User login
Learning the ICU
Although deployment of hospitalists into ICUs during the COVID-19 crisis varies widely, in that sense it reflects the pre-COVID hospital landscape of variable involvement, in which many hospitalists pressed into this role expressed discomfort practicing critical care beyond their scope of training, according to a survey published in the Journal of Hospital Medicine in 2018.1 “Hospitalists frequently deliver critical care services without adequate training or support, most prevalently in rural hospitals,” the authors concluded.
A Critical Care for the Hospitalist Series of resources and lectures developed by Eric Siegal, MD, a pulmonologist in Milwaukee, Wisc., and David Aymond, MD, a hospitalist in Alexandria, La., is available on the SHM website. They recommend that hospitalists trying to get oriented to working in the ICU start with the online courses on fluid resuscitation, mechanical ventilation, and noninvasive ventilation.
“Ninety-five percent of management of COVID-19 patients is nothing other than practicing sound critical care medicine,” Dr. Siegal said. “If you want to take effective care of sick COVID patients, you need to develop good foundational critical care skills and knowledge. Without them, you’re doing stuff without understand it.”
Dr. Aymond also encourages hospitalists to develop a stronger understanding of key physiological concepts by reviewing the critical care clinical topics compiled at SHM’s website.
References
1. Sweigart JR et al. Characterizing hospitalist practice and perceptions of critical care delivery. J Hosp Med. 2018 Jan;13(1):6-12.
Although deployment of hospitalists into ICUs during the COVID-19 crisis varies widely, in that sense it reflects the pre-COVID hospital landscape of variable involvement, in which many hospitalists pressed into this role expressed discomfort practicing critical care beyond their scope of training, according to a survey published in the Journal of Hospital Medicine in 2018.1 “Hospitalists frequently deliver critical care services without adequate training or support, most prevalently in rural hospitals,” the authors concluded.
A Critical Care for the Hospitalist Series of resources and lectures developed by Eric Siegal, MD, a pulmonologist in Milwaukee, Wisc., and David Aymond, MD, a hospitalist in Alexandria, La., is available on the SHM website. They recommend that hospitalists trying to get oriented to working in the ICU start with the online courses on fluid resuscitation, mechanical ventilation, and noninvasive ventilation.
“Ninety-five percent of management of COVID-19 patients is nothing other than practicing sound critical care medicine,” Dr. Siegal said. “If you want to take effective care of sick COVID patients, you need to develop good foundational critical care skills and knowledge. Without them, you’re doing stuff without understand it.”
Dr. Aymond also encourages hospitalists to develop a stronger understanding of key physiological concepts by reviewing the critical care clinical topics compiled at SHM’s website.
References
1. Sweigart JR et al. Characterizing hospitalist practice and perceptions of critical care delivery. J Hosp Med. 2018 Jan;13(1):6-12.
Although deployment of hospitalists into ICUs during the COVID-19 crisis varies widely, in that sense it reflects the pre-COVID hospital landscape of variable involvement, in which many hospitalists pressed into this role expressed discomfort practicing critical care beyond their scope of training, according to a survey published in the Journal of Hospital Medicine in 2018.1 “Hospitalists frequently deliver critical care services without adequate training or support, most prevalently in rural hospitals,” the authors concluded.
A Critical Care for the Hospitalist Series of resources and lectures developed by Eric Siegal, MD, a pulmonologist in Milwaukee, Wisc., and David Aymond, MD, a hospitalist in Alexandria, La., is available on the SHM website. They recommend that hospitalists trying to get oriented to working in the ICU start with the online courses on fluid resuscitation, mechanical ventilation, and noninvasive ventilation.
“Ninety-five percent of management of COVID-19 patients is nothing other than practicing sound critical care medicine,” Dr. Siegal said. “If you want to take effective care of sick COVID patients, you need to develop good foundational critical care skills and knowledge. Without them, you’re doing stuff without understand it.”
Dr. Aymond also encourages hospitalists to develop a stronger understanding of key physiological concepts by reviewing the critical care clinical topics compiled at SHM’s website.
References
1. Sweigart JR et al. Characterizing hospitalist practice and perceptions of critical care delivery. J Hosp Med. 2018 Jan;13(1):6-12.
I am part of the problem
Race is not something I’ve spent that much time contemplating. I grew up in Elizabeth, N.J., a city of just over 100,000, in the 1970s and attended public schools where people came in all shapes and colors; diversity came with the turf, it wasn’t something anyone needed to strive for.
My high school had more than 4,000 students with roughly even numbers of white, black, and Hispanic students. Armed police patrolled the halls, the thick aroma of weed settled in the stairwells and restrooms, girls brought their babies to school to show them off on half-days, and the “preppies” wore Fair Isle sweaters and played on the tennis team. The school’s campus was brand new and every lab, studio, and athletic amenity was state of the art; at the time, it was the most expensive public high school ever built in America. There were black teachers, librarians, and administrators, and segregation was something we read about in history books. I lived in a world of Technicolor and the Civil Rights movement of the 1960s, while still fresh in the minds of the adults, was something that showed up on black-and-white footage from another time.
My world became both wealthier and whiter when I went to college. There were minority students, but many of the black students at the University of Pennsylvania chose to live in the W.E.B. Du Bois College House.
People are often more comfortable being with others who share their backgrounds and this makes for an interesting conundrum: We all agree that desegregation is a good thing, but not everyone wishes to be told either where to go or not go, and there is an odd unbalance to creating a safe place for black students to be, one that both integrates and separates them from the larger community. Perhaps all our lines get fuzzy – I recall when I was on the Maryland Psychiatric Society Women’s Committee and a male psychiatrist signed up to join us – he was politely told that he could not join, but 20 years later, I’m wondering if it was okay to exclude a man who expressed interest in women’s issues.
In medical school, we were taught to note a patient’s age, race, and marital status, and we might learn that certain illnesses were more prevalent in certain populations, but there was no discussion of racial inequities in health care or anywhere else.
What was really different about the world back then, however, was what we didn’t see and what we didn’t talk about. Social media has opened a world where we can share our pain in the moment and we can band together to speak out against crimes and injustices in every realm. From the MeToo moments, to racially motivated police brutality. Cell phone cameras let us record and publicize these moments so the world can be the judge. George Floyd’s sadistic murder by a police officer, as other officers stood by and watched 8 minutes and 46 seconds of torture, left us all triggered, distressed, angry, sad, and activated. Maybe now we can make real progress on a discussion that began in 1992 with the videotape of Rodney King’s assault, a discussion we’ve had over and over to no avail.
Obviously, I have also been provoked by the events of the past weeks – like many Americans, I’ve paused to wonder how I can help the cause, both personally and as a psychiatrist. I would not normally write about racial topics – as a white woman I can listen, but I don’t feel this pain in the same way as someone who has lived with a lifetime of discrimination and oppression. Dr. Lorenzo Norris and Dr. Brandon Newsome,two black psychiatrists, put out a special edition of the MDEdge Psychcast, “The fallout from George Floyd’s death,” and Dr. Norris noted that two of his white colleagues told him they thought of checking on him, but they didn’t know what to say. Yes, I thought, that’s exactly it, I don’t know what to say and I worry that I might unintentionally say something that would worsen someone else’s pain. Staying silent has always seemed to be the safest option. With this article, I’m moving from a place of comfort.
I started my career with a mix of private practice and community psychiatry. There were things I loved about working in a community clinic: the social aspects of being part of a team, seeing a full range of psychopathology, and treating patients in which the racial and ethnic demographics mirrored that of the community. There were things I didn’t like, however. The pay was low, there were constant institutional requirements that were not relevant to the practice of psychiatry, and my relationship with the patients as their prescriber was much less fulfilling than the relationship I have with those I see for both psychotherapy and medication. Ultimately, the hospital shift to electronic medical records was the final distraction that caused me to leave community work.
Like roughly half of psychiatrists in private practice, I don’t participate with commercial or public insurance plans. Early in my career, I worked in a group setting with billing secretaries, and I did participate with Blue Cross, but even with administrative help, nothing about this was easy, and when I left to do solo practice, I left insurance participation behind. I love the autonomy of my career, I’m proud of the care I am able to give in this setting, and I don’t miss the hassles. But – the out-of-pocket cost of care is higher and the effort of trying to get reimbursed falls to the patient. It means that most of the patients I see have the means to pay for care, none are impoverished or homeless, and while I work in a city that is 62% black, black patients make up a small percentage of my caseload. I don’t think I am unique in this; I would be shocked if any white private practice psychiatrist who specializes in psychotherapy is serving a racially proportionate population. As we start to embrace the idea that people don’t neatly divide into being racist or not, and that bias affects us all, we must acknowledge that medical practices that don’t support racially balanced access to care are part of the problem.
Amy R. Greensfelder, LMSW, is the executive director of Maryland’s Pro Bono Counseling Project (PBCP), an organization that coordinates mental health professionals in private practice in Maryland to volunteer their services to those with limited resources. PBCP has found that 50% of those seeking services share that they are black or African American, and an additional 5% identify as multiracial. Of all of those seeking care approximately 65% are black, Indigenous, or People of Color (BIPOC), and and 14% are Latino/a/x/Hispanic. She says: “We see the racial composition of our clients as a direct demonstration of who is being left behind in the mental health system as it’s currently set up, as BIPOC individuals are represented to a greater degree in our clients than they are in the general population of Maryland. During our intake interview, we provide an opportunity for clients to share if there are certain characteristics they are looking for in a therapist – often black clients share that they would prefer to be matched with a black therapist or a therapist who has received specific training on working with black clients.”
While 13% of the American population is black, only 4% of physicians, 2% of psychiatrists, and 4% of psychologists are black. In her Psychology Today blog post, “Why African Americans Avoid Psychotherapy,” Monnica T. Williams, PhD, notes: “Apprehension about clashing with the values or worldview of the clinician can cause ambivalence about seeking help, and this may be especially true for the many who believe that mental health treatment was designed by white people for white people.” Dr. Williams notes that black Americans also are less likely to seek care because of increased stigma and fear of judgment, concerns about the treatment process, and fears of being involuntarily hospitalized, cost and lack of insurance, and finally logistical issues with work, transportation, and family responsibilities.
George Floyd’s tragic death has led us to a moment of crisis. It’s my hope that the dialogue is now galvanized to make meaningful changes toward fixing racial inequities. I am part of the problem and these conversations need to include more equitable access to psychiatric care.
My thanks to Rachel Donabedian and Gina Henderson for their help with this article.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Race is not something I’ve spent that much time contemplating. I grew up in Elizabeth, N.J., a city of just over 100,000, in the 1970s and attended public schools where people came in all shapes and colors; diversity came with the turf, it wasn’t something anyone needed to strive for.
My high school had more than 4,000 students with roughly even numbers of white, black, and Hispanic students. Armed police patrolled the halls, the thick aroma of weed settled in the stairwells and restrooms, girls brought their babies to school to show them off on half-days, and the “preppies” wore Fair Isle sweaters and played on the tennis team. The school’s campus was brand new and every lab, studio, and athletic amenity was state of the art; at the time, it was the most expensive public high school ever built in America. There were black teachers, librarians, and administrators, and segregation was something we read about in history books. I lived in a world of Technicolor and the Civil Rights movement of the 1960s, while still fresh in the minds of the adults, was something that showed up on black-and-white footage from another time.
My world became both wealthier and whiter when I went to college. There were minority students, but many of the black students at the University of Pennsylvania chose to live in the W.E.B. Du Bois College House.
People are often more comfortable being with others who share their backgrounds and this makes for an interesting conundrum: We all agree that desegregation is a good thing, but not everyone wishes to be told either where to go or not go, and there is an odd unbalance to creating a safe place for black students to be, one that both integrates and separates them from the larger community. Perhaps all our lines get fuzzy – I recall when I was on the Maryland Psychiatric Society Women’s Committee and a male psychiatrist signed up to join us – he was politely told that he could not join, but 20 years later, I’m wondering if it was okay to exclude a man who expressed interest in women’s issues.
In medical school, we were taught to note a patient’s age, race, and marital status, and we might learn that certain illnesses were more prevalent in certain populations, but there was no discussion of racial inequities in health care or anywhere else.
What was really different about the world back then, however, was what we didn’t see and what we didn’t talk about. Social media has opened a world where we can share our pain in the moment and we can band together to speak out against crimes and injustices in every realm. From the MeToo moments, to racially motivated police brutality. Cell phone cameras let us record and publicize these moments so the world can be the judge. George Floyd’s sadistic murder by a police officer, as other officers stood by and watched 8 minutes and 46 seconds of torture, left us all triggered, distressed, angry, sad, and activated. Maybe now we can make real progress on a discussion that began in 1992 with the videotape of Rodney King’s assault, a discussion we’ve had over and over to no avail.
Obviously, I have also been provoked by the events of the past weeks – like many Americans, I’ve paused to wonder how I can help the cause, both personally and as a psychiatrist. I would not normally write about racial topics – as a white woman I can listen, but I don’t feel this pain in the same way as someone who has lived with a lifetime of discrimination and oppression. Dr. Lorenzo Norris and Dr. Brandon Newsome,two black psychiatrists, put out a special edition of the MDEdge Psychcast, “The fallout from George Floyd’s death,” and Dr. Norris noted that two of his white colleagues told him they thought of checking on him, but they didn’t know what to say. Yes, I thought, that’s exactly it, I don’t know what to say and I worry that I might unintentionally say something that would worsen someone else’s pain. Staying silent has always seemed to be the safest option. With this article, I’m moving from a place of comfort.
I started my career with a mix of private practice and community psychiatry. There were things I loved about working in a community clinic: the social aspects of being part of a team, seeing a full range of psychopathology, and treating patients in which the racial and ethnic demographics mirrored that of the community. There were things I didn’t like, however. The pay was low, there were constant institutional requirements that were not relevant to the practice of psychiatry, and my relationship with the patients as their prescriber was much less fulfilling than the relationship I have with those I see for both psychotherapy and medication. Ultimately, the hospital shift to electronic medical records was the final distraction that caused me to leave community work.
Like roughly half of psychiatrists in private practice, I don’t participate with commercial or public insurance plans. Early in my career, I worked in a group setting with billing secretaries, and I did participate with Blue Cross, but even with administrative help, nothing about this was easy, and when I left to do solo practice, I left insurance participation behind. I love the autonomy of my career, I’m proud of the care I am able to give in this setting, and I don’t miss the hassles. But – the out-of-pocket cost of care is higher and the effort of trying to get reimbursed falls to the patient. It means that most of the patients I see have the means to pay for care, none are impoverished or homeless, and while I work in a city that is 62% black, black patients make up a small percentage of my caseload. I don’t think I am unique in this; I would be shocked if any white private practice psychiatrist who specializes in psychotherapy is serving a racially proportionate population. As we start to embrace the idea that people don’t neatly divide into being racist or not, and that bias affects us all, we must acknowledge that medical practices that don’t support racially balanced access to care are part of the problem.
Amy R. Greensfelder, LMSW, is the executive director of Maryland’s Pro Bono Counseling Project (PBCP), an organization that coordinates mental health professionals in private practice in Maryland to volunteer their services to those with limited resources. PBCP has found that 50% of those seeking services share that they are black or African American, and an additional 5% identify as multiracial. Of all of those seeking care approximately 65% are black, Indigenous, or People of Color (BIPOC), and and 14% are Latino/a/x/Hispanic. She says: “We see the racial composition of our clients as a direct demonstration of who is being left behind in the mental health system as it’s currently set up, as BIPOC individuals are represented to a greater degree in our clients than they are in the general population of Maryland. During our intake interview, we provide an opportunity for clients to share if there are certain characteristics they are looking for in a therapist – often black clients share that they would prefer to be matched with a black therapist or a therapist who has received specific training on working with black clients.”
While 13% of the American population is black, only 4% of physicians, 2% of psychiatrists, and 4% of psychologists are black. In her Psychology Today blog post, “Why African Americans Avoid Psychotherapy,” Monnica T. Williams, PhD, notes: “Apprehension about clashing with the values or worldview of the clinician can cause ambivalence about seeking help, and this may be especially true for the many who believe that mental health treatment was designed by white people for white people.” Dr. Williams notes that black Americans also are less likely to seek care because of increased stigma and fear of judgment, concerns about the treatment process, and fears of being involuntarily hospitalized, cost and lack of insurance, and finally logistical issues with work, transportation, and family responsibilities.
George Floyd’s tragic death has led us to a moment of crisis. It’s my hope that the dialogue is now galvanized to make meaningful changes toward fixing racial inequities. I am part of the problem and these conversations need to include more equitable access to psychiatric care.
My thanks to Rachel Donabedian and Gina Henderson for their help with this article.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Race is not something I’ve spent that much time contemplating. I grew up in Elizabeth, N.J., a city of just over 100,000, in the 1970s and attended public schools where people came in all shapes and colors; diversity came with the turf, it wasn’t something anyone needed to strive for.
My high school had more than 4,000 students with roughly even numbers of white, black, and Hispanic students. Armed police patrolled the halls, the thick aroma of weed settled in the stairwells and restrooms, girls brought their babies to school to show them off on half-days, and the “preppies” wore Fair Isle sweaters and played on the tennis team. The school’s campus was brand new and every lab, studio, and athletic amenity was state of the art; at the time, it was the most expensive public high school ever built in America. There were black teachers, librarians, and administrators, and segregation was something we read about in history books. I lived in a world of Technicolor and the Civil Rights movement of the 1960s, while still fresh in the minds of the adults, was something that showed up on black-and-white footage from another time.
My world became both wealthier and whiter when I went to college. There were minority students, but many of the black students at the University of Pennsylvania chose to live in the W.E.B. Du Bois College House.
People are often more comfortable being with others who share their backgrounds and this makes for an interesting conundrum: We all agree that desegregation is a good thing, but not everyone wishes to be told either where to go or not go, and there is an odd unbalance to creating a safe place for black students to be, one that both integrates and separates them from the larger community. Perhaps all our lines get fuzzy – I recall when I was on the Maryland Psychiatric Society Women’s Committee and a male psychiatrist signed up to join us – he was politely told that he could not join, but 20 years later, I’m wondering if it was okay to exclude a man who expressed interest in women’s issues.
In medical school, we were taught to note a patient’s age, race, and marital status, and we might learn that certain illnesses were more prevalent in certain populations, but there was no discussion of racial inequities in health care or anywhere else.
What was really different about the world back then, however, was what we didn’t see and what we didn’t talk about. Social media has opened a world where we can share our pain in the moment and we can band together to speak out against crimes and injustices in every realm. From the MeToo moments, to racially motivated police brutality. Cell phone cameras let us record and publicize these moments so the world can be the judge. George Floyd’s sadistic murder by a police officer, as other officers stood by and watched 8 minutes and 46 seconds of torture, left us all triggered, distressed, angry, sad, and activated. Maybe now we can make real progress on a discussion that began in 1992 with the videotape of Rodney King’s assault, a discussion we’ve had over and over to no avail.
Obviously, I have also been provoked by the events of the past weeks – like many Americans, I’ve paused to wonder how I can help the cause, both personally and as a psychiatrist. I would not normally write about racial topics – as a white woman I can listen, but I don’t feel this pain in the same way as someone who has lived with a lifetime of discrimination and oppression. Dr. Lorenzo Norris and Dr. Brandon Newsome,two black psychiatrists, put out a special edition of the MDEdge Psychcast, “The fallout from George Floyd’s death,” and Dr. Norris noted that two of his white colleagues told him they thought of checking on him, but they didn’t know what to say. Yes, I thought, that’s exactly it, I don’t know what to say and I worry that I might unintentionally say something that would worsen someone else’s pain. Staying silent has always seemed to be the safest option. With this article, I’m moving from a place of comfort.
I started my career with a mix of private practice and community psychiatry. There were things I loved about working in a community clinic: the social aspects of being part of a team, seeing a full range of psychopathology, and treating patients in which the racial and ethnic demographics mirrored that of the community. There were things I didn’t like, however. The pay was low, there were constant institutional requirements that were not relevant to the practice of psychiatry, and my relationship with the patients as their prescriber was much less fulfilling than the relationship I have with those I see for both psychotherapy and medication. Ultimately, the hospital shift to electronic medical records was the final distraction that caused me to leave community work.
Like roughly half of psychiatrists in private practice, I don’t participate with commercial or public insurance plans. Early in my career, I worked in a group setting with billing secretaries, and I did participate with Blue Cross, but even with administrative help, nothing about this was easy, and when I left to do solo practice, I left insurance participation behind. I love the autonomy of my career, I’m proud of the care I am able to give in this setting, and I don’t miss the hassles. But – the out-of-pocket cost of care is higher and the effort of trying to get reimbursed falls to the patient. It means that most of the patients I see have the means to pay for care, none are impoverished or homeless, and while I work in a city that is 62% black, black patients make up a small percentage of my caseload. I don’t think I am unique in this; I would be shocked if any white private practice psychiatrist who specializes in psychotherapy is serving a racially proportionate population. As we start to embrace the idea that people don’t neatly divide into being racist or not, and that bias affects us all, we must acknowledge that medical practices that don’t support racially balanced access to care are part of the problem.
Amy R. Greensfelder, LMSW, is the executive director of Maryland’s Pro Bono Counseling Project (PBCP), an organization that coordinates mental health professionals in private practice in Maryland to volunteer their services to those with limited resources. PBCP has found that 50% of those seeking services share that they are black or African American, and an additional 5% identify as multiracial. Of all of those seeking care approximately 65% are black, Indigenous, or People of Color (BIPOC), and and 14% are Latino/a/x/Hispanic. She says: “We see the racial composition of our clients as a direct demonstration of who is being left behind in the mental health system as it’s currently set up, as BIPOC individuals are represented to a greater degree in our clients than they are in the general population of Maryland. During our intake interview, we provide an opportunity for clients to share if there are certain characteristics they are looking for in a therapist – often black clients share that they would prefer to be matched with a black therapist or a therapist who has received specific training on working with black clients.”
While 13% of the American population is black, only 4% of physicians, 2% of psychiatrists, and 4% of psychologists are black. In her Psychology Today blog post, “Why African Americans Avoid Psychotherapy,” Monnica T. Williams, PhD, notes: “Apprehension about clashing with the values or worldview of the clinician can cause ambivalence about seeking help, and this may be especially true for the many who believe that mental health treatment was designed by white people for white people.” Dr. Williams notes that black Americans also are less likely to seek care because of increased stigma and fear of judgment, concerns about the treatment process, and fears of being involuntarily hospitalized, cost and lack of insurance, and finally logistical issues with work, transportation, and family responsibilities.
George Floyd’s tragic death has led us to a moment of crisis. It’s my hope that the dialogue is now galvanized to make meaningful changes toward fixing racial inequities. I am part of the problem and these conversations need to include more equitable access to psychiatric care.
My thanks to Rachel Donabedian and Gina Henderson for their help with this article.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Antinuclear antibody test interpretation guidance gets updated
New recommendations from the European League Against Rheumatism on interpreting the results of antinuclear antibody (ANA) testing advised taking the test methodology into account because of differences in performance.
ANA results vary not only by the test being used but also by the underlying disease they are being used to assess, warned Pier Luigi Meroni, MD, director of the Immunorheumatology Research Laboratory at the IRCCS Istituto Auxologico Italiano in Milan.
“Antinuclear antibody testing is a known diagnostic tool. But the recent advances in methodologies strongly suggests that we have to update our knowledge for a better interpretation of the results,” Dr. Meroni said in his presentation at the annual European Congress of Rheumatology, held online this year due to COVID-19.
There is “no doubt that ANA testing is useful,” he continued, adding that ANA is used as a primary screening tool in many rheumatic diseases, notably systemic lupus erythematosus (SLE), primary Sjögren’s syndrome, and systemic sclerosis. It’s also recently been suggested as an important entry criterion for the classification of SLE.
In fact, the 2019 SLE classification criteria – developed by EULAR in collaboration with the American College of Rheumatology – state that “testing by immunofluorescence on HEp-2 cells or a solid-phase ANA screening immunoassay with at least equivalent performance is highly recommended,” Dr. Meroni said.
The ideas underpinning that recommendation was that “ANA expression is invariable in SLE, and that ANA-negative lupus is quite rare,” he explained. Also, as SLE expression persists over time, ANA testing could be used for classification at any point in the disease course. These assumptions have been borne out in several studies, with very small percentages of patients (6% or less) having ANA-negative lupus, and more than 80% having a positive HEp-2 test over time, even with immunosuppressive treatment.
Which test methodology to use?
There are several methods that can be used to detect ANA, including the preferred HEp-2 indirect fluorescence assay (IFA), several solid-phase assays (SpA), and line- or dot-blot immunoassays. The issue is which assay should be used in which disease?
The performance of a particular assay can depend on the disease in which they are used. For instance, while the HEp-2 IFA and SpA are equivalent in SLE and in other connective tissue diseases, “this is not the case for other autoimmune diseases in which basically we don’t know exactly all the autoantigens,” Dr. Meroni explained. “Most of the autoantigens are undefined. They cannot be found in solid-phase kits, and we have to use the IFA for detecting all these autoantibodies.”
Importantly, neither the IFA nor the SpA is superior to the other. “We just say that one technique can detect relevant antibodies that are not detectable by the other one, and maybe the combination of the two techniques can be the right strategy to get the highest sensitivity,” Dr. Meroni said.
“Clinicians should be aware of the type of assay used for ANA detection,” he said, “because there are strong differences in the performance, for example between IFA and SpA, and such differences can have important clinical and relevant consequences.”
The test selected will depend on if the aim is to exclude or confirm a disease, and the optimal strategy will depend on pretest probability. For instance, IFA is more sensitive than SpA for SLE and scleroderma, whereas IFA is less sensitive than SpA for Sjögren’s. For SLE, it is suggested to use both the IFA and SpA. A combination of both tests is also considered optimal for scleroderma. SpA testing offers the best sensitivity for Sjögren’s.
“The story is a little bit more complicated for inflammatory myopathies in which we don’t have assays able to detect all the autoantibodies,” Dr. Meroni said. In that situation, several different techniques have to be used to check if the SpA results fit with the IFA pattern.
In 2019, the ACR released its own position statement on ANA testing, highlighting that it supported the use of the HEp-2 IFA assay as the preferred option for ANA testing and that labs should specify the methods being used to test for ANA when reporting their results. The ACR position statement also noted that “ordering health care professionals should select specific ANA subserologies based on a patient’s signs and symptoms and when there is a high pretest suspicion for a specific condition.”
Dr. Meroni disclosed serving as a consultant to Inova Diagnostics, Thermo Fisher Scientific, Pfizer, AbbVie, Merck Sharp & Dohme, and UCB.
New recommendations from the European League Against Rheumatism on interpreting the results of antinuclear antibody (ANA) testing advised taking the test methodology into account because of differences in performance.
ANA results vary not only by the test being used but also by the underlying disease they are being used to assess, warned Pier Luigi Meroni, MD, director of the Immunorheumatology Research Laboratory at the IRCCS Istituto Auxologico Italiano in Milan.
“Antinuclear antibody testing is a known diagnostic tool. But the recent advances in methodologies strongly suggests that we have to update our knowledge for a better interpretation of the results,” Dr. Meroni said in his presentation at the annual European Congress of Rheumatology, held online this year due to COVID-19.
There is “no doubt that ANA testing is useful,” he continued, adding that ANA is used as a primary screening tool in many rheumatic diseases, notably systemic lupus erythematosus (SLE), primary Sjögren’s syndrome, and systemic sclerosis. It’s also recently been suggested as an important entry criterion for the classification of SLE.
In fact, the 2019 SLE classification criteria – developed by EULAR in collaboration with the American College of Rheumatology – state that “testing by immunofluorescence on HEp-2 cells or a solid-phase ANA screening immunoassay with at least equivalent performance is highly recommended,” Dr. Meroni said.
The ideas underpinning that recommendation was that “ANA expression is invariable in SLE, and that ANA-negative lupus is quite rare,” he explained. Also, as SLE expression persists over time, ANA testing could be used for classification at any point in the disease course. These assumptions have been borne out in several studies, with very small percentages of patients (6% or less) having ANA-negative lupus, and more than 80% having a positive HEp-2 test over time, even with immunosuppressive treatment.
Which test methodology to use?
There are several methods that can be used to detect ANA, including the preferred HEp-2 indirect fluorescence assay (IFA), several solid-phase assays (SpA), and line- or dot-blot immunoassays. The issue is which assay should be used in which disease?
The performance of a particular assay can depend on the disease in which they are used. For instance, while the HEp-2 IFA and SpA are equivalent in SLE and in other connective tissue diseases, “this is not the case for other autoimmune diseases in which basically we don’t know exactly all the autoantigens,” Dr. Meroni explained. “Most of the autoantigens are undefined. They cannot be found in solid-phase kits, and we have to use the IFA for detecting all these autoantibodies.”
Importantly, neither the IFA nor the SpA is superior to the other. “We just say that one technique can detect relevant antibodies that are not detectable by the other one, and maybe the combination of the two techniques can be the right strategy to get the highest sensitivity,” Dr. Meroni said.
“Clinicians should be aware of the type of assay used for ANA detection,” he said, “because there are strong differences in the performance, for example between IFA and SpA, and such differences can have important clinical and relevant consequences.”
The test selected will depend on if the aim is to exclude or confirm a disease, and the optimal strategy will depend on pretest probability. For instance, IFA is more sensitive than SpA for SLE and scleroderma, whereas IFA is less sensitive than SpA for Sjögren’s. For SLE, it is suggested to use both the IFA and SpA. A combination of both tests is also considered optimal for scleroderma. SpA testing offers the best sensitivity for Sjögren’s.
“The story is a little bit more complicated for inflammatory myopathies in which we don’t have assays able to detect all the autoantibodies,” Dr. Meroni said. In that situation, several different techniques have to be used to check if the SpA results fit with the IFA pattern.
In 2019, the ACR released its own position statement on ANA testing, highlighting that it supported the use of the HEp-2 IFA assay as the preferred option for ANA testing and that labs should specify the methods being used to test for ANA when reporting their results. The ACR position statement also noted that “ordering health care professionals should select specific ANA subserologies based on a patient’s signs and symptoms and when there is a high pretest suspicion for a specific condition.”
Dr. Meroni disclosed serving as a consultant to Inova Diagnostics, Thermo Fisher Scientific, Pfizer, AbbVie, Merck Sharp & Dohme, and UCB.
New recommendations from the European League Against Rheumatism on interpreting the results of antinuclear antibody (ANA) testing advised taking the test methodology into account because of differences in performance.
ANA results vary not only by the test being used but also by the underlying disease they are being used to assess, warned Pier Luigi Meroni, MD, director of the Immunorheumatology Research Laboratory at the IRCCS Istituto Auxologico Italiano in Milan.
“Antinuclear antibody testing is a known diagnostic tool. But the recent advances in methodologies strongly suggests that we have to update our knowledge for a better interpretation of the results,” Dr. Meroni said in his presentation at the annual European Congress of Rheumatology, held online this year due to COVID-19.
There is “no doubt that ANA testing is useful,” he continued, adding that ANA is used as a primary screening tool in many rheumatic diseases, notably systemic lupus erythematosus (SLE), primary Sjögren’s syndrome, and systemic sclerosis. It’s also recently been suggested as an important entry criterion for the classification of SLE.
In fact, the 2019 SLE classification criteria – developed by EULAR in collaboration with the American College of Rheumatology – state that “testing by immunofluorescence on HEp-2 cells or a solid-phase ANA screening immunoassay with at least equivalent performance is highly recommended,” Dr. Meroni said.
The ideas underpinning that recommendation was that “ANA expression is invariable in SLE, and that ANA-negative lupus is quite rare,” he explained. Also, as SLE expression persists over time, ANA testing could be used for classification at any point in the disease course. These assumptions have been borne out in several studies, with very small percentages of patients (6% or less) having ANA-negative lupus, and more than 80% having a positive HEp-2 test over time, even with immunosuppressive treatment.
Which test methodology to use?
There are several methods that can be used to detect ANA, including the preferred HEp-2 indirect fluorescence assay (IFA), several solid-phase assays (SpA), and line- or dot-blot immunoassays. The issue is which assay should be used in which disease?
The performance of a particular assay can depend on the disease in which they are used. For instance, while the HEp-2 IFA and SpA are equivalent in SLE and in other connective tissue diseases, “this is not the case for other autoimmune diseases in which basically we don’t know exactly all the autoantigens,” Dr. Meroni explained. “Most of the autoantigens are undefined. They cannot be found in solid-phase kits, and we have to use the IFA for detecting all these autoantibodies.”
Importantly, neither the IFA nor the SpA is superior to the other. “We just say that one technique can detect relevant antibodies that are not detectable by the other one, and maybe the combination of the two techniques can be the right strategy to get the highest sensitivity,” Dr. Meroni said.
“Clinicians should be aware of the type of assay used for ANA detection,” he said, “because there are strong differences in the performance, for example between IFA and SpA, and such differences can have important clinical and relevant consequences.”
The test selected will depend on if the aim is to exclude or confirm a disease, and the optimal strategy will depend on pretest probability. For instance, IFA is more sensitive than SpA for SLE and scleroderma, whereas IFA is less sensitive than SpA for Sjögren’s. For SLE, it is suggested to use both the IFA and SpA. A combination of both tests is also considered optimal for scleroderma. SpA testing offers the best sensitivity for Sjögren’s.
“The story is a little bit more complicated for inflammatory myopathies in which we don’t have assays able to detect all the autoantibodies,” Dr. Meroni said. In that situation, several different techniques have to be used to check if the SpA results fit with the IFA pattern.
In 2019, the ACR released its own position statement on ANA testing, highlighting that it supported the use of the HEp-2 IFA assay as the preferred option for ANA testing and that labs should specify the methods being used to test for ANA when reporting their results. The ACR position statement also noted that “ordering health care professionals should select specific ANA subserologies based on a patient’s signs and symptoms and when there is a high pretest suspicion for a specific condition.”
Dr. Meroni disclosed serving as a consultant to Inova Diagnostics, Thermo Fisher Scientific, Pfizer, AbbVie, Merck Sharp & Dohme, and UCB.
FROM THE EULAR 2020 E-CONGRESS
Daily Recap: FDA revokes emergency use of hydroxychloroquine; Hardest hit specialties ranked in financial report
Here are the stories our MDedge editors across specialties think you need to know about today:
It’s official: COVID-19 is bad for your health care business
For the months of March and April 2020, use of medical professional services dropped by 65% and 68%, respectively, compared with last year, and estimated revenue fell by 45% and 48%, FAIR Health, a nonprofit organization that manages a database of 31 billion claim records, said in a new report.
Of the seven specialties included in the study, oral surgery was hit the hardest, followed by gastroenterology, cardiology, orthopedics, dermatology, adult primary care, and pediatric primary care, FAIR Health said.
“Even when medical practices have continued to function via telehealth, many have experienced lower reimbursements for telehealth visits than for in-person visits and more time educating patients on how to use the technology,” according to the report. Read more.
FDA revokes emergency use of hydroxychloroquine
The FDA revoked its decision from March 28 allowing use of hydroxychloroquine and chloroquine to treat people hospitalized with COVID-19 under an emergency use authorization (EUA).
"Based on its ongoing analysis of the EUA and emerging scientific data, the FDA determined that chloroquine and hydroxychloroquine are unlikely to be effective in treating COVID-19," the agency announced in a June 15 statement.
"In light of ongoing serious cardiac adverse events and other potential serious side effects, the known and potential benefits of chloroquine and hydroxychloroquine no longer outweigh the known and potential risks for the authorized use," noted the FDA. Read more.
Secondary infections common in COVID-19, implications unclear
Secondary respiratory infections appear to be highly prevalent among patients with severe COVID-19, but at this point, most physicians aren’t sure what to make of this understudied phenomenon.
“We really do not understand the implications of secondary infections on outcomes in COVID-19 patients,” David L. Bowton, MD, FCCP, said in an interview. “In most early reports the incidence of secondary infections was much higher in patients dying from COVID-19, compared to survivors, but it isn’t clear whether this indicates that the secondary infection itself led to excess mortality or was more a marker of the severity of the COVID-19 infection."
An early retrospective cohort study including 191 COVID-19 patients in Wuhan, China found that of the 54 who died in hospital, half had secondary bacterial lung infections (Lancet. 2020 Mar 28;395[10229]:1054-62). That comes as no surprise to U.S. physicians, who learned in training that many deaths during the so-called Spanish influenza epidemic were actually caused by secondary pneumonia involving Staphylococcus aureus, commented Daniel L. Ouellette, MD, FCCP. Read more.
Automated insulin delivery system ‘getting better and better’
Medtronic’s next-generation automated insulin delivery system offers significant improvements over the currently available model, particularly in young people with type 1 diabetes, new data suggest.
Data from three trials of such systems using Medtronic’s advanced hybrid closed-loop (AHCL) algorithm (trade name SmartGuard) were presented during the virtual American Diabetes Association (ADA) 80th Scientific Sessions.
Taken together, the data from the three trials showed that the AHCL-based system improved glycemic time-in-range with no increased risk for hypoglycemia, including in children and teenagers, with high patient-reported satisfaction.
“None of these devices is perfect, but they are a substantial improvement over what we’ve had ... They might make the quality of [patient] lives better. That’s really underappreciated,” session moderator Timothy S. Bailey, MD, commented. Read more.
Access more top news from the ADA virtual meeting.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
It’s official: COVID-19 is bad for your health care business
For the months of March and April 2020, use of medical professional services dropped by 65% and 68%, respectively, compared with last year, and estimated revenue fell by 45% and 48%, FAIR Health, a nonprofit organization that manages a database of 31 billion claim records, said in a new report.
Of the seven specialties included in the study, oral surgery was hit the hardest, followed by gastroenterology, cardiology, orthopedics, dermatology, adult primary care, and pediatric primary care, FAIR Health said.
“Even when medical practices have continued to function via telehealth, many have experienced lower reimbursements for telehealth visits than for in-person visits and more time educating patients on how to use the technology,” according to the report. Read more.
FDA revokes emergency use of hydroxychloroquine
The FDA revoked its decision from March 28 allowing use of hydroxychloroquine and chloroquine to treat people hospitalized with COVID-19 under an emergency use authorization (EUA).
"Based on its ongoing analysis of the EUA and emerging scientific data, the FDA determined that chloroquine and hydroxychloroquine are unlikely to be effective in treating COVID-19," the agency announced in a June 15 statement.
"In light of ongoing serious cardiac adverse events and other potential serious side effects, the known and potential benefits of chloroquine and hydroxychloroquine no longer outweigh the known and potential risks for the authorized use," noted the FDA. Read more.
Secondary infections common in COVID-19, implications unclear
Secondary respiratory infections appear to be highly prevalent among patients with severe COVID-19, but at this point, most physicians aren’t sure what to make of this understudied phenomenon.
“We really do not understand the implications of secondary infections on outcomes in COVID-19 patients,” David L. Bowton, MD, FCCP, said in an interview. “In most early reports the incidence of secondary infections was much higher in patients dying from COVID-19, compared to survivors, but it isn’t clear whether this indicates that the secondary infection itself led to excess mortality or was more a marker of the severity of the COVID-19 infection."
An early retrospective cohort study including 191 COVID-19 patients in Wuhan, China found that of the 54 who died in hospital, half had secondary bacterial lung infections (Lancet. 2020 Mar 28;395[10229]:1054-62). That comes as no surprise to U.S. physicians, who learned in training that many deaths during the so-called Spanish influenza epidemic were actually caused by secondary pneumonia involving Staphylococcus aureus, commented Daniel L. Ouellette, MD, FCCP. Read more.
Automated insulin delivery system ‘getting better and better’
Medtronic’s next-generation automated insulin delivery system offers significant improvements over the currently available model, particularly in young people with type 1 diabetes, new data suggest.
Data from three trials of such systems using Medtronic’s advanced hybrid closed-loop (AHCL) algorithm (trade name SmartGuard) were presented during the virtual American Diabetes Association (ADA) 80th Scientific Sessions.
Taken together, the data from the three trials showed that the AHCL-based system improved glycemic time-in-range with no increased risk for hypoglycemia, including in children and teenagers, with high patient-reported satisfaction.
“None of these devices is perfect, but they are a substantial improvement over what we’ve had ... They might make the quality of [patient] lives better. That’s really underappreciated,” session moderator Timothy S. Bailey, MD, commented. Read more.
Access more top news from the ADA virtual meeting.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
It’s official: COVID-19 is bad for your health care business
For the months of March and April 2020, use of medical professional services dropped by 65% and 68%, respectively, compared with last year, and estimated revenue fell by 45% and 48%, FAIR Health, a nonprofit organization that manages a database of 31 billion claim records, said in a new report.
Of the seven specialties included in the study, oral surgery was hit the hardest, followed by gastroenterology, cardiology, orthopedics, dermatology, adult primary care, and pediatric primary care, FAIR Health said.
“Even when medical practices have continued to function via telehealth, many have experienced lower reimbursements for telehealth visits than for in-person visits and more time educating patients on how to use the technology,” according to the report. Read more.
FDA revokes emergency use of hydroxychloroquine
The FDA revoked its decision from March 28 allowing use of hydroxychloroquine and chloroquine to treat people hospitalized with COVID-19 under an emergency use authorization (EUA).
"Based on its ongoing analysis of the EUA and emerging scientific data, the FDA determined that chloroquine and hydroxychloroquine are unlikely to be effective in treating COVID-19," the agency announced in a June 15 statement.
"In light of ongoing serious cardiac adverse events and other potential serious side effects, the known and potential benefits of chloroquine and hydroxychloroquine no longer outweigh the known and potential risks for the authorized use," noted the FDA. Read more.
Secondary infections common in COVID-19, implications unclear
Secondary respiratory infections appear to be highly prevalent among patients with severe COVID-19, but at this point, most physicians aren’t sure what to make of this understudied phenomenon.
“We really do not understand the implications of secondary infections on outcomes in COVID-19 patients,” David L. Bowton, MD, FCCP, said in an interview. “In most early reports the incidence of secondary infections was much higher in patients dying from COVID-19, compared to survivors, but it isn’t clear whether this indicates that the secondary infection itself led to excess mortality or was more a marker of the severity of the COVID-19 infection."
An early retrospective cohort study including 191 COVID-19 patients in Wuhan, China found that of the 54 who died in hospital, half had secondary bacterial lung infections (Lancet. 2020 Mar 28;395[10229]:1054-62). That comes as no surprise to U.S. physicians, who learned in training that many deaths during the so-called Spanish influenza epidemic were actually caused by secondary pneumonia involving Staphylococcus aureus, commented Daniel L. Ouellette, MD, FCCP. Read more.
Automated insulin delivery system ‘getting better and better’
Medtronic’s next-generation automated insulin delivery system offers significant improvements over the currently available model, particularly in young people with type 1 diabetes, new data suggest.
Data from three trials of such systems using Medtronic’s advanced hybrid closed-loop (AHCL) algorithm (trade name SmartGuard) were presented during the virtual American Diabetes Association (ADA) 80th Scientific Sessions.
Taken together, the data from the three trials showed that the AHCL-based system improved glycemic time-in-range with no increased risk for hypoglycemia, including in children and teenagers, with high patient-reported satisfaction.
“None of these devices is perfect, but they are a substantial improvement over what we’ve had ... They might make the quality of [patient] lives better. That’s really underappreciated,” session moderator Timothy S. Bailey, MD, commented. Read more.
Access more top news from the ADA virtual meeting.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
It’s official: COVID-19 was bad for the health care business
COVID-19 took a huge cut of clinicians’ business in March and April
In the first 2 months of the COVID-19 pandemic, health care professionals experienced sharp drops in both utilization and revenue, according to an analysis of the nation’s largest collection of private health care claims data.
For the months of March and April 2020, use of medical professional services dropped by 65% and 68%, respectively, compared with last year, and estimated revenue fell by 45% and 48%, FAIR Health, a nonprofit organization that manages a database of 31 billion claim records, said in a new report.
For the Northeast states – the epicenter of the pandemic in March and April – patient volume was down by 60% in March and 80% in April, while revenue fell by 55% in March and 79% in April, the organization said.
For this analysis, “a professional service was defined as any service provided by an individual (e.g., physician, nurse, nurse practitioner, physician assistant) instead of being billed by a facility,” FAIR Health noted. Figures for 2019 were adjusted using the Consumer Price Index.
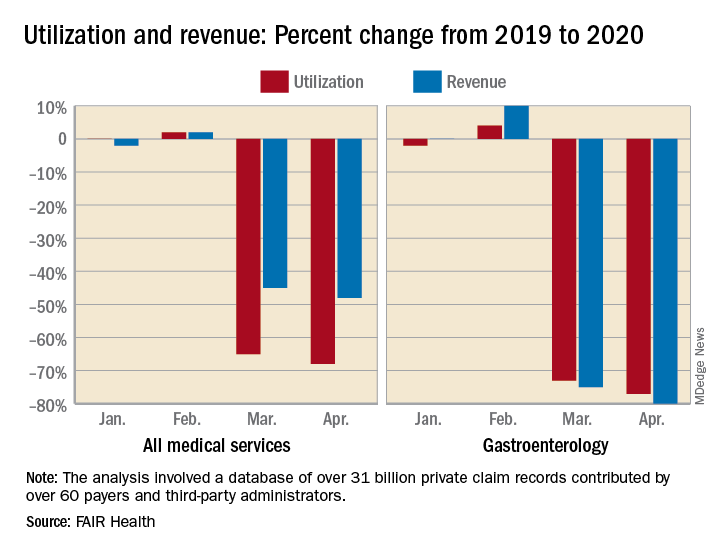
The size of the pandemic-related decreases in utilization and income varied by specialty. Of the seven specialties included in the study, oral surgery was hit the hardest, followed by gastroenterology, cardiology, orthopedics, dermatology, adult primary care, and pediatric primary care, FAIR Health said.
After experiencing a 2% drop in utilization this January and an increase of 4% in February, compared with 2019, gastroenterology saw corresponding drops of 73% in March and 77% in April. Estimated revenue for the specialty was flat in January and rose by 10% in February, but plummeted by 75% in March and 80% in April, the FAIR Health data show.
In cardiology, patient volume from 2019 to 2020 looked like this: Down by 4% in January, up 5% in February, down by 62% in March, and down by 71% in April. The earnings numbers tell a similar story: Down by 2% in January, up by 15% in February, down by 57% in March, and down by 73% in April, the organization reported.
Dermatology did the best among the non–primary care specialties, but that was just a relative success. Utilization still dropped by 62% and 68% in March and April of 2020, compared with last year, and revenue declined by 50% in March and 59% in April, FAIR Health said.
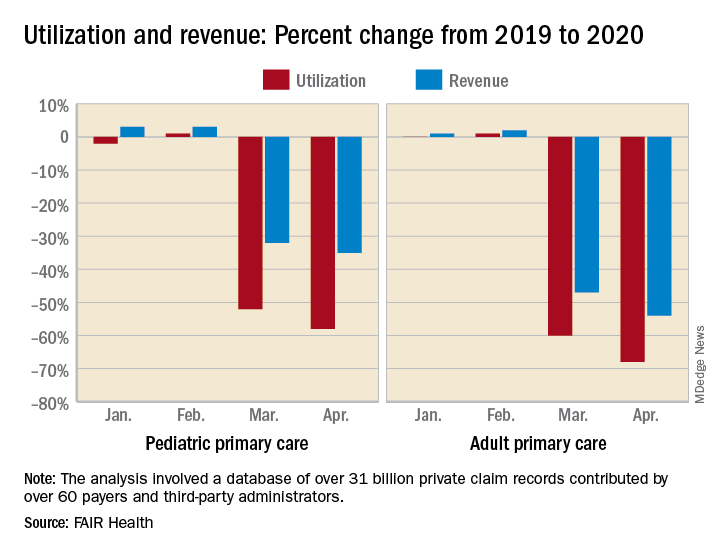
For adult primary care, the utilization numbers were similar, but revenue took a somewhat smaller hit. Patient volume from 2019 to 2020 was fairly steady in January and February, then nosedived in March (down 60%) and April (down 68%). Earnings were up initially, rising 1% in January and 2% in February, but fell 47% in March and 54% in April, FAIR Health said.
Pediatric primary care, it appears, may have been buoyed somewhat by its younger patients. The specialty as a whole saw utilization tumble by 52% in March and 58% in April, but revenue dropped by just 32% and 35%, respectively, according to the report.
A little extra data diving showed that the figures for preventive care visits for patients aged 0-4 years in March and April were –2% and 0% for volume and –2% and 1% for revenue. Meanwhile, the volume of immunizations only dropped by 14% and 10% and vaccine-related revenue slipped by just 7% and 2%, FAIR Health noted.
“Across many specialties from January to April 2020, office or other outpatient [evaluation and management] visits became more common relative to other procedures. ... This may have been due in part to the fact that many of these E&M services could be rendered via telehealth,” FAIR Health said.
Telehealth, however, was no panacea, the report explained: “Even when medical practices have continued to function via telehealth, many have experienced lower reimbursements for telehealth visits than for in-person visits and more time educating patients on how to use the technology.”
COVID-19 took a huge cut of clinicians’ business in March and April
COVID-19 took a huge cut of clinicians’ business in March and April
In the first 2 months of the COVID-19 pandemic, health care professionals experienced sharp drops in both utilization and revenue, according to an analysis of the nation’s largest collection of private health care claims data.

For the months of March and April 2020, use of medical professional services dropped by 65% and 68%, respectively, compared with last year, and estimated revenue fell by 45% and 48%, FAIR Health, a nonprofit organization that manages a database of 31 billion claim records, said in a new report.
For the Northeast states – the epicenter of the pandemic in March and April – patient volume was down by 60% in March and 80% in April, while revenue fell by 55% in March and 79% in April, the organization said.
For this analysis, “a professional service was defined as any service provided by an individual (e.g., physician, nurse, nurse practitioner, physician assistant) instead of being billed by a facility,” FAIR Health noted. Figures for 2019 were adjusted using the Consumer Price Index.
The size of the pandemic-related decreases in utilization and income varied by specialty. Of the seven specialties included in the study, oral surgery was hit the hardest, followed by gastroenterology, cardiology, orthopedics, dermatology, adult primary care, and pediatric primary care, FAIR Health said.
After experiencing a 2% drop in utilization this January and an increase of 4% in February, compared with 2019, gastroenterology saw corresponding drops of 73% in March and 77% in April. Estimated revenue for the specialty was flat in January and rose by 10% in February, but plummeted by 75% in March and 80% in April, the FAIR Health data show.
In cardiology, patient volume from 2019 to 2020 looked like this: Down by 4% in January, up 5% in February, down by 62% in March, and down by 71% in April. The earnings numbers tell a similar story: Down by 2% in January, up by 15% in February, down by 57% in March, and down by 73% in April, the organization reported.
Dermatology did the best among the non–primary care specialties, but that was just a relative success. Utilization still dropped by 62% and 68% in March and April of 2020, compared with last year, and revenue declined by 50% in March and 59% in April, FAIR Health said.
For adult primary care, the utilization numbers were similar, but revenue took a somewhat smaller hit. Patient volume from 2019 to 2020 was fairly steady in January and February, then nosedived in March (down 60%) and April (down 68%). Earnings were up initially, rising 1% in January and 2% in February, but fell 47% in March and 54% in April, FAIR Health said.
Pediatric primary care, it appears, may have been buoyed somewhat by its younger patients. The specialty as a whole saw utilization tumble by 52% in March and 58% in April, but revenue dropped by just 32% and 35%, respectively, according to the report.
A little extra data diving showed that the figures for preventive care visits for patients aged 0-4 years in March and April were –2% and 0% for volume and –2% and 1% for revenue. Meanwhile, the volume of immunizations only dropped by 14% and 10% and vaccine-related revenue slipped by just 7% and 2%, FAIR Health noted.
“Across many specialties from January to April 2020, office or other outpatient [evaluation and management] visits became more common relative to other procedures. ... This may have been due in part to the fact that many of these E&M services could be rendered via telehealth,” FAIR Health said.
Telehealth, however, was no panacea, the report explained: “Even when medical practices have continued to function via telehealth, many have experienced lower reimbursements for telehealth visits than for in-person visits and more time educating patients on how to use the technology.”
In the first 2 months of the COVID-19 pandemic, health care professionals experienced sharp drops in both utilization and revenue, according to an analysis of the nation’s largest collection of private health care claims data.

For the months of March and April 2020, use of medical professional services dropped by 65% and 68%, respectively, compared with last year, and estimated revenue fell by 45% and 48%, FAIR Health, a nonprofit organization that manages a database of 31 billion claim records, said in a new report.
For the Northeast states – the epicenter of the pandemic in March and April – patient volume was down by 60% in March and 80% in April, while revenue fell by 55% in March and 79% in April, the organization said.
For this analysis, “a professional service was defined as any service provided by an individual (e.g., physician, nurse, nurse practitioner, physician assistant) instead of being billed by a facility,” FAIR Health noted. Figures for 2019 were adjusted using the Consumer Price Index.
The size of the pandemic-related decreases in utilization and income varied by specialty. Of the seven specialties included in the study, oral surgery was hit the hardest, followed by gastroenterology, cardiology, orthopedics, dermatology, adult primary care, and pediatric primary care, FAIR Health said.
After experiencing a 2% drop in utilization this January and an increase of 4% in February, compared with 2019, gastroenterology saw corresponding drops of 73% in March and 77% in April. Estimated revenue for the specialty was flat in January and rose by 10% in February, but plummeted by 75% in March and 80% in April, the FAIR Health data show.
In cardiology, patient volume from 2019 to 2020 looked like this: Down by 4% in January, up 5% in February, down by 62% in March, and down by 71% in April. The earnings numbers tell a similar story: Down by 2% in January, up by 15% in February, down by 57% in March, and down by 73% in April, the organization reported.
Dermatology did the best among the non–primary care specialties, but that was just a relative success. Utilization still dropped by 62% and 68% in March and April of 2020, compared with last year, and revenue declined by 50% in March and 59% in April, FAIR Health said.
For adult primary care, the utilization numbers were similar, but revenue took a somewhat smaller hit. Patient volume from 2019 to 2020 was fairly steady in January and February, then nosedived in March (down 60%) and April (down 68%). Earnings were up initially, rising 1% in January and 2% in February, but fell 47% in March and 54% in April, FAIR Health said.
Pediatric primary care, it appears, may have been buoyed somewhat by its younger patients. The specialty as a whole saw utilization tumble by 52% in March and 58% in April, but revenue dropped by just 32% and 35%, respectively, according to the report.
A little extra data diving showed that the figures for preventive care visits for patients aged 0-4 years in March and April were –2% and 0% for volume and –2% and 1% for revenue. Meanwhile, the volume of immunizations only dropped by 14% and 10% and vaccine-related revenue slipped by just 7% and 2%, FAIR Health noted.
“Across many specialties from January to April 2020, office or other outpatient [evaluation and management] visits became more common relative to other procedures. ... This may have been due in part to the fact that many of these E&M services could be rendered via telehealth,” FAIR Health said.
Telehealth, however, was no panacea, the report explained: “Even when medical practices have continued to function via telehealth, many have experienced lower reimbursements for telehealth visits than for in-person visits and more time educating patients on how to use the technology.”
It’s official: COVID-19 was bad for the health care business
COVID-19 took a huge cut of clinicians’ business in March and April
In the first 2 months of the COVID-19 pandemic, health care professionals experienced sharp drops in both utilization and revenue, according to an analysis of the nation’s largest collection of private health care claims data.
For the months of March and April 2020, use of medical professional services dropped by 65% and 68%, respectively, compared with last year, and estimated revenue fell by 45% and 48%, FAIR Health, a nonprofit organization that manages a database of 31 billion claim records, said in a new report.
For the Northeast states – the epicenter of the pandemic in March and April – patient volume was down by 60% in March and 80% in April, while revenue fell by 55% in March and 79% in April, the organization said.
For this analysis, “a professional service was defined as any service provided by an individual (e.g., physician, nurse, nurse practitioner, physician assistant) instead of being billed by a facility,” FAIR Health noted. Figures for 2019 were adjusted using the Consumer Price Index.
The size of the pandemic-related decreases in utilization and income varied by specialty. Of the seven specialties included in the study, oral surgery was hit the hardest, followed by gastroenterology, cardiology, orthopedics, dermatology, adult primary care, and pediatric primary care, FAIR Health said.
After experiencing a 2% drop in utilization this January and an increase of 4% in February, compared with 2019, gastroenterology saw corresponding drops of 73% in March and 77% in April. Estimated revenue for the specialty was flat in January and rose by 10% in February, but plummeted by 75% in March and 80% in April, the FAIR Health data show.
In cardiology, patient volume from 2019 to 2020 looked like this: Down by 4% in January, up 5% in February, down by 62% in March, and down by 71% in April. The earnings numbers tell a similar story: Down by 2% in January, up by 15% in February, down by 57% in March, and down by 73% in April, the organization reported.
Dermatology did the best among the non–primary care specialties, but that was just a relative success. Utilization still dropped by 62% and 68% in March and April of 2020, compared with last year, and revenue declined by 50% in March and 59% in April, FAIR Health said.
For adult primary care, the utilization numbers were similar, but revenue took a somewhat smaller hit. Patient volume from 2019 to 2020 was fairly steady in January and February, then nosedived in March (down 60%) and April (down 68%). Earnings were up initially, rising 1% in January and 2% in February, but fell 47% in March and 54% in April, FAIR Health said.
Pediatric primary care, it appears, may have been buoyed somewhat by its younger patients. The specialty as a whole saw utilization tumble by 52% in March and 58% in April, but revenue dropped by just 32% and 35%, respectively, according to the report.
A little extra data diving showed that the figures for preventive care visits for patients aged 0-4 years in March and April were –2% and 0% for volume and –2% and 1% for revenue. Meanwhile, the volume of immunizations only dropped by 14% and 10% and vaccine-related revenue slipped by just 7% and 2%, FAIR Health noted.
“Across many specialties from January to April 2020, office or other outpatient [evaluation and management] visits became more common relative to other procedures. ... This may have been due in part to the fact that many of these E&M services could be rendered via telehealth,” FAIR Health said.
Telehealth, however, was no panacea, the report explained: “Even when medical practices have continued to function via telehealth, many have experienced lower reimbursements for telehealth visits than for in-person visits and more time educating patients on how to use the technology.”
COVID-19 took a huge cut of clinicians’ business in March and April
COVID-19 took a huge cut of clinicians’ business in March and April
In the first 2 months of the COVID-19 pandemic, health care professionals experienced sharp drops in both utilization and revenue, according to an analysis of the nation’s largest collection of private health care claims data.

For the months of March and April 2020, use of medical professional services dropped by 65% and 68%, respectively, compared with last year, and estimated revenue fell by 45% and 48%, FAIR Health, a nonprofit organization that manages a database of 31 billion claim records, said in a new report.
For the Northeast states – the epicenter of the pandemic in March and April – patient volume was down by 60% in March and 80% in April, while revenue fell by 55% in March and 79% in April, the organization said.
For this analysis, “a professional service was defined as any service provided by an individual (e.g., physician, nurse, nurse practitioner, physician assistant) instead of being billed by a facility,” FAIR Health noted. Figures for 2019 were adjusted using the Consumer Price Index.
The size of the pandemic-related decreases in utilization and income varied by specialty. Of the seven specialties included in the study, oral surgery was hit the hardest, followed by gastroenterology, cardiology, orthopedics, dermatology, adult primary care, and pediatric primary care, FAIR Health said.
After experiencing a 2% drop in utilization this January and an increase of 4% in February, compared with 2019, gastroenterology saw corresponding drops of 73% in March and 77% in April. Estimated revenue for the specialty was flat in January and rose by 10% in February, but plummeted by 75% in March and 80% in April, the FAIR Health data show.
In cardiology, patient volume from 2019 to 2020 looked like this: Down by 4% in January, up 5% in February, down by 62% in March, and down by 71% in April. The earnings numbers tell a similar story: Down by 2% in January, up by 15% in February, down by 57% in March, and down by 73% in April, the organization reported.
Dermatology did the best among the non–primary care specialties, but that was just a relative success. Utilization still dropped by 62% and 68% in March and April of 2020, compared with last year, and revenue declined by 50% in March and 59% in April, FAIR Health said.
For adult primary care, the utilization numbers were similar, but revenue took a somewhat smaller hit. Patient volume from 2019 to 2020 was fairly steady in January and February, then nosedived in March (down 60%) and April (down 68%). Earnings were up initially, rising 1% in January and 2% in February, but fell 47% in March and 54% in April, FAIR Health said.
Pediatric primary care, it appears, may have been buoyed somewhat by its younger patients. The specialty as a whole saw utilization tumble by 52% in March and 58% in April, but revenue dropped by just 32% and 35%, respectively, according to the report.
A little extra data diving showed that the figures for preventive care visits for patients aged 0-4 years in March and April were –2% and 0% for volume and –2% and 1% for revenue. Meanwhile, the volume of immunizations only dropped by 14% and 10% and vaccine-related revenue slipped by just 7% and 2%, FAIR Health noted.
“Across many specialties from January to April 2020, office or other outpatient [evaluation and management] visits became more common relative to other procedures. ... This may have been due in part to the fact that many of these E&M services could be rendered via telehealth,” FAIR Health said.
Telehealth, however, was no panacea, the report explained: “Even when medical practices have continued to function via telehealth, many have experienced lower reimbursements for telehealth visits than for in-person visits and more time educating patients on how to use the technology.”
In the first 2 months of the COVID-19 pandemic, health care professionals experienced sharp drops in both utilization and revenue, according to an analysis of the nation’s largest collection of private health care claims data.

For the months of March and April 2020, use of medical professional services dropped by 65% and 68%, respectively, compared with last year, and estimated revenue fell by 45% and 48%, FAIR Health, a nonprofit organization that manages a database of 31 billion claim records, said in a new report.
For the Northeast states – the epicenter of the pandemic in March and April – patient volume was down by 60% in March and 80% in April, while revenue fell by 55% in March and 79% in April, the organization said.
For this analysis, “a professional service was defined as any service provided by an individual (e.g., physician, nurse, nurse practitioner, physician assistant) instead of being billed by a facility,” FAIR Health noted. Figures for 2019 were adjusted using the Consumer Price Index.
The size of the pandemic-related decreases in utilization and income varied by specialty. Of the seven specialties included in the study, oral surgery was hit the hardest, followed by gastroenterology, cardiology, orthopedics, dermatology, adult primary care, and pediatric primary care, FAIR Health said.
After experiencing a 2% drop in utilization this January and an increase of 4% in February, compared with 2019, gastroenterology saw corresponding drops of 73% in March and 77% in April. Estimated revenue for the specialty was flat in January and rose by 10% in February, but plummeted by 75% in March and 80% in April, the FAIR Health data show.
In cardiology, patient volume from 2019 to 2020 looked like this: Down by 4% in January, up 5% in February, down by 62% in March, and down by 71% in April. The earnings numbers tell a similar story: Down by 2% in January, up by 15% in February, down by 57% in March, and down by 73% in April, the organization reported.
Dermatology did the best among the non–primary care specialties, but that was just a relative success. Utilization still dropped by 62% and 68% in March and April of 2020, compared with last year, and revenue declined by 50% in March and 59% in April, FAIR Health said.
For adult primary care, the utilization numbers were similar, but revenue took a somewhat smaller hit. Patient volume from 2019 to 2020 was fairly steady in January and February, then nosedived in March (down 60%) and April (down 68%). Earnings were up initially, rising 1% in January and 2% in February, but fell 47% in March and 54% in April, FAIR Health said.
Pediatric primary care, it appears, may have been buoyed somewhat by its younger patients. The specialty as a whole saw utilization tumble by 52% in March and 58% in April, but revenue dropped by just 32% and 35%, respectively, according to the report.
A little extra data diving showed that the figures for preventive care visits for patients aged 0-4 years in March and April were –2% and 0% for volume and –2% and 1% for revenue. Meanwhile, the volume of immunizations only dropped by 14% and 10% and vaccine-related revenue slipped by just 7% and 2%, FAIR Health noted.
“Across many specialties from January to April 2020, office or other outpatient [evaluation and management] visits became more common relative to other procedures. ... This may have been due in part to the fact that many of these E&M services could be rendered via telehealth,” FAIR Health said.
Telehealth, however, was no panacea, the report explained: “Even when medical practices have continued to function via telehealth, many have experienced lower reimbursements for telehealth visits than for in-person visits and more time educating patients on how to use the technology.”
Results from two phase 3 trials of bimekizumab unveiled
Results from two late-breaking
“The rapid and lasting skin clearance observed in the majority of patients in both clinical studies demonstrate bimekizumab’s strong potential to deliver across three key areas: speed, depth and durability,” Kristian Reich, MD, said in an interview during the virtual annual meeting of the American Academy of Dermatology.
Bimekizumab selectively inhibits IL-17A and IL-17F, two key cytokines that drive inflammation and tissue damage across multiple diseases. In BE VIVID, Dr. Reich, of the Center for Translational Research in Inflammatory Skin Diseases at the University Medical Center Hamburg-Eppendorf (Germany), and colleagues randomized 567 patients with moderate to severe psoriasis to bimekizumab 320 mg every 4 weeks (Q4W), ustekinumab (45/90 mg weight-based dosing at baseline and week 4, then every 12 weeks), or placebo (Q4W through week 16 then bimekizumab 320 mg Q4W). Coprimary endpoints were a Psoriasis Area and Severity Index (PASI) of at least 90 and an Investigator Global Assessment (IGA) response of 0 or 1. Secondary/other outcomes included PASI 100 at week 16; PASI 90, IGA 0/1, and PASI 100 at week 52; and safety. (Ustekinumab is an IL-12 and IL-23 antagonist.)
The mean age of patients was 46 years and 72% were male. The researchers found that the proportion of patients who achieved PASI 90 and an IGA of 0/1 was higher in the bimekizumab arm at week 16 (85.0% and 84.1%, respectively), compared with those in the ustekinumab arm (49.7% and 53.4%) and those on placebo (4.8% and 4.8%; P < .001 for all associations). In addition, 58.6% of patients in the bimekizumab arm achieved PASI 100, compared with 20.9% of those in the ustekinumab arm and none of those on placebo.
At week 52, patients in the bimekizumab arm achieved PASI 90, IGA 0/1, and PASI 100 response rates of 81.6%, 77.9%, and 64.2%, respectively, compared with 55.8%, 60.7%, and 38.0% of those in the ustekinumab arm. Over 52 weeks, incidence of serious treatment-emergent adverse events was 6.1% with bimekizumab arm, compared with 7.4% in the ustekinumab arm. Four deaths occurred (two in the bimekizumab arm, and one each in the ustekinumab and placebo arms), all considered unrelated to treatment. The most common reported adverse events in the bimekizumab arm through week 52 were nasopharyngitis (21.8%), oral candidiasis (15.2%), and upper respiratory tract infections (9.1%).
“The rapid and lasting skin clearance observed in the majority of patients treated with bimekizumab provide support for inhibiting IL-17F, in addition to IL-17A, to inhibit the IL-17 pathway,” Dr. Reich said. “This can make a meaningful difference for people living with psoriasis.” He added that the results of the head-to-head study of bimekizumab with secukinumab (an IL-17A antagonist) are expected later this year. “It will be very interesting to see if the marked differences in treatment effect seen in the BE VIVID study remain when comparing to an IL-17.”
In BE READY, a pivotal phase 3, randomized, withdrawal study, investigators led by Kenneth Gordon, MD, randomized 435 patients with moderate to severe psoriasis 4:1 to receive 320 mg Q4W or placebo, and followed them for 16 weeks. In a second part of the study, patients who had achieved at least a PASI 90 response at week 16 were rerandomized to receive continuous bimekizumab at two different dosing regimens: 320 mg Q4W or 320 mg every 8 weeks (Q8W), or to be withdrawn from treatment (placebo Q4W), and followed through week 56. Relapse was defined as a PASI score of less than 75 from week 20.
The mean age of patients was 44 years and 72% were male. At week 16, the proportion of patients who achieved a PASI 90 and an IGA of 0/1 was greatest in the bimekizumab arm (90.8% and 92.6%, respectively), compared with those on placebo. In addition, 68.2% of patients in the bimekizumab arm achieved PASI 100 at week 16, compared with only 1.2% of those on placebo (P < .001 for all associations). In the second part of the study, the researchers found that 86.8% of patients who received continuous bimekizumab 320 mg Q4W maintained PASI 90 at week 56, compared with 91% who were switched to bimekizumab 320 mg Q8W, and 16.2% of patients who were withdrawn from the trial.
“The speed of response and the number of patients who achieved clearance are extremely high, especially in a phase 3 trial,” Dr. Gordon, professor and Thomas R. Russell Family Chair of Dermatology at the Medical College of Wisconsin, Milwaukee, said in an interview. “However, the most surprising aspect may be the impressive maintenance of response in patients, even those who were treated with every-8-week dosing in the maintenance phase. While it is possible that there are some patients who may benefit from more frequent dosing in the long term, the possibility of every-8-week dosing would be a tremendous benefit for patients.”
As in the BE VIVID trial, the most frequently reported adverse events with bimekizumab between week 16 and week 56 in BE READY were nasopharyngitis (10.4% in the Q4W arm vs. 23% in the Q8W arm), oral candidiasis (11.3% Q4W vs. 9% Q8W), and upper respiratory tract infections (11.3% Q4W vs. 8% Q8W). The incidence of serious treatment-emergent adverse events with bimekizumab was 4.7% in the Q4W arm and 3% in the Q8W arm versus 3.8% in the placebo arm at week 56.
“The results from BE READY demonstrate that bimekizumab has the potential to deliver rapid and lasting skin improvement for psoriasis patients,” Dr. Gordon said. “The findings also support the hypothesis that inhibiting IL-17F, in addition to IL-17A, may be more effective in suppressing inflammation in suppressing inflammation in psoriasis than IL-17A inhibition alone.”
Both studies were funded by UCB Pharma. Dr. Reich disclosed that he has served as adviser and/or paid speaker for and/or participated in clinical trials sponsored by companies that include UCB. Dr. Gordon disclosed that he has received honoraria and/or research support from companies that include UCB..
Results from two late-breaking
“The rapid and lasting skin clearance observed in the majority of patients in both clinical studies demonstrate bimekizumab’s strong potential to deliver across three key areas: speed, depth and durability,” Kristian Reich, MD, said in an interview during the virtual annual meeting of the American Academy of Dermatology.
Bimekizumab selectively inhibits IL-17A and IL-17F, two key cytokines that drive inflammation and tissue damage across multiple diseases. In BE VIVID, Dr. Reich, of the Center for Translational Research in Inflammatory Skin Diseases at the University Medical Center Hamburg-Eppendorf (Germany), and colleagues randomized 567 patients with moderate to severe psoriasis to bimekizumab 320 mg every 4 weeks (Q4W), ustekinumab (45/90 mg weight-based dosing at baseline and week 4, then every 12 weeks), or placebo (Q4W through week 16 then bimekizumab 320 mg Q4W). Coprimary endpoints were a Psoriasis Area and Severity Index (PASI) of at least 90 and an Investigator Global Assessment (IGA) response of 0 or 1. Secondary/other outcomes included PASI 100 at week 16; PASI 90, IGA 0/1, and PASI 100 at week 52; and safety. (Ustekinumab is an IL-12 and IL-23 antagonist.)
The mean age of patients was 46 years and 72% were male. The researchers found that the proportion of patients who achieved PASI 90 and an IGA of 0/1 was higher in the bimekizumab arm at week 16 (85.0% and 84.1%, respectively), compared with those in the ustekinumab arm (49.7% and 53.4%) and those on placebo (4.8% and 4.8%; P < .001 for all associations). In addition, 58.6% of patients in the bimekizumab arm achieved PASI 100, compared with 20.9% of those in the ustekinumab arm and none of those on placebo.
At week 52, patients in the bimekizumab arm achieved PASI 90, IGA 0/1, and PASI 100 response rates of 81.6%, 77.9%, and 64.2%, respectively, compared with 55.8%, 60.7%, and 38.0% of those in the ustekinumab arm. Over 52 weeks, incidence of serious treatment-emergent adverse events was 6.1% with bimekizumab arm, compared with 7.4% in the ustekinumab arm. Four deaths occurred (two in the bimekizumab arm, and one each in the ustekinumab and placebo arms), all considered unrelated to treatment. The most common reported adverse events in the bimekizumab arm through week 52 were nasopharyngitis (21.8%), oral candidiasis (15.2%), and upper respiratory tract infections (9.1%).
“The rapid and lasting skin clearance observed in the majority of patients treated with bimekizumab provide support for inhibiting IL-17F, in addition to IL-17A, to inhibit the IL-17 pathway,” Dr. Reich said. “This can make a meaningful difference for people living with psoriasis.” He added that the results of the head-to-head study of bimekizumab with secukinumab (an IL-17A antagonist) are expected later this year. “It will be very interesting to see if the marked differences in treatment effect seen in the BE VIVID study remain when comparing to an IL-17.”
In BE READY, a pivotal phase 3, randomized, withdrawal study, investigators led by Kenneth Gordon, MD, randomized 435 patients with moderate to severe psoriasis 4:1 to receive 320 mg Q4W or placebo, and followed them for 16 weeks. In a second part of the study, patients who had achieved at least a PASI 90 response at week 16 were rerandomized to receive continuous bimekizumab at two different dosing regimens: 320 mg Q4W or 320 mg every 8 weeks (Q8W), or to be withdrawn from treatment (placebo Q4W), and followed through week 56. Relapse was defined as a PASI score of less than 75 from week 20.
The mean age of patients was 44 years and 72% were male. At week 16, the proportion of patients who achieved a PASI 90 and an IGA of 0/1 was greatest in the bimekizumab arm (90.8% and 92.6%, respectively), compared with those on placebo. In addition, 68.2% of patients in the bimekizumab arm achieved PASI 100 at week 16, compared with only 1.2% of those on placebo (P < .001 for all associations). In the second part of the study, the researchers found that 86.8% of patients who received continuous bimekizumab 320 mg Q4W maintained PASI 90 at week 56, compared with 91% who were switched to bimekizumab 320 mg Q8W, and 16.2% of patients who were withdrawn from the trial.
“The speed of response and the number of patients who achieved clearance are extremely high, especially in a phase 3 trial,” Dr. Gordon, professor and Thomas R. Russell Family Chair of Dermatology at the Medical College of Wisconsin, Milwaukee, said in an interview. “However, the most surprising aspect may be the impressive maintenance of response in patients, even those who were treated with every-8-week dosing in the maintenance phase. While it is possible that there are some patients who may benefit from more frequent dosing in the long term, the possibility of every-8-week dosing would be a tremendous benefit for patients.”
As in the BE VIVID trial, the most frequently reported adverse events with bimekizumab between week 16 and week 56 in BE READY were nasopharyngitis (10.4% in the Q4W arm vs. 23% in the Q8W arm), oral candidiasis (11.3% Q4W vs. 9% Q8W), and upper respiratory tract infections (11.3% Q4W vs. 8% Q8W). The incidence of serious treatment-emergent adverse events with bimekizumab was 4.7% in the Q4W arm and 3% in the Q8W arm versus 3.8% in the placebo arm at week 56.
“The results from BE READY demonstrate that bimekizumab has the potential to deliver rapid and lasting skin improvement for psoriasis patients,” Dr. Gordon said. “The findings also support the hypothesis that inhibiting IL-17F, in addition to IL-17A, may be more effective in suppressing inflammation in suppressing inflammation in psoriasis than IL-17A inhibition alone.”
Both studies were funded by UCB Pharma. Dr. Reich disclosed that he has served as adviser and/or paid speaker for and/or participated in clinical trials sponsored by companies that include UCB. Dr. Gordon disclosed that he has received honoraria and/or research support from companies that include UCB..
Results from two late-breaking
“The rapid and lasting skin clearance observed in the majority of patients in both clinical studies demonstrate bimekizumab’s strong potential to deliver across three key areas: speed, depth and durability,” Kristian Reich, MD, said in an interview during the virtual annual meeting of the American Academy of Dermatology.
Bimekizumab selectively inhibits IL-17A and IL-17F, two key cytokines that drive inflammation and tissue damage across multiple diseases. In BE VIVID, Dr. Reich, of the Center for Translational Research in Inflammatory Skin Diseases at the University Medical Center Hamburg-Eppendorf (Germany), and colleagues randomized 567 patients with moderate to severe psoriasis to bimekizumab 320 mg every 4 weeks (Q4W), ustekinumab (45/90 mg weight-based dosing at baseline and week 4, then every 12 weeks), or placebo (Q4W through week 16 then bimekizumab 320 mg Q4W). Coprimary endpoints were a Psoriasis Area and Severity Index (PASI) of at least 90 and an Investigator Global Assessment (IGA) response of 0 or 1. Secondary/other outcomes included PASI 100 at week 16; PASI 90, IGA 0/1, and PASI 100 at week 52; and safety. (Ustekinumab is an IL-12 and IL-23 antagonist.)
The mean age of patients was 46 years and 72% were male. The researchers found that the proportion of patients who achieved PASI 90 and an IGA of 0/1 was higher in the bimekizumab arm at week 16 (85.0% and 84.1%, respectively), compared with those in the ustekinumab arm (49.7% and 53.4%) and those on placebo (4.8% and 4.8%; P < .001 for all associations). In addition, 58.6% of patients in the bimekizumab arm achieved PASI 100, compared with 20.9% of those in the ustekinumab arm and none of those on placebo.
At week 52, patients in the bimekizumab arm achieved PASI 90, IGA 0/1, and PASI 100 response rates of 81.6%, 77.9%, and 64.2%, respectively, compared with 55.8%, 60.7%, and 38.0% of those in the ustekinumab arm. Over 52 weeks, incidence of serious treatment-emergent adverse events was 6.1% with bimekizumab arm, compared with 7.4% in the ustekinumab arm. Four deaths occurred (two in the bimekizumab arm, and one each in the ustekinumab and placebo arms), all considered unrelated to treatment. The most common reported adverse events in the bimekizumab arm through week 52 were nasopharyngitis (21.8%), oral candidiasis (15.2%), and upper respiratory tract infections (9.1%).
“The rapid and lasting skin clearance observed in the majority of patients treated with bimekizumab provide support for inhibiting IL-17F, in addition to IL-17A, to inhibit the IL-17 pathway,” Dr. Reich said. “This can make a meaningful difference for people living with psoriasis.” He added that the results of the head-to-head study of bimekizumab with secukinumab (an IL-17A antagonist) are expected later this year. “It will be very interesting to see if the marked differences in treatment effect seen in the BE VIVID study remain when comparing to an IL-17.”
In BE READY, a pivotal phase 3, randomized, withdrawal study, investigators led by Kenneth Gordon, MD, randomized 435 patients with moderate to severe psoriasis 4:1 to receive 320 mg Q4W or placebo, and followed them for 16 weeks. In a second part of the study, patients who had achieved at least a PASI 90 response at week 16 were rerandomized to receive continuous bimekizumab at two different dosing regimens: 320 mg Q4W or 320 mg every 8 weeks (Q8W), or to be withdrawn from treatment (placebo Q4W), and followed through week 56. Relapse was defined as a PASI score of less than 75 from week 20.
The mean age of patients was 44 years and 72% were male. At week 16, the proportion of patients who achieved a PASI 90 and an IGA of 0/1 was greatest in the bimekizumab arm (90.8% and 92.6%, respectively), compared with those on placebo. In addition, 68.2% of patients in the bimekizumab arm achieved PASI 100 at week 16, compared with only 1.2% of those on placebo (P < .001 for all associations). In the second part of the study, the researchers found that 86.8% of patients who received continuous bimekizumab 320 mg Q4W maintained PASI 90 at week 56, compared with 91% who were switched to bimekizumab 320 mg Q8W, and 16.2% of patients who were withdrawn from the trial.
“The speed of response and the number of patients who achieved clearance are extremely high, especially in a phase 3 trial,” Dr. Gordon, professor and Thomas R. Russell Family Chair of Dermatology at the Medical College of Wisconsin, Milwaukee, said in an interview. “However, the most surprising aspect may be the impressive maintenance of response in patients, even those who were treated with every-8-week dosing in the maintenance phase. While it is possible that there are some patients who may benefit from more frequent dosing in the long term, the possibility of every-8-week dosing would be a tremendous benefit for patients.”
As in the BE VIVID trial, the most frequently reported adverse events with bimekizumab between week 16 and week 56 in BE READY were nasopharyngitis (10.4% in the Q4W arm vs. 23% in the Q8W arm), oral candidiasis (11.3% Q4W vs. 9% Q8W), and upper respiratory tract infections (11.3% Q4W vs. 8% Q8W). The incidence of serious treatment-emergent adverse events with bimekizumab was 4.7% in the Q4W arm and 3% in the Q8W arm versus 3.8% in the placebo arm at week 56.
“The results from BE READY demonstrate that bimekizumab has the potential to deliver rapid and lasting skin improvement for psoriasis patients,” Dr. Gordon said. “The findings also support the hypothesis that inhibiting IL-17F, in addition to IL-17A, may be more effective in suppressing inflammation in suppressing inflammation in psoriasis than IL-17A inhibition alone.”
Both studies were funded by UCB Pharma. Dr. Reich disclosed that he has served as adviser and/or paid speaker for and/or participated in clinical trials sponsored by companies that include UCB. Dr. Gordon disclosed that he has received honoraria and/or research support from companies that include UCB..
FROM AAD 2020
Disseminated Erythema Induratum in a Patient With a History of Tuberculosis
To the Editor:
Erythema induratum, also known as nodular vasculitis, is a panniculitis that usually affects the lower extremities in middle-aged women. Classically, it has been described as a delayed-type hypersensitivity reaction to Mycobacterium tuberculosis, also known as a tuberculid.1,2 Other infections, however, also have been implicated as causes of erythema induratum, including bacillus Calmette-Guérin (BCG), the attenuated form of Mycobacterium bovis, which commonly is used for tuberculosis vaccination. Medications also may cause erythema induratum. The characteristic distribution of the nodules on the posterior calves helps to distinguish erythema induratum from other panniculitides. A PubMed search of articles indexed for MEDLINE using the term disseminated erythema induratum revealed few case reports documenting nodules on the arms, thighs, or chest, and only 1 case report of disseminated erythema induratum.3-8 We describe a rare combination of disseminated erythema induratum in a patient with remote exposure to tuberculosis and recent BCG exposure.
An 88-year-old woman presented for evaluation of violaceous, minimally tender, nonulcerated, subcutaneous nodules on the legs, arms, and trunk of several weeks’ duration (Figure 1). She had a remote history of tuberculosis as a child, prior to the advent of modern antituberculosis regimens. Her medical history also included hypertension, breast cancer treated with lymph node dissection, gastroesophageal reflux disease, and bladder cancer treated with intravesical BCG 10 years prior to the onset of the nodules. She reported minimal coughing and a 25-lb weight loss over the last year, but she denied night sweats, fever, or chills.
Workup included a biopsy, which showed a dense inflammatory infiltrate within the septae and lobules of the subcutaneous tissue (Figure 2A). Foci of necrosis were seen within the fat lobules (Figure 2B). The histologic diagnosis was erythema induratum. Tissue cultures for bacteria, fungi, and atypical mycobacteria were negative. Mycobacterium tuberculosis polymerase chain reaction (PCR) analysis also was negative. An IFN-γ release assay test was positive for infection with M tuberculosis, suggesting that the erythema induratum was due to tuberculosis rather than BCG exposure. A chest radiograph demonstrated a 22-mm nodule in the left lung (unchanged from a prior film) and a new 10-mm nodule in the left upper lobe.
The patient was referred to an infectious disease specialist who concurred that the erythema induratum and the new lung nodule likely represented a reactivation of tuberculosis. Sputum samples were found to be smear and culture negative for mycobacteria, but due to high clinical suspicion, she was started on a 4-drug tuberculosis regimen of isoniazid, rifampin, pyrazinamide, and ethambutol. Some lesions had started to improve prior to the institution of therapy; after initiation of treatment, all lesions resolved within 4 weeks of starting treatment without recurrence.
Erythema induratum was first described by Bazin9 in 1861. The disorder usually occurs in middle-aged women and is characterized by violaceous ulcerative plaques that classically present on the lower extremities, especially the calves. When the eruption occurs due to a nontuberculous etiology, the term nodular vasculitis is used.1,5 The distinction largely is historical, as most dermatologists today recognize erythema induratum and nodular vasculitis to be the same entity. Examples of nontuberculous causes include infections such as Nocardia, Pseudomonas, Fusarium, or other Mycobacterium species.10 Medications such as propylthiouracil also have been implicated.11 The classification of erythema induratum as a tuberculid suggests that the nodules are a reaction pattern rather than a primary infection, though the term tuberculid may be imprecise. The differential diagnosis of violaceous nodules on the lower extremities and trunk is broad and includes erythema nodosum, cutaneous polyarteritis nodosa, pancreatic panniculitis, subcutaneous T-cell lymphoma, and lupus profundus.1,11,12
Histologically, lesions classically demonstrate a mostly lobular panniculitis with varying degrees of septal fibrosis and focal necrosis. Neutrophils may predominate early, while adipocyte necrosis, epithelioid histiocytes, multinucleated giant cells, and lymphocytes may be found in older lesions. The presence of vasculitis as a requisite diagnostic criterion remains controversial.1,12
The incidence of erythema induratum has decreased since multidrug tuberculosis treatment has become more widespread.3 Our case displayed the disseminated variant of erythema induratum, an even rarer clinical entity.8 Interestingly, our patient had a history of tuberculosis and exposure to BCG prior to the development of lesions. Case reports have documented erythema induratum after BCG exposure but less frequently than in cases associated with tuberculosis.3,13
The use of BCG vaccines has necessitated the need for a more precise method of determining tuberculosis activity. The tuberculin skin test reacts positively with a history of BCG exposure, rendering it an inadequate test in a patient who is suspected of having an active or latent M tuberculosis infection.13,14 IFN-γ release assays are more specific in detecting latent or active tuberculosis than the tuberculin skin test. Such assays use early secretory antigenic target 6 and cultured filtrate protein 10 as antigens to determine sensitization to M tuberculosis.13,15 These antigens are not produced by BCG or Mycobacterium avium; however, other mycobacteria such as Mycobacterium marinum, Mycobacterium kansasii, and some strains of M bovis produce the aforementioned antigens, and exposure to these microbes may be confounding.13 Importantly, positive IFN-γ release assay results also have been documented after BCG exposure but occur at a much lower frequency than for tuberculosis.15 Thus, the combination of the positive IFN-γ release assay and new chest radiograph nodule in our patient provided strong evidence of reactivated tuberculosis as the precipitating cause of her skin disease.
Despite her negative PCR study, our patient’s presentation remains consistent with the diagnosis of disseminated erythema induratum.13,15 The value of PCR studies in establishing the diagnosis remains to be determined. Case reports have described positive PCR results detecting M tuberculosis in panniculitic nodules, suggesting that trace amounts of the organism are present in lesional tissue despite the negative culture result and immunostains.1 Tuberculid reactions, including lichen scrofulosorum, papulonecrotic tuberculid, and erythema induratum, historically are defined by the lack of positive cultures and immunostains, making positive PCR results difficult to reconcile pathophysiologically.1,13 Therefore, use of the term tuberculid altogether as a descriptor for pathogenesis of this disease may need to be avoided.16 Postulated explanations for the relationship of tuberculid diseases and negative cultures and immunostains include the presence of a small number of bacilli that escape routine laboratory detection, early destruction of organisms, or a reaction to circulating M tuberculosis fragments.2 Regardless, until the pathophysiology of erythema induratum has been fully elucidated, the value of PCR remains unclear.
Disseminated erythema induratum, an exceptionally rare variant of panniculitis, may be seen in patients with a remote history of M tuberculosis exposure and/or recent therapeutic BCG exposure. It is imperative to rule out active tuberculosis, especially in elderly patients whose disease predated the advent of modern antituberculosis therapy. Using an IFN-γ release assay in addition to chest radiographs and other clinical stigmata allows differentiation of the etiology of erythema induratum in those patients with tuberculosis who also were treated with BCG.
- Mascaro JM, Basalga E. Erythema induratum of Bazin. Dermatol Clin. 2008;28:439-445.
- Lighter J, Tse DB, Li Y, et al. Erythema induratum of Bazin in a child: evidence for a cell-mediated hyper-response to Mycobacterium tuberculosis. Pediatr Infect Dis J. 2009;28:326-328.
- Inoue T, Fukumoto T, Ansai S, et al. Erythema induratum of Bazin in an infant after bacilli Calmette-Guerin vaccination. J Dermatol. 2006;33:268-272.
- Degonda Halter M, Nebiker P, Hug B, et al. Atypical erythema induratum Bazin with tuberculous osteomyelitis. Internist. 2006;47:853-856.
- Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320-327.
- Sharma S, Sehgal VN, Bhattacharya SN, et al. Clinicopathologic spectrum of cutaneous tuberculosis: a retrospective analysis of 165 Indians. Am J Dermatopathol. 2015;37:444-450.
- Sethuraman G, Ramesh V. Cutaneous tuberculosis in children. Pediatr Dermatol. 2013;30:7-16.
- Teramura K, Fujimoto N, Nakanishi G, et al. Disseminated erythema induratum of Bazin. Eur J Dermatol. 2014;24:697-698.
- Bazin E. Extrait des Lecons Théoretiques et Cliniques sur le Scrofule. 2nd ed. Paris, France: Delhaye; 1861.
- Campbell SM, Winkelmann RR, Sammons DL. Erythema induratum caused by Mycobacterium chelonei in an immunocompetent patient. J Clin Aesthet Dermatol. 2013;6:38-40.
- Patterson JW. Panniculitis. In: Bolognia JL, Jorizzo J, Rapini RP, et al, eds. Dermatology. Barcelona, Spain: Mosby Elsevier; 2012:1641-1662.
- Segura S, Pujol R, Trinidade F, et al. Vasculitis in erythema induratum of Bazin: a histopathologic study of 101 biopsy specimens from 86 patients. J Am Acad Dermatol. 2008;59:839-851.
- Vera-Kellet C, Peters L, Elwood K, et al. Usefulness of interferon-γ release assays in the diagnosis of erythema induratum. Arch Dermatol. 2011;147:949-952.
- Prajapati V, Steed M, Grewal P, et al. Erythema induratum: case series illustrating the utility of the interferon-γ release assay in determining the association with tuberculosis. J Cutan Med Surg. 2013;17:S6-S11.
- Sim JH, Whang KU. Application of the QuantiFERON-Gold TB test in erythema induratum. J Dermatolog Treat. 2014;25:260-263.
- Wiebels D, Turnbull K, Steinkraus V, et al. Erythema induratum Bazin.”tuberculid” or tuberculosis? [in German]. Hautarzt. 2007;58:237-240.
To the Editor:
Erythema induratum, also known as nodular vasculitis, is a panniculitis that usually affects the lower extremities in middle-aged women. Classically, it has been described as a delayed-type hypersensitivity reaction to Mycobacterium tuberculosis, also known as a tuberculid.1,2 Other infections, however, also have been implicated as causes of erythema induratum, including bacillus Calmette-Guérin (BCG), the attenuated form of Mycobacterium bovis, which commonly is used for tuberculosis vaccination. Medications also may cause erythema induratum. The characteristic distribution of the nodules on the posterior calves helps to distinguish erythema induratum from other panniculitides. A PubMed search of articles indexed for MEDLINE using the term disseminated erythema induratum revealed few case reports documenting nodules on the arms, thighs, or chest, and only 1 case report of disseminated erythema induratum.3-8 We describe a rare combination of disseminated erythema induratum in a patient with remote exposure to tuberculosis and recent BCG exposure.
An 88-year-old woman presented for evaluation of violaceous, minimally tender, nonulcerated, subcutaneous nodules on the legs, arms, and trunk of several weeks’ duration (Figure 1). She had a remote history of tuberculosis as a child, prior to the advent of modern antituberculosis regimens. Her medical history also included hypertension, breast cancer treated with lymph node dissection, gastroesophageal reflux disease, and bladder cancer treated with intravesical BCG 10 years prior to the onset of the nodules. She reported minimal coughing and a 25-lb weight loss over the last year, but she denied night sweats, fever, or chills.

Workup included a biopsy, which showed a dense inflammatory infiltrate within the septae and lobules of the subcutaneous tissue (Figure 2A). Foci of necrosis were seen within the fat lobules (Figure 2B). The histologic diagnosis was erythema induratum. Tissue cultures for bacteria, fungi, and atypical mycobacteria were negative. Mycobacterium tuberculosis polymerase chain reaction (PCR) analysis also was negative. An IFN-γ release assay test was positive for infection with M tuberculosis, suggesting that the erythema induratum was due to tuberculosis rather than BCG exposure. A chest radiograph demonstrated a 22-mm nodule in the left lung (unchanged from a prior film) and a new 10-mm nodule in the left upper lobe.

The patient was referred to an infectious disease specialist who concurred that the erythema induratum and the new lung nodule likely represented a reactivation of tuberculosis. Sputum samples were found to be smear and culture negative for mycobacteria, but due to high clinical suspicion, she was started on a 4-drug tuberculosis regimen of isoniazid, rifampin, pyrazinamide, and ethambutol. Some lesions had started to improve prior to the institution of therapy; after initiation of treatment, all lesions resolved within 4 weeks of starting treatment without recurrence.
Erythema induratum was first described by Bazin9 in 1861. The disorder usually occurs in middle-aged women and is characterized by violaceous ulcerative plaques that classically present on the lower extremities, especially the calves. When the eruption occurs due to a nontuberculous etiology, the term nodular vasculitis is used.1,5 The distinction largely is historical, as most dermatologists today recognize erythema induratum and nodular vasculitis to be the same entity. Examples of nontuberculous causes include infections such as Nocardia, Pseudomonas, Fusarium, or other Mycobacterium species.10 Medications such as propylthiouracil also have been implicated.11 The classification of erythema induratum as a tuberculid suggests that the nodules are a reaction pattern rather than a primary infection, though the term tuberculid may be imprecise. The differential diagnosis of violaceous nodules on the lower extremities and trunk is broad and includes erythema nodosum, cutaneous polyarteritis nodosa, pancreatic panniculitis, subcutaneous T-cell lymphoma, and lupus profundus.1,11,12
Histologically, lesions classically demonstrate a mostly lobular panniculitis with varying degrees of septal fibrosis and focal necrosis. Neutrophils may predominate early, while adipocyte necrosis, epithelioid histiocytes, multinucleated giant cells, and lymphocytes may be found in older lesions. The presence of vasculitis as a requisite diagnostic criterion remains controversial.1,12
The incidence of erythema induratum has decreased since multidrug tuberculosis treatment has become more widespread.3 Our case displayed the disseminated variant of erythema induratum, an even rarer clinical entity.8 Interestingly, our patient had a history of tuberculosis and exposure to BCG prior to the development of lesions. Case reports have documented erythema induratum after BCG exposure but less frequently than in cases associated with tuberculosis.3,13
The use of BCG vaccines has necessitated the need for a more precise method of determining tuberculosis activity. The tuberculin skin test reacts positively with a history of BCG exposure, rendering it an inadequate test in a patient who is suspected of having an active or latent M tuberculosis infection.13,14 IFN-γ release assays are more specific in detecting latent or active tuberculosis than the tuberculin skin test. Such assays use early secretory antigenic target 6 and cultured filtrate protein 10 as antigens to determine sensitization to M tuberculosis.13,15 These antigens are not produced by BCG or Mycobacterium avium; however, other mycobacteria such as Mycobacterium marinum, Mycobacterium kansasii, and some strains of M bovis produce the aforementioned antigens, and exposure to these microbes may be confounding.13 Importantly, positive IFN-γ release assay results also have been documented after BCG exposure but occur at a much lower frequency than for tuberculosis.15 Thus, the combination of the positive IFN-γ release assay and new chest radiograph nodule in our patient provided strong evidence of reactivated tuberculosis as the precipitating cause of her skin disease.
Despite her negative PCR study, our patient’s presentation remains consistent with the diagnosis of disseminated erythema induratum.13,15 The value of PCR studies in establishing the diagnosis remains to be determined. Case reports have described positive PCR results detecting M tuberculosis in panniculitic nodules, suggesting that trace amounts of the organism are present in lesional tissue despite the negative culture result and immunostains.1 Tuberculid reactions, including lichen scrofulosorum, papulonecrotic tuberculid, and erythema induratum, historically are defined by the lack of positive cultures and immunostains, making positive PCR results difficult to reconcile pathophysiologically.1,13 Therefore, use of the term tuberculid altogether as a descriptor for pathogenesis of this disease may need to be avoided.16 Postulated explanations for the relationship of tuberculid diseases and negative cultures and immunostains include the presence of a small number of bacilli that escape routine laboratory detection, early destruction of organisms, or a reaction to circulating M tuberculosis fragments.2 Regardless, until the pathophysiology of erythema induratum has been fully elucidated, the value of PCR remains unclear.
Disseminated erythema induratum, an exceptionally rare variant of panniculitis, may be seen in patients with a remote history of M tuberculosis exposure and/or recent therapeutic BCG exposure. It is imperative to rule out active tuberculosis, especially in elderly patients whose disease predated the advent of modern antituberculosis therapy. Using an IFN-γ release assay in addition to chest radiographs and other clinical stigmata allows differentiation of the etiology of erythema induratum in those patients with tuberculosis who also were treated with BCG.
To the Editor:
Erythema induratum, also known as nodular vasculitis, is a panniculitis that usually affects the lower extremities in middle-aged women. Classically, it has been described as a delayed-type hypersensitivity reaction to Mycobacterium tuberculosis, also known as a tuberculid.1,2 Other infections, however, also have been implicated as causes of erythema induratum, including bacillus Calmette-Guérin (BCG), the attenuated form of Mycobacterium bovis, which commonly is used for tuberculosis vaccination. Medications also may cause erythema induratum. The characteristic distribution of the nodules on the posterior calves helps to distinguish erythema induratum from other panniculitides. A PubMed search of articles indexed for MEDLINE using the term disseminated erythema induratum revealed few case reports documenting nodules on the arms, thighs, or chest, and only 1 case report of disseminated erythema induratum.3-8 We describe a rare combination of disseminated erythema induratum in a patient with remote exposure to tuberculosis and recent BCG exposure.
An 88-year-old woman presented for evaluation of violaceous, minimally tender, nonulcerated, subcutaneous nodules on the legs, arms, and trunk of several weeks’ duration (Figure 1). She had a remote history of tuberculosis as a child, prior to the advent of modern antituberculosis regimens. Her medical history also included hypertension, breast cancer treated with lymph node dissection, gastroesophageal reflux disease, and bladder cancer treated with intravesical BCG 10 years prior to the onset of the nodules. She reported minimal coughing and a 25-lb weight loss over the last year, but she denied night sweats, fever, or chills.

Workup included a biopsy, which showed a dense inflammatory infiltrate within the septae and lobules of the subcutaneous tissue (Figure 2A). Foci of necrosis were seen within the fat lobules (Figure 2B). The histologic diagnosis was erythema induratum. Tissue cultures for bacteria, fungi, and atypical mycobacteria were negative. Mycobacterium tuberculosis polymerase chain reaction (PCR) analysis also was negative. An IFN-γ release assay test was positive for infection with M tuberculosis, suggesting that the erythema induratum was due to tuberculosis rather than BCG exposure. A chest radiograph demonstrated a 22-mm nodule in the left lung (unchanged from a prior film) and a new 10-mm nodule in the left upper lobe.

The patient was referred to an infectious disease specialist who concurred that the erythema induratum and the new lung nodule likely represented a reactivation of tuberculosis. Sputum samples were found to be smear and culture negative for mycobacteria, but due to high clinical suspicion, she was started on a 4-drug tuberculosis regimen of isoniazid, rifampin, pyrazinamide, and ethambutol. Some lesions had started to improve prior to the institution of therapy; after initiation of treatment, all lesions resolved within 4 weeks of starting treatment without recurrence.
Erythema induratum was first described by Bazin9 in 1861. The disorder usually occurs in middle-aged women and is characterized by violaceous ulcerative plaques that classically present on the lower extremities, especially the calves. When the eruption occurs due to a nontuberculous etiology, the term nodular vasculitis is used.1,5 The distinction largely is historical, as most dermatologists today recognize erythema induratum and nodular vasculitis to be the same entity. Examples of nontuberculous causes include infections such as Nocardia, Pseudomonas, Fusarium, or other Mycobacterium species.10 Medications such as propylthiouracil also have been implicated.11 The classification of erythema induratum as a tuberculid suggests that the nodules are a reaction pattern rather than a primary infection, though the term tuberculid may be imprecise. The differential diagnosis of violaceous nodules on the lower extremities and trunk is broad and includes erythema nodosum, cutaneous polyarteritis nodosa, pancreatic panniculitis, subcutaneous T-cell lymphoma, and lupus profundus.1,11,12
Histologically, lesions classically demonstrate a mostly lobular panniculitis with varying degrees of septal fibrosis and focal necrosis. Neutrophils may predominate early, while adipocyte necrosis, epithelioid histiocytes, multinucleated giant cells, and lymphocytes may be found in older lesions. The presence of vasculitis as a requisite diagnostic criterion remains controversial.1,12
The incidence of erythema induratum has decreased since multidrug tuberculosis treatment has become more widespread.3 Our case displayed the disseminated variant of erythema induratum, an even rarer clinical entity.8 Interestingly, our patient had a history of tuberculosis and exposure to BCG prior to the development of lesions. Case reports have documented erythema induratum after BCG exposure but less frequently than in cases associated with tuberculosis.3,13
The use of BCG vaccines has necessitated the need for a more precise method of determining tuberculosis activity. The tuberculin skin test reacts positively with a history of BCG exposure, rendering it an inadequate test in a patient who is suspected of having an active or latent M tuberculosis infection.13,14 IFN-γ release assays are more specific in detecting latent or active tuberculosis than the tuberculin skin test. Such assays use early secretory antigenic target 6 and cultured filtrate protein 10 as antigens to determine sensitization to M tuberculosis.13,15 These antigens are not produced by BCG or Mycobacterium avium; however, other mycobacteria such as Mycobacterium marinum, Mycobacterium kansasii, and some strains of M bovis produce the aforementioned antigens, and exposure to these microbes may be confounding.13 Importantly, positive IFN-γ release assay results also have been documented after BCG exposure but occur at a much lower frequency than for tuberculosis.15 Thus, the combination of the positive IFN-γ release assay and new chest radiograph nodule in our patient provided strong evidence of reactivated tuberculosis as the precipitating cause of her skin disease.
Despite her negative PCR study, our patient’s presentation remains consistent with the diagnosis of disseminated erythema induratum.13,15 The value of PCR studies in establishing the diagnosis remains to be determined. Case reports have described positive PCR results detecting M tuberculosis in panniculitic nodules, suggesting that trace amounts of the organism are present in lesional tissue despite the negative culture result and immunostains.1 Tuberculid reactions, including lichen scrofulosorum, papulonecrotic tuberculid, and erythema induratum, historically are defined by the lack of positive cultures and immunostains, making positive PCR results difficult to reconcile pathophysiologically.1,13 Therefore, use of the term tuberculid altogether as a descriptor for pathogenesis of this disease may need to be avoided.16 Postulated explanations for the relationship of tuberculid diseases and negative cultures and immunostains include the presence of a small number of bacilli that escape routine laboratory detection, early destruction of organisms, or a reaction to circulating M tuberculosis fragments.2 Regardless, until the pathophysiology of erythema induratum has been fully elucidated, the value of PCR remains unclear.
Disseminated erythema induratum, an exceptionally rare variant of panniculitis, may be seen in patients with a remote history of M tuberculosis exposure and/or recent therapeutic BCG exposure. It is imperative to rule out active tuberculosis, especially in elderly patients whose disease predated the advent of modern antituberculosis therapy. Using an IFN-γ release assay in addition to chest radiographs and other clinical stigmata allows differentiation of the etiology of erythema induratum in those patients with tuberculosis who also were treated with BCG.
- Mascaro JM, Basalga E. Erythema induratum of Bazin. Dermatol Clin. 2008;28:439-445.
- Lighter J, Tse DB, Li Y, et al. Erythema induratum of Bazin in a child: evidence for a cell-mediated hyper-response to Mycobacterium tuberculosis. Pediatr Infect Dis J. 2009;28:326-328.
- Inoue T, Fukumoto T, Ansai S, et al. Erythema induratum of Bazin in an infant after bacilli Calmette-Guerin vaccination. J Dermatol. 2006;33:268-272.
- Degonda Halter M, Nebiker P, Hug B, et al. Atypical erythema induratum Bazin with tuberculous osteomyelitis. Internist. 2006;47:853-856.
- Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320-327.
- Sharma S, Sehgal VN, Bhattacharya SN, et al. Clinicopathologic spectrum of cutaneous tuberculosis: a retrospective analysis of 165 Indians. Am J Dermatopathol. 2015;37:444-450.
- Sethuraman G, Ramesh V. Cutaneous tuberculosis in children. Pediatr Dermatol. 2013;30:7-16.
- Teramura K, Fujimoto N, Nakanishi G, et al. Disseminated erythema induratum of Bazin. Eur J Dermatol. 2014;24:697-698.
- Bazin E. Extrait des Lecons Théoretiques et Cliniques sur le Scrofule. 2nd ed. Paris, France: Delhaye; 1861.
- Campbell SM, Winkelmann RR, Sammons DL. Erythema induratum caused by Mycobacterium chelonei in an immunocompetent patient. J Clin Aesthet Dermatol. 2013;6:38-40.
- Patterson JW. Panniculitis. In: Bolognia JL, Jorizzo J, Rapini RP, et al, eds. Dermatology. Barcelona, Spain: Mosby Elsevier; 2012:1641-1662.
- Segura S, Pujol R, Trinidade F, et al. Vasculitis in erythema induratum of Bazin: a histopathologic study of 101 biopsy specimens from 86 patients. J Am Acad Dermatol. 2008;59:839-851.
- Vera-Kellet C, Peters L, Elwood K, et al. Usefulness of interferon-γ release assays in the diagnosis of erythema induratum. Arch Dermatol. 2011;147:949-952.
- Prajapati V, Steed M, Grewal P, et al. Erythema induratum: case series illustrating the utility of the interferon-γ release assay in determining the association with tuberculosis. J Cutan Med Surg. 2013;17:S6-S11.
- Sim JH, Whang KU. Application of the QuantiFERON-Gold TB test in erythema induratum. J Dermatolog Treat. 2014;25:260-263.
- Wiebels D, Turnbull K, Steinkraus V, et al. Erythema induratum Bazin.”tuberculid” or tuberculosis? [in German]. Hautarzt. 2007;58:237-240.
- Mascaro JM, Basalga E. Erythema induratum of Bazin. Dermatol Clin. 2008;28:439-445.
- Lighter J, Tse DB, Li Y, et al. Erythema induratum of Bazin in a child: evidence for a cell-mediated hyper-response to Mycobacterium tuberculosis. Pediatr Infect Dis J. 2009;28:326-328.
- Inoue T, Fukumoto T, Ansai S, et al. Erythema induratum of Bazin in an infant after bacilli Calmette-Guerin vaccination. J Dermatol. 2006;33:268-272.
- Degonda Halter M, Nebiker P, Hug B, et al. Atypical erythema induratum Bazin with tuberculous osteomyelitis. Internist. 2006;47:853-856.
- Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320-327.
- Sharma S, Sehgal VN, Bhattacharya SN, et al. Clinicopathologic spectrum of cutaneous tuberculosis: a retrospective analysis of 165 Indians. Am J Dermatopathol. 2015;37:444-450.
- Sethuraman G, Ramesh V. Cutaneous tuberculosis in children. Pediatr Dermatol. 2013;30:7-16.
- Teramura K, Fujimoto N, Nakanishi G, et al. Disseminated erythema induratum of Bazin. Eur J Dermatol. 2014;24:697-698.
- Bazin E. Extrait des Lecons Théoretiques et Cliniques sur le Scrofule. 2nd ed. Paris, France: Delhaye; 1861.
- Campbell SM, Winkelmann RR, Sammons DL. Erythema induratum caused by Mycobacterium chelonei in an immunocompetent patient. J Clin Aesthet Dermatol. 2013;6:38-40.
- Patterson JW. Panniculitis. In: Bolognia JL, Jorizzo J, Rapini RP, et al, eds. Dermatology. Barcelona, Spain: Mosby Elsevier; 2012:1641-1662.
- Segura S, Pujol R, Trinidade F, et al. Vasculitis in erythema induratum of Bazin: a histopathologic study of 101 biopsy specimens from 86 patients. J Am Acad Dermatol. 2008;59:839-851.
- Vera-Kellet C, Peters L, Elwood K, et al. Usefulness of interferon-γ release assays in the diagnosis of erythema induratum. Arch Dermatol. 2011;147:949-952.
- Prajapati V, Steed M, Grewal P, et al. Erythema induratum: case series illustrating the utility of the interferon-γ release assay in determining the association with tuberculosis. J Cutan Med Surg. 2013;17:S6-S11.
- Sim JH, Whang KU. Application of the QuantiFERON-Gold TB test in erythema induratum. J Dermatolog Treat. 2014;25:260-263.
- Wiebels D, Turnbull K, Steinkraus V, et al. Erythema induratum Bazin.”tuberculid” or tuberculosis? [in German]. Hautarzt. 2007;58:237-240.
Practice Points
- Erythema induratum is an uncommon panniculitis attributed to a delayed-type hypersensitivity reaction, classically to Mycobacterium tuberculosis.
- The workup for such patients with exposure to both M tuberculosis and bacillus Calmette-Guérin should include IFN-11γ release assays.
- Clinicians should be aware of the disseminated variant of erythema induratum and the laboratory testing needed to establish a cause and help direct treatment.
Be vigilant for scleroderma renal crisis
Scleroderma renal crisis is often the most challenging type of scleroderma emergency to identify promptly, according to Francesco Boin, MD, professor of medicine and director of the scleroderma center at the University of California, San Francisco.
“Fortunately, it’s not a frequent event. But it’s severe enough that all rheumatologists should be aware of it,” he said at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
Atypical presentations occur in 30%
Scleroderma renal crisis (SRC) occurs in 5%-10% of scleroderma patients. A vexing feature of this emergency is that not uncommonly it actually precedes the diagnosis of scleroderma. Indeed, 20% of patients with SRC present with sine scleroderma – that is, they have no skin disease and their renal crisis is their first symptom of scleroderma. In contrast, critical digital ischemia – the most common scleroderma emergency – is invariably preceded by worsening episodes of Raynaud’s, and impending intestinal pseudo-obstruction – also among the most common scleroderma emergencies – is heralded by an established history of dysmotility, loss of appetite, abdominal bloating, small intestinal bacterial overgrowth, and bowel distension.
While sine SRC often poses a formidable diagnostic challenge, SRC occurs most often in patients with early, rapidly progressing diffuse scleroderma skin disease. Indeed, the median duration of scleroderma when SRC strikes is just 8 months. The use of glucocorticoids at 15 mg or more per day, or at lower doses for a lengthy period, is an independent risk factor for SRC. Detection of anti–RNA polymerase III antibodies warrants increased vigilance, since 60% of patients with SRC are anti–RNA polymerase III antibody positive. Other autoantibodies are not a risk factor. Neither is preexisting hypertension nor a high baseline serum creatinine.
The classic textbook presentation of SRC is abrupt onset of blood pressures greater than 20 mm Hg above normal for that individual, along with sudden renal failure; a climbing creatinine; proteinuria; and expressions of malignant hypertension such as pulmonary edema, new-onset heart failure, encephalopathy, and/or development of a thrombotic microangiopathy.
Notably, however, 30% of individuals with SRC don’t fit this picture at all. They may present with abrupt-onset severe hypertension but no evidence of renal failure, at least early on. Or they may have sudden renal failure without a hypertensive crisis. Alternatively, they may have no signs of malignant hypertension, just an asymptomatic pericardial effusion or mild arrhythmias.
“Also, the thrombotic microangiopathy can be present without the other features of scleroderma renal crisis, so no renal failure or hypertensive emergency. Be aware of the possibility of atypical presentations, and always suspect this unfolding problem in the right individuals,” the rheumatologist urged.
Anyone with scleroderma who presents with new-onset hypertension needs to begin keeping a careful home blood pressure diary. If the blood pressure shoots up, or symptoms of malignant hypertension develop, or laboratory monitoring reveals evidence of thrombotic microangiopathy, the patient should immediately go to the ED because these events are often followed by accelerated progression to renal crisis.
Inpatient management of SRC is critical. “In the hospital we can monitor renal function in a more refined way, we can manage the malignant hypertension, and early on, hospitalization provides the opportunity to do a renal biopsy. I always consider doing this early. The pathologist often pushes back, but I think it’s relevant. It confirms the diagnosis. We’ve had patients where we were surprised: We thought it was scleroderma renal crisis, but instead they had interstitial nephritis or glomerulonephritis. Most important, biopsy has major prognostic implications: You can measure the extent of damage and therefore have a sense of whether the patient will be able to recover renal function,” Dr. Boin explained.
Prognosis and predictors
Outcome of SRC is often poor: the 1-year mortality is 20%-30%, with a 5-year mortality of 30%-50%. Normotensive SRC with renal crisis, which accounts for about 10% of all cases of SRC, is particularly serious in its implication, with a 1-year mortality of 60%. Half of patients with SRC require hemodialysis, and only one-quarter of them recover spontaneous renal function.
Predictors of worse outcome include older age at onset of SRC, male gender, a serum creatinine level above 3 mg/dL at presentation, incomplete blood pressure control within the first 3 days of the crisis, and normotensive SRC. Use of an ACE inhibitor prior to SRC is also an independent predictor of poor outcome, possibly because by keeping the blood pressure under control the medication blunts recognition of the unfolding renal crisis.
“This is why experts don’t recommend prophylactic ACE inhibitors in patients who are at risk for SRC,” according to Dr. Boin.
He reported having no financial conflicts regarding his presentation.
Scleroderma renal crisis is often the most challenging type of scleroderma emergency to identify promptly, according to Francesco Boin, MD, professor of medicine and director of the scleroderma center at the University of California, San Francisco.
“Fortunately, it’s not a frequent event. But it’s severe enough that all rheumatologists should be aware of it,” he said at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
Atypical presentations occur in 30%
Scleroderma renal crisis (SRC) occurs in 5%-10% of scleroderma patients. A vexing feature of this emergency is that not uncommonly it actually precedes the diagnosis of scleroderma. Indeed, 20% of patients with SRC present with sine scleroderma – that is, they have no skin disease and their renal crisis is their first symptom of scleroderma. In contrast, critical digital ischemia – the most common scleroderma emergency – is invariably preceded by worsening episodes of Raynaud’s, and impending intestinal pseudo-obstruction – also among the most common scleroderma emergencies – is heralded by an established history of dysmotility, loss of appetite, abdominal bloating, small intestinal bacterial overgrowth, and bowel distension.
While sine SRC often poses a formidable diagnostic challenge, SRC occurs most often in patients with early, rapidly progressing diffuse scleroderma skin disease. Indeed, the median duration of scleroderma when SRC strikes is just 8 months. The use of glucocorticoids at 15 mg or more per day, or at lower doses for a lengthy period, is an independent risk factor for SRC. Detection of anti–RNA polymerase III antibodies warrants increased vigilance, since 60% of patients with SRC are anti–RNA polymerase III antibody positive. Other autoantibodies are not a risk factor. Neither is preexisting hypertension nor a high baseline serum creatinine.
The classic textbook presentation of SRC is abrupt onset of blood pressures greater than 20 mm Hg above normal for that individual, along with sudden renal failure; a climbing creatinine; proteinuria; and expressions of malignant hypertension such as pulmonary edema, new-onset heart failure, encephalopathy, and/or development of a thrombotic microangiopathy.
Notably, however, 30% of individuals with SRC don’t fit this picture at all. They may present with abrupt-onset severe hypertension but no evidence of renal failure, at least early on. Or they may have sudden renal failure without a hypertensive crisis. Alternatively, they may have no signs of malignant hypertension, just an asymptomatic pericardial effusion or mild arrhythmias.
“Also, the thrombotic microangiopathy can be present without the other features of scleroderma renal crisis, so no renal failure or hypertensive emergency. Be aware of the possibility of atypical presentations, and always suspect this unfolding problem in the right individuals,” the rheumatologist urged.
Anyone with scleroderma who presents with new-onset hypertension needs to begin keeping a careful home blood pressure diary. If the blood pressure shoots up, or symptoms of malignant hypertension develop, or laboratory monitoring reveals evidence of thrombotic microangiopathy, the patient should immediately go to the ED because these events are often followed by accelerated progression to renal crisis.
Inpatient management of SRC is critical. “In the hospital we can monitor renal function in a more refined way, we can manage the malignant hypertension, and early on, hospitalization provides the opportunity to do a renal biopsy. I always consider doing this early. The pathologist often pushes back, but I think it’s relevant. It confirms the diagnosis. We’ve had patients where we were surprised: We thought it was scleroderma renal crisis, but instead they had interstitial nephritis or glomerulonephritis. Most important, biopsy has major prognostic implications: You can measure the extent of damage and therefore have a sense of whether the patient will be able to recover renal function,” Dr. Boin explained.
Prognosis and predictors
Outcome of SRC is often poor: the 1-year mortality is 20%-30%, with a 5-year mortality of 30%-50%. Normotensive SRC with renal crisis, which accounts for about 10% of all cases of SRC, is particularly serious in its implication, with a 1-year mortality of 60%. Half of patients with SRC require hemodialysis, and only one-quarter of them recover spontaneous renal function.
Predictors of worse outcome include older age at onset of SRC, male gender, a serum creatinine level above 3 mg/dL at presentation, incomplete blood pressure control within the first 3 days of the crisis, and normotensive SRC. Use of an ACE inhibitor prior to SRC is also an independent predictor of poor outcome, possibly because by keeping the blood pressure under control the medication blunts recognition of the unfolding renal crisis.
“This is why experts don’t recommend prophylactic ACE inhibitors in patients who are at risk for SRC,” according to Dr. Boin.
He reported having no financial conflicts regarding his presentation.
Scleroderma renal crisis is often the most challenging type of scleroderma emergency to identify promptly, according to Francesco Boin, MD, professor of medicine and director of the scleroderma center at the University of California, San Francisco.
“Fortunately, it’s not a frequent event. But it’s severe enough that all rheumatologists should be aware of it,” he said at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
Atypical presentations occur in 30%
Scleroderma renal crisis (SRC) occurs in 5%-10% of scleroderma patients. A vexing feature of this emergency is that not uncommonly it actually precedes the diagnosis of scleroderma. Indeed, 20% of patients with SRC present with sine scleroderma – that is, they have no skin disease and their renal crisis is their first symptom of scleroderma. In contrast, critical digital ischemia – the most common scleroderma emergency – is invariably preceded by worsening episodes of Raynaud’s, and impending intestinal pseudo-obstruction – also among the most common scleroderma emergencies – is heralded by an established history of dysmotility, loss of appetite, abdominal bloating, small intestinal bacterial overgrowth, and bowel distension.
While sine SRC often poses a formidable diagnostic challenge, SRC occurs most often in patients with early, rapidly progressing diffuse scleroderma skin disease. Indeed, the median duration of scleroderma when SRC strikes is just 8 months. The use of glucocorticoids at 15 mg or more per day, or at lower doses for a lengthy period, is an independent risk factor for SRC. Detection of anti–RNA polymerase III antibodies warrants increased vigilance, since 60% of patients with SRC are anti–RNA polymerase III antibody positive. Other autoantibodies are not a risk factor. Neither is preexisting hypertension nor a high baseline serum creatinine.
The classic textbook presentation of SRC is abrupt onset of blood pressures greater than 20 mm Hg above normal for that individual, along with sudden renal failure; a climbing creatinine; proteinuria; and expressions of malignant hypertension such as pulmonary edema, new-onset heart failure, encephalopathy, and/or development of a thrombotic microangiopathy.
Notably, however, 30% of individuals with SRC don’t fit this picture at all. They may present with abrupt-onset severe hypertension but no evidence of renal failure, at least early on. Or they may have sudden renal failure without a hypertensive crisis. Alternatively, they may have no signs of malignant hypertension, just an asymptomatic pericardial effusion or mild arrhythmias.
“Also, the thrombotic microangiopathy can be present without the other features of scleroderma renal crisis, so no renal failure or hypertensive emergency. Be aware of the possibility of atypical presentations, and always suspect this unfolding problem in the right individuals,” the rheumatologist urged.
Anyone with scleroderma who presents with new-onset hypertension needs to begin keeping a careful home blood pressure diary. If the blood pressure shoots up, or symptoms of malignant hypertension develop, or laboratory monitoring reveals evidence of thrombotic microangiopathy, the patient should immediately go to the ED because these events are often followed by accelerated progression to renal crisis.
Inpatient management of SRC is critical. “In the hospital we can monitor renal function in a more refined way, we can manage the malignant hypertension, and early on, hospitalization provides the opportunity to do a renal biopsy. I always consider doing this early. The pathologist often pushes back, but I think it’s relevant. It confirms the diagnosis. We’ve had patients where we were surprised: We thought it was scleroderma renal crisis, but instead they had interstitial nephritis or glomerulonephritis. Most important, biopsy has major prognostic implications: You can measure the extent of damage and therefore have a sense of whether the patient will be able to recover renal function,” Dr. Boin explained.
Prognosis and predictors
Outcome of SRC is often poor: the 1-year mortality is 20%-30%, with a 5-year mortality of 30%-50%. Normotensive SRC with renal crisis, which accounts for about 10% of all cases of SRC, is particularly serious in its implication, with a 1-year mortality of 60%. Half of patients with SRC require hemodialysis, and only one-quarter of them recover spontaneous renal function.
Predictors of worse outcome include older age at onset of SRC, male gender, a serum creatinine level above 3 mg/dL at presentation, incomplete blood pressure control within the first 3 days of the crisis, and normotensive SRC. Use of an ACE inhibitor prior to SRC is also an independent predictor of poor outcome, possibly because by keeping the blood pressure under control the medication blunts recognition of the unfolding renal crisis.
“This is why experts don’t recommend prophylactic ACE inhibitors in patients who are at risk for SRC,” according to Dr. Boin.
He reported having no financial conflicts regarding his presentation.
FROM SOTA 2020
Pediatric Dermatology: A Supplement to Pediatric News & Dermatology News
Content includes:
- Early onset of atopic dermatitis linked to poorer control, could signify more persistent disease
- Patients with actopic dermatitis should be routinely asked about conjunctivitis
- Hope on the horizon: New cantharidin formulation alleviates molluscum contagiosum in pivotal trials
- Patch testing in atopic dermatitis: When and how
- Topical calcineurin inhibitors are an effective treatment option for periorificial dermatitis
- Psychology consults for children’s skin issues can boost adherence, wellness
Content includes:
- Early onset of atopic dermatitis linked to poorer control, could signify more persistent disease
- Patients with actopic dermatitis should be routinely asked about conjunctivitis
- Hope on the horizon: New cantharidin formulation alleviates molluscum contagiosum in pivotal trials
- Patch testing in atopic dermatitis: When and how
- Topical calcineurin inhibitors are an effective treatment option for periorificial dermatitis
- Psychology consults for children’s skin issues can boost adherence, wellness
Content includes:
- Early onset of atopic dermatitis linked to poorer control, could signify more persistent disease
- Patients with actopic dermatitis should be routinely asked about conjunctivitis
- Hope on the horizon: New cantharidin formulation alleviates molluscum contagiosum in pivotal trials
- Patch testing in atopic dermatitis: When and how
- Topical calcineurin inhibitors are an effective treatment option for periorificial dermatitis
- Psychology consults for children’s skin issues can boost adherence, wellness