User login
Interventional psychiatry (Part 1)
Advances in the understanding of neurobiological and neuropsychiatric pathophysiology have opened new avenues of treatment for psychiatric patients. Historically, with a few exceptions, most psychiatric medications have been administered orally. However, many of the newer treatments require some form of specialized administration because they cannot be taken orally due to their chemical property (such as aducanumab); because there is the need to produce stable blood levels of the medication (such as brexanolone); because oral administration greatly diminished efficacy (such as oral vs IV magnesium or scopolamine), or because the treatment is focused on specific brain structures. This need for specialized administration has created a subspecialty called interventional psychiatry.
Part 1 of this 2-part article provides an overview of 1 type of interventional psychiatry: parenterally administered medications, including those administered via IV. We also describe 3 other interventional approaches to treatment: stellate ganglion blocks, glabellar botulinum toxin (BT) injections, and trigger point injections. In Part 2 we will review interventional approaches that involve neuromodulation.
Parenteral medications in psychiatry
In general, IV and IM medications can be more potent that oral medications due to their overall faster onset of action and higher blood concentrations. These more invasive forms of administration can have significant limitations, such as a risk of infection at the injection site, the need to be administered in a medical setting, additional time, and patient discomfort.
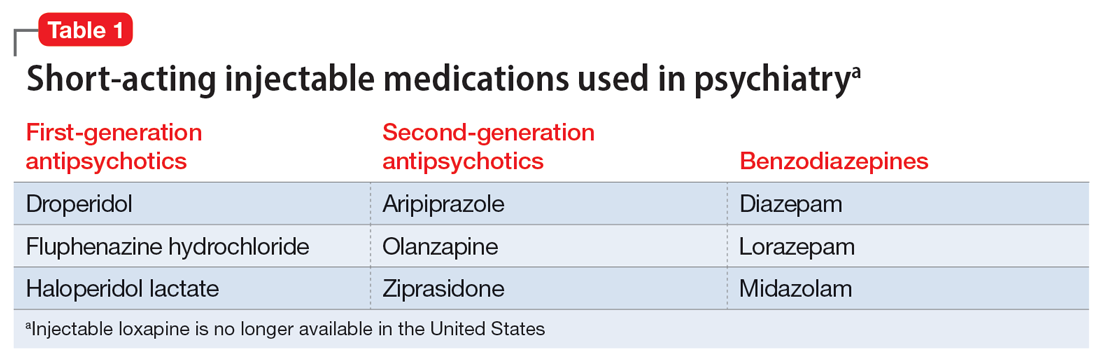
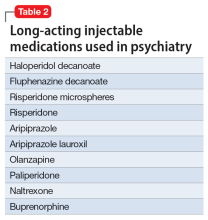
Table 1 lists short-acting injectable medications used in psychiatry, and Table 2 lists long-acting injectable medications. Parenteral administration of antipsychotics is performed to alleviate acute agitation or for chronic symptom control. These medications generally are not considered interventional treatments, but could be classified as such due to their invasive nature.1 Furthermore, inhalable loxapine—which is indicated for managing acute agitation—requires a Risk Evaluation and Mitigation Strategy program consisting of 2 hours observation and monitoring of respiratory status.2,3 Other indications for parenteral treatments include IM naltrexone extended release4 and subcutaneous injections of buprenorphine extended release5 and risperidone.6
IV administration
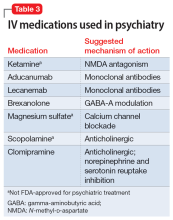
Most IV treatments described in this article are not FDA-approved for psychiatric treatment. Despite this, many interventional psychiatric treatments are part of clinical practice. IV infusion of ketamine is the most widely known and most researched of these. Table 3 lists other IV treatments that could be used as psychiatric treatment.
Ketamine
Since the early 1960s, ketamine has been used as a surgical anesthetic for animals. In the United States, it was approved for human surgical anesthesia in 1970. It was widely used during the Vietnam War due to its lack of inhibition of respiratory drive; medical staff first noticed an improvement in depressive symptoms and the resolution of suicidal ideation in patients who received ketamine. This led to further research on ketamine, particularly to determine its application in treatment-resistant depression (TRD) and other conditions.7 IV ketamine administration is most widely researched, but IM injections, intranasal sprays, and lozenges are also available. The dissociative properties of ketamine have led to its recreational use.8
Hypotheses for the mechanism of action of ketamine as an antidepressant include direct synaptic or extrasynaptic (GluN2B-selective), N-methyl-
Continue to: Ketamine is a schedule...
Ketamine is a schedule III medication with addictive properties. Delirium, panic attacks, hallucinations, nightmares, dysphoria, and paranoia may occur during and after use.13 Premedication with benzodiazepines, most notably lorazepam, is occasionally used to minimize ketamine’s adverse effects, but this generally is not recommended because doing so reduces ketamine’s antidepressant effects.14 Driving and operating heavy machinery is contraindicated after IV infusion. The usual protocol involves an IV infusion of ketamine 0.4 mg/kg to 1 mg/kg dosing over 1 hour. Doses between 0.4 mg/kg and 0.6 mg/kg are most common. Ketamine has a therapeutic window; doses >0.5 mg/kg are progressively less effective.15 Unlike the recommendation after esketamine administration, after receiving ketamine, patients remain in the care of their treatment team for <2 hours.
Esketamine, the S enantiomer of ketamine, was FDA-approved for TRD as an intranasal formulation. Esketamine is more commonly used than IV ketamine because it is FDA-approved and typically covered by insurance, but it may not be as effective.16 An economic analysis by Brendle et al17 suggested insurance companies would lower costs if they covered ketamine infusions vs intranasal esketamine.
Aducanumab and lecanemab
The most recent FDA-approved interventional agents are aducanumab and lecanemab, which are indicated for treating Alzheimer disease.18,19 Both are human monoclonal antibodies that bind selectively and with high affinity to amyloid beta plaque aggregates and promote their removal by Fc receptor–mediated phagocytosis.20
FDA approval of aducanumab and lecanemab was controversial. Initially, aducanumab’s safety monitoring board performed a futility analysis that suggested aducanumab was unlikely to separate from placebo, and the research was stopped.21 The manufacturer petitioned the FDA to consider the medication for accelerated approval on the basis of biomarker data showing that amyloid beta plaque aggregates become smaller. Current FDA approval is temporary to allow patients access to this potentially beneficial agent, but the manufacturer must supply clinical evidence that the reduction of amyloid beta plaques is associated with desirable changes in the course of Alzheimer disease, or risk losing the approval.
Lecanemab is also a human monoclonal antibody intended to remove amyloid beta plaques that was FDA-approved under the accelerated approval pathway.22 Unlike aducanumab, lecanemab demonstrated a statistically significant (although clinically imperceptible) reduction in the rate of cognitive decline; it did not show cognitive improvement.23 Lecanemab also significantly reduced amyloid beta plaques.23
Continue to: Aducanumab and lecanemab are generally...
Aducanumab and lecanemab are generally not covered by insurance and typically cost >$26,000 annually. Both are administered by IV infusion once a month. More monoclonal antibody medications for treating early Alzheimer disease are in the late stages of development, most notably donanebab.24 Observations during clinical trials found that in the later stages of Alzheimer disease, forceful removal of plaques by the autoimmune process damages neurons, while in less dense deposits of early dementia such removal is not harmful to the cells and prevents amyloid buildup.
Brexanolone is an aqueous formulation of allopregnanolone, a major metabolite of progesterone and a positive allosteric modulator of GABA-A receptors.25 Its levels are maximal at the end of the third trimester of pregnancy and fall rapidly following delivery. Research showed a 3-day infusion was rapidly and significantly effective for treating postpartum depression26 and brexanolone received FDA approval for this indication in March 2019.27 However, various administrative, economic, insurance, and other hurdles make it difficult for patients to access this treatment. Despite its rapid onset of action (usually 48 hours), brexanolone takes an average of 15 days to go through the prior authorization process.28 In addition to the need for prior authorization, the main impediment to the use of brexanolone is the 3-day infusion schedule, which greatly magnifies the cost but is partially circumvented by the availability of dedicated outpatient centers.
Magnesium
Magnesium is on the World Health Organization’s Model List of Essential Medicines.29 There has been extensive research on the use of magnesium sulfate for psychiatric indications, especially for depression.30 Magnesium functions similarly to calcium channel blockers by competitively blocking intracellular calcium channels, decreasing calcium availability, and inhibiting smooth muscle contractility.31 It also competes with calcium at the motor end plate, reducing excitation by inhibiting the release of acetylcholine.32 This property is used for high-dose IV magnesium treatment of impending preterm labor in obstetrics. Magnesium sulfate is the drug of choice in treating eclamptic seizures and preventing seizures in severe preeclampsia or gestational hypertension with severe features.33 It is also used to treat torsade de pointes, severe asthma exacerbations, constipation, and barium poisoning.34 Beneficial use in asthma treatment35 and the treatment of migraine36 have also been reported.
IV magnesium in myocardial infarction may be harmful,37 though outside of acute cardiac events, magnesium is found to be safe.38
Oral magnesium sulfate is a common over-the-counter anxiety remedy. As a general cell stabilizer (mediated by the reduction of intracellular calcium), magnesium is potentially beneficial outside of its muscle-relaxing properties, although muscle relaxing can benefit anxious patients. It is used to treat anxiety,39 alcohol withdrawal,40 and fear.41 Low magnesium blood levels are found in patients with depression, schizophrenia,42 and attention-deficit/hyperactivity disorder.43 However, it is important to note that the therapeutic effect of magnesium when treating anxiety and headache is independent of preinfusion magnesium blood levels.43
Continue to: The efficacy of oral magnesium...
The efficacy of oral magnesium is not robust. However, IV administration has a pronounced beneficial effect as an abortive and preventative treatment in many patients with anxiety.44
IV administration of magnesium can produce adverse effects, including flushing, sweating, hypotension, depressed reflexes, flaccid paralysis, hypothermia, circulatory collapse, and cardiac and CNS depression. These complications are very rare and dose-dependent.45 Magnesium is excreted by the kidneys, and dosing must be decreased in patients with kidney failure. The most common adverse effect is local burning along the vein upon infusion; small doses of IV lidocaine can remedy this. Hot flashes are also common.45
Various dosing strategies are available. In patients with anxiety, application dosing is based on the recommended preeclampsia IV dose of 4 g diluted in 250 mL of 5% dextrose. Much higher doses may be used in obstetrics. Unlike in obstetrics, for psychiatric indications, magnesium is administered over 60 to 90 minutes. Heart monitoring is recommended.
Scopolamine
Scopolamine is primarily used to relieve nausea, vomiting, and dizziness associated with motion sickness and recovery from anesthesia. It is also used in ophthalmology and in patients with excessive sweating. In off-label and experimental applications, scopolamine has been used in patients with TRD.46
Scopolamine is an anticholinergic medication. It is a nonselective antagonist at muscarinic receptors.47 Tricyclic antidepressants (TCAs) possess strong anticholinergic function. Newer generations of antidepressants were designed specifically not to have this function because it was believed to be an unwanted and potentially dangerous adverse effect. However, data suggest that anticholinergic action is important in decreasing depressive symptoms. Several hypotheses of anticholinergic effects on depression have been published since the 1970s. They include the cholinergic-adrenergic hypothesis,48 acetylcholine predominance relative to adrenergic action hypothesis,49 and insecticide poisoning observations.50 Centrally acting physostigmine (but not peripherally acting neostigmine) was reported to control mania.48,51 A genetic connection between the M2acetylcholine receptor in patients with major depressive disorder (MDD) and alcohol use disorder is also suggestive.52
Continue to: Multiple animal studies show...
Multiple animal studies show direct improvement in mobility and a decrease in despair upon introducing anticholinergic substances.53-55 The cholinergic theory of depression has been studied in several controlled clinical human studies.56,57 Use of a short-acting anticholinergic glycopyrrolate during electroconvulsive therapy (ECT) may contribute to the procedure’s efficacy.
Human research shows scopolamine has a higher efficacy as an antidepressant and anti-anxiety medication in women than in men,58 possibly because estrogen increases the activity of choline acetyltransferase and release of acetylcholine.59,60 M2receptors mediate estrogen influence on the NMDAR, which may explain the anticholinergic effects of depression treatments in women.61
Another proposed mechanism of action of scopolamine is a potent inhibition of the NMDAR.62 Rapid treatments of depression may be based on this mechanism. Examples of such treatments include IV ketamine and sleep deprivation.63 IV scopolamine shows potency in treating MDD and bipolar depression. This treatment should be reserved for patients who do not respond to or are not candidates for other usual treatment modalities of MDD and for the most severe cases. Scopolamine is 30 times more potent than amitriptyline in anticholinergic effect and reportedly produces sustained improvement in MDD.64
Scopolamine has no black-box warnings. It has not been studied in pregnant women and is not recommended for use during pregnancy. Due to possible negative cardiovascular effects, a normal electrocardiogram is required before the start of treatment. Exercise caution in patients with glaucoma, benign prostatic enlargement, gastroparesis, unstable cardiovascular status, or severe renal impairment.
Treatment with scopolamine is not indicated for patients with myasthenia gravis, psychosis, or seizures. Patients must be off potassium for 3 days before beginning scopolamine treatment. Patients should consult with their cardiologist before having a scopolamine infusion. Adverse reactions may include psychosis, tachycardia, seizures, paralytic ileus, and glaucoma exacerbation. The most common adverse effects of scopolamine infusion treatment include drowsiness, dry mouth, blurred vision, lightheadedness, and dizziness. Due to possible drowsiness, operating motor vehicles or heavy machinery must be avoided on the day of treatment.65 Overall, the adverse effects of scopolamine are preventable and manageable, and its antidepressant efficacy is noteworthy.66
Continue to: Treatment typically consists of 3 consecutive infusions...
Treatment typically consists of 3 consecutive infusions of 4 mcg/kg separated by 3 to 5 days.56 It is possible to have a longer treatment course if the patient experiences only partial improvement. Repeated courses or maintenance treatment (similar to ECT maintenance) are utilized in some patients if indicated. Cardiac monitoring is mandatory.
Clomipramine
Clomipramine, a TCA, acts as a preferential inhibitor of 5-hydroxytryptamine uptake and has proven effective in managing depression, TRD, and obsessive-compulsive disorder (OCD).67 Although this medication has reported treatment benefits for patients with phobia, panic disorder,15 chronic pain,68 Tourette syndrome,69 premature ejaculation, anorexia nervosa,70 cataplexy,49 and enuresis,71 it is FDA-approved only for the treatment of OCD.72 Clomipramine may also be beneficial for pain and headache, possibly because of its anti-inflammatory action.73 The anticholinergic effects of clomipramine may add to its efficacy in depression.
The pathophysiology of MDD is connected to hyperactivity of the HPA axis and elevated cortisol levels. Higher clomipramine plasma levels show a linear correlation with lower cortisol secretion and levels, possibly aiding in the treatment of MDD and anxiety.74 The higher the blood level, the more pronounced clomipramine’s therapeutic effect across multiple domains.75
IV infusion of clomipramine produces the highest concentration in the shortest time, but overall, research does not necessarily support increased efficacy of IV over oral administration. There is evidence suggesting that subgroups of patients with severe, treatment-refractory OCD may benefit from IV agents and research suggests a faster onset of action.76 Faster onset of symptom relief is the basis for IV clomipramine use. In patients with OCD, it can take several months for oral medications to produce therapeutic benefits; not all patients can tolerate this. In such scenarios, IV administration may be considered, though it is not appropriate for routine use until more research is available. Patients with treatment-resistant OCD who have exhausted other means of symptom relief may also be candidates for IV treatment.
The adverse effects of IV clomipramine are no different from oral use, though they may be more pronounced. A pretreatment cardiac exam is desirable because clomipramine, like other TCAs, may be cardiotoxic. The anticholinergic adverse effects of TCAs are well known to clinicians77 and partially explained in the scopolamine section of this article. It is not advisable to combine clomipramine with other TCAs or serotonin reuptake inhibitors. Clomipramine also should not be combined with monoamine oxidase inhibitors, though such a combination was reported in medical literature.78 Combination with antiarrhythmics such as lidocaine or opioids such as fentanyl or and tramadol is highly discouraged (fentanyl and tramadol also have serotonergic effects).79
Continue to: Clomipramine for IV use is not commercially available...
Clomipramine for IV use is not commercially available and must be sterilely compounded. The usual course of treatment is a series of 3 infusions: 150 mg on Day 1, 200 mg on Day 2 or Day 3, and 250 mg on Day 3, Day 4, or Day 5, depending on tolerability. A protocol with a 50 mg/d starting dose and titration up to a maximum dose of 225 mg/d over 5 to 7 days has been suggested for inpatient settings.67 Titration to 250 mg is more common.80
A longer series may be performed, but this increases the likelihood of adverse effects. Booster and maintenance treatments are also completed when required. Cardiac monitoring is mandatory.
Vortioxetine and citalopram
IV treatment of depression with
Injections and blocks
Three interventional approaches to treatment that possess psychotherapeutic potential include stellate ganglion blocks (SGBs), glabellar BT injections, and trigger point injections (TPIs). None of these are FDA-approved for psychiatric treatment.
Stellate ganglion blocks
The sympathetic nervous system is involved in autonomic hyperarousal and is at the core of posttraumatic symptomatology.83 Insomnia, anxiety, irritability, hypervigilance, and other excitatory CNS events are connected to the sympathetic nervous system and amygdala activation is commonly observed in those exposed to extreme stress or traumatic events.84
Continue to: SGBs were first performed 100 years ago...
SGBs were first performed 100 years ago and reported to have beneficial psychiatric effects at the end of the 1940s. In 1998 in Finland, improvement of posttraumatic stress disorder (PTSD) symptoms was observed accidentally via thoracic level spine blocks.85 In 2006, cervical level sympathetic blocks were shown to be effective for PTSD symptom control.86 By the end of 2010, Veterans Administration hospitals adopted SGBs to treat veterans with PTSD.87,88 The first multisite, randomized clinical trial of
Since the stellate ganglion is connected to the amygdala, SGB has also been assessed for treating anxiety and depression.89,90 Outside of PTSD, SGBs are used to treat complex regional pain syndrome,91 phantom limb pain, trigeminal neuralgia,92 intractable angina,93 and postherpetic neuralgia in the head, neck, upper chest, or arms.94 The procedure consists of an injection of a local anesthetic through a 25-gauge needle into the stellate sympathetic ganglion at the C6 or C7 vertebral levels. An injection into C6 is considered safer because of specific cervical spine anatomy. Ideally, fluoroscopic guidance or ultrasound is used to guide needle insertion.95
A severe drop in blood pressure may be associated with SGBs and is mitigated by IV hydration. Other adverse effects include red eyes, drooping of the eyelids, nasal congestion, hoarseness, difficulty swallowing, a sensation of a “lump” in the throat, and a sensation of warmth or tingling in the arm or hand. Bilateral SGB is contraindicated due to the danger of respiratory arrest.96
Glabellar BT injections
OnabotulinumtoxinA (BT) injection was first approved for therapeutic use in 1989 for eye muscle disorders such as strabismus97 and blepharospasm.98 It was later approved for several other indications, including cosmetic use, hyperhidrosis, migraine prevention, neurogenic bladder disorder, overactive bladder, urinary incontinence, and spasticity.99-104 BT is used off-label for achalasia and sialorrhea.105,106 Its mechanism of action is primarily attributed to muscle paralysis by blocking presynaptic acetylcholine release into neuromuscular junctions.107
Facial BT injections are usually administered for cosmetic purposes, but smoothing forehead wrinkles and frown lines (the glabellar region of the face) both have antidepressant effects.108 BT injections into the glabellar region also demonstrate antidepressant effects, particularly in patients with comorbid migraines and MDD.109 Early case observations supported the independent benefit of the toxin on MDD when the toxin was injected into the glabellar region.110,111 The most frequent protocol involves injections in the procerus and corrugator muscles.
Continue to: The facial feedback/emotional proprioception hypothesis...
The facial feedback/emotional proprioception hypothesis has dominated thinking about the mood-improving effects of BT. The theory is that blocking muscular expression of sadness (especially in the face) interrupts the experience of sadness; therefore, depression subsides.112,113 However, BT injections in the muscles involved in the smile and an expression of positive emotions (lateral part of the musculus orbicularis oculi) have been associated with increased MDD scores.114 Thus, the mechanism clearly involves more than the cosmetic effect, since facial muscle injections in rats also have antidepressant effects.115
The use of progressive muscle relaxation is well-established in psychiatric treatment. The investigated conditions of increased muscle tone, especially torticollis and blepharospasm, are associated with MDD, and it may be speculated that proprioceptive feedback from the affected muscles may be causally involved in this association.116-118 Activity of the corrugator muscle has been positively associated with increased amygdala activity.119 This suggests a potential similar mechanism to that hypothesized for SGB.
Alternatively, BT is commonly used to treat chronic conditions that may contribute to depression; its success in relieving the underlying problem may indirectly relieve MDD. Thus, in a postmarketing safety evaluation of BT, MDD was demonstrated 40% to 88% less often by patients treated with BT for 6 of the 8 conditions and injection sites, such as in spasms and spasticity of arms and legs, torticollis and neck pain, and axilla and palm injections for hyperhidrosis. In a parotid and submandibular glands BT injection subcohort, no patients experienced depressive symptoms.120
Medicinal BT is generally considered safe. The most common adverse effects are hypersensitivity, injection site reactions, and other adverse effects specific to the injection site.121 Additionally, the cosmetic effects are transient, given the nature of the medication.
Trigger point injections
TPIs in the neck and shoulders are frequently used to treat tension headaches and various referred pain locations in the face and arms. Tension and depression frequently overlap in clinical practice.122 Relieving muscle tension (with resulting trigger points) improves muscle function and mood.
Continue to: The higher the number of active...
The higher the number of active trigger points (TPs), the greater the physical burden of headache and the higher the anxiety level. Gender differences in TP prevalence and TPI efficacy have been described in the literature. For example, the number of active TPs seems directly associated with anxiety levels in women but not in men.123 Although TPs appear to be more closely associated with anxiety than depression,124 depression associated with muscle tension does improve with TPIs. European studies have demonstrated a decrease in scores on the Hamilton Depression Rating Scale and Hamilton Anxiety Rating Scale following TPI treatment.125 The effect may be indirect, as when a patient’s pain is relieved, sleep and other psychiatric symptoms improve.126
A randomized controlled trial by Castro Sánchez et al127 demonstrated that dry needling therapy in patients with fibromyalgia syndrome (FMS) showed improvements in pain pressure thresholds, body pain, vitality, and social function, as well as total FMS symptoms, quality of sleep, anxiety, hospital anxiety and depression, general pain intensity, and fatigue.
Myofascial pain syndrome, catastrophizing, and muscle tension are common in patients with depression, anxiety, and somatization. Local TPI therapy aimed at inactivating pain generators is supported by moderate quality evidence. All manner of therapies have been described, including injection of saline, corticosteroids, local anesthetic agents, and dry needling. BT injections in chronic TPs are also practiced, though no specific injection therapy has been reliably shown to be more advantageous than another. The benefits of TPIs may be derived from the needle itself rather than from any specific substance injected. Stimulation of a local twitch response with direct needling of the TP appears of importance. There is no established consensus regarding the number of injection points, frequency of administration, and volume or type of injectate.128
Adverse effects of TPIs relate to the nature of the invasive therapy, with the risk of tissue damage and bleeding. Pneumothorax risk is present with needle insertion at the neck and thorax.129 Patients with diabetes may experience variations in blood sugar control if steroids are used.
Bottom Line
Interventional treatment modalities that may have a role in psychiatric treatment include IV administration of ketamine, aducanumab, lecanemab, brexanolone, magnesium, scopolamine, and clomipramine. Other interventional approaches include stellate ganglion blocks, glabellar botulinum toxin injections, and trigger point injections.
Related Resources
- Dokucu ME, Janicak PG. Nontraditional therapies for treatment-resistant depression. Current Psychiatry. 2021; 20(9):38-43,49. doi:10.12788/cp.0166
- Kim J, Khoury R, Grossberg GT. Botulinum toxin: emerging psychiatric indications. Current Psychiatry. 2018;17(12):8-18.
Drug Brand Names
Aducanumab • Aduhelm
Aripiprazole • Abilify
Aripiprazole lauroxil • Aristada
Brexanolone • Zulresso
Buprenorphine • Sublocade
Citalopram • Celexa
Clomipramine • Anafranil
Diazepam • Valium
Droperidol • Inapsine
Esketamine • Spravato
Fentanyl • Actiq
Fluphenazine decanoate • Modecate
Fluphenazine hydrochloride • Prolixin
Haloperidol decanoate • Haldol decanoate
Haloperidol lactate • Haldol
Ketamine • Ketalar
Lecanemab • Leqembi
Lidocaine • Xylocaine
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Naltrexone • Vivitrol
Magnesium sulfate • Sulfamag
Midazolam • Versed
Olanzapine • Zyprexa
OnabotulinumtoxinA injection • Botox
Paliperidone • Invega Hafyera, Invega Sustenna, Invega Trinza
Rapamycin • Rapamune, Sirolimus
Risperidone • Perseris
Risperidone microspheres • Risperdal Consta, Rykindo
Scopolamine • Hyoscine
Tramadol • Conzip
Vortioxetine • Trintellix
Ziprasidone • Geodon
1. Vincent KM, Ryan M, Palmer E, et al. Interventional psychiatry. Postgrad Med. 2020;132(7):573-574.
2. Allen MH, Feifel D, Lesem MD, et al. Efficacy and safety of loxapine for inhalation in the treatment of agitation in patients with schizophrenia: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2011;72(10):1313-1321.
3. Kwentus J, Riesenberg RA, Marandi M, et al. Rapid acute treatment of agitation in patients with bipolar I disorder: a multicenter, randomized, placebo-controlled clinical trial with inhaled loxapine. Bipolar Disord. 2012;14(1):31-40.
4. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2019;393(10173):778-790.
6. Andorn A, Graham J, Csernansky J, et al. Monthly extended-release risperidone (RBP-7000) in the treatment of schizophrenia: results from the phase 3 program. J Clin Psychopharmacol. 2019;39(5):428-433.
7. Dundee TW. Twenty-five years of ketamine. A report of an international meeting. Anaesthesia. 1990;45(2):159. doi:10.1111/j.1365-2044.1990.tb14287.x
8. White PF, Way WL, Trevor AJ. Ketamine--its pharmacology and therapeutic uses. Anesthesiology. 1982;56(2):119-136. doi:10.1097/00000542-198202000-00007
9. Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23(4):801-811.
10. Molero P, Ramos-Quiroga JA, Martin-Santos R, et al. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs. 2018;32(5):411-420. doi:10.1007/s40263-018-0519-3
11. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
12. Witkin JM, Martin AE, Golani LK, et al. Rapid-acting antidepressants. Adv Pharmacol. 2019;86:47-96.
13. Strayer RJ, Nelson LS. Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med. 2008;26(9):985-1028. doi:10.1016/j.ajem.2007.12.005
14. Frye MA, Blier P, Tye SJ. Concomitant benzodiazepine use attenuates ketamine response: implications for large scale study design and clinical development. J Clin Psychopharmacol. 2015;35(3):334-336.
15. Fava M, Freeman MP, Flynn M, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. 2020;25(7):1592-1603.
16. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affect Disord. 2021;278:542-555. Erratum in: J Affect Disord. 2021;281:1001.
17. Brendle M, Robison R, Malone DC. Cost-effectiveness of esketamine nasal spray compared to intravenous ketamine for patients with treatment-resistant depression in the US utilizing clinical trial efficacy and real-world effectiveness estimates. J Affect Disord. 2022;319:388-396.
18. Dhillon S. Aducanumab: first approval. Drugs. 2021;81(12):1437-1443. Erratum in: Drugs. 2021;81(14):1701.
19. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
20. Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50-56. Update in: Nature. 2017;546(7659):564.
21. Fillit H, Green A. Aducanumab and the FDA – where are we now? Nat Rev Neurol. 2021;17(3):129-130.
22. Reardon S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature. 2023;613(7943):227-228. doi:10.1038/d41586-023-00030-3
23. McDade E, Cummings JL, Dhadda S, et al. Lecanemab in patients with early Alzheimer’s disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res Ther. 2022;14(1):191. doi:10.1186/s13195-022-01124-2
24. Mintun MA, Lo AC, Evans CD, et al. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384(18):1691-1704.
25. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433.
26. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
27. Powell JG, Garland S, Preston K, et al. Brexanolone (Zulresso): finally, an FDA-approved treatment for postpartum depression. Ann Pharmacother. 2020;54(2):157-163.
28. Patterson R, Krohn H, Richardson E, et al. A brexanolone treatment program at an academic medical center: patient selection, 90-day posttreatment outcomes, and lessons learned. J Acad Consult Liaison Psychiatry. 2022;63(1):14-22.
29. World Health Organization. WHO model list of essential medicines - 22nd list (2021). World Health Organization. September 30, 2021. Accessed April 7, 2023. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02
30. Eby GA, Eby KL, Mruk H. Magnesium and major depression. In: Vink R, Nechifor M, eds. Magnesium in the Central Nervous System. University of Adelaide Press; 2011.
31. Plant TM, Zeleznik AJ. Knobil and Neill’s Physiology of Reproduction. 4th ed. Elsevier Inc.; 2015:2503-2550.
32. Sidebotham D, Le Grice IJ. Physiology and pathophysiology. In: Sidebotham D, McKee A, Gillham M, Levy J. Cardiothoracic Critical Care. Elsevier, Inc.; 2007:3-27.
33. Duley L, Gülmezoglu AM, Henderson-Smart DJ, et al. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010;2010(11):CD000025.
34. Emergency supply of medicines. In: British National Formulary. British Medical Association, Royal Pharmaceutical Society; 2015:6. Accessed April 7, 2023. https://www.academia.edu/35076015/british_national_formulary_2015_pdf
35. Kwofie K, Wolfson AB. Intravenous magnesium sulfate for acute asthma exacerbation in children and adults. Am Fam Physician. 2021;103(4):245-246.
36. Patniyot IR, Gelfand AA. Acute treatment therapies for pediatric migraine: a qualitative systematic review. Headache. 2016;56(1):49-70.
37. Wang X, Du X, Yang H, et al. Use of intravenous magnesium sulfate among patients with acute myocardial infarction in China from 2001 to 2015: China PEACE-Retrospective AMI Study. BMJ Open. 2020;10(3):e033269.
38. Karhu E, Atlas SE, Jinrun G, et al. Intravenous infusion of magnesium sulfate is not associated with cardiovascular, liver, kidney, and metabolic toxicity in adults. J Clin Transl Res. 2018;4(1):47-55.
39. Noah L, Pickering G, Mazur A, et al. Impact of magnesium supplementation, in combination with vitamin B6, on stress and magnesium status: secondary data from a randomized controlled trial. Magnes Res. 2020;33(3):45-57.
40. Erstad BL, Cotugno CL. Management of alcohol withdrawal. Am J Health Syst Pharm. 1995;52(7):697-709.
41. Abumaria N, Luo L, Ahn M, et al. Magnesium supplement enhances spatial-context pattern separation and prevents fear overgeneralization. Behav Pharmacol. 2013;24(4):255-263.
42. Kirov GK, Tsachev KN. Magnesium, schizophrenia and manic-depressive disease. Neuropsychobiology. 1990;23(2):79-81.
43. Botturi A, Ciappolino V, Delvecchio G, et al. The role and the effect of magnesium in mental disorders: a systematic review. Nutrients. 2020;12(6):1661.
44. Kirkland AE, Sarlo GL, Holton KF. The role of magnesium in neurological disorders. Nutrients. 2018;10(6):730.
45. Magnesium sulfate intravenous side effects by likelihood and severity. WebMD. Accessed April 9, 2023. https://www.webmd.com/drugs/2/drug-149570/magnesium-sulfate-intravenous/details/list-sideeffects
46. Scopolamine base transdermal system – uses, side effects, and more. WebMD. Accessed April 9, 2023. https://www.webmd.com/drugs/2/drug-14032/scopolamine-transdermal/details
47. Bolden C, Cusack B, Richelson E. Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther. 1992;260(2):576-580.
48. Janowsky DS, el-Yousef MK, Davis JM, et al. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2(7778):632-635.
49. Janowsky DS, Risch SC, Gillin JC. Adrenergic-cholinergic balance and the treatment of affective disorders. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7(2-3):297-307.
50. Gershon S, Shaw FH. Psychiatric sequelae of chronic exposure to organophosphorous insecticides. Lancet. 1972;1(7191):1371-1374.
51. Davis KL, Berger PA, Hollister LE, et al. Physostigmine in mania. Arch Gen Psychiatry. 1978;35(1):119-122.
52. Wang JC, Hinrichs AL, Stock H, et al. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13(17):1903-1911.
53. Brown RG. Effects of antidepressants and anticholinergics in a mouse “behavioral despair” test. Eur J Pharmacol. 1979;58(3):331-334.
54. Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730-732.
55. Ji CX, Zhang JJ. Effect of scopolamine on depression in mice. Abstract in English. Yao Xue Xue Bao. 2011;46(4):400-405.
56. Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63(10):1121-1129.
57. Drevets WC, Furey ML. Replication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67(5):432-438.
58. Furey ML, Khanna A, Hoffman EM, et al. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35(12):2479-2488.
59. Gibbs RB, Gabor R, Cox T, et al. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29(6):741-748.
60. Pongrac JL, Gibbs RB, Defranco DB. Estrogen-mediated regulation of cholinergic expression in basal forebrain neurons requires extracellular-signal-regulated kinase activity. Neuroscience. 2004;124(4):809-816.
61. Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21(17):6949-6956.
62. Gerhard DM, Wohleb ES, Duman RS. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today. 2016;21(3):454-464.
63. Voderholzer U. Sleep deprivation and antidepressant treatment. Dialogues Clin Neurosci. 2003;5(4):366-369.
64. Hasselmann H. Scopolamine and depression: a role for muscarinic antagonism? CNS Neurol Disord Drug Targets. 2014;13(4):673-683.
65. Transderm scopolamine [prescribing information]. Warren, NJ: GSK Consumer Healthcare; 2019.
66. Jaffe RJ, Novakovic V, Peselow ED. Scopolamine as an antidepressant: a systematic review. Clin Neuropharmacol. 2013;36(1):24-26.
67. Karameh WK, Khani M. Intravenous clomipramine for treatment-resistant obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2015;19(2):pyv084.
68. Andrews ET, Beattie RM, Tighe MP. Functional abdominal pain: what clinicians need to know. Arch Dis Child. 2020;105(10):938-944. doi:10.1136/archdischild-2020-318825
69. Aliane V, Pérez S, Bohren Y, et al. Key role of striatal cholinergic interneurons in processes leading to arrest of motor stereotypies. Brain. 2011;134(Pt 1):110-118. doi:10.1093/brain/awq285
70. Tzavara ET, Bymaster FP, Davis RJ, et al. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 2004;18(12):1410-1412. doi:10.1096/fj.04-1575fje
71. Korczyn AD, Kish I. The mechanism of imipramine in enuresis nocturna. Clin Exp Pharmacol Physiol. 1979;6(1):31-35. doi:10.1111/j.1440-1681.1979.tb00004.x
72. Trimble MR. Worldwide use of clomipramine. J Clin Psychiatry. 1990;51(Suppl):51-54; discussion 55-58.
73. Gong W, Zhang S, Zong Y, et al. Involvement of the microglial NLRP3 inflammasome in the anti-inflammatory effect of the antidepressant clomipramine. J Affect Disord. 2019;254:15-25.
74. Piwowarska J, Wrzosek M, Radziwon’-Zaleska M. Serum cortisol concentration in patients with major depression after treatment with clomipramine. Pharmacol Rep. 2009;61(4):604-611.
75. Danish University Antidepressant Group (DUAG). Clomipramine dose-effect study in patients with depression: clinical end points and pharmacokinetics. Clin Pharmacol Ther. 1999;66(2):152-165.
76. Moukaddam NJ, Hirschfeld RMA. Intravenous antidepressants: a review. Depress Anxiety. 2004;19(1):1-9.
77. Gerretsen P, Pollock BG. Rediscovering adverse anticholinergic effects. J Clin Psychiatry. 2011;72(6):869-870. doi:10.4088/JCP.11ac07093
78. Thomas SJ, Shin M, McInnis MG, et al. Combination therapy with monoamine oxidase inhibitors and other antidepressants or stimulants: strategies for the management of treatment-resistant depression. Pharmacotherapy. 2015;35(4):433-449. doi:10.1002/phar.1576
79. Robles LA. Serotonin syndrome induced by fentanyl in a child: case report. Clin Neuropharmacol. 2015;38(5):206-208. doi:10.1097/WNF.0000000000000100
80. Fallon BA, Liebowitz MR, Campeas R, et al. Intravenous clomipramine for obsessive-compulsive disorder refractory to oral clomipramine: a placebo-controlled study. Arch Gen Psychiatry. 1998;55(10):918-924.
81. Vieta E, Florea I, Schmidt SN, et al. Intravenous vortioxetine to accelerate onset of effect in major depressive disorder: a 2-week, randomized, double-blind, placebo-controlled study. Int Clin Psychopharmacol. 2019;34(4):153-160.
82. Kasper S, Müller-Spahn F. Intravenous antidepressant treatment: focus on citalopram. Eur Arch Psychiatry Clin Neurosci. 2002;252(3):105-109.
83. Togay B, El-Mallakh RS. Posttraumatic stress disorder: from pathophysiology to pharmacology. Current Psychiatry. 2020;19(5):33-39.
84. Adhikari A, Lerner TN, Finkelstein J, et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature. 2015;527(7577):179-185. doi:10.1038/nature15698
85. Lipov E. In search of an effective treatment for combat-related post-traumatic stress disorder (PTSD): can the stellate ganglion block be the answer? Pain Pract. 2010;10(4):265-266.
86. Lipov E, Ritchie EC. A review of the use of stellate ganglion block in the treatment of PTSD. Curr Psychiatry Rep. 2015;17(8):599.
87. Olmsted KLR, Bartoszek M, McLean B, et al. Effect of stellate ganglion block treatment on posttraumatic stress disorder symptoms: a randomized clinical trial. JAMA Psychiatry. 2020;77(2):130-138.
88. Lipov E, Candido K. The successful use of left-sided stellate ganglion block in patients that fail to respond to right-sided stellate ganglion block for the treatment of post-traumatic stress disorder symptoms: a retrospective analysis of 205 patients. Mil Med. 2021;186(11-12):319-320.
89. Li Y, Loshak H. Stellate ganglion block for the treatment of post-traumatic stress disorder, depression, and anxiety. Canadian J Health Technol. 2021;1(3):1-30.
90. Kerzner J, Liu H, Demchenko I, et al. Stellate ganglion block for psychiatric disorders: a systematic review of the clinical research landscape. Chronic Stress (Thousand Oaks). 2021;5:24705470211055176.
91. Wie C, Gupta R, Maloney J, et al. Interventional modalities to treat complex regional pain syndrome. Curr Pain Headache Rep. 2021;25(2):10. doi:10.1007/s11916-020-00904-5
92. Chaturvedi A, Dash HH. Sympathetic blockade for the relief of chronic pain. J Indian Med Assoc. 2001;99(12):698-703.
93. Chester M, Hammond C. Leach A. Long-term benefits of stellate ganglion block in severe chronic refractory angina. Pain. 2000;87(1):103-105. doi:10.1016/S0304-3959(00)00270-0
94. Jeon Y. Therapeutic potential of stellate ganglion block in orofacial pain: a mini review. J Dent Anesth Pain Med. 2016;16(3):159-163. doi:10.17245/jdapm.2016.16.3.159
95. Shan HH, Chen HF, Ni Y, et al. Effects of stellate ganglion block through different approaches under guidance of ultrasound. Front Surg. 2022;8:797793. doi:10.3389/fsurg.2021.797793
96. Goel V, Patwardhan AM, Ibrahim M, et al. Complications associated with stellate ganglion nerve block: a systematic review. Reg Anesth Pain Med. 2019;rapm-2018-100127. doi:10.1136/rapm-2018-100127
97. Rowe FJ, Noonan CP. Botulinum toxin for the treatment of strabismus. Cochrane Database Syst Rev. 2017;3(3):CD006499.
98. Roggenkämper P, Jost WH, Bihari K, et al. Efficacy and safety of a new botulinum toxin type A free of complexing proteins in the treatment of blepharospasm. J Neural Transm (Vienna). 2006;113(3):303-312.
99. Heckmann M, Ceballos-Baumann AO, Plewig G; Hyperhidrosis Study Group. Botulinum toxin A for axillary hyperhidrosis (excessive sweating). N Engl J Med. 2001;344(7):488-493.
100. Carruthers JA, Lowe NJ, Menter MA, et al. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 2002;46(6):840-849.
101. Schurch B, de Sèze M, Denys P, et al. Botulinum toxin type A is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol. 2005;174:196–200.
102. Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: Pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51(9):1358-1373.
103. Dashtipour K, Chen JJ, Walker HW, et al. Systematic literature review of abobotulinumtoxinA in clinical trials for adult upper limb spasticity. Am J Phys Med Rehabil. 2015;94(3):229-238.
104. Nitti VW, Dmochowski R, Herschorn S, et al. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo-controlled trial. J Urol. 2017;197(2S):S216-S223.
105. Jongerius PH, van den Hoogen FJA, van Limbeek J, et al. Effect of botulinum toxin in the treatment of drooling: a controlled clinical trial. Pediatrics. 2004;114(3):620-627.
106. Zaninotto, G. Annese V, Costantini M, et al. Randomized controlled trial of botulinum toxin versus laparoscopic heller myotomy for esophageal achalasia. Ann Surg. 2004;239(3):364-370.
107. Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005;53:3-9.
108. Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J Cosmet Dermatol. 2009;8(1):24-26.
109. Affatato O, Moulin TC, Pisanu C, et al. High efficacy of onabotulinumtoxinA treatment in patients with comorbid migraine and depression: a meta-analysis. J Transl Med. 2021;19(1):133.
110. Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: a case series. Dermatol Surg. 2006;32(5):645-649; discussion 649-650.
111. Schulze J, Neumann I, Magid M, et al. Botulinum toxin for the management of depression: an updated review of the evidence and meta-analysis. J Psychiatr Res. 2021;135:332-340.
112. Finzi E, Rosenthal NE. Emotional proprioception: treatment of depression with afferent facial feedback. J Psychiatr Res. 2016;80:93-96.
113. Söderkvist S, Ohlén K, Dimberg U. How the experience of emotion is modulated by facial feedback. J Nonverbal Behav. 2018;42(1):129-151.
114. Lewis, MB. The interactions between botulinum-toxin-based facial treatments and embodied emotions. Sci Rep. 2018;8(1):14720.
115. Li Y, Liu J, Liu X, et al. Antidepressant-like action of single facial injection of botulinum neurotoxin A is associated with augmented 5-HT levels and BDNF/ERK/CREB pathways in mouse brain. Neurosci Bull. 2019;35(4):661-672. Erratum in: Neurosci Bull. 2019;35(4):779-780.
116. Gündel H, Wolf A, Xidara V, et al. High psychiatric comorbidity in spasmodic torticollis: a controlled study. J Nerv Ment Dis. 2003;191(7):465-473.
117. Hall TA, McGwin G Jr, Searcey K, et al. Health-related quality of life and psychosocial characteristics of patients with benign essential blepharospasm. Arch Ophthalmol. 2006;124(1):116-119.
118. Ceylan D, Erer S, Zarifog˘lu M, et al. Evaluation of anxiety and depression scales and quality of life in cervical dystonia patients on botulinum toxin therapy and their relatives. Neurol Sci. 2019;40(4):725-731.
119. Heller AS, Lapate RC, Mayer KE, et al. The face of negative affect: trial-by-trial corrugator responses to negative pictures are positively associated with amygdala and negatively associated with ventromedial prefrontal cortex activity. J Cogn Neurosci. 2014;26(9):2102-2110.
120. Makunts T, Wollmer MA, Abagyan R. Postmarketing safety surveillance data reveals antidepressant effects of botulinum toxin across various indications and injection sites. Sci Rep. 2020;10(1):12851.
121. Ahsanuddin S, Roy S, Nasser W, et al. Adverse events associated with botox as reported in a Food and Drug Administration database. Aesthetic Plast Surg. 2021;45(3):1201-1209. doi:10.1007/s00266-020-02027-z
122. Kashif M, Tahir S, Ashfaq F, et al. Association of myofascial trigger points in neck and shoulder region with depression, anxiety, and stress among university students. J Pak Med Assoc. 2021;71(9):2139-2142.
123. Cigarán-Méndez M, Jiménez-Antona C, Parás-Bravo P, et al. Active trigger points are associated with anxiety and widespread pressure pain sensitivity in women, but not men, with tension type headache. Pain Pract. 2019;19(5):522-529.
124. Palacios-Ceña M, Castaldo M, Wang K, et al. Relationship of active trigger points with related disability and anxiety in people with tension-type headache. Medicine (Baltimore). 2017;96(13):e6548.
125. Karadas Ö, Inan LE, Ulas Ü, et al. Efficacy of local lidocaine application on anxiety and depression and its curative effect on patients with chronic tension-type headache. Eur Neurol. 2013;70(1-2):95-101.
126. Gerwin RD. Classification, epidemiology and natural history of myofascial pain syndrome. Curr Pain Headache Rep. 2001;5(5):412-420.
127. Castro Sánchez AM, García López H, Fernández Sánchez M, et al. Improvement in clinical outcomes after dry needling versus myofascial release on pain pressure thresholds, quality of life, fatigue, pain intensity, quality of sleep, anxiety, and depression in patients with fibromyalgia syndrome. Disabil Rehabil. 2019;41(19):2235-2246.
128. Healy GM, Finn DP, O’Gorman DA, et al. Pretreatment anxiety and pain acceptance are associated with response to trigger point injection therapy for chronic myofascial pain. Pain Med. 2015;16(10):1955-1966.
129. Morjaria JB, Lakshminarayana UB, Liu-Shiu-Cheong P, et al. Pneumothorax: a tale of pain or spontaneity. Ther Adv Chronic Dis. 2014;5(6):269-273.
Advances in the understanding of neurobiological and neuropsychiatric pathophysiology have opened new avenues of treatment for psychiatric patients. Historically, with a few exceptions, most psychiatric medications have been administered orally. However, many of the newer treatments require some form of specialized administration because they cannot be taken orally due to their chemical property (such as aducanumab); because there is the need to produce stable blood levels of the medication (such as brexanolone); because oral administration greatly diminished efficacy (such as oral vs IV magnesium or scopolamine), or because the treatment is focused on specific brain structures. This need for specialized administration has created a subspecialty called interventional psychiatry.
Part 1 of this 2-part article provides an overview of 1 type of interventional psychiatry: parenterally administered medications, including those administered via IV. We also describe 3 other interventional approaches to treatment: stellate ganglion blocks, glabellar botulinum toxin (BT) injections, and trigger point injections. In Part 2 we will review interventional approaches that involve neuromodulation.
Parenteral medications in psychiatry
In general, IV and IM medications can be more potent that oral medications due to their overall faster onset of action and higher blood concentrations. These more invasive forms of administration can have significant limitations, such as a risk of infection at the injection site, the need to be administered in a medical setting, additional time, and patient discomfort.

Table 1 lists short-acting injectable medications used in psychiatry, and Table 2 lists long-acting injectable medications. Parenteral administration of antipsychotics is performed to alleviate acute agitation or for chronic symptom control. These medications generally are not considered interventional treatments, but could be classified as such due to their invasive nature.1 Furthermore, inhalable loxapine—which is indicated for managing acute agitation—requires a Risk Evaluation and Mitigation Strategy program consisting of 2 hours observation and monitoring of respiratory status.2,3 Other indications for parenteral treatments include IM naltrexone extended release4 and subcutaneous injections of buprenorphine extended release5 and risperidone.6
IV administration
Most IV treatments described in this article are not FDA-approved for psychiatric treatment. Despite this, many interventional psychiatric treatments are part of clinical practice. IV infusion of ketamine is the most widely known and most researched of these. Table 3 lists other IV treatments that could be used as psychiatric treatment.
Ketamine
Since the early 1960s, ketamine has been used as a surgical anesthetic for animals. In the United States, it was approved for human surgical anesthesia in 1970. It was widely used during the Vietnam War due to its lack of inhibition of respiratory drive; medical staff first noticed an improvement in depressive symptoms and the resolution of suicidal ideation in patients who received ketamine. This led to further research on ketamine, particularly to determine its application in treatment-resistant depression (TRD) and other conditions.7 IV ketamine administration is most widely researched, but IM injections, intranasal sprays, and lozenges are also available. The dissociative properties of ketamine have led to its recreational use.8
Hypotheses for the mechanism of action of ketamine as an antidepressant include direct synaptic or extrasynaptic (GluN2B-selective), N-methyl-
Continue to: Ketamine is a schedule...
Ketamine is a schedule III medication with addictive properties. Delirium, panic attacks, hallucinations, nightmares, dysphoria, and paranoia may occur during and after use.13 Premedication with benzodiazepines, most notably lorazepam, is occasionally used to minimize ketamine’s adverse effects, but this generally is not recommended because doing so reduces ketamine’s antidepressant effects.14 Driving and operating heavy machinery is contraindicated after IV infusion. The usual protocol involves an IV infusion of ketamine 0.4 mg/kg to 1 mg/kg dosing over 1 hour. Doses between 0.4 mg/kg and 0.6 mg/kg are most common. Ketamine has a therapeutic window; doses >0.5 mg/kg are progressively less effective.15 Unlike the recommendation after esketamine administration, after receiving ketamine, patients remain in the care of their treatment team for <2 hours.
Esketamine, the S enantiomer of ketamine, was FDA-approved for TRD as an intranasal formulation. Esketamine is more commonly used than IV ketamine because it is FDA-approved and typically covered by insurance, but it may not be as effective.16 An economic analysis by Brendle et al17 suggested insurance companies would lower costs if they covered ketamine infusions vs intranasal esketamine.
Aducanumab and lecanemab
The most recent FDA-approved interventional agents are aducanumab and lecanemab, which are indicated for treating Alzheimer disease.18,19 Both are human monoclonal antibodies that bind selectively and with high affinity to amyloid beta plaque aggregates and promote their removal by Fc receptor–mediated phagocytosis.20
FDA approval of aducanumab and lecanemab was controversial. Initially, aducanumab’s safety monitoring board performed a futility analysis that suggested aducanumab was unlikely to separate from placebo, and the research was stopped.21 The manufacturer petitioned the FDA to consider the medication for accelerated approval on the basis of biomarker data showing that amyloid beta plaque aggregates become smaller. Current FDA approval is temporary to allow patients access to this potentially beneficial agent, but the manufacturer must supply clinical evidence that the reduction of amyloid beta plaques is associated with desirable changes in the course of Alzheimer disease, or risk losing the approval.
Lecanemab is also a human monoclonal antibody intended to remove amyloid beta plaques that was FDA-approved under the accelerated approval pathway.22 Unlike aducanumab, lecanemab demonstrated a statistically significant (although clinically imperceptible) reduction in the rate of cognitive decline; it did not show cognitive improvement.23 Lecanemab also significantly reduced amyloid beta plaques.23
Continue to: Aducanumab and lecanemab are generally...
Aducanumab and lecanemab are generally not covered by insurance and typically cost >$26,000 annually. Both are administered by IV infusion once a month. More monoclonal antibody medications for treating early Alzheimer disease are in the late stages of development, most notably donanebab.24 Observations during clinical trials found that in the later stages of Alzheimer disease, forceful removal of plaques by the autoimmune process damages neurons, while in less dense deposits of early dementia such removal is not harmful to the cells and prevents amyloid buildup.
Brexanolone is an aqueous formulation of allopregnanolone, a major metabolite of progesterone and a positive allosteric modulator of GABA-A receptors.25 Its levels are maximal at the end of the third trimester of pregnancy and fall rapidly following delivery. Research showed a 3-day infusion was rapidly and significantly effective for treating postpartum depression26 and brexanolone received FDA approval for this indication in March 2019.27 However, various administrative, economic, insurance, and other hurdles make it difficult for patients to access this treatment. Despite its rapid onset of action (usually 48 hours), brexanolone takes an average of 15 days to go through the prior authorization process.28 In addition to the need for prior authorization, the main impediment to the use of brexanolone is the 3-day infusion schedule, which greatly magnifies the cost but is partially circumvented by the availability of dedicated outpatient centers.
Magnesium
Magnesium is on the World Health Organization’s Model List of Essential Medicines.29 There has been extensive research on the use of magnesium sulfate for psychiatric indications, especially for depression.30 Magnesium functions similarly to calcium channel blockers by competitively blocking intracellular calcium channels, decreasing calcium availability, and inhibiting smooth muscle contractility.31 It also competes with calcium at the motor end plate, reducing excitation by inhibiting the release of acetylcholine.32 This property is used for high-dose IV magnesium treatment of impending preterm labor in obstetrics. Magnesium sulfate is the drug of choice in treating eclamptic seizures and preventing seizures in severe preeclampsia or gestational hypertension with severe features.33 It is also used to treat torsade de pointes, severe asthma exacerbations, constipation, and barium poisoning.34 Beneficial use in asthma treatment35 and the treatment of migraine36 have also been reported.
IV magnesium in myocardial infarction may be harmful,37 though outside of acute cardiac events, magnesium is found to be safe.38
Oral magnesium sulfate is a common over-the-counter anxiety remedy. As a general cell stabilizer (mediated by the reduction of intracellular calcium), magnesium is potentially beneficial outside of its muscle-relaxing properties, although muscle relaxing can benefit anxious patients. It is used to treat anxiety,39 alcohol withdrawal,40 and fear.41 Low magnesium blood levels are found in patients with depression, schizophrenia,42 and attention-deficit/hyperactivity disorder.43 However, it is important to note that the therapeutic effect of magnesium when treating anxiety and headache is independent of preinfusion magnesium blood levels.43
Continue to: The efficacy of oral magnesium...
The efficacy of oral magnesium is not robust. However, IV administration has a pronounced beneficial effect as an abortive and preventative treatment in many patients with anxiety.44
IV administration of magnesium can produce adverse effects, including flushing, sweating, hypotension, depressed reflexes, flaccid paralysis, hypothermia, circulatory collapse, and cardiac and CNS depression. These complications are very rare and dose-dependent.45 Magnesium is excreted by the kidneys, and dosing must be decreased in patients with kidney failure. The most common adverse effect is local burning along the vein upon infusion; small doses of IV lidocaine can remedy this. Hot flashes are also common.45
Various dosing strategies are available. In patients with anxiety, application dosing is based on the recommended preeclampsia IV dose of 4 g diluted in 250 mL of 5% dextrose. Much higher doses may be used in obstetrics. Unlike in obstetrics, for psychiatric indications, magnesium is administered over 60 to 90 minutes. Heart monitoring is recommended.
Scopolamine
Scopolamine is primarily used to relieve nausea, vomiting, and dizziness associated with motion sickness and recovery from anesthesia. It is also used in ophthalmology and in patients with excessive sweating. In off-label and experimental applications, scopolamine has been used in patients with TRD.46
Scopolamine is an anticholinergic medication. It is a nonselective antagonist at muscarinic receptors.47 Tricyclic antidepressants (TCAs) possess strong anticholinergic function. Newer generations of antidepressants were designed specifically not to have this function because it was believed to be an unwanted and potentially dangerous adverse effect. However, data suggest that anticholinergic action is important in decreasing depressive symptoms. Several hypotheses of anticholinergic effects on depression have been published since the 1970s. They include the cholinergic-adrenergic hypothesis,48 acetylcholine predominance relative to adrenergic action hypothesis,49 and insecticide poisoning observations.50 Centrally acting physostigmine (but not peripherally acting neostigmine) was reported to control mania.48,51 A genetic connection between the M2acetylcholine receptor in patients with major depressive disorder (MDD) and alcohol use disorder is also suggestive.52
Continue to: Multiple animal studies show...
Multiple animal studies show direct improvement in mobility and a decrease in despair upon introducing anticholinergic substances.53-55 The cholinergic theory of depression has been studied in several controlled clinical human studies.56,57 Use of a short-acting anticholinergic glycopyrrolate during electroconvulsive therapy (ECT) may contribute to the procedure’s efficacy.
Human research shows scopolamine has a higher efficacy as an antidepressant and anti-anxiety medication in women than in men,58 possibly because estrogen increases the activity of choline acetyltransferase and release of acetylcholine.59,60 M2receptors mediate estrogen influence on the NMDAR, which may explain the anticholinergic effects of depression treatments in women.61
Another proposed mechanism of action of scopolamine is a potent inhibition of the NMDAR.62 Rapid treatments of depression may be based on this mechanism. Examples of such treatments include IV ketamine and sleep deprivation.63 IV scopolamine shows potency in treating MDD and bipolar depression. This treatment should be reserved for patients who do not respond to or are not candidates for other usual treatment modalities of MDD and for the most severe cases. Scopolamine is 30 times more potent than amitriptyline in anticholinergic effect and reportedly produces sustained improvement in MDD.64
Scopolamine has no black-box warnings. It has not been studied in pregnant women and is not recommended for use during pregnancy. Due to possible negative cardiovascular effects, a normal electrocardiogram is required before the start of treatment. Exercise caution in patients with glaucoma, benign prostatic enlargement, gastroparesis, unstable cardiovascular status, or severe renal impairment.
Treatment with scopolamine is not indicated for patients with myasthenia gravis, psychosis, or seizures. Patients must be off potassium for 3 days before beginning scopolamine treatment. Patients should consult with their cardiologist before having a scopolamine infusion. Adverse reactions may include psychosis, tachycardia, seizures, paralytic ileus, and glaucoma exacerbation. The most common adverse effects of scopolamine infusion treatment include drowsiness, dry mouth, blurred vision, lightheadedness, and dizziness. Due to possible drowsiness, operating motor vehicles or heavy machinery must be avoided on the day of treatment.65 Overall, the adverse effects of scopolamine are preventable and manageable, and its antidepressant efficacy is noteworthy.66
Continue to: Treatment typically consists of 3 consecutive infusions...
Treatment typically consists of 3 consecutive infusions of 4 mcg/kg separated by 3 to 5 days.56 It is possible to have a longer treatment course if the patient experiences only partial improvement. Repeated courses or maintenance treatment (similar to ECT maintenance) are utilized in some patients if indicated. Cardiac monitoring is mandatory.
Clomipramine
Clomipramine, a TCA, acts as a preferential inhibitor of 5-hydroxytryptamine uptake and has proven effective in managing depression, TRD, and obsessive-compulsive disorder (OCD).67 Although this medication has reported treatment benefits for patients with phobia, panic disorder,15 chronic pain,68 Tourette syndrome,69 premature ejaculation, anorexia nervosa,70 cataplexy,49 and enuresis,71 it is FDA-approved only for the treatment of OCD.72 Clomipramine may also be beneficial for pain and headache, possibly because of its anti-inflammatory action.73 The anticholinergic effects of clomipramine may add to its efficacy in depression.
The pathophysiology of MDD is connected to hyperactivity of the HPA axis and elevated cortisol levels. Higher clomipramine plasma levels show a linear correlation with lower cortisol secretion and levels, possibly aiding in the treatment of MDD and anxiety.74 The higher the blood level, the more pronounced clomipramine’s therapeutic effect across multiple domains.75
IV infusion of clomipramine produces the highest concentration in the shortest time, but overall, research does not necessarily support increased efficacy of IV over oral administration. There is evidence suggesting that subgroups of patients with severe, treatment-refractory OCD may benefit from IV agents and research suggests a faster onset of action.76 Faster onset of symptom relief is the basis for IV clomipramine use. In patients with OCD, it can take several months for oral medications to produce therapeutic benefits; not all patients can tolerate this. In such scenarios, IV administration may be considered, though it is not appropriate for routine use until more research is available. Patients with treatment-resistant OCD who have exhausted other means of symptom relief may also be candidates for IV treatment.
The adverse effects of IV clomipramine are no different from oral use, though they may be more pronounced. A pretreatment cardiac exam is desirable because clomipramine, like other TCAs, may be cardiotoxic. The anticholinergic adverse effects of TCAs are well known to clinicians77 and partially explained in the scopolamine section of this article. It is not advisable to combine clomipramine with other TCAs or serotonin reuptake inhibitors. Clomipramine also should not be combined with monoamine oxidase inhibitors, though such a combination was reported in medical literature.78 Combination with antiarrhythmics such as lidocaine or opioids such as fentanyl or and tramadol is highly discouraged (fentanyl and tramadol also have serotonergic effects).79
Continue to: Clomipramine for IV use is not commercially available...
Clomipramine for IV use is not commercially available and must be sterilely compounded. The usual course of treatment is a series of 3 infusions: 150 mg on Day 1, 200 mg on Day 2 or Day 3, and 250 mg on Day 3, Day 4, or Day 5, depending on tolerability. A protocol with a 50 mg/d starting dose and titration up to a maximum dose of 225 mg/d over 5 to 7 days has been suggested for inpatient settings.67 Titration to 250 mg is more common.80
A longer series may be performed, but this increases the likelihood of adverse effects. Booster and maintenance treatments are also completed when required. Cardiac monitoring is mandatory.
Vortioxetine and citalopram
IV treatment of depression with
Injections and blocks
Three interventional approaches to treatment that possess psychotherapeutic potential include stellate ganglion blocks (SGBs), glabellar BT injections, and trigger point injections (TPIs). None of these are FDA-approved for psychiatric treatment.
Stellate ganglion blocks
The sympathetic nervous system is involved in autonomic hyperarousal and is at the core of posttraumatic symptomatology.83 Insomnia, anxiety, irritability, hypervigilance, and other excitatory CNS events are connected to the sympathetic nervous system and amygdala activation is commonly observed in those exposed to extreme stress or traumatic events.84
Continue to: SGBs were first performed 100 years ago...
SGBs were first performed 100 years ago and reported to have beneficial psychiatric effects at the end of the 1940s. In 1998 in Finland, improvement of posttraumatic stress disorder (PTSD) symptoms was observed accidentally via thoracic level spine blocks.85 In 2006, cervical level sympathetic blocks were shown to be effective for PTSD symptom control.86 By the end of 2010, Veterans Administration hospitals adopted SGBs to treat veterans with PTSD.87,88 The first multisite, randomized clinical trial of
Since the stellate ganglion is connected to the amygdala, SGB has also been assessed for treating anxiety and depression.89,90 Outside of PTSD, SGBs are used to treat complex regional pain syndrome,91 phantom limb pain, trigeminal neuralgia,92 intractable angina,93 and postherpetic neuralgia in the head, neck, upper chest, or arms.94 The procedure consists of an injection of a local anesthetic through a 25-gauge needle into the stellate sympathetic ganglion at the C6 or C7 vertebral levels. An injection into C6 is considered safer because of specific cervical spine anatomy. Ideally, fluoroscopic guidance or ultrasound is used to guide needle insertion.95
A severe drop in blood pressure may be associated with SGBs and is mitigated by IV hydration. Other adverse effects include red eyes, drooping of the eyelids, nasal congestion, hoarseness, difficulty swallowing, a sensation of a “lump” in the throat, and a sensation of warmth or tingling in the arm or hand. Bilateral SGB is contraindicated due to the danger of respiratory arrest.96
Glabellar BT injections
OnabotulinumtoxinA (BT) injection was first approved for therapeutic use in 1989 for eye muscle disorders such as strabismus97 and blepharospasm.98 It was later approved for several other indications, including cosmetic use, hyperhidrosis, migraine prevention, neurogenic bladder disorder, overactive bladder, urinary incontinence, and spasticity.99-104 BT is used off-label for achalasia and sialorrhea.105,106 Its mechanism of action is primarily attributed to muscle paralysis by blocking presynaptic acetylcholine release into neuromuscular junctions.107
Facial BT injections are usually administered for cosmetic purposes, but smoothing forehead wrinkles and frown lines (the glabellar region of the face) both have antidepressant effects.108 BT injections into the glabellar region also demonstrate antidepressant effects, particularly in patients with comorbid migraines and MDD.109 Early case observations supported the independent benefit of the toxin on MDD when the toxin was injected into the glabellar region.110,111 The most frequent protocol involves injections in the procerus and corrugator muscles.
Continue to: The facial feedback/emotional proprioception hypothesis...
The facial feedback/emotional proprioception hypothesis has dominated thinking about the mood-improving effects of BT. The theory is that blocking muscular expression of sadness (especially in the face) interrupts the experience of sadness; therefore, depression subsides.112,113 However, BT injections in the muscles involved in the smile and an expression of positive emotions (lateral part of the musculus orbicularis oculi) have been associated with increased MDD scores.114 Thus, the mechanism clearly involves more than the cosmetic effect, since facial muscle injections in rats also have antidepressant effects.115
The use of progressive muscle relaxation is well-established in psychiatric treatment. The investigated conditions of increased muscle tone, especially torticollis and blepharospasm, are associated with MDD, and it may be speculated that proprioceptive feedback from the affected muscles may be causally involved in this association.116-118 Activity of the corrugator muscle has been positively associated with increased amygdala activity.119 This suggests a potential similar mechanism to that hypothesized for SGB.
Alternatively, BT is commonly used to treat chronic conditions that may contribute to depression; its success in relieving the underlying problem may indirectly relieve MDD. Thus, in a postmarketing safety evaluation of BT, MDD was demonstrated 40% to 88% less often by patients treated with BT for 6 of the 8 conditions and injection sites, such as in spasms and spasticity of arms and legs, torticollis and neck pain, and axilla and palm injections for hyperhidrosis. In a parotid and submandibular glands BT injection subcohort, no patients experienced depressive symptoms.120
Medicinal BT is generally considered safe. The most common adverse effects are hypersensitivity, injection site reactions, and other adverse effects specific to the injection site.121 Additionally, the cosmetic effects are transient, given the nature of the medication.
Trigger point injections
TPIs in the neck and shoulders are frequently used to treat tension headaches and various referred pain locations in the face and arms. Tension and depression frequently overlap in clinical practice.122 Relieving muscle tension (with resulting trigger points) improves muscle function and mood.
Continue to: The higher the number of active...
The higher the number of active trigger points (TPs), the greater the physical burden of headache and the higher the anxiety level. Gender differences in TP prevalence and TPI efficacy have been described in the literature. For example, the number of active TPs seems directly associated with anxiety levels in women but not in men.123 Although TPs appear to be more closely associated with anxiety than depression,124 depression associated with muscle tension does improve with TPIs. European studies have demonstrated a decrease in scores on the Hamilton Depression Rating Scale and Hamilton Anxiety Rating Scale following TPI treatment.125 The effect may be indirect, as when a patient’s pain is relieved, sleep and other psychiatric symptoms improve.126
A randomized controlled trial by Castro Sánchez et al127 demonstrated that dry needling therapy in patients with fibromyalgia syndrome (FMS) showed improvements in pain pressure thresholds, body pain, vitality, and social function, as well as total FMS symptoms, quality of sleep, anxiety, hospital anxiety and depression, general pain intensity, and fatigue.
Myofascial pain syndrome, catastrophizing, and muscle tension are common in patients with depression, anxiety, and somatization. Local TPI therapy aimed at inactivating pain generators is supported by moderate quality evidence. All manner of therapies have been described, including injection of saline, corticosteroids, local anesthetic agents, and dry needling. BT injections in chronic TPs are also practiced, though no specific injection therapy has been reliably shown to be more advantageous than another. The benefits of TPIs may be derived from the needle itself rather than from any specific substance injected. Stimulation of a local twitch response with direct needling of the TP appears of importance. There is no established consensus regarding the number of injection points, frequency of administration, and volume or type of injectate.128
Adverse effects of TPIs relate to the nature of the invasive therapy, with the risk of tissue damage and bleeding. Pneumothorax risk is present with needle insertion at the neck and thorax.129 Patients with diabetes may experience variations in blood sugar control if steroids are used.
Bottom Line
Interventional treatment modalities that may have a role in psychiatric treatment include IV administration of ketamine, aducanumab, lecanemab, brexanolone, magnesium, scopolamine, and clomipramine. Other interventional approaches include stellate ganglion blocks, glabellar botulinum toxin injections, and trigger point injections.
Related Resources
- Dokucu ME, Janicak PG. Nontraditional therapies for treatment-resistant depression. Current Psychiatry. 2021; 20(9):38-43,49. doi:10.12788/cp.0166
- Kim J, Khoury R, Grossberg GT. Botulinum toxin: emerging psychiatric indications. Current Psychiatry. 2018;17(12):8-18.
Drug Brand Names
Aducanumab • Aduhelm
Aripiprazole • Abilify
Aripiprazole lauroxil • Aristada
Brexanolone • Zulresso
Buprenorphine • Sublocade
Citalopram • Celexa
Clomipramine • Anafranil
Diazepam • Valium
Droperidol • Inapsine
Esketamine • Spravato
Fentanyl • Actiq
Fluphenazine decanoate • Modecate
Fluphenazine hydrochloride • Prolixin
Haloperidol decanoate • Haldol decanoate
Haloperidol lactate • Haldol
Ketamine • Ketalar
Lecanemab • Leqembi
Lidocaine • Xylocaine
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Naltrexone • Vivitrol
Magnesium sulfate • Sulfamag
Midazolam • Versed
Olanzapine • Zyprexa
OnabotulinumtoxinA injection • Botox
Paliperidone • Invega Hafyera, Invega Sustenna, Invega Trinza
Rapamycin • Rapamune, Sirolimus
Risperidone • Perseris
Risperidone microspheres • Risperdal Consta, Rykindo
Scopolamine • Hyoscine
Tramadol • Conzip
Vortioxetine • Trintellix
Ziprasidone • Geodon
Advances in the understanding of neurobiological and neuropsychiatric pathophysiology have opened new avenues of treatment for psychiatric patients. Historically, with a few exceptions, most psychiatric medications have been administered orally. However, many of the newer treatments require some form of specialized administration because they cannot be taken orally due to their chemical property (such as aducanumab); because there is the need to produce stable blood levels of the medication (such as brexanolone); because oral administration greatly diminished efficacy (such as oral vs IV magnesium or scopolamine), or because the treatment is focused on specific brain structures. This need for specialized administration has created a subspecialty called interventional psychiatry.
Part 1 of this 2-part article provides an overview of 1 type of interventional psychiatry: parenterally administered medications, including those administered via IV. We also describe 3 other interventional approaches to treatment: stellate ganglion blocks, glabellar botulinum toxin (BT) injections, and trigger point injections. In Part 2 we will review interventional approaches that involve neuromodulation.
Parenteral medications in psychiatry
In general, IV and IM medications can be more potent that oral medications due to their overall faster onset of action and higher blood concentrations. These more invasive forms of administration can have significant limitations, such as a risk of infection at the injection site, the need to be administered in a medical setting, additional time, and patient discomfort.

Table 1 lists short-acting injectable medications used in psychiatry, and Table 2 lists long-acting injectable medications. Parenteral administration of antipsychotics is performed to alleviate acute agitation or for chronic symptom control. These medications generally are not considered interventional treatments, but could be classified as such due to their invasive nature.1 Furthermore, inhalable loxapine—which is indicated for managing acute agitation—requires a Risk Evaluation and Mitigation Strategy program consisting of 2 hours observation and monitoring of respiratory status.2,3 Other indications for parenteral treatments include IM naltrexone extended release4 and subcutaneous injections of buprenorphine extended release5 and risperidone.6
IV administration
Most IV treatments described in this article are not FDA-approved for psychiatric treatment. Despite this, many interventional psychiatric treatments are part of clinical practice. IV infusion of ketamine is the most widely known and most researched of these. Table 3 lists other IV treatments that could be used as psychiatric treatment.
Ketamine
Since the early 1960s, ketamine has been used as a surgical anesthetic for animals. In the United States, it was approved for human surgical anesthesia in 1970. It was widely used during the Vietnam War due to its lack of inhibition of respiratory drive; medical staff first noticed an improvement in depressive symptoms and the resolution of suicidal ideation in patients who received ketamine. This led to further research on ketamine, particularly to determine its application in treatment-resistant depression (TRD) and other conditions.7 IV ketamine administration is most widely researched, but IM injections, intranasal sprays, and lozenges are also available. The dissociative properties of ketamine have led to its recreational use.8
Hypotheses for the mechanism of action of ketamine as an antidepressant include direct synaptic or extrasynaptic (GluN2B-selective), N-methyl-
Continue to: Ketamine is a schedule...
Ketamine is a schedule III medication with addictive properties. Delirium, panic attacks, hallucinations, nightmares, dysphoria, and paranoia may occur during and after use.13 Premedication with benzodiazepines, most notably lorazepam, is occasionally used to minimize ketamine’s adverse effects, but this generally is not recommended because doing so reduces ketamine’s antidepressant effects.14 Driving and operating heavy machinery is contraindicated after IV infusion. The usual protocol involves an IV infusion of ketamine 0.4 mg/kg to 1 mg/kg dosing over 1 hour. Doses between 0.4 mg/kg and 0.6 mg/kg are most common. Ketamine has a therapeutic window; doses >0.5 mg/kg are progressively less effective.15 Unlike the recommendation after esketamine administration, after receiving ketamine, patients remain in the care of their treatment team for <2 hours.
Esketamine, the S enantiomer of ketamine, was FDA-approved for TRD as an intranasal formulation. Esketamine is more commonly used than IV ketamine because it is FDA-approved and typically covered by insurance, but it may not be as effective.16 An economic analysis by Brendle et al17 suggested insurance companies would lower costs if they covered ketamine infusions vs intranasal esketamine.
Aducanumab and lecanemab
The most recent FDA-approved interventional agents are aducanumab and lecanemab, which are indicated for treating Alzheimer disease.18,19 Both are human monoclonal antibodies that bind selectively and with high affinity to amyloid beta plaque aggregates and promote their removal by Fc receptor–mediated phagocytosis.20
FDA approval of aducanumab and lecanemab was controversial. Initially, aducanumab’s safety monitoring board performed a futility analysis that suggested aducanumab was unlikely to separate from placebo, and the research was stopped.21 The manufacturer petitioned the FDA to consider the medication for accelerated approval on the basis of biomarker data showing that amyloid beta plaque aggregates become smaller. Current FDA approval is temporary to allow patients access to this potentially beneficial agent, but the manufacturer must supply clinical evidence that the reduction of amyloid beta plaques is associated with desirable changes in the course of Alzheimer disease, or risk losing the approval.
Lecanemab is also a human monoclonal antibody intended to remove amyloid beta plaques that was FDA-approved under the accelerated approval pathway.22 Unlike aducanumab, lecanemab demonstrated a statistically significant (although clinically imperceptible) reduction in the rate of cognitive decline; it did not show cognitive improvement.23 Lecanemab also significantly reduced amyloid beta plaques.23
Continue to: Aducanumab and lecanemab are generally...
Aducanumab and lecanemab are generally not covered by insurance and typically cost >$26,000 annually. Both are administered by IV infusion once a month. More monoclonal antibody medications for treating early Alzheimer disease are in the late stages of development, most notably donanebab.24 Observations during clinical trials found that in the later stages of Alzheimer disease, forceful removal of plaques by the autoimmune process damages neurons, while in less dense deposits of early dementia such removal is not harmful to the cells and prevents amyloid buildup.
Brexanolone is an aqueous formulation of allopregnanolone, a major metabolite of progesterone and a positive allosteric modulator of GABA-A receptors.25 Its levels are maximal at the end of the third trimester of pregnancy and fall rapidly following delivery. Research showed a 3-day infusion was rapidly and significantly effective for treating postpartum depression26 and brexanolone received FDA approval for this indication in March 2019.27 However, various administrative, economic, insurance, and other hurdles make it difficult for patients to access this treatment. Despite its rapid onset of action (usually 48 hours), brexanolone takes an average of 15 days to go through the prior authorization process.28 In addition to the need for prior authorization, the main impediment to the use of brexanolone is the 3-day infusion schedule, which greatly magnifies the cost but is partially circumvented by the availability of dedicated outpatient centers.
Magnesium
Magnesium is on the World Health Organization’s Model List of Essential Medicines.29 There has been extensive research on the use of magnesium sulfate for psychiatric indications, especially for depression.30 Magnesium functions similarly to calcium channel blockers by competitively blocking intracellular calcium channels, decreasing calcium availability, and inhibiting smooth muscle contractility.31 It also competes with calcium at the motor end plate, reducing excitation by inhibiting the release of acetylcholine.32 This property is used for high-dose IV magnesium treatment of impending preterm labor in obstetrics. Magnesium sulfate is the drug of choice in treating eclamptic seizures and preventing seizures in severe preeclampsia or gestational hypertension with severe features.33 It is also used to treat torsade de pointes, severe asthma exacerbations, constipation, and barium poisoning.34 Beneficial use in asthma treatment35 and the treatment of migraine36 have also been reported.
IV magnesium in myocardial infarction may be harmful,37 though outside of acute cardiac events, magnesium is found to be safe.38
Oral magnesium sulfate is a common over-the-counter anxiety remedy. As a general cell stabilizer (mediated by the reduction of intracellular calcium), magnesium is potentially beneficial outside of its muscle-relaxing properties, although muscle relaxing can benefit anxious patients. It is used to treat anxiety,39 alcohol withdrawal,40 and fear.41 Low magnesium blood levels are found in patients with depression, schizophrenia,42 and attention-deficit/hyperactivity disorder.43 However, it is important to note that the therapeutic effect of magnesium when treating anxiety and headache is independent of preinfusion magnesium blood levels.43
Continue to: The efficacy of oral magnesium...
The efficacy of oral magnesium is not robust. However, IV administration has a pronounced beneficial effect as an abortive and preventative treatment in many patients with anxiety.44
IV administration of magnesium can produce adverse effects, including flushing, sweating, hypotension, depressed reflexes, flaccid paralysis, hypothermia, circulatory collapse, and cardiac and CNS depression. These complications are very rare and dose-dependent.45 Magnesium is excreted by the kidneys, and dosing must be decreased in patients with kidney failure. The most common adverse effect is local burning along the vein upon infusion; small doses of IV lidocaine can remedy this. Hot flashes are also common.45
Various dosing strategies are available. In patients with anxiety, application dosing is based on the recommended preeclampsia IV dose of 4 g diluted in 250 mL of 5% dextrose. Much higher doses may be used in obstetrics. Unlike in obstetrics, for psychiatric indications, magnesium is administered over 60 to 90 minutes. Heart monitoring is recommended.
Scopolamine
Scopolamine is primarily used to relieve nausea, vomiting, and dizziness associated with motion sickness and recovery from anesthesia. It is also used in ophthalmology and in patients with excessive sweating. In off-label and experimental applications, scopolamine has been used in patients with TRD.46
Scopolamine is an anticholinergic medication. It is a nonselective antagonist at muscarinic receptors.47 Tricyclic antidepressants (TCAs) possess strong anticholinergic function. Newer generations of antidepressants were designed specifically not to have this function because it was believed to be an unwanted and potentially dangerous adverse effect. However, data suggest that anticholinergic action is important in decreasing depressive symptoms. Several hypotheses of anticholinergic effects on depression have been published since the 1970s. They include the cholinergic-adrenergic hypothesis,48 acetylcholine predominance relative to adrenergic action hypothesis,49 and insecticide poisoning observations.50 Centrally acting physostigmine (but not peripherally acting neostigmine) was reported to control mania.48,51 A genetic connection between the M2acetylcholine receptor in patients with major depressive disorder (MDD) and alcohol use disorder is also suggestive.52
Continue to: Multiple animal studies show...
Multiple animal studies show direct improvement in mobility and a decrease in despair upon introducing anticholinergic substances.53-55 The cholinergic theory of depression has been studied in several controlled clinical human studies.56,57 Use of a short-acting anticholinergic glycopyrrolate during electroconvulsive therapy (ECT) may contribute to the procedure’s efficacy.
Human research shows scopolamine has a higher efficacy as an antidepressant and anti-anxiety medication in women than in men,58 possibly because estrogen increases the activity of choline acetyltransferase and release of acetylcholine.59,60 M2receptors mediate estrogen influence on the NMDAR, which may explain the anticholinergic effects of depression treatments in women.61
Another proposed mechanism of action of scopolamine is a potent inhibition of the NMDAR.62 Rapid treatments of depression may be based on this mechanism. Examples of such treatments include IV ketamine and sleep deprivation.63 IV scopolamine shows potency in treating MDD and bipolar depression. This treatment should be reserved for patients who do not respond to or are not candidates for other usual treatment modalities of MDD and for the most severe cases. Scopolamine is 30 times more potent than amitriptyline in anticholinergic effect and reportedly produces sustained improvement in MDD.64
Scopolamine has no black-box warnings. It has not been studied in pregnant women and is not recommended for use during pregnancy. Due to possible negative cardiovascular effects, a normal electrocardiogram is required before the start of treatment. Exercise caution in patients with glaucoma, benign prostatic enlargement, gastroparesis, unstable cardiovascular status, or severe renal impairment.
Treatment with scopolamine is not indicated for patients with myasthenia gravis, psychosis, or seizures. Patients must be off potassium for 3 days before beginning scopolamine treatment. Patients should consult with their cardiologist before having a scopolamine infusion. Adverse reactions may include psychosis, tachycardia, seizures, paralytic ileus, and glaucoma exacerbation. The most common adverse effects of scopolamine infusion treatment include drowsiness, dry mouth, blurred vision, lightheadedness, and dizziness. Due to possible drowsiness, operating motor vehicles or heavy machinery must be avoided on the day of treatment.65 Overall, the adverse effects of scopolamine are preventable and manageable, and its antidepressant efficacy is noteworthy.66
Continue to: Treatment typically consists of 3 consecutive infusions...
Treatment typically consists of 3 consecutive infusions of 4 mcg/kg separated by 3 to 5 days.56 It is possible to have a longer treatment course if the patient experiences only partial improvement. Repeated courses or maintenance treatment (similar to ECT maintenance) are utilized in some patients if indicated. Cardiac monitoring is mandatory.
Clomipramine
Clomipramine, a TCA, acts as a preferential inhibitor of 5-hydroxytryptamine uptake and has proven effective in managing depression, TRD, and obsessive-compulsive disorder (OCD).67 Although this medication has reported treatment benefits for patients with phobia, panic disorder,15 chronic pain,68 Tourette syndrome,69 premature ejaculation, anorexia nervosa,70 cataplexy,49 and enuresis,71 it is FDA-approved only for the treatment of OCD.72 Clomipramine may also be beneficial for pain and headache, possibly because of its anti-inflammatory action.73 The anticholinergic effects of clomipramine may add to its efficacy in depression.
The pathophysiology of MDD is connected to hyperactivity of the HPA axis and elevated cortisol levels. Higher clomipramine plasma levels show a linear correlation with lower cortisol secretion and levels, possibly aiding in the treatment of MDD and anxiety.74 The higher the blood level, the more pronounced clomipramine’s therapeutic effect across multiple domains.75
IV infusion of clomipramine produces the highest concentration in the shortest time, but overall, research does not necessarily support increased efficacy of IV over oral administration. There is evidence suggesting that subgroups of patients with severe, treatment-refractory OCD may benefit from IV agents and research suggests a faster onset of action.76 Faster onset of symptom relief is the basis for IV clomipramine use. In patients with OCD, it can take several months for oral medications to produce therapeutic benefits; not all patients can tolerate this. In such scenarios, IV administration may be considered, though it is not appropriate for routine use until more research is available. Patients with treatment-resistant OCD who have exhausted other means of symptom relief may also be candidates for IV treatment.
The adverse effects of IV clomipramine are no different from oral use, though they may be more pronounced. A pretreatment cardiac exam is desirable because clomipramine, like other TCAs, may be cardiotoxic. The anticholinergic adverse effects of TCAs are well known to clinicians77 and partially explained in the scopolamine section of this article. It is not advisable to combine clomipramine with other TCAs or serotonin reuptake inhibitors. Clomipramine also should not be combined with monoamine oxidase inhibitors, though such a combination was reported in medical literature.78 Combination with antiarrhythmics such as lidocaine or opioids such as fentanyl or and tramadol is highly discouraged (fentanyl and tramadol also have serotonergic effects).79
Continue to: Clomipramine for IV use is not commercially available...
Clomipramine for IV use is not commercially available and must be sterilely compounded. The usual course of treatment is a series of 3 infusions: 150 mg on Day 1, 200 mg on Day 2 or Day 3, and 250 mg on Day 3, Day 4, or Day 5, depending on tolerability. A protocol with a 50 mg/d starting dose and titration up to a maximum dose of 225 mg/d over 5 to 7 days has been suggested for inpatient settings.67 Titration to 250 mg is more common.80
A longer series may be performed, but this increases the likelihood of adverse effects. Booster and maintenance treatments are also completed when required. Cardiac monitoring is mandatory.
Vortioxetine and citalopram
IV treatment of depression with
Injections and blocks
Three interventional approaches to treatment that possess psychotherapeutic potential include stellate ganglion blocks (SGBs), glabellar BT injections, and trigger point injections (TPIs). None of these are FDA-approved for psychiatric treatment.
Stellate ganglion blocks
The sympathetic nervous system is involved in autonomic hyperarousal and is at the core of posttraumatic symptomatology.83 Insomnia, anxiety, irritability, hypervigilance, and other excitatory CNS events are connected to the sympathetic nervous system and amygdala activation is commonly observed in those exposed to extreme stress or traumatic events.84
Continue to: SGBs were first performed 100 years ago...
SGBs were first performed 100 years ago and reported to have beneficial psychiatric effects at the end of the 1940s. In 1998 in Finland, improvement of posttraumatic stress disorder (PTSD) symptoms was observed accidentally via thoracic level spine blocks.85 In 2006, cervical level sympathetic blocks were shown to be effective for PTSD symptom control.86 By the end of 2010, Veterans Administration hospitals adopted SGBs to treat veterans with PTSD.87,88 The first multisite, randomized clinical trial of
Since the stellate ganglion is connected to the amygdala, SGB has also been assessed for treating anxiety and depression.89,90 Outside of PTSD, SGBs are used to treat complex regional pain syndrome,91 phantom limb pain, trigeminal neuralgia,92 intractable angina,93 and postherpetic neuralgia in the head, neck, upper chest, or arms.94 The procedure consists of an injection of a local anesthetic through a 25-gauge needle into the stellate sympathetic ganglion at the C6 or C7 vertebral levels. An injection into C6 is considered safer because of specific cervical spine anatomy. Ideally, fluoroscopic guidance or ultrasound is used to guide needle insertion.95
A severe drop in blood pressure may be associated with SGBs and is mitigated by IV hydration. Other adverse effects include red eyes, drooping of the eyelids, nasal congestion, hoarseness, difficulty swallowing, a sensation of a “lump” in the throat, and a sensation of warmth or tingling in the arm or hand. Bilateral SGB is contraindicated due to the danger of respiratory arrest.96
Glabellar BT injections
OnabotulinumtoxinA (BT) injection was first approved for therapeutic use in 1989 for eye muscle disorders such as strabismus97 and blepharospasm.98 It was later approved for several other indications, including cosmetic use, hyperhidrosis, migraine prevention, neurogenic bladder disorder, overactive bladder, urinary incontinence, and spasticity.99-104 BT is used off-label for achalasia and sialorrhea.105,106 Its mechanism of action is primarily attributed to muscle paralysis by blocking presynaptic acetylcholine release into neuromuscular junctions.107
Facial BT injections are usually administered for cosmetic purposes, but smoothing forehead wrinkles and frown lines (the glabellar region of the face) both have antidepressant effects.108 BT injections into the glabellar region also demonstrate antidepressant effects, particularly in patients with comorbid migraines and MDD.109 Early case observations supported the independent benefit of the toxin on MDD when the toxin was injected into the glabellar region.110,111 The most frequent protocol involves injections in the procerus and corrugator muscles.
Continue to: The facial feedback/emotional proprioception hypothesis...
The facial feedback/emotional proprioception hypothesis has dominated thinking about the mood-improving effects of BT. The theory is that blocking muscular expression of sadness (especially in the face) interrupts the experience of sadness; therefore, depression subsides.112,113 However, BT injections in the muscles involved in the smile and an expression of positive emotions (lateral part of the musculus orbicularis oculi) have been associated with increased MDD scores.114 Thus, the mechanism clearly involves more than the cosmetic effect, since facial muscle injections in rats also have antidepressant effects.115
The use of progressive muscle relaxation is well-established in psychiatric treatment. The investigated conditions of increased muscle tone, especially torticollis and blepharospasm, are associated with MDD, and it may be speculated that proprioceptive feedback from the affected muscles may be causally involved in this association.116-118 Activity of the corrugator muscle has been positively associated with increased amygdala activity.119 This suggests a potential similar mechanism to that hypothesized for SGB.
Alternatively, BT is commonly used to treat chronic conditions that may contribute to depression; its success in relieving the underlying problem may indirectly relieve MDD. Thus, in a postmarketing safety evaluation of BT, MDD was demonstrated 40% to 88% less often by patients treated with BT for 6 of the 8 conditions and injection sites, such as in spasms and spasticity of arms and legs, torticollis and neck pain, and axilla and palm injections for hyperhidrosis. In a parotid and submandibular glands BT injection subcohort, no patients experienced depressive symptoms.120
Medicinal BT is generally considered safe. The most common adverse effects are hypersensitivity, injection site reactions, and other adverse effects specific to the injection site.121 Additionally, the cosmetic effects are transient, given the nature of the medication.
Trigger point injections
TPIs in the neck and shoulders are frequently used to treat tension headaches and various referred pain locations in the face and arms. Tension and depression frequently overlap in clinical practice.122 Relieving muscle tension (with resulting trigger points) improves muscle function and mood.
Continue to: The higher the number of active...
The higher the number of active trigger points (TPs), the greater the physical burden of headache and the higher the anxiety level. Gender differences in TP prevalence and TPI efficacy have been described in the literature. For example, the number of active TPs seems directly associated with anxiety levels in women but not in men.123 Although TPs appear to be more closely associated with anxiety than depression,124 depression associated with muscle tension does improve with TPIs. European studies have demonstrated a decrease in scores on the Hamilton Depression Rating Scale and Hamilton Anxiety Rating Scale following TPI treatment.125 The effect may be indirect, as when a patient’s pain is relieved, sleep and other psychiatric symptoms improve.126
A randomized controlled trial by Castro Sánchez et al127 demonstrated that dry needling therapy in patients with fibromyalgia syndrome (FMS) showed improvements in pain pressure thresholds, body pain, vitality, and social function, as well as total FMS symptoms, quality of sleep, anxiety, hospital anxiety and depression, general pain intensity, and fatigue.
Myofascial pain syndrome, catastrophizing, and muscle tension are common in patients with depression, anxiety, and somatization. Local TPI therapy aimed at inactivating pain generators is supported by moderate quality evidence. All manner of therapies have been described, including injection of saline, corticosteroids, local anesthetic agents, and dry needling. BT injections in chronic TPs are also practiced, though no specific injection therapy has been reliably shown to be more advantageous than another. The benefits of TPIs may be derived from the needle itself rather than from any specific substance injected. Stimulation of a local twitch response with direct needling of the TP appears of importance. There is no established consensus regarding the number of injection points, frequency of administration, and volume or type of injectate.128
Adverse effects of TPIs relate to the nature of the invasive therapy, with the risk of tissue damage and bleeding. Pneumothorax risk is present with needle insertion at the neck and thorax.129 Patients with diabetes may experience variations in blood sugar control if steroids are used.
Bottom Line
Interventional treatment modalities that may have a role in psychiatric treatment include IV administration of ketamine, aducanumab, lecanemab, brexanolone, magnesium, scopolamine, and clomipramine. Other interventional approaches include stellate ganglion blocks, glabellar botulinum toxin injections, and trigger point injections.
Related Resources
- Dokucu ME, Janicak PG. Nontraditional therapies for treatment-resistant depression. Current Psychiatry. 2021; 20(9):38-43,49. doi:10.12788/cp.0166
- Kim J, Khoury R, Grossberg GT. Botulinum toxin: emerging psychiatric indications. Current Psychiatry. 2018;17(12):8-18.
Drug Brand Names
Aducanumab • Aduhelm
Aripiprazole • Abilify
Aripiprazole lauroxil • Aristada
Brexanolone • Zulresso
Buprenorphine • Sublocade
Citalopram • Celexa
Clomipramine • Anafranil
Diazepam • Valium
Droperidol • Inapsine
Esketamine • Spravato
Fentanyl • Actiq
Fluphenazine decanoate • Modecate
Fluphenazine hydrochloride • Prolixin
Haloperidol decanoate • Haldol decanoate
Haloperidol lactate • Haldol
Ketamine • Ketalar
Lecanemab • Leqembi
Lidocaine • Xylocaine
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Naltrexone • Vivitrol
Magnesium sulfate • Sulfamag
Midazolam • Versed
Olanzapine • Zyprexa
OnabotulinumtoxinA injection • Botox
Paliperidone • Invega Hafyera, Invega Sustenna, Invega Trinza
Rapamycin • Rapamune, Sirolimus
Risperidone • Perseris
Risperidone microspheres • Risperdal Consta, Rykindo
Scopolamine • Hyoscine
Tramadol • Conzip
Vortioxetine • Trintellix
Ziprasidone • Geodon
1. Vincent KM, Ryan M, Palmer E, et al. Interventional psychiatry. Postgrad Med. 2020;132(7):573-574.
2. Allen MH, Feifel D, Lesem MD, et al. Efficacy and safety of loxapine for inhalation in the treatment of agitation in patients with schizophrenia: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2011;72(10):1313-1321.
3. Kwentus J, Riesenberg RA, Marandi M, et al. Rapid acute treatment of agitation in patients with bipolar I disorder: a multicenter, randomized, placebo-controlled clinical trial with inhaled loxapine. Bipolar Disord. 2012;14(1):31-40.
4. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2019;393(10173):778-790.
6. Andorn A, Graham J, Csernansky J, et al. Monthly extended-release risperidone (RBP-7000) in the treatment of schizophrenia: results from the phase 3 program. J Clin Psychopharmacol. 2019;39(5):428-433.
7. Dundee TW. Twenty-five years of ketamine. A report of an international meeting. Anaesthesia. 1990;45(2):159. doi:10.1111/j.1365-2044.1990.tb14287.x
8. White PF, Way WL, Trevor AJ. Ketamine--its pharmacology and therapeutic uses. Anesthesiology. 1982;56(2):119-136. doi:10.1097/00000542-198202000-00007
9. Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23(4):801-811.
10. Molero P, Ramos-Quiroga JA, Martin-Santos R, et al. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs. 2018;32(5):411-420. doi:10.1007/s40263-018-0519-3
11. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
12. Witkin JM, Martin AE, Golani LK, et al. Rapid-acting antidepressants. Adv Pharmacol. 2019;86:47-96.
13. Strayer RJ, Nelson LS. Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med. 2008;26(9):985-1028. doi:10.1016/j.ajem.2007.12.005
14. Frye MA, Blier P, Tye SJ. Concomitant benzodiazepine use attenuates ketamine response: implications for large scale study design and clinical development. J Clin Psychopharmacol. 2015;35(3):334-336.
15. Fava M, Freeman MP, Flynn M, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. 2020;25(7):1592-1603.
16. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affect Disord. 2021;278:542-555. Erratum in: J Affect Disord. 2021;281:1001.
17. Brendle M, Robison R, Malone DC. Cost-effectiveness of esketamine nasal spray compared to intravenous ketamine for patients with treatment-resistant depression in the US utilizing clinical trial efficacy and real-world effectiveness estimates. J Affect Disord. 2022;319:388-396.
18. Dhillon S. Aducanumab: first approval. Drugs. 2021;81(12):1437-1443. Erratum in: Drugs. 2021;81(14):1701.
19. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
20. Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50-56. Update in: Nature. 2017;546(7659):564.
21. Fillit H, Green A. Aducanumab and the FDA – where are we now? Nat Rev Neurol. 2021;17(3):129-130.
22. Reardon S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature. 2023;613(7943):227-228. doi:10.1038/d41586-023-00030-3
23. McDade E, Cummings JL, Dhadda S, et al. Lecanemab in patients with early Alzheimer’s disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res Ther. 2022;14(1):191. doi:10.1186/s13195-022-01124-2
24. Mintun MA, Lo AC, Evans CD, et al. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384(18):1691-1704.
25. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433.
26. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
27. Powell JG, Garland S, Preston K, et al. Brexanolone (Zulresso): finally, an FDA-approved treatment for postpartum depression. Ann Pharmacother. 2020;54(2):157-163.
28. Patterson R, Krohn H, Richardson E, et al. A brexanolone treatment program at an academic medical center: patient selection, 90-day posttreatment outcomes, and lessons learned. J Acad Consult Liaison Psychiatry. 2022;63(1):14-22.
29. World Health Organization. WHO model list of essential medicines - 22nd list (2021). World Health Organization. September 30, 2021. Accessed April 7, 2023. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02
30. Eby GA, Eby KL, Mruk H. Magnesium and major depression. In: Vink R, Nechifor M, eds. Magnesium in the Central Nervous System. University of Adelaide Press; 2011.
31. Plant TM, Zeleznik AJ. Knobil and Neill’s Physiology of Reproduction. 4th ed. Elsevier Inc.; 2015:2503-2550.
32. Sidebotham D, Le Grice IJ. Physiology and pathophysiology. In: Sidebotham D, McKee A, Gillham M, Levy J. Cardiothoracic Critical Care. Elsevier, Inc.; 2007:3-27.
33. Duley L, Gülmezoglu AM, Henderson-Smart DJ, et al. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010;2010(11):CD000025.
34. Emergency supply of medicines. In: British National Formulary. British Medical Association, Royal Pharmaceutical Society; 2015:6. Accessed April 7, 2023. https://www.academia.edu/35076015/british_national_formulary_2015_pdf
35. Kwofie K, Wolfson AB. Intravenous magnesium sulfate for acute asthma exacerbation in children and adults. Am Fam Physician. 2021;103(4):245-246.
36. Patniyot IR, Gelfand AA. Acute treatment therapies for pediatric migraine: a qualitative systematic review. Headache. 2016;56(1):49-70.
37. Wang X, Du X, Yang H, et al. Use of intravenous magnesium sulfate among patients with acute myocardial infarction in China from 2001 to 2015: China PEACE-Retrospective AMI Study. BMJ Open. 2020;10(3):e033269.
38. Karhu E, Atlas SE, Jinrun G, et al. Intravenous infusion of magnesium sulfate is not associated with cardiovascular, liver, kidney, and metabolic toxicity in adults. J Clin Transl Res. 2018;4(1):47-55.
39. Noah L, Pickering G, Mazur A, et al. Impact of magnesium supplementation, in combination with vitamin B6, on stress and magnesium status: secondary data from a randomized controlled trial. Magnes Res. 2020;33(3):45-57.
40. Erstad BL, Cotugno CL. Management of alcohol withdrawal. Am J Health Syst Pharm. 1995;52(7):697-709.
41. Abumaria N, Luo L, Ahn M, et al. Magnesium supplement enhances spatial-context pattern separation and prevents fear overgeneralization. Behav Pharmacol. 2013;24(4):255-263.
42. Kirov GK, Tsachev KN. Magnesium, schizophrenia and manic-depressive disease. Neuropsychobiology. 1990;23(2):79-81.
43. Botturi A, Ciappolino V, Delvecchio G, et al. The role and the effect of magnesium in mental disorders: a systematic review. Nutrients. 2020;12(6):1661.
44. Kirkland AE, Sarlo GL, Holton KF. The role of magnesium in neurological disorders. Nutrients. 2018;10(6):730.
45. Magnesium sulfate intravenous side effects by likelihood and severity. WebMD. Accessed April 9, 2023. https://www.webmd.com/drugs/2/drug-149570/magnesium-sulfate-intravenous/details/list-sideeffects
46. Scopolamine base transdermal system – uses, side effects, and more. WebMD. Accessed April 9, 2023. https://www.webmd.com/drugs/2/drug-14032/scopolamine-transdermal/details
47. Bolden C, Cusack B, Richelson E. Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther. 1992;260(2):576-580.
48. Janowsky DS, el-Yousef MK, Davis JM, et al. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2(7778):632-635.
49. Janowsky DS, Risch SC, Gillin JC. Adrenergic-cholinergic balance and the treatment of affective disorders. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7(2-3):297-307.
50. Gershon S, Shaw FH. Psychiatric sequelae of chronic exposure to organophosphorous insecticides. Lancet. 1972;1(7191):1371-1374.
51. Davis KL, Berger PA, Hollister LE, et al. Physostigmine in mania. Arch Gen Psychiatry. 1978;35(1):119-122.
52. Wang JC, Hinrichs AL, Stock H, et al. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13(17):1903-1911.
53. Brown RG. Effects of antidepressants and anticholinergics in a mouse “behavioral despair” test. Eur J Pharmacol. 1979;58(3):331-334.
54. Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730-732.
55. Ji CX, Zhang JJ. Effect of scopolamine on depression in mice. Abstract in English. Yao Xue Xue Bao. 2011;46(4):400-405.
56. Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63(10):1121-1129.
57. Drevets WC, Furey ML. Replication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67(5):432-438.
58. Furey ML, Khanna A, Hoffman EM, et al. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35(12):2479-2488.
59. Gibbs RB, Gabor R, Cox T, et al. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29(6):741-748.
60. Pongrac JL, Gibbs RB, Defranco DB. Estrogen-mediated regulation of cholinergic expression in basal forebrain neurons requires extracellular-signal-regulated kinase activity. Neuroscience. 2004;124(4):809-816.
61. Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21(17):6949-6956.
62. Gerhard DM, Wohleb ES, Duman RS. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today. 2016;21(3):454-464.
63. Voderholzer U. Sleep deprivation and antidepressant treatment. Dialogues Clin Neurosci. 2003;5(4):366-369.
64. Hasselmann H. Scopolamine and depression: a role for muscarinic antagonism? CNS Neurol Disord Drug Targets. 2014;13(4):673-683.
65. Transderm scopolamine [prescribing information]. Warren, NJ: GSK Consumer Healthcare; 2019.
66. Jaffe RJ, Novakovic V, Peselow ED. Scopolamine as an antidepressant: a systematic review. Clin Neuropharmacol. 2013;36(1):24-26.
67. Karameh WK, Khani M. Intravenous clomipramine for treatment-resistant obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2015;19(2):pyv084.
68. Andrews ET, Beattie RM, Tighe MP. Functional abdominal pain: what clinicians need to know. Arch Dis Child. 2020;105(10):938-944. doi:10.1136/archdischild-2020-318825
69. Aliane V, Pérez S, Bohren Y, et al. Key role of striatal cholinergic interneurons in processes leading to arrest of motor stereotypies. Brain. 2011;134(Pt 1):110-118. doi:10.1093/brain/awq285
70. Tzavara ET, Bymaster FP, Davis RJ, et al. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 2004;18(12):1410-1412. doi:10.1096/fj.04-1575fje
71. Korczyn AD, Kish I. The mechanism of imipramine in enuresis nocturna. Clin Exp Pharmacol Physiol. 1979;6(1):31-35. doi:10.1111/j.1440-1681.1979.tb00004.x
72. Trimble MR. Worldwide use of clomipramine. J Clin Psychiatry. 1990;51(Suppl):51-54; discussion 55-58.
73. Gong W, Zhang S, Zong Y, et al. Involvement of the microglial NLRP3 inflammasome in the anti-inflammatory effect of the antidepressant clomipramine. J Affect Disord. 2019;254:15-25.
74. Piwowarska J, Wrzosek M, Radziwon’-Zaleska M. Serum cortisol concentration in patients with major depression after treatment with clomipramine. Pharmacol Rep. 2009;61(4):604-611.
75. Danish University Antidepressant Group (DUAG). Clomipramine dose-effect study in patients with depression: clinical end points and pharmacokinetics. Clin Pharmacol Ther. 1999;66(2):152-165.
76. Moukaddam NJ, Hirschfeld RMA. Intravenous antidepressants: a review. Depress Anxiety. 2004;19(1):1-9.
77. Gerretsen P, Pollock BG. Rediscovering adverse anticholinergic effects. J Clin Psychiatry. 2011;72(6):869-870. doi:10.4088/JCP.11ac07093
78. Thomas SJ, Shin M, McInnis MG, et al. Combination therapy with monoamine oxidase inhibitors and other antidepressants or stimulants: strategies for the management of treatment-resistant depression. Pharmacotherapy. 2015;35(4):433-449. doi:10.1002/phar.1576
79. Robles LA. Serotonin syndrome induced by fentanyl in a child: case report. Clin Neuropharmacol. 2015;38(5):206-208. doi:10.1097/WNF.0000000000000100
80. Fallon BA, Liebowitz MR, Campeas R, et al. Intravenous clomipramine for obsessive-compulsive disorder refractory to oral clomipramine: a placebo-controlled study. Arch Gen Psychiatry. 1998;55(10):918-924.
81. Vieta E, Florea I, Schmidt SN, et al. Intravenous vortioxetine to accelerate onset of effect in major depressive disorder: a 2-week, randomized, double-blind, placebo-controlled study. Int Clin Psychopharmacol. 2019;34(4):153-160.
82. Kasper S, Müller-Spahn F. Intravenous antidepressant treatment: focus on citalopram. Eur Arch Psychiatry Clin Neurosci. 2002;252(3):105-109.
83. Togay B, El-Mallakh RS. Posttraumatic stress disorder: from pathophysiology to pharmacology. Current Psychiatry. 2020;19(5):33-39.
84. Adhikari A, Lerner TN, Finkelstein J, et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature. 2015;527(7577):179-185. doi:10.1038/nature15698
85. Lipov E. In search of an effective treatment for combat-related post-traumatic stress disorder (PTSD): can the stellate ganglion block be the answer? Pain Pract. 2010;10(4):265-266.
86. Lipov E, Ritchie EC. A review of the use of stellate ganglion block in the treatment of PTSD. Curr Psychiatry Rep. 2015;17(8):599.
87. Olmsted KLR, Bartoszek M, McLean B, et al. Effect of stellate ganglion block treatment on posttraumatic stress disorder symptoms: a randomized clinical trial. JAMA Psychiatry. 2020;77(2):130-138.
88. Lipov E, Candido K. The successful use of left-sided stellate ganglion block in patients that fail to respond to right-sided stellate ganglion block for the treatment of post-traumatic stress disorder symptoms: a retrospective analysis of 205 patients. Mil Med. 2021;186(11-12):319-320.
89. Li Y, Loshak H. Stellate ganglion block for the treatment of post-traumatic stress disorder, depression, and anxiety. Canadian J Health Technol. 2021;1(3):1-30.
90. Kerzner J, Liu H, Demchenko I, et al. Stellate ganglion block for psychiatric disorders: a systematic review of the clinical research landscape. Chronic Stress (Thousand Oaks). 2021;5:24705470211055176.
91. Wie C, Gupta R, Maloney J, et al. Interventional modalities to treat complex regional pain syndrome. Curr Pain Headache Rep. 2021;25(2):10. doi:10.1007/s11916-020-00904-5
92. Chaturvedi A, Dash HH. Sympathetic blockade for the relief of chronic pain. J Indian Med Assoc. 2001;99(12):698-703.
93. Chester M, Hammond C. Leach A. Long-term benefits of stellate ganglion block in severe chronic refractory angina. Pain. 2000;87(1):103-105. doi:10.1016/S0304-3959(00)00270-0
94. Jeon Y. Therapeutic potential of stellate ganglion block in orofacial pain: a mini review. J Dent Anesth Pain Med. 2016;16(3):159-163. doi:10.17245/jdapm.2016.16.3.159
95. Shan HH, Chen HF, Ni Y, et al. Effects of stellate ganglion block through different approaches under guidance of ultrasound. Front Surg. 2022;8:797793. doi:10.3389/fsurg.2021.797793
96. Goel V, Patwardhan AM, Ibrahim M, et al. Complications associated with stellate ganglion nerve block: a systematic review. Reg Anesth Pain Med. 2019;rapm-2018-100127. doi:10.1136/rapm-2018-100127
97. Rowe FJ, Noonan CP. Botulinum toxin for the treatment of strabismus. Cochrane Database Syst Rev. 2017;3(3):CD006499.
98. Roggenkämper P, Jost WH, Bihari K, et al. Efficacy and safety of a new botulinum toxin type A free of complexing proteins in the treatment of blepharospasm. J Neural Transm (Vienna). 2006;113(3):303-312.
99. Heckmann M, Ceballos-Baumann AO, Plewig G; Hyperhidrosis Study Group. Botulinum toxin A for axillary hyperhidrosis (excessive sweating). N Engl J Med. 2001;344(7):488-493.
100. Carruthers JA, Lowe NJ, Menter MA, et al. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 2002;46(6):840-849.
101. Schurch B, de Sèze M, Denys P, et al. Botulinum toxin type A is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol. 2005;174:196–200.
102. Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: Pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51(9):1358-1373.
103. Dashtipour K, Chen JJ, Walker HW, et al. Systematic literature review of abobotulinumtoxinA in clinical trials for adult upper limb spasticity. Am J Phys Med Rehabil. 2015;94(3):229-238.
104. Nitti VW, Dmochowski R, Herschorn S, et al. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo-controlled trial. J Urol. 2017;197(2S):S216-S223.
105. Jongerius PH, van den Hoogen FJA, van Limbeek J, et al. Effect of botulinum toxin in the treatment of drooling: a controlled clinical trial. Pediatrics. 2004;114(3):620-627.
106. Zaninotto, G. Annese V, Costantini M, et al. Randomized controlled trial of botulinum toxin versus laparoscopic heller myotomy for esophageal achalasia. Ann Surg. 2004;239(3):364-370.
107. Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005;53:3-9.
108. Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J Cosmet Dermatol. 2009;8(1):24-26.
109. Affatato O, Moulin TC, Pisanu C, et al. High efficacy of onabotulinumtoxinA treatment in patients with comorbid migraine and depression: a meta-analysis. J Transl Med. 2021;19(1):133.
110. Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: a case series. Dermatol Surg. 2006;32(5):645-649; discussion 649-650.
111. Schulze J, Neumann I, Magid M, et al. Botulinum toxin for the management of depression: an updated review of the evidence and meta-analysis. J Psychiatr Res. 2021;135:332-340.
112. Finzi E, Rosenthal NE. Emotional proprioception: treatment of depression with afferent facial feedback. J Psychiatr Res. 2016;80:93-96.
113. Söderkvist S, Ohlén K, Dimberg U. How the experience of emotion is modulated by facial feedback. J Nonverbal Behav. 2018;42(1):129-151.
114. Lewis, MB. The interactions between botulinum-toxin-based facial treatments and embodied emotions. Sci Rep. 2018;8(1):14720.
115. Li Y, Liu J, Liu X, et al. Antidepressant-like action of single facial injection of botulinum neurotoxin A is associated with augmented 5-HT levels and BDNF/ERK/CREB pathways in mouse brain. Neurosci Bull. 2019;35(4):661-672. Erratum in: Neurosci Bull. 2019;35(4):779-780.
116. Gündel H, Wolf A, Xidara V, et al. High psychiatric comorbidity in spasmodic torticollis: a controlled study. J Nerv Ment Dis. 2003;191(7):465-473.
117. Hall TA, McGwin G Jr, Searcey K, et al. Health-related quality of life and psychosocial characteristics of patients with benign essential blepharospasm. Arch Ophthalmol. 2006;124(1):116-119.
118. Ceylan D, Erer S, Zarifog˘lu M, et al. Evaluation of anxiety and depression scales and quality of life in cervical dystonia patients on botulinum toxin therapy and their relatives. Neurol Sci. 2019;40(4):725-731.
119. Heller AS, Lapate RC, Mayer KE, et al. The face of negative affect: trial-by-trial corrugator responses to negative pictures are positively associated with amygdala and negatively associated with ventromedial prefrontal cortex activity. J Cogn Neurosci. 2014;26(9):2102-2110.
120. Makunts T, Wollmer MA, Abagyan R. Postmarketing safety surveillance data reveals antidepressant effects of botulinum toxin across various indications and injection sites. Sci Rep. 2020;10(1):12851.
121. Ahsanuddin S, Roy S, Nasser W, et al. Adverse events associated with botox as reported in a Food and Drug Administration database. Aesthetic Plast Surg. 2021;45(3):1201-1209. doi:10.1007/s00266-020-02027-z
122. Kashif M, Tahir S, Ashfaq F, et al. Association of myofascial trigger points in neck and shoulder region with depression, anxiety, and stress among university students. J Pak Med Assoc. 2021;71(9):2139-2142.
123. Cigarán-Méndez M, Jiménez-Antona C, Parás-Bravo P, et al. Active trigger points are associated with anxiety and widespread pressure pain sensitivity in women, but not men, with tension type headache. Pain Pract. 2019;19(5):522-529.
124. Palacios-Ceña M, Castaldo M, Wang K, et al. Relationship of active trigger points with related disability and anxiety in people with tension-type headache. Medicine (Baltimore). 2017;96(13):e6548.
125. Karadas Ö, Inan LE, Ulas Ü, et al. Efficacy of local lidocaine application on anxiety and depression and its curative effect on patients with chronic tension-type headache. Eur Neurol. 2013;70(1-2):95-101.
126. Gerwin RD. Classification, epidemiology and natural history of myofascial pain syndrome. Curr Pain Headache Rep. 2001;5(5):412-420.
127. Castro Sánchez AM, García López H, Fernández Sánchez M, et al. Improvement in clinical outcomes after dry needling versus myofascial release on pain pressure thresholds, quality of life, fatigue, pain intensity, quality of sleep, anxiety, and depression in patients with fibromyalgia syndrome. Disabil Rehabil. 2019;41(19):2235-2246.
128. Healy GM, Finn DP, O’Gorman DA, et al. Pretreatment anxiety and pain acceptance are associated with response to trigger point injection therapy for chronic myofascial pain. Pain Med. 2015;16(10):1955-1966.
129. Morjaria JB, Lakshminarayana UB, Liu-Shiu-Cheong P, et al. Pneumothorax: a tale of pain or spontaneity. Ther Adv Chronic Dis. 2014;5(6):269-273.
1. Vincent KM, Ryan M, Palmer E, et al. Interventional psychiatry. Postgrad Med. 2020;132(7):573-574.
2. Allen MH, Feifel D, Lesem MD, et al. Efficacy and safety of loxapine for inhalation in the treatment of agitation in patients with schizophrenia: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2011;72(10):1313-1321.
3. Kwentus J, Riesenberg RA, Marandi M, et al. Rapid acute treatment of agitation in patients with bipolar I disorder: a multicenter, randomized, placebo-controlled clinical trial with inhaled loxapine. Bipolar Disord. 2012;14(1):31-40.
4. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2019;393(10173):778-790.
6. Andorn A, Graham J, Csernansky J, et al. Monthly extended-release risperidone (RBP-7000) in the treatment of schizophrenia: results from the phase 3 program. J Clin Psychopharmacol. 2019;39(5):428-433.
7. Dundee TW. Twenty-five years of ketamine. A report of an international meeting. Anaesthesia. 1990;45(2):159. doi:10.1111/j.1365-2044.1990.tb14287.x
8. White PF, Way WL, Trevor AJ. Ketamine--its pharmacology and therapeutic uses. Anesthesiology. 1982;56(2):119-136. doi:10.1097/00000542-198202000-00007
9. Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23(4):801-811.
10. Molero P, Ramos-Quiroga JA, Martin-Santos R, et al. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs. 2018;32(5):411-420. doi:10.1007/s40263-018-0519-3
11. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
12. Witkin JM, Martin AE, Golani LK, et al. Rapid-acting antidepressants. Adv Pharmacol. 2019;86:47-96.
13. Strayer RJ, Nelson LS. Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med. 2008;26(9):985-1028. doi:10.1016/j.ajem.2007.12.005
14. Frye MA, Blier P, Tye SJ. Concomitant benzodiazepine use attenuates ketamine response: implications for large scale study design and clinical development. J Clin Psychopharmacol. 2015;35(3):334-336.
15. Fava M, Freeman MP, Flynn M, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. 2020;25(7):1592-1603.
16. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affect Disord. 2021;278:542-555. Erratum in: J Affect Disord. 2021;281:1001.
17. Brendle M, Robison R, Malone DC. Cost-effectiveness of esketamine nasal spray compared to intravenous ketamine for patients with treatment-resistant depression in the US utilizing clinical trial efficacy and real-world effectiveness estimates. J Affect Disord. 2022;319:388-396.
18. Dhillon S. Aducanumab: first approval. Drugs. 2021;81(12):1437-1443. Erratum in: Drugs. 2021;81(14):1701.
19. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
20. Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50-56. Update in: Nature. 2017;546(7659):564.
21. Fillit H, Green A. Aducanumab and the FDA – where are we now? Nat Rev Neurol. 2021;17(3):129-130.
22. Reardon S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature. 2023;613(7943):227-228. doi:10.1038/d41586-023-00030-3
23. McDade E, Cummings JL, Dhadda S, et al. Lecanemab in patients with early Alzheimer’s disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res Ther. 2022;14(1):191. doi:10.1186/s13195-022-01124-2
24. Mintun MA, Lo AC, Evans CD, et al. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384(18):1691-1704.
25. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433.
26. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
27. Powell JG, Garland S, Preston K, et al. Brexanolone (Zulresso): finally, an FDA-approved treatment for postpartum depression. Ann Pharmacother. 2020;54(2):157-163.
28. Patterson R, Krohn H, Richardson E, et al. A brexanolone treatment program at an academic medical center: patient selection, 90-day posttreatment outcomes, and lessons learned. J Acad Consult Liaison Psychiatry. 2022;63(1):14-22.
29. World Health Organization. WHO model list of essential medicines - 22nd list (2021). World Health Organization. September 30, 2021. Accessed April 7, 2023. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02
30. Eby GA, Eby KL, Mruk H. Magnesium and major depression. In: Vink R, Nechifor M, eds. Magnesium in the Central Nervous System. University of Adelaide Press; 2011.
31. Plant TM, Zeleznik AJ. Knobil and Neill’s Physiology of Reproduction. 4th ed. Elsevier Inc.; 2015:2503-2550.
32. Sidebotham D, Le Grice IJ. Physiology and pathophysiology. In: Sidebotham D, McKee A, Gillham M, Levy J. Cardiothoracic Critical Care. Elsevier, Inc.; 2007:3-27.
33. Duley L, Gülmezoglu AM, Henderson-Smart DJ, et al. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010;2010(11):CD000025.
34. Emergency supply of medicines. In: British National Formulary. British Medical Association, Royal Pharmaceutical Society; 2015:6. Accessed April 7, 2023. https://www.academia.edu/35076015/british_national_formulary_2015_pdf
35. Kwofie K, Wolfson AB. Intravenous magnesium sulfate for acute asthma exacerbation in children and adults. Am Fam Physician. 2021;103(4):245-246.
36. Patniyot IR, Gelfand AA. Acute treatment therapies for pediatric migraine: a qualitative systematic review. Headache. 2016;56(1):49-70.
37. Wang X, Du X, Yang H, et al. Use of intravenous magnesium sulfate among patients with acute myocardial infarction in China from 2001 to 2015: China PEACE-Retrospective AMI Study. BMJ Open. 2020;10(3):e033269.
38. Karhu E, Atlas SE, Jinrun G, et al. Intravenous infusion of magnesium sulfate is not associated with cardiovascular, liver, kidney, and metabolic toxicity in adults. J Clin Transl Res. 2018;4(1):47-55.
39. Noah L, Pickering G, Mazur A, et al. Impact of magnesium supplementation, in combination with vitamin B6, on stress and magnesium status: secondary data from a randomized controlled trial. Magnes Res. 2020;33(3):45-57.
40. Erstad BL, Cotugno CL. Management of alcohol withdrawal. Am J Health Syst Pharm. 1995;52(7):697-709.
41. Abumaria N, Luo L, Ahn M, et al. Magnesium supplement enhances spatial-context pattern separation and prevents fear overgeneralization. Behav Pharmacol. 2013;24(4):255-263.
42. Kirov GK, Tsachev KN. Magnesium, schizophrenia and manic-depressive disease. Neuropsychobiology. 1990;23(2):79-81.
43. Botturi A, Ciappolino V, Delvecchio G, et al. The role and the effect of magnesium in mental disorders: a systematic review. Nutrients. 2020;12(6):1661.
44. Kirkland AE, Sarlo GL, Holton KF. The role of magnesium in neurological disorders. Nutrients. 2018;10(6):730.
45. Magnesium sulfate intravenous side effects by likelihood and severity. WebMD. Accessed April 9, 2023. https://www.webmd.com/drugs/2/drug-149570/magnesium-sulfate-intravenous/details/list-sideeffects
46. Scopolamine base transdermal system – uses, side effects, and more. WebMD. Accessed April 9, 2023. https://www.webmd.com/drugs/2/drug-14032/scopolamine-transdermal/details
47. Bolden C, Cusack B, Richelson E. Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther. 1992;260(2):576-580.
48. Janowsky DS, el-Yousef MK, Davis JM, et al. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2(7778):632-635.
49. Janowsky DS, Risch SC, Gillin JC. Adrenergic-cholinergic balance and the treatment of affective disorders. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7(2-3):297-307.
50. Gershon S, Shaw FH. Psychiatric sequelae of chronic exposure to organophosphorous insecticides. Lancet. 1972;1(7191):1371-1374.
51. Davis KL, Berger PA, Hollister LE, et al. Physostigmine in mania. Arch Gen Psychiatry. 1978;35(1):119-122.
52. Wang JC, Hinrichs AL, Stock H, et al. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13(17):1903-1911.
53. Brown RG. Effects of antidepressants and anticholinergics in a mouse “behavioral despair” test. Eur J Pharmacol. 1979;58(3):331-334.
54. Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730-732.
55. Ji CX, Zhang JJ. Effect of scopolamine on depression in mice. Abstract in English. Yao Xue Xue Bao. 2011;46(4):400-405.
56. Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63(10):1121-1129.
57. Drevets WC, Furey ML. Replication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67(5):432-438.
58. Furey ML, Khanna A, Hoffman EM, et al. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35(12):2479-2488.
59. Gibbs RB, Gabor R, Cox T, et al. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29(6):741-748.
60. Pongrac JL, Gibbs RB, Defranco DB. Estrogen-mediated regulation of cholinergic expression in basal forebrain neurons requires extracellular-signal-regulated kinase activity. Neuroscience. 2004;124(4):809-816.
61. Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21(17):6949-6956.
62. Gerhard DM, Wohleb ES, Duman RS. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today. 2016;21(3):454-464.
63. Voderholzer U. Sleep deprivation and antidepressant treatment. Dialogues Clin Neurosci. 2003;5(4):366-369.
64. Hasselmann H. Scopolamine and depression: a role for muscarinic antagonism? CNS Neurol Disord Drug Targets. 2014;13(4):673-683.
65. Transderm scopolamine [prescribing information]. Warren, NJ: GSK Consumer Healthcare; 2019.
66. Jaffe RJ, Novakovic V, Peselow ED. Scopolamine as an antidepressant: a systematic review. Clin Neuropharmacol. 2013;36(1):24-26.
67. Karameh WK, Khani M. Intravenous clomipramine for treatment-resistant obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2015;19(2):pyv084.
68. Andrews ET, Beattie RM, Tighe MP. Functional abdominal pain: what clinicians need to know. Arch Dis Child. 2020;105(10):938-944. doi:10.1136/archdischild-2020-318825
69. Aliane V, Pérez S, Bohren Y, et al. Key role of striatal cholinergic interneurons in processes leading to arrest of motor stereotypies. Brain. 2011;134(Pt 1):110-118. doi:10.1093/brain/awq285
70. Tzavara ET, Bymaster FP, Davis RJ, et al. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 2004;18(12):1410-1412. doi:10.1096/fj.04-1575fje
71. Korczyn AD, Kish I. The mechanism of imipramine in enuresis nocturna. Clin Exp Pharmacol Physiol. 1979;6(1):31-35. doi:10.1111/j.1440-1681.1979.tb00004.x
72. Trimble MR. Worldwide use of clomipramine. J Clin Psychiatry. 1990;51(Suppl):51-54; discussion 55-58.
73. Gong W, Zhang S, Zong Y, et al. Involvement of the microglial NLRP3 inflammasome in the anti-inflammatory effect of the antidepressant clomipramine. J Affect Disord. 2019;254:15-25.
74. Piwowarska J, Wrzosek M, Radziwon’-Zaleska M. Serum cortisol concentration in patients with major depression after treatment with clomipramine. Pharmacol Rep. 2009;61(4):604-611.
75. Danish University Antidepressant Group (DUAG). Clomipramine dose-effect study in patients with depression: clinical end points and pharmacokinetics. Clin Pharmacol Ther. 1999;66(2):152-165.
76. Moukaddam NJ, Hirschfeld RMA. Intravenous antidepressants: a review. Depress Anxiety. 2004;19(1):1-9.
77. Gerretsen P, Pollock BG. Rediscovering adverse anticholinergic effects. J Clin Psychiatry. 2011;72(6):869-870. doi:10.4088/JCP.11ac07093
78. Thomas SJ, Shin M, McInnis MG, et al. Combination therapy with monoamine oxidase inhibitors and other antidepressants or stimulants: strategies for the management of treatment-resistant depression. Pharmacotherapy. 2015;35(4):433-449. doi:10.1002/phar.1576
79. Robles LA. Serotonin syndrome induced by fentanyl in a child: case report. Clin Neuropharmacol. 2015;38(5):206-208. doi:10.1097/WNF.0000000000000100
80. Fallon BA, Liebowitz MR, Campeas R, et al. Intravenous clomipramine for obsessive-compulsive disorder refractory to oral clomipramine: a placebo-controlled study. Arch Gen Psychiatry. 1998;55(10):918-924.
81. Vieta E, Florea I, Schmidt SN, et al. Intravenous vortioxetine to accelerate onset of effect in major depressive disorder: a 2-week, randomized, double-blind, placebo-controlled study. Int Clin Psychopharmacol. 2019;34(4):153-160.
82. Kasper S, Müller-Spahn F. Intravenous antidepressant treatment: focus on citalopram. Eur Arch Psychiatry Clin Neurosci. 2002;252(3):105-109.
83. Togay B, El-Mallakh RS. Posttraumatic stress disorder: from pathophysiology to pharmacology. Current Psychiatry. 2020;19(5):33-39.
84. Adhikari A, Lerner TN, Finkelstein J, et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature. 2015;527(7577):179-185. doi:10.1038/nature15698
85. Lipov E. In search of an effective treatment for combat-related post-traumatic stress disorder (PTSD): can the stellate ganglion block be the answer? Pain Pract. 2010;10(4):265-266.
86. Lipov E, Ritchie EC. A review of the use of stellate ganglion block in the treatment of PTSD. Curr Psychiatry Rep. 2015;17(8):599.
87. Olmsted KLR, Bartoszek M, McLean B, et al. Effect of stellate ganglion block treatment on posttraumatic stress disorder symptoms: a randomized clinical trial. JAMA Psychiatry. 2020;77(2):130-138.
88. Lipov E, Candido K. The successful use of left-sided stellate ganglion block in patients that fail to respond to right-sided stellate ganglion block for the treatment of post-traumatic stress disorder symptoms: a retrospective analysis of 205 patients. Mil Med. 2021;186(11-12):319-320.
89. Li Y, Loshak H. Stellate ganglion block for the treatment of post-traumatic stress disorder, depression, and anxiety. Canadian J Health Technol. 2021;1(3):1-30.
90. Kerzner J, Liu H, Demchenko I, et al. Stellate ganglion block for psychiatric disorders: a systematic review of the clinical research landscape. Chronic Stress (Thousand Oaks). 2021;5:24705470211055176.
91. Wie C, Gupta R, Maloney J, et al. Interventional modalities to treat complex regional pain syndrome. Curr Pain Headache Rep. 2021;25(2):10. doi:10.1007/s11916-020-00904-5
92. Chaturvedi A, Dash HH. Sympathetic blockade for the relief of chronic pain. J Indian Med Assoc. 2001;99(12):698-703.
93. Chester M, Hammond C. Leach A. Long-term benefits of stellate ganglion block in severe chronic refractory angina. Pain. 2000;87(1):103-105. doi:10.1016/S0304-3959(00)00270-0
94. Jeon Y. Therapeutic potential of stellate ganglion block in orofacial pain: a mini review. J Dent Anesth Pain Med. 2016;16(3):159-163. doi:10.17245/jdapm.2016.16.3.159
95. Shan HH, Chen HF, Ni Y, et al. Effects of stellate ganglion block through different approaches under guidance of ultrasound. Front Surg. 2022;8:797793. doi:10.3389/fsurg.2021.797793
96. Goel V, Patwardhan AM, Ibrahim M, et al. Complications associated with stellate ganglion nerve block: a systematic review. Reg Anesth Pain Med. 2019;rapm-2018-100127. doi:10.1136/rapm-2018-100127
97. Rowe FJ, Noonan CP. Botulinum toxin for the treatment of strabismus. Cochrane Database Syst Rev. 2017;3(3):CD006499.
98. Roggenkämper P, Jost WH, Bihari K, et al. Efficacy and safety of a new botulinum toxin type A free of complexing proteins in the treatment of blepharospasm. J Neural Transm (Vienna). 2006;113(3):303-312.
99. Heckmann M, Ceballos-Baumann AO, Plewig G; Hyperhidrosis Study Group. Botulinum toxin A for axillary hyperhidrosis (excessive sweating). N Engl J Med. 2001;344(7):488-493.
100. Carruthers JA, Lowe NJ, Menter MA, et al. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 2002;46(6):840-849.
101. Schurch B, de Sèze M, Denys P, et al. Botulinum toxin type A is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol. 2005;174:196–200.
102. Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: Pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51(9):1358-1373.
103. Dashtipour K, Chen JJ, Walker HW, et al. Systematic literature review of abobotulinumtoxinA in clinical trials for adult upper limb spasticity. Am J Phys Med Rehabil. 2015;94(3):229-238.
104. Nitti VW, Dmochowski R, Herschorn S, et al. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo-controlled trial. J Urol. 2017;197(2S):S216-S223.
105. Jongerius PH, van den Hoogen FJA, van Limbeek J, et al. Effect of botulinum toxin in the treatment of drooling: a controlled clinical trial. Pediatrics. 2004;114(3):620-627.
106. Zaninotto, G. Annese V, Costantini M, et al. Randomized controlled trial of botulinum toxin versus laparoscopic heller myotomy for esophageal achalasia. Ann Surg. 2004;239(3):364-370.
107. Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005;53:3-9.
108. Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J Cosmet Dermatol. 2009;8(1):24-26.
109. Affatato O, Moulin TC, Pisanu C, et al. High efficacy of onabotulinumtoxinA treatment in patients with comorbid migraine and depression: a meta-analysis. J Transl Med. 2021;19(1):133.
110. Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: a case series. Dermatol Surg. 2006;32(5):645-649; discussion 649-650.
111. Schulze J, Neumann I, Magid M, et al. Botulinum toxin for the management of depression: an updated review of the evidence and meta-analysis. J Psychiatr Res. 2021;135:332-340.
112. Finzi E, Rosenthal NE. Emotional proprioception: treatment of depression with afferent facial feedback. J Psychiatr Res. 2016;80:93-96.
113. Söderkvist S, Ohlén K, Dimberg U. How the experience of emotion is modulated by facial feedback. J Nonverbal Behav. 2018;42(1):129-151.
114. Lewis, MB. The interactions between botulinum-toxin-based facial treatments and embodied emotions. Sci Rep. 2018;8(1):14720.
115. Li Y, Liu J, Liu X, et al. Antidepressant-like action of single facial injection of botulinum neurotoxin A is associated with augmented 5-HT levels and BDNF/ERK/CREB pathways in mouse brain. Neurosci Bull. 2019;35(4):661-672. Erratum in: Neurosci Bull. 2019;35(4):779-780.
116. Gündel H, Wolf A, Xidara V, et al. High psychiatric comorbidity in spasmodic torticollis: a controlled study. J Nerv Ment Dis. 2003;191(7):465-473.
117. Hall TA, McGwin G Jr, Searcey K, et al. Health-related quality of life and psychosocial characteristics of patients with benign essential blepharospasm. Arch Ophthalmol. 2006;124(1):116-119.
118. Ceylan D, Erer S, Zarifog˘lu M, et al. Evaluation of anxiety and depression scales and quality of life in cervical dystonia patients on botulinum toxin therapy and their relatives. Neurol Sci. 2019;40(4):725-731.
119. Heller AS, Lapate RC, Mayer KE, et al. The face of negative affect: trial-by-trial corrugator responses to negative pictures are positively associated with amygdala and negatively associated with ventromedial prefrontal cortex activity. J Cogn Neurosci. 2014;26(9):2102-2110.
120. Makunts T, Wollmer MA, Abagyan R. Postmarketing safety surveillance data reveals antidepressant effects of botulinum toxin across various indications and injection sites. Sci Rep. 2020;10(1):12851.
121. Ahsanuddin S, Roy S, Nasser W, et al. Adverse events associated with botox as reported in a Food and Drug Administration database. Aesthetic Plast Surg. 2021;45(3):1201-1209. doi:10.1007/s00266-020-02027-z
122. Kashif M, Tahir S, Ashfaq F, et al. Association of myofascial trigger points in neck and shoulder region with depression, anxiety, and stress among university students. J Pak Med Assoc. 2021;71(9):2139-2142.
123. Cigarán-Méndez M, Jiménez-Antona C, Parás-Bravo P, et al. Active trigger points are associated with anxiety and widespread pressure pain sensitivity in women, but not men, with tension type headache. Pain Pract. 2019;19(5):522-529.
124. Palacios-Ceña M, Castaldo M, Wang K, et al. Relationship of active trigger points with related disability and anxiety in people with tension-type headache. Medicine (Baltimore). 2017;96(13):e6548.
125. Karadas Ö, Inan LE, Ulas Ü, et al. Efficacy of local lidocaine application on anxiety and depression and its curative effect on patients with chronic tension-type headache. Eur Neurol. 2013;70(1-2):95-101.
126. Gerwin RD. Classification, epidemiology and natural history of myofascial pain syndrome. Curr Pain Headache Rep. 2001;5(5):412-420.
127. Castro Sánchez AM, García López H, Fernández Sánchez M, et al. Improvement in clinical outcomes after dry needling versus myofascial release on pain pressure thresholds, quality of life, fatigue, pain intensity, quality of sleep, anxiety, and depression in patients with fibromyalgia syndrome. Disabil Rehabil. 2019;41(19):2235-2246.
128. Healy GM, Finn DP, O’Gorman DA, et al. Pretreatment anxiety and pain acceptance are associated with response to trigger point injection therapy for chronic myofascial pain. Pain Med. 2015;16(10):1955-1966.
129. Morjaria JB, Lakshminarayana UB, Liu-Shiu-Cheong P, et al. Pneumothorax: a tale of pain or spontaneity. Ther Adv Chronic Dis. 2014;5(6):269-273.
Strong need for eating disorder screening in patients with PTSD
WASHINGTON –
“Eating-related and body-image concerns may be more prevalent than we think, and if not considered, these concerns can make psychotherapy treatment less effective,” study author Nick Powers, a doctoral student in clinical psychology, La Salle University, Philadelphia, told this news organization.
The findings were presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
Common bedfellows
Although many patients with PTSD also have an eating disorder, they are not always properly assessed for eating pathology and related functional impairment.
Some therapists don’t feel adequately equipped to target eating-related concerns in these patients and so may refer them to other providers. This, said Mr. Powers, can prolong symptoms and further distress patients.
Mr. Powers noted childhood physical or sexual abuse may affect eating patterns in patients with PTSD. “The evidence suggests these types of trauma exposure can be risk factors for the development of an eating disorder.”
Undiagnosed eating pathology may exacerbate functional impairment from PTSD and weaken the impact of evidence-based treatment.
Such patients are challenging to treat as they may not have the requisite skills to fully engage in exposure therapy, an evidence-based approach to treat PTSD, said Mr. Powers.
To determine whether PTSD would be significantly linked to greater eating disorder impairment (EDI) compared with other anxiety-related diagnoses and whether this would impair treatment, investigators studied 748 patients with an anxiety disorder who were attending a cognitive behavioral therapy (CBT) clinic. Anxiety disorders included PTSD, obsessive-compulsive disorder (OCD), social anxiety, and panic disorder.
Participants completed the 16-item Clinical Impairment Assessment (CIA) questionnaire, which includes questions about eating habits and feelings about food, body shape, and weight over the previous 4 weeks. Participants also reported anxiety symptom severity at the beginning, during, and end of treatment.
Need for better screening
Results showed that compared with those who had other anxiety disorders, patients with PTSD were three times more likely to have disordered eating (odds ratio [OR], 3.06; 95% confidence interval [CI], 1.47-6.37; P = .003).
In addition, higher baseline CIA scores predicted poorer PTSD treatment outcome (beta = –1.4; 95% CI, –1.67 to –1.10; P < .01).
“Having higher baseline CIA scores meant that patients’ PTSD symptoms did not remit as strongly compared to those with lower scores,” said Mr. Powers.
Patients with both PTSD and an eating disorder may have difficulty with regulating emotions and tolerating distress, he said.
“They may use binge eating, purging, or food restriction as strategies to regulate emotions. These behaviors may allow patients to become numb to or avoid heightened emotions that come from having PTSD and an eating disorder.”
Prior research linked perfectionism tendencies to poorer response to PTSD treatment. Those with an eating disorder may share similar tendencies, said Mr. Powers.
“If someone is consistently thinking negatively about their eating or body to the point where it interrupts their functioning, they may not be as likely to fully engage with PTSD treatment,” he said.
Ideally, clinicians would screen all patients with PTSD for an eating disorder, said Mr. Powers. “If screening instruments aren’t feasible or available, even just inquiring about body image or history of maladaptive eating behaviors can be helpful.”
He added this could open up a conversation about a traumatic event in the patient’s past.
Confirmatory research
Commenting on the study, Karen S. Mitchell, PhD, clinical research psychologist, National Center for PTSD, VA Boston Healthcare System, and associate professor in psychiatry, Boston University, said she was “excited” to see this research.
“Very few studies have examined the impact of baseline eating disorder symptoms on PTSD treatment outcomes or vice versa,” she said.
The study findings “add to the small but growing body of evidence suggesting that comorbid PTSD and eating disorder symptoms can impact recovery from each disorder,” she said.
She noted the importance of assessing comorbidity in patients presenting for treatment and of addressing comorbidity in both eating disorders and PTSD treatment. “But we need more research on how best to do this.”
Mr. Powers and Dr. Mitchell have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WASHINGTON –
“Eating-related and body-image concerns may be more prevalent than we think, and if not considered, these concerns can make psychotherapy treatment less effective,” study author Nick Powers, a doctoral student in clinical psychology, La Salle University, Philadelphia, told this news organization.
The findings were presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
Common bedfellows
Although many patients with PTSD also have an eating disorder, they are not always properly assessed for eating pathology and related functional impairment.
Some therapists don’t feel adequately equipped to target eating-related concerns in these patients and so may refer them to other providers. This, said Mr. Powers, can prolong symptoms and further distress patients.
Mr. Powers noted childhood physical or sexual abuse may affect eating patterns in patients with PTSD. “The evidence suggests these types of trauma exposure can be risk factors for the development of an eating disorder.”
Undiagnosed eating pathology may exacerbate functional impairment from PTSD and weaken the impact of evidence-based treatment.
Such patients are challenging to treat as they may not have the requisite skills to fully engage in exposure therapy, an evidence-based approach to treat PTSD, said Mr. Powers.
To determine whether PTSD would be significantly linked to greater eating disorder impairment (EDI) compared with other anxiety-related diagnoses and whether this would impair treatment, investigators studied 748 patients with an anxiety disorder who were attending a cognitive behavioral therapy (CBT) clinic. Anxiety disorders included PTSD, obsessive-compulsive disorder (OCD), social anxiety, and panic disorder.
Participants completed the 16-item Clinical Impairment Assessment (CIA) questionnaire, which includes questions about eating habits and feelings about food, body shape, and weight over the previous 4 weeks. Participants also reported anxiety symptom severity at the beginning, during, and end of treatment.
Need for better screening
Results showed that compared with those who had other anxiety disorders, patients with PTSD were three times more likely to have disordered eating (odds ratio [OR], 3.06; 95% confidence interval [CI], 1.47-6.37; P = .003).
In addition, higher baseline CIA scores predicted poorer PTSD treatment outcome (beta = –1.4; 95% CI, –1.67 to –1.10; P < .01).
“Having higher baseline CIA scores meant that patients’ PTSD symptoms did not remit as strongly compared to those with lower scores,” said Mr. Powers.
Patients with both PTSD and an eating disorder may have difficulty with regulating emotions and tolerating distress, he said.
“They may use binge eating, purging, or food restriction as strategies to regulate emotions. These behaviors may allow patients to become numb to or avoid heightened emotions that come from having PTSD and an eating disorder.”
Prior research linked perfectionism tendencies to poorer response to PTSD treatment. Those with an eating disorder may share similar tendencies, said Mr. Powers.
“If someone is consistently thinking negatively about their eating or body to the point where it interrupts their functioning, they may not be as likely to fully engage with PTSD treatment,” he said.
Ideally, clinicians would screen all patients with PTSD for an eating disorder, said Mr. Powers. “If screening instruments aren’t feasible or available, even just inquiring about body image or history of maladaptive eating behaviors can be helpful.”
He added this could open up a conversation about a traumatic event in the patient’s past.
Confirmatory research
Commenting on the study, Karen S. Mitchell, PhD, clinical research psychologist, National Center for PTSD, VA Boston Healthcare System, and associate professor in psychiatry, Boston University, said she was “excited” to see this research.
“Very few studies have examined the impact of baseline eating disorder symptoms on PTSD treatment outcomes or vice versa,” she said.
The study findings “add to the small but growing body of evidence suggesting that comorbid PTSD and eating disorder symptoms can impact recovery from each disorder,” she said.
She noted the importance of assessing comorbidity in patients presenting for treatment and of addressing comorbidity in both eating disorders and PTSD treatment. “But we need more research on how best to do this.”
Mr. Powers and Dr. Mitchell have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WASHINGTON –
“Eating-related and body-image concerns may be more prevalent than we think, and if not considered, these concerns can make psychotherapy treatment less effective,” study author Nick Powers, a doctoral student in clinical psychology, La Salle University, Philadelphia, told this news organization.
The findings were presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
Common bedfellows
Although many patients with PTSD also have an eating disorder, they are not always properly assessed for eating pathology and related functional impairment.
Some therapists don’t feel adequately equipped to target eating-related concerns in these patients and so may refer them to other providers. This, said Mr. Powers, can prolong symptoms and further distress patients.
Mr. Powers noted childhood physical or sexual abuse may affect eating patterns in patients with PTSD. “The evidence suggests these types of trauma exposure can be risk factors for the development of an eating disorder.”
Undiagnosed eating pathology may exacerbate functional impairment from PTSD and weaken the impact of evidence-based treatment.
Such patients are challenging to treat as they may not have the requisite skills to fully engage in exposure therapy, an evidence-based approach to treat PTSD, said Mr. Powers.
To determine whether PTSD would be significantly linked to greater eating disorder impairment (EDI) compared with other anxiety-related diagnoses and whether this would impair treatment, investigators studied 748 patients with an anxiety disorder who were attending a cognitive behavioral therapy (CBT) clinic. Anxiety disorders included PTSD, obsessive-compulsive disorder (OCD), social anxiety, and panic disorder.
Participants completed the 16-item Clinical Impairment Assessment (CIA) questionnaire, which includes questions about eating habits and feelings about food, body shape, and weight over the previous 4 weeks. Participants also reported anxiety symptom severity at the beginning, during, and end of treatment.
Need for better screening
Results showed that compared with those who had other anxiety disorders, patients with PTSD were three times more likely to have disordered eating (odds ratio [OR], 3.06; 95% confidence interval [CI], 1.47-6.37; P = .003).
In addition, higher baseline CIA scores predicted poorer PTSD treatment outcome (beta = –1.4; 95% CI, –1.67 to –1.10; P < .01).
“Having higher baseline CIA scores meant that patients’ PTSD symptoms did not remit as strongly compared to those with lower scores,” said Mr. Powers.
Patients with both PTSD and an eating disorder may have difficulty with regulating emotions and tolerating distress, he said.
“They may use binge eating, purging, or food restriction as strategies to regulate emotions. These behaviors may allow patients to become numb to or avoid heightened emotions that come from having PTSD and an eating disorder.”
Prior research linked perfectionism tendencies to poorer response to PTSD treatment. Those with an eating disorder may share similar tendencies, said Mr. Powers.
“If someone is consistently thinking negatively about their eating or body to the point where it interrupts their functioning, they may not be as likely to fully engage with PTSD treatment,” he said.
Ideally, clinicians would screen all patients with PTSD for an eating disorder, said Mr. Powers. “If screening instruments aren’t feasible or available, even just inquiring about body image or history of maladaptive eating behaviors can be helpful.”
He added this could open up a conversation about a traumatic event in the patient’s past.
Confirmatory research
Commenting on the study, Karen S. Mitchell, PhD, clinical research psychologist, National Center for PTSD, VA Boston Healthcare System, and associate professor in psychiatry, Boston University, said she was “excited” to see this research.
“Very few studies have examined the impact of baseline eating disorder symptoms on PTSD treatment outcomes or vice versa,” she said.
The study findings “add to the small but growing body of evidence suggesting that comorbid PTSD and eating disorder symptoms can impact recovery from each disorder,” she said.
She noted the importance of assessing comorbidity in patients presenting for treatment and of addressing comorbidity in both eating disorders and PTSD treatment. “But we need more research on how best to do this.”
Mr. Powers and Dr. Mitchell have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ADAA 2023
Telehealth suicide prevention program safe, acceptable
WASHINGTON –
Skeptics had worried that participating in the program through telehealth would exacerbate safety and other issues veterans had about discussing suicide in a group setting, study investigator Sarah Sullivan, PhD student, Health Psychology & Clinical Science, City University of New York, told this news organization.
“But that for us was not really true. People opened up about their suicidal thoughts and triggers even on this telehealth format, and that’s really important for providers to know,” she said.
The findings were presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
Trial run
Suicide is a major public health issue, particularly for veterans. Recent data from the Veterans Administration show 17 veterans die by suicide every day.
The current study included 15 male and 2 female veterans (29.4% White, 70.6% Hispanic) from New York City and Philadelphia. Participants had an average age of 50 and all were either deemed by a clinician to be at extremely high risk for suicide or were hospitalized for this reason.
The individuals completed an online version of the Project Life Force (PLF) program, which uses dialectical behavioral therapy and psychoeducational approaches. The program includes the brief Safety Planning intervention (SPI), aimed at reducing short-term suicide risk.
Considered a best practice, the SPI includes a written list of personal suicide warning signs or triggers, internal coping strategies, social contacts who offer support and distraction from suicidal thoughts, contact information for professionals, a suicide crisis hotline, and nearby emergency services.
In addition to these steps, the PLF program focuses on sleep, exercise, and making the safety plan accessible.
The telehealth platform for the program was WebEx software. Participants were offered a “trial run” to orient them to the technology, said Ms. Sullivan.
Group sessions were held once weekly for 10 weeks, with optional “booster” sessions if needed. Each session included about five participants.
To ensure privacy, participants were provided with headphones and laptops. This was especially important for those sharing a living space, including spouses and children, said Ms. Sullivan.
High ratings
Participants completed the Acceptability of Intervention Measure (AIM), Intervention Appropriateness Measure (IAM), and Feasibility of Intervention Measure (FIM). Each of these yields scores from four items rated on a Likert scale of 1-5, for a total score ranging from 5 to 20, with higher scores indicating higher ratings.
Veterans rated PLF-T as highly acceptable (mean AIM, 17.50), appropriate (mean IAM, 17.25), and feasible (mean FIM, 18).
Study participants reported the program was convenient and noted that it decreased the burden of traveling to sessions, especially during the COVID-19 pandemic.
They also reported the program was less likely to compete with other demands such as childcare and other appointments, said Ms. Sullivan.
In addition, it helped those with comorbidities such as posttraumatic stress disorder, she added. She noted veterans with PTSD may be triggered on subways or buses when traveling to in-person treatment sessions.
“That can take away from addressing the suicidal triggers,” said Ms. Sullivan. “So, this program allows them to fully concentrate on the safety plan.”
Results showed that study participants “enjoyed the group and would recommend it to others,” said Ms. Sullivan. “I think that signifies the group was effective in its goal of mitigating loneliness, which was exacerbated during the COVID-19 pandemic, and creating a socially supportive environment, especially for the vets living alone.”
Veterans also reported that the program helped them understand the connection between depression or PTSD and suicidal thoughts, urges, and plans. In addition, they appreciated the group dynamics, where they felt connected to other veterans experiencing similar challenges.
Hopeful results
Commenting on the study, Paul E. Holtzheimer, MD, deputy director for research at the National Center for PTSD, praised the study for focusing on a very high-risk group.
“This gets you closer to the population you’re probably going to have an impact on in terms of preventing suicide,” said Dr. Holtzheimer, a professor of psychiatry and surgery at Dartmouth College’s Geisel School of Medicine, Hanover, N.H.
The fact that many of the participants had attempted suicide in the last year underlines that this was a very high-risk population, said Dr. Holtzheimer. “Not only are they thinking about suicide, but almost two-thirds had actually attempted or tried something.”
This kind of program “would be great for rural environments where people may be living like four hours away from the VA or a clinic,” said Dr. Holtzheimer, noting that many veterans are often quite isolated.
“One of the very positive outcomes of the COVID-19 pandemic was helping us strengthen our ability to do telehealth,” he said.
However, Dr. Holtzheimer noted the study was small and qualitative. “The next step ideally would be a controlled trial looking at not just ideation but at risky behavior or clear suicide attempts or preparation, like buying a gun or hoarding medication, to help determine efficacy.”
The researchers and Dr. Holtzheimer report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WASHINGTON –
Skeptics had worried that participating in the program through telehealth would exacerbate safety and other issues veterans had about discussing suicide in a group setting, study investigator Sarah Sullivan, PhD student, Health Psychology & Clinical Science, City University of New York, told this news organization.
“But that for us was not really true. People opened up about their suicidal thoughts and triggers even on this telehealth format, and that’s really important for providers to know,” she said.
The findings were presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
Trial run
Suicide is a major public health issue, particularly for veterans. Recent data from the Veterans Administration show 17 veterans die by suicide every day.
The current study included 15 male and 2 female veterans (29.4% White, 70.6% Hispanic) from New York City and Philadelphia. Participants had an average age of 50 and all were either deemed by a clinician to be at extremely high risk for suicide or were hospitalized for this reason.
The individuals completed an online version of the Project Life Force (PLF) program, which uses dialectical behavioral therapy and psychoeducational approaches. The program includes the brief Safety Planning intervention (SPI), aimed at reducing short-term suicide risk.
Considered a best practice, the SPI includes a written list of personal suicide warning signs or triggers, internal coping strategies, social contacts who offer support and distraction from suicidal thoughts, contact information for professionals, a suicide crisis hotline, and nearby emergency services.
In addition to these steps, the PLF program focuses on sleep, exercise, and making the safety plan accessible.
The telehealth platform for the program was WebEx software. Participants were offered a “trial run” to orient them to the technology, said Ms. Sullivan.
Group sessions were held once weekly for 10 weeks, with optional “booster” sessions if needed. Each session included about five participants.
To ensure privacy, participants were provided with headphones and laptops. This was especially important for those sharing a living space, including spouses and children, said Ms. Sullivan.
High ratings
Participants completed the Acceptability of Intervention Measure (AIM), Intervention Appropriateness Measure (IAM), and Feasibility of Intervention Measure (FIM). Each of these yields scores from four items rated on a Likert scale of 1-5, for a total score ranging from 5 to 20, with higher scores indicating higher ratings.
Veterans rated PLF-T as highly acceptable (mean AIM, 17.50), appropriate (mean IAM, 17.25), and feasible (mean FIM, 18).
Study participants reported the program was convenient and noted that it decreased the burden of traveling to sessions, especially during the COVID-19 pandemic.
They also reported the program was less likely to compete with other demands such as childcare and other appointments, said Ms. Sullivan.
In addition, it helped those with comorbidities such as posttraumatic stress disorder, she added. She noted veterans with PTSD may be triggered on subways or buses when traveling to in-person treatment sessions.
“That can take away from addressing the suicidal triggers,” said Ms. Sullivan. “So, this program allows them to fully concentrate on the safety plan.”
Results showed that study participants “enjoyed the group and would recommend it to others,” said Ms. Sullivan. “I think that signifies the group was effective in its goal of mitigating loneliness, which was exacerbated during the COVID-19 pandemic, and creating a socially supportive environment, especially for the vets living alone.”
Veterans also reported that the program helped them understand the connection between depression or PTSD and suicidal thoughts, urges, and plans. In addition, they appreciated the group dynamics, where they felt connected to other veterans experiencing similar challenges.
Hopeful results
Commenting on the study, Paul E. Holtzheimer, MD, deputy director for research at the National Center for PTSD, praised the study for focusing on a very high-risk group.
“This gets you closer to the population you’re probably going to have an impact on in terms of preventing suicide,” said Dr. Holtzheimer, a professor of psychiatry and surgery at Dartmouth College’s Geisel School of Medicine, Hanover, N.H.
The fact that many of the participants had attempted suicide in the last year underlines that this was a very high-risk population, said Dr. Holtzheimer. “Not only are they thinking about suicide, but almost two-thirds had actually attempted or tried something.”
This kind of program “would be great for rural environments where people may be living like four hours away from the VA or a clinic,” said Dr. Holtzheimer, noting that many veterans are often quite isolated.
“One of the very positive outcomes of the COVID-19 pandemic was helping us strengthen our ability to do telehealth,” he said.
However, Dr. Holtzheimer noted the study was small and qualitative. “The next step ideally would be a controlled trial looking at not just ideation but at risky behavior or clear suicide attempts or preparation, like buying a gun or hoarding medication, to help determine efficacy.”
The researchers and Dr. Holtzheimer report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WASHINGTON –
Skeptics had worried that participating in the program through telehealth would exacerbate safety and other issues veterans had about discussing suicide in a group setting, study investigator Sarah Sullivan, PhD student, Health Psychology & Clinical Science, City University of New York, told this news organization.
“But that for us was not really true. People opened up about their suicidal thoughts and triggers even on this telehealth format, and that’s really important for providers to know,” she said.
The findings were presented as part of the Anxiety and Depression Association of America Anxiety & Depression conference.
Trial run
Suicide is a major public health issue, particularly for veterans. Recent data from the Veterans Administration show 17 veterans die by suicide every day.
The current study included 15 male and 2 female veterans (29.4% White, 70.6% Hispanic) from New York City and Philadelphia. Participants had an average age of 50 and all were either deemed by a clinician to be at extremely high risk for suicide or were hospitalized for this reason.
The individuals completed an online version of the Project Life Force (PLF) program, which uses dialectical behavioral therapy and psychoeducational approaches. The program includes the brief Safety Planning intervention (SPI), aimed at reducing short-term suicide risk.
Considered a best practice, the SPI includes a written list of personal suicide warning signs or triggers, internal coping strategies, social contacts who offer support and distraction from suicidal thoughts, contact information for professionals, a suicide crisis hotline, and nearby emergency services.
In addition to these steps, the PLF program focuses on sleep, exercise, and making the safety plan accessible.
The telehealth platform for the program was WebEx software. Participants were offered a “trial run” to orient them to the technology, said Ms. Sullivan.
Group sessions were held once weekly for 10 weeks, with optional “booster” sessions if needed. Each session included about five participants.
To ensure privacy, participants were provided with headphones and laptops. This was especially important for those sharing a living space, including spouses and children, said Ms. Sullivan.
High ratings
Participants completed the Acceptability of Intervention Measure (AIM), Intervention Appropriateness Measure (IAM), and Feasibility of Intervention Measure (FIM). Each of these yields scores from four items rated on a Likert scale of 1-5, for a total score ranging from 5 to 20, with higher scores indicating higher ratings.
Veterans rated PLF-T as highly acceptable (mean AIM, 17.50), appropriate (mean IAM, 17.25), and feasible (mean FIM, 18).
Study participants reported the program was convenient and noted that it decreased the burden of traveling to sessions, especially during the COVID-19 pandemic.
They also reported the program was less likely to compete with other demands such as childcare and other appointments, said Ms. Sullivan.
In addition, it helped those with comorbidities such as posttraumatic stress disorder, she added. She noted veterans with PTSD may be triggered on subways or buses when traveling to in-person treatment sessions.
“That can take away from addressing the suicidal triggers,” said Ms. Sullivan. “So, this program allows them to fully concentrate on the safety plan.”
Results showed that study participants “enjoyed the group and would recommend it to others,” said Ms. Sullivan. “I think that signifies the group was effective in its goal of mitigating loneliness, which was exacerbated during the COVID-19 pandemic, and creating a socially supportive environment, especially for the vets living alone.”
Veterans also reported that the program helped them understand the connection between depression or PTSD and suicidal thoughts, urges, and plans. In addition, they appreciated the group dynamics, where they felt connected to other veterans experiencing similar challenges.
Hopeful results
Commenting on the study, Paul E. Holtzheimer, MD, deputy director for research at the National Center for PTSD, praised the study for focusing on a very high-risk group.
“This gets you closer to the population you’re probably going to have an impact on in terms of preventing suicide,” said Dr. Holtzheimer, a professor of psychiatry and surgery at Dartmouth College’s Geisel School of Medicine, Hanover, N.H.
The fact that many of the participants had attempted suicide in the last year underlines that this was a very high-risk population, said Dr. Holtzheimer. “Not only are they thinking about suicide, but almost two-thirds had actually attempted or tried something.”
This kind of program “would be great for rural environments where people may be living like four hours away from the VA or a clinic,” said Dr. Holtzheimer, noting that many veterans are often quite isolated.
“One of the very positive outcomes of the COVID-19 pandemic was helping us strengthen our ability to do telehealth,” he said.
However, Dr. Holtzheimer noted the study was small and qualitative. “The next step ideally would be a controlled trial looking at not just ideation but at risky behavior or clear suicide attempts or preparation, like buying a gun or hoarding medication, to help determine efficacy.”
The researchers and Dr. Holtzheimer report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ADAA 2023
Anger in adults a red flag for childhood trauma
PARIS –
Investigators examined data on more than 2,250 individuals who were asked about trauma during childhood and a subsequent tendency toward anger or angry outbursts 4 years later.
Results showed that emotional neglect during childhood was associated with approximately a 40% increased likelihood of subsequent anger, while psychological abuse was linked to a 30% increased likelihood.
Childhood physical abuse was also significantly associated with anger in adults, with an increased risk of approximately 40%. The researchers found no link between childhood sexual abuse and adult anger.
“We can’t definitively say that the trauma causes the anger, but the link is clear,” study investigator Nienke De Bles, PhD student, department of psychiatry, Leiden (the Netherlands) University Medical Center, said in a news release.
“Being easily angered can have several consequences,” she continued. “It can make personal interactions more difficult, and it can have consequences for your mental health and well-being, but people who get angry easily also have a greater tendency to discontinue psychiatric treatment, so this anger may mean that it reduces their chances of a better life,” she added.
Ms. De Bles believes that “it should be standard practice to ask depression and anxiety sufferers about anger and past trauma, even if the patient is not exhibiting current anger.”
The findings were presented at the European Psychiatric Association 2023 Congress.
A ‘red flag’ for abuse
“Psychiatric treatments for past trauma may differ from treatments for depression, so psychiatrists need to try to understand the cause so that they can offer the correct treatment to each patient,” said Ms. De Bles.
Ms. De Bles noted that childhood trauma has many negative consequences later in life and that it is associated with a higher prevalence of adult depression and anxiety.
“There are several potential mechanisms for psychopathology in the context of childhood trauma, and emotion regulation seems to be one of the key mechanisms,” she said.
The researchers previously found that anger was highly prevalent among patients with affective disorders. It was present in 30% of those with current anxiety or depressive disorder and in 40% of those with comorbid depression and anxiety with a tendency toward anger versus 5% of healthy control persons.
Other studies have shown that anger is associated with poor treatment outcomes and dropping out of treatment.
To further investigate the link between childhood trauma and anger in adulthood, the researchers examined data on 2,271 participants in the Netherlands Study of Depression and Anxiety (NESDA).
Childhood trauma was assessed at baseline using the semistructured Childhood Trauma Interview. Anger was measured at a 4-year follow-up using the Spielberger Trait Anger Subscale, the Anger Attacks Questionnaire, and the borderline and antisocial subscales of the Personality Disorder Questionnaire 4 to identify cluster B personality traits.
Results showed that emotional neglect during childhood was significantly associated with trait anger in adulthood, at an adjusted odds ratio of 1.42 (P < .001), anger attacks (OR, 1.35; P = .004), and borderline (OR, 1.76; P < .001) and antisocial (OR, 1.88; P = .001) personality traits.
Childhood psychological abuse was also significantly associated with later trait anger (OR, 1.28; P = .002), anger attacks (OR, 1.31; P = .024), and borderline (OR, 1.77; P < .001) and antisocial (OR, 1.69; P = .011) traits.
There was also a significant association between childhood psychical abuse and trait anger in adulthood (OR, 1.37; P < .001), anger attacks (OR, 1.48; P = .004), and borderline (OR, 1.71; P < .001) and antisocial (OR, 1.98; P = .002) traits.
There was no significant association between sexual abuse experienced in childhood and later anger or personality traits.
Ms. De Bles said the findings suggest “there is indeed a relationship between childhood trauma and anger in adulthood, and this is something that might be interesting for clinicians, as anger could be a red flag for a history of childhood trauma.”
She said in an interview that anger is a “very normal human emotion” but that it has not been as widely studied as sadness and anxiety.
She suggested that future research could examine the use of trauma-based therapies for patients with a history of childhood trauma and anger.
Overlooked, neglected
Commenting on the findings, Nur Hani Zainal, PhD, department of healthcare policy, Harvard Medical School, Boston, said the findings are “very consistent with the current biopsychosocial models in psychiatry and clinical psychology.”
Dr. Zainal, who was coauthor of a recent study that showed that anger appears to mediate the relationship between childhood trauma and adult psychopathology, said the current study offers a “good, incremental contribution” to the literature.
She noted there are “good uses” for the emotion of anger, as “sometimes we need anger to set healthy boundaries for ourselves.” However, she agreed that, as an aspect of depression, anxiety, and posttraumatic stress disorder, it is often “overlooked.”
Dr. Zainal said that the findings reinforce the importance of thoroughly evaluating adult patients’ experiences during childhood.
Julian Beezhold, MD, secretary general of the EPA and a consultant psychiatrist with the Norwich (England) Medical School, University of East Anglia, commented in the release that anger is a “somewhat neglected symptom.
“The findings are in line with what we see in day-to-day clinical practice and will hopefully help increase the awareness of the importance of both anger and associated childhood trauma.”
The infrastructure for the NESDA study is funded through the Geestkracht program of the Netherlands Organization for Health Research and Development and financial contributions by participating universities and mental health care organizations. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PARIS –
Investigators examined data on more than 2,250 individuals who were asked about trauma during childhood and a subsequent tendency toward anger or angry outbursts 4 years later.
Results showed that emotional neglect during childhood was associated with approximately a 40% increased likelihood of subsequent anger, while psychological abuse was linked to a 30% increased likelihood.
Childhood physical abuse was also significantly associated with anger in adults, with an increased risk of approximately 40%. The researchers found no link between childhood sexual abuse and adult anger.
“We can’t definitively say that the trauma causes the anger, but the link is clear,” study investigator Nienke De Bles, PhD student, department of psychiatry, Leiden (the Netherlands) University Medical Center, said in a news release.
“Being easily angered can have several consequences,” she continued. “It can make personal interactions more difficult, and it can have consequences for your mental health and well-being, but people who get angry easily also have a greater tendency to discontinue psychiatric treatment, so this anger may mean that it reduces their chances of a better life,” she added.
Ms. De Bles believes that “it should be standard practice to ask depression and anxiety sufferers about anger and past trauma, even if the patient is not exhibiting current anger.”
The findings were presented at the European Psychiatric Association 2023 Congress.
A ‘red flag’ for abuse
“Psychiatric treatments for past trauma may differ from treatments for depression, so psychiatrists need to try to understand the cause so that they can offer the correct treatment to each patient,” said Ms. De Bles.
Ms. De Bles noted that childhood trauma has many negative consequences later in life and that it is associated with a higher prevalence of adult depression and anxiety.
“There are several potential mechanisms for psychopathology in the context of childhood trauma, and emotion regulation seems to be one of the key mechanisms,” she said.
The researchers previously found that anger was highly prevalent among patients with affective disorders. It was present in 30% of those with current anxiety or depressive disorder and in 40% of those with comorbid depression and anxiety with a tendency toward anger versus 5% of healthy control persons.
Other studies have shown that anger is associated with poor treatment outcomes and dropping out of treatment.
To further investigate the link between childhood trauma and anger in adulthood, the researchers examined data on 2,271 participants in the Netherlands Study of Depression and Anxiety (NESDA).
Childhood trauma was assessed at baseline using the semistructured Childhood Trauma Interview. Anger was measured at a 4-year follow-up using the Spielberger Trait Anger Subscale, the Anger Attacks Questionnaire, and the borderline and antisocial subscales of the Personality Disorder Questionnaire 4 to identify cluster B personality traits.
Results showed that emotional neglect during childhood was significantly associated with trait anger in adulthood, at an adjusted odds ratio of 1.42 (P < .001), anger attacks (OR, 1.35; P = .004), and borderline (OR, 1.76; P < .001) and antisocial (OR, 1.88; P = .001) personality traits.
Childhood psychological abuse was also significantly associated with later trait anger (OR, 1.28; P = .002), anger attacks (OR, 1.31; P = .024), and borderline (OR, 1.77; P < .001) and antisocial (OR, 1.69; P = .011) traits.
There was also a significant association between childhood psychical abuse and trait anger in adulthood (OR, 1.37; P < .001), anger attacks (OR, 1.48; P = .004), and borderline (OR, 1.71; P < .001) and antisocial (OR, 1.98; P = .002) traits.
There was no significant association between sexual abuse experienced in childhood and later anger or personality traits.
Ms. De Bles said the findings suggest “there is indeed a relationship between childhood trauma and anger in adulthood, and this is something that might be interesting for clinicians, as anger could be a red flag for a history of childhood trauma.”
She said in an interview that anger is a “very normal human emotion” but that it has not been as widely studied as sadness and anxiety.
She suggested that future research could examine the use of trauma-based therapies for patients with a history of childhood trauma and anger.
Overlooked, neglected
Commenting on the findings, Nur Hani Zainal, PhD, department of healthcare policy, Harvard Medical School, Boston, said the findings are “very consistent with the current biopsychosocial models in psychiatry and clinical psychology.”
Dr. Zainal, who was coauthor of a recent study that showed that anger appears to mediate the relationship between childhood trauma and adult psychopathology, said the current study offers a “good, incremental contribution” to the literature.
She noted there are “good uses” for the emotion of anger, as “sometimes we need anger to set healthy boundaries for ourselves.” However, she agreed that, as an aspect of depression, anxiety, and posttraumatic stress disorder, it is often “overlooked.”
Dr. Zainal said that the findings reinforce the importance of thoroughly evaluating adult patients’ experiences during childhood.
Julian Beezhold, MD, secretary general of the EPA and a consultant psychiatrist with the Norwich (England) Medical School, University of East Anglia, commented in the release that anger is a “somewhat neglected symptom.
“The findings are in line with what we see in day-to-day clinical practice and will hopefully help increase the awareness of the importance of both anger and associated childhood trauma.”
The infrastructure for the NESDA study is funded through the Geestkracht program of the Netherlands Organization for Health Research and Development and financial contributions by participating universities and mental health care organizations. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PARIS –
Investigators examined data on more than 2,250 individuals who were asked about trauma during childhood and a subsequent tendency toward anger or angry outbursts 4 years later.
Results showed that emotional neglect during childhood was associated with approximately a 40% increased likelihood of subsequent anger, while psychological abuse was linked to a 30% increased likelihood.
Childhood physical abuse was also significantly associated with anger in adults, with an increased risk of approximately 40%. The researchers found no link between childhood sexual abuse and adult anger.
“We can’t definitively say that the trauma causes the anger, but the link is clear,” study investigator Nienke De Bles, PhD student, department of psychiatry, Leiden (the Netherlands) University Medical Center, said in a news release.
“Being easily angered can have several consequences,” she continued. “It can make personal interactions more difficult, and it can have consequences for your mental health and well-being, but people who get angry easily also have a greater tendency to discontinue psychiatric treatment, so this anger may mean that it reduces their chances of a better life,” she added.
Ms. De Bles believes that “it should be standard practice to ask depression and anxiety sufferers about anger and past trauma, even if the patient is not exhibiting current anger.”
The findings were presented at the European Psychiatric Association 2023 Congress.
A ‘red flag’ for abuse
“Psychiatric treatments for past trauma may differ from treatments for depression, so psychiatrists need to try to understand the cause so that they can offer the correct treatment to each patient,” said Ms. De Bles.
Ms. De Bles noted that childhood trauma has many negative consequences later in life and that it is associated with a higher prevalence of adult depression and anxiety.
“There are several potential mechanisms for psychopathology in the context of childhood trauma, and emotion regulation seems to be one of the key mechanisms,” she said.
The researchers previously found that anger was highly prevalent among patients with affective disorders. It was present in 30% of those with current anxiety or depressive disorder and in 40% of those with comorbid depression and anxiety with a tendency toward anger versus 5% of healthy control persons.
Other studies have shown that anger is associated with poor treatment outcomes and dropping out of treatment.
To further investigate the link between childhood trauma and anger in adulthood, the researchers examined data on 2,271 participants in the Netherlands Study of Depression and Anxiety (NESDA).
Childhood trauma was assessed at baseline using the semistructured Childhood Trauma Interview. Anger was measured at a 4-year follow-up using the Spielberger Trait Anger Subscale, the Anger Attacks Questionnaire, and the borderline and antisocial subscales of the Personality Disorder Questionnaire 4 to identify cluster B personality traits.
Results showed that emotional neglect during childhood was significantly associated with trait anger in adulthood, at an adjusted odds ratio of 1.42 (P < .001), anger attacks (OR, 1.35; P = .004), and borderline (OR, 1.76; P < .001) and antisocial (OR, 1.88; P = .001) personality traits.
Childhood psychological abuse was also significantly associated with later trait anger (OR, 1.28; P = .002), anger attacks (OR, 1.31; P = .024), and borderline (OR, 1.77; P < .001) and antisocial (OR, 1.69; P = .011) traits.
There was also a significant association between childhood psychical abuse and trait anger in adulthood (OR, 1.37; P < .001), anger attacks (OR, 1.48; P = .004), and borderline (OR, 1.71; P < .001) and antisocial (OR, 1.98; P = .002) traits.
There was no significant association between sexual abuse experienced in childhood and later anger or personality traits.
Ms. De Bles said the findings suggest “there is indeed a relationship between childhood trauma and anger in adulthood, and this is something that might be interesting for clinicians, as anger could be a red flag for a history of childhood trauma.”
She said in an interview that anger is a “very normal human emotion” but that it has not been as widely studied as sadness and anxiety.
She suggested that future research could examine the use of trauma-based therapies for patients with a history of childhood trauma and anger.
Overlooked, neglected
Commenting on the findings, Nur Hani Zainal, PhD, department of healthcare policy, Harvard Medical School, Boston, said the findings are “very consistent with the current biopsychosocial models in psychiatry and clinical psychology.”
Dr. Zainal, who was coauthor of a recent study that showed that anger appears to mediate the relationship between childhood trauma and adult psychopathology, said the current study offers a “good, incremental contribution” to the literature.
She noted there are “good uses” for the emotion of anger, as “sometimes we need anger to set healthy boundaries for ourselves.” However, she agreed that, as an aspect of depression, anxiety, and posttraumatic stress disorder, it is often “overlooked.”
Dr. Zainal said that the findings reinforce the importance of thoroughly evaluating adult patients’ experiences during childhood.
Julian Beezhold, MD, secretary general of the EPA and a consultant psychiatrist with the Norwich (England) Medical School, University of East Anglia, commented in the release that anger is a “somewhat neglected symptom.
“The findings are in line with what we see in day-to-day clinical practice and will hopefully help increase the awareness of the importance of both anger and associated childhood trauma.”
The infrastructure for the NESDA study is funded through the Geestkracht program of the Netherlands Organization for Health Research and Development and financial contributions by participating universities and mental health care organizations. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EPA 2023
Family violence after COVID: Understanding coercive relationships
Despite the ability of some couples to pull together and manage through the COVID-19 pandemic, other couples and families failed to thrive. Increasing divorce rates have been noted nationwide with many disagreements being specifically about COVID.1
A review of over 1 million tweets, between April 12 and July 16, 2020, found an increase in calls to hotlines and increased reports of a variety of types of family violence. There were also more inquiries about social services for family violence, an increased presence from social movements, and more domestic violence-related news.2
The literature addressing family violence uses a variety of terms, so here are some definitions.
Domestic violence is defined as a pattern of behaviors used to gain or maintain power and control. Broadly speaking, domestic violence includes elder abuse, sibling abuse, child abuse, intimate partner abuse, parent abuse, and can also include people who don’t necessarily live together but who have an intimate relationship. Domestic violence centers use the Power and Control Wheel (see graphic) developed by the Domestic Abuse Intervention Project in Duluth, Minn., to describe how domestic violence occurs.
Intimate partner violence is more specific, referring to violence that happens between people in an ongoing or former intimate or romantic relationship, and is a subcategory of domestic violence.
Coercive control is the use of power for control and compliance. It is a dynamic and systematic process described in the top left corner of the Power and Control Wheel. Overt control occurs with the implication that “if you don’t follow the rules, I’ll kill you.” More subtle control is when obedience is forced through monopolizing resources, dictating preferred choices, microregulating a partner’s behavior, and deprivation of supports needed to exercise independent judgment.
All interpersonal relationships have elements of persuasion and influence; however, the goal of coercive relationships is to maintain power and control. It is a dynamic of the relationship. Coercive control emphasizes the systematic, organized, multifaceted, and patterned nature of this interpersonal dynamic and can be considered to originate in the patriarchal dynamic where men control women.
Most professionals who work in this interdisciplinary area now refer to domestic violence as coercive control. Victimizers target women whom they sense they can control to get their own needs met. They are disinclined to invest in relationships with women who stress their own points of view, who do not readily accept blame when there is a disagreement, and who offer nurturing only when it is reciprocated.
In my office, if I think there are elements of coercion in a relationship, I bring out the Power and Control Wheel and the patient and I go over it. Good education is our responsibility. However, we all have met women who decide to stay in unhealthy relationships.
Assessing people who stay in coercive relationships
Fear
The most important first step is to assess safety. Are they afraid of increased violence if they challenge their partner? Restraining orders or other legal deterrents may not offer solace, as many women are clear that their spouse will come after them, if not tomorrow, then next week, or even next month. They are sure that they will not be safe.
In these cases, I go over safety steps with them so that if they decide to go, they will be prepared. I bring out the “safety box,” which includes the following action steps:
- Memorize important phone numbers of people to call in an emergency.
- If your children are old enough, teach them important phone numbers, including when to dial 911.
- If you can, open your own bank account.
- Stay in touch with friends. Get to know your neighbors. Don’t cut yourself off from people, even if you feel like you want to be alone.
- Rehearse your escape plan until you know it by heart.
- Leave a set of car keys, extra money, a change of clothes and copies of important documents with a trusted friend or relative: your own and your children’s birth certificates, children’s school and medical records, bank books, welfare identification, passport/green card, immigration papers, social security card, lease agreements or mortgage payment books, insurance papers, important addresses, and telephone numbers.
- Keep information about domestic violence in a safe place, where your abuser won’t find it, but where you can get it when you need to review it.
Some women may acknowledge that the risk of physical violence is not the determining factor in their decision to stay and have difficulty explaining why they choose to stay. I suggest that we then consider the following frames that have their origin in the study of the impact of trauma.
Shame
From this lens, abusive events are humiliating experiences, now represented as shame experiences. Humiliation and shame hide hostile feelings that the patient is not able to acknowledge.
“In shame, the self is the failure and others may reject or be critical of this exposed, flawed self.”3 Women will therefore remain attached to an abuser to avoid the exposure of their defective self.
Action steps: Empathic engagement and acknowledgment of shame and humiliation are key. For someone to overcome shame, they must face their sense of their defective self and have strategies to manage these feelings. The development of such strategies is the next step.
Trauma repetition and trauma bonding
Women subjected to domestic violence often respond with incapacitating traumatic syndromes. The concept of “trauma repetition” is suggested as a cause of vulnerability to repeated abuse, and “trauma bonding” is the term for the intense and tenacious bond that can form between abusers and victims.4
Trauma bonding implies that a sense of safety and closeness and secure attachment can only be reached through highly abusive engagement; anything else is experienced as “superficial, cold, or irrelevant.”5 Trauma bonding may have its origins in emotional neglect, according to self reports of 116 women.6Action steps: The literature on trauma is growing and many patients will benefit from good curated sources. Having a good list of books and website on hand is important. Discussion and exploration of the impact of trauma will be needed, and can be provided by someone who is available on a consistent and frequent basis. This work may be time consuming and difficult.
Some asides
1. Some psychiatrists proffer the explanation that these women who stay must be masochistic. The misogynistic concept of masochism still haunts the halls of psychiatry. It is usually offered as a way to dismiss these women’s concerns.
2. One of the obstacles to recognizing chronic mistreatment in relationships is that most abusive men simply “do not seem like abusers.” They have many good qualities, including times of kindness, warmth, and humor, especially in the initial period of a relationship. An abuser’s friends may think the world of him. He may have a successful work life and have no problems with drugs or alcohol. He may simply not fit anyone’s image of a cruel or intimidating person. So, when a woman feels her relationship spinning out of control, it may not occur to her that her partner is an abuser. Even if she does consider her partner to be overly controlling, others may question her perception.
3. Neutrality in family courts is systemic sexism/misogyny. When it comes to domestic violence, family courts tend to split the difference. Stephanie Brandt, MD, notes that The assumption that it is violence alone that matters has formed the basis of much clinical and legal confusion.7 As an analyst, she has gone against the grain of a favored neutrality and become active in the courts, noting the secondary victimization that occurs when a woman enters the legal system.
In summary, psychiatrists must reclaim our expertise in systemic dynamics and point out the role of systemic misogyny. Justices and other court officials need to be educated. Ideally, justice should be based on the equality of men and women in a society free of systemic misogyny. Unfortunately our society has not yet reached this position. In the meanwhile, we must think systemically about interpersonal dynamics. This is our lane. This should not be controversial.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at alisonheru@gmail.com. Dr. Heru would like to thank Dr. Stephanie Brandt for discussing this topic with her and supporting this work.
References
1. Ellyatt H. Arguing with your partner over Covid? You’re not alone, with the pandemic straining many relationships. 2022 Jan 21. https://www.cnbc.com/2022/01/21/covid-has-put-pressures-and-strains-on-relationships.html
2. Xue J et al. J Med Internet Res. 2020 Nov 6;22(11):e24361. doi: 10.2196/24361.
3. Dorahy MJ. J Trauma Dissociation. 2017 May-Jun;18(3):383-96. doi: 10.1080/15299732.2017.1295422.
4. Dutton DG and Painter SL. Victimology. 1981 Jan;6(1):139-55.
5. Sachs A. J Trauma Dissociation. 2017 May-Jun;18(3):319-39. doi: 10.1080/15299732.2017.1295400.
6. Krüger C and Fletcher L. J Trauma Dissociation. 2017 May-Jun;18(3):356-72. doi: 10.1080/15299732.2017.1295420.
7. Brandt S and Rudden M. Int J Appl Psychoanal Studies. 2020 Sept;17(3):215-31. doi: 10.1002/aps.1671.
Despite the ability of some couples to pull together and manage through the COVID-19 pandemic, other couples and families failed to thrive. Increasing divorce rates have been noted nationwide with many disagreements being specifically about COVID.1
A review of over 1 million tweets, between April 12 and July 16, 2020, found an increase in calls to hotlines and increased reports of a variety of types of family violence. There were also more inquiries about social services for family violence, an increased presence from social movements, and more domestic violence-related news.2
The literature addressing family violence uses a variety of terms, so here are some definitions.
Domestic violence is defined as a pattern of behaviors used to gain or maintain power and control. Broadly speaking, domestic violence includes elder abuse, sibling abuse, child abuse, intimate partner abuse, parent abuse, and can also include people who don’t necessarily live together but who have an intimate relationship. Domestic violence centers use the Power and Control Wheel (see graphic) developed by the Domestic Abuse Intervention Project in Duluth, Minn., to describe how domestic violence occurs.
Intimate partner violence is more specific, referring to violence that happens between people in an ongoing or former intimate or romantic relationship, and is a subcategory of domestic violence.
Coercive control is the use of power for control and compliance. It is a dynamic and systematic process described in the top left corner of the Power and Control Wheel. Overt control occurs with the implication that “if you don’t follow the rules, I’ll kill you.” More subtle control is when obedience is forced through monopolizing resources, dictating preferred choices, microregulating a partner’s behavior, and deprivation of supports needed to exercise independent judgment.
All interpersonal relationships have elements of persuasion and influence; however, the goal of coercive relationships is to maintain power and control. It is a dynamic of the relationship. Coercive control emphasizes the systematic, organized, multifaceted, and patterned nature of this interpersonal dynamic and can be considered to originate in the patriarchal dynamic where men control women.
Most professionals who work in this interdisciplinary area now refer to domestic violence as coercive control. Victimizers target women whom they sense they can control to get their own needs met. They are disinclined to invest in relationships with women who stress their own points of view, who do not readily accept blame when there is a disagreement, and who offer nurturing only when it is reciprocated.
In my office, if I think there are elements of coercion in a relationship, I bring out the Power and Control Wheel and the patient and I go over it. Good education is our responsibility. However, we all have met women who decide to stay in unhealthy relationships.
Assessing people who stay in coercive relationships
Fear
The most important first step is to assess safety. Are they afraid of increased violence if they challenge their partner? Restraining orders or other legal deterrents may not offer solace, as many women are clear that their spouse will come after them, if not tomorrow, then next week, or even next month. They are sure that they will not be safe.
In these cases, I go over safety steps with them so that if they decide to go, they will be prepared. I bring out the “safety box,” which includes the following action steps:
- Memorize important phone numbers of people to call in an emergency.
- If your children are old enough, teach them important phone numbers, including when to dial 911.
- If you can, open your own bank account.
- Stay in touch with friends. Get to know your neighbors. Don’t cut yourself off from people, even if you feel like you want to be alone.
- Rehearse your escape plan until you know it by heart.
- Leave a set of car keys, extra money, a change of clothes and copies of important documents with a trusted friend or relative: your own and your children’s birth certificates, children’s school and medical records, bank books, welfare identification, passport/green card, immigration papers, social security card, lease agreements or mortgage payment books, insurance papers, important addresses, and telephone numbers.
- Keep information about domestic violence in a safe place, where your abuser won’t find it, but where you can get it when you need to review it.
Some women may acknowledge that the risk of physical violence is not the determining factor in their decision to stay and have difficulty explaining why they choose to stay. I suggest that we then consider the following frames that have their origin in the study of the impact of trauma.
Shame
From this lens, abusive events are humiliating experiences, now represented as shame experiences. Humiliation and shame hide hostile feelings that the patient is not able to acknowledge.
“In shame, the self is the failure and others may reject or be critical of this exposed, flawed self.”3 Women will therefore remain attached to an abuser to avoid the exposure of their defective self.
Action steps: Empathic engagement and acknowledgment of shame and humiliation are key. For someone to overcome shame, they must face their sense of their defective self and have strategies to manage these feelings. The development of such strategies is the next step.
Trauma repetition and trauma bonding
Women subjected to domestic violence often respond with incapacitating traumatic syndromes. The concept of “trauma repetition” is suggested as a cause of vulnerability to repeated abuse, and “trauma bonding” is the term for the intense and tenacious bond that can form between abusers and victims.4
Trauma bonding implies that a sense of safety and closeness and secure attachment can only be reached through highly abusive engagement; anything else is experienced as “superficial, cold, or irrelevant.”5 Trauma bonding may have its origins in emotional neglect, according to self reports of 116 women.6Action steps: The literature on trauma is growing and many patients will benefit from good curated sources. Having a good list of books and website on hand is important. Discussion and exploration of the impact of trauma will be needed, and can be provided by someone who is available on a consistent and frequent basis. This work may be time consuming and difficult.
Some asides
1. Some psychiatrists proffer the explanation that these women who stay must be masochistic. The misogynistic concept of masochism still haunts the halls of psychiatry. It is usually offered as a way to dismiss these women’s concerns.
2. One of the obstacles to recognizing chronic mistreatment in relationships is that most abusive men simply “do not seem like abusers.” They have many good qualities, including times of kindness, warmth, and humor, especially in the initial period of a relationship. An abuser’s friends may think the world of him. He may have a successful work life and have no problems with drugs or alcohol. He may simply not fit anyone’s image of a cruel or intimidating person. So, when a woman feels her relationship spinning out of control, it may not occur to her that her partner is an abuser. Even if she does consider her partner to be overly controlling, others may question her perception.
3. Neutrality in family courts is systemic sexism/misogyny. When it comes to domestic violence, family courts tend to split the difference. Stephanie Brandt, MD, notes that The assumption that it is violence alone that matters has formed the basis of much clinical and legal confusion.7 As an analyst, she has gone against the grain of a favored neutrality and become active in the courts, noting the secondary victimization that occurs when a woman enters the legal system.
In summary, psychiatrists must reclaim our expertise in systemic dynamics and point out the role of systemic misogyny. Justices and other court officials need to be educated. Ideally, justice should be based on the equality of men and women in a society free of systemic misogyny. Unfortunately our society has not yet reached this position. In the meanwhile, we must think systemically about interpersonal dynamics. This is our lane. This should not be controversial.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at alisonheru@gmail.com. Dr. Heru would like to thank Dr. Stephanie Brandt for discussing this topic with her and supporting this work.
References
1. Ellyatt H. Arguing with your partner over Covid? You’re not alone, with the pandemic straining many relationships. 2022 Jan 21. https://www.cnbc.com/2022/01/21/covid-has-put-pressures-and-strains-on-relationships.html
2. Xue J et al. J Med Internet Res. 2020 Nov 6;22(11):e24361. doi: 10.2196/24361.
3. Dorahy MJ. J Trauma Dissociation. 2017 May-Jun;18(3):383-96. doi: 10.1080/15299732.2017.1295422.
4. Dutton DG and Painter SL. Victimology. 1981 Jan;6(1):139-55.
5. Sachs A. J Trauma Dissociation. 2017 May-Jun;18(3):319-39. doi: 10.1080/15299732.2017.1295400.
6. Krüger C and Fletcher L. J Trauma Dissociation. 2017 May-Jun;18(3):356-72. doi: 10.1080/15299732.2017.1295420.
7. Brandt S and Rudden M. Int J Appl Psychoanal Studies. 2020 Sept;17(3):215-31. doi: 10.1002/aps.1671.
Despite the ability of some couples to pull together and manage through the COVID-19 pandemic, other couples and families failed to thrive. Increasing divorce rates have been noted nationwide with many disagreements being specifically about COVID.1
A review of over 1 million tweets, between April 12 and July 16, 2020, found an increase in calls to hotlines and increased reports of a variety of types of family violence. There were also more inquiries about social services for family violence, an increased presence from social movements, and more domestic violence-related news.2
The literature addressing family violence uses a variety of terms, so here are some definitions.
Domestic violence is defined as a pattern of behaviors used to gain or maintain power and control. Broadly speaking, domestic violence includes elder abuse, sibling abuse, child abuse, intimate partner abuse, parent abuse, and can also include people who don’t necessarily live together but who have an intimate relationship. Domestic violence centers use the Power and Control Wheel (see graphic) developed by the Domestic Abuse Intervention Project in Duluth, Minn., to describe how domestic violence occurs.
Intimate partner violence is more specific, referring to violence that happens between people in an ongoing or former intimate or romantic relationship, and is a subcategory of domestic violence.
Coercive control is the use of power for control and compliance. It is a dynamic and systematic process described in the top left corner of the Power and Control Wheel. Overt control occurs with the implication that “if you don’t follow the rules, I’ll kill you.” More subtle control is when obedience is forced through monopolizing resources, dictating preferred choices, microregulating a partner’s behavior, and deprivation of supports needed to exercise independent judgment.
All interpersonal relationships have elements of persuasion and influence; however, the goal of coercive relationships is to maintain power and control. It is a dynamic of the relationship. Coercive control emphasizes the systematic, organized, multifaceted, and patterned nature of this interpersonal dynamic and can be considered to originate in the patriarchal dynamic where men control women.
Most professionals who work in this interdisciplinary area now refer to domestic violence as coercive control. Victimizers target women whom they sense they can control to get their own needs met. They are disinclined to invest in relationships with women who stress their own points of view, who do not readily accept blame when there is a disagreement, and who offer nurturing only when it is reciprocated.
In my office, if I think there are elements of coercion in a relationship, I bring out the Power and Control Wheel and the patient and I go over it. Good education is our responsibility. However, we all have met women who decide to stay in unhealthy relationships.
Assessing people who stay in coercive relationships
Fear
The most important first step is to assess safety. Are they afraid of increased violence if they challenge their partner? Restraining orders or other legal deterrents may not offer solace, as many women are clear that their spouse will come after them, if not tomorrow, then next week, or even next month. They are sure that they will not be safe.
In these cases, I go over safety steps with them so that if they decide to go, they will be prepared. I bring out the “safety box,” which includes the following action steps:
- Memorize important phone numbers of people to call in an emergency.
- If your children are old enough, teach them important phone numbers, including when to dial 911.
- If you can, open your own bank account.
- Stay in touch with friends. Get to know your neighbors. Don’t cut yourself off from people, even if you feel like you want to be alone.
- Rehearse your escape plan until you know it by heart.
- Leave a set of car keys, extra money, a change of clothes and copies of important documents with a trusted friend or relative: your own and your children’s birth certificates, children’s school and medical records, bank books, welfare identification, passport/green card, immigration papers, social security card, lease agreements or mortgage payment books, insurance papers, important addresses, and telephone numbers.
- Keep information about domestic violence in a safe place, where your abuser won’t find it, but where you can get it when you need to review it.
Some women may acknowledge that the risk of physical violence is not the determining factor in their decision to stay and have difficulty explaining why they choose to stay. I suggest that we then consider the following frames that have their origin in the study of the impact of trauma.
Shame
From this lens, abusive events are humiliating experiences, now represented as shame experiences. Humiliation and shame hide hostile feelings that the patient is not able to acknowledge.
“In shame, the self is the failure and others may reject or be critical of this exposed, flawed self.”3 Women will therefore remain attached to an abuser to avoid the exposure of their defective self.
Action steps: Empathic engagement and acknowledgment of shame and humiliation are key. For someone to overcome shame, they must face their sense of their defective self and have strategies to manage these feelings. The development of such strategies is the next step.
Trauma repetition and trauma bonding
Women subjected to domestic violence often respond with incapacitating traumatic syndromes. The concept of “trauma repetition” is suggested as a cause of vulnerability to repeated abuse, and “trauma bonding” is the term for the intense and tenacious bond that can form between abusers and victims.4
Trauma bonding implies that a sense of safety and closeness and secure attachment can only be reached through highly abusive engagement; anything else is experienced as “superficial, cold, or irrelevant.”5 Trauma bonding may have its origins in emotional neglect, according to self reports of 116 women.6Action steps: The literature on trauma is growing and many patients will benefit from good curated sources. Having a good list of books and website on hand is important. Discussion and exploration of the impact of trauma will be needed, and can be provided by someone who is available on a consistent and frequent basis. This work may be time consuming and difficult.
Some asides
1. Some psychiatrists proffer the explanation that these women who stay must be masochistic. The misogynistic concept of masochism still haunts the halls of psychiatry. It is usually offered as a way to dismiss these women’s concerns.
2. One of the obstacles to recognizing chronic mistreatment in relationships is that most abusive men simply “do not seem like abusers.” They have many good qualities, including times of kindness, warmth, and humor, especially in the initial period of a relationship. An abuser’s friends may think the world of him. He may have a successful work life and have no problems with drugs or alcohol. He may simply not fit anyone’s image of a cruel or intimidating person. So, when a woman feels her relationship spinning out of control, it may not occur to her that her partner is an abuser. Even if she does consider her partner to be overly controlling, others may question her perception.
3. Neutrality in family courts is systemic sexism/misogyny. When it comes to domestic violence, family courts tend to split the difference. Stephanie Brandt, MD, notes that The assumption that it is violence alone that matters has formed the basis of much clinical and legal confusion.7 As an analyst, she has gone against the grain of a favored neutrality and become active in the courts, noting the secondary victimization that occurs when a woman enters the legal system.
In summary, psychiatrists must reclaim our expertise in systemic dynamics and point out the role of systemic misogyny. Justices and other court officials need to be educated. Ideally, justice should be based on the equality of men and women in a society free of systemic misogyny. Unfortunately our society has not yet reached this position. In the meanwhile, we must think systemically about interpersonal dynamics. This is our lane. This should not be controversial.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at alisonheru@gmail.com. Dr. Heru would like to thank Dr. Stephanie Brandt for discussing this topic with her and supporting this work.
References
1. Ellyatt H. Arguing with your partner over Covid? You’re not alone, with the pandemic straining many relationships. 2022 Jan 21. https://www.cnbc.com/2022/01/21/covid-has-put-pressures-and-strains-on-relationships.html
2. Xue J et al. J Med Internet Res. 2020 Nov 6;22(11):e24361. doi: 10.2196/24361.
3. Dorahy MJ. J Trauma Dissociation. 2017 May-Jun;18(3):383-96. doi: 10.1080/15299732.2017.1295422.
4. Dutton DG and Painter SL. Victimology. 1981 Jan;6(1):139-55.
5. Sachs A. J Trauma Dissociation. 2017 May-Jun;18(3):319-39. doi: 10.1080/15299732.2017.1295400.
6. Krüger C and Fletcher L. J Trauma Dissociation. 2017 May-Jun;18(3):356-72. doi: 10.1080/15299732.2017.1295420.
7. Brandt S and Rudden M. Int J Appl Psychoanal Studies. 2020 Sept;17(3):215-31. doi: 10.1002/aps.1671.
Four PTSD blood biomarkers identified
“More accurate means of predicting or screening for PTSD could help to overcome the disorder by identifying individuals at high risk of developing PTSD and providing them with early intervention or prevention strategies,” said study investigator Stacy-Ann Miller, MS.
She also noted that the biomarkers could be used to monitor treatment for PTSD, identify subtypes of PTSD, and lead to a new understanding of the mechanisms underlying PTSD.
The findings were presented at Discover BMB, the annual meeting of the American Society for Biochemistry and Molecular Biology.
Toward better clinical assessment
The findings originated from research conducted by the Department of Defense–initiated PTSD Systems Biology Consortium. The consortium’s goals include developing a reproducible panel of blood-based biomarkers with high sensitivity and specificity for PTSD diagnosis and is made up of about 45 researchers, led by Marti Jett, PhD, Charles Marmar, MD, and Francis J. Doyle III, PhD.
The researchers analyzed blood samples from 1,000 active-duty Army personnel from the 101st Airborne at Fort Campbell, Ky. Participants were assessed before and after deployment to Afghanistan in February 2014 and are referred to as the Fort Campbell Cohort (FCC). Participants’ age ranged from 25 to 30 and approximately 6% were female.
Investigators collected blood samples from the service members and looked for four biomarkers: glycolytic ratio, arginine, serotonin, and glutamate. The team then divided the participants into four groups – those with PTSD (PTSD Checklist score above 30), those who were subthreshold for PTSD (PTSD Checklist score 15-30), those who had high resilience, and those who had low levels of resilience.
The resilience groups were determined based on answers to the Generalized Anxiety Disorder Questionnaire, Patient Health Questionnaire, Pittsburgh Sleep Quality Index, Intensive Combat Exposure (DRRI-D), the number of deployments, whether they had moderate or severe traumatic brain injury, and scores on the Alcohol Use Disorders Identification Test.
Those who scored in the high range at current or prior time points or who were PTSD/subthreshold at prior time points were placed in the low resilience group.
Ms. Miller noted that those in the PTSD group had more severe symptoms than those in the PTSD subthreshold group based on the longitudinal clinical assessment at 3-6 months, 5 years, and longer post deployment. The low resilience group had a much higher rate of PTSD post deployment than the high resilience group.
Investigators found participants with PTSD or subthreshold PTSD had significantly higher glycolic ratios and lower arginine than those with high resilience. They also found that those with PTSD had significantly lower serotonin and higher glutamate levels versus those with high resilience. These associations were independent of factors such as sex, age, body mass index, smoking, and caffeine consumption.
Ms. Miller said that the study results require further validation by the consortium’s labs and third-party labs.
“We are also interested in determining the most appropriate time to screen soldiers for PTSD, as it has been noted that the time period where we see the most psychological issues is around 2-3 months post return from deployment and when the soldier is preparing for their next assignment, perhaps a next deployment,” she said.
She added that previous studies have identified several promising biomarkers of PTSD. “However, like much of the research data, the study sample was comprised mainly of combat-exposed males. With more women serving on the front lines, the military faces new challenges in how combat affects females in the military,” including sex-specific biomarkers that will improve clinical assessment for female soldiers.
Eventually, the team would also like to be able to apply their research to the civilian population experiencing PTSD.
“Our research is anticipated to be useful in helping the medical provider select appropriate therapeutic interventions,” Ms. Miller said.
A version of this article first appeared on Medscape.com.
“More accurate means of predicting or screening for PTSD could help to overcome the disorder by identifying individuals at high risk of developing PTSD and providing them with early intervention or prevention strategies,” said study investigator Stacy-Ann Miller, MS.
She also noted that the biomarkers could be used to monitor treatment for PTSD, identify subtypes of PTSD, and lead to a new understanding of the mechanisms underlying PTSD.
The findings were presented at Discover BMB, the annual meeting of the American Society for Biochemistry and Molecular Biology.
Toward better clinical assessment
The findings originated from research conducted by the Department of Defense–initiated PTSD Systems Biology Consortium. The consortium’s goals include developing a reproducible panel of blood-based biomarkers with high sensitivity and specificity for PTSD diagnosis and is made up of about 45 researchers, led by Marti Jett, PhD, Charles Marmar, MD, and Francis J. Doyle III, PhD.
The researchers analyzed blood samples from 1,000 active-duty Army personnel from the 101st Airborne at Fort Campbell, Ky. Participants were assessed before and after deployment to Afghanistan in February 2014 and are referred to as the Fort Campbell Cohort (FCC). Participants’ age ranged from 25 to 30 and approximately 6% were female.
Investigators collected blood samples from the service members and looked for four biomarkers: glycolytic ratio, arginine, serotonin, and glutamate. The team then divided the participants into four groups – those with PTSD (PTSD Checklist score above 30), those who were subthreshold for PTSD (PTSD Checklist score 15-30), those who had high resilience, and those who had low levels of resilience.
The resilience groups were determined based on answers to the Generalized Anxiety Disorder Questionnaire, Patient Health Questionnaire, Pittsburgh Sleep Quality Index, Intensive Combat Exposure (DRRI-D), the number of deployments, whether they had moderate or severe traumatic brain injury, and scores on the Alcohol Use Disorders Identification Test.
Those who scored in the high range at current or prior time points or who were PTSD/subthreshold at prior time points were placed in the low resilience group.
Ms. Miller noted that those in the PTSD group had more severe symptoms than those in the PTSD subthreshold group based on the longitudinal clinical assessment at 3-6 months, 5 years, and longer post deployment. The low resilience group had a much higher rate of PTSD post deployment than the high resilience group.
Investigators found participants with PTSD or subthreshold PTSD had significantly higher glycolic ratios and lower arginine than those with high resilience. They also found that those with PTSD had significantly lower serotonin and higher glutamate levels versus those with high resilience. These associations were independent of factors such as sex, age, body mass index, smoking, and caffeine consumption.
Ms. Miller said that the study results require further validation by the consortium’s labs and third-party labs.
“We are also interested in determining the most appropriate time to screen soldiers for PTSD, as it has been noted that the time period where we see the most psychological issues is around 2-3 months post return from deployment and when the soldier is preparing for their next assignment, perhaps a next deployment,” she said.
She added that previous studies have identified several promising biomarkers of PTSD. “However, like much of the research data, the study sample was comprised mainly of combat-exposed males. With more women serving on the front lines, the military faces new challenges in how combat affects females in the military,” including sex-specific biomarkers that will improve clinical assessment for female soldiers.
Eventually, the team would also like to be able to apply their research to the civilian population experiencing PTSD.
“Our research is anticipated to be useful in helping the medical provider select appropriate therapeutic interventions,” Ms. Miller said.
A version of this article first appeared on Medscape.com.
“More accurate means of predicting or screening for PTSD could help to overcome the disorder by identifying individuals at high risk of developing PTSD and providing them with early intervention or prevention strategies,” said study investigator Stacy-Ann Miller, MS.
She also noted that the biomarkers could be used to monitor treatment for PTSD, identify subtypes of PTSD, and lead to a new understanding of the mechanisms underlying PTSD.
The findings were presented at Discover BMB, the annual meeting of the American Society for Biochemistry and Molecular Biology.
Toward better clinical assessment
The findings originated from research conducted by the Department of Defense–initiated PTSD Systems Biology Consortium. The consortium’s goals include developing a reproducible panel of blood-based biomarkers with high sensitivity and specificity for PTSD diagnosis and is made up of about 45 researchers, led by Marti Jett, PhD, Charles Marmar, MD, and Francis J. Doyle III, PhD.
The researchers analyzed blood samples from 1,000 active-duty Army personnel from the 101st Airborne at Fort Campbell, Ky. Participants were assessed before and after deployment to Afghanistan in February 2014 and are referred to as the Fort Campbell Cohort (FCC). Participants’ age ranged from 25 to 30 and approximately 6% were female.
Investigators collected blood samples from the service members and looked for four biomarkers: glycolytic ratio, arginine, serotonin, and glutamate. The team then divided the participants into four groups – those with PTSD (PTSD Checklist score above 30), those who were subthreshold for PTSD (PTSD Checklist score 15-30), those who had high resilience, and those who had low levels of resilience.
The resilience groups were determined based on answers to the Generalized Anxiety Disorder Questionnaire, Patient Health Questionnaire, Pittsburgh Sleep Quality Index, Intensive Combat Exposure (DRRI-D), the number of deployments, whether they had moderate or severe traumatic brain injury, and scores on the Alcohol Use Disorders Identification Test.
Those who scored in the high range at current or prior time points or who were PTSD/subthreshold at prior time points were placed in the low resilience group.
Ms. Miller noted that those in the PTSD group had more severe symptoms than those in the PTSD subthreshold group based on the longitudinal clinical assessment at 3-6 months, 5 years, and longer post deployment. The low resilience group had a much higher rate of PTSD post deployment than the high resilience group.
Investigators found participants with PTSD or subthreshold PTSD had significantly higher glycolic ratios and lower arginine than those with high resilience. They also found that those with PTSD had significantly lower serotonin and higher glutamate levels versus those with high resilience. These associations were independent of factors such as sex, age, body mass index, smoking, and caffeine consumption.
Ms. Miller said that the study results require further validation by the consortium’s labs and third-party labs.
“We are also interested in determining the most appropriate time to screen soldiers for PTSD, as it has been noted that the time period where we see the most psychological issues is around 2-3 months post return from deployment and when the soldier is preparing for their next assignment, perhaps a next deployment,” she said.
She added that previous studies have identified several promising biomarkers of PTSD. “However, like much of the research data, the study sample was comprised mainly of combat-exposed males. With more women serving on the front lines, the military faces new challenges in how combat affects females in the military,” including sex-specific biomarkers that will improve clinical assessment for female soldiers.
Eventually, the team would also like to be able to apply their research to the civilian population experiencing PTSD.
“Our research is anticipated to be useful in helping the medical provider select appropriate therapeutic interventions,” Ms. Miller said.
A version of this article first appeared on Medscape.com.
FROM DISCOVER BMB
Impact of child abuse differs by gender
PARIS – , new research shows.
Investigators found childhood emotional and sexual abuse had a greater effect on women than men, whereas men were more adversely affected by emotional and physical neglect.
“Our findings indicate that exposure to childhood maltreatment increases the risk of having psychiatric symptoms in both men and women,” lead researcher Thanavadee Prachason, PhD, department of psychiatry and neuropsychology, Maastricht (the Netherlands) University Medical Center, said in a press release.
“Exposure to emotionally or sexually abusive experiences during childhood increases the risk of a variety of psychiatric symptoms, particularly in women. In contrast, a history of emotional or physical neglect in childhood increases the risk of having psychiatric symptoms more in men,” Dr. Prachason added.
The findings were presented at the European Psychiatric Association 2023 Congress.
A leading mental illness risk factor
Study presenter Laura Fusar-Poli, MD, PhD, from the department of brain and behavioral sciences, University of Pavia (Italy), said that the differential impact of trauma subtypes in men and women indicate that both gender and the type of childhood adversity experienced need to be taken into account in future studies.
Dr. Fusar-Poli began by highlighting that 13%-36% of individuals have experienced some kind of childhood trauma, with 30% exposed to at least two types of trauma.
Trauma has been identified as a risk factor for a range of mental health problems.
“It is estimated that, worldwide, around one third of all psychiatric disorders are related to childhood trauma,” senior researcher Sinan Gül
Consequently, “childhood trauma is a leading preventable risk factor for mental illness,” he added.
Previous research suggests the subtype of trauma has an impact on subsequent biological changes and clinical outcomes, and that there are gender differences in the effects of childhood trauma.
To investigate, the researchers examined data from TwinssCan, a Belgian cohort of twins and siblings aged 15-35 years without a diagnosis of pervasive mental disorders.
The study included 477 females and 314 males who had completed the Childhood Trauma Questionnaire–Short Form (CTQ) and the Symptom Checklist-90 SR (SCL-90) to determine exposure to childhood adversity and levels of psychopathology, respectively.
Results showed that total CTQ scores were significantly associated with total SCL-90 scores in both men and women, as well as with each of the nine symptom domains of the SCL-90 (P < .001 for all assessments). These included psychoticism, paranoid ideation, anxiety, depression, somatization, obsessive-compulsive, interpersonal sensitivity, hostility, and phobic anxiety.
There were no significant differences in the associations with total CTQ scores between men and women.
However, when the researchers examined trauma subtypes and psychopathology, clear gender differences emerged.
Investigators found a significant association between emotional abuse on the CTQ and total SCL-90 scores in both men (P < .023) and women (P < .001), but that the association was significantly stronger in women (P = .043).
Sexual abuse was significantly associated with total SCL-90 scores in women (P < .001), while emotional neglect and physical neglect were significantly associated with psychopathology scores in men (P = .026 and P < .001, respectively).
“Physical neglect may include experiences of not having enough to eat, wearing dirty clothes, not being taken care of, and not getting taken to the doctor when the person was growing up,” said Dr. Prachason.
“Emotional neglect may include childhood experiences like not feeling loved or important, and not feeling close to the family.”
In women, emotional abuse was significantly associated with all nine symptom domains of the SCL-90, while sexual abuse was associated with seven: psychoticism, paranoid ideation, anxiety, depression, somatization, obsessive-compulsive, and hostility.
Physical neglect, in men, was significantly associated with eight of the symptom domains (all but somatization), but emotional neglect was linked only to depression, Dr. Fusar-Poli reported.
“This study showed a very important consequence of childhood trauma, and not only in people with mental disorders. I would like to underline that this is a general population, composed of adolescents and young adults, which is the age in which the majority of mental disorders starts, Dr. Fusar-Poli said in an interview.
She emphasized that psychotic disorders are only a part of the “broad range” of conditions that may be related to childhood trauma, which “can have an impact on sub-threshold symptoms that can affect functioning and quality of life in the general population.”
Addressing the differential findings in men and women, Dr. Gül
However, he said, this is “something that we really need understand,” as there is likely an underlying mechanism, “and not only a biological mechanism but probably a societal one.”
Dr. Gül
Compromised cognitive, emotional function
Commenting on the findings for this news organization, Elaine F. Walker, PhD, professor of psychology and neuroscience at Emory University in Atlanta, said stress exposure in general, including childhood trauma, “has transdiagnostic effects on vulnerability to mental disorders.”
“The effects are primarily mediated by the hypothalamic-pituitary-adrenal axis, which triggers the release of cortisol. When persistently elevated, this can result in neurobiological processes that have adverse effects on brain structure and circuitry which, in turn, compromises cognitive and emotional functioning,” said Dr. Walker, who was not associated with the study.
She noted that, “while it is possible that there are sex differences in biological sensitivity to certain subtypes of childhood trauma, it may also be the case that sex differences in the likelihood of exposure to trauma subtypes is actually the key factor.”
“At the present time, there are not specific treatment protocols aimed at addressing childhood trauma subtypes, but most experienced therapists will incorporate information about the individual’s trauma history in their treatment,” Dr. Walker added.
Also commenting on the research, Philip Gorwood, MD, PhD, head of the Clinique des Maladies Mentales et de l’Encéphale at Centre Hospitalier Sainte Anne in Paris, said the results are “important … as childhood trauma has been clearly recognized as a major risk factor for the vast majority of psychiatric disorders, but with poor knowledge of gender specificities.”
“Understanding which aspects of trauma are more damaging according to gender will facilitate research on the resilience process. Many intervention strategies will indeed benefit from a more personalized approach,” he said in a statement. Dr. Gorwood was not involved with this study.
The study authors, Dr. Gorwood, and Dr. Walker report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PARIS – , new research shows.
Investigators found childhood emotional and sexual abuse had a greater effect on women than men, whereas men were more adversely affected by emotional and physical neglect.
“Our findings indicate that exposure to childhood maltreatment increases the risk of having psychiatric symptoms in both men and women,” lead researcher Thanavadee Prachason, PhD, department of psychiatry and neuropsychology, Maastricht (the Netherlands) University Medical Center, said in a press release.
“Exposure to emotionally or sexually abusive experiences during childhood increases the risk of a variety of psychiatric symptoms, particularly in women. In contrast, a history of emotional or physical neglect in childhood increases the risk of having psychiatric symptoms more in men,” Dr. Prachason added.
The findings were presented at the European Psychiatric Association 2023 Congress.
A leading mental illness risk factor
Study presenter Laura Fusar-Poli, MD, PhD, from the department of brain and behavioral sciences, University of Pavia (Italy), said that the differential impact of trauma subtypes in men and women indicate that both gender and the type of childhood adversity experienced need to be taken into account in future studies.
Dr. Fusar-Poli began by highlighting that 13%-36% of individuals have experienced some kind of childhood trauma, with 30% exposed to at least two types of trauma.
Trauma has been identified as a risk factor for a range of mental health problems.
“It is estimated that, worldwide, around one third of all psychiatric disorders are related to childhood trauma,” senior researcher Sinan Gül
Consequently, “childhood trauma is a leading preventable risk factor for mental illness,” he added.
Previous research suggests the subtype of trauma has an impact on subsequent biological changes and clinical outcomes, and that there are gender differences in the effects of childhood trauma.
To investigate, the researchers examined data from TwinssCan, a Belgian cohort of twins and siblings aged 15-35 years without a diagnosis of pervasive mental disorders.
The study included 477 females and 314 males who had completed the Childhood Trauma Questionnaire–Short Form (CTQ) and the Symptom Checklist-90 SR (SCL-90) to determine exposure to childhood adversity and levels of psychopathology, respectively.
Results showed that total CTQ scores were significantly associated with total SCL-90 scores in both men and women, as well as with each of the nine symptom domains of the SCL-90 (P < .001 for all assessments). These included psychoticism, paranoid ideation, anxiety, depression, somatization, obsessive-compulsive, interpersonal sensitivity, hostility, and phobic anxiety.
There were no significant differences in the associations with total CTQ scores between men and women.
However, when the researchers examined trauma subtypes and psychopathology, clear gender differences emerged.
Investigators found a significant association between emotional abuse on the CTQ and total SCL-90 scores in both men (P < .023) and women (P < .001), but that the association was significantly stronger in women (P = .043).
Sexual abuse was significantly associated with total SCL-90 scores in women (P < .001), while emotional neglect and physical neglect were significantly associated with psychopathology scores in men (P = .026 and P < .001, respectively).
“Physical neglect may include experiences of not having enough to eat, wearing dirty clothes, not being taken care of, and not getting taken to the doctor when the person was growing up,” said Dr. Prachason.
“Emotional neglect may include childhood experiences like not feeling loved or important, and not feeling close to the family.”
In women, emotional abuse was significantly associated with all nine symptom domains of the SCL-90, while sexual abuse was associated with seven: psychoticism, paranoid ideation, anxiety, depression, somatization, obsessive-compulsive, and hostility.
Physical neglect, in men, was significantly associated with eight of the symptom domains (all but somatization), but emotional neglect was linked only to depression, Dr. Fusar-Poli reported.
“This study showed a very important consequence of childhood trauma, and not only in people with mental disorders. I would like to underline that this is a general population, composed of adolescents and young adults, which is the age in which the majority of mental disorders starts, Dr. Fusar-Poli said in an interview.
She emphasized that psychotic disorders are only a part of the “broad range” of conditions that may be related to childhood trauma, which “can have an impact on sub-threshold symptoms that can affect functioning and quality of life in the general population.”
Addressing the differential findings in men and women, Dr. Gül
However, he said, this is “something that we really need understand,” as there is likely an underlying mechanism, “and not only a biological mechanism but probably a societal one.”
Dr. Gül
Compromised cognitive, emotional function
Commenting on the findings for this news organization, Elaine F. Walker, PhD, professor of psychology and neuroscience at Emory University in Atlanta, said stress exposure in general, including childhood trauma, “has transdiagnostic effects on vulnerability to mental disorders.”
“The effects are primarily mediated by the hypothalamic-pituitary-adrenal axis, which triggers the release of cortisol. When persistently elevated, this can result in neurobiological processes that have adverse effects on brain structure and circuitry which, in turn, compromises cognitive and emotional functioning,” said Dr. Walker, who was not associated with the study.
She noted that, “while it is possible that there are sex differences in biological sensitivity to certain subtypes of childhood trauma, it may also be the case that sex differences in the likelihood of exposure to trauma subtypes is actually the key factor.”
“At the present time, there are not specific treatment protocols aimed at addressing childhood trauma subtypes, but most experienced therapists will incorporate information about the individual’s trauma history in their treatment,” Dr. Walker added.
Also commenting on the research, Philip Gorwood, MD, PhD, head of the Clinique des Maladies Mentales et de l’Encéphale at Centre Hospitalier Sainte Anne in Paris, said the results are “important … as childhood trauma has been clearly recognized as a major risk factor for the vast majority of psychiatric disorders, but with poor knowledge of gender specificities.”
“Understanding which aspects of trauma are more damaging according to gender will facilitate research on the resilience process. Many intervention strategies will indeed benefit from a more personalized approach,” he said in a statement. Dr. Gorwood was not involved with this study.
The study authors, Dr. Gorwood, and Dr. Walker report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PARIS – , new research shows.
Investigators found childhood emotional and sexual abuse had a greater effect on women than men, whereas men were more adversely affected by emotional and physical neglect.
“Our findings indicate that exposure to childhood maltreatment increases the risk of having psychiatric symptoms in both men and women,” lead researcher Thanavadee Prachason, PhD, department of psychiatry and neuropsychology, Maastricht (the Netherlands) University Medical Center, said in a press release.
“Exposure to emotionally or sexually abusive experiences during childhood increases the risk of a variety of psychiatric symptoms, particularly in women. In contrast, a history of emotional or physical neglect in childhood increases the risk of having psychiatric symptoms more in men,” Dr. Prachason added.
The findings were presented at the European Psychiatric Association 2023 Congress.
A leading mental illness risk factor
Study presenter Laura Fusar-Poli, MD, PhD, from the department of brain and behavioral sciences, University of Pavia (Italy), said that the differential impact of trauma subtypes in men and women indicate that both gender and the type of childhood adversity experienced need to be taken into account in future studies.
Dr. Fusar-Poli began by highlighting that 13%-36% of individuals have experienced some kind of childhood trauma, with 30% exposed to at least two types of trauma.
Trauma has been identified as a risk factor for a range of mental health problems.
“It is estimated that, worldwide, around one third of all psychiatric disorders are related to childhood trauma,” senior researcher Sinan Gül
Consequently, “childhood trauma is a leading preventable risk factor for mental illness,” he added.
Previous research suggests the subtype of trauma has an impact on subsequent biological changes and clinical outcomes, and that there are gender differences in the effects of childhood trauma.
To investigate, the researchers examined data from TwinssCan, a Belgian cohort of twins and siblings aged 15-35 years without a diagnosis of pervasive mental disorders.
The study included 477 females and 314 males who had completed the Childhood Trauma Questionnaire–Short Form (CTQ) and the Symptom Checklist-90 SR (SCL-90) to determine exposure to childhood adversity and levels of psychopathology, respectively.
Results showed that total CTQ scores were significantly associated with total SCL-90 scores in both men and women, as well as with each of the nine symptom domains of the SCL-90 (P < .001 for all assessments). These included psychoticism, paranoid ideation, anxiety, depression, somatization, obsessive-compulsive, interpersonal sensitivity, hostility, and phobic anxiety.
There were no significant differences in the associations with total CTQ scores between men and women.
However, when the researchers examined trauma subtypes and psychopathology, clear gender differences emerged.
Investigators found a significant association between emotional abuse on the CTQ and total SCL-90 scores in both men (P < .023) and women (P < .001), but that the association was significantly stronger in women (P = .043).
Sexual abuse was significantly associated with total SCL-90 scores in women (P < .001), while emotional neglect and physical neglect were significantly associated with psychopathology scores in men (P = .026 and P < .001, respectively).
“Physical neglect may include experiences of not having enough to eat, wearing dirty clothes, not being taken care of, and not getting taken to the doctor when the person was growing up,” said Dr. Prachason.
“Emotional neglect may include childhood experiences like not feeling loved or important, and not feeling close to the family.”
In women, emotional abuse was significantly associated with all nine symptom domains of the SCL-90, while sexual abuse was associated with seven: psychoticism, paranoid ideation, anxiety, depression, somatization, obsessive-compulsive, and hostility.
Physical neglect, in men, was significantly associated with eight of the symptom domains (all but somatization), but emotional neglect was linked only to depression, Dr. Fusar-Poli reported.
“This study showed a very important consequence of childhood trauma, and not only in people with mental disorders. I would like to underline that this is a general population, composed of adolescents and young adults, which is the age in which the majority of mental disorders starts, Dr. Fusar-Poli said in an interview.
She emphasized that psychotic disorders are only a part of the “broad range” of conditions that may be related to childhood trauma, which “can have an impact on sub-threshold symptoms that can affect functioning and quality of life in the general population.”
Addressing the differential findings in men and women, Dr. Gül
However, he said, this is “something that we really need understand,” as there is likely an underlying mechanism, “and not only a biological mechanism but probably a societal one.”
Dr. Gül
Compromised cognitive, emotional function
Commenting on the findings for this news organization, Elaine F. Walker, PhD, professor of psychology and neuroscience at Emory University in Atlanta, said stress exposure in general, including childhood trauma, “has transdiagnostic effects on vulnerability to mental disorders.”
“The effects are primarily mediated by the hypothalamic-pituitary-adrenal axis, which triggers the release of cortisol. When persistently elevated, this can result in neurobiological processes that have adverse effects on brain structure and circuitry which, in turn, compromises cognitive and emotional functioning,” said Dr. Walker, who was not associated with the study.
She noted that, “while it is possible that there are sex differences in biological sensitivity to certain subtypes of childhood trauma, it may also be the case that sex differences in the likelihood of exposure to trauma subtypes is actually the key factor.”
“At the present time, there are not specific treatment protocols aimed at addressing childhood trauma subtypes, but most experienced therapists will incorporate information about the individual’s trauma history in their treatment,” Dr. Walker added.
Also commenting on the research, Philip Gorwood, MD, PhD, head of the Clinique des Maladies Mentales et de l’Encéphale at Centre Hospitalier Sainte Anne in Paris, said the results are “important … as childhood trauma has been clearly recognized as a major risk factor for the vast majority of psychiatric disorders, but with poor knowledge of gender specificities.”
“Understanding which aspects of trauma are more damaging according to gender will facilitate research on the resilience process. Many intervention strategies will indeed benefit from a more personalized approach,” he said in a statement. Dr. Gorwood was not involved with this study.
The study authors, Dr. Gorwood, and Dr. Walker report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EPA 2023
A new way to gauge suicide risk?
Researchers found SDOH are risk factors for suicide among U.S. veterans and NLP can be leveraged to extract SDOH information from unstructured data in the EHR.
“Since SDOH is overwhelmingly described in EHR notes, the importance of NLP-extracted SDOH can be very significant, meaning that NLP can be used as an effective method for epidemiological and public health study,” senior investigator Hong Yu, PhD, from Miner School of Information and Computer Sciences, University of Massachusetts Lowell, told this news organization.
Although the study was conducted among U.S. veterans, the results likely hold for the general population as well.
“The NLP methods are generalizable. The SDOH categories are generalizable. There may be some variations in terms of the strength of associations in NLP-extracted SDOH and suicide death, but the overall findings are generalizable,” Dr. Yu said.
The study was published online JAMA Network Open.
Improved risk assessment
SDOH, which include factors such as socioeconomic status, access to healthy food, education, housing, and physical environment, are strong predictors of suicidal behaviors.
Several studies have identified a range of common risk factors for suicide using International Classification of Diseases (ICD) codes and other “structured” data from the EHR. However, the use of unstructured EHR data from clinician notes has received little attention in investigating potential associations between suicide and SDOH.
Using the large Veterans Health Administration EHR system, the researchers determined associations between veterans’ death by suicide and recent SDOH, identified using both structured data (ICD-10 codes and Veterans Health Administration stop codes) and unstructured data (NLP-processed clinical notes).
Participants included 8,821 veterans who committed suicide and 35,284 matched controls. The cohort was mostly male (96%) and White (79%). The mean age was 58 years.
The NLP-extracted SDOH were social isolation, job or financial insecurity, housing instability, legal problems, violence, barriers to care, transition of care, and food insecurity.
All of these unstructured clinical notes on SDOH were significantly associated with increased risk for death by suicide.
Legal problems had the largest estimated effect size, more than twice the risk of those with no exposure (adjusted odds ratio 2.62; 95% confidence interval, 2.38-2.89), followed by violence (aOR, 2.34; 95% CI, 2.17-2.52) and social isolation (aOR, 1.94; 95% CI, 1.83-2.06).
Similarly, all of the structured SDOH – social or family problems, employment or financial problems, housing instability, legal problems, violence, and nonspecific psychosocial needs – also showed significant associations with increased risk for suicide death, once again, with legal problems linked to the highest risk (aOR, 2.63; 95% CI, 2.37-2.91).
When combining the structured and NLP-extracted unstructured data, the top three risk factors for death by suicide were legal problems (aOR, 2.66; 95% CI 2.46-2.89), violence (aOR, 2.12; 95% CI, 1.98-2.27), and nonspecific psychosocial needs (aOR, 2.07; 95% CI, 1.92-2.23).
“To our knowledge, this the first large-scale study to implement and use an NLP system to extract SDOH information from unstructured EHR data,” the researchers write.
“We strongly believe that analyzing all available SDOH information, including those contained in clinical notes, can help develop a better system for risk assessment and suicide prevention. However, more studies are required to investigate ways of seamlessly incorporating SDOHs into existing health care systems,” they conclude.
Dr. Yu said it’s also important to note that their NLP system is built upon “the most advanced deep-learning technologies and therefore is more generalizable than most existing work that mainly used rule-based approaches or traditional machine learning for identifying social determinants of health.”
In an accompanying editorial, Ishanu Chattopadhyay, PhD, of the University of Chicago, said this suggests that unstructured clinical notes “may efficiently identify at-risk individuals even when structured data on the relevant variables are missing or incomplete.”
This work may provide “the foundation for addressing the key hurdles in enacting efficient universal assessment for suicide risk among the veterans and perhaps in the general population,” Dr. Chattopadhyay added.
This research was funded by a grant from the National Institute of Mental Health. The study authors and editorialist report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Researchers found SDOH are risk factors for suicide among U.S. veterans and NLP can be leveraged to extract SDOH information from unstructured data in the EHR.
“Since SDOH is overwhelmingly described in EHR notes, the importance of NLP-extracted SDOH can be very significant, meaning that NLP can be used as an effective method for epidemiological and public health study,” senior investigator Hong Yu, PhD, from Miner School of Information and Computer Sciences, University of Massachusetts Lowell, told this news organization.
Although the study was conducted among U.S. veterans, the results likely hold for the general population as well.
“The NLP methods are generalizable. The SDOH categories are generalizable. There may be some variations in terms of the strength of associations in NLP-extracted SDOH and suicide death, but the overall findings are generalizable,” Dr. Yu said.
The study was published online JAMA Network Open.
Improved risk assessment
SDOH, which include factors such as socioeconomic status, access to healthy food, education, housing, and physical environment, are strong predictors of suicidal behaviors.
Several studies have identified a range of common risk factors for suicide using International Classification of Diseases (ICD) codes and other “structured” data from the EHR. However, the use of unstructured EHR data from clinician notes has received little attention in investigating potential associations between suicide and SDOH.
Using the large Veterans Health Administration EHR system, the researchers determined associations between veterans’ death by suicide and recent SDOH, identified using both structured data (ICD-10 codes and Veterans Health Administration stop codes) and unstructured data (NLP-processed clinical notes).
Participants included 8,821 veterans who committed suicide and 35,284 matched controls. The cohort was mostly male (96%) and White (79%). The mean age was 58 years.
The NLP-extracted SDOH were social isolation, job or financial insecurity, housing instability, legal problems, violence, barriers to care, transition of care, and food insecurity.
All of these unstructured clinical notes on SDOH were significantly associated with increased risk for death by suicide.
Legal problems had the largest estimated effect size, more than twice the risk of those with no exposure (adjusted odds ratio 2.62; 95% confidence interval, 2.38-2.89), followed by violence (aOR, 2.34; 95% CI, 2.17-2.52) and social isolation (aOR, 1.94; 95% CI, 1.83-2.06).
Similarly, all of the structured SDOH – social or family problems, employment or financial problems, housing instability, legal problems, violence, and nonspecific psychosocial needs – also showed significant associations with increased risk for suicide death, once again, with legal problems linked to the highest risk (aOR, 2.63; 95% CI, 2.37-2.91).
When combining the structured and NLP-extracted unstructured data, the top three risk factors for death by suicide were legal problems (aOR, 2.66; 95% CI 2.46-2.89), violence (aOR, 2.12; 95% CI, 1.98-2.27), and nonspecific psychosocial needs (aOR, 2.07; 95% CI, 1.92-2.23).
“To our knowledge, this the first large-scale study to implement and use an NLP system to extract SDOH information from unstructured EHR data,” the researchers write.
“We strongly believe that analyzing all available SDOH information, including those contained in clinical notes, can help develop a better system for risk assessment and suicide prevention. However, more studies are required to investigate ways of seamlessly incorporating SDOHs into existing health care systems,” they conclude.
Dr. Yu said it’s also important to note that their NLP system is built upon “the most advanced deep-learning technologies and therefore is more generalizable than most existing work that mainly used rule-based approaches or traditional machine learning for identifying social determinants of health.”
In an accompanying editorial, Ishanu Chattopadhyay, PhD, of the University of Chicago, said this suggests that unstructured clinical notes “may efficiently identify at-risk individuals even when structured data on the relevant variables are missing or incomplete.”
This work may provide “the foundation for addressing the key hurdles in enacting efficient universal assessment for suicide risk among the veterans and perhaps in the general population,” Dr. Chattopadhyay added.
This research was funded by a grant from the National Institute of Mental Health. The study authors and editorialist report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Researchers found SDOH are risk factors for suicide among U.S. veterans and NLP can be leveraged to extract SDOH information from unstructured data in the EHR.
“Since SDOH is overwhelmingly described in EHR notes, the importance of NLP-extracted SDOH can be very significant, meaning that NLP can be used as an effective method for epidemiological and public health study,” senior investigator Hong Yu, PhD, from Miner School of Information and Computer Sciences, University of Massachusetts Lowell, told this news organization.
Although the study was conducted among U.S. veterans, the results likely hold for the general population as well.
“The NLP methods are generalizable. The SDOH categories are generalizable. There may be some variations in terms of the strength of associations in NLP-extracted SDOH and suicide death, but the overall findings are generalizable,” Dr. Yu said.
The study was published online JAMA Network Open.
Improved risk assessment
SDOH, which include factors such as socioeconomic status, access to healthy food, education, housing, and physical environment, are strong predictors of suicidal behaviors.
Several studies have identified a range of common risk factors for suicide using International Classification of Diseases (ICD) codes and other “structured” data from the EHR. However, the use of unstructured EHR data from clinician notes has received little attention in investigating potential associations between suicide and SDOH.
Using the large Veterans Health Administration EHR system, the researchers determined associations between veterans’ death by suicide and recent SDOH, identified using both structured data (ICD-10 codes and Veterans Health Administration stop codes) and unstructured data (NLP-processed clinical notes).
Participants included 8,821 veterans who committed suicide and 35,284 matched controls. The cohort was mostly male (96%) and White (79%). The mean age was 58 years.
The NLP-extracted SDOH were social isolation, job or financial insecurity, housing instability, legal problems, violence, barriers to care, transition of care, and food insecurity.
All of these unstructured clinical notes on SDOH were significantly associated with increased risk for death by suicide.
Legal problems had the largest estimated effect size, more than twice the risk of those with no exposure (adjusted odds ratio 2.62; 95% confidence interval, 2.38-2.89), followed by violence (aOR, 2.34; 95% CI, 2.17-2.52) and social isolation (aOR, 1.94; 95% CI, 1.83-2.06).
Similarly, all of the structured SDOH – social or family problems, employment or financial problems, housing instability, legal problems, violence, and nonspecific psychosocial needs – also showed significant associations with increased risk for suicide death, once again, with legal problems linked to the highest risk (aOR, 2.63; 95% CI, 2.37-2.91).
When combining the structured and NLP-extracted unstructured data, the top three risk factors for death by suicide were legal problems (aOR, 2.66; 95% CI 2.46-2.89), violence (aOR, 2.12; 95% CI, 1.98-2.27), and nonspecific psychosocial needs (aOR, 2.07; 95% CI, 1.92-2.23).
“To our knowledge, this the first large-scale study to implement and use an NLP system to extract SDOH information from unstructured EHR data,” the researchers write.
“We strongly believe that analyzing all available SDOH information, including those contained in clinical notes, can help develop a better system for risk assessment and suicide prevention. However, more studies are required to investigate ways of seamlessly incorporating SDOHs into existing health care systems,” they conclude.
Dr. Yu said it’s also important to note that their NLP system is built upon “the most advanced deep-learning technologies and therefore is more generalizable than most existing work that mainly used rule-based approaches or traditional machine learning for identifying social determinants of health.”
In an accompanying editorial, Ishanu Chattopadhyay, PhD, of the University of Chicago, said this suggests that unstructured clinical notes “may efficiently identify at-risk individuals even when structured data on the relevant variables are missing or incomplete.”
This work may provide “the foundation for addressing the key hurdles in enacting efficient universal assessment for suicide risk among the veterans and perhaps in the general population,” Dr. Chattopadhyay added.
This research was funded by a grant from the National Institute of Mental Health. The study authors and editorialist report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Cultivating strength: Psychological well-being after nonfatal suicide attempts
A study of three separate nationally representative samples of nearly 9,000 U.S. military veterans found psychological well-being – defined in terms of having a high sense of purpose, social connectedness, and happiness – to be significantly diminished among veteran suicide attempt survivors relative to nonattempters, even decades after their last attempt.1
Despite the trend toward diminished well-being, many veterans who survived a suicide attempt reported average to optimal levels of well-being. Specifically, 52%-60% of veterans reporting a prior suicide attempt also reported experiencing as much purpose, social connection, and happiness as veterans without a suicide attempt history. Remarkably, a small subset (2-7%) of veteran attempt survivors even reported higher levels of well-being than veterans without a suicide attempt history.
Thus,
These data are notable because, in 2021, approximately 1.4 million U.S. adults made a nonfatal suicide attempt. Historically, suicide research has understandably emphasized the study of risk factors that increase the likelihood that someone dies by suicide. Given that a prior suicide attempt is among the top risk factors for suicide, virtually all research on suicide attempt survivors has focused on their elevated risk for future suicidality. Yet, 9 out of 10 people who have made a nonfatal suicide attempt do not go on to die by suicide. It is thus critical to investigate the quality of life of the millions of suicide attempt survivors.
To date, we know little about a question keenly important to suicide attempt survivors and their loved ones: What is the possibility of rebuilding a meaningful, high-quality life after a suicide attempt?
In addition to reporting on the prevalence of high levels of psychological well-being after a nonfatal suicide attempt, it is pivotal to investigate factors that may help facilitate this outcome. To that end, we identified personal characteristics associated with high levels of well-being. Notably, it was malleable psychological strengths such as optimism and a curious mindset, more than the mere absence of symptoms, that were linked to higher levels of well-being among veteran suicide attempt survivors.
Current suicide prevention interventions and treatments, which often focus on mitigating immediate suicide risk by treating symptoms, may be overlooking the importance of cultivating and building psychological strengths that may help promote greater well-being and enriched lives. Moreover, treatments that emphasize such strengths might be particularly fruitful in mitigating suicide risk in veterans, as veterans may be more receptive to prevention and treatment initiatives that embrace the cultivation and bolstering of strengths that are inherent in military culture and values, such as resilience and perseverance in the face of life challenges.2
One notable caveat to this study is that the data were cross-sectional, meaning they were collected at a single time point. As such, the authors cannot conclude that factors such as curiosity necessarily caused higher levels of well-being in veterans, as opposed to well-being causing higher levels of curiosity.
Similarly, while one can infer that psychological well-being was near-absent at the time of a suicide attempt, well-being of attempt survivors was not assessed before their attempt. Longitudinal studies that follow attempt survivors over time are needed to understand how well-being changes over time for suicide attempt survivors and the causal chain in what predicts that change.
Nevertheless, the results of this large, multicohort study serve as an important first step toward a more comprehensive view of prognosis after a suicide attempt. Just as the process that leads to a suicide attempt is complex, so too is the process of recovery after an attempt. While this study provides sound estimates of well-being outcomes and some possible candidates that might facilitate these outcomes, a critical next step for future research is to replicate and extend these findings. To do so, it is pivotal to extend the assessment scope beyond symptom-based measures and include measures of well-being.
Additionally, the investment in resources into longer-term examinations following suicide attempts is essential to understand different pathways toward achieving greater well-being. Providing hope is vital and potentially lifesaving, as one of the most common experiences reported before a suicide attempt is an unremitting sense of hopelessness. Continued research on well-being has the potential to impart a more balanced, nuanced prognosis after a suicide attempt that challenges perceptions of an invariably bleak prospect of recovery after suicidality.
Collectively, these results highlight the importance of broadening the scope of how the mental health field views and treats psychiatric difficulties to include a greater focus on recovery-based outcomes and personal strengths that help facilitate recovery from adverse life experiences such as suicide attempts.
People desire lives that they enjoy and find meaningful, and having a history of suicide attempts does not preclude the prospect of such a life. It is time that suicide research reflects the vast landscape of potential outcomes after a suicide attempt that goes beyond the prediction of future suicide risk.
Mr. Brown is a doctoral student of clinical psychology at the University of South Florida, Tampa. Dr. Rottenberg is director of the Mood and Emotion Lab and area director of the department of clinical psychology, University of South Florida.
References
1. Brown BA et al. Psychological well-being in US veterans with non-fatal suicide attempts: A multi-cohort population-based study. J Affect Disord. 2022 Oct 1;314:34-43. doi: 10.1016/j.jad.2022.07.003.
2. Bryan CJ et al. Understanding and preventing military suicide. Arch Suicide Res. 2012;16(2):95-110. doi: 10.1080/13811118.2012.667321.
A study of three separate nationally representative samples of nearly 9,000 U.S. military veterans found psychological well-being – defined in terms of having a high sense of purpose, social connectedness, and happiness – to be significantly diminished among veteran suicide attempt survivors relative to nonattempters, even decades after their last attempt.1
Despite the trend toward diminished well-being, many veterans who survived a suicide attempt reported average to optimal levels of well-being. Specifically, 52%-60% of veterans reporting a prior suicide attempt also reported experiencing as much purpose, social connection, and happiness as veterans without a suicide attempt history. Remarkably, a small subset (2-7%) of veteran attempt survivors even reported higher levels of well-being than veterans without a suicide attempt history.
Thus,
These data are notable because, in 2021, approximately 1.4 million U.S. adults made a nonfatal suicide attempt. Historically, suicide research has understandably emphasized the study of risk factors that increase the likelihood that someone dies by suicide. Given that a prior suicide attempt is among the top risk factors for suicide, virtually all research on suicide attempt survivors has focused on their elevated risk for future suicidality. Yet, 9 out of 10 people who have made a nonfatal suicide attempt do not go on to die by suicide. It is thus critical to investigate the quality of life of the millions of suicide attempt survivors.
To date, we know little about a question keenly important to suicide attempt survivors and their loved ones: What is the possibility of rebuilding a meaningful, high-quality life after a suicide attempt?
In addition to reporting on the prevalence of high levels of psychological well-being after a nonfatal suicide attempt, it is pivotal to investigate factors that may help facilitate this outcome. To that end, we identified personal characteristics associated with high levels of well-being. Notably, it was malleable psychological strengths such as optimism and a curious mindset, more than the mere absence of symptoms, that were linked to higher levels of well-being among veteran suicide attempt survivors.
Current suicide prevention interventions and treatments, which often focus on mitigating immediate suicide risk by treating symptoms, may be overlooking the importance of cultivating and building psychological strengths that may help promote greater well-being and enriched lives. Moreover, treatments that emphasize such strengths might be particularly fruitful in mitigating suicide risk in veterans, as veterans may be more receptive to prevention and treatment initiatives that embrace the cultivation and bolstering of strengths that are inherent in military culture and values, such as resilience and perseverance in the face of life challenges.2
One notable caveat to this study is that the data were cross-sectional, meaning they were collected at a single time point. As such, the authors cannot conclude that factors such as curiosity necessarily caused higher levels of well-being in veterans, as opposed to well-being causing higher levels of curiosity.
Similarly, while one can infer that psychological well-being was near-absent at the time of a suicide attempt, well-being of attempt survivors was not assessed before their attempt. Longitudinal studies that follow attempt survivors over time are needed to understand how well-being changes over time for suicide attempt survivors and the causal chain in what predicts that change.
Nevertheless, the results of this large, multicohort study serve as an important first step toward a more comprehensive view of prognosis after a suicide attempt. Just as the process that leads to a suicide attempt is complex, so too is the process of recovery after an attempt. While this study provides sound estimates of well-being outcomes and some possible candidates that might facilitate these outcomes, a critical next step for future research is to replicate and extend these findings. To do so, it is pivotal to extend the assessment scope beyond symptom-based measures and include measures of well-being.
Additionally, the investment in resources into longer-term examinations following suicide attempts is essential to understand different pathways toward achieving greater well-being. Providing hope is vital and potentially lifesaving, as one of the most common experiences reported before a suicide attempt is an unremitting sense of hopelessness. Continued research on well-being has the potential to impart a more balanced, nuanced prognosis after a suicide attempt that challenges perceptions of an invariably bleak prospect of recovery after suicidality.
Collectively, these results highlight the importance of broadening the scope of how the mental health field views and treats psychiatric difficulties to include a greater focus on recovery-based outcomes and personal strengths that help facilitate recovery from adverse life experiences such as suicide attempts.
People desire lives that they enjoy and find meaningful, and having a history of suicide attempts does not preclude the prospect of such a life. It is time that suicide research reflects the vast landscape of potential outcomes after a suicide attempt that goes beyond the prediction of future suicide risk.
Mr. Brown is a doctoral student of clinical psychology at the University of South Florida, Tampa. Dr. Rottenberg is director of the Mood and Emotion Lab and area director of the department of clinical psychology, University of South Florida.
References
1. Brown BA et al. Psychological well-being in US veterans with non-fatal suicide attempts: A multi-cohort population-based study. J Affect Disord. 2022 Oct 1;314:34-43. doi: 10.1016/j.jad.2022.07.003.
2. Bryan CJ et al. Understanding and preventing military suicide. Arch Suicide Res. 2012;16(2):95-110. doi: 10.1080/13811118.2012.667321.
A study of three separate nationally representative samples of nearly 9,000 U.S. military veterans found psychological well-being – defined in terms of having a high sense of purpose, social connectedness, and happiness – to be significantly diminished among veteran suicide attempt survivors relative to nonattempters, even decades after their last attempt.1
Despite the trend toward diminished well-being, many veterans who survived a suicide attempt reported average to optimal levels of well-being. Specifically, 52%-60% of veterans reporting a prior suicide attempt also reported experiencing as much purpose, social connection, and happiness as veterans without a suicide attempt history. Remarkably, a small subset (2-7%) of veteran attempt survivors even reported higher levels of well-being than veterans without a suicide attempt history.
Thus,
These data are notable because, in 2021, approximately 1.4 million U.S. adults made a nonfatal suicide attempt. Historically, suicide research has understandably emphasized the study of risk factors that increase the likelihood that someone dies by suicide. Given that a prior suicide attempt is among the top risk factors for suicide, virtually all research on suicide attempt survivors has focused on their elevated risk for future suicidality. Yet, 9 out of 10 people who have made a nonfatal suicide attempt do not go on to die by suicide. It is thus critical to investigate the quality of life of the millions of suicide attempt survivors.
To date, we know little about a question keenly important to suicide attempt survivors and their loved ones: What is the possibility of rebuilding a meaningful, high-quality life after a suicide attempt?
In addition to reporting on the prevalence of high levels of psychological well-being after a nonfatal suicide attempt, it is pivotal to investigate factors that may help facilitate this outcome. To that end, we identified personal characteristics associated with high levels of well-being. Notably, it was malleable psychological strengths such as optimism and a curious mindset, more than the mere absence of symptoms, that were linked to higher levels of well-being among veteran suicide attempt survivors.
Current suicide prevention interventions and treatments, which often focus on mitigating immediate suicide risk by treating symptoms, may be overlooking the importance of cultivating and building psychological strengths that may help promote greater well-being and enriched lives. Moreover, treatments that emphasize such strengths might be particularly fruitful in mitigating suicide risk in veterans, as veterans may be more receptive to prevention and treatment initiatives that embrace the cultivation and bolstering of strengths that are inherent in military culture and values, such as resilience and perseverance in the face of life challenges.2
One notable caveat to this study is that the data were cross-sectional, meaning they were collected at a single time point. As such, the authors cannot conclude that factors such as curiosity necessarily caused higher levels of well-being in veterans, as opposed to well-being causing higher levels of curiosity.
Similarly, while one can infer that psychological well-being was near-absent at the time of a suicide attempt, well-being of attempt survivors was not assessed before their attempt. Longitudinal studies that follow attempt survivors over time are needed to understand how well-being changes over time for suicide attempt survivors and the causal chain in what predicts that change.
Nevertheless, the results of this large, multicohort study serve as an important first step toward a more comprehensive view of prognosis after a suicide attempt. Just as the process that leads to a suicide attempt is complex, so too is the process of recovery after an attempt. While this study provides sound estimates of well-being outcomes and some possible candidates that might facilitate these outcomes, a critical next step for future research is to replicate and extend these findings. To do so, it is pivotal to extend the assessment scope beyond symptom-based measures and include measures of well-being.
Additionally, the investment in resources into longer-term examinations following suicide attempts is essential to understand different pathways toward achieving greater well-being. Providing hope is vital and potentially lifesaving, as one of the most common experiences reported before a suicide attempt is an unremitting sense of hopelessness. Continued research on well-being has the potential to impart a more balanced, nuanced prognosis after a suicide attempt that challenges perceptions of an invariably bleak prospect of recovery after suicidality.
Collectively, these results highlight the importance of broadening the scope of how the mental health field views and treats psychiatric difficulties to include a greater focus on recovery-based outcomes and personal strengths that help facilitate recovery from adverse life experiences such as suicide attempts.
People desire lives that they enjoy and find meaningful, and having a history of suicide attempts does not preclude the prospect of such a life. It is time that suicide research reflects the vast landscape of potential outcomes after a suicide attempt that goes beyond the prediction of future suicide risk.
Mr. Brown is a doctoral student of clinical psychology at the University of South Florida, Tampa. Dr. Rottenberg is director of the Mood and Emotion Lab and area director of the department of clinical psychology, University of South Florida.
References
1. Brown BA et al. Psychological well-being in US veterans with non-fatal suicide attempts: A multi-cohort population-based study. J Affect Disord. 2022 Oct 1;314:34-43. doi: 10.1016/j.jad.2022.07.003.
2. Bryan CJ et al. Understanding and preventing military suicide. Arch Suicide Res. 2012;16(2):95-110. doi: 10.1080/13811118.2012.667321.
Clinician violence: Virtual reality to the rescue?
This discussion was recorded on Feb. 21, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Welcome, Dr. Salazar. It’s a pleasure to have you join us today.
Gilberto A. Salazar, MD: The pleasure is all mine, Dr. Glatter. Thank you so much for having me.
Dr. Glatter: This is such an important topic, as you can imagine. Workplace violence is affecting so many providers in hospital emergency departments but also throughout other parts of the hospital.
First, can you describe how the virtual reality (VR) program was designed that you developed and what type of situations it simulates?
Dr. Salazar: We worked in conjunction with the University of Texas at Dallas. They help people like me, subject matter experts in health care, to bring ideas to reality. I worked very closely with a group of engineers from their department in designing a module specifically designed to tackle, as you mentioned, one of our biggest threats in workplace violence.
We decided to bring in a series of competencies and proficiencies that we wanted to bring into the virtual reality space. In leveraging the technology and the expertise from UT Dallas, we were able to make that happen.
Dr. Glatter: I think it’s important to understand, in terms of virtual reality, what type of environment the program creates. Can you describe what a provider who puts the goggles on is experiencing? Do they feel anything? Is there technology that enables this?
Dr. Salazar: Yes, absolutely. We were able to bring to reality a series of scenarios very common from what you and I see in the emergency department on a daily basis. We wanted to immerse a learner into that specific environment. We didn’t feel that a module or something on a computer or a slide set could really bring the reality of what it’s like to interact with a patient who may be escalating or may be aggressive.
We are immersing learners into an actual hospital room to our specifications, very similar to exactly where we practice each and every day, and taking the learners through different situations that we designed with various levels of escalation and aggression, and asking the learner to manage that situation as best as they possibly can using the competencies and proficiencies that we taught them.
Dr. Glatter: Haptic feedback is an important part of the program and also the approach and technique that you’re using. Can you describe what haptic feedback means and what people actually feel?
Dr. Salazar: Absolutely. One of the most unfortunate things in my professional career is physical abuse suffered by people like me and you and our colleagues, nursing personnel, technicians, and others, resulting in injury.
We wanted to provide the most realistic experience that we could design. Haptics engage digital senses other than your auditory and your visuals. They really engage your tactile senses. These haptic vests and gloves and technology allow us to provide a third set of sensory stimuli for the learner.
At one of the modules, we have an actual physical assault that takes place, and the learner is actually able to feel in their body the strikes – of course, not painful – but just bringing in those senses and that stimulus, really leaving the learner with an experience that’s going to be long-lasting.
Dr. Glatter: Feeling that stimulus certainly affects your vital signs. Do you monitor a provider’s vital signs, such as their blood pressure and heart rate, as the situation and the threat escalate? That could potentially trigger some issues in people with prior PTSD or people with other mental health issues. Has that ever been considered in the design of your program?
Dr. Salazar: Yes, 100%. The beautiful thing about haptics is that they can be tailored to our specific parameters. The sensory stimulus that’s provided is actually very mild. It feels more like a tap than an actual strike. It just reminds us that when we’re having or experiencing an actual physical attack, we’re really engaging the senses.
We have an emergency physician or an EMT-paramedic on site at all times during the training so that we can monitor our subjects and make sure that they’re comfortable and healthy.
Dr. Glatter: Do they have actual sensors attached to their bodies that are part of your program or distinct in terms of monitoring their vital signs?
Dr. Salazar: It’s completely different. We have two different systems that we are planning on utilizing. Frankly, in the final version of this virtual reality module, we may not even involve the haptics. We’re going to study it and see how our learners behave and how much information they’re able to acquire and retain.
It may be very possible that just the visuals – the auditory and the immersion taking place within the hospital room – may be enough. It’s very possible that, in the next final version of this, we may find that haptics bring in quite a bit of value, and we may incorporate that. If that is the case, then we will, of course, acquire different technology to monitor the patient’s vital signs.
Dr. Glatter: Clearly, when situations escalate in the department, everyone gets more concerned about the patient, but providers are part of this equation, as you allude to.
In 2022, there was a poll by the American College of Emergency Physicians that stated that 85% of emergency physicians reported an increase in violent activity in their ERs in the past 5 years. Nearly two-thirds of nearly 3,000 emergency physicians surveyed reported being assaulted in the past year. This is an important module that we integrate into training providers in terms of these types of tense situations that can result not only in mental anguish but also in physical injury.
Dr. Salazar: One hundred percent. I frankly got tired of seeing my friends and my colleagues suffer both the physical and mental effects of verbal and physical abuse, and I wanted to design a project that was very patient centric while allowing our personnel to really manage these situations a little bit better.
Frankly, we don’t receive great training in this space, and I wanted to rewrite that narrative and make things better for our clinicians out there while remaining patient centric. I wanted to do something about it, and hopefully this dream will become a reality.
Dr. Glatter: Absolutely. There are other data from the Bureau of Labor Statistics stating that health care workers are five times more likely than employees in any other area of work to experience workplace violence. This could, again, range from verbal to physical violence. This is a very important module that you’re developing.
Are there any thoughts to extend this to active-shooter scenarios or any other high-stakes scenarios that you can imagine in the department?
Dr. Salazar: We’re actually working with the same developer that’s helping us with this VR module in developing a mass-casualty incident module so that we can get better training in responding to these very unfortunate high-stakes situations.
Dr. Glatter: In terms of using the module remotely, certainly not requiring resources or having to be in a physical place, can providers in your plan be able to take such a headset home and practice on their own in the sense of being able to deal with a situation? Would this be more reserved for in-department use?
Dr. Salazar: That’s a phenomenal question. I wanted to create the most flexible module that I possibly could. Ideally, a dream scenario is leveraging a simulation center at an academic center and not just do the VR module but also have a brief didactics incorporating a small slide set, some feedback, and some standardized patients. I wanted it to be flexible enough so that folks here in my state, a different state, or even internationally could take advantage of this technology and do it from the comfort of their home.
As you mentioned, this is going to strike some people. It’s going to hit them heavier than others in terms of prior experience as PTSD. For some people, it may be more comfortable to do it in the comfort of their homes. I wanted to create something very flexible and dynamic.
Dr. Glatter: I think that’s ideal. Just one other point. Can you discuss the different levels of competencies involved in this module and how that would be attained?
Dr. Salazar: It’s all evidence based, so we borrowed from literature and the specialties of emergency medicine. We collaborated with psychiatrists within our medical center. We looked at all available literature and methods, proficiencies, competencies, and best practices, and we took all of them together to form something that we think is organized and concise.
We were able to create our own algorithm, but it’s not brand new. We’re just borrowing what we think is the best to create something that the majority of health care personnel are going to be able to relate to and be able to really be proficient at.
This includes things like active listening, bargaining, how to respond, where to put yourself in a situation, and the best possible situation to respond to a scenario, how to prevent things – how to get out of a chokehold, for example. We’re borrowing from several different disciplines and creating something that can be very concise and organized.
Dr. Glatter: Does this program that you’ve developed allow the provider to get feedback in the sense that when they’re in such a danger, their life could be at risk? For example, if they don’t remove themselves in a certain amount of time, this could be lethal.
Dr. Salazar: Yes, 100%. Probably the one thing that differentiates our project from any others is the ability to customize the experience so that a learner who is doing the things that we ask them to do in terms of safety and response is able to get out of a situation successfully within the environment. If they don’t, they get some kind of feedback.
Not to spoil the surprise here, but we’re going to be doing things like looking at decibel meters to see what the volume in the room is doing and how you’re managing the volume and the stimulation within the room. If you are able to maintain the decibel readings at a specific level, you’re going to succeed through the module. If you don’t, we keep the patient escalation going.
Dr. Glatter: There is a debrief built into this type of approach where, in other words, learning points are emphasized – where you could have done better and such.
Dr. Salazar: Yes, absolutely. We are going to be able to get individualized data for each learner so that we can tailor the debrief to their own performance and be able to give them actionable items to work on. It’s a debrief that’s productive and individualized, and folks can walk away with something useful in the end.
Dr. Glatter: Are the data shared or confidential at present?
Dr. Salazar: At this very moment, the data are confidential. We are going to look at how to best use this. We’re hoping to eventually write this up and see how this information can be best used to train personnel.
Eventually, we may see that some of the advice that we’re giving is very common to most folks. Others may require some individualized type of feedback. That said, it remains to be seen, but right now, it’s confidential.
Dr. Glatter: Is this currently being implemented as part of your curriculum for emergency medicine residents?
Dr. Salazar: We’re going to study it first. We’re very excited to include our emergency medicine residents as one of our cohorts that’s going to be undergoing the module, and we’re going to be studying other forms of workplace violence mitigation strategies. We’re really excited about the possibility of this eventually becoming the standard of education for not only our emergency medicine residents, but also health care personnel all over the world.
Dr. Glatter: I’m glad you mentioned that, because obviously nurses, clerks in the department, and anyone who’s working in the department, for that matter, and who interfaces with patients really should undergo such training.
Dr. Salazar: Absolutely. The folks at intake, at check-in, and at kiosks. Do they go through a separate area for screening? You’re absolutely right. There are many folks who interface with patients and all of us are potential victims of workplace violence. We want to give our health care family the best opportunity to succeed in these situations.
Dr. Glatter:: Absolutely. Even EMS providers, being on the front lines and encountering patients in such situations, would benefit, in my opinion.
Dr. Salazar: Yes, absolutely. Behavioral health emergencies and organically induced altered mental status results in injury, both physical and mental, to EMS professionals as well, and there’s good evidence of that. I’ll be very glad to see this type of education make it out to our initial and continuing education efforts for EMS as well.
Dr. Glatter: I want to thank you. This has been very helpful. It’s such an important task that you’ve started to explore, and I look forward to follow-up on this. Again, thank you for your time.
Dr. Salazar: It was my pleasure. Thank you so much for having me.
Dr. Glatter is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, N.Y. He is an editorial adviser and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes. Dr. Salazar is a board-certified emergency physician and associate professor at UT Southwestern Medicine Center in Dallas. He is involved with the UTSW Emergency Medicine Education Program and serves as the medical director to teach both initial and continuing the emergency medicine education for emergency medical technicians and paramedics, which trains most of the Dallas Fire Rescue personnel and the vast majority for EMS providers in the Dallas County. In addition, he serves as an associate chief of service at Parkland’s emergency department, and liaison to surgical services. A version of this article originally appeared on Medscape.com.
This discussion was recorded on Feb. 21, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Welcome, Dr. Salazar. It’s a pleasure to have you join us today.
Gilberto A. Salazar, MD: The pleasure is all mine, Dr. Glatter. Thank you so much for having me.
Dr. Glatter: This is such an important topic, as you can imagine. Workplace violence is affecting so many providers in hospital emergency departments but also throughout other parts of the hospital.
First, can you describe how the virtual reality (VR) program was designed that you developed and what type of situations it simulates?
Dr. Salazar: We worked in conjunction with the University of Texas at Dallas. They help people like me, subject matter experts in health care, to bring ideas to reality. I worked very closely with a group of engineers from their department in designing a module specifically designed to tackle, as you mentioned, one of our biggest threats in workplace violence.
We decided to bring in a series of competencies and proficiencies that we wanted to bring into the virtual reality space. In leveraging the technology and the expertise from UT Dallas, we were able to make that happen.
Dr. Glatter: I think it’s important to understand, in terms of virtual reality, what type of environment the program creates. Can you describe what a provider who puts the goggles on is experiencing? Do they feel anything? Is there technology that enables this?
Dr. Salazar: Yes, absolutely. We were able to bring to reality a series of scenarios very common from what you and I see in the emergency department on a daily basis. We wanted to immerse a learner into that specific environment. We didn’t feel that a module or something on a computer or a slide set could really bring the reality of what it’s like to interact with a patient who may be escalating or may be aggressive.
We are immersing learners into an actual hospital room to our specifications, very similar to exactly where we practice each and every day, and taking the learners through different situations that we designed with various levels of escalation and aggression, and asking the learner to manage that situation as best as they possibly can using the competencies and proficiencies that we taught them.
Dr. Glatter: Haptic feedback is an important part of the program and also the approach and technique that you’re using. Can you describe what haptic feedback means and what people actually feel?
Dr. Salazar: Absolutely. One of the most unfortunate things in my professional career is physical abuse suffered by people like me and you and our colleagues, nursing personnel, technicians, and others, resulting in injury.
We wanted to provide the most realistic experience that we could design. Haptics engage digital senses other than your auditory and your visuals. They really engage your tactile senses. These haptic vests and gloves and technology allow us to provide a third set of sensory stimuli for the learner.
At one of the modules, we have an actual physical assault that takes place, and the learner is actually able to feel in their body the strikes – of course, not painful – but just bringing in those senses and that stimulus, really leaving the learner with an experience that’s going to be long-lasting.
Dr. Glatter: Feeling that stimulus certainly affects your vital signs. Do you monitor a provider’s vital signs, such as their blood pressure and heart rate, as the situation and the threat escalate? That could potentially trigger some issues in people with prior PTSD or people with other mental health issues. Has that ever been considered in the design of your program?
Dr. Salazar: Yes, 100%. The beautiful thing about haptics is that they can be tailored to our specific parameters. The sensory stimulus that’s provided is actually very mild. It feels more like a tap than an actual strike. It just reminds us that when we’re having or experiencing an actual physical attack, we’re really engaging the senses.
We have an emergency physician or an EMT-paramedic on site at all times during the training so that we can monitor our subjects and make sure that they’re comfortable and healthy.
Dr. Glatter: Do they have actual sensors attached to their bodies that are part of your program or distinct in terms of monitoring their vital signs?
Dr. Salazar: It’s completely different. We have two different systems that we are planning on utilizing. Frankly, in the final version of this virtual reality module, we may not even involve the haptics. We’re going to study it and see how our learners behave and how much information they’re able to acquire and retain.
It may be very possible that just the visuals – the auditory and the immersion taking place within the hospital room – may be enough. It’s very possible that, in the next final version of this, we may find that haptics bring in quite a bit of value, and we may incorporate that. If that is the case, then we will, of course, acquire different technology to monitor the patient’s vital signs.
Dr. Glatter: Clearly, when situations escalate in the department, everyone gets more concerned about the patient, but providers are part of this equation, as you allude to.
In 2022, there was a poll by the American College of Emergency Physicians that stated that 85% of emergency physicians reported an increase in violent activity in their ERs in the past 5 years. Nearly two-thirds of nearly 3,000 emergency physicians surveyed reported being assaulted in the past year. This is an important module that we integrate into training providers in terms of these types of tense situations that can result not only in mental anguish but also in physical injury.
Dr. Salazar: One hundred percent. I frankly got tired of seeing my friends and my colleagues suffer both the physical and mental effects of verbal and physical abuse, and I wanted to design a project that was very patient centric while allowing our personnel to really manage these situations a little bit better.
Frankly, we don’t receive great training in this space, and I wanted to rewrite that narrative and make things better for our clinicians out there while remaining patient centric. I wanted to do something about it, and hopefully this dream will become a reality.
Dr. Glatter: Absolutely. There are other data from the Bureau of Labor Statistics stating that health care workers are five times more likely than employees in any other area of work to experience workplace violence. This could, again, range from verbal to physical violence. This is a very important module that you’re developing.
Are there any thoughts to extend this to active-shooter scenarios or any other high-stakes scenarios that you can imagine in the department?
Dr. Salazar: We’re actually working with the same developer that’s helping us with this VR module in developing a mass-casualty incident module so that we can get better training in responding to these very unfortunate high-stakes situations.
Dr. Glatter: In terms of using the module remotely, certainly not requiring resources or having to be in a physical place, can providers in your plan be able to take such a headset home and practice on their own in the sense of being able to deal with a situation? Would this be more reserved for in-department use?
Dr. Salazar: That’s a phenomenal question. I wanted to create the most flexible module that I possibly could. Ideally, a dream scenario is leveraging a simulation center at an academic center and not just do the VR module but also have a brief didactics incorporating a small slide set, some feedback, and some standardized patients. I wanted it to be flexible enough so that folks here in my state, a different state, or even internationally could take advantage of this technology and do it from the comfort of their home.
As you mentioned, this is going to strike some people. It’s going to hit them heavier than others in terms of prior experience as PTSD. For some people, it may be more comfortable to do it in the comfort of their homes. I wanted to create something very flexible and dynamic.
Dr. Glatter: I think that’s ideal. Just one other point. Can you discuss the different levels of competencies involved in this module and how that would be attained?
Dr. Salazar: It’s all evidence based, so we borrowed from literature and the specialties of emergency medicine. We collaborated with psychiatrists within our medical center. We looked at all available literature and methods, proficiencies, competencies, and best practices, and we took all of them together to form something that we think is organized and concise.
We were able to create our own algorithm, but it’s not brand new. We’re just borrowing what we think is the best to create something that the majority of health care personnel are going to be able to relate to and be able to really be proficient at.
This includes things like active listening, bargaining, how to respond, where to put yourself in a situation, and the best possible situation to respond to a scenario, how to prevent things – how to get out of a chokehold, for example. We’re borrowing from several different disciplines and creating something that can be very concise and organized.
Dr. Glatter: Does this program that you’ve developed allow the provider to get feedback in the sense that when they’re in such a danger, their life could be at risk? For example, if they don’t remove themselves in a certain amount of time, this could be lethal.
Dr. Salazar: Yes, 100%. Probably the one thing that differentiates our project from any others is the ability to customize the experience so that a learner who is doing the things that we ask them to do in terms of safety and response is able to get out of a situation successfully within the environment. If they don’t, they get some kind of feedback.
Not to spoil the surprise here, but we’re going to be doing things like looking at decibel meters to see what the volume in the room is doing and how you’re managing the volume and the stimulation within the room. If you are able to maintain the decibel readings at a specific level, you’re going to succeed through the module. If you don’t, we keep the patient escalation going.
Dr. Glatter: There is a debrief built into this type of approach where, in other words, learning points are emphasized – where you could have done better and such.
Dr. Salazar: Yes, absolutely. We are going to be able to get individualized data for each learner so that we can tailor the debrief to their own performance and be able to give them actionable items to work on. It’s a debrief that’s productive and individualized, and folks can walk away with something useful in the end.
Dr. Glatter: Are the data shared or confidential at present?
Dr. Salazar: At this very moment, the data are confidential. We are going to look at how to best use this. We’re hoping to eventually write this up and see how this information can be best used to train personnel.
Eventually, we may see that some of the advice that we’re giving is very common to most folks. Others may require some individualized type of feedback. That said, it remains to be seen, but right now, it’s confidential.
Dr. Glatter: Is this currently being implemented as part of your curriculum for emergency medicine residents?
Dr. Salazar: We’re going to study it first. We’re very excited to include our emergency medicine residents as one of our cohorts that’s going to be undergoing the module, and we’re going to be studying other forms of workplace violence mitigation strategies. We’re really excited about the possibility of this eventually becoming the standard of education for not only our emergency medicine residents, but also health care personnel all over the world.
Dr. Glatter: I’m glad you mentioned that, because obviously nurses, clerks in the department, and anyone who’s working in the department, for that matter, and who interfaces with patients really should undergo such training.
Dr. Salazar: Absolutely. The folks at intake, at check-in, and at kiosks. Do they go through a separate area for screening? You’re absolutely right. There are many folks who interface with patients and all of us are potential victims of workplace violence. We want to give our health care family the best opportunity to succeed in these situations.
Dr. Glatter:: Absolutely. Even EMS providers, being on the front lines and encountering patients in such situations, would benefit, in my opinion.
Dr. Salazar: Yes, absolutely. Behavioral health emergencies and organically induced altered mental status results in injury, both physical and mental, to EMS professionals as well, and there’s good evidence of that. I’ll be very glad to see this type of education make it out to our initial and continuing education efforts for EMS as well.
Dr. Glatter: I want to thank you. This has been very helpful. It’s such an important task that you’ve started to explore, and I look forward to follow-up on this. Again, thank you for your time.
Dr. Salazar: It was my pleasure. Thank you so much for having me.
Dr. Glatter is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, N.Y. He is an editorial adviser and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes. Dr. Salazar is a board-certified emergency physician and associate professor at UT Southwestern Medicine Center in Dallas. He is involved with the UTSW Emergency Medicine Education Program and serves as the medical director to teach both initial and continuing the emergency medicine education for emergency medical technicians and paramedics, which trains most of the Dallas Fire Rescue personnel and the vast majority for EMS providers in the Dallas County. In addition, he serves as an associate chief of service at Parkland’s emergency department, and liaison to surgical services. A version of this article originally appeared on Medscape.com.
This discussion was recorded on Feb. 21, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Welcome, Dr. Salazar. It’s a pleasure to have you join us today.
Gilberto A. Salazar, MD: The pleasure is all mine, Dr. Glatter. Thank you so much for having me.
Dr. Glatter: This is such an important topic, as you can imagine. Workplace violence is affecting so many providers in hospital emergency departments but also throughout other parts of the hospital.
First, can you describe how the virtual reality (VR) program was designed that you developed and what type of situations it simulates?
Dr. Salazar: We worked in conjunction with the University of Texas at Dallas. They help people like me, subject matter experts in health care, to bring ideas to reality. I worked very closely with a group of engineers from their department in designing a module specifically designed to tackle, as you mentioned, one of our biggest threats in workplace violence.
We decided to bring in a series of competencies and proficiencies that we wanted to bring into the virtual reality space. In leveraging the technology and the expertise from UT Dallas, we were able to make that happen.
Dr. Glatter: I think it’s important to understand, in terms of virtual reality, what type of environment the program creates. Can you describe what a provider who puts the goggles on is experiencing? Do they feel anything? Is there technology that enables this?
Dr. Salazar: Yes, absolutely. We were able to bring to reality a series of scenarios very common from what you and I see in the emergency department on a daily basis. We wanted to immerse a learner into that specific environment. We didn’t feel that a module or something on a computer or a slide set could really bring the reality of what it’s like to interact with a patient who may be escalating or may be aggressive.
We are immersing learners into an actual hospital room to our specifications, very similar to exactly where we practice each and every day, and taking the learners through different situations that we designed with various levels of escalation and aggression, and asking the learner to manage that situation as best as they possibly can using the competencies and proficiencies that we taught them.
Dr. Glatter: Haptic feedback is an important part of the program and also the approach and technique that you’re using. Can you describe what haptic feedback means and what people actually feel?
Dr. Salazar: Absolutely. One of the most unfortunate things in my professional career is physical abuse suffered by people like me and you and our colleagues, nursing personnel, technicians, and others, resulting in injury.
We wanted to provide the most realistic experience that we could design. Haptics engage digital senses other than your auditory and your visuals. They really engage your tactile senses. These haptic vests and gloves and technology allow us to provide a third set of sensory stimuli for the learner.
At one of the modules, we have an actual physical assault that takes place, and the learner is actually able to feel in their body the strikes – of course, not painful – but just bringing in those senses and that stimulus, really leaving the learner with an experience that’s going to be long-lasting.
Dr. Glatter: Feeling that stimulus certainly affects your vital signs. Do you monitor a provider’s vital signs, such as their blood pressure and heart rate, as the situation and the threat escalate? That could potentially trigger some issues in people with prior PTSD or people with other mental health issues. Has that ever been considered in the design of your program?
Dr. Salazar: Yes, 100%. The beautiful thing about haptics is that they can be tailored to our specific parameters. The sensory stimulus that’s provided is actually very mild. It feels more like a tap than an actual strike. It just reminds us that when we’re having or experiencing an actual physical attack, we’re really engaging the senses.
We have an emergency physician or an EMT-paramedic on site at all times during the training so that we can monitor our subjects and make sure that they’re comfortable and healthy.
Dr. Glatter: Do they have actual sensors attached to their bodies that are part of your program or distinct in terms of monitoring their vital signs?
Dr. Salazar: It’s completely different. We have two different systems that we are planning on utilizing. Frankly, in the final version of this virtual reality module, we may not even involve the haptics. We’re going to study it and see how our learners behave and how much information they’re able to acquire and retain.
It may be very possible that just the visuals – the auditory and the immersion taking place within the hospital room – may be enough. It’s very possible that, in the next final version of this, we may find that haptics bring in quite a bit of value, and we may incorporate that. If that is the case, then we will, of course, acquire different technology to monitor the patient’s vital signs.
Dr. Glatter: Clearly, when situations escalate in the department, everyone gets more concerned about the patient, but providers are part of this equation, as you allude to.
In 2022, there was a poll by the American College of Emergency Physicians that stated that 85% of emergency physicians reported an increase in violent activity in their ERs in the past 5 years. Nearly two-thirds of nearly 3,000 emergency physicians surveyed reported being assaulted in the past year. This is an important module that we integrate into training providers in terms of these types of tense situations that can result not only in mental anguish but also in physical injury.
Dr. Salazar: One hundred percent. I frankly got tired of seeing my friends and my colleagues suffer both the physical and mental effects of verbal and physical abuse, and I wanted to design a project that was very patient centric while allowing our personnel to really manage these situations a little bit better.
Frankly, we don’t receive great training in this space, and I wanted to rewrite that narrative and make things better for our clinicians out there while remaining patient centric. I wanted to do something about it, and hopefully this dream will become a reality.
Dr. Glatter: Absolutely. There are other data from the Bureau of Labor Statistics stating that health care workers are five times more likely than employees in any other area of work to experience workplace violence. This could, again, range from verbal to physical violence. This is a very important module that you’re developing.
Are there any thoughts to extend this to active-shooter scenarios or any other high-stakes scenarios that you can imagine in the department?
Dr. Salazar: We’re actually working with the same developer that’s helping us with this VR module in developing a mass-casualty incident module so that we can get better training in responding to these very unfortunate high-stakes situations.
Dr. Glatter: In terms of using the module remotely, certainly not requiring resources or having to be in a physical place, can providers in your plan be able to take such a headset home and practice on their own in the sense of being able to deal with a situation? Would this be more reserved for in-department use?
Dr. Salazar: That’s a phenomenal question. I wanted to create the most flexible module that I possibly could. Ideally, a dream scenario is leveraging a simulation center at an academic center and not just do the VR module but also have a brief didactics incorporating a small slide set, some feedback, and some standardized patients. I wanted it to be flexible enough so that folks here in my state, a different state, or even internationally could take advantage of this technology and do it from the comfort of their home.
As you mentioned, this is going to strike some people. It’s going to hit them heavier than others in terms of prior experience as PTSD. For some people, it may be more comfortable to do it in the comfort of their homes. I wanted to create something very flexible and dynamic.
Dr. Glatter: I think that’s ideal. Just one other point. Can you discuss the different levels of competencies involved in this module and how that would be attained?
Dr. Salazar: It’s all evidence based, so we borrowed from literature and the specialties of emergency medicine. We collaborated with psychiatrists within our medical center. We looked at all available literature and methods, proficiencies, competencies, and best practices, and we took all of them together to form something that we think is organized and concise.
We were able to create our own algorithm, but it’s not brand new. We’re just borrowing what we think is the best to create something that the majority of health care personnel are going to be able to relate to and be able to really be proficient at.
This includes things like active listening, bargaining, how to respond, where to put yourself in a situation, and the best possible situation to respond to a scenario, how to prevent things – how to get out of a chokehold, for example. We’re borrowing from several different disciplines and creating something that can be very concise and organized.
Dr. Glatter: Does this program that you’ve developed allow the provider to get feedback in the sense that when they’re in such a danger, their life could be at risk? For example, if they don’t remove themselves in a certain amount of time, this could be lethal.
Dr. Salazar: Yes, 100%. Probably the one thing that differentiates our project from any others is the ability to customize the experience so that a learner who is doing the things that we ask them to do in terms of safety and response is able to get out of a situation successfully within the environment. If they don’t, they get some kind of feedback.
Not to spoil the surprise here, but we’re going to be doing things like looking at decibel meters to see what the volume in the room is doing and how you’re managing the volume and the stimulation within the room. If you are able to maintain the decibel readings at a specific level, you’re going to succeed through the module. If you don’t, we keep the patient escalation going.
Dr. Glatter: There is a debrief built into this type of approach where, in other words, learning points are emphasized – where you could have done better and such.
Dr. Salazar: Yes, absolutely. We are going to be able to get individualized data for each learner so that we can tailor the debrief to their own performance and be able to give them actionable items to work on. It’s a debrief that’s productive and individualized, and folks can walk away with something useful in the end.
Dr. Glatter: Are the data shared or confidential at present?
Dr. Salazar: At this very moment, the data are confidential. We are going to look at how to best use this. We’re hoping to eventually write this up and see how this information can be best used to train personnel.
Eventually, we may see that some of the advice that we’re giving is very common to most folks. Others may require some individualized type of feedback. That said, it remains to be seen, but right now, it’s confidential.
Dr. Glatter: Is this currently being implemented as part of your curriculum for emergency medicine residents?
Dr. Salazar: We’re going to study it first. We’re very excited to include our emergency medicine residents as one of our cohorts that’s going to be undergoing the module, and we’re going to be studying other forms of workplace violence mitigation strategies. We’re really excited about the possibility of this eventually becoming the standard of education for not only our emergency medicine residents, but also health care personnel all over the world.
Dr. Glatter: I’m glad you mentioned that, because obviously nurses, clerks in the department, and anyone who’s working in the department, for that matter, and who interfaces with patients really should undergo such training.
Dr. Salazar: Absolutely. The folks at intake, at check-in, and at kiosks. Do they go through a separate area for screening? You’re absolutely right. There are many folks who interface with patients and all of us are potential victims of workplace violence. We want to give our health care family the best opportunity to succeed in these situations.
Dr. Glatter:: Absolutely. Even EMS providers, being on the front lines and encountering patients in such situations, would benefit, in my opinion.
Dr. Salazar: Yes, absolutely. Behavioral health emergencies and organically induced altered mental status results in injury, both physical and mental, to EMS professionals as well, and there’s good evidence of that. I’ll be very glad to see this type of education make it out to our initial and continuing education efforts for EMS as well.
Dr. Glatter: I want to thank you. This has been very helpful. It’s such an important task that you’ve started to explore, and I look forward to follow-up on this. Again, thank you for your time.
Dr. Salazar: It was my pleasure. Thank you so much for having me.
Dr. Glatter is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, N.Y. He is an editorial adviser and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes. Dr. Salazar is a board-certified emergency physician and associate professor at UT Southwestern Medicine Center in Dallas. He is involved with the UTSW Emergency Medicine Education Program and serves as the medical director to teach both initial and continuing the emergency medicine education for emergency medical technicians and paramedics, which trains most of the Dallas Fire Rescue personnel and the vast majority for EMS providers in the Dallas County. In addition, he serves as an associate chief of service at Parkland’s emergency department, and liaison to surgical services. A version of this article originally appeared on Medscape.com.