User login
Intense stinging and burning, followed by the development of skin lesions minutes after exposure to a plant
Urticaria from stinging nettle
The stinging nettle (Urtica dioica) is a plant that grows in the United States, Eurasia, Northern Africa, and some parts of South America. It is commonly found in patches along hiking trails and near streams. The leaves are green with a characteristic tapered tip and bear tiny spines or hairs. The spines contain substances such as histamine, serotonin, and acetylcholine. Within seconds of contact with the stinging nettle, sharp stinging and burning will occur. Urticaria and pruritus may appear a few minutes later and may last up to 24 hours. The plant is eaten in some parts of the world and has been used as medicine.
The wood nettle (Laportea canadensis) is a relative of the stinging nettle that often grows in woodlands. Like the stinging nettle, the wood nettle leaves are covered with spines that sting when they come into contact with skin. However, the leaves are shorter and more oval shaped that the stinging nettle, and they lack the tapered tip that is characteristic for the stinging nettle. The reaction from the wood nettle is generally milder than that of the stinging nettle.
Plants can illicit different types of reactions in the skin: urticaria (immunologic and toxin mediated), irritant dermatitis (mechanical and chemical), phototoxic dermatitis (phytophotodermatitis), and allergic contact dermatitis. where anyone coming into contact with the hairs of the plant can be affected. Previous sensitization is not required. The reaction usually occurs immediately after exposure.
The allergic contact dermatitis seen with toxicodendron (poison ivy and poison sumac) appears 48 hours after exposure of a previously sensitized person to the plant. This type of delayed hypersensitivity reaction is known as cell-mediated hypersensitivity. Generally, no reaction is elicited upon the first exposure to the allergen. In fact, it may take years of exposure to allergens for someone to develop an allergic contact dermatitis.
The poison ivy plant can grow anywhere and is characteristically found in “leaves of three.” Skin reactions are often appear as linearly-arranged vesicles a few days after the exposure to the urushiol chemical in the sap of the plant. Poison sumac has red stems with 7-12 green, smooth leaves, and causes a similar skin reaction as poison ivy. It typically grows in wet areas.
Most stings are self-limited. Topical corticosteroid creams may be used if needed.
This case and photo were submitted by Susannah McClain, MD, of Three Rivers Dermatology in Coraopolis, Pa., and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Urticaria from stinging nettle
The stinging nettle (Urtica dioica) is a plant that grows in the United States, Eurasia, Northern Africa, and some parts of South America. It is commonly found in patches along hiking trails and near streams. The leaves are green with a characteristic tapered tip and bear tiny spines or hairs. The spines contain substances such as histamine, serotonin, and acetylcholine. Within seconds of contact with the stinging nettle, sharp stinging and burning will occur. Urticaria and pruritus may appear a few minutes later and may last up to 24 hours. The plant is eaten in some parts of the world and has been used as medicine.
The wood nettle (Laportea canadensis) is a relative of the stinging nettle that often grows in woodlands. Like the stinging nettle, the wood nettle leaves are covered with spines that sting when they come into contact with skin. However, the leaves are shorter and more oval shaped that the stinging nettle, and they lack the tapered tip that is characteristic for the stinging nettle. The reaction from the wood nettle is generally milder than that of the stinging nettle.
Plants can illicit different types of reactions in the skin: urticaria (immunologic and toxin mediated), irritant dermatitis (mechanical and chemical), phototoxic dermatitis (phytophotodermatitis), and allergic contact dermatitis. where anyone coming into contact with the hairs of the plant can be affected. Previous sensitization is not required. The reaction usually occurs immediately after exposure.
The allergic contact dermatitis seen with toxicodendron (poison ivy and poison sumac) appears 48 hours after exposure of a previously sensitized person to the plant. This type of delayed hypersensitivity reaction is known as cell-mediated hypersensitivity. Generally, no reaction is elicited upon the first exposure to the allergen. In fact, it may take years of exposure to allergens for someone to develop an allergic contact dermatitis.
The poison ivy plant can grow anywhere and is characteristically found in “leaves of three.” Skin reactions are often appear as linearly-arranged vesicles a few days after the exposure to the urushiol chemical in the sap of the plant. Poison sumac has red stems with 7-12 green, smooth leaves, and causes a similar skin reaction as poison ivy. It typically grows in wet areas.
Most stings are self-limited. Topical corticosteroid creams may be used if needed.
This case and photo were submitted by Susannah McClain, MD, of Three Rivers Dermatology in Coraopolis, Pa., and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Urticaria from stinging nettle
The stinging nettle (Urtica dioica) is a plant that grows in the United States, Eurasia, Northern Africa, and some parts of South America. It is commonly found in patches along hiking trails and near streams. The leaves are green with a characteristic tapered tip and bear tiny spines or hairs. The spines contain substances such as histamine, serotonin, and acetylcholine. Within seconds of contact with the stinging nettle, sharp stinging and burning will occur. Urticaria and pruritus may appear a few minutes later and may last up to 24 hours. The plant is eaten in some parts of the world and has been used as medicine.
The wood nettle (Laportea canadensis) is a relative of the stinging nettle that often grows in woodlands. Like the stinging nettle, the wood nettle leaves are covered with spines that sting when they come into contact with skin. However, the leaves are shorter and more oval shaped that the stinging nettle, and they lack the tapered tip that is characteristic for the stinging nettle. The reaction from the wood nettle is generally milder than that of the stinging nettle.
Plants can illicit different types of reactions in the skin: urticaria (immunologic and toxin mediated), irritant dermatitis (mechanical and chemical), phototoxic dermatitis (phytophotodermatitis), and allergic contact dermatitis. where anyone coming into contact with the hairs of the plant can be affected. Previous sensitization is not required. The reaction usually occurs immediately after exposure.
The allergic contact dermatitis seen with toxicodendron (poison ivy and poison sumac) appears 48 hours after exposure of a previously sensitized person to the plant. This type of delayed hypersensitivity reaction is known as cell-mediated hypersensitivity. Generally, no reaction is elicited upon the first exposure to the allergen. In fact, it may take years of exposure to allergens for someone to develop an allergic contact dermatitis.
The poison ivy plant can grow anywhere and is characteristically found in “leaves of three.” Skin reactions are often appear as linearly-arranged vesicles a few days after the exposure to the urushiol chemical in the sap of the plant. Poison sumac has red stems with 7-12 green, smooth leaves, and causes a similar skin reaction as poison ivy. It typically grows in wet areas.
Most stings are self-limited. Topical corticosteroid creams may be used if needed.
This case and photo were submitted by Susannah McClain, MD, of Three Rivers Dermatology in Coraopolis, Pa., and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
His Heart’s Awkward Timing
ANSWER
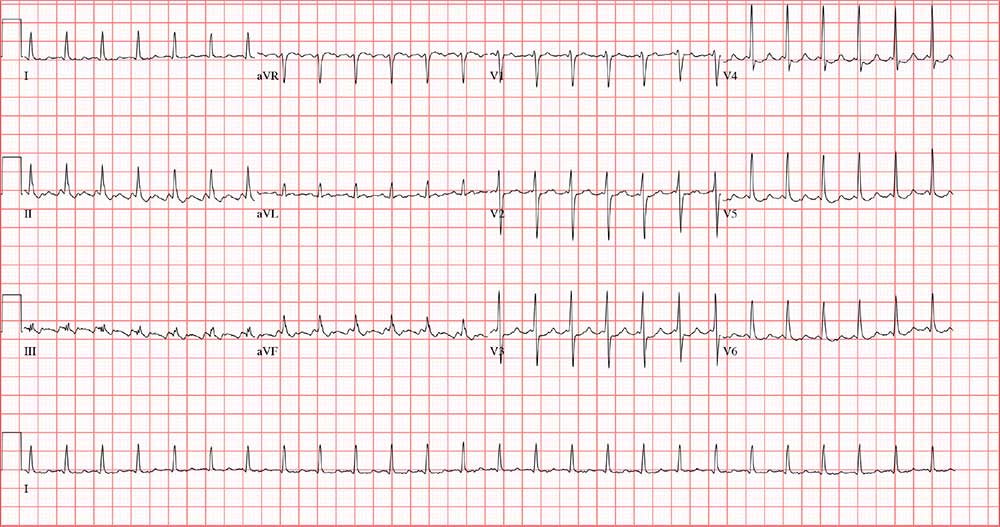
The correct answer is atrial flutter with 2:1 atrioventricular (AV) conduction. The QRS complexes are narrow and regular, indicating the rhythm originates within the atria or AV node, with conduction down the normal His-Purkinje system, and not from the ventricles.
The regular rate of the P waves and QRS complexes rules out atrial fibrillation with a rapid ventricular response. If you look carefully, you’ll see a P wave immediately before each QRS complex, and you’ll also see a P wave at the onset of the T wave (best seen in leads II, V3, and V6) resulting in what looks like a notched T wave. If you measure the duration of the P at the onset of the T wave to the P wave prior to the QRS complex, you’ll see the intervals are regular and march through the QRS complexes.
With 2 P waves for every QRS complex, the atria are contracting at 310 beats/min (193 ms), a rate consistent with atrial flutter in a 2:1 conduction pattern, compared to the ventricular rate of 155 beats/min (387 ms).
ANSWER
The correct answer is atrial flutter with 2:1 atrioventricular (AV) conduction. The QRS complexes are narrow and regular, indicating the rhythm originates within the atria or AV node, with conduction down the normal His-Purkinje system, and not from the ventricles.
The regular rate of the P waves and QRS complexes rules out atrial fibrillation with a rapid ventricular response. If you look carefully, you’ll see a P wave immediately before each QRS complex, and you’ll also see a P wave at the onset of the T wave (best seen in leads II, V3, and V6) resulting in what looks like a notched T wave. If you measure the duration of the P at the onset of the T wave to the P wave prior to the QRS complex, you’ll see the intervals are regular and march through the QRS complexes.
With 2 P waves for every QRS complex, the atria are contracting at 310 beats/min (193 ms), a rate consistent with atrial flutter in a 2:1 conduction pattern, compared to the ventricular rate of 155 beats/min (387 ms).
ANSWER
The correct answer is atrial flutter with 2:1 atrioventricular (AV) conduction. The QRS complexes are narrow and regular, indicating the rhythm originates within the atria or AV node, with conduction down the normal His-Purkinje system, and not from the ventricles.
The regular rate of the P waves and QRS complexes rules out atrial fibrillation with a rapid ventricular response. If you look carefully, you’ll see a P wave immediately before each QRS complex, and you’ll also see a P wave at the onset of the T wave (best seen in leads II, V3, and V6) resulting in what looks like a notched T wave. If you measure the duration of the P at the onset of the T wave to the P wave prior to the QRS complex, you’ll see the intervals are regular and march through the QRS complexes.
With 2 P waves for every QRS complex, the atria are contracting at 310 beats/min (193 ms), a rate consistent with atrial flutter in a 2:1 conduction pattern, compared to the ventricular rate of 155 beats/min (387 ms).
Approximately 5
Feeling fine, he went about his normal workday—but in the afternoon, while sitting at his desk, his rapid heart rate returned. He called over a coworker, who observed that he was “pale” and “sweaty.” His pulse was 130 beats/min. After “a few minutes,” the rapid heart rate spontaneously terminated, and he decided to take off the rest of the day.
This morning, he again awoke with a rapid heart rate and lightheadedness—but he also felt like the room was spinning. At that point, he called 911. By the time the paramedics arrived, his rapid heart rate had spontaneously terminated. Understandably concerned, however, he requested transport to your facility.
The patient says he is in normal health, with no prior cardiac history. He denies any chest pain, dyspnea, shortness of breath, nausea, vomiting, syncope, or near-syncope associated with his recent episodes.
Medical history is remarkable for hypertension, hyperlipidemia, and type 2 diabetes. He has had no surgical procedures. His medications include aspirin, lisinopril, and lovastatin; he says he takes his medications as prescribed and there have been no recent changes to the drugs or dosages. He has no known drug allergies.
Family history includes myocardial infarction in both parents; they are alive and well. The patient’s younger brother has Wolff-Parkinson-White syndrome and underwent an ablation at age 24.
The patient is a practicing attorney for a local firm. He is married with 2 children. He has no history of alcohol, tobacco, or illicit drug use.
Review of systems is positive for a 10-lb weight gain over the past 6 months and new-onset nocturia.
During the physical exam, the patient informs you that his heart is racing again. The exam is suspended, and a 12-lead ECG is quickly performed. It shows a ventricular rate of 155 beats/min; no measurable PR interval; QRS duration, 78 ms; QT/QTc interval, 272/437 ms; P axis, unmeasurable; R axis, 34°; and T axis, –50°.
The physical exam is completed after the tachycardia spontaneously terminates. The patient’s blood pressure is 148/88 mm Hg; pulse, 94 beats/min and regular; respiratory rate, 18 breaths/min-1; and temperature, 97.9°F. He appears frightened but otherwise healthy. Pertinent findings of the physical exam include a normal fundoscopic examination with sharp disc margins, clear breath sounds bilaterally, normal heart sounds with no murmur or rub, a soft abdomen with no palpable masses, strong and equal pulses bilaterally in both upper and lower extremities, and a normal neurologic exam with no cognitive deficits.
Now that the physical exam is complete, what is your interpretation of this ECG?
Presumptive style of conversation boosts HPV vaccination rates in adolescents
A majority of primary care physicians recommended the human papillomavirus (HPV) vaccine to children aged 11-12 years and older, and about half of them used a presumptive style to recommend the vaccine, based on survey responses from 530 clinicians.
“Because of the crucial role of provider recommendation in parental decisions to vaccinate, a great deal of research and intervention efforts have been focused on improving provider communication regarding HPV vaccination,” Allison Kempe, MD, of the University of Colorado and Children’s Hospital Colorado, Aurora, and her colleagues wrote in Pediatrics.
“A presumptive style of initiating HPV vaccine discussions uses words that convey an assumption of vaccination and does not discuss the HPV vaccine in a different manner than other adolescent vaccines,” the authors explained. By contrast, “a conversational style engages parents in an open-ended discussion about the HPV vaccine without linguistic presupposition of vaccination.” Findings from multiple studies have shown that the presumptive approach is associated with higher acceptance of the HPV vaccine, compared with the conversational approach.
The researchers examined survey responses from a nationally representative sample of 302 pediatricians and 228 family physicians. Almost all clinicians in both specialties (99% of pediatricians, 90% of FPs) said they strongly recommended the HPV vaccine for girls aged 15 years and older. Strong recommendations for the HPV vaccine were lowest in both specialties for boys aged 11-12 years (83% of pediatricians, 66% of FPs).
Significantly more pediatricians than FPs reported using a presumptive style when discussing the HPV vaccine (65% vs. 42%, respectively; P <.0001). Overall, 40% of the survey respondents used standing orders for HPV vaccination and 42% had electronic alerts in patients’ medical records to prompt an HPV vaccine discussion.
The proportion of pediatricians who reported a vaccine refusal or deferral rate of 50% or higher for patients aged 11-12 years was 10% for girls and 23% for boys; among FPs, those percentages were 27% for girls and 36% for boys.
In multivariate analysis, the factors associated with a 50% or higher refusal or deferral rate among 11- to 12-year-olds were similar for both genders and included “not strongly recommending [the vaccine] to 11- to 12-year-old patients, not … always using a presumptive recommendation style, strongly agreeing that they encounter less resistance to HPV vaccination from patients aged 13 years versus patients aged 11 years, and anticipating an uncomfortable discussion when recommending to 11- to 12-year-old patients,” the researchers wrote.
More physicians in both specialties made stronger recommendations for HPV vaccination for patients aged 13 years and older than for those aged 11 and 12 years. However, physicians might overestimate parent and patient resistance to a strong recommendation for the HPV vaccine. A strong recommendation, “delivered in the same way as for other adolescent vaccines and on same day as other adolescent vaccines, may be key to increasing acceptance among parents of 11- to 12-year-old patients,” Dr. Kempe and associates said.
The current two-dose vaccine schedule also promoted complete vaccination, according to a majority of pediatricians and FPs.
The study findings were limited by several factors, including the use of self-reports and the potential lack of generalizability of the survey responses. The results, however, were strengthened by the large, nationally representative sample and suggest that the number of physicians who strongly recommend HPV vaccination to 11- and 12-year-olds has increased over the past 5 years, they said.
“Increased use of available communication training materials and applications, as well as further development of evidence-based messages for parents, may be helpful in improving the way HPV is introduced,” the investigators concluded.
The study was supported by the Centers for Disease Control and Prevention. The researchers reported that they had no financial conflicts.
SOURCE: Kempe A et al. Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-1475.
A majority of primary care physicians recommended the human papillomavirus (HPV) vaccine to children aged 11-12 years and older, and about half of them used a presumptive style to recommend the vaccine, based on survey responses from 530 clinicians.
“Because of the crucial role of provider recommendation in parental decisions to vaccinate, a great deal of research and intervention efforts have been focused on improving provider communication regarding HPV vaccination,” Allison Kempe, MD, of the University of Colorado and Children’s Hospital Colorado, Aurora, and her colleagues wrote in Pediatrics.
“A presumptive style of initiating HPV vaccine discussions uses words that convey an assumption of vaccination and does not discuss the HPV vaccine in a different manner than other adolescent vaccines,” the authors explained. By contrast, “a conversational style engages parents in an open-ended discussion about the HPV vaccine without linguistic presupposition of vaccination.” Findings from multiple studies have shown that the presumptive approach is associated with higher acceptance of the HPV vaccine, compared with the conversational approach.
The researchers examined survey responses from a nationally representative sample of 302 pediatricians and 228 family physicians. Almost all clinicians in both specialties (99% of pediatricians, 90% of FPs) said they strongly recommended the HPV vaccine for girls aged 15 years and older. Strong recommendations for the HPV vaccine were lowest in both specialties for boys aged 11-12 years (83% of pediatricians, 66% of FPs).
Significantly more pediatricians than FPs reported using a presumptive style when discussing the HPV vaccine (65% vs. 42%, respectively; P <.0001). Overall, 40% of the survey respondents used standing orders for HPV vaccination and 42% had electronic alerts in patients’ medical records to prompt an HPV vaccine discussion.
The proportion of pediatricians who reported a vaccine refusal or deferral rate of 50% or higher for patients aged 11-12 years was 10% for girls and 23% for boys; among FPs, those percentages were 27% for girls and 36% for boys.
In multivariate analysis, the factors associated with a 50% or higher refusal or deferral rate among 11- to 12-year-olds were similar for both genders and included “not strongly recommending [the vaccine] to 11- to 12-year-old patients, not … always using a presumptive recommendation style, strongly agreeing that they encounter less resistance to HPV vaccination from patients aged 13 years versus patients aged 11 years, and anticipating an uncomfortable discussion when recommending to 11- to 12-year-old patients,” the researchers wrote.
More physicians in both specialties made stronger recommendations for HPV vaccination for patients aged 13 years and older than for those aged 11 and 12 years. However, physicians might overestimate parent and patient resistance to a strong recommendation for the HPV vaccine. A strong recommendation, “delivered in the same way as for other adolescent vaccines and on same day as other adolescent vaccines, may be key to increasing acceptance among parents of 11- to 12-year-old patients,” Dr. Kempe and associates said.
The current two-dose vaccine schedule also promoted complete vaccination, according to a majority of pediatricians and FPs.
The study findings were limited by several factors, including the use of self-reports and the potential lack of generalizability of the survey responses. The results, however, were strengthened by the large, nationally representative sample and suggest that the number of physicians who strongly recommend HPV vaccination to 11- and 12-year-olds has increased over the past 5 years, they said.
“Increased use of available communication training materials and applications, as well as further development of evidence-based messages for parents, may be helpful in improving the way HPV is introduced,” the investigators concluded.
The study was supported by the Centers for Disease Control and Prevention. The researchers reported that they had no financial conflicts.
SOURCE: Kempe A et al. Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-1475.
A majority of primary care physicians recommended the human papillomavirus (HPV) vaccine to children aged 11-12 years and older, and about half of them used a presumptive style to recommend the vaccine, based on survey responses from 530 clinicians.
“Because of the crucial role of provider recommendation in parental decisions to vaccinate, a great deal of research and intervention efforts have been focused on improving provider communication regarding HPV vaccination,” Allison Kempe, MD, of the University of Colorado and Children’s Hospital Colorado, Aurora, and her colleagues wrote in Pediatrics.
“A presumptive style of initiating HPV vaccine discussions uses words that convey an assumption of vaccination and does not discuss the HPV vaccine in a different manner than other adolescent vaccines,” the authors explained. By contrast, “a conversational style engages parents in an open-ended discussion about the HPV vaccine without linguistic presupposition of vaccination.” Findings from multiple studies have shown that the presumptive approach is associated with higher acceptance of the HPV vaccine, compared with the conversational approach.
The researchers examined survey responses from a nationally representative sample of 302 pediatricians and 228 family physicians. Almost all clinicians in both specialties (99% of pediatricians, 90% of FPs) said they strongly recommended the HPV vaccine for girls aged 15 years and older. Strong recommendations for the HPV vaccine were lowest in both specialties for boys aged 11-12 years (83% of pediatricians, 66% of FPs).
Significantly more pediatricians than FPs reported using a presumptive style when discussing the HPV vaccine (65% vs. 42%, respectively; P <.0001). Overall, 40% of the survey respondents used standing orders for HPV vaccination and 42% had electronic alerts in patients’ medical records to prompt an HPV vaccine discussion.
The proportion of pediatricians who reported a vaccine refusal or deferral rate of 50% or higher for patients aged 11-12 years was 10% for girls and 23% for boys; among FPs, those percentages were 27% for girls and 36% for boys.
In multivariate analysis, the factors associated with a 50% or higher refusal or deferral rate among 11- to 12-year-olds were similar for both genders and included “not strongly recommending [the vaccine] to 11- to 12-year-old patients, not … always using a presumptive recommendation style, strongly agreeing that they encounter less resistance to HPV vaccination from patients aged 13 years versus patients aged 11 years, and anticipating an uncomfortable discussion when recommending to 11- to 12-year-old patients,” the researchers wrote.
More physicians in both specialties made stronger recommendations for HPV vaccination for patients aged 13 years and older than for those aged 11 and 12 years. However, physicians might overestimate parent and patient resistance to a strong recommendation for the HPV vaccine. A strong recommendation, “delivered in the same way as for other adolescent vaccines and on same day as other adolescent vaccines, may be key to increasing acceptance among parents of 11- to 12-year-old patients,” Dr. Kempe and associates said.
The current two-dose vaccine schedule also promoted complete vaccination, according to a majority of pediatricians and FPs.
The study findings were limited by several factors, including the use of self-reports and the potential lack of generalizability of the survey responses. The results, however, were strengthened by the large, nationally representative sample and suggest that the number of physicians who strongly recommend HPV vaccination to 11- and 12-year-olds has increased over the past 5 years, they said.
“Increased use of available communication training materials and applications, as well as further development of evidence-based messages for parents, may be helpful in improving the way HPV is introduced,” the investigators concluded.
The study was supported by the Centers for Disease Control and Prevention. The researchers reported that they had no financial conflicts.
SOURCE: Kempe A et al. Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-1475.
FROM PEDIATRICS
Key clinical point: A presumptive style of conversation and a two-dose vaccination schedule can increase HPV vaccination rates in adolescents.
Major finding: Overall, 65% of pediatricians and 42% of FPs reported using a presumptive style to discuss HPV vaccination.
Study details: National survey of 302 pediatricians and 228 family physicians conducted July-September 2018.
Disclosures: The study was supported by the Centers for Disease Control and Prevention. The researchers reported that they had no financial conflicts.
Source: Kempe A et al. Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-1475.
Rates of off-label prescribing for children continue to increase
Physicians continue to prescribe off-label drugs for children, with rates increasing over a 10-year period from 2006 to 2015, according to findings from a new study.
The increase occurred despite recent legislation aimed at encouraging pediatric clinical trials, with the intention of improving the “quality of evidence and the number of drugs approved for children,” Divya Hoon of Rutgers University in New Brunswick, N.J., and colleagues wrote in Pediatrics.
“[Our] results can help inform ongoing education, research, and policies around efficacious, effective, and safe use of medications in children,” the researchers said.
To determine trends in, and categories of, drugs prescribed off label, the researchers used data from the National Ambulatory Medical Care Surveys for all pediatric visits and subsequent drug orders from 2006 to 2015. They focused on 141 drugs that are predominantly or exclusively used in systemic formulations and that had been ordered at least 30 times.
At least one off-label systemic drug order occurred at 18.5% of the 1.74 billion estimated ambulatory pediatric visits (95% confidence interval, 17.7%-19.3%), totaling 41.2 million off-label orders per year. The primary reason for a drug being considered off label was that it was for an unapproved condition (74.6%), followed by patient age (17.6%) and weight (0.6%). Absolute and relative rates of off-label ordering increased throughout the study, especially in regard to antihistamines and psychotropic drugs, the investigators said.
In an accompanying editorial, Katelyn Yackey, MD, of the University of Kentucky Children’s Hospital, Lexington, and Rachel Stanley, MD, of Nationwide Children’s Hospital, Columbus, Ohio, stated that “off label is not synonymous with off evidence” and emphasized the need for more clinical trials of medications for children (Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-1571).
“Although drugs are often used off label, there may be sufficient preliminary research about a medical condition and particular drugs to support their use,” they wrote. While recognizing that evaluating medications in pediatric patients has been challenging, they added that “children continue to receive medications off label and for unapproved conditions,” so studies that evaluate “safety, efficacy, pharmacokinetics, and optimal dosing in pediatric patients” remain a necessity.
Though the research featured a long study period and large study population, the authors recognized its possible limitations, including the exclusion of less commonly ordered drugs, the inability to determine drug formulation or dosage, and the fact that the survey data captured only ordered medicines and not whether they were actually dispensed or consumed.
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Rutgers Robert Wood Johnson Medical School Summer Research Fellowship. The authors reported no conflicts of interest.
SOURCE: Hoon D et al. Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-0896.
Physicians continue to prescribe off-label drugs for children, with rates increasing over a 10-year period from 2006 to 2015, according to findings from a new study.
The increase occurred despite recent legislation aimed at encouraging pediatric clinical trials, with the intention of improving the “quality of evidence and the number of drugs approved for children,” Divya Hoon of Rutgers University in New Brunswick, N.J., and colleagues wrote in Pediatrics.
“[Our] results can help inform ongoing education, research, and policies around efficacious, effective, and safe use of medications in children,” the researchers said.
To determine trends in, and categories of, drugs prescribed off label, the researchers used data from the National Ambulatory Medical Care Surveys for all pediatric visits and subsequent drug orders from 2006 to 2015. They focused on 141 drugs that are predominantly or exclusively used in systemic formulations and that had been ordered at least 30 times.
At least one off-label systemic drug order occurred at 18.5% of the 1.74 billion estimated ambulatory pediatric visits (95% confidence interval, 17.7%-19.3%), totaling 41.2 million off-label orders per year. The primary reason for a drug being considered off label was that it was for an unapproved condition (74.6%), followed by patient age (17.6%) and weight (0.6%). Absolute and relative rates of off-label ordering increased throughout the study, especially in regard to antihistamines and psychotropic drugs, the investigators said.
In an accompanying editorial, Katelyn Yackey, MD, of the University of Kentucky Children’s Hospital, Lexington, and Rachel Stanley, MD, of Nationwide Children’s Hospital, Columbus, Ohio, stated that “off label is not synonymous with off evidence” and emphasized the need for more clinical trials of medications for children (Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-1571).
“Although drugs are often used off label, there may be sufficient preliminary research about a medical condition and particular drugs to support their use,” they wrote. While recognizing that evaluating medications in pediatric patients has been challenging, they added that “children continue to receive medications off label and for unapproved conditions,” so studies that evaluate “safety, efficacy, pharmacokinetics, and optimal dosing in pediatric patients” remain a necessity.
Though the research featured a long study period and large study population, the authors recognized its possible limitations, including the exclusion of less commonly ordered drugs, the inability to determine drug formulation or dosage, and the fact that the survey data captured only ordered medicines and not whether they were actually dispensed or consumed.
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Rutgers Robert Wood Johnson Medical School Summer Research Fellowship. The authors reported no conflicts of interest.
SOURCE: Hoon D et al. Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-0896.
Physicians continue to prescribe off-label drugs for children, with rates increasing over a 10-year period from 2006 to 2015, according to findings from a new study.
The increase occurred despite recent legislation aimed at encouraging pediatric clinical trials, with the intention of improving the “quality of evidence and the number of drugs approved for children,” Divya Hoon of Rutgers University in New Brunswick, N.J., and colleagues wrote in Pediatrics.
“[Our] results can help inform ongoing education, research, and policies around efficacious, effective, and safe use of medications in children,” the researchers said.
To determine trends in, and categories of, drugs prescribed off label, the researchers used data from the National Ambulatory Medical Care Surveys for all pediatric visits and subsequent drug orders from 2006 to 2015. They focused on 141 drugs that are predominantly or exclusively used in systemic formulations and that had been ordered at least 30 times.
At least one off-label systemic drug order occurred at 18.5% of the 1.74 billion estimated ambulatory pediatric visits (95% confidence interval, 17.7%-19.3%), totaling 41.2 million off-label orders per year. The primary reason for a drug being considered off label was that it was for an unapproved condition (74.6%), followed by patient age (17.6%) and weight (0.6%). Absolute and relative rates of off-label ordering increased throughout the study, especially in regard to antihistamines and psychotropic drugs, the investigators said.
In an accompanying editorial, Katelyn Yackey, MD, of the University of Kentucky Children’s Hospital, Lexington, and Rachel Stanley, MD, of Nationwide Children’s Hospital, Columbus, Ohio, stated that “off label is not synonymous with off evidence” and emphasized the need for more clinical trials of medications for children (Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-1571).
“Although drugs are often used off label, there may be sufficient preliminary research about a medical condition and particular drugs to support their use,” they wrote. While recognizing that evaluating medications in pediatric patients has been challenging, they added that “children continue to receive medications off label and for unapproved conditions,” so studies that evaluate “safety, efficacy, pharmacokinetics, and optimal dosing in pediatric patients” remain a necessity.
Though the research featured a long study period and large study population, the authors recognized its possible limitations, including the exclusion of less commonly ordered drugs, the inability to determine drug formulation or dosage, and the fact that the survey data captured only ordered medicines and not whether they were actually dispensed or consumed.
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Rutgers Robert Wood Johnson Medical School Summer Research Fellowship. The authors reported no conflicts of interest.
SOURCE: Hoon D et al. Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-0896.
FROM PEDIATRICS
Key clinical point:
Major finding: At least one off-label systemic drug order occurred at 18.5% of the 1.74 billion estimated ambulatory pediatric visits (95% CI, 17.7%-19.3%).
Study details: A retrospective study of serial, cross-sectional data from the National Ambulatory Medical Care Surveys (2006-2015).
Disclosures: The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Rutgers Robert Wood Johnson Medical School Summer Research Fellowship. The authors reported no conflicts of interest.
Source: Hoon D et al. Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-0896.
Newer drugs provide superior disease activity control in pediatric MS
STOCKHOLM – Children with multiple sclerosis (MS) who are initially treated with one of the newer disease-modifying therapies experienced significantly better disease activity control in terms of clinical and radiologic outcomes, compared with those started on an injectable drug in a large, observational, cohort study conducted by the U.S. Network of Pediatric MS Centers.
This was the first-ever comparative effectiveness study of initial disease-modifying therapies (DMTs) in children with MS. The take-home message was clear: “This study supports the use of newer DMTs early in the course of pediatric MS,” Kristen M. Krysko, MD, said in presenting the results at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
The study was conducted because she and her coinvestigators in the network have noted increasing use of newer DMTs, even as first-line initial treatment, in the setting of pediatric MS. This represents a break with the traditional approach, which entails starting with one of the injectables – either an interferon-beta or glatiramer acetate – because of their more favorable safety profile, then escalating therapy by switching to a newer, more potent agent in the event of a disease breakthrough, explained Dr. Krysko, a clinical fellow in neurology at the University of California, San Francisco.
Until now, there has been only limited evidence on how the newer DMTs stack up in comparison with the injectables in a pediatric MS population. The chief supporting evidence for the harder-hitting initial approach, Dr. Krysko said, has come from a randomized clinical trial in 215 children showing that fingolimod had a lower relapse rate and better MRI outcomes, compared with interferon beta-1a, during 2 years of follow-up, but at the cost of a higher rate of serious adverse events (N Engl J Med. 2018;379[11]:1017-27).
Dr. Krysko presented a prospective study conducted at 12 sites participating in the network. It included 741 children, 85% of whom had MS, with clinically isolated syndrome in the remainder. For 197 patients, the first MS treatment was an injectable. The other 544 children were started on a newer DMT, most often dimethyl fumarate, rituximab, natalizumab, or fingolimod, with a smattering of patients on teriflunomide or ocrelizumab. Patients averaged roughly a 1-year disease history at the time they went on their first DMT and were then followed for a mean of 1.5-1.8 years on that drug.
The primary outcome was the propensity score–matched, annualized relapse rate during follow-up: The annualized rate was 0.2 in the group on newer DMTs, compared with 0.47 with the injectables. The propensity score matching was used because patients were not randomized by treatment. The propensity scores attempted to neutralize potential confounders, including differences in patient demographics, baseline disease activity, and severity of a first pretreatment relapse, she explained.
The between-group difference in adjusted annualized relapse rate was statistically significant. It translated to a 55% reduction in relative risk favoring children on a newer DMT. Moreover, the number needed to treat was impressively low, at 3.7.
“This can be interpreted as [needing] to treat 3.7 individuals with newer rather than injectable DMTs to prevent one relapse,” Dr. Krysko observed.
Secondary endpoints focused on brain MRI findings. The median time to development of new or enlarging T2 hyperintense lesions was 2.79 years with the newer DMTs, compared with 0.42 years with the injectables. The adjusted risk of developing such lesions was reduced by 49% with the newer DMTs.
Similarly, the median time to development of gadolinium-enhancing lesions was 2.25 years with the injectables and had not yet been reached in patients on newer DMTs when the study closed in January 2019.
“Many children on the newer DMTs never experienced a new gadolinium-positive lesion on follow-up,” she noted.
The adjusted risk of developing a new gadolinium-enhancing lesion was 62% lower in the newer-DMT group.
In terms of the safety of the newer DMTs, there were no surprises: The adverse-event profiles mirrored those that have been examined far more extensively in adults, according to Dr. Krysko.
The newer DMTs included oral agents as well as drugs given by intravenous infusion. The IV agents generally resulted in better disease control, compared with the oral agents, as one would expect, she said. The patient numbers were not sufficient to break down the results on an individual drug basis, however, even though this was a relatively large study.
Asked if these study results warranted a sweeping change in clinical practice – a move away from the conventional escalation treatment strategy in children in favor of upfront use of the newer, more effective DMTs – Dr. Krysko said that was tempting in light of a few recent studies in adults showing that even the first treatment can affect important long-term outcomes, including conversion to secondary progressive MS. However, she said she’d like to see additional studies in children that are focused on safety before making widespread changes in treatment strategy, especially because the pediatric MS network study did not include many very young children.
The study was sponsored by the Multiple Sclerosis Society. Dr. Kysko reported having no financial conflicts in regard to the study.
SOURCE: Krysko KM et al. ECTRIMS 2019, abstract 249.
STOCKHOLM – Children with multiple sclerosis (MS) who are initially treated with one of the newer disease-modifying therapies experienced significantly better disease activity control in terms of clinical and radiologic outcomes, compared with those started on an injectable drug in a large, observational, cohort study conducted by the U.S. Network of Pediatric MS Centers.
This was the first-ever comparative effectiveness study of initial disease-modifying therapies (DMTs) in children with MS. The take-home message was clear: “This study supports the use of newer DMTs early in the course of pediatric MS,” Kristen M. Krysko, MD, said in presenting the results at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
The study was conducted because she and her coinvestigators in the network have noted increasing use of newer DMTs, even as first-line initial treatment, in the setting of pediatric MS. This represents a break with the traditional approach, which entails starting with one of the injectables – either an interferon-beta or glatiramer acetate – because of their more favorable safety profile, then escalating therapy by switching to a newer, more potent agent in the event of a disease breakthrough, explained Dr. Krysko, a clinical fellow in neurology at the University of California, San Francisco.
Until now, there has been only limited evidence on how the newer DMTs stack up in comparison with the injectables in a pediatric MS population. The chief supporting evidence for the harder-hitting initial approach, Dr. Krysko said, has come from a randomized clinical trial in 215 children showing that fingolimod had a lower relapse rate and better MRI outcomes, compared with interferon beta-1a, during 2 years of follow-up, but at the cost of a higher rate of serious adverse events (N Engl J Med. 2018;379[11]:1017-27).
Dr. Krysko presented a prospective study conducted at 12 sites participating in the network. It included 741 children, 85% of whom had MS, with clinically isolated syndrome in the remainder. For 197 patients, the first MS treatment was an injectable. The other 544 children were started on a newer DMT, most often dimethyl fumarate, rituximab, natalizumab, or fingolimod, with a smattering of patients on teriflunomide or ocrelizumab. Patients averaged roughly a 1-year disease history at the time they went on their first DMT and were then followed for a mean of 1.5-1.8 years on that drug.
The primary outcome was the propensity score–matched, annualized relapse rate during follow-up: The annualized rate was 0.2 in the group on newer DMTs, compared with 0.47 with the injectables. The propensity score matching was used because patients were not randomized by treatment. The propensity scores attempted to neutralize potential confounders, including differences in patient demographics, baseline disease activity, and severity of a first pretreatment relapse, she explained.
The between-group difference in adjusted annualized relapse rate was statistically significant. It translated to a 55% reduction in relative risk favoring children on a newer DMT. Moreover, the number needed to treat was impressively low, at 3.7.
“This can be interpreted as [needing] to treat 3.7 individuals with newer rather than injectable DMTs to prevent one relapse,” Dr. Krysko observed.
Secondary endpoints focused on brain MRI findings. The median time to development of new or enlarging T2 hyperintense lesions was 2.79 years with the newer DMTs, compared with 0.42 years with the injectables. The adjusted risk of developing such lesions was reduced by 49% with the newer DMTs.
Similarly, the median time to development of gadolinium-enhancing lesions was 2.25 years with the injectables and had not yet been reached in patients on newer DMTs when the study closed in January 2019.
“Many children on the newer DMTs never experienced a new gadolinium-positive lesion on follow-up,” she noted.
The adjusted risk of developing a new gadolinium-enhancing lesion was 62% lower in the newer-DMT group.
In terms of the safety of the newer DMTs, there were no surprises: The adverse-event profiles mirrored those that have been examined far more extensively in adults, according to Dr. Krysko.
The newer DMTs included oral agents as well as drugs given by intravenous infusion. The IV agents generally resulted in better disease control, compared with the oral agents, as one would expect, she said. The patient numbers were not sufficient to break down the results on an individual drug basis, however, even though this was a relatively large study.
Asked if these study results warranted a sweeping change in clinical practice – a move away from the conventional escalation treatment strategy in children in favor of upfront use of the newer, more effective DMTs – Dr. Krysko said that was tempting in light of a few recent studies in adults showing that even the first treatment can affect important long-term outcomes, including conversion to secondary progressive MS. However, she said she’d like to see additional studies in children that are focused on safety before making widespread changes in treatment strategy, especially because the pediatric MS network study did not include many very young children.
The study was sponsored by the Multiple Sclerosis Society. Dr. Kysko reported having no financial conflicts in regard to the study.
SOURCE: Krysko KM et al. ECTRIMS 2019, abstract 249.
STOCKHOLM – Children with multiple sclerosis (MS) who are initially treated with one of the newer disease-modifying therapies experienced significantly better disease activity control in terms of clinical and radiologic outcomes, compared with those started on an injectable drug in a large, observational, cohort study conducted by the U.S. Network of Pediatric MS Centers.
This was the first-ever comparative effectiveness study of initial disease-modifying therapies (DMTs) in children with MS. The take-home message was clear: “This study supports the use of newer DMTs early in the course of pediatric MS,” Kristen M. Krysko, MD, said in presenting the results at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
The study was conducted because she and her coinvestigators in the network have noted increasing use of newer DMTs, even as first-line initial treatment, in the setting of pediatric MS. This represents a break with the traditional approach, which entails starting with one of the injectables – either an interferon-beta or glatiramer acetate – because of their more favorable safety profile, then escalating therapy by switching to a newer, more potent agent in the event of a disease breakthrough, explained Dr. Krysko, a clinical fellow in neurology at the University of California, San Francisco.
Until now, there has been only limited evidence on how the newer DMTs stack up in comparison with the injectables in a pediatric MS population. The chief supporting evidence for the harder-hitting initial approach, Dr. Krysko said, has come from a randomized clinical trial in 215 children showing that fingolimod had a lower relapse rate and better MRI outcomes, compared with interferon beta-1a, during 2 years of follow-up, but at the cost of a higher rate of serious adverse events (N Engl J Med. 2018;379[11]:1017-27).
Dr. Krysko presented a prospective study conducted at 12 sites participating in the network. It included 741 children, 85% of whom had MS, with clinically isolated syndrome in the remainder. For 197 patients, the first MS treatment was an injectable. The other 544 children were started on a newer DMT, most often dimethyl fumarate, rituximab, natalizumab, or fingolimod, with a smattering of patients on teriflunomide or ocrelizumab. Patients averaged roughly a 1-year disease history at the time they went on their first DMT and were then followed for a mean of 1.5-1.8 years on that drug.
The primary outcome was the propensity score–matched, annualized relapse rate during follow-up: The annualized rate was 0.2 in the group on newer DMTs, compared with 0.47 with the injectables. The propensity score matching was used because patients were not randomized by treatment. The propensity scores attempted to neutralize potential confounders, including differences in patient demographics, baseline disease activity, and severity of a first pretreatment relapse, she explained.
The between-group difference in adjusted annualized relapse rate was statistically significant. It translated to a 55% reduction in relative risk favoring children on a newer DMT. Moreover, the number needed to treat was impressively low, at 3.7.
“This can be interpreted as [needing] to treat 3.7 individuals with newer rather than injectable DMTs to prevent one relapse,” Dr. Krysko observed.
Secondary endpoints focused on brain MRI findings. The median time to development of new or enlarging T2 hyperintense lesions was 2.79 years with the newer DMTs, compared with 0.42 years with the injectables. The adjusted risk of developing such lesions was reduced by 49% with the newer DMTs.
Similarly, the median time to development of gadolinium-enhancing lesions was 2.25 years with the injectables and had not yet been reached in patients on newer DMTs when the study closed in January 2019.
“Many children on the newer DMTs never experienced a new gadolinium-positive lesion on follow-up,” she noted.
The adjusted risk of developing a new gadolinium-enhancing lesion was 62% lower in the newer-DMT group.
In terms of the safety of the newer DMTs, there were no surprises: The adverse-event profiles mirrored those that have been examined far more extensively in adults, according to Dr. Krysko.
The newer DMTs included oral agents as well as drugs given by intravenous infusion. The IV agents generally resulted in better disease control, compared with the oral agents, as one would expect, she said. The patient numbers were not sufficient to break down the results on an individual drug basis, however, even though this was a relatively large study.
Asked if these study results warranted a sweeping change in clinical practice – a move away from the conventional escalation treatment strategy in children in favor of upfront use of the newer, more effective DMTs – Dr. Krysko said that was tempting in light of a few recent studies in adults showing that even the first treatment can affect important long-term outcomes, including conversion to secondary progressive MS. However, she said she’d like to see additional studies in children that are focused on safety before making widespread changes in treatment strategy, especially because the pediatric MS network study did not include many very young children.
The study was sponsored by the Multiple Sclerosis Society. Dr. Kysko reported having no financial conflicts in regard to the study.
SOURCE: Krysko KM et al. ECTRIMS 2019, abstract 249.
REPORTING FROM ECTRIMS 2019
Inebilizumab looks good for neuromyelitis optica in phase 2/3 trial
STOCKHOLM – Inebilizumab, a medication being developed to treat neuromyelitis optica spectrum disorder (NMO/NMOSD), fared well against placebo in a randomized trial, according to recently presented results.
Participants in the active arm of the study saw a 77% relative reduction in the risk of an attack of NMO, compared with placebo, for a number needed to treat of 3.2 to see benefit from inebilizumab, said senior investigator Bruce Cree, MD, PhD.
The multisite, international N-MOmentum study compared inebilizumab, a B-cell depleting humanized monoclonal antibody, with placebo as monotherapy for the treatment of NMOSD. The results were presented at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
Adult patients with NMOSD were eligible if they had experienced at least one attack in the previous year, or at least two attacks in the previous 2 years, and had an Expanded Disability Status Scale (EDSS; range, 0-10; 0, normal) score of 8 or less, Dr. Cree said. Diagnostic criteria for study participation were either seropositivity for aquaporin 4-IgG or fulfillment of the 2006 Wingerchuk criteria for NMOSD if individuals were aquaporin 4-IgG negative.
Patients were about 43 years old at enrollment. More than 90% were women, and about three-quarters were white or Asian. The baseline EDSS score was about 3.5, and patients had experienced a mean of just over four attacks on study entry. The full cohort of patients was over 90% seropositive, and about 60% had been on prior immunosuppressive therapy.
“This was a monotherapy study, meaning that no background immunotherapy was permitted,” said Dr. Cree, the George A. Zimmermann Endowed Professor in Multiple Sclerosis in the department of neurology at the University of California, San Francisco. Patients were randomized 3:1 to receive either two 300-mg doses of inebilizumab by intravenous infusion on study days 1 and 13 or matched placebo infusions.
“When we think about B cells and NMO, multiple lines of evidence have suggested that NMO is a B-cell–mediated disorder resulting from pathogenic antibody production, pro-inflammatory cytokine secretion, and antigen presentation by B cells,” he explained, adding that inebilizumab targets the cell surface antigen, CD19, which is expressed “perhaps more widely than CD20.”
The randomized, double-blind, placebo-controlled phase 2/3 study was followed by an open-label extension period. “This was a time-to-event study design,” Dr. Cree explained, with the randomized period limited to 197 days. After that period, participants could enroll in the open-label extension arm and receive active treatment for at least 1 year. “In the event that a participant experienced an attack during the course of the study that was an adjudicated attack, they were pulled out and offered entry into the open-label period shortly after the attack,” he added.
Adjudicated attacks were the study’s primary endpoint, measured as the time from study day 1 to an adjudicated attack for patients in the randomized population. Dr. Cree said that study development included identifying 18 “predefined, clinically significant” attack diagnosis criteria. These included attacks of optic myelitis, neuritis, and brain-stem events.
Of the 18 criteria, 10 constituted overt clinical changes and the remaining 8 represented more moderate clinical changes that had to be accompanied by a new lesion detected on MRI. All criteria required confirmation by the adjudication committee to qualify as an attack.
By study day 197, 18 of 161 participants (11.2%) of those remaining in the randomized study arm had experienced an adjudicated NMOSD attack, compared with 22 of 52 (42.3%) of those still in the placebo arm. That drop translated into a relative risk reduction of 77.3% and a hazard ratio of 0.227 for NMOSD attack favoring inebilizumab (P less than .0001).
“That risk of attack [for participants in the inebilizumab arm] continued to be low following entry into the open-label extension, whereas patients who were initially treated with placebo experienced some attacks initially, and that looked like it began to flatten out as well” during the open-label extension arm, said Dr. Cree.
Secondary endpoints included worsening of EDSS scores, changes in low-contrast visual acuity binocular score, the cumulative number of active MRI lesions, and hospitalizations deemed to be NMOSD related. Participants receiving inebilizumab saw significant reductions in all of these endpoints except for the visual acuity measure, with no differences seen in outcomes for seropositive versus seronegative participants.
A total of 231 patients were randomized, with the eventual intention-to-treat population including 174 inebilizumab patients and 56 in the placebo arm (one patient was randomized to inebilizumab but never received a dose of study drug). All but five inebilizumab patients and two placebo patients completed the study. The independent data-monitoring committee recommended stopping enrollment in the randomized phase of the study at 231 patients for efficacy, even though there had been only 43 adjudicated attacks at that point, Dr. Cree explained.
The medication was generally well tolerated. Urinary tract infection – the most common adverse event – was experienced by 22% of patients. Infusion site reactions were more common in those receiving placebo than in those receiving inebilizumab, he noted.
Over the two total years of inebilizumab exposure to date, there have been two deaths. One was related to a severe NMO attack and the other to “an event of undetermined etiology due to a presumed inflammatory brain lesion,” Dr. Cree said, adding that “no autopsy or biopsy was performed, unfortunately.”
The investigators tracked IgG levels over the course of the study and noted that they continued to decline over the course of the study, with 14% of patients having a level less than the lower limit of normal at the 2-year mark. This suggests that IgG levels will have to be followed for patients taking the drug over the long term, he said.
Serum glial fibrillary acidic protein, a serum marker of astroglial injury, is ordinarily elevated during NMOSD attacks. For participants on inebilizumab who experienced attacks, elevations in glial fibrillary acidic protein were not as marked, which suggests that the severity of tissue injury in an attack may be attenuated by the drug, said Dr. Cree.
The study was funded by Viela Bio and Medimmune, which are developing inebilizumab. Viela Bio also funded medical writing for the presentation. Dr. Cree reported receiving consulting fees from Abbvie, Akili, Alexion, Biogen, GeNeuro, Novartis, Sanofi Genzyme, and TG Therapeutics.
SOURCE: Cree B et al. ECTRIMS 2019, abstract 139.
STOCKHOLM – Inebilizumab, a medication being developed to treat neuromyelitis optica spectrum disorder (NMO/NMOSD), fared well against placebo in a randomized trial, according to recently presented results.
Participants in the active arm of the study saw a 77% relative reduction in the risk of an attack of NMO, compared with placebo, for a number needed to treat of 3.2 to see benefit from inebilizumab, said senior investigator Bruce Cree, MD, PhD.
The multisite, international N-MOmentum study compared inebilizumab, a B-cell depleting humanized monoclonal antibody, with placebo as monotherapy for the treatment of NMOSD. The results were presented at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
Adult patients with NMOSD were eligible if they had experienced at least one attack in the previous year, or at least two attacks in the previous 2 years, and had an Expanded Disability Status Scale (EDSS; range, 0-10; 0, normal) score of 8 or less, Dr. Cree said. Diagnostic criteria for study participation were either seropositivity for aquaporin 4-IgG or fulfillment of the 2006 Wingerchuk criteria for NMOSD if individuals were aquaporin 4-IgG negative.
Patients were about 43 years old at enrollment. More than 90% were women, and about three-quarters were white or Asian. The baseline EDSS score was about 3.5, and patients had experienced a mean of just over four attacks on study entry. The full cohort of patients was over 90% seropositive, and about 60% had been on prior immunosuppressive therapy.
“This was a monotherapy study, meaning that no background immunotherapy was permitted,” said Dr. Cree, the George A. Zimmermann Endowed Professor in Multiple Sclerosis in the department of neurology at the University of California, San Francisco. Patients were randomized 3:1 to receive either two 300-mg doses of inebilizumab by intravenous infusion on study days 1 and 13 or matched placebo infusions.
“When we think about B cells and NMO, multiple lines of evidence have suggested that NMO is a B-cell–mediated disorder resulting from pathogenic antibody production, pro-inflammatory cytokine secretion, and antigen presentation by B cells,” he explained, adding that inebilizumab targets the cell surface antigen, CD19, which is expressed “perhaps more widely than CD20.”
The randomized, double-blind, placebo-controlled phase 2/3 study was followed by an open-label extension period. “This was a time-to-event study design,” Dr. Cree explained, with the randomized period limited to 197 days. After that period, participants could enroll in the open-label extension arm and receive active treatment for at least 1 year. “In the event that a participant experienced an attack during the course of the study that was an adjudicated attack, they were pulled out and offered entry into the open-label period shortly after the attack,” he added.
Adjudicated attacks were the study’s primary endpoint, measured as the time from study day 1 to an adjudicated attack for patients in the randomized population. Dr. Cree said that study development included identifying 18 “predefined, clinically significant” attack diagnosis criteria. These included attacks of optic myelitis, neuritis, and brain-stem events.
Of the 18 criteria, 10 constituted overt clinical changes and the remaining 8 represented more moderate clinical changes that had to be accompanied by a new lesion detected on MRI. All criteria required confirmation by the adjudication committee to qualify as an attack.
By study day 197, 18 of 161 participants (11.2%) of those remaining in the randomized study arm had experienced an adjudicated NMOSD attack, compared with 22 of 52 (42.3%) of those still in the placebo arm. That drop translated into a relative risk reduction of 77.3% and a hazard ratio of 0.227 for NMOSD attack favoring inebilizumab (P less than .0001).
“That risk of attack [for participants in the inebilizumab arm] continued to be low following entry into the open-label extension, whereas patients who were initially treated with placebo experienced some attacks initially, and that looked like it began to flatten out as well” during the open-label extension arm, said Dr. Cree.
Secondary endpoints included worsening of EDSS scores, changes in low-contrast visual acuity binocular score, the cumulative number of active MRI lesions, and hospitalizations deemed to be NMOSD related. Participants receiving inebilizumab saw significant reductions in all of these endpoints except for the visual acuity measure, with no differences seen in outcomes for seropositive versus seronegative participants.
A total of 231 patients were randomized, with the eventual intention-to-treat population including 174 inebilizumab patients and 56 in the placebo arm (one patient was randomized to inebilizumab but never received a dose of study drug). All but five inebilizumab patients and two placebo patients completed the study. The independent data-monitoring committee recommended stopping enrollment in the randomized phase of the study at 231 patients for efficacy, even though there had been only 43 adjudicated attacks at that point, Dr. Cree explained.
The medication was generally well tolerated. Urinary tract infection – the most common adverse event – was experienced by 22% of patients. Infusion site reactions were more common in those receiving placebo than in those receiving inebilizumab, he noted.
Over the two total years of inebilizumab exposure to date, there have been two deaths. One was related to a severe NMO attack and the other to “an event of undetermined etiology due to a presumed inflammatory brain lesion,” Dr. Cree said, adding that “no autopsy or biopsy was performed, unfortunately.”
The investigators tracked IgG levels over the course of the study and noted that they continued to decline over the course of the study, with 14% of patients having a level less than the lower limit of normal at the 2-year mark. This suggests that IgG levels will have to be followed for patients taking the drug over the long term, he said.
Serum glial fibrillary acidic protein, a serum marker of astroglial injury, is ordinarily elevated during NMOSD attacks. For participants on inebilizumab who experienced attacks, elevations in glial fibrillary acidic protein were not as marked, which suggests that the severity of tissue injury in an attack may be attenuated by the drug, said Dr. Cree.
The study was funded by Viela Bio and Medimmune, which are developing inebilizumab. Viela Bio also funded medical writing for the presentation. Dr. Cree reported receiving consulting fees from Abbvie, Akili, Alexion, Biogen, GeNeuro, Novartis, Sanofi Genzyme, and TG Therapeutics.
SOURCE: Cree B et al. ECTRIMS 2019, abstract 139.
STOCKHOLM – Inebilizumab, a medication being developed to treat neuromyelitis optica spectrum disorder (NMO/NMOSD), fared well against placebo in a randomized trial, according to recently presented results.
Participants in the active arm of the study saw a 77% relative reduction in the risk of an attack of NMO, compared with placebo, for a number needed to treat of 3.2 to see benefit from inebilizumab, said senior investigator Bruce Cree, MD, PhD.
The multisite, international N-MOmentum study compared inebilizumab, a B-cell depleting humanized monoclonal antibody, with placebo as monotherapy for the treatment of NMOSD. The results were presented at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
Adult patients with NMOSD were eligible if they had experienced at least one attack in the previous year, or at least two attacks in the previous 2 years, and had an Expanded Disability Status Scale (EDSS; range, 0-10; 0, normal) score of 8 or less, Dr. Cree said. Diagnostic criteria for study participation were either seropositivity for aquaporin 4-IgG or fulfillment of the 2006 Wingerchuk criteria for NMOSD if individuals were aquaporin 4-IgG negative.
Patients were about 43 years old at enrollment. More than 90% were women, and about three-quarters were white or Asian. The baseline EDSS score was about 3.5, and patients had experienced a mean of just over four attacks on study entry. The full cohort of patients was over 90% seropositive, and about 60% had been on prior immunosuppressive therapy.
“This was a monotherapy study, meaning that no background immunotherapy was permitted,” said Dr. Cree, the George A. Zimmermann Endowed Professor in Multiple Sclerosis in the department of neurology at the University of California, San Francisco. Patients were randomized 3:1 to receive either two 300-mg doses of inebilizumab by intravenous infusion on study days 1 and 13 or matched placebo infusions.
“When we think about B cells and NMO, multiple lines of evidence have suggested that NMO is a B-cell–mediated disorder resulting from pathogenic antibody production, pro-inflammatory cytokine secretion, and antigen presentation by B cells,” he explained, adding that inebilizumab targets the cell surface antigen, CD19, which is expressed “perhaps more widely than CD20.”
The randomized, double-blind, placebo-controlled phase 2/3 study was followed by an open-label extension period. “This was a time-to-event study design,” Dr. Cree explained, with the randomized period limited to 197 days. After that period, participants could enroll in the open-label extension arm and receive active treatment for at least 1 year. “In the event that a participant experienced an attack during the course of the study that was an adjudicated attack, they were pulled out and offered entry into the open-label period shortly after the attack,” he added.
Adjudicated attacks were the study’s primary endpoint, measured as the time from study day 1 to an adjudicated attack for patients in the randomized population. Dr. Cree said that study development included identifying 18 “predefined, clinically significant” attack diagnosis criteria. These included attacks of optic myelitis, neuritis, and brain-stem events.
Of the 18 criteria, 10 constituted overt clinical changes and the remaining 8 represented more moderate clinical changes that had to be accompanied by a new lesion detected on MRI. All criteria required confirmation by the adjudication committee to qualify as an attack.
By study day 197, 18 of 161 participants (11.2%) of those remaining in the randomized study arm had experienced an adjudicated NMOSD attack, compared with 22 of 52 (42.3%) of those still in the placebo arm. That drop translated into a relative risk reduction of 77.3% and a hazard ratio of 0.227 for NMOSD attack favoring inebilizumab (P less than .0001).
“That risk of attack [for participants in the inebilizumab arm] continued to be low following entry into the open-label extension, whereas patients who were initially treated with placebo experienced some attacks initially, and that looked like it began to flatten out as well” during the open-label extension arm, said Dr. Cree.
Secondary endpoints included worsening of EDSS scores, changes in low-contrast visual acuity binocular score, the cumulative number of active MRI lesions, and hospitalizations deemed to be NMOSD related. Participants receiving inebilizumab saw significant reductions in all of these endpoints except for the visual acuity measure, with no differences seen in outcomes for seropositive versus seronegative participants.
A total of 231 patients were randomized, with the eventual intention-to-treat population including 174 inebilizumab patients and 56 in the placebo arm (one patient was randomized to inebilizumab but never received a dose of study drug). All but five inebilizumab patients and two placebo patients completed the study. The independent data-monitoring committee recommended stopping enrollment in the randomized phase of the study at 231 patients for efficacy, even though there had been only 43 adjudicated attacks at that point, Dr. Cree explained.
The medication was generally well tolerated. Urinary tract infection – the most common adverse event – was experienced by 22% of patients. Infusion site reactions were more common in those receiving placebo than in those receiving inebilizumab, he noted.
Over the two total years of inebilizumab exposure to date, there have been two deaths. One was related to a severe NMO attack and the other to “an event of undetermined etiology due to a presumed inflammatory brain lesion,” Dr. Cree said, adding that “no autopsy or biopsy was performed, unfortunately.”
The investigators tracked IgG levels over the course of the study and noted that they continued to decline over the course of the study, with 14% of patients having a level less than the lower limit of normal at the 2-year mark. This suggests that IgG levels will have to be followed for patients taking the drug over the long term, he said.
Serum glial fibrillary acidic protein, a serum marker of astroglial injury, is ordinarily elevated during NMOSD attacks. For participants on inebilizumab who experienced attacks, elevations in glial fibrillary acidic protein were not as marked, which suggests that the severity of tissue injury in an attack may be attenuated by the drug, said Dr. Cree.
The study was funded by Viela Bio and Medimmune, which are developing inebilizumab. Viela Bio also funded medical writing for the presentation. Dr. Cree reported receiving consulting fees from Abbvie, Akili, Alexion, Biogen, GeNeuro, Novartis, Sanofi Genzyme, and TG Therapeutics.
SOURCE: Cree B et al. ECTRIMS 2019, abstract 139.
REPORTING FROM ECTRIMS 2019
Peanut allergy pill gets thumbs-up from FDA advisory panel
A pill designed to desensitize peanut-allergic children and teenagers may be on the way.
aged 4-17 years old with a confirmed peanut allergy. Conditions for approval include stipulations that a black-box warning and medication use guide are included in the packaging, the panel said. The FDA usually follows the recommendations of its advisory panels.
The committee members voted 7-2 that the drug was effective and 8-1 that it was safe.
John Kelso, MD, the sole dissenter on safety, voiced concerns about the dearth of long-term follow-up in Aimmune Therapeutic’s body of research and the finding that children who received the treatment during the dose-escalation and maintenance periods had twice the number of allergic reactions requiring epinephrine, compared with those who received placebo. There are no long-term safety data to rely on yet, he added.
“Efficacy has not been demonstrated, except on the day the peanut challenge is administered,” said Dr. Kelso, an allergist at the Scripps Clinic, San Diego, adding that only long-term follow-up data would fully convince him that the drug’s benefits outweigh the risks.
In the discussion, however, other committee members pointed out that new drugs are often approved without long-term efficacy and safety data. Those data are extrapolated from clinical trials, and only real-world experience will confirm the data, they noted.
Company representatives did not explicitly address the potential cost of the therapy, but a recent review by the Institute for Clinical and Economic Review estimated the cost to be $4,200 a year. Palforzia would have to be taken every day, for an unknown amount of time, to maintain peanut tolerance.
“Using prices from analysts for AR101 ($4,200 a year), we estimated that only 41% of eligible patients could be treated in a given year without exceeding ICER’s budget impact threshold,” the institute concluded in a publicly released analysis.
Palforzia comes in individual packs of capsules filled with peanut protein, not flour. The capsules come in doses of 0.5, 1, 10, 20, and 100, and 300 mg. A single-dose sachet contains 300 mg. Treatment begins with 0.5-6 mg over 1 day and escalates every 2 weeks until 300 mg is reached or there is a reaction requiring epinephrine. Passing at least a 300-mg dose was the requirement for exiting the escalation phase and moving on to the daily, year-long maintenance phase.
The four efficacy studies presented showed that 96% of patients tolerated 300 mg, 84% tolerated 600 mg, and 63% 1,000 mg – about 10 times the reactive dose observed in the placebo controls.
“Only 125 mg of peanut protein – the amount in about half a peanut kernel – can be enough to provoke a reaction,” said Daniel Adelman, MD, chief medical officer of Aimmune. If patients can tolerate 600 mg of protein – the equivalent of two kernels, accidental ingestion will result in a “predictable, manageable” reaction.
“This is truly a clinically significant result for patients and families who report lives dictated by the allergy,” Dr. Adelman said.
Consistent manufacturing processes and positive safety data should reassure clinicians and patients that they are receiving a safe, effective, and well-regulated treatment, he added.
The capsule, however, is not a panacea. The company advises that families continue with the peanut avoidance diet. “It’s important to remember that reactive episodes can occur with dosing, and accidental exposures can occur at unpredictable times, away from home, and despite the best efforts at avoidance,” Dr. Adelman said. “This is not a drug for everyone, but it is an effective desensitization tool and would clearly be the first therapy to treat a food allergy, providing statistically significant and clinically important improvement. Outcomes align with patients’ goals.”
Safety was assessed in 709 treated patients who received the medication and 292 who received placebo. Treatment-related adverse events were most common in initial dosing: 89% of the treatment group and 58% of the placebo group experienced at least one adverse event during that time. Adverse events were mostly mild to moderate and decreased in severity over the study period. They included abdominal pain (45% active vs. 18% placebo), throat irritation (40% vs. 17%), pruritus (33% vs. 20%), vomiting (37% vs. 16%), cough (32% vs. 24%), nausea (32% vs. 14%), urticaria (28% vs. 19%), and upper abdominal pain (30% vs. 14%).
Discontinuations caused by these adverse events were infrequent, with only 1.8% of participants who were taking the active capsule and 1% of those taking placebo discontinuing the study product during initial dose escalation. Only the severe systemic allergic reactions were considered to be anaphylaxis, and they had to affect at least two body systems.
Respiratory events were more common in those in the active group, especially in children with asthma. These events included cough, wheezing, dyspnea, dysphonia, throat irritation and tightness, and exercise-induced asthma. There was, however, no “concerning change” in asthma control.
Systemic allergic reactions and anaphylaxis were more common in the active-dose group. Systemic reactions during dose escalation occurred in 9.4% of active patients and 3.8% those taking placebo. During the maintenance phase, they occurred in 8.7% and 1.7% of patients, respectively. Three patients in the active group had a serious systemic reaction – two during up-dosing and one during maintenance.
During initial dose escalation and up-dosing combined, 6.1% of patients in the active group and 3.1% in the placebo group had a systemic reaction requiring epinephrine. This was most often administered outside of the clinic.
There were 12 cases of eosinophilic esophagitis, all of which resolved after withdrawal from the study medication.
Aimmune submitted a risk-management proposal that includes the following:
- The first dose of each progressive dose must be administered in a facility that is equipped to treat systemic allergic reactions.
- Families must have a valid prescription for injectable epinephrine before treatment starts and must demonstrate that they know how to use it.
- There must be distribution controls in every pharmacy that dispenses the product.
- Packaging will be dose specific to ensure proper at-home administration.
- Pharmacologic questionnaires will be used as data collection instruments.
- Professional and patient-focused labeling will include a medication guide and educational material.
Palforzia is not the only peanut desensitization product in the works. DBC Technology has resubmitted its biologics license application to the FDA in the hope of getting approval for its peanut allergy treatment, the Viaskin Peanut patch.
The patch is designed to desensitize allergic children aged 4-11 years through a skin-patch method known as epicutaneous immunotherapy. Results from two controlled clinical trials were included in the submission. The company is also investigating the potential of this form of skin-patch therapy for milk and egg allergies.
Correction, 9/15/19: An earlier version of this article did not clearly state that the panel recommended approval rather than granted approval for the drug.
A pill designed to desensitize peanut-allergic children and teenagers may be on the way.
aged 4-17 years old with a confirmed peanut allergy. Conditions for approval include stipulations that a black-box warning and medication use guide are included in the packaging, the panel said. The FDA usually follows the recommendations of its advisory panels.
The committee members voted 7-2 that the drug was effective and 8-1 that it was safe.
John Kelso, MD, the sole dissenter on safety, voiced concerns about the dearth of long-term follow-up in Aimmune Therapeutic’s body of research and the finding that children who received the treatment during the dose-escalation and maintenance periods had twice the number of allergic reactions requiring epinephrine, compared with those who received placebo. There are no long-term safety data to rely on yet, he added.
“Efficacy has not been demonstrated, except on the day the peanut challenge is administered,” said Dr. Kelso, an allergist at the Scripps Clinic, San Diego, adding that only long-term follow-up data would fully convince him that the drug’s benefits outweigh the risks.
In the discussion, however, other committee members pointed out that new drugs are often approved without long-term efficacy and safety data. Those data are extrapolated from clinical trials, and only real-world experience will confirm the data, they noted.
Company representatives did not explicitly address the potential cost of the therapy, but a recent review by the Institute for Clinical and Economic Review estimated the cost to be $4,200 a year. Palforzia would have to be taken every day, for an unknown amount of time, to maintain peanut tolerance.
“Using prices from analysts for AR101 ($4,200 a year), we estimated that only 41% of eligible patients could be treated in a given year without exceeding ICER’s budget impact threshold,” the institute concluded in a publicly released analysis.
Palforzia comes in individual packs of capsules filled with peanut protein, not flour. The capsules come in doses of 0.5, 1, 10, 20, and 100, and 300 mg. A single-dose sachet contains 300 mg. Treatment begins with 0.5-6 mg over 1 day and escalates every 2 weeks until 300 mg is reached or there is a reaction requiring epinephrine. Passing at least a 300-mg dose was the requirement for exiting the escalation phase and moving on to the daily, year-long maintenance phase.
The four efficacy studies presented showed that 96% of patients tolerated 300 mg, 84% tolerated 600 mg, and 63% 1,000 mg – about 10 times the reactive dose observed in the placebo controls.
“Only 125 mg of peanut protein – the amount in about half a peanut kernel – can be enough to provoke a reaction,” said Daniel Adelman, MD, chief medical officer of Aimmune. If patients can tolerate 600 mg of protein – the equivalent of two kernels, accidental ingestion will result in a “predictable, manageable” reaction.
“This is truly a clinically significant result for patients and families who report lives dictated by the allergy,” Dr. Adelman said.
Consistent manufacturing processes and positive safety data should reassure clinicians and patients that they are receiving a safe, effective, and well-regulated treatment, he added.
The capsule, however, is not a panacea. The company advises that families continue with the peanut avoidance diet. “It’s important to remember that reactive episodes can occur with dosing, and accidental exposures can occur at unpredictable times, away from home, and despite the best efforts at avoidance,” Dr. Adelman said. “This is not a drug for everyone, but it is an effective desensitization tool and would clearly be the first therapy to treat a food allergy, providing statistically significant and clinically important improvement. Outcomes align with patients’ goals.”
Safety was assessed in 709 treated patients who received the medication and 292 who received placebo. Treatment-related adverse events were most common in initial dosing: 89% of the treatment group and 58% of the placebo group experienced at least one adverse event during that time. Adverse events were mostly mild to moderate and decreased in severity over the study period. They included abdominal pain (45% active vs. 18% placebo), throat irritation (40% vs. 17%), pruritus (33% vs. 20%), vomiting (37% vs. 16%), cough (32% vs. 24%), nausea (32% vs. 14%), urticaria (28% vs. 19%), and upper abdominal pain (30% vs. 14%).
Discontinuations caused by these adverse events were infrequent, with only 1.8% of participants who were taking the active capsule and 1% of those taking placebo discontinuing the study product during initial dose escalation. Only the severe systemic allergic reactions were considered to be anaphylaxis, and they had to affect at least two body systems.
Respiratory events were more common in those in the active group, especially in children with asthma. These events included cough, wheezing, dyspnea, dysphonia, throat irritation and tightness, and exercise-induced asthma. There was, however, no “concerning change” in asthma control.
Systemic allergic reactions and anaphylaxis were more common in the active-dose group. Systemic reactions during dose escalation occurred in 9.4% of active patients and 3.8% those taking placebo. During the maintenance phase, they occurred in 8.7% and 1.7% of patients, respectively. Three patients in the active group had a serious systemic reaction – two during up-dosing and one during maintenance.
During initial dose escalation and up-dosing combined, 6.1% of patients in the active group and 3.1% in the placebo group had a systemic reaction requiring epinephrine. This was most often administered outside of the clinic.
There were 12 cases of eosinophilic esophagitis, all of which resolved after withdrawal from the study medication.
Aimmune submitted a risk-management proposal that includes the following:
- The first dose of each progressive dose must be administered in a facility that is equipped to treat systemic allergic reactions.
- Families must have a valid prescription for injectable epinephrine before treatment starts and must demonstrate that they know how to use it.
- There must be distribution controls in every pharmacy that dispenses the product.
- Packaging will be dose specific to ensure proper at-home administration.
- Pharmacologic questionnaires will be used as data collection instruments.
- Professional and patient-focused labeling will include a medication guide and educational material.
Palforzia is not the only peanut desensitization product in the works. DBC Technology has resubmitted its biologics license application to the FDA in the hope of getting approval for its peanut allergy treatment, the Viaskin Peanut patch.
The patch is designed to desensitize allergic children aged 4-11 years through a skin-patch method known as epicutaneous immunotherapy. Results from two controlled clinical trials were included in the submission. The company is also investigating the potential of this form of skin-patch therapy for milk and egg allergies.
Correction, 9/15/19: An earlier version of this article did not clearly state that the panel recommended approval rather than granted approval for the drug.
A pill designed to desensitize peanut-allergic children and teenagers may be on the way.
aged 4-17 years old with a confirmed peanut allergy. Conditions for approval include stipulations that a black-box warning and medication use guide are included in the packaging, the panel said. The FDA usually follows the recommendations of its advisory panels.
The committee members voted 7-2 that the drug was effective and 8-1 that it was safe.
John Kelso, MD, the sole dissenter on safety, voiced concerns about the dearth of long-term follow-up in Aimmune Therapeutic’s body of research and the finding that children who received the treatment during the dose-escalation and maintenance periods had twice the number of allergic reactions requiring epinephrine, compared with those who received placebo. There are no long-term safety data to rely on yet, he added.
“Efficacy has not been demonstrated, except on the day the peanut challenge is administered,” said Dr. Kelso, an allergist at the Scripps Clinic, San Diego, adding that only long-term follow-up data would fully convince him that the drug’s benefits outweigh the risks.
In the discussion, however, other committee members pointed out that new drugs are often approved without long-term efficacy and safety data. Those data are extrapolated from clinical trials, and only real-world experience will confirm the data, they noted.
Company representatives did not explicitly address the potential cost of the therapy, but a recent review by the Institute for Clinical and Economic Review estimated the cost to be $4,200 a year. Palforzia would have to be taken every day, for an unknown amount of time, to maintain peanut tolerance.
“Using prices from analysts for AR101 ($4,200 a year), we estimated that only 41% of eligible patients could be treated in a given year without exceeding ICER’s budget impact threshold,” the institute concluded in a publicly released analysis.
Palforzia comes in individual packs of capsules filled with peanut protein, not flour. The capsules come in doses of 0.5, 1, 10, 20, and 100, and 300 mg. A single-dose sachet contains 300 mg. Treatment begins with 0.5-6 mg over 1 day and escalates every 2 weeks until 300 mg is reached or there is a reaction requiring epinephrine. Passing at least a 300-mg dose was the requirement for exiting the escalation phase and moving on to the daily, year-long maintenance phase.
The four efficacy studies presented showed that 96% of patients tolerated 300 mg, 84% tolerated 600 mg, and 63% 1,000 mg – about 10 times the reactive dose observed in the placebo controls.
“Only 125 mg of peanut protein – the amount in about half a peanut kernel – can be enough to provoke a reaction,” said Daniel Adelman, MD, chief medical officer of Aimmune. If patients can tolerate 600 mg of protein – the equivalent of two kernels, accidental ingestion will result in a “predictable, manageable” reaction.
“This is truly a clinically significant result for patients and families who report lives dictated by the allergy,” Dr. Adelman said.
Consistent manufacturing processes and positive safety data should reassure clinicians and patients that they are receiving a safe, effective, and well-regulated treatment, he added.
The capsule, however, is not a panacea. The company advises that families continue with the peanut avoidance diet. “It’s important to remember that reactive episodes can occur with dosing, and accidental exposures can occur at unpredictable times, away from home, and despite the best efforts at avoidance,” Dr. Adelman said. “This is not a drug for everyone, but it is an effective desensitization tool and would clearly be the first therapy to treat a food allergy, providing statistically significant and clinically important improvement. Outcomes align with patients’ goals.”
Safety was assessed in 709 treated patients who received the medication and 292 who received placebo. Treatment-related adverse events were most common in initial dosing: 89% of the treatment group and 58% of the placebo group experienced at least one adverse event during that time. Adverse events were mostly mild to moderate and decreased in severity over the study period. They included abdominal pain (45% active vs. 18% placebo), throat irritation (40% vs. 17%), pruritus (33% vs. 20%), vomiting (37% vs. 16%), cough (32% vs. 24%), nausea (32% vs. 14%), urticaria (28% vs. 19%), and upper abdominal pain (30% vs. 14%).
Discontinuations caused by these adverse events were infrequent, with only 1.8% of participants who were taking the active capsule and 1% of those taking placebo discontinuing the study product during initial dose escalation. Only the severe systemic allergic reactions were considered to be anaphylaxis, and they had to affect at least two body systems.
Respiratory events were more common in those in the active group, especially in children with asthma. These events included cough, wheezing, dyspnea, dysphonia, throat irritation and tightness, and exercise-induced asthma. There was, however, no “concerning change” in asthma control.
Systemic allergic reactions and anaphylaxis were more common in the active-dose group. Systemic reactions during dose escalation occurred in 9.4% of active patients and 3.8% those taking placebo. During the maintenance phase, they occurred in 8.7% and 1.7% of patients, respectively. Three patients in the active group had a serious systemic reaction – two during up-dosing and one during maintenance.
During initial dose escalation and up-dosing combined, 6.1% of patients in the active group and 3.1% in the placebo group had a systemic reaction requiring epinephrine. This was most often administered outside of the clinic.
There were 12 cases of eosinophilic esophagitis, all of which resolved after withdrawal from the study medication.
Aimmune submitted a risk-management proposal that includes the following:
- The first dose of each progressive dose must be administered in a facility that is equipped to treat systemic allergic reactions.
- Families must have a valid prescription for injectable epinephrine before treatment starts and must demonstrate that they know how to use it.
- There must be distribution controls in every pharmacy that dispenses the product.
- Packaging will be dose specific to ensure proper at-home administration.
- Pharmacologic questionnaires will be used as data collection instruments.
- Professional and patient-focused labeling will include a medication guide and educational material.
Palforzia is not the only peanut desensitization product in the works. DBC Technology has resubmitted its biologics license application to the FDA in the hope of getting approval for its peanut allergy treatment, the Viaskin Peanut patch.
The patch is designed to desensitize allergic children aged 4-11 years through a skin-patch method known as epicutaneous immunotherapy. Results from two controlled clinical trials were included in the submission. The company is also investigating the potential of this form of skin-patch therapy for milk and egg allergies.
Correction, 9/15/19: An earlier version of this article did not clearly state that the panel recommended approval rather than granted approval for the drug.
FROM THE FDA ALLERGENIC PRODUCTS ADVISORY COMMITTEE MEETING
Nivolumab may be beneficial for recurrent or metastatic cervical cancer
The checkpoint inhibitor nivolumab may hold some promise for treating women with metastatic gynecologic cancers, especially cervical disease, according to cohort results from the phase 1/2 CheckMate 358 trial.
Of twenty-four patients enrolled in the recurrent/metastatic cervical and vaginal/vulvar carcinoma cohorts, 26.3% of patients with cervical cancer and 20% with vaginal/vulvar cancers experienced an objective response, R. Wendel Naumann, MD, and colleagues reported in the Journal of Clinical Oncology.
Median overall survival was 21.9 months (95% confidence interval, 15.1 months to not reached) among patients with cervical cancer. Because of the small size of the vaginal/vulvar cohort, median overall survival was not calculated.
“Given the lack of effective therapy and low survival rates for patients with metastatic disease in these gynecologic cancers, the results reported here are of strong clinical interest and underscore the growing role of immune checkpoint inhibitors in this patient population,” the investigators wrote.
Checkmate 358 is investigating nivolumab in patients with virus-associated cancers. Patients receive nivolumab monotherapy (240 mg intravenously every 2 weeks for 2 years) until disease progression, unacceptable side effects, or withdrawal of consent.
The median age was 51 years in the cervical cohort (n = 19) and 59 in the vaginal/vulvar cohort (n = 5). HPV status was positive in 83.3% of those with cervical disease and 40% of those with vaginal/vulvar disease. Ten cervical tumors and four vaginal/vulvar tumors expressed PD-L1.
The median duration of nivolumab treatment was 5.6 months in the cervical cohort and 6.7 months in the vaginal/vulvar cohort. Median follow-up was 19 months and 10 months, respectively.
At 12 months and 18 months, 40.0% of the cervical cohort and 20% of the vaginal/vulvar cohort were still alive.
The most common treatment-related adverse effects were diarrhea and decreased appetite. One patient in the cervical cohort discontinued treatment because of pneumonitis, which was considered treatment related.
Bristol-Myers Squibb funded the study. Several of Dr. Naumann’s associates reported relationships with the company and four others are employed by Bristol-Myers Squibb.
SOURCE: Naumann RW et al. J Clin Oncol. 2019 Sep 5. doi: 10.1200JCP.19.00739.
The checkpoint inhibitor nivolumab may hold some promise for treating women with metastatic gynecologic cancers, especially cervical disease, according to cohort results from the phase 1/2 CheckMate 358 trial.
Of twenty-four patients enrolled in the recurrent/metastatic cervical and vaginal/vulvar carcinoma cohorts, 26.3% of patients with cervical cancer and 20% with vaginal/vulvar cancers experienced an objective response, R. Wendel Naumann, MD, and colleagues reported in the Journal of Clinical Oncology.
Median overall survival was 21.9 months (95% confidence interval, 15.1 months to not reached) among patients with cervical cancer. Because of the small size of the vaginal/vulvar cohort, median overall survival was not calculated.
“Given the lack of effective therapy and low survival rates for patients with metastatic disease in these gynecologic cancers, the results reported here are of strong clinical interest and underscore the growing role of immune checkpoint inhibitors in this patient population,” the investigators wrote.
Checkmate 358 is investigating nivolumab in patients with virus-associated cancers. Patients receive nivolumab monotherapy (240 mg intravenously every 2 weeks for 2 years) until disease progression, unacceptable side effects, or withdrawal of consent.
The median age was 51 years in the cervical cohort (n = 19) and 59 in the vaginal/vulvar cohort (n = 5). HPV status was positive in 83.3% of those with cervical disease and 40% of those with vaginal/vulvar disease. Ten cervical tumors and four vaginal/vulvar tumors expressed PD-L1.
The median duration of nivolumab treatment was 5.6 months in the cervical cohort and 6.7 months in the vaginal/vulvar cohort. Median follow-up was 19 months and 10 months, respectively.
At 12 months and 18 months, 40.0% of the cervical cohort and 20% of the vaginal/vulvar cohort were still alive.
The most common treatment-related adverse effects were diarrhea and decreased appetite. One patient in the cervical cohort discontinued treatment because of pneumonitis, which was considered treatment related.
Bristol-Myers Squibb funded the study. Several of Dr. Naumann’s associates reported relationships with the company and four others are employed by Bristol-Myers Squibb.
SOURCE: Naumann RW et al. J Clin Oncol. 2019 Sep 5. doi: 10.1200JCP.19.00739.
The checkpoint inhibitor nivolumab may hold some promise for treating women with metastatic gynecologic cancers, especially cervical disease, according to cohort results from the phase 1/2 CheckMate 358 trial.
Of twenty-four patients enrolled in the recurrent/metastatic cervical and vaginal/vulvar carcinoma cohorts, 26.3% of patients with cervical cancer and 20% with vaginal/vulvar cancers experienced an objective response, R. Wendel Naumann, MD, and colleagues reported in the Journal of Clinical Oncology.
Median overall survival was 21.9 months (95% confidence interval, 15.1 months to not reached) among patients with cervical cancer. Because of the small size of the vaginal/vulvar cohort, median overall survival was not calculated.
“Given the lack of effective therapy and low survival rates for patients with metastatic disease in these gynecologic cancers, the results reported here are of strong clinical interest and underscore the growing role of immune checkpoint inhibitors in this patient population,” the investigators wrote.
Checkmate 358 is investigating nivolumab in patients with virus-associated cancers. Patients receive nivolumab monotherapy (240 mg intravenously every 2 weeks for 2 years) until disease progression, unacceptable side effects, or withdrawal of consent.
The median age was 51 years in the cervical cohort (n = 19) and 59 in the vaginal/vulvar cohort (n = 5). HPV status was positive in 83.3% of those with cervical disease and 40% of those with vaginal/vulvar disease. Ten cervical tumors and four vaginal/vulvar tumors expressed PD-L1.
The median duration of nivolumab treatment was 5.6 months in the cervical cohort and 6.7 months in the vaginal/vulvar cohort. Median follow-up was 19 months and 10 months, respectively.
At 12 months and 18 months, 40.0% of the cervical cohort and 20% of the vaginal/vulvar cohort were still alive.
The most common treatment-related adverse effects were diarrhea and decreased appetite. One patient in the cervical cohort discontinued treatment because of pneumonitis, which was considered treatment related.
Bristol-Myers Squibb funded the study. Several of Dr. Naumann’s associates reported relationships with the company and four others are employed by Bristol-Myers Squibb.
SOURCE: Naumann RW et al. J Clin Oncol. 2019 Sep 5. doi: 10.1200JCP.19.00739.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
States pass record number of laws to reel in drug prices
Whether Congress will act this year to address the affordability of prescription drugs – a high priority among voters – remains uncertain. But states aren’t waiting.
So far this year, 33 states have enacted a record 51 laws to address drug prices, affordability, and access. That tops the previous record of 45 laws enacted in 28 states set just last year, according to the National Academy for State Health Policy, a nonprofit advocacy group that develops model legislation and promotes such laws.
Among the new measures are those that authorize importing prescription drugs, screen for excessive price increases by drug companies, and establish oversight boards to set the prices that states will pay for drugs.
“Legislative activity in this area is escalating,” said Trish Riley, NASHP’s executive director. “This year, some states moved to launch programs that directly impact what they and consumers pay for high-cost drugs.”
And more laws could be coming before year’s end. Of the handful of states still in legislative session – including California, Massachusetts, Michigan, New Jersey, Ohio, and Pennsylvania – debate continues on dozens of prescription drug bills. In New Jersey alone, some 20 proposed laws are under consideration.
“Both Democrat and Republican leaders have shown a willingness to pursue strong measures that help consumers but also protect state taxpayer dollars,” said Hemi Tewarson, director of the National Governors Association’s health programs.
Ms. Riley, Ms. Tewarson, and others note, however, that states can go only so far in addressing rising drug prices, and that federal legislation would be necessary to have a major effect on the way the marketplace works.
Federal lawmakers are keeping a close eye on the state initiatives, Ms. Tewarson said, to gauge where legislative compromise may lie – even as Congress debates more than a dozen bills that target drug costs. Political divisiveness, a packed congressional schedule and a looming election year could stall momentum at the federal level.
The pharmaceutical industry has opposed most – though not all – state bills, said Priscilla VanderVeer, a spokeswoman for the Pharmaceutical Research and Manufacturers of America, the industry’s main trade group.
“We agree that what consumers now pay for drugs out-of-pocket is a serious problem,” said Ms. VanderVeer. “Many states have passed bills that look good on paper but that we don’t believe will save consumers money.”
Limiting gag rules for pharmacists
At least 16 states have enacted 20 laws governing the behavior of pharmacy benefit managers. The so-called PBMs serve as middlemen among drugmakers, insurance companies, and pharmacies, largely with pharmaceutical industry support.
Those laws add to the 28 passed in 2018. Most of the new laws ban “gag clauses” that some PBMs impose on pharmacists. The clauses, written into pharmacy contracts, stop pharmacists from discussing with customers whether a drug’s cash price would be lower than its out-of-pocket cost under insurance.
With widespread public outrage over gag clauses pushing states to act, federal lawmakers got the message. In October, Congress passed a federal law banning such clauses in PBM-pharmacy contracts nationwide and under the Medicare Part D prescription drug benefit. The Senate passed it 98-2.
Even so, many of this year’s PBM laws contain additional gag clause limitations that go beyond the 2018 federal law.
Importing cheaper drugs
Four states – Colorado, Florida, Maine, and Vermont – this year have enacted measures to establish programs to import cheaper prescription drugs from Canada and, in Florida’s case, potentially other countries. Six other states are considering such legislation.
Medicines from Canada and other countries are less expensive because those nations negotiate directly with drugmakers to set prices.
“This is an area where states once feared to tread,” said Jane Horvath, a consultant with NASHP who has advised Maryland and Oregon, among other states, on prescription drug policy. “Now both Republicans and Democrats view it as a way to infuse more price competition into the marketplace.”
Hurdles remain, however. A 2003 law allows states to import cheaper drugs from Canada but only if the federal Health & Human Services Department approves a state’s plan and certifies its safety. During 2004-2009, the federal government halted nascent drug import efforts in five states.
Even so, momentum for importation has built in recent years in states and Congress as drug prices have continued to rise. And the Trump administration this summer threw its support behind the idea.
Florida Gov. Ron DeSantis, a Republican and close ally of President Donald Trump, signed his state’s measure into law on June 11, claiming he did so after Trump personally promised him that the White House would back the initiative.
On July 31, HHS announced an “action plan” to “lay the foundation for safe importation of certain prescription drugs.” The plan includes a process to authorize state initiatives. It also requires formal regulatory review, including establishing Food and Drug Administration safety criteria. That process could take up to 2 years.
Two big problems remain: In the weeks since the announcement, the Canadian government has opposed any plan that would rely solely on Canada as a source of imported drugs. The pharmaceutical industry also opposes the plan.
Creating drug affordability boards
Maryland and Maine enacted laws this year that establish state agencies to review the costs of drugs and take action against those whose price increases exceed a certain threshold.
New Jersey and Massachusetts are debating similar legislation this year.
Maryland’s law establishes a five-member board to review the list prices and costs of drugs purchased by the state and Maryland’s county and local governments. The board will probe drugs that increase in price by $3,000 or more per year and new medicines that enter the market costing $30,000 or more per year or over the course of treatment.
If approved by future legislation, upper payment limits on drugs with excessive price increases or annual costs would take effect in January 2022.
“My constituents have signaled loud and clear that bringing drug prices down is one of their top priorities,” said state Sen. Katherine Klausmeier, a Democrat representing Baltimore, who sponsored the legislation.
Maine’s law also establishes a five-member board. Beginning in 2021, the board will set annual spending targets for drugs purchased by the state and local governments.
Increasing price transparency
This year, four states – Colorado, Oregon, Texas, and Washington – became the latest to enact laws requiring drug companies to provide information to states and consumers on the list prices of drugs and planned price increases.
The majority of states now have such transparency laws, and most post the data on public websites. The details vary, but all states with such laws seek to identify drugs with price increases above 10% or more a year, and drugs with price increases above set dollar values.
Oregon’s new law, for example, requires manufacturers to notify the state 60 days in advance of any planned increase of 10% or more in the price of brand-name drugs, and any 25% or greater increase in the price of generic drugs.
“That 60-days’ notice was very important to us,” said state Rep. Andrea Salinas, a Democrat and chair of the Oregon House’s health committee, who represents Lake Oswego. “It gives doctors and patients advance notice and a chance to adjust and consider what to do.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Whether Congress will act this year to address the affordability of prescription drugs – a high priority among voters – remains uncertain. But states aren’t waiting.
So far this year, 33 states have enacted a record 51 laws to address drug prices, affordability, and access. That tops the previous record of 45 laws enacted in 28 states set just last year, according to the National Academy for State Health Policy, a nonprofit advocacy group that develops model legislation and promotes such laws.
Among the new measures are those that authorize importing prescription drugs, screen for excessive price increases by drug companies, and establish oversight boards to set the prices that states will pay for drugs.
“Legislative activity in this area is escalating,” said Trish Riley, NASHP’s executive director. “This year, some states moved to launch programs that directly impact what they and consumers pay for high-cost drugs.”
And more laws could be coming before year’s end. Of the handful of states still in legislative session – including California, Massachusetts, Michigan, New Jersey, Ohio, and Pennsylvania – debate continues on dozens of prescription drug bills. In New Jersey alone, some 20 proposed laws are under consideration.
“Both Democrat and Republican leaders have shown a willingness to pursue strong measures that help consumers but also protect state taxpayer dollars,” said Hemi Tewarson, director of the National Governors Association’s health programs.
Ms. Riley, Ms. Tewarson, and others note, however, that states can go only so far in addressing rising drug prices, and that federal legislation would be necessary to have a major effect on the way the marketplace works.
Federal lawmakers are keeping a close eye on the state initiatives, Ms. Tewarson said, to gauge where legislative compromise may lie – even as Congress debates more than a dozen bills that target drug costs. Political divisiveness, a packed congressional schedule and a looming election year could stall momentum at the federal level.
The pharmaceutical industry has opposed most – though not all – state bills, said Priscilla VanderVeer, a spokeswoman for the Pharmaceutical Research and Manufacturers of America, the industry’s main trade group.
“We agree that what consumers now pay for drugs out-of-pocket is a serious problem,” said Ms. VanderVeer. “Many states have passed bills that look good on paper but that we don’t believe will save consumers money.”
Limiting gag rules for pharmacists
At least 16 states have enacted 20 laws governing the behavior of pharmacy benefit managers. The so-called PBMs serve as middlemen among drugmakers, insurance companies, and pharmacies, largely with pharmaceutical industry support.
Those laws add to the 28 passed in 2018. Most of the new laws ban “gag clauses” that some PBMs impose on pharmacists. The clauses, written into pharmacy contracts, stop pharmacists from discussing with customers whether a drug’s cash price would be lower than its out-of-pocket cost under insurance.
With widespread public outrage over gag clauses pushing states to act, federal lawmakers got the message. In October, Congress passed a federal law banning such clauses in PBM-pharmacy contracts nationwide and under the Medicare Part D prescription drug benefit. The Senate passed it 98-2.
Even so, many of this year’s PBM laws contain additional gag clause limitations that go beyond the 2018 federal law.
Importing cheaper drugs
Four states – Colorado, Florida, Maine, and Vermont – this year have enacted measures to establish programs to import cheaper prescription drugs from Canada and, in Florida’s case, potentially other countries. Six other states are considering such legislation.
Medicines from Canada and other countries are less expensive because those nations negotiate directly with drugmakers to set prices.
“This is an area where states once feared to tread,” said Jane Horvath, a consultant with NASHP who has advised Maryland and Oregon, among other states, on prescription drug policy. “Now both Republicans and Democrats view it as a way to infuse more price competition into the marketplace.”
Hurdles remain, however. A 2003 law allows states to import cheaper drugs from Canada but only if the federal Health & Human Services Department approves a state’s plan and certifies its safety. During 2004-2009, the federal government halted nascent drug import efforts in five states.
Even so, momentum for importation has built in recent years in states and Congress as drug prices have continued to rise. And the Trump administration this summer threw its support behind the idea.
Florida Gov. Ron DeSantis, a Republican and close ally of President Donald Trump, signed his state’s measure into law on June 11, claiming he did so after Trump personally promised him that the White House would back the initiative.
On July 31, HHS announced an “action plan” to “lay the foundation for safe importation of certain prescription drugs.” The plan includes a process to authorize state initiatives. It also requires formal regulatory review, including establishing Food and Drug Administration safety criteria. That process could take up to 2 years.
Two big problems remain: In the weeks since the announcement, the Canadian government has opposed any plan that would rely solely on Canada as a source of imported drugs. The pharmaceutical industry also opposes the plan.
Creating drug affordability boards
Maryland and Maine enacted laws this year that establish state agencies to review the costs of drugs and take action against those whose price increases exceed a certain threshold.
New Jersey and Massachusetts are debating similar legislation this year.
Maryland’s law establishes a five-member board to review the list prices and costs of drugs purchased by the state and Maryland’s county and local governments. The board will probe drugs that increase in price by $3,000 or more per year and new medicines that enter the market costing $30,000 or more per year or over the course of treatment.
If approved by future legislation, upper payment limits on drugs with excessive price increases or annual costs would take effect in January 2022.
“My constituents have signaled loud and clear that bringing drug prices down is one of their top priorities,” said state Sen. Katherine Klausmeier, a Democrat representing Baltimore, who sponsored the legislation.
Maine’s law also establishes a five-member board. Beginning in 2021, the board will set annual spending targets for drugs purchased by the state and local governments.
Increasing price transparency
This year, four states – Colorado, Oregon, Texas, and Washington – became the latest to enact laws requiring drug companies to provide information to states and consumers on the list prices of drugs and planned price increases.
The majority of states now have such transparency laws, and most post the data on public websites. The details vary, but all states with such laws seek to identify drugs with price increases above 10% or more a year, and drugs with price increases above set dollar values.
Oregon’s new law, for example, requires manufacturers to notify the state 60 days in advance of any planned increase of 10% or more in the price of brand-name drugs, and any 25% or greater increase in the price of generic drugs.
“That 60-days’ notice was very important to us,” said state Rep. Andrea Salinas, a Democrat and chair of the Oregon House’s health committee, who represents Lake Oswego. “It gives doctors and patients advance notice and a chance to adjust and consider what to do.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Whether Congress will act this year to address the affordability of prescription drugs – a high priority among voters – remains uncertain. But states aren’t waiting.
So far this year, 33 states have enacted a record 51 laws to address drug prices, affordability, and access. That tops the previous record of 45 laws enacted in 28 states set just last year, according to the National Academy for State Health Policy, a nonprofit advocacy group that develops model legislation and promotes such laws.
Among the new measures are those that authorize importing prescription drugs, screen for excessive price increases by drug companies, and establish oversight boards to set the prices that states will pay for drugs.
“Legislative activity in this area is escalating,” said Trish Riley, NASHP’s executive director. “This year, some states moved to launch programs that directly impact what they and consumers pay for high-cost drugs.”
And more laws could be coming before year’s end. Of the handful of states still in legislative session – including California, Massachusetts, Michigan, New Jersey, Ohio, and Pennsylvania – debate continues on dozens of prescription drug bills. In New Jersey alone, some 20 proposed laws are under consideration.
“Both Democrat and Republican leaders have shown a willingness to pursue strong measures that help consumers but also protect state taxpayer dollars,” said Hemi Tewarson, director of the National Governors Association’s health programs.
Ms. Riley, Ms. Tewarson, and others note, however, that states can go only so far in addressing rising drug prices, and that federal legislation would be necessary to have a major effect on the way the marketplace works.
Federal lawmakers are keeping a close eye on the state initiatives, Ms. Tewarson said, to gauge where legislative compromise may lie – even as Congress debates more than a dozen bills that target drug costs. Political divisiveness, a packed congressional schedule and a looming election year could stall momentum at the federal level.
The pharmaceutical industry has opposed most – though not all – state bills, said Priscilla VanderVeer, a spokeswoman for the Pharmaceutical Research and Manufacturers of America, the industry’s main trade group.
“We agree that what consumers now pay for drugs out-of-pocket is a serious problem,” said Ms. VanderVeer. “Many states have passed bills that look good on paper but that we don’t believe will save consumers money.”
Limiting gag rules for pharmacists
At least 16 states have enacted 20 laws governing the behavior of pharmacy benefit managers. The so-called PBMs serve as middlemen among drugmakers, insurance companies, and pharmacies, largely with pharmaceutical industry support.
Those laws add to the 28 passed in 2018. Most of the new laws ban “gag clauses” that some PBMs impose on pharmacists. The clauses, written into pharmacy contracts, stop pharmacists from discussing with customers whether a drug’s cash price would be lower than its out-of-pocket cost under insurance.
With widespread public outrage over gag clauses pushing states to act, federal lawmakers got the message. In October, Congress passed a federal law banning such clauses in PBM-pharmacy contracts nationwide and under the Medicare Part D prescription drug benefit. The Senate passed it 98-2.
Even so, many of this year’s PBM laws contain additional gag clause limitations that go beyond the 2018 federal law.
Importing cheaper drugs
Four states – Colorado, Florida, Maine, and Vermont – this year have enacted measures to establish programs to import cheaper prescription drugs from Canada and, in Florida’s case, potentially other countries. Six other states are considering such legislation.
Medicines from Canada and other countries are less expensive because those nations negotiate directly with drugmakers to set prices.
“This is an area where states once feared to tread,” said Jane Horvath, a consultant with NASHP who has advised Maryland and Oregon, among other states, on prescription drug policy. “Now both Republicans and Democrats view it as a way to infuse more price competition into the marketplace.”
Hurdles remain, however. A 2003 law allows states to import cheaper drugs from Canada but only if the federal Health & Human Services Department approves a state’s plan and certifies its safety. During 2004-2009, the federal government halted nascent drug import efforts in five states.
Even so, momentum for importation has built in recent years in states and Congress as drug prices have continued to rise. And the Trump administration this summer threw its support behind the idea.
Florida Gov. Ron DeSantis, a Republican and close ally of President Donald Trump, signed his state’s measure into law on June 11, claiming he did so after Trump personally promised him that the White House would back the initiative.
On July 31, HHS announced an “action plan” to “lay the foundation for safe importation of certain prescription drugs.” The plan includes a process to authorize state initiatives. It also requires formal regulatory review, including establishing Food and Drug Administration safety criteria. That process could take up to 2 years.
Two big problems remain: In the weeks since the announcement, the Canadian government has opposed any plan that would rely solely on Canada as a source of imported drugs. The pharmaceutical industry also opposes the plan.
Creating drug affordability boards
Maryland and Maine enacted laws this year that establish state agencies to review the costs of drugs and take action against those whose price increases exceed a certain threshold.
New Jersey and Massachusetts are debating similar legislation this year.
Maryland’s law establishes a five-member board to review the list prices and costs of drugs purchased by the state and Maryland’s county and local governments. The board will probe drugs that increase in price by $3,000 or more per year and new medicines that enter the market costing $30,000 or more per year or over the course of treatment.
If approved by future legislation, upper payment limits on drugs with excessive price increases or annual costs would take effect in January 2022.
“My constituents have signaled loud and clear that bringing drug prices down is one of their top priorities,” said state Sen. Katherine Klausmeier, a Democrat representing Baltimore, who sponsored the legislation.
Maine’s law also establishes a five-member board. Beginning in 2021, the board will set annual spending targets for drugs purchased by the state and local governments.
Increasing price transparency
This year, four states – Colorado, Oregon, Texas, and Washington – became the latest to enact laws requiring drug companies to provide information to states and consumers on the list prices of drugs and planned price increases.
The majority of states now have such transparency laws, and most post the data on public websites. The details vary, but all states with such laws seek to identify drugs with price increases above 10% or more a year, and drugs with price increases above set dollar values.
Oregon’s new law, for example, requires manufacturers to notify the state 60 days in advance of any planned increase of 10% or more in the price of brand-name drugs, and any 25% or greater increase in the price of generic drugs.
“That 60-days’ notice was very important to us,” said state Rep. Andrea Salinas, a Democrat and chair of the Oregon House’s health committee, who represents Lake Oswego. “It gives doctors and patients advance notice and a chance to adjust and consider what to do.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
FDA issues warning for CDK 4/6 inhibitors
The Food and Drug Administration is warning that the entire class of the cyclin-dependent kinase 4/6 (CDK 4/6) inhibitors used to treat advanced breast cancer may cause rare but severe inflammation of the lungs.
“We reviewed CDK 4/6 inhibitors cases from completed and ongoing clinical trials undertaken by manufacturers and their postmarket safety databases that described specific types of inflammation of the lungs, called interstitial lung disease and pneumonitis. Across the entire drug class, there were reports of serious cases, including fatalities,” the FDA said in a press statement.
The overall benefit of CDK 4/6 inhibitors, however, is still greater than the risks when used as prescribed, the agency said.
CDK 4/6 inhibitors are used in combination with hormone therapies to treat adults with hormone receptor–positive, human epidermal growth factor 2–negative advanced or metastatic breast cancer that has spread to other parts of the body. The FDA approved the CDK 4/6 inhibitors palbociclib (Ibrance) in 2015 and ribociclib (Kisqali) and abemaciclib (Verzenio) in 2017, based on improvements in progression-free survival.
Health care professionals should monitor patients regularly for pulmonary symptoms indicative of interstitial lung disease and/or pneumonitis. Signs and symptoms may include hypoxia, cough, dyspnea, or interstitial infiltrates on radiologic exams in patients in whom infectious, neoplastic, and other causes have been excluded. Interrupt CDK 4/6 inhibitor treatment in patients who have new or worsening respiratory symptoms, and permanently discontinue treatment in patients with severe interstitial lung disease and/or pneumonitis, the FDA said.
The Food and Drug Administration is warning that the entire class of the cyclin-dependent kinase 4/6 (CDK 4/6) inhibitors used to treat advanced breast cancer may cause rare but severe inflammation of the lungs.
“We reviewed CDK 4/6 inhibitors cases from completed and ongoing clinical trials undertaken by manufacturers and their postmarket safety databases that described specific types of inflammation of the lungs, called interstitial lung disease and pneumonitis. Across the entire drug class, there were reports of serious cases, including fatalities,” the FDA said in a press statement.
The overall benefit of CDK 4/6 inhibitors, however, is still greater than the risks when used as prescribed, the agency said.
CDK 4/6 inhibitors are used in combination with hormone therapies to treat adults with hormone receptor–positive, human epidermal growth factor 2–negative advanced or metastatic breast cancer that has spread to other parts of the body. The FDA approved the CDK 4/6 inhibitors palbociclib (Ibrance) in 2015 and ribociclib (Kisqali) and abemaciclib (Verzenio) in 2017, based on improvements in progression-free survival.
Health care professionals should monitor patients regularly for pulmonary symptoms indicative of interstitial lung disease and/or pneumonitis. Signs and symptoms may include hypoxia, cough, dyspnea, or interstitial infiltrates on radiologic exams in patients in whom infectious, neoplastic, and other causes have been excluded. Interrupt CDK 4/6 inhibitor treatment in patients who have new or worsening respiratory symptoms, and permanently discontinue treatment in patients with severe interstitial lung disease and/or pneumonitis, the FDA said.
The Food and Drug Administration is warning that the entire class of the cyclin-dependent kinase 4/6 (CDK 4/6) inhibitors used to treat advanced breast cancer may cause rare but severe inflammation of the lungs.
“We reviewed CDK 4/6 inhibitors cases from completed and ongoing clinical trials undertaken by manufacturers and their postmarket safety databases that described specific types of inflammation of the lungs, called interstitial lung disease and pneumonitis. Across the entire drug class, there were reports of serious cases, including fatalities,” the FDA said in a press statement.
The overall benefit of CDK 4/6 inhibitors, however, is still greater than the risks when used as prescribed, the agency said.
CDK 4/6 inhibitors are used in combination with hormone therapies to treat adults with hormone receptor–positive, human epidermal growth factor 2–negative advanced or metastatic breast cancer that has spread to other parts of the body. The FDA approved the CDK 4/6 inhibitors palbociclib (Ibrance) in 2015 and ribociclib (Kisqali) and abemaciclib (Verzenio) in 2017, based on improvements in progression-free survival.
Health care professionals should monitor patients regularly for pulmonary symptoms indicative of interstitial lung disease and/or pneumonitis. Signs and symptoms may include hypoxia, cough, dyspnea, or interstitial infiltrates on radiologic exams in patients in whom infectious, neoplastic, and other causes have been excluded. Interrupt CDK 4/6 inhibitor treatment in patients who have new or worsening respiratory symptoms, and permanently discontinue treatment in patients with severe interstitial lung disease and/or pneumonitis, the FDA said.