User login
Pediatric Dermatology: A Supplement to Pediatric News & Dermatology News
Content includes:
- Early onset of atopic dermatitis linked to poorer control, could signify more persistent disease
- Patients with actopic dermatitis should be routinely asked about conjunctivitis
- Hope on the horizon: New cantharidin formulation alleviates molluscum contagiosum in pivotal trials
- Patch testing in atopic dermatitis: When and how
- Topical calcineurin inhibitors are an effective treatment option for periorificial dermatitis
- Psychology consults for children’s skin issues can boost adherence, wellness
Content includes:
- Early onset of atopic dermatitis linked to poorer control, could signify more persistent disease
- Patients with actopic dermatitis should be routinely asked about conjunctivitis
- Hope on the horizon: New cantharidin formulation alleviates molluscum contagiosum in pivotal trials
- Patch testing in atopic dermatitis: When and how
- Topical calcineurin inhibitors are an effective treatment option for periorificial dermatitis
- Psychology consults for children’s skin issues can boost adherence, wellness
Content includes:
- Early onset of atopic dermatitis linked to poorer control, could signify more persistent disease
- Patients with actopic dermatitis should be routinely asked about conjunctivitis
- Hope on the horizon: New cantharidin formulation alleviates molluscum contagiosum in pivotal trials
- Patch testing in atopic dermatitis: When and how
- Topical calcineurin inhibitors are an effective treatment option for periorificial dermatitis
- Psychology consults for children’s skin issues can boost adherence, wellness
FDA approves Uplizna for treatment of anti-AQP4 antibody–positive NMOSD
The Food and Drug Administration has approved Uplizna (inebilizumab-cdon) for the treatment of adult patients with neuromyelitis optica spectrum disorder (NMOSD) who are anti-AQP4 antibody positive. Uplizna is the second approved treatment for the disorder.
Approval was based on results from the global, placebo-controlled N-MOmentum trial, which included 213 anti-AQP4 antibody–positive patients and 17 anti-AQP4 antibody–negative patients who received inebilizumab-cdon or placebo. Just under 90% of patients in the positive group remained relapse free 6 months after the initial dosing, compared with 58% of patients taking placebo. People who took inebilizumab also saw a reduction in NMOSD-related hospitalizations. There was no evidence of a benefit in patients who were anti-AQP4 antibody negative.
Inebilizumab-cdon was safe and well tolerated during the trial, with the most common adverse events being urinary tract infection (20%), nasopharyngitis (13%), infusion reaction (12%), arthralgia (11%), and headache (10%). The drug is approved as twice-yearly maintenance after initial dosing. The prescribing information for Uplizna includes a warning for infusion reactions, potential depletion of certain proteins (hypogammaglobulinemia), and potential increased risk of infection—including progressive multifocal leukoencephalopathy—and potential reactivation of hepatitis B and tuberculosis.
“NMOSD is an extremely challenging disease to treat. Patients experience unpredictable attacks that can lead to permanent disability from blindness and paralysis. In addition, each subsequent attack may result in a cumulative worsening of disability,” Bruce Cree, MD, PhD, lead investigator for the N-MOmentum trial and professor of clinical neurology at the University of California, San Francisco, said in a press release. “Uplizna is an important new treatment option that provides prescribing physicians and patients living with NMOSD a therapy with proven efficacy, a favorable safety profile and a twice-a-year maintenance dosing schedule.”
The Food and Drug Administration has approved Uplizna (inebilizumab-cdon) for the treatment of adult patients with neuromyelitis optica spectrum disorder (NMOSD) who are anti-AQP4 antibody positive. Uplizna is the second approved treatment for the disorder.
Approval was based on results from the global, placebo-controlled N-MOmentum trial, which included 213 anti-AQP4 antibody–positive patients and 17 anti-AQP4 antibody–negative patients who received inebilizumab-cdon or placebo. Just under 90% of patients in the positive group remained relapse free 6 months after the initial dosing, compared with 58% of patients taking placebo. People who took inebilizumab also saw a reduction in NMOSD-related hospitalizations. There was no evidence of a benefit in patients who were anti-AQP4 antibody negative.
Inebilizumab-cdon was safe and well tolerated during the trial, with the most common adverse events being urinary tract infection (20%), nasopharyngitis (13%), infusion reaction (12%), arthralgia (11%), and headache (10%). The drug is approved as twice-yearly maintenance after initial dosing. The prescribing information for Uplizna includes a warning for infusion reactions, potential depletion of certain proteins (hypogammaglobulinemia), and potential increased risk of infection—including progressive multifocal leukoencephalopathy—and potential reactivation of hepatitis B and tuberculosis.
“NMOSD is an extremely challenging disease to treat. Patients experience unpredictable attacks that can lead to permanent disability from blindness and paralysis. In addition, each subsequent attack may result in a cumulative worsening of disability,” Bruce Cree, MD, PhD, lead investigator for the N-MOmentum trial and professor of clinical neurology at the University of California, San Francisco, said in a press release. “Uplizna is an important new treatment option that provides prescribing physicians and patients living with NMOSD a therapy with proven efficacy, a favorable safety profile and a twice-a-year maintenance dosing schedule.”
The Food and Drug Administration has approved Uplizna (inebilizumab-cdon) for the treatment of adult patients with neuromyelitis optica spectrum disorder (NMOSD) who are anti-AQP4 antibody positive. Uplizna is the second approved treatment for the disorder.
Approval was based on results from the global, placebo-controlled N-MOmentum trial, which included 213 anti-AQP4 antibody–positive patients and 17 anti-AQP4 antibody–negative patients who received inebilizumab-cdon or placebo. Just under 90% of patients in the positive group remained relapse free 6 months after the initial dosing, compared with 58% of patients taking placebo. People who took inebilizumab also saw a reduction in NMOSD-related hospitalizations. There was no evidence of a benefit in patients who were anti-AQP4 antibody negative.
Inebilizumab-cdon was safe and well tolerated during the trial, with the most common adverse events being urinary tract infection (20%), nasopharyngitis (13%), infusion reaction (12%), arthralgia (11%), and headache (10%). The drug is approved as twice-yearly maintenance after initial dosing. The prescribing information for Uplizna includes a warning for infusion reactions, potential depletion of certain proteins (hypogammaglobulinemia), and potential increased risk of infection—including progressive multifocal leukoencephalopathy—and potential reactivation of hepatitis B and tuberculosis.
“NMOSD is an extremely challenging disease to treat. Patients experience unpredictable attacks that can lead to permanent disability from blindness and paralysis. In addition, each subsequent attack may result in a cumulative worsening of disability,” Bruce Cree, MD, PhD, lead investigator for the N-MOmentum trial and professor of clinical neurology at the University of California, San Francisco, said in a press release. “Uplizna is an important new treatment option that provides prescribing physicians and patients living with NMOSD a therapy with proven efficacy, a favorable safety profile and a twice-a-year maintenance dosing schedule.”
She Can’t Turn the Other Cheek on the Lesion
ANSWER
The correct answer is seborrheic keratosis (choice “a”).
DISCUSSION
Seborrheic keratosis could not be in the differential because it is, by definition, an epidermal lesion—that is, “stuck on” the surface of the skin. It creates a rough surface that can be easily scraped off. The lesion could have been an actual scar, but other factors (its continuous growth) and the history of excessive ultraviolet exposure pushed us away from including this condition in the differential.
The differential for this patient included sun-caused skin cancers: basal cell carcinoma (BCC; choice “b”), squamous cell carcinoma (SCC; choice “d”), and amelanotic melanoma (choice “c”). These conditions can have a colorless and scar-like appearance, and they also destroy surface adnexae. Therefore, the lack of hairs, pores, or skin lines in a circumscribed area should raise concern for possible skin cancer, especially in at-risk patients such as this one.
BCC (otherwise known as cicatricial basal cell carcinoma) is by far the most common of all sun-caused skin cancers, but it usually presents as an obvious papule or nodule, often with telltale features such as pearly, rolled borders and focal erosion or ulceration. But there are exceptions, and the scar-like BCC is one.
SCC can also occasionally present in this manner, as can amelanotic melanoma, which is a colorless melanoma and very easy to miss. This case perfectly illustrates the point I often make to the students and residents I teach: When skin cancer is suspected, pay at least as much attention to the owner as to the lesion. Also, when in doubt, biopsy will settle the matter.
TREATMENT
For the patient, shave biopsy confirmed the presence of BCC. She was then referred for Mohs micrographic surgery because of the lesion’s size, location, and uncertain visible margins.
ANSWER
The correct answer is seborrheic keratosis (choice “a”).
DISCUSSION
Seborrheic keratosis could not be in the differential because it is, by definition, an epidermal lesion—that is, “stuck on” the surface of the skin. It creates a rough surface that can be easily scraped off. The lesion could have been an actual scar, but other factors (its continuous growth) and the history of excessive ultraviolet exposure pushed us away from including this condition in the differential.
The differential for this patient included sun-caused skin cancers: basal cell carcinoma (BCC; choice “b”), squamous cell carcinoma (SCC; choice “d”), and amelanotic melanoma (choice “c”). These conditions can have a colorless and scar-like appearance, and they also destroy surface adnexae. Therefore, the lack of hairs, pores, or skin lines in a circumscribed area should raise concern for possible skin cancer, especially in at-risk patients such as this one.
BCC (otherwise known as cicatricial basal cell carcinoma) is by far the most common of all sun-caused skin cancers, but it usually presents as an obvious papule or nodule, often with telltale features such as pearly, rolled borders and focal erosion or ulceration. But there are exceptions, and the scar-like BCC is one.
SCC can also occasionally present in this manner, as can amelanotic melanoma, which is a colorless melanoma and very easy to miss. This case perfectly illustrates the point I often make to the students and residents I teach: When skin cancer is suspected, pay at least as much attention to the owner as to the lesion. Also, when in doubt, biopsy will settle the matter.
TREATMENT
For the patient, shave biopsy confirmed the presence of BCC. She was then referred for Mohs micrographic surgery because of the lesion’s size, location, and uncertain visible margins.
ANSWER
The correct answer is seborrheic keratosis (choice “a”).
DISCUSSION
Seborrheic keratosis could not be in the differential because it is, by definition, an epidermal lesion—that is, “stuck on” the surface of the skin. It creates a rough surface that can be easily scraped off. The lesion could have been an actual scar, but other factors (its continuous growth) and the history of excessive ultraviolet exposure pushed us away from including this condition in the differential.
The differential for this patient included sun-caused skin cancers: basal cell carcinoma (BCC; choice “b”), squamous cell carcinoma (SCC; choice “d”), and amelanotic melanoma (choice “c”). These conditions can have a colorless and scar-like appearance, and they also destroy surface adnexae. Therefore, the lack of hairs, pores, or skin lines in a circumscribed area should raise concern for possible skin cancer, especially in at-risk patients such as this one.
BCC (otherwise known as cicatricial basal cell carcinoma) is by far the most common of all sun-caused skin cancers, but it usually presents as an obvious papule or nodule, often with telltale features such as pearly, rolled borders and focal erosion or ulceration. But there are exceptions, and the scar-like BCC is one.
SCC can also occasionally present in this manner, as can amelanotic melanoma, which is a colorless melanoma and very easy to miss. This case perfectly illustrates the point I often make to the students and residents I teach: When skin cancer is suspected, pay at least as much attention to the owner as to the lesion. Also, when in doubt, biopsy will settle the matter.
TREATMENT
For the patient, shave biopsy confirmed the presence of BCC. She was then referred for Mohs micrographic surgery because of the lesion’s size, location, and uncertain visible margins.
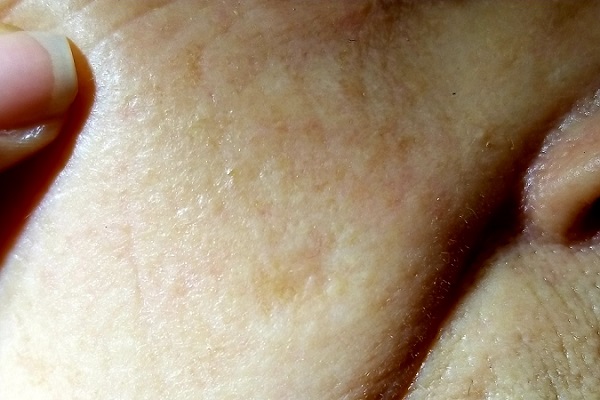
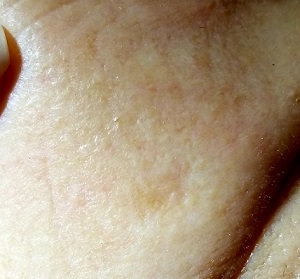
For several years, a 70-year-old woman has had an asymptomatic lesion on her cheek that has been growing slowly and steadily. Her primary care provider has reassured her at multiple visits that it should not cause her worry. Still, because of the lesion’s continued growth and her history of excessive sun exposure when she was young, she self-refers to dermatology for evaluation.
The patient has no history of skin cancer but her 2 sisters do, including a recent diagnosis of melanoma for one of them. During the 1950s, the 3 sisters were often outdoors—all burning easily and often and tanning only with difficulty. Since then, the sisters’ sun-drenched days have ended. All are in otherwise excellent health.
Examination reveals a patient with quite fair (type 2) skin and blue eyes. There is abundant evidence of past overexposure to ultraviolet light, including a weathered effect, scattered telangiectasias, and patches of white mottled skin (otherwise known as solar elastosis).
The lesion in question is quite faint and difficult to see. Magnification shows a 2-cm round patch that is slightly lighter than the surrounding skin and completely macular. No induration is felt on palpation, and no nodes are detected in the region.
An even closer and meticulous examination reveals that the surface adnexal structures—such as pores, skin lines, and even tiny hairs—that should be inside the lesion are completely missing. Slightly yellowish discoloration can be seen in the center of the patch. The rest of her skin shows no other worrisome features or lesions.
Smart phones boosted compliance for cardiac device data transmission
A phone, an app, and the next generation of implanted cardiac device data signaling produced an unprecedented level of data transmission compliance in a single-arm, multicenter, pilot study with 245 patients, adding momentum to the expanding penetration of personal smart devices into cardiac electrophysiology.
During 12-month follow-up, the 245 patients who received either a medically indicated pacemaker or cardiac resynchronization therapy (CRT)–pacemaker equipped with Bluetooth remote transmission capability had successful data transfer to their clinicians for 95% of their scheduled data uploads while using a personal phone or tablet as the link between their heart implant and the Internet. This rate significantly surpassed the transmission-success rates tallied by traditional, bedside transmitters in historical control groups, Khaldoun G. Tarakji, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19.
A related analysis by Dr. Tarakji and colleagues of 811 patients from real-world practice who received similar implanted cardiac devices with the same remote-transmission capability showed a 93% rate of successful data transfers via smart devices.
In contrast, historical performance showed a 77% success rate in matched patients drawn from a pool of more than 69,000 people in routine care who had received a pacemaker or CRT-pacemaker that automatically transmitted to a bedside monitor. Historical transmission success among matched patients from a pool of more than 128,000 routine-care patients with similar implants who used a wand to interrogate their implants before the attached monitor transmitted their data had a 56% rate of successful transmissions.
Cardiac device signals that flow directly into a patient’s phone or pad and then relay automatically via an app to the clinic “are clearly much easier,” than the methods now used, observed Dr. Tarakji, a cardiac electrophysiologist at the Cleveland Clinic. “It is truly as seamless as possible. Patients don’t really need to do anything,” he said during a press briefing. The key is that most patients tend to keep their smart devices, especially their phones, near them all the time, which minimizes the chance that the implanted cardiac device might try to file a report when the patient is not positioned near the device that’s facilitating transmission. When patients use conventional, bedside transmitters they can forget to bring them on trips, while many fewer fail to take their phone. Another advantage is that the link between a phone and a cardiac implant can be started in the clinic once the patient downloads an app. Bedside units need home setup, and “some patients never even get theirs out of the box,” Dr. Tarakji lamented.
Another feature of handheld device transmissions that run off an app is that the app can display clinical metrics, activity, device performance, and transmission history, as well as educational information. All of these features can enhance patient engagement with their implanted device, their arrhythmia, and their health status. Bedside units often give patients little feedback, and they don’t display clinical data. “The real challenge for clinicians is what data you let patients see. That’s complicated,” Dr. Tarakji said.
“This study was designed to see whether the technology works. The next step is to study how it affects risk-factor modification” or other outcomes. “There are many opportunities” to explore with this new data transmission and processing capability, he concluded.
The BlueSync Field Evaluation study enrolled patients at 20 centers in the United States, France, Italy, and the United Kingdom during 2018, and the 245 patients who received a BlueSync device and were included in the analysis sent at least one of their scheduled data transmissions during their 12 months of follow-up. Participants were eligible if they were willing to use their own smart phone or pad that could interact with their cardiac implant, and included both first-time implant recipients as well as some patients who received replacement units.
Personal device–based data transmission from cardiac implants “will no doubt change the way we manage patients,” commented Nassir F. Marrouche, MD, a cardiac electrophysiologist and professor of medicine at Tulane University in New Orleans, and a designated discussant for the report. “Every implanted cardiac device should be able to connect with a phone, which can improve adoption and adherence,” he said.
But the study has several limitations for interpreting the implications of the findings, starting with its limited size and single-arm design, noted a second discussant, Roderick Tung, MD, director of cardiac electrophysiology at the University of Chicago. Another issue is the generalizability of the findings, which are likely biased by involving only patients who own a smart phone or tablet and may be more likely to transmit their data regardless of the means. And comparing transmission success in a prospective study with rates that occurred during real-world, routine practice could have a Hawthorne effect bias, where people under study behave differently than they do in everyday life. But that effect may be mitigated by confirmatory findings from a real-world group that also used smart-device transmission included in the report. Despite these caveats, it’s valuable to develop new ways of improving data collection from cardiac devices, Dr. Tung said.
The BlueSync Field Evaluation study was sponsored by Medtronic, the company that markets Bluetooth-enabled cardiac devices. Dr. Tarakji has been a consultant to Medtronic, and also to AliveCor, Boston Scientific, and Johnson & Johnson. Dr. Marrouche has been a consultant to Medtronic as well as to Biosense Webster, Biotronik, Cardiac Design, and Preventice, and he has received research funding from Abbott, Biosense Webster, Boston Scientific, and GE Healthcare. Dr. Tung has been a speaker on behalf of Abbott, Boston Scientific, and Biosense Webster.
SOURCE: Tarakji KG. Heart Rhythm 2020, Abstract D-LBCT04-01.
A phone, an app, and the next generation of implanted cardiac device data signaling produced an unprecedented level of data transmission compliance in a single-arm, multicenter, pilot study with 245 patients, adding momentum to the expanding penetration of personal smart devices into cardiac electrophysiology.
During 12-month follow-up, the 245 patients who received either a medically indicated pacemaker or cardiac resynchronization therapy (CRT)–pacemaker equipped with Bluetooth remote transmission capability had successful data transfer to their clinicians for 95% of their scheduled data uploads while using a personal phone or tablet as the link between their heart implant and the Internet. This rate significantly surpassed the transmission-success rates tallied by traditional, bedside transmitters in historical control groups, Khaldoun G. Tarakji, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19.
A related analysis by Dr. Tarakji and colleagues of 811 patients from real-world practice who received similar implanted cardiac devices with the same remote-transmission capability showed a 93% rate of successful data transfers via smart devices.
In contrast, historical performance showed a 77% success rate in matched patients drawn from a pool of more than 69,000 people in routine care who had received a pacemaker or CRT-pacemaker that automatically transmitted to a bedside monitor. Historical transmission success among matched patients from a pool of more than 128,000 routine-care patients with similar implants who used a wand to interrogate their implants before the attached monitor transmitted their data had a 56% rate of successful transmissions.
Cardiac device signals that flow directly into a patient’s phone or pad and then relay automatically via an app to the clinic “are clearly much easier,” than the methods now used, observed Dr. Tarakji, a cardiac electrophysiologist at the Cleveland Clinic. “It is truly as seamless as possible. Patients don’t really need to do anything,” he said during a press briefing. The key is that most patients tend to keep their smart devices, especially their phones, near them all the time, which minimizes the chance that the implanted cardiac device might try to file a report when the patient is not positioned near the device that’s facilitating transmission. When patients use conventional, bedside transmitters they can forget to bring them on trips, while many fewer fail to take their phone. Another advantage is that the link between a phone and a cardiac implant can be started in the clinic once the patient downloads an app. Bedside units need home setup, and “some patients never even get theirs out of the box,” Dr. Tarakji lamented.
Another feature of handheld device transmissions that run off an app is that the app can display clinical metrics, activity, device performance, and transmission history, as well as educational information. All of these features can enhance patient engagement with their implanted device, their arrhythmia, and their health status. Bedside units often give patients little feedback, and they don’t display clinical data. “The real challenge for clinicians is what data you let patients see. That’s complicated,” Dr. Tarakji said.
“This study was designed to see whether the technology works. The next step is to study how it affects risk-factor modification” or other outcomes. “There are many opportunities” to explore with this new data transmission and processing capability, he concluded.
The BlueSync Field Evaluation study enrolled patients at 20 centers in the United States, France, Italy, and the United Kingdom during 2018, and the 245 patients who received a BlueSync device and were included in the analysis sent at least one of their scheduled data transmissions during their 12 months of follow-up. Participants were eligible if they were willing to use their own smart phone or pad that could interact with their cardiac implant, and included both first-time implant recipients as well as some patients who received replacement units.
Personal device–based data transmission from cardiac implants “will no doubt change the way we manage patients,” commented Nassir F. Marrouche, MD, a cardiac electrophysiologist and professor of medicine at Tulane University in New Orleans, and a designated discussant for the report. “Every implanted cardiac device should be able to connect with a phone, which can improve adoption and adherence,” he said.
But the study has several limitations for interpreting the implications of the findings, starting with its limited size and single-arm design, noted a second discussant, Roderick Tung, MD, director of cardiac electrophysiology at the University of Chicago. Another issue is the generalizability of the findings, which are likely biased by involving only patients who own a smart phone or tablet and may be more likely to transmit their data regardless of the means. And comparing transmission success in a prospective study with rates that occurred during real-world, routine practice could have a Hawthorne effect bias, where people under study behave differently than they do in everyday life. But that effect may be mitigated by confirmatory findings from a real-world group that also used smart-device transmission included in the report. Despite these caveats, it’s valuable to develop new ways of improving data collection from cardiac devices, Dr. Tung said.
The BlueSync Field Evaluation study was sponsored by Medtronic, the company that markets Bluetooth-enabled cardiac devices. Dr. Tarakji has been a consultant to Medtronic, and also to AliveCor, Boston Scientific, and Johnson & Johnson. Dr. Marrouche has been a consultant to Medtronic as well as to Biosense Webster, Biotronik, Cardiac Design, and Preventice, and he has received research funding from Abbott, Biosense Webster, Boston Scientific, and GE Healthcare. Dr. Tung has been a speaker on behalf of Abbott, Boston Scientific, and Biosense Webster.
SOURCE: Tarakji KG. Heart Rhythm 2020, Abstract D-LBCT04-01.
A phone, an app, and the next generation of implanted cardiac device data signaling produced an unprecedented level of data transmission compliance in a single-arm, multicenter, pilot study with 245 patients, adding momentum to the expanding penetration of personal smart devices into cardiac electrophysiology.
During 12-month follow-up, the 245 patients who received either a medically indicated pacemaker or cardiac resynchronization therapy (CRT)–pacemaker equipped with Bluetooth remote transmission capability had successful data transfer to their clinicians for 95% of their scheduled data uploads while using a personal phone or tablet as the link between their heart implant and the Internet. This rate significantly surpassed the transmission-success rates tallied by traditional, bedside transmitters in historical control groups, Khaldoun G. Tarakji, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19.
A related analysis by Dr. Tarakji and colleagues of 811 patients from real-world practice who received similar implanted cardiac devices with the same remote-transmission capability showed a 93% rate of successful data transfers via smart devices.
In contrast, historical performance showed a 77% success rate in matched patients drawn from a pool of more than 69,000 people in routine care who had received a pacemaker or CRT-pacemaker that automatically transmitted to a bedside monitor. Historical transmission success among matched patients from a pool of more than 128,000 routine-care patients with similar implants who used a wand to interrogate their implants before the attached monitor transmitted their data had a 56% rate of successful transmissions.
Cardiac device signals that flow directly into a patient’s phone or pad and then relay automatically via an app to the clinic “are clearly much easier,” than the methods now used, observed Dr. Tarakji, a cardiac electrophysiologist at the Cleveland Clinic. “It is truly as seamless as possible. Patients don’t really need to do anything,” he said during a press briefing. The key is that most patients tend to keep their smart devices, especially their phones, near them all the time, which minimizes the chance that the implanted cardiac device might try to file a report when the patient is not positioned near the device that’s facilitating transmission. When patients use conventional, bedside transmitters they can forget to bring them on trips, while many fewer fail to take their phone. Another advantage is that the link between a phone and a cardiac implant can be started in the clinic once the patient downloads an app. Bedside units need home setup, and “some patients never even get theirs out of the box,” Dr. Tarakji lamented.
Another feature of handheld device transmissions that run off an app is that the app can display clinical metrics, activity, device performance, and transmission history, as well as educational information. All of these features can enhance patient engagement with their implanted device, their arrhythmia, and their health status. Bedside units often give patients little feedback, and they don’t display clinical data. “The real challenge for clinicians is what data you let patients see. That’s complicated,” Dr. Tarakji said.
“This study was designed to see whether the technology works. The next step is to study how it affects risk-factor modification” or other outcomes. “There are many opportunities” to explore with this new data transmission and processing capability, he concluded.
The BlueSync Field Evaluation study enrolled patients at 20 centers in the United States, France, Italy, and the United Kingdom during 2018, and the 245 patients who received a BlueSync device and were included in the analysis sent at least one of their scheduled data transmissions during their 12 months of follow-up. Participants were eligible if they were willing to use their own smart phone or pad that could interact with their cardiac implant, and included both first-time implant recipients as well as some patients who received replacement units.
Personal device–based data transmission from cardiac implants “will no doubt change the way we manage patients,” commented Nassir F. Marrouche, MD, a cardiac electrophysiologist and professor of medicine at Tulane University in New Orleans, and a designated discussant for the report. “Every implanted cardiac device should be able to connect with a phone, which can improve adoption and adherence,” he said.
But the study has several limitations for interpreting the implications of the findings, starting with its limited size and single-arm design, noted a second discussant, Roderick Tung, MD, director of cardiac electrophysiology at the University of Chicago. Another issue is the generalizability of the findings, which are likely biased by involving only patients who own a smart phone or tablet and may be more likely to transmit their data regardless of the means. And comparing transmission success in a prospective study with rates that occurred during real-world, routine practice could have a Hawthorne effect bias, where people under study behave differently than they do in everyday life. But that effect may be mitigated by confirmatory findings from a real-world group that also used smart-device transmission included in the report. Despite these caveats, it’s valuable to develop new ways of improving data collection from cardiac devices, Dr. Tung said.
The BlueSync Field Evaluation study was sponsored by Medtronic, the company that markets Bluetooth-enabled cardiac devices. Dr. Tarakji has been a consultant to Medtronic, and also to AliveCor, Boston Scientific, and Johnson & Johnson. Dr. Marrouche has been a consultant to Medtronic as well as to Biosense Webster, Biotronik, Cardiac Design, and Preventice, and he has received research funding from Abbott, Biosense Webster, Boston Scientific, and GE Healthcare. Dr. Tung has been a speaker on behalf of Abbott, Boston Scientific, and Biosense Webster.
SOURCE: Tarakji KG. Heart Rhythm 2020, Abstract D-LBCT04-01.
FROM HEART RHYTHM 2020
Combo exhibits activity in metastatic mucosal melanoma
according to a presentation made as part of the American Society of Clinical Oncology virtual scientific program.
The combination was well tolerated and “the preliminary efficacy seems to be promising,” which warrants a phase 3 trial, said investigator Jun Guo, MD, of the Peking University Cancer Hospital and Institute in Beijing, who presented the findings.
Mucosal melanoma does not respond as well as cutaneous melanoma to standard programmed death-1 (PD-1) blockade, so investigators are looking for additional options, Dr. Guo noted. Earlier studies have shown that vascular endothelial growth factor expression correlates negatively with clinical outcome, so the combination of VEGF inhibition with PD-1 blockade might provide therapeutic opportunities.
To find out, Dr. Guo and colleagues tested the anti-PD-1 antibody toripalimab in combination with the VEGF inhibitor axitinib in a phase 1 trial. The trial was conducted in China, where mucosal melanoma accounts for up to a quarter of all melanoma cases and where toripalimab is approved to treat mucosal melanoma.
The trial enrolled 33 patients with pathologically confirmed metastatic mucosal melanoma. The esophagus and genital tract were the most common primary lesion sites (both 21.2%). The patients’ average age was 53.4 years, and 60.6% were women. Two patients (6.1%) had previously received systemic chemotherapy. Most (64.6%) were PD–ligand 1 (PD-L1) negative, and most (60.6%) were BRAF/RAS/NF1 wild type.
The patients received axitinib at 5 mg twice daily plus toripalimab at 3 mg/kg every 2 weeks until confirmed disease progression, unacceptable toxicity, or voluntary withdrawal.
As of May 2, 2020, the overall response rate was 48.5%. There were 15 partial responses and 1 complete response. The median duration of response was 13.7 months. The median progression-free survival was 7.5 months, and the median overall survival was 20.7 months.
Progression-free and overall survival were numerically higher in PD-L1-positive subjects and those with higher tumor mutation burdens. An expression profile of 12 genes related to inflammation and angiogenesis showed a significant correlation with response. This might help identify patients most likely to respond to the combination, but further validation is needed, Dr. Guo said.
A total of 32 subjects (97%) have had a treatment-related adverse event, including 13 (39.4%) with grade 3-5 events. The most common of these were proteinuria, hypertension, and neutropenia (all 9.1%).
“So does this study address the unmet need? In many ways, yes,” said Ryan Sullivan, MD, an assistant professor of hematology/oncology at Massachusetts General Hospital in Boston, and the discussant on Dr. Guo’s presentation.
“However, the data to date [don’t] mean we should be treating all of our mucosal melanoma patients with axitinib plus an anti-PD-1 antibody. There needs to be randomized data, but I would describe this data as very encouraging,” he said.
The study was funded by the maker of toripalimab, Shanghai Junshi Bioscience. Dr. Guo disclosed relationships with Shanghai Junshi Bioscience and Pfizer, maker of axitinib. Other investigators are employed by Shanghai Junshi Bioscience. Dr. Sullivan reported institutional research funding from Pfizer.
SOURCE: Guo J et al. ASCO 2020, Abstract 10007.
according to a presentation made as part of the American Society of Clinical Oncology virtual scientific program.
The combination was well tolerated and “the preliminary efficacy seems to be promising,” which warrants a phase 3 trial, said investigator Jun Guo, MD, of the Peking University Cancer Hospital and Institute in Beijing, who presented the findings.
Mucosal melanoma does not respond as well as cutaneous melanoma to standard programmed death-1 (PD-1) blockade, so investigators are looking for additional options, Dr. Guo noted. Earlier studies have shown that vascular endothelial growth factor expression correlates negatively with clinical outcome, so the combination of VEGF inhibition with PD-1 blockade might provide therapeutic opportunities.
To find out, Dr. Guo and colleagues tested the anti-PD-1 antibody toripalimab in combination with the VEGF inhibitor axitinib in a phase 1 trial. The trial was conducted in China, where mucosal melanoma accounts for up to a quarter of all melanoma cases and where toripalimab is approved to treat mucosal melanoma.
The trial enrolled 33 patients with pathologically confirmed metastatic mucosal melanoma. The esophagus and genital tract were the most common primary lesion sites (both 21.2%). The patients’ average age was 53.4 years, and 60.6% were women. Two patients (6.1%) had previously received systemic chemotherapy. Most (64.6%) were PD–ligand 1 (PD-L1) negative, and most (60.6%) were BRAF/RAS/NF1 wild type.
The patients received axitinib at 5 mg twice daily plus toripalimab at 3 mg/kg every 2 weeks until confirmed disease progression, unacceptable toxicity, or voluntary withdrawal.
As of May 2, 2020, the overall response rate was 48.5%. There were 15 partial responses and 1 complete response. The median duration of response was 13.7 months. The median progression-free survival was 7.5 months, and the median overall survival was 20.7 months.
Progression-free and overall survival were numerically higher in PD-L1-positive subjects and those with higher tumor mutation burdens. An expression profile of 12 genes related to inflammation and angiogenesis showed a significant correlation with response. This might help identify patients most likely to respond to the combination, but further validation is needed, Dr. Guo said.
A total of 32 subjects (97%) have had a treatment-related adverse event, including 13 (39.4%) with grade 3-5 events. The most common of these were proteinuria, hypertension, and neutropenia (all 9.1%).
“So does this study address the unmet need? In many ways, yes,” said Ryan Sullivan, MD, an assistant professor of hematology/oncology at Massachusetts General Hospital in Boston, and the discussant on Dr. Guo’s presentation.
“However, the data to date [don’t] mean we should be treating all of our mucosal melanoma patients with axitinib plus an anti-PD-1 antibody. There needs to be randomized data, but I would describe this data as very encouraging,” he said.
The study was funded by the maker of toripalimab, Shanghai Junshi Bioscience. Dr. Guo disclosed relationships with Shanghai Junshi Bioscience and Pfizer, maker of axitinib. Other investigators are employed by Shanghai Junshi Bioscience. Dr. Sullivan reported institutional research funding from Pfizer.
SOURCE: Guo J et al. ASCO 2020, Abstract 10007.
according to a presentation made as part of the American Society of Clinical Oncology virtual scientific program.
The combination was well tolerated and “the preliminary efficacy seems to be promising,” which warrants a phase 3 trial, said investigator Jun Guo, MD, of the Peking University Cancer Hospital and Institute in Beijing, who presented the findings.
Mucosal melanoma does not respond as well as cutaneous melanoma to standard programmed death-1 (PD-1) blockade, so investigators are looking for additional options, Dr. Guo noted. Earlier studies have shown that vascular endothelial growth factor expression correlates negatively with clinical outcome, so the combination of VEGF inhibition with PD-1 blockade might provide therapeutic opportunities.
To find out, Dr. Guo and colleagues tested the anti-PD-1 antibody toripalimab in combination with the VEGF inhibitor axitinib in a phase 1 trial. The trial was conducted in China, where mucosal melanoma accounts for up to a quarter of all melanoma cases and where toripalimab is approved to treat mucosal melanoma.
The trial enrolled 33 patients with pathologically confirmed metastatic mucosal melanoma. The esophagus and genital tract were the most common primary lesion sites (both 21.2%). The patients’ average age was 53.4 years, and 60.6% were women. Two patients (6.1%) had previously received systemic chemotherapy. Most (64.6%) were PD–ligand 1 (PD-L1) negative, and most (60.6%) were BRAF/RAS/NF1 wild type.
The patients received axitinib at 5 mg twice daily plus toripalimab at 3 mg/kg every 2 weeks until confirmed disease progression, unacceptable toxicity, or voluntary withdrawal.
As of May 2, 2020, the overall response rate was 48.5%. There were 15 partial responses and 1 complete response. The median duration of response was 13.7 months. The median progression-free survival was 7.5 months, and the median overall survival was 20.7 months.
Progression-free and overall survival were numerically higher in PD-L1-positive subjects and those with higher tumor mutation burdens. An expression profile of 12 genes related to inflammation and angiogenesis showed a significant correlation with response. This might help identify patients most likely to respond to the combination, but further validation is needed, Dr. Guo said.
A total of 32 subjects (97%) have had a treatment-related adverse event, including 13 (39.4%) with grade 3-5 events. The most common of these were proteinuria, hypertension, and neutropenia (all 9.1%).
“So does this study address the unmet need? In many ways, yes,” said Ryan Sullivan, MD, an assistant professor of hematology/oncology at Massachusetts General Hospital in Boston, and the discussant on Dr. Guo’s presentation.
“However, the data to date [don’t] mean we should be treating all of our mucosal melanoma patients with axitinib plus an anti-PD-1 antibody. There needs to be randomized data, but I would describe this data as very encouraging,” he said.
The study was funded by the maker of toripalimab, Shanghai Junshi Bioscience. Dr. Guo disclosed relationships with Shanghai Junshi Bioscience and Pfizer, maker of axitinib. Other investigators are employed by Shanghai Junshi Bioscience. Dr. Sullivan reported institutional research funding from Pfizer.
SOURCE: Guo J et al. ASCO 2020, Abstract 10007.
FROM ASCO 2020
Renal denervation response similar regardless of CV risks, comorbidities
In a new analysis of international registry data, renal denervation resulted in similar reduced blood pressure levels in patients with varying high-risk comorbidities and across a range of cardiovascular risk scores.
At 3 years, 24-hour systolic BP was reduced by an average of –8.9 mm Hg overall, with slightly higher or lower readings seen in those with higher cardiovascular risk scores (–10.4 mm Hg) and 65 years or older (–10.2 mm Hg). Similar reductions were seen in those with resistant hypertension (–8.7 mm Hg), diabetes (–8.6 mm Hg), isolated systolic hypertension (–10.1 mm Hg), chronic kidney disease (–10.1 mm Hg), or atrial fibrillation (–10.0 mm Hg).
“In the largest international registry of its kind, the efficacy of renal denervation was similar in patients with and without baseline conditions associated with increased sympathetic activity and irrespective of ASCVD [atherosclerotic cardiovascular disease] risk,” first author Felix Mahfoud, MD, said in an interview.
Dr. Mahfoud, from University Hospital of Saarland, Homburg, Germany, and colleagues published their analysis in the Journal of the American College of Cardiology.
The article reported a post hoc analysis of data from the Global SYMPLICITY Registry (GSR), an international, Medtronic-funded effort that includes 2,652 patients with uncontrolled hypertension treated with a Symplicity denervation system. Data were obtained from 196 centers in 45 countries.
“Blood pressure reductions were durable and sustained to 3 years and the rates of new-onset, end-stage renal disease and elevation in serum creatinine levels were very low in patients at high and low [cardiovascular] risk,” reported Dr. Mahfoud.
As expected, adverse event rates were higher for patients with higher baseline cardiovascular risk. “Elevated rates were also seen in patients with [atrial fibrillation] and diabetes, identifying these subgroups who might derive even greater clinical benefit from improved BP control using renal denervation,” said Dr. Mahfoud.
Asked which patients might be optimal candidates for renal denervation, Dr. Mahfoud recommended the technology for “patients with uncontrolled hypertension on medication, patients with nonadherence, unwillingness, or intolerability to medication, and patients with combined systolic and diastolic hypertension.”
Analyses limited by incomplete data
Stephen C. Textor, MD, has concerns over the amount of missing data in the GSR database and its continued use as a repository of information on renal denervation.
“I am a bit lukewarm on this paper in part because of the nature of the registry data they’re using,” he added in an interview. “The problem I see is that the registry is not terribly uniform as to what information they collect on each patient, not terribly uniform in terms of how the procedure is performed, and not terribly uniform on how they follow up patients.”
Indeed, the post hoc subgroup analyses represent only a limited subset because of incomplete data, added Dr. Textor, a nephrologist at the Cleveland Clinic in Rochester, Minn.
“Remarkably, only 504 [patients] had “matched” data for office [systolic BP] levels at the time points defined in the report,” he wrote in an editorial comment accompanying the registry report (J Am Coll Cardiol. 2020 Jun 16;75[23]:2889-91).
Similarly, the researchers were able to calculate baseline atherosclerotic cardiovascular risk scores in only 1,485 patients (56% of total), primarily because of missing cholesterol measurements.
“They simply did these paired comparison that may have included a couple hundred cases, and on average, there were no differences in response, but what I would have liked to see is a multivariate analysis, where you have all the data on everybody and look at what are the factors that impact response?” Dr. Textor said in the interview.
“They really couldn’t do that because they just, they’re just too many holes in the data,” he added.
On the bright side, Dr. Textor noted that, while the impact overall on systolic BP was “modest,” the standard deviations in some cases were large, indicating that some people had large reductions of systolic BP of more than 30-40 mm Hg.
“There is a belief out there that there are some people that really benefit from this, but how to identify them has been the question,” Dr. Textor said.
Enthusiasm for renal denervation plummeted after results from the SYMPLICITY HTN-3 showed the procedure failing to meet its efficacy endpoint in resistant hypertension. The procedure was associated with a 14–mm Hg fall in systolic BP, compared with an 11–mm Hg drop in the “sham” control group (N Engl J Med. 2014 Apr 10;370:1393-401). However, post hoc analysis of the trial revealed significant shortcomings in design and execution.
No renal denervation device is approved in the United States. The Symplicity device used in this registry is approved in the European Union.
In early 2020, the Food and Drug Administration promised a rigorous review of new renal denervation trials. Subsequently, primary results from the SPYRAL HTN-OFF MED pivotal trial were presented at the annual meeting of the American College of Cardiology in March and showed promising efficacy.
SPYRAL HTN OFF-MED was designed in collaboration with the FDA to obtain meaningful evidence of whether renal denervation performed with the Symplicity Spyral multielectrode catheter (Medtronic Vascular) could reduce BP in patients not taking antihypertensive medication.
Dr. Mahfoud reported he has received speaking honoraria from Medtronic and ReCor. Two other authors are employees of Medtronic. Dr. Textor reported no relationships relevant to the contents of this paper. The Global SYMPLICITY Registry is funded by Medtronic Vascular.
SOURCE: Mahfoud F et al. J Am Coll Cardiol. 2020 June 16;75:2879-88.
This article was updated 6/16/20.
In a new analysis of international registry data, renal denervation resulted in similar reduced blood pressure levels in patients with varying high-risk comorbidities and across a range of cardiovascular risk scores.
At 3 years, 24-hour systolic BP was reduced by an average of –8.9 mm Hg overall, with slightly higher or lower readings seen in those with higher cardiovascular risk scores (–10.4 mm Hg) and 65 years or older (–10.2 mm Hg). Similar reductions were seen in those with resistant hypertension (–8.7 mm Hg), diabetes (–8.6 mm Hg), isolated systolic hypertension (–10.1 mm Hg), chronic kidney disease (–10.1 mm Hg), or atrial fibrillation (–10.0 mm Hg).
“In the largest international registry of its kind, the efficacy of renal denervation was similar in patients with and without baseline conditions associated with increased sympathetic activity and irrespective of ASCVD [atherosclerotic cardiovascular disease] risk,” first author Felix Mahfoud, MD, said in an interview.
Dr. Mahfoud, from University Hospital of Saarland, Homburg, Germany, and colleagues published their analysis in the Journal of the American College of Cardiology.
The article reported a post hoc analysis of data from the Global SYMPLICITY Registry (GSR), an international, Medtronic-funded effort that includes 2,652 patients with uncontrolled hypertension treated with a Symplicity denervation system. Data were obtained from 196 centers in 45 countries.
“Blood pressure reductions were durable and sustained to 3 years and the rates of new-onset, end-stage renal disease and elevation in serum creatinine levels were very low in patients at high and low [cardiovascular] risk,” reported Dr. Mahfoud.
As expected, adverse event rates were higher for patients with higher baseline cardiovascular risk. “Elevated rates were also seen in patients with [atrial fibrillation] and diabetes, identifying these subgroups who might derive even greater clinical benefit from improved BP control using renal denervation,” said Dr. Mahfoud.
Asked which patients might be optimal candidates for renal denervation, Dr. Mahfoud recommended the technology for “patients with uncontrolled hypertension on medication, patients with nonadherence, unwillingness, or intolerability to medication, and patients with combined systolic and diastolic hypertension.”
Analyses limited by incomplete data
Stephen C. Textor, MD, has concerns over the amount of missing data in the GSR database and its continued use as a repository of information on renal denervation.
“I am a bit lukewarm on this paper in part because of the nature of the registry data they’re using,” he added in an interview. “The problem I see is that the registry is not terribly uniform as to what information they collect on each patient, not terribly uniform in terms of how the procedure is performed, and not terribly uniform on how they follow up patients.”
Indeed, the post hoc subgroup analyses represent only a limited subset because of incomplete data, added Dr. Textor, a nephrologist at the Cleveland Clinic in Rochester, Minn.
“Remarkably, only 504 [patients] had “matched” data for office [systolic BP] levels at the time points defined in the report,” he wrote in an editorial comment accompanying the registry report (J Am Coll Cardiol. 2020 Jun 16;75[23]:2889-91).
Similarly, the researchers were able to calculate baseline atherosclerotic cardiovascular risk scores in only 1,485 patients (56% of total), primarily because of missing cholesterol measurements.
“They simply did these paired comparison that may have included a couple hundred cases, and on average, there were no differences in response, but what I would have liked to see is a multivariate analysis, where you have all the data on everybody and look at what are the factors that impact response?” Dr. Textor said in the interview.
“They really couldn’t do that because they just, they’re just too many holes in the data,” he added.
On the bright side, Dr. Textor noted that, while the impact overall on systolic BP was “modest,” the standard deviations in some cases were large, indicating that some people had large reductions of systolic BP of more than 30-40 mm Hg.
“There is a belief out there that there are some people that really benefit from this, but how to identify them has been the question,” Dr. Textor said.
Enthusiasm for renal denervation plummeted after results from the SYMPLICITY HTN-3 showed the procedure failing to meet its efficacy endpoint in resistant hypertension. The procedure was associated with a 14–mm Hg fall in systolic BP, compared with an 11–mm Hg drop in the “sham” control group (N Engl J Med. 2014 Apr 10;370:1393-401). However, post hoc analysis of the trial revealed significant shortcomings in design and execution.
No renal denervation device is approved in the United States. The Symplicity device used in this registry is approved in the European Union.
In early 2020, the Food and Drug Administration promised a rigorous review of new renal denervation trials. Subsequently, primary results from the SPYRAL HTN-OFF MED pivotal trial were presented at the annual meeting of the American College of Cardiology in March and showed promising efficacy.
SPYRAL HTN OFF-MED was designed in collaboration with the FDA to obtain meaningful evidence of whether renal denervation performed with the Symplicity Spyral multielectrode catheter (Medtronic Vascular) could reduce BP in patients not taking antihypertensive medication.
Dr. Mahfoud reported he has received speaking honoraria from Medtronic and ReCor. Two other authors are employees of Medtronic. Dr. Textor reported no relationships relevant to the contents of this paper. The Global SYMPLICITY Registry is funded by Medtronic Vascular.
SOURCE: Mahfoud F et al. J Am Coll Cardiol. 2020 June 16;75:2879-88.
This article was updated 6/16/20.
In a new analysis of international registry data, renal denervation resulted in similar reduced blood pressure levels in patients with varying high-risk comorbidities and across a range of cardiovascular risk scores.
At 3 years, 24-hour systolic BP was reduced by an average of –8.9 mm Hg overall, with slightly higher or lower readings seen in those with higher cardiovascular risk scores (–10.4 mm Hg) and 65 years or older (–10.2 mm Hg). Similar reductions were seen in those with resistant hypertension (–8.7 mm Hg), diabetes (–8.6 mm Hg), isolated systolic hypertension (–10.1 mm Hg), chronic kidney disease (–10.1 mm Hg), or atrial fibrillation (–10.0 mm Hg).
“In the largest international registry of its kind, the efficacy of renal denervation was similar in patients with and without baseline conditions associated with increased sympathetic activity and irrespective of ASCVD [atherosclerotic cardiovascular disease] risk,” first author Felix Mahfoud, MD, said in an interview.
Dr. Mahfoud, from University Hospital of Saarland, Homburg, Germany, and colleagues published their analysis in the Journal of the American College of Cardiology.
The article reported a post hoc analysis of data from the Global SYMPLICITY Registry (GSR), an international, Medtronic-funded effort that includes 2,652 patients with uncontrolled hypertension treated with a Symplicity denervation system. Data were obtained from 196 centers in 45 countries.
“Blood pressure reductions were durable and sustained to 3 years and the rates of new-onset, end-stage renal disease and elevation in serum creatinine levels were very low in patients at high and low [cardiovascular] risk,” reported Dr. Mahfoud.
As expected, adverse event rates were higher for patients with higher baseline cardiovascular risk. “Elevated rates were also seen in patients with [atrial fibrillation] and diabetes, identifying these subgroups who might derive even greater clinical benefit from improved BP control using renal denervation,” said Dr. Mahfoud.
Asked which patients might be optimal candidates for renal denervation, Dr. Mahfoud recommended the technology for “patients with uncontrolled hypertension on medication, patients with nonadherence, unwillingness, or intolerability to medication, and patients with combined systolic and diastolic hypertension.”
Analyses limited by incomplete data
Stephen C. Textor, MD, has concerns over the amount of missing data in the GSR database and its continued use as a repository of information on renal denervation.
“I am a bit lukewarm on this paper in part because of the nature of the registry data they’re using,” he added in an interview. “The problem I see is that the registry is not terribly uniform as to what information they collect on each patient, not terribly uniform in terms of how the procedure is performed, and not terribly uniform on how they follow up patients.”
Indeed, the post hoc subgroup analyses represent only a limited subset because of incomplete data, added Dr. Textor, a nephrologist at the Cleveland Clinic in Rochester, Minn.
“Remarkably, only 504 [patients] had “matched” data for office [systolic BP] levels at the time points defined in the report,” he wrote in an editorial comment accompanying the registry report (J Am Coll Cardiol. 2020 Jun 16;75[23]:2889-91).
Similarly, the researchers were able to calculate baseline atherosclerotic cardiovascular risk scores in only 1,485 patients (56% of total), primarily because of missing cholesterol measurements.
“They simply did these paired comparison that may have included a couple hundred cases, and on average, there were no differences in response, but what I would have liked to see is a multivariate analysis, where you have all the data on everybody and look at what are the factors that impact response?” Dr. Textor said in the interview.
“They really couldn’t do that because they just, they’re just too many holes in the data,” he added.
On the bright side, Dr. Textor noted that, while the impact overall on systolic BP was “modest,” the standard deviations in some cases were large, indicating that some people had large reductions of systolic BP of more than 30-40 mm Hg.
“There is a belief out there that there are some people that really benefit from this, but how to identify them has been the question,” Dr. Textor said.
Enthusiasm for renal denervation plummeted after results from the SYMPLICITY HTN-3 showed the procedure failing to meet its efficacy endpoint in resistant hypertension. The procedure was associated with a 14–mm Hg fall in systolic BP, compared with an 11–mm Hg drop in the “sham” control group (N Engl J Med. 2014 Apr 10;370:1393-401). However, post hoc analysis of the trial revealed significant shortcomings in design and execution.
No renal denervation device is approved in the United States. The Symplicity device used in this registry is approved in the European Union.
In early 2020, the Food and Drug Administration promised a rigorous review of new renal denervation trials. Subsequently, primary results from the SPYRAL HTN-OFF MED pivotal trial were presented at the annual meeting of the American College of Cardiology in March and showed promising efficacy.
SPYRAL HTN OFF-MED was designed in collaboration with the FDA to obtain meaningful evidence of whether renal denervation performed with the Symplicity Spyral multielectrode catheter (Medtronic Vascular) could reduce BP in patients not taking antihypertensive medication.
Dr. Mahfoud reported he has received speaking honoraria from Medtronic and ReCor. Two other authors are employees of Medtronic. Dr. Textor reported no relationships relevant to the contents of this paper. The Global SYMPLICITY Registry is funded by Medtronic Vascular.
SOURCE: Mahfoud F et al. J Am Coll Cardiol. 2020 June 16;75:2879-88.
This article was updated 6/16/20.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Anti–PD1 Immune Checkpoint Inhibitor–Induced Bullous Pemphigoid in Metastatic Melanoma and Non–Small Cell Lung Cancer
Immune checkpoint inhibitors are used for a variety of advanced malignancies, including melanoma, non–small cell lung cancer, urothelial cancer, and renal cell carcinoma. Anti–programmed cell death 1 (PD1) targeted therapies, such as pembrolizumab and nivolumab, are improving patient survival. This class of immunotherapy is revolutionary but is associated with autoimmune adverse effects. A rare but increasingly reported adverse effect of anti-PD1 therapy is bullous pemphigoid (BP), an autoimmune blistering disease directed against
High clinical suspicion, early diagnosis, and proper management of immunotherapy-related BP are imperative for keeping patients on life-prolonging treatment. We present 3 cases of BP secondary to anti-PD1 immunotherapy in patients with melanoma or non–small cell lung cancer to highlight the diagnosis and treatment of BP as well as emphasize the importance of the dermatologist in the care of patients with immunotherapy-related skin disease.
Case Reports
Patient 1
A 72-year-old woman with metastatic BRAF-mutated melanoma from an unknown primary site presented with intensely pruritic papules on the back, chest, and extremities of 4 months’ duration. She described her symptoms as insidious in onset and refractory to clobetasol ointment, oral diphenhydramine, and over-the-counter anti-itch creams. The patient had been treated with oral dabrafenib 150 mg twice daily and trametinib 2 mg/d but was switched to pembrolizumab when the disease progressed. After 8 months, she had a complete radiologic response to pembrolizumab 2 mg/kg every 3 weeks, which was discontinued in favor of observation 3 months prior to presentation to dermatology.
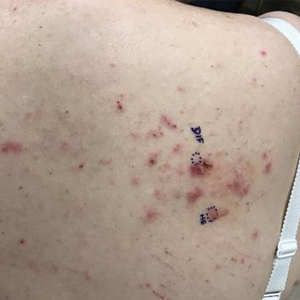
At the current presentation, physical examination revealed innumerable erythematous, excoriated, 2- to 4-mm, red papules diffusely scattered on the upper back, chest, abdomen, and thighs, with one 8×4-mm vesicle on the right side of the upper back (Figure 1). Discrete areas of depigmented macules, consistent with vitiligo, coalesced into patches on the legs, thighs, arms, and back. The patient was started on a 3-week oral prednisone taper for symptom relief. A hematoxylin and eosin (H&E)–stained punch biopsy of the back revealed a subepidermal split with eosinophils and a dense eosinophilic infiltrate in the dermis (Figure 2). Direct immunofluorescence (DIF) studies from a specimen adjacent to the biopsy collected for H&E staining showed linear deposition of IgA, IgG, and C3 along the dermoepidermal junction (Figure 3). Histologic findings were consistent with BP.
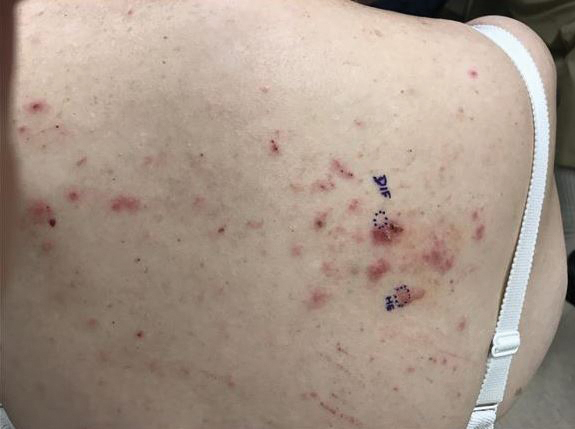
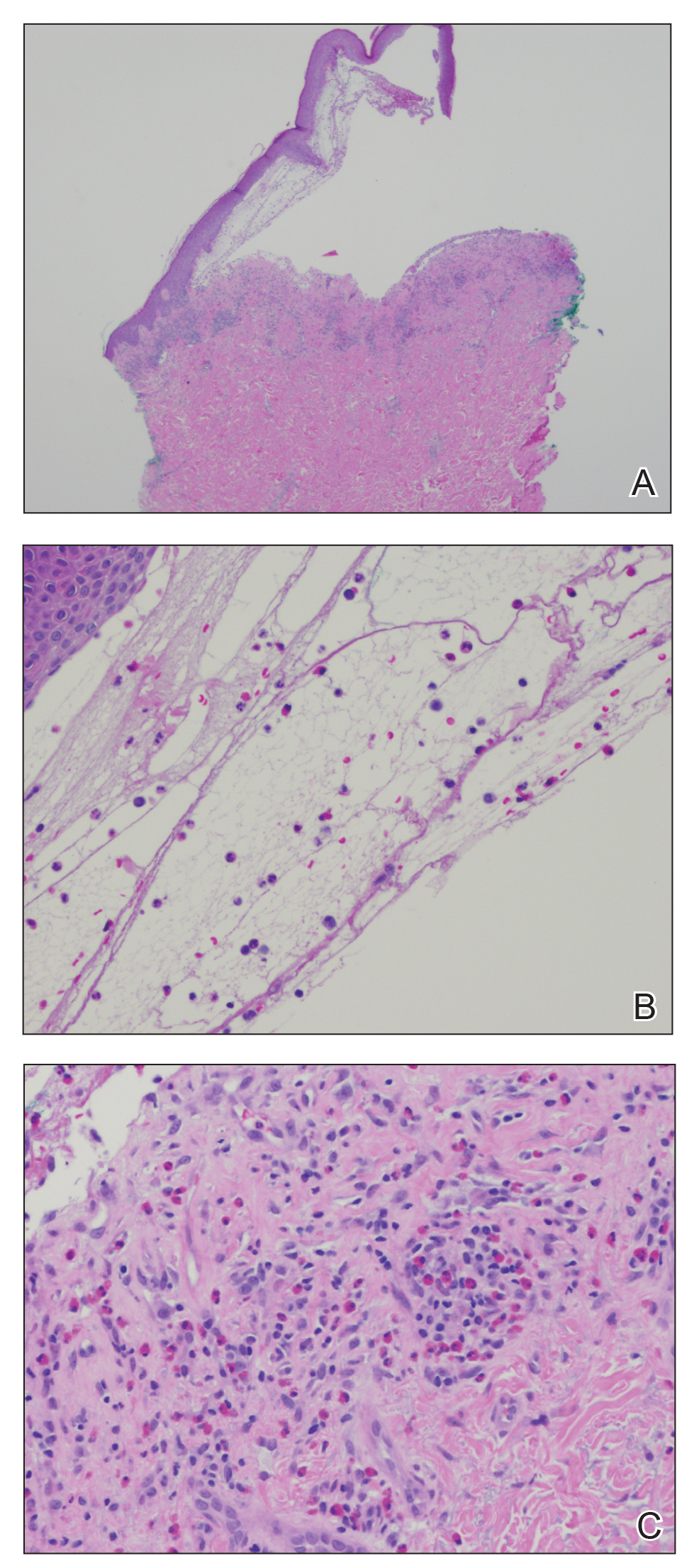
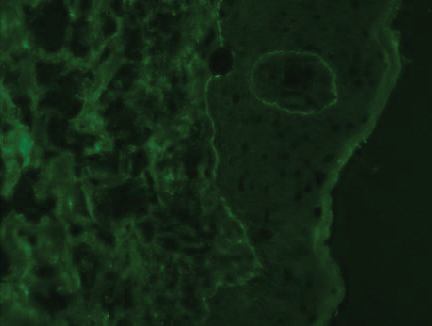
The patient was started on doxycycline 100 mg twice daily and clobetasol ointment 0.05% once daily to supplement the prednisone taper. At 3-week follow-up, she reported pruritus and a few erythematous macules but no new bullae. At 12 weeks, some papules persisted; however, the patient was averse to using systemic agents and decided that symptoms were adequately controlled with clobetasol ointment and oral doxycycline.
Because the patient currently remains in clinical and radiologic remission, anti-PD1 immune checkpoint inhibitors have not been restarted but remain an option for the future if disease recurs
Patient 2
An 82-year-old man with a history of stage IIC desmoplastic melanoma presented to dermatology with an intensely pruritic eruption on the legs, arms, waist, upper torso, and scalp of 3 weeks’ duration. Clobetasol ointment had provided minimal relief.
Six months prior to presenting to dermatology, the patient underwent immunotherapy with 4 cycles of ipilimumab 200 mg intravenous (IV) and nivolumab 240 mg IV every 2 weeks, receiving ipilimumab during the first cycle only because of a lack of availability at the pharmacy. He then received nivolumab 240 mg IV every 2 weeks as maintenance therapy. After the second dose of nivolumab maintenance therapy, however, he developed generalized bullae and pruritus. Dermatology was consulted during an oncology appointment, and his oncologist decided to hold nivolumab.
Physical examination revealed generalized tense and eroded bullae covering more than 50% of the body surface area and affecting the scalp, arms, legs, torso, and buttocks. Two punch biopsies were obtained. Hematoxylin and eosin staining revealed a subepidermal split with predominantly eosinophils and scattered neutrophils. Direct immunofluorescence studies showed linear deposition of IgG, IgA, and C3 along the dermoepidermal junction, consistent with BP.
The patient’s BP was difficult to control, requiring several hospital admissions for wound care, high-dose systemic steroids, and initiation of mycophenolate mofetil. After 4 months of waxing and waning symptoms, the BP was controlled with mycophenolate mofetil 1500 mg/d; clobetasol ointment 0.05%; and diphenhydramine for pruritus. Due to the prolonged recovery and severity of BP, the patient’s oncologist deemed that he was not a candidate for future immunotherapy.
Patient 3
A 68-year-old man with PD1-negative, metastatic, well-differentiated squamous cell carcinoma of the lung presented to dermatology with a pruritic rash of 3 weeks’ duration. He had been receiving nivolumab for 2 years after disease progressed on prior chemotherapies and experienced several grade 1 or grade 2 nivolumab-induced autoimmune reactions including thyroiditis, dermatitis, and nephritis, for which he was taking prednisone 5 mg/d for suppression.
Physical examination revealed psoriasiform pink plaques on the arms, chest, and legs. The differential diagnosis at the time favored psoriasiform dermatitis over lichenoid dermatitis. A punch biopsy revealed psoriasiform dermatitis. The patient was prescribed fluocinonide ointment 0.05% daily. His plaques improved with topical steroids.
The patient returned approximately 1 month later with a report of a new blistering rash on the legs. Physical examination revealed interval improvement of the psoriasiform plaques on the scalp, torso, and extremities, but tense bullae were seen on the thighs, with surrounding superficial erosions at sites of recent bullae. Punch biopsies of the skin for H&E staining and DIF showed BP.
Prednisone was increased to 50 mg/d for a 3-week taper. Doxycycline 100 mg twice daily was started. The patient’s skin disease continued to be difficult to control with therapy; nivolumab was held by his oncologist.
Comment
Immunotherapy with immune checkpoint blockade represents a successful application of immune recognition to treat metastatic cancers, including melanoma, non–small cell lung cancer, urothelial cancer, and renal cell carcinoma.
Anti-PD1 targeted therapies improve survival in solid and hematologic malignancies, with a response rate as high as 40% in melanoma.2 Although these medications can prolong survival, many are associated with loss of self-tolerance and severe autoimmunelike events that can limit therapy.3 An exception is PD1-induced vitiligo, which patient 1 developed and has been associated with a better response to therapy.4
Anti-PD1–induced BP is a newly reported adverse effect. In its early stages, BP can be difficult to differentiate from eczematous or urticarial dermatitis.5-8 Discontinuation of immunotherapy has been reported in more than 70% of patients who develop BP.1 There are reports of successful treatment of BP with a course of a PD1 inhibitor,9 but 2 of our patients had severe BP that led to discontinuation of immunotherapy.
Consider Prescreening
Given that development of BP often leads to cessation of therapy, identifying patients at risk prior to starting an immune checkpoint inhibitor might have clinical utility. Biopsy with DIF is the gold standard for diagnosis, but serologic testing can be a useful adjunct because enzyme-linked immunosorbent assay for BP antigen 1 and BP antigen 2 has a reported sensitivity and specificity of 87% and 98%, respectively.10 Serologic testing prior to starting therapy with an immune checkpoint inhibitor can provide a baseline for patients. A rise in titer, in conjunction with onset of a rash, might aid in earlier diagnosis, particularly because urticarial BP can be difficult to diagnose clinically.
Further study on the utility vs cost-benefit of these screening modalities is warranted. Their predictive utility might be limited, however, and positive serologic test results might have unanticipated consequences, such as hesitation in treating patients, thus leading to a delay in therapy or access to these medications.
Conclusion
The expanding use of immune checkpoint inhibitors is increasing survival in patients with metastatic melanoma and other malignancies. Adverse effects are part of the continuum of immune system stimulation, with overstimulation resulting in dermatitis; thyroiditis; pneumonitis; and less commonly hypophysitis, vitiligo, and colitis.
Rarely, immune checkpoint inhibition induces BP. Development of BP leads to discontinuation of therapy in more than half of reported cases due to lack of adequate treatment for this skin disease and its impact on quality of life. Therefore, quick diagnosis of BP in patients on immunotherapy and successful management techniques can prevent discontinuation of these lifesaving cancer therapies. For that reason, dermatologists play an important role in the management of patients on immune checkpoint inhibitors for cancer.
- Lopez AT, Khanna T, Antonov N, et al. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol. 2018;57:664-669.
- Márquez-Rodas, I, Cerezuela P, Soria A, et al. Immune checkpoint inhibitors: therapeutic advances in melanoma. Ann Transl Med. 2015;3:267.
- Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors a review. JAMA Oncol. 2016;2:1346-1353.
- Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45-51.
- Hwang SJE, Carlos G, Chou S, et al. Bullous pemphigoid, an autoantibody-mediated disease, is a novel immune-related adverse event in patients treated with anti-programmed cell death 1 antibodies. Melanoma Res. 2016;26:413-416.
- Damsky W, Kole L, Tomayko MM. Development of bullous pemphigoid during nivolumab therapy. JAAD Case Rep. 2016;2:442-444.
- Garje R, Chau JJ, Chung J, et al. Acute flare of bullous pemphigus with pembrolizumab used for treatment of metastatic urothelial cancer. J Immunother. 2018;41:42-44.
- Ito M, Hoashi T, Endo Y, et al. Atypical pemphigus developed in a patient with urothelial carcinoma treated with nivolumab. J Dermatol. 2019;46:e90-e92.
- Chen W-S, Tetzlaff MT, Diwan H, et al. Suprabasal acantholytic dermatologic toxicities associated checkpoint inhibitor therapy: a spectrum of immune reactions from paraneoplastic pemphigus-like to Grover-like lesions. J Cutan Pathol. 2018;45:764-773.
- Muglia C, Bronsnick T, Kirkorian AY, et al. Questioning the specificity and sensitivity of ELISA for bullous pemphigoid diagnosis. Cutis. 2017;99:E27-E30.
Immune checkpoint inhibitors are used for a variety of advanced malignancies, including melanoma, non–small cell lung cancer, urothelial cancer, and renal cell carcinoma. Anti–programmed cell death 1 (PD1) targeted therapies, such as pembrolizumab and nivolumab, are improving patient survival. This class of immunotherapy is revolutionary but is associated with autoimmune adverse effects. A rare but increasingly reported adverse effect of anti-PD1 therapy is bullous pemphigoid (BP), an autoimmune blistering disease directed against
High clinical suspicion, early diagnosis, and proper management of immunotherapy-related BP are imperative for keeping patients on life-prolonging treatment. We present 3 cases of BP secondary to anti-PD1 immunotherapy in patients with melanoma or non–small cell lung cancer to highlight the diagnosis and treatment of BP as well as emphasize the importance of the dermatologist in the care of patients with immunotherapy-related skin disease.
Case Reports
Patient 1
A 72-year-old woman with metastatic BRAF-mutated melanoma from an unknown primary site presented with intensely pruritic papules on the back, chest, and extremities of 4 months’ duration. She described her symptoms as insidious in onset and refractory to clobetasol ointment, oral diphenhydramine, and over-the-counter anti-itch creams. The patient had been treated with oral dabrafenib 150 mg twice daily and trametinib 2 mg/d but was switched to pembrolizumab when the disease progressed. After 8 months, she had a complete radiologic response to pembrolizumab 2 mg/kg every 3 weeks, which was discontinued in favor of observation 3 months prior to presentation to dermatology.
At the current presentation, physical examination revealed innumerable erythematous, excoriated, 2- to 4-mm, red papules diffusely scattered on the upper back, chest, abdomen, and thighs, with one 8×4-mm vesicle on the right side of the upper back (Figure 1). Discrete areas of depigmented macules, consistent with vitiligo, coalesced into patches on the legs, thighs, arms, and back. The patient was started on a 3-week oral prednisone taper for symptom relief. A hematoxylin and eosin (H&E)–stained punch biopsy of the back revealed a subepidermal split with eosinophils and a dense eosinophilic infiltrate in the dermis (Figure 2). Direct immunofluorescence (DIF) studies from a specimen adjacent to the biopsy collected for H&E staining showed linear deposition of IgA, IgG, and C3 along the dermoepidermal junction (Figure 3). Histologic findings were consistent with BP.



The patient was started on doxycycline 100 mg twice daily and clobetasol ointment 0.05% once daily to supplement the prednisone taper. At 3-week follow-up, she reported pruritus and a few erythematous macules but no new bullae. At 12 weeks, some papules persisted; however, the patient was averse to using systemic agents and decided that symptoms were adequately controlled with clobetasol ointment and oral doxycycline.
Because the patient currently remains in clinical and radiologic remission, anti-PD1 immune checkpoint inhibitors have not been restarted but remain an option for the future if disease recurs
Patient 2
An 82-year-old man with a history of stage IIC desmoplastic melanoma presented to dermatology with an intensely pruritic eruption on the legs, arms, waist, upper torso, and scalp of 3 weeks’ duration. Clobetasol ointment had provided minimal relief.
Six months prior to presenting to dermatology, the patient underwent immunotherapy with 4 cycles of ipilimumab 200 mg intravenous (IV) and nivolumab 240 mg IV every 2 weeks, receiving ipilimumab during the first cycle only because of a lack of availability at the pharmacy. He then received nivolumab 240 mg IV every 2 weeks as maintenance therapy. After the second dose of nivolumab maintenance therapy, however, he developed generalized bullae and pruritus. Dermatology was consulted during an oncology appointment, and his oncologist decided to hold nivolumab.
Physical examination revealed generalized tense and eroded bullae covering more than 50% of the body surface area and affecting the scalp, arms, legs, torso, and buttocks. Two punch biopsies were obtained. Hematoxylin and eosin staining revealed a subepidermal split with predominantly eosinophils and scattered neutrophils. Direct immunofluorescence studies showed linear deposition of IgG, IgA, and C3 along the dermoepidermal junction, consistent with BP.
The patient’s BP was difficult to control, requiring several hospital admissions for wound care, high-dose systemic steroids, and initiation of mycophenolate mofetil. After 4 months of waxing and waning symptoms, the BP was controlled with mycophenolate mofetil 1500 mg/d; clobetasol ointment 0.05%; and diphenhydramine for pruritus. Due to the prolonged recovery and severity of BP, the patient’s oncologist deemed that he was not a candidate for future immunotherapy.
Patient 3
A 68-year-old man with PD1-negative, metastatic, well-differentiated squamous cell carcinoma of the lung presented to dermatology with a pruritic rash of 3 weeks’ duration. He had been receiving nivolumab for 2 years after disease progressed on prior chemotherapies and experienced several grade 1 or grade 2 nivolumab-induced autoimmune reactions including thyroiditis, dermatitis, and nephritis, for which he was taking prednisone 5 mg/d for suppression.
Physical examination revealed psoriasiform pink plaques on the arms, chest, and legs. The differential diagnosis at the time favored psoriasiform dermatitis over lichenoid dermatitis. A punch biopsy revealed psoriasiform dermatitis. The patient was prescribed fluocinonide ointment 0.05% daily. His plaques improved with topical steroids.
The patient returned approximately 1 month later with a report of a new blistering rash on the legs. Physical examination revealed interval improvement of the psoriasiform plaques on the scalp, torso, and extremities, but tense bullae were seen on the thighs, with surrounding superficial erosions at sites of recent bullae. Punch biopsies of the skin for H&E staining and DIF showed BP.
Prednisone was increased to 50 mg/d for a 3-week taper. Doxycycline 100 mg twice daily was started. The patient’s skin disease continued to be difficult to control with therapy; nivolumab was held by his oncologist.
Comment
Immunotherapy with immune checkpoint blockade represents a successful application of immune recognition to treat metastatic cancers, including melanoma, non–small cell lung cancer, urothelial cancer, and renal cell carcinoma.
Anti-PD1 targeted therapies improve survival in solid and hematologic malignancies, with a response rate as high as 40% in melanoma.2 Although these medications can prolong survival, many are associated with loss of self-tolerance and severe autoimmunelike events that can limit therapy.3 An exception is PD1-induced vitiligo, which patient 1 developed and has been associated with a better response to therapy.4
Anti-PD1–induced BP is a newly reported adverse effect. In its early stages, BP can be difficult to differentiate from eczematous or urticarial dermatitis.5-8 Discontinuation of immunotherapy has been reported in more than 70% of patients who develop BP.1 There are reports of successful treatment of BP with a course of a PD1 inhibitor,9 but 2 of our patients had severe BP that led to discontinuation of immunotherapy.
Consider Prescreening
Given that development of BP often leads to cessation of therapy, identifying patients at risk prior to starting an immune checkpoint inhibitor might have clinical utility. Biopsy with DIF is the gold standard for diagnosis, but serologic testing can be a useful adjunct because enzyme-linked immunosorbent assay for BP antigen 1 and BP antigen 2 has a reported sensitivity and specificity of 87% and 98%, respectively.10 Serologic testing prior to starting therapy with an immune checkpoint inhibitor can provide a baseline for patients. A rise in titer, in conjunction with onset of a rash, might aid in earlier diagnosis, particularly because urticarial BP can be difficult to diagnose clinically.
Further study on the utility vs cost-benefit of these screening modalities is warranted. Their predictive utility might be limited, however, and positive serologic test results might have unanticipated consequences, such as hesitation in treating patients, thus leading to a delay in therapy or access to these medications.
Conclusion
The expanding use of immune checkpoint inhibitors is increasing survival in patients with metastatic melanoma and other malignancies. Adverse effects are part of the continuum of immune system stimulation, with overstimulation resulting in dermatitis; thyroiditis; pneumonitis; and less commonly hypophysitis, vitiligo, and colitis.
Rarely, immune checkpoint inhibition induces BP. Development of BP leads to discontinuation of therapy in more than half of reported cases due to lack of adequate treatment for this skin disease and its impact on quality of life. Therefore, quick diagnosis of BP in patients on immunotherapy and successful management techniques can prevent discontinuation of these lifesaving cancer therapies. For that reason, dermatologists play an important role in the management of patients on immune checkpoint inhibitors for cancer.
Immune checkpoint inhibitors are used for a variety of advanced malignancies, including melanoma, non–small cell lung cancer, urothelial cancer, and renal cell carcinoma. Anti–programmed cell death 1 (PD1) targeted therapies, such as pembrolizumab and nivolumab, are improving patient survival. This class of immunotherapy is revolutionary but is associated with autoimmune adverse effects. A rare but increasingly reported adverse effect of anti-PD1 therapy is bullous pemphigoid (BP), an autoimmune blistering disease directed against
High clinical suspicion, early diagnosis, and proper management of immunotherapy-related BP are imperative for keeping patients on life-prolonging treatment. We present 3 cases of BP secondary to anti-PD1 immunotherapy in patients with melanoma or non–small cell lung cancer to highlight the diagnosis and treatment of BP as well as emphasize the importance of the dermatologist in the care of patients with immunotherapy-related skin disease.
Case Reports
Patient 1
A 72-year-old woman with metastatic BRAF-mutated melanoma from an unknown primary site presented with intensely pruritic papules on the back, chest, and extremities of 4 months’ duration. She described her symptoms as insidious in onset and refractory to clobetasol ointment, oral diphenhydramine, and over-the-counter anti-itch creams. The patient had been treated with oral dabrafenib 150 mg twice daily and trametinib 2 mg/d but was switched to pembrolizumab when the disease progressed. After 8 months, she had a complete radiologic response to pembrolizumab 2 mg/kg every 3 weeks, which was discontinued in favor of observation 3 months prior to presentation to dermatology.
At the current presentation, physical examination revealed innumerable erythematous, excoriated, 2- to 4-mm, red papules diffusely scattered on the upper back, chest, abdomen, and thighs, with one 8×4-mm vesicle on the right side of the upper back (Figure 1). Discrete areas of depigmented macules, consistent with vitiligo, coalesced into patches on the legs, thighs, arms, and back. The patient was started on a 3-week oral prednisone taper for symptom relief. A hematoxylin and eosin (H&E)–stained punch biopsy of the back revealed a subepidermal split with eosinophils and a dense eosinophilic infiltrate in the dermis (Figure 2). Direct immunofluorescence (DIF) studies from a specimen adjacent to the biopsy collected for H&E staining showed linear deposition of IgA, IgG, and C3 along the dermoepidermal junction (Figure 3). Histologic findings were consistent with BP.



The patient was started on doxycycline 100 mg twice daily and clobetasol ointment 0.05% once daily to supplement the prednisone taper. At 3-week follow-up, she reported pruritus and a few erythematous macules but no new bullae. At 12 weeks, some papules persisted; however, the patient was averse to using systemic agents and decided that symptoms were adequately controlled with clobetasol ointment and oral doxycycline.
Because the patient currently remains in clinical and radiologic remission, anti-PD1 immune checkpoint inhibitors have not been restarted but remain an option for the future if disease recurs
Patient 2
An 82-year-old man with a history of stage IIC desmoplastic melanoma presented to dermatology with an intensely pruritic eruption on the legs, arms, waist, upper torso, and scalp of 3 weeks’ duration. Clobetasol ointment had provided minimal relief.
Six months prior to presenting to dermatology, the patient underwent immunotherapy with 4 cycles of ipilimumab 200 mg intravenous (IV) and nivolumab 240 mg IV every 2 weeks, receiving ipilimumab during the first cycle only because of a lack of availability at the pharmacy. He then received nivolumab 240 mg IV every 2 weeks as maintenance therapy. After the second dose of nivolumab maintenance therapy, however, he developed generalized bullae and pruritus. Dermatology was consulted during an oncology appointment, and his oncologist decided to hold nivolumab.
Physical examination revealed generalized tense and eroded bullae covering more than 50% of the body surface area and affecting the scalp, arms, legs, torso, and buttocks. Two punch biopsies were obtained. Hematoxylin and eosin staining revealed a subepidermal split with predominantly eosinophils and scattered neutrophils. Direct immunofluorescence studies showed linear deposition of IgG, IgA, and C3 along the dermoepidermal junction, consistent with BP.
The patient’s BP was difficult to control, requiring several hospital admissions for wound care, high-dose systemic steroids, and initiation of mycophenolate mofetil. After 4 months of waxing and waning symptoms, the BP was controlled with mycophenolate mofetil 1500 mg/d; clobetasol ointment 0.05%; and diphenhydramine for pruritus. Due to the prolonged recovery and severity of BP, the patient’s oncologist deemed that he was not a candidate for future immunotherapy.
Patient 3
A 68-year-old man with PD1-negative, metastatic, well-differentiated squamous cell carcinoma of the lung presented to dermatology with a pruritic rash of 3 weeks’ duration. He had been receiving nivolumab for 2 years after disease progressed on prior chemotherapies and experienced several grade 1 or grade 2 nivolumab-induced autoimmune reactions including thyroiditis, dermatitis, and nephritis, for which he was taking prednisone 5 mg/d for suppression.
Physical examination revealed psoriasiform pink plaques on the arms, chest, and legs. The differential diagnosis at the time favored psoriasiform dermatitis over lichenoid dermatitis. A punch biopsy revealed psoriasiform dermatitis. The patient was prescribed fluocinonide ointment 0.05% daily. His plaques improved with topical steroids.
The patient returned approximately 1 month later with a report of a new blistering rash on the legs. Physical examination revealed interval improvement of the psoriasiform plaques on the scalp, torso, and extremities, but tense bullae were seen on the thighs, with surrounding superficial erosions at sites of recent bullae. Punch biopsies of the skin for H&E staining and DIF showed BP.
Prednisone was increased to 50 mg/d for a 3-week taper. Doxycycline 100 mg twice daily was started. The patient’s skin disease continued to be difficult to control with therapy; nivolumab was held by his oncologist.
Comment
Immunotherapy with immune checkpoint blockade represents a successful application of immune recognition to treat metastatic cancers, including melanoma, non–small cell lung cancer, urothelial cancer, and renal cell carcinoma.
Anti-PD1 targeted therapies improve survival in solid and hematologic malignancies, with a response rate as high as 40% in melanoma.2 Although these medications can prolong survival, many are associated with loss of self-tolerance and severe autoimmunelike events that can limit therapy.3 An exception is PD1-induced vitiligo, which patient 1 developed and has been associated with a better response to therapy.4
Anti-PD1–induced BP is a newly reported adverse effect. In its early stages, BP can be difficult to differentiate from eczematous or urticarial dermatitis.5-8 Discontinuation of immunotherapy has been reported in more than 70% of patients who develop BP.1 There are reports of successful treatment of BP with a course of a PD1 inhibitor,9 but 2 of our patients had severe BP that led to discontinuation of immunotherapy.
Consider Prescreening
Given that development of BP often leads to cessation of therapy, identifying patients at risk prior to starting an immune checkpoint inhibitor might have clinical utility. Biopsy with DIF is the gold standard for diagnosis, but serologic testing can be a useful adjunct because enzyme-linked immunosorbent assay for BP antigen 1 and BP antigen 2 has a reported sensitivity and specificity of 87% and 98%, respectively.10 Serologic testing prior to starting therapy with an immune checkpoint inhibitor can provide a baseline for patients. A rise in titer, in conjunction with onset of a rash, might aid in earlier diagnosis, particularly because urticarial BP can be difficult to diagnose clinically.
Further study on the utility vs cost-benefit of these screening modalities is warranted. Their predictive utility might be limited, however, and positive serologic test results might have unanticipated consequences, such as hesitation in treating patients, thus leading to a delay in therapy or access to these medications.
Conclusion
The expanding use of immune checkpoint inhibitors is increasing survival in patients with metastatic melanoma and other malignancies. Adverse effects are part of the continuum of immune system stimulation, with overstimulation resulting in dermatitis; thyroiditis; pneumonitis; and less commonly hypophysitis, vitiligo, and colitis.
Rarely, immune checkpoint inhibition induces BP. Development of BP leads to discontinuation of therapy in more than half of reported cases due to lack of adequate treatment for this skin disease and its impact on quality of life. Therefore, quick diagnosis of BP in patients on immunotherapy and successful management techniques can prevent discontinuation of these lifesaving cancer therapies. For that reason, dermatologists play an important role in the management of patients on immune checkpoint inhibitors for cancer.
- Lopez AT, Khanna T, Antonov N, et al. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol. 2018;57:664-669.
- Márquez-Rodas, I, Cerezuela P, Soria A, et al. Immune checkpoint inhibitors: therapeutic advances in melanoma. Ann Transl Med. 2015;3:267.
- Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors a review. JAMA Oncol. 2016;2:1346-1353.
- Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45-51.
- Hwang SJE, Carlos G, Chou S, et al. Bullous pemphigoid, an autoantibody-mediated disease, is a novel immune-related adverse event in patients treated with anti-programmed cell death 1 antibodies. Melanoma Res. 2016;26:413-416.
- Damsky W, Kole L, Tomayko MM. Development of bullous pemphigoid during nivolumab therapy. JAAD Case Rep. 2016;2:442-444.
- Garje R, Chau JJ, Chung J, et al. Acute flare of bullous pemphigus with pembrolizumab used for treatment of metastatic urothelial cancer. J Immunother. 2018;41:42-44.
- Ito M, Hoashi T, Endo Y, et al. Atypical pemphigus developed in a patient with urothelial carcinoma treated with nivolumab. J Dermatol. 2019;46:e90-e92.
- Chen W-S, Tetzlaff MT, Diwan H, et al. Suprabasal acantholytic dermatologic toxicities associated checkpoint inhibitor therapy: a spectrum of immune reactions from paraneoplastic pemphigus-like to Grover-like lesions. J Cutan Pathol. 2018;45:764-773.
- Muglia C, Bronsnick T, Kirkorian AY, et al. Questioning the specificity and sensitivity of ELISA for bullous pemphigoid diagnosis. Cutis. 2017;99:E27-E30.
- Lopez AT, Khanna T, Antonov N, et al. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol. 2018;57:664-669.
- Márquez-Rodas, I, Cerezuela P, Soria A, et al. Immune checkpoint inhibitors: therapeutic advances in melanoma. Ann Transl Med. 2015;3:267.
- Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors a review. JAMA Oncol. 2016;2:1346-1353.
- Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45-51.
- Hwang SJE, Carlos G, Chou S, et al. Bullous pemphigoid, an autoantibody-mediated disease, is a novel immune-related adverse event in patients treated with anti-programmed cell death 1 antibodies. Melanoma Res. 2016;26:413-416.
- Damsky W, Kole L, Tomayko MM. Development of bullous pemphigoid during nivolumab therapy. JAAD Case Rep. 2016;2:442-444.
- Garje R, Chau JJ, Chung J, et al. Acute flare of bullous pemphigus with pembrolizumab used for treatment of metastatic urothelial cancer. J Immunother. 2018;41:42-44.
- Ito M, Hoashi T, Endo Y, et al. Atypical pemphigus developed in a patient with urothelial carcinoma treated with nivolumab. J Dermatol. 2019;46:e90-e92.
- Chen W-S, Tetzlaff MT, Diwan H, et al. Suprabasal acantholytic dermatologic toxicities associated checkpoint inhibitor therapy: a spectrum of immune reactions from paraneoplastic pemphigus-like to Grover-like lesions. J Cutan Pathol. 2018;45:764-773.
- Muglia C, Bronsnick T, Kirkorian AY, et al. Questioning the specificity and sensitivity of ELISA for bullous pemphigoid diagnosis. Cutis. 2017;99:E27-E30.
Practice Points
- Anti–programmed cell death 1 (PD1) targeted therapies improve survival in solid and hematologic malignancies but are associated with autoimmune side effects, with bullous pemphigoid (BP) being the newest reported.
- Bullous pemphigoid can develop months into immunotherapy treatment.
- Bullous pemphigoid should be on the differential diagnosis in a patient who is on an anti-PD1 immune checkpoint inhibitor and develops 1 or more of the following: pruritus, dermatitis, and vesicles.
- Early diagnosis of BP is essential for keeping patients on immunotherapy because its severity often results in temporary or permanent discontinuation of treatment.
Seropositivity in RA linked with doubled pneumonia incidence
from a single U.S. medical system.
“Patients with seropositive RA, particularly RF [rheumatoid factor]-positive RA, had increased risk for pneumonia throughout the RA disease course that was not explained by measured confounders, including smoking status, multimorbidity, medications, and [erythrocyte sedimentation rate] level,” Jeffrey A. Sparks, MD, said at the annual European Congress of Rheumatology, held online this year due to COVID-19.
“There has been much interest about the relationship between lung inflammation and the generation of RF and CCP [cyclic citrullinated protein] prior to the onset of RA. We hypothesized that patients with seropositive RA might have subclinical lung injury that could predispose them to pneumonia after clinical RA onset,” Dr. Sparks said in an interview. “Pneumonia is one of the most common serious infections in both patients with RA and the general population, and it causes serious morbidity and mortality.”
The doubled relative risk for pneumonia seen in the findings “translates into a clinically meaningful finding when considering the high rate and the many patients at risk since RA is relatively common,” said Dr. Sparks, a rheumatologist at Brigham and Women’s Hospital in Boston.
“Patients with RF-positive RA who present with symptoms concerning for pneumonia should be evaluated carefully for this and for other possible pulmonary manifestations of RA. Vaccination for pneumonia should be strongly considered for patients with RA who are on disease-modifying antirheumatic drugs, and we hope that our report encourages clinicians and patients” to undertake vaccination, he said.
His study used a database of more than 60,000 patients diagnosed with RA as of November 2013 in the records of a large Boston-area medical system that includes physicians affiliated with Brigham and Women’s Hospital and Massachusetts General Hospital. The researchers applied a validated algorithm for calculating a patient’s probability of having RA, and at the level of 97% probability they narrowed the cohort down to just under 10,000 patients. Additional winnowing because of missing data or a history of pneumonia yielded a study group of 4,110, which included 3,279 (80%) who were seropositive for either or both CCP and RF, and 831 (20%) who were seronegative. During a median follow-up of 7.8 years and total follow-up of more than 32,000 patient-years, the overall pneumonia incidence was 5.8%, with a 2.8% rate among the seronegatives and a 6.6% rate among seropositives. After adjustment for age, sex, glucocorticoid use, disease-modifying antirheumatic drug use, and several other possible confounders, the researchers calculated a 99% relative increased rate of pneumonia among all seropositive patients, compared with the seronegatives.
Further analysis looked at pneumonia incidence rates among patients positive only for CCP antibody, positive only for RF antibody, or both, compared with seronegative patients. This showed that CCP seropositivity had no statistically significant link with incident pneumonia, while RF seropositivity linked with a statistically significant, roughly twofold higher rate. Only 6% of all seropositive patients were positive only for CCP antibody, 59% were positive specifically for RF antibody, and 35% for both.
The data Dr. Sparks presented did not include information on pneumonia type, the timing of the pneumonia, compared with the onset of RA, disease activity, or smoking intensity.
“We anticipated that both RF positive and CCP positive would each be associated with pneumonia, so it was somewhat surprising that we only detected this for RF,” Dr. Sparks said. But he added that, because the number of patients with only CCP positivity was relatively so small, “it is still possible that CCP [antibody] could also increase pneumonia risk.”
The study had no commercial funding. Dr. Sparks had no disclosures.
SOURCE: Sparks JA et al. Ann Rheum Dis. 2020 Jun;79[suppl 1]:73, Abstract OP0111.
from a single U.S. medical system.
“Patients with seropositive RA, particularly RF [rheumatoid factor]-positive RA, had increased risk for pneumonia throughout the RA disease course that was not explained by measured confounders, including smoking status, multimorbidity, medications, and [erythrocyte sedimentation rate] level,” Jeffrey A. Sparks, MD, said at the annual European Congress of Rheumatology, held online this year due to COVID-19.
“There has been much interest about the relationship between lung inflammation and the generation of RF and CCP [cyclic citrullinated protein] prior to the onset of RA. We hypothesized that patients with seropositive RA might have subclinical lung injury that could predispose them to pneumonia after clinical RA onset,” Dr. Sparks said in an interview. “Pneumonia is one of the most common serious infections in both patients with RA and the general population, and it causes serious morbidity and mortality.”
The doubled relative risk for pneumonia seen in the findings “translates into a clinically meaningful finding when considering the high rate and the many patients at risk since RA is relatively common,” said Dr. Sparks, a rheumatologist at Brigham and Women’s Hospital in Boston.
“Patients with RF-positive RA who present with symptoms concerning for pneumonia should be evaluated carefully for this and for other possible pulmonary manifestations of RA. Vaccination for pneumonia should be strongly considered for patients with RA who are on disease-modifying antirheumatic drugs, and we hope that our report encourages clinicians and patients” to undertake vaccination, he said.
His study used a database of more than 60,000 patients diagnosed with RA as of November 2013 in the records of a large Boston-area medical system that includes physicians affiliated with Brigham and Women’s Hospital and Massachusetts General Hospital. The researchers applied a validated algorithm for calculating a patient’s probability of having RA, and at the level of 97% probability they narrowed the cohort down to just under 10,000 patients. Additional winnowing because of missing data or a history of pneumonia yielded a study group of 4,110, which included 3,279 (80%) who were seropositive for either or both CCP and RF, and 831 (20%) who were seronegative. During a median follow-up of 7.8 years and total follow-up of more than 32,000 patient-years, the overall pneumonia incidence was 5.8%, with a 2.8% rate among the seronegatives and a 6.6% rate among seropositives. After adjustment for age, sex, glucocorticoid use, disease-modifying antirheumatic drug use, and several other possible confounders, the researchers calculated a 99% relative increased rate of pneumonia among all seropositive patients, compared with the seronegatives.
Further analysis looked at pneumonia incidence rates among patients positive only for CCP antibody, positive only for RF antibody, or both, compared with seronegative patients. This showed that CCP seropositivity had no statistically significant link with incident pneumonia, while RF seropositivity linked with a statistically significant, roughly twofold higher rate. Only 6% of all seropositive patients were positive only for CCP antibody, 59% were positive specifically for RF antibody, and 35% for both.
The data Dr. Sparks presented did not include information on pneumonia type, the timing of the pneumonia, compared with the onset of RA, disease activity, or smoking intensity.
“We anticipated that both RF positive and CCP positive would each be associated with pneumonia, so it was somewhat surprising that we only detected this for RF,” Dr. Sparks said. But he added that, because the number of patients with only CCP positivity was relatively so small, “it is still possible that CCP [antibody] could also increase pneumonia risk.”
The study had no commercial funding. Dr. Sparks had no disclosures.
SOURCE: Sparks JA et al. Ann Rheum Dis. 2020 Jun;79[suppl 1]:73, Abstract OP0111.
from a single U.S. medical system.
“Patients with seropositive RA, particularly RF [rheumatoid factor]-positive RA, had increased risk for pneumonia throughout the RA disease course that was not explained by measured confounders, including smoking status, multimorbidity, medications, and [erythrocyte sedimentation rate] level,” Jeffrey A. Sparks, MD, said at the annual European Congress of Rheumatology, held online this year due to COVID-19.
“There has been much interest about the relationship between lung inflammation and the generation of RF and CCP [cyclic citrullinated protein] prior to the onset of RA. We hypothesized that patients with seropositive RA might have subclinical lung injury that could predispose them to pneumonia after clinical RA onset,” Dr. Sparks said in an interview. “Pneumonia is one of the most common serious infections in both patients with RA and the general population, and it causes serious morbidity and mortality.”
The doubled relative risk for pneumonia seen in the findings “translates into a clinically meaningful finding when considering the high rate and the many patients at risk since RA is relatively common,” said Dr. Sparks, a rheumatologist at Brigham and Women’s Hospital in Boston.
“Patients with RF-positive RA who present with symptoms concerning for pneumonia should be evaluated carefully for this and for other possible pulmonary manifestations of RA. Vaccination for pneumonia should be strongly considered for patients with RA who are on disease-modifying antirheumatic drugs, and we hope that our report encourages clinicians and patients” to undertake vaccination, he said.
His study used a database of more than 60,000 patients diagnosed with RA as of November 2013 in the records of a large Boston-area medical system that includes physicians affiliated with Brigham and Women’s Hospital and Massachusetts General Hospital. The researchers applied a validated algorithm for calculating a patient’s probability of having RA, and at the level of 97% probability they narrowed the cohort down to just under 10,000 patients. Additional winnowing because of missing data or a history of pneumonia yielded a study group of 4,110, which included 3,279 (80%) who were seropositive for either or both CCP and RF, and 831 (20%) who were seronegative. During a median follow-up of 7.8 years and total follow-up of more than 32,000 patient-years, the overall pneumonia incidence was 5.8%, with a 2.8% rate among the seronegatives and a 6.6% rate among seropositives. After adjustment for age, sex, glucocorticoid use, disease-modifying antirheumatic drug use, and several other possible confounders, the researchers calculated a 99% relative increased rate of pneumonia among all seropositive patients, compared with the seronegatives.
Further analysis looked at pneumonia incidence rates among patients positive only for CCP antibody, positive only for RF antibody, or both, compared with seronegative patients. This showed that CCP seropositivity had no statistically significant link with incident pneumonia, while RF seropositivity linked with a statistically significant, roughly twofold higher rate. Only 6% of all seropositive patients were positive only for CCP antibody, 59% were positive specifically for RF antibody, and 35% for both.
The data Dr. Sparks presented did not include information on pneumonia type, the timing of the pneumonia, compared with the onset of RA, disease activity, or smoking intensity.
“We anticipated that both RF positive and CCP positive would each be associated with pneumonia, so it was somewhat surprising that we only detected this for RF,” Dr. Sparks said. But he added that, because the number of patients with only CCP positivity was relatively so small, “it is still possible that CCP [antibody] could also increase pneumonia risk.”
The study had no commercial funding. Dr. Sparks had no disclosures.
SOURCE: Sparks JA et al. Ann Rheum Dis. 2020 Jun;79[suppl 1]:73, Abstract OP0111.
FROM THE EULAR 2020 E-CONGRESS
Cognitive-behavioral therapy a standout for better immune function
Psychosocial interventions, particularly cognitive-behavioral therapy (CBT), are associated with enhanced immune system function, new research suggests.
Results of a systematic review and meta-analysis that included 56 randomized controlled trials and more than 4,000 participants showed that over time, psychosocial interventions appeared to augment beneficial immune system function while concurrently decreasing harmful immune system function in comparison with control conditions.
“These associations were most reliable for cognitive-behavioral therapy and multiple or combined interventions and for studies that assessed proinflammatory cytokines or markers, which are key indicators of inflammation in the body,” study investigator George M. Slavich, PhD, said in an interview.
“The analysis helps address the question of which types of psychosocial interventions are most consistently associated with changes in immune system function, under what conditions, and for whom. This knowledge could, in turn, be used to inform research efforts and public policy aimed at using psychosocial interventions to improve immune-related health outcomes,” added Dr. Slavich, director of the Laboratory for Stress Assessment and Research, University of California, Los Angeles.
The study was published online June 3 in JAMA Psychiatry.
Link to serious physical, mental illnesses
There is substantial evidence that the immune system plays a role in a variety of mental and physical health problems. Such problems include anxiety disorders, depression, suicide, schizophrenia, cardiovascular disease, autoimmune disorders, and neurodegenerative diseases. It has been recently suggested that more than half of all deaths worldwide are attributable to inflammation-related conditions.
Although pharmacologic interventions can play a role in addressing inflammation, they are not without drawbacks, most notably, cost and adverse side effects.
The World Health Organization, the National Academy of Medicine, the National Institutes of Health, and other groups have emphasized the importance of addressing global disease burden through psychosocial interventions when possible.
Such recommendations are supported by scientific evidence. Previous research has shown that immune system processes are influenced by a variety of social, neurocognitive, and behavioral factors.
Given such findings, researchers have examined the effects of interventions that reduce stress or bolster psychological resources on immune system function.
However, such research has yielded conflicting findings. Some studies show that psychosocial interventions clearly enhance immunity, whereas others do not.
In addition, questions remain regarding which types of interventions reliably improve immune system function, under what conditions, and for whom.
“Research has shown that psychological factors – such as life stress, negative emotions, and social support – are associated with changes in immune system function,” Dr. Slavich noted.
“In addition, there is growing appreciation that immune system processes involved in inflammation may contribute to peoples’ risk for several major mental and physical health problems, including anxiety disorders, depression, heart disease, and autoimmune and neurodegenerative disorders.”
First study of its kind
To shed light on these potential links, the researchers conducted what they believe is the first systematic review and meta-analysis of randomized clinical trials of the effects of psychosocial interventions on immune system outcomes.
As part of the review, Dr. Slavich and colleagues estimated the associations between eight psychosocial interventions and seven markers of immune system function.
The eight psychosocial interventions were behavior therapy, cognitive therapy, CBT, CBT plus additive treatment or mode of delivery, bereavement or supportive therapy, multiple or combined interventions, other psychotherapy, and psychoeducation.
The seven immune outcomes that might be influenced by these interventions are proinflammatory cytokines and markers, anti-inflammatory cytokines, antibodies, immune cell counts, natural killer cell activity, viral load, and other immune outcomes.
The researchers also examined nine potential factors that might moderate the associations between psychosocial interventions and immune system function.
They searched a variety of databases for all relevant randomized controlled trials published through Dec. 31, 2018. Studies were eligible for inclusion if they included a psychosocial intervention and immune outcome, as well as preintervention and postintervention immunologic assessments.
The researchers identified 4,621 studies. Of these studies, 62 were eligible for inclusion; 56, which included 4,060 patients, were included in the final meta-analysis.
Results showed that psychosocial interventions were associated with enhanced immune system function (P < .001). There was relatively low heterogeneity between studies in these effect sizes, which, the investigators said, indicates that the association was relatively consistent across studies and conditions.
The meta-analysis showed that individuals who were assigned to a psychosocial intervention condition demonstrated a 14.7% improvement (95% confidence interval [CI], 5.7%–23.8%) in beneficial immune system function compared with their counterparts who were assigned to a control condition.
Similarly, participants who received psychosocial interventions demonstrated an 18.0% decrease (95% CI, 7.2%–28.8%) in harmful immune system function over time.
A standout
Regarding the effect of the type of intervention on the association, only CBT (31 studies; P < .001) and multiple or combined interventions (seven studies; P = .01) were significantly associated with changes in immune system outcomes.
The analysis also found that interventions that included a group component were more consistently associated with enhanced immune function than were those that did not include a group component. Nevertheless, this difference did not reach statistical significance (P = .06).
Contrary to the researchers’ expectations, the analysis also revealed that intervention length did not moderate the association between psychosocial interventions and immune system function (P = .93).
With respect to the type of immune marker studied, the meta-analysis found that psychosocial interventions had significantly different associations with the various immune markers studied. Of the seven immune outcomes investigated, only proinflammatory cytokine or marker levels (33 studies; P < .001) and immune cell counts (27 studies; P < .001) were significantly associated with the psychosocial interventions examined.
and were robust across age, sex, and intervention duration.
These results suggest that psychosocial interventions – particularly CBT and multiple or combined psychotherapeutic modalities – may play an important role in improving immune-related health outcomes.
Such interventions may not only be effective, they may also prove to be affordable alternatives to current therapeutic options. The mean length of a CBT intervention in the meta-analysis was 10.4 weeks, which the investigators equated with a total cost of $1,560 per patient.
“By comparison, the cost of using infliximab to reduce inflammation in persons with an autoimmune disorder is approximately $25,000 per patient per year,” they wrote.
“The results suggest the possibility that psychotherapy may be helpful for reducing inflammation and improving immune-related health in certain circumstances,” Dr. Slavich concluded. “However, the studies that we examined differed in terms of their quality, and we did not examine health outcomes in the present investigation.
“Therefore, more research needs to be done to determine how the present findings might be translated into treatment options or public policy.”
A path to better health
In an accompanying editorial, Veronika Engert, PhD, Joshua A. Grant, PhD, and Bernhard Strauss, PhD, noted that although infectious disease was once the primary cause of death in society, it has been supplanted by other complex and chronic illnesses, which often do not follow simple cause-and-effect associations.
“Rather,” they wrote, “these illnesses develop from a complex milieu of biological, psychological, and social factors that may also influence the disease progress and its prognosis. Against this backdrop, the meta-analysis by Shields and colleagues is an important confirmation of the biopsychosocial model.”
The editorialists explained that recent psychophysiological, neurobiological, and epigenetic research offers a glimpse into the relationship between psychological and social factors in pathogenesis. Nevertheless, the authors noted that a comprehensive examination of the potential effects of psychosocial interventions on immune parameters in various physical health conditions has been lacking.
“The evidence provided by Shields et al. is exactly what is needed to more fully shift treatment from an illness-centered to a patient-centered approach,” they wrote. “To that end, this meta-analysis may serve as a guide for policy makers aiming to improve immune-associated health.”
The research was supported by a Society in Science–Branco Weiss Fellowship, Brain and Behavior Research, and the National Institutes of Health. Dr. Slavich, Dr. Engert, Dr. Grant, and Dr. Strauss have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Psychosocial interventions, particularly cognitive-behavioral therapy (CBT), are associated with enhanced immune system function, new research suggests.
Results of a systematic review and meta-analysis that included 56 randomized controlled trials and more than 4,000 participants showed that over time, psychosocial interventions appeared to augment beneficial immune system function while concurrently decreasing harmful immune system function in comparison with control conditions.
“These associations were most reliable for cognitive-behavioral therapy and multiple or combined interventions and for studies that assessed proinflammatory cytokines or markers, which are key indicators of inflammation in the body,” study investigator George M. Slavich, PhD, said in an interview.
“The analysis helps address the question of which types of psychosocial interventions are most consistently associated with changes in immune system function, under what conditions, and for whom. This knowledge could, in turn, be used to inform research efforts and public policy aimed at using psychosocial interventions to improve immune-related health outcomes,” added Dr. Slavich, director of the Laboratory for Stress Assessment and Research, University of California, Los Angeles.
The study was published online June 3 in JAMA Psychiatry.
Link to serious physical, mental illnesses
There is substantial evidence that the immune system plays a role in a variety of mental and physical health problems. Such problems include anxiety disorders, depression, suicide, schizophrenia, cardiovascular disease, autoimmune disorders, and neurodegenerative diseases. It has been recently suggested that more than half of all deaths worldwide are attributable to inflammation-related conditions.
Although pharmacologic interventions can play a role in addressing inflammation, they are not without drawbacks, most notably, cost and adverse side effects.
The World Health Organization, the National Academy of Medicine, the National Institutes of Health, and other groups have emphasized the importance of addressing global disease burden through psychosocial interventions when possible.
Such recommendations are supported by scientific evidence. Previous research has shown that immune system processes are influenced by a variety of social, neurocognitive, and behavioral factors.
Given such findings, researchers have examined the effects of interventions that reduce stress or bolster psychological resources on immune system function.
However, such research has yielded conflicting findings. Some studies show that psychosocial interventions clearly enhance immunity, whereas others do not.
In addition, questions remain regarding which types of interventions reliably improve immune system function, under what conditions, and for whom.
“Research has shown that psychological factors – such as life stress, negative emotions, and social support – are associated with changes in immune system function,” Dr. Slavich noted.
“In addition, there is growing appreciation that immune system processes involved in inflammation may contribute to peoples’ risk for several major mental and physical health problems, including anxiety disorders, depression, heart disease, and autoimmune and neurodegenerative disorders.”
First study of its kind
To shed light on these potential links, the researchers conducted what they believe is the first systematic review and meta-analysis of randomized clinical trials of the effects of psychosocial interventions on immune system outcomes.
As part of the review, Dr. Slavich and colleagues estimated the associations between eight psychosocial interventions and seven markers of immune system function.
The eight psychosocial interventions were behavior therapy, cognitive therapy, CBT, CBT plus additive treatment or mode of delivery, bereavement or supportive therapy, multiple or combined interventions, other psychotherapy, and psychoeducation.
The seven immune outcomes that might be influenced by these interventions are proinflammatory cytokines and markers, anti-inflammatory cytokines, antibodies, immune cell counts, natural killer cell activity, viral load, and other immune outcomes.
The researchers also examined nine potential factors that might moderate the associations between psychosocial interventions and immune system function.
They searched a variety of databases for all relevant randomized controlled trials published through Dec. 31, 2018. Studies were eligible for inclusion if they included a psychosocial intervention and immune outcome, as well as preintervention and postintervention immunologic assessments.
The researchers identified 4,621 studies. Of these studies, 62 were eligible for inclusion; 56, which included 4,060 patients, were included in the final meta-analysis.
Results showed that psychosocial interventions were associated with enhanced immune system function (P < .001). There was relatively low heterogeneity between studies in these effect sizes, which, the investigators said, indicates that the association was relatively consistent across studies and conditions.
The meta-analysis showed that individuals who were assigned to a psychosocial intervention condition demonstrated a 14.7% improvement (95% confidence interval [CI], 5.7%–23.8%) in beneficial immune system function compared with their counterparts who were assigned to a control condition.
Similarly, participants who received psychosocial interventions demonstrated an 18.0% decrease (95% CI, 7.2%–28.8%) in harmful immune system function over time.
A standout
Regarding the effect of the type of intervention on the association, only CBT (31 studies; P < .001) and multiple or combined interventions (seven studies; P = .01) were significantly associated with changes in immune system outcomes.
The analysis also found that interventions that included a group component were more consistently associated with enhanced immune function than were those that did not include a group component. Nevertheless, this difference did not reach statistical significance (P = .06).
Contrary to the researchers’ expectations, the analysis also revealed that intervention length did not moderate the association between psychosocial interventions and immune system function (P = .93).
With respect to the type of immune marker studied, the meta-analysis found that psychosocial interventions had significantly different associations with the various immune markers studied. Of the seven immune outcomes investigated, only proinflammatory cytokine or marker levels (33 studies; P < .001) and immune cell counts (27 studies; P < .001) were significantly associated with the psychosocial interventions examined.
and were robust across age, sex, and intervention duration.
These results suggest that psychosocial interventions – particularly CBT and multiple or combined psychotherapeutic modalities – may play an important role in improving immune-related health outcomes.
Such interventions may not only be effective, they may also prove to be affordable alternatives to current therapeutic options. The mean length of a CBT intervention in the meta-analysis was 10.4 weeks, which the investigators equated with a total cost of $1,560 per patient.
“By comparison, the cost of using infliximab to reduce inflammation in persons with an autoimmune disorder is approximately $25,000 per patient per year,” they wrote.
“The results suggest the possibility that psychotherapy may be helpful for reducing inflammation and improving immune-related health in certain circumstances,” Dr. Slavich concluded. “However, the studies that we examined differed in terms of their quality, and we did not examine health outcomes in the present investigation.
“Therefore, more research needs to be done to determine how the present findings might be translated into treatment options or public policy.”
A path to better health
In an accompanying editorial, Veronika Engert, PhD, Joshua A. Grant, PhD, and Bernhard Strauss, PhD, noted that although infectious disease was once the primary cause of death in society, it has been supplanted by other complex and chronic illnesses, which often do not follow simple cause-and-effect associations.
“Rather,” they wrote, “these illnesses develop from a complex milieu of biological, psychological, and social factors that may also influence the disease progress and its prognosis. Against this backdrop, the meta-analysis by Shields and colleagues is an important confirmation of the biopsychosocial model.”
The editorialists explained that recent psychophysiological, neurobiological, and epigenetic research offers a glimpse into the relationship between psychological and social factors in pathogenesis. Nevertheless, the authors noted that a comprehensive examination of the potential effects of psychosocial interventions on immune parameters in various physical health conditions has been lacking.
“The evidence provided by Shields et al. is exactly what is needed to more fully shift treatment from an illness-centered to a patient-centered approach,” they wrote. “To that end, this meta-analysis may serve as a guide for policy makers aiming to improve immune-associated health.”
The research was supported by a Society in Science–Branco Weiss Fellowship, Brain and Behavior Research, and the National Institutes of Health. Dr. Slavich, Dr. Engert, Dr. Grant, and Dr. Strauss have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Psychosocial interventions, particularly cognitive-behavioral therapy (CBT), are associated with enhanced immune system function, new research suggests.
Results of a systematic review and meta-analysis that included 56 randomized controlled trials and more than 4,000 participants showed that over time, psychosocial interventions appeared to augment beneficial immune system function while concurrently decreasing harmful immune system function in comparison with control conditions.
“These associations were most reliable for cognitive-behavioral therapy and multiple or combined interventions and for studies that assessed proinflammatory cytokines or markers, which are key indicators of inflammation in the body,” study investigator George M. Slavich, PhD, said in an interview.
“The analysis helps address the question of which types of psychosocial interventions are most consistently associated with changes in immune system function, under what conditions, and for whom. This knowledge could, in turn, be used to inform research efforts and public policy aimed at using psychosocial interventions to improve immune-related health outcomes,” added Dr. Slavich, director of the Laboratory for Stress Assessment and Research, University of California, Los Angeles.
The study was published online June 3 in JAMA Psychiatry.
Link to serious physical, mental illnesses
There is substantial evidence that the immune system plays a role in a variety of mental and physical health problems. Such problems include anxiety disorders, depression, suicide, schizophrenia, cardiovascular disease, autoimmune disorders, and neurodegenerative diseases. It has been recently suggested that more than half of all deaths worldwide are attributable to inflammation-related conditions.
Although pharmacologic interventions can play a role in addressing inflammation, they are not without drawbacks, most notably, cost and adverse side effects.
The World Health Organization, the National Academy of Medicine, the National Institutes of Health, and other groups have emphasized the importance of addressing global disease burden through psychosocial interventions when possible.
Such recommendations are supported by scientific evidence. Previous research has shown that immune system processes are influenced by a variety of social, neurocognitive, and behavioral factors.
Given such findings, researchers have examined the effects of interventions that reduce stress or bolster psychological resources on immune system function.
However, such research has yielded conflicting findings. Some studies show that psychosocial interventions clearly enhance immunity, whereas others do not.
In addition, questions remain regarding which types of interventions reliably improve immune system function, under what conditions, and for whom.
“Research has shown that psychological factors – such as life stress, negative emotions, and social support – are associated with changes in immune system function,” Dr. Slavich noted.
“In addition, there is growing appreciation that immune system processes involved in inflammation may contribute to peoples’ risk for several major mental and physical health problems, including anxiety disorders, depression, heart disease, and autoimmune and neurodegenerative disorders.”
First study of its kind
To shed light on these potential links, the researchers conducted what they believe is the first systematic review and meta-analysis of randomized clinical trials of the effects of psychosocial interventions on immune system outcomes.
As part of the review, Dr. Slavich and colleagues estimated the associations between eight psychosocial interventions and seven markers of immune system function.
The eight psychosocial interventions were behavior therapy, cognitive therapy, CBT, CBT plus additive treatment or mode of delivery, bereavement or supportive therapy, multiple or combined interventions, other psychotherapy, and psychoeducation.
The seven immune outcomes that might be influenced by these interventions are proinflammatory cytokines and markers, anti-inflammatory cytokines, antibodies, immune cell counts, natural killer cell activity, viral load, and other immune outcomes.
The researchers also examined nine potential factors that might moderate the associations between psychosocial interventions and immune system function.
They searched a variety of databases for all relevant randomized controlled trials published through Dec. 31, 2018. Studies were eligible for inclusion if they included a psychosocial intervention and immune outcome, as well as preintervention and postintervention immunologic assessments.
The researchers identified 4,621 studies. Of these studies, 62 were eligible for inclusion; 56, which included 4,060 patients, were included in the final meta-analysis.
Results showed that psychosocial interventions were associated with enhanced immune system function (P < .001). There was relatively low heterogeneity between studies in these effect sizes, which, the investigators said, indicates that the association was relatively consistent across studies and conditions.
The meta-analysis showed that individuals who were assigned to a psychosocial intervention condition demonstrated a 14.7% improvement (95% confidence interval [CI], 5.7%–23.8%) in beneficial immune system function compared with their counterparts who were assigned to a control condition.
Similarly, participants who received psychosocial interventions demonstrated an 18.0% decrease (95% CI, 7.2%–28.8%) in harmful immune system function over time.
A standout
Regarding the effect of the type of intervention on the association, only CBT (31 studies; P < .001) and multiple or combined interventions (seven studies; P = .01) were significantly associated with changes in immune system outcomes.
The analysis also found that interventions that included a group component were more consistently associated with enhanced immune function than were those that did not include a group component. Nevertheless, this difference did not reach statistical significance (P = .06).
Contrary to the researchers’ expectations, the analysis also revealed that intervention length did not moderate the association between psychosocial interventions and immune system function (P = .93).
With respect to the type of immune marker studied, the meta-analysis found that psychosocial interventions had significantly different associations with the various immune markers studied. Of the seven immune outcomes investigated, only proinflammatory cytokine or marker levels (33 studies; P < .001) and immune cell counts (27 studies; P < .001) were significantly associated with the psychosocial interventions examined.
and were robust across age, sex, and intervention duration.
These results suggest that psychosocial interventions – particularly CBT and multiple or combined psychotherapeutic modalities – may play an important role in improving immune-related health outcomes.
Such interventions may not only be effective, they may also prove to be affordable alternatives to current therapeutic options. The mean length of a CBT intervention in the meta-analysis was 10.4 weeks, which the investigators equated with a total cost of $1,560 per patient.
“By comparison, the cost of using infliximab to reduce inflammation in persons with an autoimmune disorder is approximately $25,000 per patient per year,” they wrote.
“The results suggest the possibility that psychotherapy may be helpful for reducing inflammation and improving immune-related health in certain circumstances,” Dr. Slavich concluded. “However, the studies that we examined differed in terms of their quality, and we did not examine health outcomes in the present investigation.
“Therefore, more research needs to be done to determine how the present findings might be translated into treatment options or public policy.”
A path to better health
In an accompanying editorial, Veronika Engert, PhD, Joshua A. Grant, PhD, and Bernhard Strauss, PhD, noted that although infectious disease was once the primary cause of death in society, it has been supplanted by other complex and chronic illnesses, which often do not follow simple cause-and-effect associations.
“Rather,” they wrote, “these illnesses develop from a complex milieu of biological, psychological, and social factors that may also influence the disease progress and its prognosis. Against this backdrop, the meta-analysis by Shields and colleagues is an important confirmation of the biopsychosocial model.”
The editorialists explained that recent psychophysiological, neurobiological, and epigenetic research offers a glimpse into the relationship between psychological and social factors in pathogenesis. Nevertheless, the authors noted that a comprehensive examination of the potential effects of psychosocial interventions on immune parameters in various physical health conditions has been lacking.
“The evidence provided by Shields et al. is exactly what is needed to more fully shift treatment from an illness-centered to a patient-centered approach,” they wrote. “To that end, this meta-analysis may serve as a guide for policy makers aiming to improve immune-associated health.”
The research was supported by a Society in Science–Branco Weiss Fellowship, Brain and Behavior Research, and the National Institutes of Health. Dr. Slavich, Dr. Engert, Dr. Grant, and Dr. Strauss have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Compound CAR T – a double whammy with promise for AML
Six of eight relapsed/refractory acute myeloid leukemia patients, and one patient with accelerated phase chronic myelogenous leukemia, had no sign of residual disease 4 weeks after receiving compound CAR T therapy targeting both CD33 and CLL1.
Six patients moved on to subsequent hematopoietic stem cell transplantation (HSCT); the seventh responder withdrew from the study for personal reasons, according to a report at the virtual annual congress of the European Hematology Association.
Much work remains, but the initial results suggest that “CLL1-CD33 compound CAR T cell therapy could be developed as a bridge to transplant, a supplement to chemotherapy, or a standalone therapy for patients with acute myeloid leukemia” and other myeloid malignancies. The approach might also allow for reduced intensity conditioning or nonmyeloablative conditioning for HSCT, said lead investigator Fang Liu, MD, PhD, of the department of hematology at the Chengdu Military General Hospital, in Sichuan province, China.
It’s “a topic that will interest a lot of us.” For the first time, “a compound CAR with two independent CAR units induced remissions in AML,” said Pieter Sonneveld, MD, PhD, of the Erasmus Medical Center Cancer Institute, Rotterdam, the Netherlands, who introduced Dr. Liu’s presentation.
Chimeric antigen receptor (CAR) T cell therapy works well for B-cell malignancies, but translation to AML is “yet to be accomplished.” Meanwhile, despite progress against AML, about one-third of patients still relapse, “and prognosis for relapsed or refractory AML is dismal,” Dr. Liu and her team said.
CAR T is generally aimed against a single target, but AML bears heterogeneous cells that offset killing by single target therapies, resulting in disease relapse.
That problem suggested targeting multiple antigens simultaneously. CLL1 is an “ideal target,” Dr. Liu said, because the myeloid lineage antigen is highly expressed in AML, but absent in normal hematopoietic stem cells. CD33, meanwhile, is expressed on bulk AML cells in the majority of patients.
The CAR T cells were manufactured from autologous cells in eight of the subjects, and from a human leukocyte antigen-matched sibling donor cells for the ninth. The patients were lymphodepleted with fludarabine and cyclophosphamide, then infused with the therapeutic cells by a dose escalation at approximately 1-3 x 106/kg in a single or split dose.
On disease reevaluation within 4 weeks, seven of nine patients – all with relapsed or refractory disease after multiple conventional treatments – were minimal residual disease negative by flow cytometry. The other two had no response, one of whom was CD33 positive but CLL1 negative, “indicating the importance of [the] CLL1 target in CAR T treatment,” the investigators said.
All nine patients developed grade 4 pancytopenia; eight had cytokine release syndrome (CRS), which was grade 3 in two; and four subjects developed neurotoxicity, which was grade 3 in three.
Five subjects had mild liver enzyme elevations; four had a coagulation disorder; four developed diarrhea; three developed sepsis; two fungal infections; and three pneumonia. One subject had a skin rash and one developed renal insufficiency.
The adverse events resolved after treatment. “Early intervention with steroids had a positive effect on the reduction of CRS and neurotoxicity,” the team noted.
Of the six patients who went on to HCST, one had standard myeloablative conditioning, but the rest had reduced intensity conditioning. Five subjects successfully engrafted with persistent full chimerism, but one died of sepsis before engraftment.
The median age was 32 years. The median bone marrow blast count before treatment was 47%. Seven subjects had de novo AML; one – a 6-year-old girl – had juvenile myelomonocytic leukemia that transformed into AML; and one had accelerated phase chronic myelogenous leukemia.
A phase 1 trial is underway (NCT03795779).
The work was funded by iCell Gene Therapeutics. Several investigators were employees. Dr. Liu didn’t report any disclosures.
SOURCE: Liu F et al. EHA Congress. Abstract S149.
Six of eight relapsed/refractory acute myeloid leukemia patients, and one patient with accelerated phase chronic myelogenous leukemia, had no sign of residual disease 4 weeks after receiving compound CAR T therapy targeting both CD33 and CLL1.
Six patients moved on to subsequent hematopoietic stem cell transplantation (HSCT); the seventh responder withdrew from the study for personal reasons, according to a report at the virtual annual congress of the European Hematology Association.
Much work remains, but the initial results suggest that “CLL1-CD33 compound CAR T cell therapy could be developed as a bridge to transplant, a supplement to chemotherapy, or a standalone therapy for patients with acute myeloid leukemia” and other myeloid malignancies. The approach might also allow for reduced intensity conditioning or nonmyeloablative conditioning for HSCT, said lead investigator Fang Liu, MD, PhD, of the department of hematology at the Chengdu Military General Hospital, in Sichuan province, China.
It’s “a topic that will interest a lot of us.” For the first time, “a compound CAR with two independent CAR units induced remissions in AML,” said Pieter Sonneveld, MD, PhD, of the Erasmus Medical Center Cancer Institute, Rotterdam, the Netherlands, who introduced Dr. Liu’s presentation.
Chimeric antigen receptor (CAR) T cell therapy works well for B-cell malignancies, but translation to AML is “yet to be accomplished.” Meanwhile, despite progress against AML, about one-third of patients still relapse, “and prognosis for relapsed or refractory AML is dismal,” Dr. Liu and her team said.
CAR T is generally aimed against a single target, but AML bears heterogeneous cells that offset killing by single target therapies, resulting in disease relapse.
That problem suggested targeting multiple antigens simultaneously. CLL1 is an “ideal target,” Dr. Liu said, because the myeloid lineage antigen is highly expressed in AML, but absent in normal hematopoietic stem cells. CD33, meanwhile, is expressed on bulk AML cells in the majority of patients.
The CAR T cells were manufactured from autologous cells in eight of the subjects, and from a human leukocyte antigen-matched sibling donor cells for the ninth. The patients were lymphodepleted with fludarabine and cyclophosphamide, then infused with the therapeutic cells by a dose escalation at approximately 1-3 x 106/kg in a single or split dose.
On disease reevaluation within 4 weeks, seven of nine patients – all with relapsed or refractory disease after multiple conventional treatments – were minimal residual disease negative by flow cytometry. The other two had no response, one of whom was CD33 positive but CLL1 negative, “indicating the importance of [the] CLL1 target in CAR T treatment,” the investigators said.
All nine patients developed grade 4 pancytopenia; eight had cytokine release syndrome (CRS), which was grade 3 in two; and four subjects developed neurotoxicity, which was grade 3 in three.
Five subjects had mild liver enzyme elevations; four had a coagulation disorder; four developed diarrhea; three developed sepsis; two fungal infections; and three pneumonia. One subject had a skin rash and one developed renal insufficiency.
The adverse events resolved after treatment. “Early intervention with steroids had a positive effect on the reduction of CRS and neurotoxicity,” the team noted.
Of the six patients who went on to HCST, one had standard myeloablative conditioning, but the rest had reduced intensity conditioning. Five subjects successfully engrafted with persistent full chimerism, but one died of sepsis before engraftment.
The median age was 32 years. The median bone marrow blast count before treatment was 47%. Seven subjects had de novo AML; one – a 6-year-old girl – had juvenile myelomonocytic leukemia that transformed into AML; and one had accelerated phase chronic myelogenous leukemia.
A phase 1 trial is underway (NCT03795779).
The work was funded by iCell Gene Therapeutics. Several investigators were employees. Dr. Liu didn’t report any disclosures.
SOURCE: Liu F et al. EHA Congress. Abstract S149.
Six of eight relapsed/refractory acute myeloid leukemia patients, and one patient with accelerated phase chronic myelogenous leukemia, had no sign of residual disease 4 weeks after receiving compound CAR T therapy targeting both CD33 and CLL1.
Six patients moved on to subsequent hematopoietic stem cell transplantation (HSCT); the seventh responder withdrew from the study for personal reasons, according to a report at the virtual annual congress of the European Hematology Association.
Much work remains, but the initial results suggest that “CLL1-CD33 compound CAR T cell therapy could be developed as a bridge to transplant, a supplement to chemotherapy, or a standalone therapy for patients with acute myeloid leukemia” and other myeloid malignancies. The approach might also allow for reduced intensity conditioning or nonmyeloablative conditioning for HSCT, said lead investigator Fang Liu, MD, PhD, of the department of hematology at the Chengdu Military General Hospital, in Sichuan province, China.
It’s “a topic that will interest a lot of us.” For the first time, “a compound CAR with two independent CAR units induced remissions in AML,” said Pieter Sonneveld, MD, PhD, of the Erasmus Medical Center Cancer Institute, Rotterdam, the Netherlands, who introduced Dr. Liu’s presentation.
Chimeric antigen receptor (CAR) T cell therapy works well for B-cell malignancies, but translation to AML is “yet to be accomplished.” Meanwhile, despite progress against AML, about one-third of patients still relapse, “and prognosis for relapsed or refractory AML is dismal,” Dr. Liu and her team said.
CAR T is generally aimed against a single target, but AML bears heterogeneous cells that offset killing by single target therapies, resulting in disease relapse.
That problem suggested targeting multiple antigens simultaneously. CLL1 is an “ideal target,” Dr. Liu said, because the myeloid lineage antigen is highly expressed in AML, but absent in normal hematopoietic stem cells. CD33, meanwhile, is expressed on bulk AML cells in the majority of patients.
The CAR T cells were manufactured from autologous cells in eight of the subjects, and from a human leukocyte antigen-matched sibling donor cells for the ninth. The patients were lymphodepleted with fludarabine and cyclophosphamide, then infused with the therapeutic cells by a dose escalation at approximately 1-3 x 106/kg in a single or split dose.
On disease reevaluation within 4 weeks, seven of nine patients – all with relapsed or refractory disease after multiple conventional treatments – were minimal residual disease negative by flow cytometry. The other two had no response, one of whom was CD33 positive but CLL1 negative, “indicating the importance of [the] CLL1 target in CAR T treatment,” the investigators said.
All nine patients developed grade 4 pancytopenia; eight had cytokine release syndrome (CRS), which was grade 3 in two; and four subjects developed neurotoxicity, which was grade 3 in three.
Five subjects had mild liver enzyme elevations; four had a coagulation disorder; four developed diarrhea; three developed sepsis; two fungal infections; and three pneumonia. One subject had a skin rash and one developed renal insufficiency.
The adverse events resolved after treatment. “Early intervention with steroids had a positive effect on the reduction of CRS and neurotoxicity,” the team noted.
Of the six patients who went on to HCST, one had standard myeloablative conditioning, but the rest had reduced intensity conditioning. Five subjects successfully engrafted with persistent full chimerism, but one died of sepsis before engraftment.
The median age was 32 years. The median bone marrow blast count before treatment was 47%. Seven subjects had de novo AML; one – a 6-year-old girl – had juvenile myelomonocytic leukemia that transformed into AML; and one had accelerated phase chronic myelogenous leukemia.
A phase 1 trial is underway (NCT03795779).
The work was funded by iCell Gene Therapeutics. Several investigators were employees. Dr. Liu didn’t report any disclosures.
SOURCE: Liu F et al. EHA Congress. Abstract S149.
FROM EHA CONGRESS