User login
FDA grants priority review to quizartinib
The U.S. Food and Drug Administration (FDA) has accepted for priority review a new drug application (NDA) for the FLT3 inhibitor quizartinib.
With this NDA, Daiichi Sankyo is seeking approval for quizartinib to treat adults with relapsed/refractory FLT3-ITD acute myeloid leukemia (AML).
The FDA grants priority review to applications for products that are expected to provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The FDA aims to take action on a priority review application within 6 months rather than the standard 10 months.
The FDA is expected to make a decision on the quizartinib NDA by May 25, 2019.
In addition to priority review, quizartinib has breakthrough therapy designation and fast track designation from the FDA.
Trial results
The NDA for quizartinib is supported by results from the phase 3 QuANTUM-R study. Topline results from this study were presented at the 23rd Congress of the European Hematology Association in June, and new analyses are set to be presented at the 2018 ASH Annual Meeting in December (abstract 563).
QuANTUM-R enrolled adults with FLT3-ITD AML (at least 3% FLT3-ITD allelic ratio) who had refractory disease or had relapsed within 6 months of their first complete response (CR).
Patients were randomized to receive once-daily treatment with quizartinib (n=245) or a salvage chemotherapy regimen (n=122)—low-dose cytarabine (LoDAC, n=29); combination mitoxantrone, etoposide, and cytarabine (MEC, n=40); or combination fludarabine, cytarabine, and idarubicin (FLAG-IDA, n=53).
Patients who responded to treatment could proceed to hematopoietic stem cell transplant (HSCT), and those in the quizartinib arm could resume quizartinib after HSCT.
In all, 241 patients received quizartinib, and 94 received salvage chemotherapy—LoDAC (n=22), MEC (n=25), and FLAG-IDA (n=47). Of the 28 patients in the chemotherapy group who were not treated, most withdrew consent.
Thirty-two percent of quizartinib-treated patients and 12% of the chemotherapy group went on to HSCT.
Efficacy
The median follow-up was 23.5 months. The efficacy results include all randomized patients.
The overall response rate was 69% in the quizartinib arm and 30% in the chemotherapy arm. The composite CR rate was 48% in the quizartinib arm and 27% in the chemotherapy arm. This includes:
- The CR rate (4% and 1%, respectively)
- The rate of CR with incomplete platelet recovery (4% and 0%, respectively)
- The rate of CR with incomplete hematologic recovery (40% and 26%, respectively).
The median event-free survival was 6.0 weeks in the quizartinib arm and 3.7 weeks in the chemotherapy arm (hazard ratio=0.90, P=0.1071).
The median overall survival was 6.2 months in the quizartinib arm and 4.7 months in the chemotherapy arm (hazard ratio=0.76, P=0.0177). The 1-year overall survival rate was 27% and 20%, respectively.
Safety
The safety results include only patients who received their assigned treatment.
Grade 3 or higher hematologic treatment-emergent adverse events occurring in at least 5% of patients (in the quizartinib and chemotherapy groups, respectively) included:
- Thrombocytopenia (35% and 34%)
- Anemia (30% and 29%)
- Neutropenia (32% and 25%)
- Febrile neutropenia (31% and 21%)
- Leukopenia (17% and 16%).
Grade 3 or higher non-hematologic treatment-emergent adverse events occurring in at least 5% of patients (in the quizartinib and chemotherapy groups, respectively) included:
- Sepsis/septic shock (16% and 18%)
- Hypokalemia (12% and 9%)
- Pneumonia (12% and 9%)
- Fatigue (8% and 1%)
- Dyspnea (5% for both)
- Hypophosphatemia (5% for both).
The U.S. Food and Drug Administration (FDA) has accepted for priority review a new drug application (NDA) for the FLT3 inhibitor quizartinib.
With this NDA, Daiichi Sankyo is seeking approval for quizartinib to treat adults with relapsed/refractory FLT3-ITD acute myeloid leukemia (AML).
The FDA grants priority review to applications for products that are expected to provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The FDA aims to take action on a priority review application within 6 months rather than the standard 10 months.
The FDA is expected to make a decision on the quizartinib NDA by May 25, 2019.
In addition to priority review, quizartinib has breakthrough therapy designation and fast track designation from the FDA.
Trial results
The NDA for quizartinib is supported by results from the phase 3 QuANTUM-R study. Topline results from this study were presented at the 23rd Congress of the European Hematology Association in June, and new analyses are set to be presented at the 2018 ASH Annual Meeting in December (abstract 563).
QuANTUM-R enrolled adults with FLT3-ITD AML (at least 3% FLT3-ITD allelic ratio) who had refractory disease or had relapsed within 6 months of their first complete response (CR).
Patients were randomized to receive once-daily treatment with quizartinib (n=245) or a salvage chemotherapy regimen (n=122)—low-dose cytarabine (LoDAC, n=29); combination mitoxantrone, etoposide, and cytarabine (MEC, n=40); or combination fludarabine, cytarabine, and idarubicin (FLAG-IDA, n=53).
Patients who responded to treatment could proceed to hematopoietic stem cell transplant (HSCT), and those in the quizartinib arm could resume quizartinib after HSCT.
In all, 241 patients received quizartinib, and 94 received salvage chemotherapy—LoDAC (n=22), MEC (n=25), and FLAG-IDA (n=47). Of the 28 patients in the chemotherapy group who were not treated, most withdrew consent.
Thirty-two percent of quizartinib-treated patients and 12% of the chemotherapy group went on to HSCT.
Efficacy
The median follow-up was 23.5 months. The efficacy results include all randomized patients.
The overall response rate was 69% in the quizartinib arm and 30% in the chemotherapy arm. The composite CR rate was 48% in the quizartinib arm and 27% in the chemotherapy arm. This includes:
- The CR rate (4% and 1%, respectively)
- The rate of CR with incomplete platelet recovery (4% and 0%, respectively)
- The rate of CR with incomplete hematologic recovery (40% and 26%, respectively).
The median event-free survival was 6.0 weeks in the quizartinib arm and 3.7 weeks in the chemotherapy arm (hazard ratio=0.90, P=0.1071).
The median overall survival was 6.2 months in the quizartinib arm and 4.7 months in the chemotherapy arm (hazard ratio=0.76, P=0.0177). The 1-year overall survival rate was 27% and 20%, respectively.
Safety
The safety results include only patients who received their assigned treatment.
Grade 3 or higher hematologic treatment-emergent adverse events occurring in at least 5% of patients (in the quizartinib and chemotherapy groups, respectively) included:
- Thrombocytopenia (35% and 34%)
- Anemia (30% and 29%)
- Neutropenia (32% and 25%)
- Febrile neutropenia (31% and 21%)
- Leukopenia (17% and 16%).
Grade 3 or higher non-hematologic treatment-emergent adverse events occurring in at least 5% of patients (in the quizartinib and chemotherapy groups, respectively) included:
- Sepsis/septic shock (16% and 18%)
- Hypokalemia (12% and 9%)
- Pneumonia (12% and 9%)
- Fatigue (8% and 1%)
- Dyspnea (5% for both)
- Hypophosphatemia (5% for both).
The U.S. Food and Drug Administration (FDA) has accepted for priority review a new drug application (NDA) for the FLT3 inhibitor quizartinib.
With this NDA, Daiichi Sankyo is seeking approval for quizartinib to treat adults with relapsed/refractory FLT3-ITD acute myeloid leukemia (AML).
The FDA grants priority review to applications for products that are expected to provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The FDA aims to take action on a priority review application within 6 months rather than the standard 10 months.
The FDA is expected to make a decision on the quizartinib NDA by May 25, 2019.
In addition to priority review, quizartinib has breakthrough therapy designation and fast track designation from the FDA.
Trial results
The NDA for quizartinib is supported by results from the phase 3 QuANTUM-R study. Topline results from this study were presented at the 23rd Congress of the European Hematology Association in June, and new analyses are set to be presented at the 2018 ASH Annual Meeting in December (abstract 563).
QuANTUM-R enrolled adults with FLT3-ITD AML (at least 3% FLT3-ITD allelic ratio) who had refractory disease or had relapsed within 6 months of their first complete response (CR).
Patients were randomized to receive once-daily treatment with quizartinib (n=245) or a salvage chemotherapy regimen (n=122)—low-dose cytarabine (LoDAC, n=29); combination mitoxantrone, etoposide, and cytarabine (MEC, n=40); or combination fludarabine, cytarabine, and idarubicin (FLAG-IDA, n=53).
Patients who responded to treatment could proceed to hematopoietic stem cell transplant (HSCT), and those in the quizartinib arm could resume quizartinib after HSCT.
In all, 241 patients received quizartinib, and 94 received salvage chemotherapy—LoDAC (n=22), MEC (n=25), and FLAG-IDA (n=47). Of the 28 patients in the chemotherapy group who were not treated, most withdrew consent.
Thirty-two percent of quizartinib-treated patients and 12% of the chemotherapy group went on to HSCT.
Efficacy
The median follow-up was 23.5 months. The efficacy results include all randomized patients.
The overall response rate was 69% in the quizartinib arm and 30% in the chemotherapy arm. The composite CR rate was 48% in the quizartinib arm and 27% in the chemotherapy arm. This includes:
- The CR rate (4% and 1%, respectively)
- The rate of CR with incomplete platelet recovery (4% and 0%, respectively)
- The rate of CR with incomplete hematologic recovery (40% and 26%, respectively).
The median event-free survival was 6.0 weeks in the quizartinib arm and 3.7 weeks in the chemotherapy arm (hazard ratio=0.90, P=0.1071).
The median overall survival was 6.2 months in the quizartinib arm and 4.7 months in the chemotherapy arm (hazard ratio=0.76, P=0.0177). The 1-year overall survival rate was 27% and 20%, respectively.
Safety
The safety results include only patients who received their assigned treatment.
Grade 3 or higher hematologic treatment-emergent adverse events occurring in at least 5% of patients (in the quizartinib and chemotherapy groups, respectively) included:
- Thrombocytopenia (35% and 34%)
- Anemia (30% and 29%)
- Neutropenia (32% and 25%)
- Febrile neutropenia (31% and 21%)
- Leukopenia (17% and 16%).
Grade 3 or higher non-hematologic treatment-emergent adverse events occurring in at least 5% of patients (in the quizartinib and chemotherapy groups, respectively) included:
- Sepsis/septic shock (16% and 18%)
- Hypokalemia (12% and 9%)
- Pneumonia (12% and 9%)
- Fatigue (8% and 1%)
- Dyspnea (5% for both)
- Hypophosphatemia (5% for both).
Harnessing the power of urine tests in pain care
SAN DIEGO – Clinicians have few tools to in their patients. However, addiction specialist and internist Edwin Salsitz, MD, says an inexpensive and simple tool, the urine test, can provide an impressive amount of useful information.
“The urine drug test, or another matrix for testing, gives one of the only objective factors we have to see how a patient is doing, if they’re following the treatment plan,” said Dr. Salsitz, of Mount Sinai Beth Israel, New York, in a presentation at Pain Care for Primary Care, a symposium offered by the American Pain Society and the Global Academy for Medical Education.
Dr. Salsitz offered these tips about urine tests in pain care:
Consider urine tests before beginning opioid therapy
Dr. Salsitz pointed to this 2016 recommendation from the Centers for Disease Control and Prevention: “When prescribing opioids for chronic pain, clinicians should use urine drug testing before starting opioid therapy and consider urine drug testing at least annually to assess for prescribed medications as well as other controlled prescription drugs and illicit drugs.” As Dr. Salsitz puts it, these tests “can help identify misuse, which hopefully hasn’t gotten to addiction yet.”
Ask the patient what the urine test will reveal
Dr. Salsitz likes to tell patients: “If you tell me the truth, no matter what’s in the urine, it’s going to be OK. I’m not going to stop prescribing or do anything harmful to you.” But, he tells patients, if they lie, “you’re going to start breaking the trust between us. Once you do that, it becomes a problem. I don’t know what’s true or not.” In some cases, he said, patients will fess up to drug use that wouldn’t have shown up in the urine tests because it didn’t happen recently enough. “We’ll talk about whether it’s a problem,” he said.
Begin with an immunoassay panel test (IA)
The CDC recommends using an immunoassay panel first in most situations. “You can do this in the office,” Dr. Salsitz said, using a dipstick-style test. Or you can “send it out to a lab, and they’ll do the same thing.”
Understand what IA tests do and don’t do
Standard 5-drug IA screening tests detect marijuana, cocaine, amphetamine/methamphetamine, PCP, and opiates (morphine/codeine). Keep in mind, Dr. Salsitz said, that opiates and opioids aren’t the same. That means IA tests don’t pick up oxycodone use, for example, he said. More sophisticated (and more expensive) tests can distinguish between types of drugs (for example, morphine vs. codeine) and can detect drugs that aren’t included in the IA tests.
Don’t make assumptions about positive or negative tests
A positive drug test for cocaine doesn’t necessarily mean the person is addicted, Dr. Salsitz said. “It just means they used that molecule in the last 3 days. It’s up to you to figure out what it actually means.” And if a patient’s urine fails to show that he or she is taking a prescribed medication, that doesn’t necessarily indicate that the drug is being illegally diverted. The patient could have run out of the drug or lost insurance coverage, Dr. Salsitz said.
Be aware that patients may fake urine tests
“Cheating is a huge problem,” Dr. Salsitz said. “Is it their urine or not their urine?” Many kits promise to help people provide fake urine, and some have even provided fake penises to foil observed urine collection. What to do? Alternative tests that rely on hair, saliva, and even sweat are available, Dr. Salsitz said, and these make cheating more difficult. However, they have various limitations. Saliva, for example, only tells you what patients are using now, not what they used days ago, he said, and it’s not sensitive for marijuana.
Dr. Salsitz reported no disclosures.
The Global Academy for Medical Education, which offered the Pain Care for Primary Care symposium, and this news organization are owned by the same parent company.
SAN DIEGO – Clinicians have few tools to in their patients. However, addiction specialist and internist Edwin Salsitz, MD, says an inexpensive and simple tool, the urine test, can provide an impressive amount of useful information.
“The urine drug test, or another matrix for testing, gives one of the only objective factors we have to see how a patient is doing, if they’re following the treatment plan,” said Dr. Salsitz, of Mount Sinai Beth Israel, New York, in a presentation at Pain Care for Primary Care, a symposium offered by the American Pain Society and the Global Academy for Medical Education.
Dr. Salsitz offered these tips about urine tests in pain care:
Consider urine tests before beginning opioid therapy
Dr. Salsitz pointed to this 2016 recommendation from the Centers for Disease Control and Prevention: “When prescribing opioids for chronic pain, clinicians should use urine drug testing before starting opioid therapy and consider urine drug testing at least annually to assess for prescribed medications as well as other controlled prescription drugs and illicit drugs.” As Dr. Salsitz puts it, these tests “can help identify misuse, which hopefully hasn’t gotten to addiction yet.”
Ask the patient what the urine test will reveal
Dr. Salsitz likes to tell patients: “If you tell me the truth, no matter what’s in the urine, it’s going to be OK. I’m not going to stop prescribing or do anything harmful to you.” But, he tells patients, if they lie, “you’re going to start breaking the trust between us. Once you do that, it becomes a problem. I don’t know what’s true or not.” In some cases, he said, patients will fess up to drug use that wouldn’t have shown up in the urine tests because it didn’t happen recently enough. “We’ll talk about whether it’s a problem,” he said.
Begin with an immunoassay panel test (IA)
The CDC recommends using an immunoassay panel first in most situations. “You can do this in the office,” Dr. Salsitz said, using a dipstick-style test. Or you can “send it out to a lab, and they’ll do the same thing.”
Understand what IA tests do and don’t do
Standard 5-drug IA screening tests detect marijuana, cocaine, amphetamine/methamphetamine, PCP, and opiates (morphine/codeine). Keep in mind, Dr. Salsitz said, that opiates and opioids aren’t the same. That means IA tests don’t pick up oxycodone use, for example, he said. More sophisticated (and more expensive) tests can distinguish between types of drugs (for example, morphine vs. codeine) and can detect drugs that aren’t included in the IA tests.
Don’t make assumptions about positive or negative tests
A positive drug test for cocaine doesn’t necessarily mean the person is addicted, Dr. Salsitz said. “It just means they used that molecule in the last 3 days. It’s up to you to figure out what it actually means.” And if a patient’s urine fails to show that he or she is taking a prescribed medication, that doesn’t necessarily indicate that the drug is being illegally diverted. The patient could have run out of the drug or lost insurance coverage, Dr. Salsitz said.
Be aware that patients may fake urine tests
“Cheating is a huge problem,” Dr. Salsitz said. “Is it their urine or not their urine?” Many kits promise to help people provide fake urine, and some have even provided fake penises to foil observed urine collection. What to do? Alternative tests that rely on hair, saliva, and even sweat are available, Dr. Salsitz said, and these make cheating more difficult. However, they have various limitations. Saliva, for example, only tells you what patients are using now, not what they used days ago, he said, and it’s not sensitive for marijuana.
Dr. Salsitz reported no disclosures.
The Global Academy for Medical Education, which offered the Pain Care for Primary Care symposium, and this news organization are owned by the same parent company.
SAN DIEGO – Clinicians have few tools to in their patients. However, addiction specialist and internist Edwin Salsitz, MD, says an inexpensive and simple tool, the urine test, can provide an impressive amount of useful information.
“The urine drug test, or another matrix for testing, gives one of the only objective factors we have to see how a patient is doing, if they’re following the treatment plan,” said Dr. Salsitz, of Mount Sinai Beth Israel, New York, in a presentation at Pain Care for Primary Care, a symposium offered by the American Pain Society and the Global Academy for Medical Education.
Dr. Salsitz offered these tips about urine tests in pain care:
Consider urine tests before beginning opioid therapy
Dr. Salsitz pointed to this 2016 recommendation from the Centers for Disease Control and Prevention: “When prescribing opioids for chronic pain, clinicians should use urine drug testing before starting opioid therapy and consider urine drug testing at least annually to assess for prescribed medications as well as other controlled prescription drugs and illicit drugs.” As Dr. Salsitz puts it, these tests “can help identify misuse, which hopefully hasn’t gotten to addiction yet.”
Ask the patient what the urine test will reveal
Dr. Salsitz likes to tell patients: “If you tell me the truth, no matter what’s in the urine, it’s going to be OK. I’m not going to stop prescribing or do anything harmful to you.” But, he tells patients, if they lie, “you’re going to start breaking the trust between us. Once you do that, it becomes a problem. I don’t know what’s true or not.” In some cases, he said, patients will fess up to drug use that wouldn’t have shown up in the urine tests because it didn’t happen recently enough. “We’ll talk about whether it’s a problem,” he said.
Begin with an immunoassay panel test (IA)
The CDC recommends using an immunoassay panel first in most situations. “You can do this in the office,” Dr. Salsitz said, using a dipstick-style test. Or you can “send it out to a lab, and they’ll do the same thing.”
Understand what IA tests do and don’t do
Standard 5-drug IA screening tests detect marijuana, cocaine, amphetamine/methamphetamine, PCP, and opiates (morphine/codeine). Keep in mind, Dr. Salsitz said, that opiates and opioids aren’t the same. That means IA tests don’t pick up oxycodone use, for example, he said. More sophisticated (and more expensive) tests can distinguish between types of drugs (for example, morphine vs. codeine) and can detect drugs that aren’t included in the IA tests.
Don’t make assumptions about positive or negative tests
A positive drug test for cocaine doesn’t necessarily mean the person is addicted, Dr. Salsitz said. “It just means they used that molecule in the last 3 days. It’s up to you to figure out what it actually means.” And if a patient’s urine fails to show that he or she is taking a prescribed medication, that doesn’t necessarily indicate that the drug is being illegally diverted. The patient could have run out of the drug or lost insurance coverage, Dr. Salsitz said.
Be aware that patients may fake urine tests
“Cheating is a huge problem,” Dr. Salsitz said. “Is it their urine or not their urine?” Many kits promise to help people provide fake urine, and some have even provided fake penises to foil observed urine collection. What to do? Alternative tests that rely on hair, saliva, and even sweat are available, Dr. Salsitz said, and these make cheating more difficult. However, they have various limitations. Saliva, for example, only tells you what patients are using now, not what they used days ago, he said, and it’s not sensitive for marijuana.
Dr. Salsitz reported no disclosures.
The Global Academy for Medical Education, which offered the Pain Care for Primary Care symposium, and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM PAIN CARE FOR PRIMARY CARE
The powerful virus inflammatory response
Inflammation is a double-edged sword. Controlled and modest proinflammatory responses can enhance host immunity against viruses and decrease bacterial colonization and infection, whereas excessive uncontrolled proinflammatory responses may increase the susceptibility to bacterial colonization and secondary infection to facilitate disease pathogenesis. The immune system produces both proinflammatory and anti-inflammatory cytokines and chemokines. It is a balanced response that is key to maintaining good health.
Viral upper respiratory tract infections (URIs) are caused by rhinoviruses, coronaviruses, enteroviruses, respiratory syncytial viruses, influenza A and B viruses, parainfluenza viruses, adenoviruses, and human metapneumoviruses. Viruses are powerful. In the nose, they induce hypersecretion of mucus, slow cilia beating, up-regulate nasal epithelial cell receptors to facilitate bacterial attachment, suppress neutrophil function, and cause increased release of proinflammatory cytokines and chemokines. All these actions by respiratory viruses promote bacterial overgrowth in the nasopharynx and thereby facilitate bacterial superinfections. In fact, progression in pathogenesis of the common bacterial respiratory infections – acute otitis media, acute sinusitis, acute conjunctivitis, and pneumonia – almost always is preceded by a viral URI. Viruses activate multiple target cells in the upper respiratory tract to produce an array of proinflammatory cytokines and chemokines. The symptoms of a viral URI resolve coinciding with an anti-inflammatory response and adaptive immunity.
In recent work, we found a higher frequency of viral URIs in children who experienced more frequent acute otitis media (AOM). We sought to understand why this might occur by comparing levels of inflammatory cytokines/chemokines in the nose during viral URI that did not precipitate AOM versus when a viral URI precipitated an AOM episode. When a child had a viral URI but did not go on to experience an AOM, the child had higher proinflammatory responses than when the viral URI precipitated an AOM. When differences of levels of proinflammatory cytokines/chemokines were compared in otitis-prone and non–otitis-prone children, lower nasal responses were associated with higher otitis-prone classification frequency (Clin Infect Dis. 2018. doi: 10.1093/cid/ciy750).
The powerful virus and the inflammatory response it can induce also play a major role in allergy and asthma. Viral URIs enhance allergic sensitization to respiratory viruses, such as influenza and respiratory syncytial virus, cause cytopathic damage to airway epithelium, promote excessive proinflammatory cytokine/chemokine production, and increase the exposure of allergens and irritants to antigen-presenting cells. Viral infections also may induce the release of epithelial mediators and cytokines that may propagate eosinophilia. Viral URIs, particularly with respiratory syncytial virus and rhinovirus, are the most common causes of wheezing in children, and they have important influences on the development of asthma. Studies have shown that viral infections trigger up to 85% of asthma exacerbations in school-aged children.
Because this column is being published during the winter, a brief discussion of influenza as a powerful virus is appropriate. Influenza occurs in winter outbreaks of varying extent every year. The severity of the influenza season reflects the changing nature of the antigenic properties of influenza viruses, and their spread depends on susceptibility of the population. Influenza outbreaks typically peak over a 2-3 week period and last for 2-3 months. Most outbreaks have attack rates of 10%-20% in children. There may be variations in disease severity caused by different influenza virus types. The symptoms are caused by excessive proinflammatory cytokine/chemokine production in the nose and lung.
Influenza and other viruses can precipitate the systemic inflammatory response syndrome (SIRS), a manifestation of extreme immune dysregulation resulting in organ dysfunction that clinically resembles bacterial sepsis. In this syndrome, tissues remote from the original insult display the cardinal signs of inflammation, including vasodilation, increased microvascular permeability, and leukocyte accumulation. SIRS is another example of the double-edged sword of inflammation.
The onset and progression of SIRS occurs because of dysregulation of the normal inflammatory response, usually with an increase in both proinflammatory and anti-inflammatory cytokines and chemokines, initiating a chain of events that leads to organ failure.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at pdnews@mdedge.com.
Inflammation is a double-edged sword. Controlled and modest proinflammatory responses can enhance host immunity against viruses and decrease bacterial colonization and infection, whereas excessive uncontrolled proinflammatory responses may increase the susceptibility to bacterial colonization and secondary infection to facilitate disease pathogenesis. The immune system produces both proinflammatory and anti-inflammatory cytokines and chemokines. It is a balanced response that is key to maintaining good health.
Viral upper respiratory tract infections (URIs) are caused by rhinoviruses, coronaviruses, enteroviruses, respiratory syncytial viruses, influenza A and B viruses, parainfluenza viruses, adenoviruses, and human metapneumoviruses. Viruses are powerful. In the nose, they induce hypersecretion of mucus, slow cilia beating, up-regulate nasal epithelial cell receptors to facilitate bacterial attachment, suppress neutrophil function, and cause increased release of proinflammatory cytokines and chemokines. All these actions by respiratory viruses promote bacterial overgrowth in the nasopharynx and thereby facilitate bacterial superinfections. In fact, progression in pathogenesis of the common bacterial respiratory infections – acute otitis media, acute sinusitis, acute conjunctivitis, and pneumonia – almost always is preceded by a viral URI. Viruses activate multiple target cells in the upper respiratory tract to produce an array of proinflammatory cytokines and chemokines. The symptoms of a viral URI resolve coinciding with an anti-inflammatory response and adaptive immunity.
In recent work, we found a higher frequency of viral URIs in children who experienced more frequent acute otitis media (AOM). We sought to understand why this might occur by comparing levels of inflammatory cytokines/chemokines in the nose during viral URI that did not precipitate AOM versus when a viral URI precipitated an AOM episode. When a child had a viral URI but did not go on to experience an AOM, the child had higher proinflammatory responses than when the viral URI precipitated an AOM. When differences of levels of proinflammatory cytokines/chemokines were compared in otitis-prone and non–otitis-prone children, lower nasal responses were associated with higher otitis-prone classification frequency (Clin Infect Dis. 2018. doi: 10.1093/cid/ciy750).
The powerful virus and the inflammatory response it can induce also play a major role in allergy and asthma. Viral URIs enhance allergic sensitization to respiratory viruses, such as influenza and respiratory syncytial virus, cause cytopathic damage to airway epithelium, promote excessive proinflammatory cytokine/chemokine production, and increase the exposure of allergens and irritants to antigen-presenting cells. Viral infections also may induce the release of epithelial mediators and cytokines that may propagate eosinophilia. Viral URIs, particularly with respiratory syncytial virus and rhinovirus, are the most common causes of wheezing in children, and they have important influences on the development of asthma. Studies have shown that viral infections trigger up to 85% of asthma exacerbations in school-aged children.
Because this column is being published during the winter, a brief discussion of influenza as a powerful virus is appropriate. Influenza occurs in winter outbreaks of varying extent every year. The severity of the influenza season reflects the changing nature of the antigenic properties of influenza viruses, and their spread depends on susceptibility of the population. Influenza outbreaks typically peak over a 2-3 week period and last for 2-3 months. Most outbreaks have attack rates of 10%-20% in children. There may be variations in disease severity caused by different influenza virus types. The symptoms are caused by excessive proinflammatory cytokine/chemokine production in the nose and lung.
Influenza and other viruses can precipitate the systemic inflammatory response syndrome (SIRS), a manifestation of extreme immune dysregulation resulting in organ dysfunction that clinically resembles bacterial sepsis. In this syndrome, tissues remote from the original insult display the cardinal signs of inflammation, including vasodilation, increased microvascular permeability, and leukocyte accumulation. SIRS is another example of the double-edged sword of inflammation.
The onset and progression of SIRS occurs because of dysregulation of the normal inflammatory response, usually with an increase in both proinflammatory and anti-inflammatory cytokines and chemokines, initiating a chain of events that leads to organ failure.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at pdnews@mdedge.com.
Inflammation is a double-edged sword. Controlled and modest proinflammatory responses can enhance host immunity against viruses and decrease bacterial colonization and infection, whereas excessive uncontrolled proinflammatory responses may increase the susceptibility to bacterial colonization and secondary infection to facilitate disease pathogenesis. The immune system produces both proinflammatory and anti-inflammatory cytokines and chemokines. It is a balanced response that is key to maintaining good health.
Viral upper respiratory tract infections (URIs) are caused by rhinoviruses, coronaviruses, enteroviruses, respiratory syncytial viruses, influenza A and B viruses, parainfluenza viruses, adenoviruses, and human metapneumoviruses. Viruses are powerful. In the nose, they induce hypersecretion of mucus, slow cilia beating, up-regulate nasal epithelial cell receptors to facilitate bacterial attachment, suppress neutrophil function, and cause increased release of proinflammatory cytokines and chemokines. All these actions by respiratory viruses promote bacterial overgrowth in the nasopharynx and thereby facilitate bacterial superinfections. In fact, progression in pathogenesis of the common bacterial respiratory infections – acute otitis media, acute sinusitis, acute conjunctivitis, and pneumonia – almost always is preceded by a viral URI. Viruses activate multiple target cells in the upper respiratory tract to produce an array of proinflammatory cytokines and chemokines. The symptoms of a viral URI resolve coinciding with an anti-inflammatory response and adaptive immunity.
In recent work, we found a higher frequency of viral URIs in children who experienced more frequent acute otitis media (AOM). We sought to understand why this might occur by comparing levels of inflammatory cytokines/chemokines in the nose during viral URI that did not precipitate AOM versus when a viral URI precipitated an AOM episode. When a child had a viral URI but did not go on to experience an AOM, the child had higher proinflammatory responses than when the viral URI precipitated an AOM. When differences of levels of proinflammatory cytokines/chemokines were compared in otitis-prone and non–otitis-prone children, lower nasal responses were associated with higher otitis-prone classification frequency (Clin Infect Dis. 2018. doi: 10.1093/cid/ciy750).
The powerful virus and the inflammatory response it can induce also play a major role in allergy and asthma. Viral URIs enhance allergic sensitization to respiratory viruses, such as influenza and respiratory syncytial virus, cause cytopathic damage to airway epithelium, promote excessive proinflammatory cytokine/chemokine production, and increase the exposure of allergens and irritants to antigen-presenting cells. Viral infections also may induce the release of epithelial mediators and cytokines that may propagate eosinophilia. Viral URIs, particularly with respiratory syncytial virus and rhinovirus, are the most common causes of wheezing in children, and they have important influences on the development of asthma. Studies have shown that viral infections trigger up to 85% of asthma exacerbations in school-aged children.
Because this column is being published during the winter, a brief discussion of influenza as a powerful virus is appropriate. Influenza occurs in winter outbreaks of varying extent every year. The severity of the influenza season reflects the changing nature of the antigenic properties of influenza viruses, and their spread depends on susceptibility of the population. Influenza outbreaks typically peak over a 2-3 week period and last for 2-3 months. Most outbreaks have attack rates of 10%-20% in children. There may be variations in disease severity caused by different influenza virus types. The symptoms are caused by excessive proinflammatory cytokine/chemokine production in the nose and lung.
Influenza and other viruses can precipitate the systemic inflammatory response syndrome (SIRS), a manifestation of extreme immune dysregulation resulting in organ dysfunction that clinically resembles bacterial sepsis. In this syndrome, tissues remote from the original insult display the cardinal signs of inflammation, including vasodilation, increased microvascular permeability, and leukocyte accumulation. SIRS is another example of the double-edged sword of inflammation.
The onset and progression of SIRS occurs because of dysregulation of the normal inflammatory response, usually with an increase in both proinflammatory and anti-inflammatory cytokines and chemokines, initiating a chain of events that leads to organ failure.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at pdnews@mdedge.com.
Malignant olecranon bursitis in the setting of multiple myeloma relapse
Multiple myeloma is the most common plasma cell neoplasm, with an estimated 24,000 cases occurring annually.1 Symptomatic multiple myeloma most commonly presents with one or more of the cardinal CRAB phenomena of hypercalcemia, renal dysfunction, anemia, or lytic bone lesions.2 Less commonly, patients may present with plasmacytomas (focal lesions of malignant plasma cells), which may involve bony or soft tissues.1
Plasma cell neoplasms occasionally involve the joints, including the elbows, typically as plasmacytomas. The elbow is an unusual but reported location of plasmacytomas.3,4 A case of multiple myeloma and amyloid light-chain (AL) amyloidosis has been reported, with manifestations including pseudomyopathy, bone marrow plasmacytosis, and bilateral trochanteric bursitis.5Bursitis is defined as inflammation of the synovial-fluid–containing sacs that lubricate joints. The olecranon bursa is commonly affected. Etiologies include infection, inflammatory disease, trauma, and malignancy. Furthermore, there is an association between bursitis and immunosuppression.6,7 The most common modes of therapy used to treat bursitis are nonsteroidal anti-inflammatory drugs, corticosteroid injections, and surgical management.
Trochanteric bursitis has been attributed to multiple myeloma in one previous case report, but we are not aware of any previous cases of olecranon bursitis caused by multiple myeloma. Here, we present the case of a 46-year-old man with heavily pretreated multiple myeloma and amyloidosis who developed left olecranon bursitis contemporaneously with disease relapse; flow cytometric analysis of the bursal fluid demonstrated an abnormal plasma cell population, establishing the etiology.
Case presentation and summary
A 46-year-old man with a longstanding history of multiple myeloma developed swelling of the left elbow that was initially painless in September 2016. He had been diagnosed with IgA kappa multiple myeloma and AL deposition in 2011. Over the course of his disease, he was treated with the following sequence of therapies: cyclophosphamide, bortezomib, and dexamethasone, followed by melphalan-conditioned autologous peripheral blood stem cell transplant; lenalidomide and dexamethasone; carfilzomib and dexamethasone; pomalidomide, bortezomib, and dexamethasone; and bortezomib, lenalidomide, dexamethasone, doxorubicin, cyclophosphamide, and etoposide, followed by second melphalan-conditioned autologous peripheral blood stem cell transplant. In addition to treatment with numerous novel and chemotherapeutic agents, his disease course was notable for amyloid deposition in the liver, bone marrow, and kidneys, which resulted in dialysis dependence.
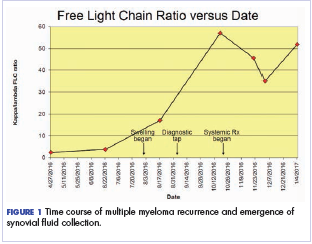
After the second autologous transplant, he achieved a very good partial response and experienced about 9 months of remission, after which laboratory evaluation indicated recurrence of IgA kappa monoclonal protein and free kappa light-chains, which increased slowly over several months without focal symptoms, cytopenias, or decline in organ function (Figure 1).
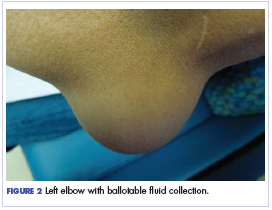
Twelve months after his second transplant, he presented in September 2016 with 4 weeks of left elbow swelling, with the appearance suggesting a fluid collection over the left olecranon process (Figure 2). The fluid collection was not painful unless bumped or pushed. The maximum pain level was 1-2 on a scale of 0-10. His daughter drained the fluid collection on 2 occasions, but it reaccumulated over 2 to 3 days. He reported no fevers, chills, or sweats. He did not have any redness at the site. He did not report any systemic symptoms.
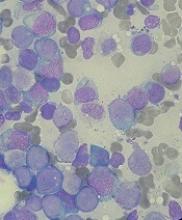
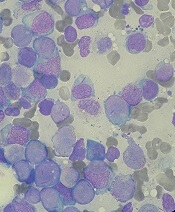
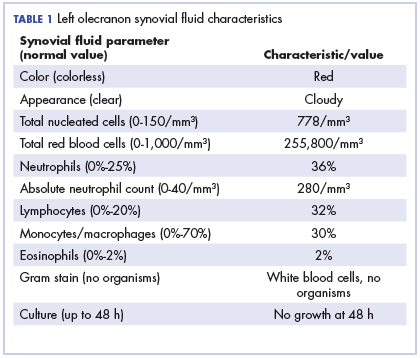
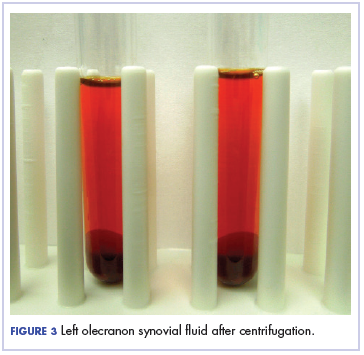
Physical examination of the left elbow demonstrated a ballotable fluid collection associated with the olecranon, with no associated warmth, tenderness, or erythema. Bursal fluid was sampled, yielding orange-colored serous fluid with bland characteristics (Figure 3). Microbiologic studies were negative (Table 1). We did not suspect a malignant cause initially.
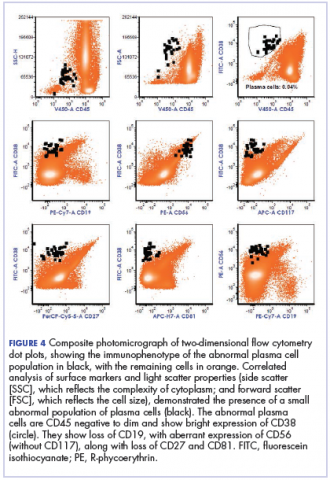
The fluid collection persisted despite treatment with nonsteroidal anti-inflammatory drugs and serial drainage procedures approximately twice per week. It became more erythematous and uncomfortable. We repeated diagnostic sampling at 13 months post-transplant. Cytospin revealed scant plasma cells. A multiparametric 8-color flow cytometric analysis was performed on the bursal fluid. It demonstrated the presence of a small abnormal population of plasma cells (0.04%). The abnormal plasma cells showed expression of CD138 and bright CD38 with aberrant expression of CD56, dim CD45, and loss of CD19, CD81 and CD27. They did not express CD117 or CD20 (Figure 4).
Because of the patient’s discomfort and his history of multidrug-refractory multiple myeloma, we obtained computed tomography imaging of the axial and appendicular skeleton, which demonstrated diffuse small lytic lesions, none larger than 3 mm, including the left elbow joint. The patient began systemic treatment with ixazomib, pomalidomide, and dexamethasone and then received radiation therapy of 20 Gy in 4 fractions to the left olecranon area. The bursal fluid collection remained stable in size but required periodic, though less frequent, drainage procedures. Unfortunately, the patient only tolerated 2 cycles of systemic therapy before experiencing hypercalcemia, exacerbation of hepatic amyloidosis, and a decline in performance status. He died 17 months after the transplant.
Discussion
Our patient experienced left olecranon bursitis simultaneously with relapse of multiple myeloma and AL amyloidosis. Evaluation for infectious causes was negative, and the bursal fluid did not have strongly inflammatory characteristics. Furthermore, a small plasma cell population was isolated from the fluid. Imaging did not reveal an underlying dominant lytic lesion. Although we do not have direct pathologic confirmation, the clinical scenario and flow cytometry findings support our interpretation that the patient’s bursitis was caused by or at least related to underlying multiple myeloma. While reactive plasma cells are also CD38 positive and CD138 positive, they maintain the expression of CD19 and CD45 without aberrant expression of CD56 or CD117 and do not show loss of expression of CD81 or CD27. In this situation, we suspect that either a plasmacytoma involving the soft tissue of the bursa or amyloid infiltration of the synovium may have occurred. Anti-myeloma therapies and radiation therapy did not result in control of the bursitis, though it should be noted that the patient’s highly refractory disease progressed despite treatment with a combination of later-generation immunomodulatory imide and proteasome inhibitor therapies.
Cases of malignant bursitis have been reported several times in the literature, though nearly all of the instances involved connective tissue or metastatic tumors. Tumor histologies include osteochondroma,8,9 malignant fibrous histiocytoma,10 synovial sarcoma,11 and metastatic breast cancer.12
Hematologic malignancies are more rare causes of bursitis; our literature search identified a report of 2 cases of non-Hodgkin lymphoma mimicking rheumatoid arthritis. The joints were the knee and elbow. Synovial fluid from one case was clear and yellow, with leukocytosis with a neutrophilic predominance (similar to our case). In both cases, pathology confirmed lymphomatous infiltration of the synovium.13 Notably, we identified a case of a previously healthy 35-year-old woman with bilateral trochanteric bursitis. Biopsy of tissue from the right trochanteric bursa demonstrated positive birefringence, diagnostic of AL amyloidosis. The patient also had a biclonal paraprotein accompanied by calvarial lytic lesions. She was treated with a corticosteroid pulse and bisphosphonates, followed by autologous hematopoietic stem cell transplant. 5 Our case shares features with the above case, including the relatively young age of the patient and the presence of AL amyloidosis.
Our patient wished to avoid a surgical biopsy procedure, and therefore we utilized flow cytometry of the bursal fluid to establish that the etiology of fluid collection was consistent with his concurrent relapse of multiple myeloma. We believe that we are reporting the second case of multiple myeloma-associated bursitis and the first case associated with multiple myeloma relapse; to our knowledge, it is the first to be diagnosed with the aid of flow cytometry.
Because of our patient’s reliance on hemodialysis beginning one year prior to his presentation with olecranon bursitis, we entertain “dialysis elbow” within the differential diagnosis. Dialysis elbow is a relatively uncommon complication of dialysis, in which patients develop olecranon bursitis on the same side as the hemodialysis access after a prolonged (months to years) duration of hemodialysis. Serositis and mechanical forces are the hypothesized etiologies14; infectious and rheumatologic causes were excluded from the reported cases. Nevertheless, we favor a malignant cause based upon the flow cytometry findings indicating involvement by immunophenotypically abnormal plasma cells.
Our patient was treated initially with serial drainage and nonsteroidals, which had little impact. After diagnosis of a plasma cell population in the fluid, we offered local treatment with radiation and systemic treatment of multiple myeloma, which offered better but suboptimal control. Possible treatments for olecranon bursitis include surgery, corticosteroid injections, anti-inflammatories, and serial drainage. Nonsurgical management may be more effective than surgical management, and corticosteroid injection carries significant risks. On the other hand, serial drainage does not confer additional infection risk in cases with aseptic etiology.15 We combined conservative measures as well as treatment of the underlying disease, but we believe that our patient did not derive significant benefit because of the refractory nature of his disease; he also expressed a preference to avoid surgical intervention.
Conclusion
Bursitis is a rare but thought-provoking potential manifestation of multiple myeloma and AL amyloidosis; we believe that our patient’s bursitis was related to plasma cell neoplasia based upon co-occurrence with disease relapse. His bursitis turned out to be an early indicator of impending systemic relapse. In this particular case, in which the patient wished to avoid surgical intervention, flow cytometry was of great value, and we believe that our case is the first report of malignant bursitis being diagnosed by flow cytometry. Our patient’s case shares similarities with other biopsy-confirmed cases of malignant bursitis, but we were able to avoid the need for surgical biopsy or bursal stripping.
The authors thank Jennifer Wilham MT (ASCP), Pat Byrd MT (ASCP), and Darlene Mann MT (ASCP) for their technical support.
1. Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443-459.
2. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–e548.
3. Gozzetti A, Coviello G, Fabbri A, et al. Unusual localizations of plasmacytoma. Leuk Res. 2011;35(7):e104-e105.
4. Kivioja AH, Karaharju EO, Elomaa I, Böhling TO. Surgical treatment of myeloma of bone. Eur J Cancer. 1992;28(11):1865-1869.
5. Santos MS, Soares B, Mendes O, Carvalho CM, Casimiro RF. Multiple myeloma-amyloidosis presenting as pseudomyopathy. Rev Bras Reumatol. 2011;51(6):651-654. 6. Blackwell JR, Hay BA, Bolt AM, May SM. Olecranon bursitis: a systematic overview. Shoulder Elbow. 2014;6(3):182-190.
7. Reilly D, Kamineni S. Olecranon bursitis. J Shoulder Elbow Surg. 2016;25(1):158-167.
8. De Groote J, Geerts B, Mermuys K, Verstraete K. Osteochondroma of the proximal humerus with frictional bursitis and secondary synovial osteochondromatosis. JBR-BTR. 2015;98(1):45-47. 9. Kumar R, Anjana, Kundan M. Retrocalcaneal bursitis due to rare calcaneal osteochrondroma in adult male: excision and outcome. J Orthop Case Rep. 2016;6(2):16-19.
10. Yoon PW, Jang WY, Yoo JJ, Yoon KS, Kim HJ. Malignant fibrous histiocytoma at the site of an alumina-on-alumina-bearing total hip arthroplasty mimicking infected trochanteric bursitis. J Arthroplasty. 2012;27(2):324.e9-324.e12.
11. Hutchison CW, Kling DH. Malignant synovioma. Am J Cancer. 1940;40(1):8-84.
12. Hutchings C, Hull R. Metastatic bone disease presenting as trochanteric bursitis. J R Soc Med. 1997;90(12):685-686.
13. Dorfman HD, Siegel HL, Perry MC, Oxenhandler R. Non-Hodgkin’s lymphoma of the synovium simulating rheumatoid arthritis. Arthritis Rheum. 1987;30(2):155-161.
14. Chao CT, Wu MS. Dialysis elbow. QJM. 2012;105(5):485-486.
15. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: a systematic review. Arch Orthop Trauma Surg. 2014;134(11):1517-1536.
Multiple myeloma is the most common plasma cell neoplasm, with an estimated 24,000 cases occurring annually.1 Symptomatic multiple myeloma most commonly presents with one or more of the cardinal CRAB phenomena of hypercalcemia, renal dysfunction, anemia, or lytic bone lesions.2 Less commonly, patients may present with plasmacytomas (focal lesions of malignant plasma cells), which may involve bony or soft tissues.1
Plasma cell neoplasms occasionally involve the joints, including the elbows, typically as plasmacytomas. The elbow is an unusual but reported location of plasmacytomas.3,4 A case of multiple myeloma and amyloid light-chain (AL) amyloidosis has been reported, with manifestations including pseudomyopathy, bone marrow plasmacytosis, and bilateral trochanteric bursitis.5Bursitis is defined as inflammation of the synovial-fluid–containing sacs that lubricate joints. The olecranon bursa is commonly affected. Etiologies include infection, inflammatory disease, trauma, and malignancy. Furthermore, there is an association between bursitis and immunosuppression.6,7 The most common modes of therapy used to treat bursitis are nonsteroidal anti-inflammatory drugs, corticosteroid injections, and surgical management.
Trochanteric bursitis has been attributed to multiple myeloma in one previous case report, but we are not aware of any previous cases of olecranon bursitis caused by multiple myeloma. Here, we present the case of a 46-year-old man with heavily pretreated multiple myeloma and amyloidosis who developed left olecranon bursitis contemporaneously with disease relapse; flow cytometric analysis of the bursal fluid demonstrated an abnormal plasma cell population, establishing the etiology.
Case presentation and summary
A 46-year-old man with a longstanding history of multiple myeloma developed swelling of the left elbow that was initially painless in September 2016. He had been diagnosed with IgA kappa multiple myeloma and AL deposition in 2011. Over the course of his disease, he was treated with the following sequence of therapies: cyclophosphamide, bortezomib, and dexamethasone, followed by melphalan-conditioned autologous peripheral blood stem cell transplant; lenalidomide and dexamethasone; carfilzomib and dexamethasone; pomalidomide, bortezomib, and dexamethasone; and bortezomib, lenalidomide, dexamethasone, doxorubicin, cyclophosphamide, and etoposide, followed by second melphalan-conditioned autologous peripheral blood stem cell transplant. In addition to treatment with numerous novel and chemotherapeutic agents, his disease course was notable for amyloid deposition in the liver, bone marrow, and kidneys, which resulted in dialysis dependence.
After the second autologous transplant, he achieved a very good partial response and experienced about 9 months of remission, after which laboratory evaluation indicated recurrence of IgA kappa monoclonal protein and free kappa light-chains, which increased slowly over several months without focal symptoms, cytopenias, or decline in organ function (Figure 1).
Twelve months after his second transplant, he presented in September 2016 with 4 weeks of left elbow swelling, with the appearance suggesting a fluid collection over the left olecranon process (Figure 2). The fluid collection was not painful unless bumped or pushed. The maximum pain level was 1-2 on a scale of 0-10. His daughter drained the fluid collection on 2 occasions, but it reaccumulated over 2 to 3 days. He reported no fevers, chills, or sweats. He did not have any redness at the site. He did not report any systemic symptoms.
Physical examination of the left elbow demonstrated a ballotable fluid collection associated with the olecranon, with no associated warmth, tenderness, or erythema. Bursal fluid was sampled, yielding orange-colored serous fluid with bland characteristics (Figure 3). Microbiologic studies were negative (Table 1). We did not suspect a malignant cause initially.
The fluid collection persisted despite treatment with nonsteroidal anti-inflammatory drugs and serial drainage procedures approximately twice per week. It became more erythematous and uncomfortable. We repeated diagnostic sampling at 13 months post-transplant. Cytospin revealed scant plasma cells. A multiparametric 8-color flow cytometric analysis was performed on the bursal fluid. It demonstrated the presence of a small abnormal population of plasma cells (0.04%). The abnormal plasma cells showed expression of CD138 and bright CD38 with aberrant expression of CD56, dim CD45, and loss of CD19, CD81 and CD27. They did not express CD117 or CD20 (Figure 4).
Because of the patient’s discomfort and his history of multidrug-refractory multiple myeloma, we obtained computed tomography imaging of the axial and appendicular skeleton, which demonstrated diffuse small lytic lesions, none larger than 3 mm, including the left elbow joint. The patient began systemic treatment with ixazomib, pomalidomide, and dexamethasone and then received radiation therapy of 20 Gy in 4 fractions to the left olecranon area. The bursal fluid collection remained stable in size but required periodic, though less frequent, drainage procedures. Unfortunately, the patient only tolerated 2 cycles of systemic therapy before experiencing hypercalcemia, exacerbation of hepatic amyloidosis, and a decline in performance status. He died 17 months after the transplant.
Discussion
Our patient experienced left olecranon bursitis simultaneously with relapse of multiple myeloma and AL amyloidosis. Evaluation for infectious causes was negative, and the bursal fluid did not have strongly inflammatory characteristics. Furthermore, a small plasma cell population was isolated from the fluid. Imaging did not reveal an underlying dominant lytic lesion. Although we do not have direct pathologic confirmation, the clinical scenario and flow cytometry findings support our interpretation that the patient’s bursitis was caused by or at least related to underlying multiple myeloma. While reactive plasma cells are also CD38 positive and CD138 positive, they maintain the expression of CD19 and CD45 without aberrant expression of CD56 or CD117 and do not show loss of expression of CD81 or CD27. In this situation, we suspect that either a plasmacytoma involving the soft tissue of the bursa or amyloid infiltration of the synovium may have occurred. Anti-myeloma therapies and radiation therapy did not result in control of the bursitis, though it should be noted that the patient’s highly refractory disease progressed despite treatment with a combination of later-generation immunomodulatory imide and proteasome inhibitor therapies.
Cases of malignant bursitis have been reported several times in the literature, though nearly all of the instances involved connective tissue or metastatic tumors. Tumor histologies include osteochondroma,8,9 malignant fibrous histiocytoma,10 synovial sarcoma,11 and metastatic breast cancer.12
Hematologic malignancies are more rare causes of bursitis; our literature search identified a report of 2 cases of non-Hodgkin lymphoma mimicking rheumatoid arthritis. The joints were the knee and elbow. Synovial fluid from one case was clear and yellow, with leukocytosis with a neutrophilic predominance (similar to our case). In both cases, pathology confirmed lymphomatous infiltration of the synovium.13 Notably, we identified a case of a previously healthy 35-year-old woman with bilateral trochanteric bursitis. Biopsy of tissue from the right trochanteric bursa demonstrated positive birefringence, diagnostic of AL amyloidosis. The patient also had a biclonal paraprotein accompanied by calvarial lytic lesions. She was treated with a corticosteroid pulse and bisphosphonates, followed by autologous hematopoietic stem cell transplant. 5 Our case shares features with the above case, including the relatively young age of the patient and the presence of AL amyloidosis.
Our patient wished to avoid a surgical biopsy procedure, and therefore we utilized flow cytometry of the bursal fluid to establish that the etiology of fluid collection was consistent with his concurrent relapse of multiple myeloma. We believe that we are reporting the second case of multiple myeloma-associated bursitis and the first case associated with multiple myeloma relapse; to our knowledge, it is the first to be diagnosed with the aid of flow cytometry.
Because of our patient’s reliance on hemodialysis beginning one year prior to his presentation with olecranon bursitis, we entertain “dialysis elbow” within the differential diagnosis. Dialysis elbow is a relatively uncommon complication of dialysis, in which patients develop olecranon bursitis on the same side as the hemodialysis access after a prolonged (months to years) duration of hemodialysis. Serositis and mechanical forces are the hypothesized etiologies14; infectious and rheumatologic causes were excluded from the reported cases. Nevertheless, we favor a malignant cause based upon the flow cytometry findings indicating involvement by immunophenotypically abnormal plasma cells.
Our patient was treated initially with serial drainage and nonsteroidals, which had little impact. After diagnosis of a plasma cell population in the fluid, we offered local treatment with radiation and systemic treatment of multiple myeloma, which offered better but suboptimal control. Possible treatments for olecranon bursitis include surgery, corticosteroid injections, anti-inflammatories, and serial drainage. Nonsurgical management may be more effective than surgical management, and corticosteroid injection carries significant risks. On the other hand, serial drainage does not confer additional infection risk in cases with aseptic etiology.15 We combined conservative measures as well as treatment of the underlying disease, but we believe that our patient did not derive significant benefit because of the refractory nature of his disease; he also expressed a preference to avoid surgical intervention.
Conclusion
Bursitis is a rare but thought-provoking potential manifestation of multiple myeloma and AL amyloidosis; we believe that our patient’s bursitis was related to plasma cell neoplasia based upon co-occurrence with disease relapse. His bursitis turned out to be an early indicator of impending systemic relapse. In this particular case, in which the patient wished to avoid surgical intervention, flow cytometry was of great value, and we believe that our case is the first report of malignant bursitis being diagnosed by flow cytometry. Our patient’s case shares similarities with other biopsy-confirmed cases of malignant bursitis, but we were able to avoid the need for surgical biopsy or bursal stripping.
The authors thank Jennifer Wilham MT (ASCP), Pat Byrd MT (ASCP), and Darlene Mann MT (ASCP) for their technical support.
Multiple myeloma is the most common plasma cell neoplasm, with an estimated 24,000 cases occurring annually.1 Symptomatic multiple myeloma most commonly presents with one or more of the cardinal CRAB phenomena of hypercalcemia, renal dysfunction, anemia, or lytic bone lesions.2 Less commonly, patients may present with plasmacytomas (focal lesions of malignant plasma cells), which may involve bony or soft tissues.1
Plasma cell neoplasms occasionally involve the joints, including the elbows, typically as plasmacytomas. The elbow is an unusual but reported location of plasmacytomas.3,4 A case of multiple myeloma and amyloid light-chain (AL) amyloidosis has been reported, with manifestations including pseudomyopathy, bone marrow plasmacytosis, and bilateral trochanteric bursitis.5Bursitis is defined as inflammation of the synovial-fluid–containing sacs that lubricate joints. The olecranon bursa is commonly affected. Etiologies include infection, inflammatory disease, trauma, and malignancy. Furthermore, there is an association between bursitis and immunosuppression.6,7 The most common modes of therapy used to treat bursitis are nonsteroidal anti-inflammatory drugs, corticosteroid injections, and surgical management.
Trochanteric bursitis has been attributed to multiple myeloma in one previous case report, but we are not aware of any previous cases of olecranon bursitis caused by multiple myeloma. Here, we present the case of a 46-year-old man with heavily pretreated multiple myeloma and amyloidosis who developed left olecranon bursitis contemporaneously with disease relapse; flow cytometric analysis of the bursal fluid demonstrated an abnormal plasma cell population, establishing the etiology.
Case presentation and summary
A 46-year-old man with a longstanding history of multiple myeloma developed swelling of the left elbow that was initially painless in September 2016. He had been diagnosed with IgA kappa multiple myeloma and AL deposition in 2011. Over the course of his disease, he was treated with the following sequence of therapies: cyclophosphamide, bortezomib, and dexamethasone, followed by melphalan-conditioned autologous peripheral blood stem cell transplant; lenalidomide and dexamethasone; carfilzomib and dexamethasone; pomalidomide, bortezomib, and dexamethasone; and bortezomib, lenalidomide, dexamethasone, doxorubicin, cyclophosphamide, and etoposide, followed by second melphalan-conditioned autologous peripheral blood stem cell transplant. In addition to treatment with numerous novel and chemotherapeutic agents, his disease course was notable for amyloid deposition in the liver, bone marrow, and kidneys, which resulted in dialysis dependence.
After the second autologous transplant, he achieved a very good partial response and experienced about 9 months of remission, after which laboratory evaluation indicated recurrence of IgA kappa monoclonal protein and free kappa light-chains, which increased slowly over several months without focal symptoms, cytopenias, or decline in organ function (Figure 1).
Twelve months after his second transplant, he presented in September 2016 with 4 weeks of left elbow swelling, with the appearance suggesting a fluid collection over the left olecranon process (Figure 2). The fluid collection was not painful unless bumped or pushed. The maximum pain level was 1-2 on a scale of 0-10. His daughter drained the fluid collection on 2 occasions, but it reaccumulated over 2 to 3 days. He reported no fevers, chills, or sweats. He did not have any redness at the site. He did not report any systemic symptoms.
Physical examination of the left elbow demonstrated a ballotable fluid collection associated with the olecranon, with no associated warmth, tenderness, or erythema. Bursal fluid was sampled, yielding orange-colored serous fluid with bland characteristics (Figure 3). Microbiologic studies were negative (Table 1). We did not suspect a malignant cause initially.
The fluid collection persisted despite treatment with nonsteroidal anti-inflammatory drugs and serial drainage procedures approximately twice per week. It became more erythematous and uncomfortable. We repeated diagnostic sampling at 13 months post-transplant. Cytospin revealed scant plasma cells. A multiparametric 8-color flow cytometric analysis was performed on the bursal fluid. It demonstrated the presence of a small abnormal population of plasma cells (0.04%). The abnormal plasma cells showed expression of CD138 and bright CD38 with aberrant expression of CD56, dim CD45, and loss of CD19, CD81 and CD27. They did not express CD117 or CD20 (Figure 4).
Because of the patient’s discomfort and his history of multidrug-refractory multiple myeloma, we obtained computed tomography imaging of the axial and appendicular skeleton, which demonstrated diffuse small lytic lesions, none larger than 3 mm, including the left elbow joint. The patient began systemic treatment with ixazomib, pomalidomide, and dexamethasone and then received radiation therapy of 20 Gy in 4 fractions to the left olecranon area. The bursal fluid collection remained stable in size but required periodic, though less frequent, drainage procedures. Unfortunately, the patient only tolerated 2 cycles of systemic therapy before experiencing hypercalcemia, exacerbation of hepatic amyloidosis, and a decline in performance status. He died 17 months after the transplant.
Discussion
Our patient experienced left olecranon bursitis simultaneously with relapse of multiple myeloma and AL amyloidosis. Evaluation for infectious causes was negative, and the bursal fluid did not have strongly inflammatory characteristics. Furthermore, a small plasma cell population was isolated from the fluid. Imaging did not reveal an underlying dominant lytic lesion. Although we do not have direct pathologic confirmation, the clinical scenario and flow cytometry findings support our interpretation that the patient’s bursitis was caused by or at least related to underlying multiple myeloma. While reactive plasma cells are also CD38 positive and CD138 positive, they maintain the expression of CD19 and CD45 without aberrant expression of CD56 or CD117 and do not show loss of expression of CD81 or CD27. In this situation, we suspect that either a plasmacytoma involving the soft tissue of the bursa or amyloid infiltration of the synovium may have occurred. Anti-myeloma therapies and radiation therapy did not result in control of the bursitis, though it should be noted that the patient’s highly refractory disease progressed despite treatment with a combination of later-generation immunomodulatory imide and proteasome inhibitor therapies.
Cases of malignant bursitis have been reported several times in the literature, though nearly all of the instances involved connective tissue or metastatic tumors. Tumor histologies include osteochondroma,8,9 malignant fibrous histiocytoma,10 synovial sarcoma,11 and metastatic breast cancer.12
Hematologic malignancies are more rare causes of bursitis; our literature search identified a report of 2 cases of non-Hodgkin lymphoma mimicking rheumatoid arthritis. The joints were the knee and elbow. Synovial fluid from one case was clear and yellow, with leukocytosis with a neutrophilic predominance (similar to our case). In both cases, pathology confirmed lymphomatous infiltration of the synovium.13 Notably, we identified a case of a previously healthy 35-year-old woman with bilateral trochanteric bursitis. Biopsy of tissue from the right trochanteric bursa demonstrated positive birefringence, diagnostic of AL amyloidosis. The patient also had a biclonal paraprotein accompanied by calvarial lytic lesions. She was treated with a corticosteroid pulse and bisphosphonates, followed by autologous hematopoietic stem cell transplant. 5 Our case shares features with the above case, including the relatively young age of the patient and the presence of AL amyloidosis.
Our patient wished to avoid a surgical biopsy procedure, and therefore we utilized flow cytometry of the bursal fluid to establish that the etiology of fluid collection was consistent with his concurrent relapse of multiple myeloma. We believe that we are reporting the second case of multiple myeloma-associated bursitis and the first case associated with multiple myeloma relapse; to our knowledge, it is the first to be diagnosed with the aid of flow cytometry.
Because of our patient’s reliance on hemodialysis beginning one year prior to his presentation with olecranon bursitis, we entertain “dialysis elbow” within the differential diagnosis. Dialysis elbow is a relatively uncommon complication of dialysis, in which patients develop olecranon bursitis on the same side as the hemodialysis access after a prolonged (months to years) duration of hemodialysis. Serositis and mechanical forces are the hypothesized etiologies14; infectious and rheumatologic causes were excluded from the reported cases. Nevertheless, we favor a malignant cause based upon the flow cytometry findings indicating involvement by immunophenotypically abnormal plasma cells.
Our patient was treated initially with serial drainage and nonsteroidals, which had little impact. After diagnosis of a plasma cell population in the fluid, we offered local treatment with radiation and systemic treatment of multiple myeloma, which offered better but suboptimal control. Possible treatments for olecranon bursitis include surgery, corticosteroid injections, anti-inflammatories, and serial drainage. Nonsurgical management may be more effective than surgical management, and corticosteroid injection carries significant risks. On the other hand, serial drainage does not confer additional infection risk in cases with aseptic etiology.15 We combined conservative measures as well as treatment of the underlying disease, but we believe that our patient did not derive significant benefit because of the refractory nature of his disease; he also expressed a preference to avoid surgical intervention.
Conclusion
Bursitis is a rare but thought-provoking potential manifestation of multiple myeloma and AL amyloidosis; we believe that our patient’s bursitis was related to plasma cell neoplasia based upon co-occurrence with disease relapse. His bursitis turned out to be an early indicator of impending systemic relapse. In this particular case, in which the patient wished to avoid surgical intervention, flow cytometry was of great value, and we believe that our case is the first report of malignant bursitis being diagnosed by flow cytometry. Our patient’s case shares similarities with other biopsy-confirmed cases of malignant bursitis, but we were able to avoid the need for surgical biopsy or bursal stripping.
The authors thank Jennifer Wilham MT (ASCP), Pat Byrd MT (ASCP), and Darlene Mann MT (ASCP) for their technical support.
1. Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443-459.
2. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–e548.
3. Gozzetti A, Coviello G, Fabbri A, et al. Unusual localizations of plasmacytoma. Leuk Res. 2011;35(7):e104-e105.
4. Kivioja AH, Karaharju EO, Elomaa I, Böhling TO. Surgical treatment of myeloma of bone. Eur J Cancer. 1992;28(11):1865-1869.
5. Santos MS, Soares B, Mendes O, Carvalho CM, Casimiro RF. Multiple myeloma-amyloidosis presenting as pseudomyopathy. Rev Bras Reumatol. 2011;51(6):651-654. 6. Blackwell JR, Hay BA, Bolt AM, May SM. Olecranon bursitis: a systematic overview. Shoulder Elbow. 2014;6(3):182-190.
7. Reilly D, Kamineni S. Olecranon bursitis. J Shoulder Elbow Surg. 2016;25(1):158-167.
8. De Groote J, Geerts B, Mermuys K, Verstraete K. Osteochondroma of the proximal humerus with frictional bursitis and secondary synovial osteochondromatosis. JBR-BTR. 2015;98(1):45-47. 9. Kumar R, Anjana, Kundan M. Retrocalcaneal bursitis due to rare calcaneal osteochrondroma in adult male: excision and outcome. J Orthop Case Rep. 2016;6(2):16-19.
10. Yoon PW, Jang WY, Yoo JJ, Yoon KS, Kim HJ. Malignant fibrous histiocytoma at the site of an alumina-on-alumina-bearing total hip arthroplasty mimicking infected trochanteric bursitis. J Arthroplasty. 2012;27(2):324.e9-324.e12.
11. Hutchison CW, Kling DH. Malignant synovioma. Am J Cancer. 1940;40(1):8-84.
12. Hutchings C, Hull R. Metastatic bone disease presenting as trochanteric bursitis. J R Soc Med. 1997;90(12):685-686.
13. Dorfman HD, Siegel HL, Perry MC, Oxenhandler R. Non-Hodgkin’s lymphoma of the synovium simulating rheumatoid arthritis. Arthritis Rheum. 1987;30(2):155-161.
14. Chao CT, Wu MS. Dialysis elbow. QJM. 2012;105(5):485-486.
15. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: a systematic review. Arch Orthop Trauma Surg. 2014;134(11):1517-1536.
1. Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443-459.
2. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–e548.
3. Gozzetti A, Coviello G, Fabbri A, et al. Unusual localizations of plasmacytoma. Leuk Res. 2011;35(7):e104-e105.
4. Kivioja AH, Karaharju EO, Elomaa I, Böhling TO. Surgical treatment of myeloma of bone. Eur J Cancer. 1992;28(11):1865-1869.
5. Santos MS, Soares B, Mendes O, Carvalho CM, Casimiro RF. Multiple myeloma-amyloidosis presenting as pseudomyopathy. Rev Bras Reumatol. 2011;51(6):651-654. 6. Blackwell JR, Hay BA, Bolt AM, May SM. Olecranon bursitis: a systematic overview. Shoulder Elbow. 2014;6(3):182-190.
7. Reilly D, Kamineni S. Olecranon bursitis. J Shoulder Elbow Surg. 2016;25(1):158-167.
8. De Groote J, Geerts B, Mermuys K, Verstraete K. Osteochondroma of the proximal humerus with frictional bursitis and secondary synovial osteochondromatosis. JBR-BTR. 2015;98(1):45-47. 9. Kumar R, Anjana, Kundan M. Retrocalcaneal bursitis due to rare calcaneal osteochrondroma in adult male: excision and outcome. J Orthop Case Rep. 2016;6(2):16-19.
10. Yoon PW, Jang WY, Yoo JJ, Yoon KS, Kim HJ. Malignant fibrous histiocytoma at the site of an alumina-on-alumina-bearing total hip arthroplasty mimicking infected trochanteric bursitis. J Arthroplasty. 2012;27(2):324.e9-324.e12.
11. Hutchison CW, Kling DH. Malignant synovioma. Am J Cancer. 1940;40(1):8-84.
12. Hutchings C, Hull R. Metastatic bone disease presenting as trochanteric bursitis. J R Soc Med. 1997;90(12):685-686.
13. Dorfman HD, Siegel HL, Perry MC, Oxenhandler R. Non-Hodgkin’s lymphoma of the synovium simulating rheumatoid arthritis. Arthritis Rheum. 1987;30(2):155-161.
14. Chao CT, Wu MS. Dialysis elbow. QJM. 2012;105(5):485-486.
15. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: a systematic review. Arch Orthop Trauma Surg. 2014;134(11):1517-1536.
Pot for chronic pain? The jury is still out
SAN DIEGO – Is pot a valid alternative to opioids for patients with chronic pain? The verdict on the use of medical marijuana is still hazy, a pain specialist told primary care colleagues. “There’s some evidence for pain, but it’s not extensive,” said Timothy Furnish, MD, of the University of California at San Diego.
Still, he said, studies suggest that the expanding legal use of medical marijuana isn’t boosting opioid use or worsening traffic accident rates. (JAMA Intern Med. 2018;178[5]:667-72; Am J Public Health. 2016 Nov;106[11]:2032-7) And, he said, two things are clear: “There is no one who has overdosed and died specifically from marijuana ... and compared to opioids, cannabinoids have a relatively good safety profile.”
Dr. Furnish spoke in a presentation at Pain Care for Primary Care, a symposium held by the American Pain Society and Global Academy for Medical Education.
Thirty-three states and the District of Columbia allow – or will soon allow – the medical use of marijuana, although their laws and policies vary widely. The newest states to join the list are Utah, Missouri, and Oklahoma, where voters passed medical marijuana measures this year.
Ten states and the District of Columbia also allow the recreational use of marijuana.
However, most states in the South, including Texas and Georgia, don’t allow medical marijuana. The other states that continue to forbid it are in the Midwest and Rocky Mountain regions.
How does cannabis fare against pain? Dr. Furnish pointed to a 2011 systematic review of 18 randomized trials in noncancer chronic pain that showed that “overall there is evidence that cannabinoids are safe and modestly effective in neuropathic pain with preliminary evidence of efficacy in fibromyalgia and rheumatoid arthritis” (Br J Clin Pharmacol. 2011 Nov;72[5]:735-44).
More recently, a 2018 systematic review and meta-analysis of 104 studies found that “it seems unlikely that cannabinoids are highly effective medicines” for chronic noncancer pain (Pain. 2018 Oct;159[10]:1932-54).
In cancer pain, Dr. Furnish said, evidence supporting marijuana is limited and mainly involves nabiximols (Sativex), a cannabis-based inhaled spray that is approved in several nations outside the United States as a treatment for muscle stiffness and spasm in MS. The drug is still an investigational medication in the United States.
How much marijuana should patients with pain take? In the clinic, Dr. Furnish said, “patients actually do best on a relatively modest dose or low dose. High doses are probably more escape than pain relief.”
Dr. Furnish said that because of safety concerns, he never advises patients to smoke marijuana. “Vaporizing may be safer,” he said, noting that the inhaled route has a relatively rapid onset (2-10 minutes) and lasts for 2-4 hours.
Be aware, he said, that cannabis taken orally may not kick in for 30-90 minutes – “it will depend on what they’ve already ingested, and if they have recently consumed a fatty meal” – and patients may have trouble titrating their doses properly. “It’s a little bit easier to ingest more than they intended,” he said.
If you do want to give patients guidance about medical marijuana, keep in mind that “we can’t write a prescription. We only make recommendations,” he said.
“Use some of the same criteria that you’d use in suggesting a patient for opioid therapy,” he said. For example, exclude patients with active psychosis and schizophrenia.
Another red flag is a significant substance abuse history, since there is “abuse and dependence” in marijuana, he said. “Regular heavy users can experience withdrawal, but it tends to be a lot lighter than opioid withdrawal.”
Also be aware, he said, that there’s no federal oversight of medical marijuana, and “production, purity, potency, and state oversight vary widely.”
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Furnish has no disclosures.
SAN DIEGO – Is pot a valid alternative to opioids for patients with chronic pain? The verdict on the use of medical marijuana is still hazy, a pain specialist told primary care colleagues. “There’s some evidence for pain, but it’s not extensive,” said Timothy Furnish, MD, of the University of California at San Diego.
Still, he said, studies suggest that the expanding legal use of medical marijuana isn’t boosting opioid use or worsening traffic accident rates. (JAMA Intern Med. 2018;178[5]:667-72; Am J Public Health. 2016 Nov;106[11]:2032-7) And, he said, two things are clear: “There is no one who has overdosed and died specifically from marijuana ... and compared to opioids, cannabinoids have a relatively good safety profile.”
Dr. Furnish spoke in a presentation at Pain Care for Primary Care, a symposium held by the American Pain Society and Global Academy for Medical Education.
Thirty-three states and the District of Columbia allow – or will soon allow – the medical use of marijuana, although their laws and policies vary widely. The newest states to join the list are Utah, Missouri, and Oklahoma, where voters passed medical marijuana measures this year.
Ten states and the District of Columbia also allow the recreational use of marijuana.
However, most states in the South, including Texas and Georgia, don’t allow medical marijuana. The other states that continue to forbid it are in the Midwest and Rocky Mountain regions.
How does cannabis fare against pain? Dr. Furnish pointed to a 2011 systematic review of 18 randomized trials in noncancer chronic pain that showed that “overall there is evidence that cannabinoids are safe and modestly effective in neuropathic pain with preliminary evidence of efficacy in fibromyalgia and rheumatoid arthritis” (Br J Clin Pharmacol. 2011 Nov;72[5]:735-44).
More recently, a 2018 systematic review and meta-analysis of 104 studies found that “it seems unlikely that cannabinoids are highly effective medicines” for chronic noncancer pain (Pain. 2018 Oct;159[10]:1932-54).
In cancer pain, Dr. Furnish said, evidence supporting marijuana is limited and mainly involves nabiximols (Sativex), a cannabis-based inhaled spray that is approved in several nations outside the United States as a treatment for muscle stiffness and spasm in MS. The drug is still an investigational medication in the United States.
How much marijuana should patients with pain take? In the clinic, Dr. Furnish said, “patients actually do best on a relatively modest dose or low dose. High doses are probably more escape than pain relief.”
Dr. Furnish said that because of safety concerns, he never advises patients to smoke marijuana. “Vaporizing may be safer,” he said, noting that the inhaled route has a relatively rapid onset (2-10 minutes) and lasts for 2-4 hours.
Be aware, he said, that cannabis taken orally may not kick in for 30-90 minutes – “it will depend on what they’ve already ingested, and if they have recently consumed a fatty meal” – and patients may have trouble titrating their doses properly. “It’s a little bit easier to ingest more than they intended,” he said.
If you do want to give patients guidance about medical marijuana, keep in mind that “we can’t write a prescription. We only make recommendations,” he said.
“Use some of the same criteria that you’d use in suggesting a patient for opioid therapy,” he said. For example, exclude patients with active psychosis and schizophrenia.
Another red flag is a significant substance abuse history, since there is “abuse and dependence” in marijuana, he said. “Regular heavy users can experience withdrawal, but it tends to be a lot lighter than opioid withdrawal.”
Also be aware, he said, that there’s no federal oversight of medical marijuana, and “production, purity, potency, and state oversight vary widely.”
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Furnish has no disclosures.
SAN DIEGO – Is pot a valid alternative to opioids for patients with chronic pain? The verdict on the use of medical marijuana is still hazy, a pain specialist told primary care colleagues. “There’s some evidence for pain, but it’s not extensive,” said Timothy Furnish, MD, of the University of California at San Diego.
Still, he said, studies suggest that the expanding legal use of medical marijuana isn’t boosting opioid use or worsening traffic accident rates. (JAMA Intern Med. 2018;178[5]:667-72; Am J Public Health. 2016 Nov;106[11]:2032-7) And, he said, two things are clear: “There is no one who has overdosed and died specifically from marijuana ... and compared to opioids, cannabinoids have a relatively good safety profile.”
Dr. Furnish spoke in a presentation at Pain Care for Primary Care, a symposium held by the American Pain Society and Global Academy for Medical Education.
Thirty-three states and the District of Columbia allow – or will soon allow – the medical use of marijuana, although their laws and policies vary widely. The newest states to join the list are Utah, Missouri, and Oklahoma, where voters passed medical marijuana measures this year.
Ten states and the District of Columbia also allow the recreational use of marijuana.
However, most states in the South, including Texas and Georgia, don’t allow medical marijuana. The other states that continue to forbid it are in the Midwest and Rocky Mountain regions.
How does cannabis fare against pain? Dr. Furnish pointed to a 2011 systematic review of 18 randomized trials in noncancer chronic pain that showed that “overall there is evidence that cannabinoids are safe and modestly effective in neuropathic pain with preliminary evidence of efficacy in fibromyalgia and rheumatoid arthritis” (Br J Clin Pharmacol. 2011 Nov;72[5]:735-44).
More recently, a 2018 systematic review and meta-analysis of 104 studies found that “it seems unlikely that cannabinoids are highly effective medicines” for chronic noncancer pain (Pain. 2018 Oct;159[10]:1932-54).
In cancer pain, Dr. Furnish said, evidence supporting marijuana is limited and mainly involves nabiximols (Sativex), a cannabis-based inhaled spray that is approved in several nations outside the United States as a treatment for muscle stiffness and spasm in MS. The drug is still an investigational medication in the United States.
How much marijuana should patients with pain take? In the clinic, Dr. Furnish said, “patients actually do best on a relatively modest dose or low dose. High doses are probably more escape than pain relief.”
Dr. Furnish said that because of safety concerns, he never advises patients to smoke marijuana. “Vaporizing may be safer,” he said, noting that the inhaled route has a relatively rapid onset (2-10 minutes) and lasts for 2-4 hours.
Be aware, he said, that cannabis taken orally may not kick in for 30-90 minutes – “it will depend on what they’ve already ingested, and if they have recently consumed a fatty meal” – and patients may have trouble titrating their doses properly. “It’s a little bit easier to ingest more than they intended,” he said.
If you do want to give patients guidance about medical marijuana, keep in mind that “we can’t write a prescription. We only make recommendations,” he said.
“Use some of the same criteria that you’d use in suggesting a patient for opioid therapy,” he said. For example, exclude patients with active psychosis and schizophrenia.
Another red flag is a significant substance abuse history, since there is “abuse and dependence” in marijuana, he said. “Regular heavy users can experience withdrawal, but it tends to be a lot lighter than opioid withdrawal.”
Also be aware, he said, that there’s no federal oversight of medical marijuana, and “production, purity, potency, and state oversight vary widely.”
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Furnish has no disclosures.
EXPERT ANALYSIS FROM PAIN CARE FOR PRIMARY CARE
Routine markers predicted histologic response to obeticholic acid in NASH
Routine clinical and laboratory markers predicted histologic response to obeticholic acid therapy among patients with nonalcoholic steatohepatitis (NASH), investigators reported in Gastroenterology.
In a secondary analysis of data from the FLINT trial, histologic response at treatment week 24 correlated significantly with baseline nonalcoholic fatty liver disease activity score (NAS) greater than 5, baseline triglycerides 154 mg/dL or less, baseline international normalized ratio no greater than 1, baseline aspartate aminotransferase (AST) level no greater than 49 U/L, and at least a 17-U/L decrease from baseline in alanine aminotransferase (ALT) level.
A stepwise logistic regression model including these variables and receipt of obeticholic acid distinguished histologic responders from nonresponders with an area under the receiver operating characteristic curve (AUROC) of 0.83 (95% confidence interval, 0.77-0.89; P less than .0001). These parameters “are readily available clinical and biochemical characteristics that are routinely available to clinicians and may be applied to daily practice,” wrote Rohit Loomba, MD, of the University of California, San Diego, with his associates. They may show “that the patients most likely to achieve histologic response are those with higher disease activity, but still with largely conserved liver function, allowing for potential healing or improvement.”
NASH is expected to become the leading reason for liver transplantation in the next few decades. Several treatments can induce histologic hepatic improvement, but none are approved for NASH. Obeticholic acid (Ocaliva) is a selective agonist of the farsenoid X receptor ligand and is indicated for treating primary biliary cholangitis (PBC) in combination with ursodeoxycholic acid (UDCA).
In the 72-week, multicenter, randomized, double-blind FLINT trial, noncirrhotic adults with biopsy-confirmed NASH received once-daily obeticholic acid (25 mg) or placebo. Blinded pathologists interpreted biopsies. The primary endpoint (improvement in liver histology) was met in the interim analysis, so the researchers stopped collecting final liver biopsies.
The secondary analysis included all patients with baseline and final biopsies, including 73 histologic responders and 127 nonresponders. “[The] trends for each of the selected predictors was the same when comparing histologic responders to nonresponders, regardless of treatment group (obeticholic acid versus placebo),” the researchers wrote. The predictors are biologically feasible, the researchers contended – for example, high baseline NAS would be more susceptible to significant improvement, while lower baseline triglyceride levels might reflect a liver that “is less burdened by triglyceride secretion” and, therefore, might have greater capacity to heal. Both AST and ALT “are metrics of liver injury,” and lower baseline AST, in combination with greater reduction in ALT at week 24, probably reflected “AST and ALT levels that are closer to normal,” they added.
Nonetheless, the researchers acknowledged several possible sources of bias. Trial participants were recruited from tertiary care settings and had complete biopsy data, which might not reflect the overall NASH population. Overfitting also could have biased the model because the number of variables assessed approached the number of events being predicted. Furthermore, the model assessed no treatment other than obeticholic acid. “A more robust model could potentially be developed if multiple pharmacological interventions could be considered simultaneously,” the researchers noted. The ongoing phase 3 REGENERATE trial aims to confirm the benefit of obeticholic acid in patients with NASH, they added. Topline results are expected in October 2022.
The FLINT trial was funded by Intercept Pharmaceuticals and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Loomba cochaired the FLINT trial protocol writing committee, is on the steering committee of the ongoing REGENERATE trial, and has received research funding from Intercept Pharmaceuticals, which developed and markets obeticholic acid. Several other coinvestigators reported ties to Intercept and to other pharmaceutical companies.
SOURCE: Loomba R et al. Gastroenterology. 2018 Sep 14. doi: 10.1053/j.gastro.2018.09.021.
Routine clinical and laboratory markers predicted histologic response to obeticholic acid therapy among patients with nonalcoholic steatohepatitis (NASH), investigators reported in Gastroenterology.
In a secondary analysis of data from the FLINT trial, histologic response at treatment week 24 correlated significantly with baseline nonalcoholic fatty liver disease activity score (NAS) greater than 5, baseline triglycerides 154 mg/dL or less, baseline international normalized ratio no greater than 1, baseline aspartate aminotransferase (AST) level no greater than 49 U/L, and at least a 17-U/L decrease from baseline in alanine aminotransferase (ALT) level.
A stepwise logistic regression model including these variables and receipt of obeticholic acid distinguished histologic responders from nonresponders with an area under the receiver operating characteristic curve (AUROC) of 0.83 (95% confidence interval, 0.77-0.89; P less than .0001). These parameters “are readily available clinical and biochemical characteristics that are routinely available to clinicians and may be applied to daily practice,” wrote Rohit Loomba, MD, of the University of California, San Diego, with his associates. They may show “that the patients most likely to achieve histologic response are those with higher disease activity, but still with largely conserved liver function, allowing for potential healing or improvement.”
NASH is expected to become the leading reason for liver transplantation in the next few decades. Several treatments can induce histologic hepatic improvement, but none are approved for NASH. Obeticholic acid (Ocaliva) is a selective agonist of the farsenoid X receptor ligand and is indicated for treating primary biliary cholangitis (PBC) in combination with ursodeoxycholic acid (UDCA).
In the 72-week, multicenter, randomized, double-blind FLINT trial, noncirrhotic adults with biopsy-confirmed NASH received once-daily obeticholic acid (25 mg) or placebo. Blinded pathologists interpreted biopsies. The primary endpoint (improvement in liver histology) was met in the interim analysis, so the researchers stopped collecting final liver biopsies.
The secondary analysis included all patients with baseline and final biopsies, including 73 histologic responders and 127 nonresponders. “[The] trends for each of the selected predictors was the same when comparing histologic responders to nonresponders, regardless of treatment group (obeticholic acid versus placebo),” the researchers wrote. The predictors are biologically feasible, the researchers contended – for example, high baseline NAS would be more susceptible to significant improvement, while lower baseline triglyceride levels might reflect a liver that “is less burdened by triglyceride secretion” and, therefore, might have greater capacity to heal. Both AST and ALT “are metrics of liver injury,” and lower baseline AST, in combination with greater reduction in ALT at week 24, probably reflected “AST and ALT levels that are closer to normal,” they added.
Nonetheless, the researchers acknowledged several possible sources of bias. Trial participants were recruited from tertiary care settings and had complete biopsy data, which might not reflect the overall NASH population. Overfitting also could have biased the model because the number of variables assessed approached the number of events being predicted. Furthermore, the model assessed no treatment other than obeticholic acid. “A more robust model could potentially be developed if multiple pharmacological interventions could be considered simultaneously,” the researchers noted. The ongoing phase 3 REGENERATE trial aims to confirm the benefit of obeticholic acid in patients with NASH, they added. Topline results are expected in October 2022.
The FLINT trial was funded by Intercept Pharmaceuticals and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Loomba cochaired the FLINT trial protocol writing committee, is on the steering committee of the ongoing REGENERATE trial, and has received research funding from Intercept Pharmaceuticals, which developed and markets obeticholic acid. Several other coinvestigators reported ties to Intercept and to other pharmaceutical companies.
SOURCE: Loomba R et al. Gastroenterology. 2018 Sep 14. doi: 10.1053/j.gastro.2018.09.021.
Routine clinical and laboratory markers predicted histologic response to obeticholic acid therapy among patients with nonalcoholic steatohepatitis (NASH), investigators reported in Gastroenterology.
In a secondary analysis of data from the FLINT trial, histologic response at treatment week 24 correlated significantly with baseline nonalcoholic fatty liver disease activity score (NAS) greater than 5, baseline triglycerides 154 mg/dL or less, baseline international normalized ratio no greater than 1, baseline aspartate aminotransferase (AST) level no greater than 49 U/L, and at least a 17-U/L decrease from baseline in alanine aminotransferase (ALT) level.
A stepwise logistic regression model including these variables and receipt of obeticholic acid distinguished histologic responders from nonresponders with an area under the receiver operating characteristic curve (AUROC) of 0.83 (95% confidence interval, 0.77-0.89; P less than .0001). These parameters “are readily available clinical and biochemical characteristics that are routinely available to clinicians and may be applied to daily practice,” wrote Rohit Loomba, MD, of the University of California, San Diego, with his associates. They may show “that the patients most likely to achieve histologic response are those with higher disease activity, but still with largely conserved liver function, allowing for potential healing or improvement.”
NASH is expected to become the leading reason for liver transplantation in the next few decades. Several treatments can induce histologic hepatic improvement, but none are approved for NASH. Obeticholic acid (Ocaliva) is a selective agonist of the farsenoid X receptor ligand and is indicated for treating primary biliary cholangitis (PBC) in combination with ursodeoxycholic acid (UDCA).
In the 72-week, multicenter, randomized, double-blind FLINT trial, noncirrhotic adults with biopsy-confirmed NASH received once-daily obeticholic acid (25 mg) or placebo. Blinded pathologists interpreted biopsies. The primary endpoint (improvement in liver histology) was met in the interim analysis, so the researchers stopped collecting final liver biopsies.
The secondary analysis included all patients with baseline and final biopsies, including 73 histologic responders and 127 nonresponders. “[The] trends for each of the selected predictors was the same when comparing histologic responders to nonresponders, regardless of treatment group (obeticholic acid versus placebo),” the researchers wrote. The predictors are biologically feasible, the researchers contended – for example, high baseline NAS would be more susceptible to significant improvement, while lower baseline triglyceride levels might reflect a liver that “is less burdened by triglyceride secretion” and, therefore, might have greater capacity to heal. Both AST and ALT “are metrics of liver injury,” and lower baseline AST, in combination with greater reduction in ALT at week 24, probably reflected “AST and ALT levels that are closer to normal,” they added.
Nonetheless, the researchers acknowledged several possible sources of bias. Trial participants were recruited from tertiary care settings and had complete biopsy data, which might not reflect the overall NASH population. Overfitting also could have biased the model because the number of variables assessed approached the number of events being predicted. Furthermore, the model assessed no treatment other than obeticholic acid. “A more robust model could potentially be developed if multiple pharmacological interventions could be considered simultaneously,” the researchers noted. The ongoing phase 3 REGENERATE trial aims to confirm the benefit of obeticholic acid in patients with NASH, they added. Topline results are expected in October 2022.
The FLINT trial was funded by Intercept Pharmaceuticals and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Loomba cochaired the FLINT trial protocol writing committee, is on the steering committee of the ongoing REGENERATE trial, and has received research funding from Intercept Pharmaceuticals, which developed and markets obeticholic acid. Several other coinvestigators reported ties to Intercept and to other pharmaceutical companies.
SOURCE: Loomba R et al. Gastroenterology. 2018 Sep 14. doi: 10.1053/j.gastro.2018.09.021.
FROM GASTROENTEROLOGY
Key clinical point: Routine clinical and laboratory parameters were significant correlates of histologic response to obeticholic acid therapy in patients with nonalcoholic steatohepatitis.
Major finding: Significant predictors included baseline nonalcoholic fatty liver disease activity score (NAS) greater than 5, baseline triglycerides up to 154 mg/dL, baseline international normalized ratio up to 1, baseline aspartate aminotransferase up to 49 U/L, and at least a 17-U/L decrease in alanine aminotransferase at week 24.
Study details: Secondary analysis of data from 200 patients with nonalcoholic steatohepatitis in the randomized, double-blind FLINT trial.
Disclosures: The FLINT trial was funded by Intercept Pharmaceuticals and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Loomba cochaired the FLINT trial protocol writing committee, is on the steering committee of the ongoing REGENERATE trial, and has received research funding from Intercept Pharmaceuticals, which developed and markets obeticholic acid. Several other coinvestigators reported ties to Intercept and to other pharmaceutical companies.
Source: Loomba R et al. Gastroenterology. 2018 Sep 14. doi: 10.1053/j.gastro.2018.09.021.
Comorbid depression, anxiety linked to doubled risk of diabetes
Comorbid symptoms of anxiety and depression are associated with twice the risk of developing type 2 diabetes, according to a research paper published in the Journal of Affective Disorders.
The researchers sampled 78,025 Dutch adults aged 30-75 years from the Lifelines Cohort Study and assessed them for depressive and anxious symptoms using the Mini-International Neuropsychiatric Interview before sorting them into groups based on whether they had both, depressive symptoms alone, anxious symptoms alone, or neither.
reported Sonya S. Deschênes, PhD, of the department of psychiatry at McGill University, Montreal, and her associates. Furthermore, in an analysis that adjusted for sociodemographic and lifestyle factors and a family history of diabetes, Dr. Deschênes and her associates found that the participants with both kinds of symptoms had an OR of 1.93 (95% CI, 1.21-3.07) of developing type 2 diabetes. Those with only depressive or anxious symptoms alone did not have a statistically significant risk of developing type 2 diabetes.
A limitation cited by the researchers is that a screening tool was used to assess depressive and anxiety symptoms. Also, glycosylated hemoglobin data were available only for a subset of the participants.
Nevertheless, Dr. Deschênes and her associates wrote, the “study extends ... prior findings and suggests that having co-occurring symptoms of [depression] and anxiety is most strongly associated with an increased risk of [type 2 diabetes]. This study also provides further support for the notion that depression with comorbid anxiety symptoms might represent a group with distinct features.”
SOURCE: Deschênes SS et al. J Affect Disorder. 2018 Oct 1. doi: 10.1016/j.jad.2018.05.029.
Comorbid symptoms of anxiety and depression are associated with twice the risk of developing type 2 diabetes, according to a research paper published in the Journal of Affective Disorders.
The researchers sampled 78,025 Dutch adults aged 30-75 years from the Lifelines Cohort Study and assessed them for depressive and anxious symptoms using the Mini-International Neuropsychiatric Interview before sorting them into groups based on whether they had both, depressive symptoms alone, anxious symptoms alone, or neither.
reported Sonya S. Deschênes, PhD, of the department of psychiatry at McGill University, Montreal, and her associates. Furthermore, in an analysis that adjusted for sociodemographic and lifestyle factors and a family history of diabetes, Dr. Deschênes and her associates found that the participants with both kinds of symptoms had an OR of 1.93 (95% CI, 1.21-3.07) of developing type 2 diabetes. Those with only depressive or anxious symptoms alone did not have a statistically significant risk of developing type 2 diabetes.
A limitation cited by the researchers is that a screening tool was used to assess depressive and anxiety symptoms. Also, glycosylated hemoglobin data were available only for a subset of the participants.
Nevertheless, Dr. Deschênes and her associates wrote, the “study extends ... prior findings and suggests that having co-occurring symptoms of [depression] and anxiety is most strongly associated with an increased risk of [type 2 diabetes]. This study also provides further support for the notion that depression with comorbid anxiety symptoms might represent a group with distinct features.”
SOURCE: Deschênes SS et al. J Affect Disorder. 2018 Oct 1. doi: 10.1016/j.jad.2018.05.029.
Comorbid symptoms of anxiety and depression are associated with twice the risk of developing type 2 diabetes, according to a research paper published in the Journal of Affective Disorders.
The researchers sampled 78,025 Dutch adults aged 30-75 years from the Lifelines Cohort Study and assessed them for depressive and anxious symptoms using the Mini-International Neuropsychiatric Interview before sorting them into groups based on whether they had both, depressive symptoms alone, anxious symptoms alone, or neither.
reported Sonya S. Deschênes, PhD, of the department of psychiatry at McGill University, Montreal, and her associates. Furthermore, in an analysis that adjusted for sociodemographic and lifestyle factors and a family history of diabetes, Dr. Deschênes and her associates found that the participants with both kinds of symptoms had an OR of 1.93 (95% CI, 1.21-3.07) of developing type 2 diabetes. Those with only depressive or anxious symptoms alone did not have a statistically significant risk of developing type 2 diabetes.
A limitation cited by the researchers is that a screening tool was used to assess depressive and anxiety symptoms. Also, glycosylated hemoglobin data were available only for a subset of the participants.
Nevertheless, Dr. Deschênes and her associates wrote, the “study extends ... prior findings and suggests that having co-occurring symptoms of [depression] and anxiety is most strongly associated with an increased risk of [type 2 diabetes]. This study also provides further support for the notion that depression with comorbid anxiety symptoms might represent a group with distinct features.”
SOURCE: Deschênes SS et al. J Affect Disorder. 2018 Oct 1. doi: 10.1016/j.jad.2018.05.029.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Open AAA repair mortality rates doubled for very-low-volume surgeons
NEW YORK – If New York State is representative, the risk of bad outcomes in patients undergoing open abdominal aortic aneurysm repair (OAR) or carotid endarterectomy (CEA), including death in the case of OAR, is about double when performed by very low- versus higher-volume surgeons, according to data presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
“What should we do to fix the problem? We could require surgeons to track their outcomes in quality improvement registry,” suggested Jack L. Cronenwett, MD, professor of surgery, Geisel School of Medicine at Dartmouth, Hanover, N.H.
The outcomes were evaluated from inpatient data gathered from patients undergoing OAR or CEA in an all-payer database involving every hospital discharge in New York State. Surgeons were defined as very-low-volume for a given procedure if they averaged one or less per year, though the results held true if very-low-volume was defined as less than three cases per year, according to Dr. Cronenwett.
The database had outcomes on 8,781 OAR procedures and 68,896 CEA procedures performed from 2000 to 2014.
Of the 614 surgeons who performed one or more OARs over this period, 318 (51.8%) were defined as low-volume surgeons. Despite their substantial representation, they performed just 7.6% of the procedures.
When outcomes from procedures performed by very-low-volume surgeons were compared to those done by higher-volume surgeons, the mortality rates without adjustments were nearly double (6.7% vs. 3.5%; P less than .001). Procedures performed by low-volume surgeons were associated with far higher rates of sepsis or shock (5.7% vs. 3.7%; P = .008), and patients treated by low-volume surgeons were more likely to spend 9 or more days in the hospital (39.3% vs. 30.1%; P less than .001).
When fully adjusted for other variables, “low-volume surgeons had twofold higher odds [OR 2.09] of postoperative death,” Dr. Cronenwett reported.
Of the 1,071 surgeons who performed CEA over this period, 512 (47.8%) were low-volume. They performed 1.3% of the procedures.
Mortality and sepsis or shock following CEA were less than 1% in procedures performed by either low- or higher-volume surgeons without significant differences. However, procedures performed by low-volume surgeons were associated with a three-times higher rate of myocardial infarction (1.5% vs. 0.5%; P less than .001) and a 65% higher rate of stroke (3.5% vs. 2.1%; P = .003).
In addition, patients who underwent CEA performed by a low-volume surgeon had a significantly higher rate of 30-day readmission (11.5% vs. 8.5%; P = .002) and a significantly longer median length of stay (2 days vs. 1 day; P less than .001) than did those treated by a higher-volume surgeon.
Whether OAR or CEA, patients treated by a low-volume surgeon were more likely to have Medicaid coverage. The fact that procedures by low-volume surgeons were more likely to be performed in New York City than other areas of the state suggest that access to care was not a variable, according to Dr. Cronenwett.
Surgeon volume was calculated in this study by dividing the total number of OAR or CEA procedures performed by the number of years that the surgeon was in practice in New York State. Surgeons were classified as vascular surgeons if 75% or more of their surgical practice involved vascular procedures, cardiac surgeons if more than 20% of their surgical practice involved cardiac procedures, and general surgeons if they did not meet either of these criteria.
Of OAR procedures were done by a higher-volume surgeon, approximately 65% were by vascular specialists, 5% were by cardiac specialists, and the remaining were by general surgeons.
Of OAR procedures were done by a low-volume surgeon, approximately 25% were by vascular surgeons, 20% were by cardiac surgeons, and the remaining were by general surgeons. For CEA, there was a somewhat greater representation of general surgeons in both categories, but the patterns were similar.
Dr. Cronenwett argued that more rigorous steps should be taken to ensure that those with proven skills perform OAR and CEA and that open abdominal aortic aneurysm repair should be performed only by high-volume surgeons and hospitals. He suggested there are a variety of incentives or disincentives that could help, but he stressed the importance of tracking results and making them available to referring physicians and to patients.
“Some of the low-volume surgeons are probably not tracking their results so are not even aware of these bad outcomes,” he added.
Dr. Cronenwett reported that he had no relevant disclosures.
NEW YORK – If New York State is representative, the risk of bad outcomes in patients undergoing open abdominal aortic aneurysm repair (OAR) or carotid endarterectomy (CEA), including death in the case of OAR, is about double when performed by very low- versus higher-volume surgeons, according to data presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
“What should we do to fix the problem? We could require surgeons to track their outcomes in quality improvement registry,” suggested Jack L. Cronenwett, MD, professor of surgery, Geisel School of Medicine at Dartmouth, Hanover, N.H.
The outcomes were evaluated from inpatient data gathered from patients undergoing OAR or CEA in an all-payer database involving every hospital discharge in New York State. Surgeons were defined as very-low-volume for a given procedure if they averaged one or less per year, though the results held true if very-low-volume was defined as less than three cases per year, according to Dr. Cronenwett.
The database had outcomes on 8,781 OAR procedures and 68,896 CEA procedures performed from 2000 to 2014.
Of the 614 surgeons who performed one or more OARs over this period, 318 (51.8%) were defined as low-volume surgeons. Despite their substantial representation, they performed just 7.6% of the procedures.
When outcomes from procedures performed by very-low-volume surgeons were compared to those done by higher-volume surgeons, the mortality rates without adjustments were nearly double (6.7% vs. 3.5%; P less than .001). Procedures performed by low-volume surgeons were associated with far higher rates of sepsis or shock (5.7% vs. 3.7%; P = .008), and patients treated by low-volume surgeons were more likely to spend 9 or more days in the hospital (39.3% vs. 30.1%; P less than .001).
When fully adjusted for other variables, “low-volume surgeons had twofold higher odds [OR 2.09] of postoperative death,” Dr. Cronenwett reported.
Of the 1,071 surgeons who performed CEA over this period, 512 (47.8%) were low-volume. They performed 1.3% of the procedures.
Mortality and sepsis or shock following CEA were less than 1% in procedures performed by either low- or higher-volume surgeons without significant differences. However, procedures performed by low-volume surgeons were associated with a three-times higher rate of myocardial infarction (1.5% vs. 0.5%; P less than .001) and a 65% higher rate of stroke (3.5% vs. 2.1%; P = .003).
In addition, patients who underwent CEA performed by a low-volume surgeon had a significantly higher rate of 30-day readmission (11.5% vs. 8.5%; P = .002) and a significantly longer median length of stay (2 days vs. 1 day; P less than .001) than did those treated by a higher-volume surgeon.
Whether OAR or CEA, patients treated by a low-volume surgeon were more likely to have Medicaid coverage. The fact that procedures by low-volume surgeons were more likely to be performed in New York City than other areas of the state suggest that access to care was not a variable, according to Dr. Cronenwett.
Surgeon volume was calculated in this study by dividing the total number of OAR or CEA procedures performed by the number of years that the surgeon was in practice in New York State. Surgeons were classified as vascular surgeons if 75% or more of their surgical practice involved vascular procedures, cardiac surgeons if more than 20% of their surgical practice involved cardiac procedures, and general surgeons if they did not meet either of these criteria.
Of OAR procedures were done by a higher-volume surgeon, approximately 65% were by vascular specialists, 5% were by cardiac specialists, and the remaining were by general surgeons.
Of OAR procedures were done by a low-volume surgeon, approximately 25% were by vascular surgeons, 20% were by cardiac surgeons, and the remaining were by general surgeons. For CEA, there was a somewhat greater representation of general surgeons in both categories, but the patterns were similar.
Dr. Cronenwett argued that more rigorous steps should be taken to ensure that those with proven skills perform OAR and CEA and that open abdominal aortic aneurysm repair should be performed only by high-volume surgeons and hospitals. He suggested there are a variety of incentives or disincentives that could help, but he stressed the importance of tracking results and making them available to referring physicians and to patients.
“Some of the low-volume surgeons are probably not tracking their results so are not even aware of these bad outcomes,” he added.
Dr. Cronenwett reported that he had no relevant disclosures.
NEW YORK – If New York State is representative, the risk of bad outcomes in patients undergoing open abdominal aortic aneurysm repair (OAR) or carotid endarterectomy (CEA), including death in the case of OAR, is about double when performed by very low- versus higher-volume surgeons, according to data presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
“What should we do to fix the problem? We could require surgeons to track their outcomes in quality improvement registry,” suggested Jack L. Cronenwett, MD, professor of surgery, Geisel School of Medicine at Dartmouth, Hanover, N.H.
The outcomes were evaluated from inpatient data gathered from patients undergoing OAR or CEA in an all-payer database involving every hospital discharge in New York State. Surgeons were defined as very-low-volume for a given procedure if they averaged one or less per year, though the results held true if very-low-volume was defined as less than three cases per year, according to Dr. Cronenwett.
The database had outcomes on 8,781 OAR procedures and 68,896 CEA procedures performed from 2000 to 2014.
Of the 614 surgeons who performed one or more OARs over this period, 318 (51.8%) were defined as low-volume surgeons. Despite their substantial representation, they performed just 7.6% of the procedures.
When outcomes from procedures performed by very-low-volume surgeons were compared to those done by higher-volume surgeons, the mortality rates without adjustments were nearly double (6.7% vs. 3.5%; P less than .001). Procedures performed by low-volume surgeons were associated with far higher rates of sepsis or shock (5.7% vs. 3.7%; P = .008), and patients treated by low-volume surgeons were more likely to spend 9 or more days in the hospital (39.3% vs. 30.1%; P less than .001).
When fully adjusted for other variables, “low-volume surgeons had twofold higher odds [OR 2.09] of postoperative death,” Dr. Cronenwett reported.
Of the 1,071 surgeons who performed CEA over this period, 512 (47.8%) were low-volume. They performed 1.3% of the procedures.
Mortality and sepsis or shock following CEA were less than 1% in procedures performed by either low- or higher-volume surgeons without significant differences. However, procedures performed by low-volume surgeons were associated with a three-times higher rate of myocardial infarction (1.5% vs. 0.5%; P less than .001) and a 65% higher rate of stroke (3.5% vs. 2.1%; P = .003).
In addition, patients who underwent CEA performed by a low-volume surgeon had a significantly higher rate of 30-day readmission (11.5% vs. 8.5%; P = .002) and a significantly longer median length of stay (2 days vs. 1 day; P less than .001) than did those treated by a higher-volume surgeon.
Whether OAR or CEA, patients treated by a low-volume surgeon were more likely to have Medicaid coverage. The fact that procedures by low-volume surgeons were more likely to be performed in New York City than other areas of the state suggest that access to care was not a variable, according to Dr. Cronenwett.
Surgeon volume was calculated in this study by dividing the total number of OAR or CEA procedures performed by the number of years that the surgeon was in practice in New York State. Surgeons were classified as vascular surgeons if 75% or more of their surgical practice involved vascular procedures, cardiac surgeons if more than 20% of their surgical practice involved cardiac procedures, and general surgeons if they did not meet either of these criteria.
Of OAR procedures were done by a higher-volume surgeon, approximately 65% were by vascular specialists, 5% were by cardiac specialists, and the remaining were by general surgeons.
Of OAR procedures were done by a low-volume surgeon, approximately 25% were by vascular surgeons, 20% were by cardiac surgeons, and the remaining were by general surgeons. For CEA, there was a somewhat greater representation of general surgeons in both categories, but the patterns were similar.
Dr. Cronenwett argued that more rigorous steps should be taken to ensure that those with proven skills perform OAR and CEA and that open abdominal aortic aneurysm repair should be performed only by high-volume surgeons and hospitals. He suggested there are a variety of incentives or disincentives that could help, but he stressed the importance of tracking results and making them available to referring physicians and to patients.
“Some of the low-volume surgeons are probably not tracking their results so are not even aware of these bad outcomes,” he added.
Dr. Cronenwett reported that he had no relevant disclosures.
REPORTING FROM VEITHSYMPOSIUM
Key clinical point:
Major finding: In-hospital mortality is approximately double (OR 2.09; P less than .001) for very low-volume relative to high-volume surgeon.
Study details: Retrospective database review.
Disclosures: Dr. Cronenwett reports no conflicts of interest.
Source: Cronenwett JL et al. 2018; 45th VEITHsymposium.
Smaller inguinal hernias linked to more postoperative pain
according to a study published in Annals of Surgery.
Researchers used data from the European Herniamed registry to look at 1-year outcomes in 57,999 male patients who underwent primary unilateral inguinal hernia repair.
While patients with larger hernias showed significantly longer operation times and had a significantly larger body mass index, those with smaller hernias had significantly higher rates of pain at rest or on exertion, and chronic pain requiring treatment.
Individuals with smaller hernias (EHS I) had a 35% higher odds of pain at rest, compared with those with medium-sized hernias (EHS II), and 84% higher odds, compared with individuals with large hernias (EHS III). Smaller hernias were also associated with a 100% higher odds of pain on exertion and greater than 100% higher odds of chronic postoperative pain requiring treatment, compared with large hernias.
“CPIP [chronic postoperative inguinal pain] has become one of the most important surgical quality parameters after elective inguinal hernia repair with significant consequences affecting patient productivity, employment, and quality of life,” wrote Henry Hoffmann, MD, of the clinic for visceral surgery at the University Hospital Basel in Switzerland, and his coauthors. “With our findings we contribute to the important discussion of prevention, risk factors, and treatment of CPIP identifying smaller inguinal hernias [EHS I-II] as a new independent, patient-related risk factor for the development of CPIP.”
Noting that an association between smaller hernias and increased postoperative pain seems counterintuitive, the authors suggested that patients’ expectations of outcomes may be higher in those with smaller hernias than in patients with larger hernias. While larger hernias are likely more bothersome rather than painful or uncomfortable, smaller hernias may be associated more with pain and discomfort. As such, patients with smaller hernias may have higher hopes for relief from surgery, and therefore may be more likely to experience disappointment.
“Based on a cognitive information processing model it has been suggested that a greater discrepancy between expected and actual pain after surgery leads to significant postoperative distress,” the authors wrote.
The study also found patients aged younger than 55 years were also at greater risk of pain at rest, pain on exertion, and chronic pain requiring treatment.
Overall, around half the patients in the study underwent transabdominal preperitoneal repair, 35.6% underwent totally extraperitoneal repair, 10.1% had Lichtenstein repair, and 3.8% had Shouldice repair.
Lichtenstein was associated with more postoperative pain at rest and on exertion, compared with other surgical methods, and there was a trend toward an increased odds for developing pain requiring treatment in patients with smaller hernias.
The Herniamed Registry is supported by Johnson & Johnson, Karl Storz, pfm medical Cologne, Dahlhausen Cologne, B. Braun Tuttlingen, MenkeMed, and Bard. No conflicts of interest were reported.
SOURCE: Hoffmann H et al. Ann Surg. 2018 Oct 10. doi: 10.1097/SLA.0000000000003065.
according to a study published in Annals of Surgery.
Researchers used data from the European Herniamed registry to look at 1-year outcomes in 57,999 male patients who underwent primary unilateral inguinal hernia repair.
While patients with larger hernias showed significantly longer operation times and had a significantly larger body mass index, those with smaller hernias had significantly higher rates of pain at rest or on exertion, and chronic pain requiring treatment.
Individuals with smaller hernias (EHS I) had a 35% higher odds of pain at rest, compared with those with medium-sized hernias (EHS II), and 84% higher odds, compared with individuals with large hernias (EHS III). Smaller hernias were also associated with a 100% higher odds of pain on exertion and greater than 100% higher odds of chronic postoperative pain requiring treatment, compared with large hernias.
“CPIP [chronic postoperative inguinal pain] has become one of the most important surgical quality parameters after elective inguinal hernia repair with significant consequences affecting patient productivity, employment, and quality of life,” wrote Henry Hoffmann, MD, of the clinic for visceral surgery at the University Hospital Basel in Switzerland, and his coauthors. “With our findings we contribute to the important discussion of prevention, risk factors, and treatment of CPIP identifying smaller inguinal hernias [EHS I-II] as a new independent, patient-related risk factor for the development of CPIP.”
Noting that an association between smaller hernias and increased postoperative pain seems counterintuitive, the authors suggested that patients’ expectations of outcomes may be higher in those with smaller hernias than in patients with larger hernias. While larger hernias are likely more bothersome rather than painful or uncomfortable, smaller hernias may be associated more with pain and discomfort. As such, patients with smaller hernias may have higher hopes for relief from surgery, and therefore may be more likely to experience disappointment.
“Based on a cognitive information processing model it has been suggested that a greater discrepancy between expected and actual pain after surgery leads to significant postoperative distress,” the authors wrote.
The study also found patients aged younger than 55 years were also at greater risk of pain at rest, pain on exertion, and chronic pain requiring treatment.
Overall, around half the patients in the study underwent transabdominal preperitoneal repair, 35.6% underwent totally extraperitoneal repair, 10.1% had Lichtenstein repair, and 3.8% had Shouldice repair.
Lichtenstein was associated with more postoperative pain at rest and on exertion, compared with other surgical methods, and there was a trend toward an increased odds for developing pain requiring treatment in patients with smaller hernias.
The Herniamed Registry is supported by Johnson & Johnson, Karl Storz, pfm medical Cologne, Dahlhausen Cologne, B. Braun Tuttlingen, MenkeMed, and Bard. No conflicts of interest were reported.
SOURCE: Hoffmann H et al. Ann Surg. 2018 Oct 10. doi: 10.1097/SLA.0000000000003065.
according to a study published in Annals of Surgery.
Researchers used data from the European Herniamed registry to look at 1-year outcomes in 57,999 male patients who underwent primary unilateral inguinal hernia repair.
While patients with larger hernias showed significantly longer operation times and had a significantly larger body mass index, those with smaller hernias had significantly higher rates of pain at rest or on exertion, and chronic pain requiring treatment.
Individuals with smaller hernias (EHS I) had a 35% higher odds of pain at rest, compared with those with medium-sized hernias (EHS II), and 84% higher odds, compared with individuals with large hernias (EHS III). Smaller hernias were also associated with a 100% higher odds of pain on exertion and greater than 100% higher odds of chronic postoperative pain requiring treatment, compared with large hernias.
“CPIP [chronic postoperative inguinal pain] has become one of the most important surgical quality parameters after elective inguinal hernia repair with significant consequences affecting patient productivity, employment, and quality of life,” wrote Henry Hoffmann, MD, of the clinic for visceral surgery at the University Hospital Basel in Switzerland, and his coauthors. “With our findings we contribute to the important discussion of prevention, risk factors, and treatment of CPIP identifying smaller inguinal hernias [EHS I-II] as a new independent, patient-related risk factor for the development of CPIP.”
Noting that an association between smaller hernias and increased postoperative pain seems counterintuitive, the authors suggested that patients’ expectations of outcomes may be higher in those with smaller hernias than in patients with larger hernias. While larger hernias are likely more bothersome rather than painful or uncomfortable, smaller hernias may be associated more with pain and discomfort. As such, patients with smaller hernias may have higher hopes for relief from surgery, and therefore may be more likely to experience disappointment.
“Based on a cognitive information processing model it has been suggested that a greater discrepancy between expected and actual pain after surgery leads to significant postoperative distress,” the authors wrote.
The study also found patients aged younger than 55 years were also at greater risk of pain at rest, pain on exertion, and chronic pain requiring treatment.
Overall, around half the patients in the study underwent transabdominal preperitoneal repair, 35.6% underwent totally extraperitoneal repair, 10.1% had Lichtenstein repair, and 3.8% had Shouldice repair.
Lichtenstein was associated with more postoperative pain at rest and on exertion, compared with other surgical methods, and there was a trend toward an increased odds for developing pain requiring treatment in patients with smaller hernias.
The Herniamed Registry is supported by Johnson & Johnson, Karl Storz, pfm medical Cologne, Dahlhausen Cologne, B. Braun Tuttlingen, MenkeMed, and Bard. No conflicts of interest were reported.
SOURCE: Hoffmann H et al. Ann Surg. 2018 Oct 10. doi: 10.1097/SLA.0000000000003065.
FROM ANNALS OF SURGERY
Key clinical point: Smaller inguinal hernias associated with more postoperative pain than larger hernias.
Major finding: Smaller hernias are associated with a 100% higher odds of pain on exertion after surgery than those with large hernias.
Study details: An analysis of registry data from 57,999 patients who underwent hernia repair.
Disclosures: The Herniamed registry is supported by Johnson & Johnson, Karl Storz, pfm medical Cologne, Dahlhausen Cologne, B. Braun Tuttlingen, MenkeMed, and Bard. No conflicts of interest were reported.
Source: Hoffmann H et al. Ann Surg. 2018 Oct 10. doi: 10.1097/SLA.0000000000003065.
Rheumatologists underscreened RA patients for hyperlipidemia
Patients with rheumatoid arthritis had low rates of screening for primary hyperlipidemia, but patients who visited both a rheumatologist and a nonrheumatology clinician for follow-up were more likely to be screened, according to research published in Arthritis Care & Research.
Iris Navarro-Millán, MD, MSPH, from Cornell University, New York, and her colleagues performed a retrospective study of roughly 244,000 participants with private insurance, Medicare, or Medicaid who had rheumatoid arthritis (13,000 patients), diabetes (63,000), both rheumatoid arthritis (RA) and diabetes (1,000), and neither RA nor diabetes (168,000) during 2006-2010. Patients were between the ages of 41 years and 85 years. There were similar rates of hypertension among the RA patients (40%) and patients with neither RA nor diabetes (39%), as well as among the diabetes patients (79%) and the both patients with both RA and diabetes (70%).
The researchers found 37% of RA patients, 60% of diabetes patients, 55% of patients with both RA and diabetes, and 41% of patients with neither RA nor diabetes received primary lipid screening over 2 years’ follow-up. For RA-only patients, 22% saw a rheumatologist only, while 56% saw both a rheumatologist and then a nonrheumatology clinician at 2-years’ follow-up. Patients who visited a rheumatologist and a nonrheumatology clinician for follow-up were 55% more likely to receive primary lipid screening, and RA patients who visited a nonrheumatology clinician only were 22% more likely to receive screening than RA patients who saw only a rheumatologist.
Dr. Navarro-Millán and her colleagues said European RA patients may be more likely to receive screening for hyperlipidemia because their health care systems follow European League Against Rheumatism (EULAR) recommendations for management of cardiovascular disease (CVD) risk, while many rheumatologists based in the United States are “still reluctant to take responsibility to assess and mitigate (if needed) CVD risk for patients with RA.”
“Measures to achieve this goal must be implemented and may include defining specific roles for rheumatologists, nonrheumatology practitioners, and patients to determine who should be responsible for hyperlipidemia screening and treatment for patients with RA,” Dr. Navarro-Millán and her colleagues wrote.
Limitations to the study include lack of information on uninsured patients, lack of some clinical and demographic data, and potential misclassification of some clinicians who may have provided rheumatology-specific care but may have been in primary care settings.
This study is funded by grants and contracts from the National Institutes of Health and the Department of Health and Human Services. The authors report no relevant financial disclosures.
SOURCE: Navarro-Millán I et al. Arthritis Care Res. 2018. doi: 10.1002/acr.23810.
Patients with rheumatoid arthritis had low rates of screening for primary hyperlipidemia, but patients who visited both a rheumatologist and a nonrheumatology clinician for follow-up were more likely to be screened, according to research published in Arthritis Care & Research.
Iris Navarro-Millán, MD, MSPH, from Cornell University, New York, and her colleagues performed a retrospective study of roughly 244,000 participants with private insurance, Medicare, or Medicaid who had rheumatoid arthritis (13,000 patients), diabetes (63,000), both rheumatoid arthritis (RA) and diabetes (1,000), and neither RA nor diabetes (168,000) during 2006-2010. Patients were between the ages of 41 years and 85 years. There were similar rates of hypertension among the RA patients (40%) and patients with neither RA nor diabetes (39%), as well as among the diabetes patients (79%) and the both patients with both RA and diabetes (70%).
The researchers found 37% of RA patients, 60% of diabetes patients, 55% of patients with both RA and diabetes, and 41% of patients with neither RA nor diabetes received primary lipid screening over 2 years’ follow-up. For RA-only patients, 22% saw a rheumatologist only, while 56% saw both a rheumatologist and then a nonrheumatology clinician at 2-years’ follow-up. Patients who visited a rheumatologist and a nonrheumatology clinician for follow-up were 55% more likely to receive primary lipid screening, and RA patients who visited a nonrheumatology clinician only were 22% more likely to receive screening than RA patients who saw only a rheumatologist.
Dr. Navarro-Millán and her colleagues said European RA patients may be more likely to receive screening for hyperlipidemia because their health care systems follow European League Against Rheumatism (EULAR) recommendations for management of cardiovascular disease (CVD) risk, while many rheumatologists based in the United States are “still reluctant to take responsibility to assess and mitigate (if needed) CVD risk for patients with RA.”
“Measures to achieve this goal must be implemented and may include defining specific roles for rheumatologists, nonrheumatology practitioners, and patients to determine who should be responsible for hyperlipidemia screening and treatment for patients with RA,” Dr. Navarro-Millán and her colleagues wrote.
Limitations to the study include lack of information on uninsured patients, lack of some clinical and demographic data, and potential misclassification of some clinicians who may have provided rheumatology-specific care but may have been in primary care settings.
This study is funded by grants and contracts from the National Institutes of Health and the Department of Health and Human Services. The authors report no relevant financial disclosures.
SOURCE: Navarro-Millán I et al. Arthritis Care Res. 2018. doi: 10.1002/acr.23810.
Patients with rheumatoid arthritis had low rates of screening for primary hyperlipidemia, but patients who visited both a rheumatologist and a nonrheumatology clinician for follow-up were more likely to be screened, according to research published in Arthritis Care & Research.
Iris Navarro-Millán, MD, MSPH, from Cornell University, New York, and her colleagues performed a retrospective study of roughly 244,000 participants with private insurance, Medicare, or Medicaid who had rheumatoid arthritis (13,000 patients), diabetes (63,000), both rheumatoid arthritis (RA) and diabetes (1,000), and neither RA nor diabetes (168,000) during 2006-2010. Patients were between the ages of 41 years and 85 years. There were similar rates of hypertension among the RA patients (40%) and patients with neither RA nor diabetes (39%), as well as among the diabetes patients (79%) and the both patients with both RA and diabetes (70%).
The researchers found 37% of RA patients, 60% of diabetes patients, 55% of patients with both RA and diabetes, and 41% of patients with neither RA nor diabetes received primary lipid screening over 2 years’ follow-up. For RA-only patients, 22% saw a rheumatologist only, while 56% saw both a rheumatologist and then a nonrheumatology clinician at 2-years’ follow-up. Patients who visited a rheumatologist and a nonrheumatology clinician for follow-up were 55% more likely to receive primary lipid screening, and RA patients who visited a nonrheumatology clinician only were 22% more likely to receive screening than RA patients who saw only a rheumatologist.
Dr. Navarro-Millán and her colleagues said European RA patients may be more likely to receive screening for hyperlipidemia because their health care systems follow European League Against Rheumatism (EULAR) recommendations for management of cardiovascular disease (CVD) risk, while many rheumatologists based in the United States are “still reluctant to take responsibility to assess and mitigate (if needed) CVD risk for patients with RA.”
“Measures to achieve this goal must be implemented and may include defining specific roles for rheumatologists, nonrheumatology practitioners, and patients to determine who should be responsible for hyperlipidemia screening and treatment for patients with RA,” Dr. Navarro-Millán and her colleagues wrote.
Limitations to the study include lack of information on uninsured patients, lack of some clinical and demographic data, and potential misclassification of some clinicians who may have provided rheumatology-specific care but may have been in primary care settings.
This study is funded by grants and contracts from the National Institutes of Health and the Department of Health and Human Services. The authors report no relevant financial disclosures.
SOURCE: Navarro-Millán I et al. Arthritis Care Res. 2018. doi: 10.1002/acr.23810.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Patients with rheumatoid arthritis who saw only a rheumatologist were less likely to receive primary lipid screening than those who saw both a rheumatologist and then a clinician at 2-year follow-up.
Major finding: 37% of RA patients, 60% of diabetes patients, 55% of patients with both RA and diabetes, and 41% of patients with neither RA nor diabetes received primary lipid screening over 2-year follow-up.
Study details: A retrospective study of claims data from 243,909 participants aged 41-85 years in private and public health plans during 2006-2010.
Disclosures: This study is funded by grants and contracts from the National Institutes of Health and the Department of Health and Human Services. The authors report no relevant financial disclosures.
Source: Navarro-Millán I et al. Arthritis Care Res. 2018. doi: 10.1002/acr.23810.