User login
Headache may predict clinical evolution of COVID-19
new research suggests. An observational study of more than 100 patients showed that headache onset could occur during the presymptomatic or symptomatic phase of COVID-19 and could resemble tension-type or migraine headache.
Headache itself was associated with a shorter symptomatic period, while headache and anosmia were associated with a shorter hospitalization period. In a subgroup of participants, headache persisted even after the symptoms of COVID-19 had been resolved.
Investigators noted that understanding the pathophysiology of headache in COVID-19 could improve understanding of migraine and other headache disorders. “It seems that those patients who start early on, during the asymptomatic or early symptomatic period of COVID-19, with headache have a more localized inflammatory response that may reflect the ability of the body to better control and respond to the infection by SARS-CoV-2,” lead investigator Patricia Pozo-Rosich, MD, PhD, head of the headache and craniofacial pain unit at Vall d’Hebron University Hospital, Barcelona, said in an interview.
She presented the findings at the virtual annual meeting of the American Headache Society.
Systemic inflammation
Headache is one of the main symptoms of COVID-19. A recent study of 214 patients with COVID-19 showed that approximately 13% of the participants had headache and 5% had anosmia.
SARS-CoV-2 penetrates the cells through the ACE2 receptor, which is present throughout the body. “SARS-CoV-2 enters the body through the nasal cavity and it probably penetrates the nervous system in the periphery through afferent branches of the olfactory and trigeminal nerve,” Dr. Pozo-Rosich said. It travels to the lungs and, later, the bloodstream. This generates systemic inflammation that may turn into a cytokine storm. Evidence has identified cortical hyperintensities and olfactory bulb hyperintensities in patients with COVID-19, suggesting that the virus directly infects the CNS.
Interleukin-6, one of the main inflammatory molecules, has been proven to be related to COVID-19 and has become a therapeutic target. Levels of IL-6 may be lower and tend to be more stable in patients with both COVID-19 and headache than in patients with COVID-19 only.
The researchers observed 130 patients (51% women; mean age, 54 years) with COVID-19 who were attended by neurologists at Vall d’Hebron. In this group, 74.4% had headache. Patients with headache tended to be younger than those without headache (mean age, 50 years vs. 63 years, respectively) and tended to be women (58.6% vs. 29.4%).
Approximately one-third of patients with headache had a history of migraine. Most reported mild to moderate pain that resembled tension-type headache. In participants with severe pain and migraine-like features, headache more often began during the asymptomatic phase of COVID-19.
Disease evolution predictor?
The investigators followed up on 100 of the 130 patients with COVID-19, of whom 74 had headache. About 38% of these patients had ongoing headache after 6 weeks, which suggests that some patients may develop a new daily persistent headache once a 3-month period has elapsed. Half of this group had no previous headache history. Headache had been the prodromal symptom of COVID-19 for 21.4% of these patients.
Results showed that headache predicted the clinical evolution of COVID-19. The symptomatic phase of COVID-19 was 7 days shorter for patients with headache than for those without headache. In addition, the period of hospitalization was 7 days shorter for patients with headache and anosmia, compared with patients who had neither headache nor anosmia.
Most therapies, including ibuprofen, candesartan, and anti–calcitonin gene–related peptide (CGRP) monoclonal antibodies, are safe for treating headache in COVID-19, the investigators noted. “We should just try to initially avoid steroids to avoid interference with the body’s reaction to SARS-CoV-2,” Dr. Pozo-Rosich said.
Researchers at Sidney Kimmel Medical College, Philadelphia, are currently studying intranasal vazegepant, an anti-CGRP therapy, as a way to potentially blunt the severe inflammatory response in the lungs of patients with COVID-19, she noted, adding that this peptide may have a future role not only in headache, but also in COVID-19.
Historical link to viral infections
Commenting on the study, Matthew S. Robbins, MD, associate professor of neurology at Weill Cornell Medicine, New York, said the findings associating headache with a shorter symptomatic phase of COVID-19 were “interesting.”
“Headache is common with mild viral infections. More severe viral infections may simply feature more overwhelming respiratory symptoms and fever that lead to underreporting or underascertainment of headache,” said Dr. Robbins, who was not involved with the research.
He noted that the finding showing an association of headache and COVID-19 with a younger age and in women “may be related to a higher prevalence of migraine biology in such patients, and being triggered by the virus or the psychological stress associated with it.”
Dr. Robbins added that viral illnesses have long been associated with new daily persistent headache, “dating back to the early 1980s,” when it was first described in association with Epstein-Barr virus. These infections have also been implicated in the progression of migraine to chronic migraine in adolescents.
“In my view, treatment should be aimed at the symptomatic headache type for which new daily persistent headache resembles, regardless of the potential inciting factor,” Dr. Robbins said.
Dr. Pozo-Rosich has received consulting fees from Allergan, Amgen, Almirall, Biohaven, Chiesi, Eli Lilly, Medscape, Novartis, and Teva Pharmaceuticals. Dr. Robbins has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. An observational study of more than 100 patients showed that headache onset could occur during the presymptomatic or symptomatic phase of COVID-19 and could resemble tension-type or migraine headache.
Headache itself was associated with a shorter symptomatic period, while headache and anosmia were associated with a shorter hospitalization period. In a subgroup of participants, headache persisted even after the symptoms of COVID-19 had been resolved.
Investigators noted that understanding the pathophysiology of headache in COVID-19 could improve understanding of migraine and other headache disorders. “It seems that those patients who start early on, during the asymptomatic or early symptomatic period of COVID-19, with headache have a more localized inflammatory response that may reflect the ability of the body to better control and respond to the infection by SARS-CoV-2,” lead investigator Patricia Pozo-Rosich, MD, PhD, head of the headache and craniofacial pain unit at Vall d’Hebron University Hospital, Barcelona, said in an interview.
She presented the findings at the virtual annual meeting of the American Headache Society.
Systemic inflammation
Headache is one of the main symptoms of COVID-19. A recent study of 214 patients with COVID-19 showed that approximately 13% of the participants had headache and 5% had anosmia.
SARS-CoV-2 penetrates the cells through the ACE2 receptor, which is present throughout the body. “SARS-CoV-2 enters the body through the nasal cavity and it probably penetrates the nervous system in the periphery through afferent branches of the olfactory and trigeminal nerve,” Dr. Pozo-Rosich said. It travels to the lungs and, later, the bloodstream. This generates systemic inflammation that may turn into a cytokine storm. Evidence has identified cortical hyperintensities and olfactory bulb hyperintensities in patients with COVID-19, suggesting that the virus directly infects the CNS.
Interleukin-6, one of the main inflammatory molecules, has been proven to be related to COVID-19 and has become a therapeutic target. Levels of IL-6 may be lower and tend to be more stable in patients with both COVID-19 and headache than in patients with COVID-19 only.
The researchers observed 130 patients (51% women; mean age, 54 years) with COVID-19 who were attended by neurologists at Vall d’Hebron. In this group, 74.4% had headache. Patients with headache tended to be younger than those without headache (mean age, 50 years vs. 63 years, respectively) and tended to be women (58.6% vs. 29.4%).
Approximately one-third of patients with headache had a history of migraine. Most reported mild to moderate pain that resembled tension-type headache. In participants with severe pain and migraine-like features, headache more often began during the asymptomatic phase of COVID-19.
Disease evolution predictor?
The investigators followed up on 100 of the 130 patients with COVID-19, of whom 74 had headache. About 38% of these patients had ongoing headache after 6 weeks, which suggests that some patients may develop a new daily persistent headache once a 3-month period has elapsed. Half of this group had no previous headache history. Headache had been the prodromal symptom of COVID-19 for 21.4% of these patients.
Results showed that headache predicted the clinical evolution of COVID-19. The symptomatic phase of COVID-19 was 7 days shorter for patients with headache than for those without headache. In addition, the period of hospitalization was 7 days shorter for patients with headache and anosmia, compared with patients who had neither headache nor anosmia.
Most therapies, including ibuprofen, candesartan, and anti–calcitonin gene–related peptide (CGRP) monoclonal antibodies, are safe for treating headache in COVID-19, the investigators noted. “We should just try to initially avoid steroids to avoid interference with the body’s reaction to SARS-CoV-2,” Dr. Pozo-Rosich said.
Researchers at Sidney Kimmel Medical College, Philadelphia, are currently studying intranasal vazegepant, an anti-CGRP therapy, as a way to potentially blunt the severe inflammatory response in the lungs of patients with COVID-19, she noted, adding that this peptide may have a future role not only in headache, but also in COVID-19.
Historical link to viral infections
Commenting on the study, Matthew S. Robbins, MD, associate professor of neurology at Weill Cornell Medicine, New York, said the findings associating headache with a shorter symptomatic phase of COVID-19 were “interesting.”
“Headache is common with mild viral infections. More severe viral infections may simply feature more overwhelming respiratory symptoms and fever that lead to underreporting or underascertainment of headache,” said Dr. Robbins, who was not involved with the research.
He noted that the finding showing an association of headache and COVID-19 with a younger age and in women “may be related to a higher prevalence of migraine biology in such patients, and being triggered by the virus or the psychological stress associated with it.”
Dr. Robbins added that viral illnesses have long been associated with new daily persistent headache, “dating back to the early 1980s,” when it was first described in association with Epstein-Barr virus. These infections have also been implicated in the progression of migraine to chronic migraine in adolescents.
“In my view, treatment should be aimed at the symptomatic headache type for which new daily persistent headache resembles, regardless of the potential inciting factor,” Dr. Robbins said.
Dr. Pozo-Rosich has received consulting fees from Allergan, Amgen, Almirall, Biohaven, Chiesi, Eli Lilly, Medscape, Novartis, and Teva Pharmaceuticals. Dr. Robbins has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. An observational study of more than 100 patients showed that headache onset could occur during the presymptomatic or symptomatic phase of COVID-19 and could resemble tension-type or migraine headache.
Headache itself was associated with a shorter symptomatic period, while headache and anosmia were associated with a shorter hospitalization period. In a subgroup of participants, headache persisted even after the symptoms of COVID-19 had been resolved.
Investigators noted that understanding the pathophysiology of headache in COVID-19 could improve understanding of migraine and other headache disorders. “It seems that those patients who start early on, during the asymptomatic or early symptomatic period of COVID-19, with headache have a more localized inflammatory response that may reflect the ability of the body to better control and respond to the infection by SARS-CoV-2,” lead investigator Patricia Pozo-Rosich, MD, PhD, head of the headache and craniofacial pain unit at Vall d’Hebron University Hospital, Barcelona, said in an interview.
She presented the findings at the virtual annual meeting of the American Headache Society.
Systemic inflammation
Headache is one of the main symptoms of COVID-19. A recent study of 214 patients with COVID-19 showed that approximately 13% of the participants had headache and 5% had anosmia.
SARS-CoV-2 penetrates the cells through the ACE2 receptor, which is present throughout the body. “SARS-CoV-2 enters the body through the nasal cavity and it probably penetrates the nervous system in the periphery through afferent branches of the olfactory and trigeminal nerve,” Dr. Pozo-Rosich said. It travels to the lungs and, later, the bloodstream. This generates systemic inflammation that may turn into a cytokine storm. Evidence has identified cortical hyperintensities and olfactory bulb hyperintensities in patients with COVID-19, suggesting that the virus directly infects the CNS.
Interleukin-6, one of the main inflammatory molecules, has been proven to be related to COVID-19 and has become a therapeutic target. Levels of IL-6 may be lower and tend to be more stable in patients with both COVID-19 and headache than in patients with COVID-19 only.
The researchers observed 130 patients (51% women; mean age, 54 years) with COVID-19 who were attended by neurologists at Vall d’Hebron. In this group, 74.4% had headache. Patients with headache tended to be younger than those without headache (mean age, 50 years vs. 63 years, respectively) and tended to be women (58.6% vs. 29.4%).
Approximately one-third of patients with headache had a history of migraine. Most reported mild to moderate pain that resembled tension-type headache. In participants with severe pain and migraine-like features, headache more often began during the asymptomatic phase of COVID-19.
Disease evolution predictor?
The investigators followed up on 100 of the 130 patients with COVID-19, of whom 74 had headache. About 38% of these patients had ongoing headache after 6 weeks, which suggests that some patients may develop a new daily persistent headache once a 3-month period has elapsed. Half of this group had no previous headache history. Headache had been the prodromal symptom of COVID-19 for 21.4% of these patients.
Results showed that headache predicted the clinical evolution of COVID-19. The symptomatic phase of COVID-19 was 7 days shorter for patients with headache than for those without headache. In addition, the period of hospitalization was 7 days shorter for patients with headache and anosmia, compared with patients who had neither headache nor anosmia.
Most therapies, including ibuprofen, candesartan, and anti–calcitonin gene–related peptide (CGRP) monoclonal antibodies, are safe for treating headache in COVID-19, the investigators noted. “We should just try to initially avoid steroids to avoid interference with the body’s reaction to SARS-CoV-2,” Dr. Pozo-Rosich said.
Researchers at Sidney Kimmel Medical College, Philadelphia, are currently studying intranasal vazegepant, an anti-CGRP therapy, as a way to potentially blunt the severe inflammatory response in the lungs of patients with COVID-19, she noted, adding that this peptide may have a future role not only in headache, but also in COVID-19.
Historical link to viral infections
Commenting on the study, Matthew S. Robbins, MD, associate professor of neurology at Weill Cornell Medicine, New York, said the findings associating headache with a shorter symptomatic phase of COVID-19 were “interesting.”
“Headache is common with mild viral infections. More severe viral infections may simply feature more overwhelming respiratory symptoms and fever that lead to underreporting or underascertainment of headache,” said Dr. Robbins, who was not involved with the research.
He noted that the finding showing an association of headache and COVID-19 with a younger age and in women “may be related to a higher prevalence of migraine biology in such patients, and being triggered by the virus or the psychological stress associated with it.”
Dr. Robbins added that viral illnesses have long been associated with new daily persistent headache, “dating back to the early 1980s,” when it was first described in association with Epstein-Barr virus. These infections have also been implicated in the progression of migraine to chronic migraine in adolescents.
“In my view, treatment should be aimed at the symptomatic headache type for which new daily persistent headache resembles, regardless of the potential inciting factor,” Dr. Robbins said.
Dr. Pozo-Rosich has received consulting fees from Allergan, Amgen, Almirall, Biohaven, Chiesi, Eli Lilly, Medscape, Novartis, and Teva Pharmaceuticals. Dr. Robbins has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM AHS 2020
The plague of racism in our society
Here we are, faced with history in real time. A plague upon a plague. A new one and a longstanding one. COVID-19 and racial injustice. Both are plagues upon our medical house, and it’s time for some spring cleaning.
Initially, COVID-19 concerns brought news of an infection coming for anyone and everyone. Like the Black Death, it was supposedly “the great equalizer,” the “Triumph of Death,” regardless of station in life.
Yet, as the COVID-19 story unfurls, it is clear that minorities are disproportionately affected, just as they always seem to be. In Chicago, for example, African Americans make up 70% of the COVID-19 deaths, yet only 29% of the population. Similar results have been found in Milwaukee and Louisiana, and other parts of the country. As an article in the Journal of Law and the Biosciences indicated, “These racial and ethnic disparities in COVID-19 infections and deaths are a result of historical and current practices of racism that cause disparities in exposure, susceptibility and treatment.”
People of color are disproportionately affected, an outcome of racial health disparities. And these disparities are a public health crisis, sitting in the living room of our house. Disparities continue to exist in national infant mortality, maternal health, and deaths from premature heart disease and stroke. They exist in access to care and are playing out in real time during this pandemic.
COVID-19 and racial injustice, in addition to being sociological and economic crises, are both public health crises that are plaguing African American communities. Consider the case of police violence as a public health issue. Black males are three times more likely to be killed by police than are non-Hispanic white males.
This is a dying room in our medical house, with our patient lying alongside a history of medicine littered with racial injustice, telling us, “I can’t breathe.”
As professionals, we must run to that patient. Professionalism bears the pillars of our ethical principles of primacy of patient welfare, patient autonomy, and social justice. We work hard on the first two. Medical errors, quality improvement, communication, patient safety. All important. But we too often dance, or sit silently, about the third.
Here is an excerpt from “Moral choices for today’s physician”, by Donald M. Berwick, MD:
“The work of a physician as healer cannot stop at the door of an office, the threshold of an operating room, or the front gate of a hospital. The rescue of a society and the restoration of a political ethos that remembers to heal have become the physician’s jobs, too. Professional silence in the face of social injustice is wrong.”
It is chilling to see the great institutions of health care, hospitals, physician groups, and scientific bodies assume that the seat of bystander is available. That seat is gone. To try to avoid the political fray through silence is impossible because silence is now political. Either engage or assist the harm. There is no third choice.
Dr. Berwick echoed the words of Rev. Dr. Martin Luther King Jr., from 1959, in Birmingham, Ala.:
“If you fail to act now, history will have to record that the greatest tragedy of this period of social transition was not the strident clamor of the bad people, but the appalling silence of the good people.”
I have this space to write and speak up, and I urge many of you to do the same. Write to your local newspaper. Share your stories. Listen to others. Engage with your society. Create the space in your practice, your group, your hospital, your department for listening, learning, relearning, educating, and acting.
It’s not easy speaking up and speaking out. Yet, this is our foundation, our call, our professional obligation. We must remember George Floyd, Breonna Taylor, Sandra Bland, Eric Garner, Tamir Rice, Trayvon Martin, and too many others. To recognize the humanity behind the injustices, and to call out their names.
This is lesson 101 on the wards. It’s not the heart failure in bed 1 or the sepsis in bed 2, but the mother, brother, father, and sister who seek out just care. Humanity reaching out their hand, and we must grab it.
I came to medicine for the compassion, for the love, for the comforting hand offered to our patients. That compassion, by definition, requires action.
In his book “Altruism: The Power of Compassion to Change Yourself and the World,” Matthieu Ricard wrote “If compassion without wisdom is blind, compassion without action is hypocritical.”
Silence is inaction. Let’s act.
Dr. Messler is the executive director, quality initiatives, at Glytec and works as a hospitalist at Morton Plant Hospitalist group in Clearwater, Fla. This essay appeared first at SHM’s official blog The Hospital Leader.
Here we are, faced with history in real time. A plague upon a plague. A new one and a longstanding one. COVID-19 and racial injustice. Both are plagues upon our medical house, and it’s time for some spring cleaning.
Initially, COVID-19 concerns brought news of an infection coming for anyone and everyone. Like the Black Death, it was supposedly “the great equalizer,” the “Triumph of Death,” regardless of station in life.
Yet, as the COVID-19 story unfurls, it is clear that minorities are disproportionately affected, just as they always seem to be. In Chicago, for example, African Americans make up 70% of the COVID-19 deaths, yet only 29% of the population. Similar results have been found in Milwaukee and Louisiana, and other parts of the country. As an article in the Journal of Law and the Biosciences indicated, “These racial and ethnic disparities in COVID-19 infections and deaths are a result of historical and current practices of racism that cause disparities in exposure, susceptibility and treatment.”
People of color are disproportionately affected, an outcome of racial health disparities. And these disparities are a public health crisis, sitting in the living room of our house. Disparities continue to exist in national infant mortality, maternal health, and deaths from premature heart disease and stroke. They exist in access to care and are playing out in real time during this pandemic.
COVID-19 and racial injustice, in addition to being sociological and economic crises, are both public health crises that are plaguing African American communities. Consider the case of police violence as a public health issue. Black males are three times more likely to be killed by police than are non-Hispanic white males.
This is a dying room in our medical house, with our patient lying alongside a history of medicine littered with racial injustice, telling us, “I can’t breathe.”
As professionals, we must run to that patient. Professionalism bears the pillars of our ethical principles of primacy of patient welfare, patient autonomy, and social justice. We work hard on the first two. Medical errors, quality improvement, communication, patient safety. All important. But we too often dance, or sit silently, about the third.
Here is an excerpt from “Moral choices for today’s physician”, by Donald M. Berwick, MD:
“The work of a physician as healer cannot stop at the door of an office, the threshold of an operating room, or the front gate of a hospital. The rescue of a society and the restoration of a political ethos that remembers to heal have become the physician’s jobs, too. Professional silence in the face of social injustice is wrong.”
It is chilling to see the great institutions of health care, hospitals, physician groups, and scientific bodies assume that the seat of bystander is available. That seat is gone. To try to avoid the political fray through silence is impossible because silence is now political. Either engage or assist the harm. There is no third choice.
Dr. Berwick echoed the words of Rev. Dr. Martin Luther King Jr., from 1959, in Birmingham, Ala.:
“If you fail to act now, history will have to record that the greatest tragedy of this period of social transition was not the strident clamor of the bad people, but the appalling silence of the good people.”
I have this space to write and speak up, and I urge many of you to do the same. Write to your local newspaper. Share your stories. Listen to others. Engage with your society. Create the space in your practice, your group, your hospital, your department for listening, learning, relearning, educating, and acting.
It’s not easy speaking up and speaking out. Yet, this is our foundation, our call, our professional obligation. We must remember George Floyd, Breonna Taylor, Sandra Bland, Eric Garner, Tamir Rice, Trayvon Martin, and too many others. To recognize the humanity behind the injustices, and to call out their names.
This is lesson 101 on the wards. It’s not the heart failure in bed 1 or the sepsis in bed 2, but the mother, brother, father, and sister who seek out just care. Humanity reaching out their hand, and we must grab it.
I came to medicine for the compassion, for the love, for the comforting hand offered to our patients. That compassion, by definition, requires action.
In his book “Altruism: The Power of Compassion to Change Yourself and the World,” Matthieu Ricard wrote “If compassion without wisdom is blind, compassion without action is hypocritical.”
Silence is inaction. Let’s act.
Dr. Messler is the executive director, quality initiatives, at Glytec and works as a hospitalist at Morton Plant Hospitalist group in Clearwater, Fla. This essay appeared first at SHM’s official blog The Hospital Leader.
Here we are, faced with history in real time. A plague upon a plague. A new one and a longstanding one. COVID-19 and racial injustice. Both are plagues upon our medical house, and it’s time for some spring cleaning.
Initially, COVID-19 concerns brought news of an infection coming for anyone and everyone. Like the Black Death, it was supposedly “the great equalizer,” the “Triumph of Death,” regardless of station in life.
Yet, as the COVID-19 story unfurls, it is clear that minorities are disproportionately affected, just as they always seem to be. In Chicago, for example, African Americans make up 70% of the COVID-19 deaths, yet only 29% of the population. Similar results have been found in Milwaukee and Louisiana, and other parts of the country. As an article in the Journal of Law and the Biosciences indicated, “These racial and ethnic disparities in COVID-19 infections and deaths are a result of historical and current practices of racism that cause disparities in exposure, susceptibility and treatment.”
People of color are disproportionately affected, an outcome of racial health disparities. And these disparities are a public health crisis, sitting in the living room of our house. Disparities continue to exist in national infant mortality, maternal health, and deaths from premature heart disease and stroke. They exist in access to care and are playing out in real time during this pandemic.
COVID-19 and racial injustice, in addition to being sociological and economic crises, are both public health crises that are plaguing African American communities. Consider the case of police violence as a public health issue. Black males are three times more likely to be killed by police than are non-Hispanic white males.
This is a dying room in our medical house, with our patient lying alongside a history of medicine littered with racial injustice, telling us, “I can’t breathe.”
As professionals, we must run to that patient. Professionalism bears the pillars of our ethical principles of primacy of patient welfare, patient autonomy, and social justice. We work hard on the first two. Medical errors, quality improvement, communication, patient safety. All important. But we too often dance, or sit silently, about the third.
Here is an excerpt from “Moral choices for today’s physician”, by Donald M. Berwick, MD:
“The work of a physician as healer cannot stop at the door of an office, the threshold of an operating room, or the front gate of a hospital. The rescue of a society and the restoration of a political ethos that remembers to heal have become the physician’s jobs, too. Professional silence in the face of social injustice is wrong.”
It is chilling to see the great institutions of health care, hospitals, physician groups, and scientific bodies assume that the seat of bystander is available. That seat is gone. To try to avoid the political fray through silence is impossible because silence is now political. Either engage or assist the harm. There is no third choice.
Dr. Berwick echoed the words of Rev. Dr. Martin Luther King Jr., from 1959, in Birmingham, Ala.:
“If you fail to act now, history will have to record that the greatest tragedy of this period of social transition was not the strident clamor of the bad people, but the appalling silence of the good people.”
I have this space to write and speak up, and I urge many of you to do the same. Write to your local newspaper. Share your stories. Listen to others. Engage with your society. Create the space in your practice, your group, your hospital, your department for listening, learning, relearning, educating, and acting.
It’s not easy speaking up and speaking out. Yet, this is our foundation, our call, our professional obligation. We must remember George Floyd, Breonna Taylor, Sandra Bland, Eric Garner, Tamir Rice, Trayvon Martin, and too many others. To recognize the humanity behind the injustices, and to call out their names.
This is lesson 101 on the wards. It’s not the heart failure in bed 1 or the sepsis in bed 2, but the mother, brother, father, and sister who seek out just care. Humanity reaching out their hand, and we must grab it.
I came to medicine for the compassion, for the love, for the comforting hand offered to our patients. That compassion, by definition, requires action.
In his book “Altruism: The Power of Compassion to Change Yourself and the World,” Matthieu Ricard wrote “If compassion without wisdom is blind, compassion without action is hypocritical.”
Silence is inaction. Let’s act.
Dr. Messler is the executive director, quality initiatives, at Glytec and works as a hospitalist at Morton Plant Hospitalist group in Clearwater, Fla. This essay appeared first at SHM’s official blog The Hospital Leader.
Highlights in Non–Small Cell Lung Cancer From ASCO 2020
Presented during the ASCO 2020 plenary session, the results of the phase 3 ADAURA trial will prove practice-changing, according to Dr. Mark Kris of Memorial Sloan Kettering Cancer Center. Over 600 patients whose resected tumors were found to have EGFR mutations were treated with osimertinib. The results more than doubled disease-free survival rates, from 44% to 90% at 2 years.
Among other adjuvant trials, the phase 2 VISION study looked at tepotinib, a once-daily, highly selective oral MET inhibitor. The study showed durable responses coupled with acceptable side effects. The drug has been given fast-track status by the US Food and Drug Administration.
Dr. Kris notes that the DESTINY study introduces trastuzumab deruxtecan, an antibody-drug conjugate, as a promising new class of drugs for lung cancer patients. Interim results presented at ASCO further support the HER2 mutation as another potential target for patients with lung cancer.
Finally, the phase 2 CITYSCAPE study provides preliminary evidence for a new checkpoint inhibitor. The monoclonal antibody tiragolumab was developed to block TIGIT. The study showed that the combination of tiragolumab and atezolizumab can improve both rates of response and time to disease recurrence — results Dr. Kris considers encouraging for patients with advanced lung cancer.
Mark G. Kris, MD
Mark G. Kris, MD, Professor, Department of Medicine, Weill Cornell Medical College; Attending Physician, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY.
Presented during the ASCO 2020 plenary session, the results of the phase 3 ADAURA trial will prove practice-changing, according to Dr. Mark Kris of Memorial Sloan Kettering Cancer Center. Over 600 patients whose resected tumors were found to have EGFR mutations were treated with osimertinib. The results more than doubled disease-free survival rates, from 44% to 90% at 2 years.
Among other adjuvant trials, the phase 2 VISION study looked at tepotinib, a once-daily, highly selective oral MET inhibitor. The study showed durable responses coupled with acceptable side effects. The drug has been given fast-track status by the US Food and Drug Administration.
Dr. Kris notes that the DESTINY study introduces trastuzumab deruxtecan, an antibody-drug conjugate, as a promising new class of drugs for lung cancer patients. Interim results presented at ASCO further support the HER2 mutation as another potential target for patients with lung cancer.
Finally, the phase 2 CITYSCAPE study provides preliminary evidence for a new checkpoint inhibitor. The monoclonal antibody tiragolumab was developed to block TIGIT. The study showed that the combination of tiragolumab and atezolizumab can improve both rates of response and time to disease recurrence — results Dr. Kris considers encouraging for patients with advanced lung cancer.
Mark G. Kris, MD
Mark G. Kris, MD, Professor, Department of Medicine, Weill Cornell Medical College; Attending Physician, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY.
Presented during the ASCO 2020 plenary session, the results of the phase 3 ADAURA trial will prove practice-changing, according to Dr. Mark Kris of Memorial Sloan Kettering Cancer Center. Over 600 patients whose resected tumors were found to have EGFR mutations were treated with osimertinib. The results more than doubled disease-free survival rates, from 44% to 90% at 2 years.
Among other adjuvant trials, the phase 2 VISION study looked at tepotinib, a once-daily, highly selective oral MET inhibitor. The study showed durable responses coupled with acceptable side effects. The drug has been given fast-track status by the US Food and Drug Administration.
Dr. Kris notes that the DESTINY study introduces trastuzumab deruxtecan, an antibody-drug conjugate, as a promising new class of drugs for lung cancer patients. Interim results presented at ASCO further support the HER2 mutation as another potential target for patients with lung cancer.
Finally, the phase 2 CITYSCAPE study provides preliminary evidence for a new checkpoint inhibitor. The monoclonal antibody tiragolumab was developed to block TIGIT. The study showed that the combination of tiragolumab and atezolizumab can improve both rates of response and time to disease recurrence — results Dr. Kris considers encouraging for patients with advanced lung cancer.
Mark G. Kris, MD
Mark G. Kris, MD, Professor, Department of Medicine, Weill Cornell Medical College; Attending Physician, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY.

Lipid-lowering bempedoic acid does not hasten or worsen diabetes
In an analysis of four phase 3 trials, the oral lipid-lowering drug bempedoic acid (Nexletol; Esperion) did not worsen glycemic control or increase the incidence of type 2 diabetes.
As previously reported, this first-in-class drug, which acts by inhibiting ATP-citrate lyase, was approved by the Food and Drug Administration in February 2020.
Lawrence A. Leiter MD, from the University of Toronto, delivered the findings of this latest analysis in an oral presentation at the virtual American Diabetes Association 80th Scientific Sessions.
“The current study is important as it shows overall consistent efficacy and safety regardless of glycemic status and no increase in new-onset diabetes,” Dr. Leiter said in an interview.
There is interest in how lipid-lowering drugs might affect glycemia because “meta-analyses have shown about a 10% increased risk of new-onset diabetes in statin users, although the absolute increased risk is 1 extra case per 255 treated patients [in whom one would expect 5.4 cardiovascular events to be prevented by the statin],” he noted.
In a comment, John R. Guyton, MD, from Duke University Medical Center, Durham, N.C., agreed that the new study demonstrates that “patients with diabetes and prediabetes respond to bempedoic acid with LDL cholesterol lowering that is similar to that in patients with normal glucose tolerance.”
Although “statins have a slight effect of worsening glucose tolerance and a modest effect of increasing cases of new-onset diabetes,” the current research shows that “bempedoic acid appears to be free of these effects,” said Dr. Guyton, who discussed this drug in another symposium at the meeting where he also discussed how the agent will “fit” into prescribing patterns.
How do patients with diabetes, prediabetes fare?
“Current guidelines support aggressive LDL cholesterol lowering in patients with diabetes, given the increased risk of cardiovascular morbidity and mortality,” said Dr. Leiter.
Bempedoic acid was approved as an adjunct to diet and maximally tolerated statin therapy to treat adults with atherosclerotic cardiovascular disease (ASCVD) and/or heterozygous familial hypercholesterolemia (HeFH) who require additional lowering of LDL cholesterol, although its effect on cardiovascular morbidity and mortality has not been determined, the prescribing information states.
However, it has been unknown how bempedoic acid affects LDL cholesterol or hemoglobin A1c levels in patients with diabetes, prediabetes, or normoglycemia.
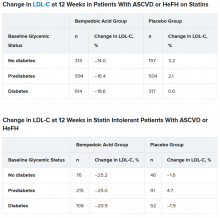
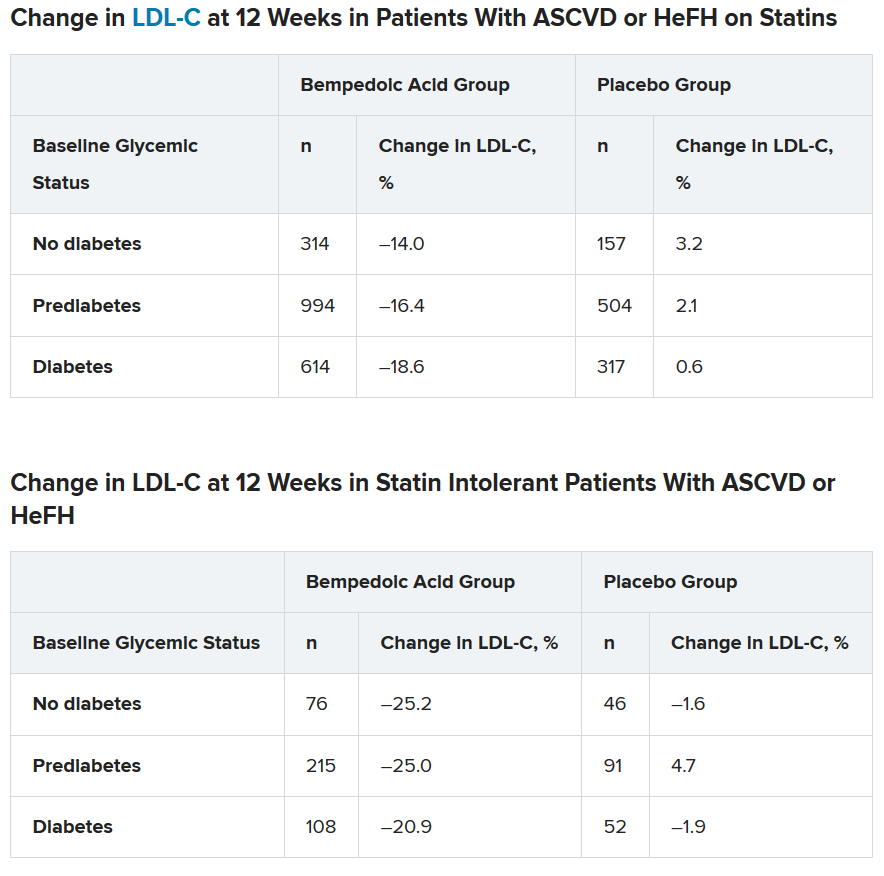
To examine this, the researchers pooled data from four phase 3 trials in 3623 patients with ASCVD or HeFH who had been randomized 2:1 to bempedoic acid 180 mg/day or placebo for 12 or 24 weeks (if they were statin intolerant) or 52 weeks (if they were also on statins).
In the pooled sample, about half the patients had prediabetes (52%), and the rest had diabetes (31%) or normoglycemia (17%). Overall, 75%-84% of patients had a history of ASCVD.
Mean LDL cholesterol levels were higher in patients with normoglycemia (119 mg/dL) or prediabetes (115 mg/dL) than in patients with diabetes (110 mg/dL).
The primary outcome was percent change in LDL cholesterol from baseline to week 12.
In the two types of patients (all with ASCVD or HeFH) – those on statins and those with statin intolerance – LDL cholesterol at 12 weeks was significantly lower in patients who received bempedoic acid, compared with placebo, regardless of whether they had no diabetes, prediabetes, or diabetes (all P < .001).
Similarly, patients who received bempedoic acid also had significant reductions in total cholesterol, non–HDL cholesterol, apolipoprotein B, and high-sensitivity C-reactive protein (hsCRP) at 12 weeks, compared with patients who received placebo (all P < .01).
The safety profile of bempedoic acid was similar to placebo and did not vary by glycemic status.
“Of course, with any lipid-lowering therapy, there’s lots of interest in changes in glycemic parameters,” said Dr. Leiter. “A1c did not increase. In fact, it was significantly lower in patients with prediabetes and diabetes on bempedoic acid versus placebo.”
In addition, “statin trials have shown small increases in body weight. We did not observe this,” he reported.
Where does bempedoic acid ‘fit?’
“Bempedoic acid will be a useful add-on to any patient who requires additional LDL cholesterol lowering,” according to Dr. Leiter. “It will typically be used as an add-on to statins, but will also be very useful in the statin-intolerant patient, especially when used in combination with ezetimib.”
The fixed-dose combination of bempedoic acid plus ezetimibe (Nexlizet; Esperion), was also approved in the United States in February, just days after bempedoic acid as a solo agent was cleared for marketing.
“Bempedoic acid would not be chosen in preference to a statin, ezetimibe, or PCSK9 inhibitor,” Dr. Guyton said. Rather, “its chief use will be in patients with statin intolerance and either FH or ASCVD when LDL-cholesterol is poorly controlled despite maximum tolerated lipid-lowering therapy.”
According to Dr. Guyton, “use of bempedoic acid should be undertaken only when provider-patient discussion acknowledges that it has not been shown to reduce cardiovascular events, although preliminary evidence from genetic analysis [Mendelian randomization study] suggests that it will,” as previously reported.
The CLEAR Outcomes cardiovascular outcomes trial of bempedoic acid completed enrollment in August 2019, involving 14,032 patients with hypercholesterolemia and high CVD risk according to a company statement.
The study was funded by Esperion. Dr. Leiter has reported being on advisory panels for Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, HLS Therapeutics, Janssen, Merck, Novo Nordisk, Sanofi, and Servier, receiving research support from Amgen, AstraZeneca, Kowa Pharmaceuticals, and the Medicines Company, and being on speakers bureaus for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, HLS Therapeutics, Janssen, Medscape, Merck, Novo Nordisk, Sanofi, and Servier. Disclosures for the other authors are listed with the abstract. Dr. Guyton has reported being a consultant for Amarin and receiving research support form Regeneron.
A version of this article originally appeared on Medscape.com.
In an analysis of four phase 3 trials, the oral lipid-lowering drug bempedoic acid (Nexletol; Esperion) did not worsen glycemic control or increase the incidence of type 2 diabetes.
As previously reported, this first-in-class drug, which acts by inhibiting ATP-citrate lyase, was approved by the Food and Drug Administration in February 2020.
Lawrence A. Leiter MD, from the University of Toronto, delivered the findings of this latest analysis in an oral presentation at the virtual American Diabetes Association 80th Scientific Sessions.
“The current study is important as it shows overall consistent efficacy and safety regardless of glycemic status and no increase in new-onset diabetes,” Dr. Leiter said in an interview.
There is interest in how lipid-lowering drugs might affect glycemia because “meta-analyses have shown about a 10% increased risk of new-onset diabetes in statin users, although the absolute increased risk is 1 extra case per 255 treated patients [in whom one would expect 5.4 cardiovascular events to be prevented by the statin],” he noted.
In a comment, John R. Guyton, MD, from Duke University Medical Center, Durham, N.C., agreed that the new study demonstrates that “patients with diabetes and prediabetes respond to bempedoic acid with LDL cholesterol lowering that is similar to that in patients with normal glucose tolerance.”
Although “statins have a slight effect of worsening glucose tolerance and a modest effect of increasing cases of new-onset diabetes,” the current research shows that “bempedoic acid appears to be free of these effects,” said Dr. Guyton, who discussed this drug in another symposium at the meeting where he also discussed how the agent will “fit” into prescribing patterns.
How do patients with diabetes, prediabetes fare?
“Current guidelines support aggressive LDL cholesterol lowering in patients with diabetes, given the increased risk of cardiovascular morbidity and mortality,” said Dr. Leiter.
Bempedoic acid was approved as an adjunct to diet and maximally tolerated statin therapy to treat adults with atherosclerotic cardiovascular disease (ASCVD) and/or heterozygous familial hypercholesterolemia (HeFH) who require additional lowering of LDL cholesterol, although its effect on cardiovascular morbidity and mortality has not been determined, the prescribing information states.
However, it has been unknown how bempedoic acid affects LDL cholesterol or hemoglobin A1c levels in patients with diabetes, prediabetes, or normoglycemia.
To examine this, the researchers pooled data from four phase 3 trials in 3623 patients with ASCVD or HeFH who had been randomized 2:1 to bempedoic acid 180 mg/day or placebo for 12 or 24 weeks (if they were statin intolerant) or 52 weeks (if they were also on statins).
In the pooled sample, about half the patients had prediabetes (52%), and the rest had diabetes (31%) or normoglycemia (17%). Overall, 75%-84% of patients had a history of ASCVD.
Mean LDL cholesterol levels were higher in patients with normoglycemia (119 mg/dL) or prediabetes (115 mg/dL) than in patients with diabetes (110 mg/dL).
The primary outcome was percent change in LDL cholesterol from baseline to week 12.
In the two types of patients (all with ASCVD or HeFH) – those on statins and those with statin intolerance – LDL cholesterol at 12 weeks was significantly lower in patients who received bempedoic acid, compared with placebo, regardless of whether they had no diabetes, prediabetes, or diabetes (all P < .001).
Similarly, patients who received bempedoic acid also had significant reductions in total cholesterol, non–HDL cholesterol, apolipoprotein B, and high-sensitivity C-reactive protein (hsCRP) at 12 weeks, compared with patients who received placebo (all P < .01).
The safety profile of bempedoic acid was similar to placebo and did not vary by glycemic status.
“Of course, with any lipid-lowering therapy, there’s lots of interest in changes in glycemic parameters,” said Dr. Leiter. “A1c did not increase. In fact, it was significantly lower in patients with prediabetes and diabetes on bempedoic acid versus placebo.”
In addition, “statin trials have shown small increases in body weight. We did not observe this,” he reported.
Where does bempedoic acid ‘fit?’
“Bempedoic acid will be a useful add-on to any patient who requires additional LDL cholesterol lowering,” according to Dr. Leiter. “It will typically be used as an add-on to statins, but will also be very useful in the statin-intolerant patient, especially when used in combination with ezetimib.”
The fixed-dose combination of bempedoic acid plus ezetimibe (Nexlizet; Esperion), was also approved in the United States in February, just days after bempedoic acid as a solo agent was cleared for marketing.
“Bempedoic acid would not be chosen in preference to a statin, ezetimibe, or PCSK9 inhibitor,” Dr. Guyton said. Rather, “its chief use will be in patients with statin intolerance and either FH or ASCVD when LDL-cholesterol is poorly controlled despite maximum tolerated lipid-lowering therapy.”
According to Dr. Guyton, “use of bempedoic acid should be undertaken only when provider-patient discussion acknowledges that it has not been shown to reduce cardiovascular events, although preliminary evidence from genetic analysis [Mendelian randomization study] suggests that it will,” as previously reported.
The CLEAR Outcomes cardiovascular outcomes trial of bempedoic acid completed enrollment in August 2019, involving 14,032 patients with hypercholesterolemia and high CVD risk according to a company statement.
The study was funded by Esperion. Dr. Leiter has reported being on advisory panels for Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, HLS Therapeutics, Janssen, Merck, Novo Nordisk, Sanofi, and Servier, receiving research support from Amgen, AstraZeneca, Kowa Pharmaceuticals, and the Medicines Company, and being on speakers bureaus for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, HLS Therapeutics, Janssen, Medscape, Merck, Novo Nordisk, Sanofi, and Servier. Disclosures for the other authors are listed with the abstract. Dr. Guyton has reported being a consultant for Amarin and receiving research support form Regeneron.
A version of this article originally appeared on Medscape.com.
In an analysis of four phase 3 trials, the oral lipid-lowering drug bempedoic acid (Nexletol; Esperion) did not worsen glycemic control or increase the incidence of type 2 diabetes.
As previously reported, this first-in-class drug, which acts by inhibiting ATP-citrate lyase, was approved by the Food and Drug Administration in February 2020.
Lawrence A. Leiter MD, from the University of Toronto, delivered the findings of this latest analysis in an oral presentation at the virtual American Diabetes Association 80th Scientific Sessions.
“The current study is important as it shows overall consistent efficacy and safety regardless of glycemic status and no increase in new-onset diabetes,” Dr. Leiter said in an interview.
There is interest in how lipid-lowering drugs might affect glycemia because “meta-analyses have shown about a 10% increased risk of new-onset diabetes in statin users, although the absolute increased risk is 1 extra case per 255 treated patients [in whom one would expect 5.4 cardiovascular events to be prevented by the statin],” he noted.
In a comment, John R. Guyton, MD, from Duke University Medical Center, Durham, N.C., agreed that the new study demonstrates that “patients with diabetes and prediabetes respond to bempedoic acid with LDL cholesterol lowering that is similar to that in patients with normal glucose tolerance.”
Although “statins have a slight effect of worsening glucose tolerance and a modest effect of increasing cases of new-onset diabetes,” the current research shows that “bempedoic acid appears to be free of these effects,” said Dr. Guyton, who discussed this drug in another symposium at the meeting where he also discussed how the agent will “fit” into prescribing patterns.
How do patients with diabetes, prediabetes fare?
“Current guidelines support aggressive LDL cholesterol lowering in patients with diabetes, given the increased risk of cardiovascular morbidity and mortality,” said Dr. Leiter.
Bempedoic acid was approved as an adjunct to diet and maximally tolerated statin therapy to treat adults with atherosclerotic cardiovascular disease (ASCVD) and/or heterozygous familial hypercholesterolemia (HeFH) who require additional lowering of LDL cholesterol, although its effect on cardiovascular morbidity and mortality has not been determined, the prescribing information states.
However, it has been unknown how bempedoic acid affects LDL cholesterol or hemoglobin A1c levels in patients with diabetes, prediabetes, or normoglycemia.
To examine this, the researchers pooled data from four phase 3 trials in 3623 patients with ASCVD or HeFH who had been randomized 2:1 to bempedoic acid 180 mg/day or placebo for 12 or 24 weeks (if they were statin intolerant) or 52 weeks (if they were also on statins).
In the pooled sample, about half the patients had prediabetes (52%), and the rest had diabetes (31%) or normoglycemia (17%). Overall, 75%-84% of patients had a history of ASCVD.
Mean LDL cholesterol levels were higher in patients with normoglycemia (119 mg/dL) or prediabetes (115 mg/dL) than in patients with diabetes (110 mg/dL).
The primary outcome was percent change in LDL cholesterol from baseline to week 12.
In the two types of patients (all with ASCVD or HeFH) – those on statins and those with statin intolerance – LDL cholesterol at 12 weeks was significantly lower in patients who received bempedoic acid, compared with placebo, regardless of whether they had no diabetes, prediabetes, or diabetes (all P < .001).
Similarly, patients who received bempedoic acid also had significant reductions in total cholesterol, non–HDL cholesterol, apolipoprotein B, and high-sensitivity C-reactive protein (hsCRP) at 12 weeks, compared with patients who received placebo (all P < .01).
The safety profile of bempedoic acid was similar to placebo and did not vary by glycemic status.
“Of course, with any lipid-lowering therapy, there’s lots of interest in changes in glycemic parameters,” said Dr. Leiter. “A1c did not increase. In fact, it was significantly lower in patients with prediabetes and diabetes on bempedoic acid versus placebo.”
In addition, “statin trials have shown small increases in body weight. We did not observe this,” he reported.
Where does bempedoic acid ‘fit?’
“Bempedoic acid will be a useful add-on to any patient who requires additional LDL cholesterol lowering,” according to Dr. Leiter. “It will typically be used as an add-on to statins, but will also be very useful in the statin-intolerant patient, especially when used in combination with ezetimib.”
The fixed-dose combination of bempedoic acid plus ezetimibe (Nexlizet; Esperion), was also approved in the United States in February, just days after bempedoic acid as a solo agent was cleared for marketing.
“Bempedoic acid would not be chosen in preference to a statin, ezetimibe, or PCSK9 inhibitor,” Dr. Guyton said. Rather, “its chief use will be in patients with statin intolerance and either FH or ASCVD when LDL-cholesterol is poorly controlled despite maximum tolerated lipid-lowering therapy.”
According to Dr. Guyton, “use of bempedoic acid should be undertaken only when provider-patient discussion acknowledges that it has not been shown to reduce cardiovascular events, although preliminary evidence from genetic analysis [Mendelian randomization study] suggests that it will,” as previously reported.
The CLEAR Outcomes cardiovascular outcomes trial of bempedoic acid completed enrollment in August 2019, involving 14,032 patients with hypercholesterolemia and high CVD risk according to a company statement.
The study was funded by Esperion. Dr. Leiter has reported being on advisory panels for Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, HLS Therapeutics, Janssen, Merck, Novo Nordisk, Sanofi, and Servier, receiving research support from Amgen, AstraZeneca, Kowa Pharmaceuticals, and the Medicines Company, and being on speakers bureaus for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, HLS Therapeutics, Janssen, Medscape, Merck, Novo Nordisk, Sanofi, and Servier. Disclosures for the other authors are listed with the abstract. Dr. Guyton has reported being a consultant for Amarin and receiving research support form Regeneron.
A version of this article originally appeared on Medscape.com.
Lyumjev ultra-rapid-acting insulin gets FDA nod
The US Food and Drug Administration has approved rapid-acting insulin lispro-aabc injection 100 and 200 units/mL (Lyumjev, Eli Lilly) for the treatment of adults with type 1 and type 2 diabetes.
The product is a novel formulation of insulin lispro developed to speed absorption of insulin into the bloodstream. It will be available in two strengths: U-100 (100 units/mL) and U-200 (200 units/mL). The Lyumjev U-200 prefilled pen contains twice as much insulin per 1 mL as standard (U-100) insulin.
Approval was based on data from two phase 3 randomized, active-controlled, treat-to-target studies comparing lispro-aabc with insulin lispro injection 100 units/mL (Humalog, Lilly) in people with type 1 diabetes (PRONTO-T1D) and type 2 diabetes (PRONTO-T2D).
In both studies, noninferiority in A1c reduction was demonstrated when the two insulins were dosed at mealtime, but lispro-aabc showed superior blood glucose reduction at 1-hour and 2-hours post-meal compared with lispro.
Lyumjev is approved only in the United States for use as part of a multiple daily injection regimen, not for use in insulin pumps. Lilly intends to submit for this latter indication later in 2020.
Lyumjev will compete with Novo Nordisk’s fast-acting insulin aspart injection 100 units/mL (Fiasp).
Fiasp had a big head start: It was approved for use in adults in the United States in September 2017, for use in insulin pumps in October 2019, and for use in children with diabetes in January 2020.
However, in a poster presented at the American Diabetes Association 79th Scientific Sessions in 2019, lispro-aabb demonstrated faster insulin absorption than lispro, insulin aspart (Novolog/Novorapid, Novo Nordisk), or Fiasp.
Early half-maximal drug concentration was reached at 13 minutes with lispro-aabb, compared with 19 minutes with faster aspart and 25-27 minutes with the two conventional insulins (P < .05 for lispro-aabb vs other insulins).
Insulin lispro-aabc was approved in the European Union and Japan in March 2020.
Lilly is currently working to make Lyumjev available to adults with diabetes in the United States as quickly as possible and says it will be included in the Lilly Insulin Value Program, allowing anyone with commercial insurance and those without insurance to fill their monthly prescription of Lyumjev for $35.
The list price of Lyumjev will be the same as the list price for Humalog, it adds.
This article first appeared on Medscape.com.
The US Food and Drug Administration has approved rapid-acting insulin lispro-aabc injection 100 and 200 units/mL (Lyumjev, Eli Lilly) for the treatment of adults with type 1 and type 2 diabetes.
The product is a novel formulation of insulin lispro developed to speed absorption of insulin into the bloodstream. It will be available in two strengths: U-100 (100 units/mL) and U-200 (200 units/mL). The Lyumjev U-200 prefilled pen contains twice as much insulin per 1 mL as standard (U-100) insulin.
Approval was based on data from two phase 3 randomized, active-controlled, treat-to-target studies comparing lispro-aabc with insulin lispro injection 100 units/mL (Humalog, Lilly) in people with type 1 diabetes (PRONTO-T1D) and type 2 diabetes (PRONTO-T2D).
In both studies, noninferiority in A1c reduction was demonstrated when the two insulins were dosed at mealtime, but lispro-aabc showed superior blood glucose reduction at 1-hour and 2-hours post-meal compared with lispro.
Lyumjev is approved only in the United States for use as part of a multiple daily injection regimen, not for use in insulin pumps. Lilly intends to submit for this latter indication later in 2020.
Lyumjev will compete with Novo Nordisk’s fast-acting insulin aspart injection 100 units/mL (Fiasp).
Fiasp had a big head start: It was approved for use in adults in the United States in September 2017, for use in insulin pumps in October 2019, and for use in children with diabetes in January 2020.
However, in a poster presented at the American Diabetes Association 79th Scientific Sessions in 2019, lispro-aabb demonstrated faster insulin absorption than lispro, insulin aspart (Novolog/Novorapid, Novo Nordisk), or Fiasp.
Early half-maximal drug concentration was reached at 13 minutes with lispro-aabb, compared with 19 minutes with faster aspart and 25-27 minutes with the two conventional insulins (P < .05 for lispro-aabb vs other insulins).
Insulin lispro-aabc was approved in the European Union and Japan in March 2020.
Lilly is currently working to make Lyumjev available to adults with diabetes in the United States as quickly as possible and says it will be included in the Lilly Insulin Value Program, allowing anyone with commercial insurance and those without insurance to fill their monthly prescription of Lyumjev for $35.
The list price of Lyumjev will be the same as the list price for Humalog, it adds.
This article first appeared on Medscape.com.
The US Food and Drug Administration has approved rapid-acting insulin lispro-aabc injection 100 and 200 units/mL (Lyumjev, Eli Lilly) for the treatment of adults with type 1 and type 2 diabetes.
The product is a novel formulation of insulin lispro developed to speed absorption of insulin into the bloodstream. It will be available in two strengths: U-100 (100 units/mL) and U-200 (200 units/mL). The Lyumjev U-200 prefilled pen contains twice as much insulin per 1 mL as standard (U-100) insulin.
Approval was based on data from two phase 3 randomized, active-controlled, treat-to-target studies comparing lispro-aabc with insulin lispro injection 100 units/mL (Humalog, Lilly) in people with type 1 diabetes (PRONTO-T1D) and type 2 diabetes (PRONTO-T2D).
In both studies, noninferiority in A1c reduction was demonstrated when the two insulins were dosed at mealtime, but lispro-aabc showed superior blood glucose reduction at 1-hour and 2-hours post-meal compared with lispro.
Lyumjev is approved only in the United States for use as part of a multiple daily injection regimen, not for use in insulin pumps. Lilly intends to submit for this latter indication later in 2020.
Lyumjev will compete with Novo Nordisk’s fast-acting insulin aspart injection 100 units/mL (Fiasp).
Fiasp had a big head start: It was approved for use in adults in the United States in September 2017, for use in insulin pumps in October 2019, and for use in children with diabetes in January 2020.
However, in a poster presented at the American Diabetes Association 79th Scientific Sessions in 2019, lispro-aabb demonstrated faster insulin absorption than lispro, insulin aspart (Novolog/Novorapid, Novo Nordisk), or Fiasp.
Early half-maximal drug concentration was reached at 13 minutes with lispro-aabb, compared with 19 minutes with faster aspart and 25-27 minutes with the two conventional insulins (P < .05 for lispro-aabb vs other insulins).
Insulin lispro-aabc was approved in the European Union and Japan in March 2020.
Lilly is currently working to make Lyumjev available to adults with diabetes in the United States as quickly as possible and says it will be included in the Lilly Insulin Value Program, allowing anyone with commercial insurance and those without insurance to fill their monthly prescription of Lyumjev for $35.
The list price of Lyumjev will be the same as the list price for Humalog, it adds.
This article first appeared on Medscape.com.
Where does dexamethasone fit in with diabetic ketoacidosis in COVID-19?
A new article in the Journal of Clinical Endocrinology & Metabolism (JCEM) addresses unique concerns and considerations regarding diabetic ketoacidosis (DKA) in the setting of COVID-19.
Corresponding author Marie E. McDonnell, MD, director of the diabetes program at Brigham and Women’s Hospital, Boston, Massachusetts, discussed the recommendations with Medscape Medical News and also spoke about the news this week that the corticosteroid dexamethasone reduced death rates in severely ill patients with COVID-19.
The full JCEM article, by lead author Nadine E. Palermo, DO, Division of Endocrinology, Diabetes, and Hypertension, also at Brigham and Women’s Hospital, covers DKA diagnosis and triage, and emphasizes that usual hospital protocols for DKA management may need to be adjusted during COVID-19 to help preserve personal protective equipment and ICU beds.
“Hospitals and clinicians need to be able to quickly identify and manage DKA in COVID patients to save lives. This involves determining the options for management, including when less intensive subcutaneous insulin is indicated, and understanding how to guide patients on avoiding this serious complication,” McDonnell said in an Endocrine Society statement.
What about dexamethasone for severe COVID-19 in diabetes?
The new article briefly touches on the fact that upward adjustments to intensive intravenous insulin therapy for DKA may be necessary in patients with COVID-19 who are receiving concomitant corticosteroids or vasopressors.
But it was written prior to the June 16 announcement of the “RECOVERY” trial results with dexamethasone. The UK National Health Service immediately approved the drug’s use in the COVID-19 setting, despite the fact that there has been no published article on the findings yet.
McDonnell told Medscape Medical News that she would need to see formal results to better understand exactly which patients were studied and which ones benefited.
“The peer review will be critical. It looks as if it only benefits people who need respiratory support, but I want to understand that in much more detail,” she said. “If they all had acute respiratory distress syndrome [ARDS],” that’s different.
“There are already some data supporting steroid use in ARDS,” she noted, but added that not all of it suggests benefit.
She pointed to one of several studies now showing that diabetes, and hyperglycemia among people without a prior diabetes diagnosis, are both strong predictors of mortality in hospitalized patients with COVID-19.
“There was a very clear relationship between hyperglycemia and outcomes. We really shouldn’t put people at risk until we have clear data,” she said.
If, once the data are reviewed and appropriate dexamethasone becomes an established treatment for severe COVID-19, hyperglycemia would be a concern among all patients, not just those with previously diagnosed diabetes, she noted.
“We know a good number of people with prediabetes develop hyperglycemia when put on steroids. They can push people over the edge. We’re not going to miss anybody, but treating steroid-induced hyperglycemia is really hard,” McDonnell explained.
She also recommended 2014 guidance from Diabetes UK and the Association of British Clinical Diabetologists, which addresses management of inpatient steroid-induced DKA in patients with and without pre-existing diabetes.
Another major concern, she said, is “patients trying to get dexamethasone when they start to get sick” because this is not the right population to use this agent.
“We worry about people who do not need this drug. If they have diabetes, they put themselves at risk of hyperglycemia, which then increases the risk of severe COVID-19. And then they’re also putting themselves at risk of DKA. It would just be bad medicine,” she said.
Managing DKA in the face of COVID-19: Flexibility is key
In the JCEM article, Palermo and colleagues emphasize that the usual hospital protocols for DKA management may need to be adjusted during COVID-19 in the interest of reducing transmission risk and preserving scare resources.
They provide evidence for alternative treatment strategies, such as the use of subcutaneous rather than intravenous insulin when appropriate.
“We wanted to outline when exactly you should consider nonintensive management strategies for DKA,” McDonnell further explained to Medscape Medical News.
“That would include those with mild or some with moderate DKA. ... The idea is to remind our colleagues about that because hospitals tend to operate on a protocol-driven algorithmic methodology, they can forget to step off the usual care pathway even if evidence supports that,” she said.
But on the other hand, she also said that, in some very complex or severely ill patients with COVID-19, classical intravenous insulin therapy makes the most sense even if their DKA is mild.
The outpatient setting: Prevention and preparation
The new article also addresses several concerns regarding DKA prevention in the outpatient setting.
As with other guidelines, it includes a reminder that patients with diabetes should be advised to discontinue sodium-glucose cotransporter 2 (SGLT2) inhibitors if they become ill with COVID-19, especially if they’re not eating or drinking normally, because they raise the risk for DKA.
Also, for patients with type 1 diabetes, particularly those with a history of repeated DKA, “this is the time to make sure we reach out to patients to refill their insulin prescriptions and address issues related to cost and other access difficulties,” McDonnell said.
The authors also emphasize that insulin starts and education should not be postponed during the pandemic. “Patients identified as meeting criteria to start insulin should be referred for urgent education, either in person or, whenever possible and practical, via video teleconferencing,” they urge.
McDonnell has reported receiving research funding from Novo Nordisk. The other two authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
A new article in the Journal of Clinical Endocrinology & Metabolism (JCEM) addresses unique concerns and considerations regarding diabetic ketoacidosis (DKA) in the setting of COVID-19.
Corresponding author Marie E. McDonnell, MD, director of the diabetes program at Brigham and Women’s Hospital, Boston, Massachusetts, discussed the recommendations with Medscape Medical News and also spoke about the news this week that the corticosteroid dexamethasone reduced death rates in severely ill patients with COVID-19.
The full JCEM article, by lead author Nadine E. Palermo, DO, Division of Endocrinology, Diabetes, and Hypertension, also at Brigham and Women’s Hospital, covers DKA diagnosis and triage, and emphasizes that usual hospital protocols for DKA management may need to be adjusted during COVID-19 to help preserve personal protective equipment and ICU beds.
“Hospitals and clinicians need to be able to quickly identify and manage DKA in COVID patients to save lives. This involves determining the options for management, including when less intensive subcutaneous insulin is indicated, and understanding how to guide patients on avoiding this serious complication,” McDonnell said in an Endocrine Society statement.
What about dexamethasone for severe COVID-19 in diabetes?
The new article briefly touches on the fact that upward adjustments to intensive intravenous insulin therapy for DKA may be necessary in patients with COVID-19 who are receiving concomitant corticosteroids or vasopressors.
But it was written prior to the June 16 announcement of the “RECOVERY” trial results with dexamethasone. The UK National Health Service immediately approved the drug’s use in the COVID-19 setting, despite the fact that there has been no published article on the findings yet.
McDonnell told Medscape Medical News that she would need to see formal results to better understand exactly which patients were studied and which ones benefited.
“The peer review will be critical. It looks as if it only benefits people who need respiratory support, but I want to understand that in much more detail,” she said. “If they all had acute respiratory distress syndrome [ARDS],” that’s different.
“There are already some data supporting steroid use in ARDS,” she noted, but added that not all of it suggests benefit.
She pointed to one of several studies now showing that diabetes, and hyperglycemia among people without a prior diabetes diagnosis, are both strong predictors of mortality in hospitalized patients with COVID-19.
“There was a very clear relationship between hyperglycemia and outcomes. We really shouldn’t put people at risk until we have clear data,” she said.
If, once the data are reviewed and appropriate dexamethasone becomes an established treatment for severe COVID-19, hyperglycemia would be a concern among all patients, not just those with previously diagnosed diabetes, she noted.
“We know a good number of people with prediabetes develop hyperglycemia when put on steroids. They can push people over the edge. We’re not going to miss anybody, but treating steroid-induced hyperglycemia is really hard,” McDonnell explained.
She also recommended 2014 guidance from Diabetes UK and the Association of British Clinical Diabetologists, which addresses management of inpatient steroid-induced DKA in patients with and without pre-existing diabetes.
Another major concern, she said, is “patients trying to get dexamethasone when they start to get sick” because this is not the right population to use this agent.
“We worry about people who do not need this drug. If they have diabetes, they put themselves at risk of hyperglycemia, which then increases the risk of severe COVID-19. And then they’re also putting themselves at risk of DKA. It would just be bad medicine,” she said.
Managing DKA in the face of COVID-19: Flexibility is key
In the JCEM article, Palermo and colleagues emphasize that the usual hospital protocols for DKA management may need to be adjusted during COVID-19 in the interest of reducing transmission risk and preserving scare resources.
They provide evidence for alternative treatment strategies, such as the use of subcutaneous rather than intravenous insulin when appropriate.
“We wanted to outline when exactly you should consider nonintensive management strategies for DKA,” McDonnell further explained to Medscape Medical News.
“That would include those with mild or some with moderate DKA. ... The idea is to remind our colleagues about that because hospitals tend to operate on a protocol-driven algorithmic methodology, they can forget to step off the usual care pathway even if evidence supports that,” she said.
But on the other hand, she also said that, in some very complex or severely ill patients with COVID-19, classical intravenous insulin therapy makes the most sense even if their DKA is mild.
The outpatient setting: Prevention and preparation
The new article also addresses several concerns regarding DKA prevention in the outpatient setting.
As with other guidelines, it includes a reminder that patients with diabetes should be advised to discontinue sodium-glucose cotransporter 2 (SGLT2) inhibitors if they become ill with COVID-19, especially if they’re not eating or drinking normally, because they raise the risk for DKA.
Also, for patients with type 1 diabetes, particularly those with a history of repeated DKA, “this is the time to make sure we reach out to patients to refill their insulin prescriptions and address issues related to cost and other access difficulties,” McDonnell said.
The authors also emphasize that insulin starts and education should not be postponed during the pandemic. “Patients identified as meeting criteria to start insulin should be referred for urgent education, either in person or, whenever possible and practical, via video teleconferencing,” they urge.
McDonnell has reported receiving research funding from Novo Nordisk. The other two authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
A new article in the Journal of Clinical Endocrinology & Metabolism (JCEM) addresses unique concerns and considerations regarding diabetic ketoacidosis (DKA) in the setting of COVID-19.
Corresponding author Marie E. McDonnell, MD, director of the diabetes program at Brigham and Women’s Hospital, Boston, Massachusetts, discussed the recommendations with Medscape Medical News and also spoke about the news this week that the corticosteroid dexamethasone reduced death rates in severely ill patients with COVID-19.
The full JCEM article, by lead author Nadine E. Palermo, DO, Division of Endocrinology, Diabetes, and Hypertension, also at Brigham and Women’s Hospital, covers DKA diagnosis and triage, and emphasizes that usual hospital protocols for DKA management may need to be adjusted during COVID-19 to help preserve personal protective equipment and ICU beds.
“Hospitals and clinicians need to be able to quickly identify and manage DKA in COVID patients to save lives. This involves determining the options for management, including when less intensive subcutaneous insulin is indicated, and understanding how to guide patients on avoiding this serious complication,” McDonnell said in an Endocrine Society statement.
What about dexamethasone for severe COVID-19 in diabetes?
The new article briefly touches on the fact that upward adjustments to intensive intravenous insulin therapy for DKA may be necessary in patients with COVID-19 who are receiving concomitant corticosteroids or vasopressors.
But it was written prior to the June 16 announcement of the “RECOVERY” trial results with dexamethasone. The UK National Health Service immediately approved the drug’s use in the COVID-19 setting, despite the fact that there has been no published article on the findings yet.
McDonnell told Medscape Medical News that she would need to see formal results to better understand exactly which patients were studied and which ones benefited.
“The peer review will be critical. It looks as if it only benefits people who need respiratory support, but I want to understand that in much more detail,” she said. “If they all had acute respiratory distress syndrome [ARDS],” that’s different.
“There are already some data supporting steroid use in ARDS,” she noted, but added that not all of it suggests benefit.
She pointed to one of several studies now showing that diabetes, and hyperglycemia among people without a prior diabetes diagnosis, are both strong predictors of mortality in hospitalized patients with COVID-19.
“There was a very clear relationship between hyperglycemia and outcomes. We really shouldn’t put people at risk until we have clear data,” she said.
If, once the data are reviewed and appropriate dexamethasone becomes an established treatment for severe COVID-19, hyperglycemia would be a concern among all patients, not just those with previously diagnosed diabetes, she noted.
“We know a good number of people with prediabetes develop hyperglycemia when put on steroids. They can push people over the edge. We’re not going to miss anybody, but treating steroid-induced hyperglycemia is really hard,” McDonnell explained.
She also recommended 2014 guidance from Diabetes UK and the Association of British Clinical Diabetologists, which addresses management of inpatient steroid-induced DKA in patients with and without pre-existing diabetes.
Another major concern, she said, is “patients trying to get dexamethasone when they start to get sick” because this is not the right population to use this agent.
“We worry about people who do not need this drug. If they have diabetes, they put themselves at risk of hyperglycemia, which then increases the risk of severe COVID-19. And then they’re also putting themselves at risk of DKA. It would just be bad medicine,” she said.
Managing DKA in the face of COVID-19: Flexibility is key
In the JCEM article, Palermo and colleagues emphasize that the usual hospital protocols for DKA management may need to be adjusted during COVID-19 in the interest of reducing transmission risk and preserving scare resources.
They provide evidence for alternative treatment strategies, such as the use of subcutaneous rather than intravenous insulin when appropriate.
“We wanted to outline when exactly you should consider nonintensive management strategies for DKA,” McDonnell further explained to Medscape Medical News.
“That would include those with mild or some with moderate DKA. ... The idea is to remind our colleagues about that because hospitals tend to operate on a protocol-driven algorithmic methodology, they can forget to step off the usual care pathway even if evidence supports that,” she said.
But on the other hand, she also said that, in some very complex or severely ill patients with COVID-19, classical intravenous insulin therapy makes the most sense even if their DKA is mild.
The outpatient setting: Prevention and preparation
The new article also addresses several concerns regarding DKA prevention in the outpatient setting.
As with other guidelines, it includes a reminder that patients with diabetes should be advised to discontinue sodium-glucose cotransporter 2 (SGLT2) inhibitors if they become ill with COVID-19, especially if they’re not eating or drinking normally, because they raise the risk for DKA.
Also, for patients with type 1 diabetes, particularly those with a history of repeated DKA, “this is the time to make sure we reach out to patients to refill their insulin prescriptions and address issues related to cost and other access difficulties,” McDonnell said.
The authors also emphasize that insulin starts and education should not be postponed during the pandemic. “Patients identified as meeting criteria to start insulin should be referred for urgent education, either in person or, whenever possible and practical, via video teleconferencing,” they urge.
McDonnell has reported receiving research funding from Novo Nordisk. The other two authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Pilot study shows apremilast effective for severe recurrent canker sores
showed.
“Canker sores [aphthous ulcers] are very common, yet are often not well managed as the diagnosis is not always correctly made,” lead study author Alison J. Bruce, MB, ChB, said in an interview following the virtual annual meeting of the American Academy of Dermatology. “They’re often mistaken for herpes infection and therefore treated with antiviral therapy. Of the available therapies, several have common side effects or require lab monitoring or are not uniformly effective.”
In their poster abstract, Dr. Bruce, of the division of dermatology at the Mayo Clinic, Jacksonville, Fla., and colleagues noted that, while no principal etiology has been established for recurrent aphthous stomatitis (RAS), immune up-regulation plays a role in the pathogenesis of the condition. “Attacks of RAS may be precipitated by local trauma, stress, food intake, drugs, hormonal changes and vitamin and trace element deficiencies,” they wrote. “Local and systemic conditions and genetic, immunological and microbial factors all may play a role in the pathogenesis.”
Apremilast, a phosphodiesterase-4 inhibitor, down-regulates inflammatory response by modulating expression of tumor necrosis factor–alpha; interferon-gamma; and interleukin-2, IL-12, IL-17, and IL-23. It is approved by the Food and Drug Administration for treating plaque psoriasis and psoriatic arthritis, and in July 2019, was approved for treating ulcers associated with Behçet’s disease, in adults.*
For the pilot study, the researchers enrolled 15 patients with RAS to receive apremilast 30 mg twice daily for 15 weeks after 1 week titration. To be eligible for the trial, patients must have had monthly oral ulcers in preceding 6 months, at least two ulcers in previous 4 weeks prior to enrollment at baseline, at least three ulcers during flares, inadequate control with topical therapy, and no evidence of systemic disease. They excluded patients on immune-modulating therapy or systemic steroids, pregnant or breastfeeding women, those with a systemic infection, those with a history of recurrent bacterial, viral, fungal, or mycobacterial infection, those with a history of depression, as well as those with a known malignancy or vitamin deficiencies. Patients were assessed monthly, evaluating number of ulcers, visual analog pain scale, physician’s global assessment and Chronic Oral Mucosal Disease Questionnaire (COMDQ).
Dr. Bruce and colleagues found that, within 4 weeks of therapy, complete clearance of RAS lesions occurred in all patients except one in whom ulcers were reported to be less severe. That patient had considerable reduction in number, size, and duration of oral ulcers. Remission in all patients was sustained during 16 weeks of treatment. COMDQ responses improved considerably from baseline to week 8, and this was continued until week 16.
“Onset of response [to apremilast] was rapid,” Dr. Bruce said. “For many other therapies, patients are counseled that [they] may take several weeks to become effective. Response was also dramatic. Almost all patients had complete remission from their ulcers, compared with other therapies where oftentimes reduction or attenuation is achieved, as opposed to complete resolution. There was a suggestion that a lower dose [of apremilast] may still be effective. This adds to our ‘toolbox’ of therapeutic options.”
The most common adverse effects were nausea/vomiting and headache, but these were mild and tolerable and generally resolved by week 4.
The researchers acknowledged certain limitations of the study, including its small sample size. “The challenge will most likely be insurance coverage,” Dr. Bruce said. “This is unfortunate, as it would be ideal to offer a safe treatment without the need for monitoring.”
The investigator-initiated study was supported by Celgene. The researchers reported having no financial disclosures.
SOURCE: Bruce AJ et al. AAD 20, Abstract 17701.
*Correction 6/23/2020: An earlier version of this story misstated the approved indications for apremilast.
showed.
“Canker sores [aphthous ulcers] are very common, yet are often not well managed as the diagnosis is not always correctly made,” lead study author Alison J. Bruce, MB, ChB, said in an interview following the virtual annual meeting of the American Academy of Dermatology. “They’re often mistaken for herpes infection and therefore treated with antiviral therapy. Of the available therapies, several have common side effects or require lab monitoring or are not uniformly effective.”
In their poster abstract, Dr. Bruce, of the division of dermatology at the Mayo Clinic, Jacksonville, Fla., and colleagues noted that, while no principal etiology has been established for recurrent aphthous stomatitis (RAS), immune up-regulation plays a role in the pathogenesis of the condition. “Attacks of RAS may be precipitated by local trauma, stress, food intake, drugs, hormonal changes and vitamin and trace element deficiencies,” they wrote. “Local and systemic conditions and genetic, immunological and microbial factors all may play a role in the pathogenesis.”
Apremilast, a phosphodiesterase-4 inhibitor, down-regulates inflammatory response by modulating expression of tumor necrosis factor–alpha; interferon-gamma; and interleukin-2, IL-12, IL-17, and IL-23. It is approved by the Food and Drug Administration for treating plaque psoriasis and psoriatic arthritis, and in July 2019, was approved for treating ulcers associated with Behçet’s disease, in adults.*
For the pilot study, the researchers enrolled 15 patients with RAS to receive apremilast 30 mg twice daily for 15 weeks after 1 week titration. To be eligible for the trial, patients must have had monthly oral ulcers in preceding 6 months, at least two ulcers in previous 4 weeks prior to enrollment at baseline, at least three ulcers during flares, inadequate control with topical therapy, and no evidence of systemic disease. They excluded patients on immune-modulating therapy or systemic steroids, pregnant or breastfeeding women, those with a systemic infection, those with a history of recurrent bacterial, viral, fungal, or mycobacterial infection, those with a history of depression, as well as those with a known malignancy or vitamin deficiencies. Patients were assessed monthly, evaluating number of ulcers, visual analog pain scale, physician’s global assessment and Chronic Oral Mucosal Disease Questionnaire (COMDQ).
Dr. Bruce and colleagues found that, within 4 weeks of therapy, complete clearance of RAS lesions occurred in all patients except one in whom ulcers were reported to be less severe. That patient had considerable reduction in number, size, and duration of oral ulcers. Remission in all patients was sustained during 16 weeks of treatment. COMDQ responses improved considerably from baseline to week 8, and this was continued until week 16.
“Onset of response [to apremilast] was rapid,” Dr. Bruce said. “For many other therapies, patients are counseled that [they] may take several weeks to become effective. Response was also dramatic. Almost all patients had complete remission from their ulcers, compared with other therapies where oftentimes reduction or attenuation is achieved, as opposed to complete resolution. There was a suggestion that a lower dose [of apremilast] may still be effective. This adds to our ‘toolbox’ of therapeutic options.”
The most common adverse effects were nausea/vomiting and headache, but these were mild and tolerable and generally resolved by week 4.
The researchers acknowledged certain limitations of the study, including its small sample size. “The challenge will most likely be insurance coverage,” Dr. Bruce said. “This is unfortunate, as it would be ideal to offer a safe treatment without the need for monitoring.”
The investigator-initiated study was supported by Celgene. The researchers reported having no financial disclosures.
SOURCE: Bruce AJ et al. AAD 20, Abstract 17701.
*Correction 6/23/2020: An earlier version of this story misstated the approved indications for apremilast.
showed.
“Canker sores [aphthous ulcers] are very common, yet are often not well managed as the diagnosis is not always correctly made,” lead study author Alison J. Bruce, MB, ChB, said in an interview following the virtual annual meeting of the American Academy of Dermatology. “They’re often mistaken for herpes infection and therefore treated with antiviral therapy. Of the available therapies, several have common side effects or require lab monitoring or are not uniformly effective.”
In their poster abstract, Dr. Bruce, of the division of dermatology at the Mayo Clinic, Jacksonville, Fla., and colleagues noted that, while no principal etiology has been established for recurrent aphthous stomatitis (RAS), immune up-regulation plays a role in the pathogenesis of the condition. “Attacks of RAS may be precipitated by local trauma, stress, food intake, drugs, hormonal changes and vitamin and trace element deficiencies,” they wrote. “Local and systemic conditions and genetic, immunological and microbial factors all may play a role in the pathogenesis.”
Apremilast, a phosphodiesterase-4 inhibitor, down-regulates inflammatory response by modulating expression of tumor necrosis factor–alpha; interferon-gamma; and interleukin-2, IL-12, IL-17, and IL-23. It is approved by the Food and Drug Administration for treating plaque psoriasis and psoriatic arthritis, and in July 2019, was approved for treating ulcers associated with Behçet’s disease, in adults.*
For the pilot study, the researchers enrolled 15 patients with RAS to receive apremilast 30 mg twice daily for 15 weeks after 1 week titration. To be eligible for the trial, patients must have had monthly oral ulcers in preceding 6 months, at least two ulcers in previous 4 weeks prior to enrollment at baseline, at least three ulcers during flares, inadequate control with topical therapy, and no evidence of systemic disease. They excluded patients on immune-modulating therapy or systemic steroids, pregnant or breastfeeding women, those with a systemic infection, those with a history of recurrent bacterial, viral, fungal, or mycobacterial infection, those with a history of depression, as well as those with a known malignancy or vitamin deficiencies. Patients were assessed monthly, evaluating number of ulcers, visual analog pain scale, physician’s global assessment and Chronic Oral Mucosal Disease Questionnaire (COMDQ).
Dr. Bruce and colleagues found that, within 4 weeks of therapy, complete clearance of RAS lesions occurred in all patients except one in whom ulcers were reported to be less severe. That patient had considerable reduction in number, size, and duration of oral ulcers. Remission in all patients was sustained during 16 weeks of treatment. COMDQ responses improved considerably from baseline to week 8, and this was continued until week 16.
“Onset of response [to apremilast] was rapid,” Dr. Bruce said. “For many other therapies, patients are counseled that [they] may take several weeks to become effective. Response was also dramatic. Almost all patients had complete remission from their ulcers, compared with other therapies where oftentimes reduction or attenuation is achieved, as opposed to complete resolution. There was a suggestion that a lower dose [of apremilast] may still be effective. This adds to our ‘toolbox’ of therapeutic options.”
The most common adverse effects were nausea/vomiting and headache, but these were mild and tolerable and generally resolved by week 4.
The researchers acknowledged certain limitations of the study, including its small sample size. “The challenge will most likely be insurance coverage,” Dr. Bruce said. “This is unfortunate, as it would be ideal to offer a safe treatment without the need for monitoring.”
The investigator-initiated study was supported by Celgene. The researchers reported having no financial disclosures.
SOURCE: Bruce AJ et al. AAD 20, Abstract 17701.
*Correction 6/23/2020: An earlier version of this story misstated the approved indications for apremilast.
FROM AAD 20
Amid pandemic, prison psychiatrists adjust and persist
Maryland psychiatrist Annette Hanson, MD, hasn’t changed her morning routine much since the coronavirus pandemic began. She still avoids putting on a necklace or earrings, which could be torn away or used as a ligature, and heads to work.
The only difference is that Dr. Hanson wears easy-to-clean scrubs instead of business attire. “That way I can strip down and shower as soon as I get home. I’m not sure that’s necessary, but I’m being cautious,” said Dr. Hanson, a forensic psychiatrist who is an assistant professor of psychiatry at the University of Maryland and at Johns Hopkins University, both in Baltimore.
As many of her colleagues shelter in place and work from home with the help of telemedicine, prison psychiatrists such as Dr. Hanson continue to evaluate and treat patients in person – behind bars. That hasn’t changed. But so much else has, from the elimination of family visits to the suspension of many court hearings, leaving already vulnerable inmates in limbo.
“Prisons continue to be a poor place to receive mental health care. The setting is destructive to physical and mental health, and the pandemic has made it worse,” said Bandy X. Lee, MD, MDiv, of Yale University, New Haven, Conn., who treats inmates in several states.
Like the inmates they treat, “The most challenging part is to continue care in a system that has essentially been frozen in place,” Dr. Hanson said.
As of June 9, nearly 44,000 inmates in federal and state prisons had tested positive for coronavirus, according to the Associated Press and the Marshall Project. At least 500 people have died. Those numbers do not include inmates and staff members in local jails or juvenile detention centers.
Statistics about COVID-19 in prison staff members are incomplete since only 20 states reported them, and it’s not clear where they contracted the virus. Even so, at least 9,180 cases in staff members were reported, along with 38 deaths, the AP/Marshall Project report.
Using telemedicine is impossible at many jails and prisons, forcing many psychiatrists to protect themselves and their patients as best they can. At the Los Angeles County Jail, which does not use telemedicine, group sessions have been greatly reduced. Instead, psychiatrists are spending more time talking to inmates at the doors to cells or modules, said supervising psychiatrist Joseph R. Simpson, MD, PhD.
The risk of transmission still exists, he said. “Our health system has a comprehensive testing, monitoring, and isolation system in place now to slow the spread and flatten the curve,” Dr. Simpson said. “However, once COVID enters any correctional facility, preventing it from spreading entirely is difficult or impossible given the nature of the living arrangements.”
In interviews, psychiatrists said inmates are more stressed by the limitations spawned by the pandemic than the risk of infection. Many facilities have banned in-person visits, and telephone calls are an expensive alternative, said Nicolas Badre, MD, who treats inmates at jails in the San Diego region.
“That one lifeline you had is no longer there. The second lifeline is that your public defender will get you a plea deal, but they’ve postponed hearings,” he said. “I’ve seen cases of folks who are more anxious and more depressed because COVID is delaying their case or because they’re unable to speak with their families and friends.”
Restrictions on contact with people on the outside are especially difficult for inmates at risk of psychosis, Dr. Badre said. “You add those two [limitations], and how does that not sound to someone with schizophrenia like the government is out to get you? And when someone asks you to wear a mask, how do you trust them?”
According to Dr. Lee, some patients with severe mental illness are unable to comprehend the risk of the pandemic, and they fail to protect themselves. While she’s begun to rely on telemedicine, “it’s a very blunt instrument. Many of my patients are very sick and less able to interact with a screen. And sometimes you’re exhausted at the end of the day because you’ve been yelling at the screen and trying different ways to gain the attention of individuals who are responding to external stimuli and can’t engage.”
The pandemic has improved conditions in prisons and jails on one front: Many are releasing inmates to lower the risk of spreading infection. And Dr. Badre said, “a lot of people are doing just fine, finding themselves to be completely resilient and finding meaning at this time.”
Other than anxiety, the psychiatrists did not report seeing higher percentages of any specific conditions. And they said they are not prescribing any more medications than before COVID-19. But many of the perennial treatments for anxiety – improving the diet, getting out and exercising, developing a hobby, reaching out to others – can be difficult at the best of times behind bars. Those treatments might be impossible now.
At the juvenile justice system in the Chicago area, for example, the pandemic has forced the cancellation of activities such as writing, taking art classes, and barber training. In-person visits are banned, too. “For a lot of them, seeing their family relieves stress, makes them feel more hopeful. It gives them a sense of normalcy to hug their mom,” said Yana Oskin, MD.
But it’s still possible to urge the young people to read, write, work with puzzles, and exercise daily even if it’s just in their rooms, she said. “While their movements have been limited, they do still get to go outside. If they can’t go to the gym, the recreation specialist comes to their pod.”
And while some psychiatrists and older inmates might not be thrilled to have to adjust to therapy via screen during the pandemic, young people are a different story. Dr. Oskin is working with them via telemedicine, which allowed at least one inmate to gain a kind of victory.
“We have an assistant who sets up Skype visits, and the camera was not angled properly,” she recalled. “She couldn’t figure out. The kid sat down and fixed it in 2 seconds.”
Dr. Hanson is the coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has no other disclosures. Dr. Lee is the author of “Violence: An Interdisciplinary Approach to Causes, Consequences and Cures” (Wiley Blackwell, 2019). She has no other disclosures. Dr. Simpson is coauthor of “Neuroimaging in Forensic Psychiatry: From the Clinic to the Courtroom” (Wiley Blackwell, 2012). He has no other disclosures. Dr. Badre and Dr. Oskin reported no disclosures.
Maryland psychiatrist Annette Hanson, MD, hasn’t changed her morning routine much since the coronavirus pandemic began. She still avoids putting on a necklace or earrings, which could be torn away or used as a ligature, and heads to work.
The only difference is that Dr. Hanson wears easy-to-clean scrubs instead of business attire. “That way I can strip down and shower as soon as I get home. I’m not sure that’s necessary, but I’m being cautious,” said Dr. Hanson, a forensic psychiatrist who is an assistant professor of psychiatry at the University of Maryland and at Johns Hopkins University, both in Baltimore.
As many of her colleagues shelter in place and work from home with the help of telemedicine, prison psychiatrists such as Dr. Hanson continue to evaluate and treat patients in person – behind bars. That hasn’t changed. But so much else has, from the elimination of family visits to the suspension of many court hearings, leaving already vulnerable inmates in limbo.
“Prisons continue to be a poor place to receive mental health care. The setting is destructive to physical and mental health, and the pandemic has made it worse,” said Bandy X. Lee, MD, MDiv, of Yale University, New Haven, Conn., who treats inmates in several states.
Like the inmates they treat, “The most challenging part is to continue care in a system that has essentially been frozen in place,” Dr. Hanson said.
As of June 9, nearly 44,000 inmates in federal and state prisons had tested positive for coronavirus, according to the Associated Press and the Marshall Project. At least 500 people have died. Those numbers do not include inmates and staff members in local jails or juvenile detention centers.
Statistics about COVID-19 in prison staff members are incomplete since only 20 states reported them, and it’s not clear where they contracted the virus. Even so, at least 9,180 cases in staff members were reported, along with 38 deaths, the AP/Marshall Project report.
Using telemedicine is impossible at many jails and prisons, forcing many psychiatrists to protect themselves and their patients as best they can. At the Los Angeles County Jail, which does not use telemedicine, group sessions have been greatly reduced. Instead, psychiatrists are spending more time talking to inmates at the doors to cells or modules, said supervising psychiatrist Joseph R. Simpson, MD, PhD.
The risk of transmission still exists, he said. “Our health system has a comprehensive testing, monitoring, and isolation system in place now to slow the spread and flatten the curve,” Dr. Simpson said. “However, once COVID enters any correctional facility, preventing it from spreading entirely is difficult or impossible given the nature of the living arrangements.”
In interviews, psychiatrists said inmates are more stressed by the limitations spawned by the pandemic than the risk of infection. Many facilities have banned in-person visits, and telephone calls are an expensive alternative, said Nicolas Badre, MD, who treats inmates at jails in the San Diego region.
“That one lifeline you had is no longer there. The second lifeline is that your public defender will get you a plea deal, but they’ve postponed hearings,” he said. “I’ve seen cases of folks who are more anxious and more depressed because COVID is delaying their case or because they’re unable to speak with their families and friends.”
Restrictions on contact with people on the outside are especially difficult for inmates at risk of psychosis, Dr. Badre said. “You add those two [limitations], and how does that not sound to someone with schizophrenia like the government is out to get you? And when someone asks you to wear a mask, how do you trust them?”
According to Dr. Lee, some patients with severe mental illness are unable to comprehend the risk of the pandemic, and they fail to protect themselves. While she’s begun to rely on telemedicine, “it’s a very blunt instrument. Many of my patients are very sick and less able to interact with a screen. And sometimes you’re exhausted at the end of the day because you’ve been yelling at the screen and trying different ways to gain the attention of individuals who are responding to external stimuli and can’t engage.”
The pandemic has improved conditions in prisons and jails on one front: Many are releasing inmates to lower the risk of spreading infection. And Dr. Badre said, “a lot of people are doing just fine, finding themselves to be completely resilient and finding meaning at this time.”
Other than anxiety, the psychiatrists did not report seeing higher percentages of any specific conditions. And they said they are not prescribing any more medications than before COVID-19. But many of the perennial treatments for anxiety – improving the diet, getting out and exercising, developing a hobby, reaching out to others – can be difficult at the best of times behind bars. Those treatments might be impossible now.
At the juvenile justice system in the Chicago area, for example, the pandemic has forced the cancellation of activities such as writing, taking art classes, and barber training. In-person visits are banned, too. “For a lot of them, seeing their family relieves stress, makes them feel more hopeful. It gives them a sense of normalcy to hug their mom,” said Yana Oskin, MD.
But it’s still possible to urge the young people to read, write, work with puzzles, and exercise daily even if it’s just in their rooms, she said. “While their movements have been limited, they do still get to go outside. If they can’t go to the gym, the recreation specialist comes to their pod.”
And while some psychiatrists and older inmates might not be thrilled to have to adjust to therapy via screen during the pandemic, young people are a different story. Dr. Oskin is working with them via telemedicine, which allowed at least one inmate to gain a kind of victory.
“We have an assistant who sets up Skype visits, and the camera was not angled properly,” she recalled. “She couldn’t figure out. The kid sat down and fixed it in 2 seconds.”
Dr. Hanson is the coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has no other disclosures. Dr. Lee is the author of “Violence: An Interdisciplinary Approach to Causes, Consequences and Cures” (Wiley Blackwell, 2019). She has no other disclosures. Dr. Simpson is coauthor of “Neuroimaging in Forensic Psychiatry: From the Clinic to the Courtroom” (Wiley Blackwell, 2012). He has no other disclosures. Dr. Badre and Dr. Oskin reported no disclosures.
Maryland psychiatrist Annette Hanson, MD, hasn’t changed her morning routine much since the coronavirus pandemic began. She still avoids putting on a necklace or earrings, which could be torn away or used as a ligature, and heads to work.
The only difference is that Dr. Hanson wears easy-to-clean scrubs instead of business attire. “That way I can strip down and shower as soon as I get home. I’m not sure that’s necessary, but I’m being cautious,” said Dr. Hanson, a forensic psychiatrist who is an assistant professor of psychiatry at the University of Maryland and at Johns Hopkins University, both in Baltimore.
As many of her colleagues shelter in place and work from home with the help of telemedicine, prison psychiatrists such as Dr. Hanson continue to evaluate and treat patients in person – behind bars. That hasn’t changed. But so much else has, from the elimination of family visits to the suspension of many court hearings, leaving already vulnerable inmates in limbo.
“Prisons continue to be a poor place to receive mental health care. The setting is destructive to physical and mental health, and the pandemic has made it worse,” said Bandy X. Lee, MD, MDiv, of Yale University, New Haven, Conn., who treats inmates in several states.
Like the inmates they treat, “The most challenging part is to continue care in a system that has essentially been frozen in place,” Dr. Hanson said.
As of June 9, nearly 44,000 inmates in federal and state prisons had tested positive for coronavirus, according to the Associated Press and the Marshall Project. At least 500 people have died. Those numbers do not include inmates and staff members in local jails or juvenile detention centers.
Statistics about COVID-19 in prison staff members are incomplete since only 20 states reported them, and it’s not clear where they contracted the virus. Even so, at least 9,180 cases in staff members were reported, along with 38 deaths, the AP/Marshall Project report.
Using telemedicine is impossible at many jails and prisons, forcing many psychiatrists to protect themselves and their patients as best they can. At the Los Angeles County Jail, which does not use telemedicine, group sessions have been greatly reduced. Instead, psychiatrists are spending more time talking to inmates at the doors to cells or modules, said supervising psychiatrist Joseph R. Simpson, MD, PhD.
The risk of transmission still exists, he said. “Our health system has a comprehensive testing, monitoring, and isolation system in place now to slow the spread and flatten the curve,” Dr. Simpson said. “However, once COVID enters any correctional facility, preventing it from spreading entirely is difficult or impossible given the nature of the living arrangements.”
In interviews, psychiatrists said inmates are more stressed by the limitations spawned by the pandemic than the risk of infection. Many facilities have banned in-person visits, and telephone calls are an expensive alternative, said Nicolas Badre, MD, who treats inmates at jails in the San Diego region.
“That one lifeline you had is no longer there. The second lifeline is that your public defender will get you a plea deal, but they’ve postponed hearings,” he said. “I’ve seen cases of folks who are more anxious and more depressed because COVID is delaying their case or because they’re unable to speak with their families and friends.”
Restrictions on contact with people on the outside are especially difficult for inmates at risk of psychosis, Dr. Badre said. “You add those two [limitations], and how does that not sound to someone with schizophrenia like the government is out to get you? And when someone asks you to wear a mask, how do you trust them?”
According to Dr. Lee, some patients with severe mental illness are unable to comprehend the risk of the pandemic, and they fail to protect themselves. While she’s begun to rely on telemedicine, “it’s a very blunt instrument. Many of my patients are very sick and less able to interact with a screen. And sometimes you’re exhausted at the end of the day because you’ve been yelling at the screen and trying different ways to gain the attention of individuals who are responding to external stimuli and can’t engage.”
The pandemic has improved conditions in prisons and jails on one front: Many are releasing inmates to lower the risk of spreading infection. And Dr. Badre said, “a lot of people are doing just fine, finding themselves to be completely resilient and finding meaning at this time.”
Other than anxiety, the psychiatrists did not report seeing higher percentages of any specific conditions. And they said they are not prescribing any more medications than before COVID-19. But many of the perennial treatments for anxiety – improving the diet, getting out and exercising, developing a hobby, reaching out to others – can be difficult at the best of times behind bars. Those treatments might be impossible now.
At the juvenile justice system in the Chicago area, for example, the pandemic has forced the cancellation of activities such as writing, taking art classes, and barber training. In-person visits are banned, too. “For a lot of them, seeing their family relieves stress, makes them feel more hopeful. It gives them a sense of normalcy to hug their mom,” said Yana Oskin, MD.
But it’s still possible to urge the young people to read, write, work with puzzles, and exercise daily even if it’s just in their rooms, she said. “While their movements have been limited, they do still get to go outside. If they can’t go to the gym, the recreation specialist comes to their pod.”
And while some psychiatrists and older inmates might not be thrilled to have to adjust to therapy via screen during the pandemic, young people are a different story. Dr. Oskin is working with them via telemedicine, which allowed at least one inmate to gain a kind of victory.
“We have an assistant who sets up Skype visits, and the camera was not angled properly,” she recalled. “She couldn’t figure out. The kid sat down and fixed it in 2 seconds.”
Dr. Hanson is the coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has no other disclosures. Dr. Lee is the author of “Violence: An Interdisciplinary Approach to Causes, Consequences and Cures” (Wiley Blackwell, 2019). She has no other disclosures. Dr. Simpson is coauthor of “Neuroimaging in Forensic Psychiatry: From the Clinic to the Courtroom” (Wiley Blackwell, 2012). He has no other disclosures. Dr. Badre and Dr. Oskin reported no disclosures.
Sequential chemoradiation improves DFS in early-stage cervical cancer
In the study’s intent-to-treat (ITT) population, the 3-year DFS rate was significantly higher among patients who received sequential chemoradiation than among patients who received concurrent chemoradiation or radiation alone.
“After an adjustment for baseline presence of lymph node metastasis, we found that sequential chemoradiation decreased, by 40% to 50%, the recurrence risk, compared with the other two groups,” said investigator He Huang, MD, of Sun Yat-Sen University Cancer Center in Guangzhou, China.
Dr. Huang presented these findings as part of the American Society of Clinical Oncology virtual scientific program.
Study rationale and details
“As we know, early-stage cervical cancer can be cured after treatment,” Dr. Huang said. “However, the risk of recurrence remains higher among patients with high-risk factors, including lymph node metastases, positive surgical margins, and parametrial invasion. Postoperative adjuvant radiation and chemotherapy may result in favorable survival for patients with high-risk factors.”
Although the efficacy of concurrent radiation with single-agent cisplatin has been demonstrated in primary treatment for local advanced cervical cancer, it has not been assessed as adjuvant treatment for early-stage cervical cancer in a randomized, controlled trial, Dr. Huang explained.
“Another question is whether patients with intermediate-risk factors could benefit from the concurrent chemotherapy [with] radiation,” he said. “So far, we don’t have enough evidence.”
To gain some insight, Dr. Huang and colleagues initiated the STARS trial. The trial enrolled patients with stage IB1-IIA2 cervical cancer and at least one adverse risk factor after radical hysterectomy. Patients had a median age of 48 years, and most had stage IB1 or IIA1 disease.
The patients were randomized 1:1:1 to receive radiation alone, concurrent chemoradiation, or sequential chemoradiation.
All patients received pelvic radiation at 45-50 Gy. Patients in the concurrent chemoradiation arm also received five doses of weekly cisplatin at 30-40 mg/m2.
Patients in the sequential chemoradiation arm received 60-75 mg/m2 of weekly cisplatin plus 135-175 mg/m2 of paclitaxel in 21-day cycles, with two cycles given before and two cycles given after radiotherapy.
The treatment groups were comparable with respect to histologic subtypes, lymphovascular invasion rates, parametrial or surgical margin involvement, deep stromal involvement, tumor grade, minimally invasive surgery rates, and neoadjuvant chemotherapy, Dr. Huang said. He added, however, that lymph node metastasis was lowest in the radiation-only arm.
DFS results
In the ITT population, patients who received sequential chemoradiation had significantly better DFS compared with patients in the other treatment arms. The ITT population included 353 patients in the sequential chemoradiation arm, 345 patients in the concurrent chemoradiation arm, and 350 patients in the radiation-only arm.
The 3-year DFS rates were 90% in patients randomized to sequential chemoradiation, 85% in patients randomized to concurrent chemoradiation, and 82% in patients randomized to radiation alone (hazard ratios, 0.52 for sequential chemoradiation vs. radiation alone and 0.65 for sequential vs. concurrent chemoradiation; P = .03).
Subgroup analyses showed a DFS benefit with sequential chemoradiation versus radiation alone across histology types, tumor grades, and tumor size, Dr. Huang noted.
In the per-protocol population, sequential chemoradiation was associated with better DFS when compared with radiation alone. However, there were no significant differences between the sequential and concurrent chemoradiation arms or the concurrent chemoradiation and radiation-only arms.
The per-protocol population included 235 patients in the sequential chemoradiation arm, 190 patients in the concurrent chemoradiation arm, and 324 patients in the radiation-only arm.
In the per-protocol population, the 3-year DFS rates were 91% in patients randomized to sequential chemoradiation, 86% in patients randomized to concurrent chemoradiation, and 82% in patients randomized to radiation alone (HRs, 0.47 for sequential vs. radiation alone and 0.67 for sequential vs. concurrent; P = .026).
In the overall population, DFS was superior among patients with intermediate-risk versus high-risk factors. The 3-year DFS rates were 90% and 74%, respectively (P < .05).
Overall survival and safety
As for overall survival (OS), sequential chemoradiation showed a trend toward improvement at 5 years, compared with the other treatment groups. However, the differences were not statistically significant in either the ITT or per-protocol populations.
In the ITT population, the 5-year OS was 92% in the sequential chemoradiation arm, 89% in the concurrent chemoradiation arm, and 88% in the radiation-only arm. In the per-protocol population, the 5-year OS rates were 93%, 90%, and 88%, respectively.
Patients with intermediate-risk factors had a more favorable OS. The 5-year OS rate was 93% in patients with intermediate-risk factors and 81% in patients with high-risk factors (P < .05).
Dr. Huang noted that patients in the radiation-only arm experienced fewer grade 3-4 adverse events than the other patients. Less neutropenia, nausea, and vomiting were observed in the radiation-only arm.
There was more grade 3-4 nausea and vomiting in the concurrent chemoradiation arm than in the sequential chemoradiation arm.
However, Dr. Huang noted that “adding chemotherapy to radiation did not impact the patients’ quality of life in the long-term compared with radiation alone.
“In this study, sequential chemoradiation resulted in a better disease-free survival and a lower risk of cancer death for cervical cancer patients with adverse pathological factors,” he said. “We believe that sequential chemoradiation could be an optimal option for post-operative adjuvant treatment.”
This study was sponsored by Sun Yat-sen University in collaboration with Guangdong Provincial People’s Hospital, First Affiliated Hospital/Sun Yat-Sen University, Shenzhen People’s Hospital, and Cancer Hospital of Guangxi Medical University. Dr. Huang reported receiving honoraria, travel, accommodations, and expenses from AstraZeneca.
SOURCE: Huang H et al. ASCO 2020, Abstract 6007.
In the study’s intent-to-treat (ITT) population, the 3-year DFS rate was significantly higher among patients who received sequential chemoradiation than among patients who received concurrent chemoradiation or radiation alone.
“After an adjustment for baseline presence of lymph node metastasis, we found that sequential chemoradiation decreased, by 40% to 50%, the recurrence risk, compared with the other two groups,” said investigator He Huang, MD, of Sun Yat-Sen University Cancer Center in Guangzhou, China.
Dr. Huang presented these findings as part of the American Society of Clinical Oncology virtual scientific program.
Study rationale and details
“As we know, early-stage cervical cancer can be cured after treatment,” Dr. Huang said. “However, the risk of recurrence remains higher among patients with high-risk factors, including lymph node metastases, positive surgical margins, and parametrial invasion. Postoperative adjuvant radiation and chemotherapy may result in favorable survival for patients with high-risk factors.”
Although the efficacy of concurrent radiation with single-agent cisplatin has been demonstrated in primary treatment for local advanced cervical cancer, it has not been assessed as adjuvant treatment for early-stage cervical cancer in a randomized, controlled trial, Dr. Huang explained.
“Another question is whether patients with intermediate-risk factors could benefit from the concurrent chemotherapy [with] radiation,” he said. “So far, we don’t have enough evidence.”
To gain some insight, Dr. Huang and colleagues initiated the STARS trial. The trial enrolled patients with stage IB1-IIA2 cervical cancer and at least one adverse risk factor after radical hysterectomy. Patients had a median age of 48 years, and most had stage IB1 or IIA1 disease.
The patients were randomized 1:1:1 to receive radiation alone, concurrent chemoradiation, or sequential chemoradiation.
All patients received pelvic radiation at 45-50 Gy. Patients in the concurrent chemoradiation arm also received five doses of weekly cisplatin at 30-40 mg/m2.
Patients in the sequential chemoradiation arm received 60-75 mg/m2 of weekly cisplatin plus 135-175 mg/m2 of paclitaxel in 21-day cycles, with two cycles given before and two cycles given after radiotherapy.
The treatment groups were comparable with respect to histologic subtypes, lymphovascular invasion rates, parametrial or surgical margin involvement, deep stromal involvement, tumor grade, minimally invasive surgery rates, and neoadjuvant chemotherapy, Dr. Huang said. He added, however, that lymph node metastasis was lowest in the radiation-only arm.
DFS results
In the ITT population, patients who received sequential chemoradiation had significantly better DFS compared with patients in the other treatment arms. The ITT population included 353 patients in the sequential chemoradiation arm, 345 patients in the concurrent chemoradiation arm, and 350 patients in the radiation-only arm.
The 3-year DFS rates were 90% in patients randomized to sequential chemoradiation, 85% in patients randomized to concurrent chemoradiation, and 82% in patients randomized to radiation alone (hazard ratios, 0.52 for sequential chemoradiation vs. radiation alone and 0.65 for sequential vs. concurrent chemoradiation; P = .03).
Subgroup analyses showed a DFS benefit with sequential chemoradiation versus radiation alone across histology types, tumor grades, and tumor size, Dr. Huang noted.
In the per-protocol population, sequential chemoradiation was associated with better DFS when compared with radiation alone. However, there were no significant differences between the sequential and concurrent chemoradiation arms or the concurrent chemoradiation and radiation-only arms.
The per-protocol population included 235 patients in the sequential chemoradiation arm, 190 patients in the concurrent chemoradiation arm, and 324 patients in the radiation-only arm.
In the per-protocol population, the 3-year DFS rates were 91% in patients randomized to sequential chemoradiation, 86% in patients randomized to concurrent chemoradiation, and 82% in patients randomized to radiation alone (HRs, 0.47 for sequential vs. radiation alone and 0.67 for sequential vs. concurrent; P = .026).
In the overall population, DFS was superior among patients with intermediate-risk versus high-risk factors. The 3-year DFS rates were 90% and 74%, respectively (P < .05).
Overall survival and safety
As for overall survival (OS), sequential chemoradiation showed a trend toward improvement at 5 years, compared with the other treatment groups. However, the differences were not statistically significant in either the ITT or per-protocol populations.
In the ITT population, the 5-year OS was 92% in the sequential chemoradiation arm, 89% in the concurrent chemoradiation arm, and 88% in the radiation-only arm. In the per-protocol population, the 5-year OS rates were 93%, 90%, and 88%, respectively.
Patients with intermediate-risk factors had a more favorable OS. The 5-year OS rate was 93% in patients with intermediate-risk factors and 81% in patients with high-risk factors (P < .05).
Dr. Huang noted that patients in the radiation-only arm experienced fewer grade 3-4 adverse events than the other patients. Less neutropenia, nausea, and vomiting were observed in the radiation-only arm.
There was more grade 3-4 nausea and vomiting in the concurrent chemoradiation arm than in the sequential chemoradiation arm.
However, Dr. Huang noted that “adding chemotherapy to radiation did not impact the patients’ quality of life in the long-term compared with radiation alone.
“In this study, sequential chemoradiation resulted in a better disease-free survival and a lower risk of cancer death for cervical cancer patients with adverse pathological factors,” he said. “We believe that sequential chemoradiation could be an optimal option for post-operative adjuvant treatment.”
This study was sponsored by Sun Yat-sen University in collaboration with Guangdong Provincial People’s Hospital, First Affiliated Hospital/Sun Yat-Sen University, Shenzhen People’s Hospital, and Cancer Hospital of Guangxi Medical University. Dr. Huang reported receiving honoraria, travel, accommodations, and expenses from AstraZeneca.
SOURCE: Huang H et al. ASCO 2020, Abstract 6007.
In the study’s intent-to-treat (ITT) population, the 3-year DFS rate was significantly higher among patients who received sequential chemoradiation than among patients who received concurrent chemoradiation or radiation alone.
“After an adjustment for baseline presence of lymph node metastasis, we found that sequential chemoradiation decreased, by 40% to 50%, the recurrence risk, compared with the other two groups,” said investigator He Huang, MD, of Sun Yat-Sen University Cancer Center in Guangzhou, China.
Dr. Huang presented these findings as part of the American Society of Clinical Oncology virtual scientific program.
Study rationale and details
“As we know, early-stage cervical cancer can be cured after treatment,” Dr. Huang said. “However, the risk of recurrence remains higher among patients with high-risk factors, including lymph node metastases, positive surgical margins, and parametrial invasion. Postoperative adjuvant radiation and chemotherapy may result in favorable survival for patients with high-risk factors.”
Although the efficacy of concurrent radiation with single-agent cisplatin has been demonstrated in primary treatment for local advanced cervical cancer, it has not been assessed as adjuvant treatment for early-stage cervical cancer in a randomized, controlled trial, Dr. Huang explained.
“Another question is whether patients with intermediate-risk factors could benefit from the concurrent chemotherapy [with] radiation,” he said. “So far, we don’t have enough evidence.”
To gain some insight, Dr. Huang and colleagues initiated the STARS trial. The trial enrolled patients with stage IB1-IIA2 cervical cancer and at least one adverse risk factor after radical hysterectomy. Patients had a median age of 48 years, and most had stage IB1 or IIA1 disease.
The patients were randomized 1:1:1 to receive radiation alone, concurrent chemoradiation, or sequential chemoradiation.
All patients received pelvic radiation at 45-50 Gy. Patients in the concurrent chemoradiation arm also received five doses of weekly cisplatin at 30-40 mg/m2.
Patients in the sequential chemoradiation arm received 60-75 mg/m2 of weekly cisplatin plus 135-175 mg/m2 of paclitaxel in 21-day cycles, with two cycles given before and two cycles given after radiotherapy.
The treatment groups were comparable with respect to histologic subtypes, lymphovascular invasion rates, parametrial or surgical margin involvement, deep stromal involvement, tumor grade, minimally invasive surgery rates, and neoadjuvant chemotherapy, Dr. Huang said. He added, however, that lymph node metastasis was lowest in the radiation-only arm.
DFS results
In the ITT population, patients who received sequential chemoradiation had significantly better DFS compared with patients in the other treatment arms. The ITT population included 353 patients in the sequential chemoradiation arm, 345 patients in the concurrent chemoradiation arm, and 350 patients in the radiation-only arm.
The 3-year DFS rates were 90% in patients randomized to sequential chemoradiation, 85% in patients randomized to concurrent chemoradiation, and 82% in patients randomized to radiation alone (hazard ratios, 0.52 for sequential chemoradiation vs. radiation alone and 0.65 for sequential vs. concurrent chemoradiation; P = .03).
Subgroup analyses showed a DFS benefit with sequential chemoradiation versus radiation alone across histology types, tumor grades, and tumor size, Dr. Huang noted.
In the per-protocol population, sequential chemoradiation was associated with better DFS when compared with radiation alone. However, there were no significant differences between the sequential and concurrent chemoradiation arms or the concurrent chemoradiation and radiation-only arms.
The per-protocol population included 235 patients in the sequential chemoradiation arm, 190 patients in the concurrent chemoradiation arm, and 324 patients in the radiation-only arm.
In the per-protocol population, the 3-year DFS rates were 91% in patients randomized to sequential chemoradiation, 86% in patients randomized to concurrent chemoradiation, and 82% in patients randomized to radiation alone (HRs, 0.47 for sequential vs. radiation alone and 0.67 for sequential vs. concurrent; P = .026).
In the overall population, DFS was superior among patients with intermediate-risk versus high-risk factors. The 3-year DFS rates were 90% and 74%, respectively (P < .05).
Overall survival and safety
As for overall survival (OS), sequential chemoradiation showed a trend toward improvement at 5 years, compared with the other treatment groups. However, the differences were not statistically significant in either the ITT or per-protocol populations.
In the ITT population, the 5-year OS was 92% in the sequential chemoradiation arm, 89% in the concurrent chemoradiation arm, and 88% in the radiation-only arm. In the per-protocol population, the 5-year OS rates were 93%, 90%, and 88%, respectively.
Patients with intermediate-risk factors had a more favorable OS. The 5-year OS rate was 93% in patients with intermediate-risk factors and 81% in patients with high-risk factors (P < .05).
Dr. Huang noted that patients in the radiation-only arm experienced fewer grade 3-4 adverse events than the other patients. Less neutropenia, nausea, and vomiting were observed in the radiation-only arm.
There was more grade 3-4 nausea and vomiting in the concurrent chemoradiation arm than in the sequential chemoradiation arm.
However, Dr. Huang noted that “adding chemotherapy to radiation did not impact the patients’ quality of life in the long-term compared with radiation alone.
“In this study, sequential chemoradiation resulted in a better disease-free survival and a lower risk of cancer death for cervical cancer patients with adverse pathological factors,” he said. “We believe that sequential chemoradiation could be an optimal option for post-operative adjuvant treatment.”
This study was sponsored by Sun Yat-sen University in collaboration with Guangdong Provincial People’s Hospital, First Affiliated Hospital/Sun Yat-Sen University, Shenzhen People’s Hospital, and Cancer Hospital of Guangxi Medical University. Dr. Huang reported receiving honoraria, travel, accommodations, and expenses from AstraZeneca.
SOURCE: Huang H et al. ASCO 2020, Abstract 6007.
FROM ASCO 2020
Rituximab safe and effective in pregnant women with MS
Key clinical point: Periconceptional rituximab is a highly effective and safe treatment for women with suboptimally controlled multiple sclerosis (MS).
Major finding: Among 38 live births, 3 deliveries were preterm (including 1 set of twins), 1 baby was admitted for perinatal ischemic stroke, and 1 twin died after 6 days from an intraventricular hemorrhage. Fifteen women reported at least one first-trimester miscarriage, of whom 8 had a history of infertility. No stillbirths, chorioamnionitis, or major malformations were recorded. Only 2 women experienced relapses, one during pregnancy and the other postpartum.
Study details: The data come from a study of 55 women with MS (74 pregnancies) treated with at least 1 dose of rituximab infusion before conception.
Disclosures: This study was supported in part by the Patient-Centered Outcomes Research Institute.
Some of the investigators reported receiving research grants from multiple pharmaceutical companies.
Citation: Smith JB et al. Neurol Neuroimmunol Neuroinflamm. 2020 May 01. doi: 10.1212/NXI.0000000000000734.
Key clinical point: Periconceptional rituximab is a highly effective and safe treatment for women with suboptimally controlled multiple sclerosis (MS).
Major finding: Among 38 live births, 3 deliveries were preterm (including 1 set of twins), 1 baby was admitted for perinatal ischemic stroke, and 1 twin died after 6 days from an intraventricular hemorrhage. Fifteen women reported at least one first-trimester miscarriage, of whom 8 had a history of infertility. No stillbirths, chorioamnionitis, or major malformations were recorded. Only 2 women experienced relapses, one during pregnancy and the other postpartum.
Study details: The data come from a study of 55 women with MS (74 pregnancies) treated with at least 1 dose of rituximab infusion before conception.
Disclosures: This study was supported in part by the Patient-Centered Outcomes Research Institute.
Some of the investigators reported receiving research grants from multiple pharmaceutical companies.
Citation: Smith JB et al. Neurol Neuroimmunol Neuroinflamm. 2020 May 01. doi: 10.1212/NXI.0000000000000734.
Key clinical point: Periconceptional rituximab is a highly effective and safe treatment for women with suboptimally controlled multiple sclerosis (MS).
Major finding: Among 38 live births, 3 deliveries were preterm (including 1 set of twins), 1 baby was admitted for perinatal ischemic stroke, and 1 twin died after 6 days from an intraventricular hemorrhage. Fifteen women reported at least one first-trimester miscarriage, of whom 8 had a history of infertility. No stillbirths, chorioamnionitis, or major malformations were recorded. Only 2 women experienced relapses, one during pregnancy and the other postpartum.
Study details: The data come from a study of 55 women with MS (74 pregnancies) treated with at least 1 dose of rituximab infusion before conception.
Disclosures: This study was supported in part by the Patient-Centered Outcomes Research Institute.
Some of the investigators reported receiving research grants from multiple pharmaceutical companies.
Citation: Smith JB et al. Neurol Neuroimmunol Neuroinflamm. 2020 May 01. doi: 10.1212/NXI.0000000000000734.