User login
Improving Antibiotic Utilization among Hospitalists
Inappropriate antibiotic use is a major public health concern and demonstrates the need for quality improvement initiatives in the delivery of health care.16 Each year nearly 2 million patients in the United States acquire an infection in the hospital, and about 90,000 of them die from these infections.7 More than 70% of the bacteria that cause hospital‐acquired infections are resistant to at least one commonly used drug.7 Persons infected with drug‐resistant organisms have longer hospital stays and higher mortality rates.7
Inappropriate antibiotic use in the inpatient hospital setting can be classified into 5 categories. First, antibiotics may be given for illnesses for which they are not indicated (eg, viral infections). Second, broad‐spectrum antibiotics (such as piperacillin‐tazobactam and quinolones) may be overused in the empiric treatment of common infections.8 Overuse of broad‐spectrum drugs increases selective pressure for antimicrobial resistance and exposes patients to the side effects of some of these drugs, such as Clostridium difficile colitis.8 Third, clinicians occasionally prescribe intravenous (IV) antibiotics when the efficacy of oral agents would be similar. Inappropriate intravenous therapy increases the cost of care and also exposes the patient to the risk of intravenous catheters.8 Fourth, when the correct antibiotic choice is made, inappropriate antibiotic dosage, schedule, and/or duration of treatment can threaten patient safety.8 Fifth, bug‐drug mismatch occurs when susceptibility studies indicate that the drug being used is ineffective or only marginally effective.8 Beyond antimicrobial resistance and safety, these practices also usually increase costs to both the patient and the hospital.7, 910
Influencing providers' prescribing patterns is difficult.11 In this project we assessed the prescribing patterns of hospitalists in an active inpatient environment and then developed an intervention to improve the providers' use of antibiotics. The intervention utilized public health methodologyprior to implementation, we defined the problem, determined its magnitude, identified a behavior change model, and constructed a conceptual framework that identifyied the key determinants. A pilot academic detailing project addressing many determinants was developed, implemented, and evaluated.
Conceptual Model
To change prescribing behaviors is to change learned behaviors. Changing behavior is a complex process affected by several factors including beliefs, expectations, motivations, and the psychosocial environments of the target groups.12 Each of these factors must be considered when attempting to bring about behavior changes. In doing so, a theory that can be depicted in a model often emerges.13 This approach is widely used in understanding and developing public health interventions.
Formulating the Model
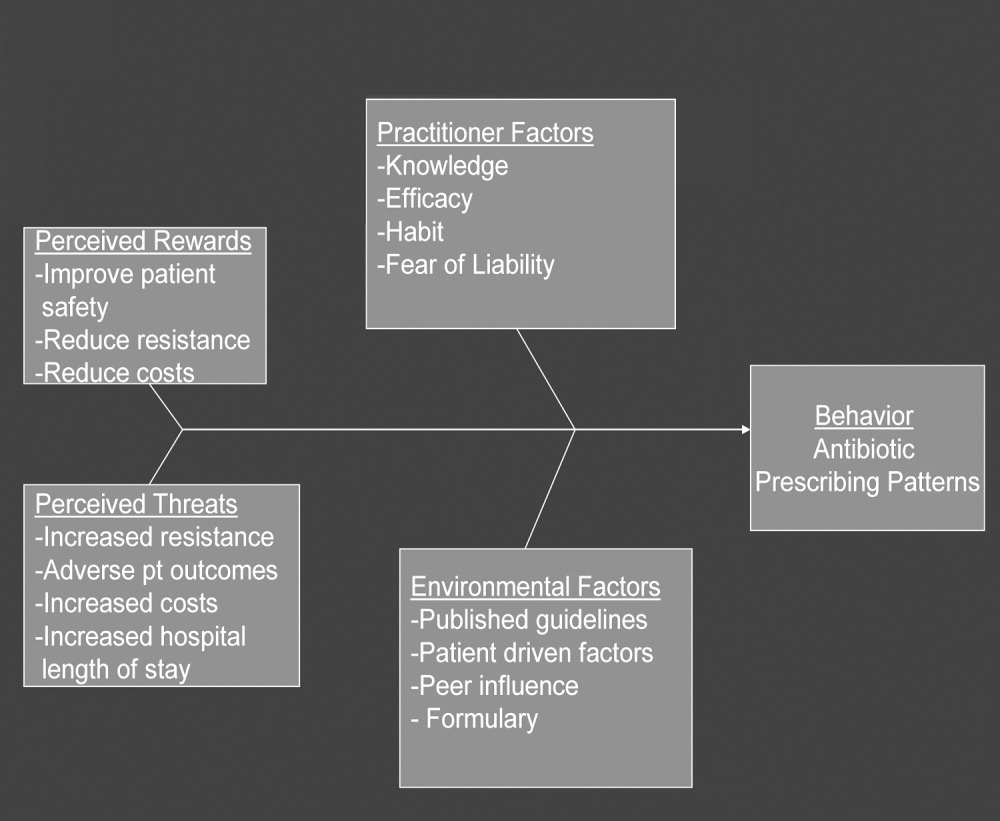
In any public health intervention, recognizing and engaging key stakeholders is a critical step. We identified the following stakeholders: (1) hospitalist practitioners and other prescribing providers including residents and infectious disease specialists; (2) nurses; (3) administrators who are focused on cost effectiveness; (4) patients and their families, who want to get well affordably, without side effects; (5) pharmacists; (6) risk management; and (7) society, which is fearful of the propagation of resistant microbes. In consulting with some of the stakeholders, 4 factors that influence hospitalists' prescribing patterns became apparent. These are practitioner factors, environmental factors, perceived rewards, and perceived threats (Fig. 1).
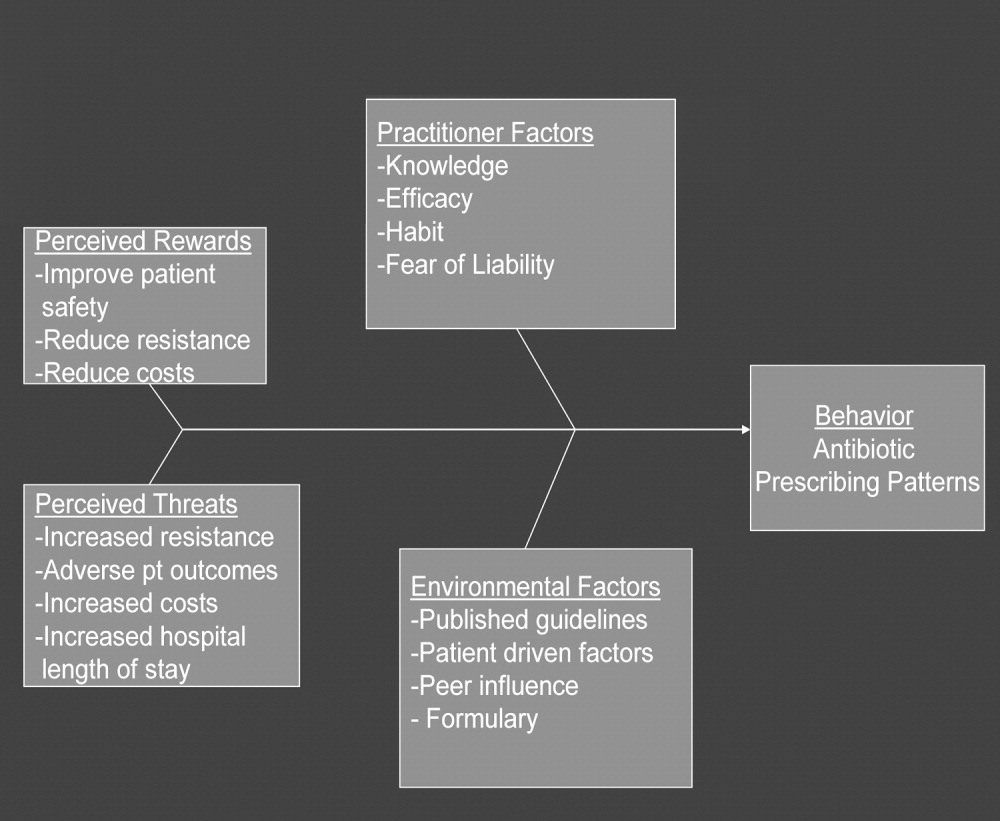
The practitioner factors shaping prescribing are: (1) knowledge of current best care; (2) self‐efficacy, which determines whether a provider is confident in his or her knowledge to adequately treat a specific infection; (3) habit, which causes providers to pick from a narrow repertoire of antibiotics when treating an infection; and (4) fear of liability, which forces some providers to be cautious. Four environmental factors affecting antibiotic prescriptions are: (1) published guidelines regarding organisms' sensitivity to antibiotics; (2) patient‐driven factors such as affordability, compliance with dosing regimens, side effects, and interactions between the antibiotics and other medications; (3) peer influence, in that providers are reluctant to change a prescription started by another provider (eg, emergency room physician); and (4) the formulary of the hospital, as it forces providers to prescribe within specific parameters. The perceived rewards of specific prescribing practices may include improving patient safety and reducing antibiotic resistance and costs, whereas the perceived threats are increasing antimicrobial resistance, having adverse patient outcomes, and increasing costs and hospital length of stay. We selected a high‐yield, low‐effort intervention in order to have an impact on some of the factors underlying hospitalists' prescribing patterns.
METHODS
Participants
The study participants were 17 hospitalist practitioners including physicians, nurse‐practitioners, and physician assistants who make up the Collaborative Inpatient Medical Service (CIMS) at Johns Hopkins Bayview Medical Center (JHBMC; Table 1). All consented to participate. The study was approved by the institutional review board.
| Age in years, mean (SD) | 36 (6) |
| Female, n (%) | 13 (76%) |
| Physician, n (%) | 9 (53%) |
| Nurse‐practitioner, n (%) | 5 (29%) |
| Physician assistant, n (%) | 3 (18%) |
| Years in practice, mean (SD) | 5.1 (2.8) |
| Number of pharmaceutical representatives exposed to in past year, mean | 1 |
| Number of shifts worked per month, mean (SD) | 14 (4) |
| Primarily works days, n (%) | 13 (76%) |
Data Collection
We collected and assessed prescription patterns over 3 periods: preintervention, interim, and postintervention.
Assessing Appropriateness of Antibiotics
For each order that was assessed in the preintervention, interim, and postintervention periods, the following information was collected: (1) drug ordered, (2) clinical diagnosis, (3) microbiology results available at the time of the order (including relevant results from recent cultures), (4) other medical diagnoses (ICD9 codes), (5) allergies, and (6) exposure to health care facilities (within the past 30 days). The computerized medical record allowed access to the discharge summaries of a patient's hospitalization. These records summarized the patient's hospitalization, allowing the investigators to understand the reasons for a provider's choice of antibiotics. If the rationale was not clear about how to categorize a prescription from reading the data, the investigators performed a chart review. From the information culled from these reviews, the primary investigator and an infectious disease specialist classified each prescription order by consensus as appropriate, effective but inappropriate, or inappropriate therapy.
Prescriptions were classified as appropriate when they were indicated and correlated with sensitivities, if available, or were of a narrow‐enough spectrum and recommended as a first‐line treatment for specific illnesses by either the Johns Hopkins Antibiotic Guide14 or the Stanford Guide to Antimicrobial Therapy.15 For example, cephalexin to treat uncomplicated cellulitis was considered appropriate therapy. Effective but inappropriate prescriptions were broad‐spectrum antibiotics used to treat an infection when a narrower‐spectrum antibiotic would have sufficed. For example, piperacillin‐tazobactam would be effective in treating a simple urinary tract infection but inappropriate to use because of its broad spectrum. Other examples of effective but inappropriate prescriptions were giving an IV when an oral alternative would be equally effective and tolerated or prescribing antibiotic treatment whose duration was too long. Finally, inappropriate prescriptions were those written for conditions for which antibiotics are not indicated or for which the prescribed antibiotic was ineffective for the specified infection (bug‐drug mismatch).
Preintervention
In January 2006 the investigators retrospectively reviewed the prescribing patterns of the 17 providers over the previous year. Using the computerized medical record and physician order entry, consecutive prescriptions of each provider were evaluated, beginning December 31, 2005, going back reverse chronologically until 20 prescriptions had been identified. For 12 of the providers, it was actually possible to review 20 prescriptions. For 2 other providers, both new, part‐time additions to the hospitalist group, only 1 and 7 prescriptions were found for the entire year. The prescribing history of the 3 remaining providers who participated in the study, all physician assistants, could not be evaluated (during any period) because all their orders were linked only to physicians, making it impossible to determine their specific prescriptions using the physician order entry system.
Interim
During the interim period between obtaining informed consent and completing the academic detailing (January 3, 2006, to March 23, 2006), provider prescribing patterns were reviewed to determine if the mere knowledge of the project would produce changes in prescribing behavior.
Postintervention
After the academic detailing was completed (March 23, 2006), the prescribing patterns of the hospitalists were followed through April 23, 2006. Each week after the detailing session, the hospitalists received reminders to prescribe appropriately (including pens with the message Reduce the Overuse).
Detailing Procedures
After the review, a profile was assembled for each of the CIMS providers. The study team detailers (a physician and a pharmacist) met with the individual providers for 30 to 45 minutes. Each hospitalist participant completed a short survey that collected demographic information and was asked about the rationale for his or her antibiotic prescribing pattern. Next, the appraisal of the provider's prescribing pattern was reviewed. This review included looking at the costs of the prescribed antibiotics compared with those of the appropriate alternatives and a reexamination of the guidelines for the selected target drugspiperacillin‐tazobactam, vancomycin, and extended‐spectrum quinolones. These 3 antibiotics were picked because our providers had been particularly vulnerable to inappropriately prescribing them. The hospitalists were provided an antibiotic guide developed specifically for this project and based on the Johns Hopkins Antibiotic Guide14 that summarizes the consensus guidelines.
Data Analysis
The primary outcome variable was the aggregate proportion of inappropriate antibiotic prescribed (as defined earlier) before the intervention, during the interim between obtaining informed consent and intervening on all study subjects, and after the intervention. The percentage of appropriate prescriptions versus total not appropriate prescriptions (combining of the effective but inappropriate and inappropriate categories) were compared across the 3 periods. Ninety‐five percent confidence intervals for comparisons of the proportions were determined using Stata 9.0 (College Station, TX). The difference between the proportions of total not appropriate prescriptions before and after academic detailing was computed in Stata using Fisher's exact test to assess significance.
RESULTS
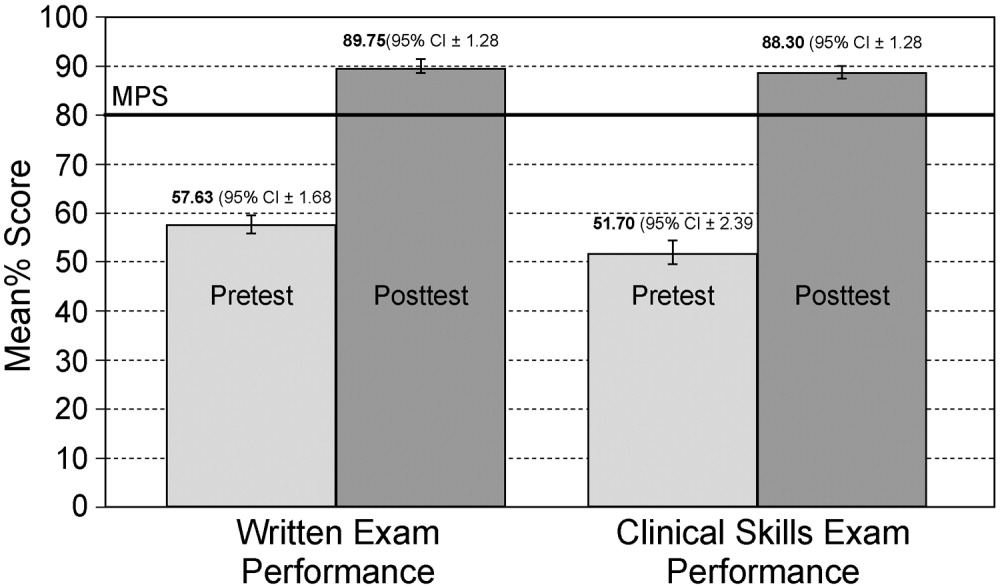
Demographic information and professional characteristics of the 17 providers are shown in Table 1. Their mean age was 36 years, and 76% were female. The top 4 reasons the providers gave for their prescribing practices were: (1) published guidelines, (2) easier dosing schedule for patient when discharged, (3) continuing an antibiotic course initiated in the emergency room, and (4) broad‐spectrum antibiotics cover all possible microbes.
Comparison of Preintervention, Interim, and Postintervention Periods
Table 2 depicts the results of the prescription appraisals from the retrospective reviews. Of the 14 providers who had ordered antibiotics, 8 (57%) had more prescriptions that were total not appropriate than were appropriate in the preintervention period compared with 3 providers (25%) with this prescribing pattern in the postintervention period (P = .13).
| Provider | Preintervention | Postintervention | ||||
|---|---|---|---|---|---|---|
| Prescriptions (n) | Appropriate, n (%) | Total not appropriate, n (%) | Prescriptions (n) | Appropriate, n (%) | Total not appropriate, n (%) | |
| ||||||
| 1 | 20 | 7 (35%) | 13 (65%) | 24 | 17 (70.8%) | 7 (29.2%) |
| 2 | 20 | 10 (50%) | 10 (50%) | 12 | 11 (91.7%) | 1 (8.3%) |
| 3 | 20 | 6 (30%) | 14 (70%) | 8 | 8 (100%) | 0 (0%) |
| 4* | 19 | 10 (52.6%) | 9 (47.4%) | 4 | 3 (75%) | 1 (25%) |
| 5 | 20 | 9 (45%) | 11 (55%) | 10 | 4 (40%) | 6 (60%) |
| 6 | 20 | 5 (25%) | 15 (75%) | 3 | 1 (33.3%) | 2 (66.7%) |
| 7 | 20 | 8 (40%) | 12 (60%) | 8 | 7 (87.5%) | 1 (12.5%) |
| 8* | 1 | 0 (0%) | 1 (100%) | 0 | 0 (0%) | 0 (0%) |
| 9 | 20 | 11 (55%) | 9 (45%) | 5 | 2 (40%) | 3 (60%) |
| 10* | 7 | 3 (42.9%) | 4 (57.1%) | 0 | 0 (0%) | 0 (0%) |
| 11 | 20 | 10 (50%) | 10 (50%) | 17 | 13 (76.5%) | 4 (23.5%) |
| 12 | 20 | 6 (30%) | 14 (70%) | 16 | 14 (87.5%) | 2 (12.5%) |
| 13 | 20 | 12 (60%) | 8 (40%) | 15 | 11 (73.3%) | 4 (26.7%) |
| 14 | 20 | 10 (50%) | 10 (50%) | 7 | 4 (57.1%) | 3 (42.9%) |
| Total | 247 | 107 (43%) | 140 (57%) | 129 | 95 (73.6%) | 34 (26.4%) |
Table 3 shows the proportions of appropriate, effective but inappropriate, and total not appropriate prescriptions in the retrospective, interim, and postintervention periods. Forty‐three percent (95% CI 37%‐49%) of prescriptions were judged to be appropriate, and 57% (95% CI 51%‐63%) to be not appropriate prior to the academic detailing. In the interim period, 59% (95% CI 52%‐65%) of the prescriptions were appropriate, and 41% (95% CI 35%‐48%) were not appropriate; P = .0003. After the intervention, 74% (95% CI 65%‐81%) of the prescriptions were appropriate, and 26% (95% CI 19%‐35%) were not appropriate; P < .0001.
| Period | Appropriate, n (%) | 95% CI | Effective but inappropriate, n (%) | Inappropriate, n (%) | Total not appropriate, n (%) | 95% CI | P value* |
|---|---|---|---|---|---|---|---|
| |||||||
| Retrospective review (pre) | 107 (43%) | 37%‐49% | 75 (30.4%) | 65 (26.6%) | 140 (57%) | 51%‐63% | |
| Interim | 146 (59%) | 52%‐65% | 37 (15%) | 65 (26%) | 102 (41%) | 35%‐48% | .0003 |
| Postintervention | 95 (74%) | 65%‐81% | 8 (6%) | 26 (20%) | 34 (26%) | 19%‐35% | < .0001 |
DISCUSSION
We have demonstrated that academic detailing had a positive impact on the prescribing patterns of hospitalists. The aggregated improvement in antibiotic prescribing patterns can be attributed to improvement in the prescribing patterns of almost every hospitalist practitioner (Table 2). This study focused on aggregate prescriptions as the primary outcome measure because the hospitalists at JHBMC, like at many other institutions, function as a team, with a patient routinely having multiple providers over the course of the hospital stay. The improved prescribing patterns noted during the interim period suggest that the mere knowledge of a project can have an impact on providers. Providers informed the investigators that they were more thoughtful about their choice of antibiotics when they knew that they were being studied. The further statistically significant improvement in prescribing patterns with the intervention shows that the academic detailing itself was successful.
The greatest absolute change in practice was seen in effective but inappropriate prescribing (from 30.4% to 6%), whereas inappropriate prescribing only decreased from 26.6% to 20.6%. Although we aimed to have an impact on all inappropriate antibiotic prescribing patterns, we specifically reviewed the prescribing guidelines for piperacillin‐tazobactam, extended‐spectrum quinolones, and vancomycin. These 3 antibiotics were targeted because our providers had been particularly susceptible to inappropriately prescribing them. The focus on these antibiotics may have resulted in the larger absolute change noted in effective but inappropriate prescribing. We did not collect any data to determine if having an impact on effective but inappropriate prescribing changed the clinical course of the patients, such as shortening their hospital stays. Anecdotal evidence, however, suggests that it does. At our institution it is not uncommon for patients to be kept in the hospital for an extra day to ensure they are stable when transitioned from extended‐spectrum to narrower‐spectrum antibiotics prior to discharge. The effect of reducing effective but inappropriate prescriptions on the clinical course of patients could be an outcome measure assessed by a future, larger study.
Our one‐on‐one appraisal of each provider's prescribing patterns included a review of the cost of the prescribed antibiotics compared with that of the appropriate alternatives. Although decisions on antibiotic choice should be driven by clinical guidelines and appropriateness rather than price, we believed it was relevant to include education about costs and pricing so that providers would be reminded to ascertain whether patients would be able to afford their antibiotics. Antibiotic resistance is influenced by a patient's failure to complete the course of treatment, and noncompliance may be caused by an inability to afford the medication. Often, there are affordable, appropriate alternatives to the newest and most expensive drugs.
A hospitalist‐based academic detailing approach to improving antibiotic prescribing may have far‐reaching benefits and influence. First, it has the potential to affect other practitioners by setting an example and role modeling. In addition to that with their immediate peer group, hospitalists have close and repeated contact with house officers and emergency room physicians and often act as consultants to physicians in other departments such as surgery and psychiatry. Furthermore, some community hospitals have no infectious disease specialists readily available. So this represents an opportunity for hospitalists to promote quality in antibiotic prescribing. Practice‐based learning was very effective because it brought the practitioners face to face with their prescribing patterns. Although intellectually everyone agreed that antibiotics are often misused, this approach forced the providers to stop and reflect on their individual practices. This peer‐delivered intervention allowed for a collaborative approach to solving the problem; the peer (detailer) was approachable, nonjudgmental, and available for further discussion and guidance.
The public health quality improvement approach that we used for our intervention helped us to realize and appreciate the factors underlying prescribing patterns. Only by understanding the motivations for prescribing patterns can we hope to make sustainable changes. This coincides with our previous assertion that hospitalists are engaging in some public health practice.16 In pubic health, the programs, services, and institutions involved emphasize the prevention of disease and the health needs of the population as a whole.17 Hospitalist teams aim to make sure that the high‐quality services needed for protecting the health of their community (hospitalized patients) are available and that this population receives proper consideration in the allocation of resources. Antibiotic optimization is a key role that could fall within the mantra of public health practice for the hospitalist.
Several limitations of this pilot should be considered. First, the intervention is labor intensive. However, it is essential to use the problem‐solving paradigm and incorporate behavior change theories in order to identify interventions that can lead to sustainable change. Second, this was not a randomized controlled trial, and it is possible that there might have been some contamination by external forces. However, in reviewing the educational events at our institution, the press, and articles published during the study period, we could not identify any external factors that would have influenced antibiotic prescribing patterns. It would not have been possible to conduct a randomized trial at our institution because the hospitalists work so closely together that we could not ensure complete separation if the subjects were randomized. There would have been contamination from the intervention group to the control group. A trial with randomization at the institution level is the next step. Third, the number of months retrospectively reviewed in order to identify 20 prescriptions of a provider varied. This study assumed there were no other differences during those months that could have affected provider prescribing behavior; this may have introduced some bias. Fourth, the sustainability of this intervention's positive impact is unknown. We assessed outcome soon after the intervention, and it is unknown whether continual booster sessions are required to maintain the positive impact on prescribing patterns.
This pilot was a good starting place to show that behavior change can be realized with a well‐conceived and methodically executed intervention, even among the busiest of physicians. Audit and feedback, or practice‐based learning, appears to be a powerful educational intervention among professionals who take great pride in their work.
- ,.Improving antibiotic use in low‐income countries: an overview of evidence on determinants.Soc Sci Med.2003;57:733–744.
- .Mechanisms of antimicrobial resistance in bacteria.Am J Med.2006;119(6A):S3–S10.
- .Antimicrobial resistance in gram‐positive bacteria.Am J Med.2006;119(6A):S11–S19.
- .Resistance in Gram‐negative bacteria: enterobacteriaceae.Am J Med.2006;119(6A):S20–S28.
- .Pharmacodynamics: relation to antimicrobial resistance.Am J Med.2006;119(6A):S37–S44.
- .Managing methicillin‐resistant staphylococci: a paradigm for preventing nosocomial transmission of resistant organisms.Am J Med.2006;119(6A):S45–S52.
- NIH. The Problem of Antibiotic Resistance. Available at: http://www.niaid.nih.gov.
- ,,,.Educational interventions to improve antibiotic use in the community: report from the International Forum on Antibiotic Resistance (IFAR) colloquium, 2002.Lancet Infect Dis.2004;4:44–53.
- ,,, et al.The rate and cost of hospital‐acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposed.J Hosp Infect.2001;47:198–209.
- ,.The impact of hospital‐acquired bloodstream infections.Emerg Infect Dis.2001;7(2):174–177.
- .Antimicrobial stewardship.Am J Med.2006;119(6A):S53–S61
- ,,, et al.Changing provider behavior: an overview of systemic reviews of interventions.Med Care.2001;39:II–2‐II‐45.
- .A review of current health education theories.Calif J Health Promot.2004;2:74–87
- The Johns Hopkins Hospital Antibiotic Management Program. 2005 Antibiotic Guidelines: Treatment Recommendations for Adult Inpatients. Johns Hopkins Medicine.
- ,,,.The Sanford Guide to Antimicrobial Therapy 2005.35th ed.Hyde Park, VT:Antimicrobial Therapy, Inc.;2005.
- ,,,.Expanding the roles of hospitalist physicians to include public health.J Hosp Med.2007;2:93–101.
- ,.Principles of Public Health Practice.Albany, NY:Delmar Publishing;1997.
Inappropriate antibiotic use is a major public health concern and demonstrates the need for quality improvement initiatives in the delivery of health care.16 Each year nearly 2 million patients in the United States acquire an infection in the hospital, and about 90,000 of them die from these infections.7 More than 70% of the bacteria that cause hospital‐acquired infections are resistant to at least one commonly used drug.7 Persons infected with drug‐resistant organisms have longer hospital stays and higher mortality rates.7
Inappropriate antibiotic use in the inpatient hospital setting can be classified into 5 categories. First, antibiotics may be given for illnesses for which they are not indicated (eg, viral infections). Second, broad‐spectrum antibiotics (such as piperacillin‐tazobactam and quinolones) may be overused in the empiric treatment of common infections.8 Overuse of broad‐spectrum drugs increases selective pressure for antimicrobial resistance and exposes patients to the side effects of some of these drugs, such as Clostridium difficile colitis.8 Third, clinicians occasionally prescribe intravenous (IV) antibiotics when the efficacy of oral agents would be similar. Inappropriate intravenous therapy increases the cost of care and also exposes the patient to the risk of intravenous catheters.8 Fourth, when the correct antibiotic choice is made, inappropriate antibiotic dosage, schedule, and/or duration of treatment can threaten patient safety.8 Fifth, bug‐drug mismatch occurs when susceptibility studies indicate that the drug being used is ineffective or only marginally effective.8 Beyond antimicrobial resistance and safety, these practices also usually increase costs to both the patient and the hospital.7, 910
Influencing providers' prescribing patterns is difficult.11 In this project we assessed the prescribing patterns of hospitalists in an active inpatient environment and then developed an intervention to improve the providers' use of antibiotics. The intervention utilized public health methodologyprior to implementation, we defined the problem, determined its magnitude, identified a behavior change model, and constructed a conceptual framework that identifyied the key determinants. A pilot academic detailing project addressing many determinants was developed, implemented, and evaluated.
Conceptual Model
To change prescribing behaviors is to change learned behaviors. Changing behavior is a complex process affected by several factors including beliefs, expectations, motivations, and the psychosocial environments of the target groups.12 Each of these factors must be considered when attempting to bring about behavior changes. In doing so, a theory that can be depicted in a model often emerges.13 This approach is widely used in understanding and developing public health interventions.
Formulating the Model
In any public health intervention, recognizing and engaging key stakeholders is a critical step. We identified the following stakeholders: (1) hospitalist practitioners and other prescribing providers including residents and infectious disease specialists; (2) nurses; (3) administrators who are focused on cost effectiveness; (4) patients and their families, who want to get well affordably, without side effects; (5) pharmacists; (6) risk management; and (7) society, which is fearful of the propagation of resistant microbes. In consulting with some of the stakeholders, 4 factors that influence hospitalists' prescribing patterns became apparent. These are practitioner factors, environmental factors, perceived rewards, and perceived threats (Fig. 1).

The practitioner factors shaping prescribing are: (1) knowledge of current best care; (2) self‐efficacy, which determines whether a provider is confident in his or her knowledge to adequately treat a specific infection; (3) habit, which causes providers to pick from a narrow repertoire of antibiotics when treating an infection; and (4) fear of liability, which forces some providers to be cautious. Four environmental factors affecting antibiotic prescriptions are: (1) published guidelines regarding organisms' sensitivity to antibiotics; (2) patient‐driven factors such as affordability, compliance with dosing regimens, side effects, and interactions between the antibiotics and other medications; (3) peer influence, in that providers are reluctant to change a prescription started by another provider (eg, emergency room physician); and (4) the formulary of the hospital, as it forces providers to prescribe within specific parameters. The perceived rewards of specific prescribing practices may include improving patient safety and reducing antibiotic resistance and costs, whereas the perceived threats are increasing antimicrobial resistance, having adverse patient outcomes, and increasing costs and hospital length of stay. We selected a high‐yield, low‐effort intervention in order to have an impact on some of the factors underlying hospitalists' prescribing patterns.
METHODS
Participants
The study participants were 17 hospitalist practitioners including physicians, nurse‐practitioners, and physician assistants who make up the Collaborative Inpatient Medical Service (CIMS) at Johns Hopkins Bayview Medical Center (JHBMC; Table 1). All consented to participate. The study was approved by the institutional review board.
| Age in years, mean (SD) | 36 (6) |
| Female, n (%) | 13 (76%) |
| Physician, n (%) | 9 (53%) |
| Nurse‐practitioner, n (%) | 5 (29%) |
| Physician assistant, n (%) | 3 (18%) |
| Years in practice, mean (SD) | 5.1 (2.8) |
| Number of pharmaceutical representatives exposed to in past year, mean | 1 |
| Number of shifts worked per month, mean (SD) | 14 (4) |
| Primarily works days, n (%) | 13 (76%) |
Data Collection
We collected and assessed prescription patterns over 3 periods: preintervention, interim, and postintervention.
Assessing Appropriateness of Antibiotics
For each order that was assessed in the preintervention, interim, and postintervention periods, the following information was collected: (1) drug ordered, (2) clinical diagnosis, (3) microbiology results available at the time of the order (including relevant results from recent cultures), (4) other medical diagnoses (ICD9 codes), (5) allergies, and (6) exposure to health care facilities (within the past 30 days). The computerized medical record allowed access to the discharge summaries of a patient's hospitalization. These records summarized the patient's hospitalization, allowing the investigators to understand the reasons for a provider's choice of antibiotics. If the rationale was not clear about how to categorize a prescription from reading the data, the investigators performed a chart review. From the information culled from these reviews, the primary investigator and an infectious disease specialist classified each prescription order by consensus as appropriate, effective but inappropriate, or inappropriate therapy.
Prescriptions were classified as appropriate when they were indicated and correlated with sensitivities, if available, or were of a narrow‐enough spectrum and recommended as a first‐line treatment for specific illnesses by either the Johns Hopkins Antibiotic Guide14 or the Stanford Guide to Antimicrobial Therapy.15 For example, cephalexin to treat uncomplicated cellulitis was considered appropriate therapy. Effective but inappropriate prescriptions were broad‐spectrum antibiotics used to treat an infection when a narrower‐spectrum antibiotic would have sufficed. For example, piperacillin‐tazobactam would be effective in treating a simple urinary tract infection but inappropriate to use because of its broad spectrum. Other examples of effective but inappropriate prescriptions were giving an IV when an oral alternative would be equally effective and tolerated or prescribing antibiotic treatment whose duration was too long. Finally, inappropriate prescriptions were those written for conditions for which antibiotics are not indicated or for which the prescribed antibiotic was ineffective for the specified infection (bug‐drug mismatch).
Preintervention
In January 2006 the investigators retrospectively reviewed the prescribing patterns of the 17 providers over the previous year. Using the computerized medical record and physician order entry, consecutive prescriptions of each provider were evaluated, beginning December 31, 2005, going back reverse chronologically until 20 prescriptions had been identified. For 12 of the providers, it was actually possible to review 20 prescriptions. For 2 other providers, both new, part‐time additions to the hospitalist group, only 1 and 7 prescriptions were found for the entire year. The prescribing history of the 3 remaining providers who participated in the study, all physician assistants, could not be evaluated (during any period) because all their orders were linked only to physicians, making it impossible to determine their specific prescriptions using the physician order entry system.
Interim
During the interim period between obtaining informed consent and completing the academic detailing (January 3, 2006, to March 23, 2006), provider prescribing patterns were reviewed to determine if the mere knowledge of the project would produce changes in prescribing behavior.
Postintervention
After the academic detailing was completed (March 23, 2006), the prescribing patterns of the hospitalists were followed through April 23, 2006. Each week after the detailing session, the hospitalists received reminders to prescribe appropriately (including pens with the message Reduce the Overuse).
Detailing Procedures
After the review, a profile was assembled for each of the CIMS providers. The study team detailers (a physician and a pharmacist) met with the individual providers for 30 to 45 minutes. Each hospitalist participant completed a short survey that collected demographic information and was asked about the rationale for his or her antibiotic prescribing pattern. Next, the appraisal of the provider's prescribing pattern was reviewed. This review included looking at the costs of the prescribed antibiotics compared with those of the appropriate alternatives and a reexamination of the guidelines for the selected target drugspiperacillin‐tazobactam, vancomycin, and extended‐spectrum quinolones. These 3 antibiotics were picked because our providers had been particularly vulnerable to inappropriately prescribing them. The hospitalists were provided an antibiotic guide developed specifically for this project and based on the Johns Hopkins Antibiotic Guide14 that summarizes the consensus guidelines.
Data Analysis
The primary outcome variable was the aggregate proportion of inappropriate antibiotic prescribed (as defined earlier) before the intervention, during the interim between obtaining informed consent and intervening on all study subjects, and after the intervention. The percentage of appropriate prescriptions versus total not appropriate prescriptions (combining of the effective but inappropriate and inappropriate categories) were compared across the 3 periods. Ninety‐five percent confidence intervals for comparisons of the proportions were determined using Stata 9.0 (College Station, TX). The difference between the proportions of total not appropriate prescriptions before and after academic detailing was computed in Stata using Fisher's exact test to assess significance.
RESULTS
Demographic information and professional characteristics of the 17 providers are shown in Table 1. Their mean age was 36 years, and 76% were female. The top 4 reasons the providers gave for their prescribing practices were: (1) published guidelines, (2) easier dosing schedule for patient when discharged, (3) continuing an antibiotic course initiated in the emergency room, and (4) broad‐spectrum antibiotics cover all possible microbes.
Comparison of Preintervention, Interim, and Postintervention Periods
Table 2 depicts the results of the prescription appraisals from the retrospective reviews. Of the 14 providers who had ordered antibiotics, 8 (57%) had more prescriptions that were total not appropriate than were appropriate in the preintervention period compared with 3 providers (25%) with this prescribing pattern in the postintervention period (P = .13).
| Provider | Preintervention | Postintervention | ||||
|---|---|---|---|---|---|---|
| Prescriptions (n) | Appropriate, n (%) | Total not appropriate, n (%) | Prescriptions (n) | Appropriate, n (%) | Total not appropriate, n (%) | |
| ||||||
| 1 | 20 | 7 (35%) | 13 (65%) | 24 | 17 (70.8%) | 7 (29.2%) |
| 2 | 20 | 10 (50%) | 10 (50%) | 12 | 11 (91.7%) | 1 (8.3%) |
| 3 | 20 | 6 (30%) | 14 (70%) | 8 | 8 (100%) | 0 (0%) |
| 4* | 19 | 10 (52.6%) | 9 (47.4%) | 4 | 3 (75%) | 1 (25%) |
| 5 | 20 | 9 (45%) | 11 (55%) | 10 | 4 (40%) | 6 (60%) |
| 6 | 20 | 5 (25%) | 15 (75%) | 3 | 1 (33.3%) | 2 (66.7%) |
| 7 | 20 | 8 (40%) | 12 (60%) | 8 | 7 (87.5%) | 1 (12.5%) |
| 8* | 1 | 0 (0%) | 1 (100%) | 0 | 0 (0%) | 0 (0%) |
| 9 | 20 | 11 (55%) | 9 (45%) | 5 | 2 (40%) | 3 (60%) |
| 10* | 7 | 3 (42.9%) | 4 (57.1%) | 0 | 0 (0%) | 0 (0%) |
| 11 | 20 | 10 (50%) | 10 (50%) | 17 | 13 (76.5%) | 4 (23.5%) |
| 12 | 20 | 6 (30%) | 14 (70%) | 16 | 14 (87.5%) | 2 (12.5%) |
| 13 | 20 | 12 (60%) | 8 (40%) | 15 | 11 (73.3%) | 4 (26.7%) |
| 14 | 20 | 10 (50%) | 10 (50%) | 7 | 4 (57.1%) | 3 (42.9%) |
| Total | 247 | 107 (43%) | 140 (57%) | 129 | 95 (73.6%) | 34 (26.4%) |
Table 3 shows the proportions of appropriate, effective but inappropriate, and total not appropriate prescriptions in the retrospective, interim, and postintervention periods. Forty‐three percent (95% CI 37%‐49%) of prescriptions were judged to be appropriate, and 57% (95% CI 51%‐63%) to be not appropriate prior to the academic detailing. In the interim period, 59% (95% CI 52%‐65%) of the prescriptions were appropriate, and 41% (95% CI 35%‐48%) were not appropriate; P = .0003. After the intervention, 74% (95% CI 65%‐81%) of the prescriptions were appropriate, and 26% (95% CI 19%‐35%) were not appropriate; P < .0001.
| Period | Appropriate, n (%) | 95% CI | Effective but inappropriate, n (%) | Inappropriate, n (%) | Total not appropriate, n (%) | 95% CI | P value* |
|---|---|---|---|---|---|---|---|
| |||||||
| Retrospective review (pre) | 107 (43%) | 37%‐49% | 75 (30.4%) | 65 (26.6%) | 140 (57%) | 51%‐63% | |
| Interim | 146 (59%) | 52%‐65% | 37 (15%) | 65 (26%) | 102 (41%) | 35%‐48% | .0003 |
| Postintervention | 95 (74%) | 65%‐81% | 8 (6%) | 26 (20%) | 34 (26%) | 19%‐35% | < .0001 |
DISCUSSION
We have demonstrated that academic detailing had a positive impact on the prescribing patterns of hospitalists. The aggregated improvement in antibiotic prescribing patterns can be attributed to improvement in the prescribing patterns of almost every hospitalist practitioner (Table 2). This study focused on aggregate prescriptions as the primary outcome measure because the hospitalists at JHBMC, like at many other institutions, function as a team, with a patient routinely having multiple providers over the course of the hospital stay. The improved prescribing patterns noted during the interim period suggest that the mere knowledge of a project can have an impact on providers. Providers informed the investigators that they were more thoughtful about their choice of antibiotics when they knew that they were being studied. The further statistically significant improvement in prescribing patterns with the intervention shows that the academic detailing itself was successful.
The greatest absolute change in practice was seen in effective but inappropriate prescribing (from 30.4% to 6%), whereas inappropriate prescribing only decreased from 26.6% to 20.6%. Although we aimed to have an impact on all inappropriate antibiotic prescribing patterns, we specifically reviewed the prescribing guidelines for piperacillin‐tazobactam, extended‐spectrum quinolones, and vancomycin. These 3 antibiotics were targeted because our providers had been particularly susceptible to inappropriately prescribing them. The focus on these antibiotics may have resulted in the larger absolute change noted in effective but inappropriate prescribing. We did not collect any data to determine if having an impact on effective but inappropriate prescribing changed the clinical course of the patients, such as shortening their hospital stays. Anecdotal evidence, however, suggests that it does. At our institution it is not uncommon for patients to be kept in the hospital for an extra day to ensure they are stable when transitioned from extended‐spectrum to narrower‐spectrum antibiotics prior to discharge. The effect of reducing effective but inappropriate prescriptions on the clinical course of patients could be an outcome measure assessed by a future, larger study.
Our one‐on‐one appraisal of each provider's prescribing patterns included a review of the cost of the prescribed antibiotics compared with that of the appropriate alternatives. Although decisions on antibiotic choice should be driven by clinical guidelines and appropriateness rather than price, we believed it was relevant to include education about costs and pricing so that providers would be reminded to ascertain whether patients would be able to afford their antibiotics. Antibiotic resistance is influenced by a patient's failure to complete the course of treatment, and noncompliance may be caused by an inability to afford the medication. Often, there are affordable, appropriate alternatives to the newest and most expensive drugs.
A hospitalist‐based academic detailing approach to improving antibiotic prescribing may have far‐reaching benefits and influence. First, it has the potential to affect other practitioners by setting an example and role modeling. In addition to that with their immediate peer group, hospitalists have close and repeated contact with house officers and emergency room physicians and often act as consultants to physicians in other departments such as surgery and psychiatry. Furthermore, some community hospitals have no infectious disease specialists readily available. So this represents an opportunity for hospitalists to promote quality in antibiotic prescribing. Practice‐based learning was very effective because it brought the practitioners face to face with their prescribing patterns. Although intellectually everyone agreed that antibiotics are often misused, this approach forced the providers to stop and reflect on their individual practices. This peer‐delivered intervention allowed for a collaborative approach to solving the problem; the peer (detailer) was approachable, nonjudgmental, and available for further discussion and guidance.
The public health quality improvement approach that we used for our intervention helped us to realize and appreciate the factors underlying prescribing patterns. Only by understanding the motivations for prescribing patterns can we hope to make sustainable changes. This coincides with our previous assertion that hospitalists are engaging in some public health practice.16 In pubic health, the programs, services, and institutions involved emphasize the prevention of disease and the health needs of the population as a whole.17 Hospitalist teams aim to make sure that the high‐quality services needed for protecting the health of their community (hospitalized patients) are available and that this population receives proper consideration in the allocation of resources. Antibiotic optimization is a key role that could fall within the mantra of public health practice for the hospitalist.
Several limitations of this pilot should be considered. First, the intervention is labor intensive. However, it is essential to use the problem‐solving paradigm and incorporate behavior change theories in order to identify interventions that can lead to sustainable change. Second, this was not a randomized controlled trial, and it is possible that there might have been some contamination by external forces. However, in reviewing the educational events at our institution, the press, and articles published during the study period, we could not identify any external factors that would have influenced antibiotic prescribing patterns. It would not have been possible to conduct a randomized trial at our institution because the hospitalists work so closely together that we could not ensure complete separation if the subjects were randomized. There would have been contamination from the intervention group to the control group. A trial with randomization at the institution level is the next step. Third, the number of months retrospectively reviewed in order to identify 20 prescriptions of a provider varied. This study assumed there were no other differences during those months that could have affected provider prescribing behavior; this may have introduced some bias. Fourth, the sustainability of this intervention's positive impact is unknown. We assessed outcome soon after the intervention, and it is unknown whether continual booster sessions are required to maintain the positive impact on prescribing patterns.
This pilot was a good starting place to show that behavior change can be realized with a well‐conceived and methodically executed intervention, even among the busiest of physicians. Audit and feedback, or practice‐based learning, appears to be a powerful educational intervention among professionals who take great pride in their work.
Inappropriate antibiotic use is a major public health concern and demonstrates the need for quality improvement initiatives in the delivery of health care.16 Each year nearly 2 million patients in the United States acquire an infection in the hospital, and about 90,000 of them die from these infections.7 More than 70% of the bacteria that cause hospital‐acquired infections are resistant to at least one commonly used drug.7 Persons infected with drug‐resistant organisms have longer hospital stays and higher mortality rates.7
Inappropriate antibiotic use in the inpatient hospital setting can be classified into 5 categories. First, antibiotics may be given for illnesses for which they are not indicated (eg, viral infections). Second, broad‐spectrum antibiotics (such as piperacillin‐tazobactam and quinolones) may be overused in the empiric treatment of common infections.8 Overuse of broad‐spectrum drugs increases selective pressure for antimicrobial resistance and exposes patients to the side effects of some of these drugs, such as Clostridium difficile colitis.8 Third, clinicians occasionally prescribe intravenous (IV) antibiotics when the efficacy of oral agents would be similar. Inappropriate intravenous therapy increases the cost of care and also exposes the patient to the risk of intravenous catheters.8 Fourth, when the correct antibiotic choice is made, inappropriate antibiotic dosage, schedule, and/or duration of treatment can threaten patient safety.8 Fifth, bug‐drug mismatch occurs when susceptibility studies indicate that the drug being used is ineffective or only marginally effective.8 Beyond antimicrobial resistance and safety, these practices also usually increase costs to both the patient and the hospital.7, 910
Influencing providers' prescribing patterns is difficult.11 In this project we assessed the prescribing patterns of hospitalists in an active inpatient environment and then developed an intervention to improve the providers' use of antibiotics. The intervention utilized public health methodologyprior to implementation, we defined the problem, determined its magnitude, identified a behavior change model, and constructed a conceptual framework that identifyied the key determinants. A pilot academic detailing project addressing many determinants was developed, implemented, and evaluated.
Conceptual Model
To change prescribing behaviors is to change learned behaviors. Changing behavior is a complex process affected by several factors including beliefs, expectations, motivations, and the psychosocial environments of the target groups.12 Each of these factors must be considered when attempting to bring about behavior changes. In doing so, a theory that can be depicted in a model often emerges.13 This approach is widely used in understanding and developing public health interventions.
Formulating the Model
In any public health intervention, recognizing and engaging key stakeholders is a critical step. We identified the following stakeholders: (1) hospitalist practitioners and other prescribing providers including residents and infectious disease specialists; (2) nurses; (3) administrators who are focused on cost effectiveness; (4) patients and their families, who want to get well affordably, without side effects; (5) pharmacists; (6) risk management; and (7) society, which is fearful of the propagation of resistant microbes. In consulting with some of the stakeholders, 4 factors that influence hospitalists' prescribing patterns became apparent. These are practitioner factors, environmental factors, perceived rewards, and perceived threats (Fig. 1).

The practitioner factors shaping prescribing are: (1) knowledge of current best care; (2) self‐efficacy, which determines whether a provider is confident in his or her knowledge to adequately treat a specific infection; (3) habit, which causes providers to pick from a narrow repertoire of antibiotics when treating an infection; and (4) fear of liability, which forces some providers to be cautious. Four environmental factors affecting antibiotic prescriptions are: (1) published guidelines regarding organisms' sensitivity to antibiotics; (2) patient‐driven factors such as affordability, compliance with dosing regimens, side effects, and interactions between the antibiotics and other medications; (3) peer influence, in that providers are reluctant to change a prescription started by another provider (eg, emergency room physician); and (4) the formulary of the hospital, as it forces providers to prescribe within specific parameters. The perceived rewards of specific prescribing practices may include improving patient safety and reducing antibiotic resistance and costs, whereas the perceived threats are increasing antimicrobial resistance, having adverse patient outcomes, and increasing costs and hospital length of stay. We selected a high‐yield, low‐effort intervention in order to have an impact on some of the factors underlying hospitalists' prescribing patterns.
METHODS
Participants
The study participants were 17 hospitalist practitioners including physicians, nurse‐practitioners, and physician assistants who make up the Collaborative Inpatient Medical Service (CIMS) at Johns Hopkins Bayview Medical Center (JHBMC; Table 1). All consented to participate. The study was approved by the institutional review board.
| Age in years, mean (SD) | 36 (6) |
| Female, n (%) | 13 (76%) |
| Physician, n (%) | 9 (53%) |
| Nurse‐practitioner, n (%) | 5 (29%) |
| Physician assistant, n (%) | 3 (18%) |
| Years in practice, mean (SD) | 5.1 (2.8) |
| Number of pharmaceutical representatives exposed to in past year, mean | 1 |
| Number of shifts worked per month, mean (SD) | 14 (4) |
| Primarily works days, n (%) | 13 (76%) |
Data Collection
We collected and assessed prescription patterns over 3 periods: preintervention, interim, and postintervention.
Assessing Appropriateness of Antibiotics
For each order that was assessed in the preintervention, interim, and postintervention periods, the following information was collected: (1) drug ordered, (2) clinical diagnosis, (3) microbiology results available at the time of the order (including relevant results from recent cultures), (4) other medical diagnoses (ICD9 codes), (5) allergies, and (6) exposure to health care facilities (within the past 30 days). The computerized medical record allowed access to the discharge summaries of a patient's hospitalization. These records summarized the patient's hospitalization, allowing the investigators to understand the reasons for a provider's choice of antibiotics. If the rationale was not clear about how to categorize a prescription from reading the data, the investigators performed a chart review. From the information culled from these reviews, the primary investigator and an infectious disease specialist classified each prescription order by consensus as appropriate, effective but inappropriate, or inappropriate therapy.
Prescriptions were classified as appropriate when they were indicated and correlated with sensitivities, if available, or were of a narrow‐enough spectrum and recommended as a first‐line treatment for specific illnesses by either the Johns Hopkins Antibiotic Guide14 or the Stanford Guide to Antimicrobial Therapy.15 For example, cephalexin to treat uncomplicated cellulitis was considered appropriate therapy. Effective but inappropriate prescriptions were broad‐spectrum antibiotics used to treat an infection when a narrower‐spectrum antibiotic would have sufficed. For example, piperacillin‐tazobactam would be effective in treating a simple urinary tract infection but inappropriate to use because of its broad spectrum. Other examples of effective but inappropriate prescriptions were giving an IV when an oral alternative would be equally effective and tolerated or prescribing antibiotic treatment whose duration was too long. Finally, inappropriate prescriptions were those written for conditions for which antibiotics are not indicated or for which the prescribed antibiotic was ineffective for the specified infection (bug‐drug mismatch).
Preintervention
In January 2006 the investigators retrospectively reviewed the prescribing patterns of the 17 providers over the previous year. Using the computerized medical record and physician order entry, consecutive prescriptions of each provider were evaluated, beginning December 31, 2005, going back reverse chronologically until 20 prescriptions had been identified. For 12 of the providers, it was actually possible to review 20 prescriptions. For 2 other providers, both new, part‐time additions to the hospitalist group, only 1 and 7 prescriptions were found for the entire year. The prescribing history of the 3 remaining providers who participated in the study, all physician assistants, could not be evaluated (during any period) because all their orders were linked only to physicians, making it impossible to determine their specific prescriptions using the physician order entry system.
Interim
During the interim period between obtaining informed consent and completing the academic detailing (January 3, 2006, to March 23, 2006), provider prescribing patterns were reviewed to determine if the mere knowledge of the project would produce changes in prescribing behavior.
Postintervention
After the academic detailing was completed (March 23, 2006), the prescribing patterns of the hospitalists were followed through April 23, 2006. Each week after the detailing session, the hospitalists received reminders to prescribe appropriately (including pens with the message Reduce the Overuse).
Detailing Procedures
After the review, a profile was assembled for each of the CIMS providers. The study team detailers (a physician and a pharmacist) met with the individual providers for 30 to 45 minutes. Each hospitalist participant completed a short survey that collected demographic information and was asked about the rationale for his or her antibiotic prescribing pattern. Next, the appraisal of the provider's prescribing pattern was reviewed. This review included looking at the costs of the prescribed antibiotics compared with those of the appropriate alternatives and a reexamination of the guidelines for the selected target drugspiperacillin‐tazobactam, vancomycin, and extended‐spectrum quinolones. These 3 antibiotics were picked because our providers had been particularly vulnerable to inappropriately prescribing them. The hospitalists were provided an antibiotic guide developed specifically for this project and based on the Johns Hopkins Antibiotic Guide14 that summarizes the consensus guidelines.
Data Analysis
The primary outcome variable was the aggregate proportion of inappropriate antibiotic prescribed (as defined earlier) before the intervention, during the interim between obtaining informed consent and intervening on all study subjects, and after the intervention. The percentage of appropriate prescriptions versus total not appropriate prescriptions (combining of the effective but inappropriate and inappropriate categories) were compared across the 3 periods. Ninety‐five percent confidence intervals for comparisons of the proportions were determined using Stata 9.0 (College Station, TX). The difference between the proportions of total not appropriate prescriptions before and after academic detailing was computed in Stata using Fisher's exact test to assess significance.
RESULTS
Demographic information and professional characteristics of the 17 providers are shown in Table 1. Their mean age was 36 years, and 76% were female. The top 4 reasons the providers gave for their prescribing practices were: (1) published guidelines, (2) easier dosing schedule for patient when discharged, (3) continuing an antibiotic course initiated in the emergency room, and (4) broad‐spectrum antibiotics cover all possible microbes.
Comparison of Preintervention, Interim, and Postintervention Periods
Table 2 depicts the results of the prescription appraisals from the retrospective reviews. Of the 14 providers who had ordered antibiotics, 8 (57%) had more prescriptions that were total not appropriate than were appropriate in the preintervention period compared with 3 providers (25%) with this prescribing pattern in the postintervention period (P = .13).
| Provider | Preintervention | Postintervention | ||||
|---|---|---|---|---|---|---|
| Prescriptions (n) | Appropriate, n (%) | Total not appropriate, n (%) | Prescriptions (n) | Appropriate, n (%) | Total not appropriate, n (%) | |
| ||||||
| 1 | 20 | 7 (35%) | 13 (65%) | 24 | 17 (70.8%) | 7 (29.2%) |
| 2 | 20 | 10 (50%) | 10 (50%) | 12 | 11 (91.7%) | 1 (8.3%) |
| 3 | 20 | 6 (30%) | 14 (70%) | 8 | 8 (100%) | 0 (0%) |
| 4* | 19 | 10 (52.6%) | 9 (47.4%) | 4 | 3 (75%) | 1 (25%) |
| 5 | 20 | 9 (45%) | 11 (55%) | 10 | 4 (40%) | 6 (60%) |
| 6 | 20 | 5 (25%) | 15 (75%) | 3 | 1 (33.3%) | 2 (66.7%) |
| 7 | 20 | 8 (40%) | 12 (60%) | 8 | 7 (87.5%) | 1 (12.5%) |
| 8* | 1 | 0 (0%) | 1 (100%) | 0 | 0 (0%) | 0 (0%) |
| 9 | 20 | 11 (55%) | 9 (45%) | 5 | 2 (40%) | 3 (60%) |
| 10* | 7 | 3 (42.9%) | 4 (57.1%) | 0 | 0 (0%) | 0 (0%) |
| 11 | 20 | 10 (50%) | 10 (50%) | 17 | 13 (76.5%) | 4 (23.5%) |
| 12 | 20 | 6 (30%) | 14 (70%) | 16 | 14 (87.5%) | 2 (12.5%) |
| 13 | 20 | 12 (60%) | 8 (40%) | 15 | 11 (73.3%) | 4 (26.7%) |
| 14 | 20 | 10 (50%) | 10 (50%) | 7 | 4 (57.1%) | 3 (42.9%) |
| Total | 247 | 107 (43%) | 140 (57%) | 129 | 95 (73.6%) | 34 (26.4%) |
Table 3 shows the proportions of appropriate, effective but inappropriate, and total not appropriate prescriptions in the retrospective, interim, and postintervention periods. Forty‐three percent (95% CI 37%‐49%) of prescriptions were judged to be appropriate, and 57% (95% CI 51%‐63%) to be not appropriate prior to the academic detailing. In the interim period, 59% (95% CI 52%‐65%) of the prescriptions were appropriate, and 41% (95% CI 35%‐48%) were not appropriate; P = .0003. After the intervention, 74% (95% CI 65%‐81%) of the prescriptions were appropriate, and 26% (95% CI 19%‐35%) were not appropriate; P < .0001.
| Period | Appropriate, n (%) | 95% CI | Effective but inappropriate, n (%) | Inappropriate, n (%) | Total not appropriate, n (%) | 95% CI | P value* |
|---|---|---|---|---|---|---|---|
| |||||||
| Retrospective review (pre) | 107 (43%) | 37%‐49% | 75 (30.4%) | 65 (26.6%) | 140 (57%) | 51%‐63% | |
| Interim | 146 (59%) | 52%‐65% | 37 (15%) | 65 (26%) | 102 (41%) | 35%‐48% | .0003 |
| Postintervention | 95 (74%) | 65%‐81% | 8 (6%) | 26 (20%) | 34 (26%) | 19%‐35% | < .0001 |
DISCUSSION
We have demonstrated that academic detailing had a positive impact on the prescribing patterns of hospitalists. The aggregated improvement in antibiotic prescribing patterns can be attributed to improvement in the prescribing patterns of almost every hospitalist practitioner (Table 2). This study focused on aggregate prescriptions as the primary outcome measure because the hospitalists at JHBMC, like at many other institutions, function as a team, with a patient routinely having multiple providers over the course of the hospital stay. The improved prescribing patterns noted during the interim period suggest that the mere knowledge of a project can have an impact on providers. Providers informed the investigators that they were more thoughtful about their choice of antibiotics when they knew that they were being studied. The further statistically significant improvement in prescribing patterns with the intervention shows that the academic detailing itself was successful.
The greatest absolute change in practice was seen in effective but inappropriate prescribing (from 30.4% to 6%), whereas inappropriate prescribing only decreased from 26.6% to 20.6%. Although we aimed to have an impact on all inappropriate antibiotic prescribing patterns, we specifically reviewed the prescribing guidelines for piperacillin‐tazobactam, extended‐spectrum quinolones, and vancomycin. These 3 antibiotics were targeted because our providers had been particularly susceptible to inappropriately prescribing them. The focus on these antibiotics may have resulted in the larger absolute change noted in effective but inappropriate prescribing. We did not collect any data to determine if having an impact on effective but inappropriate prescribing changed the clinical course of the patients, such as shortening their hospital stays. Anecdotal evidence, however, suggests that it does. At our institution it is not uncommon for patients to be kept in the hospital for an extra day to ensure they are stable when transitioned from extended‐spectrum to narrower‐spectrum antibiotics prior to discharge. The effect of reducing effective but inappropriate prescriptions on the clinical course of patients could be an outcome measure assessed by a future, larger study.
Our one‐on‐one appraisal of each provider's prescribing patterns included a review of the cost of the prescribed antibiotics compared with that of the appropriate alternatives. Although decisions on antibiotic choice should be driven by clinical guidelines and appropriateness rather than price, we believed it was relevant to include education about costs and pricing so that providers would be reminded to ascertain whether patients would be able to afford their antibiotics. Antibiotic resistance is influenced by a patient's failure to complete the course of treatment, and noncompliance may be caused by an inability to afford the medication. Often, there are affordable, appropriate alternatives to the newest and most expensive drugs.
A hospitalist‐based academic detailing approach to improving antibiotic prescribing may have far‐reaching benefits and influence. First, it has the potential to affect other practitioners by setting an example and role modeling. In addition to that with their immediate peer group, hospitalists have close and repeated contact with house officers and emergency room physicians and often act as consultants to physicians in other departments such as surgery and psychiatry. Furthermore, some community hospitals have no infectious disease specialists readily available. So this represents an opportunity for hospitalists to promote quality in antibiotic prescribing. Practice‐based learning was very effective because it brought the practitioners face to face with their prescribing patterns. Although intellectually everyone agreed that antibiotics are often misused, this approach forced the providers to stop and reflect on their individual practices. This peer‐delivered intervention allowed for a collaborative approach to solving the problem; the peer (detailer) was approachable, nonjudgmental, and available for further discussion and guidance.
The public health quality improvement approach that we used for our intervention helped us to realize and appreciate the factors underlying prescribing patterns. Only by understanding the motivations for prescribing patterns can we hope to make sustainable changes. This coincides with our previous assertion that hospitalists are engaging in some public health practice.16 In pubic health, the programs, services, and institutions involved emphasize the prevention of disease and the health needs of the population as a whole.17 Hospitalist teams aim to make sure that the high‐quality services needed for protecting the health of their community (hospitalized patients) are available and that this population receives proper consideration in the allocation of resources. Antibiotic optimization is a key role that could fall within the mantra of public health practice for the hospitalist.
Several limitations of this pilot should be considered. First, the intervention is labor intensive. However, it is essential to use the problem‐solving paradigm and incorporate behavior change theories in order to identify interventions that can lead to sustainable change. Second, this was not a randomized controlled trial, and it is possible that there might have been some contamination by external forces. However, in reviewing the educational events at our institution, the press, and articles published during the study period, we could not identify any external factors that would have influenced antibiotic prescribing patterns. It would not have been possible to conduct a randomized trial at our institution because the hospitalists work so closely together that we could not ensure complete separation if the subjects were randomized. There would have been contamination from the intervention group to the control group. A trial with randomization at the institution level is the next step. Third, the number of months retrospectively reviewed in order to identify 20 prescriptions of a provider varied. This study assumed there were no other differences during those months that could have affected provider prescribing behavior; this may have introduced some bias. Fourth, the sustainability of this intervention's positive impact is unknown. We assessed outcome soon after the intervention, and it is unknown whether continual booster sessions are required to maintain the positive impact on prescribing patterns.
This pilot was a good starting place to show that behavior change can be realized with a well‐conceived and methodically executed intervention, even among the busiest of physicians. Audit and feedback, or practice‐based learning, appears to be a powerful educational intervention among professionals who take great pride in their work.
- ,.Improving antibiotic use in low‐income countries: an overview of evidence on determinants.Soc Sci Med.2003;57:733–744.
- .Mechanisms of antimicrobial resistance in bacteria.Am J Med.2006;119(6A):S3–S10.
- .Antimicrobial resistance in gram‐positive bacteria.Am J Med.2006;119(6A):S11–S19.
- .Resistance in Gram‐negative bacteria: enterobacteriaceae.Am J Med.2006;119(6A):S20–S28.
- .Pharmacodynamics: relation to antimicrobial resistance.Am J Med.2006;119(6A):S37–S44.
- .Managing methicillin‐resistant staphylococci: a paradigm for preventing nosocomial transmission of resistant organisms.Am J Med.2006;119(6A):S45–S52.
- NIH. The Problem of Antibiotic Resistance. Available at: http://www.niaid.nih.gov.
- ,,,.Educational interventions to improve antibiotic use in the community: report from the International Forum on Antibiotic Resistance (IFAR) colloquium, 2002.Lancet Infect Dis.2004;4:44–53.
- ,,, et al.The rate and cost of hospital‐acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposed.J Hosp Infect.2001;47:198–209.
- ,.The impact of hospital‐acquired bloodstream infections.Emerg Infect Dis.2001;7(2):174–177.
- .Antimicrobial stewardship.Am J Med.2006;119(6A):S53–S61
- ,,, et al.Changing provider behavior: an overview of systemic reviews of interventions.Med Care.2001;39:II–2‐II‐45.
- .A review of current health education theories.Calif J Health Promot.2004;2:74–87
- The Johns Hopkins Hospital Antibiotic Management Program. 2005 Antibiotic Guidelines: Treatment Recommendations for Adult Inpatients. Johns Hopkins Medicine.
- ,,,.The Sanford Guide to Antimicrobial Therapy 2005.35th ed.Hyde Park, VT:Antimicrobial Therapy, Inc.;2005.
- ,,,.Expanding the roles of hospitalist physicians to include public health.J Hosp Med.2007;2:93–101.
- ,.Principles of Public Health Practice.Albany, NY:Delmar Publishing;1997.
- ,.Improving antibiotic use in low‐income countries: an overview of evidence on determinants.Soc Sci Med.2003;57:733–744.
- .Mechanisms of antimicrobial resistance in bacteria.Am J Med.2006;119(6A):S3–S10.
- .Antimicrobial resistance in gram‐positive bacteria.Am J Med.2006;119(6A):S11–S19.
- .Resistance in Gram‐negative bacteria: enterobacteriaceae.Am J Med.2006;119(6A):S20–S28.
- .Pharmacodynamics: relation to antimicrobial resistance.Am J Med.2006;119(6A):S37–S44.
- .Managing methicillin‐resistant staphylococci: a paradigm for preventing nosocomial transmission of resistant organisms.Am J Med.2006;119(6A):S45–S52.
- NIH. The Problem of Antibiotic Resistance. Available at: http://www.niaid.nih.gov.
- ,,,.Educational interventions to improve antibiotic use in the community: report from the International Forum on Antibiotic Resistance (IFAR) colloquium, 2002.Lancet Infect Dis.2004;4:44–53.
- ,,, et al.The rate and cost of hospital‐acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposed.J Hosp Infect.2001;47:198–209.
- ,.The impact of hospital‐acquired bloodstream infections.Emerg Infect Dis.2001;7(2):174–177.
- .Antimicrobial stewardship.Am J Med.2006;119(6A):S53–S61
- ,,, et al.Changing provider behavior: an overview of systemic reviews of interventions.Med Care.2001;39:II–2‐II‐45.
- .A review of current health education theories.Calif J Health Promot.2004;2:74–87
- The Johns Hopkins Hospital Antibiotic Management Program. 2005 Antibiotic Guidelines: Treatment Recommendations for Adult Inpatients. Johns Hopkins Medicine.
- ,,,.The Sanford Guide to Antimicrobial Therapy 2005.35th ed.Hyde Park, VT:Antimicrobial Therapy, Inc.;2005.
- ,,,.Expanding the roles of hospitalist physicians to include public health.J Hosp Med.2007;2:93–101.
- ,.Principles of Public Health Practice.Albany, NY:Delmar Publishing;1997.
Copyright © 2008 Society of Hospital Medicine
CD‐ROM‐Based Education on Anticoagulation
Given recent changes in the goals and objectives of residency training as well as changes in the functioning of teaching hospitals, traditional educational formats may need to be supplemented or replaced.1 The Accreditation Council for Graduate Medical Education (ACGME) is promoting changes in resident education with the goal of not only enhancing trainee competency using innovative methods but also of demonstrating that these educational innovations result in enhanced quality of patient care and improved patient safety.1 A challenging aspect of these initiatives is that programs are working to implement them at a time when there are greater nonteaching demands on faculty time, mandated resident work‐hour limitations have been instituted, in some states by law, and resident patient care and educational activities are prone to disruptions inherent in caring for patients in a complex health system. Various solutions have been proposed including increased incorporation of self‐directed learning as a means of meeting modern resident educational challenges, yet the ideal tools with which to accomplish this are unknown.
Computer‐based instruction in medicine has been available since the 1960s, and although its use had initially been more widespread in medical student, nursing, allied health professional, and patient education,2, 3 it is being increasingly incorporated into resident education as well. Some studies have shown that for medical students, computer‐based teaching is at least as effective in improving knowledge as conventional lectures4 and that learners' satisfaction with computer‐based formats appears comparable with that of traditional didactic lectures.5 In recent years computer‐based teaching has been applied to resident education in various fields including surgery and surgical subspecialties, pediatrics, and obstetrics and gynecology.612 Little is known, however, about how computer‐based educational methods affect resident knowledge and especially how these methods might affect clinical practice.
Venous thromboembolism (VTE) is a common and hazardous complication of acute inpatient hospitalization.13 Recognizing that errors in proper prescribing and monitoring of anticoagulants are a major cause of acute inpatient morbidity and mortality,14 we began an initiative to educate our residents and improve their patient care practices regarding the proper use of anticoagulants. To accomplish this, we developed a CD‐ROM‐based learning module with the aim of increasing resident knowledge of anticoagulation as well as compliance with national standards for VTE prevention. In this study we assessed the impact of the CD‐ROM intervention on resident knowledge and their appropriate use of VTE prophylaxis.
METHODS
The study was approved by the institutional review board. With the participation of faculty educators in the departments of medicine, surgery, and neurology, one of the authors (H.K.) coordinated the development of a CD‐ROM containing concise modules on core topics in anticoagulation (Table 1). The presenters for these topics included the director of clinical hematology, 2 cardiologists including the director of the coronary care unit, the director of the medical intensive care unit, the director of cerebrovascular diseases, and 2 vascular surgeons, one of whom serves as vice chair of surgery. These modules, each lasting about 1 hour, had audio and slide components detailing the proper indications, monitoring, and efficacy of anticoagulants in atrial fibrillation, acute ischemic stroke, acute coronary syndromes, and VTE prevention in acutely ill hospitalized patients. The guidelines presented were based on the sixth (2000) ACCP guidelines for antithrombotic therapy for the prevention and treatment of thrombosis.15 The content of the CD‐ROM was reviewed for accuracy by the authors, though none of them were speakers. We asked that before all current residents in the departments of cardiothoracic surgery, emergency medicine, otolaryngology, internal medicine, neurosurgery, dental medicine, neurology, obstetrics and gynecology, orthopedics, surgery, and urology viewed the CD‐ROM, they complete a pretest to determine their baseline knowledge of this subject. After completing the pretest, the residents were required to view the CD‐ROM and retake the same test. We then compared pre‐ and posttest scores.
| Overview of anticoagulation |
| Venous thromboembolism |
| Atrial fibrillation |
| Unfractionated heparin in acute coronary syndrome |
| Treatment of thromboembolic events with intravenous heparin |
| Anticoagulants in the management of patients with acute ischemic stroke |
| Deep venous thrombosis prophylaxis |
To determine whether an increase in knowledge was secondary to the CD‐ROM intervention or simply a consequence of acquired clinical experience during training, we compared test scores of residents who did and did not receive the CD‐ROM intervention. In the academic year following our initial testing, we asked the incoming categorical medical PGY‐1 classes at our hospital and at a comparable local tertiary‐care hospital in our health system 2 miles away to take an anticoagulation pretest (different from the examination given for the initial testing) during their PGY‐1 orientations. The 2 institutions are comparable in many ways including in patient demographics and size and most residents come from the same medical schools, have a similar rotation structure, and use a comparable curriculum under a unified graduate medical education office. The CD‐ROMs were only given to categorical PGY‐1 residents at our institution. Both groups then retook the same test (posttest) 3 months into their clinical training, a time chosen because it is when all PGY‐1s would be expected to have gained significant clinical experience on the medical wards and or in the intensive care units. The exam questions were generated by one of the authors (B.M.) and covered all the topics in the CD‐ROM.
An Anticoagulation Steering Committee was formed to assess whether the CD‐ROM intervention affected our residents' patient care practices. None of the members of this committee were authors of this work. Members of this committee reviewed inpatient charts and documented resident compliance with VTE prevention standards during periods before and after they had viewed the CD‐ROMs. We chose this particular portion of the CD‐ROM because at both the test and control hospitals, initiatives were underway using order sets to improve anticoagulation in cardiac, neurological, and surgical patients but not in VTE prophylaxis. Charts from the same 2 nursing units on the medical service were reviewed in each period and included patients with a discharge diagnosis of congestive heart failure, any oncologic diagnosis, or sepsis. The chart review tool was developed by the anticoagulation committee and included a thrombosis risk factor assessment section as well as a list of contraindications to anticoagulation to determine if anticoagulation was appropriately implemented. Charts were reviewed for compliance with VTE prophylaxis after the CD‐ROM intervention (given in July 2004) in August 2004. To have a comparable pre‐CD‐ROM comparison, charts of patients with the diagnoses stated above were reviewed from August of the preceding year. The same month was chosen in the previous year to minimize any impact of resident experience, which would likely be a confounding factor if charts from May or June of the academic year were used as a control, for example. To determine whether an improvement in adherence to VTE prophylaxis standards was sustained, an additional chart review was carried out 7 months after the initial CD‐ROM viewing. The same group of observers, none of whom were authors, did all the chart assessments.
Statistics
Continuous variables are reported as means SDs. Comparisons of test scores before and after the CD‐ROM intervention were carried out using paired t testing. Comparisons of pre‐ and posttest scores between both institutions were carried out using analysis of variance with Tukey‐Kramer multiple‐comparisons testing (GraphPad InStat Statistical Software, version 3.01, GraphPad Software, Inc.). We calculated that 13 residents would need to be tested in order to have a statistical power of 80% to detect a 25% increase in test scores with a type I error of 0.05. Comparisons of the proportions of patients who received appropriate VTE prophylaxis were carried out using chi‐square testing. Statistical significance was defined as a 2‐tailed P value less than 0.05.
RESULTS
Overall and Departmental Resident Test Results
One hundred and seventeen residents from all departments participated in the project including taking the pre‐ and posttests. The response rate was 44% overall and ranged from 10% to 100% for individual departments. For all residents combined, there was a statistically significant increase in scores (pretest 46.7% 15.1%, posttest 77.8% 15.1%, P < .005). Overall scores and those for individual departments are summarized in Table 2. As can be seen, there was a significant increase in test results for each department. The only exception was a department that already had a high baseline score and that had only 4 residents, limiting the power of statistical analysis. These findings suggest that the CD‐ROM intervention favorably affected resident knowledge of anticoagulation across all medical specialties tested.
| Department | n | Prescore | Postscore | P value* |
|---|---|---|---|---|
| ||||
| Cardiothoracic surgery | 1 | 72 | 83 | NA |
| Dentistry | 22 | 34.9 10.3 | 72.3 12.4 | < .0001 |
| Surgery | 19 | 52.6 14.5 | 77.1 14.5 | < .0001 |
| Medicine | 21 | 54.3 11.6 | 84.0 8.9 | < .0001 |
| Emergency medicine | 4 | 61.3 4.5 | 94.3 8.0 | < .05 |
| Otolaryngology | 5 | 48.8 5.0 | 80.0 11.6 | < .01 |
| Urology | 4 | 66.5 23.6 | 84.5 15.8 | 0.15 |
| Neurology | 10 | 42.1 11.5 | 68.8 18.9 | < .01 |
| Orthopedics | 12 | 43.4 16.0 | 70.4 24.1 | < .01 |
| Obstetrics/gynecology | 19 | 41.4 13.1 | 81.8 11.0 | < .0001 |
| ALL | 117 | 46.7 15.1 | 77.8 15.1 | < .005 |
Assessment of Independent Effect of CD‐ROM Intervention
To determine what independent effect the CD‐ROM intervention might have, given that scores may improve with the acquisition of clinical experience alone, in July 2004 we tested internal medicine categorical PGY‐1s at our institution and at another tertiary‐care hospital, as described in the Methods section. The results of testing both groups are shown in Figure 1. Nineteen medical PGY‐1s at our hospital (hospital A) completed the anticoagulation pretest, and 16 completed the posttest. Twenty‐two medical PGY‐1s completed the pretest, and 17 completed the posttest at our neighboring hospital (hospital B). Although posttest scores were higher at both institutions, the increase in scores at our institution, which received the CD‐ROM intervention, was statistically significant, whereas the increase for the group not receiving the intervention was not significant. These findings suggest that the CD‐ROM intervention may have had an independent effect on resident knowledge of anticoagulation.
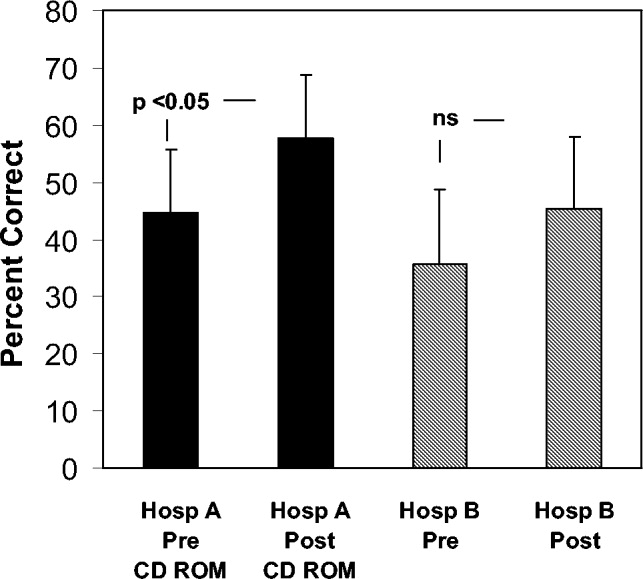
Effect of CD‐ROM Intervention on Resident Use of VTE Prophylaxis
Appropriate use of VTE prophylaxis by residents was assessed at 3 points, as detailed in the Methods section: 1 year before the CD‐ROM intervention (baseline), immediately after the CD‐ROM intervention, and 7 months after the CD‐ROM intervention. VTE prophylaxis, one element of the CD‐ROM, was chosen as a surrogate marker for the impact of the CD‐ROM initiative. A review of 40 charts of patients with the specified diagnoses (100% of the patients with the specified diagnoses, which represented about one third of admissions to the unit) before the CD‐ROM intervention revealed that 30 patients (75%) received appropriate VTE prophylaxis. A review of 38 charts after the CD‐ROM intervention showed that 36 patients (95%) received appropriate prophylaxis; similar findings were obtained 7 months after the CD‐ROM intervention (33 of 35 patients, 94%, P = .0107). These findings, which are shown in Figure 2, suggest that the CD‐ROM intervention enhanced resident compliance with VTE prophylaxis guidelines and that this effect was sustained for at least 7 months.
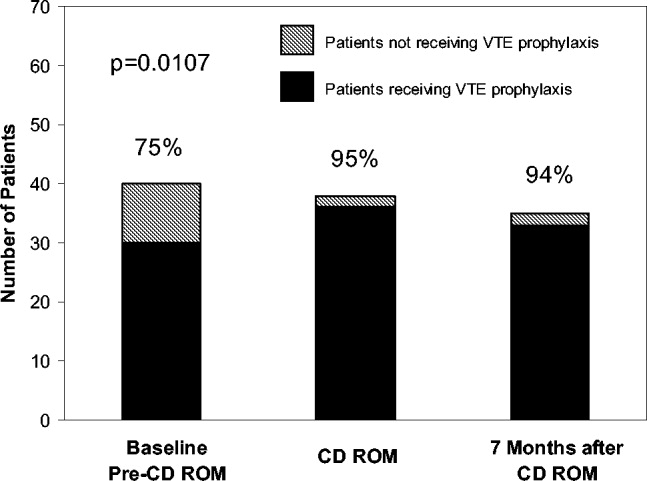
DISCUSSION
Residency training is facing challenges on several fronts. In addition to substantially changed educational requirements, strict limits on the amount of time that trainees can spend in the hospital have resulted from ACGME requirements and several state laws. Residents who are on night rotations or were on call the night before often miss educational conferences or must choose between attending patient carerelated activities and educational sessions. Time constraints on faculty have compounded this problem, and for residents to effectively learn, the focus of graduate medical education may need to shift somewhat from teaching medical information toward teaching the practice of self‐directed learning, with CD‐ROMs one such mode by which this can take place. Accomplishing this will require novel teaching approaches, and residency programs will need to document their effectiveness.
In this study we demonstrated that our residents increased their knowledge and improved their patient care practices using a CD‐ROM‐based educational tool. Residents frequently make use of computer‐based educational resources in the form of journals, textbooks, informational databases such as comprehensive drug listings, and personal digital assistantbased tools. Advantages of the computer‐based learning format include increased accessibility and flexibility in viewing the material. Residents have the option of repeated screening as desired and of viewing the CD‐ROM in segments if necessary. Although residents often must choose to attend a scheduled traditional lecture or engage in a patient carerelated activity, the CD‐ROM format allows the resident to choose the ideal time and setting to engage in structured educational activities. Other advantages of the CD‐ROM format would be ease of monitoring for accuracy, applicability, and comprehensiveness as well as more flexibility in faculty time commitments. It should be noted that we have no information about how much time residents devoted to the CD‐ROM program and how often they may have returned to the module for review. It should also be noted that although there have been some reports suggesting that CD‐ROM‐based education may play a useful role in student and perhaps resident education,1618 there is no evidence to date demonstrating that widespread use of CD‐ROMs in residency training can differentially affect resident behavior compared with the use of traditional methods.
A number of variables could have affected our results. For overall test scores, the response rate was less than 50%, with variability between departments suggesting that perhaps it was more motivated residents who participated and were therefore more likely to demonstrate improvement. Although our data comparing institutions with and without the CD‐ROM intervention suggested that the CD‐ROM intervention had a discernable effect on resident knowledge, we must also consider the possibility that the 2 groups might not have been comparable, as attitudes, expectations, and other variables might have differed. All the residents were categorical trainees, and given the similarities in many aspects of the training programs in these 2 tertiary‐care hospitals, as described in the Methods section, it is hoped that any such differences were minor. Nevertheless, this must be considered a limitation of our study. Also of note, the number of trainees was small, as was the patient population studied with VTE prophylaxis; hence, we recognize that our work can best be regarded as a pilot study using an alternative learning method. We also realize that giving a group of residents a test followed by distribution of a CD‐ROM might have suggested that we were directing them toward a goal, and this may have affected the results. Heightened awareness of the importance of anticoagulation from the introduction of new guidelines and other variables also could have affected our findings. The taking of an examination itself might also have had an impact on knowledge that could affect subsequent test scores. An additional point to consider is that if knowledge and patient care did improve, we do not know whether this affects residents acquiring other knowledge or whether this will translate into improved patient care in other areas.
Although CD‐ROM‐based learning could serve a useful function in the increasingly complex environment of residency training, this learning method also has disadvantages, including not providing personal contact or having the capability of question‐and‐answer sessions between teacher and resident. This could be overcome by providing time for faculty‐precepted question‐and‐answer sessions or perhaps creating a Web‐based venue for questions to be submitted and answered. In addition, the CD‐ROMs themselves can be designed in an interactive format in which residents can provide answers to clinical questions with feedback based on their selections provided as part of the CD‐ROM program.
In summary, the CD‐ROM‐based program in this study appears to have had an effect on not only knowledge but also patient care practice and suggests that this type of format could serve a useful role in residency training. Studies of additional interventions such as this one might allow for more extensive evaluation of the utility of CD‐ROM‐based learning as a residency training tool.
- ,,, et al., for theResidency Review Committee for Internal Medicine.A new model for accreditation of residency programs in internal medicine.Ann Intern Med.2004;140:902–909.
- ,,.Technology‐based vs. traditional instruction. A comparison of two methods for teaching the skill of performing a 12‐lead ECG.Nurs Educ Perspect.2003;24:70–74.
- ,,.Canadian physical therapists' interest in web‐based and computer‐assisted continuing education.Phys Ther.2005;85:226–237.
- ,,,,,.Computer Assisted Learning is an effective way of teaching endocrinology.Clin Endocrinol.2001;55:537–542.
- ,.Web‐based minimally invasive surgery training: competency assessment in PGY 1‐2 surgical residents.Curr Surg.2004;61:120–124.
- ,.Use of computer‐assisted learning module to achieve ACGME competencies in orthopaedic foot and ankle surgery.Foot Ankle Int.2003;24:938–941.
- ,,,.Tutor versus computer: a prospective comparison of interactive tutorial and computer‐assisted instruction in radiology education.Acad Radiol.2002;9:40–49.
- ,,, et al.Using simulation to instruct emergency medicine residents in cognitive forcing strategies.Acad Med.2004;79:438–446
- ,,, et al.Successful implementation of a novel internet hybrid surgery curriculum: the early phase outcome of thoracic surgery prerequisite curriculum e‐learning project.Ann Surg.2004;240:499–507.
- ,.Development and evaluation of a CD‐ROM computer program to teach residents telephone management.Pediatrics.1998;101:E2.
- ,,,,.The Pediatric Residency Training on Tobacco Project: baseline findings from the resident tobacco survey and observed structured clinical examinations.Prev Med.2004;39:507–516.
- ,,,.Development of a CD‐ROM Internet hybrid: a new thoracic surgery curriculum.Ann Thorac Surg.2002;74:1741–1746
- ,,, et al.A population‐based perspective of the hospital incidence and case fatality rates of deep venous thrombosis and pulmonary embolism. The Worcester DVT Study.Arch Intern Med.1991;151:933–938.
- ,,,,.Relationship between time to achieve the lower limit of the APTT therapeutic range and recurrent venous thromboembolism during heparin treatment for deep vein thrombosis.Arch Intern Med.1997;22:2562–2568.
- ,,;American College of Chest Physicians.The sixth (2000) ACCP guidelines for antithrombotic therapy for prevention and treatment of thrombosis.American College of Chest Physicians.Chest.2001;119(1 Suppl):1S–2S.
- ,,.Student assessment of the educational benefits of using a CD‐ROM for instruction of basic surgical skills.J Vet Med Educ.2005;32:138–143.
- .A multimedia CD‐ROM tool to improve student understanding of bile salts and bilirubin metabolism: evaluation of its use in a medical hybrid PBL course.Adv Physiol Educ.2005;29:40–50.
- ,,,.Testing a multimedia module in cancer pain management.J Cancer Educ.1999;14:161–163.
Given recent changes in the goals and objectives of residency training as well as changes in the functioning of teaching hospitals, traditional educational formats may need to be supplemented or replaced.1 The Accreditation Council for Graduate Medical Education (ACGME) is promoting changes in resident education with the goal of not only enhancing trainee competency using innovative methods but also of demonstrating that these educational innovations result in enhanced quality of patient care and improved patient safety.1 A challenging aspect of these initiatives is that programs are working to implement them at a time when there are greater nonteaching demands on faculty time, mandated resident work‐hour limitations have been instituted, in some states by law, and resident patient care and educational activities are prone to disruptions inherent in caring for patients in a complex health system. Various solutions have been proposed including increased incorporation of self‐directed learning as a means of meeting modern resident educational challenges, yet the ideal tools with which to accomplish this are unknown.
Computer‐based instruction in medicine has been available since the 1960s, and although its use had initially been more widespread in medical student, nursing, allied health professional, and patient education,2, 3 it is being increasingly incorporated into resident education as well. Some studies have shown that for medical students, computer‐based teaching is at least as effective in improving knowledge as conventional lectures4 and that learners' satisfaction with computer‐based formats appears comparable with that of traditional didactic lectures.5 In recent years computer‐based teaching has been applied to resident education in various fields including surgery and surgical subspecialties, pediatrics, and obstetrics and gynecology.612 Little is known, however, about how computer‐based educational methods affect resident knowledge and especially how these methods might affect clinical practice.
Venous thromboembolism (VTE) is a common and hazardous complication of acute inpatient hospitalization.13 Recognizing that errors in proper prescribing and monitoring of anticoagulants are a major cause of acute inpatient morbidity and mortality,14 we began an initiative to educate our residents and improve their patient care practices regarding the proper use of anticoagulants. To accomplish this, we developed a CD‐ROM‐based learning module with the aim of increasing resident knowledge of anticoagulation as well as compliance with national standards for VTE prevention. In this study we assessed the impact of the CD‐ROM intervention on resident knowledge and their appropriate use of VTE prophylaxis.
METHODS
The study was approved by the institutional review board. With the participation of faculty educators in the departments of medicine, surgery, and neurology, one of the authors (H.K.) coordinated the development of a CD‐ROM containing concise modules on core topics in anticoagulation (Table 1). The presenters for these topics included the director of clinical hematology, 2 cardiologists including the director of the coronary care unit, the director of the medical intensive care unit, the director of cerebrovascular diseases, and 2 vascular surgeons, one of whom serves as vice chair of surgery. These modules, each lasting about 1 hour, had audio and slide components detailing the proper indications, monitoring, and efficacy of anticoagulants in atrial fibrillation, acute ischemic stroke, acute coronary syndromes, and VTE prevention in acutely ill hospitalized patients. The guidelines presented were based on the sixth (2000) ACCP guidelines for antithrombotic therapy for the prevention and treatment of thrombosis.15 The content of the CD‐ROM was reviewed for accuracy by the authors, though none of them were speakers. We asked that before all current residents in the departments of cardiothoracic surgery, emergency medicine, otolaryngology, internal medicine, neurosurgery, dental medicine, neurology, obstetrics and gynecology, orthopedics, surgery, and urology viewed the CD‐ROM, they complete a pretest to determine their baseline knowledge of this subject. After completing the pretest, the residents were required to view the CD‐ROM and retake the same test. We then compared pre‐ and posttest scores.
| Overview of anticoagulation |
| Venous thromboembolism |
| Atrial fibrillation |
| Unfractionated heparin in acute coronary syndrome |
| Treatment of thromboembolic events with intravenous heparin |
| Anticoagulants in the management of patients with acute ischemic stroke |
| Deep venous thrombosis prophylaxis |
To determine whether an increase in knowledge was secondary to the CD‐ROM intervention or simply a consequence of acquired clinical experience during training, we compared test scores of residents who did and did not receive the CD‐ROM intervention. In the academic year following our initial testing, we asked the incoming categorical medical PGY‐1 classes at our hospital and at a comparable local tertiary‐care hospital in our health system 2 miles away to take an anticoagulation pretest (different from the examination given for the initial testing) during their PGY‐1 orientations. The 2 institutions are comparable in many ways including in patient demographics and size and most residents come from the same medical schools, have a similar rotation structure, and use a comparable curriculum under a unified graduate medical education office. The CD‐ROMs were only given to categorical PGY‐1 residents at our institution. Both groups then retook the same test (posttest) 3 months into their clinical training, a time chosen because it is when all PGY‐1s would be expected to have gained significant clinical experience on the medical wards and or in the intensive care units. The exam questions were generated by one of the authors (B.M.) and covered all the topics in the CD‐ROM.
An Anticoagulation Steering Committee was formed to assess whether the CD‐ROM intervention affected our residents' patient care practices. None of the members of this committee were authors of this work. Members of this committee reviewed inpatient charts and documented resident compliance with VTE prevention standards during periods before and after they had viewed the CD‐ROMs. We chose this particular portion of the CD‐ROM because at both the test and control hospitals, initiatives were underway using order sets to improve anticoagulation in cardiac, neurological, and surgical patients but not in VTE prophylaxis. Charts from the same 2 nursing units on the medical service were reviewed in each period and included patients with a discharge diagnosis of congestive heart failure, any oncologic diagnosis, or sepsis. The chart review tool was developed by the anticoagulation committee and included a thrombosis risk factor assessment section as well as a list of contraindications to anticoagulation to determine if anticoagulation was appropriately implemented. Charts were reviewed for compliance with VTE prophylaxis after the CD‐ROM intervention (given in July 2004) in August 2004. To have a comparable pre‐CD‐ROM comparison, charts of patients with the diagnoses stated above were reviewed from August of the preceding year. The same month was chosen in the previous year to minimize any impact of resident experience, which would likely be a confounding factor if charts from May or June of the academic year were used as a control, for example. To determine whether an improvement in adherence to VTE prophylaxis standards was sustained, an additional chart review was carried out 7 months after the initial CD‐ROM viewing. The same group of observers, none of whom were authors, did all the chart assessments.
Statistics
Continuous variables are reported as means SDs. Comparisons of test scores before and after the CD‐ROM intervention were carried out using paired t testing. Comparisons of pre‐ and posttest scores between both institutions were carried out using analysis of variance with Tukey‐Kramer multiple‐comparisons testing (GraphPad InStat Statistical Software, version 3.01, GraphPad Software, Inc.). We calculated that 13 residents would need to be tested in order to have a statistical power of 80% to detect a 25% increase in test scores with a type I error of 0.05. Comparisons of the proportions of patients who received appropriate VTE prophylaxis were carried out using chi‐square testing. Statistical significance was defined as a 2‐tailed P value less than 0.05.
RESULTS
Overall and Departmental Resident Test Results
One hundred and seventeen residents from all departments participated in the project including taking the pre‐ and posttests. The response rate was 44% overall and ranged from 10% to 100% for individual departments. For all residents combined, there was a statistically significant increase in scores (pretest 46.7% 15.1%, posttest 77.8% 15.1%, P < .005). Overall scores and those for individual departments are summarized in Table 2. As can be seen, there was a significant increase in test results for each department. The only exception was a department that already had a high baseline score and that had only 4 residents, limiting the power of statistical analysis. These findings suggest that the CD‐ROM intervention favorably affected resident knowledge of anticoagulation across all medical specialties tested.
| Department | n | Prescore | Postscore | P value* |
|---|---|---|---|---|
| ||||
| Cardiothoracic surgery | 1 | 72 | 83 | NA |
| Dentistry | 22 | 34.9 10.3 | 72.3 12.4 | < .0001 |
| Surgery | 19 | 52.6 14.5 | 77.1 14.5 | < .0001 |
| Medicine | 21 | 54.3 11.6 | 84.0 8.9 | < .0001 |
| Emergency medicine | 4 | 61.3 4.5 | 94.3 8.0 | < .05 |
| Otolaryngology | 5 | 48.8 5.0 | 80.0 11.6 | < .01 |
| Urology | 4 | 66.5 23.6 | 84.5 15.8 | 0.15 |
| Neurology | 10 | 42.1 11.5 | 68.8 18.9 | < .01 |
| Orthopedics | 12 | 43.4 16.0 | 70.4 24.1 | < .01 |
| Obstetrics/gynecology | 19 | 41.4 13.1 | 81.8 11.0 | < .0001 |
| ALL | 117 | 46.7 15.1 | 77.8 15.1 | < .005 |
Assessment of Independent Effect of CD‐ROM Intervention
To determine what independent effect the CD‐ROM intervention might have, given that scores may improve with the acquisition of clinical experience alone, in July 2004 we tested internal medicine categorical PGY‐1s at our institution and at another tertiary‐care hospital, as described in the Methods section. The results of testing both groups are shown in Figure 1. Nineteen medical PGY‐1s at our hospital (hospital A) completed the anticoagulation pretest, and 16 completed the posttest. Twenty‐two medical PGY‐1s completed the pretest, and 17 completed the posttest at our neighboring hospital (hospital B). Although posttest scores were higher at both institutions, the increase in scores at our institution, which received the CD‐ROM intervention, was statistically significant, whereas the increase for the group not receiving the intervention was not significant. These findings suggest that the CD‐ROM intervention may have had an independent effect on resident knowledge of anticoagulation.

Effect of CD‐ROM Intervention on Resident Use of VTE Prophylaxis
Appropriate use of VTE prophylaxis by residents was assessed at 3 points, as detailed in the Methods section: 1 year before the CD‐ROM intervention (baseline), immediately after the CD‐ROM intervention, and 7 months after the CD‐ROM intervention. VTE prophylaxis, one element of the CD‐ROM, was chosen as a surrogate marker for the impact of the CD‐ROM initiative. A review of 40 charts of patients with the specified diagnoses (100% of the patients with the specified diagnoses, which represented about one third of admissions to the unit) before the CD‐ROM intervention revealed that 30 patients (75%) received appropriate VTE prophylaxis. A review of 38 charts after the CD‐ROM intervention showed that 36 patients (95%) received appropriate prophylaxis; similar findings were obtained 7 months after the CD‐ROM intervention (33 of 35 patients, 94%, P = .0107). These findings, which are shown in Figure 2, suggest that the CD‐ROM intervention enhanced resident compliance with VTE prophylaxis guidelines and that this effect was sustained for at least 7 months.

DISCUSSION
Residency training is facing challenges on several fronts. In addition to substantially changed educational requirements, strict limits on the amount of time that trainees can spend in the hospital have resulted from ACGME requirements and several state laws. Residents who are on night rotations or were on call the night before often miss educational conferences or must choose between attending patient carerelated activities and educational sessions. Time constraints on faculty have compounded this problem, and for residents to effectively learn, the focus of graduate medical education may need to shift somewhat from teaching medical information toward teaching the practice of self‐directed learning, with CD‐ROMs one such mode by which this can take place. Accomplishing this will require novel teaching approaches, and residency programs will need to document their effectiveness.
In this study we demonstrated that our residents increased their knowledge and improved their patient care practices using a CD‐ROM‐based educational tool. Residents frequently make use of computer‐based educational resources in the form of journals, textbooks, informational databases such as comprehensive drug listings, and personal digital assistantbased tools. Advantages of the computer‐based learning format include increased accessibility and flexibility in viewing the material. Residents have the option of repeated screening as desired and of viewing the CD‐ROM in segments if necessary. Although residents often must choose to attend a scheduled traditional lecture or engage in a patient carerelated activity, the CD‐ROM format allows the resident to choose the ideal time and setting to engage in structured educational activities. Other advantages of the CD‐ROM format would be ease of monitoring for accuracy, applicability, and comprehensiveness as well as more flexibility in faculty time commitments. It should be noted that we have no information about how much time residents devoted to the CD‐ROM program and how often they may have returned to the module for review. It should also be noted that although there have been some reports suggesting that CD‐ROM‐based education may play a useful role in student and perhaps resident education,1618 there is no evidence to date demonstrating that widespread use of CD‐ROMs in residency training can differentially affect resident behavior compared with the use of traditional methods.
A number of variables could have affected our results. For overall test scores, the response rate was less than 50%, with variability between departments suggesting that perhaps it was more motivated residents who participated and were therefore more likely to demonstrate improvement. Although our data comparing institutions with and without the CD‐ROM intervention suggested that the CD‐ROM intervention had a discernable effect on resident knowledge, we must also consider the possibility that the 2 groups might not have been comparable, as attitudes, expectations, and other variables might have differed. All the residents were categorical trainees, and given the similarities in many aspects of the training programs in these 2 tertiary‐care hospitals, as described in the Methods section, it is hoped that any such differences were minor. Nevertheless, this must be considered a limitation of our study. Also of note, the number of trainees was small, as was the patient population studied with VTE prophylaxis; hence, we recognize that our work can best be regarded as a pilot study using an alternative learning method. We also realize that giving a group of residents a test followed by distribution of a CD‐ROM might have suggested that we were directing them toward a goal, and this may have affected the results. Heightened awareness of the importance of anticoagulation from the introduction of new guidelines and other variables also could have affected our findings. The taking of an examination itself might also have had an impact on knowledge that could affect subsequent test scores. An additional point to consider is that if knowledge and patient care did improve, we do not know whether this affects residents acquiring other knowledge or whether this will translate into improved patient care in other areas.
Although CD‐ROM‐based learning could serve a useful function in the increasingly complex environment of residency training, this learning method also has disadvantages, including not providing personal contact or having the capability of question‐and‐answer sessions between teacher and resident. This could be overcome by providing time for faculty‐precepted question‐and‐answer sessions or perhaps creating a Web‐based venue for questions to be submitted and answered. In addition, the CD‐ROMs themselves can be designed in an interactive format in which residents can provide answers to clinical questions with feedback based on their selections provided as part of the CD‐ROM program.
In summary, the CD‐ROM‐based program in this study appears to have had an effect on not only knowledge but also patient care practice and suggests that this type of format could serve a useful role in residency training. Studies of additional interventions such as this one might allow for more extensive evaluation of the utility of CD‐ROM‐based learning as a residency training tool.
Given recent changes in the goals and objectives of residency training as well as changes in the functioning of teaching hospitals, traditional educational formats may need to be supplemented or replaced.1 The Accreditation Council for Graduate Medical Education (ACGME) is promoting changes in resident education with the goal of not only enhancing trainee competency using innovative methods but also of demonstrating that these educational innovations result in enhanced quality of patient care and improved patient safety.1 A challenging aspect of these initiatives is that programs are working to implement them at a time when there are greater nonteaching demands on faculty time, mandated resident work‐hour limitations have been instituted, in some states by law, and resident patient care and educational activities are prone to disruptions inherent in caring for patients in a complex health system. Various solutions have been proposed including increased incorporation of self‐directed learning as a means of meeting modern resident educational challenges, yet the ideal tools with which to accomplish this are unknown.
Computer‐based instruction in medicine has been available since the 1960s, and although its use had initially been more widespread in medical student, nursing, allied health professional, and patient education,2, 3 it is being increasingly incorporated into resident education as well. Some studies have shown that for medical students, computer‐based teaching is at least as effective in improving knowledge as conventional lectures4 and that learners' satisfaction with computer‐based formats appears comparable with that of traditional didactic lectures.5 In recent years computer‐based teaching has been applied to resident education in various fields including surgery and surgical subspecialties, pediatrics, and obstetrics and gynecology.612 Little is known, however, about how computer‐based educational methods affect resident knowledge and especially how these methods might affect clinical practice.
Venous thromboembolism (VTE) is a common and hazardous complication of acute inpatient hospitalization.13 Recognizing that errors in proper prescribing and monitoring of anticoagulants are a major cause of acute inpatient morbidity and mortality,14 we began an initiative to educate our residents and improve their patient care practices regarding the proper use of anticoagulants. To accomplish this, we developed a CD‐ROM‐based learning module with the aim of increasing resident knowledge of anticoagulation as well as compliance with national standards for VTE prevention. In this study we assessed the impact of the CD‐ROM intervention on resident knowledge and their appropriate use of VTE prophylaxis.
METHODS
The study was approved by the institutional review board. With the participation of faculty educators in the departments of medicine, surgery, and neurology, one of the authors (H.K.) coordinated the development of a CD‐ROM containing concise modules on core topics in anticoagulation (Table 1). The presenters for these topics included the director of clinical hematology, 2 cardiologists including the director of the coronary care unit, the director of the medical intensive care unit, the director of cerebrovascular diseases, and 2 vascular surgeons, one of whom serves as vice chair of surgery. These modules, each lasting about 1 hour, had audio and slide components detailing the proper indications, monitoring, and efficacy of anticoagulants in atrial fibrillation, acute ischemic stroke, acute coronary syndromes, and VTE prevention in acutely ill hospitalized patients. The guidelines presented were based on the sixth (2000) ACCP guidelines for antithrombotic therapy for the prevention and treatment of thrombosis.15 The content of the CD‐ROM was reviewed for accuracy by the authors, though none of them were speakers. We asked that before all current residents in the departments of cardiothoracic surgery, emergency medicine, otolaryngology, internal medicine, neurosurgery, dental medicine, neurology, obstetrics and gynecology, orthopedics, surgery, and urology viewed the CD‐ROM, they complete a pretest to determine their baseline knowledge of this subject. After completing the pretest, the residents were required to view the CD‐ROM and retake the same test. We then compared pre‐ and posttest scores.
| Overview of anticoagulation |
| Venous thromboembolism |
| Atrial fibrillation |
| Unfractionated heparin in acute coronary syndrome |
| Treatment of thromboembolic events with intravenous heparin |
| Anticoagulants in the management of patients with acute ischemic stroke |
| Deep venous thrombosis prophylaxis |
To determine whether an increase in knowledge was secondary to the CD‐ROM intervention or simply a consequence of acquired clinical experience during training, we compared test scores of residents who did and did not receive the CD‐ROM intervention. In the academic year following our initial testing, we asked the incoming categorical medical PGY‐1 classes at our hospital and at a comparable local tertiary‐care hospital in our health system 2 miles away to take an anticoagulation pretest (different from the examination given for the initial testing) during their PGY‐1 orientations. The 2 institutions are comparable in many ways including in patient demographics and size and most residents come from the same medical schools, have a similar rotation structure, and use a comparable curriculum under a unified graduate medical education office. The CD‐ROMs were only given to categorical PGY‐1 residents at our institution. Both groups then retook the same test (posttest) 3 months into their clinical training, a time chosen because it is when all PGY‐1s would be expected to have gained significant clinical experience on the medical wards and or in the intensive care units. The exam questions were generated by one of the authors (B.M.) and covered all the topics in the CD‐ROM.
An Anticoagulation Steering Committee was formed to assess whether the CD‐ROM intervention affected our residents' patient care practices. None of the members of this committee were authors of this work. Members of this committee reviewed inpatient charts and documented resident compliance with VTE prevention standards during periods before and after they had viewed the CD‐ROMs. We chose this particular portion of the CD‐ROM because at both the test and control hospitals, initiatives were underway using order sets to improve anticoagulation in cardiac, neurological, and surgical patients but not in VTE prophylaxis. Charts from the same 2 nursing units on the medical service were reviewed in each period and included patients with a discharge diagnosis of congestive heart failure, any oncologic diagnosis, or sepsis. The chart review tool was developed by the anticoagulation committee and included a thrombosis risk factor assessment section as well as a list of contraindications to anticoagulation to determine if anticoagulation was appropriately implemented. Charts were reviewed for compliance with VTE prophylaxis after the CD‐ROM intervention (given in July 2004) in August 2004. To have a comparable pre‐CD‐ROM comparison, charts of patients with the diagnoses stated above were reviewed from August of the preceding year. The same month was chosen in the previous year to minimize any impact of resident experience, which would likely be a confounding factor if charts from May or June of the academic year were used as a control, for example. To determine whether an improvement in adherence to VTE prophylaxis standards was sustained, an additional chart review was carried out 7 months after the initial CD‐ROM viewing. The same group of observers, none of whom were authors, did all the chart assessments.
Statistics
Continuous variables are reported as means SDs. Comparisons of test scores before and after the CD‐ROM intervention were carried out using paired t testing. Comparisons of pre‐ and posttest scores between both institutions were carried out using analysis of variance with Tukey‐Kramer multiple‐comparisons testing (GraphPad InStat Statistical Software, version 3.01, GraphPad Software, Inc.). We calculated that 13 residents would need to be tested in order to have a statistical power of 80% to detect a 25% increase in test scores with a type I error of 0.05. Comparisons of the proportions of patients who received appropriate VTE prophylaxis were carried out using chi‐square testing. Statistical significance was defined as a 2‐tailed P value less than 0.05.
RESULTS
Overall and Departmental Resident Test Results
One hundred and seventeen residents from all departments participated in the project including taking the pre‐ and posttests. The response rate was 44% overall and ranged from 10% to 100% for individual departments. For all residents combined, there was a statistically significant increase in scores (pretest 46.7% 15.1%, posttest 77.8% 15.1%, P < .005). Overall scores and those for individual departments are summarized in Table 2. As can be seen, there was a significant increase in test results for each department. The only exception was a department that already had a high baseline score and that had only 4 residents, limiting the power of statistical analysis. These findings suggest that the CD‐ROM intervention favorably affected resident knowledge of anticoagulation across all medical specialties tested.
| Department | n | Prescore | Postscore | P value* |
|---|---|---|---|---|
| ||||
| Cardiothoracic surgery | 1 | 72 | 83 | NA |
| Dentistry | 22 | 34.9 10.3 | 72.3 12.4 | < .0001 |
| Surgery | 19 | 52.6 14.5 | 77.1 14.5 | < .0001 |
| Medicine | 21 | 54.3 11.6 | 84.0 8.9 | < .0001 |
| Emergency medicine | 4 | 61.3 4.5 | 94.3 8.0 | < .05 |
| Otolaryngology | 5 | 48.8 5.0 | 80.0 11.6 | < .01 |
| Urology | 4 | 66.5 23.6 | 84.5 15.8 | 0.15 |
| Neurology | 10 | 42.1 11.5 | 68.8 18.9 | < .01 |
| Orthopedics | 12 | 43.4 16.0 | 70.4 24.1 | < .01 |
| Obstetrics/gynecology | 19 | 41.4 13.1 | 81.8 11.0 | < .0001 |
| ALL | 117 | 46.7 15.1 | 77.8 15.1 | < .005 |
Assessment of Independent Effect of CD‐ROM Intervention
To determine what independent effect the CD‐ROM intervention might have, given that scores may improve with the acquisition of clinical experience alone, in July 2004 we tested internal medicine categorical PGY‐1s at our institution and at another tertiary‐care hospital, as described in the Methods section. The results of testing both groups are shown in Figure 1. Nineteen medical PGY‐1s at our hospital (hospital A) completed the anticoagulation pretest, and 16 completed the posttest. Twenty‐two medical PGY‐1s completed the pretest, and 17 completed the posttest at our neighboring hospital (hospital B). Although posttest scores were higher at both institutions, the increase in scores at our institution, which received the CD‐ROM intervention, was statistically significant, whereas the increase for the group not receiving the intervention was not significant. These findings suggest that the CD‐ROM intervention may have had an independent effect on resident knowledge of anticoagulation.

Effect of CD‐ROM Intervention on Resident Use of VTE Prophylaxis
Appropriate use of VTE prophylaxis by residents was assessed at 3 points, as detailed in the Methods section: 1 year before the CD‐ROM intervention (baseline), immediately after the CD‐ROM intervention, and 7 months after the CD‐ROM intervention. VTE prophylaxis, one element of the CD‐ROM, was chosen as a surrogate marker for the impact of the CD‐ROM initiative. A review of 40 charts of patients with the specified diagnoses (100% of the patients with the specified diagnoses, which represented about one third of admissions to the unit) before the CD‐ROM intervention revealed that 30 patients (75%) received appropriate VTE prophylaxis. A review of 38 charts after the CD‐ROM intervention showed that 36 patients (95%) received appropriate prophylaxis; similar findings were obtained 7 months after the CD‐ROM intervention (33 of 35 patients, 94%, P = .0107). These findings, which are shown in Figure 2, suggest that the CD‐ROM intervention enhanced resident compliance with VTE prophylaxis guidelines and that this effect was sustained for at least 7 months.

DISCUSSION
Residency training is facing challenges on several fronts. In addition to substantially changed educational requirements, strict limits on the amount of time that trainees can spend in the hospital have resulted from ACGME requirements and several state laws. Residents who are on night rotations or were on call the night before often miss educational conferences or must choose between attending patient carerelated activities and educational sessions. Time constraints on faculty have compounded this problem, and for residents to effectively learn, the focus of graduate medical education may need to shift somewhat from teaching medical information toward teaching the practice of self‐directed learning, with CD‐ROMs one such mode by which this can take place. Accomplishing this will require novel teaching approaches, and residency programs will need to document their effectiveness.
In this study we demonstrated that our residents increased their knowledge and improved their patient care practices using a CD‐ROM‐based educational tool. Residents frequently make use of computer‐based educational resources in the form of journals, textbooks, informational databases such as comprehensive drug listings, and personal digital assistantbased tools. Advantages of the computer‐based learning format include increased accessibility and flexibility in viewing the material. Residents have the option of repeated screening as desired and of viewing the CD‐ROM in segments if necessary. Although residents often must choose to attend a scheduled traditional lecture or engage in a patient carerelated activity, the CD‐ROM format allows the resident to choose the ideal time and setting to engage in structured educational activities. Other advantages of the CD‐ROM format would be ease of monitoring for accuracy, applicability, and comprehensiveness as well as more flexibility in faculty time commitments. It should be noted that we have no information about how much time residents devoted to the CD‐ROM program and how often they may have returned to the module for review. It should also be noted that although there have been some reports suggesting that CD‐ROM‐based education may play a useful role in student and perhaps resident education,1618 there is no evidence to date demonstrating that widespread use of CD‐ROMs in residency training can differentially affect resident behavior compared with the use of traditional methods.
A number of variables could have affected our results. For overall test scores, the response rate was less than 50%, with variability between departments suggesting that perhaps it was more motivated residents who participated and were therefore more likely to demonstrate improvement. Although our data comparing institutions with and without the CD‐ROM intervention suggested that the CD‐ROM intervention had a discernable effect on resident knowledge, we must also consider the possibility that the 2 groups might not have been comparable, as attitudes, expectations, and other variables might have differed. All the residents were categorical trainees, and given the similarities in many aspects of the training programs in these 2 tertiary‐care hospitals, as described in the Methods section, it is hoped that any such differences were minor. Nevertheless, this must be considered a limitation of our study. Also of note, the number of trainees was small, as was the patient population studied with VTE prophylaxis; hence, we recognize that our work can best be regarded as a pilot study using an alternative learning method. We also realize that giving a group of residents a test followed by distribution of a CD‐ROM might have suggested that we were directing them toward a goal, and this may have affected the results. Heightened awareness of the importance of anticoagulation from the introduction of new guidelines and other variables also could have affected our findings. The taking of an examination itself might also have had an impact on knowledge that could affect subsequent test scores. An additional point to consider is that if knowledge and patient care did improve, we do not know whether this affects residents acquiring other knowledge or whether this will translate into improved patient care in other areas.
Although CD‐ROM‐based learning could serve a useful function in the increasingly complex environment of residency training, this learning method also has disadvantages, including not providing personal contact or having the capability of question‐and‐answer sessions between teacher and resident. This could be overcome by providing time for faculty‐precepted question‐and‐answer sessions or perhaps creating a Web‐based venue for questions to be submitted and answered. In addition, the CD‐ROMs themselves can be designed in an interactive format in which residents can provide answers to clinical questions with feedback based on their selections provided as part of the CD‐ROM program.
In summary, the CD‐ROM‐based program in this study appears to have had an effect on not only knowledge but also patient care practice and suggests that this type of format could serve a useful role in residency training. Studies of additional interventions such as this one might allow for more extensive evaluation of the utility of CD‐ROM‐based learning as a residency training tool.
- ,,, et al., for theResidency Review Committee for Internal Medicine.A new model for accreditation of residency programs in internal medicine.Ann Intern Med.2004;140:902–909.
- ,,.Technology‐based vs. traditional instruction. A comparison of two methods for teaching the skill of performing a 12‐lead ECG.Nurs Educ Perspect.2003;24:70–74.
- ,,.Canadian physical therapists' interest in web‐based and computer‐assisted continuing education.Phys Ther.2005;85:226–237.
- ,,,,,.Computer Assisted Learning is an effective way of teaching endocrinology.Clin Endocrinol.2001;55:537–542.
- ,.Web‐based minimally invasive surgery training: competency assessment in PGY 1‐2 surgical residents.Curr Surg.2004;61:120–124.
- ,.Use of computer‐assisted learning module to achieve ACGME competencies in orthopaedic foot and ankle surgery.Foot Ankle Int.2003;24:938–941.
- ,,,.Tutor versus computer: a prospective comparison of interactive tutorial and computer‐assisted instruction in radiology education.Acad Radiol.2002;9:40–49.
- ,,, et al.Using simulation to instruct emergency medicine residents in cognitive forcing strategies.Acad Med.2004;79:438–446
- ,,, et al.Successful implementation of a novel internet hybrid surgery curriculum: the early phase outcome of thoracic surgery prerequisite curriculum e‐learning project.Ann Surg.2004;240:499–507.
- ,.Development and evaluation of a CD‐ROM computer program to teach residents telephone management.Pediatrics.1998;101:E2.
- ,,,,.The Pediatric Residency Training on Tobacco Project: baseline findings from the resident tobacco survey and observed structured clinical examinations.Prev Med.2004;39:507–516.
- ,,,.Development of a CD‐ROM Internet hybrid: a new thoracic surgery curriculum.Ann Thorac Surg.2002;74:1741–1746
- ,,, et al.A population‐based perspective of the hospital incidence and case fatality rates of deep venous thrombosis and pulmonary embolism. The Worcester DVT Study.Arch Intern Med.1991;151:933–938.
- ,,,,.Relationship between time to achieve the lower limit of the APTT therapeutic range and recurrent venous thromboembolism during heparin treatment for deep vein thrombosis.Arch Intern Med.1997;22:2562–2568.
- ,,;American College of Chest Physicians.The sixth (2000) ACCP guidelines for antithrombotic therapy for prevention and treatment of thrombosis.American College of Chest Physicians.Chest.2001;119(1 Suppl):1S–2S.
- ,,.Student assessment of the educational benefits of using a CD‐ROM for instruction of basic surgical skills.J Vet Med Educ.2005;32:138–143.
- .A multimedia CD‐ROM tool to improve student understanding of bile salts and bilirubin metabolism: evaluation of its use in a medical hybrid PBL course.Adv Physiol Educ.2005;29:40–50.
- ,,,.Testing a multimedia module in cancer pain management.J Cancer Educ.1999;14:161–163.
- ,,, et al., for theResidency Review Committee for Internal Medicine.A new model for accreditation of residency programs in internal medicine.Ann Intern Med.2004;140:902–909.
- ,,.Technology‐based vs. traditional instruction. A comparison of two methods for teaching the skill of performing a 12‐lead ECG.Nurs Educ Perspect.2003;24:70–74.
- ,,.Canadian physical therapists' interest in web‐based and computer‐assisted continuing education.Phys Ther.2005;85:226–237.
- ,,,,,.Computer Assisted Learning is an effective way of teaching endocrinology.Clin Endocrinol.2001;55:537–542.
- ,.Web‐based minimally invasive surgery training: competency assessment in PGY 1‐2 surgical residents.Curr Surg.2004;61:120–124.
- ,.Use of computer‐assisted learning module to achieve ACGME competencies in orthopaedic foot and ankle surgery.Foot Ankle Int.2003;24:938–941.
- ,,,.Tutor versus computer: a prospective comparison of interactive tutorial and computer‐assisted instruction in radiology education.Acad Radiol.2002;9:40–49.
- ,,, et al.Using simulation to instruct emergency medicine residents in cognitive forcing strategies.Acad Med.2004;79:438–446
- ,,, et al.Successful implementation of a novel internet hybrid surgery curriculum: the early phase outcome of thoracic surgery prerequisite curriculum e‐learning project.Ann Surg.2004;240:499–507.
- ,.Development and evaluation of a CD‐ROM computer program to teach residents telephone management.Pediatrics.1998;101:E2.
- ,,,,.The Pediatric Residency Training on Tobacco Project: baseline findings from the resident tobacco survey and observed structured clinical examinations.Prev Med.2004;39:507–516.
- ,,,.Development of a CD‐ROM Internet hybrid: a new thoracic surgery curriculum.Ann Thorac Surg.2002;74:1741–1746
- ,,, et al.A population‐based perspective of the hospital incidence and case fatality rates of deep venous thrombosis and pulmonary embolism. The Worcester DVT Study.Arch Intern Med.1991;151:933–938.
- ,,,,.Relationship between time to achieve the lower limit of the APTT therapeutic range and recurrent venous thromboembolism during heparin treatment for deep vein thrombosis.Arch Intern Med.1997;22:2562–2568.
- ,,;American College of Chest Physicians.The sixth (2000) ACCP guidelines for antithrombotic therapy for prevention and treatment of thrombosis.American College of Chest Physicians.Chest.2001;119(1 Suppl):1S–2S.
- ,,.Student assessment of the educational benefits of using a CD‐ROM for instruction of basic surgical skills.J Vet Med Educ.2005;32:138–143.
- .A multimedia CD‐ROM tool to improve student understanding of bile salts and bilirubin metabolism: evaluation of its use in a medical hybrid PBL course.Adv Physiol Educ.2005;29:40–50.
- ,,,.Testing a multimedia module in cancer pain management.J Cancer Educ.1999;14:161–163.
Copyright © 2008 Society of Hospital Medicine
Case Report
Hyperhemolysis syndrome is a form of atypical hemolytic transfusion reaction (HTR). It is characterized by a significant drop in hemoglobin (Hb) after seemingly compatible red blood cell transfusions, suggesting destruction of both transfused and autologous red blood cells. Its pathophysiology is not well understood, and a serologic cause is often not identified.14 In contrast, delayed HTRs are typically characterized by a positive direct antiglobulin test (DAT), suggesting that the patient's red blood cells are coated by immunoglobulin G and/or complement components and by the appearance of previously undetected red blood cell alloantibody or antibodies that developed from a secondary anamnestic response; however, autologous red cells are not destroyed.
CASE
A 48‐year‐old African American woman with sickle cell disease (SCD) was readmitted for pain crisis. Her medical history included stroke, pulmonary hypertension, and congestive heart failure. She had received several transfusions and consequently had developed antibodies to seven clinically significant red blood cell antigens. A week prior to readmission, she was discharged from the hospital with an Hb of 6.9 g/dL after a sickle cell crisis precipitated by pneumonia. She was treated with hydration, pain medications, antibiotics, and a unit of cross‐match‐compatible red blood cells (RBCs) that was antigen negative for her antibodies.
On readmission, she had an Hb of 5.6 g/dL and an uncorrected reticulocyte count of 17.6%. Her reticulocyte production index, a reticulocyte count corrected for the degree of anemia and reticulocyte maturation time, was elevated at 2.6. She was transfused with 1 unit of phenotypically matched and cross‐match‐compatible RBCs. Three hours after transfusion, she developed dark‐colored urine. The transfusion reaction investigation revealed no clerical error or incompatibility, a negative DAT, and an antibody panel identical to that from pretransfusion testing. During hospitalization, the hemolytic anemia worsened (Fig. 1). On the 10th hospital day, she became severely dyspneic as her Hb reached its nadir of 3.6 g/dL despite ongoing erythropoiesis. She developed decompensated heart failure and renal insufficiency, precipitated by the acutely worsening anemia. Along with diuretic and vasodilator therapies, she was treated with methylprednisolone at 125 mg twice daily for 3 days followed by tapering doses of prednisone for 2 weeks, intravenous immunoglobulin (IVIG) at 400 mg/kg a day for 5 days, and 4 cross‐match‐compatible RBC transfusions that were antigen negative for her antibodies. The hemolysis resolved and the patient improved. Throughout hospitalization, her DAT remained negative. The Hb remained stable at 7 g/dL until she was discharged. Ten months of follow‐up showed no new red blood cell antibody in her serum or recurrence of hyperhemolysis syndrome despite receiving subsequent transfusions.
DISCUSSION
Hyperhemolysis syndrome has been described in patients with SCD,14, 6, 7 suggesting that an underlying hemoglobinopathy may be a risk factor; however, a patient with anemia of chronic disease was recently described in the literature to have developed hyperhemolysis syndrome.5 Possible mechanisms include innocent bystander hemolysis through complement‐mediated lysis and/or formation of red blood cell alloantibody or autoantibody;1, 2 and hyperactive macrophages of the reticuloendothelial system that recognize Hb S RBCs of patients with SCD more avidly than normal RBCs because of the exposure of aminophosphatides in the outer layer of the sickled RBC membrane.3 In effect, red blood cells may be destroyed regardless of whether they are autologous or transfused. Additionally, transfusion‐related suppression of erythropoiesis may worsen the severity of anemia.2 Recent studies of patients with SCD suggest that the presence of free plasma Hb, as a consequence of hemolysis, reduces nitric oxide bioavailability, promotes endothelial dysfunction, and contributes to the development of pulmonary hypertension and the varying presentations of vasoocclusion.6 A common observation among patients who experience hyperhemolysis syndrome is that withholding transfusion seems beneficial, probably because immunologic reactions are not exacerbated, whereas treatment with steroids1, 2, 4 and/or IVIG3, 7 resolves hemolysis because of their immunomodulatory effects.
CONCLUSIONS
Hyperhemolysis syndrome is a potentially life‐threatening complication of RBC transfusion. It is important to recognize this syndrome when managing patients with SCD who present with worsening anemia after RBC transfusions. Although further transfusions can exacerbate hemolysis4, 7 and may be relatively contraindicated, in severe and desperate situations, simultaneous treatment with steroids and IVIG, together with RBC transfusions, may be lifesaving.
- ,,,,.Delayed hemolytic transfusion reactions in sickle cell disease: simultaneous destruction of recipients' red cells.Transfusion.1997;37:376–381.
- ,,,,.The sickle cell hemolytic transfusion reaction syndrome.Transfusion.1997;37:382–392.
- ,,,,.Hyperhemolytic transfusion reaction in sickle cell disease.Transfusion.2001;41:323–328.
- ,,,,.Delayed hemolytic transfusion reaction/hyperhemolysis syndrome in children with sickle cell disease.Pediatrics.2003;111(6 Pt 1):e661–e665.
- ,.Hyperhemolysis syndrome in anemia of chronic disease.Transfusion.2005;45:1930–1933.
- and.Hyperhemolysis during the evolution of uncomplicated acute painful episodes in patients with sickle cell anemia.Transfusion.2006;46:105–110.
- ,,,.Post‐transfusion hyperhemolysis in a patient with sickle cell disease: use of steroids and intravenous immunoglobulin to prevent further red cell destruction.Vox Sang.1995;69:355–357.
Hyperhemolysis syndrome is a form of atypical hemolytic transfusion reaction (HTR). It is characterized by a significant drop in hemoglobin (Hb) after seemingly compatible red blood cell transfusions, suggesting destruction of both transfused and autologous red blood cells. Its pathophysiology is not well understood, and a serologic cause is often not identified.14 In contrast, delayed HTRs are typically characterized by a positive direct antiglobulin test (DAT), suggesting that the patient's red blood cells are coated by immunoglobulin G and/or complement components and by the appearance of previously undetected red blood cell alloantibody or antibodies that developed from a secondary anamnestic response; however, autologous red cells are not destroyed.
CASE
A 48‐year‐old African American woman with sickle cell disease (SCD) was readmitted for pain crisis. Her medical history included stroke, pulmonary hypertension, and congestive heart failure. She had received several transfusions and consequently had developed antibodies to seven clinically significant red blood cell antigens. A week prior to readmission, she was discharged from the hospital with an Hb of 6.9 g/dL after a sickle cell crisis precipitated by pneumonia. She was treated with hydration, pain medications, antibiotics, and a unit of cross‐match‐compatible red blood cells (RBCs) that was antigen negative for her antibodies.
On readmission, she had an Hb of 5.6 g/dL and an uncorrected reticulocyte count of 17.6%. Her reticulocyte production index, a reticulocyte count corrected for the degree of anemia and reticulocyte maturation time, was elevated at 2.6. She was transfused with 1 unit of phenotypically matched and cross‐match‐compatible RBCs. Three hours after transfusion, she developed dark‐colored urine. The transfusion reaction investigation revealed no clerical error or incompatibility, a negative DAT, and an antibody panel identical to that from pretransfusion testing. During hospitalization, the hemolytic anemia worsened (Fig. 1). On the 10th hospital day, she became severely dyspneic as her Hb reached its nadir of 3.6 g/dL despite ongoing erythropoiesis. She developed decompensated heart failure and renal insufficiency, precipitated by the acutely worsening anemia. Along with diuretic and vasodilator therapies, she was treated with methylprednisolone at 125 mg twice daily for 3 days followed by tapering doses of prednisone for 2 weeks, intravenous immunoglobulin (IVIG) at 400 mg/kg a day for 5 days, and 4 cross‐match‐compatible RBC transfusions that were antigen negative for her antibodies. The hemolysis resolved and the patient improved. Throughout hospitalization, her DAT remained negative. The Hb remained stable at 7 g/dL until she was discharged. Ten months of follow‐up showed no new red blood cell antibody in her serum or recurrence of hyperhemolysis syndrome despite receiving subsequent transfusions.

DISCUSSION
Hyperhemolysis syndrome has been described in patients with SCD,14, 6, 7 suggesting that an underlying hemoglobinopathy may be a risk factor; however, a patient with anemia of chronic disease was recently described in the literature to have developed hyperhemolysis syndrome.5 Possible mechanisms include innocent bystander hemolysis through complement‐mediated lysis and/or formation of red blood cell alloantibody or autoantibody;1, 2 and hyperactive macrophages of the reticuloendothelial system that recognize Hb S RBCs of patients with SCD more avidly than normal RBCs because of the exposure of aminophosphatides in the outer layer of the sickled RBC membrane.3 In effect, red blood cells may be destroyed regardless of whether they are autologous or transfused. Additionally, transfusion‐related suppression of erythropoiesis may worsen the severity of anemia.2 Recent studies of patients with SCD suggest that the presence of free plasma Hb, as a consequence of hemolysis, reduces nitric oxide bioavailability, promotes endothelial dysfunction, and contributes to the development of pulmonary hypertension and the varying presentations of vasoocclusion.6 A common observation among patients who experience hyperhemolysis syndrome is that withholding transfusion seems beneficial, probably because immunologic reactions are not exacerbated, whereas treatment with steroids1, 2, 4 and/or IVIG3, 7 resolves hemolysis because of their immunomodulatory effects.
CONCLUSIONS
Hyperhemolysis syndrome is a potentially life‐threatening complication of RBC transfusion. It is important to recognize this syndrome when managing patients with SCD who present with worsening anemia after RBC transfusions. Although further transfusions can exacerbate hemolysis4, 7 and may be relatively contraindicated, in severe and desperate situations, simultaneous treatment with steroids and IVIG, together with RBC transfusions, may be lifesaving.
Hyperhemolysis syndrome is a form of atypical hemolytic transfusion reaction (HTR). It is characterized by a significant drop in hemoglobin (Hb) after seemingly compatible red blood cell transfusions, suggesting destruction of both transfused and autologous red blood cells. Its pathophysiology is not well understood, and a serologic cause is often not identified.14 In contrast, delayed HTRs are typically characterized by a positive direct antiglobulin test (DAT), suggesting that the patient's red blood cells are coated by immunoglobulin G and/or complement components and by the appearance of previously undetected red blood cell alloantibody or antibodies that developed from a secondary anamnestic response; however, autologous red cells are not destroyed.
CASE
A 48‐year‐old African American woman with sickle cell disease (SCD) was readmitted for pain crisis. Her medical history included stroke, pulmonary hypertension, and congestive heart failure. She had received several transfusions and consequently had developed antibodies to seven clinically significant red blood cell antigens. A week prior to readmission, she was discharged from the hospital with an Hb of 6.9 g/dL after a sickle cell crisis precipitated by pneumonia. She was treated with hydration, pain medications, antibiotics, and a unit of cross‐match‐compatible red blood cells (RBCs) that was antigen negative for her antibodies.
On readmission, she had an Hb of 5.6 g/dL and an uncorrected reticulocyte count of 17.6%. Her reticulocyte production index, a reticulocyte count corrected for the degree of anemia and reticulocyte maturation time, was elevated at 2.6. She was transfused with 1 unit of phenotypically matched and cross‐match‐compatible RBCs. Three hours after transfusion, she developed dark‐colored urine. The transfusion reaction investigation revealed no clerical error or incompatibility, a negative DAT, and an antibody panel identical to that from pretransfusion testing. During hospitalization, the hemolytic anemia worsened (Fig. 1). On the 10th hospital day, she became severely dyspneic as her Hb reached its nadir of 3.6 g/dL despite ongoing erythropoiesis. She developed decompensated heart failure and renal insufficiency, precipitated by the acutely worsening anemia. Along with diuretic and vasodilator therapies, she was treated with methylprednisolone at 125 mg twice daily for 3 days followed by tapering doses of prednisone for 2 weeks, intravenous immunoglobulin (IVIG) at 400 mg/kg a day for 5 days, and 4 cross‐match‐compatible RBC transfusions that were antigen negative for her antibodies. The hemolysis resolved and the patient improved. Throughout hospitalization, her DAT remained negative. The Hb remained stable at 7 g/dL until she was discharged. Ten months of follow‐up showed no new red blood cell antibody in her serum or recurrence of hyperhemolysis syndrome despite receiving subsequent transfusions.

DISCUSSION
Hyperhemolysis syndrome has been described in patients with SCD,14, 6, 7 suggesting that an underlying hemoglobinopathy may be a risk factor; however, a patient with anemia of chronic disease was recently described in the literature to have developed hyperhemolysis syndrome.5 Possible mechanisms include innocent bystander hemolysis through complement‐mediated lysis and/or formation of red blood cell alloantibody or autoantibody;1, 2 and hyperactive macrophages of the reticuloendothelial system that recognize Hb S RBCs of patients with SCD more avidly than normal RBCs because of the exposure of aminophosphatides in the outer layer of the sickled RBC membrane.3 In effect, red blood cells may be destroyed regardless of whether they are autologous or transfused. Additionally, transfusion‐related suppression of erythropoiesis may worsen the severity of anemia.2 Recent studies of patients with SCD suggest that the presence of free plasma Hb, as a consequence of hemolysis, reduces nitric oxide bioavailability, promotes endothelial dysfunction, and contributes to the development of pulmonary hypertension and the varying presentations of vasoocclusion.6 A common observation among patients who experience hyperhemolysis syndrome is that withholding transfusion seems beneficial, probably because immunologic reactions are not exacerbated, whereas treatment with steroids1, 2, 4 and/or IVIG3, 7 resolves hemolysis because of their immunomodulatory effects.
CONCLUSIONS
Hyperhemolysis syndrome is a potentially life‐threatening complication of RBC transfusion. It is important to recognize this syndrome when managing patients with SCD who present with worsening anemia after RBC transfusions. Although further transfusions can exacerbate hemolysis4, 7 and may be relatively contraindicated, in severe and desperate situations, simultaneous treatment with steroids and IVIG, together with RBC transfusions, may be lifesaving.
- ,,,,.Delayed hemolytic transfusion reactions in sickle cell disease: simultaneous destruction of recipients' red cells.Transfusion.1997;37:376–381.
- ,,,,.The sickle cell hemolytic transfusion reaction syndrome.Transfusion.1997;37:382–392.
- ,,,,.Hyperhemolytic transfusion reaction in sickle cell disease.Transfusion.2001;41:323–328.
- ,,,,.Delayed hemolytic transfusion reaction/hyperhemolysis syndrome in children with sickle cell disease.Pediatrics.2003;111(6 Pt 1):e661–e665.
- ,.Hyperhemolysis syndrome in anemia of chronic disease.Transfusion.2005;45:1930–1933.
- and.Hyperhemolysis during the evolution of uncomplicated acute painful episodes in patients with sickle cell anemia.Transfusion.2006;46:105–110.
- ,,,.Post‐transfusion hyperhemolysis in a patient with sickle cell disease: use of steroids and intravenous immunoglobulin to prevent further red cell destruction.Vox Sang.1995;69:355–357.
- ,,,,.Delayed hemolytic transfusion reactions in sickle cell disease: simultaneous destruction of recipients' red cells.Transfusion.1997;37:376–381.
- ,,,,.The sickle cell hemolytic transfusion reaction syndrome.Transfusion.1997;37:382–392.
- ,,,,.Hyperhemolytic transfusion reaction in sickle cell disease.Transfusion.2001;41:323–328.
- ,,,,.Delayed hemolytic transfusion reaction/hyperhemolysis syndrome in children with sickle cell disease.Pediatrics.2003;111(6 Pt 1):e661–e665.
- ,.Hyperhemolysis syndrome in anemia of chronic disease.Transfusion.2005;45:1930–1933.
- and.Hyperhemolysis during the evolution of uncomplicated acute painful episodes in patients with sickle cell anemia.Transfusion.2006;46:105–110.
- ,,,.Post‐transfusion hyperhemolysis in a patient with sickle cell disease: use of steroids and intravenous immunoglobulin to prevent further red cell destruction.Vox Sang.1995;69:355–357.
Practice Patterns of Hospitalists and Community Physicians
The use of hospitalists, physicians who specialize in inpatient care, has seen a rapid expansion over the last decade.1 Several studies have shown that with hospitalists there is a shorter length of stay (LOS) and decreased utilization of resources and that hospitalists play a positive role in medical education.24 However, only a few studies have examined the specific strategies employed by hospitalists to achieve improved efficiency and outcomes.
Congestive heart failure (CHF) is the most common diagnosis of hospitalized patients older than age 65, with more Medicare spending devoted to patients with CHF than to any other diagnosis‐related group (DRG).5, 6 Over the last 2 decades hospital discharges for congestive heart failure increased by 165%.7 In addition, the rate of hospital readmission of patients with CHF remains high: 2%, 20%, and 50% within 2 days, 1 month, and 6 months, respectively.8
Several previous studies have shown that patients cared for by hospitalists had improved clinical outcomes. Meltzer et al. found that 30‐day mortality of hospitalists' patients was lower than that of non‐hospitalists' patients, 4.2% versus 6.0%, respectively, in the second year of implementation of a hospitalist program.3 A study by Huddleston et al. showed a reduction of 11.8% in the rate of complications experienced by postsurgical orthopedic patients with the involvement of hospitalists in their care in conjunction with the surgeons.4
Many previous studies have pointed to improvements in economic outcomes such as LOS and costs for patients followed by hospitalists. Kulaga et al. showed that patients cared for by hospitalists had reductions of approximately 20% in LOS and 18% in total costs per case compared with those cared for by community‐based physicians.2 Meltzer et al. found a decrease in the average adjusted LOS of 0.49 days in the second year of implementation of a hospitalist program.3 Rifkin et al. found that patients with pneumonia cared for by hospitalists had a mean adjusted LOS of 5.6 days versus 6.5 days for those cared for by non‐hospitalists.9
Few previous studies have looked at specific practice patterns of hospitalists that result in improved efficiency and better outcomes. Rifkin et al., who found that patients with pneumonia cared for by hospitalists had a shorter LOS, suggested this finding was a result of the earlier recognition by hospitalists that patients were stable and more rapid conversion to oral antibiotics.9 Likewise, Stein et al. found that community‐acquired pneumonia patients treated by hospitalists had a shorter LOS than those treated by non‐hospitalists. However, they were unable to assess the differences in patient management that led to this result because of the design of the study.10
Lindenauer et al. compared quality‐of‐care indicators and resource utilization for patients with congestive heart failure treated by hospitalists and non‐hospitalist general internists. They found that patients under the care of hospitalists had a shorter LOS than those cared for by general internists but that the overall costs of care were similar between the groups.11 They compared the quality indicators developed by the Joint Commission on Accreditation of Healthcare Organizations in the Core Measures Initiative, but did not focus on patterns of practices of hospitalists and nonhospitalists. Moreover, they did not look at full‐time hospitalists but focused on physicians who spent at least 25% of their practice caring for inpatients.
We sought to identify distinct, quantifiable practices of full‐time hospitalists in the management of their patients with CHF. We hypothesized that hospitalists would adhere more closely to the current congestive heart failure guidelines and would utilize available resources more judiciously, leading to improved clinical and economic outcomes. To identify these practices, we compared utilization of well‐established therapeutic and diagnostic modalities such as use of ACE‐I, ARB, and beta‐blockers; ordering of chest x‐rays; measurement of brain natriuretic peptide (BNP); and use of medical subspecialty consultants. We also compared standard clinical and economic outcomes such as in‐hospital mortality, readmission rate, LOS, and costs per case between hospitalists and community‐based physicians.
METHODS
Design and Setting
The study was a retrospective chart review of 447 patients treated for CHF from July 1, 2003, through June 30, 2004, at the Queen's Medical Center, a 505‐bed community‐based teaching hospital in Honolulu, Hawaii, and the leading medical referral center in the Pacific Basin. All patients had been cared for by either a community‐based physician or a hospitalist. The community‐based physicians (referred to as non‐hospitalists from here on) were a diverse group of internists and subspecialists, in solo or group practice, who provided inpatient and ambulatory care. The non‐hospitalist group included 119 cardiologists (55%), 83 general internists (38%), and 3 family practitioners (1%), with the other 6% made up of clinicians in the medical oncology, pediatrics, pulmonary, radiation oncology, and thoracic/cardiovascular surgery subspecialties.
The hospitalist group comprised 10 full‐time internists employed by the hospital who provided care for patients only in the inpatient setting and 3 part‐time hospitalists who practiced in the ambulatory setting in addition to providing inpatient night coverage for the group. During the study period, 2 hospitalists left the group, and 2 hospitalists were hired. On average the length of involvement of a full‐time hospitalist in the study was 9 months. Permission to conduct this study was granted by the Queen's Medical Center Institutional Review Board.
Patient Population
Patients were included in the study if they were admitted to Queen's Medical Center during the 18‐month study period, were at least 18 years old, and were coded on discharge by the medical records department with a principal diagnosis of congestive heart failure (International Classification of Diseases, 9th Revision, codes 428, 428.1, 428.9, 402.01, 402.11, 402.91, 404.01, 404.11, and 404.91). Baseline characteristics of patients collected were age, sex, insurance status, comorbidities, and code status on admission. Comorbidities included coronary artery disease, diabetes mellitus (type 1 or 2), hypertension, chronic renal insufficiency (creatinine > 2 mg/dL), and chronic obstructive pulmonary disease (COPD). Patients were excluded if they had initially been admitted to the medical intensive care unit, required ventilatory support, had end‐stage renal disease requiring hemodialysis, or had an LOS greater than 14 days.
Data Collection
Medical records were reviewed by research nurses not directly involved with the hospitalist group. Training to ensure high‐level reliability of data collection was provided, and reliability was verified by the primary author (M.M.R.). The following data were collected: use of ACE‐I, ARB, and beta‐blockers on admission and discharge; use of intravenous and oral diuretics; time to switch to oral diuretic; rates of utilization of medical consultants, physical therapy, dietary consults, social work, and sodium and fluid restriction; and number of repeat chest radiographs, echocardiograms, and BNP measurements. These criteria were developed based on ACC/AHA 2005 guidelines for diagnosis and management of congestive heart failure in adults,11 several studies delineating the importance of initiating therapy in the inpatient setting, and the experience of the Cardiovascular Hospital Atherosclerosis Management Program (CHAMP) for patients with established coronary artery disease.1315 Data on medical resident involvement in patient care were collected for hospitalists and non‐hospitalists.
Additional outcomes included in‐hospital mortality, rate of acute renal failure, readmission rate, LOS, expense, revenue, and margin per case. Acute renal failure was defined as a doubling of the admission creatinine value. The rate of readmissiondefined as readmission to Queen's Medical Center for any reasonwas evaluated after 7, 14, and 30 days and was stratified further for readmissions for CHF. Expense was defined as costs directly related to patient care plus costs related to operating a hospital facility. Revenue was defined as the compensation the hospital expected to collect for service rendered adjusted for bad debt/charity care. Margin was defined as revenue minus expense.
Data Analysis
Descriptive statistics are reported for baseline patient characteristics (age, sex, insurance status, etc.), quality‐of‐care measures (ACE‐I, ARB, diuretic, and beta‐blocker use, time to oral diuretic, etc.), and outcome measures (readmission rate, in‐hospital mortality, LOS, cost data) using frequencies and proportions for categorical variables (eg, sex, ethnicity, insurance status), means and standard deviations (SDs) for continuous variables (age), and medians and interquartile ranges (Q1‐Q3) for skewed variables (eg, LOS, cost data). The patients cared for by hospitalists were compared with those cared for by non‐hospitalists using the chi‐square test or Fisher's exact test for categorical data and the Student t test for continuous data. All‐Payer Severity‐adjusted Diagnosis Related Groups (APS‐DRGs) were used to control for severity of patient illness. The severity of illness codes were taken from 3M APR Benchmarking software for DRGs adjusted for severity of illness and risk of mortality. 3M defined severity of illness as the extent of physiologic decompensation or organ system loss of function. Each diagnosis was assigned 1 of 4 severity levels: minor, moderate, major, or extreme. Kruskal‐Wallis analysis of covariance was used for LOS and cost outcomes, adjusting for age, insurance status, comorbidities, and severity of illness. Multivariate logistic regression was performed for binary outcomes (eg, ACE‐I, ARB, beta‐blocker use) to adjust for confounding variables. Statistical analysis was performed using SAS version 9 (SAS Institute Inc., Cary, NC). All tests were 2‐sided, and differences with a P value < .05 were considered significant.
RESULTS
Patient Characteristics
Table 1 shows the patient characteristic data. There were 447 admissions for congestive heart failure during the study period, 342 of which met study inclusion criteria. Hospitalists provided care for 126 of these patients and non‐hospitalists for 216 patients. Mean age of patients in the hospitalist and nonhospitalist groups was 63 and 73 years, respectively. There were significant differences in insurance status, with hospitalists more frequently caring for patients covered by Medicaid (26% vs. 7%; P < .001) and patients who were uninsured (6% vs. 1%; P = .04). Patients cared for by hospitalists had a lower incidence of coronary artery disease (42% vs. 59%; P = .003) and prior CHF (44% vs. 56%; P = .05). The hospitalists' patients were more likely to have a full resuscitation code status on admission; however, this difference did not reach statistical significance (90% vs. 81%; P = .07). There were no significant differences between patients cared for by hospitalists and non‐hospitalists in sex, ethnic background, other comorbidities, or house staff involvement.
| Non‐hospitalist cases (%) (n = 216) | Hospitalist cases (%) (n = 126) | P value | |
|---|---|---|---|
| |||
| Age (years, mean SD) | 73 15 | 63 16 | < .001 |
| Male sex | 124 (57) | 78 (62) | .41 |
| Caucasian ethnicity | 41 (19) | 30 (24) | .29 |
| Insurance status | |||
| Medicare | 119 (55) | 58 (46) | .11 |
| Medicaid/Quest | 16 (7) | 33 (26) | < .001 |
| HMSA | 68 (31) | 19 (15) | < .001 |
| Self‐pay | 3 (1) | 7 (6) | .04 |
| Other | 10(5) | 9(7) | .33 |
| Comorbidy | |||
| CAD | 127 (59) | 53 (42) | .003 |
| DM | 78 (36) | 53 (4) | .27 |
| HTN | 139 (64) | 80 (63) | .87 |
| CRI | 43 (20) | 28 (22) | .61 |
| COPD | 30 (14) | 26 (21) | .10 |
| Prior CHF | 120 (56) | 56 (44) | .05 |
| Full code | 174 (81) | 113 (90) | .07 |
| House staff involvement | 42 (19) | 20 (16) | .41 |
Practice Patterns and Resource Utilization
Practice patterns and resource utilization are shown in Table 2. Hospitalists used more ACE‐I/ARBs, with 86% of patients receiving these interventions within 24 hours of admission versus 72% of the patients of non‐hospitalists (adjusted P = .001). Hospitalists treated fewer patients with beta‐blockers on admission and on discharge and more patients with intravenous diuretics (90% vs. 73%; adjusted P = .001). The rate of beta‐blocker use did not change significantly after controlling for patients with COPD (data not shown).
| Non‐hospitalist cases (%) (n = 216) | Hospitalist cases (%) (n = 126) | P value* | |
|---|---|---|---|
| |||
| ACE‐I/ARB within 24 hours | 155 (72) | 108 (86) | .001 |
| Beta‐blocker within 24 hours | 119 (55) | 50 (40) | .004 |
| ACE‐I/ARB at discharge | 147 (69) | 95 (75) | .24 |
| Beta‐blocker at discharge | 116 (54) | 52 (41) | .03 |
| Echocardiogram 1 | 125 (58) | 81 (64) | .50 |
| MD Consultants 2 | 35 (16) | 10 (8) | .01 |
| Chest x‐ray 2 | 27 (13) | 5 (4) | .02 |
| BNP 1 | 128 (59) | 95 (75) | .005 |
| BNP > 1 | 22 (10) | 7 (6) | .14 |
| Physical therapy | 35 (16) | 17 (13) | .48 |
| Dietary consult | 29 (13) | 19 (15) | .67 |
| Social work | 62 (29) | 60 (48) | .003 |
| Sodium restriction | 184 (85) | 102 (81) | .31 |
| Fluid restriction | 47 (22) | 35 (28) | .21 |
| IV diuretic | 158 (73) | 114 (90) | .001 |
| Time to oral diuretic (days), median (Q1,Q3) | 1 (1, 3) | 1 (0, 2) | .30 |
Hospitalists were less likely to obtain 2 or more chest x‐rays (4% vs. 13%; adjusted P = .02) or to obtain 2 or more medical consultations (8% vs. 16%; adjusted P = .01). In addition, they obtained more initial measurements of BNP; however, there was a trend toward fewer repeat BNP measurements (6% vs. 10%; P = .14). There was a significantly higher rate of social work utilization by hospitalists than by nonhospitalists (48% vs. 29%; adjusted P = .003). There were no differences between the groups in the rates of obtaining echocardiograms, physical therapy, and dietary consults or in sodium and fluid restrictions.
Outcomes
Significant differences were noted in LOS and cost outcomes between hospitalists and non‐hospitalists after adjusting for age, insurance status, comorbidities, and severity of illness (Tables 3 and 4). Patients cared for by hospitalists had a shorter overall LOS than did patients cared for by non‐hospitalists (adjusted P = .002). A shorter LOS was noted for patients in the minor (median 3 vs. 5 days), moderate (median 4 vs. 5 days), and extreme (7 vs. 8 days) severity categories. Overall adjusted expense was significantly lower for the care of hospitalists' patients across all severity categories (P < .001). There was a trend toward lower adjusted revenue for patients of hospitalists than those of non‐hospitalist (P = .06). The adjusted profit margin did not significantly differ between the groups (P =.14).
| Severity | Nonhospitalist cases (n = 216) | Hospitalist cases (n = 126) | P value | |
|---|---|---|---|---|
| ||||
| Severity (%) | Minor | 40 (19) | 30 (24) | .13 |
| Moderate | 99 (46) | 64 (51) | ||
| Major | 72 (33) | 27 (21) | ||
| Extreme | 5 (2) | 4 (3) | ||
| LOS (days) | Minor | 5 (3, 6) | 3 (2, 4) | .002 |
| Moderate | 5 (3, 7) | 4 (3, 6) | ||
| Major | 6 (4,10) | 6 (4, 10) | ||
| Extreme | 8 (2, 8) | 7 (6, 8) | ||
| Expense ($) | Minor | 5792 (4414, 6715) | 4164 (2401, 5499) | < .001 |
| Moderate | 6953 (4273, 10,224) | 5951 (4301, 8621) | ||
| Major | 13,622 (8219, 28,553) | 10,519 (5249, 15,581) | ||
| Extreme | 18,908 (12913, 24,688) | 16,192 (6135, 26,147) | ||
| Revenue ($) | Minor | 7095 (6611, 7212) | 7116 (4160, 7218) | .06 |
| Moderate | 7118 (7025, 7215) | 6893 (3755, 7164) | ||
| Major | 9601 (6972, 16,668) | 6743 (4612, 7116) | ||
| Extreme | 11,019 (10,009, 24,897) | 9184 (5783, 13,931) | ||
| Margin ($) | Minor | 786 (162, 2997) | 2290 (409, 4768) | .14 |
| Moderate | 256 (1999, 3366) | 796 (2741, 1565) | ||
| Major | 2314 (7870, 1448) | 3499 (8818, 1008) | ||
| Extreme | 1263 (2904, 4012) | 6537 (15,617, 3050) | ||
| Nonhospitalist cases (%) (n = 216) | Hospitalist cases (%) (n = 126) | P value | |
|---|---|---|---|
| |||
| Acute renal failure | 2 (1) | 0 (0) | 0.53 |
| In‐hospital mortality | 9 (4) | 0 (0) | 0.03 |
| Readmission for any reason | 53 (25) | 35 (28) | 0.52* |
| Readmission for CHF | 19 (9) | 18 (14) | 0.16* |
In‐hospital mortality of patients treated by hospitalists was lower than that of non‐hospitalist‐treated patients (0% vs. 4%; P =.03). Rates of acute renal failure, overall readmissions and readmissions specifically for congestive heart failure did not differ significantly. Notably, severity of illness assessed by APS‐DRG did not differ between hospitalists' and nonhospitalists' patients (P = .13).
DISCUSSION
Practice Patterns
Our study identified specific practices that hospitalists use more than non‐hospitalists in the management of patients with CHF. These practices, which may have resulted in decreased LOS and lower costs, included higher use of ACE‐I/ARB within 24 hours of admission and of intravenous diuretics. We hypothesized that earlier and more aggressive use of ACE‐I/ARB contributed to after‐load reduction and alteration of cardiac remodeling5 and may have led to faster recovery and improved outcomes. Greater use of intravenous diuretics may signify that hospitalists have a more aggressive approach to managing exacerbations of acute congestive heart failure, which may also lead to faster recovery.
Hospitalists used fewer beta‐blockers on admission and at discharge. Reasons for this finding remain unclear; however, it may have been a result of the practice of avoiding beta‐blockers during exacerbations of acute CHF and the subsequent reliance on primary care providers to restart beta‐blockers after discharge. Lower use of beta‐blockers did not appear to have a negative impact on mortality or readmission rates.
Resource Utilization
Hospitalists used fewer serial chest x‐rays, more initial BNP measurements, and more social work consults, and there was a trend toward their using fewer repeat BNP measurements. The less frequent use of serial chest x‐rays may be a result of hospitalists being able to assess patients more frequently and to rely less on imaging. Higher rates of initial BNP measurement by hospitalists may reflect the ordering patterns of the emergency room physicians because most patients are admitted to the hospitalists via the emergency room. The trend toward fewer repeat BNP measurements by hospitalists may again reflect their ability to perform more frequent clinical assessments and to rely less on laboratory data. The higher rate of utilization of social workers by hospitalists is likely a reflection of a population in need of such interventions rather than the hospitalists having a lower threshold before requesting a social work consultation. There were no differences in the rates of obtaining echocardiograms, physical therapy, and dietary consults and of sodium and fluid restrictions.
Clinical Outcomes
Severity of illness assessed by APS‐DRG did not differ between the patients cared for by hospitalists and those care for by non‐hospitalists (P = .13) despite the hospitalists caring for a younger population. In‐hospital mortality of hospitalist‐treated patients was lower (0% vs. 4%), whereas the rates of readmission and renal failure did not differ between the 2 groups. A slight advantage in the mortality rate appears to be in agreement with prior findings3, 4; however, this may have been a result of the non‐hospitalists caring for an older patient population.
Economic Outcomes
The shorter LOS and lower overall costs of patients followed by hospitalists supports previous findings.2, 3, 10 The LOS in our study was found to be shorter for hospitalist‐treated patients whose illnesses were in the minor, moderate, and extreme severity categories by 40%, 20%, and 13%, respectively. The median expense per case was less across all severity categories, ranging from $1000 to $3100 for the patients followed by hospitalists compared with those followed by non‐hospitalists. There was a trend toward lower adjusted median revenue in all categories except for minor severity for hospitalists' patients (P = .06). The profit margin per case did not differ significantly between patients cared for by hospitalists and non‐hospitalists. The shorter LOS and lower expenses per case of patients under the care of hospitalists should have led to higher revenue and profit margin. However, our study showed lower revenue and no significant differences in profit margin, which may be explained by the fact that the hospitalists' patients had a worse insurance mix with a higher proportion of uninsured and Medicaid patients. It is also possible that non‐hospitalists, in particular, cardiologists, generate higher revenue by performing more procedures such as cardiac catheterizations, thus offsetting the costs.
As noted above, the analysis of LOS, expenses, revenue, and margin controlled for age, comorbidities, severity of illness, and insurance status (Table 3). The results were not significantly affected by adjusting for age, insurance status, and comorbidities after controlling for severity. The difference in age may in part be a result of older patients having established relationships with primary care physicians and being less likely to be admitted by hospitalists. It may also reflect the high prevalence of methamphetamine abuse, which has reached epidemic proportions in Hawaii, and methamphetamine‐induced cardiomyopathy in a younger population of patients followed by hospitalists. Further studies would be necessary to estimate the impact of drug‐induced congestive heart failure in these populations.
Although our study provided a detailed look at practice patterns of a coherent hospitalist group, it had several important limitations. It was a retrospective study conducted at a single institution, making the findings difficult to generalize to hospitalist practices nationwide. It included an unusually large number of non‐Caucasian patients, reflecting the demographics of the state of Hawaii. Data on contraindications to ACE‐I/ARB were not collected because the degree of renal dysfunction that would serve as a contraindication was difficult to define. The primary mode of adjustment was APS, which may have been a limiting factor in assessing severity of illness. The inability to follow patients' course after discharge limited collection of long‐term outcomes data.
In agreement with previous studies, we showed a decreased LOS and lower expenses per case of patients cared for by full‐time hospitalists while preserving quality of care and improving clinical outcomes. We identified specific practices of hospitalists in the management of patients with CHF that differ from those of non‐hospitalists. These practices include early use of ACE‐I/ARB, aggressive approach to diuresis, higher utilization of social work services, and decreased utilization of serial chest x‐rays, medical consultants, and serial BNP measurements. Our study was not designed to identify a direct causal relationship between hospitalist practices and improved outcomes; however, we believe it to be the first step in understanding practice patterns and the impact of the hospitalist movement.
- ,,,,.Advances in hospital medicine: a review of key articles from the literature.Med Clin North Am.2002;86:797–823.
- ,,, et al.The positive impact of initiation of hospitalist clinician educators.J Gen Intern Med.2004;19:293–301.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty.Ann Intern Med.2004;141:28–38.
- ,,.Advances in the management of acute and chronic decompensated heart failure.Lippincotts Case Manag.2004;9:S1–S15.
- ,,,, et al.ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult.Circulation.2001;104:2996–3007.
- American Heart Association.Heart disease and stroke statistics—2003 update.2003.
- .Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency department.Rev Cardiovasc Med.2002;3(suppl):S3–S9.
- ,,,.Comparison of processes and outcomes of pneumonia care between hospitalists and community‐based primary care physicians.Mayo Clin Proc.2002;77:1053–1058.
- ,,,,.Economic effects of community versus hospital‐based faculty pneumonia care.J Gen Intern Med.1998;13:774–777.
- ,,,,.Quality of care for patients hospitalized with heart failure. Assessing the impact of hospitalists.Arch Intern Med.2002;162:1251–1256.
- ,,, et al.ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in adult.ACC/AHA Pract Guidel.2005:1–82
- ,,.Importance of in‐hospital initiation of evidence‐based medical therapies for heart failure—a review.Am J Cardiol.2004;94:1155–1160.
- .Role of in‐hospital initiation of carvedilol to improve treatment rates and clinical outcomes.Am J Cardiol.2004;93(suppl):77B–81B.
- ,.Rationale and design of the Cardiac Hospitalization Atherosclerosis Management Program at the University of California Los Angeles.Am J Cardiol.2000;85:10A–17A.
The use of hospitalists, physicians who specialize in inpatient care, has seen a rapid expansion over the last decade.1 Several studies have shown that with hospitalists there is a shorter length of stay (LOS) and decreased utilization of resources and that hospitalists play a positive role in medical education.24 However, only a few studies have examined the specific strategies employed by hospitalists to achieve improved efficiency and outcomes.
Congestive heart failure (CHF) is the most common diagnosis of hospitalized patients older than age 65, with more Medicare spending devoted to patients with CHF than to any other diagnosis‐related group (DRG).5, 6 Over the last 2 decades hospital discharges for congestive heart failure increased by 165%.7 In addition, the rate of hospital readmission of patients with CHF remains high: 2%, 20%, and 50% within 2 days, 1 month, and 6 months, respectively.8
Several previous studies have shown that patients cared for by hospitalists had improved clinical outcomes. Meltzer et al. found that 30‐day mortality of hospitalists' patients was lower than that of non‐hospitalists' patients, 4.2% versus 6.0%, respectively, in the second year of implementation of a hospitalist program.3 A study by Huddleston et al. showed a reduction of 11.8% in the rate of complications experienced by postsurgical orthopedic patients with the involvement of hospitalists in their care in conjunction with the surgeons.4
Many previous studies have pointed to improvements in economic outcomes such as LOS and costs for patients followed by hospitalists. Kulaga et al. showed that patients cared for by hospitalists had reductions of approximately 20% in LOS and 18% in total costs per case compared with those cared for by community‐based physicians.2 Meltzer et al. found a decrease in the average adjusted LOS of 0.49 days in the second year of implementation of a hospitalist program.3 Rifkin et al. found that patients with pneumonia cared for by hospitalists had a mean adjusted LOS of 5.6 days versus 6.5 days for those cared for by non‐hospitalists.9
Few previous studies have looked at specific practice patterns of hospitalists that result in improved efficiency and better outcomes. Rifkin et al., who found that patients with pneumonia cared for by hospitalists had a shorter LOS, suggested this finding was a result of the earlier recognition by hospitalists that patients were stable and more rapid conversion to oral antibiotics.9 Likewise, Stein et al. found that community‐acquired pneumonia patients treated by hospitalists had a shorter LOS than those treated by non‐hospitalists. However, they were unable to assess the differences in patient management that led to this result because of the design of the study.10
Lindenauer et al. compared quality‐of‐care indicators and resource utilization for patients with congestive heart failure treated by hospitalists and non‐hospitalist general internists. They found that patients under the care of hospitalists had a shorter LOS than those cared for by general internists but that the overall costs of care were similar between the groups.11 They compared the quality indicators developed by the Joint Commission on Accreditation of Healthcare Organizations in the Core Measures Initiative, but did not focus on patterns of practices of hospitalists and nonhospitalists. Moreover, they did not look at full‐time hospitalists but focused on physicians who spent at least 25% of their practice caring for inpatients.
We sought to identify distinct, quantifiable practices of full‐time hospitalists in the management of their patients with CHF. We hypothesized that hospitalists would adhere more closely to the current congestive heart failure guidelines and would utilize available resources more judiciously, leading to improved clinical and economic outcomes. To identify these practices, we compared utilization of well‐established therapeutic and diagnostic modalities such as use of ACE‐I, ARB, and beta‐blockers; ordering of chest x‐rays; measurement of brain natriuretic peptide (BNP); and use of medical subspecialty consultants. We also compared standard clinical and economic outcomes such as in‐hospital mortality, readmission rate, LOS, and costs per case between hospitalists and community‐based physicians.
METHODS
Design and Setting
The study was a retrospective chart review of 447 patients treated for CHF from July 1, 2003, through June 30, 2004, at the Queen's Medical Center, a 505‐bed community‐based teaching hospital in Honolulu, Hawaii, and the leading medical referral center in the Pacific Basin. All patients had been cared for by either a community‐based physician or a hospitalist. The community‐based physicians (referred to as non‐hospitalists from here on) were a diverse group of internists and subspecialists, in solo or group practice, who provided inpatient and ambulatory care. The non‐hospitalist group included 119 cardiologists (55%), 83 general internists (38%), and 3 family practitioners (1%), with the other 6% made up of clinicians in the medical oncology, pediatrics, pulmonary, radiation oncology, and thoracic/cardiovascular surgery subspecialties.
The hospitalist group comprised 10 full‐time internists employed by the hospital who provided care for patients only in the inpatient setting and 3 part‐time hospitalists who practiced in the ambulatory setting in addition to providing inpatient night coverage for the group. During the study period, 2 hospitalists left the group, and 2 hospitalists were hired. On average the length of involvement of a full‐time hospitalist in the study was 9 months. Permission to conduct this study was granted by the Queen's Medical Center Institutional Review Board.
Patient Population
Patients were included in the study if they were admitted to Queen's Medical Center during the 18‐month study period, were at least 18 years old, and were coded on discharge by the medical records department with a principal diagnosis of congestive heart failure (International Classification of Diseases, 9th Revision, codes 428, 428.1, 428.9, 402.01, 402.11, 402.91, 404.01, 404.11, and 404.91). Baseline characteristics of patients collected were age, sex, insurance status, comorbidities, and code status on admission. Comorbidities included coronary artery disease, diabetes mellitus (type 1 or 2), hypertension, chronic renal insufficiency (creatinine > 2 mg/dL), and chronic obstructive pulmonary disease (COPD). Patients were excluded if they had initially been admitted to the medical intensive care unit, required ventilatory support, had end‐stage renal disease requiring hemodialysis, or had an LOS greater than 14 days.
Data Collection
Medical records were reviewed by research nurses not directly involved with the hospitalist group. Training to ensure high‐level reliability of data collection was provided, and reliability was verified by the primary author (M.M.R.). The following data were collected: use of ACE‐I, ARB, and beta‐blockers on admission and discharge; use of intravenous and oral diuretics; time to switch to oral diuretic; rates of utilization of medical consultants, physical therapy, dietary consults, social work, and sodium and fluid restriction; and number of repeat chest radiographs, echocardiograms, and BNP measurements. These criteria were developed based on ACC/AHA 2005 guidelines for diagnosis and management of congestive heart failure in adults,11 several studies delineating the importance of initiating therapy in the inpatient setting, and the experience of the Cardiovascular Hospital Atherosclerosis Management Program (CHAMP) for patients with established coronary artery disease.1315 Data on medical resident involvement in patient care were collected for hospitalists and non‐hospitalists.
Additional outcomes included in‐hospital mortality, rate of acute renal failure, readmission rate, LOS, expense, revenue, and margin per case. Acute renal failure was defined as a doubling of the admission creatinine value. The rate of readmissiondefined as readmission to Queen's Medical Center for any reasonwas evaluated after 7, 14, and 30 days and was stratified further for readmissions for CHF. Expense was defined as costs directly related to patient care plus costs related to operating a hospital facility. Revenue was defined as the compensation the hospital expected to collect for service rendered adjusted for bad debt/charity care. Margin was defined as revenue minus expense.
Data Analysis
Descriptive statistics are reported for baseline patient characteristics (age, sex, insurance status, etc.), quality‐of‐care measures (ACE‐I, ARB, diuretic, and beta‐blocker use, time to oral diuretic, etc.), and outcome measures (readmission rate, in‐hospital mortality, LOS, cost data) using frequencies and proportions for categorical variables (eg, sex, ethnicity, insurance status), means and standard deviations (SDs) for continuous variables (age), and medians and interquartile ranges (Q1‐Q3) for skewed variables (eg, LOS, cost data). The patients cared for by hospitalists were compared with those cared for by non‐hospitalists using the chi‐square test or Fisher's exact test for categorical data and the Student t test for continuous data. All‐Payer Severity‐adjusted Diagnosis Related Groups (APS‐DRGs) were used to control for severity of patient illness. The severity of illness codes were taken from 3M APR Benchmarking software for DRGs adjusted for severity of illness and risk of mortality. 3M defined severity of illness as the extent of physiologic decompensation or organ system loss of function. Each diagnosis was assigned 1 of 4 severity levels: minor, moderate, major, or extreme. Kruskal‐Wallis analysis of covariance was used for LOS and cost outcomes, adjusting for age, insurance status, comorbidities, and severity of illness. Multivariate logistic regression was performed for binary outcomes (eg, ACE‐I, ARB, beta‐blocker use) to adjust for confounding variables. Statistical analysis was performed using SAS version 9 (SAS Institute Inc., Cary, NC). All tests were 2‐sided, and differences with a P value < .05 were considered significant.
RESULTS
Patient Characteristics
Table 1 shows the patient characteristic data. There were 447 admissions for congestive heart failure during the study period, 342 of which met study inclusion criteria. Hospitalists provided care for 126 of these patients and non‐hospitalists for 216 patients. Mean age of patients in the hospitalist and nonhospitalist groups was 63 and 73 years, respectively. There were significant differences in insurance status, with hospitalists more frequently caring for patients covered by Medicaid (26% vs. 7%; P < .001) and patients who were uninsured (6% vs. 1%; P = .04). Patients cared for by hospitalists had a lower incidence of coronary artery disease (42% vs. 59%; P = .003) and prior CHF (44% vs. 56%; P = .05). The hospitalists' patients were more likely to have a full resuscitation code status on admission; however, this difference did not reach statistical significance (90% vs. 81%; P = .07). There were no significant differences between patients cared for by hospitalists and non‐hospitalists in sex, ethnic background, other comorbidities, or house staff involvement.
| Non‐hospitalist cases (%) (n = 216) | Hospitalist cases (%) (n = 126) | P value | |
|---|---|---|---|
| |||
| Age (years, mean SD) | 73 15 | 63 16 | < .001 |
| Male sex | 124 (57) | 78 (62) | .41 |
| Caucasian ethnicity | 41 (19) | 30 (24) | .29 |
| Insurance status | |||
| Medicare | 119 (55) | 58 (46) | .11 |
| Medicaid/Quest | 16 (7) | 33 (26) | < .001 |
| HMSA | 68 (31) | 19 (15) | < .001 |
| Self‐pay | 3 (1) | 7 (6) | .04 |
| Other | 10(5) | 9(7) | .33 |
| Comorbidy | |||
| CAD | 127 (59) | 53 (42) | .003 |
| DM | 78 (36) | 53 (4) | .27 |
| HTN | 139 (64) | 80 (63) | .87 |
| CRI | 43 (20) | 28 (22) | .61 |
| COPD | 30 (14) | 26 (21) | .10 |
| Prior CHF | 120 (56) | 56 (44) | .05 |
| Full code | 174 (81) | 113 (90) | .07 |
| House staff involvement | 42 (19) | 20 (16) | .41 |
Practice Patterns and Resource Utilization
Practice patterns and resource utilization are shown in Table 2. Hospitalists used more ACE‐I/ARBs, with 86% of patients receiving these interventions within 24 hours of admission versus 72% of the patients of non‐hospitalists (adjusted P = .001). Hospitalists treated fewer patients with beta‐blockers on admission and on discharge and more patients with intravenous diuretics (90% vs. 73%; adjusted P = .001). The rate of beta‐blocker use did not change significantly after controlling for patients with COPD (data not shown).
| Non‐hospitalist cases (%) (n = 216) | Hospitalist cases (%) (n = 126) | P value* | |
|---|---|---|---|
| |||
| ACE‐I/ARB within 24 hours | 155 (72) | 108 (86) | .001 |
| Beta‐blocker within 24 hours | 119 (55) | 50 (40) | .004 |
| ACE‐I/ARB at discharge | 147 (69) | 95 (75) | .24 |
| Beta‐blocker at discharge | 116 (54) | 52 (41) | .03 |
| Echocardiogram 1 | 125 (58) | 81 (64) | .50 |
| MD Consultants 2 | 35 (16) | 10 (8) | .01 |
| Chest x‐ray 2 | 27 (13) | 5 (4) | .02 |
| BNP 1 | 128 (59) | 95 (75) | .005 |
| BNP > 1 | 22 (10) | 7 (6) | .14 |
| Physical therapy | 35 (16) | 17 (13) | .48 |
| Dietary consult | 29 (13) | 19 (15) | .67 |
| Social work | 62 (29) | 60 (48) | .003 |
| Sodium restriction | 184 (85) | 102 (81) | .31 |
| Fluid restriction | 47 (22) | 35 (28) | .21 |
| IV diuretic | 158 (73) | 114 (90) | .001 |
| Time to oral diuretic (days), median (Q1,Q3) | 1 (1, 3) | 1 (0, 2) | .30 |
Hospitalists were less likely to obtain 2 or more chest x‐rays (4% vs. 13%; adjusted P = .02) or to obtain 2 or more medical consultations (8% vs. 16%; adjusted P = .01). In addition, they obtained more initial measurements of BNP; however, there was a trend toward fewer repeat BNP measurements (6% vs. 10%; P = .14). There was a significantly higher rate of social work utilization by hospitalists than by nonhospitalists (48% vs. 29%; adjusted P = .003). There were no differences between the groups in the rates of obtaining echocardiograms, physical therapy, and dietary consults or in sodium and fluid restrictions.
Outcomes
Significant differences were noted in LOS and cost outcomes between hospitalists and non‐hospitalists after adjusting for age, insurance status, comorbidities, and severity of illness (Tables 3 and 4). Patients cared for by hospitalists had a shorter overall LOS than did patients cared for by non‐hospitalists (adjusted P = .002). A shorter LOS was noted for patients in the minor (median 3 vs. 5 days), moderate (median 4 vs. 5 days), and extreme (7 vs. 8 days) severity categories. Overall adjusted expense was significantly lower for the care of hospitalists' patients across all severity categories (P < .001). There was a trend toward lower adjusted revenue for patients of hospitalists than those of non‐hospitalist (P = .06). The adjusted profit margin did not significantly differ between the groups (P =.14).
| Severity | Nonhospitalist cases (n = 216) | Hospitalist cases (n = 126) | P value | |
|---|---|---|---|---|
| ||||
| Severity (%) | Minor | 40 (19) | 30 (24) | .13 |
| Moderate | 99 (46) | 64 (51) | ||
| Major | 72 (33) | 27 (21) | ||
| Extreme | 5 (2) | 4 (3) | ||
| LOS (days) | Minor | 5 (3, 6) | 3 (2, 4) | .002 |
| Moderate | 5 (3, 7) | 4 (3, 6) | ||
| Major | 6 (4,10) | 6 (4, 10) | ||
| Extreme | 8 (2, 8) | 7 (6, 8) | ||
| Expense ($) | Minor | 5792 (4414, 6715) | 4164 (2401, 5499) | < .001 |
| Moderate | 6953 (4273, 10,224) | 5951 (4301, 8621) | ||
| Major | 13,622 (8219, 28,553) | 10,519 (5249, 15,581) | ||
| Extreme | 18,908 (12913, 24,688) | 16,192 (6135, 26,147) | ||
| Revenue ($) | Minor | 7095 (6611, 7212) | 7116 (4160, 7218) | .06 |
| Moderate | 7118 (7025, 7215) | 6893 (3755, 7164) | ||
| Major | 9601 (6972, 16,668) | 6743 (4612, 7116) | ||
| Extreme | 11,019 (10,009, 24,897) | 9184 (5783, 13,931) | ||
| Margin ($) | Minor | 786 (162, 2997) | 2290 (409, 4768) | .14 |
| Moderate | 256 (1999, 3366) | 796 (2741, 1565) | ||
| Major | 2314 (7870, 1448) | 3499 (8818, 1008) | ||
| Extreme | 1263 (2904, 4012) | 6537 (15,617, 3050) | ||
| Nonhospitalist cases (%) (n = 216) | Hospitalist cases (%) (n = 126) | P value | |
|---|---|---|---|
| |||
| Acute renal failure | 2 (1) | 0 (0) | 0.53 |
| In‐hospital mortality | 9 (4) | 0 (0) | 0.03 |
| Readmission for any reason | 53 (25) | 35 (28) | 0.52* |
| Readmission for CHF | 19 (9) | 18 (14) | 0.16* |
In‐hospital mortality of patients treated by hospitalists was lower than that of non‐hospitalist‐treated patients (0% vs. 4%; P =.03). Rates of acute renal failure, overall readmissions and readmissions specifically for congestive heart failure did not differ significantly. Notably, severity of illness assessed by APS‐DRG did not differ between hospitalists' and nonhospitalists' patients (P = .13).
DISCUSSION
Practice Patterns
Our study identified specific practices that hospitalists use more than non‐hospitalists in the management of patients with CHF. These practices, which may have resulted in decreased LOS and lower costs, included higher use of ACE‐I/ARB within 24 hours of admission and of intravenous diuretics. We hypothesized that earlier and more aggressive use of ACE‐I/ARB contributed to after‐load reduction and alteration of cardiac remodeling5 and may have led to faster recovery and improved outcomes. Greater use of intravenous diuretics may signify that hospitalists have a more aggressive approach to managing exacerbations of acute congestive heart failure, which may also lead to faster recovery.
Hospitalists used fewer beta‐blockers on admission and at discharge. Reasons for this finding remain unclear; however, it may have been a result of the practice of avoiding beta‐blockers during exacerbations of acute CHF and the subsequent reliance on primary care providers to restart beta‐blockers after discharge. Lower use of beta‐blockers did not appear to have a negative impact on mortality or readmission rates.
Resource Utilization
Hospitalists used fewer serial chest x‐rays, more initial BNP measurements, and more social work consults, and there was a trend toward their using fewer repeat BNP measurements. The less frequent use of serial chest x‐rays may be a result of hospitalists being able to assess patients more frequently and to rely less on imaging. Higher rates of initial BNP measurement by hospitalists may reflect the ordering patterns of the emergency room physicians because most patients are admitted to the hospitalists via the emergency room. The trend toward fewer repeat BNP measurements by hospitalists may again reflect their ability to perform more frequent clinical assessments and to rely less on laboratory data. The higher rate of utilization of social workers by hospitalists is likely a reflection of a population in need of such interventions rather than the hospitalists having a lower threshold before requesting a social work consultation. There were no differences in the rates of obtaining echocardiograms, physical therapy, and dietary consults and of sodium and fluid restrictions.
Clinical Outcomes
Severity of illness assessed by APS‐DRG did not differ between the patients cared for by hospitalists and those care for by non‐hospitalists (P = .13) despite the hospitalists caring for a younger population. In‐hospital mortality of hospitalist‐treated patients was lower (0% vs. 4%), whereas the rates of readmission and renal failure did not differ between the 2 groups. A slight advantage in the mortality rate appears to be in agreement with prior findings3, 4; however, this may have been a result of the non‐hospitalists caring for an older patient population.
Economic Outcomes
The shorter LOS and lower overall costs of patients followed by hospitalists supports previous findings.2, 3, 10 The LOS in our study was found to be shorter for hospitalist‐treated patients whose illnesses were in the minor, moderate, and extreme severity categories by 40%, 20%, and 13%, respectively. The median expense per case was less across all severity categories, ranging from $1000 to $3100 for the patients followed by hospitalists compared with those followed by non‐hospitalists. There was a trend toward lower adjusted median revenue in all categories except for minor severity for hospitalists' patients (P = .06). The profit margin per case did not differ significantly between patients cared for by hospitalists and non‐hospitalists. The shorter LOS and lower expenses per case of patients under the care of hospitalists should have led to higher revenue and profit margin. However, our study showed lower revenue and no significant differences in profit margin, which may be explained by the fact that the hospitalists' patients had a worse insurance mix with a higher proportion of uninsured and Medicaid patients. It is also possible that non‐hospitalists, in particular, cardiologists, generate higher revenue by performing more procedures such as cardiac catheterizations, thus offsetting the costs.
As noted above, the analysis of LOS, expenses, revenue, and margin controlled for age, comorbidities, severity of illness, and insurance status (Table 3). The results were not significantly affected by adjusting for age, insurance status, and comorbidities after controlling for severity. The difference in age may in part be a result of older patients having established relationships with primary care physicians and being less likely to be admitted by hospitalists. It may also reflect the high prevalence of methamphetamine abuse, which has reached epidemic proportions in Hawaii, and methamphetamine‐induced cardiomyopathy in a younger population of patients followed by hospitalists. Further studies would be necessary to estimate the impact of drug‐induced congestive heart failure in these populations.
Although our study provided a detailed look at practice patterns of a coherent hospitalist group, it had several important limitations. It was a retrospective study conducted at a single institution, making the findings difficult to generalize to hospitalist practices nationwide. It included an unusually large number of non‐Caucasian patients, reflecting the demographics of the state of Hawaii. Data on contraindications to ACE‐I/ARB were not collected because the degree of renal dysfunction that would serve as a contraindication was difficult to define. The primary mode of adjustment was APS, which may have been a limiting factor in assessing severity of illness. The inability to follow patients' course after discharge limited collection of long‐term outcomes data.
In agreement with previous studies, we showed a decreased LOS and lower expenses per case of patients cared for by full‐time hospitalists while preserving quality of care and improving clinical outcomes. We identified specific practices of hospitalists in the management of patients with CHF that differ from those of non‐hospitalists. These practices include early use of ACE‐I/ARB, aggressive approach to diuresis, higher utilization of social work services, and decreased utilization of serial chest x‐rays, medical consultants, and serial BNP measurements. Our study was not designed to identify a direct causal relationship between hospitalist practices and improved outcomes; however, we believe it to be the first step in understanding practice patterns and the impact of the hospitalist movement.
The use of hospitalists, physicians who specialize in inpatient care, has seen a rapid expansion over the last decade.1 Several studies have shown that with hospitalists there is a shorter length of stay (LOS) and decreased utilization of resources and that hospitalists play a positive role in medical education.24 However, only a few studies have examined the specific strategies employed by hospitalists to achieve improved efficiency and outcomes.
Congestive heart failure (CHF) is the most common diagnosis of hospitalized patients older than age 65, with more Medicare spending devoted to patients with CHF than to any other diagnosis‐related group (DRG).5, 6 Over the last 2 decades hospital discharges for congestive heart failure increased by 165%.7 In addition, the rate of hospital readmission of patients with CHF remains high: 2%, 20%, and 50% within 2 days, 1 month, and 6 months, respectively.8
Several previous studies have shown that patients cared for by hospitalists had improved clinical outcomes. Meltzer et al. found that 30‐day mortality of hospitalists' patients was lower than that of non‐hospitalists' patients, 4.2% versus 6.0%, respectively, in the second year of implementation of a hospitalist program.3 A study by Huddleston et al. showed a reduction of 11.8% in the rate of complications experienced by postsurgical orthopedic patients with the involvement of hospitalists in their care in conjunction with the surgeons.4
Many previous studies have pointed to improvements in economic outcomes such as LOS and costs for patients followed by hospitalists. Kulaga et al. showed that patients cared for by hospitalists had reductions of approximately 20% in LOS and 18% in total costs per case compared with those cared for by community‐based physicians.2 Meltzer et al. found a decrease in the average adjusted LOS of 0.49 days in the second year of implementation of a hospitalist program.3 Rifkin et al. found that patients with pneumonia cared for by hospitalists had a mean adjusted LOS of 5.6 days versus 6.5 days for those cared for by non‐hospitalists.9
Few previous studies have looked at specific practice patterns of hospitalists that result in improved efficiency and better outcomes. Rifkin et al., who found that patients with pneumonia cared for by hospitalists had a shorter LOS, suggested this finding was a result of the earlier recognition by hospitalists that patients were stable and more rapid conversion to oral antibiotics.9 Likewise, Stein et al. found that community‐acquired pneumonia patients treated by hospitalists had a shorter LOS than those treated by non‐hospitalists. However, they were unable to assess the differences in patient management that led to this result because of the design of the study.10
Lindenauer et al. compared quality‐of‐care indicators and resource utilization for patients with congestive heart failure treated by hospitalists and non‐hospitalist general internists. They found that patients under the care of hospitalists had a shorter LOS than those cared for by general internists but that the overall costs of care were similar between the groups.11 They compared the quality indicators developed by the Joint Commission on Accreditation of Healthcare Organizations in the Core Measures Initiative, but did not focus on patterns of practices of hospitalists and nonhospitalists. Moreover, they did not look at full‐time hospitalists but focused on physicians who spent at least 25% of their practice caring for inpatients.
We sought to identify distinct, quantifiable practices of full‐time hospitalists in the management of their patients with CHF. We hypothesized that hospitalists would adhere more closely to the current congestive heart failure guidelines and would utilize available resources more judiciously, leading to improved clinical and economic outcomes. To identify these practices, we compared utilization of well‐established therapeutic and diagnostic modalities such as use of ACE‐I, ARB, and beta‐blockers; ordering of chest x‐rays; measurement of brain natriuretic peptide (BNP); and use of medical subspecialty consultants. We also compared standard clinical and economic outcomes such as in‐hospital mortality, readmission rate, LOS, and costs per case between hospitalists and community‐based physicians.
METHODS
Design and Setting
The study was a retrospective chart review of 447 patients treated for CHF from July 1, 2003, through June 30, 2004, at the Queen's Medical Center, a 505‐bed community‐based teaching hospital in Honolulu, Hawaii, and the leading medical referral center in the Pacific Basin. All patients had been cared for by either a community‐based physician or a hospitalist. The community‐based physicians (referred to as non‐hospitalists from here on) were a diverse group of internists and subspecialists, in solo or group practice, who provided inpatient and ambulatory care. The non‐hospitalist group included 119 cardiologists (55%), 83 general internists (38%), and 3 family practitioners (1%), with the other 6% made up of clinicians in the medical oncology, pediatrics, pulmonary, radiation oncology, and thoracic/cardiovascular surgery subspecialties.
The hospitalist group comprised 10 full‐time internists employed by the hospital who provided care for patients only in the inpatient setting and 3 part‐time hospitalists who practiced in the ambulatory setting in addition to providing inpatient night coverage for the group. During the study period, 2 hospitalists left the group, and 2 hospitalists were hired. On average the length of involvement of a full‐time hospitalist in the study was 9 months. Permission to conduct this study was granted by the Queen's Medical Center Institutional Review Board.
Patient Population
Patients were included in the study if they were admitted to Queen's Medical Center during the 18‐month study period, were at least 18 years old, and were coded on discharge by the medical records department with a principal diagnosis of congestive heart failure (International Classification of Diseases, 9th Revision, codes 428, 428.1, 428.9, 402.01, 402.11, 402.91, 404.01, 404.11, and 404.91). Baseline characteristics of patients collected were age, sex, insurance status, comorbidities, and code status on admission. Comorbidities included coronary artery disease, diabetes mellitus (type 1 or 2), hypertension, chronic renal insufficiency (creatinine > 2 mg/dL), and chronic obstructive pulmonary disease (COPD). Patients were excluded if they had initially been admitted to the medical intensive care unit, required ventilatory support, had end‐stage renal disease requiring hemodialysis, or had an LOS greater than 14 days.
Data Collection
Medical records were reviewed by research nurses not directly involved with the hospitalist group. Training to ensure high‐level reliability of data collection was provided, and reliability was verified by the primary author (M.M.R.). The following data were collected: use of ACE‐I, ARB, and beta‐blockers on admission and discharge; use of intravenous and oral diuretics; time to switch to oral diuretic; rates of utilization of medical consultants, physical therapy, dietary consults, social work, and sodium and fluid restriction; and number of repeat chest radiographs, echocardiograms, and BNP measurements. These criteria were developed based on ACC/AHA 2005 guidelines for diagnosis and management of congestive heart failure in adults,11 several studies delineating the importance of initiating therapy in the inpatient setting, and the experience of the Cardiovascular Hospital Atherosclerosis Management Program (CHAMP) for patients with established coronary artery disease.1315 Data on medical resident involvement in patient care were collected for hospitalists and non‐hospitalists.
Additional outcomes included in‐hospital mortality, rate of acute renal failure, readmission rate, LOS, expense, revenue, and margin per case. Acute renal failure was defined as a doubling of the admission creatinine value. The rate of readmissiondefined as readmission to Queen's Medical Center for any reasonwas evaluated after 7, 14, and 30 days and was stratified further for readmissions for CHF. Expense was defined as costs directly related to patient care plus costs related to operating a hospital facility. Revenue was defined as the compensation the hospital expected to collect for service rendered adjusted for bad debt/charity care. Margin was defined as revenue minus expense.
Data Analysis
Descriptive statistics are reported for baseline patient characteristics (age, sex, insurance status, etc.), quality‐of‐care measures (ACE‐I, ARB, diuretic, and beta‐blocker use, time to oral diuretic, etc.), and outcome measures (readmission rate, in‐hospital mortality, LOS, cost data) using frequencies and proportions for categorical variables (eg, sex, ethnicity, insurance status), means and standard deviations (SDs) for continuous variables (age), and medians and interquartile ranges (Q1‐Q3) for skewed variables (eg, LOS, cost data). The patients cared for by hospitalists were compared with those cared for by non‐hospitalists using the chi‐square test or Fisher's exact test for categorical data and the Student t test for continuous data. All‐Payer Severity‐adjusted Diagnosis Related Groups (APS‐DRGs) were used to control for severity of patient illness. The severity of illness codes were taken from 3M APR Benchmarking software for DRGs adjusted for severity of illness and risk of mortality. 3M defined severity of illness as the extent of physiologic decompensation or organ system loss of function. Each diagnosis was assigned 1 of 4 severity levels: minor, moderate, major, or extreme. Kruskal‐Wallis analysis of covariance was used for LOS and cost outcomes, adjusting for age, insurance status, comorbidities, and severity of illness. Multivariate logistic regression was performed for binary outcomes (eg, ACE‐I, ARB, beta‐blocker use) to adjust for confounding variables. Statistical analysis was performed using SAS version 9 (SAS Institute Inc., Cary, NC). All tests were 2‐sided, and differences with a P value < .05 were considered significant.
RESULTS
Patient Characteristics
Table 1 shows the patient characteristic data. There were 447 admissions for congestive heart failure during the study period, 342 of which met study inclusion criteria. Hospitalists provided care for 126 of these patients and non‐hospitalists for 216 patients. Mean age of patients in the hospitalist and nonhospitalist groups was 63 and 73 years, respectively. There were significant differences in insurance status, with hospitalists more frequently caring for patients covered by Medicaid (26% vs. 7%; P < .001) and patients who were uninsured (6% vs. 1%; P = .04). Patients cared for by hospitalists had a lower incidence of coronary artery disease (42% vs. 59%; P = .003) and prior CHF (44% vs. 56%; P = .05). The hospitalists' patients were more likely to have a full resuscitation code status on admission; however, this difference did not reach statistical significance (90% vs. 81%; P = .07). There were no significant differences between patients cared for by hospitalists and non‐hospitalists in sex, ethnic background, other comorbidities, or house staff involvement.
| Non‐hospitalist cases (%) (n = 216) | Hospitalist cases (%) (n = 126) | P value | |
|---|---|---|---|
| |||
| Age (years, mean SD) | 73 15 | 63 16 | < .001 |
| Male sex | 124 (57) | 78 (62) | .41 |
| Caucasian ethnicity | 41 (19) | 30 (24) | .29 |
| Insurance status | |||
| Medicare | 119 (55) | 58 (46) | .11 |
| Medicaid/Quest | 16 (7) | 33 (26) | < .001 |
| HMSA | 68 (31) | 19 (15) | < .001 |
| Self‐pay | 3 (1) | 7 (6) | .04 |
| Other | 10(5) | 9(7) | .33 |
| Comorbidy | |||
| CAD | 127 (59) | 53 (42) | .003 |
| DM | 78 (36) | 53 (4) | .27 |
| HTN | 139 (64) | 80 (63) | .87 |
| CRI | 43 (20) | 28 (22) | .61 |
| COPD | 30 (14) | 26 (21) | .10 |
| Prior CHF | 120 (56) | 56 (44) | .05 |
| Full code | 174 (81) | 113 (90) | .07 |
| House staff involvement | 42 (19) | 20 (16) | .41 |
Practice Patterns and Resource Utilization
Practice patterns and resource utilization are shown in Table 2. Hospitalists used more ACE‐I/ARBs, with 86% of patients receiving these interventions within 24 hours of admission versus 72% of the patients of non‐hospitalists (adjusted P = .001). Hospitalists treated fewer patients with beta‐blockers on admission and on discharge and more patients with intravenous diuretics (90% vs. 73%; adjusted P = .001). The rate of beta‐blocker use did not change significantly after controlling for patients with COPD (data not shown).
| Non‐hospitalist cases (%) (n = 216) | Hospitalist cases (%) (n = 126) | P value* | |
|---|---|---|---|
| |||
| ACE‐I/ARB within 24 hours | 155 (72) | 108 (86) | .001 |
| Beta‐blocker within 24 hours | 119 (55) | 50 (40) | .004 |
| ACE‐I/ARB at discharge | 147 (69) | 95 (75) | .24 |
| Beta‐blocker at discharge | 116 (54) | 52 (41) | .03 |
| Echocardiogram 1 | 125 (58) | 81 (64) | .50 |
| MD Consultants 2 | 35 (16) | 10 (8) | .01 |
| Chest x‐ray 2 | 27 (13) | 5 (4) | .02 |
| BNP 1 | 128 (59) | 95 (75) | .005 |
| BNP > 1 | 22 (10) | 7 (6) | .14 |
| Physical therapy | 35 (16) | 17 (13) | .48 |
| Dietary consult | 29 (13) | 19 (15) | .67 |
| Social work | 62 (29) | 60 (48) | .003 |
| Sodium restriction | 184 (85) | 102 (81) | .31 |
| Fluid restriction | 47 (22) | 35 (28) | .21 |
| IV diuretic | 158 (73) | 114 (90) | .001 |
| Time to oral diuretic (days), median (Q1,Q3) | 1 (1, 3) | 1 (0, 2) | .30 |
Hospitalists were less likely to obtain 2 or more chest x‐rays (4% vs. 13%; adjusted P = .02) or to obtain 2 or more medical consultations (8% vs. 16%; adjusted P = .01). In addition, they obtained more initial measurements of BNP; however, there was a trend toward fewer repeat BNP measurements (6% vs. 10%; P = .14). There was a significantly higher rate of social work utilization by hospitalists than by nonhospitalists (48% vs. 29%; adjusted P = .003). There were no differences between the groups in the rates of obtaining echocardiograms, physical therapy, and dietary consults or in sodium and fluid restrictions.
Outcomes
Significant differences were noted in LOS and cost outcomes between hospitalists and non‐hospitalists after adjusting for age, insurance status, comorbidities, and severity of illness (Tables 3 and 4). Patients cared for by hospitalists had a shorter overall LOS than did patients cared for by non‐hospitalists (adjusted P = .002). A shorter LOS was noted for patients in the minor (median 3 vs. 5 days), moderate (median 4 vs. 5 days), and extreme (7 vs. 8 days) severity categories. Overall adjusted expense was significantly lower for the care of hospitalists' patients across all severity categories (P < .001). There was a trend toward lower adjusted revenue for patients of hospitalists than those of non‐hospitalist (P = .06). The adjusted profit margin did not significantly differ between the groups (P =.14).
| Severity | Nonhospitalist cases (n = 216) | Hospitalist cases (n = 126) | P value | |
|---|---|---|---|---|
| ||||
| Severity (%) | Minor | 40 (19) | 30 (24) | .13 |
| Moderate | 99 (46) | 64 (51) | ||
| Major | 72 (33) | 27 (21) | ||
| Extreme | 5 (2) | 4 (3) | ||
| LOS (days) | Minor | 5 (3, 6) | 3 (2, 4) | .002 |
| Moderate | 5 (3, 7) | 4 (3, 6) | ||
| Major | 6 (4,10) | 6 (4, 10) | ||
| Extreme | 8 (2, 8) | 7 (6, 8) | ||
| Expense ($) | Minor | 5792 (4414, 6715) | 4164 (2401, 5499) | < .001 |
| Moderate | 6953 (4273, 10,224) | 5951 (4301, 8621) | ||
| Major | 13,622 (8219, 28,553) | 10,519 (5249, 15,581) | ||
| Extreme | 18,908 (12913, 24,688) | 16,192 (6135, 26,147) | ||
| Revenue ($) | Minor | 7095 (6611, 7212) | 7116 (4160, 7218) | .06 |
| Moderate | 7118 (7025, 7215) | 6893 (3755, 7164) | ||
| Major | 9601 (6972, 16,668) | 6743 (4612, 7116) | ||
| Extreme | 11,019 (10,009, 24,897) | 9184 (5783, 13,931) | ||
| Margin ($) | Minor | 786 (162, 2997) | 2290 (409, 4768) | .14 |
| Moderate | 256 (1999, 3366) | 796 (2741, 1565) | ||
| Major | 2314 (7870, 1448) | 3499 (8818, 1008) | ||
| Extreme | 1263 (2904, 4012) | 6537 (15,617, 3050) | ||
| Nonhospitalist cases (%) (n = 216) | Hospitalist cases (%) (n = 126) | P value | |
|---|---|---|---|
| |||
| Acute renal failure | 2 (1) | 0 (0) | 0.53 |
| In‐hospital mortality | 9 (4) | 0 (0) | 0.03 |
| Readmission for any reason | 53 (25) | 35 (28) | 0.52* |
| Readmission for CHF | 19 (9) | 18 (14) | 0.16* |
In‐hospital mortality of patients treated by hospitalists was lower than that of non‐hospitalist‐treated patients (0% vs. 4%; P =.03). Rates of acute renal failure, overall readmissions and readmissions specifically for congestive heart failure did not differ significantly. Notably, severity of illness assessed by APS‐DRG did not differ between hospitalists' and nonhospitalists' patients (P = .13).
DISCUSSION
Practice Patterns
Our study identified specific practices that hospitalists use more than non‐hospitalists in the management of patients with CHF. These practices, which may have resulted in decreased LOS and lower costs, included higher use of ACE‐I/ARB within 24 hours of admission and of intravenous diuretics. We hypothesized that earlier and more aggressive use of ACE‐I/ARB contributed to after‐load reduction and alteration of cardiac remodeling5 and may have led to faster recovery and improved outcomes. Greater use of intravenous diuretics may signify that hospitalists have a more aggressive approach to managing exacerbations of acute congestive heart failure, which may also lead to faster recovery.
Hospitalists used fewer beta‐blockers on admission and at discharge. Reasons for this finding remain unclear; however, it may have been a result of the practice of avoiding beta‐blockers during exacerbations of acute CHF and the subsequent reliance on primary care providers to restart beta‐blockers after discharge. Lower use of beta‐blockers did not appear to have a negative impact on mortality or readmission rates.
Resource Utilization
Hospitalists used fewer serial chest x‐rays, more initial BNP measurements, and more social work consults, and there was a trend toward their using fewer repeat BNP measurements. The less frequent use of serial chest x‐rays may be a result of hospitalists being able to assess patients more frequently and to rely less on imaging. Higher rates of initial BNP measurement by hospitalists may reflect the ordering patterns of the emergency room physicians because most patients are admitted to the hospitalists via the emergency room. The trend toward fewer repeat BNP measurements by hospitalists may again reflect their ability to perform more frequent clinical assessments and to rely less on laboratory data. The higher rate of utilization of social workers by hospitalists is likely a reflection of a population in need of such interventions rather than the hospitalists having a lower threshold before requesting a social work consultation. There were no differences in the rates of obtaining echocardiograms, physical therapy, and dietary consults and of sodium and fluid restrictions.
Clinical Outcomes
Severity of illness assessed by APS‐DRG did not differ between the patients cared for by hospitalists and those care for by non‐hospitalists (P = .13) despite the hospitalists caring for a younger population. In‐hospital mortality of hospitalist‐treated patients was lower (0% vs. 4%), whereas the rates of readmission and renal failure did not differ between the 2 groups. A slight advantage in the mortality rate appears to be in agreement with prior findings3, 4; however, this may have been a result of the non‐hospitalists caring for an older patient population.
Economic Outcomes
The shorter LOS and lower overall costs of patients followed by hospitalists supports previous findings.2, 3, 10 The LOS in our study was found to be shorter for hospitalist‐treated patients whose illnesses were in the minor, moderate, and extreme severity categories by 40%, 20%, and 13%, respectively. The median expense per case was less across all severity categories, ranging from $1000 to $3100 for the patients followed by hospitalists compared with those followed by non‐hospitalists. There was a trend toward lower adjusted median revenue in all categories except for minor severity for hospitalists' patients (P = .06). The profit margin per case did not differ significantly between patients cared for by hospitalists and non‐hospitalists. The shorter LOS and lower expenses per case of patients under the care of hospitalists should have led to higher revenue and profit margin. However, our study showed lower revenue and no significant differences in profit margin, which may be explained by the fact that the hospitalists' patients had a worse insurance mix with a higher proportion of uninsured and Medicaid patients. It is also possible that non‐hospitalists, in particular, cardiologists, generate higher revenue by performing more procedures such as cardiac catheterizations, thus offsetting the costs.
As noted above, the analysis of LOS, expenses, revenue, and margin controlled for age, comorbidities, severity of illness, and insurance status (Table 3). The results were not significantly affected by adjusting for age, insurance status, and comorbidities after controlling for severity. The difference in age may in part be a result of older patients having established relationships with primary care physicians and being less likely to be admitted by hospitalists. It may also reflect the high prevalence of methamphetamine abuse, which has reached epidemic proportions in Hawaii, and methamphetamine‐induced cardiomyopathy in a younger population of patients followed by hospitalists. Further studies would be necessary to estimate the impact of drug‐induced congestive heart failure in these populations.
Although our study provided a detailed look at practice patterns of a coherent hospitalist group, it had several important limitations. It was a retrospective study conducted at a single institution, making the findings difficult to generalize to hospitalist practices nationwide. It included an unusually large number of non‐Caucasian patients, reflecting the demographics of the state of Hawaii. Data on contraindications to ACE‐I/ARB were not collected because the degree of renal dysfunction that would serve as a contraindication was difficult to define. The primary mode of adjustment was APS, which may have been a limiting factor in assessing severity of illness. The inability to follow patients' course after discharge limited collection of long‐term outcomes data.
In agreement with previous studies, we showed a decreased LOS and lower expenses per case of patients cared for by full‐time hospitalists while preserving quality of care and improving clinical outcomes. We identified specific practices of hospitalists in the management of patients with CHF that differ from those of non‐hospitalists. These practices include early use of ACE‐I/ARB, aggressive approach to diuresis, higher utilization of social work services, and decreased utilization of serial chest x‐rays, medical consultants, and serial BNP measurements. Our study was not designed to identify a direct causal relationship between hospitalist practices and improved outcomes; however, we believe it to be the first step in understanding practice patterns and the impact of the hospitalist movement.
- ,,,,.Advances in hospital medicine: a review of key articles from the literature.Med Clin North Am.2002;86:797–823.
- ,,, et al.The positive impact of initiation of hospitalist clinician educators.J Gen Intern Med.2004;19:293–301.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty.Ann Intern Med.2004;141:28–38.
- ,,.Advances in the management of acute and chronic decompensated heart failure.Lippincotts Case Manag.2004;9:S1–S15.
- ,,,, et al.ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult.Circulation.2001;104:2996–3007.
- American Heart Association.Heart disease and stroke statistics—2003 update.2003.
- .Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency department.Rev Cardiovasc Med.2002;3(suppl):S3–S9.
- ,,,.Comparison of processes and outcomes of pneumonia care between hospitalists and community‐based primary care physicians.Mayo Clin Proc.2002;77:1053–1058.
- ,,,,.Economic effects of community versus hospital‐based faculty pneumonia care.J Gen Intern Med.1998;13:774–777.
- ,,,,.Quality of care for patients hospitalized with heart failure. Assessing the impact of hospitalists.Arch Intern Med.2002;162:1251–1256.
- ,,, et al.ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in adult.ACC/AHA Pract Guidel.2005:1–82
- ,,.Importance of in‐hospital initiation of evidence‐based medical therapies for heart failure—a review.Am J Cardiol.2004;94:1155–1160.
- .Role of in‐hospital initiation of carvedilol to improve treatment rates and clinical outcomes.Am J Cardiol.2004;93(suppl):77B–81B.
- ,.Rationale and design of the Cardiac Hospitalization Atherosclerosis Management Program at the University of California Los Angeles.Am J Cardiol.2000;85:10A–17A.
- ,,,,.Advances in hospital medicine: a review of key articles from the literature.Med Clin North Am.2002;86:797–823.
- ,,, et al.The positive impact of initiation of hospitalist clinician educators.J Gen Intern Med.2004;19:293–301.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty.Ann Intern Med.2004;141:28–38.
- ,,.Advances in the management of acute and chronic decompensated heart failure.Lippincotts Case Manag.2004;9:S1–S15.
- ,,,, et al.ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult.Circulation.2001;104:2996–3007.
- American Heart Association.Heart disease and stroke statistics—2003 update.2003.
- .Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency department.Rev Cardiovasc Med.2002;3(suppl):S3–S9.
- ,,,.Comparison of processes and outcomes of pneumonia care between hospitalists and community‐based primary care physicians.Mayo Clin Proc.2002;77:1053–1058.
- ,,,,.Economic effects of community versus hospital‐based faculty pneumonia care.J Gen Intern Med.1998;13:774–777.
- ,,,,.Quality of care for patients hospitalized with heart failure. Assessing the impact of hospitalists.Arch Intern Med.2002;162:1251–1256.
- ,,, et al.ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in adult.ACC/AHA Pract Guidel.2005:1–82
- ,,.Importance of in‐hospital initiation of evidence‐based medical therapies for heart failure—a review.Am J Cardiol.2004;94:1155–1160.
- .Role of in‐hospital initiation of carvedilol to improve treatment rates and clinical outcomes.Am J Cardiol.2004;93(suppl):77B–81B.
- ,.Rationale and design of the Cardiac Hospitalization Atherosclerosis Management Program at the University of California Los Angeles.Am J Cardiol.2000;85:10A–17A.
Copyright © 2008 Society of Hospital Medicine
Glycemic Control in Medical Inpatients
Diabetes mellitus is a common comorbid condition in hospitalized patients. In 2003, diabetes was listed as a diagnosis in 17.2% of hospital discharges in the United States.1 Because these diagnosis codes do not account for undiagnosed diabetes or hospital‐related hyperglycemia, the true prevalence of diabetes or hyperglycemia in hospitalized patients is likely higher and has been estimated to be as great as 38%.2 Hyperglycemia has been associated with adverse outcomes among hospitalized patients, including infectious complications, increased length of stay, and increased mortality.27 However, because hyperglycemia is not usually the primary reason patients with diabetes are hospitalized, its management is often not a focus in the inpatient setting. Sliding‐scale insulin alone continues to be commonly prescribed despite clinical evidence showing it to be ineffective in achieving glycemic control.8, 9
Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units10, 11 and in patients admitted for myocardial infarction.12, 13 Based on this clinical evidence and strong observational data linking hyperglycemia to poor patient outcomes in the non‐ICU setting,27 the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90‐130 mg/dL and peak postprandial glucose levels < 180 mg/dL in hospitalized non‐ICU patients with hyperglycemia14 (note that these targets are less aggressive than those for ICU patients, for whom randomized controlled trials showed the benefits of reduced mortality provided by tight glucose control).11 To reach these targets, the ADA and American College of Endocrinology suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.15 Protocols should promote the use of continuous intravenous insulin or scheduled subcutaneous insulin as opposed to the use of sliding‐scale insulin alone. Subcutaneous insulin protocols should include target glucose levels; basal, nutritional, and supplemental insulin; and daily adjustments based on previous glucose levels, insulin sensitivity, nutritional intake, illness, and medications.6, 15 To date, few published protocols or algorithms for inpatient subcutaneous insulin have been shown to be effective.16, 17 It is therefore not known how best to design and implement an inpatient diabetes management protocol that is effective, efficient, and self‐perpetuating. The aims of our pilot study were to develop and implement a subcutaneous insulin protocol on a general medicine service, to identify barriers to implementation, and to determine the effect of this protocol on glycemic control.
METHODS
Setting and Participants
This prospective quality‐improvement pilot study was conducted at Brigham and Women's Hospital (BWH) from January 10, 2005, through June 23, 2005. Patients were eligible to participate if they were admitted to either of 2 General Medicine Service (GMS) teams with either a known diagnosis of type 2 diabetes or inpatient hyperglycemia (random laboratory glucose level > 180 mg/dL) and at least 1 fasting point‐of‐care glucose reading > 140 mg/dL. Patients were excluded if they had diabetic ketoacidosis, hyperosmolar hyperglycemic state, another absolute indication for intravenous insulin, or fasting glucose < 60 mg/dL on no insulin or if they were pregnant. Each GMS team consisted of a teaching attending, a junior or senior resident, 2 interns, and a clinical pharmacist. Twenty‐six physicians attended on these 2 teams during the study period, 13 of whom were hospitalists. This study was approved by the BWH Institutional Review Board; patient consent to participate in this study was deemed not necessary because of the relatively nonsensitive nature of the data (eg, glucose control, insulin orders), the noninvasive means of data collection (eg, chart review), and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
A multidisciplinary team composed of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (J.M.T.) developed a subcutaneous insulin protocol that was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee. The protocol consisted of a set of treatment recommendations made by a pharmacist to be carried out by the medical team. The primary components are shown in Table 1 (a full description can be found in the Appendix). The main emphasis of the protocol was on discontinuing oral antihyperglycemic agents during hospitalization, initiating basal insulin in most patients, and adjusting basal insulin daily as needed.
|
| Oral agents |
| 1. Stop oral agents in most patients |
| Glucose testing |
| 2. Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if not eating |
| Insulin |
| 3. Start basal insulin Patient's home dose or NPH 0.1 units/kg before breakfast and at bedtime or insulin glargine 0.2 units/kg at bedtime (max dose 20 units) If NPO, consider half dose unless hyperglycemic |
| 4. Start nutritional insulin Discrete meals: insulin aspart 0.05‐0.1 units/kg per meal or home dose 0‐15 minutes prior to eating Continuous tube feeds: regular insulin every 6 hours or NPH every morning and at bedtime (0.1‐0.2 units/kg per day in addition to basal insulin) Hold if NPO |
| 5. Start correctional insulin Scale provided based on blood glucose and daily scheduled insulin requirements |
| Daily Adjustments |
6. Adjust scheduled insulin daily
|
| Other Considerations |
| 7. Hypoglycemia management (protocols for fruit juice, glucagons, IV dextrose, and when to call physician) |
| 8. Discharge orders (recommendations to discharge most patients on admission medication regimen, avoid sliding scale insulin, simplify dosing for patients requiring new insulin regimens, ensure adequate patient education and prompt outpatient follow‐up) |
All medical residents received general instructions regarding inpatient diabetes control by the research team's diabetologist (M.L.P.) through a 1‐hour department‐wide didactic lecture. The standards of care taught were identical to those in the protocol. In addition, the research team's hospitalist (J.L.S.) contacted each medical resident assigned to the 2 GMS teams electronically to introduce the protocol and describe the purpose and logistics of the pilot study.
A research assistant prospectively identified eligible patients each weekday by screening all patients admitted to the 2 GMS teams using the daily computerized sign‐out system used by all medical residents. Specifically, laboratory random glucose levels, inpatient medications, and medical history were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were confirmed by medical record review. The pharmacist recommended to the primary team that the protocol be initiated for eligible patients. In addition, the pharmacist recommended daily adjustment of the insulin dose according to the protocol as appropriate. A chronologically organized summary of clinical data relevant to glycemic management for each patient, including bedside blood glucose measurements, general dietary intake, use of intravenous dextrose solutions, and administration of systemic steroids, oral diabetes medications, and all insulins, was provided to the team each day by the research assistant.
Measurements
The resident's acceptance of the protocol or reasons for declining it were recorded by the pharmacist on the day the protocol was recommended. Protocol acceptance was categorized as yes, no, or partial. Partial acceptance was defined as resident agreement to use the protocol, but with stated caveats or modifications. Clinical data were collected on each eligible patient for up to 7 days on GMS. Several data sources were used, including physician admission notes, the hospital's computerized clinical data system, vital‐sign sheets, medication administration records, and personal communication with nurses regarding any missing or discrepant data.
All insulin use (prescribed drug, dose, route, schedule and actual administered drug, dose, route, and time) was recorded each day by the research assistant. Use of basal and nutritional insulin and daily dose adjustments if previous hypo‐ or hyperglycemia (categorized as yes, no, or not applicable for each patient each day) were determined by the study pharmacist (J.M.T.) through retrospective review of all orders.
Up to 4 routine bedside blood glucose measurements were recorded each day: for patients eating discrete meals, these were the measurements taken before meals and at bedtime; for patients not eating or receiving continuous nutrition, these were the measurements taken closest to 6 AM, noon, 6 PM, and midnight. Additional measurements were not recorded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Glucose readings on the day of admission were excluded from analysis because these values are not amenable to inpatient ordering practices.
Study outcomes included overall protocol acceptance rate, insulin prescribing practices including use of basal insulin (ie, long‐acting agents such as NPH and insulin glargine), nutritional insulin (ie, scheduled regular, lispro, or aspart insulin given before each meal), daily dose adjustments under the protocol, and mean percentage of glucose readings per person greater than 180 mg/dL (hyperglycemia) and below 60 mg/dL (hypoglycemia). Comparable data from a previous cohort study of 91 GMS patients were used as baseline data for comparisons with the results of the present study.9
Other patient data collected included age, sex, weight, baseline A1C (taken at or within 6 months of admission), diabetic medications used prior to admission (none, oral agents only, or any insulin use); daily inpatient use of oral or intravenous steroids, oral diabetic medications, dextrose‐containing intravenous fluids, tube feeds, total parenteral nutrition, and general nutritional intake (nothing by mouth, clear diet, low carbohydrate diet, house diet).
Statistical Analysis
Characteristics of the study subjects and process and outcome measures were analyzed descriptively using rates, means, and standard deviations or medians with interquartile ranges as appropriate. Comparisons between the pilot study and baseline cohorts were performed using Fisher's exact test for dichotomous outcomes (eg, use of basal insulin). For rates of hyperglycemia (ie, fraction of readings > 180 mg/dL), we used binomial logistic regression, accounting for potential correlation among repeated events by individual patients with a dispersion parameter18 (note that we did not use the same analysis for rates of hypoglycemia because it was such a rare event; for analysis of hypoglycemia, the variables were dichotomized). We also analyzed outcomes by hospital day (through hospital day 5, the limit used in the baseline study) to determine daily trends during the course of hospitalization; for these analyses we used the Mantel‐Haenszel chi‐square test for dichotomous variables and binomial logistic regression with hospital day as the independent variable for rates of hyperglycemia. Two‐sided P values < .05 were considered significant. SAS version 9.1 (Cary, NC) was used for all analyses.
RESULTS
After screening all 785 admissions to the 2 medical teams during the study period, we prospectively identified 109 patients (14%) for the pilot study. Twenty patients were subsequently excluded: 7 patients who were discharged the same day they were identified, 4 who did not have a fasting blood glucose value greater than 140 mg/dL, 4 patients who had type 1 diabetes, 2 patients who were admitted with diabetic ketoacidosis, and 3 patients whose data could not be accessed because of repeated unavailability of the medical record. Characteristics of the remaining 89 study subjects are shown in Table 2 and are compared to 91 baseline subjects. The mean age of the study subjects was 68.7 years; 45% were men. Five patients (6%) did not have a previous diagnosis of diabetes, and 51% were taking insulin prior to admission; the median A1C was 6.8%.
| Characteristic | Baseline (n = 91) | Pilot (n = 89) |
|---|---|---|
| ||
| Age (years), mean (SD) | 66.0 (14.5) | 68.7 (14.7) |
| Male | 53/91 (58%) | 40/89 (45%) |
| No diagnosis of diabetes at admission | 7/91 (8%) | 5/89 (6%) |
| Preadmission diabetes regimen | ||
| None | 15/91 (16%) | 14/78 (18%) |
| Oral medications only | 32/91 (35%) | 24/78 (31%) |
| Insulin | 44/91 (48%) | 40/78 (51%) |
| A1C (IQR) | 7.0 (6.0, 8.0) | 6.8 (6.3, 7.8) |
| Hospital length of stay (days), median (IQR) | 5 (3, 7) | 5 (3, 7) |
The medical residents agreed, at least in theory, to follow the subcutaneous insulin protocol for 50 patients (56%), partially accepted it for 8 (9%), and declined for 31 (35%). Reasons for declining the protocol included fear of hypoglycemia, severity of patient's other disease states or overall poor health of patient, concern for the effects of renal insufficiency on insulin clearance, concern for the effect of steroid tapers on glucose levels, desire to titrate oral medications, and anticipation of patient's imminent discharge. Other reasons such as the glucose levels are not that bad and let's watch the glucose levels for one more day suggest that some residents did not view hyperglycemia as an acute problem requiring immediate attention.
Regarding insulin‐ordering practices (Table 3), basal insulin was prescribed for 57 patients (64%) in the pilot group compared to 45 patients (49%) in the baseline group (P = .05). Nutritional insulin was prescribed to 12 patients (13%) in the pilot group compared to no patients in the baseline group (P < .001). Oral hypoglycemic agents were prescribed less often in the pilot study than at baseline (20% vs. 38%, P = .01). The use of a standard default sliding scale from the hospital computer order set was high and was not significantly different in the pilot study compared with that at baseline (93% vs. 90%, P = .78). Twenty‐four of the 83 patients in the pilot group (29%) received sliding‐scale insulin without ever receiving basal or nutritional insulin during hospitalization compared to 45 of 91 patients in the baseline group (49%; P = .01 for comparison). Among patients started on basal insulin, 42% (24 of 57) were started after the first full hospital day. The initial basal insulin dose was appropriate according to the protocol (within 20%) in 38 of 57 patients (67%). Only 20 of 61 patients (33%) who had any hypo‐ or hyperglycemia had any change to their insulin regimen made during days 2 through 7 of their hospitalization on GMS, similar to the rate noted at baseline (36%).
| Measure | Baseline | Pilot | P value |
|---|---|---|---|
| |||
| Process | |||
| Any basal insulin during hospitalization | 45/91 (49%) | 57/89 (64%) | 0.05 |
| Any nutritional insulin during hospitalization | 0/91 (0%) | 12/89 (13%) | < 0.001 |
| Change in dose to any insulin order during hospitalization | 24/66 (36%) | 20/61 (33%) | 0.71 |
| Standard sliding scale from hospital computer order set | 75/83 (90%) | 76/82 (93%) | 0.78 |
| Any oral antihyperglycemic agents during hospitalization | 35/91 (38%) | 18/89 (20%) | 0.01 |
| Outcome | |||
| Mean percentage of glucose readings > 180 mg/dL (SD) | 33.3% (33.3%) | 31.6% (29.6%) | 0.85 |
| Any hyperglycemia (glucose > 180 mg/dL) | 66/89 (74%) | 59/78 (76%) | 0.86 |
| 1%‐20% of readings | 17/89 (19%) | 15/78 (19%) | 0.85 for trend |
| 20%‐40% | 15/89 (17%) | 15/78 (19%) | |
| 40%‐60% | 15/89 (17%) | 15/78 (19%) | |
| 60%‐80% | 7/89 (8%) | 6/78 (8%) | |
| >80% | 12/89 (13%) | 8/78 (10%) | |
| Any hypoglycemia (glucose < 60 mg/dL) | 6/89 (7%) | 10/78 (13%) | 0.20 |
Regarding glucose control (Table 3), the mean percentage of glucose readings per patient greater than 180 mg/dL was not significantly different in the pilot study compared to baseline (31.6% vs. 33.3%, P = .85). Despite implementation of the protocol and increased use of basal and nutritional insulin, 76% of patients had at least 1 routine glucose reading greater than 180 mg/dL, and 37% of patients had at least 40% of their routine glucose readings greater than 180 mg/dL, comparable to baseline (74% and 38%, respectively, P = NS for both comparisons). At least 1 hypoglycemic event (glucose reading below 60 mg/dL) occurred in 7% of patients at baseline and 13% during the pilot study (P = .20). Eleven hypoglycemic events in the pilot study were between 50 and 59 mg/dL (55%), 6 were between 40 and 49 mg/dL (30%), 3 were between 30 and 39 mg/dL (15%), and none were less than 30 mg/dL. Nine occurred before breakfast (45%), 5 before dinner (25%), 3 before lunch (15%), and 3 at bedtime (15%).
During the pilot study, the use of basal insulin did improve over the first 5 days of hospitalization (Fig. 1), in both the percentage of patients prescribed any basal insulin and the percentage of each patient's total insulin dose (basal, nutritional, and supplemental) given as basal (both P < .001 for trend). Hyperglycemia rates also improved during hospitalization (Fig. 1), decreasing from 48% on hospital day 1 to 34% on hospital day 5 (P = .004 for trend). These trends were not observed in the baseline group, with hyperglycemia rates of 37% on hospital day 1 and 34% on hospital day 5 (P = .16 for trend).
Patients for whom the resident accepted or partially accepted the protocol had higher use of basal insulin (91% vs. 13%, P < .0001), higher use of nutritional insulin (21% vs. 0%, P = .01), and more frequent dose adjustments (47% vs. 7%, P = .01) compared with patients for whom the resident declined the protocol. However, the rate of hyperglycemia was higher in patients for whom the protocol was accepted or partially accepted than in patients for whom the protocol was declined (37% vs. 20%, P = .02).
DISCUSSION
Our subcutaneous insulin protocol focused on increasing the use of basal and nutritional insulin, avoiding the use of sliding‐scale insulin by itself, and performing daily insulin adjustments in response to the hypo‐ or hyperglycemia of general medical inpatients with diabetes or hyperglycemia.
The most notable finding of our pilot study was that residents were resistant to using the protocol, both in general and in its specific recommendations. Despite receiving education about inpatient diabetes control and protocol recommendations from the team pharmacist, and despite being on a hospitalist‐run medical service, the residents accepted use of the protocol for only half the eligible patients. Patients who were started on basal insulin were often underdosed or started after the first day of hospitalization, and daily dose adjustments were not consistently made despite persistent hypo‐ or hyperglycemia. Although the use of nutritional insulin was greater compared with that in the baseline group, it was still only prescribed for 13% of patients. Use of a standard sliding scale from the hospital computer order set was common in the pilot study and similar to that in the baseline group. These results suggest significant resistance to changing the current standard of practice.
Despite this lack of adherence to the protocol, some modest improvements in processes of care were seen. Basal insulin was ordered more often during the pilot study than at baseline, especially over the course of a hospital stay. Nutritional insulin was also ordered more often during the pilot study than at baseline, but was still infrequent. Oral antihyperglycemic agents were ordered less often during the pilot study than at baseline. This demonstrates that use of the protocol may be able to improve process outcomes. However, the modest improvements in process outcomes could have simply been a result of increased awareness and education, not the protocol itself.
Regarding patient outcomes, the overall hyperglycemia rate did not improve in the pilot study relative to that at baseline. Importantly, hypoglycemia rates did not increase significantly compared with those at baseline. However, because of the small number of hypoglycemia events, the sample size may not have been sufficient to detect a true difference between groups.
The most likely reason that the protocol did not show an effect on glycemic control was that its recommendations were not adhered to. In turn, this may have been a result of incomplete education, training, and implementation measures and/or inherent problems with the protocol that made its recommendations difficult to follow. Another possibility is that the protocol itself may not have been capable of improving glucose control, even when properly used. However, we do know that resident agreement to use the protocol did lead to higher rates of recommended best practices being carried out, such as basal insulin use and daily insulin dose adjustments, and that use of the protocol was associated with improvements in glucose control over the hospital stay. A larger study with a higher degree of protocol adherence would be better able to evaluate the merits of the protocol itself, as would a randomized controlled trial using instrumental variables to measure treatment efficacy. Another possibility explanation for the lack of effect is that glucose control on admission happened to be worse in the pilot group than in the control group: rates of hyperglycemia on day 1 were 48% in the pilot group compared with 37% in the baseline group (Fig. 1). Also, the decreased use of oral agents in the pilot group, a purposeful change to decrease the risk of hypoglycemia, may have counteracted the beneficial effects of more appropriate insulin use. Finally, there were few patients with poorly controlled diabetes at baseline (18 patients with A1C 8.0 in the baseline group and 12 such patients in the pilot group), arguably those most likely to benefit.
There is a pressing need to identify protocols that can improve glucose control in the non‐ICU inpatient setting and successfully implement these protocols with a minimum of resources and effort. To date, most studies that have improved glucose control in the non‐ICU setting have relied on frequent input from diabetologists or nurse‐practitioners.14, 15
The results of this study should be viewed in light of its limitations, including its relatively small sample size (thus limiting our ability to detect possible significant differences between groups) and that it was conducted at a single institution (thus limiting its generalizability). Patients were enrolled on weekdays, so patients admitted and discharged over a weekend or on a holiday may have been missed. Also, because of the nonrandomized design of the study, we cannot exclude the possibility that the improvements noted in the pilot study were a result of the increased education provided or of increased awareness and general improvement in diabetes management over the course of the study. Finally, implementation of the protocol was somewhat labor intensive and required staff support that could be difficult to replicate in other institutions. However, most of the study staff's effort was necessary either to implement the protocol in the absence of an order set or to evaluate barriers to implementation. Widespread implementation of a protocol with an order set, education, and the use of highly reliable tools should be possible with much less effort and resources. The strengths of this study include its prospective data collection methods, which included rigorous inclusion criteria and collection of detailed clinical data.
Our study findings suggest several approaches to improve care in the future. To combat resistance to change, the American Association of Clinical Endocrinologists strongly recommends that each institution ensure that all its clinicians involved agree about general philosophies of diabetes management.19 A more expansive, hospital‐wide educational and promotional plan may increase the initial acceptance of the protocol. Interviews with residents also indicated there was unfamiliarity with diabetes management and significant concerns about the harmful affects of tight glucose control (ie, risk of hypoglycemia), especially in certain patient subgroups. These results confirmed the need for more practical individualized training and sparked the implementation of small‐group, case‐based educational sessions on inpatient diabetes management for all house officers, with a particular focus on patients with multiple comorbidities, on steroid tapers, and/or with renal failure.
The lack of nutritional insulin orders, delays in ordering basal insulin, and use of inadequate doses of insulin may be counteracted by the use of an order set, in our case built into our computer physician order entry (CPOE) system. The use of CPOE also allows reminders to be automatically sent to clinicians if eligible patients are not started on these orders. Clinical inertia (eg, failure to adjust the insulin doses of specific patients despite hyperglycemia) is more difficult to combat but may be addressed through better organization of clinical data, individualized, case‐based education, and CPOE reminders and eventually through culture change.
As a result of our pilot study, additional revisions were made to the protocol in hopes of increasing protocol adherence. For example, for patients eating discrete meals who are not taking insulin at home, the pilot protocol had suggested a starting insulin dose range for basal and nutritional insulin that required 2 separate calculations. The revised protocol was simplified to recommend a total daily insulin dose to be split evenly between basal and nutritional insulin. The daily adjustment instructions were also simplified. The pilot protocol had included a complicated table of adjustment recommendations based on bedside glucose trends. The revised protocol recommends adjusting the new daily dose by adding the total units of insulin given the previous day (including supplemental doses), making minor adjustments for hyper‐ or hypoglycemia and other clinical factors (like renal failure), and splitting this dose evenly between scheduled basal and nutritional insulin. In addition, 3 order sets were built into our computerized physician order entry system to facilitate early and appropriate insulin orders for patients with different diets (discrete meals, continuous tube feeds, and nothing by mouth); 3 different insulin sliding scales were created for patients with different degrees of insulin resistance; a diabetes management page for our electronic medication administration record is being developed to better organize clinical data; and hospital‐wide education and individualized training are ongoing.
In conclusion, the adherence to an inpatient glycemic management protocol that focused on increasing use of basal insulin and performing daily insulin adjustments was only fair. Barriers to successful implementation included clinical inertia regarding individual patients, unfamiliarity with inpatient diabetes management strategies, fear of hypoglycemia, and resistance to changing the current standard of practice. Targeted education, standard order sets, better organization of clinical data, protocol simplification, and institutional culture changes may be necessary for successful protocol implementation and improved inpatient glucose control.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. 8/17/05; http://www.ahrq.gove/HCUPnet/. Accessed 7/17/06,2006.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity.Eur Heart J.2005;26:650–661.
- American Diabetes Association.Standards of Medical Care in Diabetes ‐ 2006.Diabetes Care.2006;29:S4–S42.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control: A call to action.Diabetes Care.2006;29:1955–1962.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–11.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial).Diabetes Care.2007;30:2181–2186.
- .Extra‐binomial variation in logistic linear models.Appl Stat.1982;31:144–148.
- ,.Hospital management of diabetes.Endocrinol Metab Clin North Am.2005;34:99–116.
Diabetes mellitus is a common comorbid condition in hospitalized patients. In 2003, diabetes was listed as a diagnosis in 17.2% of hospital discharges in the United States.1 Because these diagnosis codes do not account for undiagnosed diabetes or hospital‐related hyperglycemia, the true prevalence of diabetes or hyperglycemia in hospitalized patients is likely higher and has been estimated to be as great as 38%.2 Hyperglycemia has been associated with adverse outcomes among hospitalized patients, including infectious complications, increased length of stay, and increased mortality.27 However, because hyperglycemia is not usually the primary reason patients with diabetes are hospitalized, its management is often not a focus in the inpatient setting. Sliding‐scale insulin alone continues to be commonly prescribed despite clinical evidence showing it to be ineffective in achieving glycemic control.8, 9
Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units10, 11 and in patients admitted for myocardial infarction.12, 13 Based on this clinical evidence and strong observational data linking hyperglycemia to poor patient outcomes in the non‐ICU setting,27 the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90‐130 mg/dL and peak postprandial glucose levels < 180 mg/dL in hospitalized non‐ICU patients with hyperglycemia14 (note that these targets are less aggressive than those for ICU patients, for whom randomized controlled trials showed the benefits of reduced mortality provided by tight glucose control).11 To reach these targets, the ADA and American College of Endocrinology suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.15 Protocols should promote the use of continuous intravenous insulin or scheduled subcutaneous insulin as opposed to the use of sliding‐scale insulin alone. Subcutaneous insulin protocols should include target glucose levels; basal, nutritional, and supplemental insulin; and daily adjustments based on previous glucose levels, insulin sensitivity, nutritional intake, illness, and medications.6, 15 To date, few published protocols or algorithms for inpatient subcutaneous insulin have been shown to be effective.16, 17 It is therefore not known how best to design and implement an inpatient diabetes management protocol that is effective, efficient, and self‐perpetuating. The aims of our pilot study were to develop and implement a subcutaneous insulin protocol on a general medicine service, to identify barriers to implementation, and to determine the effect of this protocol on glycemic control.
METHODS
Setting and Participants
This prospective quality‐improvement pilot study was conducted at Brigham and Women's Hospital (BWH) from January 10, 2005, through June 23, 2005. Patients were eligible to participate if they were admitted to either of 2 General Medicine Service (GMS) teams with either a known diagnosis of type 2 diabetes or inpatient hyperglycemia (random laboratory glucose level > 180 mg/dL) and at least 1 fasting point‐of‐care glucose reading > 140 mg/dL. Patients were excluded if they had diabetic ketoacidosis, hyperosmolar hyperglycemic state, another absolute indication for intravenous insulin, or fasting glucose < 60 mg/dL on no insulin or if they were pregnant. Each GMS team consisted of a teaching attending, a junior or senior resident, 2 interns, and a clinical pharmacist. Twenty‐six physicians attended on these 2 teams during the study period, 13 of whom were hospitalists. This study was approved by the BWH Institutional Review Board; patient consent to participate in this study was deemed not necessary because of the relatively nonsensitive nature of the data (eg, glucose control, insulin orders), the noninvasive means of data collection (eg, chart review), and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
A multidisciplinary team composed of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (J.M.T.) developed a subcutaneous insulin protocol that was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee. The protocol consisted of a set of treatment recommendations made by a pharmacist to be carried out by the medical team. The primary components are shown in Table 1 (a full description can be found in the Appendix). The main emphasis of the protocol was on discontinuing oral antihyperglycemic agents during hospitalization, initiating basal insulin in most patients, and adjusting basal insulin daily as needed.
|
| Oral agents |
| 1. Stop oral agents in most patients |
| Glucose testing |
| 2. Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if not eating |
| Insulin |
| 3. Start basal insulin Patient's home dose or NPH 0.1 units/kg before breakfast and at bedtime or insulin glargine 0.2 units/kg at bedtime (max dose 20 units) If NPO, consider half dose unless hyperglycemic |
| 4. Start nutritional insulin Discrete meals: insulin aspart 0.05‐0.1 units/kg per meal or home dose 0‐15 minutes prior to eating Continuous tube feeds: regular insulin every 6 hours or NPH every morning and at bedtime (0.1‐0.2 units/kg per day in addition to basal insulin) Hold if NPO |
| 5. Start correctional insulin Scale provided based on blood glucose and daily scheduled insulin requirements |
| Daily Adjustments |
6. Adjust scheduled insulin daily
|
| Other Considerations |
| 7. Hypoglycemia management (protocols for fruit juice, glucagons, IV dextrose, and when to call physician) |
| 8. Discharge orders (recommendations to discharge most patients on admission medication regimen, avoid sliding scale insulin, simplify dosing for patients requiring new insulin regimens, ensure adequate patient education and prompt outpatient follow‐up) |
All medical residents received general instructions regarding inpatient diabetes control by the research team's diabetologist (M.L.P.) through a 1‐hour department‐wide didactic lecture. The standards of care taught were identical to those in the protocol. In addition, the research team's hospitalist (J.L.S.) contacted each medical resident assigned to the 2 GMS teams electronically to introduce the protocol and describe the purpose and logistics of the pilot study.
A research assistant prospectively identified eligible patients each weekday by screening all patients admitted to the 2 GMS teams using the daily computerized sign‐out system used by all medical residents. Specifically, laboratory random glucose levels, inpatient medications, and medical history were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were confirmed by medical record review. The pharmacist recommended to the primary team that the protocol be initiated for eligible patients. In addition, the pharmacist recommended daily adjustment of the insulin dose according to the protocol as appropriate. A chronologically organized summary of clinical data relevant to glycemic management for each patient, including bedside blood glucose measurements, general dietary intake, use of intravenous dextrose solutions, and administration of systemic steroids, oral diabetes medications, and all insulins, was provided to the team each day by the research assistant.
Measurements
The resident's acceptance of the protocol or reasons for declining it were recorded by the pharmacist on the day the protocol was recommended. Protocol acceptance was categorized as yes, no, or partial. Partial acceptance was defined as resident agreement to use the protocol, but with stated caveats or modifications. Clinical data were collected on each eligible patient for up to 7 days on GMS. Several data sources were used, including physician admission notes, the hospital's computerized clinical data system, vital‐sign sheets, medication administration records, and personal communication with nurses regarding any missing or discrepant data.
All insulin use (prescribed drug, dose, route, schedule and actual administered drug, dose, route, and time) was recorded each day by the research assistant. Use of basal and nutritional insulin and daily dose adjustments if previous hypo‐ or hyperglycemia (categorized as yes, no, or not applicable for each patient each day) were determined by the study pharmacist (J.M.T.) through retrospective review of all orders.
Up to 4 routine bedside blood glucose measurements were recorded each day: for patients eating discrete meals, these were the measurements taken before meals and at bedtime; for patients not eating or receiving continuous nutrition, these were the measurements taken closest to 6 AM, noon, 6 PM, and midnight. Additional measurements were not recorded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Glucose readings on the day of admission were excluded from analysis because these values are not amenable to inpatient ordering practices.
Study outcomes included overall protocol acceptance rate, insulin prescribing practices including use of basal insulin (ie, long‐acting agents such as NPH and insulin glargine), nutritional insulin (ie, scheduled regular, lispro, or aspart insulin given before each meal), daily dose adjustments under the protocol, and mean percentage of glucose readings per person greater than 180 mg/dL (hyperglycemia) and below 60 mg/dL (hypoglycemia). Comparable data from a previous cohort study of 91 GMS patients were used as baseline data for comparisons with the results of the present study.9
Other patient data collected included age, sex, weight, baseline A1C (taken at or within 6 months of admission), diabetic medications used prior to admission (none, oral agents only, or any insulin use); daily inpatient use of oral or intravenous steroids, oral diabetic medications, dextrose‐containing intravenous fluids, tube feeds, total parenteral nutrition, and general nutritional intake (nothing by mouth, clear diet, low carbohydrate diet, house diet).
Statistical Analysis
Characteristics of the study subjects and process and outcome measures were analyzed descriptively using rates, means, and standard deviations or medians with interquartile ranges as appropriate. Comparisons between the pilot study and baseline cohorts were performed using Fisher's exact test for dichotomous outcomes (eg, use of basal insulin). For rates of hyperglycemia (ie, fraction of readings > 180 mg/dL), we used binomial logistic regression, accounting for potential correlation among repeated events by individual patients with a dispersion parameter18 (note that we did not use the same analysis for rates of hypoglycemia because it was such a rare event; for analysis of hypoglycemia, the variables were dichotomized). We also analyzed outcomes by hospital day (through hospital day 5, the limit used in the baseline study) to determine daily trends during the course of hospitalization; for these analyses we used the Mantel‐Haenszel chi‐square test for dichotomous variables and binomial logistic regression with hospital day as the independent variable for rates of hyperglycemia. Two‐sided P values < .05 were considered significant. SAS version 9.1 (Cary, NC) was used for all analyses.
RESULTS
After screening all 785 admissions to the 2 medical teams during the study period, we prospectively identified 109 patients (14%) for the pilot study. Twenty patients were subsequently excluded: 7 patients who were discharged the same day they were identified, 4 who did not have a fasting blood glucose value greater than 140 mg/dL, 4 patients who had type 1 diabetes, 2 patients who were admitted with diabetic ketoacidosis, and 3 patients whose data could not be accessed because of repeated unavailability of the medical record. Characteristics of the remaining 89 study subjects are shown in Table 2 and are compared to 91 baseline subjects. The mean age of the study subjects was 68.7 years; 45% were men. Five patients (6%) did not have a previous diagnosis of diabetes, and 51% were taking insulin prior to admission; the median A1C was 6.8%.
| Characteristic | Baseline (n = 91) | Pilot (n = 89) |
|---|---|---|
| ||
| Age (years), mean (SD) | 66.0 (14.5) | 68.7 (14.7) |
| Male | 53/91 (58%) | 40/89 (45%) |
| No diagnosis of diabetes at admission | 7/91 (8%) | 5/89 (6%) |
| Preadmission diabetes regimen | ||
| None | 15/91 (16%) | 14/78 (18%) |
| Oral medications only | 32/91 (35%) | 24/78 (31%) |
| Insulin | 44/91 (48%) | 40/78 (51%) |
| A1C (IQR) | 7.0 (6.0, 8.0) | 6.8 (6.3, 7.8) |
| Hospital length of stay (days), median (IQR) | 5 (3, 7) | 5 (3, 7) |
The medical residents agreed, at least in theory, to follow the subcutaneous insulin protocol for 50 patients (56%), partially accepted it for 8 (9%), and declined for 31 (35%). Reasons for declining the protocol included fear of hypoglycemia, severity of patient's other disease states or overall poor health of patient, concern for the effects of renal insufficiency on insulin clearance, concern for the effect of steroid tapers on glucose levels, desire to titrate oral medications, and anticipation of patient's imminent discharge. Other reasons such as the glucose levels are not that bad and let's watch the glucose levels for one more day suggest that some residents did not view hyperglycemia as an acute problem requiring immediate attention.
Regarding insulin‐ordering practices (Table 3), basal insulin was prescribed for 57 patients (64%) in the pilot group compared to 45 patients (49%) in the baseline group (P = .05). Nutritional insulin was prescribed to 12 patients (13%) in the pilot group compared to no patients in the baseline group (P < .001). Oral hypoglycemic agents were prescribed less often in the pilot study than at baseline (20% vs. 38%, P = .01). The use of a standard default sliding scale from the hospital computer order set was high and was not significantly different in the pilot study compared with that at baseline (93% vs. 90%, P = .78). Twenty‐four of the 83 patients in the pilot group (29%) received sliding‐scale insulin without ever receiving basal or nutritional insulin during hospitalization compared to 45 of 91 patients in the baseline group (49%; P = .01 for comparison). Among patients started on basal insulin, 42% (24 of 57) were started after the first full hospital day. The initial basal insulin dose was appropriate according to the protocol (within 20%) in 38 of 57 patients (67%). Only 20 of 61 patients (33%) who had any hypo‐ or hyperglycemia had any change to their insulin regimen made during days 2 through 7 of their hospitalization on GMS, similar to the rate noted at baseline (36%).
| Measure | Baseline | Pilot | P value |
|---|---|---|---|
| |||
| Process | |||
| Any basal insulin during hospitalization | 45/91 (49%) | 57/89 (64%) | 0.05 |
| Any nutritional insulin during hospitalization | 0/91 (0%) | 12/89 (13%) | < 0.001 |
| Change in dose to any insulin order during hospitalization | 24/66 (36%) | 20/61 (33%) | 0.71 |
| Standard sliding scale from hospital computer order set | 75/83 (90%) | 76/82 (93%) | 0.78 |
| Any oral antihyperglycemic agents during hospitalization | 35/91 (38%) | 18/89 (20%) | 0.01 |
| Outcome | |||
| Mean percentage of glucose readings > 180 mg/dL (SD) | 33.3% (33.3%) | 31.6% (29.6%) | 0.85 |
| Any hyperglycemia (glucose > 180 mg/dL) | 66/89 (74%) | 59/78 (76%) | 0.86 |
| 1%‐20% of readings | 17/89 (19%) | 15/78 (19%) | 0.85 for trend |
| 20%‐40% | 15/89 (17%) | 15/78 (19%) | |
| 40%‐60% | 15/89 (17%) | 15/78 (19%) | |
| 60%‐80% | 7/89 (8%) | 6/78 (8%) | |
| >80% | 12/89 (13%) | 8/78 (10%) | |
| Any hypoglycemia (glucose < 60 mg/dL) | 6/89 (7%) | 10/78 (13%) | 0.20 |
Regarding glucose control (Table 3), the mean percentage of glucose readings per patient greater than 180 mg/dL was not significantly different in the pilot study compared to baseline (31.6% vs. 33.3%, P = .85). Despite implementation of the protocol and increased use of basal and nutritional insulin, 76% of patients had at least 1 routine glucose reading greater than 180 mg/dL, and 37% of patients had at least 40% of their routine glucose readings greater than 180 mg/dL, comparable to baseline (74% and 38%, respectively, P = NS for both comparisons). At least 1 hypoglycemic event (glucose reading below 60 mg/dL) occurred in 7% of patients at baseline and 13% during the pilot study (P = .20). Eleven hypoglycemic events in the pilot study were between 50 and 59 mg/dL (55%), 6 were between 40 and 49 mg/dL (30%), 3 were between 30 and 39 mg/dL (15%), and none were less than 30 mg/dL. Nine occurred before breakfast (45%), 5 before dinner (25%), 3 before lunch (15%), and 3 at bedtime (15%).
During the pilot study, the use of basal insulin did improve over the first 5 days of hospitalization (Fig. 1), in both the percentage of patients prescribed any basal insulin and the percentage of each patient's total insulin dose (basal, nutritional, and supplemental) given as basal (both P < .001 for trend). Hyperglycemia rates also improved during hospitalization (Fig. 1), decreasing from 48% on hospital day 1 to 34% on hospital day 5 (P = .004 for trend). These trends were not observed in the baseline group, with hyperglycemia rates of 37% on hospital day 1 and 34% on hospital day 5 (P = .16 for trend).

Patients for whom the resident accepted or partially accepted the protocol had higher use of basal insulin (91% vs. 13%, P < .0001), higher use of nutritional insulin (21% vs. 0%, P = .01), and more frequent dose adjustments (47% vs. 7%, P = .01) compared with patients for whom the resident declined the protocol. However, the rate of hyperglycemia was higher in patients for whom the protocol was accepted or partially accepted than in patients for whom the protocol was declined (37% vs. 20%, P = .02).
DISCUSSION
Our subcutaneous insulin protocol focused on increasing the use of basal and nutritional insulin, avoiding the use of sliding‐scale insulin by itself, and performing daily insulin adjustments in response to the hypo‐ or hyperglycemia of general medical inpatients with diabetes or hyperglycemia.
The most notable finding of our pilot study was that residents were resistant to using the protocol, both in general and in its specific recommendations. Despite receiving education about inpatient diabetes control and protocol recommendations from the team pharmacist, and despite being on a hospitalist‐run medical service, the residents accepted use of the protocol for only half the eligible patients. Patients who were started on basal insulin were often underdosed or started after the first day of hospitalization, and daily dose adjustments were not consistently made despite persistent hypo‐ or hyperglycemia. Although the use of nutritional insulin was greater compared with that in the baseline group, it was still only prescribed for 13% of patients. Use of a standard sliding scale from the hospital computer order set was common in the pilot study and similar to that in the baseline group. These results suggest significant resistance to changing the current standard of practice.
Despite this lack of adherence to the protocol, some modest improvements in processes of care were seen. Basal insulin was ordered more often during the pilot study than at baseline, especially over the course of a hospital stay. Nutritional insulin was also ordered more often during the pilot study than at baseline, but was still infrequent. Oral antihyperglycemic agents were ordered less often during the pilot study than at baseline. This demonstrates that use of the protocol may be able to improve process outcomes. However, the modest improvements in process outcomes could have simply been a result of increased awareness and education, not the protocol itself.
Regarding patient outcomes, the overall hyperglycemia rate did not improve in the pilot study relative to that at baseline. Importantly, hypoglycemia rates did not increase significantly compared with those at baseline. However, because of the small number of hypoglycemia events, the sample size may not have been sufficient to detect a true difference between groups.
The most likely reason that the protocol did not show an effect on glycemic control was that its recommendations were not adhered to. In turn, this may have been a result of incomplete education, training, and implementation measures and/or inherent problems with the protocol that made its recommendations difficult to follow. Another possibility is that the protocol itself may not have been capable of improving glucose control, even when properly used. However, we do know that resident agreement to use the protocol did lead to higher rates of recommended best practices being carried out, such as basal insulin use and daily insulin dose adjustments, and that use of the protocol was associated with improvements in glucose control over the hospital stay. A larger study with a higher degree of protocol adherence would be better able to evaluate the merits of the protocol itself, as would a randomized controlled trial using instrumental variables to measure treatment efficacy. Another possibility explanation for the lack of effect is that glucose control on admission happened to be worse in the pilot group than in the control group: rates of hyperglycemia on day 1 were 48% in the pilot group compared with 37% in the baseline group (Fig. 1). Also, the decreased use of oral agents in the pilot group, a purposeful change to decrease the risk of hypoglycemia, may have counteracted the beneficial effects of more appropriate insulin use. Finally, there were few patients with poorly controlled diabetes at baseline (18 patients with A1C 8.0 in the baseline group and 12 such patients in the pilot group), arguably those most likely to benefit.
There is a pressing need to identify protocols that can improve glucose control in the non‐ICU inpatient setting and successfully implement these protocols with a minimum of resources and effort. To date, most studies that have improved glucose control in the non‐ICU setting have relied on frequent input from diabetologists or nurse‐practitioners.14, 15
The results of this study should be viewed in light of its limitations, including its relatively small sample size (thus limiting our ability to detect possible significant differences between groups) and that it was conducted at a single institution (thus limiting its generalizability). Patients were enrolled on weekdays, so patients admitted and discharged over a weekend or on a holiday may have been missed. Also, because of the nonrandomized design of the study, we cannot exclude the possibility that the improvements noted in the pilot study were a result of the increased education provided or of increased awareness and general improvement in diabetes management over the course of the study. Finally, implementation of the protocol was somewhat labor intensive and required staff support that could be difficult to replicate in other institutions. However, most of the study staff's effort was necessary either to implement the protocol in the absence of an order set or to evaluate barriers to implementation. Widespread implementation of a protocol with an order set, education, and the use of highly reliable tools should be possible with much less effort and resources. The strengths of this study include its prospective data collection methods, which included rigorous inclusion criteria and collection of detailed clinical data.
Our study findings suggest several approaches to improve care in the future. To combat resistance to change, the American Association of Clinical Endocrinologists strongly recommends that each institution ensure that all its clinicians involved agree about general philosophies of diabetes management.19 A more expansive, hospital‐wide educational and promotional plan may increase the initial acceptance of the protocol. Interviews with residents also indicated there was unfamiliarity with diabetes management and significant concerns about the harmful affects of tight glucose control (ie, risk of hypoglycemia), especially in certain patient subgroups. These results confirmed the need for more practical individualized training and sparked the implementation of small‐group, case‐based educational sessions on inpatient diabetes management for all house officers, with a particular focus on patients with multiple comorbidities, on steroid tapers, and/or with renal failure.
The lack of nutritional insulin orders, delays in ordering basal insulin, and use of inadequate doses of insulin may be counteracted by the use of an order set, in our case built into our computer physician order entry (CPOE) system. The use of CPOE also allows reminders to be automatically sent to clinicians if eligible patients are not started on these orders. Clinical inertia (eg, failure to adjust the insulin doses of specific patients despite hyperglycemia) is more difficult to combat but may be addressed through better organization of clinical data, individualized, case‐based education, and CPOE reminders and eventually through culture change.
As a result of our pilot study, additional revisions were made to the protocol in hopes of increasing protocol adherence. For example, for patients eating discrete meals who are not taking insulin at home, the pilot protocol had suggested a starting insulin dose range for basal and nutritional insulin that required 2 separate calculations. The revised protocol was simplified to recommend a total daily insulin dose to be split evenly between basal and nutritional insulin. The daily adjustment instructions were also simplified. The pilot protocol had included a complicated table of adjustment recommendations based on bedside glucose trends. The revised protocol recommends adjusting the new daily dose by adding the total units of insulin given the previous day (including supplemental doses), making minor adjustments for hyper‐ or hypoglycemia and other clinical factors (like renal failure), and splitting this dose evenly between scheduled basal and nutritional insulin. In addition, 3 order sets were built into our computerized physician order entry system to facilitate early and appropriate insulin orders for patients with different diets (discrete meals, continuous tube feeds, and nothing by mouth); 3 different insulin sliding scales were created for patients with different degrees of insulin resistance; a diabetes management page for our electronic medication administration record is being developed to better organize clinical data; and hospital‐wide education and individualized training are ongoing.
In conclusion, the adherence to an inpatient glycemic management protocol that focused on increasing use of basal insulin and performing daily insulin adjustments was only fair. Barriers to successful implementation included clinical inertia regarding individual patients, unfamiliarity with inpatient diabetes management strategies, fear of hypoglycemia, and resistance to changing the current standard of practice. Targeted education, standard order sets, better organization of clinical data, protocol simplification, and institutional culture changes may be necessary for successful protocol implementation and improved inpatient glucose control.
Diabetes mellitus is a common comorbid condition in hospitalized patients. In 2003, diabetes was listed as a diagnosis in 17.2% of hospital discharges in the United States.1 Because these diagnosis codes do not account for undiagnosed diabetes or hospital‐related hyperglycemia, the true prevalence of diabetes or hyperglycemia in hospitalized patients is likely higher and has been estimated to be as great as 38%.2 Hyperglycemia has been associated with adverse outcomes among hospitalized patients, including infectious complications, increased length of stay, and increased mortality.27 However, because hyperglycemia is not usually the primary reason patients with diabetes are hospitalized, its management is often not a focus in the inpatient setting. Sliding‐scale insulin alone continues to be commonly prescribed despite clinical evidence showing it to be ineffective in achieving glycemic control.8, 9
Recent randomized controlled trials have demonstrated that aggressive treatment of inpatient hyperglycemia improves outcomes in surgical and medical intensive care units10, 11 and in patients admitted for myocardial infarction.12, 13 Based on this clinical evidence and strong observational data linking hyperglycemia to poor patient outcomes in the non‐ICU setting,27 the American Diabetes Association (ADA) now advocates good metabolic control, defined as preprandial glucose levels of 90‐130 mg/dL and peak postprandial glucose levels < 180 mg/dL in hospitalized non‐ICU patients with hyperglycemia14 (note that these targets are less aggressive than those for ICU patients, for whom randomized controlled trials showed the benefits of reduced mortality provided by tight glucose control).11 To reach these targets, the ADA and American College of Endocrinology suggest that multidisciplinary teams develop and implement hyperglycemia management guidelines and protocols.15 Protocols should promote the use of continuous intravenous insulin or scheduled subcutaneous insulin as opposed to the use of sliding‐scale insulin alone. Subcutaneous insulin protocols should include target glucose levels; basal, nutritional, and supplemental insulin; and daily adjustments based on previous glucose levels, insulin sensitivity, nutritional intake, illness, and medications.6, 15 To date, few published protocols or algorithms for inpatient subcutaneous insulin have been shown to be effective.16, 17 It is therefore not known how best to design and implement an inpatient diabetes management protocol that is effective, efficient, and self‐perpetuating. The aims of our pilot study were to develop and implement a subcutaneous insulin protocol on a general medicine service, to identify barriers to implementation, and to determine the effect of this protocol on glycemic control.
METHODS
Setting and Participants
This prospective quality‐improvement pilot study was conducted at Brigham and Women's Hospital (BWH) from January 10, 2005, through June 23, 2005. Patients were eligible to participate if they were admitted to either of 2 General Medicine Service (GMS) teams with either a known diagnosis of type 2 diabetes or inpatient hyperglycemia (random laboratory glucose level > 180 mg/dL) and at least 1 fasting point‐of‐care glucose reading > 140 mg/dL. Patients were excluded if they had diabetic ketoacidosis, hyperosmolar hyperglycemic state, another absolute indication for intravenous insulin, or fasting glucose < 60 mg/dL on no insulin or if they were pregnant. Each GMS team consisted of a teaching attending, a junior or senior resident, 2 interns, and a clinical pharmacist. Twenty‐six physicians attended on these 2 teams during the study period, 13 of whom were hospitalists. This study was approved by the BWH Institutional Review Board; patient consent to participate in this study was deemed not necessary because of the relatively nonsensitive nature of the data (eg, glucose control, insulin orders), the noninvasive means of data collection (eg, chart review), and the steps taken by research personnel to minimize any breach in patient confidentiality.
Intervention
A multidisciplinary team composed of a diabetologist (M.L.P.), a hospitalist (J.L.S.), and a pharmacist (J.M.T.) developed a subcutaneous insulin protocol that was approved by the BWH Pharmacy and Therapeutics Diabetes Subcommittee. The protocol consisted of a set of treatment recommendations made by a pharmacist to be carried out by the medical team. The primary components are shown in Table 1 (a full description can be found in the Appendix). The main emphasis of the protocol was on discontinuing oral antihyperglycemic agents during hospitalization, initiating basal insulin in most patients, and adjusting basal insulin daily as needed.
|
| Oral agents |
| 1. Stop oral agents in most patients |
| Glucose testing |
| 2. Check bedside blood glucose before meals and at bedtime if eating, or every 6 hours if not eating |
| Insulin |
| 3. Start basal insulin Patient's home dose or NPH 0.1 units/kg before breakfast and at bedtime or insulin glargine 0.2 units/kg at bedtime (max dose 20 units) If NPO, consider half dose unless hyperglycemic |
| 4. Start nutritional insulin Discrete meals: insulin aspart 0.05‐0.1 units/kg per meal or home dose 0‐15 minutes prior to eating Continuous tube feeds: regular insulin every 6 hours or NPH every morning and at bedtime (0.1‐0.2 units/kg per day in addition to basal insulin) Hold if NPO |
| 5. Start correctional insulin Scale provided based on blood glucose and daily scheduled insulin requirements |
| Daily Adjustments |
6. Adjust scheduled insulin daily
|
| Other Considerations |
| 7. Hypoglycemia management (protocols for fruit juice, glucagons, IV dextrose, and when to call physician) |
| 8. Discharge orders (recommendations to discharge most patients on admission medication regimen, avoid sliding scale insulin, simplify dosing for patients requiring new insulin regimens, ensure adequate patient education and prompt outpatient follow‐up) |
All medical residents received general instructions regarding inpatient diabetes control by the research team's diabetologist (M.L.P.) through a 1‐hour department‐wide didactic lecture. The standards of care taught were identical to those in the protocol. In addition, the research team's hospitalist (J.L.S.) contacted each medical resident assigned to the 2 GMS teams electronically to introduce the protocol and describe the purpose and logistics of the pilot study.
A research assistant prospectively identified eligible patients each weekday by screening all patients admitted to the 2 GMS teams using the daily computerized sign‐out system used by all medical residents. Specifically, laboratory random glucose levels, inpatient medications, and medical history were reviewed to determine if each patient met eligibility criteria. Eligibility criteria were confirmed by medical record review. The pharmacist recommended to the primary team that the protocol be initiated for eligible patients. In addition, the pharmacist recommended daily adjustment of the insulin dose according to the protocol as appropriate. A chronologically organized summary of clinical data relevant to glycemic management for each patient, including bedside blood glucose measurements, general dietary intake, use of intravenous dextrose solutions, and administration of systemic steroids, oral diabetes medications, and all insulins, was provided to the team each day by the research assistant.
Measurements
The resident's acceptance of the protocol or reasons for declining it were recorded by the pharmacist on the day the protocol was recommended. Protocol acceptance was categorized as yes, no, or partial. Partial acceptance was defined as resident agreement to use the protocol, but with stated caveats or modifications. Clinical data were collected on each eligible patient for up to 7 days on GMS. Several data sources were used, including physician admission notes, the hospital's computerized clinical data system, vital‐sign sheets, medication administration records, and personal communication with nurses regarding any missing or discrepant data.
All insulin use (prescribed drug, dose, route, schedule and actual administered drug, dose, route, and time) was recorded each day by the research assistant. Use of basal and nutritional insulin and daily dose adjustments if previous hypo‐ or hyperglycemia (categorized as yes, no, or not applicable for each patient each day) were determined by the study pharmacist (J.M.T.) through retrospective review of all orders.
Up to 4 routine bedside blood glucose measurements were recorded each day: for patients eating discrete meals, these were the measurements taken before meals and at bedtime; for patients not eating or receiving continuous nutrition, these were the measurements taken closest to 6 AM, noon, 6 PM, and midnight. Additional measurements were not recorded to avoid ascertainment bias caused by follow‐up testing of abnormal glucose values. Glucose readings on the day of admission were excluded from analysis because these values are not amenable to inpatient ordering practices.
Study outcomes included overall protocol acceptance rate, insulin prescribing practices including use of basal insulin (ie, long‐acting agents such as NPH and insulin glargine), nutritional insulin (ie, scheduled regular, lispro, or aspart insulin given before each meal), daily dose adjustments under the protocol, and mean percentage of glucose readings per person greater than 180 mg/dL (hyperglycemia) and below 60 mg/dL (hypoglycemia). Comparable data from a previous cohort study of 91 GMS patients were used as baseline data for comparisons with the results of the present study.9
Other patient data collected included age, sex, weight, baseline A1C (taken at or within 6 months of admission), diabetic medications used prior to admission (none, oral agents only, or any insulin use); daily inpatient use of oral or intravenous steroids, oral diabetic medications, dextrose‐containing intravenous fluids, tube feeds, total parenteral nutrition, and general nutritional intake (nothing by mouth, clear diet, low carbohydrate diet, house diet).
Statistical Analysis
Characteristics of the study subjects and process and outcome measures were analyzed descriptively using rates, means, and standard deviations or medians with interquartile ranges as appropriate. Comparisons between the pilot study and baseline cohorts were performed using Fisher's exact test for dichotomous outcomes (eg, use of basal insulin). For rates of hyperglycemia (ie, fraction of readings > 180 mg/dL), we used binomial logistic regression, accounting for potential correlation among repeated events by individual patients with a dispersion parameter18 (note that we did not use the same analysis for rates of hypoglycemia because it was such a rare event; for analysis of hypoglycemia, the variables were dichotomized). We also analyzed outcomes by hospital day (through hospital day 5, the limit used in the baseline study) to determine daily trends during the course of hospitalization; for these analyses we used the Mantel‐Haenszel chi‐square test for dichotomous variables and binomial logistic regression with hospital day as the independent variable for rates of hyperglycemia. Two‐sided P values < .05 were considered significant. SAS version 9.1 (Cary, NC) was used for all analyses.
RESULTS
After screening all 785 admissions to the 2 medical teams during the study period, we prospectively identified 109 patients (14%) for the pilot study. Twenty patients were subsequently excluded: 7 patients who were discharged the same day they were identified, 4 who did not have a fasting blood glucose value greater than 140 mg/dL, 4 patients who had type 1 diabetes, 2 patients who were admitted with diabetic ketoacidosis, and 3 patients whose data could not be accessed because of repeated unavailability of the medical record. Characteristics of the remaining 89 study subjects are shown in Table 2 and are compared to 91 baseline subjects. The mean age of the study subjects was 68.7 years; 45% were men. Five patients (6%) did not have a previous diagnosis of diabetes, and 51% were taking insulin prior to admission; the median A1C was 6.8%.
| Characteristic | Baseline (n = 91) | Pilot (n = 89) |
|---|---|---|
| ||
| Age (years), mean (SD) | 66.0 (14.5) | 68.7 (14.7) |
| Male | 53/91 (58%) | 40/89 (45%) |
| No diagnosis of diabetes at admission | 7/91 (8%) | 5/89 (6%) |
| Preadmission diabetes regimen | ||
| None | 15/91 (16%) | 14/78 (18%) |
| Oral medications only | 32/91 (35%) | 24/78 (31%) |
| Insulin | 44/91 (48%) | 40/78 (51%) |
| A1C (IQR) | 7.0 (6.0, 8.0) | 6.8 (6.3, 7.8) |
| Hospital length of stay (days), median (IQR) | 5 (3, 7) | 5 (3, 7) |
The medical residents agreed, at least in theory, to follow the subcutaneous insulin protocol for 50 patients (56%), partially accepted it for 8 (9%), and declined for 31 (35%). Reasons for declining the protocol included fear of hypoglycemia, severity of patient's other disease states or overall poor health of patient, concern for the effects of renal insufficiency on insulin clearance, concern for the effect of steroid tapers on glucose levels, desire to titrate oral medications, and anticipation of patient's imminent discharge. Other reasons such as the glucose levels are not that bad and let's watch the glucose levels for one more day suggest that some residents did not view hyperglycemia as an acute problem requiring immediate attention.
Regarding insulin‐ordering practices (Table 3), basal insulin was prescribed for 57 patients (64%) in the pilot group compared to 45 patients (49%) in the baseline group (P = .05). Nutritional insulin was prescribed to 12 patients (13%) in the pilot group compared to no patients in the baseline group (P < .001). Oral hypoglycemic agents were prescribed less often in the pilot study than at baseline (20% vs. 38%, P = .01). The use of a standard default sliding scale from the hospital computer order set was high and was not significantly different in the pilot study compared with that at baseline (93% vs. 90%, P = .78). Twenty‐four of the 83 patients in the pilot group (29%) received sliding‐scale insulin without ever receiving basal or nutritional insulin during hospitalization compared to 45 of 91 patients in the baseline group (49%; P = .01 for comparison). Among patients started on basal insulin, 42% (24 of 57) were started after the first full hospital day. The initial basal insulin dose was appropriate according to the protocol (within 20%) in 38 of 57 patients (67%). Only 20 of 61 patients (33%) who had any hypo‐ or hyperglycemia had any change to their insulin regimen made during days 2 through 7 of their hospitalization on GMS, similar to the rate noted at baseline (36%).
| Measure | Baseline | Pilot | P value |
|---|---|---|---|
| |||
| Process | |||
| Any basal insulin during hospitalization | 45/91 (49%) | 57/89 (64%) | 0.05 |
| Any nutritional insulin during hospitalization | 0/91 (0%) | 12/89 (13%) | < 0.001 |
| Change in dose to any insulin order during hospitalization | 24/66 (36%) | 20/61 (33%) | 0.71 |
| Standard sliding scale from hospital computer order set | 75/83 (90%) | 76/82 (93%) | 0.78 |
| Any oral antihyperglycemic agents during hospitalization | 35/91 (38%) | 18/89 (20%) | 0.01 |
| Outcome | |||
| Mean percentage of glucose readings > 180 mg/dL (SD) | 33.3% (33.3%) | 31.6% (29.6%) | 0.85 |
| Any hyperglycemia (glucose > 180 mg/dL) | 66/89 (74%) | 59/78 (76%) | 0.86 |
| 1%‐20% of readings | 17/89 (19%) | 15/78 (19%) | 0.85 for trend |
| 20%‐40% | 15/89 (17%) | 15/78 (19%) | |
| 40%‐60% | 15/89 (17%) | 15/78 (19%) | |
| 60%‐80% | 7/89 (8%) | 6/78 (8%) | |
| >80% | 12/89 (13%) | 8/78 (10%) | |
| Any hypoglycemia (glucose < 60 mg/dL) | 6/89 (7%) | 10/78 (13%) | 0.20 |
Regarding glucose control (Table 3), the mean percentage of glucose readings per patient greater than 180 mg/dL was not significantly different in the pilot study compared to baseline (31.6% vs. 33.3%, P = .85). Despite implementation of the protocol and increased use of basal and nutritional insulin, 76% of patients had at least 1 routine glucose reading greater than 180 mg/dL, and 37% of patients had at least 40% of their routine glucose readings greater than 180 mg/dL, comparable to baseline (74% and 38%, respectively, P = NS for both comparisons). At least 1 hypoglycemic event (glucose reading below 60 mg/dL) occurred in 7% of patients at baseline and 13% during the pilot study (P = .20). Eleven hypoglycemic events in the pilot study were between 50 and 59 mg/dL (55%), 6 were between 40 and 49 mg/dL (30%), 3 were between 30 and 39 mg/dL (15%), and none were less than 30 mg/dL. Nine occurred before breakfast (45%), 5 before dinner (25%), 3 before lunch (15%), and 3 at bedtime (15%).
During the pilot study, the use of basal insulin did improve over the first 5 days of hospitalization (Fig. 1), in both the percentage of patients prescribed any basal insulin and the percentage of each patient's total insulin dose (basal, nutritional, and supplemental) given as basal (both P < .001 for trend). Hyperglycemia rates also improved during hospitalization (Fig. 1), decreasing from 48% on hospital day 1 to 34% on hospital day 5 (P = .004 for trend). These trends were not observed in the baseline group, with hyperglycemia rates of 37% on hospital day 1 and 34% on hospital day 5 (P = .16 for trend).

Patients for whom the resident accepted or partially accepted the protocol had higher use of basal insulin (91% vs. 13%, P < .0001), higher use of nutritional insulin (21% vs. 0%, P = .01), and more frequent dose adjustments (47% vs. 7%, P = .01) compared with patients for whom the resident declined the protocol. However, the rate of hyperglycemia was higher in patients for whom the protocol was accepted or partially accepted than in patients for whom the protocol was declined (37% vs. 20%, P = .02).
DISCUSSION
Our subcutaneous insulin protocol focused on increasing the use of basal and nutritional insulin, avoiding the use of sliding‐scale insulin by itself, and performing daily insulin adjustments in response to the hypo‐ or hyperglycemia of general medical inpatients with diabetes or hyperglycemia.
The most notable finding of our pilot study was that residents were resistant to using the protocol, both in general and in its specific recommendations. Despite receiving education about inpatient diabetes control and protocol recommendations from the team pharmacist, and despite being on a hospitalist‐run medical service, the residents accepted use of the protocol for only half the eligible patients. Patients who were started on basal insulin were often underdosed or started after the first day of hospitalization, and daily dose adjustments were not consistently made despite persistent hypo‐ or hyperglycemia. Although the use of nutritional insulin was greater compared with that in the baseline group, it was still only prescribed for 13% of patients. Use of a standard sliding scale from the hospital computer order set was common in the pilot study and similar to that in the baseline group. These results suggest significant resistance to changing the current standard of practice.
Despite this lack of adherence to the protocol, some modest improvements in processes of care were seen. Basal insulin was ordered more often during the pilot study than at baseline, especially over the course of a hospital stay. Nutritional insulin was also ordered more often during the pilot study than at baseline, but was still infrequent. Oral antihyperglycemic agents were ordered less often during the pilot study than at baseline. This demonstrates that use of the protocol may be able to improve process outcomes. However, the modest improvements in process outcomes could have simply been a result of increased awareness and education, not the protocol itself.
Regarding patient outcomes, the overall hyperglycemia rate did not improve in the pilot study relative to that at baseline. Importantly, hypoglycemia rates did not increase significantly compared with those at baseline. However, because of the small number of hypoglycemia events, the sample size may not have been sufficient to detect a true difference between groups.
The most likely reason that the protocol did not show an effect on glycemic control was that its recommendations were not adhered to. In turn, this may have been a result of incomplete education, training, and implementation measures and/or inherent problems with the protocol that made its recommendations difficult to follow. Another possibility is that the protocol itself may not have been capable of improving glucose control, even when properly used. However, we do know that resident agreement to use the protocol did lead to higher rates of recommended best practices being carried out, such as basal insulin use and daily insulin dose adjustments, and that use of the protocol was associated with improvements in glucose control over the hospital stay. A larger study with a higher degree of protocol adherence would be better able to evaluate the merits of the protocol itself, as would a randomized controlled trial using instrumental variables to measure treatment efficacy. Another possibility explanation for the lack of effect is that glucose control on admission happened to be worse in the pilot group than in the control group: rates of hyperglycemia on day 1 were 48% in the pilot group compared with 37% in the baseline group (Fig. 1). Also, the decreased use of oral agents in the pilot group, a purposeful change to decrease the risk of hypoglycemia, may have counteracted the beneficial effects of more appropriate insulin use. Finally, there were few patients with poorly controlled diabetes at baseline (18 patients with A1C 8.0 in the baseline group and 12 such patients in the pilot group), arguably those most likely to benefit.
There is a pressing need to identify protocols that can improve glucose control in the non‐ICU inpatient setting and successfully implement these protocols with a minimum of resources and effort. To date, most studies that have improved glucose control in the non‐ICU setting have relied on frequent input from diabetologists or nurse‐practitioners.14, 15
The results of this study should be viewed in light of its limitations, including its relatively small sample size (thus limiting our ability to detect possible significant differences between groups) and that it was conducted at a single institution (thus limiting its generalizability). Patients were enrolled on weekdays, so patients admitted and discharged over a weekend or on a holiday may have been missed. Also, because of the nonrandomized design of the study, we cannot exclude the possibility that the improvements noted in the pilot study were a result of the increased education provided or of increased awareness and general improvement in diabetes management over the course of the study. Finally, implementation of the protocol was somewhat labor intensive and required staff support that could be difficult to replicate in other institutions. However, most of the study staff's effort was necessary either to implement the protocol in the absence of an order set or to evaluate barriers to implementation. Widespread implementation of a protocol with an order set, education, and the use of highly reliable tools should be possible with much less effort and resources. The strengths of this study include its prospective data collection methods, which included rigorous inclusion criteria and collection of detailed clinical data.
Our study findings suggest several approaches to improve care in the future. To combat resistance to change, the American Association of Clinical Endocrinologists strongly recommends that each institution ensure that all its clinicians involved agree about general philosophies of diabetes management.19 A more expansive, hospital‐wide educational and promotional plan may increase the initial acceptance of the protocol. Interviews with residents also indicated there was unfamiliarity with diabetes management and significant concerns about the harmful affects of tight glucose control (ie, risk of hypoglycemia), especially in certain patient subgroups. These results confirmed the need for more practical individualized training and sparked the implementation of small‐group, case‐based educational sessions on inpatient diabetes management for all house officers, with a particular focus on patients with multiple comorbidities, on steroid tapers, and/or with renal failure.
The lack of nutritional insulin orders, delays in ordering basal insulin, and use of inadequate doses of insulin may be counteracted by the use of an order set, in our case built into our computer physician order entry (CPOE) system. The use of CPOE also allows reminders to be automatically sent to clinicians if eligible patients are not started on these orders. Clinical inertia (eg, failure to adjust the insulin doses of specific patients despite hyperglycemia) is more difficult to combat but may be addressed through better organization of clinical data, individualized, case‐based education, and CPOE reminders and eventually through culture change.
As a result of our pilot study, additional revisions were made to the protocol in hopes of increasing protocol adherence. For example, for patients eating discrete meals who are not taking insulin at home, the pilot protocol had suggested a starting insulin dose range for basal and nutritional insulin that required 2 separate calculations. The revised protocol was simplified to recommend a total daily insulin dose to be split evenly between basal and nutritional insulin. The daily adjustment instructions were also simplified. The pilot protocol had included a complicated table of adjustment recommendations based on bedside glucose trends. The revised protocol recommends adjusting the new daily dose by adding the total units of insulin given the previous day (including supplemental doses), making minor adjustments for hyper‐ or hypoglycemia and other clinical factors (like renal failure), and splitting this dose evenly between scheduled basal and nutritional insulin. In addition, 3 order sets were built into our computerized physician order entry system to facilitate early and appropriate insulin orders for patients with different diets (discrete meals, continuous tube feeds, and nothing by mouth); 3 different insulin sliding scales were created for patients with different degrees of insulin resistance; a diabetes management page for our electronic medication administration record is being developed to better organize clinical data; and hospital‐wide education and individualized training are ongoing.
In conclusion, the adherence to an inpatient glycemic management protocol that focused on increasing use of basal insulin and performing daily insulin adjustments was only fair. Barriers to successful implementation included clinical inertia regarding individual patients, unfamiliarity with inpatient diabetes management strategies, fear of hypoglycemia, and resistance to changing the current standard of practice. Targeted education, standard order sets, better organization of clinical data, protocol simplification, and institutional culture changes may be necessary for successful protocol implementation and improved inpatient glucose control.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. 8/17/05; http://www.ahrq.gove/HCUPnet/. Accessed 7/17/06,2006.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity.Eur Heart J.2005;26:650–661.
- American Diabetes Association.Standards of Medical Care in Diabetes ‐ 2006.Diabetes Care.2006;29:S4–S42.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control: A call to action.Diabetes Care.2006;29:1955–1962.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–11.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial).Diabetes Care.2007;30:2181–2186.
- .Extra‐binomial variation in logistic linear models.Appl Stat.1982;31:144–148.
- ,.Hospital management of diabetes.Endocrinol Metab Clin North Am.2005;34:99–116.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. 8/17/05; http://www.ahrq.gove/HCUPnet/. Accessed 7/17/06,2006.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease.Thorax2006;61:284–289.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition.Diabetes Care.2005;28:2367–2371.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity.Eur Heart J.2005;26:650–661.
- American Diabetes Association.Standards of Medical Care in Diabetes ‐ 2006.Diabetes Care.2006;29:S4–S42.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control: A call to action.Diabetes Care.2006;29:1955–1962.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–11.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial).Diabetes Care.2007;30:2181–2186.
- .Extra‐binomial variation in logistic linear models.Appl Stat.1982;31:144–148.
- ,.Hospital management of diabetes.Endocrinol Metab Clin North Am.2005;34:99–116.
Mastery Learning of Procedural Skills
In a supplement to its inaugural issue, the Journal of Hospital Medicine published core competencies for hospitalists covering 3 areas: clinical conditions, systems in health care, and procedures.1 Completion of a traditional internal medicine residency may not provide hospitalists with the skills necessary to safely perform necessary procedures such as thoracentesis. A recent article reported that most internal medicine residents surveyed were uncomfortable performing common procedures, and their discomfort was higher for thoracentesis than for central line insertion, lumbar puncture, or paracentesis.2 This confirmed a previous report that family practice residents had low confidence in performing thoracenteses.3 Thoracentesis also carries the risk of the potentially life‐threatening complication of pneumothorax, which may be increased when performed by physicians‐in‐training.4
One method for improving training and assessment is the use of simulation technology. Simulation has been used to increase knowledge, provide opportunities for deliberate and safe practice, and shape the development of clinical skills.5, 6 Simulation has also been advocated for assessing competence in procedures including carotid angiography,7 emergency airway management,8 basic bronchoscopy,9 and advanced cardiac life support (ACLS).10, 11
Recently, we used simulation technology to help residents reach mastery learning standards for ACLS.11 Mastery learning,12 an extreme form of competency‐based education,13 implies that learners have acquired the clinical knowledge and skill measured against rigorous achievement standards. In mastery learning, educational results are equivalent, whereas educational practice time differs. To demonstrate mastery learning, we first documented a 38% improvement in skill after a simulation‐based educational intervention10 and used a multidisciplinary panel to determine mastery achievement standards for ACLS skills in 6 clinical scenarios.14 These standards were used in a study in which the amount of time needed to achieve skill mastery was allowed to vary while the skill outcomes of the residents were identical clinically.11
The present study had 4 aims. The first was to assess the baseline skill and knowledge of third‐year residents in thoracentesis. The second was to compare the thoracentesis‐related knowledge and skills of residents before and after an educational intervention. The third was to assess the correlation of medical knowledge and clinical experience with performance on a clinical skills examination after simulation training. The last was to document the feasibility of incorporating simulation‐based education into a training program.
METHODS
Objectives and Design
The study, which had a pretestposttest design without a control group,15 was of a simulation‐based, mastery learning educational intervention in thoracentesis. Primary measurements were obtained at baseline (pretest) and after the educational intervention (posttest).
Participants
Study participants were all 40 third‐year residents in the internal medicine residency program at Northwestern University's Chicago campus from January to May 2006. The Northwestern University Institutional Review Board approved the study. Participants provided informed consent before baseline assessment.
This residency program is based at Northwestern Memorial Hospital (NMH) and the Jesse Brown Veteran's Affairs Medical Center. Residents perform thoracenteses under the supervision of second‐ or third‐year residents or faculty members who are credentialed to perform the procedure. A didactic lecture on thoracentesis is part of the annual lecture series.
Procedure
The residents were kept as an intact group during the study period. The research procedure had 2 phases. First, the knowledge and clinical skills of participants at baseline were measured. Second, residents received two 2‐hour education sessions featuring didactic content and deliberate practice using a thoracentesis model. Between 4 and 6 weeks after the pretest, all residents were retested and were expected to meet or exceed a minimum passing score (MPS) on the clinical skills exam. Those who scored below the MPS engaged in more clinical skills practice until the mastery standard was reached. The amount of extra time needed to achieve the MPS was documented.
Educational Intervention
The intervention was designed to help residents acquire the knowledge and skills needed to perform a competent thoracentesis. The necessary components for mastery skill development were contained in the intervention. These included deliberate practice, rigorous skills assessment, and the provision of feedback in a supportive environment.16
The study was conducted in the Northwestern University Center for Advanced Surgical Education (N‐CASE) using the thoracentesis simulator developed by MediSim Inc. (Alton, Ontario) (
Teaching and testing sessions were standardized. In teaching sessions, groups of 2‐4 residents had 4 hours to practice and ask questions, and to receive structured education and feedback from 1 of 2 hospitalist faculty instructors (J.H.B., K.J.O.). One of the 4 hours was devoted to the presentation of didactic material on indications, complications, and interpretation of results and a step‐by‐step demonstration of a thoracentesis. This presentation was videotaped to ensure standardization of content. The remaining 3 hours were devoted to clinical skills exam education, deliberate practice, and feedback.
One resident was present at each pretest and posttest session with 1 of the 2 faculty instructors who gave standardized instructions. The resident was expected to obtain a relevant history; perform a limited physical examination; review PA, lateral, and decubitus chest radiographs; perform a simulated thoracentesis; and order appropriate diagnostic tests. Written examinations were completed at the pretest and posttest sessions.
Measurements
A 25‐item checklist was developed for the thoracentesis procedure using relevant sources17, 18 and rigorous step‐by‐step procedures.19 Each skill or other action was listed in order and given equal weight. Each skill or action was scored dichotomouslyeither 0 = done correctly or 1 = done incorrectly. Checklists were reviewed for completeness and accuracy by 2 authors who frequently perform and supervise thoracenteses (J.H.B., K.J.O.), 2 authors with expertise in checklist design (D.B.W., W.C.M.), and the physician director of the medical intensive care unit at NMH. The checklist was used in a pilot clinical skills examination of 4 chief medical residents to estimate checklist reliability and face validity.
The MPS for the thoracentesis clinical skills examination was determined by 10 clinical experts using the Angoff and Hofstee standard setting methods. The panel was composed of clinical pulmonary critical care medicine faculty (n = 7) and senior fellows (n = 3). Each panel member was given instruction on standard setting and asked to use the Angoff and Hofstee methods to assign pass/fail standards. The Angoff method asks expert judges to estimate the percentage of borderline examinees who would answer each test item correctly. The Hofstee method requires judges to estimate 4 properties of an evaluation's passing scores and failure rates. The panel was asked to repeat their judgments 6 weeks later to assure stability of the MPS. Details about the use of a standard setting exercise to set an MPS for clinical skills examinations have been published previously.14, 20
Evaluation of each resident's skill was recorded on the checklist by 1 of the 2 faculty raters at the pretest and posttest sessions. A random sample of 50% of the pretest sessions was rescored by a third rater with expertise in scoring clinical skills examinations (D.B.W.) to assess interrater reliability. The rescorer was blinded to the results of the first evaluation.
A multiple choice written examination was prepared according to examination development guidelines21 using appropriate reference articles and texts.17, 18, 22 The examination was prepared by 1 author (J.H.B.) and reviewed for accuracy and clarity by 2 others (K.J.O., D.B.W.) and by the director of the medical intensive care unit at NMH. The examination had questions on knowledge and comprehension of the procedure as well as data interpretation and application. It was administered to 9 fourth‐year medical students and 5 pulmonary/critical care fellows to obtain pilot data. Results of the pilot allowed creation of a pretest and a posttest that were equivalent in content and difficulty.23 The Kuder Richardson Formula 20 (KR‐20) reliability coefficients for the 20‐item pretest and the 20‐item posttest were .72 and .74, respectively.
Demographic data were obtained from the participants including age, gender, ethnicity, medical school, and scores on the United States Medical Licensing Examination (USMLE) Steps 1 and 2. Each resident's experience performing the procedure was also collected at pretest.
Primary outcome measures were performance on the posttest written and clinical examinations. Secondary outcome measures were the total training time needed to reach the MPS (minimum = 240 minutes) and a course evaluation questionnaire.
Data Analysis
Checklist score reliability was estimated by calculating interrater reliability, the preferred method for assessments that depend on human judges,24 using the kappa () coefficient25 adjusted using the formula of Brennan and Prediger.26 Within‐group differences from pretest (baseline) to posttest (outcome) were analyzed using paired t tests. Multiple regression analysis was used to assess the correlation of posttest performance on thoracentesis skills with (1) performance on pretest thoracentesis skills, (2) medical knowledge measured by the thoracentesis pretest and posttest and USMLE Steps 1 and 2, (3) clinical experience in performing thoracentesis, (4) clinical self‐confidence about performing thoracentesis, and (5) whether additional training was needed to master the procedure.
RESULTS
All residents consented to participate and completed the entire training protocol. Table 1 presents demographic data about the residents. Most had limited experience performing and supervising thoracenteses.
| Characteristic | PGY‐3 Resident |
|---|---|
| Age (years), mean (SD) | 28.88 (1.57) |
| Male | 23 (57.5%) |
| Female | 17 (42.5%) |
| African American | 1 (2.5%) |
| White | 21 (52.5%) |
| Asian | 14 (35.0%) |
| Other | 4 (10.0%) |
| U.S. medical school graduate | 39 (97.5%) |
| Foreign medical school graduate | 1 (2.5%) |
| Number of thoracentesis procedures | |
| Performed as an intern | |
| 0‐1 | 27.5% |
| 2‐4 | 60.0% |
| 5 | 12.5% |
| Performed as a PGY‐2 and PGY‐3 resident | |
| 0‐1 | 25.0% |
| 2‐4 | 55.0% |
| 5 | 20.0% |
| Supervised others as a PGY‐2 and PGY‐3 resident | |
| 0‐1 | 27.5% |
| 2‐4 | 57.5% |
| 5 | 15.0% |
Interrater reliability for the thoracentesis checklist data was calculated at pretest. Across the 25 checklist items, the mean kappa coefficient was very high (n = .94). The MPS used as the mastery achievement standard was 80% (eg, 20 of 25 checklist items). This was the mean of the Angoff and Hofstee ratings obtained from the first judgment of the expert panel and is displayed in Figure 1.
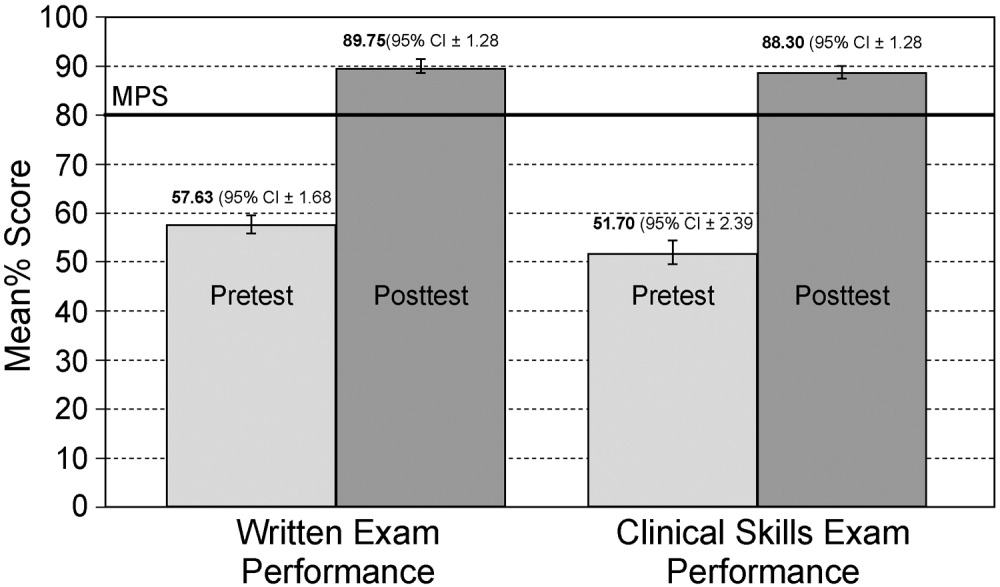
No resident achieved mastery at pretest. However, 37 of the 40 medicine residents (93%) achieved mastery within the standard 4‐hour thoracentesis curriculum. The remaining 3 residents (7%) needed extra time ranging from 20 to 90 minutes to reach mastery.
Figure 1 is a graphic portrait with descriptive statistics of the residents' pretest and posttest performance on the thoracentesis written and clinical skills exams. For the written exam, the mean score rose from 57.63% to 89.75%, a statistically significant improvement of 56% from pretest to posttest (t[39] = 17.0, P < .0001). The clinical skills exam also showed a highly significant 71% pretest‐to‐posttest gain, as the mean score rose from 51.70% to 88.3% (t[39] = 15.6, P < .0001).
Results from the regression analysis indicate that neither pretest performance, medical knowledge measured by local or USMLE examinations, nor thoracentesis clinical experience was correlated with the posttest measure of thoracentesis clinical skills. However, the need for additional practice to reach the mastery standard on the posttest was a powerful negative predictor of posttest performance: b = .27 (95% CI = .46 to .09; P < .006; r2 = .28). For those residents who required extra practice time, the initial clinical skills posttest score was 20% lower than that of their peers. Although the need for extra deliberate practice was associated with relatively lower initial posttest scores, all residents ultimately met or exceeded the rigorous thoracentesis MPS.
The responses of the 40 residents on a course evaluation questionnaire were uniformly positive. Responses were recorded on a Likert scale where 1 = strongly disagree, 2 = disagree, 3 = uncertain, 4 = agree, and 5 = strongly agree (Table 2). The data show that residents strongly agreed that practice with the medical simulator boosts clinical skills and self‐confidence, that they received useful feedback from the training sessions, and that deliberate practice using the simulator is a valuable educational experience. Residents were uncertain whether practice with the medical simulator has more educational value than patient care.
| Mean | SD | |
|---|---|---|
| Practice with the thoracentesis model boosts my skills to perform this procedure. | 4.3 | 0.8 |
| I receive useful educational feedback from the training sessions. | 4.0 | 0.6 |
| Practice with the thoracentesis model boosts my clinical self‐confidence. | 4.1 | 0.9 |
| Practice with the thoracentesis model has more educational value than patient care experience. | 2.3 | 1.0 |
| The Skills Center staff are competent. | 4.3 | 0.6 |
| Practice sessions in the Skills Center are a good use of my time. | 3.7 | 1.0 |
| Practice sessions using procedural models should be a required component of residency education. | 3.8 | 0.8 |
| Deliberate practice using models is a valuable educational experience. | 4.0 | 0.9 |
| Practice sessions using models are hard work. | 2.1 | 0.7 |
| Increasing the difficulty of simulated clinical problems helps me become a better doctor. | 3.9 | 0.7 |
| The controlled environment in the Skills Center helps me focus on clinical education problems. | 3.9 | 0.8 |
| Practice with the thoracentesis model has helped to prepare me to perform the procedure better than clinical experience alone. | 4.0 | 1.0 |
DISCUSSION
This study demonstrates the use of a mastery learning model to develop the thoracentesis skills of internal medicine residents to a high level. Use of a thoracentesis model in a structured educational program offering an opportunity for deliberate practice with feedback produced large and consistent improvements in residents' skills. An important finding of our study is that despite having completed most of their internal medicine training, residents displayed poor knowledge and clinical skill in thoracentesis procedures at baseline. This is similar to previous studies showing that the procedural skills and knowledge of physicians at all stages of training are often poor. Examples of areas in which significant gaps were found include basic skills such as chest radiography,27 emergency airway management,8 and pulmonary auscultation.28 In contrast, after the mastery learning program, all the residents met or exceeded the MPS for the thoracentesis clinical procedure and scored much higher on the posttest written examination.
Our data also demonstrate that medical knowledge measured by procedure‐specific pretests and posttests and USMLE Steps 1 and 2 scores were not correlated with thoracentesis skill acquisition. This reinforces findings from our previous studies of ACLS skill acquisition10, 11 and supports the difference between professional and academic achievement. Pretest skill performance and clinical experience also were not correlated with posttest outcomes. However, the amount of deliberate practice needed to reach the mastery standard was a powerful negative predictor of posttest thoracentesis skill scores, replicating our research on ACLS.11 We believe that clinical experience was not correlated with posttest outcomes because residents infrequently performed thoracenteses procedures during their training.
This project demonstrates a practical model for outcomes‐based education, certification, and program accreditation. Given the need to move procedural training in internal medicine beyond such historical methods as see one, do one, teach one,29 extension of the mastery model to other invasive procedures deserves further study. At our institution we have been encouraged by the ability of simulation‐based education in ACLS to promote long‐term skill retention30 and improvement in the quality of actual patient care.31 In addition to studying these outcomes for thoracentesis, we plan to incorporate the use of ultrasound when training residents to perform procedures such as thoracentesis and central venous catheter insertion.
Given concerns about the quality of resident preparation to perform invasive procedures, programs such as this should be considered as part of the procedural certification process. As shown by our experience with several classes of residents (n = 158), use of simulation technology to reach high procedural skill levels is effective and feasible in internal medicine residency training. In addition, our residents have consistently enjoyed participating in the simulated training programs. Postcourse questionnaires show that residents agree that deliberate practice with simulation technology complements but does not replace patient care in graduate medical education.5, 10
An important question needing more research is whether performance in a simulated environment transfers to actual clinical settings. Several small studies have demonstrated such a relationship,8, 9, 31, 32 yet the transfer of simulated training to clinical practice requires further study. More work should also be done to assess long‐term retention of skills30 and to determine the utility and benefit of simulation‐based training in procedural certification and credentialing.
This study had several limitations. It was conducted in 1 training program at a single medical center. The sample size (n = 40) was relatively small. The thoracentesis model was used for both education and testing, potentially confounding the events. However, these limitations do not diminish the pronounced impact that the simulation‐based training had on the skills and knowledge of our residents.
In conclusion, this study has demonstrated the ability of deliberate practice using a thoracentesis model to produce high‐level performance of simulated thoracenteses. The project received high ratings from learners and provides reliable assessments of procedural competence. Although internists are performing fewer invasive procedures now than in years past, procedural training is still an important component of internal medicine training.29, 33 Attainment of high procedural skill levels may be especially important for residents who plan to practice hospital medicine. We believe that simulation‐based training using deliberate practice should be a key contributor to future internal medicine residency education, certification, and accreditation.
Acknowledgements
The authors thank Charles Watts, MD, and J. Larry Jameson, MD, PhD, for their support of this work. We recognize and appreciate the Northwestern University internal medicine residents for their dedication to patient care and education.
- ,,,,.Core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1:48–56.
- ,,, et al.Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures.Am J Med.2006;119:71.e17–71.e24.
- ,,.Perception of competency to perform procedures and future practice intent: a national survey of family practice residents.Acad Med.2003;78:926–932.
- ,,,,,.Lower risk and higher yield for thoracentesis when performed by experienced operators.Chest.1993;103:1873–1876.
- ,,, et al.Simulation technology for health care professional skills training and assessment.JAMA.1999;282:861–866.
- ,,, et al.Reliability and validity of a simulation‐based acute care skills assessment for medical students and residents.Anesthesiology.2003;99:1270–1280.
- ,,,.Learning curves and reliability measures for virtual reality simulation in the performance assessment of carotid angiography.J Am Coll Cardiol.2006;47:1796–1802.
- ,,,,.Achieving house staff competence in emergency airway management: results of a teaching program using a computerized patient simulator.Crit Care Med.2004;32:2422–2427.
- ,,.Bronchoscopy simulator effectively prepares junior residents to competently perform basic clinical bronchoscopy.Ann Thorac Surg.2004;78:287–291.
- ,,, et al.Simulation‐based training of internal medicine residents in advanced cardiac life support protocols: a randomized trial.Teach Learn Med.2005;17:210–216.
- ,,, et al.Mastery learning of advanced cardiac life support skills by internal medicine residents using simulation technology and deliberate practice.J Gen Intern Med.2006;21:251–256.
- Block JH, ed.Mastery Learning: Theory and Practice.New York:Holt, Rinehart and Winston;1971.
- ,,,.Competency‐Based Curriculum Development in Medical Education. Public Health Paper No. 68.Geneva, Switzerland:World Health Organization;1978.
- ,,, et al.Comparison of two standard‐setting methods for advanced cardiac life support training.Acad Med.2005;80(10 Suppl):S63–S66.
- ,,.Experimental and Quasi‐Experimental Designs for Generalized Causal Inference.Boston:Houghton Mifflin;2002.
- .Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains.Acad Med.2004;79(10 Suppl):S70–S81.
- ,,,,.Guidelines for thoracentesis and needle biopsy of the pleura. This position paper of the American Thoracic Society was adopted by the ATS Board of Directors June 1988.Am Rev Resp Dis.1989;140:257–258.
- .Clinical practice. Pleural effusion.N Engl J Med2002;346:1971–1977.
- . The Checklists Development Checklist. Western Michigan University Evaluation Center, July 2000. Available at: http://www.wmich.edu/evalctr/checklists/cdc.htm. Accessed December 15,2005.
- ,,.Procedures for establishing defensible absolute passing scores on performance examinations in health professions education.Teach Learn Med2006;18:50–57.
- ,.Measurement and Assessment in Teaching.8th ed.Upper Saddle River, NJ:Prentice Hall;2000.
- .Pleural Diseases.4th ed.Philadelphia, PA:Lippincott Williams 2000:821–829.
- .Reliability: on the reproducibility of assessment data.Med Educ.2004;38:1006–1012.
- ,,.Statistical Methods for Rates and Proportions.3rd ed.New York:John Wiley 2003.
- ,.Coefficient kappa: some uses, misuses, and alternatives.Educ Psychol Meas.1981;41:687–699.
- ,,,.Competency in chest radiography: a comparison of medical students, residents and fellows.J Gen Intern Med.2006;21:460–465.
- ,.Pulmonary auscultatory skills during training in internal medicine and family practice.Am J Resp Crit Care Med.1999;159:1119–1124.
- ,.What procedures should internists do?Ann Intern Med.2007;146:392–3.
- ,,, et al.A longitudinal study of internal medicine residents' retention of advanced cardiac life support (ACLS) skills.Acad Med.2006;81(10 Suppl):S9–S12.
- ,,,,,.Simulation‐based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case‐control study.Chest.2008;[Epub ahead of print].
- ,,, et al.Virtual reality training improves operating room performance: results of a randomized, double‐blinded study.Ann Surg.2002;236:458–464.
- ,.The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians.Ann Intern Med.2007;146:355–360.
In a supplement to its inaugural issue, the Journal of Hospital Medicine published core competencies for hospitalists covering 3 areas: clinical conditions, systems in health care, and procedures.1 Completion of a traditional internal medicine residency may not provide hospitalists with the skills necessary to safely perform necessary procedures such as thoracentesis. A recent article reported that most internal medicine residents surveyed were uncomfortable performing common procedures, and their discomfort was higher for thoracentesis than for central line insertion, lumbar puncture, or paracentesis.2 This confirmed a previous report that family practice residents had low confidence in performing thoracenteses.3 Thoracentesis also carries the risk of the potentially life‐threatening complication of pneumothorax, which may be increased when performed by physicians‐in‐training.4
One method for improving training and assessment is the use of simulation technology. Simulation has been used to increase knowledge, provide opportunities for deliberate and safe practice, and shape the development of clinical skills.5, 6 Simulation has also been advocated for assessing competence in procedures including carotid angiography,7 emergency airway management,8 basic bronchoscopy,9 and advanced cardiac life support (ACLS).10, 11
Recently, we used simulation technology to help residents reach mastery learning standards for ACLS.11 Mastery learning,12 an extreme form of competency‐based education,13 implies that learners have acquired the clinical knowledge and skill measured against rigorous achievement standards. In mastery learning, educational results are equivalent, whereas educational practice time differs. To demonstrate mastery learning, we first documented a 38% improvement in skill after a simulation‐based educational intervention10 and used a multidisciplinary panel to determine mastery achievement standards for ACLS skills in 6 clinical scenarios.14 These standards were used in a study in which the amount of time needed to achieve skill mastery was allowed to vary while the skill outcomes of the residents were identical clinically.11
The present study had 4 aims. The first was to assess the baseline skill and knowledge of third‐year residents in thoracentesis. The second was to compare the thoracentesis‐related knowledge and skills of residents before and after an educational intervention. The third was to assess the correlation of medical knowledge and clinical experience with performance on a clinical skills examination after simulation training. The last was to document the feasibility of incorporating simulation‐based education into a training program.
METHODS
Objectives and Design
The study, which had a pretestposttest design without a control group,15 was of a simulation‐based, mastery learning educational intervention in thoracentesis. Primary measurements were obtained at baseline (pretest) and after the educational intervention (posttest).
Participants
Study participants were all 40 third‐year residents in the internal medicine residency program at Northwestern University's Chicago campus from January to May 2006. The Northwestern University Institutional Review Board approved the study. Participants provided informed consent before baseline assessment.
This residency program is based at Northwestern Memorial Hospital (NMH) and the Jesse Brown Veteran's Affairs Medical Center. Residents perform thoracenteses under the supervision of second‐ or third‐year residents or faculty members who are credentialed to perform the procedure. A didactic lecture on thoracentesis is part of the annual lecture series.
Procedure
The residents were kept as an intact group during the study period. The research procedure had 2 phases. First, the knowledge and clinical skills of participants at baseline were measured. Second, residents received two 2‐hour education sessions featuring didactic content and deliberate practice using a thoracentesis model. Between 4 and 6 weeks after the pretest, all residents were retested and were expected to meet or exceed a minimum passing score (MPS) on the clinical skills exam. Those who scored below the MPS engaged in more clinical skills practice until the mastery standard was reached. The amount of extra time needed to achieve the MPS was documented.
Educational Intervention
The intervention was designed to help residents acquire the knowledge and skills needed to perform a competent thoracentesis. The necessary components for mastery skill development were contained in the intervention. These included deliberate practice, rigorous skills assessment, and the provision of feedback in a supportive environment.16
The study was conducted in the Northwestern University Center for Advanced Surgical Education (N‐CASE) using the thoracentesis simulator developed by MediSim Inc. (Alton, Ontario) (
Teaching and testing sessions were standardized. In teaching sessions, groups of 2‐4 residents had 4 hours to practice and ask questions, and to receive structured education and feedback from 1 of 2 hospitalist faculty instructors (J.H.B., K.J.O.). One of the 4 hours was devoted to the presentation of didactic material on indications, complications, and interpretation of results and a step‐by‐step demonstration of a thoracentesis. This presentation was videotaped to ensure standardization of content. The remaining 3 hours were devoted to clinical skills exam education, deliberate practice, and feedback.
One resident was present at each pretest and posttest session with 1 of the 2 faculty instructors who gave standardized instructions. The resident was expected to obtain a relevant history; perform a limited physical examination; review PA, lateral, and decubitus chest radiographs; perform a simulated thoracentesis; and order appropriate diagnostic tests. Written examinations were completed at the pretest and posttest sessions.
Measurements
A 25‐item checklist was developed for the thoracentesis procedure using relevant sources17, 18 and rigorous step‐by‐step procedures.19 Each skill or other action was listed in order and given equal weight. Each skill or action was scored dichotomouslyeither 0 = done correctly or 1 = done incorrectly. Checklists were reviewed for completeness and accuracy by 2 authors who frequently perform and supervise thoracenteses (J.H.B., K.J.O.), 2 authors with expertise in checklist design (D.B.W., W.C.M.), and the physician director of the medical intensive care unit at NMH. The checklist was used in a pilot clinical skills examination of 4 chief medical residents to estimate checklist reliability and face validity.
The MPS for the thoracentesis clinical skills examination was determined by 10 clinical experts using the Angoff and Hofstee standard setting methods. The panel was composed of clinical pulmonary critical care medicine faculty (n = 7) and senior fellows (n = 3). Each panel member was given instruction on standard setting and asked to use the Angoff and Hofstee methods to assign pass/fail standards. The Angoff method asks expert judges to estimate the percentage of borderline examinees who would answer each test item correctly. The Hofstee method requires judges to estimate 4 properties of an evaluation's passing scores and failure rates. The panel was asked to repeat their judgments 6 weeks later to assure stability of the MPS. Details about the use of a standard setting exercise to set an MPS for clinical skills examinations have been published previously.14, 20
Evaluation of each resident's skill was recorded on the checklist by 1 of the 2 faculty raters at the pretest and posttest sessions. A random sample of 50% of the pretest sessions was rescored by a third rater with expertise in scoring clinical skills examinations (D.B.W.) to assess interrater reliability. The rescorer was blinded to the results of the first evaluation.
A multiple choice written examination was prepared according to examination development guidelines21 using appropriate reference articles and texts.17, 18, 22 The examination was prepared by 1 author (J.H.B.) and reviewed for accuracy and clarity by 2 others (K.J.O., D.B.W.) and by the director of the medical intensive care unit at NMH. The examination had questions on knowledge and comprehension of the procedure as well as data interpretation and application. It was administered to 9 fourth‐year medical students and 5 pulmonary/critical care fellows to obtain pilot data. Results of the pilot allowed creation of a pretest and a posttest that were equivalent in content and difficulty.23 The Kuder Richardson Formula 20 (KR‐20) reliability coefficients for the 20‐item pretest and the 20‐item posttest were .72 and .74, respectively.
Demographic data were obtained from the participants including age, gender, ethnicity, medical school, and scores on the United States Medical Licensing Examination (USMLE) Steps 1 and 2. Each resident's experience performing the procedure was also collected at pretest.
Primary outcome measures were performance on the posttest written and clinical examinations. Secondary outcome measures were the total training time needed to reach the MPS (minimum = 240 minutes) and a course evaluation questionnaire.
Data Analysis
Checklist score reliability was estimated by calculating interrater reliability, the preferred method for assessments that depend on human judges,24 using the kappa () coefficient25 adjusted using the formula of Brennan and Prediger.26 Within‐group differences from pretest (baseline) to posttest (outcome) were analyzed using paired t tests. Multiple regression analysis was used to assess the correlation of posttest performance on thoracentesis skills with (1) performance on pretest thoracentesis skills, (2) medical knowledge measured by the thoracentesis pretest and posttest and USMLE Steps 1 and 2, (3) clinical experience in performing thoracentesis, (4) clinical self‐confidence about performing thoracentesis, and (5) whether additional training was needed to master the procedure.
RESULTS
All residents consented to participate and completed the entire training protocol. Table 1 presents demographic data about the residents. Most had limited experience performing and supervising thoracenteses.
| Characteristic | PGY‐3 Resident |
|---|---|
| Age (years), mean (SD) | 28.88 (1.57) |
| Male | 23 (57.5%) |
| Female | 17 (42.5%) |
| African American | 1 (2.5%) |
| White | 21 (52.5%) |
| Asian | 14 (35.0%) |
| Other | 4 (10.0%) |
| U.S. medical school graduate | 39 (97.5%) |
| Foreign medical school graduate | 1 (2.5%) |
| Number of thoracentesis procedures | |
| Performed as an intern | |
| 0‐1 | 27.5% |
| 2‐4 | 60.0% |
| 5 | 12.5% |
| Performed as a PGY‐2 and PGY‐3 resident | |
| 0‐1 | 25.0% |
| 2‐4 | 55.0% |
| 5 | 20.0% |
| Supervised others as a PGY‐2 and PGY‐3 resident | |
| 0‐1 | 27.5% |
| 2‐4 | 57.5% |
| 5 | 15.0% |
Interrater reliability for the thoracentesis checklist data was calculated at pretest. Across the 25 checklist items, the mean kappa coefficient was very high (n = .94). The MPS used as the mastery achievement standard was 80% (eg, 20 of 25 checklist items). This was the mean of the Angoff and Hofstee ratings obtained from the first judgment of the expert panel and is displayed in Figure 1.

No resident achieved mastery at pretest. However, 37 of the 40 medicine residents (93%) achieved mastery within the standard 4‐hour thoracentesis curriculum. The remaining 3 residents (7%) needed extra time ranging from 20 to 90 minutes to reach mastery.
Figure 1 is a graphic portrait with descriptive statistics of the residents' pretest and posttest performance on the thoracentesis written and clinical skills exams. For the written exam, the mean score rose from 57.63% to 89.75%, a statistically significant improvement of 56% from pretest to posttest (t[39] = 17.0, P < .0001). The clinical skills exam also showed a highly significant 71% pretest‐to‐posttest gain, as the mean score rose from 51.70% to 88.3% (t[39] = 15.6, P < .0001).
Results from the regression analysis indicate that neither pretest performance, medical knowledge measured by local or USMLE examinations, nor thoracentesis clinical experience was correlated with the posttest measure of thoracentesis clinical skills. However, the need for additional practice to reach the mastery standard on the posttest was a powerful negative predictor of posttest performance: b = .27 (95% CI = .46 to .09; P < .006; r2 = .28). For those residents who required extra practice time, the initial clinical skills posttest score was 20% lower than that of their peers. Although the need for extra deliberate practice was associated with relatively lower initial posttest scores, all residents ultimately met or exceeded the rigorous thoracentesis MPS.
The responses of the 40 residents on a course evaluation questionnaire were uniformly positive. Responses were recorded on a Likert scale where 1 = strongly disagree, 2 = disagree, 3 = uncertain, 4 = agree, and 5 = strongly agree (Table 2). The data show that residents strongly agreed that practice with the medical simulator boosts clinical skills and self‐confidence, that they received useful feedback from the training sessions, and that deliberate practice using the simulator is a valuable educational experience. Residents were uncertain whether practice with the medical simulator has more educational value than patient care.
| Mean | SD | |
|---|---|---|
| Practice with the thoracentesis model boosts my skills to perform this procedure. | 4.3 | 0.8 |
| I receive useful educational feedback from the training sessions. | 4.0 | 0.6 |
| Practice with the thoracentesis model boosts my clinical self‐confidence. | 4.1 | 0.9 |
| Practice with the thoracentesis model has more educational value than patient care experience. | 2.3 | 1.0 |
| The Skills Center staff are competent. | 4.3 | 0.6 |
| Practice sessions in the Skills Center are a good use of my time. | 3.7 | 1.0 |
| Practice sessions using procedural models should be a required component of residency education. | 3.8 | 0.8 |
| Deliberate practice using models is a valuable educational experience. | 4.0 | 0.9 |
| Practice sessions using models are hard work. | 2.1 | 0.7 |
| Increasing the difficulty of simulated clinical problems helps me become a better doctor. | 3.9 | 0.7 |
| The controlled environment in the Skills Center helps me focus on clinical education problems. | 3.9 | 0.8 |
| Practice with the thoracentesis model has helped to prepare me to perform the procedure better than clinical experience alone. | 4.0 | 1.0 |
DISCUSSION
This study demonstrates the use of a mastery learning model to develop the thoracentesis skills of internal medicine residents to a high level. Use of a thoracentesis model in a structured educational program offering an opportunity for deliberate practice with feedback produced large and consistent improvements in residents' skills. An important finding of our study is that despite having completed most of their internal medicine training, residents displayed poor knowledge and clinical skill in thoracentesis procedures at baseline. This is similar to previous studies showing that the procedural skills and knowledge of physicians at all stages of training are often poor. Examples of areas in which significant gaps were found include basic skills such as chest radiography,27 emergency airway management,8 and pulmonary auscultation.28 In contrast, after the mastery learning program, all the residents met or exceeded the MPS for the thoracentesis clinical procedure and scored much higher on the posttest written examination.
Our data also demonstrate that medical knowledge measured by procedure‐specific pretests and posttests and USMLE Steps 1 and 2 scores were not correlated with thoracentesis skill acquisition. This reinforces findings from our previous studies of ACLS skill acquisition10, 11 and supports the difference between professional and academic achievement. Pretest skill performance and clinical experience also were not correlated with posttest outcomes. However, the amount of deliberate practice needed to reach the mastery standard was a powerful negative predictor of posttest thoracentesis skill scores, replicating our research on ACLS.11 We believe that clinical experience was not correlated with posttest outcomes because residents infrequently performed thoracenteses procedures during their training.
This project demonstrates a practical model for outcomes‐based education, certification, and program accreditation. Given the need to move procedural training in internal medicine beyond such historical methods as see one, do one, teach one,29 extension of the mastery model to other invasive procedures deserves further study. At our institution we have been encouraged by the ability of simulation‐based education in ACLS to promote long‐term skill retention30 and improvement in the quality of actual patient care.31 In addition to studying these outcomes for thoracentesis, we plan to incorporate the use of ultrasound when training residents to perform procedures such as thoracentesis and central venous catheter insertion.
Given concerns about the quality of resident preparation to perform invasive procedures, programs such as this should be considered as part of the procedural certification process. As shown by our experience with several classes of residents (n = 158), use of simulation technology to reach high procedural skill levels is effective and feasible in internal medicine residency training. In addition, our residents have consistently enjoyed participating in the simulated training programs. Postcourse questionnaires show that residents agree that deliberate practice with simulation technology complements but does not replace patient care in graduate medical education.5, 10
An important question needing more research is whether performance in a simulated environment transfers to actual clinical settings. Several small studies have demonstrated such a relationship,8, 9, 31, 32 yet the transfer of simulated training to clinical practice requires further study. More work should also be done to assess long‐term retention of skills30 and to determine the utility and benefit of simulation‐based training in procedural certification and credentialing.
This study had several limitations. It was conducted in 1 training program at a single medical center. The sample size (n = 40) was relatively small. The thoracentesis model was used for both education and testing, potentially confounding the events. However, these limitations do not diminish the pronounced impact that the simulation‐based training had on the skills and knowledge of our residents.
In conclusion, this study has demonstrated the ability of deliberate practice using a thoracentesis model to produce high‐level performance of simulated thoracenteses. The project received high ratings from learners and provides reliable assessments of procedural competence. Although internists are performing fewer invasive procedures now than in years past, procedural training is still an important component of internal medicine training.29, 33 Attainment of high procedural skill levels may be especially important for residents who plan to practice hospital medicine. We believe that simulation‐based training using deliberate practice should be a key contributor to future internal medicine residency education, certification, and accreditation.
Acknowledgements
The authors thank Charles Watts, MD, and J. Larry Jameson, MD, PhD, for their support of this work. We recognize and appreciate the Northwestern University internal medicine residents for their dedication to patient care and education.
In a supplement to its inaugural issue, the Journal of Hospital Medicine published core competencies for hospitalists covering 3 areas: clinical conditions, systems in health care, and procedures.1 Completion of a traditional internal medicine residency may not provide hospitalists with the skills necessary to safely perform necessary procedures such as thoracentesis. A recent article reported that most internal medicine residents surveyed were uncomfortable performing common procedures, and their discomfort was higher for thoracentesis than for central line insertion, lumbar puncture, or paracentesis.2 This confirmed a previous report that family practice residents had low confidence in performing thoracenteses.3 Thoracentesis also carries the risk of the potentially life‐threatening complication of pneumothorax, which may be increased when performed by physicians‐in‐training.4
One method for improving training and assessment is the use of simulation technology. Simulation has been used to increase knowledge, provide opportunities for deliberate and safe practice, and shape the development of clinical skills.5, 6 Simulation has also been advocated for assessing competence in procedures including carotid angiography,7 emergency airway management,8 basic bronchoscopy,9 and advanced cardiac life support (ACLS).10, 11
Recently, we used simulation technology to help residents reach mastery learning standards for ACLS.11 Mastery learning,12 an extreme form of competency‐based education,13 implies that learners have acquired the clinical knowledge and skill measured against rigorous achievement standards. In mastery learning, educational results are equivalent, whereas educational practice time differs. To demonstrate mastery learning, we first documented a 38% improvement in skill after a simulation‐based educational intervention10 and used a multidisciplinary panel to determine mastery achievement standards for ACLS skills in 6 clinical scenarios.14 These standards were used in a study in which the amount of time needed to achieve skill mastery was allowed to vary while the skill outcomes of the residents were identical clinically.11
The present study had 4 aims. The first was to assess the baseline skill and knowledge of third‐year residents in thoracentesis. The second was to compare the thoracentesis‐related knowledge and skills of residents before and after an educational intervention. The third was to assess the correlation of medical knowledge and clinical experience with performance on a clinical skills examination after simulation training. The last was to document the feasibility of incorporating simulation‐based education into a training program.
METHODS
Objectives and Design
The study, which had a pretestposttest design without a control group,15 was of a simulation‐based, mastery learning educational intervention in thoracentesis. Primary measurements were obtained at baseline (pretest) and after the educational intervention (posttest).
Participants
Study participants were all 40 third‐year residents in the internal medicine residency program at Northwestern University's Chicago campus from January to May 2006. The Northwestern University Institutional Review Board approved the study. Participants provided informed consent before baseline assessment.
This residency program is based at Northwestern Memorial Hospital (NMH) and the Jesse Brown Veteran's Affairs Medical Center. Residents perform thoracenteses under the supervision of second‐ or third‐year residents or faculty members who are credentialed to perform the procedure. A didactic lecture on thoracentesis is part of the annual lecture series.
Procedure
The residents were kept as an intact group during the study period. The research procedure had 2 phases. First, the knowledge and clinical skills of participants at baseline were measured. Second, residents received two 2‐hour education sessions featuring didactic content and deliberate practice using a thoracentesis model. Between 4 and 6 weeks after the pretest, all residents were retested and were expected to meet or exceed a minimum passing score (MPS) on the clinical skills exam. Those who scored below the MPS engaged in more clinical skills practice until the mastery standard was reached. The amount of extra time needed to achieve the MPS was documented.
Educational Intervention
The intervention was designed to help residents acquire the knowledge and skills needed to perform a competent thoracentesis. The necessary components for mastery skill development were contained in the intervention. These included deliberate practice, rigorous skills assessment, and the provision of feedback in a supportive environment.16
The study was conducted in the Northwestern University Center for Advanced Surgical Education (N‐CASE) using the thoracentesis simulator developed by MediSim Inc. (Alton, Ontario) (
Teaching and testing sessions were standardized. In teaching sessions, groups of 2‐4 residents had 4 hours to practice and ask questions, and to receive structured education and feedback from 1 of 2 hospitalist faculty instructors (J.H.B., K.J.O.). One of the 4 hours was devoted to the presentation of didactic material on indications, complications, and interpretation of results and a step‐by‐step demonstration of a thoracentesis. This presentation was videotaped to ensure standardization of content. The remaining 3 hours were devoted to clinical skills exam education, deliberate practice, and feedback.
One resident was present at each pretest and posttest session with 1 of the 2 faculty instructors who gave standardized instructions. The resident was expected to obtain a relevant history; perform a limited physical examination; review PA, lateral, and decubitus chest radiographs; perform a simulated thoracentesis; and order appropriate diagnostic tests. Written examinations were completed at the pretest and posttest sessions.
Measurements
A 25‐item checklist was developed for the thoracentesis procedure using relevant sources17, 18 and rigorous step‐by‐step procedures.19 Each skill or other action was listed in order and given equal weight. Each skill or action was scored dichotomouslyeither 0 = done correctly or 1 = done incorrectly. Checklists were reviewed for completeness and accuracy by 2 authors who frequently perform and supervise thoracenteses (J.H.B., K.J.O.), 2 authors with expertise in checklist design (D.B.W., W.C.M.), and the physician director of the medical intensive care unit at NMH. The checklist was used in a pilot clinical skills examination of 4 chief medical residents to estimate checklist reliability and face validity.
The MPS for the thoracentesis clinical skills examination was determined by 10 clinical experts using the Angoff and Hofstee standard setting methods. The panel was composed of clinical pulmonary critical care medicine faculty (n = 7) and senior fellows (n = 3). Each panel member was given instruction on standard setting and asked to use the Angoff and Hofstee methods to assign pass/fail standards. The Angoff method asks expert judges to estimate the percentage of borderline examinees who would answer each test item correctly. The Hofstee method requires judges to estimate 4 properties of an evaluation's passing scores and failure rates. The panel was asked to repeat their judgments 6 weeks later to assure stability of the MPS. Details about the use of a standard setting exercise to set an MPS for clinical skills examinations have been published previously.14, 20
Evaluation of each resident's skill was recorded on the checklist by 1 of the 2 faculty raters at the pretest and posttest sessions. A random sample of 50% of the pretest sessions was rescored by a third rater with expertise in scoring clinical skills examinations (D.B.W.) to assess interrater reliability. The rescorer was blinded to the results of the first evaluation.
A multiple choice written examination was prepared according to examination development guidelines21 using appropriate reference articles and texts.17, 18, 22 The examination was prepared by 1 author (J.H.B.) and reviewed for accuracy and clarity by 2 others (K.J.O., D.B.W.) and by the director of the medical intensive care unit at NMH. The examination had questions on knowledge and comprehension of the procedure as well as data interpretation and application. It was administered to 9 fourth‐year medical students and 5 pulmonary/critical care fellows to obtain pilot data. Results of the pilot allowed creation of a pretest and a posttest that were equivalent in content and difficulty.23 The Kuder Richardson Formula 20 (KR‐20) reliability coefficients for the 20‐item pretest and the 20‐item posttest were .72 and .74, respectively.
Demographic data were obtained from the participants including age, gender, ethnicity, medical school, and scores on the United States Medical Licensing Examination (USMLE) Steps 1 and 2. Each resident's experience performing the procedure was also collected at pretest.
Primary outcome measures were performance on the posttest written and clinical examinations. Secondary outcome measures were the total training time needed to reach the MPS (minimum = 240 minutes) and a course evaluation questionnaire.
Data Analysis
Checklist score reliability was estimated by calculating interrater reliability, the preferred method for assessments that depend on human judges,24 using the kappa () coefficient25 adjusted using the formula of Brennan and Prediger.26 Within‐group differences from pretest (baseline) to posttest (outcome) were analyzed using paired t tests. Multiple regression analysis was used to assess the correlation of posttest performance on thoracentesis skills with (1) performance on pretest thoracentesis skills, (2) medical knowledge measured by the thoracentesis pretest and posttest and USMLE Steps 1 and 2, (3) clinical experience in performing thoracentesis, (4) clinical self‐confidence about performing thoracentesis, and (5) whether additional training was needed to master the procedure.
RESULTS
All residents consented to participate and completed the entire training protocol. Table 1 presents demographic data about the residents. Most had limited experience performing and supervising thoracenteses.
| Characteristic | PGY‐3 Resident |
|---|---|
| Age (years), mean (SD) | 28.88 (1.57) |
| Male | 23 (57.5%) |
| Female | 17 (42.5%) |
| African American | 1 (2.5%) |
| White | 21 (52.5%) |
| Asian | 14 (35.0%) |
| Other | 4 (10.0%) |
| U.S. medical school graduate | 39 (97.5%) |
| Foreign medical school graduate | 1 (2.5%) |
| Number of thoracentesis procedures | |
| Performed as an intern | |
| 0‐1 | 27.5% |
| 2‐4 | 60.0% |
| 5 | 12.5% |
| Performed as a PGY‐2 and PGY‐3 resident | |
| 0‐1 | 25.0% |
| 2‐4 | 55.0% |
| 5 | 20.0% |
| Supervised others as a PGY‐2 and PGY‐3 resident | |
| 0‐1 | 27.5% |
| 2‐4 | 57.5% |
| 5 | 15.0% |
Interrater reliability for the thoracentesis checklist data was calculated at pretest. Across the 25 checklist items, the mean kappa coefficient was very high (n = .94). The MPS used as the mastery achievement standard was 80% (eg, 20 of 25 checklist items). This was the mean of the Angoff and Hofstee ratings obtained from the first judgment of the expert panel and is displayed in Figure 1.

No resident achieved mastery at pretest. However, 37 of the 40 medicine residents (93%) achieved mastery within the standard 4‐hour thoracentesis curriculum. The remaining 3 residents (7%) needed extra time ranging from 20 to 90 minutes to reach mastery.
Figure 1 is a graphic portrait with descriptive statistics of the residents' pretest and posttest performance on the thoracentesis written and clinical skills exams. For the written exam, the mean score rose from 57.63% to 89.75%, a statistically significant improvement of 56% from pretest to posttest (t[39] = 17.0, P < .0001). The clinical skills exam also showed a highly significant 71% pretest‐to‐posttest gain, as the mean score rose from 51.70% to 88.3% (t[39] = 15.6, P < .0001).
Results from the regression analysis indicate that neither pretest performance, medical knowledge measured by local or USMLE examinations, nor thoracentesis clinical experience was correlated with the posttest measure of thoracentesis clinical skills. However, the need for additional practice to reach the mastery standard on the posttest was a powerful negative predictor of posttest performance: b = .27 (95% CI = .46 to .09; P < .006; r2 = .28). For those residents who required extra practice time, the initial clinical skills posttest score was 20% lower than that of their peers. Although the need for extra deliberate practice was associated with relatively lower initial posttest scores, all residents ultimately met or exceeded the rigorous thoracentesis MPS.
The responses of the 40 residents on a course evaluation questionnaire were uniformly positive. Responses were recorded on a Likert scale where 1 = strongly disagree, 2 = disagree, 3 = uncertain, 4 = agree, and 5 = strongly agree (Table 2). The data show that residents strongly agreed that practice with the medical simulator boosts clinical skills and self‐confidence, that they received useful feedback from the training sessions, and that deliberate practice using the simulator is a valuable educational experience. Residents were uncertain whether practice with the medical simulator has more educational value than patient care.
| Mean | SD | |
|---|---|---|
| Practice with the thoracentesis model boosts my skills to perform this procedure. | 4.3 | 0.8 |
| I receive useful educational feedback from the training sessions. | 4.0 | 0.6 |
| Practice with the thoracentesis model boosts my clinical self‐confidence. | 4.1 | 0.9 |
| Practice with the thoracentesis model has more educational value than patient care experience. | 2.3 | 1.0 |
| The Skills Center staff are competent. | 4.3 | 0.6 |
| Practice sessions in the Skills Center are a good use of my time. | 3.7 | 1.0 |
| Practice sessions using procedural models should be a required component of residency education. | 3.8 | 0.8 |
| Deliberate practice using models is a valuable educational experience. | 4.0 | 0.9 |
| Practice sessions using models are hard work. | 2.1 | 0.7 |
| Increasing the difficulty of simulated clinical problems helps me become a better doctor. | 3.9 | 0.7 |
| The controlled environment in the Skills Center helps me focus on clinical education problems. | 3.9 | 0.8 |
| Practice with the thoracentesis model has helped to prepare me to perform the procedure better than clinical experience alone. | 4.0 | 1.0 |
DISCUSSION
This study demonstrates the use of a mastery learning model to develop the thoracentesis skills of internal medicine residents to a high level. Use of a thoracentesis model in a structured educational program offering an opportunity for deliberate practice with feedback produced large and consistent improvements in residents' skills. An important finding of our study is that despite having completed most of their internal medicine training, residents displayed poor knowledge and clinical skill in thoracentesis procedures at baseline. This is similar to previous studies showing that the procedural skills and knowledge of physicians at all stages of training are often poor. Examples of areas in which significant gaps were found include basic skills such as chest radiography,27 emergency airway management,8 and pulmonary auscultation.28 In contrast, after the mastery learning program, all the residents met or exceeded the MPS for the thoracentesis clinical procedure and scored much higher on the posttest written examination.
Our data also demonstrate that medical knowledge measured by procedure‐specific pretests and posttests and USMLE Steps 1 and 2 scores were not correlated with thoracentesis skill acquisition. This reinforces findings from our previous studies of ACLS skill acquisition10, 11 and supports the difference between professional and academic achievement. Pretest skill performance and clinical experience also were not correlated with posttest outcomes. However, the amount of deliberate practice needed to reach the mastery standard was a powerful negative predictor of posttest thoracentesis skill scores, replicating our research on ACLS.11 We believe that clinical experience was not correlated with posttest outcomes because residents infrequently performed thoracenteses procedures during their training.
This project demonstrates a practical model for outcomes‐based education, certification, and program accreditation. Given the need to move procedural training in internal medicine beyond such historical methods as see one, do one, teach one,29 extension of the mastery model to other invasive procedures deserves further study. At our institution we have been encouraged by the ability of simulation‐based education in ACLS to promote long‐term skill retention30 and improvement in the quality of actual patient care.31 In addition to studying these outcomes for thoracentesis, we plan to incorporate the use of ultrasound when training residents to perform procedures such as thoracentesis and central venous catheter insertion.
Given concerns about the quality of resident preparation to perform invasive procedures, programs such as this should be considered as part of the procedural certification process. As shown by our experience with several classes of residents (n = 158), use of simulation technology to reach high procedural skill levels is effective and feasible in internal medicine residency training. In addition, our residents have consistently enjoyed participating in the simulated training programs. Postcourse questionnaires show that residents agree that deliberate practice with simulation technology complements but does not replace patient care in graduate medical education.5, 10
An important question needing more research is whether performance in a simulated environment transfers to actual clinical settings. Several small studies have demonstrated such a relationship,8, 9, 31, 32 yet the transfer of simulated training to clinical practice requires further study. More work should also be done to assess long‐term retention of skills30 and to determine the utility and benefit of simulation‐based training in procedural certification and credentialing.
This study had several limitations. It was conducted in 1 training program at a single medical center. The sample size (n = 40) was relatively small. The thoracentesis model was used for both education and testing, potentially confounding the events. However, these limitations do not diminish the pronounced impact that the simulation‐based training had on the skills and knowledge of our residents.
In conclusion, this study has demonstrated the ability of deliberate practice using a thoracentesis model to produce high‐level performance of simulated thoracenteses. The project received high ratings from learners and provides reliable assessments of procedural competence. Although internists are performing fewer invasive procedures now than in years past, procedural training is still an important component of internal medicine training.29, 33 Attainment of high procedural skill levels may be especially important for residents who plan to practice hospital medicine. We believe that simulation‐based training using deliberate practice should be a key contributor to future internal medicine residency education, certification, and accreditation.
Acknowledgements
The authors thank Charles Watts, MD, and J. Larry Jameson, MD, PhD, for their support of this work. We recognize and appreciate the Northwestern University internal medicine residents for their dedication to patient care and education.
- ,,,,.Core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1:48–56.
- ,,, et al.Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures.Am J Med.2006;119:71.e17–71.e24.
- ,,.Perception of competency to perform procedures and future practice intent: a national survey of family practice residents.Acad Med.2003;78:926–932.
- ,,,,,.Lower risk and higher yield for thoracentesis when performed by experienced operators.Chest.1993;103:1873–1876.
- ,,, et al.Simulation technology for health care professional skills training and assessment.JAMA.1999;282:861–866.
- ,,, et al.Reliability and validity of a simulation‐based acute care skills assessment for medical students and residents.Anesthesiology.2003;99:1270–1280.
- ,,,.Learning curves and reliability measures for virtual reality simulation in the performance assessment of carotid angiography.J Am Coll Cardiol.2006;47:1796–1802.
- ,,,,.Achieving house staff competence in emergency airway management: results of a teaching program using a computerized patient simulator.Crit Care Med.2004;32:2422–2427.
- ,,.Bronchoscopy simulator effectively prepares junior residents to competently perform basic clinical bronchoscopy.Ann Thorac Surg.2004;78:287–291.
- ,,, et al.Simulation‐based training of internal medicine residents in advanced cardiac life support protocols: a randomized trial.Teach Learn Med.2005;17:210–216.
- ,,, et al.Mastery learning of advanced cardiac life support skills by internal medicine residents using simulation technology and deliberate practice.J Gen Intern Med.2006;21:251–256.
- Block JH, ed.Mastery Learning: Theory and Practice.New York:Holt, Rinehart and Winston;1971.
- ,,,.Competency‐Based Curriculum Development in Medical Education. Public Health Paper No. 68.Geneva, Switzerland:World Health Organization;1978.
- ,,, et al.Comparison of two standard‐setting methods for advanced cardiac life support training.Acad Med.2005;80(10 Suppl):S63–S66.
- ,,.Experimental and Quasi‐Experimental Designs for Generalized Causal Inference.Boston:Houghton Mifflin;2002.
- .Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains.Acad Med.2004;79(10 Suppl):S70–S81.
- ,,,,.Guidelines for thoracentesis and needle biopsy of the pleura. This position paper of the American Thoracic Society was adopted by the ATS Board of Directors June 1988.Am Rev Resp Dis.1989;140:257–258.
- .Clinical practice. Pleural effusion.N Engl J Med2002;346:1971–1977.
- . The Checklists Development Checklist. Western Michigan University Evaluation Center, July 2000. Available at: http://www.wmich.edu/evalctr/checklists/cdc.htm. Accessed December 15,2005.
- ,,.Procedures for establishing defensible absolute passing scores on performance examinations in health professions education.Teach Learn Med2006;18:50–57.
- ,.Measurement and Assessment in Teaching.8th ed.Upper Saddle River, NJ:Prentice Hall;2000.
- .Pleural Diseases.4th ed.Philadelphia, PA:Lippincott Williams 2000:821–829.
- .Reliability: on the reproducibility of assessment data.Med Educ.2004;38:1006–1012.
- ,,.Statistical Methods for Rates and Proportions.3rd ed.New York:John Wiley 2003.
- ,.Coefficient kappa: some uses, misuses, and alternatives.Educ Psychol Meas.1981;41:687–699.
- ,,,.Competency in chest radiography: a comparison of medical students, residents and fellows.J Gen Intern Med.2006;21:460–465.
- ,.Pulmonary auscultatory skills during training in internal medicine and family practice.Am J Resp Crit Care Med.1999;159:1119–1124.
- ,.What procedures should internists do?Ann Intern Med.2007;146:392–3.
- ,,, et al.A longitudinal study of internal medicine residents' retention of advanced cardiac life support (ACLS) skills.Acad Med.2006;81(10 Suppl):S9–S12.
- ,,,,,.Simulation‐based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case‐control study.Chest.2008;[Epub ahead of print].
- ,,, et al.Virtual reality training improves operating room performance: results of a randomized, double‐blinded study.Ann Surg.2002;236:458–464.
- ,.The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians.Ann Intern Med.2007;146:355–360.
- ,,,,.Core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1:48–56.
- ,,, et al.Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures.Am J Med.2006;119:71.e17–71.e24.
- ,,.Perception of competency to perform procedures and future practice intent: a national survey of family practice residents.Acad Med.2003;78:926–932.
- ,,,,,.Lower risk and higher yield for thoracentesis when performed by experienced operators.Chest.1993;103:1873–1876.
- ,,, et al.Simulation technology for health care professional skills training and assessment.JAMA.1999;282:861–866.
- ,,, et al.Reliability and validity of a simulation‐based acute care skills assessment for medical students and residents.Anesthesiology.2003;99:1270–1280.
- ,,,.Learning curves and reliability measures for virtual reality simulation in the performance assessment of carotid angiography.J Am Coll Cardiol.2006;47:1796–1802.
- ,,,,.Achieving house staff competence in emergency airway management: results of a teaching program using a computerized patient simulator.Crit Care Med.2004;32:2422–2427.
- ,,.Bronchoscopy simulator effectively prepares junior residents to competently perform basic clinical bronchoscopy.Ann Thorac Surg.2004;78:287–291.
- ,,, et al.Simulation‐based training of internal medicine residents in advanced cardiac life support protocols: a randomized trial.Teach Learn Med.2005;17:210–216.
- ,,, et al.Mastery learning of advanced cardiac life support skills by internal medicine residents using simulation technology and deliberate practice.J Gen Intern Med.2006;21:251–256.
- Block JH, ed.Mastery Learning: Theory and Practice.New York:Holt, Rinehart and Winston;1971.
- ,,,.Competency‐Based Curriculum Development in Medical Education. Public Health Paper No. 68.Geneva, Switzerland:World Health Organization;1978.
- ,,, et al.Comparison of two standard‐setting methods for advanced cardiac life support training.Acad Med.2005;80(10 Suppl):S63–S66.
- ,,.Experimental and Quasi‐Experimental Designs for Generalized Causal Inference.Boston:Houghton Mifflin;2002.
- .Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains.Acad Med.2004;79(10 Suppl):S70–S81.
- ,,,,.Guidelines for thoracentesis and needle biopsy of the pleura. This position paper of the American Thoracic Society was adopted by the ATS Board of Directors June 1988.Am Rev Resp Dis.1989;140:257–258.
- .Clinical practice. Pleural effusion.N Engl J Med2002;346:1971–1977.
- . The Checklists Development Checklist. Western Michigan University Evaluation Center, July 2000. Available at: http://www.wmich.edu/evalctr/checklists/cdc.htm. Accessed December 15,2005.
- ,,.Procedures for establishing defensible absolute passing scores on performance examinations in health professions education.Teach Learn Med2006;18:50–57.
- ,.Measurement and Assessment in Teaching.8th ed.Upper Saddle River, NJ:Prentice Hall;2000.
- .Pleural Diseases.4th ed.Philadelphia, PA:Lippincott Williams 2000:821–829.
- .Reliability: on the reproducibility of assessment data.Med Educ.2004;38:1006–1012.
- ,,.Statistical Methods for Rates and Proportions.3rd ed.New York:John Wiley 2003.
- ,.Coefficient kappa: some uses, misuses, and alternatives.Educ Psychol Meas.1981;41:687–699.
- ,,,.Competency in chest radiography: a comparison of medical students, residents and fellows.J Gen Intern Med.2006;21:460–465.
- ,.Pulmonary auscultatory skills during training in internal medicine and family practice.Am J Resp Crit Care Med.1999;159:1119–1124.
- ,.What procedures should internists do?Ann Intern Med.2007;146:392–3.
- ,,, et al.A longitudinal study of internal medicine residents' retention of advanced cardiac life support (ACLS) skills.Acad Med.2006;81(10 Suppl):S9–S12.
- ,,,,,.Simulation‐based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case‐control study.Chest.2008;[Epub ahead of print].
- ,,, et al.Virtual reality training improves operating room performance: results of a randomized, double‐blinded study.Ann Surg.2002;236:458–464.
- ,.The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians.Ann Intern Med.2007;146:355–360.
Copyright © 2008 Society of Hospital Medicine
Postdischarge Follow‐Up Visits for Medical/Pharmacy Students
The increasing burden of chronic illness has prompted concerns about the traditional education model that focuses on management of acute disease.13 Chronic illness has replaced acute disease as the major cause of disability and total national health care expenditures.46 Medical educators have called for improved chronic disease curricula,2, 3 and the Institute of Medicine has asserted that health professions, including medicine and pharmacy, must reexamine how students are educated to manage patients with complex illnesses.7, 8 Despite the rising prevalence of chronic illness, the positive attitudes of medical students toward providing care to such patients decline during training.2, 9 One theory is that the current model of core clerkship training excessively exposes students to highly complex, seriously ill hospitalized patients. Students may become disillusioned and overwhelmed by these encounters, particularly without the opportunity to see improvement or thriving in the outpatient setting.2
There are few curricula on how to transition chronically ill patients from an inpatient to an outpatient setting and the inherent safety risks of this transition. For these patients, the posthospital discharge period is particularly confusing because of the sudden change in health status and new medication regimens.1012 It is very likely that communication among providers and patients will be insufficient during the discharge process,11, 1315 yet physicians tend to overestimate patients' understanding of postdischarge treatment plans and thereby underanticipate problems.16 One intervention to address these concerns is a postdischarge visit. Home visits have been shown to improve students' understanding of continuity of care and of the impact of chronic illness on their patients' medical and psychosocial situations.1719
There is scant structured teaching of third‐year medical students about another critical aspect of transitional care: the role of different health care disciplines. Although research about the impact of undergraduate interdisciplinary education on patient outcomes is limited, training students in interdisciplinary collaboration may improve their ability to provide quality care.2022 Multiple disciplines are critical for a smooth transition of chronically ill patients from an inpatient to an outpatient setting. In particular, pharmacist involvement in a predischarge medication review, patient counseling, and telephone follow‐up has been associated with improved outcomes.11, 12, 23, 24 Early introduction of interdisciplinary team training can improve student attitudes about working within a team.25
To teach the importance of safe discharges and interdisciplinary collaboration in caring for chronically ill patients, we developed an inpatient medicine clerkship curriculum for medical and pharmacy students that included postdischarge visits to students' own team patients. The purpose of the study was to assess the impact of this didactic and experiential curriculum on students' attitudes and self‐assessed skills in the interdisciplinary care and transitional care of chronically ill patients. We hypothesized that the discharge curriculum would improve student attitudes and self‐assessed skills in these domains. Finally, we hypothesized that visiting a patient's home would highlight for students the potential challenges of care transitions for patients.
METHODS
Participants and Setting
Participants were third‐year medical students on an 8‐week internal medicine (IM) clerkship and fourth‐year pharmacy students on a 6‐week pharmacy practice clerkship at a tertiary‐care university‐based hospital between April 2005 and April 2006. The hospital is 1 of 3 IM clerkship sites for medical students and 1 of 9 for pharmacy students. This site was selected because it included both medical and pharmacy students on most inpatient teams.
Clerkship students were assigned to all 7 medical teams, each consisting of an attending physician, a senior IM resident (postgraduate year 2 or 3), 2 IM interns (postgraduate year 1), 1 or 2 medical students, and up to 1 pharmacy student. Hospitalists covered 52% of inpatient months, with the remainder staffed by faculty primary care physicians, specialists, or chief residents. Although only three‐quarters of the medical teams were randomly assigned a pharmacy student at any given point, each team had a pharmacy student for a portion of time that overlapped with the rotation of the medical students. Over the year, 810 medical students rotated on the service during each of 6 blocks, and 46 pharmacy students and 1 pharmacy practice resident rotated during each of 8 blocks. The pharmacy students rotated on a different schedule than the medical students, and thus the curriculum was scheduled around the medical students' clerkship.
The Institutional Review Board of the University of California at San Francisco approved the study.
Intervention (Curriculum Description)
We developed a 3‐part pilot interdisciplinary curriculum (Fig. 1). During the first 2 weeks of the IM clerkship, interdisciplinary faculty, including 3 pharmacists, 2 hospitalists, and occasionally a social worker and geriatric clinical nurse specialist, led a 1‐hour interactive workshop on transitional care. The 3 workshop topics were: roles that various disciplines such as social work and pharmacy play in discharge care; the challenges a patient faces around the time of discharge, using a typical case; and discussion of elements of a postdischarge visit.
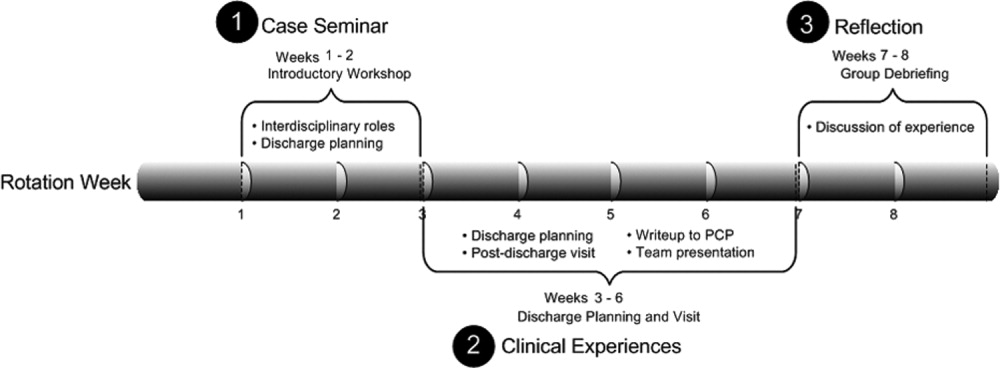
Medical and pharmacy students were partnered based on clerkship team assignments in teams of up to 3 student partners (1 or 2 medical students and 1 pharmacy student). Partners were advised to select a consenting patient known to them from the ward team for 1 postdischarge visit. Suggested selection criteria were at least 1 chronic illness, 1 prior hospitalization, and older than age 65 because patients fitting these criteria are most at risk for readmission or adverse outcomes following discharge.15, 26, 27 The student partners scheduled a postdischarge visit by the end of the rotation to the patient's home, nursing home, or subacute care facility. Each patient and the patient's primary care provider (PCP) gave informed consent.
During the postdischarge visit, student partners assessed medication discrepancies, environmental safety, and clinical status using structured data collection protocols developed by the investigators after review of the literature.28, 29 After the visit, students reported back to the ward teams on the patient's status and wrote a visit summary letter to the patient's PCP. The letter described the patient's clinical status and home environment, any medication discrepancies, and follow‐up plans and included a reflection piece. Reflection questions included, How did the visit change your perspective of patient discharge? What were the most critical aspects of this or any discharge? How do you think this experience will affect your future practice? What was the best thing about this experience?
During the last 2 weeks of the rotation, all student participants met with faculty preceptors for an hour‐long group debriefing session on the postdischarge visits.
Survey Instrument and Procedure
Students were asked to complete a presurvey at the beginning of the first workshop and a postsurvey at the end of the second (debriefing) workshop. The surveys contained self‐assessment questions on attitudes and skills in 3 domains: interdisciplinary care, chronic illness management, and transitional care. Questions were developed and tested with IM faculty with experience in student education and with ineligible students on previous rotations, and questions were revised for clarity and comprehensiveness. Students had the option to write a 4‐digit identifier on the pre‐ and postsurveys to allow matched analysis.
The 10‐item presurvey contained 4 items on interdisciplinary care and 3 each on chronic care and follow‐up visits. We reviewed surveys in the literature regarding home care and chronic illness to inform the development of our survey.30, 31 Students rated each item on a 5‐point Likert scale, ranging from 1 (strongly disagree) to 5 (strongly agree). The 22‐item postsurvey included the same 10 items and additional Likert‐scaled questions on satisfaction with the curriculum. Two open‐ended questions solicited opinions about the value of the program and lessons learned for future patient encounters.
Statistical Analysis
We assessed the mean Likert score ( SD) for each presurvey and postsurvey question and compared means ( SD). We evaluated the differences between medical students and for pharmacy students in mean Likert score on the surveys using a dependent‐samples t test and set the level of significance at 0.05.
Change in scores between prepost survey variables were calculated overall and within student type (medicine vs. pharmacy). Because no intercorrelations and possible patterns indicating a structure were found, a factor analysis was not conducted.
Two investigators (C.L., H.N.) read all written responses to the open‐ended questions and independently generated a list of themes. The list was reconciled through discussion and was used to code all comments in order to determine the frequency of each theme. Discrepancies were discussed until consensus was reached.
RESULTS
Participants
Ninety‐seven percent of eligible students (37 of 39 medical students and 22 of 22 pharmacy students) completed the curriculum. Two medical students did not complete the home visit because their patients did not keep the appointment. The presurvey response was 100% for medical students and 91% for pharmacy students. The postsurvey response was 92% for medical students and 86% for pharmacy students; 58% of medical students and 59% of pharmacy students wrote in matching prepost survey identifiers for statistical analysis. Prepost survey responses showed an increase for both student groups in positive attitudes and self‐assessed skill in interdisciplinary collaboration, chronic illness management, and transitional care. Trends over time were highly significant for individual items on matched surveys (P < 0.05; Table 1a,b).
| Question | Medical students (matched respondents n = 23) | Pharmacy students (matched respondents n = 13) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Presurvey, mean (SD) | Postsurvey, mean (SD) | Mean difference | P value | Effect size | Presurvey, mean (SD) | Postsurvey, mean (SD) | Mean difference | P value | Effect size | |
| ||||||||||
| 1. I am able to state the various roles of the pharmacy students and/or pharmacists (or medical students and/or physicians) in taking care of hospitalized patients. | 2.83 (0.89) | 4.35 (0.57) | 1.52 | < .001* | 1.72 | 3.69 (0.63) | 4.15 (0.38) | 0.46 | .03* | 0.73 |
| 2. I am able to state the various roles of the case manager and/or social worker in taking care of hospitalized patients. | 2.83 (0.78) | 3.91 (0.42) | 1.09 | < .001* | 1.40 | 2.77 (0.83) | 3.54 (0.97) | 0.77 | .01* | 0.92 |
| 3. I am confident in my ability to work with a pharmacy student or pharmacist (or medical student and/or physician) in taking care of inpatients with chronic illness. | 3.22 (1.00) | 4.52 (0.51) | 1.30 | < .001* | 1.31 | 3.62 (0.87) | 4.23 (0.44) | 0.62 | .04* | 0.71 |
| 4. I am confident in my ability to work with a case manager and/or social worker in taking care of inpatients with chronic illness. | 2.96 (0.71) | 3.96 (0.56) | 1.00 | < .001* | 1.42 | 3.08 (0.95) | 3.38 (0.87) | 0.31 | .34 | 0.32 |
| 5. I am confident in my ability to involve patients in making a plan for their care. | 3.74 (0.62) | 4.26 (0.54) | 0.52 | < .001* | 0.84 | 3.23 (0.60) | 4.15 (0.55) | 0.92 | < .001* | 1.54 |
| 6. I am able to assist patients in solving problems they encounter in self‐management of their chronic illness. | 3.30 (0.70) | 3.91 (0.60) | 0.61 | < .001* | 0.87 | 3.75 (0.87) | 3.92 (0.49) | 0.17 | .50 | 0.20 |
| 7. I am confident in my ability to review patients' medications and side effects. | 3.00 (0.85) | 3.70 (0.76) | 0.70 | < .001* | 0.82 | 3.92 (0.76) | 4.46 (0.52) | 0.54 | .03* | 0.71 |
| 8. I am able to review the goals of a follow‐up visit with a patient. | 3.52 (0.95) | 4.43 (0.51) | 0.91 | < .001* | 0.96 | 3.08 (0.76) | 3.62 (0.77) | 0.54 | .05 | 0.71 |
| 9. I can identify factors that may facilitate or impede a patient's transition to an outpatient setting. | 3.48 (0.51) | 4.35 (0.49) | 0.87 | < .001* | 1.70 | 3.00 (0.82) | 3.85 (0.69) | 0.85 | .01* | 1.04 |
| 10. I can identify several topics for review at a follow‐up visit to confirm a safe transition to an outpatient setting. | 3.39 (0.94) | 4.52 (0.59) | 1.13 | < .001* | 1.20 | 3.23 (0.73) | 3.77 (0.73) | 0.54 | .11 | 0.74 |
Twenty‐two student partners of 1 or 2 medical students and 1 pharmacy student visited 22 patients (64% women; mean age 71 years). Most visits (91%) occurred at patients' homes.
Students were satisfied with the curriculum (Table 2). Both the medical and the pharmacy students perceived the 2 most valuable components to be the interdisciplinary collaboration on patient care and the postdischarge visit, followed by the debriefing session. The least useful were the initial workshop on interdisciplinary roles and the write‐up to the PCP. Ninety‐one percent of students agreed that they learned skills valuable for future patient care (medical students 4.4, SD 0.61; pharmacy students 4.1, SD 0.62; Table 3). Most students agreed that the program enhanced their learning about interdisciplinary care (4.3, SD 0.72), discharge planning (4.4, SD 0.70), and humanism (4.4, SD 0.63). Ninety‐three percent agreed that this curriculum was valuable to their education.
| Component | Mean score* (SD) | Rated very good or excellent (%) |
|---|---|---|
| ||
| Joint patient care with medical/pharmacy student | 4.5 (1.04) | 94% |
| Postdischarge visit | 4.3 (0.68) | 91% |
| Debriefing session | 3.9 (1.04) | 75% |
| Team presentation after patient visit | 3.7 (1.32) | 63% |
| Case‐based workshop | 3.6 (1.18) | 54% |
| Write‐up on experience | 3.4 (0.81) | 48% |
| Overall program | 4.1 (1.14) | 86% |
| Medical students (n = 35) | Pharmacy students (n = 18) | All students (n = 53) | ||||
|---|---|---|---|---|---|---|
| Mean score* (SD) | Agree/strongly agree (%) | Mean score (SD) | Agree/strongly agree (%) | Mean score (SD) | Agree/strongly agree (%) | |
| ||||||
| I have learned skills from this program that I plan to apply to future patient care experiences. | 4.4 (0.61) | 94% | 4.1 (0.62) | 84% | 4.3 (0.63) | 91% |
| This program added to my learning about an interdisciplinary approach to patient care beyond the other experiences of this clerkship. | 4.3 (0.74) | 91% | 4.2 (0.71) | 84% | 4.3 (0.72) | 89% |
| This program added to my learning about discharge planning and transitional care beyond the other experiences of this clerkship. | 4.4 (0.66) | 91% | 4.2 (0.79) | 89% | 4.4 (0.70) | 91% |
| This program added to my understanding of a patient as a whole person beyond the other experiences of this clerkship. | 4.3 (0.69) | 89% | 4.5 (0.51) | 100% | 4.4 (0.63) | 93% |
| This program was valuable to my medical education. | 4.3 (0.74) | 91% | 4.4 (0.60) | 95% | 4.4 (0.68) | 93% |
Open‐Ended Comments on Educational Value
Twenty‐nine medical students and 15 pharmacy students wrote responses to the open‐ended questions. Students identified the most valuable component of the curriculum as seeing patients at home in their social context (30 total comments). In the reflection write‐up, one student explained,
I was unaware of the types of living conditions many patients face, especially in the setting of chronic disease. In the future I will try to gain a more detailed understanding of my patients' social situations in order to help identify and anticipate problems in the management of their medical issues.
Thirteen students commented that working as an interdisciplinary team was a valuable experience. Eight students expressed appreciation at learning about transitional care and the components of discharge planning.
I was a little surprised during this home visit to find how much Ms. C had altered her medication regimen. She didn't like how she was feeling on the higher blood pressure medications, so she halved them. She doesn't really like taking pills, in general, so she stopped taking the aspirin, Senna, and Colace. I suppose something that might have made this discharge more successful would have been if we had really elicited her preferences regarding medications while she was in the hospital, such that we could have been more selective in what we prescribed and very clear with her with respect to what exactly we were hoping to accomplish with each.
During group debriefing, students reinforced the themes in their written comments and shared additional reflections. Students observed a shift in dynamics between patient and student provider; the patients appeared more comfortable in familiar settings. Students were also surprised that many of their patients did not have a clear understanding of medication regimens at home. In addition, they discussed the importance of communicating with patients' PCPs about the hospital course and follow‐up.
Also during the debriefing, students expressed the value of the postdischarge visit and interdisciplinary collaboration. Medical students appreciated seeing how the pharmacy students reviewed medications and taught patients how to use their medications. However, the students thought that preparation of paperwork prior to the visit and the write‐up seemed less valuable.
DISCUSSION
A discharge curriculum that included a postdischarge visit to a recently hospitalized patient improved the attitudes and self‐assessed skills of third‐year medical students and fourth‐year pharmacy students about interdisciplinary collaboration and transitions in care. It also deepened their appreciation of the impact of chronic illness on individual patients. To our knowledge, this is the first study to report an interdisciplinary curriculum with postdischarge home visits for students on their inpatient medicine clerkship.
Our curriculum was unique because its activities were linked to patients the students had cared for in the inpatient setting, a relationship that was key to students accepting the curriculum, as was the autonomy they had in selecting one of their patients for a visit. Although home visits are often part of medical school training, they generally occur in the preclinical years5, 19 or during third‐year primary care rotations, during which students are assigned patients at home or in outpatient facilities.17, 32 Home visits have been qualitatively reported to be a valuable aspect of geriatric, primary care, and other ambulatory‐based rotations of medical students.17, 19, 32 Postdischarge visits in graduate medical education have been shown to improve residents' awareness of and skills with transitions in care.28, 33, 34
Another novel aspect of this curriculum was the interdisciplinary collaboration in discharge planning and postdischarge visits. Although educators have implemented conferences on interdisciplinary education in preclinical medical education,3537 patient‐centered curricula in real‐time allow realistic interdisciplinary collaboration between medical and pharmacy students in their core clerkships. In our study, quantitative and qualitative data showed that the student partners valued each other's expertise in the context of a clinically relevant activitydischarge planning and a follow‐up home visit. Students reported confidence in their collaborative abilities after completing the curriculum, and comments supported a broadened understanding of other professionals' roles in patient care. Given that pharmacist involvement in discharge planning has been shown to improve patient outcomes,11, 24 our study supports the idea that medical educators should develop structured curricula on interdisciplinary training in core clerkships.
By evaluating the impact of hospitalization and chronic illness on their patients after discharge, our students developed an appreciation for safe transitions and opportunities to improve patients' health and level of function. We observed that students also appreciated the positive effect of the home environment on patient health and well‐being. From their postdischarge visit, students also became aware of the need for communication with primary care providers, particularly for patients with comorbidities. This type of transitional care experience may help to counter the negative attitudes toward chronic illness that students typically develop during clerkships.2, 9, 38, 39
Of note, although pharmacy students reported improvement in their attitudes and skills with transitional care, the trend toward significance was less than that for medical students. This difference was consistent with the broader rotation goals of each group. At the end of the curriculum, the pharmacy students expressed more comfort with medication review than did medical students, although the latter were better able to conduct transitional care including postdischarge visits and identification of barriers or facilitators to a safe discharge. Another interesting note is that pharmacy students came into the curriculum with a better understanding of the roles of physicians, whereas the medical students had a less clear idea of the pharmacist's role. A possible explanation is that pharmacy students are better trained in their preclinical years to work as a team with medical personnel. The pharmacy school curriculum places an emphasis on independent learning and interdisciplinary collaboration, which may lead to the greater comfort felt by the pharmacy students.
This study had several limitations. The absolute number of visits was small overall; however, nearly all student partners completed their visits. Although the response rate to the postcurriculum survey was high, the response rate to matched prepost surveys was lower. In addition, the survey questions were not validated. Further, although there was significant improvement in students' attitudes and self‐assessed skills after completion of the curriculum, we cannot be certain whether this improvement was a result of the curriculum or of other rotation experiences. We attempted to clarify this effect by asking if the curriculum added to their learning beyond other clerkship experiences, and students perceived that our curriculum was responsible for the positive effect. Also, the curriculum was used at 1 academic site and may not be generalizable to other hospitals, student populations, or team structures. The patients were selected by students, and thus the results may not be reproducible for every population; in some situations, students had to ask several patients until a patient consented to a postdischarge visit.
In implementing this interdisciplinary curriculum, we were challenged by the discordant schedules of the medical and pharmacy students. Initially, it was also difficult to overcome students' concerns about adding an additional expectation to an already busy rotation. The medical students, in particular, voiced concerns about having to leave the hospital during their inpatient rotation. However, this has become much less of an issue with time as the value of the postdischarge visit has become clear to students and team members, with the latter now aware of and supportive of the program.
This discharge curriculum represents a clinically relevant experience that addresses national educational mandates regarding interdisciplinary care and chronic illness across care settings. We are now expanding the curriculum from the original site to our other clerkship sites and are evaluating its impact on patient safety and clinical outcomes. Future research should focus on whether these interdisciplinary postdischarge patient visits lead to improved attitudes and skills during residency training or practice and whether, ultimately, they lead to improved patient outcomes.
Acknowledgements
The authors gratefully acknowledge Deborah Airo for editorial review and Kathleen Kerr for statistical support.
- ,,,,,.Medical students' experiences with and perceptions of chronic illness prior to medical school.Med Educ.1993;27:355–359.
- ,,,,,.Do clerkship experiences affect medical students' attitudes toward chronically ill patients?Acad Med.2001;76:815–820.
- ,,,,.More training needed in chronic care: a survey of US physicians.Acad Med.2004;79:541–548.
- .Chronic disease—the need for a new clinical education.JAMA.2004;292:1057–1059.
- ,,.Medical students as health coaches.Acad Med.2002;77:1164–1166.
- ,,.Persons with chronic conditions. Their prevalence and costs.JAMA.1996;276:1473–1479.
- ,,.To Err Is Human.Washington, DC:National Academy Press;2000.
- Institute of Medicine.Health Professions Education: A Bridge to Quality.Washington DC:National Academy Press;2000.
- ,.The loss of student idealism in the 3rd‐year clinical clerkships.Eval Health Prof.2001:24:61–71.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20:317–323.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- ,,,,.Does the addition of a pharmacist transition coordinator improve evidence‐based medication management and health outcomes in older adults moving from the hospital to a long‐term care facility? Results of a randomized, controlled trial.Am J Geriatr Pharmacother.2004;2:257–264.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,.Posthospital medication discrepancies: Prevalence and contributing factors.Arch Intern Med.2005;165:1842–1847.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170:345–349.
- ,,, et al.Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan.Arch Intern Med.1997;157:1026–1030.
- ,,,,,.Reflections of medical students on visiting chronically ill older patients in the home.J Am Geriatr Soc.2006;54:1778–1783.
- ,,.Home care.JAMA.2003;290:1203–1207.
- ,,,,,.Mi casa o su casa? Assessing function and values in the home.J Am Geriatr Soc.2005;53:336–342.
- ,.Interdisciplinary education, and teamwork: a long and winding road.Med Educ.2001;35:867–875.
- ,,.Interdisciplinary education: evaluation of a palliative care training intervention for pre‐professionals.Acad Med.2004;79:769–776.
- ,.Effective interdisciplinary training: Lessons from the University of North Carolina's Student Health Action Coalition.Acad Med.2006;81:749–759.
- ,,,.The impact of follow‐up telephone calls to patients after hospitalization.Am J Med.2001;111(9B):26S–30S.
- ,,,.Clinical pharmacists and inpatient medical care: A systematic review.Arch Intern Med.2006;166:955–964.
- ,,,.Developing an evidence base for interdisciplinary learning: a systematic review.J Adv Nurs.2001;35:228–237.
- ,,,,,.Factors associated with unplanned hospital readmission among patients 65 years of age and older in a Medicare managed care plan.Am J Med.1999;107:13–17.
- ,,.Risk factors for early unplanned hospital readmission in the elderly.J Gen Intern Med.1991;6:223–228.
- ,,, et al.Hospital to home: Improving internal medicine residents' understanding of the needs of older persons after a hospital stay.Acad Med.2003;78:793–797.
- . Care transitions program. University of Colorado at Denver, Health Sciences Center. Available at: http://www.caretransitions.org/index.asp. Accessed April 9,2007.
- ,,,,,.Validation of an instrument designed to assess medical student attitudes toward home care.J Am Geriatr Soc.2001;49:470–473.
- Patient Assessment of Chronic Illness Care (PACIC) from Improving Chronic Illness Care, a national program of the Robert Wood Johnson Foundation. Available at: http://improvingchroniccare.org/tools/pacic.htm. Accessed April 9,2007.
- ,,,,.The determinants of attitudinal change among medical students participating in home care training: A multi‐center study.Acad Med.2002;77:336–343.
- ,,,,.There's no place like home: Evaluating family medicine residents' training in home care.Home Health Care Serv Q.2002;21:1–17.
- ,,.Geriatric follow‐up by home visits after discharge from hospital: a randomized controlled trial.Age Ageing.1992;21:445–450.
- ,,.Multiprofessional learning: the attitudes of medical nursing, and pharmacy students to shared learning.Med Educ.2001;35:876–883.
- ,,.Can participation in a health affairs interdisciplinary case conference improve medical students' knowledge and attitudes?Acad Med.2006;81:257–261.
- ,,, et al.Bringing interdisciplinary and multicultural team building to health care education: the Downstate Team‐Building Initiative.Acad Med.2005;80:74–83.
- , and.“It's always continuing”: First year medical students' perspectives on chronic illness and the care of chronically ill patients.Acad Med.2005;80:183–188.
- ,,,,,.Training U.S. medical students to care for the chronically ill.Acad Med.2004;79:32–40.
The increasing burden of chronic illness has prompted concerns about the traditional education model that focuses on management of acute disease.13 Chronic illness has replaced acute disease as the major cause of disability and total national health care expenditures.46 Medical educators have called for improved chronic disease curricula,2, 3 and the Institute of Medicine has asserted that health professions, including medicine and pharmacy, must reexamine how students are educated to manage patients with complex illnesses.7, 8 Despite the rising prevalence of chronic illness, the positive attitudes of medical students toward providing care to such patients decline during training.2, 9 One theory is that the current model of core clerkship training excessively exposes students to highly complex, seriously ill hospitalized patients. Students may become disillusioned and overwhelmed by these encounters, particularly without the opportunity to see improvement or thriving in the outpatient setting.2
There are few curricula on how to transition chronically ill patients from an inpatient to an outpatient setting and the inherent safety risks of this transition. For these patients, the posthospital discharge period is particularly confusing because of the sudden change in health status and new medication regimens.1012 It is very likely that communication among providers and patients will be insufficient during the discharge process,11, 1315 yet physicians tend to overestimate patients' understanding of postdischarge treatment plans and thereby underanticipate problems.16 One intervention to address these concerns is a postdischarge visit. Home visits have been shown to improve students' understanding of continuity of care and of the impact of chronic illness on their patients' medical and psychosocial situations.1719
There is scant structured teaching of third‐year medical students about another critical aspect of transitional care: the role of different health care disciplines. Although research about the impact of undergraduate interdisciplinary education on patient outcomes is limited, training students in interdisciplinary collaboration may improve their ability to provide quality care.2022 Multiple disciplines are critical for a smooth transition of chronically ill patients from an inpatient to an outpatient setting. In particular, pharmacist involvement in a predischarge medication review, patient counseling, and telephone follow‐up has been associated with improved outcomes.11, 12, 23, 24 Early introduction of interdisciplinary team training can improve student attitudes about working within a team.25
To teach the importance of safe discharges and interdisciplinary collaboration in caring for chronically ill patients, we developed an inpatient medicine clerkship curriculum for medical and pharmacy students that included postdischarge visits to students' own team patients. The purpose of the study was to assess the impact of this didactic and experiential curriculum on students' attitudes and self‐assessed skills in the interdisciplinary care and transitional care of chronically ill patients. We hypothesized that the discharge curriculum would improve student attitudes and self‐assessed skills in these domains. Finally, we hypothesized that visiting a patient's home would highlight for students the potential challenges of care transitions for patients.
METHODS
Participants and Setting
Participants were third‐year medical students on an 8‐week internal medicine (IM) clerkship and fourth‐year pharmacy students on a 6‐week pharmacy practice clerkship at a tertiary‐care university‐based hospital between April 2005 and April 2006. The hospital is 1 of 3 IM clerkship sites for medical students and 1 of 9 for pharmacy students. This site was selected because it included both medical and pharmacy students on most inpatient teams.
Clerkship students were assigned to all 7 medical teams, each consisting of an attending physician, a senior IM resident (postgraduate year 2 or 3), 2 IM interns (postgraduate year 1), 1 or 2 medical students, and up to 1 pharmacy student. Hospitalists covered 52% of inpatient months, with the remainder staffed by faculty primary care physicians, specialists, or chief residents. Although only three‐quarters of the medical teams were randomly assigned a pharmacy student at any given point, each team had a pharmacy student for a portion of time that overlapped with the rotation of the medical students. Over the year, 810 medical students rotated on the service during each of 6 blocks, and 46 pharmacy students and 1 pharmacy practice resident rotated during each of 8 blocks. The pharmacy students rotated on a different schedule than the medical students, and thus the curriculum was scheduled around the medical students' clerkship.
The Institutional Review Board of the University of California at San Francisco approved the study.
Intervention (Curriculum Description)
We developed a 3‐part pilot interdisciplinary curriculum (Fig. 1). During the first 2 weeks of the IM clerkship, interdisciplinary faculty, including 3 pharmacists, 2 hospitalists, and occasionally a social worker and geriatric clinical nurse specialist, led a 1‐hour interactive workshop on transitional care. The 3 workshop topics were: roles that various disciplines such as social work and pharmacy play in discharge care; the challenges a patient faces around the time of discharge, using a typical case; and discussion of elements of a postdischarge visit.

Medical and pharmacy students were partnered based on clerkship team assignments in teams of up to 3 student partners (1 or 2 medical students and 1 pharmacy student). Partners were advised to select a consenting patient known to them from the ward team for 1 postdischarge visit. Suggested selection criteria were at least 1 chronic illness, 1 prior hospitalization, and older than age 65 because patients fitting these criteria are most at risk for readmission or adverse outcomes following discharge.15, 26, 27 The student partners scheduled a postdischarge visit by the end of the rotation to the patient's home, nursing home, or subacute care facility. Each patient and the patient's primary care provider (PCP) gave informed consent.
During the postdischarge visit, student partners assessed medication discrepancies, environmental safety, and clinical status using structured data collection protocols developed by the investigators after review of the literature.28, 29 After the visit, students reported back to the ward teams on the patient's status and wrote a visit summary letter to the patient's PCP. The letter described the patient's clinical status and home environment, any medication discrepancies, and follow‐up plans and included a reflection piece. Reflection questions included, How did the visit change your perspective of patient discharge? What were the most critical aspects of this or any discharge? How do you think this experience will affect your future practice? What was the best thing about this experience?
During the last 2 weeks of the rotation, all student participants met with faculty preceptors for an hour‐long group debriefing session on the postdischarge visits.
Survey Instrument and Procedure
Students were asked to complete a presurvey at the beginning of the first workshop and a postsurvey at the end of the second (debriefing) workshop. The surveys contained self‐assessment questions on attitudes and skills in 3 domains: interdisciplinary care, chronic illness management, and transitional care. Questions were developed and tested with IM faculty with experience in student education and with ineligible students on previous rotations, and questions were revised for clarity and comprehensiveness. Students had the option to write a 4‐digit identifier on the pre‐ and postsurveys to allow matched analysis.
The 10‐item presurvey contained 4 items on interdisciplinary care and 3 each on chronic care and follow‐up visits. We reviewed surveys in the literature regarding home care and chronic illness to inform the development of our survey.30, 31 Students rated each item on a 5‐point Likert scale, ranging from 1 (strongly disagree) to 5 (strongly agree). The 22‐item postsurvey included the same 10 items and additional Likert‐scaled questions on satisfaction with the curriculum. Two open‐ended questions solicited opinions about the value of the program and lessons learned for future patient encounters.
Statistical Analysis
We assessed the mean Likert score ( SD) for each presurvey and postsurvey question and compared means ( SD). We evaluated the differences between medical students and for pharmacy students in mean Likert score on the surveys using a dependent‐samples t test and set the level of significance at 0.05.
Change in scores between prepost survey variables were calculated overall and within student type (medicine vs. pharmacy). Because no intercorrelations and possible patterns indicating a structure were found, a factor analysis was not conducted.
Two investigators (C.L., H.N.) read all written responses to the open‐ended questions and independently generated a list of themes. The list was reconciled through discussion and was used to code all comments in order to determine the frequency of each theme. Discrepancies were discussed until consensus was reached.
RESULTS
Participants
Ninety‐seven percent of eligible students (37 of 39 medical students and 22 of 22 pharmacy students) completed the curriculum. Two medical students did not complete the home visit because their patients did not keep the appointment. The presurvey response was 100% for medical students and 91% for pharmacy students. The postsurvey response was 92% for medical students and 86% for pharmacy students; 58% of medical students and 59% of pharmacy students wrote in matching prepost survey identifiers for statistical analysis. Prepost survey responses showed an increase for both student groups in positive attitudes and self‐assessed skill in interdisciplinary collaboration, chronic illness management, and transitional care. Trends over time were highly significant for individual items on matched surveys (P < 0.05; Table 1a,b).
| Question | Medical students (matched respondents n = 23) | Pharmacy students (matched respondents n = 13) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Presurvey, mean (SD) | Postsurvey, mean (SD) | Mean difference | P value | Effect size | Presurvey, mean (SD) | Postsurvey, mean (SD) | Mean difference | P value | Effect size | |
| ||||||||||
| 1. I am able to state the various roles of the pharmacy students and/or pharmacists (or medical students and/or physicians) in taking care of hospitalized patients. | 2.83 (0.89) | 4.35 (0.57) | 1.52 | < .001* | 1.72 | 3.69 (0.63) | 4.15 (0.38) | 0.46 | .03* | 0.73 |
| 2. I am able to state the various roles of the case manager and/or social worker in taking care of hospitalized patients. | 2.83 (0.78) | 3.91 (0.42) | 1.09 | < .001* | 1.40 | 2.77 (0.83) | 3.54 (0.97) | 0.77 | .01* | 0.92 |
| 3. I am confident in my ability to work with a pharmacy student or pharmacist (or medical student and/or physician) in taking care of inpatients with chronic illness. | 3.22 (1.00) | 4.52 (0.51) | 1.30 | < .001* | 1.31 | 3.62 (0.87) | 4.23 (0.44) | 0.62 | .04* | 0.71 |
| 4. I am confident in my ability to work with a case manager and/or social worker in taking care of inpatients with chronic illness. | 2.96 (0.71) | 3.96 (0.56) | 1.00 | < .001* | 1.42 | 3.08 (0.95) | 3.38 (0.87) | 0.31 | .34 | 0.32 |
| 5. I am confident in my ability to involve patients in making a plan for their care. | 3.74 (0.62) | 4.26 (0.54) | 0.52 | < .001* | 0.84 | 3.23 (0.60) | 4.15 (0.55) | 0.92 | < .001* | 1.54 |
| 6. I am able to assist patients in solving problems they encounter in self‐management of their chronic illness. | 3.30 (0.70) | 3.91 (0.60) | 0.61 | < .001* | 0.87 | 3.75 (0.87) | 3.92 (0.49) | 0.17 | .50 | 0.20 |
| 7. I am confident in my ability to review patients' medications and side effects. | 3.00 (0.85) | 3.70 (0.76) | 0.70 | < .001* | 0.82 | 3.92 (0.76) | 4.46 (0.52) | 0.54 | .03* | 0.71 |
| 8. I am able to review the goals of a follow‐up visit with a patient. | 3.52 (0.95) | 4.43 (0.51) | 0.91 | < .001* | 0.96 | 3.08 (0.76) | 3.62 (0.77) | 0.54 | .05 | 0.71 |
| 9. I can identify factors that may facilitate or impede a patient's transition to an outpatient setting. | 3.48 (0.51) | 4.35 (0.49) | 0.87 | < .001* | 1.70 | 3.00 (0.82) | 3.85 (0.69) | 0.85 | .01* | 1.04 |
| 10. I can identify several topics for review at a follow‐up visit to confirm a safe transition to an outpatient setting. | 3.39 (0.94) | 4.52 (0.59) | 1.13 | < .001* | 1.20 | 3.23 (0.73) | 3.77 (0.73) | 0.54 | .11 | 0.74 |
Twenty‐two student partners of 1 or 2 medical students and 1 pharmacy student visited 22 patients (64% women; mean age 71 years). Most visits (91%) occurred at patients' homes.
Students were satisfied with the curriculum (Table 2). Both the medical and the pharmacy students perceived the 2 most valuable components to be the interdisciplinary collaboration on patient care and the postdischarge visit, followed by the debriefing session. The least useful were the initial workshop on interdisciplinary roles and the write‐up to the PCP. Ninety‐one percent of students agreed that they learned skills valuable for future patient care (medical students 4.4, SD 0.61; pharmacy students 4.1, SD 0.62; Table 3). Most students agreed that the program enhanced their learning about interdisciplinary care (4.3, SD 0.72), discharge planning (4.4, SD 0.70), and humanism (4.4, SD 0.63). Ninety‐three percent agreed that this curriculum was valuable to their education.
| Component | Mean score* (SD) | Rated very good or excellent (%) |
|---|---|---|
| ||
| Joint patient care with medical/pharmacy student | 4.5 (1.04) | 94% |
| Postdischarge visit | 4.3 (0.68) | 91% |
| Debriefing session | 3.9 (1.04) | 75% |
| Team presentation after patient visit | 3.7 (1.32) | 63% |
| Case‐based workshop | 3.6 (1.18) | 54% |
| Write‐up on experience | 3.4 (0.81) | 48% |
| Overall program | 4.1 (1.14) | 86% |
| Medical students (n = 35) | Pharmacy students (n = 18) | All students (n = 53) | ||||
|---|---|---|---|---|---|---|
| Mean score* (SD) | Agree/strongly agree (%) | Mean score (SD) | Agree/strongly agree (%) | Mean score (SD) | Agree/strongly agree (%) | |
| ||||||
| I have learned skills from this program that I plan to apply to future patient care experiences. | 4.4 (0.61) | 94% | 4.1 (0.62) | 84% | 4.3 (0.63) | 91% |
| This program added to my learning about an interdisciplinary approach to patient care beyond the other experiences of this clerkship. | 4.3 (0.74) | 91% | 4.2 (0.71) | 84% | 4.3 (0.72) | 89% |
| This program added to my learning about discharge planning and transitional care beyond the other experiences of this clerkship. | 4.4 (0.66) | 91% | 4.2 (0.79) | 89% | 4.4 (0.70) | 91% |
| This program added to my understanding of a patient as a whole person beyond the other experiences of this clerkship. | 4.3 (0.69) | 89% | 4.5 (0.51) | 100% | 4.4 (0.63) | 93% |
| This program was valuable to my medical education. | 4.3 (0.74) | 91% | 4.4 (0.60) | 95% | 4.4 (0.68) | 93% |
Open‐Ended Comments on Educational Value
Twenty‐nine medical students and 15 pharmacy students wrote responses to the open‐ended questions. Students identified the most valuable component of the curriculum as seeing patients at home in their social context (30 total comments). In the reflection write‐up, one student explained,
I was unaware of the types of living conditions many patients face, especially in the setting of chronic disease. In the future I will try to gain a more detailed understanding of my patients' social situations in order to help identify and anticipate problems in the management of their medical issues.
Thirteen students commented that working as an interdisciplinary team was a valuable experience. Eight students expressed appreciation at learning about transitional care and the components of discharge planning.
I was a little surprised during this home visit to find how much Ms. C had altered her medication regimen. She didn't like how she was feeling on the higher blood pressure medications, so she halved them. She doesn't really like taking pills, in general, so she stopped taking the aspirin, Senna, and Colace. I suppose something that might have made this discharge more successful would have been if we had really elicited her preferences regarding medications while she was in the hospital, such that we could have been more selective in what we prescribed and very clear with her with respect to what exactly we were hoping to accomplish with each.
During group debriefing, students reinforced the themes in their written comments and shared additional reflections. Students observed a shift in dynamics between patient and student provider; the patients appeared more comfortable in familiar settings. Students were also surprised that many of their patients did not have a clear understanding of medication regimens at home. In addition, they discussed the importance of communicating with patients' PCPs about the hospital course and follow‐up.
Also during the debriefing, students expressed the value of the postdischarge visit and interdisciplinary collaboration. Medical students appreciated seeing how the pharmacy students reviewed medications and taught patients how to use their medications. However, the students thought that preparation of paperwork prior to the visit and the write‐up seemed less valuable.
DISCUSSION
A discharge curriculum that included a postdischarge visit to a recently hospitalized patient improved the attitudes and self‐assessed skills of third‐year medical students and fourth‐year pharmacy students about interdisciplinary collaboration and transitions in care. It also deepened their appreciation of the impact of chronic illness on individual patients. To our knowledge, this is the first study to report an interdisciplinary curriculum with postdischarge home visits for students on their inpatient medicine clerkship.
Our curriculum was unique because its activities were linked to patients the students had cared for in the inpatient setting, a relationship that was key to students accepting the curriculum, as was the autonomy they had in selecting one of their patients for a visit. Although home visits are often part of medical school training, they generally occur in the preclinical years5, 19 or during third‐year primary care rotations, during which students are assigned patients at home or in outpatient facilities.17, 32 Home visits have been qualitatively reported to be a valuable aspect of geriatric, primary care, and other ambulatory‐based rotations of medical students.17, 19, 32 Postdischarge visits in graduate medical education have been shown to improve residents' awareness of and skills with transitions in care.28, 33, 34
Another novel aspect of this curriculum was the interdisciplinary collaboration in discharge planning and postdischarge visits. Although educators have implemented conferences on interdisciplinary education in preclinical medical education,3537 patient‐centered curricula in real‐time allow realistic interdisciplinary collaboration between medical and pharmacy students in their core clerkships. In our study, quantitative and qualitative data showed that the student partners valued each other's expertise in the context of a clinically relevant activitydischarge planning and a follow‐up home visit. Students reported confidence in their collaborative abilities after completing the curriculum, and comments supported a broadened understanding of other professionals' roles in patient care. Given that pharmacist involvement in discharge planning has been shown to improve patient outcomes,11, 24 our study supports the idea that medical educators should develop structured curricula on interdisciplinary training in core clerkships.
By evaluating the impact of hospitalization and chronic illness on their patients after discharge, our students developed an appreciation for safe transitions and opportunities to improve patients' health and level of function. We observed that students also appreciated the positive effect of the home environment on patient health and well‐being. From their postdischarge visit, students also became aware of the need for communication with primary care providers, particularly for patients with comorbidities. This type of transitional care experience may help to counter the negative attitudes toward chronic illness that students typically develop during clerkships.2, 9, 38, 39
Of note, although pharmacy students reported improvement in their attitudes and skills with transitional care, the trend toward significance was less than that for medical students. This difference was consistent with the broader rotation goals of each group. At the end of the curriculum, the pharmacy students expressed more comfort with medication review than did medical students, although the latter were better able to conduct transitional care including postdischarge visits and identification of barriers or facilitators to a safe discharge. Another interesting note is that pharmacy students came into the curriculum with a better understanding of the roles of physicians, whereas the medical students had a less clear idea of the pharmacist's role. A possible explanation is that pharmacy students are better trained in their preclinical years to work as a team with medical personnel. The pharmacy school curriculum places an emphasis on independent learning and interdisciplinary collaboration, which may lead to the greater comfort felt by the pharmacy students.
This study had several limitations. The absolute number of visits was small overall; however, nearly all student partners completed their visits. Although the response rate to the postcurriculum survey was high, the response rate to matched prepost surveys was lower. In addition, the survey questions were not validated. Further, although there was significant improvement in students' attitudes and self‐assessed skills after completion of the curriculum, we cannot be certain whether this improvement was a result of the curriculum or of other rotation experiences. We attempted to clarify this effect by asking if the curriculum added to their learning beyond other clerkship experiences, and students perceived that our curriculum was responsible for the positive effect. Also, the curriculum was used at 1 academic site and may not be generalizable to other hospitals, student populations, or team structures. The patients were selected by students, and thus the results may not be reproducible for every population; in some situations, students had to ask several patients until a patient consented to a postdischarge visit.
In implementing this interdisciplinary curriculum, we were challenged by the discordant schedules of the medical and pharmacy students. Initially, it was also difficult to overcome students' concerns about adding an additional expectation to an already busy rotation. The medical students, in particular, voiced concerns about having to leave the hospital during their inpatient rotation. However, this has become much less of an issue with time as the value of the postdischarge visit has become clear to students and team members, with the latter now aware of and supportive of the program.
This discharge curriculum represents a clinically relevant experience that addresses national educational mandates regarding interdisciplinary care and chronic illness across care settings. We are now expanding the curriculum from the original site to our other clerkship sites and are evaluating its impact on patient safety and clinical outcomes. Future research should focus on whether these interdisciplinary postdischarge patient visits lead to improved attitudes and skills during residency training or practice and whether, ultimately, they lead to improved patient outcomes.
Acknowledgements
The authors gratefully acknowledge Deborah Airo for editorial review and Kathleen Kerr for statistical support.
The increasing burden of chronic illness has prompted concerns about the traditional education model that focuses on management of acute disease.13 Chronic illness has replaced acute disease as the major cause of disability and total national health care expenditures.46 Medical educators have called for improved chronic disease curricula,2, 3 and the Institute of Medicine has asserted that health professions, including medicine and pharmacy, must reexamine how students are educated to manage patients with complex illnesses.7, 8 Despite the rising prevalence of chronic illness, the positive attitudes of medical students toward providing care to such patients decline during training.2, 9 One theory is that the current model of core clerkship training excessively exposes students to highly complex, seriously ill hospitalized patients. Students may become disillusioned and overwhelmed by these encounters, particularly without the opportunity to see improvement or thriving in the outpatient setting.2
There are few curricula on how to transition chronically ill patients from an inpatient to an outpatient setting and the inherent safety risks of this transition. For these patients, the posthospital discharge period is particularly confusing because of the sudden change in health status and new medication regimens.1012 It is very likely that communication among providers and patients will be insufficient during the discharge process,11, 1315 yet physicians tend to overestimate patients' understanding of postdischarge treatment plans and thereby underanticipate problems.16 One intervention to address these concerns is a postdischarge visit. Home visits have been shown to improve students' understanding of continuity of care and of the impact of chronic illness on their patients' medical and psychosocial situations.1719
There is scant structured teaching of third‐year medical students about another critical aspect of transitional care: the role of different health care disciplines. Although research about the impact of undergraduate interdisciplinary education on patient outcomes is limited, training students in interdisciplinary collaboration may improve their ability to provide quality care.2022 Multiple disciplines are critical for a smooth transition of chronically ill patients from an inpatient to an outpatient setting. In particular, pharmacist involvement in a predischarge medication review, patient counseling, and telephone follow‐up has been associated with improved outcomes.11, 12, 23, 24 Early introduction of interdisciplinary team training can improve student attitudes about working within a team.25
To teach the importance of safe discharges and interdisciplinary collaboration in caring for chronically ill patients, we developed an inpatient medicine clerkship curriculum for medical and pharmacy students that included postdischarge visits to students' own team patients. The purpose of the study was to assess the impact of this didactic and experiential curriculum on students' attitudes and self‐assessed skills in the interdisciplinary care and transitional care of chronically ill patients. We hypothesized that the discharge curriculum would improve student attitudes and self‐assessed skills in these domains. Finally, we hypothesized that visiting a patient's home would highlight for students the potential challenges of care transitions for patients.
METHODS
Participants and Setting
Participants were third‐year medical students on an 8‐week internal medicine (IM) clerkship and fourth‐year pharmacy students on a 6‐week pharmacy practice clerkship at a tertiary‐care university‐based hospital between April 2005 and April 2006. The hospital is 1 of 3 IM clerkship sites for medical students and 1 of 9 for pharmacy students. This site was selected because it included both medical and pharmacy students on most inpatient teams.
Clerkship students were assigned to all 7 medical teams, each consisting of an attending physician, a senior IM resident (postgraduate year 2 or 3), 2 IM interns (postgraduate year 1), 1 or 2 medical students, and up to 1 pharmacy student. Hospitalists covered 52% of inpatient months, with the remainder staffed by faculty primary care physicians, specialists, or chief residents. Although only three‐quarters of the medical teams were randomly assigned a pharmacy student at any given point, each team had a pharmacy student for a portion of time that overlapped with the rotation of the medical students. Over the year, 810 medical students rotated on the service during each of 6 blocks, and 46 pharmacy students and 1 pharmacy practice resident rotated during each of 8 blocks. The pharmacy students rotated on a different schedule than the medical students, and thus the curriculum was scheduled around the medical students' clerkship.
The Institutional Review Board of the University of California at San Francisco approved the study.
Intervention (Curriculum Description)
We developed a 3‐part pilot interdisciplinary curriculum (Fig. 1). During the first 2 weeks of the IM clerkship, interdisciplinary faculty, including 3 pharmacists, 2 hospitalists, and occasionally a social worker and geriatric clinical nurse specialist, led a 1‐hour interactive workshop on transitional care. The 3 workshop topics were: roles that various disciplines such as social work and pharmacy play in discharge care; the challenges a patient faces around the time of discharge, using a typical case; and discussion of elements of a postdischarge visit.

Medical and pharmacy students were partnered based on clerkship team assignments in teams of up to 3 student partners (1 or 2 medical students and 1 pharmacy student). Partners were advised to select a consenting patient known to them from the ward team for 1 postdischarge visit. Suggested selection criteria were at least 1 chronic illness, 1 prior hospitalization, and older than age 65 because patients fitting these criteria are most at risk for readmission or adverse outcomes following discharge.15, 26, 27 The student partners scheduled a postdischarge visit by the end of the rotation to the patient's home, nursing home, or subacute care facility. Each patient and the patient's primary care provider (PCP) gave informed consent.
During the postdischarge visit, student partners assessed medication discrepancies, environmental safety, and clinical status using structured data collection protocols developed by the investigators after review of the literature.28, 29 After the visit, students reported back to the ward teams on the patient's status and wrote a visit summary letter to the patient's PCP. The letter described the patient's clinical status and home environment, any medication discrepancies, and follow‐up plans and included a reflection piece. Reflection questions included, How did the visit change your perspective of patient discharge? What were the most critical aspects of this or any discharge? How do you think this experience will affect your future practice? What was the best thing about this experience?
During the last 2 weeks of the rotation, all student participants met with faculty preceptors for an hour‐long group debriefing session on the postdischarge visits.
Survey Instrument and Procedure
Students were asked to complete a presurvey at the beginning of the first workshop and a postsurvey at the end of the second (debriefing) workshop. The surveys contained self‐assessment questions on attitudes and skills in 3 domains: interdisciplinary care, chronic illness management, and transitional care. Questions were developed and tested with IM faculty with experience in student education and with ineligible students on previous rotations, and questions were revised for clarity and comprehensiveness. Students had the option to write a 4‐digit identifier on the pre‐ and postsurveys to allow matched analysis.
The 10‐item presurvey contained 4 items on interdisciplinary care and 3 each on chronic care and follow‐up visits. We reviewed surveys in the literature regarding home care and chronic illness to inform the development of our survey.30, 31 Students rated each item on a 5‐point Likert scale, ranging from 1 (strongly disagree) to 5 (strongly agree). The 22‐item postsurvey included the same 10 items and additional Likert‐scaled questions on satisfaction with the curriculum. Two open‐ended questions solicited opinions about the value of the program and lessons learned for future patient encounters.
Statistical Analysis
We assessed the mean Likert score ( SD) for each presurvey and postsurvey question and compared means ( SD). We evaluated the differences between medical students and for pharmacy students in mean Likert score on the surveys using a dependent‐samples t test and set the level of significance at 0.05.
Change in scores between prepost survey variables were calculated overall and within student type (medicine vs. pharmacy). Because no intercorrelations and possible patterns indicating a structure were found, a factor analysis was not conducted.
Two investigators (C.L., H.N.) read all written responses to the open‐ended questions and independently generated a list of themes. The list was reconciled through discussion and was used to code all comments in order to determine the frequency of each theme. Discrepancies were discussed until consensus was reached.
RESULTS
Participants
Ninety‐seven percent of eligible students (37 of 39 medical students and 22 of 22 pharmacy students) completed the curriculum. Two medical students did not complete the home visit because their patients did not keep the appointment. The presurvey response was 100% for medical students and 91% for pharmacy students. The postsurvey response was 92% for medical students and 86% for pharmacy students; 58% of medical students and 59% of pharmacy students wrote in matching prepost survey identifiers for statistical analysis. Prepost survey responses showed an increase for both student groups in positive attitudes and self‐assessed skill in interdisciplinary collaboration, chronic illness management, and transitional care. Trends over time were highly significant for individual items on matched surveys (P < 0.05; Table 1a,b).
| Question | Medical students (matched respondents n = 23) | Pharmacy students (matched respondents n = 13) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Presurvey, mean (SD) | Postsurvey, mean (SD) | Mean difference | P value | Effect size | Presurvey, mean (SD) | Postsurvey, mean (SD) | Mean difference | P value | Effect size | |
| ||||||||||
| 1. I am able to state the various roles of the pharmacy students and/or pharmacists (or medical students and/or physicians) in taking care of hospitalized patients. | 2.83 (0.89) | 4.35 (0.57) | 1.52 | < .001* | 1.72 | 3.69 (0.63) | 4.15 (0.38) | 0.46 | .03* | 0.73 |
| 2. I am able to state the various roles of the case manager and/or social worker in taking care of hospitalized patients. | 2.83 (0.78) | 3.91 (0.42) | 1.09 | < .001* | 1.40 | 2.77 (0.83) | 3.54 (0.97) | 0.77 | .01* | 0.92 |
| 3. I am confident in my ability to work with a pharmacy student or pharmacist (or medical student and/or physician) in taking care of inpatients with chronic illness. | 3.22 (1.00) | 4.52 (0.51) | 1.30 | < .001* | 1.31 | 3.62 (0.87) | 4.23 (0.44) | 0.62 | .04* | 0.71 |
| 4. I am confident in my ability to work with a case manager and/or social worker in taking care of inpatients with chronic illness. | 2.96 (0.71) | 3.96 (0.56) | 1.00 | < .001* | 1.42 | 3.08 (0.95) | 3.38 (0.87) | 0.31 | .34 | 0.32 |
| 5. I am confident in my ability to involve patients in making a plan for their care. | 3.74 (0.62) | 4.26 (0.54) | 0.52 | < .001* | 0.84 | 3.23 (0.60) | 4.15 (0.55) | 0.92 | < .001* | 1.54 |
| 6. I am able to assist patients in solving problems they encounter in self‐management of their chronic illness. | 3.30 (0.70) | 3.91 (0.60) | 0.61 | < .001* | 0.87 | 3.75 (0.87) | 3.92 (0.49) | 0.17 | .50 | 0.20 |
| 7. I am confident in my ability to review patients' medications and side effects. | 3.00 (0.85) | 3.70 (0.76) | 0.70 | < .001* | 0.82 | 3.92 (0.76) | 4.46 (0.52) | 0.54 | .03* | 0.71 |
| 8. I am able to review the goals of a follow‐up visit with a patient. | 3.52 (0.95) | 4.43 (0.51) | 0.91 | < .001* | 0.96 | 3.08 (0.76) | 3.62 (0.77) | 0.54 | .05 | 0.71 |
| 9. I can identify factors that may facilitate or impede a patient's transition to an outpatient setting. | 3.48 (0.51) | 4.35 (0.49) | 0.87 | < .001* | 1.70 | 3.00 (0.82) | 3.85 (0.69) | 0.85 | .01* | 1.04 |
| 10. I can identify several topics for review at a follow‐up visit to confirm a safe transition to an outpatient setting. | 3.39 (0.94) | 4.52 (0.59) | 1.13 | < .001* | 1.20 | 3.23 (0.73) | 3.77 (0.73) | 0.54 | .11 | 0.74 |
Twenty‐two student partners of 1 or 2 medical students and 1 pharmacy student visited 22 patients (64% women; mean age 71 years). Most visits (91%) occurred at patients' homes.
Students were satisfied with the curriculum (Table 2). Both the medical and the pharmacy students perceived the 2 most valuable components to be the interdisciplinary collaboration on patient care and the postdischarge visit, followed by the debriefing session. The least useful were the initial workshop on interdisciplinary roles and the write‐up to the PCP. Ninety‐one percent of students agreed that they learned skills valuable for future patient care (medical students 4.4, SD 0.61; pharmacy students 4.1, SD 0.62; Table 3). Most students agreed that the program enhanced their learning about interdisciplinary care (4.3, SD 0.72), discharge planning (4.4, SD 0.70), and humanism (4.4, SD 0.63). Ninety‐three percent agreed that this curriculum was valuable to their education.
| Component | Mean score* (SD) | Rated very good or excellent (%) |
|---|---|---|
| ||
| Joint patient care with medical/pharmacy student | 4.5 (1.04) | 94% |
| Postdischarge visit | 4.3 (0.68) | 91% |
| Debriefing session | 3.9 (1.04) | 75% |
| Team presentation after patient visit | 3.7 (1.32) | 63% |
| Case‐based workshop | 3.6 (1.18) | 54% |
| Write‐up on experience | 3.4 (0.81) | 48% |
| Overall program | 4.1 (1.14) | 86% |
| Medical students (n = 35) | Pharmacy students (n = 18) | All students (n = 53) | ||||
|---|---|---|---|---|---|---|
| Mean score* (SD) | Agree/strongly agree (%) | Mean score (SD) | Agree/strongly agree (%) | Mean score (SD) | Agree/strongly agree (%) | |
| ||||||
| I have learned skills from this program that I plan to apply to future patient care experiences. | 4.4 (0.61) | 94% | 4.1 (0.62) | 84% | 4.3 (0.63) | 91% |
| This program added to my learning about an interdisciplinary approach to patient care beyond the other experiences of this clerkship. | 4.3 (0.74) | 91% | 4.2 (0.71) | 84% | 4.3 (0.72) | 89% |
| This program added to my learning about discharge planning and transitional care beyond the other experiences of this clerkship. | 4.4 (0.66) | 91% | 4.2 (0.79) | 89% | 4.4 (0.70) | 91% |
| This program added to my understanding of a patient as a whole person beyond the other experiences of this clerkship. | 4.3 (0.69) | 89% | 4.5 (0.51) | 100% | 4.4 (0.63) | 93% |
| This program was valuable to my medical education. | 4.3 (0.74) | 91% | 4.4 (0.60) | 95% | 4.4 (0.68) | 93% |
Open‐Ended Comments on Educational Value
Twenty‐nine medical students and 15 pharmacy students wrote responses to the open‐ended questions. Students identified the most valuable component of the curriculum as seeing patients at home in their social context (30 total comments). In the reflection write‐up, one student explained,
I was unaware of the types of living conditions many patients face, especially in the setting of chronic disease. In the future I will try to gain a more detailed understanding of my patients' social situations in order to help identify and anticipate problems in the management of their medical issues.
Thirteen students commented that working as an interdisciplinary team was a valuable experience. Eight students expressed appreciation at learning about transitional care and the components of discharge planning.
I was a little surprised during this home visit to find how much Ms. C had altered her medication regimen. She didn't like how she was feeling on the higher blood pressure medications, so she halved them. She doesn't really like taking pills, in general, so she stopped taking the aspirin, Senna, and Colace. I suppose something that might have made this discharge more successful would have been if we had really elicited her preferences regarding medications while she was in the hospital, such that we could have been more selective in what we prescribed and very clear with her with respect to what exactly we were hoping to accomplish with each.
During group debriefing, students reinforced the themes in their written comments and shared additional reflections. Students observed a shift in dynamics between patient and student provider; the patients appeared more comfortable in familiar settings. Students were also surprised that many of their patients did not have a clear understanding of medication regimens at home. In addition, they discussed the importance of communicating with patients' PCPs about the hospital course and follow‐up.
Also during the debriefing, students expressed the value of the postdischarge visit and interdisciplinary collaboration. Medical students appreciated seeing how the pharmacy students reviewed medications and taught patients how to use their medications. However, the students thought that preparation of paperwork prior to the visit and the write‐up seemed less valuable.
DISCUSSION
A discharge curriculum that included a postdischarge visit to a recently hospitalized patient improved the attitudes and self‐assessed skills of third‐year medical students and fourth‐year pharmacy students about interdisciplinary collaboration and transitions in care. It also deepened their appreciation of the impact of chronic illness on individual patients. To our knowledge, this is the first study to report an interdisciplinary curriculum with postdischarge home visits for students on their inpatient medicine clerkship.
Our curriculum was unique because its activities were linked to patients the students had cared for in the inpatient setting, a relationship that was key to students accepting the curriculum, as was the autonomy they had in selecting one of their patients for a visit. Although home visits are often part of medical school training, they generally occur in the preclinical years5, 19 or during third‐year primary care rotations, during which students are assigned patients at home or in outpatient facilities.17, 32 Home visits have been qualitatively reported to be a valuable aspect of geriatric, primary care, and other ambulatory‐based rotations of medical students.17, 19, 32 Postdischarge visits in graduate medical education have been shown to improve residents' awareness of and skills with transitions in care.28, 33, 34
Another novel aspect of this curriculum was the interdisciplinary collaboration in discharge planning and postdischarge visits. Although educators have implemented conferences on interdisciplinary education in preclinical medical education,3537 patient‐centered curricula in real‐time allow realistic interdisciplinary collaboration between medical and pharmacy students in their core clerkships. In our study, quantitative and qualitative data showed that the student partners valued each other's expertise in the context of a clinically relevant activitydischarge planning and a follow‐up home visit. Students reported confidence in their collaborative abilities after completing the curriculum, and comments supported a broadened understanding of other professionals' roles in patient care. Given that pharmacist involvement in discharge planning has been shown to improve patient outcomes,11, 24 our study supports the idea that medical educators should develop structured curricula on interdisciplinary training in core clerkships.
By evaluating the impact of hospitalization and chronic illness on their patients after discharge, our students developed an appreciation for safe transitions and opportunities to improve patients' health and level of function. We observed that students also appreciated the positive effect of the home environment on patient health and well‐being. From their postdischarge visit, students also became aware of the need for communication with primary care providers, particularly for patients with comorbidities. This type of transitional care experience may help to counter the negative attitudes toward chronic illness that students typically develop during clerkships.2, 9, 38, 39
Of note, although pharmacy students reported improvement in their attitudes and skills with transitional care, the trend toward significance was less than that for medical students. This difference was consistent with the broader rotation goals of each group. At the end of the curriculum, the pharmacy students expressed more comfort with medication review than did medical students, although the latter were better able to conduct transitional care including postdischarge visits and identification of barriers or facilitators to a safe discharge. Another interesting note is that pharmacy students came into the curriculum with a better understanding of the roles of physicians, whereas the medical students had a less clear idea of the pharmacist's role. A possible explanation is that pharmacy students are better trained in their preclinical years to work as a team with medical personnel. The pharmacy school curriculum places an emphasis on independent learning and interdisciplinary collaboration, which may lead to the greater comfort felt by the pharmacy students.
This study had several limitations. The absolute number of visits was small overall; however, nearly all student partners completed their visits. Although the response rate to the postcurriculum survey was high, the response rate to matched prepost surveys was lower. In addition, the survey questions were not validated. Further, although there was significant improvement in students' attitudes and self‐assessed skills after completion of the curriculum, we cannot be certain whether this improvement was a result of the curriculum or of other rotation experiences. We attempted to clarify this effect by asking if the curriculum added to their learning beyond other clerkship experiences, and students perceived that our curriculum was responsible for the positive effect. Also, the curriculum was used at 1 academic site and may not be generalizable to other hospitals, student populations, or team structures. The patients were selected by students, and thus the results may not be reproducible for every population; in some situations, students had to ask several patients until a patient consented to a postdischarge visit.
In implementing this interdisciplinary curriculum, we were challenged by the discordant schedules of the medical and pharmacy students. Initially, it was also difficult to overcome students' concerns about adding an additional expectation to an already busy rotation. The medical students, in particular, voiced concerns about having to leave the hospital during their inpatient rotation. However, this has become much less of an issue with time as the value of the postdischarge visit has become clear to students and team members, with the latter now aware of and supportive of the program.
This discharge curriculum represents a clinically relevant experience that addresses national educational mandates regarding interdisciplinary care and chronic illness across care settings. We are now expanding the curriculum from the original site to our other clerkship sites and are evaluating its impact on patient safety and clinical outcomes. Future research should focus on whether these interdisciplinary postdischarge patient visits lead to improved attitudes and skills during residency training or practice and whether, ultimately, they lead to improved patient outcomes.
Acknowledgements
The authors gratefully acknowledge Deborah Airo for editorial review and Kathleen Kerr for statistical support.
- ,,,,,.Medical students' experiences with and perceptions of chronic illness prior to medical school.Med Educ.1993;27:355–359.
- ,,,,,.Do clerkship experiences affect medical students' attitudes toward chronically ill patients?Acad Med.2001;76:815–820.
- ,,,,.More training needed in chronic care: a survey of US physicians.Acad Med.2004;79:541–548.
- .Chronic disease—the need for a new clinical education.JAMA.2004;292:1057–1059.
- ,,.Medical students as health coaches.Acad Med.2002;77:1164–1166.
- ,,.Persons with chronic conditions. Their prevalence and costs.JAMA.1996;276:1473–1479.
- ,,.To Err Is Human.Washington, DC:National Academy Press;2000.
- Institute of Medicine.Health Professions Education: A Bridge to Quality.Washington DC:National Academy Press;2000.
- ,.The loss of student idealism in the 3rd‐year clinical clerkships.Eval Health Prof.2001:24:61–71.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20:317–323.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- ,,,,.Does the addition of a pharmacist transition coordinator improve evidence‐based medication management and health outcomes in older adults moving from the hospital to a long‐term care facility? Results of a randomized, controlled trial.Am J Geriatr Pharmacother.2004;2:257–264.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,.Posthospital medication discrepancies: Prevalence and contributing factors.Arch Intern Med.2005;165:1842–1847.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170:345–349.
- ,,, et al.Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan.Arch Intern Med.1997;157:1026–1030.
- ,,,,,.Reflections of medical students on visiting chronically ill older patients in the home.J Am Geriatr Soc.2006;54:1778–1783.
- ,,.Home care.JAMA.2003;290:1203–1207.
- ,,,,,.Mi casa o su casa? Assessing function and values in the home.J Am Geriatr Soc.2005;53:336–342.
- ,.Interdisciplinary education, and teamwork: a long and winding road.Med Educ.2001;35:867–875.
- ,,.Interdisciplinary education: evaluation of a palliative care training intervention for pre‐professionals.Acad Med.2004;79:769–776.
- ,.Effective interdisciplinary training: Lessons from the University of North Carolina's Student Health Action Coalition.Acad Med.2006;81:749–759.
- ,,,.The impact of follow‐up telephone calls to patients after hospitalization.Am J Med.2001;111(9B):26S–30S.
- ,,,.Clinical pharmacists and inpatient medical care: A systematic review.Arch Intern Med.2006;166:955–964.
- ,,,.Developing an evidence base for interdisciplinary learning: a systematic review.J Adv Nurs.2001;35:228–237.
- ,,,,,.Factors associated with unplanned hospital readmission among patients 65 years of age and older in a Medicare managed care plan.Am J Med.1999;107:13–17.
- ,,.Risk factors for early unplanned hospital readmission in the elderly.J Gen Intern Med.1991;6:223–228.
- ,,, et al.Hospital to home: Improving internal medicine residents' understanding of the needs of older persons after a hospital stay.Acad Med.2003;78:793–797.
- . Care transitions program. University of Colorado at Denver, Health Sciences Center. Available at: http://www.caretransitions.org/index.asp. Accessed April 9,2007.
- ,,,,,.Validation of an instrument designed to assess medical student attitudes toward home care.J Am Geriatr Soc.2001;49:470–473.
- Patient Assessment of Chronic Illness Care (PACIC) from Improving Chronic Illness Care, a national program of the Robert Wood Johnson Foundation. Available at: http://improvingchroniccare.org/tools/pacic.htm. Accessed April 9,2007.
- ,,,,.The determinants of attitudinal change among medical students participating in home care training: A multi‐center study.Acad Med.2002;77:336–343.
- ,,,,.There's no place like home: Evaluating family medicine residents' training in home care.Home Health Care Serv Q.2002;21:1–17.
- ,,.Geriatric follow‐up by home visits after discharge from hospital: a randomized controlled trial.Age Ageing.1992;21:445–450.
- ,,.Multiprofessional learning: the attitudes of medical nursing, and pharmacy students to shared learning.Med Educ.2001;35:876–883.
- ,,.Can participation in a health affairs interdisciplinary case conference improve medical students' knowledge and attitudes?Acad Med.2006;81:257–261.
- ,,, et al.Bringing interdisciplinary and multicultural team building to health care education: the Downstate Team‐Building Initiative.Acad Med.2005;80:74–83.
- , and.“It's always continuing”: First year medical students' perspectives on chronic illness and the care of chronically ill patients.Acad Med.2005;80:183–188.
- ,,,,,.Training U.S. medical students to care for the chronically ill.Acad Med.2004;79:32–40.
- ,,,,,.Medical students' experiences with and perceptions of chronic illness prior to medical school.Med Educ.1993;27:355–359.
- ,,,,,.Do clerkship experiences affect medical students' attitudes toward chronically ill patients?Acad Med.2001;76:815–820.
- ,,,,.More training needed in chronic care: a survey of US physicians.Acad Med.2004;79:541–548.
- .Chronic disease—the need for a new clinical education.JAMA.2004;292:1057–1059.
- ,,.Medical students as health coaches.Acad Med.2002;77:1164–1166.
- ,,.Persons with chronic conditions. Their prevalence and costs.JAMA.1996;276:1473–1479.
- ,,.To Err Is Human.Washington, DC:National Academy Press;2000.
- Institute of Medicine.Health Professions Education: A Bridge to Quality.Washington DC:National Academy Press;2000.
- ,.The loss of student idealism in the 3rd‐year clinical clerkships.Eval Health Prof.2001:24:61–71.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20:317–323.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- ,,,,.Does the addition of a pharmacist transition coordinator improve evidence‐based medication management and health outcomes in older adults moving from the hospital to a long‐term care facility? Results of a randomized, controlled trial.Am J Geriatr Pharmacother.2004;2:257–264.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,.Posthospital medication discrepancies: Prevalence and contributing factors.Arch Intern Med.2005;165:1842–1847.
- ,,, et al.Adverse events among medical patients after discharge from hospital.CMAJ.2004;170:345–349.
- ,,, et al.Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan.Arch Intern Med.1997;157:1026–1030.
- ,,,,,.Reflections of medical students on visiting chronically ill older patients in the home.J Am Geriatr Soc.2006;54:1778–1783.
- ,,.Home care.JAMA.2003;290:1203–1207.
- ,,,,,.Mi casa o su casa? Assessing function and values in the home.J Am Geriatr Soc.2005;53:336–342.
- ,.Interdisciplinary education, and teamwork: a long and winding road.Med Educ.2001;35:867–875.
- ,,.Interdisciplinary education: evaluation of a palliative care training intervention for pre‐professionals.Acad Med.2004;79:769–776.
- ,.Effective interdisciplinary training: Lessons from the University of North Carolina's Student Health Action Coalition.Acad Med.2006;81:749–759.
- ,,,.The impact of follow‐up telephone calls to patients after hospitalization.Am J Med.2001;111(9B):26S–30S.
- ,,,.Clinical pharmacists and inpatient medical care: A systematic review.Arch Intern Med.2006;166:955–964.
- ,,,.Developing an evidence base for interdisciplinary learning: a systematic review.J Adv Nurs.2001;35:228–237.
- ,,,,,.Factors associated with unplanned hospital readmission among patients 65 years of age and older in a Medicare managed care plan.Am J Med.1999;107:13–17.
- ,,.Risk factors for early unplanned hospital readmission in the elderly.J Gen Intern Med.1991;6:223–228.
- ,,, et al.Hospital to home: Improving internal medicine residents' understanding of the needs of older persons after a hospital stay.Acad Med.2003;78:793–797.
- . Care transitions program. University of Colorado at Denver, Health Sciences Center. Available at: http://www.caretransitions.org/index.asp. Accessed April 9,2007.
- ,,,,,.Validation of an instrument designed to assess medical student attitudes toward home care.J Am Geriatr Soc.2001;49:470–473.
- Patient Assessment of Chronic Illness Care (PACIC) from Improving Chronic Illness Care, a national program of the Robert Wood Johnson Foundation. Available at: http://improvingchroniccare.org/tools/pacic.htm. Accessed April 9,2007.
- ,,,,.The determinants of attitudinal change among medical students participating in home care training: A multi‐center study.Acad Med.2002;77:336–343.
- ,,,,.There's no place like home: Evaluating family medicine residents' training in home care.Home Health Care Serv Q.2002;21:1–17.
- ,,.Geriatric follow‐up by home visits after discharge from hospital: a randomized controlled trial.Age Ageing.1992;21:445–450.
- ,,.Multiprofessional learning: the attitudes of medical nursing, and pharmacy students to shared learning.Med Educ.2001;35:876–883.
- ,,.Can participation in a health affairs interdisciplinary case conference improve medical students' knowledge and attitudes?Acad Med.2006;81:257–261.
- ,,, et al.Bringing interdisciplinary and multicultural team building to health care education: the Downstate Team‐Building Initiative.Acad Med.2005;80:74–83.
- , and.“It's always continuing”: First year medical students' perspectives on chronic illness and the care of chronically ill patients.Acad Med.2005;80:183–188.
- ,,,,,.Training U.S. medical students to care for the chronically ill.Acad Med.2004;79:32–40.
Copyright © 2008 Society of Hospital Medicine
Camplyobacter Empyema
A 72‐year‐old man had been suffering from low‐grade fever, minimally productive cough, and shortness of breath for 1 week when he experienced sudden, moderately severe right‐sided chest pain. His local primary care physician found no abnormalities on physical exam and laboratory testing. A chest x‐ray, however, did reveal a small right‐sided pleural effusion. The patient was empirically started on levofloxacin but noticed no improvement. Two weeks into his illness, he was referred to our institution for further management. By this time, he reported a rapid 10‐pound weight loss and a daily low‐grade fever. Chest examination revealed dullness to percussion along with decreased breath sounds in the right posterior lung fields. A complete blood count showed an elevated white count of 17,000/mL with 14,000 neutrophils. Hemoglobin was 13.5 g/dL. A repeat chest x‐ ray and then a CT scan showed a multiloculated pleural effusion in the right lower hemithorax. Ultrasound‐guided tap of this effusion showed cloudy fluid consistent with pus, with a protein of 4.8 g/dL and total nucleated cells of 6000/mL. A gram stain on this fluid was negative.
The patient had a history remarkable for severe underlying chronic obstructive pulmonary disease (COPD). His forced expiratory volume in 1 second (FEV1) was 21%, and his diffusing capacity of carbon monoxide (DLCO) was 27%. Therefore, decortication under general anesthesia was not an option. So the largest pus pocket was drained under CT guidance, and the patient was dismissed home on levofloxacin.
He returned for follow‐up after 3 weeks and reported daily low‐grade fever, night sweats, and an additional weight loss of 14 pounds. His white count had risen to 18,300/mL with a neutrophil count of 16,600. Hemoglobin had fallen to 11.9 g/dL. A repeat CT scan showed that although the previously drained fluid pocket had resolved, a moderate amount of fluid had reaccumulated in other pockets. Delayed anaerobic culture results from the hospitalization 3 weeks earlier were now available and, interestingly, showed 2+ growth of Campylobacter jejuni, broadly sensitive to all antibiotics including penicillin. Piperacillin/tazobactam was started intravenously, and CT‐guided drainage of the largest pus pocket was again performed.
We carefully reexamined the patient's CT scan, and there appeared to be a lesion in the right main‐stem bronchus. We decided to perform a bronchoscopy, which revealed a foreign body in the right main‐stem bronchus. The foreign body turned out to be a piece of chicken and a peanut. On specific questioning of the patient again, he admitted that at times he coughed after eating too quickly. Specifically, he remembered that a few days before falling sick he was at a village fair, where he had had chicken, and he thought he might have coughed after eating it. He denied any diarrheal illness in the recent past. We obtained a swallow study and upper gastrointestinal endoscopy, both of which were unremarkable.
He improved remarkably after removal of the foreign body and was sent home on amoxicillin‐clavulanic acid for 3 weeks.
DISCUSSION
Campylobacter is one of the most common zoonoses in the world.1 Commercially raised poultry is nearly always colonized with Campylobacter jejuni, and therefore, not surprisingly, 50% to 70% of C. jejuni infection in humans is caused by undercooked poultry.2 The most common presentation of C. jejuni in humans is acute enteritis or colitis, but it can have numerous extraintestinal manifestations.3 Bacteremia occurs in fewer than 1% of patients, but C. jejuni meningitis and endocarditis have been reported. Hepatitis, interstitial nephritis, hemolytic‐uremic syndrome, and IgA nephropathy are other reported complications. Our patient probably aspirated a piece of undercooked chicken that likely was the source of the C. jejuni, causing a persistent empyema.
Most patients fully recover from C. jejuni infections without medications, but if illness is severe or prolonged, antibiotics are recommended. Macrolides are usually the first‐line treatment, but their increasing veterinary use is leading to their being resistant to these drugs.4 Most isolates are not susceptible to cephalosporins or penicillins, except amoxicillin or ticarcillin plus clavulanic acid. The C. jejuni isolated in culture in our lab from this patient was unusual in being broadly sensitive.
Our patient aspirated a foreign body in the form of chicken and a peanut without even realizing it. This is extremely uncommon, although foreign‐body aspiration in otherwise healthy and alert adults sometimes does occur. The most common presentation is sudden choking, coughing, and vomiting, followed by wheezing and breathlessness. Patients may also present with persistent cough, hemoptysis, fever, breathlessness, or wheezing. Children may present with cyanosis.
Inorganic foreign bodies tend to be from dental accidents, and organic aspirated foreign bodies tend to depend on the types of food eaten in a particular population, with bones, nuts, and apple pips the most common. In adults, all foreign bodies tend to lodge in the right bronchial tree. Aspiration of organic material is usually diagnosed later than aspiration of nonorganic material.5 In either case, airway foreign‐body aspiration is a common cause of recurrent bacterial pneumonia, and long delays in diagnosis are quite typical.6
Plain x‐rays may be entirely normal. A CT may demonstrate an aspirated foreign body in the lumen of the tracheobronchial tree. Other common findings are atelectasis, hyperlucency, bronchiectasis, lobar consolidation, ipsilateral pleural effusion, and lymphadenopathy and a thickened bronchial wall adjacent to the foreign body.7 Newer methods such as CT virtual bronchoscopy are being evaluated for use in selected cases when clinical suspicion is high.8 0
- ,,,.Campylobacter jejuni—an emerging foodborne pathogen.Emerg Infect Dis.1999;5(1):28–35.
- ,.Sources of Campylobacter colonization in broiler chickens.Appl Environ Microbiol.2003;69:4343–4351.
- .Clinical aspects of Campylobacter jejuni infections in adults.West J Med.1994;161(2):148–152.
- ,.Macrolide resistance in Campylobacter jejuni and Campylobacter coli.J Antimicrob Chemother.2006;58:243–255.
- ,,,.Bronchoscopic removal of foreign bodies in adults: experience with 62 patients from 1974–1998.Eur Respir J.1999;14:792–795.
- ,,,,.Foreign body aspiration into the lower airway in Chinese adults.Chest.1997;112(1):129–133.
- ,,,,,.CT findings of the chest in adults with aspirated foreign bodies.Eur Radiol.2001;11:606–611.
- ,,, et al.CT virtual bronchoscopy in the evaluation of children with suspected foreign body aspiration.Eur J Radiol.2003;48:188–192.
A 72‐year‐old man had been suffering from low‐grade fever, minimally productive cough, and shortness of breath for 1 week when he experienced sudden, moderately severe right‐sided chest pain. His local primary care physician found no abnormalities on physical exam and laboratory testing. A chest x‐ray, however, did reveal a small right‐sided pleural effusion. The patient was empirically started on levofloxacin but noticed no improvement. Two weeks into his illness, he was referred to our institution for further management. By this time, he reported a rapid 10‐pound weight loss and a daily low‐grade fever. Chest examination revealed dullness to percussion along with decreased breath sounds in the right posterior lung fields. A complete blood count showed an elevated white count of 17,000/mL with 14,000 neutrophils. Hemoglobin was 13.5 g/dL. A repeat chest x‐ ray and then a CT scan showed a multiloculated pleural effusion in the right lower hemithorax. Ultrasound‐guided tap of this effusion showed cloudy fluid consistent with pus, with a protein of 4.8 g/dL and total nucleated cells of 6000/mL. A gram stain on this fluid was negative.
The patient had a history remarkable for severe underlying chronic obstructive pulmonary disease (COPD). His forced expiratory volume in 1 second (FEV1) was 21%, and his diffusing capacity of carbon monoxide (DLCO) was 27%. Therefore, decortication under general anesthesia was not an option. So the largest pus pocket was drained under CT guidance, and the patient was dismissed home on levofloxacin.
He returned for follow‐up after 3 weeks and reported daily low‐grade fever, night sweats, and an additional weight loss of 14 pounds. His white count had risen to 18,300/mL with a neutrophil count of 16,600. Hemoglobin had fallen to 11.9 g/dL. A repeat CT scan showed that although the previously drained fluid pocket had resolved, a moderate amount of fluid had reaccumulated in other pockets. Delayed anaerobic culture results from the hospitalization 3 weeks earlier were now available and, interestingly, showed 2+ growth of Campylobacter jejuni, broadly sensitive to all antibiotics including penicillin. Piperacillin/tazobactam was started intravenously, and CT‐guided drainage of the largest pus pocket was again performed.
We carefully reexamined the patient's CT scan, and there appeared to be a lesion in the right main‐stem bronchus. We decided to perform a bronchoscopy, which revealed a foreign body in the right main‐stem bronchus. The foreign body turned out to be a piece of chicken and a peanut. On specific questioning of the patient again, he admitted that at times he coughed after eating too quickly. Specifically, he remembered that a few days before falling sick he was at a village fair, where he had had chicken, and he thought he might have coughed after eating it. He denied any diarrheal illness in the recent past. We obtained a swallow study and upper gastrointestinal endoscopy, both of which were unremarkable.
He improved remarkably after removal of the foreign body and was sent home on amoxicillin‐clavulanic acid for 3 weeks.
DISCUSSION
Campylobacter is one of the most common zoonoses in the world.1 Commercially raised poultry is nearly always colonized with Campylobacter jejuni, and therefore, not surprisingly, 50% to 70% of C. jejuni infection in humans is caused by undercooked poultry.2 The most common presentation of C. jejuni in humans is acute enteritis or colitis, but it can have numerous extraintestinal manifestations.3 Bacteremia occurs in fewer than 1% of patients, but C. jejuni meningitis and endocarditis have been reported. Hepatitis, interstitial nephritis, hemolytic‐uremic syndrome, and IgA nephropathy are other reported complications. Our patient probably aspirated a piece of undercooked chicken that likely was the source of the C. jejuni, causing a persistent empyema.
Most patients fully recover from C. jejuni infections without medications, but if illness is severe or prolonged, antibiotics are recommended. Macrolides are usually the first‐line treatment, but their increasing veterinary use is leading to their being resistant to these drugs.4 Most isolates are not susceptible to cephalosporins or penicillins, except amoxicillin or ticarcillin plus clavulanic acid. The C. jejuni isolated in culture in our lab from this patient was unusual in being broadly sensitive.
Our patient aspirated a foreign body in the form of chicken and a peanut without even realizing it. This is extremely uncommon, although foreign‐body aspiration in otherwise healthy and alert adults sometimes does occur. The most common presentation is sudden choking, coughing, and vomiting, followed by wheezing and breathlessness. Patients may also present with persistent cough, hemoptysis, fever, breathlessness, or wheezing. Children may present with cyanosis.
Inorganic foreign bodies tend to be from dental accidents, and organic aspirated foreign bodies tend to depend on the types of food eaten in a particular population, with bones, nuts, and apple pips the most common. In adults, all foreign bodies tend to lodge in the right bronchial tree. Aspiration of organic material is usually diagnosed later than aspiration of nonorganic material.5 In either case, airway foreign‐body aspiration is a common cause of recurrent bacterial pneumonia, and long delays in diagnosis are quite typical.6
Plain x‐rays may be entirely normal. A CT may demonstrate an aspirated foreign body in the lumen of the tracheobronchial tree. Other common findings are atelectasis, hyperlucency, bronchiectasis, lobar consolidation, ipsilateral pleural effusion, and lymphadenopathy and a thickened bronchial wall adjacent to the foreign body.7 Newer methods such as CT virtual bronchoscopy are being evaluated for use in selected cases when clinical suspicion is high.8 0

A 72‐year‐old man had been suffering from low‐grade fever, minimally productive cough, and shortness of breath for 1 week when he experienced sudden, moderately severe right‐sided chest pain. His local primary care physician found no abnormalities on physical exam and laboratory testing. A chest x‐ray, however, did reveal a small right‐sided pleural effusion. The patient was empirically started on levofloxacin but noticed no improvement. Two weeks into his illness, he was referred to our institution for further management. By this time, he reported a rapid 10‐pound weight loss and a daily low‐grade fever. Chest examination revealed dullness to percussion along with decreased breath sounds in the right posterior lung fields. A complete blood count showed an elevated white count of 17,000/mL with 14,000 neutrophils. Hemoglobin was 13.5 g/dL. A repeat chest x‐ ray and then a CT scan showed a multiloculated pleural effusion in the right lower hemithorax. Ultrasound‐guided tap of this effusion showed cloudy fluid consistent with pus, with a protein of 4.8 g/dL and total nucleated cells of 6000/mL. A gram stain on this fluid was negative.
The patient had a history remarkable for severe underlying chronic obstructive pulmonary disease (COPD). His forced expiratory volume in 1 second (FEV1) was 21%, and his diffusing capacity of carbon monoxide (DLCO) was 27%. Therefore, decortication under general anesthesia was not an option. So the largest pus pocket was drained under CT guidance, and the patient was dismissed home on levofloxacin.
He returned for follow‐up after 3 weeks and reported daily low‐grade fever, night sweats, and an additional weight loss of 14 pounds. His white count had risen to 18,300/mL with a neutrophil count of 16,600. Hemoglobin had fallen to 11.9 g/dL. A repeat CT scan showed that although the previously drained fluid pocket had resolved, a moderate amount of fluid had reaccumulated in other pockets. Delayed anaerobic culture results from the hospitalization 3 weeks earlier were now available and, interestingly, showed 2+ growth of Campylobacter jejuni, broadly sensitive to all antibiotics including penicillin. Piperacillin/tazobactam was started intravenously, and CT‐guided drainage of the largest pus pocket was again performed.
We carefully reexamined the patient's CT scan, and there appeared to be a lesion in the right main‐stem bronchus. We decided to perform a bronchoscopy, which revealed a foreign body in the right main‐stem bronchus. The foreign body turned out to be a piece of chicken and a peanut. On specific questioning of the patient again, he admitted that at times he coughed after eating too quickly. Specifically, he remembered that a few days before falling sick he was at a village fair, where he had had chicken, and he thought he might have coughed after eating it. He denied any diarrheal illness in the recent past. We obtained a swallow study and upper gastrointestinal endoscopy, both of which were unremarkable.
He improved remarkably after removal of the foreign body and was sent home on amoxicillin‐clavulanic acid for 3 weeks.
DISCUSSION
Campylobacter is one of the most common zoonoses in the world.1 Commercially raised poultry is nearly always colonized with Campylobacter jejuni, and therefore, not surprisingly, 50% to 70% of C. jejuni infection in humans is caused by undercooked poultry.2 The most common presentation of C. jejuni in humans is acute enteritis or colitis, but it can have numerous extraintestinal manifestations.3 Bacteremia occurs in fewer than 1% of patients, but C. jejuni meningitis and endocarditis have been reported. Hepatitis, interstitial nephritis, hemolytic‐uremic syndrome, and IgA nephropathy are other reported complications. Our patient probably aspirated a piece of undercooked chicken that likely was the source of the C. jejuni, causing a persistent empyema.
Most patients fully recover from C. jejuni infections without medications, but if illness is severe or prolonged, antibiotics are recommended. Macrolides are usually the first‐line treatment, but their increasing veterinary use is leading to their being resistant to these drugs.4 Most isolates are not susceptible to cephalosporins or penicillins, except amoxicillin or ticarcillin plus clavulanic acid. The C. jejuni isolated in culture in our lab from this patient was unusual in being broadly sensitive.
Our patient aspirated a foreign body in the form of chicken and a peanut without even realizing it. This is extremely uncommon, although foreign‐body aspiration in otherwise healthy and alert adults sometimes does occur. The most common presentation is sudden choking, coughing, and vomiting, followed by wheezing and breathlessness. Patients may also present with persistent cough, hemoptysis, fever, breathlessness, or wheezing. Children may present with cyanosis.
Inorganic foreign bodies tend to be from dental accidents, and organic aspirated foreign bodies tend to depend on the types of food eaten in a particular population, with bones, nuts, and apple pips the most common. In adults, all foreign bodies tend to lodge in the right bronchial tree. Aspiration of organic material is usually diagnosed later than aspiration of nonorganic material.5 In either case, airway foreign‐body aspiration is a common cause of recurrent bacterial pneumonia, and long delays in diagnosis are quite typical.6
Plain x‐rays may be entirely normal. A CT may demonstrate an aspirated foreign body in the lumen of the tracheobronchial tree. Other common findings are atelectasis, hyperlucency, bronchiectasis, lobar consolidation, ipsilateral pleural effusion, and lymphadenopathy and a thickened bronchial wall adjacent to the foreign body.7 Newer methods such as CT virtual bronchoscopy are being evaluated for use in selected cases when clinical suspicion is high.8 0

- ,,,.Campylobacter jejuni—an emerging foodborne pathogen.Emerg Infect Dis.1999;5(1):28–35.
- ,.Sources of Campylobacter colonization in broiler chickens.Appl Environ Microbiol.2003;69:4343–4351.
- .Clinical aspects of Campylobacter jejuni infections in adults.West J Med.1994;161(2):148–152.
- ,.Macrolide resistance in Campylobacter jejuni and Campylobacter coli.J Antimicrob Chemother.2006;58:243–255.
- ,,,.Bronchoscopic removal of foreign bodies in adults: experience with 62 patients from 1974–1998.Eur Respir J.1999;14:792–795.
- ,,,,.Foreign body aspiration into the lower airway in Chinese adults.Chest.1997;112(1):129–133.
- ,,,,,.CT findings of the chest in adults with aspirated foreign bodies.Eur Radiol.2001;11:606–611.
- ,,, et al.CT virtual bronchoscopy in the evaluation of children with suspected foreign body aspiration.Eur J Radiol.2003;48:188–192.
- ,,,.Campylobacter jejuni—an emerging foodborne pathogen.Emerg Infect Dis.1999;5(1):28–35.
- ,.Sources of Campylobacter colonization in broiler chickens.Appl Environ Microbiol.2003;69:4343–4351.
- .Clinical aspects of Campylobacter jejuni infections in adults.West J Med.1994;161(2):148–152.
- ,.Macrolide resistance in Campylobacter jejuni and Campylobacter coli.J Antimicrob Chemother.2006;58:243–255.
- ,,,.Bronchoscopic removal of foreign bodies in adults: experience with 62 patients from 1974–1998.Eur Respir J.1999;14:792–795.
- ,,,,.Foreign body aspiration into the lower airway in Chinese adults.Chest.1997;112(1):129–133.
- ,,,,,.CT findings of the chest in adults with aspirated foreign bodies.Eur Radiol.2001;11:606–611.
- ,,, et al.CT virtual bronchoscopy in the evaluation of children with suspected foreign body aspiration.Eur J Radiol.2003;48:188–192.
Editorial
Autonomy is one of the most familiar principles in Western bioethics, whereas informed consent is probably its most practical expression.1 Autonomy's modern formulation was particularly shaped by political philosophers like John Locke (1632‐1704), who worried about the coercive powers of the state.2 As Lockean‐inspired governments evolved over the last 3 centuries, their legislatures became increasingly disposed to granting citizens an ever‐increasing number of individual rights and freedoms. In American medicine, that sensibility began to take a determinate shape early in the 20th century, such as in Judge Benjamin Cardozo's famous declaration in 1914 that:
Every human being of adult years and sound mind has a right to determine what shall be done with his body, and a surgeon who performs an operation without his patient's consent commits an assault for which he is liable in damages.3
Another half century would be required, however, to agree on the informational content, or scope of disclosure, that would reasonably educate patients on what they would be consenting to. Precedent‐setting decisions in the 1960s and 1970s, such as in Natanson v. Kline4 and Canterbury v. Spence,5 ultimately held that informing a patient about a proposed clinical intervention must include an explanation as to why the intervention is recommended and what particular benefits might accrue from it. Most important, however, is informing the patient about any significant risks the intervention poses. Not associated with or pertaining to error or negligence, but rather understood as foreseeable complications or adverse events that could occur even if the standard of care was scrupulously followed, risk information must be imparted to decisionally able patients or their surrogates to honor their autonomy, or right of bodily ownership.6
The problem with determining whether a risk should be disclosed is that it is often reduced to a judgment call about a risk's severity and frequency. The common understanding is that risks whose severity and frequency are both extremely low need not be discussed. Risk disclosure becomes complex when either of these variables begins to increase, but even then, a significant likelihood of temporary headache or gastrointestinal upset associated with some treatment might not be mentioned. On the other hand, courts have awarded damages to plaintiffs who experienced the materialization of a 1 in 2500 chance of a serious but undisclosed risk.7 The ethical challenge in judging whether a particular risk needs to be disclosed involves the difficulty inherent in determining at what point in the comingling of risk severity and likelihood of materialization does disclosure become required.8
The article by Upadhyay et al. investigates a related facet about risk disclosure.9 For a long time, hospitals have exhibited inconsistent policies for securing informed consent for certain common but nevertheless risky procedures or treatments, especially those involving medications. Many hospitals, for example, would have staff members simply tell patients that they needed diuretics or thrombolytics, even though in certain instances, and especially with thrombolytic agents, the risk of a significant adverse event could well exceed some reasonable disclosure threshold (which is often set at 1%).8
The article by Upadhyay et al. suggests at least 3 issues meriting serious ethical consideration. The first is that the risk scenario primarily discussed in the articlea serious cerebral bleed from thrombolysis with a frequency of from 1% to 20%would most certainly require formal informed consent from patients. To the extent that hospitals recognize such risk scenarios but fail to secure informed consent, they are violating their patients' autonomous rights. The article by Upadhyay et al. is therefore a clarion call to these institutions to become more aggressive and conscientious in honoring their informed consent duties to patients.
A second issue is that the patients surveyed in the study overwhelmingly desired risk disclosure. Notice that if a treatment's risk magnitude is such that it would normally obligate disclosure, the only factors that would preclude disclosure in nonemergent cases would be (1) if the patient was deemed judgmentally or psychologically impaired (and even then, next of kin or the patient's proxy would need to be contacted and informed) or (2) if the patient refused to hear a recitation of the risks (perhaps because it would cause him or her excessive anxiety).10 Otherwise, and as implied by the empirical findings reported in the article, disclosure in an instance like thrombolysis would not only be consistent with (and therefore obligated by) more familiar instances of disclosure such as occur in surgical interventions, it would also be consistent with patient centeredness, as indicated by the responses of the research participants themselves.
But a third issue raises a serious ethical complication. Many patients interviewed in this study also wanted informed consent (or at least wanted to provide permission) for seemingly banal medical interventions. Although respecting patient autonomy is an enduring tenet of medical ethics, it can be argued that it could be limited by other ethical constraints. If respecting a patient's autonomy becomes synonymous with an ethical obligation to disclose all potential risks of every possible treatment regardless of their likelihood or severity, the physician's time might be unreasonably compromised.11 For example, it seems fair to say that many physicians would think it ethically excessive or unreasonable to demand that busy hospitalists discuss the risks, benefits, alternatives, and likelihood of success before ordering intravenous furosemide, potassium supplementation, or routine phlebotomy.
In the general care of hospitalized patients, virtually all physicians will obtain specific, written informed consent prior to invasive procedures, but many might assume that consent for routine medical care has been secured during the consent documentation process of the patient's admission to hospital. Upadhyay et al.'s findings, however, make us question the extent to which consent on admission is ethically sufficient. If it is not, then we must ask what other opportunities exist for effecting patient‐centered explanations of proposed interventions without unduly compromising a health professional's duties and commitments during the workday.
A solution may consist in the way that artful communication skills are key to the physicianpatient relationship. The Accreditation Council on Graduate Medical Education outlines 6 core competencies that all resident physicians should attain during training. One core measure is communication skills: Residents must be able to demonstrate interpersonal and communication skills that result in effective information exchange and teaming with patients, their patients' families, and professional associates.12
Perhaps the individuals surveyed in this study would not require explicit informed consent from a physician if they enjoyed an appropriate number of informational exchanges with all their treating professionals. Their daily treatment plan with its attendant risks and benefits could be discussed in reasonable detail, their comprehension could be elicited through teach back, and their remaining concerns could be explored through empathic communication techniques. This process, which would fold informed consent into a more elaborate, transparent, and humanistically oriented sharing of information, might ease the tension over autonomy versus time constraints by spreading informational responsibilities throughout the health care system. Achieving that quality of informational exchange, however, will require a serious institutional and especially educational commitment in our undergraduate and graduate training programs because it is unlikely that most physicians or other health professionals would seek such skill development on their own.
- ,,.Clinical Ethics: a Practical Approach to Ethical Decisions in Clinical Medicine.6th ed.New York:McGraw‐Hill;2006.
- .Two Treatises of Government.Cambridge, UK:Cambridge University Press;1988.
- . Society of New York Hospital, 105 N.E. 92 (1914).
- ,350 P.2d1093 (1960).
- ,464 F.2d772 (1972).
- ,.Principles of Biomedical Ethics.5th ed.Oxford, UK:Oxford University Press;2001.
- ,286 A.2d647 (1971).
- .Informed Consent: A Guide for Health Care Providers.Rockville, MD:Aspen Systems Corporation;1981.
- ,,,,.Patients' predilections regarding informed consent for hospital treatments.J Hosp Med.2008;3:6–11.
- Council on Ethical and Judicial Affairs.Code of Medical Ethics: Current Opinions with Annotations.2002–2003 ed.Chicago, IL:AMA Press;2002:8.08.
- ,.Physicians' silent decisions: Because patient autonomy does not always come first.Am J Bioeth.2007;7:33–38.
- Available at http://www.acgme.org/outcome/comp/compFull.asp#4 (emphasis added). Accessed on November 6,2007.
Autonomy is one of the most familiar principles in Western bioethics, whereas informed consent is probably its most practical expression.1 Autonomy's modern formulation was particularly shaped by political philosophers like John Locke (1632‐1704), who worried about the coercive powers of the state.2 As Lockean‐inspired governments evolved over the last 3 centuries, their legislatures became increasingly disposed to granting citizens an ever‐increasing number of individual rights and freedoms. In American medicine, that sensibility began to take a determinate shape early in the 20th century, such as in Judge Benjamin Cardozo's famous declaration in 1914 that:
Every human being of adult years and sound mind has a right to determine what shall be done with his body, and a surgeon who performs an operation without his patient's consent commits an assault for which he is liable in damages.3
Another half century would be required, however, to agree on the informational content, or scope of disclosure, that would reasonably educate patients on what they would be consenting to. Precedent‐setting decisions in the 1960s and 1970s, such as in Natanson v. Kline4 and Canterbury v. Spence,5 ultimately held that informing a patient about a proposed clinical intervention must include an explanation as to why the intervention is recommended and what particular benefits might accrue from it. Most important, however, is informing the patient about any significant risks the intervention poses. Not associated with or pertaining to error or negligence, but rather understood as foreseeable complications or adverse events that could occur even if the standard of care was scrupulously followed, risk information must be imparted to decisionally able patients or their surrogates to honor their autonomy, or right of bodily ownership.6
The problem with determining whether a risk should be disclosed is that it is often reduced to a judgment call about a risk's severity and frequency. The common understanding is that risks whose severity and frequency are both extremely low need not be discussed. Risk disclosure becomes complex when either of these variables begins to increase, but even then, a significant likelihood of temporary headache or gastrointestinal upset associated with some treatment might not be mentioned. On the other hand, courts have awarded damages to plaintiffs who experienced the materialization of a 1 in 2500 chance of a serious but undisclosed risk.7 The ethical challenge in judging whether a particular risk needs to be disclosed involves the difficulty inherent in determining at what point in the comingling of risk severity and likelihood of materialization does disclosure become required.8
The article by Upadhyay et al. investigates a related facet about risk disclosure.9 For a long time, hospitals have exhibited inconsistent policies for securing informed consent for certain common but nevertheless risky procedures or treatments, especially those involving medications. Many hospitals, for example, would have staff members simply tell patients that they needed diuretics or thrombolytics, even though in certain instances, and especially with thrombolytic agents, the risk of a significant adverse event could well exceed some reasonable disclosure threshold (which is often set at 1%).8
The article by Upadhyay et al. suggests at least 3 issues meriting serious ethical consideration. The first is that the risk scenario primarily discussed in the articlea serious cerebral bleed from thrombolysis with a frequency of from 1% to 20%would most certainly require formal informed consent from patients. To the extent that hospitals recognize such risk scenarios but fail to secure informed consent, they are violating their patients' autonomous rights. The article by Upadhyay et al. is therefore a clarion call to these institutions to become more aggressive and conscientious in honoring their informed consent duties to patients.
A second issue is that the patients surveyed in the study overwhelmingly desired risk disclosure. Notice that if a treatment's risk magnitude is such that it would normally obligate disclosure, the only factors that would preclude disclosure in nonemergent cases would be (1) if the patient was deemed judgmentally or psychologically impaired (and even then, next of kin or the patient's proxy would need to be contacted and informed) or (2) if the patient refused to hear a recitation of the risks (perhaps because it would cause him or her excessive anxiety).10 Otherwise, and as implied by the empirical findings reported in the article, disclosure in an instance like thrombolysis would not only be consistent with (and therefore obligated by) more familiar instances of disclosure such as occur in surgical interventions, it would also be consistent with patient centeredness, as indicated by the responses of the research participants themselves.
But a third issue raises a serious ethical complication. Many patients interviewed in this study also wanted informed consent (or at least wanted to provide permission) for seemingly banal medical interventions. Although respecting patient autonomy is an enduring tenet of medical ethics, it can be argued that it could be limited by other ethical constraints. If respecting a patient's autonomy becomes synonymous with an ethical obligation to disclose all potential risks of every possible treatment regardless of their likelihood or severity, the physician's time might be unreasonably compromised.11 For example, it seems fair to say that many physicians would think it ethically excessive or unreasonable to demand that busy hospitalists discuss the risks, benefits, alternatives, and likelihood of success before ordering intravenous furosemide, potassium supplementation, or routine phlebotomy.
In the general care of hospitalized patients, virtually all physicians will obtain specific, written informed consent prior to invasive procedures, but many might assume that consent for routine medical care has been secured during the consent documentation process of the patient's admission to hospital. Upadhyay et al.'s findings, however, make us question the extent to which consent on admission is ethically sufficient. If it is not, then we must ask what other opportunities exist for effecting patient‐centered explanations of proposed interventions without unduly compromising a health professional's duties and commitments during the workday.
A solution may consist in the way that artful communication skills are key to the physicianpatient relationship. The Accreditation Council on Graduate Medical Education outlines 6 core competencies that all resident physicians should attain during training. One core measure is communication skills: Residents must be able to demonstrate interpersonal and communication skills that result in effective information exchange and teaming with patients, their patients' families, and professional associates.12
Perhaps the individuals surveyed in this study would not require explicit informed consent from a physician if they enjoyed an appropriate number of informational exchanges with all their treating professionals. Their daily treatment plan with its attendant risks and benefits could be discussed in reasonable detail, their comprehension could be elicited through teach back, and their remaining concerns could be explored through empathic communication techniques. This process, which would fold informed consent into a more elaborate, transparent, and humanistically oriented sharing of information, might ease the tension over autonomy versus time constraints by spreading informational responsibilities throughout the health care system. Achieving that quality of informational exchange, however, will require a serious institutional and especially educational commitment in our undergraduate and graduate training programs because it is unlikely that most physicians or other health professionals would seek such skill development on their own.
Autonomy is one of the most familiar principles in Western bioethics, whereas informed consent is probably its most practical expression.1 Autonomy's modern formulation was particularly shaped by political philosophers like John Locke (1632‐1704), who worried about the coercive powers of the state.2 As Lockean‐inspired governments evolved over the last 3 centuries, their legislatures became increasingly disposed to granting citizens an ever‐increasing number of individual rights and freedoms. In American medicine, that sensibility began to take a determinate shape early in the 20th century, such as in Judge Benjamin Cardozo's famous declaration in 1914 that:
Every human being of adult years and sound mind has a right to determine what shall be done with his body, and a surgeon who performs an operation without his patient's consent commits an assault for which he is liable in damages.3
Another half century would be required, however, to agree on the informational content, or scope of disclosure, that would reasonably educate patients on what they would be consenting to. Precedent‐setting decisions in the 1960s and 1970s, such as in Natanson v. Kline4 and Canterbury v. Spence,5 ultimately held that informing a patient about a proposed clinical intervention must include an explanation as to why the intervention is recommended and what particular benefits might accrue from it. Most important, however, is informing the patient about any significant risks the intervention poses. Not associated with or pertaining to error or negligence, but rather understood as foreseeable complications or adverse events that could occur even if the standard of care was scrupulously followed, risk information must be imparted to decisionally able patients or their surrogates to honor their autonomy, or right of bodily ownership.6
The problem with determining whether a risk should be disclosed is that it is often reduced to a judgment call about a risk's severity and frequency. The common understanding is that risks whose severity and frequency are both extremely low need not be discussed. Risk disclosure becomes complex when either of these variables begins to increase, but even then, a significant likelihood of temporary headache or gastrointestinal upset associated with some treatment might not be mentioned. On the other hand, courts have awarded damages to plaintiffs who experienced the materialization of a 1 in 2500 chance of a serious but undisclosed risk.7 The ethical challenge in judging whether a particular risk needs to be disclosed involves the difficulty inherent in determining at what point in the comingling of risk severity and likelihood of materialization does disclosure become required.8
The article by Upadhyay et al. investigates a related facet about risk disclosure.9 For a long time, hospitals have exhibited inconsistent policies for securing informed consent for certain common but nevertheless risky procedures or treatments, especially those involving medications. Many hospitals, for example, would have staff members simply tell patients that they needed diuretics or thrombolytics, even though in certain instances, and especially with thrombolytic agents, the risk of a significant adverse event could well exceed some reasonable disclosure threshold (which is often set at 1%).8
The article by Upadhyay et al. suggests at least 3 issues meriting serious ethical consideration. The first is that the risk scenario primarily discussed in the articlea serious cerebral bleed from thrombolysis with a frequency of from 1% to 20%would most certainly require formal informed consent from patients. To the extent that hospitals recognize such risk scenarios but fail to secure informed consent, they are violating their patients' autonomous rights. The article by Upadhyay et al. is therefore a clarion call to these institutions to become more aggressive and conscientious in honoring their informed consent duties to patients.
A second issue is that the patients surveyed in the study overwhelmingly desired risk disclosure. Notice that if a treatment's risk magnitude is such that it would normally obligate disclosure, the only factors that would preclude disclosure in nonemergent cases would be (1) if the patient was deemed judgmentally or psychologically impaired (and even then, next of kin or the patient's proxy would need to be contacted and informed) or (2) if the patient refused to hear a recitation of the risks (perhaps because it would cause him or her excessive anxiety).10 Otherwise, and as implied by the empirical findings reported in the article, disclosure in an instance like thrombolysis would not only be consistent with (and therefore obligated by) more familiar instances of disclosure such as occur in surgical interventions, it would also be consistent with patient centeredness, as indicated by the responses of the research participants themselves.
But a third issue raises a serious ethical complication. Many patients interviewed in this study also wanted informed consent (or at least wanted to provide permission) for seemingly banal medical interventions. Although respecting patient autonomy is an enduring tenet of medical ethics, it can be argued that it could be limited by other ethical constraints. If respecting a patient's autonomy becomes synonymous with an ethical obligation to disclose all potential risks of every possible treatment regardless of their likelihood or severity, the physician's time might be unreasonably compromised.11 For example, it seems fair to say that many physicians would think it ethically excessive or unreasonable to demand that busy hospitalists discuss the risks, benefits, alternatives, and likelihood of success before ordering intravenous furosemide, potassium supplementation, or routine phlebotomy.
In the general care of hospitalized patients, virtually all physicians will obtain specific, written informed consent prior to invasive procedures, but many might assume that consent for routine medical care has been secured during the consent documentation process of the patient's admission to hospital. Upadhyay et al.'s findings, however, make us question the extent to which consent on admission is ethically sufficient. If it is not, then we must ask what other opportunities exist for effecting patient‐centered explanations of proposed interventions without unduly compromising a health professional's duties and commitments during the workday.
A solution may consist in the way that artful communication skills are key to the physicianpatient relationship. The Accreditation Council on Graduate Medical Education outlines 6 core competencies that all resident physicians should attain during training. One core measure is communication skills: Residents must be able to demonstrate interpersonal and communication skills that result in effective information exchange and teaming with patients, their patients' families, and professional associates.12
Perhaps the individuals surveyed in this study would not require explicit informed consent from a physician if they enjoyed an appropriate number of informational exchanges with all their treating professionals. Their daily treatment plan with its attendant risks and benefits could be discussed in reasonable detail, their comprehension could be elicited through teach back, and their remaining concerns could be explored through empathic communication techniques. This process, which would fold informed consent into a more elaborate, transparent, and humanistically oriented sharing of information, might ease the tension over autonomy versus time constraints by spreading informational responsibilities throughout the health care system. Achieving that quality of informational exchange, however, will require a serious institutional and especially educational commitment in our undergraduate and graduate training programs because it is unlikely that most physicians or other health professionals would seek such skill development on their own.
- ,,.Clinical Ethics: a Practical Approach to Ethical Decisions in Clinical Medicine.6th ed.New York:McGraw‐Hill;2006.
- .Two Treatises of Government.Cambridge, UK:Cambridge University Press;1988.
- . Society of New York Hospital, 105 N.E. 92 (1914).
- ,350 P.2d1093 (1960).
- ,464 F.2d772 (1972).
- ,.Principles of Biomedical Ethics.5th ed.Oxford, UK:Oxford University Press;2001.
- ,286 A.2d647 (1971).
- .Informed Consent: A Guide for Health Care Providers.Rockville, MD:Aspen Systems Corporation;1981.
- ,,,,.Patients' predilections regarding informed consent for hospital treatments.J Hosp Med.2008;3:6–11.
- Council on Ethical and Judicial Affairs.Code of Medical Ethics: Current Opinions with Annotations.2002–2003 ed.Chicago, IL:AMA Press;2002:8.08.
- ,.Physicians' silent decisions: Because patient autonomy does not always come first.Am J Bioeth.2007;7:33–38.
- Available at http://www.acgme.org/outcome/comp/compFull.asp#4 (emphasis added). Accessed on November 6,2007.
- ,,.Clinical Ethics: a Practical Approach to Ethical Decisions in Clinical Medicine.6th ed.New York:McGraw‐Hill;2006.
- .Two Treatises of Government.Cambridge, UK:Cambridge University Press;1988.
- . Society of New York Hospital, 105 N.E. 92 (1914).
- ,350 P.2d1093 (1960).
- ,464 F.2d772 (1972).
- ,.Principles of Biomedical Ethics.5th ed.Oxford, UK:Oxford University Press;2001.
- ,286 A.2d647 (1971).
- .Informed Consent: A Guide for Health Care Providers.Rockville, MD:Aspen Systems Corporation;1981.
- ,,,,.Patients' predilections regarding informed consent for hospital treatments.J Hosp Med.2008;3:6–11.
- Council on Ethical and Judicial Affairs.Code of Medical Ethics: Current Opinions with Annotations.2002–2003 ed.Chicago, IL:AMA Press;2002:8.08.
- ,.Physicians' silent decisions: Because patient autonomy does not always come first.Am J Bioeth.2007;7:33–38.
- Available at http://www.acgme.org/outcome/comp/compFull.asp#4 (emphasis added). Accessed on November 6,2007.
Prescription Issues after Hospital Discharge
The period immediately following hospital discharge is a vulnerable time for patients, who must assume responsibilities for their own care as they return home.1 The process of hospital discharge may be a rushed event, and patients often have difficulty understanding and following their postdischarge treatment plan.2, 3 Medication‐related problems after hospital discharge, which include patients not filling or refilling prescriptions,46 not understanding how to take medications,2, 3 showing discrepancies between what they are and what they should be taking,79 and having adverse drug events,1012 are a major cause of morbidity and mortality.13
According to prior studies, elderly patients and patients taking more than 5 medications are more likely to experience problems with their medications.5, 14 Adverse drug events are more common with certain high‐risk drugs, including cardiovascular agents, anticoagulants, insulin, antibiotics, and steroids.11, 14, 15 Beyond this, however, patient management of prescription medications after hospital discharge has not been well described. In particular, studies in the community setting and in the immediate postdischarge period are needed.
We conducted a large observational study of patients at 170 community hospitals in order to examine the frequency of prescription‐related issues 4872 hours after hospital discharge. These issues included problems with filling or taking medications prescribed at discharge. We hypothesized that age and number of medications would be independently associated with prescription‐related problems. We also examined the effects of other factors, including insurance type, length of stay, severity of illness (SOI), clinical diagnosis, and use of certain high‐risk drugs.
METHODS
Setting and Population
Information for the present analysis consisted of deidentified clinical, administrative, and survey data provided by a national hospitalist management group, IPCThe Hospitalist Company. At the time of the study, IPC employed more than 300 physicians working at 170 community hospitals in 18 regions across the United States. As part of their daily patient management, physicians entered clinical and administrative data into a proprietary Web‐based program. At discharge, physicians completed discharge summaries in the same program. These summaries were faxed to the outpatient physicians scheduled to see the patients and were transmitted electronically to a call center. The call center attempted to contact all patients at home to assess their clinical status and satisfaction and to assist with any postdischarge needs. The call center staff made up to 2 attempts to reach each patient by telephone within 3 days of discharge. Patients who were reached were interviewed using a scripted survey. Any identified medical needs were addressed in a separate follow‐up call by a nurse.
Patients were included in this analysis if they were at least 18 years old, were treated by an IPC hospitalist, had been discharged between January 1, 2005, and December 31, 2005, and were successfully surveyed by telephone 4872 hours after hospital discharge. If patients had more than 1 discharge during the study period, only the first survey and its corresponding hospital stay were included. The analytic plan was approved by the Emory University Institutional Review Board.
Data Collection
Hospitalists recorded the age, sex, and insurance coverage of each hospitalized patient and noted the discharge diagnoses and medications on the discharge summary. Primary diagnosis and length of stay were determined from hospitalist billing data, which were entered daily into the Web‐based program. Each patient's severity of illness was classified as minor, moderate, major, or extreme using a commercially available program (3M Health Information Systems) that considered patient age, primary diagnosis, diagnosis‐related group (DRG), and nonoperating room procedures.
A common patient identifier code linked these data with patient‐reported information obtained from the call center. Patients indicated whether they picked up their prescribed medications and if they had any trouble understanding how to take their medications. For the present analysis, patients were considered to have a prescription‐related issue if they had problems filling or taking medications prescribed at discharge, a composite variable defined as including not picking up discharge medications, not knowing whether discharge medications had been picked up, not taking discharge medications, or not understanding how to take discharge medications.
Statistical Analysis
Initial analyses included construction of frequency tables to estimate the distribution of prescription‐related issues across patient demographic characteristics, insurance type, clinical diagnosis, and number of medications, as well as among users of certain high‐risk classes of medication. Some continuous variables, such as age and number of medications, were categorized (age into clinically relevant categories, number of medications into tertiles). Separate variables were created for clinical diagnoses by mapping DRGs to 26 major diagnostic categories (MDCs) so that comparisons could be made based on the frequency of prescription‐related issues for those with a primary diagnosis pertaining to a particular organ system versus those with a primary diagnosis outside that organ system. The 10 most common MDCs were circulatory, digestive, respiratory, nervous, skin‐subcutaneous‐breast, kidney‐urinary, musculoskeletal‐connective, hepatobiliary‐pancreas, endocrine‐nutrition‐metabolic, and infectious. The categories of the severity of illness variable were reduced to 3 by combining major and extreme because there were so few in the extreme category.
Unadjusted odds ratios were calculated based on a logistic regression model relating any prescription‐related issues to each possible covariate 1 at a time (ie, not adjusted for any of the other covariates). Adjusted odds ratios were obtained through stepwise building of a logistic regression model. Initially, all possible covariates were entered into the model, and the model was then reduced using Wald test results to assess the significance of dropped parameters. All analyses were conducted using SAS version 9.1 (Cary, NC) and a significance level of 0.05.
RESULTS
In 2005, there were 104,506 eligible adult hospital discharges, corresponding to 96,179 patients. Excluding discharged patients who could not be contacted by the call center or who refused to complete the survey (n = 67,084), multiple surveys of the same patient (n = 3156), and surveys with insufficient data to determine whether there were prescription‐related issues (n = 3067) left 31,199 patients available for analysis (effective response rate 32.4%).
More than half the participants (57.0%) were women, and the mean age was 61.1 years (SD 17.8 years). The median number of discharge medications was 4 (range 128). The most frequently prescribed drugs were antibiotics and analgesics, followed by several cardiovascular drug classes (Table 1). About 60% of the primary diagnoses were of circulatory, digestive, and respiratory disorders (Table 1). Compared with nonparticipants, the study sample was more likely to be female, older, and covered by Medicare. Study patients also had greater comorbidity, as indicated by greater severity of illness rating and number of discharge medications.
| Characteristic | Study sample (n = 31,199) | Excluded patients (n = 65,060) |
|---|---|---|
| ||
| Age (years), n (%) | ||
| 34 | 2712 (8.7%) | 9064 (13.9%) |
| 3549 | 5645 (18.1%) | 16,327 (25.1%) |
| 5064 | 8359 (26.8%) | 17,583 (27.0%) |
| 6579 | 9192 (29.5%) | 13,660 (21.0%) |
| 80 | 5291 (17.0%) | 8426 (13.0%) |
| Sex, n (% female) | 17,450 (57.0%) | 34,298 (52.7%) |
| Insurance type, n (%) | ||
| Medicare | 12,455 (39.9%) | 21,255 (32.7%) |
| Medicare HMO | 966 (3.1%) | 1462 (2.2%) |
| HMO (non‐Medicare) | 11,076 (35.5%) | 22,666 (34.8%) |
| Medicaid | 1599 (5.1%) | 4315 (6.6%) |
| Self‐pay/uninsured | 2151 (6.9%) | 7717 (11.9%) |
| Commercial | 2952 (9.5%) | 7645 (11.8%) |
| Severity of illness, n (%) | ||
| Minor | 12,097 (39.1%) | 27,958 (43.6%) |
| Moderate | 14,800 (47.9%) | 29,020 (45.1%) |
| Major/extreme | 4020 (13.0%) | 7353 (11.4%) |
| Length of stay (days), mean (SD) | 3.9 (3.9) | 3.6 (4.0) |
| Discharge medications, n (%) | ||
| 12 | 8100 (26.0%) | 22,060 (33.9%) |
| 35 | 13,299 (42.6%) | 26,654 (41.0%) |
| 6 | 9800 (31.4%) | 16,346 (25.1%) |
| Medication class, n (%) | ||
| Antibiotics | 9927 (31.8%) | 17,721 (27.2%) |
| Analgesics | 9153 (29.3%) | 18,660 (28.7%) |
| Beta‐blockers | 8398 (26.9%) | 14,733 (22.6%) |
| Aspirin | 7028 (22.5%) | 13,040 (20.0%) |
| ACE inhibitors | 6493 (20.8%) | 11,640 (17.9%) |
| Lipid‐lowering agents | 5661 (18.1%) | 9421 (14.5%) |
| Diuretics | 5100 (16.3%) | 8393 (12.9%) |
| Inhalers | 4352 (13.9%) | 7297 (11.2%) |
| Oral hypoglycemics | 3705 (11.9%) | 6819 (10.5%) |
| Steroids | 3521 (11.3%) | 6056 (9.3%) |
| Anticoagulants | 3152 (10.1%) | 4820 (7.4%) |
| Insulins | 2236 (7.2%) | 4589 (7.1%) |
| Angiotensin II receptor blockers | 2034 (6.5%) | 3285 (5.0%) |
| Major diagnostic category, n (%) | ||
| Circulatory | 7971 (25.7%) | 16,963 (26.3%) |
| Digestive | 4990 (16.1%) | 10,211 (15.8%) |
| Respiratory | 4955 (16.0%) | 8482 (13.1%) |
| Nervous | 2469 (8.0%) | 5505 (8.5%) |
| Skin‐subcutaneous‐breast | 1738 (5.6%) | 3664 (5.7%) |
| Renal | 1610 (5.2%) | 3216 (5.0%) |
| Musculoskeletal‐connective | 1357 (4.4%) | 2592 (4.0%) |
| Hepatobiliary‐pancreas | 1273 (4.1%) | 2844 (4.4%) |
| Endocrine‐nutrition‐metabolic | 1195 (3.9%) | 2666 (4.1%) |
| Infectious | 771 (2.5%) | 1429 (2.2%) |
Overall, 7.2% of patients (n = 2253) had prescription‐related issues 4872 hours after hospital discharge. This included not picking up prescribed discharge medications (n = 1797, or 79.8% of issues), not knowing if they were picked up (n = 55 or 2.4%), and admitting to not taking (n = 154, or 6.8%) or not understanding how to take (n = 247, or 11%) medications.
In unadjusted analyses, prescription‐related issues were significantly associated with age, sex, insurance type, severity of illness rating, length of stay, number of discharge medications, certain medication types, and major diagnostic category (Table 2). Except for the youngest patients (age < 35 years), having prescription‐related issues appeared to be inversely related to patient age. Adults 3549 years old had the highest frequency of problems filling or taking medications (9.3%), whereas patients 80 years or older had the lowest frequency (5.6%). Analysis by insurance status showed that patients with Medicaid (12.6%) or self‐pay/uninsured status (11.9%) had significantly higher rates of prescription issues and patients with non‐Medicare HMO or commercial insurance had significantly lower rates (6.1% and 4.9%, respectively). Being prescribed at least 6 medications or taking ACE inhibitors, inhalers, oral hypoglycemics, or insulins was also associated with a higher frequency of prescription‐related problems in unadjusted analyses. Patients prescribed antibiotics or anticoagulants were less likely to report problems in unadjusted analyses.
| Characteristic | Prescription‐related issues, n (%) | Unadjusted OR (95% CI) | P value |
|---|---|---|---|
| |||
| Age | < .0001 | ||
| 34 | 195 (7.2%) | ||
| 3549 | 523 (9.3%) | 1.32 (1.111.56) | |
| 5064 | 646 (7.7%) | 1.08 (0.921.28) | |
| 6579 | 592 (6.4%) | 0.89 (0.751.05) | |
| 80 | 297 (5.6%) | 0.77 (0.640.93) | |
| Sex | .035 | ||
| Male | 906 (6.9%) | ||
| Female | 1310 (7.5%) | 1.10 (1.011.20) | |
| Insurance type | < .0001 | ||
| Medicare | 891 (7.2%) | ||
| Medicare HMO | 86 (8.9%) | 1.27 (1.011.60) | |
| HMO (non‐Medicare) | 674 (6.1%) | 0.84 (0.760.93) | |
| Medicaid | 201 (12.6%) | 1.87 (1.592.20) | |
| Self‐pay/uninsured | 256 (11.9%) | 1.75 (1.512.03) | |
| Commercial | 145 (4.9%) | 0.66 (0.550.79) | |
| Severity of illness | .0007 | ||
| Minor | 794 (6.6%) | ||
| Moderate | 1107 (7.5%) | 1.15 (1.051.27) | |
| Major/extreme | 328 (8.2%) | 1.27 (1.111.45) | |
| Length of stay (days) | 1.01 (1.001.02) | .014 | |
| Discharge medications | < .0001 | ||
| 12 | 526 (6.5%) | ||
| 35 | 928 (7.0%) | 1.08 (0.971.21) | |
| 6 | 799 (8.2%) | 1.28 (1.141.43) | |
| Medication class | |||
| Antibiotics | 639 (6.4%) | 0.84 (0.760.92) | .0003 |
| Analgesics | 656 (7.2%) | 0.99 (0.901.09) | .8 |
| Beta‐blockers | 608 (7.2%) | 1.00 (0.911.11) | .9 |
| Aspirin | 539 (7.7%) | 1.09 (0.981.20) | .1 |
| ACE inhibitors | 520 (8.0%) | 1.15 (1.041.28) | .006 |
| Lipid‐lowering agents | 438 (7.7%) | 1.10 (0.981.22) | .1 |
| Diuretics | 370 (7.3%) | 1.00 (0.901.13) | .9 |
| Inhalers | 373 (8.6%) | 1.25 (1.111.40) | .0002 |
| Oral hypoglycemics | 295 (8.0%) | 1.23 (0.991.28) | .06 |
| Steroids | 261 (7.4%) | 1.03 (0.901.18) | .64 |
| Anticoagulants | 189 (6.0%) | 0.80 (0.690.94) | .005 |
| Insulins | 210 (9.4%) | 1.37 (1.181.59) | < .0001 |
| Angiotensin II receptor blockers | 126 (6.2%) | 0.84 (0.701.01) | .06 |
| Major diagnostic category | |||
| Circulatory | 619 (7.8%) | 1.12 (1.011.23) | .025 |
| Digestive | 398 (8.0%) | 1.14 (1.021.28) | .02 |
| Respiratory | 354 (7.1%) | 0.99 (0.881.11) | .86 |
| Nervous | 188 (7.6%) | 1.07 (0.911.25) | .41 |
| Skin‐subcutaneous‐breast | 71 (4.1%) | 0.53 (0.420.68) | < .0001 |
| Renal | 98 (6.1%) | 0.83 (0.671.02) | .075 |
| Musculoskeletal‐connective | 74 (5.5%) | 0.73 (0.580.93) | .01 |
| Hepatobiliary‐pancreas | 105 (8.3%) | 1.17 (0.951.43) | .14 |
| Endocrine‐nutrition‐metabolic | 93 (7.8%) | 1.09 (0.881.35) | .43 |
| Infectious | 45 (5.8%) | 0.79 (0.591.08) | .14 |
In multivariable models, age, sex, insurance type, severity of illness, number of medications, and certain medication types were independently associated with prescription‐related issues after discharge (Table 3). Seniors reported significantly fewer problems than the youngest patients (6579 years, OR 0.69; 80 years, OR 0.59). Those with Medicare HMOs, Medicaid, or no insurance had more difficulty obtaining and taking prescription medications (OR 1.29, 1.33, and 1.31, respectively), whereas patients with HMO or commercial insurance plans had less difficulty (OR 0.68 and 0.51, respectively). Prescription‐related problems were also more common among women (OR 1.11), patients with higher severity of illness (moderate SOI, OR 1.12; major/extreme SOI, OR 1.23), and those with 6 or more discharge medications (OR 1.35). In adjusted analyses, inhalers were the only type of medication associated with a significantly higher frequency of problems (OR 1.14).
| Characteristic | Adjusted OR (95% CI) | P value |
|---|---|---|
| ||
| Age | < .0001 | |
| 34 | ||
| 3549 | 1.27 (1.071.51) | |
| 5064 | 1.02 (0.861.21) | |
| 6579 | 0.69 (0.570.84) | |
| 80 | 0.59 (0.480.73) | |
| Sex | .03 | |
| Male | ||
| Female | 1.11 (1.011.21) | |
| Insurance type | < .0001 | |
| Medicare | ||
| Medicare HMO | 1.29 (1.021.63) | |
| HMO (non‐Medicare) | 0.68 (0.600.76) | |
| Medicaid | 1.33 (1.111.60) | |
| Self‐pay/uninsured | 1.31 (1.101.56) | |
| Commercial | 0.51 (0.420.62) | |
| Severity of illness | .008 | |
| Minor | ||
| Moderate | 1.12 (1.021.24) | |
| Major/extreme | 1.23 (1.071.42) | |
| Discharge medications | < .0001 | |
| 12 | ||
| 35 | 1.11 (0.991.24) | |
| 6 | 1.35 (1.191.54) | |
| Medication class | ||
| Antibiotic | 0.78 (0.710.86) | < .0001 |
| Inhalers | 1.14 (1.011.29) | .04 |
| Anticoagulants | 0.81 (0.690.95) | .009 |
| Angiotensin II receptor blockers | 0.81 (0.670.98) | .03 |
| Major diagnostic category | ||
| Skin‐subcutaneous‐breast | 0.52 (0.410.67) | < .0001 |
| Musculoskeletal‐connective | 0.74 (0.580.94) | .01 |
Analyses were repeated using only failure to pick up medications as the dependent variable, and results were similar (not shown).
DISCUSSION
In this large multicenter study, 7.2% of patients reported problems obtaining or taking prescribed medications in the 4872 hours following hospital discharge. In about 80% of cases, the problem was failure to pick up discharge medications. Multivariable analyses showed adults 3549 years old; women; patients with Medicare HMO insurance, Medicaid, or no coverage (self‐pay); adults with high severity of illness rating; and those prescribed more than 5 medications or an inhaler had significantly greater odds of prescription‐related issues. Other factors were protective including age 65 or older; HMO or commercial insurance; prescription of antibiotics, anticoagulants, or angiotensin II receptor blockers; and major diagnosis in the skin or musculoskeletal category.
Among all the groups studied, patients with Medicaid or no insurance had the highest frequency of problems filling and taking discharge medications (12.6% and 11.9%, respectively). This was likely related to their having less prescription drug coverage or experiencing other financial constraints. In previous studies, patients have expressed concern over the rising cost of medications and have admitted to not filling prescriptions or stretching out the use of medications to make them last longer because of high out‐of‐pocket costs.5, 16 Prescriptions given at hospital discharge may pose a significant unexpected expense for patients who have a fixed monthly income, rely on samples from outpatient physicians for their medications, or need time to research cost‐saving measures such as discount plans. Greater attention by physicians to knowing the cost of discharge medications, to prescribing only those drugs that are truly necessary, and to discussing cost‐saving strategies with patients may help to minimize financial concerns and improve the ability of patients to fill discharge prescriptions.17
The finding that polypharmacy is associated with greater odds of prescription‐related issues is consistent with research that found that other medication problems such as adverse drug events and nonadherence were more prevalent among patients prescribed more than 5 medications.5, 14 Polypharmacy may have contributed to prescription‐related difficulties in this study by increasing medication costs or by increasing the chance that patients had a problem with at least 1 medication.
The higher frequency of prescription‐related issues among patients prescribed inhalers indicates that this category of medication may be associated with lower fill rates or greater confusion after discharge. This would be concerning, given that repeat exacerbations of obstructive lung disease may lead to rehospitalization. Other medications, including anticoagulants and antibiotics, were associated with a lower frequency of problems. This may have been the result of better education at discharge about the importance of promptly filling prescriptions for these agents in order to avoid a lapse in therapy following acute treatment for thromboembolic disorders or acute infections. It is hoped that a similar educational effort about filling prescriptions for inhalers also would have occurred. These effects have not been noted in prior research and require further substantiation.11, 14 Also, the observed relationships may be related to the size of the data set and the number of variables considered, rather than to a true effect.
The main strength of this study was that the data from which conclusions were drawn came from a large and geographically diverse patient population. However, the study also had several limitations. First, the response rate was relatively low, primarily because this study was a retrospective analysis performed using data collected for clinical and administrative reasons. Patient contact number was missing or incorrect in 16% of cases. Also, because of the narrow window of time during which the survey was administered, the call center, which was following up an average of 370 discharged patients per day, was only able to make 1 or 2 attempts to reach each patient. This contrasts with prospective research on postdischarge medication use such as the study by Forster and colleagues, in which the investigator made up to 20 attempts to reach patients at different times and on different days.11 Despite these efforts, the follow‐up rate was only 69%, underscoring the challenge of data collection in this setting.
The low response rate raises the possibility that the estimated prevalence of prescription‐related issues may be inaccurate. Although highly unlikely, if all the nonresponders had problems with their prescriptions, the true event rate would be 69.9%. Conversely, if none of the nonresponders had problems, the true event rate would be 2.3%. Given the characteristics of responders and nonresponders, however, we expect that a higher survey completion rate would have yielded similar results. Nonresponders had certain characteristics that would be expected to be associated with a higher frequency of prescription‐related issues (younger age, uninsured, covered by Medicaid), but these were balanced by others that would be expected to be associated with a lower frequency of problems (higher percentage of men, lower severity of illness, fewer medications).
Another study limitation concerns the self‐reported nature of the composite outcome variable. After reviewing the structure of the call center data, we chose this composite measure because it conceptually represented difficulties in obtaining or taking prescribed discharge medications. When we analyzed results using only the most prevalent component of this composite variable, the results were similar. However, all these findings could have been influenced by social desirability bias. Patients may have underreported not filling their discharge prescriptions and also may not acknowledged difficulties in understanding how to take the medications. We would therefore expect the true prevalence of prescription‐related concerns after hospital discharge to be higher than that found in this study.
These limitations notwithstanding, the findings from this large, multicenter study show that prescription‐related issues are common after hospital discharge and, further, that they usually take the form of not filling discharge prescriptions. The highest‐risk patients appear to be those without insurance and those covered by Medicaid or Medicare HMOs, as well as adults age 3549, patients prescribed 6 or more medications, and patients with a higher severity of illness. When preparing patients to leave the hospital, physicians and other health care providers should strive to identify financial, behavioral, and other barriers to proper medication use so that appropriate assistance or counseling may be offered prior to discharge.18, 19 Close follow‐up of patients by telephone may also be a helpful approach to promptly identifying prescription‐related issues and other problems so that providers can intervene before more serious complications arise.
- ,.Lost in transition: challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;141:533–536.
- ,,, et al.Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan.Arch Intern Med.1997;157:1026–1030.
- ,.Patients' understanding of their treatment plans and diagnosis at discharge.Mayo Clin Proc2005;80:991–994.
- ,,.Medication adherence in elderly patients receiving home health services following hospital discharge.Ann Pharmacother.2001;35:539–545.
- ,.Uncovering a multitude of sins: medication management in the home post acute hospitalisation among the chronically ill.Aust N Z J Med.1999;29(2):220–227.
- ,,, et al.Impact of medication therapy discontinuation on mortality after myocardial infarction.Arch Intern Med.2006;166:1842–1847.
- ,,.A new tool for identifying discrepancies in postacute medications for community‐dwelling older adults.Am J Geriatr Pharmacother.2004;2(2):141–147.
- ,,,.Posthospital medication discrepancies: prevalence and contributing factors.Arch Intern Med.2005;165:1842–1847.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- ,,, et al.Adverse events among medical patients after discharge from hospital.Can Med Assoc J.2004;170:345–349.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20:317–323.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med2003;18:646–651.
- ,,, et al.Adverse drug events in ambulatory care.N Engl J Med.2003;348:1556–1564.
- MA Coalition for the Prevention of Medical Errors. Reconciling medications. Recommended practices. Available at: http://www.macoalition.org/documents/RecMedPractices.pdf. Accessed July 27,2005.
- ,,.Cost‐related medication underuse: do patients with chronic illnesses tell their doctors?Arch Intern Med.2004;164:1749–1755.
- ,,,,,.Physician communication about the cost and acquisition of newly prescribed medications.Am J Manag Care.2006;12:657–664.
- ,,.Medication education of acutely hospitalized older patients.J Gen Intern Med.1999;14:610–616.
- ,,.Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure.Med Care.2005;43:246–255.
The period immediately following hospital discharge is a vulnerable time for patients, who must assume responsibilities for their own care as they return home.1 The process of hospital discharge may be a rushed event, and patients often have difficulty understanding and following their postdischarge treatment plan.2, 3 Medication‐related problems after hospital discharge, which include patients not filling or refilling prescriptions,46 not understanding how to take medications,2, 3 showing discrepancies between what they are and what they should be taking,79 and having adverse drug events,1012 are a major cause of morbidity and mortality.13
According to prior studies, elderly patients and patients taking more than 5 medications are more likely to experience problems with their medications.5, 14 Adverse drug events are more common with certain high‐risk drugs, including cardiovascular agents, anticoagulants, insulin, antibiotics, and steroids.11, 14, 15 Beyond this, however, patient management of prescription medications after hospital discharge has not been well described. In particular, studies in the community setting and in the immediate postdischarge period are needed.
We conducted a large observational study of patients at 170 community hospitals in order to examine the frequency of prescription‐related issues 4872 hours after hospital discharge. These issues included problems with filling or taking medications prescribed at discharge. We hypothesized that age and number of medications would be independently associated with prescription‐related problems. We also examined the effects of other factors, including insurance type, length of stay, severity of illness (SOI), clinical diagnosis, and use of certain high‐risk drugs.
METHODS
Setting and Population
Information for the present analysis consisted of deidentified clinical, administrative, and survey data provided by a national hospitalist management group, IPCThe Hospitalist Company. At the time of the study, IPC employed more than 300 physicians working at 170 community hospitals in 18 regions across the United States. As part of their daily patient management, physicians entered clinical and administrative data into a proprietary Web‐based program. At discharge, physicians completed discharge summaries in the same program. These summaries were faxed to the outpatient physicians scheduled to see the patients and were transmitted electronically to a call center. The call center attempted to contact all patients at home to assess their clinical status and satisfaction and to assist with any postdischarge needs. The call center staff made up to 2 attempts to reach each patient by telephone within 3 days of discharge. Patients who were reached were interviewed using a scripted survey. Any identified medical needs were addressed in a separate follow‐up call by a nurse.
Patients were included in this analysis if they were at least 18 years old, were treated by an IPC hospitalist, had been discharged between January 1, 2005, and December 31, 2005, and were successfully surveyed by telephone 4872 hours after hospital discharge. If patients had more than 1 discharge during the study period, only the first survey and its corresponding hospital stay were included. The analytic plan was approved by the Emory University Institutional Review Board.
Data Collection
Hospitalists recorded the age, sex, and insurance coverage of each hospitalized patient and noted the discharge diagnoses and medications on the discharge summary. Primary diagnosis and length of stay were determined from hospitalist billing data, which were entered daily into the Web‐based program. Each patient's severity of illness was classified as minor, moderate, major, or extreme using a commercially available program (3M Health Information Systems) that considered patient age, primary diagnosis, diagnosis‐related group (DRG), and nonoperating room procedures.
A common patient identifier code linked these data with patient‐reported information obtained from the call center. Patients indicated whether they picked up their prescribed medications and if they had any trouble understanding how to take their medications. For the present analysis, patients were considered to have a prescription‐related issue if they had problems filling or taking medications prescribed at discharge, a composite variable defined as including not picking up discharge medications, not knowing whether discharge medications had been picked up, not taking discharge medications, or not understanding how to take discharge medications.
Statistical Analysis
Initial analyses included construction of frequency tables to estimate the distribution of prescription‐related issues across patient demographic characteristics, insurance type, clinical diagnosis, and number of medications, as well as among users of certain high‐risk classes of medication. Some continuous variables, such as age and number of medications, were categorized (age into clinically relevant categories, number of medications into tertiles). Separate variables were created for clinical diagnoses by mapping DRGs to 26 major diagnostic categories (MDCs) so that comparisons could be made based on the frequency of prescription‐related issues for those with a primary diagnosis pertaining to a particular organ system versus those with a primary diagnosis outside that organ system. The 10 most common MDCs were circulatory, digestive, respiratory, nervous, skin‐subcutaneous‐breast, kidney‐urinary, musculoskeletal‐connective, hepatobiliary‐pancreas, endocrine‐nutrition‐metabolic, and infectious. The categories of the severity of illness variable were reduced to 3 by combining major and extreme because there were so few in the extreme category.
Unadjusted odds ratios were calculated based on a logistic regression model relating any prescription‐related issues to each possible covariate 1 at a time (ie, not adjusted for any of the other covariates). Adjusted odds ratios were obtained through stepwise building of a logistic regression model. Initially, all possible covariates were entered into the model, and the model was then reduced using Wald test results to assess the significance of dropped parameters. All analyses were conducted using SAS version 9.1 (Cary, NC) and a significance level of 0.05.
RESULTS
In 2005, there were 104,506 eligible adult hospital discharges, corresponding to 96,179 patients. Excluding discharged patients who could not be contacted by the call center or who refused to complete the survey (n = 67,084), multiple surveys of the same patient (n = 3156), and surveys with insufficient data to determine whether there were prescription‐related issues (n = 3067) left 31,199 patients available for analysis (effective response rate 32.4%).
More than half the participants (57.0%) were women, and the mean age was 61.1 years (SD 17.8 years). The median number of discharge medications was 4 (range 128). The most frequently prescribed drugs were antibiotics and analgesics, followed by several cardiovascular drug classes (Table 1). About 60% of the primary diagnoses were of circulatory, digestive, and respiratory disorders (Table 1). Compared with nonparticipants, the study sample was more likely to be female, older, and covered by Medicare. Study patients also had greater comorbidity, as indicated by greater severity of illness rating and number of discharge medications.
| Characteristic | Study sample (n = 31,199) | Excluded patients (n = 65,060) |
|---|---|---|
| ||
| Age (years), n (%) | ||
| 34 | 2712 (8.7%) | 9064 (13.9%) |
| 3549 | 5645 (18.1%) | 16,327 (25.1%) |
| 5064 | 8359 (26.8%) | 17,583 (27.0%) |
| 6579 | 9192 (29.5%) | 13,660 (21.0%) |
| 80 | 5291 (17.0%) | 8426 (13.0%) |
| Sex, n (% female) | 17,450 (57.0%) | 34,298 (52.7%) |
| Insurance type, n (%) | ||
| Medicare | 12,455 (39.9%) | 21,255 (32.7%) |
| Medicare HMO | 966 (3.1%) | 1462 (2.2%) |
| HMO (non‐Medicare) | 11,076 (35.5%) | 22,666 (34.8%) |
| Medicaid | 1599 (5.1%) | 4315 (6.6%) |
| Self‐pay/uninsured | 2151 (6.9%) | 7717 (11.9%) |
| Commercial | 2952 (9.5%) | 7645 (11.8%) |
| Severity of illness, n (%) | ||
| Minor | 12,097 (39.1%) | 27,958 (43.6%) |
| Moderate | 14,800 (47.9%) | 29,020 (45.1%) |
| Major/extreme | 4020 (13.0%) | 7353 (11.4%) |
| Length of stay (days), mean (SD) | 3.9 (3.9) | 3.6 (4.0) |
| Discharge medications, n (%) | ||
| 12 | 8100 (26.0%) | 22,060 (33.9%) |
| 35 | 13,299 (42.6%) | 26,654 (41.0%) |
| 6 | 9800 (31.4%) | 16,346 (25.1%) |
| Medication class, n (%) | ||
| Antibiotics | 9927 (31.8%) | 17,721 (27.2%) |
| Analgesics | 9153 (29.3%) | 18,660 (28.7%) |
| Beta‐blockers | 8398 (26.9%) | 14,733 (22.6%) |
| Aspirin | 7028 (22.5%) | 13,040 (20.0%) |
| ACE inhibitors | 6493 (20.8%) | 11,640 (17.9%) |
| Lipid‐lowering agents | 5661 (18.1%) | 9421 (14.5%) |
| Diuretics | 5100 (16.3%) | 8393 (12.9%) |
| Inhalers | 4352 (13.9%) | 7297 (11.2%) |
| Oral hypoglycemics | 3705 (11.9%) | 6819 (10.5%) |
| Steroids | 3521 (11.3%) | 6056 (9.3%) |
| Anticoagulants | 3152 (10.1%) | 4820 (7.4%) |
| Insulins | 2236 (7.2%) | 4589 (7.1%) |
| Angiotensin II receptor blockers | 2034 (6.5%) | 3285 (5.0%) |
| Major diagnostic category, n (%) | ||
| Circulatory | 7971 (25.7%) | 16,963 (26.3%) |
| Digestive | 4990 (16.1%) | 10,211 (15.8%) |
| Respiratory | 4955 (16.0%) | 8482 (13.1%) |
| Nervous | 2469 (8.0%) | 5505 (8.5%) |
| Skin‐subcutaneous‐breast | 1738 (5.6%) | 3664 (5.7%) |
| Renal | 1610 (5.2%) | 3216 (5.0%) |
| Musculoskeletal‐connective | 1357 (4.4%) | 2592 (4.0%) |
| Hepatobiliary‐pancreas | 1273 (4.1%) | 2844 (4.4%) |
| Endocrine‐nutrition‐metabolic | 1195 (3.9%) | 2666 (4.1%) |
| Infectious | 771 (2.5%) | 1429 (2.2%) |
Overall, 7.2% of patients (n = 2253) had prescription‐related issues 4872 hours after hospital discharge. This included not picking up prescribed discharge medications (n = 1797, or 79.8% of issues), not knowing if they were picked up (n = 55 or 2.4%), and admitting to not taking (n = 154, or 6.8%) or not understanding how to take (n = 247, or 11%) medications.
In unadjusted analyses, prescription‐related issues were significantly associated with age, sex, insurance type, severity of illness rating, length of stay, number of discharge medications, certain medication types, and major diagnostic category (Table 2). Except for the youngest patients (age < 35 years), having prescription‐related issues appeared to be inversely related to patient age. Adults 3549 years old had the highest frequency of problems filling or taking medications (9.3%), whereas patients 80 years or older had the lowest frequency (5.6%). Analysis by insurance status showed that patients with Medicaid (12.6%) or self‐pay/uninsured status (11.9%) had significantly higher rates of prescription issues and patients with non‐Medicare HMO or commercial insurance had significantly lower rates (6.1% and 4.9%, respectively). Being prescribed at least 6 medications or taking ACE inhibitors, inhalers, oral hypoglycemics, or insulins was also associated with a higher frequency of prescription‐related problems in unadjusted analyses. Patients prescribed antibiotics or anticoagulants were less likely to report problems in unadjusted analyses.
| Characteristic | Prescription‐related issues, n (%) | Unadjusted OR (95% CI) | P value |
|---|---|---|---|
| |||
| Age | < .0001 | ||
| 34 | 195 (7.2%) | ||
| 3549 | 523 (9.3%) | 1.32 (1.111.56) | |
| 5064 | 646 (7.7%) | 1.08 (0.921.28) | |
| 6579 | 592 (6.4%) | 0.89 (0.751.05) | |
| 80 | 297 (5.6%) | 0.77 (0.640.93) | |
| Sex | .035 | ||
| Male | 906 (6.9%) | ||
| Female | 1310 (7.5%) | 1.10 (1.011.20) | |
| Insurance type | < .0001 | ||
| Medicare | 891 (7.2%) | ||
| Medicare HMO | 86 (8.9%) | 1.27 (1.011.60) | |
| HMO (non‐Medicare) | 674 (6.1%) | 0.84 (0.760.93) | |
| Medicaid | 201 (12.6%) | 1.87 (1.592.20) | |
| Self‐pay/uninsured | 256 (11.9%) | 1.75 (1.512.03) | |
| Commercial | 145 (4.9%) | 0.66 (0.550.79) | |
| Severity of illness | .0007 | ||
| Minor | 794 (6.6%) | ||
| Moderate | 1107 (7.5%) | 1.15 (1.051.27) | |
| Major/extreme | 328 (8.2%) | 1.27 (1.111.45) | |
| Length of stay (days) | 1.01 (1.001.02) | .014 | |
| Discharge medications | < .0001 | ||
| 12 | 526 (6.5%) | ||
| 35 | 928 (7.0%) | 1.08 (0.971.21) | |
| 6 | 799 (8.2%) | 1.28 (1.141.43) | |
| Medication class | |||
| Antibiotics | 639 (6.4%) | 0.84 (0.760.92) | .0003 |
| Analgesics | 656 (7.2%) | 0.99 (0.901.09) | .8 |
| Beta‐blockers | 608 (7.2%) | 1.00 (0.911.11) | .9 |
| Aspirin | 539 (7.7%) | 1.09 (0.981.20) | .1 |
| ACE inhibitors | 520 (8.0%) | 1.15 (1.041.28) | .006 |
| Lipid‐lowering agents | 438 (7.7%) | 1.10 (0.981.22) | .1 |
| Diuretics | 370 (7.3%) | 1.00 (0.901.13) | .9 |
| Inhalers | 373 (8.6%) | 1.25 (1.111.40) | .0002 |
| Oral hypoglycemics | 295 (8.0%) | 1.23 (0.991.28) | .06 |
| Steroids | 261 (7.4%) | 1.03 (0.901.18) | .64 |
| Anticoagulants | 189 (6.0%) | 0.80 (0.690.94) | .005 |
| Insulins | 210 (9.4%) | 1.37 (1.181.59) | < .0001 |
| Angiotensin II receptor blockers | 126 (6.2%) | 0.84 (0.701.01) | .06 |
| Major diagnostic category | |||
| Circulatory | 619 (7.8%) | 1.12 (1.011.23) | .025 |
| Digestive | 398 (8.0%) | 1.14 (1.021.28) | .02 |
| Respiratory | 354 (7.1%) | 0.99 (0.881.11) | .86 |
| Nervous | 188 (7.6%) | 1.07 (0.911.25) | .41 |
| Skin‐subcutaneous‐breast | 71 (4.1%) | 0.53 (0.420.68) | < .0001 |
| Renal | 98 (6.1%) | 0.83 (0.671.02) | .075 |
| Musculoskeletal‐connective | 74 (5.5%) | 0.73 (0.580.93) | .01 |
| Hepatobiliary‐pancreas | 105 (8.3%) | 1.17 (0.951.43) | .14 |
| Endocrine‐nutrition‐metabolic | 93 (7.8%) | 1.09 (0.881.35) | .43 |
| Infectious | 45 (5.8%) | 0.79 (0.591.08) | .14 |
In multivariable models, age, sex, insurance type, severity of illness, number of medications, and certain medication types were independently associated with prescription‐related issues after discharge (Table 3). Seniors reported significantly fewer problems than the youngest patients (6579 years, OR 0.69; 80 years, OR 0.59). Those with Medicare HMOs, Medicaid, or no insurance had more difficulty obtaining and taking prescription medications (OR 1.29, 1.33, and 1.31, respectively), whereas patients with HMO or commercial insurance plans had less difficulty (OR 0.68 and 0.51, respectively). Prescription‐related problems were also more common among women (OR 1.11), patients with higher severity of illness (moderate SOI, OR 1.12; major/extreme SOI, OR 1.23), and those with 6 or more discharge medications (OR 1.35). In adjusted analyses, inhalers were the only type of medication associated with a significantly higher frequency of problems (OR 1.14).
| Characteristic | Adjusted OR (95% CI) | P value |
|---|---|---|
| ||
| Age | < .0001 | |
| 34 | ||
| 3549 | 1.27 (1.071.51) | |
| 5064 | 1.02 (0.861.21) | |
| 6579 | 0.69 (0.570.84) | |
| 80 | 0.59 (0.480.73) | |
| Sex | .03 | |
| Male | ||
| Female | 1.11 (1.011.21) | |
| Insurance type | < .0001 | |
| Medicare | ||
| Medicare HMO | 1.29 (1.021.63) | |
| HMO (non‐Medicare) | 0.68 (0.600.76) | |
| Medicaid | 1.33 (1.111.60) | |
| Self‐pay/uninsured | 1.31 (1.101.56) | |
| Commercial | 0.51 (0.420.62) | |
| Severity of illness | .008 | |
| Minor | ||
| Moderate | 1.12 (1.021.24) | |
| Major/extreme | 1.23 (1.071.42) | |
| Discharge medications | < .0001 | |
| 12 | ||
| 35 | 1.11 (0.991.24) | |
| 6 | 1.35 (1.191.54) | |
| Medication class | ||
| Antibiotic | 0.78 (0.710.86) | < .0001 |
| Inhalers | 1.14 (1.011.29) | .04 |
| Anticoagulants | 0.81 (0.690.95) | .009 |
| Angiotensin II receptor blockers | 0.81 (0.670.98) | .03 |
| Major diagnostic category | ||
| Skin‐subcutaneous‐breast | 0.52 (0.410.67) | < .0001 |
| Musculoskeletal‐connective | 0.74 (0.580.94) | .01 |
Analyses were repeated using only failure to pick up medications as the dependent variable, and results were similar (not shown).
DISCUSSION
In this large multicenter study, 7.2% of patients reported problems obtaining or taking prescribed medications in the 4872 hours following hospital discharge. In about 80% of cases, the problem was failure to pick up discharge medications. Multivariable analyses showed adults 3549 years old; women; patients with Medicare HMO insurance, Medicaid, or no coverage (self‐pay); adults with high severity of illness rating; and those prescribed more than 5 medications or an inhaler had significantly greater odds of prescription‐related issues. Other factors were protective including age 65 or older; HMO or commercial insurance; prescription of antibiotics, anticoagulants, or angiotensin II receptor blockers; and major diagnosis in the skin or musculoskeletal category.
Among all the groups studied, patients with Medicaid or no insurance had the highest frequency of problems filling and taking discharge medications (12.6% and 11.9%, respectively). This was likely related to their having less prescription drug coverage or experiencing other financial constraints. In previous studies, patients have expressed concern over the rising cost of medications and have admitted to not filling prescriptions or stretching out the use of medications to make them last longer because of high out‐of‐pocket costs.5, 16 Prescriptions given at hospital discharge may pose a significant unexpected expense for patients who have a fixed monthly income, rely on samples from outpatient physicians for their medications, or need time to research cost‐saving measures such as discount plans. Greater attention by physicians to knowing the cost of discharge medications, to prescribing only those drugs that are truly necessary, and to discussing cost‐saving strategies with patients may help to minimize financial concerns and improve the ability of patients to fill discharge prescriptions.17
The finding that polypharmacy is associated with greater odds of prescription‐related issues is consistent with research that found that other medication problems such as adverse drug events and nonadherence were more prevalent among patients prescribed more than 5 medications.5, 14 Polypharmacy may have contributed to prescription‐related difficulties in this study by increasing medication costs or by increasing the chance that patients had a problem with at least 1 medication.
The higher frequency of prescription‐related issues among patients prescribed inhalers indicates that this category of medication may be associated with lower fill rates or greater confusion after discharge. This would be concerning, given that repeat exacerbations of obstructive lung disease may lead to rehospitalization. Other medications, including anticoagulants and antibiotics, were associated with a lower frequency of problems. This may have been the result of better education at discharge about the importance of promptly filling prescriptions for these agents in order to avoid a lapse in therapy following acute treatment for thromboembolic disorders or acute infections. It is hoped that a similar educational effort about filling prescriptions for inhalers also would have occurred. These effects have not been noted in prior research and require further substantiation.11, 14 Also, the observed relationships may be related to the size of the data set and the number of variables considered, rather than to a true effect.
The main strength of this study was that the data from which conclusions were drawn came from a large and geographically diverse patient population. However, the study also had several limitations. First, the response rate was relatively low, primarily because this study was a retrospective analysis performed using data collected for clinical and administrative reasons. Patient contact number was missing or incorrect in 16% of cases. Also, because of the narrow window of time during which the survey was administered, the call center, which was following up an average of 370 discharged patients per day, was only able to make 1 or 2 attempts to reach each patient. This contrasts with prospective research on postdischarge medication use such as the study by Forster and colleagues, in which the investigator made up to 20 attempts to reach patients at different times and on different days.11 Despite these efforts, the follow‐up rate was only 69%, underscoring the challenge of data collection in this setting.
The low response rate raises the possibility that the estimated prevalence of prescription‐related issues may be inaccurate. Although highly unlikely, if all the nonresponders had problems with their prescriptions, the true event rate would be 69.9%. Conversely, if none of the nonresponders had problems, the true event rate would be 2.3%. Given the characteristics of responders and nonresponders, however, we expect that a higher survey completion rate would have yielded similar results. Nonresponders had certain characteristics that would be expected to be associated with a higher frequency of prescription‐related issues (younger age, uninsured, covered by Medicaid), but these were balanced by others that would be expected to be associated with a lower frequency of problems (higher percentage of men, lower severity of illness, fewer medications).
Another study limitation concerns the self‐reported nature of the composite outcome variable. After reviewing the structure of the call center data, we chose this composite measure because it conceptually represented difficulties in obtaining or taking prescribed discharge medications. When we analyzed results using only the most prevalent component of this composite variable, the results were similar. However, all these findings could have been influenced by social desirability bias. Patients may have underreported not filling their discharge prescriptions and also may not acknowledged difficulties in understanding how to take the medications. We would therefore expect the true prevalence of prescription‐related concerns after hospital discharge to be higher than that found in this study.
These limitations notwithstanding, the findings from this large, multicenter study show that prescription‐related issues are common after hospital discharge and, further, that they usually take the form of not filling discharge prescriptions. The highest‐risk patients appear to be those without insurance and those covered by Medicaid or Medicare HMOs, as well as adults age 3549, patients prescribed 6 or more medications, and patients with a higher severity of illness. When preparing patients to leave the hospital, physicians and other health care providers should strive to identify financial, behavioral, and other barriers to proper medication use so that appropriate assistance or counseling may be offered prior to discharge.18, 19 Close follow‐up of patients by telephone may also be a helpful approach to promptly identifying prescription‐related issues and other problems so that providers can intervene before more serious complications arise.
The period immediately following hospital discharge is a vulnerable time for patients, who must assume responsibilities for their own care as they return home.1 The process of hospital discharge may be a rushed event, and patients often have difficulty understanding and following their postdischarge treatment plan.2, 3 Medication‐related problems after hospital discharge, which include patients not filling or refilling prescriptions,46 not understanding how to take medications,2, 3 showing discrepancies between what they are and what they should be taking,79 and having adverse drug events,1012 are a major cause of morbidity and mortality.13
According to prior studies, elderly patients and patients taking more than 5 medications are more likely to experience problems with their medications.5, 14 Adverse drug events are more common with certain high‐risk drugs, including cardiovascular agents, anticoagulants, insulin, antibiotics, and steroids.11, 14, 15 Beyond this, however, patient management of prescription medications after hospital discharge has not been well described. In particular, studies in the community setting and in the immediate postdischarge period are needed.
We conducted a large observational study of patients at 170 community hospitals in order to examine the frequency of prescription‐related issues 4872 hours after hospital discharge. These issues included problems with filling or taking medications prescribed at discharge. We hypothesized that age and number of medications would be independently associated with prescription‐related problems. We also examined the effects of other factors, including insurance type, length of stay, severity of illness (SOI), clinical diagnosis, and use of certain high‐risk drugs.
METHODS
Setting and Population
Information for the present analysis consisted of deidentified clinical, administrative, and survey data provided by a national hospitalist management group, IPCThe Hospitalist Company. At the time of the study, IPC employed more than 300 physicians working at 170 community hospitals in 18 regions across the United States. As part of their daily patient management, physicians entered clinical and administrative data into a proprietary Web‐based program. At discharge, physicians completed discharge summaries in the same program. These summaries were faxed to the outpatient physicians scheduled to see the patients and were transmitted electronically to a call center. The call center attempted to contact all patients at home to assess their clinical status and satisfaction and to assist with any postdischarge needs. The call center staff made up to 2 attempts to reach each patient by telephone within 3 days of discharge. Patients who were reached were interviewed using a scripted survey. Any identified medical needs were addressed in a separate follow‐up call by a nurse.
Patients were included in this analysis if they were at least 18 years old, were treated by an IPC hospitalist, had been discharged between January 1, 2005, and December 31, 2005, and were successfully surveyed by telephone 4872 hours after hospital discharge. If patients had more than 1 discharge during the study period, only the first survey and its corresponding hospital stay were included. The analytic plan was approved by the Emory University Institutional Review Board.
Data Collection
Hospitalists recorded the age, sex, and insurance coverage of each hospitalized patient and noted the discharge diagnoses and medications on the discharge summary. Primary diagnosis and length of stay were determined from hospitalist billing data, which were entered daily into the Web‐based program. Each patient's severity of illness was classified as minor, moderate, major, or extreme using a commercially available program (3M Health Information Systems) that considered patient age, primary diagnosis, diagnosis‐related group (DRG), and nonoperating room procedures.
A common patient identifier code linked these data with patient‐reported information obtained from the call center. Patients indicated whether they picked up their prescribed medications and if they had any trouble understanding how to take their medications. For the present analysis, patients were considered to have a prescription‐related issue if they had problems filling or taking medications prescribed at discharge, a composite variable defined as including not picking up discharge medications, not knowing whether discharge medications had been picked up, not taking discharge medications, or not understanding how to take discharge medications.
Statistical Analysis
Initial analyses included construction of frequency tables to estimate the distribution of prescription‐related issues across patient demographic characteristics, insurance type, clinical diagnosis, and number of medications, as well as among users of certain high‐risk classes of medication. Some continuous variables, such as age and number of medications, were categorized (age into clinically relevant categories, number of medications into tertiles). Separate variables were created for clinical diagnoses by mapping DRGs to 26 major diagnostic categories (MDCs) so that comparisons could be made based on the frequency of prescription‐related issues for those with a primary diagnosis pertaining to a particular organ system versus those with a primary diagnosis outside that organ system. The 10 most common MDCs were circulatory, digestive, respiratory, nervous, skin‐subcutaneous‐breast, kidney‐urinary, musculoskeletal‐connective, hepatobiliary‐pancreas, endocrine‐nutrition‐metabolic, and infectious. The categories of the severity of illness variable were reduced to 3 by combining major and extreme because there were so few in the extreme category.
Unadjusted odds ratios were calculated based on a logistic regression model relating any prescription‐related issues to each possible covariate 1 at a time (ie, not adjusted for any of the other covariates). Adjusted odds ratios were obtained through stepwise building of a logistic regression model. Initially, all possible covariates were entered into the model, and the model was then reduced using Wald test results to assess the significance of dropped parameters. All analyses were conducted using SAS version 9.1 (Cary, NC) and a significance level of 0.05.
RESULTS
In 2005, there were 104,506 eligible adult hospital discharges, corresponding to 96,179 patients. Excluding discharged patients who could not be contacted by the call center or who refused to complete the survey (n = 67,084), multiple surveys of the same patient (n = 3156), and surveys with insufficient data to determine whether there were prescription‐related issues (n = 3067) left 31,199 patients available for analysis (effective response rate 32.4%).
More than half the participants (57.0%) were women, and the mean age was 61.1 years (SD 17.8 years). The median number of discharge medications was 4 (range 128). The most frequently prescribed drugs were antibiotics and analgesics, followed by several cardiovascular drug classes (Table 1). About 60% of the primary diagnoses were of circulatory, digestive, and respiratory disorders (Table 1). Compared with nonparticipants, the study sample was more likely to be female, older, and covered by Medicare. Study patients also had greater comorbidity, as indicated by greater severity of illness rating and number of discharge medications.
| Characteristic | Study sample (n = 31,199) | Excluded patients (n = 65,060) |
|---|---|---|
| ||
| Age (years), n (%) | ||
| 34 | 2712 (8.7%) | 9064 (13.9%) |
| 3549 | 5645 (18.1%) | 16,327 (25.1%) |
| 5064 | 8359 (26.8%) | 17,583 (27.0%) |
| 6579 | 9192 (29.5%) | 13,660 (21.0%) |
| 80 | 5291 (17.0%) | 8426 (13.0%) |
| Sex, n (% female) | 17,450 (57.0%) | 34,298 (52.7%) |
| Insurance type, n (%) | ||
| Medicare | 12,455 (39.9%) | 21,255 (32.7%) |
| Medicare HMO | 966 (3.1%) | 1462 (2.2%) |
| HMO (non‐Medicare) | 11,076 (35.5%) | 22,666 (34.8%) |
| Medicaid | 1599 (5.1%) | 4315 (6.6%) |
| Self‐pay/uninsured | 2151 (6.9%) | 7717 (11.9%) |
| Commercial | 2952 (9.5%) | 7645 (11.8%) |
| Severity of illness, n (%) | ||
| Minor | 12,097 (39.1%) | 27,958 (43.6%) |
| Moderate | 14,800 (47.9%) | 29,020 (45.1%) |
| Major/extreme | 4020 (13.0%) | 7353 (11.4%) |
| Length of stay (days), mean (SD) | 3.9 (3.9) | 3.6 (4.0) |
| Discharge medications, n (%) | ||
| 12 | 8100 (26.0%) | 22,060 (33.9%) |
| 35 | 13,299 (42.6%) | 26,654 (41.0%) |
| 6 | 9800 (31.4%) | 16,346 (25.1%) |
| Medication class, n (%) | ||
| Antibiotics | 9927 (31.8%) | 17,721 (27.2%) |
| Analgesics | 9153 (29.3%) | 18,660 (28.7%) |
| Beta‐blockers | 8398 (26.9%) | 14,733 (22.6%) |
| Aspirin | 7028 (22.5%) | 13,040 (20.0%) |
| ACE inhibitors | 6493 (20.8%) | 11,640 (17.9%) |
| Lipid‐lowering agents | 5661 (18.1%) | 9421 (14.5%) |
| Diuretics | 5100 (16.3%) | 8393 (12.9%) |
| Inhalers | 4352 (13.9%) | 7297 (11.2%) |
| Oral hypoglycemics | 3705 (11.9%) | 6819 (10.5%) |
| Steroids | 3521 (11.3%) | 6056 (9.3%) |
| Anticoagulants | 3152 (10.1%) | 4820 (7.4%) |
| Insulins | 2236 (7.2%) | 4589 (7.1%) |
| Angiotensin II receptor blockers | 2034 (6.5%) | 3285 (5.0%) |
| Major diagnostic category, n (%) | ||
| Circulatory | 7971 (25.7%) | 16,963 (26.3%) |
| Digestive | 4990 (16.1%) | 10,211 (15.8%) |
| Respiratory | 4955 (16.0%) | 8482 (13.1%) |
| Nervous | 2469 (8.0%) | 5505 (8.5%) |
| Skin‐subcutaneous‐breast | 1738 (5.6%) | 3664 (5.7%) |
| Renal | 1610 (5.2%) | 3216 (5.0%) |
| Musculoskeletal‐connective | 1357 (4.4%) | 2592 (4.0%) |
| Hepatobiliary‐pancreas | 1273 (4.1%) | 2844 (4.4%) |
| Endocrine‐nutrition‐metabolic | 1195 (3.9%) | 2666 (4.1%) |
| Infectious | 771 (2.5%) | 1429 (2.2%) |
Overall, 7.2% of patients (n = 2253) had prescription‐related issues 4872 hours after hospital discharge. This included not picking up prescribed discharge medications (n = 1797, or 79.8% of issues), not knowing if they were picked up (n = 55 or 2.4%), and admitting to not taking (n = 154, or 6.8%) or not understanding how to take (n = 247, or 11%) medications.
In unadjusted analyses, prescription‐related issues were significantly associated with age, sex, insurance type, severity of illness rating, length of stay, number of discharge medications, certain medication types, and major diagnostic category (Table 2). Except for the youngest patients (age < 35 years), having prescription‐related issues appeared to be inversely related to patient age. Adults 3549 years old had the highest frequency of problems filling or taking medications (9.3%), whereas patients 80 years or older had the lowest frequency (5.6%). Analysis by insurance status showed that patients with Medicaid (12.6%) or self‐pay/uninsured status (11.9%) had significantly higher rates of prescription issues and patients with non‐Medicare HMO or commercial insurance had significantly lower rates (6.1% and 4.9%, respectively). Being prescribed at least 6 medications or taking ACE inhibitors, inhalers, oral hypoglycemics, or insulins was also associated with a higher frequency of prescription‐related problems in unadjusted analyses. Patients prescribed antibiotics or anticoagulants were less likely to report problems in unadjusted analyses.
| Characteristic | Prescription‐related issues, n (%) | Unadjusted OR (95% CI) | P value |
|---|---|---|---|
| |||
| Age | < .0001 | ||
| 34 | 195 (7.2%) | ||
| 3549 | 523 (9.3%) | 1.32 (1.111.56) | |
| 5064 | 646 (7.7%) | 1.08 (0.921.28) | |
| 6579 | 592 (6.4%) | 0.89 (0.751.05) | |
| 80 | 297 (5.6%) | 0.77 (0.640.93) | |
| Sex | .035 | ||
| Male | 906 (6.9%) | ||
| Female | 1310 (7.5%) | 1.10 (1.011.20) | |
| Insurance type | < .0001 | ||
| Medicare | 891 (7.2%) | ||
| Medicare HMO | 86 (8.9%) | 1.27 (1.011.60) | |
| HMO (non‐Medicare) | 674 (6.1%) | 0.84 (0.760.93) | |
| Medicaid | 201 (12.6%) | 1.87 (1.592.20) | |
| Self‐pay/uninsured | 256 (11.9%) | 1.75 (1.512.03) | |
| Commercial | 145 (4.9%) | 0.66 (0.550.79) | |
| Severity of illness | .0007 | ||
| Minor | 794 (6.6%) | ||
| Moderate | 1107 (7.5%) | 1.15 (1.051.27) | |
| Major/extreme | 328 (8.2%) | 1.27 (1.111.45) | |
| Length of stay (days) | 1.01 (1.001.02) | .014 | |
| Discharge medications | < .0001 | ||
| 12 | 526 (6.5%) | ||
| 35 | 928 (7.0%) | 1.08 (0.971.21) | |
| 6 | 799 (8.2%) | 1.28 (1.141.43) | |
| Medication class | |||
| Antibiotics | 639 (6.4%) | 0.84 (0.760.92) | .0003 |
| Analgesics | 656 (7.2%) | 0.99 (0.901.09) | .8 |
| Beta‐blockers | 608 (7.2%) | 1.00 (0.911.11) | .9 |
| Aspirin | 539 (7.7%) | 1.09 (0.981.20) | .1 |
| ACE inhibitors | 520 (8.0%) | 1.15 (1.041.28) | .006 |
| Lipid‐lowering agents | 438 (7.7%) | 1.10 (0.981.22) | .1 |
| Diuretics | 370 (7.3%) | 1.00 (0.901.13) | .9 |
| Inhalers | 373 (8.6%) | 1.25 (1.111.40) | .0002 |
| Oral hypoglycemics | 295 (8.0%) | 1.23 (0.991.28) | .06 |
| Steroids | 261 (7.4%) | 1.03 (0.901.18) | .64 |
| Anticoagulants | 189 (6.0%) | 0.80 (0.690.94) | .005 |
| Insulins | 210 (9.4%) | 1.37 (1.181.59) | < .0001 |
| Angiotensin II receptor blockers | 126 (6.2%) | 0.84 (0.701.01) | .06 |
| Major diagnostic category | |||
| Circulatory | 619 (7.8%) | 1.12 (1.011.23) | .025 |
| Digestive | 398 (8.0%) | 1.14 (1.021.28) | .02 |
| Respiratory | 354 (7.1%) | 0.99 (0.881.11) | .86 |
| Nervous | 188 (7.6%) | 1.07 (0.911.25) | .41 |
| Skin‐subcutaneous‐breast | 71 (4.1%) | 0.53 (0.420.68) | < .0001 |
| Renal | 98 (6.1%) | 0.83 (0.671.02) | .075 |
| Musculoskeletal‐connective | 74 (5.5%) | 0.73 (0.580.93) | .01 |
| Hepatobiliary‐pancreas | 105 (8.3%) | 1.17 (0.951.43) | .14 |
| Endocrine‐nutrition‐metabolic | 93 (7.8%) | 1.09 (0.881.35) | .43 |
| Infectious | 45 (5.8%) | 0.79 (0.591.08) | .14 |
In multivariable models, age, sex, insurance type, severity of illness, number of medications, and certain medication types were independently associated with prescription‐related issues after discharge (Table 3). Seniors reported significantly fewer problems than the youngest patients (6579 years, OR 0.69; 80 years, OR 0.59). Those with Medicare HMOs, Medicaid, or no insurance had more difficulty obtaining and taking prescription medications (OR 1.29, 1.33, and 1.31, respectively), whereas patients with HMO or commercial insurance plans had less difficulty (OR 0.68 and 0.51, respectively). Prescription‐related problems were also more common among women (OR 1.11), patients with higher severity of illness (moderate SOI, OR 1.12; major/extreme SOI, OR 1.23), and those with 6 or more discharge medications (OR 1.35). In adjusted analyses, inhalers were the only type of medication associated with a significantly higher frequency of problems (OR 1.14).
| Characteristic | Adjusted OR (95% CI) | P value |
|---|---|---|
| ||
| Age | < .0001 | |
| 34 | ||
| 3549 | 1.27 (1.071.51) | |
| 5064 | 1.02 (0.861.21) | |
| 6579 | 0.69 (0.570.84) | |
| 80 | 0.59 (0.480.73) | |
| Sex | .03 | |
| Male | ||
| Female | 1.11 (1.011.21) | |
| Insurance type | < .0001 | |
| Medicare | ||
| Medicare HMO | 1.29 (1.021.63) | |
| HMO (non‐Medicare) | 0.68 (0.600.76) | |
| Medicaid | 1.33 (1.111.60) | |
| Self‐pay/uninsured | 1.31 (1.101.56) | |
| Commercial | 0.51 (0.420.62) | |
| Severity of illness | .008 | |
| Minor | ||
| Moderate | 1.12 (1.021.24) | |
| Major/extreme | 1.23 (1.071.42) | |
| Discharge medications | < .0001 | |
| 12 | ||
| 35 | 1.11 (0.991.24) | |
| 6 | 1.35 (1.191.54) | |
| Medication class | ||
| Antibiotic | 0.78 (0.710.86) | < .0001 |
| Inhalers | 1.14 (1.011.29) | .04 |
| Anticoagulants | 0.81 (0.690.95) | .009 |
| Angiotensin II receptor blockers | 0.81 (0.670.98) | .03 |
| Major diagnostic category | ||
| Skin‐subcutaneous‐breast | 0.52 (0.410.67) | < .0001 |
| Musculoskeletal‐connective | 0.74 (0.580.94) | .01 |
Analyses were repeated using only failure to pick up medications as the dependent variable, and results were similar (not shown).
DISCUSSION
In this large multicenter study, 7.2% of patients reported problems obtaining or taking prescribed medications in the 4872 hours following hospital discharge. In about 80% of cases, the problem was failure to pick up discharge medications. Multivariable analyses showed adults 3549 years old; women; patients with Medicare HMO insurance, Medicaid, or no coverage (self‐pay); adults with high severity of illness rating; and those prescribed more than 5 medications or an inhaler had significantly greater odds of prescription‐related issues. Other factors were protective including age 65 or older; HMO or commercial insurance; prescription of antibiotics, anticoagulants, or angiotensin II receptor blockers; and major diagnosis in the skin or musculoskeletal category.
Among all the groups studied, patients with Medicaid or no insurance had the highest frequency of problems filling and taking discharge medications (12.6% and 11.9%, respectively). This was likely related to their having less prescription drug coverage or experiencing other financial constraints. In previous studies, patients have expressed concern over the rising cost of medications and have admitted to not filling prescriptions or stretching out the use of medications to make them last longer because of high out‐of‐pocket costs.5, 16 Prescriptions given at hospital discharge may pose a significant unexpected expense for patients who have a fixed monthly income, rely on samples from outpatient physicians for their medications, or need time to research cost‐saving measures such as discount plans. Greater attention by physicians to knowing the cost of discharge medications, to prescribing only those drugs that are truly necessary, and to discussing cost‐saving strategies with patients may help to minimize financial concerns and improve the ability of patients to fill discharge prescriptions.17
The finding that polypharmacy is associated with greater odds of prescription‐related issues is consistent with research that found that other medication problems such as adverse drug events and nonadherence were more prevalent among patients prescribed more than 5 medications.5, 14 Polypharmacy may have contributed to prescription‐related difficulties in this study by increasing medication costs or by increasing the chance that patients had a problem with at least 1 medication.
The higher frequency of prescription‐related issues among patients prescribed inhalers indicates that this category of medication may be associated with lower fill rates or greater confusion after discharge. This would be concerning, given that repeat exacerbations of obstructive lung disease may lead to rehospitalization. Other medications, including anticoagulants and antibiotics, were associated with a lower frequency of problems. This may have been the result of better education at discharge about the importance of promptly filling prescriptions for these agents in order to avoid a lapse in therapy following acute treatment for thromboembolic disorders or acute infections. It is hoped that a similar educational effort about filling prescriptions for inhalers also would have occurred. These effects have not been noted in prior research and require further substantiation.11, 14 Also, the observed relationships may be related to the size of the data set and the number of variables considered, rather than to a true effect.
The main strength of this study was that the data from which conclusions were drawn came from a large and geographically diverse patient population. However, the study also had several limitations. First, the response rate was relatively low, primarily because this study was a retrospective analysis performed using data collected for clinical and administrative reasons. Patient contact number was missing or incorrect in 16% of cases. Also, because of the narrow window of time during which the survey was administered, the call center, which was following up an average of 370 discharged patients per day, was only able to make 1 or 2 attempts to reach each patient. This contrasts with prospective research on postdischarge medication use such as the study by Forster and colleagues, in which the investigator made up to 20 attempts to reach patients at different times and on different days.11 Despite these efforts, the follow‐up rate was only 69%, underscoring the challenge of data collection in this setting.
The low response rate raises the possibility that the estimated prevalence of prescription‐related issues may be inaccurate. Although highly unlikely, if all the nonresponders had problems with their prescriptions, the true event rate would be 69.9%. Conversely, if none of the nonresponders had problems, the true event rate would be 2.3%. Given the characteristics of responders and nonresponders, however, we expect that a higher survey completion rate would have yielded similar results. Nonresponders had certain characteristics that would be expected to be associated with a higher frequency of prescription‐related issues (younger age, uninsured, covered by Medicaid), but these were balanced by others that would be expected to be associated with a lower frequency of problems (higher percentage of men, lower severity of illness, fewer medications).
Another study limitation concerns the self‐reported nature of the composite outcome variable. After reviewing the structure of the call center data, we chose this composite measure because it conceptually represented difficulties in obtaining or taking prescribed discharge medications. When we analyzed results using only the most prevalent component of this composite variable, the results were similar. However, all these findings could have been influenced by social desirability bias. Patients may have underreported not filling their discharge prescriptions and also may not acknowledged difficulties in understanding how to take the medications. We would therefore expect the true prevalence of prescription‐related concerns after hospital discharge to be higher than that found in this study.
These limitations notwithstanding, the findings from this large, multicenter study show that prescription‐related issues are common after hospital discharge and, further, that they usually take the form of not filling discharge prescriptions. The highest‐risk patients appear to be those without insurance and those covered by Medicaid or Medicare HMOs, as well as adults age 3549, patients prescribed 6 or more medications, and patients with a higher severity of illness. When preparing patients to leave the hospital, physicians and other health care providers should strive to identify financial, behavioral, and other barriers to proper medication use so that appropriate assistance or counseling may be offered prior to discharge.18, 19 Close follow‐up of patients by telephone may also be a helpful approach to promptly identifying prescription‐related issues and other problems so that providers can intervene before more serious complications arise.
- ,.Lost in transition: challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;141:533–536.
- ,,, et al.Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan.Arch Intern Med.1997;157:1026–1030.
- ,.Patients' understanding of their treatment plans and diagnosis at discharge.Mayo Clin Proc2005;80:991–994.
- ,,.Medication adherence in elderly patients receiving home health services following hospital discharge.Ann Pharmacother.2001;35:539–545.
- ,.Uncovering a multitude of sins: medication management in the home post acute hospitalisation among the chronically ill.Aust N Z J Med.1999;29(2):220–227.
- ,,, et al.Impact of medication therapy discontinuation on mortality after myocardial infarction.Arch Intern Med.2006;166:1842–1847.
- ,,.A new tool for identifying discrepancies in postacute medications for community‐dwelling older adults.Am J Geriatr Pharmacother.2004;2(2):141–147.
- ,,,.Posthospital medication discrepancies: prevalence and contributing factors.Arch Intern Med.2005;165:1842–1847.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- ,,, et al.Adverse events among medical patients after discharge from hospital.Can Med Assoc J.2004;170:345–349.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20:317–323.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med2003;18:646–651.
- ,,, et al.Adverse drug events in ambulatory care.N Engl J Med.2003;348:1556–1564.
- MA Coalition for the Prevention of Medical Errors. Reconciling medications. Recommended practices. Available at: http://www.macoalition.org/documents/RecMedPractices.pdf. Accessed July 27,2005.
- ,,.Cost‐related medication underuse: do patients with chronic illnesses tell their doctors?Arch Intern Med.2004;164:1749–1755.
- ,,,,,.Physician communication about the cost and acquisition of newly prescribed medications.Am J Manag Care.2006;12:657–664.
- ,,.Medication education of acutely hospitalized older patients.J Gen Intern Med.1999;14:610–616.
- ,,.Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure.Med Care.2005;43:246–255.
- ,.Lost in transition: challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;141:533–536.
- ,,, et al.Patient‐physician communication at hospital discharge and patients' understanding of the postdischarge treatment plan.Arch Intern Med.1997;157:1026–1030.
- ,.Patients' understanding of their treatment plans and diagnosis at discharge.Mayo Clin Proc2005;80:991–994.
- ,,.Medication adherence in elderly patients receiving home health services following hospital discharge.Ann Pharmacother.2001;35:539–545.
- ,.Uncovering a multitude of sins: medication management in the home post acute hospitalisation among the chronically ill.Aust N Z J Med.1999;29(2):220–227.
- ,,, et al.Impact of medication therapy discontinuation on mortality after myocardial infarction.Arch Intern Med.2006;166:1842–1847.
- ,,.A new tool for identifying discrepancies in postacute medications for community‐dwelling older adults.Am J Geriatr Pharmacother.2004;2(2):141–147.
- ,,,.Posthospital medication discrepancies: prevalence and contributing factors.Arch Intern Med.2005;165:1842–1847.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- ,,, et al.Adverse events among medical patients after discharge from hospital.Can Med Assoc J.2004;170:345–349.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20:317–323.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med2003;18:646–651.
- ,,, et al.Adverse drug events in ambulatory care.N Engl J Med.2003;348:1556–1564.
- MA Coalition for the Prevention of Medical Errors. Reconciling medications. Recommended practices. Available at: http://www.macoalition.org/documents/RecMedPractices.pdf. Accessed July 27,2005.
- ,,.Cost‐related medication underuse: do patients with chronic illnesses tell their doctors?Arch Intern Med.2004;164:1749–1755.
- ,,,,,.Physician communication about the cost and acquisition of newly prescribed medications.Am J Manag Care.2006;12:657–664.
- ,,.Medication education of acutely hospitalized older patients.J Gen Intern Med.1999;14:610–616.
- ,,.Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure.Med Care.2005;43:246–255.
Copyright © 2008 Society of Hospital Medicine