User login
Benzodiazapines for anxiety: Psychcast Masterclass
Also in this episode, what happens when you have plans to get some work done, but Alexa has other ideas? Dr. RK offers her thoughts on just such a situation – and on practicing what she preaches. You can contact the Psychcast by emailing podcasts@mdedge.com.
Also in this episode, what happens when you have plans to get some work done, but Alexa has other ideas? Dr. RK offers her thoughts on just such a situation – and on practicing what she preaches. You can contact the Psychcast by emailing podcasts@mdedge.com.
Also in this episode, what happens when you have plans to get some work done, but Alexa has other ideas? Dr. RK offers her thoughts on just such a situation – and on practicing what she preaches. You can contact the Psychcast by emailing podcasts@mdedge.com.
Automated office BP readings
Also today, a positive fecal immunochemical test should prompt colonoscopy, mild aerobic exercise speeds recovery for sports concussions, and phase 2 studies of the antiamyloid Alzheimer’s drug crenezumab are stopped.
Also today, a positive fecal immunochemical test should prompt colonoscopy, mild aerobic exercise speeds recovery for sports concussions, and phase 2 studies of the antiamyloid Alzheimer’s drug crenezumab are stopped.
Also today, a positive fecal immunochemical test should prompt colonoscopy, mild aerobic exercise speeds recovery for sports concussions, and phase 2 studies of the antiamyloid Alzheimer’s drug crenezumab are stopped.
Barriers and Facilitators to the Use of Genomic-Based Targeted Therapy in the VA: Qualitative Findings(FULL)
Lung cancer is the most frequent cause of cancer-related mortality worldwide.1 The most prevalent type of lung cancer is non-small cell lung cancer (NSCLC), which comprises about 85% of lung cancer cases.2 As there are no cost-effective approaches to screening for lung cancer, most lung cancers are identified at an advanced stage (stage IIIB or IV).
New approaches to managing advanced lung cancer have emerged in recent years, including drugs designed to target specific genetic mutations in some tumors.3 The National Comprehensive Cancer Network (NCCN) recommends erlotinib, a receptor tyrosine kinase inhibitor of the epidermal growth factor receptor (EGFR) for first-line treatment of advanced NSCLC with EGFR mutation.4 Crizotinib is recommended to treat cancers that test positive for the anaplastic lymphoma kinase (ALK) mutation.4 Utilization of targeting agents has been found to extend the survival times for patients with the specified mutations.5 Both erlotinib and crizotinib are available at the VHA.
Previous research showed that VHA providers expressed overall favorable attitudes about genomic medicine.6 Providers perceived genomic medicine to have an important and possibly transformative role in medicine. Barriers to utilization of genomic medicine involved concerns about coordination of care, changes in workload, and increased length of patient visits. In addition to these system-level barriers, many providers had concerns about the proficiency of VHA-based practitioners to appropriately use genomic medicine.
Previous research has evaluated utilization of genomic testing and genomic-based targeted therapy (GBTT) in VA and community settings.5-8 It is unclear whether VHA-based providers are following clinical guidelines regarding genomic testing and utilization of GBTT.4 The authors set out to identify factors that impede and encourage guideline-consistent care in the management of NSCLC at the VHA. The authors specifically sought information about oncologists’ perceptions and experiences with EGFR and ALK mutation testing in patients with advanced NSCLC, as well as use of erlotinib and crizotinib in treating such patients.
Methods
This study was approved by the institutional review boards at Michael E. DeBakey VAMC in Houston, Texas and Baylor College of Medicine. In-depth qualitative interviews were conducted with VHA oncologists to examine their reported barriers and facilitators to mutation testing and prescribing of genomic-based treatment in patients with advanced NSCLC.
The sample of participants was recruited from a list of VHA medical oncologists, compiled by the study project coordinator. Investigators stratified the list by American College of Surgeons Commission on Cancer (CoC) accreditation status (yes/no) and used a stratified purposive sampling technique to recruit participants from CoC-accredited facilities and nonaccredited facilities. Recruitment and data collection occurred between March 2015 and February 2016. Oncologists were considered for inclusion if they (1) were specialists in oncology; (2) practiced at the VHA during the time of recruitment; and (3) had experience treating lung cancer at a VHA facility. During recruitment, potential participants were told that the investigators were interested in learning about oncologists’ experiences and decisions about using GBTT to treat advanced lung cancer in the VHA. Participants were scheduled for telephone-based interviews, and verbal consent was obtained prior to all interviews. Interviews ranged from 19 to 90 minutes (average, 40 min).
Recruitment was stopped at the point of thematic saturation, defined a priori as the point when 2 independent coders agreed that 3 consecutive transcripts for a given interview category (see below) rendered no new thematic concepts.9,10 Consistent with the theoretical framework developed by Cabana and colleagues, interviews were designed to elicit information about oncologists’ knowledge, attitudes, intent to use GBTT, and perceived facilitators and barriers to using GBTT in the VHA.11 Additional findings are presented elsewhere.12 The interview guide was pilot tested and revised prior to initiating data collection. All interviews were recorded, transcribed, and analyzed for content.
Analysis
Data were analyzed using framework analysis methodology, which allows for the inclusion of existing concepts as well as emergent themes within an established theoretical framework.13 Two independent coders with expertise in framework analysis independently created codes and indexed the data using Atlas.ti 6.2 (Scientific Software Development, Berlin, Germany). Disagreements about coding decisions were resolved through group consensus. Coding centered on 2 themes:
- Barriers and facilitators to mutation testing. This includes system or facility factors and testing weaknesses that act as barriers to ordering mutation testing, system or facility factors that facilitate ordering mutation testing, and oncologists’ suggestions for ways to encourage more testing in the VHA.
- Barriers and facilitators to prescribing GBTT. This includes system or facility factors that act as barriers to prescribing GBTT, system or facility factors that facilitate prescribing GBTT, and oncologists’ suggestions for ways to encourage more prescribing of GBTT in the VHA.
Thirty medical oncologists were interviewed. Participant demographics are presented in the Table.
Barriers to testing
The 2 most commonly cited barriers to ordering mutation testing can be considered weaknesses in the testing process: lack of tissue and wait time for results. Almost all providers identified lack of tissue as a barrier to ordering a mutation test.
Another frequently cited testing weakness involved the wait time for results. Because the mutation analysis is not conducted in the VHA facility, providers often must wait 2 to 4 weeks to receive results. This can present a problem because some providers do not want to wait for the results before recommending a course of treatment.
Several providers cited system and facility factors as barriers to mutation testing. The most common of these involves the ordering process. Oncology providers often remarked that ordering the mutation test is cumbersome or inconvenient because there is no ordering mechanism in the Computerized Patient Record System (CPRS). Many different approaches for ordering a mutation test exist, including e-mailing the pathology department, calling to place the order, or requesting the test in person. As providers can order many, if not most, other tests via CPRS, it is clear that this presents an inconvenient exception.
Budgetary constraints were another frequently cited system or facility-level barrier. Providers sometimes were unable to access the test due to the cost
Finally, several providers noted that in some cases patients did not wish to undergo a biopsy. Thus, patient preference can act as a barrier to mutation testing. Some patients wish to forgo treatment, which eliminates the need for a mutation test. Other patients believe that due to their smoking history, they are unlikely to have an ALK or EGFR mutation and instead immediately opt for chemotherapy. Only a small minority of participants identified no barriers to mutation testing.
Facilitators for Testing
Many providers complimented the availability of the mutation test. Interestingly, while some providers mentioned that lack of CPRS ordering was a barrier to testing, several also listed access to a CPRS order as a facilitator. These providers commented that ordering a test was streamlined and easy, given the mechanism in CPRS. Some VHA facilities offer CPRS order capabilities, and others do not. Other oncologists commented more generally on the cooperativeness of the pathology department in ordering mutation tests. It seems that facilities may use different ordering procedures, but in most of these facilities, a high degree of cooperation exists between departments to send out for tests that are requested.
Providers offered many ideas for ways to improve mutation testing or to facilitate the testing. By far, the most commonly cited way to improve the testing process was to make mutation testing reflexive for metastatic nonsquamous NSCLC. Some acknowledged that to achieve this would require a change to the budgeting process such that the test would not drain the pathology department’s budget. Implementing reflexive testing of patients, as recommended by guidelines, would understandably address several of the barriers that were identified in this study. Other providers recommended standardizing the ordering procedure and location of results. Specifically, providers recommended creating a button in CPRS for ordering and always reporting the results in the same place in CPRS.
Barriers to GBTT Prescribing
The clear majority of providers identified no barriers to prescribing GBTTs. A few mentioned that they were required to submit a nonformulary consult. A representative quote described this as “more out of a formality, and the pharmacist basically is there with me and he approves it on the spot and provides the prescription on the day, right when I’m seeing the patient.” Only a very small minority of providers identified medication cost as a barrier, but even those respondents did not indicate that cost prevented them from offering GBTTs to their patients. Rather, cost consciousness simply made them more mindful and judicious when making decisions about prescribing GBTTs.
Facilitators to GBTT Prescribing
Several providers listed availability of the costly medication in the VHA as a facilitator to prescribing. Veterans can obtain GBTTs with little to no insurance cost or copayment, which is not always the case outside the VHA.
One recommendation for further facilitating prescribing of GBTTs involved eliminating the preauthorization requirement, particularly in first-line use for patients testing positive for ALK or EGFR mutations. Although the preauthorization was not seen as a significant barrier, removal of this formality could make prescribing easier.
Discussion
Although in some cases, testing weaknesses (lack of tissue, wait time to receive results) can interrupt a treatment trajectory, many of the barriers identified in this study are modifiable. Overwhelmingly, oncologists recommended making mutation testing reflexive for metastatic nonsquamous NSCLC. Implementing reflexive testing of patients, as recommended by guidelines, would understandably address issues related to variable utilization of genomic testing in VHA.12 Additionally, in response to system and facility barriers to mutation testing, other providers recommended standardizing the ordering procedure and location of results. Utilization of GBTT can be facilitated by eliminating the preauthorization requirement, particularly in first-line use for patients with positive mutations. Although the preauthorization was not seen as a significant barrier, removal of this formality could make prescribing easier.
This study extends previous research that identified underuse of genomic testing in community-based practices. The authors sought to interview a broad sample of providers from various facilities (small, large, CoC accredited, nonaccredited) to understand the range of conditions faced by VA providers. Some providers face more barriers than do others, whereas some face few or no barriers. This wide range of experiences can help to better understand the factors that facilitate guideline-adherent care.
Limitations
The authors recognize that availability of resources and testing and prescribing practices are constantly evolving and perhaps have improved since the data were collected. Thus, the age of the study data might be a limitation to the study. Like most qualitative studies, these findings are limited in their generalizability beyond the study population. Additionally, the authors were limited to recruiting oncologists with reliable contact information listed in the VHA directory. Although this could have introduced some degree of sampling bias, the authors are confident that the sample sufficiently represents the population of VHA-based medical oncologists who treat lung cancer. Despite these limitations, these findings provide novel perspectives on barriers and facilitators to genomic testing GBTT prescribing in the VHA. The authors identify modifiable barriers to testing and prescribing that can be addressed to improve and standardize care of advanced lung cancer in the VHA.
Conclusion
Efforts should be made to address modifiable barriers to mutation testing and guideline-consistent prescribing of GBTT in the VA setting. Implementation of specific practices like reflexive testing for all metastatic nonsquamous NSCLC, standardization of the mutation test ordering procedure, standardization of results reporting, and elimination of the preauthorization to prescribe GBTT could impact the utilization of GBTT in VHA.
Click here to read the digital edition.
. , , , Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
2. American Cancer Society. What is non-small cell lung cancer? https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/what-is-non-small-cell-lung-cancer.html. Updated May 16, 2016. Accessed January 19, 2018.
3. , , . New targetable oncogenes in non-small-cell lung cancer. J Clin Oncol. 2013;31(8):1097-1104.
4. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). non-small cell lung cancer 2. 2018. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Updated December 19, 2017. Accessed Jan
5. Rosell R, Moran T, Queralt C, et al; Spanish Lung Cancer Group. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958-967.
6. Arar N, Seo J, Abboud HE, Parchman M, Noel P. Providers’ behavioral beliefs regarding the delivery of genomic medicine at the Veterans Health Administration. Per Med. 2010;7(5):485-494.
7. Lynch JA, Berse B, Dotson D, Khoury MJ, Coomer N, Kautter J. Utilization of genetic tests: analysis of gene-specific billing in Medicare claims data. Genet Med. 2017; 19(8):890-899.
8. Gutierrez ME, Choi K, Lanman RB, et al. Genomic profiling of advanced non-small cell lung cancer in community settings: gaps and opportunities. Clin Lung Cancer, 2017;18(6):651-659.
9. Morse JM. The significance of saturation. Qual Health Res.1995;5(2):147-149.
10. Aita VA, McIlvain HE. An armchair adventure in case study research. In: Crabtree BF, Miller WF, eds. Doing Qualitative Research. Thousand Oaks, CA: Sage; 1999:253-268.
11. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465.
12. Arney JB, Helm A, Crook T, Braun U, Chen GJ, Hayes TG. Utilization of genomic testing in advanced non-small cell lung cancer among oncologists in the Veterans Health Administration. Lung Cancer, 2018;116:25-29.
13. Ritchie J, Spencer L. Qualitative data analysis for applied policy research. In: Bryman A, Burgess RG, eds. Analyzing Qualitative Data. New York, NY: Routledge; 1994:173-194.
Lung cancer is the most frequent cause of cancer-related mortality worldwide.1 The most prevalent type of lung cancer is non-small cell lung cancer (NSCLC), which comprises about 85% of lung cancer cases.2 As there are no cost-effective approaches to screening for lung cancer, most lung cancers are identified at an advanced stage (stage IIIB or IV).
New approaches to managing advanced lung cancer have emerged in recent years, including drugs designed to target specific genetic mutations in some tumors.3 The National Comprehensive Cancer Network (NCCN) recommends erlotinib, a receptor tyrosine kinase inhibitor of the epidermal growth factor receptor (EGFR) for first-line treatment of advanced NSCLC with EGFR mutation.4 Crizotinib is recommended to treat cancers that test positive for the anaplastic lymphoma kinase (ALK) mutation.4 Utilization of targeting agents has been found to extend the survival times for patients with the specified mutations.5 Both erlotinib and crizotinib are available at the VHA.
Previous research showed that VHA providers expressed overall favorable attitudes about genomic medicine.6 Providers perceived genomic medicine to have an important and possibly transformative role in medicine. Barriers to utilization of genomic medicine involved concerns about coordination of care, changes in workload, and increased length of patient visits. In addition to these system-level barriers, many providers had concerns about the proficiency of VHA-based practitioners to appropriately use genomic medicine.
Previous research has evaluated utilization of genomic testing and genomic-based targeted therapy (GBTT) in VA and community settings.5-8 It is unclear whether VHA-based providers are following clinical guidelines regarding genomic testing and utilization of GBTT.4 The authors set out to identify factors that impede and encourage guideline-consistent care in the management of NSCLC at the VHA. The authors specifically sought information about oncologists’ perceptions and experiences with EGFR and ALK mutation testing in patients with advanced NSCLC, as well as use of erlotinib and crizotinib in treating such patients.
Methods
This study was approved by the institutional review boards at Michael E. DeBakey VAMC in Houston, Texas and Baylor College of Medicine. In-depth qualitative interviews were conducted with VHA oncologists to examine their reported barriers and facilitators to mutation testing and prescribing of genomic-based treatment in patients with advanced NSCLC.
The sample of participants was recruited from a list of VHA medical oncologists, compiled by the study project coordinator. Investigators stratified the list by American College of Surgeons Commission on Cancer (CoC) accreditation status (yes/no) and used a stratified purposive sampling technique to recruit participants from CoC-accredited facilities and nonaccredited facilities. Recruitment and data collection occurred between March 2015 and February 2016. Oncologists were considered for inclusion if they (1) were specialists in oncology; (2) practiced at the VHA during the time of recruitment; and (3) had experience treating lung cancer at a VHA facility. During recruitment, potential participants were told that the investigators were interested in learning about oncologists’ experiences and decisions about using GBTT to treat advanced lung cancer in the VHA. Participants were scheduled for telephone-based interviews, and verbal consent was obtained prior to all interviews. Interviews ranged from 19 to 90 minutes (average, 40 min).
Recruitment was stopped at the point of thematic saturation, defined a priori as the point when 2 independent coders agreed that 3 consecutive transcripts for a given interview category (see below) rendered no new thematic concepts.9,10 Consistent with the theoretical framework developed by Cabana and colleagues, interviews were designed to elicit information about oncologists’ knowledge, attitudes, intent to use GBTT, and perceived facilitators and barriers to using GBTT in the VHA.11 Additional findings are presented elsewhere.12 The interview guide was pilot tested and revised prior to initiating data collection. All interviews were recorded, transcribed, and analyzed for content.
Analysis
Data were analyzed using framework analysis methodology, which allows for the inclusion of existing concepts as well as emergent themes within an established theoretical framework.13 Two independent coders with expertise in framework analysis independently created codes and indexed the data using Atlas.ti 6.2 (Scientific Software Development, Berlin, Germany). Disagreements about coding decisions were resolved through group consensus. Coding centered on 2 themes:
- Barriers and facilitators to mutation testing. This includes system or facility factors and testing weaknesses that act as barriers to ordering mutation testing, system or facility factors that facilitate ordering mutation testing, and oncologists’ suggestions for ways to encourage more testing in the VHA.
- Barriers and facilitators to prescribing GBTT. This includes system or facility factors that act as barriers to prescribing GBTT, system or facility factors that facilitate prescribing GBTT, and oncologists’ suggestions for ways to encourage more prescribing of GBTT in the VHA.
Thirty medical oncologists were interviewed. Participant demographics are presented in the Table.
Barriers to testing
The 2 most commonly cited barriers to ordering mutation testing can be considered weaknesses in the testing process: lack of tissue and wait time for results. Almost all providers identified lack of tissue as a barrier to ordering a mutation test.
Another frequently cited testing weakness involved the wait time for results. Because the mutation analysis is not conducted in the VHA facility, providers often must wait 2 to 4 weeks to receive results. This can present a problem because some providers do not want to wait for the results before recommending a course of treatment.
Several providers cited system and facility factors as barriers to mutation testing. The most common of these involves the ordering process. Oncology providers often remarked that ordering the mutation test is cumbersome or inconvenient because there is no ordering mechanism in the Computerized Patient Record System (CPRS). Many different approaches for ordering a mutation test exist, including e-mailing the pathology department, calling to place the order, or requesting the test in person. As providers can order many, if not most, other tests via CPRS, it is clear that this presents an inconvenient exception.
Budgetary constraints were another frequently cited system or facility-level barrier. Providers sometimes were unable to access the test due to the cost
Finally, several providers noted that in some cases patients did not wish to undergo a biopsy. Thus, patient preference can act as a barrier to mutation testing. Some patients wish to forgo treatment, which eliminates the need for a mutation test. Other patients believe that due to their smoking history, they are unlikely to have an ALK or EGFR mutation and instead immediately opt for chemotherapy. Only a small minority of participants identified no barriers to mutation testing.
Facilitators for Testing
Many providers complimented the availability of the mutation test. Interestingly, while some providers mentioned that lack of CPRS ordering was a barrier to testing, several also listed access to a CPRS order as a facilitator. These providers commented that ordering a test was streamlined and easy, given the mechanism in CPRS. Some VHA facilities offer CPRS order capabilities, and others do not. Other oncologists commented more generally on the cooperativeness of the pathology department in ordering mutation tests. It seems that facilities may use different ordering procedures, but in most of these facilities, a high degree of cooperation exists between departments to send out for tests that are requested.
Providers offered many ideas for ways to improve mutation testing or to facilitate the testing. By far, the most commonly cited way to improve the testing process was to make mutation testing reflexive for metastatic nonsquamous NSCLC. Some acknowledged that to achieve this would require a change to the budgeting process such that the test would not drain the pathology department’s budget. Implementing reflexive testing of patients, as recommended by guidelines, would understandably address several of the barriers that were identified in this study. Other providers recommended standardizing the ordering procedure and location of results. Specifically, providers recommended creating a button in CPRS for ordering and always reporting the results in the same place in CPRS.
Barriers to GBTT Prescribing
The clear majority of providers identified no barriers to prescribing GBTTs. A few mentioned that they were required to submit a nonformulary consult. A representative quote described this as “more out of a formality, and the pharmacist basically is there with me and he approves it on the spot and provides the prescription on the day, right when I’m seeing the patient.” Only a very small minority of providers identified medication cost as a barrier, but even those respondents did not indicate that cost prevented them from offering GBTTs to their patients. Rather, cost consciousness simply made them more mindful and judicious when making decisions about prescribing GBTTs.
Facilitators to GBTT Prescribing
Several providers listed availability of the costly medication in the VHA as a facilitator to prescribing. Veterans can obtain GBTTs with little to no insurance cost or copayment, which is not always the case outside the VHA.
One recommendation for further facilitating prescribing of GBTTs involved eliminating the preauthorization requirement, particularly in first-line use for patients testing positive for ALK or EGFR mutations. Although the preauthorization was not seen as a significant barrier, removal of this formality could make prescribing easier.
Discussion
Although in some cases, testing weaknesses (lack of tissue, wait time to receive results) can interrupt a treatment trajectory, many of the barriers identified in this study are modifiable. Overwhelmingly, oncologists recommended making mutation testing reflexive for metastatic nonsquamous NSCLC. Implementing reflexive testing of patients, as recommended by guidelines, would understandably address issues related to variable utilization of genomic testing in VHA.12 Additionally, in response to system and facility barriers to mutation testing, other providers recommended standardizing the ordering procedure and location of results. Utilization of GBTT can be facilitated by eliminating the preauthorization requirement, particularly in first-line use for patients with positive mutations. Although the preauthorization was not seen as a significant barrier, removal of this formality could make prescribing easier.
This study extends previous research that identified underuse of genomic testing in community-based practices. The authors sought to interview a broad sample of providers from various facilities (small, large, CoC accredited, nonaccredited) to understand the range of conditions faced by VA providers. Some providers face more barriers than do others, whereas some face few or no barriers. This wide range of experiences can help to better understand the factors that facilitate guideline-adherent care.
Limitations
The authors recognize that availability of resources and testing and prescribing practices are constantly evolving and perhaps have improved since the data were collected. Thus, the age of the study data might be a limitation to the study. Like most qualitative studies, these findings are limited in their generalizability beyond the study population. Additionally, the authors were limited to recruiting oncologists with reliable contact information listed in the VHA directory. Although this could have introduced some degree of sampling bias, the authors are confident that the sample sufficiently represents the population of VHA-based medical oncologists who treat lung cancer. Despite these limitations, these findings provide novel perspectives on barriers and facilitators to genomic testing GBTT prescribing in the VHA. The authors identify modifiable barriers to testing and prescribing that can be addressed to improve and standardize care of advanced lung cancer in the VHA.
Conclusion
Efforts should be made to address modifiable barriers to mutation testing and guideline-consistent prescribing of GBTT in the VA setting. Implementation of specific practices like reflexive testing for all metastatic nonsquamous NSCLC, standardization of the mutation test ordering procedure, standardization of results reporting, and elimination of the preauthorization to prescribe GBTT could impact the utilization of GBTT in VHA.
Click here to read the digital edition.
Lung cancer is the most frequent cause of cancer-related mortality worldwide.1 The most prevalent type of lung cancer is non-small cell lung cancer (NSCLC), which comprises about 85% of lung cancer cases.2 As there are no cost-effective approaches to screening for lung cancer, most lung cancers are identified at an advanced stage (stage IIIB or IV).
New approaches to managing advanced lung cancer have emerged in recent years, including drugs designed to target specific genetic mutations in some tumors.3 The National Comprehensive Cancer Network (NCCN) recommends erlotinib, a receptor tyrosine kinase inhibitor of the epidermal growth factor receptor (EGFR) for first-line treatment of advanced NSCLC with EGFR mutation.4 Crizotinib is recommended to treat cancers that test positive for the anaplastic lymphoma kinase (ALK) mutation.4 Utilization of targeting agents has been found to extend the survival times for patients with the specified mutations.5 Both erlotinib and crizotinib are available at the VHA.
Previous research showed that VHA providers expressed overall favorable attitudes about genomic medicine.6 Providers perceived genomic medicine to have an important and possibly transformative role in medicine. Barriers to utilization of genomic medicine involved concerns about coordination of care, changes in workload, and increased length of patient visits. In addition to these system-level barriers, many providers had concerns about the proficiency of VHA-based practitioners to appropriately use genomic medicine.
Previous research has evaluated utilization of genomic testing and genomic-based targeted therapy (GBTT) in VA and community settings.5-8 It is unclear whether VHA-based providers are following clinical guidelines regarding genomic testing and utilization of GBTT.4 The authors set out to identify factors that impede and encourage guideline-consistent care in the management of NSCLC at the VHA. The authors specifically sought information about oncologists’ perceptions and experiences with EGFR and ALK mutation testing in patients with advanced NSCLC, as well as use of erlotinib and crizotinib in treating such patients.
Methods
This study was approved by the institutional review boards at Michael E. DeBakey VAMC in Houston, Texas and Baylor College of Medicine. In-depth qualitative interviews were conducted with VHA oncologists to examine their reported barriers and facilitators to mutation testing and prescribing of genomic-based treatment in patients with advanced NSCLC.
The sample of participants was recruited from a list of VHA medical oncologists, compiled by the study project coordinator. Investigators stratified the list by American College of Surgeons Commission on Cancer (CoC) accreditation status (yes/no) and used a stratified purposive sampling technique to recruit participants from CoC-accredited facilities and nonaccredited facilities. Recruitment and data collection occurred between March 2015 and February 2016. Oncologists were considered for inclusion if they (1) were specialists in oncology; (2) practiced at the VHA during the time of recruitment; and (3) had experience treating lung cancer at a VHA facility. During recruitment, potential participants were told that the investigators were interested in learning about oncologists’ experiences and decisions about using GBTT to treat advanced lung cancer in the VHA. Participants were scheduled for telephone-based interviews, and verbal consent was obtained prior to all interviews. Interviews ranged from 19 to 90 minutes (average, 40 min).
Recruitment was stopped at the point of thematic saturation, defined a priori as the point when 2 independent coders agreed that 3 consecutive transcripts for a given interview category (see below) rendered no new thematic concepts.9,10 Consistent with the theoretical framework developed by Cabana and colleagues, interviews were designed to elicit information about oncologists’ knowledge, attitudes, intent to use GBTT, and perceived facilitators and barriers to using GBTT in the VHA.11 Additional findings are presented elsewhere.12 The interview guide was pilot tested and revised prior to initiating data collection. All interviews were recorded, transcribed, and analyzed for content.
Analysis
Data were analyzed using framework analysis methodology, which allows for the inclusion of existing concepts as well as emergent themes within an established theoretical framework.13 Two independent coders with expertise in framework analysis independently created codes and indexed the data using Atlas.ti 6.2 (Scientific Software Development, Berlin, Germany). Disagreements about coding decisions were resolved through group consensus. Coding centered on 2 themes:
- Barriers and facilitators to mutation testing. This includes system or facility factors and testing weaknesses that act as barriers to ordering mutation testing, system or facility factors that facilitate ordering mutation testing, and oncologists’ suggestions for ways to encourage more testing in the VHA.
- Barriers and facilitators to prescribing GBTT. This includes system or facility factors that act as barriers to prescribing GBTT, system or facility factors that facilitate prescribing GBTT, and oncologists’ suggestions for ways to encourage more prescribing of GBTT in the VHA.
Thirty medical oncologists were interviewed. Participant demographics are presented in the Table.
Barriers to testing
The 2 most commonly cited barriers to ordering mutation testing can be considered weaknesses in the testing process: lack of tissue and wait time for results. Almost all providers identified lack of tissue as a barrier to ordering a mutation test.
Another frequently cited testing weakness involved the wait time for results. Because the mutation analysis is not conducted in the VHA facility, providers often must wait 2 to 4 weeks to receive results. This can present a problem because some providers do not want to wait for the results before recommending a course of treatment.
Several providers cited system and facility factors as barriers to mutation testing. The most common of these involves the ordering process. Oncology providers often remarked that ordering the mutation test is cumbersome or inconvenient because there is no ordering mechanism in the Computerized Patient Record System (CPRS). Many different approaches for ordering a mutation test exist, including e-mailing the pathology department, calling to place the order, or requesting the test in person. As providers can order many, if not most, other tests via CPRS, it is clear that this presents an inconvenient exception.
Budgetary constraints were another frequently cited system or facility-level barrier. Providers sometimes were unable to access the test due to the cost
Finally, several providers noted that in some cases patients did not wish to undergo a biopsy. Thus, patient preference can act as a barrier to mutation testing. Some patients wish to forgo treatment, which eliminates the need for a mutation test. Other patients believe that due to their smoking history, they are unlikely to have an ALK or EGFR mutation and instead immediately opt for chemotherapy. Only a small minority of participants identified no barriers to mutation testing.
Facilitators for Testing
Many providers complimented the availability of the mutation test. Interestingly, while some providers mentioned that lack of CPRS ordering was a barrier to testing, several also listed access to a CPRS order as a facilitator. These providers commented that ordering a test was streamlined and easy, given the mechanism in CPRS. Some VHA facilities offer CPRS order capabilities, and others do not. Other oncologists commented more generally on the cooperativeness of the pathology department in ordering mutation tests. It seems that facilities may use different ordering procedures, but in most of these facilities, a high degree of cooperation exists between departments to send out for tests that are requested.
Providers offered many ideas for ways to improve mutation testing or to facilitate the testing. By far, the most commonly cited way to improve the testing process was to make mutation testing reflexive for metastatic nonsquamous NSCLC. Some acknowledged that to achieve this would require a change to the budgeting process such that the test would not drain the pathology department’s budget. Implementing reflexive testing of patients, as recommended by guidelines, would understandably address several of the barriers that were identified in this study. Other providers recommended standardizing the ordering procedure and location of results. Specifically, providers recommended creating a button in CPRS for ordering and always reporting the results in the same place in CPRS.
Barriers to GBTT Prescribing
The clear majority of providers identified no barriers to prescribing GBTTs. A few mentioned that they were required to submit a nonformulary consult. A representative quote described this as “more out of a formality, and the pharmacist basically is there with me and he approves it on the spot and provides the prescription on the day, right when I’m seeing the patient.” Only a very small minority of providers identified medication cost as a barrier, but even those respondents did not indicate that cost prevented them from offering GBTTs to their patients. Rather, cost consciousness simply made them more mindful and judicious when making decisions about prescribing GBTTs.
Facilitators to GBTT Prescribing
Several providers listed availability of the costly medication in the VHA as a facilitator to prescribing. Veterans can obtain GBTTs with little to no insurance cost or copayment, which is not always the case outside the VHA.
One recommendation for further facilitating prescribing of GBTTs involved eliminating the preauthorization requirement, particularly in first-line use for patients testing positive for ALK or EGFR mutations. Although the preauthorization was not seen as a significant barrier, removal of this formality could make prescribing easier.
Discussion
Although in some cases, testing weaknesses (lack of tissue, wait time to receive results) can interrupt a treatment trajectory, many of the barriers identified in this study are modifiable. Overwhelmingly, oncologists recommended making mutation testing reflexive for metastatic nonsquamous NSCLC. Implementing reflexive testing of patients, as recommended by guidelines, would understandably address issues related to variable utilization of genomic testing in VHA.12 Additionally, in response to system and facility barriers to mutation testing, other providers recommended standardizing the ordering procedure and location of results. Utilization of GBTT can be facilitated by eliminating the preauthorization requirement, particularly in first-line use for patients with positive mutations. Although the preauthorization was not seen as a significant barrier, removal of this formality could make prescribing easier.
This study extends previous research that identified underuse of genomic testing in community-based practices. The authors sought to interview a broad sample of providers from various facilities (small, large, CoC accredited, nonaccredited) to understand the range of conditions faced by VA providers. Some providers face more barriers than do others, whereas some face few or no barriers. This wide range of experiences can help to better understand the factors that facilitate guideline-adherent care.
Limitations
The authors recognize that availability of resources and testing and prescribing practices are constantly evolving and perhaps have improved since the data were collected. Thus, the age of the study data might be a limitation to the study. Like most qualitative studies, these findings are limited in their generalizability beyond the study population. Additionally, the authors were limited to recruiting oncologists with reliable contact information listed in the VHA directory. Although this could have introduced some degree of sampling bias, the authors are confident that the sample sufficiently represents the population of VHA-based medical oncologists who treat lung cancer. Despite these limitations, these findings provide novel perspectives on barriers and facilitators to genomic testing GBTT prescribing in the VHA. The authors identify modifiable barriers to testing and prescribing that can be addressed to improve and standardize care of advanced lung cancer in the VHA.
Conclusion
Efforts should be made to address modifiable barriers to mutation testing and guideline-consistent prescribing of GBTT in the VA setting. Implementation of specific practices like reflexive testing for all metastatic nonsquamous NSCLC, standardization of the mutation test ordering procedure, standardization of results reporting, and elimination of the preauthorization to prescribe GBTT could impact the utilization of GBTT in VHA.
Click here to read the digital edition.
. , , , Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
2. American Cancer Society. What is non-small cell lung cancer? https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/what-is-non-small-cell-lung-cancer.html. Updated May 16, 2016. Accessed January 19, 2018.
3. , , . New targetable oncogenes in non-small-cell lung cancer. J Clin Oncol. 2013;31(8):1097-1104.
4. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). non-small cell lung cancer 2. 2018. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Updated December 19, 2017. Accessed Jan
5. Rosell R, Moran T, Queralt C, et al; Spanish Lung Cancer Group. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958-967.
6. Arar N, Seo J, Abboud HE, Parchman M, Noel P. Providers’ behavioral beliefs regarding the delivery of genomic medicine at the Veterans Health Administration. Per Med. 2010;7(5):485-494.
7. Lynch JA, Berse B, Dotson D, Khoury MJ, Coomer N, Kautter J. Utilization of genetic tests: analysis of gene-specific billing in Medicare claims data. Genet Med. 2017; 19(8):890-899.
8. Gutierrez ME, Choi K, Lanman RB, et al. Genomic profiling of advanced non-small cell lung cancer in community settings: gaps and opportunities. Clin Lung Cancer, 2017;18(6):651-659.
9. Morse JM. The significance of saturation. Qual Health Res.1995;5(2):147-149.
10. Aita VA, McIlvain HE. An armchair adventure in case study research. In: Crabtree BF, Miller WF, eds. Doing Qualitative Research. Thousand Oaks, CA: Sage; 1999:253-268.
11. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465.
12. Arney JB, Helm A, Crook T, Braun U, Chen GJ, Hayes TG. Utilization of genomic testing in advanced non-small cell lung cancer among oncologists in the Veterans Health Administration. Lung Cancer, 2018;116:25-29.
13. Ritchie J, Spencer L. Qualitative data analysis for applied policy research. In: Bryman A, Burgess RG, eds. Analyzing Qualitative Data. New York, NY: Routledge; 1994:173-194.
. , , , Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
2. American Cancer Society. What is non-small cell lung cancer? https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/what-is-non-small-cell-lung-cancer.html. Updated May 16, 2016. Accessed January 19, 2018.
3. , , . New targetable oncogenes in non-small-cell lung cancer. J Clin Oncol. 2013;31(8):1097-1104.
4. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). non-small cell lung cancer 2. 2018. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Updated December 19, 2017. Accessed Jan
5. Rosell R, Moran T, Queralt C, et al; Spanish Lung Cancer Group. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958-967.
6. Arar N, Seo J, Abboud HE, Parchman M, Noel P. Providers’ behavioral beliefs regarding the delivery of genomic medicine at the Veterans Health Administration. Per Med. 2010;7(5):485-494.
7. Lynch JA, Berse B, Dotson D, Khoury MJ, Coomer N, Kautter J. Utilization of genetic tests: analysis of gene-specific billing in Medicare claims data. Genet Med. 2017; 19(8):890-899.
8. Gutierrez ME, Choi K, Lanman RB, et al. Genomic profiling of advanced non-small cell lung cancer in community settings: gaps and opportunities. Clin Lung Cancer, 2017;18(6):651-659.
9. Morse JM. The significance of saturation. Qual Health Res.1995;5(2):147-149.
10. Aita VA, McIlvain HE. An armchair adventure in case study research. In: Crabtree BF, Miller WF, eds. Doing Qualitative Research. Thousand Oaks, CA: Sage; 1999:253-268.
11. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465.
12. Arney JB, Helm A, Crook T, Braun U, Chen GJ, Hayes TG. Utilization of genomic testing in advanced non-small cell lung cancer among oncologists in the Veterans Health Administration. Lung Cancer, 2018;116:25-29.
13. Ritchie J, Spencer L. Qualitative data analysis for applied policy research. In: Bryman A, Burgess RG, eds. Analyzing Qualitative Data. New York, NY: Routledge; 1994:173-194.
Chronic Myeloid Leukemia: Evaluation and Diagnosis
Chronic myeloid leukemia (CML) is a rare myeloproliferative neoplasm that is characterized by the presence of the Philadelphia (Ph) chromosome and uninhibited expansion of bone marrow stem cells. The Ph chromosome arises from a reciprocal translocation between the Abelson (ABL) region on chromosome 9 and the breakpoint cluster region (BCR) of chromosome 22 (t(9;22)(q34;q11.2), resulting in the BCR-ABL1 fusion gene.1BCR-ABL1 encodes an oncoprotein with constitutive tyrosine kinase activity that promotes growth and replication through downstream pathways, which is the driving factor in the pathogenesis of CML.1
Typical treatment for CML involves life-long use of oral BCR-ABL tyrosine kinase inhibitors (TKI). Currently, 5 TKIs have regulatory approval for treatment of this disease. With the introduction of imatinib in 2001 and the subsequent development of second- (dasatinib, nilotinib, bosutinib) and third-generation (ponatinib) TKIs, CML has become a chronic disease with a life-expectancy that is similar to that of the general population. This article reviews the diagnosis of CML and the parameters used for monitoring response to TKI therapy; the selection of initial TKI therapy is reviewed in a separate follow-up article.
Epidemiology
According to SEER data estimates, 8430 new cases of CML were diagnosed in the United States in 2018. CML is a disease of older adults, with a median age of 65 years at diagnosis, and there is a slight male predominance. Between 2011 and 2015, the number of new CML cases was 1.8 per 100,000 persons. The median overall survival (OS) in patients with newly diagnosed chronic-phase CML (CP-CML) has not been reached.2 Given the effective treatments available for managing CML, it is estimated that the prevalence of CML in the United States will plateau at 180,000 patients by 2050.3
Diagnosis
Case Presentation
A 53-year-old woman presents to her primary care physician with complaints of fatigue, early satiety, left upper quadrant abdominal pain, and an 8-lb unintentional weight loss over the prior month. Her past medical history is significant for uncontrolled diabetes, coronary artery disease requiring placement of 3 cardiac stents 2 years prior, and chronic obstructive pulmonary disease (COPD) related to a 30-pack-year history of smoking. On physicial exam her spleen is palpated 8 cm below the left costal margin. A complete blood count (CBC) with differential identifies a total white blood cell (WBC) count of 124,000/μL, with a left-shifted differential including 6% basophils, 3% eosinophils, and 3% blasts; hemoglobin is 12.4 g/dL and platelet count is 801 × 103/µL.
- How is the diagnosis of CML made?
Clinical Features
The diagnosis of CML is often suspected based on an incidental finding of leukocytosis and, in some cases, thrombocytosis. In many cases, this is an incidental finding on routine blood work, but approximately 50% of patients will present with constitutional symptoms associated with the disease. Characteristic features of the WBC differential include left-shifted maturation with neutrophilia and immature circulating myeloid cells. Basophilia and eosinophilia are often present as well. Splenomegaly is a common sign, present in 50% to 90% of patients at diagnosis. In those patients with symptoms related to CML at diagnosis, the most common presentation includes increasing fatigue, fevers, night sweats, early satiety, and weight loss. The diagnosis is confirmed by cytogenetic studies showing the Ph chromosome abnormality, t(9; 22)(q3.4;q1.1), and/or reverse transcriptase polymerase chain reaction (PCR) showing BCR-ABL1 transcripts.
- What further testing is needed when evaluating a patient for CML?
There are 3 distinct phases of CML: chronic phase (CP), accelerated phase (AP), and blast phase (BP). Bone marrow biopsy and aspiration at diagnosis are mandatory in order to determine the phase of the disease at diagnosis. This distinction is based on the percentage of blasts, promyelocytes, and basophils present as well as the platelet count and presence or absence of extramedullary disease.4 The vast majority of patients at diagnosis have CML that is in the chronic phase. The typical appearance in CP-CML is a hypercellular marrow with granulocytic and occasionally megakaryocytic hyperplasia. In many cases, basophilia and/or eosinophilia are noted as well. Dysplasia is not a typical finding in CML.5 Bone marrow fibrosis can be seen in up to one-third of patients at diagnosis, and may indicate a slightly worse prognosis.6 Although a diagnosis of CML can be made without a bone marrow biopsy, complete staging and prognostication are only possible with information gained from this test, including baseline karyotype and confirmation of CP versus a more advanced phase of CML.
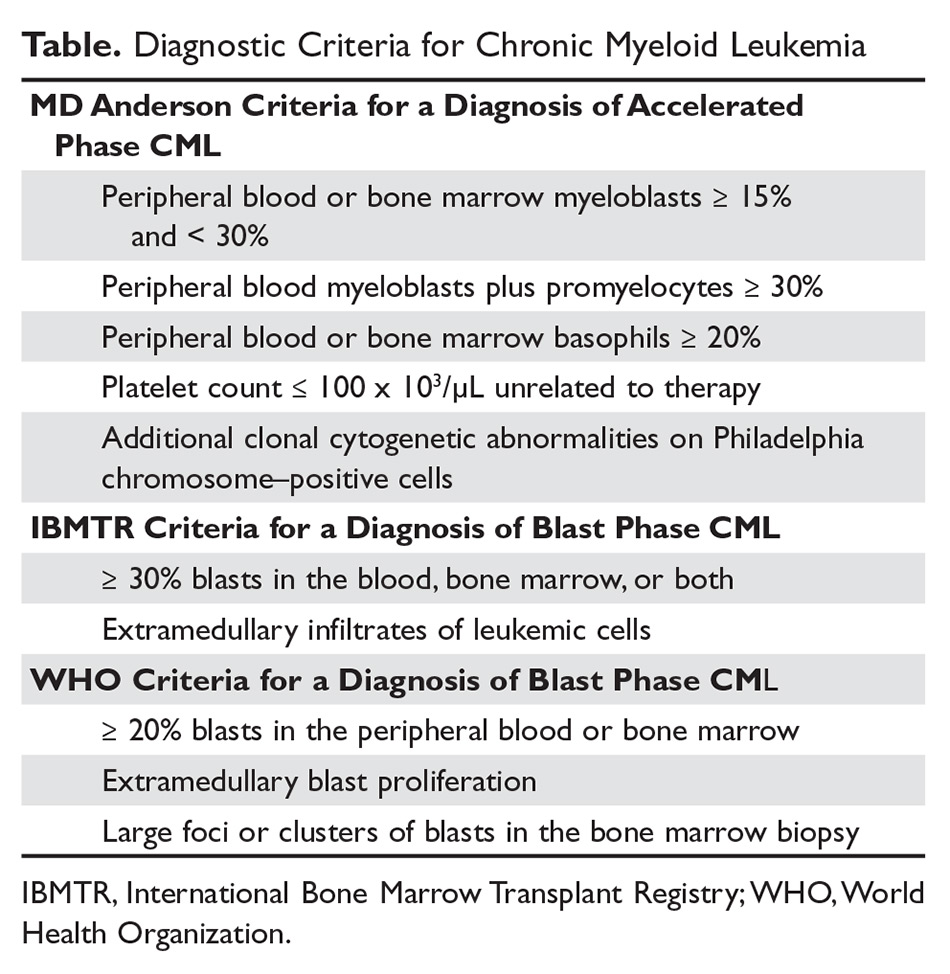
The criteria for diagnosing AP-CML has not been agreed upon by various groups, but the modified MD Anderson Cancer Center (MDACC) criteria are used in the majority of clinical trials evaluating the efficacy of TKIs in preventing progression to advanced phases of CML. MDACC criteria define AP-CML as the presence of one of the following: 15% to 29% blasts in the peripheral blood or bone marrow, ≥ 30% peripheral blasts plus promyelocytes, ≥ 20% basophils in the blood or bone marrow, platelet count ≤ 100 × 103/μL unrelated to therapy, and clonal cytogenetic evolution in Ph-positive metaphases (Table).7
BP-CML is typically defined using the criteria developed by the International Bone Marrow Transplant Registry (IBMTR): ≥ 30% blasts in the peripheral blood and/or the bone marrow or the presence of extramedullary disease.8 Although not typically used in clinical trials, the revised World Health Organization (WHO) criteria for BP-CML include ≥ 20% blasts in the peripheral blood or bone marrow, extramedullary blast proliferation, and large foci or clusters of blasts in the bone marrow biopsy (Table).9 The defining feature of CML is the presence of the Ph chromosome abnormality. In a small subset of patients, additional chromosomal abnormalities (ACA) in the Ph-positive cells may be identified at diagnosis. Some reports indicate that the presence of “major route” ACA (trisomy 8, isochromosome 17q, a second Ph chromosome, or trisomy 19) at diagnosis may negatively impact prognosis, but other reports contradict these findings.10,11
The typical BCR breakpoint in CML is the major breakpoint cluster region (M-BCR), which results in a 210-kDa protein (p210). Alternate breakpoints that are less frequently identified are the minor BCR (mBCR or p190), which is more commonly found in Ph-positive acute lymphoblastic leukemia (ALL), and the micro BCR (µBCR or p230), which is much less common and is often characterized by chronic neutrophilia.12 Identifying which BCR-ABL1 transcript is present in each patient using qualitative PCR is crucial in order to ensure proper monitoring during treatment.
The most sensitive method for detecting BCR-ABL1 mRNA transcripts is the quantitative real-time PCR (RQ-PCR) assay, which is typically done on peripheral blood. RQ-PCR is capable of detecting a single CML cell in the presence of ≥ 100,000 normal cells. This test should be done during the initial diagnostic workup in order to confirm the presence of BCR-ABL1 transcripts, and it is used as a standard method for monitoring response to TKI therapy.13 The International Scale (IS) is a standardized approach to reporting RQ-PCR results that was developed to allow comparison of results across various laboratories and has become the gold standard for reporting BCR-ABL1 transcript values.14
Determining Risk Scores
Calculating a patient’s Sokal score or EURO risk score at diagnosis remains an important component of the diagnostic workup in CP-CML, as this information has prognostic and therapeutic implications (an online calculator is available through European LeukemiaNet [ELN]). The risk for disease progression to the accelerated or blast phases is higher in patients with intermediate- or high-risk scores compared to those with a low-risk score at diagnosis. The risk of progression in intermediate- or high-risk patients is lower when a second-generation TKI (dasatinib, nilotinib, or bosutinib) is used as frontline therapy compared to imatinib, and therefore, the National Comprehensive Cancer Network (NCCN) CML Panel recommends starting with a second-generation TKI in these patients.15-19
Monitoring Response to Therapy
Case Continued
Fluorescent in-situ hybridization using a peripheral blood sample to detect the BCR-ABL gene rearrangement is performed and is positive in 87% of cells. Bone marrow biopsy and aspiration show a 95% cellular bone marrow with granulocytic hyperplasia and 1% blasts. Cytogenetics are 46,XX,t(9;22)(q34;q11.2).20 RQ-PCR assay performed to measure BCR-ABL1 transcripts in the peripheral blood shows a value of 98% IS. The patient is ultimately given a diagnosis of CP-CML. Her Sokal risk score is 1.42, making her disease high risk.
How is response to TKI therapy measured and monitored?
After confirming a diagnosis of CML and selecting the most appropriate TKI for first-line therapy, the successful management of a CML patient relies on close monitoring and follow-up to ensure patients are meeting the desired treatment milestones. Responses in CML can be assessed based on hematologic parameters, cytogenetic results, and molecular responses. A complete hematologic response (CHR) implies complete normalization of peripheral blood counts (with the exception of TKI-induced cytopenias) and resolution of any palpable splenomegaly. The majority of patients will achieve a CHR within 4 to 6 weeks after initiating CML-directed therapy.21
Cytogenetic Response
Cytogenetic responses are defined by the decrease in the number of Ph chromosome–positive metaphases when assessed on bone marrow cytogenetics. A partial cytogenetic response (PCyR) is defined as having 1% to 35% Ph-positive metaphases, a major cytogenetic response (MCyR) as having 0% to 35% Ph-positive metaphases, and a CCyR implies that no Ph-positive metaphases are identified on bone marrow cytogenetics. An ideal response is the achievement of PCyR after 3 months on a TKI and a CCyR after 12 months on a TKI.22
Molecular Response
Once a patient has achieved a CCyR, monitoring their response to therapy can only be done using RQ-PCR to measure BCR-ABL1 transcripts in the peripheral blood. The NCCN and the ELN recommend monitoring RQ-PCR from the peripheral blood every 3 months in order to assess response to TKIs.19,23 As noted, the International Scale (IS) has become the gold standard reporting system for all BCR-ABL1 transcript levels in the majority of laboratories worldwide.14,24 Molecular responses are based on a log-reduction in BCR-ABL1 transcripts from a standardized baseline. Many molecular responses can be correlated with cytogenetic responses such that if reliable RQ-PCR testing is available, monitoring can be done using only peripheral blood RQ-PCR rather than repeat bone marrow biopsies. For example, an early molecular response (EMR) is defined as a RQ-PCR value of ≤ 10% IS, which is approximately equivalent to a PCyR.25 A value of 1% IS is approximately equivalent to CCyR. A major molecular response (MMR) is a ≥ 3-log reduction in BCR-ABL1 transcripts from baseline and is a value of ≤ 0.1% IS. Deeper levels of molecular response are best described by the log-reduction in BCR-ABL1 transcripts, with a 4-log reduction denoted as MR4.0, a 4.5-log reduction as MR4.5, and so forth. Complete molecular response (CMR) is defined by the level of sensitivity of the RQ-PCR assay being used.14
The definition of relapsed disease in CML is dependent on the type of response the patient had previously achieved. Relapse could be the loss of a hematologic or cytogenetic response, but fluctuations in BCR-ABL1 transcripts on routine RQ-PCR do not necessarily indicate relapsed CML. A 1-log increase in the level of BCR-ABL1 transcripts with a concurrent loss of MMR should prompt a bone marrow biopsy in order to assess for the loss of CCyR, and thus a cytogenetic relapse; however, this loss of MMR does not define relapse in and of itself. In the setting of relapsed disease, testing should be done to look for possible ABL kinase domain mutations, and alternate therapy should be selected.19
Multiple reports have identified the prognostic relevance of achieving an EMR at 3 and 6 months after starting TKI therapy. Marin and colleagues reported that in 282 imatinib-treated patients, there was a significant improvement in 8-year OS, progression-free survival, and cumulative incidence of CCyR and CMR in patients who had BCR-ABL1 transcripts < 9.84% IS after 3 months on treatment.25 This data highlights the importance of early molecular monitoring in order to ensure the best outcomes for patients with CP-CML.
The NCCN CML guidelines and ELN recommendations both agree that an ideal response after 3 months on a TKI is BCR-ABL1 transcripts < 10% IS, but treatment is not considered to be failing at this point if the patient marginally misses this milestone. After 6 months on treatment, an ideal response is considered BCR-ABL1 transcripts < 1%–10% IS. Ideally, patients will have BCR-ABL1 transcripts < 0.1%–1% IS by the time they complete 12 months of TKI therapy, suggesting that these patients have at least achieved a CCyR.19,23 Even after patients achieve these early milestones, frequent monitoring by RQ-PCR is required to ensure that they are maintaining their response to treatment. This will help to ensure patient compliance with treatment and will also help to identify a select subset of patients who could potentially be considered for an attempt at TKI cessation (not discussed in detail here) after a minimum of 3 years on therapy.19,26
Conclusion
Given the successful treatments available for patients with CML, it is crucial to identify patients with this disease, ensure they receive a complete, appropriate diagnostic workup including a bone marrow biopsy and aspiration with cytogenetic testing, and select the best therapy for each individual patient. Once on treatment, the importance of frequent monitoring cannot be overstated.
1. Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164-172.
2. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Leukemia - Chronic Myeloid Leukemia (CML). 2018.
3. Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer. 2012;118:3123-3127.
4. Savage DG, Szydlo RM, Chase A, et al. Bone marrow transplantation for chronic myeloid leukaemia: the effects of differing criteria for defining chronic phase on probabilities of survival and relapse. Br J Haematol. 1997;99:30-35.
5. Knox WF, Bhavnani M, Davson J, Geary CG. Histological classification of chronic granulocytic leukaemia. Clin Lab Haematol. 1984;6:171-175.
6. Kvasnicka HM, Thiele J, Schmitt-Graeff A, et al. Impact of bone marrow morphology on multivariate risk classification in chronic myelogenous leukemia. Acta Haematol. 2003;109:53-56.
7. Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306-1315.
8. Druker BJ. Chronic myeloid leukemia In: DeVita VT, Lawrence TS, Rosenburg SA, eds. DeVita, Hellman, and Rosenberg’s Cancer Principles & Practice of Oncology. 8th ed. Philadelphia, PA: Lippincott, Williams and Wilkins; 2007:2267-2304.
9. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405.
10. Fabarius A, Leitner A, Hochhaus A, et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118:6760-6768.
11. Alhuraiji A, Kantarjian H, Boddu P, et al. Prognostic significance of additional chromosomal abnormalities at the time of diagnosis in patients with chronic myeloid leukemia treated with frontline tyrosine kinase inhibitors. Am J Hematol. 2018;93:84-90.
12. Melo JV. BCR-ABL gene variants. Baillieres Clin Haematol. 1997;10:203-222.
13. Kantarjian HM, Talpaz M, Cortes J, et al. Quantitative polymerase chain reaction monitoring of BCR-ABL during therapy with imatinib mesylate (STI571; gleevec) in chronic-phase chronic myelogenous leukemia. Clin Cancer Res. 2003;9:160-166.
14. Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28-37.
15. Hochhaus A, Larson RA, Guilhot F, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917-927.
16. Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients trial. J Clin Oncol. 2016;34:2333-3340.
17. Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044-1054.
18. Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36:231-237.
19. Radich JP, Deininger M, Abboud CN, et al. Chronic Myeloid Leukemia, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1108-1135.
20. Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037.
21. Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Ann Intern Med. 1999;131:207-219.
22. O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994-1004.
23. Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872-884.
24. Larripa I, Ruiz MS, Gutierrez M, Bianchini M. [Guidelines for molecular monitoring of BCR-ABL1 in chronic myeloid leukemia patients by RT-qPCR.] Medicina (B Aires). 2017;77:61-72.
25. Marin D, Ibrahim AR, Lucas C, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012;30:232-238.
26. Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128:17-23.
Chronic myeloid leukemia (CML) is a rare myeloproliferative neoplasm that is characterized by the presence of the Philadelphia (Ph) chromosome and uninhibited expansion of bone marrow stem cells. The Ph chromosome arises from a reciprocal translocation between the Abelson (ABL) region on chromosome 9 and the breakpoint cluster region (BCR) of chromosome 22 (t(9;22)(q34;q11.2), resulting in the BCR-ABL1 fusion gene.1BCR-ABL1 encodes an oncoprotein with constitutive tyrosine kinase activity that promotes growth and replication through downstream pathways, which is the driving factor in the pathogenesis of CML.1
Typical treatment for CML involves life-long use of oral BCR-ABL tyrosine kinase inhibitors (TKI). Currently, 5 TKIs have regulatory approval for treatment of this disease. With the introduction of imatinib in 2001 and the subsequent development of second- (dasatinib, nilotinib, bosutinib) and third-generation (ponatinib) TKIs, CML has become a chronic disease with a life-expectancy that is similar to that of the general population. This article reviews the diagnosis of CML and the parameters used for monitoring response to TKI therapy; the selection of initial TKI therapy is reviewed in a separate follow-up article.
Epidemiology
According to SEER data estimates, 8430 new cases of CML were diagnosed in the United States in 2018. CML is a disease of older adults, with a median age of 65 years at diagnosis, and there is a slight male predominance. Between 2011 and 2015, the number of new CML cases was 1.8 per 100,000 persons. The median overall survival (OS) in patients with newly diagnosed chronic-phase CML (CP-CML) has not been reached.2 Given the effective treatments available for managing CML, it is estimated that the prevalence of CML in the United States will plateau at 180,000 patients by 2050.3
Diagnosis
Case Presentation
A 53-year-old woman presents to her primary care physician with complaints of fatigue, early satiety, left upper quadrant abdominal pain, and an 8-lb unintentional weight loss over the prior month. Her past medical history is significant for uncontrolled diabetes, coronary artery disease requiring placement of 3 cardiac stents 2 years prior, and chronic obstructive pulmonary disease (COPD) related to a 30-pack-year history of smoking. On physicial exam her spleen is palpated 8 cm below the left costal margin. A complete blood count (CBC) with differential identifies a total white blood cell (WBC) count of 124,000/μL, with a left-shifted differential including 6% basophils, 3% eosinophils, and 3% blasts; hemoglobin is 12.4 g/dL and platelet count is 801 × 103/µL.
- How is the diagnosis of CML made?
Clinical Features
The diagnosis of CML is often suspected based on an incidental finding of leukocytosis and, in some cases, thrombocytosis. In many cases, this is an incidental finding on routine blood work, but approximately 50% of patients will present with constitutional symptoms associated with the disease. Characteristic features of the WBC differential include left-shifted maturation with neutrophilia and immature circulating myeloid cells. Basophilia and eosinophilia are often present as well. Splenomegaly is a common sign, present in 50% to 90% of patients at diagnosis. In those patients with symptoms related to CML at diagnosis, the most common presentation includes increasing fatigue, fevers, night sweats, early satiety, and weight loss. The diagnosis is confirmed by cytogenetic studies showing the Ph chromosome abnormality, t(9; 22)(q3.4;q1.1), and/or reverse transcriptase polymerase chain reaction (PCR) showing BCR-ABL1 transcripts.
- What further testing is needed when evaluating a patient for CML?
There are 3 distinct phases of CML: chronic phase (CP), accelerated phase (AP), and blast phase (BP). Bone marrow biopsy and aspiration at diagnosis are mandatory in order to determine the phase of the disease at diagnosis. This distinction is based on the percentage of blasts, promyelocytes, and basophils present as well as the platelet count and presence or absence of extramedullary disease.4 The vast majority of patients at diagnosis have CML that is in the chronic phase. The typical appearance in CP-CML is a hypercellular marrow with granulocytic and occasionally megakaryocytic hyperplasia. In many cases, basophilia and/or eosinophilia are noted as well. Dysplasia is not a typical finding in CML.5 Bone marrow fibrosis can be seen in up to one-third of patients at diagnosis, and may indicate a slightly worse prognosis.6 Although a diagnosis of CML can be made without a bone marrow biopsy, complete staging and prognostication are only possible with information gained from this test, including baseline karyotype and confirmation of CP versus a more advanced phase of CML.
The criteria for diagnosing AP-CML has not been agreed upon by various groups, but the modified MD Anderson Cancer Center (MDACC) criteria are used in the majority of clinical trials evaluating the efficacy of TKIs in preventing progression to advanced phases of CML. MDACC criteria define AP-CML as the presence of one of the following: 15% to 29% blasts in the peripheral blood or bone marrow, ≥ 30% peripheral blasts plus promyelocytes, ≥ 20% basophils in the blood or bone marrow, platelet count ≤ 100 × 103/μL unrelated to therapy, and clonal cytogenetic evolution in Ph-positive metaphases (Table).7
BP-CML is typically defined using the criteria developed by the International Bone Marrow Transplant Registry (IBMTR): ≥ 30% blasts in the peripheral blood and/or the bone marrow or the presence of extramedullary disease.8 Although not typically used in clinical trials, the revised World Health Organization (WHO) criteria for BP-CML include ≥ 20% blasts in the peripheral blood or bone marrow, extramedullary blast proliferation, and large foci or clusters of blasts in the bone marrow biopsy (Table).9 The defining feature of CML is the presence of the Ph chromosome abnormality. In a small subset of patients, additional chromosomal abnormalities (ACA) in the Ph-positive cells may be identified at diagnosis. Some reports indicate that the presence of “major route” ACA (trisomy 8, isochromosome 17q, a second Ph chromosome, or trisomy 19) at diagnosis may negatively impact prognosis, but other reports contradict these findings.10,11
The typical BCR breakpoint in CML is the major breakpoint cluster region (M-BCR), which results in a 210-kDa protein (p210). Alternate breakpoints that are less frequently identified are the minor BCR (mBCR or p190), which is more commonly found in Ph-positive acute lymphoblastic leukemia (ALL), and the micro BCR (µBCR or p230), which is much less common and is often characterized by chronic neutrophilia.12 Identifying which BCR-ABL1 transcript is present in each patient using qualitative PCR is crucial in order to ensure proper monitoring during treatment.
The most sensitive method for detecting BCR-ABL1 mRNA transcripts is the quantitative real-time PCR (RQ-PCR) assay, which is typically done on peripheral blood. RQ-PCR is capable of detecting a single CML cell in the presence of ≥ 100,000 normal cells. This test should be done during the initial diagnostic workup in order to confirm the presence of BCR-ABL1 transcripts, and it is used as a standard method for monitoring response to TKI therapy.13 The International Scale (IS) is a standardized approach to reporting RQ-PCR results that was developed to allow comparison of results across various laboratories and has become the gold standard for reporting BCR-ABL1 transcript values.14
Determining Risk Scores
Calculating a patient’s Sokal score or EURO risk score at diagnosis remains an important component of the diagnostic workup in CP-CML, as this information has prognostic and therapeutic implications (an online calculator is available through European LeukemiaNet [ELN]). The risk for disease progression to the accelerated or blast phases is higher in patients with intermediate- or high-risk scores compared to those with a low-risk score at diagnosis. The risk of progression in intermediate- or high-risk patients is lower when a second-generation TKI (dasatinib, nilotinib, or bosutinib) is used as frontline therapy compared to imatinib, and therefore, the National Comprehensive Cancer Network (NCCN) CML Panel recommends starting with a second-generation TKI in these patients.15-19
Monitoring Response to Therapy
Case Continued
Fluorescent in-situ hybridization using a peripheral blood sample to detect the BCR-ABL gene rearrangement is performed and is positive in 87% of cells. Bone marrow biopsy and aspiration show a 95% cellular bone marrow with granulocytic hyperplasia and 1% blasts. Cytogenetics are 46,XX,t(9;22)(q34;q11.2).20 RQ-PCR assay performed to measure BCR-ABL1 transcripts in the peripheral blood shows a value of 98% IS. The patient is ultimately given a diagnosis of CP-CML. Her Sokal risk score is 1.42, making her disease high risk.
How is response to TKI therapy measured and monitored?
After confirming a diagnosis of CML and selecting the most appropriate TKI for first-line therapy, the successful management of a CML patient relies on close monitoring and follow-up to ensure patients are meeting the desired treatment milestones. Responses in CML can be assessed based on hematologic parameters, cytogenetic results, and molecular responses. A complete hematologic response (CHR) implies complete normalization of peripheral blood counts (with the exception of TKI-induced cytopenias) and resolution of any palpable splenomegaly. The majority of patients will achieve a CHR within 4 to 6 weeks after initiating CML-directed therapy.21
Cytogenetic Response
Cytogenetic responses are defined by the decrease in the number of Ph chromosome–positive metaphases when assessed on bone marrow cytogenetics. A partial cytogenetic response (PCyR) is defined as having 1% to 35% Ph-positive metaphases, a major cytogenetic response (MCyR) as having 0% to 35% Ph-positive metaphases, and a CCyR implies that no Ph-positive metaphases are identified on bone marrow cytogenetics. An ideal response is the achievement of PCyR after 3 months on a TKI and a CCyR after 12 months on a TKI.22
Molecular Response
Once a patient has achieved a CCyR, monitoring their response to therapy can only be done using RQ-PCR to measure BCR-ABL1 transcripts in the peripheral blood. The NCCN and the ELN recommend monitoring RQ-PCR from the peripheral blood every 3 months in order to assess response to TKIs.19,23 As noted, the International Scale (IS) has become the gold standard reporting system for all BCR-ABL1 transcript levels in the majority of laboratories worldwide.14,24 Molecular responses are based on a log-reduction in BCR-ABL1 transcripts from a standardized baseline. Many molecular responses can be correlated with cytogenetic responses such that if reliable RQ-PCR testing is available, monitoring can be done using only peripheral blood RQ-PCR rather than repeat bone marrow biopsies. For example, an early molecular response (EMR) is defined as a RQ-PCR value of ≤ 10% IS, which is approximately equivalent to a PCyR.25 A value of 1% IS is approximately equivalent to CCyR. A major molecular response (MMR) is a ≥ 3-log reduction in BCR-ABL1 transcripts from baseline and is a value of ≤ 0.1% IS. Deeper levels of molecular response are best described by the log-reduction in BCR-ABL1 transcripts, with a 4-log reduction denoted as MR4.0, a 4.5-log reduction as MR4.5, and so forth. Complete molecular response (CMR) is defined by the level of sensitivity of the RQ-PCR assay being used.14
The definition of relapsed disease in CML is dependent on the type of response the patient had previously achieved. Relapse could be the loss of a hematologic or cytogenetic response, but fluctuations in BCR-ABL1 transcripts on routine RQ-PCR do not necessarily indicate relapsed CML. A 1-log increase in the level of BCR-ABL1 transcripts with a concurrent loss of MMR should prompt a bone marrow biopsy in order to assess for the loss of CCyR, and thus a cytogenetic relapse; however, this loss of MMR does not define relapse in and of itself. In the setting of relapsed disease, testing should be done to look for possible ABL kinase domain mutations, and alternate therapy should be selected.19
Multiple reports have identified the prognostic relevance of achieving an EMR at 3 and 6 months after starting TKI therapy. Marin and colleagues reported that in 282 imatinib-treated patients, there was a significant improvement in 8-year OS, progression-free survival, and cumulative incidence of CCyR and CMR in patients who had BCR-ABL1 transcripts < 9.84% IS after 3 months on treatment.25 This data highlights the importance of early molecular monitoring in order to ensure the best outcomes for patients with CP-CML.
The NCCN CML guidelines and ELN recommendations both agree that an ideal response after 3 months on a TKI is BCR-ABL1 transcripts < 10% IS, but treatment is not considered to be failing at this point if the patient marginally misses this milestone. After 6 months on treatment, an ideal response is considered BCR-ABL1 transcripts < 1%–10% IS. Ideally, patients will have BCR-ABL1 transcripts < 0.1%–1% IS by the time they complete 12 months of TKI therapy, suggesting that these patients have at least achieved a CCyR.19,23 Even after patients achieve these early milestones, frequent monitoring by RQ-PCR is required to ensure that they are maintaining their response to treatment. This will help to ensure patient compliance with treatment and will also help to identify a select subset of patients who could potentially be considered for an attempt at TKI cessation (not discussed in detail here) after a minimum of 3 years on therapy.19,26
Conclusion
Given the successful treatments available for patients with CML, it is crucial to identify patients with this disease, ensure they receive a complete, appropriate diagnostic workup including a bone marrow biopsy and aspiration with cytogenetic testing, and select the best therapy for each individual patient. Once on treatment, the importance of frequent monitoring cannot be overstated.
Chronic myeloid leukemia (CML) is a rare myeloproliferative neoplasm that is characterized by the presence of the Philadelphia (Ph) chromosome and uninhibited expansion of bone marrow stem cells. The Ph chromosome arises from a reciprocal translocation between the Abelson (ABL) region on chromosome 9 and the breakpoint cluster region (BCR) of chromosome 22 (t(9;22)(q34;q11.2), resulting in the BCR-ABL1 fusion gene.1BCR-ABL1 encodes an oncoprotein with constitutive tyrosine kinase activity that promotes growth and replication through downstream pathways, which is the driving factor in the pathogenesis of CML.1
Typical treatment for CML involves life-long use of oral BCR-ABL tyrosine kinase inhibitors (TKI). Currently, 5 TKIs have regulatory approval for treatment of this disease. With the introduction of imatinib in 2001 and the subsequent development of second- (dasatinib, nilotinib, bosutinib) and third-generation (ponatinib) TKIs, CML has become a chronic disease with a life-expectancy that is similar to that of the general population. This article reviews the diagnosis of CML and the parameters used for monitoring response to TKI therapy; the selection of initial TKI therapy is reviewed in a separate follow-up article.
Epidemiology
According to SEER data estimates, 8430 new cases of CML were diagnosed in the United States in 2018. CML is a disease of older adults, with a median age of 65 years at diagnosis, and there is a slight male predominance. Between 2011 and 2015, the number of new CML cases was 1.8 per 100,000 persons. The median overall survival (OS) in patients with newly diagnosed chronic-phase CML (CP-CML) has not been reached.2 Given the effective treatments available for managing CML, it is estimated that the prevalence of CML in the United States will plateau at 180,000 patients by 2050.3
Diagnosis
Case Presentation
A 53-year-old woman presents to her primary care physician with complaints of fatigue, early satiety, left upper quadrant abdominal pain, and an 8-lb unintentional weight loss over the prior month. Her past medical history is significant for uncontrolled diabetes, coronary artery disease requiring placement of 3 cardiac stents 2 years prior, and chronic obstructive pulmonary disease (COPD) related to a 30-pack-year history of smoking. On physicial exam her spleen is palpated 8 cm below the left costal margin. A complete blood count (CBC) with differential identifies a total white blood cell (WBC) count of 124,000/μL, with a left-shifted differential including 6% basophils, 3% eosinophils, and 3% blasts; hemoglobin is 12.4 g/dL and platelet count is 801 × 103/µL.
- How is the diagnosis of CML made?
Clinical Features
The diagnosis of CML is often suspected based on an incidental finding of leukocytosis and, in some cases, thrombocytosis. In many cases, this is an incidental finding on routine blood work, but approximately 50% of patients will present with constitutional symptoms associated with the disease. Characteristic features of the WBC differential include left-shifted maturation with neutrophilia and immature circulating myeloid cells. Basophilia and eosinophilia are often present as well. Splenomegaly is a common sign, present in 50% to 90% of patients at diagnosis. In those patients with symptoms related to CML at diagnosis, the most common presentation includes increasing fatigue, fevers, night sweats, early satiety, and weight loss. The diagnosis is confirmed by cytogenetic studies showing the Ph chromosome abnormality, t(9; 22)(q3.4;q1.1), and/or reverse transcriptase polymerase chain reaction (PCR) showing BCR-ABL1 transcripts.
- What further testing is needed when evaluating a patient for CML?
There are 3 distinct phases of CML: chronic phase (CP), accelerated phase (AP), and blast phase (BP). Bone marrow biopsy and aspiration at diagnosis are mandatory in order to determine the phase of the disease at diagnosis. This distinction is based on the percentage of blasts, promyelocytes, and basophils present as well as the platelet count and presence or absence of extramedullary disease.4 The vast majority of patients at diagnosis have CML that is in the chronic phase. The typical appearance in CP-CML is a hypercellular marrow with granulocytic and occasionally megakaryocytic hyperplasia. In many cases, basophilia and/or eosinophilia are noted as well. Dysplasia is not a typical finding in CML.5 Bone marrow fibrosis can be seen in up to one-third of patients at diagnosis, and may indicate a slightly worse prognosis.6 Although a diagnosis of CML can be made without a bone marrow biopsy, complete staging and prognostication are only possible with information gained from this test, including baseline karyotype and confirmation of CP versus a more advanced phase of CML.
The criteria for diagnosing AP-CML has not been agreed upon by various groups, but the modified MD Anderson Cancer Center (MDACC) criteria are used in the majority of clinical trials evaluating the efficacy of TKIs in preventing progression to advanced phases of CML. MDACC criteria define AP-CML as the presence of one of the following: 15% to 29% blasts in the peripheral blood or bone marrow, ≥ 30% peripheral blasts plus promyelocytes, ≥ 20% basophils in the blood or bone marrow, platelet count ≤ 100 × 103/μL unrelated to therapy, and clonal cytogenetic evolution in Ph-positive metaphases (Table).7
BP-CML is typically defined using the criteria developed by the International Bone Marrow Transplant Registry (IBMTR): ≥ 30% blasts in the peripheral blood and/or the bone marrow or the presence of extramedullary disease.8 Although not typically used in clinical trials, the revised World Health Organization (WHO) criteria for BP-CML include ≥ 20% blasts in the peripheral blood or bone marrow, extramedullary blast proliferation, and large foci or clusters of blasts in the bone marrow biopsy (Table).9 The defining feature of CML is the presence of the Ph chromosome abnormality. In a small subset of patients, additional chromosomal abnormalities (ACA) in the Ph-positive cells may be identified at diagnosis. Some reports indicate that the presence of “major route” ACA (trisomy 8, isochromosome 17q, a second Ph chromosome, or trisomy 19) at diagnosis may negatively impact prognosis, but other reports contradict these findings.10,11
The typical BCR breakpoint in CML is the major breakpoint cluster region (M-BCR), which results in a 210-kDa protein (p210). Alternate breakpoints that are less frequently identified are the minor BCR (mBCR or p190), which is more commonly found in Ph-positive acute lymphoblastic leukemia (ALL), and the micro BCR (µBCR or p230), which is much less common and is often characterized by chronic neutrophilia.12 Identifying which BCR-ABL1 transcript is present in each patient using qualitative PCR is crucial in order to ensure proper monitoring during treatment.
The most sensitive method for detecting BCR-ABL1 mRNA transcripts is the quantitative real-time PCR (RQ-PCR) assay, which is typically done on peripheral blood. RQ-PCR is capable of detecting a single CML cell in the presence of ≥ 100,000 normal cells. This test should be done during the initial diagnostic workup in order to confirm the presence of BCR-ABL1 transcripts, and it is used as a standard method for monitoring response to TKI therapy.13 The International Scale (IS) is a standardized approach to reporting RQ-PCR results that was developed to allow comparison of results across various laboratories and has become the gold standard for reporting BCR-ABL1 transcript values.14
Determining Risk Scores
Calculating a patient’s Sokal score or EURO risk score at diagnosis remains an important component of the diagnostic workup in CP-CML, as this information has prognostic and therapeutic implications (an online calculator is available through European LeukemiaNet [ELN]). The risk for disease progression to the accelerated or blast phases is higher in patients with intermediate- or high-risk scores compared to those with a low-risk score at diagnosis. The risk of progression in intermediate- or high-risk patients is lower when a second-generation TKI (dasatinib, nilotinib, or bosutinib) is used as frontline therapy compared to imatinib, and therefore, the National Comprehensive Cancer Network (NCCN) CML Panel recommends starting with a second-generation TKI in these patients.15-19
Monitoring Response to Therapy
Case Continued
Fluorescent in-situ hybridization using a peripheral blood sample to detect the BCR-ABL gene rearrangement is performed and is positive in 87% of cells. Bone marrow biopsy and aspiration show a 95% cellular bone marrow with granulocytic hyperplasia and 1% blasts. Cytogenetics are 46,XX,t(9;22)(q34;q11.2).20 RQ-PCR assay performed to measure BCR-ABL1 transcripts in the peripheral blood shows a value of 98% IS. The patient is ultimately given a diagnosis of CP-CML. Her Sokal risk score is 1.42, making her disease high risk.
How is response to TKI therapy measured and monitored?
After confirming a diagnosis of CML and selecting the most appropriate TKI for first-line therapy, the successful management of a CML patient relies on close monitoring and follow-up to ensure patients are meeting the desired treatment milestones. Responses in CML can be assessed based on hematologic parameters, cytogenetic results, and molecular responses. A complete hematologic response (CHR) implies complete normalization of peripheral blood counts (with the exception of TKI-induced cytopenias) and resolution of any palpable splenomegaly. The majority of patients will achieve a CHR within 4 to 6 weeks after initiating CML-directed therapy.21
Cytogenetic Response
Cytogenetic responses are defined by the decrease in the number of Ph chromosome–positive metaphases when assessed on bone marrow cytogenetics. A partial cytogenetic response (PCyR) is defined as having 1% to 35% Ph-positive metaphases, a major cytogenetic response (MCyR) as having 0% to 35% Ph-positive metaphases, and a CCyR implies that no Ph-positive metaphases are identified on bone marrow cytogenetics. An ideal response is the achievement of PCyR after 3 months on a TKI and a CCyR after 12 months on a TKI.22
Molecular Response
Once a patient has achieved a CCyR, monitoring their response to therapy can only be done using RQ-PCR to measure BCR-ABL1 transcripts in the peripheral blood. The NCCN and the ELN recommend monitoring RQ-PCR from the peripheral blood every 3 months in order to assess response to TKIs.19,23 As noted, the International Scale (IS) has become the gold standard reporting system for all BCR-ABL1 transcript levels in the majority of laboratories worldwide.14,24 Molecular responses are based on a log-reduction in BCR-ABL1 transcripts from a standardized baseline. Many molecular responses can be correlated with cytogenetic responses such that if reliable RQ-PCR testing is available, monitoring can be done using only peripheral blood RQ-PCR rather than repeat bone marrow biopsies. For example, an early molecular response (EMR) is defined as a RQ-PCR value of ≤ 10% IS, which is approximately equivalent to a PCyR.25 A value of 1% IS is approximately equivalent to CCyR. A major molecular response (MMR) is a ≥ 3-log reduction in BCR-ABL1 transcripts from baseline and is a value of ≤ 0.1% IS. Deeper levels of molecular response are best described by the log-reduction in BCR-ABL1 transcripts, with a 4-log reduction denoted as MR4.0, a 4.5-log reduction as MR4.5, and so forth. Complete molecular response (CMR) is defined by the level of sensitivity of the RQ-PCR assay being used.14
The definition of relapsed disease in CML is dependent on the type of response the patient had previously achieved. Relapse could be the loss of a hematologic or cytogenetic response, but fluctuations in BCR-ABL1 transcripts on routine RQ-PCR do not necessarily indicate relapsed CML. A 1-log increase in the level of BCR-ABL1 transcripts with a concurrent loss of MMR should prompt a bone marrow biopsy in order to assess for the loss of CCyR, and thus a cytogenetic relapse; however, this loss of MMR does not define relapse in and of itself. In the setting of relapsed disease, testing should be done to look for possible ABL kinase domain mutations, and alternate therapy should be selected.19
Multiple reports have identified the prognostic relevance of achieving an EMR at 3 and 6 months after starting TKI therapy. Marin and colleagues reported that in 282 imatinib-treated patients, there was a significant improvement in 8-year OS, progression-free survival, and cumulative incidence of CCyR and CMR in patients who had BCR-ABL1 transcripts < 9.84% IS after 3 months on treatment.25 This data highlights the importance of early molecular monitoring in order to ensure the best outcomes for patients with CP-CML.
The NCCN CML guidelines and ELN recommendations both agree that an ideal response after 3 months on a TKI is BCR-ABL1 transcripts < 10% IS, but treatment is not considered to be failing at this point if the patient marginally misses this milestone. After 6 months on treatment, an ideal response is considered BCR-ABL1 transcripts < 1%–10% IS. Ideally, patients will have BCR-ABL1 transcripts < 0.1%–1% IS by the time they complete 12 months of TKI therapy, suggesting that these patients have at least achieved a CCyR.19,23 Even after patients achieve these early milestones, frequent monitoring by RQ-PCR is required to ensure that they are maintaining their response to treatment. This will help to ensure patient compliance with treatment and will also help to identify a select subset of patients who could potentially be considered for an attempt at TKI cessation (not discussed in detail here) after a minimum of 3 years on therapy.19,26
Conclusion
Given the successful treatments available for patients with CML, it is crucial to identify patients with this disease, ensure they receive a complete, appropriate diagnostic workup including a bone marrow biopsy and aspiration with cytogenetic testing, and select the best therapy for each individual patient. Once on treatment, the importance of frequent monitoring cannot be overstated.
1. Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164-172.
2. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Leukemia - Chronic Myeloid Leukemia (CML). 2018.
3. Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer. 2012;118:3123-3127.
4. Savage DG, Szydlo RM, Chase A, et al. Bone marrow transplantation for chronic myeloid leukaemia: the effects of differing criteria for defining chronic phase on probabilities of survival and relapse. Br J Haematol. 1997;99:30-35.
5. Knox WF, Bhavnani M, Davson J, Geary CG. Histological classification of chronic granulocytic leukaemia. Clin Lab Haematol. 1984;6:171-175.
6. Kvasnicka HM, Thiele J, Schmitt-Graeff A, et al. Impact of bone marrow morphology on multivariate risk classification in chronic myelogenous leukemia. Acta Haematol. 2003;109:53-56.
7. Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306-1315.
8. Druker BJ. Chronic myeloid leukemia In: DeVita VT, Lawrence TS, Rosenburg SA, eds. DeVita, Hellman, and Rosenberg’s Cancer Principles & Practice of Oncology. 8th ed. Philadelphia, PA: Lippincott, Williams and Wilkins; 2007:2267-2304.
9. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405.
10. Fabarius A, Leitner A, Hochhaus A, et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118:6760-6768.
11. Alhuraiji A, Kantarjian H, Boddu P, et al. Prognostic significance of additional chromosomal abnormalities at the time of diagnosis in patients with chronic myeloid leukemia treated with frontline tyrosine kinase inhibitors. Am J Hematol. 2018;93:84-90.
12. Melo JV. BCR-ABL gene variants. Baillieres Clin Haematol. 1997;10:203-222.
13. Kantarjian HM, Talpaz M, Cortes J, et al. Quantitative polymerase chain reaction monitoring of BCR-ABL during therapy with imatinib mesylate (STI571; gleevec) in chronic-phase chronic myelogenous leukemia. Clin Cancer Res. 2003;9:160-166.
14. Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28-37.
15. Hochhaus A, Larson RA, Guilhot F, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917-927.
16. Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients trial. J Clin Oncol. 2016;34:2333-3340.
17. Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044-1054.
18. Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36:231-237.
19. Radich JP, Deininger M, Abboud CN, et al. Chronic Myeloid Leukemia, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1108-1135.
20. Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037.
21. Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Ann Intern Med. 1999;131:207-219.
22. O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994-1004.
23. Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872-884.
24. Larripa I, Ruiz MS, Gutierrez M, Bianchini M. [Guidelines for molecular monitoring of BCR-ABL1 in chronic myeloid leukemia patients by RT-qPCR.] Medicina (B Aires). 2017;77:61-72.
25. Marin D, Ibrahim AR, Lucas C, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012;30:232-238.
26. Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128:17-23.
1. Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164-172.
2. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Leukemia - Chronic Myeloid Leukemia (CML). 2018.
3. Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer. 2012;118:3123-3127.
4. Savage DG, Szydlo RM, Chase A, et al. Bone marrow transplantation for chronic myeloid leukaemia: the effects of differing criteria for defining chronic phase on probabilities of survival and relapse. Br J Haematol. 1997;99:30-35.
5. Knox WF, Bhavnani M, Davson J, Geary CG. Histological classification of chronic granulocytic leukaemia. Clin Lab Haematol. 1984;6:171-175.
6. Kvasnicka HM, Thiele J, Schmitt-Graeff A, et al. Impact of bone marrow morphology on multivariate risk classification in chronic myelogenous leukemia. Acta Haematol. 2003;109:53-56.
7. Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306-1315.
8. Druker BJ. Chronic myeloid leukemia In: DeVita VT, Lawrence TS, Rosenburg SA, eds. DeVita, Hellman, and Rosenberg’s Cancer Principles & Practice of Oncology. 8th ed. Philadelphia, PA: Lippincott, Williams and Wilkins; 2007:2267-2304.
9. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405.
10. Fabarius A, Leitner A, Hochhaus A, et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118:6760-6768.
11. Alhuraiji A, Kantarjian H, Boddu P, et al. Prognostic significance of additional chromosomal abnormalities at the time of diagnosis in patients with chronic myeloid leukemia treated with frontline tyrosine kinase inhibitors. Am J Hematol. 2018;93:84-90.
12. Melo JV. BCR-ABL gene variants. Baillieres Clin Haematol. 1997;10:203-222.
13. Kantarjian HM, Talpaz M, Cortes J, et al. Quantitative polymerase chain reaction monitoring of BCR-ABL during therapy with imatinib mesylate (STI571; gleevec) in chronic-phase chronic myelogenous leukemia. Clin Cancer Res. 2003;9:160-166.
14. Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28-37.
15. Hochhaus A, Larson RA, Guilhot F, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917-927.
16. Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients trial. J Clin Oncol. 2016;34:2333-3340.
17. Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044-1054.
18. Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36:231-237.
19. Radich JP, Deininger M, Abboud CN, et al. Chronic Myeloid Leukemia, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1108-1135.
20. Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037.
21. Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Ann Intern Med. 1999;131:207-219.
22. O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994-1004.
23. Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872-884.
24. Larripa I, Ruiz MS, Gutierrez M, Bianchini M. [Guidelines for molecular monitoring of BCR-ABL1 in chronic myeloid leukemia patients by RT-qPCR.] Medicina (B Aires). 2017;77:61-72.
25. Marin D, Ibrahim AR, Lucas C, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012;30:232-238.
26. Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128:17-23.
Researchers compare focused ultrasound and DBS for essential tremor
LAS VEGAS – according to two presentations delivered at the annual meeting of the North American Neuromodulation Society. The techniques’ surgical procedures, associated risks, and adverse event profiles may influence neurologists and patients in their choice of treatment.
FUS allows neurosurgeons to apply thermal ablation to create a lesion on the thalamus. MRI guidance enables precise control of the lesion location (within approximately 1 mm) and of the treatment intensity. The surgery can be performed with high-resolution stereotactic framing.
DBS entails the surgical implantation of a neurostimulator and attached leads and electrodes. The neurosurgeon drills a hole of approximately 14 mm in diameter into the skull so that the electrode can be inserted stereotactically while the patient is awake or asleep. The neurostimulator is installed separately.
Both treatments provide functional benefits
In 2016, W. Jeff Elias, MD, director of stereotactic and functional neurosurgery at the University of Virginia in Charlottesville, and his colleagues published the results of a randomized controlled trial that compared FUS with sham treatment in 76 patients with essential tremor. At three months, hand tremor had improved by approximately 50% among treated patients, but controls had no significant benefit(N Engl J Med. 2016 Aug 25;375[8]:730-9). The improvement among treated patients was maintained for 12 months. Disability and quality of life also improved after FUS.
A study by Schuurman et al. published in 2000 (N Engl J Med. 2000 Feb 17;342[7]:461-8) showed that DBS and FUS had similar efficacy at 1 year, said Kathryn L. Holloway, MD, professor of neurosurgery at Virginia Commonwealth University in Richmond. It included 45 patients with Parkinson’s disease, 13 with essential tremor, and 10 with multiple sclerosis who were randomized 1:1 to FUS or DBS. The primary outcome was activities of daily living, and blinded physicians assessed patient videos. Most of the patients who improved had received DBS, and most of the ones who worsened had received FUS, said Dr. Holloway. Among patients with essential tremor, tremor improved by between 94% and 100% with either treatment.
To find more recent data about these treatments, Dr. Holloway searched the literature for studies of FUS or DBS for essential tremor. She analyzed only studies that included unselected populations, blinded evaluations within 1 or 2 years of surgery, and tremor scores for the treated side. She found two studies of FUS, including Dr. Elias’s 2016 trial and a 2018 follow-up (Ann Neurol. 2018 Jan;83[1]:107-14). Dr. Holloway also identified three trials of DBS.
In these studies, reduction of hand tremor was 55% with FUS and between 63% and 69% with DBS. Reduction of postural tremor was approximately 72% with FUS and approximately 67% with DBS. Reduction of action tremor was about 52% with FUS and between 65% and 71% with DBS. Overall, DBS appears to be more effective, said Dr. Holloway.
A 2015 study (Mov Disord. 2015 Dec;30[14]:1937-43) that compared bilateral DBS, unilateral DBS, and unilateral FUS for essential tremor indicated that the treatments provide similar benefits on hand tremor, disability, and quality of life, said Dr. Elias. FUS is inferior to DBS, however, for total tremor and axial tremor.
Furthermore, the efficacy of FUS wanes over time, said Dr. Elias. He and his colleagues conducted a pilot study of 15 patients with essential tremor who received FUS (N Engl J Med. 2013 Aug 15;369[7]:640-8). At 6 years, 6 of 13 patients whose data were available still had a 50% improvement in tremor. “Some went on to [receive] DBS,” said Dr. Elias. “Functional improvements persisted more than the tremor improvement.”
Adverse events
In their 2016 trial of FUS, Dr. Elias and his colleagues observed 210 adverse events, which is approximately “what you would expect with a modern day, FDA-monitored clinical trial.” Sensory effects and gait disturbance accounted for most of the thalamotomy-related adverse events. Sensory problems such as numbness or parestheisa persisted at 1 year in 14% of treated patients, and gait disturbance persisted at 1 year in 9%. The investigators did not observe any hemorrhages, infections, or cavitation-related effects from FUS.
In a 2018 analysis of five clinical trials of FUS for essential tremor, Fishman et al. found that 79% of adverse events were mild and 1% were severe (Mov Disord. 2018 May;33[5]:843-7). The risk of a severe adverse event therefore can be considered low, and it may decrease as neurosurgeons gain experience with the procedure, said Dr. Elias.
In the 2000 Schuurman et al. study, the researchers observed significantly fewer adverse events overall among patients with Parkinson’s disease or essential tremor who received DBS, compared with patients who received FUS. Cognitive deterioration, severe dysarthria, and severe ataxia were more common in the FUS group than in the DBS group. Dr. Holloway’s analysis of adverse events in the five more recent trials that she identified yielded similar results.
Although MRI-guided FUS is a precise way to make lesions, functional areas in the thalamus overlap, which makes it more difficult to target only the intended region, said Dr. Holloway. The functional overlap thus increases the risk of adverse events (e.g., sensory impairments, dysarthria, or ataxia). The adverse events that result from FUS may last as long as a year. “Patients will put up anything for about a month after surgery, and then they start to get annoyed,” said Dr. Holloway.
In addition, Schuurman et al. found that FUS entailed a greater risk of permanent side effects, compared with DBS. “That’s the key point here,” said Dr. Holloway. Most of the adverse effects in the DBS group were resolved by adjusting or turning off the stimulator. Hardware issues resulting from DBS are frustrating, but reversible, but a patient with an adverse event after FUS often is “stuck with it,” said Dr. Holloway. The Schuurman et al. data indicated that, in terms of adverse events, “thalamotomy was inferior to DBS,” she added.
Implantation of DBS entails the risks inherent to surgeries that open the skull (such as seizures, air embolism, and hemorrhage). DBS entails a 2% risk of hemorrhage or infection, said Dr. Elias. Furthermore, as much as 15% of patients who undergo DBS implantation require additional surgery.
“FUS is not going to cause a life-threatening hemorrhage, but DBS certainly can,” said Dr. Holloway.
Managing disease progression
Essential tremor is a progressive disease, and older patients are more likely to have exponential progression than linear progression. Data, such as those published by Zhang et al. (J Neurosurg. 2010 Jun;112[6]:1271-6), indicate that DBS can “keep up with the progression of the disease,” said Dr. Holloway. The authors found that tremor scores did not change significantly over approximately 5 years when patients with essential tremor who had received DBS implantation had periodic assessments and increases in stimulation parameters when appropriate.
If a patient with essential tremor undergoes FUS thalamotomy and has subsequent disease progression, DBS may be considered for reducing tremor, said Dr. Holloway. Most adverse events resulting from DBS implantation are reversible with adjustment of the stimulation parameters. A second thalamotomy, however, could cause severe dysarthria and other irreversible adverse events. “Only DBS can safely address tremor progression,” said Dr. Holloway.
LAS VEGAS – according to two presentations delivered at the annual meeting of the North American Neuromodulation Society. The techniques’ surgical procedures, associated risks, and adverse event profiles may influence neurologists and patients in their choice of treatment.
FUS allows neurosurgeons to apply thermal ablation to create a lesion on the thalamus. MRI guidance enables precise control of the lesion location (within approximately 1 mm) and of the treatment intensity. The surgery can be performed with high-resolution stereotactic framing.
DBS entails the surgical implantation of a neurostimulator and attached leads and electrodes. The neurosurgeon drills a hole of approximately 14 mm in diameter into the skull so that the electrode can be inserted stereotactically while the patient is awake or asleep. The neurostimulator is installed separately.
Both treatments provide functional benefits
In 2016, W. Jeff Elias, MD, director of stereotactic and functional neurosurgery at the University of Virginia in Charlottesville, and his colleagues published the results of a randomized controlled trial that compared FUS with sham treatment in 76 patients with essential tremor. At three months, hand tremor had improved by approximately 50% among treated patients, but controls had no significant benefit(N Engl J Med. 2016 Aug 25;375[8]:730-9). The improvement among treated patients was maintained for 12 months. Disability and quality of life also improved after FUS.
A study by Schuurman et al. published in 2000 (N Engl J Med. 2000 Feb 17;342[7]:461-8) showed that DBS and FUS had similar efficacy at 1 year, said Kathryn L. Holloway, MD, professor of neurosurgery at Virginia Commonwealth University in Richmond. It included 45 patients with Parkinson’s disease, 13 with essential tremor, and 10 with multiple sclerosis who were randomized 1:1 to FUS or DBS. The primary outcome was activities of daily living, and blinded physicians assessed patient videos. Most of the patients who improved had received DBS, and most of the ones who worsened had received FUS, said Dr. Holloway. Among patients with essential tremor, tremor improved by between 94% and 100% with either treatment.
To find more recent data about these treatments, Dr. Holloway searched the literature for studies of FUS or DBS for essential tremor. She analyzed only studies that included unselected populations, blinded evaluations within 1 or 2 years of surgery, and tremor scores for the treated side. She found two studies of FUS, including Dr. Elias’s 2016 trial and a 2018 follow-up (Ann Neurol. 2018 Jan;83[1]:107-14). Dr. Holloway also identified three trials of DBS.
In these studies, reduction of hand tremor was 55% with FUS and between 63% and 69% with DBS. Reduction of postural tremor was approximately 72% with FUS and approximately 67% with DBS. Reduction of action tremor was about 52% with FUS and between 65% and 71% with DBS. Overall, DBS appears to be more effective, said Dr. Holloway.
A 2015 study (Mov Disord. 2015 Dec;30[14]:1937-43) that compared bilateral DBS, unilateral DBS, and unilateral FUS for essential tremor indicated that the treatments provide similar benefits on hand tremor, disability, and quality of life, said Dr. Elias. FUS is inferior to DBS, however, for total tremor and axial tremor.
Furthermore, the efficacy of FUS wanes over time, said Dr. Elias. He and his colleagues conducted a pilot study of 15 patients with essential tremor who received FUS (N Engl J Med. 2013 Aug 15;369[7]:640-8). At 6 years, 6 of 13 patients whose data were available still had a 50% improvement in tremor. “Some went on to [receive] DBS,” said Dr. Elias. “Functional improvements persisted more than the tremor improvement.”
Adverse events
In their 2016 trial of FUS, Dr. Elias and his colleagues observed 210 adverse events, which is approximately “what you would expect with a modern day, FDA-monitored clinical trial.” Sensory effects and gait disturbance accounted for most of the thalamotomy-related adverse events. Sensory problems such as numbness or parestheisa persisted at 1 year in 14% of treated patients, and gait disturbance persisted at 1 year in 9%. The investigators did not observe any hemorrhages, infections, or cavitation-related effects from FUS.
In a 2018 analysis of five clinical trials of FUS for essential tremor, Fishman et al. found that 79% of adverse events were mild and 1% were severe (Mov Disord. 2018 May;33[5]:843-7). The risk of a severe adverse event therefore can be considered low, and it may decrease as neurosurgeons gain experience with the procedure, said Dr. Elias.
In the 2000 Schuurman et al. study, the researchers observed significantly fewer adverse events overall among patients with Parkinson’s disease or essential tremor who received DBS, compared with patients who received FUS. Cognitive deterioration, severe dysarthria, and severe ataxia were more common in the FUS group than in the DBS group. Dr. Holloway’s analysis of adverse events in the five more recent trials that she identified yielded similar results.
Although MRI-guided FUS is a precise way to make lesions, functional areas in the thalamus overlap, which makes it more difficult to target only the intended region, said Dr. Holloway. The functional overlap thus increases the risk of adverse events (e.g., sensory impairments, dysarthria, or ataxia). The adverse events that result from FUS may last as long as a year. “Patients will put up anything for about a month after surgery, and then they start to get annoyed,” said Dr. Holloway.
In addition, Schuurman et al. found that FUS entailed a greater risk of permanent side effects, compared with DBS. “That’s the key point here,” said Dr. Holloway. Most of the adverse effects in the DBS group were resolved by adjusting or turning off the stimulator. Hardware issues resulting from DBS are frustrating, but reversible, but a patient with an adverse event after FUS often is “stuck with it,” said Dr. Holloway. The Schuurman et al. data indicated that, in terms of adverse events, “thalamotomy was inferior to DBS,” she added.
Implantation of DBS entails the risks inherent to surgeries that open the skull (such as seizures, air embolism, and hemorrhage). DBS entails a 2% risk of hemorrhage or infection, said Dr. Elias. Furthermore, as much as 15% of patients who undergo DBS implantation require additional surgery.
“FUS is not going to cause a life-threatening hemorrhage, but DBS certainly can,” said Dr. Holloway.
Managing disease progression
Essential tremor is a progressive disease, and older patients are more likely to have exponential progression than linear progression. Data, such as those published by Zhang et al. (J Neurosurg. 2010 Jun;112[6]:1271-6), indicate that DBS can “keep up with the progression of the disease,” said Dr. Holloway. The authors found that tremor scores did not change significantly over approximately 5 years when patients with essential tremor who had received DBS implantation had periodic assessments and increases in stimulation parameters when appropriate.
If a patient with essential tremor undergoes FUS thalamotomy and has subsequent disease progression, DBS may be considered for reducing tremor, said Dr. Holloway. Most adverse events resulting from DBS implantation are reversible with adjustment of the stimulation parameters. A second thalamotomy, however, could cause severe dysarthria and other irreversible adverse events. “Only DBS can safely address tremor progression,” said Dr. Holloway.
LAS VEGAS – according to two presentations delivered at the annual meeting of the North American Neuromodulation Society. The techniques’ surgical procedures, associated risks, and adverse event profiles may influence neurologists and patients in their choice of treatment.
FUS allows neurosurgeons to apply thermal ablation to create a lesion on the thalamus. MRI guidance enables precise control of the lesion location (within approximately 1 mm) and of the treatment intensity. The surgery can be performed with high-resolution stereotactic framing.
DBS entails the surgical implantation of a neurostimulator and attached leads and electrodes. The neurosurgeon drills a hole of approximately 14 mm in diameter into the skull so that the electrode can be inserted stereotactically while the patient is awake or asleep. The neurostimulator is installed separately.
Both treatments provide functional benefits
In 2016, W. Jeff Elias, MD, director of stereotactic and functional neurosurgery at the University of Virginia in Charlottesville, and his colleagues published the results of a randomized controlled trial that compared FUS with sham treatment in 76 patients with essential tremor. At three months, hand tremor had improved by approximately 50% among treated patients, but controls had no significant benefit(N Engl J Med. 2016 Aug 25;375[8]:730-9). The improvement among treated patients was maintained for 12 months. Disability and quality of life also improved after FUS.
A study by Schuurman et al. published in 2000 (N Engl J Med. 2000 Feb 17;342[7]:461-8) showed that DBS and FUS had similar efficacy at 1 year, said Kathryn L. Holloway, MD, professor of neurosurgery at Virginia Commonwealth University in Richmond. It included 45 patients with Parkinson’s disease, 13 with essential tremor, and 10 with multiple sclerosis who were randomized 1:1 to FUS or DBS. The primary outcome was activities of daily living, and blinded physicians assessed patient videos. Most of the patients who improved had received DBS, and most of the ones who worsened had received FUS, said Dr. Holloway. Among patients with essential tremor, tremor improved by between 94% and 100% with either treatment.
To find more recent data about these treatments, Dr. Holloway searched the literature for studies of FUS or DBS for essential tremor. She analyzed only studies that included unselected populations, blinded evaluations within 1 or 2 years of surgery, and tremor scores for the treated side. She found two studies of FUS, including Dr. Elias’s 2016 trial and a 2018 follow-up (Ann Neurol. 2018 Jan;83[1]:107-14). Dr. Holloway also identified three trials of DBS.
In these studies, reduction of hand tremor was 55% with FUS and between 63% and 69% with DBS. Reduction of postural tremor was approximately 72% with FUS and approximately 67% with DBS. Reduction of action tremor was about 52% with FUS and between 65% and 71% with DBS. Overall, DBS appears to be more effective, said Dr. Holloway.
A 2015 study (Mov Disord. 2015 Dec;30[14]:1937-43) that compared bilateral DBS, unilateral DBS, and unilateral FUS for essential tremor indicated that the treatments provide similar benefits on hand tremor, disability, and quality of life, said Dr. Elias. FUS is inferior to DBS, however, for total tremor and axial tremor.
Furthermore, the efficacy of FUS wanes over time, said Dr. Elias. He and his colleagues conducted a pilot study of 15 patients with essential tremor who received FUS (N Engl J Med. 2013 Aug 15;369[7]:640-8). At 6 years, 6 of 13 patients whose data were available still had a 50% improvement in tremor. “Some went on to [receive] DBS,” said Dr. Elias. “Functional improvements persisted more than the tremor improvement.”
Adverse events
In their 2016 trial of FUS, Dr. Elias and his colleagues observed 210 adverse events, which is approximately “what you would expect with a modern day, FDA-monitored clinical trial.” Sensory effects and gait disturbance accounted for most of the thalamotomy-related adverse events. Sensory problems such as numbness or parestheisa persisted at 1 year in 14% of treated patients, and gait disturbance persisted at 1 year in 9%. The investigators did not observe any hemorrhages, infections, or cavitation-related effects from FUS.
In a 2018 analysis of five clinical trials of FUS for essential tremor, Fishman et al. found that 79% of adverse events were mild and 1% were severe (Mov Disord. 2018 May;33[5]:843-7). The risk of a severe adverse event therefore can be considered low, and it may decrease as neurosurgeons gain experience with the procedure, said Dr. Elias.
In the 2000 Schuurman et al. study, the researchers observed significantly fewer adverse events overall among patients with Parkinson’s disease or essential tremor who received DBS, compared with patients who received FUS. Cognitive deterioration, severe dysarthria, and severe ataxia were more common in the FUS group than in the DBS group. Dr. Holloway’s analysis of adverse events in the five more recent trials that she identified yielded similar results.
Although MRI-guided FUS is a precise way to make lesions, functional areas in the thalamus overlap, which makes it more difficult to target only the intended region, said Dr. Holloway. The functional overlap thus increases the risk of adverse events (e.g., sensory impairments, dysarthria, or ataxia). The adverse events that result from FUS may last as long as a year. “Patients will put up anything for about a month after surgery, and then they start to get annoyed,” said Dr. Holloway.
In addition, Schuurman et al. found that FUS entailed a greater risk of permanent side effects, compared with DBS. “That’s the key point here,” said Dr. Holloway. Most of the adverse effects in the DBS group were resolved by adjusting or turning off the stimulator. Hardware issues resulting from DBS are frustrating, but reversible, but a patient with an adverse event after FUS often is “stuck with it,” said Dr. Holloway. The Schuurman et al. data indicated that, in terms of adverse events, “thalamotomy was inferior to DBS,” she added.
Implantation of DBS entails the risks inherent to surgeries that open the skull (such as seizures, air embolism, and hemorrhage). DBS entails a 2% risk of hemorrhage or infection, said Dr. Elias. Furthermore, as much as 15% of patients who undergo DBS implantation require additional surgery.
“FUS is not going to cause a life-threatening hemorrhage, but DBS certainly can,” said Dr. Holloway.
Managing disease progression
Essential tremor is a progressive disease, and older patients are more likely to have exponential progression than linear progression. Data, such as those published by Zhang et al. (J Neurosurg. 2010 Jun;112[6]:1271-6), indicate that DBS can “keep up with the progression of the disease,” said Dr. Holloway. The authors found that tremor scores did not change significantly over approximately 5 years when patients with essential tremor who had received DBS implantation had periodic assessments and increases in stimulation parameters when appropriate.
If a patient with essential tremor undergoes FUS thalamotomy and has subsequent disease progression, DBS may be considered for reducing tremor, said Dr. Holloway. Most adverse events resulting from DBS implantation are reversible with adjustment of the stimulation parameters. A second thalamotomy, however, could cause severe dysarthria and other irreversible adverse events. “Only DBS can safely address tremor progression,” said Dr. Holloway.
REPORTING FROM NANS 2019
Is vaginal estrogen used for GSM associated with a higher risk of CVD or cancer?
Expert Commentary
Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. December 17, 2018. doi: 10.1097/GME.0000000000001284.
GSM, a chronic and often progressive condition, occurs in almost 50% of postmenopausal women and has been shown to impair sexual function and quality of life.1 Symptoms include vaginal dryness, vulvar or vaginal itching, dyspareunia, urinary urgency or frequency, and increased urinary tract infections. Although lubricants or vaginal moisturizers may be sufficient to treat GSM, targeted hormonal therapy may be needed to improve the symptoms and resolve the underlying cause, due to vaginal hormone loss.
Despite lack of any observational or clinical trial evidence for chronic health disease risks related to low-dose vaginal estrogen use, there remains an US Food and Drug Administration boxed warning on the package label for low-dose vaginal estrogen related to risks of heart disease, stroke, venous thromboembolism, pdementia, and breast cancer. The objective of the investigation by Bhupathiraju and colleagues was to evaluate associations between vaginal estrogen use and health outcomes, including CVD (myocardial infarction, stroke, and pulmonary embolism/deep vein thrombosis), cancer (total invasive, breast, endometrial, ovarian, and colorectal), and hip fracture.
Details of the study
The prospective analysis included 896 postmenopausal current users of vaginal estrogen in the Nurses’ Health Study (NHS; 1982–2012), compared with 52,901 nonusers. Eighteen years of follow-up was evaluated. Users of systemic hormone therapy were excluded from the analysis. For the NHS, self-reported data were collected every 2 years on questionnaires for vaginal estrogen use and health outcomes. Investigators used medical records to confirm health outcomes.
After adjusting for covariates, no significant differences in risks were found for CVD, cancer, and hip fracture between users and nonusers of vaginal estrogen, regardless of hysterectomy status.
Key findings
After adjusting for multiple variables (including age, race, physical activity, age at menopause, hysterectomy, aspirin use, parental history of cancer, etc), health outcomes for CVDs, all cancers, and hip fracture were:
- myocardial infarction: hazard ratio (HR), 0.73 (95% confidence interval [CI], 0.47–1.13)
- stroke: HR, 0.85 (95% CI, 0.56–1.29)
- pulmonary embolism/deep vein thrombosis: HR, 1.06 (95% CI, 0.58–1.93)
- hip fracture: HR, 0.91 (95% CI, 0.60–1.38)
- all cancers: HR, 1.05 (95% CI, 0.89–1.25).
Continue to: Health outcomes for specific invasive cancers
Health outcomes for specific invasive cancers (risk for endometrial cancer included only women with an intact uterus) were:
- invasive breast cancer: HR, 1.07 (95% CI, 0.78–1.47)
- ovarian cancer: HR, 1.17 (95% CI, 0.52–2.65)
- endometrial cancer: HR, 1.62 (95% CI, 0.88–2.97)
- colorectal cancer: HR, 0.77 (95% CI, 0.45–1.34).
Study strengths and weaknesses
A causal relationship cannot be proven as the study was observational. However, a strength included the 18 years of follow-up. Women used vaginal estrogen for an average of 3 years, which provided longer-term safety data than available 12-month clinical trial data. Data were collected through self-report on questionnaires every 2 years, which is a drawback; however, participants were registered nurses, who have been shown to provide reliable health-related information. Comparisons between therapies were not possible as data were not collected about type or dosage of vaginal estrogen. Available therapies during the NHS included vaginal estrogen tablets, creams, and an estradiol ring, with higher doses available during earlier parts of the study than the lower doses commonly prescribed in current day.
Overall
The findings from this long-term follow-up of the NHS provide support for the safety of vaginal estrogen for treatment of GSM. No statistically significant increased health risks were found for users of vaginal estrogen, similar to earlier reported findings from the large Women’s Health Initiative.2 Low-dose vaginal estrogen is recommended for treatment of GSM by The North American Menopause Society, the American College of Obstetricians and Gynecologists, and the Endocrine Society.
Absorption of low-dose vaginal estrogen preparations appears minimal, and they are effective and generally safe for the treatment of GSM for women at any age. Progesterone is not recommended with low-dose vaginal estrogen therapies, based primarily on randomized clinical trial safety data of 12 months.3 Postmenopausal bleeding, however, needs to be thoroughly evaluated. For women with breast cancer, include the oncologist in decision making about the use of low-dose vaginal estrogen.
Despite the boxed warning on vaginal estrogen, the findings from this study support the safety of vaginal estrogen use for effective relief of GSM in women with and without a uterus.
JOANN V. PINKERTON, MD, NCMP
- Gandhi J, Chen A, Dagur G, et al. Genitourinary syndrome of menopause: an overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am J Obstet Gynecol. 2016;251:704-711.
- Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women's Health Initiative Observational Study. Menopause. 2018;25:11-20.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
Expert Commentary
Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. December 17, 2018. doi: 10.1097/GME.0000000000001284.
GSM, a chronic and often progressive condition, occurs in almost 50% of postmenopausal women and has been shown to impair sexual function and quality of life.1 Symptoms include vaginal dryness, vulvar or vaginal itching, dyspareunia, urinary urgency or frequency, and increased urinary tract infections. Although lubricants or vaginal moisturizers may be sufficient to treat GSM, targeted hormonal therapy may be needed to improve the symptoms and resolve the underlying cause, due to vaginal hormone loss.
Despite lack of any observational or clinical trial evidence for chronic health disease risks related to low-dose vaginal estrogen use, there remains an US Food and Drug Administration boxed warning on the package label for low-dose vaginal estrogen related to risks of heart disease, stroke, venous thromboembolism, pdementia, and breast cancer. The objective of the investigation by Bhupathiraju and colleagues was to evaluate associations between vaginal estrogen use and health outcomes, including CVD (myocardial infarction, stroke, and pulmonary embolism/deep vein thrombosis), cancer (total invasive, breast, endometrial, ovarian, and colorectal), and hip fracture.
Details of the study
The prospective analysis included 896 postmenopausal current users of vaginal estrogen in the Nurses’ Health Study (NHS; 1982–2012), compared with 52,901 nonusers. Eighteen years of follow-up was evaluated. Users of systemic hormone therapy were excluded from the analysis. For the NHS, self-reported data were collected every 2 years on questionnaires for vaginal estrogen use and health outcomes. Investigators used medical records to confirm health outcomes.
After adjusting for covariates, no significant differences in risks were found for CVD, cancer, and hip fracture between users and nonusers of vaginal estrogen, regardless of hysterectomy status.
Key findings
After adjusting for multiple variables (including age, race, physical activity, age at menopause, hysterectomy, aspirin use, parental history of cancer, etc), health outcomes for CVDs, all cancers, and hip fracture were:
- myocardial infarction: hazard ratio (HR), 0.73 (95% confidence interval [CI], 0.47–1.13)
- stroke: HR, 0.85 (95% CI, 0.56–1.29)
- pulmonary embolism/deep vein thrombosis: HR, 1.06 (95% CI, 0.58–1.93)
- hip fracture: HR, 0.91 (95% CI, 0.60–1.38)
- all cancers: HR, 1.05 (95% CI, 0.89–1.25).
Continue to: Health outcomes for specific invasive cancers
Health outcomes for specific invasive cancers (risk for endometrial cancer included only women with an intact uterus) were:
- invasive breast cancer: HR, 1.07 (95% CI, 0.78–1.47)
- ovarian cancer: HR, 1.17 (95% CI, 0.52–2.65)
- endometrial cancer: HR, 1.62 (95% CI, 0.88–2.97)
- colorectal cancer: HR, 0.77 (95% CI, 0.45–1.34).
Study strengths and weaknesses
A causal relationship cannot be proven as the study was observational. However, a strength included the 18 years of follow-up. Women used vaginal estrogen for an average of 3 years, which provided longer-term safety data than available 12-month clinical trial data. Data were collected through self-report on questionnaires every 2 years, which is a drawback; however, participants were registered nurses, who have been shown to provide reliable health-related information. Comparisons between therapies were not possible as data were not collected about type or dosage of vaginal estrogen. Available therapies during the NHS included vaginal estrogen tablets, creams, and an estradiol ring, with higher doses available during earlier parts of the study than the lower doses commonly prescribed in current day.
Overall
The findings from this long-term follow-up of the NHS provide support for the safety of vaginal estrogen for treatment of GSM. No statistically significant increased health risks were found for users of vaginal estrogen, similar to earlier reported findings from the large Women’s Health Initiative.2 Low-dose vaginal estrogen is recommended for treatment of GSM by The North American Menopause Society, the American College of Obstetricians and Gynecologists, and the Endocrine Society.
Absorption of low-dose vaginal estrogen preparations appears minimal, and they are effective and generally safe for the treatment of GSM for women at any age. Progesterone is not recommended with low-dose vaginal estrogen therapies, based primarily on randomized clinical trial safety data of 12 months.3 Postmenopausal bleeding, however, needs to be thoroughly evaluated. For women with breast cancer, include the oncologist in decision making about the use of low-dose vaginal estrogen.
Despite the boxed warning on vaginal estrogen, the findings from this study support the safety of vaginal estrogen use for effective relief of GSM in women with and without a uterus.
JOANN V. PINKERTON, MD, NCMP
Expert Commentary
Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. December 17, 2018. doi: 10.1097/GME.0000000000001284.
GSM, a chronic and often progressive condition, occurs in almost 50% of postmenopausal women and has been shown to impair sexual function and quality of life.1 Symptoms include vaginal dryness, vulvar or vaginal itching, dyspareunia, urinary urgency or frequency, and increased urinary tract infections. Although lubricants or vaginal moisturizers may be sufficient to treat GSM, targeted hormonal therapy may be needed to improve the symptoms and resolve the underlying cause, due to vaginal hormone loss.
Despite lack of any observational or clinical trial evidence for chronic health disease risks related to low-dose vaginal estrogen use, there remains an US Food and Drug Administration boxed warning on the package label for low-dose vaginal estrogen related to risks of heart disease, stroke, venous thromboembolism, pdementia, and breast cancer. The objective of the investigation by Bhupathiraju and colleagues was to evaluate associations between vaginal estrogen use and health outcomes, including CVD (myocardial infarction, stroke, and pulmonary embolism/deep vein thrombosis), cancer (total invasive, breast, endometrial, ovarian, and colorectal), and hip fracture.
Details of the study
The prospective analysis included 896 postmenopausal current users of vaginal estrogen in the Nurses’ Health Study (NHS; 1982–2012), compared with 52,901 nonusers. Eighteen years of follow-up was evaluated. Users of systemic hormone therapy were excluded from the analysis. For the NHS, self-reported data were collected every 2 years on questionnaires for vaginal estrogen use and health outcomes. Investigators used medical records to confirm health outcomes.
After adjusting for covariates, no significant differences in risks were found for CVD, cancer, and hip fracture between users and nonusers of vaginal estrogen, regardless of hysterectomy status.
Key findings
After adjusting for multiple variables (including age, race, physical activity, age at menopause, hysterectomy, aspirin use, parental history of cancer, etc), health outcomes for CVDs, all cancers, and hip fracture were:
- myocardial infarction: hazard ratio (HR), 0.73 (95% confidence interval [CI], 0.47–1.13)
- stroke: HR, 0.85 (95% CI, 0.56–1.29)
- pulmonary embolism/deep vein thrombosis: HR, 1.06 (95% CI, 0.58–1.93)
- hip fracture: HR, 0.91 (95% CI, 0.60–1.38)
- all cancers: HR, 1.05 (95% CI, 0.89–1.25).
Continue to: Health outcomes for specific invasive cancers
Health outcomes for specific invasive cancers (risk for endometrial cancer included only women with an intact uterus) were:
- invasive breast cancer: HR, 1.07 (95% CI, 0.78–1.47)
- ovarian cancer: HR, 1.17 (95% CI, 0.52–2.65)
- endometrial cancer: HR, 1.62 (95% CI, 0.88–2.97)
- colorectal cancer: HR, 0.77 (95% CI, 0.45–1.34).
Study strengths and weaknesses
A causal relationship cannot be proven as the study was observational. However, a strength included the 18 years of follow-up. Women used vaginal estrogen for an average of 3 years, which provided longer-term safety data than available 12-month clinical trial data. Data were collected through self-report on questionnaires every 2 years, which is a drawback; however, participants were registered nurses, who have been shown to provide reliable health-related information. Comparisons between therapies were not possible as data were not collected about type or dosage of vaginal estrogen. Available therapies during the NHS included vaginal estrogen tablets, creams, and an estradiol ring, with higher doses available during earlier parts of the study than the lower doses commonly prescribed in current day.
Overall
The findings from this long-term follow-up of the NHS provide support for the safety of vaginal estrogen for treatment of GSM. No statistically significant increased health risks were found for users of vaginal estrogen, similar to earlier reported findings from the large Women’s Health Initiative.2 Low-dose vaginal estrogen is recommended for treatment of GSM by The North American Menopause Society, the American College of Obstetricians and Gynecologists, and the Endocrine Society.
Absorption of low-dose vaginal estrogen preparations appears minimal, and they are effective and generally safe for the treatment of GSM for women at any age. Progesterone is not recommended with low-dose vaginal estrogen therapies, based primarily on randomized clinical trial safety data of 12 months.3 Postmenopausal bleeding, however, needs to be thoroughly evaluated. For women with breast cancer, include the oncologist in decision making about the use of low-dose vaginal estrogen.
Despite the boxed warning on vaginal estrogen, the findings from this study support the safety of vaginal estrogen use for effective relief of GSM in women with and without a uterus.
JOANN V. PINKERTON, MD, NCMP
- Gandhi J, Chen A, Dagur G, et al. Genitourinary syndrome of menopause: an overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am J Obstet Gynecol. 2016;251:704-711.
- Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women's Health Initiative Observational Study. Menopause. 2018;25:11-20.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Gandhi J, Chen A, Dagur G, et al. Genitourinary syndrome of menopause: an overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am J Obstet Gynecol. 2016;251:704-711.
- Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women's Health Initiative Observational Study. Menopause. 2018;25:11-20.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
Maltodextrin may increase colitis risk
The food additive maltodextrin may increase risk of inflammatory bowel disease, according to a recent study.
Compared with control subjects, mice given drinking water that contained 5% maltodextrin were significantly more likely to develop colitis and lose weight when challenged with dextran sodium sulfate (DSS), reported lead author Federica Laudisi, PhD, of the department of systems medicine at the University of Rome Tor Vergata in Rome, and her colleagues.
Further experiments with murine intestinal crypts and a human cell line echoed these results and offered mechanistic insight. Treatment with maltodextrin stressed the endoplasmic reticulum of goblet cells, predisposing the intestinal epithelium to mucus depletion and inflammation. With these results, maltodextrin joins polysorbate 80 and carboxymethylcellulose on a growing list of food additives in the Western diet with proinflammatory potential.
“Although the U.S. Food and Drug Administration recognizes these dietary elements as safe,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology, “their use has been linked to the development of intestinal pathologies in both animals and human beings.
“It also has been shown that the polysaccharide maltodextrin, which is commonly used as a filler and thickener during food processing, can alter microbial phenotype and host antibacterial defenses. Maltodextrin expands the Escherichia coli population in the ileum and induces necrotizing enterocolitis in preterm piglets (Am J Physiol Gastrointest Liver Physiol. 2009 Dec;297:G1115-25).”
The present study began by administering three compounds dissolved in drinking water to wild-type Balb/c mice for 45 days: 5% maltodextrin, 0.5% propylene glycol, or 5 g/L animal gelatin. Control mice drank plain water. None of the treatments triggered clinical or histologic signs of colitis, and stool levels of lipocalin-2 (Lcn-2), a biomarker of intestinal inflammation, remained comparable with that of control mice. However, outcomes changed when mice were challenged with DSS (1.75% in drinking water) on days 35-45 or injected subcutaneously with indomethacin (5 mg/kg) on day 35 and sacrificed 24 hours later. When challenged with DSS, mice in the maltodextrin group developed severe colitis and lost 10%-15% of body weight, compared with minimal colitis and negligible weight loss in the other groups. In addition, compared with other mice, maltodextrin-fed mice had increased colon tissue expression of Lcn-2 and inflammatory cytokine interleukin (IL)-1beta. These initial findings suggested that dietary maltodextrin could increase susceptibility to clinical colitis.
To determine the pathophysiology of this phenomenon, the investigators performed microarray analysis of colonic samples. Multiple genes associated with carbohydrate and lipid metabolism were upregulated in maltodextrin-fed mice, including genes that controlled the unfolded protein response (UPR), a process in which unfolded proteins accumulate in the endoplasmic reticulum (ER) during ER stress. The most prominently expressed among the UPR-related genes was Ern-2, which regulates inositol-requiring enzyme 1beta, found exclusively in the ER of goblet cells in the small intestine and colon. When maltodextrin causes ER stress in goblet cells, it leads to misfolding of mucin glycoprotein Mucin-2 (Muc-2), a major component of gut mucus, causing gut mucus levels to drop. A diminished mucus barrier exposes the intestine to infection and damage, as demonstrated by higher rates of pathogenic bacteria in Muc-2–deficient mice than in control mice, and more severe intestinal damage than in controls when Muc-2 mice are deliberately infected with pathogens.
The investigators found that humans likely have similar responses to dietary maltodextrin. Treating the mucus-secreting HT29-methotrexate treated (HT29-MTX) cell line with 5% maltodextrin resulted in upregulation of Ern-2, which is the same mechanism observed in mice. Additional testing showed that this process was mediated by p38 mitogen-activated protein kinase, and pharmacologic inhibition or knockdown of p38 suppressed RNA expression of Ern-2. The investigators found that p38 was similarly involved in maltodextrin-fed mice.
To show that maltodextrin enhances susceptibility to inflammation via ER stress, the investigators used tauroursodeoxycholic acid (TUDCA) to inhibit ER stress. Indeed, inhibition led to reduced Ern-2 expression in HT29-MTX cells and in mice treated with maltodextrin. Giving TUDCA to maltodextrin-fed mice resulted in less weight loss, improved histology, and lower expression of Lcn-2 and IL-1beta.
The study concluded with three final experiments: The first showed that maltodextrin did not alter mucosa-associated microbiota; the second showed that mice fed 5% maltodextrin long term (for 10 weeks) had low-grade intestinal inflammation on histology, albeit without clinical colitis or weight loss; and the third showed that mice consuming maltodextrin long term had higher 15-hour fasting blood glycemic levels than control mice, supporting recent research suggesting that food additives can disrupt metabolism in a nonsusceptible host.
“In conclusion,” the investigators wrote, “this study shows that a maltodextrin-enriched diet reduces the intestinal content of Muc-2, thus making the host more sensitive to colitogenic stimuli. These data, together with the demonstration that maltodextrin can promote epithelial intestinal adhesion of pathogenic bacteria, supports the hypothesis that Western diets rich in maltodextrin can contribute to gut disease susceptibility.”
The study was funded by the Italian Ministry of Education, Universities, and Research. The authors reported no conflicts of interest.
SOURCE: Laudisi F et al. CMGH. 2019 Jan 18. doi: 10.1016/j.jcmgh.2018.09.002.
Maltodextrin is a polysaccharide derived from starch hydrolysis and broadly used as a thickener and filler in processed food. While it is regarded as inert and considered “generally regarded as safe” by the U.S. Food and Drug Administration, multiple recent studies have demonstrated detrimental roles played by maltodextrin in the intestinal environment, suggesting that this broadly used food additive may play a role in chronic inflammatory diseases.
Importantly, in addition to the use of a murine model of colitis, Laudisi and colleagues also investigated the impact that maltodextrin may have on a “normal” host, i.e. without genetic susceptibility nor induced colitis. While maltodextrin did not induce visible levels of intestinal inflammation, it led to the development of low-grade intestinal inflammation, characterized by subtle but nonetheless consistent elevation in intestinal inflammatory markers, ultimately leading to metabolic abnormalities.
Altogether, these recent results, together with previous reports, suggest that consumption of the food additive maltodextrin may be a risk factor for the IBD-prone population, as well as a factor promoting chronic low-grade intestinal inflammation leading to metabolic abnormalities in the general population. These findings further support the concept that FDA testing of food additives should be performed in disease-prone and resistant host models, designed to detect chronic and low-grade inflammation, as well as consider impacts on the gut microbiota.
Benoit Chassaing, PhD, is an assistant professor in the Neuroscience Institute and Institute for Biomedical Sciences, Georgia State University, Atlanta. He has no conflicts. These remarks are excerpted from an editorial accompanying Dr. Laudisi’s article (CMGH. 2019 Jan 18. doi.org/10.1016/j.jcmgh.2018.09.002).
Maltodextrin is a polysaccharide derived from starch hydrolysis and broadly used as a thickener and filler in processed food. While it is regarded as inert and considered “generally regarded as safe” by the U.S. Food and Drug Administration, multiple recent studies have demonstrated detrimental roles played by maltodextrin in the intestinal environment, suggesting that this broadly used food additive may play a role in chronic inflammatory diseases.
Importantly, in addition to the use of a murine model of colitis, Laudisi and colleagues also investigated the impact that maltodextrin may have on a “normal” host, i.e. without genetic susceptibility nor induced colitis. While maltodextrin did not induce visible levels of intestinal inflammation, it led to the development of low-grade intestinal inflammation, characterized by subtle but nonetheless consistent elevation in intestinal inflammatory markers, ultimately leading to metabolic abnormalities.
Altogether, these recent results, together with previous reports, suggest that consumption of the food additive maltodextrin may be a risk factor for the IBD-prone population, as well as a factor promoting chronic low-grade intestinal inflammation leading to metabolic abnormalities in the general population. These findings further support the concept that FDA testing of food additives should be performed in disease-prone and resistant host models, designed to detect chronic and low-grade inflammation, as well as consider impacts on the gut microbiota.
Benoit Chassaing, PhD, is an assistant professor in the Neuroscience Institute and Institute for Biomedical Sciences, Georgia State University, Atlanta. He has no conflicts. These remarks are excerpted from an editorial accompanying Dr. Laudisi’s article (CMGH. 2019 Jan 18. doi.org/10.1016/j.jcmgh.2018.09.002).
Maltodextrin is a polysaccharide derived from starch hydrolysis and broadly used as a thickener and filler in processed food. While it is regarded as inert and considered “generally regarded as safe” by the U.S. Food and Drug Administration, multiple recent studies have demonstrated detrimental roles played by maltodextrin in the intestinal environment, suggesting that this broadly used food additive may play a role in chronic inflammatory diseases.
Importantly, in addition to the use of a murine model of colitis, Laudisi and colleagues also investigated the impact that maltodextrin may have on a “normal” host, i.e. without genetic susceptibility nor induced colitis. While maltodextrin did not induce visible levels of intestinal inflammation, it led to the development of low-grade intestinal inflammation, characterized by subtle but nonetheless consistent elevation in intestinal inflammatory markers, ultimately leading to metabolic abnormalities.
Altogether, these recent results, together with previous reports, suggest that consumption of the food additive maltodextrin may be a risk factor for the IBD-prone population, as well as a factor promoting chronic low-grade intestinal inflammation leading to metabolic abnormalities in the general population. These findings further support the concept that FDA testing of food additives should be performed in disease-prone and resistant host models, designed to detect chronic and low-grade inflammation, as well as consider impacts on the gut microbiota.
Benoit Chassaing, PhD, is an assistant professor in the Neuroscience Institute and Institute for Biomedical Sciences, Georgia State University, Atlanta. He has no conflicts. These remarks are excerpted from an editorial accompanying Dr. Laudisi’s article (CMGH. 2019 Jan 18. doi.org/10.1016/j.jcmgh.2018.09.002).
The food additive maltodextrin may increase risk of inflammatory bowel disease, according to a recent study.
Compared with control subjects, mice given drinking water that contained 5% maltodextrin were significantly more likely to develop colitis and lose weight when challenged with dextran sodium sulfate (DSS), reported lead author Federica Laudisi, PhD, of the department of systems medicine at the University of Rome Tor Vergata in Rome, and her colleagues.
Further experiments with murine intestinal crypts and a human cell line echoed these results and offered mechanistic insight. Treatment with maltodextrin stressed the endoplasmic reticulum of goblet cells, predisposing the intestinal epithelium to mucus depletion and inflammation. With these results, maltodextrin joins polysorbate 80 and carboxymethylcellulose on a growing list of food additives in the Western diet with proinflammatory potential.
“Although the U.S. Food and Drug Administration recognizes these dietary elements as safe,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology, “their use has been linked to the development of intestinal pathologies in both animals and human beings.
“It also has been shown that the polysaccharide maltodextrin, which is commonly used as a filler and thickener during food processing, can alter microbial phenotype and host antibacterial defenses. Maltodextrin expands the Escherichia coli population in the ileum and induces necrotizing enterocolitis in preterm piglets (Am J Physiol Gastrointest Liver Physiol. 2009 Dec;297:G1115-25).”
The present study began by administering three compounds dissolved in drinking water to wild-type Balb/c mice for 45 days: 5% maltodextrin, 0.5% propylene glycol, or 5 g/L animal gelatin. Control mice drank plain water. None of the treatments triggered clinical or histologic signs of colitis, and stool levels of lipocalin-2 (Lcn-2), a biomarker of intestinal inflammation, remained comparable with that of control mice. However, outcomes changed when mice were challenged with DSS (1.75% in drinking water) on days 35-45 or injected subcutaneously with indomethacin (5 mg/kg) on day 35 and sacrificed 24 hours later. When challenged with DSS, mice in the maltodextrin group developed severe colitis and lost 10%-15% of body weight, compared with minimal colitis and negligible weight loss in the other groups. In addition, compared with other mice, maltodextrin-fed mice had increased colon tissue expression of Lcn-2 and inflammatory cytokine interleukin (IL)-1beta. These initial findings suggested that dietary maltodextrin could increase susceptibility to clinical colitis.
To determine the pathophysiology of this phenomenon, the investigators performed microarray analysis of colonic samples. Multiple genes associated with carbohydrate and lipid metabolism were upregulated in maltodextrin-fed mice, including genes that controlled the unfolded protein response (UPR), a process in which unfolded proteins accumulate in the endoplasmic reticulum (ER) during ER stress. The most prominently expressed among the UPR-related genes was Ern-2, which regulates inositol-requiring enzyme 1beta, found exclusively in the ER of goblet cells in the small intestine and colon. When maltodextrin causes ER stress in goblet cells, it leads to misfolding of mucin glycoprotein Mucin-2 (Muc-2), a major component of gut mucus, causing gut mucus levels to drop. A diminished mucus barrier exposes the intestine to infection and damage, as demonstrated by higher rates of pathogenic bacteria in Muc-2–deficient mice than in control mice, and more severe intestinal damage than in controls when Muc-2 mice are deliberately infected with pathogens.
The investigators found that humans likely have similar responses to dietary maltodextrin. Treating the mucus-secreting HT29-methotrexate treated (HT29-MTX) cell line with 5% maltodextrin resulted in upregulation of Ern-2, which is the same mechanism observed in mice. Additional testing showed that this process was mediated by p38 mitogen-activated protein kinase, and pharmacologic inhibition or knockdown of p38 suppressed RNA expression of Ern-2. The investigators found that p38 was similarly involved in maltodextrin-fed mice.
To show that maltodextrin enhances susceptibility to inflammation via ER stress, the investigators used tauroursodeoxycholic acid (TUDCA) to inhibit ER stress. Indeed, inhibition led to reduced Ern-2 expression in HT29-MTX cells and in mice treated with maltodextrin. Giving TUDCA to maltodextrin-fed mice resulted in less weight loss, improved histology, and lower expression of Lcn-2 and IL-1beta.
The study concluded with three final experiments: The first showed that maltodextrin did not alter mucosa-associated microbiota; the second showed that mice fed 5% maltodextrin long term (for 10 weeks) had low-grade intestinal inflammation on histology, albeit without clinical colitis or weight loss; and the third showed that mice consuming maltodextrin long term had higher 15-hour fasting blood glycemic levels than control mice, supporting recent research suggesting that food additives can disrupt metabolism in a nonsusceptible host.
“In conclusion,” the investigators wrote, “this study shows that a maltodextrin-enriched diet reduces the intestinal content of Muc-2, thus making the host more sensitive to colitogenic stimuli. These data, together with the demonstration that maltodextrin can promote epithelial intestinal adhesion of pathogenic bacteria, supports the hypothesis that Western diets rich in maltodextrin can contribute to gut disease susceptibility.”
The study was funded by the Italian Ministry of Education, Universities, and Research. The authors reported no conflicts of interest.
SOURCE: Laudisi F et al. CMGH. 2019 Jan 18. doi: 10.1016/j.jcmgh.2018.09.002.
The food additive maltodextrin may increase risk of inflammatory bowel disease, according to a recent study.
Compared with control subjects, mice given drinking water that contained 5% maltodextrin were significantly more likely to develop colitis and lose weight when challenged with dextran sodium sulfate (DSS), reported lead author Federica Laudisi, PhD, of the department of systems medicine at the University of Rome Tor Vergata in Rome, and her colleagues.
Further experiments with murine intestinal crypts and a human cell line echoed these results and offered mechanistic insight. Treatment with maltodextrin stressed the endoplasmic reticulum of goblet cells, predisposing the intestinal epithelium to mucus depletion and inflammation. With these results, maltodextrin joins polysorbate 80 and carboxymethylcellulose on a growing list of food additives in the Western diet with proinflammatory potential.
“Although the U.S. Food and Drug Administration recognizes these dietary elements as safe,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology, “their use has been linked to the development of intestinal pathologies in both animals and human beings.
“It also has been shown that the polysaccharide maltodextrin, which is commonly used as a filler and thickener during food processing, can alter microbial phenotype and host antibacterial defenses. Maltodextrin expands the Escherichia coli population in the ileum and induces necrotizing enterocolitis in preterm piglets (Am J Physiol Gastrointest Liver Physiol. 2009 Dec;297:G1115-25).”
The present study began by administering three compounds dissolved in drinking water to wild-type Balb/c mice for 45 days: 5% maltodextrin, 0.5% propylene glycol, or 5 g/L animal gelatin. Control mice drank plain water. None of the treatments triggered clinical or histologic signs of colitis, and stool levels of lipocalin-2 (Lcn-2), a biomarker of intestinal inflammation, remained comparable with that of control mice. However, outcomes changed when mice were challenged with DSS (1.75% in drinking water) on days 35-45 or injected subcutaneously with indomethacin (5 mg/kg) on day 35 and sacrificed 24 hours later. When challenged with DSS, mice in the maltodextrin group developed severe colitis and lost 10%-15% of body weight, compared with minimal colitis and negligible weight loss in the other groups. In addition, compared with other mice, maltodextrin-fed mice had increased colon tissue expression of Lcn-2 and inflammatory cytokine interleukin (IL)-1beta. These initial findings suggested that dietary maltodextrin could increase susceptibility to clinical colitis.
To determine the pathophysiology of this phenomenon, the investigators performed microarray analysis of colonic samples. Multiple genes associated with carbohydrate and lipid metabolism were upregulated in maltodextrin-fed mice, including genes that controlled the unfolded protein response (UPR), a process in which unfolded proteins accumulate in the endoplasmic reticulum (ER) during ER stress. The most prominently expressed among the UPR-related genes was Ern-2, which regulates inositol-requiring enzyme 1beta, found exclusively in the ER of goblet cells in the small intestine and colon. When maltodextrin causes ER stress in goblet cells, it leads to misfolding of mucin glycoprotein Mucin-2 (Muc-2), a major component of gut mucus, causing gut mucus levels to drop. A diminished mucus barrier exposes the intestine to infection and damage, as demonstrated by higher rates of pathogenic bacteria in Muc-2–deficient mice than in control mice, and more severe intestinal damage than in controls when Muc-2 mice are deliberately infected with pathogens.
The investigators found that humans likely have similar responses to dietary maltodextrin. Treating the mucus-secreting HT29-methotrexate treated (HT29-MTX) cell line with 5% maltodextrin resulted in upregulation of Ern-2, which is the same mechanism observed in mice. Additional testing showed that this process was mediated by p38 mitogen-activated protein kinase, and pharmacologic inhibition or knockdown of p38 suppressed RNA expression of Ern-2. The investigators found that p38 was similarly involved in maltodextrin-fed mice.
To show that maltodextrin enhances susceptibility to inflammation via ER stress, the investigators used tauroursodeoxycholic acid (TUDCA) to inhibit ER stress. Indeed, inhibition led to reduced Ern-2 expression in HT29-MTX cells and in mice treated with maltodextrin. Giving TUDCA to maltodextrin-fed mice resulted in less weight loss, improved histology, and lower expression of Lcn-2 and IL-1beta.
The study concluded with three final experiments: The first showed that maltodextrin did not alter mucosa-associated microbiota; the second showed that mice fed 5% maltodextrin long term (for 10 weeks) had low-grade intestinal inflammation on histology, albeit without clinical colitis or weight loss; and the third showed that mice consuming maltodextrin long term had higher 15-hour fasting blood glycemic levels than control mice, supporting recent research suggesting that food additives can disrupt metabolism in a nonsusceptible host.
“In conclusion,” the investigators wrote, “this study shows that a maltodextrin-enriched diet reduces the intestinal content of Muc-2, thus making the host more sensitive to colitogenic stimuli. These data, together with the demonstration that maltodextrin can promote epithelial intestinal adhesion of pathogenic bacteria, supports the hypothesis that Western diets rich in maltodextrin can contribute to gut disease susceptibility.”
The study was funded by the Italian Ministry of Education, Universities, and Research. The authors reported no conflicts of interest.
SOURCE: Laudisi F et al. CMGH. 2019 Jan 18. doi: 10.1016/j.jcmgh.2018.09.002.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: The food additive maltodextrin may increase risk of inflammatory bowel disease.
Major finding: When challenged with dextran sulfate sodium, mice consuming maltodextrin developed colitis and lost about 10%-15% of original body weight, compared with negligible inflammation and weight loss in mice not receiving maltodextrin.
Study details: A prospective study involving in vivo experiments with wild-type Balb/c mice and in vitro experiments with murine intestinal crypts and a human intestinal cell line.
Disclosures: The study was funded by the Italian Ministry of Education, Universities, and Research. The investigators reported no conflicts of interest.
Source: Laudisi F et al. CMGH. 2019 Jan 18. doi: 10.1016/j.jcmgh.2018.09.002.
Interview with Joseph R. Berger, MD, on the Financial Contribution of the MS Specialist
Dr. Joseph Berger’s article The Financial Contribution of the Multiple Sclerosis Specialist
(Neurol Clin Pract. 2017;7:246-255) is an eye-opening examination of how multiple sclerosis (MS) specialists fit into the economic framework of large academic institutions. We sat down with Dr. Berger to discuss his findings.
How would you describe the downstream revenue generated from MS specialists at large academic institutions?
The downstream revenue generated from MS is highly dependent on whether the drugs prescribed to patients are provided by the academic institution, and whether the infusions and imaging studies are done at the academic institution.
Another component is whether that institution operates under a Medicare 340B, a law that enables the institutions that are providing care to the underserved to acquire drugs at a steeply discounted price. And, as cited in the paper, the Office of the Inspector General of the Department of Health and Human Services estimated that the profit margin is 58% for all the drugs being provided under 340B. It’s important to note that that statistic accounts for all drugs, not a specific drug or a specific class of drugs. But, for the sake of argument let’s assume that it’s 50% for MS drugs.
The typical MS drug costs $60,000 a year if not more, and that means that 50% of that total goes to the contribution margin of the MS provider or the MS clinic. If there are 1000 patients for whom the specialty pharmacy within the institution is providing drugs, that means an enormous amount of money is returning to the institution as a result of the contribution by the MS practitioners.
In addition to the cost of the drugs, there’s also the cost of infusions associated with the drug that contributes substantially to the bottom line of the institution.
MS specialists tend to do more imaging studies than any other discipline. At the time of diagnosis, individuals with MS get MRIs of brain, cervical and thoracic spine, and frequently the orbits. The frequency with which these images are repeated depends on the nature of the patient’s illness, the activity of their disease, etc.
How do you make a case to administrators for more funding in MS centers?
Unfortunately, the downstream revenue does not always find its way back to the MS centers. Moreover, MS practitioners are forced to prove their value to the institution before they can receive the resources that they need.
There are only two things that administrators in medical institutions respond to. The first is need. The second is the financial impact of the activity.
Here is an example from my own personal experience. I prescribed a specific drug for a patient who did not live close to the institution. It took three months for the patient to receive the drug. This was due, in large measure, to problems with the insurance company and the outside specialty pharmacies that we were dealing with. In that course of time the patient suffered two relapses from which she never fully recovered.
I thought we could do a much better job treating our patients if our own specialty pharmacy was providing the drug. Eventually, after some negotiating with the administration, we were able to provide all those drugs through our specialty pharmacy. That change resulted in a significant increase in terms of contribution margin for the MS team, and it was a great benefit for our patients.
How does MS compare with other neurology disciplines?
If you look at the contribution margin from MS and compare it to in any other division in neurology, it exceeds all of them combined by a significant percentage.
For example, the current contribution margin in the MS division at the University of Pennsylvania exceeds that of virtually any other line within the Neuroscience Center Service, which includes neurosurgery. It is on par with, and may exceed, that of spine surgery, which in the past had always been the biggest driver of the contribution margin from the Neuroscience service line.
Often, MS specialists aren’t getting the resources needed despite the fact that their growing practice would enhance the contribution margin.
Since this has been brought to the attention of the administration at the University of Pennsylvania, there have been increased resources available for the division; we now have more nurses and nurse practitioners, and we have pharmacists within the division. All of this has made a big difference in helping to provide the best care for our patients.
How would you characterize the compensation of the MS specialist?
One of the things that I did address in the article, but only obliquely, is the compensation of the MS neurologist.
Historically, the MS neurologist was among the least compensated of all the neurology disciplines, in academics as well as in private practice. The reason for this was simple. Until the early 1990s, there were very few drugs to treat MS. It was more a matter of diagnosing people and treating the symptoms as they arose. When drugs for MS emerged, they were not particularly complex to manage.
However, as new drugs have become available, and the efficacy of these drugs increased, so did their side effect profiles. A need arose for specialists to manage the treatment of patients with MS.
I hope to address this further in a future publication, but the underlying assertion is that the compensation of the MS neurologist needs to be revisited at both academic institutions and in the community.
Final thoughts?
The article was an attempt to educate not just the MS community, but the broader neurologic community as to the value of an MS specialist to an institution.
The purpose of this article was to encourage people to think about their worth and the worth of what they do as it applies to the financial well-being of the institution with which they’re associated.
Dr. Joseph Berger’s article The Financial Contribution of the Multiple Sclerosis Specialist
(Neurol Clin Pract. 2017;7:246-255) is an eye-opening examination of how multiple sclerosis (MS) specialists fit into the economic framework of large academic institutions. We sat down with Dr. Berger to discuss his findings.
How would you describe the downstream revenue generated from MS specialists at large academic institutions?
The downstream revenue generated from MS is highly dependent on whether the drugs prescribed to patients are provided by the academic institution, and whether the infusions and imaging studies are done at the academic institution.
Another component is whether that institution operates under a Medicare 340B, a law that enables the institutions that are providing care to the underserved to acquire drugs at a steeply discounted price. And, as cited in the paper, the Office of the Inspector General of the Department of Health and Human Services estimated that the profit margin is 58% for all the drugs being provided under 340B. It’s important to note that that statistic accounts for all drugs, not a specific drug or a specific class of drugs. But, for the sake of argument let’s assume that it’s 50% for MS drugs.
The typical MS drug costs $60,000 a year if not more, and that means that 50% of that total goes to the contribution margin of the MS provider or the MS clinic. If there are 1000 patients for whom the specialty pharmacy within the institution is providing drugs, that means an enormous amount of money is returning to the institution as a result of the contribution by the MS practitioners.
In addition to the cost of the drugs, there’s also the cost of infusions associated with the drug that contributes substantially to the bottom line of the institution.
MS specialists tend to do more imaging studies than any other discipline. At the time of diagnosis, individuals with MS get MRIs of brain, cervical and thoracic spine, and frequently the orbits. The frequency with which these images are repeated depends on the nature of the patient’s illness, the activity of their disease, etc.
How do you make a case to administrators for more funding in MS centers?
Unfortunately, the downstream revenue does not always find its way back to the MS centers. Moreover, MS practitioners are forced to prove their value to the institution before they can receive the resources that they need.
There are only two things that administrators in medical institutions respond to. The first is need. The second is the financial impact of the activity.
Here is an example from my own personal experience. I prescribed a specific drug for a patient who did not live close to the institution. It took three months for the patient to receive the drug. This was due, in large measure, to problems with the insurance company and the outside specialty pharmacies that we were dealing with. In that course of time the patient suffered two relapses from which she never fully recovered.
I thought we could do a much better job treating our patients if our own specialty pharmacy was providing the drug. Eventually, after some negotiating with the administration, we were able to provide all those drugs through our specialty pharmacy. That change resulted in a significant increase in terms of contribution margin for the MS team, and it was a great benefit for our patients.
How does MS compare with other neurology disciplines?
If you look at the contribution margin from MS and compare it to in any other division in neurology, it exceeds all of them combined by a significant percentage.
For example, the current contribution margin in the MS division at the University of Pennsylvania exceeds that of virtually any other line within the Neuroscience Center Service, which includes neurosurgery. It is on par with, and may exceed, that of spine surgery, which in the past had always been the biggest driver of the contribution margin from the Neuroscience service line.
Often, MS specialists aren’t getting the resources needed despite the fact that their growing practice would enhance the contribution margin.
Since this has been brought to the attention of the administration at the University of Pennsylvania, there have been increased resources available for the division; we now have more nurses and nurse practitioners, and we have pharmacists within the division. All of this has made a big difference in helping to provide the best care for our patients.
How would you characterize the compensation of the MS specialist?
One of the things that I did address in the article, but only obliquely, is the compensation of the MS neurologist.
Historically, the MS neurologist was among the least compensated of all the neurology disciplines, in academics as well as in private practice. The reason for this was simple. Until the early 1990s, there were very few drugs to treat MS. It was more a matter of diagnosing people and treating the symptoms as they arose. When drugs for MS emerged, they were not particularly complex to manage.
However, as new drugs have become available, and the efficacy of these drugs increased, so did their side effect profiles. A need arose for specialists to manage the treatment of patients with MS.
I hope to address this further in a future publication, but the underlying assertion is that the compensation of the MS neurologist needs to be revisited at both academic institutions and in the community.
Final thoughts?
The article was an attempt to educate not just the MS community, but the broader neurologic community as to the value of an MS specialist to an institution.
The purpose of this article was to encourage people to think about their worth and the worth of what they do as it applies to the financial well-being of the institution with which they’re associated.
Dr. Joseph Berger’s article The Financial Contribution of the Multiple Sclerosis Specialist
(Neurol Clin Pract. 2017;7:246-255) is an eye-opening examination of how multiple sclerosis (MS) specialists fit into the economic framework of large academic institutions. We sat down with Dr. Berger to discuss his findings.
How would you describe the downstream revenue generated from MS specialists at large academic institutions?
The downstream revenue generated from MS is highly dependent on whether the drugs prescribed to patients are provided by the academic institution, and whether the infusions and imaging studies are done at the academic institution.
Another component is whether that institution operates under a Medicare 340B, a law that enables the institutions that are providing care to the underserved to acquire drugs at a steeply discounted price. And, as cited in the paper, the Office of the Inspector General of the Department of Health and Human Services estimated that the profit margin is 58% for all the drugs being provided under 340B. It’s important to note that that statistic accounts for all drugs, not a specific drug or a specific class of drugs. But, for the sake of argument let’s assume that it’s 50% for MS drugs.
The typical MS drug costs $60,000 a year if not more, and that means that 50% of that total goes to the contribution margin of the MS provider or the MS clinic. If there are 1000 patients for whom the specialty pharmacy within the institution is providing drugs, that means an enormous amount of money is returning to the institution as a result of the contribution by the MS practitioners.
In addition to the cost of the drugs, there’s also the cost of infusions associated with the drug that contributes substantially to the bottom line of the institution.
MS specialists tend to do more imaging studies than any other discipline. At the time of diagnosis, individuals with MS get MRIs of brain, cervical and thoracic spine, and frequently the orbits. The frequency with which these images are repeated depends on the nature of the patient’s illness, the activity of their disease, etc.
How do you make a case to administrators for more funding in MS centers?
Unfortunately, the downstream revenue does not always find its way back to the MS centers. Moreover, MS practitioners are forced to prove their value to the institution before they can receive the resources that they need.
There are only two things that administrators in medical institutions respond to. The first is need. The second is the financial impact of the activity.
Here is an example from my own personal experience. I prescribed a specific drug for a patient who did not live close to the institution. It took three months for the patient to receive the drug. This was due, in large measure, to problems with the insurance company and the outside specialty pharmacies that we were dealing with. In that course of time the patient suffered two relapses from which she never fully recovered.
I thought we could do a much better job treating our patients if our own specialty pharmacy was providing the drug. Eventually, after some negotiating with the administration, we were able to provide all those drugs through our specialty pharmacy. That change resulted in a significant increase in terms of contribution margin for the MS team, and it was a great benefit for our patients.
How does MS compare with other neurology disciplines?
If you look at the contribution margin from MS and compare it to in any other division in neurology, it exceeds all of them combined by a significant percentage.
For example, the current contribution margin in the MS division at the University of Pennsylvania exceeds that of virtually any other line within the Neuroscience Center Service, which includes neurosurgery. It is on par with, and may exceed, that of spine surgery, which in the past had always been the biggest driver of the contribution margin from the Neuroscience service line.
Often, MS specialists aren’t getting the resources needed despite the fact that their growing practice would enhance the contribution margin.
Since this has been brought to the attention of the administration at the University of Pennsylvania, there have been increased resources available for the division; we now have more nurses and nurse practitioners, and we have pharmacists within the division. All of this has made a big difference in helping to provide the best care for our patients.
How would you characterize the compensation of the MS specialist?
One of the things that I did address in the article, but only obliquely, is the compensation of the MS neurologist.
Historically, the MS neurologist was among the least compensated of all the neurology disciplines, in academics as well as in private practice. The reason for this was simple. Until the early 1990s, there were very few drugs to treat MS. It was more a matter of diagnosing people and treating the symptoms as they arose. When drugs for MS emerged, they were not particularly complex to manage.
However, as new drugs have become available, and the efficacy of these drugs increased, so did their side effect profiles. A need arose for specialists to manage the treatment of patients with MS.
I hope to address this further in a future publication, but the underlying assertion is that the compensation of the MS neurologist needs to be revisited at both academic institutions and in the community.
Final thoughts?
The article was an attempt to educate not just the MS community, but the broader neurologic community as to the value of an MS specialist to an institution.
The purpose of this article was to encourage people to think about their worth and the worth of what they do as it applies to the financial well-being of the institution with which they’re associated.
2019 Update on fertility
Professional societies, global organizations, and advocacy groups are continually working toward the goal of having the costs of infertility care covered by insurance carriers. Paramount to that effort is obtaining recognition of infertility as a burdensome disease. In this Update, we summarize national and international initiatives and societal trends that are helping to move us closer to that goal, and we encourage ObGyns to lead advocacy efforts.
Next, we detail several notable new features available in the annual report of the Society for Assisted Reproductive Technology (SART), an online interactive document that can be used to assist clinicians and patients in treatment decisions.
We also tackle the complexities of embryo selection for in vitro fertilization (IVF) and describe a potentially promising aneuploidy screening test, and explore its limitations.
Advances in recognizing infertility as a disease that merits insurance coverage
Major reproductive medicine organizations globally have endorsed the definition of infertility as a disease that "generates disability as an impairment of function" (TABLE 1).2 Fortunately, medical, societal, and judicial changes have resulted in progress for the 6.1 million women (and equivalent number of men) affected by infertility in the United States.3

Professional group advocacy efforts, and judicial rulings
The World Health Organization (WHO) has addressed infertility over the past several decades, with the organization's standards on semen analysis being the most recognized outcome. Progress has been limited, however, regarding global or national policy that recognizes the importance of infertility as a medical and public health problem.
In 2009, the glossary published by the WHO with the International Committee for Monitoring Assisted Reproductive Technology (ICMART) defined infertility as a disease.4 This recognition is important because it aids policy making, insurance coverage, and/or other payments for services.
The WHO also has begun the process of developing new infertility guidelines. Recently, the WHO held a summit on safety and access to fertility care, which was attended by many representatives of nation-state governments and international experts. It is hoped that a document from those proceedings will reinforce the public health importance of infertility and support the need to promote equality in access to safe fertility care. WHO initiatives matter because they apply to nation-states.
In the United States, the American Society for Reproductive Medicine (ASRM) for many years has recognized infertility as a disease. Only in 2017, however, did delegates at the American Medical Association's annual meeting vote to support the WHO's designation of infertility as a disease.
Continue to: Judicial views
Judicial views. In 1998, the US Supreme Court held that infertility is a disability under the Americans with Disabilities Act (ADA). The Court subsequently held, however, that a person is not considered disabled under the act if the disability can be overcome by mitigating or corrective measures. In 2000, a lower court held that, while infertility is a disability, an employer's health plan that excludes treatment for it is not discriminatory under the ADA if it applies to all employees.
Societal recognition. Interestingly, improved technology for oocyte cryopreservation has resulted in greater recognition of reproductive issues and the disparity in reproductive health societal norms and rights between men and women.
Media stories and gender issues in employment, especially in such high-profile industries as technology and finance, have highlighted long-standing inequities, many of which concern reproductive issues. These issues have been further disseminated by the #metoo movement. Some employers are beginning to respond by recognizing their employees' reproductive needs and providing improved benefits for reproductive care.
ObGyns must continue to lead advocacy
Not all has been progress. Personhood bills in several states threaten basic reproductive rights of women and men. The ASRM and Resolve (the National Infertility Association) have taken leading roles in opposing these legislative initiatives and supporting reproductive rights.5
Advocacy efforts through events and trends have resulted in gradually improving the recognition of the burden of infertility, inadequate insurance coverage, and continuing gender inequalities in reproduction. Today, patients, professionals, and national and international organizations are coalescing around demands for recognition, access to care, and gender and diversity equality. While much remains to be done, progress is being made in society, government, the workplace, and the health care system.
ObGyns and other women's health care providers can help continue the progress toward equality in reproductive rights, including access to infertility care, by discussing insurance inequities with patients, informing insurance companies that infertility is a disease, and encouraging patients to challenge inadequate and unequal insurance coverage of needed reproductive health care.
The time is now for ObGyns and other women’s health care providers to advocate for insurance coverage of infertility care. When our patients have inadequate coverage, we should encourage them to take action by contacting their insurance company and their employers to explain the reasons and argue for better coverage. Also, contact RESOLVE for additional information.
Latest SART report offers new features to aid in treatment decision making
Knowledge of the prognosis and its various treatment options is an important aspect of infertility treatment. The SART recently updated its annual Clinic Summary Report (CSR), which includes valuable new features for patients and physicians considering assisted reproductive technology (ART) treatment.6
SART compiles complex data and reports outcomes
The SART has been reporting IVF outcomes and other ART outcomes since 1988. The society's annual report is widely read by consumers, patients, physicians, and policy makers, and it has many important uses. However, the report is complicated and difficult to interpret for many reasons. For example, treatments are complex and varied (especially with application of new cryopreservation technology), and there are variations among clinics with respect to patient selection, protocols used, philosophy of practice, and numerous other variables.
Continue to: Because of this...
Because of this, the SART states, "The SART Clinic Summary Report (CSR) allows patients to view national and individual clinic IVF success rates. The data presented in this report should not be used for comparing clinics. Clinics may have differences in patient selection and treatment approaches which may artificially inflate or lower pregnancy rates relative to another clinic. Please discuss this with your doctor."6
Nevertheless, the CSR is extremely useful because it reports outcomes, which can lead to more informed patients and physicians and thus better access to safe and effective use of ART. The SART has redesigned the CSR to make it more useful.
Redesigned CSR focuses on outcomes important to patients
In recent years, new technologies have increased dramatically the use of embryo cryopreservation, genetic testing, and single embryo transfer (SET). The new CSR format is more patient focused and identifies more directly the treatment burden: ovarian stimulation, egg retrieval, intracytoplasmic sperm injection, preimplantation genetic testing (PGT), cryopreservation, frozen embryo transfer, and multiple cycles. It also focuses on the important patient outcomes, including live birth of a healthy child, multiple pregnancy, number of cycles, and chances of success per patient over time (including both fresh and frozen embryo transfers).
Notable changes
A major change in the CSR is that there is a preliminary report for a given year and then a final report the following year. This helps to more accurately report cycles that have been "delayed" because of egg retrieval and embryo freezing performed in the reported year but then transferred in the following reporting year.
Cycle counting. A cycle is counted when a woman has started medications for an ART procedure or, in a "natural" cycle when no medications are used, the first day of menses of the ART cycle. If several cycles are performed to bank eggs or embryos, each will be counted in the denominator when calculating the pregnancy rate. This more accurately reflects the patient treatment burden and costs. A cycle cancelled before egg retrieval is still counted as a cycle.
Defining success. Success is characterized as delivery of a child, since this is the outcome patients desire. Singleton deliveries are emphasized, since twin and higher-order multiple pregnancies have a higher risk of prematurity, morbidity, mortality, and cost. The percentages of triplet, twin, and singleton births contributing to the live birth rate are provided for each cycle group, as is prematurity (TABLE 2).6
The end point of a treatment cycle can vary. The new CSR captures the success rate following one or more egg retrievals and the first embryo transfer (primary outcome), the success of subsequent cycles using frozen eggs or embryos not transferred in the first embryo transfer, and the combined contribution of the primary and subsequent cycles to the cumulative live birth rate for a patient both in the preliminary report and the final report for any given year. The live birth rate per patient also is reported and includes the outcomes for patients who are new to an infertility center and starting their first cycle for retrieval of their own eggs during the reporting year.
Continue to: Outcomes and prognostic factors...
Outcomes and prognostic factors. Outcomes are reported by multiple factors, including patient age and source of the eggs. These are important prognostic factors; separating the data allows you to obtain a better idea of both national and individual clinic experience by these factors.
The CSR also contains filters for infertility diagnosis, stimulation type, and other treatment details (FIGURE).6 The filter is a useful feature because multiple types of treatment can be included or excluded. The outcome of different treatment interventions can then be estimated based on outcomes from the entire sample of US patients with similar characteristics and interventions. This powerful tool can help patients and physicians choose the best treatment based on prognosis.
Personalized prognosis. An important new feature is the SART Patient Predictor (https://www.sartcorsonline.com/predictor/patient), a model that permits an individual patient to obtain a more personalized prognosis. While the SART predictor uses only basic patient information, such as age, body mass index, and diagnosis, its estimate is based on the entire US sample of reported ART experience and therefore can help patients in decision making. Furthermore, the predictor calculates percentages for the outcome of one transfer of 2 embryos, and 2 transfers of a single embryo, to demonstrate the advantages of SET that result in a higher live birth rate but a significantly lower multiple pregnancy rate.
Summing up
The SART's new CSR is extremely useful to patients and to any physician who cares for infertility patients. It can help users both understand the expected results from different ART treatments and enable better physician-patient communication and decision making.
The updated annual SART Clinic Summary Report is an exceptionally valuable and easy-to-use online tool for you and your infertility patients.
Embryo selection techniques refined with use of newer technologies
Since the introduction of IVF in 1978, the final cumulative live birth rates per cycle initiated for oocyte retrieval after all resulting embryos have been trasferred continue to rise, currently standing at 54% for women younger than age 35 in the United States.7 A number of achievements have contributed to this remarkable success, namely, improvements in IVF laboratory and embryo culture systems, advances in cryopreservation technology, availability of highly effective gonadotropins and gonadotropin-releasing hormone analogues, improved ultrasound technology, and the introduction of soft catheters for atraumatic embryo transfers.
Treatment now focuses on improved embryo selection
Now that excellent success rates have been attained, the focus of optimizing efforts in fertility treatment has shifted to improving safety by reducing the rates of multiple pregnancy through elective single embryo transfer (eSET), reducing the rates of miscarriage, and shortening the time to live birth. Methods to improve embryo selection lie at the forefront of these initiatives. These vary and include extended culture to blastocyst stage, standard morphologic evaluation as well as morphokinetic assessment of embryonic development via time-lapse imaging, and more recently the reintroduction of preimplantation genetic testing for aneuploidy (PGT-A), formerly known as preimplantation genetic screening (PGS).
Chromosomal abnormalities of the embryo, or embryo aneuploidies, are the most common cause of treatment failure following embryo transfer in IVF. The proportion of embryos affected with aneuploidies significantly increases with advancing maternal age: 40% to 50% of blastocysts in women younger than age 35 and about 90% of blastocysts in women older than age 42.8 The premise with PGT-A is to identify these aneuploid embryos and increase the chances of success per embryo transfer by transferring euploid embryos.
Continue to: That concept was initially applied...
That concept was initially applied to cleavage-stage embryos through the use of fluorescence in situ hybridization (FISH) technology to interrogate a maximum of 5 to 9 chromosomes in a single cell (single blastomere); however, although initial results from observational studies were encouraging, subsequent randomized controlled studies unexpectedly showed a reduction in pregnancy rates.9 This was attributed to several factors, including biopsy-related damage to the cleavage-stage embryo, inability of FISH technology to assess aneuploidies of more than 5 to 9 chromosomes, mosaicism, and technical limitations associated with single-cell analysis.
Second-generation PGT-A testing has promise, and limitations
The newer PGT-A tests the embryos at the blastocyst stage by using biopsy samples from the trophectoderm (which will form the future placenta); this is expected to spare the inner cell mass ([ICM] which will give rise to the embryo proper) from biopsy-related injury.
On the genetics side, newer technologies, such as array comparative genomic hybridization, single nucleotide polymorphism arrays, quantitative polymerase chain reaction, and next-generation sequencing, offer the opportunity to assess all 24 chromosomes in a single biopsy specimen. Although a detailed discussion of these testing platforms is beyond the scope of this Update, certain points are worth mentioning. All these technologies require some form of genetic material amplification (most commonly whole genome amplification or multiplex polymerase chain reaction) to increase the relatively scant amount of DNA obtained from a sample of 4 to 6 cells. These amplification techniques have limitations that can subsequently impact the validity of the test results.
Furthermore, there is no consistency in depth of coverage for various parts of the genome, and subchromosomal (segmental) copy number variations below 3 to 5 Mb may not be detected. The threshold used in bioinformatics algorithms employed to interpret the raw data is subject to several biases and is not consistent among laboratories. As a result, the same sample assessed in different laboratories can potentially yield different results.
In addition to these technical limitations, mosaicism can pose another biologic limitation, as the biopsied trophectoderm cells may not accurately represent the chromosomal makeup of the ICM. Also, an embryo may be able to undergo self-correction during subsequent stages of development, and therefore even a documented trophectoderm abnormality at the blastocyst stage may not necessarily preclude that embryo from developing into a healthy baby.
Standardization is needed. Despite widespread promotion of PGT-A, well-designed randomized clinical trials (RCTs) have not yet consistently shown its benefits in improving pregnancy rates or reducing miscarriage rates. Although the initial small RCTs in a selected group of good prognosis patients suggested a beneficial effect in ongoing pregnancy rates per transfer, the largest multicenter RCT to date did not show any improvement in pregnancy rates or reduction in miscarriage rates.10 In that study, a post hoc subgroup analysis suggested a possible beneficial effect in women aged 35 to 40. However, those results must be validated and reproduced with randomization at the start of stimulation, with the primary outcome being the live birth rate per initiated cycle, instead of per transfer, before PGT-A can be adopted universally in clinical practice.
Continue to: With all the above considerations...
With all the above considerations, the ASRM has appropriately concluded that "the value of preimplantation genetic testing for aneuploidy (PGT-A) as a screening test for IVF patients has yet to be determined."11
Standardization of clinical and laboratory protocols and additional studies to assess the effects of PGT-A on live birth rates per initiated cycles are recommended before this new technology is widely adopted in routine clinical practice. In our practice, we routinely offer and perform extended culture to blastocyst stage and standard morphologic assessment. After a thorough counseling on the current status of PGT-A, about 15% to 20% of our patients opt to undergo PGT-A.
- United Nations website. General Assembly resolution 217A: Declaration of human rights. December 10, 1948. http://www.un.org/en/universal-declara tion-human-rights/. Accessed January 11, 2019.
- Zegers-Hochschild F, Adamson GD, Dyer S, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108:393-406.
- US Department of Health and Human Services Office on Women's Health website. Infertility. https://www.womenshealth.gov/a-z-topics/infertility. Accessed January 24, 2019.
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al; International Committee for Monitoring Assisted Reproductive Technology, World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92:1520-1524.
- RESOLVE: The National Infertility Association website. Opposing personhood: Resolve fights to keep fertility medical treatments legal in the US. https://resolve.org/get-involved/advocate-for-access/our-issues/opposing-personhood/. Accessed January 11, 2019.
- Society for Assisted Reproductive Technology website. National summary report. 2016 Preliminary national data. https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?reportingYear=2016 . Accessed January 12, 2019.
- Society for Assisted Reproductive Technology website. National summary report 2015. https://www.sartcorsonline,com/rptCSR_PublicMultYear.aspx ?reportingYear=2015. Accessed January 12, 2019.
- Harton GL, Munne S, Surrey M, et al; PGD Practitioners Group. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril. 2013;100:1695-1703.
- Mastenbroek S, Twisk M, van Echten-Arends, et al. In vitro fertilization with preimplantation genetic screening. N Engl J Med. 2007;357:9-17
- Munne S, Kaplan B, Frattarelli JL, et al. Global multicenter randomized controlled trial comparing single embryo transfer with embryo selected by preimplantation genetic screening using next-generation sequencing versus morphologic assessment [abstract O-43]. Fertil Steril. 2017;108(suppl):e19.
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018;109:429-436.
Professional societies, global organizations, and advocacy groups are continually working toward the goal of having the costs of infertility care covered by insurance carriers. Paramount to that effort is obtaining recognition of infertility as a burdensome disease. In this Update, we summarize national and international initiatives and societal trends that are helping to move us closer to that goal, and we encourage ObGyns to lead advocacy efforts.
Next, we detail several notable new features available in the annual report of the Society for Assisted Reproductive Technology (SART), an online interactive document that can be used to assist clinicians and patients in treatment decisions.
We also tackle the complexities of embryo selection for in vitro fertilization (IVF) and describe a potentially promising aneuploidy screening test, and explore its limitations.
Advances in recognizing infertility as a disease that merits insurance coverage
Major reproductive medicine organizations globally have endorsed the definition of infertility as a disease that "generates disability as an impairment of function" (TABLE 1).2 Fortunately, medical, societal, and judicial changes have resulted in progress for the 6.1 million women (and equivalent number of men) affected by infertility in the United States.3

Professional group advocacy efforts, and judicial rulings
The World Health Organization (WHO) has addressed infertility over the past several decades, with the organization's standards on semen analysis being the most recognized outcome. Progress has been limited, however, regarding global or national policy that recognizes the importance of infertility as a medical and public health problem.
In 2009, the glossary published by the WHO with the International Committee for Monitoring Assisted Reproductive Technology (ICMART) defined infertility as a disease.4 This recognition is important because it aids policy making, insurance coverage, and/or other payments for services.
The WHO also has begun the process of developing new infertility guidelines. Recently, the WHO held a summit on safety and access to fertility care, which was attended by many representatives of nation-state governments and international experts. It is hoped that a document from those proceedings will reinforce the public health importance of infertility and support the need to promote equality in access to safe fertility care. WHO initiatives matter because they apply to nation-states.
In the United States, the American Society for Reproductive Medicine (ASRM) for many years has recognized infertility as a disease. Only in 2017, however, did delegates at the American Medical Association's annual meeting vote to support the WHO's designation of infertility as a disease.
Continue to: Judicial views
Judicial views. In 1998, the US Supreme Court held that infertility is a disability under the Americans with Disabilities Act (ADA). The Court subsequently held, however, that a person is not considered disabled under the act if the disability can be overcome by mitigating or corrective measures. In 2000, a lower court held that, while infertility is a disability, an employer's health plan that excludes treatment for it is not discriminatory under the ADA if it applies to all employees.
Societal recognition. Interestingly, improved technology for oocyte cryopreservation has resulted in greater recognition of reproductive issues and the disparity in reproductive health societal norms and rights between men and women.
Media stories and gender issues in employment, especially in such high-profile industries as technology and finance, have highlighted long-standing inequities, many of which concern reproductive issues. These issues have been further disseminated by the #metoo movement. Some employers are beginning to respond by recognizing their employees' reproductive needs and providing improved benefits for reproductive care.
ObGyns must continue to lead advocacy
Not all has been progress. Personhood bills in several states threaten basic reproductive rights of women and men. The ASRM and Resolve (the National Infertility Association) have taken leading roles in opposing these legislative initiatives and supporting reproductive rights.5
Advocacy efforts through events and trends have resulted in gradually improving the recognition of the burden of infertility, inadequate insurance coverage, and continuing gender inequalities in reproduction. Today, patients, professionals, and national and international organizations are coalescing around demands for recognition, access to care, and gender and diversity equality. While much remains to be done, progress is being made in society, government, the workplace, and the health care system.
ObGyns and other women's health care providers can help continue the progress toward equality in reproductive rights, including access to infertility care, by discussing insurance inequities with patients, informing insurance companies that infertility is a disease, and encouraging patients to challenge inadequate and unequal insurance coverage of needed reproductive health care.
The time is now for ObGyns and other women’s health care providers to advocate for insurance coverage of infertility care. When our patients have inadequate coverage, we should encourage them to take action by contacting their insurance company and their employers to explain the reasons and argue for better coverage. Also, contact RESOLVE for additional information.
Latest SART report offers new features to aid in treatment decision making
Knowledge of the prognosis and its various treatment options is an important aspect of infertility treatment. The SART recently updated its annual Clinic Summary Report (CSR), which includes valuable new features for patients and physicians considering assisted reproductive technology (ART) treatment.6
SART compiles complex data and reports outcomes
The SART has been reporting IVF outcomes and other ART outcomes since 1988. The society's annual report is widely read by consumers, patients, physicians, and policy makers, and it has many important uses. However, the report is complicated and difficult to interpret for many reasons. For example, treatments are complex and varied (especially with application of new cryopreservation technology), and there are variations among clinics with respect to patient selection, protocols used, philosophy of practice, and numerous other variables.
Continue to: Because of this...
Because of this, the SART states, "The SART Clinic Summary Report (CSR) allows patients to view national and individual clinic IVF success rates. The data presented in this report should not be used for comparing clinics. Clinics may have differences in patient selection and treatment approaches which may artificially inflate or lower pregnancy rates relative to another clinic. Please discuss this with your doctor."6
Nevertheless, the CSR is extremely useful because it reports outcomes, which can lead to more informed patients and physicians and thus better access to safe and effective use of ART. The SART has redesigned the CSR to make it more useful.
Redesigned CSR focuses on outcomes important to patients
In recent years, new technologies have increased dramatically the use of embryo cryopreservation, genetic testing, and single embryo transfer (SET). The new CSR format is more patient focused and identifies more directly the treatment burden: ovarian stimulation, egg retrieval, intracytoplasmic sperm injection, preimplantation genetic testing (PGT), cryopreservation, frozen embryo transfer, and multiple cycles. It also focuses on the important patient outcomes, including live birth of a healthy child, multiple pregnancy, number of cycles, and chances of success per patient over time (including both fresh and frozen embryo transfers).
Notable changes
A major change in the CSR is that there is a preliminary report for a given year and then a final report the following year. This helps to more accurately report cycles that have been "delayed" because of egg retrieval and embryo freezing performed in the reported year but then transferred in the following reporting year.
Cycle counting. A cycle is counted when a woman has started medications for an ART procedure or, in a "natural" cycle when no medications are used, the first day of menses of the ART cycle. If several cycles are performed to bank eggs or embryos, each will be counted in the denominator when calculating the pregnancy rate. This more accurately reflects the patient treatment burden and costs. A cycle cancelled before egg retrieval is still counted as a cycle.
Defining success. Success is characterized as delivery of a child, since this is the outcome patients desire. Singleton deliveries are emphasized, since twin and higher-order multiple pregnancies have a higher risk of prematurity, morbidity, mortality, and cost. The percentages of triplet, twin, and singleton births contributing to the live birth rate are provided for each cycle group, as is prematurity (TABLE 2).6
The end point of a treatment cycle can vary. The new CSR captures the success rate following one or more egg retrievals and the first embryo transfer (primary outcome), the success of subsequent cycles using frozen eggs or embryos not transferred in the first embryo transfer, and the combined contribution of the primary and subsequent cycles to the cumulative live birth rate for a patient both in the preliminary report and the final report for any given year. The live birth rate per patient also is reported and includes the outcomes for patients who are new to an infertility center and starting their first cycle for retrieval of their own eggs during the reporting year.
Continue to: Outcomes and prognostic factors...
Outcomes and prognostic factors. Outcomes are reported by multiple factors, including patient age and source of the eggs. These are important prognostic factors; separating the data allows you to obtain a better idea of both national and individual clinic experience by these factors.
The CSR also contains filters for infertility diagnosis, stimulation type, and other treatment details (FIGURE).6 The filter is a useful feature because multiple types of treatment can be included or excluded. The outcome of different treatment interventions can then be estimated based on outcomes from the entire sample of US patients with similar characteristics and interventions. This powerful tool can help patients and physicians choose the best treatment based on prognosis.

Personalized prognosis. An important new feature is the SART Patient Predictor (https://www.sartcorsonline.com/predictor/patient), a model that permits an individual patient to obtain a more personalized prognosis. While the SART predictor uses only basic patient information, such as age, body mass index, and diagnosis, its estimate is based on the entire US sample of reported ART experience and therefore can help patients in decision making. Furthermore, the predictor calculates percentages for the outcome of one transfer of 2 embryos, and 2 transfers of a single embryo, to demonstrate the advantages of SET that result in a higher live birth rate but a significantly lower multiple pregnancy rate.

Summing up
The SART's new CSR is extremely useful to patients and to any physician who cares for infertility patients. It can help users both understand the expected results from different ART treatments and enable better physician-patient communication and decision making.
The updated annual SART Clinic Summary Report is an exceptionally valuable and easy-to-use online tool for you and your infertility patients.
Embryo selection techniques refined with use of newer technologies
Since the introduction of IVF in 1978, the final cumulative live birth rates per cycle initiated for oocyte retrieval after all resulting embryos have been trasferred continue to rise, currently standing at 54% for women younger than age 35 in the United States.7 A number of achievements have contributed to this remarkable success, namely, improvements in IVF laboratory and embryo culture systems, advances in cryopreservation technology, availability of highly effective gonadotropins and gonadotropin-releasing hormone analogues, improved ultrasound technology, and the introduction of soft catheters for atraumatic embryo transfers.
Treatment now focuses on improved embryo selection
Now that excellent success rates have been attained, the focus of optimizing efforts in fertility treatment has shifted to improving safety by reducing the rates of multiple pregnancy through elective single embryo transfer (eSET), reducing the rates of miscarriage, and shortening the time to live birth. Methods to improve embryo selection lie at the forefront of these initiatives. These vary and include extended culture to blastocyst stage, standard morphologic evaluation as well as morphokinetic assessment of embryonic development via time-lapse imaging, and more recently the reintroduction of preimplantation genetic testing for aneuploidy (PGT-A), formerly known as preimplantation genetic screening (PGS).
Chromosomal abnormalities of the embryo, or embryo aneuploidies, are the most common cause of treatment failure following embryo transfer in IVF. The proportion of embryos affected with aneuploidies significantly increases with advancing maternal age: 40% to 50% of blastocysts in women younger than age 35 and about 90% of blastocysts in women older than age 42.8 The premise with PGT-A is to identify these aneuploid embryos and increase the chances of success per embryo transfer by transferring euploid embryos.
Continue to: That concept was initially applied...
That concept was initially applied to cleavage-stage embryos through the use of fluorescence in situ hybridization (FISH) technology to interrogate a maximum of 5 to 9 chromosomes in a single cell (single blastomere); however, although initial results from observational studies were encouraging, subsequent randomized controlled studies unexpectedly showed a reduction in pregnancy rates.9 This was attributed to several factors, including biopsy-related damage to the cleavage-stage embryo, inability of FISH technology to assess aneuploidies of more than 5 to 9 chromosomes, mosaicism, and technical limitations associated with single-cell analysis.
Second-generation PGT-A testing has promise, and limitations
The newer PGT-A tests the embryos at the blastocyst stage by using biopsy samples from the trophectoderm (which will form the future placenta); this is expected to spare the inner cell mass ([ICM] which will give rise to the embryo proper) from biopsy-related injury.
On the genetics side, newer technologies, such as array comparative genomic hybridization, single nucleotide polymorphism arrays, quantitative polymerase chain reaction, and next-generation sequencing, offer the opportunity to assess all 24 chromosomes in a single biopsy specimen. Although a detailed discussion of these testing platforms is beyond the scope of this Update, certain points are worth mentioning. All these technologies require some form of genetic material amplification (most commonly whole genome amplification or multiplex polymerase chain reaction) to increase the relatively scant amount of DNA obtained from a sample of 4 to 6 cells. These amplification techniques have limitations that can subsequently impact the validity of the test results.
Furthermore, there is no consistency in depth of coverage for various parts of the genome, and subchromosomal (segmental) copy number variations below 3 to 5 Mb may not be detected. The threshold used in bioinformatics algorithms employed to interpret the raw data is subject to several biases and is not consistent among laboratories. As a result, the same sample assessed in different laboratories can potentially yield different results.
In addition to these technical limitations, mosaicism can pose another biologic limitation, as the biopsied trophectoderm cells may not accurately represent the chromosomal makeup of the ICM. Also, an embryo may be able to undergo self-correction during subsequent stages of development, and therefore even a documented trophectoderm abnormality at the blastocyst stage may not necessarily preclude that embryo from developing into a healthy baby.
Standardization is needed. Despite widespread promotion of PGT-A, well-designed randomized clinical trials (RCTs) have not yet consistently shown its benefits in improving pregnancy rates or reducing miscarriage rates. Although the initial small RCTs in a selected group of good prognosis patients suggested a beneficial effect in ongoing pregnancy rates per transfer, the largest multicenter RCT to date did not show any improvement in pregnancy rates or reduction in miscarriage rates.10 In that study, a post hoc subgroup analysis suggested a possible beneficial effect in women aged 35 to 40. However, those results must be validated and reproduced with randomization at the start of stimulation, with the primary outcome being the live birth rate per initiated cycle, instead of per transfer, before PGT-A can be adopted universally in clinical practice.
Continue to: With all the above considerations...
With all the above considerations, the ASRM has appropriately concluded that "the value of preimplantation genetic testing for aneuploidy (PGT-A) as a screening test for IVF patients has yet to be determined."11
Standardization of clinical and laboratory protocols and additional studies to assess the effects of PGT-A on live birth rates per initiated cycles are recommended before this new technology is widely adopted in routine clinical practice. In our practice, we routinely offer and perform extended culture to blastocyst stage and standard morphologic assessment. After a thorough counseling on the current status of PGT-A, about 15% to 20% of our patients opt to undergo PGT-A.
Professional societies, global organizations, and advocacy groups are continually working toward the goal of having the costs of infertility care covered by insurance carriers. Paramount to that effort is obtaining recognition of infertility as a burdensome disease. In this Update, we summarize national and international initiatives and societal trends that are helping to move us closer to that goal, and we encourage ObGyns to lead advocacy efforts.
Next, we detail several notable new features available in the annual report of the Society for Assisted Reproductive Technology (SART), an online interactive document that can be used to assist clinicians and patients in treatment decisions.
We also tackle the complexities of embryo selection for in vitro fertilization (IVF) and describe a potentially promising aneuploidy screening test, and explore its limitations.
Advances in recognizing infertility as a disease that merits insurance coverage
Major reproductive medicine organizations globally have endorsed the definition of infertility as a disease that "generates disability as an impairment of function" (TABLE 1).2 Fortunately, medical, societal, and judicial changes have resulted in progress for the 6.1 million women (and equivalent number of men) affected by infertility in the United States.3

Professional group advocacy efforts, and judicial rulings
The World Health Organization (WHO) has addressed infertility over the past several decades, with the organization's standards on semen analysis being the most recognized outcome. Progress has been limited, however, regarding global or national policy that recognizes the importance of infertility as a medical and public health problem.
In 2009, the glossary published by the WHO with the International Committee for Monitoring Assisted Reproductive Technology (ICMART) defined infertility as a disease.4 This recognition is important because it aids policy making, insurance coverage, and/or other payments for services.
The WHO also has begun the process of developing new infertility guidelines. Recently, the WHO held a summit on safety and access to fertility care, which was attended by many representatives of nation-state governments and international experts. It is hoped that a document from those proceedings will reinforce the public health importance of infertility and support the need to promote equality in access to safe fertility care. WHO initiatives matter because they apply to nation-states.
In the United States, the American Society for Reproductive Medicine (ASRM) for many years has recognized infertility as a disease. Only in 2017, however, did delegates at the American Medical Association's annual meeting vote to support the WHO's designation of infertility as a disease.
Continue to: Judicial views
Judicial views. In 1998, the US Supreme Court held that infertility is a disability under the Americans with Disabilities Act (ADA). The Court subsequently held, however, that a person is not considered disabled under the act if the disability can be overcome by mitigating or corrective measures. In 2000, a lower court held that, while infertility is a disability, an employer's health plan that excludes treatment for it is not discriminatory under the ADA if it applies to all employees.
Societal recognition. Interestingly, improved technology for oocyte cryopreservation has resulted in greater recognition of reproductive issues and the disparity in reproductive health societal norms and rights between men and women.
Media stories and gender issues in employment, especially in such high-profile industries as technology and finance, have highlighted long-standing inequities, many of which concern reproductive issues. These issues have been further disseminated by the #metoo movement. Some employers are beginning to respond by recognizing their employees' reproductive needs and providing improved benefits for reproductive care.
ObGyns must continue to lead advocacy
Not all has been progress. Personhood bills in several states threaten basic reproductive rights of women and men. The ASRM and Resolve (the National Infertility Association) have taken leading roles in opposing these legislative initiatives and supporting reproductive rights.5
Advocacy efforts through events and trends have resulted in gradually improving the recognition of the burden of infertility, inadequate insurance coverage, and continuing gender inequalities in reproduction. Today, patients, professionals, and national and international organizations are coalescing around demands for recognition, access to care, and gender and diversity equality. While much remains to be done, progress is being made in society, government, the workplace, and the health care system.
ObGyns and other women's health care providers can help continue the progress toward equality in reproductive rights, including access to infertility care, by discussing insurance inequities with patients, informing insurance companies that infertility is a disease, and encouraging patients to challenge inadequate and unequal insurance coverage of needed reproductive health care.
The time is now for ObGyns and other women’s health care providers to advocate for insurance coverage of infertility care. When our patients have inadequate coverage, we should encourage them to take action by contacting their insurance company and their employers to explain the reasons and argue for better coverage. Also, contact RESOLVE for additional information.
Latest SART report offers new features to aid in treatment decision making
Knowledge of the prognosis and its various treatment options is an important aspect of infertility treatment. The SART recently updated its annual Clinic Summary Report (CSR), which includes valuable new features for patients and physicians considering assisted reproductive technology (ART) treatment.6
SART compiles complex data and reports outcomes
The SART has been reporting IVF outcomes and other ART outcomes since 1988. The society's annual report is widely read by consumers, patients, physicians, and policy makers, and it has many important uses. However, the report is complicated and difficult to interpret for many reasons. For example, treatments are complex and varied (especially with application of new cryopreservation technology), and there are variations among clinics with respect to patient selection, protocols used, philosophy of practice, and numerous other variables.
Continue to: Because of this...
Because of this, the SART states, "The SART Clinic Summary Report (CSR) allows patients to view national and individual clinic IVF success rates. The data presented in this report should not be used for comparing clinics. Clinics may have differences in patient selection and treatment approaches which may artificially inflate or lower pregnancy rates relative to another clinic. Please discuss this with your doctor."6
Nevertheless, the CSR is extremely useful because it reports outcomes, which can lead to more informed patients and physicians and thus better access to safe and effective use of ART. The SART has redesigned the CSR to make it more useful.
Redesigned CSR focuses on outcomes important to patients
In recent years, new technologies have increased dramatically the use of embryo cryopreservation, genetic testing, and single embryo transfer (SET). The new CSR format is more patient focused and identifies more directly the treatment burden: ovarian stimulation, egg retrieval, intracytoplasmic sperm injection, preimplantation genetic testing (PGT), cryopreservation, frozen embryo transfer, and multiple cycles. It also focuses on the important patient outcomes, including live birth of a healthy child, multiple pregnancy, number of cycles, and chances of success per patient over time (including both fresh and frozen embryo transfers).
Notable changes
A major change in the CSR is that there is a preliminary report for a given year and then a final report the following year. This helps to more accurately report cycles that have been "delayed" because of egg retrieval and embryo freezing performed in the reported year but then transferred in the following reporting year.
Cycle counting. A cycle is counted when a woman has started medications for an ART procedure or, in a "natural" cycle when no medications are used, the first day of menses of the ART cycle. If several cycles are performed to bank eggs or embryos, each will be counted in the denominator when calculating the pregnancy rate. This more accurately reflects the patient treatment burden and costs. A cycle cancelled before egg retrieval is still counted as a cycle.
Defining success. Success is characterized as delivery of a child, since this is the outcome patients desire. Singleton deliveries are emphasized, since twin and higher-order multiple pregnancies have a higher risk of prematurity, morbidity, mortality, and cost. The percentages of triplet, twin, and singleton births contributing to the live birth rate are provided for each cycle group, as is prematurity (TABLE 2).6
The end point of a treatment cycle can vary. The new CSR captures the success rate following one or more egg retrievals and the first embryo transfer (primary outcome), the success of subsequent cycles using frozen eggs or embryos not transferred in the first embryo transfer, and the combined contribution of the primary and subsequent cycles to the cumulative live birth rate for a patient both in the preliminary report and the final report for any given year. The live birth rate per patient also is reported and includes the outcomes for patients who are new to an infertility center and starting their first cycle for retrieval of their own eggs during the reporting year.
Continue to: Outcomes and prognostic factors...
Outcomes and prognostic factors. Outcomes are reported by multiple factors, including patient age and source of the eggs. These are important prognostic factors; separating the data allows you to obtain a better idea of both national and individual clinic experience by these factors.
The CSR also contains filters for infertility diagnosis, stimulation type, and other treatment details (FIGURE).6 The filter is a useful feature because multiple types of treatment can be included or excluded. The outcome of different treatment interventions can then be estimated based on outcomes from the entire sample of US patients with similar characteristics and interventions. This powerful tool can help patients and physicians choose the best treatment based on prognosis.

Personalized prognosis. An important new feature is the SART Patient Predictor (https://www.sartcorsonline.com/predictor/patient), a model that permits an individual patient to obtain a more personalized prognosis. While the SART predictor uses only basic patient information, such as age, body mass index, and diagnosis, its estimate is based on the entire US sample of reported ART experience and therefore can help patients in decision making. Furthermore, the predictor calculates percentages for the outcome of one transfer of 2 embryos, and 2 transfers of a single embryo, to demonstrate the advantages of SET that result in a higher live birth rate but a significantly lower multiple pregnancy rate.

Summing up
The SART's new CSR is extremely useful to patients and to any physician who cares for infertility patients. It can help users both understand the expected results from different ART treatments and enable better physician-patient communication and decision making.
The updated annual SART Clinic Summary Report is an exceptionally valuable and easy-to-use online tool for you and your infertility patients.
Embryo selection techniques refined with use of newer technologies
Since the introduction of IVF in 1978, the final cumulative live birth rates per cycle initiated for oocyte retrieval after all resulting embryos have been trasferred continue to rise, currently standing at 54% for women younger than age 35 in the United States.7 A number of achievements have contributed to this remarkable success, namely, improvements in IVF laboratory and embryo culture systems, advances in cryopreservation technology, availability of highly effective gonadotropins and gonadotropin-releasing hormone analogues, improved ultrasound technology, and the introduction of soft catheters for atraumatic embryo transfers.
Treatment now focuses on improved embryo selection
Now that excellent success rates have been attained, the focus of optimizing efforts in fertility treatment has shifted to improving safety by reducing the rates of multiple pregnancy through elective single embryo transfer (eSET), reducing the rates of miscarriage, and shortening the time to live birth. Methods to improve embryo selection lie at the forefront of these initiatives. These vary and include extended culture to blastocyst stage, standard morphologic evaluation as well as morphokinetic assessment of embryonic development via time-lapse imaging, and more recently the reintroduction of preimplantation genetic testing for aneuploidy (PGT-A), formerly known as preimplantation genetic screening (PGS).
Chromosomal abnormalities of the embryo, or embryo aneuploidies, are the most common cause of treatment failure following embryo transfer in IVF. The proportion of embryos affected with aneuploidies significantly increases with advancing maternal age: 40% to 50% of blastocysts in women younger than age 35 and about 90% of blastocysts in women older than age 42.8 The premise with PGT-A is to identify these aneuploid embryos and increase the chances of success per embryo transfer by transferring euploid embryos.
Continue to: That concept was initially applied...
That concept was initially applied to cleavage-stage embryos through the use of fluorescence in situ hybridization (FISH) technology to interrogate a maximum of 5 to 9 chromosomes in a single cell (single blastomere); however, although initial results from observational studies were encouraging, subsequent randomized controlled studies unexpectedly showed a reduction in pregnancy rates.9 This was attributed to several factors, including biopsy-related damage to the cleavage-stage embryo, inability of FISH technology to assess aneuploidies of more than 5 to 9 chromosomes, mosaicism, and technical limitations associated with single-cell analysis.
Second-generation PGT-A testing has promise, and limitations
The newer PGT-A tests the embryos at the blastocyst stage by using biopsy samples from the trophectoderm (which will form the future placenta); this is expected to spare the inner cell mass ([ICM] which will give rise to the embryo proper) from biopsy-related injury.
On the genetics side, newer technologies, such as array comparative genomic hybridization, single nucleotide polymorphism arrays, quantitative polymerase chain reaction, and next-generation sequencing, offer the opportunity to assess all 24 chromosomes in a single biopsy specimen. Although a detailed discussion of these testing platforms is beyond the scope of this Update, certain points are worth mentioning. All these technologies require some form of genetic material amplification (most commonly whole genome amplification or multiplex polymerase chain reaction) to increase the relatively scant amount of DNA obtained from a sample of 4 to 6 cells. These amplification techniques have limitations that can subsequently impact the validity of the test results.
Furthermore, there is no consistency in depth of coverage for various parts of the genome, and subchromosomal (segmental) copy number variations below 3 to 5 Mb may not be detected. The threshold used in bioinformatics algorithms employed to interpret the raw data is subject to several biases and is not consistent among laboratories. As a result, the same sample assessed in different laboratories can potentially yield different results.
In addition to these technical limitations, mosaicism can pose another biologic limitation, as the biopsied trophectoderm cells may not accurately represent the chromosomal makeup of the ICM. Also, an embryo may be able to undergo self-correction during subsequent stages of development, and therefore even a documented trophectoderm abnormality at the blastocyst stage may not necessarily preclude that embryo from developing into a healthy baby.
Standardization is needed. Despite widespread promotion of PGT-A, well-designed randomized clinical trials (RCTs) have not yet consistently shown its benefits in improving pregnancy rates or reducing miscarriage rates. Although the initial small RCTs in a selected group of good prognosis patients suggested a beneficial effect in ongoing pregnancy rates per transfer, the largest multicenter RCT to date did not show any improvement in pregnancy rates or reduction in miscarriage rates.10 In that study, a post hoc subgroup analysis suggested a possible beneficial effect in women aged 35 to 40. However, those results must be validated and reproduced with randomization at the start of stimulation, with the primary outcome being the live birth rate per initiated cycle, instead of per transfer, before PGT-A can be adopted universally in clinical practice.
Continue to: With all the above considerations...
With all the above considerations, the ASRM has appropriately concluded that "the value of preimplantation genetic testing for aneuploidy (PGT-A) as a screening test for IVF patients has yet to be determined."11
Standardization of clinical and laboratory protocols and additional studies to assess the effects of PGT-A on live birth rates per initiated cycles are recommended before this new technology is widely adopted in routine clinical practice. In our practice, we routinely offer and perform extended culture to blastocyst stage and standard morphologic assessment. After a thorough counseling on the current status of PGT-A, about 15% to 20% of our patients opt to undergo PGT-A.
- United Nations website. General Assembly resolution 217A: Declaration of human rights. December 10, 1948. http://www.un.org/en/universal-declara tion-human-rights/. Accessed January 11, 2019.
- Zegers-Hochschild F, Adamson GD, Dyer S, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108:393-406.
- US Department of Health and Human Services Office on Women's Health website. Infertility. https://www.womenshealth.gov/a-z-topics/infertility. Accessed January 24, 2019.
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al; International Committee for Monitoring Assisted Reproductive Technology, World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92:1520-1524.
- RESOLVE: The National Infertility Association website. Opposing personhood: Resolve fights to keep fertility medical treatments legal in the US. https://resolve.org/get-involved/advocate-for-access/our-issues/opposing-personhood/. Accessed January 11, 2019.
- Society for Assisted Reproductive Technology website. National summary report. 2016 Preliminary national data. https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?reportingYear=2016 . Accessed January 12, 2019.
- Society for Assisted Reproductive Technology website. National summary report 2015. https://www.sartcorsonline,com/rptCSR_PublicMultYear.aspx ?reportingYear=2015. Accessed January 12, 2019.
- Harton GL, Munne S, Surrey M, et al; PGD Practitioners Group. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril. 2013;100:1695-1703.
- Mastenbroek S, Twisk M, van Echten-Arends, et al. In vitro fertilization with preimplantation genetic screening. N Engl J Med. 2007;357:9-17
- Munne S, Kaplan B, Frattarelli JL, et al. Global multicenter randomized controlled trial comparing single embryo transfer with embryo selected by preimplantation genetic screening using next-generation sequencing versus morphologic assessment [abstract O-43]. Fertil Steril. 2017;108(suppl):e19.
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018;109:429-436.
- United Nations website. General Assembly resolution 217A: Declaration of human rights. December 10, 1948. http://www.un.org/en/universal-declara tion-human-rights/. Accessed January 11, 2019.
- Zegers-Hochschild F, Adamson GD, Dyer S, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108:393-406.
- US Department of Health and Human Services Office on Women's Health website. Infertility. https://www.womenshealth.gov/a-z-topics/infertility. Accessed January 24, 2019.
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al; International Committee for Monitoring Assisted Reproductive Technology, World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92:1520-1524.
- RESOLVE: The National Infertility Association website. Opposing personhood: Resolve fights to keep fertility medical treatments legal in the US. https://resolve.org/get-involved/advocate-for-access/our-issues/opposing-personhood/. Accessed January 11, 2019.
- Society for Assisted Reproductive Technology website. National summary report. 2016 Preliminary national data. https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?reportingYear=2016 . Accessed January 12, 2019.
- Society for Assisted Reproductive Technology website. National summary report 2015. https://www.sartcorsonline,com/rptCSR_PublicMultYear.aspx ?reportingYear=2015. Accessed January 12, 2019.
- Harton GL, Munne S, Surrey M, et al; PGD Practitioners Group. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril. 2013;100:1695-1703.
- Mastenbroek S, Twisk M, van Echten-Arends, et al. In vitro fertilization with preimplantation genetic screening. N Engl J Med. 2007;357:9-17
- Munne S, Kaplan B, Frattarelli JL, et al. Global multicenter randomized controlled trial comparing single embryo transfer with embryo selected by preimplantation genetic screening using next-generation sequencing versus morphologic assessment [abstract O-43]. Fertil Steril. 2017;108(suppl):e19.
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018;109:429-436.
Meta-analysis: IVIG bests anti-D on platelet count in pediatric ITP
For patients with pediatric immune thrombocytopenia (ITP), treatment with intravenous immunoglobulins (IVIG) is more likely to raise platelet count in the short-term, compared with anti-D immunoglobulins (anti-D), according the authors of a recent systematic review and meta-analysis.
Although findings from the meta-analysis support recommendations for first-line IVIG, not all studies reported bleeding symptoms, so the clinical effects of differing platelet responses remain unknown, reported lead author Bertrand Lioger, MD, of François-Rabelais University in Tours, France, and his colleagues.
“To date, no meta-analysis has compared the efficacy and safety of IVIG vs. anti-D,” the investigators wrote in The Journal of Pediatrics.
Each treatment approach has strengths and weaknesses, the investigators noted. Namely, IVIG is more expensive, while anti-D is more likely to cause adverse drugs reactions (ADRs), such as disseminated intravascular coagulation and hemolysis.
The present review evaluated 11 studies comparing the efficacy of IVIG with that of anti-D in 704 children with ITP. Platelet response and bleeding were the main efficacy outcomes. The investigators used response thresholds defined by each study because several did not use standardized levels. Other outcomes considered were mortality, disease course, splenectomy, and ADRs. The ADRs included serious adverse reactions, infusion reactions, transfusions, hemoglobin loss, and hemolysis.
In alignment with previous guidelines, anti-D therapy was most often given to RhD positive, nonsplenectomized children at a dose of 50-75 mcg/kg, whereas IVIG was dosed at 0.8-1 g/kg for 1 or 2 consecutive days.
Results showed that patients treated with IVIG were 15% more likely to have platelet counts greater than 20 × 109/L within 24-72 hours, compared with those given anti-D. This disparity rose to 25% in favor of IVIG when using a threshold of 50 × 109/L.
Treatment risk was lower and general symptoms were less common after treatment with anti-D infusion, compared with IVIG (24.6% vs. 31.4%), but this was only true for trials foregoing premedication. Anti-D was more often associated with hemolysis, making transfusion necessary for some patients.
Although platelet count is often used as a surrogate measure of bleeding risk, the investigators decided that a lack of bleeding data among the studies precluded an accurate determination of clinical superiority between the treatments.
“Severe hemolysis remains an important issue when using anti-D immunoglobulins and premedication reduces the incidence of general symptoms observed with IVIG,” the investigators wrote. “Our conclusions should, however, be cautiously considered due to the poor overall quality of included studies and to limited data about clinically relevant outcomes.”
The study was not supported by outside funding. The investigators reported financial relationships with Amgen, Novartis, Roche Pharma, Sanofi, and others.
SOURCE: Lioger B et al. J Pediatr. 2019;204:225-33.
For patients with pediatric immune thrombocytopenia (ITP), treatment with intravenous immunoglobulins (IVIG) is more likely to raise platelet count in the short-term, compared with anti-D immunoglobulins (anti-D), according the authors of a recent systematic review and meta-analysis.
Although findings from the meta-analysis support recommendations for first-line IVIG, not all studies reported bleeding symptoms, so the clinical effects of differing platelet responses remain unknown, reported lead author Bertrand Lioger, MD, of François-Rabelais University in Tours, France, and his colleagues.
“To date, no meta-analysis has compared the efficacy and safety of IVIG vs. anti-D,” the investigators wrote in The Journal of Pediatrics.
Each treatment approach has strengths and weaknesses, the investigators noted. Namely, IVIG is more expensive, while anti-D is more likely to cause adverse drugs reactions (ADRs), such as disseminated intravascular coagulation and hemolysis.
The present review evaluated 11 studies comparing the efficacy of IVIG with that of anti-D in 704 children with ITP. Platelet response and bleeding were the main efficacy outcomes. The investigators used response thresholds defined by each study because several did not use standardized levels. Other outcomes considered were mortality, disease course, splenectomy, and ADRs. The ADRs included serious adverse reactions, infusion reactions, transfusions, hemoglobin loss, and hemolysis.
In alignment with previous guidelines, anti-D therapy was most often given to RhD positive, nonsplenectomized children at a dose of 50-75 mcg/kg, whereas IVIG was dosed at 0.8-1 g/kg for 1 or 2 consecutive days.
Results showed that patients treated with IVIG were 15% more likely to have platelet counts greater than 20 × 109/L within 24-72 hours, compared with those given anti-D. This disparity rose to 25% in favor of IVIG when using a threshold of 50 × 109/L.
Treatment risk was lower and general symptoms were less common after treatment with anti-D infusion, compared with IVIG (24.6% vs. 31.4%), but this was only true for trials foregoing premedication. Anti-D was more often associated with hemolysis, making transfusion necessary for some patients.
Although platelet count is often used as a surrogate measure of bleeding risk, the investigators decided that a lack of bleeding data among the studies precluded an accurate determination of clinical superiority between the treatments.
“Severe hemolysis remains an important issue when using anti-D immunoglobulins and premedication reduces the incidence of general symptoms observed with IVIG,” the investigators wrote. “Our conclusions should, however, be cautiously considered due to the poor overall quality of included studies and to limited data about clinically relevant outcomes.”
The study was not supported by outside funding. The investigators reported financial relationships with Amgen, Novartis, Roche Pharma, Sanofi, and others.
SOURCE: Lioger B et al. J Pediatr. 2019;204:225-33.
For patients with pediatric immune thrombocytopenia (ITP), treatment with intravenous immunoglobulins (IVIG) is more likely to raise platelet count in the short-term, compared with anti-D immunoglobulins (anti-D), according the authors of a recent systematic review and meta-analysis.
Although findings from the meta-analysis support recommendations for first-line IVIG, not all studies reported bleeding symptoms, so the clinical effects of differing platelet responses remain unknown, reported lead author Bertrand Lioger, MD, of François-Rabelais University in Tours, France, and his colleagues.
“To date, no meta-analysis has compared the efficacy and safety of IVIG vs. anti-D,” the investigators wrote in The Journal of Pediatrics.
Each treatment approach has strengths and weaknesses, the investigators noted. Namely, IVIG is more expensive, while anti-D is more likely to cause adverse drugs reactions (ADRs), such as disseminated intravascular coagulation and hemolysis.
The present review evaluated 11 studies comparing the efficacy of IVIG with that of anti-D in 704 children with ITP. Platelet response and bleeding were the main efficacy outcomes. The investigators used response thresholds defined by each study because several did not use standardized levels. Other outcomes considered were mortality, disease course, splenectomy, and ADRs. The ADRs included serious adverse reactions, infusion reactions, transfusions, hemoglobin loss, and hemolysis.
In alignment with previous guidelines, anti-D therapy was most often given to RhD positive, nonsplenectomized children at a dose of 50-75 mcg/kg, whereas IVIG was dosed at 0.8-1 g/kg for 1 or 2 consecutive days.
Results showed that patients treated with IVIG were 15% more likely to have platelet counts greater than 20 × 109/L within 24-72 hours, compared with those given anti-D. This disparity rose to 25% in favor of IVIG when using a threshold of 50 × 109/L.
Treatment risk was lower and general symptoms were less common after treatment with anti-D infusion, compared with IVIG (24.6% vs. 31.4%), but this was only true for trials foregoing premedication. Anti-D was more often associated with hemolysis, making transfusion necessary for some patients.
Although platelet count is often used as a surrogate measure of bleeding risk, the investigators decided that a lack of bleeding data among the studies precluded an accurate determination of clinical superiority between the treatments.
“Severe hemolysis remains an important issue when using anti-D immunoglobulins and premedication reduces the incidence of general symptoms observed with IVIG,” the investigators wrote. “Our conclusions should, however, be cautiously considered due to the poor overall quality of included studies and to limited data about clinically relevant outcomes.”
The study was not supported by outside funding. The investigators reported financial relationships with Amgen, Novartis, Roche Pharma, Sanofi, and others.
SOURCE: Lioger B et al. J Pediatr. 2019;204:225-33.
FROM THE JOURNAL OF PEDIATRICS
Key clinical point:
Major finding: Treatment with IVIG was 15% more likely than anti-D immunoglobulin to raise platelet counts higher than 20 × 109/L within 24-72 hours.
Study details: A systematic review and meta-analysis of 11 studies comparing the efficacy of IVIG with that of anti-D in 704 children with ITP.
Disclosures: The meta-analysis did not have outside funding. The investigators reported financial relationships with Amgen, Novartis, Roche Pharma, Sanofi, and others.
Source: Lioger B et al. J Pediatr. 2019; 204:225-33.