User login
Indwelling endoscopic biliary stents reduced risk of recurrent strictures in chronic pancreatitis
Sundeep Lakhtakia, MD, and colleagues reported in Gastrointestinal Endoscopy.
Patients with severe disease at baseline were more than twice as likely to develop a postprocedural stricture (odds ratio, 2.4). Longer baseline stricture length was less predictive, but it was still significantly associated with increased risk (OR, 1.2), according to Dr. Lakhtakia of the Asian Institute of Gastroenterology, Hyderabad, India, and coauthors.
The results indicate that indwelling biliary stenting is a reasonable and beneficial procedure for many of these patients, wrote Dr. Lakhtakia and coauthors.
“The major message to be taken from this study is that in patients with chronic [symptomatic] pancreatitis ... associated with benign biliary strictures, the single placement of a fully covered self-expanding metal stent for an intended indwell of 10-12 months allows more than 60% to remain free of symptoms up to 5 years later without additional intervention.”
The prospective nonrandomized study comprised 118 patients with chronic symptomatic pancreatitis and benign biliary strictures. All received a stent with removal scheduled for 10-12 months later. Patients were followed for 5 years. The primary endpoints were stricture resolution and freedom from recurrence at the end of follow-up.
Patients were a mean of 52 years old; most (83%) were male. At baseline, the mean total bilirubin was 1.4 mg/dL, and the mean alkaline phosphate level was 338.7 IU/L. Mean stricture length was 23.7 mm, but varied from 7.2 to 40 mm. Severe disease was present in 70%.
Among the cohort, five cases (4.2%) were considered treatment failures, with four lost to follow-up and one treated surgically for chronic pancreatitis progression. Another five experienced a spontaneous complete distal stent migration. The rest of the cohort (108) had their scheduled stent removal. At that time, 95 of the 118 experienced successful stent removal, without serious adverse events or the need for immediate replacement.
At 5 years, patients were reassessed, with the primary follow-up endpoint of stricture resolution. Secondary endpoints were time to stricture recurrence and/or changes in liver function tests. Overall, 79.7% (94) of the overall cohort showed stricture resolution at 5 years.
Among the 108 who had a successful removal, a longer time of stent indwell was associated with a decreased chance of recurrent placement. Among those with the longer indwell (median, 344 days), the risk reduction was 34% (OR, 0.66). Of the 94 patients with stricture resolution at stent removal, 77.4% remained stent free at 5 years.
At the end of follow-up, 56 patients had symptomatic data available. Most (53) had not experienced symptoms of biliary obstruction and/or cholestasis. The other three had been symptom free at 48 months but had incomplete or missing 5-year data.
By 5 years, 19 patients needed a new stent. Of these, 13 had symptoms of biliary obstruction.
About 23% of stented patients had a stent-related serious adverse event. These included cholangitis (9.3%), abdominal pain (5%), pancreatitis (3.4%), cholecystitis (2%), and cholestasis (1.7%).
About 80% of the 19 patients who had a stricture recurrence experienced a serious adverse event in the month before recurrent stent placement. The most common were cholangitis, cholestasis, abdominal pain, and cholelithiasis.
In a univariate analysis, recurrence risk was significantly associated with severe baseline disease and longer stricture length. The associations remained significant in the multivariate model.
“Strikingly, patients with initial stricture resolution at [stent] removal ... were very likely to have long-term stricture resolution” the authors noted.
Dr. Lakhtakia had no financial disclosures.
SOURCE: Lakhtakia S et al. Gastrointest Endosc. 2019. doi: 10.1016/j.gie.2019.08.037.
Sundeep Lakhtakia, MD, and colleagues reported in Gastrointestinal Endoscopy.
Patients with severe disease at baseline were more than twice as likely to develop a postprocedural stricture (odds ratio, 2.4). Longer baseline stricture length was less predictive, but it was still significantly associated with increased risk (OR, 1.2), according to Dr. Lakhtakia of the Asian Institute of Gastroenterology, Hyderabad, India, and coauthors.
The results indicate that indwelling biliary stenting is a reasonable and beneficial procedure for many of these patients, wrote Dr. Lakhtakia and coauthors.
“The major message to be taken from this study is that in patients with chronic [symptomatic] pancreatitis ... associated with benign biliary strictures, the single placement of a fully covered self-expanding metal stent for an intended indwell of 10-12 months allows more than 60% to remain free of symptoms up to 5 years later without additional intervention.”
The prospective nonrandomized study comprised 118 patients with chronic symptomatic pancreatitis and benign biliary strictures. All received a stent with removal scheduled for 10-12 months later. Patients were followed for 5 years. The primary endpoints were stricture resolution and freedom from recurrence at the end of follow-up.
Patients were a mean of 52 years old; most (83%) were male. At baseline, the mean total bilirubin was 1.4 mg/dL, and the mean alkaline phosphate level was 338.7 IU/L. Mean stricture length was 23.7 mm, but varied from 7.2 to 40 mm. Severe disease was present in 70%.
Among the cohort, five cases (4.2%) were considered treatment failures, with four lost to follow-up and one treated surgically for chronic pancreatitis progression. Another five experienced a spontaneous complete distal stent migration. The rest of the cohort (108) had their scheduled stent removal. At that time, 95 of the 118 experienced successful stent removal, without serious adverse events or the need for immediate replacement.
At 5 years, patients were reassessed, with the primary follow-up endpoint of stricture resolution. Secondary endpoints were time to stricture recurrence and/or changes in liver function tests. Overall, 79.7% (94) of the overall cohort showed stricture resolution at 5 years.
Among the 108 who had a successful removal, a longer time of stent indwell was associated with a decreased chance of recurrent placement. Among those with the longer indwell (median, 344 days), the risk reduction was 34% (OR, 0.66). Of the 94 patients with stricture resolution at stent removal, 77.4% remained stent free at 5 years.
At the end of follow-up, 56 patients had symptomatic data available. Most (53) had not experienced symptoms of biliary obstruction and/or cholestasis. The other three had been symptom free at 48 months but had incomplete or missing 5-year data.
By 5 years, 19 patients needed a new stent. Of these, 13 had symptoms of biliary obstruction.
About 23% of stented patients had a stent-related serious adverse event. These included cholangitis (9.3%), abdominal pain (5%), pancreatitis (3.4%), cholecystitis (2%), and cholestasis (1.7%).
About 80% of the 19 patients who had a stricture recurrence experienced a serious adverse event in the month before recurrent stent placement. The most common were cholangitis, cholestasis, abdominal pain, and cholelithiasis.
In a univariate analysis, recurrence risk was significantly associated with severe baseline disease and longer stricture length. The associations remained significant in the multivariate model.
“Strikingly, patients with initial stricture resolution at [stent] removal ... were very likely to have long-term stricture resolution” the authors noted.
Dr. Lakhtakia had no financial disclosures.
SOURCE: Lakhtakia S et al. Gastrointest Endosc. 2019. doi: 10.1016/j.gie.2019.08.037.
Sundeep Lakhtakia, MD, and colleagues reported in Gastrointestinal Endoscopy.
Patients with severe disease at baseline were more than twice as likely to develop a postprocedural stricture (odds ratio, 2.4). Longer baseline stricture length was less predictive, but it was still significantly associated with increased risk (OR, 1.2), according to Dr. Lakhtakia of the Asian Institute of Gastroenterology, Hyderabad, India, and coauthors.
The results indicate that indwelling biliary stenting is a reasonable and beneficial procedure for many of these patients, wrote Dr. Lakhtakia and coauthors.
“The major message to be taken from this study is that in patients with chronic [symptomatic] pancreatitis ... associated with benign biliary strictures, the single placement of a fully covered self-expanding metal stent for an intended indwell of 10-12 months allows more than 60% to remain free of symptoms up to 5 years later without additional intervention.”
The prospective nonrandomized study comprised 118 patients with chronic symptomatic pancreatitis and benign biliary strictures. All received a stent with removal scheduled for 10-12 months later. Patients were followed for 5 years. The primary endpoints were stricture resolution and freedom from recurrence at the end of follow-up.
Patients were a mean of 52 years old; most (83%) were male. At baseline, the mean total bilirubin was 1.4 mg/dL, and the mean alkaline phosphate level was 338.7 IU/L. Mean stricture length was 23.7 mm, but varied from 7.2 to 40 mm. Severe disease was present in 70%.
Among the cohort, five cases (4.2%) were considered treatment failures, with four lost to follow-up and one treated surgically for chronic pancreatitis progression. Another five experienced a spontaneous complete distal stent migration. The rest of the cohort (108) had their scheduled stent removal. At that time, 95 of the 118 experienced successful stent removal, without serious adverse events or the need for immediate replacement.
At 5 years, patients were reassessed, with the primary follow-up endpoint of stricture resolution. Secondary endpoints were time to stricture recurrence and/or changes in liver function tests. Overall, 79.7% (94) of the overall cohort showed stricture resolution at 5 years.
Among the 108 who had a successful removal, a longer time of stent indwell was associated with a decreased chance of recurrent placement. Among those with the longer indwell (median, 344 days), the risk reduction was 34% (OR, 0.66). Of the 94 patients with stricture resolution at stent removal, 77.4% remained stent free at 5 years.
At the end of follow-up, 56 patients had symptomatic data available. Most (53) had not experienced symptoms of biliary obstruction and/or cholestasis. The other three had been symptom free at 48 months but had incomplete or missing 5-year data.
By 5 years, 19 patients needed a new stent. Of these, 13 had symptoms of biliary obstruction.
About 23% of stented patients had a stent-related serious adverse event. These included cholangitis (9.3%), abdominal pain (5%), pancreatitis (3.4%), cholecystitis (2%), and cholestasis (1.7%).
About 80% of the 19 patients who had a stricture recurrence experienced a serious adverse event in the month before recurrent stent placement. The most common were cholangitis, cholestasis, abdominal pain, and cholelithiasis.
In a univariate analysis, recurrence risk was significantly associated with severe baseline disease and longer stricture length. The associations remained significant in the multivariate model.
“Strikingly, patients with initial stricture resolution at [stent] removal ... were very likely to have long-term stricture resolution” the authors noted.
Dr. Lakhtakia had no financial disclosures.
SOURCE: Lakhtakia S et al. Gastrointest Endosc. 2019. doi: 10.1016/j.gie.2019.08.037.
FROM GASTROINTESTINAL ENDOSCOPY
Genetic analysis highlights value of germline variants in MDS, AML
Germline DDX41 mutations were found to be relatively prevalent and showed favorable outcomes in a cohort of patients with myelodysplastic syndromes or acute myeloid leukemia, according to a genetic analysis.
The results demonstrate that systematic genetic testing for DDX41 mutations may aid in clinical decision making for adults with myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML).
“We screened a large, unselected cohort of adult patients diagnosed with MDS/AML to analyze the biological and clinical features of DDX41-related myeloid malignancies,” wrote Marie Sébert, MD, PhD, of Hôpital Saint Louis in Paris and colleagues. The results were published in Blood.
The researchers used next-generation sequencing to analyze blood and bone marrow samples from 1,385 patients with MDS or AML to detect DDX41 mutations. A variant allele frequency of greater than 0.4 was regarded as indicative of a germline origin, and only specific variants (minor allele frequency of less than 0.01) were included.
The team analyzed various parameters relevant to DDX41-related myeloid disorders, including patient demographics, karyotyping, and treatment response rates, in addition to the prevalence of DDX41-related malignancies.
A total of 28 distinct germline DDX41 variants were detected among 43 unrelated patients. The researchers classified 21 of the variants as causal, with the rest being of unknown significance.
“We focused on the 33 patients having causal variants, representing 2.4% of our cohort,” they wrote.
The majority of patients with DDX41-related MDS/AML were male, with a median age of 69 years. Few patients (27%) had a family history of blood malignancies, while the majority of patients (85%) had a normal karyotype.
With respect to treatment, most high-risk patients received either azacitidine or intensive chemotherapy, with overall response rates of 73% and 100%, respectively. The median overall survival was 5.2 years.
There are currently no consensus recommendations on genetic counseling and follow-up of asymptomatic carriers and more studies are needed to refine clinical management and genetic counseling, the researchers wrote. “However, in our experience, DDX41-mutated patients frequently presented mild cytopenias years before overt hematological myeloid malignancy, suggesting that watchful surveillance would allow the detection of disease evolution.”
No funding sources were reported, and the authors reported having no conflicts of interest.
SOURCE: Sébert M et al. Blood. 2019 Sep 4. doi: 10.1182/blood.2019000909.
Germline DDX41 mutations were found to be relatively prevalent and showed favorable outcomes in a cohort of patients with myelodysplastic syndromes or acute myeloid leukemia, according to a genetic analysis.
The results demonstrate that systematic genetic testing for DDX41 mutations may aid in clinical decision making for adults with myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML).
“We screened a large, unselected cohort of adult patients diagnosed with MDS/AML to analyze the biological and clinical features of DDX41-related myeloid malignancies,” wrote Marie Sébert, MD, PhD, of Hôpital Saint Louis in Paris and colleagues. The results were published in Blood.
The researchers used next-generation sequencing to analyze blood and bone marrow samples from 1,385 patients with MDS or AML to detect DDX41 mutations. A variant allele frequency of greater than 0.4 was regarded as indicative of a germline origin, and only specific variants (minor allele frequency of less than 0.01) were included.
The team analyzed various parameters relevant to DDX41-related myeloid disorders, including patient demographics, karyotyping, and treatment response rates, in addition to the prevalence of DDX41-related malignancies.
A total of 28 distinct germline DDX41 variants were detected among 43 unrelated patients. The researchers classified 21 of the variants as causal, with the rest being of unknown significance.
“We focused on the 33 patients having causal variants, representing 2.4% of our cohort,” they wrote.
The majority of patients with DDX41-related MDS/AML were male, with a median age of 69 years. Few patients (27%) had a family history of blood malignancies, while the majority of patients (85%) had a normal karyotype.
With respect to treatment, most high-risk patients received either azacitidine or intensive chemotherapy, with overall response rates of 73% and 100%, respectively. The median overall survival was 5.2 years.
There are currently no consensus recommendations on genetic counseling and follow-up of asymptomatic carriers and more studies are needed to refine clinical management and genetic counseling, the researchers wrote. “However, in our experience, DDX41-mutated patients frequently presented mild cytopenias years before overt hematological myeloid malignancy, suggesting that watchful surveillance would allow the detection of disease evolution.”
No funding sources were reported, and the authors reported having no conflicts of interest.
SOURCE: Sébert M et al. Blood. 2019 Sep 4. doi: 10.1182/blood.2019000909.
Germline DDX41 mutations were found to be relatively prevalent and showed favorable outcomes in a cohort of patients with myelodysplastic syndromes or acute myeloid leukemia, according to a genetic analysis.
The results demonstrate that systematic genetic testing for DDX41 mutations may aid in clinical decision making for adults with myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML).
“We screened a large, unselected cohort of adult patients diagnosed with MDS/AML to analyze the biological and clinical features of DDX41-related myeloid malignancies,” wrote Marie Sébert, MD, PhD, of Hôpital Saint Louis in Paris and colleagues. The results were published in Blood.
The researchers used next-generation sequencing to analyze blood and bone marrow samples from 1,385 patients with MDS or AML to detect DDX41 mutations. A variant allele frequency of greater than 0.4 was regarded as indicative of a germline origin, and only specific variants (minor allele frequency of less than 0.01) were included.
The team analyzed various parameters relevant to DDX41-related myeloid disorders, including patient demographics, karyotyping, and treatment response rates, in addition to the prevalence of DDX41-related malignancies.
A total of 28 distinct germline DDX41 variants were detected among 43 unrelated patients. The researchers classified 21 of the variants as causal, with the rest being of unknown significance.
“We focused on the 33 patients having causal variants, representing 2.4% of our cohort,” they wrote.
The majority of patients with DDX41-related MDS/AML were male, with a median age of 69 years. Few patients (27%) had a family history of blood malignancies, while the majority of patients (85%) had a normal karyotype.
With respect to treatment, most high-risk patients received either azacitidine or intensive chemotherapy, with overall response rates of 73% and 100%, respectively. The median overall survival was 5.2 years.
There are currently no consensus recommendations on genetic counseling and follow-up of asymptomatic carriers and more studies are needed to refine clinical management and genetic counseling, the researchers wrote. “However, in our experience, DDX41-mutated patients frequently presented mild cytopenias years before overt hematological myeloid malignancy, suggesting that watchful surveillance would allow the detection of disease evolution.”
No funding sources were reported, and the authors reported having no conflicts of interest.
SOURCE: Sébert M et al. Blood. 2019 Sep 4. doi: 10.1182/blood.2019000909.
FROM BLOOD
Juvenile dermatomyositis derails growth and pubertal development
Children with juvenile dermatomyositis showed significant growth failure and pubertal delay, based on data from a longitudinal cohort study.
“Both the inflammatory activity of this severe chronic rheumatic disease and the well-known side effects of corticosteroid treatment may interfere with normal growth and pubertal development of children,” wrote Ellen Nordal, MD, of the University Hospital of Northern Norway, Tromsø, and colleagues.
The goal in treating juvenile dermatomyositis (JDM) is to achieve inactive disease and prevent permanent damage, but long-term data on growth and puberty in JDM patients are limited, they wrote.
In a study published in Arthritis Care & Research, the investigators reviewed data from 196 children and followed them for 2 years. The patients were part of the Paediatric Rheumatology International Trials Organisation (PRINTO) observational cohort study.
Overall, the researchers identified growth failure, height deflection, and/or delayed puberty in 94 children (48%) at the last study visit.
Growth failure was present at baseline in 17% of girls and 10% of boys. Over the 2-year study period, height deflection increased to 25% of girls and 31% of boys, but this change was not significant. Height deflection was defined as a change in the height z score of less than –0.25 per year from baseline. However, body mass index increased significantly from baseline during the study.
Catch-up growth had occurred by the final study visit in some patients, based on parent-adjusted z scores over time. Girls with a disease duration of 12 months or more showed no catch-up growth at 2 years and had significantly lower parent-adjusted height z scores.
In addition, the researchers observed a delay in the onset of puberty (including pubertal tempo and menarche) in approximately 36% of both boys and girls. However, neither growth failure nor height deflection was significantly associated with delayed puberty in either sex.
“In follow-up, clinicians should therefore be aware of both the pubertal development and the growth of the child, assess the milestones of development, and ensure that the children reach as much as possible of their genetic potential,” the researchers wrote.
The study participants were younger than 18 years at study enrollment, and all were in an active disease phase, defined as needing to start or receive a major dose increase of corticosteroids and/or immunosuppressants. Patients were assessed at baseline, at 6 months and/or at 12 months, and during a final visit at approximately 26 months. During the study, approximately half of the participants (50.5%) received methotrexate, 30 (15.3%) received cyclosporine A, 10 (5.1%) received cyclophosphamide, and 27 (13.8%) received intravenous immunoglobulin.
The study findings were limited by several factors, including the short follow-up period for assessing pubertal development and the inability to analyze any impact of corticosteroid use prior to the study, the researchers noted. However, “the overall frequency of growth failure was not significantly higher at the final study visit 2 years after baseline, indicating that the very high doses of corticosteroid treatment given during the study period is reasonably well tolerated with regards to growth,” they wrote. But monitoring remains essential, especially for children with previous growth failure or with disease onset early in pubertal development.
The study was supported by the European Union, Helse Nord Research grants, and by IRCCS Istituto Giannina Gaslini. Five authors of the study reported financial relationships with pharmaceutical companies.
SOURCE: Nordal E et al. Arthritis Care Res. 2019 Sep 10. doi: 10.1002/acr.24065.
Children with juvenile dermatomyositis showed significant growth failure and pubertal delay, based on data from a longitudinal cohort study.
“Both the inflammatory activity of this severe chronic rheumatic disease and the well-known side effects of corticosteroid treatment may interfere with normal growth and pubertal development of children,” wrote Ellen Nordal, MD, of the University Hospital of Northern Norway, Tromsø, and colleagues.
The goal in treating juvenile dermatomyositis (JDM) is to achieve inactive disease and prevent permanent damage, but long-term data on growth and puberty in JDM patients are limited, they wrote.
In a study published in Arthritis Care & Research, the investigators reviewed data from 196 children and followed them for 2 years. The patients were part of the Paediatric Rheumatology International Trials Organisation (PRINTO) observational cohort study.
Overall, the researchers identified growth failure, height deflection, and/or delayed puberty in 94 children (48%) at the last study visit.
Growth failure was present at baseline in 17% of girls and 10% of boys. Over the 2-year study period, height deflection increased to 25% of girls and 31% of boys, but this change was not significant. Height deflection was defined as a change in the height z score of less than –0.25 per year from baseline. However, body mass index increased significantly from baseline during the study.
Catch-up growth had occurred by the final study visit in some patients, based on parent-adjusted z scores over time. Girls with a disease duration of 12 months or more showed no catch-up growth at 2 years and had significantly lower parent-adjusted height z scores.
In addition, the researchers observed a delay in the onset of puberty (including pubertal tempo and menarche) in approximately 36% of both boys and girls. However, neither growth failure nor height deflection was significantly associated with delayed puberty in either sex.
“In follow-up, clinicians should therefore be aware of both the pubertal development and the growth of the child, assess the milestones of development, and ensure that the children reach as much as possible of their genetic potential,” the researchers wrote.
The study participants were younger than 18 years at study enrollment, and all were in an active disease phase, defined as needing to start or receive a major dose increase of corticosteroids and/or immunosuppressants. Patients were assessed at baseline, at 6 months and/or at 12 months, and during a final visit at approximately 26 months. During the study, approximately half of the participants (50.5%) received methotrexate, 30 (15.3%) received cyclosporine A, 10 (5.1%) received cyclophosphamide, and 27 (13.8%) received intravenous immunoglobulin.
The study findings were limited by several factors, including the short follow-up period for assessing pubertal development and the inability to analyze any impact of corticosteroid use prior to the study, the researchers noted. However, “the overall frequency of growth failure was not significantly higher at the final study visit 2 years after baseline, indicating that the very high doses of corticosteroid treatment given during the study period is reasonably well tolerated with regards to growth,” they wrote. But monitoring remains essential, especially for children with previous growth failure or with disease onset early in pubertal development.
The study was supported by the European Union, Helse Nord Research grants, and by IRCCS Istituto Giannina Gaslini. Five authors of the study reported financial relationships with pharmaceutical companies.
SOURCE: Nordal E et al. Arthritis Care Res. 2019 Sep 10. doi: 10.1002/acr.24065.
Children with juvenile dermatomyositis showed significant growth failure and pubertal delay, based on data from a longitudinal cohort study.
“Both the inflammatory activity of this severe chronic rheumatic disease and the well-known side effects of corticosteroid treatment may interfere with normal growth and pubertal development of children,” wrote Ellen Nordal, MD, of the University Hospital of Northern Norway, Tromsø, and colleagues.
The goal in treating juvenile dermatomyositis (JDM) is to achieve inactive disease and prevent permanent damage, but long-term data on growth and puberty in JDM patients are limited, they wrote.
In a study published in Arthritis Care & Research, the investigators reviewed data from 196 children and followed them for 2 years. The patients were part of the Paediatric Rheumatology International Trials Organisation (PRINTO) observational cohort study.
Overall, the researchers identified growth failure, height deflection, and/or delayed puberty in 94 children (48%) at the last study visit.
Growth failure was present at baseline in 17% of girls and 10% of boys. Over the 2-year study period, height deflection increased to 25% of girls and 31% of boys, but this change was not significant. Height deflection was defined as a change in the height z score of less than –0.25 per year from baseline. However, body mass index increased significantly from baseline during the study.
Catch-up growth had occurred by the final study visit in some patients, based on parent-adjusted z scores over time. Girls with a disease duration of 12 months or more showed no catch-up growth at 2 years and had significantly lower parent-adjusted height z scores.
In addition, the researchers observed a delay in the onset of puberty (including pubertal tempo and menarche) in approximately 36% of both boys and girls. However, neither growth failure nor height deflection was significantly associated with delayed puberty in either sex.
“In follow-up, clinicians should therefore be aware of both the pubertal development and the growth of the child, assess the milestones of development, and ensure that the children reach as much as possible of their genetic potential,” the researchers wrote.
The study participants were younger than 18 years at study enrollment, and all were in an active disease phase, defined as needing to start or receive a major dose increase of corticosteroids and/or immunosuppressants. Patients were assessed at baseline, at 6 months and/or at 12 months, and during a final visit at approximately 26 months. During the study, approximately half of the participants (50.5%) received methotrexate, 30 (15.3%) received cyclosporine A, 10 (5.1%) received cyclophosphamide, and 27 (13.8%) received intravenous immunoglobulin.
The study findings were limited by several factors, including the short follow-up period for assessing pubertal development and the inability to analyze any impact of corticosteroid use prior to the study, the researchers noted. However, “the overall frequency of growth failure was not significantly higher at the final study visit 2 years after baseline, indicating that the very high doses of corticosteroid treatment given during the study period is reasonably well tolerated with regards to growth,” they wrote. But monitoring remains essential, especially for children with previous growth failure or with disease onset early in pubertal development.
The study was supported by the European Union, Helse Nord Research grants, and by IRCCS Istituto Giannina Gaslini. Five authors of the study reported financial relationships with pharmaceutical companies.
SOURCE: Nordal E et al. Arthritis Care Res. 2019 Sep 10. doi: 10.1002/acr.24065.
FROM ARTHRITIS CARE & RESEARCH
Not all labs are created equal: What to look for when searching for the right lab
Introduction
As researchers, we spend a substantial period of our careers as trainees in a lab. As graduate students or postdoctoral fellows, selecting the “right” lab has the potential to make a huge difference for your future career and your mental health. Although much of the decision is based on instinct, there is still some logic and reason that can be applied to ensure the choice is a good fit.
PI/mentor
PI funding. The history of funding and the funding of your specific project are important factors to consider. No matter how significant the scientific question, not having adequate funding can be paralyzing. For both graduate students and postdoctoral fellows, it is in your best interest to proactively inquire about the funding status of the lab. This can be accomplished using online reporters (such as NIH Reporter or Grantome) and asking the PI. It is equally important to identify which grant application/fellowship will fund your stipend and ensure that the funding is secure for 4-6 years.
PI personality. For better or for worse, the PI largely sets the tone for the lab. The personality of the PI can be a key determining factor in finding the right “fit” for a trainee. Ask your peers and current and past lab members about working with the PI. As you speak to lab members and alumni, don’t disregard warning signs of unethical behavior, bullying, or harassment. Even with the rise of movements such as the #MeTooSTEM, academic misconduct is hard to correct. It is far better to avoid these labs. If available, examine the PI’s social networks (LinkedIn, Twitter, Facebook, etc.) or read previously published interviews and look for the PI’s attitude toward its lab members.
Mentoring style. The NIH emphasizes mentorship in numerous funding mechanisms; thus, it isn’t surprising that selecting an appropriate mentor is paramount for a successful training experience. A lot of this decision requires information about you as a trainee. Reflect on what type of scientific mentor will be the best fit for your needs and honestly evaluate your own communication style, expectations, and final career goals. Ask questions of the PI and lab members to identify the mentoring style. Find a mentor who takes his or her responsibility to train you seriously and who genuinely cares about the well-being of the lab personnel. Good mentors advocate for their trainees inside and outside of the lab. A mentor’s vocal support of talented trainees can help propel them toward their career goals.
Career support. One of the most important questions you need to consider is whether your mentor can help you accomplish your career goals. Be prepared to have an honest conversation with the PI about your goals. The majority of PhD scientists will pursue careers outside of academia, so choose a mentor who supports diverse career paths. A mentor who is invested in your success will work with you to finish papers in a timely manner, encourage you to apply for awards and grants, aid in the identification of fruitful collaborations, help develop new skills, and expand your professional network. A good mentor will also ensure that you have freedom in your project to pursue your own scientific interests, and ultimately allow you to carve out a project for you to take with you when your training is complete.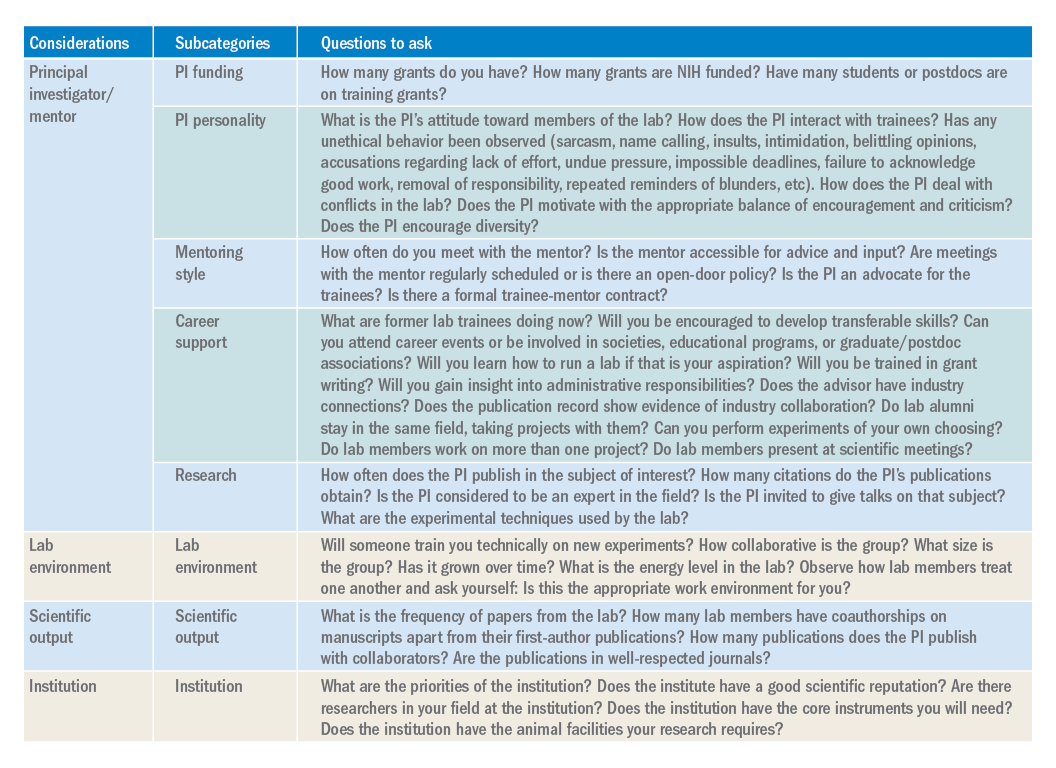
Research. The research done in your lab will also factor into your decision. Especially if you are a postdoctoral fellow, this will be the field/subject/niche in which you will likely establish your career and expertise. If there is a field you want to pursue, identify mentors who have a publication record in that subject. Examine the PIs for publications, citations, and overall contributions to the field. If the PI in question is young, you may not find many citations of papers. However, you can identify the quality of the papers, the lab’s experimental techniques, and how often the PI is invited to give talks on that subject.
Lab environment
The composition of a lab can play a huge role in your overall productivity and mental well-being. Your coworkers will be the ones you interact with on a day-to-day basis. Your lab mates can be a huge asset in terms of mastering new techniques, collaborating on a project, or receiving helpful feedback. Thus, it is to your benefit to get along with your lab mates and to choose a lab that has people who you want to work with or don’t mind working with. The reality of postdoctoral training is that you will spend a large amount of time working in the lab, so choosing a lab that has an environment that fits your personality and desired workload and schedule is important. Considering the valuable contribution of lab members toward your research progression, you should give considerable weight to this factor when choosing a lab.
Scientific output
Although labs with high-impact publications are attractive, it is more important to consider the frequency with which a lab comes out with papers. Search Pubmed to obtain the complete publication roster of the PI. This output will give you an idea about the consistency and regularity of publications and overall lab productivity. When querying the lab’s publication list, check if the lab members have coauthorships on manuscripts apart from their first-author ones. It is desirable to be a coauthor on other manuscripts, as this will help increase your publication record. In addition, look for collaborators of the PI. These collaborations might benefit you by broadening your skill set and experience. Be intentional about reviewing the papers from the lab over the last 5 years. If you want to pursue academic research, you will want to be in a lab that publishes frequently in well-respected journals.
Institution
The institutional environment should also influence your decision. Top universities can attract some of the best researchers. Moreover, funding agencies examine the “Environment and Institutional Commitment” as one of the criteria for awarding grants. When choosing an institution, consider its priorities and ensure that it aligns with your priorities (i.e., research, undergraduate education, etc.). Consider the number of PIs conducting research in your field. These other labs may benefit your career development through collaborations, scientific discussions, letters of recommendation, career support, and feedback.
Conclusion
While there are certain key criteria that should be prioritized when choosing a lab, the path to finding (and joining) the right lab will vary from individual to individual. Gather data, ask questions, research what you can online (lab website, publication records, LinkedIn, ResearchGate, or Twitter), and be ready for the interview with a long list of written questions for the PI and lab members. One of the best ways to begin looking for a new lab is to ask your current mentor, committee members, peers, and others in your professional network for their suggestions. Table 1 has questions to keep in mind as you are searching for a lab. You will spend much of your time in the lab of your choosing, so choose wisely.
Dr. Engevik is an instructor in pathology and immunology, Baylor College of Medicine, Texas Children’s Hospital, Houston.
Introduction
As researchers, we spend a substantial period of our careers as trainees in a lab. As graduate students or postdoctoral fellows, selecting the “right” lab has the potential to make a huge difference for your future career and your mental health. Although much of the decision is based on instinct, there is still some logic and reason that can be applied to ensure the choice is a good fit.
PI/mentor
PI funding. The history of funding and the funding of your specific project are important factors to consider. No matter how significant the scientific question, not having adequate funding can be paralyzing. For both graduate students and postdoctoral fellows, it is in your best interest to proactively inquire about the funding status of the lab. This can be accomplished using online reporters (such as NIH Reporter or Grantome) and asking the PI. It is equally important to identify which grant application/fellowship will fund your stipend and ensure that the funding is secure for 4-6 years.
PI personality. For better or for worse, the PI largely sets the tone for the lab. The personality of the PI can be a key determining factor in finding the right “fit” for a trainee. Ask your peers and current and past lab members about working with the PI. As you speak to lab members and alumni, don’t disregard warning signs of unethical behavior, bullying, or harassment. Even with the rise of movements such as the #MeTooSTEM, academic misconduct is hard to correct. It is far better to avoid these labs. If available, examine the PI’s social networks (LinkedIn, Twitter, Facebook, etc.) or read previously published interviews and look for the PI’s attitude toward its lab members.
Mentoring style. The NIH emphasizes mentorship in numerous funding mechanisms; thus, it isn’t surprising that selecting an appropriate mentor is paramount for a successful training experience. A lot of this decision requires information about you as a trainee. Reflect on what type of scientific mentor will be the best fit for your needs and honestly evaluate your own communication style, expectations, and final career goals. Ask questions of the PI and lab members to identify the mentoring style. Find a mentor who takes his or her responsibility to train you seriously and who genuinely cares about the well-being of the lab personnel. Good mentors advocate for their trainees inside and outside of the lab. A mentor’s vocal support of talented trainees can help propel them toward their career goals.
Career support. One of the most important questions you need to consider is whether your mentor can help you accomplish your career goals. Be prepared to have an honest conversation with the PI about your goals. The majority of PhD scientists will pursue careers outside of academia, so choose a mentor who supports diverse career paths. A mentor who is invested in your success will work with you to finish papers in a timely manner, encourage you to apply for awards and grants, aid in the identification of fruitful collaborations, help develop new skills, and expand your professional network. A good mentor will also ensure that you have freedom in your project to pursue your own scientific interests, and ultimately allow you to carve out a project for you to take with you when your training is complete.
Research. The research done in your lab will also factor into your decision. Especially if you are a postdoctoral fellow, this will be the field/subject/niche in which you will likely establish your career and expertise. If there is a field you want to pursue, identify mentors who have a publication record in that subject. Examine the PIs for publications, citations, and overall contributions to the field. If the PI in question is young, you may not find many citations of papers. However, you can identify the quality of the papers, the lab’s experimental techniques, and how often the PI is invited to give talks on that subject.
Lab environment
The composition of a lab can play a huge role in your overall productivity and mental well-being. Your coworkers will be the ones you interact with on a day-to-day basis. Your lab mates can be a huge asset in terms of mastering new techniques, collaborating on a project, or receiving helpful feedback. Thus, it is to your benefit to get along with your lab mates and to choose a lab that has people who you want to work with or don’t mind working with. The reality of postdoctoral training is that you will spend a large amount of time working in the lab, so choosing a lab that has an environment that fits your personality and desired workload and schedule is important. Considering the valuable contribution of lab members toward your research progression, you should give considerable weight to this factor when choosing a lab.
Scientific output
Although labs with high-impact publications are attractive, it is more important to consider the frequency with which a lab comes out with papers. Search Pubmed to obtain the complete publication roster of the PI. This output will give you an idea about the consistency and regularity of publications and overall lab productivity. When querying the lab’s publication list, check if the lab members have coauthorships on manuscripts apart from their first-author ones. It is desirable to be a coauthor on other manuscripts, as this will help increase your publication record. In addition, look for collaborators of the PI. These collaborations might benefit you by broadening your skill set and experience. Be intentional about reviewing the papers from the lab over the last 5 years. If you want to pursue academic research, you will want to be in a lab that publishes frequently in well-respected journals.
Institution
The institutional environment should also influence your decision. Top universities can attract some of the best researchers. Moreover, funding agencies examine the “Environment and Institutional Commitment” as one of the criteria for awarding grants. When choosing an institution, consider its priorities and ensure that it aligns with your priorities (i.e., research, undergraduate education, etc.). Consider the number of PIs conducting research in your field. These other labs may benefit your career development through collaborations, scientific discussions, letters of recommendation, career support, and feedback.
Conclusion
While there are certain key criteria that should be prioritized when choosing a lab, the path to finding (and joining) the right lab will vary from individual to individual. Gather data, ask questions, research what you can online (lab website, publication records, LinkedIn, ResearchGate, or Twitter), and be ready for the interview with a long list of written questions for the PI and lab members. One of the best ways to begin looking for a new lab is to ask your current mentor, committee members, peers, and others in your professional network for their suggestions. Table 1 has questions to keep in mind as you are searching for a lab. You will spend much of your time in the lab of your choosing, so choose wisely.
Dr. Engevik is an instructor in pathology and immunology, Baylor College of Medicine, Texas Children’s Hospital, Houston.
Introduction
As researchers, we spend a substantial period of our careers as trainees in a lab. As graduate students or postdoctoral fellows, selecting the “right” lab has the potential to make a huge difference for your future career and your mental health. Although much of the decision is based on instinct, there is still some logic and reason that can be applied to ensure the choice is a good fit.
PI/mentor
PI funding. The history of funding and the funding of your specific project are important factors to consider. No matter how significant the scientific question, not having adequate funding can be paralyzing. For both graduate students and postdoctoral fellows, it is in your best interest to proactively inquire about the funding status of the lab. This can be accomplished using online reporters (such as NIH Reporter or Grantome) and asking the PI. It is equally important to identify which grant application/fellowship will fund your stipend and ensure that the funding is secure for 4-6 years.
PI personality. For better or for worse, the PI largely sets the tone for the lab. The personality of the PI can be a key determining factor in finding the right “fit” for a trainee. Ask your peers and current and past lab members about working with the PI. As you speak to lab members and alumni, don’t disregard warning signs of unethical behavior, bullying, or harassment. Even with the rise of movements such as the #MeTooSTEM, academic misconduct is hard to correct. It is far better to avoid these labs. If available, examine the PI’s social networks (LinkedIn, Twitter, Facebook, etc.) or read previously published interviews and look for the PI’s attitude toward its lab members.
Mentoring style. The NIH emphasizes mentorship in numerous funding mechanisms; thus, it isn’t surprising that selecting an appropriate mentor is paramount for a successful training experience. A lot of this decision requires information about you as a trainee. Reflect on what type of scientific mentor will be the best fit for your needs and honestly evaluate your own communication style, expectations, and final career goals. Ask questions of the PI and lab members to identify the mentoring style. Find a mentor who takes his or her responsibility to train you seriously and who genuinely cares about the well-being of the lab personnel. Good mentors advocate for their trainees inside and outside of the lab. A mentor’s vocal support of talented trainees can help propel them toward their career goals.
Career support. One of the most important questions you need to consider is whether your mentor can help you accomplish your career goals. Be prepared to have an honest conversation with the PI about your goals. The majority of PhD scientists will pursue careers outside of academia, so choose a mentor who supports diverse career paths. A mentor who is invested in your success will work with you to finish papers in a timely manner, encourage you to apply for awards and grants, aid in the identification of fruitful collaborations, help develop new skills, and expand your professional network. A good mentor will also ensure that you have freedom in your project to pursue your own scientific interests, and ultimately allow you to carve out a project for you to take with you when your training is complete.
Research. The research done in your lab will also factor into your decision. Especially if you are a postdoctoral fellow, this will be the field/subject/niche in which you will likely establish your career and expertise. If there is a field you want to pursue, identify mentors who have a publication record in that subject. Examine the PIs for publications, citations, and overall contributions to the field. If the PI in question is young, you may not find many citations of papers. However, you can identify the quality of the papers, the lab’s experimental techniques, and how often the PI is invited to give talks on that subject.
Lab environment
The composition of a lab can play a huge role in your overall productivity and mental well-being. Your coworkers will be the ones you interact with on a day-to-day basis. Your lab mates can be a huge asset in terms of mastering new techniques, collaborating on a project, or receiving helpful feedback. Thus, it is to your benefit to get along with your lab mates and to choose a lab that has people who you want to work with or don’t mind working with. The reality of postdoctoral training is that you will spend a large amount of time working in the lab, so choosing a lab that has an environment that fits your personality and desired workload and schedule is important. Considering the valuable contribution of lab members toward your research progression, you should give considerable weight to this factor when choosing a lab.
Scientific output
Although labs with high-impact publications are attractive, it is more important to consider the frequency with which a lab comes out with papers. Search Pubmed to obtain the complete publication roster of the PI. This output will give you an idea about the consistency and regularity of publications and overall lab productivity. When querying the lab’s publication list, check if the lab members have coauthorships on manuscripts apart from their first-author ones. It is desirable to be a coauthor on other manuscripts, as this will help increase your publication record. In addition, look for collaborators of the PI. These collaborations might benefit you by broadening your skill set and experience. Be intentional about reviewing the papers from the lab over the last 5 years. If you want to pursue academic research, you will want to be in a lab that publishes frequently in well-respected journals.
Institution
The institutional environment should also influence your decision. Top universities can attract some of the best researchers. Moreover, funding agencies examine the “Environment and Institutional Commitment” as one of the criteria for awarding grants. When choosing an institution, consider its priorities and ensure that it aligns with your priorities (i.e., research, undergraduate education, etc.). Consider the number of PIs conducting research in your field. These other labs may benefit your career development through collaborations, scientific discussions, letters of recommendation, career support, and feedback.
Conclusion
While there are certain key criteria that should be prioritized when choosing a lab, the path to finding (and joining) the right lab will vary from individual to individual. Gather data, ask questions, research what you can online (lab website, publication records, LinkedIn, ResearchGate, or Twitter), and be ready for the interview with a long list of written questions for the PI and lab members. One of the best ways to begin looking for a new lab is to ask your current mentor, committee members, peers, and others in your professional network for their suggestions. Table 1 has questions to keep in mind as you are searching for a lab. You will spend much of your time in the lab of your choosing, so choose wisely.
Dr. Engevik is an instructor in pathology and immunology, Baylor College of Medicine, Texas Children’s Hospital, Houston.
Three factors predict 6-month mortality in patients with DILI
Medical comorbidity burden is significantly associated with 6-month and overall mortality in individuals with suspected drug-induced liver injury (DILI). In addition,
Those are key findings from a study which set out to investigate the association between comorbidity burden and outcomes of patients with DILI and to develop a model to calculate risk of death within 6 months.
“Drug-induced liver injury is an important cause of liver-related morbidity and mortality that is likely under-recognized,” investigators led by Marwan S. Ghabril, MD, of the division of gastroenterology and hepatology at Indiana University, Indianapolis, wrote in a study published in Gastroenterology. “Its diagnosis depends on high index of suspicion, compatible temporal relationship, and thorough exclusion of competing etiologies. DILI by an implicated drug commonly occurs in patients with one or several comorbid conditions such as hypertension, diabetes mellitus, cardiovascular disease, renal disease, and malignancy. However, the impact of comorbidity burden on mortality in patients with suspected DILI has not been previously investigated.”
For the current analysis and model development, the researchers drew from 306 patients enrolled in the multicenter Drug-Induced Liver Injury Network Prospective Study at Indiana University between 2003 and 2017 (discovery cohort; Drug Saf. 2009;32:55-68). To validate their model, they used data from 247 patients who were enrolled in the same study at the University of North Carolina (validation cohort). The primary outcome of interest was mortality within 6 months of onset of liver injury.
The mean ages of the discovery and validation cohorts were 49 years and 51 years, respectively. Dr. Ghabril and colleagues found that 6-month mortality was 8.5% in the discovery cohort and 4.5% in the validation cohort. “The most common class of implicated agent was antimicrobials with no significant differences between groups,” they wrote. “However, herbal and dietary supplements were predominantly implicated in patients with none to mild comorbidity, while cardiovascular agents were predominantly implicated in patients with significant comorbidity.”
Among patients in the discovery cohort, the presence of significant comorbidities, defined as a Charlson Comorbidity Index score greater than 2, was independently associated with 6-month mortality (odds ratio, 5.22), as was model for end-stage liver disease score (OR, 1.11) and serum level of albumin at presentation (OR, 0.39). When the researchers created a morbidity risk model based on those three clinical variables, it performed well, identifying patients who died within 6 months with a C statistic value of 0.89 in the discovery cohort and 0.91 in the validation cohort. This spurred the development of a web-based risk calculator, which clinicians can access at http://gihep.com/calculators/hepatology/dili-cam/.
“Since DILI is not a unique cause of liver injury, it is conceivable that models incorporating comorbidity burden and severity of liver injury could prove useful in improving the prediction of mortality in a variety of liver injuries and diseases, and as such warrants further studies,” the researchers wrote.
The study was funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Ghabril reported having no financial disclosures, but two coauthors reported having numerous financial ties to industry.
SOURCE: Ghabril M et al. Gastroenterology. 2019 Jul 11. doi: 10/1053/j.gastro.2019.07.006.
Medical comorbidity burden is significantly associated with 6-month and overall mortality in individuals with suspected drug-induced liver injury (DILI). In addition,
Those are key findings from a study which set out to investigate the association between comorbidity burden and outcomes of patients with DILI and to develop a model to calculate risk of death within 6 months.
“Drug-induced liver injury is an important cause of liver-related morbidity and mortality that is likely under-recognized,” investigators led by Marwan S. Ghabril, MD, of the division of gastroenterology and hepatology at Indiana University, Indianapolis, wrote in a study published in Gastroenterology. “Its diagnosis depends on high index of suspicion, compatible temporal relationship, and thorough exclusion of competing etiologies. DILI by an implicated drug commonly occurs in patients with one or several comorbid conditions such as hypertension, diabetes mellitus, cardiovascular disease, renal disease, and malignancy. However, the impact of comorbidity burden on mortality in patients with suspected DILI has not been previously investigated.”
For the current analysis and model development, the researchers drew from 306 patients enrolled in the multicenter Drug-Induced Liver Injury Network Prospective Study at Indiana University between 2003 and 2017 (discovery cohort; Drug Saf. 2009;32:55-68). To validate their model, they used data from 247 patients who were enrolled in the same study at the University of North Carolina (validation cohort). The primary outcome of interest was mortality within 6 months of onset of liver injury.
The mean ages of the discovery and validation cohorts were 49 years and 51 years, respectively. Dr. Ghabril and colleagues found that 6-month mortality was 8.5% in the discovery cohort and 4.5% in the validation cohort. “The most common class of implicated agent was antimicrobials with no significant differences between groups,” they wrote. “However, herbal and dietary supplements were predominantly implicated in patients with none to mild comorbidity, while cardiovascular agents were predominantly implicated in patients with significant comorbidity.”
Among patients in the discovery cohort, the presence of significant comorbidities, defined as a Charlson Comorbidity Index score greater than 2, was independently associated with 6-month mortality (odds ratio, 5.22), as was model for end-stage liver disease score (OR, 1.11) and serum level of albumin at presentation (OR, 0.39). When the researchers created a morbidity risk model based on those three clinical variables, it performed well, identifying patients who died within 6 months with a C statistic value of 0.89 in the discovery cohort and 0.91 in the validation cohort. This spurred the development of a web-based risk calculator, which clinicians can access at http://gihep.com/calculators/hepatology/dili-cam/.
“Since DILI is not a unique cause of liver injury, it is conceivable that models incorporating comorbidity burden and severity of liver injury could prove useful in improving the prediction of mortality in a variety of liver injuries and diseases, and as such warrants further studies,” the researchers wrote.
The study was funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Ghabril reported having no financial disclosures, but two coauthors reported having numerous financial ties to industry.
SOURCE: Ghabril M et al. Gastroenterology. 2019 Jul 11. doi: 10/1053/j.gastro.2019.07.006.
Medical comorbidity burden is significantly associated with 6-month and overall mortality in individuals with suspected drug-induced liver injury (DILI). In addition,
Those are key findings from a study which set out to investigate the association between comorbidity burden and outcomes of patients with DILI and to develop a model to calculate risk of death within 6 months.
“Drug-induced liver injury is an important cause of liver-related morbidity and mortality that is likely under-recognized,” investigators led by Marwan S. Ghabril, MD, of the division of gastroenterology and hepatology at Indiana University, Indianapolis, wrote in a study published in Gastroenterology. “Its diagnosis depends on high index of suspicion, compatible temporal relationship, and thorough exclusion of competing etiologies. DILI by an implicated drug commonly occurs in patients with one or several comorbid conditions such as hypertension, diabetes mellitus, cardiovascular disease, renal disease, and malignancy. However, the impact of comorbidity burden on mortality in patients with suspected DILI has not been previously investigated.”
For the current analysis and model development, the researchers drew from 306 patients enrolled in the multicenter Drug-Induced Liver Injury Network Prospective Study at Indiana University between 2003 and 2017 (discovery cohort; Drug Saf. 2009;32:55-68). To validate their model, they used data from 247 patients who were enrolled in the same study at the University of North Carolina (validation cohort). The primary outcome of interest was mortality within 6 months of onset of liver injury.
The mean ages of the discovery and validation cohorts were 49 years and 51 years, respectively. Dr. Ghabril and colleagues found that 6-month mortality was 8.5% in the discovery cohort and 4.5% in the validation cohort. “The most common class of implicated agent was antimicrobials with no significant differences between groups,” they wrote. “However, herbal and dietary supplements were predominantly implicated in patients with none to mild comorbidity, while cardiovascular agents were predominantly implicated in patients with significant comorbidity.”
Among patients in the discovery cohort, the presence of significant comorbidities, defined as a Charlson Comorbidity Index score greater than 2, was independently associated with 6-month mortality (odds ratio, 5.22), as was model for end-stage liver disease score (OR, 1.11) and serum level of albumin at presentation (OR, 0.39). When the researchers created a morbidity risk model based on those three clinical variables, it performed well, identifying patients who died within 6 months with a C statistic value of 0.89 in the discovery cohort and 0.91 in the validation cohort. This spurred the development of a web-based risk calculator, which clinicians can access at http://gihep.com/calculators/hepatology/dili-cam/.
“Since DILI is not a unique cause of liver injury, it is conceivable that models incorporating comorbidity burden and severity of liver injury could prove useful in improving the prediction of mortality in a variety of liver injuries and diseases, and as such warrants further studies,” the researchers wrote.
The study was funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Ghabril reported having no financial disclosures, but two coauthors reported having numerous financial ties to industry.
SOURCE: Ghabril M et al. Gastroenterology. 2019 Jul 11. doi: 10/1053/j.gastro.2019.07.006.
FROM GASTROENTEROLOGY
Australia’s rotavirus outbreak wasn’t caused by vaccine effectiveness decline
In 2017, the Australian state of New South Wales experienced an outbreak of rotavirus gastroenteritis in children despite a high level of rotavirus immunization. In a new study, researchers reported evidence that suggests a decline in vaccine effectiveness (VE) isn’t the cause, although they found that VE declines over time as children age.
“More analysis is required to investigate how novel or unusual strains ... interact with rotavirus vaccines and whether antigenic changes affect VE and challenge vaccination programs,” the study authors wrote in Pediatrics.
Researchers led by Julia E. Maguire, BSc, MSci(Epi), of Australia’s National Center for Immunization Research and the Australian National University, Canberra, launched the analysis in the wake of a 2017 outbreak of 2,319 rotavirus cases in New South Wales, a 210% increase over the rate in 2016. (The state, the largest in Australia, has about 7.5 million residents.)
The study authors tracked VE from 2010 to 2017 by analyzing 9,517 rotavirus cases in the state (50% male; median age, 5 years). Half weren’t eligible for rotavirus immunization because of their age; of the rest, 31% weren’t vaccinated.
Ms. Maguire and associates found that “In our study, two doses of RV1 [the Rotarix vaccine] was 73.7% effective in protecting children aged 6 months to 9 years against laboratory-confirmed rotavirus over our 8-year study period. Somewhat surprisingly in the 2017 outbreak year, a high two-dose VE of 88.4% in those aged 6-11 months was also observed.”
They added that “the median age of rotavirus cases has increased in Australia over the last 8 years from 3.9 years in 2010 to 7.1 years in 2017. Adults and older children born before the availability of vaccination in Australia are unimmunized and may have been less likely to have repeated subclinical infections because of reductions in virus circulation overall, resulting in less immune boosting.”
Going forward, the study authors wrote that “investigation of population-level VE in relation to rotavirus genotype data should continue in a range of settings to improve our understanding of rotavirus vaccines and the impact they have on disease across the age spectrum over time.”
In an accompanying commentary, Benjamin Lee, MD, and E. Ross Colgate, PhD, of the University of Vermont, Burlington, wrote that Australia’s adoption of rotavirus immunization in 2017 “with state-level implementation of either Rotarix or RotaTeq ... enabled a fascinating natural experiment of VE and strain selection.”
Pressure from vaccines “potentially enables the emergence of novel strains,” they wrote. “Despite this, large-scale strain replacement has not been demonstrated in rotaviruses, in contrast to the development of pneumococcal serotype replacement that was seen after pneumococcal conjugate vaccine introduction. Similarly, there has been no evidence of widespread vaccine escape due to antigenic drift or shift, as occurs with another important segmented RNA virus, influenza A.”
As Dr. Lee and Dr. Colgate noted, 100 million children worldwide remain unvaccinated against rotavirus, and more than 128,000 die because of rotavirus-associated gastroenteritis each year. “Improving vaccine access and coverage and solving the riddle of [oral rotavirus vaccine] underperformance in low-income countries are urgent priorities, which may ultimately require next-generation oral and/or parenteral vaccines, a number of which are under development and in clinical trials. In addition, because the emergence of novel strains of disease-causing pathogens is always a possibility, vigilance in rotavirus surveillance, including genotype assessment, should remain a priority for public health programs.”
The study was funded by Australia’s National Center for Immunization Research and Surveillance, which receives government funding. The Australian Rotavirus Surveillance Program is supported by government funding and the vaccine companies Commonwealth Serum Laboratories and GlaxoSmithKline. Ms. Maguire is supported by an Australian Government Research Training Program Scholarship. One author is director of the Australian Rotavirus Surveillance Program, which received funding as above. The other study authors and the commentary authors reported no relevant financial disclosures.
SOURCES: Maguire JE et al. Pediatrics. 2019 Sep 17. doi: 10.1542/peds.2019-1024; Lee B, Colgate ER. Pediatrics. 2019 Sep 17. doi: 10.1542/peds.2019-2426.
In 2017, the Australian state of New South Wales experienced an outbreak of rotavirus gastroenteritis in children despite a high level of rotavirus immunization. In a new study, researchers reported evidence that suggests a decline in vaccine effectiveness (VE) isn’t the cause, although they found that VE declines over time as children age.
“More analysis is required to investigate how novel or unusual strains ... interact with rotavirus vaccines and whether antigenic changes affect VE and challenge vaccination programs,” the study authors wrote in Pediatrics.
Researchers led by Julia E. Maguire, BSc, MSci(Epi), of Australia’s National Center for Immunization Research and the Australian National University, Canberra, launched the analysis in the wake of a 2017 outbreak of 2,319 rotavirus cases in New South Wales, a 210% increase over the rate in 2016. (The state, the largest in Australia, has about 7.5 million residents.)
The study authors tracked VE from 2010 to 2017 by analyzing 9,517 rotavirus cases in the state (50% male; median age, 5 years). Half weren’t eligible for rotavirus immunization because of their age; of the rest, 31% weren’t vaccinated.
Ms. Maguire and associates found that “In our study, two doses of RV1 [the Rotarix vaccine] was 73.7% effective in protecting children aged 6 months to 9 years against laboratory-confirmed rotavirus over our 8-year study period. Somewhat surprisingly in the 2017 outbreak year, a high two-dose VE of 88.4% in those aged 6-11 months was also observed.”
They added that “the median age of rotavirus cases has increased in Australia over the last 8 years from 3.9 years in 2010 to 7.1 years in 2017. Adults and older children born before the availability of vaccination in Australia are unimmunized and may have been less likely to have repeated subclinical infections because of reductions in virus circulation overall, resulting in less immune boosting.”
Going forward, the study authors wrote that “investigation of population-level VE in relation to rotavirus genotype data should continue in a range of settings to improve our understanding of rotavirus vaccines and the impact they have on disease across the age spectrum over time.”
In an accompanying commentary, Benjamin Lee, MD, and E. Ross Colgate, PhD, of the University of Vermont, Burlington, wrote that Australia’s adoption of rotavirus immunization in 2017 “with state-level implementation of either Rotarix or RotaTeq ... enabled a fascinating natural experiment of VE and strain selection.”
Pressure from vaccines “potentially enables the emergence of novel strains,” they wrote. “Despite this, large-scale strain replacement has not been demonstrated in rotaviruses, in contrast to the development of pneumococcal serotype replacement that was seen after pneumococcal conjugate vaccine introduction. Similarly, there has been no evidence of widespread vaccine escape due to antigenic drift or shift, as occurs with another important segmented RNA virus, influenza A.”
As Dr. Lee and Dr. Colgate noted, 100 million children worldwide remain unvaccinated against rotavirus, and more than 128,000 die because of rotavirus-associated gastroenteritis each year. “Improving vaccine access and coverage and solving the riddle of [oral rotavirus vaccine] underperformance in low-income countries are urgent priorities, which may ultimately require next-generation oral and/or parenteral vaccines, a number of which are under development and in clinical trials. In addition, because the emergence of novel strains of disease-causing pathogens is always a possibility, vigilance in rotavirus surveillance, including genotype assessment, should remain a priority for public health programs.”
The study was funded by Australia’s National Center for Immunization Research and Surveillance, which receives government funding. The Australian Rotavirus Surveillance Program is supported by government funding and the vaccine companies Commonwealth Serum Laboratories and GlaxoSmithKline. Ms. Maguire is supported by an Australian Government Research Training Program Scholarship. One author is director of the Australian Rotavirus Surveillance Program, which received funding as above. The other study authors and the commentary authors reported no relevant financial disclosures.
SOURCES: Maguire JE et al. Pediatrics. 2019 Sep 17. doi: 10.1542/peds.2019-1024; Lee B, Colgate ER. Pediatrics. 2019 Sep 17. doi: 10.1542/peds.2019-2426.
In 2017, the Australian state of New South Wales experienced an outbreak of rotavirus gastroenteritis in children despite a high level of rotavirus immunization. In a new study, researchers reported evidence that suggests a decline in vaccine effectiveness (VE) isn’t the cause, although they found that VE declines over time as children age.
“More analysis is required to investigate how novel or unusual strains ... interact with rotavirus vaccines and whether antigenic changes affect VE and challenge vaccination programs,” the study authors wrote in Pediatrics.
Researchers led by Julia E. Maguire, BSc, MSci(Epi), of Australia’s National Center for Immunization Research and the Australian National University, Canberra, launched the analysis in the wake of a 2017 outbreak of 2,319 rotavirus cases in New South Wales, a 210% increase over the rate in 2016. (The state, the largest in Australia, has about 7.5 million residents.)
The study authors tracked VE from 2010 to 2017 by analyzing 9,517 rotavirus cases in the state (50% male; median age, 5 years). Half weren’t eligible for rotavirus immunization because of their age; of the rest, 31% weren’t vaccinated.
Ms. Maguire and associates found that “In our study, two doses of RV1 [the Rotarix vaccine] was 73.7% effective in protecting children aged 6 months to 9 years against laboratory-confirmed rotavirus over our 8-year study period. Somewhat surprisingly in the 2017 outbreak year, a high two-dose VE of 88.4% in those aged 6-11 months was also observed.”
They added that “the median age of rotavirus cases has increased in Australia over the last 8 years from 3.9 years in 2010 to 7.1 years in 2017. Adults and older children born before the availability of vaccination in Australia are unimmunized and may have been less likely to have repeated subclinical infections because of reductions in virus circulation overall, resulting in less immune boosting.”
Going forward, the study authors wrote that “investigation of population-level VE in relation to rotavirus genotype data should continue in a range of settings to improve our understanding of rotavirus vaccines and the impact they have on disease across the age spectrum over time.”
In an accompanying commentary, Benjamin Lee, MD, and E. Ross Colgate, PhD, of the University of Vermont, Burlington, wrote that Australia’s adoption of rotavirus immunization in 2017 “with state-level implementation of either Rotarix or RotaTeq ... enabled a fascinating natural experiment of VE and strain selection.”
Pressure from vaccines “potentially enables the emergence of novel strains,” they wrote. “Despite this, large-scale strain replacement has not been demonstrated in rotaviruses, in contrast to the development of pneumococcal serotype replacement that was seen after pneumococcal conjugate vaccine introduction. Similarly, there has been no evidence of widespread vaccine escape due to antigenic drift or shift, as occurs with another important segmented RNA virus, influenza A.”
As Dr. Lee and Dr. Colgate noted, 100 million children worldwide remain unvaccinated against rotavirus, and more than 128,000 die because of rotavirus-associated gastroenteritis each year. “Improving vaccine access and coverage and solving the riddle of [oral rotavirus vaccine] underperformance in low-income countries are urgent priorities, which may ultimately require next-generation oral and/or parenteral vaccines, a number of which are under development and in clinical trials. In addition, because the emergence of novel strains of disease-causing pathogens is always a possibility, vigilance in rotavirus surveillance, including genotype assessment, should remain a priority for public health programs.”
The study was funded by Australia’s National Center for Immunization Research and Surveillance, which receives government funding. The Australian Rotavirus Surveillance Program is supported by government funding and the vaccine companies Commonwealth Serum Laboratories and GlaxoSmithKline. Ms. Maguire is supported by an Australian Government Research Training Program Scholarship. One author is director of the Australian Rotavirus Surveillance Program, which received funding as above. The other study authors and the commentary authors reported no relevant financial disclosures.
SOURCES: Maguire JE et al. Pediatrics. 2019 Sep 17. doi: 10.1542/peds.2019-1024; Lee B, Colgate ER. Pediatrics. 2019 Sep 17. doi: 10.1542/peds.2019-2426.
FROM PEDIATRICS
Laser treatment of basal cell carcinoma continues to be refined
SAN DIEGO – Using laser and light sources to treat nonaggressive basal cell carcinoma (BCC) is emerging as a promising treatment option, especially for those with multiple tumors and those who are poor surgical candidates, Arisa E. Ortiz, MD, said at the annual Masters of Aesthetics Symposium.
“Topical therapies often result in recurrence, so there really is a need for an alternative [to surgery] that’s effective, efficient, and carries a low risk of side effects,” said Dr. Ortiz, who is director of laser and cosmetic dermatology at the University of California, San Diego,
“The prototypic feature of BCC is the presence of telangiectatic vessels,” she explained, and the postulated mechanism of action is selective photothermolysis of the tumor vasculature. “These vessels are slightly larger in caliber, compared with normal skin – 40 micrometers versus 15 micrometers – and more fragile. You can tailor your pulse duration to the size of the vessels. Theoretically, by targeting the vasculature then you get tumor regression with sparing of normal tissue.”
Initial studies of this approach have used the 595-nm pulsed-dye laser, which is well absorbed by oxyhemoglobin, but more recent studies have used the 1064-nm Nd:YAG to reach deep arterial vessels. In a prospective, open-label study, 10 patients with 13 BCCs less than 1.5 cm in diameter received one treatment with a 10-ms pulsed 1064-nm Nd:YAG laser delivered on the trunk or extremities at a fluence of 80-120 J/cm2 (Lasers Surg Med. 2015;47[2]:106-10). Dr. Ortiz and her colleagues observed a 92% clearance rate overall.
She described other earlier studies of the approach as flawed, because they relied on confirmation of clearance rates with clinical exam or biopsy rather than with surgical excision. “Also, some of the protocols weren’t standardized, multiple treatments were required, and subjects with suboptimal response were currently on anticoagulation,” she said. “Intravascular coagulation is important for effective treatment with vascular lasers, so anticoagulation may interfere with efficacy.”
In a more recent multicenter study, Dr. Ortiz and her colleagues treated 33 BCCs once with the long-pulsed 1064-nm Nd:YAG laser delivered with a 5-6 mm spot size at a fluence of 125-140 J/cm2 and a 7-10 ms pulse duration (Laser Surgery Med. Feb 13 2018. doi: 10.1002/lsm.22803). Standard surgical excision with 5-mm margins was performed 4 weeks after laser treatment. Among 31 subjects who completed the study, 28 of 31 BCC tumors (90%) cleared after one treatment.
“The treatments were performed without anesthesia, because we didn’t want the vasculature to be affected, but in clinical practice I am now using lidocaine with no epinephrine,” Dr. Ortiz said. She characterized the results as “at least comparable to, if not superior to” common modalities including methyl aminolevulinate–PDT (72.8%), imiquimod cream (83.4%), and fluorouracil cream (80.1%). “One criticism I hear is that with such high fluences, you’re probably getting some bulk heating,” she said. “Maybe so, but it seems to work and there’s no scarring, which suggests otherwise.”
Advantages of using a 1064-nm Nd:YAG for treating nonaggressive BCCs are that it requires just one treatment, it takes about 5 minutes, and there is no significant downtime, with no limitations in posttreatment activity. “Potentially there is a relatively decreased risk for complications, including infection and bleeding,” she added. “It’s a good alternative for treating patients with multiple tumors or those who are poor surgical candidates.”
She and her colleagues are currently performing a long-term follow-up study of 35 BCC lesions. Only one has potentially recurred, but that recurrence has not yet been confirmed.
Dr. Ortiz treats BCCs with a standard 5-mm margin and uses lidocaine without epinephrine to avoid vasoconstriction. She typically uses a 1064-nm Cutera excel V laser delivered at a pulse duration of 8 ms and a fluence of 140 J/cm2, with no cooling. “Theoretically, any 1064-nm pulsed-dye laser could work, but the way the pulse is delivered is different, depending on which device” is used, she said.
“I always like waiting between passes to avoid any bulk heating. The immediate endpoint to strive for is slight graying and slight contraction,” she said. Billing codes for malignant destruction/electrodesiccation and curettage can be used (codes 17260-17266 for the trunk and 17280-17283 for the face).
In order to determine the mechanism of cell death and to optimize results, Dr. Ortiz said that further studies need to be conducted in vitro and in vivo. In order to determine treatment efficacy, clinical studies involving various heat sources and low concentrations of lidocaine are also required.
Dr. Ortiz disclosed having financial relationships with numerous pharmaceutical and device companies. She is also cochair of the MOAS.
SAN DIEGO – Using laser and light sources to treat nonaggressive basal cell carcinoma (BCC) is emerging as a promising treatment option, especially for those with multiple tumors and those who are poor surgical candidates, Arisa E. Ortiz, MD, said at the annual Masters of Aesthetics Symposium.
“Topical therapies often result in recurrence, so there really is a need for an alternative [to surgery] that’s effective, efficient, and carries a low risk of side effects,” said Dr. Ortiz, who is director of laser and cosmetic dermatology at the University of California, San Diego,
“The prototypic feature of BCC is the presence of telangiectatic vessels,” she explained, and the postulated mechanism of action is selective photothermolysis of the tumor vasculature. “These vessels are slightly larger in caliber, compared with normal skin – 40 micrometers versus 15 micrometers – and more fragile. You can tailor your pulse duration to the size of the vessels. Theoretically, by targeting the vasculature then you get tumor regression with sparing of normal tissue.”
Initial studies of this approach have used the 595-nm pulsed-dye laser, which is well absorbed by oxyhemoglobin, but more recent studies have used the 1064-nm Nd:YAG to reach deep arterial vessels. In a prospective, open-label study, 10 patients with 13 BCCs less than 1.5 cm in diameter received one treatment with a 10-ms pulsed 1064-nm Nd:YAG laser delivered on the trunk or extremities at a fluence of 80-120 J/cm2 (Lasers Surg Med. 2015;47[2]:106-10). Dr. Ortiz and her colleagues observed a 92% clearance rate overall.
She described other earlier studies of the approach as flawed, because they relied on confirmation of clearance rates with clinical exam or biopsy rather than with surgical excision. “Also, some of the protocols weren’t standardized, multiple treatments were required, and subjects with suboptimal response were currently on anticoagulation,” she said. “Intravascular coagulation is important for effective treatment with vascular lasers, so anticoagulation may interfere with efficacy.”
In a more recent multicenter study, Dr. Ortiz and her colleagues treated 33 BCCs once with the long-pulsed 1064-nm Nd:YAG laser delivered with a 5-6 mm spot size at a fluence of 125-140 J/cm2 and a 7-10 ms pulse duration (Laser Surgery Med. Feb 13 2018. doi: 10.1002/lsm.22803). Standard surgical excision with 5-mm margins was performed 4 weeks after laser treatment. Among 31 subjects who completed the study, 28 of 31 BCC tumors (90%) cleared after one treatment.
“The treatments were performed without anesthesia, because we didn’t want the vasculature to be affected, but in clinical practice I am now using lidocaine with no epinephrine,” Dr. Ortiz said. She characterized the results as “at least comparable to, if not superior to” common modalities including methyl aminolevulinate–PDT (72.8%), imiquimod cream (83.4%), and fluorouracil cream (80.1%). “One criticism I hear is that with such high fluences, you’re probably getting some bulk heating,” she said. “Maybe so, but it seems to work and there’s no scarring, which suggests otherwise.”
Advantages of using a 1064-nm Nd:YAG for treating nonaggressive BCCs are that it requires just one treatment, it takes about 5 minutes, and there is no significant downtime, with no limitations in posttreatment activity. “Potentially there is a relatively decreased risk for complications, including infection and bleeding,” she added. “It’s a good alternative for treating patients with multiple tumors or those who are poor surgical candidates.”
She and her colleagues are currently performing a long-term follow-up study of 35 BCC lesions. Only one has potentially recurred, but that recurrence has not yet been confirmed.
Dr. Ortiz treats BCCs with a standard 5-mm margin and uses lidocaine without epinephrine to avoid vasoconstriction. She typically uses a 1064-nm Cutera excel V laser delivered at a pulse duration of 8 ms and a fluence of 140 J/cm2, with no cooling. “Theoretically, any 1064-nm pulsed-dye laser could work, but the way the pulse is delivered is different, depending on which device” is used, she said.
“I always like waiting between passes to avoid any bulk heating. The immediate endpoint to strive for is slight graying and slight contraction,” she said. Billing codes for malignant destruction/electrodesiccation and curettage can be used (codes 17260-17266 for the trunk and 17280-17283 for the face).
In order to determine the mechanism of cell death and to optimize results, Dr. Ortiz said that further studies need to be conducted in vitro and in vivo. In order to determine treatment efficacy, clinical studies involving various heat sources and low concentrations of lidocaine are also required.
Dr. Ortiz disclosed having financial relationships with numerous pharmaceutical and device companies. She is also cochair of the MOAS.
SAN DIEGO – Using laser and light sources to treat nonaggressive basal cell carcinoma (BCC) is emerging as a promising treatment option, especially for those with multiple tumors and those who are poor surgical candidates, Arisa E. Ortiz, MD, said at the annual Masters of Aesthetics Symposium.
“Topical therapies often result in recurrence, so there really is a need for an alternative [to surgery] that’s effective, efficient, and carries a low risk of side effects,” said Dr. Ortiz, who is director of laser and cosmetic dermatology at the University of California, San Diego,
“The prototypic feature of BCC is the presence of telangiectatic vessels,” she explained, and the postulated mechanism of action is selective photothermolysis of the tumor vasculature. “These vessels are slightly larger in caliber, compared with normal skin – 40 micrometers versus 15 micrometers – and more fragile. You can tailor your pulse duration to the size of the vessels. Theoretically, by targeting the vasculature then you get tumor regression with sparing of normal tissue.”
Initial studies of this approach have used the 595-nm pulsed-dye laser, which is well absorbed by oxyhemoglobin, but more recent studies have used the 1064-nm Nd:YAG to reach deep arterial vessels. In a prospective, open-label study, 10 patients with 13 BCCs less than 1.5 cm in diameter received one treatment with a 10-ms pulsed 1064-nm Nd:YAG laser delivered on the trunk or extremities at a fluence of 80-120 J/cm2 (Lasers Surg Med. 2015;47[2]:106-10). Dr. Ortiz and her colleagues observed a 92% clearance rate overall.
She described other earlier studies of the approach as flawed, because they relied on confirmation of clearance rates with clinical exam or biopsy rather than with surgical excision. “Also, some of the protocols weren’t standardized, multiple treatments were required, and subjects with suboptimal response were currently on anticoagulation,” she said. “Intravascular coagulation is important for effective treatment with vascular lasers, so anticoagulation may interfere with efficacy.”
In a more recent multicenter study, Dr. Ortiz and her colleagues treated 33 BCCs once with the long-pulsed 1064-nm Nd:YAG laser delivered with a 5-6 mm spot size at a fluence of 125-140 J/cm2 and a 7-10 ms pulse duration (Laser Surgery Med. Feb 13 2018. doi: 10.1002/lsm.22803). Standard surgical excision with 5-mm margins was performed 4 weeks after laser treatment. Among 31 subjects who completed the study, 28 of 31 BCC tumors (90%) cleared after one treatment.
“The treatments were performed without anesthesia, because we didn’t want the vasculature to be affected, but in clinical practice I am now using lidocaine with no epinephrine,” Dr. Ortiz said. She characterized the results as “at least comparable to, if not superior to” common modalities including methyl aminolevulinate–PDT (72.8%), imiquimod cream (83.4%), and fluorouracil cream (80.1%). “One criticism I hear is that with such high fluences, you’re probably getting some bulk heating,” she said. “Maybe so, but it seems to work and there’s no scarring, which suggests otherwise.”
Advantages of using a 1064-nm Nd:YAG for treating nonaggressive BCCs are that it requires just one treatment, it takes about 5 minutes, and there is no significant downtime, with no limitations in posttreatment activity. “Potentially there is a relatively decreased risk for complications, including infection and bleeding,” she added. “It’s a good alternative for treating patients with multiple tumors or those who are poor surgical candidates.”
She and her colleagues are currently performing a long-term follow-up study of 35 BCC lesions. Only one has potentially recurred, but that recurrence has not yet been confirmed.
Dr. Ortiz treats BCCs with a standard 5-mm margin and uses lidocaine without epinephrine to avoid vasoconstriction. She typically uses a 1064-nm Cutera excel V laser delivered at a pulse duration of 8 ms and a fluence of 140 J/cm2, with no cooling. “Theoretically, any 1064-nm pulsed-dye laser could work, but the way the pulse is delivered is different, depending on which device” is used, she said.
“I always like waiting between passes to avoid any bulk heating. The immediate endpoint to strive for is slight graying and slight contraction,” she said. Billing codes for malignant destruction/electrodesiccation and curettage can be used (codes 17260-17266 for the trunk and 17280-17283 for the face).
In order to determine the mechanism of cell death and to optimize results, Dr. Ortiz said that further studies need to be conducted in vitro and in vivo. In order to determine treatment efficacy, clinical studies involving various heat sources and low concentrations of lidocaine are also required.
Dr. Ortiz disclosed having financial relationships with numerous pharmaceutical and device companies. She is also cochair of the MOAS.
EXPERT ANALYSIS FROM MOAS 2019
Society of Hospital Medicine Position on the American Board of Pediatrics Response to the Pediatric Hospital Medicine Petition
The first Pediatric Hospital Medicine (PHM) fellowships in the United States were established in 2003;1 and since then, the field has expanded and matured dramatically. This growth, accompanied by greater definition of the role and recommended competencies of pediatric hospitalists,2 culminated in the submission of a petition to the American Board of Pediatrics (ABP) in August 2014 to consider recognition of PHM as a new pediatric subspecialty.3 After an 18-month iterative process requiring extensive input from the Joint Council of Pediatric Hospital Medicine, ABP subcommittees, the Association of Medical School Pediatric Department Chairs, the Association of Pediatric Program Directors, and other prominent pediatric professional societies, the ABP voted in December 2015 to recommend that the American Board of Medical Subspecialties (ABMS) recognize PHM as a new subspecialty.3
The ABP subsequently announced three pathways for board certification in PHM:
- Training pathway for those completing an Accreditation Council for Graduate Medical Education–accredited two-year PHM fellowship program;
- Practice pathway for those satisfying ABP criteria for clinical activity in PHM for four years prior to exam dates (in 2019, 2021, and 2023), initially described as “direct patient care of hospitalized children ≥25% full-time equivalent (FTE) defined as ≥450-500 hours per year every year for the preceding four years”;4
- Combined pathway for those completing less than two years of fellowship, who would be required to complete two years of practice experience that satisfy the same criteria as each year of the practice pathway.5
While the training pathway met near-uniform acceptance, concerns were raised through the American Academy of Pediatrics Section of Hospital Medicine (AAP SOHM) Listserv regarding the practice pathway, and by extension, the combined pathway. Specifically, language describing the necessary characteristics of acceptable PHM practice was felt to be vague and not transparent. Listserv posts also raised concerns regarding the potential exclusion of “niche” practices such as subspecialty hospitalists and newborn hospitalists. As applicants in the practice pathway began to receive denials, opinions voiced in listserv posts were increasingly critical of the ABP’s lack of transparency regarding the specific criteria adjudicating applications.
ORIGIN OF THE PHM PETITION
A group of hospitalists, led by Dr. David Skey, a pediatric hospitalist at Arnold Palmer Children’s Hospital in Orlando, Florida, created a petition which was submitted to the ABP on August 6, 2019, and raised the following issues:
- “A perception of unfairness/bias in the practice pathway criteria and the way these criteria have been applied.
- Denials based on gaps in employment without reasonable consideration of mitigating factors.
- Lack of transparency, accountability, and responsiveness from the ABP.”6
The petition, posted on the AAP SOHM listserv and signed by 1,479 individuals,7 raised concerns of anecdotal evidence that the practice pathway criteria disproportionately disadvantaged women, although intentional bias was not suspected by the signers of the letter. The petition’s signers submitted the following demands to the ABP:
- “Facilitate a timely analysis to determine if gender bias is present or perform this analysis internally and release the findings publicly.
- Revise the practice pathway criteria to be more inclusive of applicants with interrupted practice and varied clinical experience, to include clear-cut parameters rather than considering these applications on a closed-door ‘case-by-case basis...at the discretion of the ABP’.
- Clarify the appeals process and improve responsiveness to appeals and inquiries regarding denials.
- Provide a formal response to this petition letter through the PHM ListServ and/or the ABP website within one week of receiving the signed petition.”6
THE ABP RESPONSE TO THE PHM PETITION
A formal response to the petition was released on the AAP SOHM Listserv on August 29, 2019, to address the concerns raised and is published in this issue of the Journal of Hospital Medicine.4 In response to the allegation of gender bias, the ABP maintained that the data did not support this, as the denial rate for females (4.0%) was not significantly different than that for males (3.7%). The response acknowledged that once clear-cut criteria were decided upon to augment the general practice pathway criteria published at the outset, these criteria should have been disseminated. The ABP maintained, however, that these criteria, once established, were used consistently in adjudicating all applications. To clarify and simplify the eligibility criteria, the percentage of the full-time equivalent and practice interruption criteria were removed, as the work-hours criteria (direct patient care of hospitalized children ≥450-500 hours per year every year for the preceding four years)8 were deemed sufficient to ensure adequate clinical participation.
SHM’S POSITION REGARDING THE PHM PETITION AND ABP RESPONSE
The Society of Hospital Medicine (SHM), through pediatric hospitalists and pediatricians on its Board, committees, and the Executive Council of the Pediatric Special Interest Group, has followed with great interest the public debate surrounding the PHM certification process and the subsequent PHM petition to the ABP. The ABP responded swiftly and with full transparency to the petition, and SHM supports these efforts by the ABP to provide a timely, honest, data-driven response to the concerns raised by the PHM petition. SHM recognizes that the mission of the ABP is to provide the public with confidence that physicians with ABP board certifications meet appropriate “standards of excellence”. While the revisions implemented by the ABP in its response still may not satisfy the concerns of all members of the PHM community, SHM recognizes that the revised requirements remain true to the mission of the ABP.
SHM applauds the authors and signatories of the PHM petition for bravely raising their concerns of gender bias and lack of transparency. The response of the ABP to this petition by further improving transparency serves as an example of continuous improvement in collaborative practice to all medical specialty boards.
While SHM supports the ABP response to the PHM petition, it is clear that excellent physicians caring for hospitalized children will be unable to achieve PHM board certification for a variety of reasons. For these physicians who are not PHM board certified as pediatric hospitalists by the ABP, SHM supports providing these physicians with recognition as hospitalists. These include “niche” hospitalists, such as newborn hospitalists, subacute hospitalists, and subspecialty hospitalists. SHM will also continue to support and recognize community-based hospitalists, family medicine-trained hospitalists, and Med-Peds hospitalists whose practice may not comply with criteria laid out by the ABP. For these physicians, receiving Fellow designation through SHM, a merit-based distinction requiring demonstration of clinical excellence and commitment to hospital medicine, is another route whereby physicians can achieve designation as a hospitalist.
FUTURE DIRECTIONS FOR PEDIATRIC HOSPITALISTS
SHM supports future efforts by the ABP to be vigilant for bias of any sort in the certification process. Other future considerations for the PHM community include the possibility of a focused practice pathway in hospital medicine (FPHM) for pediatrics as is currently jointly offered by the American Board of Internal Medicine (ABIM) and the American Board of Family Medicine (ABFM). This maintenance of certification program is a variation of internal medicine or family medicine recertification, not a subspecialty, but allows physicians practicing primarily in inpatient settings to focus continuing education efforts on skills and attitudes needed for inpatient practice.9 While this possibility was discounted by the ABP in the past based on initially low numbers of physicians choosing this pathway, this pathway has grown from initially attracting 150 internal medicine applicants yearly to 265 in 2015.10 The ABMS approved the ABIM/ABFM FPHM as its first approved designation in March 2017 after more than 2,500 physicians earned this designation.11 Of the >2,800 pediatric residency graduates (not including combined programs) each year, 10% report planning on becoming pediatric hospitalists,12 and currently only 72-74 fellows graduate from PHM fellowships yearly.13 FPHM for pediatric hospital medicine would provide focused maintenance of certification and hospitalist designation for those who cannot match to fellowship programs.
Acknowledgments
The authors would like to acknowledge the input and support from the Executive Council of the Society of Hospital Medicine Pediatric Special Interest Group in writing this statement.
Disclosures
Dr. Chang served as an author of the Pediatric Hospital Medicine Petition to the American Board of Pediatrics for Subspecialty Certification. Drs. Hopkins, Rehm, Gage, and Shen have nothing to disclose.
1. Freed GL, Dunham KM, Research Advisory Committee of the American Board of P. Characteristics of pediatric hospital medicine fellowships and training programs. J Hosp Med. 2009;4(3):157-163. https://doi.org/10.1002/jhm.409.
2. Stucky ER, Maniscalco J, Ottolini MC, et al. The Pediatric Hospital Medicine Core Competencies Supplement: a Framework for Curriculum Development by the Society of Hospital Medicine with acknowledgement to pediatric hospitalists from the American Academy of Pediatrics and the Academic Pediatric Association. J Hosp Med. 2010;5 Suppl 2:i-xv, 1-114. https://doi.org/10.1002/jhm.776.
3. Barrett DJ, McGuinness GA, Cunha CA, et al. Pediatric Hospital Medicine: A Proposed New Subspecialty. Pediatrics. 2017;139(3). https://doi.org/10.1542/peds.2016-1823.
4. Nichols DG WS. The American Board of Pediatrics response to the Pediatric Hospital Medicine petition. J Hosp Med. 2019;14(10):586-588. https://doi.org/10.12788/jhm.3322.
5. Pediatric hospital medicine certification. American Board of Pediatrics. https://www.abp.org/content/pediatric-hospital-medicine-certification#training. Accessed 3 September, 2019.
6. Skey D. Pediatric Hospitalists, It’s time to take a stand on the PHM Boards Application Process! Five Dog Development, LLC. https://www.phmpetition.com/. Accessed 3 September, 2019.
7. Skey D. Petition Update. In: AAP SOHM Listserv: American Academy of Pediatrics; 2019.
8. The American Board of Pediatrics Response to the Pediatric Hospital Medicine Petition. The American Board of Pediatrics. https://www.abp.org/sites/abp/files/phm-petition-response.pdf. Published 2019. Accessed September 4, 2019.
9. Focused practice in hospital medicine. American Board of Internal Medicine. https://www.abim.org/maintenance-of-certification/moc-requirements/focused-practice-hospital-medicine.aspx. Published 2019 Accessed September 4, 2019.
10. Butterfield S. Following the focused practice pathway. American College of Physicians. Your career Web site. https://acphospitalist.org/archives/2016/09/focused-practice-hospital-medicine.htm. Published 2016. Accessed September 4, 2019.
11. American Board of Medical Specialties Announces New, Focused Practice Designation [press release]. American Board of Medical Specialties, 14 Mar 2017.
12. Leyenaar JK, Frintner MP. Graduating Pediatric Residents Entering the Hospital Medicine Workforce, 2006-2015. Acad Pediatr. 2018;18(2):200-207. https://doi.org/10.1016/j.acap.2017.05.001.
13. PHM Fellowship Programs. PHMFellows.org. http://phmfellows.org/phm-programs/. Published 2019. Accessed September 4, 2019.
The first Pediatric Hospital Medicine (PHM) fellowships in the United States were established in 2003;1 and since then, the field has expanded and matured dramatically. This growth, accompanied by greater definition of the role and recommended competencies of pediatric hospitalists,2 culminated in the submission of a petition to the American Board of Pediatrics (ABP) in August 2014 to consider recognition of PHM as a new pediatric subspecialty.3 After an 18-month iterative process requiring extensive input from the Joint Council of Pediatric Hospital Medicine, ABP subcommittees, the Association of Medical School Pediatric Department Chairs, the Association of Pediatric Program Directors, and other prominent pediatric professional societies, the ABP voted in December 2015 to recommend that the American Board of Medical Subspecialties (ABMS) recognize PHM as a new subspecialty.3
The ABP subsequently announced three pathways for board certification in PHM:
- Training pathway for those completing an Accreditation Council for Graduate Medical Education–accredited two-year PHM fellowship program;
- Practice pathway for those satisfying ABP criteria for clinical activity in PHM for four years prior to exam dates (in 2019, 2021, and 2023), initially described as “direct patient care of hospitalized children ≥25% full-time equivalent (FTE) defined as ≥450-500 hours per year every year for the preceding four years”;4
- Combined pathway for those completing less than two years of fellowship, who would be required to complete two years of practice experience that satisfy the same criteria as each year of the practice pathway.5
While the training pathway met near-uniform acceptance, concerns were raised through the American Academy of Pediatrics Section of Hospital Medicine (AAP SOHM) Listserv regarding the practice pathway, and by extension, the combined pathway. Specifically, language describing the necessary characteristics of acceptable PHM practice was felt to be vague and not transparent. Listserv posts also raised concerns regarding the potential exclusion of “niche” practices such as subspecialty hospitalists and newborn hospitalists. As applicants in the practice pathway began to receive denials, opinions voiced in listserv posts were increasingly critical of the ABP’s lack of transparency regarding the specific criteria adjudicating applications.
ORIGIN OF THE PHM PETITION
A group of hospitalists, led by Dr. David Skey, a pediatric hospitalist at Arnold Palmer Children’s Hospital in Orlando, Florida, created a petition which was submitted to the ABP on August 6, 2019, and raised the following issues:
- “A perception of unfairness/bias in the practice pathway criteria and the way these criteria have been applied.
- Denials based on gaps in employment without reasonable consideration of mitigating factors.
- Lack of transparency, accountability, and responsiveness from the ABP.”6
The petition, posted on the AAP SOHM listserv and signed by 1,479 individuals,7 raised concerns of anecdotal evidence that the practice pathway criteria disproportionately disadvantaged women, although intentional bias was not suspected by the signers of the letter. The petition’s signers submitted the following demands to the ABP:
- “Facilitate a timely analysis to determine if gender bias is present or perform this analysis internally and release the findings publicly.
- Revise the practice pathway criteria to be more inclusive of applicants with interrupted practice and varied clinical experience, to include clear-cut parameters rather than considering these applications on a closed-door ‘case-by-case basis...at the discretion of the ABP’.
- Clarify the appeals process and improve responsiveness to appeals and inquiries regarding denials.
- Provide a formal response to this petition letter through the PHM ListServ and/or the ABP website within one week of receiving the signed petition.”6
THE ABP RESPONSE TO THE PHM PETITION
A formal response to the petition was released on the AAP SOHM Listserv on August 29, 2019, to address the concerns raised and is published in this issue of the Journal of Hospital Medicine.4 In response to the allegation of gender bias, the ABP maintained that the data did not support this, as the denial rate for females (4.0%) was not significantly different than that for males (3.7%). The response acknowledged that once clear-cut criteria were decided upon to augment the general practice pathway criteria published at the outset, these criteria should have been disseminated. The ABP maintained, however, that these criteria, once established, were used consistently in adjudicating all applications. To clarify and simplify the eligibility criteria, the percentage of the full-time equivalent and practice interruption criteria were removed, as the work-hours criteria (direct patient care of hospitalized children ≥450-500 hours per year every year for the preceding four years)8 were deemed sufficient to ensure adequate clinical participation.
SHM’S POSITION REGARDING THE PHM PETITION AND ABP RESPONSE
The Society of Hospital Medicine (SHM), through pediatric hospitalists and pediatricians on its Board, committees, and the Executive Council of the Pediatric Special Interest Group, has followed with great interest the public debate surrounding the PHM certification process and the subsequent PHM petition to the ABP. The ABP responded swiftly and with full transparency to the petition, and SHM supports these efforts by the ABP to provide a timely, honest, data-driven response to the concerns raised by the PHM petition. SHM recognizes that the mission of the ABP is to provide the public with confidence that physicians with ABP board certifications meet appropriate “standards of excellence”. While the revisions implemented by the ABP in its response still may not satisfy the concerns of all members of the PHM community, SHM recognizes that the revised requirements remain true to the mission of the ABP.
SHM applauds the authors and signatories of the PHM petition for bravely raising their concerns of gender bias and lack of transparency. The response of the ABP to this petition by further improving transparency serves as an example of continuous improvement in collaborative practice to all medical specialty boards.
While SHM supports the ABP response to the PHM petition, it is clear that excellent physicians caring for hospitalized children will be unable to achieve PHM board certification for a variety of reasons. For these physicians who are not PHM board certified as pediatric hospitalists by the ABP, SHM supports providing these physicians with recognition as hospitalists. These include “niche” hospitalists, such as newborn hospitalists, subacute hospitalists, and subspecialty hospitalists. SHM will also continue to support and recognize community-based hospitalists, family medicine-trained hospitalists, and Med-Peds hospitalists whose practice may not comply with criteria laid out by the ABP. For these physicians, receiving Fellow designation through SHM, a merit-based distinction requiring demonstration of clinical excellence and commitment to hospital medicine, is another route whereby physicians can achieve designation as a hospitalist.
FUTURE DIRECTIONS FOR PEDIATRIC HOSPITALISTS
SHM supports future efforts by the ABP to be vigilant for bias of any sort in the certification process. Other future considerations for the PHM community include the possibility of a focused practice pathway in hospital medicine (FPHM) for pediatrics as is currently jointly offered by the American Board of Internal Medicine (ABIM) and the American Board of Family Medicine (ABFM). This maintenance of certification program is a variation of internal medicine or family medicine recertification, not a subspecialty, but allows physicians practicing primarily in inpatient settings to focus continuing education efforts on skills and attitudes needed for inpatient practice.9 While this possibility was discounted by the ABP in the past based on initially low numbers of physicians choosing this pathway, this pathway has grown from initially attracting 150 internal medicine applicants yearly to 265 in 2015.10 The ABMS approved the ABIM/ABFM FPHM as its first approved designation in March 2017 after more than 2,500 physicians earned this designation.11 Of the >2,800 pediatric residency graduates (not including combined programs) each year, 10% report planning on becoming pediatric hospitalists,12 and currently only 72-74 fellows graduate from PHM fellowships yearly.13 FPHM for pediatric hospital medicine would provide focused maintenance of certification and hospitalist designation for those who cannot match to fellowship programs.
Acknowledgments
The authors would like to acknowledge the input and support from the Executive Council of the Society of Hospital Medicine Pediatric Special Interest Group in writing this statement.
Disclosures
Dr. Chang served as an author of the Pediatric Hospital Medicine Petition to the American Board of Pediatrics for Subspecialty Certification. Drs. Hopkins, Rehm, Gage, and Shen have nothing to disclose.
The first Pediatric Hospital Medicine (PHM) fellowships in the United States were established in 2003;1 and since then, the field has expanded and matured dramatically. This growth, accompanied by greater definition of the role and recommended competencies of pediatric hospitalists,2 culminated in the submission of a petition to the American Board of Pediatrics (ABP) in August 2014 to consider recognition of PHM as a new pediatric subspecialty.3 After an 18-month iterative process requiring extensive input from the Joint Council of Pediatric Hospital Medicine, ABP subcommittees, the Association of Medical School Pediatric Department Chairs, the Association of Pediatric Program Directors, and other prominent pediatric professional societies, the ABP voted in December 2015 to recommend that the American Board of Medical Subspecialties (ABMS) recognize PHM as a new subspecialty.3
The ABP subsequently announced three pathways for board certification in PHM:
- Training pathway for those completing an Accreditation Council for Graduate Medical Education–accredited two-year PHM fellowship program;
- Practice pathway for those satisfying ABP criteria for clinical activity in PHM for four years prior to exam dates (in 2019, 2021, and 2023), initially described as “direct patient care of hospitalized children ≥25% full-time equivalent (FTE) defined as ≥450-500 hours per year every year for the preceding four years”;4
- Combined pathway for those completing less than two years of fellowship, who would be required to complete two years of practice experience that satisfy the same criteria as each year of the practice pathway.5
While the training pathway met near-uniform acceptance, concerns were raised through the American Academy of Pediatrics Section of Hospital Medicine (AAP SOHM) Listserv regarding the practice pathway, and by extension, the combined pathway. Specifically, language describing the necessary characteristics of acceptable PHM practice was felt to be vague and not transparent. Listserv posts also raised concerns regarding the potential exclusion of “niche” practices such as subspecialty hospitalists and newborn hospitalists. As applicants in the practice pathway began to receive denials, opinions voiced in listserv posts were increasingly critical of the ABP’s lack of transparency regarding the specific criteria adjudicating applications.
ORIGIN OF THE PHM PETITION
A group of hospitalists, led by Dr. David Skey, a pediatric hospitalist at Arnold Palmer Children’s Hospital in Orlando, Florida, created a petition which was submitted to the ABP on August 6, 2019, and raised the following issues:
- “A perception of unfairness/bias in the practice pathway criteria and the way these criteria have been applied.
- Denials based on gaps in employment without reasonable consideration of mitigating factors.
- Lack of transparency, accountability, and responsiveness from the ABP.”6
The petition, posted on the AAP SOHM listserv and signed by 1,479 individuals,7 raised concerns of anecdotal evidence that the practice pathway criteria disproportionately disadvantaged women, although intentional bias was not suspected by the signers of the letter. The petition’s signers submitted the following demands to the ABP:
- “Facilitate a timely analysis to determine if gender bias is present or perform this analysis internally and release the findings publicly.
- Revise the practice pathway criteria to be more inclusive of applicants with interrupted practice and varied clinical experience, to include clear-cut parameters rather than considering these applications on a closed-door ‘case-by-case basis...at the discretion of the ABP’.
- Clarify the appeals process and improve responsiveness to appeals and inquiries regarding denials.
- Provide a formal response to this petition letter through the PHM ListServ and/or the ABP website within one week of receiving the signed petition.”6
THE ABP RESPONSE TO THE PHM PETITION
A formal response to the petition was released on the AAP SOHM Listserv on August 29, 2019, to address the concerns raised and is published in this issue of the Journal of Hospital Medicine.4 In response to the allegation of gender bias, the ABP maintained that the data did not support this, as the denial rate for females (4.0%) was not significantly different than that for males (3.7%). The response acknowledged that once clear-cut criteria were decided upon to augment the general practice pathway criteria published at the outset, these criteria should have been disseminated. The ABP maintained, however, that these criteria, once established, were used consistently in adjudicating all applications. To clarify and simplify the eligibility criteria, the percentage of the full-time equivalent and practice interruption criteria were removed, as the work-hours criteria (direct patient care of hospitalized children ≥450-500 hours per year every year for the preceding four years)8 were deemed sufficient to ensure adequate clinical participation.
SHM’S POSITION REGARDING THE PHM PETITION AND ABP RESPONSE
The Society of Hospital Medicine (SHM), through pediatric hospitalists and pediatricians on its Board, committees, and the Executive Council of the Pediatric Special Interest Group, has followed with great interest the public debate surrounding the PHM certification process and the subsequent PHM petition to the ABP. The ABP responded swiftly and with full transparency to the petition, and SHM supports these efforts by the ABP to provide a timely, honest, data-driven response to the concerns raised by the PHM petition. SHM recognizes that the mission of the ABP is to provide the public with confidence that physicians with ABP board certifications meet appropriate “standards of excellence”. While the revisions implemented by the ABP in its response still may not satisfy the concerns of all members of the PHM community, SHM recognizes that the revised requirements remain true to the mission of the ABP.
SHM applauds the authors and signatories of the PHM petition for bravely raising their concerns of gender bias and lack of transparency. The response of the ABP to this petition by further improving transparency serves as an example of continuous improvement in collaborative practice to all medical specialty boards.
While SHM supports the ABP response to the PHM petition, it is clear that excellent physicians caring for hospitalized children will be unable to achieve PHM board certification for a variety of reasons. For these physicians who are not PHM board certified as pediatric hospitalists by the ABP, SHM supports providing these physicians with recognition as hospitalists. These include “niche” hospitalists, such as newborn hospitalists, subacute hospitalists, and subspecialty hospitalists. SHM will also continue to support and recognize community-based hospitalists, family medicine-trained hospitalists, and Med-Peds hospitalists whose practice may not comply with criteria laid out by the ABP. For these physicians, receiving Fellow designation through SHM, a merit-based distinction requiring demonstration of clinical excellence and commitment to hospital medicine, is another route whereby physicians can achieve designation as a hospitalist.
FUTURE DIRECTIONS FOR PEDIATRIC HOSPITALISTS
SHM supports future efforts by the ABP to be vigilant for bias of any sort in the certification process. Other future considerations for the PHM community include the possibility of a focused practice pathway in hospital medicine (FPHM) for pediatrics as is currently jointly offered by the American Board of Internal Medicine (ABIM) and the American Board of Family Medicine (ABFM). This maintenance of certification program is a variation of internal medicine or family medicine recertification, not a subspecialty, but allows physicians practicing primarily in inpatient settings to focus continuing education efforts on skills and attitudes needed for inpatient practice.9 While this possibility was discounted by the ABP in the past based on initially low numbers of physicians choosing this pathway, this pathway has grown from initially attracting 150 internal medicine applicants yearly to 265 in 2015.10 The ABMS approved the ABIM/ABFM FPHM as its first approved designation in March 2017 after more than 2,500 physicians earned this designation.11 Of the >2,800 pediatric residency graduates (not including combined programs) each year, 10% report planning on becoming pediatric hospitalists,12 and currently only 72-74 fellows graduate from PHM fellowships yearly.13 FPHM for pediatric hospital medicine would provide focused maintenance of certification and hospitalist designation for those who cannot match to fellowship programs.
Acknowledgments
The authors would like to acknowledge the input and support from the Executive Council of the Society of Hospital Medicine Pediatric Special Interest Group in writing this statement.
Disclosures
Dr. Chang served as an author of the Pediatric Hospital Medicine Petition to the American Board of Pediatrics for Subspecialty Certification. Drs. Hopkins, Rehm, Gage, and Shen have nothing to disclose.
1. Freed GL, Dunham KM, Research Advisory Committee of the American Board of P. Characteristics of pediatric hospital medicine fellowships and training programs. J Hosp Med. 2009;4(3):157-163. https://doi.org/10.1002/jhm.409.
2. Stucky ER, Maniscalco J, Ottolini MC, et al. The Pediatric Hospital Medicine Core Competencies Supplement: a Framework for Curriculum Development by the Society of Hospital Medicine with acknowledgement to pediatric hospitalists from the American Academy of Pediatrics and the Academic Pediatric Association. J Hosp Med. 2010;5 Suppl 2:i-xv, 1-114. https://doi.org/10.1002/jhm.776.
3. Barrett DJ, McGuinness GA, Cunha CA, et al. Pediatric Hospital Medicine: A Proposed New Subspecialty. Pediatrics. 2017;139(3). https://doi.org/10.1542/peds.2016-1823.
4. Nichols DG WS. The American Board of Pediatrics response to the Pediatric Hospital Medicine petition. J Hosp Med. 2019;14(10):586-588. https://doi.org/10.12788/jhm.3322.
5. Pediatric hospital medicine certification. American Board of Pediatrics. https://www.abp.org/content/pediatric-hospital-medicine-certification#training. Accessed 3 September, 2019.
6. Skey D. Pediatric Hospitalists, It’s time to take a stand on the PHM Boards Application Process! Five Dog Development, LLC. https://www.phmpetition.com/. Accessed 3 September, 2019.
7. Skey D. Petition Update. In: AAP SOHM Listserv: American Academy of Pediatrics; 2019.
8. The American Board of Pediatrics Response to the Pediatric Hospital Medicine Petition. The American Board of Pediatrics. https://www.abp.org/sites/abp/files/phm-petition-response.pdf. Published 2019. Accessed September 4, 2019.
9. Focused practice in hospital medicine. American Board of Internal Medicine. https://www.abim.org/maintenance-of-certification/moc-requirements/focused-practice-hospital-medicine.aspx. Published 2019 Accessed September 4, 2019.
10. Butterfield S. Following the focused practice pathway. American College of Physicians. Your career Web site. https://acphospitalist.org/archives/2016/09/focused-practice-hospital-medicine.htm. Published 2016. Accessed September 4, 2019.
11. American Board of Medical Specialties Announces New, Focused Practice Designation [press release]. American Board of Medical Specialties, 14 Mar 2017.
12. Leyenaar JK, Frintner MP. Graduating Pediatric Residents Entering the Hospital Medicine Workforce, 2006-2015. Acad Pediatr. 2018;18(2):200-207. https://doi.org/10.1016/j.acap.2017.05.001.
13. PHM Fellowship Programs. PHMFellows.org. http://phmfellows.org/phm-programs/. Published 2019. Accessed September 4, 2019.
1. Freed GL, Dunham KM, Research Advisory Committee of the American Board of P. Characteristics of pediatric hospital medicine fellowships and training programs. J Hosp Med. 2009;4(3):157-163. https://doi.org/10.1002/jhm.409.
2. Stucky ER, Maniscalco J, Ottolini MC, et al. The Pediatric Hospital Medicine Core Competencies Supplement: a Framework for Curriculum Development by the Society of Hospital Medicine with acknowledgement to pediatric hospitalists from the American Academy of Pediatrics and the Academic Pediatric Association. J Hosp Med. 2010;5 Suppl 2:i-xv, 1-114. https://doi.org/10.1002/jhm.776.
3. Barrett DJ, McGuinness GA, Cunha CA, et al. Pediatric Hospital Medicine: A Proposed New Subspecialty. Pediatrics. 2017;139(3). https://doi.org/10.1542/peds.2016-1823.
4. Nichols DG WS. The American Board of Pediatrics response to the Pediatric Hospital Medicine petition. J Hosp Med. 2019;14(10):586-588. https://doi.org/10.12788/jhm.3322.
5. Pediatric hospital medicine certification. American Board of Pediatrics. https://www.abp.org/content/pediatric-hospital-medicine-certification#training. Accessed 3 September, 2019.
6. Skey D. Pediatric Hospitalists, It’s time to take a stand on the PHM Boards Application Process! Five Dog Development, LLC. https://www.phmpetition.com/. Accessed 3 September, 2019.
7. Skey D. Petition Update. In: AAP SOHM Listserv: American Academy of Pediatrics; 2019.
8. The American Board of Pediatrics Response to the Pediatric Hospital Medicine Petition. The American Board of Pediatrics. https://www.abp.org/sites/abp/files/phm-petition-response.pdf. Published 2019. Accessed September 4, 2019.
9. Focused practice in hospital medicine. American Board of Internal Medicine. https://www.abim.org/maintenance-of-certification/moc-requirements/focused-practice-hospital-medicine.aspx. Published 2019 Accessed September 4, 2019.
10. Butterfield S. Following the focused practice pathway. American College of Physicians. Your career Web site. https://acphospitalist.org/archives/2016/09/focused-practice-hospital-medicine.htm. Published 2016. Accessed September 4, 2019.
11. American Board of Medical Specialties Announces New, Focused Practice Designation [press release]. American Board of Medical Specialties, 14 Mar 2017.
12. Leyenaar JK, Frintner MP. Graduating Pediatric Residents Entering the Hospital Medicine Workforce, 2006-2015. Acad Pediatr. 2018;18(2):200-207. https://doi.org/10.1016/j.acap.2017.05.001.
13. PHM Fellowship Programs. PHMFellows.org. http://phmfellows.org/phm-programs/. Published 2019. Accessed September 4, 2019.
© 2019 Society of Hospital Medicine
Could home care replace inpatient HSCT?
Can receiving all posttransplant care at home benefit patients undergoing hematopoietic stem cell transplant (HSCT)? Researchers are conducting phase 2 trials to find out.
Nelson Chao, MD, and colleagues at Duke University in Durham, N.C., completed a phase 1 trial that suggested post-HSCT care at home was feasible and safe (Blood. 2017;130:745).
Now, the team is conducting phase 2 trials – NCT01725022 and NCT02218151 – comparing patients who receive all posttransplant care at home with patients treated in the hospital or in the outpatient setting with daily visits to the clinic.
The main goal is to determine if allogeneic HSCT recipients treated at home can maintain their normal microbiome and, as a result, have a lower risk of graft-versus-host disease (GVHD). The researchers are also looking at other outcomes such as quality of life, treatment-related morbidities and mortality, and the cost of care for both allogeneic and autologous transplant recipients.
To be eligible for home care after HSCT, a patient must live within a 90-minute driving distance of Duke and have a caregiver available at home. The patient’s home must pass an inspection, showing it to be free of sources for potential infection, such as mold or pets that sleep in the patient’s bed.
When the time comes for treatment, the patient receives conditioning at the hospital but can return home the day before or the day of transplant. After discharge, the patient is visited by a nurse practitioner or physician assistant each morning for a physical examination and blood draw.
In the afternoon, the patient is visited by a clinic nurse who brings any necessary supplies or treatments, such as blood products or intravenous antibiotics. The patient also has daily video calls with an attending physician and can be admitted to the hospital for any events that cannot be managed in the home setting.
Patients can have visitors and spend time away from home, but precautions are necessary. Friends or family who are sick should not be allowed to visit, and patients should avoid crowds when they go out.

Initial findings
The Duke team has treated 41 HSCT recipients at home so far. Dr. Chao said it’s still too early to draw any conclusions about differences in outcomes between home care and inpatient/outpatient HSCT.
However, a preliminary analysis of costs suggests home care is cheaper than inpatient HSCT. The researchers found that, for the first several transplants, at day 60, the cost of home care was roughly half that of inpatient HSCT.
In addition, patients seem to be happy with posttransplant care at home.
“The patients love being at home, in their own environment, with their families,” Dr. Chao said. “Almost every single patient [in the phase 1 trial] said that he or she liked it much better. There was one patient in the phase 1 that felt a little isolated, and I can see why because we say, ‘You can stay home, but don’t have a whole lot of people in.’ ”
One patient’s experience
Beth Vanderkin said it was “a blessing” to receive care at home after undergoing HSCT at Duke.
Ms. Vanderkin was diagnosed with diffuse large B-cell lymphoma in 2014. After two chemotherapy regimens failed to shrink the tumor in her chest, she underwent radiotherapy and responded well. When a PET scan revealed the tumor had gone completely, she proceeded to transplant.
She received a haploidentical HSCT using cells donated by her eldest daughter, Hannah Eichhorst. Ms. Vanderkin received the transplant in the hospital, and for 2 weeks after that, she made daily visits to the transplant clinic.
After those 2 weeks, Ms. Vanderkin continued her treatment at home. Like other patients eligible for home care, Ms. Vanderkin lived close to Duke, had a caregiver available, and had passed a home inspection. The Duke team shipped the needed medical supplies to her house and arranged twice-daily visits from nurses and daily video calls with a doctor.
Ms. Vanderkin said receiving care at home was “a game changer.” She derived comfort from recovering in her own environment, could spend more time with her family, and didn’t have to miss special events. While receiving care at home, Ms. Vanderkin attended the homecoming event where her son, Josiah, was part of the court. Wearing a face mask and carrying a portable pump in her purse, Ms. Vanderkin joined other mothers in escorting their children onto the football field.
“I got to escort my son out onto the field, and he was crowned king that night,” Ms. Vanderkin said. “I didn’t do a lot of things [while receiving care at home], but there were things I didn’t have to miss because I was at home and not in the hospital.”
Ms. Vanderkin said home care was also beneficial for her husband, who was her caregiver. Thomas Vanderkin was able to work from home while caring for his wife, and the daily nurses’ visits allowed him to run errands without having to leave Ms. Vanderkin alone.
Since her experience with home care, Ms. Vanderkin has spent many more days in the hospital and clinic. She experienced a relapse after the transplant and went on to receive more chemotherapy as well as ipilimumab. She responded to that treatment and has now been cancer-free for 3 years.
The ipilimumab did cause side effects, including intestinal problems that resulted in the need for parenteral nutrition. This side effect was made more bearable, Ms. Vanderkin said, because she was able to receive the parenteral nutrition at home. She and her husband were comfortable with additional home care because of their positive experience with posttransplant care.
“I think we’re conditioned to think that, to receive the best care, we have to be sitting in a hospital room or a clinic, but I think there’s a lot of things we can probably do at home,” Ms. Vanderkin said. “And we might fare a lot better as patients if we’re in an environment that we feel comfortable in.”
Experience at other centers
The team at Duke is not the first to study HSCT care at home. In fact, researchers in Sweden have been studying posttransplant home care since 1998.
A pilot trial the group published in 2000 suggested that home care was safe and, in some ways, superior to inpatient HSCT (Bone Marrow Transplant. 2000 Nov;26[10]:1057-60). Patients treated at home had a lower rate of bacteremia, fewer days of total parenteral nutrition, fewer erythrocyte transfusions, and fewer days on antibiotics and analgesics. Rates of fever, engraftment time, and acute GVHD were similar between the inpatient and home-care groups.
A study published by the same researchers in 2002 showed that patients who received home care had lower rates of grade 2-4 acute GVHD and transplant-related mortality compared to inpatients (Blood. 2002 Dec 15;100[13]:4317-24). Two-year overall survival was superior with home care as well.
On the other hand, a study the group published in 2013 showed no significant differences in 5-year survival, transplant-related mortality, relapse, or chronic GVHD between inpatients and those who received care at home (Biol Blood Marrow Transplant. 2013. doi: 10.1016/j.bbmt.2012.11.5189).
The phase 2 trials at Duke should provide more insight into patient outcomes, but results probably won’t be available for 2 more years, Dr. Chao said.
In the meantime, other U.S. researchers are studying home care as well. Memorial Sloan Kettering Cancer Center is conducting a pilot study to determine if HSCT care at home is feasible (NCT02671448).
Dr. Chao said home care should be possible for other centers, particularly those that already perform outpatient HSCT.
“Having the outpatient infrastructure to support these patients is a big step,” he said. “And I think we were able to do that mainly because we do most of our transplants in the outpatient setting already. So that jump to the home is a little less compared to a center that does no outpatient transplants.”
He added, “There’s a certain amount of inertia to overcome and a certain amount of apprehension from the caregivers initially because [patients aren’t] sitting in your unit all the time, but I don’t see this as a huge barrier.”
In fact, Dr. Chao said, if results with home care are favorable, it could potentially replace inpatient HSCT for certain patients.
Dr. Chao’s research is supported by Duke University, and he reported having no relevant financial disclosures.
Can receiving all posttransplant care at home benefit patients undergoing hematopoietic stem cell transplant (HSCT)? Researchers are conducting phase 2 trials to find out.
Nelson Chao, MD, and colleagues at Duke University in Durham, N.C., completed a phase 1 trial that suggested post-HSCT care at home was feasible and safe (Blood. 2017;130:745).
Now, the team is conducting phase 2 trials – NCT01725022 and NCT02218151 – comparing patients who receive all posttransplant care at home with patients treated in the hospital or in the outpatient setting with daily visits to the clinic.
The main goal is to determine if allogeneic HSCT recipients treated at home can maintain their normal microbiome and, as a result, have a lower risk of graft-versus-host disease (GVHD). The researchers are also looking at other outcomes such as quality of life, treatment-related morbidities and mortality, and the cost of care for both allogeneic and autologous transplant recipients.
To be eligible for home care after HSCT, a patient must live within a 90-minute driving distance of Duke and have a caregiver available at home. The patient’s home must pass an inspection, showing it to be free of sources for potential infection, such as mold or pets that sleep in the patient’s bed.
When the time comes for treatment, the patient receives conditioning at the hospital but can return home the day before or the day of transplant. After discharge, the patient is visited by a nurse practitioner or physician assistant each morning for a physical examination and blood draw.
In the afternoon, the patient is visited by a clinic nurse who brings any necessary supplies or treatments, such as blood products or intravenous antibiotics. The patient also has daily video calls with an attending physician and can be admitted to the hospital for any events that cannot be managed in the home setting.
Patients can have visitors and spend time away from home, but precautions are necessary. Friends or family who are sick should not be allowed to visit, and patients should avoid crowds when they go out.

Initial findings
The Duke team has treated 41 HSCT recipients at home so far. Dr. Chao said it’s still too early to draw any conclusions about differences in outcomes between home care and inpatient/outpatient HSCT.
However, a preliminary analysis of costs suggests home care is cheaper than inpatient HSCT. The researchers found that, for the first several transplants, at day 60, the cost of home care was roughly half that of inpatient HSCT.
In addition, patients seem to be happy with posttransplant care at home.
“The patients love being at home, in their own environment, with their families,” Dr. Chao said. “Almost every single patient [in the phase 1 trial] said that he or she liked it much better. There was one patient in the phase 1 that felt a little isolated, and I can see why because we say, ‘You can stay home, but don’t have a whole lot of people in.’ ”
One patient’s experience
Beth Vanderkin said it was “a blessing” to receive care at home after undergoing HSCT at Duke.
Ms. Vanderkin was diagnosed with diffuse large B-cell lymphoma in 2014. After two chemotherapy regimens failed to shrink the tumor in her chest, she underwent radiotherapy and responded well. When a PET scan revealed the tumor had gone completely, she proceeded to transplant.
She received a haploidentical HSCT using cells donated by her eldest daughter, Hannah Eichhorst. Ms. Vanderkin received the transplant in the hospital, and for 2 weeks after that, she made daily visits to the transplant clinic.
After those 2 weeks, Ms. Vanderkin continued her treatment at home. Like other patients eligible for home care, Ms. Vanderkin lived close to Duke, had a caregiver available, and had passed a home inspection. The Duke team shipped the needed medical supplies to her house and arranged twice-daily visits from nurses and daily video calls with a doctor.
Ms. Vanderkin said receiving care at home was “a game changer.” She derived comfort from recovering in her own environment, could spend more time with her family, and didn’t have to miss special events. While receiving care at home, Ms. Vanderkin attended the homecoming event where her son, Josiah, was part of the court. Wearing a face mask and carrying a portable pump in her purse, Ms. Vanderkin joined other mothers in escorting their children onto the football field.
“I got to escort my son out onto the field, and he was crowned king that night,” Ms. Vanderkin said. “I didn’t do a lot of things [while receiving care at home], but there were things I didn’t have to miss because I was at home and not in the hospital.”
Ms. Vanderkin said home care was also beneficial for her husband, who was her caregiver. Thomas Vanderkin was able to work from home while caring for his wife, and the daily nurses’ visits allowed him to run errands without having to leave Ms. Vanderkin alone.
Since her experience with home care, Ms. Vanderkin has spent many more days in the hospital and clinic. She experienced a relapse after the transplant and went on to receive more chemotherapy as well as ipilimumab. She responded to that treatment and has now been cancer-free for 3 years.
The ipilimumab did cause side effects, including intestinal problems that resulted in the need for parenteral nutrition. This side effect was made more bearable, Ms. Vanderkin said, because she was able to receive the parenteral nutrition at home. She and her husband were comfortable with additional home care because of their positive experience with posttransplant care.
“I think we’re conditioned to think that, to receive the best care, we have to be sitting in a hospital room or a clinic, but I think there’s a lot of things we can probably do at home,” Ms. Vanderkin said. “And we might fare a lot better as patients if we’re in an environment that we feel comfortable in.”
Experience at other centers
The team at Duke is not the first to study HSCT care at home. In fact, researchers in Sweden have been studying posttransplant home care since 1998.
A pilot trial the group published in 2000 suggested that home care was safe and, in some ways, superior to inpatient HSCT (Bone Marrow Transplant. 2000 Nov;26[10]:1057-60). Patients treated at home had a lower rate of bacteremia, fewer days of total parenteral nutrition, fewer erythrocyte transfusions, and fewer days on antibiotics and analgesics. Rates of fever, engraftment time, and acute GVHD were similar between the inpatient and home-care groups.
A study published by the same researchers in 2002 showed that patients who received home care had lower rates of grade 2-4 acute GVHD and transplant-related mortality compared to inpatients (Blood. 2002 Dec 15;100[13]:4317-24). Two-year overall survival was superior with home care as well.
On the other hand, a study the group published in 2013 showed no significant differences in 5-year survival, transplant-related mortality, relapse, or chronic GVHD between inpatients and those who received care at home (Biol Blood Marrow Transplant. 2013. doi: 10.1016/j.bbmt.2012.11.5189).
The phase 2 trials at Duke should provide more insight into patient outcomes, but results probably won’t be available for 2 more years, Dr. Chao said.
In the meantime, other U.S. researchers are studying home care as well. Memorial Sloan Kettering Cancer Center is conducting a pilot study to determine if HSCT care at home is feasible (NCT02671448).
Dr. Chao said home care should be possible for other centers, particularly those that already perform outpatient HSCT.
“Having the outpatient infrastructure to support these patients is a big step,” he said. “And I think we were able to do that mainly because we do most of our transplants in the outpatient setting already. So that jump to the home is a little less compared to a center that does no outpatient transplants.”
He added, “There’s a certain amount of inertia to overcome and a certain amount of apprehension from the caregivers initially because [patients aren’t] sitting in your unit all the time, but I don’t see this as a huge barrier.”
In fact, Dr. Chao said, if results with home care are favorable, it could potentially replace inpatient HSCT for certain patients.
Dr. Chao’s research is supported by Duke University, and he reported having no relevant financial disclosures.
Can receiving all posttransplant care at home benefit patients undergoing hematopoietic stem cell transplant (HSCT)? Researchers are conducting phase 2 trials to find out.
Nelson Chao, MD, and colleagues at Duke University in Durham, N.C., completed a phase 1 trial that suggested post-HSCT care at home was feasible and safe (Blood. 2017;130:745).
Now, the team is conducting phase 2 trials – NCT01725022 and NCT02218151 – comparing patients who receive all posttransplant care at home with patients treated in the hospital or in the outpatient setting with daily visits to the clinic.
The main goal is to determine if allogeneic HSCT recipients treated at home can maintain their normal microbiome and, as a result, have a lower risk of graft-versus-host disease (GVHD). The researchers are also looking at other outcomes such as quality of life, treatment-related morbidities and mortality, and the cost of care for both allogeneic and autologous transplant recipients.
To be eligible for home care after HSCT, a patient must live within a 90-minute driving distance of Duke and have a caregiver available at home. The patient’s home must pass an inspection, showing it to be free of sources for potential infection, such as mold or pets that sleep in the patient’s bed.
When the time comes for treatment, the patient receives conditioning at the hospital but can return home the day before or the day of transplant. After discharge, the patient is visited by a nurse practitioner or physician assistant each morning for a physical examination and blood draw.
In the afternoon, the patient is visited by a clinic nurse who brings any necessary supplies or treatments, such as blood products or intravenous antibiotics. The patient also has daily video calls with an attending physician and can be admitted to the hospital for any events that cannot be managed in the home setting.
Patients can have visitors and spend time away from home, but precautions are necessary. Friends or family who are sick should not be allowed to visit, and patients should avoid crowds when they go out.

Initial findings
The Duke team has treated 41 HSCT recipients at home so far. Dr. Chao said it’s still too early to draw any conclusions about differences in outcomes between home care and inpatient/outpatient HSCT.
However, a preliminary analysis of costs suggests home care is cheaper than inpatient HSCT. The researchers found that, for the first several transplants, at day 60, the cost of home care was roughly half that of inpatient HSCT.
In addition, patients seem to be happy with posttransplant care at home.
“The patients love being at home, in their own environment, with their families,” Dr. Chao said. “Almost every single patient [in the phase 1 trial] said that he or she liked it much better. There was one patient in the phase 1 that felt a little isolated, and I can see why because we say, ‘You can stay home, but don’t have a whole lot of people in.’ ”
One patient’s experience
Beth Vanderkin said it was “a blessing” to receive care at home after undergoing HSCT at Duke.
Ms. Vanderkin was diagnosed with diffuse large B-cell lymphoma in 2014. After two chemotherapy regimens failed to shrink the tumor in her chest, she underwent radiotherapy and responded well. When a PET scan revealed the tumor had gone completely, she proceeded to transplant.
She received a haploidentical HSCT using cells donated by her eldest daughter, Hannah Eichhorst. Ms. Vanderkin received the transplant in the hospital, and for 2 weeks after that, she made daily visits to the transplant clinic.
After those 2 weeks, Ms. Vanderkin continued her treatment at home. Like other patients eligible for home care, Ms. Vanderkin lived close to Duke, had a caregiver available, and had passed a home inspection. The Duke team shipped the needed medical supplies to her house and arranged twice-daily visits from nurses and daily video calls with a doctor.
Ms. Vanderkin said receiving care at home was “a game changer.” She derived comfort from recovering in her own environment, could spend more time with her family, and didn’t have to miss special events. While receiving care at home, Ms. Vanderkin attended the homecoming event where her son, Josiah, was part of the court. Wearing a face mask and carrying a portable pump in her purse, Ms. Vanderkin joined other mothers in escorting their children onto the football field.
“I got to escort my son out onto the field, and he was crowned king that night,” Ms. Vanderkin said. “I didn’t do a lot of things [while receiving care at home], but there were things I didn’t have to miss because I was at home and not in the hospital.”
Ms. Vanderkin said home care was also beneficial for her husband, who was her caregiver. Thomas Vanderkin was able to work from home while caring for his wife, and the daily nurses’ visits allowed him to run errands without having to leave Ms. Vanderkin alone.
Since her experience with home care, Ms. Vanderkin has spent many more days in the hospital and clinic. She experienced a relapse after the transplant and went on to receive more chemotherapy as well as ipilimumab. She responded to that treatment and has now been cancer-free for 3 years.
The ipilimumab did cause side effects, including intestinal problems that resulted in the need for parenteral nutrition. This side effect was made more bearable, Ms. Vanderkin said, because she was able to receive the parenteral nutrition at home. She and her husband were comfortable with additional home care because of their positive experience with posttransplant care.
“I think we’re conditioned to think that, to receive the best care, we have to be sitting in a hospital room or a clinic, but I think there’s a lot of things we can probably do at home,” Ms. Vanderkin said. “And we might fare a lot better as patients if we’re in an environment that we feel comfortable in.”
Experience at other centers
The team at Duke is not the first to study HSCT care at home. In fact, researchers in Sweden have been studying posttransplant home care since 1998.
A pilot trial the group published in 2000 suggested that home care was safe and, in some ways, superior to inpatient HSCT (Bone Marrow Transplant. 2000 Nov;26[10]:1057-60). Patients treated at home had a lower rate of bacteremia, fewer days of total parenteral nutrition, fewer erythrocyte transfusions, and fewer days on antibiotics and analgesics. Rates of fever, engraftment time, and acute GVHD were similar between the inpatient and home-care groups.
A study published by the same researchers in 2002 showed that patients who received home care had lower rates of grade 2-4 acute GVHD and transplant-related mortality compared to inpatients (Blood. 2002 Dec 15;100[13]:4317-24). Two-year overall survival was superior with home care as well.
On the other hand, a study the group published in 2013 showed no significant differences in 5-year survival, transplant-related mortality, relapse, or chronic GVHD between inpatients and those who received care at home (Biol Blood Marrow Transplant. 2013. doi: 10.1016/j.bbmt.2012.11.5189).
The phase 2 trials at Duke should provide more insight into patient outcomes, but results probably won’t be available for 2 more years, Dr. Chao said.
In the meantime, other U.S. researchers are studying home care as well. Memorial Sloan Kettering Cancer Center is conducting a pilot study to determine if HSCT care at home is feasible (NCT02671448).
Dr. Chao said home care should be possible for other centers, particularly those that already perform outpatient HSCT.
“Having the outpatient infrastructure to support these patients is a big step,” he said. “And I think we were able to do that mainly because we do most of our transplants in the outpatient setting already. So that jump to the home is a little less compared to a center that does no outpatient transplants.”
He added, “There’s a certain amount of inertia to overcome and a certain amount of apprehension from the caregivers initially because [patients aren’t] sitting in your unit all the time, but I don’t see this as a huge barrier.”
In fact, Dr. Chao said, if results with home care are favorable, it could potentially replace inpatient HSCT for certain patients.
Dr. Chao’s research is supported by Duke University, and he reported having no relevant financial disclosures.
Drug abuse–linked infective endocarditis spiking in U.S.
Hospitalizations for infective endocarditis associated with drug abuse doubled in the United States from 2002 to 2016, in a trend investigators call “alarming,” and link to a concurrent rise in opioid abuse.
Patients tend to be younger, poorer white males, according to findings published online in the Journal of the American Heart Association.
For their research, Amer N. Kadri, MD, of the Cleveland Clinic and colleagues looked at records for nearly a million hospitalizations for infective endocarditis (IE) in the National Inpatient Sample registry. All U.S. regions saw increases in drug abuse–linked cases of IE as a share of IE hospitalizations. Incidence of drug abuse–associated IC rose from 48 cases/100,000 population in 2002 to 79/100,000 in 2016. The Midwest saw the highest rate of change, with an annual percent increase of 4.9%.
While most IE hospitalizations in the study cohort were of white men (including 68% for drug-linked cases), the drug abuse–related cases were younger (median age, 38 vs. 70 years for nondrug-related IE), and more likely male (55.5% vs. 50%). About 45% of the drug-related cases were in people receiving Medicaid, and 42% were in the lowest quartile of median household income.
The drug abuse cases had fewer renal and cardiovascular comorbidities, compared with the nondrug cases, but were significantly more likely to present with HIV, hepatitis C, alcohol abuse, and liver disease. Inpatient mortality was lower among the drug-linked cases – 6% vs. 9% – but the drug cases saw significantly more cardiac or valve surgeries, longer hospital stays, and higher costs.
“Hospitalizations for IE have been increasing side by side with the opioid epidemic,” the investigators wrote in their analysis. “The opioid crisis has reached epidemic levels, and now drug overdoses have been the leading cause of injury-related death in the U.S. Heroin deaths had remained relatively low from 1999 until 2010 whereas it then increased threefold from 2010-2015.” The analysis showed a rise in drug abuse–associated IE “that corresponds to this general period.” The findings argue, the investigators said, for better treatment for opioid addiction after hospitalization and greater efforts to make drug rehabilitation available after discharge. The researchers described as a limitation of their study the use of billing codes that changed late in the study period, increasing detection of drug abuse cases after 2015. They reported no outside funding or conflicts of interest.
SOURCE: Kadri AN et al. J Am Heart Assoc. 2019 Sep 18.
Hospitalizations for infective endocarditis associated with drug abuse doubled in the United States from 2002 to 2016, in a trend investigators call “alarming,” and link to a concurrent rise in opioid abuse.
Patients tend to be younger, poorer white males, according to findings published online in the Journal of the American Heart Association.
For their research, Amer N. Kadri, MD, of the Cleveland Clinic and colleagues looked at records for nearly a million hospitalizations for infective endocarditis (IE) in the National Inpatient Sample registry. All U.S. regions saw increases in drug abuse–linked cases of IE as a share of IE hospitalizations. Incidence of drug abuse–associated IC rose from 48 cases/100,000 population in 2002 to 79/100,000 in 2016. The Midwest saw the highest rate of change, with an annual percent increase of 4.9%.
While most IE hospitalizations in the study cohort were of white men (including 68% for drug-linked cases), the drug abuse–related cases were younger (median age, 38 vs. 70 years for nondrug-related IE), and more likely male (55.5% vs. 50%). About 45% of the drug-related cases were in people receiving Medicaid, and 42% were in the lowest quartile of median household income.
The drug abuse cases had fewer renal and cardiovascular comorbidities, compared with the nondrug cases, but were significantly more likely to present with HIV, hepatitis C, alcohol abuse, and liver disease. Inpatient mortality was lower among the drug-linked cases – 6% vs. 9% – but the drug cases saw significantly more cardiac or valve surgeries, longer hospital stays, and higher costs.
“Hospitalizations for IE have been increasing side by side with the opioid epidemic,” the investigators wrote in their analysis. “The opioid crisis has reached epidemic levels, and now drug overdoses have been the leading cause of injury-related death in the U.S. Heroin deaths had remained relatively low from 1999 until 2010 whereas it then increased threefold from 2010-2015.” The analysis showed a rise in drug abuse–associated IE “that corresponds to this general period.” The findings argue, the investigators said, for better treatment for opioid addiction after hospitalization and greater efforts to make drug rehabilitation available after discharge. The researchers described as a limitation of their study the use of billing codes that changed late in the study period, increasing detection of drug abuse cases after 2015. They reported no outside funding or conflicts of interest.
SOURCE: Kadri AN et al. J Am Heart Assoc. 2019 Sep 18.
Hospitalizations for infective endocarditis associated with drug abuse doubled in the United States from 2002 to 2016, in a trend investigators call “alarming,” and link to a concurrent rise in opioid abuse.
Patients tend to be younger, poorer white males, according to findings published online in the Journal of the American Heart Association.
For their research, Amer N. Kadri, MD, of the Cleveland Clinic and colleagues looked at records for nearly a million hospitalizations for infective endocarditis (IE) in the National Inpatient Sample registry. All U.S. regions saw increases in drug abuse–linked cases of IE as a share of IE hospitalizations. Incidence of drug abuse–associated IC rose from 48 cases/100,000 population in 2002 to 79/100,000 in 2016. The Midwest saw the highest rate of change, with an annual percent increase of 4.9%.
While most IE hospitalizations in the study cohort were of white men (including 68% for drug-linked cases), the drug abuse–related cases were younger (median age, 38 vs. 70 years for nondrug-related IE), and more likely male (55.5% vs. 50%). About 45% of the drug-related cases were in people receiving Medicaid, and 42% were in the lowest quartile of median household income.
The drug abuse cases had fewer renal and cardiovascular comorbidities, compared with the nondrug cases, but were significantly more likely to present with HIV, hepatitis C, alcohol abuse, and liver disease. Inpatient mortality was lower among the drug-linked cases – 6% vs. 9% – but the drug cases saw significantly more cardiac or valve surgeries, longer hospital stays, and higher costs.
“Hospitalizations for IE have been increasing side by side with the opioid epidemic,” the investigators wrote in their analysis. “The opioid crisis has reached epidemic levels, and now drug overdoses have been the leading cause of injury-related death in the U.S. Heroin deaths had remained relatively low from 1999 until 2010 whereas it then increased threefold from 2010-2015.” The analysis showed a rise in drug abuse–associated IE “that corresponds to this general period.” The findings argue, the investigators said, for better treatment for opioid addiction after hospitalization and greater efforts to make drug rehabilitation available after discharge. The researchers described as a limitation of their study the use of billing codes that changed late in the study period, increasing detection of drug abuse cases after 2015. They reported no outside funding or conflicts of interest.
SOURCE: Kadri AN et al. J Am Heart Assoc. 2019 Sep 18.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Key clinical point:
Major finding: Incidence of drug abuse–associated IC increased from 48 cases/100,000 in 2002 to 79/100,000 in 2016.
Study details: A retrospective cohort study identifying about a more than 950,000 cases of IC from the National Inpatient Sample registry.
Disclosures: None.
Source: Kadri AN et al. J Am Heart Assoc. 2019 Sep 18.











