User login
Post-MI angina in stable patients flags high risk
PARIS – Patients with angina who were also more than 3 months out from a prior MI had a significantly increased rate of subsequent cardiovascular death or nonfatal MI in a study of nearly 33,000 patients, a finding that identified angina as a new red flag when following post-MI patients.
“Angina and prior MI was a higher-risk subgroup that may warrant more intensive management. This is new,” Emmanuel Sorbets, MD, said at the annual congress of the European Society of Cardiology.
The finding came from review of 32,703 patients from 45 countries with stable coronary artery disease enrolled in the CLARIFY (Prospective Observational Longitudinal Registry of Patients with Stable Coronary Artery Disease) registry during 2009-2010, a population that appeared to uniformly meet the new definition of chronic chromic syndromes recently published by a task force of the society (Eur Heart J. 2019 Aug 31. doi: 10.1093/eurheartj/ehz425).
Among the CLARIFY patients, 60% had an MI more than 3 months prior to enrollment (the registry excluded patients with more proximate MIs), and in this subgroup angina at baseline was linked with a 3.6% absolute increase in the rate of cardiovascular death or nonfatal MI compared with post-MI patients without angina at baseline during a median 5 year follow-up. This translated into a 44% relative increase that remained statistically significant after adjustment for several demographic and clinical factors, said Dr. Sorbets, a cardiologist at Avicenne Hospital in Bobigny, France. The cumulative incidence of the combined endpoint was 11.8% among post-MI patients with baseline angina and 8.2% in those without angina. Among patients without a prior MI, the presence or absence of angina at entry into the registry had no link with the incidence of later outcomes. Concurrently with Dr. Sorbets’ report at the congress, the results appeared in an article published online (Eur Heart J. 2019 Sep 3;doi: 10.1093/eurheartj/ehz660).
This finding should immediately influence practice, said Sanjay Sharma, MD, professor of inherited cardiac diseases and sports cardiology at St. George’s University, London. “One of the messages from this study is that we now have a very easy way to measure a high-risk factor,” and that patients who present with angina more than 3 months after an MI “require intensive investigation and aggressive management,” he said. The new evidence identified post-MI patients with angina as having a “semi-urgent” condition that needs added anti-anginal treatment and, if symptoms persist, possible revascularization, especially patients without prior revascularization, he said in a video interview. Although the study did not analyze the type of MI linked with these poor outcomes, Dr. Sharma speculated that certain patients with a prior non ST-elevation MI may face the greatest danger it they did not undergo percutaneous coronary revascularization at the time of their MI.
CLARIFY is sponsored by Servier. Dr. Sorbets has received personal fees from Servier, AstraZeneca, Bayer, Bristol-Myers Squibb, Merck Sharpe & Dohme, and Novartis. Dr. Sharma had no relevant disclosures.
This analysis of data collected in the CLARIFY registry introduces a new scenario that may be of interest: Post-MI patients who develop angina. However, this registry comes with several caveats. Most importantly, the endpoints recorded in the CLARIFY registry were based on predefined events that did not undergo routine adjudication. Cardiovascular disease death is hard to identify, and only 1% of events underwent an audit. It is also hard to contextualize the findings as no other randomized trial or registry has enrolled an entirely similar population. The patients enrolled in CLARIFY show the diversity of patients who fall under the new category recently defined by a task force of the European Society of Cardiology: chronic coronary syndromes (Eur Heart J. 2019 Aug 31. doi: 10.1093/eurheartj/ehz425).
David Hasdai, MD , is professor of medicine at Rabin Medical Center in Peta Tikva, Israel. He had no disclosures. He made these comments as designated discussant for the CLARIFY report.
This analysis of data collected in the CLARIFY registry introduces a new scenario that may be of interest: Post-MI patients who develop angina. However, this registry comes with several caveats. Most importantly, the endpoints recorded in the CLARIFY registry were based on predefined events that did not undergo routine adjudication. Cardiovascular disease death is hard to identify, and only 1% of events underwent an audit. It is also hard to contextualize the findings as no other randomized trial or registry has enrolled an entirely similar population. The patients enrolled in CLARIFY show the diversity of patients who fall under the new category recently defined by a task force of the European Society of Cardiology: chronic coronary syndromes (Eur Heart J. 2019 Aug 31. doi: 10.1093/eurheartj/ehz425).
David Hasdai, MD , is professor of medicine at Rabin Medical Center in Peta Tikva, Israel. He had no disclosures. He made these comments as designated discussant for the CLARIFY report.
This analysis of data collected in the CLARIFY registry introduces a new scenario that may be of interest: Post-MI patients who develop angina. However, this registry comes with several caveats. Most importantly, the endpoints recorded in the CLARIFY registry were based on predefined events that did not undergo routine adjudication. Cardiovascular disease death is hard to identify, and only 1% of events underwent an audit. It is also hard to contextualize the findings as no other randomized trial or registry has enrolled an entirely similar population. The patients enrolled in CLARIFY show the diversity of patients who fall under the new category recently defined by a task force of the European Society of Cardiology: chronic coronary syndromes (Eur Heart J. 2019 Aug 31. doi: 10.1093/eurheartj/ehz425).
David Hasdai, MD , is professor of medicine at Rabin Medical Center in Peta Tikva, Israel. He had no disclosures. He made these comments as designated discussant for the CLARIFY report.
PARIS – Patients with angina who were also more than 3 months out from a prior MI had a significantly increased rate of subsequent cardiovascular death or nonfatal MI in a study of nearly 33,000 patients, a finding that identified angina as a new red flag when following post-MI patients.
“Angina and prior MI was a higher-risk subgroup that may warrant more intensive management. This is new,” Emmanuel Sorbets, MD, said at the annual congress of the European Society of Cardiology.
The finding came from review of 32,703 patients from 45 countries with stable coronary artery disease enrolled in the CLARIFY (Prospective Observational Longitudinal Registry of Patients with Stable Coronary Artery Disease) registry during 2009-2010, a population that appeared to uniformly meet the new definition of chronic chromic syndromes recently published by a task force of the society (Eur Heart J. 2019 Aug 31. doi: 10.1093/eurheartj/ehz425).
Among the CLARIFY patients, 60% had an MI more than 3 months prior to enrollment (the registry excluded patients with more proximate MIs), and in this subgroup angina at baseline was linked with a 3.6% absolute increase in the rate of cardiovascular death or nonfatal MI compared with post-MI patients without angina at baseline during a median 5 year follow-up. This translated into a 44% relative increase that remained statistically significant after adjustment for several demographic and clinical factors, said Dr. Sorbets, a cardiologist at Avicenne Hospital in Bobigny, France. The cumulative incidence of the combined endpoint was 11.8% among post-MI patients with baseline angina and 8.2% in those without angina. Among patients without a prior MI, the presence or absence of angina at entry into the registry had no link with the incidence of later outcomes. Concurrently with Dr. Sorbets’ report at the congress, the results appeared in an article published online (Eur Heart J. 2019 Sep 3;doi: 10.1093/eurheartj/ehz660).
This finding should immediately influence practice, said Sanjay Sharma, MD, professor of inherited cardiac diseases and sports cardiology at St. George’s University, London. “One of the messages from this study is that we now have a very easy way to measure a high-risk factor,” and that patients who present with angina more than 3 months after an MI “require intensive investigation and aggressive management,” he said. The new evidence identified post-MI patients with angina as having a “semi-urgent” condition that needs added anti-anginal treatment and, if symptoms persist, possible revascularization, especially patients without prior revascularization, he said in a video interview. Although the study did not analyze the type of MI linked with these poor outcomes, Dr. Sharma speculated that certain patients with a prior non ST-elevation MI may face the greatest danger it they did not undergo percutaneous coronary revascularization at the time of their MI.
CLARIFY is sponsored by Servier. Dr. Sorbets has received personal fees from Servier, AstraZeneca, Bayer, Bristol-Myers Squibb, Merck Sharpe & Dohme, and Novartis. Dr. Sharma had no relevant disclosures.
PARIS – Patients with angina who were also more than 3 months out from a prior MI had a significantly increased rate of subsequent cardiovascular death or nonfatal MI in a study of nearly 33,000 patients, a finding that identified angina as a new red flag when following post-MI patients.
“Angina and prior MI was a higher-risk subgroup that may warrant more intensive management. This is new,” Emmanuel Sorbets, MD, said at the annual congress of the European Society of Cardiology.
The finding came from review of 32,703 patients from 45 countries with stable coronary artery disease enrolled in the CLARIFY (Prospective Observational Longitudinal Registry of Patients with Stable Coronary Artery Disease) registry during 2009-2010, a population that appeared to uniformly meet the new definition of chronic chromic syndromes recently published by a task force of the society (Eur Heart J. 2019 Aug 31. doi: 10.1093/eurheartj/ehz425).
Among the CLARIFY patients, 60% had an MI more than 3 months prior to enrollment (the registry excluded patients with more proximate MIs), and in this subgroup angina at baseline was linked with a 3.6% absolute increase in the rate of cardiovascular death or nonfatal MI compared with post-MI patients without angina at baseline during a median 5 year follow-up. This translated into a 44% relative increase that remained statistically significant after adjustment for several demographic and clinical factors, said Dr. Sorbets, a cardiologist at Avicenne Hospital in Bobigny, France. The cumulative incidence of the combined endpoint was 11.8% among post-MI patients with baseline angina and 8.2% in those without angina. Among patients without a prior MI, the presence or absence of angina at entry into the registry had no link with the incidence of later outcomes. Concurrently with Dr. Sorbets’ report at the congress, the results appeared in an article published online (Eur Heart J. 2019 Sep 3;doi: 10.1093/eurheartj/ehz660).
This finding should immediately influence practice, said Sanjay Sharma, MD, professor of inherited cardiac diseases and sports cardiology at St. George’s University, London. “One of the messages from this study is that we now have a very easy way to measure a high-risk factor,” and that patients who present with angina more than 3 months after an MI “require intensive investigation and aggressive management,” he said. The new evidence identified post-MI patients with angina as having a “semi-urgent” condition that needs added anti-anginal treatment and, if symptoms persist, possible revascularization, especially patients without prior revascularization, he said in a video interview. Although the study did not analyze the type of MI linked with these poor outcomes, Dr. Sharma speculated that certain patients with a prior non ST-elevation MI may face the greatest danger it they did not undergo percutaneous coronary revascularization at the time of their MI.
CLARIFY is sponsored by Servier. Dr. Sorbets has received personal fees from Servier, AstraZeneca, Bayer, Bristol-Myers Squibb, Merck Sharpe & Dohme, and Novartis. Dr. Sharma had no relevant disclosures.
REPORTING FROM THE ESC CONGRESS 2019
Trial of mesh vs. hysterectomy for prolapse yields inconclusive results
Transvaginal mesh hysteropexy for symptomatic uterovaginal prolapse may not significantly reduce treatment failure after 3 years, compared with vaginal hysterectomy with uterosacral ligament suspension, according to randomized trial results.
Nevertheless, “the point estimate favored hysteropexy,” the study authors wrote in JAMA. The 36-month cumulative treatment failure outcomes – defined as retreatment of prolapse, prolapse beyond the hymen, or prolapse symptoms – were 33% for patients who underwent hysteropexy, compared with 42% for patients who underwent hysterectomy. In addition, mean operative time was 45 minutes less for patients who underwent hysteropexy.
The publication follows the Food and Drug Administration’s ruling in April 2019 that manufacturers must cease marketing transvaginal mesh kits for repair of anterior or apical compartment prolapse. The investigators plan to continue evaluating patient outcomes to 5 years, and they noted that longer follow-up may lead to different conclusions.
From a class II device to class III
Surgical repair of uterovaginal prolapse is common. Although vaginal hysterectomy is the procedure of choice for many surgeons, “uterine-sparing suspension techniques ... are increasing in usage,” wrote Charles W. Nager, MD, chair and professor of obstetrics, gynecology, and reproductive sciences at the University of California, San Diego, and coauthors. However, few high-quality, long-term studies have compared apical transvaginal mesh with native tissue procedures.
The FDA first approved a mesh device for transvaginal repair of prolapse in 2002. In 2008, the agency notified clinicians and patients about an increase in adverse event reports related to vaginal mesh. It later advised that mesh for the treatment of pelvic organ prolapse does not conclusively improve clinical outcomes and that serious adverse events are not rare.
In 2016, the FDA reclassified surgical mesh to repair pelvic organ prolapse transvaginally as high risk, citing safety concerns such as severe pelvic pain and organ perforation. And in April 2019, the FDA ordered companies to stop selling transvaginal mesh intended for pelvic organ prolapse repair. “Even though these products can no longer be used in patients moving forward, [manufacturers] are required to continue follow-up” of patients in post–market surveillance studies, the FDA said in a statement.
An FDA panel had concluded that 3-year outcomes for prolapse repair with mesh should be better than the outcomes for repair with native tissue, and that the procedures should have comparable safety profiles.
The SUPeR trial
To compare the efficacy and adverse events of vaginal hysterectomy with suture apical suspension and transvaginal mesh hysteropexy, Dr. Nager and colleagues conducted the Study of Uterine Prolapse Procedures Randomized (SUPeR) trial.
Researchers enrolled 183 postmenopausal women with symptomatic uterovaginal prolapse undergoing surgical intervention at nine sites between April 2013 and February 2015. Investigators randomized 93 women to undergo vaginal mesh hysteropexy and 90 to undergo vaginal hysterectomy with uterosacral ligament suspension. Hysteropexy used the UpholdLITE transvaginal mesh support system (Boston Scientific). Uterosacral ligament suspension required one permanent and one delayed absorbable suture on each side. The primary analysis included data from 175 patients.
Compared with hysterectomy, hysteropexy resulted in an adjusted hazard ratio of treatment failure of 0.62 after 3 years, which was not statistically significant (P = .06). The 95% confidence interval of 0.38-1.02 “was wide and only slightly crossed the null value,” the researchers said. “The remaining uncertainty is too great” to establish or rule out the benefit of vaginal mesh hysteropexy.
Mean operative time was about 45 minutes shorter in the hysteropexy group versus the hysterectomy group (111.5 minutes vs. 156.7 minutes). Adverse events in the hysteropexy versus hysterectomy groups included mesh exposure (8% vs. 0%), ureteral kinking managed intraoperatively (0% vs. 7%), excessive granulation tissue after 12 weeks (1% vs. 11%), and suture exposure after 12 weeks (3% vs. 21%).
“Both groups reported improvements in sexual function, and dyspareunia and pain and de novo dyspareunia rates were low,” Dr. Nager and colleagues wrote. “All other complications with long-term sequelae were not different between groups.”
“Patients in the current study are being followed up for 60 months and the results and conclusions at 36 months could change with extended follow-up,” they added.
A role for mesh?
“The report ... by Nager and colleagues is particularly timely and important,” Cynthia A. Brincat, MD, PhD, wrote in an accompanying editorial. Dr. Brincat is affiliated with the division of female pelvic medicine and reconstructive surgery at Rush Medical College, Chicago.
Although the mesh exposures, granulation tissue, or suture exposures during the trial did not require reoperation, “management of these adverse events was not described,” the editorialist noted. “Clinically important differences could exist between the management of these reported adverse events.”
Based on the findings, gynecologic surgeons “will need to reconsider several important questions regarding the repair of pelvic organ prolapse. For instance, is hysterectomy a necessary component for the repair? What is the role of mesh, and can its use reduce the use of otherwise unnecessary procedures (i.e., hysterectomy) without increasing risk to patients?” she wrote. Other questions center on what constitutes operative failure and how surgeons should augment prolapse repair.
“This study also provides a potential new and well-defined role for the use of mesh in pelvic prolapse surgery, with no significant difference, and perhaps some benefit (i.e., no hysterectomy), compared with a native tissue repair,” Dr. Brincat wrote. “The study also provides useful information for shared decision-making discussions between patients and gynecologic surgeons with respect to selection of procedures and use of mesh for treatment of women with symptomatic uterovaginal prolapse undergoing vaginal surgery.”
The trial was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health Office of Research on Women’s Health. Boston Scientific provided support through an unrestricted grant. One author reported stock ownership in a medical device company, and others reported grants from medical device companies outside the submitted work. Dr. Brincat reported no conflicts of interest.
SOURCES: Nager CW et al. JAMA. 2019 Sep 17;322(11):1054-65; Brincat CA. JAMA. 2019 Sep 17;322(11):1047-8.
Transvaginal mesh hysteropexy for symptomatic uterovaginal prolapse may not significantly reduce treatment failure after 3 years, compared with vaginal hysterectomy with uterosacral ligament suspension, according to randomized trial results.
Nevertheless, “the point estimate favored hysteropexy,” the study authors wrote in JAMA. The 36-month cumulative treatment failure outcomes – defined as retreatment of prolapse, prolapse beyond the hymen, or prolapse symptoms – were 33% for patients who underwent hysteropexy, compared with 42% for patients who underwent hysterectomy. In addition, mean operative time was 45 minutes less for patients who underwent hysteropexy.
The publication follows the Food and Drug Administration’s ruling in April 2019 that manufacturers must cease marketing transvaginal mesh kits for repair of anterior or apical compartment prolapse. The investigators plan to continue evaluating patient outcomes to 5 years, and they noted that longer follow-up may lead to different conclusions.
From a class II device to class III
Surgical repair of uterovaginal prolapse is common. Although vaginal hysterectomy is the procedure of choice for many surgeons, “uterine-sparing suspension techniques ... are increasing in usage,” wrote Charles W. Nager, MD, chair and professor of obstetrics, gynecology, and reproductive sciences at the University of California, San Diego, and coauthors. However, few high-quality, long-term studies have compared apical transvaginal mesh with native tissue procedures.
The FDA first approved a mesh device for transvaginal repair of prolapse in 2002. In 2008, the agency notified clinicians and patients about an increase in adverse event reports related to vaginal mesh. It later advised that mesh for the treatment of pelvic organ prolapse does not conclusively improve clinical outcomes and that serious adverse events are not rare.
In 2016, the FDA reclassified surgical mesh to repair pelvic organ prolapse transvaginally as high risk, citing safety concerns such as severe pelvic pain and organ perforation. And in April 2019, the FDA ordered companies to stop selling transvaginal mesh intended for pelvic organ prolapse repair. “Even though these products can no longer be used in patients moving forward, [manufacturers] are required to continue follow-up” of patients in post–market surveillance studies, the FDA said in a statement.
An FDA panel had concluded that 3-year outcomes for prolapse repair with mesh should be better than the outcomes for repair with native tissue, and that the procedures should have comparable safety profiles.
The SUPeR trial
To compare the efficacy and adverse events of vaginal hysterectomy with suture apical suspension and transvaginal mesh hysteropexy, Dr. Nager and colleagues conducted the Study of Uterine Prolapse Procedures Randomized (SUPeR) trial.
Researchers enrolled 183 postmenopausal women with symptomatic uterovaginal prolapse undergoing surgical intervention at nine sites between April 2013 and February 2015. Investigators randomized 93 women to undergo vaginal mesh hysteropexy and 90 to undergo vaginal hysterectomy with uterosacral ligament suspension. Hysteropexy used the UpholdLITE transvaginal mesh support system (Boston Scientific). Uterosacral ligament suspension required one permanent and one delayed absorbable suture on each side. The primary analysis included data from 175 patients.
Compared with hysterectomy, hysteropexy resulted in an adjusted hazard ratio of treatment failure of 0.62 after 3 years, which was not statistically significant (P = .06). The 95% confidence interval of 0.38-1.02 “was wide and only slightly crossed the null value,” the researchers said. “The remaining uncertainty is too great” to establish or rule out the benefit of vaginal mesh hysteropexy.
Mean operative time was about 45 minutes shorter in the hysteropexy group versus the hysterectomy group (111.5 minutes vs. 156.7 minutes). Adverse events in the hysteropexy versus hysterectomy groups included mesh exposure (8% vs. 0%), ureteral kinking managed intraoperatively (0% vs. 7%), excessive granulation tissue after 12 weeks (1% vs. 11%), and suture exposure after 12 weeks (3% vs. 21%).
“Both groups reported improvements in sexual function, and dyspareunia and pain and de novo dyspareunia rates were low,” Dr. Nager and colleagues wrote. “All other complications with long-term sequelae were not different between groups.”
“Patients in the current study are being followed up for 60 months and the results and conclusions at 36 months could change with extended follow-up,” they added.
A role for mesh?
“The report ... by Nager and colleagues is particularly timely and important,” Cynthia A. Brincat, MD, PhD, wrote in an accompanying editorial. Dr. Brincat is affiliated with the division of female pelvic medicine and reconstructive surgery at Rush Medical College, Chicago.
Although the mesh exposures, granulation tissue, or suture exposures during the trial did not require reoperation, “management of these adverse events was not described,” the editorialist noted. “Clinically important differences could exist between the management of these reported adverse events.”
Based on the findings, gynecologic surgeons “will need to reconsider several important questions regarding the repair of pelvic organ prolapse. For instance, is hysterectomy a necessary component for the repair? What is the role of mesh, and can its use reduce the use of otherwise unnecessary procedures (i.e., hysterectomy) without increasing risk to patients?” she wrote. Other questions center on what constitutes operative failure and how surgeons should augment prolapse repair.
“This study also provides a potential new and well-defined role for the use of mesh in pelvic prolapse surgery, with no significant difference, and perhaps some benefit (i.e., no hysterectomy), compared with a native tissue repair,” Dr. Brincat wrote. “The study also provides useful information for shared decision-making discussions between patients and gynecologic surgeons with respect to selection of procedures and use of mesh for treatment of women with symptomatic uterovaginal prolapse undergoing vaginal surgery.”
The trial was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health Office of Research on Women’s Health. Boston Scientific provided support through an unrestricted grant. One author reported stock ownership in a medical device company, and others reported grants from medical device companies outside the submitted work. Dr. Brincat reported no conflicts of interest.
SOURCES: Nager CW et al. JAMA. 2019 Sep 17;322(11):1054-65; Brincat CA. JAMA. 2019 Sep 17;322(11):1047-8.
Transvaginal mesh hysteropexy for symptomatic uterovaginal prolapse may not significantly reduce treatment failure after 3 years, compared with vaginal hysterectomy with uterosacral ligament suspension, according to randomized trial results.
Nevertheless, “the point estimate favored hysteropexy,” the study authors wrote in JAMA. The 36-month cumulative treatment failure outcomes – defined as retreatment of prolapse, prolapse beyond the hymen, or prolapse symptoms – were 33% for patients who underwent hysteropexy, compared with 42% for patients who underwent hysterectomy. In addition, mean operative time was 45 minutes less for patients who underwent hysteropexy.
The publication follows the Food and Drug Administration’s ruling in April 2019 that manufacturers must cease marketing transvaginal mesh kits for repair of anterior or apical compartment prolapse. The investigators plan to continue evaluating patient outcomes to 5 years, and they noted that longer follow-up may lead to different conclusions.
From a class II device to class III
Surgical repair of uterovaginal prolapse is common. Although vaginal hysterectomy is the procedure of choice for many surgeons, “uterine-sparing suspension techniques ... are increasing in usage,” wrote Charles W. Nager, MD, chair and professor of obstetrics, gynecology, and reproductive sciences at the University of California, San Diego, and coauthors. However, few high-quality, long-term studies have compared apical transvaginal mesh with native tissue procedures.
The FDA first approved a mesh device for transvaginal repair of prolapse in 2002. In 2008, the agency notified clinicians and patients about an increase in adverse event reports related to vaginal mesh. It later advised that mesh for the treatment of pelvic organ prolapse does not conclusively improve clinical outcomes and that serious adverse events are not rare.
In 2016, the FDA reclassified surgical mesh to repair pelvic organ prolapse transvaginally as high risk, citing safety concerns such as severe pelvic pain and organ perforation. And in April 2019, the FDA ordered companies to stop selling transvaginal mesh intended for pelvic organ prolapse repair. “Even though these products can no longer be used in patients moving forward, [manufacturers] are required to continue follow-up” of patients in post–market surveillance studies, the FDA said in a statement.
An FDA panel had concluded that 3-year outcomes for prolapse repair with mesh should be better than the outcomes for repair with native tissue, and that the procedures should have comparable safety profiles.
The SUPeR trial
To compare the efficacy and adverse events of vaginal hysterectomy with suture apical suspension and transvaginal mesh hysteropexy, Dr. Nager and colleagues conducted the Study of Uterine Prolapse Procedures Randomized (SUPeR) trial.
Researchers enrolled 183 postmenopausal women with symptomatic uterovaginal prolapse undergoing surgical intervention at nine sites between April 2013 and February 2015. Investigators randomized 93 women to undergo vaginal mesh hysteropexy and 90 to undergo vaginal hysterectomy with uterosacral ligament suspension. Hysteropexy used the UpholdLITE transvaginal mesh support system (Boston Scientific). Uterosacral ligament suspension required one permanent and one delayed absorbable suture on each side. The primary analysis included data from 175 patients.
Compared with hysterectomy, hysteropexy resulted in an adjusted hazard ratio of treatment failure of 0.62 after 3 years, which was not statistically significant (P = .06). The 95% confidence interval of 0.38-1.02 “was wide and only slightly crossed the null value,” the researchers said. “The remaining uncertainty is too great” to establish or rule out the benefit of vaginal mesh hysteropexy.
Mean operative time was about 45 minutes shorter in the hysteropexy group versus the hysterectomy group (111.5 minutes vs. 156.7 minutes). Adverse events in the hysteropexy versus hysterectomy groups included mesh exposure (8% vs. 0%), ureteral kinking managed intraoperatively (0% vs. 7%), excessive granulation tissue after 12 weeks (1% vs. 11%), and suture exposure after 12 weeks (3% vs. 21%).
“Both groups reported improvements in sexual function, and dyspareunia and pain and de novo dyspareunia rates were low,” Dr. Nager and colleagues wrote. “All other complications with long-term sequelae were not different between groups.”
“Patients in the current study are being followed up for 60 months and the results and conclusions at 36 months could change with extended follow-up,” they added.
A role for mesh?
“The report ... by Nager and colleagues is particularly timely and important,” Cynthia A. Brincat, MD, PhD, wrote in an accompanying editorial. Dr. Brincat is affiliated with the division of female pelvic medicine and reconstructive surgery at Rush Medical College, Chicago.
Although the mesh exposures, granulation tissue, or suture exposures during the trial did not require reoperation, “management of these adverse events was not described,” the editorialist noted. “Clinically important differences could exist between the management of these reported adverse events.”
Based on the findings, gynecologic surgeons “will need to reconsider several important questions regarding the repair of pelvic organ prolapse. For instance, is hysterectomy a necessary component for the repair? What is the role of mesh, and can its use reduce the use of otherwise unnecessary procedures (i.e., hysterectomy) without increasing risk to patients?” she wrote. Other questions center on what constitutes operative failure and how surgeons should augment prolapse repair.
“This study also provides a potential new and well-defined role for the use of mesh in pelvic prolapse surgery, with no significant difference, and perhaps some benefit (i.e., no hysterectomy), compared with a native tissue repair,” Dr. Brincat wrote. “The study also provides useful information for shared decision-making discussions between patients and gynecologic surgeons with respect to selection of procedures and use of mesh for treatment of women with symptomatic uterovaginal prolapse undergoing vaginal surgery.”
The trial was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health Office of Research on Women’s Health. Boston Scientific provided support through an unrestricted grant. One author reported stock ownership in a medical device company, and others reported grants from medical device companies outside the submitted work. Dr. Brincat reported no conflicts of interest.
SOURCES: Nager CW et al. JAMA. 2019 Sep 17;322(11):1054-65; Brincat CA. JAMA. 2019 Sep 17;322(11):1047-8.
FROM JAMA
SFA awards grants to 15 researchers
Since its inception, the Sarcoma Foundation of America (SFA) has awarded research grants for the best, most promising research to cure sarcoma. This year SFA awarded $750,000 in research funds to 15 scientists as part of its 2019 SFA Research Grant program. The grants, worth $50,000 each, explore numerous sarcoma subtypes, multiple strategies, and different approaches to find effective treatments for many forms of the disease. Research projects are listed below alphabetically by investigator last name. More details are available on the SFA’s grant pages, available at https://www.curesarcoma.org/grant

Since its inception, the Sarcoma Foundation of America (SFA) has awarded research grants for the best, most promising research to cure sarcoma. This year SFA awarded $750,000 in research funds to 15 scientists as part of its 2019 SFA Research Grant program. The grants, worth $50,000 each, explore numerous sarcoma subtypes, multiple strategies, and different approaches to find effective treatments for many forms of the disease. Research projects are listed below alphabetically by investigator last name. More details are available on the SFA’s grant pages, available at https://www.curesarcoma.org/grant

Since its inception, the Sarcoma Foundation of America (SFA) has awarded research grants for the best, most promising research to cure sarcoma. This year SFA awarded $750,000 in research funds to 15 scientists as part of its 2019 SFA Research Grant program. The grants, worth $50,000 each, explore numerous sarcoma subtypes, multiple strategies, and different approaches to find effective treatments for many forms of the disease. Research projects are listed below alphabetically by investigator last name. More details are available on the SFA’s grant pages, available at https://www.curesarcoma.org/grant

Significant clinical response induced by vismodegib in advanced sarcoma: Hedgehog pathway inhibition
Spindle cell sarcomas are part of a rare, heterogeneous family of connective tissue tumors. These tumors are primarily treated with surgery and have a high risk of recurrence and distant metastasis with elevated mortality rates.1 Other than the evidence for first-line therapy with doxorubicin in advanced soft tissue sarcoma, little evidence exists to point to an optimal second-line therapy. This is due to the diversity of soft tissue sarcomas, which encompass approximately 70 different histologic subtypes that can each respond differently to treatment.2 As such, newer strategies, including immunotherapy and targeted molecular drugs, are being developed.
Quiescent in most adult tissues, the Hedgehog signaling pathway, when inappropriately activated, has been implicated in the development of multiple types of cancers, including basal cell, breast, prostate, hepatocellular, pancreatic, and brain cancer.3 The Hedgehog signaling pathway is an important regulator of cell growth and differentiation in early development, but when inappropriately activated can lead to cell proliferation and increased angiogenic factors, decreased apoptosis, and breakdown of tight junctions promoting cancer growth and metastasis.4 Recent data reveal that the Hedgehog pathway plays a specific role in activation of satellite cells, proliferation of myoblasts, and differentiation of skeletal muscle.5 Activation of this embryonic pathway has been implicated in embryonal rhabdoymyosarcoma, osteosarcoma, and chondrosarcoma.5-7
This pathway has recently been recognized as a therapeutic target, with the development of vismodegib, a targeted Hedgehog pathway inhibitor. This novel agent is in active use for treatment of advanced basal cell carcinoma and is currently undergoing trials for various other malignancies. Recently, a phase 2a basket study, called MyPathway, evaluated the use of targeted therapies in 35 different advanced refractory solid tumors harboring specific molecular alterations. Out of 21 patients with mutations in the Hedgehog pathway, 3 had a partial response to vismodegib—one had an unknown primary tumor, another a squamous skin cancer, and the third a salivary gland cancer.8 Vismodegib (GDC-0449) was also evaluated in a phase 2 multicenter clinical trial in patients with progressive advanced chondrosarcoma.7 Although the study did not meet its primary endpoint, the proportion of patients with non-progressive disease was 25.6% at 6 months. Investigators observed that the benefit occurred in the subset of patients with overexpression of the Hedgehog ligand. Genomic studies for mutations in SMO and PTCH genes were available for only 28 and 26 patients, respectively, of the 45 patients enrolled on the trial. While there were no mutations identified, expression data revealed that overexpression of the Hedgehog ligand was present in 65% of cases tested (13 out of 20 patients). In patients with stable disease at 6 months, all had overexpression of the Hedgehog ligand.7 These studies point to the potential use of vismodegib in both bone and soft tissue sarcomas, and more specifically, to the importance of genomic testing in these cases.
Case Presentation and Summary
This report describes the novel use of vismodegib, an oral Hedgehog signaling pathway inhibitor, in the treatment of a patient with metastatic soft tissue sarcoma.
An 18-year-old female with no particular previous illnesses was initially diagnosed with superficial soft tissue sarcoma overlying the right hip in 2013. Due to the complexity of pathology, a second opinion was requested and revealed atypical cellular spindle and epithelioid cells, morphologically and immunohistochemically suggestive of spindle cell sarcoma, not otherwise specified. She underwent negative-margin resection in January 2014. Her course was complicated by two recurrences in the right inguinal lymph nodes in July 2014 and July 2015. She was treated with lymph node dissection in 2014, followed by numerous right lymph node dissections and adjuvant radiation in 2015.
A routine computerized tomography (CT) scan of the thorax-abdomen and pelvis in August 2016 revealed recurrence of disease, with multiple lung nodules as well as metastases in the retroperitoneum. She received 6 cycles of gemcitabine and docetaxel with stability of disease. The patient was then started on a PI3K inhibitor as part of a clinical trial, as genotypic analysis of the tumor revealed an activating mutation of the PI3K gene. The patient’s course was complicated by acute obstructive renal failure requiring a double J stent for right-sided hydronephrosis.
Repeat imaging revealed disease progression, and the patient was then switched to liposomal doxorubicin alone for 4 months and then in combination with olaratumab. She received the combined treatment for a total of 3 months, which was then stopped when she was found to have new peritoneal implants and worsening ascites. At this time, tissue was sent for FoundationOne® next generation sequencing (NGS)-based genomic testing, and the patient received one dose of nivolumab.
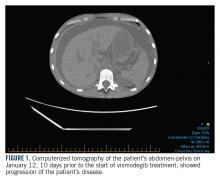
In January 2018, 2 days after receiving her first dose of nivolumab, the patient required admission for worsening abdominal pain secondary to progression of her disease (FIGURE 1). She was found to have acute kidney injury on top of chronic kidney disease due to hydronephrosis requiring a left-sided double J stent. She also had transaminitis resulting from a common bile duct stricture treated with a biliary stent and worsening ascites requiring regular paracentesis. This was all in the context of new or growing metastatic implants.
At this time, the result of the FoundationOne genomic testing revealed PTCH1 loss of exons 1-24 and CDKN2A/B loss. Mutation of tumor suppressor gene PTCH1 leads to Hedgehog pathway activation and therefore the patient was started on vismodegib on January 22, 2018. She was discharged from the hospital in stable condition a day later, on January 23.
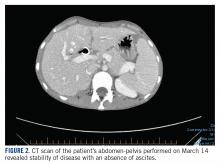
The patient’s clinical status subsequently improved, with significant reduction in her chronic abdominal pain and very minimal side effects. Clinically, the patient’s acute kidney injury resolved (from a creatinine of 272 μmol/L at discharge to 85 μmol/L after a week of treatment) and her liver enzymes normalized (from an alkaline phosphatase of 301 U/L to 83 U/L, and alanine transaminase of 111 U/L to 38 U/L). CT scan of her chest and abdomen, which was performed 1 month post treatment, revealed stability of disease with absence of ascites (FIGURE 2). The patient continued to have a good response to treatment for 6 months, with no recurrence of pain or ascites.
Six months later, in July 2018, the patient developed increasing pain and a CT scan revealed worsening of abdominopelvic carcinomatosis. In this context, vismodegib was discontinued on July 17. In the next 5 months, she went on to receive carboplatin and paclitaxel, gemcitabine, and nivolumab consecutively with no response. She was admitted to hospital on December 30 for a pain crisis. She passed away on January 9, 2019, from fecal peritonitis.
Discussion
To the best of our knowledge, this is the first patient with metastatic sarcoma to receive vismodegib, a Hedgehog signaling pathway inhibitor. She achieved an excellent clinical response with progression- free disease for approximately 6 months after starting treatment.
There is no current standard second- line treatment for metastatic soft tissue sarcoma. The choice of systemic therapy is histology-driven and therefore treatment is individualized for each patient. The future of oncology is heading towards an even more personalized approach with molecular profiling. Our case report highlights the relevance of genomic testing and targeted therapies, especially in cases of diverse clinical and biological disease behavior.
Molecular targeting is even more necessary in patients with advanced cancer who have failed multiple lines of treatment. As in our study, these patients can obtain a significant response with meaningful improvement in their quality of life. Future research is currently focusing on identifying new molecular targets in patients with advanced refractory cancers. Further studies will need to be done to determine whether these molecular targeting agents, such as vismodegib, lead to significant outcome changes in these patients.
1. Collini P, Sorensen PHB, Patel S, et al. Sarcomas with spindle cell morphology. Semin Oncol. 2009;36(4):324-337.
2. Frezza AM, Stacchiotti S, Gronchi A. Systemic treatment in advanced soft tissue sarcoma: what is standard, what is new. BMC Med. 2017;15(1):109.
3. Hanna A, Shevde LA. Hedgehog signaling: modulation of cancer properties and tumor microenvironment. Mol Cancer. 2016;15:24.
4. Abidi A. Hedgehog signaling pathway: a novel target for cancer therapy: vismodegib, a promising therapeutic option in treatment of basal cell carcinomas. Indian J Pharmacol. 2014;46(1): 3-12.
5. Belyea B, Kephart JG, Blum J, Kirsch DG, Linardic CM. Embryonic signaling pathways and rhabdomyosarcoma: contributions to cancer development and opportunities for therapeutic targeting. Sarcoma. 2012;2012:13.
6. Yao Z, Han L, Chen Y, et al. Hedgehog signalling in the tumourigenesis and metastasis of osteosarcoma, and its potential value in the clinical therapy of osteosarcoma. Cell Death Dis. 2018;9(6):701.
7. Italiano A, Le Cesne A, Bellera C, et al. GDC- 0449 in patients with advanced chondrosarcomas: a French Sarcoma Group/US and French National Cancer Institute Single-Arm Phase II Collaborative Study. Ann Oncol. 2013;24(11):2922-2926.
8. Hainsworth JD, Meric-Bernstam F, Swanton C, et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from MyPathway, an open-label, phase IIa multiple basket study. J Clin Oncol. 2018;36(6): 536-542.
Spindle cell sarcomas are part of a rare, heterogeneous family of connective tissue tumors. These tumors are primarily treated with surgery and have a high risk of recurrence and distant metastasis with elevated mortality rates.1 Other than the evidence for first-line therapy with doxorubicin in advanced soft tissue sarcoma, little evidence exists to point to an optimal second-line therapy. This is due to the diversity of soft tissue sarcomas, which encompass approximately 70 different histologic subtypes that can each respond differently to treatment.2 As such, newer strategies, including immunotherapy and targeted molecular drugs, are being developed.
Quiescent in most adult tissues, the Hedgehog signaling pathway, when inappropriately activated, has been implicated in the development of multiple types of cancers, including basal cell, breast, prostate, hepatocellular, pancreatic, and brain cancer.3 The Hedgehog signaling pathway is an important regulator of cell growth and differentiation in early development, but when inappropriately activated can lead to cell proliferation and increased angiogenic factors, decreased apoptosis, and breakdown of tight junctions promoting cancer growth and metastasis.4 Recent data reveal that the Hedgehog pathway plays a specific role in activation of satellite cells, proliferation of myoblasts, and differentiation of skeletal muscle.5 Activation of this embryonic pathway has been implicated in embryonal rhabdoymyosarcoma, osteosarcoma, and chondrosarcoma.5-7
This pathway has recently been recognized as a therapeutic target, with the development of vismodegib, a targeted Hedgehog pathway inhibitor. This novel agent is in active use for treatment of advanced basal cell carcinoma and is currently undergoing trials for various other malignancies. Recently, a phase 2a basket study, called MyPathway, evaluated the use of targeted therapies in 35 different advanced refractory solid tumors harboring specific molecular alterations. Out of 21 patients with mutations in the Hedgehog pathway, 3 had a partial response to vismodegib—one had an unknown primary tumor, another a squamous skin cancer, and the third a salivary gland cancer.8 Vismodegib (GDC-0449) was also evaluated in a phase 2 multicenter clinical trial in patients with progressive advanced chondrosarcoma.7 Although the study did not meet its primary endpoint, the proportion of patients with non-progressive disease was 25.6% at 6 months. Investigators observed that the benefit occurred in the subset of patients with overexpression of the Hedgehog ligand. Genomic studies for mutations in SMO and PTCH genes were available for only 28 and 26 patients, respectively, of the 45 patients enrolled on the trial. While there were no mutations identified, expression data revealed that overexpression of the Hedgehog ligand was present in 65% of cases tested (13 out of 20 patients). In patients with stable disease at 6 months, all had overexpression of the Hedgehog ligand.7 These studies point to the potential use of vismodegib in both bone and soft tissue sarcomas, and more specifically, to the importance of genomic testing in these cases.
Case Presentation and Summary
This report describes the novel use of vismodegib, an oral Hedgehog signaling pathway inhibitor, in the treatment of a patient with metastatic soft tissue sarcoma.
An 18-year-old female with no particular previous illnesses was initially diagnosed with superficial soft tissue sarcoma overlying the right hip in 2013. Due to the complexity of pathology, a second opinion was requested and revealed atypical cellular spindle and epithelioid cells, morphologically and immunohistochemically suggestive of spindle cell sarcoma, not otherwise specified. She underwent negative-margin resection in January 2014. Her course was complicated by two recurrences in the right inguinal lymph nodes in July 2014 and July 2015. She was treated with lymph node dissection in 2014, followed by numerous right lymph node dissections and adjuvant radiation in 2015.
A routine computerized tomography (CT) scan of the thorax-abdomen and pelvis in August 2016 revealed recurrence of disease, with multiple lung nodules as well as metastases in the retroperitoneum. She received 6 cycles of gemcitabine and docetaxel with stability of disease. The patient was then started on a PI3K inhibitor as part of a clinical trial, as genotypic analysis of the tumor revealed an activating mutation of the PI3K gene. The patient’s course was complicated by acute obstructive renal failure requiring a double J stent for right-sided hydronephrosis.
Repeat imaging revealed disease progression, and the patient was then switched to liposomal doxorubicin alone for 4 months and then in combination with olaratumab. She received the combined treatment for a total of 3 months, which was then stopped when she was found to have new peritoneal implants and worsening ascites. At this time, tissue was sent for FoundationOne® next generation sequencing (NGS)-based genomic testing, and the patient received one dose of nivolumab.
In January 2018, 2 days after receiving her first dose of nivolumab, the patient required admission for worsening abdominal pain secondary to progression of her disease (FIGURE 1). She was found to have acute kidney injury on top of chronic kidney disease due to hydronephrosis requiring a left-sided double J stent. She also had transaminitis resulting from a common bile duct stricture treated with a biliary stent and worsening ascites requiring regular paracentesis. This was all in the context of new or growing metastatic implants.
At this time, the result of the FoundationOne genomic testing revealed PTCH1 loss of exons 1-24 and CDKN2A/B loss. Mutation of tumor suppressor gene PTCH1 leads to Hedgehog pathway activation and therefore the patient was started on vismodegib on January 22, 2018. She was discharged from the hospital in stable condition a day later, on January 23.
The patient’s clinical status subsequently improved, with significant reduction in her chronic abdominal pain and very minimal side effects. Clinically, the patient’s acute kidney injury resolved (from a creatinine of 272 μmol/L at discharge to 85 μmol/L after a week of treatment) and her liver enzymes normalized (from an alkaline phosphatase of 301 U/L to 83 U/L, and alanine transaminase of 111 U/L to 38 U/L). CT scan of her chest and abdomen, which was performed 1 month post treatment, revealed stability of disease with absence of ascites (FIGURE 2). The patient continued to have a good response to treatment for 6 months, with no recurrence of pain or ascites.
Six months later, in July 2018, the patient developed increasing pain and a CT scan revealed worsening of abdominopelvic carcinomatosis. In this context, vismodegib was discontinued on July 17. In the next 5 months, she went on to receive carboplatin and paclitaxel, gemcitabine, and nivolumab consecutively with no response. She was admitted to hospital on December 30 for a pain crisis. She passed away on January 9, 2019, from fecal peritonitis.
Discussion
To the best of our knowledge, this is the first patient with metastatic sarcoma to receive vismodegib, a Hedgehog signaling pathway inhibitor. She achieved an excellent clinical response with progression- free disease for approximately 6 months after starting treatment.
There is no current standard second- line treatment for metastatic soft tissue sarcoma. The choice of systemic therapy is histology-driven and therefore treatment is individualized for each patient. The future of oncology is heading towards an even more personalized approach with molecular profiling. Our case report highlights the relevance of genomic testing and targeted therapies, especially in cases of diverse clinical and biological disease behavior.
Molecular targeting is even more necessary in patients with advanced cancer who have failed multiple lines of treatment. As in our study, these patients can obtain a significant response with meaningful improvement in their quality of life. Future research is currently focusing on identifying new molecular targets in patients with advanced refractory cancers. Further studies will need to be done to determine whether these molecular targeting agents, such as vismodegib, lead to significant outcome changes in these patients.
Spindle cell sarcomas are part of a rare, heterogeneous family of connective tissue tumors. These tumors are primarily treated with surgery and have a high risk of recurrence and distant metastasis with elevated mortality rates.1 Other than the evidence for first-line therapy with doxorubicin in advanced soft tissue sarcoma, little evidence exists to point to an optimal second-line therapy. This is due to the diversity of soft tissue sarcomas, which encompass approximately 70 different histologic subtypes that can each respond differently to treatment.2 As such, newer strategies, including immunotherapy and targeted molecular drugs, are being developed.
Quiescent in most adult tissues, the Hedgehog signaling pathway, when inappropriately activated, has been implicated in the development of multiple types of cancers, including basal cell, breast, prostate, hepatocellular, pancreatic, and brain cancer.3 The Hedgehog signaling pathway is an important regulator of cell growth and differentiation in early development, but when inappropriately activated can lead to cell proliferation and increased angiogenic factors, decreased apoptosis, and breakdown of tight junctions promoting cancer growth and metastasis.4 Recent data reveal that the Hedgehog pathway plays a specific role in activation of satellite cells, proliferation of myoblasts, and differentiation of skeletal muscle.5 Activation of this embryonic pathway has been implicated in embryonal rhabdoymyosarcoma, osteosarcoma, and chondrosarcoma.5-7
This pathway has recently been recognized as a therapeutic target, with the development of vismodegib, a targeted Hedgehog pathway inhibitor. This novel agent is in active use for treatment of advanced basal cell carcinoma and is currently undergoing trials for various other malignancies. Recently, a phase 2a basket study, called MyPathway, evaluated the use of targeted therapies in 35 different advanced refractory solid tumors harboring specific molecular alterations. Out of 21 patients with mutations in the Hedgehog pathway, 3 had a partial response to vismodegib—one had an unknown primary tumor, another a squamous skin cancer, and the third a salivary gland cancer.8 Vismodegib (GDC-0449) was also evaluated in a phase 2 multicenter clinical trial in patients with progressive advanced chondrosarcoma.7 Although the study did not meet its primary endpoint, the proportion of patients with non-progressive disease was 25.6% at 6 months. Investigators observed that the benefit occurred in the subset of patients with overexpression of the Hedgehog ligand. Genomic studies for mutations in SMO and PTCH genes were available for only 28 and 26 patients, respectively, of the 45 patients enrolled on the trial. While there were no mutations identified, expression data revealed that overexpression of the Hedgehog ligand was present in 65% of cases tested (13 out of 20 patients). In patients with stable disease at 6 months, all had overexpression of the Hedgehog ligand.7 These studies point to the potential use of vismodegib in both bone and soft tissue sarcomas, and more specifically, to the importance of genomic testing in these cases.
Case Presentation and Summary
This report describes the novel use of vismodegib, an oral Hedgehog signaling pathway inhibitor, in the treatment of a patient with metastatic soft tissue sarcoma.
An 18-year-old female with no particular previous illnesses was initially diagnosed with superficial soft tissue sarcoma overlying the right hip in 2013. Due to the complexity of pathology, a second opinion was requested and revealed atypical cellular spindle and epithelioid cells, morphologically and immunohistochemically suggestive of spindle cell sarcoma, not otherwise specified. She underwent negative-margin resection in January 2014. Her course was complicated by two recurrences in the right inguinal lymph nodes in July 2014 and July 2015. She was treated with lymph node dissection in 2014, followed by numerous right lymph node dissections and adjuvant radiation in 2015.
A routine computerized tomography (CT) scan of the thorax-abdomen and pelvis in August 2016 revealed recurrence of disease, with multiple lung nodules as well as metastases in the retroperitoneum. She received 6 cycles of gemcitabine and docetaxel with stability of disease. The patient was then started on a PI3K inhibitor as part of a clinical trial, as genotypic analysis of the tumor revealed an activating mutation of the PI3K gene. The patient’s course was complicated by acute obstructive renal failure requiring a double J stent for right-sided hydronephrosis.
Repeat imaging revealed disease progression, and the patient was then switched to liposomal doxorubicin alone for 4 months and then in combination with olaratumab. She received the combined treatment for a total of 3 months, which was then stopped when she was found to have new peritoneal implants and worsening ascites. At this time, tissue was sent for FoundationOne® next generation sequencing (NGS)-based genomic testing, and the patient received one dose of nivolumab.
In January 2018, 2 days after receiving her first dose of nivolumab, the patient required admission for worsening abdominal pain secondary to progression of her disease (FIGURE 1). She was found to have acute kidney injury on top of chronic kidney disease due to hydronephrosis requiring a left-sided double J stent. She also had transaminitis resulting from a common bile duct stricture treated with a biliary stent and worsening ascites requiring regular paracentesis. This was all in the context of new or growing metastatic implants.
At this time, the result of the FoundationOne genomic testing revealed PTCH1 loss of exons 1-24 and CDKN2A/B loss. Mutation of tumor suppressor gene PTCH1 leads to Hedgehog pathway activation and therefore the patient was started on vismodegib on January 22, 2018. She was discharged from the hospital in stable condition a day later, on January 23.
The patient’s clinical status subsequently improved, with significant reduction in her chronic abdominal pain and very minimal side effects. Clinically, the patient’s acute kidney injury resolved (from a creatinine of 272 μmol/L at discharge to 85 μmol/L after a week of treatment) and her liver enzymes normalized (from an alkaline phosphatase of 301 U/L to 83 U/L, and alanine transaminase of 111 U/L to 38 U/L). CT scan of her chest and abdomen, which was performed 1 month post treatment, revealed stability of disease with absence of ascites (FIGURE 2). The patient continued to have a good response to treatment for 6 months, with no recurrence of pain or ascites.
Six months later, in July 2018, the patient developed increasing pain and a CT scan revealed worsening of abdominopelvic carcinomatosis. In this context, vismodegib was discontinued on July 17. In the next 5 months, she went on to receive carboplatin and paclitaxel, gemcitabine, and nivolumab consecutively with no response. She was admitted to hospital on December 30 for a pain crisis. She passed away on January 9, 2019, from fecal peritonitis.
Discussion
To the best of our knowledge, this is the first patient with metastatic sarcoma to receive vismodegib, a Hedgehog signaling pathway inhibitor. She achieved an excellent clinical response with progression- free disease for approximately 6 months after starting treatment.
There is no current standard second- line treatment for metastatic soft tissue sarcoma. The choice of systemic therapy is histology-driven and therefore treatment is individualized for each patient. The future of oncology is heading towards an even more personalized approach with molecular profiling. Our case report highlights the relevance of genomic testing and targeted therapies, especially in cases of diverse clinical and biological disease behavior.
Molecular targeting is even more necessary in patients with advanced cancer who have failed multiple lines of treatment. As in our study, these patients can obtain a significant response with meaningful improvement in their quality of life. Future research is currently focusing on identifying new molecular targets in patients with advanced refractory cancers. Further studies will need to be done to determine whether these molecular targeting agents, such as vismodegib, lead to significant outcome changes in these patients.
1. Collini P, Sorensen PHB, Patel S, et al. Sarcomas with spindle cell morphology. Semin Oncol. 2009;36(4):324-337.
2. Frezza AM, Stacchiotti S, Gronchi A. Systemic treatment in advanced soft tissue sarcoma: what is standard, what is new. BMC Med. 2017;15(1):109.
3. Hanna A, Shevde LA. Hedgehog signaling: modulation of cancer properties and tumor microenvironment. Mol Cancer. 2016;15:24.
4. Abidi A. Hedgehog signaling pathway: a novel target for cancer therapy: vismodegib, a promising therapeutic option in treatment of basal cell carcinomas. Indian J Pharmacol. 2014;46(1): 3-12.
5. Belyea B, Kephart JG, Blum J, Kirsch DG, Linardic CM. Embryonic signaling pathways and rhabdomyosarcoma: contributions to cancer development and opportunities for therapeutic targeting. Sarcoma. 2012;2012:13.
6. Yao Z, Han L, Chen Y, et al. Hedgehog signalling in the tumourigenesis and metastasis of osteosarcoma, and its potential value in the clinical therapy of osteosarcoma. Cell Death Dis. 2018;9(6):701.
7. Italiano A, Le Cesne A, Bellera C, et al. GDC- 0449 in patients with advanced chondrosarcomas: a French Sarcoma Group/US and French National Cancer Institute Single-Arm Phase II Collaborative Study. Ann Oncol. 2013;24(11):2922-2926.
8. Hainsworth JD, Meric-Bernstam F, Swanton C, et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from MyPathway, an open-label, phase IIa multiple basket study. J Clin Oncol. 2018;36(6): 536-542.
1. Collini P, Sorensen PHB, Patel S, et al. Sarcomas with spindle cell morphology. Semin Oncol. 2009;36(4):324-337.
2. Frezza AM, Stacchiotti S, Gronchi A. Systemic treatment in advanced soft tissue sarcoma: what is standard, what is new. BMC Med. 2017;15(1):109.
3. Hanna A, Shevde LA. Hedgehog signaling: modulation of cancer properties and tumor microenvironment. Mol Cancer. 2016;15:24.
4. Abidi A. Hedgehog signaling pathway: a novel target for cancer therapy: vismodegib, a promising therapeutic option in treatment of basal cell carcinomas. Indian J Pharmacol. 2014;46(1): 3-12.
5. Belyea B, Kephart JG, Blum J, Kirsch DG, Linardic CM. Embryonic signaling pathways and rhabdomyosarcoma: contributions to cancer development and opportunities for therapeutic targeting. Sarcoma. 2012;2012:13.
6. Yao Z, Han L, Chen Y, et al. Hedgehog signalling in the tumourigenesis and metastasis of osteosarcoma, and its potential value in the clinical therapy of osteosarcoma. Cell Death Dis. 2018;9(6):701.
7. Italiano A, Le Cesne A, Bellera C, et al. GDC- 0449 in patients with advanced chondrosarcomas: a French Sarcoma Group/US and French National Cancer Institute Single-Arm Phase II Collaborative Study. Ann Oncol. 2013;24(11):2922-2926.
8. Hainsworth JD, Meric-Bernstam F, Swanton C, et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from MyPathway, an open-label, phase IIa multiple basket study. J Clin Oncol. 2018;36(6): 536-542.
Even with no disease activity, recurrence risk near 50% when stopping DMTs for MS
STOCKHOLM – according to data presented at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
Of 49 patients who had been on DMT for at least 5 years and had shown no evidence of disease activity (NEDA) during that time, 26 continued to have NEDA through at least 5 years of follow-up, explained Tobias Monschein, MD, of the department of neurology at the Medical University of Vienna.
The cohort of patients had all been taking either interferon beta or glatiramer acetate after a first clinical episode leading to an initial diagnosis of MS. Patients all met Barkhof criteria for MS diagnosis on MRI, and all but six patients had oligoclonal bands found on examination of cerebrospinal fluid.
All patients in the cohort thus met criteria for clinically isolated syndrome (CIS) and MS under the 2017 revisions to the McDonald diagnostic criteria. “To our knowledge, this is the first study determining the risk of disease recurrence in a homogenous cohort of patients with CIS,” reported Dr. Monschein and collaborators.
Before stopping DMT, patients had to show at least 5 years of NEDA status; at that point, patients were offered the opportunity to discontinue medication. The decision to stop or continue taking a DMT was left to individual patient choice, Dr. Monschein said in an interview.
The cohort of patients who decided to discontinue DMT was seen yearly; they received a clinical examination that included expanded disability status scale (EDSS) rating. Patients also received an annual MRI.
Age at DMT discontinuation was predictive of remaining disease free, found Dr. Monschein and collaborators. The 26 patients who continued disease free after DMT discontinuation were a mean 29.7 years old, while patients who had disease recurrence were a mean 22.7 years old.
Looking at age as a dichotomous variable, the investigators found that the 16 patients who were 40 years or older when they stopped DMT had an 18.8% risk of MS recurrence, while the 33 patients younger than 40 years at the time of ceasing DMT had a 60.6% risk of recurrence.
Age, in fact, was the only patient, disease, or therapy characteristic that Dr. Monschein and colleagues found predictive of relapse: “Gender, type of DMT, treatment duration, and CIS symptom did not differ significantly between groups,” they reported.
The data “should not encourage patients to generally stop DMTs after a long NEDA period,” they noted.
In the context of shared patient decision-making, though, the data can inform discussion with patients who wish to discontinue DMTS after long disease-free periods, Dr. Monschein said.
“Physicians should encourage younger people to stay on DMTS despite long lasting NEDA status while in patients [older than] 40 years stopping DMTs together with regular clinical and radiological monitoring could be a reasonable option,” he and his colleagues advised.
Dr. Monschein reported no outside sources of funding and no conflicts of interest.
SOURCE: Monschein T et al. ECTRIMS 2019, Abstract P654.
STOCKHOLM – according to data presented at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
Of 49 patients who had been on DMT for at least 5 years and had shown no evidence of disease activity (NEDA) during that time, 26 continued to have NEDA through at least 5 years of follow-up, explained Tobias Monschein, MD, of the department of neurology at the Medical University of Vienna.
The cohort of patients had all been taking either interferon beta or glatiramer acetate after a first clinical episode leading to an initial diagnosis of MS. Patients all met Barkhof criteria for MS diagnosis on MRI, and all but six patients had oligoclonal bands found on examination of cerebrospinal fluid.
All patients in the cohort thus met criteria for clinically isolated syndrome (CIS) and MS under the 2017 revisions to the McDonald diagnostic criteria. “To our knowledge, this is the first study determining the risk of disease recurrence in a homogenous cohort of patients with CIS,” reported Dr. Monschein and collaborators.
Before stopping DMT, patients had to show at least 5 years of NEDA status; at that point, patients were offered the opportunity to discontinue medication. The decision to stop or continue taking a DMT was left to individual patient choice, Dr. Monschein said in an interview.
The cohort of patients who decided to discontinue DMT was seen yearly; they received a clinical examination that included expanded disability status scale (EDSS) rating. Patients also received an annual MRI.
Age at DMT discontinuation was predictive of remaining disease free, found Dr. Monschein and collaborators. The 26 patients who continued disease free after DMT discontinuation were a mean 29.7 years old, while patients who had disease recurrence were a mean 22.7 years old.
Looking at age as a dichotomous variable, the investigators found that the 16 patients who were 40 years or older when they stopped DMT had an 18.8% risk of MS recurrence, while the 33 patients younger than 40 years at the time of ceasing DMT had a 60.6% risk of recurrence.
Age, in fact, was the only patient, disease, or therapy characteristic that Dr. Monschein and colleagues found predictive of relapse: “Gender, type of DMT, treatment duration, and CIS symptom did not differ significantly between groups,” they reported.
The data “should not encourage patients to generally stop DMTs after a long NEDA period,” they noted.
In the context of shared patient decision-making, though, the data can inform discussion with patients who wish to discontinue DMTS after long disease-free periods, Dr. Monschein said.
“Physicians should encourage younger people to stay on DMTS despite long lasting NEDA status while in patients [older than] 40 years stopping DMTs together with regular clinical and radiological monitoring could be a reasonable option,” he and his colleagues advised.
Dr. Monschein reported no outside sources of funding and no conflicts of interest.
SOURCE: Monschein T et al. ECTRIMS 2019, Abstract P654.
STOCKHOLM – according to data presented at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
Of 49 patients who had been on DMT for at least 5 years and had shown no evidence of disease activity (NEDA) during that time, 26 continued to have NEDA through at least 5 years of follow-up, explained Tobias Monschein, MD, of the department of neurology at the Medical University of Vienna.
The cohort of patients had all been taking either interferon beta or glatiramer acetate after a first clinical episode leading to an initial diagnosis of MS. Patients all met Barkhof criteria for MS diagnosis on MRI, and all but six patients had oligoclonal bands found on examination of cerebrospinal fluid.
All patients in the cohort thus met criteria for clinically isolated syndrome (CIS) and MS under the 2017 revisions to the McDonald diagnostic criteria. “To our knowledge, this is the first study determining the risk of disease recurrence in a homogenous cohort of patients with CIS,” reported Dr. Monschein and collaborators.
Before stopping DMT, patients had to show at least 5 years of NEDA status; at that point, patients were offered the opportunity to discontinue medication. The decision to stop or continue taking a DMT was left to individual patient choice, Dr. Monschein said in an interview.
The cohort of patients who decided to discontinue DMT was seen yearly; they received a clinical examination that included expanded disability status scale (EDSS) rating. Patients also received an annual MRI.
Age at DMT discontinuation was predictive of remaining disease free, found Dr. Monschein and collaborators. The 26 patients who continued disease free after DMT discontinuation were a mean 29.7 years old, while patients who had disease recurrence were a mean 22.7 years old.
Looking at age as a dichotomous variable, the investigators found that the 16 patients who were 40 years or older when they stopped DMT had an 18.8% risk of MS recurrence, while the 33 patients younger than 40 years at the time of ceasing DMT had a 60.6% risk of recurrence.
Age, in fact, was the only patient, disease, or therapy characteristic that Dr. Monschein and colleagues found predictive of relapse: “Gender, type of DMT, treatment duration, and CIS symptom did not differ significantly between groups,” they reported.
The data “should not encourage patients to generally stop DMTs after a long NEDA period,” they noted.
In the context of shared patient decision-making, though, the data can inform discussion with patients who wish to discontinue DMTS after long disease-free periods, Dr. Monschein said.
“Physicians should encourage younger people to stay on DMTS despite long lasting NEDA status while in patients [older than] 40 years stopping DMTs together with regular clinical and radiological monitoring could be a reasonable option,” he and his colleagues advised.
Dr. Monschein reported no outside sources of funding and no conflicts of interest.
SOURCE: Monschein T et al. ECTRIMS 2019, Abstract P654.
REPORTING FROM ECTRIMS 2019
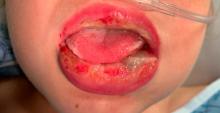
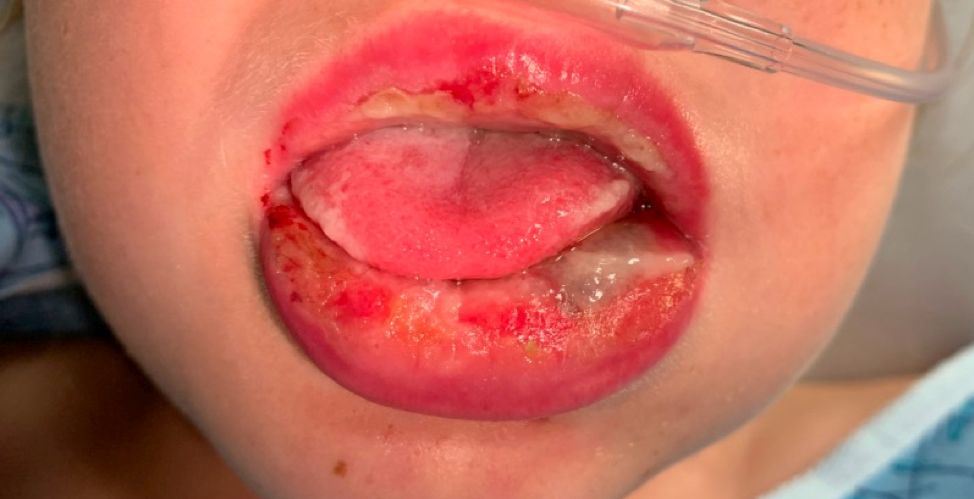
Pneumonia with tender, dry, crusted lips
Mycoplasma pneumoniae infection commonly manifests as an upper or lower respiratory tract infection with associated fever, dyspnea, cough, and coryza. However, patients can present with extrapulmonary complications with dermatologic findings including mucocutaneous eruptions. M. pneumoniae–associated mucocutaneous disease has prominent mucositis and typically sparse cutaneous involvement. The mucositis usually involves the lips and oral mucosa, eye conjunctivae, and nasal mucosa and can involve urogenital lesions. It predominantly is observed in children and adolescents. This condition is essentially a subtype of Stevens-Johnson syndrome, with a specific infection-associated etiology, and has been called “Mycoplasma pneumoniae–induced rash and mucositis,” shortened to “MIRM.”
Severe reactive mucocutaneous eruptions include erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). While there has been semantic confusion over the years, there are some distinctive characteristics.
EM is characterized by typical three-ringed target papules that are predominantly acral in location and often without mucosal involvement. The lesions are “multiforme” in that they can appear polymorphous and evolve during an episode, with erythematous macules progressing to edematous papules, sometimes with a halo of pallor and concentric “target-like” appearance. Lesions of EM are fixed, meaning individual lesions last 7-10 days, unlike urticarial lesions that last hours. EM classically is associated with herpes simplex virus infections which usually precede its development.
SJS and TEN display atypical macules and papules which develop into erythematous vesicles, bullae, and potentially extensive desquamation, usually presenting with fever and systemic symptoms, with multiple mucosal sites involved. SJS usually is defined by having bullae restricted to less than 10% of body surface area (BSA), TEN as greater than 30% BSA, and “overlap SJS-TEN” as 20%-30% skin detachment.1 SJS and TEN commonly are induced by medications and on a spectrum of drug hypersensitivity–induced epidermal necrolysis.
MIRM has been highlighted as a distinct, common condition, usually mucous-membrane predominant with involvement of two or more mucosal sites, less than 10% total BSA, the presence of few vesiculobullous lesions or scattered atypical targets with or without targetoid lesions (without rash is called MIRM sine rash), and clinical and laboratory evidence of atypical pneumonia.2 Other infections can cause similar eruptions (for example, Chlamydia pneumoniae), and a recent proposal by the Pediatric Dermatology Research Alliance has suggested the term “Reactive Infectious Mucocutaneous Eruption” (RIME) to include MIRM and other infection-induced reactions.
Laboratory diagnosis of M. pneumoniae is via serology or polymerase chain reaction. Antibody titers begin to rise approximately 7-9 days after infection and peak at 3-4 weeks. Enzyme immunoassay is more sensitive in detecting acute infection than culture and has sensitivity comparable to the polymerase chain reaction if there has been sufficient time to develop an antibody response.
The differential diagnosis between RIME/MIRM, SJS, and TEN may be difficult to distinguish in the first few days of presentation, and consideration of infections and possible medication causes is important. DRESS syndrome (drug reaction with eosinophilia and systemic symptoms) also is in the differential diagnosis. DRESS usually has a long latency (2-8 weeks) between drug exposure and disease onset.
Treatment of RIME/MIRM is supportive care and treatment of any underlying infection. Steroids and intravenous immune globulin (IVIG) have been used to treat reactive mucositis, as well as cyclosporine and biologic agents (such as etanercept), in an attempt to minimize the extent and duration of mucous membrane vesiculation and denudation. While these drugs may help shorten the duration of the disease course, controlled trials are lacking and there is little comparative literature on efficacy or safety of these agents.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. They said they have no financial disclosures. Email Dr. Eichenfield and Dr. Bhatti at pdnews@mdedge.com.
References
1. Arch Dermatol. 1993 Jan;129(1):92-6.
2. J Am Acad Dermatol. 2015 Feb;72(2):239-45.
Mycoplasma pneumoniae infection commonly manifests as an upper or lower respiratory tract infection with associated fever, dyspnea, cough, and coryza. However, patients can present with extrapulmonary complications with dermatologic findings including mucocutaneous eruptions. M. pneumoniae–associated mucocutaneous disease has prominent mucositis and typically sparse cutaneous involvement. The mucositis usually involves the lips and oral mucosa, eye conjunctivae, and nasal mucosa and can involve urogenital lesions. It predominantly is observed in children and adolescents. This condition is essentially a subtype of Stevens-Johnson syndrome, with a specific infection-associated etiology, and has been called “Mycoplasma pneumoniae–induced rash and mucositis,” shortened to “MIRM.”
Severe reactive mucocutaneous eruptions include erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). While there has been semantic confusion over the years, there are some distinctive characteristics.
EM is characterized by typical three-ringed target papules that are predominantly acral in location and often without mucosal involvement. The lesions are “multiforme” in that they can appear polymorphous and evolve during an episode, with erythematous macules progressing to edematous papules, sometimes with a halo of pallor and concentric “target-like” appearance. Lesions of EM are fixed, meaning individual lesions last 7-10 days, unlike urticarial lesions that last hours. EM classically is associated with herpes simplex virus infections which usually precede its development.
SJS and TEN display atypical macules and papules which develop into erythematous vesicles, bullae, and potentially extensive desquamation, usually presenting with fever and systemic symptoms, with multiple mucosal sites involved. SJS usually is defined by having bullae restricted to less than 10% of body surface area (BSA), TEN as greater than 30% BSA, and “overlap SJS-TEN” as 20%-30% skin detachment.1 SJS and TEN commonly are induced by medications and on a spectrum of drug hypersensitivity–induced epidermal necrolysis.
MIRM has been highlighted as a distinct, common condition, usually mucous-membrane predominant with involvement of two or more mucosal sites, less than 10% total BSA, the presence of few vesiculobullous lesions or scattered atypical targets with or without targetoid lesions (without rash is called MIRM sine rash), and clinical and laboratory evidence of atypical pneumonia.2 Other infections can cause similar eruptions (for example, Chlamydia pneumoniae), and a recent proposal by the Pediatric Dermatology Research Alliance has suggested the term “Reactive Infectious Mucocutaneous Eruption” (RIME) to include MIRM and other infection-induced reactions.
Laboratory diagnosis of M. pneumoniae is via serology or polymerase chain reaction. Antibody titers begin to rise approximately 7-9 days after infection and peak at 3-4 weeks. Enzyme immunoassay is more sensitive in detecting acute infection than culture and has sensitivity comparable to the polymerase chain reaction if there has been sufficient time to develop an antibody response.
The differential diagnosis between RIME/MIRM, SJS, and TEN may be difficult to distinguish in the first few days of presentation, and consideration of infections and possible medication causes is important. DRESS syndrome (drug reaction with eosinophilia and systemic symptoms) also is in the differential diagnosis. DRESS usually has a long latency (2-8 weeks) between drug exposure and disease onset.
Treatment of RIME/MIRM is supportive care and treatment of any underlying infection. Steroids and intravenous immune globulin (IVIG) have been used to treat reactive mucositis, as well as cyclosporine and biologic agents (such as etanercept), in an attempt to minimize the extent and duration of mucous membrane vesiculation and denudation. While these drugs may help shorten the duration of the disease course, controlled trials are lacking and there is little comparative literature on efficacy or safety of these agents.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. They said they have no financial disclosures. Email Dr. Eichenfield and Dr. Bhatti at pdnews@mdedge.com.
References
1. Arch Dermatol. 1993 Jan;129(1):92-6.
2. J Am Acad Dermatol. 2015 Feb;72(2):239-45.
Mycoplasma pneumoniae infection commonly manifests as an upper or lower respiratory tract infection with associated fever, dyspnea, cough, and coryza. However, patients can present with extrapulmonary complications with dermatologic findings including mucocutaneous eruptions. M. pneumoniae–associated mucocutaneous disease has prominent mucositis and typically sparse cutaneous involvement. The mucositis usually involves the lips and oral mucosa, eye conjunctivae, and nasal mucosa and can involve urogenital lesions. It predominantly is observed in children and adolescents. This condition is essentially a subtype of Stevens-Johnson syndrome, with a specific infection-associated etiology, and has been called “Mycoplasma pneumoniae–induced rash and mucositis,” shortened to “MIRM.”
Severe reactive mucocutaneous eruptions include erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). While there has been semantic confusion over the years, there are some distinctive characteristics.
EM is characterized by typical three-ringed target papules that are predominantly acral in location and often without mucosal involvement. The lesions are “multiforme” in that they can appear polymorphous and evolve during an episode, with erythematous macules progressing to edematous papules, sometimes with a halo of pallor and concentric “target-like” appearance. Lesions of EM are fixed, meaning individual lesions last 7-10 days, unlike urticarial lesions that last hours. EM classically is associated with herpes simplex virus infections which usually precede its development.
SJS and TEN display atypical macules and papules which develop into erythematous vesicles, bullae, and potentially extensive desquamation, usually presenting with fever and systemic symptoms, with multiple mucosal sites involved. SJS usually is defined by having bullae restricted to less than 10% of body surface area (BSA), TEN as greater than 30% BSA, and “overlap SJS-TEN” as 20%-30% skin detachment.1 SJS and TEN commonly are induced by medications and on a spectrum of drug hypersensitivity–induced epidermal necrolysis.
MIRM has been highlighted as a distinct, common condition, usually mucous-membrane predominant with involvement of two or more mucosal sites, less than 10% total BSA, the presence of few vesiculobullous lesions or scattered atypical targets with or without targetoid lesions (without rash is called MIRM sine rash), and clinical and laboratory evidence of atypical pneumonia.2 Other infections can cause similar eruptions (for example, Chlamydia pneumoniae), and a recent proposal by the Pediatric Dermatology Research Alliance has suggested the term “Reactive Infectious Mucocutaneous Eruption” (RIME) to include MIRM and other infection-induced reactions.
Laboratory diagnosis of M. pneumoniae is via serology or polymerase chain reaction. Antibody titers begin to rise approximately 7-9 days after infection and peak at 3-4 weeks. Enzyme immunoassay is more sensitive in detecting acute infection than culture and has sensitivity comparable to the polymerase chain reaction if there has been sufficient time to develop an antibody response.
The differential diagnosis between RIME/MIRM, SJS, and TEN may be difficult to distinguish in the first few days of presentation, and consideration of infections and possible medication causes is important. DRESS syndrome (drug reaction with eosinophilia and systemic symptoms) also is in the differential diagnosis. DRESS usually has a long latency (2-8 weeks) between drug exposure and disease onset.
Treatment of RIME/MIRM is supportive care and treatment of any underlying infection. Steroids and intravenous immune globulin (IVIG) have been used to treat reactive mucositis, as well as cyclosporine and biologic agents (such as etanercept), in an attempt to minimize the extent and duration of mucous membrane vesiculation and denudation. While these drugs may help shorten the duration of the disease course, controlled trials are lacking and there is little comparative literature on efficacy or safety of these agents.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. They said they have no financial disclosures. Email Dr. Eichenfield and Dr. Bhatti at pdnews@mdedge.com.
References
1. Arch Dermatol. 1993 Jan;129(1):92-6.
2. J Am Acad Dermatol. 2015 Feb;72(2):239-45.
Clinical trials in sarcoma bring hope and promise
In this issue of The Sarcoma Journal, we highlight research and developments presented at the 2019 ASCO annual meeting. Despite the rarity of sarcoma, it was not lost among the thousands of abstracts, posters, and talks presented during the four-and-a-half days of the meeting.
In the past 5 years, there has been a resurgence of phase 3 clinical trials in sarcoma, including several large first-line studies comparing combination therapies to doxorubicin—the gold standard since the mid-1970s. None have shown superiority. Despite this, there has been a gradual improvement in overall survival. This is attributed to advances in the multidisciplinary management of sarcomas and available supportive care services as well as a better understanding of and emergent therapies for individual sarcoma subtypes.
In the United States, we have seen the approval of several agents in sarcoma: pazopanib, with a 3-month improvement in progression-free survival (PFS) over placebo; trabectedin in liposarcoma and leiomyosarcoma, with a 2.7-month improvement in PFS over dacarbazine; and eribulin, based on a liposarcoma subgroup analysis that showed a 7-month improvement in overall survival over dacarbazine.None of these therapies are approved in the US in the front-line setting; rather, all after a patient generally receives a doxorubicin- based therapy.
We have learned a great deal from these studies. They highlight some of the challenges in designing clinical trials in a rare and heterogeneous group of malignancies. The sarcoma community is very much focused on overcoming these challenges by designing clinical trials appropriate to the disease and the therapy that is being studied. This includes the incorporation of novel endpoints, imaging modalities, patient-reported outcome measures, and statistical methodologies to best serve the patient and to determine whether and how the therapy is helping them.
There is tremendous hope and promise in sarcoma due to significant advancements in our understanding of mesenchymal biology and of the genetic diversity in these diseases. This has led to an influx of promising agents and trials, many of which have transformed treatment paradigms on specific sarcoma subtypes. This issue provides a glimpse into the progress being made.
From Dr. Tap’s plenary presentation at ASCO 2019
In this issue of The Sarcoma Journal, we highlight research and developments presented at the 2019 ASCO annual meeting. Despite the rarity of sarcoma, it was not lost among the thousands of abstracts, posters, and talks presented during the four-and-a-half days of the meeting.
In the past 5 years, there has been a resurgence of phase 3 clinical trials in sarcoma, including several large first-line studies comparing combination therapies to doxorubicin—the gold standard since the mid-1970s. None have shown superiority. Despite this, there has been a gradual improvement in overall survival. This is attributed to advances in the multidisciplinary management of sarcomas and available supportive care services as well as a better understanding of and emergent therapies for individual sarcoma subtypes.
In the United States, we have seen the approval of several agents in sarcoma: pazopanib, with a 3-month improvement in progression-free survival (PFS) over placebo; trabectedin in liposarcoma and leiomyosarcoma, with a 2.7-month improvement in PFS over dacarbazine; and eribulin, based on a liposarcoma subgroup analysis that showed a 7-month improvement in overall survival over dacarbazine.None of these therapies are approved in the US in the front-line setting; rather, all after a patient generally receives a doxorubicin- based therapy.
We have learned a great deal from these studies. They highlight some of the challenges in designing clinical trials in a rare and heterogeneous group of malignancies. The sarcoma community is very much focused on overcoming these challenges by designing clinical trials appropriate to the disease and the therapy that is being studied. This includes the incorporation of novel endpoints, imaging modalities, patient-reported outcome measures, and statistical methodologies to best serve the patient and to determine whether and how the therapy is helping them.
There is tremendous hope and promise in sarcoma due to significant advancements in our understanding of mesenchymal biology and of the genetic diversity in these diseases. This has led to an influx of promising agents and trials, many of which have transformed treatment paradigms on specific sarcoma subtypes. This issue provides a glimpse into the progress being made.
From Dr. Tap’s plenary presentation at ASCO 2019
In this issue of The Sarcoma Journal, we highlight research and developments presented at the 2019 ASCO annual meeting. Despite the rarity of sarcoma, it was not lost among the thousands of abstracts, posters, and talks presented during the four-and-a-half days of the meeting.
In the past 5 years, there has been a resurgence of phase 3 clinical trials in sarcoma, including several large first-line studies comparing combination therapies to doxorubicin—the gold standard since the mid-1970s. None have shown superiority. Despite this, there has been a gradual improvement in overall survival. This is attributed to advances in the multidisciplinary management of sarcomas and available supportive care services as well as a better understanding of and emergent therapies for individual sarcoma subtypes.
In the United States, we have seen the approval of several agents in sarcoma: pazopanib, with a 3-month improvement in progression-free survival (PFS) over placebo; trabectedin in liposarcoma and leiomyosarcoma, with a 2.7-month improvement in PFS over dacarbazine; and eribulin, based on a liposarcoma subgroup analysis that showed a 7-month improvement in overall survival over dacarbazine.None of these therapies are approved in the US in the front-line setting; rather, all after a patient generally receives a doxorubicin- based therapy.
We have learned a great deal from these studies. They highlight some of the challenges in designing clinical trials in a rare and heterogeneous group of malignancies. The sarcoma community is very much focused on overcoming these challenges by designing clinical trials appropriate to the disease and the therapy that is being studied. This includes the incorporation of novel endpoints, imaging modalities, patient-reported outcome measures, and statistical methodologies to best serve the patient and to determine whether and how the therapy is helping them.
There is tremendous hope and promise in sarcoma due to significant advancements in our understanding of mesenchymal biology and of the genetic diversity in these diseases. This has led to an influx of promising agents and trials, many of which have transformed treatment paradigms on specific sarcoma subtypes. This issue provides a glimpse into the progress being made.
From Dr. Tap’s plenary presentation at ASCO 2019
Meta-analysis finds platelet-rich plasma may improve hair growth
Treatment with intradermal injections of autologous compared with placebo, according to a meta-analysis of seven studies.
In the report of the results, published in the Journal of Cosmetic Dermatology, Gezim Dervishi, from University Hospital Cologne (Germany), and coauthors noted that autologous PRP contains cytokines and growth factors – including platelet‐derived growth factor, vascular endothelial growth factor, and insulinlike growth factor–1 – that are believed to play a role in new hair growth. While the evidence to support the clinical efficacy of this approach is currently limited and PRP is not approved for hair-loss treatment, it is being used in clinical trials and off label for hair restoration in Europe and the United States.
The authors reviewed 13 randomized, controlled trials involving 343 people with pattern hair loss randomized to PRP control treatment, or placebo in a simple-parallel or half-head design, and assessed at 3-6 months after starting treatment. The studies were conducted in countries that included the United States, Brazil, Spain, and Italy.
The meta-analysis included seven studies that used change in hair density as the primary outcome. Six of these studies compared PRP with placebo and one compared PRP plus minoxidil or finasteride versus placebo plus minoxidil or finasteride. Five evaluated outcomes 6 months after the intervention, and two studies evaluated outcomes 3 months afterwards.
Of these seven studies, five reported statistically significant increases in hair density in favor of PRP over placebo, while the remaining two did not find any significant treatment effect. In the seven studies, the pooled mean difference in increasing hair density between treatment and placebo was 30.35 (95% confidence interval, 1.77-58.93; P less than .00001).
The 13 studies involved applications of platelet-rich plasma one to eight times over treatment periods of 1-5 months. Three studies compared PRP in conjunction with either minoxidil, finasteride, or polydeoxyribonucleotide, compared with those same therapies without PRP, and 10 studies compared PRP with either normal saline or distilled water. Five studies were judged to be at high risk of bias for at least one item, but none of these studies were included in the meta-analysis.
None of the studies identified any significant short-term treatment-related adverse effects, but the authors suggested that future studies look at potential long-term adverse effects of treatment.
“We recommend further [randomized, controlled trials] that should ensure adequate random sequence generation, adequate allocation concealment, blinding of performance and detection, and that should prevent incomplete data and selective reporting,” they wrote.
Funding and conflicts of interest statements were not available.
SOURCE: Dervishi G et al. J Cosmet Dermatol. 2019 Aug 12. doi: 10.1111/jocd.13113.
Treatment with intradermal injections of autologous compared with placebo, according to a meta-analysis of seven studies.
In the report of the results, published in the Journal of Cosmetic Dermatology, Gezim Dervishi, from University Hospital Cologne (Germany), and coauthors noted that autologous PRP contains cytokines and growth factors – including platelet‐derived growth factor, vascular endothelial growth factor, and insulinlike growth factor–1 – that are believed to play a role in new hair growth. While the evidence to support the clinical efficacy of this approach is currently limited and PRP is not approved for hair-loss treatment, it is being used in clinical trials and off label for hair restoration in Europe and the United States.
The authors reviewed 13 randomized, controlled trials involving 343 people with pattern hair loss randomized to PRP control treatment, or placebo in a simple-parallel or half-head design, and assessed at 3-6 months after starting treatment. The studies were conducted in countries that included the United States, Brazil, Spain, and Italy.
The meta-analysis included seven studies that used change in hair density as the primary outcome. Six of these studies compared PRP with placebo and one compared PRP plus minoxidil or finasteride versus placebo plus minoxidil or finasteride. Five evaluated outcomes 6 months after the intervention, and two studies evaluated outcomes 3 months afterwards.
Of these seven studies, five reported statistically significant increases in hair density in favor of PRP over placebo, while the remaining two did not find any significant treatment effect. In the seven studies, the pooled mean difference in increasing hair density between treatment and placebo was 30.35 (95% confidence interval, 1.77-58.93; P less than .00001).
The 13 studies involved applications of platelet-rich plasma one to eight times over treatment periods of 1-5 months. Three studies compared PRP in conjunction with either minoxidil, finasteride, or polydeoxyribonucleotide, compared with those same therapies without PRP, and 10 studies compared PRP with either normal saline or distilled water. Five studies were judged to be at high risk of bias for at least one item, but none of these studies were included in the meta-analysis.
None of the studies identified any significant short-term treatment-related adverse effects, but the authors suggested that future studies look at potential long-term adverse effects of treatment.
“We recommend further [randomized, controlled trials] that should ensure adequate random sequence generation, adequate allocation concealment, blinding of performance and detection, and that should prevent incomplete data and selective reporting,” they wrote.
Funding and conflicts of interest statements were not available.
SOURCE: Dervishi G et al. J Cosmet Dermatol. 2019 Aug 12. doi: 10.1111/jocd.13113.
Treatment with intradermal injections of autologous compared with placebo, according to a meta-analysis of seven studies.
In the report of the results, published in the Journal of Cosmetic Dermatology, Gezim Dervishi, from University Hospital Cologne (Germany), and coauthors noted that autologous PRP contains cytokines and growth factors – including platelet‐derived growth factor, vascular endothelial growth factor, and insulinlike growth factor–1 – that are believed to play a role in new hair growth. While the evidence to support the clinical efficacy of this approach is currently limited and PRP is not approved for hair-loss treatment, it is being used in clinical trials and off label for hair restoration in Europe and the United States.
The authors reviewed 13 randomized, controlled trials involving 343 people with pattern hair loss randomized to PRP control treatment, or placebo in a simple-parallel or half-head design, and assessed at 3-6 months after starting treatment. The studies were conducted in countries that included the United States, Brazil, Spain, and Italy.
The meta-analysis included seven studies that used change in hair density as the primary outcome. Six of these studies compared PRP with placebo and one compared PRP plus minoxidil or finasteride versus placebo plus minoxidil or finasteride. Five evaluated outcomes 6 months after the intervention, and two studies evaluated outcomes 3 months afterwards.
Of these seven studies, five reported statistically significant increases in hair density in favor of PRP over placebo, while the remaining two did not find any significant treatment effect. In the seven studies, the pooled mean difference in increasing hair density between treatment and placebo was 30.35 (95% confidence interval, 1.77-58.93; P less than .00001).
The 13 studies involved applications of platelet-rich plasma one to eight times over treatment periods of 1-5 months. Three studies compared PRP in conjunction with either minoxidil, finasteride, or polydeoxyribonucleotide, compared with those same therapies without PRP, and 10 studies compared PRP with either normal saline or distilled water. Five studies were judged to be at high risk of bias for at least one item, but none of these studies were included in the meta-analysis.
None of the studies identified any significant short-term treatment-related adverse effects, but the authors suggested that future studies look at potential long-term adverse effects of treatment.
“We recommend further [randomized, controlled trials] that should ensure adequate random sequence generation, adequate allocation concealment, blinding of performance and detection, and that should prevent incomplete data and selective reporting,” they wrote.
Funding and conflicts of interest statements were not available.
SOURCE: Dervishi G et al. J Cosmet Dermatol. 2019 Aug 12. doi: 10.1111/jocd.13113.
FROM THE JOURNAL OF COSMETIC DERMATOLOGY
Benefits of peanut desensitization may not last
based on data from a phase 2 randomized trial of individuals with confirmed peanut allergies.
Previous studies have shown that desensitization to peanuts can be successful, but sustained response to oral immunotherapy after treatment reduction or discontinuation has not been well studied, wrote R. Sharon Chinthrajah, MD, of Stanford (Calif.)University, and colleagues.
“We found that OIT with peanut was able to desensitise people with peanut allergy to 4,000 mg of peanut protein, but that discontinuation of peanut, or even a reduction to 300 mg daily, increased the likelihood of regaining clinical reactivity to peanut,” they wrote. “With peanut allergy therapies in varying stages of clinical development, and some nearing [Food and Drug Administration] approval, vital questions remain regarding the durability of treatment effects and the appropriate maintenance doses.”
In the Peanut Oral Immunotherapy Study: Safety Efficacy and Discovery (POISED), published in The Lancet, the researchers randomized 120 participants to three groups:
• 60 patients built up to a maintenance dose of 4,000 mg of peanut protein for 104 weeks followed by total discontinuation (peanut-0).
• 35 patients built up to a maintenance dose of 4,000 mg of peanut protein for 104 weeks followed by a 300-mg maintenance dose of peanut protein in the form of peanut flour (peanut-300).
• 25 patients to an oat flour placebo.
All participants were trained on how and when to use epinephrine autoinjector devices to treat allergic symptoms such as respiratory problems (cough, shortness of breath, or change in voice), widespread hives or erythema, repetitive vomiting, persistent abdominal pain, angioedema of the face, or feeling faint.
The primary outcome was passing a double-blind, placebo-controlled, food challenge (DBPCFC) to 4,000 mg of peanut protein, which was measured at baseline and at weeks 104, 117, 130, 143, and 156.
Overall, 35% of the peanut-0 group passed the challenge at 104 and 117 weeks, compared with 4% of the placebo group. At week 156 after discontinuing OIT, 13% of the peanut-0 group met the DBPCFC challenge, compared with 4% of the placebo group. However, 37% of participants randomized to a reduced peanut protein dose of 300 mg passed the challenge at 156 weeks, suggesting that more data are needed on optimal maintenance dosing strategies.
Baseline demographics were similar across all groups. The median age at study enrollment was 11 years and the median allergy duration was 9 years. The most common adverse events were mild gastrointestinal and respiratory problems. Adverse events decreased over time in all three groups.
“Higher levels of peanut-specific IgE to total IgE ratio, peanut sIgE, Ara h 1, Ara h 2, and Ara h 1 IgE to peanut-specific IgE ratio at baseline in participants were associated with increased frequencies of adverse events during active peanut OIT,” the researchers noted.
The study findings were limited by several factors including the ability of participants to tolerate 4,000 mg of peanut protein after achieving a maintenance dose but conducting serial testing only for those who passed the challenge. In addition, the results may be limited to peanut and not generalizable to other food allergies, the researchers said.
However, the results suggest that OIT remains a promising treatment for peanut allergies, and the association of biomarkers with clinical outcomes “might help the practitioner in identifying good candidates for OIT and those individuals who warrant increased vigilance against allergic reactions during OIT,” they said.
The National Institutes of Health supported the study. The researchers had no financial conflicts to disclose.
SOURCE: Chinthrajah RS et al. Lancet. 2019 Sep 12. doi: 10.1016/S0140-6736(19)31793-3.
based on data from a phase 2 randomized trial of individuals with confirmed peanut allergies.
Previous studies have shown that desensitization to peanuts can be successful, but sustained response to oral immunotherapy after treatment reduction or discontinuation has not been well studied, wrote R. Sharon Chinthrajah, MD, of Stanford (Calif.)University, and colleagues.
“We found that OIT with peanut was able to desensitise people with peanut allergy to 4,000 mg of peanut protein, but that discontinuation of peanut, or even a reduction to 300 mg daily, increased the likelihood of regaining clinical reactivity to peanut,” they wrote. “With peanut allergy therapies in varying stages of clinical development, and some nearing [Food and Drug Administration] approval, vital questions remain regarding the durability of treatment effects and the appropriate maintenance doses.”
In the Peanut Oral Immunotherapy Study: Safety Efficacy and Discovery (POISED), published in The Lancet, the researchers randomized 120 participants to three groups:
• 60 patients built up to a maintenance dose of 4,000 mg of peanut protein for 104 weeks followed by total discontinuation (peanut-0).
• 35 patients built up to a maintenance dose of 4,000 mg of peanut protein for 104 weeks followed by a 300-mg maintenance dose of peanut protein in the form of peanut flour (peanut-300).
• 25 patients to an oat flour placebo.
All participants were trained on how and when to use epinephrine autoinjector devices to treat allergic symptoms such as respiratory problems (cough, shortness of breath, or change in voice), widespread hives or erythema, repetitive vomiting, persistent abdominal pain, angioedema of the face, or feeling faint.
The primary outcome was passing a double-blind, placebo-controlled, food challenge (DBPCFC) to 4,000 mg of peanut protein, which was measured at baseline and at weeks 104, 117, 130, 143, and 156.
Overall, 35% of the peanut-0 group passed the challenge at 104 and 117 weeks, compared with 4% of the placebo group. At week 156 after discontinuing OIT, 13% of the peanut-0 group met the DBPCFC challenge, compared with 4% of the placebo group. However, 37% of participants randomized to a reduced peanut protein dose of 300 mg passed the challenge at 156 weeks, suggesting that more data are needed on optimal maintenance dosing strategies.
Baseline demographics were similar across all groups. The median age at study enrollment was 11 years and the median allergy duration was 9 years. The most common adverse events were mild gastrointestinal and respiratory problems. Adverse events decreased over time in all three groups.
“Higher levels of peanut-specific IgE to total IgE ratio, peanut sIgE, Ara h 1, Ara h 2, and Ara h 1 IgE to peanut-specific IgE ratio at baseline in participants were associated with increased frequencies of adverse events during active peanut OIT,” the researchers noted.
The study findings were limited by several factors including the ability of participants to tolerate 4,000 mg of peanut protein after achieving a maintenance dose but conducting serial testing only for those who passed the challenge. In addition, the results may be limited to peanut and not generalizable to other food allergies, the researchers said.
However, the results suggest that OIT remains a promising treatment for peanut allergies, and the association of biomarkers with clinical outcomes “might help the practitioner in identifying good candidates for OIT and those individuals who warrant increased vigilance against allergic reactions during OIT,” they said.
The National Institutes of Health supported the study. The researchers had no financial conflicts to disclose.
SOURCE: Chinthrajah RS et al. Lancet. 2019 Sep 12. doi: 10.1016/S0140-6736(19)31793-3.
based on data from a phase 2 randomized trial of individuals with confirmed peanut allergies.
Previous studies have shown that desensitization to peanuts can be successful, but sustained response to oral immunotherapy after treatment reduction or discontinuation has not been well studied, wrote R. Sharon Chinthrajah, MD, of Stanford (Calif.)University, and colleagues.
“We found that OIT with peanut was able to desensitise people with peanut allergy to 4,000 mg of peanut protein, but that discontinuation of peanut, or even a reduction to 300 mg daily, increased the likelihood of regaining clinical reactivity to peanut,” they wrote. “With peanut allergy therapies in varying stages of clinical development, and some nearing [Food and Drug Administration] approval, vital questions remain regarding the durability of treatment effects and the appropriate maintenance doses.”
In the Peanut Oral Immunotherapy Study: Safety Efficacy and Discovery (POISED), published in The Lancet, the researchers randomized 120 participants to three groups:
• 60 patients built up to a maintenance dose of 4,000 mg of peanut protein for 104 weeks followed by total discontinuation (peanut-0).
• 35 patients built up to a maintenance dose of 4,000 mg of peanut protein for 104 weeks followed by a 300-mg maintenance dose of peanut protein in the form of peanut flour (peanut-300).
• 25 patients to an oat flour placebo.
All participants were trained on how and when to use epinephrine autoinjector devices to treat allergic symptoms such as respiratory problems (cough, shortness of breath, or change in voice), widespread hives or erythema, repetitive vomiting, persistent abdominal pain, angioedema of the face, or feeling faint.
The primary outcome was passing a double-blind, placebo-controlled, food challenge (DBPCFC) to 4,000 mg of peanut protein, which was measured at baseline and at weeks 104, 117, 130, 143, and 156.
Overall, 35% of the peanut-0 group passed the challenge at 104 and 117 weeks, compared with 4% of the placebo group. At week 156 after discontinuing OIT, 13% of the peanut-0 group met the DBPCFC challenge, compared with 4% of the placebo group. However, 37% of participants randomized to a reduced peanut protein dose of 300 mg passed the challenge at 156 weeks, suggesting that more data are needed on optimal maintenance dosing strategies.
Baseline demographics were similar across all groups. The median age at study enrollment was 11 years and the median allergy duration was 9 years. The most common adverse events were mild gastrointestinal and respiratory problems. Adverse events decreased over time in all three groups.
“Higher levels of peanut-specific IgE to total IgE ratio, peanut sIgE, Ara h 1, Ara h 2, and Ara h 1 IgE to peanut-specific IgE ratio at baseline in participants were associated with increased frequencies of adverse events during active peanut OIT,” the researchers noted.
The study findings were limited by several factors including the ability of participants to tolerate 4,000 mg of peanut protein after achieving a maintenance dose but conducting serial testing only for those who passed the challenge. In addition, the results may be limited to peanut and not generalizable to other food allergies, the researchers said.
However, the results suggest that OIT remains a promising treatment for peanut allergies, and the association of biomarkers with clinical outcomes “might help the practitioner in identifying good candidates for OIT and those individuals who warrant increased vigilance against allergic reactions during OIT,” they said.
The National Institutes of Health supported the study. The researchers had no financial conflicts to disclose.
SOURCE: Chinthrajah RS et al. Lancet. 2019 Sep 12. doi: 10.1016/S0140-6736(19)31793-3.
FROM THE LANCET
Prolonged Antibiotic Treatment in Newborns May Promote Multidrug Resistance
Antibiotics given to preterm infants can set them up for health problems later in life; research has shown, including allergies, psoriasis, diabetes, and inflammatory bowel disease. Researchers who conducted a National Institutes of Health (NIH)-funded study have added to that body of knowledge with their finding that treating preterm infants with long-term antibiotics could have lasting effects by promoting multidrug-resistant gut bacteria.
They used high-speed DNA sequencing and advanced computational analysis to study stool samples from 32 infants born very preterm who received antibiotic treatment for 21 months in the hospital and after discharge, then compared those with results from 9 very preterm infants treated with antibiotics for > 1 week and 17 healthy term and late-term infants who had not received antibiotics.
The infants on long-term antibiotics had less diverse bacterial populations in their gut, and those bacteria contained more antibiotic-resistant genes.
Strikingly, the genomes of the high-antibiotic-use samples contained genes for resistance to antibiotics typically not given to newborns, such as ciprofloxacin and chloramphenicol. The researchers say this may mean that the genes originate in multidrug-resistant bacteria. Using a particular antibiotic may trigger resistance to other antibiotics even if they were not used.
“The collateral damage of early-life antibiotic treatment and hospitalization in preterm infants is long lasting,” the researchers say. They urge development of strategies to protect these highly vulnerable patients.
Antibiotics given to preterm infants can set them up for health problems later in life; research has shown, including allergies, psoriasis, diabetes, and inflammatory bowel disease. Researchers who conducted a National Institutes of Health (NIH)-funded study have added to that body of knowledge with their finding that treating preterm infants with long-term antibiotics could have lasting effects by promoting multidrug-resistant gut bacteria.
They used high-speed DNA sequencing and advanced computational analysis to study stool samples from 32 infants born very preterm who received antibiotic treatment for 21 months in the hospital and after discharge, then compared those with results from 9 very preterm infants treated with antibiotics for > 1 week and 17 healthy term and late-term infants who had not received antibiotics.
The infants on long-term antibiotics had less diverse bacterial populations in their gut, and those bacteria contained more antibiotic-resistant genes.
Strikingly, the genomes of the high-antibiotic-use samples contained genes for resistance to antibiotics typically not given to newborns, such as ciprofloxacin and chloramphenicol. The researchers say this may mean that the genes originate in multidrug-resistant bacteria. Using a particular antibiotic may trigger resistance to other antibiotics even if they were not used.
“The collateral damage of early-life antibiotic treatment and hospitalization in preterm infants is long lasting,” the researchers say. They urge development of strategies to protect these highly vulnerable patients.
Antibiotics given to preterm infants can set them up for health problems later in life; research has shown, including allergies, psoriasis, diabetes, and inflammatory bowel disease. Researchers who conducted a National Institutes of Health (NIH)-funded study have added to that body of knowledge with their finding that treating preterm infants with long-term antibiotics could have lasting effects by promoting multidrug-resistant gut bacteria.
They used high-speed DNA sequencing and advanced computational analysis to study stool samples from 32 infants born very preterm who received antibiotic treatment for 21 months in the hospital and after discharge, then compared those with results from 9 very preterm infants treated with antibiotics for > 1 week and 17 healthy term and late-term infants who had not received antibiotics.
The infants on long-term antibiotics had less diverse bacterial populations in their gut, and those bacteria contained more antibiotic-resistant genes.
Strikingly, the genomes of the high-antibiotic-use samples contained genes for resistance to antibiotics typically not given to newborns, such as ciprofloxacin and chloramphenicol. The researchers say this may mean that the genes originate in multidrug-resistant bacteria. Using a particular antibiotic may trigger resistance to other antibiotics even if they were not used.
“The collateral damage of early-life antibiotic treatment and hospitalization in preterm infants is long lasting,” the researchers say. They urge development of strategies to protect these highly vulnerable patients.