives, which often provide avenues for external facilitation.25,34,35 National networks, such as the VIP network, lead national learning collaboratives that provide external facilitation as well as other resources (eg, educational materials, data analysis support) to community hospitals seeking to improve pediatric care.24 Previous work by McDaniel et al. identified that intentional partnerships between children’s and community hospitals can also potentially provide access to resources for education and training in pediatric care and support in navigating organizational and resource challenges.22
Our results characterize the key determinants of pediatric asthma pathway implementation using a national sample of community hospitals that were diverse in geography, size, and structure. This imparts greater transferability of our findings. We also used strategies to promote the rigor of our findings, including triangulation and reflexivity. However, our study has several limitations. First, we analyzed only the meetings that occurred during the early months of pathway implementation. As such, we did not capture any key determinants that may have arisen later in implementation. However, process analyses of implementation indicate that the majority of implementation efforts occurred within these first three to four months.36 Second, we did not elicit input from hospital administration or leadership. The lack of administrative/leadership input probably affected the CFIR themes we found, as no themes from the outer setting were elicited. However, the goal of our study was to characterize the experiences of those leading implementation efforts, and focusing on these leaders allows our work to better guide those doing similar work in the future. Third, we used CFIR to guide the development of our interview guide and as a reference during analysis, which may have skewed our findings to preferentially reflect CFIR constructs. However, our overall analysis was grounded in the primary data and we employed reflexivity during all stages of our analysis. In addition, having the facilitators conduct the qualitative interviews may have biased our findings toward the perspectives of the facilitators; however, the facilitators represented quite diverse clinical and QI backgrounds. Finally, our findings do not necessarily correlate with improvements in clinical outcomes. As such, they are not meant to serve as explicit recommendations for improving patient outcomes, but rather as a characterization of the context, processes, and experiences of implementing pathways in the community setting to inform others doing this important work.
CONCLUSIONS
We identified the key determinants of pediatric asthma pathway implementation in community hospitals, which may help inform QI efforts in these settings. We also identified organizational and resource limitations that are probably unique to these adult-focused hospitals. Participating in national learning collaboratives and/or working with facilitators may support pathway implementation and improved quality of care for children with asthma in community hospitals.
Future work should seek to correlate these and other determinants of pathway implementation with health outcomes for hospitalized children, as well as integrate broader and more diverse samples of community hospitals.
1. National Asthma E, Prevention P. Expert Panel Report 3 (EPR-3): Guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(5):S94-S138. https://doi.org/10.1016/j.jaci.2007.09.043.
2. Bekmezian A, Hersh AL, Maselli JH, Cabana MD. Pediatric emergency departments are more likely than general emergency departments to treat asthma exacerbation with systemic corticosteroids. J Asthma. 2011;48(1):69-74. https://doi.org/10.3109/02770903.2010.535884.
3. Biagini Myers JM, Simmons JM, Kercsmar CM, et al. Heterogeneity in asthma care in a statewide collaborative: the Ohio Pediatric Asthma Repository. Pediatrics. 2015;135(2):271-279. https://doi.org/10.1542/peds.2014-2230.
4. Kharbanda AB, Hall M, Shah SS, et al. Variation in resource utilization across a national sample of pediatric emergency departments. J Pediatr. 2013;163(1):230-236. https://doi.org/10.1016/j.jpeds.2012.12.013.
5. O’Con
6. Lougheed MD, Garvey N, Chapman KR, et al. Variations and gaps in management of acute asthma in Ontario emergency departments. Chest. 2009;135(3):724-736. https://doi.org/10.1378/chest.08-0371.
7. Bekmezian A, Fee C, Weber E. Clinical pathway improves pediatrics asthma management in the emergency department and reduces admissions. J Asthma. 2015;52(8):806-814. https://doi.org/10.3109/02770903.2015.1019086.
8. Chen KH, Chen CC, Liu HE, Tzeng PC, Glasziou PP. Effectiveness of paediatric asthma clinical pathways: a narrative systematic review. J Asthma. 2014;51(5):480-492. https://doi.org/10.3109/02770903.2014.887728.
9. Johnson KB, Blaisdell CJ, Walker A, Eggleston P. Effectiveness of a clinical pathway for inpatient asthma management. Pediatrics. 2000;106(5):1006-1012. https://doi.org/10.1542/peds.106.5.1006.
10. Kelly CS, Andersen CL, Pestian JP, et al. Improved outcomes for hospitalized asthmatic children using a clinical pathway. Ann Allergy Asthma Immunol. 2000;84(5):509-516. https://doi.org/10.1016/S1081-1206(10)62514-8.
11. McDowell KM, Chatburn RL, Myers TR, O’Riordan MA, Kercsmar CM. A cost-saving algorithm for children hospitalized for status asthmaticus. Arch Pediatr Adolesc Med. 1998;152(10):977-984. https://doi.org/10.1001/archpedi.152.10.977.
12. Miller AG, Breslin ME, Pineda LC, Fox JW. An asthma protocol improved adherence to evidence-based guidelines for pediatric subjects with status asthmaticus in the emergency department. Respir Care. 2015;60(12):1759-1764. https://doi.org/10.4187/respcare.04011.
13. Nkoy F, Fassl B, Stone B, et al. Improving pediatric asthma care and outcomes across multiple hospitals. Pediatrics. 2015;136(6):e1602-e1610. https://doi.org/10.1542/peds.2015-0285.
14. Rutman L, Atkins RC, Migita R, et al. Modification of an established pediatric asthma pathway improves evidence-based, efficient care. Pediatrics. 2016;138(6). https://doi.org/10.1542/peds.2016-1248.
15. Glauber JH, Farber HJ, Homer CJ. Asthma clinical pathways: toward what end? Pediatrics. 2001;107(3):590-592. https://doi.org/10.1542/peds.107.3.590.
16. Grimshaw J, Eccles M, Thomas R, et al. Toward evidence-based quality improvement. Evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966-1998. J Gen Intern Med. 2006;21(2):S14-S20. https://doi.org/10.1111/j.1525-1497.2006.00357.x.
17. Scott SD, Grimshaw J, Klassen TP, Nettel-Aguirre A, Johnson DW. Understanding implementation processes of clinical pathways and clinical practice guidelines in pediatric contexts: a study protocol. Implement Sci. 2011;6:133. https://doi.org/10.1186/1748-5908-6-133.
18. Walls TA, Hughes NT, Mullan PC, Chamberlain JM, Brown K. Improving pediatric asthma outcomes in a community emergency department. Pediatrics. 2017;139(1). https://doi.org/10.1542/peds.2016-0088.
19. Kaiser SV, Lam R, Cabana MD, et al. Best practices in implementing inpatient pediatric asthma pathways: a qualitative study. J Asthma. 2019:1-11. https://doi.org/10.1080/02770903.2019.1606237.
20. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624.
21. Franca UL, McManus ML. Availability of definitive hospital care for children. JAMA Pediatr. 2017;171(9):e171096. https://doi.org/10.1001/jamapediatrics.2017.1096.
22. McDaniel CE, Jennings R, Schroeder AR, Paciorkowski N, Hofmann M, Leyenaar J. Aligning inpatient pediatric research with settings of care: a call to action. Pediatrics. 2019;143(5). https://doi.org/10.1542/peds.2018-2648.
23. Kaiser SV JB. Value in inpatient pediatrics network launches National Asthma Project. In: AAP Quality Connections 2018; 26:8-9. Retrieved from: https://www.aap.org/en-us/Documents/coqips_newsletter_2018_winter_26.pdf
24. Value in Inpatient Pediatrics. https://www.aap.org/en-us/professional-resources/quality-improvement/Pages/Value-in-Inpatient-Pediatrics.aspx. Accessed December 1, 2017.
25. The Breakthrough Series: IHI’s Collaborative Model for Achieving Breakthrough Improvement. IHI Innovation Series white paper. Boston: Institute for Healthcare Improvement; 2003. Retrieved from: www.IHI.org
26. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21. https://doi.org/10.1186/s13012-015-0209-1.
27. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. https://doi.org/10.1186/1748-5908-4-50.
28. Braun VaC, V. Thematic analysis. In: H. Cooper PC, Long DL, Panter AT, Rindskopf E, Sher KJ, eds. APA handbook of research methods in psychology, Vol 2. Research designs: Quantitative, qualitative, neuropsychologial, and biological. Washington, DC, US: American Psychological Association; 2012. https://doi.org/10.1037/13620-000.
29. Charmaz K. Grounded Theory. 2nd ed. Thousand Oaks, CA: SAGE Publications; 2014.
30. Creswell JW, Poth CNCN CJaP. Qualitative Inquiry and Research Design: Choosing Among Five Approaches. Thousand Oaks, CA: Sage; 2017.
31. Kaiser SV, Rodean J, Bekmezian A, et al. Effectiveness of pediatric asthma pathways for hospitalized children: a multicenter, national analysis. J. Pediatr. 2018;197:165-171. https://doi.org/10.1016/j.jpeds.2018.01.084.
32. Leyenaar JK, Andrews CB, Tyksinski ER, Biondi E, Parikh K, Ralston S. Facilitators of interdepartmental quality improvement: a mixed-methods analysis of a collaborative to improve pediatric community-acquired pneumonia management. BMJ Qual Saf. 2019;28(3):215-222. https://doi.org/10.1136/bmjqs-2018-008065. 33. Ralston SL, Atwood EC, Garber MD, Holmes AV. What works to reduce unnecessary care for bronchiolitis? A qualitative analysis of a national collaborative. Acad Pediatr. 2017;17(2):198-204. https://doi.org/10.1016/j.acap.2016.07.001.
33. Ralston SL, Atwood EC, Garber MD, Holmes AV. What works to reduce unnecessary care for bronchiolitis? A qualitative analysis of a national collaborative. Acad Pediatr. 2017;17(2):198-204. https://doi.org/10.1016/j.acap.2016.07.001.
34. Parikh K, Biondi E, Nazif J, et al. A multicenter collaborative to improve care of community acquired pneumonia in hospitalized children. Pediatrics. 2017;139(2). https://doi.org/10.1542/peds.2016-1411.
35. Ralston S, Garber M, Narang S, et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8(1):25-30. https://doi.org/10.1002/jhm.1982.
36. Gupta N CA, Cabana MD, Jennings B, Parikh K, Kaiser SV. PIPA (Pathways for Improving Pediatric Asthma Care): Process Evaluation of a National Collaborative to Implement Pathways. Platform presented at Pediatric Academic Society National Meeting. Baltimore, Maryland; 2019.
Despite the widespread availability of evidence-based guidelines,1 there is inappropriate variation in the care and outcomes for children with asthma in both the emergency department (ED) and the inpatient setting.2-6 Operational versions of evidence-based guidelines known as “pathways” have been shown to improve adoption of evidence-based guidelines, quality of care, and health outcomes for children with asthma.7-14 However, little is known about how to successfully implement pathways outside of free-standing children’s hospitals.15-19
The majority of children with asthma in the United States are cared for in community hospitals, which provide services for both adults and children.20 However, prior studies of pediatric asthma pathways have largely excluded community hospitals. These studies primarily focused on determining clinical effectiveness, rather than detailing the implementation process. These approaches have left critical gaps that hinder our ability to implement pathways and improve care in community hospitals, which have unique barriers and less resources.21,22 Therefore, understanding the process of pathway implementation in community hospitals is critical to improving care for children.22 Our objective was to identify the key determinants of successful pediatric asthma pathway implementation using a national sample of community hospitals. This knowledge can guide hospital leaders and healthcare providers in efforts to improve pediatric care and outcomes in these settings.
METHODS
Study Setting, Design, and Population
In Fall 2017, the Value in Inpatient Pediatrics (VIP) network launched PIPA, Pathways to Improving Pediatric Asthma care.23 The VIP network, a part of the American Academy of Pediatrics (AAP), aims to improve the value of care delivered to any pediatric patient in a hospital bed, from rural to free-standing children’s hospitals.24 PIPA used a learning collaborative model25 and recruited local project leaders (physicians, nurses, respiratory therapists (RT), and pharmacists) from 89 hospitals around the country. PIPA provided these hospital teams with asthma pathways and several resources for implementation support, including educational meetings, quality improvement (QI) training, audit and feedback, and facilitation. Facilitation is a process of interactive problem-solving and support that occurs in the context of a supportive interpersonal relationship and a recognized need for improvement.26 A facilitator, or a “coach”, is an external expert who provides project mentorship and assists the process of making meaningful changes to improve patient care.26 Facilitation was provided by external consultants with QI expertise.
For this qualitative study, facilitators conducted semi-structured interviews with a convenience sample of project leaders from community hospitals participating in PIPA, with some interviews including multiple project leaders (eg, nursing, inpatient, and Emergency Department [ED] leaders). Verbal consent was obtained from all participants. No incentives were provided. This study was approved by the AAP institutional review board.
Data Collection
We used the constructs described in the Consolidated Framework for Implementation Research (CFIR)27 and adapted those salient to pediatric asthma pathways to develop an interview guide that was used with all participants (Appendix 1). The CFIR offers an overarching typology to understand what works where and why across five major domains that influence implementation: intervention characteristics, inner setting (hospital), outer setting (economic, political, and social context of the hospital), characteristics of the individuals involved, and the process of implementation. Data were collected across these domains to inform our analysis of the key determinants of pediatric asthma pathway implementation in community hospitals.
Interviews were conducted by phone from December 2017 to April 2018 (first four months of pathway implementation). Interviews lasted 30-60 minutes and were recorded and transcribed verbatim. Transcripts were edited for accuracy using the audio recordings. As data collection occurred concurrently with analysis, the interview guide was iteratively revised to reflect new insights and patterns that emerged from our analysis. All sites were anonymized in the data analysis. New interviews were coded until thematic saturation was reached.
Analysis
We conducted an inductive thematic analysis using the CFIR as our conceptual framework.28,29 Four investigators (CM, MJ, ES, and SK) performed the initial open coding of the data. Investigators met twice during the open coding process to develop and then finalize a codebook of standard definitions for codes. This codebook facilitated coding consistency through the remainder of the analytic process. Two investigators (CM and MJ) then independently read and coded all data to ensure intercoder reliability. During this process, CM and MJ met every two weeks to compare coding consistency, resolve discrepancies, and discuss preliminary findings. When the coding was complete, all investigators met to explore and develop themes that encompassed related common codes.
The CFIR was used at two stages of the study: (1) developing the interview guide and (2) cross-checking for any potentially important codes that were missing/needed to be explored further. Thus, the investigators maintained an inductive approach grounded in the data. To assure study rigor, we employed investigator triangulation (use of multiple investigators and participants from multiple clinical roles) and reflexivity (ongoing critique and critical reflection of the individual biases of the investigators).30 Coding was performed using Dedoose (version 7.0.23; Los Angeles, California).
RESULTS
A total of 34 community hospitals completed the PIPA project, of which the project leaders of 25 hospitals connected with the facilitators and were approached to participate; 18 (72%) hospitals’ project leaders participated in the study. We analyzed 18 interviews conducted between facilitators and project leaders, which included a total of 32 project leaders (one to five leaders per interview). The hospitals represented were diverse in geographic location and size (range 4-50 pediatric beds per hospital), and the majority of sites (78%) supported the trainees (Table 1).
We identified four overarching themes that described the key determinants of pathway implementation in community hospitals. These themes are presented in order of their frequency of occurrence in the data. They included (1) building an implementation infrastructure, (2) engaging and motivating providers, (3) addressing organizational and resource limitations, and (4) devising implementation solutions with facilitators. Descriptions and exemplary quotations for each theme are provided in Table 2 and Appendix Figure 1.
Building an Implementation Infrastructure
“So, I’m going to sit down with the primary nursing staff and the other four physicians in the group to go over the expectations…We’re not going to have the actual EMR [changes] and we’re not going to have the nursing documentation field built right away but [we want to] make sure that people are documenting the respiratory score in their generic nursing note so that the information is easily accessible.” (Physician leader, Hospital G)
Participants also described the need to deliver education on the evidence supporting changes in practice and skills training specific to pediatric asthma care:
“Once we realized that we were going to be doing this pathway, we started training our nurses on the inpatient side on [pediatric respiratory scoring].” (Nursing leader, Hospital P)
In addition, pathway implementation required modification of clinical workflows via changes to hospital policies or guidelines, electronic medical records (EMR), and/or the physical environment (eg, placing supplies in proximity to care delivery):
“I think it can help if we could get an order set or a nursing protocol where asthmatics over a certain severity can just get steroids in triage.” (Physician leader, Hospital A)
Engaging and Motivating Providers
Another crucial step in pathway implementation was engaging and motivating providers. This included overcoming inertia to practice change, facilitating multidisciplinary collaboration, and handling conflicts regarding practice changes. Participants discussed the excitement of participating in a national collaborative as especially motivating to help drive engagement and overcome barriers to change, particularly the ability to compare local hospital performance to national peers.
“I think everyone is a little competitive. So I think that when we see how we compare to other institutions—both our group and the ER—I think it also adds a little oomph…I think for our nurses too; we’re able to say, ‘[look how we compare to] most of the other hospitals.’ I think that helps.” (Physician leader, Hospital B)
Multidisciplinary collaboration across a wide variety of frontline pediatric and nonpediatric providers was key to understanding current workflows and identifying needed modifications for pathway implementation:
“I do think clearly our biggest obstacles are the fact that we have adult ED providers. We have the opportunity on the inpatient side [with nursing and respiratory therapy], who really do awesome with pediatric changes, to take our wins where we can and make the changes with the ED. In the ED we have an RN educator. She’s very on board with doing the respiratory scoring and getting this whole thing started.” (Physician leader, Hospital L)
Intentional communication and leadership skills also played key roles in engaging hesitant providers and handling conflict:
“Just sitting and talking with our respiratory therapist about the ability to provide this type of service or support and seeing what their reservations have been, at least it’s open to conversation so that we could provide these types of therapies in the future and we’re able to see like what people’s concerns are. I think just basically increasing familiarity with not only these processes, but different types of therapy will hopefully in the future help us provide better care to our patients.” (Physician leader, Hospital Q)
Addressing Organizational and Resource Limitations
Participants recognized organizational and resource limitations, some of which may be unique to community hospitals that prioritize resources for adult care. The limitations described included EMR staff support, healthcare provider staffing/capacity, navigating IRBs, and addressing administrative processes. Competing demands for information technology staff support and lack of prioritization of pediatric-specific initiatives often hindered efforts to modify the EMR.
“Resource wise, we are hoping to implement an order set in our Epic EMR, [but] finding the availability from the Epic team may be a challenge.” (Physician Leader, Hospital A)
Participants also reported that limited staff capacity (eg, nursing, RT) hindered pathway implementation efforts. This limited capacity hindered workflow changes and limited the time available for education and training on pathways:
“[Respiratory scoring for asthma is] an added responsibility for the [nursing] staff and we don’t have patient technicians. So they’re doing everything from changing the sheets to bringing water to all of the medical patients. So, that I think may be a barrier.” (Physician leader, Hospital B)
Across sites, navigating the IRB posed various challenges. Some sites were required to obtain approval from regional IRBs, which did not have resources to devote to pediatric projects. Other sites did not have IRBs at all, but instead required separate approvals for the project from hospital leadership or other entities:
“On the IRB, I contacted the manager of the IRB and she’s said, ‘No, it’s not an IRB project,’ but she sent it to another director for review, and it took forever to be able to get a data agreement with [the local university hospital] so that we can pull the data. I just couldn’t believe it took months to get done.” (Physician Leader, Hospital K)
Finally, administrative barriers such as addressing formulary changes in the context of adult-focused settings were challenging. For example, at one hospital, metered dose inhalers (MDIs) were not used for adult patients, and the hospital administration was resistant to incorporate their use into practice for pediatric patients due to the cost of such changes.
“The [general hospital] didn’t have MDI’s anymore because of cost reasons, and when we started the pediatric work, we really made it a point to get the MDI’s for pediatric patients back in the formulary.” (Physician leader, Hospital A)
Devising Implementation Solutions with Practice Facilitators
Participants often devised pathway implementation solutions with facilitators in-the-moment during meetings. This problem-solving included figuring out work-arounds, proactive coaching by external facilitators, and just-in-time solution building. Furthermore, in meetings that included more than one project leader, leaders would often work with each other to devise solutions. Meetings provided forums that stimulated identification of implementation barriers, brainstorming, and subsequently solution building.
Physician leader: I’m wondering if we could, as an interim solution, try out an algorithm on paper, I don’t know if that’s allowed, until we get Epic approval. Do you know?
Nurse Leader: You mean having an algorithm posted in triage? Yeah, I don’t see why not. (Hospital A)
Next, problem solving was often driven by the facilitator’s experience and knowledge, drawn from their interactions with other collaborative sites or their own prior experiences with asthma, QI, or pathway implementation. The facilitators brought an outside perspective, not bound by that particular hospital’s local culture or structural intricacies. This proactive coaching spurred the identification of creative, yet practical solutions:
Project Leader: We’re still trying to get all our templates [for the EMR]…because [currently they are] all adult templates.
Facilitator: If you’re making templates right now, could you also add the three asterisks? Like smoking or exposure to second hand tobacco smoke or marijuana…then have the three asterisks there and then “Referral made?***”. That would force people to document in a certain place in the template as well.Project Leader: That’s definitely something we could add right now. (Hospital O)
Check-in meetings with facilitators offered an opportunity to trouble shoot, brainstorm work-arounds, devise in-the-moment site-specific solutions to enable successful pathway implementation, and provide ongoing support throughout implementation.
DISCUSSION
Pathways can improve the quality of care for children with asthma.31 However, there is little evidence-based guidance on how to implement pathways and improve pediatric care in community hospitals,17-20 where the majority of children are cared for nationally. This is the first study to our knowledge that details the key determinants of pediatric asthma pathway implementation in community hospital settings. We identified four key determinants of implementation that can help guide others in similar settings. These include building an implementation infrastructure, engaging and motivating multidisciplinary providers, addressing organizational and resource limitations, and using external facilitators to devise implementation solutions.
Existing frameworks such as the CFIR outline the potential determinants of implementation success but do not provide population- or setting-specific guidance.27 There have been prior studies detailing pathway implementation for pediatric populations, but these studies did not focus on community hospitals.32,33 Our findings align with these prior studies, which highlight the importance of identifying implementation champions, engaging and motivating multidisciplinary providers, establishing a QI infrastructure, and addressing organizational and resource limitations, such as EMR integration.32,33 However, our study provides unique insights into issues that are important to successful pathway implementation in community hospitals, including engagement of adult-focused healthcare providers, reprioritization of resources toward the care of children, and the potentially critical role of external facilitators.
Our findings indicate that community hospitals seeking to improve care for children may particularly benefit from using external facilitators and/or partnering with external organizations. We found that external facilitators played a significant and proactive role in community hospitals’ efforts to improve care for children. Facilitators helped devise work-arounds and engaged in just-in-time solution building with local project leaders. For instance, facilitators helped develop strategies for training healthcare providers in performing new clinical tasks, building reminders of pathway recommendations into clinical workflows, and overcoming resource barriers. Thus, community hospitals may uniquely benefit from participation in national learning collaboratives, which often provide avenues for external facilitation.25,34,35 National networks, such as the VIP network, lead national learning collaboratives that provide external facilitation as well as other resources (eg, educational materials, data analysis support) to community hospitals seeking to improve pediatric care.24 Previous work by McDaniel et al. identified that intentional partnerships between children’s and community hospitals can also potentially provide access to resources for education and training in pediatric care and support in navigating organizational and resource challenges.22
Our results characterize the key determinants of pediatric asthma pathway implementation using a national sample of community hospitals that were diverse in geography, size, and structure. This imparts greater transferability of our findings. We also used strategies to promote the rigor of our findings, including triangulation and reflexivity. However, our study has several limitations. First, we analyzed only the meetings that occurred during the early months of pathway implementation. As such, we did not capture any key determinants that may have arisen later in implementation. However, process analyses of implementation indicate that the majority of implementation efforts occurred within these first three to four months.36 Second, we did not elicit input from hospital administration or leadership. The lack of administrative/leadership input probably affected the CFIR themes we found, as no themes from the outer setting were elicited. However, the goal of our study was to characterize the experiences of those leading implementation efforts, and focusing on these leaders allows our work to better guide those doing similar work in the future. Third, we used CFIR to guide the development of our interview guide and as a reference during analysis, which may have skewed our findings to preferentially reflect CFIR constructs. However, our overall analysis was grounded in the primary data and we employed reflexivity during all stages of our analysis. In addition, having the facilitators conduct the qualitative interviews may have biased our findings toward the perspectives of the facilitators; however, the facilitators represented quite diverse clinical and QI backgrounds. Finally, our findings do not necessarily correlate with improvements in clinical outcomes. As such, they are not meant to serve as explicit recommendations for improving patient outcomes, but rather as a characterization of the context, processes, and experiences of implementing pathways in the community setting to inform others doing this important work.
CONCLUSIONS
We identified the key determinants of pediatric asthma pathway implementation in community hospitals, which may help inform QI efforts in these settings. We also identified organizational and resource limitations that are probably unique to these adult-focused hospitals. Participating in national learning collaboratives and/or working with facilitators may support pathway implementation and improved quality of care for children with asthma in community hospitals.
Future work should seek to correlate these and other determinants of pathway implementation with health outcomes for hospitalized children, as well as integrate broader and more diverse samples of community hospitals.
Despite the widespread availability of evidence-based guidelines,1 there is inappropriate variation in the care and outcomes for children with asthma in both the emergency department (ED) and the inpatient setting.2-6 Operational versions of evidence-based guidelines known as “pathways” have been shown to improve adoption of evidence-based guidelines, quality of care, and health outcomes for children with asthma.7-14 However, little is known about how to successfully implement pathways outside of free-standing children’s hospitals.15-19
The majority of children with asthma in the United States are cared for in community hospitals, which provide services for both adults and children.20 However, prior studies of pediatric asthma pathways have largely excluded community hospitals. These studies primarily focused on determining clinical effectiveness, rather than detailing the implementation process. These approaches have left critical gaps that hinder our ability to implement pathways and improve care in community hospitals, which have unique barriers and less resources.21,22 Therefore, understanding the process of pathway implementation in community hospitals is critical to improving care for children.22 Our objective was to identify the key determinants of successful pediatric asthma pathway implementation using a national sample of community hospitals. This knowledge can guide hospital leaders and healthcare providers in efforts to improve pediatric care and outcomes in these settings.
METHODS
Study Setting, Design, and Population
In Fall 2017, the Value in Inpatient Pediatrics (VIP) network launched PIPA, Pathways to Improving Pediatric Asthma care.23 The VIP network, a part of the American Academy of Pediatrics (AAP), aims to improve the value of care delivered to any pediatric patient in a hospital bed, from rural to free-standing children’s hospitals.24 PIPA used a learning collaborative model25 and recruited local project leaders (physicians, nurses, respiratory therapists (RT), and pharmacists) from 89 hospitals around the country. PIPA provided these hospital teams with asthma pathways and several resources for implementation support, including educational meetings, quality improvement (QI) training, audit and feedback, and facilitation. Facilitation is a process of interactive problem-solving and support that occurs in the context of a supportive interpersonal relationship and a recognized need for improvement.26 A facilitator, or a “coach”, is an external expert who provides project mentorship and assists the process of making meaningful changes to improve patient care.26 Facilitation was provided by external consultants with QI expertise.
For this qualitative study, facilitators conducted semi-structured interviews with a convenience sample of project leaders from community hospitals participating in PIPA, with some interviews including multiple project leaders (eg, nursing, inpatient, and Emergency Department [ED] leaders). Verbal consent was obtained from all participants. No incentives were provided. This study was approved by the AAP institutional review board.
Data Collection
We used the constructs described in the Consolidated Framework for Implementation Research (CFIR)27 and adapted those salient to pediatric asthma pathways to develop an interview guide that was used with all participants (Appendix 1). The CFIR offers an overarching typology to understand what works where and why across five major domains that influence implementation: intervention characteristics, inner setting (hospital), outer setting (economic, political, and social context of the hospital), characteristics of the individuals involved, and the process of implementation. Data were collected across these domains to inform our analysis of the key determinants of pediatric asthma pathway implementation in community hospitals.
Interviews were conducted by phone from December 2017 to April 2018 (first four months of pathway implementation). Interviews lasted 30-60 minutes and were recorded and transcribed verbatim. Transcripts were edited for accuracy using the audio recordings. As data collection occurred concurrently with analysis, the interview guide was iteratively revised to reflect new insights and patterns that emerged from our analysis. All sites were anonymized in the data analysis. New interviews were coded until thematic saturation was reached.
Analysis
We conducted an inductive thematic analysis using the CFIR as our conceptual framework.28,29 Four investigators (CM, MJ, ES, and SK) performed the initial open coding of the data. Investigators met twice during the open coding process to develop and then finalize a codebook of standard definitions for codes. This codebook facilitated coding consistency through the remainder of the analytic process. Two investigators (CM and MJ) then independently read and coded all data to ensure intercoder reliability. During this process, CM and MJ met every two weeks to compare coding consistency, resolve discrepancies, and discuss preliminary findings. When the coding was complete, all investigators met to explore and develop themes that encompassed related common codes.
The CFIR was used at two stages of the study: (1) developing the interview guide and (2) cross-checking for any potentially important codes that were missing/needed to be explored further. Thus, the investigators maintained an inductive approach grounded in the data. To assure study rigor, we employed investigator triangulation (use of multiple investigators and participants from multiple clinical roles) and reflexivity (ongoing critique and critical reflection of the individual biases of the investigators).30 Coding was performed using Dedoose (version 7.0.23; Los Angeles, California).
RESULTS
A total of 34 community hospitals completed the PIPA project, of which the project leaders of 25 hospitals connected with the facilitators and were approached to participate; 18 (72%) hospitals’ project leaders participated in the study. We analyzed 18 interviews conducted between facilitators and project leaders, which included a total of 32 project leaders (one to five leaders per interview). The hospitals represented were diverse in geographic location and size (range 4-50 pediatric beds per hospital), and the majority of sites (78%) supported the trainees (Table 1).
We identified four overarching themes that described the key determinants of pathway implementation in community hospitals. These themes are presented in order of their frequency of occurrence in the data. They included (1) building an implementation infrastructure, (2) engaging and motivating providers, (3) addressing organizational and resource limitations, and (4) devising implementation solutions with facilitators. Descriptions and exemplary quotations for each theme are provided in Table 2 and Appendix Figure 1.
Building an Implementation Infrastructure
“So, I’m going to sit down with the primary nursing staff and the other four physicians in the group to go over the expectations…We’re not going to have the actual EMR [changes] and we’re not going to have the nursing documentation field built right away but [we want to] make sure that people are documenting the respiratory score in their generic nursing note so that the information is easily accessible.” (Physician leader, Hospital G)
Participants also described the need to deliver education on the evidence supporting changes in practice and skills training specific to pediatric asthma care:
“Once we realized that we were going to be doing this pathway, we started training our nurses on the inpatient side on [pediatric respiratory scoring].” (Nursing leader, Hospital P)
In addition, pathway implementation required modification of clinical workflows via changes to hospital policies or guidelines, electronic medical records (EMR), and/or the physical environment (eg, placing supplies in proximity to care delivery):
“I think it can help if we could get an order set or a nursing protocol where asthmatics over a certain severity can just get steroids in triage.” (Physician leader, Hospital A)
Engaging and Motivating Providers
Another crucial step in pathway implementation was engaging and motivating providers. This included overcoming inertia to practice change, facilitating multidisciplinary collaboration, and handling conflicts regarding practice changes. Participants discussed the excitement of participating in a national collaborative as especially motivating to help drive engagement and overcome barriers to change, particularly the ability to compare local hospital performance to national peers.
“I think everyone is a little competitive. So I think that when we see how we compare to other institutions—both our group and the ER—I think it also adds a little oomph…I think for our nurses too; we’re able to say, ‘[look how we compare to] most of the other hospitals.’ I think that helps.” (Physician leader, Hospital B)
Multidisciplinary collaboration across a wide variety of frontline pediatric and nonpediatric providers was key to understanding current workflows and identifying needed modifications for pathway implementation:
“I do think clearly our biggest obstacles are the fact that we have adult ED providers. We have the opportunity on the inpatient side [with nursing and respiratory therapy], who really do awesome with pediatric changes, to take our wins where we can and make the changes with the ED. In the ED we have an RN educator. She’s very on board with doing the respiratory scoring and getting this whole thing started.” (Physician leader, Hospital L)
Intentional communication and leadership skills also played key roles in engaging hesitant providers and handling conflict:
“Just sitting and talking with our respiratory therapist about the ability to provide this type of service or support and seeing what their reservations have been, at least it’s open to conversation so that we could provide these types of therapies in the future and we’re able to see like what people’s concerns are. I think just basically increasing familiarity with not only these processes, but different types of therapy will hopefully in the future help us provide better care to our patients.” (Physician leader, Hospital Q)
Addressing Organizational and Resource Limitations
Participants recognized organizational and resource limitations, some of which may be unique to community hospitals that prioritize resources for adult care. The limitations described included EMR staff support, healthcare provider staffing/capacity, navigating IRBs, and addressing administrative processes. Competing demands for information technology staff support and lack of prioritization of pediatric-specific initiatives often hindered efforts to modify the EMR.
“Resource wise, we are hoping to implement an order set in our Epic EMR, [but] finding the availability from the Epic team may be a challenge.” (Physician Leader, Hospital A)
Participants also reported that limited staff capacity (eg, nursing, RT) hindered pathway implementation efforts. This limited capacity hindered workflow changes and limited the time available for education and training on pathways:
“[Respiratory scoring for asthma is] an added responsibility for the [nursing] staff and we don’t have patient technicians. So they’re doing everything from changing the sheets to bringing water to all of the medical patients. So, that I think may be a barrier.” (Physician leader, Hospital B)
Across sites, navigating the IRB posed various challenges. Some sites were required to obtain approval from regional IRBs, which did not have resources to devote to pediatric projects. Other sites did not have IRBs at all, but instead required separate approvals for the project from hospital leadership or other entities:
“On the IRB, I contacted the manager of the IRB and she’s said, ‘No, it’s not an IRB project,’ but she sent it to another director for review, and it took forever to be able to get a data agreement with [the local university hospital] so that we can pull the data. I just couldn’t believe it took months to get done.” (Physician Leader, Hospital K)
Finally, administrative barriers such as addressing formulary changes in the context of adult-focused settings were challenging. For example, at one hospital, metered dose inhalers (MDIs) were not used for adult patients, and the hospital administration was resistant to incorporate their use into practice for pediatric patients due to the cost of such changes.
“The [general hospital] didn’t have MDI’s anymore because of cost reasons, and when we started the pediatric work, we really made it a point to get the MDI’s for pediatric patients back in the formulary.” (Physician leader, Hospital A)
Devising Implementation Solutions with Practice Facilitators
Participants often devised pathway implementation solutions with facilitators in-the-moment during meetings. This problem-solving included figuring out work-arounds, proactive coaching by external facilitators, and just-in-time solution building. Furthermore, in meetings that included more than one project leader, leaders would often work with each other to devise solutions. Meetings provided forums that stimulated identification of implementation barriers, brainstorming, and subsequently solution building.
Physician leader: I’m wondering if we could, as an interim solution, try out an algorithm on paper, I don’t know if that’s allowed, until we get Epic approval. Do you know?
Nurse Leader: You mean having an algorithm posted in triage? Yeah, I don’t see why not. (Hospital A)
Next, problem solving was often driven by the facilitator’s experience and knowledge, drawn from their interactions with other collaborative sites or their own prior experiences with asthma, QI, or pathway implementation. The facilitators brought an outside perspective, not bound by that particular hospital’s local culture or structural intricacies. This proactive coaching spurred the identification of creative, yet practical solutions:
Project Leader: We’re still trying to get all our templates [for the EMR]…because [currently they are] all adult templates.
Facilitator: If you’re making templates right now, could you also add the three asterisks? Like smoking or exposure to second hand tobacco smoke or marijuana…then have the three asterisks there and then “Referral made?***”. That would force people to document in a certain place in the template as well.Project Leader: That’s definitely something we could add right now. (Hospital O)
Check-in meetings with facilitators offered an opportunity to trouble shoot, brainstorm work-arounds, devise in-the-moment site-specific solutions to enable successful pathway implementation, and provide ongoing support throughout implementation.
DISCUSSION
Pathways can improve the quality of care for children with asthma.31 However, there is little evidence-based guidance on how to implement pathways and improve pediatric care in community hospitals,17-20 where the majority of children are cared for nationally. This is the first study to our knowledge that details the key determinants of pediatric asthma pathway implementation in community hospital settings. We identified four key determinants of implementation that can help guide others in similar settings. These include building an implementation infrastructure, engaging and motivating multidisciplinary providers, addressing organizational and resource limitations, and using external facilitators to devise implementation solutions.
Existing frameworks such as the CFIR outline the potential determinants of implementation success but do not provide population- or setting-specific guidance.27 There have been prior studies detailing pathway implementation for pediatric populations, but these studies did not focus on community hospitals.32,33 Our findings align with these prior studies, which highlight the importance of identifying implementation champions, engaging and motivating multidisciplinary providers, establishing a QI infrastructure, and addressing organizational and resource limitations, such as EMR integration.32,33 However, our study provides unique insights into issues that are important to successful pathway implementation in community hospitals, including engagement of adult-focused healthcare providers, reprioritization of resources toward the care of children, and the potentially critical role of external facilitators.
Our findings indicate that community hospitals seeking to improve care for children may particularly benefit from using external facilitators and/or partnering with external organizations. We found that external facilitators played a significant and proactive role in community hospitals’ efforts to improve care for children. Facilitators helped devise work-arounds and engaged in just-in-time solution building with local project leaders. For instance, facilitators helped develop strategies for training healthcare providers in performing new clinical tasks, building reminders of pathway recommendations into clinical workflows, and overcoming resource barriers. Thus, community hospitals may uniquely benefit from participation in national learning collaboratives, which often provide avenues for external facilitation.25,34,35 National networks, such as the VIP network, lead national learning collaboratives that provide external facilitation as well as other resources (eg, educational materials, data analysis support) to community hospitals seeking to improve pediatric care.24 Previous work by McDaniel et al. identified that intentional partnerships between children’s and community hospitals can also potentially provide access to resources for education and training in pediatric care and support in navigating organizational and resource challenges.22
Our results characterize the key determinants of pediatric asthma pathway implementation using a national sample of community hospitals that were diverse in geography, size, and structure. This imparts greater transferability of our findings. We also used strategies to promote the rigor of our findings, including triangulation and reflexivity. However, our study has several limitations. First, we analyzed only the meetings that occurred during the early months of pathway implementation. As such, we did not capture any key determinants that may have arisen later in implementation. However, process analyses of implementation indicate that the majority of implementation efforts occurred within these first three to four months.36 Second, we did not elicit input from hospital administration or leadership. The lack of administrative/leadership input probably affected the CFIR themes we found, as no themes from the outer setting were elicited. However, the goal of our study was to characterize the experiences of those leading implementation efforts, and focusing on these leaders allows our work to better guide those doing similar work in the future. Third, we used CFIR to guide the development of our interview guide and as a reference during analysis, which may have skewed our findings to preferentially reflect CFIR constructs. However, our overall analysis was grounded in the primary data and we employed reflexivity during all stages of our analysis. In addition, having the facilitators conduct the qualitative interviews may have biased our findings toward the perspectives of the facilitators; however, the facilitators represented quite diverse clinical and QI backgrounds. Finally, our findings do not necessarily correlate with improvements in clinical outcomes. As such, they are not meant to serve as explicit recommendations for improving patient outcomes, but rather as a characterization of the context, processes, and experiences of implementing pathways in the community setting to inform others doing this important work.
CONCLUSIONS
We identified the key determinants of pediatric asthma pathway implementation in community hospitals, which may help inform QI efforts in these settings. We also identified organizational and resource limitations that are probably unique to these adult-focused hospitals. Participating in national learning collaboratives and/or working with facilitators may support pathway implementation and improved quality of care for children with asthma in community hospitals.
Future work should seek to correlate these and other determinants of pathway implementation with health outcomes for hospitalized children, as well as integrate broader and more diverse samples of community hospitals.
1. National Asthma E, Prevention P. Expert Panel Report 3 (EPR-3): Guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(5):S94-S138. https://doi.org/10.1016/j.jaci.2007.09.043.
2. Bekmezian A, Hersh AL, Maselli JH, Cabana MD. Pediatric emergency departments are more likely than general emergency departments to treat asthma exacerbation with systemic corticosteroids. J Asthma. 2011;48(1):69-74. https://doi.org/10.3109/02770903.2010.535884.
3. Biagini Myers JM, Simmons JM, Kercsmar CM, et al. Heterogeneity in asthma care in a statewide collaborative: the Ohio Pediatric Asthma Repository. Pediatrics. 2015;135(2):271-279. https://doi.org/10.1542/peds.2014-2230.
4. Kharbanda AB, Hall M, Shah SS, et al. Variation in resource utilization across a national sample of pediatric emergency departments. J Pediatr. 2013;163(1):230-236. https://doi.org/10.1016/j.jpeds.2012.12.013.
5. O’Con
6. Lougheed MD, Garvey N, Chapman KR, et al. Variations and gaps in management of acute asthma in Ontario emergency departments. Chest. 2009;135(3):724-736. https://doi.org/10.1378/chest.08-0371.
7. Bekmezian A, Fee C, Weber E. Clinical pathway improves pediatrics asthma management in the emergency department and reduces admissions. J Asthma. 2015;52(8):806-814. https://doi.org/10.3109/02770903.2015.1019086.
8. Chen KH, Chen CC, Liu HE, Tzeng PC, Glasziou PP. Effectiveness of paediatric asthma clinical pathways: a narrative systematic review. J Asthma. 2014;51(5):480-492. https://doi.org/10.3109/02770903.2014.887728.
9. Johnson KB, Blaisdell CJ, Walker A, Eggleston P. Effectiveness of a clinical pathway for inpatient asthma management. Pediatrics. 2000;106(5):1006-1012. https://doi.org/10.1542/peds.106.5.1006.
10. Kelly CS, Andersen CL, Pestian JP, et al. Improved outcomes for hospitalized asthmatic children using a clinical pathway. Ann Allergy Asthma Immunol. 2000;84(5):509-516. https://doi.org/10.1016/S1081-1206(10)62514-8.
11. McDowell KM, Chatburn RL, Myers TR, O’Riordan MA, Kercsmar CM. A cost-saving algorithm for children hospitalized for status asthmaticus. Arch Pediatr Adolesc Med. 1998;152(10):977-984. https://doi.org/10.1001/archpedi.152.10.977.
12. Miller AG, Breslin ME, Pineda LC, Fox JW. An asthma protocol improved adherence to evidence-based guidelines for pediatric subjects with status asthmaticus in the emergency department. Respir Care. 2015;60(12):1759-1764. https://doi.org/10.4187/respcare.04011.
13. Nkoy F, Fassl B, Stone B, et al. Improving pediatric asthma care and outcomes across multiple hospitals. Pediatrics. 2015;136(6):e1602-e1610. https://doi.org/10.1542/peds.2015-0285.
14. Rutman L, Atkins RC, Migita R, et al. Modification of an established pediatric asthma pathway improves evidence-based, efficient care. Pediatrics. 2016;138(6). https://doi.org/10.1542/peds.2016-1248.
15. Glauber JH, Farber HJ, Homer CJ. Asthma clinical pathways: toward what end? Pediatrics. 2001;107(3):590-592. https://doi.org/10.1542/peds.107.3.590.
16. Grimshaw J, Eccles M, Thomas R, et al. Toward evidence-based quality improvement. Evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966-1998. J Gen Intern Med. 2006;21(2):S14-S20. https://doi.org/10.1111/j.1525-1497.2006.00357.x.
17. Scott SD, Grimshaw J, Klassen TP, Nettel-Aguirre A, Johnson DW. Understanding implementation processes of clinical pathways and clinical practice guidelines in pediatric contexts: a study protocol. Implement Sci. 2011;6:133. https://doi.org/10.1186/1748-5908-6-133.
18. Walls TA, Hughes NT, Mullan PC, Chamberlain JM, Brown K. Improving pediatric asthma outcomes in a community emergency department. Pediatrics. 2017;139(1). https://doi.org/10.1542/peds.2016-0088.
19. Kaiser SV, Lam R, Cabana MD, et al. Best practices in implementing inpatient pediatric asthma pathways: a qualitative study. J Asthma. 2019:1-11. https://doi.org/10.1080/02770903.2019.1606237.
20. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624.
21. Franca UL, McManus ML. Availability of definitive hospital care for children. JAMA Pediatr. 2017;171(9):e171096. https://doi.org/10.1001/jamapediatrics.2017.1096.
22. McDaniel CE, Jennings R, Schroeder AR, Paciorkowski N, Hofmann M, Leyenaar J. Aligning inpatient pediatric research with settings of care: a call to action. Pediatrics. 2019;143(5). https://doi.org/10.1542/peds.2018-2648.
23. Kaiser SV JB. Value in inpatient pediatrics network launches National Asthma Project. In: AAP Quality Connections 2018; 26:8-9. Retrieved from: https://www.aap.org/en-us/Documents/coqips_newsletter_2018_winter_26.pdf
24. Value in Inpatient Pediatrics. https://www.aap.org/en-us/professional-resources/quality-improvement/Pages/Value-in-Inpatient-Pediatrics.aspx. Accessed December 1, 2017.
25. The Breakthrough Series: IHI’s Collaborative Model for Achieving Breakthrough Improvement. IHI Innovation Series white paper. Boston: Institute for Healthcare Improvement; 2003. Retrieved from: www.IHI.org
26. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21. https://doi.org/10.1186/s13012-015-0209-1.
27. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. https://doi.org/10.1186/1748-5908-4-50.
28. Braun VaC, V. Thematic analysis. In: H. Cooper PC, Long DL, Panter AT, Rindskopf E, Sher KJ, eds. APA handbook of research methods in psychology, Vol 2. Research designs: Quantitative, qualitative, neuropsychologial, and biological. Washington, DC, US: American Psychological Association; 2012. https://doi.org/10.1037/13620-000.
29. Charmaz K. Grounded Theory. 2nd ed. Thousand Oaks, CA: SAGE Publications; 2014.
30. Creswell JW, Poth CNCN CJaP. Qualitative Inquiry and Research Design: Choosing Among Five Approaches. Thousand Oaks, CA: Sage; 2017.
31. Kaiser SV, Rodean J, Bekmezian A, et al. Effectiveness of pediatric asthma pathways for hospitalized children: a multicenter, national analysis. J. Pediatr. 2018;197:165-171. https://doi.org/10.1016/j.jpeds.2018.01.084.
32. Leyenaar JK, Andrews CB, Tyksinski ER, Biondi E, Parikh K, Ralston S. Facilitators of interdepartmental quality improvement: a mixed-methods analysis of a collaborative to improve pediatric community-acquired pneumonia management. BMJ Qual Saf. 2019;28(3):215-222. https://doi.org/10.1136/bmjqs-2018-008065. 33. Ralston SL, Atwood EC, Garber MD, Holmes AV. What works to reduce unnecessary care for bronchiolitis? A qualitative analysis of a national collaborative. Acad Pediatr. 2017;17(2):198-204. https://doi.org/10.1016/j.acap.2016.07.001.
33. Ralston SL, Atwood EC, Garber MD, Holmes AV. What works to reduce unnecessary care for bronchiolitis? A qualitative analysis of a national collaborative. Acad Pediatr. 2017;17(2):198-204. https://doi.org/10.1016/j.acap.2016.07.001.
34. Parikh K, Biondi E, Nazif J, et al. A multicenter collaborative to improve care of community acquired pneumonia in hospitalized children. Pediatrics. 2017;139(2). https://doi.org/10.1542/peds.2016-1411.
35. Ralston S, Garber M, Narang S, et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8(1):25-30. https://doi.org/10.1002/jhm.1982.
36. Gupta N CA, Cabana MD, Jennings B, Parikh K, Kaiser SV. PIPA (Pathways for Improving Pediatric Asthma Care): Process Evaluation of a National Collaborative to Implement Pathways. Platform presented at Pediatric Academic Society National Meeting. Baltimore, Maryland; 2019.
1. National Asthma E, Prevention P. Expert Panel Report 3 (EPR-3): Guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(5):S94-S138. https://doi.org/10.1016/j.jaci.2007.09.043.
2. Bekmezian A, Hersh AL, Maselli JH, Cabana MD. Pediatric emergency departments are more likely than general emergency departments to treat asthma exacerbation with systemic corticosteroids. J Asthma. 2011;48(1):69-74. https://doi.org/10.3109/02770903.2010.535884.
3. Biagini Myers JM, Simmons JM, Kercsmar CM, et al. Heterogeneity in asthma care in a statewide collaborative: the Ohio Pediatric Asthma Repository. Pediatrics. 2015;135(2):271-279. https://doi.org/10.1542/peds.2014-2230.
4. Kharbanda AB, Hall M, Shah SS, et al. Variation in resource utilization across a national sample of pediatric emergency departments. J Pediatr. 2013;163(1):230-236. https://doi.org/10.1016/j.jpeds.2012.12.013.
5. O’Con
6. Lougheed MD, Garvey N, Chapman KR, et al. Variations and gaps in management of acute asthma in Ontario emergency departments. Chest. 2009;135(3):724-736. https://doi.org/10.1378/chest.08-0371.
7. Bekmezian A, Fee C, Weber E. Clinical pathway improves pediatrics asthma management in the emergency department and reduces admissions. J Asthma. 2015;52(8):806-814. https://doi.org/10.3109/02770903.2015.1019086.
8. Chen KH, Chen CC, Liu HE, Tzeng PC, Glasziou PP. Effectiveness of paediatric asthma clinical pathways: a narrative systematic review. J Asthma. 2014;51(5):480-492. https://doi.org/10.3109/02770903.2014.887728.
9. Johnson KB, Blaisdell CJ, Walker A, Eggleston P. Effectiveness of a clinical pathway for inpatient asthma management. Pediatrics. 2000;106(5):1006-1012. https://doi.org/10.1542/peds.106.5.1006.
10. Kelly CS, Andersen CL, Pestian JP, et al. Improved outcomes for hospitalized asthmatic children using a clinical pathway. Ann Allergy Asthma Immunol. 2000;84(5):509-516. https://doi.org/10.1016/S1081-1206(10)62514-8.
11. McDowell KM, Chatburn RL, Myers TR, O’Riordan MA, Kercsmar CM. A cost-saving algorithm for children hospitalized for status asthmaticus. Arch Pediatr Adolesc Med. 1998;152(10):977-984. https://doi.org/10.1001/archpedi.152.10.977.
12. Miller AG, Breslin ME, Pineda LC, Fox JW. An asthma protocol improved adherence to evidence-based guidelines for pediatric subjects with status asthmaticus in the emergency department. Respir Care. 2015;60(12):1759-1764. https://doi.org/10.4187/respcare.04011.
13. Nkoy F, Fassl B, Stone B, et al. Improving pediatric asthma care and outcomes across multiple hospitals. Pediatrics. 2015;136(6):e1602-e1610. https://doi.org/10.1542/peds.2015-0285.
14. Rutman L, Atkins RC, Migita R, et al. Modification of an established pediatric asthma pathway improves evidence-based, efficient care. Pediatrics. 2016;138(6). https://doi.org/10.1542/peds.2016-1248.
15. Glauber JH, Farber HJ, Homer CJ. Asthma clinical pathways: toward what end? Pediatrics. 2001;107(3):590-592. https://doi.org/10.1542/peds.107.3.590.
16. Grimshaw J, Eccles M, Thomas R, et al. Toward evidence-based quality improvement. Evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966-1998. J Gen Intern Med. 2006;21(2):S14-S20. https://doi.org/10.1111/j.1525-1497.2006.00357.x.
17. Scott SD, Grimshaw J, Klassen TP, Nettel-Aguirre A, Johnson DW. Understanding implementation processes of clinical pathways and clinical practice guidelines in pediatric contexts: a study protocol. Implement Sci. 2011;6:133. https://doi.org/10.1186/1748-5908-6-133.
18. Walls TA, Hughes NT, Mullan PC, Chamberlain JM, Brown K. Improving pediatric asthma outcomes in a community emergency department. Pediatrics. 2017;139(1). https://doi.org/10.1542/peds.2016-0088.
19. Kaiser SV, Lam R, Cabana MD, et al. Best practices in implementing inpatient pediatric asthma pathways: a qualitative study. J Asthma. 2019:1-11. https://doi.org/10.1080/02770903.2019.1606237.
20. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624.
21. Franca UL, McManus ML. Availability of definitive hospital care for children. JAMA Pediatr. 2017;171(9):e171096. https://doi.org/10.1001/jamapediatrics.2017.1096.
22. McDaniel CE, Jennings R, Schroeder AR, Paciorkowski N, Hofmann M, Leyenaar J. Aligning inpatient pediatric research with settings of care: a call to action. Pediatrics. 2019;143(5). https://doi.org/10.1542/peds.2018-2648.
23. Kaiser SV JB. Value in inpatient pediatrics network launches National Asthma Project. In: AAP Quality Connections 2018; 26:8-9. Retrieved from: https://www.aap.org/en-us/Documents/coqips_newsletter_2018_winter_26.pdf
24. Value in Inpatient Pediatrics. https://www.aap.org/en-us/professional-resources/quality-improvement/Pages/Value-in-Inpatient-Pediatrics.aspx. Accessed December 1, 2017.
25. The Breakthrough Series: IHI’s Collaborative Model for Achieving Breakthrough Improvement. IHI Innovation Series white paper. Boston: Institute for Healthcare Improvement; 2003. Retrieved from: www.IHI.org
26. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21. https://doi.org/10.1186/s13012-015-0209-1.
27. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. https://doi.org/10.1186/1748-5908-4-50.
28. Braun VaC, V. Thematic analysis. In: H. Cooper PC, Long DL, Panter AT, Rindskopf E, Sher KJ, eds. APA handbook of research methods in psychology, Vol 2. Research designs: Quantitative, qualitative, neuropsychologial, and biological. Washington, DC, US: American Psychological Association; 2012. https://doi.org/10.1037/13620-000.
29. Charmaz K. Grounded Theory. 2nd ed. Thousand Oaks, CA: SAGE Publications; 2014.
30. Creswell JW, Poth CNCN CJaP. Qualitative Inquiry and Research Design: Choosing Among Five Approaches. Thousand Oaks, CA: Sage; 2017.
31. Kaiser SV, Rodean J, Bekmezian A, et al. Effectiveness of pediatric asthma pathways for hospitalized children: a multicenter, national analysis. J. Pediatr. 2018;197:165-171. https://doi.org/10.1016/j.jpeds.2018.01.084.
32. Leyenaar JK, Andrews CB, Tyksinski ER, Biondi E, Parikh K, Ralston S. Facilitators of interdepartmental quality improvement: a mixed-methods analysis of a collaborative to improve pediatric community-acquired pneumonia management. BMJ Qual Saf. 2019;28(3):215-222. https://doi.org/10.1136/bmjqs-2018-008065. 33. Ralston SL, Atwood EC, Garber MD, Holmes AV. What works to reduce unnecessary care for bronchiolitis? A qualitative analysis of a national collaborative. Acad Pediatr. 2017;17(2):198-204. https://doi.org/10.1016/j.acap.2016.07.001.
33. Ralston SL, Atwood EC, Garber MD, Holmes AV. What works to reduce unnecessary care for bronchiolitis? A qualitative analysis of a national collaborative. Acad Pediatr. 2017;17(2):198-204. https://doi.org/10.1016/j.acap.2016.07.001.
34. Parikh K, Biondi E, Nazif J, et al. A multicenter collaborative to improve care of community acquired pneumonia in hospitalized children. Pediatrics. 2017;139(2). https://doi.org/10.1542/peds.2016-1411.
35. Ralston S, Garber M, Narang S, et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8(1):25-30. https://doi.org/10.1002/jhm.1982.
36. Gupta N CA, Cabana MD, Jennings B, Parikh K, Kaiser SV. PIPA (Pathways for Improving Pediatric Asthma Care): Process Evaluation of a National Collaborative to Implement Pathways. Platform presented at Pediatric Academic Society National Meeting. Baltimore, Maryland; 2019.
© 2020 Society of Hospital Medicine
Things We Do For No Reason: Routine Blood Culture Acquisition for Children Hospitalized with Community-Acquired Pneumonia

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 4-year-old previously healthy, fully immunized boy presented to the emergency department (ED) with three days of worsening cough, fever to 103oF, dyspnea, and decreased oral intake. In the ED, he was febrile, temperature 102.7oF, heart rate 115 beats/min, respiratory rate 30 breaths/min, and O2 saturation 86%. Pertinent findings identified on examination included tachypnea, dry mucous membranes, and decreased breath sounds in the posterior right lung fields. Chest radiograph revealed a right lower lobe opacification concerning for community-acquired pneumonia (CAP). He was admitted to the hospital due to hypoxemia and dehydration. A blood culture was obtained, and treatment with ampicillin was initiated. The following morning, he was afebrile, clinically improved, and no longer hypoxemic, but the blood culture grew Gram-positive cocci. Another blood culture was performed, and he was switched to vancomycin. The next day, penicillin-susceptible Streptococcus pneumoniae was confirmed from the original culture, and he was discharged home on high-dose amoxicillin.
WHY YOU MIGHT THINK A BLOOD CULTURE IS HELPFUL
CAP is a prominent cause of childhood morbidity and among the most common causes for acute childhood hospitalizations in the United States, with 124,900 hospital stays documented in 2012.1 In 2011, the Infectious Diseases Society of America (IDSA) released recommendations for pediatric CAP in immunocompetent children aged >3 months without chronic medical conditions. The recommendations clearly discourage blood cultures in the outpatient setting but are less direct in the inpatient setting. The recommendations state that providers should obtain blood cultures “in children requiring hospitalization for presumed bacterial CAP that is moderate to severe, particularly those with complicated pneumonia.”2 The recommendation is graded as “strong”, though the IDSA acknowledged the “low” quality of supporting evidence. Although the organization provides a classification for “complicated pneumonia,” it does not define what constitutes mild versus moderate or severe pneumonia.
Without clear recommendations, decisions to obtain blood cultures for hospitalized children with CAP vary among providers and institutions, with the reported hospital-to-hospital variation being as large as 0%-78.7%.3 Some believe that any child hospitalized with CAP meets the definition of moderate to severe pneumonia and have implemented projects to increase blood culture acquisition for this population.4 The decision to err on the side of routinely obtaining a blood culture may come from providers’ prevalent worry of “missing” a diagnosis, desire to target antibacterial therapy, and assumption that it will provide additional information for patients lacking improvement.
WHY A ROUTINE BLOOD CULTURE ON PEDIATRIC CAP ADMISSIONS IS NOT HELPFUL
Since the publication of the 2011 IDSA guidelines, new evidence has revealed a decreasing incidence of bacteremia in pediatric populations.5 Moreover, viruses were the most frequently identified pathogens in children hospitalized with CAP in a large study, which were isolated in 66% of patients, whereas typical bacteria (either alone or in combination with a virus) were identified in only 7% of cases.6 When blood cultures are obtained for pediatric CAP, the incidence of a true bacterial bloodstream pathogen is 1.4%-7% of patients in the United States in the conjugate vaccine era.7-11 Given that the practice of obtaining blood cultures varies widely among hospitalized patients and that cultures are often obtained based on perceived severity of presentation,8,9,12 the true incidence of bacteremia in children with CAP would likely be lower if blood cultures were performed in all patients.
Since the introduction of the first conjugated pneumococcal vaccine, the prevalence of penicillin resistance among pneumococcal isolates dramatically declined,13 though with geographic variability.14 Therefore, when we isolate pneumococcus strains, resistance prevalence requires that we alter treatment much less frequently in the majority of patients with CAP receiving IDSA-recommended ampicillin/amoxicillin.2 In a large six-center, geographically dispersed retrospective cohort study, Neuman et al. reported a rate of true bacteremia of 2.53%; 82% of all pathogens and 92% of pneumococcal isolates were susceptible to penicillin. Therefore, the authors estimated that 667 children hospitalized with CAP would need blood cultures to identify one child requiring an antibiotic other than an aminopenicillin.9 Staphylococcus aureus was identified only in 1% (23/2,138) of patients in the EPIC cohort; the pathogen was identified via blood culture in only 26% (6/23) of these patients.15 Therefore, the concern about the possibility of S. aureus may be a common reason for physicians straying from IDSA-recommended therapy, but it is an uncommon cause of CAP and infrequently identified via blood culture.
Blood culture contaminants have been reported to approach the rate of true pathogens in some studies8,9,11 and be equal or exceed the rates in others.7,16 While awaiting bacterial speciation, antibiotic coverage is often broadened, even for contaminants,8 which can result in unnecessary exposure to nephrotoxic agents such as vancomycin, cause rare adverse events such as Stevens-Johnson syndrome, contribute to antibiotic resistance and unnecessary costs, and increase the length of stay and laboratory utilization.17-19
WHEN MIGHT A BLOOD CULTURE BE HELPFUL
Given the low penicillin resistance prevalence among pneumococcal isolates in several parts of the United States, blood cultures should be used to identify patients with nonpneumococcal CAP as these patients are more likely to require antibiotics other than penicillin or aminopenicillin. Children with complicated pneumonia are more likely to have nonpneumococcal etiologies than children with uncomplicated pneumonia.2 Moreover, literature published since the IDSA guidelines continues to indicate that the incidence of bacteremia in complicated pneumonia is significantly higher than that in uncomplicated pneumonia (Table). This further supports the IDSA guideline recommendation for blood culture acquisition in children with complicated pneumonia.2
One difficulty in interpreting these data is that each publication used a different definition of “complicated” pneumonia, probably due to differences in data sources. Neuman et al. incorporated the narrowest definition of severe and complicated pneumonia as patients who were either admitted to an intensive care unit (ICU) or who underwent a pleural drainage procedure.9 Myers’ and Shah’s definitions were similar to each other but much broader than that of Neuman et al. Shah et al. included lung abscess/necrosis, parapneumonic effusion/empyema, or bronchopleural fistula.11 Myers et al. included the same indications but qualified their pleural fluid effusions as “moderate-to-large” and any effusion that required pleural drainage procedure.8 Myers et al. also reported bacteremia in 75% of patients with metastatic complications, including osteomyelitis.8 These definitions of complicated pneumonia may at least partially explain the differences noted in the rates of bacteremia in complicated pneumonia, with the patients in the study of Myers et al. potentially representing the most severe cohort and with the highest rate of bacteremia8,9 (Table).
These studies not only support the definition of complicated pneumonia put forward by the IDSA but also provide further information, though imperfect, on how to define “moderate to severe.” All the patients with bacteremia in the report of Heine et al. had complicated pneumonia and were described on chart review as either toxic-appearing or requiring ICU care.7 This, in addition to the inclusion of ICU care in the definition of complicated pneumonia of Neuman et al.,9 indicates that children with CAP requiring ICU care may be at higher risk of bacteremia. In fact, the British Thoracic Society guidelines do not recommend microbiological investigations of children with CAP, including blood culture, unless a child requires ICU care.20
WHAT YOU SHOULD DO INSTEAD
Given the low rate of bacteremia in CAP, the risk of blood culture contaminants, and the small likelihood that isolation of a pathogen alters treatment for children, we recommend not using hospital admission as the determining factor for blood culture acquisition. Instead, we recommend a more targeted approach. To achieve a higher rate of true-positive bacteremia in immunocompetent children with up-to-date vaccinations, we recommend acquiring a blood culture in children with complicated pneumonia, metastatic complications, or with ICU needs. By initiating the IDSA-recommended ampicillin/amoxicillin in the remaining hospitalized patients and acquiring blood cultures for the minority of patients who do not improve, we can increase the likelihood of isolating penicillin-resistant bacteria.
Attempting to balance the importance of identifying clinically important bacteremia for children hospitalized with CAP and the inherent risks of obtaining blood cultures for all hospitalized patients, Andrews et al. created and analyzed a cost-effectiveness model. The authors compared universal acquisition of blood cultures for hospitalized children with CAP versus a targeted approach with blood cultures obtained in patients with effusion or empyema, requiring ICU care, or who are immunosuppressed. Based on this model, a targeted approach could save more than $187 million annually, reduce the number of cultures needed to result in a meaningful change in antibiotic therapy for one patient from 122 to 42, and would “miss” only approximately one case of bacteremia resulting in treatment failure per 1,400 patients.17
RECOMMENDATIONS
- Do not obtain blood culture routinely for children aged >3 months hospitalized for uncomplicated CAP.
- Obtain a blood culture for the following hospitalized patients with CAP:
a. Patients with complicated CAP as defined by the IDSA, particularly those with empyema, abscess, or fistula, or metastatic complications of pneumonia (Table); or
b. Patients with CAP requiring ICU care20 for the management of shock and/or advanced respiratory support.
c. Patients with CAP judged to need antibiotic treatment with an agent other than the IDSA-recommended ampicillin/penicillin (concern for pathogens other than penicillin-sensitive S. pneumonia, immunocompromised or under-immunized status, or inadequate clinical response to empiric ampicillin therapy).
CONCLUSION
Implementing a more targeted approach to blood culture acquisition for hospitalized children with CAP will hopefully increase the yield of true bacterial pathogens that alter management decisions. A targeted approach for the child in the opening vignette would have saved him from the pain of unnecessary phlebotomy (repeat culture), exposure to vancomycin as a nephrotoxic agent, and an additional hospital day.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?™” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by e-mailing TWDFNR@hospitalmedicine.org.
1. Whitney P, Whitt AJW, Elixhauser A. Overview of hospital stays for children in the United States, 2012. Statistical Brief 187. 2014;187. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb187-Hospital-Stays-Children-2012.jsp. Accessed December 21, 2017.
2. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1093/cid/cir531.
3. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. https://doi.org/10.1097/INF.0b013e31825f2b10.
4. Murtagh Kurowski E, Shah SS, Thomson J, et al. Improvement methodology increases guideline recommended blood cultures in children with pneumonia. Pediatrics. 2015;135(4):e1052-e1059. https://doi.org/10.1542/peds.2014-2077.
5. Greenhow TL, Hung YY, Herz A. Bacteremia in children 3 to 36 months old after introduction of conjugated pneumococcal vaccines. Pediatrics. 2017;139(4):e20162098. https://doi.org/10.1542/peds.2016-2098.
6. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. https://doi.org/10.1056/NEJMoa1405870.
7. Heine D, Cochran C, Moore M, Titus MO, Andrews AL. The prevalence of bacteremia in pediatric patients with community-acquired pneumonia: guidelines to reduce the frequency of obtaining blood cultures. Hosp Pediatr. 2013;3(2):92-96. https://doi.org/10.1542/hpeds.2012-0050.
8. Myers AL, Hall M, Williams DJ, et al. Prevalence of bacteremia in hospitalized pediatric patients with community-acquired pneumonia. Pediatr Infect Dis J. 2013;32(7):736-740. https://doi.org/10.1097/INF.0b013e318290bf63.
9. Neuman MI, Hall M, Lipsett SC, et al. Utility of blood culture among children hospitalized with community-acquired pneumonia. Pediatrics. 2017;140(3). https://doi.org/10.1542/peds.2017-1013.
10. Sandora TJ, Desai R, Miko BA, Harper MB. Assessing quality indicators for pediatric community-acquired pneumonia. Am J Med Qual. 2009;24(5):419-427. https://doi.org/10.1177/1062860609337900.
11. Shah SS, Dugan MH, Bell LM, et al. Blood cultures in the emergency department evaluation of childhood pneumonia. Pediatr Infect Dis J. 2011;30(6):475-479. https://doi.org/10.1097/INF.0b013e31820a5adb.
12. Davis TR, Evans HR, Murtas J et al. Utility of blood cultures in children admitted to hospital with community-acquired pneumonia. J Paediatr Child Health. 2017;53(3):232-236. https://doi.org/10.1111/jpc.13376.
13. Williams DJ, Shah SS. Community-acquired pneumonia in the conjugate vaccine era. J Pediatr Infect Dis Soc. 2012;1(4):314-328. https://doi.org/10.1093/jpids/pis101.
14. Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354(14):1455-1463. https://doi.org/10.1056/NEJMoa051642.
15. Frush JM, Zhu Y, Edwards KM, et al. Prevalence of Staphylococcus aureus and use of antistaphylococcal therapy in children hospitalized with pneumonia. J Hosp Med. 2018;13(12):848-852. https://doi.org/10.12788/jhm.3093.
16. Mendoza-Paredes A, Bastos J, Leber M, Erickson E, Waseem M. Utility of blood culture in uncomplicated pneumonia in children. Clin Med Insights Pediatr. 2013;7:1-5. https://doi.org/10.4137/CMPed.S8051.
17. Andrews AL, Simpson AN, Heine D, Teufel II RJ. A cost-effectiveness analysis of obtaining blood cultures in children hospitalized for community-acquired pneumonia. J Pediatr. 2015;167(6):1280-1286. https://doi.org/10.1016/j.jpeds.2015.09.025.
18. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062.
19. McCulloh RJ, Koster MP, Yin DE, et al. Evaluating the use of blood cultures in the management of children hospitalized for community-acquired pneumonia. PloS One. 2015;10(2):e0117462. https://doi.org/10.1371/journal.pone.0117462.
20. Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(2):ii1-ii23. https://doi.org/10.1136/thoraxjnl-2011-200598.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 4-year-old previously healthy, fully immunized boy presented to the emergency department (ED) with three days of worsening cough, fever to 103oF, dyspnea, and decreased oral intake. In the ED, he was febrile, temperature 102.7oF, heart rate 115 beats/min, respiratory rate 30 breaths/min, and O2 saturation 86%. Pertinent findings identified on examination included tachypnea, dry mucous membranes, and decreased breath sounds in the posterior right lung fields. Chest radiograph revealed a right lower lobe opacification concerning for community-acquired pneumonia (CAP). He was admitted to the hospital due to hypoxemia and dehydration. A blood culture was obtained, and treatment with ampicillin was initiated. The following morning, he was afebrile, clinically improved, and no longer hypoxemic, but the blood culture grew Gram-positive cocci. Another blood culture was performed, and he was switched to vancomycin. The next day, penicillin-susceptible Streptococcus pneumoniae was confirmed from the original culture, and he was discharged home on high-dose amoxicillin.
WHY YOU MIGHT THINK A BLOOD CULTURE IS HELPFUL
CAP is a prominent cause of childhood morbidity and among the most common causes for acute childhood hospitalizations in the United States, with 124,900 hospital stays documented in 2012.1 In 2011, the Infectious Diseases Society of America (IDSA) released recommendations for pediatric CAP in immunocompetent children aged >3 months without chronic medical conditions. The recommendations clearly discourage blood cultures in the outpatient setting but are less direct in the inpatient setting. The recommendations state that providers should obtain blood cultures “in children requiring hospitalization for presumed bacterial CAP that is moderate to severe, particularly those with complicated pneumonia.”2 The recommendation is graded as “strong”, though the IDSA acknowledged the “low” quality of supporting evidence. Although the organization provides a classification for “complicated pneumonia,” it does not define what constitutes mild versus moderate or severe pneumonia.
Without clear recommendations, decisions to obtain blood cultures for hospitalized children with CAP vary among providers and institutions, with the reported hospital-to-hospital variation being as large as 0%-78.7%.3 Some believe that any child hospitalized with CAP meets the definition of moderate to severe pneumonia and have implemented projects to increase blood culture acquisition for this population.4 The decision to err on the side of routinely obtaining a blood culture may come from providers’ prevalent worry of “missing” a diagnosis, desire to target antibacterial therapy, and assumption that it will provide additional information for patients lacking improvement.
WHY A ROUTINE BLOOD CULTURE ON PEDIATRIC CAP ADMISSIONS IS NOT HELPFUL
Since the publication of the 2011 IDSA guidelines, new evidence has revealed a decreasing incidence of bacteremia in pediatric populations.5 Moreover, viruses were the most frequently identified pathogens in children hospitalized with CAP in a large study, which were isolated in 66% of patients, whereas typical bacteria (either alone or in combination with a virus) were identified in only 7% of cases.6 When blood cultures are obtained for pediatric CAP, the incidence of a true bacterial bloodstream pathogen is 1.4%-7% of patients in the United States in the conjugate vaccine era.7-11 Given that the practice of obtaining blood cultures varies widely among hospitalized patients and that cultures are often obtained based on perceived severity of presentation,8,9,12 the true incidence of bacteremia in children with CAP would likely be lower if blood cultures were performed in all patients.
Since the introduction of the first conjugated pneumococcal vaccine, the prevalence of penicillin resistance among pneumococcal isolates dramatically declined,13 though with geographic variability.14 Therefore, when we isolate pneumococcus strains, resistance prevalence requires that we alter treatment much less frequently in the majority of patients with CAP receiving IDSA-recommended ampicillin/amoxicillin.2 In a large six-center, geographically dispersed retrospective cohort study, Neuman et al. reported a rate of true bacteremia of 2.53%; 82% of all pathogens and 92% of pneumococcal isolates were susceptible to penicillin. Therefore, the authors estimated that 667 children hospitalized with CAP would need blood cultures to identify one child requiring an antibiotic other than an aminopenicillin.9 Staphylococcus aureus was identified only in 1% (23/2,138) of patients in the EPIC cohort; the pathogen was identified via blood culture in only 26% (6/23) of these patients.15 Therefore, the concern about the possibility of S. aureus may be a common reason for physicians straying from IDSA-recommended therapy, but it is an uncommon cause of CAP and infrequently identified via blood culture.
Blood culture contaminants have been reported to approach the rate of true pathogens in some studies8,9,11 and be equal or exceed the rates in others.7,16 While awaiting bacterial speciation, antibiotic coverage is often broadened, even for contaminants,8 which can result in unnecessary exposure to nephrotoxic agents such as vancomycin, cause rare adverse events such as Stevens-Johnson syndrome, contribute to antibiotic resistance and unnecessary costs, and increase the length of stay and laboratory utilization.17-19
WHEN MIGHT A BLOOD CULTURE BE HELPFUL
Given the low penicillin resistance prevalence among pneumococcal isolates in several parts of the United States, blood cultures should be used to identify patients with nonpneumococcal CAP as these patients are more likely to require antibiotics other than penicillin or aminopenicillin. Children with complicated pneumonia are more likely to have nonpneumococcal etiologies than children with uncomplicated pneumonia.2 Moreover, literature published since the IDSA guidelines continues to indicate that the incidence of bacteremia in complicated pneumonia is significantly higher than that in uncomplicated pneumonia (Table). This further supports the IDSA guideline recommendation for blood culture acquisition in children with complicated pneumonia.2
One difficulty in interpreting these data is that each publication used a different definition of “complicated” pneumonia, probably due to differences in data sources. Neuman et al. incorporated the narrowest definition of severe and complicated pneumonia as patients who were either admitted to an intensive care unit (ICU) or who underwent a pleural drainage procedure.9 Myers’ and Shah’s definitions were similar to each other but much broader than that of Neuman et al. Shah et al. included lung abscess/necrosis, parapneumonic effusion/empyema, or bronchopleural fistula.11 Myers et al. included the same indications but qualified their pleural fluid effusions as “moderate-to-large” and any effusion that required pleural drainage procedure.8 Myers et al. also reported bacteremia in 75% of patients with metastatic complications, including osteomyelitis.8 These definitions of complicated pneumonia may at least partially explain the differences noted in the rates of bacteremia in complicated pneumonia, with the patients in the study of Myers et al. potentially representing the most severe cohort and with the highest rate of bacteremia8,9 (Table).
These studies not only support the definition of complicated pneumonia put forward by the IDSA but also provide further information, though imperfect, on how to define “moderate to severe.” All the patients with bacteremia in the report of Heine et al. had complicated pneumonia and were described on chart review as either toxic-appearing or requiring ICU care.7 This, in addition to the inclusion of ICU care in the definition of complicated pneumonia of Neuman et al.,9 indicates that children with CAP requiring ICU care may be at higher risk of bacteremia. In fact, the British Thoracic Society guidelines do not recommend microbiological investigations of children with CAP, including blood culture, unless a child requires ICU care.20
WHAT YOU SHOULD DO INSTEAD
Given the low rate of bacteremia in CAP, the risk of blood culture contaminants, and the small likelihood that isolation of a pathogen alters treatment for children, we recommend not using hospital admission as the determining factor for blood culture acquisition. Instead, we recommend a more targeted approach. To achieve a higher rate of true-positive bacteremia in immunocompetent children with up-to-date vaccinations, we recommend acquiring a blood culture in children with complicated pneumonia, metastatic complications, or with ICU needs. By initiating the IDSA-recommended ampicillin/amoxicillin in the remaining hospitalized patients and acquiring blood cultures for the minority of patients who do not improve, we can increase the likelihood of isolating penicillin-resistant bacteria.
Attempting to balance the importance of identifying clinically important bacteremia for children hospitalized with CAP and the inherent risks of obtaining blood cultures for all hospitalized patients, Andrews et al. created and analyzed a cost-effectiveness model. The authors compared universal acquisition of blood cultures for hospitalized children with CAP versus a targeted approach with blood cultures obtained in patients with effusion or empyema, requiring ICU care, or who are immunosuppressed. Based on this model, a targeted approach could save more than $187 million annually, reduce the number of cultures needed to result in a meaningful change in antibiotic therapy for one patient from 122 to 42, and would “miss” only approximately one case of bacteremia resulting in treatment failure per 1,400 patients.17
RECOMMENDATIONS
- Do not obtain blood culture routinely for children aged >3 months hospitalized for uncomplicated CAP.
- Obtain a blood culture for the following hospitalized patients with CAP:
a. Patients with complicated CAP as defined by the IDSA, particularly those with empyema, abscess, or fistula, or metastatic complications of pneumonia (Table); or
b. Patients with CAP requiring ICU care20 for the management of shock and/or advanced respiratory support.
c. Patients with CAP judged to need antibiotic treatment with an agent other than the IDSA-recommended ampicillin/penicillin (concern for pathogens other than penicillin-sensitive S. pneumonia, immunocompromised or under-immunized status, or inadequate clinical response to empiric ampicillin therapy).
CONCLUSION
Implementing a more targeted approach to blood culture acquisition for hospitalized children with CAP will hopefully increase the yield of true bacterial pathogens that alter management decisions. A targeted approach for the child in the opening vignette would have saved him from the pain of unnecessary phlebotomy (repeat culture), exposure to vancomycin as a nephrotoxic agent, and an additional hospital day.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?™” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by e-mailing TWDFNR@hospitalmedicine.org.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 4-year-old previously healthy, fully immunized boy presented to the emergency department (ED) with three days of worsening cough, fever to 103oF, dyspnea, and decreased oral intake. In the ED, he was febrile, temperature 102.7oF, heart rate 115 beats/min, respiratory rate 30 breaths/min, and O2 saturation 86%. Pertinent findings identified on examination included tachypnea, dry mucous membranes, and decreased breath sounds in the posterior right lung fields. Chest radiograph revealed a right lower lobe opacification concerning for community-acquired pneumonia (CAP). He was admitted to the hospital due to hypoxemia and dehydration. A blood culture was obtained, and treatment with ampicillin was initiated. The following morning, he was afebrile, clinically improved, and no longer hypoxemic, but the blood culture grew Gram-positive cocci. Another blood culture was performed, and he was switched to vancomycin. The next day, penicillin-susceptible Streptococcus pneumoniae was confirmed from the original culture, and he was discharged home on high-dose amoxicillin.
WHY YOU MIGHT THINK A BLOOD CULTURE IS HELPFUL
CAP is a prominent cause of childhood morbidity and among the most common causes for acute childhood hospitalizations in the United States, with 124,900 hospital stays documented in 2012.1 In 2011, the Infectious Diseases Society of America (IDSA) released recommendations for pediatric CAP in immunocompetent children aged >3 months without chronic medical conditions. The recommendations clearly discourage blood cultures in the outpatient setting but are less direct in the inpatient setting. The recommendations state that providers should obtain blood cultures “in children requiring hospitalization for presumed bacterial CAP that is moderate to severe, particularly those with complicated pneumonia.”2 The recommendation is graded as “strong”, though the IDSA acknowledged the “low” quality of supporting evidence. Although the organization provides a classification for “complicated pneumonia,” it does not define what constitutes mild versus moderate or severe pneumonia.
Without clear recommendations, decisions to obtain blood cultures for hospitalized children with CAP vary among providers and institutions, with the reported hospital-to-hospital variation being as large as 0%-78.7%.3 Some believe that any child hospitalized with CAP meets the definition of moderate to severe pneumonia and have implemented projects to increase blood culture acquisition for this population.4 The decision to err on the side of routinely obtaining a blood culture may come from providers’ prevalent worry of “missing” a diagnosis, desire to target antibacterial therapy, and assumption that it will provide additional information for patients lacking improvement.
WHY A ROUTINE BLOOD CULTURE ON PEDIATRIC CAP ADMISSIONS IS NOT HELPFUL
Since the publication of the 2011 IDSA guidelines, new evidence has revealed a decreasing incidence of bacteremia in pediatric populations.5 Moreover, viruses were the most frequently identified pathogens in children hospitalized with CAP in a large study, which were isolated in 66% of patients, whereas typical bacteria (either alone or in combination with a virus) were identified in only 7% of cases.6 When blood cultures are obtained for pediatric CAP, the incidence of a true bacterial bloodstream pathogen is 1.4%-7% of patients in the United States in the conjugate vaccine era.7-11 Given that the practice of obtaining blood cultures varies widely among hospitalized patients and that cultures are often obtained based on perceived severity of presentation,8,9,12 the true incidence of bacteremia in children with CAP would likely be lower if blood cultures were performed in all patients.
Since the introduction of the first conjugated pneumococcal vaccine, the prevalence of penicillin resistance among pneumococcal isolates dramatically declined,13 though with geographic variability.14 Therefore, when we isolate pneumococcus strains, resistance prevalence requires that we alter treatment much less frequently in the majority of patients with CAP receiving IDSA-recommended ampicillin/amoxicillin.2 In a large six-center, geographically dispersed retrospective cohort study, Neuman et al. reported a rate of true bacteremia of 2.53%; 82% of all pathogens and 92% of pneumococcal isolates were susceptible to penicillin. Therefore, the authors estimated that 667 children hospitalized with CAP would need blood cultures to identify one child requiring an antibiotic other than an aminopenicillin.9 Staphylococcus aureus was identified only in 1% (23/2,138) of patients in the EPIC cohort; the pathogen was identified via blood culture in only 26% (6/23) of these patients.15 Therefore, the concern about the possibility of S. aureus may be a common reason for physicians straying from IDSA-recommended therapy, but it is an uncommon cause of CAP and infrequently identified via blood culture.
Blood culture contaminants have been reported to approach the rate of true pathogens in some studies8,9,11 and be equal or exceed the rates in others.7,16 While awaiting bacterial speciation, antibiotic coverage is often broadened, even for contaminants,8 which can result in unnecessary exposure to nephrotoxic agents such as vancomycin, cause rare adverse events such as Stevens-Johnson syndrome, contribute to antibiotic resistance and unnecessary costs, and increase the length of stay and laboratory utilization.17-19
WHEN MIGHT A BLOOD CULTURE BE HELPFUL
Given the low penicillin resistance prevalence among pneumococcal isolates in several parts of the United States, blood cultures should be used to identify patients with nonpneumococcal CAP as these patients are more likely to require antibiotics other than penicillin or aminopenicillin. Children with complicated pneumonia are more likely to have nonpneumococcal etiologies than children with uncomplicated pneumonia.2 Moreover, literature published since the IDSA guidelines continues to indicate that the incidence of bacteremia in complicated pneumonia is significantly higher than that in uncomplicated pneumonia (Table). This further supports the IDSA guideline recommendation for blood culture acquisition in children with complicated pneumonia.2
One difficulty in interpreting these data is that each publication used a different definition of “complicated” pneumonia, probably due to differences in data sources. Neuman et al. incorporated the narrowest definition of severe and complicated pneumonia as patients who were either admitted to an intensive care unit (ICU) or who underwent a pleural drainage procedure.9 Myers’ and Shah’s definitions were similar to each other but much broader than that of Neuman et al. Shah et al. included lung abscess/necrosis, parapneumonic effusion/empyema, or bronchopleural fistula.11 Myers et al. included the same indications but qualified their pleural fluid effusions as “moderate-to-large” and any effusion that required pleural drainage procedure.8 Myers et al. also reported bacteremia in 75% of patients with metastatic complications, including osteomyelitis.8 These definitions of complicated pneumonia may at least partially explain the differences noted in the rates of bacteremia in complicated pneumonia, with the patients in the study of Myers et al. potentially representing the most severe cohort and with the highest rate of bacteremia8,9 (Table).
These studies not only support the definition of complicated pneumonia put forward by the IDSA but also provide further information, though imperfect, on how to define “moderate to severe.” All the patients with bacteremia in the report of Heine et al. had complicated pneumonia and were described on chart review as either toxic-appearing or requiring ICU care.7 This, in addition to the inclusion of ICU care in the definition of complicated pneumonia of Neuman et al.,9 indicates that children with CAP requiring ICU care may be at higher risk of bacteremia. In fact, the British Thoracic Society guidelines do not recommend microbiological investigations of children with CAP, including blood culture, unless a child requires ICU care.20
WHAT YOU SHOULD DO INSTEAD
Given the low rate of bacteremia in CAP, the risk of blood culture contaminants, and the small likelihood that isolation of a pathogen alters treatment for children, we recommend not using hospital admission as the determining factor for blood culture acquisition. Instead, we recommend a more targeted approach. To achieve a higher rate of true-positive bacteremia in immunocompetent children with up-to-date vaccinations, we recommend acquiring a blood culture in children with complicated pneumonia, metastatic complications, or with ICU needs. By initiating the IDSA-recommended ampicillin/amoxicillin in the remaining hospitalized patients and acquiring blood cultures for the minority of patients who do not improve, we can increase the likelihood of isolating penicillin-resistant bacteria.
Attempting to balance the importance of identifying clinically important bacteremia for children hospitalized with CAP and the inherent risks of obtaining blood cultures for all hospitalized patients, Andrews et al. created and analyzed a cost-effectiveness model. The authors compared universal acquisition of blood cultures for hospitalized children with CAP versus a targeted approach with blood cultures obtained in patients with effusion or empyema, requiring ICU care, or who are immunosuppressed. Based on this model, a targeted approach could save more than $187 million annually, reduce the number of cultures needed to result in a meaningful change in antibiotic therapy for one patient from 122 to 42, and would “miss” only approximately one case of bacteremia resulting in treatment failure per 1,400 patients.17
RECOMMENDATIONS
- Do not obtain blood culture routinely for children aged >3 months hospitalized for uncomplicated CAP.
- Obtain a blood culture for the following hospitalized patients with CAP:
a. Patients with complicated CAP as defined by the IDSA, particularly those with empyema, abscess, or fistula, or metastatic complications of pneumonia (Table); or
b. Patients with CAP requiring ICU care20 for the management of shock and/or advanced respiratory support.
c. Patients with CAP judged to need antibiotic treatment with an agent other than the IDSA-recommended ampicillin/penicillin (concern for pathogens other than penicillin-sensitive S. pneumonia, immunocompromised or under-immunized status, or inadequate clinical response to empiric ampicillin therapy).
CONCLUSION
Implementing a more targeted approach to blood culture acquisition for hospitalized children with CAP will hopefully increase the yield of true bacterial pathogens that alter management decisions. A targeted approach for the child in the opening vignette would have saved him from the pain of unnecessary phlebotomy (repeat culture), exposure to vancomycin as a nephrotoxic agent, and an additional hospital day.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?™” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by e-mailing TWDFNR@hospitalmedicine.org.
1. Whitney P, Whitt AJW, Elixhauser A. Overview of hospital stays for children in the United States, 2012. Statistical Brief 187. 2014;187. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb187-Hospital-Stays-Children-2012.jsp. Accessed December 21, 2017.
2. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1093/cid/cir531.
3. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. https://doi.org/10.1097/INF.0b013e31825f2b10.
4. Murtagh Kurowski E, Shah SS, Thomson J, et al. Improvement methodology increases guideline recommended blood cultures in children with pneumonia. Pediatrics. 2015;135(4):e1052-e1059. https://doi.org/10.1542/peds.2014-2077.
5. Greenhow TL, Hung YY, Herz A. Bacteremia in children 3 to 36 months old after introduction of conjugated pneumococcal vaccines. Pediatrics. 2017;139(4):e20162098. https://doi.org/10.1542/peds.2016-2098.
6. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. https://doi.org/10.1056/NEJMoa1405870.
7. Heine D, Cochran C, Moore M, Titus MO, Andrews AL. The prevalence of bacteremia in pediatric patients with community-acquired pneumonia: guidelines to reduce the frequency of obtaining blood cultures. Hosp Pediatr. 2013;3(2):92-96. https://doi.org/10.1542/hpeds.2012-0050.
8. Myers AL, Hall M, Williams DJ, et al. Prevalence of bacteremia in hospitalized pediatric patients with community-acquired pneumonia. Pediatr Infect Dis J. 2013;32(7):736-740. https://doi.org/10.1097/INF.0b013e318290bf63.
9. Neuman MI, Hall M, Lipsett SC, et al. Utility of blood culture among children hospitalized with community-acquired pneumonia. Pediatrics. 2017;140(3). https://doi.org/10.1542/peds.2017-1013.
10. Sandora TJ, Desai R, Miko BA, Harper MB. Assessing quality indicators for pediatric community-acquired pneumonia. Am J Med Qual. 2009;24(5):419-427. https://doi.org/10.1177/1062860609337900.
11. Shah SS, Dugan MH, Bell LM, et al. Blood cultures in the emergency department evaluation of childhood pneumonia. Pediatr Infect Dis J. 2011;30(6):475-479. https://doi.org/10.1097/INF.0b013e31820a5adb.
12. Davis TR, Evans HR, Murtas J et al. Utility of blood cultures in children admitted to hospital with community-acquired pneumonia. J Paediatr Child Health. 2017;53(3):232-236. https://doi.org/10.1111/jpc.13376.
13. Williams DJ, Shah SS. Community-acquired pneumonia in the conjugate vaccine era. J Pediatr Infect Dis Soc. 2012;1(4):314-328. https://doi.org/10.1093/jpids/pis101.
14. Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354(14):1455-1463. https://doi.org/10.1056/NEJMoa051642.
15. Frush JM, Zhu Y, Edwards KM, et al. Prevalence of Staphylococcus aureus and use of antistaphylococcal therapy in children hospitalized with pneumonia. J Hosp Med. 2018;13(12):848-852. https://doi.org/10.12788/jhm.3093.
16. Mendoza-Paredes A, Bastos J, Leber M, Erickson E, Waseem M. Utility of blood culture in uncomplicated pneumonia in children. Clin Med Insights Pediatr. 2013;7:1-5. https://doi.org/10.4137/CMPed.S8051.
17. Andrews AL, Simpson AN, Heine D, Teufel II RJ. A cost-effectiveness analysis of obtaining blood cultures in children hospitalized for community-acquired pneumonia. J Pediatr. 2015;167(6):1280-1286. https://doi.org/10.1016/j.jpeds.2015.09.025.
18. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062.
19. McCulloh RJ, Koster MP, Yin DE, et al. Evaluating the use of blood cultures in the management of children hospitalized for community-acquired pneumonia. PloS One. 2015;10(2):e0117462. https://doi.org/10.1371/journal.pone.0117462.
20. Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(2):ii1-ii23. https://doi.org/10.1136/thoraxjnl-2011-200598.
1. Whitney P, Whitt AJW, Elixhauser A. Overview of hospital stays for children in the United States, 2012. Statistical Brief 187. 2014;187. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb187-Hospital-Stays-Children-2012.jsp. Accessed December 21, 2017.
2. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1093/cid/cir531.
3. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. https://doi.org/10.1097/INF.0b013e31825f2b10.
4. Murtagh Kurowski E, Shah SS, Thomson J, et al. Improvement methodology increases guideline recommended blood cultures in children with pneumonia. Pediatrics. 2015;135(4):e1052-e1059. https://doi.org/10.1542/peds.2014-2077.
5. Greenhow TL, Hung YY, Herz A. Bacteremia in children 3 to 36 months old after introduction of conjugated pneumococcal vaccines. Pediatrics. 2017;139(4):e20162098. https://doi.org/10.1542/peds.2016-2098.
6. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. https://doi.org/10.1056/NEJMoa1405870.
7. Heine D, Cochran C, Moore M, Titus MO, Andrews AL. The prevalence of bacteremia in pediatric patients with community-acquired pneumonia: guidelines to reduce the frequency of obtaining blood cultures. Hosp Pediatr. 2013;3(2):92-96. https://doi.org/10.1542/hpeds.2012-0050.
8. Myers AL, Hall M, Williams DJ, et al. Prevalence of bacteremia in hospitalized pediatric patients with community-acquired pneumonia. Pediatr Infect Dis J. 2013;32(7):736-740. https://doi.org/10.1097/INF.0b013e318290bf63.
9. Neuman MI, Hall M, Lipsett SC, et al. Utility of blood culture among children hospitalized with community-acquired pneumonia. Pediatrics. 2017;140(3). https://doi.org/10.1542/peds.2017-1013.
10. Sandora TJ, Desai R, Miko BA, Harper MB. Assessing quality indicators for pediatric community-acquired pneumonia. Am J Med Qual. 2009;24(5):419-427. https://doi.org/10.1177/1062860609337900.
11. Shah SS, Dugan MH, Bell LM, et al. Blood cultures in the emergency department evaluation of childhood pneumonia. Pediatr Infect Dis J. 2011;30(6):475-479. https://doi.org/10.1097/INF.0b013e31820a5adb.
12. Davis TR, Evans HR, Murtas J et al. Utility of blood cultures in children admitted to hospital with community-acquired pneumonia. J Paediatr Child Health. 2017;53(3):232-236. https://doi.org/10.1111/jpc.13376.
13. Williams DJ, Shah SS. Community-acquired pneumonia in the conjugate vaccine era. J Pediatr Infect Dis Soc. 2012;1(4):314-328. https://doi.org/10.1093/jpids/pis101.
14. Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354(14):1455-1463. https://doi.org/10.1056/NEJMoa051642.
15. Frush JM, Zhu Y, Edwards KM, et al. Prevalence of Staphylococcus aureus and use of antistaphylococcal therapy in children hospitalized with pneumonia. J Hosp Med. 2018;13(12):848-852. https://doi.org/10.12788/jhm.3093.
16. Mendoza-Paredes A, Bastos J, Leber M, Erickson E, Waseem M. Utility of blood culture in uncomplicated pneumonia in children. Clin Med Insights Pediatr. 2013;7:1-5. https://doi.org/10.4137/CMPed.S8051.
17. Andrews AL, Simpson AN, Heine D, Teufel II RJ. A cost-effectiveness analysis of obtaining blood cultures in children hospitalized for community-acquired pneumonia. J Pediatr. 2015;167(6):1280-1286. https://doi.org/10.1016/j.jpeds.2015.09.025.
18. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062.
19. McCulloh RJ, Koster MP, Yin DE, et al. Evaluating the use of blood cultures in the management of children hospitalized for community-acquired pneumonia. PloS One. 2015;10(2):e0117462. https://doi.org/10.1371/journal.pone.0117462.
20. Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(2):ii1-ii23. https://doi.org/10.1136/thoraxjnl-2011-200598.
© 2020 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: Diagnosis and Management of Clostridium difficile in Adults
Clostridium difficile, now referred to as Clostridioides difficile (C. difficile), is the most commonly identified cause of healthcare-associated infection among adults in the United States.1 Because C. difficile infection results in significant mortality and inpatient costs, its persistence threatens to undermine patient safety and the value of healthcare delivery.1 A standardized, evidence-based approach to diagnosis and management is crucial. However, inconsistencies remain with regard to the appropriate threshold for testing, the type of diagnostic tests used, and treatment. Knowledge of these areas has progressed since the publication of the previous C. difficile guidelines in 2010. These guidelines contain 53 recommendations across 35 sections based on a systematic weighting of the strength of recommendation and quality of evidence using the Grading of Recommendations Assessment, Development, and Evaluation system. Herein, we have chosen to highlight five of these recommendations most relevant to hospitalists.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. Patients with unexplained and new-onset ≥3 unformed stools within 24 hours are the preferred target population for testing for C. difficile infection (weak recommendation, very low quality of evidence). Do not perform repeat testing (within seven days) during the same episode of diarrhea and do not test stool from asymptomatic patients (strong recommendation, moderate quality of evidence).
In the recent past, healthcare facilities employed C. difficile tests with limited sensitivity, leading to frequent and repeat testing of hospitalized patients. Excess testing puts patients at risk for false positive results and unnecessary or prolonged treatment courses. Proper testing requires consideration of pretest probability, including analysis of the alternative causes of diarrhea. Duration of hospitalization and antibiotic exposure are the most significant modifiable risk factors for C. difficile infection in adult inpatients.2 Laxative use within the previous 48 hours, enteral tube feeding, and underlying medical conditions, such as inflammatory bowel disease (IBD), are common causes of improper testing.3 This decision may be difficult, as some underlying causes of diarrhea, such as IBD and enteral tube feeding, also increase the risk of C. difficile infection.3 Laboratories can help by rejecting specimens that are not liquid or soft and employing a multistep algorithm using a combination of nucleic acid testing, antigen testing, and toxin detection to maximize sensitivity and specificity. Because recurrent C. difficile infection is relatively common, repeat testing is appropriate only for recurrence of symptoms following successful treatment and should focus on detection of C. difficile toxin because the persistence of the organism itself can occur after successful treatment.4
Recommendation 2. Either vancomycin (125 mg orally four times per day for 10 days) or fidaxomicin (200 mg twice daily for 10 days) is recommended over metronidazole for an initial episode of nonsevere or severe C. difficile infection (strong recommendation, high quality of evidence). For fulminant C. difficile infection, the regimen of choice is a vancomycin dosage of 500 mg orally four times per day (per rectum every six hours if with ileus) in addition to intravenous metronidazole (strong recommendation, moderate quality of evidence).
For several decades now, metronidazole has been the primary antibiotic agent for initial treatment of nonsevere C. difficile infection. Two recent randomized, placebo-controlled trials, however, have found oral vancomycin to be superior to metronidazole for producing a clinical cure and resolution of diarrhea without recurrence.5,6 Oral vancomycin remains the treatment of choice for severe C. difficile infection. Fidaxomicin, a recently FDA-approved antibiotic, can also be used as initial treatment in place of oral vancomycin. One study found fidaxomicin to be superior to oral vancomycin for producing a sustained clinical response, that is, resolution of diarrhea at the end of treatment without recurrence 25 days later.7 Fulminant disease, which is characterized by hypotension or shock, ileus, or megacolon, requires a higher dose of oral vancomycin (or vancomycin enema if with ileus) in addition to intravenous metronidazole.
Recommendation 3. Treat a first recurrence of C. difficile infection with oral vancomycin as a tapered and pulsed regimen rather than a second standard 10-day course of vancomycin or metronidazole (weak recommendation, low quality of evidence).
Despite the improved treatment response with oral vancomycin, one in four patients will experience recurrence. For a first recurrence of C. difficile infection after a 10-day course of oral vancomycin, an extended taper or pulsed course of vancomycin should be attempted. Various regimens have been tried and found to be effective. For a second recurrence, providers can consider addition of rifaximin following oral vancomycin. Fecal microbiota transplantation is recommended for patients with multiple recurrences of C. difficile infection who have failed these antibiotic treatments.
Recommendation 4. Minimize the frequency and duration of high-risk antibiotic therapy (based on local epidemiology) and the number of antibiotic agents prescribed to reduce C. difficile infection risk (strong recommendation, moderate quality of evidence).
Antibiotic stewardship is a necessary component of any successful effort to reduce C. difficile infections. Antibiotic stewardship programs, which are now commonplace in US hospitals, largely rely on educational initiatives or committee-based order review. Hospitalists should take a structured approach emphasizing the four critical questions of antibiotic prescribing: Does this infection require antibiotics? Have I ordered appropriate cultures and the correct empiric therapy? Can I stop, narrow, or switch to oral agents? Finally, what duration of therapy is needed at discharge?8 Initial efforts should focus on the restriction of fluoroquinolones, clindamycin, and cephalosporins (except for surgical antibiotic prophylaxis) given their known risk to cause C. difficile infection.
Recommendation 5. Contact precautions should be maintained for at least 48 hours after diarrhea has resolved (weak recommendation, low quality of evidence).
Although C. difficile is undetectable in stool samples from most patients by the time diarrhea has resolved, skin and environmental contaminations remain high. No studies demonstrating a benefit to further extending contact precautions beyond 48 hours after resolution of diarrhea are yet available.
CRITIQUE
Methods in Preparing Guidelines
The guideline committee consisted of an interdisciplinary team of healthcare providers with extensive experience in the diagnosis, infection control, treatment, and management of C. difficile. The literature search accessed five different databases (Medline, Embase, Cochrane, Health Technology Assessment, and Database of Abstracts of Reviews and Effects), relevant journals, conference proceedings, and regulatory websites published over the search period of 2009-2016.
A major strength of these guidelines is the extensive work that went into their preparation. The committee reviewed over 14,000 pieces of literature and performed a detailed analysis of each one to determine the quality of evidence in support of each recommendation.
Sources of Potential Conflict of Interest or Bias
To reduce bias, the committee’s work was funded by Infectious Disease Society of America and Society for Healthcare Epidemiology of America. Some authors received funding for work outside of this guideline by companies that manufacture diagnostic assays, vancomycin, and fidaxomicin. These potential conflicts were listed at the end of the article.
Generalizability of the Guideline
Not all studies included in the guideline contain exclusively hospitalized patients, but much of the guideline content is applicable to hospitalized patients. Because C. difficile infection is such a widespread public health problem and these guidelines represent a significant update in knowledge since 2010, the specific recommendations highlighted in this review will impact numerous hospitalists, regardless of the practice setting.
Areas in Need of Future Study
Based on the current literature, as well as statements in the guideline, we expect future guidance around potential screening for and isolation of asymptomatic carriers, including closer guidance on stool transplantation focusing on timing and route, as further data emerge in these areas.
Other Resources
- Grading of Recommendations Assessment, Development, and Evaluation system (http://www.gradeworkinggroup.org)
- Universal Screening for C. difficile in a Tertiary Hospital: risk factors for carriage and clinical disease (Color/Blackhttps://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(19)30048-5/fulltext)
- Effectiveness of Isolating Clostridium Difficile Asymptomatic Carriers on the Incidence of Infections (Color/Blackhttps://clinicaltrials.gov/ct2/show/NCT03223415)
- Effect of Detecting and Isolating Clostridium difficile Carriers at Hospital Admission on the Incidence of C difficile Infections (Color/Blackhttps://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2516765)
- Clinical Trial Testing Fecal Microbiota Transplant for Recurrent Diarrheal Disease Begins (Color/Blackhttps://www.nih.gov/news-events/news-releases/clinical-trial-testing-fecal-microbiota-transplant-recurrent-diarrheal-disease-begins)
1. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(2):S88-S92. https://doi.org/10.1093/cid/cis335.
2. Loo VG, Bourgault AM, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365(18):1693-703. https://doi.org/10.1056/NEJMoa1012413.
3. O’Keefe SJ. Tube feeding, the microbiota, and Clostridium difficile infection. World J Gastroenterol. 2010;16(2):139-142. https://doi.org/10.3748/wjg.v16.i2.139
4. Zacharioudakis IM, Zervou FN, Pliakos EE, Ziakas PD, Mylonakis E. Colonization with toxinogenic C. difficile upon hospital admission, and risk of infection: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110(3):381-90; quiz 391. https://doi.org/10.1038/ajg.2015.22.
5. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59(3):345-354. https://doi.org/10.1093/cid/ciu313.
6. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302-307. https://doi.org/10.1086/519265.
7. Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55(2):S93-S103. https://doi.org/10.1093/cid/cis499.
8. Tamma, PD, Miller MA, Cosgrove SE. Rethinking how antibiotics are prescribed: incorporating the 4 moments of antibiotic decision making into clinical practice. JAMA. 2018;321(2):139-140. https://doi.org/10.1001/jama.2018.19509.
Clostridium difficile, now referred to as Clostridioides difficile (C. difficile), is the most commonly identified cause of healthcare-associated infection among adults in the United States.1 Because C. difficile infection results in significant mortality and inpatient costs, its persistence threatens to undermine patient safety and the value of healthcare delivery.1 A standardized, evidence-based approach to diagnosis and management is crucial. However, inconsistencies remain with regard to the appropriate threshold for testing, the type of diagnostic tests used, and treatment. Knowledge of these areas has progressed since the publication of the previous C. difficile guidelines in 2010. These guidelines contain 53 recommendations across 35 sections based on a systematic weighting of the strength of recommendation and quality of evidence using the Grading of Recommendations Assessment, Development, and Evaluation system. Herein, we have chosen to highlight five of these recommendations most relevant to hospitalists.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. Patients with unexplained and new-onset ≥3 unformed stools within 24 hours are the preferred target population for testing for C. difficile infection (weak recommendation, very low quality of evidence). Do not perform repeat testing (within seven days) during the same episode of diarrhea and do not test stool from asymptomatic patients (strong recommendation, moderate quality of evidence).
In the recent past, healthcare facilities employed C. difficile tests with limited sensitivity, leading to frequent and repeat testing of hospitalized patients. Excess testing puts patients at risk for false positive results and unnecessary or prolonged treatment courses. Proper testing requires consideration of pretest probability, including analysis of the alternative causes of diarrhea. Duration of hospitalization and antibiotic exposure are the most significant modifiable risk factors for C. difficile infection in adult inpatients.2 Laxative use within the previous 48 hours, enteral tube feeding, and underlying medical conditions, such as inflammatory bowel disease (IBD), are common causes of improper testing.3 This decision may be difficult, as some underlying causes of diarrhea, such as IBD and enteral tube feeding, also increase the risk of C. difficile infection.3 Laboratories can help by rejecting specimens that are not liquid or soft and employing a multistep algorithm using a combination of nucleic acid testing, antigen testing, and toxin detection to maximize sensitivity and specificity. Because recurrent C. difficile infection is relatively common, repeat testing is appropriate only for recurrence of symptoms following successful treatment and should focus on detection of C. difficile toxin because the persistence of the organism itself can occur after successful treatment.4
Recommendation 2. Either vancomycin (125 mg orally four times per day for 10 days) or fidaxomicin (200 mg twice daily for 10 days) is recommended over metronidazole for an initial episode of nonsevere or severe C. difficile infection (strong recommendation, high quality of evidence). For fulminant C. difficile infection, the regimen of choice is a vancomycin dosage of 500 mg orally four times per day (per rectum every six hours if with ileus) in addition to intravenous metronidazole (strong recommendation, moderate quality of evidence).
For several decades now, metronidazole has been the primary antibiotic agent for initial treatment of nonsevere C. difficile infection. Two recent randomized, placebo-controlled trials, however, have found oral vancomycin to be superior to metronidazole for producing a clinical cure and resolution of diarrhea without recurrence.5,6 Oral vancomycin remains the treatment of choice for severe C. difficile infection. Fidaxomicin, a recently FDA-approved antibiotic, can also be used as initial treatment in place of oral vancomycin. One study found fidaxomicin to be superior to oral vancomycin for producing a sustained clinical response, that is, resolution of diarrhea at the end of treatment without recurrence 25 days later.7 Fulminant disease, which is characterized by hypotension or shock, ileus, or megacolon, requires a higher dose of oral vancomycin (or vancomycin enema if with ileus) in addition to intravenous metronidazole.
Recommendation 3. Treat a first recurrence of C. difficile infection with oral vancomycin as a tapered and pulsed regimen rather than a second standard 10-day course of vancomycin or metronidazole (weak recommendation, low quality of evidence).
Despite the improved treatment response with oral vancomycin, one in four patients will experience recurrence. For a first recurrence of C. difficile infection after a 10-day course of oral vancomycin, an extended taper or pulsed course of vancomycin should be attempted. Various regimens have been tried and found to be effective. For a second recurrence, providers can consider addition of rifaximin following oral vancomycin. Fecal microbiota transplantation is recommended for patients with multiple recurrences of C. difficile infection who have failed these antibiotic treatments.
Recommendation 4. Minimize the frequency and duration of high-risk antibiotic therapy (based on local epidemiology) and the number of antibiotic agents prescribed to reduce C. difficile infection risk (strong recommendation, moderate quality of evidence).
Antibiotic stewardship is a necessary component of any successful effort to reduce C. difficile infections. Antibiotic stewardship programs, which are now commonplace in US hospitals, largely rely on educational initiatives or committee-based order review. Hospitalists should take a structured approach emphasizing the four critical questions of antibiotic prescribing: Does this infection require antibiotics? Have I ordered appropriate cultures and the correct empiric therapy? Can I stop, narrow, or switch to oral agents? Finally, what duration of therapy is needed at discharge?8 Initial efforts should focus on the restriction of fluoroquinolones, clindamycin, and cephalosporins (except for surgical antibiotic prophylaxis) given their known risk to cause C. difficile infection.
Recommendation 5. Contact precautions should be maintained for at least 48 hours after diarrhea has resolved (weak recommendation, low quality of evidence).
Although C. difficile is undetectable in stool samples from most patients by the time diarrhea has resolved, skin and environmental contaminations remain high. No studies demonstrating a benefit to further extending contact precautions beyond 48 hours after resolution of diarrhea are yet available.
CRITIQUE
Methods in Preparing Guidelines
The guideline committee consisted of an interdisciplinary team of healthcare providers with extensive experience in the diagnosis, infection control, treatment, and management of C. difficile. The literature search accessed five different databases (Medline, Embase, Cochrane, Health Technology Assessment, and Database of Abstracts of Reviews and Effects), relevant journals, conference proceedings, and regulatory websites published over the search period of 2009-2016.
A major strength of these guidelines is the extensive work that went into their preparation. The committee reviewed over 14,000 pieces of literature and performed a detailed analysis of each one to determine the quality of evidence in support of each recommendation.
Sources of Potential Conflict of Interest or Bias
To reduce bias, the committee’s work was funded by Infectious Disease Society of America and Society for Healthcare Epidemiology of America. Some authors received funding for work outside of this guideline by companies that manufacture diagnostic assays, vancomycin, and fidaxomicin. These potential conflicts were listed at the end of the article.
Generalizability of the Guideline
Not all studies included in the guideline contain exclusively hospitalized patients, but much of the guideline content is applicable to hospitalized patients. Because C. difficile infection is such a widespread public health problem and these guidelines represent a significant update in knowledge since 2010, the specific recommendations highlighted in this review will impact numerous hospitalists, regardless of the practice setting.
Areas in Need of Future Study
Based on the current literature, as well as statements in the guideline, we expect future guidance around potential screening for and isolation of asymptomatic carriers, including closer guidance on stool transplantation focusing on timing and route, as further data emerge in these areas.
Other Resources
- Grading of Recommendations Assessment, Development, and Evaluation system (http://www.gradeworkinggroup.org)
- Universal Screening for C. difficile in a Tertiary Hospital: risk factors for carriage and clinical disease (Color/Blackhttps://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(19)30048-5/fulltext)
- Effectiveness of Isolating Clostridium Difficile Asymptomatic Carriers on the Incidence of Infections (Color/Blackhttps://clinicaltrials.gov/ct2/show/NCT03223415)
- Effect of Detecting and Isolating Clostridium difficile Carriers at Hospital Admission on the Incidence of C difficile Infections (Color/Blackhttps://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2516765)
- Clinical Trial Testing Fecal Microbiota Transplant for Recurrent Diarrheal Disease Begins (Color/Blackhttps://www.nih.gov/news-events/news-releases/clinical-trial-testing-fecal-microbiota-transplant-recurrent-diarrheal-disease-begins)
Clostridium difficile, now referred to as Clostridioides difficile (C. difficile), is the most commonly identified cause of healthcare-associated infection among adults in the United States.1 Because C. difficile infection results in significant mortality and inpatient costs, its persistence threatens to undermine patient safety and the value of healthcare delivery.1 A standardized, evidence-based approach to diagnosis and management is crucial. However, inconsistencies remain with regard to the appropriate threshold for testing, the type of diagnostic tests used, and treatment. Knowledge of these areas has progressed since the publication of the previous C. difficile guidelines in 2010. These guidelines contain 53 recommendations across 35 sections based on a systematic weighting of the strength of recommendation and quality of evidence using the Grading of Recommendations Assessment, Development, and Evaluation system. Herein, we have chosen to highlight five of these recommendations most relevant to hospitalists.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. Patients with unexplained and new-onset ≥3 unformed stools within 24 hours are the preferred target population for testing for C. difficile infection (weak recommendation, very low quality of evidence). Do not perform repeat testing (within seven days) during the same episode of diarrhea and do not test stool from asymptomatic patients (strong recommendation, moderate quality of evidence).
In the recent past, healthcare facilities employed C. difficile tests with limited sensitivity, leading to frequent and repeat testing of hospitalized patients. Excess testing puts patients at risk for false positive results and unnecessary or prolonged treatment courses. Proper testing requires consideration of pretest probability, including analysis of the alternative causes of diarrhea. Duration of hospitalization and antibiotic exposure are the most significant modifiable risk factors for C. difficile infection in adult inpatients.2 Laxative use within the previous 48 hours, enteral tube feeding, and underlying medical conditions, such as inflammatory bowel disease (IBD), are common causes of improper testing.3 This decision may be difficult, as some underlying causes of diarrhea, such as IBD and enteral tube feeding, also increase the risk of C. difficile infection.3 Laboratories can help by rejecting specimens that are not liquid or soft and employing a multistep algorithm using a combination of nucleic acid testing, antigen testing, and toxin detection to maximize sensitivity and specificity. Because recurrent C. difficile infection is relatively common, repeat testing is appropriate only for recurrence of symptoms following successful treatment and should focus on detection of C. difficile toxin because the persistence of the organism itself can occur after successful treatment.4
Recommendation 2. Either vancomycin (125 mg orally four times per day for 10 days) or fidaxomicin (200 mg twice daily for 10 days) is recommended over metronidazole for an initial episode of nonsevere or severe C. difficile infection (strong recommendation, high quality of evidence). For fulminant C. difficile infection, the regimen of choice is a vancomycin dosage of 500 mg orally four times per day (per rectum every six hours if with ileus) in addition to intravenous metronidazole (strong recommendation, moderate quality of evidence).
For several decades now, metronidazole has been the primary antibiotic agent for initial treatment of nonsevere C. difficile infection. Two recent randomized, placebo-controlled trials, however, have found oral vancomycin to be superior to metronidazole for producing a clinical cure and resolution of diarrhea without recurrence.5,6 Oral vancomycin remains the treatment of choice for severe C. difficile infection. Fidaxomicin, a recently FDA-approved antibiotic, can also be used as initial treatment in place of oral vancomycin. One study found fidaxomicin to be superior to oral vancomycin for producing a sustained clinical response, that is, resolution of diarrhea at the end of treatment without recurrence 25 days later.7 Fulminant disease, which is characterized by hypotension or shock, ileus, or megacolon, requires a higher dose of oral vancomycin (or vancomycin enema if with ileus) in addition to intravenous metronidazole.
Recommendation 3. Treat a first recurrence of C. difficile infection with oral vancomycin as a tapered and pulsed regimen rather than a second standard 10-day course of vancomycin or metronidazole (weak recommendation, low quality of evidence).
Despite the improved treatment response with oral vancomycin, one in four patients will experience recurrence. For a first recurrence of C. difficile infection after a 10-day course of oral vancomycin, an extended taper or pulsed course of vancomycin should be attempted. Various regimens have been tried and found to be effective. For a second recurrence, providers can consider addition of rifaximin following oral vancomycin. Fecal microbiota transplantation is recommended for patients with multiple recurrences of C. difficile infection who have failed these antibiotic treatments.
Recommendation 4. Minimize the frequency and duration of high-risk antibiotic therapy (based on local epidemiology) and the number of antibiotic agents prescribed to reduce C. difficile infection risk (strong recommendation, moderate quality of evidence).
Antibiotic stewardship is a necessary component of any successful effort to reduce C. difficile infections. Antibiotic stewardship programs, which are now commonplace in US hospitals, largely rely on educational initiatives or committee-based order review. Hospitalists should take a structured approach emphasizing the four critical questions of antibiotic prescribing: Does this infection require antibiotics? Have I ordered appropriate cultures and the correct empiric therapy? Can I stop, narrow, or switch to oral agents? Finally, what duration of therapy is needed at discharge?8 Initial efforts should focus on the restriction of fluoroquinolones, clindamycin, and cephalosporins (except for surgical antibiotic prophylaxis) given their known risk to cause C. difficile infection.
Recommendation 5. Contact precautions should be maintained for at least 48 hours after diarrhea has resolved (weak recommendation, low quality of evidence).
Although C. difficile is undetectable in stool samples from most patients by the time diarrhea has resolved, skin and environmental contaminations remain high. No studies demonstrating a benefit to further extending contact precautions beyond 48 hours after resolution of diarrhea are yet available.
CRITIQUE
Methods in Preparing Guidelines
The guideline committee consisted of an interdisciplinary team of healthcare providers with extensive experience in the diagnosis, infection control, treatment, and management of C. difficile. The literature search accessed five different databases (Medline, Embase, Cochrane, Health Technology Assessment, and Database of Abstracts of Reviews and Effects), relevant journals, conference proceedings, and regulatory websites published over the search period of 2009-2016.
A major strength of these guidelines is the extensive work that went into their preparation. The committee reviewed over 14,000 pieces of literature and performed a detailed analysis of each one to determine the quality of evidence in support of each recommendation.
Sources of Potential Conflict of Interest or Bias
To reduce bias, the committee’s work was funded by Infectious Disease Society of America and Society for Healthcare Epidemiology of America. Some authors received funding for work outside of this guideline by companies that manufacture diagnostic assays, vancomycin, and fidaxomicin. These potential conflicts were listed at the end of the article.
Generalizability of the Guideline
Not all studies included in the guideline contain exclusively hospitalized patients, but much of the guideline content is applicable to hospitalized patients. Because C. difficile infection is such a widespread public health problem and these guidelines represent a significant update in knowledge since 2010, the specific recommendations highlighted in this review will impact numerous hospitalists, regardless of the practice setting.
Areas in Need of Future Study
Based on the current literature, as well as statements in the guideline, we expect future guidance around potential screening for and isolation of asymptomatic carriers, including closer guidance on stool transplantation focusing on timing and route, as further data emerge in these areas.
Other Resources
- Grading of Recommendations Assessment, Development, and Evaluation system (http://www.gradeworkinggroup.org)
- Universal Screening for C. difficile in a Tertiary Hospital: risk factors for carriage and clinical disease (Color/Blackhttps://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(19)30048-5/fulltext)
- Effectiveness of Isolating Clostridium Difficile Asymptomatic Carriers on the Incidence of Infections (Color/Blackhttps://clinicaltrials.gov/ct2/show/NCT03223415)
- Effect of Detecting and Isolating Clostridium difficile Carriers at Hospital Admission on the Incidence of C difficile Infections (Color/Blackhttps://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2516765)
- Clinical Trial Testing Fecal Microbiota Transplant for Recurrent Diarrheal Disease Begins (Color/Blackhttps://www.nih.gov/news-events/news-releases/clinical-trial-testing-fecal-microbiota-transplant-recurrent-diarrheal-disease-begins)
1. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(2):S88-S92. https://doi.org/10.1093/cid/cis335.
2. Loo VG, Bourgault AM, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365(18):1693-703. https://doi.org/10.1056/NEJMoa1012413.
3. O’Keefe SJ. Tube feeding, the microbiota, and Clostridium difficile infection. World J Gastroenterol. 2010;16(2):139-142. https://doi.org/10.3748/wjg.v16.i2.139
4. Zacharioudakis IM, Zervou FN, Pliakos EE, Ziakas PD, Mylonakis E. Colonization with toxinogenic C. difficile upon hospital admission, and risk of infection: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110(3):381-90; quiz 391. https://doi.org/10.1038/ajg.2015.22.
5. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59(3):345-354. https://doi.org/10.1093/cid/ciu313.
6. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302-307. https://doi.org/10.1086/519265.
7. Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55(2):S93-S103. https://doi.org/10.1093/cid/cis499.
8. Tamma, PD, Miller MA, Cosgrove SE. Rethinking how antibiotics are prescribed: incorporating the 4 moments of antibiotic decision making into clinical practice. JAMA. 2018;321(2):139-140. https://doi.org/10.1001/jama.2018.19509.
1. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(2):S88-S92. https://doi.org/10.1093/cid/cis335.
2. Loo VG, Bourgault AM, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365(18):1693-703. https://doi.org/10.1056/NEJMoa1012413.
3. O’Keefe SJ. Tube feeding, the microbiota, and Clostridium difficile infection. World J Gastroenterol. 2010;16(2):139-142. https://doi.org/10.3748/wjg.v16.i2.139
4. Zacharioudakis IM, Zervou FN, Pliakos EE, Ziakas PD, Mylonakis E. Colonization with toxinogenic C. difficile upon hospital admission, and risk of infection: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110(3):381-90; quiz 391. https://doi.org/10.1038/ajg.2015.22.
5. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59(3):345-354. https://doi.org/10.1093/cid/ciu313.
6. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302-307. https://doi.org/10.1086/519265.
7. Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55(2):S93-S103. https://doi.org/10.1093/cid/cis499.
8. Tamma, PD, Miller MA, Cosgrove SE. Rethinking how antibiotics are prescribed: incorporating the 4 moments of antibiotic decision making into clinical practice. JAMA. 2018;321(2):139-140. https://doi.org/10.1001/jama.2018.19509.
© 2019 Society of Hospital Medicine
All in the Stream
A 67-year-old man presented to the emergency department with four days of nausea, vomiting, and chills. He was originally from the Philippines but lived in the United States for six years. His past medical history was notable for nephrolithiasis for which a ureteral stent had been placed and was subsequently removed three years prior. He reported no abdominal pain, diarrhea, dysuria, urinary frequency, hematuria, cough, headache, or fever. He was a retired high school teacher and a lifelong nonsmoker.
This patient presents with a nonspecific constellation of constitutional and gastrointestinal (GI) symptoms. A system-based approach may be helpful when considering the differential diagnosis. Chills most often suggest infection, especially in older patients. With regard to GI causes, acute gastroenteritis and other food-borne infections can cause nausea, vomiting, and chills, but these are typically accompanied by abdominal pain and diarrhea and often resolve in less than four days. Abdominal pain would also be expected with cholecystitis as well as more life-threatening causes of nausea such as acute pancreatitis, mesenteric ischemia, and bowel obstruction. In contrast, abdominal pain would not be expected with a central nervous system (CNS) infection such as a brain abscess, which may cause nausea from increased intracranial pressure. Headaches occur in a majority of these cases, making CNS etiologies of nausea less likely. Cardiovascular causes, including myocardial ischemia and infarction, may lead to nausea and vomiting, but these are less likely given the absence of chest pain. Genitourinary causes must be considered, especially given his history of both nephrolithiasis and instrumentation. A stricture or recurrence of nephrolithiasis could lead to acute pyelonephritis or perinephric abscess, although both commonly present with urinary tract symptoms. Uremia, possibly from obstructive uropathy given the patient’s history of nephrolithiasis, could also lead to this constellation of symptoms.
On examination, temperature was 101.6 °F, heart rate 126 beats per minute, respiratory rate 18 breaths per minute, blood pressure 120/76 mm Hg, and oxygen saturation 98% on room air. The oral mucosa was moist, heart sounds were normal without murmurs, lungs were clear to auscultation, and abdomen was soft, nontender, and nondistended. There was no flank tenderness, and penile, testicular, and prostate examination findings were normal.
Laboratory studies revealed a serum sodium of 126 mEq/L, potassium 5.0 mEq/L, chloride 98 mEq/L, bicarbonate 15 mEq/L, blood urea nitrogen 88 mg/dL, creatinine 9.0 mg/dL, calcium 8 mg/dL, glucose 110 mg/dL, and albumin 3.3 g/dL. One year prior, serum creatinine was 1.4 mg/dL. His white blood cell (WBC) count was 8.0 k/uL with normal differential, hemoglobin 11.4 g/dL with normal MCV, and platelet count was normal. Serum osmolality was 286 mOsm/kg and serum parathyroid hormone (PTH) level 63 pg/mL (normal, 10-65). The urinalysis revealed cloudy urine with a specific gravity 1.009, 54 red blood cells (RBC), 236 WBC, large leukocyte esterase, negative nitrite, trace protein, and no casts or dysmorphic RBCs. A random urine specimen revealed sodium of 86 mEq/L, potassium 16 mEq/L, chloride 80 mEq/L, and creatinine 70 mg/dL.
Fever and tachycardia support an infectious cause of his symptoms. Absent flank tenderness and a normal genitourinary examination have only moderate negative predictive values for acute pyelonephritis and prostatitis, respectively. The most striking laboratory finding is his azotemia. Acute kidney injury (AKI) is more likely than chronic kidney disease (CKD) given that the PTH level is normal and the serum creatinine from a year ago was near normal. The most useful finding to differentiate AKI from CKD is the presence of atrophic kidneys on imaging. The low bicarbonate level indicates a metabolic acidosis. His serum anion gap is 13 mEq/L, which falls above most normal ranges. A mildly elevated serum anion gap together with a “delta serum anion gap/delta serum bicarbonate” ratio less than one suggest concomitant anion gap metabolic acidosis and non anion gap metabolic acidosis. The latter, coupled with a history of nephrolithiasis, may point to the possibility of renal tubular acidosis and AKI caused by urinary tract obstruction. This could also account for the marked hyponatremia. Moreover, his high fractional excretion of sodium (9%) is not suggestive of prerenal injury, the most common acute renal injury among patients who present to the emergency department. Hematuria carries a broad differential diagnosis, but most common causes include nephrolithiasis, urinary tract infection (UTI), prostatitis, neoplasm, and glomerulonephritis (GN). The lack of casts and dysmorphic RBCs makes GN unlikely. Taken together, his vital signs, examination, and laboratory studies suggest a high likelihood of an upper UTI (acute obstructive pyelonephritis) in the context of AKI due to obstructive uropathy.
Despite both a normal serum WBC count (which has only a moderate negative predictive value) and his low risk of developing life-threating organ dysfunction from sepsis based on a quick Sequential Organ Failure Assessment (qSOFA) score of zero, it is appropriate to start antibiotics after drawing blood and doing urine cultures. The next step should include administration of a broad-spectrum regimen that is appropriately dose-adjusted for renal dysfunction, such as an antipseudomonal carbapenem and vancomycin to cover extended-spectrum beta-lactamase (ESBL)-producing organisms, Pseudomonas aeruginosa, and methicillin-resistant Staphylococcus aureus (MRSA). This broad coverage is indicated for empiric treatment of complicated obstructive pyelonephritis, a condition that may arise from significant urinary obstruction and that carries a high risk of rapid clinical deterioration. He should undergo a rapid bedside test to assess for urethral or bladder outlet obstruction: either a bladder ultrasound or temporary insertion of a bladder catheter. He should also have an urgent computed tomography (CT) of his abdomen and pelvis without intravenous (IV) contrast, looking for evidence of urinary tract obstruction. A CT is preferred over ultrasound of the kidneys and bladder as CT has higher sensitivity and specificity for nephrolithiasis and neoplasm.
A CT of the abdomen and pelvis without IV contrast revealed bilateral hydroureter and hydronephrosis with multiple punctate calcified stones within the right calyces and the distal right ureter (Figure 1, Figure 2). However, these appeared too small to cause the degree of obstruction visualized. There were no stones noted in the left ureter to account for the obstruction, though small stones were noted in the left calyces. The bladder appeared normal.
Rarely are both ureters obstructed proximal to the ureterovesical junctions in the retroperitoneum. When they are, CT scans usually reveal culprit lesions that are extrinsic to the urinary tract, such as masses or retroperitoneal fibrosis, the latter of which can be associated with IgG4-related disease. Intrinsic causes of urinary tract obstructions include ureteral strictures (from infections, nephrolithiasis, instrumentation, prior radiotherapy, or rarely urothelial cancer), blood clots, metastatic ureteral deposits, or nephrolithiasis. While most intrinsic causes are unilateral, the patient is predisposed to strictures given his history of ureteral instrumentation. A preexisting unilateral obstruction due to a stricture may now, therefore, be unmasked by a second intrinsic obstruction in the contralateral ureter. Alternatively, given his remote history of living in the Philippines, a site where Schistosoma haematobium is endemic, chronic genitourinary schistosomiasis may have caused ureteral strictures due to granulomas, fibrosis, or pseudopolyps.
More commonly, bilateral hydroureter with bilateral hydronephrosis is caused by an obstruction of the bladder or urethra. CT scans can reveal prostatic hyperplasia (occasionally with protrusion into the bladder) and increased bladder wall thickness as a result of chronic bladder outlet obstruction, but the negative predictive value of either finding is modest. More revealing is that the patient reported neither an inability to pass urine (in fact, a “random” urine sample was obtained) nor suprapubic discomfort. Both symptoms would be pronounced with acute bladder obstruction but may be minimal with slowly progressive obstruction. In either case, a distended bladder would have been seen on the CT scan.
Regardless of the cause or whether the obstruction is in the upper or lower urinary tract, emergency intervention is needed to relieve the obstruction when AKI presents with bilateral hydronephrosis. A urology consultation should be sought urgently to determine the best strategy to relieve the obstruction. This may include bilateral percutaneous nephrostomy tubes given that the obstruction appears to be above the level of the bladder. Their findings will also direct additional diagnostic workup.
The patient received ceftriaxone and underwent cystoscopy, which revealed a stricture of the distal bulbar urethra. The ureters and bladder could not be completely visualized due to hematuria. The urethral stricture was dilated, and a Foley catheter was placed. In the operating room, the patient had a fever of 103 °F and developed severe hypotension unresponsive to 3 L of intravenous normal saline. Norepinephrine infusion was initiated for refractory hypotension.
Except for transurethral prostate resections, endoscopic urologic procedures rarely lead to a stricture of any segment of the urethra, suggesting that the previous ureteral stent placement and retrieval were not causal. Longstanding Mycobacterium tuberculosis or Schistosoma haematobium infection occasionally causes urethral strictures presenting as bilateral hydronephrosis. The multiple punctate calcified “stones” demonstrated on CT may suggest either diagnosis if they were actually calcified granulomas.
Regardless of the cause, most patients with a urethral stricture have chronic lower urinary tract symptoms such as decreased stream and the feeling of incomplete bladder emptying. Since this patient does not report these symptoms, it is important to consider if the stricture might be merely incidental. The absence of pain is more telling than the absence of chronic or recurrent symptoms. Lack of pain argues strongly against a pure de novo acute obstruction because abrupt stretching of the renal capsules and the walls of the collecting system is usually painful. Slow stretching caused by a progressive stricture may mask the pain of a superimposed acute obstruction. A blood clot, for example, may have precipitated an acute-on-chronic obstruction upon lodging at the urethral stricture.
The worsening systemic response to the procedure may be due to increased intravesical pressure by dilation and cystoscopy, which may have caused subsequent backflow of bacteria from the renal parenchyma into the circulation (pyelorenal backflow). The broad-spectrum antibiotic regimen suggested above and IV crystalloid infusion should be continued with close hemodynamic monitoring.
Treatment for severe sepsis was initiated with empiric piperacillin-tazobactam, ceftriaxone was discontinued, and the patient was transferred to the intensive care unit (ICU). Norepinephrine was discontinued after 24 hours. Despite the indwelling Foley catheter, his kidney function worsened (creatinine increased to 10.5 over the next 36 hours, and he remained oliguric). Therefore, bilateral percutaneous nephrostomy tubes were placed to relieve the ongoing obstruction. In the ICU, he remained febrile, despite receiving piperacillin-tazobactam, through hospital day 7. Serial blood and urine cultures all remained negative. HIV testing was negative. His chest radiograph was unremarkable, and transthoracic echocardiogram was normal. His creatinine improved but plateaued at 2.5 mg/dL by day 7.
Worsening renal function (alongside oliguria or anuria) despite a functioning Foley catheter suggests either intrinsic renal disease or bilateral ureteral obstructions. The initial attempt at relieving the obstruction with a Foley catheter did not take into consideration the bilateral ureteral strictures. As a result, soon thereafter, the insertion of percutaneous nephrostomy tubes was necessary. Given the severity of his illness, underlying obstructive uropathy, and persistent fever, suggesting an ongoing infection, one strategy would be to continue antibiotics with broader coverage than piperacillin-tazobactam. This approach may be reasonable, given the emergence of ESBL organisms and the possibility of MRSA due to instrumentation. However, it is important to note that only sterile pyuria has been identified to date, which raises the possibility of nonbacterial infections. Although chronic infection with Schistosoma haematobium can cause bilateral ureteral strictures, associated fever is limited to the acute phase of infection and not the chronic obstructive phase, unless there is a superimposed infection. Genitourinary Mycobacterium tuberculosis remains a likely possibility, regardless of the unrevealing chest radiograph. Urine nucleic acid amplification and acid-fast bacilli (AFB) smear and culture, the best initial diagnostic test, should be sent. Although less definitive, a tuberculin skin test and an interferon-gamma release assay should also be conducted. Histopathology of the ureters obtained by repeat cystoscopy may be diagnostic, but given the limited visualization during the last cystoscopy and the recent dilation of the urethra, this option should be kept in reserve for now.
Antibiotics were discontinued on day 7, but the patient continued to experience ongoing fever. Urine Histoplasma and serum cryptococcal antigens were negative. His urine AFB smear was 1+ positive. Liver function tests revealed a total protein of 7.0 g/dL, albumin 3.0 g/dL, total bilirubin 1.2 mg/dL, direct bilirubin 0.3 mg/dL, alkaline phosphatase 418 U/L, aspartate aminotransferase 65 U/L, alanine aminotransferase 88 U/L, gamma-glutamyltransferase (GGT) 609 U/L (normal, 3-60), and lactate dehydrogenase 284 U/L (normal, 85-210).
Acid-fast bacillus in the urine strongly suggests Mycobacterium tuberculosis (MTB) with several reports of likelihood ratios greater than 10. Nevertheless, confirmation is needed to rule out nontuberculous mycobacteria because of potential hepatotoxicity from treatment. Up to six urine samples should be sent for mycobacterium culture. However, false negative rates of up to 90% are reported, and final test results can take up to two months, so other methods of confirmation should be simultaneously sought. A nucleic acid amplification test of urine could rule in a pathogenic species within 24 hours. Alternatively, the probability of a nonpathogenic colonizing species would be negligible if a caseating granuloma was found. Biopsy could be obtained from the ureters, as suggested above. Liver biopsy should also be considered, especially if the moderate elevations in alkaline phosphatase and GGT (the most common liver enzyme abnormalities in hepatic tuberculosis) did not merely wax and wane with sepsis.
A CT of the thorax without IV contrast was done to evaluate for evidence of pulmonary disease given the positive urine AFB. This demonstrated bilateral fibrotic upper lobe opacities suggestive of prior granulomatous disease but no cavitary lung lesions (Figure 3). Three sputum smears were negative for AFB, but one sample showed Mycobacterium tuberculosis detected by a polymerase chain reaction (PCR) probe.
Given the concern for genitourinary tuberculosis (GUTB), it is appropriate to place the patient in respiratory isolation to exclude concomitant pulmonary tuberculosis (TB). AFB smears were negative, but the sputum PCR probe was positive, confirming pathogenic MTB. However, the negative AFB smears make the likelihood of pulmonary infectivity low. As a result, contact tracing is often deemed unnecessary by hospital infection control teams. Though his chest radiograph was normal, CT showed bilateral upper lobe fibrotic disease suggestive of prior pulmonary TB, thus making it likely that the current GUTB represents reactivation.
The two-month initiation phase of treatment with four antituberculosis drugs should begin while drug susceptibility tests are pending. Potential hepatotoxicity should be closely monitored, ideally by a clinician with experience treating tuberculosis in patients with existing liver disease. As a general precaution, alcohol should be avoided as should medications such as acetaminophen that are known to be hepatotoxic. Urology follow-up is also needed because about one-third of tuberculous ureteral strictures treated initially with percutaneous nephrostomy do not resolve with antituberculosis therapy.
The patient was started on weight-based antituberculosis treatment with four antimicrobial agents (rifampin, ethambutol, pyrazinamide, and isoniazid). He was seen in the infectious disease clinic two weeks later; his fever had resolved, and his liver function tests showed normalization of AST and LDH as well as a 45% reduction in his GGT and alkaline phosphatase levels. Two months following discharge, a nuclear medicine radionuclide angiogram renal flow scan showed normal right kidney function. The right nephrostomy tube was subsequently removed. He continued to have left kidney outflow obstruction due to a residual ureteral stricture (Figure 4). Repeat cystoscopy and attempted left ureteral stenting was unsuccessful. The left nephrostomy tube remained in place.
DISCUSSION
According to the Centers for Disease Control, in 2017, 10 million people became sick with TB, and there were 1.3 million TB-related deaths worldwide with 9,150 cases reported in the United States. Extrapulmonary TB (EPTB) constitutes 10% of all TB cases globally.1-4 GUTB is the second most common form of EPTB after lymph node TB, and it occurs in up to 20% of all pulmonary TB cases.2,3
Mycobacteria reach the genitourinary (GU) tract via hematogenous spread during primary infection or reactivation of TB. This leads to cortical and medullary lesions, which can heal spontaneously or eventually (average of 22 years) rupture into the tubules and onto the collecting system, ureters, and bladder.5,6 Spread to the ureter and bladder leads to multiple ureteral strictures and contracture of the bladder with disruption of the ureterovesical junction (UVJ), which causes hydroureter and hydronephrosis.7 Unilateral kidney involvement is most common, but bilateral involvement can occur following exacerbated hematogenous spread, particularly in immune deficient patients. Bilateral kidney involvement is also possible from retrograde spread to the good kidney due to bladder contracture and UVJ disruption.8,9 Distal infection can involve all aspects of the male and female genital tracts, but urethral strictures are extremely rare.10,11
GUTB affects males more than females (2:1) and presents insidiously at 40 to 60 years of age.12 Other risk factors for TB include birth in TB endemic areas, prior TB infection, immunosuppression, malnutrition, severe systemic disease, diabetes, and cirrhosis. It is crucial to assess these risk factors when creating and refining differential diagnoses. Many patients have hematuria and sterile pyuria as incidental initial findings. The most common symptoms arise from bladder involvement, including frequency, urgency, and dysuria. Low back pain and gross hematuria are also common, but fever and constitutional symptoms are uncommon.10 Bilateral ureteral strictures can lead to obstructive renal failure, and involvement of the genital tracts can lead to pelvic or scrotal pain, swelling, and fistula formation.10
Diagnosis involves the demonstration of TB bacilli in urine or GU tissue. The urinalysis reveals hematuria and sterile pyuria.13 Urine AFB stains are positive in 52% of cases but are not diagnostic as nontuberculous mycobacteria may also cause a positive test result.13,14 Urine cultures for Mycobacterium tuberculosis are positive in up to 90% of cases after six to eight weeks. As many as three to six morning urine samples are required to achieve diagnostic accuracy.10,14 Urine PCR for Mycobacterium tuberculosis has 96% sensitivity and up to 98% specificity,14 while PCR on GU tissue has a sensitivity of 88% and specificity of 87%.15 The rapid nucleic acid amplification assay Xpert MTB/RIF in urine has a sensitivity of 83%, and specificity of 98%.16 Imaging is required to evaluate for obstruction, and the CT scan is abnormal in up to 90% of cases, showing multiple ureteral stenoses, hydroureter and hydronephrosis, and a contracted bladder.10,17
GUTB is treated with standard antituberculosis regimens.18 Patients with urinary obstruction benefit from ureteral stenting or percutaneous nephrostomy, bladder diversion, or ureteral reconstruction surgery. Unilateral nephrectomy for a nonfunctioning kidney with extensive disease is occasionally required.19 Following treatment, relapse occurs in up to 6% of patients over five years, and long-term follow-up with urine cultures and PCR every six months is recommended.10,20 Appropriate screening and treatment for latent tuberculosis infection greatly reduces the risk of reactivation GUTB.
This patient presented with features of an infection, which, combined with his history of renal stones and his urinalysis, led to an appropriate suspicion of and empiric treatment for an upper UTI. Given the AKI and nephrolithiasis, imaging was done to exclude obstruction. The CT finding of bilateral hydroureters and hydronephrosis absent an obstructing stone or mass or abnormal bladder was the initial clue that this was not a typical bacterial infection and that there was likely an underlying infectious pathologic process such as TB involving the GU tract diffusely. The care team treated the patient as an individual with fever and sterile pyuria in the context of multiple urinary tract strictures and an initial unrevealing infectious diagnostic workup. They recognized that the clues to the ultimate diagnosis of GUTB were all in the stream.
KEY TEACHING POINTS
- GUTB is a significant cause of sterile pyuria.
- In the presence of bilateral hydronephrosis, it is vital to determine the level of obstruction. If the bladder is not distended or contracted, then obstruction is likely at the level of the ureters and initial use of percutaneous nephrostomy tubes to relieve obstruction may be preferred.
- Imaging abnormalities such as multiple ureteral strictures, hydroureter and hydronephrosis (absent an obstructing stone or mass), and the finding of a contracted bladder are highly suggestive of GUTB.
- The mainstay of treatment for GUTB is standard antituberculosis treatment regimens in combination with the relief of urinary obstruction by ureteral stenting, percutaneous nephrostomy or open surgery.
- GUTB can relapse in up to 6% of treated cases over five years, and long-term follow-up and surveillance with urine culture and PCR every six months are recommended.
Disclosures
Benjamin Mba, Nathan Houchens, Marie Jennifer Seares, and Udit Joshi have no financial conflicts of interest and no disclosures.
Funding
Brian P. Lucas receives funding from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, and National Center for Translational Science (UL1TR001086).
1. Forssbohm M, Zwahlen M, Loddenkemper R, Rieder HL. Demographic characteristics of patients with extrapulmonary tuberculosis in Germany. Eur Resp J. 2008;31(1):99-105. https://doi.org/10.1183/09031936.00020607.
2. French CE, Antoine D, Gelb D, Jones JA, Gilbert RL, Watson JM. Tuberculosis in non-UK-born persons, England and Wales, 2001-2003. Int J Tuberc Lung Dis. 2007;11(5):577-584.
3. Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis. 2009;49(9):1350-1357. https://doi.org/10.1086/605559.
4. Alvarez S, McCabe WR. Extrapulmonary tuberculosis revisited: a review of experience at Boston City and other hospitals. Medicine. 1984;63(1):25-55.
5. Simon HB, Weinstein AJ, Pasternak MS, Swartz MN, Kunz LJ. Genitourinary tuberculosis. Clinical features in a general hospital population. Am J Med. 1977;63(3):410-420. https://doi.org/10.1016/0002-9343(77)90279-0.
6. Christensen WI. Genitourinary tuberculosis: review of 102 cases. Medicine. 1974;53(5):377-390. https://doi.org/10.1016/0002-9343(77)90279-0.
7. Eastwood JB, Corbishley CM, Grange JM. Tuberculosis and the kidney. J Am Soc Nephrol. 2001;12(6):1307-1314.
8. Garcia-Rodriguez JA, Garcia Sanchez JE, Munoz Bellido JL, et al. Genitourinary tuberculosis in Spain: review of 81 cases. Clin Infect Dis.1994;18(4):557-561. https://doi.org/10.1093/clinids/18.4.557.
9. de Figueiredo AA, Lucon AM, Srougi M. Bladder augmentation for the treatment of chronic tuberculous cystitis. Clinical and urodynamic evaluation of 25 patients after long term follow-up. Neurourol Urodyn. 2006;25(5):433-440. https://doi.org/10.1002/nau.20264.
10. Figueiredo AA, Lucon AM, Srougi M. Urogenital Tuberculosis. Microbiol Spectr. 2017;5. https://doi.org/10.1128/microbiolspec.TNMI7-0015-2016.
11. Gupta N, Mandal AK, Singh SK. Tuberculosis of the prostate and urethra: A review. Indian J Urol. 2008;24(3):388-391. https://doi.org/10.4103/0970-1591.42623.
12. Figueiredo AA, Lucon AM, Junior RF, Srougi M. Epidemiology of urogenital tuberculosis worldwide. Int J Urol. 2008;15(9):827-832. https://doi.org/10.1111/j.1442-2042.2008.02099.x.
13. Mortier E, Pouchot J, Girard L, Boussougant Y, Vinceneux P. Assessment of urine analysis for the diagnosis of tuberculosis. BMJ (Clinical research ed). 1996;312:27-28. https://doi.org/10.1136/bmj.312.7022.27.
14. Moussa OM, Eraky I, El-Far MA, et al. Rapid diagnosis of genitourinary tuberculosis by polymerase chain reaction and non-radioactive DNA hybridization. J Urol. 2000;164(2):584-588. https://doi.org/10.1016/S0022-5347(05)67427-7.
15. Chawla A, Chawla K, Reddy S, et al. Can tissue PCR augment the diagnostic accuracy in genitourinary tract tuberculosis? Urol Int. 2012;88(1):34-38. https://doi.org/10.1159/000327039.
16. Kohli M, Schiller I, Dendukuri N, et al. Xpert((R)) MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database Syst Rev. 2018;8:Cd012768. https://doi.org/10.1002/14651858.CD012768.pub2.
17. Figueiredo AA, Lucon AM, Arvellos AN, et al. A better understanding of urogenital tuberculosis pathophysiology based on radiological findings. Eur J Radiol. 2010;76(2):246-257. https://doi.org/10.1016/j.ejrad.2009.05.049.
18. Treatment of Tuberculosis: Guidelines. 4th edition. Geneva: World Health Organization. 2010.
19. O’Flynn D. Surgical treatment of genito-urinary tuberculosis. A report on 762 cases. Br J Urol. 1970;42(6):667-671. https://doi.org/10.1111/j.1464-410X.1970.tb06789.x.
20. Butler MR, O’Flynn JD. Reactivation of genito-urinary tuberculosis: a retrospective review of 838 cases. Eur Urol. 1975;1:14-17. https://doi.org/10.1159/000455566.
A 67-year-old man presented to the emergency department with four days of nausea, vomiting, and chills. He was originally from the Philippines but lived in the United States for six years. His past medical history was notable for nephrolithiasis for which a ureteral stent had been placed and was subsequently removed three years prior. He reported no abdominal pain, diarrhea, dysuria, urinary frequency, hematuria, cough, headache, or fever. He was a retired high school teacher and a lifelong nonsmoker.
This patient presents with a nonspecific constellation of constitutional and gastrointestinal (GI) symptoms. A system-based approach may be helpful when considering the differential diagnosis. Chills most often suggest infection, especially in older patients. With regard to GI causes, acute gastroenteritis and other food-borne infections can cause nausea, vomiting, and chills, but these are typically accompanied by abdominal pain and diarrhea and often resolve in less than four days. Abdominal pain would also be expected with cholecystitis as well as more life-threatening causes of nausea such as acute pancreatitis, mesenteric ischemia, and bowel obstruction. In contrast, abdominal pain would not be expected with a central nervous system (CNS) infection such as a brain abscess, which may cause nausea from increased intracranial pressure. Headaches occur in a majority of these cases, making CNS etiologies of nausea less likely. Cardiovascular causes, including myocardial ischemia and infarction, may lead to nausea and vomiting, but these are less likely given the absence of chest pain. Genitourinary causes must be considered, especially given his history of both nephrolithiasis and instrumentation. A stricture or recurrence of nephrolithiasis could lead to acute pyelonephritis or perinephric abscess, although both commonly present with urinary tract symptoms. Uremia, possibly from obstructive uropathy given the patient’s history of nephrolithiasis, could also lead to this constellation of symptoms.
On examination, temperature was 101.6 °F, heart rate 126 beats per minute, respiratory rate 18 breaths per minute, blood pressure 120/76 mm Hg, and oxygen saturation 98% on room air. The oral mucosa was moist, heart sounds were normal without murmurs, lungs were clear to auscultation, and abdomen was soft, nontender, and nondistended. There was no flank tenderness, and penile, testicular, and prostate examination findings were normal.
Laboratory studies revealed a serum sodium of 126 mEq/L, potassium 5.0 mEq/L, chloride 98 mEq/L, bicarbonate 15 mEq/L, blood urea nitrogen 88 mg/dL, creatinine 9.0 mg/dL, calcium 8 mg/dL, glucose 110 mg/dL, and albumin 3.3 g/dL. One year prior, serum creatinine was 1.4 mg/dL. His white blood cell (WBC) count was 8.0 k/uL with normal differential, hemoglobin 11.4 g/dL with normal MCV, and platelet count was normal. Serum osmolality was 286 mOsm/kg and serum parathyroid hormone (PTH) level 63 pg/mL (normal, 10-65). The urinalysis revealed cloudy urine with a specific gravity 1.009, 54 red blood cells (RBC), 236 WBC, large leukocyte esterase, negative nitrite, trace protein, and no casts or dysmorphic RBCs. A random urine specimen revealed sodium of 86 mEq/L, potassium 16 mEq/L, chloride 80 mEq/L, and creatinine 70 mg/dL.
Fever and tachycardia support an infectious cause of his symptoms. Absent flank tenderness and a normal genitourinary examination have only moderate negative predictive values for acute pyelonephritis and prostatitis, respectively. The most striking laboratory finding is his azotemia. Acute kidney injury (AKI) is more likely than chronic kidney disease (CKD) given that the PTH level is normal and the serum creatinine from a year ago was near normal. The most useful finding to differentiate AKI from CKD is the presence of atrophic kidneys on imaging. The low bicarbonate level indicates a metabolic acidosis. His serum anion gap is 13 mEq/L, which falls above most normal ranges. A mildly elevated serum anion gap together with a “delta serum anion gap/delta serum bicarbonate” ratio less than one suggest concomitant anion gap metabolic acidosis and non anion gap metabolic acidosis. The latter, coupled with a history of nephrolithiasis, may point to the possibility of renal tubular acidosis and AKI caused by urinary tract obstruction. This could also account for the marked hyponatremia. Moreover, his high fractional excretion of sodium (9%) is not suggestive of prerenal injury, the most common acute renal injury among patients who present to the emergency department. Hematuria carries a broad differential diagnosis, but most common causes include nephrolithiasis, urinary tract infection (UTI), prostatitis, neoplasm, and glomerulonephritis (GN). The lack of casts and dysmorphic RBCs makes GN unlikely. Taken together, his vital signs, examination, and laboratory studies suggest a high likelihood of an upper UTI (acute obstructive pyelonephritis) in the context of AKI due to obstructive uropathy.
Despite both a normal serum WBC count (which has only a moderate negative predictive value) and his low risk of developing life-threating organ dysfunction from sepsis based on a quick Sequential Organ Failure Assessment (qSOFA) score of zero, it is appropriate to start antibiotics after drawing blood and doing urine cultures. The next step should include administration of a broad-spectrum regimen that is appropriately dose-adjusted for renal dysfunction, such as an antipseudomonal carbapenem and vancomycin to cover extended-spectrum beta-lactamase (ESBL)-producing organisms, Pseudomonas aeruginosa, and methicillin-resistant Staphylococcus aureus (MRSA). This broad coverage is indicated for empiric treatment of complicated obstructive pyelonephritis, a condition that may arise from significant urinary obstruction and that carries a high risk of rapid clinical deterioration. He should undergo a rapid bedside test to assess for urethral or bladder outlet obstruction: either a bladder ultrasound or temporary insertion of a bladder catheter. He should also have an urgent computed tomography (CT) of his abdomen and pelvis without intravenous (IV) contrast, looking for evidence of urinary tract obstruction. A CT is preferred over ultrasound of the kidneys and bladder as CT has higher sensitivity and specificity for nephrolithiasis and neoplasm.
A CT of the abdomen and pelvis without IV contrast revealed bilateral hydroureter and hydronephrosis with multiple punctate calcified stones within the right calyces and the distal right ureter (Figure 1, Figure 2). However, these appeared too small to cause the degree of obstruction visualized. There were no stones noted in the left ureter to account for the obstruction, though small stones were noted in the left calyces. The bladder appeared normal.
Rarely are both ureters obstructed proximal to the ureterovesical junctions in the retroperitoneum. When they are, CT scans usually reveal culprit lesions that are extrinsic to the urinary tract, such as masses or retroperitoneal fibrosis, the latter of which can be associated with IgG4-related disease. Intrinsic causes of urinary tract obstructions include ureteral strictures (from infections, nephrolithiasis, instrumentation, prior radiotherapy, or rarely urothelial cancer), blood clots, metastatic ureteral deposits, or nephrolithiasis. While most intrinsic causes are unilateral, the patient is predisposed to strictures given his history of ureteral instrumentation. A preexisting unilateral obstruction due to a stricture may now, therefore, be unmasked by a second intrinsic obstruction in the contralateral ureter. Alternatively, given his remote history of living in the Philippines, a site where Schistosoma haematobium is endemic, chronic genitourinary schistosomiasis may have caused ureteral strictures due to granulomas, fibrosis, or pseudopolyps.
More commonly, bilateral hydroureter with bilateral hydronephrosis is caused by an obstruction of the bladder or urethra. CT scans can reveal prostatic hyperplasia (occasionally with protrusion into the bladder) and increased bladder wall thickness as a result of chronic bladder outlet obstruction, but the negative predictive value of either finding is modest. More revealing is that the patient reported neither an inability to pass urine (in fact, a “random” urine sample was obtained) nor suprapubic discomfort. Both symptoms would be pronounced with acute bladder obstruction but may be minimal with slowly progressive obstruction. In either case, a distended bladder would have been seen on the CT scan.
Regardless of the cause or whether the obstruction is in the upper or lower urinary tract, emergency intervention is needed to relieve the obstruction when AKI presents with bilateral hydronephrosis. A urology consultation should be sought urgently to determine the best strategy to relieve the obstruction. This may include bilateral percutaneous nephrostomy tubes given that the obstruction appears to be above the level of the bladder. Their findings will also direct additional diagnostic workup.
The patient received ceftriaxone and underwent cystoscopy, which revealed a stricture of the distal bulbar urethra. The ureters and bladder could not be completely visualized due to hematuria. The urethral stricture was dilated, and a Foley catheter was placed. In the operating room, the patient had a fever of 103 °F and developed severe hypotension unresponsive to 3 L of intravenous normal saline. Norepinephrine infusion was initiated for refractory hypotension.
Except for transurethral prostate resections, endoscopic urologic procedures rarely lead to a stricture of any segment of the urethra, suggesting that the previous ureteral stent placement and retrieval were not causal. Longstanding Mycobacterium tuberculosis or Schistosoma haematobium infection occasionally causes urethral strictures presenting as bilateral hydronephrosis. The multiple punctate calcified “stones” demonstrated on CT may suggest either diagnosis if they were actually calcified granulomas.
Regardless of the cause, most patients with a urethral stricture have chronic lower urinary tract symptoms such as decreased stream and the feeling of incomplete bladder emptying. Since this patient does not report these symptoms, it is important to consider if the stricture might be merely incidental. The absence of pain is more telling than the absence of chronic or recurrent symptoms. Lack of pain argues strongly against a pure de novo acute obstruction because abrupt stretching of the renal capsules and the walls of the collecting system is usually painful. Slow stretching caused by a progressive stricture may mask the pain of a superimposed acute obstruction. A blood clot, for example, may have precipitated an acute-on-chronic obstruction upon lodging at the urethral stricture.
The worsening systemic response to the procedure may be due to increased intravesical pressure by dilation and cystoscopy, which may have caused subsequent backflow of bacteria from the renal parenchyma into the circulation (pyelorenal backflow). The broad-spectrum antibiotic regimen suggested above and IV crystalloid infusion should be continued with close hemodynamic monitoring.
Treatment for severe sepsis was initiated with empiric piperacillin-tazobactam, ceftriaxone was discontinued, and the patient was transferred to the intensive care unit (ICU). Norepinephrine was discontinued after 24 hours. Despite the indwelling Foley catheter, his kidney function worsened (creatinine increased to 10.5 over the next 36 hours, and he remained oliguric). Therefore, bilateral percutaneous nephrostomy tubes were placed to relieve the ongoing obstruction. In the ICU, he remained febrile, despite receiving piperacillin-tazobactam, through hospital day 7. Serial blood and urine cultures all remained negative. HIV testing was negative. His chest radiograph was unremarkable, and transthoracic echocardiogram was normal. His creatinine improved but plateaued at 2.5 mg/dL by day 7.
Worsening renal function (alongside oliguria or anuria) despite a functioning Foley catheter suggests either intrinsic renal disease or bilateral ureteral obstructions. The initial attempt at relieving the obstruction with a Foley catheter did not take into consideration the bilateral ureteral strictures. As a result, soon thereafter, the insertion of percutaneous nephrostomy tubes was necessary. Given the severity of his illness, underlying obstructive uropathy, and persistent fever, suggesting an ongoing infection, one strategy would be to continue antibiotics with broader coverage than piperacillin-tazobactam. This approach may be reasonable, given the emergence of ESBL organisms and the possibility of MRSA due to instrumentation. However, it is important to note that only sterile pyuria has been identified to date, which raises the possibility of nonbacterial infections. Although chronic infection with Schistosoma haematobium can cause bilateral ureteral strictures, associated fever is limited to the acute phase of infection and not the chronic obstructive phase, unless there is a superimposed infection. Genitourinary Mycobacterium tuberculosis remains a likely possibility, regardless of the unrevealing chest radiograph. Urine nucleic acid amplification and acid-fast bacilli (AFB) smear and culture, the best initial diagnostic test, should be sent. Although less definitive, a tuberculin skin test and an interferon-gamma release assay should also be conducted. Histopathology of the ureters obtained by repeat cystoscopy may be diagnostic, but given the limited visualization during the last cystoscopy and the recent dilation of the urethra, this option should be kept in reserve for now.
Antibiotics were discontinued on day 7, but the patient continued to experience ongoing fever. Urine Histoplasma and serum cryptococcal antigens were negative. His urine AFB smear was 1+ positive. Liver function tests revealed a total protein of 7.0 g/dL, albumin 3.0 g/dL, total bilirubin 1.2 mg/dL, direct bilirubin 0.3 mg/dL, alkaline phosphatase 418 U/L, aspartate aminotransferase 65 U/L, alanine aminotransferase 88 U/L, gamma-glutamyltransferase (GGT) 609 U/L (normal, 3-60), and lactate dehydrogenase 284 U/L (normal, 85-210).
Acid-fast bacillus in the urine strongly suggests Mycobacterium tuberculosis (MTB) with several reports of likelihood ratios greater than 10. Nevertheless, confirmation is needed to rule out nontuberculous mycobacteria because of potential hepatotoxicity from treatment. Up to six urine samples should be sent for mycobacterium culture. However, false negative rates of up to 90% are reported, and final test results can take up to two months, so other methods of confirmation should be simultaneously sought. A nucleic acid amplification test of urine could rule in a pathogenic species within 24 hours. Alternatively, the probability of a nonpathogenic colonizing species would be negligible if a caseating granuloma was found. Biopsy could be obtained from the ureters, as suggested above. Liver biopsy should also be considered, especially if the moderate elevations in alkaline phosphatase and GGT (the most common liver enzyme abnormalities in hepatic tuberculosis) did not merely wax and wane with sepsis.
A CT of the thorax without IV contrast was done to evaluate for evidence of pulmonary disease given the positive urine AFB. This demonstrated bilateral fibrotic upper lobe opacities suggestive of prior granulomatous disease but no cavitary lung lesions (Figure 3). Three sputum smears were negative for AFB, but one sample showed Mycobacterium tuberculosis detected by a polymerase chain reaction (PCR) probe.
Given the concern for genitourinary tuberculosis (GUTB), it is appropriate to place the patient in respiratory isolation to exclude concomitant pulmonary tuberculosis (TB). AFB smears were negative, but the sputum PCR probe was positive, confirming pathogenic MTB. However, the negative AFB smears make the likelihood of pulmonary infectivity low. As a result, contact tracing is often deemed unnecessary by hospital infection control teams. Though his chest radiograph was normal, CT showed bilateral upper lobe fibrotic disease suggestive of prior pulmonary TB, thus making it likely that the current GUTB represents reactivation.
The two-month initiation phase of treatment with four antituberculosis drugs should begin while drug susceptibility tests are pending. Potential hepatotoxicity should be closely monitored, ideally by a clinician with experience treating tuberculosis in patients with existing liver disease. As a general precaution, alcohol should be avoided as should medications such as acetaminophen that are known to be hepatotoxic. Urology follow-up is also needed because about one-third of tuberculous ureteral strictures treated initially with percutaneous nephrostomy do not resolve with antituberculosis therapy.
The patient was started on weight-based antituberculosis treatment with four antimicrobial agents (rifampin, ethambutol, pyrazinamide, and isoniazid). He was seen in the infectious disease clinic two weeks later; his fever had resolved, and his liver function tests showed normalization of AST and LDH as well as a 45% reduction in his GGT and alkaline phosphatase levels. Two months following discharge, a nuclear medicine radionuclide angiogram renal flow scan showed normal right kidney function. The right nephrostomy tube was subsequently removed. He continued to have left kidney outflow obstruction due to a residual ureteral stricture (Figure 4). Repeat cystoscopy and attempted left ureteral stenting was unsuccessful. The left nephrostomy tube remained in place.
DISCUSSION
According to the Centers for Disease Control, in 2017, 10 million people became sick with TB, and there were 1.3 million TB-related deaths worldwide with 9,150 cases reported in the United States. Extrapulmonary TB (EPTB) constitutes 10% of all TB cases globally.1-4 GUTB is the second most common form of EPTB after lymph node TB, and it occurs in up to 20% of all pulmonary TB cases.2,3
Mycobacteria reach the genitourinary (GU) tract via hematogenous spread during primary infection or reactivation of TB. This leads to cortical and medullary lesions, which can heal spontaneously or eventually (average of 22 years) rupture into the tubules and onto the collecting system, ureters, and bladder.5,6 Spread to the ureter and bladder leads to multiple ureteral strictures and contracture of the bladder with disruption of the ureterovesical junction (UVJ), which causes hydroureter and hydronephrosis.7 Unilateral kidney involvement is most common, but bilateral involvement can occur following exacerbated hematogenous spread, particularly in immune deficient patients. Bilateral kidney involvement is also possible from retrograde spread to the good kidney due to bladder contracture and UVJ disruption.8,9 Distal infection can involve all aspects of the male and female genital tracts, but urethral strictures are extremely rare.10,11
GUTB affects males more than females (2:1) and presents insidiously at 40 to 60 years of age.12 Other risk factors for TB include birth in TB endemic areas, prior TB infection, immunosuppression, malnutrition, severe systemic disease, diabetes, and cirrhosis. It is crucial to assess these risk factors when creating and refining differential diagnoses. Many patients have hematuria and sterile pyuria as incidental initial findings. The most common symptoms arise from bladder involvement, including frequency, urgency, and dysuria. Low back pain and gross hematuria are also common, but fever and constitutional symptoms are uncommon.10 Bilateral ureteral strictures can lead to obstructive renal failure, and involvement of the genital tracts can lead to pelvic or scrotal pain, swelling, and fistula formation.10
Diagnosis involves the demonstration of TB bacilli in urine or GU tissue. The urinalysis reveals hematuria and sterile pyuria.13 Urine AFB stains are positive in 52% of cases but are not diagnostic as nontuberculous mycobacteria may also cause a positive test result.13,14 Urine cultures for Mycobacterium tuberculosis are positive in up to 90% of cases after six to eight weeks. As many as three to six morning urine samples are required to achieve diagnostic accuracy.10,14 Urine PCR for Mycobacterium tuberculosis has 96% sensitivity and up to 98% specificity,14 while PCR on GU tissue has a sensitivity of 88% and specificity of 87%.15 The rapid nucleic acid amplification assay Xpert MTB/RIF in urine has a sensitivity of 83%, and specificity of 98%.16 Imaging is required to evaluate for obstruction, and the CT scan is abnormal in up to 90% of cases, showing multiple ureteral stenoses, hydroureter and hydronephrosis, and a contracted bladder.10,17
GUTB is treated with standard antituberculosis regimens.18 Patients with urinary obstruction benefit from ureteral stenting or percutaneous nephrostomy, bladder diversion, or ureteral reconstruction surgery. Unilateral nephrectomy for a nonfunctioning kidney with extensive disease is occasionally required.19 Following treatment, relapse occurs in up to 6% of patients over five years, and long-term follow-up with urine cultures and PCR every six months is recommended.10,20 Appropriate screening and treatment for latent tuberculosis infection greatly reduces the risk of reactivation GUTB.
This patient presented with features of an infection, which, combined with his history of renal stones and his urinalysis, led to an appropriate suspicion of and empiric treatment for an upper UTI. Given the AKI and nephrolithiasis, imaging was done to exclude obstruction. The CT finding of bilateral hydroureters and hydronephrosis absent an obstructing stone or mass or abnormal bladder was the initial clue that this was not a typical bacterial infection and that there was likely an underlying infectious pathologic process such as TB involving the GU tract diffusely. The care team treated the patient as an individual with fever and sterile pyuria in the context of multiple urinary tract strictures and an initial unrevealing infectious diagnostic workup. They recognized that the clues to the ultimate diagnosis of GUTB were all in the stream.
KEY TEACHING POINTS
- GUTB is a significant cause of sterile pyuria.
- In the presence of bilateral hydronephrosis, it is vital to determine the level of obstruction. If the bladder is not distended or contracted, then obstruction is likely at the level of the ureters and initial use of percutaneous nephrostomy tubes to relieve obstruction may be preferred.
- Imaging abnormalities such as multiple ureteral strictures, hydroureter and hydronephrosis (absent an obstructing stone or mass), and the finding of a contracted bladder are highly suggestive of GUTB.
- The mainstay of treatment for GUTB is standard antituberculosis treatment regimens in combination with the relief of urinary obstruction by ureteral stenting, percutaneous nephrostomy or open surgery.
- GUTB can relapse in up to 6% of treated cases over five years, and long-term follow-up and surveillance with urine culture and PCR every six months are recommended.
Disclosures
Benjamin Mba, Nathan Houchens, Marie Jennifer Seares, and Udit Joshi have no financial conflicts of interest and no disclosures.
Funding
Brian P. Lucas receives funding from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, and National Center for Translational Science (UL1TR001086).
A 67-year-old man presented to the emergency department with four days of nausea, vomiting, and chills. He was originally from the Philippines but lived in the United States for six years. His past medical history was notable for nephrolithiasis for which a ureteral stent had been placed and was subsequently removed three years prior. He reported no abdominal pain, diarrhea, dysuria, urinary frequency, hematuria, cough, headache, or fever. He was a retired high school teacher and a lifelong nonsmoker.
This patient presents with a nonspecific constellation of constitutional and gastrointestinal (GI) symptoms. A system-based approach may be helpful when considering the differential diagnosis. Chills most often suggest infection, especially in older patients. With regard to GI causes, acute gastroenteritis and other food-borne infections can cause nausea, vomiting, and chills, but these are typically accompanied by abdominal pain and diarrhea and often resolve in less than four days. Abdominal pain would also be expected with cholecystitis as well as more life-threatening causes of nausea such as acute pancreatitis, mesenteric ischemia, and bowel obstruction. In contrast, abdominal pain would not be expected with a central nervous system (CNS) infection such as a brain abscess, which may cause nausea from increased intracranial pressure. Headaches occur in a majority of these cases, making CNS etiologies of nausea less likely. Cardiovascular causes, including myocardial ischemia and infarction, may lead to nausea and vomiting, but these are less likely given the absence of chest pain. Genitourinary causes must be considered, especially given his history of both nephrolithiasis and instrumentation. A stricture or recurrence of nephrolithiasis could lead to acute pyelonephritis or perinephric abscess, although both commonly present with urinary tract symptoms. Uremia, possibly from obstructive uropathy given the patient’s history of nephrolithiasis, could also lead to this constellation of symptoms.
On examination, temperature was 101.6 °F, heart rate 126 beats per minute, respiratory rate 18 breaths per minute, blood pressure 120/76 mm Hg, and oxygen saturation 98% on room air. The oral mucosa was moist, heart sounds were normal without murmurs, lungs were clear to auscultation, and abdomen was soft, nontender, and nondistended. There was no flank tenderness, and penile, testicular, and prostate examination findings were normal.
Laboratory studies revealed a serum sodium of 126 mEq/L, potassium 5.0 mEq/L, chloride 98 mEq/L, bicarbonate 15 mEq/L, blood urea nitrogen 88 mg/dL, creatinine 9.0 mg/dL, calcium 8 mg/dL, glucose 110 mg/dL, and albumin 3.3 g/dL. One year prior, serum creatinine was 1.4 mg/dL. His white blood cell (WBC) count was 8.0 k/uL with normal differential, hemoglobin 11.4 g/dL with normal MCV, and platelet count was normal. Serum osmolality was 286 mOsm/kg and serum parathyroid hormone (PTH) level 63 pg/mL (normal, 10-65). The urinalysis revealed cloudy urine with a specific gravity 1.009, 54 red blood cells (RBC), 236 WBC, large leukocyte esterase, negative nitrite, trace protein, and no casts or dysmorphic RBCs. A random urine specimen revealed sodium of 86 mEq/L, potassium 16 mEq/L, chloride 80 mEq/L, and creatinine 70 mg/dL.
Fever and tachycardia support an infectious cause of his symptoms. Absent flank tenderness and a normal genitourinary examination have only moderate negative predictive values for acute pyelonephritis and prostatitis, respectively. The most striking laboratory finding is his azotemia. Acute kidney injury (AKI) is more likely than chronic kidney disease (CKD) given that the PTH level is normal and the serum creatinine from a year ago was near normal. The most useful finding to differentiate AKI from CKD is the presence of atrophic kidneys on imaging. The low bicarbonate level indicates a metabolic acidosis. His serum anion gap is 13 mEq/L, which falls above most normal ranges. A mildly elevated serum anion gap together with a “delta serum anion gap/delta serum bicarbonate” ratio less than one suggest concomitant anion gap metabolic acidosis and non anion gap metabolic acidosis. The latter, coupled with a history of nephrolithiasis, may point to the possibility of renal tubular acidosis and AKI caused by urinary tract obstruction. This could also account for the marked hyponatremia. Moreover, his high fractional excretion of sodium (9%) is not suggestive of prerenal injury, the most common acute renal injury among patients who present to the emergency department. Hematuria carries a broad differential diagnosis, but most common causes include nephrolithiasis, urinary tract infection (UTI), prostatitis, neoplasm, and glomerulonephritis (GN). The lack of casts and dysmorphic RBCs makes GN unlikely. Taken together, his vital signs, examination, and laboratory studies suggest a high likelihood of an upper UTI (acute obstructive pyelonephritis) in the context of AKI due to obstructive uropathy.
Despite both a normal serum WBC count (which has only a moderate negative predictive value) and his low risk of developing life-threating organ dysfunction from sepsis based on a quick Sequential Organ Failure Assessment (qSOFA) score of zero, it is appropriate to start antibiotics after drawing blood and doing urine cultures. The next step should include administration of a broad-spectrum regimen that is appropriately dose-adjusted for renal dysfunction, such as an antipseudomonal carbapenem and vancomycin to cover extended-spectrum beta-lactamase (ESBL)-producing organisms, Pseudomonas aeruginosa, and methicillin-resistant Staphylococcus aureus (MRSA). This broad coverage is indicated for empiric treatment of complicated obstructive pyelonephritis, a condition that may arise from significant urinary obstruction and that carries a high risk of rapid clinical deterioration. He should undergo a rapid bedside test to assess for urethral or bladder outlet obstruction: either a bladder ultrasound or temporary insertion of a bladder catheter. He should also have an urgent computed tomography (CT) of his abdomen and pelvis without intravenous (IV) contrast, looking for evidence of urinary tract obstruction. A CT is preferred over ultrasound of the kidneys and bladder as CT has higher sensitivity and specificity for nephrolithiasis and neoplasm.
A CT of the abdomen and pelvis without IV contrast revealed bilateral hydroureter and hydronephrosis with multiple punctate calcified stones within the right calyces and the distal right ureter (Figure 1, Figure 2). However, these appeared too small to cause the degree of obstruction visualized. There were no stones noted in the left ureter to account for the obstruction, though small stones were noted in the left calyces. The bladder appeared normal.
Rarely are both ureters obstructed proximal to the ureterovesical junctions in the retroperitoneum. When they are, CT scans usually reveal culprit lesions that are extrinsic to the urinary tract, such as masses or retroperitoneal fibrosis, the latter of which can be associated with IgG4-related disease. Intrinsic causes of urinary tract obstructions include ureteral strictures (from infections, nephrolithiasis, instrumentation, prior radiotherapy, or rarely urothelial cancer), blood clots, metastatic ureteral deposits, or nephrolithiasis. While most intrinsic causes are unilateral, the patient is predisposed to strictures given his history of ureteral instrumentation. A preexisting unilateral obstruction due to a stricture may now, therefore, be unmasked by a second intrinsic obstruction in the contralateral ureter. Alternatively, given his remote history of living in the Philippines, a site where Schistosoma haematobium is endemic, chronic genitourinary schistosomiasis may have caused ureteral strictures due to granulomas, fibrosis, or pseudopolyps.
More commonly, bilateral hydroureter with bilateral hydronephrosis is caused by an obstruction of the bladder or urethra. CT scans can reveal prostatic hyperplasia (occasionally with protrusion into the bladder) and increased bladder wall thickness as a result of chronic bladder outlet obstruction, but the negative predictive value of either finding is modest. More revealing is that the patient reported neither an inability to pass urine (in fact, a “random” urine sample was obtained) nor suprapubic discomfort. Both symptoms would be pronounced with acute bladder obstruction but may be minimal with slowly progressive obstruction. In either case, a distended bladder would have been seen on the CT scan.
Regardless of the cause or whether the obstruction is in the upper or lower urinary tract, emergency intervention is needed to relieve the obstruction when AKI presents with bilateral hydronephrosis. A urology consultation should be sought urgently to determine the best strategy to relieve the obstruction. This may include bilateral percutaneous nephrostomy tubes given that the obstruction appears to be above the level of the bladder. Their findings will also direct additional diagnostic workup.
The patient received ceftriaxone and underwent cystoscopy, which revealed a stricture of the distal bulbar urethra. The ureters and bladder could not be completely visualized due to hematuria. The urethral stricture was dilated, and a Foley catheter was placed. In the operating room, the patient had a fever of 103 °F and developed severe hypotension unresponsive to 3 L of intravenous normal saline. Norepinephrine infusion was initiated for refractory hypotension.
Except for transurethral prostate resections, endoscopic urologic procedures rarely lead to a stricture of any segment of the urethra, suggesting that the previous ureteral stent placement and retrieval were not causal. Longstanding Mycobacterium tuberculosis or Schistosoma haematobium infection occasionally causes urethral strictures presenting as bilateral hydronephrosis. The multiple punctate calcified “stones” demonstrated on CT may suggest either diagnosis if they were actually calcified granulomas.
Regardless of the cause, most patients with a urethral stricture have chronic lower urinary tract symptoms such as decreased stream and the feeling of incomplete bladder emptying. Since this patient does not report these symptoms, it is important to consider if the stricture might be merely incidental. The absence of pain is more telling than the absence of chronic or recurrent symptoms. Lack of pain argues strongly against a pure de novo acute obstruction because abrupt stretching of the renal capsules and the walls of the collecting system is usually painful. Slow stretching caused by a progressive stricture may mask the pain of a superimposed acute obstruction. A blood clot, for example, may have precipitated an acute-on-chronic obstruction upon lodging at the urethral stricture.
The worsening systemic response to the procedure may be due to increased intravesical pressure by dilation and cystoscopy, which may have caused subsequent backflow of bacteria from the renal parenchyma into the circulation (pyelorenal backflow). The broad-spectrum antibiotic regimen suggested above and IV crystalloid infusion should be continued with close hemodynamic monitoring.
Treatment for severe sepsis was initiated with empiric piperacillin-tazobactam, ceftriaxone was discontinued, and the patient was transferred to the intensive care unit (ICU). Norepinephrine was discontinued after 24 hours. Despite the indwelling Foley catheter, his kidney function worsened (creatinine increased to 10.5 over the next 36 hours, and he remained oliguric). Therefore, bilateral percutaneous nephrostomy tubes were placed to relieve the ongoing obstruction. In the ICU, he remained febrile, despite receiving piperacillin-tazobactam, through hospital day 7. Serial blood and urine cultures all remained negative. HIV testing was negative. His chest radiograph was unremarkable, and transthoracic echocardiogram was normal. His creatinine improved but plateaued at 2.5 mg/dL by day 7.
Worsening renal function (alongside oliguria or anuria) despite a functioning Foley catheter suggests either intrinsic renal disease or bilateral ureteral obstructions. The initial attempt at relieving the obstruction with a Foley catheter did not take into consideration the bilateral ureteral strictures. As a result, soon thereafter, the insertion of percutaneous nephrostomy tubes was necessary. Given the severity of his illness, underlying obstructive uropathy, and persistent fever, suggesting an ongoing infection, one strategy would be to continue antibiotics with broader coverage than piperacillin-tazobactam. This approach may be reasonable, given the emergence of ESBL organisms and the possibility of MRSA due to instrumentation. However, it is important to note that only sterile pyuria has been identified to date, which raises the possibility of nonbacterial infections. Although chronic infection with Schistosoma haematobium can cause bilateral ureteral strictures, associated fever is limited to the acute phase of infection and not the chronic obstructive phase, unless there is a superimposed infection. Genitourinary Mycobacterium tuberculosis remains a likely possibility, regardless of the unrevealing chest radiograph. Urine nucleic acid amplification and acid-fast bacilli (AFB) smear and culture, the best initial diagnostic test, should be sent. Although less definitive, a tuberculin skin test and an interferon-gamma release assay should also be conducted. Histopathology of the ureters obtained by repeat cystoscopy may be diagnostic, but given the limited visualization during the last cystoscopy and the recent dilation of the urethra, this option should be kept in reserve for now.
Antibiotics were discontinued on day 7, but the patient continued to experience ongoing fever. Urine Histoplasma and serum cryptococcal antigens were negative. His urine AFB smear was 1+ positive. Liver function tests revealed a total protein of 7.0 g/dL, albumin 3.0 g/dL, total bilirubin 1.2 mg/dL, direct bilirubin 0.3 mg/dL, alkaline phosphatase 418 U/L, aspartate aminotransferase 65 U/L, alanine aminotransferase 88 U/L, gamma-glutamyltransferase (GGT) 609 U/L (normal, 3-60), and lactate dehydrogenase 284 U/L (normal, 85-210).
Acid-fast bacillus in the urine strongly suggests Mycobacterium tuberculosis (MTB) with several reports of likelihood ratios greater than 10. Nevertheless, confirmation is needed to rule out nontuberculous mycobacteria because of potential hepatotoxicity from treatment. Up to six urine samples should be sent for mycobacterium culture. However, false negative rates of up to 90% are reported, and final test results can take up to two months, so other methods of confirmation should be simultaneously sought. A nucleic acid amplification test of urine could rule in a pathogenic species within 24 hours. Alternatively, the probability of a nonpathogenic colonizing species would be negligible if a caseating granuloma was found. Biopsy could be obtained from the ureters, as suggested above. Liver biopsy should also be considered, especially if the moderate elevations in alkaline phosphatase and GGT (the most common liver enzyme abnormalities in hepatic tuberculosis) did not merely wax and wane with sepsis.
A CT of the thorax without IV contrast was done to evaluate for evidence of pulmonary disease given the positive urine AFB. This demonstrated bilateral fibrotic upper lobe opacities suggestive of prior granulomatous disease but no cavitary lung lesions (Figure 3). Three sputum smears were negative for AFB, but one sample showed Mycobacterium tuberculosis detected by a polymerase chain reaction (PCR) probe.
Given the concern for genitourinary tuberculosis (GUTB), it is appropriate to place the patient in respiratory isolation to exclude concomitant pulmonary tuberculosis (TB). AFB smears were negative, but the sputum PCR probe was positive, confirming pathogenic MTB. However, the negative AFB smears make the likelihood of pulmonary infectivity low. As a result, contact tracing is often deemed unnecessary by hospital infection control teams. Though his chest radiograph was normal, CT showed bilateral upper lobe fibrotic disease suggestive of prior pulmonary TB, thus making it likely that the current GUTB represents reactivation.
The two-month initiation phase of treatment with four antituberculosis drugs should begin while drug susceptibility tests are pending. Potential hepatotoxicity should be closely monitored, ideally by a clinician with experience treating tuberculosis in patients with existing liver disease. As a general precaution, alcohol should be avoided as should medications such as acetaminophen that are known to be hepatotoxic. Urology follow-up is also needed because about one-third of tuberculous ureteral strictures treated initially with percutaneous nephrostomy do not resolve with antituberculosis therapy.
The patient was started on weight-based antituberculosis treatment with four antimicrobial agents (rifampin, ethambutol, pyrazinamide, and isoniazid). He was seen in the infectious disease clinic two weeks later; his fever had resolved, and his liver function tests showed normalization of AST and LDH as well as a 45% reduction in his GGT and alkaline phosphatase levels. Two months following discharge, a nuclear medicine radionuclide angiogram renal flow scan showed normal right kidney function. The right nephrostomy tube was subsequently removed. He continued to have left kidney outflow obstruction due to a residual ureteral stricture (Figure 4). Repeat cystoscopy and attempted left ureteral stenting was unsuccessful. The left nephrostomy tube remained in place.
DISCUSSION
According to the Centers for Disease Control, in 2017, 10 million people became sick with TB, and there were 1.3 million TB-related deaths worldwide with 9,150 cases reported in the United States. Extrapulmonary TB (EPTB) constitutes 10% of all TB cases globally.1-4 GUTB is the second most common form of EPTB after lymph node TB, and it occurs in up to 20% of all pulmonary TB cases.2,3
Mycobacteria reach the genitourinary (GU) tract via hematogenous spread during primary infection or reactivation of TB. This leads to cortical and medullary lesions, which can heal spontaneously or eventually (average of 22 years) rupture into the tubules and onto the collecting system, ureters, and bladder.5,6 Spread to the ureter and bladder leads to multiple ureteral strictures and contracture of the bladder with disruption of the ureterovesical junction (UVJ), which causes hydroureter and hydronephrosis.7 Unilateral kidney involvement is most common, but bilateral involvement can occur following exacerbated hematogenous spread, particularly in immune deficient patients. Bilateral kidney involvement is also possible from retrograde spread to the good kidney due to bladder contracture and UVJ disruption.8,9 Distal infection can involve all aspects of the male and female genital tracts, but urethral strictures are extremely rare.10,11
GUTB affects males more than females (2:1) and presents insidiously at 40 to 60 years of age.12 Other risk factors for TB include birth in TB endemic areas, prior TB infection, immunosuppression, malnutrition, severe systemic disease, diabetes, and cirrhosis. It is crucial to assess these risk factors when creating and refining differential diagnoses. Many patients have hematuria and sterile pyuria as incidental initial findings. The most common symptoms arise from bladder involvement, including frequency, urgency, and dysuria. Low back pain and gross hematuria are also common, but fever and constitutional symptoms are uncommon.10 Bilateral ureteral strictures can lead to obstructive renal failure, and involvement of the genital tracts can lead to pelvic or scrotal pain, swelling, and fistula formation.10
Diagnosis involves the demonstration of TB bacilli in urine or GU tissue. The urinalysis reveals hematuria and sterile pyuria.13 Urine AFB stains are positive in 52% of cases but are not diagnostic as nontuberculous mycobacteria may also cause a positive test result.13,14 Urine cultures for Mycobacterium tuberculosis are positive in up to 90% of cases after six to eight weeks. As many as three to six morning urine samples are required to achieve diagnostic accuracy.10,14 Urine PCR for Mycobacterium tuberculosis has 96% sensitivity and up to 98% specificity,14 while PCR on GU tissue has a sensitivity of 88% and specificity of 87%.15 The rapid nucleic acid amplification assay Xpert MTB/RIF in urine has a sensitivity of 83%, and specificity of 98%.16 Imaging is required to evaluate for obstruction, and the CT scan is abnormal in up to 90% of cases, showing multiple ureteral stenoses, hydroureter and hydronephrosis, and a contracted bladder.10,17
GUTB is treated with standard antituberculosis regimens.18 Patients with urinary obstruction benefit from ureteral stenting or percutaneous nephrostomy, bladder diversion, or ureteral reconstruction surgery. Unilateral nephrectomy for a nonfunctioning kidney with extensive disease is occasionally required.19 Following treatment, relapse occurs in up to 6% of patients over five years, and long-term follow-up with urine cultures and PCR every six months is recommended.10,20 Appropriate screening and treatment for latent tuberculosis infection greatly reduces the risk of reactivation GUTB.
This patient presented with features of an infection, which, combined with his history of renal stones and his urinalysis, led to an appropriate suspicion of and empiric treatment for an upper UTI. Given the AKI and nephrolithiasis, imaging was done to exclude obstruction. The CT finding of bilateral hydroureters and hydronephrosis absent an obstructing stone or mass or abnormal bladder was the initial clue that this was not a typical bacterial infection and that there was likely an underlying infectious pathologic process such as TB involving the GU tract diffusely. The care team treated the patient as an individual with fever and sterile pyuria in the context of multiple urinary tract strictures and an initial unrevealing infectious diagnostic workup. They recognized that the clues to the ultimate diagnosis of GUTB were all in the stream.
KEY TEACHING POINTS
- GUTB is a significant cause of sterile pyuria.
- In the presence of bilateral hydronephrosis, it is vital to determine the level of obstruction. If the bladder is not distended or contracted, then obstruction is likely at the level of the ureters and initial use of percutaneous nephrostomy tubes to relieve obstruction may be preferred.
- Imaging abnormalities such as multiple ureteral strictures, hydroureter and hydronephrosis (absent an obstructing stone or mass), and the finding of a contracted bladder are highly suggestive of GUTB.
- The mainstay of treatment for GUTB is standard antituberculosis treatment regimens in combination with the relief of urinary obstruction by ureteral stenting, percutaneous nephrostomy or open surgery.
- GUTB can relapse in up to 6% of treated cases over five years, and long-term follow-up and surveillance with urine culture and PCR every six months are recommended.
Disclosures
Benjamin Mba, Nathan Houchens, Marie Jennifer Seares, and Udit Joshi have no financial conflicts of interest and no disclosures.
Funding
Brian P. Lucas receives funding from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, and National Center for Translational Science (UL1TR001086).
1. Forssbohm M, Zwahlen M, Loddenkemper R, Rieder HL. Demographic characteristics of patients with extrapulmonary tuberculosis in Germany. Eur Resp J. 2008;31(1):99-105. https://doi.org/10.1183/09031936.00020607.
2. French CE, Antoine D, Gelb D, Jones JA, Gilbert RL, Watson JM. Tuberculosis in non-UK-born persons, England and Wales, 2001-2003. Int J Tuberc Lung Dis. 2007;11(5):577-584.
3. Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis. 2009;49(9):1350-1357. https://doi.org/10.1086/605559.
4. Alvarez S, McCabe WR. Extrapulmonary tuberculosis revisited: a review of experience at Boston City and other hospitals. Medicine. 1984;63(1):25-55.
5. Simon HB, Weinstein AJ, Pasternak MS, Swartz MN, Kunz LJ. Genitourinary tuberculosis. Clinical features in a general hospital population. Am J Med. 1977;63(3):410-420. https://doi.org/10.1016/0002-9343(77)90279-0.
6. Christensen WI. Genitourinary tuberculosis: review of 102 cases. Medicine. 1974;53(5):377-390. https://doi.org/10.1016/0002-9343(77)90279-0.
7. Eastwood JB, Corbishley CM, Grange JM. Tuberculosis and the kidney. J Am Soc Nephrol. 2001;12(6):1307-1314.
8. Garcia-Rodriguez JA, Garcia Sanchez JE, Munoz Bellido JL, et al. Genitourinary tuberculosis in Spain: review of 81 cases. Clin Infect Dis.1994;18(4):557-561. https://doi.org/10.1093/clinids/18.4.557.
9. de Figueiredo AA, Lucon AM, Srougi M. Bladder augmentation for the treatment of chronic tuberculous cystitis. Clinical and urodynamic evaluation of 25 patients after long term follow-up. Neurourol Urodyn. 2006;25(5):433-440. https://doi.org/10.1002/nau.20264.
10. Figueiredo AA, Lucon AM, Srougi M. Urogenital Tuberculosis. Microbiol Spectr. 2017;5. https://doi.org/10.1128/microbiolspec.TNMI7-0015-2016.
11. Gupta N, Mandal AK, Singh SK. Tuberculosis of the prostate and urethra: A review. Indian J Urol. 2008;24(3):388-391. https://doi.org/10.4103/0970-1591.42623.
12. Figueiredo AA, Lucon AM, Junior RF, Srougi M. Epidemiology of urogenital tuberculosis worldwide. Int J Urol. 2008;15(9):827-832. https://doi.org/10.1111/j.1442-2042.2008.02099.x.
13. Mortier E, Pouchot J, Girard L, Boussougant Y, Vinceneux P. Assessment of urine analysis for the diagnosis of tuberculosis. BMJ (Clinical research ed). 1996;312:27-28. https://doi.org/10.1136/bmj.312.7022.27.
14. Moussa OM, Eraky I, El-Far MA, et al. Rapid diagnosis of genitourinary tuberculosis by polymerase chain reaction and non-radioactive DNA hybridization. J Urol. 2000;164(2):584-588. https://doi.org/10.1016/S0022-5347(05)67427-7.
15. Chawla A, Chawla K, Reddy S, et al. Can tissue PCR augment the diagnostic accuracy in genitourinary tract tuberculosis? Urol Int. 2012;88(1):34-38. https://doi.org/10.1159/000327039.
16. Kohli M, Schiller I, Dendukuri N, et al. Xpert((R)) MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database Syst Rev. 2018;8:Cd012768. https://doi.org/10.1002/14651858.CD012768.pub2.
17. Figueiredo AA, Lucon AM, Arvellos AN, et al. A better understanding of urogenital tuberculosis pathophysiology based on radiological findings. Eur J Radiol. 2010;76(2):246-257. https://doi.org/10.1016/j.ejrad.2009.05.049.
18. Treatment of Tuberculosis: Guidelines. 4th edition. Geneva: World Health Organization. 2010.
19. O’Flynn D. Surgical treatment of genito-urinary tuberculosis. A report on 762 cases. Br J Urol. 1970;42(6):667-671. https://doi.org/10.1111/j.1464-410X.1970.tb06789.x.
20. Butler MR, O’Flynn JD. Reactivation of genito-urinary tuberculosis: a retrospective review of 838 cases. Eur Urol. 1975;1:14-17. https://doi.org/10.1159/000455566.
1. Forssbohm M, Zwahlen M, Loddenkemper R, Rieder HL. Demographic characteristics of patients with extrapulmonary tuberculosis in Germany. Eur Resp J. 2008;31(1):99-105. https://doi.org/10.1183/09031936.00020607.
2. French CE, Antoine D, Gelb D, Jones JA, Gilbert RL, Watson JM. Tuberculosis in non-UK-born persons, England and Wales, 2001-2003. Int J Tuberc Lung Dis. 2007;11(5):577-584.
3. Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis. 2009;49(9):1350-1357. https://doi.org/10.1086/605559.
4. Alvarez S, McCabe WR. Extrapulmonary tuberculosis revisited: a review of experience at Boston City and other hospitals. Medicine. 1984;63(1):25-55.
5. Simon HB, Weinstein AJ, Pasternak MS, Swartz MN, Kunz LJ. Genitourinary tuberculosis. Clinical features in a general hospital population. Am J Med. 1977;63(3):410-420. https://doi.org/10.1016/0002-9343(77)90279-0.
6. Christensen WI. Genitourinary tuberculosis: review of 102 cases. Medicine. 1974;53(5):377-390. https://doi.org/10.1016/0002-9343(77)90279-0.
7. Eastwood JB, Corbishley CM, Grange JM. Tuberculosis and the kidney. J Am Soc Nephrol. 2001;12(6):1307-1314.
8. Garcia-Rodriguez JA, Garcia Sanchez JE, Munoz Bellido JL, et al. Genitourinary tuberculosis in Spain: review of 81 cases. Clin Infect Dis.1994;18(4):557-561. https://doi.org/10.1093/clinids/18.4.557.
9. de Figueiredo AA, Lucon AM, Srougi M. Bladder augmentation for the treatment of chronic tuberculous cystitis. Clinical and urodynamic evaluation of 25 patients after long term follow-up. Neurourol Urodyn. 2006;25(5):433-440. https://doi.org/10.1002/nau.20264.
10. Figueiredo AA, Lucon AM, Srougi M. Urogenital Tuberculosis. Microbiol Spectr. 2017;5. https://doi.org/10.1128/microbiolspec.TNMI7-0015-2016.
11. Gupta N, Mandal AK, Singh SK. Tuberculosis of the prostate and urethra: A review. Indian J Urol. 2008;24(3):388-391. https://doi.org/10.4103/0970-1591.42623.
12. Figueiredo AA, Lucon AM, Junior RF, Srougi M. Epidemiology of urogenital tuberculosis worldwide. Int J Urol. 2008;15(9):827-832. https://doi.org/10.1111/j.1442-2042.2008.02099.x.
13. Mortier E, Pouchot J, Girard L, Boussougant Y, Vinceneux P. Assessment of urine analysis for the diagnosis of tuberculosis. BMJ (Clinical research ed). 1996;312:27-28. https://doi.org/10.1136/bmj.312.7022.27.
14. Moussa OM, Eraky I, El-Far MA, et al. Rapid diagnosis of genitourinary tuberculosis by polymerase chain reaction and non-radioactive DNA hybridization. J Urol. 2000;164(2):584-588. https://doi.org/10.1016/S0022-5347(05)67427-7.
15. Chawla A, Chawla K, Reddy S, et al. Can tissue PCR augment the diagnostic accuracy in genitourinary tract tuberculosis? Urol Int. 2012;88(1):34-38. https://doi.org/10.1159/000327039.
16. Kohli M, Schiller I, Dendukuri N, et al. Xpert((R)) MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database Syst Rev. 2018;8:Cd012768. https://doi.org/10.1002/14651858.CD012768.pub2.
17. Figueiredo AA, Lucon AM, Arvellos AN, et al. A better understanding of urogenital tuberculosis pathophysiology based on radiological findings. Eur J Radiol. 2010;76(2):246-257. https://doi.org/10.1016/j.ejrad.2009.05.049.
18. Treatment of Tuberculosis: Guidelines. 4th edition. Geneva: World Health Organization. 2010.
19. O’Flynn D. Surgical treatment of genito-urinary tuberculosis. A report on 762 cases. Br J Urol. 1970;42(6):667-671. https://doi.org/10.1111/j.1464-410X.1970.tb06789.x.
20. Butler MR, O’Flynn JD. Reactivation of genito-urinary tuberculosis: a retrospective review of 838 cases. Eur Urol. 1975;1:14-17. https://doi.org/10.1159/000455566.
© 2019 Society of Hospital Medicine
Hospital Medicine Has a Specialty Code. Is the Memo Still in the Mail?
In recognizing the importance of Hospital Medicine (HM) and its practitioners, the Centers for Medicare and Medicaid Services (CMS) awarded the field a specialty designation in 2016. The code is self-selected by hospitalists and used by the CMS for programmatic and claims processing purposes. The HM code (“C6”), submitted to the CMS by the provider or their designee through the Provider Enrollment Chain and Ownership System (PECOS), in turn links to the National Provider Identification provider data.
The Society of Hospital Medicine® sought the designation given the growth of hospitalists practicing nationally, their impact on the practice of medicine in the inpatient setting,1 and their secondary effects on global care.2 In fact, early efforts by the CMS to transition physician payments to the value-based payment used specialty designations to create benchmarks in cost metrics, heightening the importance for hospitalists to be able to assess their performance. The need to identify any shifts in resource utilization and workforce mix in the broader context of health reforms necessitated action. Essentially, to understand the “why’s” of hospital medicine, the field required an accounting of the “who’s” and “where’s.”
The CMS granted the C6 designation in 2016, and it went live in April 2017. Despite the code’s brief two-year tenure, calls for its creation long predated its existence. As such, the new modifier requires an initial look to help steer the role of HM in any future CMS and managed care organization (MCO) quality, payment, or practice improvement activities.
METHODS
We analyzed publicly available 2017 Medicare Part B utilization data3 to explore the rates of Evaluation & Management (E&M) codes used across specialties, using the C6 designation to identify hospitalists.
To try to estimate the percentage of hospitalists who were likely billing under the C6 designation, we then compared the rates of C6 billing to expected rates of hospitalist E&M billing based on an analysis of hospitalist prevalence in the 2012 Medicare physician payment data. Prior work to identify hospitalists before the implementation of the C6 designation relied on thresholds of inpatient codes for various inpatient E&M services.4,5 We used our previously published approach of a threshold of 60% of inpatient E&M hospital services to differentiate hospitalists from their parent specialties.6 We also calculated the expected rates of E&M billing for other select specialty services by applying the 2012 E&M coding trends to the 2017 data.
RESULTS
Table 1 shows the distribution of inpatient E&M codes billed by hospitalists using the C6 identification, as well as the use of those codes by other specialists. Hospitalists identified by the C6 designation billed only 2%-5% of inpatient and 6% of observation codes. As an example, in 2017, discharge CPT codes 99238 and 99239 were used 7,872,323 times. However, C6-identified hospitalists accounted for only 441,420 of these codes.
Table 2 compares the observed billing rates by specialty using the C6 designation to identify hospitalists with what would be the expected rates with the 2012 threshold-based specialty billing designation applied to the 2017 data. This comparison demonstrates that hospitalist billing based on the C6 modifier use is approximately one-tenth of what would have been their expected volume of E&M services.
DISCUSSION
We examined the patterns of hospitalist billing using the C6 hospital medicine specialty modifier, comparing billing patterns with what we would expect hospitalist activity to be if we had used a threshold-based approach. The difference between the C6 and the threshold-based approaches to assessing hospitalist activity suggests that as few as 10% of hospitalists have adopted the C6 code.
Why is the adoption of the C6 modifier so low? Although administrative data do not allow us to identify the reasons why providers chose to disregard the C6 designation, we can speculate on causes. There are, to date, low direct risks and recognized benefits with using the code. We hypothesize that several factors could be impeding whether providers use the modifier to bring about potential gains. The first may be knowledge-related; ie, hospitalists might not be familiar with the specialty code or unaware of the importance of accurately capturing hospitalist practice patterns. They may also wrongly assume that their practices are aware of the revision or have submitted the appropriate paperwork. Similarly, practice personnel may lack knowledge regarding the code or the importance of its use. The second factor may be logistical; ie, administrative barriers such as difficulty accessing the Provider Enrollment, Chain and Ownership System (PECOS) and out-of-date paper registration forms impede fast uptake. The final reason might be related to professionals whose tenures as hospitalists will be brief, and their unease of carrying an identifier into their next non-HM position prompts hesitation. Providers may have a misperception that using the C6 code may somehow impact or limit their future scope of practice, when, in fact, they may change their Medicare specialty designation at any time.
Changes in reimbursement models, including the Bundled Payments for Care Improvement Advanced (BPCI-A) and other value-based initiatives, heighten the need for a more accurate identification of the specialty. Classifying individual providers and groups to make valid performance comparisons is relevant for the same reasons. The CMS continues to advance cost and efficiency measures in its publicly accessible physiciancompare.gov website.7 Without an improved ability to identify services provided by hospitalists—by both CMS and commercial entities—the potential benefits delivered by hospitalists in terms of improved care quality, safety, or efficiency could go undetected by payers and policymakers. Moreover, C6 may be used in other ways by the CMS throughout its payment systems and programmatic efforts that use specialty to differentiate between Medicare providers.8 Finally, the C6 is an identifier for the Medicare fee-for-service system; state programs and MCOs may not identify hospitalists in the same manner, or at all. Therefore, it may make it more difficult for those groups and HM researchers to study the trends in care delivery changes. The specialty needs to engage with these other payers to assist in revising their information systems to better account for how hospitalists care for their insured populations.
Although we would expect a natural increase in C6 adoption over time, optimally meeting stakeholders’ data needs requires more rapid uptake. Our analysis is limited by our assumption that specialty patterns of code use remain similar from 2012 to 2017. Regardless, the magnitude of the difference between the estimate of hospitalists using the C6 versus billing thresholds strongly suggests underuse of the C6 designation. The CMS and MCOs have an increasing need for valid and representative data, and C6 can be used to assess “HM-adjusted” resource utilization, relative value units (RVUs), and performance evaluations. Therefore, hospitalists may see more incentives to use the C6 specialty code in a manner consistent with other recognized subspecialties.
Disclaimer
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, and the Health Services Research and Development Service. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
1. Wachter RM, Goldman L. Zero to 50,000—The 20th Anniversary of the Hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958.
2. Quinn R. HM 2016: A year in review. The Hospitalist. 2016;12. https://www.the-hospitalist.org/hospitalist/article/121419/everything-you-need-know-about-bundled-payments-care-improvement
3. Centers for Medicare and Medicaid Services. Medicare Utilization for Part B. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/medicarefeeforsvcpartsab/medicareutilizationforpartb.html. Accessed June 14, 2019.
4. Saint S, Christakis DA, Baldwin L-M, Rosenblatt R. Is hospitalism new? An analysis of Medicare data from Washington State in 1994. Eff Clin Pract. 2000;3(1):35-39.
5. Welch WP, Stearns SC, Cuellar AE, Bindman AB. Use of hospitalists by Medicare beneficiaries: a national picture. Medicare Medicaid Res Rev. 2014;4(2). https://doi.org/10.5600/mmrr2014-004-02-b01.
6. Lapps J, Flansbaum B, Leykum L, Boswell J, Haines L. Updating threshold-based identification of hospitalists in 2012 medicare pay data. J Hosp Med. 2016;11(1):45-47. https://doi.org/10.1002/jhm.2480.
7. Centers for Medicare & Medicaid Services. Physician Compare Initiative. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/physician-compare-initiative/index.html. Accessed June 14, 2019.
8. Centers for Medicare & Medicaid Services. Revisions to Payment Policies under the Medicare Physician Fee Schedule, Quality Payment Program and Other Revisions to Part B for CY 2020 (CMS-1715-P). Accessed prior to publishing in the Federal Register through www.regulations.gov.
In recognizing the importance of Hospital Medicine (HM) and its practitioners, the Centers for Medicare and Medicaid Services (CMS) awarded the field a specialty designation in 2016. The code is self-selected by hospitalists and used by the CMS for programmatic and claims processing purposes. The HM code (“C6”), submitted to the CMS by the provider or their designee through the Provider Enrollment Chain and Ownership System (PECOS), in turn links to the National Provider Identification provider data.
The Society of Hospital Medicine® sought the designation given the growth of hospitalists practicing nationally, their impact on the practice of medicine in the inpatient setting,1 and their secondary effects on global care.2 In fact, early efforts by the CMS to transition physician payments to the value-based payment used specialty designations to create benchmarks in cost metrics, heightening the importance for hospitalists to be able to assess their performance. The need to identify any shifts in resource utilization and workforce mix in the broader context of health reforms necessitated action. Essentially, to understand the “why’s” of hospital medicine, the field required an accounting of the “who’s” and “where’s.”
The CMS granted the C6 designation in 2016, and it went live in April 2017. Despite the code’s brief two-year tenure, calls for its creation long predated its existence. As such, the new modifier requires an initial look to help steer the role of HM in any future CMS and managed care organization (MCO) quality, payment, or practice improvement activities.
METHODS
We analyzed publicly available 2017 Medicare Part B utilization data3 to explore the rates of Evaluation & Management (E&M) codes used across specialties, using the C6 designation to identify hospitalists.
To try to estimate the percentage of hospitalists who were likely billing under the C6 designation, we then compared the rates of C6 billing to expected rates of hospitalist E&M billing based on an analysis of hospitalist prevalence in the 2012 Medicare physician payment data. Prior work to identify hospitalists before the implementation of the C6 designation relied on thresholds of inpatient codes for various inpatient E&M services.4,5 We used our previously published approach of a threshold of 60% of inpatient E&M hospital services to differentiate hospitalists from their parent specialties.6 We also calculated the expected rates of E&M billing for other select specialty services by applying the 2012 E&M coding trends to the 2017 data.
RESULTS
Table 1 shows the distribution of inpatient E&M codes billed by hospitalists using the C6 identification, as well as the use of those codes by other specialists. Hospitalists identified by the C6 designation billed only 2%-5% of inpatient and 6% of observation codes. As an example, in 2017, discharge CPT codes 99238 and 99239 were used 7,872,323 times. However, C6-identified hospitalists accounted for only 441,420 of these codes.
Table 2 compares the observed billing rates by specialty using the C6 designation to identify hospitalists with what would be the expected rates with the 2012 threshold-based specialty billing designation applied to the 2017 data. This comparison demonstrates that hospitalist billing based on the C6 modifier use is approximately one-tenth of what would have been their expected volume of E&M services.
DISCUSSION
We examined the patterns of hospitalist billing using the C6 hospital medicine specialty modifier, comparing billing patterns with what we would expect hospitalist activity to be if we had used a threshold-based approach. The difference between the C6 and the threshold-based approaches to assessing hospitalist activity suggests that as few as 10% of hospitalists have adopted the C6 code.
Why is the adoption of the C6 modifier so low? Although administrative data do not allow us to identify the reasons why providers chose to disregard the C6 designation, we can speculate on causes. There are, to date, low direct risks and recognized benefits with using the code. We hypothesize that several factors could be impeding whether providers use the modifier to bring about potential gains. The first may be knowledge-related; ie, hospitalists might not be familiar with the specialty code or unaware of the importance of accurately capturing hospitalist practice patterns. They may also wrongly assume that their practices are aware of the revision or have submitted the appropriate paperwork. Similarly, practice personnel may lack knowledge regarding the code or the importance of its use. The second factor may be logistical; ie, administrative barriers such as difficulty accessing the Provider Enrollment, Chain and Ownership System (PECOS) and out-of-date paper registration forms impede fast uptake. The final reason might be related to professionals whose tenures as hospitalists will be brief, and their unease of carrying an identifier into their next non-HM position prompts hesitation. Providers may have a misperception that using the C6 code may somehow impact or limit their future scope of practice, when, in fact, they may change their Medicare specialty designation at any time.
Changes in reimbursement models, including the Bundled Payments for Care Improvement Advanced (BPCI-A) and other value-based initiatives, heighten the need for a more accurate identification of the specialty. Classifying individual providers and groups to make valid performance comparisons is relevant for the same reasons. The CMS continues to advance cost and efficiency measures in its publicly accessible physiciancompare.gov website.7 Without an improved ability to identify services provided by hospitalists—by both CMS and commercial entities—the potential benefits delivered by hospitalists in terms of improved care quality, safety, or efficiency could go undetected by payers and policymakers. Moreover, C6 may be used in other ways by the CMS throughout its payment systems and programmatic efforts that use specialty to differentiate between Medicare providers.8 Finally, the C6 is an identifier for the Medicare fee-for-service system; state programs and MCOs may not identify hospitalists in the same manner, or at all. Therefore, it may make it more difficult for those groups and HM researchers to study the trends in care delivery changes. The specialty needs to engage with these other payers to assist in revising their information systems to better account for how hospitalists care for their insured populations.
Although we would expect a natural increase in C6 adoption over time, optimally meeting stakeholders’ data needs requires more rapid uptake. Our analysis is limited by our assumption that specialty patterns of code use remain similar from 2012 to 2017. Regardless, the magnitude of the difference between the estimate of hospitalists using the C6 versus billing thresholds strongly suggests underuse of the C6 designation. The CMS and MCOs have an increasing need for valid and representative data, and C6 can be used to assess “HM-adjusted” resource utilization, relative value units (RVUs), and performance evaluations. Therefore, hospitalists may see more incentives to use the C6 specialty code in a manner consistent with other recognized subspecialties.
Disclaimer
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, and the Health Services Research and Development Service. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
In recognizing the importance of Hospital Medicine (HM) and its practitioners, the Centers for Medicare and Medicaid Services (CMS) awarded the field a specialty designation in 2016. The code is self-selected by hospitalists and used by the CMS for programmatic and claims processing purposes. The HM code (“C6”), submitted to the CMS by the provider or their designee through the Provider Enrollment Chain and Ownership System (PECOS), in turn links to the National Provider Identification provider data.
The Society of Hospital Medicine® sought the designation given the growth of hospitalists practicing nationally, their impact on the practice of medicine in the inpatient setting,1 and their secondary effects on global care.2 In fact, early efforts by the CMS to transition physician payments to the value-based payment used specialty designations to create benchmarks in cost metrics, heightening the importance for hospitalists to be able to assess their performance. The need to identify any shifts in resource utilization and workforce mix in the broader context of health reforms necessitated action. Essentially, to understand the “why’s” of hospital medicine, the field required an accounting of the “who’s” and “where’s.”
The CMS granted the C6 designation in 2016, and it went live in April 2017. Despite the code’s brief two-year tenure, calls for its creation long predated its existence. As such, the new modifier requires an initial look to help steer the role of HM in any future CMS and managed care organization (MCO) quality, payment, or practice improvement activities.
METHODS
We analyzed publicly available 2017 Medicare Part B utilization data3 to explore the rates of Evaluation & Management (E&M) codes used across specialties, using the C6 designation to identify hospitalists.
To try to estimate the percentage of hospitalists who were likely billing under the C6 designation, we then compared the rates of C6 billing to expected rates of hospitalist E&M billing based on an analysis of hospitalist prevalence in the 2012 Medicare physician payment data. Prior work to identify hospitalists before the implementation of the C6 designation relied on thresholds of inpatient codes for various inpatient E&M services.4,5 We used our previously published approach of a threshold of 60% of inpatient E&M hospital services to differentiate hospitalists from their parent specialties.6 We also calculated the expected rates of E&M billing for other select specialty services by applying the 2012 E&M coding trends to the 2017 data.
RESULTS
Table 1 shows the distribution of inpatient E&M codes billed by hospitalists using the C6 identification, as well as the use of those codes by other specialists. Hospitalists identified by the C6 designation billed only 2%-5% of inpatient and 6% of observation codes. As an example, in 2017, discharge CPT codes 99238 and 99239 were used 7,872,323 times. However, C6-identified hospitalists accounted for only 441,420 of these codes.
Table 2 compares the observed billing rates by specialty using the C6 designation to identify hospitalists with what would be the expected rates with the 2012 threshold-based specialty billing designation applied to the 2017 data. This comparison demonstrates that hospitalist billing based on the C6 modifier use is approximately one-tenth of what would have been their expected volume of E&M services.
DISCUSSION
We examined the patterns of hospitalist billing using the C6 hospital medicine specialty modifier, comparing billing patterns with what we would expect hospitalist activity to be if we had used a threshold-based approach. The difference between the C6 and the threshold-based approaches to assessing hospitalist activity suggests that as few as 10% of hospitalists have adopted the C6 code.
Why is the adoption of the C6 modifier so low? Although administrative data do not allow us to identify the reasons why providers chose to disregard the C6 designation, we can speculate on causes. There are, to date, low direct risks and recognized benefits with using the code. We hypothesize that several factors could be impeding whether providers use the modifier to bring about potential gains. The first may be knowledge-related; ie, hospitalists might not be familiar with the specialty code or unaware of the importance of accurately capturing hospitalist practice patterns. They may also wrongly assume that their practices are aware of the revision or have submitted the appropriate paperwork. Similarly, practice personnel may lack knowledge regarding the code or the importance of its use. The second factor may be logistical; ie, administrative barriers such as difficulty accessing the Provider Enrollment, Chain and Ownership System (PECOS) and out-of-date paper registration forms impede fast uptake. The final reason might be related to professionals whose tenures as hospitalists will be brief, and their unease of carrying an identifier into their next non-HM position prompts hesitation. Providers may have a misperception that using the C6 code may somehow impact or limit their future scope of practice, when, in fact, they may change their Medicare specialty designation at any time.
Changes in reimbursement models, including the Bundled Payments for Care Improvement Advanced (BPCI-A) and other value-based initiatives, heighten the need for a more accurate identification of the specialty. Classifying individual providers and groups to make valid performance comparisons is relevant for the same reasons. The CMS continues to advance cost and efficiency measures in its publicly accessible physiciancompare.gov website.7 Without an improved ability to identify services provided by hospitalists—by both CMS and commercial entities—the potential benefits delivered by hospitalists in terms of improved care quality, safety, or efficiency could go undetected by payers and policymakers. Moreover, C6 may be used in other ways by the CMS throughout its payment systems and programmatic efforts that use specialty to differentiate between Medicare providers.8 Finally, the C6 is an identifier for the Medicare fee-for-service system; state programs and MCOs may not identify hospitalists in the same manner, or at all. Therefore, it may make it more difficult for those groups and HM researchers to study the trends in care delivery changes. The specialty needs to engage with these other payers to assist in revising their information systems to better account for how hospitalists care for their insured populations.
Although we would expect a natural increase in C6 adoption over time, optimally meeting stakeholders’ data needs requires more rapid uptake. Our analysis is limited by our assumption that specialty patterns of code use remain similar from 2012 to 2017. Regardless, the magnitude of the difference between the estimate of hospitalists using the C6 versus billing thresholds strongly suggests underuse of the C6 designation. The CMS and MCOs have an increasing need for valid and representative data, and C6 can be used to assess “HM-adjusted” resource utilization, relative value units (RVUs), and performance evaluations. Therefore, hospitalists may see more incentives to use the C6 specialty code in a manner consistent with other recognized subspecialties.
Disclaimer
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, and the Health Services Research and Development Service. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
1. Wachter RM, Goldman L. Zero to 50,000—The 20th Anniversary of the Hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958.
2. Quinn R. HM 2016: A year in review. The Hospitalist. 2016;12. https://www.the-hospitalist.org/hospitalist/article/121419/everything-you-need-know-about-bundled-payments-care-improvement
3. Centers for Medicare and Medicaid Services. Medicare Utilization for Part B. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/medicarefeeforsvcpartsab/medicareutilizationforpartb.html. Accessed June 14, 2019.
4. Saint S, Christakis DA, Baldwin L-M, Rosenblatt R. Is hospitalism new? An analysis of Medicare data from Washington State in 1994. Eff Clin Pract. 2000;3(1):35-39.
5. Welch WP, Stearns SC, Cuellar AE, Bindman AB. Use of hospitalists by Medicare beneficiaries: a national picture. Medicare Medicaid Res Rev. 2014;4(2). https://doi.org/10.5600/mmrr2014-004-02-b01.
6. Lapps J, Flansbaum B, Leykum L, Boswell J, Haines L. Updating threshold-based identification of hospitalists in 2012 medicare pay data. J Hosp Med. 2016;11(1):45-47. https://doi.org/10.1002/jhm.2480.
7. Centers for Medicare & Medicaid Services. Physician Compare Initiative. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/physician-compare-initiative/index.html. Accessed June 14, 2019.
8. Centers for Medicare & Medicaid Services. Revisions to Payment Policies under the Medicare Physician Fee Schedule, Quality Payment Program and Other Revisions to Part B for CY 2020 (CMS-1715-P). Accessed prior to publishing in the Federal Register through www.regulations.gov.
1. Wachter RM, Goldman L. Zero to 50,000—The 20th Anniversary of the Hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958.
2. Quinn R. HM 2016: A year in review. The Hospitalist. 2016;12. https://www.the-hospitalist.org/hospitalist/article/121419/everything-you-need-know-about-bundled-payments-care-improvement
3. Centers for Medicare and Medicaid Services. Medicare Utilization for Part B. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/medicarefeeforsvcpartsab/medicareutilizationforpartb.html. Accessed June 14, 2019.
4. Saint S, Christakis DA, Baldwin L-M, Rosenblatt R. Is hospitalism new? An analysis of Medicare data from Washington State in 1994. Eff Clin Pract. 2000;3(1):35-39.
5. Welch WP, Stearns SC, Cuellar AE, Bindman AB. Use of hospitalists by Medicare beneficiaries: a national picture. Medicare Medicaid Res Rev. 2014;4(2). https://doi.org/10.5600/mmrr2014-004-02-b01.
6. Lapps J, Flansbaum B, Leykum L, Boswell J, Haines L. Updating threshold-based identification of hospitalists in 2012 medicare pay data. J Hosp Med. 2016;11(1):45-47. https://doi.org/10.1002/jhm.2480.
7. Centers for Medicare & Medicaid Services. Physician Compare Initiative. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/physician-compare-initiative/index.html. Accessed June 14, 2019.
8. Centers for Medicare & Medicaid Services. Revisions to Payment Policies under the Medicare Physician Fee Schedule, Quality Payment Program and Other Revisions to Part B for CY 2020 (CMS-1715-P). Accessed prior to publishing in the Federal Register through www.regulations.gov.
© 2019 Society of Hospital Medicine
Part 3: Leadership Is a Team Effort
“Lead, follow, or get out of the way” sounds pejorative—even arrogant—but it ultimately speaks truth about most situations involving a team. A leader must know, or at least sense, the right action to take at any given moment; sometimes that action entails yielding leadership to another team member. So let’s break down this quote to identify the functional behavioral requirements of leadership.
Northouse presents the notion that leadership is a relationship or process of collaboration in which each team member is needed. The leader should be cognizant of each member’s interests, ideas, passions, attitudes, and motivations.1
As a leader, you must reflect on all of your output. This includes how you relate to those you lead; how you collaborate with and affect the actions of your teammates; and how you communicate the process and influence a team toward a goal—which is crucial to the entire team’s success. Allow me to illustrate these core principles.
Relating
Early in the NP movement, it was necessary to develop a collective vision for the profession’s future. What was the purpose of an NP? What could we add to the existing health care landscape? The founders and early proponents of our profession, recognizing that there was power in numbers and strength in collaboration, identified a mission: Provide health care services for those who were underserved. Working as a group, NPs leveraged strength in numbers, creating a more efficient way to move forward and achieve that mission.2 In those early NP pioneers, I recognize the leadership skills—ability to engage individuals and coordinate activities to move an agenda forward—that are key components of any relationship.
Collaborating
Later, in 1984, a small group of like-minded NPs (of which I was one) joined together to investigate the possibility of a starting an organization dedicated to NPs. As a profession, we were woefully underrepresented nationally. Our role was not fully understood, especially by legislators, and there were laws in place that impeded patients’ access to care by NPs. The existing nursing organizations were in no position to dedicate their resources to represent us professionally or politically.
Several colleagues and I were willing to take a risk to move our profession forward, even if it meant alienating other NPs. Each of us was able to work autonomously, as well as in a team, and we all viewed adversity as an opportunity. This gave us the impetus and motivation to carry out the footwork needed to achieve our goal. These skills—determination, energy, persistence—are essential for anyone looking to start a business or get involved in an organization.
Maybe when my colleagues and I formed the American Academy of Nurse Practitioners (AANP), we weren’t all leaders … but our relationship consisted of the passion and collective vision needed to work together and achieve. We knew we had to build on each other’s strengths and remain open and respectful of each other’s ideas. We believed we had nothing to lose and everything to gain and—honestly—we succeeded on all fronts!
Continue to: Influencing
Influencing
One success story happened in 1988 when Title VIII of the Public Health Service Act—the Nurse Education Act—was under review. New provisions in the bill included specific penalties for NPs and nurses if they defaulted on their student loans—penalties that did not apply to other health care professionals. My colleagues and I were outraged! Like many others, I had such a loan, which had allowed me to pursue my dream of becoming an NP.
The AANP got the word out, and we bombarded our legislators’ offices with calls and a threat to “march on Washington.” For my part, I personally spoke with Senator Edward “Ted” Kennedy and asked him if he realized the revisions made him look like a “loan shark.” I told him that NPs were in direct competition with physicians in settings identified as “loan repayments sites” and that physicians were more apt to be hired in these settings than NPs. I quickly offered up alternatives to increase the number of eligible sites where NPs could work for loan repayment, such as community health centers—a system for which he had secured funding decades earlier.
The end result of our influence? Community health centers throughout the country would be considered “loan repayment” sites, which helped to expand the opportunities for NPs to fulfill their financial obligations. If that doesn’t show you that being a leader requires you to challenge unfairness and identify solutions to correct inequity, I don’t know what does.
In a health care organization, we all have multiple roles that require us to be leaders. We are collaborators, providers of care, advocates for our patients, problem-solvers, and idealists. We are also role models for nascent health care providers. A leader’s responsibility spans the breadth of the organization, and today’s health care system continues to demand strong leaders capable of utilizing a variety of skills.
Next Thursday, I will continue my investigation of how to become an effective leader. In our fourth and final part of this series, we will discuss how acknowledging our specific personality traits can strengthen the efforts of a leader.
1. Northouse PG. Introduction to Leadership: Concepts and Practice. Thousand Oaks, CA: SAGE Publications; 2009.
2. Resnick B, Sheer B, McArthur DB, et al. The world is our oyster: celebrating our past and anticipating our future. J Am Acad Nurse Pract. 2002;14(11):484-491.
“Lead, follow, or get out of the way” sounds pejorative—even arrogant—but it ultimately speaks truth about most situations involving a team. A leader must know, or at least sense, the right action to take at any given moment; sometimes that action entails yielding leadership to another team member. So let’s break down this quote to identify the functional behavioral requirements of leadership.
Northouse presents the notion that leadership is a relationship or process of collaboration in which each team member is needed. The leader should be cognizant of each member’s interests, ideas, passions, attitudes, and motivations.1
As a leader, you must reflect on all of your output. This includes how you relate to those you lead; how you collaborate with and affect the actions of your teammates; and how you communicate the process and influence a team toward a goal—which is crucial to the entire team’s success. Allow me to illustrate these core principles.
Relating
Early in the NP movement, it was necessary to develop a collective vision for the profession’s future. What was the purpose of an NP? What could we add to the existing health care landscape? The founders and early proponents of our profession, recognizing that there was power in numbers and strength in collaboration, identified a mission: Provide health care services for those who were underserved. Working as a group, NPs leveraged strength in numbers, creating a more efficient way to move forward and achieve that mission.2 In those early NP pioneers, I recognize the leadership skills—ability to engage individuals and coordinate activities to move an agenda forward—that are key components of any relationship.
Collaborating
Later, in 1984, a small group of like-minded NPs (of which I was one) joined together to investigate the possibility of a starting an organization dedicated to NPs. As a profession, we were woefully underrepresented nationally. Our role was not fully understood, especially by legislators, and there were laws in place that impeded patients’ access to care by NPs. The existing nursing organizations were in no position to dedicate their resources to represent us professionally or politically.
Several colleagues and I were willing to take a risk to move our profession forward, even if it meant alienating other NPs. Each of us was able to work autonomously, as well as in a team, and we all viewed adversity as an opportunity. This gave us the impetus and motivation to carry out the footwork needed to achieve our goal. These skills—determination, energy, persistence—are essential for anyone looking to start a business or get involved in an organization.
Maybe when my colleagues and I formed the American Academy of Nurse Practitioners (AANP), we weren’t all leaders … but our relationship consisted of the passion and collective vision needed to work together and achieve. We knew we had to build on each other’s strengths and remain open and respectful of each other’s ideas. We believed we had nothing to lose and everything to gain and—honestly—we succeeded on all fronts!
Continue to: Influencing
Influencing
One success story happened in 1988 when Title VIII of the Public Health Service Act—the Nurse Education Act—was under review. New provisions in the bill included specific penalties for NPs and nurses if they defaulted on their student loans—penalties that did not apply to other health care professionals. My colleagues and I were outraged! Like many others, I had such a loan, which had allowed me to pursue my dream of becoming an NP.
The AANP got the word out, and we bombarded our legislators’ offices with calls and a threat to “march on Washington.” For my part, I personally spoke with Senator Edward “Ted” Kennedy and asked him if he realized the revisions made him look like a “loan shark.” I told him that NPs were in direct competition with physicians in settings identified as “loan repayments sites” and that physicians were more apt to be hired in these settings than NPs. I quickly offered up alternatives to increase the number of eligible sites where NPs could work for loan repayment, such as community health centers—a system for which he had secured funding decades earlier.
The end result of our influence? Community health centers throughout the country would be considered “loan repayment” sites, which helped to expand the opportunities for NPs to fulfill their financial obligations. If that doesn’t show you that being a leader requires you to challenge unfairness and identify solutions to correct inequity, I don’t know what does.
In a health care organization, we all have multiple roles that require us to be leaders. We are collaborators, providers of care, advocates for our patients, problem-solvers, and idealists. We are also role models for nascent health care providers. A leader’s responsibility spans the breadth of the organization, and today’s health care system continues to demand strong leaders capable of utilizing a variety of skills.
Next Thursday, I will continue my investigation of how to become an effective leader. In our fourth and final part of this series, we will discuss how acknowledging our specific personality traits can strengthen the efforts of a leader.
“Lead, follow, or get out of the way” sounds pejorative—even arrogant—but it ultimately speaks truth about most situations involving a team. A leader must know, or at least sense, the right action to take at any given moment; sometimes that action entails yielding leadership to another team member. So let’s break down this quote to identify the functional behavioral requirements of leadership.
Northouse presents the notion that leadership is a relationship or process of collaboration in which each team member is needed. The leader should be cognizant of each member’s interests, ideas, passions, attitudes, and motivations.1
As a leader, you must reflect on all of your output. This includes how you relate to those you lead; how you collaborate with and affect the actions of your teammates; and how you communicate the process and influence a team toward a goal—which is crucial to the entire team’s success. Allow me to illustrate these core principles.
Relating
Early in the NP movement, it was necessary to develop a collective vision for the profession’s future. What was the purpose of an NP? What could we add to the existing health care landscape? The founders and early proponents of our profession, recognizing that there was power in numbers and strength in collaboration, identified a mission: Provide health care services for those who were underserved. Working as a group, NPs leveraged strength in numbers, creating a more efficient way to move forward and achieve that mission.2 In those early NP pioneers, I recognize the leadership skills—ability to engage individuals and coordinate activities to move an agenda forward—that are key components of any relationship.
Collaborating
Later, in 1984, a small group of like-minded NPs (of which I was one) joined together to investigate the possibility of a starting an organization dedicated to NPs. As a profession, we were woefully underrepresented nationally. Our role was not fully understood, especially by legislators, and there were laws in place that impeded patients’ access to care by NPs. The existing nursing organizations were in no position to dedicate their resources to represent us professionally or politically.
Several colleagues and I were willing to take a risk to move our profession forward, even if it meant alienating other NPs. Each of us was able to work autonomously, as well as in a team, and we all viewed adversity as an opportunity. This gave us the impetus and motivation to carry out the footwork needed to achieve our goal. These skills—determination, energy, persistence—are essential for anyone looking to start a business or get involved in an organization.
Maybe when my colleagues and I formed the American Academy of Nurse Practitioners (AANP), we weren’t all leaders … but our relationship consisted of the passion and collective vision needed to work together and achieve. We knew we had to build on each other’s strengths and remain open and respectful of each other’s ideas. We believed we had nothing to lose and everything to gain and—honestly—we succeeded on all fronts!
Continue to: Influencing
Influencing
One success story happened in 1988 when Title VIII of the Public Health Service Act—the Nurse Education Act—was under review. New provisions in the bill included specific penalties for NPs and nurses if they defaulted on their student loans—penalties that did not apply to other health care professionals. My colleagues and I were outraged! Like many others, I had such a loan, which had allowed me to pursue my dream of becoming an NP.
The AANP got the word out, and we bombarded our legislators’ offices with calls and a threat to “march on Washington.” For my part, I personally spoke with Senator Edward “Ted” Kennedy and asked him if he realized the revisions made him look like a “loan shark.” I told him that NPs were in direct competition with physicians in settings identified as “loan repayments sites” and that physicians were more apt to be hired in these settings than NPs. I quickly offered up alternatives to increase the number of eligible sites where NPs could work for loan repayment, such as community health centers—a system for which he had secured funding decades earlier.
The end result of our influence? Community health centers throughout the country would be considered “loan repayment” sites, which helped to expand the opportunities for NPs to fulfill their financial obligations. If that doesn’t show you that being a leader requires you to challenge unfairness and identify solutions to correct inequity, I don’t know what does.
In a health care organization, we all have multiple roles that require us to be leaders. We are collaborators, providers of care, advocates for our patients, problem-solvers, and idealists. We are also role models for nascent health care providers. A leader’s responsibility spans the breadth of the organization, and today’s health care system continues to demand strong leaders capable of utilizing a variety of skills.
Next Thursday, I will continue my investigation of how to become an effective leader. In our fourth and final part of this series, we will discuss how acknowledging our specific personality traits can strengthen the efforts of a leader.
1. Northouse PG. Introduction to Leadership: Concepts and Practice. Thousand Oaks, CA: SAGE Publications; 2009.
2. Resnick B, Sheer B, McArthur DB, et al. The world is our oyster: celebrating our past and anticipating our future. J Am Acad Nurse Pract. 2002;14(11):484-491.
1. Northouse PG. Introduction to Leadership: Concepts and Practice. Thousand Oaks, CA: SAGE Publications; 2009.
2. Resnick B, Sheer B, McArthur DB, et al. The world is our oyster: celebrating our past and anticipating our future. J Am Acad Nurse Pract. 2002;14(11):484-491.
FDA grants sirolimus-eluting balloon breakthrough device designation for PAD
The Food and Drug Administration has granted the Breakthrough Device Designation to the Virtue sirolimus-eluting balloon (SEB) for below-the-knee peripheral arterial disease, according to a statement from Orchestra BioMed.
According to the FDA, this designation indicates that the Virtue SEB could provide a “more effective treatment option ... for a life-threatening or irreversibly debilitating disease”; as the release notes, below-the-knee atherosclerosis presents a high rate of amputation and poor survival outcomes but has limited treatment options. The designation leads to expedited development, assessment, and review.
Darren R. Sherman, president, CEO, and cofounder of Orchestra BioMed, noted that the Virtue SEB “has the potential to improve long-term outcomes and reduce periprocedural complications” that can “extend hospital stay and increase cost of treatment.” The system had previously received this designation for coronary in-stent restenosis based upon the 3-year results of the European SABRE trial.
The Food and Drug Administration has granted the Breakthrough Device Designation to the Virtue sirolimus-eluting balloon (SEB) for below-the-knee peripheral arterial disease, according to a statement from Orchestra BioMed.
According to the FDA, this designation indicates that the Virtue SEB could provide a “more effective treatment option ... for a life-threatening or irreversibly debilitating disease”; as the release notes, below-the-knee atherosclerosis presents a high rate of amputation and poor survival outcomes but has limited treatment options. The designation leads to expedited development, assessment, and review.
Darren R. Sherman, president, CEO, and cofounder of Orchestra BioMed, noted that the Virtue SEB “has the potential to improve long-term outcomes and reduce periprocedural complications” that can “extend hospital stay and increase cost of treatment.” The system had previously received this designation for coronary in-stent restenosis based upon the 3-year results of the European SABRE trial.
The Food and Drug Administration has granted the Breakthrough Device Designation to the Virtue sirolimus-eluting balloon (SEB) for below-the-knee peripheral arterial disease, according to a statement from Orchestra BioMed.
According to the FDA, this designation indicates that the Virtue SEB could provide a “more effective treatment option ... for a life-threatening or irreversibly debilitating disease”; as the release notes, below-the-knee atherosclerosis presents a high rate of amputation and poor survival outcomes but has limited treatment options. The designation leads to expedited development, assessment, and review.
Darren R. Sherman, president, CEO, and cofounder of Orchestra BioMed, noted that the Virtue SEB “has the potential to improve long-term outcomes and reduce periprocedural complications” that can “extend hospital stay and increase cost of treatment.” The system had previously received this designation for coronary in-stent restenosis based upon the 3-year results of the European SABRE trial.
U.S. increases in RA burden outpace global averages
according to a new analysis of RA activity in 195 countries.
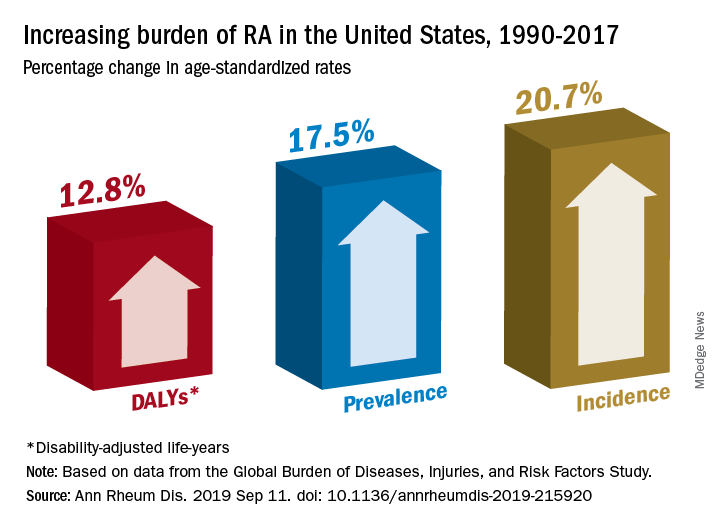
Percentage changes in the incidence, prevalence, and disability-adjusted life-year (DALY) rates for RA all reached double digits in the United States over the study period, but global increases for those measures stayed in the single digits, except for DALY, which did not increase, Saeid Safiri, PhD, of Tabriz (Iran) University of Medical Sciences and associates wrote in Annals of the Rheumatic Diseases.
Data from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2017 show that RA incidence in the United States had the largest increase (20.7%) among the three measures, rising from 19.3 cases per 100,000 population in 1990 to 23.4 in 2017. Overall incidence for the 195 countries included in the study went from 13.7 to 14.9 cases per 100,000, for an increase of 8.2%, the investigators reported.
That pattern largely repeats for prevalence: an increase of 17.5% in the United States as the number of cases went from 336.5 per 100,000 in 1990 to 395.5 in 2017, and an increase of 7.4% globally, with the number of prevalent cases rising from 229.6 to 246.5 per 100,000, they said.
The DALY numbers – think of each DALY as 1 lost year of “healthy” life, the World Health Organization says – tell a somewhat different story. The United States had DALY rate of 53.2 per 100,000 in 1990, but by 2017 it had climbed to 60 per 100,000, an increase of 12.8%. Over that same time period, the global rate fell by 3.6% as it went from 44.9 to 43.3, Dr. Safiri and associates reported.
That long-term decline does, however, disguise a more recent trend. The global DALY rate “decreased from 1990 to 2012 but then increased and reached higher than expected levels in the following 5 years to 2017,” they wrote.
RA rates in the United States in 2017 were, as noted, above average, but they were not the highest. The United Kingdom achieved the RA trifecta of highest incidence (27.5 per 100,000), highest prevalence (471.8 per 100,000), and highest DALY rate (73 per 100,000) among the 195 countries in the study. At the other end of the three scales, Indonesia had the lowest incidence (5.6 per 100,000) and prevalence (91.1 per 100,000), and Sri Lanka had the lowest DALY rate (14.2 per 100,000), they said.
“Age-standardized prevalence and incidence rates are overall increasing globally. Increasing population awareness regarding RA, its risk factors and the importance of early diagnosis and treatment with disease-modifying agents is warranted to reduce the future burden of this condition,” the research team concluded.
GBD is funded by the Bill and Melinda Gates Foundation. The current analysis also was supported by Social Determinants of Health Research Center, Shahid Beheshti University of Medical Sciences in Tehran, Iran. The investigators did not declare any conflicts of interest.
SOURCE: Safiri S et al. Ann Rheum Dis. 2019 Sep 11. doi: 10.1136/annrheumdis-2019-215920.
according to a new analysis of RA activity in 195 countries.

Percentage changes in the incidence, prevalence, and disability-adjusted life-year (DALY) rates for RA all reached double digits in the United States over the study period, but global increases for those measures stayed in the single digits, except for DALY, which did not increase, Saeid Safiri, PhD, of Tabriz (Iran) University of Medical Sciences and associates wrote in Annals of the Rheumatic Diseases.
Data from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2017 show that RA incidence in the United States had the largest increase (20.7%) among the three measures, rising from 19.3 cases per 100,000 population in 1990 to 23.4 in 2017. Overall incidence for the 195 countries included in the study went from 13.7 to 14.9 cases per 100,000, for an increase of 8.2%, the investigators reported.
That pattern largely repeats for prevalence: an increase of 17.5% in the United States as the number of cases went from 336.5 per 100,000 in 1990 to 395.5 in 2017, and an increase of 7.4% globally, with the number of prevalent cases rising from 229.6 to 246.5 per 100,000, they said.
The DALY numbers – think of each DALY as 1 lost year of “healthy” life, the World Health Organization says – tell a somewhat different story. The United States had DALY rate of 53.2 per 100,000 in 1990, but by 2017 it had climbed to 60 per 100,000, an increase of 12.8%. Over that same time period, the global rate fell by 3.6% as it went from 44.9 to 43.3, Dr. Safiri and associates reported.
That long-term decline does, however, disguise a more recent trend. The global DALY rate “decreased from 1990 to 2012 but then increased and reached higher than expected levels in the following 5 years to 2017,” they wrote.
RA rates in the United States in 2017 were, as noted, above average, but they were not the highest. The United Kingdom achieved the RA trifecta of highest incidence (27.5 per 100,000), highest prevalence (471.8 per 100,000), and highest DALY rate (73 per 100,000) among the 195 countries in the study. At the other end of the three scales, Indonesia had the lowest incidence (5.6 per 100,000) and prevalence (91.1 per 100,000), and Sri Lanka had the lowest DALY rate (14.2 per 100,000), they said.
“Age-standardized prevalence and incidence rates are overall increasing globally. Increasing population awareness regarding RA, its risk factors and the importance of early diagnosis and treatment with disease-modifying agents is warranted to reduce the future burden of this condition,” the research team concluded.
GBD is funded by the Bill and Melinda Gates Foundation. The current analysis also was supported by Social Determinants of Health Research Center, Shahid Beheshti University of Medical Sciences in Tehran, Iran. The investigators did not declare any conflicts of interest.
SOURCE: Safiri S et al. Ann Rheum Dis. 2019 Sep 11. doi: 10.1136/annrheumdis-2019-215920.
according to a new analysis of RA activity in 195 countries.

Percentage changes in the incidence, prevalence, and disability-adjusted life-year (DALY) rates for RA all reached double digits in the United States over the study period, but global increases for those measures stayed in the single digits, except for DALY, which did not increase, Saeid Safiri, PhD, of Tabriz (Iran) University of Medical Sciences and associates wrote in Annals of the Rheumatic Diseases.
Data from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2017 show that RA incidence in the United States had the largest increase (20.7%) among the three measures, rising from 19.3 cases per 100,000 population in 1990 to 23.4 in 2017. Overall incidence for the 195 countries included in the study went from 13.7 to 14.9 cases per 100,000, for an increase of 8.2%, the investigators reported.
That pattern largely repeats for prevalence: an increase of 17.5% in the United States as the number of cases went from 336.5 per 100,000 in 1990 to 395.5 in 2017, and an increase of 7.4% globally, with the number of prevalent cases rising from 229.6 to 246.5 per 100,000, they said.
The DALY numbers – think of each DALY as 1 lost year of “healthy” life, the World Health Organization says – tell a somewhat different story. The United States had DALY rate of 53.2 per 100,000 in 1990, but by 2017 it had climbed to 60 per 100,000, an increase of 12.8%. Over that same time period, the global rate fell by 3.6% as it went from 44.9 to 43.3, Dr. Safiri and associates reported.
That long-term decline does, however, disguise a more recent trend. The global DALY rate “decreased from 1990 to 2012 but then increased and reached higher than expected levels in the following 5 years to 2017,” they wrote.
RA rates in the United States in 2017 were, as noted, above average, but they were not the highest. The United Kingdom achieved the RA trifecta of highest incidence (27.5 per 100,000), highest prevalence (471.8 per 100,000), and highest DALY rate (73 per 100,000) among the 195 countries in the study. At the other end of the three scales, Indonesia had the lowest incidence (5.6 per 100,000) and prevalence (91.1 per 100,000), and Sri Lanka had the lowest DALY rate (14.2 per 100,000), they said.
“Age-standardized prevalence and incidence rates are overall increasing globally. Increasing population awareness regarding RA, its risk factors and the importance of early diagnosis and treatment with disease-modifying agents is warranted to reduce the future burden of this condition,” the research team concluded.
GBD is funded by the Bill and Melinda Gates Foundation. The current analysis also was supported by Social Determinants of Health Research Center, Shahid Beheshti University of Medical Sciences in Tehran, Iran. The investigators did not declare any conflicts of interest.
SOURCE: Safiri S et al. Ann Rheum Dis. 2019 Sep 11. doi: 10.1136/annrheumdis-2019-215920.
FROM ANNALS OF THE RHEUMATIC DISEASES
FDA approves pembrolizumab/lenvatinib combo for advanced endometrial carcinoma
The Food and Drug Administration has granted accelerated approval to a pembrolizumab (Keytruda) plus lenvatinib (Lenvima) combination for the treatment of patients with advanced endometrial carcinoma that is not microsatellite instability high or mismatch repair deficient, and who have disease progression following prior systemic therapy but are not candidates for curative surgery or radiation.
The approval was based on results of KEYNOTE-146, a single-arm, multicenter, open-label, multicohort trial with 108 patients with metastatic endometrial carcinoma; 94 of these were not microsatellite instability high or mismatch repair deficient. The objective response rate in those 94 patients was 38.3% (95% confidence interval, 29%-49%), with 10 complete responses and 26 partial responses. The median duration of response was not reached over the trial period, and 69% of those who responded had a response duration of at least 6 months.
The most common adverse events reported during the trial were fatigue, hypertension, musculoskeletal pain, diarrhea, decreased appetite, hypothyroidism, nausea, stomatitis, vomiting, decreased weight, abdominal pain, headache, constipation, urinary tract infection, dysphonia, hemorrhagic events, hypomagnesemia, palmar-plantar erythrodysesthesia, dyspnea, cough, and rash.
The recommended dosage is lenvatinib 20 mg orally once daily with pembrolizumab 200 mg administered as an intravenous infusion over 30 minutes every 3 weeks, according to the FDA.
The Food and Drug Administration has granted accelerated approval to a pembrolizumab (Keytruda) plus lenvatinib (Lenvima) combination for the treatment of patients with advanced endometrial carcinoma that is not microsatellite instability high or mismatch repair deficient, and who have disease progression following prior systemic therapy but are not candidates for curative surgery or radiation.
The approval was based on results of KEYNOTE-146, a single-arm, multicenter, open-label, multicohort trial with 108 patients with metastatic endometrial carcinoma; 94 of these were not microsatellite instability high or mismatch repair deficient. The objective response rate in those 94 patients was 38.3% (95% confidence interval, 29%-49%), with 10 complete responses and 26 partial responses. The median duration of response was not reached over the trial period, and 69% of those who responded had a response duration of at least 6 months.
The most common adverse events reported during the trial were fatigue, hypertension, musculoskeletal pain, diarrhea, decreased appetite, hypothyroidism, nausea, stomatitis, vomiting, decreased weight, abdominal pain, headache, constipation, urinary tract infection, dysphonia, hemorrhagic events, hypomagnesemia, palmar-plantar erythrodysesthesia, dyspnea, cough, and rash.
The recommended dosage is lenvatinib 20 mg orally once daily with pembrolizumab 200 mg administered as an intravenous infusion over 30 minutes every 3 weeks, according to the FDA.
The Food and Drug Administration has granted accelerated approval to a pembrolizumab (Keytruda) plus lenvatinib (Lenvima) combination for the treatment of patients with advanced endometrial carcinoma that is not microsatellite instability high or mismatch repair deficient, and who have disease progression following prior systemic therapy but are not candidates for curative surgery or radiation.
The approval was based on results of KEYNOTE-146, a single-arm, multicenter, open-label, multicohort trial with 108 patients with metastatic endometrial carcinoma; 94 of these were not microsatellite instability high or mismatch repair deficient. The objective response rate in those 94 patients was 38.3% (95% confidence interval, 29%-49%), with 10 complete responses and 26 partial responses. The median duration of response was not reached over the trial period, and 69% of those who responded had a response duration of at least 6 months.
The most common adverse events reported during the trial were fatigue, hypertension, musculoskeletal pain, diarrhea, decreased appetite, hypothyroidism, nausea, stomatitis, vomiting, decreased weight, abdominal pain, headache, constipation, urinary tract infection, dysphonia, hemorrhagic events, hypomagnesemia, palmar-plantar erythrodysesthesia, dyspnea, cough, and rash.
The recommended dosage is lenvatinib 20 mg orally once daily with pembrolizumab 200 mg administered as an intravenous infusion over 30 minutes every 3 weeks, according to the FDA.
Rivaroxaban bests combo therapy in post-PCI AFib
PARIS – Rivaroxaban monotherapy bested combination therapy with rivaroxaban and an antiplatelet agent for patients with atrial fibrillation and stable coronary artery disease, with significantly more deaths and bleeding events seen with combination therapy.
The pronounced imbalance in all-cause and cardiovascular mortality (the hazard ratio favoring rivaroxaban monotherapy was 9.72) came as a surprise, and led to early cessation of the multisite Japanese trial, lead investigator Satoshi Yasuda, MD, said at the annual congress of the European Society of Cardiology.
Several previous clinical trials had studied a reduced antithrombotic regimen for patients with atrial fibrillation (AFib) after percutaneous coronary intervention (PCI), said Dr. Yasuda, professor of medicine at Tohoku University, Sendai, Japan. Current guidelines recommend triple therapy with an oral anticoagulant plus aspirin and a P2Y12 inhibitor for the shortest duration possible, with combination therapy of an anticoagulant plus a P2Y12 inhibitor for up to 12 months. Once the 1-year post-PCI mark is reached, current European and American guidelines or consensus documents recommend monotherapy with an oral anticoagulant if AFib persists and the patient has stable coronary artery disease (CAD), explained Dr. Yasuda. “However, this approach has yet to be supported by evidence from randomized, controlled trials,” he said, adding “substantial numbers of patients in this situation continue to be treated with combination therapy, which indicates a gap between guidelines and clinical practice.”
The Atrial Fibrillation and Ischemic events with Rivaroxaban in Patients With Stable Coronary Artery Disease Study (AFIRE), he said, was designed to address this practice gap, randomizing 2,200 individuals to receive monotherapy with rivaroxaban or combination therapy. A total of 1,973 patients completed follow-up.
Patients were included in the randomized, open-label, parallel-group trial if they had AFib and stable CAD and were more than 1 year out from revascularization, or if they had angiographically confirmed CAD that did not need revascularization. All 294 AFIRE study sites were in Japan.
The study’s primary endpoint for efficacy was a composite of stroke, systemic embolism, myocardial infarction, unstable angina requiring revascularization, and all-cause death.
Most of the patients (79%) were male, and the mean age was 74 years. About 70% of patients in each treatment arm had received prior PCI, and 11% had undergone previous coronary artery bypass grafting (CABG).
The monotherapy arm received rivaroxaban 10 or 15 mg once daily depending on renal status. Patients in the combination therapy arm received rivaroxaban, plus a single antiplatelet drug. This could be 81 or 100 mg aspirin daily, clopidogrel at 50 or 75 mg/day, or prasugrel at 2.5 or 3.5 mg/day.
On the recommendation of the data and safety monitoring committee, the trial was terminated about 3 months early because significantly more all-cause deaths were being seen in the combination therapy group, said Dr. Yasuda. In the end, patients were treated under the study protocol for a median 23 months and followed up for a median 24.1 months.
Kaplan-Meier estimates for the first occurrence of the composite efficacy endpoint showed that monotherapy had a rate of 4.14% per patient-year, while combination therapy had a rate of occurrence for the efficacy endpoint of 5.75% per patient-year.
These figures yielded a statistically significant hazard ratio (HR) of 9.72 favoring monotherapy (P less than .001) for the prespecified noninferiority endpoint. In a post hoc analysis, rivaroxaban monotherapy achieved superiority over dual therapy (P = .02).
Breaking down the composite efficacy endpoint into its constituents, deaths by any cause and cardiovascular deaths primarily drove the difference in treatment arms. Seventy-three patients in the combo therapy arm and 41 in the rivaroxaban arm died of any cause, and the cause of death was cardiovascular for 43 combination therapy patients and 26 monotherapy patients. This yielded HRs favoring rivaroxaban of 0.55 for all-cause mortality and 0.59 for cardiovascular deaths.
Hazard ratios for individual cardiovascular events were not statistically significantly different between treatment arms, except for hemorrhagic stroke, which was seen in 13 patients receiving dual therapy and 4 receiving rivaroxaban alone, for a hazard ratio of 0.30.
Rivaroxaban monotherapy also bested dual therapy in safety: The HR was 0.59 for the incidence of a major bleed on rivaroxaban versus combination therapy, using International Society on Thrombosis and Haemostasis–established criteria for major bleeding. In the dual therapy arm, 58 individuals experienced major bleeding – the study’s primary safety endpoint – compared with 35 in the monotherapy arm, for a hazard ratio of 0.59; nonmajor bleeding occurred in 198 dual therapy patients and 121 monotherapy patients, yielding a hazard ratio of 0.58.
The Kaplan-Meier estimate for major bleeding on monotherapy was 1.62% per patient-year, compared with 2.76% per patient-year for those on combination therapy. These findings, said Dr. Yasuda, were “generally” consistent across prespecified subgroups that included participant stratification by age, sex, and bleeding risk, among others.
Dr. Yasuda acknowledged the many limitations of the trial. First, early termination introduced the possibility of overestimating the benefit of rivaroxaban monotherapy. Indeed, said Dr. Yasuda, “the reductions in rate of ischemic events and death from any cause with rivaroxaban monotherapy were unanticipated and are difficult to explain.”
Furthermore, the open-label trial design could be a source of bias and the use of both aspirin and P2Y12 inhibitors for antiplatelet therapy “makes it uncertain whether the benefit of rivaroxaban monotherapy applies equally to the two combination regimens,” said Dr. Yasuda.
Rivaroxaban dosing in AFIRE was tailored to the Japanese study population, noted Dr. Yasuda. This means that the study is not immediately generalizable to non-Asian populations, needing replication before fully closing the knowledge gap about best long-term management of patients with AFib and stable CAD in the United States and Western Europe.
However, Dr. Yasuda pointed out, serum rivaroxaban levels in Japanese patients taking the 10- or 15-mg dose are generally similar to those seen in white patients taking a 20-mg rivaroxaban dose.
Freek Verheugt, MD, of Onze Lieve Vrouwe Gasthuis Hospital, Amsterdam, was the discussant for the presentation. He raised an additional concern: “East Asian patients are poor metabolizers of clopidogrel, which may have resulted also in underestimation of bleeding.” He cautioned that the AFIRE results may not be applicable to patients on a novel anticoagulant other than clopidogrel, or on vitamin K antagonists.
In his detailed critique of the AFIRE results, Dr. Verheugt cited the OAC ALONE trial, which used a similar study design and was also conducted in Japan. For OAC ALONE, Dr. Verheugt pointed out that “You can see ... that it was not harmful in this 700-patient study to stop aspirin therapy 1 year after an intervention.” However, he said, “the net clinical benefit is not very different, either” between treatment arms in the OAC ALONE trial. “Given the low number of patients and the low number of events, this trial was not conclusive whatsoever” he added, so AFIRE’s findings were needed.
The safety data from AFIRE, with a study population triple that of OAC ALONE, makes the safety argument for monotherapy “a very easy winner,” said Dr. Verheugt.
Dr. Verheugt was not mystified by the lower all-cause and cardiovascular death rate in the monotherapy group. “What are the mechanisms that if you stop antiplatelet therapy you have a better ischemic outcome? How come?” asked Dr. Verheugt.
“Very likely, it is the bleeding ... that you prevent if you stop antiplatelet therapy,” he said, adding that it’s known from previous studies in individuals with acute coronary syndromes and AFib that “bleeding is correlated with mortality, and that’s also proven here.”
Though Dr. Verheugt joined Dr. Yasuda in calling for replication of the results in a non-Asian population, he concurred that the AFIRE results validate current practice for anticoagulation in AFib with stable CAD. “Stopping at 1 year is safer than continuation and, most of all, it saves lives,” he said.
Full results of AFIRE were published online at the time of Dr. Yasuda’s presentation (N Engl J Med. 2019 Sep 2. doi: 10.1056/NEJMoa1904143).
The study was funded by the Japanese Cardiovascular Research Foundation. Dr. Yasuda reported financial relationships with Abbott, Bristol-Myers, Daiichi-Sankyo, and Takeda. Dr. Verheugt reported financial relationships with BayerHealthcare, BMS/Pfizer, Boehringer-Ingelheim, and Daiichi-Sankyo.
SOURCE: Yasuda S. et al. ESC 2019, Hot Line Session 3, Abstract 3175.
PARIS – Rivaroxaban monotherapy bested combination therapy with rivaroxaban and an antiplatelet agent for patients with atrial fibrillation and stable coronary artery disease, with significantly more deaths and bleeding events seen with combination therapy.
The pronounced imbalance in all-cause and cardiovascular mortality (the hazard ratio favoring rivaroxaban monotherapy was 9.72) came as a surprise, and led to early cessation of the multisite Japanese trial, lead investigator Satoshi Yasuda, MD, said at the annual congress of the European Society of Cardiology.
Several previous clinical trials had studied a reduced antithrombotic regimen for patients with atrial fibrillation (AFib) after percutaneous coronary intervention (PCI), said Dr. Yasuda, professor of medicine at Tohoku University, Sendai, Japan. Current guidelines recommend triple therapy with an oral anticoagulant plus aspirin and a P2Y12 inhibitor for the shortest duration possible, with combination therapy of an anticoagulant plus a P2Y12 inhibitor for up to 12 months. Once the 1-year post-PCI mark is reached, current European and American guidelines or consensus documents recommend monotherapy with an oral anticoagulant if AFib persists and the patient has stable coronary artery disease (CAD), explained Dr. Yasuda. “However, this approach has yet to be supported by evidence from randomized, controlled trials,” he said, adding “substantial numbers of patients in this situation continue to be treated with combination therapy, which indicates a gap between guidelines and clinical practice.”
The Atrial Fibrillation and Ischemic events with Rivaroxaban in Patients With Stable Coronary Artery Disease Study (AFIRE), he said, was designed to address this practice gap, randomizing 2,200 individuals to receive monotherapy with rivaroxaban or combination therapy. A total of 1,973 patients completed follow-up.
Patients were included in the randomized, open-label, parallel-group trial if they had AFib and stable CAD and were more than 1 year out from revascularization, or if they had angiographically confirmed CAD that did not need revascularization. All 294 AFIRE study sites were in Japan.
The study’s primary endpoint for efficacy was a composite of stroke, systemic embolism, myocardial infarction, unstable angina requiring revascularization, and all-cause death.
Most of the patients (79%) were male, and the mean age was 74 years. About 70% of patients in each treatment arm had received prior PCI, and 11% had undergone previous coronary artery bypass grafting (CABG).
The monotherapy arm received rivaroxaban 10 or 15 mg once daily depending on renal status. Patients in the combination therapy arm received rivaroxaban, plus a single antiplatelet drug. This could be 81 or 100 mg aspirin daily, clopidogrel at 50 or 75 mg/day, or prasugrel at 2.5 or 3.5 mg/day.
On the recommendation of the data and safety monitoring committee, the trial was terminated about 3 months early because significantly more all-cause deaths were being seen in the combination therapy group, said Dr. Yasuda. In the end, patients were treated under the study protocol for a median 23 months and followed up for a median 24.1 months.
Kaplan-Meier estimates for the first occurrence of the composite efficacy endpoint showed that monotherapy had a rate of 4.14% per patient-year, while combination therapy had a rate of occurrence for the efficacy endpoint of 5.75% per patient-year.
These figures yielded a statistically significant hazard ratio (HR) of 9.72 favoring monotherapy (P less than .001) for the prespecified noninferiority endpoint. In a post hoc analysis, rivaroxaban monotherapy achieved superiority over dual therapy (P = .02).
Breaking down the composite efficacy endpoint into its constituents, deaths by any cause and cardiovascular deaths primarily drove the difference in treatment arms. Seventy-three patients in the combo therapy arm and 41 in the rivaroxaban arm died of any cause, and the cause of death was cardiovascular for 43 combination therapy patients and 26 monotherapy patients. This yielded HRs favoring rivaroxaban of 0.55 for all-cause mortality and 0.59 for cardiovascular deaths.
Hazard ratios for individual cardiovascular events were not statistically significantly different between treatment arms, except for hemorrhagic stroke, which was seen in 13 patients receiving dual therapy and 4 receiving rivaroxaban alone, for a hazard ratio of 0.30.
Rivaroxaban monotherapy also bested dual therapy in safety: The HR was 0.59 for the incidence of a major bleed on rivaroxaban versus combination therapy, using International Society on Thrombosis and Haemostasis–established criteria for major bleeding. In the dual therapy arm, 58 individuals experienced major bleeding – the study’s primary safety endpoint – compared with 35 in the monotherapy arm, for a hazard ratio of 0.59; nonmajor bleeding occurred in 198 dual therapy patients and 121 monotherapy patients, yielding a hazard ratio of 0.58.
The Kaplan-Meier estimate for major bleeding on monotherapy was 1.62% per patient-year, compared with 2.76% per patient-year for those on combination therapy. These findings, said Dr. Yasuda, were “generally” consistent across prespecified subgroups that included participant stratification by age, sex, and bleeding risk, among others.
Dr. Yasuda acknowledged the many limitations of the trial. First, early termination introduced the possibility of overestimating the benefit of rivaroxaban monotherapy. Indeed, said Dr. Yasuda, “the reductions in rate of ischemic events and death from any cause with rivaroxaban monotherapy were unanticipated and are difficult to explain.”
Furthermore, the open-label trial design could be a source of bias and the use of both aspirin and P2Y12 inhibitors for antiplatelet therapy “makes it uncertain whether the benefit of rivaroxaban monotherapy applies equally to the two combination regimens,” said Dr. Yasuda.
Rivaroxaban dosing in AFIRE was tailored to the Japanese study population, noted Dr. Yasuda. This means that the study is not immediately generalizable to non-Asian populations, needing replication before fully closing the knowledge gap about best long-term management of patients with AFib and stable CAD in the United States and Western Europe.
However, Dr. Yasuda pointed out, serum rivaroxaban levels in Japanese patients taking the 10- or 15-mg dose are generally similar to those seen in white patients taking a 20-mg rivaroxaban dose.
Freek Verheugt, MD, of Onze Lieve Vrouwe Gasthuis Hospital, Amsterdam, was the discussant for the presentation. He raised an additional concern: “East Asian patients are poor metabolizers of clopidogrel, which may have resulted also in underestimation of bleeding.” He cautioned that the AFIRE results may not be applicable to patients on a novel anticoagulant other than clopidogrel, or on vitamin K antagonists.
In his detailed critique of the AFIRE results, Dr. Verheugt cited the OAC ALONE trial, which used a similar study design and was also conducted in Japan. For OAC ALONE, Dr. Verheugt pointed out that “You can see ... that it was not harmful in this 700-patient study to stop aspirin therapy 1 year after an intervention.” However, he said, “the net clinical benefit is not very different, either” between treatment arms in the OAC ALONE trial. “Given the low number of patients and the low number of events, this trial was not conclusive whatsoever” he added, so AFIRE’s findings were needed.
The safety data from AFIRE, with a study population triple that of OAC ALONE, makes the safety argument for monotherapy “a very easy winner,” said Dr. Verheugt.
Dr. Verheugt was not mystified by the lower all-cause and cardiovascular death rate in the monotherapy group. “What are the mechanisms that if you stop antiplatelet therapy you have a better ischemic outcome? How come?” asked Dr. Verheugt.
“Very likely, it is the bleeding ... that you prevent if you stop antiplatelet therapy,” he said, adding that it’s known from previous studies in individuals with acute coronary syndromes and AFib that “bleeding is correlated with mortality, and that’s also proven here.”
Though Dr. Verheugt joined Dr. Yasuda in calling for replication of the results in a non-Asian population, he concurred that the AFIRE results validate current practice for anticoagulation in AFib with stable CAD. “Stopping at 1 year is safer than continuation and, most of all, it saves lives,” he said.
Full results of AFIRE were published online at the time of Dr. Yasuda’s presentation (N Engl J Med. 2019 Sep 2. doi: 10.1056/NEJMoa1904143).
The study was funded by the Japanese Cardiovascular Research Foundation. Dr. Yasuda reported financial relationships with Abbott, Bristol-Myers, Daiichi-Sankyo, and Takeda. Dr. Verheugt reported financial relationships with BayerHealthcare, BMS/Pfizer, Boehringer-Ingelheim, and Daiichi-Sankyo.
SOURCE: Yasuda S. et al. ESC 2019, Hot Line Session 3, Abstract 3175.
PARIS – Rivaroxaban monotherapy bested combination therapy with rivaroxaban and an antiplatelet agent for patients with atrial fibrillation and stable coronary artery disease, with significantly more deaths and bleeding events seen with combination therapy.
The pronounced imbalance in all-cause and cardiovascular mortality (the hazard ratio favoring rivaroxaban monotherapy was 9.72) came as a surprise, and led to early cessation of the multisite Japanese trial, lead investigator Satoshi Yasuda, MD, said at the annual congress of the European Society of Cardiology.
Several previous clinical trials had studied a reduced antithrombotic regimen for patients with atrial fibrillation (AFib) after percutaneous coronary intervention (PCI), said Dr. Yasuda, professor of medicine at Tohoku University, Sendai, Japan. Current guidelines recommend triple therapy with an oral anticoagulant plus aspirin and a P2Y12 inhibitor for the shortest duration possible, with combination therapy of an anticoagulant plus a P2Y12 inhibitor for up to 12 months. Once the 1-year post-PCI mark is reached, current European and American guidelines or consensus documents recommend monotherapy with an oral anticoagulant if AFib persists and the patient has stable coronary artery disease (CAD), explained Dr. Yasuda. “However, this approach has yet to be supported by evidence from randomized, controlled trials,” he said, adding “substantial numbers of patients in this situation continue to be treated with combination therapy, which indicates a gap between guidelines and clinical practice.”
The Atrial Fibrillation and Ischemic events with Rivaroxaban in Patients With Stable Coronary Artery Disease Study (AFIRE), he said, was designed to address this practice gap, randomizing 2,200 individuals to receive monotherapy with rivaroxaban or combination therapy. A total of 1,973 patients completed follow-up.
Patients were included in the randomized, open-label, parallel-group trial if they had AFib and stable CAD and were more than 1 year out from revascularization, or if they had angiographically confirmed CAD that did not need revascularization. All 294 AFIRE study sites were in Japan.
The study’s primary endpoint for efficacy was a composite of stroke, systemic embolism, myocardial infarction, unstable angina requiring revascularization, and all-cause death.
Most of the patients (79%) were male, and the mean age was 74 years. About 70% of patients in each treatment arm had received prior PCI, and 11% had undergone previous coronary artery bypass grafting (CABG).
The monotherapy arm received rivaroxaban 10 or 15 mg once daily depending on renal status. Patients in the combination therapy arm received rivaroxaban, plus a single antiplatelet drug. This could be 81 or 100 mg aspirin daily, clopidogrel at 50 or 75 mg/day, or prasugrel at 2.5 or 3.5 mg/day.
On the recommendation of the data and safety monitoring committee, the trial was terminated about 3 months early because significantly more all-cause deaths were being seen in the combination therapy group, said Dr. Yasuda. In the end, patients were treated under the study protocol for a median 23 months and followed up for a median 24.1 months.
Kaplan-Meier estimates for the first occurrence of the composite efficacy endpoint showed that monotherapy had a rate of 4.14% per patient-year, while combination therapy had a rate of occurrence for the efficacy endpoint of 5.75% per patient-year.
These figures yielded a statistically significant hazard ratio (HR) of 9.72 favoring monotherapy (P less than .001) for the prespecified noninferiority endpoint. In a post hoc analysis, rivaroxaban monotherapy achieved superiority over dual therapy (P = .02).
Breaking down the composite efficacy endpoint into its constituents, deaths by any cause and cardiovascular deaths primarily drove the difference in treatment arms. Seventy-three patients in the combo therapy arm and 41 in the rivaroxaban arm died of any cause, and the cause of death was cardiovascular for 43 combination therapy patients and 26 monotherapy patients. This yielded HRs favoring rivaroxaban of 0.55 for all-cause mortality and 0.59 for cardiovascular deaths.
Hazard ratios for individual cardiovascular events were not statistically significantly different between treatment arms, except for hemorrhagic stroke, which was seen in 13 patients receiving dual therapy and 4 receiving rivaroxaban alone, for a hazard ratio of 0.30.
Rivaroxaban monotherapy also bested dual therapy in safety: The HR was 0.59 for the incidence of a major bleed on rivaroxaban versus combination therapy, using International Society on Thrombosis and Haemostasis–established criteria for major bleeding. In the dual therapy arm, 58 individuals experienced major bleeding – the study’s primary safety endpoint – compared with 35 in the monotherapy arm, for a hazard ratio of 0.59; nonmajor bleeding occurred in 198 dual therapy patients and 121 monotherapy patients, yielding a hazard ratio of 0.58.
The Kaplan-Meier estimate for major bleeding on monotherapy was 1.62% per patient-year, compared with 2.76% per patient-year for those on combination therapy. These findings, said Dr. Yasuda, were “generally” consistent across prespecified subgroups that included participant stratification by age, sex, and bleeding risk, among others.
Dr. Yasuda acknowledged the many limitations of the trial. First, early termination introduced the possibility of overestimating the benefit of rivaroxaban monotherapy. Indeed, said Dr. Yasuda, “the reductions in rate of ischemic events and death from any cause with rivaroxaban monotherapy were unanticipated and are difficult to explain.”
Furthermore, the open-label trial design could be a source of bias and the use of both aspirin and P2Y12 inhibitors for antiplatelet therapy “makes it uncertain whether the benefit of rivaroxaban monotherapy applies equally to the two combination regimens,” said Dr. Yasuda.
Rivaroxaban dosing in AFIRE was tailored to the Japanese study population, noted Dr. Yasuda. This means that the study is not immediately generalizable to non-Asian populations, needing replication before fully closing the knowledge gap about best long-term management of patients with AFib and stable CAD in the United States and Western Europe.
However, Dr. Yasuda pointed out, serum rivaroxaban levels in Japanese patients taking the 10- or 15-mg dose are generally similar to those seen in white patients taking a 20-mg rivaroxaban dose.
Freek Verheugt, MD, of Onze Lieve Vrouwe Gasthuis Hospital, Amsterdam, was the discussant for the presentation. He raised an additional concern: “East Asian patients are poor metabolizers of clopidogrel, which may have resulted also in underestimation of bleeding.” He cautioned that the AFIRE results may not be applicable to patients on a novel anticoagulant other than clopidogrel, or on vitamin K antagonists.
In his detailed critique of the AFIRE results, Dr. Verheugt cited the OAC ALONE trial, which used a similar study design and was also conducted in Japan. For OAC ALONE, Dr. Verheugt pointed out that “You can see ... that it was not harmful in this 700-patient study to stop aspirin therapy 1 year after an intervention.” However, he said, “the net clinical benefit is not very different, either” between treatment arms in the OAC ALONE trial. “Given the low number of patients and the low number of events, this trial was not conclusive whatsoever” he added, so AFIRE’s findings were needed.
The safety data from AFIRE, with a study population triple that of OAC ALONE, makes the safety argument for monotherapy “a very easy winner,” said Dr. Verheugt.
Dr. Verheugt was not mystified by the lower all-cause and cardiovascular death rate in the monotherapy group. “What are the mechanisms that if you stop antiplatelet therapy you have a better ischemic outcome? How come?” asked Dr. Verheugt.
“Very likely, it is the bleeding ... that you prevent if you stop antiplatelet therapy,” he said, adding that it’s known from previous studies in individuals with acute coronary syndromes and AFib that “bleeding is correlated with mortality, and that’s also proven here.”
Though Dr. Verheugt joined Dr. Yasuda in calling for replication of the results in a non-Asian population, he concurred that the AFIRE results validate current practice for anticoagulation in AFib with stable CAD. “Stopping at 1 year is safer than continuation and, most of all, it saves lives,” he said.
Full results of AFIRE were published online at the time of Dr. Yasuda’s presentation (N Engl J Med. 2019 Sep 2. doi: 10.1056/NEJMoa1904143).
The study was funded by the Japanese Cardiovascular Research Foundation. Dr. Yasuda reported financial relationships with Abbott, Bristol-Myers, Daiichi-Sankyo, and Takeda. Dr. Verheugt reported financial relationships with BayerHealthcare, BMS/Pfizer, Boehringer-Ingelheim, and Daiichi-Sankyo.
SOURCE: Yasuda S. et al. ESC 2019, Hot Line Session 3, Abstract 3175.
AT THE ESC CONGRESS 2019