User login
SABCS 2018: PHARE, KATHERINE, and KATE2 in HER2+ breast cancer
Revisiting the old and enhancing with the new might describe the range of results in HER2+ breast cancer studies to be presented at the upcoming San Antonio Breast Cancer Symposium, which will be held Dec. 4-8 in San Antonio.
Since 2005, 12 months of trastuzumab added to chemotherapy alone has been the standard of care in patients with HER2-positive early breast cancer. PHARE (Protocol for Herceptin as Adjuvant Therapy With Reduced Exposure) was the first trial evaluating a reduced schedule of trastuzumab, a noninferiority trial comparing 6 with 12 months of adjuvant trastuzumab. Results published in 2013 in Lancet Oncology demonstrated a failure to prove that 6 months of treatment was non-inferior to 12 months. The final analysis of PHARE will be presented on Wednesday at SABCS 2018 by Xavier Pivot, MD, PhD, of Paul-Strauss Cancer Centre, Université de Strasbourg (France).
In a more recent study, trastuzumab emtansine (T-DM1) was pitted against trastuzumab as adjuvant therapy in patients with HER2-positive early breast cancer with residual invasive disease after neoadjuvant chemotherapy and HER2-targeted therapy including trastuzumab. The primary results of the phase 3 study (KATHERINE) will be presented by Charles E. Geyer, MD, of Virginia Commonwealth University and the Massey Cancer Center, both in Richmond.
As for the new, KATE2 is a phase 2 randomized trial evaluating the addition of checkpoint inhibitor atezolizumab to T-DM1 for patients with locally advanced or metastatic HER2-positive breast cancer who received prior trastuzumab and taxane-based therapy. Results will be presented by Leisha A. Emens, MD, PhD, professor at the University of Pittsburgh and director of translational immunotherapy for the Women’s Cancer Research Center there.
Revisiting the old and enhancing with the new might describe the range of results in HER2+ breast cancer studies to be presented at the upcoming San Antonio Breast Cancer Symposium, which will be held Dec. 4-8 in San Antonio.
Since 2005, 12 months of trastuzumab added to chemotherapy alone has been the standard of care in patients with HER2-positive early breast cancer. PHARE (Protocol for Herceptin as Adjuvant Therapy With Reduced Exposure) was the first trial evaluating a reduced schedule of trastuzumab, a noninferiority trial comparing 6 with 12 months of adjuvant trastuzumab. Results published in 2013 in Lancet Oncology demonstrated a failure to prove that 6 months of treatment was non-inferior to 12 months. The final analysis of PHARE will be presented on Wednesday at SABCS 2018 by Xavier Pivot, MD, PhD, of Paul-Strauss Cancer Centre, Université de Strasbourg (France).
In a more recent study, trastuzumab emtansine (T-DM1) was pitted against trastuzumab as adjuvant therapy in patients with HER2-positive early breast cancer with residual invasive disease after neoadjuvant chemotherapy and HER2-targeted therapy including trastuzumab. The primary results of the phase 3 study (KATHERINE) will be presented by Charles E. Geyer, MD, of Virginia Commonwealth University and the Massey Cancer Center, both in Richmond.
As for the new, KATE2 is a phase 2 randomized trial evaluating the addition of checkpoint inhibitor atezolizumab to T-DM1 for patients with locally advanced or metastatic HER2-positive breast cancer who received prior trastuzumab and taxane-based therapy. Results will be presented by Leisha A. Emens, MD, PhD, professor at the University of Pittsburgh and director of translational immunotherapy for the Women’s Cancer Research Center there.
Revisiting the old and enhancing with the new might describe the range of results in HER2+ breast cancer studies to be presented at the upcoming San Antonio Breast Cancer Symposium, which will be held Dec. 4-8 in San Antonio.
Since 2005, 12 months of trastuzumab added to chemotherapy alone has been the standard of care in patients with HER2-positive early breast cancer. PHARE (Protocol for Herceptin as Adjuvant Therapy With Reduced Exposure) was the first trial evaluating a reduced schedule of trastuzumab, a noninferiority trial comparing 6 with 12 months of adjuvant trastuzumab. Results published in 2013 in Lancet Oncology demonstrated a failure to prove that 6 months of treatment was non-inferior to 12 months. The final analysis of PHARE will be presented on Wednesday at SABCS 2018 by Xavier Pivot, MD, PhD, of Paul-Strauss Cancer Centre, Université de Strasbourg (France).
In a more recent study, trastuzumab emtansine (T-DM1) was pitted against trastuzumab as adjuvant therapy in patients with HER2-positive early breast cancer with residual invasive disease after neoadjuvant chemotherapy and HER2-targeted therapy including trastuzumab. The primary results of the phase 3 study (KATHERINE) will be presented by Charles E. Geyer, MD, of Virginia Commonwealth University and the Massey Cancer Center, both in Richmond.
As for the new, KATE2 is a phase 2 randomized trial evaluating the addition of checkpoint inhibitor atezolizumab to T-DM1 for patients with locally advanced or metastatic HER2-positive breast cancer who received prior trastuzumab and taxane-based therapy. Results will be presented by Leisha A. Emens, MD, PhD, professor at the University of Pittsburgh and director of translational immunotherapy for the Women’s Cancer Research Center there.
Three drugs disappoint in SSc trials, but show some promise
CHICAGO – Recent randomized, placebo-controlled, phase 3 trials of tocilizumab, abatacept, and riociguat for the treatment of systemic sclerosis each failed to reach its primary endpoint of change from baseline in modified Rodnan Skin Score (mRSS).
Still, findings with respect to secondary endpoints and certain exploratory outcomes suggest each of the agents holds some promise in the systemic sclerosis (SSc) arena, according to the data presented at the annual meeting of the American College of Rheumatology.
Tocilizumab (Actemra)
In the double-blind portion of the phase 3 focuSSced trial of 212 patients with SSc, numerical improvement was observed for the primary endpoint of mean change in mRSS from baseline to week 48 with tocilizumab versus placebo (–6.14 vs. –4.41 points, respectively). The change in the treatment group was comparable with what was seen in the phase 2 faSScinate trial, but the decline in mRSS in the placebo group was much greater in phase 3 than in phase 2, and so the difference between the groups in the current study failed to reach statistical significance (P = .098), reported Dinesh Khanna, MBBS, a professor of medicine and director of the scleroderma program at the University of Michigan, Ann Arbor.
The interleukin-6 (IL-6) receptor–alpha antibody was previously shown in the faSScinate trial to lead to numeric improvements in skin thickening as measured by the mRSS, as well as to clinically meaningful lung function preservation as measured by percent predicted forced vital capacity (FVC).
In the current phase 3 study, key secondary end points also appeared to favor tocilizumab, but since the primary endpoint for mRSS was not met, all other P values cannot be considered statistically significant despite the strength of the evidence and were reported for informational purposes only, he noted.
The median cumulative distribution of change from baseline to week 48 in percent predicted FVC with tocilizumab versus placebo was –0.6 vs. –3.9, respectively (descriptive P = .0015), and the mean change from baseline in FVC at week 48 was –24 mL vs. –190 mL (difference of 167 mL in favor of tocilizumab; descriptive P = .0001).
Time to treatment failure also favored tocilizumab, he said (hazard ratio, 0.63; descriptive P = .082), he said.
Patients were randomly assigned to receive either weekly 162-mg injections of subcutaneous tocilizumab or placebo for 48 weeks. Escape therapy was allowed beginning at week 16 if patients experienced declines in FVC or beginning at week 24 if they experienced worsened mRSS or worsened SSc complications, Dr. Khanna said.
“The key part is that no immunotherapy was allowed. ... So it’s a true randomized, placebo-controlled trial,” he said.
Most (81%) of the patients were women, and they had a mean age of 48 years, mean SSc duration of 23 months, mean mRSS of 20.4 units on a 0-51 scale, and a normal mean percent predicted FVC of 82.1%.
“HAQ-DI showed moderate disability of 1.2,” he noted.
Safety in the study was consistent with that seen in prior tocilizumab studies; no new safety signals were identified. Serious adverse events occurred in 13% and 17% of tocilizumab and placebo group patients , respectively, and serious infections were reported by 7% and 2%.
Although clinically meaningful and consistent differences in FVC favoring tocilizumab were shown in this study, the primary endpoint was not met, Dr. Khanna said.
“There were no statistically significant differences, largely driven by unexpected improvement in the placebo group, which was different than what we found in [the faSScinate] trial,” he said, noting, however, that the FVC findings in the current study were clinically meaningful.
Also, in a separate presentation at the meeting, he explained that the differences favoring tocilizumab were statistically significant when patient-level data from the trial were analyzed based on the ACR Composite Response Index in Systemic Sclerosis (CRISS). Those findings provide validation of the novel outcomes measure, he said.
Abatacept (Orencia)
Dr. Khanna also reported results of the 12-month, double-blind, randomized, placebo-controlled phase 2 ASSET trial of abatacept, which showed no significant difference in mRSS in patients with early diffuse cutaneous SSc (dfSSc) who were treated with 125 mg of the recombinant fusion protein weekly and those who received placebo. However, certain secondary outcomes favored abatacept. No concomitant immunotherapy was allowed.
The adjusted mean decrease in the mRSS among patients who completed the 12-month treatment period was –6.24 vs. –4.49 in 34 patients in the abatacept group and 35 in the placebo group, respectively (P = .28).
The secondary outcome measures of mean change in Health Assessment Questionnaire Disability Index (HAQ-DI), patients global assessment, physician global assessment, and ACR CRISS scores were statistically significant or showed numerical results favoring abatacept over placebo: mean decrease in HAQ-DI, –0.17 vs. –0.11 (P = .05), respectively; mean change in physician global assessment scores, –1.30 vs. –0.35 (P = .03); median ACR CRISS index, 0.68 vs. 0.01 (P = .03), decline in percent predicted FVC of 4.13% and 1.34% (P = .11).
Escape therapy was allowed at 6 months for worsening SSc, but it did not change the outcomes trajectory, he said. A larger proportion of placebo vs. abatacept subjects required escape immunosuppressive therapy (36% vs. 16%; P = .03).
Patients were enrolled between 2014 and 2018 at 27 U.S., Canadian, and U.K. sites. At baseline, participants had a mean age of 49 years, 75% were women, and mean disease duration was very short at 1.59 years, with 60% having disease duration of 18 months or less. The mean baseline mRSS was 22.4, mean percent predicted FVC was 85.3%, and mean HAQ-DI was 1.0.
Compliance with both treatments was greater than 98%. Abatacept was well tolerated with comparable adverse events (AEs), serious AEs, and AEs of special interest such as infections and malignancies between treatments, Dr. Khanna said, noting that two deaths occurred in the abatacept group (caused by scleroderma renal crisis in both cases at days 11 and 46) and one occurred in a placebo group patient who experienced sudden cardiac arrest at day 310.
Of note, mRSS showed large variability, despite recruiting an early dcSSc population, Dr. Khanna said.
The finding with respect to the primary outcome is consistent with other recent trials because of improvement in mRSS that’s part of the natural history of the disease, including the tocilizumab findings that he reported at the meeting. The findings with respect to secondary endpoints and safety show promise.
“Stay tuned for robust ongoing work on the relationship between clinical changes and ongoing mechanistic work,” he said.
Riociguat (Adempas)
Similarly, in the randomized, placebo-controlled phase 2b RISE-SSc study comparing riociguat and placebo for early dcSSc, the primary efficacy endpoint of mean change in mRSS did not reach statistical significance, but exploratory data suggested that the soluble guanylate cyclase stimulator prevented disease progression in patients with early dcSSc, reported Oliver Distler, MD, head of the connective tissue diseases program at University Hospital Zurich (Switzerland).
The mean mRSS at baseline was comparable in 60 patients randomized to receive riociguat and 61 in the placebo group (16.8 and 16.71, respectively). These mean values at week 52 dropped to 14.63 vs. 15.73, respectively (P = .08).
“So it was close, but it didn’t reach significance,” he said.
The difference in the mRSS progression rate, however, suggested significant effects favoring riociguat (descriptive P = .02), he said.
Further, mean change from baseline to week 52 in percent predicted FVC was not different overall between the groups, but a large difference favoring riociguat was seen among patients with scleroderma interstitial lung disease at baseline (mean change of –2.7 vs. –8.9), he said.
No differences were seen between the groups in HAQ-DI or patient and physician global assessment. The proportion of patients with probability of improvement at 52 weeks as measured using ACR CRISS was also the same at 18% in both treatment arms, he noted, ”but the CRISS is designed more for assessing disease regression than for assessing prevention of progression.”
Treatment was, however, well tolerated. At week 52, fewer serious adverse events occurred with riociguat group than in the placebo group (15% vs. 25%, respectively), and no new safety signals were observed, he said.
Riociguat has previously shown antifibrotic effects in animal models and efficacy in patients with pulmonary arterial hypertension associated with connective tissue disease, so it was hypothesized that patients with dcSSc might benefit from riociguat therapy, Dr. Distler explained.
Study subjects had very early dcSSc (duration of 18 months or less; mean of 9 months), mRSS of 10-22 units, FVC of 45% predicted or greater, and diffusion capacity of the lung for carbon monoxide of at least 40% of predicted at screening.
Riociguat was given at an individually adjusted dose between 0.5 mg and 2.5 mg three times daily.
The findings demonstrate a numeric decrease in mRSS over time with riociguat versus placebo and a prevention of progression with riociguat; the failure to reach the primary endpoint may be related to the small study size and the higher than expected regression rate in the placebo group, Dr. Distler said.
Dr. Khanna is a consultant to Roche/Genentech and Bayer, which markets riociguat, and other companies. He has received research grants from Bayer, Bristol-Myers Squibb (which markets abatacept), and Pfizer. The ASSET trial he presented was sponsored by an National Institutes of Health/National Institute of Allergy and Infectious Diseases Clinical ACE grant and an investigator-initiated grant by Bristol-Myers Squibb. Dr. Distler has a consultancy relationship and/or has received research funding from Bayer, Roche/Genentech, and other companies. In addition, he has a patent on mir-29 for the treatment of systemic sclerosis.
SOURCES: Khanna D et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 898 and Abstract 900; Distler O et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 903.
CHICAGO – Recent randomized, placebo-controlled, phase 3 trials of tocilizumab, abatacept, and riociguat for the treatment of systemic sclerosis each failed to reach its primary endpoint of change from baseline in modified Rodnan Skin Score (mRSS).
Still, findings with respect to secondary endpoints and certain exploratory outcomes suggest each of the agents holds some promise in the systemic sclerosis (SSc) arena, according to the data presented at the annual meeting of the American College of Rheumatology.
Tocilizumab (Actemra)
In the double-blind portion of the phase 3 focuSSced trial of 212 patients with SSc, numerical improvement was observed for the primary endpoint of mean change in mRSS from baseline to week 48 with tocilizumab versus placebo (–6.14 vs. –4.41 points, respectively). The change in the treatment group was comparable with what was seen in the phase 2 faSScinate trial, but the decline in mRSS in the placebo group was much greater in phase 3 than in phase 2, and so the difference between the groups in the current study failed to reach statistical significance (P = .098), reported Dinesh Khanna, MBBS, a professor of medicine and director of the scleroderma program at the University of Michigan, Ann Arbor.
The interleukin-6 (IL-6) receptor–alpha antibody was previously shown in the faSScinate trial to lead to numeric improvements in skin thickening as measured by the mRSS, as well as to clinically meaningful lung function preservation as measured by percent predicted forced vital capacity (FVC).
In the current phase 3 study, key secondary end points also appeared to favor tocilizumab, but since the primary endpoint for mRSS was not met, all other P values cannot be considered statistically significant despite the strength of the evidence and were reported for informational purposes only, he noted.
The median cumulative distribution of change from baseline to week 48 in percent predicted FVC with tocilizumab versus placebo was –0.6 vs. –3.9, respectively (descriptive P = .0015), and the mean change from baseline in FVC at week 48 was –24 mL vs. –190 mL (difference of 167 mL in favor of tocilizumab; descriptive P = .0001).
Time to treatment failure also favored tocilizumab, he said (hazard ratio, 0.63; descriptive P = .082), he said.
Patients were randomly assigned to receive either weekly 162-mg injections of subcutaneous tocilizumab or placebo for 48 weeks. Escape therapy was allowed beginning at week 16 if patients experienced declines in FVC or beginning at week 24 if they experienced worsened mRSS or worsened SSc complications, Dr. Khanna said.
“The key part is that no immunotherapy was allowed. ... So it’s a true randomized, placebo-controlled trial,” he said.
Most (81%) of the patients were women, and they had a mean age of 48 years, mean SSc duration of 23 months, mean mRSS of 20.4 units on a 0-51 scale, and a normal mean percent predicted FVC of 82.1%.
“HAQ-DI showed moderate disability of 1.2,” he noted.
Safety in the study was consistent with that seen in prior tocilizumab studies; no new safety signals were identified. Serious adverse events occurred in 13% and 17% of tocilizumab and placebo group patients , respectively, and serious infections were reported by 7% and 2%.
Although clinically meaningful and consistent differences in FVC favoring tocilizumab were shown in this study, the primary endpoint was not met, Dr. Khanna said.
“There were no statistically significant differences, largely driven by unexpected improvement in the placebo group, which was different than what we found in [the faSScinate] trial,” he said, noting, however, that the FVC findings in the current study were clinically meaningful.
Also, in a separate presentation at the meeting, he explained that the differences favoring tocilizumab were statistically significant when patient-level data from the trial were analyzed based on the ACR Composite Response Index in Systemic Sclerosis (CRISS). Those findings provide validation of the novel outcomes measure, he said.
Abatacept (Orencia)
Dr. Khanna also reported results of the 12-month, double-blind, randomized, placebo-controlled phase 2 ASSET trial of abatacept, which showed no significant difference in mRSS in patients with early diffuse cutaneous SSc (dfSSc) who were treated with 125 mg of the recombinant fusion protein weekly and those who received placebo. However, certain secondary outcomes favored abatacept. No concomitant immunotherapy was allowed.
The adjusted mean decrease in the mRSS among patients who completed the 12-month treatment period was –6.24 vs. –4.49 in 34 patients in the abatacept group and 35 in the placebo group, respectively (P = .28).
The secondary outcome measures of mean change in Health Assessment Questionnaire Disability Index (HAQ-DI), patients global assessment, physician global assessment, and ACR CRISS scores were statistically significant or showed numerical results favoring abatacept over placebo: mean decrease in HAQ-DI, –0.17 vs. –0.11 (P = .05), respectively; mean change in physician global assessment scores, –1.30 vs. –0.35 (P = .03); median ACR CRISS index, 0.68 vs. 0.01 (P = .03), decline in percent predicted FVC of 4.13% and 1.34% (P = .11).
Escape therapy was allowed at 6 months for worsening SSc, but it did not change the outcomes trajectory, he said. A larger proportion of placebo vs. abatacept subjects required escape immunosuppressive therapy (36% vs. 16%; P = .03).
Patients were enrolled between 2014 and 2018 at 27 U.S., Canadian, and U.K. sites. At baseline, participants had a mean age of 49 years, 75% were women, and mean disease duration was very short at 1.59 years, with 60% having disease duration of 18 months or less. The mean baseline mRSS was 22.4, mean percent predicted FVC was 85.3%, and mean HAQ-DI was 1.0.
Compliance with both treatments was greater than 98%. Abatacept was well tolerated with comparable adverse events (AEs), serious AEs, and AEs of special interest such as infections and malignancies between treatments, Dr. Khanna said, noting that two deaths occurred in the abatacept group (caused by scleroderma renal crisis in both cases at days 11 and 46) and one occurred in a placebo group patient who experienced sudden cardiac arrest at day 310.
Of note, mRSS showed large variability, despite recruiting an early dcSSc population, Dr. Khanna said.
The finding with respect to the primary outcome is consistent with other recent trials because of improvement in mRSS that’s part of the natural history of the disease, including the tocilizumab findings that he reported at the meeting. The findings with respect to secondary endpoints and safety show promise.
“Stay tuned for robust ongoing work on the relationship between clinical changes and ongoing mechanistic work,” he said.
Riociguat (Adempas)
Similarly, in the randomized, placebo-controlled phase 2b RISE-SSc study comparing riociguat and placebo for early dcSSc, the primary efficacy endpoint of mean change in mRSS did not reach statistical significance, but exploratory data suggested that the soluble guanylate cyclase stimulator prevented disease progression in patients with early dcSSc, reported Oliver Distler, MD, head of the connective tissue diseases program at University Hospital Zurich (Switzerland).
The mean mRSS at baseline was comparable in 60 patients randomized to receive riociguat and 61 in the placebo group (16.8 and 16.71, respectively). These mean values at week 52 dropped to 14.63 vs. 15.73, respectively (P = .08).
“So it was close, but it didn’t reach significance,” he said.
The difference in the mRSS progression rate, however, suggested significant effects favoring riociguat (descriptive P = .02), he said.
Further, mean change from baseline to week 52 in percent predicted FVC was not different overall between the groups, but a large difference favoring riociguat was seen among patients with scleroderma interstitial lung disease at baseline (mean change of –2.7 vs. –8.9), he said.
No differences were seen between the groups in HAQ-DI or patient and physician global assessment. The proportion of patients with probability of improvement at 52 weeks as measured using ACR CRISS was also the same at 18% in both treatment arms, he noted, ”but the CRISS is designed more for assessing disease regression than for assessing prevention of progression.”
Treatment was, however, well tolerated. At week 52, fewer serious adverse events occurred with riociguat group than in the placebo group (15% vs. 25%, respectively), and no new safety signals were observed, he said.
Riociguat has previously shown antifibrotic effects in animal models and efficacy in patients with pulmonary arterial hypertension associated with connective tissue disease, so it was hypothesized that patients with dcSSc might benefit from riociguat therapy, Dr. Distler explained.
Study subjects had very early dcSSc (duration of 18 months or less; mean of 9 months), mRSS of 10-22 units, FVC of 45% predicted or greater, and diffusion capacity of the lung for carbon monoxide of at least 40% of predicted at screening.
Riociguat was given at an individually adjusted dose between 0.5 mg and 2.5 mg three times daily.
The findings demonstrate a numeric decrease in mRSS over time with riociguat versus placebo and a prevention of progression with riociguat; the failure to reach the primary endpoint may be related to the small study size and the higher than expected regression rate in the placebo group, Dr. Distler said.
Dr. Khanna is a consultant to Roche/Genentech and Bayer, which markets riociguat, and other companies. He has received research grants from Bayer, Bristol-Myers Squibb (which markets abatacept), and Pfizer. The ASSET trial he presented was sponsored by an National Institutes of Health/National Institute of Allergy and Infectious Diseases Clinical ACE grant and an investigator-initiated grant by Bristol-Myers Squibb. Dr. Distler has a consultancy relationship and/or has received research funding from Bayer, Roche/Genentech, and other companies. In addition, he has a patent on mir-29 for the treatment of systemic sclerosis.
SOURCES: Khanna D et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 898 and Abstract 900; Distler O et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 903.
CHICAGO – Recent randomized, placebo-controlled, phase 3 trials of tocilizumab, abatacept, and riociguat for the treatment of systemic sclerosis each failed to reach its primary endpoint of change from baseline in modified Rodnan Skin Score (mRSS).
Still, findings with respect to secondary endpoints and certain exploratory outcomes suggest each of the agents holds some promise in the systemic sclerosis (SSc) arena, according to the data presented at the annual meeting of the American College of Rheumatology.
Tocilizumab (Actemra)
In the double-blind portion of the phase 3 focuSSced trial of 212 patients with SSc, numerical improvement was observed for the primary endpoint of mean change in mRSS from baseline to week 48 with tocilizumab versus placebo (–6.14 vs. –4.41 points, respectively). The change in the treatment group was comparable with what was seen in the phase 2 faSScinate trial, but the decline in mRSS in the placebo group was much greater in phase 3 than in phase 2, and so the difference between the groups in the current study failed to reach statistical significance (P = .098), reported Dinesh Khanna, MBBS, a professor of medicine and director of the scleroderma program at the University of Michigan, Ann Arbor.
The interleukin-6 (IL-6) receptor–alpha antibody was previously shown in the faSScinate trial to lead to numeric improvements in skin thickening as measured by the mRSS, as well as to clinically meaningful lung function preservation as measured by percent predicted forced vital capacity (FVC).
In the current phase 3 study, key secondary end points also appeared to favor tocilizumab, but since the primary endpoint for mRSS was not met, all other P values cannot be considered statistically significant despite the strength of the evidence and were reported for informational purposes only, he noted.
The median cumulative distribution of change from baseline to week 48 in percent predicted FVC with tocilizumab versus placebo was –0.6 vs. –3.9, respectively (descriptive P = .0015), and the mean change from baseline in FVC at week 48 was –24 mL vs. –190 mL (difference of 167 mL in favor of tocilizumab; descriptive P = .0001).
Time to treatment failure also favored tocilizumab, he said (hazard ratio, 0.63; descriptive P = .082), he said.
Patients were randomly assigned to receive either weekly 162-mg injections of subcutaneous tocilizumab or placebo for 48 weeks. Escape therapy was allowed beginning at week 16 if patients experienced declines in FVC or beginning at week 24 if they experienced worsened mRSS or worsened SSc complications, Dr. Khanna said.
“The key part is that no immunotherapy was allowed. ... So it’s a true randomized, placebo-controlled trial,” he said.
Most (81%) of the patients were women, and they had a mean age of 48 years, mean SSc duration of 23 months, mean mRSS of 20.4 units on a 0-51 scale, and a normal mean percent predicted FVC of 82.1%.
“HAQ-DI showed moderate disability of 1.2,” he noted.
Safety in the study was consistent with that seen in prior tocilizumab studies; no new safety signals were identified. Serious adverse events occurred in 13% and 17% of tocilizumab and placebo group patients , respectively, and serious infections were reported by 7% and 2%.
Although clinically meaningful and consistent differences in FVC favoring tocilizumab were shown in this study, the primary endpoint was not met, Dr. Khanna said.
“There were no statistically significant differences, largely driven by unexpected improvement in the placebo group, which was different than what we found in [the faSScinate] trial,” he said, noting, however, that the FVC findings in the current study were clinically meaningful.
Also, in a separate presentation at the meeting, he explained that the differences favoring tocilizumab were statistically significant when patient-level data from the trial were analyzed based on the ACR Composite Response Index in Systemic Sclerosis (CRISS). Those findings provide validation of the novel outcomes measure, he said.
Abatacept (Orencia)
Dr. Khanna also reported results of the 12-month, double-blind, randomized, placebo-controlled phase 2 ASSET trial of abatacept, which showed no significant difference in mRSS in patients with early diffuse cutaneous SSc (dfSSc) who were treated with 125 mg of the recombinant fusion protein weekly and those who received placebo. However, certain secondary outcomes favored abatacept. No concomitant immunotherapy was allowed.
The adjusted mean decrease in the mRSS among patients who completed the 12-month treatment period was –6.24 vs. –4.49 in 34 patients in the abatacept group and 35 in the placebo group, respectively (P = .28).
The secondary outcome measures of mean change in Health Assessment Questionnaire Disability Index (HAQ-DI), patients global assessment, physician global assessment, and ACR CRISS scores were statistically significant or showed numerical results favoring abatacept over placebo: mean decrease in HAQ-DI, –0.17 vs. –0.11 (P = .05), respectively; mean change in physician global assessment scores, –1.30 vs. –0.35 (P = .03); median ACR CRISS index, 0.68 vs. 0.01 (P = .03), decline in percent predicted FVC of 4.13% and 1.34% (P = .11).
Escape therapy was allowed at 6 months for worsening SSc, but it did not change the outcomes trajectory, he said. A larger proportion of placebo vs. abatacept subjects required escape immunosuppressive therapy (36% vs. 16%; P = .03).
Patients were enrolled between 2014 and 2018 at 27 U.S., Canadian, and U.K. sites. At baseline, participants had a mean age of 49 years, 75% were women, and mean disease duration was very short at 1.59 years, with 60% having disease duration of 18 months or less. The mean baseline mRSS was 22.4, mean percent predicted FVC was 85.3%, and mean HAQ-DI was 1.0.
Compliance with both treatments was greater than 98%. Abatacept was well tolerated with comparable adverse events (AEs), serious AEs, and AEs of special interest such as infections and malignancies between treatments, Dr. Khanna said, noting that two deaths occurred in the abatacept group (caused by scleroderma renal crisis in both cases at days 11 and 46) and one occurred in a placebo group patient who experienced sudden cardiac arrest at day 310.
Of note, mRSS showed large variability, despite recruiting an early dcSSc population, Dr. Khanna said.
The finding with respect to the primary outcome is consistent with other recent trials because of improvement in mRSS that’s part of the natural history of the disease, including the tocilizumab findings that he reported at the meeting. The findings with respect to secondary endpoints and safety show promise.
“Stay tuned for robust ongoing work on the relationship between clinical changes and ongoing mechanistic work,” he said.
Riociguat (Adempas)
Similarly, in the randomized, placebo-controlled phase 2b RISE-SSc study comparing riociguat and placebo for early dcSSc, the primary efficacy endpoint of mean change in mRSS did not reach statistical significance, but exploratory data suggested that the soluble guanylate cyclase stimulator prevented disease progression in patients with early dcSSc, reported Oliver Distler, MD, head of the connective tissue diseases program at University Hospital Zurich (Switzerland).
The mean mRSS at baseline was comparable in 60 patients randomized to receive riociguat and 61 in the placebo group (16.8 and 16.71, respectively). These mean values at week 52 dropped to 14.63 vs. 15.73, respectively (P = .08).
“So it was close, but it didn’t reach significance,” he said.
The difference in the mRSS progression rate, however, suggested significant effects favoring riociguat (descriptive P = .02), he said.
Further, mean change from baseline to week 52 in percent predicted FVC was not different overall between the groups, but a large difference favoring riociguat was seen among patients with scleroderma interstitial lung disease at baseline (mean change of –2.7 vs. –8.9), he said.
No differences were seen between the groups in HAQ-DI or patient and physician global assessment. The proportion of patients with probability of improvement at 52 weeks as measured using ACR CRISS was also the same at 18% in both treatment arms, he noted, ”but the CRISS is designed more for assessing disease regression than for assessing prevention of progression.”
Treatment was, however, well tolerated. At week 52, fewer serious adverse events occurred with riociguat group than in the placebo group (15% vs. 25%, respectively), and no new safety signals were observed, he said.
Riociguat has previously shown antifibrotic effects in animal models and efficacy in patients with pulmonary arterial hypertension associated with connective tissue disease, so it was hypothesized that patients with dcSSc might benefit from riociguat therapy, Dr. Distler explained.
Study subjects had very early dcSSc (duration of 18 months or less; mean of 9 months), mRSS of 10-22 units, FVC of 45% predicted or greater, and diffusion capacity of the lung for carbon monoxide of at least 40% of predicted at screening.
Riociguat was given at an individually adjusted dose between 0.5 mg and 2.5 mg three times daily.
The findings demonstrate a numeric decrease in mRSS over time with riociguat versus placebo and a prevention of progression with riociguat; the failure to reach the primary endpoint may be related to the small study size and the higher than expected regression rate in the placebo group, Dr. Distler said.
Dr. Khanna is a consultant to Roche/Genentech and Bayer, which markets riociguat, and other companies. He has received research grants from Bayer, Bristol-Myers Squibb (which markets abatacept), and Pfizer. The ASSET trial he presented was sponsored by an National Institutes of Health/National Institute of Allergy and Infectious Diseases Clinical ACE grant and an investigator-initiated grant by Bristol-Myers Squibb. Dr. Distler has a consultancy relationship and/or has received research funding from Bayer, Roche/Genentech, and other companies. In addition, he has a patent on mir-29 for the treatment of systemic sclerosis.
SOURCES: Khanna D et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 898 and Abstract 900; Distler O et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 903.
REPORTING FROM THE ACR ANNUAL MEETING
Temixys plus other antiretrovirals approved for HIV-1
The Food and Drug Administration has approved the combination of lamivudine (3TC) and tenofovir disoproxil fumarate (TDF) known as Temixys for treatment of HIV-1 when used with other antiretrovirals. The approval is for adult and pediatric patients with HIV-1 who weigh at least 35 kg.
The approval is based on data through 144 weeks in a double-blind, active-controlled, multicenter trial in 600 antiretroviral-naive patients. The trial compared TDF/3TC plus efavirenz (EFV) with 3TC/EFV plus stavudine (d4T). The results showed similar responses at 144 weeks between both groups: 62% of patients taking TDF/3TC/EFV and 58% of patients taking d4T/3TC/EFV achieved and maintained fewer than 50 copies/mL of HIV-1 RNA.
The most common adverse events include headache, pain, depression, rash, and diarrhea. Prior to initiating treatment, patients should be tested for hepatitis B virus because there have been reports of 3TC-resistant strains of hepatitis B virus associated with treatment of HIV-1 with 3TC-containing regimens in coinfected patients. Patients should also be tested for estimated creatinine clearance, urine glucose, and urine protein because TDF/3TC is not recommended for patients with renal impairment.
The full prescribing information can be found on the FDA website.
The Food and Drug Administration has approved the combination of lamivudine (3TC) and tenofovir disoproxil fumarate (TDF) known as Temixys for treatment of HIV-1 when used with other antiretrovirals. The approval is for adult and pediatric patients with HIV-1 who weigh at least 35 kg.
The approval is based on data through 144 weeks in a double-blind, active-controlled, multicenter trial in 600 antiretroviral-naive patients. The trial compared TDF/3TC plus efavirenz (EFV) with 3TC/EFV plus stavudine (d4T). The results showed similar responses at 144 weeks between both groups: 62% of patients taking TDF/3TC/EFV and 58% of patients taking d4T/3TC/EFV achieved and maintained fewer than 50 copies/mL of HIV-1 RNA.
The most common adverse events include headache, pain, depression, rash, and diarrhea. Prior to initiating treatment, patients should be tested for hepatitis B virus because there have been reports of 3TC-resistant strains of hepatitis B virus associated with treatment of HIV-1 with 3TC-containing regimens in coinfected patients. Patients should also be tested for estimated creatinine clearance, urine glucose, and urine protein because TDF/3TC is not recommended for patients with renal impairment.
The full prescribing information can be found on the FDA website.
The Food and Drug Administration has approved the combination of lamivudine (3TC) and tenofovir disoproxil fumarate (TDF) known as Temixys for treatment of HIV-1 when used with other antiretrovirals. The approval is for adult and pediatric patients with HIV-1 who weigh at least 35 kg.
The approval is based on data through 144 weeks in a double-blind, active-controlled, multicenter trial in 600 antiretroviral-naive patients. The trial compared TDF/3TC plus efavirenz (EFV) with 3TC/EFV plus stavudine (d4T). The results showed similar responses at 144 weeks between both groups: 62% of patients taking TDF/3TC/EFV and 58% of patients taking d4T/3TC/EFV achieved and maintained fewer than 50 copies/mL of HIV-1 RNA.
The most common adverse events include headache, pain, depression, rash, and diarrhea. Prior to initiating treatment, patients should be tested for hepatitis B virus because there have been reports of 3TC-resistant strains of hepatitis B virus associated with treatment of HIV-1 with 3TC-containing regimens in coinfected patients. Patients should also be tested for estimated creatinine clearance, urine glucose, and urine protein because TDF/3TC is not recommended for patients with renal impairment.
The full prescribing information can be found on the FDA website.
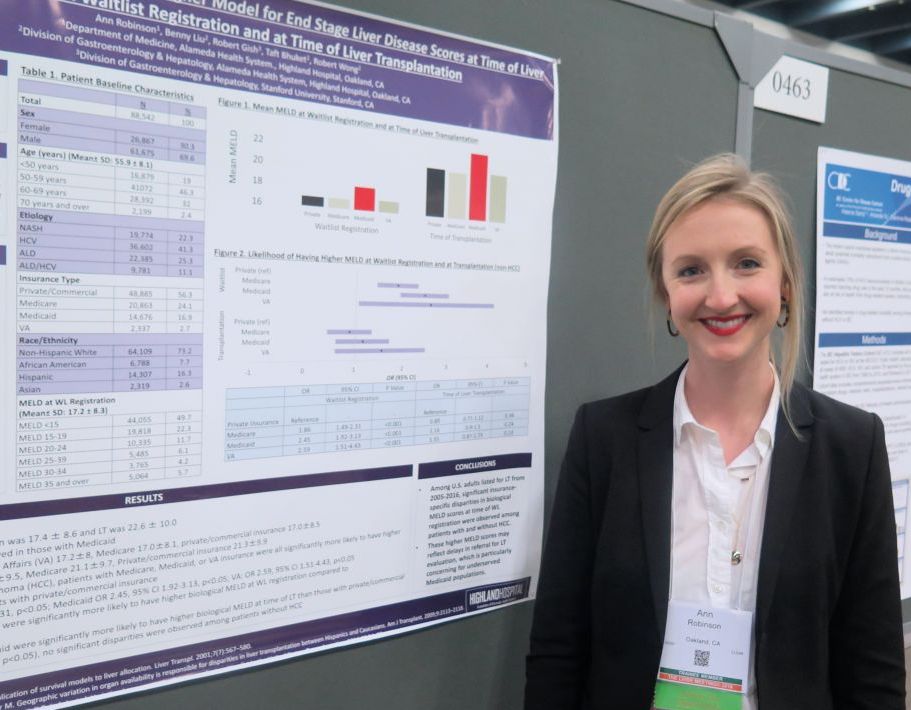
Medicaid patients have higher MELD scores at time of liver transplantation
SAN FRANCISCO – Despite implementation of the Model for End Stage Liver Disease score to prioritize liver transplantation, .
“It can be difficult for patients with Medicaid to access liver transplantation,” lead study author Ann Robinson, MD, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases. “These patients may be living in underserved areas with limited resources.”
In an effort to evaluate insurance-specific disparities in severity of liver disease at the time of liver transplantation wait-list registration and at the time of liver transplantation, Dr. Robinson and her colleagues retrospectively evaluated the 2005-2016 United Network for Organ Sharing/Organ Procurement and Transplant Network liver transplant registry. They used multivariate linear regression models to make insurance-specific comparisons of MELD scores at wait-list registration and at liver transplantation, which included adjustments for age, sex, year, etiology of liver disease, body mass index, ascites, hepatocellular carcinoma (HCC), and hepatic encephalopathy.
Dr. Robinson, who is a third-year internal medicine resident at Highland Hospital, Oakland, Calif., reported findings from 88,542 liver transplantation wait-list registrants with a mean age of 56 years. Their overall mean MELD score was 17.4 at wait-list registration and 22.6 at time of liver transplantation. The greatest mean MELD score at the time of wait-list registration was observed in Medicaid patients (18.4, compared with 17.2 among Veterans Affairs patients, 17 among Medicare patients, and 17 among privately/commercially insured patients; P less than .01). Meanwhile, the greatest mean MELD score at the time of liver transplantation was observed in Medicaid patients (23.5, compared with 21.4 among VA patients, 21.3 among privately/commercially insured patients, and 21.1 among Medicare patients; P less than .01).
Multivariate regression analysis revealed that, among patients without hepatocellular carcinoma, those with coverage other than private or commercial insurance had significantly higher MELD scores at wait-list registration (P less than .01). Specifically, the odds ratio was highest for VA patients (odds ratio, 2.59), followed by those covered by Medicaid (OR, 2.45), and Medicare (OR, 1.86). Similar trends were observed in hepatocellular carcinoma patients, with the highest biological MELD score at wait-list seen in those covered by Medicaid.
On regression analysis, while Medicaid patients with hepatocellular carcinoma had significantly higher biological MELD scores at time of liver transplantation, compared with those with private/commercial insurance (Medicaid OR, 2.06; P less than .05), no differences were observed among patients without hepatocellular carcinoma.
Dr. Robinson reported having no financial disclosures.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 464.
SAN FRANCISCO – Despite implementation of the Model for End Stage Liver Disease score to prioritize liver transplantation, .
“It can be difficult for patients with Medicaid to access liver transplantation,” lead study author Ann Robinson, MD, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases. “These patients may be living in underserved areas with limited resources.”
In an effort to evaluate insurance-specific disparities in severity of liver disease at the time of liver transplantation wait-list registration and at the time of liver transplantation, Dr. Robinson and her colleagues retrospectively evaluated the 2005-2016 United Network for Organ Sharing/Organ Procurement and Transplant Network liver transplant registry. They used multivariate linear regression models to make insurance-specific comparisons of MELD scores at wait-list registration and at liver transplantation, which included adjustments for age, sex, year, etiology of liver disease, body mass index, ascites, hepatocellular carcinoma (HCC), and hepatic encephalopathy.
Dr. Robinson, who is a third-year internal medicine resident at Highland Hospital, Oakland, Calif., reported findings from 88,542 liver transplantation wait-list registrants with a mean age of 56 years. Their overall mean MELD score was 17.4 at wait-list registration and 22.6 at time of liver transplantation. The greatest mean MELD score at the time of wait-list registration was observed in Medicaid patients (18.4, compared with 17.2 among Veterans Affairs patients, 17 among Medicare patients, and 17 among privately/commercially insured patients; P less than .01). Meanwhile, the greatest mean MELD score at the time of liver transplantation was observed in Medicaid patients (23.5, compared with 21.4 among VA patients, 21.3 among privately/commercially insured patients, and 21.1 among Medicare patients; P less than .01).
Multivariate regression analysis revealed that, among patients without hepatocellular carcinoma, those with coverage other than private or commercial insurance had significantly higher MELD scores at wait-list registration (P less than .01). Specifically, the odds ratio was highest for VA patients (odds ratio, 2.59), followed by those covered by Medicaid (OR, 2.45), and Medicare (OR, 1.86). Similar trends were observed in hepatocellular carcinoma patients, with the highest biological MELD score at wait-list seen in those covered by Medicaid.
On regression analysis, while Medicaid patients with hepatocellular carcinoma had significantly higher biological MELD scores at time of liver transplantation, compared with those with private/commercial insurance (Medicaid OR, 2.06; P less than .05), no differences were observed among patients without hepatocellular carcinoma.
Dr. Robinson reported having no financial disclosures.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 464.
SAN FRANCISCO – Despite implementation of the Model for End Stage Liver Disease score to prioritize liver transplantation, .
“It can be difficult for patients with Medicaid to access liver transplantation,” lead study author Ann Robinson, MD, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases. “These patients may be living in underserved areas with limited resources.”
In an effort to evaluate insurance-specific disparities in severity of liver disease at the time of liver transplantation wait-list registration and at the time of liver transplantation, Dr. Robinson and her colleagues retrospectively evaluated the 2005-2016 United Network for Organ Sharing/Organ Procurement and Transplant Network liver transplant registry. They used multivariate linear regression models to make insurance-specific comparisons of MELD scores at wait-list registration and at liver transplantation, which included adjustments for age, sex, year, etiology of liver disease, body mass index, ascites, hepatocellular carcinoma (HCC), and hepatic encephalopathy.
Dr. Robinson, who is a third-year internal medicine resident at Highland Hospital, Oakland, Calif., reported findings from 88,542 liver transplantation wait-list registrants with a mean age of 56 years. Their overall mean MELD score was 17.4 at wait-list registration and 22.6 at time of liver transplantation. The greatest mean MELD score at the time of wait-list registration was observed in Medicaid patients (18.4, compared with 17.2 among Veterans Affairs patients, 17 among Medicare patients, and 17 among privately/commercially insured patients; P less than .01). Meanwhile, the greatest mean MELD score at the time of liver transplantation was observed in Medicaid patients (23.5, compared with 21.4 among VA patients, 21.3 among privately/commercially insured patients, and 21.1 among Medicare patients; P less than .01).
Multivariate regression analysis revealed that, among patients without hepatocellular carcinoma, those with coverage other than private or commercial insurance had significantly higher MELD scores at wait-list registration (P less than .01). Specifically, the odds ratio was highest for VA patients (odds ratio, 2.59), followed by those covered by Medicaid (OR, 2.45), and Medicare (OR, 1.86). Similar trends were observed in hepatocellular carcinoma patients, with the highest biological MELD score at wait-list seen in those covered by Medicaid.
On regression analysis, while Medicaid patients with hepatocellular carcinoma had significantly higher biological MELD scores at time of liver transplantation, compared with those with private/commercial insurance (Medicaid OR, 2.06; P less than .05), no differences were observed among patients without hepatocellular carcinoma.
Dr. Robinson reported having no financial disclosures.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 464.
AT THE LIVER MEETING 2018
Key clinical point: Significant insurance-specific disparities in MELD scores at time of wait-list registration were observed among patients with and without hepatocellular carcinoma.
Major finding: Among patients without hepatocellular carcinoma, those with Medicaid coverage were 2.45 times more likely to have higher MELD scores at wait-list registration, compared with those covered by commercial or private insurance (P less than .01).
Study details: A retrospective analysis of 88,542 liver transplantation wait-list registrants.
Disclosures: Dr. Robinson reported having no disclosures.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 464.
AAP speaker emphasizes importance of understanding patients’ ‘lived experience’
ORLANDO – Pediatricians who learn about their patients’ lived experience have the potential to encourage patients and help them overcome biases, assumptions, and barriers of opioid use disorder, Tamela Milan said at the American Academy of Pediatrics annual meeting.
After her five children were taken into state welfare custody and she began a sixth pregnancy while struggling with opioid use disorder and as a survivor of domestic violence, Ms. Milan’s pediatrician was the one to encourage her to take steps to improve her life. She went on to regain custody of her children and complete college, and has given back by working in community health programs for over 20 years.
In a video interview, Ms. Milan said she would not have been able to overcome these barriers had it not been for the support of her pediatrician, who saw her as a person instead of a mother with opioid use disorder.
“I’ve been on both sides of the fence,” Ms. Milan said. “As someone who’s had to receive treatment and to provide it, it’s really important that we start looking at people for who they are and where they are.”
Tamela Milan has no relevant conflicts of interest.
ORLANDO – Pediatricians who learn about their patients’ lived experience have the potential to encourage patients and help them overcome biases, assumptions, and barriers of opioid use disorder, Tamela Milan said at the American Academy of Pediatrics annual meeting.
After her five children were taken into state welfare custody and she began a sixth pregnancy while struggling with opioid use disorder and as a survivor of domestic violence, Ms. Milan’s pediatrician was the one to encourage her to take steps to improve her life. She went on to regain custody of her children and complete college, and has given back by working in community health programs for over 20 years.
In a video interview, Ms. Milan said she would not have been able to overcome these barriers had it not been for the support of her pediatrician, who saw her as a person instead of a mother with opioid use disorder.
“I’ve been on both sides of the fence,” Ms. Milan said. “As someone who’s had to receive treatment and to provide it, it’s really important that we start looking at people for who they are and where they are.”
Tamela Milan has no relevant conflicts of interest.
ORLANDO – Pediatricians who learn about their patients’ lived experience have the potential to encourage patients and help them overcome biases, assumptions, and barriers of opioid use disorder, Tamela Milan said at the American Academy of Pediatrics annual meeting.
After her five children were taken into state welfare custody and she began a sixth pregnancy while struggling with opioid use disorder and as a survivor of domestic violence, Ms. Milan’s pediatrician was the one to encourage her to take steps to improve her life. She went on to regain custody of her children and complete college, and has given back by working in community health programs for over 20 years.
In a video interview, Ms. Milan said she would not have been able to overcome these barriers had it not been for the support of her pediatrician, who saw her as a person instead of a mother with opioid use disorder.
“I’ve been on both sides of the fence,” Ms. Milan said. “As someone who’s had to receive treatment and to provide it, it’s really important that we start looking at people for who they are and where they are.”
Tamela Milan has no relevant conflicts of interest.
REPORTING FROM AAP 2018
Use of smartphone app improves pain outcomes
SAN DIEGO – A smartphone app that included artificial intelligence elements was associated with improved pain outcomes and reduced hospital admissions in patients with advanced cancers.
Pain severity significantly decreased among patients randomized to use the app versus control patients who received only palliative care, researchers reported at the Palliative and Supportive Care in Oncology Symposium.
The risk of pain-related hospital admissions was significantly lower for those who used the pain tracking app, called ePAL, though anxiety scores were higher in the app users, the investigators said, and no difference between arms was noted in quality of life or global symptom scores.
The ePAL app prompts patients three times per week to track their pain levels and, depending on the severity of pain, will use an algorithm to guide patients through their symptoms, or, in patients with persistent or worsening pain, connect them directly with the palliative care service for additional assessment.
The app also includes pain management tips, among other educational content, provides the ability to request pain prescription refills, and creates a summary of the patient’s pain condition for the provider, said Mihir M. Kamdar, MD, associate director of palliative care at Massachusetts General Hospital in Boston.
“The provider can actually start the visit with that information, instead of having to spend several minutes trying to recap what might or might not have happened since the last clinic visit,” Dr. Kamdar said.
The study included 112 English-speaking adult patients with stage IV solid cancers and moderate to severe pain who were being followed in a palliative care clinic. They were randomly assigned to receive the ePAL app plus standard of care or standard of care alone; 39 patients in the app group and 40 in the control arm completed the 8-week evaluation.
Pain severity, the primary study endpoint, decreased over time in the intervention group, from a composite Brief Pain Inventory score of 3.74 at enrollment to 2.99 at 8 weeks, while in the control group, the scores were 4.02 at enrollment and 4.05 at 8 weeks (P = .017 for intervention versus control), Dr. Kamdar reported.
Risk of pain-related hospital admissions was significantly lower in the intervention group, according to Dr. Kamdar. The per-patient risk of an inpatient admission was 0.071 and 0.232 for the intervention and controls groups, respectively, with a risk ratio of 0.31 (95% CI, 0.11-0.89; P = .018).
Anxiety was increased in the app users, as measured by the Generalized Anxiety Disorder 7-item scale, with a significant difference between the app and control groups at 8 weeks (P = .015). However, the change was in a range considered mild and was not seen in patients who used the app more than two times per week.
Negative attitudes toward cancer pain treatment decreased significantly in the app group, as shown by a lower score on the Barriers Questionnaire II at 8 weeks (P = .042), Dr. Kamdar reported.
The app and study were supported by the McKesson Foundation’s Mobilizing for Health Initiative. Dr. Kamdar reported stock/ownership and consulting/advisory role disclosures related to Amorsa Therapeutics.
SOURCE: Kamdar MM et al. PallOnc 2018, Abstract 76.
SAN DIEGO – A smartphone app that included artificial intelligence elements was associated with improved pain outcomes and reduced hospital admissions in patients with advanced cancers.
Pain severity significantly decreased among patients randomized to use the app versus control patients who received only palliative care, researchers reported at the Palliative and Supportive Care in Oncology Symposium.
The risk of pain-related hospital admissions was significantly lower for those who used the pain tracking app, called ePAL, though anxiety scores were higher in the app users, the investigators said, and no difference between arms was noted in quality of life or global symptom scores.
The ePAL app prompts patients three times per week to track their pain levels and, depending on the severity of pain, will use an algorithm to guide patients through their symptoms, or, in patients with persistent or worsening pain, connect them directly with the palliative care service for additional assessment.
The app also includes pain management tips, among other educational content, provides the ability to request pain prescription refills, and creates a summary of the patient’s pain condition for the provider, said Mihir M. Kamdar, MD, associate director of palliative care at Massachusetts General Hospital in Boston.
“The provider can actually start the visit with that information, instead of having to spend several minutes trying to recap what might or might not have happened since the last clinic visit,” Dr. Kamdar said.
The study included 112 English-speaking adult patients with stage IV solid cancers and moderate to severe pain who were being followed in a palliative care clinic. They were randomly assigned to receive the ePAL app plus standard of care or standard of care alone; 39 patients in the app group and 40 in the control arm completed the 8-week evaluation.
Pain severity, the primary study endpoint, decreased over time in the intervention group, from a composite Brief Pain Inventory score of 3.74 at enrollment to 2.99 at 8 weeks, while in the control group, the scores were 4.02 at enrollment and 4.05 at 8 weeks (P = .017 for intervention versus control), Dr. Kamdar reported.
Risk of pain-related hospital admissions was significantly lower in the intervention group, according to Dr. Kamdar. The per-patient risk of an inpatient admission was 0.071 and 0.232 for the intervention and controls groups, respectively, with a risk ratio of 0.31 (95% CI, 0.11-0.89; P = .018).
Anxiety was increased in the app users, as measured by the Generalized Anxiety Disorder 7-item scale, with a significant difference between the app and control groups at 8 weeks (P = .015). However, the change was in a range considered mild and was not seen in patients who used the app more than two times per week.
Negative attitudes toward cancer pain treatment decreased significantly in the app group, as shown by a lower score on the Barriers Questionnaire II at 8 weeks (P = .042), Dr. Kamdar reported.
The app and study were supported by the McKesson Foundation’s Mobilizing for Health Initiative. Dr. Kamdar reported stock/ownership and consulting/advisory role disclosures related to Amorsa Therapeutics.
SOURCE: Kamdar MM et al. PallOnc 2018, Abstract 76.
SAN DIEGO – A smartphone app that included artificial intelligence elements was associated with improved pain outcomes and reduced hospital admissions in patients with advanced cancers.
Pain severity significantly decreased among patients randomized to use the app versus control patients who received only palliative care, researchers reported at the Palliative and Supportive Care in Oncology Symposium.
The risk of pain-related hospital admissions was significantly lower for those who used the pain tracking app, called ePAL, though anxiety scores were higher in the app users, the investigators said, and no difference between arms was noted in quality of life or global symptom scores.
The ePAL app prompts patients three times per week to track their pain levels and, depending on the severity of pain, will use an algorithm to guide patients through their symptoms, or, in patients with persistent or worsening pain, connect them directly with the palliative care service for additional assessment.
The app also includes pain management tips, among other educational content, provides the ability to request pain prescription refills, and creates a summary of the patient’s pain condition for the provider, said Mihir M. Kamdar, MD, associate director of palliative care at Massachusetts General Hospital in Boston.
“The provider can actually start the visit with that information, instead of having to spend several minutes trying to recap what might or might not have happened since the last clinic visit,” Dr. Kamdar said.
The study included 112 English-speaking adult patients with stage IV solid cancers and moderate to severe pain who were being followed in a palliative care clinic. They were randomly assigned to receive the ePAL app plus standard of care or standard of care alone; 39 patients in the app group and 40 in the control arm completed the 8-week evaluation.
Pain severity, the primary study endpoint, decreased over time in the intervention group, from a composite Brief Pain Inventory score of 3.74 at enrollment to 2.99 at 8 weeks, while in the control group, the scores were 4.02 at enrollment and 4.05 at 8 weeks (P = .017 for intervention versus control), Dr. Kamdar reported.
Risk of pain-related hospital admissions was significantly lower in the intervention group, according to Dr. Kamdar. The per-patient risk of an inpatient admission was 0.071 and 0.232 for the intervention and controls groups, respectively, with a risk ratio of 0.31 (95% CI, 0.11-0.89; P = .018).
Anxiety was increased in the app users, as measured by the Generalized Anxiety Disorder 7-item scale, with a significant difference between the app and control groups at 8 weeks (P = .015). However, the change was in a range considered mild and was not seen in patients who used the app more than two times per week.
Negative attitudes toward cancer pain treatment decreased significantly in the app group, as shown by a lower score on the Barriers Questionnaire II at 8 weeks (P = .042), Dr. Kamdar reported.
The app and study were supported by the McKesson Foundation’s Mobilizing for Health Initiative. Dr. Kamdar reported stock/ownership and consulting/advisory role disclosures related to Amorsa Therapeutics.
SOURCE: Kamdar MM et al. PallOnc 2018, Abstract 76.
REPORTING FROM PALLONC 2018
Key clinical point: Use of a smartphone app with artificial intelligence elements improved pain outcomes and reduced hospital admissions in patients with advanced cancers.
Major finding: Pain severity decreased over time from a composite Brief Pain Inventory score of 3.74 at enrollment to 2.99 at 8 weeks, compared with baseline and 8-week values of 4.02 and 4.05 in the control group (P = .017).
Study details: A randomized study including 112 English-speaking adult patients with stage IV solid cancers and moderate to severe pain who were followed in a palliative care clinic.
Disclosures: The research was supported by the McKesson Foundation. The presenting author reported stock/ownership and consulting/advisory role disclosures related to Amorsa Therapeutic.
Source: Kamdar MM et al. PallOnc 2018, Abstract 76.
ACR CRISS: A way forward for scleroderma treatment trials?
CHICAGO – At least three phase 3 randomized scleroderma treatment trials presented at the annual meeting of the American College of Rheumatology failed to meet modified Rodnan Skin Score–based primary endpoints, but a different story emerged when data from the trials were analyzed using the novel ACR Composite Response Index in Systemic Sclerosis (CRISS).
The differences highlight the limitations of individual outcome measures like the modified Rodnan Skin Score (mRSS) and underscore the need for new measures that capture the complexity of systemic sclerosis (SSc) and are more sensitive to changes in disease severity, according to Robert Spiera, MD, director of the vasculitis and scleroderma program at the Hospital for Special Surgery in New York.
Such measures are needed to better assess the effects of treatment interventions in scleroderma trials, Dr. Spiera said in an interview.
The ACR CRISS
Development of the ACR CRISS was led by Dinesh Khanna, MBBS, a professor of medicine and director of the scleroderma program at the University of Michigan, Ann Arbor. He and his colleagues described the measure in 2016 (Arthritis Rheumatol. 2016 Feb;68[2]:299-311. doi: 10.1002/art.39501).
Its use involves a two-step process of identifying any significant disease worsening or new end-organ damage, and then calculating the probability of patient improvement after 1 year of treatment on a 0- to 1-point scale based on changes from baseline in five variables: the mRSS, percent predicted forced vital capacity (FVC), patient and physician global assessments, and the Health Assessment Questionnaire Disability Index (HAQ-DI).
A CRISS score of 0.6 or higher indicates likelihood that a patient improved on treatment. Of note, subjects with significant worsening of renal or cardiopulmonary involvement are classified as not improved (score of 0), regardless of improvements in other core items.
To devise the CRISS, the investigators compiled 150 patient profiles with standardized clinical outcome elements using patients with diffuse cutaneous systemic sclerosis (dcSSc). The profiles were assessed by 40 scleroderma experts who rated patient improvement or lack thereof over 12 months.
Using the 79% of profiles for which a consensus was reached, the investigators “fit logistic regression models in which the binary outcome referred to whether the patient was improved or not, and the changes in the core set items from baseline to follow-up were entered as covariates,” they explained.
This led to the selection of the five measures included in the final version, which was found to have sensitivity of 0.982 and specificity of 0.931. When evaluated in a previously completed 1-year randomized controlled trial, the index differentiated the effect of methotrexate from the effect of placebo (P = .02), they reported.
Based on these findings, the ACR board of directors granted “provisional” endorsement of the CRISS for use in SSc clinical studies, signifying that it had been quantitatively validated using patient data, but had not undergone validation using an external data set.
New data presented at the 2018 ACR meeting will likely lead to full approval of the measure once the studies validating the measure are published, according to Dr. Spiera.
The focuSSced study of tocilizumab
For example, in the phase 3 focuSSced study comparing the interleukin-6 (IL-6) receptor–alpha antibody tocilizumab (Actemra) with placebo in patients with SSc, the primary endpoint of mean change from baseline in mRSS was not met (–6.14 vs. –4.41 points with tocilizumab vs. placebo, respectively; P = .098), Dr. Khanna said during a report of findings from the double-blind portion of the study.
However, when the CRISS was applied and its performance prospectively assessed as an exploratory outcome at week 48 in the focuSSced trial, the differences between the groups were statistically significant. Dr. Khanna reported those findings in a separate presentation at the meeting, noting that they marked the first prospective evaluation of the ACR CRISS in a phase 3 trial in SSc.
The CRISS was applied – using a blinded review of adverse events and serious adverse events to complete step 1 – in all patients who received study treatment, stratified by baseline IL-6 levels.
Of 104 patients in the tocilizumab arm and 106 in the placebo arm, 6 and 13 patients, respectively, had cardio-pulmonary-renal involvement and therefore received a score of 0.
Using the ACR CRISS as a continuous measure, scores favored tocilizumab over placebo at week 48 with a median increase of 0.89 vs. 0.25 points (P = .023), and using the binary form of CRISS, 51% vs. 37% of patients in the treatment and placebo arms achieved the score cutoff of 0.60 or higher at week 48 (P = .035), he said.
“The focuSSced study validates the ACR CRISS endpoint for the first time in an independent prospective clinical trial and highlights the importance of step 1 as an indicator of reduced organ progression during 48 weeks of treatment,” Dr. Khanna concluded.
The ASSET trial of abatacept
Dr. Khanna also reported results from the 12-month phase 2 ASSET trial comparing abatacept (Orencia) and placebo in early dcSSc.
Again, the change from baseline in mRSS – the primary endpoint of the study – did not differ significantly with treatment vs. placebo (–6.24 vs. –4.49; P = .28).
And again, as presented separately in a poster session at ACR, application of CRISS at 12 months – a secondary outcome measure in the trial – showed a significant difference between the groups.
The investigators prospectively performed step 1 of the CRISS using a case report form. Of 63 patients with complete data available for relevant outcomes at 12 months, 10 (5 in each group) had cardio-pulmonary-renal involvement and thus were given a score of 0.
Overall, there was evidence of significantly improved CRISS scores in the abatacept group vs. the placebo group (P = .03), he said.
Most of the individual CRISS variables, except HAQ-DI and patient global assessment, had statistically significant correlations with the CRISS, he noted, adding that “although the degree of correlation is high between the mRSS and CRISS, there is evidence that CRISS may be more sensitive to clinically meaningful treatment changes than the standard skin score endpoint.”
“This suggests further validation of CRISS as an independent primary endpoint for scleroderma clinical trials,” he concluded.
The phase 2b RISE-SSc study of riociguat
As in the focuSSced and ASSET studies, the primary efficacy endpoint of mean change from baseline in mRSS was not met in the randomized, double-blind, placebo-controlled RISE-SSc study evaluating the safety and efficacy of the soluble guanylate cyclase stimulator riociguat (Adempas) vs. placebo in 121 patients with early dcSSc, reported Oliver Distler, MD, a professor at University Hospital Zurich.
The mean change in mRSS at 52 weeks was 2.25 vs. 0.97 with riociguat vs. placebo, respectively (P = .08), although the difference in mRSS progression rate showed significant effects favoring riociguat (P = .02), he noted.
In this study, however, the proportion of patients with ACR CRISS probability of improvement (score of 0.60 or higher) at week 52 – a secondary study outcome – was the same at 18% in both arms, Dr. Distler said.
In a way, it’s helpful that the CRISS findings as well as the mRSS outcome in the RISE-SSc study were negative because it shows that “not everything comes up positive using the CRISS,” Dr. Spiera said.
An open-label extension trial of lenabasum
In the open-label extension of a phase 2 trial of lenabasum, Dr. Spiera and his colleagues also found the CRISS useful for assessing response in 36 patients. Lenabasum is a synthetic, nonimunosuppressive, selective cannabinoid receptor type 2 agonist that activates resolution of innate immune responses.
The agent continued to demonstrate acceptable safety and tolerability in dcSSc with no severe or serious adverse events or study discontinuations related to treatment during 12 months of open-label extension dosing, both from baseline and from the start of the extension, they reported in a poster at the ACR meeting. These assessments were based on ACR CRISS score, mRSS, physician global assessment, and multiple patient-reported outcomes.
The median CRISS score was 92% at week 52, and mRSS declined by a mean of 9.4 points (41.3% from baseline). More than a third of patients (35%) achieved a low mRSS of 10 or less.
The investigators noted, however, that definitive attribution of the findings to lenabasum is limited by the use of background therapy, the potential for spontaneous improvement in patients, and open-label dosing.
Evaluating immunosuppressive therapy in SSc
In another study presented at the ACR meeting, Boyang Zheng, MD, a second-year rheumatology fellow at McGill University in Montreal and his colleagues evaluated the effect of current immunosuppressive therapy on the ACR CRISS in 301 adult dcSSc patients without prior immunosuppression who were part of the Canadian Scleroderma Research Group (CSRG) registry.
Patients newly treated with methotrexate, azathioprine, mycophenolate and/or cyclophosphamide for at least 2 years (47 patients) were considered “exposed patients,” and untreated patients with at least the same follow-up duration were considered control subjects (254 patients).
Inverse probability of treatment weighting (IPTW) was performed to balance potential confounders in the two groups, including age, sex, disease duration, and CRISS variables, in an effort “to create a statistical cohort that would resemble a randomized, controlled trial,” Dr Zheng explained.
Prior to IPTW, treated patients trended towards more improvement after 1 year, but with “unimpressive absolute values.”
“But [after IPTW], when you look at overall CRISS at 1 year, more of the treated patients had actually improved – 23% vs. 11.8% in the untreated patients. After adjusting for age, sex, and disease duration, immunosuppression was associated with an almost twofold higher likelihood of improvement, although this was not statistically significant,” he said.
“Most importantly, after our balancing in the statistical cohort – so after balancing and adjusting for covariates ... immunosuppression use was still associated with a higher likelihood of improving [with an] odds ratio of 1.85 and P-value of 0.018,” he said.
The treated patients were sicker, and the CRISS was still able to capture individual patient improvement, he added.
Though limited by the observational design and the fact that “IPTW balancing cannot correct for all possible confounders,” the findings “provide novel evidence to support the use of immunosuppression in diffuse systemic sclerosis, and this reassures us in our current practice; it provides evidence that the CRISS seems to have better sensitivity to change than the mean of individual disease measures,” he said.
However, it also shows that “we have a long way to go to have better treatment for our patients,” he added, noting that only a minority (23%) of the patients in this study improved on the CRISS.
Moving forward in systemic sclerosis
Indeed, in order to move forward in developing better treatment for SSc, it is important to have the best possible means for assessing the effects of the potential new treatments, Dr. Spiera said.
“The mRSS is a good, reliable outcome measure in terms of intra- and inter-rater reliability, and we know it has meaning in the real world; in a patient with rapidly progressive skin thickening and a skin score that is getting higher and higher, we know they have worse prognosis in terms of function and even survival,” he said. “On the other hand, it’s just one piece of this very complicated story when you’re dealing with patients with systemic sclerosis, some of whom can have important internal organ involvement and not even have skin involvement.”
The main challenge with the skin score is how it performs in clinical trials when it comes to demonstrating whether a drug works, he added.
“This is particularly relevant in an era where our better understanding of the disease has led to candidate therapeutic agents that we have reason to think might work, but where clinical trials haven’t shown a significant difference between the groups using the skin score.”
Conversely, there have been studies in which skin scores improve, but the patient is doing terribly, he noted.
“That patient would not be [shown to be] doing well using the CRISS,” he said, explaining that the complex formula used to determine the CRISS score, which is “heavily weighted toward skin score,” allows for an overall score that is “greater than the sum of its parts.” That is, if a patient is improving in multiple domains indicating that the patient is responding to therapy, more credit is given for each of those parts.
“So my sense, and I think it’s the consensus in the community of clinical investigators, is that the skin score is not adequately sensitive to change in the context of a trial and doesn’t capture the disease holistically enough to be the optimal measure for scleroderma clinical trials,” he said. “We think the CRISS score works better in terms of capturing improvement in the course of a trial, and also in capturing other outcomes that are really, really important to patients and to their physicians, like disability or lung function.”
The hope is that, given the evidence, regulatory agencies will look favorably on the ACR CRISS as an outcome measure in clinical trials moving forward, and that understanding of its use and value will increase across the rheumatology community, he said.
Dr. Spiera has received research grants, consulting fees, and/or other payments from many companies involved in SSc treatments, including Roche/Genentech, which markets tocilizumab, and Corbus Pharmaceuticals, which is developing lenabasum.
Dr. Khanna is a consultant to Roche/Genentech and Bayer, which markets riociguat, and other companies. He has received research grants from Bayer, Bristol-Myers Squibb (which markets abatacept), and Pfizer. The ASSET trial he presented was sponsored by an NIH/NIAID Clinical ACE grant and an investigator-initiated grant by Bristol-Myers Squibb.
Dr. Distler has a consultancy relationship and/or has received research funding from Bayer, Roche/Genentech, and other companies. He also has a patent for a treatment for systemic sclerosis.
Dr. Zheng reported having no disclosures.
sworcester@mdedge.com
CHICAGO – At least three phase 3 randomized scleroderma treatment trials presented at the annual meeting of the American College of Rheumatology failed to meet modified Rodnan Skin Score–based primary endpoints, but a different story emerged when data from the trials were analyzed using the novel ACR Composite Response Index in Systemic Sclerosis (CRISS).
The differences highlight the limitations of individual outcome measures like the modified Rodnan Skin Score (mRSS) and underscore the need for new measures that capture the complexity of systemic sclerosis (SSc) and are more sensitive to changes in disease severity, according to Robert Spiera, MD, director of the vasculitis and scleroderma program at the Hospital for Special Surgery in New York.
Such measures are needed to better assess the effects of treatment interventions in scleroderma trials, Dr. Spiera said in an interview.
The ACR CRISS
Development of the ACR CRISS was led by Dinesh Khanna, MBBS, a professor of medicine and director of the scleroderma program at the University of Michigan, Ann Arbor. He and his colleagues described the measure in 2016 (Arthritis Rheumatol. 2016 Feb;68[2]:299-311. doi: 10.1002/art.39501).
Its use involves a two-step process of identifying any significant disease worsening or new end-organ damage, and then calculating the probability of patient improvement after 1 year of treatment on a 0- to 1-point scale based on changes from baseline in five variables: the mRSS, percent predicted forced vital capacity (FVC), patient and physician global assessments, and the Health Assessment Questionnaire Disability Index (HAQ-DI).
A CRISS score of 0.6 or higher indicates likelihood that a patient improved on treatment. Of note, subjects with significant worsening of renal or cardiopulmonary involvement are classified as not improved (score of 0), regardless of improvements in other core items.
To devise the CRISS, the investigators compiled 150 patient profiles with standardized clinical outcome elements using patients with diffuse cutaneous systemic sclerosis (dcSSc). The profiles were assessed by 40 scleroderma experts who rated patient improvement or lack thereof over 12 months.
Using the 79% of profiles for which a consensus was reached, the investigators “fit logistic regression models in which the binary outcome referred to whether the patient was improved or not, and the changes in the core set items from baseline to follow-up were entered as covariates,” they explained.
This led to the selection of the five measures included in the final version, which was found to have sensitivity of 0.982 and specificity of 0.931. When evaluated in a previously completed 1-year randomized controlled trial, the index differentiated the effect of methotrexate from the effect of placebo (P = .02), they reported.
Based on these findings, the ACR board of directors granted “provisional” endorsement of the CRISS for use in SSc clinical studies, signifying that it had been quantitatively validated using patient data, but had not undergone validation using an external data set.
New data presented at the 2018 ACR meeting will likely lead to full approval of the measure once the studies validating the measure are published, according to Dr. Spiera.
The focuSSced study of tocilizumab
For example, in the phase 3 focuSSced study comparing the interleukin-6 (IL-6) receptor–alpha antibody tocilizumab (Actemra) with placebo in patients with SSc, the primary endpoint of mean change from baseline in mRSS was not met (–6.14 vs. –4.41 points with tocilizumab vs. placebo, respectively; P = .098), Dr. Khanna said during a report of findings from the double-blind portion of the study.
However, when the CRISS was applied and its performance prospectively assessed as an exploratory outcome at week 48 in the focuSSced trial, the differences between the groups were statistically significant. Dr. Khanna reported those findings in a separate presentation at the meeting, noting that they marked the first prospective evaluation of the ACR CRISS in a phase 3 trial in SSc.
The CRISS was applied – using a blinded review of adverse events and serious adverse events to complete step 1 – in all patients who received study treatment, stratified by baseline IL-6 levels.
Of 104 patients in the tocilizumab arm and 106 in the placebo arm, 6 and 13 patients, respectively, had cardio-pulmonary-renal involvement and therefore received a score of 0.
Using the ACR CRISS as a continuous measure, scores favored tocilizumab over placebo at week 48 with a median increase of 0.89 vs. 0.25 points (P = .023), and using the binary form of CRISS, 51% vs. 37% of patients in the treatment and placebo arms achieved the score cutoff of 0.60 or higher at week 48 (P = .035), he said.
“The focuSSced study validates the ACR CRISS endpoint for the first time in an independent prospective clinical trial and highlights the importance of step 1 as an indicator of reduced organ progression during 48 weeks of treatment,” Dr. Khanna concluded.
The ASSET trial of abatacept
Dr. Khanna also reported results from the 12-month phase 2 ASSET trial comparing abatacept (Orencia) and placebo in early dcSSc.
Again, the change from baseline in mRSS – the primary endpoint of the study – did not differ significantly with treatment vs. placebo (–6.24 vs. –4.49; P = .28).
And again, as presented separately in a poster session at ACR, application of CRISS at 12 months – a secondary outcome measure in the trial – showed a significant difference between the groups.
The investigators prospectively performed step 1 of the CRISS using a case report form. Of 63 patients with complete data available for relevant outcomes at 12 months, 10 (5 in each group) had cardio-pulmonary-renal involvement and thus were given a score of 0.
Overall, there was evidence of significantly improved CRISS scores in the abatacept group vs. the placebo group (P = .03), he said.
Most of the individual CRISS variables, except HAQ-DI and patient global assessment, had statistically significant correlations with the CRISS, he noted, adding that “although the degree of correlation is high between the mRSS and CRISS, there is evidence that CRISS may be more sensitive to clinically meaningful treatment changes than the standard skin score endpoint.”
“This suggests further validation of CRISS as an independent primary endpoint for scleroderma clinical trials,” he concluded.
The phase 2b RISE-SSc study of riociguat
As in the focuSSced and ASSET studies, the primary efficacy endpoint of mean change from baseline in mRSS was not met in the randomized, double-blind, placebo-controlled RISE-SSc study evaluating the safety and efficacy of the soluble guanylate cyclase stimulator riociguat (Adempas) vs. placebo in 121 patients with early dcSSc, reported Oliver Distler, MD, a professor at University Hospital Zurich.
The mean change in mRSS at 52 weeks was 2.25 vs. 0.97 with riociguat vs. placebo, respectively (P = .08), although the difference in mRSS progression rate showed significant effects favoring riociguat (P = .02), he noted.
In this study, however, the proportion of patients with ACR CRISS probability of improvement (score of 0.60 or higher) at week 52 – a secondary study outcome – was the same at 18% in both arms, Dr. Distler said.
In a way, it’s helpful that the CRISS findings as well as the mRSS outcome in the RISE-SSc study were negative because it shows that “not everything comes up positive using the CRISS,” Dr. Spiera said.
An open-label extension trial of lenabasum
In the open-label extension of a phase 2 trial of lenabasum, Dr. Spiera and his colleagues also found the CRISS useful for assessing response in 36 patients. Lenabasum is a synthetic, nonimunosuppressive, selective cannabinoid receptor type 2 agonist that activates resolution of innate immune responses.
The agent continued to demonstrate acceptable safety and tolerability in dcSSc with no severe or serious adverse events or study discontinuations related to treatment during 12 months of open-label extension dosing, both from baseline and from the start of the extension, they reported in a poster at the ACR meeting. These assessments were based on ACR CRISS score, mRSS, physician global assessment, and multiple patient-reported outcomes.
The median CRISS score was 92% at week 52, and mRSS declined by a mean of 9.4 points (41.3% from baseline). More than a third of patients (35%) achieved a low mRSS of 10 or less.
The investigators noted, however, that definitive attribution of the findings to lenabasum is limited by the use of background therapy, the potential for spontaneous improvement in patients, and open-label dosing.
Evaluating immunosuppressive therapy in SSc
In another study presented at the ACR meeting, Boyang Zheng, MD, a second-year rheumatology fellow at McGill University in Montreal and his colleagues evaluated the effect of current immunosuppressive therapy on the ACR CRISS in 301 adult dcSSc patients without prior immunosuppression who were part of the Canadian Scleroderma Research Group (CSRG) registry.
Patients newly treated with methotrexate, azathioprine, mycophenolate and/or cyclophosphamide for at least 2 years (47 patients) were considered “exposed patients,” and untreated patients with at least the same follow-up duration were considered control subjects (254 patients).
Inverse probability of treatment weighting (IPTW) was performed to balance potential confounders in the two groups, including age, sex, disease duration, and CRISS variables, in an effort “to create a statistical cohort that would resemble a randomized, controlled trial,” Dr Zheng explained.
Prior to IPTW, treated patients trended towards more improvement after 1 year, but with “unimpressive absolute values.”
“But [after IPTW], when you look at overall CRISS at 1 year, more of the treated patients had actually improved – 23% vs. 11.8% in the untreated patients. After adjusting for age, sex, and disease duration, immunosuppression was associated with an almost twofold higher likelihood of improvement, although this was not statistically significant,” he said.
“Most importantly, after our balancing in the statistical cohort – so after balancing and adjusting for covariates ... immunosuppression use was still associated with a higher likelihood of improving [with an] odds ratio of 1.85 and P-value of 0.018,” he said.
The treated patients were sicker, and the CRISS was still able to capture individual patient improvement, he added.
Though limited by the observational design and the fact that “IPTW balancing cannot correct for all possible confounders,” the findings “provide novel evidence to support the use of immunosuppression in diffuse systemic sclerosis, and this reassures us in our current practice; it provides evidence that the CRISS seems to have better sensitivity to change than the mean of individual disease measures,” he said.
However, it also shows that “we have a long way to go to have better treatment for our patients,” he added, noting that only a minority (23%) of the patients in this study improved on the CRISS.
Moving forward in systemic sclerosis
Indeed, in order to move forward in developing better treatment for SSc, it is important to have the best possible means for assessing the effects of the potential new treatments, Dr. Spiera said.
“The mRSS is a good, reliable outcome measure in terms of intra- and inter-rater reliability, and we know it has meaning in the real world; in a patient with rapidly progressive skin thickening and a skin score that is getting higher and higher, we know they have worse prognosis in terms of function and even survival,” he said. “On the other hand, it’s just one piece of this very complicated story when you’re dealing with patients with systemic sclerosis, some of whom can have important internal organ involvement and not even have skin involvement.”
The main challenge with the skin score is how it performs in clinical trials when it comes to demonstrating whether a drug works, he added.
“This is particularly relevant in an era where our better understanding of the disease has led to candidate therapeutic agents that we have reason to think might work, but where clinical trials haven’t shown a significant difference between the groups using the skin score.”
Conversely, there have been studies in which skin scores improve, but the patient is doing terribly, he noted.
“That patient would not be [shown to be] doing well using the CRISS,” he said, explaining that the complex formula used to determine the CRISS score, which is “heavily weighted toward skin score,” allows for an overall score that is “greater than the sum of its parts.” That is, if a patient is improving in multiple domains indicating that the patient is responding to therapy, more credit is given for each of those parts.
“So my sense, and I think it’s the consensus in the community of clinical investigators, is that the skin score is not adequately sensitive to change in the context of a trial and doesn’t capture the disease holistically enough to be the optimal measure for scleroderma clinical trials,” he said. “We think the CRISS score works better in terms of capturing improvement in the course of a trial, and also in capturing other outcomes that are really, really important to patients and to their physicians, like disability or lung function.”
The hope is that, given the evidence, regulatory agencies will look favorably on the ACR CRISS as an outcome measure in clinical trials moving forward, and that understanding of its use and value will increase across the rheumatology community, he said.
Dr. Spiera has received research grants, consulting fees, and/or other payments from many companies involved in SSc treatments, including Roche/Genentech, which markets tocilizumab, and Corbus Pharmaceuticals, which is developing lenabasum.
Dr. Khanna is a consultant to Roche/Genentech and Bayer, which markets riociguat, and other companies. He has received research grants from Bayer, Bristol-Myers Squibb (which markets abatacept), and Pfizer. The ASSET trial he presented was sponsored by an NIH/NIAID Clinical ACE grant and an investigator-initiated grant by Bristol-Myers Squibb.
Dr. Distler has a consultancy relationship and/or has received research funding from Bayer, Roche/Genentech, and other companies. He also has a patent for a treatment for systemic sclerosis.
Dr. Zheng reported having no disclosures.
sworcester@mdedge.com
CHICAGO – At least three phase 3 randomized scleroderma treatment trials presented at the annual meeting of the American College of Rheumatology failed to meet modified Rodnan Skin Score–based primary endpoints, but a different story emerged when data from the trials were analyzed using the novel ACR Composite Response Index in Systemic Sclerosis (CRISS).
The differences highlight the limitations of individual outcome measures like the modified Rodnan Skin Score (mRSS) and underscore the need for new measures that capture the complexity of systemic sclerosis (SSc) and are more sensitive to changes in disease severity, according to Robert Spiera, MD, director of the vasculitis and scleroderma program at the Hospital for Special Surgery in New York.
Such measures are needed to better assess the effects of treatment interventions in scleroderma trials, Dr. Spiera said in an interview.
The ACR CRISS
Development of the ACR CRISS was led by Dinesh Khanna, MBBS, a professor of medicine and director of the scleroderma program at the University of Michigan, Ann Arbor. He and his colleagues described the measure in 2016 (Arthritis Rheumatol. 2016 Feb;68[2]:299-311. doi: 10.1002/art.39501).
Its use involves a two-step process of identifying any significant disease worsening or new end-organ damage, and then calculating the probability of patient improvement after 1 year of treatment on a 0- to 1-point scale based on changes from baseline in five variables: the mRSS, percent predicted forced vital capacity (FVC), patient and physician global assessments, and the Health Assessment Questionnaire Disability Index (HAQ-DI).
A CRISS score of 0.6 or higher indicates likelihood that a patient improved on treatment. Of note, subjects with significant worsening of renal or cardiopulmonary involvement are classified as not improved (score of 0), regardless of improvements in other core items.
To devise the CRISS, the investigators compiled 150 patient profiles with standardized clinical outcome elements using patients with diffuse cutaneous systemic sclerosis (dcSSc). The profiles were assessed by 40 scleroderma experts who rated patient improvement or lack thereof over 12 months.
Using the 79% of profiles for which a consensus was reached, the investigators “fit logistic regression models in which the binary outcome referred to whether the patient was improved or not, and the changes in the core set items from baseline to follow-up were entered as covariates,” they explained.
This led to the selection of the five measures included in the final version, which was found to have sensitivity of 0.982 and specificity of 0.931. When evaluated in a previously completed 1-year randomized controlled trial, the index differentiated the effect of methotrexate from the effect of placebo (P = .02), they reported.
Based on these findings, the ACR board of directors granted “provisional” endorsement of the CRISS for use in SSc clinical studies, signifying that it had been quantitatively validated using patient data, but had not undergone validation using an external data set.
New data presented at the 2018 ACR meeting will likely lead to full approval of the measure once the studies validating the measure are published, according to Dr. Spiera.
The focuSSced study of tocilizumab
For example, in the phase 3 focuSSced study comparing the interleukin-6 (IL-6) receptor–alpha antibody tocilizumab (Actemra) with placebo in patients with SSc, the primary endpoint of mean change from baseline in mRSS was not met (–6.14 vs. –4.41 points with tocilizumab vs. placebo, respectively; P = .098), Dr. Khanna said during a report of findings from the double-blind portion of the study.
However, when the CRISS was applied and its performance prospectively assessed as an exploratory outcome at week 48 in the focuSSced trial, the differences between the groups were statistically significant. Dr. Khanna reported those findings in a separate presentation at the meeting, noting that they marked the first prospective evaluation of the ACR CRISS in a phase 3 trial in SSc.
The CRISS was applied – using a blinded review of adverse events and serious adverse events to complete step 1 – in all patients who received study treatment, stratified by baseline IL-6 levels.
Of 104 patients in the tocilizumab arm and 106 in the placebo arm, 6 and 13 patients, respectively, had cardio-pulmonary-renal involvement and therefore received a score of 0.
Using the ACR CRISS as a continuous measure, scores favored tocilizumab over placebo at week 48 with a median increase of 0.89 vs. 0.25 points (P = .023), and using the binary form of CRISS, 51% vs. 37% of patients in the treatment and placebo arms achieved the score cutoff of 0.60 or higher at week 48 (P = .035), he said.
“The focuSSced study validates the ACR CRISS endpoint for the first time in an independent prospective clinical trial and highlights the importance of step 1 as an indicator of reduced organ progression during 48 weeks of treatment,” Dr. Khanna concluded.
The ASSET trial of abatacept
Dr. Khanna also reported results from the 12-month phase 2 ASSET trial comparing abatacept (Orencia) and placebo in early dcSSc.
Again, the change from baseline in mRSS – the primary endpoint of the study – did not differ significantly with treatment vs. placebo (–6.24 vs. –4.49; P = .28).
And again, as presented separately in a poster session at ACR, application of CRISS at 12 months – a secondary outcome measure in the trial – showed a significant difference between the groups.
The investigators prospectively performed step 1 of the CRISS using a case report form. Of 63 patients with complete data available for relevant outcomes at 12 months, 10 (5 in each group) had cardio-pulmonary-renal involvement and thus were given a score of 0.
Overall, there was evidence of significantly improved CRISS scores in the abatacept group vs. the placebo group (P = .03), he said.
Most of the individual CRISS variables, except HAQ-DI and patient global assessment, had statistically significant correlations with the CRISS, he noted, adding that “although the degree of correlation is high between the mRSS and CRISS, there is evidence that CRISS may be more sensitive to clinically meaningful treatment changes than the standard skin score endpoint.”
“This suggests further validation of CRISS as an independent primary endpoint for scleroderma clinical trials,” he concluded.
The phase 2b RISE-SSc study of riociguat
As in the focuSSced and ASSET studies, the primary efficacy endpoint of mean change from baseline in mRSS was not met in the randomized, double-blind, placebo-controlled RISE-SSc study evaluating the safety and efficacy of the soluble guanylate cyclase stimulator riociguat (Adempas) vs. placebo in 121 patients with early dcSSc, reported Oliver Distler, MD, a professor at University Hospital Zurich.
The mean change in mRSS at 52 weeks was 2.25 vs. 0.97 with riociguat vs. placebo, respectively (P = .08), although the difference in mRSS progression rate showed significant effects favoring riociguat (P = .02), he noted.
In this study, however, the proportion of patients with ACR CRISS probability of improvement (score of 0.60 or higher) at week 52 – a secondary study outcome – was the same at 18% in both arms, Dr. Distler said.
In a way, it’s helpful that the CRISS findings as well as the mRSS outcome in the RISE-SSc study were negative because it shows that “not everything comes up positive using the CRISS,” Dr. Spiera said.
An open-label extension trial of lenabasum
In the open-label extension of a phase 2 trial of lenabasum, Dr. Spiera and his colleagues also found the CRISS useful for assessing response in 36 patients. Lenabasum is a synthetic, nonimunosuppressive, selective cannabinoid receptor type 2 agonist that activates resolution of innate immune responses.
The agent continued to demonstrate acceptable safety and tolerability in dcSSc with no severe or serious adverse events or study discontinuations related to treatment during 12 months of open-label extension dosing, both from baseline and from the start of the extension, they reported in a poster at the ACR meeting. These assessments were based on ACR CRISS score, mRSS, physician global assessment, and multiple patient-reported outcomes.
The median CRISS score was 92% at week 52, and mRSS declined by a mean of 9.4 points (41.3% from baseline). More than a third of patients (35%) achieved a low mRSS of 10 or less.
The investigators noted, however, that definitive attribution of the findings to lenabasum is limited by the use of background therapy, the potential for spontaneous improvement in patients, and open-label dosing.
Evaluating immunosuppressive therapy in SSc
In another study presented at the ACR meeting, Boyang Zheng, MD, a second-year rheumatology fellow at McGill University in Montreal and his colleagues evaluated the effect of current immunosuppressive therapy on the ACR CRISS in 301 adult dcSSc patients without prior immunosuppression who were part of the Canadian Scleroderma Research Group (CSRG) registry.
Patients newly treated with methotrexate, azathioprine, mycophenolate and/or cyclophosphamide for at least 2 years (47 patients) were considered “exposed patients,” and untreated patients with at least the same follow-up duration were considered control subjects (254 patients).
Inverse probability of treatment weighting (IPTW) was performed to balance potential confounders in the two groups, including age, sex, disease duration, and CRISS variables, in an effort “to create a statistical cohort that would resemble a randomized, controlled trial,” Dr Zheng explained.
Prior to IPTW, treated patients trended towards more improvement after 1 year, but with “unimpressive absolute values.”
“But [after IPTW], when you look at overall CRISS at 1 year, more of the treated patients had actually improved – 23% vs. 11.8% in the untreated patients. After adjusting for age, sex, and disease duration, immunosuppression was associated with an almost twofold higher likelihood of improvement, although this was not statistically significant,” he said.
“Most importantly, after our balancing in the statistical cohort – so after balancing and adjusting for covariates ... immunosuppression use was still associated with a higher likelihood of improving [with an] odds ratio of 1.85 and P-value of 0.018,” he said.
The treated patients were sicker, and the CRISS was still able to capture individual patient improvement, he added.
Though limited by the observational design and the fact that “IPTW balancing cannot correct for all possible confounders,” the findings “provide novel evidence to support the use of immunosuppression in diffuse systemic sclerosis, and this reassures us in our current practice; it provides evidence that the CRISS seems to have better sensitivity to change than the mean of individual disease measures,” he said.
However, it also shows that “we have a long way to go to have better treatment for our patients,” he added, noting that only a minority (23%) of the patients in this study improved on the CRISS.
Moving forward in systemic sclerosis
Indeed, in order to move forward in developing better treatment for SSc, it is important to have the best possible means for assessing the effects of the potential new treatments, Dr. Spiera said.
“The mRSS is a good, reliable outcome measure in terms of intra- and inter-rater reliability, and we know it has meaning in the real world; in a patient with rapidly progressive skin thickening and a skin score that is getting higher and higher, we know they have worse prognosis in terms of function and even survival,” he said. “On the other hand, it’s just one piece of this very complicated story when you’re dealing with patients with systemic sclerosis, some of whom can have important internal organ involvement and not even have skin involvement.”
The main challenge with the skin score is how it performs in clinical trials when it comes to demonstrating whether a drug works, he added.
“This is particularly relevant in an era where our better understanding of the disease has led to candidate therapeutic agents that we have reason to think might work, but where clinical trials haven’t shown a significant difference between the groups using the skin score.”
Conversely, there have been studies in which skin scores improve, but the patient is doing terribly, he noted.
“That patient would not be [shown to be] doing well using the CRISS,” he said, explaining that the complex formula used to determine the CRISS score, which is “heavily weighted toward skin score,” allows for an overall score that is “greater than the sum of its parts.” That is, if a patient is improving in multiple domains indicating that the patient is responding to therapy, more credit is given for each of those parts.
“So my sense, and I think it’s the consensus in the community of clinical investigators, is that the skin score is not adequately sensitive to change in the context of a trial and doesn’t capture the disease holistically enough to be the optimal measure for scleroderma clinical trials,” he said. “We think the CRISS score works better in terms of capturing improvement in the course of a trial, and also in capturing other outcomes that are really, really important to patients and to their physicians, like disability or lung function.”
The hope is that, given the evidence, regulatory agencies will look favorably on the ACR CRISS as an outcome measure in clinical trials moving forward, and that understanding of its use and value will increase across the rheumatology community, he said.
Dr. Spiera has received research grants, consulting fees, and/or other payments from many companies involved in SSc treatments, including Roche/Genentech, which markets tocilizumab, and Corbus Pharmaceuticals, which is developing lenabasum.
Dr. Khanna is a consultant to Roche/Genentech and Bayer, which markets riociguat, and other companies. He has received research grants from Bayer, Bristol-Myers Squibb (which markets abatacept), and Pfizer. The ASSET trial he presented was sponsored by an NIH/NIAID Clinical ACE grant and an investigator-initiated grant by Bristol-Myers Squibb.
Dr. Distler has a consultancy relationship and/or has received research funding from Bayer, Roche/Genentech, and other companies. He also has a patent for a treatment for systemic sclerosis.
Dr. Zheng reported having no disclosures.
sworcester@mdedge.com
REPORTING FROM THE ACR ANNUAL MEETING
The Liver Meeting 2018: Hepatitis B novel therapies debrief – key abstracts
SAN FRANCISCO – Most years, when it comes to research to treat hepatitis viral infections, hepatitis C has been front and center at the annual meeting of the American Association for the Study of Liver Diseases. But things have changed in the past couple of years, with hepatitis C curative treatment maturing and altering the therapeutic landscape.
“Hepatitis C has been where all the action is, but that’s clearly changed in the last few years,” said Jordan Feld, MD, MPH, who summed up the hepatitis B findings during a wrap-up session on the final day of the conference. Dr. Feld is a clinician-scientist at the Toronto Western Hospital Liver Clinic and the McLaughlin-Rotman Centre for Global Health.
An analysis (Abstract 212) of a mixed North American and Asian cohort of more than 10,000 untreated patients showed just a 1.3% annual clearance rate of surface antigen, with little guidance for risk stratification. “This leaves us really needing new therapies,” said Dr. Feld.
Fortunately, the hepatitis B virus (HBV) life cycle offers various opportunities for therapeutic intervention, including blocking entry, targeting assembly and export of the virus, targeting HBV RNA, and targeting the capsid protein and viral packaging.
One study (Abstract 16) examined a hepatitis B entry inhibitor’s effect on hepatitis D virus (HDV), which requires coinfection with HBV to replicate. A phase 2 clinical trial found that treatment with Myrcludex B alone or in combination with interferon led to a decline in HDV RNA, but the result was most pronounced in patients who received the combination therapy. The combination was also associated with a greater probability of surface antigen decline. “I think that’s really important, that we see this synergistic effect. This is really promising phase 2 data that raises the possibility of curative therapy for this troubling infection,” said Dr. Feld.
Another study (Abstract LB-25) looked at an RNA inhibitor that targets both integrated and covalently closed circular DNA (cccDNA)–derived HBV RNA. The drug was given to 11 HBV patients who were positive for HBeAg (hepatitis B e-antigen) and 13 HBV patients who were HBeAg negative. It had similar effects in reducing HBV surface antigens and other correlated antigens in both groups of patients, and no evidence of a dose-response relationship. “Seeing a similar effect is quite important and suggests that it’s targeting both cccDNA-derived and integrated HBV DNA, and although there were some mild injection reactions, it generally seemed to be safe and pretty well tolerated,” said Dr. Feld.
Further down the life cycle, capsid assembly modulators (CAMs) have the potential to counter HBV by two mechanisms; blocking encapsulation of pregenomic RNA, and degrading capsids, which could prevent the replenishment of cccDNA. The latter effect could be important for achieving a cure, according to Dr. Feld.
A novel CAM, JNJ-6379 (Abstract 74), was tested at three different doses, and was well tolerated at higher doses, but it had limited dose response at the higher dose with respect to HBV DNA suppression. However, it could be that the two CAM mechanisms may require different doses. “These two things are hard to tease apart, and hopefully, we’ll see more data to separate them in the future,” said Dr. Feld.
Another CAM, ABI-HO731 (Abstract 73), had a potent effect on HBV DNA and HBV RNA, showing that it blocks encapsulation of both pregenomic RNA related to reverse transcription and pregenomic RNAs within the capsid. Stopping the medication led to some HBV DNA rebound, though no alanine aminotransferase flares, which Dr. Feld found reassuring. One patient had baseline resistance but was nevertheless able to achieve some suppression on the drug.
Another therapeutic approach used a nucleic acid polymer to block subviral particle release (Abstract 393). The study treated immunosuppressed patients with the polymer alone or in combination with interferon or tenofovir disoproxil, and led to “striking reductions in hepatitis B surface antigen quantities during therapy,” said Dr. Feld. An alanine aminotransferase flair did occur, which may signify an immune response, and it will be important to determine if this is indeed the case, he said. After stopping therapy there was a gain of anti–hepatitis B antibodies, which suggests that functional clearance of surface antigen is occurring.
Researchers also are recruiting the immune system to combat HBV. The novel agent inarigivir is a retinoic acid-inducible gene-1 agonist, which has both a direct antiviral effect and an indirect effect via the intrahepatic innate immune response, which it accomplishes by activating the interferon signaling pathway. It also directly interferes with the interaction between pregenomic RNA and the HBV polymerase, preventing replication. In the ACHIEVE trial, the researchers noted greater reduction in HBV RNA and DNA at higher doses (Abstract 75). “This is certainly an interesting molecule and an interesting proof of concept that you can potentially target HBV using two different pathways, and we’ll be interested to see more data with this approach,” said Dr. Feld.
Dr. Feld wrapped up the discussion of novel therapies with an animal model study (Abstract 77) that suggests future strategies for a cure. In it, the researchers combined a therapeutic vaccine with a stabilized, liver-targeted small interfering RNA to suppress surface antigen. Animals that received only the vaccine saw little benefit, but the combined approach led to viral clearance. The treatment also restored the immune response. “It gives us an inkling that we may need to both reduce the antigen load and stimulate immunity,” said Dr. Feld.
Dr. Feld has consulted for AbbVie, Gilead, ContraVir, MedImmune, and Merck. He has received funding from AbbVie, Gilead, Merck, and Janssen.
SAN FRANCISCO – Most years, when it comes to research to treat hepatitis viral infections, hepatitis C has been front and center at the annual meeting of the American Association for the Study of Liver Diseases. But things have changed in the past couple of years, with hepatitis C curative treatment maturing and altering the therapeutic landscape.
“Hepatitis C has been where all the action is, but that’s clearly changed in the last few years,” said Jordan Feld, MD, MPH, who summed up the hepatitis B findings during a wrap-up session on the final day of the conference. Dr. Feld is a clinician-scientist at the Toronto Western Hospital Liver Clinic and the McLaughlin-Rotman Centre for Global Health.
An analysis (Abstract 212) of a mixed North American and Asian cohort of more than 10,000 untreated patients showed just a 1.3% annual clearance rate of surface antigen, with little guidance for risk stratification. “This leaves us really needing new therapies,” said Dr. Feld.
Fortunately, the hepatitis B virus (HBV) life cycle offers various opportunities for therapeutic intervention, including blocking entry, targeting assembly and export of the virus, targeting HBV RNA, and targeting the capsid protein and viral packaging.
One study (Abstract 16) examined a hepatitis B entry inhibitor’s effect on hepatitis D virus (HDV), which requires coinfection with HBV to replicate. A phase 2 clinical trial found that treatment with Myrcludex B alone or in combination with interferon led to a decline in HDV RNA, but the result was most pronounced in patients who received the combination therapy. The combination was also associated with a greater probability of surface antigen decline. “I think that’s really important, that we see this synergistic effect. This is really promising phase 2 data that raises the possibility of curative therapy for this troubling infection,” said Dr. Feld.
Another study (Abstract LB-25) looked at an RNA inhibitor that targets both integrated and covalently closed circular DNA (cccDNA)–derived HBV RNA. The drug was given to 11 HBV patients who were positive for HBeAg (hepatitis B e-antigen) and 13 HBV patients who were HBeAg negative. It had similar effects in reducing HBV surface antigens and other correlated antigens in both groups of patients, and no evidence of a dose-response relationship. “Seeing a similar effect is quite important and suggests that it’s targeting both cccDNA-derived and integrated HBV DNA, and although there were some mild injection reactions, it generally seemed to be safe and pretty well tolerated,” said Dr. Feld.
Further down the life cycle, capsid assembly modulators (CAMs) have the potential to counter HBV by two mechanisms; blocking encapsulation of pregenomic RNA, and degrading capsids, which could prevent the replenishment of cccDNA. The latter effect could be important for achieving a cure, according to Dr. Feld.
A novel CAM, JNJ-6379 (Abstract 74), was tested at three different doses, and was well tolerated at higher doses, but it had limited dose response at the higher dose with respect to HBV DNA suppression. However, it could be that the two CAM mechanisms may require different doses. “These two things are hard to tease apart, and hopefully, we’ll see more data to separate them in the future,” said Dr. Feld.
Another CAM, ABI-HO731 (Abstract 73), had a potent effect on HBV DNA and HBV RNA, showing that it blocks encapsulation of both pregenomic RNA related to reverse transcription and pregenomic RNAs within the capsid. Stopping the medication led to some HBV DNA rebound, though no alanine aminotransferase flares, which Dr. Feld found reassuring. One patient had baseline resistance but was nevertheless able to achieve some suppression on the drug.
Another therapeutic approach used a nucleic acid polymer to block subviral particle release (Abstract 393). The study treated immunosuppressed patients with the polymer alone or in combination with interferon or tenofovir disoproxil, and led to “striking reductions in hepatitis B surface antigen quantities during therapy,” said Dr. Feld. An alanine aminotransferase flair did occur, which may signify an immune response, and it will be important to determine if this is indeed the case, he said. After stopping therapy there was a gain of anti–hepatitis B antibodies, which suggests that functional clearance of surface antigen is occurring.
Researchers also are recruiting the immune system to combat HBV. The novel agent inarigivir is a retinoic acid-inducible gene-1 agonist, which has both a direct antiviral effect and an indirect effect via the intrahepatic innate immune response, which it accomplishes by activating the interferon signaling pathway. It also directly interferes with the interaction between pregenomic RNA and the HBV polymerase, preventing replication. In the ACHIEVE trial, the researchers noted greater reduction in HBV RNA and DNA at higher doses (Abstract 75). “This is certainly an interesting molecule and an interesting proof of concept that you can potentially target HBV using two different pathways, and we’ll be interested to see more data with this approach,” said Dr. Feld.
Dr. Feld wrapped up the discussion of novel therapies with an animal model study (Abstract 77) that suggests future strategies for a cure. In it, the researchers combined a therapeutic vaccine with a stabilized, liver-targeted small interfering RNA to suppress surface antigen. Animals that received only the vaccine saw little benefit, but the combined approach led to viral clearance. The treatment also restored the immune response. “It gives us an inkling that we may need to both reduce the antigen load and stimulate immunity,” said Dr. Feld.
Dr. Feld has consulted for AbbVie, Gilead, ContraVir, MedImmune, and Merck. He has received funding from AbbVie, Gilead, Merck, and Janssen.
SAN FRANCISCO – Most years, when it comes to research to treat hepatitis viral infections, hepatitis C has been front and center at the annual meeting of the American Association for the Study of Liver Diseases. But things have changed in the past couple of years, with hepatitis C curative treatment maturing and altering the therapeutic landscape.
“Hepatitis C has been where all the action is, but that’s clearly changed in the last few years,” said Jordan Feld, MD, MPH, who summed up the hepatitis B findings during a wrap-up session on the final day of the conference. Dr. Feld is a clinician-scientist at the Toronto Western Hospital Liver Clinic and the McLaughlin-Rotman Centre for Global Health.
An analysis (Abstract 212) of a mixed North American and Asian cohort of more than 10,000 untreated patients showed just a 1.3% annual clearance rate of surface antigen, with little guidance for risk stratification. “This leaves us really needing new therapies,” said Dr. Feld.
Fortunately, the hepatitis B virus (HBV) life cycle offers various opportunities for therapeutic intervention, including blocking entry, targeting assembly and export of the virus, targeting HBV RNA, and targeting the capsid protein and viral packaging.
One study (Abstract 16) examined a hepatitis B entry inhibitor’s effect on hepatitis D virus (HDV), which requires coinfection with HBV to replicate. A phase 2 clinical trial found that treatment with Myrcludex B alone or in combination with interferon led to a decline in HDV RNA, but the result was most pronounced in patients who received the combination therapy. The combination was also associated with a greater probability of surface antigen decline. “I think that’s really important, that we see this synergistic effect. This is really promising phase 2 data that raises the possibility of curative therapy for this troubling infection,” said Dr. Feld.
Another study (Abstract LB-25) looked at an RNA inhibitor that targets both integrated and covalently closed circular DNA (cccDNA)–derived HBV RNA. The drug was given to 11 HBV patients who were positive for HBeAg (hepatitis B e-antigen) and 13 HBV patients who were HBeAg negative. It had similar effects in reducing HBV surface antigens and other correlated antigens in both groups of patients, and no evidence of a dose-response relationship. “Seeing a similar effect is quite important and suggests that it’s targeting both cccDNA-derived and integrated HBV DNA, and although there were some mild injection reactions, it generally seemed to be safe and pretty well tolerated,” said Dr. Feld.
Further down the life cycle, capsid assembly modulators (CAMs) have the potential to counter HBV by two mechanisms; blocking encapsulation of pregenomic RNA, and degrading capsids, which could prevent the replenishment of cccDNA. The latter effect could be important for achieving a cure, according to Dr. Feld.
A novel CAM, JNJ-6379 (Abstract 74), was tested at three different doses, and was well tolerated at higher doses, but it had limited dose response at the higher dose with respect to HBV DNA suppression. However, it could be that the two CAM mechanisms may require different doses. “These two things are hard to tease apart, and hopefully, we’ll see more data to separate them in the future,” said Dr. Feld.
Another CAM, ABI-HO731 (Abstract 73), had a potent effect on HBV DNA and HBV RNA, showing that it blocks encapsulation of both pregenomic RNA related to reverse transcription and pregenomic RNAs within the capsid. Stopping the medication led to some HBV DNA rebound, though no alanine aminotransferase flares, which Dr. Feld found reassuring. One patient had baseline resistance but was nevertheless able to achieve some suppression on the drug.
Another therapeutic approach used a nucleic acid polymer to block subviral particle release (Abstract 393). The study treated immunosuppressed patients with the polymer alone or in combination with interferon or tenofovir disoproxil, and led to “striking reductions in hepatitis B surface antigen quantities during therapy,” said Dr. Feld. An alanine aminotransferase flair did occur, which may signify an immune response, and it will be important to determine if this is indeed the case, he said. After stopping therapy there was a gain of anti–hepatitis B antibodies, which suggests that functional clearance of surface antigen is occurring.
Researchers also are recruiting the immune system to combat HBV. The novel agent inarigivir is a retinoic acid-inducible gene-1 agonist, which has both a direct antiviral effect and an indirect effect via the intrahepatic innate immune response, which it accomplishes by activating the interferon signaling pathway. It also directly interferes with the interaction between pregenomic RNA and the HBV polymerase, preventing replication. In the ACHIEVE trial, the researchers noted greater reduction in HBV RNA and DNA at higher doses (Abstract 75). “This is certainly an interesting molecule and an interesting proof of concept that you can potentially target HBV using two different pathways, and we’ll be interested to see more data with this approach,” said Dr. Feld.
Dr. Feld wrapped up the discussion of novel therapies with an animal model study (Abstract 77) that suggests future strategies for a cure. In it, the researchers combined a therapeutic vaccine with a stabilized, liver-targeted small interfering RNA to suppress surface antigen. Animals that received only the vaccine saw little benefit, but the combined approach led to viral clearance. The treatment also restored the immune response. “It gives us an inkling that we may need to both reduce the antigen load and stimulate immunity,” said Dr. Feld.
Dr. Feld has consulted for AbbVie, Gilead, ContraVir, MedImmune, and Merck. He has received funding from AbbVie, Gilead, Merck, and Janssen.
REPORTING FROM THE LIVER MEETING 2018
Point of Care Ultrasound (POCUS) for Small Bowel Obstruction in the ED
Small bowel obstruction (SBO) accounts for 2% of all cases of abdominal pain presenting to the ED and 15% of abdominal pain admissions to surgical units from the ED.1,2 SBO can be a difficult diagnosis; the most common symptoms include nausea, vomiting, abdominal pain, obstipation, and constipation. The symptomatology depends on multiple factors: the area of the blockage, length of obstruction, and degree of the obstruction (either partial or complete).3 An upper gastrointestinal (GI) blockage classically presents with nausea and vomiting, while a lower GI blockage often presents with abdominal pain, constipation, and obstipation. Complications of obstruction range from significant morbidity—such as bowel strangulation (23%) and sepsis (31%)—to mortality (9%).4 ED POCUS allows for rapid and accurate diagnosis of SBO.
CASE
A 60-year-old female with a past medical history of peptic ulcer disease and multiple abdominal surgeries, including umbilical hernia repair, appendectomy, and total abdominal hysterectomy, presented to the ED with an 8-hour history of nausea and vomiting. She reported that her abdomen felt bloated. She had experienced non-bloody, watery stools for the prior 3 weeks. She also reported three to four weeks of epigastric abdominal pain similar to her previous “ulcer pain.” Of note, she was evaluated in GI clinic one day prior to her ED visit for dysphagia, abdominal distention, and diarrhea and was scheduled for an outpatient upper endoscopy. Initial vitals were significant for a heart rate of 100 beats/min. Physical exam was significant for a mildly distended abdomen, tender to palpation at epigastrium without rebound or guarding. Labs showed a white blood cell count of 11.8 K/uL and otherwise unremarkable complete blood count, basic metabolic panel, liver function tests, and lactate measurement. Given the patient’s history of multiple abdominal surgeries and clinical presentation, POCUS was performed to evaluate for SBO. Dilated loops of small bowel were visualized in the lower abdomen gas, suggestive of SBO.
Since the small bowel encompasses a large portion of the abdomen, to fully evaluate for SBO, multiple views are necessary. These include the epigastrium, bilateral colic gutters, and suprapubic regions.5 Use the low-frequency curvilinear transducer to obtain these views, scanning in the transverse and sagittal planes (see Figures 1 and 2). Scan while moving the transducer in columns (ie, “mowing the lawn”), making sure to cover the entire abdomen. To assure that you are evaluating the small bowel, and not the large bowel, look for the characteristic plicae circularis of the small bowel (shown in Figure 3). In children and very slender adults, the high-frequency linear probe may provide enough depth to obtain adequate views.
A fluid-filled small intestinal segment >2.5 cm is consistent with a diagnosis of SBO. Measuring the diameter of the small bowel is both the most sensitive and specific sign; a measurement of greater than 2.5 cm is diagnostic, with a sensitivity of 97% and specificity of 91% (see Figure 4).6 This can be somewhat difficult to visualize, as bowel loops are multidirectional and diameters can mistakenly be taken on an indirect cut; to avoid over- or underestimation of bowel diameter, you may want to measure in the short axis using a transverse cross-sectional view of the bowel.
Lack of peristalsis is suggestive of a closed-loop obstruction. However, this finding may be more difficult to visualize, as it requires several continuous minutes of scanning or repeated exams to truly establish absent peristalsis. In prolonged courses of SBO, the bowel wall can measure >3 mm, which suggests necrosis, warranting accelerated surgical intervention. In addition, the detection of extraluminal peritoneal fluid can help determine the severity of the SBO, and small versus large fluid amounts can help determine whether medical or surgical management is warranted (see Figure 5).7
DISCUSSION
Increased time to diagnosis of SBO can lead to prolonged patient suffering and greater complication rates. The gold standard for diagnosing SBO—CT with intravenous and oral contrast—can take hours, requiring patients, who are often nauseated, to ingest and tolerate oral contrast. In the past, an “obstructive series” of x-rays would have been used early in the work-up of possible SBO.6
Recent literature suggests that POCUS is not only faster, more cost effective, and advantageous (involving no ionizing radiation), but also more accurate than x-rays. Specifically, a meta-analysis by Taylor et al showed pooled estimates for obstructive series x-rays have a sensitivity (Sn) of 75%, a specificity (Sp) of 66%, a positive likelihood ratio (+LR) of 1.6, and a negative likelihood ratio (-LR) of 0.43.1 On the other hand, pooled results from ED studies of emergency medicine (EM) residents performing POCUS in patients with signs and symptoms suspicious for SBO showed POCUS had a Sn of 97%, Sp of 90%, +LR of 9.5, and a -LR of 0.04.1,5,8 While detractors point to the operator-dependent nature of POCUS, literature suggests that with EM residents novice to POCUS for SBO (defined as less than 5 previous scans for SBO) were given a 10-minute didactic session and yielded Sn 94%, Sp 81%, +LR 5.0, -LR 0.07.5 Unluer et al trained novice EM residents for 6 hours and found them to yield Sn 98%, Sp 95%, +LR 19.5, and -LR 0.02.8 Thus, while it is no surprise that those with more training attain better results, both studies show it does not take much time for EM providers to surpass the accuracy of x-rays with POCUS.
CASE CONCLUSION
The findings on POCUS highly suggested the diagnosis of an SBO. A CT scan of the abdomen and pelvis with intravenous and oral contrast was ordered to further evaluate obstruction, transition point, and possible complications, including signs of ischemia per surgical request. CT demonstrated dilated loops of small bowel with transition point in the right lower quadrant, with a small amount of mesenteric fluid consistent with SBO with possible early bowel compromise due to ischemia. General surgery admitted the patient; conservative treatment with serial abdominal exams, nasogastric tube, NPO and bowel rest was ordered. The patient’s diet was gradually advanced, and she was discharged on the eleventh day of hospitalization.
SUMMARY
POCUS is a useful non-invasive tool that can accurately diagnose SBO. POCUS has increased sensitivity and specificity when compared to abdominal X-rays. This bedside imaging will not only give the ED provider rapid diagnostic information but also lead to expedited surgical intervention.
- Taylor MR, Lalani N. Adult small bowel obstruction. Acad Emerg Med. 2013;20(6):528-544.
- Hastings RS, Powers RD. Abdominal pain in the ED: a 35-year retrospective. Am J Emerg Med.2011;29:711-716.
- Markogiannakis H, Messaris E, Dardamanis D, et al. Acute mechanical bowel obstruction: clinical presentation, etiology, management and outcome. World J Gastroenterol. 2007;13:432.
- Bickell N, Federman A, Aufses A. Influence of time on risk of bowel resection in complete small bowel obstruction. J Am Coll Surg. 2005;201(6):847-854.
- Jang TB, Chandler D, Kaji AH. Bedside ultrasonography for the detection of small bowel obstruction in the emergency department. Emerg Med J. 2011;28:676-678.
- Carpenter CR, Pines JM. The end of X-rays for suspected small bowel obstruction? Using evidence-based diagnostics to inform best practices in emergency medicine. Acad Emerg Med. 2013;20:618-20.
- Grassi R, Romano S, D’Amario F, et al. The relevance of free fluid between intestinal loops detected by sonography in the clinical assessment of small bowel obstruction in adults. Eur J Radiol. 2004;50(1):5-14.
- Unlüer E, Yavaşi O, Eroğlu O, Yilmaz C, Akarca F. Ultrasonography by emergency medicine and radiology residents for the diagnosis of small bowel obstruction. Eur J Emerg Med. 2010;17(5):260-264.
Small bowel obstruction (SBO) accounts for 2% of all cases of abdominal pain presenting to the ED and 15% of abdominal pain admissions to surgical units from the ED.1,2 SBO can be a difficult diagnosis; the most common symptoms include nausea, vomiting, abdominal pain, obstipation, and constipation. The symptomatology depends on multiple factors: the area of the blockage, length of obstruction, and degree of the obstruction (either partial or complete).3 An upper gastrointestinal (GI) blockage classically presents with nausea and vomiting, while a lower GI blockage often presents with abdominal pain, constipation, and obstipation. Complications of obstruction range from significant morbidity—such as bowel strangulation (23%) and sepsis (31%)—to mortality (9%).4 ED POCUS allows for rapid and accurate diagnosis of SBO.
CASE
A 60-year-old female with a past medical history of peptic ulcer disease and multiple abdominal surgeries, including umbilical hernia repair, appendectomy, and total abdominal hysterectomy, presented to the ED with an 8-hour history of nausea and vomiting. She reported that her abdomen felt bloated. She had experienced non-bloody, watery stools for the prior 3 weeks. She also reported three to four weeks of epigastric abdominal pain similar to her previous “ulcer pain.” Of note, she was evaluated in GI clinic one day prior to her ED visit for dysphagia, abdominal distention, and diarrhea and was scheduled for an outpatient upper endoscopy. Initial vitals were significant for a heart rate of 100 beats/min. Physical exam was significant for a mildly distended abdomen, tender to palpation at epigastrium without rebound or guarding. Labs showed a white blood cell count of 11.8 K/uL and otherwise unremarkable complete blood count, basic metabolic panel, liver function tests, and lactate measurement. Given the patient’s history of multiple abdominal surgeries and clinical presentation, POCUS was performed to evaluate for SBO. Dilated loops of small bowel were visualized in the lower abdomen gas, suggestive of SBO.
Since the small bowel encompasses a large portion of the abdomen, to fully evaluate for SBO, multiple views are necessary. These include the epigastrium, bilateral colic gutters, and suprapubic regions.5 Use the low-frequency curvilinear transducer to obtain these views, scanning in the transverse and sagittal planes (see Figures 1 and 2). Scan while moving the transducer in columns (ie, “mowing the lawn”), making sure to cover the entire abdomen. To assure that you are evaluating the small bowel, and not the large bowel, look for the characteristic plicae circularis of the small bowel (shown in Figure 3). In children and very slender adults, the high-frequency linear probe may provide enough depth to obtain adequate views.


A fluid-filled small intestinal segment >2.5 cm is consistent with a diagnosis of SBO. Measuring the diameter of the small bowel is both the most sensitive and specific sign; a measurement of greater than 2.5 cm is diagnostic, with a sensitivity of 97% and specificity of 91% (see Figure 4).6 This can be somewhat difficult to visualize, as bowel loops are multidirectional and diameters can mistakenly be taken on an indirect cut; to avoid over- or underestimation of bowel diameter, you may want to measure in the short axis using a transverse cross-sectional view of the bowel.

Lack of peristalsis is suggestive of a closed-loop obstruction. However, this finding may be more difficult to visualize, as it requires several continuous minutes of scanning or repeated exams to truly establish absent peristalsis. In prolonged courses of SBO, the bowel wall can measure >3 mm, which suggests necrosis, warranting accelerated surgical intervention. In addition, the detection of extraluminal peritoneal fluid can help determine the severity of the SBO, and small versus large fluid amounts can help determine whether medical or surgical management is warranted (see Figure 5).7

DISCUSSION
Increased time to diagnosis of SBO can lead to prolonged patient suffering and greater complication rates. The gold standard for diagnosing SBO—CT with intravenous and oral contrast—can take hours, requiring patients, who are often nauseated, to ingest and tolerate oral contrast. In the past, an “obstructive series” of x-rays would have been used early in the work-up of possible SBO.6
Recent literature suggests that POCUS is not only faster, more cost effective, and advantageous (involving no ionizing radiation), but also more accurate than x-rays. Specifically, a meta-analysis by Taylor et al showed pooled estimates for obstructive series x-rays have a sensitivity (Sn) of 75%, a specificity (Sp) of 66%, a positive likelihood ratio (+LR) of 1.6, and a negative likelihood ratio (-LR) of 0.43.1 On the other hand, pooled results from ED studies of emergency medicine (EM) residents performing POCUS in patients with signs and symptoms suspicious for SBO showed POCUS had a Sn of 97%, Sp of 90%, +LR of 9.5, and a -LR of 0.04.1,5,8 While detractors point to the operator-dependent nature of POCUS, literature suggests that with EM residents novice to POCUS for SBO (defined as less than 5 previous scans for SBO) were given a 10-minute didactic session and yielded Sn 94%, Sp 81%, +LR 5.0, -LR 0.07.5 Unluer et al trained novice EM residents for 6 hours and found them to yield Sn 98%, Sp 95%, +LR 19.5, and -LR 0.02.8 Thus, while it is no surprise that those with more training attain better results, both studies show it does not take much time for EM providers to surpass the accuracy of x-rays with POCUS.
CASE CONCLUSION
The findings on POCUS highly suggested the diagnosis of an SBO. A CT scan of the abdomen and pelvis with intravenous and oral contrast was ordered to further evaluate obstruction, transition point, and possible complications, including signs of ischemia per surgical request. CT demonstrated dilated loops of small bowel with transition point in the right lower quadrant, with a small amount of mesenteric fluid consistent with SBO with possible early bowel compromise due to ischemia. General surgery admitted the patient; conservative treatment with serial abdominal exams, nasogastric tube, NPO and bowel rest was ordered. The patient’s diet was gradually advanced, and she was discharged on the eleventh day of hospitalization.
SUMMARY
POCUS is a useful non-invasive tool that can accurately diagnose SBO. POCUS has increased sensitivity and specificity when compared to abdominal X-rays. This bedside imaging will not only give the ED provider rapid diagnostic information but also lead to expedited surgical intervention.
Small bowel obstruction (SBO) accounts for 2% of all cases of abdominal pain presenting to the ED and 15% of abdominal pain admissions to surgical units from the ED.1,2 SBO can be a difficult diagnosis; the most common symptoms include nausea, vomiting, abdominal pain, obstipation, and constipation. The symptomatology depends on multiple factors: the area of the blockage, length of obstruction, and degree of the obstruction (either partial or complete).3 An upper gastrointestinal (GI) blockage classically presents with nausea and vomiting, while a lower GI blockage often presents with abdominal pain, constipation, and obstipation. Complications of obstruction range from significant morbidity—such as bowel strangulation (23%) and sepsis (31%)—to mortality (9%).4 ED POCUS allows for rapid and accurate diagnosis of SBO.
CASE
A 60-year-old female with a past medical history of peptic ulcer disease and multiple abdominal surgeries, including umbilical hernia repair, appendectomy, and total abdominal hysterectomy, presented to the ED with an 8-hour history of nausea and vomiting. She reported that her abdomen felt bloated. She had experienced non-bloody, watery stools for the prior 3 weeks. She also reported three to four weeks of epigastric abdominal pain similar to her previous “ulcer pain.” Of note, she was evaluated in GI clinic one day prior to her ED visit for dysphagia, abdominal distention, and diarrhea and was scheduled for an outpatient upper endoscopy. Initial vitals were significant for a heart rate of 100 beats/min. Physical exam was significant for a mildly distended abdomen, tender to palpation at epigastrium without rebound or guarding. Labs showed a white blood cell count of 11.8 K/uL and otherwise unremarkable complete blood count, basic metabolic panel, liver function tests, and lactate measurement. Given the patient’s history of multiple abdominal surgeries and clinical presentation, POCUS was performed to evaluate for SBO. Dilated loops of small bowel were visualized in the lower abdomen gas, suggestive of SBO.
Since the small bowel encompasses a large portion of the abdomen, to fully evaluate for SBO, multiple views are necessary. These include the epigastrium, bilateral colic gutters, and suprapubic regions.5 Use the low-frequency curvilinear transducer to obtain these views, scanning in the transverse and sagittal planes (see Figures 1 and 2). Scan while moving the transducer in columns (ie, “mowing the lawn”), making sure to cover the entire abdomen. To assure that you are evaluating the small bowel, and not the large bowel, look for the characteristic plicae circularis of the small bowel (shown in Figure 3). In children and very slender adults, the high-frequency linear probe may provide enough depth to obtain adequate views.


A fluid-filled small intestinal segment >2.5 cm is consistent with a diagnosis of SBO. Measuring the diameter of the small bowel is both the most sensitive and specific sign; a measurement of greater than 2.5 cm is diagnostic, with a sensitivity of 97% and specificity of 91% (see Figure 4).6 This can be somewhat difficult to visualize, as bowel loops are multidirectional and diameters can mistakenly be taken on an indirect cut; to avoid over- or underestimation of bowel diameter, you may want to measure in the short axis using a transverse cross-sectional view of the bowel.

Lack of peristalsis is suggestive of a closed-loop obstruction. However, this finding may be more difficult to visualize, as it requires several continuous minutes of scanning or repeated exams to truly establish absent peristalsis. In prolonged courses of SBO, the bowel wall can measure >3 mm, which suggests necrosis, warranting accelerated surgical intervention. In addition, the detection of extraluminal peritoneal fluid can help determine the severity of the SBO, and small versus large fluid amounts can help determine whether medical or surgical management is warranted (see Figure 5).7

DISCUSSION
Increased time to diagnosis of SBO can lead to prolonged patient suffering and greater complication rates. The gold standard for diagnosing SBO—CT with intravenous and oral contrast—can take hours, requiring patients, who are often nauseated, to ingest and tolerate oral contrast. In the past, an “obstructive series” of x-rays would have been used early in the work-up of possible SBO.6
Recent literature suggests that POCUS is not only faster, more cost effective, and advantageous (involving no ionizing radiation), but also more accurate than x-rays. Specifically, a meta-analysis by Taylor et al showed pooled estimates for obstructive series x-rays have a sensitivity (Sn) of 75%, a specificity (Sp) of 66%, a positive likelihood ratio (+LR) of 1.6, and a negative likelihood ratio (-LR) of 0.43.1 On the other hand, pooled results from ED studies of emergency medicine (EM) residents performing POCUS in patients with signs and symptoms suspicious for SBO showed POCUS had a Sn of 97%, Sp of 90%, +LR of 9.5, and a -LR of 0.04.1,5,8 While detractors point to the operator-dependent nature of POCUS, literature suggests that with EM residents novice to POCUS for SBO (defined as less than 5 previous scans for SBO) were given a 10-minute didactic session and yielded Sn 94%, Sp 81%, +LR 5.0, -LR 0.07.5 Unluer et al trained novice EM residents for 6 hours and found them to yield Sn 98%, Sp 95%, +LR 19.5, and -LR 0.02.8 Thus, while it is no surprise that those with more training attain better results, both studies show it does not take much time for EM providers to surpass the accuracy of x-rays with POCUS.
CASE CONCLUSION
The findings on POCUS highly suggested the diagnosis of an SBO. A CT scan of the abdomen and pelvis with intravenous and oral contrast was ordered to further evaluate obstruction, transition point, and possible complications, including signs of ischemia per surgical request. CT demonstrated dilated loops of small bowel with transition point in the right lower quadrant, with a small amount of mesenteric fluid consistent with SBO with possible early bowel compromise due to ischemia. General surgery admitted the patient; conservative treatment with serial abdominal exams, nasogastric tube, NPO and bowel rest was ordered. The patient’s diet was gradually advanced, and she was discharged on the eleventh day of hospitalization.
SUMMARY
POCUS is a useful non-invasive tool that can accurately diagnose SBO. POCUS has increased sensitivity and specificity when compared to abdominal X-rays. This bedside imaging will not only give the ED provider rapid diagnostic information but also lead to expedited surgical intervention.
- Taylor MR, Lalani N. Adult small bowel obstruction. Acad Emerg Med. 2013;20(6):528-544.
- Hastings RS, Powers RD. Abdominal pain in the ED: a 35-year retrospective. Am J Emerg Med.2011;29:711-716.
- Markogiannakis H, Messaris E, Dardamanis D, et al. Acute mechanical bowel obstruction: clinical presentation, etiology, management and outcome. World J Gastroenterol. 2007;13:432.
- Bickell N, Federman A, Aufses A. Influence of time on risk of bowel resection in complete small bowel obstruction. J Am Coll Surg. 2005;201(6):847-854.
- Jang TB, Chandler D, Kaji AH. Bedside ultrasonography for the detection of small bowel obstruction in the emergency department. Emerg Med J. 2011;28:676-678.
- Carpenter CR, Pines JM. The end of X-rays for suspected small bowel obstruction? Using evidence-based diagnostics to inform best practices in emergency medicine. Acad Emerg Med. 2013;20:618-20.
- Grassi R, Romano S, D’Amario F, et al. The relevance of free fluid between intestinal loops detected by sonography in the clinical assessment of small bowel obstruction in adults. Eur J Radiol. 2004;50(1):5-14.
- Unlüer E, Yavaşi O, Eroğlu O, Yilmaz C, Akarca F. Ultrasonography by emergency medicine and radiology residents for the diagnosis of small bowel obstruction. Eur J Emerg Med. 2010;17(5):260-264.
- Taylor MR, Lalani N. Adult small bowel obstruction. Acad Emerg Med. 2013;20(6):528-544.
- Hastings RS, Powers RD. Abdominal pain in the ED: a 35-year retrospective. Am J Emerg Med.2011;29:711-716.
- Markogiannakis H, Messaris E, Dardamanis D, et al. Acute mechanical bowel obstruction: clinical presentation, etiology, management and outcome. World J Gastroenterol. 2007;13:432.
- Bickell N, Federman A, Aufses A. Influence of time on risk of bowel resection in complete small bowel obstruction. J Am Coll Surg. 2005;201(6):847-854.
- Jang TB, Chandler D, Kaji AH. Bedside ultrasonography for the detection of small bowel obstruction in the emergency department. Emerg Med J. 2011;28:676-678.
- Carpenter CR, Pines JM. The end of X-rays for suspected small bowel obstruction? Using evidence-based diagnostics to inform best practices in emergency medicine. Acad Emerg Med. 2013;20:618-20.
- Grassi R, Romano S, D’Amario F, et al. The relevance of free fluid between intestinal loops detected by sonography in the clinical assessment of small bowel obstruction in adults. Eur J Radiol. 2004;50(1):5-14.
- Unlüer E, Yavaşi O, Eroğlu O, Yilmaz C, Akarca F. Ultrasonography by emergency medicine and radiology residents for the diagnosis of small bowel obstruction. Eur J Emerg Med. 2010;17(5):260-264.
A deep commitment to veterans’ medical needs
VA hospitalist Dr. Mel Anderson loves his work
Mel C. Anderson, MD, FACP, section chief of hospital medicine for the Veterans Administration of Eastern Colorado, and his hospitalist colleagues share a mission to care for the men and women who served their country in the armed forces and are now being served by the VA.
“That mission binds us together in a deep and impactful way,” he said. “One of the greatest joys of my life has been to dedicate, with the teams I lead, our hearts and minds to serving this population of veterans.”
Approximately 400 hospitalists work nationwide in the VA, the country’s largest integrated health system, typically in groups of about a dozen. Not every VA medical center employs hospitalists; this depends on local tradition and size of the facility. Dr. Anderson was for several years the lone hospitalist at the VA Medical Center in Denver, starting in 2005, and now he heads a group of 17. The Denver facility employs five inpatient teams plus nocturnists, supported by residents, interns, and medical students in training from the University of Colorado at Denver, Aurora, to deliver all of its inpatient medical care.
“We also have an open ICU here. Hospitalists are able to follow their patients across the hospital, and we can make the decision to move them to the ICU,” Dr. Anderson said. The Denver group also established a hospitalist-staffed postdischarge clinic, where patients can reconnect with their hospital team. “It’s not to supplant primary care but to help promote safe transit as the patient moves back to the community,” he said. “We’ve also developed a surgery consult service for orthopedics and other surgical subspecialties.”
The VA’s integrated electronic medical record facilitates communication between hospitalists and primary care physicians, with instant messaging for updating the PCPs on the patient’s hospital stay.
The Denver VA hospitalists value their collegial culture, Dr. Anderson said. “We are invested in our group and in one another and in life-long learning. I often ask my group for their feedback. It’s one of the singular joys of my career to lead such a wonderful group, which has been built up person by person. I hired every single member. As much as their clinical skills and the achievements on their curriculum vitae were important, I also paid attention to their interpersonal communication skills.”
Members of the Denver hospitalist group also share an academic focus and commitment to scholarship and research. Dr. Anderson’s academic emphasis is on how to promote teaching and faculty development through organized bedside rounding and how to orient students to teaching as a potential career path. He is associate program director for medicine residencies at the University of Colorado and leads its Clinician/Educator Pathway.
The VA hospital’s interdisciplinary bedside rounding initiative involves the medicine team – students, residents, attending – and pharmacist, plus the patient’s bedside nurse and nurse care coordinator. “We have worked on fostering an interdisciplinary culture, and we’re very proud of the rounding model we developed here. We all round together at the bedside, and typically that might include 7 or 8 people,” Dr. Anderson explained.
“In planning this program, we used a Rapid Performance Improvement Project team with a nurse, pharmacist, and physical therapist helping us envision how to redesign rounds to overcome the time constraints,” he said. “We altered nurses’ work flow to permit them to join the rounding for their patients, and we moved morning medication administration to 7 a.m., so it wouldn’t get in the way of the rounding. We now audit rates of physician-to-nurse communication on rounds and how often we successfully achieve the nurse’s participation.”1 This approach has also cut rates of phone pages from nurses to house staff, and substantially increased job satisfaction.
What’s different in the VA?
The work of hospitalists in the VA is mostly similar to other hospital settings, but perhaps with more intensity, Dr. Anderson said. There are comorbidities such as higher rates of PTSD, alcohol use disorder, substance abuse, and mental health issues – all of which have an effect over time on patients. But veterans also have different attitudes about, for example, pain.
“When patients are asked to rate their pain on a scale of 0 to 10, for a veteran of a foreign war, 2 out of 10 is not the same as someone else’s 2 out of 10. How do we compensate for that difference?” he said. “And while awareness of PTSD and efforts to mitigate its impact have made incredible gains over the past 15 years, we still see a lot of these issues and their manifestations in social challenges such as homelessness. We are fortunate to have VA outpatient services and homeless veteran programs to help with these issues.”
There is a different paradigm for care at the VA, Dr. Anderson said. “We are a not-for-profit institution with the welfare of veterans as our primary aim. Beyond their health and wellness, that means supporting them in other ways and reaching out into the community. As doctors and nurses we feel a kinship around that mission, although we also have to be stewards of taxpayer dollars. We recognize that the VA is a large and complicated, somewhat inertia-laden organization in which making changes can be very challenging. But there are also opportunities as a national organization to effect changes on a national scale.”
Dr. Anderson chairs the VA’s Hospitalist Field Advisory Committee (HFAC), a group of about eight hospitalists empaneled to advise the system’s Office of Specialty Care Services on clinical policy and program development. They serve 3-year terms and meet monthly by phone and annually in Washington. The HFAC’s last annual meeting occurred in mid-September 2018 in Washington with a focus on developing a hospital medicine annual survey and needs assessment, revisiting strategic goals, and convening multilateral meetings with the chiefs of medicine and emergency medicine FACs.
“Our biggest emphasis is clinical – this includes clinical pathways, best practices for managing PTSD or acute coronary syndrome, and the like. We also share management issues, such as how to configure medical records or arrange night coverage. This is a national conversation to share what some sites have already experienced and learned,” Dr. Anderson said.
“We also have a VA Academic Hospitalist Subcommittee, working together on multisite research studies and on resident education protocols. Because we’re a large system, we’re able to connect with one another and leverage what we’ve learned. I get emails almost every day about research topics from colleagues across the country,” he said. A collaborative website and email distribution list allows doctors to post questions to their peers nationwide.
A calling for hospital medicine
Before moving to Denver, Dr. Anderson served as a major in the Air Force Medical Corps and was based at the David Grant US Air Force Medical Center on Travis Air Force Base in California – which is where he did his residency. In the course of a “traditionalist” internal medical training, including 4-month stints on hospital wards in addition to outpatient services, he realized he had a calling for hospital medicine.
In a job at the Providence (R.I.) VA Medical Center, he exclusively practiced outpatient care, but he found that he missed key aspects of inpatient work, such as the intensity of the clinical issues and teaching encounters. “I cold-called the hospital’s chief of medicine and volunteered to start mentoring inpatient residents,” Dr. Anderson said. “That was 17 years ago.”
Another abiding interest derived from Dr. Anderson’s military service is travel medicine. While a physician in the Air Force, he was deployed to Haiti in 1995 and to Nicaragua in 2000, where he treated thousands of patients – both U.S. service personnel and local populations.
“In Haiti, our primary mission was for U.S. troops who were still based there following the 1994 Operation Uphold Democracy intervention, but there were a lot fewer of them, so we mostly kept busy providing care to Haitian nationals,” he said. “That work was eye opening, to say the least,” and led to a professional interest in tropical illnesses. “Since then, I’ve been a visiting professor for the University of Colorado posted to the University of Zimbabwe in Harare in 2012 and 2016.”
What gives Dr. Anderson such joy and enthusiasm for his VA work? “I am a curious lifelong learner. Every day, there are 10 new things I need to learn, whether clinically or operationally in a big hospital system or just the day-to-day realities of leading a group of physicians. I never feel like I’m treading water,” he said. He is also energized by teaching – seeing “the light bulb go on” for the students he is instructing – and by serving as a role model for doctors in training.
“As I contemplate all the simultaneous balls I have in the air, including our recent move into a new hospital building, sometimes I think it is kind of crazy to be doing as much as I do,” he said. “But I also take time away, balancing work versus nonwork.” He spends quality time with his wife of 21 years, 17-year-old daughter, other relatives, and friends, as well as on physical activity, reading books about philosophy, and his hobby of rebuilding motorcycles, which he says offers a kind of meditative calm.
“I also feel a deep sense of service – to patients, colleagues, students, and to the mission of the VA,” Dr. Anderson said. “There is truly something special about caring for the veteran. It’s hard to articulate, but it really keeps us coming back for more. I’ve had vets sing to me, tell jokes, do magic tricks, share their war stories. I’ve had patients open up to me in ways that were both profound and humbling.”
References
1. Young E et al. Impact of altered medication administration time on interdisciplinary bedside rounds on academic medical ward. J Nurs Care Qual. 2017 Jul/Sep;32(3):218-225.
VA hospitalist Dr. Mel Anderson loves his work
VA hospitalist Dr. Mel Anderson loves his work
Mel C. Anderson, MD, FACP, section chief of hospital medicine for the Veterans Administration of Eastern Colorado, and his hospitalist colleagues share a mission to care for the men and women who served their country in the armed forces and are now being served by the VA.
“That mission binds us together in a deep and impactful way,” he said. “One of the greatest joys of my life has been to dedicate, with the teams I lead, our hearts and minds to serving this population of veterans.”
Approximately 400 hospitalists work nationwide in the VA, the country’s largest integrated health system, typically in groups of about a dozen. Not every VA medical center employs hospitalists; this depends on local tradition and size of the facility. Dr. Anderson was for several years the lone hospitalist at the VA Medical Center in Denver, starting in 2005, and now he heads a group of 17. The Denver facility employs five inpatient teams plus nocturnists, supported by residents, interns, and medical students in training from the University of Colorado at Denver, Aurora, to deliver all of its inpatient medical care.
“We also have an open ICU here. Hospitalists are able to follow their patients across the hospital, and we can make the decision to move them to the ICU,” Dr. Anderson said. The Denver group also established a hospitalist-staffed postdischarge clinic, where patients can reconnect with their hospital team. “It’s not to supplant primary care but to help promote safe transit as the patient moves back to the community,” he said. “We’ve also developed a surgery consult service for orthopedics and other surgical subspecialties.”
The VA’s integrated electronic medical record facilitates communication between hospitalists and primary care physicians, with instant messaging for updating the PCPs on the patient’s hospital stay.
The Denver VA hospitalists value their collegial culture, Dr. Anderson said. “We are invested in our group and in one another and in life-long learning. I often ask my group for their feedback. It’s one of the singular joys of my career to lead such a wonderful group, which has been built up person by person. I hired every single member. As much as their clinical skills and the achievements on their curriculum vitae were important, I also paid attention to their interpersonal communication skills.”
Members of the Denver hospitalist group also share an academic focus and commitment to scholarship and research. Dr. Anderson’s academic emphasis is on how to promote teaching and faculty development through organized bedside rounding and how to orient students to teaching as a potential career path. He is associate program director for medicine residencies at the University of Colorado and leads its Clinician/Educator Pathway.
The VA hospital’s interdisciplinary bedside rounding initiative involves the medicine team – students, residents, attending – and pharmacist, plus the patient’s bedside nurse and nurse care coordinator. “We have worked on fostering an interdisciplinary culture, and we’re very proud of the rounding model we developed here. We all round together at the bedside, and typically that might include 7 or 8 people,” Dr. Anderson explained.
“In planning this program, we used a Rapid Performance Improvement Project team with a nurse, pharmacist, and physical therapist helping us envision how to redesign rounds to overcome the time constraints,” he said. “We altered nurses’ work flow to permit them to join the rounding for their patients, and we moved morning medication administration to 7 a.m., so it wouldn’t get in the way of the rounding. We now audit rates of physician-to-nurse communication on rounds and how often we successfully achieve the nurse’s participation.”1 This approach has also cut rates of phone pages from nurses to house staff, and substantially increased job satisfaction.
What’s different in the VA?
The work of hospitalists in the VA is mostly similar to other hospital settings, but perhaps with more intensity, Dr. Anderson said. There are comorbidities such as higher rates of PTSD, alcohol use disorder, substance abuse, and mental health issues – all of which have an effect over time on patients. But veterans also have different attitudes about, for example, pain.
“When patients are asked to rate their pain on a scale of 0 to 10, for a veteran of a foreign war, 2 out of 10 is not the same as someone else’s 2 out of 10. How do we compensate for that difference?” he said. “And while awareness of PTSD and efforts to mitigate its impact have made incredible gains over the past 15 years, we still see a lot of these issues and their manifestations in social challenges such as homelessness. We are fortunate to have VA outpatient services and homeless veteran programs to help with these issues.”
There is a different paradigm for care at the VA, Dr. Anderson said. “We are a not-for-profit institution with the welfare of veterans as our primary aim. Beyond their health and wellness, that means supporting them in other ways and reaching out into the community. As doctors and nurses we feel a kinship around that mission, although we also have to be stewards of taxpayer dollars. We recognize that the VA is a large and complicated, somewhat inertia-laden organization in which making changes can be very challenging. But there are also opportunities as a national organization to effect changes on a national scale.”
Dr. Anderson chairs the VA’s Hospitalist Field Advisory Committee (HFAC), a group of about eight hospitalists empaneled to advise the system’s Office of Specialty Care Services on clinical policy and program development. They serve 3-year terms and meet monthly by phone and annually in Washington. The HFAC’s last annual meeting occurred in mid-September 2018 in Washington with a focus on developing a hospital medicine annual survey and needs assessment, revisiting strategic goals, and convening multilateral meetings with the chiefs of medicine and emergency medicine FACs.
“Our biggest emphasis is clinical – this includes clinical pathways, best practices for managing PTSD or acute coronary syndrome, and the like. We also share management issues, such as how to configure medical records or arrange night coverage. This is a national conversation to share what some sites have already experienced and learned,” Dr. Anderson said.
“We also have a VA Academic Hospitalist Subcommittee, working together on multisite research studies and on resident education protocols. Because we’re a large system, we’re able to connect with one another and leverage what we’ve learned. I get emails almost every day about research topics from colleagues across the country,” he said. A collaborative website and email distribution list allows doctors to post questions to their peers nationwide.
A calling for hospital medicine
Before moving to Denver, Dr. Anderson served as a major in the Air Force Medical Corps and was based at the David Grant US Air Force Medical Center on Travis Air Force Base in California – which is where he did his residency. In the course of a “traditionalist” internal medical training, including 4-month stints on hospital wards in addition to outpatient services, he realized he had a calling for hospital medicine.
In a job at the Providence (R.I.) VA Medical Center, he exclusively practiced outpatient care, but he found that he missed key aspects of inpatient work, such as the intensity of the clinical issues and teaching encounters. “I cold-called the hospital’s chief of medicine and volunteered to start mentoring inpatient residents,” Dr. Anderson said. “That was 17 years ago.”
Another abiding interest derived from Dr. Anderson’s military service is travel medicine. While a physician in the Air Force, he was deployed to Haiti in 1995 and to Nicaragua in 2000, where he treated thousands of patients – both U.S. service personnel and local populations.
“In Haiti, our primary mission was for U.S. troops who were still based there following the 1994 Operation Uphold Democracy intervention, but there were a lot fewer of them, so we mostly kept busy providing care to Haitian nationals,” he said. “That work was eye opening, to say the least,” and led to a professional interest in tropical illnesses. “Since then, I’ve been a visiting professor for the University of Colorado posted to the University of Zimbabwe in Harare in 2012 and 2016.”
What gives Dr. Anderson such joy and enthusiasm for his VA work? “I am a curious lifelong learner. Every day, there are 10 new things I need to learn, whether clinically or operationally in a big hospital system or just the day-to-day realities of leading a group of physicians. I never feel like I’m treading water,” he said. He is also energized by teaching – seeing “the light bulb go on” for the students he is instructing – and by serving as a role model for doctors in training.
“As I contemplate all the simultaneous balls I have in the air, including our recent move into a new hospital building, sometimes I think it is kind of crazy to be doing as much as I do,” he said. “But I also take time away, balancing work versus nonwork.” He spends quality time with his wife of 21 years, 17-year-old daughter, other relatives, and friends, as well as on physical activity, reading books about philosophy, and his hobby of rebuilding motorcycles, which he says offers a kind of meditative calm.
“I also feel a deep sense of service – to patients, colleagues, students, and to the mission of the VA,” Dr. Anderson said. “There is truly something special about caring for the veteran. It’s hard to articulate, but it really keeps us coming back for more. I’ve had vets sing to me, tell jokes, do magic tricks, share their war stories. I’ve had patients open up to me in ways that were both profound and humbling.”
References
1. Young E et al. Impact of altered medication administration time on interdisciplinary bedside rounds on academic medical ward. J Nurs Care Qual. 2017 Jul/Sep;32(3):218-225.
Mel C. Anderson, MD, FACP, section chief of hospital medicine for the Veterans Administration of Eastern Colorado, and his hospitalist colleagues share a mission to care for the men and women who served their country in the armed forces and are now being served by the VA.
“That mission binds us together in a deep and impactful way,” he said. “One of the greatest joys of my life has been to dedicate, with the teams I lead, our hearts and minds to serving this population of veterans.”
Approximately 400 hospitalists work nationwide in the VA, the country’s largest integrated health system, typically in groups of about a dozen. Not every VA medical center employs hospitalists; this depends on local tradition and size of the facility. Dr. Anderson was for several years the lone hospitalist at the VA Medical Center in Denver, starting in 2005, and now he heads a group of 17. The Denver facility employs five inpatient teams plus nocturnists, supported by residents, interns, and medical students in training from the University of Colorado at Denver, Aurora, to deliver all of its inpatient medical care.
“We also have an open ICU here. Hospitalists are able to follow their patients across the hospital, and we can make the decision to move them to the ICU,” Dr. Anderson said. The Denver group also established a hospitalist-staffed postdischarge clinic, where patients can reconnect with their hospital team. “It’s not to supplant primary care but to help promote safe transit as the patient moves back to the community,” he said. “We’ve also developed a surgery consult service for orthopedics and other surgical subspecialties.”
The VA’s integrated electronic medical record facilitates communication between hospitalists and primary care physicians, with instant messaging for updating the PCPs on the patient’s hospital stay.
The Denver VA hospitalists value their collegial culture, Dr. Anderson said. “We are invested in our group and in one another and in life-long learning. I often ask my group for their feedback. It’s one of the singular joys of my career to lead such a wonderful group, which has been built up person by person. I hired every single member. As much as their clinical skills and the achievements on their curriculum vitae were important, I also paid attention to their interpersonal communication skills.”
Members of the Denver hospitalist group also share an academic focus and commitment to scholarship and research. Dr. Anderson’s academic emphasis is on how to promote teaching and faculty development through organized bedside rounding and how to orient students to teaching as a potential career path. He is associate program director for medicine residencies at the University of Colorado and leads its Clinician/Educator Pathway.
The VA hospital’s interdisciplinary bedside rounding initiative involves the medicine team – students, residents, attending – and pharmacist, plus the patient’s bedside nurse and nurse care coordinator. “We have worked on fostering an interdisciplinary culture, and we’re very proud of the rounding model we developed here. We all round together at the bedside, and typically that might include 7 or 8 people,” Dr. Anderson explained.
“In planning this program, we used a Rapid Performance Improvement Project team with a nurse, pharmacist, and physical therapist helping us envision how to redesign rounds to overcome the time constraints,” he said. “We altered nurses’ work flow to permit them to join the rounding for their patients, and we moved morning medication administration to 7 a.m., so it wouldn’t get in the way of the rounding. We now audit rates of physician-to-nurse communication on rounds and how often we successfully achieve the nurse’s participation.”1 This approach has also cut rates of phone pages from nurses to house staff, and substantially increased job satisfaction.
What’s different in the VA?
The work of hospitalists in the VA is mostly similar to other hospital settings, but perhaps with more intensity, Dr. Anderson said. There are comorbidities such as higher rates of PTSD, alcohol use disorder, substance abuse, and mental health issues – all of which have an effect over time on patients. But veterans also have different attitudes about, for example, pain.
“When patients are asked to rate their pain on a scale of 0 to 10, for a veteran of a foreign war, 2 out of 10 is not the same as someone else’s 2 out of 10. How do we compensate for that difference?” he said. “And while awareness of PTSD and efforts to mitigate its impact have made incredible gains over the past 15 years, we still see a lot of these issues and their manifestations in social challenges such as homelessness. We are fortunate to have VA outpatient services and homeless veteran programs to help with these issues.”
There is a different paradigm for care at the VA, Dr. Anderson said. “We are a not-for-profit institution with the welfare of veterans as our primary aim. Beyond their health and wellness, that means supporting them in other ways and reaching out into the community. As doctors and nurses we feel a kinship around that mission, although we also have to be stewards of taxpayer dollars. We recognize that the VA is a large and complicated, somewhat inertia-laden organization in which making changes can be very challenging. But there are also opportunities as a national organization to effect changes on a national scale.”
Dr. Anderson chairs the VA’s Hospitalist Field Advisory Committee (HFAC), a group of about eight hospitalists empaneled to advise the system’s Office of Specialty Care Services on clinical policy and program development. They serve 3-year terms and meet monthly by phone and annually in Washington. The HFAC’s last annual meeting occurred in mid-September 2018 in Washington with a focus on developing a hospital medicine annual survey and needs assessment, revisiting strategic goals, and convening multilateral meetings with the chiefs of medicine and emergency medicine FACs.
“Our biggest emphasis is clinical – this includes clinical pathways, best practices for managing PTSD or acute coronary syndrome, and the like. We also share management issues, such as how to configure medical records or arrange night coverage. This is a national conversation to share what some sites have already experienced and learned,” Dr. Anderson said.
“We also have a VA Academic Hospitalist Subcommittee, working together on multisite research studies and on resident education protocols. Because we’re a large system, we’re able to connect with one another and leverage what we’ve learned. I get emails almost every day about research topics from colleagues across the country,” he said. A collaborative website and email distribution list allows doctors to post questions to their peers nationwide.
A calling for hospital medicine
Before moving to Denver, Dr. Anderson served as a major in the Air Force Medical Corps and was based at the David Grant US Air Force Medical Center on Travis Air Force Base in California – which is where he did his residency. In the course of a “traditionalist” internal medical training, including 4-month stints on hospital wards in addition to outpatient services, he realized he had a calling for hospital medicine.
In a job at the Providence (R.I.) VA Medical Center, he exclusively practiced outpatient care, but he found that he missed key aspects of inpatient work, such as the intensity of the clinical issues and teaching encounters. “I cold-called the hospital’s chief of medicine and volunteered to start mentoring inpatient residents,” Dr. Anderson said. “That was 17 years ago.”
Another abiding interest derived from Dr. Anderson’s military service is travel medicine. While a physician in the Air Force, he was deployed to Haiti in 1995 and to Nicaragua in 2000, where he treated thousands of patients – both U.S. service personnel and local populations.
“In Haiti, our primary mission was for U.S. troops who were still based there following the 1994 Operation Uphold Democracy intervention, but there were a lot fewer of them, so we mostly kept busy providing care to Haitian nationals,” he said. “That work was eye opening, to say the least,” and led to a professional interest in tropical illnesses. “Since then, I’ve been a visiting professor for the University of Colorado posted to the University of Zimbabwe in Harare in 2012 and 2016.”
What gives Dr. Anderson such joy and enthusiasm for his VA work? “I am a curious lifelong learner. Every day, there are 10 new things I need to learn, whether clinically or operationally in a big hospital system or just the day-to-day realities of leading a group of physicians. I never feel like I’m treading water,” he said. He is also energized by teaching – seeing “the light bulb go on” for the students he is instructing – and by serving as a role model for doctors in training.
“As I contemplate all the simultaneous balls I have in the air, including our recent move into a new hospital building, sometimes I think it is kind of crazy to be doing as much as I do,” he said. “But I also take time away, balancing work versus nonwork.” He spends quality time with his wife of 21 years, 17-year-old daughter, other relatives, and friends, as well as on physical activity, reading books about philosophy, and his hobby of rebuilding motorcycles, which he says offers a kind of meditative calm.
“I also feel a deep sense of service – to patients, colleagues, students, and to the mission of the VA,” Dr. Anderson said. “There is truly something special about caring for the veteran. It’s hard to articulate, but it really keeps us coming back for more. I’ve had vets sing to me, tell jokes, do magic tricks, share their war stories. I’ve had patients open up to me in ways that were both profound and humbling.”
References
1. Young E et al. Impact of altered medication administration time on interdisciplinary bedside rounds on academic medical ward. J Nurs Care Qual. 2017 Jul/Sep;32(3):218-225.