User login
Blue Nevi: A Case Report and Review of the Literature
What's Eating You? Bees, Part 2: Venom Immunotherapy and Mastocytosis
Sun Sensitivity in 5 US Ethnoracial Groups
Want a bonus check? CMS has a program for you
Quality measures are reported on the CMS claim form just as any other service would be, except that no charge is billed for the reported measure. The time frame established for the reporting of these measures is July 1 through December 31 of this year. Although there are plans to continue the program in 2008, it is unclear whether funds will be available for a bonus in 2009, and the measures for 2008 will be different from those used in 2007.
To calculate the potential bonus amount when at least 3 measures are successfully reported, use your total Medicare income for the past 6 months. If you received $60,000 for treating Medicare patients from January 1 through May 31, for example, and Medicare income has been steady, expect a lump sum bonus of $900 in mid-2008.
How do I report an intervention?
Good news: You do not have to register to participate in PQRI; you need only report the selected quality measures each time you submit a claim for the patient service to which the quality measure applies. Criteria for reporting (and then receiving the bonus in mid-2008) for these quality measures are as follows:
- Select the quality measures that apply most often to your practice (see the TABLE)
- Enter the PQRI codes on block 24D of the CMS 1500 claim form with a $0.00 dollar amount; if your system does not allow this amount to be entered, change it to $0.01
- There must be a match between the acceptable CPT or ICD-9 code reported for the overall service with a CPT Category II or HCPCS “G” code designated as the quality measure, as listed in the Medicare specifications file (www.cms.hhs.gov/PQRI/15_MeasuresCodes.asp#TopOfPage)
- Apply any applicable allowed modifier that explains why the quality measure was not assessed:
- Measure title
- Description
- Instructions on reporting, including frequency, time frames, and applicability
- Numerator coding
- Definition of terms
- Coding instructions
The numerator part of the measure is represented by a CPT Category II code with or without a modifier. CPT code 1090F (presence or absence of urinary stress incontinence assessed) would be reported if the presence or absence of urinary incontinence was assessed, but a modifier 1P is placed in box 24E of the claim form if you have documented a medical reason why this was not assessed, or modifier 8P if it was not assessed but the reason was not documented.
TABLE
The Physician Quality Reporting Initiative: 10 measures may apply to ObGyn practice in 2007
| MEASURE | CONSTRAINTS AND COMMENTS | ||
|---|---|---|---|
| #20 Perioperative care: Timing of antibiotic prophylaxis—ordering physician |
| ||
| #21 Perioperative care: Selection of prophylactic antibiotic—first- or second-generation cephalosporin |
| ||
| #22 Perioperative care: Discontinuation of prophylactic antibiotic (non-cardiac procedures) |
| ||
| #23 Perioperative care: venous thromboembolism prophylaxis (when indicated in all patients) |
| ||
| #39 Screening or therapy for osteoporosis for women 65 years and older |
| ||
| #41 Osteoporosis: Pharmacotherapy |
| ||
| #42 Osteoporosis: Counseling for vitamin D and calcium intake, and exercise |
| ||
| #48 Assessment of presence or absence of urinary incontinence in women aged 65 years and older |
| ||
| #49 Characterization of urinary incontinence in women aged 65 years and older |
| ||
| #50 Plan of care for urinary incontinence in women aged 65 years and older |
| ||
Quality measures are reported on the CMS claim form just as any other service would be, except that no charge is billed for the reported measure. The time frame established for the reporting of these measures is July 1 through December 31 of this year. Although there are plans to continue the program in 2008, it is unclear whether funds will be available for a bonus in 2009, and the measures for 2008 will be different from those used in 2007.
To calculate the potential bonus amount when at least 3 measures are successfully reported, use your total Medicare income for the past 6 months. If you received $60,000 for treating Medicare patients from January 1 through May 31, for example, and Medicare income has been steady, expect a lump sum bonus of $900 in mid-2008.
How do I report an intervention?
Good news: You do not have to register to participate in PQRI; you need only report the selected quality measures each time you submit a claim for the patient service to which the quality measure applies. Criteria for reporting (and then receiving the bonus in mid-2008) for these quality measures are as follows:
- Select the quality measures that apply most often to your practice (see the TABLE)
- Enter the PQRI codes on block 24D of the CMS 1500 claim form with a $0.00 dollar amount; if your system does not allow this amount to be entered, change it to $0.01
- There must be a match between the acceptable CPT or ICD-9 code reported for the overall service with a CPT Category II or HCPCS “G” code designated as the quality measure, as listed in the Medicare specifications file (www.cms.hhs.gov/PQRI/15_MeasuresCodes.asp#TopOfPage)
- Apply any applicable allowed modifier that explains why the quality measure was not assessed:
- Measure title
- Description
- Instructions on reporting, including frequency, time frames, and applicability
- Numerator coding
- Definition of terms
- Coding instructions
The numerator part of the measure is represented by a CPT Category II code with or without a modifier. CPT code 1090F (presence or absence of urinary stress incontinence assessed) would be reported if the presence or absence of urinary incontinence was assessed, but a modifier 1P is placed in box 24E of the claim form if you have documented a medical reason why this was not assessed, or modifier 8P if it was not assessed but the reason was not documented.
TABLE
The Physician Quality Reporting Initiative: 10 measures may apply to ObGyn practice in 2007
| MEASURE | CONSTRAINTS AND COMMENTS | ||
|---|---|---|---|
| #20 Perioperative care: Timing of antibiotic prophylaxis—ordering physician |
| ||
| #21 Perioperative care: Selection of prophylactic antibiotic—first- or second-generation cephalosporin |
| ||
| #22 Perioperative care: Discontinuation of prophylactic antibiotic (non-cardiac procedures) |
| ||
| #23 Perioperative care: venous thromboembolism prophylaxis (when indicated in all patients) |
| ||
| #39 Screening or therapy for osteoporosis for women 65 years and older |
| ||
| #41 Osteoporosis: Pharmacotherapy |
| ||
| #42 Osteoporosis: Counseling for vitamin D and calcium intake, and exercise |
| ||
| #48 Assessment of presence or absence of urinary incontinence in women aged 65 years and older |
| ||
| #49 Characterization of urinary incontinence in women aged 65 years and older |
| ||
| #50 Plan of care for urinary incontinence in women aged 65 years and older |
| ||
Quality measures are reported on the CMS claim form just as any other service would be, except that no charge is billed for the reported measure. The time frame established for the reporting of these measures is July 1 through December 31 of this year. Although there are plans to continue the program in 2008, it is unclear whether funds will be available for a bonus in 2009, and the measures for 2008 will be different from those used in 2007.
To calculate the potential bonus amount when at least 3 measures are successfully reported, use your total Medicare income for the past 6 months. If you received $60,000 for treating Medicare patients from January 1 through May 31, for example, and Medicare income has been steady, expect a lump sum bonus of $900 in mid-2008.
How do I report an intervention?
Good news: You do not have to register to participate in PQRI; you need only report the selected quality measures each time you submit a claim for the patient service to which the quality measure applies. Criteria for reporting (and then receiving the bonus in mid-2008) for these quality measures are as follows:
- Select the quality measures that apply most often to your practice (see the TABLE)
- Enter the PQRI codes on block 24D of the CMS 1500 claim form with a $0.00 dollar amount; if your system does not allow this amount to be entered, change it to $0.01
- There must be a match between the acceptable CPT or ICD-9 code reported for the overall service with a CPT Category II or HCPCS “G” code designated as the quality measure, as listed in the Medicare specifications file (www.cms.hhs.gov/PQRI/15_MeasuresCodes.asp#TopOfPage)
- Apply any applicable allowed modifier that explains why the quality measure was not assessed:
- Measure title
- Description
- Instructions on reporting, including frequency, time frames, and applicability
- Numerator coding
- Definition of terms
- Coding instructions
The numerator part of the measure is represented by a CPT Category II code with or without a modifier. CPT code 1090F (presence or absence of urinary stress incontinence assessed) would be reported if the presence or absence of urinary incontinence was assessed, but a modifier 1P is placed in box 24E of the claim form if you have documented a medical reason why this was not assessed, or modifier 8P if it was not assessed but the reason was not documented.
TABLE
The Physician Quality Reporting Initiative: 10 measures may apply to ObGyn practice in 2007
| MEASURE | CONSTRAINTS AND COMMENTS | ||
|---|---|---|---|
| #20 Perioperative care: Timing of antibiotic prophylaxis—ordering physician |
| ||
| #21 Perioperative care: Selection of prophylactic antibiotic—first- or second-generation cephalosporin |
| ||
| #22 Perioperative care: Discontinuation of prophylactic antibiotic (non-cardiac procedures) |
| ||
| #23 Perioperative care: venous thromboembolism prophylaxis (when indicated in all patients) |
| ||
| #39 Screening or therapy for osteoporosis for women 65 years and older |
| ||
| #41 Osteoporosis: Pharmacotherapy |
| ||
| #42 Osteoporosis: Counseling for vitamin D and calcium intake, and exercise |
| ||
| #48 Assessment of presence or absence of urinary incontinence in women aged 65 years and older |
| ||
| #49 Characterization of urinary incontinence in women aged 65 years and older |
| ||
| #50 Plan of care for urinary incontinence in women aged 65 years and older |
| ||
Do our talks with patients meet their expectations?
- Patients want an attentive, friendly, frank and empathic doctor who listens well.
- To enhance quality of health care, consider asking patients at the end of a visit whether their communication preferences were met.
One physician has written that good patient-doctor communication, like jazz, calls for improvisation.1 We agree. And improvise we must when patients’ expectations for how we will communicate with them vary between visits and individuals.
For example, those who are ill may prefer that their doctor communicate with them in a way that is less important to those who are healthy. Patients with biomedical problems may have different preferences than persons with psychosocial problems. And older individuals may have communication desires that differ from those who are younger.2-4
Do patients want cure or care, or both?
Depending on the reason for a visit—eg, biomedical or psychosocial—patient preferences may fit either the cure or the care dimension.
Cure dimension. On one hand, patients expect their doctor to be task-oriented and to find a cure for what ails them. They want an explanation of what is wrong and advice about possible treatments, and they want the doctor to do whatever is needed to get answers.5
Care dimension. On the other hand, patients may feel anxious and want reassurance. They expect the doctor to listen to their story and encourage them to disclose all health problems, concerns, and worries. They also expect friendliness and empathy. They want to be taken seriously. The extent to which the doctor shows this affect-oriented (and patient-centered) behavior will determine how fulfilled patients feel in their preference for care.6,7
Why does it matter? Good communication serves a patient’s need to understand and to be understood.6,8,9 And communication aimed at matching patient preferences enhances satisfaction with care, compliance with medical instructions, and health status.10-13
How well do we assess patients’ communication preferences?
Patient-centered behavior is a necessary tool for discovering and fulfilling patients’ task-oriented (cure dimension) and affect-oriented (care dimension) communication preferences.14-17 It’s important to know how well primary-care physicians interpret patients’ preferences for clinical encounters and if they respond in a manner that satisfies those expectations.
Reassuringly, patients indicate on surveys that their physicians do a fairly good job of interpreting their communication preferences and acting accordingly.18-20 They also report that their desires and expectations from consultations are increasingly met.
There is always the worry, though, that physicians in certain positions—eg, non-gatekeeper roles or positions involving only part-time clinical responsibilities—would be challenged to assess patient preferences as accurately as others.21
The aim of our study
While it’s encouraging that physicians by and large understand their patients and communicate with them meaningfully, we wondered whether communication could improve further. Our purpose in this study was to gain detailed insight into patients’ preferences in physician communication and, through patients’ subjective perspectives and observed real practice consultations, learn how well physicians communicate according to those preferences.
Methods
Design
We derived physician data from the Second Dutch National Survey of General Practice (2001). This study was carried out in practices representative of Dutch general practice.22 We asked patients for permission to videotape consultations with the general practitioner (GP), and asked them to sign a consent form. Collected data were kept private as per regulations.
We videotaped consultations of 142 GPs (76.1% male) and 2784 patients (41.2% male). The number of patients cared for by each GP ranged between 17 and 21 (mean=19.6). Each patient was videotaped just once. We rated roughly 15 patient-consultations per GP (13–15, mean=14.8), excluding the first 3 to correct for possible bias because of the video camera. Before and immediately after the consultation, patients 18 years of age and older answered a questionnaire. We used data from 1787 patient consultations.
Patients rate their communication preferences
The patient questionnaire covered demographic characteristics (gender, age, education); health problems (psychosocial or not [ICPC-coded]);23 overall health during the past 2 weeks (1=excellent, 2=very good, 3=good, 4=fair, 5=poor); and depressive feelings during the past 2 weeks (1=not at all, 2=slightly, 3=moderately, 4=quite a bit, 5=extremely) (COOP-WONCA charts24).
We defined communication preferences as “the extent of importance patients attach to communication aspects.”25 Patients’ preferences and the actual performance by the GP were measured using the conceptual framework of the QUOTE scale (quality of care through the patient’s eyes).5,25
Before consultation, patients recorded how important they considered different aspects of communication for the coming visit (1=not important, 2=rather important, 3=important, 4=utmost important). Following consultation, they rated the GP’s performance in meeting their expectations for these aspects (1=not, 2=really not, 3=really yes, 4=yes).
Factor analysis of both the pre- and post-visit lists of questions on preference and performance revealed 2 relevant subscales: an affect-oriented scale of 7 communication aspects and a task-oriented scale of 6 communication aspects (Cronbach’s alpha between 0.74 and 0.89).
We also used communication aspects from the original 4-point scale to present 4 new categories that compared and contrasted preferences and relevance. These categories included: important and performed; important and not performed; not important and performed; not important and not performed. In the multilevel analysis, we included the 2 subscales using the original 4-point scale.
Socio-demographic and practice variables were derived from the GP questionnaires in the Second Dutch National Survey of General Practice (2001).
Video observations
Nine observers measured verbal behavior during the videotaped visits using the Roter Method of Interaction Process Analysis (RIAS26), a well-documented, widely used system in the US and Netherlands. This observation system distinguishes both affect-oriented (socio-emotional) and task-oriented (instrumental) verbal behavior of doctors and patients, reflecting the care and cure dimensions, respectively. The RIAS categories are mutually exclusive and exhaustive.
Affect-oriented communication consists of personal remarks, agreements, concerns, reassurances, paraphrases, and disagreements.
Task-oriented talk includes asking questions, giving information, and (only GPs) counseling about medical/therapeutic and psychosocial, social context and lifestyle issues, and process-oriented talk (instructions, asking for understanding).
After finishing the RIAS-coding, we calculated the total numbers of affect-oriented and task-oriented verbal behaviors separately for GPs and patients.
The relevance and performance items and the RIAS-categories all measured the affect-oriented and task-oriented aspects.
We used the Noldus Observer-Video-Pro computer program for the observation study,27 including measurement of consultation length. The interobserver reliabilities were good to excellent: between r=0.80 to r=0.95 per category, except for personal remarks (0.72).
Patient-centeredness measured in 3 ways
The observers, using a 5-point scale, also rated the extent to which GPs communicated in a patient-centered way in 3 areas: patient’s involvement in the problem-defining process; patient’s involvement in the decision-making process; and doctor’s overall responsiveness to the patient.
Based on ratings in these 3 areas, we determined an overall magnitude of patient-centeredness (Cronbach’s alpha=0.75). Observers and the responsible researcher met weekly to validate the quality of rating. The same was done for the RIAS coding.
Controlling variables
For GPs, controlling variables were gender, age, and number of full-time equivalents (FTEs) working. For patients, GP and patient gender were included in the variable “gender-dyad”—male GPs/male patients, male GPs/female patients, female GPs/male patients, female GPs/female patients. Other patient variables were age; education (low=no/primary school, middle=secondary school, high=higher vocational training/university); health problems: somatic or psychosocial (ICPC chapters); overall physical health and mental health during the past 2 weeks; and consultation length.
Data analysis
We used descriptive and multilevel analyses. The intra-class correlations of the affect-oriented and task-oriented communication and patient-centeredness were significant (between .05 and 0.23), which made it clear that consultations of the same GP did indeed exhibit a greater degree of similarity than the consultations of different GPs. Therefore, multilevel analyses were necessary to account for the clustering of patients with the same GP.28 We applied a significance level of ≤0.05 (2-sided).
Results
Response rate
The overall patient response rate was 88%. Analysis of non-responders’ gender, age, and type of insurance showed no bias resulting from patients’ refusal.
GP response rate was 72.8%. Respondents were representative of all Dutch GPs with respect to gender, age, working hours, practice experience (mean=15.6 years, SD=8.3, range=1–32), and location (58% in an urban area). More GPs worked in a partnership or group practice than in a solo practice. We analyzed the influence of the practice type on doctor-patient communication and deemed it insignificant.
Study population
GP and patient characteristics appear in TABLE 1. Among patients, 22% had little education, 62% had an average education, and 16% had higher education. Nearly 10% had a psychosocial problem. GP-patient gender dyads were as follows: 32.1% male GP/male patient; 45.3% male GP/female patient; 6.9% female GP/male patient; 15.8% female GP/female patient.
TABLE 1
General practitioner and patient characteristics (N GPs=142, N patients=1787)
| MEAN | SD | RANGE | |
|---|---|---|---|
| GP characteristics | |||
| Age (yrs) | 46.9 | 6.2 | 32–62 |
| Full-time equivalents | 0.8 | 0.2 | 0.2–1 |
| Patient characteristics | |||
| Age (yrs) | 49.5 | 17.4 | 18–95 |
| Psychosocial problem (1=yes) | 9.8% | — | — |
| Overall health (1=excellent, 5=poor) | 3.2 | 1.1 | 1–5 |
| Depressive feelings (1=not at all, 5=extremely) | 2.2 | 1.2 | 1–5 |
| Consultation length (min) | 10.1 | 4.8 | 1.3–33.0 |
| Patients’ preferences | |||
| Affect-oriented preference (1=not, 4=utmost important) | 3.2 | 0.5 | 1–4 |
| Task-oriented preference (1=not, 4=utmost important) | 3.1 | 0.6 | 1–4 |
Preference and performance of communication aspects
GPs good with affect-oriented communication aspects. Patients considered 6 of 7 affect-oriented communication aspects as very important (87%–96%, TABLE 2). The item “Doctor was empathic to me” was less important (61%) than items like “Doctor listened well to me” (96%) and “Doctor took enough time for me” (93%). We noted only a few discrepancies between preference and performance of the GPs’ affect-oriented behavior. If patients said beforehand that a communication aspect was important, the doctors nearly always performed that aspect. For instance, 87% wanted enough attention from the doctor and received it, while 99% of all patients received GP’s attention, whether it was important to them or not.
GPs less successful with task-oriented communication aspects. Many patients wanted information, explanations, advice, and help with their problems (85%–94%, TABLE 2). Knowing the diagnosis was less important (77%) than, say, receiving advice on what to do and having details of treatment explained.
GPs also performed most of the task-oriented aspects, if patients considered these aspects important.
Subjectively, preferences for GP task-oriented behavior and perceived performance often went together, though more discrepancies were visible than with affect-oriented behavior. One fifth of patients said their problems were not helped, though they had said this was important. Similarly, GPs did not give a diagnosis to nearly 15% of patients who considered it important.
TABLE 2
Care vs cure-centered communication: Physicians fared better on the care side (N=1787)
| AFFECT-ORIENTED ASPECTS (CARE DIMENSION) | PERFORMED | NOT PERFORMED | TOTAL* | |||
|---|---|---|---|---|---|---|
| N | % | N % | % | N | % | |
| Doctor gave me enough attention | ||||||
| Important | 1304 | 87.5 | 9 | 0.6 | 1313 | 88.1 |
| Not important | 174 | 11.7 | 4 | 0.3 | 178 | 11.9 |
| Doctor listened well to me | ||||||
| Important | 1456 | 95.3 | 10 | 0.7 | 1466 | 95.9 |
| Not important | 61 | 4.0 | 1 | 0.1 | 62 | 4.1 |
| Doctor took enough time for me | ||||||
| Important | 1412 | 92.3 | 11 | 0.7 | 1423 | 93.1 |
| Not important | 105 | 6.9 | 1 | 0.1 | 106 | 6.9 |
| Doctor was friendly | ||||||
| Important | 1331 | 87.2 | 3 | 0.2 | 1334 | 87.4 |
| Not important | 193 | 12.6 | 0 | 0.0 | 193 | 12.6 |
| Doctor was frank to me | ||||||
| Important | 1451 | 95.5 | 5 | 0.3 | 1456 | 95.8 |
| Not important | 63 | 4.15 | 0 | 0.0 | 63 | 4.2 |
| Doctor took my problem seriously | ||||||
| Important | 1455 | 95.8 | 7 | 0.5 | 1462 | 96.3 |
| Not important | 55 | 3.6 | 1 | 0.1 | 56 | 3.7 |
| Doctor was empathic to me | ||||||
| Important | 846 | 58.4 | 36 | 2.5 | 882 | 60.9 |
| Not important | 492 | 34.0 | 74 | 5.1 | 566 | 19.1 |
| TASK-ORIENTED ASPECTS (CURE DIMENSION) | ||||||
| Doctor diagnosed what’s wrong | ||||||
| Important | 921 | 62.8 | 209 | 14.2 | 1130 | 77.0 |
| Not important | 197 | 13.4 | 140 | 9.5 | 337 | 23.0 |
| Doctor explained well what’s wrong | ||||||
| Important | 1166 | 78.3 | 101 | 6.8 | 1267 | 85.0 |
| Not important | 175 | 11.7 | 48 | 3.2 | 223 | 15.0 |
| Doctor informed well on treatment | ||||||
| Important | 1304 | 86.6 | 109 | 7.2 | 1413 | 93.9 |
| Not important | 75 | 5.0 | 17 | 1.1 | 92 | 6.1 |
| Doctor gave advice on what to do | ||||||
| Important | 1294 | 85.9 | 121 | 8.0 | 1415 | 94.0 |
| Not important | 75 | 5.0 | 16 | 1.1 | 91 | 6.0 |
| Doctor helped me with my problem | ||||||
| Important | 1031 | 70.0 | 121 | 18.9 | 1152 | 88.9 |
| Not important | 94 | 6.4 | 16 | 4.7 | 110 | 11.1 |
| Doctor examined me | ||||||
| Important | 902 | 59.9 | 132 | 8.8 | 1034 | 62.8 |
| Not important | 228 | 15.1 | 244 | 16.2 | 472 | 27.2 |
| * Totals do not always add up to 1787 because of missing data. | ||||||
GP communication varies by doctor gender, patient characteristics
GPs engaged less in affect-oriented than in task-oriented communication (48.6 and 70.0 utterances on average, respectively, P≤.001).
The more patients regarded affect-oriented talk by GPs as important, the more the GPs actually showed affective and patient-centered behavior (TABLE 3). Preferences for task-oriented behavior (question-asking, information-giving, and counseling) were mirrored in their doctors’ talk.
When taking into account other GP and patient characteristics, female doctors were more often affect-oriented as well as task-oriented when communicating with patients than were male doctors, especially with female patients. In consultations with older patients and those in poor health, the doctors were more affective than in consultations with younger and healthy patients.
TABLE 3
On observation, physician communication corresponded to patient preferences (N GPs=142, N patients=1787)
| REGRESSION COEFFICIENTS | |||
|---|---|---|---|
| AFFECT-ORIENTED TALK GPs | TASK-ORIENTED TALK GPs | PATIENT-CENTEREDNESS | |
| GP characteristics | |||
| Age (yrs) | –0.20 | –0.37* | –0.01* |
| Full-time equivalents | –12.13* | 2.45 | 0.03 |
| Patient characteristics | |||
| Gender-dyad: | |||
| - Male/female | –0.89c | 0.17d | 0.01 |
| - Female/male | 9.40a,b,d | 6.24a,d | 0.10 |
| - Female/female | 5.73a,c | 6.85a,b,c | 0.02 |
| Age (yrs) | 0.09* | –0.15 | –0.00* |
| Education (1=low, 2=middle, 3=high) | –0.70 | 0.15 | 0.05 |
| Psychosocial problems (1=yes) | 7.93* | –4.62* | 0.13* |
| Overall health (1=excellent, 5=poor) | 1.13* | 0.96 | –0.01 |
| Depressive feelings (1=not at all, 5=extremely) | 0.78 | –0.72 | 0.01 |
| Consultation length (min) | 4.03* | 4.30* | 0.04* |
| Patients’ preferences | |||
| Affect-oriented preference (1=not, 4=utmost important) | 2.81* | –1.94 | 0.16* |
| Task-oriented preference (1=not, 4=utmost important) | –4.23 * | 3.62* | –0.15* |
| * P<.05 | |||
| a. Score differs significantly from score of male GP/male patient dyad (reference group). | |||
| b. Score differs significantly from score of male GP/female patient dyad. | |||
| c. Score differs significantly from score of female GP/male patient dyad. | |||
| d. Score differs significantly from score of female GP/female patient dyad. | |||
Discussion
Our study suggests most patients receive from their GPs the kind of communication they prefer in a consultation. In general, patients consider both affect- and task-oriented communication aspects important, and believe they are often performed. Our findings agree with most of the literature.5,14,20 Furthermore, patients’ preferences are for the greater part reflected in the GPs’ observed communication during the visit, which agrees with one earlier study18 but not with others.5,20
Patient preference for an affective doctor is very often met. GPs are generally considered attentive, friendly, frank, empathic, and good listeners. Patients seem satisfied in this respect. However, the task-oriented communication of the GPs is sometimes less satisfying. Contrary to patient preference, for example, GPs are not always able to make a diagnosis.
Observed physician behavior: patients usually get what they want. Looking at the relationship between preferences and actual GP communication, it appears that the more patients prefer an affective or caring doctor, the more they are likely to get an empathic, concerned, interested, and patient-centered doctor, especially when psychosocial problems are expressed. An affective GP was patient centered, involving patients in problem definition and decision making. This relationship between affective behavior and patient-centeredness was also found in earlier studies.22,29 However, Swenson found that not all patients wanted the doctor to exhibit a patient-centered approach.30
Likewise, the more patients prefer a task-oriented doctor, the better the chance they will have a doctor who explains things well, and who gives information and advice to their satisfaction. However, task-oriented doctors are usually less affective and less patient-centered when talking with patients. In view of the postulate that a doctor has to be curing as well as caring,6 doctors would be wise to give attention to both aspects.
GPs do improvise while communicating with patients. The study shows that GPs and patients working together can create the type of encounter both want. GPs are able to change their behavior in response to real-time cues they believe patients are giving in an encounter.
Physician gender often makes a difference. Our findings suggest that female doctors are more affective and task-oriented when talking with their patients than are male doctors, especially with female patients. In view of the steady increase of female doctors in general practice, this combined communication style may become more common in the future.
Psychosocial complaints prompt affective communication. Patients with a psychosocial problem are more likely to encounter an affective doctor than those with a biomedical problem. The growing number of psychosocial problems in the population may lead to a more affective communication.
Eventually the demand and the supply of affective communication may coincide. However, it is a challenge for every doctor to keep his or her mind open to both biomedical (task-oriented) and psychosocial (affective-oriented) information.31
Study caveats. Because we used scale scores for affect- and task-oriented preferences instead of the separate item scores for patient preferences, the reflection of preferences for GP communicative behavior might be somewhat overestimated. Likewise, we used total observation scores for affect- and task-oriented talk instead of the separate RIAS categories. More detailed measures of such communication aspects as empathy might give better insight into patient preferences.
Final thoughts on personal application. Primary care physicians would do well to take notice of patients’ preferences for communication. GPs in our study were often able to grasp what patients considered important to talk about, and there seemed to be only modest mismatches between patient expectations and physician behavior. To increase the quality of health care, consider asking patients at the end of a visit whether their preferences were met.
Acknowledgments
We acknowledge the participating general practitioners and patients, the observers of the videotaped consultations, and the Ministry of Health, Welfare and Sports for funding (for the greater part) the research project.
Correspondence
A. van den Brink-Muinen, PhD, NIVEL, PO Box 1568, 3500 BN Utrecht, The Netherlands; a.vandenbrink@nivel.nl
1. Haidet P. Jazz and the ‘Art’ of Medicine: Improvisation in the Medical Encounter. Ann Fam Med 2007;5:164-169.
2. Jung HP, Baerveldt C, Olesen F, Grol R, Wensing M. Patient characteristics as predictors of primary health care p: a systematic literature analysis. Health Expect 2003;6:160-181.
3. Bartholomew L, Schneiderman LJ. Attitudes of patients towards family care in a family practice group. J Fam Pract 1982;15:477-481.
4. Wetzels R, Geest TA, Wensing M, Lopes Ferreira P, Grol R, Baker R. GPs’ views on involvement of older patients: a European qualitative study. Pat Educ Couns 2004;53:183-188.
5. Brink-Muinen A, Verhaak PFM, Bensing JM, et al. Doctor-patient communication in different European health care systems: relevance and performance from the patients’ perspective. Pat Educ Counsel 2000;39:115-127.
6. Bensing JM, Dronkers J. Instrumental and affective aspects of physician behaviour. Med Care 1992;30:283-
7. Little P, Everitt H, Williamson I, et al. observational study of effect of patient centeredness and positive approach on outcomes of general practice. BMJ 2001;323:908-911.
8. Roter DL, Hall JA. Doctors Talking with Patients/Patients Talking with Doctors: Improving Communication in Medical Visits Westport/London: Auburn House; 1992.
9. Roter DL, Stewart M, Putnam SM, Lipkin M, Stiles W, Inui TS. Communication patterns of primary care physicians. JAMA 1997;277:350-356.
10. Sixma H, Spreeuwenberg P, Pasch M van der. Patient satisfaction with the general practitioner: a two-level analysis. Med Care 1998;2:212-229.
11. Dulmen AM van, Bensing JM. The Effect of Context in Health Care: A Programming Study The Hague: RGO; 2001.
12. Wensing M, Baker R. Patient involvement in general practice care: a pragmatic framework. Eur J Gen Pract 2003;9:62-65.
13. Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract 2000;49:796-804.
14. Bensing JM, Langewitz W. Die ärztliche Konsultation. In: Uexkull: Psychosomatische Medizin. 2002:415-424.
15. Brown J, Stewart M, Ryan B. Assessing communication between patients and physicians: the measure of patient-centred communication (MPCC). London, Ontario, Canada: Centre for Studies in Family Medicine, 2001.
16. Sullivan M. The new subjective medicine: taking the patients’ view on health care and health. Soc Sci Med 2003;56:1995-1604.
17. Mead N, Bower P. Patient-centeredness: a conceptual framework and review of the empirical literature. Soc Sci Med 2000;51:1087-1110.
18. Jung HP, Wensing M, Grol R. What makes a good general practitioner: do patients and doctors have different views? Br J Gen Pract 1997;47:805—809.
19. Wensing M, Jung HP, Mainz J, Olesen F, Grol R. A systematic review of the literature in patient priorities for general practice care. Part 1: description of the research domain. Soc Sci Med 1998;47:1573-1588.
20. Thorsen H, Witt K, Hollnagel H, Malterud K. The purpose of the general practice consultation from the patients’ perspective—theoretical aspects. Fam Pract 2001;18:638-643.
21. Brink-Muinen A, Verhaak PFM, Bensing JM, et al. Communication in general practice: differences between European countries. Fam Pract 2003;20:478-485.
22. Schellevis FG, Westert GP, Bakker DH de, Groenewegen PP, Zee J van der, Bensing JM. De tweede Nationale studie naar ziekten en verrichtingen in de huisartsenpraktijk: aanleiding en methoden [Second Dutch National Survey of general practice: background and methods]. H&W 2003;46:7-12.
23. Lamberts H, Wood M (eds). International Classification of Primary Care Oxford: Oxford University Press; 1987.
24. Weel C van, König-Zahn C, Touw-Otten FWMM, Duijn NP van, Meyboom-de Jong B. Measuring Functional Health Status with the COOP/WONCA Charts. A Manual WoNCA, ERGHo, NCH, university of Groningen, Netherlands; 1995.
25. Sixma HJ, Kerssens JJ, Campen C van, Peters L. Quality of care from the patients’ perspective: from theoretical concept to a new measurement instrument. Health Expect 1998;1:82-95.
26. Roter DL. The Roter Method of Interaction Process Analysis Baltimore, Md: Johns Hopkins University; 2001.
27. Noldus LP, Trienes RJ, Hendriksen AH, Janden H, Jansen RG. The observer video-Pro: new software for the collection, management and presentation of time-structured data from videotapes and digital media films. Behavioral Research Methods, Instruments & Computers 2000;32:197-206.
28. Leyland AH, Groenewegen PP. Multilevel modelling and public health policy. Scand J Public Health 2003;31:267-274
29. Jung HP, Horne F van, Wensing M, Hearnshaw H, Grol R. Which aspects of general practitioners’ behaviour determine patients’ evaluations of care? Soc Sci Med 1998;47:1077-1087.
30. Swenson SL, Buell S, Zettler P, White M, Ruston DC, Lo B. Patient-centred communication: do patients really prefer it? J Gen Intern Med 2004;19:1069-1079.
31. Epstein RM, Campbell TL, Cohen-Cole SA, MaWhinney IR, Smilkstein G. Perspectives on patient-doctor communication. J Fam Pract 1993;37:377-388.
- Patients want an attentive, friendly, frank and empathic doctor who listens well.
- To enhance quality of health care, consider asking patients at the end of a visit whether their communication preferences were met.
One physician has written that good patient-doctor communication, like jazz, calls for improvisation.1 We agree. And improvise we must when patients’ expectations for how we will communicate with them vary between visits and individuals.
For example, those who are ill may prefer that their doctor communicate with them in a way that is less important to those who are healthy. Patients with biomedical problems may have different preferences than persons with psychosocial problems. And older individuals may have communication desires that differ from those who are younger.2-4
Do patients want cure or care, or both?
Depending on the reason for a visit—eg, biomedical or psychosocial—patient preferences may fit either the cure or the care dimension.
Cure dimension. On one hand, patients expect their doctor to be task-oriented and to find a cure for what ails them. They want an explanation of what is wrong and advice about possible treatments, and they want the doctor to do whatever is needed to get answers.5
Care dimension. On the other hand, patients may feel anxious and want reassurance. They expect the doctor to listen to their story and encourage them to disclose all health problems, concerns, and worries. They also expect friendliness and empathy. They want to be taken seriously. The extent to which the doctor shows this affect-oriented (and patient-centered) behavior will determine how fulfilled patients feel in their preference for care.6,7
Why does it matter? Good communication serves a patient’s need to understand and to be understood.6,8,9 And communication aimed at matching patient preferences enhances satisfaction with care, compliance with medical instructions, and health status.10-13
How well do we assess patients’ communication preferences?
Patient-centered behavior is a necessary tool for discovering and fulfilling patients’ task-oriented (cure dimension) and affect-oriented (care dimension) communication preferences.14-17 It’s important to know how well primary-care physicians interpret patients’ preferences for clinical encounters and if they respond in a manner that satisfies those expectations.
Reassuringly, patients indicate on surveys that their physicians do a fairly good job of interpreting their communication preferences and acting accordingly.18-20 They also report that their desires and expectations from consultations are increasingly met.
There is always the worry, though, that physicians in certain positions—eg, non-gatekeeper roles or positions involving only part-time clinical responsibilities—would be challenged to assess patient preferences as accurately as others.21
The aim of our study
While it’s encouraging that physicians by and large understand their patients and communicate with them meaningfully, we wondered whether communication could improve further. Our purpose in this study was to gain detailed insight into patients’ preferences in physician communication and, through patients’ subjective perspectives and observed real practice consultations, learn how well physicians communicate according to those preferences.
Methods
Design
We derived physician data from the Second Dutch National Survey of General Practice (2001). This study was carried out in practices representative of Dutch general practice.22 We asked patients for permission to videotape consultations with the general practitioner (GP), and asked them to sign a consent form. Collected data were kept private as per regulations.
We videotaped consultations of 142 GPs (76.1% male) and 2784 patients (41.2% male). The number of patients cared for by each GP ranged between 17 and 21 (mean=19.6). Each patient was videotaped just once. We rated roughly 15 patient-consultations per GP (13–15, mean=14.8), excluding the first 3 to correct for possible bias because of the video camera. Before and immediately after the consultation, patients 18 years of age and older answered a questionnaire. We used data from 1787 patient consultations.
Patients rate their communication preferences
The patient questionnaire covered demographic characteristics (gender, age, education); health problems (psychosocial or not [ICPC-coded]);23 overall health during the past 2 weeks (1=excellent, 2=very good, 3=good, 4=fair, 5=poor); and depressive feelings during the past 2 weeks (1=not at all, 2=slightly, 3=moderately, 4=quite a bit, 5=extremely) (COOP-WONCA charts24).
We defined communication preferences as “the extent of importance patients attach to communication aspects.”25 Patients’ preferences and the actual performance by the GP were measured using the conceptual framework of the QUOTE scale (quality of care through the patient’s eyes).5,25
Before consultation, patients recorded how important they considered different aspects of communication for the coming visit (1=not important, 2=rather important, 3=important, 4=utmost important). Following consultation, they rated the GP’s performance in meeting their expectations for these aspects (1=not, 2=really not, 3=really yes, 4=yes).
Factor analysis of both the pre- and post-visit lists of questions on preference and performance revealed 2 relevant subscales: an affect-oriented scale of 7 communication aspects and a task-oriented scale of 6 communication aspects (Cronbach’s alpha between 0.74 and 0.89).
We also used communication aspects from the original 4-point scale to present 4 new categories that compared and contrasted preferences and relevance. These categories included: important and performed; important and not performed; not important and performed; not important and not performed. In the multilevel analysis, we included the 2 subscales using the original 4-point scale.
Socio-demographic and practice variables were derived from the GP questionnaires in the Second Dutch National Survey of General Practice (2001).
Video observations
Nine observers measured verbal behavior during the videotaped visits using the Roter Method of Interaction Process Analysis (RIAS26), a well-documented, widely used system in the US and Netherlands. This observation system distinguishes both affect-oriented (socio-emotional) and task-oriented (instrumental) verbal behavior of doctors and patients, reflecting the care and cure dimensions, respectively. The RIAS categories are mutually exclusive and exhaustive.
Affect-oriented communication consists of personal remarks, agreements, concerns, reassurances, paraphrases, and disagreements.
Task-oriented talk includes asking questions, giving information, and (only GPs) counseling about medical/therapeutic and psychosocial, social context and lifestyle issues, and process-oriented talk (instructions, asking for understanding).
After finishing the RIAS-coding, we calculated the total numbers of affect-oriented and task-oriented verbal behaviors separately for GPs and patients.
The relevance and performance items and the RIAS-categories all measured the affect-oriented and task-oriented aspects.
We used the Noldus Observer-Video-Pro computer program for the observation study,27 including measurement of consultation length. The interobserver reliabilities were good to excellent: between r=0.80 to r=0.95 per category, except for personal remarks (0.72).
Patient-centeredness measured in 3 ways
The observers, using a 5-point scale, also rated the extent to which GPs communicated in a patient-centered way in 3 areas: patient’s involvement in the problem-defining process; patient’s involvement in the decision-making process; and doctor’s overall responsiveness to the patient.
Based on ratings in these 3 areas, we determined an overall magnitude of patient-centeredness (Cronbach’s alpha=0.75). Observers and the responsible researcher met weekly to validate the quality of rating. The same was done for the RIAS coding.
Controlling variables
For GPs, controlling variables were gender, age, and number of full-time equivalents (FTEs) working. For patients, GP and patient gender were included in the variable “gender-dyad”—male GPs/male patients, male GPs/female patients, female GPs/male patients, female GPs/female patients. Other patient variables were age; education (low=no/primary school, middle=secondary school, high=higher vocational training/university); health problems: somatic or psychosocial (ICPC chapters); overall physical health and mental health during the past 2 weeks; and consultation length.
Data analysis
We used descriptive and multilevel analyses. The intra-class correlations of the affect-oriented and task-oriented communication and patient-centeredness were significant (between .05 and 0.23), which made it clear that consultations of the same GP did indeed exhibit a greater degree of similarity than the consultations of different GPs. Therefore, multilevel analyses were necessary to account for the clustering of patients with the same GP.28 We applied a significance level of ≤0.05 (2-sided).
Results
Response rate
The overall patient response rate was 88%. Analysis of non-responders’ gender, age, and type of insurance showed no bias resulting from patients’ refusal.
GP response rate was 72.8%. Respondents were representative of all Dutch GPs with respect to gender, age, working hours, practice experience (mean=15.6 years, SD=8.3, range=1–32), and location (58% in an urban area). More GPs worked in a partnership or group practice than in a solo practice. We analyzed the influence of the practice type on doctor-patient communication and deemed it insignificant.
Study population
GP and patient characteristics appear in TABLE 1. Among patients, 22% had little education, 62% had an average education, and 16% had higher education. Nearly 10% had a psychosocial problem. GP-patient gender dyads were as follows: 32.1% male GP/male patient; 45.3% male GP/female patient; 6.9% female GP/male patient; 15.8% female GP/female patient.
TABLE 1
General practitioner and patient characteristics (N GPs=142, N patients=1787)
| MEAN | SD | RANGE | |
|---|---|---|---|
| GP characteristics | |||
| Age (yrs) | 46.9 | 6.2 | 32–62 |
| Full-time equivalents | 0.8 | 0.2 | 0.2–1 |
| Patient characteristics | |||
| Age (yrs) | 49.5 | 17.4 | 18–95 |
| Psychosocial problem (1=yes) | 9.8% | — | — |
| Overall health (1=excellent, 5=poor) | 3.2 | 1.1 | 1–5 |
| Depressive feelings (1=not at all, 5=extremely) | 2.2 | 1.2 | 1–5 |
| Consultation length (min) | 10.1 | 4.8 | 1.3–33.0 |
| Patients’ preferences | |||
| Affect-oriented preference (1=not, 4=utmost important) | 3.2 | 0.5 | 1–4 |
| Task-oriented preference (1=not, 4=utmost important) | 3.1 | 0.6 | 1–4 |
Preference and performance of communication aspects
GPs good with affect-oriented communication aspects. Patients considered 6 of 7 affect-oriented communication aspects as very important (87%–96%, TABLE 2). The item “Doctor was empathic to me” was less important (61%) than items like “Doctor listened well to me” (96%) and “Doctor took enough time for me” (93%). We noted only a few discrepancies between preference and performance of the GPs’ affect-oriented behavior. If patients said beforehand that a communication aspect was important, the doctors nearly always performed that aspect. For instance, 87% wanted enough attention from the doctor and received it, while 99% of all patients received GP’s attention, whether it was important to them or not.
GPs less successful with task-oriented communication aspects. Many patients wanted information, explanations, advice, and help with their problems (85%–94%, TABLE 2). Knowing the diagnosis was less important (77%) than, say, receiving advice on what to do and having details of treatment explained.
GPs also performed most of the task-oriented aspects, if patients considered these aspects important.
Subjectively, preferences for GP task-oriented behavior and perceived performance often went together, though more discrepancies were visible than with affect-oriented behavior. One fifth of patients said their problems were not helped, though they had said this was important. Similarly, GPs did not give a diagnosis to nearly 15% of patients who considered it important.
TABLE 2
Care vs cure-centered communication: Physicians fared better on the care side (N=1787)
| AFFECT-ORIENTED ASPECTS (CARE DIMENSION) | PERFORMED | NOT PERFORMED | TOTAL* | |||
|---|---|---|---|---|---|---|
| N | % | N % | % | N | % | |
| Doctor gave me enough attention | ||||||
| Important | 1304 | 87.5 | 9 | 0.6 | 1313 | 88.1 |
| Not important | 174 | 11.7 | 4 | 0.3 | 178 | 11.9 |
| Doctor listened well to me | ||||||
| Important | 1456 | 95.3 | 10 | 0.7 | 1466 | 95.9 |
| Not important | 61 | 4.0 | 1 | 0.1 | 62 | 4.1 |
| Doctor took enough time for me | ||||||
| Important | 1412 | 92.3 | 11 | 0.7 | 1423 | 93.1 |
| Not important | 105 | 6.9 | 1 | 0.1 | 106 | 6.9 |
| Doctor was friendly | ||||||
| Important | 1331 | 87.2 | 3 | 0.2 | 1334 | 87.4 |
| Not important | 193 | 12.6 | 0 | 0.0 | 193 | 12.6 |
| Doctor was frank to me | ||||||
| Important | 1451 | 95.5 | 5 | 0.3 | 1456 | 95.8 |
| Not important | 63 | 4.15 | 0 | 0.0 | 63 | 4.2 |
| Doctor took my problem seriously | ||||||
| Important | 1455 | 95.8 | 7 | 0.5 | 1462 | 96.3 |
| Not important | 55 | 3.6 | 1 | 0.1 | 56 | 3.7 |
| Doctor was empathic to me | ||||||
| Important | 846 | 58.4 | 36 | 2.5 | 882 | 60.9 |
| Not important | 492 | 34.0 | 74 | 5.1 | 566 | 19.1 |
| TASK-ORIENTED ASPECTS (CURE DIMENSION) | ||||||
| Doctor diagnosed what’s wrong | ||||||
| Important | 921 | 62.8 | 209 | 14.2 | 1130 | 77.0 |
| Not important | 197 | 13.4 | 140 | 9.5 | 337 | 23.0 |
| Doctor explained well what’s wrong | ||||||
| Important | 1166 | 78.3 | 101 | 6.8 | 1267 | 85.0 |
| Not important | 175 | 11.7 | 48 | 3.2 | 223 | 15.0 |
| Doctor informed well on treatment | ||||||
| Important | 1304 | 86.6 | 109 | 7.2 | 1413 | 93.9 |
| Not important | 75 | 5.0 | 17 | 1.1 | 92 | 6.1 |
| Doctor gave advice on what to do | ||||||
| Important | 1294 | 85.9 | 121 | 8.0 | 1415 | 94.0 |
| Not important | 75 | 5.0 | 16 | 1.1 | 91 | 6.0 |
| Doctor helped me with my problem | ||||||
| Important | 1031 | 70.0 | 121 | 18.9 | 1152 | 88.9 |
| Not important | 94 | 6.4 | 16 | 4.7 | 110 | 11.1 |
| Doctor examined me | ||||||
| Important | 902 | 59.9 | 132 | 8.8 | 1034 | 62.8 |
| Not important | 228 | 15.1 | 244 | 16.2 | 472 | 27.2 |
| * Totals do not always add up to 1787 because of missing data. | ||||||
GP communication varies by doctor gender, patient characteristics
GPs engaged less in affect-oriented than in task-oriented communication (48.6 and 70.0 utterances on average, respectively, P≤.001).
The more patients regarded affect-oriented talk by GPs as important, the more the GPs actually showed affective and patient-centered behavior (TABLE 3). Preferences for task-oriented behavior (question-asking, information-giving, and counseling) were mirrored in their doctors’ talk.
When taking into account other GP and patient characteristics, female doctors were more often affect-oriented as well as task-oriented when communicating with patients than were male doctors, especially with female patients. In consultations with older patients and those in poor health, the doctors were more affective than in consultations with younger and healthy patients.
TABLE 3
On observation, physician communication corresponded to patient preferences (N GPs=142, N patients=1787)
| REGRESSION COEFFICIENTS | |||
|---|---|---|---|
| AFFECT-ORIENTED TALK GPs | TASK-ORIENTED TALK GPs | PATIENT-CENTEREDNESS | |
| GP characteristics | |||
| Age (yrs) | –0.20 | –0.37* | –0.01* |
| Full-time equivalents | –12.13* | 2.45 | 0.03 |
| Patient characteristics | |||
| Gender-dyad: | |||
| - Male/female | –0.89c | 0.17d | 0.01 |
| - Female/male | 9.40a,b,d | 6.24a,d | 0.10 |
| - Female/female | 5.73a,c | 6.85a,b,c | 0.02 |
| Age (yrs) | 0.09* | –0.15 | –0.00* |
| Education (1=low, 2=middle, 3=high) | –0.70 | 0.15 | 0.05 |
| Psychosocial problems (1=yes) | 7.93* | –4.62* | 0.13* |
| Overall health (1=excellent, 5=poor) | 1.13* | 0.96 | –0.01 |
| Depressive feelings (1=not at all, 5=extremely) | 0.78 | –0.72 | 0.01 |
| Consultation length (min) | 4.03* | 4.30* | 0.04* |
| Patients’ preferences | |||
| Affect-oriented preference (1=not, 4=utmost important) | 2.81* | –1.94 | 0.16* |
| Task-oriented preference (1=not, 4=utmost important) | –4.23 * | 3.62* | –0.15* |
| * P<.05 | |||
| a. Score differs significantly from score of male GP/male patient dyad (reference group). | |||
| b. Score differs significantly from score of male GP/female patient dyad. | |||
| c. Score differs significantly from score of female GP/male patient dyad. | |||
| d. Score differs significantly from score of female GP/female patient dyad. | |||
Discussion
Our study suggests most patients receive from their GPs the kind of communication they prefer in a consultation. In general, patients consider both affect- and task-oriented communication aspects important, and believe they are often performed. Our findings agree with most of the literature.5,14,20 Furthermore, patients’ preferences are for the greater part reflected in the GPs’ observed communication during the visit, which agrees with one earlier study18 but not with others.5,20
Patient preference for an affective doctor is very often met. GPs are generally considered attentive, friendly, frank, empathic, and good listeners. Patients seem satisfied in this respect. However, the task-oriented communication of the GPs is sometimes less satisfying. Contrary to patient preference, for example, GPs are not always able to make a diagnosis.
Observed physician behavior: patients usually get what they want. Looking at the relationship between preferences and actual GP communication, it appears that the more patients prefer an affective or caring doctor, the more they are likely to get an empathic, concerned, interested, and patient-centered doctor, especially when psychosocial problems are expressed. An affective GP was patient centered, involving patients in problem definition and decision making. This relationship between affective behavior and patient-centeredness was also found in earlier studies.22,29 However, Swenson found that not all patients wanted the doctor to exhibit a patient-centered approach.30
Likewise, the more patients prefer a task-oriented doctor, the better the chance they will have a doctor who explains things well, and who gives information and advice to their satisfaction. However, task-oriented doctors are usually less affective and less patient-centered when talking with patients. In view of the postulate that a doctor has to be curing as well as caring,6 doctors would be wise to give attention to both aspects.
GPs do improvise while communicating with patients. The study shows that GPs and patients working together can create the type of encounter both want. GPs are able to change their behavior in response to real-time cues they believe patients are giving in an encounter.
Physician gender often makes a difference. Our findings suggest that female doctors are more affective and task-oriented when talking with their patients than are male doctors, especially with female patients. In view of the steady increase of female doctors in general practice, this combined communication style may become more common in the future.
Psychosocial complaints prompt affective communication. Patients with a psychosocial problem are more likely to encounter an affective doctor than those with a biomedical problem. The growing number of psychosocial problems in the population may lead to a more affective communication.
Eventually the demand and the supply of affective communication may coincide. However, it is a challenge for every doctor to keep his or her mind open to both biomedical (task-oriented) and psychosocial (affective-oriented) information.31
Study caveats. Because we used scale scores for affect- and task-oriented preferences instead of the separate item scores for patient preferences, the reflection of preferences for GP communicative behavior might be somewhat overestimated. Likewise, we used total observation scores for affect- and task-oriented talk instead of the separate RIAS categories. More detailed measures of such communication aspects as empathy might give better insight into patient preferences.
Final thoughts on personal application. Primary care physicians would do well to take notice of patients’ preferences for communication. GPs in our study were often able to grasp what patients considered important to talk about, and there seemed to be only modest mismatches between patient expectations and physician behavior. To increase the quality of health care, consider asking patients at the end of a visit whether their preferences were met.
Acknowledgments
We acknowledge the participating general practitioners and patients, the observers of the videotaped consultations, and the Ministry of Health, Welfare and Sports for funding (for the greater part) the research project.
Correspondence
A. van den Brink-Muinen, PhD, NIVEL, PO Box 1568, 3500 BN Utrecht, The Netherlands; a.vandenbrink@nivel.nl
- Patients want an attentive, friendly, frank and empathic doctor who listens well.
- To enhance quality of health care, consider asking patients at the end of a visit whether their communication preferences were met.
One physician has written that good patient-doctor communication, like jazz, calls for improvisation.1 We agree. And improvise we must when patients’ expectations for how we will communicate with them vary between visits and individuals.
For example, those who are ill may prefer that their doctor communicate with them in a way that is less important to those who are healthy. Patients with biomedical problems may have different preferences than persons with psychosocial problems. And older individuals may have communication desires that differ from those who are younger.2-4
Do patients want cure or care, or both?
Depending on the reason for a visit—eg, biomedical or psychosocial—patient preferences may fit either the cure or the care dimension.
Cure dimension. On one hand, patients expect their doctor to be task-oriented and to find a cure for what ails them. They want an explanation of what is wrong and advice about possible treatments, and they want the doctor to do whatever is needed to get answers.5
Care dimension. On the other hand, patients may feel anxious and want reassurance. They expect the doctor to listen to their story and encourage them to disclose all health problems, concerns, and worries. They also expect friendliness and empathy. They want to be taken seriously. The extent to which the doctor shows this affect-oriented (and patient-centered) behavior will determine how fulfilled patients feel in their preference for care.6,7
Why does it matter? Good communication serves a patient’s need to understand and to be understood.6,8,9 And communication aimed at matching patient preferences enhances satisfaction with care, compliance with medical instructions, and health status.10-13
How well do we assess patients’ communication preferences?
Patient-centered behavior is a necessary tool for discovering and fulfilling patients’ task-oriented (cure dimension) and affect-oriented (care dimension) communication preferences.14-17 It’s important to know how well primary-care physicians interpret patients’ preferences for clinical encounters and if they respond in a manner that satisfies those expectations.
Reassuringly, patients indicate on surveys that their physicians do a fairly good job of interpreting their communication preferences and acting accordingly.18-20 They also report that their desires and expectations from consultations are increasingly met.
There is always the worry, though, that physicians in certain positions—eg, non-gatekeeper roles or positions involving only part-time clinical responsibilities—would be challenged to assess patient preferences as accurately as others.21
The aim of our study
While it’s encouraging that physicians by and large understand their patients and communicate with them meaningfully, we wondered whether communication could improve further. Our purpose in this study was to gain detailed insight into patients’ preferences in physician communication and, through patients’ subjective perspectives and observed real practice consultations, learn how well physicians communicate according to those preferences.
Methods
Design
We derived physician data from the Second Dutch National Survey of General Practice (2001). This study was carried out in practices representative of Dutch general practice.22 We asked patients for permission to videotape consultations with the general practitioner (GP), and asked them to sign a consent form. Collected data were kept private as per regulations.
We videotaped consultations of 142 GPs (76.1% male) and 2784 patients (41.2% male). The number of patients cared for by each GP ranged between 17 and 21 (mean=19.6). Each patient was videotaped just once. We rated roughly 15 patient-consultations per GP (13–15, mean=14.8), excluding the first 3 to correct for possible bias because of the video camera. Before and immediately after the consultation, patients 18 years of age and older answered a questionnaire. We used data from 1787 patient consultations.
Patients rate their communication preferences
The patient questionnaire covered demographic characteristics (gender, age, education); health problems (psychosocial or not [ICPC-coded]);23 overall health during the past 2 weeks (1=excellent, 2=very good, 3=good, 4=fair, 5=poor); and depressive feelings during the past 2 weeks (1=not at all, 2=slightly, 3=moderately, 4=quite a bit, 5=extremely) (COOP-WONCA charts24).
We defined communication preferences as “the extent of importance patients attach to communication aspects.”25 Patients’ preferences and the actual performance by the GP were measured using the conceptual framework of the QUOTE scale (quality of care through the patient’s eyes).5,25
Before consultation, patients recorded how important they considered different aspects of communication for the coming visit (1=not important, 2=rather important, 3=important, 4=utmost important). Following consultation, they rated the GP’s performance in meeting their expectations for these aspects (1=not, 2=really not, 3=really yes, 4=yes).
Factor analysis of both the pre- and post-visit lists of questions on preference and performance revealed 2 relevant subscales: an affect-oriented scale of 7 communication aspects and a task-oriented scale of 6 communication aspects (Cronbach’s alpha between 0.74 and 0.89).
We also used communication aspects from the original 4-point scale to present 4 new categories that compared and contrasted preferences and relevance. These categories included: important and performed; important and not performed; not important and performed; not important and not performed. In the multilevel analysis, we included the 2 subscales using the original 4-point scale.
Socio-demographic and practice variables were derived from the GP questionnaires in the Second Dutch National Survey of General Practice (2001).
Video observations
Nine observers measured verbal behavior during the videotaped visits using the Roter Method of Interaction Process Analysis (RIAS26), a well-documented, widely used system in the US and Netherlands. This observation system distinguishes both affect-oriented (socio-emotional) and task-oriented (instrumental) verbal behavior of doctors and patients, reflecting the care and cure dimensions, respectively. The RIAS categories are mutually exclusive and exhaustive.
Affect-oriented communication consists of personal remarks, agreements, concerns, reassurances, paraphrases, and disagreements.
Task-oriented talk includes asking questions, giving information, and (only GPs) counseling about medical/therapeutic and psychosocial, social context and lifestyle issues, and process-oriented talk (instructions, asking for understanding).
After finishing the RIAS-coding, we calculated the total numbers of affect-oriented and task-oriented verbal behaviors separately for GPs and patients.
The relevance and performance items and the RIAS-categories all measured the affect-oriented and task-oriented aspects.
We used the Noldus Observer-Video-Pro computer program for the observation study,27 including measurement of consultation length. The interobserver reliabilities were good to excellent: between r=0.80 to r=0.95 per category, except for personal remarks (0.72).
Patient-centeredness measured in 3 ways
The observers, using a 5-point scale, also rated the extent to which GPs communicated in a patient-centered way in 3 areas: patient’s involvement in the problem-defining process; patient’s involvement in the decision-making process; and doctor’s overall responsiveness to the patient.
Based on ratings in these 3 areas, we determined an overall magnitude of patient-centeredness (Cronbach’s alpha=0.75). Observers and the responsible researcher met weekly to validate the quality of rating. The same was done for the RIAS coding.
Controlling variables
For GPs, controlling variables were gender, age, and number of full-time equivalents (FTEs) working. For patients, GP and patient gender were included in the variable “gender-dyad”—male GPs/male patients, male GPs/female patients, female GPs/male patients, female GPs/female patients. Other patient variables were age; education (low=no/primary school, middle=secondary school, high=higher vocational training/university); health problems: somatic or psychosocial (ICPC chapters); overall physical health and mental health during the past 2 weeks; and consultation length.
Data analysis
We used descriptive and multilevel analyses. The intra-class correlations of the affect-oriented and task-oriented communication and patient-centeredness were significant (between .05 and 0.23), which made it clear that consultations of the same GP did indeed exhibit a greater degree of similarity than the consultations of different GPs. Therefore, multilevel analyses were necessary to account for the clustering of patients with the same GP.28 We applied a significance level of ≤0.05 (2-sided).
Results
Response rate
The overall patient response rate was 88%. Analysis of non-responders’ gender, age, and type of insurance showed no bias resulting from patients’ refusal.
GP response rate was 72.8%. Respondents were representative of all Dutch GPs with respect to gender, age, working hours, practice experience (mean=15.6 years, SD=8.3, range=1–32), and location (58% in an urban area). More GPs worked in a partnership or group practice than in a solo practice. We analyzed the influence of the practice type on doctor-patient communication and deemed it insignificant.
Study population
GP and patient characteristics appear in TABLE 1. Among patients, 22% had little education, 62% had an average education, and 16% had higher education. Nearly 10% had a psychosocial problem. GP-patient gender dyads were as follows: 32.1% male GP/male patient; 45.3% male GP/female patient; 6.9% female GP/male patient; 15.8% female GP/female patient.
TABLE 1
General practitioner and patient characteristics (N GPs=142, N patients=1787)
| MEAN | SD | RANGE | |
|---|---|---|---|
| GP characteristics | |||
| Age (yrs) | 46.9 | 6.2 | 32–62 |
| Full-time equivalents | 0.8 | 0.2 | 0.2–1 |
| Patient characteristics | |||
| Age (yrs) | 49.5 | 17.4 | 18–95 |
| Psychosocial problem (1=yes) | 9.8% | — | — |
| Overall health (1=excellent, 5=poor) | 3.2 | 1.1 | 1–5 |
| Depressive feelings (1=not at all, 5=extremely) | 2.2 | 1.2 | 1–5 |
| Consultation length (min) | 10.1 | 4.8 | 1.3–33.0 |
| Patients’ preferences | |||
| Affect-oriented preference (1=not, 4=utmost important) | 3.2 | 0.5 | 1–4 |
| Task-oriented preference (1=not, 4=utmost important) | 3.1 | 0.6 | 1–4 |
Preference and performance of communication aspects
GPs good with affect-oriented communication aspects. Patients considered 6 of 7 affect-oriented communication aspects as very important (87%–96%, TABLE 2). The item “Doctor was empathic to me” was less important (61%) than items like “Doctor listened well to me” (96%) and “Doctor took enough time for me” (93%). We noted only a few discrepancies between preference and performance of the GPs’ affect-oriented behavior. If patients said beforehand that a communication aspect was important, the doctors nearly always performed that aspect. For instance, 87% wanted enough attention from the doctor and received it, while 99% of all patients received GP’s attention, whether it was important to them or not.
GPs less successful with task-oriented communication aspects. Many patients wanted information, explanations, advice, and help with their problems (85%–94%, TABLE 2). Knowing the diagnosis was less important (77%) than, say, receiving advice on what to do and having details of treatment explained.
GPs also performed most of the task-oriented aspects, if patients considered these aspects important.
Subjectively, preferences for GP task-oriented behavior and perceived performance often went together, though more discrepancies were visible than with affect-oriented behavior. One fifth of patients said their problems were not helped, though they had said this was important. Similarly, GPs did not give a diagnosis to nearly 15% of patients who considered it important.
TABLE 2
Care vs cure-centered communication: Physicians fared better on the care side (N=1787)
| AFFECT-ORIENTED ASPECTS (CARE DIMENSION) | PERFORMED | NOT PERFORMED | TOTAL* | |||
|---|---|---|---|---|---|---|
| N | % | N % | % | N | % | |
| Doctor gave me enough attention | ||||||
| Important | 1304 | 87.5 | 9 | 0.6 | 1313 | 88.1 |
| Not important | 174 | 11.7 | 4 | 0.3 | 178 | 11.9 |
| Doctor listened well to me | ||||||
| Important | 1456 | 95.3 | 10 | 0.7 | 1466 | 95.9 |
| Not important | 61 | 4.0 | 1 | 0.1 | 62 | 4.1 |
| Doctor took enough time for me | ||||||
| Important | 1412 | 92.3 | 11 | 0.7 | 1423 | 93.1 |
| Not important | 105 | 6.9 | 1 | 0.1 | 106 | 6.9 |
| Doctor was friendly | ||||||
| Important | 1331 | 87.2 | 3 | 0.2 | 1334 | 87.4 |
| Not important | 193 | 12.6 | 0 | 0.0 | 193 | 12.6 |
| Doctor was frank to me | ||||||
| Important | 1451 | 95.5 | 5 | 0.3 | 1456 | 95.8 |
| Not important | 63 | 4.15 | 0 | 0.0 | 63 | 4.2 |
| Doctor took my problem seriously | ||||||
| Important | 1455 | 95.8 | 7 | 0.5 | 1462 | 96.3 |
| Not important | 55 | 3.6 | 1 | 0.1 | 56 | 3.7 |
| Doctor was empathic to me | ||||||
| Important | 846 | 58.4 | 36 | 2.5 | 882 | 60.9 |
| Not important | 492 | 34.0 | 74 | 5.1 | 566 | 19.1 |
| TASK-ORIENTED ASPECTS (CURE DIMENSION) | ||||||
| Doctor diagnosed what’s wrong | ||||||
| Important | 921 | 62.8 | 209 | 14.2 | 1130 | 77.0 |
| Not important | 197 | 13.4 | 140 | 9.5 | 337 | 23.0 |
| Doctor explained well what’s wrong | ||||||
| Important | 1166 | 78.3 | 101 | 6.8 | 1267 | 85.0 |
| Not important | 175 | 11.7 | 48 | 3.2 | 223 | 15.0 |
| Doctor informed well on treatment | ||||||
| Important | 1304 | 86.6 | 109 | 7.2 | 1413 | 93.9 |
| Not important | 75 | 5.0 | 17 | 1.1 | 92 | 6.1 |
| Doctor gave advice on what to do | ||||||
| Important | 1294 | 85.9 | 121 | 8.0 | 1415 | 94.0 |
| Not important | 75 | 5.0 | 16 | 1.1 | 91 | 6.0 |
| Doctor helped me with my problem | ||||||
| Important | 1031 | 70.0 | 121 | 18.9 | 1152 | 88.9 |
| Not important | 94 | 6.4 | 16 | 4.7 | 110 | 11.1 |
| Doctor examined me | ||||||
| Important | 902 | 59.9 | 132 | 8.8 | 1034 | 62.8 |
| Not important | 228 | 15.1 | 244 | 16.2 | 472 | 27.2 |
| * Totals do not always add up to 1787 because of missing data. | ||||||
GP communication varies by doctor gender, patient characteristics
GPs engaged less in affect-oriented than in task-oriented communication (48.6 and 70.0 utterances on average, respectively, P≤.001).
The more patients regarded affect-oriented talk by GPs as important, the more the GPs actually showed affective and patient-centered behavior (TABLE 3). Preferences for task-oriented behavior (question-asking, information-giving, and counseling) were mirrored in their doctors’ talk.
When taking into account other GP and patient characteristics, female doctors were more often affect-oriented as well as task-oriented when communicating with patients than were male doctors, especially with female patients. In consultations with older patients and those in poor health, the doctors were more affective than in consultations with younger and healthy patients.
TABLE 3
On observation, physician communication corresponded to patient preferences (N GPs=142, N patients=1787)
| REGRESSION COEFFICIENTS | |||
|---|---|---|---|
| AFFECT-ORIENTED TALK GPs | TASK-ORIENTED TALK GPs | PATIENT-CENTEREDNESS | |
| GP characteristics | |||
| Age (yrs) | –0.20 | –0.37* | –0.01* |
| Full-time equivalents | –12.13* | 2.45 | 0.03 |
| Patient characteristics | |||
| Gender-dyad: | |||
| - Male/female | –0.89c | 0.17d | 0.01 |
| - Female/male | 9.40a,b,d | 6.24a,d | 0.10 |
| - Female/female | 5.73a,c | 6.85a,b,c | 0.02 |
| Age (yrs) | 0.09* | –0.15 | –0.00* |
| Education (1=low, 2=middle, 3=high) | –0.70 | 0.15 | 0.05 |
| Psychosocial problems (1=yes) | 7.93* | –4.62* | 0.13* |
| Overall health (1=excellent, 5=poor) | 1.13* | 0.96 | –0.01 |
| Depressive feelings (1=not at all, 5=extremely) | 0.78 | –0.72 | 0.01 |
| Consultation length (min) | 4.03* | 4.30* | 0.04* |
| Patients’ preferences | |||
| Affect-oriented preference (1=not, 4=utmost important) | 2.81* | –1.94 | 0.16* |
| Task-oriented preference (1=not, 4=utmost important) | –4.23 * | 3.62* | –0.15* |
| * P<.05 | |||
| a. Score differs significantly from score of male GP/male patient dyad (reference group). | |||
| b. Score differs significantly from score of male GP/female patient dyad. | |||
| c. Score differs significantly from score of female GP/male patient dyad. | |||
| d. Score differs significantly from score of female GP/female patient dyad. | |||
Discussion
Our study suggests most patients receive from their GPs the kind of communication they prefer in a consultation. In general, patients consider both affect- and task-oriented communication aspects important, and believe they are often performed. Our findings agree with most of the literature.5,14,20 Furthermore, patients’ preferences are for the greater part reflected in the GPs’ observed communication during the visit, which agrees with one earlier study18 but not with others.5,20
Patient preference for an affective doctor is very often met. GPs are generally considered attentive, friendly, frank, empathic, and good listeners. Patients seem satisfied in this respect. However, the task-oriented communication of the GPs is sometimes less satisfying. Contrary to patient preference, for example, GPs are not always able to make a diagnosis.
Observed physician behavior: patients usually get what they want. Looking at the relationship between preferences and actual GP communication, it appears that the more patients prefer an affective or caring doctor, the more they are likely to get an empathic, concerned, interested, and patient-centered doctor, especially when psychosocial problems are expressed. An affective GP was patient centered, involving patients in problem definition and decision making. This relationship between affective behavior and patient-centeredness was also found in earlier studies.22,29 However, Swenson found that not all patients wanted the doctor to exhibit a patient-centered approach.30
Likewise, the more patients prefer a task-oriented doctor, the better the chance they will have a doctor who explains things well, and who gives information and advice to their satisfaction. However, task-oriented doctors are usually less affective and less patient-centered when talking with patients. In view of the postulate that a doctor has to be curing as well as caring,6 doctors would be wise to give attention to both aspects.
GPs do improvise while communicating with patients. The study shows that GPs and patients working together can create the type of encounter both want. GPs are able to change their behavior in response to real-time cues they believe patients are giving in an encounter.
Physician gender often makes a difference. Our findings suggest that female doctors are more affective and task-oriented when talking with their patients than are male doctors, especially with female patients. In view of the steady increase of female doctors in general practice, this combined communication style may become more common in the future.
Psychosocial complaints prompt affective communication. Patients with a psychosocial problem are more likely to encounter an affective doctor than those with a biomedical problem. The growing number of psychosocial problems in the population may lead to a more affective communication.
Eventually the demand and the supply of affective communication may coincide. However, it is a challenge for every doctor to keep his or her mind open to both biomedical (task-oriented) and psychosocial (affective-oriented) information.31
Study caveats. Because we used scale scores for affect- and task-oriented preferences instead of the separate item scores for patient preferences, the reflection of preferences for GP communicative behavior might be somewhat overestimated. Likewise, we used total observation scores for affect- and task-oriented talk instead of the separate RIAS categories. More detailed measures of such communication aspects as empathy might give better insight into patient preferences.
Final thoughts on personal application. Primary care physicians would do well to take notice of patients’ preferences for communication. GPs in our study were often able to grasp what patients considered important to talk about, and there seemed to be only modest mismatches between patient expectations and physician behavior. To increase the quality of health care, consider asking patients at the end of a visit whether their preferences were met.
Acknowledgments
We acknowledge the participating general practitioners and patients, the observers of the videotaped consultations, and the Ministry of Health, Welfare and Sports for funding (for the greater part) the research project.
Correspondence
A. van den Brink-Muinen, PhD, NIVEL, PO Box 1568, 3500 BN Utrecht, The Netherlands; a.vandenbrink@nivel.nl
1. Haidet P. Jazz and the ‘Art’ of Medicine: Improvisation in the Medical Encounter. Ann Fam Med 2007;5:164-169.
2. Jung HP, Baerveldt C, Olesen F, Grol R, Wensing M. Patient characteristics as predictors of primary health care p: a systematic literature analysis. Health Expect 2003;6:160-181.
3. Bartholomew L, Schneiderman LJ. Attitudes of patients towards family care in a family practice group. J Fam Pract 1982;15:477-481.
4. Wetzels R, Geest TA, Wensing M, Lopes Ferreira P, Grol R, Baker R. GPs’ views on involvement of older patients: a European qualitative study. Pat Educ Couns 2004;53:183-188.
5. Brink-Muinen A, Verhaak PFM, Bensing JM, et al. Doctor-patient communication in different European health care systems: relevance and performance from the patients’ perspective. Pat Educ Counsel 2000;39:115-127.
6. Bensing JM, Dronkers J. Instrumental and affective aspects of physician behaviour. Med Care 1992;30:283-
7. Little P, Everitt H, Williamson I, et al. observational study of effect of patient centeredness and positive approach on outcomes of general practice. BMJ 2001;323:908-911.
8. Roter DL, Hall JA. Doctors Talking with Patients/Patients Talking with Doctors: Improving Communication in Medical Visits Westport/London: Auburn House; 1992.
9. Roter DL, Stewart M, Putnam SM, Lipkin M, Stiles W, Inui TS. Communication patterns of primary care physicians. JAMA 1997;277:350-356.
10. Sixma H, Spreeuwenberg P, Pasch M van der. Patient satisfaction with the general practitioner: a two-level analysis. Med Care 1998;2:212-229.
11. Dulmen AM van, Bensing JM. The Effect of Context in Health Care: A Programming Study The Hague: RGO; 2001.
12. Wensing M, Baker R. Patient involvement in general practice care: a pragmatic framework. Eur J Gen Pract 2003;9:62-65.
13. Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract 2000;49:796-804.
14. Bensing JM, Langewitz W. Die ärztliche Konsultation. In: Uexkull: Psychosomatische Medizin. 2002:415-424.
15. Brown J, Stewart M, Ryan B. Assessing communication between patients and physicians: the measure of patient-centred communication (MPCC). London, Ontario, Canada: Centre for Studies in Family Medicine, 2001.
16. Sullivan M. The new subjective medicine: taking the patients’ view on health care and health. Soc Sci Med 2003;56:1995-1604.
17. Mead N, Bower P. Patient-centeredness: a conceptual framework and review of the empirical literature. Soc Sci Med 2000;51:1087-1110.
18. Jung HP, Wensing M, Grol R. What makes a good general practitioner: do patients and doctors have different views? Br J Gen Pract 1997;47:805—809.
19. Wensing M, Jung HP, Mainz J, Olesen F, Grol R. A systematic review of the literature in patient priorities for general practice care. Part 1: description of the research domain. Soc Sci Med 1998;47:1573-1588.
20. Thorsen H, Witt K, Hollnagel H, Malterud K. The purpose of the general practice consultation from the patients’ perspective—theoretical aspects. Fam Pract 2001;18:638-643.
21. Brink-Muinen A, Verhaak PFM, Bensing JM, et al. Communication in general practice: differences between European countries. Fam Pract 2003;20:478-485.
22. Schellevis FG, Westert GP, Bakker DH de, Groenewegen PP, Zee J van der, Bensing JM. De tweede Nationale studie naar ziekten en verrichtingen in de huisartsenpraktijk: aanleiding en methoden [Second Dutch National Survey of general practice: background and methods]. H&W 2003;46:7-12.
23. Lamberts H, Wood M (eds). International Classification of Primary Care Oxford: Oxford University Press; 1987.
24. Weel C van, König-Zahn C, Touw-Otten FWMM, Duijn NP van, Meyboom-de Jong B. Measuring Functional Health Status with the COOP/WONCA Charts. A Manual WoNCA, ERGHo, NCH, university of Groningen, Netherlands; 1995.
25. Sixma HJ, Kerssens JJ, Campen C van, Peters L. Quality of care from the patients’ perspective: from theoretical concept to a new measurement instrument. Health Expect 1998;1:82-95.
26. Roter DL. The Roter Method of Interaction Process Analysis Baltimore, Md: Johns Hopkins University; 2001.
27. Noldus LP, Trienes RJ, Hendriksen AH, Janden H, Jansen RG. The observer video-Pro: new software for the collection, management and presentation of time-structured data from videotapes and digital media films. Behavioral Research Methods, Instruments & Computers 2000;32:197-206.
28. Leyland AH, Groenewegen PP. Multilevel modelling and public health policy. Scand J Public Health 2003;31:267-274
29. Jung HP, Horne F van, Wensing M, Hearnshaw H, Grol R. Which aspects of general practitioners’ behaviour determine patients’ evaluations of care? Soc Sci Med 1998;47:1077-1087.
30. Swenson SL, Buell S, Zettler P, White M, Ruston DC, Lo B. Patient-centred communication: do patients really prefer it? J Gen Intern Med 2004;19:1069-1079.
31. Epstein RM, Campbell TL, Cohen-Cole SA, MaWhinney IR, Smilkstein G. Perspectives on patient-doctor communication. J Fam Pract 1993;37:377-388.
1. Haidet P. Jazz and the ‘Art’ of Medicine: Improvisation in the Medical Encounter. Ann Fam Med 2007;5:164-169.
2. Jung HP, Baerveldt C, Olesen F, Grol R, Wensing M. Patient characteristics as predictors of primary health care p: a systematic literature analysis. Health Expect 2003;6:160-181.
3. Bartholomew L, Schneiderman LJ. Attitudes of patients towards family care in a family practice group. J Fam Pract 1982;15:477-481.
4. Wetzels R, Geest TA, Wensing M, Lopes Ferreira P, Grol R, Baker R. GPs’ views on involvement of older patients: a European qualitative study. Pat Educ Couns 2004;53:183-188.
5. Brink-Muinen A, Verhaak PFM, Bensing JM, et al. Doctor-patient communication in different European health care systems: relevance and performance from the patients’ perspective. Pat Educ Counsel 2000;39:115-127.
6. Bensing JM, Dronkers J. Instrumental and affective aspects of physician behaviour. Med Care 1992;30:283-
7. Little P, Everitt H, Williamson I, et al. observational study of effect of patient centeredness and positive approach on outcomes of general practice. BMJ 2001;323:908-911.
8. Roter DL, Hall JA. Doctors Talking with Patients/Patients Talking with Doctors: Improving Communication in Medical Visits Westport/London: Auburn House; 1992.
9. Roter DL, Stewart M, Putnam SM, Lipkin M, Stiles W, Inui TS. Communication patterns of primary care physicians. JAMA 1997;277:350-356.
10. Sixma H, Spreeuwenberg P, Pasch M van der. Patient satisfaction with the general practitioner: a two-level analysis. Med Care 1998;2:212-229.
11. Dulmen AM van, Bensing JM. The Effect of Context in Health Care: A Programming Study The Hague: RGO; 2001.
12. Wensing M, Baker R. Patient involvement in general practice care: a pragmatic framework. Eur J Gen Pract 2003;9:62-65.
13. Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract 2000;49:796-804.
14. Bensing JM, Langewitz W. Die ärztliche Konsultation. In: Uexkull: Psychosomatische Medizin. 2002:415-424.
15. Brown J, Stewart M, Ryan B. Assessing communication between patients and physicians: the measure of patient-centred communication (MPCC). London, Ontario, Canada: Centre for Studies in Family Medicine, 2001.
16. Sullivan M. The new subjective medicine: taking the patients’ view on health care and health. Soc Sci Med 2003;56:1995-1604.
17. Mead N, Bower P. Patient-centeredness: a conceptual framework and review of the empirical literature. Soc Sci Med 2000;51:1087-1110.
18. Jung HP, Wensing M, Grol R. What makes a good general practitioner: do patients and doctors have different views? Br J Gen Pract 1997;47:805—809.
19. Wensing M, Jung HP, Mainz J, Olesen F, Grol R. A systematic review of the literature in patient priorities for general practice care. Part 1: description of the research domain. Soc Sci Med 1998;47:1573-1588.
20. Thorsen H, Witt K, Hollnagel H, Malterud K. The purpose of the general practice consultation from the patients’ perspective—theoretical aspects. Fam Pract 2001;18:638-643.
21. Brink-Muinen A, Verhaak PFM, Bensing JM, et al. Communication in general practice: differences between European countries. Fam Pract 2003;20:478-485.
22. Schellevis FG, Westert GP, Bakker DH de, Groenewegen PP, Zee J van der, Bensing JM. De tweede Nationale studie naar ziekten en verrichtingen in de huisartsenpraktijk: aanleiding en methoden [Second Dutch National Survey of general practice: background and methods]. H&W 2003;46:7-12.
23. Lamberts H, Wood M (eds). International Classification of Primary Care Oxford: Oxford University Press; 1987.
24. Weel C van, König-Zahn C, Touw-Otten FWMM, Duijn NP van, Meyboom-de Jong B. Measuring Functional Health Status with the COOP/WONCA Charts. A Manual WoNCA, ERGHo, NCH, university of Groningen, Netherlands; 1995.
25. Sixma HJ, Kerssens JJ, Campen C van, Peters L. Quality of care from the patients’ perspective: from theoretical concept to a new measurement instrument. Health Expect 1998;1:82-95.
26. Roter DL. The Roter Method of Interaction Process Analysis Baltimore, Md: Johns Hopkins University; 2001.
27. Noldus LP, Trienes RJ, Hendriksen AH, Janden H, Jansen RG. The observer video-Pro: new software for the collection, management and presentation of time-structured data from videotapes and digital media films. Behavioral Research Methods, Instruments & Computers 2000;32:197-206.
28. Leyland AH, Groenewegen PP. Multilevel modelling and public health policy. Scand J Public Health 2003;31:267-274
29. Jung HP, Horne F van, Wensing M, Hearnshaw H, Grol R. Which aspects of general practitioners’ behaviour determine patients’ evaluations of care? Soc Sci Med 1998;47:1077-1087.
30. Swenson SL, Buell S, Zettler P, White M, Ruston DC, Lo B. Patient-centred communication: do patients really prefer it? J Gen Intern Med 2004;19:1069-1079.
31. Epstein RM, Campbell TL, Cohen-Cole SA, MaWhinney IR, Smilkstein G. Perspectives on patient-doctor communication. J Fam Pract 1993;37:377-388.
Practice alert: CDC no longer recommends quinolones for treatment of gonorrhea
- The CDC no longer recommends the use of fluoroquinolones for the treatment of gonococcal infections and associated conditions such as pelvic inflammatory disease (PID).
- Consequently, only one class of drugs, the cephalosporins, is still recommended and available for the treatment of gonorrhea.
- The CDC now recommends ceftriaxone, 125 mg IM, in a single dose, as the preferred treatment.
- For patients with cephalosporin allergies, azithromycin, 2 g orally, as a single dose, remains an option. The CDC discourages widespread use, however, because of concerns about resistance.
The Centers for Disease Control and Prevention (CDC) recently released an update to its treatment guidelines for sexually transmitted diseases, stating that fluoroquinolones are no longer recommended for treatment of gonococcal infections.1 This change resulted from a progressive increase in the rate of resistance to quinolones among gonorrhea isolated from publicly funded treatment centers across the country.
The new advisory applies to all quinolones previously recommended: ciprofloxacin, ofloxacin, and levofloxacin.
Epidemiology. Gonorrhea remains common in the United States, with nearly 340,000 cases reported in 2005. Since it is under-reported, estimates are that more than 600,000 cases occur each year.2
Neisseria gonorrhoeae causes infection of the cervix, urethra, rectum, pharynx, and adnexa. It can also cause disseminated disease that can affect joints, heart, and the meninges.
Tracking the spread of resistant cases
Since the early 1990s, fluoroquinolones have been one of the recommended treatments for gonorrhea because of their availability as effective, single-dose oral regimens. Fluoroquinolone-resistant N gonorrhea began to emerge at the end of the century and has progressed rapidly since. FIGURE 1 illustrates the proportion of fluoroquinolone-resistant N gonorrhea from the CDC’s Gonococcal Isolate Surveillance Project (GISP) by year, from 1990 to 2006.
Resistance began to emerge first among gonorrhea isolates from men who have sex with men (MSM), and resistance rates among MSM continue to be higher than in heterosexual men (FIGURE 2).
Geographic trends. In 2000, the CDC recommended that quinolones should no longer be used to treat gonorrhea in persons who contracted the infection in Asia or the Pacific. In 2002, California was added to this list. In 2004, the recommendation against quinolone use was extended to all MSM in the US.
The new recommendation against general use is based on resistance surpassing 5% of total isolates.
FIGURE 1
Percentage of N gonorrhoeae isolates with intermediate resistance or resistance to ciprofloxacin
Data for 2006 are preliminary (January-June only).
* Demonstrating ciprofloxacin minimum inhibitory concentration of 0.125–0.500 mcg/mL.
† Demonstrating ciprofloxacin minimum inhibitory concentration of ≥1.0 mcg/ml.
Source: Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinalones no longer recommended for treatment of gonococcal infections.
MMWR Recomm Rep 2007; 56:332-336.
FIGURE 2
Progressive increase of fluoroquinolone resistance
Percent of isolates from the CDC Gonococcal Isolate Surveillance Project found to be resistant to fluoroquinalones, 2002 through June 2006
Source: GISP report. Centers for Disease Control and Prevention.
Sexually Transmitted Disease Surveillance 2005 Supplement, Gonoccal Isolate Surveillance Project (GISP) Annual Report 2005. Atlanta, Ga: US Department of Health and Human Services, Centers for Disease Control and Prevention, January 2007.
Ceftriaxone, the default treatment of choice
The loss of quinolones as a recommended gonorrhea treatment leaves only ceftriaxone, 125 mg intramuscularly (IM), as the only readily available treatment for urogenital, anorectal, and pharyngeal gonorrhea. Cefixime 400 mg as a single dose is also recommended, but is not currently available in tablet form in the US. It is available as a suspension with 100 mg per 5 cc.
Other options
Possible oral options include cefpodoxime 400 mg or cefuroxime axetil 1 g. However, neither has the official endorsement of the CDC, and neither appears effective against pharyngeal infection.
Spectinomycin 2 g intramuscularly is recommended for those with cephalosporin allergy—but, like cefixime, it is not currently available in the US, and it also is not considered effective against pharyngeal infection.
Azithromycin 2 g orally as a single dose is currently effective against gonorrhea and is an option for those with cephalosporin allergies. The CDC discourages its widespread use because of concerns about resistance.
New information regarding the availability of spectinomycin and cefixime can be obtained from local health departments or the CDC’s sexually transmitted diseases web site (www.cdc.gov/std).3
Recommended regimens for treatment of gonorrhea
| Uncomplicated gonococcal infections of the cervix, urethra, and rectum* |
| Recommended regimens† |
| Ceftriaxone 125 mg in a single IM dose |
| or |
| Cefixime‡ 400 mg in a single oral dose |
| plus |
| Treatment for chlamydia if chlamydial infection has not been ruled out |
| Uncomplicated gonococcal infections of the pharynx* |
| Recommended regimens |
| Ceftriaxone 125 mg in a single IM dose |
| plus |
| Treatment for chlamydia if chlamydial infection has not been ruled out |
| * For all adult and adolescent patients, regardless of travel history or sexual behavior. For those allergic to penicillins or cephalosporins, or for treatment of disseminated gonococcal infections, PID, and epididymitis, see www.cdc.gov/std/treatment. |
| † Alternative regimens: Spectinomycin 2 g in a single IM dose (not currently available in US) or cephalosporin single-dose regimens. |
| Other single-dose cephalosporin regimens that are considered alternative treatment regimens against uncomplicated urogenital and anorectal gonococcal infections include ceftizoxime 500 mg IM; or cefoxitin 2 g IM, administered with probenecid 1 g orally; or cefotaxime 500 mg IM. Some evidence indicates that cefpodoxime 400 mg and cefuroxime axetil 1 g might be oral alternatives. |
| ‡ 400 mg by suspension; tablets are no longer available in the US. |
| Source: www.cdc.gov/mmwr/PDF/rr/rr5511.pdf.2 |
Associated conditions
Treat for chlamydia if chlamydial infection is not ruled out
The CDC continues to recommend concurrent treatment for chlamydia for all persons who have gonorrhea, unless coinfection has been ruled out.
Therapies for chlamydia include azithromycin 1 g as a single dose or doxycycline 100 mg twice a day for 7 days.
Pelvic inflammatory disease and epididymitis
The treatment of both pelvic inflammatory disease (PID) and epididymitis include an option of ceftriaxone 250 mg IM plus doxycycline for either 7 days (for epididymitis) or 10 days (for PID). There are several parenteral options for PID and disseminated gonorrhea; these can be found on the CDC’s STD web site.3
Should you always retest to ensure a cure?
It is still not necessary to retest patients who have had the recommended treatments. However, patients with persistent symptoms or rapidly recurring symptoms should be retested by cultures so that drug-resistance patterns can be checked if gonorrhea is documented.
Retest for recurrence
Consider retesting all treated patients after 3 to 6 months, since anyone with a sexually transmitted infection is at risk of being reinfected.
Summary
The ongoing challenges with the evolving resistance patterns of gonorrhea illustrate the importance of physicians accurately diagnosing gonorrhea, treating with recommended regimens, reporting positive cases to the local public health department, and assisting with partner evaluation and treatment.
1. CDC. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep 2007;56:332-336.Available at: www.cdc.gov/mmwr/pdf/wk/mm5614.pdf. Accessed on June 15, 2007.
2. CDC. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep 2006;55(RR-11).-Available at www.cdc.gov/mmwr/PDF/rr/rr5511.pdf. Accessed on June 15, 2007.
3. Updated recommended treatment regimens for gonococcal infections and associated conditions—United States, April 2007. Available at: www.cdc.gov/std/treatment/2006/updated-regimens.htm. Accessed on June 15, 2007.
- The CDC no longer recommends the use of fluoroquinolones for the treatment of gonococcal infections and associated conditions such as pelvic inflammatory disease (PID).
- Consequently, only one class of drugs, the cephalosporins, is still recommended and available for the treatment of gonorrhea.
- The CDC now recommends ceftriaxone, 125 mg IM, in a single dose, as the preferred treatment.
- For patients with cephalosporin allergies, azithromycin, 2 g orally, as a single dose, remains an option. The CDC discourages widespread use, however, because of concerns about resistance.
The Centers for Disease Control and Prevention (CDC) recently released an update to its treatment guidelines for sexually transmitted diseases, stating that fluoroquinolones are no longer recommended for treatment of gonococcal infections.1 This change resulted from a progressive increase in the rate of resistance to quinolones among gonorrhea isolated from publicly funded treatment centers across the country.
The new advisory applies to all quinolones previously recommended: ciprofloxacin, ofloxacin, and levofloxacin.
Epidemiology. Gonorrhea remains common in the United States, with nearly 340,000 cases reported in 2005. Since it is under-reported, estimates are that more than 600,000 cases occur each year.2
Neisseria gonorrhoeae causes infection of the cervix, urethra, rectum, pharynx, and adnexa. It can also cause disseminated disease that can affect joints, heart, and the meninges.
Tracking the spread of resistant cases
Since the early 1990s, fluoroquinolones have been one of the recommended treatments for gonorrhea because of their availability as effective, single-dose oral regimens. Fluoroquinolone-resistant N gonorrhea began to emerge at the end of the century and has progressed rapidly since. FIGURE 1 illustrates the proportion of fluoroquinolone-resistant N gonorrhea from the CDC’s Gonococcal Isolate Surveillance Project (GISP) by year, from 1990 to 2006.
Resistance began to emerge first among gonorrhea isolates from men who have sex with men (MSM), and resistance rates among MSM continue to be higher than in heterosexual men (FIGURE 2).
Geographic trends. In 2000, the CDC recommended that quinolones should no longer be used to treat gonorrhea in persons who contracted the infection in Asia or the Pacific. In 2002, California was added to this list. In 2004, the recommendation against quinolone use was extended to all MSM in the US.
The new recommendation against general use is based on resistance surpassing 5% of total isolates.
FIGURE 1
Percentage of N gonorrhoeae isolates with intermediate resistance or resistance to ciprofloxacin
Data for 2006 are preliminary (January-June only).
* Demonstrating ciprofloxacin minimum inhibitory concentration of 0.125–0.500 mcg/mL.
† Demonstrating ciprofloxacin minimum inhibitory concentration of ≥1.0 mcg/ml.
Source: Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinalones no longer recommended for treatment of gonococcal infections.
MMWR Recomm Rep 2007; 56:332-336.
FIGURE 2
Progressive increase of fluoroquinolone resistance
Percent of isolates from the CDC Gonococcal Isolate Surveillance Project found to be resistant to fluoroquinalones, 2002 through June 2006
Source: GISP report. Centers for Disease Control and Prevention.
Sexually Transmitted Disease Surveillance 2005 Supplement, Gonoccal Isolate Surveillance Project (GISP) Annual Report 2005. Atlanta, Ga: US Department of Health and Human Services, Centers for Disease Control and Prevention, January 2007.
Ceftriaxone, the default treatment of choice
The loss of quinolones as a recommended gonorrhea treatment leaves only ceftriaxone, 125 mg intramuscularly (IM), as the only readily available treatment for urogenital, anorectal, and pharyngeal gonorrhea. Cefixime 400 mg as a single dose is also recommended, but is not currently available in tablet form in the US. It is available as a suspension with 100 mg per 5 cc.
Other options
Possible oral options include cefpodoxime 400 mg or cefuroxime axetil 1 g. However, neither has the official endorsement of the CDC, and neither appears effective against pharyngeal infection.
Spectinomycin 2 g intramuscularly is recommended for those with cephalosporin allergy—but, like cefixime, it is not currently available in the US, and it also is not considered effective against pharyngeal infection.
Azithromycin 2 g orally as a single dose is currently effective against gonorrhea and is an option for those with cephalosporin allergies. The CDC discourages its widespread use because of concerns about resistance.
New information regarding the availability of spectinomycin and cefixime can be obtained from local health departments or the CDC’s sexually transmitted diseases web site (www.cdc.gov/std).3
Recommended regimens for treatment of gonorrhea
| Uncomplicated gonococcal infections of the cervix, urethra, and rectum* |
| Recommended regimens† |
| Ceftriaxone 125 mg in a single IM dose |
| or |
| Cefixime‡ 400 mg in a single oral dose |
| plus |
| Treatment for chlamydia if chlamydial infection has not been ruled out |
| Uncomplicated gonococcal infections of the pharynx* |
| Recommended regimens |
| Ceftriaxone 125 mg in a single IM dose |
| plus |
| Treatment for chlamydia if chlamydial infection has not been ruled out |
| * For all adult and adolescent patients, regardless of travel history or sexual behavior. For those allergic to penicillins or cephalosporins, or for treatment of disseminated gonococcal infections, PID, and epididymitis, see www.cdc.gov/std/treatment. |
| † Alternative regimens: Spectinomycin 2 g in a single IM dose (not currently available in US) or cephalosporin single-dose regimens. |
| Other single-dose cephalosporin regimens that are considered alternative treatment regimens against uncomplicated urogenital and anorectal gonococcal infections include ceftizoxime 500 mg IM; or cefoxitin 2 g IM, administered with probenecid 1 g orally; or cefotaxime 500 mg IM. Some evidence indicates that cefpodoxime 400 mg and cefuroxime axetil 1 g might be oral alternatives. |
| ‡ 400 mg by suspension; tablets are no longer available in the US. |
| Source: www.cdc.gov/mmwr/PDF/rr/rr5511.pdf.2 |
Associated conditions
Treat for chlamydia if chlamydial infection is not ruled out
The CDC continues to recommend concurrent treatment for chlamydia for all persons who have gonorrhea, unless coinfection has been ruled out.
Therapies for chlamydia include azithromycin 1 g as a single dose or doxycycline 100 mg twice a day for 7 days.
Pelvic inflammatory disease and epididymitis
The treatment of both pelvic inflammatory disease (PID) and epididymitis include an option of ceftriaxone 250 mg IM plus doxycycline for either 7 days (for epididymitis) or 10 days (for PID). There are several parenteral options for PID and disseminated gonorrhea; these can be found on the CDC’s STD web site.3
Should you always retest to ensure a cure?
It is still not necessary to retest patients who have had the recommended treatments. However, patients with persistent symptoms or rapidly recurring symptoms should be retested by cultures so that drug-resistance patterns can be checked if gonorrhea is documented.
Retest for recurrence
Consider retesting all treated patients after 3 to 6 months, since anyone with a sexually transmitted infection is at risk of being reinfected.
Summary
The ongoing challenges with the evolving resistance patterns of gonorrhea illustrate the importance of physicians accurately diagnosing gonorrhea, treating with recommended regimens, reporting positive cases to the local public health department, and assisting with partner evaluation and treatment.
- The CDC no longer recommends the use of fluoroquinolones for the treatment of gonococcal infections and associated conditions such as pelvic inflammatory disease (PID).
- Consequently, only one class of drugs, the cephalosporins, is still recommended and available for the treatment of gonorrhea.
- The CDC now recommends ceftriaxone, 125 mg IM, in a single dose, as the preferred treatment.
- For patients with cephalosporin allergies, azithromycin, 2 g orally, as a single dose, remains an option. The CDC discourages widespread use, however, because of concerns about resistance.
The Centers for Disease Control and Prevention (CDC) recently released an update to its treatment guidelines for sexually transmitted diseases, stating that fluoroquinolones are no longer recommended for treatment of gonococcal infections.1 This change resulted from a progressive increase in the rate of resistance to quinolones among gonorrhea isolated from publicly funded treatment centers across the country.
The new advisory applies to all quinolones previously recommended: ciprofloxacin, ofloxacin, and levofloxacin.
Epidemiology. Gonorrhea remains common in the United States, with nearly 340,000 cases reported in 2005. Since it is under-reported, estimates are that more than 600,000 cases occur each year.2
Neisseria gonorrhoeae causes infection of the cervix, urethra, rectum, pharynx, and adnexa. It can also cause disseminated disease that can affect joints, heart, and the meninges.
Tracking the spread of resistant cases
Since the early 1990s, fluoroquinolones have been one of the recommended treatments for gonorrhea because of their availability as effective, single-dose oral regimens. Fluoroquinolone-resistant N gonorrhea began to emerge at the end of the century and has progressed rapidly since. FIGURE 1 illustrates the proportion of fluoroquinolone-resistant N gonorrhea from the CDC’s Gonococcal Isolate Surveillance Project (GISP) by year, from 1990 to 2006.
Resistance began to emerge first among gonorrhea isolates from men who have sex with men (MSM), and resistance rates among MSM continue to be higher than in heterosexual men (FIGURE 2).
Geographic trends. In 2000, the CDC recommended that quinolones should no longer be used to treat gonorrhea in persons who contracted the infection in Asia or the Pacific. In 2002, California was added to this list. In 2004, the recommendation against quinolone use was extended to all MSM in the US.
The new recommendation against general use is based on resistance surpassing 5% of total isolates.
FIGURE 1
Percentage of N gonorrhoeae isolates with intermediate resistance or resistance to ciprofloxacin
Data for 2006 are preliminary (January-June only).
* Demonstrating ciprofloxacin minimum inhibitory concentration of 0.125–0.500 mcg/mL.
† Demonstrating ciprofloxacin minimum inhibitory concentration of ≥1.0 mcg/ml.
Source: Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinalones no longer recommended for treatment of gonococcal infections.
MMWR Recomm Rep 2007; 56:332-336.
FIGURE 2
Progressive increase of fluoroquinolone resistance
Percent of isolates from the CDC Gonococcal Isolate Surveillance Project found to be resistant to fluoroquinalones, 2002 through June 2006
Source: GISP report. Centers for Disease Control and Prevention.
Sexually Transmitted Disease Surveillance 2005 Supplement, Gonoccal Isolate Surveillance Project (GISP) Annual Report 2005. Atlanta, Ga: US Department of Health and Human Services, Centers for Disease Control and Prevention, January 2007.
Ceftriaxone, the default treatment of choice
The loss of quinolones as a recommended gonorrhea treatment leaves only ceftriaxone, 125 mg intramuscularly (IM), as the only readily available treatment for urogenital, anorectal, and pharyngeal gonorrhea. Cefixime 400 mg as a single dose is also recommended, but is not currently available in tablet form in the US. It is available as a suspension with 100 mg per 5 cc.
Other options
Possible oral options include cefpodoxime 400 mg or cefuroxime axetil 1 g. However, neither has the official endorsement of the CDC, and neither appears effective against pharyngeal infection.
Spectinomycin 2 g intramuscularly is recommended for those with cephalosporin allergy—but, like cefixime, it is not currently available in the US, and it also is not considered effective against pharyngeal infection.
Azithromycin 2 g orally as a single dose is currently effective against gonorrhea and is an option for those with cephalosporin allergies. The CDC discourages its widespread use because of concerns about resistance.
New information regarding the availability of spectinomycin and cefixime can be obtained from local health departments or the CDC’s sexually transmitted diseases web site (www.cdc.gov/std).3
Recommended regimens for treatment of gonorrhea
| Uncomplicated gonococcal infections of the cervix, urethra, and rectum* |
| Recommended regimens† |
| Ceftriaxone 125 mg in a single IM dose |
| or |
| Cefixime‡ 400 mg in a single oral dose |
| plus |
| Treatment for chlamydia if chlamydial infection has not been ruled out |
| Uncomplicated gonococcal infections of the pharynx* |
| Recommended regimens |
| Ceftriaxone 125 mg in a single IM dose |
| plus |
| Treatment for chlamydia if chlamydial infection has not been ruled out |
| * For all adult and adolescent patients, regardless of travel history or sexual behavior. For those allergic to penicillins or cephalosporins, or for treatment of disseminated gonococcal infections, PID, and epididymitis, see www.cdc.gov/std/treatment. |
| † Alternative regimens: Spectinomycin 2 g in a single IM dose (not currently available in US) or cephalosporin single-dose regimens. |
| Other single-dose cephalosporin regimens that are considered alternative treatment regimens against uncomplicated urogenital and anorectal gonococcal infections include ceftizoxime 500 mg IM; or cefoxitin 2 g IM, administered with probenecid 1 g orally; or cefotaxime 500 mg IM. Some evidence indicates that cefpodoxime 400 mg and cefuroxime axetil 1 g might be oral alternatives. |
| ‡ 400 mg by suspension; tablets are no longer available in the US. |
| Source: www.cdc.gov/mmwr/PDF/rr/rr5511.pdf.2 |
Associated conditions
Treat for chlamydia if chlamydial infection is not ruled out
The CDC continues to recommend concurrent treatment for chlamydia for all persons who have gonorrhea, unless coinfection has been ruled out.
Therapies for chlamydia include azithromycin 1 g as a single dose or doxycycline 100 mg twice a day for 7 days.
Pelvic inflammatory disease and epididymitis
The treatment of both pelvic inflammatory disease (PID) and epididymitis include an option of ceftriaxone 250 mg IM plus doxycycline for either 7 days (for epididymitis) or 10 days (for PID). There are several parenteral options for PID and disseminated gonorrhea; these can be found on the CDC’s STD web site.3
Should you always retest to ensure a cure?
It is still not necessary to retest patients who have had the recommended treatments. However, patients with persistent symptoms or rapidly recurring symptoms should be retested by cultures so that drug-resistance patterns can be checked if gonorrhea is documented.
Retest for recurrence
Consider retesting all treated patients after 3 to 6 months, since anyone with a sexually transmitted infection is at risk of being reinfected.
Summary
The ongoing challenges with the evolving resistance patterns of gonorrhea illustrate the importance of physicians accurately diagnosing gonorrhea, treating with recommended regimens, reporting positive cases to the local public health department, and assisting with partner evaluation and treatment.
1. CDC. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep 2007;56:332-336.Available at: www.cdc.gov/mmwr/pdf/wk/mm5614.pdf. Accessed on June 15, 2007.
2. CDC. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep 2006;55(RR-11).-Available at www.cdc.gov/mmwr/PDF/rr/rr5511.pdf. Accessed on June 15, 2007.
3. Updated recommended treatment regimens for gonococcal infections and associated conditions—United States, April 2007. Available at: www.cdc.gov/std/treatment/2006/updated-regimens.htm. Accessed on June 15, 2007.
1. CDC. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep 2007;56:332-336.Available at: www.cdc.gov/mmwr/pdf/wk/mm5614.pdf. Accessed on June 15, 2007.
2. CDC. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep 2006;55(RR-11).-Available at www.cdc.gov/mmwr/PDF/rr/rr5511.pdf. Accessed on June 15, 2007.
3. Updated recommended treatment regimens for gonococcal infections and associated conditions—United States, April 2007. Available at: www.cdc.gov/std/treatment/2006/updated-regimens.htm. Accessed on June 15, 2007.
Curbing nocturnal binges in sleep-related eating disorder
Ms. G, age 39, has a body mass index (BMI) >35 kg/m2 and is pursuing bariatric surgery to treat obesity. She is frustrated with dieting and describes a decade of unconscious nocturnal eating, including peanut butter and uncooked spaghetti.
This behavior began after her divorce 10 years ago. Initially she had partial recall of the nocturnal binges, but now describes full amnesia. Treatment for a depressive episode did not control her nocturnal eating.
Sleep-related eating disorder (SRED) can be associated with disrupted sleep, weight gain, and major chronic morbidity. In SRED—involuntary eating while asleep, with partial or complete amnesia—the normal suppression of eating during the sleep period is disinhibited. The disorder can be idiopathic, associated with medication use, or linked to other sleep disorders such as somnambulism (sleepwalking), restless legs syndrome (RLS), periodic limb movement disorder (PLMD), or obstructive sleep apnea (OSA).
SRED is more common in women than men; it usually begins in the third decade of life but can begin in childhood or middle age. About one-half of SRED patients also have a psychiatric illness, usually a mood disorder. Unremitting SRED may lead to psychopathology, as the onset of sleep-related eating usually precedes the onset of a psychiatric disorder by years.
SRED often is unrecognized, but it can be effectively identified and treated. This article examines how to:
- distinguish SRED from nocturnal eating syndrome (NES) and other disorders
- identify precipitating causes
- select effective pharmacologic therapy.
Because hormones that regulate appetite, food intake, and body weight also play a role in sleep regulation, patients with eating disorders often have associated sleep disorders. For example, obesity is related to obstructive sleep apnea (OSA)—weight gain is a risk factor for OSA, and weight loss often is an effective treatment.1 Moreover, patients with anorexia nervosa frequently demonstrate sleep initiation and maintenance insomnia.2
Conversely, epidemiologic studies have demonstrated that sleep duration is inversely correlated with body mass index. In particular, individuals with shorter sleep times are more likely to be overweight.3 The nature of this association is unclear; however, hormones that normally regulate appetite are disrupted in patients with sleep deprivation. For instance, leptin is an appetite suppressant that is normally released from adipocytes during sleep, so sleep deprivation may promote hunger by restricting its secretion.4
Differentiating SRED from NES
Eating and sleeping—and disorders of each—are closely linked (Box).1-4 SRED and night eating syndrome (NES) are 2 principal night eating disorders. SRED is characterized by inappropriately consuming food after falling asleep,5 whereas NES is characterized by hyperphagia after the evening meal, either before bedtime or after fully awakening during the night.6
To meet diagnostic criteria for SRED, patients must experience involuntary nocturnal eating and demonstrate at least 1 other symptom, such as:
- eating peculiar, inedible, or toxic substances
- engaging in dangerous behavior while preparing food (Table 1).
Level of consciousness. In both SRED and NES, patients demonstrate morning anorexia. However, patients with NES report being awake and alert during their nocturnal eating, whereas patients with SRED describe partial or complete amnesia. SRED patients with partial awareness often describe the experience as being involuntary, dream-like, and “out-of-control.” Interestingly, hunger is notably absent during most episodes in which patients have at least partial awareness.
Typically, patients cannot be awakened easily from a sleep-eating episode. In this regard, SRED resembles sleepwalking. Sleepwalking without eating often precedes SRED, but once eating develops it often becomes the predominant or exclusive sleepwalking behavior. This pattern has led many researchers to consider SRED a “sleepwalking variant disorder.”
Eating episodes in SRED are often characterized by binge eating, and many patients describe at least one episode per night.5 They usually eat high-calorie foods. The spectrum of cuisine is broad, ranging from dry cereal to hot meals that require more than 30 minutes to prepare. Patients treated at our sleep center report eating foods that are high in simple carbohydrates, fats, and—to a lesser extent—protein. Peanut butter—a preferred item—can lead to near-choking episodes when patients fall asleep with peanut butter in their mouths and wake up gasping for air. Alcohol consumption is rare.
SRED episodes can be hazardous, with risks of drinking or eating excessively hot liquids or solids, choking on thick foods, or receiving lacerations while using knives to prepare food. Patients may consume foods to which they are allergic or eat inedible or even toxic substances (Table 2).5,7-9
Table 1
Differences between expressive and supportive psychotherapy
|
| Source: International classification of sleep disorders: diagnostic and coding manual, 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005:174-5. |
Table 2
Typical foods consumed while sleep-eating
| Simple | Peanut butter, dry cereal, candy, bread/toast |
| Peculiar | Uncooked spaghetti, sugar/ salt sandwiches, cat/dog food, frozen food |
| Inedible/toxic | Egg shells, coffee beans, sunfl ower shells, buttered cigarettes, glue/cleaning solutions |
Chain of consequences
Repeated nocturnal binge eating episodes can have multiple adverse health effects.5,7 Patients often wake up with painful abdominal distention. Weight gain and subsequent increased BMI may compromise the control of medical complications such as diabetes mellitus, hyperlipidemia, hypertriglyceridemia, hypertension, OSA, and cardiovascular disease. Patients with SRED also report dental problems such as tooth chipping and increased incidence of caries.
Failure to control nocturnal eating can lead to secondary depressive disorders related to excessive weight gain. Moreover, SRED patients’ nighttime behaviors may disrupt their bed partners’ sleep and cause interpersonal and marital problems.
Untreated SRED is usually unremitting. In our experience, most patients describe suffering for years before seeking treatment. Many report that their symptoms have been dismissed by other physicians or wrongly attributed to a mood disorder. Not surprisingly, patients in obesity clinics and eating disorder groups regularly report SRED.
Multiple causes
Medication-induced. The commonly prescribed hypnotic zolpidem can induce SRED.10,11 Sporadic cases of SRED have been reported with other psychotropics, such as tricyclic antidepressants, anticholinergics, lithium, triazolam, olanzapine, and risperidone.12
Life stressors. For a subgroup of patients, such as Ms. G, a life stressor such as a death or divorce precipitates the disorder. Others report SRED onset with cessation of cigarette smoking, ethanol abuse, or amphetamine/cocaine abuse.5,7 Thus, SRED can be viewed as a “final common pathway disorder” that can be triggered by a variety of sleep disorders, medical-neurologic disorders, medications, and stress. It also can be idiopathic (Table 3).12
Table 3
Sleep disorders and medications associated with SRED
| Sleep disorders | Sleepwalking, obstructive sleep apnea, restless legs syndrome, circadian rhythm disorder, narcolepsy |
| Medications | Zolpidem, lithium, triazolam, olanzapine, risperidone, anticholinergics |
| Source: References 5,7-9 | |
CASE CONTINUED: Reaching a diagnosis
Ms. G’s psychiatrist refers her to an accredited sleep center, where she is instructed to keep a diary of her eating and sleeping behaviors for 2 weeks. She returns to the center and undergoes overnight video polysomnography (PSG). During this test, Ms. G demonstrates recurrent confusional arousals arising from non-rapid eye movement sleep (NREM) and eating binges while asleep with no subsequent recall.
Sleep studies aid diagnosis
Diagnosing a patient with SRED requires taking a diligent history to:
- characterize nocturnal eating
- identify predisposing or precipitating factors
- differentiate the behavior from other sleep-related or eating disorders.
At our sleep center, we frequently ask patients and their families to track the patient’s sleep and nocturnal eating behavior 2 weeks before a clinic visit. These diaries help document sleep and eating patterns and assess the patient’s awareness and subsequent recall.
As described above, recurrent nighttime eating with full awareness and control would be best characterized as NES. How-ever, there is some debate as to the extent that SRED can manifest with substantial or full alertness and subsequent recall.13 SRED and NES might be at opposite poles of a pathology continuum, in which a sub-group of patients falls into a “gray area” of mixed SRED/NES features.13,14
Self-induced emesis or other purging behavior usually is not seen in SRED. If a patient presents with this symptom, consider an alternate diagnosis such as bulimia nervosa. A patient with SRED may be diagnosed with a coexisting eating disorder, however, as long as the diagnostic features of the eating disorder are not associated with the nocturnal episodes of SRED.
Finally, at least 2 reports exist of a nocturnal dissociative disorder, in which a recurrent nocturnal “eating personality” emerges.7
Sleep laboratory testing. Overnight video PSG—recording the biophysiologic changes that occur during sleep—often is valuable in characterizing SRED and identifying other sleep disorders. To facilitate the eating behavior, we ask patients to bring to the sleep laboratory commonly consumed food to be placed within reach of their bed.
If the patient does eat during the study, we identify the sleep state (non-REM sleep or REM sleep) that precipitates the behavior. Confusional arousals, both with and without eating, usually arise from nonREM sleep.
In patients with SRED, PSG often helps to identify other sleep abnormalities that trigger arousal. Reversible disorders such as RLS, PLMD, and OSA or more subtle sleep disordered breathing are especially important to identify so they can be properly treated. Recently, PSG found rhythmic masticatory muscle activity in stages 1 and 2 non-REM sleep in 29 of 35 patients diagnosed with SRED.15
CASE CONTINUED: Adding medication
After diagnosing SRED, Ms. G’s psychiatrist initiates the anticonvulsant topiramate, 25 mg at bedtime. After the dose is gradually increased in 25-mg increments to 100 mg at bedtime, Ms. G achieves full control of recurrent nocturnal eating. She loses 40 pounds within the next 6 months.
Pharmacotherapy
SRED is treatable and a reversible cause of obesity. The choice of medication depends on:
- which form of SRED the patient exhibits (drug-induced or idiopathic)
- whether the patient has treatable comorbid conditions.
Temazepam. Switch patients whose SRED is triggered by zolpidem or another hypnotic to a different agent. We have had excellent success with temazepam, 15 to 30 mg at bedtime.
Topiramate. For idiopathic SRED or the sleepwalking variant of SRED, trials from 2 academic institutions suggest that off-label use of topiramate, 25 to 150 mg at bedtime, may be the treatment of choice.16-18
Start topiramate at 25 mg, and increase in 25-mg increments every 5 to 7 days until the night eating episodes are eliminated. Paresthesias, visual symptoms, and (rarely) renal calculus are reported side effects.
Other medications. Other agents that have shown at least some benefit in patients with SRED include dopaminergic agonists, opiates, and clonazepam.14 Patients with SRED and a history of chemical dependency may respond to combined levodopa, trazodone, and bupropion (dopaminergic/noradrenergic antidepressant) therapy at bedtime.19 Also focus treatment on any coexisting sleep disorder, such as RLS or OSA.
Related resources
- American Obesity Association. www.obesity.org.
- American Insomnia Association. www.americaninsomniaassociation.org.
- Schenck CH. Paradox lost: midnight in the battleground of sleep and dreams. Minneapolis, MN: Extreme-Nights, LLC; 2006.
Drug brand names
- Bupropion • Wellbutrin
- Clonazepam • Klonopin
- Levodopa/carbidopa • Sinemet
- Lithium • Eskalith, Lithobid
- Olanzapine • Zyprexa
- Risperidone • Risperdal
- Temazepam • Restoril
- Topiramate • Topamax
- Trazodone • Desyrel
- Triazolam • Halcion
- Zolpidem • Ambien
Disclosures
Drs. Howell and Schenck report no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products.
Dr. Crow has received grants or research support from Bristol-Myers Squibb and Pfizer Inc. and served as a consultant to Eli Lilly and Company.
1. Flemons WW. Obstructive sleep apnea. N Engl J Med 2002;347:498-504.
2. Levy AB, Dixon KN, Schmidt H. Sleep architecture in anorexia nervosa and bulimia. Biol Psychiatry 1988;23:99-101.
3. Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep 2005;28:1289-96.
4. Mullington JM, Chan JL, Van Dongen HP, et al. Sleep loss reduces the diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol 2003;15:851-4.
5. International classification of sleep disorders: diagnostic and coding manual, 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005.
6. Rogers NL, Dinges DF, Allison KC, et al. Assessment of sleep in women with night eating syndrome. Sleep 2006;29:814-19.
7. Schenck CH, Mahowald MW. Review of nocturnal sleep-related eating disorders. Int J Eat Disord 1994;15:343-56.
8. Winkelman JW. Clinical and polysomnographic features of sleep-related eating disorder. J Clin Psychiatry 1998;59:14-9.
9. Schenck CH. Paradox lost: midnight in the battleground of sleep and dreams. Minneapolis, MN: Extreme-Nights, LLC; 2006.
10. Morgenthaler TI, Silber MH. Amnestic sleep-related eating disorder associated with zolpidem. Sleep Med 2002;3:323-7.
11. Schenck CH, Connoy DA, Castellanos M, et al. Zolpidem-induced sleep-related eating disorder (SRED) in 19 patients. Sleep 2005;28:A259.-
12. Schenck CH, Hurwitz TD, O’Connor KA, Mahowald MW. Additional categories of sleep-related eating disorders and the current status of treatment. Sleep 1993;16:457-66.
13. Winkelman JW. Sleep-related eating disorder and night eating syndrome: sleep disorders, eating disorders, or both? Sleep 2006;29:876-7.
14. Schenck CH. Journal search and commentary: a study of circadian eating and sleeping patterns in night eating syndrome (NES) points the way to future studies on NES and sleep-related eating disorder. Sleep Medicine 2006;7:653-6.
15. Vetrugno R, Manconi M, Ferini-Strambi L, et al. Nocturnal eating: sleep-related eating disorder or night eating syndrome? A videopolysomnographic study. Sleep 2006;29:949-54.
16. Winkelman JW. Treatment of nocturnal eating syndrome and sleep-related eating disorder with topiramate. Sleep Medicine 2003;4:243-6.
17. Schenck CH, Mahowald MW. Topiramate therapy of sleep related eating disorder. Sleep 2006;29:A268.-
18. Winkelman JW. Efficacy and tolerability of topiramate in the treatment of sleep related eating disorders: an open-label, retrospective case series. J Clin Psychiatry In press.
19. Schenck CH, Mahowald MW. Combined bupropionlevodopa-trazodone therapy of sleep-related eating and sleep disruption in two adults with chemical dependency. Sleep 2000;23:587-8.
Ms. G, age 39, has a body mass index (BMI) >35 kg/m2 and is pursuing bariatric surgery to treat obesity. She is frustrated with dieting and describes a decade of unconscious nocturnal eating, including peanut butter and uncooked spaghetti.
This behavior began after her divorce 10 years ago. Initially she had partial recall of the nocturnal binges, but now describes full amnesia. Treatment for a depressive episode did not control her nocturnal eating.
Sleep-related eating disorder (SRED) can be associated with disrupted sleep, weight gain, and major chronic morbidity. In SRED—involuntary eating while asleep, with partial or complete amnesia—the normal suppression of eating during the sleep period is disinhibited. The disorder can be idiopathic, associated with medication use, or linked to other sleep disorders such as somnambulism (sleepwalking), restless legs syndrome (RLS), periodic limb movement disorder (PLMD), or obstructive sleep apnea (OSA).
SRED is more common in women than men; it usually begins in the third decade of life but can begin in childhood or middle age. About one-half of SRED patients also have a psychiatric illness, usually a mood disorder. Unremitting SRED may lead to psychopathology, as the onset of sleep-related eating usually precedes the onset of a psychiatric disorder by years.
SRED often is unrecognized, but it can be effectively identified and treated. This article examines how to:
- distinguish SRED from nocturnal eating syndrome (NES) and other disorders
- identify precipitating causes
- select effective pharmacologic therapy.
Because hormones that regulate appetite, food intake, and body weight also play a role in sleep regulation, patients with eating disorders often have associated sleep disorders. For example, obesity is related to obstructive sleep apnea (OSA)—weight gain is a risk factor for OSA, and weight loss often is an effective treatment.1 Moreover, patients with anorexia nervosa frequently demonstrate sleep initiation and maintenance insomnia.2
Conversely, epidemiologic studies have demonstrated that sleep duration is inversely correlated with body mass index. In particular, individuals with shorter sleep times are more likely to be overweight.3 The nature of this association is unclear; however, hormones that normally regulate appetite are disrupted in patients with sleep deprivation. For instance, leptin is an appetite suppressant that is normally released from adipocytes during sleep, so sleep deprivation may promote hunger by restricting its secretion.4
Differentiating SRED from NES
Eating and sleeping—and disorders of each—are closely linked (Box).1-4 SRED and night eating syndrome (NES) are 2 principal night eating disorders. SRED is characterized by inappropriately consuming food after falling asleep,5 whereas NES is characterized by hyperphagia after the evening meal, either before bedtime or after fully awakening during the night.6
To meet diagnostic criteria for SRED, patients must experience involuntary nocturnal eating and demonstrate at least 1 other symptom, such as:
- eating peculiar, inedible, or toxic substances
- engaging in dangerous behavior while preparing food (Table 1).
Level of consciousness. In both SRED and NES, patients demonstrate morning anorexia. However, patients with NES report being awake and alert during their nocturnal eating, whereas patients with SRED describe partial or complete amnesia. SRED patients with partial awareness often describe the experience as being involuntary, dream-like, and “out-of-control.” Interestingly, hunger is notably absent during most episodes in which patients have at least partial awareness.
Typically, patients cannot be awakened easily from a sleep-eating episode. In this regard, SRED resembles sleepwalking. Sleepwalking without eating often precedes SRED, but once eating develops it often becomes the predominant or exclusive sleepwalking behavior. This pattern has led many researchers to consider SRED a “sleepwalking variant disorder.”
Eating episodes in SRED are often characterized by binge eating, and many patients describe at least one episode per night.5 They usually eat high-calorie foods. The spectrum of cuisine is broad, ranging from dry cereal to hot meals that require more than 30 minutes to prepare. Patients treated at our sleep center report eating foods that are high in simple carbohydrates, fats, and—to a lesser extent—protein. Peanut butter—a preferred item—can lead to near-choking episodes when patients fall asleep with peanut butter in their mouths and wake up gasping for air. Alcohol consumption is rare.
SRED episodes can be hazardous, with risks of drinking or eating excessively hot liquids or solids, choking on thick foods, or receiving lacerations while using knives to prepare food. Patients may consume foods to which they are allergic or eat inedible or even toxic substances (Table 2).5,7-9
Table 1
Differences between expressive and supportive psychotherapy
|
| Source: International classification of sleep disorders: diagnostic and coding manual, 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005:174-5. |
Table 2
Typical foods consumed while sleep-eating
| Simple | Peanut butter, dry cereal, candy, bread/toast |
| Peculiar | Uncooked spaghetti, sugar/ salt sandwiches, cat/dog food, frozen food |
| Inedible/toxic | Egg shells, coffee beans, sunfl ower shells, buttered cigarettes, glue/cleaning solutions |
Chain of consequences
Repeated nocturnal binge eating episodes can have multiple adverse health effects.5,7 Patients often wake up with painful abdominal distention. Weight gain and subsequent increased BMI may compromise the control of medical complications such as diabetes mellitus, hyperlipidemia, hypertriglyceridemia, hypertension, OSA, and cardiovascular disease. Patients with SRED also report dental problems such as tooth chipping and increased incidence of caries.
Failure to control nocturnal eating can lead to secondary depressive disorders related to excessive weight gain. Moreover, SRED patients’ nighttime behaviors may disrupt their bed partners’ sleep and cause interpersonal and marital problems.
Untreated SRED is usually unremitting. In our experience, most patients describe suffering for years before seeking treatment. Many report that their symptoms have been dismissed by other physicians or wrongly attributed to a mood disorder. Not surprisingly, patients in obesity clinics and eating disorder groups regularly report SRED.
Multiple causes
Medication-induced. The commonly prescribed hypnotic zolpidem can induce SRED.10,11 Sporadic cases of SRED have been reported with other psychotropics, such as tricyclic antidepressants, anticholinergics, lithium, triazolam, olanzapine, and risperidone.12
Life stressors. For a subgroup of patients, such as Ms. G, a life stressor such as a death or divorce precipitates the disorder. Others report SRED onset with cessation of cigarette smoking, ethanol abuse, or amphetamine/cocaine abuse.5,7 Thus, SRED can be viewed as a “final common pathway disorder” that can be triggered by a variety of sleep disorders, medical-neurologic disorders, medications, and stress. It also can be idiopathic (Table 3).12
Table 3
Sleep disorders and medications associated with SRED
| Sleep disorders | Sleepwalking, obstructive sleep apnea, restless legs syndrome, circadian rhythm disorder, narcolepsy |
| Medications | Zolpidem, lithium, triazolam, olanzapine, risperidone, anticholinergics |
| Source: References 5,7-9 | |
CASE CONTINUED: Reaching a diagnosis
Ms. G’s psychiatrist refers her to an accredited sleep center, where she is instructed to keep a diary of her eating and sleeping behaviors for 2 weeks. She returns to the center and undergoes overnight video polysomnography (PSG). During this test, Ms. G demonstrates recurrent confusional arousals arising from non-rapid eye movement sleep (NREM) and eating binges while asleep with no subsequent recall.
Sleep studies aid diagnosis
Diagnosing a patient with SRED requires taking a diligent history to:
- characterize nocturnal eating
- identify predisposing or precipitating factors
- differentiate the behavior from other sleep-related or eating disorders.
At our sleep center, we frequently ask patients and their families to track the patient’s sleep and nocturnal eating behavior 2 weeks before a clinic visit. These diaries help document sleep and eating patterns and assess the patient’s awareness and subsequent recall.
As described above, recurrent nighttime eating with full awareness and control would be best characterized as NES. How-ever, there is some debate as to the extent that SRED can manifest with substantial or full alertness and subsequent recall.13 SRED and NES might be at opposite poles of a pathology continuum, in which a sub-group of patients falls into a “gray area” of mixed SRED/NES features.13,14
Self-induced emesis or other purging behavior usually is not seen in SRED. If a patient presents with this symptom, consider an alternate diagnosis such as bulimia nervosa. A patient with SRED may be diagnosed with a coexisting eating disorder, however, as long as the diagnostic features of the eating disorder are not associated with the nocturnal episodes of SRED.
Finally, at least 2 reports exist of a nocturnal dissociative disorder, in which a recurrent nocturnal “eating personality” emerges.7
Sleep laboratory testing. Overnight video PSG—recording the biophysiologic changes that occur during sleep—often is valuable in characterizing SRED and identifying other sleep disorders. To facilitate the eating behavior, we ask patients to bring to the sleep laboratory commonly consumed food to be placed within reach of their bed.
If the patient does eat during the study, we identify the sleep state (non-REM sleep or REM sleep) that precipitates the behavior. Confusional arousals, both with and without eating, usually arise from nonREM sleep.
In patients with SRED, PSG often helps to identify other sleep abnormalities that trigger arousal. Reversible disorders such as RLS, PLMD, and OSA or more subtle sleep disordered breathing are especially important to identify so they can be properly treated. Recently, PSG found rhythmic masticatory muscle activity in stages 1 and 2 non-REM sleep in 29 of 35 patients diagnosed with SRED.15
CASE CONTINUED: Adding medication
After diagnosing SRED, Ms. G’s psychiatrist initiates the anticonvulsant topiramate, 25 mg at bedtime. After the dose is gradually increased in 25-mg increments to 100 mg at bedtime, Ms. G achieves full control of recurrent nocturnal eating. She loses 40 pounds within the next 6 months.
Pharmacotherapy
SRED is treatable and a reversible cause of obesity. The choice of medication depends on:
- which form of SRED the patient exhibits (drug-induced or idiopathic)
- whether the patient has treatable comorbid conditions.
Temazepam. Switch patients whose SRED is triggered by zolpidem or another hypnotic to a different agent. We have had excellent success with temazepam, 15 to 30 mg at bedtime.
Topiramate. For idiopathic SRED or the sleepwalking variant of SRED, trials from 2 academic institutions suggest that off-label use of topiramate, 25 to 150 mg at bedtime, may be the treatment of choice.16-18
Start topiramate at 25 mg, and increase in 25-mg increments every 5 to 7 days until the night eating episodes are eliminated. Paresthesias, visual symptoms, and (rarely) renal calculus are reported side effects.
Other medications. Other agents that have shown at least some benefit in patients with SRED include dopaminergic agonists, opiates, and clonazepam.14 Patients with SRED and a history of chemical dependency may respond to combined levodopa, trazodone, and bupropion (dopaminergic/noradrenergic antidepressant) therapy at bedtime.19 Also focus treatment on any coexisting sleep disorder, such as RLS or OSA.
Related resources
- American Obesity Association. www.obesity.org.
- American Insomnia Association. www.americaninsomniaassociation.org.
- Schenck CH. Paradox lost: midnight in the battleground of sleep and dreams. Minneapolis, MN: Extreme-Nights, LLC; 2006.
Drug brand names
- Bupropion • Wellbutrin
- Clonazepam • Klonopin
- Levodopa/carbidopa • Sinemet
- Lithium • Eskalith, Lithobid
- Olanzapine • Zyprexa
- Risperidone • Risperdal
- Temazepam • Restoril
- Topiramate • Topamax
- Trazodone • Desyrel
- Triazolam • Halcion
- Zolpidem • Ambien
Disclosures
Drs. Howell and Schenck report no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products.
Dr. Crow has received grants or research support from Bristol-Myers Squibb and Pfizer Inc. and served as a consultant to Eli Lilly and Company.
Ms. G, age 39, has a body mass index (BMI) >35 kg/m2 and is pursuing bariatric surgery to treat obesity. She is frustrated with dieting and describes a decade of unconscious nocturnal eating, including peanut butter and uncooked spaghetti.
This behavior began after her divorce 10 years ago. Initially she had partial recall of the nocturnal binges, but now describes full amnesia. Treatment for a depressive episode did not control her nocturnal eating.
Sleep-related eating disorder (SRED) can be associated with disrupted sleep, weight gain, and major chronic morbidity. In SRED—involuntary eating while asleep, with partial or complete amnesia—the normal suppression of eating during the sleep period is disinhibited. The disorder can be idiopathic, associated with medication use, or linked to other sleep disorders such as somnambulism (sleepwalking), restless legs syndrome (RLS), periodic limb movement disorder (PLMD), or obstructive sleep apnea (OSA).
SRED is more common in women than men; it usually begins in the third decade of life but can begin in childhood or middle age. About one-half of SRED patients also have a psychiatric illness, usually a mood disorder. Unremitting SRED may lead to psychopathology, as the onset of sleep-related eating usually precedes the onset of a psychiatric disorder by years.
SRED often is unrecognized, but it can be effectively identified and treated. This article examines how to:
- distinguish SRED from nocturnal eating syndrome (NES) and other disorders
- identify precipitating causes
- select effective pharmacologic therapy.
Because hormones that regulate appetite, food intake, and body weight also play a role in sleep regulation, patients with eating disorders often have associated sleep disorders. For example, obesity is related to obstructive sleep apnea (OSA)—weight gain is a risk factor for OSA, and weight loss often is an effective treatment.1 Moreover, patients with anorexia nervosa frequently demonstrate sleep initiation and maintenance insomnia.2
Conversely, epidemiologic studies have demonstrated that sleep duration is inversely correlated with body mass index. In particular, individuals with shorter sleep times are more likely to be overweight.3 The nature of this association is unclear; however, hormones that normally regulate appetite are disrupted in patients with sleep deprivation. For instance, leptin is an appetite suppressant that is normally released from adipocytes during sleep, so sleep deprivation may promote hunger by restricting its secretion.4
Differentiating SRED from NES
Eating and sleeping—and disorders of each—are closely linked (Box).1-4 SRED and night eating syndrome (NES) are 2 principal night eating disorders. SRED is characterized by inappropriately consuming food after falling asleep,5 whereas NES is characterized by hyperphagia after the evening meal, either before bedtime or after fully awakening during the night.6
To meet diagnostic criteria for SRED, patients must experience involuntary nocturnal eating and demonstrate at least 1 other symptom, such as:
- eating peculiar, inedible, or toxic substances
- engaging in dangerous behavior while preparing food (Table 1).
Level of consciousness. In both SRED and NES, patients demonstrate morning anorexia. However, patients with NES report being awake and alert during their nocturnal eating, whereas patients with SRED describe partial or complete amnesia. SRED patients with partial awareness often describe the experience as being involuntary, dream-like, and “out-of-control.” Interestingly, hunger is notably absent during most episodes in which patients have at least partial awareness.
Typically, patients cannot be awakened easily from a sleep-eating episode. In this regard, SRED resembles sleepwalking. Sleepwalking without eating often precedes SRED, but once eating develops it often becomes the predominant or exclusive sleepwalking behavior. This pattern has led many researchers to consider SRED a “sleepwalking variant disorder.”
Eating episodes in SRED are often characterized by binge eating, and many patients describe at least one episode per night.5 They usually eat high-calorie foods. The spectrum of cuisine is broad, ranging from dry cereal to hot meals that require more than 30 minutes to prepare. Patients treated at our sleep center report eating foods that are high in simple carbohydrates, fats, and—to a lesser extent—protein. Peanut butter—a preferred item—can lead to near-choking episodes when patients fall asleep with peanut butter in their mouths and wake up gasping for air. Alcohol consumption is rare.
SRED episodes can be hazardous, with risks of drinking or eating excessively hot liquids or solids, choking on thick foods, or receiving lacerations while using knives to prepare food. Patients may consume foods to which they are allergic or eat inedible or even toxic substances (Table 2).5,7-9
Table 1
Differences between expressive and supportive psychotherapy
|
| Source: International classification of sleep disorders: diagnostic and coding manual, 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005:174-5. |
Table 2
Typical foods consumed while sleep-eating
| Simple | Peanut butter, dry cereal, candy, bread/toast |
| Peculiar | Uncooked spaghetti, sugar/ salt sandwiches, cat/dog food, frozen food |
| Inedible/toxic | Egg shells, coffee beans, sunfl ower shells, buttered cigarettes, glue/cleaning solutions |
Chain of consequences
Repeated nocturnal binge eating episodes can have multiple adverse health effects.5,7 Patients often wake up with painful abdominal distention. Weight gain and subsequent increased BMI may compromise the control of medical complications such as diabetes mellitus, hyperlipidemia, hypertriglyceridemia, hypertension, OSA, and cardiovascular disease. Patients with SRED also report dental problems such as tooth chipping and increased incidence of caries.
Failure to control nocturnal eating can lead to secondary depressive disorders related to excessive weight gain. Moreover, SRED patients’ nighttime behaviors may disrupt their bed partners’ sleep and cause interpersonal and marital problems.
Untreated SRED is usually unremitting. In our experience, most patients describe suffering for years before seeking treatment. Many report that their symptoms have been dismissed by other physicians or wrongly attributed to a mood disorder. Not surprisingly, patients in obesity clinics and eating disorder groups regularly report SRED.
Multiple causes
Medication-induced. The commonly prescribed hypnotic zolpidem can induce SRED.10,11 Sporadic cases of SRED have been reported with other psychotropics, such as tricyclic antidepressants, anticholinergics, lithium, triazolam, olanzapine, and risperidone.12
Life stressors. For a subgroup of patients, such as Ms. G, a life stressor such as a death or divorce precipitates the disorder. Others report SRED onset with cessation of cigarette smoking, ethanol abuse, or amphetamine/cocaine abuse.5,7 Thus, SRED can be viewed as a “final common pathway disorder” that can be triggered by a variety of sleep disorders, medical-neurologic disorders, medications, and stress. It also can be idiopathic (Table 3).12
Table 3
Sleep disorders and medications associated with SRED
| Sleep disorders | Sleepwalking, obstructive sleep apnea, restless legs syndrome, circadian rhythm disorder, narcolepsy |
| Medications | Zolpidem, lithium, triazolam, olanzapine, risperidone, anticholinergics |
| Source: References 5,7-9 | |
CASE CONTINUED: Reaching a diagnosis
Ms. G’s psychiatrist refers her to an accredited sleep center, where she is instructed to keep a diary of her eating and sleeping behaviors for 2 weeks. She returns to the center and undergoes overnight video polysomnography (PSG). During this test, Ms. G demonstrates recurrent confusional arousals arising from non-rapid eye movement sleep (NREM) and eating binges while asleep with no subsequent recall.
Sleep studies aid diagnosis
Diagnosing a patient with SRED requires taking a diligent history to:
- characterize nocturnal eating
- identify predisposing or precipitating factors
- differentiate the behavior from other sleep-related or eating disorders.
At our sleep center, we frequently ask patients and their families to track the patient’s sleep and nocturnal eating behavior 2 weeks before a clinic visit. These diaries help document sleep and eating patterns and assess the patient’s awareness and subsequent recall.
As described above, recurrent nighttime eating with full awareness and control would be best characterized as NES. How-ever, there is some debate as to the extent that SRED can manifest with substantial or full alertness and subsequent recall.13 SRED and NES might be at opposite poles of a pathology continuum, in which a sub-group of patients falls into a “gray area” of mixed SRED/NES features.13,14
Self-induced emesis or other purging behavior usually is not seen in SRED. If a patient presents with this symptom, consider an alternate diagnosis such as bulimia nervosa. A patient with SRED may be diagnosed with a coexisting eating disorder, however, as long as the diagnostic features of the eating disorder are not associated with the nocturnal episodes of SRED.
Finally, at least 2 reports exist of a nocturnal dissociative disorder, in which a recurrent nocturnal “eating personality” emerges.7
Sleep laboratory testing. Overnight video PSG—recording the biophysiologic changes that occur during sleep—often is valuable in characterizing SRED and identifying other sleep disorders. To facilitate the eating behavior, we ask patients to bring to the sleep laboratory commonly consumed food to be placed within reach of their bed.
If the patient does eat during the study, we identify the sleep state (non-REM sleep or REM sleep) that precipitates the behavior. Confusional arousals, both with and without eating, usually arise from nonREM sleep.
In patients with SRED, PSG often helps to identify other sleep abnormalities that trigger arousal. Reversible disorders such as RLS, PLMD, and OSA or more subtle sleep disordered breathing are especially important to identify so they can be properly treated. Recently, PSG found rhythmic masticatory muscle activity in stages 1 and 2 non-REM sleep in 29 of 35 patients diagnosed with SRED.15
CASE CONTINUED: Adding medication
After diagnosing SRED, Ms. G’s psychiatrist initiates the anticonvulsant topiramate, 25 mg at bedtime. After the dose is gradually increased in 25-mg increments to 100 mg at bedtime, Ms. G achieves full control of recurrent nocturnal eating. She loses 40 pounds within the next 6 months.
Pharmacotherapy
SRED is treatable and a reversible cause of obesity. The choice of medication depends on:
- which form of SRED the patient exhibits (drug-induced or idiopathic)
- whether the patient has treatable comorbid conditions.
Temazepam. Switch patients whose SRED is triggered by zolpidem or another hypnotic to a different agent. We have had excellent success with temazepam, 15 to 30 mg at bedtime.
Topiramate. For idiopathic SRED or the sleepwalking variant of SRED, trials from 2 academic institutions suggest that off-label use of topiramate, 25 to 150 mg at bedtime, may be the treatment of choice.16-18
Start topiramate at 25 mg, and increase in 25-mg increments every 5 to 7 days until the night eating episodes are eliminated. Paresthesias, visual symptoms, and (rarely) renal calculus are reported side effects.
Other medications. Other agents that have shown at least some benefit in patients with SRED include dopaminergic agonists, opiates, and clonazepam.14 Patients with SRED and a history of chemical dependency may respond to combined levodopa, trazodone, and bupropion (dopaminergic/noradrenergic antidepressant) therapy at bedtime.19 Also focus treatment on any coexisting sleep disorder, such as RLS or OSA.
Related resources
- American Obesity Association. www.obesity.org.
- American Insomnia Association. www.americaninsomniaassociation.org.
- Schenck CH. Paradox lost: midnight in the battleground of sleep and dreams. Minneapolis, MN: Extreme-Nights, LLC; 2006.
Drug brand names
- Bupropion • Wellbutrin
- Clonazepam • Klonopin
- Levodopa/carbidopa • Sinemet
- Lithium • Eskalith, Lithobid
- Olanzapine • Zyprexa
- Risperidone • Risperdal
- Temazepam • Restoril
- Topiramate • Topamax
- Trazodone • Desyrel
- Triazolam • Halcion
- Zolpidem • Ambien
Disclosures
Drs. Howell and Schenck report no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products.
Dr. Crow has received grants or research support from Bristol-Myers Squibb and Pfizer Inc. and served as a consultant to Eli Lilly and Company.
1. Flemons WW. Obstructive sleep apnea. N Engl J Med 2002;347:498-504.
2. Levy AB, Dixon KN, Schmidt H. Sleep architecture in anorexia nervosa and bulimia. Biol Psychiatry 1988;23:99-101.
3. Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep 2005;28:1289-96.
4. Mullington JM, Chan JL, Van Dongen HP, et al. Sleep loss reduces the diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol 2003;15:851-4.
5. International classification of sleep disorders: diagnostic and coding manual, 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005.
6. Rogers NL, Dinges DF, Allison KC, et al. Assessment of sleep in women with night eating syndrome. Sleep 2006;29:814-19.
7. Schenck CH, Mahowald MW. Review of nocturnal sleep-related eating disorders. Int J Eat Disord 1994;15:343-56.
8. Winkelman JW. Clinical and polysomnographic features of sleep-related eating disorder. J Clin Psychiatry 1998;59:14-9.
9. Schenck CH. Paradox lost: midnight in the battleground of sleep and dreams. Minneapolis, MN: Extreme-Nights, LLC; 2006.
10. Morgenthaler TI, Silber MH. Amnestic sleep-related eating disorder associated with zolpidem. Sleep Med 2002;3:323-7.
11. Schenck CH, Connoy DA, Castellanos M, et al. Zolpidem-induced sleep-related eating disorder (SRED) in 19 patients. Sleep 2005;28:A259.-
12. Schenck CH, Hurwitz TD, O’Connor KA, Mahowald MW. Additional categories of sleep-related eating disorders and the current status of treatment. Sleep 1993;16:457-66.
13. Winkelman JW. Sleep-related eating disorder and night eating syndrome: sleep disorders, eating disorders, or both? Sleep 2006;29:876-7.
14. Schenck CH. Journal search and commentary: a study of circadian eating and sleeping patterns in night eating syndrome (NES) points the way to future studies on NES and sleep-related eating disorder. Sleep Medicine 2006;7:653-6.
15. Vetrugno R, Manconi M, Ferini-Strambi L, et al. Nocturnal eating: sleep-related eating disorder or night eating syndrome? A videopolysomnographic study. Sleep 2006;29:949-54.
16. Winkelman JW. Treatment of nocturnal eating syndrome and sleep-related eating disorder with topiramate. Sleep Medicine 2003;4:243-6.
17. Schenck CH, Mahowald MW. Topiramate therapy of sleep related eating disorder. Sleep 2006;29:A268.-
18. Winkelman JW. Efficacy and tolerability of topiramate in the treatment of sleep related eating disorders: an open-label, retrospective case series. J Clin Psychiatry In press.
19. Schenck CH, Mahowald MW. Combined bupropionlevodopa-trazodone therapy of sleep-related eating and sleep disruption in two adults with chemical dependency. Sleep 2000;23:587-8.
1. Flemons WW. Obstructive sleep apnea. N Engl J Med 2002;347:498-504.
2. Levy AB, Dixon KN, Schmidt H. Sleep architecture in anorexia nervosa and bulimia. Biol Psychiatry 1988;23:99-101.
3. Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep 2005;28:1289-96.
4. Mullington JM, Chan JL, Van Dongen HP, et al. Sleep loss reduces the diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol 2003;15:851-4.
5. International classification of sleep disorders: diagnostic and coding manual, 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005.
6. Rogers NL, Dinges DF, Allison KC, et al. Assessment of sleep in women with night eating syndrome. Sleep 2006;29:814-19.
7. Schenck CH, Mahowald MW. Review of nocturnal sleep-related eating disorders. Int J Eat Disord 1994;15:343-56.
8. Winkelman JW. Clinical and polysomnographic features of sleep-related eating disorder. J Clin Psychiatry 1998;59:14-9.
9. Schenck CH. Paradox lost: midnight in the battleground of sleep and dreams. Minneapolis, MN: Extreme-Nights, LLC; 2006.
10. Morgenthaler TI, Silber MH. Amnestic sleep-related eating disorder associated with zolpidem. Sleep Med 2002;3:323-7.
11. Schenck CH, Connoy DA, Castellanos M, et al. Zolpidem-induced sleep-related eating disorder (SRED) in 19 patients. Sleep 2005;28:A259.-
12. Schenck CH, Hurwitz TD, O’Connor KA, Mahowald MW. Additional categories of sleep-related eating disorders and the current status of treatment. Sleep 1993;16:457-66.
13. Winkelman JW. Sleep-related eating disorder and night eating syndrome: sleep disorders, eating disorders, or both? Sleep 2006;29:876-7.
14. Schenck CH. Journal search and commentary: a study of circadian eating and sleeping patterns in night eating syndrome (NES) points the way to future studies on NES and sleep-related eating disorder. Sleep Medicine 2006;7:653-6.
15. Vetrugno R, Manconi M, Ferini-Strambi L, et al. Nocturnal eating: sleep-related eating disorder or night eating syndrome? A videopolysomnographic study. Sleep 2006;29:949-54.
16. Winkelman JW. Treatment of nocturnal eating syndrome and sleep-related eating disorder with topiramate. Sleep Medicine 2003;4:243-6.
17. Schenck CH, Mahowald MW. Topiramate therapy of sleep related eating disorder. Sleep 2006;29:A268.-
18. Winkelman JW. Efficacy and tolerability of topiramate in the treatment of sleep related eating disorders: an open-label, retrospective case series. J Clin Psychiatry In press.
19. Schenck CH, Mahowald MW. Combined bupropionlevodopa-trazodone therapy of sleep-related eating and sleep disruption in two adults with chemical dependency. Sleep 2000;23:587-8.
Severe tophaceous gout
A 55‐year‐old man was admitted to the hospital for amputation of multiple toes secondarily infected in the setting of severe tophaceous gout. He had been wheelchair bound for several years because of severe gouty arthritis. His medical history was remarkable for previous arthroscopies of his hands, knees, and Achilles tendons to remove uric acid deposits, in addition to multiple episodes of nephrolithiasis from uric acid stones. His family history was remarkable for severe, debilitating gout in multiple first‐degree relatives. On further examination he was noted to have severe tophaceous gouty involvement of numerous joints (arrows in Figs. 1 and 2). His uric acid level was 11.6 mg/dL despite receiving 900 mg of allopurinol daily. Because his creatinine was 1.7 mg/dL on admission, his dose of allopurinol was reduced. Renal ultrasound revealed multiple bilateral renal stones (arrows in Fig. 3).

He underwent surgery, and was subsequently transferred to a skilled nursing facility for wound care and physical therapy, where he recuperated uneventfully.
A 55‐year‐old man was admitted to the hospital for amputation of multiple toes secondarily infected in the setting of severe tophaceous gout. He had been wheelchair bound for several years because of severe gouty arthritis. His medical history was remarkable for previous arthroscopies of his hands, knees, and Achilles tendons to remove uric acid deposits, in addition to multiple episodes of nephrolithiasis from uric acid stones. His family history was remarkable for severe, debilitating gout in multiple first‐degree relatives. On further examination he was noted to have severe tophaceous gouty involvement of numerous joints (arrows in Figs. 1 and 2). His uric acid level was 11.6 mg/dL despite receiving 900 mg of allopurinol daily. Because his creatinine was 1.7 mg/dL on admission, his dose of allopurinol was reduced. Renal ultrasound revealed multiple bilateral renal stones (arrows in Fig. 3).



He underwent surgery, and was subsequently transferred to a skilled nursing facility for wound care and physical therapy, where he recuperated uneventfully.
A 55‐year‐old man was admitted to the hospital for amputation of multiple toes secondarily infected in the setting of severe tophaceous gout. He had been wheelchair bound for several years because of severe gouty arthritis. His medical history was remarkable for previous arthroscopies of his hands, knees, and Achilles tendons to remove uric acid deposits, in addition to multiple episodes of nephrolithiasis from uric acid stones. His family history was remarkable for severe, debilitating gout in multiple first‐degree relatives. On further examination he was noted to have severe tophaceous gouty involvement of numerous joints (arrows in Figs. 1 and 2). His uric acid level was 11.6 mg/dL despite receiving 900 mg of allopurinol daily. Because his creatinine was 1.7 mg/dL on admission, his dose of allopurinol was reduced. Renal ultrasound revealed multiple bilateral renal stones (arrows in Fig. 3).



He underwent surgery, and was subsequently transferred to a skilled nursing facility for wound care and physical therapy, where he recuperated uneventfully.
Clinical Conundrum
A 62‐year‐old man with psoriasis for more than 30 years presented to the emergency department with a scaly, pruritic rash involving his face, trunk, and extremities that he had had for the past 10 days. The rash was spreading and not responding to application of clobetasol ointment, which had helped his psoriasis in the past. He also reported mild pharyngitis, headache, and myalgias.
A patient with a chronic skin condition presenting with a new rash means the clinician must consider whether it is an alternative manifestation of the chronic disorder or a new illness. Psoriasis takes many forms including guttate psoriasis, which presents with small, droplike plaques and frequently follows respiratory infections (particularly those caused by Streptococcus). Well‐controlled psoriasis rarely transforms after 3 decades, so I would consider other conditions. The tempo of illness makes certain life‐threatening syndromes, including Stevens‐Johnson, toxic shock, and purpura fulminans, unlikely. An allergic reaction, atopic dermatitis, or medication reaction is possible. Infections, either systemic (eg, syphilis) or dermatologic (eg, scabies), should be considered. Photosensitivity could involve the sun‐exposed areas, such as the extremities and face. Seborrheic dermatitis can cause scaling lesions of the face and trunk but not the extremities. Vasculitis merits consideration, but dependent regions are typically affected more than the head. Mycosis fungoides or a paraneoplastic phenomenon could cause a diffuse rash in this age group.
The patient had diabetes mellitus, hypertension, diverticulosis, and depression. Three months earlier he had undergone surgical drainage of a perirectal abscess. His usual medications were lovastatin, paroxetine, insulin, hydrochlorothiazide, and lisinopril. Three weeks previously he had completed a 10‐day course of trimethoprim/sulfamethoxazole for an upper respiratory infection. Otherwise, he was taking no new medications. He was allergic to penicillin. He denied substance abuse, recent travel, or risk factors for human immunodeficiency virus (HIV) infection. He worked as an automobile painter, lived with his wife, and had a pet dog.
Physical examination revealed a well‐appearing man with normal vital signs. His skin had well‐defined circumscribed pink plaques, mostly 1‐2 cm in size, with thick, silvery scales in the ears and on the dorsal and ventral arms and legs, chest, back, face, and scalp. There were no pustules or other signs of infection (Figs. 1and 2). The nails exhibited distal onycholysis, oil spots, and rare pits. His posterior pharynx was mildly erythematous. The results of cardiovascular, pulmonary, and abdominal examinations were normal.

Although other scaling skin conditions such as eczema, irritant dermatitis, or malignancy remain possible, his rash is most consistent with widespread psoriasis. I would consider immunological changes that may have caused a remarkably altered and more severe expression of his chronic disease, for example, recent steroid therapy or HIV infection. The company a rash keeps helps frame the differential diagnosis. Based on the patient's well appearance, the time course, his minimal systemic symptoms, and the appearance of the rash, my leading considerations are psoriasis or an allergic dermatitis. Cutaneous T‐cell malignancy, with its indolent and sometimes protean manifestations, remains possible in a patient of his age. I would now consult a dermatologist for 3 reasons: this patient has a chronic disease that I do not manage beyond basic treatments (eg, topical steroids), he has an undiagnosed illness with substantial dermatologic manifestations, and he may need a skin biopsy for definitive diagnosis.
The dermatology team diagnosed a guttate psoriasis flare, possibly associated with streptococcal pharyngitis. The differential diagnosis included secondary syphilis, although the team believed this was less likely. The dermatology team recommended obtaining a throat culture, streptozyme assay, and rapid plasma reagin and prescribed oral erythromycin and topical steroid ointment under a sauna suit.
I would follow his response to the prescribed steroid treatments. If the patient's course deviates from the dermatologists' expectations, I would request a skin biopsy and undertake further evaluations in search of an underlying systemic disease.
The patient followed up in the dermatology clinic 3 weeks later. His rash had worsened, and he had developed patchy alopecia and progressive edema of the face, ears, and eyes. He denied mouth or tongue swelling, difficulty breathing, or hives. The streptozyme assay was positive, but the other laboratory test results were negative.
The dermatology team diagnosed a severely inflammatory psoriasis flare and prescribed an oral retinoid, acitretin, and referred him for ultraviolet light therapy. He was unable to travel for phototherapy, and acitretin was discontinued after 1 week because of elevated serum transaminase levels. The dermatologists then prescribed oral cyclosporine.
The progression of disease despite standard treatment suggests a nonpsoriatic condition. Although medications could cause the abnormal liver tests, so could another underlying illness that involves the liver. An infiltrative disorder of the skin with hair follicle destruction and local lymphedema could explain both alopecia and facial edema.
I am unable account for his clinical features with a single disease, so the differential remains broad, including severe psoriasis, an infiltrating cutaneous malignancy, or a toxic exposure. Arsenic poisoning causes hyperkeratotic skin lesions, although he lacks the associated gastrointestinal and neurological symptoms. I would not have added the potentially toxic cyclosporine.
When he returned to dermatology clinic 1 week later, his rash and facial swelling had worsened. He also reported muscle and joint aches, fatigue, lightheadedness, anorexia, nausea, abdominal pain, diarrhea, and dyspnea on exertion. He denied fever, chills, and night sweats.
He appeared ill and used a cane to arise and walk. His vital signs and oxygen saturation were normal. He had marked swelling of his face, diffuse erythema and swelling on the chest, and widespread scaly, erythematous plaques (Fig. 3). The proximal nail folds of his fingers were erythematous, with ragged cuticles. His abdomen was mildly distended, but the rest of the physical examination was normal.
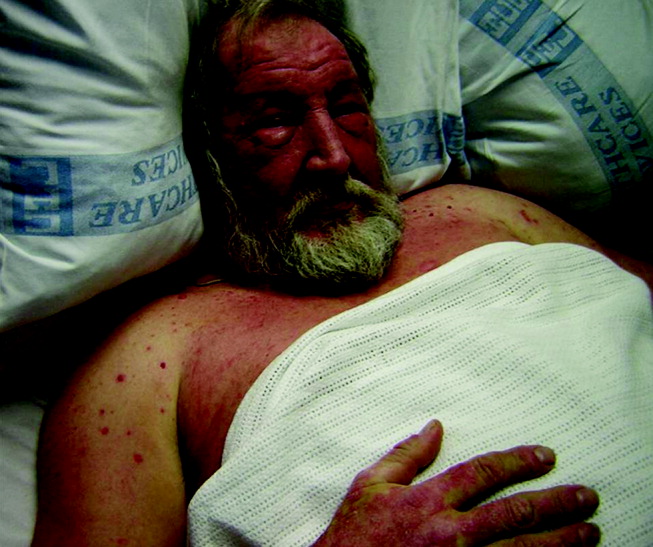
He has become too systemically ill to attribute his condition to psoriasis. The nail findings suggest dermatomyositis, which could explain many of his findings. The diffuse erythema and his difficulty walking are consistent with its skin and muscle involvement. Dyspnea could be explained by dermatomyositis‐associated interstitial lung disease. A dermatomyositis‐associated hematological or solid malignancy could account for his multisystem ailments and functional decline. A point against dermatomyositis is the relatively explosive onset of his disease. He should be carefully examined for any motor weakness. With his progressive erythroderma, I am also concerned about an advancing cutaneous T‐cell lymphoma (with leukemic transformation).
Blood tests revealed the following values: white‐blood‐cell count, 8700/L; hematocrit, 46%; platelet count, 172,000/L; blood urea nitrogen, 26 mg/dL; creatinine, 1.0 mg/dL; glucose, 199 mg/dL; albumin, 3.1 g/dL; alkaline phosphatase, 172 U/L (normal range 45‐129); alanine aminotransferase, 75 U/L (normal range 0‐39 U/L); aspartate aminotransferase, 263 U/L (normal range 0‐37 U/L); total bilirubin, 1.1 mg/dL; prothrombin time, 16 seconds (normal range 11.7‐14.3 seconds), and serum creatinine, kinase, 4253 U/L (normal range 0‐194 U/L). HIV serology was negative. Urinalysis revealed trace protein. The results of chest radiographs and an electrocardiogram were normal.
The liver function tests results are consistent with medication effects or liver involvement in a systemic disease. The creatinine kinase elevation is consistent with a myopathy such as dermatomyositis. A skin biopsy would still be useful. Depending on those results, he may need a muscle biopsy, urine heavy metal testing, and computed tomography body imaging. Considering his transaminase and creatinine kinase elevations, I would discontinue lovastatin.
The patient was hospitalized. Further questioning revealed that he had typical Raynaud's phenomenon and odynophagia. A detailed neurological examination showed weakness (3/5) of the triceps and iliopsoas muscles and difficulty rising from a chair without using his arms. Dermatoscopic examination of the proximal nail folds showed dilated capillary loops and foci of hemorrhage.
Blood tests showed a lactate dehydrogenase level of 456 U/L (normal range 0‐249 U/L) and an aldolase of 38 U/L (normal range 1.2‐7.6 U/L). Tests for antinuclear antibodies, anti‐Jo antibody, and antimyeloperoxidase antibodies were negative. Two skin biopsies were interpreted by general pathology as consistent with partially treated psoriasis, whereas another showed nonspecific changes with minimal superficial perivascular lymphohistiocytic inflammation (Fig. 4). Lisinopril was discontinued because of its possible contribution to the facial edema.
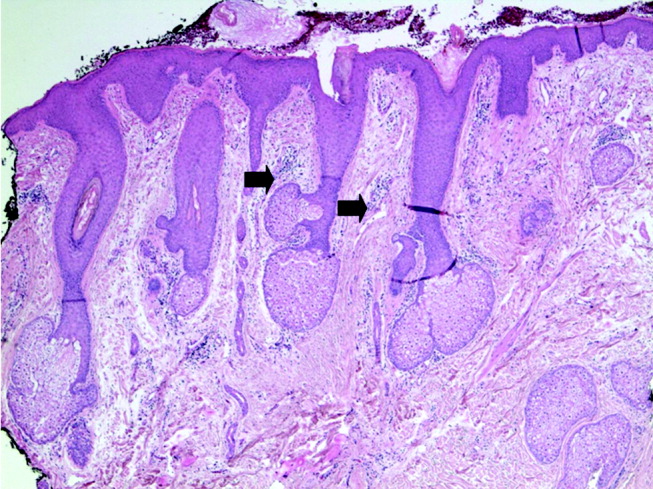
Dermatomyositis is now the leading diagnosis. Characteristic features include his proximal muscle weakness, Raynaud's phenomenon, and dilated nailfold capillary loops. I am not overly dissuaded by the negative antinuclear antibodies, but because of additional atypical features (ie, extensive cutaneous edema, rapid onset, illness severity, prominent gastrointestinal symptoms), a confirmatory muscle biopsy is needed. Endoscopy of the proximal aerodigestive tract would help evaluate the odynophagia. There is little to suggest infection, malignancy, or metabolic derangement.
The inpatient medical team considered myositis related to retinoid or cyclosporine therapy. They discontinued cyclosporine and began systemic corticosteroid therapy. Within a few days, the patient's rash, muscle pain, and weakness improved, and the elevated transaminase and creatinine kinase levels decreased.
Dermatology recommended an evaluation for dermatomyositis‐associated malignancy, but the medicine team and rheumatology consultants, noting the lack of classic skin findings (heliotrope rash and Gottron's papules) and the uncharacteristically rapid onset and improvement of myositis, suggested delaying the evaluation until dermatomyositis was proven.
An immediate improvement in symptoms with steroids is nonspecific, often occurring in autoimmune, infectious, and neoplastic diseases. This juncture in the case is common in complex multisystem illnesses, where various consultants may arrive at differing conclusions. With both typical and atypical features of dermatomyositis, where should one set the therapeutic threshold, that is, the point where one ends testing, accepts a diagnosis, and initiates treatment? Several factors raise the level of certainty I would require. First, dermatomyositis is quite rare. Adding atypical features further increases the burden of proof for that illness. Second, the existence of alternative possibilities (admittedly of equal uncertainty) gives me some pause. Finally, the toxicity of the proposed treatments raises the therapeutic threshold. Acknowledging that empiric treatment may be indicated for a severely ill patient at a lower level of certainty, I would hesitate to commit a patient to long‐term steroids without being confident of the diagnosis. I would therefore require a muscle biopsy, or at least electromyography to support or exclude dermatomyositis.
The patient was discharged from the hospital on high‐dose prednisone. He underwent electromyography, which revealed inflammatory myopathic changes more apparent in the proximal than distal muscles. These findings were thought to be compatible with dermatomyositis, although the fibrillations and positive sharp waves characteristic of acute inflammation were absent, perhaps because of corticosteroid therapy.
The patient mistakenly stopped taking his prednisone. Within days, his weakness and skin rash worsened, and he developed nausea with vomiting. He returned to clinic, where his creatinine kinase level was again found to be elevated, and he was rehospitalized. Oral corticosteroid therapy was restarted with prompt improvement. On review of the original skin biopsies, a dermatopathologist observed areas of thickened dermal collagen and a superficial and deep perivascular lymphocytic infiltrate, both consistent with connective tissue disease.
These 3 additional findings (ie, electromyography results, temporally established steroid responsiveness, and the new skin biopsy interpretation) in aggregate support the diagnosis of dermatomyositis, but the nausea and vomiting are unusual. I would discuss these results with a rheumatologist and still request a confirmatory muscle biopsy. Because diagnosing dermatomyositis should prompt consideration of seeking an underlying malignancy in a patient of this age group, I would repeat a targeted history and physical examination along with age‐ and risk‐factor‐appropriate screening. If muscle biopsy results are not definitive, finding an underlying malignancy would lend support to dermatomyositis.
While hospitalized, the patient complained of continued odynophagia and was noted to have oral candidiasis. Upper endoscopy, undertaken to evaluate for esophageal candidiasis, revealed a mass at the gastroesophageal junction. Biopsy revealed gastric‐type adenocarcinoma. An abdominal computed tomography scan demonstrated 3 hypodense hepatic lesions, evidence of cirrhosis, and ascites. Cytology of paracentesis fluid revealed cells compatible with adenocarcinoma. The patient died in hospice care 2 weeks later.
At autopsy, he had metastatic gastric‐type adenocarcinoma. A muscle biopsy (Fig. 5) revealed muscle atrophy with small foci of lymphocytic infiltrates, most compatible with dermatomyositis. Another dermatopathologist reviewed the skin biopsies and noted interface dermatitis, which is typical of connective tissue diseases like dermatomyositis (Fig. 6A,B).
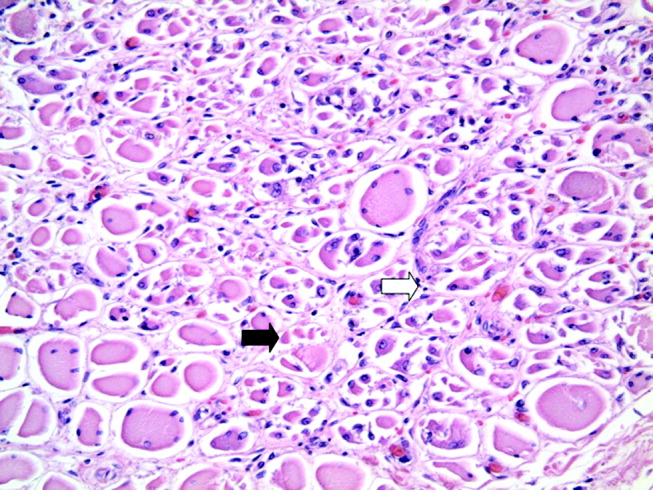
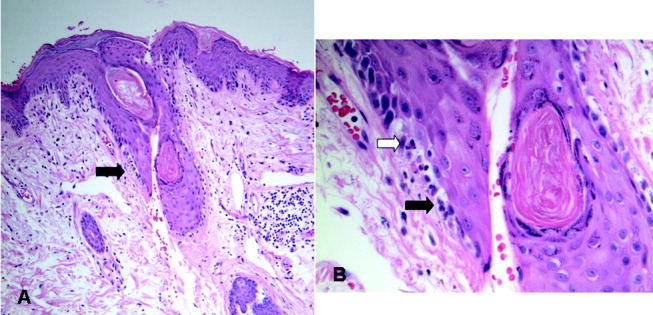
COMMENTARY
Dermatomyositis is an idiopathic inflammatory myopathy characterized by endomysial inflammation and muscle weakness and differentiated from other myopathies by the presence of a rash.1 Muscle disease may manifest with or precede the rash, but up to 40% of patients present with skin manifestations alone, an entity called amyopathic dermatomyositis.2 When present, the myositis generally develops over months, but the onset can be acute.1 The weakness is typically symmetrical and proximal,1 and many patients have oropharyngeal dysphagia.3
The characteristic rash is erythematous, symmetrical, and photodistributed.4 Classic cutaneous findings are the heliotrope rash (violaceous eyelid erythema), which is pathognomonic but uncommon, and the more common Gottron's papules (violaceous, slightly elevated papules and plaques on bony prominences and extensor surfaces, especially the knuckles).4 Other findings include periorbital edema, scalp dermatitis, poikiloderma (ie, hyperpigmentation, hypopigmentation, atrophy, and telangiectasia), periungual erythema, and dystrophic cuticles.2 The cutaneous manifestations of dermatomyositis may be similar to those of psoriasis, systemic lupus erythematosus, lichen planus, rosacea, polymorphous light eruption, drug eruption, atopic dermatitis, seborrheic dermatitis, or allergic contact dermatitis.4
Diagnosing dermatomyositis requires considering clinical, laboratory, electromyographical, and histological evidence, as there are no widely accepted, validated diagnostic criteria.1, 5 The diagnosis is usually suspected if there is a characteristic rash and symptoms of myositis (eg, proximal muscle weakness, myalgias, fatigue, or an inability to swallow). When the patient has an atypical rash, skin biopsy can differentiate dermatomyositis from other conditions, except lupus, which shares the key finding of interface dermatitis.2 The histological findings can be variable and subtle,6 so consultation with a dermatopathologist may be helpful.
Myositis may be confirmed by various studies. Most patients have elevated muscle enzymes (ie, creatinine kinase, aldolase, lactate dehydrogenase, or transaminases)1; for those who do not, magnetic resonance imaging can be helpful in detecting muscle involvement and locating the best site for muscle biopsy.7 Electromyography reveals nonspecific muscle membrane instability.8 Muscle biopsy shows muscle fiber necrosis, perifascicular atrophy, and perivascular and perifascicular lymphocytic infiltrates. These can be patchy, diminished by steroid use, and occasionally seen in noninflammatory muscular dystrophies.8 For a patient with typical myositis and a characteristic rash, muscle biopsy may be unnecessary.1
The clinical utility of serologic testing for diagnosing dermatomyositis is controversial.2 Myositis‐specific antibody testing is insensitive but specific; these antibodies include Jo‐1, an antisynthetase antibody that predicts incomplete response to therapy and lung involvement, and Mi‐2, which is associated with better response to therapy.2, 9, 10 The sensitivity and specificity of antinuclear antibodies are both approximately 60%.10
Patients with dermatomyositis have higher rates of cancers than age‐matched controls, and nearly 25% of patients are diagnosed with a malignancy at some point during the course of the disease.11 Malignancies are typically solid tumors that manifest within 3 years of the diagnosis,1214 although the increased risk may exist for at least 5 years.14 There is a 10‐fold higher risk of ovarian cancer in women with dermatomyositis.12, 15 Other associated malignancies include lung, gastric, colorectal, pancreatic, and breast carcinomas and non‐Hodgkin's lymphoma.14
Recommendations for screening affected patients for cancer have changed over the years, with increasing evidence of an association between dermatomyositis and malignancy and evolving improvements in diagnostic techniques.16 Many authorities recommend that all adult patients with dermatomyositis be evaluated for cancer, including a complete physical examination, basic hematological tests, age‐ and sex‐appropriate screening (eg, mammography, pap smear, and colonoscopy), and chest x‐ray.16 Some would add upper endoscopy; imaging of the chest, abdomen, and pelvis; gynecological examination; and serum CA‐125 level to better evaluate for the most common malignancies (ie, ovarian, gastric, lung, and pancreatic carcinomas and non‐Hodgkins lymphoma).12, 1720
In 19% of adults, dermatomyositis overlaps with other autoimmune disorders, usually systemic lupus erythematosus and systemic sclerosis.21 These manifest as Raynaud's phenomenon, arthritis, esophageal dysmotility, renal disease, or neuropathy.21 Other potentially serious systemic manifestations of dermatomyositis include proximal dysphagia from pharyngeal myopathy; distal dysphagia from esophageal dysmotility in systemic sclerosis overlap; pulmonary disease from autoimmune interstitial lung disease or aspiration; cardiac disease from conduction abnormalities, myocarditis, pericarditis, and valvular disease; and rhabdomyolysis.2
Treatment of dermatomyositis requires systemic immunosuppression with 1 or more agents. The prognosis of dermatomyositis is variable. Mortality at 5 years ranges from 23% to 73%. At least a third of patients are left with mild to severe disability.1 In addition to older age, predictors of poor outcome include male sex, dysphagia, longstanding symptoms before treatment, pulmonary or cardiac involvement, and presence of antisynthetase antibodies.22
Dermatomyositis is often treated in the outpatient setting, but there are many reasons for hospitalization. Complications of treatment, like infection or adverse effects of medications, could result in hospitalization. Treatment with intravenous pulse corticosteroids or IVIG may require inpatient administration if no infusion center is available. Other indications for inpatient evaluation include the consequences of various malignancies and the more severe expression of systemic complications of dermatomyositis (eg, dysphagia and pulmonary, cardiac, or renal disease).
Every parent knows the plaintive backseat whine, Are we there, yet? Clinicians may also experience this feeling when attempting to diagnose a perplexing illness, especially one that lacks a definitive diagnostic test. It was easy for this patient's doctors to assume initially that his new rash was a manifestation of his long‐standing psoriasis. Having done so, they could understandably attribute the subsequent findings to either evolution of this disease or to consequences of the prescribed treatments, rather than considering a novel diagnosis. Only when faced with new (or newly appreciated) findings suggesting myopathy did the clinicians (and our discussant) consider the diagnosis of dermatomyositis. Even then, the primary inpatient medical team and their consultants were unsure when they had sufficient evidence to be certain.
Several factors compounded the difficulty of making a diagnosis in this case: the clinicians were dealing with a rare disease; they were considering alternative diagnoses (ie, psoriasis or a toxic effect of medication); and the disease presented somewhat atypically. The clinicians initially failed to consider and then accept the correct diagnosis because the patient's rash was not classic, his biopsy was interpreted as nonspecific, and he lacked myositis at presentation. Furthermore, when the generalists sought expert assistance, they encountered a difference of opinion among the consultants. These complex situations should goad the clinician into carefully considering the therapeutic threshold, that is, the transition point from diagnostic testing to therapeutic intervention.23 With complex cases like this, it may be difficult to know when one has reached a strongly supported diagnosis, and frequently asking whether we are there yet may be appropriate.
Take‐Home Points for the Hospitalist
-
A skin rash, which may have typical or atypical features, distinguishes dermatomyositis from other acquired myopathies.
-
Consider consultation with pathology specialists for skin and muscle biopsies.
-
Ovarian, lung, gastric, colorectal, pancreatic, and breast carcinomas and non‐Hodgkin's lymphoma are the most common cancers associated with dermatomyositis.
-
In addition to age‐appropriate cancer screening, consider obtaining upper endoscopy, imaging of the chest/abdomen/pelvis, and CA‐125.
-
Patients with dermatomyositis and no obvious concurrent malignancy need long‐term outpatient follow‐up for repeated malignancy screening.
- ,.Polymyositis and dermatomyositis.Lancet.2003;362:971–982.
- .Dermatomyositis.Lancet.2000;355:53–47.
- ,,,.Oropharyngeal dysphagia in polymyositis/dermatomyositis.Clin Neurol Neurosurg.2004;107(1):32–37.
- ,.Skin involvement in dermatomyositis.Curr Opin Rheumatol.2003;15:714–22.
- ,,,,,.Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: analysis of 100 French Canadian patients.Medicine (Baltimore).2005;84:231–249.
- .Skin Pathology.2nd ed.New York:Churchill Livingstone;2002.
- ,.Utility of magnetic resonance imaging in the evaluation of patients with inflammatory myopathies.Curr Rheumatol Rep.2001;3:334–245.
- ,,.Is it really myositis? A consideration of the differential diagnosis.Curr Opin Rheumatol2004;16:684–691.
- .Idiopathic inflammatory myopathy: autoantibody update.Curr Rheumatol Rep.2002;4:434–441.
- ,,.Laboratory assessment in musculoskeletal disorders.Best Pract Res Clin Rheumatol.2003;17:475–494.
- ,.Dermatomyositis.Clin Dermatol.2006;24:363–373.
- ,,, et al.Frequency of specific cancer types in dermatomyositis and polymyositis: a population‐based study.Lancet.2001;357:96–100.
- ,,, et al.Cancer‐associated myositis: clinical features and prognostic signs.Ann N Y Acad Sci.2005;1051:64–71.
- ,,,,.Incidence of malignant disease in biopsy‐proven inflammatory myopathy. A population‐based cohort study.Ann Intern Med.2001;134:1087–1095.
- ,,.Risk of cancer in patients with dermatomyositis or polymyositis, and follow‐up implications: a Scottish population‐based cohort study.Br J Cancer.2001;85 (1):41–45.
- .When and how should the patient with dermatomyositis or amyopathic dermatomyositis be assessed for possible cancer?Arch Dermatol.2002;138:969–971.
- ,,.Ovarian cancer in patients with dermatomyositis.Medicine (Baltimore).1994;73(3):153–160.
- ,,,.Dermatomyositis sine myositis: association with malignancy.J Rheumatol.1996;23 (1):101–105.
- ,,, et al.Tumor antigen markers for the detection of solid cancers in inflammatory myopathies.Cancer Epidemiol Biomarkers Prev.2005;14:1279–1282.
- ,,, et al.Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: a study of 40 patients.Arch Dermatol.2002;138:885–890.
- ,,,,,.Dermatomyositis: a dermatology‐based case series.J Am Acad Dermatol.1998;38:397–404.
- ,,, et al.Long‐term outcome in polymyositis and dermatomyositis.Ann Rheum Dis.2006;65:1456–1461.
- .Our stubborn quest for diagnostic certainty. A cause of excessive testing.N Engl J Med.1989;320:1489–1491.
A 62‐year‐old man with psoriasis for more than 30 years presented to the emergency department with a scaly, pruritic rash involving his face, trunk, and extremities that he had had for the past 10 days. The rash was spreading and not responding to application of clobetasol ointment, which had helped his psoriasis in the past. He also reported mild pharyngitis, headache, and myalgias.
A patient with a chronic skin condition presenting with a new rash means the clinician must consider whether it is an alternative manifestation of the chronic disorder or a new illness. Psoriasis takes many forms including guttate psoriasis, which presents with small, droplike plaques and frequently follows respiratory infections (particularly those caused by Streptococcus). Well‐controlled psoriasis rarely transforms after 3 decades, so I would consider other conditions. The tempo of illness makes certain life‐threatening syndromes, including Stevens‐Johnson, toxic shock, and purpura fulminans, unlikely. An allergic reaction, atopic dermatitis, or medication reaction is possible. Infections, either systemic (eg, syphilis) or dermatologic (eg, scabies), should be considered. Photosensitivity could involve the sun‐exposed areas, such as the extremities and face. Seborrheic dermatitis can cause scaling lesions of the face and trunk but not the extremities. Vasculitis merits consideration, but dependent regions are typically affected more than the head. Mycosis fungoides or a paraneoplastic phenomenon could cause a diffuse rash in this age group.
The patient had diabetes mellitus, hypertension, diverticulosis, and depression. Three months earlier he had undergone surgical drainage of a perirectal abscess. His usual medications were lovastatin, paroxetine, insulin, hydrochlorothiazide, and lisinopril. Three weeks previously he had completed a 10‐day course of trimethoprim/sulfamethoxazole for an upper respiratory infection. Otherwise, he was taking no new medications. He was allergic to penicillin. He denied substance abuse, recent travel, or risk factors for human immunodeficiency virus (HIV) infection. He worked as an automobile painter, lived with his wife, and had a pet dog.
Physical examination revealed a well‐appearing man with normal vital signs. His skin had well‐defined circumscribed pink plaques, mostly 1‐2 cm in size, with thick, silvery scales in the ears and on the dorsal and ventral arms and legs, chest, back, face, and scalp. There were no pustules or other signs of infection (Figs. 1and 2). The nails exhibited distal onycholysis, oil spots, and rare pits. His posterior pharynx was mildly erythematous. The results of cardiovascular, pulmonary, and abdominal examinations were normal.


Although other scaling skin conditions such as eczema, irritant dermatitis, or malignancy remain possible, his rash is most consistent with widespread psoriasis. I would consider immunological changes that may have caused a remarkably altered and more severe expression of his chronic disease, for example, recent steroid therapy or HIV infection. The company a rash keeps helps frame the differential diagnosis. Based on the patient's well appearance, the time course, his minimal systemic symptoms, and the appearance of the rash, my leading considerations are psoriasis or an allergic dermatitis. Cutaneous T‐cell malignancy, with its indolent and sometimes protean manifestations, remains possible in a patient of his age. I would now consult a dermatologist for 3 reasons: this patient has a chronic disease that I do not manage beyond basic treatments (eg, topical steroids), he has an undiagnosed illness with substantial dermatologic manifestations, and he may need a skin biopsy for definitive diagnosis.
The dermatology team diagnosed a guttate psoriasis flare, possibly associated with streptococcal pharyngitis. The differential diagnosis included secondary syphilis, although the team believed this was less likely. The dermatology team recommended obtaining a throat culture, streptozyme assay, and rapid plasma reagin and prescribed oral erythromycin and topical steroid ointment under a sauna suit.
I would follow his response to the prescribed steroid treatments. If the patient's course deviates from the dermatologists' expectations, I would request a skin biopsy and undertake further evaluations in search of an underlying systemic disease.
The patient followed up in the dermatology clinic 3 weeks later. His rash had worsened, and he had developed patchy alopecia and progressive edema of the face, ears, and eyes. He denied mouth or tongue swelling, difficulty breathing, or hives. The streptozyme assay was positive, but the other laboratory test results were negative.
The dermatology team diagnosed a severely inflammatory psoriasis flare and prescribed an oral retinoid, acitretin, and referred him for ultraviolet light therapy. He was unable to travel for phototherapy, and acitretin was discontinued after 1 week because of elevated serum transaminase levels. The dermatologists then prescribed oral cyclosporine.
The progression of disease despite standard treatment suggests a nonpsoriatic condition. Although medications could cause the abnormal liver tests, so could another underlying illness that involves the liver. An infiltrative disorder of the skin with hair follicle destruction and local lymphedema could explain both alopecia and facial edema.
I am unable account for his clinical features with a single disease, so the differential remains broad, including severe psoriasis, an infiltrating cutaneous malignancy, or a toxic exposure. Arsenic poisoning causes hyperkeratotic skin lesions, although he lacks the associated gastrointestinal and neurological symptoms. I would not have added the potentially toxic cyclosporine.
When he returned to dermatology clinic 1 week later, his rash and facial swelling had worsened. He also reported muscle and joint aches, fatigue, lightheadedness, anorexia, nausea, abdominal pain, diarrhea, and dyspnea on exertion. He denied fever, chills, and night sweats.
He appeared ill and used a cane to arise and walk. His vital signs and oxygen saturation were normal. He had marked swelling of his face, diffuse erythema and swelling on the chest, and widespread scaly, erythematous plaques (Fig. 3). The proximal nail folds of his fingers were erythematous, with ragged cuticles. His abdomen was mildly distended, but the rest of the physical examination was normal.

He has become too systemically ill to attribute his condition to psoriasis. The nail findings suggest dermatomyositis, which could explain many of his findings. The diffuse erythema and his difficulty walking are consistent with its skin and muscle involvement. Dyspnea could be explained by dermatomyositis‐associated interstitial lung disease. A dermatomyositis‐associated hematological or solid malignancy could account for his multisystem ailments and functional decline. A point against dermatomyositis is the relatively explosive onset of his disease. He should be carefully examined for any motor weakness. With his progressive erythroderma, I am also concerned about an advancing cutaneous T‐cell lymphoma (with leukemic transformation).
Blood tests revealed the following values: white‐blood‐cell count, 8700/L; hematocrit, 46%; platelet count, 172,000/L; blood urea nitrogen, 26 mg/dL; creatinine, 1.0 mg/dL; glucose, 199 mg/dL; albumin, 3.1 g/dL; alkaline phosphatase, 172 U/L (normal range 45‐129); alanine aminotransferase, 75 U/L (normal range 0‐39 U/L); aspartate aminotransferase, 263 U/L (normal range 0‐37 U/L); total bilirubin, 1.1 mg/dL; prothrombin time, 16 seconds (normal range 11.7‐14.3 seconds), and serum creatinine, kinase, 4253 U/L (normal range 0‐194 U/L). HIV serology was negative. Urinalysis revealed trace protein. The results of chest radiographs and an electrocardiogram were normal.
The liver function tests results are consistent with medication effects or liver involvement in a systemic disease. The creatinine kinase elevation is consistent with a myopathy such as dermatomyositis. A skin biopsy would still be useful. Depending on those results, he may need a muscle biopsy, urine heavy metal testing, and computed tomography body imaging. Considering his transaminase and creatinine kinase elevations, I would discontinue lovastatin.
The patient was hospitalized. Further questioning revealed that he had typical Raynaud's phenomenon and odynophagia. A detailed neurological examination showed weakness (3/5) of the triceps and iliopsoas muscles and difficulty rising from a chair without using his arms. Dermatoscopic examination of the proximal nail folds showed dilated capillary loops and foci of hemorrhage.
Blood tests showed a lactate dehydrogenase level of 456 U/L (normal range 0‐249 U/L) and an aldolase of 38 U/L (normal range 1.2‐7.6 U/L). Tests for antinuclear antibodies, anti‐Jo antibody, and antimyeloperoxidase antibodies were negative. Two skin biopsies were interpreted by general pathology as consistent with partially treated psoriasis, whereas another showed nonspecific changes with minimal superficial perivascular lymphohistiocytic inflammation (Fig. 4). Lisinopril was discontinued because of its possible contribution to the facial edema.

Dermatomyositis is now the leading diagnosis. Characteristic features include his proximal muscle weakness, Raynaud's phenomenon, and dilated nailfold capillary loops. I am not overly dissuaded by the negative antinuclear antibodies, but because of additional atypical features (ie, extensive cutaneous edema, rapid onset, illness severity, prominent gastrointestinal symptoms), a confirmatory muscle biopsy is needed. Endoscopy of the proximal aerodigestive tract would help evaluate the odynophagia. There is little to suggest infection, malignancy, or metabolic derangement.
The inpatient medical team considered myositis related to retinoid or cyclosporine therapy. They discontinued cyclosporine and began systemic corticosteroid therapy. Within a few days, the patient's rash, muscle pain, and weakness improved, and the elevated transaminase and creatinine kinase levels decreased.
Dermatology recommended an evaluation for dermatomyositis‐associated malignancy, but the medicine team and rheumatology consultants, noting the lack of classic skin findings (heliotrope rash and Gottron's papules) and the uncharacteristically rapid onset and improvement of myositis, suggested delaying the evaluation until dermatomyositis was proven.
An immediate improvement in symptoms with steroids is nonspecific, often occurring in autoimmune, infectious, and neoplastic diseases. This juncture in the case is common in complex multisystem illnesses, where various consultants may arrive at differing conclusions. With both typical and atypical features of dermatomyositis, where should one set the therapeutic threshold, that is, the point where one ends testing, accepts a diagnosis, and initiates treatment? Several factors raise the level of certainty I would require. First, dermatomyositis is quite rare. Adding atypical features further increases the burden of proof for that illness. Second, the existence of alternative possibilities (admittedly of equal uncertainty) gives me some pause. Finally, the toxicity of the proposed treatments raises the therapeutic threshold. Acknowledging that empiric treatment may be indicated for a severely ill patient at a lower level of certainty, I would hesitate to commit a patient to long‐term steroids without being confident of the diagnosis. I would therefore require a muscle biopsy, or at least electromyography to support or exclude dermatomyositis.
The patient was discharged from the hospital on high‐dose prednisone. He underwent electromyography, which revealed inflammatory myopathic changes more apparent in the proximal than distal muscles. These findings were thought to be compatible with dermatomyositis, although the fibrillations and positive sharp waves characteristic of acute inflammation were absent, perhaps because of corticosteroid therapy.
The patient mistakenly stopped taking his prednisone. Within days, his weakness and skin rash worsened, and he developed nausea with vomiting. He returned to clinic, where his creatinine kinase level was again found to be elevated, and he was rehospitalized. Oral corticosteroid therapy was restarted with prompt improvement. On review of the original skin biopsies, a dermatopathologist observed areas of thickened dermal collagen and a superficial and deep perivascular lymphocytic infiltrate, both consistent with connective tissue disease.
These 3 additional findings (ie, electromyography results, temporally established steroid responsiveness, and the new skin biopsy interpretation) in aggregate support the diagnosis of dermatomyositis, but the nausea and vomiting are unusual. I would discuss these results with a rheumatologist and still request a confirmatory muscle biopsy. Because diagnosing dermatomyositis should prompt consideration of seeking an underlying malignancy in a patient of this age group, I would repeat a targeted history and physical examination along with age‐ and risk‐factor‐appropriate screening. If muscle biopsy results are not definitive, finding an underlying malignancy would lend support to dermatomyositis.
While hospitalized, the patient complained of continued odynophagia and was noted to have oral candidiasis. Upper endoscopy, undertaken to evaluate for esophageal candidiasis, revealed a mass at the gastroesophageal junction. Biopsy revealed gastric‐type adenocarcinoma. An abdominal computed tomography scan demonstrated 3 hypodense hepatic lesions, evidence of cirrhosis, and ascites. Cytology of paracentesis fluid revealed cells compatible with adenocarcinoma. The patient died in hospice care 2 weeks later.
At autopsy, he had metastatic gastric‐type adenocarcinoma. A muscle biopsy (Fig. 5) revealed muscle atrophy with small foci of lymphocytic infiltrates, most compatible with dermatomyositis. Another dermatopathologist reviewed the skin biopsies and noted interface dermatitis, which is typical of connective tissue diseases like dermatomyositis (Fig. 6A,B).


COMMENTARY
Dermatomyositis is an idiopathic inflammatory myopathy characterized by endomysial inflammation and muscle weakness and differentiated from other myopathies by the presence of a rash.1 Muscle disease may manifest with or precede the rash, but up to 40% of patients present with skin manifestations alone, an entity called amyopathic dermatomyositis.2 When present, the myositis generally develops over months, but the onset can be acute.1 The weakness is typically symmetrical and proximal,1 and many patients have oropharyngeal dysphagia.3
The characteristic rash is erythematous, symmetrical, and photodistributed.4 Classic cutaneous findings are the heliotrope rash (violaceous eyelid erythema), which is pathognomonic but uncommon, and the more common Gottron's papules (violaceous, slightly elevated papules and plaques on bony prominences and extensor surfaces, especially the knuckles).4 Other findings include periorbital edema, scalp dermatitis, poikiloderma (ie, hyperpigmentation, hypopigmentation, atrophy, and telangiectasia), periungual erythema, and dystrophic cuticles.2 The cutaneous manifestations of dermatomyositis may be similar to those of psoriasis, systemic lupus erythematosus, lichen planus, rosacea, polymorphous light eruption, drug eruption, atopic dermatitis, seborrheic dermatitis, or allergic contact dermatitis.4
Diagnosing dermatomyositis requires considering clinical, laboratory, electromyographical, and histological evidence, as there are no widely accepted, validated diagnostic criteria.1, 5 The diagnosis is usually suspected if there is a characteristic rash and symptoms of myositis (eg, proximal muscle weakness, myalgias, fatigue, or an inability to swallow). When the patient has an atypical rash, skin biopsy can differentiate dermatomyositis from other conditions, except lupus, which shares the key finding of interface dermatitis.2 The histological findings can be variable and subtle,6 so consultation with a dermatopathologist may be helpful.
Myositis may be confirmed by various studies. Most patients have elevated muscle enzymes (ie, creatinine kinase, aldolase, lactate dehydrogenase, or transaminases)1; for those who do not, magnetic resonance imaging can be helpful in detecting muscle involvement and locating the best site for muscle biopsy.7 Electromyography reveals nonspecific muscle membrane instability.8 Muscle biopsy shows muscle fiber necrosis, perifascicular atrophy, and perivascular and perifascicular lymphocytic infiltrates. These can be patchy, diminished by steroid use, and occasionally seen in noninflammatory muscular dystrophies.8 For a patient with typical myositis and a characteristic rash, muscle biopsy may be unnecessary.1
The clinical utility of serologic testing for diagnosing dermatomyositis is controversial.2 Myositis‐specific antibody testing is insensitive but specific; these antibodies include Jo‐1, an antisynthetase antibody that predicts incomplete response to therapy and lung involvement, and Mi‐2, which is associated with better response to therapy.2, 9, 10 The sensitivity and specificity of antinuclear antibodies are both approximately 60%.10
Patients with dermatomyositis have higher rates of cancers than age‐matched controls, and nearly 25% of patients are diagnosed with a malignancy at some point during the course of the disease.11 Malignancies are typically solid tumors that manifest within 3 years of the diagnosis,1214 although the increased risk may exist for at least 5 years.14 There is a 10‐fold higher risk of ovarian cancer in women with dermatomyositis.12, 15 Other associated malignancies include lung, gastric, colorectal, pancreatic, and breast carcinomas and non‐Hodgkin's lymphoma.14
Recommendations for screening affected patients for cancer have changed over the years, with increasing evidence of an association between dermatomyositis and malignancy and evolving improvements in diagnostic techniques.16 Many authorities recommend that all adult patients with dermatomyositis be evaluated for cancer, including a complete physical examination, basic hematological tests, age‐ and sex‐appropriate screening (eg, mammography, pap smear, and colonoscopy), and chest x‐ray.16 Some would add upper endoscopy; imaging of the chest, abdomen, and pelvis; gynecological examination; and serum CA‐125 level to better evaluate for the most common malignancies (ie, ovarian, gastric, lung, and pancreatic carcinomas and non‐Hodgkins lymphoma).12, 1720
In 19% of adults, dermatomyositis overlaps with other autoimmune disorders, usually systemic lupus erythematosus and systemic sclerosis.21 These manifest as Raynaud's phenomenon, arthritis, esophageal dysmotility, renal disease, or neuropathy.21 Other potentially serious systemic manifestations of dermatomyositis include proximal dysphagia from pharyngeal myopathy; distal dysphagia from esophageal dysmotility in systemic sclerosis overlap; pulmonary disease from autoimmune interstitial lung disease or aspiration; cardiac disease from conduction abnormalities, myocarditis, pericarditis, and valvular disease; and rhabdomyolysis.2
Treatment of dermatomyositis requires systemic immunosuppression with 1 or more agents. The prognosis of dermatomyositis is variable. Mortality at 5 years ranges from 23% to 73%. At least a third of patients are left with mild to severe disability.1 In addition to older age, predictors of poor outcome include male sex, dysphagia, longstanding symptoms before treatment, pulmonary or cardiac involvement, and presence of antisynthetase antibodies.22
Dermatomyositis is often treated in the outpatient setting, but there are many reasons for hospitalization. Complications of treatment, like infection or adverse effects of medications, could result in hospitalization. Treatment with intravenous pulse corticosteroids or IVIG may require inpatient administration if no infusion center is available. Other indications for inpatient evaluation include the consequences of various malignancies and the more severe expression of systemic complications of dermatomyositis (eg, dysphagia and pulmonary, cardiac, or renal disease).
Every parent knows the plaintive backseat whine, Are we there, yet? Clinicians may also experience this feeling when attempting to diagnose a perplexing illness, especially one that lacks a definitive diagnostic test. It was easy for this patient's doctors to assume initially that his new rash was a manifestation of his long‐standing psoriasis. Having done so, they could understandably attribute the subsequent findings to either evolution of this disease or to consequences of the prescribed treatments, rather than considering a novel diagnosis. Only when faced with new (or newly appreciated) findings suggesting myopathy did the clinicians (and our discussant) consider the diagnosis of dermatomyositis. Even then, the primary inpatient medical team and their consultants were unsure when they had sufficient evidence to be certain.
Several factors compounded the difficulty of making a diagnosis in this case: the clinicians were dealing with a rare disease; they were considering alternative diagnoses (ie, psoriasis or a toxic effect of medication); and the disease presented somewhat atypically. The clinicians initially failed to consider and then accept the correct diagnosis because the patient's rash was not classic, his biopsy was interpreted as nonspecific, and he lacked myositis at presentation. Furthermore, when the generalists sought expert assistance, they encountered a difference of opinion among the consultants. These complex situations should goad the clinician into carefully considering the therapeutic threshold, that is, the transition point from diagnostic testing to therapeutic intervention.23 With complex cases like this, it may be difficult to know when one has reached a strongly supported diagnosis, and frequently asking whether we are there yet may be appropriate.
Take‐Home Points for the Hospitalist
-
A skin rash, which may have typical or atypical features, distinguishes dermatomyositis from other acquired myopathies.
-
Consider consultation with pathology specialists for skin and muscle biopsies.
-
Ovarian, lung, gastric, colorectal, pancreatic, and breast carcinomas and non‐Hodgkin's lymphoma are the most common cancers associated with dermatomyositis.
-
In addition to age‐appropriate cancer screening, consider obtaining upper endoscopy, imaging of the chest/abdomen/pelvis, and CA‐125.
-
Patients with dermatomyositis and no obvious concurrent malignancy need long‐term outpatient follow‐up for repeated malignancy screening.
A 62‐year‐old man with psoriasis for more than 30 years presented to the emergency department with a scaly, pruritic rash involving his face, trunk, and extremities that he had had for the past 10 days. The rash was spreading and not responding to application of clobetasol ointment, which had helped his psoriasis in the past. He also reported mild pharyngitis, headache, and myalgias.
A patient with a chronic skin condition presenting with a new rash means the clinician must consider whether it is an alternative manifestation of the chronic disorder or a new illness. Psoriasis takes many forms including guttate psoriasis, which presents with small, droplike plaques and frequently follows respiratory infections (particularly those caused by Streptococcus). Well‐controlled psoriasis rarely transforms after 3 decades, so I would consider other conditions. The tempo of illness makes certain life‐threatening syndromes, including Stevens‐Johnson, toxic shock, and purpura fulminans, unlikely. An allergic reaction, atopic dermatitis, or medication reaction is possible. Infections, either systemic (eg, syphilis) or dermatologic (eg, scabies), should be considered. Photosensitivity could involve the sun‐exposed areas, such as the extremities and face. Seborrheic dermatitis can cause scaling lesions of the face and trunk but not the extremities. Vasculitis merits consideration, but dependent regions are typically affected more than the head. Mycosis fungoides or a paraneoplastic phenomenon could cause a diffuse rash in this age group.
The patient had diabetes mellitus, hypertension, diverticulosis, and depression. Three months earlier he had undergone surgical drainage of a perirectal abscess. His usual medications were lovastatin, paroxetine, insulin, hydrochlorothiazide, and lisinopril. Three weeks previously he had completed a 10‐day course of trimethoprim/sulfamethoxazole for an upper respiratory infection. Otherwise, he was taking no new medications. He was allergic to penicillin. He denied substance abuse, recent travel, or risk factors for human immunodeficiency virus (HIV) infection. He worked as an automobile painter, lived with his wife, and had a pet dog.
Physical examination revealed a well‐appearing man with normal vital signs. His skin had well‐defined circumscribed pink plaques, mostly 1‐2 cm in size, with thick, silvery scales in the ears and on the dorsal and ventral arms and legs, chest, back, face, and scalp. There were no pustules or other signs of infection (Figs. 1and 2). The nails exhibited distal onycholysis, oil spots, and rare pits. His posterior pharynx was mildly erythematous. The results of cardiovascular, pulmonary, and abdominal examinations were normal.


Although other scaling skin conditions such as eczema, irritant dermatitis, or malignancy remain possible, his rash is most consistent with widespread psoriasis. I would consider immunological changes that may have caused a remarkably altered and more severe expression of his chronic disease, for example, recent steroid therapy or HIV infection. The company a rash keeps helps frame the differential diagnosis. Based on the patient's well appearance, the time course, his minimal systemic symptoms, and the appearance of the rash, my leading considerations are psoriasis or an allergic dermatitis. Cutaneous T‐cell malignancy, with its indolent and sometimes protean manifestations, remains possible in a patient of his age. I would now consult a dermatologist for 3 reasons: this patient has a chronic disease that I do not manage beyond basic treatments (eg, topical steroids), he has an undiagnosed illness with substantial dermatologic manifestations, and he may need a skin biopsy for definitive diagnosis.
The dermatology team diagnosed a guttate psoriasis flare, possibly associated with streptococcal pharyngitis. The differential diagnosis included secondary syphilis, although the team believed this was less likely. The dermatology team recommended obtaining a throat culture, streptozyme assay, and rapid plasma reagin and prescribed oral erythromycin and topical steroid ointment under a sauna suit.
I would follow his response to the prescribed steroid treatments. If the patient's course deviates from the dermatologists' expectations, I would request a skin biopsy and undertake further evaluations in search of an underlying systemic disease.
The patient followed up in the dermatology clinic 3 weeks later. His rash had worsened, and he had developed patchy alopecia and progressive edema of the face, ears, and eyes. He denied mouth or tongue swelling, difficulty breathing, or hives. The streptozyme assay was positive, but the other laboratory test results were negative.
The dermatology team diagnosed a severely inflammatory psoriasis flare and prescribed an oral retinoid, acitretin, and referred him for ultraviolet light therapy. He was unable to travel for phototherapy, and acitretin was discontinued after 1 week because of elevated serum transaminase levels. The dermatologists then prescribed oral cyclosporine.
The progression of disease despite standard treatment suggests a nonpsoriatic condition. Although medications could cause the abnormal liver tests, so could another underlying illness that involves the liver. An infiltrative disorder of the skin with hair follicle destruction and local lymphedema could explain both alopecia and facial edema.
I am unable account for his clinical features with a single disease, so the differential remains broad, including severe psoriasis, an infiltrating cutaneous malignancy, or a toxic exposure. Arsenic poisoning causes hyperkeratotic skin lesions, although he lacks the associated gastrointestinal and neurological symptoms. I would not have added the potentially toxic cyclosporine.
When he returned to dermatology clinic 1 week later, his rash and facial swelling had worsened. He also reported muscle and joint aches, fatigue, lightheadedness, anorexia, nausea, abdominal pain, diarrhea, and dyspnea on exertion. He denied fever, chills, and night sweats.
He appeared ill and used a cane to arise and walk. His vital signs and oxygen saturation were normal. He had marked swelling of his face, diffuse erythema and swelling on the chest, and widespread scaly, erythematous plaques (Fig. 3). The proximal nail folds of his fingers were erythematous, with ragged cuticles. His abdomen was mildly distended, but the rest of the physical examination was normal.

He has become too systemically ill to attribute his condition to psoriasis. The nail findings suggest dermatomyositis, which could explain many of his findings. The diffuse erythema and his difficulty walking are consistent with its skin and muscle involvement. Dyspnea could be explained by dermatomyositis‐associated interstitial lung disease. A dermatomyositis‐associated hematological or solid malignancy could account for his multisystem ailments and functional decline. A point against dermatomyositis is the relatively explosive onset of his disease. He should be carefully examined for any motor weakness. With his progressive erythroderma, I am also concerned about an advancing cutaneous T‐cell lymphoma (with leukemic transformation).
Blood tests revealed the following values: white‐blood‐cell count, 8700/L; hematocrit, 46%; platelet count, 172,000/L; blood urea nitrogen, 26 mg/dL; creatinine, 1.0 mg/dL; glucose, 199 mg/dL; albumin, 3.1 g/dL; alkaline phosphatase, 172 U/L (normal range 45‐129); alanine aminotransferase, 75 U/L (normal range 0‐39 U/L); aspartate aminotransferase, 263 U/L (normal range 0‐37 U/L); total bilirubin, 1.1 mg/dL; prothrombin time, 16 seconds (normal range 11.7‐14.3 seconds), and serum creatinine, kinase, 4253 U/L (normal range 0‐194 U/L). HIV serology was negative. Urinalysis revealed trace protein. The results of chest radiographs and an electrocardiogram were normal.
The liver function tests results are consistent with medication effects or liver involvement in a systemic disease. The creatinine kinase elevation is consistent with a myopathy such as dermatomyositis. A skin biopsy would still be useful. Depending on those results, he may need a muscle biopsy, urine heavy metal testing, and computed tomography body imaging. Considering his transaminase and creatinine kinase elevations, I would discontinue lovastatin.
The patient was hospitalized. Further questioning revealed that he had typical Raynaud's phenomenon and odynophagia. A detailed neurological examination showed weakness (3/5) of the triceps and iliopsoas muscles and difficulty rising from a chair without using his arms. Dermatoscopic examination of the proximal nail folds showed dilated capillary loops and foci of hemorrhage.
Blood tests showed a lactate dehydrogenase level of 456 U/L (normal range 0‐249 U/L) and an aldolase of 38 U/L (normal range 1.2‐7.6 U/L). Tests for antinuclear antibodies, anti‐Jo antibody, and antimyeloperoxidase antibodies were negative. Two skin biopsies were interpreted by general pathology as consistent with partially treated psoriasis, whereas another showed nonspecific changes with minimal superficial perivascular lymphohistiocytic inflammation (Fig. 4). Lisinopril was discontinued because of its possible contribution to the facial edema.

Dermatomyositis is now the leading diagnosis. Characteristic features include his proximal muscle weakness, Raynaud's phenomenon, and dilated nailfold capillary loops. I am not overly dissuaded by the negative antinuclear antibodies, but because of additional atypical features (ie, extensive cutaneous edema, rapid onset, illness severity, prominent gastrointestinal symptoms), a confirmatory muscle biopsy is needed. Endoscopy of the proximal aerodigestive tract would help evaluate the odynophagia. There is little to suggest infection, malignancy, or metabolic derangement.
The inpatient medical team considered myositis related to retinoid or cyclosporine therapy. They discontinued cyclosporine and began systemic corticosteroid therapy. Within a few days, the patient's rash, muscle pain, and weakness improved, and the elevated transaminase and creatinine kinase levels decreased.
Dermatology recommended an evaluation for dermatomyositis‐associated malignancy, but the medicine team and rheumatology consultants, noting the lack of classic skin findings (heliotrope rash and Gottron's papules) and the uncharacteristically rapid onset and improvement of myositis, suggested delaying the evaluation until dermatomyositis was proven.
An immediate improvement in symptoms with steroids is nonspecific, often occurring in autoimmune, infectious, and neoplastic diseases. This juncture in the case is common in complex multisystem illnesses, where various consultants may arrive at differing conclusions. With both typical and atypical features of dermatomyositis, where should one set the therapeutic threshold, that is, the point where one ends testing, accepts a diagnosis, and initiates treatment? Several factors raise the level of certainty I would require. First, dermatomyositis is quite rare. Adding atypical features further increases the burden of proof for that illness. Second, the existence of alternative possibilities (admittedly of equal uncertainty) gives me some pause. Finally, the toxicity of the proposed treatments raises the therapeutic threshold. Acknowledging that empiric treatment may be indicated for a severely ill patient at a lower level of certainty, I would hesitate to commit a patient to long‐term steroids without being confident of the diagnosis. I would therefore require a muscle biopsy, or at least electromyography to support or exclude dermatomyositis.
The patient was discharged from the hospital on high‐dose prednisone. He underwent electromyography, which revealed inflammatory myopathic changes more apparent in the proximal than distal muscles. These findings were thought to be compatible with dermatomyositis, although the fibrillations and positive sharp waves characteristic of acute inflammation were absent, perhaps because of corticosteroid therapy.
The patient mistakenly stopped taking his prednisone. Within days, his weakness and skin rash worsened, and he developed nausea with vomiting. He returned to clinic, where his creatinine kinase level was again found to be elevated, and he was rehospitalized. Oral corticosteroid therapy was restarted with prompt improvement. On review of the original skin biopsies, a dermatopathologist observed areas of thickened dermal collagen and a superficial and deep perivascular lymphocytic infiltrate, both consistent with connective tissue disease.
These 3 additional findings (ie, electromyography results, temporally established steroid responsiveness, and the new skin biopsy interpretation) in aggregate support the diagnosis of dermatomyositis, but the nausea and vomiting are unusual. I would discuss these results with a rheumatologist and still request a confirmatory muscle biopsy. Because diagnosing dermatomyositis should prompt consideration of seeking an underlying malignancy in a patient of this age group, I would repeat a targeted history and physical examination along with age‐ and risk‐factor‐appropriate screening. If muscle biopsy results are not definitive, finding an underlying malignancy would lend support to dermatomyositis.
While hospitalized, the patient complained of continued odynophagia and was noted to have oral candidiasis. Upper endoscopy, undertaken to evaluate for esophageal candidiasis, revealed a mass at the gastroesophageal junction. Biopsy revealed gastric‐type adenocarcinoma. An abdominal computed tomography scan demonstrated 3 hypodense hepatic lesions, evidence of cirrhosis, and ascites. Cytology of paracentesis fluid revealed cells compatible with adenocarcinoma. The patient died in hospice care 2 weeks later.
At autopsy, he had metastatic gastric‐type adenocarcinoma. A muscle biopsy (Fig. 5) revealed muscle atrophy with small foci of lymphocytic infiltrates, most compatible with dermatomyositis. Another dermatopathologist reviewed the skin biopsies and noted interface dermatitis, which is typical of connective tissue diseases like dermatomyositis (Fig. 6A,B).


COMMENTARY
Dermatomyositis is an idiopathic inflammatory myopathy characterized by endomysial inflammation and muscle weakness and differentiated from other myopathies by the presence of a rash.1 Muscle disease may manifest with or precede the rash, but up to 40% of patients present with skin manifestations alone, an entity called amyopathic dermatomyositis.2 When present, the myositis generally develops over months, but the onset can be acute.1 The weakness is typically symmetrical and proximal,1 and many patients have oropharyngeal dysphagia.3
The characteristic rash is erythematous, symmetrical, and photodistributed.4 Classic cutaneous findings are the heliotrope rash (violaceous eyelid erythema), which is pathognomonic but uncommon, and the more common Gottron's papules (violaceous, slightly elevated papules and plaques on bony prominences and extensor surfaces, especially the knuckles).4 Other findings include periorbital edema, scalp dermatitis, poikiloderma (ie, hyperpigmentation, hypopigmentation, atrophy, and telangiectasia), periungual erythema, and dystrophic cuticles.2 The cutaneous manifestations of dermatomyositis may be similar to those of psoriasis, systemic lupus erythematosus, lichen planus, rosacea, polymorphous light eruption, drug eruption, atopic dermatitis, seborrheic dermatitis, or allergic contact dermatitis.4
Diagnosing dermatomyositis requires considering clinical, laboratory, electromyographical, and histological evidence, as there are no widely accepted, validated diagnostic criteria.1, 5 The diagnosis is usually suspected if there is a characteristic rash and symptoms of myositis (eg, proximal muscle weakness, myalgias, fatigue, or an inability to swallow). When the patient has an atypical rash, skin biopsy can differentiate dermatomyositis from other conditions, except lupus, which shares the key finding of interface dermatitis.2 The histological findings can be variable and subtle,6 so consultation with a dermatopathologist may be helpful.
Myositis may be confirmed by various studies. Most patients have elevated muscle enzymes (ie, creatinine kinase, aldolase, lactate dehydrogenase, or transaminases)1; for those who do not, magnetic resonance imaging can be helpful in detecting muscle involvement and locating the best site for muscle biopsy.7 Electromyography reveals nonspecific muscle membrane instability.8 Muscle biopsy shows muscle fiber necrosis, perifascicular atrophy, and perivascular and perifascicular lymphocytic infiltrates. These can be patchy, diminished by steroid use, and occasionally seen in noninflammatory muscular dystrophies.8 For a patient with typical myositis and a characteristic rash, muscle biopsy may be unnecessary.1
The clinical utility of serologic testing for diagnosing dermatomyositis is controversial.2 Myositis‐specific antibody testing is insensitive but specific; these antibodies include Jo‐1, an antisynthetase antibody that predicts incomplete response to therapy and lung involvement, and Mi‐2, which is associated with better response to therapy.2, 9, 10 The sensitivity and specificity of antinuclear antibodies are both approximately 60%.10
Patients with dermatomyositis have higher rates of cancers than age‐matched controls, and nearly 25% of patients are diagnosed with a malignancy at some point during the course of the disease.11 Malignancies are typically solid tumors that manifest within 3 years of the diagnosis,1214 although the increased risk may exist for at least 5 years.14 There is a 10‐fold higher risk of ovarian cancer in women with dermatomyositis.12, 15 Other associated malignancies include lung, gastric, colorectal, pancreatic, and breast carcinomas and non‐Hodgkin's lymphoma.14
Recommendations for screening affected patients for cancer have changed over the years, with increasing evidence of an association between dermatomyositis and malignancy and evolving improvements in diagnostic techniques.16 Many authorities recommend that all adult patients with dermatomyositis be evaluated for cancer, including a complete physical examination, basic hematological tests, age‐ and sex‐appropriate screening (eg, mammography, pap smear, and colonoscopy), and chest x‐ray.16 Some would add upper endoscopy; imaging of the chest, abdomen, and pelvis; gynecological examination; and serum CA‐125 level to better evaluate for the most common malignancies (ie, ovarian, gastric, lung, and pancreatic carcinomas and non‐Hodgkins lymphoma).12, 1720
In 19% of adults, dermatomyositis overlaps with other autoimmune disorders, usually systemic lupus erythematosus and systemic sclerosis.21 These manifest as Raynaud's phenomenon, arthritis, esophageal dysmotility, renal disease, or neuropathy.21 Other potentially serious systemic manifestations of dermatomyositis include proximal dysphagia from pharyngeal myopathy; distal dysphagia from esophageal dysmotility in systemic sclerosis overlap; pulmonary disease from autoimmune interstitial lung disease or aspiration; cardiac disease from conduction abnormalities, myocarditis, pericarditis, and valvular disease; and rhabdomyolysis.2
Treatment of dermatomyositis requires systemic immunosuppression with 1 or more agents. The prognosis of dermatomyositis is variable. Mortality at 5 years ranges from 23% to 73%. At least a third of patients are left with mild to severe disability.1 In addition to older age, predictors of poor outcome include male sex, dysphagia, longstanding symptoms before treatment, pulmonary or cardiac involvement, and presence of antisynthetase antibodies.22
Dermatomyositis is often treated in the outpatient setting, but there are many reasons for hospitalization. Complications of treatment, like infection or adverse effects of medications, could result in hospitalization. Treatment with intravenous pulse corticosteroids or IVIG may require inpatient administration if no infusion center is available. Other indications for inpatient evaluation include the consequences of various malignancies and the more severe expression of systemic complications of dermatomyositis (eg, dysphagia and pulmonary, cardiac, or renal disease).
Every parent knows the plaintive backseat whine, Are we there, yet? Clinicians may also experience this feeling when attempting to diagnose a perplexing illness, especially one that lacks a definitive diagnostic test. It was easy for this patient's doctors to assume initially that his new rash was a manifestation of his long‐standing psoriasis. Having done so, they could understandably attribute the subsequent findings to either evolution of this disease or to consequences of the prescribed treatments, rather than considering a novel diagnosis. Only when faced with new (or newly appreciated) findings suggesting myopathy did the clinicians (and our discussant) consider the diagnosis of dermatomyositis. Even then, the primary inpatient medical team and their consultants were unsure when they had sufficient evidence to be certain.
Several factors compounded the difficulty of making a diagnosis in this case: the clinicians were dealing with a rare disease; they were considering alternative diagnoses (ie, psoriasis or a toxic effect of medication); and the disease presented somewhat atypically. The clinicians initially failed to consider and then accept the correct diagnosis because the patient's rash was not classic, his biopsy was interpreted as nonspecific, and he lacked myositis at presentation. Furthermore, when the generalists sought expert assistance, they encountered a difference of opinion among the consultants. These complex situations should goad the clinician into carefully considering the therapeutic threshold, that is, the transition point from diagnostic testing to therapeutic intervention.23 With complex cases like this, it may be difficult to know when one has reached a strongly supported diagnosis, and frequently asking whether we are there yet may be appropriate.
Take‐Home Points for the Hospitalist
-
A skin rash, which may have typical or atypical features, distinguishes dermatomyositis from other acquired myopathies.
-
Consider consultation with pathology specialists for skin and muscle biopsies.
-
Ovarian, lung, gastric, colorectal, pancreatic, and breast carcinomas and non‐Hodgkin's lymphoma are the most common cancers associated with dermatomyositis.
-
In addition to age‐appropriate cancer screening, consider obtaining upper endoscopy, imaging of the chest/abdomen/pelvis, and CA‐125.
-
Patients with dermatomyositis and no obvious concurrent malignancy need long‐term outpatient follow‐up for repeated malignancy screening.
- ,.Polymyositis and dermatomyositis.Lancet.2003;362:971–982.
- .Dermatomyositis.Lancet.2000;355:53–47.
- ,,,.Oropharyngeal dysphagia in polymyositis/dermatomyositis.Clin Neurol Neurosurg.2004;107(1):32–37.
- ,.Skin involvement in dermatomyositis.Curr Opin Rheumatol.2003;15:714–22.
- ,,,,,.Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: analysis of 100 French Canadian patients.Medicine (Baltimore).2005;84:231–249.
- .Skin Pathology.2nd ed.New York:Churchill Livingstone;2002.
- ,.Utility of magnetic resonance imaging in the evaluation of patients with inflammatory myopathies.Curr Rheumatol Rep.2001;3:334–245.
- ,,.Is it really myositis? A consideration of the differential diagnosis.Curr Opin Rheumatol2004;16:684–691.
- .Idiopathic inflammatory myopathy: autoantibody update.Curr Rheumatol Rep.2002;4:434–441.
- ,,.Laboratory assessment in musculoskeletal disorders.Best Pract Res Clin Rheumatol.2003;17:475–494.
- ,.Dermatomyositis.Clin Dermatol.2006;24:363–373.
- ,,, et al.Frequency of specific cancer types in dermatomyositis and polymyositis: a population‐based study.Lancet.2001;357:96–100.
- ,,, et al.Cancer‐associated myositis: clinical features and prognostic signs.Ann N Y Acad Sci.2005;1051:64–71.
- ,,,,.Incidence of malignant disease in biopsy‐proven inflammatory myopathy. A population‐based cohort study.Ann Intern Med.2001;134:1087–1095.
- ,,.Risk of cancer in patients with dermatomyositis or polymyositis, and follow‐up implications: a Scottish population‐based cohort study.Br J Cancer.2001;85 (1):41–45.
- .When and how should the patient with dermatomyositis or amyopathic dermatomyositis be assessed for possible cancer?Arch Dermatol.2002;138:969–971.
- ,,.Ovarian cancer in patients with dermatomyositis.Medicine (Baltimore).1994;73(3):153–160.
- ,,,.Dermatomyositis sine myositis: association with malignancy.J Rheumatol.1996;23 (1):101–105.
- ,,, et al.Tumor antigen markers for the detection of solid cancers in inflammatory myopathies.Cancer Epidemiol Biomarkers Prev.2005;14:1279–1282.
- ,,, et al.Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: a study of 40 patients.Arch Dermatol.2002;138:885–890.
- ,,,,,.Dermatomyositis: a dermatology‐based case series.J Am Acad Dermatol.1998;38:397–404.
- ,,, et al.Long‐term outcome in polymyositis and dermatomyositis.Ann Rheum Dis.2006;65:1456–1461.
- .Our stubborn quest for diagnostic certainty. A cause of excessive testing.N Engl J Med.1989;320:1489–1491.
- ,.Polymyositis and dermatomyositis.Lancet.2003;362:971–982.
- .Dermatomyositis.Lancet.2000;355:53–47.
- ,,,.Oropharyngeal dysphagia in polymyositis/dermatomyositis.Clin Neurol Neurosurg.2004;107(1):32–37.
- ,.Skin involvement in dermatomyositis.Curr Opin Rheumatol.2003;15:714–22.
- ,,,,,.Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: analysis of 100 French Canadian patients.Medicine (Baltimore).2005;84:231–249.
- .Skin Pathology.2nd ed.New York:Churchill Livingstone;2002.
- ,.Utility of magnetic resonance imaging in the evaluation of patients with inflammatory myopathies.Curr Rheumatol Rep.2001;3:334–245.
- ,,.Is it really myositis? A consideration of the differential diagnosis.Curr Opin Rheumatol2004;16:684–691.
- .Idiopathic inflammatory myopathy: autoantibody update.Curr Rheumatol Rep.2002;4:434–441.
- ,,.Laboratory assessment in musculoskeletal disorders.Best Pract Res Clin Rheumatol.2003;17:475–494.
- ,.Dermatomyositis.Clin Dermatol.2006;24:363–373.
- ,,, et al.Frequency of specific cancer types in dermatomyositis and polymyositis: a population‐based study.Lancet.2001;357:96–100.
- ,,, et al.Cancer‐associated myositis: clinical features and prognostic signs.Ann N Y Acad Sci.2005;1051:64–71.
- ,,,,.Incidence of malignant disease in biopsy‐proven inflammatory myopathy. A population‐based cohort study.Ann Intern Med.2001;134:1087–1095.
- ,,.Risk of cancer in patients with dermatomyositis or polymyositis, and follow‐up implications: a Scottish population‐based cohort study.Br J Cancer.2001;85 (1):41–45.
- .When and how should the patient with dermatomyositis or amyopathic dermatomyositis be assessed for possible cancer?Arch Dermatol.2002;138:969–971.
- ,,.Ovarian cancer in patients with dermatomyositis.Medicine (Baltimore).1994;73(3):153–160.
- ,,,.Dermatomyositis sine myositis: association with malignancy.J Rheumatol.1996;23 (1):101–105.
- ,,, et al.Tumor antigen markers for the detection of solid cancers in inflammatory myopathies.Cancer Epidemiol Biomarkers Prev.2005;14:1279–1282.
- ,,, et al.Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: a study of 40 patients.Arch Dermatol.2002;138:885–890.
- ,,,,,.Dermatomyositis: a dermatology‐based case series.J Am Acad Dermatol.1998;38:397–404.
- ,,, et al.Long‐term outcome in polymyositis and dermatomyositis.Ann Rheum Dis.2006;65:1456–1461.
- .Our stubborn quest for diagnostic certainty. A cause of excessive testing.N Engl J Med.1989;320:1489–1491.
Case Report
A 5 year‐old girl presented to an emergency department (ED) with a 2‐day history of more than 12 episodes of nonbilious emesis. She received intravenous (IV) promethazine and 400 cc of normal saline and was discharged home. When emesis recurred the next morning, her pediatrician referred her for admission to our hospital for further hydration. The patient had neither fever nor rash but did report periumbilical pain and a few loose stools. She did not receive any medications other than the single dose of promethazine. Parents denied other toxic ingestion. The patient's family members had had a diarrheal illness over the past days.
The patient was born at term, small for gestational age, with uncomplicated pregnancy and delivery. She had normal growth and development and previously had been healthy, although her mother reported several prior ED visits for vomiting, including emesis with upper respiratory illnesses (URIs). There was no family history of gastrointestinal, metabolic, or renal disease. The patient lived with her parents and brother and attended kindergarten.
On examination, her temperature was 98.6F, blood pressure was 103/66, heart rate was 88, and respiratory rate was 24. She weighed 17.8 kg and was 115 cm tall (35th and 75th percentiles, respectively). She was alert and cooperative. Her breath had a ketotic odor. She had somewhat sunken eyes and dry mucous membranes. Her neck was supple, without lymphadenopathy. Capillary refill time was 3 seconds. Her lungs were clear to auscultation. Her abdomen was soft, nontender to palpation, and nondistended, and bowel sounds were hyperactive. Liver and spleen were of normal size. There was no edema, clubbing, nor cyanosis in the extremities. The patient was very fair‐skinned and did not have rashes, bruises, or other skin lesions. The results of her neurologic exam were entirely normal. Initial serum chemistry results were: sodium, 140 mEq/L; potassium, 5.9 mEq/L (hemolyzed); chloride, 107 mEq/L; bicarbonate, 13 mEq/L; glucose, 52 mg/dL; BUN, 19 mg/dL; creatinine, 0.5 mg/dL; and calcium, 10.5 mg/dL. Other laboratory analyses were ordered including urinalysis (UA), serum lactate, stool culture, rotaviral test, and fecal white blood cell count.
RESULTS
Further laboratory analysis revealed a urine pH of 5.5, specific gravity of 1.023, and 3+ ketones, with an otherwise normal UA. Blood lactic acid and pyruvic acid levels were normal at 0.8 and 0.11 mmol/L, respectively. All stool studies were negative. Tests to determine plasma quantitative amino acid and urine quantitative organic acid levels were ordered. The clinical course was benign, with recovery and normalization of blood chemistry values within 24 hours of IV hydration. The patient was discharged home the next day. Results of biochemical genetics laboratory testing were available 5 days later (Table 1). Plasma amino acids showed a decreased level of alanine, with elevated levels of leucine, isoleucine, and valine. L‐alloisoleucine, which is not normally in plasma but is pathognomic for maple syrup urine disease (MSUD), was detected. Urine organic acid test results were notable for ketonuria, with elevated branched‐chain 2‐hydroxy and 2‐oxo acids consistent with a diagnosis of MSUD (Table 1).
| Measured (mol/L) | Normal Range | |
|---|---|---|
| Plasma amino acids | ||
| Alanine | 103 | 246‐486 |
| Leucine | 434 | 61‐168 |
| Valine | 576 | 110‐279 |
| Isoleucine | 280 | 39‐88 |
| L‐Alloisoleucine | 28 | 0 |
| Measured (mmol/mol creatinine) | Normal Range | |
| Urine organic acids | ||
| 3‐Hydroxybutyric | 13,000 | 0‐10 |
| 2‐Hydroxyisovaleric | 16 | 0‐6 |
| 2‐Hydroxyisocaproic | 0 | 0‐2 |
| 2‐Hydroxy‐3‐methylvaleric | 0 | 0‐2 |
| 2‐Oxoisovaleric | 0 | 0‐2 |
| 2‐Oxoisocaproic | 0 | 0‐2 |
| 2‐Oxo‐3‐methylvaleric | 41 | 0‐2 |
The patient has done very well on follow‐up. Modest restriction of protein intake was instituted (<2 g/kg daily), and approximately 1 month after hospitalization a trial of thiamine was started. Plasma amino acid and urine organic acids have been normal on subsequent testing while the patient has been clinically well. Whole‐body leucine oxidation was estimated by quantitation of 13CO2 after administration of an oral bolus of 1‐13C‐leucine, following the protocol of Elsas et al.1 The enrichment of 13CO2 indicated oxidation of approximately 11% of the administered leucine over 3 hours, which was at the lower end of the normal range, with no increase after thiamine supplementation for 1 month. The patient did have 2 episodes of vomiting associated with mild intercurrent illness 1 month and 1 year after hospitalization. Care was sought promptly at urgent care centers. Ketonuria was documented; however, no blood tests were ordered. There were no changes in mental status, and oral rehydration was successful. Molecular analysis of the branched chain ketoacid dehydrogenase complex E1, E1, and E2 subunit genes did not reveal any mutations in the coding regions, although 3 sequence variations were observed in E1 (2 silent changes, c.972C>T [Phe324Phe] and c.1221A>G [Leu407Leu], and c.376G>T [Gly126Cys], at this time of unclear significance).
DISCUSSION
We report the case of a young girl who presented with what was initially labeled simple gastroenteritis. It is important to note, however, that she had several days of repeated emesis, no fever, and minimal diarrhea, along with multiple previous episodes of vomiting illnesses requiring ED visits. Combined, these prompted further evaluation of her acidosis. Maple syrup urine disease, a congenital condition that can be lethal, went undiagnosed in this patient until this admission when she was 5 years old.
Metabolic Acidosis
Metabolic acidosis is a very common laboratory abnormality caused by 1 of 3 basic mechanisms: loss of bicarbonate, impaired renal acid excretion, or the addition of either endogenous or exogenous acids to the body. Common causes of nonanion gap metabolic acidosis in children include diarrhea and renal tubular acidosis (RTA). Increased anion gap is associated with lactic acidosis, ketoacidosis, and ingestion of such substances as methanol, ethylene glycol, acetylsalicylic acid, and bismuth subsalicylate. Inborn errors of metabolism cause production of ketoacids, lactic acid, and other organic anions. This can occur chronically or during acute decompensation with illness, stress, or therapy noncompliance.2 Serum anion gap, glucose, ketones, lactate, and ammonia can help to elucidate the specific etiology of metabolic acidosis (Fig. 1).
In our patient whose anion gap was at the upper limit of normal, both increased gap and non‐anion gap were considered as causes of the metabolic acidosis. Diarrhea was minimal, and there was no history of toxin or medication ingestion other than promethazine. RTA was unlikely given the borderline high serum anion gap and normal UA. Lactate level was normal, and examination did not find evidence of profound dehydration, both refuting that she had lactic acidosis severe enough to account for a serum bicarbonate of 13 mEq/L. Inborn errors of metabolism were therefore strongly considered.
Maple Syrup Urine Disease
Background
MSUD, or branched‐chain ketoaciduria, is a disease resulting from defects in the catabolic pathway of the branched‐chain amino acids (BCAAs) isoleucine, leucine, and valine. The deficient enzyme is the branched‐chain alpha‐ketoacid dehydrogenase complex (BCKDC), an enzyme system responsible for oxidative decarboxylation of the 2‐oxoacid transamination products of isoleucine, leucine, and valine. BCKDC is made up of 4 subunits (E1, E1, E2, and E3); its coenzymes include thiamine (vitamin B1) and lipoic acid. Deficiency of BCKDC leads to accumulation of BCAAs and the related branched chain oxoacids and organic acid intermediates, including one (sotalone) that lends a sweet odor reminiscent of maple syrup to sweat, cerumen, and urine. MSUD is autosomal recessive, with more than 100 specific mutations identified in the 4 genes encoding BCKDC.3 MSUD is a rare disease, occurring in 1 of every 180,000 newborns in the United States.2
Clinical Phenotypes
MSUD has been divided into at least 5 clinical phenotypes,4 although in several cases distinctions are not clear. Differences result from variation in the severity of enzyme deficiency. Classic MSUD is the most severe form, with less than 2% of normal BCKDC function; it presents in the first week of life with poor feeding and neurologic signs such as hypo/hypertonia, seizures, lethargy, and coma.5 General laboratory findings are nonspecific except for ketoacidosis. This form is rapidly fatal in the first months of life if not treated. Intermediate MSUD is milder, having 3%‐30% of BCKDC activity. Patients manifest variable degrees of retardation, developmental delay, and failure to thrive, often without signs of ketoacidosis. Thiamine‐responsive MSUD is distinguished by the favorable response to high‐dose thiamine supplementation with significant reduction in BCAA level.6 Although it is reasonable to try treatment with thiamine in most cases of MSUD, responsive patients are very rare. MSUD due to a deficiency of the E3 subunit is the rarest form, described in fewer than 10 patients.7 Intermittent MSUD is the least severe form, with 5%‐20% BCKDC activity. Children develop with normal growth and intelligence but are at risk of acute metabolic decompensation during catabolic states such as stress, infection, or surgery. Recurrent episodes of ketoacidosis, ataxia, and lethargy can lead to coma and death if untreated.8 Initial symptoms usually occur by 2 years of age but have appeared as late as the fifth decade of life. Between episodes, a normal diet is tolerated without elevation of BCAA level.
Long‐term morbidity and mortality in MSUD is neurologic. Death is often a result of brain edema that is generally attributed to the osmotic effects of leucine and amino acid imbalance; however, pathophysiologic mechanisms remain unclear.9 Progressive white matter changes are thought to result from chronic exposure to leucine. Levels of some amino acids and neurotransmitters are reduced in MSUD, which may play a role in causing encephalopathies and coma.10
Diagnosis
Elevation of plasma BCAA level can be directly assessed by standard plasma amino acid analysis; reduction of alanine is also characteristic. L‐alloisoleucine is the most sensitive and specific marker of MSUD and is pathognomic for MSUD.1112 In most cases of intermittent MSUD it is detectable at all times, including when BCAA level is normal, but in some cases it may be absent between episodes. Urine organic acids show elevation of the 2‐oxoacids corresponding to leucine, valine, and isoleucine and the corresponding 2‐hydroxy‐acids. Modern newborn screening programs generally ascertain MSUD by liquid chromatographytandem mass spectrometry, which detects elevated levels of leucine, isoleucine, and L‐alloisoleucine or the ratio of these to alanine. BCAA concentration is elevated in plasma within hours of birth; however, it is unclear how often intermittent MSUD might be missed in newborn screening.
Treatment
Therapy consists of dietary control (limited protein intake) and, in some cases, thiamine supplementation. The goal is to limit BCAA so as not to overwhelm the capacity of the BCKDC. However, BCAAs are essential, and the challenge is therefore to provide appropriate amounts of protein to sustain growth without exceeding the individual's metabolic capacity. Generally, the amount of dietary protein tolerated is insufficient to provide enough of the other essential amino acids, so supplemental amino acids are needed. During acute episodes a BCAA‐free diet, sometimes with insulin and glucose, is used to encourage BCAA removal. If this anabolic approach is unsuccessful, dialysis may be used. Orthotopic liver transplantation has also been performed.1315 In all cases so far, BCAA level normalized after transplantation, and metabolic control was sustainable on a regular diet without protein restriction.13, 1618
Lessons for the Physician
Vomiting without true diarrhea deserves careful evaluation. Inborn errors of metabolism are individually rare but as a group are fairly common. One study noted an incidence of 15.7 per 100,000 births detected on tandem mass spectrometry screening, whereas rates of clinical detection are lower.19 This case illustrates how symptoms may be nonspecific and can easily mimic simple gastroenteritis. The following may be helpful when evaluating a patient with isolated emesis:
-
History: Does the patient have a history of emesis with illnesses that usually do not cause vomiting, such as an asthma flare or URI? Has the patient required emergency treatment or IV fluids for bouts of emesis in the past? Could toxins or medications that cause metabolic acidosis have been ingested?
-
Physical exam: Skin lesions, odors, and hepatomegaly are sometimes found in metabolic disorders. Commonly recognized odors include the musty smell of phenylketonuria and the sweet maple syrup smell of MSUD.
-
Laboratory studies: UA and calculation of anion gap are first steps to take to eliminate more common causes of metabolic acidosis in children.
-
Reliance on newborn screening: Patients with intermittent MSUD may have normal BCAA levels between acute episodes. Newborn screening tests may not identify these patients. The physician must maintain a degree of suspicion in approaching an acute illness that might indicate a metabolic disease, even in a child who has had negative expanded newborn screening.
Unusual disorders may masquerade as a simple problem. Common laboratory tests and a thorough history and exam can help to differentiate between simple gastroenteritis and inborn metabolic error and guide further diagnostic evaluation.
- ,,.Practical methods to estimate whole body leucine oxidation in maple syrup urine disease.Pediatr Res.1993;33:445–451.
- Behrman RE,Kliegman RM,Jenson HB, editors.Nelson Textbook of Pediatrics.Philadelphia:Saunders;2004:224–235,409–418.
- ,,.Lessons from genetic disorders of branched‐chain amino acid metabolism.J Nutr.2006;136(suppl 1):243S–249S.
- ,.Maple syrup urine disease (branched‐chain ketoaciduria). In:Scriver CR,Beaudet AL,Sly WS,Valle D,Vogelstein B,Childs B, editors.The Metabolic and Molecular Basis of Inherited Disease.New York:McGraw‐Hill;2001:1971–2006.
- ,,.A new syndrome: progressive familial infantile cerebral dysfunction associated with an unusual urinary substance.Pediatrics.1954;14:462–467.
- ,,,.Thiamine‐responsive maple‐syrup‐urine disease.Lancet.1971;1:310–312.
- ,,.Deficiency of dihydrolipoyl dehydrogenase (a component of the pyruvate and ‐ketoglutarate dehydrogenase complexes): a cause of congenital chronic lactic acidosis in infancy.Pediatr Res.1977;11:1198–202.
- ,,.Late‐onset branched‐chain ketoaciduria: (maple syrup urine disease).J Lancet.1966;86(3):149–152.
- ,,,.Cerebral edema causing death in children with maple syrup urine disease.J Pediatr.1991;119:42–45.
- ,,,,,,.Glutamate and γ‐aminobutyric acid neurotransmitter systems in the acute phase of maple syrup urine disease and citrullinemia encephalopathies in newborn calves.J Neurochem.1992;59:582–590.
- ,,,Maple syrup urine disease, with particular reference to dietotherapy.Pediatrics.1964;34:454–472.
- ,,,.Significance of L‐alloisoleucine in plasma for diagnosis of maple syrup urine disease.Clin Chem.1999;45:1734–40.
- ,,, et al.Mid‐term outcome of 2 cases with maple syrup urine disease: role of liver transplantation in the treatment.Arch Pediatr.1994;1:730–734.
- , et al.Transplantation for maple syrup urine disease (MSUD) and methylmalonic acidopathy (MMA).J Inherit Metab Dis.1997;20(suppl 1):37.
- ,,,.Liver transplantation in maple syrup urine disease.Eur J Pediatrics.1999;158(suppl 2):S60–S64.
- ,,, et al.Elective liver transplantation for the treatment of classical maple syrup urine disease.Am J Transplant.2006;6:557–564.
- ,,,,,.Domino liver transplantation in maple syrup urine disease.Liver Transpl.2006;12:876–882.
- ,,,.Branched‐chain L‐amino acid metabolism in classical maple syrup urine disease after orthotopic liver transplantation.J Inherit Metab Dis.2000;23:805–818.
- ,,,.Screening newborns for inborn errors of metabolism by tandem mass spectrometry.N Engl J Med.2003;348:2304–2312.
A 5 year‐old girl presented to an emergency department (ED) with a 2‐day history of more than 12 episodes of nonbilious emesis. She received intravenous (IV) promethazine and 400 cc of normal saline and was discharged home. When emesis recurred the next morning, her pediatrician referred her for admission to our hospital for further hydration. The patient had neither fever nor rash but did report periumbilical pain and a few loose stools. She did not receive any medications other than the single dose of promethazine. Parents denied other toxic ingestion. The patient's family members had had a diarrheal illness over the past days.
The patient was born at term, small for gestational age, with uncomplicated pregnancy and delivery. She had normal growth and development and previously had been healthy, although her mother reported several prior ED visits for vomiting, including emesis with upper respiratory illnesses (URIs). There was no family history of gastrointestinal, metabolic, or renal disease. The patient lived with her parents and brother and attended kindergarten.
On examination, her temperature was 98.6F, blood pressure was 103/66, heart rate was 88, and respiratory rate was 24. She weighed 17.8 kg and was 115 cm tall (35th and 75th percentiles, respectively). She was alert and cooperative. Her breath had a ketotic odor. She had somewhat sunken eyes and dry mucous membranes. Her neck was supple, without lymphadenopathy. Capillary refill time was 3 seconds. Her lungs were clear to auscultation. Her abdomen was soft, nontender to palpation, and nondistended, and bowel sounds were hyperactive. Liver and spleen were of normal size. There was no edema, clubbing, nor cyanosis in the extremities. The patient was very fair‐skinned and did not have rashes, bruises, or other skin lesions. The results of her neurologic exam were entirely normal. Initial serum chemistry results were: sodium, 140 mEq/L; potassium, 5.9 mEq/L (hemolyzed); chloride, 107 mEq/L; bicarbonate, 13 mEq/L; glucose, 52 mg/dL; BUN, 19 mg/dL; creatinine, 0.5 mg/dL; and calcium, 10.5 mg/dL. Other laboratory analyses were ordered including urinalysis (UA), serum lactate, stool culture, rotaviral test, and fecal white blood cell count.
RESULTS
Further laboratory analysis revealed a urine pH of 5.5, specific gravity of 1.023, and 3+ ketones, with an otherwise normal UA. Blood lactic acid and pyruvic acid levels were normal at 0.8 and 0.11 mmol/L, respectively. All stool studies were negative. Tests to determine plasma quantitative amino acid and urine quantitative organic acid levels were ordered. The clinical course was benign, with recovery and normalization of blood chemistry values within 24 hours of IV hydration. The patient was discharged home the next day. Results of biochemical genetics laboratory testing were available 5 days later (Table 1). Plasma amino acids showed a decreased level of alanine, with elevated levels of leucine, isoleucine, and valine. L‐alloisoleucine, which is not normally in plasma but is pathognomic for maple syrup urine disease (MSUD), was detected. Urine organic acid test results were notable for ketonuria, with elevated branched‐chain 2‐hydroxy and 2‐oxo acids consistent with a diagnosis of MSUD (Table 1).
| Measured (mol/L) | Normal Range | |
|---|---|---|
| Plasma amino acids | ||
| Alanine | 103 | 246‐486 |
| Leucine | 434 | 61‐168 |
| Valine | 576 | 110‐279 |
| Isoleucine | 280 | 39‐88 |
| L‐Alloisoleucine | 28 | 0 |
| Measured (mmol/mol creatinine) | Normal Range | |
| Urine organic acids | ||
| 3‐Hydroxybutyric | 13,000 | 0‐10 |
| 2‐Hydroxyisovaleric | 16 | 0‐6 |
| 2‐Hydroxyisocaproic | 0 | 0‐2 |
| 2‐Hydroxy‐3‐methylvaleric | 0 | 0‐2 |
| 2‐Oxoisovaleric | 0 | 0‐2 |
| 2‐Oxoisocaproic | 0 | 0‐2 |
| 2‐Oxo‐3‐methylvaleric | 41 | 0‐2 |
The patient has done very well on follow‐up. Modest restriction of protein intake was instituted (<2 g/kg daily), and approximately 1 month after hospitalization a trial of thiamine was started. Plasma amino acid and urine organic acids have been normal on subsequent testing while the patient has been clinically well. Whole‐body leucine oxidation was estimated by quantitation of 13CO2 after administration of an oral bolus of 1‐13C‐leucine, following the protocol of Elsas et al.1 The enrichment of 13CO2 indicated oxidation of approximately 11% of the administered leucine over 3 hours, which was at the lower end of the normal range, with no increase after thiamine supplementation for 1 month. The patient did have 2 episodes of vomiting associated with mild intercurrent illness 1 month and 1 year after hospitalization. Care was sought promptly at urgent care centers. Ketonuria was documented; however, no blood tests were ordered. There were no changes in mental status, and oral rehydration was successful. Molecular analysis of the branched chain ketoacid dehydrogenase complex E1, E1, and E2 subunit genes did not reveal any mutations in the coding regions, although 3 sequence variations were observed in E1 (2 silent changes, c.972C>T [Phe324Phe] and c.1221A>G [Leu407Leu], and c.376G>T [Gly126Cys], at this time of unclear significance).
DISCUSSION
We report the case of a young girl who presented with what was initially labeled simple gastroenteritis. It is important to note, however, that she had several days of repeated emesis, no fever, and minimal diarrhea, along with multiple previous episodes of vomiting illnesses requiring ED visits. Combined, these prompted further evaluation of her acidosis. Maple syrup urine disease, a congenital condition that can be lethal, went undiagnosed in this patient until this admission when she was 5 years old.
Metabolic Acidosis
Metabolic acidosis is a very common laboratory abnormality caused by 1 of 3 basic mechanisms: loss of bicarbonate, impaired renal acid excretion, or the addition of either endogenous or exogenous acids to the body. Common causes of nonanion gap metabolic acidosis in children include diarrhea and renal tubular acidosis (RTA). Increased anion gap is associated with lactic acidosis, ketoacidosis, and ingestion of such substances as methanol, ethylene glycol, acetylsalicylic acid, and bismuth subsalicylate. Inborn errors of metabolism cause production of ketoacids, lactic acid, and other organic anions. This can occur chronically or during acute decompensation with illness, stress, or therapy noncompliance.2 Serum anion gap, glucose, ketones, lactate, and ammonia can help to elucidate the specific etiology of metabolic acidosis (Fig. 1).

In our patient whose anion gap was at the upper limit of normal, both increased gap and non‐anion gap were considered as causes of the metabolic acidosis. Diarrhea was minimal, and there was no history of toxin or medication ingestion other than promethazine. RTA was unlikely given the borderline high serum anion gap and normal UA. Lactate level was normal, and examination did not find evidence of profound dehydration, both refuting that she had lactic acidosis severe enough to account for a serum bicarbonate of 13 mEq/L. Inborn errors of metabolism were therefore strongly considered.
Maple Syrup Urine Disease
Background
MSUD, or branched‐chain ketoaciduria, is a disease resulting from defects in the catabolic pathway of the branched‐chain amino acids (BCAAs) isoleucine, leucine, and valine. The deficient enzyme is the branched‐chain alpha‐ketoacid dehydrogenase complex (BCKDC), an enzyme system responsible for oxidative decarboxylation of the 2‐oxoacid transamination products of isoleucine, leucine, and valine. BCKDC is made up of 4 subunits (E1, E1, E2, and E3); its coenzymes include thiamine (vitamin B1) and lipoic acid. Deficiency of BCKDC leads to accumulation of BCAAs and the related branched chain oxoacids and organic acid intermediates, including one (sotalone) that lends a sweet odor reminiscent of maple syrup to sweat, cerumen, and urine. MSUD is autosomal recessive, with more than 100 specific mutations identified in the 4 genes encoding BCKDC.3 MSUD is a rare disease, occurring in 1 of every 180,000 newborns in the United States.2
Clinical Phenotypes
MSUD has been divided into at least 5 clinical phenotypes,4 although in several cases distinctions are not clear. Differences result from variation in the severity of enzyme deficiency. Classic MSUD is the most severe form, with less than 2% of normal BCKDC function; it presents in the first week of life with poor feeding and neurologic signs such as hypo/hypertonia, seizures, lethargy, and coma.5 General laboratory findings are nonspecific except for ketoacidosis. This form is rapidly fatal in the first months of life if not treated. Intermediate MSUD is milder, having 3%‐30% of BCKDC activity. Patients manifest variable degrees of retardation, developmental delay, and failure to thrive, often without signs of ketoacidosis. Thiamine‐responsive MSUD is distinguished by the favorable response to high‐dose thiamine supplementation with significant reduction in BCAA level.6 Although it is reasonable to try treatment with thiamine in most cases of MSUD, responsive patients are very rare. MSUD due to a deficiency of the E3 subunit is the rarest form, described in fewer than 10 patients.7 Intermittent MSUD is the least severe form, with 5%‐20% BCKDC activity. Children develop with normal growth and intelligence but are at risk of acute metabolic decompensation during catabolic states such as stress, infection, or surgery. Recurrent episodes of ketoacidosis, ataxia, and lethargy can lead to coma and death if untreated.8 Initial symptoms usually occur by 2 years of age but have appeared as late as the fifth decade of life. Between episodes, a normal diet is tolerated without elevation of BCAA level.
Long‐term morbidity and mortality in MSUD is neurologic. Death is often a result of brain edema that is generally attributed to the osmotic effects of leucine and amino acid imbalance; however, pathophysiologic mechanisms remain unclear.9 Progressive white matter changes are thought to result from chronic exposure to leucine. Levels of some amino acids and neurotransmitters are reduced in MSUD, which may play a role in causing encephalopathies and coma.10
Diagnosis
Elevation of plasma BCAA level can be directly assessed by standard plasma amino acid analysis; reduction of alanine is also characteristic. L‐alloisoleucine is the most sensitive and specific marker of MSUD and is pathognomic for MSUD.1112 In most cases of intermittent MSUD it is detectable at all times, including when BCAA level is normal, but in some cases it may be absent between episodes. Urine organic acids show elevation of the 2‐oxoacids corresponding to leucine, valine, and isoleucine and the corresponding 2‐hydroxy‐acids. Modern newborn screening programs generally ascertain MSUD by liquid chromatographytandem mass spectrometry, which detects elevated levels of leucine, isoleucine, and L‐alloisoleucine or the ratio of these to alanine. BCAA concentration is elevated in plasma within hours of birth; however, it is unclear how often intermittent MSUD might be missed in newborn screening.
Treatment
Therapy consists of dietary control (limited protein intake) and, in some cases, thiamine supplementation. The goal is to limit BCAA so as not to overwhelm the capacity of the BCKDC. However, BCAAs are essential, and the challenge is therefore to provide appropriate amounts of protein to sustain growth without exceeding the individual's metabolic capacity. Generally, the amount of dietary protein tolerated is insufficient to provide enough of the other essential amino acids, so supplemental amino acids are needed. During acute episodes a BCAA‐free diet, sometimes with insulin and glucose, is used to encourage BCAA removal. If this anabolic approach is unsuccessful, dialysis may be used. Orthotopic liver transplantation has also been performed.1315 In all cases so far, BCAA level normalized after transplantation, and metabolic control was sustainable on a regular diet without protein restriction.13, 1618
Lessons for the Physician
Vomiting without true diarrhea deserves careful evaluation. Inborn errors of metabolism are individually rare but as a group are fairly common. One study noted an incidence of 15.7 per 100,000 births detected on tandem mass spectrometry screening, whereas rates of clinical detection are lower.19 This case illustrates how symptoms may be nonspecific and can easily mimic simple gastroenteritis. The following may be helpful when evaluating a patient with isolated emesis:
-
History: Does the patient have a history of emesis with illnesses that usually do not cause vomiting, such as an asthma flare or URI? Has the patient required emergency treatment or IV fluids for bouts of emesis in the past? Could toxins or medications that cause metabolic acidosis have been ingested?
-
Physical exam: Skin lesions, odors, and hepatomegaly are sometimes found in metabolic disorders. Commonly recognized odors include the musty smell of phenylketonuria and the sweet maple syrup smell of MSUD.
-
Laboratory studies: UA and calculation of anion gap are first steps to take to eliminate more common causes of metabolic acidosis in children.
-
Reliance on newborn screening: Patients with intermittent MSUD may have normal BCAA levels between acute episodes. Newborn screening tests may not identify these patients. The physician must maintain a degree of suspicion in approaching an acute illness that might indicate a metabolic disease, even in a child who has had negative expanded newborn screening.
Unusual disorders may masquerade as a simple problem. Common laboratory tests and a thorough history and exam can help to differentiate between simple gastroenteritis and inborn metabolic error and guide further diagnostic evaluation.
A 5 year‐old girl presented to an emergency department (ED) with a 2‐day history of more than 12 episodes of nonbilious emesis. She received intravenous (IV) promethazine and 400 cc of normal saline and was discharged home. When emesis recurred the next morning, her pediatrician referred her for admission to our hospital for further hydration. The patient had neither fever nor rash but did report periumbilical pain and a few loose stools. She did not receive any medications other than the single dose of promethazine. Parents denied other toxic ingestion. The patient's family members had had a diarrheal illness over the past days.
The patient was born at term, small for gestational age, with uncomplicated pregnancy and delivery. She had normal growth and development and previously had been healthy, although her mother reported several prior ED visits for vomiting, including emesis with upper respiratory illnesses (URIs). There was no family history of gastrointestinal, metabolic, or renal disease. The patient lived with her parents and brother and attended kindergarten.
On examination, her temperature was 98.6F, blood pressure was 103/66, heart rate was 88, and respiratory rate was 24. She weighed 17.8 kg and was 115 cm tall (35th and 75th percentiles, respectively). She was alert and cooperative. Her breath had a ketotic odor. She had somewhat sunken eyes and dry mucous membranes. Her neck was supple, without lymphadenopathy. Capillary refill time was 3 seconds. Her lungs were clear to auscultation. Her abdomen was soft, nontender to palpation, and nondistended, and bowel sounds were hyperactive. Liver and spleen were of normal size. There was no edema, clubbing, nor cyanosis in the extremities. The patient was very fair‐skinned and did not have rashes, bruises, or other skin lesions. The results of her neurologic exam were entirely normal. Initial serum chemistry results were: sodium, 140 mEq/L; potassium, 5.9 mEq/L (hemolyzed); chloride, 107 mEq/L; bicarbonate, 13 mEq/L; glucose, 52 mg/dL; BUN, 19 mg/dL; creatinine, 0.5 mg/dL; and calcium, 10.5 mg/dL. Other laboratory analyses were ordered including urinalysis (UA), serum lactate, stool culture, rotaviral test, and fecal white blood cell count.
RESULTS
Further laboratory analysis revealed a urine pH of 5.5, specific gravity of 1.023, and 3+ ketones, with an otherwise normal UA. Blood lactic acid and pyruvic acid levels were normal at 0.8 and 0.11 mmol/L, respectively. All stool studies were negative. Tests to determine plasma quantitative amino acid and urine quantitative organic acid levels were ordered. The clinical course was benign, with recovery and normalization of blood chemistry values within 24 hours of IV hydration. The patient was discharged home the next day. Results of biochemical genetics laboratory testing were available 5 days later (Table 1). Plasma amino acids showed a decreased level of alanine, with elevated levels of leucine, isoleucine, and valine. L‐alloisoleucine, which is not normally in plasma but is pathognomic for maple syrup urine disease (MSUD), was detected. Urine organic acid test results were notable for ketonuria, with elevated branched‐chain 2‐hydroxy and 2‐oxo acids consistent with a diagnosis of MSUD (Table 1).
| Measured (mol/L) | Normal Range | |
|---|---|---|
| Plasma amino acids | ||
| Alanine | 103 | 246‐486 |
| Leucine | 434 | 61‐168 |
| Valine | 576 | 110‐279 |
| Isoleucine | 280 | 39‐88 |
| L‐Alloisoleucine | 28 | 0 |
| Measured (mmol/mol creatinine) | Normal Range | |
| Urine organic acids | ||
| 3‐Hydroxybutyric | 13,000 | 0‐10 |
| 2‐Hydroxyisovaleric | 16 | 0‐6 |
| 2‐Hydroxyisocaproic | 0 | 0‐2 |
| 2‐Hydroxy‐3‐methylvaleric | 0 | 0‐2 |
| 2‐Oxoisovaleric | 0 | 0‐2 |
| 2‐Oxoisocaproic | 0 | 0‐2 |
| 2‐Oxo‐3‐methylvaleric | 41 | 0‐2 |
The patient has done very well on follow‐up. Modest restriction of protein intake was instituted (<2 g/kg daily), and approximately 1 month after hospitalization a trial of thiamine was started. Plasma amino acid and urine organic acids have been normal on subsequent testing while the patient has been clinically well. Whole‐body leucine oxidation was estimated by quantitation of 13CO2 after administration of an oral bolus of 1‐13C‐leucine, following the protocol of Elsas et al.1 The enrichment of 13CO2 indicated oxidation of approximately 11% of the administered leucine over 3 hours, which was at the lower end of the normal range, with no increase after thiamine supplementation for 1 month. The patient did have 2 episodes of vomiting associated with mild intercurrent illness 1 month and 1 year after hospitalization. Care was sought promptly at urgent care centers. Ketonuria was documented; however, no blood tests were ordered. There were no changes in mental status, and oral rehydration was successful. Molecular analysis of the branched chain ketoacid dehydrogenase complex E1, E1, and E2 subunit genes did not reveal any mutations in the coding regions, although 3 sequence variations were observed in E1 (2 silent changes, c.972C>T [Phe324Phe] and c.1221A>G [Leu407Leu], and c.376G>T [Gly126Cys], at this time of unclear significance).
DISCUSSION
We report the case of a young girl who presented with what was initially labeled simple gastroenteritis. It is important to note, however, that she had several days of repeated emesis, no fever, and minimal diarrhea, along with multiple previous episodes of vomiting illnesses requiring ED visits. Combined, these prompted further evaluation of her acidosis. Maple syrup urine disease, a congenital condition that can be lethal, went undiagnosed in this patient until this admission when she was 5 years old.
Metabolic Acidosis
Metabolic acidosis is a very common laboratory abnormality caused by 1 of 3 basic mechanisms: loss of bicarbonate, impaired renal acid excretion, or the addition of either endogenous or exogenous acids to the body. Common causes of nonanion gap metabolic acidosis in children include diarrhea and renal tubular acidosis (RTA). Increased anion gap is associated with lactic acidosis, ketoacidosis, and ingestion of such substances as methanol, ethylene glycol, acetylsalicylic acid, and bismuth subsalicylate. Inborn errors of metabolism cause production of ketoacids, lactic acid, and other organic anions. This can occur chronically or during acute decompensation with illness, stress, or therapy noncompliance.2 Serum anion gap, glucose, ketones, lactate, and ammonia can help to elucidate the specific etiology of metabolic acidosis (Fig. 1).

In our patient whose anion gap was at the upper limit of normal, both increased gap and non‐anion gap were considered as causes of the metabolic acidosis. Diarrhea was minimal, and there was no history of toxin or medication ingestion other than promethazine. RTA was unlikely given the borderline high serum anion gap and normal UA. Lactate level was normal, and examination did not find evidence of profound dehydration, both refuting that she had lactic acidosis severe enough to account for a serum bicarbonate of 13 mEq/L. Inborn errors of metabolism were therefore strongly considered.
Maple Syrup Urine Disease
Background
MSUD, or branched‐chain ketoaciduria, is a disease resulting from defects in the catabolic pathway of the branched‐chain amino acids (BCAAs) isoleucine, leucine, and valine. The deficient enzyme is the branched‐chain alpha‐ketoacid dehydrogenase complex (BCKDC), an enzyme system responsible for oxidative decarboxylation of the 2‐oxoacid transamination products of isoleucine, leucine, and valine. BCKDC is made up of 4 subunits (E1, E1, E2, and E3); its coenzymes include thiamine (vitamin B1) and lipoic acid. Deficiency of BCKDC leads to accumulation of BCAAs and the related branched chain oxoacids and organic acid intermediates, including one (sotalone) that lends a sweet odor reminiscent of maple syrup to sweat, cerumen, and urine. MSUD is autosomal recessive, with more than 100 specific mutations identified in the 4 genes encoding BCKDC.3 MSUD is a rare disease, occurring in 1 of every 180,000 newborns in the United States.2
Clinical Phenotypes
MSUD has been divided into at least 5 clinical phenotypes,4 although in several cases distinctions are not clear. Differences result from variation in the severity of enzyme deficiency. Classic MSUD is the most severe form, with less than 2% of normal BCKDC function; it presents in the first week of life with poor feeding and neurologic signs such as hypo/hypertonia, seizures, lethargy, and coma.5 General laboratory findings are nonspecific except for ketoacidosis. This form is rapidly fatal in the first months of life if not treated. Intermediate MSUD is milder, having 3%‐30% of BCKDC activity. Patients manifest variable degrees of retardation, developmental delay, and failure to thrive, often without signs of ketoacidosis. Thiamine‐responsive MSUD is distinguished by the favorable response to high‐dose thiamine supplementation with significant reduction in BCAA level.6 Although it is reasonable to try treatment with thiamine in most cases of MSUD, responsive patients are very rare. MSUD due to a deficiency of the E3 subunit is the rarest form, described in fewer than 10 patients.7 Intermittent MSUD is the least severe form, with 5%‐20% BCKDC activity. Children develop with normal growth and intelligence but are at risk of acute metabolic decompensation during catabolic states such as stress, infection, or surgery. Recurrent episodes of ketoacidosis, ataxia, and lethargy can lead to coma and death if untreated.8 Initial symptoms usually occur by 2 years of age but have appeared as late as the fifth decade of life. Between episodes, a normal diet is tolerated without elevation of BCAA level.
Long‐term morbidity and mortality in MSUD is neurologic. Death is often a result of brain edema that is generally attributed to the osmotic effects of leucine and amino acid imbalance; however, pathophysiologic mechanisms remain unclear.9 Progressive white matter changes are thought to result from chronic exposure to leucine. Levels of some amino acids and neurotransmitters are reduced in MSUD, which may play a role in causing encephalopathies and coma.10
Diagnosis
Elevation of plasma BCAA level can be directly assessed by standard plasma amino acid analysis; reduction of alanine is also characteristic. L‐alloisoleucine is the most sensitive and specific marker of MSUD and is pathognomic for MSUD.1112 In most cases of intermittent MSUD it is detectable at all times, including when BCAA level is normal, but in some cases it may be absent between episodes. Urine organic acids show elevation of the 2‐oxoacids corresponding to leucine, valine, and isoleucine and the corresponding 2‐hydroxy‐acids. Modern newborn screening programs generally ascertain MSUD by liquid chromatographytandem mass spectrometry, which detects elevated levels of leucine, isoleucine, and L‐alloisoleucine or the ratio of these to alanine. BCAA concentration is elevated in plasma within hours of birth; however, it is unclear how often intermittent MSUD might be missed in newborn screening.
Treatment
Therapy consists of dietary control (limited protein intake) and, in some cases, thiamine supplementation. The goal is to limit BCAA so as not to overwhelm the capacity of the BCKDC. However, BCAAs are essential, and the challenge is therefore to provide appropriate amounts of protein to sustain growth without exceeding the individual's metabolic capacity. Generally, the amount of dietary protein tolerated is insufficient to provide enough of the other essential amino acids, so supplemental amino acids are needed. During acute episodes a BCAA‐free diet, sometimes with insulin and glucose, is used to encourage BCAA removal. If this anabolic approach is unsuccessful, dialysis may be used. Orthotopic liver transplantation has also been performed.1315 In all cases so far, BCAA level normalized after transplantation, and metabolic control was sustainable on a regular diet without protein restriction.13, 1618
Lessons for the Physician
Vomiting without true diarrhea deserves careful evaluation. Inborn errors of metabolism are individually rare but as a group are fairly common. One study noted an incidence of 15.7 per 100,000 births detected on tandem mass spectrometry screening, whereas rates of clinical detection are lower.19 This case illustrates how symptoms may be nonspecific and can easily mimic simple gastroenteritis. The following may be helpful when evaluating a patient with isolated emesis:
-
History: Does the patient have a history of emesis with illnesses that usually do not cause vomiting, such as an asthma flare or URI? Has the patient required emergency treatment or IV fluids for bouts of emesis in the past? Could toxins or medications that cause metabolic acidosis have been ingested?
-
Physical exam: Skin lesions, odors, and hepatomegaly are sometimes found in metabolic disorders. Commonly recognized odors include the musty smell of phenylketonuria and the sweet maple syrup smell of MSUD.
-
Laboratory studies: UA and calculation of anion gap are first steps to take to eliminate more common causes of metabolic acidosis in children.
-
Reliance on newborn screening: Patients with intermittent MSUD may have normal BCAA levels between acute episodes. Newborn screening tests may not identify these patients. The physician must maintain a degree of suspicion in approaching an acute illness that might indicate a metabolic disease, even in a child who has had negative expanded newborn screening.
Unusual disorders may masquerade as a simple problem. Common laboratory tests and a thorough history and exam can help to differentiate between simple gastroenteritis and inborn metabolic error and guide further diagnostic evaluation.
- ,,.Practical methods to estimate whole body leucine oxidation in maple syrup urine disease.Pediatr Res.1993;33:445–451.
- Behrman RE,Kliegman RM,Jenson HB, editors.Nelson Textbook of Pediatrics.Philadelphia:Saunders;2004:224–235,409–418.
- ,,.Lessons from genetic disorders of branched‐chain amino acid metabolism.J Nutr.2006;136(suppl 1):243S–249S.
- ,.Maple syrup urine disease (branched‐chain ketoaciduria). In:Scriver CR,Beaudet AL,Sly WS,Valle D,Vogelstein B,Childs B, editors.The Metabolic and Molecular Basis of Inherited Disease.New York:McGraw‐Hill;2001:1971–2006.
- ,,.A new syndrome: progressive familial infantile cerebral dysfunction associated with an unusual urinary substance.Pediatrics.1954;14:462–467.
- ,,,.Thiamine‐responsive maple‐syrup‐urine disease.Lancet.1971;1:310–312.
- ,,.Deficiency of dihydrolipoyl dehydrogenase (a component of the pyruvate and ‐ketoglutarate dehydrogenase complexes): a cause of congenital chronic lactic acidosis in infancy.Pediatr Res.1977;11:1198–202.
- ,,.Late‐onset branched‐chain ketoaciduria: (maple syrup urine disease).J Lancet.1966;86(3):149–152.
- ,,,.Cerebral edema causing death in children with maple syrup urine disease.J Pediatr.1991;119:42–45.
- ,,,,,,.Glutamate and γ‐aminobutyric acid neurotransmitter systems in the acute phase of maple syrup urine disease and citrullinemia encephalopathies in newborn calves.J Neurochem.1992;59:582–590.
- ,,,Maple syrup urine disease, with particular reference to dietotherapy.Pediatrics.1964;34:454–472.
- ,,,.Significance of L‐alloisoleucine in plasma for diagnosis of maple syrup urine disease.Clin Chem.1999;45:1734–40.
- ,,, et al.Mid‐term outcome of 2 cases with maple syrup urine disease: role of liver transplantation in the treatment.Arch Pediatr.1994;1:730–734.
- , et al.Transplantation for maple syrup urine disease (MSUD) and methylmalonic acidopathy (MMA).J Inherit Metab Dis.1997;20(suppl 1):37.
- ,,,.Liver transplantation in maple syrup urine disease.Eur J Pediatrics.1999;158(suppl 2):S60–S64.
- ,,, et al.Elective liver transplantation for the treatment of classical maple syrup urine disease.Am J Transplant.2006;6:557–564.
- ,,,,,.Domino liver transplantation in maple syrup urine disease.Liver Transpl.2006;12:876–882.
- ,,,.Branched‐chain L‐amino acid metabolism in classical maple syrup urine disease after orthotopic liver transplantation.J Inherit Metab Dis.2000;23:805–818.
- ,,,.Screening newborns for inborn errors of metabolism by tandem mass spectrometry.N Engl J Med.2003;348:2304–2312.
- ,,.Practical methods to estimate whole body leucine oxidation in maple syrup urine disease.Pediatr Res.1993;33:445–451.
- Behrman RE,Kliegman RM,Jenson HB, editors.Nelson Textbook of Pediatrics.Philadelphia:Saunders;2004:224–235,409–418.
- ,,.Lessons from genetic disorders of branched‐chain amino acid metabolism.J Nutr.2006;136(suppl 1):243S–249S.
- ,.Maple syrup urine disease (branched‐chain ketoaciduria). In:Scriver CR,Beaudet AL,Sly WS,Valle D,Vogelstein B,Childs B, editors.The Metabolic and Molecular Basis of Inherited Disease.New York:McGraw‐Hill;2001:1971–2006.
- ,,.A new syndrome: progressive familial infantile cerebral dysfunction associated with an unusual urinary substance.Pediatrics.1954;14:462–467.
- ,,,.Thiamine‐responsive maple‐syrup‐urine disease.Lancet.1971;1:310–312.
- ,,.Deficiency of dihydrolipoyl dehydrogenase (a component of the pyruvate and ‐ketoglutarate dehydrogenase complexes): a cause of congenital chronic lactic acidosis in infancy.Pediatr Res.1977;11:1198–202.
- ,,.Late‐onset branched‐chain ketoaciduria: (maple syrup urine disease).J Lancet.1966;86(3):149–152.
- ,,,.Cerebral edema causing death in children with maple syrup urine disease.J Pediatr.1991;119:42–45.
- ,,,,,,.Glutamate and γ‐aminobutyric acid neurotransmitter systems in the acute phase of maple syrup urine disease and citrullinemia encephalopathies in newborn calves.J Neurochem.1992;59:582–590.
- ,,,Maple syrup urine disease, with particular reference to dietotherapy.Pediatrics.1964;34:454–472.
- ,,,.Significance of L‐alloisoleucine in plasma for diagnosis of maple syrup urine disease.Clin Chem.1999;45:1734–40.
- ,,, et al.Mid‐term outcome of 2 cases with maple syrup urine disease: role of liver transplantation in the treatment.Arch Pediatr.1994;1:730–734.
- , et al.Transplantation for maple syrup urine disease (MSUD) and methylmalonic acidopathy (MMA).J Inherit Metab Dis.1997;20(suppl 1):37.
- ,,,.Liver transplantation in maple syrup urine disease.Eur J Pediatrics.1999;158(suppl 2):S60–S64.
- ,,, et al.Elective liver transplantation for the treatment of classical maple syrup urine disease.Am J Transplant.2006;6:557–564.
- ,,,,,.Domino liver transplantation in maple syrup urine disease.Liver Transpl.2006;12:876–882.
- ,,,.Branched‐chain L‐amino acid metabolism in classical maple syrup urine disease after orthotopic liver transplantation.J Inherit Metab Dis.2000;23:805–818.
- ,,,.Screening newborns for inborn errors of metabolism by tandem mass spectrometry.N Engl J Med.2003;348:2304–2312.