User login
Quality of Life of Children with NI after Fundoplication for GERD
Aspiration pneumonia is the most common cause of death in children with severe neurological impairment (NI).13 For several reasons (eg, improved survival of extremely low‐birth‐weight infants, technological advances, and changing societal attitudes), the number of children with severe NI is increasing. Many children with severe NI have dysfunctional swallowing and gastroesophageal reflux disease (GERD).46 This combination places them at high risk for recurrent aspiration that, in turn, leads to aspiration pneumonia, repeated hospitalization, respiratory failure, compromised quality of life, and death.7, 8
The most common treatment approach for the combination of dysfunctional swallowing and GERD is surgical fundoplication with a gastrostomy feeding tube. Fundoplication is the third most common procedure performed in children by pediatric surgeons in the United States.9 Fifty percent of the children who receive a fundoplication have neurological impairment.10, 11 The goals of the surgery to treat GERD unresponsive to medical management are to reduce the risk of aspiration pneumonia, improve nutritional status, and improve the quality of life of the children and their families. However, few prospective longitudinal studies have determined whether the quality of life of the children or their caregivers actually improves over time.
The importance of caregiver and child quality of life is increasingly recognized as a critical outcome of any intervention in this population.12, 13 Previous studies of fundoplication in children with NI, GERD, and dysfunctional swallowing reported surgical mortality rates between 1% and 3% and other complications between 4% and 39%, reflecting the medical fragility of these children.5, 1418 Some of these studies had longitudinal follow‐up and reported long‐term data. Recurrence of symptoms was reported in up to 56% of patients, recurrence of AP in up to 39%, further surgical procedures in up to 19%, and mortality in up to 17%.14, 1921 Few case series of children with neurological impairment who have had a fundoplication have addressed child and caregiver quality of life following either a fundoplication or placement of a feeding tube.2224 In their study of 16 patients who had a fundoplication and gastrostomy tube placed, Tawfik et al. found improvements in children's happiness, ease of giving medicines, and time to devote to other children. Sullivan et al. found improvement in caregiver quality of life following placement of a gastrostomy tube in a child; however, they did not specifically identify those children who had been treated with a fundoplication. In their retrospective study, O'Neill et al. found improved child and caregiver quality of life following a fundoplication. Collectively, these studies have found that parents report improvement of both their own and their child's quality of life after either intervention. However, not having baseline measurements, not controlling for degree of functional impairment of the children, small sample sizes, and large loss to follow‐up limit the utility of these studies. In this ongoing, long‐term prospective longitudinal study, we report the initial impact of a fundoplication on the quality of life of both children and their caregivers.
The primary objective of this study was to determine change over time in the quality of life of children with neurological impairment who received surgical treatment of their GERD and of the caregivers of these children, controlling for the degree of functional impairment of the children. We hypothesized that child and caregiver quality of life would both improve after primary fundoplication and gastrostomy tube placement. Secondary objectives included describing rates of complications in this population.
METHODS
Setting and Study Population
We enrolled patients from newborn to 18 years of age who had a diagnosis compatible with neurological impairment and who received their first fundoplication for GERD between January 2005 and July 2006 at Primary Children's Medical Center (PCMC), in Salt Lake City, Utah. PCMC is a 232‐bed children's hospital in the Intermountain West owned and operated by Intermountain Healthcare, Inc., a large vertically integrated health care delivery system that serves as both the primary hospital for Salt Lake County and as the tertiary‐care hospital for 4 additional states (Wyoming, Nevada, Idaho, and Montana).25 Patients who had a previous gastrojejunal feeding tube were excluded as were patients who had a previous fundoplication, as these procedures may have biased their reported quality of life, our main outcome measure.
Patients were included in the study if they had GERD (based on clinical history or testing) that had been refractory to medical management (defined as continued gastroesophageal reflux symptoms despite antireflux medications). GERD was defined using the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) criteria.26 These include: the presence of clinical symptoms and at least 1 abnormal result from an upper gastrointestinal x‐ray series (recognizing that this test is neither sensitive nor specific for reflux), pH probe, upper gastrointestinal endoscopy with biopsy, nuclear medicine scan, or a modified barium swallow. As this was a prospective observational study, physicians were allowed to order testing as their practice dictated. Patients were excluded if they had neurological impairment but lacked objective documentation of GERD using the NASPGHAN recommendations, unless there were obvious clinical indications such as witnessed vomiting and aspiration (N = 3). No patient received a prophylactic fundoplication (fundoplication without documented GERD).
Study Design
This is an ongoing prospective longitudinal study. Patients who had a first fundoplication at PCMC were identified by the surgical service, with weekly lists shared with the research team. Patients were approached by a research assistant during that initial hospitalization to see if they met inclusion criteria for the study using data from the medical records and surgical team when necessary.
Data Variables and Sources
Indications for the fundoplication, performance and results of diagnostic testing for GERD, complications of the fundoplication, and reasons for the neurological impairment were obtained through review of the electronic and paper medical records. Mortality data, subsequent emergency department visits, and admissions to the hospital were obtained using Intermountain Healthcare's electronic data warehouse, which merges clinical, financial, and administrative data including the Utah Vital Statistics database.
Neurological impairment was defined from 2 sources: (1) clinical diagnoses as identified by providers and (2) International Classification of Diseases Codes Modified, version 9 (ICD‐9 CM) identified a priori as indicating neurological impairment.
Instruments and Study Outcomes
Functional status was measured using the WeeFIM. This instrument has been tested and shown to be valid and reliable for children more than 6 months old with neurodevelopmental disabilities including spina bifida and Down syndrome.2732 WeeFIM is a self‐administered parent instrument composed of 18 items and 6 domains (self‐care, sphincter control, transfers, locomotion, communication, and social cognition).33 The WeeFIM allows patients to be stratified into areas of function from severely impaired to normal.
The primary outcome was child quality of life as measured by the Child Health Questionnaire Parental Form 50 (CHQ‐PF50). Caregiver quality of life was measured using the Short‐Form Health Status Survey (SF‐36) and Parenting Stress Index/Short Form (PSI/SF). The CHQ‐PF50 is a self‐administered parent questionnaire of 50 questions that measures 6 domains, including physical function and abilities, pain and discomfort, general health perception, behavior, temperament and moods, and satisfaction with growth and development.34 This instrument has been tested for validity and reliability in children with cerebral palsy.35 The SF‐36 is a widely accepted measure of health status that measures 8 domains of health: physical functioning, role limitations due to physical problems, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health. The SF‐36 has been well studied and has been used to measure the effect on a caregiver's quality of life associated with caring for a chronically ill child with significant medical problems.36, 37 Higher scores in each domain of both the CHQPF50 and the SF‐36 reflect better quality of life. Caregiver stress was measured using the PSI/SF (Psychological Assessment Resources Inc, Odessa, FL).38 In the PSI/SF a parent rates the parentchild dyad on 36 items that are summarized in 3 subscales: parental distress, parentchild dysfunctional interaction, and difficult child. A parent scores each item as strongly agree, agree, not sure, disagree, or strongly disagree. The sum of the 3 subscale scores is the total stress score. Higher scores denote a greater degree of stress. The PSI/SF has been validated in several studies for caregivers of children with chronic diseases.3943
The CHQ‐PF50, SF‐36, and PSI/SF questionnaires and the WeeFIM functional status measure were administered to each study patient and caregiver in person at enrollment (baseline) and by mail 1 month after fundoplication. A follow‐up postcard reminder was mailed 1 week after the initial mailing. Second and final mailings were sent to nonresponders 3 and 5 weeks, respectively, after the initial mailing.
Secondary outcomes included rates of complications including failure of the fundoplication. Complications were defined as a subsequent emergency department visit, hospitalization, or death related to the surgery, gastrostomy tube, or aspiration pneumonia. Failures were defined as a second fundoplication or the insertion of a gastrojejunal feeding tube as nonsurgical management of recurrent GERD and/or paraesophageal hernia. Secondary outcomes were followed from the time of the first fundoplication until 1 month after surgery.
Analyses
The differential effect of the fundoplication on the quality of life measures was assessed and quantified through statistical analysis. Because the primary interest was to measure change in baseline characteristics over time, repeated‐measures models were used to compare the group relative to changes in functional status. In particular, changes from baseline values were modeled 1 month after the procedure. The Kenward‐Roger approximation of degrees of freedom was used to compute P values from the overall tests.44 Repeated‐measures models were fit to the data using restricted maximum likelihood estimation. An autoregressive covariance matrix was assumed for the multiple measurements of each individual, thus limiting the number of restrictions forced by the model on the data. The repeated‐measures models used all the available data on participants, including those who dropped out of the study. To obtain the most accurate comparison of the study group, the covariate of functional status at baseline was taken into account in the fitted models. Statistical analyses were performed with SAS statistical software (version 9.13; SAS Institute, Cary NC). Student t tests were performed for comparison of means of the quality‐of‐life domains for the study cohort compared to either the general population or specific groups of patients for comparative purposes.
The study was approved by the institutional review boards of the University of Utah Health Sciences Center and Primary Children's Medical Center.
RESULTS
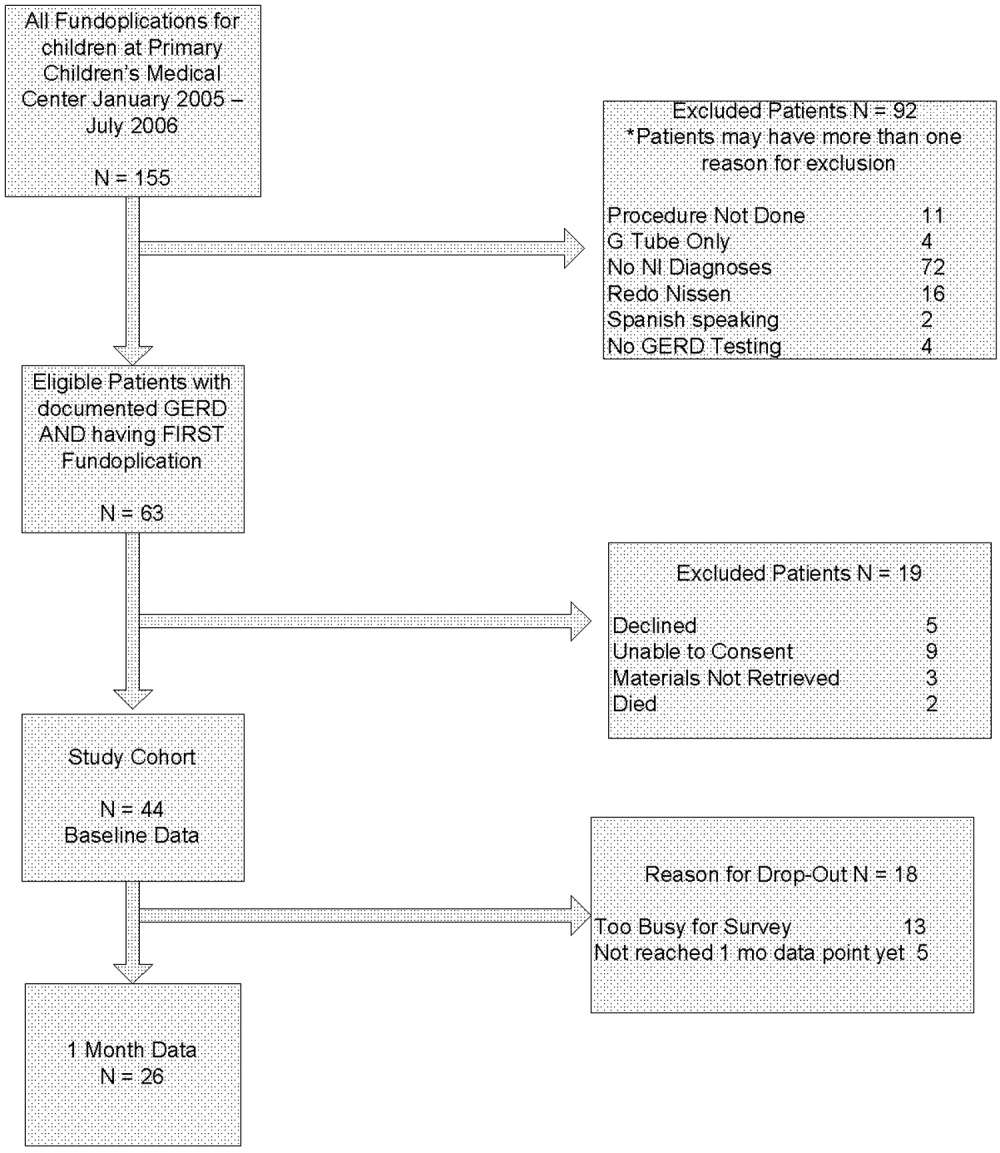
Sixty‐three children met eligibility criteria. Forty‐four families (70%) initially agreed to participate in the study and completed the baseline questionnaires (see Fig. 1). The mean age of the children was 2.2 years. Twenty‐six parents of children completed the 1‐month postfundoplication quality‐of‐life questionnaires. Thirteen patients were lost to follow‐up, 5 of whom had not reached the 1‐month postfundoplication time point. The median WeeFIM (functional status) score of the whole group was 31.2 (95% confidence interval [CI] 11‐71) compared with a childhood matched‐age norm of 83 (95% CI 60‐110), P = .001. WeeFIM scores did not change significantly from baseline to 1 month, P = .98 (Kruskall‐Wallis test).
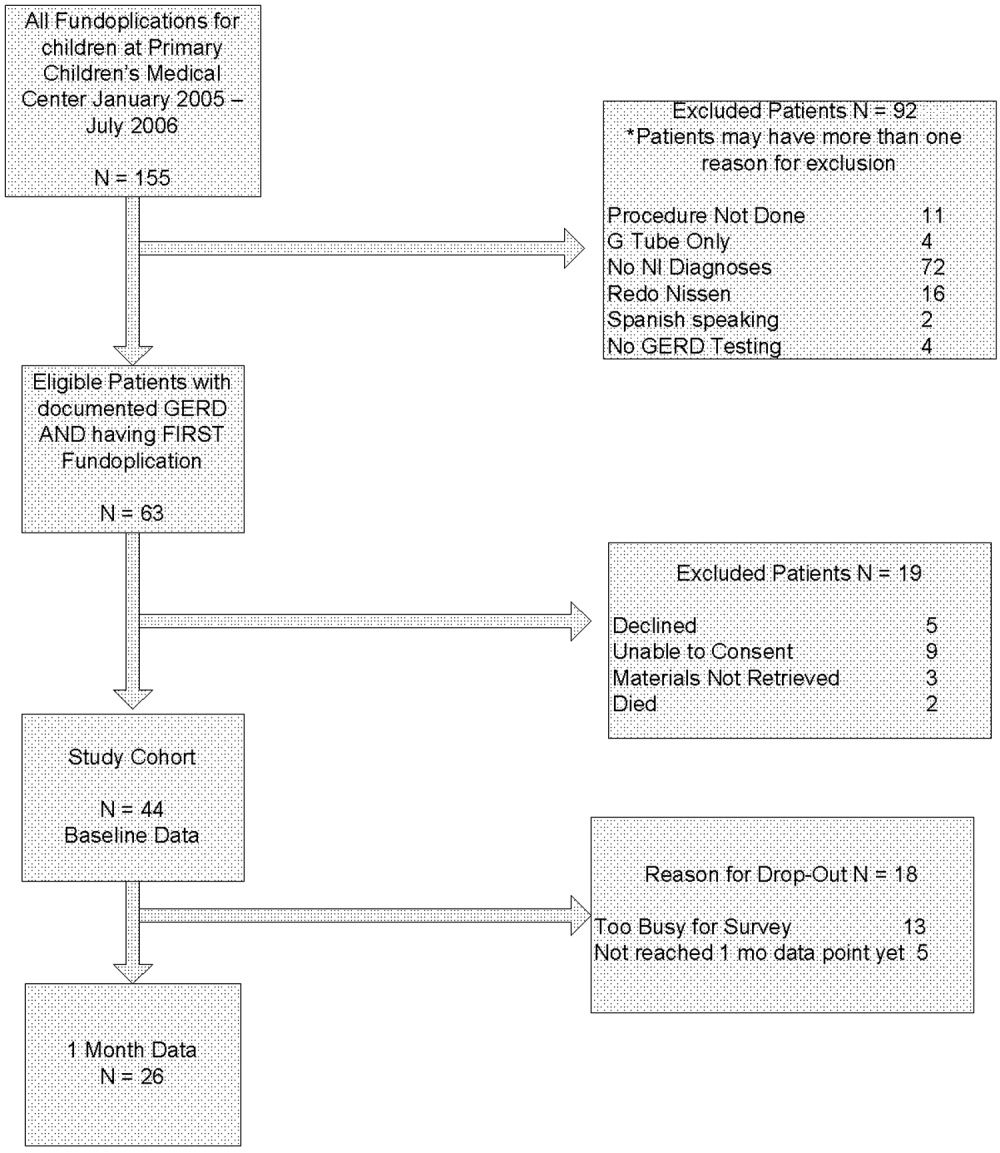
Data for the 13 parents and children (30%) who gave baseline data but were subsequently lost to follow‐up are shown in Table 1. Reasons for loss to follow‐up were caregiver reporting being too busy to fill out the questionnaires (n = 8) and no reason stated (n = 5).
In addition to the diagnosis of GERD, clinical indications for fundoplication were vomiting (55%), feeding‐related issues (47%), and failure to thrive (39%). Diagnosis of GERD was confirmed for 41 of 44 patients77% had an abnormal upper GI, 26% an abnormal pH probe, 14% an abnormal endoscopy, and 24% an abnormal swallow study. The remaining 3 had obvious clinical symptoms for GERD and did not require further testing according to their attending surgeon (2 with witnessed vomiting leading to aspiration and 1 who was exclusively gastrostomy‐fed and was witnessed having feeds coming from the tracheostomy). Various medications had been tried and were considered to have failed in these patients: 39% had been treated with acid‐suppressive agents; 80% with acid blocking agents; and 61% with prokinetic agents. Fourteen patients (32%) had cerebral palsy, and 14 (32%) had a brain or spinal cord abnormality (see Table 2).
| Diagnostic category | ICD‐9 codes used |
|---|---|
| |
| Brain or spinal cord anomaly | 335.22, 742.0, 742.1, 742.2, 742.4, 742.53 |
| Cerebral palsy | 343.0, 343.1, 343.2, 343.8, 343.9, 344.00 |
| Hydrocephalus | 331.3, 331.4, 742.3 |
| Down syndrome | 758.0 |
| Seizures | 345.10, 345.11, 345.3, 345.41, 345.50, 345.81, 345.90, 345.91 |
| Muscular dystrophy or myopathy | 359.0, 359.1, 359.2, 359.9 |
| Nervous system anomaly | 742.8, 742.9 |
| Cerebral degeneration | 330.8, 331.9 |
| Chromosomal anomaly | 758.2, 758.3, 758.5, 758.89 |
| Infantile spasms | 345.60, 345.61 |
| Menial retardation | 317.0, 318.1, 318.2 |
| Spinal muscle atrophy | 335.0, 335.10 |
Thirty‐four children underwent a laparoscopic Nissen fundoplication, and 10 had an open Nissen fundoplication. All had gastrostomy tubes placed or replaced at the time of surgery.
Analysis of the mean bodily pain scores from the CHQ‐PF50 revealed that bodily pain of patients in the study cohort had improved from baseline after 1 month of follow‐up (mean score at baseline, 32.8; after 1 month of follow‐up, 47.5; P = .01), after adjusting for functional status. However, these mean bodily pain scores were significantly lower than those of children with cerebral palsy (mean score, 73.9; P < .001).34, 35 After adjusting for functional status, scores were improved for role/social‐physical limitations (mean baseline score, 30.6; 1‐month follow‐up score, 56.6; P = .01), mental health (mean baseline score, 62.7; 1‐month follow‐up score, 70.6; P = .01), family limitation of activities (mean baseline score, 43.3; 1‐month follow‐up score, 55.1; P = .03), and parental time (mean baseline score, 43.0; 1‐month follow‐up score, 55.3; P = .03). Scores were unchanged for physical function, global health, general health perception, physical summary, role/social‐emotional, mental health, self‐esteem, and psychological summary (see Table 3).
| Domain of Quality of Life | Baseline (Mean and SD) | 1‐Month Follow‐Up (Mean and SD) | P Value |
|---|---|---|---|
| Physical functioning | 19.3 (34.1) | 16.7 (30.8) | 0.77 |
| Role physical* | 30.6 (44.4) | 56.6 (40.5) | 0.01 |
| Bodily pain* | 32.8 (24.4) | 47.5 (25.7) | 0.01 |
| Global health | 42.0 (23.7) | 44.1 (22.6) | 0.19 |
| General behavior | 72.1 (29.3) | 78.7 (14.5) | 0.21 |
| Self‐esteem | 39.9 (21.1) | 32.8 (19.4) | 0.36 |
| Mental health | 62.7 (15.9) | 70.6 (16.6) | 0.01 |
| Family limitation of activity* | 43.3 (23.7) | 55.1 (21.3) | 0.03 |
| Parental time* | 43.0 (35.5) | 55.3 (32.5) | 0.03 |
| Physical summary | 23.1 (21.2) | 17.8 (13.9) | 0.17 |
| Psychological summary | 39.0 (11.8) | 39.6 (10.8) | 0.76 |
Analysis of the SF‐36 of the parents of these children revealed mean scores significantly lower than those in general U.S. population for all quality‐of‐life domains except physical function (see Table 4). Many baseline domain scores were similar to those of adults with clinical depression. The only domain that showed improvement in quality of life of the caregivers over the 1‐month follow‐up period was vitality (mean baseline score, 41.3; 1‐month follow‐up score, 48.2; P = .001).
| Quality‐of‐life domain | Study group mean (SD) | U.S. population norm mean (SD) | P value |
|---|---|---|---|
| |||
| Physical functioning | 89.35 (14.60) | 84.15 (23.26) | 0.10 |
| Role physical | 71.02 (39.96) | 80.96 (34.00) | 0.05 |
| Bodily pain | 82.50 (24.00) | 75.15 (23.69) | 0.04 |
| General Health* | 59.07 (18.75) | 71.95 (20.34) | 0.001 |
| Vitality* | 41.33 (19.49) | 60.86 (33.04) | 0.001 |
| Social functioning* | 63.33 (34.48) | 83.28 (22.69) | 0.001 |
| Role emotional | 60.60 (40.20) | 81.26 (33.04) | 0.001 |
| Mental health | 67.00 (19.61) | 74.74 (18.05) | 0.004 |
Total stress as measured by the PSI/SF mean was 79.1 at baseline and 77.6 1 month after fundoplication (P = .54). This was significantly higher stress than the parental norm of 71.0 (P = .01). One in 4 parents expressed clinically significant levels of stress (scores > 90, 90th percentile).
Patients suffered the following complications in the month after fundoplication. Eight children had at least 1 subsequent emergency department visit related to a complication of the gastrostomy tube (8 visits), to respiratory distress (1 visit), or tovomiting (1 visit). Seven children had a subsequent admission to the hospital related to a complication of the gastrostomy tube (4 admissions), complication of surgery (2 admissions), or aspiration pneumonia (1 admission). None of the children had a repeat fundoplication or subsequently underwent placement of a gastrojejunal feeding tube. One patient died. She was 10 months old when she died, which was 3 weeks after she had received a fundoplication. She had obstructive hydrocephalus, cortical blindness, and developmental delay, and respiratory arrest and subsequent tonsillar herniation led to her death.
DISCUSSION
Parents of children with neurological impairment and GERD who underwent their first fundoplication reported improved quality of life of their children in the domains of bodily pain, role/social‐physical limitations, mental health, family limitation of activities, and parental time over the first month after surgery, when controlling for the children's degree of functional impairment. The only significant similar improvement in the parent self‐reported quality of life was in the domain of vitality.
This study had several limitations. Loss to follow‐up may have led to a bias reflecting the phenomenon that patients who have poorer quality of life are less likely to report this, or even to be able to participate in the follow‐up component of a study like this. In survival analyses, this incomplete follow‐up of patients is called informative dropout and may be minimized by applying a statistical technique that accounts for this, using the Q‐TWiST.45 However, our current study design and analysis plan precluded using this methodology. As shown in Table 1, we did not find any differences between those patients who stayed in the study and those who dropped out. Also, we were able to contact most parents who reported being too busy to fill out the surveys. Patient heterogeneity is also a concern: Table 2 shows the wide array of diagnoses responsible for the children's neurological impairment. However, we used a standardized functional status measure to ensure we were analyzing similarly disabled patients. Also, the standard deviation of the mean WeeFIM score was small, implying little variability in the study cohort. Our study analyzed data from a single center, which reflects care in the western United States. However, our hospital is similar to other medium and large children's hospitals and our patient population similar to others that perform fundoplication for children with neurological impairment.46 We believe our findings are generalizable to other surgical centers that perform a similar volume of fundoplications in such children with NI.
Our study findings are similar to those reported by O'Neill et al., whose study found that parents reported improved quality of life of their children in ease of feedings, physical comfort during feeding, and ability of the child to enjoy life.23 The CHQPF50 does not specifically ask about feeding, but we did find similar improvement in the domain of role/social‐physical limitations. O'Neill et al. also found that after the children in their study received a fundoplication, caregivers reported their own quality of life improved in the areas of being able to spend more time caring for their child's needs, which is similar to our findings of fewer family limitations of activities and more parental time. Our findings were somewhat dissimilar to the O'Neill et al. study, as parents in their study found several additional areas of improvement in caregiver own quality of life. One explanation for the differing results may be differences in the populations studied. Parents in our study had SF‐36 scores for general health, vitality, and social functioning that were similar to those of adults with depression,47 whereas parents in the O'Neill study did not. Although the O'Neill et al. study was the first to examine these critical quality‐of‐life outcomes for children with NI who have received fundoplication, it had several methodological limitations. We have had the opportunity to build on the work of O'Neill et al. and in a prospective study to capture standardized baseline data (therefore not subject to recall bias, as was likely in the O'Neill et al. study) and collect long‐term data on this population. We also controlled for functional status, which did not improve over the 1 month and by itself could be responsible for the already poor caregiver quality of life. Some aspects of the children's care did improve, but perhaps not enough to overcome the severe disabilities the children and their caregivers live with on a daily basis. We found some evidence to support that the parents' PSI/SF scores were similar to those of parents of children with heart disease, other enterally fed children, and children with traumatic brain injury (who make up between 1 in 3 and 1 in 5 parents with severe stress).39, 41, 43 Future interventions should address the stress and quality of life of these caregivers, especially if surgery does not improve caregiver quality of life or decrease stress.
Contrary to an emerging body of literature in pediatrics that describes a positive correlation between the health of children with chronic illnesses and their caregivers' quality of life,12, 42 we did not find large immediate improvements in caregiver quality of life and decrease in stress as their children's quality of life improved. This may be related to the number of parents in our sample being too small to detect such changes or that changes in longer‐term (greater than 6 months or 1 year) quality of life not being reflected by short‐term assessment. Caregiver and child quality of life following fundoplication needs to be studied over the long term (eg, over many years). We are continuing to follow these patients and their families and will repeat the quality‐of‐life measures 6 and 12 months after fundoplication and report these findings.
Additional studies of treatments for neurologically impaired children with GERD are needed. Randomized trials of alternatives to fundoplication such as gastrojejunal feeding tubes have been proposed, with which we strongly agree.46, 48 We believe that any randomized, controlled trial of children with neurological impairment and GERD must measure child and caregiver quality of life and functional status outcomes. 0
| Variables | Study patients at baseline (N = 44) | Study patients at 1‐month follow‐up (N = 26) | P value |
|---|---|---|---|
| Functional Status Measure | |||
| WeeFIM Score | 24 | 36 | NS |
| Child CHQ‐PF50 Quality‐of‐Life Scores | |||
| Role physical | 30.6 | 56.6 | 0.01 |
| Bodily pain | 32.8 | 47.5 | 0.01 |
| Mental health | 62.7 | 70.6 | 0.01 |
| Family limitation of activity | 43.3 | 55.1 | 0.03 |
| Parental time | 43.0 | 55.3 | 0.03 |
| Global health | 42.0 | 44.1 | NS |
| Physical functioning | 19.3 | 16.7 | NS |
| General behavior | 72.1 | 78.7 | NS |
| Self‐esteem | 39.9 | 32.8 | NS |
| Role emotional | 27.1 | 37.1 | NS |
| Physical summary | 23.1 | 17.8 | NS |
| Psychological summary | 39.0 | 39.6 | NS |
| Caregiver SF‐36 Quality‐of‐Life Scores | |||
| Vitality | 41.3 | 46.9 | 0.001 |
| Role physical | 89.9 | 92.5 | NS |
| Bodily pain | 71.0 | 78.7 | NS |
| General health | 82.5 | 81.1 | NS |
| Social functioning | 59.1 | 59.5 | NS |
| Role emotional | 60.6 | 65.6 | NS |
| Mental health | 67.0 | 73.5 | NS |
| Parenting stress index | 79.1 | 77.7 | NS |
Acknowledgements
The authors thank Tanner Coleman and Matthew Swenson for their invaluable help in recruiting patients. Dr. Srivastava was supported in part by the Children's Health Research Center, University of Utah and Primary Children's Medical Center Foundation.
- ,,.Comparison of respiratory mortality in the profoundly mentally retarded and in the less retarded.J Ment Defic Res.1979;23(1):1–7.
- ,,.Cause of death in cerebral palsy: a descriptive study.Arch Dis Child.1999;81:390–394.
- .Survival rates of children with severe neurologic disabilities: a review.Semin Pediatr Neurol.2003;10(2):120–129.
- ,.Gastroesophageal reflux among severely retarded children.J Pediatr.1979;94:710–714.
- ,,,,,.Operation for gastro‐oesophageal reflux associated with severe mental retardation.Arch Dis Child.1993;68:347–351.
- ,,,,,.Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford Feeding Study.Dev Med Child Neurol.2000;42:674–680.
- ,,, et al.Aspiration pneumonia in pediatric age group: etiology, predisposing factors and clinical outcome.J Pak Med Assoc.1999;49(4):105–108.
- ,.Respiratory problems in children with neurological impairment.Arch Dis Child.2003;88(1):75–78.
- ,.Gastroesophageal reflux in childhood.Curr Probl Surg.1996;33(1):1–70.
- ,.Minimally invasive surgical techniques in reoperative surgery for gastroesophageal reflux disease in infants and children.Am Surg.2002;68:989–992.
- .Laparoscopic Nissen procedure in children.Semin Laparosc Surg.2002;9(3):146–152.
- ,,, et al.Caregiving process and caregiver burden: conceptual models to guide research and practice.BMC Pediatr.2004;4(1):1.
- ,.Theoretical and psychometric analysis of caregiver strain.Res Nurs Health.1996;19:499–510.
- ,,,,,.Complications and reoperation after Nissen fundoplication in childhood.Am J Surg.1987;153(2):177–183.
- ,,, et al.Surgical treatment of gastroesophageal reflux in children: a combined hospital study of 7467 patients.Pediatrics.1998;101:419–422.
- ,,,,,.Outcomes of surgical fundoplication in children.Clin Gastroenterol Hepatol.2004;2:978–984.
- ,,, et al.The respiratory advantage of laparoscopic Nissen fundoplication.J Pediatr Surg.2003;38:886–891.
- ,,,.Laparoscopic Nissen fundoplication in children: 2‐5‐year follow‐up.Pediatr Surg Int.2003;19:537–539.
- ,,.Recognition of recurrent gastroesophageal reflux following antireflux surgery in the neurologically disabled child: high index of suspicion and definitive evaluation.J Pediatr Surg.1992;27:983–988; discussion988–990.
- ,,.Sequelae of antireflux surgery in profoundly disabled children.J Pediatr Surg.1992;27(2):267–271; discussion271–263.
- ,,.Efficacy of the Nissen fundoplication in the management of gastroesophageal reflux following esophageal atresia repair.J Pediatr Surg.1993;28(1):53–55.
- ,,,.Caregivers' perceptions following gastrostomy in severely disabled children with feeding problems.Dev Med Child Neurol.1997;39:746–751.
- ,,,,.Care‐giver evaluation of anti‐gastroesophageal reflux procedures in neurologically impaired children: what is the real‐life outcome?J Pediatr Surg.1996;31:375–380.
- ,,, et al.Impact of gastrostomy tube feeding on the quality of life of carers of children with cerebral palsy.Dev Med Child Neurol.2004;46:796–800.
- ,.Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101:805–811; discussion811–802.
- Children's Digestive Health and Nutrition Foundation Website. Gastroesophageal Reflux Disease in the Neurologically Impaired Child. Available at: http://www.cdhnf.org/PractitionerSeries.asp. Accessed August 30,2006.
- ,,,,.Functional status of school‐aged children with Down syndrome.J Paediatr Child Health.2002;38(2):160–165.
- ,,,,,.Concurrent validity of the Functional Independence Measure for Children (WeeFIM) and the Pediatric Evaluation of Disabilities Inventory in children with developmental disabilities and acquired brain injuries.Phys Occup Ther Pediatr.2001;21(2–3):91–101.
- ,,, et al.The WeeFIM instrument: its utility in detecting change in children with developmental disabilities.Arch Phys Med Rehabil.2000;81:1317–1326.
- ,,, et al.Functional assessment and care of children with neurodevelopmental disabilities.Am J Phys Med Rehabil.2000;79(2):114–123.
- ,,,,,.Predictors of mortality, morbidity, and disability in a cohort of infants < or = 28 weeks' gestation.Clin Pediatr (Phila).1993;32:521–527.
- ,.Content validity of a pediatric functional independence measure.Appl Nurs Res.1990;3(3):120–122.
- ,,, et al.The Functional Independence Measure for Children (WeeFIM). Conceptual basis and pilot use in children with developmental disabilities.Clin Pediatr (Phila). Jul1994;33:421–430.
- ,,.The CHQ User's Manual.1st ed.Boston, MA:The Health Institute, New England Medical Center,1996.
- ,,,,,.Comparing reliability and validity of pediatric instruments for measuring health and well‐being of children with spastic cerebral palsy.Dev Med Child Neurol.2002;44:468–476.
- ,,,,.Needs of carers of severely disabled people: are they identified and met adequately?Health Soc Care Community.2001;9(4):235–243.
- ,,.The realities of postoperative disability and the carer's burden.Ann R Coll Surg Engl.2001;83(3):215–218.
- Abdidin.Parenting Stress Index.3rd ed.Lutz, FL:Psychological Assessment Resources, Inc.;1995.
- ,,,.Parental stress and burden following traumatic brain injury amongst children and adolescents.Brain Inj. Jan2003;17(1):1–23.
- ,,.Comparing stress levels of parents of children with cancer and parents of children with physical disabilities.Psychooncology. Dec2004;13(12):898–903.
- ,,.Stress levels experienced by the parents of enterally fed children.Child Care Health Dev.2004;30:507–513.
- ,,, et al.The health and well‐being of caregivers of children with cerebral palsy.Pediatrics.2005;115:e626–e636.
- ,.Parenting stress and children with heart disease.J Pediatr Health Care.2003;17(4):163–168.
- ,.Small sample inference for fixed effects from restricted maximum likelihood.Biometrics.1997;53:983–997.
- ,,.Methods for the analysis of quality‐of‐life and survival data in health technology assessment.Health Technol Assess.1999;3(10):1–152.
- ,,, et al.Fundoplication and gastrostomy versus image‐guided gastrojejunal tube for enteral feeding in neurologically impaired children with gastroesophageal reflux.J Pediatr Surg.2002;37:407–412.
- SF‐36 Health Survey: Manual and Interpretation Guide.Lincoln, RI:QualityMetric Inc.;1993,year="2000"2000.
- ,,.The role of protective antireflux procedures in neurologically impaired children: a decision analysis.J Pediatr Surg. Mar2002;37:500–506.
Aspiration pneumonia is the most common cause of death in children with severe neurological impairment (NI).13 For several reasons (eg, improved survival of extremely low‐birth‐weight infants, technological advances, and changing societal attitudes), the number of children with severe NI is increasing. Many children with severe NI have dysfunctional swallowing and gastroesophageal reflux disease (GERD).46 This combination places them at high risk for recurrent aspiration that, in turn, leads to aspiration pneumonia, repeated hospitalization, respiratory failure, compromised quality of life, and death.7, 8
The most common treatment approach for the combination of dysfunctional swallowing and GERD is surgical fundoplication with a gastrostomy feeding tube. Fundoplication is the third most common procedure performed in children by pediatric surgeons in the United States.9 Fifty percent of the children who receive a fundoplication have neurological impairment.10, 11 The goals of the surgery to treat GERD unresponsive to medical management are to reduce the risk of aspiration pneumonia, improve nutritional status, and improve the quality of life of the children and their families. However, few prospective longitudinal studies have determined whether the quality of life of the children or their caregivers actually improves over time.
The importance of caregiver and child quality of life is increasingly recognized as a critical outcome of any intervention in this population.12, 13 Previous studies of fundoplication in children with NI, GERD, and dysfunctional swallowing reported surgical mortality rates between 1% and 3% and other complications between 4% and 39%, reflecting the medical fragility of these children.5, 1418 Some of these studies had longitudinal follow‐up and reported long‐term data. Recurrence of symptoms was reported in up to 56% of patients, recurrence of AP in up to 39%, further surgical procedures in up to 19%, and mortality in up to 17%.14, 1921 Few case series of children with neurological impairment who have had a fundoplication have addressed child and caregiver quality of life following either a fundoplication or placement of a feeding tube.2224 In their study of 16 patients who had a fundoplication and gastrostomy tube placed, Tawfik et al. found improvements in children's happiness, ease of giving medicines, and time to devote to other children. Sullivan et al. found improvement in caregiver quality of life following placement of a gastrostomy tube in a child; however, they did not specifically identify those children who had been treated with a fundoplication. In their retrospective study, O'Neill et al. found improved child and caregiver quality of life following a fundoplication. Collectively, these studies have found that parents report improvement of both their own and their child's quality of life after either intervention. However, not having baseline measurements, not controlling for degree of functional impairment of the children, small sample sizes, and large loss to follow‐up limit the utility of these studies. In this ongoing, long‐term prospective longitudinal study, we report the initial impact of a fundoplication on the quality of life of both children and their caregivers.
The primary objective of this study was to determine change over time in the quality of life of children with neurological impairment who received surgical treatment of their GERD and of the caregivers of these children, controlling for the degree of functional impairment of the children. We hypothesized that child and caregiver quality of life would both improve after primary fundoplication and gastrostomy tube placement. Secondary objectives included describing rates of complications in this population.
METHODS
Setting and Study Population
We enrolled patients from newborn to 18 years of age who had a diagnosis compatible with neurological impairment and who received their first fundoplication for GERD between January 2005 and July 2006 at Primary Children's Medical Center (PCMC), in Salt Lake City, Utah. PCMC is a 232‐bed children's hospital in the Intermountain West owned and operated by Intermountain Healthcare, Inc., a large vertically integrated health care delivery system that serves as both the primary hospital for Salt Lake County and as the tertiary‐care hospital for 4 additional states (Wyoming, Nevada, Idaho, and Montana).25 Patients who had a previous gastrojejunal feeding tube were excluded as were patients who had a previous fundoplication, as these procedures may have biased their reported quality of life, our main outcome measure.
Patients were included in the study if they had GERD (based on clinical history or testing) that had been refractory to medical management (defined as continued gastroesophageal reflux symptoms despite antireflux medications). GERD was defined using the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) criteria.26 These include: the presence of clinical symptoms and at least 1 abnormal result from an upper gastrointestinal x‐ray series (recognizing that this test is neither sensitive nor specific for reflux), pH probe, upper gastrointestinal endoscopy with biopsy, nuclear medicine scan, or a modified barium swallow. As this was a prospective observational study, physicians were allowed to order testing as their practice dictated. Patients were excluded if they had neurological impairment but lacked objective documentation of GERD using the NASPGHAN recommendations, unless there were obvious clinical indications such as witnessed vomiting and aspiration (N = 3). No patient received a prophylactic fundoplication (fundoplication without documented GERD).
Study Design
This is an ongoing prospective longitudinal study. Patients who had a first fundoplication at PCMC were identified by the surgical service, with weekly lists shared with the research team. Patients were approached by a research assistant during that initial hospitalization to see if they met inclusion criteria for the study using data from the medical records and surgical team when necessary.
Data Variables and Sources
Indications for the fundoplication, performance and results of diagnostic testing for GERD, complications of the fundoplication, and reasons for the neurological impairment were obtained through review of the electronic and paper medical records. Mortality data, subsequent emergency department visits, and admissions to the hospital were obtained using Intermountain Healthcare's electronic data warehouse, which merges clinical, financial, and administrative data including the Utah Vital Statistics database.
Neurological impairment was defined from 2 sources: (1) clinical diagnoses as identified by providers and (2) International Classification of Diseases Codes Modified, version 9 (ICD‐9 CM) identified a priori as indicating neurological impairment.
Instruments and Study Outcomes
Functional status was measured using the WeeFIM. This instrument has been tested and shown to be valid and reliable for children more than 6 months old with neurodevelopmental disabilities including spina bifida and Down syndrome.2732 WeeFIM is a self‐administered parent instrument composed of 18 items and 6 domains (self‐care, sphincter control, transfers, locomotion, communication, and social cognition).33 The WeeFIM allows patients to be stratified into areas of function from severely impaired to normal.
The primary outcome was child quality of life as measured by the Child Health Questionnaire Parental Form 50 (CHQ‐PF50). Caregiver quality of life was measured using the Short‐Form Health Status Survey (SF‐36) and Parenting Stress Index/Short Form (PSI/SF). The CHQ‐PF50 is a self‐administered parent questionnaire of 50 questions that measures 6 domains, including physical function and abilities, pain and discomfort, general health perception, behavior, temperament and moods, and satisfaction with growth and development.34 This instrument has been tested for validity and reliability in children with cerebral palsy.35 The SF‐36 is a widely accepted measure of health status that measures 8 domains of health: physical functioning, role limitations due to physical problems, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health. The SF‐36 has been well studied and has been used to measure the effect on a caregiver's quality of life associated with caring for a chronically ill child with significant medical problems.36, 37 Higher scores in each domain of both the CHQPF50 and the SF‐36 reflect better quality of life. Caregiver stress was measured using the PSI/SF (Psychological Assessment Resources Inc, Odessa, FL).38 In the PSI/SF a parent rates the parentchild dyad on 36 items that are summarized in 3 subscales: parental distress, parentchild dysfunctional interaction, and difficult child. A parent scores each item as strongly agree, agree, not sure, disagree, or strongly disagree. The sum of the 3 subscale scores is the total stress score. Higher scores denote a greater degree of stress. The PSI/SF has been validated in several studies for caregivers of children with chronic diseases.3943
The CHQ‐PF50, SF‐36, and PSI/SF questionnaires and the WeeFIM functional status measure were administered to each study patient and caregiver in person at enrollment (baseline) and by mail 1 month after fundoplication. A follow‐up postcard reminder was mailed 1 week after the initial mailing. Second and final mailings were sent to nonresponders 3 and 5 weeks, respectively, after the initial mailing.
Secondary outcomes included rates of complications including failure of the fundoplication. Complications were defined as a subsequent emergency department visit, hospitalization, or death related to the surgery, gastrostomy tube, or aspiration pneumonia. Failures were defined as a second fundoplication or the insertion of a gastrojejunal feeding tube as nonsurgical management of recurrent GERD and/or paraesophageal hernia. Secondary outcomes were followed from the time of the first fundoplication until 1 month after surgery.
Analyses
The differential effect of the fundoplication on the quality of life measures was assessed and quantified through statistical analysis. Because the primary interest was to measure change in baseline characteristics over time, repeated‐measures models were used to compare the group relative to changes in functional status. In particular, changes from baseline values were modeled 1 month after the procedure. The Kenward‐Roger approximation of degrees of freedom was used to compute P values from the overall tests.44 Repeated‐measures models were fit to the data using restricted maximum likelihood estimation. An autoregressive covariance matrix was assumed for the multiple measurements of each individual, thus limiting the number of restrictions forced by the model on the data. The repeated‐measures models used all the available data on participants, including those who dropped out of the study. To obtain the most accurate comparison of the study group, the covariate of functional status at baseline was taken into account in the fitted models. Statistical analyses were performed with SAS statistical software (version 9.13; SAS Institute, Cary NC). Student t tests were performed for comparison of means of the quality‐of‐life domains for the study cohort compared to either the general population or specific groups of patients for comparative purposes.
The study was approved by the institutional review boards of the University of Utah Health Sciences Center and Primary Children's Medical Center.
RESULTS
Sixty‐three children met eligibility criteria. Forty‐four families (70%) initially agreed to participate in the study and completed the baseline questionnaires (see Fig. 1). The mean age of the children was 2.2 years. Twenty‐six parents of children completed the 1‐month postfundoplication quality‐of‐life questionnaires. Thirteen patients were lost to follow‐up, 5 of whom had not reached the 1‐month postfundoplication time point. The median WeeFIM (functional status) score of the whole group was 31.2 (95% confidence interval [CI] 11‐71) compared with a childhood matched‐age norm of 83 (95% CI 60‐110), P = .001. WeeFIM scores did not change significantly from baseline to 1 month, P = .98 (Kruskall‐Wallis test).

Data for the 13 parents and children (30%) who gave baseline data but were subsequently lost to follow‐up are shown in Table 1. Reasons for loss to follow‐up were caregiver reporting being too busy to fill out the questionnaires (n = 8) and no reason stated (n = 5).
In addition to the diagnosis of GERD, clinical indications for fundoplication were vomiting (55%), feeding‐related issues (47%), and failure to thrive (39%). Diagnosis of GERD was confirmed for 41 of 44 patients77% had an abnormal upper GI, 26% an abnormal pH probe, 14% an abnormal endoscopy, and 24% an abnormal swallow study. The remaining 3 had obvious clinical symptoms for GERD and did not require further testing according to their attending surgeon (2 with witnessed vomiting leading to aspiration and 1 who was exclusively gastrostomy‐fed and was witnessed having feeds coming from the tracheostomy). Various medications had been tried and were considered to have failed in these patients: 39% had been treated with acid‐suppressive agents; 80% with acid blocking agents; and 61% with prokinetic agents. Fourteen patients (32%) had cerebral palsy, and 14 (32%) had a brain or spinal cord abnormality (see Table 2).
| Diagnostic category | ICD‐9 codes used |
|---|---|
| |
| Brain or spinal cord anomaly | 335.22, 742.0, 742.1, 742.2, 742.4, 742.53 |
| Cerebral palsy | 343.0, 343.1, 343.2, 343.8, 343.9, 344.00 |
| Hydrocephalus | 331.3, 331.4, 742.3 |
| Down syndrome | 758.0 |
| Seizures | 345.10, 345.11, 345.3, 345.41, 345.50, 345.81, 345.90, 345.91 |
| Muscular dystrophy or myopathy | 359.0, 359.1, 359.2, 359.9 |
| Nervous system anomaly | 742.8, 742.9 |
| Cerebral degeneration | 330.8, 331.9 |
| Chromosomal anomaly | 758.2, 758.3, 758.5, 758.89 |
| Infantile spasms | 345.60, 345.61 |
| Menial retardation | 317.0, 318.1, 318.2 |
| Spinal muscle atrophy | 335.0, 335.10 |
Thirty‐four children underwent a laparoscopic Nissen fundoplication, and 10 had an open Nissen fundoplication. All had gastrostomy tubes placed or replaced at the time of surgery.
Analysis of the mean bodily pain scores from the CHQ‐PF50 revealed that bodily pain of patients in the study cohort had improved from baseline after 1 month of follow‐up (mean score at baseline, 32.8; after 1 month of follow‐up, 47.5; P = .01), after adjusting for functional status. However, these mean bodily pain scores were significantly lower than those of children with cerebral palsy (mean score, 73.9; P < .001).34, 35 After adjusting for functional status, scores were improved for role/social‐physical limitations (mean baseline score, 30.6; 1‐month follow‐up score, 56.6; P = .01), mental health (mean baseline score, 62.7; 1‐month follow‐up score, 70.6; P = .01), family limitation of activities (mean baseline score, 43.3; 1‐month follow‐up score, 55.1; P = .03), and parental time (mean baseline score, 43.0; 1‐month follow‐up score, 55.3; P = .03). Scores were unchanged for physical function, global health, general health perception, physical summary, role/social‐emotional, mental health, self‐esteem, and psychological summary (see Table 3).
| Domain of Quality of Life | Baseline (Mean and SD) | 1‐Month Follow‐Up (Mean and SD) | P Value |
|---|---|---|---|
| Physical functioning | 19.3 (34.1) | 16.7 (30.8) | 0.77 |
| Role physical* | 30.6 (44.4) | 56.6 (40.5) | 0.01 |
| Bodily pain* | 32.8 (24.4) | 47.5 (25.7) | 0.01 |
| Global health | 42.0 (23.7) | 44.1 (22.6) | 0.19 |
| General behavior | 72.1 (29.3) | 78.7 (14.5) | 0.21 |
| Self‐esteem | 39.9 (21.1) | 32.8 (19.4) | 0.36 |
| Mental health | 62.7 (15.9) | 70.6 (16.6) | 0.01 |
| Family limitation of activity* | 43.3 (23.7) | 55.1 (21.3) | 0.03 |
| Parental time* | 43.0 (35.5) | 55.3 (32.5) | 0.03 |
| Physical summary | 23.1 (21.2) | 17.8 (13.9) | 0.17 |
| Psychological summary | 39.0 (11.8) | 39.6 (10.8) | 0.76 |
Analysis of the SF‐36 of the parents of these children revealed mean scores significantly lower than those in general U.S. population for all quality‐of‐life domains except physical function (see Table 4). Many baseline domain scores were similar to those of adults with clinical depression. The only domain that showed improvement in quality of life of the caregivers over the 1‐month follow‐up period was vitality (mean baseline score, 41.3; 1‐month follow‐up score, 48.2; P = .001).
| Quality‐of‐life domain | Study group mean (SD) | U.S. population norm mean (SD) | P value |
|---|---|---|---|
| |||
| Physical functioning | 89.35 (14.60) | 84.15 (23.26) | 0.10 |
| Role physical | 71.02 (39.96) | 80.96 (34.00) | 0.05 |
| Bodily pain | 82.50 (24.00) | 75.15 (23.69) | 0.04 |
| General Health* | 59.07 (18.75) | 71.95 (20.34) | 0.001 |
| Vitality* | 41.33 (19.49) | 60.86 (33.04) | 0.001 |
| Social functioning* | 63.33 (34.48) | 83.28 (22.69) | 0.001 |
| Role emotional | 60.60 (40.20) | 81.26 (33.04) | 0.001 |
| Mental health | 67.00 (19.61) | 74.74 (18.05) | 0.004 |
Total stress as measured by the PSI/SF mean was 79.1 at baseline and 77.6 1 month after fundoplication (P = .54). This was significantly higher stress than the parental norm of 71.0 (P = .01). One in 4 parents expressed clinically significant levels of stress (scores > 90, 90th percentile).
Patients suffered the following complications in the month after fundoplication. Eight children had at least 1 subsequent emergency department visit related to a complication of the gastrostomy tube (8 visits), to respiratory distress (1 visit), or tovomiting (1 visit). Seven children had a subsequent admission to the hospital related to a complication of the gastrostomy tube (4 admissions), complication of surgery (2 admissions), or aspiration pneumonia (1 admission). None of the children had a repeat fundoplication or subsequently underwent placement of a gastrojejunal feeding tube. One patient died. She was 10 months old when she died, which was 3 weeks after she had received a fundoplication. She had obstructive hydrocephalus, cortical blindness, and developmental delay, and respiratory arrest and subsequent tonsillar herniation led to her death.
DISCUSSION
Parents of children with neurological impairment and GERD who underwent their first fundoplication reported improved quality of life of their children in the domains of bodily pain, role/social‐physical limitations, mental health, family limitation of activities, and parental time over the first month after surgery, when controlling for the children's degree of functional impairment. The only significant similar improvement in the parent self‐reported quality of life was in the domain of vitality.
This study had several limitations. Loss to follow‐up may have led to a bias reflecting the phenomenon that patients who have poorer quality of life are less likely to report this, or even to be able to participate in the follow‐up component of a study like this. In survival analyses, this incomplete follow‐up of patients is called informative dropout and may be minimized by applying a statistical technique that accounts for this, using the Q‐TWiST.45 However, our current study design and analysis plan precluded using this methodology. As shown in Table 1, we did not find any differences between those patients who stayed in the study and those who dropped out. Also, we were able to contact most parents who reported being too busy to fill out the surveys. Patient heterogeneity is also a concern: Table 2 shows the wide array of diagnoses responsible for the children's neurological impairment. However, we used a standardized functional status measure to ensure we were analyzing similarly disabled patients. Also, the standard deviation of the mean WeeFIM score was small, implying little variability in the study cohort. Our study analyzed data from a single center, which reflects care in the western United States. However, our hospital is similar to other medium and large children's hospitals and our patient population similar to others that perform fundoplication for children with neurological impairment.46 We believe our findings are generalizable to other surgical centers that perform a similar volume of fundoplications in such children with NI.
Our study findings are similar to those reported by O'Neill et al., whose study found that parents reported improved quality of life of their children in ease of feedings, physical comfort during feeding, and ability of the child to enjoy life.23 The CHQPF50 does not specifically ask about feeding, but we did find similar improvement in the domain of role/social‐physical limitations. O'Neill et al. also found that after the children in their study received a fundoplication, caregivers reported their own quality of life improved in the areas of being able to spend more time caring for their child's needs, which is similar to our findings of fewer family limitations of activities and more parental time. Our findings were somewhat dissimilar to the O'Neill et al. study, as parents in their study found several additional areas of improvement in caregiver own quality of life. One explanation for the differing results may be differences in the populations studied. Parents in our study had SF‐36 scores for general health, vitality, and social functioning that were similar to those of adults with depression,47 whereas parents in the O'Neill study did not. Although the O'Neill et al. study was the first to examine these critical quality‐of‐life outcomes for children with NI who have received fundoplication, it had several methodological limitations. We have had the opportunity to build on the work of O'Neill et al. and in a prospective study to capture standardized baseline data (therefore not subject to recall bias, as was likely in the O'Neill et al. study) and collect long‐term data on this population. We also controlled for functional status, which did not improve over the 1 month and by itself could be responsible for the already poor caregiver quality of life. Some aspects of the children's care did improve, but perhaps not enough to overcome the severe disabilities the children and their caregivers live with on a daily basis. We found some evidence to support that the parents' PSI/SF scores were similar to those of parents of children with heart disease, other enterally fed children, and children with traumatic brain injury (who make up between 1 in 3 and 1 in 5 parents with severe stress).39, 41, 43 Future interventions should address the stress and quality of life of these caregivers, especially if surgery does not improve caregiver quality of life or decrease stress.
Contrary to an emerging body of literature in pediatrics that describes a positive correlation between the health of children with chronic illnesses and their caregivers' quality of life,12, 42 we did not find large immediate improvements in caregiver quality of life and decrease in stress as their children's quality of life improved. This may be related to the number of parents in our sample being too small to detect such changes or that changes in longer‐term (greater than 6 months or 1 year) quality of life not being reflected by short‐term assessment. Caregiver and child quality of life following fundoplication needs to be studied over the long term (eg, over many years). We are continuing to follow these patients and their families and will repeat the quality‐of‐life measures 6 and 12 months after fundoplication and report these findings.
Additional studies of treatments for neurologically impaired children with GERD are needed. Randomized trials of alternatives to fundoplication such as gastrojejunal feeding tubes have been proposed, with which we strongly agree.46, 48 We believe that any randomized, controlled trial of children with neurological impairment and GERD must measure child and caregiver quality of life and functional status outcomes. 0
| Variables | Study patients at baseline (N = 44) | Study patients at 1‐month follow‐up (N = 26) | P value |
|---|---|---|---|
| Functional Status Measure | |||
| WeeFIM Score | 24 | 36 | NS |
| Child CHQ‐PF50 Quality‐of‐Life Scores | |||
| Role physical | 30.6 | 56.6 | 0.01 |
| Bodily pain | 32.8 | 47.5 | 0.01 |
| Mental health | 62.7 | 70.6 | 0.01 |
| Family limitation of activity | 43.3 | 55.1 | 0.03 |
| Parental time | 43.0 | 55.3 | 0.03 |
| Global health | 42.0 | 44.1 | NS |
| Physical functioning | 19.3 | 16.7 | NS |
| General behavior | 72.1 | 78.7 | NS |
| Self‐esteem | 39.9 | 32.8 | NS |
| Role emotional | 27.1 | 37.1 | NS |
| Physical summary | 23.1 | 17.8 | NS |
| Psychological summary | 39.0 | 39.6 | NS |
| Caregiver SF‐36 Quality‐of‐Life Scores | |||
| Vitality | 41.3 | 46.9 | 0.001 |
| Role physical | 89.9 | 92.5 | NS |
| Bodily pain | 71.0 | 78.7 | NS |
| General health | 82.5 | 81.1 | NS |
| Social functioning | 59.1 | 59.5 | NS |
| Role emotional | 60.6 | 65.6 | NS |
| Mental health | 67.0 | 73.5 | NS |
| Parenting stress index | 79.1 | 77.7 | NS |
Acknowledgements
The authors thank Tanner Coleman and Matthew Swenson for their invaluable help in recruiting patients. Dr. Srivastava was supported in part by the Children's Health Research Center, University of Utah and Primary Children's Medical Center Foundation.
Aspiration pneumonia is the most common cause of death in children with severe neurological impairment (NI).13 For several reasons (eg, improved survival of extremely low‐birth‐weight infants, technological advances, and changing societal attitudes), the number of children with severe NI is increasing. Many children with severe NI have dysfunctional swallowing and gastroesophageal reflux disease (GERD).46 This combination places them at high risk for recurrent aspiration that, in turn, leads to aspiration pneumonia, repeated hospitalization, respiratory failure, compromised quality of life, and death.7, 8
The most common treatment approach for the combination of dysfunctional swallowing and GERD is surgical fundoplication with a gastrostomy feeding tube. Fundoplication is the third most common procedure performed in children by pediatric surgeons in the United States.9 Fifty percent of the children who receive a fundoplication have neurological impairment.10, 11 The goals of the surgery to treat GERD unresponsive to medical management are to reduce the risk of aspiration pneumonia, improve nutritional status, and improve the quality of life of the children and their families. However, few prospective longitudinal studies have determined whether the quality of life of the children or their caregivers actually improves over time.
The importance of caregiver and child quality of life is increasingly recognized as a critical outcome of any intervention in this population.12, 13 Previous studies of fundoplication in children with NI, GERD, and dysfunctional swallowing reported surgical mortality rates between 1% and 3% and other complications between 4% and 39%, reflecting the medical fragility of these children.5, 1418 Some of these studies had longitudinal follow‐up and reported long‐term data. Recurrence of symptoms was reported in up to 56% of patients, recurrence of AP in up to 39%, further surgical procedures in up to 19%, and mortality in up to 17%.14, 1921 Few case series of children with neurological impairment who have had a fundoplication have addressed child and caregiver quality of life following either a fundoplication or placement of a feeding tube.2224 In their study of 16 patients who had a fundoplication and gastrostomy tube placed, Tawfik et al. found improvements in children's happiness, ease of giving medicines, and time to devote to other children. Sullivan et al. found improvement in caregiver quality of life following placement of a gastrostomy tube in a child; however, they did not specifically identify those children who had been treated with a fundoplication. In their retrospective study, O'Neill et al. found improved child and caregiver quality of life following a fundoplication. Collectively, these studies have found that parents report improvement of both their own and their child's quality of life after either intervention. However, not having baseline measurements, not controlling for degree of functional impairment of the children, small sample sizes, and large loss to follow‐up limit the utility of these studies. In this ongoing, long‐term prospective longitudinal study, we report the initial impact of a fundoplication on the quality of life of both children and their caregivers.
The primary objective of this study was to determine change over time in the quality of life of children with neurological impairment who received surgical treatment of their GERD and of the caregivers of these children, controlling for the degree of functional impairment of the children. We hypothesized that child and caregiver quality of life would both improve after primary fundoplication and gastrostomy tube placement. Secondary objectives included describing rates of complications in this population.
METHODS
Setting and Study Population
We enrolled patients from newborn to 18 years of age who had a diagnosis compatible with neurological impairment and who received their first fundoplication for GERD between January 2005 and July 2006 at Primary Children's Medical Center (PCMC), in Salt Lake City, Utah. PCMC is a 232‐bed children's hospital in the Intermountain West owned and operated by Intermountain Healthcare, Inc., a large vertically integrated health care delivery system that serves as both the primary hospital for Salt Lake County and as the tertiary‐care hospital for 4 additional states (Wyoming, Nevada, Idaho, and Montana).25 Patients who had a previous gastrojejunal feeding tube were excluded as were patients who had a previous fundoplication, as these procedures may have biased their reported quality of life, our main outcome measure.
Patients were included in the study if they had GERD (based on clinical history or testing) that had been refractory to medical management (defined as continued gastroesophageal reflux symptoms despite antireflux medications). GERD was defined using the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) criteria.26 These include: the presence of clinical symptoms and at least 1 abnormal result from an upper gastrointestinal x‐ray series (recognizing that this test is neither sensitive nor specific for reflux), pH probe, upper gastrointestinal endoscopy with biopsy, nuclear medicine scan, or a modified barium swallow. As this was a prospective observational study, physicians were allowed to order testing as their practice dictated. Patients were excluded if they had neurological impairment but lacked objective documentation of GERD using the NASPGHAN recommendations, unless there were obvious clinical indications such as witnessed vomiting and aspiration (N = 3). No patient received a prophylactic fundoplication (fundoplication without documented GERD).
Study Design
This is an ongoing prospective longitudinal study. Patients who had a first fundoplication at PCMC were identified by the surgical service, with weekly lists shared with the research team. Patients were approached by a research assistant during that initial hospitalization to see if they met inclusion criteria for the study using data from the medical records and surgical team when necessary.
Data Variables and Sources
Indications for the fundoplication, performance and results of diagnostic testing for GERD, complications of the fundoplication, and reasons for the neurological impairment were obtained through review of the electronic and paper medical records. Mortality data, subsequent emergency department visits, and admissions to the hospital were obtained using Intermountain Healthcare's electronic data warehouse, which merges clinical, financial, and administrative data including the Utah Vital Statistics database.
Neurological impairment was defined from 2 sources: (1) clinical diagnoses as identified by providers and (2) International Classification of Diseases Codes Modified, version 9 (ICD‐9 CM) identified a priori as indicating neurological impairment.
Instruments and Study Outcomes
Functional status was measured using the WeeFIM. This instrument has been tested and shown to be valid and reliable for children more than 6 months old with neurodevelopmental disabilities including spina bifida and Down syndrome.2732 WeeFIM is a self‐administered parent instrument composed of 18 items and 6 domains (self‐care, sphincter control, transfers, locomotion, communication, and social cognition).33 The WeeFIM allows patients to be stratified into areas of function from severely impaired to normal.
The primary outcome was child quality of life as measured by the Child Health Questionnaire Parental Form 50 (CHQ‐PF50). Caregiver quality of life was measured using the Short‐Form Health Status Survey (SF‐36) and Parenting Stress Index/Short Form (PSI/SF). The CHQ‐PF50 is a self‐administered parent questionnaire of 50 questions that measures 6 domains, including physical function and abilities, pain and discomfort, general health perception, behavior, temperament and moods, and satisfaction with growth and development.34 This instrument has been tested for validity and reliability in children with cerebral palsy.35 The SF‐36 is a widely accepted measure of health status that measures 8 domains of health: physical functioning, role limitations due to physical problems, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health. The SF‐36 has been well studied and has been used to measure the effect on a caregiver's quality of life associated with caring for a chronically ill child with significant medical problems.36, 37 Higher scores in each domain of both the CHQPF50 and the SF‐36 reflect better quality of life. Caregiver stress was measured using the PSI/SF (Psychological Assessment Resources Inc, Odessa, FL).38 In the PSI/SF a parent rates the parentchild dyad on 36 items that are summarized in 3 subscales: parental distress, parentchild dysfunctional interaction, and difficult child. A parent scores each item as strongly agree, agree, not sure, disagree, or strongly disagree. The sum of the 3 subscale scores is the total stress score. Higher scores denote a greater degree of stress. The PSI/SF has been validated in several studies for caregivers of children with chronic diseases.3943
The CHQ‐PF50, SF‐36, and PSI/SF questionnaires and the WeeFIM functional status measure were administered to each study patient and caregiver in person at enrollment (baseline) and by mail 1 month after fundoplication. A follow‐up postcard reminder was mailed 1 week after the initial mailing. Second and final mailings were sent to nonresponders 3 and 5 weeks, respectively, after the initial mailing.
Secondary outcomes included rates of complications including failure of the fundoplication. Complications were defined as a subsequent emergency department visit, hospitalization, or death related to the surgery, gastrostomy tube, or aspiration pneumonia. Failures were defined as a second fundoplication or the insertion of a gastrojejunal feeding tube as nonsurgical management of recurrent GERD and/or paraesophageal hernia. Secondary outcomes were followed from the time of the first fundoplication until 1 month after surgery.
Analyses
The differential effect of the fundoplication on the quality of life measures was assessed and quantified through statistical analysis. Because the primary interest was to measure change in baseline characteristics over time, repeated‐measures models were used to compare the group relative to changes in functional status. In particular, changes from baseline values were modeled 1 month after the procedure. The Kenward‐Roger approximation of degrees of freedom was used to compute P values from the overall tests.44 Repeated‐measures models were fit to the data using restricted maximum likelihood estimation. An autoregressive covariance matrix was assumed for the multiple measurements of each individual, thus limiting the number of restrictions forced by the model on the data. The repeated‐measures models used all the available data on participants, including those who dropped out of the study. To obtain the most accurate comparison of the study group, the covariate of functional status at baseline was taken into account in the fitted models. Statistical analyses were performed with SAS statistical software (version 9.13; SAS Institute, Cary NC). Student t tests were performed for comparison of means of the quality‐of‐life domains for the study cohort compared to either the general population or specific groups of patients for comparative purposes.
The study was approved by the institutional review boards of the University of Utah Health Sciences Center and Primary Children's Medical Center.
RESULTS
Sixty‐three children met eligibility criteria. Forty‐four families (70%) initially agreed to participate in the study and completed the baseline questionnaires (see Fig. 1). The mean age of the children was 2.2 years. Twenty‐six parents of children completed the 1‐month postfundoplication quality‐of‐life questionnaires. Thirteen patients were lost to follow‐up, 5 of whom had not reached the 1‐month postfundoplication time point. The median WeeFIM (functional status) score of the whole group was 31.2 (95% confidence interval [CI] 11‐71) compared with a childhood matched‐age norm of 83 (95% CI 60‐110), P = .001. WeeFIM scores did not change significantly from baseline to 1 month, P = .98 (Kruskall‐Wallis test).

Data for the 13 parents and children (30%) who gave baseline data but were subsequently lost to follow‐up are shown in Table 1. Reasons for loss to follow‐up were caregiver reporting being too busy to fill out the questionnaires (n = 8) and no reason stated (n = 5).
In addition to the diagnosis of GERD, clinical indications for fundoplication were vomiting (55%), feeding‐related issues (47%), and failure to thrive (39%). Diagnosis of GERD was confirmed for 41 of 44 patients77% had an abnormal upper GI, 26% an abnormal pH probe, 14% an abnormal endoscopy, and 24% an abnormal swallow study. The remaining 3 had obvious clinical symptoms for GERD and did not require further testing according to their attending surgeon (2 with witnessed vomiting leading to aspiration and 1 who was exclusively gastrostomy‐fed and was witnessed having feeds coming from the tracheostomy). Various medications had been tried and were considered to have failed in these patients: 39% had been treated with acid‐suppressive agents; 80% with acid blocking agents; and 61% with prokinetic agents. Fourteen patients (32%) had cerebral palsy, and 14 (32%) had a brain or spinal cord abnormality (see Table 2).
| Diagnostic category | ICD‐9 codes used |
|---|---|
| |
| Brain or spinal cord anomaly | 335.22, 742.0, 742.1, 742.2, 742.4, 742.53 |
| Cerebral palsy | 343.0, 343.1, 343.2, 343.8, 343.9, 344.00 |
| Hydrocephalus | 331.3, 331.4, 742.3 |
| Down syndrome | 758.0 |
| Seizures | 345.10, 345.11, 345.3, 345.41, 345.50, 345.81, 345.90, 345.91 |
| Muscular dystrophy or myopathy | 359.0, 359.1, 359.2, 359.9 |
| Nervous system anomaly | 742.8, 742.9 |
| Cerebral degeneration | 330.8, 331.9 |
| Chromosomal anomaly | 758.2, 758.3, 758.5, 758.89 |
| Infantile spasms | 345.60, 345.61 |
| Menial retardation | 317.0, 318.1, 318.2 |
| Spinal muscle atrophy | 335.0, 335.10 |
Thirty‐four children underwent a laparoscopic Nissen fundoplication, and 10 had an open Nissen fundoplication. All had gastrostomy tubes placed or replaced at the time of surgery.
Analysis of the mean bodily pain scores from the CHQ‐PF50 revealed that bodily pain of patients in the study cohort had improved from baseline after 1 month of follow‐up (mean score at baseline, 32.8; after 1 month of follow‐up, 47.5; P = .01), after adjusting for functional status. However, these mean bodily pain scores were significantly lower than those of children with cerebral palsy (mean score, 73.9; P < .001).34, 35 After adjusting for functional status, scores were improved for role/social‐physical limitations (mean baseline score, 30.6; 1‐month follow‐up score, 56.6; P = .01), mental health (mean baseline score, 62.7; 1‐month follow‐up score, 70.6; P = .01), family limitation of activities (mean baseline score, 43.3; 1‐month follow‐up score, 55.1; P = .03), and parental time (mean baseline score, 43.0; 1‐month follow‐up score, 55.3; P = .03). Scores were unchanged for physical function, global health, general health perception, physical summary, role/social‐emotional, mental health, self‐esteem, and psychological summary (see Table 3).
| Domain of Quality of Life | Baseline (Mean and SD) | 1‐Month Follow‐Up (Mean and SD) | P Value |
|---|---|---|---|
| Physical functioning | 19.3 (34.1) | 16.7 (30.8) | 0.77 |
| Role physical* | 30.6 (44.4) | 56.6 (40.5) | 0.01 |
| Bodily pain* | 32.8 (24.4) | 47.5 (25.7) | 0.01 |
| Global health | 42.0 (23.7) | 44.1 (22.6) | 0.19 |
| General behavior | 72.1 (29.3) | 78.7 (14.5) | 0.21 |
| Self‐esteem | 39.9 (21.1) | 32.8 (19.4) | 0.36 |
| Mental health | 62.7 (15.9) | 70.6 (16.6) | 0.01 |
| Family limitation of activity* | 43.3 (23.7) | 55.1 (21.3) | 0.03 |
| Parental time* | 43.0 (35.5) | 55.3 (32.5) | 0.03 |
| Physical summary | 23.1 (21.2) | 17.8 (13.9) | 0.17 |
| Psychological summary | 39.0 (11.8) | 39.6 (10.8) | 0.76 |
Analysis of the SF‐36 of the parents of these children revealed mean scores significantly lower than those in general U.S. population for all quality‐of‐life domains except physical function (see Table 4). Many baseline domain scores were similar to those of adults with clinical depression. The only domain that showed improvement in quality of life of the caregivers over the 1‐month follow‐up period was vitality (mean baseline score, 41.3; 1‐month follow‐up score, 48.2; P = .001).
| Quality‐of‐life domain | Study group mean (SD) | U.S. population norm mean (SD) | P value |
|---|---|---|---|
| |||
| Physical functioning | 89.35 (14.60) | 84.15 (23.26) | 0.10 |
| Role physical | 71.02 (39.96) | 80.96 (34.00) | 0.05 |
| Bodily pain | 82.50 (24.00) | 75.15 (23.69) | 0.04 |
| General Health* | 59.07 (18.75) | 71.95 (20.34) | 0.001 |
| Vitality* | 41.33 (19.49) | 60.86 (33.04) | 0.001 |
| Social functioning* | 63.33 (34.48) | 83.28 (22.69) | 0.001 |
| Role emotional | 60.60 (40.20) | 81.26 (33.04) | 0.001 |
| Mental health | 67.00 (19.61) | 74.74 (18.05) | 0.004 |
Total stress as measured by the PSI/SF mean was 79.1 at baseline and 77.6 1 month after fundoplication (P = .54). This was significantly higher stress than the parental norm of 71.0 (P = .01). One in 4 parents expressed clinically significant levels of stress (scores > 90, 90th percentile).
Patients suffered the following complications in the month after fundoplication. Eight children had at least 1 subsequent emergency department visit related to a complication of the gastrostomy tube (8 visits), to respiratory distress (1 visit), or tovomiting (1 visit). Seven children had a subsequent admission to the hospital related to a complication of the gastrostomy tube (4 admissions), complication of surgery (2 admissions), or aspiration pneumonia (1 admission). None of the children had a repeat fundoplication or subsequently underwent placement of a gastrojejunal feeding tube. One patient died. She was 10 months old when she died, which was 3 weeks after she had received a fundoplication. She had obstructive hydrocephalus, cortical blindness, and developmental delay, and respiratory arrest and subsequent tonsillar herniation led to her death.
DISCUSSION
Parents of children with neurological impairment and GERD who underwent their first fundoplication reported improved quality of life of their children in the domains of bodily pain, role/social‐physical limitations, mental health, family limitation of activities, and parental time over the first month after surgery, when controlling for the children's degree of functional impairment. The only significant similar improvement in the parent self‐reported quality of life was in the domain of vitality.
This study had several limitations. Loss to follow‐up may have led to a bias reflecting the phenomenon that patients who have poorer quality of life are less likely to report this, or even to be able to participate in the follow‐up component of a study like this. In survival analyses, this incomplete follow‐up of patients is called informative dropout and may be minimized by applying a statistical technique that accounts for this, using the Q‐TWiST.45 However, our current study design and analysis plan precluded using this methodology. As shown in Table 1, we did not find any differences between those patients who stayed in the study and those who dropped out. Also, we were able to contact most parents who reported being too busy to fill out the surveys. Patient heterogeneity is also a concern: Table 2 shows the wide array of diagnoses responsible for the children's neurological impairment. However, we used a standardized functional status measure to ensure we were analyzing similarly disabled patients. Also, the standard deviation of the mean WeeFIM score was small, implying little variability in the study cohort. Our study analyzed data from a single center, which reflects care in the western United States. However, our hospital is similar to other medium and large children's hospitals and our patient population similar to others that perform fundoplication for children with neurological impairment.46 We believe our findings are generalizable to other surgical centers that perform a similar volume of fundoplications in such children with NI.
Our study findings are similar to those reported by O'Neill et al., whose study found that parents reported improved quality of life of their children in ease of feedings, physical comfort during feeding, and ability of the child to enjoy life.23 The CHQPF50 does not specifically ask about feeding, but we did find similar improvement in the domain of role/social‐physical limitations. O'Neill et al. also found that after the children in their study received a fundoplication, caregivers reported their own quality of life improved in the areas of being able to spend more time caring for their child's needs, which is similar to our findings of fewer family limitations of activities and more parental time. Our findings were somewhat dissimilar to the O'Neill et al. study, as parents in their study found several additional areas of improvement in caregiver own quality of life. One explanation for the differing results may be differences in the populations studied. Parents in our study had SF‐36 scores for general health, vitality, and social functioning that were similar to those of adults with depression,47 whereas parents in the O'Neill study did not. Although the O'Neill et al. study was the first to examine these critical quality‐of‐life outcomes for children with NI who have received fundoplication, it had several methodological limitations. We have had the opportunity to build on the work of O'Neill et al. and in a prospective study to capture standardized baseline data (therefore not subject to recall bias, as was likely in the O'Neill et al. study) and collect long‐term data on this population. We also controlled for functional status, which did not improve over the 1 month and by itself could be responsible for the already poor caregiver quality of life. Some aspects of the children's care did improve, but perhaps not enough to overcome the severe disabilities the children and their caregivers live with on a daily basis. We found some evidence to support that the parents' PSI/SF scores were similar to those of parents of children with heart disease, other enterally fed children, and children with traumatic brain injury (who make up between 1 in 3 and 1 in 5 parents with severe stress).39, 41, 43 Future interventions should address the stress and quality of life of these caregivers, especially if surgery does not improve caregiver quality of life or decrease stress.
Contrary to an emerging body of literature in pediatrics that describes a positive correlation between the health of children with chronic illnesses and their caregivers' quality of life,12, 42 we did not find large immediate improvements in caregiver quality of life and decrease in stress as their children's quality of life improved. This may be related to the number of parents in our sample being too small to detect such changes or that changes in longer‐term (greater than 6 months or 1 year) quality of life not being reflected by short‐term assessment. Caregiver and child quality of life following fundoplication needs to be studied over the long term (eg, over many years). We are continuing to follow these patients and their families and will repeat the quality‐of‐life measures 6 and 12 months after fundoplication and report these findings.
Additional studies of treatments for neurologically impaired children with GERD are needed. Randomized trials of alternatives to fundoplication such as gastrojejunal feeding tubes have been proposed, with which we strongly agree.46, 48 We believe that any randomized, controlled trial of children with neurological impairment and GERD must measure child and caregiver quality of life and functional status outcomes. 0
| Variables | Study patients at baseline (N = 44) | Study patients at 1‐month follow‐up (N = 26) | P value |
|---|---|---|---|
| Functional Status Measure | |||
| WeeFIM Score | 24 | 36 | NS |
| Child CHQ‐PF50 Quality‐of‐Life Scores | |||
| Role physical | 30.6 | 56.6 | 0.01 |
| Bodily pain | 32.8 | 47.5 | 0.01 |
| Mental health | 62.7 | 70.6 | 0.01 |
| Family limitation of activity | 43.3 | 55.1 | 0.03 |
| Parental time | 43.0 | 55.3 | 0.03 |
| Global health | 42.0 | 44.1 | NS |
| Physical functioning | 19.3 | 16.7 | NS |
| General behavior | 72.1 | 78.7 | NS |
| Self‐esteem | 39.9 | 32.8 | NS |
| Role emotional | 27.1 | 37.1 | NS |
| Physical summary | 23.1 | 17.8 | NS |
| Psychological summary | 39.0 | 39.6 | NS |
| Caregiver SF‐36 Quality‐of‐Life Scores | |||
| Vitality | 41.3 | 46.9 | 0.001 |
| Role physical | 89.9 | 92.5 | NS |
| Bodily pain | 71.0 | 78.7 | NS |
| General health | 82.5 | 81.1 | NS |
| Social functioning | 59.1 | 59.5 | NS |
| Role emotional | 60.6 | 65.6 | NS |
| Mental health | 67.0 | 73.5 | NS |
| Parenting stress index | 79.1 | 77.7 | NS |
Acknowledgements
The authors thank Tanner Coleman and Matthew Swenson for their invaluable help in recruiting patients. Dr. Srivastava was supported in part by the Children's Health Research Center, University of Utah and Primary Children's Medical Center Foundation.
- ,,.Comparison of respiratory mortality in the profoundly mentally retarded and in the less retarded.J Ment Defic Res.1979;23(1):1–7.
- ,,.Cause of death in cerebral palsy: a descriptive study.Arch Dis Child.1999;81:390–394.
- .Survival rates of children with severe neurologic disabilities: a review.Semin Pediatr Neurol.2003;10(2):120–129.
- ,.Gastroesophageal reflux among severely retarded children.J Pediatr.1979;94:710–714.
- ,,,,,.Operation for gastro‐oesophageal reflux associated with severe mental retardation.Arch Dis Child.1993;68:347–351.
- ,,,,,.Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford Feeding Study.Dev Med Child Neurol.2000;42:674–680.
- ,,, et al.Aspiration pneumonia in pediatric age group: etiology, predisposing factors and clinical outcome.J Pak Med Assoc.1999;49(4):105–108.
- ,.Respiratory problems in children with neurological impairment.Arch Dis Child.2003;88(1):75–78.
- ,.Gastroesophageal reflux in childhood.Curr Probl Surg.1996;33(1):1–70.
- ,.Minimally invasive surgical techniques in reoperative surgery for gastroesophageal reflux disease in infants and children.Am Surg.2002;68:989–992.
- .Laparoscopic Nissen procedure in children.Semin Laparosc Surg.2002;9(3):146–152.
- ,,, et al.Caregiving process and caregiver burden: conceptual models to guide research and practice.BMC Pediatr.2004;4(1):1.
- ,.Theoretical and psychometric analysis of caregiver strain.Res Nurs Health.1996;19:499–510.
- ,,,,,.Complications and reoperation after Nissen fundoplication in childhood.Am J Surg.1987;153(2):177–183.
- ,,, et al.Surgical treatment of gastroesophageal reflux in children: a combined hospital study of 7467 patients.Pediatrics.1998;101:419–422.
- ,,,,,.Outcomes of surgical fundoplication in children.Clin Gastroenterol Hepatol.2004;2:978–984.
- ,,, et al.The respiratory advantage of laparoscopic Nissen fundoplication.J Pediatr Surg.2003;38:886–891.
- ,,,.Laparoscopic Nissen fundoplication in children: 2‐5‐year follow‐up.Pediatr Surg Int.2003;19:537–539.
- ,,.Recognition of recurrent gastroesophageal reflux following antireflux surgery in the neurologically disabled child: high index of suspicion and definitive evaluation.J Pediatr Surg.1992;27:983–988; discussion988–990.
- ,,.Sequelae of antireflux surgery in profoundly disabled children.J Pediatr Surg.1992;27(2):267–271; discussion271–263.
- ,,.Efficacy of the Nissen fundoplication in the management of gastroesophageal reflux following esophageal atresia repair.J Pediatr Surg.1993;28(1):53–55.
- ,,,.Caregivers' perceptions following gastrostomy in severely disabled children with feeding problems.Dev Med Child Neurol.1997;39:746–751.
- ,,,,.Care‐giver evaluation of anti‐gastroesophageal reflux procedures in neurologically impaired children: what is the real‐life outcome?J Pediatr Surg.1996;31:375–380.
- ,,, et al.Impact of gastrostomy tube feeding on the quality of life of carers of children with cerebral palsy.Dev Med Child Neurol.2004;46:796–800.
- ,.Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101:805–811; discussion811–802.
- Children's Digestive Health and Nutrition Foundation Website. Gastroesophageal Reflux Disease in the Neurologically Impaired Child. Available at: http://www.cdhnf.org/PractitionerSeries.asp. Accessed August 30,2006.
- ,,,,.Functional status of school‐aged children with Down syndrome.J Paediatr Child Health.2002;38(2):160–165.
- ,,,,,.Concurrent validity of the Functional Independence Measure for Children (WeeFIM) and the Pediatric Evaluation of Disabilities Inventory in children with developmental disabilities and acquired brain injuries.Phys Occup Ther Pediatr.2001;21(2–3):91–101.
- ,,, et al.The WeeFIM instrument: its utility in detecting change in children with developmental disabilities.Arch Phys Med Rehabil.2000;81:1317–1326.
- ,,, et al.Functional assessment and care of children with neurodevelopmental disabilities.Am J Phys Med Rehabil.2000;79(2):114–123.
- ,,,,,.Predictors of mortality, morbidity, and disability in a cohort of infants < or = 28 weeks' gestation.Clin Pediatr (Phila).1993;32:521–527.
- ,.Content validity of a pediatric functional independence measure.Appl Nurs Res.1990;3(3):120–122.
- ,,, et al.The Functional Independence Measure for Children (WeeFIM). Conceptual basis and pilot use in children with developmental disabilities.Clin Pediatr (Phila). Jul1994;33:421–430.
- ,,.The CHQ User's Manual.1st ed.Boston, MA:The Health Institute, New England Medical Center,1996.
- ,,,,,.Comparing reliability and validity of pediatric instruments for measuring health and well‐being of children with spastic cerebral palsy.Dev Med Child Neurol.2002;44:468–476.
- ,,,,.Needs of carers of severely disabled people: are they identified and met adequately?Health Soc Care Community.2001;9(4):235–243.
- ,,.The realities of postoperative disability and the carer's burden.Ann R Coll Surg Engl.2001;83(3):215–218.
- Abdidin.Parenting Stress Index.3rd ed.Lutz, FL:Psychological Assessment Resources, Inc.;1995.
- ,,,.Parental stress and burden following traumatic brain injury amongst children and adolescents.Brain Inj. Jan2003;17(1):1–23.
- ,,.Comparing stress levels of parents of children with cancer and parents of children with physical disabilities.Psychooncology. Dec2004;13(12):898–903.
- ,,.Stress levels experienced by the parents of enterally fed children.Child Care Health Dev.2004;30:507–513.
- ,,, et al.The health and well‐being of caregivers of children with cerebral palsy.Pediatrics.2005;115:e626–e636.
- ,.Parenting stress and children with heart disease.J Pediatr Health Care.2003;17(4):163–168.
- ,.Small sample inference for fixed effects from restricted maximum likelihood.Biometrics.1997;53:983–997.
- ,,.Methods for the analysis of quality‐of‐life and survival data in health technology assessment.Health Technol Assess.1999;3(10):1–152.
- ,,, et al.Fundoplication and gastrostomy versus image‐guided gastrojejunal tube for enteral feeding in neurologically impaired children with gastroesophageal reflux.J Pediatr Surg.2002;37:407–412.
- SF‐36 Health Survey: Manual and Interpretation Guide.Lincoln, RI:QualityMetric Inc.;1993,year="2000"2000.
- ,,.The role of protective antireflux procedures in neurologically impaired children: a decision analysis.J Pediatr Surg. Mar2002;37:500–506.
- ,,.Comparison of respiratory mortality in the profoundly mentally retarded and in the less retarded.J Ment Defic Res.1979;23(1):1–7.
- ,,.Cause of death in cerebral palsy: a descriptive study.Arch Dis Child.1999;81:390–394.
- .Survival rates of children with severe neurologic disabilities: a review.Semin Pediatr Neurol.2003;10(2):120–129.
- ,.Gastroesophageal reflux among severely retarded children.J Pediatr.1979;94:710–714.
- ,,,,,.Operation for gastro‐oesophageal reflux associated with severe mental retardation.Arch Dis Child.1993;68:347–351.
- ,,,,,.Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford Feeding Study.Dev Med Child Neurol.2000;42:674–680.
- ,,, et al.Aspiration pneumonia in pediatric age group: etiology, predisposing factors and clinical outcome.J Pak Med Assoc.1999;49(4):105–108.
- ,.Respiratory problems in children with neurological impairment.Arch Dis Child.2003;88(1):75–78.
- ,.Gastroesophageal reflux in childhood.Curr Probl Surg.1996;33(1):1–70.
- ,.Minimally invasive surgical techniques in reoperative surgery for gastroesophageal reflux disease in infants and children.Am Surg.2002;68:989–992.
- .Laparoscopic Nissen procedure in children.Semin Laparosc Surg.2002;9(3):146–152.
- ,,, et al.Caregiving process and caregiver burden: conceptual models to guide research and practice.BMC Pediatr.2004;4(1):1.
- ,.Theoretical and psychometric analysis of caregiver strain.Res Nurs Health.1996;19:499–510.
- ,,,,,.Complications and reoperation after Nissen fundoplication in childhood.Am J Surg.1987;153(2):177–183.
- ,,, et al.Surgical treatment of gastroesophageal reflux in children: a combined hospital study of 7467 patients.Pediatrics.1998;101:419–422.
- ,,,,,.Outcomes of surgical fundoplication in children.Clin Gastroenterol Hepatol.2004;2:978–984.
- ,,, et al.The respiratory advantage of laparoscopic Nissen fundoplication.J Pediatr Surg.2003;38:886–891.
- ,,,.Laparoscopic Nissen fundoplication in children: 2‐5‐year follow‐up.Pediatr Surg Int.2003;19:537–539.
- ,,.Recognition of recurrent gastroesophageal reflux following antireflux surgery in the neurologically disabled child: high index of suspicion and definitive evaluation.J Pediatr Surg.1992;27:983–988; discussion988–990.
- ,,.Sequelae of antireflux surgery in profoundly disabled children.J Pediatr Surg.1992;27(2):267–271; discussion271–263.
- ,,.Efficacy of the Nissen fundoplication in the management of gastroesophageal reflux following esophageal atresia repair.J Pediatr Surg.1993;28(1):53–55.
- ,,,.Caregivers' perceptions following gastrostomy in severely disabled children with feeding problems.Dev Med Child Neurol.1997;39:746–751.
- ,,,,.Care‐giver evaluation of anti‐gastroesophageal reflux procedures in neurologically impaired children: what is the real‐life outcome?J Pediatr Surg.1996;31:375–380.
- ,,, et al.Impact of gastrostomy tube feeding on the quality of life of carers of children with cerebral palsy.Dev Med Child Neurol.2004;46:796–800.
- ,.Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101:805–811; discussion811–802.
- Children's Digestive Health and Nutrition Foundation Website. Gastroesophageal Reflux Disease in the Neurologically Impaired Child. Available at: http://www.cdhnf.org/PractitionerSeries.asp. Accessed August 30,2006.
- ,,,,.Functional status of school‐aged children with Down syndrome.J Paediatr Child Health.2002;38(2):160–165.
- ,,,,,.Concurrent validity of the Functional Independence Measure for Children (WeeFIM) and the Pediatric Evaluation of Disabilities Inventory in children with developmental disabilities and acquired brain injuries.Phys Occup Ther Pediatr.2001;21(2–3):91–101.
- ,,, et al.The WeeFIM instrument: its utility in detecting change in children with developmental disabilities.Arch Phys Med Rehabil.2000;81:1317–1326.
- ,,, et al.Functional assessment and care of children with neurodevelopmental disabilities.Am J Phys Med Rehabil.2000;79(2):114–123.
- ,,,,,.Predictors of mortality, morbidity, and disability in a cohort of infants < or = 28 weeks' gestation.Clin Pediatr (Phila).1993;32:521–527.
- ,.Content validity of a pediatric functional independence measure.Appl Nurs Res.1990;3(3):120–122.
- ,,, et al.The Functional Independence Measure for Children (WeeFIM). Conceptual basis and pilot use in children with developmental disabilities.Clin Pediatr (Phila). Jul1994;33:421–430.
- ,,.The CHQ User's Manual.1st ed.Boston, MA:The Health Institute, New England Medical Center,1996.
- ,,,,,.Comparing reliability and validity of pediatric instruments for measuring health and well‐being of children with spastic cerebral palsy.Dev Med Child Neurol.2002;44:468–476.
- ,,,,.Needs of carers of severely disabled people: are they identified and met adequately?Health Soc Care Community.2001;9(4):235–243.
- ,,.The realities of postoperative disability and the carer's burden.Ann R Coll Surg Engl.2001;83(3):215–218.
- Abdidin.Parenting Stress Index.3rd ed.Lutz, FL:Psychological Assessment Resources, Inc.;1995.
- ,,,.Parental stress and burden following traumatic brain injury amongst children and adolescents.Brain Inj. Jan2003;17(1):1–23.
- ,,.Comparing stress levels of parents of children with cancer and parents of children with physical disabilities.Psychooncology. Dec2004;13(12):898–903.
- ,,.Stress levels experienced by the parents of enterally fed children.Child Care Health Dev.2004;30:507–513.
- ,,, et al.The health and well‐being of caregivers of children with cerebral palsy.Pediatrics.2005;115:e626–e636.
- ,.Parenting stress and children with heart disease.J Pediatr Health Care.2003;17(4):163–168.
- ,.Small sample inference for fixed effects from restricted maximum likelihood.Biometrics.1997;53:983–997.
- ,,.Methods for the analysis of quality‐of‐life and survival data in health technology assessment.Health Technol Assess.1999;3(10):1–152.
- ,,, et al.Fundoplication and gastrostomy versus image‐guided gastrojejunal tube for enteral feeding in neurologically impaired children with gastroesophageal reflux.J Pediatr Surg.2002;37:407–412.
- SF‐36 Health Survey: Manual and Interpretation Guide.Lincoln, RI:QualityMetric Inc.;1993,year="2000"2000.
- ,,.The role of protective antireflux procedures in neurologically impaired children: a decision analysis.J Pediatr Surg. Mar2002;37:500–506.
Once Upon a Tenens
Having finished my family practice residency, and not being tied down by friends or family, I thought a round of working locum tenens would be a good way to see some different styles of practice.
I contacted a company called Locum-motion, filled out my paper work and was on my way. I had graduated from the University of Hamlin a few weeks earlier, armed with stethoscope and hammer. Dr. Claudio Prince (that’s me) was ready to stamp out disease and save lives.
There were several practice options available, but I chose a small hospital in Bremen. The three doctors who ran the hospital were going on a musical cruise for a week, and I would cover the entire facility for that time—ED and floor. I met with Drs. Baker, Butcher, and Maker briefly before they left for a trip. There were only a few inpatients, and one patient in the ICU. Then they were gone, and I was on my own.
My First Patient: I thought I’d start with the inpatients. The first was a Mr. B.B. Wolfe. He had been admitted for myalgia.
I was shocked when I walked into the room. He had severe hypertrichosis, prominent dentition, and proptosis. I briefly considered porphyria. I noted his history of muscle aches, high fever, facial swelling, and visual disturbance. His admit lab had showed a high CPK and LDH.
It sounded like an infestation of Trichinella to me. I questioned him about the ingestion of raw pork. He looked me in the eye and asked if his answer would be part of his medical record. I told him he needed to tell the truth for me to help him. Finally, he admitted he had eaten several portions of uncooked pig. I began to explain to him about the workup, the need for a muscle biopsy, and treatment options like mebendazole or steroids. I described the intestinal stage, which occurs between two and seven days after ingestion, when encysted larvae are liberated from the meat by gastric juices. I told him how the larvae mature into adult worms that burrow into the intestinal mucosa. I described the muscle stage, which develops after the first week and represents the period when adult-derived larvae in the intestines enter the bloodstream and disseminate hematogenously, then enter skeletal muscle causing pain.
He jumped out of bed and said he was leaving AMA; he had an appointment with a red-hooded girl. Whatever. I let him go; not much I could do to prevent his departure.
In the ED: I got a call from the ED about an old lady who had come in, having nearly choked on a bug. She looked fine to me, and I let her go. While down there, another older woman came in—in active labor. She admitted to having taken her friend’s Clomid, and had had little antepartum care secondary to a dearth of health insurance. Before I knew it, we were in the labor suite. First came one boy, then another, then another. I thought I was done, then two girls, another boy, then another girl. Seven babies—incredible! She moaned, not knowing what she was going to do with all these children.
I headed back to the floor to see more patients. The second one for the day was simple: a scrotal burn on a Mr. J.B. Nimble, who had been injured jumping over a flame. He was ready for discharge. The third patient was interesting, a Mrs. Spratt. I had been called by the lab with word that her serum looked like mayonnaise. She had abdominal pain, hepatosplenomegaly, memory loss, dyspnea and eruptive xanthomas. It sounded like type V hyperlipoproteinemia with chylomicronemia syndrome. What an interesting case, probably worse secondary to her very high-fat diet.
I walked down the hall to see my last inpatient, billed as a young man with psychotic depression pending psychiatric placement. I heard him yelling about burning witches and eating the walls. It sounded pretty psychotic. (I learned that his sister had been arraigned on manslaughter charges.) I entered the room and was struck by the smell of his breath, like cherries. A fruity smell, could he be ketotic? I instantly thought about ethylene glycol toxicity, maybe he had been sipping antifreeze, but there were no oxalate crystals in his urine. Was it delirium? I checked an O2 saturation; 95%, that wasn’t it. His blood pressure was 120/60. How about checking his finger-stick glucose? It read >400. This boy was in DKA! I started an insulin drip and hydration. It was only later I learned he had been on a starvation diet for weeks, then had binged on candy. His story about burning an old woman in a stove was unfortunately true. I called child protective services.
The ED pager went off again. That old lady who had swallowed a bug had ingested another, possibly a spider. I was worried about brown recluse or black widow envenomation, but it seemed to have been a simple barn spider, as she was now feeling OK. Again I discharged her with stern warnings to limit her invertebrate consumption. I stopped by the multiparous patient. She was happy to have the children and that they were all healthy, but what was she going to do? Where would she go? I called a social work consult.
ICU Time: I headed to the ICU. A sad story: a 24-year-old woman was unresponsive. She had a living will and did not want prolonged life support. She had choked on a piece of fruit. I walked into a room crowded with her family; it looked like seven very short uncles—one of whom was a doctor. They watched sadly as I pulled the endotracheal tube and the IVs. I told them I’d give them some time with her alone and would be back in an hour. It was a somber and tearful affair. She was so young and so beautiful. What a tragic end.
Another ED call; this bug-swallowing lady was driving them nuts. Now she was claiming she had swallowed a thrush, or maybe had thrush. Either way, she was gone before I got down there to see her.
I met with the social worker. She was dressed in a fancy gown and said she had just been at a party. She heard the story of the lady who had had so many children. She thought for a moment then said she had a few leads to check and would get back to me later that day.
We had a rush in the ED: a boy I thought might have rhinophyma and stiff-man syndrome, a girl with warts on her lips she attributed to kissing a frog, another with a glass splinter in her foot. There was a Mr. W.W. Winkee with hypothermia, and a young girl named Mary who thought she had contracted anthrax from a sheep. There was a boy named Jackie Horner with a tenosynovitis of the thumb.
The old lady came back in again. Now she was complaining of abdominal distention. When I finally laid eyes on her, I noted she certainly had a large abdomen. I grabbed a quick X-ray. Apparently she had taken my warnings against consuming avian and invertebrate entities, as now she had radiographic evidence of a feline skeleton. I planned to send her to a tertiary-care facility; perhaps they could do an endoscopic cat removal. Whatever they did, I was afraid if she kept this up she was bound to die.
I headed back to the ICU. They had dressed the girl, Miss White, in her street clothes and done her hair and makeup, but nothing could hide her severe pallor. She looked so peaceful.
Her “uncles” expectantly greeted me. They said in unison, “Welcome, Dr. Prince.” They were all inappropriately smiling. What was going on here? I went to declare her dead. I held a mirror up to her face, no signs of breathing, no lung sounds with auscultation. I laid my fingers gently across her throat. No pulse, but her skin was strangely pliant and warm.
I stared at her lovely face and the rest of the world suddenly shut itself off from me. I felt like singing. I could not stop myself … how unprofessional. But I bent over and gently kissed her goodbye. Suddenly there was music playing and—even more strangely—woodland animals frolicking at my feet. The uncles, who turned out to be roommates not relatives (had I known that I would never have stopped life support!), danced merrily. I looked back at her, and her eyes were open. She was smiling and gazing at her future husband, me.
Epilogue: The masticatory old lady eventually died from eating tainted horse meat. Mrs. Sprat improved with a low-fat diet. Mr. Wolfe died in a tragic logging accident, killed by the swing of an ax. The housing issues of the old lady with so many kids she did not know what to do was settled by the social worker, who became the septuplets’ godmother and found them housing in a refurbished, oversize shoe. As for Snow and me, we plan on living happily ever after. TH
Dr. Newman is the physician editor of The Hospitalist. He’s also consultant, Hospital Internal Medicine, and assistant professor of internal medicine and medical history, Mayo Clinic College of Medicine, Rochester, Minn.
Having finished my family practice residency, and not being tied down by friends or family, I thought a round of working locum tenens would be a good way to see some different styles of practice.
I contacted a company called Locum-motion, filled out my paper work and was on my way. I had graduated from the University of Hamlin a few weeks earlier, armed with stethoscope and hammer. Dr. Claudio Prince (that’s me) was ready to stamp out disease and save lives.
There were several practice options available, but I chose a small hospital in Bremen. The three doctors who ran the hospital were going on a musical cruise for a week, and I would cover the entire facility for that time—ED and floor. I met with Drs. Baker, Butcher, and Maker briefly before they left for a trip. There were only a few inpatients, and one patient in the ICU. Then they were gone, and I was on my own.
My First Patient: I thought I’d start with the inpatients. The first was a Mr. B.B. Wolfe. He had been admitted for myalgia.
I was shocked when I walked into the room. He had severe hypertrichosis, prominent dentition, and proptosis. I briefly considered porphyria. I noted his history of muscle aches, high fever, facial swelling, and visual disturbance. His admit lab had showed a high CPK and LDH.
It sounded like an infestation of Trichinella to me. I questioned him about the ingestion of raw pork. He looked me in the eye and asked if his answer would be part of his medical record. I told him he needed to tell the truth for me to help him. Finally, he admitted he had eaten several portions of uncooked pig. I began to explain to him about the workup, the need for a muscle biopsy, and treatment options like mebendazole or steroids. I described the intestinal stage, which occurs between two and seven days after ingestion, when encysted larvae are liberated from the meat by gastric juices. I told him how the larvae mature into adult worms that burrow into the intestinal mucosa. I described the muscle stage, which develops after the first week and represents the period when adult-derived larvae in the intestines enter the bloodstream and disseminate hematogenously, then enter skeletal muscle causing pain.
He jumped out of bed and said he was leaving AMA; he had an appointment with a red-hooded girl. Whatever. I let him go; not much I could do to prevent his departure.
In the ED: I got a call from the ED about an old lady who had come in, having nearly choked on a bug. She looked fine to me, and I let her go. While down there, another older woman came in—in active labor. She admitted to having taken her friend’s Clomid, and had had little antepartum care secondary to a dearth of health insurance. Before I knew it, we were in the labor suite. First came one boy, then another, then another. I thought I was done, then two girls, another boy, then another girl. Seven babies—incredible! She moaned, not knowing what she was going to do with all these children.
I headed back to the floor to see more patients. The second one for the day was simple: a scrotal burn on a Mr. J.B. Nimble, who had been injured jumping over a flame. He was ready for discharge. The third patient was interesting, a Mrs. Spratt. I had been called by the lab with word that her serum looked like mayonnaise. She had abdominal pain, hepatosplenomegaly, memory loss, dyspnea and eruptive xanthomas. It sounded like type V hyperlipoproteinemia with chylomicronemia syndrome. What an interesting case, probably worse secondary to her very high-fat diet.
I walked down the hall to see my last inpatient, billed as a young man with psychotic depression pending psychiatric placement. I heard him yelling about burning witches and eating the walls. It sounded pretty psychotic. (I learned that his sister had been arraigned on manslaughter charges.) I entered the room and was struck by the smell of his breath, like cherries. A fruity smell, could he be ketotic? I instantly thought about ethylene glycol toxicity, maybe he had been sipping antifreeze, but there were no oxalate crystals in his urine. Was it delirium? I checked an O2 saturation; 95%, that wasn’t it. His blood pressure was 120/60. How about checking his finger-stick glucose? It read >400. This boy was in DKA! I started an insulin drip and hydration. It was only later I learned he had been on a starvation diet for weeks, then had binged on candy. His story about burning an old woman in a stove was unfortunately true. I called child protective services.
The ED pager went off again. That old lady who had swallowed a bug had ingested another, possibly a spider. I was worried about brown recluse or black widow envenomation, but it seemed to have been a simple barn spider, as she was now feeling OK. Again I discharged her with stern warnings to limit her invertebrate consumption. I stopped by the multiparous patient. She was happy to have the children and that they were all healthy, but what was she going to do? Where would she go? I called a social work consult.
ICU Time: I headed to the ICU. A sad story: a 24-year-old woman was unresponsive. She had a living will and did not want prolonged life support. She had choked on a piece of fruit. I walked into a room crowded with her family; it looked like seven very short uncles—one of whom was a doctor. They watched sadly as I pulled the endotracheal tube and the IVs. I told them I’d give them some time with her alone and would be back in an hour. It was a somber and tearful affair. She was so young and so beautiful. What a tragic end.
Another ED call; this bug-swallowing lady was driving them nuts. Now she was claiming she had swallowed a thrush, or maybe had thrush. Either way, she was gone before I got down there to see her.
I met with the social worker. She was dressed in a fancy gown and said she had just been at a party. She heard the story of the lady who had had so many children. She thought for a moment then said she had a few leads to check and would get back to me later that day.
We had a rush in the ED: a boy I thought might have rhinophyma and stiff-man syndrome, a girl with warts on her lips she attributed to kissing a frog, another with a glass splinter in her foot. There was a Mr. W.W. Winkee with hypothermia, and a young girl named Mary who thought she had contracted anthrax from a sheep. There was a boy named Jackie Horner with a tenosynovitis of the thumb.
The old lady came back in again. Now she was complaining of abdominal distention. When I finally laid eyes on her, I noted she certainly had a large abdomen. I grabbed a quick X-ray. Apparently she had taken my warnings against consuming avian and invertebrate entities, as now she had radiographic evidence of a feline skeleton. I planned to send her to a tertiary-care facility; perhaps they could do an endoscopic cat removal. Whatever they did, I was afraid if she kept this up she was bound to die.
I headed back to the ICU. They had dressed the girl, Miss White, in her street clothes and done her hair and makeup, but nothing could hide her severe pallor. She looked so peaceful.
Her “uncles” expectantly greeted me. They said in unison, “Welcome, Dr. Prince.” They were all inappropriately smiling. What was going on here? I went to declare her dead. I held a mirror up to her face, no signs of breathing, no lung sounds with auscultation. I laid my fingers gently across her throat. No pulse, but her skin was strangely pliant and warm.
I stared at her lovely face and the rest of the world suddenly shut itself off from me. I felt like singing. I could not stop myself … how unprofessional. But I bent over and gently kissed her goodbye. Suddenly there was music playing and—even more strangely—woodland animals frolicking at my feet. The uncles, who turned out to be roommates not relatives (had I known that I would never have stopped life support!), danced merrily. I looked back at her, and her eyes were open. She was smiling and gazing at her future husband, me.
Epilogue: The masticatory old lady eventually died from eating tainted horse meat. Mrs. Sprat improved with a low-fat diet. Mr. Wolfe died in a tragic logging accident, killed by the swing of an ax. The housing issues of the old lady with so many kids she did not know what to do was settled by the social worker, who became the septuplets’ godmother and found them housing in a refurbished, oversize shoe. As for Snow and me, we plan on living happily ever after. TH
Dr. Newman is the physician editor of The Hospitalist. He’s also consultant, Hospital Internal Medicine, and assistant professor of internal medicine and medical history, Mayo Clinic College of Medicine, Rochester, Minn.
Having finished my family practice residency, and not being tied down by friends or family, I thought a round of working locum tenens would be a good way to see some different styles of practice.
I contacted a company called Locum-motion, filled out my paper work and was on my way. I had graduated from the University of Hamlin a few weeks earlier, armed with stethoscope and hammer. Dr. Claudio Prince (that’s me) was ready to stamp out disease and save lives.
There were several practice options available, but I chose a small hospital in Bremen. The three doctors who ran the hospital were going on a musical cruise for a week, and I would cover the entire facility for that time—ED and floor. I met with Drs. Baker, Butcher, and Maker briefly before they left for a trip. There were only a few inpatients, and one patient in the ICU. Then they were gone, and I was on my own.
My First Patient: I thought I’d start with the inpatients. The first was a Mr. B.B. Wolfe. He had been admitted for myalgia.
I was shocked when I walked into the room. He had severe hypertrichosis, prominent dentition, and proptosis. I briefly considered porphyria. I noted his history of muscle aches, high fever, facial swelling, and visual disturbance. His admit lab had showed a high CPK and LDH.
It sounded like an infestation of Trichinella to me. I questioned him about the ingestion of raw pork. He looked me in the eye and asked if his answer would be part of his medical record. I told him he needed to tell the truth for me to help him. Finally, he admitted he had eaten several portions of uncooked pig. I began to explain to him about the workup, the need for a muscle biopsy, and treatment options like mebendazole or steroids. I described the intestinal stage, which occurs between two and seven days after ingestion, when encysted larvae are liberated from the meat by gastric juices. I told him how the larvae mature into adult worms that burrow into the intestinal mucosa. I described the muscle stage, which develops after the first week and represents the period when adult-derived larvae in the intestines enter the bloodstream and disseminate hematogenously, then enter skeletal muscle causing pain.
He jumped out of bed and said he was leaving AMA; he had an appointment with a red-hooded girl. Whatever. I let him go; not much I could do to prevent his departure.
In the ED: I got a call from the ED about an old lady who had come in, having nearly choked on a bug. She looked fine to me, and I let her go. While down there, another older woman came in—in active labor. She admitted to having taken her friend’s Clomid, and had had little antepartum care secondary to a dearth of health insurance. Before I knew it, we were in the labor suite. First came one boy, then another, then another. I thought I was done, then two girls, another boy, then another girl. Seven babies—incredible! She moaned, not knowing what she was going to do with all these children.
I headed back to the floor to see more patients. The second one for the day was simple: a scrotal burn on a Mr. J.B. Nimble, who had been injured jumping over a flame. He was ready for discharge. The third patient was interesting, a Mrs. Spratt. I had been called by the lab with word that her serum looked like mayonnaise. She had abdominal pain, hepatosplenomegaly, memory loss, dyspnea and eruptive xanthomas. It sounded like type V hyperlipoproteinemia with chylomicronemia syndrome. What an interesting case, probably worse secondary to her very high-fat diet.
I walked down the hall to see my last inpatient, billed as a young man with psychotic depression pending psychiatric placement. I heard him yelling about burning witches and eating the walls. It sounded pretty psychotic. (I learned that his sister had been arraigned on manslaughter charges.) I entered the room and was struck by the smell of his breath, like cherries. A fruity smell, could he be ketotic? I instantly thought about ethylene glycol toxicity, maybe he had been sipping antifreeze, but there were no oxalate crystals in his urine. Was it delirium? I checked an O2 saturation; 95%, that wasn’t it. His blood pressure was 120/60. How about checking his finger-stick glucose? It read >400. This boy was in DKA! I started an insulin drip and hydration. It was only later I learned he had been on a starvation diet for weeks, then had binged on candy. His story about burning an old woman in a stove was unfortunately true. I called child protective services.
The ED pager went off again. That old lady who had swallowed a bug had ingested another, possibly a spider. I was worried about brown recluse or black widow envenomation, but it seemed to have been a simple barn spider, as she was now feeling OK. Again I discharged her with stern warnings to limit her invertebrate consumption. I stopped by the multiparous patient. She was happy to have the children and that they were all healthy, but what was she going to do? Where would she go? I called a social work consult.
ICU Time: I headed to the ICU. A sad story: a 24-year-old woman was unresponsive. She had a living will and did not want prolonged life support. She had choked on a piece of fruit. I walked into a room crowded with her family; it looked like seven very short uncles—one of whom was a doctor. They watched sadly as I pulled the endotracheal tube and the IVs. I told them I’d give them some time with her alone and would be back in an hour. It was a somber and tearful affair. She was so young and so beautiful. What a tragic end.
Another ED call; this bug-swallowing lady was driving them nuts. Now she was claiming she had swallowed a thrush, or maybe had thrush. Either way, she was gone before I got down there to see her.
I met with the social worker. She was dressed in a fancy gown and said she had just been at a party. She heard the story of the lady who had had so many children. She thought for a moment then said she had a few leads to check and would get back to me later that day.
We had a rush in the ED: a boy I thought might have rhinophyma and stiff-man syndrome, a girl with warts on her lips she attributed to kissing a frog, another with a glass splinter in her foot. There was a Mr. W.W. Winkee with hypothermia, and a young girl named Mary who thought she had contracted anthrax from a sheep. There was a boy named Jackie Horner with a tenosynovitis of the thumb.
The old lady came back in again. Now she was complaining of abdominal distention. When I finally laid eyes on her, I noted she certainly had a large abdomen. I grabbed a quick X-ray. Apparently she had taken my warnings against consuming avian and invertebrate entities, as now she had radiographic evidence of a feline skeleton. I planned to send her to a tertiary-care facility; perhaps they could do an endoscopic cat removal. Whatever they did, I was afraid if she kept this up she was bound to die.
I headed back to the ICU. They had dressed the girl, Miss White, in her street clothes and done her hair and makeup, but nothing could hide her severe pallor. She looked so peaceful.
Her “uncles” expectantly greeted me. They said in unison, “Welcome, Dr. Prince.” They were all inappropriately smiling. What was going on here? I went to declare her dead. I held a mirror up to her face, no signs of breathing, no lung sounds with auscultation. I laid my fingers gently across her throat. No pulse, but her skin was strangely pliant and warm.
I stared at her lovely face and the rest of the world suddenly shut itself off from me. I felt like singing. I could not stop myself … how unprofessional. But I bent over and gently kissed her goodbye. Suddenly there was music playing and—even more strangely—woodland animals frolicking at my feet. The uncles, who turned out to be roommates not relatives (had I known that I would never have stopped life support!), danced merrily. I looked back at her, and her eyes were open. She was smiling and gazing at her future husband, me.
Epilogue: The masticatory old lady eventually died from eating tainted horse meat. Mrs. Sprat improved with a low-fat diet. Mr. Wolfe died in a tragic logging accident, killed by the swing of an ax. The housing issues of the old lady with so many kids she did not know what to do was settled by the social worker, who became the septuplets’ godmother and found them housing in a refurbished, oversize shoe. As for Snow and me, we plan on living happily ever after. TH
Dr. Newman is the physician editor of The Hospitalist. He’s also consultant, Hospital Internal Medicine, and assistant professor of internal medicine and medical history, Mayo Clinic College of Medicine, Rochester, Minn.
How to Hire and Use Clerical Staff
For the first few years of my career I was my own secretary. The hospitalist group I was part of ranged in size from two to nine doctors, and each of us handled all our own telephone correspondence and paperwork without clerical help. If you looked up our “office” phone number in the hospital’s physician directory you would find each individual’s pager number.
As a result, each of us got many pages every day regarding routine administrative issues such as hospital medical records, death certificates, and billing questions. Sometimes I felt as though I were answering nearly as many calls via pager as the hospital operator. And the pages about important clinical issues were mixed with all these routine inquiries.
While doing without clerical support in a hospitalist practice can help keep your overhead really low (ours was always well under 10%), it is not an efficient way to operate. A nonclinical support person is nearly always worthwhile. But, while the group I was part of made the mistake of trying to do without such a person (a problem we eventually fixed), a number of groups make the opposite mistake and hire too much clerical help, making it difficult or impossible to justify the cost.
Think carefully about clerical support positions. Unfortunately, in many practices in which the hospitalists are employees of the hospital, the doctors may not be engaged in deciding the optimal role and staffing (number of fulltime employees, or FTEs) for this position. To the doctors, it feels as though this person doesn’t cost them anything (in many cases the doctors aren’t paying for it directly, the hospital is), so they might not spend a lot of time thinking about whether they’re really getting good value for the money. But the doctors are in a much better position than other hospital administrators to know whether that position optimally supports the practice.
The amount of staffing and precise job descriptions will vary tremendously from one practice to another. I want to offer some general guidelines worth consideration by nearly all practices. This discussion is not about support personnel, such as case managers dedicated to the hospitalist practice, midlevel providers, or other clinical support staff. This discussion is really about the front-office support staff for your practice.
How Many to Hire?
My experience suggests a hospitalist practice should have about one FTE of clerical support for every five to 15 FTE hospitalists. The optimal staffing for a particular practice will vary depending on the person’s precise responsibilities. A practice that operates at more than one site (e.g., one hospitalist group covers two hospitals) will usually need more support than one that operates in one hospital.
Practices smaller than five or six FTE hospitalists often need less than full-time support. They might work well using part-time clerical support from an existing member of the hospital’s staff, such as someone in administration or the medical staff office. In many cases this might mean the person has one incoming phone line dedicated to hospitalist calls and another dedicated to the other activity. Depending on which line rings, he/she answers by saying, “hospitalist office,” or “medical staff office.” Usually it is best for the person to be responsible for both activities all day long and not divide his/her time into working for the hospitalists only until noon, then spending the rest of each day supporting the other activity. Until the group I am currently part of grew to eight FTE hospitalists, our clerical support person had a full-time job—half of which was devoted to supporting our practice and the other half to supporting the hospital’s Institutional Review Board (IRB).
Define the Job
There are a number of common ways for a support person to contribute to the practice, which I have grouped into several broad categories:
Handle telephone correspondence. This person should answer all calls to the practice’s main office number. Most practices will have a separate number for billing inquiries, and clinical calls from the hospital’s nursing staff are usually paged directly to the doctor by a nurse. But that still leaves a lot of calls that will go to the support person, such as administrative questions about the practice, calls from former patients (who have been discharged) and families, pharmacies (e.g., asking about refills), funeral homes, and others.
Some practices use a “triage pager” system in which all calls about new referrals to the practice (e.g., from ED doctors, referring PCPs, surgeons requesting consults) always go to the triage pager—day or night. Usually the individual doctors take turns carrying and responding to the triage pager, and after hearing about a referral to the practice they will call the doctor who is up next for new patients and pass the information along. In a large practice, that pager can generate a huge number of daytime calls, making it difficult or impossible for the person holding the triage pager to also care for patients.
Some practices have found that the practice clerical support person can take all those calls during the daytime Monday through Friday and pass them along to the appropriate hospitalist. The clerical person would typically get only the patient name and location and the referring doctor’s name and contact information, then page it to the hospitalist next in line for a new referral. That hospitalist would then call back the referring physician to get more clinical information. That relieves a member of the practice from taking all the calls. And, it puts the referring physician directly in contact with the hospitalist who will see the patient, rather than a triage doctor who won’t be caring for the patient. This should mean a better handoff.
Handle paper correspondence. This person can sort all the faxes, mail, and medical records that come to the practice, and put them in each doctor’s mail box in the office. He/she might initiate work on some forms. For example, upon arrival of a form to certify medical necessity for a piece of equipment (e.g., home oxygen ordered on a patient recently discharged) he might open the envelope, complete as much of the form as possible, attach the relevant records from the hospital stay, and leave all this for the doctor to sign.
Another potentially critical function is to request and pursue outside clinical records requested by one of the hospitalists. For example, a hospitalist admits Ms. Smith at 1 a.m. and realizes it will be helpful to get previous creatinine values from the PCP’s office and the report of a prior cardiac cath from an outside hospital. The hospitalist could simply record a voice mail (at 1 a.m., while seeing the patient) requesting that the practice assistant track down these things the next morning. That might include ensuring an appropriate release-of-information form is signed by the patient and faxed to the outside facility. When the records arrive, the assistant would place them on the patient’s chart (and, if necessary, page the hospitalist to report that the records have arrived).
Support billing functions. Practices use many strategies to ensure good documentation, coding, charge capture, and billing. The assistant might play an important role in this process. For example, the doctors might first report all charge data to the practice assistant who reviews it to make sure there are no conflicting charges (e.g., two doctors bill the same service to a patient on the same day) and no missing charges (e.g., a doctor forgot to submit a charge for one day of a patient’s stay). The assistant can be the principle connection between the doctors and the billing service and might be the first person to troubleshoot problems encountered by the billing service (e.g., getting additional documentation, figuring out which doctor can best address an ICD-9 code that lacks a fifth digit).
Perform general practice administrative functions. The assistant can keep track of when each doctor needs to renew his or her state license, DEA certificate, ACLS certificate, as well as keep track of total hours of CME (e.g., know how many more CME hours each doctor needs this year for state licensing requirements). He/she could also assist in various human resource functions such as ensuring each doctor responds during the open-enrollment period for benefits each year.
In some practices it is appropriate for the assistant to create the physician work schedule for the next month, quarter, or year, and serve as the main point of contact for any schedule change the doctor’s need to make. However, for groups that use a complicated scheduling system, the doctors will often need to take an active role in its creation. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is a co-founder and past-president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. This column represents his views and is not intended to reflect an official position of SHM.
For the first few years of my career I was my own secretary. The hospitalist group I was part of ranged in size from two to nine doctors, and each of us handled all our own telephone correspondence and paperwork without clerical help. If you looked up our “office” phone number in the hospital’s physician directory you would find each individual’s pager number.
As a result, each of us got many pages every day regarding routine administrative issues such as hospital medical records, death certificates, and billing questions. Sometimes I felt as though I were answering nearly as many calls via pager as the hospital operator. And the pages about important clinical issues were mixed with all these routine inquiries.
While doing without clerical support in a hospitalist practice can help keep your overhead really low (ours was always well under 10%), it is not an efficient way to operate. A nonclinical support person is nearly always worthwhile. But, while the group I was part of made the mistake of trying to do without such a person (a problem we eventually fixed), a number of groups make the opposite mistake and hire too much clerical help, making it difficult or impossible to justify the cost.
Think carefully about clerical support positions. Unfortunately, in many practices in which the hospitalists are employees of the hospital, the doctors may not be engaged in deciding the optimal role and staffing (number of fulltime employees, or FTEs) for this position. To the doctors, it feels as though this person doesn’t cost them anything (in many cases the doctors aren’t paying for it directly, the hospital is), so they might not spend a lot of time thinking about whether they’re really getting good value for the money. But the doctors are in a much better position than other hospital administrators to know whether that position optimally supports the practice.
The amount of staffing and precise job descriptions will vary tremendously from one practice to another. I want to offer some general guidelines worth consideration by nearly all practices. This discussion is not about support personnel, such as case managers dedicated to the hospitalist practice, midlevel providers, or other clinical support staff. This discussion is really about the front-office support staff for your practice.
How Many to Hire?
My experience suggests a hospitalist practice should have about one FTE of clerical support for every five to 15 FTE hospitalists. The optimal staffing for a particular practice will vary depending on the person’s precise responsibilities. A practice that operates at more than one site (e.g., one hospitalist group covers two hospitals) will usually need more support than one that operates in one hospital.
Practices smaller than five or six FTE hospitalists often need less than full-time support. They might work well using part-time clerical support from an existing member of the hospital’s staff, such as someone in administration or the medical staff office. In many cases this might mean the person has one incoming phone line dedicated to hospitalist calls and another dedicated to the other activity. Depending on which line rings, he/she answers by saying, “hospitalist office,” or “medical staff office.” Usually it is best for the person to be responsible for both activities all day long and not divide his/her time into working for the hospitalists only until noon, then spending the rest of each day supporting the other activity. Until the group I am currently part of grew to eight FTE hospitalists, our clerical support person had a full-time job—half of which was devoted to supporting our practice and the other half to supporting the hospital’s Institutional Review Board (IRB).
Define the Job
There are a number of common ways for a support person to contribute to the practice, which I have grouped into several broad categories:
Handle telephone correspondence. This person should answer all calls to the practice’s main office number. Most practices will have a separate number for billing inquiries, and clinical calls from the hospital’s nursing staff are usually paged directly to the doctor by a nurse. But that still leaves a lot of calls that will go to the support person, such as administrative questions about the practice, calls from former patients (who have been discharged) and families, pharmacies (e.g., asking about refills), funeral homes, and others.
Some practices use a “triage pager” system in which all calls about new referrals to the practice (e.g., from ED doctors, referring PCPs, surgeons requesting consults) always go to the triage pager—day or night. Usually the individual doctors take turns carrying and responding to the triage pager, and after hearing about a referral to the practice they will call the doctor who is up next for new patients and pass the information along. In a large practice, that pager can generate a huge number of daytime calls, making it difficult or impossible for the person holding the triage pager to also care for patients.
Some practices have found that the practice clerical support person can take all those calls during the daytime Monday through Friday and pass them along to the appropriate hospitalist. The clerical person would typically get only the patient name and location and the referring doctor’s name and contact information, then page it to the hospitalist next in line for a new referral. That hospitalist would then call back the referring physician to get more clinical information. That relieves a member of the practice from taking all the calls. And, it puts the referring physician directly in contact with the hospitalist who will see the patient, rather than a triage doctor who won’t be caring for the patient. This should mean a better handoff.
Handle paper correspondence. This person can sort all the faxes, mail, and medical records that come to the practice, and put them in each doctor’s mail box in the office. He/she might initiate work on some forms. For example, upon arrival of a form to certify medical necessity for a piece of equipment (e.g., home oxygen ordered on a patient recently discharged) he might open the envelope, complete as much of the form as possible, attach the relevant records from the hospital stay, and leave all this for the doctor to sign.
Another potentially critical function is to request and pursue outside clinical records requested by one of the hospitalists. For example, a hospitalist admits Ms. Smith at 1 a.m. and realizes it will be helpful to get previous creatinine values from the PCP’s office and the report of a prior cardiac cath from an outside hospital. The hospitalist could simply record a voice mail (at 1 a.m., while seeing the patient) requesting that the practice assistant track down these things the next morning. That might include ensuring an appropriate release-of-information form is signed by the patient and faxed to the outside facility. When the records arrive, the assistant would place them on the patient’s chart (and, if necessary, page the hospitalist to report that the records have arrived).
Support billing functions. Practices use many strategies to ensure good documentation, coding, charge capture, and billing. The assistant might play an important role in this process. For example, the doctors might first report all charge data to the practice assistant who reviews it to make sure there are no conflicting charges (e.g., two doctors bill the same service to a patient on the same day) and no missing charges (e.g., a doctor forgot to submit a charge for one day of a patient’s stay). The assistant can be the principle connection between the doctors and the billing service and might be the first person to troubleshoot problems encountered by the billing service (e.g., getting additional documentation, figuring out which doctor can best address an ICD-9 code that lacks a fifth digit).
Perform general practice administrative functions. The assistant can keep track of when each doctor needs to renew his or her state license, DEA certificate, ACLS certificate, as well as keep track of total hours of CME (e.g., know how many more CME hours each doctor needs this year for state licensing requirements). He/she could also assist in various human resource functions such as ensuring each doctor responds during the open-enrollment period for benefits each year.
In some practices it is appropriate for the assistant to create the physician work schedule for the next month, quarter, or year, and serve as the main point of contact for any schedule change the doctor’s need to make. However, for groups that use a complicated scheduling system, the doctors will often need to take an active role in its creation. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is a co-founder and past-president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. This column represents his views and is not intended to reflect an official position of SHM.
For the first few years of my career I was my own secretary. The hospitalist group I was part of ranged in size from two to nine doctors, and each of us handled all our own telephone correspondence and paperwork without clerical help. If you looked up our “office” phone number in the hospital’s physician directory you would find each individual’s pager number.
As a result, each of us got many pages every day regarding routine administrative issues such as hospital medical records, death certificates, and billing questions. Sometimes I felt as though I were answering nearly as many calls via pager as the hospital operator. And the pages about important clinical issues were mixed with all these routine inquiries.
While doing without clerical support in a hospitalist practice can help keep your overhead really low (ours was always well under 10%), it is not an efficient way to operate. A nonclinical support person is nearly always worthwhile. But, while the group I was part of made the mistake of trying to do without such a person (a problem we eventually fixed), a number of groups make the opposite mistake and hire too much clerical help, making it difficult or impossible to justify the cost.
Think carefully about clerical support positions. Unfortunately, in many practices in which the hospitalists are employees of the hospital, the doctors may not be engaged in deciding the optimal role and staffing (number of fulltime employees, or FTEs) for this position. To the doctors, it feels as though this person doesn’t cost them anything (in many cases the doctors aren’t paying for it directly, the hospital is), so they might not spend a lot of time thinking about whether they’re really getting good value for the money. But the doctors are in a much better position than other hospital administrators to know whether that position optimally supports the practice.
The amount of staffing and precise job descriptions will vary tremendously from one practice to another. I want to offer some general guidelines worth consideration by nearly all practices. This discussion is not about support personnel, such as case managers dedicated to the hospitalist practice, midlevel providers, or other clinical support staff. This discussion is really about the front-office support staff for your practice.
How Many to Hire?
My experience suggests a hospitalist practice should have about one FTE of clerical support for every five to 15 FTE hospitalists. The optimal staffing for a particular practice will vary depending on the person’s precise responsibilities. A practice that operates at more than one site (e.g., one hospitalist group covers two hospitals) will usually need more support than one that operates in one hospital.
Practices smaller than five or six FTE hospitalists often need less than full-time support. They might work well using part-time clerical support from an existing member of the hospital’s staff, such as someone in administration or the medical staff office. In many cases this might mean the person has one incoming phone line dedicated to hospitalist calls and another dedicated to the other activity. Depending on which line rings, he/she answers by saying, “hospitalist office,” or “medical staff office.” Usually it is best for the person to be responsible for both activities all day long and not divide his/her time into working for the hospitalists only until noon, then spending the rest of each day supporting the other activity. Until the group I am currently part of grew to eight FTE hospitalists, our clerical support person had a full-time job—half of which was devoted to supporting our practice and the other half to supporting the hospital’s Institutional Review Board (IRB).
Define the Job
There are a number of common ways for a support person to contribute to the practice, which I have grouped into several broad categories:
Handle telephone correspondence. This person should answer all calls to the practice’s main office number. Most practices will have a separate number for billing inquiries, and clinical calls from the hospital’s nursing staff are usually paged directly to the doctor by a nurse. But that still leaves a lot of calls that will go to the support person, such as administrative questions about the practice, calls from former patients (who have been discharged) and families, pharmacies (e.g., asking about refills), funeral homes, and others.
Some practices use a “triage pager” system in which all calls about new referrals to the practice (e.g., from ED doctors, referring PCPs, surgeons requesting consults) always go to the triage pager—day or night. Usually the individual doctors take turns carrying and responding to the triage pager, and after hearing about a referral to the practice they will call the doctor who is up next for new patients and pass the information along. In a large practice, that pager can generate a huge number of daytime calls, making it difficult or impossible for the person holding the triage pager to also care for patients.
Some practices have found that the practice clerical support person can take all those calls during the daytime Monday through Friday and pass them along to the appropriate hospitalist. The clerical person would typically get only the patient name and location and the referring doctor’s name and contact information, then page it to the hospitalist next in line for a new referral. That hospitalist would then call back the referring physician to get more clinical information. That relieves a member of the practice from taking all the calls. And, it puts the referring physician directly in contact with the hospitalist who will see the patient, rather than a triage doctor who won’t be caring for the patient. This should mean a better handoff.
Handle paper correspondence. This person can sort all the faxes, mail, and medical records that come to the practice, and put them in each doctor’s mail box in the office. He/she might initiate work on some forms. For example, upon arrival of a form to certify medical necessity for a piece of equipment (e.g., home oxygen ordered on a patient recently discharged) he might open the envelope, complete as much of the form as possible, attach the relevant records from the hospital stay, and leave all this for the doctor to sign.
Another potentially critical function is to request and pursue outside clinical records requested by one of the hospitalists. For example, a hospitalist admits Ms. Smith at 1 a.m. and realizes it will be helpful to get previous creatinine values from the PCP’s office and the report of a prior cardiac cath from an outside hospital. The hospitalist could simply record a voice mail (at 1 a.m., while seeing the patient) requesting that the practice assistant track down these things the next morning. That might include ensuring an appropriate release-of-information form is signed by the patient and faxed to the outside facility. When the records arrive, the assistant would place them on the patient’s chart (and, if necessary, page the hospitalist to report that the records have arrived).
Support billing functions. Practices use many strategies to ensure good documentation, coding, charge capture, and billing. The assistant might play an important role in this process. For example, the doctors might first report all charge data to the practice assistant who reviews it to make sure there are no conflicting charges (e.g., two doctors bill the same service to a patient on the same day) and no missing charges (e.g., a doctor forgot to submit a charge for one day of a patient’s stay). The assistant can be the principle connection between the doctors and the billing service and might be the first person to troubleshoot problems encountered by the billing service (e.g., getting additional documentation, figuring out which doctor can best address an ICD-9 code that lacks a fifth digit).
Perform general practice administrative functions. The assistant can keep track of when each doctor needs to renew his or her state license, DEA certificate, ACLS certificate, as well as keep track of total hours of CME (e.g., know how many more CME hours each doctor needs this year for state licensing requirements). He/she could also assist in various human resource functions such as ensuring each doctor responds during the open-enrollment period for benefits each year.
In some practices it is appropriate for the assistant to create the physician work schedule for the next month, quarter, or year, and serve as the main point of contact for any schedule change the doctor’s need to make. However, for groups that use a complicated scheduling system, the doctors will often need to take an active role in its creation. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is a co-founder and past-president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. This column represents his views and is not intended to reflect an official position of SHM.
In the Literature
Thrombocytopenia Reaction to Vancomycin
Von Drygalski A, Curtis BR, Bougie DW, et al. Vancomycin-induced immune thrombocytopenia. N Engl J Med. 2007 Mar 1;356(9):904-910
The use of vancomycin has grown exponentially in the past 20 years.1 Physicians have become increasingly aware of its major side effects, such as red man syndrome, hypersensitivity, neutropenia, and nephrotoxicity. But there have been only a few case reports of thrombocytopenia associated with this drug. This article looked at cases of thrombocytopenia in patients referred for clinical suspicion of vancomycin-induced thrombocytopenia.
From 2001-2005, serum samples were sent to the Platelet and Neutrophil Immunology Laboratory at the BloodCenter of Wisconsin in Milwaukee for testing for vancomycin-dependent antibodies from several sites. Clinical information regarding these patients was obtained from their referring physicians and one of the authors. Platelet reactive antibodies were detected by flow cytometry.
IgG and IgM vancomycin-dependent antibodies were detected in 34 patients. It was found that platelets dropped an average of 93% from pretreatment levels, and the average nadir occurred on day eight. The mean platelet count was 13,600. After vancomycin was discontinued, the platelet count returned to normal in all patients except for the three who died. The average time for resolution of thrombocytopenia was 7.5 days.
Unlike other drug-induced thrombocytopenia, these cases of thrombocytopenia associated with vancomycin appear to be more prone to significant hemorrhage. In this group 34% were found to have had severe hemorrhage defined in this study as florid petechial hemorrhages, ecchymoses, and oozing form the buccal mucosa. Three patients who had renal insufficiency were found to be profoundly thrombocytopenic for a longer duration, presumably due to delayed clearance of vancomycin in this setting.
Based on this study, it appears thrombocytopenia is a significant adverse reaction that can be attributed to vancomycin. Unlike other drug-induced thrombocytopenias, it appears to be associated with a higher likelihood of significant hemorrhage, as well.
Thrombocytopenia is a common occurrence in the acutely ill hospitalized patient and has been linked to increased hospital mortality and increased length of stay.2 Many drugs and diseases that hospitalists treat are associated with thrombocytopenia. The indications for usage of vancomycin continues to grow with the increasing number of patients with prosthetic devices and intravascular access, and the increasing prevalence of MRSA. This study raises awareness of a significant side effect that can be associated with vancomycin.
References
- Ena J, Dick RW, Jones RN, et al. The epidemiology of intravenous vancomycin usage in a university hospital: a 10-year study. JAMA. 1993 Feb 3;269(5):598-602. Comment in JAMA. 1993 Sep 22-29;270(12):1426.
- Crowther MA, Cook DJ, Meade M, et al. Thrombocytopenia in medical-surgical critically ill patients: prevalence, incidence, and risk factors. J Crit Care. 2005 Dec;20(4):248-253.
Can the mBRS Stratify Pts Admitted for Nonvariceal Upper GI Bleeds?
Romagnuolo J, Barkun AN, Enns R, et al. Simple clinical predictors may obviate urgent endoscopy in selected patients with nonvariceal upper gastrointestinal tract bleeding. Arch Intern Med. 2007 Feb 12;167(3):265-270.
Nonvariceal upper gastrointestinal bleeding is one of the top 10 admission diagnoses based on reviews of diagnosis-related groups. Patients with low-risk lesions on endoscopy, such as ulcers with a clean base, esophagitis, gastritis, duodenitis, or Mallory-Weiss tears, are felt to have less than a 5% chance of recurrent bleeding. In some instances, these patients can be treated successfully and discharged to home.1
Unfortunately, endoscopy is not always available—especially late at night and on weekends. It would be helpful to have a clinical prediction rule to identify patients at low risk for bleeding who could be safely discharged to get endoscopy within a few days.
In the study, 1,869 patients who had undergone upper endoscopy for upper gastrointestinal bleeding were entered into a Canadian national Registry for Upper GI Bleeding and Endoscopy (RUGBE). A modified Blatchford risk score (mBRS) was calculated to see if it could predict the presence of high-risk stigmata of bleeding, rebleeding rates, and mortality.
This mBRS was also compared with another scoring system—the Rockall score. The mBRS uses clinical and laboratory data to risk assess nonvariceal bleeding. The variables included in the scoring system include hemoglobin, systolic blood pressure, heart rate, melena, liver disease, and heart failure. High-risk endoscopic stigmata were defined as adherent clot after irrigation, a bleeding, oozing or spurting vessel, or a nonbleeding visible vessel. Rebleeding was defined as hematemesis, melena, or a bloody nasogastric aspirate in the presence of shock or a decrease in hemoglobin of 2 g/dL or more.
Patients who had a modified Blatchford risk score of <1 were found to have a lower likelihood of high-risk stigmata on endoscopy and were at a low risk for rebleeding (5%). Patients who had high-risk stigmata on endoscopy but an mBRS score of <1 were also found to have low rebleeding rates. The mBRS seemed to a better predictor than the Rockall score for high-risk stigmata and for rebleeding rates.
Patients with nonvariceal upper gastrointestinal tract bleeding may be identified as low risk for re-bleeding if they are normotensive, not tachycardic, not anemic, and do not have active melena, liver disease, or heart failure. It is conceivable that if endoscopy were not available, these patients could be sent home on high-dose proton pump inhibitor and asked to return for outpatient upper endoscopy within a few days.
The study certainly raises interesting questions. Whether it is acceptable practice to discharge a “low-risk” patient with an upper gastrointestinal hemorrhage on a high-dose proton pump inhibitor with good social support and close outpatient follow-up, but without diagnostic endoscopy is still unclear.
The study is limited by the fact that it is a retrospective analysis; however, it does examine a large cohort of patients. The authors acknowledge this, and this work could lead to a prospective randomized trial that would help answer this question. In the meantime, the mBRS may be a helpful tool to help risk stratify patients admitted for nonvariceal upper gastrointestinal bleeding.
References
- Cipolletta L, Bianco M, Rotondano G, et al. Outpatient management for low-risk nonvariceal upper GI bleeding: a randomized controlled trial. Gastrointest Endosc. 2002;55(1):1-5.
Lumbar Puncture to Reduce Adverse Events
Straus SE, Thorpe KE, Holroyd-Leduc J. How do I perform a lumbar puncture and analyze the results to diagnose bacterial meningitis? JAMA. 2006 Oct 25;296(16):2012-2022.
Lumbar punctures (LPs) remain a common diagnostic test performed by physicians to rule out meningitis. This procedure may be associated with adverse events, with headache and backache the most commonly reported. This systematic review and meta-analysis sought to review the evidence regarding diagnostic lumbar puncture techniques that might reduce the risk of adverse events, and to examine the accuracy of cerebrospinal fluid (CSF) analysis in the diagnosis of bacterial meningitis.
Studies were identified through searches of the Cochrane Library (www3.interscience.wiley.com/cgi-bin/mrwhome/106568753/AboutCochrane.html), MEDLINE from 1966 to January 2006, and EMBASE from 1980 to January 2006 (without language restrictions) to identify relevant studies. Bibliographies of retrieved articles were also used as data sources.
Randomized controlled trials of patients 18 or older undergoing lumbar puncture testing interventions to facilitate a successful diagnostic procedure or reduce adverse events were identified and selected. As a secondary outcome, trials that assessed the accuracy of CSF biochemical analysis for the diagnosis of bacterial meningitis were also identified and included. Trials that studied spinal anesthesia or myelography were excluded.
Study appraisals for quality (randomization, blinding, and outcome assessment) and data extraction were performed by two investigators independently. Fifteen randomized trials of interventions to reduce adverse events met criteria for inclusion, and four studies of the diagnostic test characteristics of CSF analysis met criteria and were included.
Meta-analysis with a random effects model of five studies (total of 587 patients) comparing atraumatic needles with standard needles yielded a nonsignificant decrease in the odds of headache with an atraumatic needle (absolute risk reduction [ARR], 12.3%; 95% confidence interval [CI], –1.72% to 26.2%). A single study of reinsertion of the stylet before needle removal (600 patients) showed a decreased risk of headache (ARR, 11.3%; 95% CI, 6.50%-16.2%). Meta-analysis of four studies (717 patients) revealed a nonsignificant decrease in headache in patients mobilized after LP (ARR 2.9%; 95% CI, –3.4 to 9.3%).
Data from the diagnostic test studies yielded the following likelihood ratios for diagnosing bacterial meningitis: A CSF–blood glucose ratio of 0.4 or less with a likelihood ratio of 18 (95% CI, 12-27); CSF white blood cell count of 500/µL or higher with a likelihood ratio of 15 (95% CI, 10-22); and CSF lactate level of >31.53 mg/dL with a likelihood ration of 21 (95% CI, 14-32) in accurately diagnosed bacterial meningitis.
These data support the reinsertion of the stylet before needle removal to reduce the risk of headache after lumbar puncture and that patients do not require bed rest after diagnostic lumbar puncture. Biochemical analyses, including CSF-blood glucose ratio, CSF leukocyte count and lactate level are useful in diagnosing bacterial meningitis.
This Rational Clinical Examination systematic review and meta-analysis provides a nice review of the available data on optimizing diagnostic lumbar puncture technique to reduce adverse events. It is somewhat remarkable so little has changed in our knowledge about this long-standing diagnostic procedure. Post-lumbar puncture headaches remain a challenge that may affect patient satisfaction as well as hospital (or observation unit) course particularly for patients who do not have evidence of bacterial meningitis once the analysis is complete.
This review seems to provide some useful answers for physicians performing lumbar puncture, who should consider selecting a small gauge needle and reinserting the stylet prior to removal. Future studies of other maneuvers to reduce post-procedure adverse events should be considered for the question of atraumatic needles, which may be technically more difficult to use. The review confirms and helps quantify the utility of CSF biochemical analysis in the diagnosis of bacterial meningitis.
Who’s Performing Procedures?
Wigton RS, Alguire P. The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians. Ann Intern Med. 2007 Mar 6;146(5):355-360. Comment in Ann Intern Med. 2007 Mar 6; 146(5):392-393.
Prior surveys of physicians documented that general internists performed a variety and significant number of procedures in their practice. Much has changed since those prior assessments, including physician training, practice settings, availability of subspecialists, and regulatory requirements that have altered physician’s practice with regard to procedures. This study sought to reassess the volume and variety of procedures performed by general internists compared with the prior survey of 1986. The final sample included 990 completed surveys from general internists from 1,389 returned questionnaires for a successful completion rate of 39.6%.
The median number of different procedures performed in practice decreased from 16 in 1986 to seven in 2004. Internists who practiced in smaller hospitals or smaller towns reported performing almost twice as many procedures as physicians in the largest hospitals and cities. Hours spent in the care of hospitalized patients were also associated with an increased number of different procedures—in particular mechanical ventilation, central venous catheter placement, and thoracentesis. For all but one of the 34 procedures common to both surveys, fewer general internists performed them in 2004 compared with 1986. Remarkably, for 22 of the 34 procedures, a greater than 50% reduction in the proportion of respondents who performed the procedure was noted.
In the 1986 survey, the majority of internists performed all but one of the six procedures required by the American Board of Internal Medicine (ABIM) for certification (abdominal paracentesis, arterial puncture for blood gases, central venous catheter placement, joint aspiration, lumbar puncture, and thoracentesis). Except for joint aspiration, in 2004 these required procedures were performed by 25% or fewer of the respondents.
The 2004 survey demonstrated a striking reduction in the number of different procedures performed by general internists, and a decrease in the proportion of internists who do most procedures. These reductions may stem from a variety of changes in physician practices, including the emergence of hospitalists, availability of subspecialty physicians and proceduralists, and changes in technology and regulatory environments.
Regardless of the forces behind these changes, internal medicine residents’ training in procedures should be re-examined.
Many of those in academic hospital medicine have noted a decline in procedures performed by general internists at large academic centers. This study affirms this trend overall and in particular for physicians in large urban settings or in the largest hospitals. The emergence of hospital medicine may have played a role in reducing the procedures performed by primary care (outpatient) physicians who now spend less time caring for medically ill hospitalized patients.
Residency programs now must consider how to incorporate procedure skills and training to align with the needs of internists. The rising interest in careers in hospital medicine (as opposed to outpatient primary care) necessitates a new approach and individualized plans for gaining procedural skills to match career goals and practice settings. The new ABIM policy acknowledges this greater variability in the procedures performed by internists in practice, and takes steps to more closely align procedure requirements and core manual skills with physician practice.
These changes and new flexibility in requirements provide another opportunity for academic hospital medicine programs to provide leadership, and help shape the training of inpatient physicians. TH
Thrombocytopenia Reaction to Vancomycin
Von Drygalski A, Curtis BR, Bougie DW, et al. Vancomycin-induced immune thrombocytopenia. N Engl J Med. 2007 Mar 1;356(9):904-910
The use of vancomycin has grown exponentially in the past 20 years.1 Physicians have become increasingly aware of its major side effects, such as red man syndrome, hypersensitivity, neutropenia, and nephrotoxicity. But there have been only a few case reports of thrombocytopenia associated with this drug. This article looked at cases of thrombocytopenia in patients referred for clinical suspicion of vancomycin-induced thrombocytopenia.
From 2001-2005, serum samples were sent to the Platelet and Neutrophil Immunology Laboratory at the BloodCenter of Wisconsin in Milwaukee for testing for vancomycin-dependent antibodies from several sites. Clinical information regarding these patients was obtained from their referring physicians and one of the authors. Platelet reactive antibodies were detected by flow cytometry.
IgG and IgM vancomycin-dependent antibodies were detected in 34 patients. It was found that platelets dropped an average of 93% from pretreatment levels, and the average nadir occurred on day eight. The mean platelet count was 13,600. After vancomycin was discontinued, the platelet count returned to normal in all patients except for the three who died. The average time for resolution of thrombocytopenia was 7.5 days.
Unlike other drug-induced thrombocytopenia, these cases of thrombocytopenia associated with vancomycin appear to be more prone to significant hemorrhage. In this group 34% were found to have had severe hemorrhage defined in this study as florid petechial hemorrhages, ecchymoses, and oozing form the buccal mucosa. Three patients who had renal insufficiency were found to be profoundly thrombocytopenic for a longer duration, presumably due to delayed clearance of vancomycin in this setting.
Based on this study, it appears thrombocytopenia is a significant adverse reaction that can be attributed to vancomycin. Unlike other drug-induced thrombocytopenias, it appears to be associated with a higher likelihood of significant hemorrhage, as well.
Thrombocytopenia is a common occurrence in the acutely ill hospitalized patient and has been linked to increased hospital mortality and increased length of stay.2 Many drugs and diseases that hospitalists treat are associated with thrombocytopenia. The indications for usage of vancomycin continues to grow with the increasing number of patients with prosthetic devices and intravascular access, and the increasing prevalence of MRSA. This study raises awareness of a significant side effect that can be associated with vancomycin.
References
- Ena J, Dick RW, Jones RN, et al. The epidemiology of intravenous vancomycin usage in a university hospital: a 10-year study. JAMA. 1993 Feb 3;269(5):598-602. Comment in JAMA. 1993 Sep 22-29;270(12):1426.
- Crowther MA, Cook DJ, Meade M, et al. Thrombocytopenia in medical-surgical critically ill patients: prevalence, incidence, and risk factors. J Crit Care. 2005 Dec;20(4):248-253.
Can the mBRS Stratify Pts Admitted for Nonvariceal Upper GI Bleeds?
Romagnuolo J, Barkun AN, Enns R, et al. Simple clinical predictors may obviate urgent endoscopy in selected patients with nonvariceal upper gastrointestinal tract bleeding. Arch Intern Med. 2007 Feb 12;167(3):265-270.
Nonvariceal upper gastrointestinal bleeding is one of the top 10 admission diagnoses based on reviews of diagnosis-related groups. Patients with low-risk lesions on endoscopy, such as ulcers with a clean base, esophagitis, gastritis, duodenitis, or Mallory-Weiss tears, are felt to have less than a 5% chance of recurrent bleeding. In some instances, these patients can be treated successfully and discharged to home.1
Unfortunately, endoscopy is not always available—especially late at night and on weekends. It would be helpful to have a clinical prediction rule to identify patients at low risk for bleeding who could be safely discharged to get endoscopy within a few days.
In the study, 1,869 patients who had undergone upper endoscopy for upper gastrointestinal bleeding were entered into a Canadian national Registry for Upper GI Bleeding and Endoscopy (RUGBE). A modified Blatchford risk score (mBRS) was calculated to see if it could predict the presence of high-risk stigmata of bleeding, rebleeding rates, and mortality.
This mBRS was also compared with another scoring system—the Rockall score. The mBRS uses clinical and laboratory data to risk assess nonvariceal bleeding. The variables included in the scoring system include hemoglobin, systolic blood pressure, heart rate, melena, liver disease, and heart failure. High-risk endoscopic stigmata were defined as adherent clot after irrigation, a bleeding, oozing or spurting vessel, or a nonbleeding visible vessel. Rebleeding was defined as hematemesis, melena, or a bloody nasogastric aspirate in the presence of shock or a decrease in hemoglobin of 2 g/dL or more.
Patients who had a modified Blatchford risk score of <1 were found to have a lower likelihood of high-risk stigmata on endoscopy and were at a low risk for rebleeding (5%). Patients who had high-risk stigmata on endoscopy but an mBRS score of <1 were also found to have low rebleeding rates. The mBRS seemed to a better predictor than the Rockall score for high-risk stigmata and for rebleeding rates.
Patients with nonvariceal upper gastrointestinal tract bleeding may be identified as low risk for re-bleeding if they are normotensive, not tachycardic, not anemic, and do not have active melena, liver disease, or heart failure. It is conceivable that if endoscopy were not available, these patients could be sent home on high-dose proton pump inhibitor and asked to return for outpatient upper endoscopy within a few days.
The study certainly raises interesting questions. Whether it is acceptable practice to discharge a “low-risk” patient with an upper gastrointestinal hemorrhage on a high-dose proton pump inhibitor with good social support and close outpatient follow-up, but without diagnostic endoscopy is still unclear.
The study is limited by the fact that it is a retrospective analysis; however, it does examine a large cohort of patients. The authors acknowledge this, and this work could lead to a prospective randomized trial that would help answer this question. In the meantime, the mBRS may be a helpful tool to help risk stratify patients admitted for nonvariceal upper gastrointestinal bleeding.
References
- Cipolletta L, Bianco M, Rotondano G, et al. Outpatient management for low-risk nonvariceal upper GI bleeding: a randomized controlled trial. Gastrointest Endosc. 2002;55(1):1-5.
Lumbar Puncture to Reduce Adverse Events
Straus SE, Thorpe KE, Holroyd-Leduc J. How do I perform a lumbar puncture and analyze the results to diagnose bacterial meningitis? JAMA. 2006 Oct 25;296(16):2012-2022.
Lumbar punctures (LPs) remain a common diagnostic test performed by physicians to rule out meningitis. This procedure may be associated with adverse events, with headache and backache the most commonly reported. This systematic review and meta-analysis sought to review the evidence regarding diagnostic lumbar puncture techniques that might reduce the risk of adverse events, and to examine the accuracy of cerebrospinal fluid (CSF) analysis in the diagnosis of bacterial meningitis.
Studies were identified through searches of the Cochrane Library (www3.interscience.wiley.com/cgi-bin/mrwhome/106568753/AboutCochrane.html), MEDLINE from 1966 to January 2006, and EMBASE from 1980 to January 2006 (without language restrictions) to identify relevant studies. Bibliographies of retrieved articles were also used as data sources.
Randomized controlled trials of patients 18 or older undergoing lumbar puncture testing interventions to facilitate a successful diagnostic procedure or reduce adverse events were identified and selected. As a secondary outcome, trials that assessed the accuracy of CSF biochemical analysis for the diagnosis of bacterial meningitis were also identified and included. Trials that studied spinal anesthesia or myelography were excluded.
Study appraisals for quality (randomization, blinding, and outcome assessment) and data extraction were performed by two investigators independently. Fifteen randomized trials of interventions to reduce adverse events met criteria for inclusion, and four studies of the diagnostic test characteristics of CSF analysis met criteria and were included.
Meta-analysis with a random effects model of five studies (total of 587 patients) comparing atraumatic needles with standard needles yielded a nonsignificant decrease in the odds of headache with an atraumatic needle (absolute risk reduction [ARR], 12.3%; 95% confidence interval [CI], –1.72% to 26.2%). A single study of reinsertion of the stylet before needle removal (600 patients) showed a decreased risk of headache (ARR, 11.3%; 95% CI, 6.50%-16.2%). Meta-analysis of four studies (717 patients) revealed a nonsignificant decrease in headache in patients mobilized after LP (ARR 2.9%; 95% CI, –3.4 to 9.3%).
Data from the diagnostic test studies yielded the following likelihood ratios for diagnosing bacterial meningitis: A CSF–blood glucose ratio of 0.4 or less with a likelihood ratio of 18 (95% CI, 12-27); CSF white blood cell count of 500/µL or higher with a likelihood ratio of 15 (95% CI, 10-22); and CSF lactate level of >31.53 mg/dL with a likelihood ration of 21 (95% CI, 14-32) in accurately diagnosed bacterial meningitis.
These data support the reinsertion of the stylet before needle removal to reduce the risk of headache after lumbar puncture and that patients do not require bed rest after diagnostic lumbar puncture. Biochemical analyses, including CSF-blood glucose ratio, CSF leukocyte count and lactate level are useful in diagnosing bacterial meningitis.
This Rational Clinical Examination systematic review and meta-analysis provides a nice review of the available data on optimizing diagnostic lumbar puncture technique to reduce adverse events. It is somewhat remarkable so little has changed in our knowledge about this long-standing diagnostic procedure. Post-lumbar puncture headaches remain a challenge that may affect patient satisfaction as well as hospital (or observation unit) course particularly for patients who do not have evidence of bacterial meningitis once the analysis is complete.
This review seems to provide some useful answers for physicians performing lumbar puncture, who should consider selecting a small gauge needle and reinserting the stylet prior to removal. Future studies of other maneuvers to reduce post-procedure adverse events should be considered for the question of atraumatic needles, which may be technically more difficult to use. The review confirms and helps quantify the utility of CSF biochemical analysis in the diagnosis of bacterial meningitis.
Who’s Performing Procedures?
Wigton RS, Alguire P. The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians. Ann Intern Med. 2007 Mar 6;146(5):355-360. Comment in Ann Intern Med. 2007 Mar 6; 146(5):392-393.
Prior surveys of physicians documented that general internists performed a variety and significant number of procedures in their practice. Much has changed since those prior assessments, including physician training, practice settings, availability of subspecialists, and regulatory requirements that have altered physician’s practice with regard to procedures. This study sought to reassess the volume and variety of procedures performed by general internists compared with the prior survey of 1986. The final sample included 990 completed surveys from general internists from 1,389 returned questionnaires for a successful completion rate of 39.6%.
The median number of different procedures performed in practice decreased from 16 in 1986 to seven in 2004. Internists who practiced in smaller hospitals or smaller towns reported performing almost twice as many procedures as physicians in the largest hospitals and cities. Hours spent in the care of hospitalized patients were also associated with an increased number of different procedures—in particular mechanical ventilation, central venous catheter placement, and thoracentesis. For all but one of the 34 procedures common to both surveys, fewer general internists performed them in 2004 compared with 1986. Remarkably, for 22 of the 34 procedures, a greater than 50% reduction in the proportion of respondents who performed the procedure was noted.
In the 1986 survey, the majority of internists performed all but one of the six procedures required by the American Board of Internal Medicine (ABIM) for certification (abdominal paracentesis, arterial puncture for blood gases, central venous catheter placement, joint aspiration, lumbar puncture, and thoracentesis). Except for joint aspiration, in 2004 these required procedures were performed by 25% or fewer of the respondents.
The 2004 survey demonstrated a striking reduction in the number of different procedures performed by general internists, and a decrease in the proportion of internists who do most procedures. These reductions may stem from a variety of changes in physician practices, including the emergence of hospitalists, availability of subspecialty physicians and proceduralists, and changes in technology and regulatory environments.
Regardless of the forces behind these changes, internal medicine residents’ training in procedures should be re-examined.
Many of those in academic hospital medicine have noted a decline in procedures performed by general internists at large academic centers. This study affirms this trend overall and in particular for physicians in large urban settings or in the largest hospitals. The emergence of hospital medicine may have played a role in reducing the procedures performed by primary care (outpatient) physicians who now spend less time caring for medically ill hospitalized patients.
Residency programs now must consider how to incorporate procedure skills and training to align with the needs of internists. The rising interest in careers in hospital medicine (as opposed to outpatient primary care) necessitates a new approach and individualized plans for gaining procedural skills to match career goals and practice settings. The new ABIM policy acknowledges this greater variability in the procedures performed by internists in practice, and takes steps to more closely align procedure requirements and core manual skills with physician practice.
These changes and new flexibility in requirements provide another opportunity for academic hospital medicine programs to provide leadership, and help shape the training of inpatient physicians. TH
Thrombocytopenia Reaction to Vancomycin
Von Drygalski A, Curtis BR, Bougie DW, et al. Vancomycin-induced immune thrombocytopenia. N Engl J Med. 2007 Mar 1;356(9):904-910
The use of vancomycin has grown exponentially in the past 20 years.1 Physicians have become increasingly aware of its major side effects, such as red man syndrome, hypersensitivity, neutropenia, and nephrotoxicity. But there have been only a few case reports of thrombocytopenia associated with this drug. This article looked at cases of thrombocytopenia in patients referred for clinical suspicion of vancomycin-induced thrombocytopenia.
From 2001-2005, serum samples were sent to the Platelet and Neutrophil Immunology Laboratory at the BloodCenter of Wisconsin in Milwaukee for testing for vancomycin-dependent antibodies from several sites. Clinical information regarding these patients was obtained from their referring physicians and one of the authors. Platelet reactive antibodies were detected by flow cytometry.
IgG and IgM vancomycin-dependent antibodies were detected in 34 patients. It was found that platelets dropped an average of 93% from pretreatment levels, and the average nadir occurred on day eight. The mean platelet count was 13,600. After vancomycin was discontinued, the platelet count returned to normal in all patients except for the three who died. The average time for resolution of thrombocytopenia was 7.5 days.
Unlike other drug-induced thrombocytopenia, these cases of thrombocytopenia associated with vancomycin appear to be more prone to significant hemorrhage. In this group 34% were found to have had severe hemorrhage defined in this study as florid petechial hemorrhages, ecchymoses, and oozing form the buccal mucosa. Three patients who had renal insufficiency were found to be profoundly thrombocytopenic for a longer duration, presumably due to delayed clearance of vancomycin in this setting.
Based on this study, it appears thrombocytopenia is a significant adverse reaction that can be attributed to vancomycin. Unlike other drug-induced thrombocytopenias, it appears to be associated with a higher likelihood of significant hemorrhage, as well.
Thrombocytopenia is a common occurrence in the acutely ill hospitalized patient and has been linked to increased hospital mortality and increased length of stay.2 Many drugs and diseases that hospitalists treat are associated with thrombocytopenia. The indications for usage of vancomycin continues to grow with the increasing number of patients with prosthetic devices and intravascular access, and the increasing prevalence of MRSA. This study raises awareness of a significant side effect that can be associated with vancomycin.
References
- Ena J, Dick RW, Jones RN, et al. The epidemiology of intravenous vancomycin usage in a university hospital: a 10-year study. JAMA. 1993 Feb 3;269(5):598-602. Comment in JAMA. 1993 Sep 22-29;270(12):1426.
- Crowther MA, Cook DJ, Meade M, et al. Thrombocytopenia in medical-surgical critically ill patients: prevalence, incidence, and risk factors. J Crit Care. 2005 Dec;20(4):248-253.
Can the mBRS Stratify Pts Admitted for Nonvariceal Upper GI Bleeds?
Romagnuolo J, Barkun AN, Enns R, et al. Simple clinical predictors may obviate urgent endoscopy in selected patients with nonvariceal upper gastrointestinal tract bleeding. Arch Intern Med. 2007 Feb 12;167(3):265-270.
Nonvariceal upper gastrointestinal bleeding is one of the top 10 admission diagnoses based on reviews of diagnosis-related groups. Patients with low-risk lesions on endoscopy, such as ulcers with a clean base, esophagitis, gastritis, duodenitis, or Mallory-Weiss tears, are felt to have less than a 5% chance of recurrent bleeding. In some instances, these patients can be treated successfully and discharged to home.1
Unfortunately, endoscopy is not always available—especially late at night and on weekends. It would be helpful to have a clinical prediction rule to identify patients at low risk for bleeding who could be safely discharged to get endoscopy within a few days.
In the study, 1,869 patients who had undergone upper endoscopy for upper gastrointestinal bleeding were entered into a Canadian national Registry for Upper GI Bleeding and Endoscopy (RUGBE). A modified Blatchford risk score (mBRS) was calculated to see if it could predict the presence of high-risk stigmata of bleeding, rebleeding rates, and mortality.
This mBRS was also compared with another scoring system—the Rockall score. The mBRS uses clinical and laboratory data to risk assess nonvariceal bleeding. The variables included in the scoring system include hemoglobin, systolic blood pressure, heart rate, melena, liver disease, and heart failure. High-risk endoscopic stigmata were defined as adherent clot after irrigation, a bleeding, oozing or spurting vessel, or a nonbleeding visible vessel. Rebleeding was defined as hematemesis, melena, or a bloody nasogastric aspirate in the presence of shock or a decrease in hemoglobin of 2 g/dL or more.
Patients who had a modified Blatchford risk score of <1 were found to have a lower likelihood of high-risk stigmata on endoscopy and were at a low risk for rebleeding (5%). Patients who had high-risk stigmata on endoscopy but an mBRS score of <1 were also found to have low rebleeding rates. The mBRS seemed to a better predictor than the Rockall score for high-risk stigmata and for rebleeding rates.
Patients with nonvariceal upper gastrointestinal tract bleeding may be identified as low risk for re-bleeding if they are normotensive, not tachycardic, not anemic, and do not have active melena, liver disease, or heart failure. It is conceivable that if endoscopy were not available, these patients could be sent home on high-dose proton pump inhibitor and asked to return for outpatient upper endoscopy within a few days.
The study certainly raises interesting questions. Whether it is acceptable practice to discharge a “low-risk” patient with an upper gastrointestinal hemorrhage on a high-dose proton pump inhibitor with good social support and close outpatient follow-up, but without diagnostic endoscopy is still unclear.
The study is limited by the fact that it is a retrospective analysis; however, it does examine a large cohort of patients. The authors acknowledge this, and this work could lead to a prospective randomized trial that would help answer this question. In the meantime, the mBRS may be a helpful tool to help risk stratify patients admitted for nonvariceal upper gastrointestinal bleeding.
References
- Cipolletta L, Bianco M, Rotondano G, et al. Outpatient management for low-risk nonvariceal upper GI bleeding: a randomized controlled trial. Gastrointest Endosc. 2002;55(1):1-5.
Lumbar Puncture to Reduce Adverse Events
Straus SE, Thorpe KE, Holroyd-Leduc J. How do I perform a lumbar puncture and analyze the results to diagnose bacterial meningitis? JAMA. 2006 Oct 25;296(16):2012-2022.
Lumbar punctures (LPs) remain a common diagnostic test performed by physicians to rule out meningitis. This procedure may be associated with adverse events, with headache and backache the most commonly reported. This systematic review and meta-analysis sought to review the evidence regarding diagnostic lumbar puncture techniques that might reduce the risk of adverse events, and to examine the accuracy of cerebrospinal fluid (CSF) analysis in the diagnosis of bacterial meningitis.
Studies were identified through searches of the Cochrane Library (www3.interscience.wiley.com/cgi-bin/mrwhome/106568753/AboutCochrane.html), MEDLINE from 1966 to January 2006, and EMBASE from 1980 to January 2006 (without language restrictions) to identify relevant studies. Bibliographies of retrieved articles were also used as data sources.
Randomized controlled trials of patients 18 or older undergoing lumbar puncture testing interventions to facilitate a successful diagnostic procedure or reduce adverse events were identified and selected. As a secondary outcome, trials that assessed the accuracy of CSF biochemical analysis for the diagnosis of bacterial meningitis were also identified and included. Trials that studied spinal anesthesia or myelography were excluded.
Study appraisals for quality (randomization, blinding, and outcome assessment) and data extraction were performed by two investigators independently. Fifteen randomized trials of interventions to reduce adverse events met criteria for inclusion, and four studies of the diagnostic test characteristics of CSF analysis met criteria and were included.
Meta-analysis with a random effects model of five studies (total of 587 patients) comparing atraumatic needles with standard needles yielded a nonsignificant decrease in the odds of headache with an atraumatic needle (absolute risk reduction [ARR], 12.3%; 95% confidence interval [CI], –1.72% to 26.2%). A single study of reinsertion of the stylet before needle removal (600 patients) showed a decreased risk of headache (ARR, 11.3%; 95% CI, 6.50%-16.2%). Meta-analysis of four studies (717 patients) revealed a nonsignificant decrease in headache in patients mobilized after LP (ARR 2.9%; 95% CI, –3.4 to 9.3%).
Data from the diagnostic test studies yielded the following likelihood ratios for diagnosing bacterial meningitis: A CSF–blood glucose ratio of 0.4 or less with a likelihood ratio of 18 (95% CI, 12-27); CSF white blood cell count of 500/µL or higher with a likelihood ratio of 15 (95% CI, 10-22); and CSF lactate level of >31.53 mg/dL with a likelihood ration of 21 (95% CI, 14-32) in accurately diagnosed bacterial meningitis.
These data support the reinsertion of the stylet before needle removal to reduce the risk of headache after lumbar puncture and that patients do not require bed rest after diagnostic lumbar puncture. Biochemical analyses, including CSF-blood glucose ratio, CSF leukocyte count and lactate level are useful in diagnosing bacterial meningitis.
This Rational Clinical Examination systematic review and meta-analysis provides a nice review of the available data on optimizing diagnostic lumbar puncture technique to reduce adverse events. It is somewhat remarkable so little has changed in our knowledge about this long-standing diagnostic procedure. Post-lumbar puncture headaches remain a challenge that may affect patient satisfaction as well as hospital (or observation unit) course particularly for patients who do not have evidence of bacterial meningitis once the analysis is complete.
This review seems to provide some useful answers for physicians performing lumbar puncture, who should consider selecting a small gauge needle and reinserting the stylet prior to removal. Future studies of other maneuvers to reduce post-procedure adverse events should be considered for the question of atraumatic needles, which may be technically more difficult to use. The review confirms and helps quantify the utility of CSF biochemical analysis in the diagnosis of bacterial meningitis.
Who’s Performing Procedures?
Wigton RS, Alguire P. The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians. Ann Intern Med. 2007 Mar 6;146(5):355-360. Comment in Ann Intern Med. 2007 Mar 6; 146(5):392-393.
Prior surveys of physicians documented that general internists performed a variety and significant number of procedures in their practice. Much has changed since those prior assessments, including physician training, practice settings, availability of subspecialists, and regulatory requirements that have altered physician’s practice with regard to procedures. This study sought to reassess the volume and variety of procedures performed by general internists compared with the prior survey of 1986. The final sample included 990 completed surveys from general internists from 1,389 returned questionnaires for a successful completion rate of 39.6%.
The median number of different procedures performed in practice decreased from 16 in 1986 to seven in 2004. Internists who practiced in smaller hospitals or smaller towns reported performing almost twice as many procedures as physicians in the largest hospitals and cities. Hours spent in the care of hospitalized patients were also associated with an increased number of different procedures—in particular mechanical ventilation, central venous catheter placement, and thoracentesis. For all but one of the 34 procedures common to both surveys, fewer general internists performed them in 2004 compared with 1986. Remarkably, for 22 of the 34 procedures, a greater than 50% reduction in the proportion of respondents who performed the procedure was noted.
In the 1986 survey, the majority of internists performed all but one of the six procedures required by the American Board of Internal Medicine (ABIM) for certification (abdominal paracentesis, arterial puncture for blood gases, central venous catheter placement, joint aspiration, lumbar puncture, and thoracentesis). Except for joint aspiration, in 2004 these required procedures were performed by 25% or fewer of the respondents.
The 2004 survey demonstrated a striking reduction in the number of different procedures performed by general internists, and a decrease in the proportion of internists who do most procedures. These reductions may stem from a variety of changes in physician practices, including the emergence of hospitalists, availability of subspecialty physicians and proceduralists, and changes in technology and regulatory environments.
Regardless of the forces behind these changes, internal medicine residents’ training in procedures should be re-examined.
Many of those in academic hospital medicine have noted a decline in procedures performed by general internists at large academic centers. This study affirms this trend overall and in particular for physicians in large urban settings or in the largest hospitals. The emergence of hospital medicine may have played a role in reducing the procedures performed by primary care (outpatient) physicians who now spend less time caring for medically ill hospitalized patients.
Residency programs now must consider how to incorporate procedure skills and training to align with the needs of internists. The rising interest in careers in hospital medicine (as opposed to outpatient primary care) necessitates a new approach and individualized plans for gaining procedural skills to match career goals and practice settings. The new ABIM policy acknowledges this greater variability in the procedures performed by internists in practice, and takes steps to more closely align procedure requirements and core manual skills with physician practice.
These changes and new flexibility in requirements provide another opportunity for academic hospital medicine programs to provide leadership, and help shape the training of inpatient physicians. TH
Vigilant Awareness
In hospitals, clinicians constantly encounter conflicting and ambiguous information,” says Ronald M. Epstein, MD, professor of family medicine, psychiatry, and oncology at the University of Rochester Medical Center (URMC) N.Y. “This information often gets processed tacitly, outside of awareness, and often results in various undesired consequences. For example, premature closure of diagnostic thinking or ordering a test rather than inquiring further of the patient.” In the average hospital, distractions and sensory inputs, including smells, sights, sounds, and tactile sensations, as well as multiple tasks to complete, can all seem pretty overwhelming. Faced with so much data, says Dr. Epstein, the tendency of the mind is to simplify and reduce it in some way. And that’s when error can rear its ugly head.
“Simplification is often arbitrary and unconscious,” he says, and thus “the trick of working in hospital is to develop a vigilant awareness of the ambient stimuli that are all around you, making choices as to what you attend to, relegating other stimuli to the background, and in that way avoiding becoming overwhelmed or controlled by them. In that way, you have the capacity for making better judgments.”
Some clinical decisions can be made fairly easily and routinely (low-level decisions), he says, whereas other patient situations require a fair bit of deliberation (high-level decisions). (See Tables 1 and 2, right.) The human mind tends to avoid the unpleasant and to give more attention to what is compelling. Also, the ambiguity of role and responsibility—especially in large hospitals—may further confound a hospitalist’s mental capacity. Keen attention to each moment also boosts physician well being.
“Hospitalists are often working in crowded, stressful, high-paced, windowless environments in which there is no natural form of respite,” says Dr. Epstein. Therefore, all physicians need ways of keeping themselves from being overwhelmed by the challenges of sensory input and intense emotions caused by exposure to suffering, conflicts, imperatives for critical thinking, and so on.
“If practitioners were able to be more mindful,” he says, “they might experience greater well-being, because they would be able to make more choices about what they attend to and how they react to them.”
Dr. Epstein and his colleagues at the University of Rochester Medical Center—Timothy Quill, MD, Michael Krasner, MD, and Howard Beckman, MD—have studied the qualities of mind required to exercise that awareness extensively, especially as they relate to clinical practice and education.2 They were recently awarded three complementary grants to teach mindfulness to physicians: one from the Arthur Vining Davis Foundations, another from the Physicians’ Foundation for Health Systems Excellence, and the Mannix Award for Excellence in Medical Education.
But just what does mindfulness in medicine entail?
Defining Mindful Practice
“Mindful practice is recognizing where you are at every moment. If you’re distressed, if you’re content or unhappy, if you’re comfortable or in pain, if you’re experiencing some kind of positive or negative effect, if you’re feeling in tune or disconnected from yourself. It’s that monitoring function to be able to say, I’m angry or I’m uncomfortable, or, possibly, I’m in the flow,” says Dr. Epstein.
For physicians to be able to exercise those qualities of mind, to watch and deconstruct their own behavior (what Dr. Epstein describes as “the ability to observe the observer observing the observed”) is something that goes back a long way for him.3 “There’s nothing really mystical about it,” he says. “People do this all the time. It’s part of being an excellent professional in lots of fields. It’s just that no one has organized the science of doing so in the context of medical training.”
In the late 1990s, Dr. Epstein and his coworkers implemented a curriculum reform process at URMC, and his particular charge was to assess the competence of medical students. To accomplish this, he did two things. First, he reviewed the literature on the assessment and definitions of special competence. Second, he turned the magnifying glass on himself. “I thought that it might be a useful exercise to try to understand what made me practice at my best and what barriers there were to doing so.”
The resulting article from this self-monitoring and evaluation was published in JAMA in 1999, before the review article on defining and assessing professional competence appeared in that same journal.3,4 Exploring the nature of his own mindful practice reacquainted him with two areas in which he had participated as a teenager: music and the study of mind—particularly the use of meditation to enhance mental capacities. Those inquiries led him to explore the psychology of a number of qualities of mind: attentiveness, curiosity, decision-making, and the use of cognitive knowledge. The literature was convergent in a number of ways, he says, and “seemed to point to the fact that a lot of competence is not a matter of book knowledge or the kind of knowledge we can explain but tacit knowledge, things we do semi-automatically that really take some effort to deconstruct.”
He realized that “what distinguished an excellent clinician from someone who wasn’t quite so excellent had to do with some of those same qualities that one sees in accomplished musicians, athletes, and meditators, which is the ability to make fine distinctions, lower one’s own level of reactivity, respond in a more conscious way, and pay attention to the unexpected—the surprises that are part of everyday work but that we often ignore.”
All of this rather radicalized his view of what medical education should be doing. He came to believe that—on top of a foundation of knowledge and skills—physicians need to be attentive to their own mental processes and alert to the effects of bias or prior experience.
Writing about excellent clinical practice in this way drew a crescendo of response from readers of the JAMA. The JAMA editors had thoroughly engaged in helping him refine and present the ideas in a way that would really speak to clinical practitioners and educators.3 After publication, he was amazed to receive hundreds of letters from all over the world from physicians in different specialties expressing their appreciation “for having articulated something that was really at the heart of medicine,” he says. “For me, that was incredibly gratifying.”
Hospitalist Qualities of Mind
What qualities of mind are important for a hospitalist to have?
“You have to be enthusiastic, fast-paced individuals,” says Yousaf Ali, MD, hospitalist at URMC and assistant professor of medicine in the Hospital Medicine Division. You also have to be able to immediately connect with patients and families and to have the knowledge and passion that makes that possible. Further, he says, you need to quickly access knowledge pertaining to caring for patients with multiple problems.
Traci Ferguson, MD, is a hospitalist at Boca Raton Community Hospital in Florida, which, by affiliating with Florida Atlantic University (the regional campus for the University of Miami School of Medicine), is moving from community hospital to teaching hospital. Dr. Ferguson believes the qualities of mind necessary to be a good hospitalist are the capacity to be aware of reactions and biases toward patients in order to avoid being judgmental.
“I think the major thing is being present and being attentive when you are caring for patients,” she says, “and that occurs when you’re writing a chart, when you’re talking to family members, [and] when you’re talking to nurses, just as it does when you’re at the bedside.”
Other qualities of mind, in Dr. Ferguson’s view, include the whole spectrum of empathy and compassion, being personable in the sense of being open to what patients and families have to say, and being patient. She also believes the quality of mind necessary to express a human touch is sometimes missing.
Valerie Lang, MD, is also a hospitalist at URMC and has studied mindfulness with Dr. Epstein. She is enrolled in Dr. Krasner’s class for healthcare providers on being mindful. What qualities of mind does she think are important for a hospitalist to have?
“I want to say an open mind, but that’s such a broad term,” she says. “Dr. Epstein uses the term ‘beginner’s mind’ [to refer to] when you’re willing to consider many alternatives, where you don’t necessarily jump to a conclusion and then just stick with it. As a hospitalist, you start making those conclusions as soon as you hear what the patient’s chief complaint is. I think that having [a] beginner’s mind … is so important because we don’t know these patients, and it’s easy to jump to conclusions because we have to make decisions very quickly and … repeatedly.” She also believes that “being able to reflect on how you are communicating with another person is incredibly important to their care.”
—Valerie Lang, MD, hospitalist, University of Rochester Medical Center
Operationalizing Mindfulness
In 2004, after the publication of two of Dr. Epstein’s articles on mindful practice in action, the Arthur Vining Davis Foundation approached him and requested a proposal.5,6 At that time, he was in the process of writing an article on reflection and mindfulness in the context of preventing errors.1 (See Table 3, left.)
“This [proposal] was an intriguing possibility,” says Dr. Epstein, “and galvanized my putting together a curriculum that would not just be elective experiences for preclinical students, which is what the offerings related to mindfulness currently are, but something that was really going to influence clinical training.”
In Dr. Epstein’s view, placing educational reform in the first two years of medical school is teaching it when it matters the least. “Where it matters the most is when students are interacting with patients and using the knowledge and skills and doing work that they’ll ultimately end up doing for the next 30 or 40 years,” says Dr. Epstein.
One project plan is to train practicing primary care physicians to communicate more mindfully with their patients. Outcomes of the intervention will be measured by how it has affected the physicians as well as the patients’ ratings of their physicians and their practice styles.
The second project is a series of annual workshops for 100 third-year medical students and about 250 residents in the nine largest programs at the medical center. All participants will take five seminars that include mindfulness techniques to improve the capacity for paying attention and observing, and narrative exercises, whose themes will include, for instance, suffering, meaningful experience, professionalism, physician self-care, and avoiding burnout. The coursework, which will include both cognitive and experiential content, will also involve training a cadre of about 20 faculty members to teach these sessions, and educational outcomes will ultimately be measured for all participants.
Focus on Metacognition
Dr. Epstein, director of The Rochester Center to Improve Communication in Health Care, says metacognition builds on other approaches, such as the Healer’s Art, a course designed by Rachel Remen, MD, and colleagues, which a number of medical centers are incorporating into their curricula.7
“We are building on Dr. Remen’s wonderful work,” he says. Both curricula include self-awareness, humanism, caring, compassion, meaningful experiences, and physician well being. Both address the “informal curriculum”—a term used to refer to the social environment in which medical trainees adopt values, expectations, and clinical habits. In addition, Dr. Epstein and his colleagues focus on quality of clinical care, including medical decision-making and preventing errors.
“Importantly, our initiative is part of the required curriculum,” says Dr. Epstein. “It targets students and residents working in clinical settings at an advanced level, and it also has a faculty component. … We are trying to transform and heal the informal curriculum, not just immunize students against its toxicity.”
In the Thick of It
All this sounds as if it might benefit hospital practice, according to the hospitalists interviewed for this story. All three believe that mindfulness can be cultivated. Dr. Ali believes the aforementioned forces acting on hospitalists require that hospitalists work at their top capacities, but prioritizing remains essential. He believes one way a hospitalist can cultivate mindfulness in the patient-physician relationship is to avoid burnout in any way that works. Having been a hospitalist for almost 10 years, he discusses this with his medical students and residents. In addition to his hospitalist practice and teaching, Dr. Ali does patient-related quality work, which refreshes his energy.
Dr. Ferguson also thinks mindful practice can be cultivated. “I took cues from the nursing profession in realizing that you do have to care for all aspects of the patient,” she says. “But you can learn this from mentors and people who are successful: people you can emulate, shadow, and follow.”
For her, such a person is Lisa Cooper, MD, MPH, an associate professor in the department of medicine at Johns Hopkins University School of Medicine. Dr. Cooper, both a practicing internist and a researcher, studies and teaches about communication between physicians and minorities—that is, how physicians interact with people of the same or different races and ethnicities. Dr. Ferguson says she feels fortunate to have adopted a mindful awareness in that regard.
As director of the medicine clerkship, Dr. Lang came into contact with Dr. Epstein’s project through her Dean’s Teaching Fellowship, a competitive program at the URSM for faculty members who have a special interest in education.
“The discussions with other educators and clinicians really got me thinking about how my own feelings, whether they had to do with a patient or anything else in life, affect my decision-making,” says Dr. Lang. “You see the phenomenon in residency where you’re in morning report when the residents present a patient and everyone is sitting around a table—not involved with the patient—making judgments about what they should have done. It’s so much easier when you’re not involved [in the situation].”
Though Dr. Lang thinks there are a lot of reasons for that, “part of it is that you are not in the excitement of the moment. And the other factor is that when you’re presenting a patient to a group, you wouldn’t convey your own emotions, what else was going on, what were the competing pressures. Even if you have a wonderful intellect and clinical reasoning skills, you might make the wrong decision when you’re in the thick of the situation.”
Mindful Hospital Practice
Dr. Lang has seen a number of outcomes from her study of mindful practice. It has made her aware of her biases and has taught her to say, in certain cases, “OK, I need to think through the problem again to make sure I’m not changing my judgment about what we should be doing clinically based on how I’m feeling about a patient.”
Dr. Lang sometimes asks herself, “How am I feeling about this? Did that wear me down?” Or, sometimes the opposite can occur. A patient can make you feel “puffed up, where they are so complimentary and make you feel so good that you think that every decision you make is perfect,” she explains.
What Dr. Lang has learned about herself has helped her recognize when she might have prematurely closed a differential diagnosis or come to a conclusion too quickly simply because the patient appeared to agree with her clinical assessment.
Dr. Lang also thinks being a mindful physician has made her a better physician and that she is providing better care that results in better outcomes. “I definitely communicate better with my patients. … I think my relationships with my patients have significantly improved.”
What is her recommendation for how her hospitalist colleagues can learn to practice mindfully? “It’s a practice, and it’s a matter of practice,” says Dr. Lang. “It’s not something you get overnight. It’s a matter of every day, every encounter, taking the time before entering the patient’s room to pause, put things aside, and be present with the patient. And then, at the end of the day, take some time to reflect.”
How does education for mindfulness differ from her original medical training? “I don’t think you’re really ever taught how to manage your emotions when you’ve just made a medical error and you are distraught,” says Dr. Lang, “or how to manage doing that when your pager is going off like crazy and yet you need to sit down and be present with your patient. And that’s the kind of thing that ends up being in your way of being the best physician you can be.” TH
References
- Borrell-Carrió F, Epstein RM. Preventing errors in clinical practice: a call for self-awareness. Ann Fam Med. 2004;2:310-316.
- Epstein RM. Assessment in medical education. N Engl J Med. 2007;356(4):387-396.
- Epstein RM. Mindful practice. JAMA. 1999 Sep;282(9):833-839.
- Epstein RM, Hundert EM. Defining and assessing professional competence. JAMA. 2002 Jan 9;287(2):226-235.
- Epstein RM. Mindful practice in action (I): technical competence, evidence-based medicine and relationship-centered care. Fam Syst Health. 2003;21:1-9.
- Epstein RM. Mindful practice in action (II): cultivating habits of mind. Fam Syst Health. 2003;21:11-17.
- O’Donnell JF, Rabow MW, Remen RN. The healer’s art: awakening the heart of medicine. Medical Encounter: Newsletter of the American Academy on Communication in Healthcare. 2007;21, No 1.
In hospitals, clinicians constantly encounter conflicting and ambiguous information,” says Ronald M. Epstein, MD, professor of family medicine, psychiatry, and oncology at the University of Rochester Medical Center (URMC) N.Y. “This information often gets processed tacitly, outside of awareness, and often results in various undesired consequences. For example, premature closure of diagnostic thinking or ordering a test rather than inquiring further of the patient.” In the average hospital, distractions and sensory inputs, including smells, sights, sounds, and tactile sensations, as well as multiple tasks to complete, can all seem pretty overwhelming. Faced with so much data, says Dr. Epstein, the tendency of the mind is to simplify and reduce it in some way. And that’s when error can rear its ugly head.
“Simplification is often arbitrary and unconscious,” he says, and thus “the trick of working in hospital is to develop a vigilant awareness of the ambient stimuli that are all around you, making choices as to what you attend to, relegating other stimuli to the background, and in that way avoiding becoming overwhelmed or controlled by them. In that way, you have the capacity for making better judgments.”
Some clinical decisions can be made fairly easily and routinely (low-level decisions), he says, whereas other patient situations require a fair bit of deliberation (high-level decisions). (See Tables 1 and 2, right.) The human mind tends to avoid the unpleasant and to give more attention to what is compelling. Also, the ambiguity of role and responsibility—especially in large hospitals—may further confound a hospitalist’s mental capacity. Keen attention to each moment also boosts physician well being.
“Hospitalists are often working in crowded, stressful, high-paced, windowless environments in which there is no natural form of respite,” says Dr. Epstein. Therefore, all physicians need ways of keeping themselves from being overwhelmed by the challenges of sensory input and intense emotions caused by exposure to suffering, conflicts, imperatives for critical thinking, and so on.
“If practitioners were able to be more mindful,” he says, “they might experience greater well-being, because they would be able to make more choices about what they attend to and how they react to them.”
Dr. Epstein and his colleagues at the University of Rochester Medical Center—Timothy Quill, MD, Michael Krasner, MD, and Howard Beckman, MD—have studied the qualities of mind required to exercise that awareness extensively, especially as they relate to clinical practice and education.2 They were recently awarded three complementary grants to teach mindfulness to physicians: one from the Arthur Vining Davis Foundations, another from the Physicians’ Foundation for Health Systems Excellence, and the Mannix Award for Excellence in Medical Education.
But just what does mindfulness in medicine entail?
Defining Mindful Practice
“Mindful practice is recognizing where you are at every moment. If you’re distressed, if you’re content or unhappy, if you’re comfortable or in pain, if you’re experiencing some kind of positive or negative effect, if you’re feeling in tune or disconnected from yourself. It’s that monitoring function to be able to say, I’m angry or I’m uncomfortable, or, possibly, I’m in the flow,” says Dr. Epstein.
For physicians to be able to exercise those qualities of mind, to watch and deconstruct their own behavior (what Dr. Epstein describes as “the ability to observe the observer observing the observed”) is something that goes back a long way for him.3 “There’s nothing really mystical about it,” he says. “People do this all the time. It’s part of being an excellent professional in lots of fields. It’s just that no one has organized the science of doing so in the context of medical training.”
In the late 1990s, Dr. Epstein and his coworkers implemented a curriculum reform process at URMC, and his particular charge was to assess the competence of medical students. To accomplish this, he did two things. First, he reviewed the literature on the assessment and definitions of special competence. Second, he turned the magnifying glass on himself. “I thought that it might be a useful exercise to try to understand what made me practice at my best and what barriers there were to doing so.”
The resulting article from this self-monitoring and evaluation was published in JAMA in 1999, before the review article on defining and assessing professional competence appeared in that same journal.3,4 Exploring the nature of his own mindful practice reacquainted him with two areas in which he had participated as a teenager: music and the study of mind—particularly the use of meditation to enhance mental capacities. Those inquiries led him to explore the psychology of a number of qualities of mind: attentiveness, curiosity, decision-making, and the use of cognitive knowledge. The literature was convergent in a number of ways, he says, and “seemed to point to the fact that a lot of competence is not a matter of book knowledge or the kind of knowledge we can explain but tacit knowledge, things we do semi-automatically that really take some effort to deconstruct.”
He realized that “what distinguished an excellent clinician from someone who wasn’t quite so excellent had to do with some of those same qualities that one sees in accomplished musicians, athletes, and meditators, which is the ability to make fine distinctions, lower one’s own level of reactivity, respond in a more conscious way, and pay attention to the unexpected—the surprises that are part of everyday work but that we often ignore.”
All of this rather radicalized his view of what medical education should be doing. He came to believe that—on top of a foundation of knowledge and skills—physicians need to be attentive to their own mental processes and alert to the effects of bias or prior experience.
Writing about excellent clinical practice in this way drew a crescendo of response from readers of the JAMA. The JAMA editors had thoroughly engaged in helping him refine and present the ideas in a way that would really speak to clinical practitioners and educators.3 After publication, he was amazed to receive hundreds of letters from all over the world from physicians in different specialties expressing their appreciation “for having articulated something that was really at the heart of medicine,” he says. “For me, that was incredibly gratifying.”
Hospitalist Qualities of Mind
What qualities of mind are important for a hospitalist to have?
“You have to be enthusiastic, fast-paced individuals,” says Yousaf Ali, MD, hospitalist at URMC and assistant professor of medicine in the Hospital Medicine Division. You also have to be able to immediately connect with patients and families and to have the knowledge and passion that makes that possible. Further, he says, you need to quickly access knowledge pertaining to caring for patients with multiple problems.
Traci Ferguson, MD, is a hospitalist at Boca Raton Community Hospital in Florida, which, by affiliating with Florida Atlantic University (the regional campus for the University of Miami School of Medicine), is moving from community hospital to teaching hospital. Dr. Ferguson believes the qualities of mind necessary to be a good hospitalist are the capacity to be aware of reactions and biases toward patients in order to avoid being judgmental.
“I think the major thing is being present and being attentive when you are caring for patients,” she says, “and that occurs when you’re writing a chart, when you’re talking to family members, [and] when you’re talking to nurses, just as it does when you’re at the bedside.”
Other qualities of mind, in Dr. Ferguson’s view, include the whole spectrum of empathy and compassion, being personable in the sense of being open to what patients and families have to say, and being patient. She also believes the quality of mind necessary to express a human touch is sometimes missing.
Valerie Lang, MD, is also a hospitalist at URMC and has studied mindfulness with Dr. Epstein. She is enrolled in Dr. Krasner’s class for healthcare providers on being mindful. What qualities of mind does she think are important for a hospitalist to have?
“I want to say an open mind, but that’s such a broad term,” she says. “Dr. Epstein uses the term ‘beginner’s mind’ [to refer to] when you’re willing to consider many alternatives, where you don’t necessarily jump to a conclusion and then just stick with it. As a hospitalist, you start making those conclusions as soon as you hear what the patient’s chief complaint is. I think that having [a] beginner’s mind … is so important because we don’t know these patients, and it’s easy to jump to conclusions because we have to make decisions very quickly and … repeatedly.” She also believes that “being able to reflect on how you are communicating with another person is incredibly important to their care.”
—Valerie Lang, MD, hospitalist, University of Rochester Medical Center
Operationalizing Mindfulness
In 2004, after the publication of two of Dr. Epstein’s articles on mindful practice in action, the Arthur Vining Davis Foundation approached him and requested a proposal.5,6 At that time, he was in the process of writing an article on reflection and mindfulness in the context of preventing errors.1 (See Table 3, left.)
“This [proposal] was an intriguing possibility,” says Dr. Epstein, “and galvanized my putting together a curriculum that would not just be elective experiences for preclinical students, which is what the offerings related to mindfulness currently are, but something that was really going to influence clinical training.”
In Dr. Epstein’s view, placing educational reform in the first two years of medical school is teaching it when it matters the least. “Where it matters the most is when students are interacting with patients and using the knowledge and skills and doing work that they’ll ultimately end up doing for the next 30 or 40 years,” says Dr. Epstein.
One project plan is to train practicing primary care physicians to communicate more mindfully with their patients. Outcomes of the intervention will be measured by how it has affected the physicians as well as the patients’ ratings of their physicians and their practice styles.
The second project is a series of annual workshops for 100 third-year medical students and about 250 residents in the nine largest programs at the medical center. All participants will take five seminars that include mindfulness techniques to improve the capacity for paying attention and observing, and narrative exercises, whose themes will include, for instance, suffering, meaningful experience, professionalism, physician self-care, and avoiding burnout. The coursework, which will include both cognitive and experiential content, will also involve training a cadre of about 20 faculty members to teach these sessions, and educational outcomes will ultimately be measured for all participants.
Focus on Metacognition
Dr. Epstein, director of The Rochester Center to Improve Communication in Health Care, says metacognition builds on other approaches, such as the Healer’s Art, a course designed by Rachel Remen, MD, and colleagues, which a number of medical centers are incorporating into their curricula.7
“We are building on Dr. Remen’s wonderful work,” he says. Both curricula include self-awareness, humanism, caring, compassion, meaningful experiences, and physician well being. Both address the “informal curriculum”—a term used to refer to the social environment in which medical trainees adopt values, expectations, and clinical habits. In addition, Dr. Epstein and his colleagues focus on quality of clinical care, including medical decision-making and preventing errors.
“Importantly, our initiative is part of the required curriculum,” says Dr. Epstein. “It targets students and residents working in clinical settings at an advanced level, and it also has a faculty component. … We are trying to transform and heal the informal curriculum, not just immunize students against its toxicity.”
In the Thick of It
All this sounds as if it might benefit hospital practice, according to the hospitalists interviewed for this story. All three believe that mindfulness can be cultivated. Dr. Ali believes the aforementioned forces acting on hospitalists require that hospitalists work at their top capacities, but prioritizing remains essential. He believes one way a hospitalist can cultivate mindfulness in the patient-physician relationship is to avoid burnout in any way that works. Having been a hospitalist for almost 10 years, he discusses this with his medical students and residents. In addition to his hospitalist practice and teaching, Dr. Ali does patient-related quality work, which refreshes his energy.
Dr. Ferguson also thinks mindful practice can be cultivated. “I took cues from the nursing profession in realizing that you do have to care for all aspects of the patient,” she says. “But you can learn this from mentors and people who are successful: people you can emulate, shadow, and follow.”
For her, such a person is Lisa Cooper, MD, MPH, an associate professor in the department of medicine at Johns Hopkins University School of Medicine. Dr. Cooper, both a practicing internist and a researcher, studies and teaches about communication between physicians and minorities—that is, how physicians interact with people of the same or different races and ethnicities. Dr. Ferguson says she feels fortunate to have adopted a mindful awareness in that regard.
As director of the medicine clerkship, Dr. Lang came into contact with Dr. Epstein’s project through her Dean’s Teaching Fellowship, a competitive program at the URSM for faculty members who have a special interest in education.
“The discussions with other educators and clinicians really got me thinking about how my own feelings, whether they had to do with a patient or anything else in life, affect my decision-making,” says Dr. Lang. “You see the phenomenon in residency where you’re in morning report when the residents present a patient and everyone is sitting around a table—not involved with the patient—making judgments about what they should have done. It’s so much easier when you’re not involved [in the situation].”
Though Dr. Lang thinks there are a lot of reasons for that, “part of it is that you are not in the excitement of the moment. And the other factor is that when you’re presenting a patient to a group, you wouldn’t convey your own emotions, what else was going on, what were the competing pressures. Even if you have a wonderful intellect and clinical reasoning skills, you might make the wrong decision when you’re in the thick of the situation.”
Mindful Hospital Practice
Dr. Lang has seen a number of outcomes from her study of mindful practice. It has made her aware of her biases and has taught her to say, in certain cases, “OK, I need to think through the problem again to make sure I’m not changing my judgment about what we should be doing clinically based on how I’m feeling about a patient.”
Dr. Lang sometimes asks herself, “How am I feeling about this? Did that wear me down?” Or, sometimes the opposite can occur. A patient can make you feel “puffed up, where they are so complimentary and make you feel so good that you think that every decision you make is perfect,” she explains.
What Dr. Lang has learned about herself has helped her recognize when she might have prematurely closed a differential diagnosis or come to a conclusion too quickly simply because the patient appeared to agree with her clinical assessment.
Dr. Lang also thinks being a mindful physician has made her a better physician and that she is providing better care that results in better outcomes. “I definitely communicate better with my patients. … I think my relationships with my patients have significantly improved.”
What is her recommendation for how her hospitalist colleagues can learn to practice mindfully? “It’s a practice, and it’s a matter of practice,” says Dr. Lang. “It’s not something you get overnight. It’s a matter of every day, every encounter, taking the time before entering the patient’s room to pause, put things aside, and be present with the patient. And then, at the end of the day, take some time to reflect.”
How does education for mindfulness differ from her original medical training? “I don’t think you’re really ever taught how to manage your emotions when you’ve just made a medical error and you are distraught,” says Dr. Lang, “or how to manage doing that when your pager is going off like crazy and yet you need to sit down and be present with your patient. And that’s the kind of thing that ends up being in your way of being the best physician you can be.” TH
References
- Borrell-Carrió F, Epstein RM. Preventing errors in clinical practice: a call for self-awareness. Ann Fam Med. 2004;2:310-316.
- Epstein RM. Assessment in medical education. N Engl J Med. 2007;356(4):387-396.
- Epstein RM. Mindful practice. JAMA. 1999 Sep;282(9):833-839.
- Epstein RM, Hundert EM. Defining and assessing professional competence. JAMA. 2002 Jan 9;287(2):226-235.
- Epstein RM. Mindful practice in action (I): technical competence, evidence-based medicine and relationship-centered care. Fam Syst Health. 2003;21:1-9.
- Epstein RM. Mindful practice in action (II): cultivating habits of mind. Fam Syst Health. 2003;21:11-17.
- O’Donnell JF, Rabow MW, Remen RN. The healer’s art: awakening the heart of medicine. Medical Encounter: Newsletter of the American Academy on Communication in Healthcare. 2007;21, No 1.
In hospitals, clinicians constantly encounter conflicting and ambiguous information,” says Ronald M. Epstein, MD, professor of family medicine, psychiatry, and oncology at the University of Rochester Medical Center (URMC) N.Y. “This information often gets processed tacitly, outside of awareness, and often results in various undesired consequences. For example, premature closure of diagnostic thinking or ordering a test rather than inquiring further of the patient.” In the average hospital, distractions and sensory inputs, including smells, sights, sounds, and tactile sensations, as well as multiple tasks to complete, can all seem pretty overwhelming. Faced with so much data, says Dr. Epstein, the tendency of the mind is to simplify and reduce it in some way. And that’s when error can rear its ugly head.
“Simplification is often arbitrary and unconscious,” he says, and thus “the trick of working in hospital is to develop a vigilant awareness of the ambient stimuli that are all around you, making choices as to what you attend to, relegating other stimuli to the background, and in that way avoiding becoming overwhelmed or controlled by them. In that way, you have the capacity for making better judgments.”
Some clinical decisions can be made fairly easily and routinely (low-level decisions), he says, whereas other patient situations require a fair bit of deliberation (high-level decisions). (See Tables 1 and 2, right.) The human mind tends to avoid the unpleasant and to give more attention to what is compelling. Also, the ambiguity of role and responsibility—especially in large hospitals—may further confound a hospitalist’s mental capacity. Keen attention to each moment also boosts physician well being.
“Hospitalists are often working in crowded, stressful, high-paced, windowless environments in which there is no natural form of respite,” says Dr. Epstein. Therefore, all physicians need ways of keeping themselves from being overwhelmed by the challenges of sensory input and intense emotions caused by exposure to suffering, conflicts, imperatives for critical thinking, and so on.
“If practitioners were able to be more mindful,” he says, “they might experience greater well-being, because they would be able to make more choices about what they attend to and how they react to them.”
Dr. Epstein and his colleagues at the University of Rochester Medical Center—Timothy Quill, MD, Michael Krasner, MD, and Howard Beckman, MD—have studied the qualities of mind required to exercise that awareness extensively, especially as they relate to clinical practice and education.2 They were recently awarded three complementary grants to teach mindfulness to physicians: one from the Arthur Vining Davis Foundations, another from the Physicians’ Foundation for Health Systems Excellence, and the Mannix Award for Excellence in Medical Education.
But just what does mindfulness in medicine entail?
Defining Mindful Practice
“Mindful practice is recognizing where you are at every moment. If you’re distressed, if you’re content or unhappy, if you’re comfortable or in pain, if you’re experiencing some kind of positive or negative effect, if you’re feeling in tune or disconnected from yourself. It’s that monitoring function to be able to say, I’m angry or I’m uncomfortable, or, possibly, I’m in the flow,” says Dr. Epstein.
For physicians to be able to exercise those qualities of mind, to watch and deconstruct their own behavior (what Dr. Epstein describes as “the ability to observe the observer observing the observed”) is something that goes back a long way for him.3 “There’s nothing really mystical about it,” he says. “People do this all the time. It’s part of being an excellent professional in lots of fields. It’s just that no one has organized the science of doing so in the context of medical training.”
In the late 1990s, Dr. Epstein and his coworkers implemented a curriculum reform process at URMC, and his particular charge was to assess the competence of medical students. To accomplish this, he did two things. First, he reviewed the literature on the assessment and definitions of special competence. Second, he turned the magnifying glass on himself. “I thought that it might be a useful exercise to try to understand what made me practice at my best and what barriers there were to doing so.”
The resulting article from this self-monitoring and evaluation was published in JAMA in 1999, before the review article on defining and assessing professional competence appeared in that same journal.3,4 Exploring the nature of his own mindful practice reacquainted him with two areas in which he had participated as a teenager: music and the study of mind—particularly the use of meditation to enhance mental capacities. Those inquiries led him to explore the psychology of a number of qualities of mind: attentiveness, curiosity, decision-making, and the use of cognitive knowledge. The literature was convergent in a number of ways, he says, and “seemed to point to the fact that a lot of competence is not a matter of book knowledge or the kind of knowledge we can explain but tacit knowledge, things we do semi-automatically that really take some effort to deconstruct.”
He realized that “what distinguished an excellent clinician from someone who wasn’t quite so excellent had to do with some of those same qualities that one sees in accomplished musicians, athletes, and meditators, which is the ability to make fine distinctions, lower one’s own level of reactivity, respond in a more conscious way, and pay attention to the unexpected—the surprises that are part of everyday work but that we often ignore.”
All of this rather radicalized his view of what medical education should be doing. He came to believe that—on top of a foundation of knowledge and skills—physicians need to be attentive to their own mental processes and alert to the effects of bias or prior experience.
Writing about excellent clinical practice in this way drew a crescendo of response from readers of the JAMA. The JAMA editors had thoroughly engaged in helping him refine and present the ideas in a way that would really speak to clinical practitioners and educators.3 After publication, he was amazed to receive hundreds of letters from all over the world from physicians in different specialties expressing their appreciation “for having articulated something that was really at the heart of medicine,” he says. “For me, that was incredibly gratifying.”
Hospitalist Qualities of Mind
What qualities of mind are important for a hospitalist to have?
“You have to be enthusiastic, fast-paced individuals,” says Yousaf Ali, MD, hospitalist at URMC and assistant professor of medicine in the Hospital Medicine Division. You also have to be able to immediately connect with patients and families and to have the knowledge and passion that makes that possible. Further, he says, you need to quickly access knowledge pertaining to caring for patients with multiple problems.
Traci Ferguson, MD, is a hospitalist at Boca Raton Community Hospital in Florida, which, by affiliating with Florida Atlantic University (the regional campus for the University of Miami School of Medicine), is moving from community hospital to teaching hospital. Dr. Ferguson believes the qualities of mind necessary to be a good hospitalist are the capacity to be aware of reactions and biases toward patients in order to avoid being judgmental.
“I think the major thing is being present and being attentive when you are caring for patients,” she says, “and that occurs when you’re writing a chart, when you’re talking to family members, [and] when you’re talking to nurses, just as it does when you’re at the bedside.”
Other qualities of mind, in Dr. Ferguson’s view, include the whole spectrum of empathy and compassion, being personable in the sense of being open to what patients and families have to say, and being patient. She also believes the quality of mind necessary to express a human touch is sometimes missing.
Valerie Lang, MD, is also a hospitalist at URMC and has studied mindfulness with Dr. Epstein. She is enrolled in Dr. Krasner’s class for healthcare providers on being mindful. What qualities of mind does she think are important for a hospitalist to have?
“I want to say an open mind, but that’s such a broad term,” she says. “Dr. Epstein uses the term ‘beginner’s mind’ [to refer to] when you’re willing to consider many alternatives, where you don’t necessarily jump to a conclusion and then just stick with it. As a hospitalist, you start making those conclusions as soon as you hear what the patient’s chief complaint is. I think that having [a] beginner’s mind … is so important because we don’t know these patients, and it’s easy to jump to conclusions because we have to make decisions very quickly and … repeatedly.” She also believes that “being able to reflect on how you are communicating with another person is incredibly important to their care.”
—Valerie Lang, MD, hospitalist, University of Rochester Medical Center
Operationalizing Mindfulness
In 2004, after the publication of two of Dr. Epstein’s articles on mindful practice in action, the Arthur Vining Davis Foundation approached him and requested a proposal.5,6 At that time, he was in the process of writing an article on reflection and mindfulness in the context of preventing errors.1 (See Table 3, left.)
“This [proposal] was an intriguing possibility,” says Dr. Epstein, “and galvanized my putting together a curriculum that would not just be elective experiences for preclinical students, which is what the offerings related to mindfulness currently are, but something that was really going to influence clinical training.”
In Dr. Epstein’s view, placing educational reform in the first two years of medical school is teaching it when it matters the least. “Where it matters the most is when students are interacting with patients and using the knowledge and skills and doing work that they’ll ultimately end up doing for the next 30 or 40 years,” says Dr. Epstein.
One project plan is to train practicing primary care physicians to communicate more mindfully with their patients. Outcomes of the intervention will be measured by how it has affected the physicians as well as the patients’ ratings of their physicians and their practice styles.
The second project is a series of annual workshops for 100 third-year medical students and about 250 residents in the nine largest programs at the medical center. All participants will take five seminars that include mindfulness techniques to improve the capacity for paying attention and observing, and narrative exercises, whose themes will include, for instance, suffering, meaningful experience, professionalism, physician self-care, and avoiding burnout. The coursework, which will include both cognitive and experiential content, will also involve training a cadre of about 20 faculty members to teach these sessions, and educational outcomes will ultimately be measured for all participants.
Focus on Metacognition
Dr. Epstein, director of The Rochester Center to Improve Communication in Health Care, says metacognition builds on other approaches, such as the Healer’s Art, a course designed by Rachel Remen, MD, and colleagues, which a number of medical centers are incorporating into their curricula.7
“We are building on Dr. Remen’s wonderful work,” he says. Both curricula include self-awareness, humanism, caring, compassion, meaningful experiences, and physician well being. Both address the “informal curriculum”—a term used to refer to the social environment in which medical trainees adopt values, expectations, and clinical habits. In addition, Dr. Epstein and his colleagues focus on quality of clinical care, including medical decision-making and preventing errors.
“Importantly, our initiative is part of the required curriculum,” says Dr. Epstein. “It targets students and residents working in clinical settings at an advanced level, and it also has a faculty component. … We are trying to transform and heal the informal curriculum, not just immunize students against its toxicity.”
In the Thick of It
All this sounds as if it might benefit hospital practice, according to the hospitalists interviewed for this story. All three believe that mindfulness can be cultivated. Dr. Ali believes the aforementioned forces acting on hospitalists require that hospitalists work at their top capacities, but prioritizing remains essential. He believes one way a hospitalist can cultivate mindfulness in the patient-physician relationship is to avoid burnout in any way that works. Having been a hospitalist for almost 10 years, he discusses this with his medical students and residents. In addition to his hospitalist practice and teaching, Dr. Ali does patient-related quality work, which refreshes his energy.
Dr. Ferguson also thinks mindful practice can be cultivated. “I took cues from the nursing profession in realizing that you do have to care for all aspects of the patient,” she says. “But you can learn this from mentors and people who are successful: people you can emulate, shadow, and follow.”
For her, such a person is Lisa Cooper, MD, MPH, an associate professor in the department of medicine at Johns Hopkins University School of Medicine. Dr. Cooper, both a practicing internist and a researcher, studies and teaches about communication between physicians and minorities—that is, how physicians interact with people of the same or different races and ethnicities. Dr. Ferguson says she feels fortunate to have adopted a mindful awareness in that regard.
As director of the medicine clerkship, Dr. Lang came into contact with Dr. Epstein’s project through her Dean’s Teaching Fellowship, a competitive program at the URSM for faculty members who have a special interest in education.
“The discussions with other educators and clinicians really got me thinking about how my own feelings, whether they had to do with a patient or anything else in life, affect my decision-making,” says Dr. Lang. “You see the phenomenon in residency where you’re in morning report when the residents present a patient and everyone is sitting around a table—not involved with the patient—making judgments about what they should have done. It’s so much easier when you’re not involved [in the situation].”
Though Dr. Lang thinks there are a lot of reasons for that, “part of it is that you are not in the excitement of the moment. And the other factor is that when you’re presenting a patient to a group, you wouldn’t convey your own emotions, what else was going on, what were the competing pressures. Even if you have a wonderful intellect and clinical reasoning skills, you might make the wrong decision when you’re in the thick of the situation.”
Mindful Hospital Practice
Dr. Lang has seen a number of outcomes from her study of mindful practice. It has made her aware of her biases and has taught her to say, in certain cases, “OK, I need to think through the problem again to make sure I’m not changing my judgment about what we should be doing clinically based on how I’m feeling about a patient.”
Dr. Lang sometimes asks herself, “How am I feeling about this? Did that wear me down?” Or, sometimes the opposite can occur. A patient can make you feel “puffed up, where they are so complimentary and make you feel so good that you think that every decision you make is perfect,” she explains.
What Dr. Lang has learned about herself has helped her recognize when she might have prematurely closed a differential diagnosis or come to a conclusion too quickly simply because the patient appeared to agree with her clinical assessment.
Dr. Lang also thinks being a mindful physician has made her a better physician and that she is providing better care that results in better outcomes. “I definitely communicate better with my patients. … I think my relationships with my patients have significantly improved.”
What is her recommendation for how her hospitalist colleagues can learn to practice mindfully? “It’s a practice, and it’s a matter of practice,” says Dr. Lang. “It’s not something you get overnight. It’s a matter of every day, every encounter, taking the time before entering the patient’s room to pause, put things aside, and be present with the patient. And then, at the end of the day, take some time to reflect.”
How does education for mindfulness differ from her original medical training? “I don’t think you’re really ever taught how to manage your emotions when you’ve just made a medical error and you are distraught,” says Dr. Lang, “or how to manage doing that when your pager is going off like crazy and yet you need to sit down and be present with your patient. And that’s the kind of thing that ends up being in your way of being the best physician you can be.” TH
References
- Borrell-Carrió F, Epstein RM. Preventing errors in clinical practice: a call for self-awareness. Ann Fam Med. 2004;2:310-316.
- Epstein RM. Assessment in medical education. N Engl J Med. 2007;356(4):387-396.
- Epstein RM. Mindful practice. JAMA. 1999 Sep;282(9):833-839.
- Epstein RM, Hundert EM. Defining and assessing professional competence. JAMA. 2002 Jan 9;287(2):226-235.
- Epstein RM. Mindful practice in action (I): technical competence, evidence-based medicine and relationship-centered care. Fam Syst Health. 2003;21:1-9.
- Epstein RM. Mindful practice in action (II): cultivating habits of mind. Fam Syst Health. 2003;21:11-17.
- O’Donnell JF, Rabow MW, Remen RN. The healer’s art: awakening the heart of medicine. Medical Encounter: Newsletter of the American Academy on Communication in Healthcare. 2007;21, No 1.
Pitfalls in Pain Treatment
Note: This is Part 2 of The Hospitalist’s series on pain and hospital medicine. Part 1 appeared on p. 45 of the April issue.
Welcome to Part 2 of our three-part series on managing the pain of hospitalized patients. Last month’s article presented the context for pain management in the hospital—a core competency identified by SHM. It emphasized techniques for assessing patients’ pain, ranging from a zero-to-10 pain score to more complex pain histories addressing type, source, duration, and intensity as well as psychosocial and spiritual factors.
Part 2 delves into some difficult cases and dilemmas of pain management—situations that can take hospitalists out of their comfort zone and challenge their confidence in managing their patients’ pain.
Some of these dilemmas arise from misconceptions about pain and pain treatments and from the fact that, historically, physicians have not been well trained in optimal pain management. General barriers to pain management in the U.S. healthcare system, as identified by the National Association of Attorneys General, include patients’ beliefs, physician and institutional practices, restrictive state polices, and racial and socioeconomic disparities.1
Many of these issues relate specifically to the most common treatments for severe pain, opioid analgesics, which have all sorts of negative associations based on misconceptions about abuse, addiction, and overdose. In other cases, physicians face real challenges in balancing analgesic benefits with side effects and in determining the right medication, dose, and schedule to meet the patient’s need for pain relief.
Hospitalists confronting difficult pain cases work under the added pressure of trying to bring their patients’ acute illnesses under control so they can discharge them to a lower level of care as soon as prudently possible. This time pressure, along with demands arising from the rest of the hospitalist’s caseload, may impose limits on what can be accomplished in difficult situations or with medications that require time to stabilize.
Challenges also arise when the customary approach to pain management—the drug and dosing schedule the hospitalist is most comfortable using for most patients—fails to bring the pain under control. This is often a red flag for the need to try something new, says Stephen Bekanich, MD, a hospitalist at the University of Utah Medical Center in Salt Lake City and a consultant on the medical center’s palliative care service. In some cases, that means calling in a specialist in pain treatment, palliative medicine, psychiatry, or substance abuse.
“You need to work into the equation that there are pitfalls and caveats to everything we say about pain,” Dr. Bekanich observes. “Plus, the common pain treatments are controlled substances, with obvious legal implications and a professional duty for physicians to handle them safely and appropriately.”
When Dr. Bekanich finds himself confronting a difficult pain situation that has caused a conflict with a patient, he often involves one of the hospital’s customer service patient advocates. They are trained to mediate disagreements between patients and the treatment team.
Is This Patient’s Pain Real?
Physicians sometimes wonder if their patients’ reports of pain are accurate. Is the pain really as bad as the patient says it is? “Residents, frequently, are more skeptical of patients’ claims of pain, doubting whether they are truly experiencing that level of pain,” reports Jean Youngwerth, MD, a hospitalist, palliative care consultant, and fellowship associate program director at the University of Colorado Health Sciences Center in Aurora.
“I tell my residents that malingering is rare, and those few cases where it happens really tend to stand out,” Dr. Youngwerth says. “I also tell them that our default position is always to trust the patient, unless given a good reason not to. I have been burned more often when I questioned my patients’ reports of pain than when I didn’t.”
Pain experts emphasize that the patient’s self-report is the most reliable source of information on pain—based on an understanding of pain as a complex, subjective phenomenon associated with actual or potential tissue damage and the patient’s perception of and emotional reaction to that sensation. The phenomenon of pain also includes emotional, social, psychological, even spiritual components and can be mediated by a host of other factors. But that doesn’t mean it isn’t real to the patient.
“Often, younger physicians take the attitude that if the pain is real, then administration of morphine will make it go away,” says Porter Storey, MD, FACP, FAAHPM. “In reality, pain doesn’t always respond to opioids, for all sorts of reasons. Hospitalists value clarity, and they use pain as a screen for all sorts of other problems. Their goal, often, is not so much the comfort of the patient as it is diagnosing, treating, and then discharging the patient from the hospital.” Dr. Storey is a palliative care physician in Boulder, Colo., and executive vice president for Medical Affairs at the American Academy of Hospice and Palliative Medicine (AAHPM).
Physicians need to be reminded, however, that unresolved pain in hospitalized patients has many negative consequences. These range from resistance to rehabilitation to depression to delayed hospital discharge, as well as reduced job satisfaction for the healthcare professionals who care for them.
Will Prescribing Analgesics Cause Addiction?
Fears about causing addiction haunt many pain management discussions. Requests for more medications, obsessing over the next scheduled analgesic dose, and even manipulative or drug-seeking behaviors can be misunderstood by physicians who lack training in the real nature of drug addiction. Actual cases of drug addiction created by appropriate, sufficient, and well-monitored opioid analgesic treatment are rare, pain experts say. There is an important caveat: the patient who brings a prior history of drug abuse to the current acute medical episode.
“There are no good data about iatrogenic addiction,” says Robert Brody, MD, chief of the pain consultation clinic at San Francisco General Hospital and a frequent presenter on pain management topics at clinical workshops for hospitalists. “People who do pain management, certainly including hospice and palliative care physicians, don’t really believe in it. In my own clinical experience, most patients don’t like pain medications and stop them as soon as they can.”
Addiction is more accurately understood as the inappropriate use of a drug for non-medical purposes. It refers to disruptive, drug-seeking behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving.2 Addiction experts also describe addiction as a disease syndrome in its own right. Although that concept can sometimes be hard to accept by those who don’t have a lot of experience working with it, it is a useful paradigm to treat addiction as if it were a disease, says Ronald Crossno, MD, Rockdale, Texas-based area medical director for the VistaCare hospice chain.
Pain experts use the term pseudoaddiction for behaviors that are reminiscent of addiction but in fact reflect the pursuit of pain relief. Examples might include hoarding drugs, clock-watching, and exaggerated complaints of pain, such as moaning or crying. If it is pseudoaddiction, once the pain is brought under control, these behaviors cease. The term was coined in 1989 to describe an iatrogenic syndrome resulting from poorly treated pain.3-5
“Pseudoaddiction is a term you need to know,” Dr. Crossno asserted during a presentation on addiction pain at the recent annual conference of AAHPM in Salt Lake City in February. “It is at least as prevalent as addiction—and an indictment of how our healthcare system deals with pain.”
Dr. Youngwerth offers some advice.“We often see pseudoaddiction in response to undertreatment and inadequately managed pain,” she says. “If you treat the pain appropriately, these behaviors go away.” She tries to teach this concept to residents and hospital staff, who sometimes find it hard to put themselves in the shoes of patients experiencing severe pain.
“If you have a 68-year-old patient with no history of addiction or substance abuse who is in the hospital [with the] status post-hip replacement and is now clock-watching and routinely pressing the call button before her next dose of opioids is due, staff may feel that she is displaying addictive behaviors,” Dr. Youngwerth says. “Why would they think that this situation evolved into addiction during her brief hospital stay? It’s more likely that she’s just afraid of having pain.”
The solution to pseudoaddiction is to prescribe opioids at pharmacologically appropriate doses and schedules. Then, titrate up until analgesia is achieved or toxicities necessitate alternative approaches. Use all the techniques described in the first article of this series. It is also important to restore trust and the patient’s confidence in the medical system’s ability to manage his or her pain. Opioid pain regimens in the hospital should also be coordinated with plans for post-discharge medications and with the patient’s primary-care physician.
Two other concepts that often come up in discussions of opioid treatments are tolerance, which is a diminution of the drug’s effects over time, resulting in a need to increase doses of the medication to achieve the same analgesic effect, and physical dependence, in which the abrupt discontinuation of an analgesic after a period of continuous use causes physical symptoms of withdrawal from the drug. Both of these issues can be addressed with proper assessment and management, and neither is diagnostic of addiction.
Pain experts say tolerance, though a real phenomenon of opioids, is not often a serious problem with pain management in the hospital. Instead, the need for escalating analgesic doses may reflect changes in the underlying disease process. Tolerance can also include positive benefits such as its emergence for opioid side effects like nausea or sedation. Physical dependence on opioids is predictable but can be managed if the original cause of the pain is resolved and the analgesic is no longer needed. Most opioids can be gradually reduced, with each day’s dose at 75% of the previous day’s dose, until the drug is tapered off.6
What if the Patient Is an Addict?
Although pain experts believe that drug addiction caused by appropriate and adequate prescribing of opioids for analgesia is rare, this does not mean that hospitalists won’t face the problem of patients who are addicted to pain medications. “You are already treating patients with addiction,” said Dr. Crossno in his presentation at the AAHPM meeting in Salt Lake City.
Given that pre-existing addictions are relatively common in American society (estimates range from 5% to 17% of the population, depending on whether alcohol abuse is included), it is reasonable to expect this segment of the population will be represented among acutely ill, hospitalized patients.7 Sometimes, the substance abuse problem of a friend or family member affects the patient’s care, such as when pain medications are stolen from the patient.
“Some hospitalized patients do abuse opioids,” says Dr. Bekanich. “We catch people with drug paraphernalia or actually shooting up in their rooms.” Providers can exercise some control over what patients do in the hospital, but it is probably not realistic to expect that a hospitalist will be able to resolve long-standing substance abuse problems during the patient’s brief stay in the hospital.
As part of a comprehensive pain assessment, it is appropriate to ask if the patient has a history of drug use. Many patients will freely admit to such a history, may be actively in recovery or on a methadone maintenance program, or may even resist opioid analgesics despite severe pain because of their commitment to recovery. Without the benefit of such candor, however, it will be difficult to reach a conclusive diagnosis of drug addiction during the patient’s acute inpatient stay, because that ordinarily requires observations over time.
“It is not our job as hospitalists to get patients off opioids; there are other institutions and services for that,” Dr. Bekanich adds. “For us to try to do it in a few days in the hospital seems like a hopeless task. That is not to say we shouldn’t be mindful of the issues involved, talking to the patient or even offering a referral to a drug rehabilitation program. But we should not be trying to do drug rehab.”
The basic principles of believing patients’ reports of pain and providing analgesic doses sufficient to relieve the pain still apply—unless side effects or the patient’s problematic behavior demand a modification in this approach. Pain physicians often cite the maxim “trust but verify.” There are various screening tools that can be used for indicating the possibility of substance abuse, and it is imperative the use of controlled substances always be closely monitored.
Urine drug screening tests are easy to order in the hospital and may encourage compliance for patients who have a drug history when presented up front as a routine aspect of pain management. The urine test can detect prescribed medicines that are being taken by the patient as well as non-prescribed opioids, but it is important to be aware of false positives and negatives and opportunities for gaming the system by those who are determined to do so.
“Just as it is a myth that treating pain appropriately leads to addiction, it is also a myth that people with drug histories can’t have their pain treated effectively,” says Scott Irwin, MD, PhD, medical director of palliative care psychiatry at San Diego Hospice and Palliative Care. “The first thing to ask these patients is what are their goals for pain management. Get as much objective information as you can about the pain and the patient’s history. Fully inform the patient about options. Treat the pain just as you would for anyone else.”
Then, if things don’t add up, Dr. Irwin says, it may be necessary to go back and reassess the patient’s pain and history. Is there psychological distress? Perhaps the analgesic dose isn’t adequate. Maybe financial pressures or complicated social relationships are leading to drug diversion.
If the patient is participating in a methadone maintenance program or similar protocol, it is advisable for the hospitalist to speak to the medical director of that program. But effective pain control also supports maintenance. Emphasize long-acting analgesics, add non-opioid adjuvants and, when possible, find alternatives to intravenous administration. But if the patient is addicted, trying to minimize adverse effects from analgesic treatments might be the best the hospitalist can do.
Another approach to managing the patient with a history of drug abuse is the use of a contract or opioid agreement, in which the patient promises to do certain things with a clear understanding of the consequences for not doing so. Establish the rules early and be prepared to enforce them. Explain expectations for the patient and the physician’s role, designate a single pharmacy and a single physician responsible for pain prescribing, and get consent for treatment and drug testing. If a repeat offender breaks the agreement, it may be time to call in an addiction specialist. Such agreements should be negotiated in person by the physician, not delegated to nurses or other professionals, but then make sure other team members are in the loop. For an example of such an agreement, see http://tinyurl.com/y2bbh6.
Will Pain Medications Cause Respiratory Suppression?
Another common fear related to opioid use is that prescribing sufficient analgesic doses for patients with advanced illnesses could lead to toxicities, suppress their breathing, cause an overdose, or even prematurely end their lives. This scenario is often luridly presented as turning up the morphine drip. Pain management experts question the truth of this scenario, arguing that morphine often is falsely credited with deaths that result from advanced disease processes. Morphine is a common treatment for the sensation of dyspnea, while morphine-related toxicity likely will present with drowsiness, confusion, and loss of consciousness before respiratory compromise.8
A main concern of hospitalists is appreciating the need to balance pain relief with the side effects of analgesics, including opioid toxicities, which can be addressed through careful titration and frequent assessments. Respiratory suppression can be a side effect of opioids, and there are special groups of patients for whom any sedation is a major concern. An example is a lung transplant patient, for whom somnolence may suppress the important cough reflex.
Respiratory suppression from morphine is an area without a large evidence base. But a recent study of 725 patients nearing death in 13 hospice programs analyzed those who were receiving opioids and had at least one change in opioid dose prior to death to see if escalating opioid doses was associated with premature death.9 The authors conclude that “final opioid dose, but not percentage change in dose, was one of several factors associated with survival, but the association is very weak … (and explains) only a very small percentage in variation in survival.” They also found support for their conclusion that opioid use is not a major contributor to premature death in the few other published studies on the subject.
“I tell residents that the fear of respiratory suppression is overrated,” Dr. Youngwerth says. “As long as you follow World Health Organization and other recognized guidelines for dosing and titrating opioids, you can safely prescribe pain medications and control the patient’s pain. They get this fear ingrained during residency. In reality, it is not very common. I remind them that there is much more evidence of under-dosing.”
Dr. Bekanich describes a recent patient, a young woman suffering from severe abdominal pain following the birth of her baby. The pain was so difficult to manage that her hospital in rural Idaho transferred her to his medical center in Salt Lake City. She had also experienced respiratory arrest twice secondary to the application of fentanyl analgesic patches. “But she was relatively easy to manage once we tried a different drug, appropriately titrated,” he relates.
Dr. Bekanich spent two hours in the patient’s room adjusting the intravenous analgesic dose and monitoring the patient’s pulse oxygen level and neurological status. “These medicines don’t have to cause respiratory suppression, although it will happen occasionally, especially when there are multiple co-morbidities,” he says. “Hospitalists don’t realize that most of these problems can be avoided if you are meticulous in prescribing.”
Does Regulatory Scrutiny Chill Pain Treatment?
The ubiquitous fear of opioids and their potential side effects, including some unfounded or unrealistic fears, is also reflected in the regulation of controlled substances and physicians’ fears that they will be subjected to oppressive regulatory scrutiny.
Widely publicized cases of physicians being disciplined or prosecuted for over-prescribing opioids have only added to these fears, while the rare case of a physician being sued or sanctioned for under-prescribing pain medications does little to allay them.10
Growing attention to the inadequacies of and barriers to pain management—and the role of controlled substances regulation in those barriers—led to the 1998 promulgation of “Model Guidelines for the Use of Controlled Substances for the Treatment of Pain” by the Federation of State Medical Boards.11 These guidelines, promoting the legitimate role of opioids in relieving pain and acknowledging providers’ concerns about being disciplined, were revised in 2004 and have been adopted by 21 states.12
The effect remains, however. “For decades, physicians have reported being reluctant to prescribe opioids because of the fear of the stress, expense, and consequences of being investigated by licensing agencies or law enforcement,” states a 2006 state report card issued by the Pain & Policy Studies Group at the University of Wisconsin in Madison.13 “Some states—but far from all—have adopted policies which recognize that controlled substances are necessary for public health. … But in some states, pain treatment using opioids is unduly restricted by policies reflecting medical opinions that were discarded decades ago.”
The Pain & Policy Studies Group’s report card, which advocates for a balanced approach to the regulation and prescribing of controlled substances, has given every state a grade for how well it meets this goal. According to the 2006 report card, Michigan and Virginia get top grades for achieving balance in pain policy, while Georgia gets the lowest grade.
“Regulation is a real concern,” says Daniel Burkhardt, MD, associate professor and director of the Acute Pain Service at the University of California-San Francisco. “Every time a prosecutor arrests someone for prescribing too much pain medication, these things travel, adding to the extra regulatory burden on physicians.”
Carol Jessop, MD, a hospitalist and palliative care consultant at Alta Bates Summit Medical Center in Berkeley, Calif., says the burden has lessened somewhat in California because that state eliminated its requirements for triplicate paper prescribing forms for controlled substances.
A related concern involves the potential diversion of controlled substances by impaired healthcare professionals for personal use and abuse. This is another of the fears that have driven archaic pain regulation in many states. In fact, current estimates suggest that a substance abuse-related impairment will affect between 8% and 18% percent of physicians sometime in their lives, and that 2% of physicians are dealing with an active substance abuse problem.14
A recent medical journal letter to the editor from the Wisconsin Pain & Policy Studies Group suggests public policies on opioid diversion should focus more on sources of diversion such as “thefts, including armed robberies, night break-ins, and employee and customer pilferage,” rather than just the doctor-patient prescribing relationship.15
Physician diversion data don’t break out hospital medicine as a category, but some hospitalists say they have not heard of diversion problems involving hospitalist colleagues. That may reflect the fact that hospitalists, unlike some other health professionals, generally don’t administer controlled substances directly to the patient or have ready access to hospital drug storage facilities. TH
Larry Beresford is a regular contributor to The Hospitalist.
References
- Joranson D, Payne R. Will my pain be managed? In Improving End-of-Life Care: The Role of Attorneys General. National Association of Attorneys General. Washington, D.C. 2003. Available at www.naag.org/end-of-life_healthcare.php. Last accessed April 13, 2007.
- American Pain Society. Definitions related to the use of opioids for the treatment of pain: a consensus document from the American Academy of Pain Medicine, American Pain Society, and American Society of Addiction Medicine. Available at www.ampainsoc.org/advocacy/opioids2.htm. Last accessed April 13, 2007.
- Weissman DE, Haddox JD. Opioid pseudoaddiction. Pain. 1989 Mar;36(3):363-366.
- Weissman DE. Fast Fact and Concept #68: Is it pain or addiction? [The End of Life/Palliative Education Resource Center.] Available at www.eperc.mcw.edu/fastFact/ff_68.htm. Last accessed April 13, 2007.
- Weissman DE. Fast Fact and Concept #69: Pseudoaddiction. [The End of Life/Palliative Education Resource Center.] Available at www.eperc.mcw.edu/fastFact/ff_69.htm. Last accessed April 13, 2007.
- Doyle D, Hanks G, Cherny N, et al, eds. The Oxford Textbook of Palliative Medicine. 3rd ed. Oxford, England: Oxford University Press;2005:336.
- Passik SD, Kirsh KL. Chapter 56: Pain in patients with alcohol and drug dependence. In Bruera E, Higginson I, von Gunten C, et al. Textbook of Palliative Medicine. London, England: Hodder Arnold;2006:517-524.
- Von Gunten CF. Fast Fact and Concept #8: Morphine and hastened death. [The End of Life/Palliative Education Resource Center.] Available at www.eperc.mcw.edu/fastFact/ff_008.htm. Last accessed April 13, 2007.
- Portenoy RK, Siberceva U, Smout R, et al. Opioid use and survival at the end of life: a survey of a hospice population. J Pain Symptom Manage. 2006;32:532-540.
- Warm EJ, Weissman DE. Fast Fact and Concept #63: The legal liability of under-treatment of pain. [The End of Life/Palliative Education Resource Center.] Available at: www.eperc.mcw.edu/fastFact/ff_63.htm. Last accessed April 13, 2007.
- Federation of the State Medical Boards of the United States. Dallas, Texas. Available at www.fsmb.org. Accessed April 13, 2007.
- National Association of Attorneys General. Improving End-of-Life Care: The Role of Attorneys General. National Association of Attorneys General. Washington, D.C. 2003. Available at www.naag.org/end-of-life_healthcare.php. Last accessed April 13, 2007.
- Pain & Policy Studies Group. University of Wisconsin Paul P. Carbone Comprehensive Cancer Center. Available at: www.painpolicy.wisc.edu. Accessed April 13, 2007.
- Blondell RD. Taking a proactive approach to physician impairment. Postgrad Med. 2005 Jul;118(1):16-18.
- Joranson DE, Gilson AM. Drug crime is a source of abused pain medications in the United States. J Pain Symptom Manage. 2005 Oct;30(4):299-301.
Note: This is Part 2 of The Hospitalist’s series on pain and hospital medicine. Part 1 appeared on p. 45 of the April issue.
Welcome to Part 2 of our three-part series on managing the pain of hospitalized patients. Last month’s article presented the context for pain management in the hospital—a core competency identified by SHM. It emphasized techniques for assessing patients’ pain, ranging from a zero-to-10 pain score to more complex pain histories addressing type, source, duration, and intensity as well as psychosocial and spiritual factors.
Part 2 delves into some difficult cases and dilemmas of pain management—situations that can take hospitalists out of their comfort zone and challenge their confidence in managing their patients’ pain.
Some of these dilemmas arise from misconceptions about pain and pain treatments and from the fact that, historically, physicians have not been well trained in optimal pain management. General barriers to pain management in the U.S. healthcare system, as identified by the National Association of Attorneys General, include patients’ beliefs, physician and institutional practices, restrictive state polices, and racial and socioeconomic disparities.1
Many of these issues relate specifically to the most common treatments for severe pain, opioid analgesics, which have all sorts of negative associations based on misconceptions about abuse, addiction, and overdose. In other cases, physicians face real challenges in balancing analgesic benefits with side effects and in determining the right medication, dose, and schedule to meet the patient’s need for pain relief.
Hospitalists confronting difficult pain cases work under the added pressure of trying to bring their patients’ acute illnesses under control so they can discharge them to a lower level of care as soon as prudently possible. This time pressure, along with demands arising from the rest of the hospitalist’s caseload, may impose limits on what can be accomplished in difficult situations or with medications that require time to stabilize.
Challenges also arise when the customary approach to pain management—the drug and dosing schedule the hospitalist is most comfortable using for most patients—fails to bring the pain under control. This is often a red flag for the need to try something new, says Stephen Bekanich, MD, a hospitalist at the University of Utah Medical Center in Salt Lake City and a consultant on the medical center’s palliative care service. In some cases, that means calling in a specialist in pain treatment, palliative medicine, psychiatry, or substance abuse.
“You need to work into the equation that there are pitfalls and caveats to everything we say about pain,” Dr. Bekanich observes. “Plus, the common pain treatments are controlled substances, with obvious legal implications and a professional duty for physicians to handle them safely and appropriately.”
When Dr. Bekanich finds himself confronting a difficult pain situation that has caused a conflict with a patient, he often involves one of the hospital’s customer service patient advocates. They are trained to mediate disagreements between patients and the treatment team.
Is This Patient’s Pain Real?
Physicians sometimes wonder if their patients’ reports of pain are accurate. Is the pain really as bad as the patient says it is? “Residents, frequently, are more skeptical of patients’ claims of pain, doubting whether they are truly experiencing that level of pain,” reports Jean Youngwerth, MD, a hospitalist, palliative care consultant, and fellowship associate program director at the University of Colorado Health Sciences Center in Aurora.
“I tell my residents that malingering is rare, and those few cases where it happens really tend to stand out,” Dr. Youngwerth says. “I also tell them that our default position is always to trust the patient, unless given a good reason not to. I have been burned more often when I questioned my patients’ reports of pain than when I didn’t.”
Pain experts emphasize that the patient’s self-report is the most reliable source of information on pain—based on an understanding of pain as a complex, subjective phenomenon associated with actual or potential tissue damage and the patient’s perception of and emotional reaction to that sensation. The phenomenon of pain also includes emotional, social, psychological, even spiritual components and can be mediated by a host of other factors. But that doesn’t mean it isn’t real to the patient.
“Often, younger physicians take the attitude that if the pain is real, then administration of morphine will make it go away,” says Porter Storey, MD, FACP, FAAHPM. “In reality, pain doesn’t always respond to opioids, for all sorts of reasons. Hospitalists value clarity, and they use pain as a screen for all sorts of other problems. Their goal, often, is not so much the comfort of the patient as it is diagnosing, treating, and then discharging the patient from the hospital.” Dr. Storey is a palliative care physician in Boulder, Colo., and executive vice president for Medical Affairs at the American Academy of Hospice and Palliative Medicine (AAHPM).
Physicians need to be reminded, however, that unresolved pain in hospitalized patients has many negative consequences. These range from resistance to rehabilitation to depression to delayed hospital discharge, as well as reduced job satisfaction for the healthcare professionals who care for them.
Will Prescribing Analgesics Cause Addiction?
Fears about causing addiction haunt many pain management discussions. Requests for more medications, obsessing over the next scheduled analgesic dose, and even manipulative or drug-seeking behaviors can be misunderstood by physicians who lack training in the real nature of drug addiction. Actual cases of drug addiction created by appropriate, sufficient, and well-monitored opioid analgesic treatment are rare, pain experts say. There is an important caveat: the patient who brings a prior history of drug abuse to the current acute medical episode.
“There are no good data about iatrogenic addiction,” says Robert Brody, MD, chief of the pain consultation clinic at San Francisco General Hospital and a frequent presenter on pain management topics at clinical workshops for hospitalists. “People who do pain management, certainly including hospice and palliative care physicians, don’t really believe in it. In my own clinical experience, most patients don’t like pain medications and stop them as soon as they can.”
Addiction is more accurately understood as the inappropriate use of a drug for non-medical purposes. It refers to disruptive, drug-seeking behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving.2 Addiction experts also describe addiction as a disease syndrome in its own right. Although that concept can sometimes be hard to accept by those who don’t have a lot of experience working with it, it is a useful paradigm to treat addiction as if it were a disease, says Ronald Crossno, MD, Rockdale, Texas-based area medical director for the VistaCare hospice chain.
Pain experts use the term pseudoaddiction for behaviors that are reminiscent of addiction but in fact reflect the pursuit of pain relief. Examples might include hoarding drugs, clock-watching, and exaggerated complaints of pain, such as moaning or crying. If it is pseudoaddiction, once the pain is brought under control, these behaviors cease. The term was coined in 1989 to describe an iatrogenic syndrome resulting from poorly treated pain.3-5
“Pseudoaddiction is a term you need to know,” Dr. Crossno asserted during a presentation on addiction pain at the recent annual conference of AAHPM in Salt Lake City in February. “It is at least as prevalent as addiction—and an indictment of how our healthcare system deals with pain.”
Dr. Youngwerth offers some advice.“We often see pseudoaddiction in response to undertreatment and inadequately managed pain,” she says. “If you treat the pain appropriately, these behaviors go away.” She tries to teach this concept to residents and hospital staff, who sometimes find it hard to put themselves in the shoes of patients experiencing severe pain.
“If you have a 68-year-old patient with no history of addiction or substance abuse who is in the hospital [with the] status post-hip replacement and is now clock-watching and routinely pressing the call button before her next dose of opioids is due, staff may feel that she is displaying addictive behaviors,” Dr. Youngwerth says. “Why would they think that this situation evolved into addiction during her brief hospital stay? It’s more likely that she’s just afraid of having pain.”
The solution to pseudoaddiction is to prescribe opioids at pharmacologically appropriate doses and schedules. Then, titrate up until analgesia is achieved or toxicities necessitate alternative approaches. Use all the techniques described in the first article of this series. It is also important to restore trust and the patient’s confidence in the medical system’s ability to manage his or her pain. Opioid pain regimens in the hospital should also be coordinated with plans for post-discharge medications and with the patient’s primary-care physician.
Two other concepts that often come up in discussions of opioid treatments are tolerance, which is a diminution of the drug’s effects over time, resulting in a need to increase doses of the medication to achieve the same analgesic effect, and physical dependence, in which the abrupt discontinuation of an analgesic after a period of continuous use causes physical symptoms of withdrawal from the drug. Both of these issues can be addressed with proper assessment and management, and neither is diagnostic of addiction.
Pain experts say tolerance, though a real phenomenon of opioids, is not often a serious problem with pain management in the hospital. Instead, the need for escalating analgesic doses may reflect changes in the underlying disease process. Tolerance can also include positive benefits such as its emergence for opioid side effects like nausea or sedation. Physical dependence on opioids is predictable but can be managed if the original cause of the pain is resolved and the analgesic is no longer needed. Most opioids can be gradually reduced, with each day’s dose at 75% of the previous day’s dose, until the drug is tapered off.6
What if the Patient Is an Addict?
Although pain experts believe that drug addiction caused by appropriate and adequate prescribing of opioids for analgesia is rare, this does not mean that hospitalists won’t face the problem of patients who are addicted to pain medications. “You are already treating patients with addiction,” said Dr. Crossno in his presentation at the AAHPM meeting in Salt Lake City.
Given that pre-existing addictions are relatively common in American society (estimates range from 5% to 17% of the population, depending on whether alcohol abuse is included), it is reasonable to expect this segment of the population will be represented among acutely ill, hospitalized patients.7 Sometimes, the substance abuse problem of a friend or family member affects the patient’s care, such as when pain medications are stolen from the patient.
“Some hospitalized patients do abuse opioids,” says Dr. Bekanich. “We catch people with drug paraphernalia or actually shooting up in their rooms.” Providers can exercise some control over what patients do in the hospital, but it is probably not realistic to expect that a hospitalist will be able to resolve long-standing substance abuse problems during the patient’s brief stay in the hospital.
As part of a comprehensive pain assessment, it is appropriate to ask if the patient has a history of drug use. Many patients will freely admit to such a history, may be actively in recovery or on a methadone maintenance program, or may even resist opioid analgesics despite severe pain because of their commitment to recovery. Without the benefit of such candor, however, it will be difficult to reach a conclusive diagnosis of drug addiction during the patient’s acute inpatient stay, because that ordinarily requires observations over time.
“It is not our job as hospitalists to get patients off opioids; there are other institutions and services for that,” Dr. Bekanich adds. “For us to try to do it in a few days in the hospital seems like a hopeless task. That is not to say we shouldn’t be mindful of the issues involved, talking to the patient or even offering a referral to a drug rehabilitation program. But we should not be trying to do drug rehab.”
The basic principles of believing patients’ reports of pain and providing analgesic doses sufficient to relieve the pain still apply—unless side effects or the patient’s problematic behavior demand a modification in this approach. Pain physicians often cite the maxim “trust but verify.” There are various screening tools that can be used for indicating the possibility of substance abuse, and it is imperative the use of controlled substances always be closely monitored.
Urine drug screening tests are easy to order in the hospital and may encourage compliance for patients who have a drug history when presented up front as a routine aspect of pain management. The urine test can detect prescribed medicines that are being taken by the patient as well as non-prescribed opioids, but it is important to be aware of false positives and negatives and opportunities for gaming the system by those who are determined to do so.
“Just as it is a myth that treating pain appropriately leads to addiction, it is also a myth that people with drug histories can’t have their pain treated effectively,” says Scott Irwin, MD, PhD, medical director of palliative care psychiatry at San Diego Hospice and Palliative Care. “The first thing to ask these patients is what are their goals for pain management. Get as much objective information as you can about the pain and the patient’s history. Fully inform the patient about options. Treat the pain just as you would for anyone else.”
Then, if things don’t add up, Dr. Irwin says, it may be necessary to go back and reassess the patient’s pain and history. Is there psychological distress? Perhaps the analgesic dose isn’t adequate. Maybe financial pressures or complicated social relationships are leading to drug diversion.
If the patient is participating in a methadone maintenance program or similar protocol, it is advisable for the hospitalist to speak to the medical director of that program. But effective pain control also supports maintenance. Emphasize long-acting analgesics, add non-opioid adjuvants and, when possible, find alternatives to intravenous administration. But if the patient is addicted, trying to minimize adverse effects from analgesic treatments might be the best the hospitalist can do.
Another approach to managing the patient with a history of drug abuse is the use of a contract or opioid agreement, in which the patient promises to do certain things with a clear understanding of the consequences for not doing so. Establish the rules early and be prepared to enforce them. Explain expectations for the patient and the physician’s role, designate a single pharmacy and a single physician responsible for pain prescribing, and get consent for treatment and drug testing. If a repeat offender breaks the agreement, it may be time to call in an addiction specialist. Such agreements should be negotiated in person by the physician, not delegated to nurses or other professionals, but then make sure other team members are in the loop. For an example of such an agreement, see http://tinyurl.com/y2bbh6.
Will Pain Medications Cause Respiratory Suppression?
Another common fear related to opioid use is that prescribing sufficient analgesic doses for patients with advanced illnesses could lead to toxicities, suppress their breathing, cause an overdose, or even prematurely end their lives. This scenario is often luridly presented as turning up the morphine drip. Pain management experts question the truth of this scenario, arguing that morphine often is falsely credited with deaths that result from advanced disease processes. Morphine is a common treatment for the sensation of dyspnea, while morphine-related toxicity likely will present with drowsiness, confusion, and loss of consciousness before respiratory compromise.8
A main concern of hospitalists is appreciating the need to balance pain relief with the side effects of analgesics, including opioid toxicities, which can be addressed through careful titration and frequent assessments. Respiratory suppression can be a side effect of opioids, and there are special groups of patients for whom any sedation is a major concern. An example is a lung transplant patient, for whom somnolence may suppress the important cough reflex.
Respiratory suppression from morphine is an area without a large evidence base. But a recent study of 725 patients nearing death in 13 hospice programs analyzed those who were receiving opioids and had at least one change in opioid dose prior to death to see if escalating opioid doses was associated with premature death.9 The authors conclude that “final opioid dose, but not percentage change in dose, was one of several factors associated with survival, but the association is very weak … (and explains) only a very small percentage in variation in survival.” They also found support for their conclusion that opioid use is not a major contributor to premature death in the few other published studies on the subject.
“I tell residents that the fear of respiratory suppression is overrated,” Dr. Youngwerth says. “As long as you follow World Health Organization and other recognized guidelines for dosing and titrating opioids, you can safely prescribe pain medications and control the patient’s pain. They get this fear ingrained during residency. In reality, it is not very common. I remind them that there is much more evidence of under-dosing.”
Dr. Bekanich describes a recent patient, a young woman suffering from severe abdominal pain following the birth of her baby. The pain was so difficult to manage that her hospital in rural Idaho transferred her to his medical center in Salt Lake City. She had also experienced respiratory arrest twice secondary to the application of fentanyl analgesic patches. “But she was relatively easy to manage once we tried a different drug, appropriately titrated,” he relates.
Dr. Bekanich spent two hours in the patient’s room adjusting the intravenous analgesic dose and monitoring the patient’s pulse oxygen level and neurological status. “These medicines don’t have to cause respiratory suppression, although it will happen occasionally, especially when there are multiple co-morbidities,” he says. “Hospitalists don’t realize that most of these problems can be avoided if you are meticulous in prescribing.”
Does Regulatory Scrutiny Chill Pain Treatment?
The ubiquitous fear of opioids and their potential side effects, including some unfounded or unrealistic fears, is also reflected in the regulation of controlled substances and physicians’ fears that they will be subjected to oppressive regulatory scrutiny.
Widely publicized cases of physicians being disciplined or prosecuted for over-prescribing opioids have only added to these fears, while the rare case of a physician being sued or sanctioned for under-prescribing pain medications does little to allay them.10
Growing attention to the inadequacies of and barriers to pain management—and the role of controlled substances regulation in those barriers—led to the 1998 promulgation of “Model Guidelines for the Use of Controlled Substances for the Treatment of Pain” by the Federation of State Medical Boards.11 These guidelines, promoting the legitimate role of opioids in relieving pain and acknowledging providers’ concerns about being disciplined, were revised in 2004 and have been adopted by 21 states.12
The effect remains, however. “For decades, physicians have reported being reluctant to prescribe opioids because of the fear of the stress, expense, and consequences of being investigated by licensing agencies or law enforcement,” states a 2006 state report card issued by the Pain & Policy Studies Group at the University of Wisconsin in Madison.13 “Some states—but far from all—have adopted policies which recognize that controlled substances are necessary for public health. … But in some states, pain treatment using opioids is unduly restricted by policies reflecting medical opinions that were discarded decades ago.”
The Pain & Policy Studies Group’s report card, which advocates for a balanced approach to the regulation and prescribing of controlled substances, has given every state a grade for how well it meets this goal. According to the 2006 report card, Michigan and Virginia get top grades for achieving balance in pain policy, while Georgia gets the lowest grade.
“Regulation is a real concern,” says Daniel Burkhardt, MD, associate professor and director of the Acute Pain Service at the University of California-San Francisco. “Every time a prosecutor arrests someone for prescribing too much pain medication, these things travel, adding to the extra regulatory burden on physicians.”
Carol Jessop, MD, a hospitalist and palliative care consultant at Alta Bates Summit Medical Center in Berkeley, Calif., says the burden has lessened somewhat in California because that state eliminated its requirements for triplicate paper prescribing forms for controlled substances.
A related concern involves the potential diversion of controlled substances by impaired healthcare professionals for personal use and abuse. This is another of the fears that have driven archaic pain regulation in many states. In fact, current estimates suggest that a substance abuse-related impairment will affect between 8% and 18% percent of physicians sometime in their lives, and that 2% of physicians are dealing with an active substance abuse problem.14
A recent medical journal letter to the editor from the Wisconsin Pain & Policy Studies Group suggests public policies on opioid diversion should focus more on sources of diversion such as “thefts, including armed robberies, night break-ins, and employee and customer pilferage,” rather than just the doctor-patient prescribing relationship.15
Physician diversion data don’t break out hospital medicine as a category, but some hospitalists say they have not heard of diversion problems involving hospitalist colleagues. That may reflect the fact that hospitalists, unlike some other health professionals, generally don’t administer controlled substances directly to the patient or have ready access to hospital drug storage facilities. TH
Larry Beresford is a regular contributor to The Hospitalist.
References
- Joranson D, Payne R. Will my pain be managed? In Improving End-of-Life Care: The Role of Attorneys General. National Association of Attorneys General. Washington, D.C. 2003. Available at www.naag.org/end-of-life_healthcare.php. Last accessed April 13, 2007.
- American Pain Society. Definitions related to the use of opioids for the treatment of pain: a consensus document from the American Academy of Pain Medicine, American Pain Society, and American Society of Addiction Medicine. Available at www.ampainsoc.org/advocacy/opioids2.htm. Last accessed April 13, 2007.
- Weissman DE, Haddox JD. Opioid pseudoaddiction. Pain. 1989 Mar;36(3):363-366.
- Weissman DE. Fast Fact and Concept #68: Is it pain or addiction? [The End of Life/Palliative Education Resource Center.] Available at www.eperc.mcw.edu/fastFact/ff_68.htm. Last accessed April 13, 2007.
- Weissman DE. Fast Fact and Concept #69: Pseudoaddiction. [The End of Life/Palliative Education Resource Center.] Available at www.eperc.mcw.edu/fastFact/ff_69.htm. Last accessed April 13, 2007.
- Doyle D, Hanks G, Cherny N, et al, eds. The Oxford Textbook of Palliative Medicine. 3rd ed. Oxford, England: Oxford University Press;2005:336.
- Passik SD, Kirsh KL. Chapter 56: Pain in patients with alcohol and drug dependence. In Bruera E, Higginson I, von Gunten C, et al. Textbook of Palliative Medicine. London, England: Hodder Arnold;2006:517-524.
- Von Gunten CF. Fast Fact and Concept #8: Morphine and hastened death. [The End of Life/Palliative Education Resource Center.] Available at www.eperc.mcw.edu/fastFact/ff_008.htm. Last accessed April 13, 2007.
- Portenoy RK, Siberceva U, Smout R, et al. Opioid use and survival at the end of life: a survey of a hospice population. J Pain Symptom Manage. 2006;32:532-540.
- Warm EJ, Weissman DE. Fast Fact and Concept #63: The legal liability of under-treatment of pain. [The End of Life/Palliative Education Resource Center.] Available at: www.eperc.mcw.edu/fastFact/ff_63.htm. Last accessed April 13, 2007.
- Federation of the State Medical Boards of the United States. Dallas, Texas. Available at www.fsmb.org. Accessed April 13, 2007.
- National Association of Attorneys General. Improving End-of-Life Care: The Role of Attorneys General. National Association of Attorneys General. Washington, D.C. 2003. Available at www.naag.org/end-of-life_healthcare.php. Last accessed April 13, 2007.
- Pain & Policy Studies Group. University of Wisconsin Paul P. Carbone Comprehensive Cancer Center. Available at: www.painpolicy.wisc.edu. Accessed April 13, 2007.
- Blondell RD. Taking a proactive approach to physician impairment. Postgrad Med. 2005 Jul;118(1):16-18.
- Joranson DE, Gilson AM. Drug crime is a source of abused pain medications in the United States. J Pain Symptom Manage. 2005 Oct;30(4):299-301.
Note: This is Part 2 of The Hospitalist’s series on pain and hospital medicine. Part 1 appeared on p. 45 of the April issue.
Welcome to Part 2 of our three-part series on managing the pain of hospitalized patients. Last month’s article presented the context for pain management in the hospital—a core competency identified by SHM. It emphasized techniques for assessing patients’ pain, ranging from a zero-to-10 pain score to more complex pain histories addressing type, source, duration, and intensity as well as psychosocial and spiritual factors.
Part 2 delves into some difficult cases and dilemmas of pain management—situations that can take hospitalists out of their comfort zone and challenge their confidence in managing their patients’ pain.
Some of these dilemmas arise from misconceptions about pain and pain treatments and from the fact that, historically, physicians have not been well trained in optimal pain management. General barriers to pain management in the U.S. healthcare system, as identified by the National Association of Attorneys General, include patients’ beliefs, physician and institutional practices, restrictive state polices, and racial and socioeconomic disparities.1
Many of these issues relate specifically to the most common treatments for severe pain, opioid analgesics, which have all sorts of negative associations based on misconceptions about abuse, addiction, and overdose. In other cases, physicians face real challenges in balancing analgesic benefits with side effects and in determining the right medication, dose, and schedule to meet the patient’s need for pain relief.
Hospitalists confronting difficult pain cases work under the added pressure of trying to bring their patients’ acute illnesses under control so they can discharge them to a lower level of care as soon as prudently possible. This time pressure, along with demands arising from the rest of the hospitalist’s caseload, may impose limits on what can be accomplished in difficult situations or with medications that require time to stabilize.
Challenges also arise when the customary approach to pain management—the drug and dosing schedule the hospitalist is most comfortable using for most patients—fails to bring the pain under control. This is often a red flag for the need to try something new, says Stephen Bekanich, MD, a hospitalist at the University of Utah Medical Center in Salt Lake City and a consultant on the medical center’s palliative care service. In some cases, that means calling in a specialist in pain treatment, palliative medicine, psychiatry, or substance abuse.
“You need to work into the equation that there are pitfalls and caveats to everything we say about pain,” Dr. Bekanich observes. “Plus, the common pain treatments are controlled substances, with obvious legal implications and a professional duty for physicians to handle them safely and appropriately.”
When Dr. Bekanich finds himself confronting a difficult pain situation that has caused a conflict with a patient, he often involves one of the hospital’s customer service patient advocates. They are trained to mediate disagreements between patients and the treatment team.
Is This Patient’s Pain Real?
Physicians sometimes wonder if their patients’ reports of pain are accurate. Is the pain really as bad as the patient says it is? “Residents, frequently, are more skeptical of patients’ claims of pain, doubting whether they are truly experiencing that level of pain,” reports Jean Youngwerth, MD, a hospitalist, palliative care consultant, and fellowship associate program director at the University of Colorado Health Sciences Center in Aurora.
“I tell my residents that malingering is rare, and those few cases where it happens really tend to stand out,” Dr. Youngwerth says. “I also tell them that our default position is always to trust the patient, unless given a good reason not to. I have been burned more often when I questioned my patients’ reports of pain than when I didn’t.”
Pain experts emphasize that the patient’s self-report is the most reliable source of information on pain—based on an understanding of pain as a complex, subjective phenomenon associated with actual or potential tissue damage and the patient’s perception of and emotional reaction to that sensation. The phenomenon of pain also includes emotional, social, psychological, even spiritual components and can be mediated by a host of other factors. But that doesn’t mean it isn’t real to the patient.
“Often, younger physicians take the attitude that if the pain is real, then administration of morphine will make it go away,” says Porter Storey, MD, FACP, FAAHPM. “In reality, pain doesn’t always respond to opioids, for all sorts of reasons. Hospitalists value clarity, and they use pain as a screen for all sorts of other problems. Their goal, often, is not so much the comfort of the patient as it is diagnosing, treating, and then discharging the patient from the hospital.” Dr. Storey is a palliative care physician in Boulder, Colo., and executive vice president for Medical Affairs at the American Academy of Hospice and Palliative Medicine (AAHPM).
Physicians need to be reminded, however, that unresolved pain in hospitalized patients has many negative consequences. These range from resistance to rehabilitation to depression to delayed hospital discharge, as well as reduced job satisfaction for the healthcare professionals who care for them.
Will Prescribing Analgesics Cause Addiction?
Fears about causing addiction haunt many pain management discussions. Requests for more medications, obsessing over the next scheduled analgesic dose, and even manipulative or drug-seeking behaviors can be misunderstood by physicians who lack training in the real nature of drug addiction. Actual cases of drug addiction created by appropriate, sufficient, and well-monitored opioid analgesic treatment are rare, pain experts say. There is an important caveat: the patient who brings a prior history of drug abuse to the current acute medical episode.
“There are no good data about iatrogenic addiction,” says Robert Brody, MD, chief of the pain consultation clinic at San Francisco General Hospital and a frequent presenter on pain management topics at clinical workshops for hospitalists. “People who do pain management, certainly including hospice and palliative care physicians, don’t really believe in it. In my own clinical experience, most patients don’t like pain medications and stop them as soon as they can.”
Addiction is more accurately understood as the inappropriate use of a drug for non-medical purposes. It refers to disruptive, drug-seeking behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving.2 Addiction experts also describe addiction as a disease syndrome in its own right. Although that concept can sometimes be hard to accept by those who don’t have a lot of experience working with it, it is a useful paradigm to treat addiction as if it were a disease, says Ronald Crossno, MD, Rockdale, Texas-based area medical director for the VistaCare hospice chain.
Pain experts use the term pseudoaddiction for behaviors that are reminiscent of addiction but in fact reflect the pursuit of pain relief. Examples might include hoarding drugs, clock-watching, and exaggerated complaints of pain, such as moaning or crying. If it is pseudoaddiction, once the pain is brought under control, these behaviors cease. The term was coined in 1989 to describe an iatrogenic syndrome resulting from poorly treated pain.3-5
“Pseudoaddiction is a term you need to know,” Dr. Crossno asserted during a presentation on addiction pain at the recent annual conference of AAHPM in Salt Lake City in February. “It is at least as prevalent as addiction—and an indictment of how our healthcare system deals with pain.”
Dr. Youngwerth offers some advice.“We often see pseudoaddiction in response to undertreatment and inadequately managed pain,” she says. “If you treat the pain appropriately, these behaviors go away.” She tries to teach this concept to residents and hospital staff, who sometimes find it hard to put themselves in the shoes of patients experiencing severe pain.
“If you have a 68-year-old patient with no history of addiction or substance abuse who is in the hospital [with the] status post-hip replacement and is now clock-watching and routinely pressing the call button before her next dose of opioids is due, staff may feel that she is displaying addictive behaviors,” Dr. Youngwerth says. “Why would they think that this situation evolved into addiction during her brief hospital stay? It’s more likely that she’s just afraid of having pain.”
The solution to pseudoaddiction is to prescribe opioids at pharmacologically appropriate doses and schedules. Then, titrate up until analgesia is achieved or toxicities necessitate alternative approaches. Use all the techniques described in the first article of this series. It is also important to restore trust and the patient’s confidence in the medical system’s ability to manage his or her pain. Opioid pain regimens in the hospital should also be coordinated with plans for post-discharge medications and with the patient’s primary-care physician.
Two other concepts that often come up in discussions of opioid treatments are tolerance, which is a diminution of the drug’s effects over time, resulting in a need to increase doses of the medication to achieve the same analgesic effect, and physical dependence, in which the abrupt discontinuation of an analgesic after a period of continuous use causes physical symptoms of withdrawal from the drug. Both of these issues can be addressed with proper assessment and management, and neither is diagnostic of addiction.
Pain experts say tolerance, though a real phenomenon of opioids, is not often a serious problem with pain management in the hospital. Instead, the need for escalating analgesic doses may reflect changes in the underlying disease process. Tolerance can also include positive benefits such as its emergence for opioid side effects like nausea or sedation. Physical dependence on opioids is predictable but can be managed if the original cause of the pain is resolved and the analgesic is no longer needed. Most opioids can be gradually reduced, with each day’s dose at 75% of the previous day’s dose, until the drug is tapered off.6
What if the Patient Is an Addict?
Although pain experts believe that drug addiction caused by appropriate and adequate prescribing of opioids for analgesia is rare, this does not mean that hospitalists won’t face the problem of patients who are addicted to pain medications. “You are already treating patients with addiction,” said Dr. Crossno in his presentation at the AAHPM meeting in Salt Lake City.
Given that pre-existing addictions are relatively common in American society (estimates range from 5% to 17% of the population, depending on whether alcohol abuse is included), it is reasonable to expect this segment of the population will be represented among acutely ill, hospitalized patients.7 Sometimes, the substance abuse problem of a friend or family member affects the patient’s care, such as when pain medications are stolen from the patient.
“Some hospitalized patients do abuse opioids,” says Dr. Bekanich. “We catch people with drug paraphernalia or actually shooting up in their rooms.” Providers can exercise some control over what patients do in the hospital, but it is probably not realistic to expect that a hospitalist will be able to resolve long-standing substance abuse problems during the patient’s brief stay in the hospital.
As part of a comprehensive pain assessment, it is appropriate to ask if the patient has a history of drug use. Many patients will freely admit to such a history, may be actively in recovery or on a methadone maintenance program, or may even resist opioid analgesics despite severe pain because of their commitment to recovery. Without the benefit of such candor, however, it will be difficult to reach a conclusive diagnosis of drug addiction during the patient’s acute inpatient stay, because that ordinarily requires observations over time.
“It is not our job as hospitalists to get patients off opioids; there are other institutions and services for that,” Dr. Bekanich adds. “For us to try to do it in a few days in the hospital seems like a hopeless task. That is not to say we shouldn’t be mindful of the issues involved, talking to the patient or even offering a referral to a drug rehabilitation program. But we should not be trying to do drug rehab.”
The basic principles of believing patients’ reports of pain and providing analgesic doses sufficient to relieve the pain still apply—unless side effects or the patient’s problematic behavior demand a modification in this approach. Pain physicians often cite the maxim “trust but verify.” There are various screening tools that can be used for indicating the possibility of substance abuse, and it is imperative the use of controlled substances always be closely monitored.
Urine drug screening tests are easy to order in the hospital and may encourage compliance for patients who have a drug history when presented up front as a routine aspect of pain management. The urine test can detect prescribed medicines that are being taken by the patient as well as non-prescribed opioids, but it is important to be aware of false positives and negatives and opportunities for gaming the system by those who are determined to do so.
“Just as it is a myth that treating pain appropriately leads to addiction, it is also a myth that people with drug histories can’t have their pain treated effectively,” says Scott Irwin, MD, PhD, medical director of palliative care psychiatry at San Diego Hospice and Palliative Care. “The first thing to ask these patients is what are their goals for pain management. Get as much objective information as you can about the pain and the patient’s history. Fully inform the patient about options. Treat the pain just as you would for anyone else.”
Then, if things don’t add up, Dr. Irwin says, it may be necessary to go back and reassess the patient’s pain and history. Is there psychological distress? Perhaps the analgesic dose isn’t adequate. Maybe financial pressures or complicated social relationships are leading to drug diversion.
If the patient is participating in a methadone maintenance program or similar protocol, it is advisable for the hospitalist to speak to the medical director of that program. But effective pain control also supports maintenance. Emphasize long-acting analgesics, add non-opioid adjuvants and, when possible, find alternatives to intravenous administration. But if the patient is addicted, trying to minimize adverse effects from analgesic treatments might be the best the hospitalist can do.
Another approach to managing the patient with a history of drug abuse is the use of a contract or opioid agreement, in which the patient promises to do certain things with a clear understanding of the consequences for not doing so. Establish the rules early and be prepared to enforce them. Explain expectations for the patient and the physician’s role, designate a single pharmacy and a single physician responsible for pain prescribing, and get consent for treatment and drug testing. If a repeat offender breaks the agreement, it may be time to call in an addiction specialist. Such agreements should be negotiated in person by the physician, not delegated to nurses or other professionals, but then make sure other team members are in the loop. For an example of such an agreement, see http://tinyurl.com/y2bbh6.
Will Pain Medications Cause Respiratory Suppression?
Another common fear related to opioid use is that prescribing sufficient analgesic doses for patients with advanced illnesses could lead to toxicities, suppress their breathing, cause an overdose, or even prematurely end their lives. This scenario is often luridly presented as turning up the morphine drip. Pain management experts question the truth of this scenario, arguing that morphine often is falsely credited with deaths that result from advanced disease processes. Morphine is a common treatment for the sensation of dyspnea, while morphine-related toxicity likely will present with drowsiness, confusion, and loss of consciousness before respiratory compromise.8
A main concern of hospitalists is appreciating the need to balance pain relief with the side effects of analgesics, including opioid toxicities, which can be addressed through careful titration and frequent assessments. Respiratory suppression can be a side effect of opioids, and there are special groups of patients for whom any sedation is a major concern. An example is a lung transplant patient, for whom somnolence may suppress the important cough reflex.
Respiratory suppression from morphine is an area without a large evidence base. But a recent study of 725 patients nearing death in 13 hospice programs analyzed those who were receiving opioids and had at least one change in opioid dose prior to death to see if escalating opioid doses was associated with premature death.9 The authors conclude that “final opioid dose, but not percentage change in dose, was one of several factors associated with survival, but the association is very weak … (and explains) only a very small percentage in variation in survival.” They also found support for their conclusion that opioid use is not a major contributor to premature death in the few other published studies on the subject.
“I tell residents that the fear of respiratory suppression is overrated,” Dr. Youngwerth says. “As long as you follow World Health Organization and other recognized guidelines for dosing and titrating opioids, you can safely prescribe pain medications and control the patient’s pain. They get this fear ingrained during residency. In reality, it is not very common. I remind them that there is much more evidence of under-dosing.”
Dr. Bekanich describes a recent patient, a young woman suffering from severe abdominal pain following the birth of her baby. The pain was so difficult to manage that her hospital in rural Idaho transferred her to his medical center in Salt Lake City. She had also experienced respiratory arrest twice secondary to the application of fentanyl analgesic patches. “But she was relatively easy to manage once we tried a different drug, appropriately titrated,” he relates.
Dr. Bekanich spent two hours in the patient’s room adjusting the intravenous analgesic dose and monitoring the patient’s pulse oxygen level and neurological status. “These medicines don’t have to cause respiratory suppression, although it will happen occasionally, especially when there are multiple co-morbidities,” he says. “Hospitalists don’t realize that most of these problems can be avoided if you are meticulous in prescribing.”
Does Regulatory Scrutiny Chill Pain Treatment?
The ubiquitous fear of opioids and their potential side effects, including some unfounded or unrealistic fears, is also reflected in the regulation of controlled substances and physicians’ fears that they will be subjected to oppressive regulatory scrutiny.
Widely publicized cases of physicians being disciplined or prosecuted for over-prescribing opioids have only added to these fears, while the rare case of a physician being sued or sanctioned for under-prescribing pain medications does little to allay them.10
Growing attention to the inadequacies of and barriers to pain management—and the role of controlled substances regulation in those barriers—led to the 1998 promulgation of “Model Guidelines for the Use of Controlled Substances for the Treatment of Pain” by the Federation of State Medical Boards.11 These guidelines, promoting the legitimate role of opioids in relieving pain and acknowledging providers’ concerns about being disciplined, were revised in 2004 and have been adopted by 21 states.12
The effect remains, however. “For decades, physicians have reported being reluctant to prescribe opioids because of the fear of the stress, expense, and consequences of being investigated by licensing agencies or law enforcement,” states a 2006 state report card issued by the Pain & Policy Studies Group at the University of Wisconsin in Madison.13 “Some states—but far from all—have adopted policies which recognize that controlled substances are necessary for public health. … But in some states, pain treatment using opioids is unduly restricted by policies reflecting medical opinions that were discarded decades ago.”
The Pain & Policy Studies Group’s report card, which advocates for a balanced approach to the regulation and prescribing of controlled substances, has given every state a grade for how well it meets this goal. According to the 2006 report card, Michigan and Virginia get top grades for achieving balance in pain policy, while Georgia gets the lowest grade.
“Regulation is a real concern,” says Daniel Burkhardt, MD, associate professor and director of the Acute Pain Service at the University of California-San Francisco. “Every time a prosecutor arrests someone for prescribing too much pain medication, these things travel, adding to the extra regulatory burden on physicians.”
Carol Jessop, MD, a hospitalist and palliative care consultant at Alta Bates Summit Medical Center in Berkeley, Calif., says the burden has lessened somewhat in California because that state eliminated its requirements for triplicate paper prescribing forms for controlled substances.
A related concern involves the potential diversion of controlled substances by impaired healthcare professionals for personal use and abuse. This is another of the fears that have driven archaic pain regulation in many states. In fact, current estimates suggest that a substance abuse-related impairment will affect between 8% and 18% percent of physicians sometime in their lives, and that 2% of physicians are dealing with an active substance abuse problem.14
A recent medical journal letter to the editor from the Wisconsin Pain & Policy Studies Group suggests public policies on opioid diversion should focus more on sources of diversion such as “thefts, including armed robberies, night break-ins, and employee and customer pilferage,” rather than just the doctor-patient prescribing relationship.15
Physician diversion data don’t break out hospital medicine as a category, but some hospitalists say they have not heard of diversion problems involving hospitalist colleagues. That may reflect the fact that hospitalists, unlike some other health professionals, generally don’t administer controlled substances directly to the patient or have ready access to hospital drug storage facilities. TH
Larry Beresford is a regular contributor to The Hospitalist.
References
- Joranson D, Payne R. Will my pain be managed? In Improving End-of-Life Care: The Role of Attorneys General. National Association of Attorneys General. Washington, D.C. 2003. Available at www.naag.org/end-of-life_healthcare.php. Last accessed April 13, 2007.
- American Pain Society. Definitions related to the use of opioids for the treatment of pain: a consensus document from the American Academy of Pain Medicine, American Pain Society, and American Society of Addiction Medicine. Available at www.ampainsoc.org/advocacy/opioids2.htm. Last accessed April 13, 2007.
- Weissman DE, Haddox JD. Opioid pseudoaddiction. Pain. 1989 Mar;36(3):363-366.
- Weissman DE. Fast Fact and Concept #68: Is it pain or addiction? [The End of Life/Palliative Education Resource Center.] Available at www.eperc.mcw.edu/fastFact/ff_68.htm. Last accessed April 13, 2007.
- Weissman DE. Fast Fact and Concept #69: Pseudoaddiction. [The End of Life/Palliative Education Resource Center.] Available at www.eperc.mcw.edu/fastFact/ff_69.htm. Last accessed April 13, 2007.
- Doyle D, Hanks G, Cherny N, et al, eds. The Oxford Textbook of Palliative Medicine. 3rd ed. Oxford, England: Oxford University Press;2005:336.
- Passik SD, Kirsh KL. Chapter 56: Pain in patients with alcohol and drug dependence. In Bruera E, Higginson I, von Gunten C, et al. Textbook of Palliative Medicine. London, England: Hodder Arnold;2006:517-524.
- Von Gunten CF. Fast Fact and Concept #8: Morphine and hastened death. [The End of Life/Palliative Education Resource Center.] Available at www.eperc.mcw.edu/fastFact/ff_008.htm. Last accessed April 13, 2007.
- Portenoy RK, Siberceva U, Smout R, et al. Opioid use and survival at the end of life: a survey of a hospice population. J Pain Symptom Manage. 2006;32:532-540.
- Warm EJ, Weissman DE. Fast Fact and Concept #63: The legal liability of under-treatment of pain. [The End of Life/Palliative Education Resource Center.] Available at: www.eperc.mcw.edu/fastFact/ff_63.htm. Last accessed April 13, 2007.
- Federation of the State Medical Boards of the United States. Dallas, Texas. Available at www.fsmb.org. Accessed April 13, 2007.
- National Association of Attorneys General. Improving End-of-Life Care: The Role of Attorneys General. National Association of Attorneys General. Washington, D.C. 2003. Available at www.naag.org/end-of-life_healthcare.php. Last accessed April 13, 2007.
- Pain & Policy Studies Group. University of Wisconsin Paul P. Carbone Comprehensive Cancer Center. Available at: www.painpolicy.wisc.edu. Accessed April 13, 2007.
- Blondell RD. Taking a proactive approach to physician impairment. Postgrad Med. 2005 Jul;118(1):16-18.
- Joranson DE, Gilson AM. Drug crime is a source of abused pain medications in the United States. J Pain Symptom Manage. 2005 Oct;30(4):299-301.
A Wolf in Sheep's Clothing
Adverse drug reactions are a major clinical problem, accounting for 2%-6% of all hospital admissions. And, 6%-15% of hospitalized patients experience a serious adverse drug reaction that contributes to longer hospital stays and higher costs. It is crucial for clinicians to detect, diagnose, and report adverse drug reactions to ensure safe prescribing and continued drug safety monitoring, as illustrated by this brief case presentation.
The Patient
A 72-year-old male presented to the emergency department in acute respiratory distress due to severe angioedema of the face and tongue; the patient required intubation. He denied prior episodes of angioedema. A careful evaluation of all possible causes of angioedema, including a thorough assessment of the medications used by the patient, led to the conclusion that this life-threatening incident could be attributed only to a reaction to an angiotensin-converting enzyme (ACE) inhibitor. The patient had been on ACE inhibitor therapy for hypertension for more than five years and at the time of admission had been taking a combination of benazepril and amlodipine for more than two years. This medication was immediately discontinued, and he recovered fully after five days in the ICU on mechanical ventilation.
ACE Inhibitor-Associated Angioedema
ACE inhibitors are used by more than 35 million people worldwide to treat hypertension, heart failure, and diabetes mellitus; still, many physicians believe they are underprescribed.1 Angioedema is a serious complication of ACE inhibitor therapy that occurs in 0.1% to 0.68% of patients taking ACE inhibitors.2,3
Angioedema presents with a non-pitting swelling of subcutaneous or submucosal tissue without desquamation. Angioedema associated with ACE inhibitor use is rapid in onset, occurring minutes to hours after ingestion, does not present with urticaria, and usually lasts no more than 48 hours.4 At times, angioedema related to ACE inhibitor therapy occurs in the intestine, causing abdominal pain, diarrhea, and vomiting without mucocutaneous signs.1,4
Certain risk factors for developing ACE inhibitor-related angioedema include age older than 65, seasonal allergies, and black ethnicity. Another risk factor pertinent to our case presentation is the patient’s length of time on ACE inhibitor therapy. One study found that ACE inhibitor-associated angioedema occurred at a rate that was nine times higher during the first month of therapy than during subsequent months of therapy.2 Agostoni and colleagues found that ACE inhibitor-associated angioedema could occur in patients who had been on ACE inhibitor therapy for as long as eight years.5
The Process
ACE inhibitor-induced angioedema is probably a multifactorial process. Angiotensin-converting enzyme (ACE) metabolizes angiotensin I to angiotensin II in vivo and is a major inactivator of bradykinin. ACE and aminopeptidase P are the major pathways of bradykinin metabolism. A minor pathway uses carboxypeptidase N, which metabolizes bradykinin to its active metabolite, des-Arg-bradykinin. Des-Arg-bradykinin can then be inactivated by ACE and aminopeptidase P. In patients who had angioedema caused by ACE inhibitors, higher levels of des-Arg-bradykinin were found due to decreased activity of aminopeptidase P, which normally plays a major role in bradykinin breakdown when an ACE inhibitor is present.6
Bradykinin is a beta2 receptor agonist, but, when it is metabolized by carboxypeptidase N to des-Arg-bradykinin, it becomes a beta1 receptor agonist.6
During ACE inhibitor therapy, bradykinin can be inactivated by aminopeptidase P or metabolized into a beta1 receptor agonist by carboxypeptidase N, which is then broken down by aminopeptidase P. If aminopeptidase P is not active, then bradykinin can be converted to des-Arg-bradykinin, which can then act on upregulated beta1 receptors in the oropharynx and tongue, producing vasodilation, increased capillary permeability, and pain.
Treatment
Treatment of ACE inhibitor-induced angioedema includes discontinuing the ACE inhibitor and providing symptomatic support. Although some ACE inhibitors are more likely than others to cause angioedema, a patient who has had an episode of ACE inhibitor-associated angioedema should never again use any ACE inhibitor.3 Angiotensin receptor blockers (ARBs) do not affect the bradykinin system; however, they can cause angioedema (0.13% in one trial of ARBs), and it is not known if ARBs should be avoided in patients who have had ACE inhibitor-induced angioedema.7 Therapy with a bradykinin receptor antagonist to prevent or resolve ACE inhibitor-associated angioedema has not yet been studied in detail.1
Summary
Adverse drug reactions can present clinically in many different ways, and, indeed, these reactions have deposed syphilis and tuberculosis as the mimic of disease. Many adverse drug reactions are mild, but others can be severe and, occasionally, life-threatening. This variability in manifestations means clinicians always have to consider that the drug may be the cause of the patient’s symptoms. TH
Johnson is a medical student at the Kansas City University of Medicine and Biosciences, Kansas City, Mo. Dr. Egger is a consultant in general internal medicine at the Mayo Clinic, Rochester, Minn.
References
- Byrd JB, Adam A, Brown NJ. Angiotensin-converting enzyme inhibitor-associated angioedema. Immunol Allergy Clin North Am. 2006;26(4):725-737.
- Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med. 2005 Jul;165(14):1637-1642.
- Kostis JB, Packer M, Black HR, et al. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004 Feb;17(2):103-111.
- Kaplan AP, Greaves MW. Angioedema. J Am Acad Dermatol. 2005;53:373-388.
- Agostoni A, Cicardi M, Cugno M, et al. Angioedema due to angiotensin-converting enzyme inhibitors. Immunopharmacology. 1999;44:21-25.
- Molinaro G, Cugno M, Perez M, et al. Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J Pharmacol Exp Ther. 2002;303:232-237.
- Ward KE, Hume AL. Olmesartan (benicar) for hypertension. Am Fam Physician. 2005;72:673-674.
Adverse drug reactions are a major clinical problem, accounting for 2%-6% of all hospital admissions. And, 6%-15% of hospitalized patients experience a serious adverse drug reaction that contributes to longer hospital stays and higher costs. It is crucial for clinicians to detect, diagnose, and report adverse drug reactions to ensure safe prescribing and continued drug safety monitoring, as illustrated by this brief case presentation.
The Patient
A 72-year-old male presented to the emergency department in acute respiratory distress due to severe angioedema of the face and tongue; the patient required intubation. He denied prior episodes of angioedema. A careful evaluation of all possible causes of angioedema, including a thorough assessment of the medications used by the patient, led to the conclusion that this life-threatening incident could be attributed only to a reaction to an angiotensin-converting enzyme (ACE) inhibitor. The patient had been on ACE inhibitor therapy for hypertension for more than five years and at the time of admission had been taking a combination of benazepril and amlodipine for more than two years. This medication was immediately discontinued, and he recovered fully after five days in the ICU on mechanical ventilation.
ACE Inhibitor-Associated Angioedema
ACE inhibitors are used by more than 35 million people worldwide to treat hypertension, heart failure, and diabetes mellitus; still, many physicians believe they are underprescribed.1 Angioedema is a serious complication of ACE inhibitor therapy that occurs in 0.1% to 0.68% of patients taking ACE inhibitors.2,3
Angioedema presents with a non-pitting swelling of subcutaneous or submucosal tissue without desquamation. Angioedema associated with ACE inhibitor use is rapid in onset, occurring minutes to hours after ingestion, does not present with urticaria, and usually lasts no more than 48 hours.4 At times, angioedema related to ACE inhibitor therapy occurs in the intestine, causing abdominal pain, diarrhea, and vomiting without mucocutaneous signs.1,4
Certain risk factors for developing ACE inhibitor-related angioedema include age older than 65, seasonal allergies, and black ethnicity. Another risk factor pertinent to our case presentation is the patient’s length of time on ACE inhibitor therapy. One study found that ACE inhibitor-associated angioedema occurred at a rate that was nine times higher during the first month of therapy than during subsequent months of therapy.2 Agostoni and colleagues found that ACE inhibitor-associated angioedema could occur in patients who had been on ACE inhibitor therapy for as long as eight years.5
The Process
ACE inhibitor-induced angioedema is probably a multifactorial process. Angiotensin-converting enzyme (ACE) metabolizes angiotensin I to angiotensin II in vivo and is a major inactivator of bradykinin. ACE and aminopeptidase P are the major pathways of bradykinin metabolism. A minor pathway uses carboxypeptidase N, which metabolizes bradykinin to its active metabolite, des-Arg-bradykinin. Des-Arg-bradykinin can then be inactivated by ACE and aminopeptidase P. In patients who had angioedema caused by ACE inhibitors, higher levels of des-Arg-bradykinin were found due to decreased activity of aminopeptidase P, which normally plays a major role in bradykinin breakdown when an ACE inhibitor is present.6
Bradykinin is a beta2 receptor agonist, but, when it is metabolized by carboxypeptidase N to des-Arg-bradykinin, it becomes a beta1 receptor agonist.6
During ACE inhibitor therapy, bradykinin can be inactivated by aminopeptidase P or metabolized into a beta1 receptor agonist by carboxypeptidase N, which is then broken down by aminopeptidase P. If aminopeptidase P is not active, then bradykinin can be converted to des-Arg-bradykinin, which can then act on upregulated beta1 receptors in the oropharynx and tongue, producing vasodilation, increased capillary permeability, and pain.
Treatment
Treatment of ACE inhibitor-induced angioedema includes discontinuing the ACE inhibitor and providing symptomatic support. Although some ACE inhibitors are more likely than others to cause angioedema, a patient who has had an episode of ACE inhibitor-associated angioedema should never again use any ACE inhibitor.3 Angiotensin receptor blockers (ARBs) do not affect the bradykinin system; however, they can cause angioedema (0.13% in one trial of ARBs), and it is not known if ARBs should be avoided in patients who have had ACE inhibitor-induced angioedema.7 Therapy with a bradykinin receptor antagonist to prevent or resolve ACE inhibitor-associated angioedema has not yet been studied in detail.1
Summary
Adverse drug reactions can present clinically in many different ways, and, indeed, these reactions have deposed syphilis and tuberculosis as the mimic of disease. Many adverse drug reactions are mild, but others can be severe and, occasionally, life-threatening. This variability in manifestations means clinicians always have to consider that the drug may be the cause of the patient’s symptoms. TH
Johnson is a medical student at the Kansas City University of Medicine and Biosciences, Kansas City, Mo. Dr. Egger is a consultant in general internal medicine at the Mayo Clinic, Rochester, Minn.
References
- Byrd JB, Adam A, Brown NJ. Angiotensin-converting enzyme inhibitor-associated angioedema. Immunol Allergy Clin North Am. 2006;26(4):725-737.
- Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med. 2005 Jul;165(14):1637-1642.
- Kostis JB, Packer M, Black HR, et al. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004 Feb;17(2):103-111.
- Kaplan AP, Greaves MW. Angioedema. J Am Acad Dermatol. 2005;53:373-388.
- Agostoni A, Cicardi M, Cugno M, et al. Angioedema due to angiotensin-converting enzyme inhibitors. Immunopharmacology. 1999;44:21-25.
- Molinaro G, Cugno M, Perez M, et al. Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J Pharmacol Exp Ther. 2002;303:232-237.
- Ward KE, Hume AL. Olmesartan (benicar) for hypertension. Am Fam Physician. 2005;72:673-674.
Adverse drug reactions are a major clinical problem, accounting for 2%-6% of all hospital admissions. And, 6%-15% of hospitalized patients experience a serious adverse drug reaction that contributes to longer hospital stays and higher costs. It is crucial for clinicians to detect, diagnose, and report adverse drug reactions to ensure safe prescribing and continued drug safety monitoring, as illustrated by this brief case presentation.
The Patient
A 72-year-old male presented to the emergency department in acute respiratory distress due to severe angioedema of the face and tongue; the patient required intubation. He denied prior episodes of angioedema. A careful evaluation of all possible causes of angioedema, including a thorough assessment of the medications used by the patient, led to the conclusion that this life-threatening incident could be attributed only to a reaction to an angiotensin-converting enzyme (ACE) inhibitor. The patient had been on ACE inhibitor therapy for hypertension for more than five years and at the time of admission had been taking a combination of benazepril and amlodipine for more than two years. This medication was immediately discontinued, and he recovered fully after five days in the ICU on mechanical ventilation.
ACE Inhibitor-Associated Angioedema
ACE inhibitors are used by more than 35 million people worldwide to treat hypertension, heart failure, and diabetes mellitus; still, many physicians believe they are underprescribed.1 Angioedema is a serious complication of ACE inhibitor therapy that occurs in 0.1% to 0.68% of patients taking ACE inhibitors.2,3
Angioedema presents with a non-pitting swelling of subcutaneous or submucosal tissue without desquamation. Angioedema associated with ACE inhibitor use is rapid in onset, occurring minutes to hours after ingestion, does not present with urticaria, and usually lasts no more than 48 hours.4 At times, angioedema related to ACE inhibitor therapy occurs in the intestine, causing abdominal pain, diarrhea, and vomiting without mucocutaneous signs.1,4
Certain risk factors for developing ACE inhibitor-related angioedema include age older than 65, seasonal allergies, and black ethnicity. Another risk factor pertinent to our case presentation is the patient’s length of time on ACE inhibitor therapy. One study found that ACE inhibitor-associated angioedema occurred at a rate that was nine times higher during the first month of therapy than during subsequent months of therapy.2 Agostoni and colleagues found that ACE inhibitor-associated angioedema could occur in patients who had been on ACE inhibitor therapy for as long as eight years.5
The Process
ACE inhibitor-induced angioedema is probably a multifactorial process. Angiotensin-converting enzyme (ACE) metabolizes angiotensin I to angiotensin II in vivo and is a major inactivator of bradykinin. ACE and aminopeptidase P are the major pathways of bradykinin metabolism. A minor pathway uses carboxypeptidase N, which metabolizes bradykinin to its active metabolite, des-Arg-bradykinin. Des-Arg-bradykinin can then be inactivated by ACE and aminopeptidase P. In patients who had angioedema caused by ACE inhibitors, higher levels of des-Arg-bradykinin were found due to decreased activity of aminopeptidase P, which normally plays a major role in bradykinin breakdown when an ACE inhibitor is present.6
Bradykinin is a beta2 receptor agonist, but, when it is metabolized by carboxypeptidase N to des-Arg-bradykinin, it becomes a beta1 receptor agonist.6
During ACE inhibitor therapy, bradykinin can be inactivated by aminopeptidase P or metabolized into a beta1 receptor agonist by carboxypeptidase N, which is then broken down by aminopeptidase P. If aminopeptidase P is not active, then bradykinin can be converted to des-Arg-bradykinin, which can then act on upregulated beta1 receptors in the oropharynx and tongue, producing vasodilation, increased capillary permeability, and pain.
Treatment
Treatment of ACE inhibitor-induced angioedema includes discontinuing the ACE inhibitor and providing symptomatic support. Although some ACE inhibitors are more likely than others to cause angioedema, a patient who has had an episode of ACE inhibitor-associated angioedema should never again use any ACE inhibitor.3 Angiotensin receptor blockers (ARBs) do not affect the bradykinin system; however, they can cause angioedema (0.13% in one trial of ARBs), and it is not known if ARBs should be avoided in patients who have had ACE inhibitor-induced angioedema.7 Therapy with a bradykinin receptor antagonist to prevent or resolve ACE inhibitor-associated angioedema has not yet been studied in detail.1
Summary
Adverse drug reactions can present clinically in many different ways, and, indeed, these reactions have deposed syphilis and tuberculosis as the mimic of disease. Many adverse drug reactions are mild, but others can be severe and, occasionally, life-threatening. This variability in manifestations means clinicians always have to consider that the drug may be the cause of the patient’s symptoms. TH
Johnson is a medical student at the Kansas City University of Medicine and Biosciences, Kansas City, Mo. Dr. Egger is a consultant in general internal medicine at the Mayo Clinic, Rochester, Minn.
References
- Byrd JB, Adam A, Brown NJ. Angiotensin-converting enzyme inhibitor-associated angioedema. Immunol Allergy Clin North Am. 2006;26(4):725-737.
- Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med. 2005 Jul;165(14):1637-1642.
- Kostis JB, Packer M, Black HR, et al. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004 Feb;17(2):103-111.
- Kaplan AP, Greaves MW. Angioedema. J Am Acad Dermatol. 2005;53:373-388.
- Agostoni A, Cicardi M, Cugno M, et al. Angioedema due to angiotensin-converting enzyme inhibitors. Immunopharmacology. 1999;44:21-25.
- Molinaro G, Cugno M, Perez M, et al. Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J Pharmacol Exp Ther. 2002;303:232-237.
- Ward KE, Hume AL. Olmesartan (benicar) for hypertension. Am Fam Physician. 2005;72:673-674.
The New Institutional Leadership
Dr. Z has practiced hospital medicine at his local community hospital for the past three years. When he came on staff he quickly volunteered for a committee, and today he is its chair. Though he has a heavy workload, he has found the time to take several business courses at a nearby college, and he rarely turns down an opportunity to address a group.
Since he began his practice, he has never missed a local or national meeting of the professional associations to which he belongs. Dr. Z is a hospitalist with an ardent desire to make a difference. He believes he can be most effective in a hospital-wide administrative position, and he is preparing himself.
Dr. Z is a hypothetical example of a growing number of practicing hospitalists who are moving—or desire to move—into hospital-wide decision-making positions. What is the likelihood for their advancement to the higher echelons of hospital administration? Very good for those who have the right stuff for leadership, according to Larry Wellikson, MD, CEO of SHM.
Consider this: Just a decade ago, there were about 100 pioneering hospitalists caring for patients in 20 hospitals. Today there are 20,000 hospitalists serving patients in 2,500 hospitals across the country. Hospital medicine is the fastest growing medical specialty in the United States. The time is right for hospitalists to rise to the fore—not just as leaders of their hospitalist groups but also as system-wide decision makers.
Stacy Goldsholl, MD, represents the new breed: hospitalist as leader. Dr. Goldsholl has been a hospitalist for 12 years. “I got my first job as a community hospitalist before the term was even coined,” she says. “After about five years, I really hit a low point in my career. I was dissatisfied with the way the system was working; I didn’t feel there was enough emphasis on quality patient care. I was at a crossroad. I even considered giving up medicine altogether and going to rabbinical school,” she says, laughing. “But I was very passionate about making things better, so instead of quitting medicine, I embarked on a solo jaunt around the country trying to interest people in improving hospital medicine.”
Today Dr. Goldsholl is president of the Hospital Medicine Division of TeamHealth (Knoxville, Tenn.), a nationwide outsourcing provider of hospitalists in areas as far flung as Puerto Rico and Hawaii.
Though there are no hard-and-fast statistics on how many hospitalists now have leadership roles, Dr. Wellikson says the numbers are swelling. “It’s getting harder and harder to find someone who has strong leadership skills who’s five years into practice who is still just seeing patients,” he says. “There’s such a crying need for leadership in managing the team, leading the hospital medicine group, improving the hospital, improving the quality of care … there’s an enormous void. The first time somebody shows up who has an interest or an aptitude, someone will say, ‘Why don’t you be in charge?’ ”
What Hospitalists Bring to the Leadership Table
Study after study indicates hospitals employing hospitalists experience an improvement in the bottom line that is due, in part, to greater efficiency. This is an important consideration in the current economic crunch in which many hospitals find themselves, but today there is an increased focus on improving patient care as well.
In both efficiency and patient care, hospitalists are uniquely positioned to bring something to the leadership table that other candidates might not. “Hospitalists have a holistic view of the hospital,” says Dr. Goldsholl. “Private physicians don’t have the same connectedness to all the parts. That kind of experience is very valuable. After all, that’s what the hospital is all about.”
Jack M. Percelay, MD, a member of SHM’s Board of Directors, puts it another way. “Clearly, administrative positions require some knowledge of hospital function,” he says. “On-site physicians are certainly more aware of where the problems are. They face them on a daily basis.”
Dr. Wellikson adds, “From the very beginning of their medical careers, even when their main role is seeing patients, hospitalists are looking at the hospital as a system as an institution. They may be members of a quality-improvement team or a group that looks at the flow of patients from the emergency room to the hospital. It becomes sort of second nature to them. Our doctors learn these competencies from the beginning. In their training and their involvement with SHM, whole sections are devoted to systems improvement, leadership, and things like that.”
Dr. Wellikson suggests a demographic reason so many hospitalists look forward to climbing the administrative ladder: “It’s the times. Older doctors may be counting the days till retirement, whereas most hospitalists are younger—the average age is 37—and they say, ‘Hey, I’m going to have 20 more years of this. If I don’t change things, who will?’
“I’ll give you an analogy. When you go to a hotel and your towels aren’t delivered, you might complain until you get them, but you don’t try to manage the hotel. That’s the way practicing doctors felt 20 years ago. But hospitalists, because they’re going to go to work every day in that hospital, if it’s not working tip-top, they’re going to get involved. It’s an evolution in healthcare.”
How to Become a Hospitalist Leader
We asked our experts what advice they would give hospitalists who aspire to critical decision-making positions. “Even if you have natural skills as a leader—you’re charismatic, you take on responsibility—leadership is a skill like any other,” says Dr. Wellikson. “Take the time early in your career to develop that skill. Get involved in some project you feel passionate about. Part of leadership is getting other people to move in the right direction and part is dealing with the people who won’t follow. That can be frustrating. See how this feels to you. Get the education you need, and don’t be afraid to fail. Everybody has failures.”
Dr. Percelay suggests finding activities in your group where you can take initiative. “Clinical legitimacy is key, too, together with a systems viewpoint,” he says. “I would also recommend CME [continuing medical education]-type activities to develop leadership skills. There are the [SHM] Leadership Academy, local business schools, and the American College of Physician Executives, but learn mostly by doing and participating. Joining national organizations is also helpful because you will interact with other like-minded individuals.”
Dr. Goldsholl advises hospitalists to be passionate about their beliefs and to have confidence in what they do. She remembers a seminar she attended at which the speaker—a prominent business executive—gave this advice: “If you’ve never been fired, you are afraid to stand up for what you believe.”
Decision-making positions require hard work and long hours, Dr. Goldsholl cautions. “You have to keep a balance between your personal life and your career. And never underestimate the power of networking at regional and national levels as well as locally. Make your voice heard in print and [at] speaking engagements too. Get published in the Journal of Hospital Medicine. People do read these articles.”
Trends for Hospitalists as Decision Makers
Everyone with whom we spoke predicts a bright future for hospitalists who want to become critical decision makers. “Hospitalists understand the important manifestations of the way all pieces fit together to impact even a single event,” says Dr. Goldsholl. “Already hospitalists are vice presidents of medical affairs, chief operating officers, and so forth. This trend toward placing hospitalists in management roles is being driven, in large part, by the institutional knowledge that hospitalists have. I expect the trend will only expand.”
“There is so much overlap in medicine that what I see developing is a spirit of collaboration,” says Dr. Percelay. “What’s good for the hospitalist is good for the hospital and ultimately good for the patient. I believe that an alignment of incentives is driving the trend toward the appointment of hospitalists to general leadership positions.”
Hospitalists can create a healthcare system driven by teams of healthcare professionals and based on delivering measurable quality, according to Dr. Wellikson, who says hospitalists will play a major role in leading the quality revolution in this country. “We are moving to a time when business and Medicare are driving toward pay for performance,” he says. “Not just, ‘Did you do the surgery?’ but, ‘How well did you do the surgery?’ ”
Most importantly, Dr. Wellikson believes hospitalists are positioned incredibly well to be leaders in this movement. “The first thing people have to do is agree there is a problem. Then they have to measure the performance, try to improve the situation, and then measure the performance again. This is the wave of the future. We [hospitalists] are working with the government, with the National Quality Forum, with the Institute for Healthcare Improvement. Improvement in quality of care is the number one trend upon which hospitalist leaders will have an impact.”
Another emerging trend: Hospitalist decision makers will influence a redesigned hospital of the future. (Dr. Wellikson is one of 20 people on the Joint Commission Hospital of the Future Work Group.) “The hospital of the future will be very different,” explains Dr. Wellikson. “There’s going to be a home team in the new hospital. It will consist of the ED doctors, critical-care doctors, and hospitalists, working with nurses, pharmacists, and the administration as a team to deliver more technology and do more for sicker people.”
These efforts will be on a collision course with the hospital’s ability to afford them, he believes, so hospitalist leadership will be key to creating an efficient hospital that uses its resources in the best way possible and works as a team.
As more and more hospitalists gravitate toward hospitalwide leadership positions, they will confront some of their own. “It’s going to be very interesting,” says Dr. Percelay, “when the hospital medicine group leader differs with the vice president of medical affairs and they both share the same background. The hospital medicine group leader will no longer be able to say, ‘You don’t know where I’m coming from.’ ” TH
Joen Kinnan is a medical journalist based in Chicago.
Dr. Z has practiced hospital medicine at his local community hospital for the past three years. When he came on staff he quickly volunteered for a committee, and today he is its chair. Though he has a heavy workload, he has found the time to take several business courses at a nearby college, and he rarely turns down an opportunity to address a group.
Since he began his practice, he has never missed a local or national meeting of the professional associations to which he belongs. Dr. Z is a hospitalist with an ardent desire to make a difference. He believes he can be most effective in a hospital-wide administrative position, and he is preparing himself.
Dr. Z is a hypothetical example of a growing number of practicing hospitalists who are moving—or desire to move—into hospital-wide decision-making positions. What is the likelihood for their advancement to the higher echelons of hospital administration? Very good for those who have the right stuff for leadership, according to Larry Wellikson, MD, CEO of SHM.
Consider this: Just a decade ago, there were about 100 pioneering hospitalists caring for patients in 20 hospitals. Today there are 20,000 hospitalists serving patients in 2,500 hospitals across the country. Hospital medicine is the fastest growing medical specialty in the United States. The time is right for hospitalists to rise to the fore—not just as leaders of their hospitalist groups but also as system-wide decision makers.
Stacy Goldsholl, MD, represents the new breed: hospitalist as leader. Dr. Goldsholl has been a hospitalist for 12 years. “I got my first job as a community hospitalist before the term was even coined,” she says. “After about five years, I really hit a low point in my career. I was dissatisfied with the way the system was working; I didn’t feel there was enough emphasis on quality patient care. I was at a crossroad. I even considered giving up medicine altogether and going to rabbinical school,” she says, laughing. “But I was very passionate about making things better, so instead of quitting medicine, I embarked on a solo jaunt around the country trying to interest people in improving hospital medicine.”
Today Dr. Goldsholl is president of the Hospital Medicine Division of TeamHealth (Knoxville, Tenn.), a nationwide outsourcing provider of hospitalists in areas as far flung as Puerto Rico and Hawaii.
Though there are no hard-and-fast statistics on how many hospitalists now have leadership roles, Dr. Wellikson says the numbers are swelling. “It’s getting harder and harder to find someone who has strong leadership skills who’s five years into practice who is still just seeing patients,” he says. “There’s such a crying need for leadership in managing the team, leading the hospital medicine group, improving the hospital, improving the quality of care … there’s an enormous void. The first time somebody shows up who has an interest or an aptitude, someone will say, ‘Why don’t you be in charge?’ ”
What Hospitalists Bring to the Leadership Table
Study after study indicates hospitals employing hospitalists experience an improvement in the bottom line that is due, in part, to greater efficiency. This is an important consideration in the current economic crunch in which many hospitals find themselves, but today there is an increased focus on improving patient care as well.
In both efficiency and patient care, hospitalists are uniquely positioned to bring something to the leadership table that other candidates might not. “Hospitalists have a holistic view of the hospital,” says Dr. Goldsholl. “Private physicians don’t have the same connectedness to all the parts. That kind of experience is very valuable. After all, that’s what the hospital is all about.”
Jack M. Percelay, MD, a member of SHM’s Board of Directors, puts it another way. “Clearly, administrative positions require some knowledge of hospital function,” he says. “On-site physicians are certainly more aware of where the problems are. They face them on a daily basis.”
Dr. Wellikson adds, “From the very beginning of their medical careers, even when their main role is seeing patients, hospitalists are looking at the hospital as a system as an institution. They may be members of a quality-improvement team or a group that looks at the flow of patients from the emergency room to the hospital. It becomes sort of second nature to them. Our doctors learn these competencies from the beginning. In their training and their involvement with SHM, whole sections are devoted to systems improvement, leadership, and things like that.”
Dr. Wellikson suggests a demographic reason so many hospitalists look forward to climbing the administrative ladder: “It’s the times. Older doctors may be counting the days till retirement, whereas most hospitalists are younger—the average age is 37—and they say, ‘Hey, I’m going to have 20 more years of this. If I don’t change things, who will?’
“I’ll give you an analogy. When you go to a hotel and your towels aren’t delivered, you might complain until you get them, but you don’t try to manage the hotel. That’s the way practicing doctors felt 20 years ago. But hospitalists, because they’re going to go to work every day in that hospital, if it’s not working tip-top, they’re going to get involved. It’s an evolution in healthcare.”
How to Become a Hospitalist Leader
We asked our experts what advice they would give hospitalists who aspire to critical decision-making positions. “Even if you have natural skills as a leader—you’re charismatic, you take on responsibility—leadership is a skill like any other,” says Dr. Wellikson. “Take the time early in your career to develop that skill. Get involved in some project you feel passionate about. Part of leadership is getting other people to move in the right direction and part is dealing with the people who won’t follow. That can be frustrating. See how this feels to you. Get the education you need, and don’t be afraid to fail. Everybody has failures.”
Dr. Percelay suggests finding activities in your group where you can take initiative. “Clinical legitimacy is key, too, together with a systems viewpoint,” he says. “I would also recommend CME [continuing medical education]-type activities to develop leadership skills. There are the [SHM] Leadership Academy, local business schools, and the American College of Physician Executives, but learn mostly by doing and participating. Joining national organizations is also helpful because you will interact with other like-minded individuals.”
Dr. Goldsholl advises hospitalists to be passionate about their beliefs and to have confidence in what they do. She remembers a seminar she attended at which the speaker—a prominent business executive—gave this advice: “If you’ve never been fired, you are afraid to stand up for what you believe.”
Decision-making positions require hard work and long hours, Dr. Goldsholl cautions. “You have to keep a balance between your personal life and your career. And never underestimate the power of networking at regional and national levels as well as locally. Make your voice heard in print and [at] speaking engagements too. Get published in the Journal of Hospital Medicine. People do read these articles.”
Trends for Hospitalists as Decision Makers
Everyone with whom we spoke predicts a bright future for hospitalists who want to become critical decision makers. “Hospitalists understand the important manifestations of the way all pieces fit together to impact even a single event,” says Dr. Goldsholl. “Already hospitalists are vice presidents of medical affairs, chief operating officers, and so forth. This trend toward placing hospitalists in management roles is being driven, in large part, by the institutional knowledge that hospitalists have. I expect the trend will only expand.”
“There is so much overlap in medicine that what I see developing is a spirit of collaboration,” says Dr. Percelay. “What’s good for the hospitalist is good for the hospital and ultimately good for the patient. I believe that an alignment of incentives is driving the trend toward the appointment of hospitalists to general leadership positions.”
Hospitalists can create a healthcare system driven by teams of healthcare professionals and based on delivering measurable quality, according to Dr. Wellikson, who says hospitalists will play a major role in leading the quality revolution in this country. “We are moving to a time when business and Medicare are driving toward pay for performance,” he says. “Not just, ‘Did you do the surgery?’ but, ‘How well did you do the surgery?’ ”
Most importantly, Dr. Wellikson believes hospitalists are positioned incredibly well to be leaders in this movement. “The first thing people have to do is agree there is a problem. Then they have to measure the performance, try to improve the situation, and then measure the performance again. This is the wave of the future. We [hospitalists] are working with the government, with the National Quality Forum, with the Institute for Healthcare Improvement. Improvement in quality of care is the number one trend upon which hospitalist leaders will have an impact.”
Another emerging trend: Hospitalist decision makers will influence a redesigned hospital of the future. (Dr. Wellikson is one of 20 people on the Joint Commission Hospital of the Future Work Group.) “The hospital of the future will be very different,” explains Dr. Wellikson. “There’s going to be a home team in the new hospital. It will consist of the ED doctors, critical-care doctors, and hospitalists, working with nurses, pharmacists, and the administration as a team to deliver more technology and do more for sicker people.”
These efforts will be on a collision course with the hospital’s ability to afford them, he believes, so hospitalist leadership will be key to creating an efficient hospital that uses its resources in the best way possible and works as a team.
As more and more hospitalists gravitate toward hospitalwide leadership positions, they will confront some of their own. “It’s going to be very interesting,” says Dr. Percelay, “when the hospital medicine group leader differs with the vice president of medical affairs and they both share the same background. The hospital medicine group leader will no longer be able to say, ‘You don’t know where I’m coming from.’ ” TH
Joen Kinnan is a medical journalist based in Chicago.
Dr. Z has practiced hospital medicine at his local community hospital for the past three years. When he came on staff he quickly volunteered for a committee, and today he is its chair. Though he has a heavy workload, he has found the time to take several business courses at a nearby college, and he rarely turns down an opportunity to address a group.
Since he began his practice, he has never missed a local or national meeting of the professional associations to which he belongs. Dr. Z is a hospitalist with an ardent desire to make a difference. He believes he can be most effective in a hospital-wide administrative position, and he is preparing himself.
Dr. Z is a hypothetical example of a growing number of practicing hospitalists who are moving—or desire to move—into hospital-wide decision-making positions. What is the likelihood for their advancement to the higher echelons of hospital administration? Very good for those who have the right stuff for leadership, according to Larry Wellikson, MD, CEO of SHM.
Consider this: Just a decade ago, there were about 100 pioneering hospitalists caring for patients in 20 hospitals. Today there are 20,000 hospitalists serving patients in 2,500 hospitals across the country. Hospital medicine is the fastest growing medical specialty in the United States. The time is right for hospitalists to rise to the fore—not just as leaders of their hospitalist groups but also as system-wide decision makers.
Stacy Goldsholl, MD, represents the new breed: hospitalist as leader. Dr. Goldsholl has been a hospitalist for 12 years. “I got my first job as a community hospitalist before the term was even coined,” she says. “After about five years, I really hit a low point in my career. I was dissatisfied with the way the system was working; I didn’t feel there was enough emphasis on quality patient care. I was at a crossroad. I even considered giving up medicine altogether and going to rabbinical school,” she says, laughing. “But I was very passionate about making things better, so instead of quitting medicine, I embarked on a solo jaunt around the country trying to interest people in improving hospital medicine.”
Today Dr. Goldsholl is president of the Hospital Medicine Division of TeamHealth (Knoxville, Tenn.), a nationwide outsourcing provider of hospitalists in areas as far flung as Puerto Rico and Hawaii.
Though there are no hard-and-fast statistics on how many hospitalists now have leadership roles, Dr. Wellikson says the numbers are swelling. “It’s getting harder and harder to find someone who has strong leadership skills who’s five years into practice who is still just seeing patients,” he says. “There’s such a crying need for leadership in managing the team, leading the hospital medicine group, improving the hospital, improving the quality of care … there’s an enormous void. The first time somebody shows up who has an interest or an aptitude, someone will say, ‘Why don’t you be in charge?’ ”
What Hospitalists Bring to the Leadership Table
Study after study indicates hospitals employing hospitalists experience an improvement in the bottom line that is due, in part, to greater efficiency. This is an important consideration in the current economic crunch in which many hospitals find themselves, but today there is an increased focus on improving patient care as well.
In both efficiency and patient care, hospitalists are uniquely positioned to bring something to the leadership table that other candidates might not. “Hospitalists have a holistic view of the hospital,” says Dr. Goldsholl. “Private physicians don’t have the same connectedness to all the parts. That kind of experience is very valuable. After all, that’s what the hospital is all about.”
Jack M. Percelay, MD, a member of SHM’s Board of Directors, puts it another way. “Clearly, administrative positions require some knowledge of hospital function,” he says. “On-site physicians are certainly more aware of where the problems are. They face them on a daily basis.”
Dr. Wellikson adds, “From the very beginning of their medical careers, even when their main role is seeing patients, hospitalists are looking at the hospital as a system as an institution. They may be members of a quality-improvement team or a group that looks at the flow of patients from the emergency room to the hospital. It becomes sort of second nature to them. Our doctors learn these competencies from the beginning. In their training and their involvement with SHM, whole sections are devoted to systems improvement, leadership, and things like that.”
Dr. Wellikson suggests a demographic reason so many hospitalists look forward to climbing the administrative ladder: “It’s the times. Older doctors may be counting the days till retirement, whereas most hospitalists are younger—the average age is 37—and they say, ‘Hey, I’m going to have 20 more years of this. If I don’t change things, who will?’
“I’ll give you an analogy. When you go to a hotel and your towels aren’t delivered, you might complain until you get them, but you don’t try to manage the hotel. That’s the way practicing doctors felt 20 years ago. But hospitalists, because they’re going to go to work every day in that hospital, if it’s not working tip-top, they’re going to get involved. It’s an evolution in healthcare.”
How to Become a Hospitalist Leader
We asked our experts what advice they would give hospitalists who aspire to critical decision-making positions. “Even if you have natural skills as a leader—you’re charismatic, you take on responsibility—leadership is a skill like any other,” says Dr. Wellikson. “Take the time early in your career to develop that skill. Get involved in some project you feel passionate about. Part of leadership is getting other people to move in the right direction and part is dealing with the people who won’t follow. That can be frustrating. See how this feels to you. Get the education you need, and don’t be afraid to fail. Everybody has failures.”
Dr. Percelay suggests finding activities in your group where you can take initiative. “Clinical legitimacy is key, too, together with a systems viewpoint,” he says. “I would also recommend CME [continuing medical education]-type activities to develop leadership skills. There are the [SHM] Leadership Academy, local business schools, and the American College of Physician Executives, but learn mostly by doing and participating. Joining national organizations is also helpful because you will interact with other like-minded individuals.”
Dr. Goldsholl advises hospitalists to be passionate about their beliefs and to have confidence in what they do. She remembers a seminar she attended at which the speaker—a prominent business executive—gave this advice: “If you’ve never been fired, you are afraid to stand up for what you believe.”
Decision-making positions require hard work and long hours, Dr. Goldsholl cautions. “You have to keep a balance between your personal life and your career. And never underestimate the power of networking at regional and national levels as well as locally. Make your voice heard in print and [at] speaking engagements too. Get published in the Journal of Hospital Medicine. People do read these articles.”
Trends for Hospitalists as Decision Makers
Everyone with whom we spoke predicts a bright future for hospitalists who want to become critical decision makers. “Hospitalists understand the important manifestations of the way all pieces fit together to impact even a single event,” says Dr. Goldsholl. “Already hospitalists are vice presidents of medical affairs, chief operating officers, and so forth. This trend toward placing hospitalists in management roles is being driven, in large part, by the institutional knowledge that hospitalists have. I expect the trend will only expand.”
“There is so much overlap in medicine that what I see developing is a spirit of collaboration,” says Dr. Percelay. “What’s good for the hospitalist is good for the hospital and ultimately good for the patient. I believe that an alignment of incentives is driving the trend toward the appointment of hospitalists to general leadership positions.”
Hospitalists can create a healthcare system driven by teams of healthcare professionals and based on delivering measurable quality, according to Dr. Wellikson, who says hospitalists will play a major role in leading the quality revolution in this country. “We are moving to a time when business and Medicare are driving toward pay for performance,” he says. “Not just, ‘Did you do the surgery?’ but, ‘How well did you do the surgery?’ ”
Most importantly, Dr. Wellikson believes hospitalists are positioned incredibly well to be leaders in this movement. “The first thing people have to do is agree there is a problem. Then they have to measure the performance, try to improve the situation, and then measure the performance again. This is the wave of the future. We [hospitalists] are working with the government, with the National Quality Forum, with the Institute for Healthcare Improvement. Improvement in quality of care is the number one trend upon which hospitalist leaders will have an impact.”
Another emerging trend: Hospitalist decision makers will influence a redesigned hospital of the future. (Dr. Wellikson is one of 20 people on the Joint Commission Hospital of the Future Work Group.) “The hospital of the future will be very different,” explains Dr. Wellikson. “There’s going to be a home team in the new hospital. It will consist of the ED doctors, critical-care doctors, and hospitalists, working with nurses, pharmacists, and the administration as a team to deliver more technology and do more for sicker people.”
These efforts will be on a collision course with the hospital’s ability to afford them, he believes, so hospitalist leadership will be key to creating an efficient hospital that uses its resources in the best way possible and works as a team.
As more and more hospitalists gravitate toward hospitalwide leadership positions, they will confront some of their own. “It’s going to be very interesting,” says Dr. Percelay, “when the hospital medicine group leader differs with the vice president of medical affairs and they both share the same background. The hospital medicine group leader will no longer be able to say, ‘You don’t know where I’m coming from.’ ” TH
Joen Kinnan is a medical journalist based in Chicago.
A Night with Venus, a Lifetime with Mercury
When you think of mercury, what comes to mind? Do you think about the small, hot planet in our solar system nearest the sun? Perhaps the Roman god with winged feet?
I know some people who would surely think about the car. But how about heavy metal?
No, not rock music; the transition metal with the atomic number 80. Mercury is one of only five elements that is a liquid at room temperature.1 The periodic table symbol for mercury, Hg, comes from the Greek name hydrargyrum, a combination of the words for water (hydro) and silver (argyros) forming a word meaning “watery silver.’’ It’s an apt physical description of elemental mercury. The other common name for mercury is quicksilver.
Mercury, an element with a storied past, still presents dangers hospitalists must be aware of.
Organic compounds of mercury especially are toxic at even low levels. Human exposure must be limited when possible. Many elements and heavy metals are used by living organisms in trace amounts. Not mercury. Mercury is a toxin at any dose. Pure elemental mercury is less toxic than the organic compounds and salts. Exposed to water, mercury is quickly converted to the more toxic form, methylmercury. Because of its volatility, any open container of mercury is a biohazard and presents a risk of poisoning from mercury vapor. Inhaled vaporized mercury is readily absorbed through the alveoli.2
Historical Uses
Mankind has used mercury-containing products for a long time. Some of the red color in ancient cave drawings is cinnabar, or mercury sulfide (HgS). Cinnabar has also been used as the red pigment in tattoos. When I was in the Navy, we were told any skin rash that spared the red color in a tattoo was sure to be syphilis; according to the information we were given, the red pigment in a tattoo was created by mercury. Actually, tattoo parlors use several forms of red pigment, but mercury sulfide is certainly one of the choices.
Historically, many cultures used mercury in a variety of ways. More than two millennia ago, Greeks used mercury in medicines; Romans incorporated the element in their cosmetics.3 Medicinal uses of mercury have included the treatment of syphilis and use as a diuretic, antiseptic, and laxative.4 In the mid-20th century, mercury-containing compounds were popular as a remedy for infant-teething pain.
From the 17th to 19th centuries, mercury was used in the process of making felt for hats; hat makers were subjected to excessive exposure to the element. Mercury toxicity manifested as psychiatric illness and was widely recognized as an occupational hazard of hat-making.4,5 Reflecting this observation, “mad as a hatter” became a figure of speech and even found its way into the famous book “Through the Looking Glass (And What Alice Found There)’’ by Lewis Carroll.
Gold mining presents another occupational exposure to mercury. During 19th-century gold mining in California, mercury was used to extract gold from ore. Today, much controversy and ecological protest is focused on the South American gold mining industry, particularly in Brazil. In the past 20 years or so, it is estimated that 2,000 tons of mercury have been released into the environment in the Brazilian Amazon.6 Watchdog groups have been sounding the alarm for people who live in the villages along the Amazon River and consume a diet of fish caught from the river. Hair samples of those villagers revealed high levels of mercury.7 To date, clinical disease has not been reported there.
Infamous Occurrences
The most noted occurrence of clinical mercury poisoning was in Minamata, Japan. Starting before World War II and continuing until 1968, industrial mercury-containing waste was dumped into the sea near Minamata. The townspeople’s diet consisted of locally caught fish; the fish were heavily contaminated with mercury. Many people suffered permanent disability, and more than a thousand died.8 The effect of mercury poisoning was so great the disease was called Minamata’s disease.
Another epidemic occurred in the early 1970s in Iraq. Grain intended for use as seed for planting was treated with a mercury-containing fungicide. Accidentally, the local population used the grain for making bread, resulting in many cases of clinical mercury poisoning.8
One of the most historically interesting stories of mercury poisoning involves the first emperor of the Qin Dynasty in China, Qin Shi Huang Di, who lived from 259 to 210 B.C. At age 12 when Qin Shi Huang ascended to the throne, China was divided into warring feudal states. By asserting ruthless power, he succeeded in conquering the last warring province and unified China in 221 B.C. Although his rule was brutal and tyrannical, his accomplishments included standardization of the systems of measurement and weights, currency, and Chinese script. He was also a builder, conscripting labor for the development of roads, canals, and—most famously—his own mausoleum. The tomb of Qin Shi Huang at Xian is famous for its size and contents, including the army of more than 8,000 life-size, terracotta warriors.9
Qin Shi Huang Di (Di means supreme god) set out on a tour in 210 B.C. seeking eternal life in the “Islands of the Immortals.” It is not clear if his entourage found the site, but he was given a potion or pills, made by his court scientists, to make him immortal. The medication contained a toxic compound of mercury, and he died.9 The mausoleum was completed just in time to accommodate the emperor.
Exposure and Toxicity
Other than occupational exposure to mercury, the most important mercury exposures in the United States are dietary, the use of mercury amalgam in dentistry, and mercury used as a stabilizing agent in vaccines. The risk of toxicity depends on the form of mercury involved. Elemental mercury is poorly absorbed through the gastrointestinal tract; methylmercury, on the other hand, is readily absorbed if ingested. As previously noted, inhaled elemental mercury vapor is easily absorbed in the lungs.
Consensus supports the fact that dietary ingestion of methylmercury as found in large game fish—tuna, swordfish, shark, king mackerel, and tilefish—is the most important exposure to organic mercury. Small fish eat the mercury-contaminated plant life on the ocean bottom and are consumed by larger fish, and those fish are consumed by larger fish. Thus, the large game fish in the food chain accumulate high concentrations of mercury. It is recommended that children and pregnant women limit consumption of these types of fish.8
Dental silver for tooth fillings is a mixture of 50% mercury and 50% metal powder, such as silver, copper, or zinc. Because dental amalgam uses elemental mercury, gastrointestinal absorption is limited. Two studies examining more than 500 children each followed the neuro-psychiatric development of children with mercury amalgam fillings with a control group. No statistical differences were identified in IQ, memory, visual/motor, and renal function between the two groups over five- and seven-year follow-ups. Yet, doubts linger concerning long-term follow-up and questions raised with respect to the incidence of multiple sclerosis and Alzheimer’s disease.10 Overall, dental mercury amalgam is believed to be relatively safe with no evidence of acute toxicity.
Controversy has swirled around the use of thimerosal, a mercury-containing stabilizer and antibacterial agent used in vaccines. Used since the 1930s, thimerosal is metabolized to ethyl mercury in the body and has been implicated in a host of ailments, including the marked increase in incidences of autism. Although no dose-dependent toxicity has been established, questions concerning genetic vulnerability to mercury have been raised and considered significant.11-12 In 1999, the Department of Health and Human Services recommended thimerosal be decreased or eliminated from childhood vaccines.
Telltale Signs
Clinical symptoms of mercury toxicity are often insidious in onset and nonspecific, making diagnosis difficult without a high index of suspicion. Complaints may include gastrointestinal symptoms, headaches, insomnia, visual disturbances, peripheral neuropathy, or ataxia.2 Exposure to inorganic mercury from mercurial salves (merthiolate) or the chronic use of mercury-based cathartics may present with the constellation of symptoms known as acrodynia or pink disease, Feer disease, Feer’s disease, Swift syndrome, Swift’s disease, Swift disease, Swift-Feer disease, vegetative neurosis, dermatopolyneuritis, erythredema polyneuritis, and trophodermatoneurosis. This is characterized by a desquamating rash, hair loss, erythema of the palms and soles, anorexia, and gastrointestinal complaints. Elemental mercury does not readily cross the blood-brain barrier, so neurological complaints are not dominant.2
Organic mercury poisoning typically presents with neurological symptoms. In-utero exposure may result in spontaneous abortion; or the infant, if delivered, may suffer mental retardation. Adult toxicity presents with sensory and motor-neurological complaints, visual field loss, hearing loss, dysarthria, or cerebellar symptoms of ataxia. Severe toxicity results in movement disorders, paralysis, and seizures.2 Evidence of kidney damage and reproductive failure are also commonly associated.
In suspected cases, obtain blood and urine levels of mercury. Levels of mercury in hair may be helpful in some instances, but false-positive findings make this method of testing less reliable.
Treatment is supportive. Employ chelating agents if the patient is acutely symptomatic. The agent of choice is BAL (dimercaprol). Administer it as directed by consultation with a poison control team. BAL is not recommended for children. TH
References
- Wikipedia. The mercury element page. Available at http://en.wikipedia.org/wiki/Mercury_%28element%29. Last accessed April 19, 2007.
- Diner B. Toxicity, mercury. Available at http://emedicine.com/EMERGE/topic813.htm.
- WebElements. Periodic table. www.webelements.com
- Elinder CG. Epidemiology and toxicity of mercury. UpToDate, Nov. 2006. Available at member. www.patients.uptodate.com/topic.asp?file=renldis/14240]
- Mercury: useful metal or toxic chemical? Available at www.ci.vancouver.wa.us/solidwaste/pbt_site/usefulmetal.asp. Last accessed April 19, 2007.
- Malm O. Gold mining as a source of mercury exposure in the Brazilian Amazon. Environ Res 1998 May;77(2):73-78.
- Harada M, Nakanishi J, Yasoda E, et al. Mercury pollution in the Tapajos River basin, Amazon: mercury level of head hair and health effects. Environ Int. 2001;27(4):285-290
- Wikipedia. Mercury poisoning. Available at http://en.wikipedia.org/wiki/Mercury_poisoning. Last accessed April 19, 2007.
- Answers.com. Qin Shi Huang. Available at www.answers.com/topic/qin-shi-huang. Last accessed April 19, 2007.
- Needleman HL. Mercury in dental amalgam—a neurotoxic risk? JAMA. 2006 Apr 19;295(15):1835-1836.
- National Institutes of Allergy and Infectious Diseases. NAID research on thimerosal. December 2006. Available at www.niaid.nih.gov/factsheets/thimerosal.htm. Last accessed April 19, 2007.
- Environmental Working Group. Executive summary. December 13, 2004. Available at www.ewg.org/reports/autism/execsumm.php. Last accessed April 19, 2007.
When you think of mercury, what comes to mind? Do you think about the small, hot planet in our solar system nearest the sun? Perhaps the Roman god with winged feet?
I know some people who would surely think about the car. But how about heavy metal?
No, not rock music; the transition metal with the atomic number 80. Mercury is one of only five elements that is a liquid at room temperature.1 The periodic table symbol for mercury, Hg, comes from the Greek name hydrargyrum, a combination of the words for water (hydro) and silver (argyros) forming a word meaning “watery silver.’’ It’s an apt physical description of elemental mercury. The other common name for mercury is quicksilver.
Mercury, an element with a storied past, still presents dangers hospitalists must be aware of.
Organic compounds of mercury especially are toxic at even low levels. Human exposure must be limited when possible. Many elements and heavy metals are used by living organisms in trace amounts. Not mercury. Mercury is a toxin at any dose. Pure elemental mercury is less toxic than the organic compounds and salts. Exposed to water, mercury is quickly converted to the more toxic form, methylmercury. Because of its volatility, any open container of mercury is a biohazard and presents a risk of poisoning from mercury vapor. Inhaled vaporized mercury is readily absorbed through the alveoli.2
Historical Uses
Mankind has used mercury-containing products for a long time. Some of the red color in ancient cave drawings is cinnabar, or mercury sulfide (HgS). Cinnabar has also been used as the red pigment in tattoos. When I was in the Navy, we were told any skin rash that spared the red color in a tattoo was sure to be syphilis; according to the information we were given, the red pigment in a tattoo was created by mercury. Actually, tattoo parlors use several forms of red pigment, but mercury sulfide is certainly one of the choices.
Historically, many cultures used mercury in a variety of ways. More than two millennia ago, Greeks used mercury in medicines; Romans incorporated the element in their cosmetics.3 Medicinal uses of mercury have included the treatment of syphilis and use as a diuretic, antiseptic, and laxative.4 In the mid-20th century, mercury-containing compounds were popular as a remedy for infant-teething pain.
From the 17th to 19th centuries, mercury was used in the process of making felt for hats; hat makers were subjected to excessive exposure to the element. Mercury toxicity manifested as psychiatric illness and was widely recognized as an occupational hazard of hat-making.4,5 Reflecting this observation, “mad as a hatter” became a figure of speech and even found its way into the famous book “Through the Looking Glass (And What Alice Found There)’’ by Lewis Carroll.
Gold mining presents another occupational exposure to mercury. During 19th-century gold mining in California, mercury was used to extract gold from ore. Today, much controversy and ecological protest is focused on the South American gold mining industry, particularly in Brazil. In the past 20 years or so, it is estimated that 2,000 tons of mercury have been released into the environment in the Brazilian Amazon.6 Watchdog groups have been sounding the alarm for people who live in the villages along the Amazon River and consume a diet of fish caught from the river. Hair samples of those villagers revealed high levels of mercury.7 To date, clinical disease has not been reported there.
Infamous Occurrences
The most noted occurrence of clinical mercury poisoning was in Minamata, Japan. Starting before World War II and continuing until 1968, industrial mercury-containing waste was dumped into the sea near Minamata. The townspeople’s diet consisted of locally caught fish; the fish were heavily contaminated with mercury. Many people suffered permanent disability, and more than a thousand died.8 The effect of mercury poisoning was so great the disease was called Minamata’s disease.
Another epidemic occurred in the early 1970s in Iraq. Grain intended for use as seed for planting was treated with a mercury-containing fungicide. Accidentally, the local population used the grain for making bread, resulting in many cases of clinical mercury poisoning.8
One of the most historically interesting stories of mercury poisoning involves the first emperor of the Qin Dynasty in China, Qin Shi Huang Di, who lived from 259 to 210 B.C. At age 12 when Qin Shi Huang ascended to the throne, China was divided into warring feudal states. By asserting ruthless power, he succeeded in conquering the last warring province and unified China in 221 B.C. Although his rule was brutal and tyrannical, his accomplishments included standardization of the systems of measurement and weights, currency, and Chinese script. He was also a builder, conscripting labor for the development of roads, canals, and—most famously—his own mausoleum. The tomb of Qin Shi Huang at Xian is famous for its size and contents, including the army of more than 8,000 life-size, terracotta warriors.9
Qin Shi Huang Di (Di means supreme god) set out on a tour in 210 B.C. seeking eternal life in the “Islands of the Immortals.” It is not clear if his entourage found the site, but he was given a potion or pills, made by his court scientists, to make him immortal. The medication contained a toxic compound of mercury, and he died.9 The mausoleum was completed just in time to accommodate the emperor.
Exposure and Toxicity
Other than occupational exposure to mercury, the most important mercury exposures in the United States are dietary, the use of mercury amalgam in dentistry, and mercury used as a stabilizing agent in vaccines. The risk of toxicity depends on the form of mercury involved. Elemental mercury is poorly absorbed through the gastrointestinal tract; methylmercury, on the other hand, is readily absorbed if ingested. As previously noted, inhaled elemental mercury vapor is easily absorbed in the lungs.
Consensus supports the fact that dietary ingestion of methylmercury as found in large game fish—tuna, swordfish, shark, king mackerel, and tilefish—is the most important exposure to organic mercury. Small fish eat the mercury-contaminated plant life on the ocean bottom and are consumed by larger fish, and those fish are consumed by larger fish. Thus, the large game fish in the food chain accumulate high concentrations of mercury. It is recommended that children and pregnant women limit consumption of these types of fish.8
Dental silver for tooth fillings is a mixture of 50% mercury and 50% metal powder, such as silver, copper, or zinc. Because dental amalgam uses elemental mercury, gastrointestinal absorption is limited. Two studies examining more than 500 children each followed the neuro-psychiatric development of children with mercury amalgam fillings with a control group. No statistical differences were identified in IQ, memory, visual/motor, and renal function between the two groups over five- and seven-year follow-ups. Yet, doubts linger concerning long-term follow-up and questions raised with respect to the incidence of multiple sclerosis and Alzheimer’s disease.10 Overall, dental mercury amalgam is believed to be relatively safe with no evidence of acute toxicity.
Controversy has swirled around the use of thimerosal, a mercury-containing stabilizer and antibacterial agent used in vaccines. Used since the 1930s, thimerosal is metabolized to ethyl mercury in the body and has been implicated in a host of ailments, including the marked increase in incidences of autism. Although no dose-dependent toxicity has been established, questions concerning genetic vulnerability to mercury have been raised and considered significant.11-12 In 1999, the Department of Health and Human Services recommended thimerosal be decreased or eliminated from childhood vaccines.
Telltale Signs
Clinical symptoms of mercury toxicity are often insidious in onset and nonspecific, making diagnosis difficult without a high index of suspicion. Complaints may include gastrointestinal symptoms, headaches, insomnia, visual disturbances, peripheral neuropathy, or ataxia.2 Exposure to inorganic mercury from mercurial salves (merthiolate) or the chronic use of mercury-based cathartics may present with the constellation of symptoms known as acrodynia or pink disease, Feer disease, Feer’s disease, Swift syndrome, Swift’s disease, Swift disease, Swift-Feer disease, vegetative neurosis, dermatopolyneuritis, erythredema polyneuritis, and trophodermatoneurosis. This is characterized by a desquamating rash, hair loss, erythema of the palms and soles, anorexia, and gastrointestinal complaints. Elemental mercury does not readily cross the blood-brain barrier, so neurological complaints are not dominant.2
Organic mercury poisoning typically presents with neurological symptoms. In-utero exposure may result in spontaneous abortion; or the infant, if delivered, may suffer mental retardation. Adult toxicity presents with sensory and motor-neurological complaints, visual field loss, hearing loss, dysarthria, or cerebellar symptoms of ataxia. Severe toxicity results in movement disorders, paralysis, and seizures.2 Evidence of kidney damage and reproductive failure are also commonly associated.
In suspected cases, obtain blood and urine levels of mercury. Levels of mercury in hair may be helpful in some instances, but false-positive findings make this method of testing less reliable.
Treatment is supportive. Employ chelating agents if the patient is acutely symptomatic. The agent of choice is BAL (dimercaprol). Administer it as directed by consultation with a poison control team. BAL is not recommended for children. TH
References
- Wikipedia. The mercury element page. Available at http://en.wikipedia.org/wiki/Mercury_%28element%29. Last accessed April 19, 2007.
- Diner B. Toxicity, mercury. Available at http://emedicine.com/EMERGE/topic813.htm.
- WebElements. Periodic table. www.webelements.com
- Elinder CG. Epidemiology and toxicity of mercury. UpToDate, Nov. 2006. Available at member. www.patients.uptodate.com/topic.asp?file=renldis/14240]
- Mercury: useful metal or toxic chemical? Available at www.ci.vancouver.wa.us/solidwaste/pbt_site/usefulmetal.asp. Last accessed April 19, 2007.
- Malm O. Gold mining as a source of mercury exposure in the Brazilian Amazon. Environ Res 1998 May;77(2):73-78.
- Harada M, Nakanishi J, Yasoda E, et al. Mercury pollution in the Tapajos River basin, Amazon: mercury level of head hair and health effects. Environ Int. 2001;27(4):285-290
- Wikipedia. Mercury poisoning. Available at http://en.wikipedia.org/wiki/Mercury_poisoning. Last accessed April 19, 2007.
- Answers.com. Qin Shi Huang. Available at www.answers.com/topic/qin-shi-huang. Last accessed April 19, 2007.
- Needleman HL. Mercury in dental amalgam—a neurotoxic risk? JAMA. 2006 Apr 19;295(15):1835-1836.
- National Institutes of Allergy and Infectious Diseases. NAID research on thimerosal. December 2006. Available at www.niaid.nih.gov/factsheets/thimerosal.htm. Last accessed April 19, 2007.
- Environmental Working Group. Executive summary. December 13, 2004. Available at www.ewg.org/reports/autism/execsumm.php. Last accessed April 19, 2007.
When you think of mercury, what comes to mind? Do you think about the small, hot planet in our solar system nearest the sun? Perhaps the Roman god with winged feet?
I know some people who would surely think about the car. But how about heavy metal?
No, not rock music; the transition metal with the atomic number 80. Mercury is one of only five elements that is a liquid at room temperature.1 The periodic table symbol for mercury, Hg, comes from the Greek name hydrargyrum, a combination of the words for water (hydro) and silver (argyros) forming a word meaning “watery silver.’’ It’s an apt physical description of elemental mercury. The other common name for mercury is quicksilver.
Mercury, an element with a storied past, still presents dangers hospitalists must be aware of.
Organic compounds of mercury especially are toxic at even low levels. Human exposure must be limited when possible. Many elements and heavy metals are used by living organisms in trace amounts. Not mercury. Mercury is a toxin at any dose. Pure elemental mercury is less toxic than the organic compounds and salts. Exposed to water, mercury is quickly converted to the more toxic form, methylmercury. Because of its volatility, any open container of mercury is a biohazard and presents a risk of poisoning from mercury vapor. Inhaled vaporized mercury is readily absorbed through the alveoli.2
Historical Uses
Mankind has used mercury-containing products for a long time. Some of the red color in ancient cave drawings is cinnabar, or mercury sulfide (HgS). Cinnabar has also been used as the red pigment in tattoos. When I was in the Navy, we were told any skin rash that spared the red color in a tattoo was sure to be syphilis; according to the information we were given, the red pigment in a tattoo was created by mercury. Actually, tattoo parlors use several forms of red pigment, but mercury sulfide is certainly one of the choices.
Historically, many cultures used mercury in a variety of ways. More than two millennia ago, Greeks used mercury in medicines; Romans incorporated the element in their cosmetics.3 Medicinal uses of mercury have included the treatment of syphilis and use as a diuretic, antiseptic, and laxative.4 In the mid-20th century, mercury-containing compounds were popular as a remedy for infant-teething pain.
From the 17th to 19th centuries, mercury was used in the process of making felt for hats; hat makers were subjected to excessive exposure to the element. Mercury toxicity manifested as psychiatric illness and was widely recognized as an occupational hazard of hat-making.4,5 Reflecting this observation, “mad as a hatter” became a figure of speech and even found its way into the famous book “Through the Looking Glass (And What Alice Found There)’’ by Lewis Carroll.
Gold mining presents another occupational exposure to mercury. During 19th-century gold mining in California, mercury was used to extract gold from ore. Today, much controversy and ecological protest is focused on the South American gold mining industry, particularly in Brazil. In the past 20 years or so, it is estimated that 2,000 tons of mercury have been released into the environment in the Brazilian Amazon.6 Watchdog groups have been sounding the alarm for people who live in the villages along the Amazon River and consume a diet of fish caught from the river. Hair samples of those villagers revealed high levels of mercury.7 To date, clinical disease has not been reported there.
Infamous Occurrences
The most noted occurrence of clinical mercury poisoning was in Minamata, Japan. Starting before World War II and continuing until 1968, industrial mercury-containing waste was dumped into the sea near Minamata. The townspeople’s diet consisted of locally caught fish; the fish were heavily contaminated with mercury. Many people suffered permanent disability, and more than a thousand died.8 The effect of mercury poisoning was so great the disease was called Minamata’s disease.
Another epidemic occurred in the early 1970s in Iraq. Grain intended for use as seed for planting was treated with a mercury-containing fungicide. Accidentally, the local population used the grain for making bread, resulting in many cases of clinical mercury poisoning.8
One of the most historically interesting stories of mercury poisoning involves the first emperor of the Qin Dynasty in China, Qin Shi Huang Di, who lived from 259 to 210 B.C. At age 12 when Qin Shi Huang ascended to the throne, China was divided into warring feudal states. By asserting ruthless power, he succeeded in conquering the last warring province and unified China in 221 B.C. Although his rule was brutal and tyrannical, his accomplishments included standardization of the systems of measurement and weights, currency, and Chinese script. He was also a builder, conscripting labor for the development of roads, canals, and—most famously—his own mausoleum. The tomb of Qin Shi Huang at Xian is famous for its size and contents, including the army of more than 8,000 life-size, terracotta warriors.9
Qin Shi Huang Di (Di means supreme god) set out on a tour in 210 B.C. seeking eternal life in the “Islands of the Immortals.” It is not clear if his entourage found the site, but he was given a potion or pills, made by his court scientists, to make him immortal. The medication contained a toxic compound of mercury, and he died.9 The mausoleum was completed just in time to accommodate the emperor.
Exposure and Toxicity
Other than occupational exposure to mercury, the most important mercury exposures in the United States are dietary, the use of mercury amalgam in dentistry, and mercury used as a stabilizing agent in vaccines. The risk of toxicity depends on the form of mercury involved. Elemental mercury is poorly absorbed through the gastrointestinal tract; methylmercury, on the other hand, is readily absorbed if ingested. As previously noted, inhaled elemental mercury vapor is easily absorbed in the lungs.
Consensus supports the fact that dietary ingestion of methylmercury as found in large game fish—tuna, swordfish, shark, king mackerel, and tilefish—is the most important exposure to organic mercury. Small fish eat the mercury-contaminated plant life on the ocean bottom and are consumed by larger fish, and those fish are consumed by larger fish. Thus, the large game fish in the food chain accumulate high concentrations of mercury. It is recommended that children and pregnant women limit consumption of these types of fish.8
Dental silver for tooth fillings is a mixture of 50% mercury and 50% metal powder, such as silver, copper, or zinc. Because dental amalgam uses elemental mercury, gastrointestinal absorption is limited. Two studies examining more than 500 children each followed the neuro-psychiatric development of children with mercury amalgam fillings with a control group. No statistical differences were identified in IQ, memory, visual/motor, and renal function between the two groups over five- and seven-year follow-ups. Yet, doubts linger concerning long-term follow-up and questions raised with respect to the incidence of multiple sclerosis and Alzheimer’s disease.10 Overall, dental mercury amalgam is believed to be relatively safe with no evidence of acute toxicity.
Controversy has swirled around the use of thimerosal, a mercury-containing stabilizer and antibacterial agent used in vaccines. Used since the 1930s, thimerosal is metabolized to ethyl mercury in the body and has been implicated in a host of ailments, including the marked increase in incidences of autism. Although no dose-dependent toxicity has been established, questions concerning genetic vulnerability to mercury have been raised and considered significant.11-12 In 1999, the Department of Health and Human Services recommended thimerosal be decreased or eliminated from childhood vaccines.
Telltale Signs
Clinical symptoms of mercury toxicity are often insidious in onset and nonspecific, making diagnosis difficult without a high index of suspicion. Complaints may include gastrointestinal symptoms, headaches, insomnia, visual disturbances, peripheral neuropathy, or ataxia.2 Exposure to inorganic mercury from mercurial salves (merthiolate) or the chronic use of mercury-based cathartics may present with the constellation of symptoms known as acrodynia or pink disease, Feer disease, Feer’s disease, Swift syndrome, Swift’s disease, Swift disease, Swift-Feer disease, vegetative neurosis, dermatopolyneuritis, erythredema polyneuritis, and trophodermatoneurosis. This is characterized by a desquamating rash, hair loss, erythema of the palms and soles, anorexia, and gastrointestinal complaints. Elemental mercury does not readily cross the blood-brain barrier, so neurological complaints are not dominant.2
Organic mercury poisoning typically presents with neurological symptoms. In-utero exposure may result in spontaneous abortion; or the infant, if delivered, may suffer mental retardation. Adult toxicity presents with sensory and motor-neurological complaints, visual field loss, hearing loss, dysarthria, or cerebellar symptoms of ataxia. Severe toxicity results in movement disorders, paralysis, and seizures.2 Evidence of kidney damage and reproductive failure are also commonly associated.
In suspected cases, obtain blood and urine levels of mercury. Levels of mercury in hair may be helpful in some instances, but false-positive findings make this method of testing less reliable.
Treatment is supportive. Employ chelating agents if the patient is acutely symptomatic. The agent of choice is BAL (dimercaprol). Administer it as directed by consultation with a poison control team. BAL is not recommended for children. TH
References
- Wikipedia. The mercury element page. Available at http://en.wikipedia.org/wiki/Mercury_%28element%29. Last accessed April 19, 2007.
- Diner B. Toxicity, mercury. Available at http://emedicine.com/EMERGE/topic813.htm.
- WebElements. Periodic table. www.webelements.com
- Elinder CG. Epidemiology and toxicity of mercury. UpToDate, Nov. 2006. Available at member. www.patients.uptodate.com/topic.asp?file=renldis/14240]
- Mercury: useful metal or toxic chemical? Available at www.ci.vancouver.wa.us/solidwaste/pbt_site/usefulmetal.asp. Last accessed April 19, 2007.
- Malm O. Gold mining as a source of mercury exposure in the Brazilian Amazon. Environ Res 1998 May;77(2):73-78.
- Harada M, Nakanishi J, Yasoda E, et al. Mercury pollution in the Tapajos River basin, Amazon: mercury level of head hair and health effects. Environ Int. 2001;27(4):285-290
- Wikipedia. Mercury poisoning. Available at http://en.wikipedia.org/wiki/Mercury_poisoning. Last accessed April 19, 2007.
- Answers.com. Qin Shi Huang. Available at www.answers.com/topic/qin-shi-huang. Last accessed April 19, 2007.
- Needleman HL. Mercury in dental amalgam—a neurotoxic risk? JAMA. 2006 Apr 19;295(15):1835-1836.
- National Institutes of Allergy and Infectious Diseases. NAID research on thimerosal. December 2006. Available at www.niaid.nih.gov/factsheets/thimerosal.htm. Last accessed April 19, 2007.
- Environmental Working Group. Executive summary. December 13, 2004. Available at www.ewg.org/reports/autism/execsumm.php. Last accessed April 19, 2007.
The Sumter Tornado
On the evening of March 1, severe thunderstorms rumbled over southwestern Georgia, spawning a tornado that ripped through seven counties. Classified an EF 3 with winds ranging from approximately 136 to 165 miles per hour, the twister tore a path of destruction 37 miles long and as much as a mile wide, reaching its maximum strength and width as it reached Americus, the seat of Sumter county. Along the way, the Sumter tornado—part of a storm system that killed eight in a high school in Enterprise, Ala.—demolished or seriously damaged more than 200 homes and dozens of businesses in Americus and the surrounding area, causing two deaths and many more injuries.
Not only did it leave the local Winn-Dixie grocery store without its façade, but it also reduced a 1,600-foot-tall public television tower to a 150-foot stump, sheared the tops off trees, downed power lines, knocked out telephone service, and deposited a burning tractor in the middle of Highway 520.
More important, it destroyed Sumter Regional Hospital in Americus, a rural city of 17,000 residents. Thanks to Sumter Regional’s staff, including its four hospitalists, all 70 patients were evacuated from the 265,000-square-foot, 143-bed complex.
The Terror Begins
The tornado, which struck Americus between 9 and 9:30 p.m., did not arrive without warning—but the town did not sound its tornado siren. A firefighter dispatched to activate the warning was called back because it was too late to do any good. Sumter Regional’s staff was alerted a tornado might strike, and they had moved patients away from the windows.
Hospitalist Mukesh Kumar, MD, who had joined Sumter Regional two weeks before the tornado struck, rode the storm out in the hospitalists’ office—a small space on the same corridor with a number of patient rooms.
“[Dr. Kumar] was the poor guy on duty that night,” says Amanda Davis, MD, head of the hospitalist program, who was not in town that evening.
As soon as the storm passed, an emergency call went out via the local broadcast media, requesting that physicians and nurses report to the severely damaged hospital to aid in the evacuation and treat the injured.
Hospitalists Kathy Hudson, MD, and Rick Oster, MD, among others, rushed to help. Getting to the hospital wasn’t easy. The surrounding area had become a maze of downed trees and power lines. First responders who could use their cars had to park outside the devastated area and hike to the hospital in the dark. Others arrived on bicycles. Some could not make it because they were trapped in their driveways.
With the power out at the tornado-ravaged hospital, as it was in much of Americus, first responders helped those on duty carry many of the 70 patients down the stairs of the four-story complex. Outside, Dr. Hudson worked with the staff, suturing the injured. Sumter Regional Hospital was evacuated by about 1:30 the following morning.
Patients were loaded into the town’s four ambulances and others from around the region and transferred to facilities in southwest Georgia. The closest of these was Phoebe Putney Memorial Hospital in Albany, about 40 miles from Americus.
Taking Stock
Wind and water had compromised nearly every part of Sumter Regional, leaving only the chapel intact. Much of the roof was ripped off, nearly all the windows were blown out, and part of the complex collapsed. Damage was so severe the entire health center was rendered unsafe.
Not only did the storm leave Americus without its hospital, it also devastated virtually all the town’s private doctor offices, as well as the Sumter HealthPlex—a new, 8,000-square-foot, $3.1 million facility owned by the hospital. The HealthPlex provided outpatient imaging and laboratory services to the community.
In the days immediately following the tornado, the Middle Flint chapter of the American Red Cross—whose headquarters were also badly damaged—set up an emergency response center in the First Baptist Church of Americus that featured a makeshift emergency room. Dr. Davis and her staff of hospitalists, employed by TeamHealth’s Hospital Medicine Division, helped get the Red Cross center up and running. They also played a significant role in setting up a temporary urgent-care center in tents provided by the Federal Emergency Management Agency (FEMA), supplemented by two tents belonging to the Boy Scouts.
By Any Means Necessary
Although local physicians with their own practices were well represented among the first responders, they were (for the most part) not involved with staffing the tent hospital, according to Dr. Davis. Most of the work in the tents was done by the emergency department (ED) physicians and TeamHealth doctors, in conjunction with Sumter Regional’s four hospitalists.
As soon as the tents opened, the ED “regulars” started showing up in droves,” explains Dr. Davis. Because a significant number of private physicians’ offices in town were destroyed there were no other healthcare options for many residents.
“It’s a difficult situation,” says Dr. Davis. “There are people in the community without cars, for example, whose cars were destroyed. So they have no other way to get to another hospital. They were astonishingly grateful to have us there.”
As residents cleaned up more than a month after the disaster, the urgent-care facility in the tents still functions as Sumter Regional’s ED. Routine lab work and X-rays were provided, and staff also monitored patients on blood thinners and gave injections to cancer patients.
In a pinch, the tents have served as an impromptu obstetrics suite. One woman delivered her child there after being unable to travel to Phoebe Putney Memorial Hospital, which is providing obstetrical services to mothers who would have gone to Sumter Regional.
Care of the center’s cancer patients—a number of whom must take a bus to Albany for treatment—is now overseen by Phoebe Putney’s oncology department. Surgeons, too, have had to travel to other hospitals in order to operate. “It is a logistical nightmare,” says Dr. Davis.
While the urgent-care unit has been able to provide basic services, it has not been an easy task for the physicians and nurses who work there. Telephone lines have not been restored, making it virtually impossible to send faxes. Hospitalists and ED physicians staffing the tents have had to read their own films, essentially serving as their own radiologists. As Dr Davis notes, “You have to do the best with what you have.” Often, that is a bare minimum. Indeed, she once had to put in a central line to stabilize a patient. “It was pretty surreal treating (the patient) in a tent,” she says.
What’s Next?
FEMA Director David Paulison, who toured the devastated hospital with President Bush, says the immediate response was indicative of the “new FEMA.”
Bush declared Sumter County a disaster area the day after the tornado. Residents were urged to apply as quickly as possible for grants and low-cost loans to aid in recovery. Local, state, and federal officials opened a disaster recovery center March 5 in Americus, staffed by representatives of FEMA, the Georgia Emergency Management Agency (GEMA), and a number of other agencies. As of April 4, FEMA, GEMA, and the U.S. Small Business Association (SBA), had approved more than $6.58 million for disaster recovery.
A May 16 update by Sumter Regional on its Web site indicated progress in the construction of modular care facilities.
“By the end of May, there will be five modular buildings in the Mayo Street parking lot for use by physicians, each consisting of two single-wide modular buildings joined together,” the memo stated. “Currently, two of the five physician modular buildings are in place with both halves assembled, and three of the single units are on site ready for placement and assembly. The other three halves that will be used to construct the remaining three buildings are on the way. We are still seeking bids from contractors to do the plumbing, electrical and other utility work, and anticipate having the buildings functional by the end of May for physicians to begin seeing patients.”
On April 30, Sumter Regional opened its Sumter Regional East facility. Sumter East provides 24-hour urgent care, radiology services, clinical lab services, physical therapy, speech therapy, occupational therapy, and cardiopulmonary services, according to the hospital’s Web site.
An interim hospital is slated to open in mid-September, according to a May 10 statement by the hospital. The facility, to be built on the former site of the HealthPlex, is to include approximately 65 inpatient rooms, nine LDRP/obstetrics/nursery rooms, eight CCU rooms, four operating suites, and a fully functional ER.
Whether or not the center’s hospitalists will have a place in the modular health center is unclear. Maintaining the hospitalist program is expensive, explained Dr. Davis. Although she has been assured Sumter Regional’s hospitalist program will continue in some capacity, she is still unsure of the program’s status. Currently, Sumter Regional’s four TeamHealth hospitalists are without a hospital.
Eventually, Sumter Regional Hospital will be rebuilt. Shortly after the tornado destroyed the health center, its president and CEO, David Seagraves issued the following message to the community: “Sumter Regional Hospital is not closing. We are currently assessing the damage to our facility from the tornado, and we are also looking at temporary alternative sites to provide services. We will update our situation in the current days, but I repeat that we fully intend for Sumter Regional Hospital to be back better than ever as soon as humanly possible.”
But reconstructing the hospital will be costly, with damage estimated at more than $100 million. The center and its equipment are covered by a $90 million insurance policy, as well as $37 million in service interruption insurance—some of which is slated to pay the hospital’s 700 employees. The state Senate has authorized $11 million in emergency funding for the town, some of which may go toward re-establishing healthcare in the region. It will take far more than that to restore a fully functional hospital. Sumter Regional is seeking financial donations through its Web site (www.sumterregional.org) to help community members and to go toward rebuilding. Citizen’s Bank of Americus has donated $100,000, half to the hospital and half to aid the community. Wachovia is also active in the reconstruction effort.
Dr. Davis could not be more pleased with how Sumter Regional’s hospitalists responded to the disaster. “I am real proud of the doctors I work with,” she says. “They really stepped up to the plate—above and beyond the call of duty. No one complained. No one worried about getting paid. No one worried about their malpractice insurance. I am very proud.” TH
Roberta Newman is a frequent contributor to The Hospitalist.
On the evening of March 1, severe thunderstorms rumbled over southwestern Georgia, spawning a tornado that ripped through seven counties. Classified an EF 3 with winds ranging from approximately 136 to 165 miles per hour, the twister tore a path of destruction 37 miles long and as much as a mile wide, reaching its maximum strength and width as it reached Americus, the seat of Sumter county. Along the way, the Sumter tornado—part of a storm system that killed eight in a high school in Enterprise, Ala.—demolished or seriously damaged more than 200 homes and dozens of businesses in Americus and the surrounding area, causing two deaths and many more injuries.
Not only did it leave the local Winn-Dixie grocery store without its façade, but it also reduced a 1,600-foot-tall public television tower to a 150-foot stump, sheared the tops off trees, downed power lines, knocked out telephone service, and deposited a burning tractor in the middle of Highway 520.
More important, it destroyed Sumter Regional Hospital in Americus, a rural city of 17,000 residents. Thanks to Sumter Regional’s staff, including its four hospitalists, all 70 patients were evacuated from the 265,000-square-foot, 143-bed complex.
The Terror Begins
The tornado, which struck Americus between 9 and 9:30 p.m., did not arrive without warning—but the town did not sound its tornado siren. A firefighter dispatched to activate the warning was called back because it was too late to do any good. Sumter Regional’s staff was alerted a tornado might strike, and they had moved patients away from the windows.
Hospitalist Mukesh Kumar, MD, who had joined Sumter Regional two weeks before the tornado struck, rode the storm out in the hospitalists’ office—a small space on the same corridor with a number of patient rooms.
“[Dr. Kumar] was the poor guy on duty that night,” says Amanda Davis, MD, head of the hospitalist program, who was not in town that evening.
As soon as the storm passed, an emergency call went out via the local broadcast media, requesting that physicians and nurses report to the severely damaged hospital to aid in the evacuation and treat the injured.
Hospitalists Kathy Hudson, MD, and Rick Oster, MD, among others, rushed to help. Getting to the hospital wasn’t easy. The surrounding area had become a maze of downed trees and power lines. First responders who could use their cars had to park outside the devastated area and hike to the hospital in the dark. Others arrived on bicycles. Some could not make it because they were trapped in their driveways.
With the power out at the tornado-ravaged hospital, as it was in much of Americus, first responders helped those on duty carry many of the 70 patients down the stairs of the four-story complex. Outside, Dr. Hudson worked with the staff, suturing the injured. Sumter Regional Hospital was evacuated by about 1:30 the following morning.
Patients were loaded into the town’s four ambulances and others from around the region and transferred to facilities in southwest Georgia. The closest of these was Phoebe Putney Memorial Hospital in Albany, about 40 miles from Americus.
Taking Stock
Wind and water had compromised nearly every part of Sumter Regional, leaving only the chapel intact. Much of the roof was ripped off, nearly all the windows were blown out, and part of the complex collapsed. Damage was so severe the entire health center was rendered unsafe.
Not only did the storm leave Americus without its hospital, it also devastated virtually all the town’s private doctor offices, as well as the Sumter HealthPlex—a new, 8,000-square-foot, $3.1 million facility owned by the hospital. The HealthPlex provided outpatient imaging and laboratory services to the community.
In the days immediately following the tornado, the Middle Flint chapter of the American Red Cross—whose headquarters were also badly damaged—set up an emergency response center in the First Baptist Church of Americus that featured a makeshift emergency room. Dr. Davis and her staff of hospitalists, employed by TeamHealth’s Hospital Medicine Division, helped get the Red Cross center up and running. They also played a significant role in setting up a temporary urgent-care center in tents provided by the Federal Emergency Management Agency (FEMA), supplemented by two tents belonging to the Boy Scouts.
By Any Means Necessary
Although local physicians with their own practices were well represented among the first responders, they were (for the most part) not involved with staffing the tent hospital, according to Dr. Davis. Most of the work in the tents was done by the emergency department (ED) physicians and TeamHealth doctors, in conjunction with Sumter Regional’s four hospitalists.
As soon as the tents opened, the ED “regulars” started showing up in droves,” explains Dr. Davis. Because a significant number of private physicians’ offices in town were destroyed there were no other healthcare options for many residents.
“It’s a difficult situation,” says Dr. Davis. “There are people in the community without cars, for example, whose cars were destroyed. So they have no other way to get to another hospital. They were astonishingly grateful to have us there.”
As residents cleaned up more than a month after the disaster, the urgent-care facility in the tents still functions as Sumter Regional’s ED. Routine lab work and X-rays were provided, and staff also monitored patients on blood thinners and gave injections to cancer patients.
In a pinch, the tents have served as an impromptu obstetrics suite. One woman delivered her child there after being unable to travel to Phoebe Putney Memorial Hospital, which is providing obstetrical services to mothers who would have gone to Sumter Regional.
Care of the center’s cancer patients—a number of whom must take a bus to Albany for treatment—is now overseen by Phoebe Putney’s oncology department. Surgeons, too, have had to travel to other hospitals in order to operate. “It is a logistical nightmare,” says Dr. Davis.
While the urgent-care unit has been able to provide basic services, it has not been an easy task for the physicians and nurses who work there. Telephone lines have not been restored, making it virtually impossible to send faxes. Hospitalists and ED physicians staffing the tents have had to read their own films, essentially serving as their own radiologists. As Dr Davis notes, “You have to do the best with what you have.” Often, that is a bare minimum. Indeed, she once had to put in a central line to stabilize a patient. “It was pretty surreal treating (the patient) in a tent,” she says.
What’s Next?
FEMA Director David Paulison, who toured the devastated hospital with President Bush, says the immediate response was indicative of the “new FEMA.”
Bush declared Sumter County a disaster area the day after the tornado. Residents were urged to apply as quickly as possible for grants and low-cost loans to aid in recovery. Local, state, and federal officials opened a disaster recovery center March 5 in Americus, staffed by representatives of FEMA, the Georgia Emergency Management Agency (GEMA), and a number of other agencies. As of April 4, FEMA, GEMA, and the U.S. Small Business Association (SBA), had approved more than $6.58 million for disaster recovery.
A May 16 update by Sumter Regional on its Web site indicated progress in the construction of modular care facilities.
“By the end of May, there will be five modular buildings in the Mayo Street parking lot for use by physicians, each consisting of two single-wide modular buildings joined together,” the memo stated. “Currently, two of the five physician modular buildings are in place with both halves assembled, and three of the single units are on site ready for placement and assembly. The other three halves that will be used to construct the remaining three buildings are on the way. We are still seeking bids from contractors to do the plumbing, electrical and other utility work, and anticipate having the buildings functional by the end of May for physicians to begin seeing patients.”
On April 30, Sumter Regional opened its Sumter Regional East facility. Sumter East provides 24-hour urgent care, radiology services, clinical lab services, physical therapy, speech therapy, occupational therapy, and cardiopulmonary services, according to the hospital’s Web site.
An interim hospital is slated to open in mid-September, according to a May 10 statement by the hospital. The facility, to be built on the former site of the HealthPlex, is to include approximately 65 inpatient rooms, nine LDRP/obstetrics/nursery rooms, eight CCU rooms, four operating suites, and a fully functional ER.
Whether or not the center’s hospitalists will have a place in the modular health center is unclear. Maintaining the hospitalist program is expensive, explained Dr. Davis. Although she has been assured Sumter Regional’s hospitalist program will continue in some capacity, she is still unsure of the program’s status. Currently, Sumter Regional’s four TeamHealth hospitalists are without a hospital.
Eventually, Sumter Regional Hospital will be rebuilt. Shortly after the tornado destroyed the health center, its president and CEO, David Seagraves issued the following message to the community: “Sumter Regional Hospital is not closing. We are currently assessing the damage to our facility from the tornado, and we are also looking at temporary alternative sites to provide services. We will update our situation in the current days, but I repeat that we fully intend for Sumter Regional Hospital to be back better than ever as soon as humanly possible.”
But reconstructing the hospital will be costly, with damage estimated at more than $100 million. The center and its equipment are covered by a $90 million insurance policy, as well as $37 million in service interruption insurance—some of which is slated to pay the hospital’s 700 employees. The state Senate has authorized $11 million in emergency funding for the town, some of which may go toward re-establishing healthcare in the region. It will take far more than that to restore a fully functional hospital. Sumter Regional is seeking financial donations through its Web site (www.sumterregional.org) to help community members and to go toward rebuilding. Citizen’s Bank of Americus has donated $100,000, half to the hospital and half to aid the community. Wachovia is also active in the reconstruction effort.
Dr. Davis could not be more pleased with how Sumter Regional’s hospitalists responded to the disaster. “I am real proud of the doctors I work with,” she says. “They really stepped up to the plate—above and beyond the call of duty. No one complained. No one worried about getting paid. No one worried about their malpractice insurance. I am very proud.” TH
Roberta Newman is a frequent contributor to The Hospitalist.
On the evening of March 1, severe thunderstorms rumbled over southwestern Georgia, spawning a tornado that ripped through seven counties. Classified an EF 3 with winds ranging from approximately 136 to 165 miles per hour, the twister tore a path of destruction 37 miles long and as much as a mile wide, reaching its maximum strength and width as it reached Americus, the seat of Sumter county. Along the way, the Sumter tornado—part of a storm system that killed eight in a high school in Enterprise, Ala.—demolished or seriously damaged more than 200 homes and dozens of businesses in Americus and the surrounding area, causing two deaths and many more injuries.
Not only did it leave the local Winn-Dixie grocery store without its façade, but it also reduced a 1,600-foot-tall public television tower to a 150-foot stump, sheared the tops off trees, downed power lines, knocked out telephone service, and deposited a burning tractor in the middle of Highway 520.
More important, it destroyed Sumter Regional Hospital in Americus, a rural city of 17,000 residents. Thanks to Sumter Regional’s staff, including its four hospitalists, all 70 patients were evacuated from the 265,000-square-foot, 143-bed complex.
The Terror Begins
The tornado, which struck Americus between 9 and 9:30 p.m., did not arrive without warning—but the town did not sound its tornado siren. A firefighter dispatched to activate the warning was called back because it was too late to do any good. Sumter Regional’s staff was alerted a tornado might strike, and they had moved patients away from the windows.
Hospitalist Mukesh Kumar, MD, who had joined Sumter Regional two weeks before the tornado struck, rode the storm out in the hospitalists’ office—a small space on the same corridor with a number of patient rooms.
“[Dr. Kumar] was the poor guy on duty that night,” says Amanda Davis, MD, head of the hospitalist program, who was not in town that evening.
As soon as the storm passed, an emergency call went out via the local broadcast media, requesting that physicians and nurses report to the severely damaged hospital to aid in the evacuation and treat the injured.
Hospitalists Kathy Hudson, MD, and Rick Oster, MD, among others, rushed to help. Getting to the hospital wasn’t easy. The surrounding area had become a maze of downed trees and power lines. First responders who could use their cars had to park outside the devastated area and hike to the hospital in the dark. Others arrived on bicycles. Some could not make it because they were trapped in their driveways.
With the power out at the tornado-ravaged hospital, as it was in much of Americus, first responders helped those on duty carry many of the 70 patients down the stairs of the four-story complex. Outside, Dr. Hudson worked with the staff, suturing the injured. Sumter Regional Hospital was evacuated by about 1:30 the following morning.
Patients were loaded into the town’s four ambulances and others from around the region and transferred to facilities in southwest Georgia. The closest of these was Phoebe Putney Memorial Hospital in Albany, about 40 miles from Americus.
Taking Stock
Wind and water had compromised nearly every part of Sumter Regional, leaving only the chapel intact. Much of the roof was ripped off, nearly all the windows were blown out, and part of the complex collapsed. Damage was so severe the entire health center was rendered unsafe.
Not only did the storm leave Americus without its hospital, it also devastated virtually all the town’s private doctor offices, as well as the Sumter HealthPlex—a new, 8,000-square-foot, $3.1 million facility owned by the hospital. The HealthPlex provided outpatient imaging and laboratory services to the community.
In the days immediately following the tornado, the Middle Flint chapter of the American Red Cross—whose headquarters were also badly damaged—set up an emergency response center in the First Baptist Church of Americus that featured a makeshift emergency room. Dr. Davis and her staff of hospitalists, employed by TeamHealth’s Hospital Medicine Division, helped get the Red Cross center up and running. They also played a significant role in setting up a temporary urgent-care center in tents provided by the Federal Emergency Management Agency (FEMA), supplemented by two tents belonging to the Boy Scouts.
By Any Means Necessary
Although local physicians with their own practices were well represented among the first responders, they were (for the most part) not involved with staffing the tent hospital, according to Dr. Davis. Most of the work in the tents was done by the emergency department (ED) physicians and TeamHealth doctors, in conjunction with Sumter Regional’s four hospitalists.
As soon as the tents opened, the ED “regulars” started showing up in droves,” explains Dr. Davis. Because a significant number of private physicians’ offices in town were destroyed there were no other healthcare options for many residents.
“It’s a difficult situation,” says Dr. Davis. “There are people in the community without cars, for example, whose cars were destroyed. So they have no other way to get to another hospital. They were astonishingly grateful to have us there.”
As residents cleaned up more than a month after the disaster, the urgent-care facility in the tents still functions as Sumter Regional’s ED. Routine lab work and X-rays were provided, and staff also monitored patients on blood thinners and gave injections to cancer patients.
In a pinch, the tents have served as an impromptu obstetrics suite. One woman delivered her child there after being unable to travel to Phoebe Putney Memorial Hospital, which is providing obstetrical services to mothers who would have gone to Sumter Regional.
Care of the center’s cancer patients—a number of whom must take a bus to Albany for treatment—is now overseen by Phoebe Putney’s oncology department. Surgeons, too, have had to travel to other hospitals in order to operate. “It is a logistical nightmare,” says Dr. Davis.
While the urgent-care unit has been able to provide basic services, it has not been an easy task for the physicians and nurses who work there. Telephone lines have not been restored, making it virtually impossible to send faxes. Hospitalists and ED physicians staffing the tents have had to read their own films, essentially serving as their own radiologists. As Dr Davis notes, “You have to do the best with what you have.” Often, that is a bare minimum. Indeed, she once had to put in a central line to stabilize a patient. “It was pretty surreal treating (the patient) in a tent,” she says.
What’s Next?
FEMA Director David Paulison, who toured the devastated hospital with President Bush, says the immediate response was indicative of the “new FEMA.”
Bush declared Sumter County a disaster area the day after the tornado. Residents were urged to apply as quickly as possible for grants and low-cost loans to aid in recovery. Local, state, and federal officials opened a disaster recovery center March 5 in Americus, staffed by representatives of FEMA, the Georgia Emergency Management Agency (GEMA), and a number of other agencies. As of April 4, FEMA, GEMA, and the U.S. Small Business Association (SBA), had approved more than $6.58 million for disaster recovery.
A May 16 update by Sumter Regional on its Web site indicated progress in the construction of modular care facilities.
“By the end of May, there will be five modular buildings in the Mayo Street parking lot for use by physicians, each consisting of two single-wide modular buildings joined together,” the memo stated. “Currently, two of the five physician modular buildings are in place with both halves assembled, and three of the single units are on site ready for placement and assembly. The other three halves that will be used to construct the remaining three buildings are on the way. We are still seeking bids from contractors to do the plumbing, electrical and other utility work, and anticipate having the buildings functional by the end of May for physicians to begin seeing patients.”
On April 30, Sumter Regional opened its Sumter Regional East facility. Sumter East provides 24-hour urgent care, radiology services, clinical lab services, physical therapy, speech therapy, occupational therapy, and cardiopulmonary services, according to the hospital’s Web site.
An interim hospital is slated to open in mid-September, according to a May 10 statement by the hospital. The facility, to be built on the former site of the HealthPlex, is to include approximately 65 inpatient rooms, nine LDRP/obstetrics/nursery rooms, eight CCU rooms, four operating suites, and a fully functional ER.
Whether or not the center’s hospitalists will have a place in the modular health center is unclear. Maintaining the hospitalist program is expensive, explained Dr. Davis. Although she has been assured Sumter Regional’s hospitalist program will continue in some capacity, she is still unsure of the program’s status. Currently, Sumter Regional’s four TeamHealth hospitalists are without a hospital.
Eventually, Sumter Regional Hospital will be rebuilt. Shortly after the tornado destroyed the health center, its president and CEO, David Seagraves issued the following message to the community: “Sumter Regional Hospital is not closing. We are currently assessing the damage to our facility from the tornado, and we are also looking at temporary alternative sites to provide services. We will update our situation in the current days, but I repeat that we fully intend for Sumter Regional Hospital to be back better than ever as soon as humanly possible.”
But reconstructing the hospital will be costly, with damage estimated at more than $100 million. The center and its equipment are covered by a $90 million insurance policy, as well as $37 million in service interruption insurance—some of which is slated to pay the hospital’s 700 employees. The state Senate has authorized $11 million in emergency funding for the town, some of which may go toward re-establishing healthcare in the region. It will take far more than that to restore a fully functional hospital. Sumter Regional is seeking financial donations through its Web site (www.sumterregional.org) to help community members and to go toward rebuilding. Citizen’s Bank of Americus has donated $100,000, half to the hospital and half to aid the community. Wachovia is also active in the reconstruction effort.
Dr. Davis could not be more pleased with how Sumter Regional’s hospitalists responded to the disaster. “I am real proud of the doctors I work with,” she says. “They really stepped up to the plate—above and beyond the call of duty. No one complained. No one worried about getting paid. No one worried about their malpractice insurance. I am very proud.” TH
Roberta Newman is a frequent contributor to The Hospitalist.