User login
Some telepsychiatry ‘here to stay’ post COVID
The COVID-19 pandemic has changed life in numerous ways, including use of telehealth services for patients in all specialties. But telepsychiatry is an area not likely to go away even after the pandemic is over, according to Sanjay Gupta, MD.
The use of telepsychiatry has escalated significantly,” said Dr. Gupta, of the DENT Neurologic Institute, in Amherst, N.Y., in a bonus virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
About 90% of clinicians are performing telepsychiatry, Dr. Gupta noted, through methods such as phone consults, email, and video chat. As patients with psychiatric issues grapple with issues related to COVID-19 involving lockdowns, restrictions on travel, and consumption of news, they are presenting with addiction, depression, paranoia, mood lability, and other problems.
One issue immediately facing clinicians is whether to keep patients on long-acting injectables as a way to maintain psychological stability in patients with bipolar disorder, schizophrenia, and alcoholism – something Dr. Gupta and session moderator Henry A. Nasrallah, MD, advocated. “We should never stop the long-acting injectable to switch them to oral medication. Those patients are very likely to relapse,” Dr. Nasrallah said.
During the pandemic, clinicians need to find “safe and novel ways of providing the injection,” and several methods have been pioneered. For example, if a patient with schizophrenia is on lockdown, a nurse can visit monthly or bimonthly to administer an injection, check on the patient’s mental status, and assess whether that patient needs an adjustment to their medication. Other clinics are offering “drive-by” injections to patients who arrive by car, and a nurse wearing a mask and a face shield administers the injection from the car window. Monthly naltrexone also can be administered using one of these methods, and telepsychiatry can be used to monitor patients, Dr. Gupta noted at the meeting, presented by Global Academy for Medical Education.
“In my clinic, what happens is the injection room is set up just next to the door, so they don’t have to walk deep into the clinic,” Dr. Gupta said. “They walk in, go to the left, [and] there’s the injection room. They sit, get an injection, they’re out. It’s kept smooth.”
Choosing the right telehealth option
Clinicians should be aware of important regulatory changes that occurred that made widespread telehealth more appealing during the COVID-19 pandemic. Payment parity with in-office visits makes telehealth a viable consideration, while some states have begun offering telehealth licenses to practice across state lines. There is wide variation with regard to which states provide licensure and prescribing privileges for out-of-state clinicians without seeing those patients in person. “The most important thing: The psychiatry service is provided in the state where the patient is located,” Dr. Gupta said. Clinicians should check with that state’s board to figure out specific requirements. “Preferably if you get it in writing, it’s good for you,” he said.
Deciding who the clinician is seeing – consulting with patients or other physicians/clinicians – and what type of visits a clinician will conduct is an important step in transitioning to telepsychiatry. Visits from evaluation through ongoing care are possible through telepsychiatry, or a clinician can opt to see just second opinion visits, Dr. Gupta said. It is also important to consider the technical ability of the patient to do video conferencing.
As HIPAA requirements for privacy have relaxed, clinicians now have an array of teleconferencing options to choose from; platforms such as FaceTime, Doximity, Vidyo, Doxy.me, Zoom, and video chat through EMR are popular options. However, when regular HIPAA requirements are reinstated after the pandemic, clinicians will need to find a compliant platform and sign a business associate agreement to stay within the law.
“Right now, my preferred use is FaceTime,” Dr. Gupta said. “Quick, simple, easy to use. A lot of people have an iPhone, and they know how to do it. I usually have the patient call me and I don’t use my personal iPhone; my clinic has an iPhone.”
How a clinician looks during a telepsychiatry visit is also important. Lighting, position of the camera, and clothing should all be considered. Keep the camera at eye level, test the lighting in the room where the call will take place, and use artificial lighting sources behind a computer, Dr. Gupta said. Other tips for telepsychiatry visits include silencing devices and microphones before a session begins, wearing solid-colored clothes, and having an identification badge visible to the patient. Sessions should be free of background distractions, such as a dog barking or a child interrupting, with the goal of creating an environment where the patient feels free to answer questions.
Contingency planning is a must for video visits, Dr. Gupta said. “I think the simplest thing is to see the patient. But all the stuff that’s the wraparound is really hard, because issues can arise suddenly, and we need to plan.” If a patient has a medical issue or becomes actively suicidal during a session, it is important to know contact information for the local police and crisis services. Clinicians also must plan for technology failure and provide alternative options for continuing the sessions, such as by phone.
Selecting patients for telepsychiatry
Not all patients will make the transition to telepsychiatry. “You can’t do telepsychiatry with everyone. It is a risk, so pick and choose,” Dr. Gupta said.
“Safety is a big consideration for conducting a telepsychiatry visit, especially when other health care providers are present. For example, when performing telehealth visits in a clinic, nursing home, or correctional facility, “I feel a lot more comfortable if there’s another health care clinician there,” Dr. Gupta said.
Clinicians may want to avoid a telepsychiatry visit for a patient in their own home for reasons of safety, reliability, and privacy. A longitudinal history with collateral information from friends or relatives can be helpful, but some subtle signs and body language may get missed over video, compared with an in-person visit. Sometimes you may not see if the patient is using substances. You have to really reconsider if [there] is violence and self-injurious behavior,” he said.
Discussing the pros and cons of telepsychiatry is important to obtaining patient consent. While consent requirements have relaxed under the COVID-19 pandemic, consent should ideally be obtained in writing, but can also be obtained verbally during a crisis. A plan should be developed for what will happen in the case of technology failure. “The patient should also know you’re maintaining privacy, you’re maintaining confidentiality, but there is a risk of hacking,” Dr. Gupta said. “Those things can happen, [and] there are no guarantees.”
If a patient is uncomfortable after beginning telepsychiatry, moving to in-person visits is also an option. “Many times, I do that if I’m not getting a good handle on things,” Dr. Gupta said. Situations where patients insist on in-patient visits over telepsychiatry are rare in his experience, Dr. Gupta noted, and are usually the result of the patient being unfamiliar with the technology. In cases where a patient cannot be talked through a technology barrier, visits can be done in the clinic while taking proper precautions.
“If it is a first-time visit, then I do it in the clinic,” Dr. Gupta said. “They come in, they have a face mask, and we use our group therapy room. The patients sit in a social-distanced fashion. But then, you document why you did this in-person visit like that.”
Documentation during COVID-19 also includes identifying the patient at the first visit, the nature of the visit (teleconference or other), parties present, referencing the pandemic, writing the location of the patient and the clinician, noting the patient’s satisfaction, evaluating the patient’s mental status, and recording what technology was used and any technical issues that were encountered.
Some populations of patients are better suited to telepsychiatry than others. It is more convenient for chronically psychiatrically ill patients in group homes and their staff to communicate through telepsychiatry, Dr. Gupta said. Consultation liaison in hospitals and emergency departments through telepsychiatry can limit the spread of infection, while increased access and convenience occurs as telepsychiatry is implemented in correctional facilities and nursing homes.
“What we are doing now, some of it is here to stay,” Dr. Gupta said.
In situations where a patient needs to switch providers, clinicians should continue to follow that patient until his first patient visit with that new provider. It is also important to set boundaries and apply some level of formality to the telepsychiatry visit, which means seeing the patient in a secure location where he can speak freely and privately.
“The best practices are [to] maintain faith [and] fidelity of the psychiatric assessment,” Dr. Gupta said. “Keep the trust and do your best to maintain patient privacy, because the privacy is not the same as it may be in a face-to-face session when you use televideo.”
Global Academy and this news organization are owned by the same parent company.
Dr. Gupta reported no relevant financial disclosures. Dr. Nasrallah disclosed serving as a consultant for and on the speakers bureaus of several pharmaceutical companies, including Alkermes, Janssen, and Lundbeck. He also disclosed serving on the speakers bureau of Otsuka.
The COVID-19 pandemic has changed life in numerous ways, including use of telehealth services for patients in all specialties. But telepsychiatry is an area not likely to go away even after the pandemic is over, according to Sanjay Gupta, MD.
The use of telepsychiatry has escalated significantly,” said Dr. Gupta, of the DENT Neurologic Institute, in Amherst, N.Y., in a bonus virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
About 90% of clinicians are performing telepsychiatry, Dr. Gupta noted, through methods such as phone consults, email, and video chat. As patients with psychiatric issues grapple with issues related to COVID-19 involving lockdowns, restrictions on travel, and consumption of news, they are presenting with addiction, depression, paranoia, mood lability, and other problems.
One issue immediately facing clinicians is whether to keep patients on long-acting injectables as a way to maintain psychological stability in patients with bipolar disorder, schizophrenia, and alcoholism – something Dr. Gupta and session moderator Henry A. Nasrallah, MD, advocated. “We should never stop the long-acting injectable to switch them to oral medication. Those patients are very likely to relapse,” Dr. Nasrallah said.
During the pandemic, clinicians need to find “safe and novel ways of providing the injection,” and several methods have been pioneered. For example, if a patient with schizophrenia is on lockdown, a nurse can visit monthly or bimonthly to administer an injection, check on the patient’s mental status, and assess whether that patient needs an adjustment to their medication. Other clinics are offering “drive-by” injections to patients who arrive by car, and a nurse wearing a mask and a face shield administers the injection from the car window. Monthly naltrexone also can be administered using one of these methods, and telepsychiatry can be used to monitor patients, Dr. Gupta noted at the meeting, presented by Global Academy for Medical Education.
“In my clinic, what happens is the injection room is set up just next to the door, so they don’t have to walk deep into the clinic,” Dr. Gupta said. “They walk in, go to the left, [and] there’s the injection room. They sit, get an injection, they’re out. It’s kept smooth.”
Choosing the right telehealth option
Clinicians should be aware of important regulatory changes that occurred that made widespread telehealth more appealing during the COVID-19 pandemic. Payment parity with in-office visits makes telehealth a viable consideration, while some states have begun offering telehealth licenses to practice across state lines. There is wide variation with regard to which states provide licensure and prescribing privileges for out-of-state clinicians without seeing those patients in person. “The most important thing: The psychiatry service is provided in the state where the patient is located,” Dr. Gupta said. Clinicians should check with that state’s board to figure out specific requirements. “Preferably if you get it in writing, it’s good for you,” he said.
Deciding who the clinician is seeing – consulting with patients or other physicians/clinicians – and what type of visits a clinician will conduct is an important step in transitioning to telepsychiatry. Visits from evaluation through ongoing care are possible through telepsychiatry, or a clinician can opt to see just second opinion visits, Dr. Gupta said. It is also important to consider the technical ability of the patient to do video conferencing.
As HIPAA requirements for privacy have relaxed, clinicians now have an array of teleconferencing options to choose from; platforms such as FaceTime, Doximity, Vidyo, Doxy.me, Zoom, and video chat through EMR are popular options. However, when regular HIPAA requirements are reinstated after the pandemic, clinicians will need to find a compliant platform and sign a business associate agreement to stay within the law.
“Right now, my preferred use is FaceTime,” Dr. Gupta said. “Quick, simple, easy to use. A lot of people have an iPhone, and they know how to do it. I usually have the patient call me and I don’t use my personal iPhone; my clinic has an iPhone.”
How a clinician looks during a telepsychiatry visit is also important. Lighting, position of the camera, and clothing should all be considered. Keep the camera at eye level, test the lighting in the room where the call will take place, and use artificial lighting sources behind a computer, Dr. Gupta said. Other tips for telepsychiatry visits include silencing devices and microphones before a session begins, wearing solid-colored clothes, and having an identification badge visible to the patient. Sessions should be free of background distractions, such as a dog barking or a child interrupting, with the goal of creating an environment where the patient feels free to answer questions.
Contingency planning is a must for video visits, Dr. Gupta said. “I think the simplest thing is to see the patient. But all the stuff that’s the wraparound is really hard, because issues can arise suddenly, and we need to plan.” If a patient has a medical issue or becomes actively suicidal during a session, it is important to know contact information for the local police and crisis services. Clinicians also must plan for technology failure and provide alternative options for continuing the sessions, such as by phone.
Selecting patients for telepsychiatry
Not all patients will make the transition to telepsychiatry. “You can’t do telepsychiatry with everyone. It is a risk, so pick and choose,” Dr. Gupta said.
“Safety is a big consideration for conducting a telepsychiatry visit, especially when other health care providers are present. For example, when performing telehealth visits in a clinic, nursing home, or correctional facility, “I feel a lot more comfortable if there’s another health care clinician there,” Dr. Gupta said.
Clinicians may want to avoid a telepsychiatry visit for a patient in their own home for reasons of safety, reliability, and privacy. A longitudinal history with collateral information from friends or relatives can be helpful, but some subtle signs and body language may get missed over video, compared with an in-person visit. Sometimes you may not see if the patient is using substances. You have to really reconsider if [there] is violence and self-injurious behavior,” he said.
Discussing the pros and cons of telepsychiatry is important to obtaining patient consent. While consent requirements have relaxed under the COVID-19 pandemic, consent should ideally be obtained in writing, but can also be obtained verbally during a crisis. A plan should be developed for what will happen in the case of technology failure. “The patient should also know you’re maintaining privacy, you’re maintaining confidentiality, but there is a risk of hacking,” Dr. Gupta said. “Those things can happen, [and] there are no guarantees.”
If a patient is uncomfortable after beginning telepsychiatry, moving to in-person visits is also an option. “Many times, I do that if I’m not getting a good handle on things,” Dr. Gupta said. Situations where patients insist on in-patient visits over telepsychiatry are rare in his experience, Dr. Gupta noted, and are usually the result of the patient being unfamiliar with the technology. In cases where a patient cannot be talked through a technology barrier, visits can be done in the clinic while taking proper precautions.
“If it is a first-time visit, then I do it in the clinic,” Dr. Gupta said. “They come in, they have a face mask, and we use our group therapy room. The patients sit in a social-distanced fashion. But then, you document why you did this in-person visit like that.”
Documentation during COVID-19 also includes identifying the patient at the first visit, the nature of the visit (teleconference or other), parties present, referencing the pandemic, writing the location of the patient and the clinician, noting the patient’s satisfaction, evaluating the patient’s mental status, and recording what technology was used and any technical issues that were encountered.
Some populations of patients are better suited to telepsychiatry than others. It is more convenient for chronically psychiatrically ill patients in group homes and their staff to communicate through telepsychiatry, Dr. Gupta said. Consultation liaison in hospitals and emergency departments through telepsychiatry can limit the spread of infection, while increased access and convenience occurs as telepsychiatry is implemented in correctional facilities and nursing homes.
“What we are doing now, some of it is here to stay,” Dr. Gupta said.
In situations where a patient needs to switch providers, clinicians should continue to follow that patient until his first patient visit with that new provider. It is also important to set boundaries and apply some level of formality to the telepsychiatry visit, which means seeing the patient in a secure location where he can speak freely and privately.
“The best practices are [to] maintain faith [and] fidelity of the psychiatric assessment,” Dr. Gupta said. “Keep the trust and do your best to maintain patient privacy, because the privacy is not the same as it may be in a face-to-face session when you use televideo.”
Global Academy and this news organization are owned by the same parent company.
Dr. Gupta reported no relevant financial disclosures. Dr. Nasrallah disclosed serving as a consultant for and on the speakers bureaus of several pharmaceutical companies, including Alkermes, Janssen, and Lundbeck. He also disclosed serving on the speakers bureau of Otsuka.
The COVID-19 pandemic has changed life in numerous ways, including use of telehealth services for patients in all specialties. But telepsychiatry is an area not likely to go away even after the pandemic is over, according to Sanjay Gupta, MD.
The use of telepsychiatry has escalated significantly,” said Dr. Gupta, of the DENT Neurologic Institute, in Amherst, N.Y., in a bonus virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
About 90% of clinicians are performing telepsychiatry, Dr. Gupta noted, through methods such as phone consults, email, and video chat. As patients with psychiatric issues grapple with issues related to COVID-19 involving lockdowns, restrictions on travel, and consumption of news, they are presenting with addiction, depression, paranoia, mood lability, and other problems.
One issue immediately facing clinicians is whether to keep patients on long-acting injectables as a way to maintain psychological stability in patients with bipolar disorder, schizophrenia, and alcoholism – something Dr. Gupta and session moderator Henry A. Nasrallah, MD, advocated. “We should never stop the long-acting injectable to switch them to oral medication. Those patients are very likely to relapse,” Dr. Nasrallah said.
During the pandemic, clinicians need to find “safe and novel ways of providing the injection,” and several methods have been pioneered. For example, if a patient with schizophrenia is on lockdown, a nurse can visit monthly or bimonthly to administer an injection, check on the patient’s mental status, and assess whether that patient needs an adjustment to their medication. Other clinics are offering “drive-by” injections to patients who arrive by car, and a nurse wearing a mask and a face shield administers the injection from the car window. Monthly naltrexone also can be administered using one of these methods, and telepsychiatry can be used to monitor patients, Dr. Gupta noted at the meeting, presented by Global Academy for Medical Education.
“In my clinic, what happens is the injection room is set up just next to the door, so they don’t have to walk deep into the clinic,” Dr. Gupta said. “They walk in, go to the left, [and] there’s the injection room. They sit, get an injection, they’re out. It’s kept smooth.”
Choosing the right telehealth option
Clinicians should be aware of important regulatory changes that occurred that made widespread telehealth more appealing during the COVID-19 pandemic. Payment parity with in-office visits makes telehealth a viable consideration, while some states have begun offering telehealth licenses to practice across state lines. There is wide variation with regard to which states provide licensure and prescribing privileges for out-of-state clinicians without seeing those patients in person. “The most important thing: The psychiatry service is provided in the state where the patient is located,” Dr. Gupta said. Clinicians should check with that state’s board to figure out specific requirements. “Preferably if you get it in writing, it’s good for you,” he said.
Deciding who the clinician is seeing – consulting with patients or other physicians/clinicians – and what type of visits a clinician will conduct is an important step in transitioning to telepsychiatry. Visits from evaluation through ongoing care are possible through telepsychiatry, or a clinician can opt to see just second opinion visits, Dr. Gupta said. It is also important to consider the technical ability of the patient to do video conferencing.
As HIPAA requirements for privacy have relaxed, clinicians now have an array of teleconferencing options to choose from; platforms such as FaceTime, Doximity, Vidyo, Doxy.me, Zoom, and video chat through EMR are popular options. However, when regular HIPAA requirements are reinstated after the pandemic, clinicians will need to find a compliant platform and sign a business associate agreement to stay within the law.
“Right now, my preferred use is FaceTime,” Dr. Gupta said. “Quick, simple, easy to use. A lot of people have an iPhone, and they know how to do it. I usually have the patient call me and I don’t use my personal iPhone; my clinic has an iPhone.”
How a clinician looks during a telepsychiatry visit is also important. Lighting, position of the camera, and clothing should all be considered. Keep the camera at eye level, test the lighting in the room where the call will take place, and use artificial lighting sources behind a computer, Dr. Gupta said. Other tips for telepsychiatry visits include silencing devices and microphones before a session begins, wearing solid-colored clothes, and having an identification badge visible to the patient. Sessions should be free of background distractions, such as a dog barking or a child interrupting, with the goal of creating an environment where the patient feels free to answer questions.
Contingency planning is a must for video visits, Dr. Gupta said. “I think the simplest thing is to see the patient. But all the stuff that’s the wraparound is really hard, because issues can arise suddenly, and we need to plan.” If a patient has a medical issue or becomes actively suicidal during a session, it is important to know contact information for the local police and crisis services. Clinicians also must plan for technology failure and provide alternative options for continuing the sessions, such as by phone.
Selecting patients for telepsychiatry
Not all patients will make the transition to telepsychiatry. “You can’t do telepsychiatry with everyone. It is a risk, so pick and choose,” Dr. Gupta said.
“Safety is a big consideration for conducting a telepsychiatry visit, especially when other health care providers are present. For example, when performing telehealth visits in a clinic, nursing home, or correctional facility, “I feel a lot more comfortable if there’s another health care clinician there,” Dr. Gupta said.
Clinicians may want to avoid a telepsychiatry visit for a patient in their own home for reasons of safety, reliability, and privacy. A longitudinal history with collateral information from friends or relatives can be helpful, but some subtle signs and body language may get missed over video, compared with an in-person visit. Sometimes you may not see if the patient is using substances. You have to really reconsider if [there] is violence and self-injurious behavior,” he said.
Discussing the pros and cons of telepsychiatry is important to obtaining patient consent. While consent requirements have relaxed under the COVID-19 pandemic, consent should ideally be obtained in writing, but can also be obtained verbally during a crisis. A plan should be developed for what will happen in the case of technology failure. “The patient should also know you’re maintaining privacy, you’re maintaining confidentiality, but there is a risk of hacking,” Dr. Gupta said. “Those things can happen, [and] there are no guarantees.”
If a patient is uncomfortable after beginning telepsychiatry, moving to in-person visits is also an option. “Many times, I do that if I’m not getting a good handle on things,” Dr. Gupta said. Situations where patients insist on in-patient visits over telepsychiatry are rare in his experience, Dr. Gupta noted, and are usually the result of the patient being unfamiliar with the technology. In cases where a patient cannot be talked through a technology barrier, visits can be done in the clinic while taking proper precautions.
“If it is a first-time visit, then I do it in the clinic,” Dr. Gupta said. “They come in, they have a face mask, and we use our group therapy room. The patients sit in a social-distanced fashion. But then, you document why you did this in-person visit like that.”
Documentation during COVID-19 also includes identifying the patient at the first visit, the nature of the visit (teleconference or other), parties present, referencing the pandemic, writing the location of the patient and the clinician, noting the patient’s satisfaction, evaluating the patient’s mental status, and recording what technology was used and any technical issues that were encountered.
Some populations of patients are better suited to telepsychiatry than others. It is more convenient for chronically psychiatrically ill patients in group homes and their staff to communicate through telepsychiatry, Dr. Gupta said. Consultation liaison in hospitals and emergency departments through telepsychiatry can limit the spread of infection, while increased access and convenience occurs as telepsychiatry is implemented in correctional facilities and nursing homes.
“What we are doing now, some of it is here to stay,” Dr. Gupta said.
In situations where a patient needs to switch providers, clinicians should continue to follow that patient until his first patient visit with that new provider. It is also important to set boundaries and apply some level of formality to the telepsychiatry visit, which means seeing the patient in a secure location where he can speak freely and privately.
“The best practices are [to] maintain faith [and] fidelity of the psychiatric assessment,” Dr. Gupta said. “Keep the trust and do your best to maintain patient privacy, because the privacy is not the same as it may be in a face-to-face session when you use televideo.”
Global Academy and this news organization are owned by the same parent company.
Dr. Gupta reported no relevant financial disclosures. Dr. Nasrallah disclosed serving as a consultant for and on the speakers bureaus of several pharmaceutical companies, including Alkermes, Janssen, and Lundbeck. He also disclosed serving on the speakers bureau of Otsuka.
EXPERT ANALYSIS FROM CP/AACP 2020 PSYCHIATRY UPDATE
Improving Healthcare Value: COVID-19 Emergency Regulatory Relief and Implications for Post-Acute Skilled Nursing Facility Care
Medicare beneficiary who requires skilled care in a nursing home? Better be admitted for at least 3 days in the hospital first if you want the nursing home paid for. Govt doesn’t always make sense. We’re listening to feedback.
—Centers for Medicare & Medicaid Services Administrator Seema Verma, @SeemaCMS, August 4, 2019, via Twitter.1
On March 13, 2020, the president of the United States declared a national health emergency, granting the secretary of the United States Department of Health & Human Services authority to grant waivers intended to ease certain Medicare and Medicaid program requirements.2 Broad waiver categories include those that may be requested by an individual institution, as well as “COVID-19 Emergency Declaration Blanket Waivers,” which automatically apply across all facilities and providers. As stated by the Centers for Medicare & Medicaid Services (CMS), waivers are intended to create “regulatory flexibilities to help healthcare providers contain the spread of 2019 Novel Coronavirus Disease (COVID-19).” These provisions are retroactive to March 1, 2020, expire at the end of the “emergency period or 60 days from the date the waiver . . . is first published” and can be extended by the secretary.2
The issued blanket waivers remove administrative requirements in a wide range of care settings including home health, hospice, hospitals, and skilled nursing facilities (SNF), among others. The waiving of many of these administrative requirements are welcomed by providers and administrators alike in this time of national crisis. For example, relaxation of verbal order signage requirements and expanded coverage of telehealth will, almost certainly, improve accessibility, efficiency, and requisite coordination and care across settings. Emergence of these new “COVID-19” waivers also present rare and valuable opportunities to examine care improvement in areas long believed to need permanent regulatory change. Perhaps the most important of these long over-due changes is the current CMS process for determining Part A eligibility for post-acute skilled nursing facility coverage for traditional Medicare beneficiaries following an inpatient hospitalization. Under COVID-19, CMS has now granted a waiver that “authorizes the Secretary to provide for Skilled Nursing Facilities (SNF) coverage in the absence of a qualifying [three consecutive inpatient midnight] hospital stay. . . .”2 Although demand for SNF placement may shift during the pandemic, hospitals facing capacity issues will more easily be able to discharge Medicare beneficiaries ready for post-acute care.
POST-ACUTE SKILLED NURSING FACILITY COVERAGE
When Medicare was established in 1965, approximately half of Americans over age 65 did not have health insurance, and older adults were the most likely demographic to be living in poverty.3 Originally called “Hospital Insurance” or “Medicare Part A,” these “Inpatient Hospital Services” are described in Social Security statute as “items and services furnished to an inpatient of a hospital” including room and board, nursing services, pharmaceuticals, and medical and surgical services delivered in the hospital.4 In 1967, Medicare beneficiaries staying three consecutive inpatient hospital midnights were also afforded post-acute SNF coverage for up to 100 days. As expected, hospital use increased as seniors had coverage for hospital care and were also, in many cases, able to access higher quality post-hospital care.5
Over the past 50 years, two important changes have shifted Medicare beneficiary SNF coverage. First, due to efficiencies and changes in care delivery, average length of hospital stay for Americans over age 65 has shrunk from 14 days in 1965 to approximately 5 days currently.5,6 Now, fewer beneficiaries spend the necessary three or more nights in the hospital to qualify for post-acute SNF coverage. Second, and most importantly, CMS created “observation status” in the 1980s, which allowed for patients to be observed as “outpatients” in a hospital instead of as inpatients. Notably, these observation nights fall under outpatient status (Part B), and therefore do not count toward the statutory SNF coverage requirement of three inpatient midnights.
According to CMS, observation should be used so that a “decision can be made regarding whether patients will require further treatment as hospital inpatients or if they are able to be discharged from the hospital. . . . In the majority of cases, the decision can be made in less than 48 hours, usually in less than 24 hours.”7 At the time of its development, this concept fit the growing use of Emergency Department observation units, in which patients presented for an acute issue but could usually discharge home in the stated time frame.
OBSERVATION CARE
In reality, outpatient (observation) status is not synonymous with observation units. Because observation is a billing determination, not a specific type of clinical care, observation care may be delivered anywhere in a hospital—including an observation unit, a hospital ward, or even an intensive care unit (ICU). While all hospitals may deliver observation care, only about one-third of hospitals have observation units, and even hospitals with observation units deliver observation care outside of these units. Traditional Medicare beneficiaries who stay three or more nights in the hospital but cannot meet the three inpatient midnight requirement to access their SNF coverage benefits because of outpatient (observation) nights are often left vulnerable and confused, saddling them with an average of $10,503 for each uncovered SNF stay.8 As emergent evidence demonstrates striking racial, geographic, and socioeconomic-based health disparities in COVID-19, renewal of the “three-midnight rule” could have disproportionate and long-lasting ramifications for these populations in particular.9
Hospital observation stays (or observation nights) can look identical to inpatient hospital stays, as defined by the Social Security statute4; yet never count toward the three-inpatient-midnight tally. In 2014, the Office of Inspector General (OIG) found there were 633,148 hospital stays that lasted three midnights or longer but did not contain three consecutive inpatient midnights, which resulted in nonqualifying stays for purposes of SNF coverage, if that coverage was needed.10 A more recent OIG report found that Medicare was paying erroneously for some SNF stays because even CMS could not distinguish between three midnights that were all inpatient or a combination of inpatient and observation.11 Additionally, because care provided is often indistinguishable, status changes between outpatient and inpatient are common; in 2014, 40% of Medicare observation stays occurring within 30 days of an inpatient stay changed to inpatient over the course of a single hospitalization.12 Now, in the time of COVID-19, this untenable decades-long problem has the potential to be definitively addressed by a permanent removal of the three midnight requirement altogether.
PROGRESS TOWARD REFORM
Several recent signals suggest that change is supported by a diverse group of stakeholders. In their 2019 Top 25 Unimplemented Recommendations, the OIG acknowledged the similarity in observation and inpatient care, recommending that “CMS . . . analyze the potential impacts of counting time spent as an outpatient toward the 3-night requirement for skilled nursing facility (SNF) services so that beneficiaries receiving similar hospital care have similar access to these services.”13 The “Improving Access to Medicare Coverage Act of 2019,” reintroduced in the 116th Congress, would count all midnights spent in the hospital, whether those nights are inpatient or observation, toward the three midnight requirement.14 This bill has bipartisan, bicameral support, which demonstrates unified legislative interest across the political spectrum. More recently in March 2020, a federal judge in the class action lawsuit Alexander v Azar determined that Medicare beneficiaries had the right to appeal to Medicare if a physician placed a patient in inpatient status and this decision was overturned administratively by a hospital, resulting in loss of a beneficiary’s SNF coverage.15 Although now under appeal, this judicial decision signals the importance of beneficiary rights to appeal directly to CMS.
Given the mounting support for reform, it is probable that cost concerns and allocation of resources to the Part A vs Part B “buckets” remain the only barrier to permanently reforming the three-midnight inpatient stay policy. Pilot programs testing Medicare SNF waivers more than 30 years ago suggested increased cost and SNF usage.16 However, more contemporary experience from Medicare Advantage programs suggest just the opposite. Grebla et al showed there was no increased SNF use nor SNF length of stay for beneficiaries in Medicare Advantage plans that waived the three inpatient midnight requirement.17
Arguably, the current COVID-19 emergency blanket SNF waiver is not a perfect test of short- or long-term Medicare costs. First, factors such as reduced hospital elective surgeries that may typically drive post-acute SNF admissions, as well as potentially reduced SNF utilization caused by fear of COVID-19 outbreaks, may temporarily lower SNF use and associated Medicare expenditures. The existing waiver of statute is also financially constrained, stipulating that “this action does not increase overall program payments. . . .”2 Longer term, innovations in care delivery prompted by accelerated telehealth reforms may shift more post-acute care from SNFs to the home setting, changing patterns of SNF utilization altogether. Despite these limitations, this regulatory relief will still provide valuable utilization and cost information on SNF use under a system absent the three-midnight requirement.
CONCLUSION
Rarely, if ever, does a national healthcare system experience such a rapid and marked change as that seen with the COVID-19 pandemic. Despite the tragic emergency circumstances prompting CMS’s blanket waivers, it provides CMS and stakeholders with a rare opportunity to evaluate potential improvements revealed by each individual aspect of COVID-19 regulatory relief. CMS has in the past argued the three-midnight SNF requirement is a statutory issue and thus not within their control, yet they have used their regulatory authority to waive this policy to facilitate efficient care in a national health crisis. This is a change that many believe is long overdue, and one that should be maintained even after COVID-19 abates. “Govt doesn’t always make sense,” as Administrator Verma wrote,1 should be a cry for government to make better sense of existing legislation and regulation. Reform of the three-midnight inpatient rule is the right place to start.
1. @SeemaCMS. #Medicare beneficiary who requires skilled care in a nursing home? Better be admitted for at least 3 days in the hospital first if you want the nursing home paid for. [Flushed face emoji] Govt doesn’t always make sense. We’re listening to feedback. #RedTapeTales #TheBoldAndTheBureaucratic. August 4, 2019. Accessed April 17, 2020. https://twitter.com/SeemaCMS/status/1158029830056828928
2. COVID-19 Emergency Declaration Blanket Waivers for Health Care Providers. Centers for Medicare & Medicaid Services, US Dept of Health & Human Services; 2020. Accessed April 17, 2020. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf
3. Medicare & Medicaid Milestones, 1937 to 2015. Centers for Medicare and Medicaid Services, US Dept of Health & Human Services; 2015. Accessed April 17, 2020. https://www.cms.gov/About-CMS/Agency-Information/History/Downloads/Medicare-and-Medicaid-Milestones-1937-2015.pdf
4. Social Security Laws, 42 USC 1395x §1861 (1965). Accessed April 17, 2020. https://www.ssa.gov/OP_Home/ssact/title18/1861.htm
5. Loewenstein R. Early effects of Medicare on the health care of the aged. Social Security Bulletin. April 1971; pp 3-20, 42. Accessed April 14, 2020. https://www.ssa.gov/policy/docs/ssb/v34n4/v34n4p3.pdf
6. Weiss AJ, Elixhauser A. Overview of Hospital Stays in the United States, 2012. Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality, US Dept of Health & Human Services; 2014. Accessed April 16, 2020. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf
7. Medicare Benefits Policy Manual, Internet-Only Manuals. Centers for Medicare & Medicaid Services. Pub. 100-02, Chapter 6, § 20.6. Updated April 5, 2012. Accessed April 17, 2020. http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Internet-Only-Manuals-IOMs.html
8. Wright S. Hospitals’ Use of Observation Stays and Short Inpatient Stays for Medicare Beneficiaries. Office of the Inspector General, US Dept of Health & Human Services; 2014. Accessed April 16, 2020. https://oig.hhs.gov/oei/reports/oei-02-12-00040.asp
9. Yancy CW. COVID-19 and African Americans. JAMA. Published online April 15, 2020. https://doi.org/10.1001/jama.2020.6548
10. Levinson DR. Vulnerabilities Remain Under Medicare’s 2-Midnight Hospital Policy. Office of the Inspector General, US Dept of Health & Human Services; 2016. Accessed April 18, 2020. https://oig.hhs.gov/oei/reports/oei-02-15-00020.pdf
11. Levinson DR. CMS Improperly Paid Millions of Dollars for Skilled Nursing Facility Services When the Medicare 3-Day Inpatient Hospital Stay Requirement Was Not Met. Office of the Inspector General, US Dept of Health & Human Services; 2019. Accessed April 16, 2020. https://www.oig.hhs.gov/oas/reports/region5/51600043.pdf
12. Sheehy A, Shi F, Kind A. Identifying observation stays in Medicare data: policy implications of a definition. J Hosp Med. 2019;14(2):96-100. https://doi.org/10.12788/jhm.3038
13. Solutions to Reduce Fraud, Waste, and Abuse in HHS Programs: OIG’s Top Recommendations. Office of the Inspector General, US Dept of Health & Human Services; 2019. Accessed April 18, 2020. https://oig.hhs.gov/reports-and-publications/compendium/files/compendium2019.pdf
14. Improving Access to Medicare Coverage Act of 2019, HR 1682, 116th Congress (2019). Accessed April 16, 2020. https://www.congress.gov/bill/116th-congress/house-bill/1682
15. Alexander v Azar, 396 F Supp 3d 242 (D CT 2019). Accessed May 26, 2020. https://casetext.com/case/alexander-v-azar-1?
16. Lipsitz L. The 3-night hospital stay and Medicare coverage for skilled nursing care. JAMA. 2013;310(14):1441-1442. https://doi.org/10.1001/jama.2013.254845
17. Grebla R, Keohane L, Lee Y, Lipsitz L, Rahman M, Trevedi A. Waiving the three-day rule: admissions and length-of-stay at hospitals and skilled nursing facilities did not increase. Health Affairs (Millwood). 2015;34(8):1324-1330. https://doi.org/10.1377/hlthaff.2015.0054
Medicare beneficiary who requires skilled care in a nursing home? Better be admitted for at least 3 days in the hospital first if you want the nursing home paid for. Govt doesn’t always make sense. We’re listening to feedback.
—Centers for Medicare & Medicaid Services Administrator Seema Verma, @SeemaCMS, August 4, 2019, via Twitter.1
On March 13, 2020, the president of the United States declared a national health emergency, granting the secretary of the United States Department of Health & Human Services authority to grant waivers intended to ease certain Medicare and Medicaid program requirements.2 Broad waiver categories include those that may be requested by an individual institution, as well as “COVID-19 Emergency Declaration Blanket Waivers,” which automatically apply across all facilities and providers. As stated by the Centers for Medicare & Medicaid Services (CMS), waivers are intended to create “regulatory flexibilities to help healthcare providers contain the spread of 2019 Novel Coronavirus Disease (COVID-19).” These provisions are retroactive to March 1, 2020, expire at the end of the “emergency period or 60 days from the date the waiver . . . is first published” and can be extended by the secretary.2
The issued blanket waivers remove administrative requirements in a wide range of care settings including home health, hospice, hospitals, and skilled nursing facilities (SNF), among others. The waiving of many of these administrative requirements are welcomed by providers and administrators alike in this time of national crisis. For example, relaxation of verbal order signage requirements and expanded coverage of telehealth will, almost certainly, improve accessibility, efficiency, and requisite coordination and care across settings. Emergence of these new “COVID-19” waivers also present rare and valuable opportunities to examine care improvement in areas long believed to need permanent regulatory change. Perhaps the most important of these long over-due changes is the current CMS process for determining Part A eligibility for post-acute skilled nursing facility coverage for traditional Medicare beneficiaries following an inpatient hospitalization. Under COVID-19, CMS has now granted a waiver that “authorizes the Secretary to provide for Skilled Nursing Facilities (SNF) coverage in the absence of a qualifying [three consecutive inpatient midnight] hospital stay. . . .”2 Although demand for SNF placement may shift during the pandemic, hospitals facing capacity issues will more easily be able to discharge Medicare beneficiaries ready for post-acute care.
POST-ACUTE SKILLED NURSING FACILITY COVERAGE
When Medicare was established in 1965, approximately half of Americans over age 65 did not have health insurance, and older adults were the most likely demographic to be living in poverty.3 Originally called “Hospital Insurance” or “Medicare Part A,” these “Inpatient Hospital Services” are described in Social Security statute as “items and services furnished to an inpatient of a hospital” including room and board, nursing services, pharmaceuticals, and medical and surgical services delivered in the hospital.4 In 1967, Medicare beneficiaries staying three consecutive inpatient hospital midnights were also afforded post-acute SNF coverage for up to 100 days. As expected, hospital use increased as seniors had coverage for hospital care and were also, in many cases, able to access higher quality post-hospital care.5
Over the past 50 years, two important changes have shifted Medicare beneficiary SNF coverage. First, due to efficiencies and changes in care delivery, average length of hospital stay for Americans over age 65 has shrunk from 14 days in 1965 to approximately 5 days currently.5,6 Now, fewer beneficiaries spend the necessary three or more nights in the hospital to qualify for post-acute SNF coverage. Second, and most importantly, CMS created “observation status” in the 1980s, which allowed for patients to be observed as “outpatients” in a hospital instead of as inpatients. Notably, these observation nights fall under outpatient status (Part B), and therefore do not count toward the statutory SNF coverage requirement of three inpatient midnights.
According to CMS, observation should be used so that a “decision can be made regarding whether patients will require further treatment as hospital inpatients or if they are able to be discharged from the hospital. . . . In the majority of cases, the decision can be made in less than 48 hours, usually in less than 24 hours.”7 At the time of its development, this concept fit the growing use of Emergency Department observation units, in which patients presented for an acute issue but could usually discharge home in the stated time frame.
OBSERVATION CARE
In reality, outpatient (observation) status is not synonymous with observation units. Because observation is a billing determination, not a specific type of clinical care, observation care may be delivered anywhere in a hospital—including an observation unit, a hospital ward, or even an intensive care unit (ICU). While all hospitals may deliver observation care, only about one-third of hospitals have observation units, and even hospitals with observation units deliver observation care outside of these units. Traditional Medicare beneficiaries who stay three or more nights in the hospital but cannot meet the three inpatient midnight requirement to access their SNF coverage benefits because of outpatient (observation) nights are often left vulnerable and confused, saddling them with an average of $10,503 for each uncovered SNF stay.8 As emergent evidence demonstrates striking racial, geographic, and socioeconomic-based health disparities in COVID-19, renewal of the “three-midnight rule” could have disproportionate and long-lasting ramifications for these populations in particular.9
Hospital observation stays (or observation nights) can look identical to inpatient hospital stays, as defined by the Social Security statute4; yet never count toward the three-inpatient-midnight tally. In 2014, the Office of Inspector General (OIG) found there were 633,148 hospital stays that lasted three midnights or longer but did not contain three consecutive inpatient midnights, which resulted in nonqualifying stays for purposes of SNF coverage, if that coverage was needed.10 A more recent OIG report found that Medicare was paying erroneously for some SNF stays because even CMS could not distinguish between three midnights that were all inpatient or a combination of inpatient and observation.11 Additionally, because care provided is often indistinguishable, status changes between outpatient and inpatient are common; in 2014, 40% of Medicare observation stays occurring within 30 days of an inpatient stay changed to inpatient over the course of a single hospitalization.12 Now, in the time of COVID-19, this untenable decades-long problem has the potential to be definitively addressed by a permanent removal of the three midnight requirement altogether.
PROGRESS TOWARD REFORM
Several recent signals suggest that change is supported by a diverse group of stakeholders. In their 2019 Top 25 Unimplemented Recommendations, the OIG acknowledged the similarity in observation and inpatient care, recommending that “CMS . . . analyze the potential impacts of counting time spent as an outpatient toward the 3-night requirement for skilled nursing facility (SNF) services so that beneficiaries receiving similar hospital care have similar access to these services.”13 The “Improving Access to Medicare Coverage Act of 2019,” reintroduced in the 116th Congress, would count all midnights spent in the hospital, whether those nights are inpatient or observation, toward the three midnight requirement.14 This bill has bipartisan, bicameral support, which demonstrates unified legislative interest across the political spectrum. More recently in March 2020, a federal judge in the class action lawsuit Alexander v Azar determined that Medicare beneficiaries had the right to appeal to Medicare if a physician placed a patient in inpatient status and this decision was overturned administratively by a hospital, resulting in loss of a beneficiary’s SNF coverage.15 Although now under appeal, this judicial decision signals the importance of beneficiary rights to appeal directly to CMS.
Given the mounting support for reform, it is probable that cost concerns and allocation of resources to the Part A vs Part B “buckets” remain the only barrier to permanently reforming the three-midnight inpatient stay policy. Pilot programs testing Medicare SNF waivers more than 30 years ago suggested increased cost and SNF usage.16 However, more contemporary experience from Medicare Advantage programs suggest just the opposite. Grebla et al showed there was no increased SNF use nor SNF length of stay for beneficiaries in Medicare Advantage plans that waived the three inpatient midnight requirement.17
Arguably, the current COVID-19 emergency blanket SNF waiver is not a perfect test of short- or long-term Medicare costs. First, factors such as reduced hospital elective surgeries that may typically drive post-acute SNF admissions, as well as potentially reduced SNF utilization caused by fear of COVID-19 outbreaks, may temporarily lower SNF use and associated Medicare expenditures. The existing waiver of statute is also financially constrained, stipulating that “this action does not increase overall program payments. . . .”2 Longer term, innovations in care delivery prompted by accelerated telehealth reforms may shift more post-acute care from SNFs to the home setting, changing patterns of SNF utilization altogether. Despite these limitations, this regulatory relief will still provide valuable utilization and cost information on SNF use under a system absent the three-midnight requirement.
CONCLUSION
Rarely, if ever, does a national healthcare system experience such a rapid and marked change as that seen with the COVID-19 pandemic. Despite the tragic emergency circumstances prompting CMS’s blanket waivers, it provides CMS and stakeholders with a rare opportunity to evaluate potential improvements revealed by each individual aspect of COVID-19 regulatory relief. CMS has in the past argued the three-midnight SNF requirement is a statutory issue and thus not within their control, yet they have used their regulatory authority to waive this policy to facilitate efficient care in a national health crisis. This is a change that many believe is long overdue, and one that should be maintained even after COVID-19 abates. “Govt doesn’t always make sense,” as Administrator Verma wrote,1 should be a cry for government to make better sense of existing legislation and regulation. Reform of the three-midnight inpatient rule is the right place to start.
Medicare beneficiary who requires skilled care in a nursing home? Better be admitted for at least 3 days in the hospital first if you want the nursing home paid for. Govt doesn’t always make sense. We’re listening to feedback.
—Centers for Medicare & Medicaid Services Administrator Seema Verma, @SeemaCMS, August 4, 2019, via Twitter.1
On March 13, 2020, the president of the United States declared a national health emergency, granting the secretary of the United States Department of Health & Human Services authority to grant waivers intended to ease certain Medicare and Medicaid program requirements.2 Broad waiver categories include those that may be requested by an individual institution, as well as “COVID-19 Emergency Declaration Blanket Waivers,” which automatically apply across all facilities and providers. As stated by the Centers for Medicare & Medicaid Services (CMS), waivers are intended to create “regulatory flexibilities to help healthcare providers contain the spread of 2019 Novel Coronavirus Disease (COVID-19).” These provisions are retroactive to March 1, 2020, expire at the end of the “emergency period or 60 days from the date the waiver . . . is first published” and can be extended by the secretary.2
The issued blanket waivers remove administrative requirements in a wide range of care settings including home health, hospice, hospitals, and skilled nursing facilities (SNF), among others. The waiving of many of these administrative requirements are welcomed by providers and administrators alike in this time of national crisis. For example, relaxation of verbal order signage requirements and expanded coverage of telehealth will, almost certainly, improve accessibility, efficiency, and requisite coordination and care across settings. Emergence of these new “COVID-19” waivers also present rare and valuable opportunities to examine care improvement in areas long believed to need permanent regulatory change. Perhaps the most important of these long over-due changes is the current CMS process for determining Part A eligibility for post-acute skilled nursing facility coverage for traditional Medicare beneficiaries following an inpatient hospitalization. Under COVID-19, CMS has now granted a waiver that “authorizes the Secretary to provide for Skilled Nursing Facilities (SNF) coverage in the absence of a qualifying [three consecutive inpatient midnight] hospital stay. . . .”2 Although demand for SNF placement may shift during the pandemic, hospitals facing capacity issues will more easily be able to discharge Medicare beneficiaries ready for post-acute care.
POST-ACUTE SKILLED NURSING FACILITY COVERAGE
When Medicare was established in 1965, approximately half of Americans over age 65 did not have health insurance, and older adults were the most likely demographic to be living in poverty.3 Originally called “Hospital Insurance” or “Medicare Part A,” these “Inpatient Hospital Services” are described in Social Security statute as “items and services furnished to an inpatient of a hospital” including room and board, nursing services, pharmaceuticals, and medical and surgical services delivered in the hospital.4 In 1967, Medicare beneficiaries staying three consecutive inpatient hospital midnights were also afforded post-acute SNF coverage for up to 100 days. As expected, hospital use increased as seniors had coverage for hospital care and were also, in many cases, able to access higher quality post-hospital care.5
Over the past 50 years, two important changes have shifted Medicare beneficiary SNF coverage. First, due to efficiencies and changes in care delivery, average length of hospital stay for Americans over age 65 has shrunk from 14 days in 1965 to approximately 5 days currently.5,6 Now, fewer beneficiaries spend the necessary three or more nights in the hospital to qualify for post-acute SNF coverage. Second, and most importantly, CMS created “observation status” in the 1980s, which allowed for patients to be observed as “outpatients” in a hospital instead of as inpatients. Notably, these observation nights fall under outpatient status (Part B), and therefore do not count toward the statutory SNF coverage requirement of three inpatient midnights.
According to CMS, observation should be used so that a “decision can be made regarding whether patients will require further treatment as hospital inpatients or if they are able to be discharged from the hospital. . . . In the majority of cases, the decision can be made in less than 48 hours, usually in less than 24 hours.”7 At the time of its development, this concept fit the growing use of Emergency Department observation units, in which patients presented for an acute issue but could usually discharge home in the stated time frame.
OBSERVATION CARE
In reality, outpatient (observation) status is not synonymous with observation units. Because observation is a billing determination, not a specific type of clinical care, observation care may be delivered anywhere in a hospital—including an observation unit, a hospital ward, or even an intensive care unit (ICU). While all hospitals may deliver observation care, only about one-third of hospitals have observation units, and even hospitals with observation units deliver observation care outside of these units. Traditional Medicare beneficiaries who stay three or more nights in the hospital but cannot meet the three inpatient midnight requirement to access their SNF coverage benefits because of outpatient (observation) nights are often left vulnerable and confused, saddling them with an average of $10,503 for each uncovered SNF stay.8 As emergent evidence demonstrates striking racial, geographic, and socioeconomic-based health disparities in COVID-19, renewal of the “three-midnight rule” could have disproportionate and long-lasting ramifications for these populations in particular.9
Hospital observation stays (or observation nights) can look identical to inpatient hospital stays, as defined by the Social Security statute4; yet never count toward the three-inpatient-midnight tally. In 2014, the Office of Inspector General (OIG) found there were 633,148 hospital stays that lasted three midnights or longer but did not contain three consecutive inpatient midnights, which resulted in nonqualifying stays for purposes of SNF coverage, if that coverage was needed.10 A more recent OIG report found that Medicare was paying erroneously for some SNF stays because even CMS could not distinguish between three midnights that were all inpatient or a combination of inpatient and observation.11 Additionally, because care provided is often indistinguishable, status changes between outpatient and inpatient are common; in 2014, 40% of Medicare observation stays occurring within 30 days of an inpatient stay changed to inpatient over the course of a single hospitalization.12 Now, in the time of COVID-19, this untenable decades-long problem has the potential to be definitively addressed by a permanent removal of the three midnight requirement altogether.
PROGRESS TOWARD REFORM
Several recent signals suggest that change is supported by a diverse group of stakeholders. In their 2019 Top 25 Unimplemented Recommendations, the OIG acknowledged the similarity in observation and inpatient care, recommending that “CMS . . . analyze the potential impacts of counting time spent as an outpatient toward the 3-night requirement for skilled nursing facility (SNF) services so that beneficiaries receiving similar hospital care have similar access to these services.”13 The “Improving Access to Medicare Coverage Act of 2019,” reintroduced in the 116th Congress, would count all midnights spent in the hospital, whether those nights are inpatient or observation, toward the three midnight requirement.14 This bill has bipartisan, bicameral support, which demonstrates unified legislative interest across the political spectrum. More recently in March 2020, a federal judge in the class action lawsuit Alexander v Azar determined that Medicare beneficiaries had the right to appeal to Medicare if a physician placed a patient in inpatient status and this decision was overturned administratively by a hospital, resulting in loss of a beneficiary’s SNF coverage.15 Although now under appeal, this judicial decision signals the importance of beneficiary rights to appeal directly to CMS.
Given the mounting support for reform, it is probable that cost concerns and allocation of resources to the Part A vs Part B “buckets” remain the only barrier to permanently reforming the three-midnight inpatient stay policy. Pilot programs testing Medicare SNF waivers more than 30 years ago suggested increased cost and SNF usage.16 However, more contemporary experience from Medicare Advantage programs suggest just the opposite. Grebla et al showed there was no increased SNF use nor SNF length of stay for beneficiaries in Medicare Advantage plans that waived the three inpatient midnight requirement.17
Arguably, the current COVID-19 emergency blanket SNF waiver is not a perfect test of short- or long-term Medicare costs. First, factors such as reduced hospital elective surgeries that may typically drive post-acute SNF admissions, as well as potentially reduced SNF utilization caused by fear of COVID-19 outbreaks, may temporarily lower SNF use and associated Medicare expenditures. The existing waiver of statute is also financially constrained, stipulating that “this action does not increase overall program payments. . . .”2 Longer term, innovations in care delivery prompted by accelerated telehealth reforms may shift more post-acute care from SNFs to the home setting, changing patterns of SNF utilization altogether. Despite these limitations, this regulatory relief will still provide valuable utilization and cost information on SNF use under a system absent the three-midnight requirement.
CONCLUSION
Rarely, if ever, does a national healthcare system experience such a rapid and marked change as that seen with the COVID-19 pandemic. Despite the tragic emergency circumstances prompting CMS’s blanket waivers, it provides CMS and stakeholders with a rare opportunity to evaluate potential improvements revealed by each individual aspect of COVID-19 regulatory relief. CMS has in the past argued the three-midnight SNF requirement is a statutory issue and thus not within their control, yet they have used their regulatory authority to waive this policy to facilitate efficient care in a national health crisis. This is a change that many believe is long overdue, and one that should be maintained even after COVID-19 abates. “Govt doesn’t always make sense,” as Administrator Verma wrote,1 should be a cry for government to make better sense of existing legislation and regulation. Reform of the three-midnight inpatient rule is the right place to start.
1. @SeemaCMS. #Medicare beneficiary who requires skilled care in a nursing home? Better be admitted for at least 3 days in the hospital first if you want the nursing home paid for. [Flushed face emoji] Govt doesn’t always make sense. We’re listening to feedback. #RedTapeTales #TheBoldAndTheBureaucratic. August 4, 2019. Accessed April 17, 2020. https://twitter.com/SeemaCMS/status/1158029830056828928
2. COVID-19 Emergency Declaration Blanket Waivers for Health Care Providers. Centers for Medicare & Medicaid Services, US Dept of Health & Human Services; 2020. Accessed April 17, 2020. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf
3. Medicare & Medicaid Milestones, 1937 to 2015. Centers for Medicare and Medicaid Services, US Dept of Health & Human Services; 2015. Accessed April 17, 2020. https://www.cms.gov/About-CMS/Agency-Information/History/Downloads/Medicare-and-Medicaid-Milestones-1937-2015.pdf
4. Social Security Laws, 42 USC 1395x §1861 (1965). Accessed April 17, 2020. https://www.ssa.gov/OP_Home/ssact/title18/1861.htm
5. Loewenstein R. Early effects of Medicare on the health care of the aged. Social Security Bulletin. April 1971; pp 3-20, 42. Accessed April 14, 2020. https://www.ssa.gov/policy/docs/ssb/v34n4/v34n4p3.pdf
6. Weiss AJ, Elixhauser A. Overview of Hospital Stays in the United States, 2012. Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality, US Dept of Health & Human Services; 2014. Accessed April 16, 2020. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf
7. Medicare Benefits Policy Manual, Internet-Only Manuals. Centers for Medicare & Medicaid Services. Pub. 100-02, Chapter 6, § 20.6. Updated April 5, 2012. Accessed April 17, 2020. http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Internet-Only-Manuals-IOMs.html
8. Wright S. Hospitals’ Use of Observation Stays and Short Inpatient Stays for Medicare Beneficiaries. Office of the Inspector General, US Dept of Health & Human Services; 2014. Accessed April 16, 2020. https://oig.hhs.gov/oei/reports/oei-02-12-00040.asp
9. Yancy CW. COVID-19 and African Americans. JAMA. Published online April 15, 2020. https://doi.org/10.1001/jama.2020.6548
10. Levinson DR. Vulnerabilities Remain Under Medicare’s 2-Midnight Hospital Policy. Office of the Inspector General, US Dept of Health & Human Services; 2016. Accessed April 18, 2020. https://oig.hhs.gov/oei/reports/oei-02-15-00020.pdf
11. Levinson DR. CMS Improperly Paid Millions of Dollars for Skilled Nursing Facility Services When the Medicare 3-Day Inpatient Hospital Stay Requirement Was Not Met. Office of the Inspector General, US Dept of Health & Human Services; 2019. Accessed April 16, 2020. https://www.oig.hhs.gov/oas/reports/region5/51600043.pdf
12. Sheehy A, Shi F, Kind A. Identifying observation stays in Medicare data: policy implications of a definition. J Hosp Med. 2019;14(2):96-100. https://doi.org/10.12788/jhm.3038
13. Solutions to Reduce Fraud, Waste, and Abuse in HHS Programs: OIG’s Top Recommendations. Office of the Inspector General, US Dept of Health & Human Services; 2019. Accessed April 18, 2020. https://oig.hhs.gov/reports-and-publications/compendium/files/compendium2019.pdf
14. Improving Access to Medicare Coverage Act of 2019, HR 1682, 116th Congress (2019). Accessed April 16, 2020. https://www.congress.gov/bill/116th-congress/house-bill/1682
15. Alexander v Azar, 396 F Supp 3d 242 (D CT 2019). Accessed May 26, 2020. https://casetext.com/case/alexander-v-azar-1?
16. Lipsitz L. The 3-night hospital stay and Medicare coverage for skilled nursing care. JAMA. 2013;310(14):1441-1442. https://doi.org/10.1001/jama.2013.254845
17. Grebla R, Keohane L, Lee Y, Lipsitz L, Rahman M, Trevedi A. Waiving the three-day rule: admissions and length-of-stay at hospitals and skilled nursing facilities did not increase. Health Affairs (Millwood). 2015;34(8):1324-1330. https://doi.org/10.1377/hlthaff.2015.0054
1. @SeemaCMS. #Medicare beneficiary who requires skilled care in a nursing home? Better be admitted for at least 3 days in the hospital first if you want the nursing home paid for. [Flushed face emoji] Govt doesn’t always make sense. We’re listening to feedback. #RedTapeTales #TheBoldAndTheBureaucratic. August 4, 2019. Accessed April 17, 2020. https://twitter.com/SeemaCMS/status/1158029830056828928
2. COVID-19 Emergency Declaration Blanket Waivers for Health Care Providers. Centers for Medicare & Medicaid Services, US Dept of Health & Human Services; 2020. Accessed April 17, 2020. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf
3. Medicare & Medicaid Milestones, 1937 to 2015. Centers for Medicare and Medicaid Services, US Dept of Health & Human Services; 2015. Accessed April 17, 2020. https://www.cms.gov/About-CMS/Agency-Information/History/Downloads/Medicare-and-Medicaid-Milestones-1937-2015.pdf
4. Social Security Laws, 42 USC 1395x §1861 (1965). Accessed April 17, 2020. https://www.ssa.gov/OP_Home/ssact/title18/1861.htm
5. Loewenstein R. Early effects of Medicare on the health care of the aged. Social Security Bulletin. April 1971; pp 3-20, 42. Accessed April 14, 2020. https://www.ssa.gov/policy/docs/ssb/v34n4/v34n4p3.pdf
6. Weiss AJ, Elixhauser A. Overview of Hospital Stays in the United States, 2012. Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality, US Dept of Health & Human Services; 2014. Accessed April 16, 2020. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf
7. Medicare Benefits Policy Manual, Internet-Only Manuals. Centers for Medicare & Medicaid Services. Pub. 100-02, Chapter 6, § 20.6. Updated April 5, 2012. Accessed April 17, 2020. http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Internet-Only-Manuals-IOMs.html
8. Wright S. Hospitals’ Use of Observation Stays and Short Inpatient Stays for Medicare Beneficiaries. Office of the Inspector General, US Dept of Health & Human Services; 2014. Accessed April 16, 2020. https://oig.hhs.gov/oei/reports/oei-02-12-00040.asp
9. Yancy CW. COVID-19 and African Americans. JAMA. Published online April 15, 2020. https://doi.org/10.1001/jama.2020.6548
10. Levinson DR. Vulnerabilities Remain Under Medicare’s 2-Midnight Hospital Policy. Office of the Inspector General, US Dept of Health & Human Services; 2016. Accessed April 18, 2020. https://oig.hhs.gov/oei/reports/oei-02-15-00020.pdf
11. Levinson DR. CMS Improperly Paid Millions of Dollars for Skilled Nursing Facility Services When the Medicare 3-Day Inpatient Hospital Stay Requirement Was Not Met. Office of the Inspector General, US Dept of Health & Human Services; 2019. Accessed April 16, 2020. https://www.oig.hhs.gov/oas/reports/region5/51600043.pdf
12. Sheehy A, Shi F, Kind A. Identifying observation stays in Medicare data: policy implications of a definition. J Hosp Med. 2019;14(2):96-100. https://doi.org/10.12788/jhm.3038
13. Solutions to Reduce Fraud, Waste, and Abuse in HHS Programs: OIG’s Top Recommendations. Office of the Inspector General, US Dept of Health & Human Services; 2019. Accessed April 18, 2020. https://oig.hhs.gov/reports-and-publications/compendium/files/compendium2019.pdf
14. Improving Access to Medicare Coverage Act of 2019, HR 1682, 116th Congress (2019). Accessed April 16, 2020. https://www.congress.gov/bill/116th-congress/house-bill/1682
15. Alexander v Azar, 396 F Supp 3d 242 (D CT 2019). Accessed May 26, 2020. https://casetext.com/case/alexander-v-azar-1?
16. Lipsitz L. The 3-night hospital stay and Medicare coverage for skilled nursing care. JAMA. 2013;310(14):1441-1442. https://doi.org/10.1001/jama.2013.254845
17. Grebla R, Keohane L, Lee Y, Lipsitz L, Rahman M, Trevedi A. Waiving the three-day rule: admissions and length-of-stay at hospitals and skilled nursing facilities did not increase. Health Affairs (Millwood). 2015;34(8):1324-1330. https://doi.org/10.1377/hlthaff.2015.0054
© 2020 Society of Hospital Medicine
Effect of Systemic Glucocorticoids on Mortality or Mechanical Ventilation in Patients With COVID-19
Coronavirus disease 2019 (COVID-19) is the most important public health emergency of the 21st century. The pandemic has devastated New York City, where over 17,000 confirmed deaths have occurred as of June 5, 2020.1 The most common cause of death in COVID-19 patients is respiratory failure from acute respiratory distress syndrome (ARDS). A recent study reported high mortality rates among COVID-19 patients who received mechanical ventilation (MV).2
Glucocorticoids are useful as adjunctive treatment for some infections with inflammatory responses, but their efficacy in COVID-19 is unclear. Prior experience with influenza and other coronaviruses may be relevant. A recent meta-analysis of influenza pneumonia showed increased mortality and a higher rate of secondary infections in patients who were administered glucocorticoids.3 For Middle East respiratory syndrome, severe acute respiratory syndrome, and influenza, some studies have demonstrated an association between glucocorticoid use and delayed viral clearance.4-7 However, a recent retrospective series of patients with COVID-19 and ARDS demonstrated a decrease in mortality with glucocorticoid use.8 Glucocorticoids are easily obtained and familiar to providers caring for COVID-19 patients. Hence their empiric use is widespread.8,9
The primary goal of this study was to determine whether early glucocorticoid treatment is associated with reduced mortality or need for MV in COVID-19 patients.
METHODS
Study Setting and Overview
Montefiore Medical Center comprises four hospitals totaling 1,536 beds in the Bronx borough of New York, New York. Based upon early experience, some clinicians began prescribing systemic glucocorticoids to patients with COVID-19 while others did not. We leveraged this variation in practice to examine the effectiveness of glucocorticoids in reducing mortality and the rate of MV in hospitalized COVID-19 patients.
Study Populations
There were 2,998 patients admitted with a positive COVID-19 test between March 11, 2020, and April 13, 2020. An a priori decision was made to include all hospitalized COVID-19 patients, including children. Because the outcomes of in-hospital mortality and in-hospital MV cannot be assessed in patients still hospitalized, we included only patients who either died or had been discharged from the hospital. Patients who died or were placed on MV within the first 48 hours of admission were excluded because outcome events occurred before having the opportunity for glucocorticoid treatment. To ensure treatment preceded outcome measurement, we included only patients treated with glucocorticoids within the first 48 hours of admission (treatment group) and compared them with patients never treated with glucocorticoids (control group).
Outcomes and Independent Variables
The primary outcome was a composite of in-hospital mortality or in-hospital MV. Secondary outcomes were the components of the primary. Timing of MV was determined using the first documentation of a ventilator respiratory rate or tidal volume. The independent variable of interest was treatment with glucocorticoids within the first 48 hours of admission. Formulations included are described in the Appendix.
To compare treatment and control groups and to perform adjusted analyses, we also examined the demographic and clinical characteristics, comorbidities, and laboratory values of each admission. For the comparison of study populations, missing values for each variable were ignored. In the primary (unstratified) multivariable analysis, continuous variables were categorized, with missing values assumed to be normal when used as an adjustment variable. All variables extracted, number of missing values, candidates for inclusion in the multivariable analysis, and those that fell out of the model are presented in the Appendix. Several subgroup analyses were predefined including age, diabetes, admission glucose, C-reactive protein (CRP), D-dimer, and troponin T levels.
Statistical Analysis
The treated and control groups were compared with respect to demographics, clinical characteristics, comorbidities, and laboratory values. Primary and secondary outcomes in the groups were compared in unadjusted and adjusted analyses using univariable and multivariable logistic regression models. All patient characteristics that were candidates for inclusion in the adjustment models are listed in the Appendix. Variables were included in the final model if they were associated with the primary outcome (Wald test P < .20) in univariable regression. A sensitivity analysis excluded all variables missing greater than 10% of data, including CRP. Interactions between treatment and six predefined subgroups were tested using logistic regression with interaction terms (eg, [steroids]*[age]). Stratified logistic regression was used to test the association between treatment and the primary outcome in each of the predefined subgroups. Patients who were missing CRP were excluded from the stratified analysis. Because a significant interaction between treatment and initial CRP level was discovered, we undertook a post hoc adjusted analysis within each of the 15 predefined subgroup variables. Because there were fewer outcome events in each subgroup, we constructed a parsimonious logistic regression model that included all variables independently associated with the exposure (P < .05). The same seven adjustment variables were used in each of the predefined subgroups. The study was approved by the Albert Einstein College of Medicine Institutional Review Board. Stata 15.1 software (StataCorp) was used for data analysis.
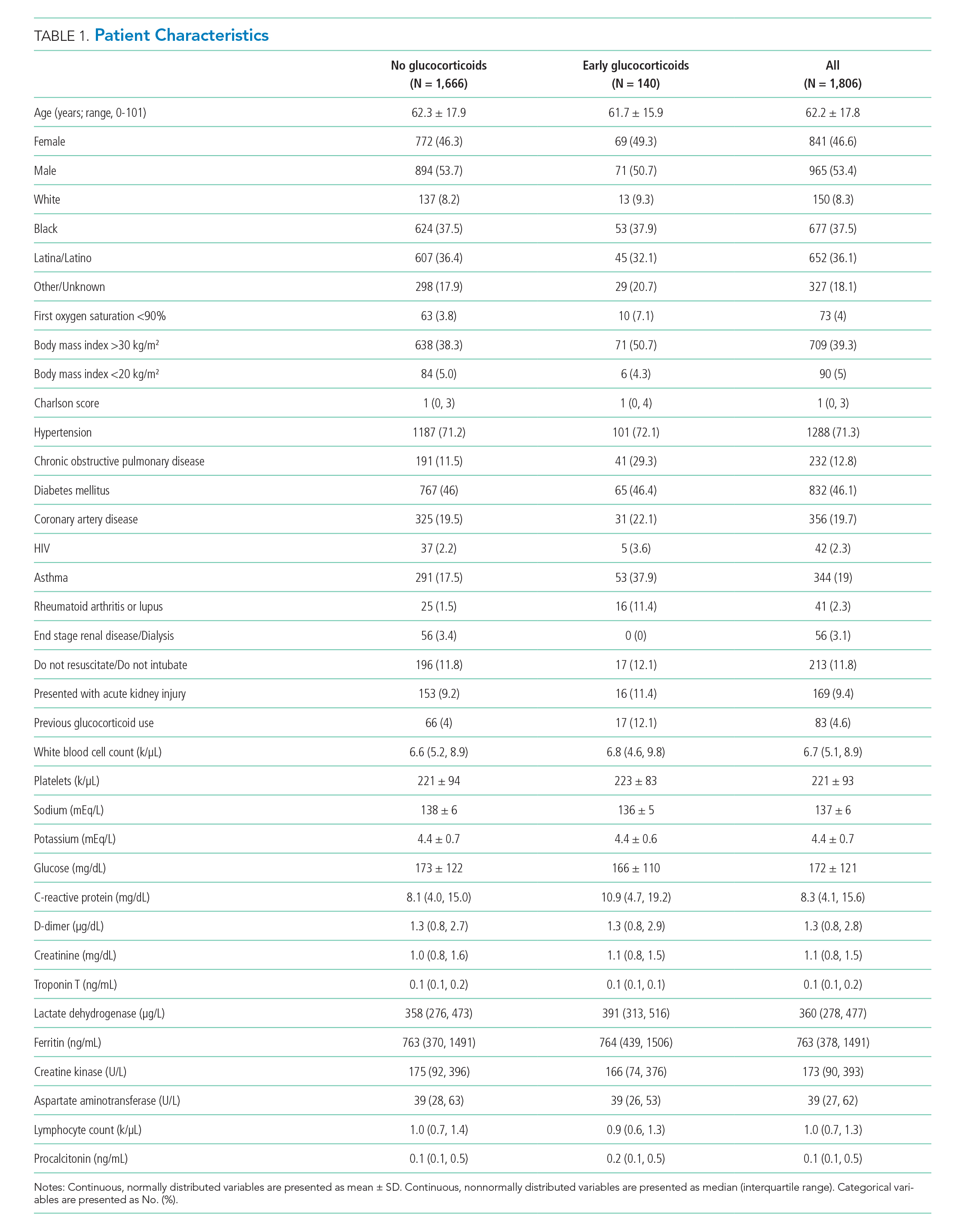
RESULTS
Of 2,998 patients examined, 1,806 met inclusion criteria and included 140 (7.7%) treated with glucocorticoids within 48 hours of admission and 1,666 who never received glucocorticoids. Reasons for exclusion of 1,192 patients are provided in the Appendix. Among patients who remained hospitalized and were excluded, 169 of 962 (17.6%) received glucocorticoids. Characteristics of the study population are presented in Table 1. Treatment and control groups were similar except that glucocorticoid-treated patients were more likely to have chronic obstructive pulmonary disease (COPD), asthma, rheumatoid arthritis or lupus, or to have received glucocorticoids in the year prior to admission.
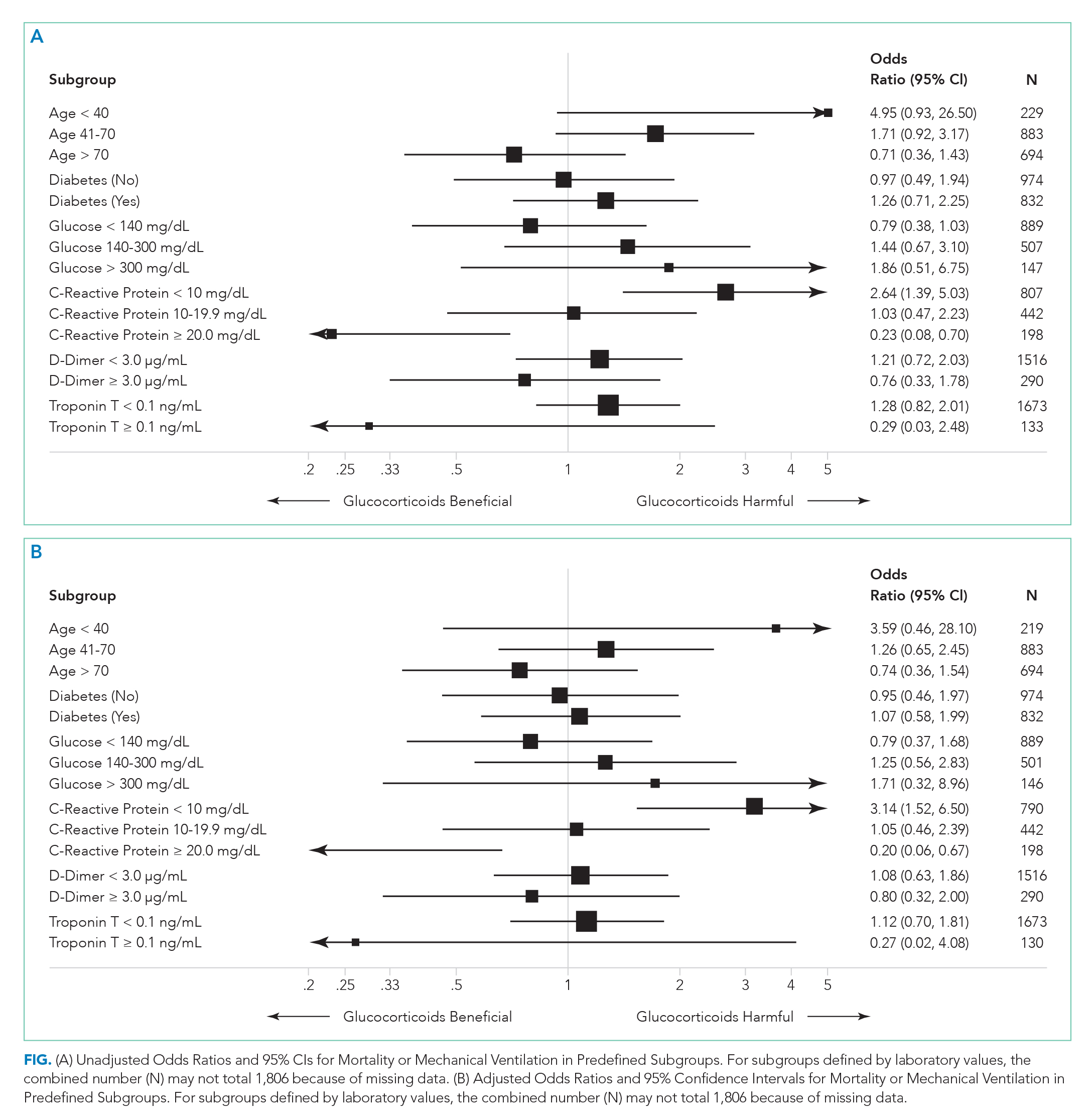
There were 318 who met the primary outcome of death or mechanical ventilation, 270 of whom died and 135 of whom required mechanical ventilation. Overall, early use of glucocorticoids was not associated with in-hospital mortality or MV as a composite outcome or as separate outcomes in both unadjusted and adjusted models (Table 2A). However, there was significant heterogeneity of treatment effect in the subgroups defined by CRP levels (P for interaction = .008; Figure). Early glucocorticoid use and an initial CRP of 20 mg/dL or higher was associated with a significantly reduced risk of mortality or MV in unadjusted (odds ratio, 0.23; 95% CI, 0.08-0.70) and adjusted (aOR, 0.20; 95% CI, 0.06-0.67) analyses (Table 2B). Conversely, glucocorticoid treatment in patients with CRP levels less than 10 mg/dL was associated with a significantly increased risk of mortality or MV in unadjusted (OR, 2.64; 95% CI, 1.39-5.03) and adjusted (aOR, 3.14; 95% CI, 1.52-6.50) analyses.
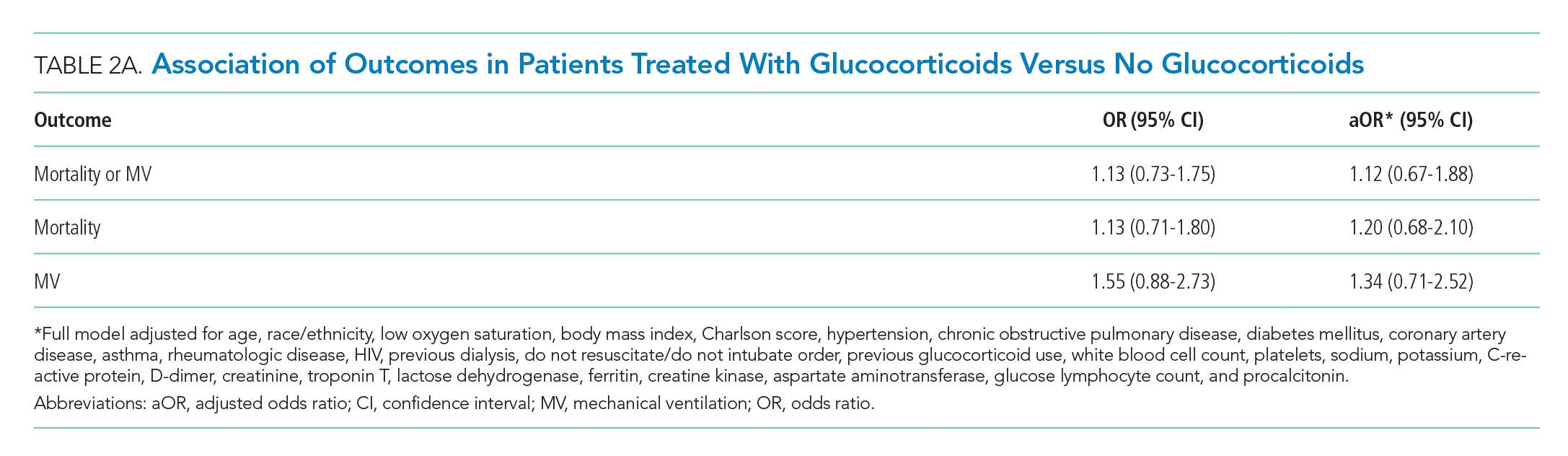
DISCUSSION
The results of this study indicate that early treatment with glucocorticoids is not associated with mortality or need for MV in unselected patients with COVID-19. Subgroup analyses suggest that glucocorticoid-treated patients with markedly elevated CRP may benefit from glucocorticoid treatment, whereas those patients with lower CRP may be harmed. Our findings were consistent after adjustment for clinical characteristics. The public health implications of these findings are hard to overestimate. Given the global growth of the pandemic and that glucocorticoids are widely available and inexpensive, glucocorticoid therapy may save many thousands of lives. Equally important because we have been able to identify a group that may be harmed, some patients may be saved because glucocorticoids will not be given.
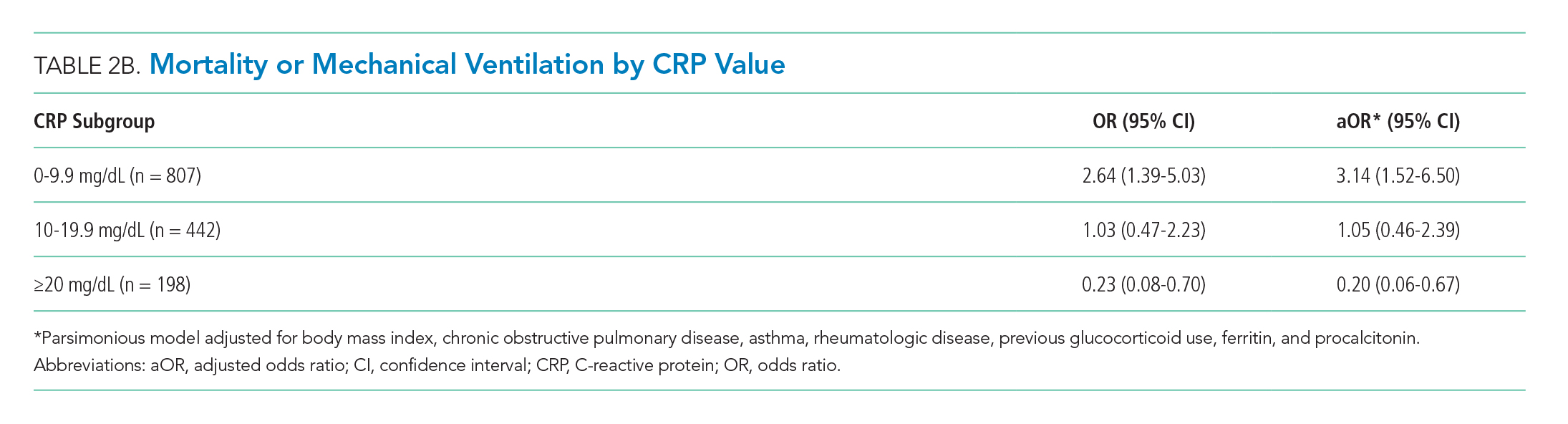
Our study reaffirms the finding of the as yet unpublished Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial that there is a subset of patients with COVID-19 who benefit from treatment with glucocorticoids.10 Our study extends the findings of the RECOVERY trial in two important ways. First, in addition to finding some patients who may benefit, we also have identified patient groups that may experience harm from treatment with glucocorticoids. This finding suggests choosing the right patients for glucocorticoid treatment is critical to maximize the likelihood of benefit and minimize the risk of harm. Second, we have identified patient groups who are likely to benefit (or be harmed) on the basis of a widely available lab test (CRP).
Our results are also consistent with previous studies of patients with SARS-CoV and MERS-CoV, in which no associations between glucocorticoid treatment and mortality were found.7 However, the results of studies examining the effect of glucocorticoids in patients with COVID-19 are less consistent.8,11,12
Few of the previous studies examined the effects of glucocorticoids in subgroups of patients. In our study, the improved outcomes associated with glucocorticoid use in patients with elevated CRPs is intriguing and may be clinically important. Proinflammatory cytokines, especially interleukin-6, acutely increase CRP levels. Cytokine storm syndrome (CSS) is a hyperinflammatory condition that occurs in a subset of COVID-19 patients, often resulting in multiorgan dysfunction.13 CRP is markedly elevated in CSS,14 and improved outcomes with glucocorticoid therapy in this subgroup may indicate benefit in this inflammatory phenotype. Patients with lower CRP are less likely to have CSS and may experience more harm than benefit associated with glucocorticoid treatment.
Several limitations are inherent to this study. Since it was done at a single center, the results may not be generalizable. As a retrospective analysis, it is subject to confounding and bias. In addition, because patients were included only if they had reached the outcome of death/MV or hospital discharge, the sample size was truncated. We believe glucocorticoid use in hospitalized patients excluded from the study reflects increased use with time because of a growing belief in their effectiveness.
Preliminary analysis from the RECOVERY study showed a reduced rate of mortality in patients randomized to dexamethasone, compared with those who received standard of care.10 These results led to the National Institutes for Health COVID-19 Treatment Guidelines Panel recommendation for dexamethasone treatment in patients with COVID-19 who require supplemental oxygen or MV.15 Our findings suggest a role for CRP to identify patients who may benefit from glucocorticoid therapy, as well as those in whom it may be harmful. Additional studies to further elucidate the role of CRP in guiding glucocorticoid therapy and to predict clinical response are needed.
1. COVID-19: Data. 2020. New York City Health. Accessed June 5, 2020. https://www1.nyc.gov/site/doh/covid/covid-19-data.page
2. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
3. Ni YN, Chen G, Sun J, Liang BM, Liang ZA. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care. 2019;23(1):99. https://doi.org/10.1186/s13054-019-2395-8
4. Arabi YM, Alothman A, Balkhy HH, et al. Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-beta1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19(1):81. https://doi.org/10.1186/s13063-017-2427-0
5. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304-309. https://doi.org/10.1016/j.jcv.2004.07.006
6. Lee N, Chan PK, Hui DS, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200(4):492-500. https://doi.org/10.1086/600383
7. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473-475. https://doi.org/10.1016/s0140-6736(20)30317-2
8. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. Published online March 13, 2020. https://doi.org/10.1001/jamainternmed.2020.0994
9. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032
10. Horby P, Lim WS, Emberson J, et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv. Preprint posted June 22, 2020. https://doi.org/10.1101/2020.06.22.20137273
11. Cao J, Tu WJ, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. Published online April 2, 2020. https://doi.org/10.1093/cid/ciaa243
12. Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5(1):57. https://doi.org/10.1038/s41392-020-0158-2
13. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620-2629. https://doi.org/10.1172/jci137244
14. McGonagle D, Sharif K, O’Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6):102537. https://doi.org/10.1016/j.autrev.2020.102537
15. The National Institutes of Health COVID-19 Treatment Guidelines Panel Provides Recommendations for Dexamethasone in Patients with COVID-19. National Institutes of Health. Updated June 25, 2020. Accessed June 25, 2020. https://www.covid19treatmentguidelines.nih.gov/dexamethasone/
Coronavirus disease 2019 (COVID-19) is the most important public health emergency of the 21st century. The pandemic has devastated New York City, where over 17,000 confirmed deaths have occurred as of June 5, 2020.1 The most common cause of death in COVID-19 patients is respiratory failure from acute respiratory distress syndrome (ARDS). A recent study reported high mortality rates among COVID-19 patients who received mechanical ventilation (MV).2
Glucocorticoids are useful as adjunctive treatment for some infections with inflammatory responses, but their efficacy in COVID-19 is unclear. Prior experience with influenza and other coronaviruses may be relevant. A recent meta-analysis of influenza pneumonia showed increased mortality and a higher rate of secondary infections in patients who were administered glucocorticoids.3 For Middle East respiratory syndrome, severe acute respiratory syndrome, and influenza, some studies have demonstrated an association between glucocorticoid use and delayed viral clearance.4-7 However, a recent retrospective series of patients with COVID-19 and ARDS demonstrated a decrease in mortality with glucocorticoid use.8 Glucocorticoids are easily obtained and familiar to providers caring for COVID-19 patients. Hence their empiric use is widespread.8,9
The primary goal of this study was to determine whether early glucocorticoid treatment is associated with reduced mortality or need for MV in COVID-19 patients.
METHODS
Study Setting and Overview
Montefiore Medical Center comprises four hospitals totaling 1,536 beds in the Bronx borough of New York, New York. Based upon early experience, some clinicians began prescribing systemic glucocorticoids to patients with COVID-19 while others did not. We leveraged this variation in practice to examine the effectiveness of glucocorticoids in reducing mortality and the rate of MV in hospitalized COVID-19 patients.
Study Populations
There were 2,998 patients admitted with a positive COVID-19 test between March 11, 2020, and April 13, 2020. An a priori decision was made to include all hospitalized COVID-19 patients, including children. Because the outcomes of in-hospital mortality and in-hospital MV cannot be assessed in patients still hospitalized, we included only patients who either died or had been discharged from the hospital. Patients who died or were placed on MV within the first 48 hours of admission were excluded because outcome events occurred before having the opportunity for glucocorticoid treatment. To ensure treatment preceded outcome measurement, we included only patients treated with glucocorticoids within the first 48 hours of admission (treatment group) and compared them with patients never treated with glucocorticoids (control group).
Outcomes and Independent Variables
The primary outcome was a composite of in-hospital mortality or in-hospital MV. Secondary outcomes were the components of the primary. Timing of MV was determined using the first documentation of a ventilator respiratory rate or tidal volume. The independent variable of interest was treatment with glucocorticoids within the first 48 hours of admission. Formulations included are described in the Appendix.
To compare treatment and control groups and to perform adjusted analyses, we also examined the demographic and clinical characteristics, comorbidities, and laboratory values of each admission. For the comparison of study populations, missing values for each variable were ignored. In the primary (unstratified) multivariable analysis, continuous variables were categorized, with missing values assumed to be normal when used as an adjustment variable. All variables extracted, number of missing values, candidates for inclusion in the multivariable analysis, and those that fell out of the model are presented in the Appendix. Several subgroup analyses were predefined including age, diabetes, admission glucose, C-reactive protein (CRP), D-dimer, and troponin T levels.
Statistical Analysis
The treated and control groups were compared with respect to demographics, clinical characteristics, comorbidities, and laboratory values. Primary and secondary outcomes in the groups were compared in unadjusted and adjusted analyses using univariable and multivariable logistic regression models. All patient characteristics that were candidates for inclusion in the adjustment models are listed in the Appendix. Variables were included in the final model if they were associated with the primary outcome (Wald test P < .20) in univariable regression. A sensitivity analysis excluded all variables missing greater than 10% of data, including CRP. Interactions between treatment and six predefined subgroups were tested using logistic regression with interaction terms (eg, [steroids]*[age]). Stratified logistic regression was used to test the association between treatment and the primary outcome in each of the predefined subgroups. Patients who were missing CRP were excluded from the stratified analysis. Because a significant interaction between treatment and initial CRP level was discovered, we undertook a post hoc adjusted analysis within each of the 15 predefined subgroup variables. Because there were fewer outcome events in each subgroup, we constructed a parsimonious logistic regression model that included all variables independently associated with the exposure (P < .05). The same seven adjustment variables were used in each of the predefined subgroups. The study was approved by the Albert Einstein College of Medicine Institutional Review Board. Stata 15.1 software (StataCorp) was used for data analysis.

RESULTS
Of 2,998 patients examined, 1,806 met inclusion criteria and included 140 (7.7%) treated with glucocorticoids within 48 hours of admission and 1,666 who never received glucocorticoids. Reasons for exclusion of 1,192 patients are provided in the Appendix. Among patients who remained hospitalized and were excluded, 169 of 962 (17.6%) received glucocorticoids. Characteristics of the study population are presented in Table 1. Treatment and control groups were similar except that glucocorticoid-treated patients were more likely to have chronic obstructive pulmonary disease (COPD), asthma, rheumatoid arthritis or lupus, or to have received glucocorticoids in the year prior to admission.

There were 318 who met the primary outcome of death or mechanical ventilation, 270 of whom died and 135 of whom required mechanical ventilation. Overall, early use of glucocorticoids was not associated with in-hospital mortality or MV as a composite outcome or as separate outcomes in both unadjusted and adjusted models (Table 2A). However, there was significant heterogeneity of treatment effect in the subgroups defined by CRP levels (P for interaction = .008; Figure). Early glucocorticoid use and an initial CRP of 20 mg/dL or higher was associated with a significantly reduced risk of mortality or MV in unadjusted (odds ratio, 0.23; 95% CI, 0.08-0.70) and adjusted (aOR, 0.20; 95% CI, 0.06-0.67) analyses (Table 2B). Conversely, glucocorticoid treatment in patients with CRP levels less than 10 mg/dL was associated with a significantly increased risk of mortality or MV in unadjusted (OR, 2.64; 95% CI, 1.39-5.03) and adjusted (aOR, 3.14; 95% CI, 1.52-6.50) analyses.

DISCUSSION
The results of this study indicate that early treatment with glucocorticoids is not associated with mortality or need for MV in unselected patients with COVID-19. Subgroup analyses suggest that glucocorticoid-treated patients with markedly elevated CRP may benefit from glucocorticoid treatment, whereas those patients with lower CRP may be harmed. Our findings were consistent after adjustment for clinical characteristics. The public health implications of these findings are hard to overestimate. Given the global growth of the pandemic and that glucocorticoids are widely available and inexpensive, glucocorticoid therapy may save many thousands of lives. Equally important because we have been able to identify a group that may be harmed, some patients may be saved because glucocorticoids will not be given.

Our study reaffirms the finding of the as yet unpublished Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial that there is a subset of patients with COVID-19 who benefit from treatment with glucocorticoids.10 Our study extends the findings of the RECOVERY trial in two important ways. First, in addition to finding some patients who may benefit, we also have identified patient groups that may experience harm from treatment with glucocorticoids. This finding suggests choosing the right patients for glucocorticoid treatment is critical to maximize the likelihood of benefit and minimize the risk of harm. Second, we have identified patient groups who are likely to benefit (or be harmed) on the basis of a widely available lab test (CRP).
Our results are also consistent with previous studies of patients with SARS-CoV and MERS-CoV, in which no associations between glucocorticoid treatment and mortality were found.7 However, the results of studies examining the effect of glucocorticoids in patients with COVID-19 are less consistent.8,11,12
Few of the previous studies examined the effects of glucocorticoids in subgroups of patients. In our study, the improved outcomes associated with glucocorticoid use in patients with elevated CRPs is intriguing and may be clinically important. Proinflammatory cytokines, especially interleukin-6, acutely increase CRP levels. Cytokine storm syndrome (CSS) is a hyperinflammatory condition that occurs in a subset of COVID-19 patients, often resulting in multiorgan dysfunction.13 CRP is markedly elevated in CSS,14 and improved outcomes with glucocorticoid therapy in this subgroup may indicate benefit in this inflammatory phenotype. Patients with lower CRP are less likely to have CSS and may experience more harm than benefit associated with glucocorticoid treatment.
Several limitations are inherent to this study. Since it was done at a single center, the results may not be generalizable. As a retrospective analysis, it is subject to confounding and bias. In addition, because patients were included only if they had reached the outcome of death/MV or hospital discharge, the sample size was truncated. We believe glucocorticoid use in hospitalized patients excluded from the study reflects increased use with time because of a growing belief in their effectiveness.
Preliminary analysis from the RECOVERY study showed a reduced rate of mortality in patients randomized to dexamethasone, compared with those who received standard of care.10 These results led to the National Institutes for Health COVID-19 Treatment Guidelines Panel recommendation for dexamethasone treatment in patients with COVID-19 who require supplemental oxygen or MV.15 Our findings suggest a role for CRP to identify patients who may benefit from glucocorticoid therapy, as well as those in whom it may be harmful. Additional studies to further elucidate the role of CRP in guiding glucocorticoid therapy and to predict clinical response are needed.
Coronavirus disease 2019 (COVID-19) is the most important public health emergency of the 21st century. The pandemic has devastated New York City, where over 17,000 confirmed deaths have occurred as of June 5, 2020.1 The most common cause of death in COVID-19 patients is respiratory failure from acute respiratory distress syndrome (ARDS). A recent study reported high mortality rates among COVID-19 patients who received mechanical ventilation (MV).2
Glucocorticoids are useful as adjunctive treatment for some infections with inflammatory responses, but their efficacy in COVID-19 is unclear. Prior experience with influenza and other coronaviruses may be relevant. A recent meta-analysis of influenza pneumonia showed increased mortality and a higher rate of secondary infections in patients who were administered glucocorticoids.3 For Middle East respiratory syndrome, severe acute respiratory syndrome, and influenza, some studies have demonstrated an association between glucocorticoid use and delayed viral clearance.4-7 However, a recent retrospective series of patients with COVID-19 and ARDS demonstrated a decrease in mortality with glucocorticoid use.8 Glucocorticoids are easily obtained and familiar to providers caring for COVID-19 patients. Hence their empiric use is widespread.8,9
The primary goal of this study was to determine whether early glucocorticoid treatment is associated with reduced mortality or need for MV in COVID-19 patients.
METHODS
Study Setting and Overview
Montefiore Medical Center comprises four hospitals totaling 1,536 beds in the Bronx borough of New York, New York. Based upon early experience, some clinicians began prescribing systemic glucocorticoids to patients with COVID-19 while others did not. We leveraged this variation in practice to examine the effectiveness of glucocorticoids in reducing mortality and the rate of MV in hospitalized COVID-19 patients.
Study Populations
There were 2,998 patients admitted with a positive COVID-19 test between March 11, 2020, and April 13, 2020. An a priori decision was made to include all hospitalized COVID-19 patients, including children. Because the outcomes of in-hospital mortality and in-hospital MV cannot be assessed in patients still hospitalized, we included only patients who either died or had been discharged from the hospital. Patients who died or were placed on MV within the first 48 hours of admission were excluded because outcome events occurred before having the opportunity for glucocorticoid treatment. To ensure treatment preceded outcome measurement, we included only patients treated with glucocorticoids within the first 48 hours of admission (treatment group) and compared them with patients never treated with glucocorticoids (control group).
Outcomes and Independent Variables
The primary outcome was a composite of in-hospital mortality or in-hospital MV. Secondary outcomes were the components of the primary. Timing of MV was determined using the first documentation of a ventilator respiratory rate or tidal volume. The independent variable of interest was treatment with glucocorticoids within the first 48 hours of admission. Formulations included are described in the Appendix.
To compare treatment and control groups and to perform adjusted analyses, we also examined the demographic and clinical characteristics, comorbidities, and laboratory values of each admission. For the comparison of study populations, missing values for each variable were ignored. In the primary (unstratified) multivariable analysis, continuous variables were categorized, with missing values assumed to be normal when used as an adjustment variable. All variables extracted, number of missing values, candidates for inclusion in the multivariable analysis, and those that fell out of the model are presented in the Appendix. Several subgroup analyses were predefined including age, diabetes, admission glucose, C-reactive protein (CRP), D-dimer, and troponin T levels.
Statistical Analysis
The treated and control groups were compared with respect to demographics, clinical characteristics, comorbidities, and laboratory values. Primary and secondary outcomes in the groups were compared in unadjusted and adjusted analyses using univariable and multivariable logistic regression models. All patient characteristics that were candidates for inclusion in the adjustment models are listed in the Appendix. Variables were included in the final model if they were associated with the primary outcome (Wald test P < .20) in univariable regression. A sensitivity analysis excluded all variables missing greater than 10% of data, including CRP. Interactions between treatment and six predefined subgroups were tested using logistic regression with interaction terms (eg, [steroids]*[age]). Stratified logistic regression was used to test the association between treatment and the primary outcome in each of the predefined subgroups. Patients who were missing CRP were excluded from the stratified analysis. Because a significant interaction between treatment and initial CRP level was discovered, we undertook a post hoc adjusted analysis within each of the 15 predefined subgroup variables. Because there were fewer outcome events in each subgroup, we constructed a parsimonious logistic regression model that included all variables independently associated with the exposure (P < .05). The same seven adjustment variables were used in each of the predefined subgroups. The study was approved by the Albert Einstein College of Medicine Institutional Review Board. Stata 15.1 software (StataCorp) was used for data analysis.

RESULTS
Of 2,998 patients examined, 1,806 met inclusion criteria and included 140 (7.7%) treated with glucocorticoids within 48 hours of admission and 1,666 who never received glucocorticoids. Reasons for exclusion of 1,192 patients are provided in the Appendix. Among patients who remained hospitalized and were excluded, 169 of 962 (17.6%) received glucocorticoids. Characteristics of the study population are presented in Table 1. Treatment and control groups were similar except that glucocorticoid-treated patients were more likely to have chronic obstructive pulmonary disease (COPD), asthma, rheumatoid arthritis or lupus, or to have received glucocorticoids in the year prior to admission.

There were 318 who met the primary outcome of death or mechanical ventilation, 270 of whom died and 135 of whom required mechanical ventilation. Overall, early use of glucocorticoids was not associated with in-hospital mortality or MV as a composite outcome or as separate outcomes in both unadjusted and adjusted models (Table 2A). However, there was significant heterogeneity of treatment effect in the subgroups defined by CRP levels (P for interaction = .008; Figure). Early glucocorticoid use and an initial CRP of 20 mg/dL or higher was associated with a significantly reduced risk of mortality or MV in unadjusted (odds ratio, 0.23; 95% CI, 0.08-0.70) and adjusted (aOR, 0.20; 95% CI, 0.06-0.67) analyses (Table 2B). Conversely, glucocorticoid treatment in patients with CRP levels less than 10 mg/dL was associated with a significantly increased risk of mortality or MV in unadjusted (OR, 2.64; 95% CI, 1.39-5.03) and adjusted (aOR, 3.14; 95% CI, 1.52-6.50) analyses.

DISCUSSION
The results of this study indicate that early treatment with glucocorticoids is not associated with mortality or need for MV in unselected patients with COVID-19. Subgroup analyses suggest that glucocorticoid-treated patients with markedly elevated CRP may benefit from glucocorticoid treatment, whereas those patients with lower CRP may be harmed. Our findings were consistent after adjustment for clinical characteristics. The public health implications of these findings are hard to overestimate. Given the global growth of the pandemic and that glucocorticoids are widely available and inexpensive, glucocorticoid therapy may save many thousands of lives. Equally important because we have been able to identify a group that may be harmed, some patients may be saved because glucocorticoids will not be given.

Our study reaffirms the finding of the as yet unpublished Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial that there is a subset of patients with COVID-19 who benefit from treatment with glucocorticoids.10 Our study extends the findings of the RECOVERY trial in two important ways. First, in addition to finding some patients who may benefit, we also have identified patient groups that may experience harm from treatment with glucocorticoids. This finding suggests choosing the right patients for glucocorticoid treatment is critical to maximize the likelihood of benefit and minimize the risk of harm. Second, we have identified patient groups who are likely to benefit (or be harmed) on the basis of a widely available lab test (CRP).
Our results are also consistent with previous studies of patients with SARS-CoV and MERS-CoV, in which no associations between glucocorticoid treatment and mortality were found.7 However, the results of studies examining the effect of glucocorticoids in patients with COVID-19 are less consistent.8,11,12
Few of the previous studies examined the effects of glucocorticoids in subgroups of patients. In our study, the improved outcomes associated with glucocorticoid use in patients with elevated CRPs is intriguing and may be clinically important. Proinflammatory cytokines, especially interleukin-6, acutely increase CRP levels. Cytokine storm syndrome (CSS) is a hyperinflammatory condition that occurs in a subset of COVID-19 patients, often resulting in multiorgan dysfunction.13 CRP is markedly elevated in CSS,14 and improved outcomes with glucocorticoid therapy in this subgroup may indicate benefit in this inflammatory phenotype. Patients with lower CRP are less likely to have CSS and may experience more harm than benefit associated with glucocorticoid treatment.
Several limitations are inherent to this study. Since it was done at a single center, the results may not be generalizable. As a retrospective analysis, it is subject to confounding and bias. In addition, because patients were included only if they had reached the outcome of death/MV or hospital discharge, the sample size was truncated. We believe glucocorticoid use in hospitalized patients excluded from the study reflects increased use with time because of a growing belief in their effectiveness.
Preliminary analysis from the RECOVERY study showed a reduced rate of mortality in patients randomized to dexamethasone, compared with those who received standard of care.10 These results led to the National Institutes for Health COVID-19 Treatment Guidelines Panel recommendation for dexamethasone treatment in patients with COVID-19 who require supplemental oxygen or MV.15 Our findings suggest a role for CRP to identify patients who may benefit from glucocorticoid therapy, as well as those in whom it may be harmful. Additional studies to further elucidate the role of CRP in guiding glucocorticoid therapy and to predict clinical response are needed.
1. COVID-19: Data. 2020. New York City Health. Accessed June 5, 2020. https://www1.nyc.gov/site/doh/covid/covid-19-data.page
2. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
3. Ni YN, Chen G, Sun J, Liang BM, Liang ZA. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care. 2019;23(1):99. https://doi.org/10.1186/s13054-019-2395-8
4. Arabi YM, Alothman A, Balkhy HH, et al. Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-beta1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19(1):81. https://doi.org/10.1186/s13063-017-2427-0
5. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304-309. https://doi.org/10.1016/j.jcv.2004.07.006
6. Lee N, Chan PK, Hui DS, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200(4):492-500. https://doi.org/10.1086/600383
7. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473-475. https://doi.org/10.1016/s0140-6736(20)30317-2
8. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. Published online March 13, 2020. https://doi.org/10.1001/jamainternmed.2020.0994
9. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032
10. Horby P, Lim WS, Emberson J, et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv. Preprint posted June 22, 2020. https://doi.org/10.1101/2020.06.22.20137273
11. Cao J, Tu WJ, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. Published online April 2, 2020. https://doi.org/10.1093/cid/ciaa243
12. Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5(1):57. https://doi.org/10.1038/s41392-020-0158-2
13. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620-2629. https://doi.org/10.1172/jci137244
14. McGonagle D, Sharif K, O’Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6):102537. https://doi.org/10.1016/j.autrev.2020.102537
15. The National Institutes of Health COVID-19 Treatment Guidelines Panel Provides Recommendations for Dexamethasone in Patients with COVID-19. National Institutes of Health. Updated June 25, 2020. Accessed June 25, 2020. https://www.covid19treatmentguidelines.nih.gov/dexamethasone/
1. COVID-19: Data. 2020. New York City Health. Accessed June 5, 2020. https://www1.nyc.gov/site/doh/covid/covid-19-data.page
2. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
3. Ni YN, Chen G, Sun J, Liang BM, Liang ZA. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care. 2019;23(1):99. https://doi.org/10.1186/s13054-019-2395-8
4. Arabi YM, Alothman A, Balkhy HH, et al. Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-beta1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19(1):81. https://doi.org/10.1186/s13063-017-2427-0
5. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304-309. https://doi.org/10.1016/j.jcv.2004.07.006
6. Lee N, Chan PK, Hui DS, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200(4):492-500. https://doi.org/10.1086/600383
7. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473-475. https://doi.org/10.1016/s0140-6736(20)30317-2
8. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. Published online March 13, 2020. https://doi.org/10.1001/jamainternmed.2020.0994
9. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032
10. Horby P, Lim WS, Emberson J, et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv. Preprint posted June 22, 2020. https://doi.org/10.1101/2020.06.22.20137273
11. Cao J, Tu WJ, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. Published online April 2, 2020. https://doi.org/10.1093/cid/ciaa243
12. Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5(1):57. https://doi.org/10.1038/s41392-020-0158-2
13. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620-2629. https://doi.org/10.1172/jci137244
14. McGonagle D, Sharif K, O’Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6):102537. https://doi.org/10.1016/j.autrev.2020.102537
15. The National Institutes of Health COVID-19 Treatment Guidelines Panel Provides Recommendations for Dexamethasone in Patients with COVID-19. National Institutes of Health. Updated June 25, 2020. Accessed June 25, 2020. https://www.covid19treatmentguidelines.nih.gov/dexamethasone/
© 2020 Society of Hospital Medicine
Immigrant Physicians Fill a Critical Need in COVID-19 Response
Immigrant physicians and international medical graduates (IMGs) have for decades been very important to the healthcare delivery in the United States. For many currently serving on the front lines, the path has been full of challenges and uncertainties, now acutely worsened by the pandemic at hand. Manpreet Malik, MD, is one of those hospitalists. He grew up in a small city in India. He completed medical school in South India where he met students from all over the world and learned to speak a new language to serve local patients. The multicultural experience inspired him to pursue residency in the United States. Manpreet obtained a J-1 visa for residency and subsequently applied for a J-1 waiver for his first hospitalist job in 2013. Then his employer, a nonprofit organization, applied for H-1B and permanent resident status. He continues on an H-1B status but awaits his green card 7 years later. His wife, a dentist, is also an H-1B visa holder and they have two children. While they have assimilated into American society and flourished professionally, a sense of security eludes them. The COVID-19 pandemic has amplified this for their family. Like many other families, they are both in high-risk occupations and worry about the future, including what would happen if either or both of them contracted the virus. Their carefully planned life feels like a wobbly house of cards.
Immigrant healthcare workers are on the front lines in the fight against COVID-19 in the United States, accounting for 16.4% of healthcare workers amid this pandemic.1 Of physicians in the United States, 29% are not born in the United States,and of the practicing hospitalists, 32% are IMGs.1,2 IMGs are physicians who have graduated from medical schools outside of the United States and Canada who lack accreditation by the Liaison Committee on Medical Education.3 IMGs are a heterogeneous group with widely varying cultural, educational, and linguistic backgrounds with around 12,000 IMGs applying yearly for US residency positions.4 IMG hospitalists are uniquely positioned at the front lines facing arguably more risks with less recognition.5 The top five countries sending physicians to the United States are India, China, the Philippines, South Korea, and Pakistan.6 Yet many of these doctors—more than a third of those practicing in this country who graduated from international medical schools—have visa restrictions that limit their ability to work in communities with the greatest need.7 Another group of approximately 65,000 IMGs currently living in the United States are not licensed; they have not passed the board exam because they haven’t matched into a residency program to be eligible to take it.8 Many are working other jobs such as medical research, even though they could be deployed to serve as scribes or work in triage via telemedicine if their visas permitted.
During the COVID-19 pandemic, immigrant doctors are putting their lives on the line daily to care for patients. Immigrant doctors on visas are not eligible for Medicaid or Social Security benefits. Further, their partners and children are often dependent on them for legal resident status in the United States because of employer-based visa sponsorship. As the primary visa holder, if a non–US-born physician in the United States gets severely ill while fighting the virus, or gets disabled, they may have no benefits to fall back on. These physicians have houses, families, and children who are American citizens, and they are contributing members of society. Physicians on visas pay taxes the same way US citizens do. If their health or employment is jeopardized, their families would be unable to stay in the US legally, becoming undocumented and risking deportation. These physicians, who are fighting COVID-19 today, are helpless to provide a stable structure for their own loved ones.
With the COVID-19 pandemic unfolding, there is a risk of more physician shortages. The US healthcare workforce relies on immigrant physicians to help provide high-quality and accessible patient care. There are challenges for IMGs for getting into residency programs, and this limits the potential workforce during COVID-19. This year, according to the National Resident Matching Program, 4,222 non–US-born IMGs are due to start their US residency training on July 1.9 These doctors have the opportunity to serve across the country during this pandemic. According to data from the matching program, IMGs make up a large proportion of the workforce, obtaining 23% of the total number of US residency positions filled, and are in many leading academic institutions. These doctors, many of whom are waiting for their visas to be processed, need to be admitted in order to provide the care that Americans need during this pandemic. A similar number of IMGs will be completing their specialty training and are due to become attending physicians in their chosen field, including areas with critical shortages in this pandemic, such as critical care medicine. These skilled physicians depend on the processing of visa extensions or green cards in order to remain in the United States. Subspecialties like internal medicine and family medicine have a large proportion of actively practicing IMGs,7 and therefore provide primary care and inpatient care across the nation, especially in underserved areas. However, the geographic location of their practice is limited to the place that sponsored their visa. So a physician in rural Minnesota, where the outbreak of COVID-19 is not severe, cannot travel to hot spots such as New York or Detroit to provide care, even if they have a desire to serve.
For IMGs, the process of obtaining legal status in the US and pertinent immigration policies includes utilizing the H-1B visa program for highly skilled workers10 or J-1 visas for residencies.11 H-1B visas are usually granted for sponsored positions in underserved or rural areas for at least 3 years, and the healthcare sector must compete with other industries, such as tech, engineering, and other specialty occupations. Physicians working on H-1B visas may apply for permanent work permits, though there is an annual cap for each country and candidates may wait decades to receive one. As a J-1 visa (cultural exchange program) holder, physicians are required to practice in their home country for 2 years prior to working again in the United States. This requirement could be waived by turning to the Conrad 30 Waiver Program12 or J-1 waivers if they agreed to work in an underserved area in the United States. A limited number of J-1 waivers for each state are dispensed on a first-come, first-served basis (30 IMGs per state per year). This program currently is only authorized through the end of 2020, although legislation has been introduced to extend it, which could expand the slots.13 Applying for a J-1 waiver thus becomes a race against time with high-stakes suspense and anxiety for many IMGs. Most, regardless of visa status, dream of a stable and secure life, with permanent resident status as they serve their communities. For some, however, the endgame could mean deportation and the premature demise of dreams.
Permanent resident status is allotted by country, and there is a long wait for green cards. Three-quarters of skilled workers waiting for green cards are from India. That translates to more than 700,000 people, of which approximately 200,000 are expected to die of old age before being granted green cards.14,15 In the meantime, while they live with restrictions on both their employment and mobility, many physicians are doing essential medical work in underserved and rural areas throughout the United States.
We urge immigration reform to increase the physician workforce by providing immigrant doctors and IMGs with more flexibility to travel to areas where they are needed the most during this pandemic. There should be a blanket extension of visa deadlines. IMGs on J-1 student visas and H-1B specialty work visas should be exempt from any future immigration bans or limitations during the COVID-19 pandemic. The time is right for accelerating permanent resident status for these highly skilled IMGs. Green cards soon after finishing residency or fellowship training or satisfying a condition of initial visa approval should be the norm instead of a stressful unending wait. Clinicians who serve in underserved communities should be incentivized, and this should include health benefits. Restrictions related to primary and secondary work sites, as well as number of J-1 waivers, should also be relaxed. This flexibility would allow immigrant physicians to care at a variety of locations or by means of telemedicine.
A physician’s role is to heal and to serve their patients, regardless of their own origin. We are the voices of America’s immigrant physicians, particularly hospitalists, serving as frontline workers in our nation’s response to the COVID-19 crisis. The battle against COVID-19 has strained many of our resources, including the need for physicians. Uncertainty and chaos reign professionally and personally for many healthcare workers across America, and more challenges lie ahead for the foreseeable future. Healthcare workers are the unselfish and unwavering wall that stands between COVID-19 and more lives lost in our country. Every effort should be made to preserve and strengthen the healthcare workforce. Immigrant hospitalists, shackled by visa restrictions, could play an even bigger role if their obstacles were removed. It is time to provide them with the sense of security they deserve and rebuild the house of cards into something with a stronger foundation and more stability for our future.
1. New American Economy Research Fund. Immigration and Covid-19. March 26, 2020. Accessed May 5, 2020. https://research.newamericaneconomy.org/report/immigration-and-covid-19/
2. Compensation and Career Survey. Today’s Hospitalist. November 1, 2008. Accessed May 29, 2020. https://www.todayshospitalist.com/survey/16_salary_survey/index.php
3. Rao NR. “A little more than kin, and less than kind”: US immigration policy on international medical graduates. Virtual Mentor. 2012;14(4):329-337. https://doi.org/10.1001/virtualmentor.2012.14.4.pfor1-1204
4. ECFMG Fact Card: Summary Data Related to ECFMG Certification. Educational Commission for Foreign Medical Graduates (ECFMG). March 20, 2019. Accessed April 22, 2020. https://www.ecfmg.org/forms/factcard.pdf
5. Compensation and Career Survey. Today’s Hospitalist. November 1, 2016. Accessed May 29, 2020. https://www.todayshospitalist.com/survey/08_salary_survey/index.php
6. Harker YS. In rural towns, immigrant doctors fill a critical need. Health Affairs. 2018;37(1):161-164. https://doi.org/10.1377/hlthaff.2017.1094
7. Ahmed AA, Hwang WT, Thomas CR Jr, Deville C Jr. International medical graduates in the US physician workforce and graduate medical education: current and historical trends. J Grad Med Educ. 2018;10(2):214‐218. https://doi.org/10.4300/jgme-d-17-00580.1
8. Peters J. Highly trained and educated, some foreign-born doctors still can’t practice medicine in the US. Public Radio International. March 28, 2018. Accessed April 22, 2020. https://www.pri.org/stories/2018-03-26/highly-trained-and-educated-some-foreign-born-doctors-still-can-t-practice
9. Results and Data: 2020 Main Residency Match. National Resident Matching Program. 2020. Accessed May 15, 2020. http://www.nrmp.org/main-residency-match-data/
10. H-1B Specialty Occupations, DOD Cooperative Research and Development Project Workers, and Fashion Models. U.S. Citizenship and Immigration Services. March 27, 2020. Accessed April 22, 2020. https://www.uscis.gov/working-united-states/temporary-workers/h-1b-specialty-occupations-dod-cooperative-research-and-development-project-workers-and-fashion-models
11. J-1 Visa Sponsorship Fact Sheet. Educational Commission for Foreign Medical Graduates (ECFMG). May 2017. Accessed April 22, 2020. https://www.ecfmg.org/evsp/j1fact.pdf
12. Conrad 30 Waiver Program. U.S. Citizenship and Immigration Services. August 25, 2011. Accessed April 22, 2020. https://www.uscis.gov/working-united-states/students-and-exchange-visitors/conrad-30-waiver-program
13. Conrad State 30 and Physician Access Reauthorization Act, S 948, 116th Congress (2019). Accessed April 22, 2020. https://www.congress.gov/bill/116thcongress/senate-bill/948/text
14. Bhattacharya A. For over 200,000 Indians, the wait for a green card is longer than their lifetimes. Quartz India. March 31, 2020. Accessed April 22, 2020. https://qz.com/india/1828970/over-200000-indians-could-die-waiting-for-a-us-green-card/
15. Bier DJ. Immigration Research and Policy Brief: Backlog for Skilled Immigrants Tops 1 Million: Over 200,000 Indians Could Die of Old Age While Awaiting Green Cards. Cato Institute: Immigration Research and Policy Brief, No. 18. March 30, 2020. Accessed April 26, 2020. https://www.cato.org/sites/cato.org/files/2020-03/irpb-18-updated.pdf
Immigrant physicians and international medical graduates (IMGs) have for decades been very important to the healthcare delivery in the United States. For many currently serving on the front lines, the path has been full of challenges and uncertainties, now acutely worsened by the pandemic at hand. Manpreet Malik, MD, is one of those hospitalists. He grew up in a small city in India. He completed medical school in South India where he met students from all over the world and learned to speak a new language to serve local patients. The multicultural experience inspired him to pursue residency in the United States. Manpreet obtained a J-1 visa for residency and subsequently applied for a J-1 waiver for his first hospitalist job in 2013. Then his employer, a nonprofit organization, applied for H-1B and permanent resident status. He continues on an H-1B status but awaits his green card 7 years later. His wife, a dentist, is also an H-1B visa holder and they have two children. While they have assimilated into American society and flourished professionally, a sense of security eludes them. The COVID-19 pandemic has amplified this for their family. Like many other families, they are both in high-risk occupations and worry about the future, including what would happen if either or both of them contracted the virus. Their carefully planned life feels like a wobbly house of cards.
Immigrant healthcare workers are on the front lines in the fight against COVID-19 in the United States, accounting for 16.4% of healthcare workers amid this pandemic.1 Of physicians in the United States, 29% are not born in the United States,and of the practicing hospitalists, 32% are IMGs.1,2 IMGs are physicians who have graduated from medical schools outside of the United States and Canada who lack accreditation by the Liaison Committee on Medical Education.3 IMGs are a heterogeneous group with widely varying cultural, educational, and linguistic backgrounds with around 12,000 IMGs applying yearly for US residency positions.4 IMG hospitalists are uniquely positioned at the front lines facing arguably more risks with less recognition.5 The top five countries sending physicians to the United States are India, China, the Philippines, South Korea, and Pakistan.6 Yet many of these doctors—more than a third of those practicing in this country who graduated from international medical schools—have visa restrictions that limit their ability to work in communities with the greatest need.7 Another group of approximately 65,000 IMGs currently living in the United States are not licensed; they have not passed the board exam because they haven’t matched into a residency program to be eligible to take it.8 Many are working other jobs such as medical research, even though they could be deployed to serve as scribes or work in triage via telemedicine if their visas permitted.
During the COVID-19 pandemic, immigrant doctors are putting their lives on the line daily to care for patients. Immigrant doctors on visas are not eligible for Medicaid or Social Security benefits. Further, their partners and children are often dependent on them for legal resident status in the United States because of employer-based visa sponsorship. As the primary visa holder, if a non–US-born physician in the United States gets severely ill while fighting the virus, or gets disabled, they may have no benefits to fall back on. These physicians have houses, families, and children who are American citizens, and they are contributing members of society. Physicians on visas pay taxes the same way US citizens do. If their health or employment is jeopardized, their families would be unable to stay in the US legally, becoming undocumented and risking deportation. These physicians, who are fighting COVID-19 today, are helpless to provide a stable structure for their own loved ones.
With the COVID-19 pandemic unfolding, there is a risk of more physician shortages. The US healthcare workforce relies on immigrant physicians to help provide high-quality and accessible patient care. There are challenges for IMGs for getting into residency programs, and this limits the potential workforce during COVID-19. This year, according to the National Resident Matching Program, 4,222 non–US-born IMGs are due to start their US residency training on July 1.9 These doctors have the opportunity to serve across the country during this pandemic. According to data from the matching program, IMGs make up a large proportion of the workforce, obtaining 23% of the total number of US residency positions filled, and are in many leading academic institutions. These doctors, many of whom are waiting for their visas to be processed, need to be admitted in order to provide the care that Americans need during this pandemic. A similar number of IMGs will be completing their specialty training and are due to become attending physicians in their chosen field, including areas with critical shortages in this pandemic, such as critical care medicine. These skilled physicians depend on the processing of visa extensions or green cards in order to remain in the United States. Subspecialties like internal medicine and family medicine have a large proportion of actively practicing IMGs,7 and therefore provide primary care and inpatient care across the nation, especially in underserved areas. However, the geographic location of their practice is limited to the place that sponsored their visa. So a physician in rural Minnesota, where the outbreak of COVID-19 is not severe, cannot travel to hot spots such as New York or Detroit to provide care, even if they have a desire to serve.
For IMGs, the process of obtaining legal status in the US and pertinent immigration policies includes utilizing the H-1B visa program for highly skilled workers10 or J-1 visas for residencies.11 H-1B visas are usually granted for sponsored positions in underserved or rural areas for at least 3 years, and the healthcare sector must compete with other industries, such as tech, engineering, and other specialty occupations. Physicians working on H-1B visas may apply for permanent work permits, though there is an annual cap for each country and candidates may wait decades to receive one. As a J-1 visa (cultural exchange program) holder, physicians are required to practice in their home country for 2 years prior to working again in the United States. This requirement could be waived by turning to the Conrad 30 Waiver Program12 or J-1 waivers if they agreed to work in an underserved area in the United States. A limited number of J-1 waivers for each state are dispensed on a first-come, first-served basis (30 IMGs per state per year). This program currently is only authorized through the end of 2020, although legislation has been introduced to extend it, which could expand the slots.13 Applying for a J-1 waiver thus becomes a race against time with high-stakes suspense and anxiety for many IMGs. Most, regardless of visa status, dream of a stable and secure life, with permanent resident status as they serve their communities. For some, however, the endgame could mean deportation and the premature demise of dreams.
Permanent resident status is allotted by country, and there is a long wait for green cards. Three-quarters of skilled workers waiting for green cards are from India. That translates to more than 700,000 people, of which approximately 200,000 are expected to die of old age before being granted green cards.14,15 In the meantime, while they live with restrictions on both their employment and mobility, many physicians are doing essential medical work in underserved and rural areas throughout the United States.
We urge immigration reform to increase the physician workforce by providing immigrant doctors and IMGs with more flexibility to travel to areas where they are needed the most during this pandemic. There should be a blanket extension of visa deadlines. IMGs on J-1 student visas and H-1B specialty work visas should be exempt from any future immigration bans or limitations during the COVID-19 pandemic. The time is right for accelerating permanent resident status for these highly skilled IMGs. Green cards soon after finishing residency or fellowship training or satisfying a condition of initial visa approval should be the norm instead of a stressful unending wait. Clinicians who serve in underserved communities should be incentivized, and this should include health benefits. Restrictions related to primary and secondary work sites, as well as number of J-1 waivers, should also be relaxed. This flexibility would allow immigrant physicians to care at a variety of locations or by means of telemedicine.
A physician’s role is to heal and to serve their patients, regardless of their own origin. We are the voices of America’s immigrant physicians, particularly hospitalists, serving as frontline workers in our nation’s response to the COVID-19 crisis. The battle against COVID-19 has strained many of our resources, including the need for physicians. Uncertainty and chaos reign professionally and personally for many healthcare workers across America, and more challenges lie ahead for the foreseeable future. Healthcare workers are the unselfish and unwavering wall that stands between COVID-19 and more lives lost in our country. Every effort should be made to preserve and strengthen the healthcare workforce. Immigrant hospitalists, shackled by visa restrictions, could play an even bigger role if their obstacles were removed. It is time to provide them with the sense of security they deserve and rebuild the house of cards into something with a stronger foundation and more stability for our future.
Immigrant physicians and international medical graduates (IMGs) have for decades been very important to the healthcare delivery in the United States. For many currently serving on the front lines, the path has been full of challenges and uncertainties, now acutely worsened by the pandemic at hand. Manpreet Malik, MD, is one of those hospitalists. He grew up in a small city in India. He completed medical school in South India where he met students from all over the world and learned to speak a new language to serve local patients. The multicultural experience inspired him to pursue residency in the United States. Manpreet obtained a J-1 visa for residency and subsequently applied for a J-1 waiver for his first hospitalist job in 2013. Then his employer, a nonprofit organization, applied for H-1B and permanent resident status. He continues on an H-1B status but awaits his green card 7 years later. His wife, a dentist, is also an H-1B visa holder and they have two children. While they have assimilated into American society and flourished professionally, a sense of security eludes them. The COVID-19 pandemic has amplified this for their family. Like many other families, they are both in high-risk occupations and worry about the future, including what would happen if either or both of them contracted the virus. Their carefully planned life feels like a wobbly house of cards.
Immigrant healthcare workers are on the front lines in the fight against COVID-19 in the United States, accounting for 16.4% of healthcare workers amid this pandemic.1 Of physicians in the United States, 29% are not born in the United States,and of the practicing hospitalists, 32% are IMGs.1,2 IMGs are physicians who have graduated from medical schools outside of the United States and Canada who lack accreditation by the Liaison Committee on Medical Education.3 IMGs are a heterogeneous group with widely varying cultural, educational, and linguistic backgrounds with around 12,000 IMGs applying yearly for US residency positions.4 IMG hospitalists are uniquely positioned at the front lines facing arguably more risks with less recognition.5 The top five countries sending physicians to the United States are India, China, the Philippines, South Korea, and Pakistan.6 Yet many of these doctors—more than a third of those practicing in this country who graduated from international medical schools—have visa restrictions that limit their ability to work in communities with the greatest need.7 Another group of approximately 65,000 IMGs currently living in the United States are not licensed; they have not passed the board exam because they haven’t matched into a residency program to be eligible to take it.8 Many are working other jobs such as medical research, even though they could be deployed to serve as scribes or work in triage via telemedicine if their visas permitted.
During the COVID-19 pandemic, immigrant doctors are putting their lives on the line daily to care for patients. Immigrant doctors on visas are not eligible for Medicaid or Social Security benefits. Further, their partners and children are often dependent on them for legal resident status in the United States because of employer-based visa sponsorship. As the primary visa holder, if a non–US-born physician in the United States gets severely ill while fighting the virus, or gets disabled, they may have no benefits to fall back on. These physicians have houses, families, and children who are American citizens, and they are contributing members of society. Physicians on visas pay taxes the same way US citizens do. If their health or employment is jeopardized, their families would be unable to stay in the US legally, becoming undocumented and risking deportation. These physicians, who are fighting COVID-19 today, are helpless to provide a stable structure for their own loved ones.
With the COVID-19 pandemic unfolding, there is a risk of more physician shortages. The US healthcare workforce relies on immigrant physicians to help provide high-quality and accessible patient care. There are challenges for IMGs for getting into residency programs, and this limits the potential workforce during COVID-19. This year, according to the National Resident Matching Program, 4,222 non–US-born IMGs are due to start their US residency training on July 1.9 These doctors have the opportunity to serve across the country during this pandemic. According to data from the matching program, IMGs make up a large proportion of the workforce, obtaining 23% of the total number of US residency positions filled, and are in many leading academic institutions. These doctors, many of whom are waiting for their visas to be processed, need to be admitted in order to provide the care that Americans need during this pandemic. A similar number of IMGs will be completing their specialty training and are due to become attending physicians in their chosen field, including areas with critical shortages in this pandemic, such as critical care medicine. These skilled physicians depend on the processing of visa extensions or green cards in order to remain in the United States. Subspecialties like internal medicine and family medicine have a large proportion of actively practicing IMGs,7 and therefore provide primary care and inpatient care across the nation, especially in underserved areas. However, the geographic location of their practice is limited to the place that sponsored their visa. So a physician in rural Minnesota, where the outbreak of COVID-19 is not severe, cannot travel to hot spots such as New York or Detroit to provide care, even if they have a desire to serve.
For IMGs, the process of obtaining legal status in the US and pertinent immigration policies includes utilizing the H-1B visa program for highly skilled workers10 or J-1 visas for residencies.11 H-1B visas are usually granted for sponsored positions in underserved or rural areas for at least 3 years, and the healthcare sector must compete with other industries, such as tech, engineering, and other specialty occupations. Physicians working on H-1B visas may apply for permanent work permits, though there is an annual cap for each country and candidates may wait decades to receive one. As a J-1 visa (cultural exchange program) holder, physicians are required to practice in their home country for 2 years prior to working again in the United States. This requirement could be waived by turning to the Conrad 30 Waiver Program12 or J-1 waivers if they agreed to work in an underserved area in the United States. A limited number of J-1 waivers for each state are dispensed on a first-come, first-served basis (30 IMGs per state per year). This program currently is only authorized through the end of 2020, although legislation has been introduced to extend it, which could expand the slots.13 Applying for a J-1 waiver thus becomes a race against time with high-stakes suspense and anxiety for many IMGs. Most, regardless of visa status, dream of a stable and secure life, with permanent resident status as they serve their communities. For some, however, the endgame could mean deportation and the premature demise of dreams.
Permanent resident status is allotted by country, and there is a long wait for green cards. Three-quarters of skilled workers waiting for green cards are from India. That translates to more than 700,000 people, of which approximately 200,000 are expected to die of old age before being granted green cards.14,15 In the meantime, while they live with restrictions on both their employment and mobility, many physicians are doing essential medical work in underserved and rural areas throughout the United States.
We urge immigration reform to increase the physician workforce by providing immigrant doctors and IMGs with more flexibility to travel to areas where they are needed the most during this pandemic. There should be a blanket extension of visa deadlines. IMGs on J-1 student visas and H-1B specialty work visas should be exempt from any future immigration bans or limitations during the COVID-19 pandemic. The time is right for accelerating permanent resident status for these highly skilled IMGs. Green cards soon after finishing residency or fellowship training or satisfying a condition of initial visa approval should be the norm instead of a stressful unending wait. Clinicians who serve in underserved communities should be incentivized, and this should include health benefits. Restrictions related to primary and secondary work sites, as well as number of J-1 waivers, should also be relaxed. This flexibility would allow immigrant physicians to care at a variety of locations or by means of telemedicine.
A physician’s role is to heal and to serve their patients, regardless of their own origin. We are the voices of America’s immigrant physicians, particularly hospitalists, serving as frontline workers in our nation’s response to the COVID-19 crisis. The battle against COVID-19 has strained many of our resources, including the need for physicians. Uncertainty and chaos reign professionally and personally for many healthcare workers across America, and more challenges lie ahead for the foreseeable future. Healthcare workers are the unselfish and unwavering wall that stands between COVID-19 and more lives lost in our country. Every effort should be made to preserve and strengthen the healthcare workforce. Immigrant hospitalists, shackled by visa restrictions, could play an even bigger role if their obstacles were removed. It is time to provide them with the sense of security they deserve and rebuild the house of cards into something with a stronger foundation and more stability for our future.
1. New American Economy Research Fund. Immigration and Covid-19. March 26, 2020. Accessed May 5, 2020. https://research.newamericaneconomy.org/report/immigration-and-covid-19/
2. Compensation and Career Survey. Today’s Hospitalist. November 1, 2008. Accessed May 29, 2020. https://www.todayshospitalist.com/survey/16_salary_survey/index.php
3. Rao NR. “A little more than kin, and less than kind”: US immigration policy on international medical graduates. Virtual Mentor. 2012;14(4):329-337. https://doi.org/10.1001/virtualmentor.2012.14.4.pfor1-1204
4. ECFMG Fact Card: Summary Data Related to ECFMG Certification. Educational Commission for Foreign Medical Graduates (ECFMG). March 20, 2019. Accessed April 22, 2020. https://www.ecfmg.org/forms/factcard.pdf
5. Compensation and Career Survey. Today’s Hospitalist. November 1, 2016. Accessed May 29, 2020. https://www.todayshospitalist.com/survey/08_salary_survey/index.php
6. Harker YS. In rural towns, immigrant doctors fill a critical need. Health Affairs. 2018;37(1):161-164. https://doi.org/10.1377/hlthaff.2017.1094
7. Ahmed AA, Hwang WT, Thomas CR Jr, Deville C Jr. International medical graduates in the US physician workforce and graduate medical education: current and historical trends. J Grad Med Educ. 2018;10(2):214‐218. https://doi.org/10.4300/jgme-d-17-00580.1
8. Peters J. Highly trained and educated, some foreign-born doctors still can’t practice medicine in the US. Public Radio International. March 28, 2018. Accessed April 22, 2020. https://www.pri.org/stories/2018-03-26/highly-trained-and-educated-some-foreign-born-doctors-still-can-t-practice
9. Results and Data: 2020 Main Residency Match. National Resident Matching Program. 2020. Accessed May 15, 2020. http://www.nrmp.org/main-residency-match-data/
10. H-1B Specialty Occupations, DOD Cooperative Research and Development Project Workers, and Fashion Models. U.S. Citizenship and Immigration Services. March 27, 2020. Accessed April 22, 2020. https://www.uscis.gov/working-united-states/temporary-workers/h-1b-specialty-occupations-dod-cooperative-research-and-development-project-workers-and-fashion-models
11. J-1 Visa Sponsorship Fact Sheet. Educational Commission for Foreign Medical Graduates (ECFMG). May 2017. Accessed April 22, 2020. https://www.ecfmg.org/evsp/j1fact.pdf
12. Conrad 30 Waiver Program. U.S. Citizenship and Immigration Services. August 25, 2011. Accessed April 22, 2020. https://www.uscis.gov/working-united-states/students-and-exchange-visitors/conrad-30-waiver-program
13. Conrad State 30 and Physician Access Reauthorization Act, S 948, 116th Congress (2019). Accessed April 22, 2020. https://www.congress.gov/bill/116thcongress/senate-bill/948/text
14. Bhattacharya A. For over 200,000 Indians, the wait for a green card is longer than their lifetimes. Quartz India. March 31, 2020. Accessed April 22, 2020. https://qz.com/india/1828970/over-200000-indians-could-die-waiting-for-a-us-green-card/
15. Bier DJ. Immigration Research and Policy Brief: Backlog for Skilled Immigrants Tops 1 Million: Over 200,000 Indians Could Die of Old Age While Awaiting Green Cards. Cato Institute: Immigration Research and Policy Brief, No. 18. March 30, 2020. Accessed April 26, 2020. https://www.cato.org/sites/cato.org/files/2020-03/irpb-18-updated.pdf
1. New American Economy Research Fund. Immigration and Covid-19. March 26, 2020. Accessed May 5, 2020. https://research.newamericaneconomy.org/report/immigration-and-covid-19/
2. Compensation and Career Survey. Today’s Hospitalist. November 1, 2008. Accessed May 29, 2020. https://www.todayshospitalist.com/survey/16_salary_survey/index.php
3. Rao NR. “A little more than kin, and less than kind”: US immigration policy on international medical graduates. Virtual Mentor. 2012;14(4):329-337. https://doi.org/10.1001/virtualmentor.2012.14.4.pfor1-1204
4. ECFMG Fact Card: Summary Data Related to ECFMG Certification. Educational Commission for Foreign Medical Graduates (ECFMG). March 20, 2019. Accessed April 22, 2020. https://www.ecfmg.org/forms/factcard.pdf
5. Compensation and Career Survey. Today’s Hospitalist. November 1, 2016. Accessed May 29, 2020. https://www.todayshospitalist.com/survey/08_salary_survey/index.php
6. Harker YS. In rural towns, immigrant doctors fill a critical need. Health Affairs. 2018;37(1):161-164. https://doi.org/10.1377/hlthaff.2017.1094
7. Ahmed AA, Hwang WT, Thomas CR Jr, Deville C Jr. International medical graduates in the US physician workforce and graduate medical education: current and historical trends. J Grad Med Educ. 2018;10(2):214‐218. https://doi.org/10.4300/jgme-d-17-00580.1
8. Peters J. Highly trained and educated, some foreign-born doctors still can’t practice medicine in the US. Public Radio International. March 28, 2018. Accessed April 22, 2020. https://www.pri.org/stories/2018-03-26/highly-trained-and-educated-some-foreign-born-doctors-still-can-t-practice
9. Results and Data: 2020 Main Residency Match. National Resident Matching Program. 2020. Accessed May 15, 2020. http://www.nrmp.org/main-residency-match-data/
10. H-1B Specialty Occupations, DOD Cooperative Research and Development Project Workers, and Fashion Models. U.S. Citizenship and Immigration Services. March 27, 2020. Accessed April 22, 2020. https://www.uscis.gov/working-united-states/temporary-workers/h-1b-specialty-occupations-dod-cooperative-research-and-development-project-workers-and-fashion-models
11. J-1 Visa Sponsorship Fact Sheet. Educational Commission for Foreign Medical Graduates (ECFMG). May 2017. Accessed April 22, 2020. https://www.ecfmg.org/evsp/j1fact.pdf
12. Conrad 30 Waiver Program. U.S. Citizenship and Immigration Services. August 25, 2011. Accessed April 22, 2020. https://www.uscis.gov/working-united-states/students-and-exchange-visitors/conrad-30-waiver-program
13. Conrad State 30 and Physician Access Reauthorization Act, S 948, 116th Congress (2019). Accessed April 22, 2020. https://www.congress.gov/bill/116thcongress/senate-bill/948/text
14. Bhattacharya A. For over 200,000 Indians, the wait for a green card is longer than their lifetimes. Quartz India. March 31, 2020. Accessed April 22, 2020. https://qz.com/india/1828970/over-200000-indians-could-die-waiting-for-a-us-green-card/
15. Bier DJ. Immigration Research and Policy Brief: Backlog for Skilled Immigrants Tops 1 Million: Over 200,000 Indians Could Die of Old Age While Awaiting Green Cards. Cato Institute: Immigration Research and Policy Brief, No. 18. March 30, 2020. Accessed April 26, 2020. https://www.cato.org/sites/cato.org/files/2020-03/irpb-18-updated.pdf
© 2020 Society of Hospital Medicine
Hospital Ward Adaptation During the COVID-19 Pandemic: A National Survey of Academic Medical Centers
The coronavirus disease of 2019 (COVID-19) pandemic has resulted in a surge in hospitalizations of patients with a novel, serious, and highly contagious infectious disease for which there is yet no proven treatment. Currently, much of the focus has been on intensive care unit (ICU) and ventilator capacity for the sickest of these patients who develop respiratory failure. However, most hospitalized patients are being cared for in general medical units.1 Some evidence exists to describe adaptations to capacity needs outside of medical wards,2-4 but few studies have specifically addressed the ward setting. Therefore, there is a pressing need for evidence to describe how to expand capacity and deliver medical ward–based care.
To better understand how inpatient care in the United States is adapting to the COVID-19 pandemic, we surveyed 72 sites participating in the Hospital Medicine Reengineering Network (HOMERuN), a national consortium of hospital medicine groups.5 We report results of this survey, carried out between April 3 and April 5, 2020.
METHODS
Sites and Subjects
HOMERuN is a collaborative network of hospitalists from across the United States whose primary goal is to catalyze research and share best practices across hospital medicine groups. Using surveys of Hospital Medicine leaders, targeted medical record review, and other methods, HOMERuN’s funded research interests to date have included care transitions, workforce issues, patient and family engagement, and diagnostic errors. Sites participating in HOMERuN sites are relatively large urban academic medical centers (Appendix).
Survey Development and Deployment
We designed a focused survey that aimed to provide a snapshot of evolving operational and clinical aspects of COVID-19 care (Appendix). Domains included COVID-19 testing turnaround times, personal protective equipment (PPE) stewardship,6 features of respiratory isolation units (RIUs; ie, dedicated units for patients with known or suspected COVID-19), and observed effects on clinical care. We tested the instrument to ensure feasibility and clarity internally, performed brief cognitive testing with several hospital medicine leaders in HOMERuN, then disseminated the survey by email on April 3, with two follow-up emails on 2 subsequent days. Our study was deemed non–human subjects research by the University of California, San Francisco, Committee on Human Research. Descriptive statistics were used to characterize survey responses.
RESULTS
Of 72 hospitals surveyed, 51 (71%) responded. Mean hospital bed count was 940, three were safety-net hospitals, and one was a community-based teaching center; responding and nonresponding hospitals did not differ significantly in terms of bed count (Appendix).
Health System Adaptations, Testing, and PPE Status
Nearly all responding hospitals (46 of 51; 90%) had RIUs for patients with known or suspected COVID-19 (Table 1). Nearly all hospitals took steps to keep potentially sick healthcare providers from infecting others (eg, staying home if sick or exposed). Among respondents, 32% had rapid response teams, 24% had respiratory therapy teams, and 29% had case management teams that were dedicated to COVID-19 care. Thirty-two (63%) had developed models, such as ethics or palliative care consult services, to assist with difficult resource-allocation decisions (eg, how to prioritize ventilator use if demand exceeded supply). Twenty-three (45%) had developed post-acute care monitoring programs dedicated to COVID-19 patients.

At the time of our survey, only 2 sites (4%) reported COVID-19 test time turnaround under 1 hour, and 15 (30%) reported turnaround in less than 6 hours. Of the 29 sites able to provide estimates of PPE stockpile, 14 (48%) reported a supply of 2 weeks or less. The most common approaches to PPE stewardship focused on reuse of masks and face shields if not obviously soiled, centralizing PPE distribution, and disinfecting or sterilizing masks. Ten sites (20%) were utilizing 3-D printed masks, while 10% used homemade face shields or masks.
Characteristics of COVID-19 RIUs
Forty-six hospitals (90% of all respondents) in our cohort had developed RIUs at the time of survey administration. The earliest RIU implementation date was February 10, 2020, and the most recent was launched on the day of our survey. Admission to RIUs was primarily based on clinical factors associated with known or suspected COVID-19 infection (Table 2). The number of non–critical care RIU beds among locations at that time ranged from 10 or less to more than 50. The mean number of hospitalist attendings caring for patients in the RIUs was 10.2, with a mean 4.1 advanced practice providers, 5.5 residents, and 0 medical students. The number of planned patients per attending was typically 5 to 15. Nurses and physicians typically rounded separately. Medical distancing (eg, reducing patient room entry) was accomplished most commonly by grouped timing of medication administration (76% of sites), video links to room outside of rounding times (54% of sites), the use of video or telemedicine during rounds (17%), and clustering of activities such as medication administration or phlebotomy. The most common criteria prompting discharge from the RIU were a negative COVID-19 test (59%) and hospital discharge (57%), though comments from many respondents suggested that discharge criteria were changing rapidly.

Effects of Isolation Measures on In-Room Encounters and Diagnostic Processes
More than 90% of sites reported decreases in in-room encounter frequency across all provider types whether as a result of policies in place or not. Reductions were reported among hospitalists, advanced practice providers, residents, consultants, and therapists (Table 3). Reduced room entry most often resulted from an established or developing policy, but many noted reduced room entry without formal policies in place. Nearly all sites reported moving specialty consultations to phone or video evaluations. Diagnostic error was commonly reported, with missed non–COVID-19 medical diagnoses among COVID-19 infected patients being reported by 22 sites (46%) and missed COVID-19 diagnoses in patients admitted for other reasons by 22 sites (45%).

DISCUSSION
In this study of medical wards at academic medical centers, we found that, in response to the COVID-19 pandemic, hospitals made several changes in a short period of time to adapt to the crisis. These included implementation and rapid expansion of dedicated RIUs, greatly expanded use of inpatient telehealth for patient assessments and consultation, implementation of other approaches to minimize room entry (such as grouping in-room activities), and deployment of ethics consultation services to help manage issues around potential scarcity of life-saving measures such as ventilators. We also found that availability of PPE and timely testing was limited. Finally, a large proportion of sites reported potential diagnostic problems in the assessment of both patients suspected and those not suspected of having COVID-19.
RIUs are emerging as a primary modality for caring for non-ICU COVID-19 patients, though they never involved medical students; we hope the role of students in particular will increase as new models of training emerge in response to the pandemic.7 In contrast, telemedicine evolved rapidly to hold a substantial role in RIUs, with both ward and specialty teams using video visit technology to communicate with patients. COVID-19 has been viewed as a perfect use case for outpatient telemedicine,8 and a growing number of studies are examining its outpatient use9,10; however, to date, somewhat less attention has been paid to inpatient deployment. Although our data suggest telemedicine has found a prominent place in RIUs, it remains to be seen whether it is associated with differences in patient or provider outcomes. For example, deficiencies in the physical examination, limited face-to-face contact, and lack of physical presence could all affect the patient–provider relationship, patient engagement, and the accuracy of the diagnostic process.
Our data suggest the possibility of missing non–COVID-19 diagnoses in patients suspected of COVID-19 and missing COVID-19 in those admitted for nonrespiratory reasons. The latter may be addressed as routine COVID-19 screening of admitted patients becomes commonplace. For the former, however, it is possible that physicians are “anchoring” their thinking on COVID-19 to the exclusion of other diagnoses, that physicians are not fully aware of complications unique to COVID-19 infection (such as thromboembolism), and/or that the above-mentioned limitations of telemedicine have decreased diagnostic performance.
Although PPE stockpile data were not easily available for some sites, a distressingly large number reported stockpiles of 2 weeks or less, with reuse being the most common approach to extending PPE supply. We also found it concerning that 43% of hospital leaders did not know their stockpile data; we believe this is an important question that hospital leaders need to be asking. Most sites in our study reported test turnaround times of longer than 6 hours; lack of rapid COVID-19 testing further stresses PPE stockpile and may slow patients’ transition out of the RIU or discharge to home.
Our study has several limitations, including the evolving nature of the pandemic and rapid adaptations of care systems in the pandemic’s surge phase. However, we attempted to frame our questions in ways that provided a focused snapshot of care. Furthermore, respondents may not have had exhaustive knowledge of their institution’s COVID-19 response strategies, but most were the directors of their hospitalist services, and we encouraged the respondents to confer with others to gather high-fidelity data. Finally, as a survey of large academic medical centers, our results may not apply to nonacademic centers.
Approaches to caring for non-ICU patients during the COVID-19 pandemic are rapidly evolving. Expansion of RIUs and developing the workforce to support them has been a primary focus, with rapid innovation in use of technology emerging as a critical adaptation while PPE limitations persist and needs for “medical distancing” continue to grow. Although rates of missed COVID-19 diagnoses will likely be reduced with testing and systems improvements, physicians and systems will also need to consider how to utilize emerging technology in ways that can improve clinical care and provider safety while aiding diagnostic thinking. This survey illustrates the rapid adaptations made by our hospitals in response to the pandemic; ongoing adaptation will likely be needed to optimally care for hospitalized patients with COVID-19 while the pandemic continues to evolve.
Acknowledgment
Thanks to members of the HOMERuN COVID-19 Collaborative Group: Baylor Scott & White Medical Center – Temple, Texas - Tresa McNeal MD; Beth Israel Deaconess Medical Center - Shani Herzig MD MPH, Joseph Li MD, Julius Yang MD PhD; Brigham and Women’s Hospital - Christopher Roy MD, Jeffrey Schnipper MD MPH; Cedars-Sinai Medical Center - Ed Seferian MD, ; ChristianaCare - Surekha Bhamidipati MD; Cleveland Clinic - Matthew Pappas MD MPH; Dartmouth-Hitchcock Medical Center - Jonathan Lurie MD MS; Dell Medical School at The University of Texas at Austin - Chris Moriates MD, Luci Leykum MD MBA MSc; Denver Health and Hospitals Authority - Diana Mancini MD; Emory University Hospital - Dan Hunt MD; Johns Hopkins Hospital - Daniel J Brotman MD, Zishan K Siddiqui MD, Shaker Eid MD MBA; Maine Medical Center - Daniel A Meyer MD, Robert Trowbridge MD; Massachusetts General Hospital - Melissa Mattison MD; Mayo Clinic Rochester – Caroline Burton MD, Sagar Dugani MD PhD; Medical College of Wisconsin - Sanjay Bhandari MD; Miriam Hospital - Kwame Dapaah-Afriyie MD MBA; Mount Sinai Hospital - Andrew Dunn MD; NorthShore - David Lovinger MD; Northwestern Memorial Hospital - Kevin O’Leary MD MS; Ohio State University Wexner Medical Center - Eric Schumacher DO; Oregon Health & Science University - Angela Alday MD; Penn Medicine - Ryan Greysen MD MHS MA; Rutgers- Robert Wood Johnson University Hospital - Michael Steinberg MD MPH; Stanford University School of Medicine - Neera Ahuja MD; Tulane Hospital and University Medical Center - Geraldine Ménard MD; UC San Diego Health - Ian Jenkins MD; UC Los Angeles Health - Michael Lazarus MD, Magdalena E. Ptaszny, MD; UC San Francisco Health - Bradley A Sharpe, MD, Margaret Fang MD MPH; UK HealthCare - Mark Williams MD MHM, John Romond MD; University of Chicago – David Meltzer MD PhD, Gregory Ruhnke MD; University of Colorado - Marisha Burden MD; University of Florida - Nila Radhakrishnan MD; University of Iowa Hospitals and Clinics - Kevin Glenn MD MS; University of Miami - Efren Manjarrez MD; University of Michigan - Vineet Chopra MD MSc, Valerie Vaughn MD MSc; University of Missouri-Columbia Hospital - Hasan Naqvi MD; University of Nebraska Medical Center - Chad Vokoun MD; University of North Carolina at Chapel Hill - David Hemsey MD; University of Pittsburgh Medical Center - Gena Marie Walker MD; University of Vermont Medical Center - Steven Grant MD; University of Washington Medical Center - Christopher Kim MD MBA, Andrew White MD; University of Washington-Harborview Medical Center - Maralyssa Bann MD; University of Wisconsin Hospital and Clinics - David Sterken MD, Farah Kaiksow MD MPP, Ann Sheehy MD MS, Jordan Kenik MD MPH; UW Northwest Campus - Ben Wolpaw MD; Vanderbilt University Medical Center - Sunil Kripalani MD MSc, Eduard E Vasilevskis MD, Kathleene T Wooldridge MD MPH; Wake Forest Baptist Health - Erik Summers MD; Washington University St. Louis - Michael Lin MD; Weill Cornell - Justin Choi MD; Yale New Haven Hospital - William Cushing MA, Chris Sankey MD; Zuckerberg San Francisco General Hospital - Sumant Ranji MD.
1. Institute for Health Metrics and Evaluation. COVID-19 Projections: United States of America. 2020. Accessed May 5, 2020. https://covid19.healthdata.org/united-states-of-america
2. Iserson KV. Alternative care sites: an option in disasters. West J Emerg Med. 2020;21(3):484‐489. https://doi.org/10.5811/westjem.2020.4.47552
3. Paganini M, Conti A, Weinstein E, Della Corte F, Ragazzoni L. Translating COVID-19 pandemic surge theory to practice in the emergency department: how to expand structure [online first]. Disaster Med Public Health Prep. 2020:1-10. https://doi.org/10.1017/dmp.2020.57
4. Kumaraiah D, Yip N, Ivascu N, Hill L. Innovative ICU Physician Care Models: Covid-19 Pandemic at NewYork-Presbyterian. NEJM: Catalyst. April 28, 2020. Accessed May 5, 2020. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0158
5. Auerbach AD, Patel MS, Metlay JP, et al. The Hospital Medicine Reengineering Network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. https://doi.org/10.1097/acm.0000000000000139
6. Livingston E, Desai A, Berkwits M. Sourcing personal protective equipment during the COVID-19 pandemic [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.5317
7. Bauchner H, Sharfstein J. A bold response to the COVID-19 pandemic: medical students, national service, and public health [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.6166
8. Hollander JE, Carr BG. Virtually perfect? telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679‐1681. https://doi.org/10.1056/nejmp2003539
9. Hau YS, Kim JK, Hur J, Chang MC. How about actively using telemedicine during the COVID-19 pandemic? J Med Syst. 2020;44(6):108. https://doi.org/10.1007/s10916-020-01580-z
10. Smith WR, Atala AJ, Terlecki RP, Kelly EE, Matthews CA. Implementation guide for rapid integration of an outpatient telemedicine program during the COVID-19 pandemic [online first]. J Am Coll Surg. 2020. https://doi.org/10.1016/j.jamcollsurg.2020.04.030
The coronavirus disease of 2019 (COVID-19) pandemic has resulted in a surge in hospitalizations of patients with a novel, serious, and highly contagious infectious disease for which there is yet no proven treatment. Currently, much of the focus has been on intensive care unit (ICU) and ventilator capacity for the sickest of these patients who develop respiratory failure. However, most hospitalized patients are being cared for in general medical units.1 Some evidence exists to describe adaptations to capacity needs outside of medical wards,2-4 but few studies have specifically addressed the ward setting. Therefore, there is a pressing need for evidence to describe how to expand capacity and deliver medical ward–based care.
To better understand how inpatient care in the United States is adapting to the COVID-19 pandemic, we surveyed 72 sites participating in the Hospital Medicine Reengineering Network (HOMERuN), a national consortium of hospital medicine groups.5 We report results of this survey, carried out between April 3 and April 5, 2020.
METHODS
Sites and Subjects
HOMERuN is a collaborative network of hospitalists from across the United States whose primary goal is to catalyze research and share best practices across hospital medicine groups. Using surveys of Hospital Medicine leaders, targeted medical record review, and other methods, HOMERuN’s funded research interests to date have included care transitions, workforce issues, patient and family engagement, and diagnostic errors. Sites participating in HOMERuN sites are relatively large urban academic medical centers (Appendix).
Survey Development and Deployment
We designed a focused survey that aimed to provide a snapshot of evolving operational and clinical aspects of COVID-19 care (Appendix). Domains included COVID-19 testing turnaround times, personal protective equipment (PPE) stewardship,6 features of respiratory isolation units (RIUs; ie, dedicated units for patients with known or suspected COVID-19), and observed effects on clinical care. We tested the instrument to ensure feasibility and clarity internally, performed brief cognitive testing with several hospital medicine leaders in HOMERuN, then disseminated the survey by email on April 3, with two follow-up emails on 2 subsequent days. Our study was deemed non–human subjects research by the University of California, San Francisco, Committee on Human Research. Descriptive statistics were used to characterize survey responses.
RESULTS
Of 72 hospitals surveyed, 51 (71%) responded. Mean hospital bed count was 940, three were safety-net hospitals, and one was a community-based teaching center; responding and nonresponding hospitals did not differ significantly in terms of bed count (Appendix).
Health System Adaptations, Testing, and PPE Status
Nearly all responding hospitals (46 of 51; 90%) had RIUs for patients with known or suspected COVID-19 (Table 1). Nearly all hospitals took steps to keep potentially sick healthcare providers from infecting others (eg, staying home if sick or exposed). Among respondents, 32% had rapid response teams, 24% had respiratory therapy teams, and 29% had case management teams that were dedicated to COVID-19 care. Thirty-two (63%) had developed models, such as ethics or palliative care consult services, to assist with difficult resource-allocation decisions (eg, how to prioritize ventilator use if demand exceeded supply). Twenty-three (45%) had developed post-acute care monitoring programs dedicated to COVID-19 patients.

At the time of our survey, only 2 sites (4%) reported COVID-19 test time turnaround under 1 hour, and 15 (30%) reported turnaround in less than 6 hours. Of the 29 sites able to provide estimates of PPE stockpile, 14 (48%) reported a supply of 2 weeks or less. The most common approaches to PPE stewardship focused on reuse of masks and face shields if not obviously soiled, centralizing PPE distribution, and disinfecting or sterilizing masks. Ten sites (20%) were utilizing 3-D printed masks, while 10% used homemade face shields or masks.
Characteristics of COVID-19 RIUs
Forty-six hospitals (90% of all respondents) in our cohort had developed RIUs at the time of survey administration. The earliest RIU implementation date was February 10, 2020, and the most recent was launched on the day of our survey. Admission to RIUs was primarily based on clinical factors associated with known or suspected COVID-19 infection (Table 2). The number of non–critical care RIU beds among locations at that time ranged from 10 or less to more than 50. The mean number of hospitalist attendings caring for patients in the RIUs was 10.2, with a mean 4.1 advanced practice providers, 5.5 residents, and 0 medical students. The number of planned patients per attending was typically 5 to 15. Nurses and physicians typically rounded separately. Medical distancing (eg, reducing patient room entry) was accomplished most commonly by grouped timing of medication administration (76% of sites), video links to room outside of rounding times (54% of sites), the use of video or telemedicine during rounds (17%), and clustering of activities such as medication administration or phlebotomy. The most common criteria prompting discharge from the RIU were a negative COVID-19 test (59%) and hospital discharge (57%), though comments from many respondents suggested that discharge criteria were changing rapidly.

Effects of Isolation Measures on In-Room Encounters and Diagnostic Processes
More than 90% of sites reported decreases in in-room encounter frequency across all provider types whether as a result of policies in place or not. Reductions were reported among hospitalists, advanced practice providers, residents, consultants, and therapists (Table 3). Reduced room entry most often resulted from an established or developing policy, but many noted reduced room entry without formal policies in place. Nearly all sites reported moving specialty consultations to phone or video evaluations. Diagnostic error was commonly reported, with missed non–COVID-19 medical diagnoses among COVID-19 infected patients being reported by 22 sites (46%) and missed COVID-19 diagnoses in patients admitted for other reasons by 22 sites (45%).

DISCUSSION
In this study of medical wards at academic medical centers, we found that, in response to the COVID-19 pandemic, hospitals made several changes in a short period of time to adapt to the crisis. These included implementation and rapid expansion of dedicated RIUs, greatly expanded use of inpatient telehealth for patient assessments and consultation, implementation of other approaches to minimize room entry (such as grouping in-room activities), and deployment of ethics consultation services to help manage issues around potential scarcity of life-saving measures such as ventilators. We also found that availability of PPE and timely testing was limited. Finally, a large proportion of sites reported potential diagnostic problems in the assessment of both patients suspected and those not suspected of having COVID-19.
RIUs are emerging as a primary modality for caring for non-ICU COVID-19 patients, though they never involved medical students; we hope the role of students in particular will increase as new models of training emerge in response to the pandemic.7 In contrast, telemedicine evolved rapidly to hold a substantial role in RIUs, with both ward and specialty teams using video visit technology to communicate with patients. COVID-19 has been viewed as a perfect use case for outpatient telemedicine,8 and a growing number of studies are examining its outpatient use9,10; however, to date, somewhat less attention has been paid to inpatient deployment. Although our data suggest telemedicine has found a prominent place in RIUs, it remains to be seen whether it is associated with differences in patient or provider outcomes. For example, deficiencies in the physical examination, limited face-to-face contact, and lack of physical presence could all affect the patient–provider relationship, patient engagement, and the accuracy of the diagnostic process.
Our data suggest the possibility of missing non–COVID-19 diagnoses in patients suspected of COVID-19 and missing COVID-19 in those admitted for nonrespiratory reasons. The latter may be addressed as routine COVID-19 screening of admitted patients becomes commonplace. For the former, however, it is possible that physicians are “anchoring” their thinking on COVID-19 to the exclusion of other diagnoses, that physicians are not fully aware of complications unique to COVID-19 infection (such as thromboembolism), and/or that the above-mentioned limitations of telemedicine have decreased diagnostic performance.
Although PPE stockpile data were not easily available for some sites, a distressingly large number reported stockpiles of 2 weeks or less, with reuse being the most common approach to extending PPE supply. We also found it concerning that 43% of hospital leaders did not know their stockpile data; we believe this is an important question that hospital leaders need to be asking. Most sites in our study reported test turnaround times of longer than 6 hours; lack of rapid COVID-19 testing further stresses PPE stockpile and may slow patients’ transition out of the RIU or discharge to home.
Our study has several limitations, including the evolving nature of the pandemic and rapid adaptations of care systems in the pandemic’s surge phase. However, we attempted to frame our questions in ways that provided a focused snapshot of care. Furthermore, respondents may not have had exhaustive knowledge of their institution’s COVID-19 response strategies, but most were the directors of their hospitalist services, and we encouraged the respondents to confer with others to gather high-fidelity data. Finally, as a survey of large academic medical centers, our results may not apply to nonacademic centers.
Approaches to caring for non-ICU patients during the COVID-19 pandemic are rapidly evolving. Expansion of RIUs and developing the workforce to support them has been a primary focus, with rapid innovation in use of technology emerging as a critical adaptation while PPE limitations persist and needs for “medical distancing” continue to grow. Although rates of missed COVID-19 diagnoses will likely be reduced with testing and systems improvements, physicians and systems will also need to consider how to utilize emerging technology in ways that can improve clinical care and provider safety while aiding diagnostic thinking. This survey illustrates the rapid adaptations made by our hospitals in response to the pandemic; ongoing adaptation will likely be needed to optimally care for hospitalized patients with COVID-19 while the pandemic continues to evolve.
Acknowledgment
Thanks to members of the HOMERuN COVID-19 Collaborative Group: Baylor Scott & White Medical Center – Temple, Texas - Tresa McNeal MD; Beth Israel Deaconess Medical Center - Shani Herzig MD MPH, Joseph Li MD, Julius Yang MD PhD; Brigham and Women’s Hospital - Christopher Roy MD, Jeffrey Schnipper MD MPH; Cedars-Sinai Medical Center - Ed Seferian MD, ; ChristianaCare - Surekha Bhamidipati MD; Cleveland Clinic - Matthew Pappas MD MPH; Dartmouth-Hitchcock Medical Center - Jonathan Lurie MD MS; Dell Medical School at The University of Texas at Austin - Chris Moriates MD, Luci Leykum MD MBA MSc; Denver Health and Hospitals Authority - Diana Mancini MD; Emory University Hospital - Dan Hunt MD; Johns Hopkins Hospital - Daniel J Brotman MD, Zishan K Siddiqui MD, Shaker Eid MD MBA; Maine Medical Center - Daniel A Meyer MD, Robert Trowbridge MD; Massachusetts General Hospital - Melissa Mattison MD; Mayo Clinic Rochester – Caroline Burton MD, Sagar Dugani MD PhD; Medical College of Wisconsin - Sanjay Bhandari MD; Miriam Hospital - Kwame Dapaah-Afriyie MD MBA; Mount Sinai Hospital - Andrew Dunn MD; NorthShore - David Lovinger MD; Northwestern Memorial Hospital - Kevin O’Leary MD MS; Ohio State University Wexner Medical Center - Eric Schumacher DO; Oregon Health & Science University - Angela Alday MD; Penn Medicine - Ryan Greysen MD MHS MA; Rutgers- Robert Wood Johnson University Hospital - Michael Steinberg MD MPH; Stanford University School of Medicine - Neera Ahuja MD; Tulane Hospital and University Medical Center - Geraldine Ménard MD; UC San Diego Health - Ian Jenkins MD; UC Los Angeles Health - Michael Lazarus MD, Magdalena E. Ptaszny, MD; UC San Francisco Health - Bradley A Sharpe, MD, Margaret Fang MD MPH; UK HealthCare - Mark Williams MD MHM, John Romond MD; University of Chicago – David Meltzer MD PhD, Gregory Ruhnke MD; University of Colorado - Marisha Burden MD; University of Florida - Nila Radhakrishnan MD; University of Iowa Hospitals and Clinics - Kevin Glenn MD MS; University of Miami - Efren Manjarrez MD; University of Michigan - Vineet Chopra MD MSc, Valerie Vaughn MD MSc; University of Missouri-Columbia Hospital - Hasan Naqvi MD; University of Nebraska Medical Center - Chad Vokoun MD; University of North Carolina at Chapel Hill - David Hemsey MD; University of Pittsburgh Medical Center - Gena Marie Walker MD; University of Vermont Medical Center - Steven Grant MD; University of Washington Medical Center - Christopher Kim MD MBA, Andrew White MD; University of Washington-Harborview Medical Center - Maralyssa Bann MD; University of Wisconsin Hospital and Clinics - David Sterken MD, Farah Kaiksow MD MPP, Ann Sheehy MD MS, Jordan Kenik MD MPH; UW Northwest Campus - Ben Wolpaw MD; Vanderbilt University Medical Center - Sunil Kripalani MD MSc, Eduard E Vasilevskis MD, Kathleene T Wooldridge MD MPH; Wake Forest Baptist Health - Erik Summers MD; Washington University St. Louis - Michael Lin MD; Weill Cornell - Justin Choi MD; Yale New Haven Hospital - William Cushing MA, Chris Sankey MD; Zuckerberg San Francisco General Hospital - Sumant Ranji MD.
The coronavirus disease of 2019 (COVID-19) pandemic has resulted in a surge in hospitalizations of patients with a novel, serious, and highly contagious infectious disease for which there is yet no proven treatment. Currently, much of the focus has been on intensive care unit (ICU) and ventilator capacity for the sickest of these patients who develop respiratory failure. However, most hospitalized patients are being cared for in general medical units.1 Some evidence exists to describe adaptations to capacity needs outside of medical wards,2-4 but few studies have specifically addressed the ward setting. Therefore, there is a pressing need for evidence to describe how to expand capacity and deliver medical ward–based care.
To better understand how inpatient care in the United States is adapting to the COVID-19 pandemic, we surveyed 72 sites participating in the Hospital Medicine Reengineering Network (HOMERuN), a national consortium of hospital medicine groups.5 We report results of this survey, carried out between April 3 and April 5, 2020.
METHODS
Sites and Subjects
HOMERuN is a collaborative network of hospitalists from across the United States whose primary goal is to catalyze research and share best practices across hospital medicine groups. Using surveys of Hospital Medicine leaders, targeted medical record review, and other methods, HOMERuN’s funded research interests to date have included care transitions, workforce issues, patient and family engagement, and diagnostic errors. Sites participating in HOMERuN sites are relatively large urban academic medical centers (Appendix).
Survey Development and Deployment
We designed a focused survey that aimed to provide a snapshot of evolving operational and clinical aspects of COVID-19 care (Appendix). Domains included COVID-19 testing turnaround times, personal protective equipment (PPE) stewardship,6 features of respiratory isolation units (RIUs; ie, dedicated units for patients with known or suspected COVID-19), and observed effects on clinical care. We tested the instrument to ensure feasibility and clarity internally, performed brief cognitive testing with several hospital medicine leaders in HOMERuN, then disseminated the survey by email on April 3, with two follow-up emails on 2 subsequent days. Our study was deemed non–human subjects research by the University of California, San Francisco, Committee on Human Research. Descriptive statistics were used to characterize survey responses.
RESULTS
Of 72 hospitals surveyed, 51 (71%) responded. Mean hospital bed count was 940, three were safety-net hospitals, and one was a community-based teaching center; responding and nonresponding hospitals did not differ significantly in terms of bed count (Appendix).
Health System Adaptations, Testing, and PPE Status
Nearly all responding hospitals (46 of 51; 90%) had RIUs for patients with known or suspected COVID-19 (Table 1). Nearly all hospitals took steps to keep potentially sick healthcare providers from infecting others (eg, staying home if sick or exposed). Among respondents, 32% had rapid response teams, 24% had respiratory therapy teams, and 29% had case management teams that were dedicated to COVID-19 care. Thirty-two (63%) had developed models, such as ethics or palliative care consult services, to assist with difficult resource-allocation decisions (eg, how to prioritize ventilator use if demand exceeded supply). Twenty-three (45%) had developed post-acute care monitoring programs dedicated to COVID-19 patients.

At the time of our survey, only 2 sites (4%) reported COVID-19 test time turnaround under 1 hour, and 15 (30%) reported turnaround in less than 6 hours. Of the 29 sites able to provide estimates of PPE stockpile, 14 (48%) reported a supply of 2 weeks or less. The most common approaches to PPE stewardship focused on reuse of masks and face shields if not obviously soiled, centralizing PPE distribution, and disinfecting or sterilizing masks. Ten sites (20%) were utilizing 3-D printed masks, while 10% used homemade face shields or masks.
Characteristics of COVID-19 RIUs
Forty-six hospitals (90% of all respondents) in our cohort had developed RIUs at the time of survey administration. The earliest RIU implementation date was February 10, 2020, and the most recent was launched on the day of our survey. Admission to RIUs was primarily based on clinical factors associated with known or suspected COVID-19 infection (Table 2). The number of non–critical care RIU beds among locations at that time ranged from 10 or less to more than 50. The mean number of hospitalist attendings caring for patients in the RIUs was 10.2, with a mean 4.1 advanced practice providers, 5.5 residents, and 0 medical students. The number of planned patients per attending was typically 5 to 15. Nurses and physicians typically rounded separately. Medical distancing (eg, reducing patient room entry) was accomplished most commonly by grouped timing of medication administration (76% of sites), video links to room outside of rounding times (54% of sites), the use of video or telemedicine during rounds (17%), and clustering of activities such as medication administration or phlebotomy. The most common criteria prompting discharge from the RIU were a negative COVID-19 test (59%) and hospital discharge (57%), though comments from many respondents suggested that discharge criteria were changing rapidly.

Effects of Isolation Measures on In-Room Encounters and Diagnostic Processes
More than 90% of sites reported decreases in in-room encounter frequency across all provider types whether as a result of policies in place or not. Reductions were reported among hospitalists, advanced practice providers, residents, consultants, and therapists (Table 3). Reduced room entry most often resulted from an established or developing policy, but many noted reduced room entry without formal policies in place. Nearly all sites reported moving specialty consultations to phone or video evaluations. Diagnostic error was commonly reported, with missed non–COVID-19 medical diagnoses among COVID-19 infected patients being reported by 22 sites (46%) and missed COVID-19 diagnoses in patients admitted for other reasons by 22 sites (45%).

DISCUSSION
In this study of medical wards at academic medical centers, we found that, in response to the COVID-19 pandemic, hospitals made several changes in a short period of time to adapt to the crisis. These included implementation and rapid expansion of dedicated RIUs, greatly expanded use of inpatient telehealth for patient assessments and consultation, implementation of other approaches to minimize room entry (such as grouping in-room activities), and deployment of ethics consultation services to help manage issues around potential scarcity of life-saving measures such as ventilators. We also found that availability of PPE and timely testing was limited. Finally, a large proportion of sites reported potential diagnostic problems in the assessment of both patients suspected and those not suspected of having COVID-19.
RIUs are emerging as a primary modality for caring for non-ICU COVID-19 patients, though they never involved medical students; we hope the role of students in particular will increase as new models of training emerge in response to the pandemic.7 In contrast, telemedicine evolved rapidly to hold a substantial role in RIUs, with both ward and specialty teams using video visit technology to communicate with patients. COVID-19 has been viewed as a perfect use case for outpatient telemedicine,8 and a growing number of studies are examining its outpatient use9,10; however, to date, somewhat less attention has been paid to inpatient deployment. Although our data suggest telemedicine has found a prominent place in RIUs, it remains to be seen whether it is associated with differences in patient or provider outcomes. For example, deficiencies in the physical examination, limited face-to-face contact, and lack of physical presence could all affect the patient–provider relationship, patient engagement, and the accuracy of the diagnostic process.
Our data suggest the possibility of missing non–COVID-19 diagnoses in patients suspected of COVID-19 and missing COVID-19 in those admitted for nonrespiratory reasons. The latter may be addressed as routine COVID-19 screening of admitted patients becomes commonplace. For the former, however, it is possible that physicians are “anchoring” their thinking on COVID-19 to the exclusion of other diagnoses, that physicians are not fully aware of complications unique to COVID-19 infection (such as thromboembolism), and/or that the above-mentioned limitations of telemedicine have decreased diagnostic performance.
Although PPE stockpile data were not easily available for some sites, a distressingly large number reported stockpiles of 2 weeks or less, with reuse being the most common approach to extending PPE supply. We also found it concerning that 43% of hospital leaders did not know their stockpile data; we believe this is an important question that hospital leaders need to be asking. Most sites in our study reported test turnaround times of longer than 6 hours; lack of rapid COVID-19 testing further stresses PPE stockpile and may slow patients’ transition out of the RIU or discharge to home.
Our study has several limitations, including the evolving nature of the pandemic and rapid adaptations of care systems in the pandemic’s surge phase. However, we attempted to frame our questions in ways that provided a focused snapshot of care. Furthermore, respondents may not have had exhaustive knowledge of their institution’s COVID-19 response strategies, but most were the directors of their hospitalist services, and we encouraged the respondents to confer with others to gather high-fidelity data. Finally, as a survey of large academic medical centers, our results may not apply to nonacademic centers.
Approaches to caring for non-ICU patients during the COVID-19 pandemic are rapidly evolving. Expansion of RIUs and developing the workforce to support them has been a primary focus, with rapid innovation in use of technology emerging as a critical adaptation while PPE limitations persist and needs for “medical distancing” continue to grow. Although rates of missed COVID-19 diagnoses will likely be reduced with testing and systems improvements, physicians and systems will also need to consider how to utilize emerging technology in ways that can improve clinical care and provider safety while aiding diagnostic thinking. This survey illustrates the rapid adaptations made by our hospitals in response to the pandemic; ongoing adaptation will likely be needed to optimally care for hospitalized patients with COVID-19 while the pandemic continues to evolve.
Acknowledgment
Thanks to members of the HOMERuN COVID-19 Collaborative Group: Baylor Scott & White Medical Center – Temple, Texas - Tresa McNeal MD; Beth Israel Deaconess Medical Center - Shani Herzig MD MPH, Joseph Li MD, Julius Yang MD PhD; Brigham and Women’s Hospital - Christopher Roy MD, Jeffrey Schnipper MD MPH; Cedars-Sinai Medical Center - Ed Seferian MD, ; ChristianaCare - Surekha Bhamidipati MD; Cleveland Clinic - Matthew Pappas MD MPH; Dartmouth-Hitchcock Medical Center - Jonathan Lurie MD MS; Dell Medical School at The University of Texas at Austin - Chris Moriates MD, Luci Leykum MD MBA MSc; Denver Health and Hospitals Authority - Diana Mancini MD; Emory University Hospital - Dan Hunt MD; Johns Hopkins Hospital - Daniel J Brotman MD, Zishan K Siddiqui MD, Shaker Eid MD MBA; Maine Medical Center - Daniel A Meyer MD, Robert Trowbridge MD; Massachusetts General Hospital - Melissa Mattison MD; Mayo Clinic Rochester – Caroline Burton MD, Sagar Dugani MD PhD; Medical College of Wisconsin - Sanjay Bhandari MD; Miriam Hospital - Kwame Dapaah-Afriyie MD MBA; Mount Sinai Hospital - Andrew Dunn MD; NorthShore - David Lovinger MD; Northwestern Memorial Hospital - Kevin O’Leary MD MS; Ohio State University Wexner Medical Center - Eric Schumacher DO; Oregon Health & Science University - Angela Alday MD; Penn Medicine - Ryan Greysen MD MHS MA; Rutgers- Robert Wood Johnson University Hospital - Michael Steinberg MD MPH; Stanford University School of Medicine - Neera Ahuja MD; Tulane Hospital and University Medical Center - Geraldine Ménard MD; UC San Diego Health - Ian Jenkins MD; UC Los Angeles Health - Michael Lazarus MD, Magdalena E. Ptaszny, MD; UC San Francisco Health - Bradley A Sharpe, MD, Margaret Fang MD MPH; UK HealthCare - Mark Williams MD MHM, John Romond MD; University of Chicago – David Meltzer MD PhD, Gregory Ruhnke MD; University of Colorado - Marisha Burden MD; University of Florida - Nila Radhakrishnan MD; University of Iowa Hospitals and Clinics - Kevin Glenn MD MS; University of Miami - Efren Manjarrez MD; University of Michigan - Vineet Chopra MD MSc, Valerie Vaughn MD MSc; University of Missouri-Columbia Hospital - Hasan Naqvi MD; University of Nebraska Medical Center - Chad Vokoun MD; University of North Carolina at Chapel Hill - David Hemsey MD; University of Pittsburgh Medical Center - Gena Marie Walker MD; University of Vermont Medical Center - Steven Grant MD; University of Washington Medical Center - Christopher Kim MD MBA, Andrew White MD; University of Washington-Harborview Medical Center - Maralyssa Bann MD; University of Wisconsin Hospital and Clinics - David Sterken MD, Farah Kaiksow MD MPP, Ann Sheehy MD MS, Jordan Kenik MD MPH; UW Northwest Campus - Ben Wolpaw MD; Vanderbilt University Medical Center - Sunil Kripalani MD MSc, Eduard E Vasilevskis MD, Kathleene T Wooldridge MD MPH; Wake Forest Baptist Health - Erik Summers MD; Washington University St. Louis - Michael Lin MD; Weill Cornell - Justin Choi MD; Yale New Haven Hospital - William Cushing MA, Chris Sankey MD; Zuckerberg San Francisco General Hospital - Sumant Ranji MD.
1. Institute for Health Metrics and Evaluation. COVID-19 Projections: United States of America. 2020. Accessed May 5, 2020. https://covid19.healthdata.org/united-states-of-america
2. Iserson KV. Alternative care sites: an option in disasters. West J Emerg Med. 2020;21(3):484‐489. https://doi.org/10.5811/westjem.2020.4.47552
3. Paganini M, Conti A, Weinstein E, Della Corte F, Ragazzoni L. Translating COVID-19 pandemic surge theory to practice in the emergency department: how to expand structure [online first]. Disaster Med Public Health Prep. 2020:1-10. https://doi.org/10.1017/dmp.2020.57
4. Kumaraiah D, Yip N, Ivascu N, Hill L. Innovative ICU Physician Care Models: Covid-19 Pandemic at NewYork-Presbyterian. NEJM: Catalyst. April 28, 2020. Accessed May 5, 2020. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0158
5. Auerbach AD, Patel MS, Metlay JP, et al. The Hospital Medicine Reengineering Network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. https://doi.org/10.1097/acm.0000000000000139
6. Livingston E, Desai A, Berkwits M. Sourcing personal protective equipment during the COVID-19 pandemic [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.5317
7. Bauchner H, Sharfstein J. A bold response to the COVID-19 pandemic: medical students, national service, and public health [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.6166
8. Hollander JE, Carr BG. Virtually perfect? telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679‐1681. https://doi.org/10.1056/nejmp2003539
9. Hau YS, Kim JK, Hur J, Chang MC. How about actively using telemedicine during the COVID-19 pandemic? J Med Syst. 2020;44(6):108. https://doi.org/10.1007/s10916-020-01580-z
10. Smith WR, Atala AJ, Terlecki RP, Kelly EE, Matthews CA. Implementation guide for rapid integration of an outpatient telemedicine program during the COVID-19 pandemic [online first]. J Am Coll Surg. 2020. https://doi.org/10.1016/j.jamcollsurg.2020.04.030
1. Institute for Health Metrics and Evaluation. COVID-19 Projections: United States of America. 2020. Accessed May 5, 2020. https://covid19.healthdata.org/united-states-of-america
2. Iserson KV. Alternative care sites: an option in disasters. West J Emerg Med. 2020;21(3):484‐489. https://doi.org/10.5811/westjem.2020.4.47552
3. Paganini M, Conti A, Weinstein E, Della Corte F, Ragazzoni L. Translating COVID-19 pandemic surge theory to practice in the emergency department: how to expand structure [online first]. Disaster Med Public Health Prep. 2020:1-10. https://doi.org/10.1017/dmp.2020.57
4. Kumaraiah D, Yip N, Ivascu N, Hill L. Innovative ICU Physician Care Models: Covid-19 Pandemic at NewYork-Presbyterian. NEJM: Catalyst. April 28, 2020. Accessed May 5, 2020. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0158
5. Auerbach AD, Patel MS, Metlay JP, et al. The Hospital Medicine Reengineering Network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. https://doi.org/10.1097/acm.0000000000000139
6. Livingston E, Desai A, Berkwits M. Sourcing personal protective equipment during the COVID-19 pandemic [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.5317
7. Bauchner H, Sharfstein J. A bold response to the COVID-19 pandemic: medical students, national service, and public health [online first]. JAMA. 2020. https://doi.org/10.1001/jama.2020.6166
8. Hollander JE, Carr BG. Virtually perfect? telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679‐1681. https://doi.org/10.1056/nejmp2003539
9. Hau YS, Kim JK, Hur J, Chang MC. How about actively using telemedicine during the COVID-19 pandemic? J Med Syst. 2020;44(6):108. https://doi.org/10.1007/s10916-020-01580-z
10. Smith WR, Atala AJ, Terlecki RP, Kelly EE, Matthews CA. Implementation guide for rapid integration of an outpatient telemedicine program during the COVID-19 pandemic [online first]. J Am Coll Surg. 2020. https://doi.org/10.1016/j.jamcollsurg.2020.04.030
© 2020 Society of Hospital Medicine
Evaluation of the Order SMARTT: An Initiative to Reduce Phlebotomy and Improve Sleep-Friendly Labs on General Medicine Services
Frequent daily laboratory testing for inpatients contributes to excessive costs,1 anemia,2 and unnecessary testing.3 The ABIM Foundation’s Choosing Wisely® campaign recommends avoiding routine labs, like complete blood counts (CBCs) and basic metabolic panels (BMP), in the face of clinical and laboratory stability.4,5 Prior interventions have reduced unnecessary labs without adverse outcomes.6-8
In addition to lab frequency, hospitalized patients face suboptimal lab timing. Labs are often ordered as early as 4
METHODS
Setting
This study was conducted on the University of Chicago Medicine (UCM) general medicine services, which consisted of a resident-covered service supervised by general medicine, subspecialist, or hospitalist attendings and a hospitalist service staffed by hospitalists and advanced practice providers.
Development of Order SMARTT
To inform intervention development, we surveyed providers about lab-ordering preferences with use of questions from a prior survey to provide a benchmark (Appendix Table 2).15 While reducing lab frequency was supported, the modal response for how frequently a stable patient should receive routine labs was every 48 hours (Appendix Table 2). Therefore, we hypothesized that labs ordered every 48 hours may be popular. Taking labs every 48 hours would not require an urgent 4
Physician Education
We created a 20-minute presentation on the harms of excessive labs and the benefits of sleep-friendly ordering. Instructional Order SMARTT posters were posted in clinician workrooms that emphasized forgoing labs on stable patients and using the “Order Sleep” shortcut when nonurgent labs were needed.
Labs Utilization Data
We used Epic Systems software (Verona, Wisconsin) and our institutional Tableau scorecard to obtain data on CBC and BMP ordering, patient census, and demographics for medical inpatients between July 1, 2017, and November 1, 2018.
Cost Analysis
Costs of lab tests (actual cost to our institution) were obtained from our institutional phlebotomy services’ estimates of direct variable labor and benefits costs and direct variable supplies cost.
Statistical Analysis
Data analysis was performed with SAS version 9.4 statistical software (Cary, North Carolina, USA) and R version 3.6.2 (Vienna, Austria). Descriptive statistics were used to summarize data. Surveys were analyzed using chi-square tests for categorical variables and two-sample t tests for continuous variables. For lab ordering data, interrupted time series analyses (ITSA) were used to determine the changes in ordering practices with the implementation of the two interventions controlling for service lines (resident vs hospitalist service). ITSA enables examination of changes in lab ordering while controlling for time. The AUTOREG function in SAS was used to build the model and estimate final parameters. This function automatically tests for autocorrelation, heteroscedasticity, and estimates any autoregressive parameters required in the model. Our main model tested the association between our two separate interventions on ordering practices, controlling for service (hospitalist or resident).16
RESULTS
Of 125 residents, 82 (65.6%) attended the session and completed the survey. Attendance and response rate for hospitalists was 80% (16 of 20). Similar to a prior study, many residents (73.1%) reported they would be comfortable if patients received less daily laboratory testing (Appendix Table 2).
We reviewed data from 7,045 total patients over 50,951 total patient days between July1, 2017, and November 1, 2018 (Appendix Table 3).
Total Lab Draws
After accounting for total patient days, we saw 26.3% reduction on average in total lab draws per patient-day per week postintervention (4.68 before vs 3.45 after; difference, 1.23; 95% CI, 0.82-1.63; P < .05; Appendix Table 3). When total lab draws were stratified by service, we saw 28% reduction on average in total lab draws per patient-day per week on resident services (4.67 before vs 3.36 after; difference, 1.31; 95% CI, 0.88-1.74; P < .05) and 23.9% reduction on average in lab draws/patient-day per week on the hospitalist service (4.73 before vs 3.60 after; difference, 1.13; 95% CI, 0.61-1.64; P < .05; Appendix Table 3).
Sleep-Friendly Labs by Intervention
For patients with routine labs, the proportion of sleep-friendly labs drawn per patient-day increased from 6% preintervention to 21% postintervention (P < .001). ITSA demonstrated both interventions were associated with improving lab timing. There was a statistically significant increase in sleep-friendly labs ordered per patient encounter per week immediately after the launch of “Order Sleep” (intercept, 0.49; standard error (SE), 0.14; P = .001) and the “4

Sleep-Friendly Lab Orders by Service
Over the study period, there was no significant difference in total sleep-friendly labs ordered/month between resident and hospitalist services (84.88 vs 86.19; P = .95).
In ITSA, “Order Sleep” was associated with a statistically significant immediate increase in sleep-friendly lab orders per patient encounter per week on resident services (intercept, 1.03; SE, 0.29; P < .001). However, this initial increase was followed by a decrease over time in sleep-friendly lab orders per week (slope change, –0.1; SE, 0.04; P = .02; Table, Figure B). There was no statistically significant change observed on the hospitalist service with “Order Sleep.”
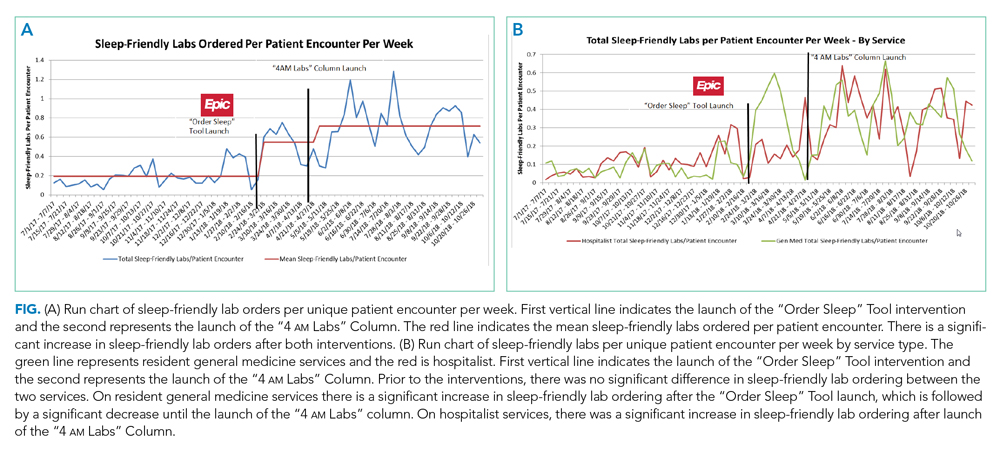
In contrast, the “4
Cost Savings
Using an estimated cost of $7.70 for CBCs and $8.01 for BMPs from our laboratory, our intervention saved an estimated $60,278 in lab costs alone over the 16-month study period (Appendix Table 4).
DISCUSSION
To our knowledge, this is the first study showing a multicomponent intervention using EHR tools can both reduce frequency and optimize timing of routine lab ordering. Our project had two interventions implemented at two different times: First, an “Order Sleep” shortcut was introduced to select sleep-friendly lab timing, including a 6
While the “Order Sleep” tool was initially associated with significant increases in sleep-friendly orders on resident services, this change was not sustained. This could have been caused by the short-lived effect of education more than sustained adoption of the tool. In contrast, the “4
The “4
While other institutions have attempted to shift lab-timing by altering phlebotomy workflows10 or via conscious decision-making on rounds,9 our study differs in several ways. We avoided default options and allowed clinicians to select sleep-friendly labs to promote buy-in. It is sometimes necessary to order 4
Our study had several limitations. First, this was a single center study on adult medicine services, which limits generalizability. Although we considered surgical services, their early rounds made deviations from 4
In conclusion, a multicomponent intervention using EHR tools can reduce inpatient daily lab frequency and optimize lab timing to help promote patient sleep.
Acknowledgments
The authors would like to thank The University of Chicago Center for Healthcare Delivery Science and Innovation for sponsoring their annual Choosing Wisely Challenge, which allowed for access to institutional support and resources for this study. We would also like to thank Mary Kate Springman, MHA, and John Fahrenbach, PhD, for their assistance with this project. Dr Tapaskar also received mentorship through the Future Leader Program for the High Value Practice Academic Alliance.
1. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-1839. https://doi.org/10.1001/jamainternmed.2017.5152
2. Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? J Gen Intern Med. 2005;20(6):520-524. https://doi.org/10.1111/j.1525-1497.2005.0094.x
3. Korenstein D, Husain S, Gennarelli RL, White C, Masciale JN, Roman BR. Impact of clinical specialty on attitudes regarding overuse of inpatient laboratory testing. J Hosp Med. 2018;13(12):844-847. https://doi.org/10.12788/jhm.2978
4. Choosing Wisely. 2020. Accessed January 10, 2020. http://www.choosingwisely.org/getting-started/
5. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. https://doi.org/10.1002/jhm.2063
6. Stuebing EA, Miner TJ. Surgical vampires and rising health care expenditure: reducing the cost of daily phlebotomy. Arch Surg. 2011;146(5):524-527. https://doi.org/10.1001/archsurg.2011.103
7. Attali M, Barel Y, Somin M, et al. A cost-effective method for reducing the volume of laboratory tests in a university-associated teaching hospital. Mt Sinai J Med. 2006;73(5):787-794.
8. Vidyarthi AR, Hamill T, Green AL, Rosenbluth G, Baron RB. Changing resident test ordering behavior: a multilevel intervention to decrease laboratory utilization at an academic medical center. Am J Med Qual. 2015;30(1):81-87. https://doi.org/10.1177/1062860613517502
9. Krafft CA, Biondi EA, Leonard MS, et al. Ending the 4 AM Blood Draw. Presented at: American Academy of Pediatrics Experience; October 25, 2015, Washington, DC. Accessed January 10, 2020. https://aap.confex.com/aap/2015/webprogrampress/Paper31640.html
10. Ramarajan V, Chima HS, Young L. Implementation of later morning specimen draws to improve patient health and satisfaction. Lab Med. 2016;47(1):e1-e4. https://doi.org/10.1093/labmed/lmv013
11. Delaney LJ, Van Haren F, Lopez V. Sleeping on a problem: the impact of sleep disturbance on intensive care patients - a clinical review. Ann Intensive Care. 2015;5:3. https://doi.org/10.1186/s13613-015-0043-2
12. Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163-178. https://doi.org/10.1016/j.smrv.2007.01.002
13. Ho A, Raja B, Waldhorn R, Baez V, Mohammed I. New onset of insomnia in hospitalized patients in general medical wards: incidence, causes, and resolution rate. J Community Hosp Int. 2017;7(5):309-313. https://doi.org/10.1080/20009666.2017.1374108
14. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091
15. Roman BR, Yang A, Masciale J, Korenstein D. Association of Attitudes Regarding Overuse of Inpatient Laboratory Testing With Health Care Provider Type. JAMA Intern Med. 2017;177(8):1205-1207. https://doi.org/10.1001/jamainternmed.2017.1634
16. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38-S44. https://doi.org/10.1016/j.acap.2013.08.002
Frequent daily laboratory testing for inpatients contributes to excessive costs,1 anemia,2 and unnecessary testing.3 The ABIM Foundation’s Choosing Wisely® campaign recommends avoiding routine labs, like complete blood counts (CBCs) and basic metabolic panels (BMP), in the face of clinical and laboratory stability.4,5 Prior interventions have reduced unnecessary labs without adverse outcomes.6-8
In addition to lab frequency, hospitalized patients face suboptimal lab timing. Labs are often ordered as early as 4
METHODS
Setting
This study was conducted on the University of Chicago Medicine (UCM) general medicine services, which consisted of a resident-covered service supervised by general medicine, subspecialist, or hospitalist attendings and a hospitalist service staffed by hospitalists and advanced practice providers.
Development of Order SMARTT
To inform intervention development, we surveyed providers about lab-ordering preferences with use of questions from a prior survey to provide a benchmark (Appendix Table 2).15 While reducing lab frequency was supported, the modal response for how frequently a stable patient should receive routine labs was every 48 hours (Appendix Table 2). Therefore, we hypothesized that labs ordered every 48 hours may be popular. Taking labs every 48 hours would not require an urgent 4
Physician Education
We created a 20-minute presentation on the harms of excessive labs and the benefits of sleep-friendly ordering. Instructional Order SMARTT posters were posted in clinician workrooms that emphasized forgoing labs on stable patients and using the “Order Sleep” shortcut when nonurgent labs were needed.
Labs Utilization Data
We used Epic Systems software (Verona, Wisconsin) and our institutional Tableau scorecard to obtain data on CBC and BMP ordering, patient census, and demographics for medical inpatients between July 1, 2017, and November 1, 2018.
Cost Analysis
Costs of lab tests (actual cost to our institution) were obtained from our institutional phlebotomy services’ estimates of direct variable labor and benefits costs and direct variable supplies cost.
Statistical Analysis
Data analysis was performed with SAS version 9.4 statistical software (Cary, North Carolina, USA) and R version 3.6.2 (Vienna, Austria). Descriptive statistics were used to summarize data. Surveys were analyzed using chi-square tests for categorical variables and two-sample t tests for continuous variables. For lab ordering data, interrupted time series analyses (ITSA) were used to determine the changes in ordering practices with the implementation of the two interventions controlling for service lines (resident vs hospitalist service). ITSA enables examination of changes in lab ordering while controlling for time. The AUTOREG function in SAS was used to build the model and estimate final parameters. This function automatically tests for autocorrelation, heteroscedasticity, and estimates any autoregressive parameters required in the model. Our main model tested the association between our two separate interventions on ordering practices, controlling for service (hospitalist or resident).16
RESULTS
Of 125 residents, 82 (65.6%) attended the session and completed the survey. Attendance and response rate for hospitalists was 80% (16 of 20). Similar to a prior study, many residents (73.1%) reported they would be comfortable if patients received less daily laboratory testing (Appendix Table 2).
We reviewed data from 7,045 total patients over 50,951 total patient days between July1, 2017, and November 1, 2018 (Appendix Table 3).
Total Lab Draws
After accounting for total patient days, we saw 26.3% reduction on average in total lab draws per patient-day per week postintervention (4.68 before vs 3.45 after; difference, 1.23; 95% CI, 0.82-1.63; P < .05; Appendix Table 3). When total lab draws were stratified by service, we saw 28% reduction on average in total lab draws per patient-day per week on resident services (4.67 before vs 3.36 after; difference, 1.31; 95% CI, 0.88-1.74; P < .05) and 23.9% reduction on average in lab draws/patient-day per week on the hospitalist service (4.73 before vs 3.60 after; difference, 1.13; 95% CI, 0.61-1.64; P < .05; Appendix Table 3).
Sleep-Friendly Labs by Intervention
For patients with routine labs, the proportion of sleep-friendly labs drawn per patient-day increased from 6% preintervention to 21% postintervention (P < .001). ITSA demonstrated both interventions were associated with improving lab timing. There was a statistically significant increase in sleep-friendly labs ordered per patient encounter per week immediately after the launch of “Order Sleep” (intercept, 0.49; standard error (SE), 0.14; P = .001) and the “4

Sleep-Friendly Lab Orders by Service
Over the study period, there was no significant difference in total sleep-friendly labs ordered/month between resident and hospitalist services (84.88 vs 86.19; P = .95).
In ITSA, “Order Sleep” was associated with a statistically significant immediate increase in sleep-friendly lab orders per patient encounter per week on resident services (intercept, 1.03; SE, 0.29; P < .001). However, this initial increase was followed by a decrease over time in sleep-friendly lab orders per week (slope change, –0.1; SE, 0.04; P = .02; Table, Figure B). There was no statistically significant change observed on the hospitalist service with “Order Sleep.”

In contrast, the “4
Cost Savings
Using an estimated cost of $7.70 for CBCs and $8.01 for BMPs from our laboratory, our intervention saved an estimated $60,278 in lab costs alone over the 16-month study period (Appendix Table 4).
DISCUSSION
To our knowledge, this is the first study showing a multicomponent intervention using EHR tools can both reduce frequency and optimize timing of routine lab ordering. Our project had two interventions implemented at two different times: First, an “Order Sleep” shortcut was introduced to select sleep-friendly lab timing, including a 6
While the “Order Sleep” tool was initially associated with significant increases in sleep-friendly orders on resident services, this change was not sustained. This could have been caused by the short-lived effect of education more than sustained adoption of the tool. In contrast, the “4
The “4
While other institutions have attempted to shift lab-timing by altering phlebotomy workflows10 or via conscious decision-making on rounds,9 our study differs in several ways. We avoided default options and allowed clinicians to select sleep-friendly labs to promote buy-in. It is sometimes necessary to order 4
Our study had several limitations. First, this was a single center study on adult medicine services, which limits generalizability. Although we considered surgical services, their early rounds made deviations from 4
In conclusion, a multicomponent intervention using EHR tools can reduce inpatient daily lab frequency and optimize lab timing to help promote patient sleep.
Acknowledgments
The authors would like to thank The University of Chicago Center for Healthcare Delivery Science and Innovation for sponsoring their annual Choosing Wisely Challenge, which allowed for access to institutional support and resources for this study. We would also like to thank Mary Kate Springman, MHA, and John Fahrenbach, PhD, for their assistance with this project. Dr Tapaskar also received mentorship through the Future Leader Program for the High Value Practice Academic Alliance.
Frequent daily laboratory testing for inpatients contributes to excessive costs,1 anemia,2 and unnecessary testing.3 The ABIM Foundation’s Choosing Wisely® campaign recommends avoiding routine labs, like complete blood counts (CBCs) and basic metabolic panels (BMP), in the face of clinical and laboratory stability.4,5 Prior interventions have reduced unnecessary labs without adverse outcomes.6-8
In addition to lab frequency, hospitalized patients face suboptimal lab timing. Labs are often ordered as early as 4
METHODS
Setting
This study was conducted on the University of Chicago Medicine (UCM) general medicine services, which consisted of a resident-covered service supervised by general medicine, subspecialist, or hospitalist attendings and a hospitalist service staffed by hospitalists and advanced practice providers.
Development of Order SMARTT
To inform intervention development, we surveyed providers about lab-ordering preferences with use of questions from a prior survey to provide a benchmark (Appendix Table 2).15 While reducing lab frequency was supported, the modal response for how frequently a stable patient should receive routine labs was every 48 hours (Appendix Table 2). Therefore, we hypothesized that labs ordered every 48 hours may be popular. Taking labs every 48 hours would not require an urgent 4
Physician Education
We created a 20-minute presentation on the harms of excessive labs and the benefits of sleep-friendly ordering. Instructional Order SMARTT posters were posted in clinician workrooms that emphasized forgoing labs on stable patients and using the “Order Sleep” shortcut when nonurgent labs were needed.
Labs Utilization Data
We used Epic Systems software (Verona, Wisconsin) and our institutional Tableau scorecard to obtain data on CBC and BMP ordering, patient census, and demographics for medical inpatients between July 1, 2017, and November 1, 2018.
Cost Analysis
Costs of lab tests (actual cost to our institution) were obtained from our institutional phlebotomy services’ estimates of direct variable labor and benefits costs and direct variable supplies cost.
Statistical Analysis
Data analysis was performed with SAS version 9.4 statistical software (Cary, North Carolina, USA) and R version 3.6.2 (Vienna, Austria). Descriptive statistics were used to summarize data. Surveys were analyzed using chi-square tests for categorical variables and two-sample t tests for continuous variables. For lab ordering data, interrupted time series analyses (ITSA) were used to determine the changes in ordering practices with the implementation of the two interventions controlling for service lines (resident vs hospitalist service). ITSA enables examination of changes in lab ordering while controlling for time. The AUTOREG function in SAS was used to build the model and estimate final parameters. This function automatically tests for autocorrelation, heteroscedasticity, and estimates any autoregressive parameters required in the model. Our main model tested the association between our two separate interventions on ordering practices, controlling for service (hospitalist or resident).16
RESULTS
Of 125 residents, 82 (65.6%) attended the session and completed the survey. Attendance and response rate for hospitalists was 80% (16 of 20). Similar to a prior study, many residents (73.1%) reported they would be comfortable if patients received less daily laboratory testing (Appendix Table 2).
We reviewed data from 7,045 total patients over 50,951 total patient days between July1, 2017, and November 1, 2018 (Appendix Table 3).
Total Lab Draws
After accounting for total patient days, we saw 26.3% reduction on average in total lab draws per patient-day per week postintervention (4.68 before vs 3.45 after; difference, 1.23; 95% CI, 0.82-1.63; P < .05; Appendix Table 3). When total lab draws were stratified by service, we saw 28% reduction on average in total lab draws per patient-day per week on resident services (4.67 before vs 3.36 after; difference, 1.31; 95% CI, 0.88-1.74; P < .05) and 23.9% reduction on average in lab draws/patient-day per week on the hospitalist service (4.73 before vs 3.60 after; difference, 1.13; 95% CI, 0.61-1.64; P < .05; Appendix Table 3).
Sleep-Friendly Labs by Intervention
For patients with routine labs, the proportion of sleep-friendly labs drawn per patient-day increased from 6% preintervention to 21% postintervention (P < .001). ITSA demonstrated both interventions were associated with improving lab timing. There was a statistically significant increase in sleep-friendly labs ordered per patient encounter per week immediately after the launch of “Order Sleep” (intercept, 0.49; standard error (SE), 0.14; P = .001) and the “4

Sleep-Friendly Lab Orders by Service
Over the study period, there was no significant difference in total sleep-friendly labs ordered/month between resident and hospitalist services (84.88 vs 86.19; P = .95).
In ITSA, “Order Sleep” was associated with a statistically significant immediate increase in sleep-friendly lab orders per patient encounter per week on resident services (intercept, 1.03; SE, 0.29; P < .001). However, this initial increase was followed by a decrease over time in sleep-friendly lab orders per week (slope change, –0.1; SE, 0.04; P = .02; Table, Figure B). There was no statistically significant change observed on the hospitalist service with “Order Sleep.”

In contrast, the “4
Cost Savings
Using an estimated cost of $7.70 for CBCs and $8.01 for BMPs from our laboratory, our intervention saved an estimated $60,278 in lab costs alone over the 16-month study period (Appendix Table 4).
DISCUSSION
To our knowledge, this is the first study showing a multicomponent intervention using EHR tools can both reduce frequency and optimize timing of routine lab ordering. Our project had two interventions implemented at two different times: First, an “Order Sleep” shortcut was introduced to select sleep-friendly lab timing, including a 6
While the “Order Sleep” tool was initially associated with significant increases in sleep-friendly orders on resident services, this change was not sustained. This could have been caused by the short-lived effect of education more than sustained adoption of the tool. In contrast, the “4
The “4
While other institutions have attempted to shift lab-timing by altering phlebotomy workflows10 or via conscious decision-making on rounds,9 our study differs in several ways. We avoided default options and allowed clinicians to select sleep-friendly labs to promote buy-in. It is sometimes necessary to order 4
Our study had several limitations. First, this was a single center study on adult medicine services, which limits generalizability. Although we considered surgical services, their early rounds made deviations from 4
In conclusion, a multicomponent intervention using EHR tools can reduce inpatient daily lab frequency and optimize lab timing to help promote patient sleep.
Acknowledgments
The authors would like to thank The University of Chicago Center for Healthcare Delivery Science and Innovation for sponsoring their annual Choosing Wisely Challenge, which allowed for access to institutional support and resources for this study. We would also like to thank Mary Kate Springman, MHA, and John Fahrenbach, PhD, for their assistance with this project. Dr Tapaskar also received mentorship through the Future Leader Program for the High Value Practice Academic Alliance.
1. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-1839. https://doi.org/10.1001/jamainternmed.2017.5152
2. Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? J Gen Intern Med. 2005;20(6):520-524. https://doi.org/10.1111/j.1525-1497.2005.0094.x
3. Korenstein D, Husain S, Gennarelli RL, White C, Masciale JN, Roman BR. Impact of clinical specialty on attitudes regarding overuse of inpatient laboratory testing. J Hosp Med. 2018;13(12):844-847. https://doi.org/10.12788/jhm.2978
4. Choosing Wisely. 2020. Accessed January 10, 2020. http://www.choosingwisely.org/getting-started/
5. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. https://doi.org/10.1002/jhm.2063
6. Stuebing EA, Miner TJ. Surgical vampires and rising health care expenditure: reducing the cost of daily phlebotomy. Arch Surg. 2011;146(5):524-527. https://doi.org/10.1001/archsurg.2011.103
7. Attali M, Barel Y, Somin M, et al. A cost-effective method for reducing the volume of laboratory tests in a university-associated teaching hospital. Mt Sinai J Med. 2006;73(5):787-794.
8. Vidyarthi AR, Hamill T, Green AL, Rosenbluth G, Baron RB. Changing resident test ordering behavior: a multilevel intervention to decrease laboratory utilization at an academic medical center. Am J Med Qual. 2015;30(1):81-87. https://doi.org/10.1177/1062860613517502
9. Krafft CA, Biondi EA, Leonard MS, et al. Ending the 4 AM Blood Draw. Presented at: American Academy of Pediatrics Experience; October 25, 2015, Washington, DC. Accessed January 10, 2020. https://aap.confex.com/aap/2015/webprogrampress/Paper31640.html
10. Ramarajan V, Chima HS, Young L. Implementation of later morning specimen draws to improve patient health and satisfaction. Lab Med. 2016;47(1):e1-e4. https://doi.org/10.1093/labmed/lmv013
11. Delaney LJ, Van Haren F, Lopez V. Sleeping on a problem: the impact of sleep disturbance on intensive care patients - a clinical review. Ann Intensive Care. 2015;5:3. https://doi.org/10.1186/s13613-015-0043-2
12. Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163-178. https://doi.org/10.1016/j.smrv.2007.01.002
13. Ho A, Raja B, Waldhorn R, Baez V, Mohammed I. New onset of insomnia in hospitalized patients in general medical wards: incidence, causes, and resolution rate. J Community Hosp Int. 2017;7(5):309-313. https://doi.org/10.1080/20009666.2017.1374108
14. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091
15. Roman BR, Yang A, Masciale J, Korenstein D. Association of Attitudes Regarding Overuse of Inpatient Laboratory Testing With Health Care Provider Type. JAMA Intern Med. 2017;177(8):1205-1207. https://doi.org/10.1001/jamainternmed.2017.1634
16. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38-S44. https://doi.org/10.1016/j.acap.2013.08.002
1. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-1839. https://doi.org/10.1001/jamainternmed.2017.5152
2. Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? J Gen Intern Med. 2005;20(6):520-524. https://doi.org/10.1111/j.1525-1497.2005.0094.x
3. Korenstein D, Husain S, Gennarelli RL, White C, Masciale JN, Roman BR. Impact of clinical specialty on attitudes regarding overuse of inpatient laboratory testing. J Hosp Med. 2018;13(12):844-847. https://doi.org/10.12788/jhm.2978
4. Choosing Wisely. 2020. Accessed January 10, 2020. http://www.choosingwisely.org/getting-started/
5. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. https://doi.org/10.1002/jhm.2063
6. Stuebing EA, Miner TJ. Surgical vampires and rising health care expenditure: reducing the cost of daily phlebotomy. Arch Surg. 2011;146(5):524-527. https://doi.org/10.1001/archsurg.2011.103
7. Attali M, Barel Y, Somin M, et al. A cost-effective method for reducing the volume of laboratory tests in a university-associated teaching hospital. Mt Sinai J Med. 2006;73(5):787-794.
8. Vidyarthi AR, Hamill T, Green AL, Rosenbluth G, Baron RB. Changing resident test ordering behavior: a multilevel intervention to decrease laboratory utilization at an academic medical center. Am J Med Qual. 2015;30(1):81-87. https://doi.org/10.1177/1062860613517502
9. Krafft CA, Biondi EA, Leonard MS, et al. Ending the 4 AM Blood Draw. Presented at: American Academy of Pediatrics Experience; October 25, 2015, Washington, DC. Accessed January 10, 2020. https://aap.confex.com/aap/2015/webprogrampress/Paper31640.html
10. Ramarajan V, Chima HS, Young L. Implementation of later morning specimen draws to improve patient health and satisfaction. Lab Med. 2016;47(1):e1-e4. https://doi.org/10.1093/labmed/lmv013
11. Delaney LJ, Van Haren F, Lopez V. Sleeping on a problem: the impact of sleep disturbance on intensive care patients - a clinical review. Ann Intensive Care. 2015;5:3. https://doi.org/10.1186/s13613-015-0043-2
12. Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163-178. https://doi.org/10.1016/j.smrv.2007.01.002
13. Ho A, Raja B, Waldhorn R, Baez V, Mohammed I. New onset of insomnia in hospitalized patients in general medical wards: incidence, causes, and resolution rate. J Community Hosp Int. 2017;7(5):309-313. https://doi.org/10.1080/20009666.2017.1374108
14. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091
15. Roman BR, Yang A, Masciale J, Korenstein D. Association of Attitudes Regarding Overuse of Inpatient Laboratory Testing With Health Care Provider Type. JAMA Intern Med. 2017;177(8):1205-1207. https://doi.org/10.1001/jamainternmed.2017.1634
16. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38-S44. https://doi.org/10.1016/j.acap.2013.08.002
© 2020 Society of Hospital Medicine
Gender Differences in Authorship of Clinical Problem-Solving Articles
A large body of evidence has demonstrated significant gender disparities in academic medicine. Women are less likely than men to reach the rank of full professor, be speakers at Grand Rounds, and author studies in medical journals.1-4 Gender-based differences in these achievements reduce the visibility of women role models in all academic medicine domains, including research, education, health systems leadership, and clinical excellence. Clinical problem-solving exercises are an opportunity to highlight the skills of women physicians as master clinicians and to establish women as clinician role models.
Clinical problem-solving exercises are highly visible demonstrations of clinical excellence in the medical literature. These exercises follow a specific format in which a clinician analyzes a diagnostic dilemma in a step-by-step manner in response to sequential segments of clinical data. The clinical problem-solving format was introduced in 1992 in the New England Journal of Medicine and has been adopted by other journals.5 (The clinical problem-solving format differs from the clinical pathologic conference format, in which an entire case is presented followed by an extended analysis). Clinical problem-solving publications are forums for learners of all levels to witness an expert clinician reason through a case.
Authorship teams on clinical reasoning exercises typically include the patient’s physician(s), specialists relevant to the final diagnosis, and the invited discussant who analyzes the clinical dilemma. Journals stipulate in the author instructions, series introductions, or standardized manuscript text of the series that the discussant be a skilled and experienced clinician.5,6 The patient’s physicians who initiate the clinical reasoning manuscript typically select the discussant; in some journals, the series editors may provide input on discussant choice. To our knowledge, this is the only author role in the medical literature in which authors are invited specifically for their diagnostic reasoning ability.
While women have been authors on fewer original research articles and guest editorials than men have,3 the proportion of women among authors of published clinical reasoning exercises is unknown. This represents a gap in our understanding of the landscape of gender inequity in academic medicine. We sought to determine the proportion of women authors in major clinical problem-solving series and examine the change in women authorship over time.
METHODS
We selected published clinical problem-solving series targeting a general medicine audience. We excluded general medicine journals in which authors were restricted to one institution or those in which the clinical problem-solving format was not a regular series. Series which met these criteria were the Clinical Problem-Solving series in the New England Journal of Medicine (NEJM), the Clinical Care Conundrums series in the Journal of Hospital Medicine (JHM), and the Exercises in Clinical Reasoning series in the Journal of General Internal Medicine (JGIM). We analyzed the proportion of women authors in each clinical reasoning series from the inaugural articles (1992 for NEJM, 2006 for JHM, and 2010 for JGIM) until July 2019. We also analyzed the change in proportion of women authors from year to year by using data up to 2018 to avoid including a partial year.
We used the gender-guesser python library7 to categorize the gender of first, last, and all authors based on their first names. The library uses a database of approximately 40,000 names8 and maps first names to the genders they are associated with across languages, classifying each name as “man,” “woman,” “mostly man,” ”mostly woman,” “androgynous,” or “unknown.” When a name is commonly associated with multiple genders, or is associated with different genders in different languages, it is classified either as mostly man, mostly woman, or androgynous. When a name is not found in the database, it is classified as unknown. For all names classified by the database as unknown, androgynous, or mostly man/mostly woman, we determined gender identities by finding the authors’ institutional webpages and consulting their listed gender pronouns. We used gender based on first name to best approximate what a reader would interpret as the author’s gender. We used gender rather than biological sex because authors may have changed their names to better express their gender identity, which may differ from sex assigned at birth.
To test for the statistical significance of changes in the proportion of women authors over time, we performed the Cochran-Armitage trend test. A P value less than .05 was considered significant.
RESULTS
We analyzed 402 articles: 280 from NEJM, 83 from JHM, and 39 from JGIM. There were 1,026 authors of clinical reasoning articles from NEJM, 362 from JHM, and 168 from JGIM. The Table shows the number of total articles, total authors, and women among first, last, and all authors by journal and by year (inaugural year and 2018). Data for all years are shown in the Appendix Table.
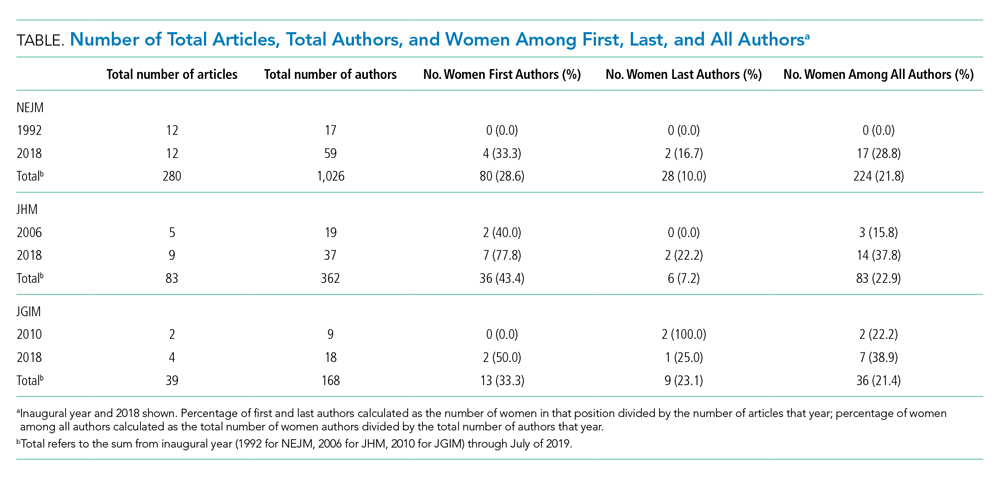
Over the entire time period studied, the percentage of women across the three journals was lowest for last authors (28/280 [10.0%] for NEJM, 6/83 [7.2%] for JHM, and 9/39 [23.1%] for JGIM) and highest for first authors (80/280 [28.6%] for NEJM, 36/83 [43.4%] for JHM, and 13/39 [33.3%] for JGIM). The percentage of women among all authors was similar for all three journals: 224/1,026 (21.8%) for NEJM, 83/362 (22.9%) for JHM, and 36/168 (21.4%) for JGIM.
The Figure shows the change in percentage of women authors from year to year through 2018. There was a significant increase in the proportion of women first authors in NEJM (from 0/12 [0.0%] in 1992 to 4/12 [33.3%] in 2018; P < .0001) and JHM (from 2/5 [40.0%] in 2006 to 7/9 [77.8%] in 2018 P = .01). There was also a significant increase in the proportion of women among all authors in NEJM (from 0/17 [0.0%] in 1992 to 17/59 [28.8%] in 2018; P < .0001) and JHM (from 3/19 [15.8%] in 2006 to 14/37 [37.8%] in 2018; P = .005). There was no significant change in the proportion of women last authors in any of the three journals. There were no statistically significant changes in JGIM authorship over time.
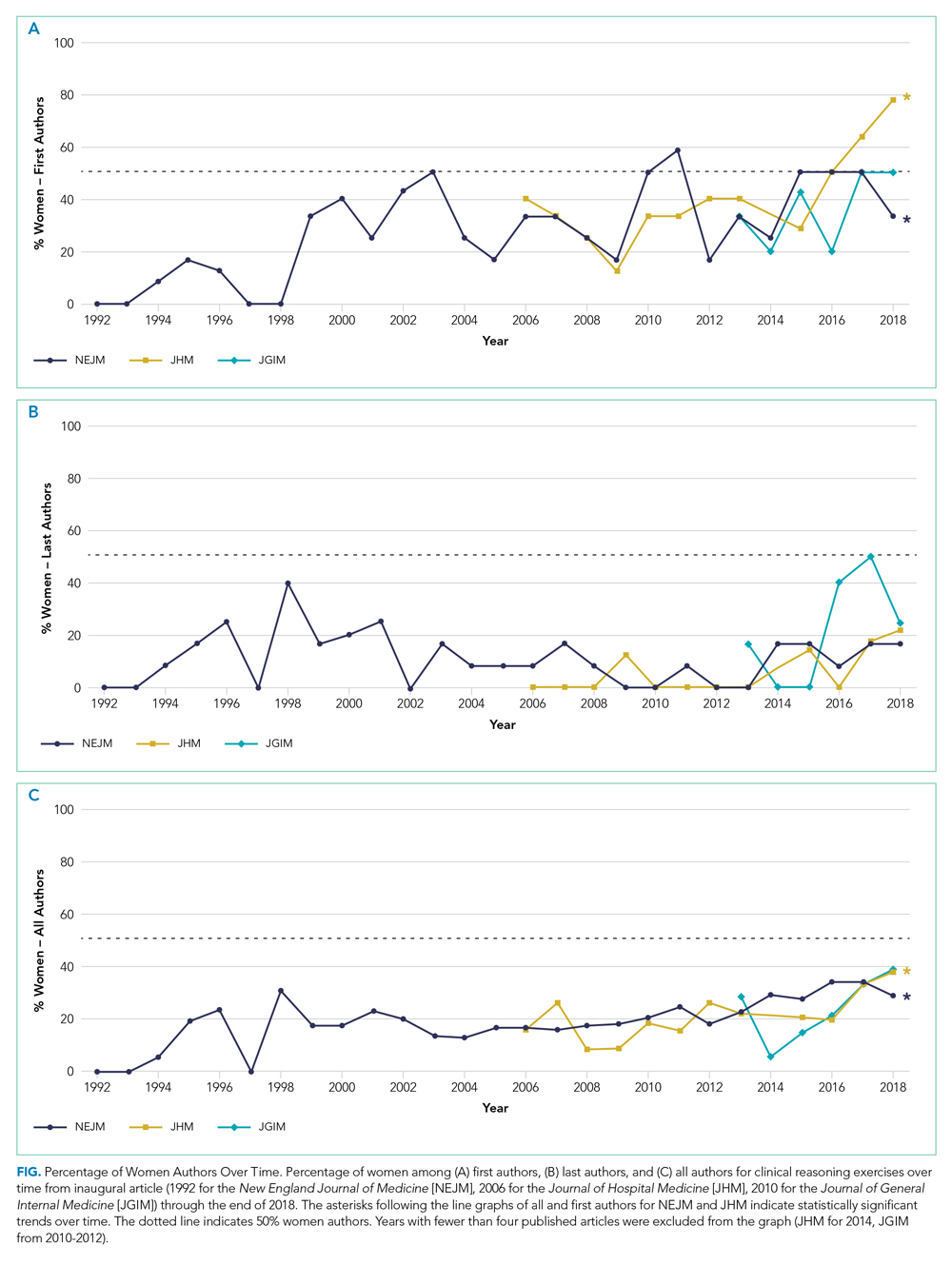
DISCUSSION
Clinical problem-solving exercises provide a forum for physicians to demonstrate diagnostic reasoning skills and clinical acumen. In this study, we focused on three prominent clinical problem-solving series in general medicine journals. We found that women authors were underrepresented in each series. The percentage of women authors has increased over time, especially among first and all authors; however, there was no change in the last author position. In all three series women still constituted less than 40% of all authors and less than 25% of last authors. In comparison, women currently constitute about 40% of general internal medicine physicians, and this proportion has been rapidly growing over time; women now represent over half of all medical school graduates as opposed to 6% in 1960.9,10 Our findings are consistent with the large body of evidence that describes gender-based differences in opportunities within academic medicine.
Prior studies have shown that gender inequities in academic medicine stem from a longstanding culture of sexism; these inequities are perpetuated in part by having too few visible women role models and mentors.11 These factors may lead to editorial practices that favor articles written by men. In addition, women may be less likely to be invited as expert discussants if other authors have a bias of associating clinical expertise with men physicians. This is consistent with data showing that women are less likely to be invited to write commentaries in peer-reviewed journals.12
Gender-based differences in authorship of clinical problem-solving publications also have important implications for women in medicine. In order to address the gender gap in academic achievement, women need visible role models and mentors.13 Including more women authors of clinical reasoning publications has the potential to establish more women as master clinicians and role models.
There are a number of actions that can help establish more women clinical problem-solving authors. Editorial boards and editors in chief should track their review and publication practices to hold themselves accountable to author diversity. For example, JHM has announced plans to analyze author representation of women and racial and ethnic minorities, including those among first and senior authors.14 Clinicians who are assembling author teams for clinical problem-solving manuscripts should also strongly consider if an equal number of men and women have been invited to serve as specialty consultants and case discussants.
Our study has limitations. We used a python library to classify author gender based on first name (supplemented by internet searches), which may have misclassified authors and did not take into account nonbinary gender identities. Because there is no convention for assigning the expert discussant to a specific author position, we could not determine the gender distribution of the discussants. However, given that women were underrepresented among first, last, and all authors in all three journals, they are likely a minority of discussants as well.
CONCLUSION
A preponderance of male voices in clinical reasoning exercises, in which learners see clinical role models, may perpetuate a culture in which women are not seen—and do not see themselves—as having the potential to be master clinicians. Including more women in clinical reasoning exercises is an opportunity to amplify the voices of women as master clinicians and combat gender discrimination in medicine.
1. Jena AB, Khullar D, Ho O, Olenski AR, Blumenthal DM. Sex differences in academic rank in US medical schools in 2014. JAMA. 2015;314(11):1149-1158. https://doi.org/10.1001/jama.2015.10680
2. Boiko JR, Anderson AJM, Gordon RA. Representation of women among academic grand rounds speakers. JAMA Intern Med. 2017;177(5):722-724. https://doi.org/10.1001/jamainternmed.2016.9646
3. Jagsi R, Guancial EA, Worobey CC, et al. The “gender gap” in authorship of academic medical literature--a 35-year perspective. N Engl J Med. 2006;355(3):281-287. https://doi.org/10.1056/nejmsa053910
4. González-Alvarez J. Author gender in The Lancet journals. Lancet. 2018;391(10140):2601. https://doi.org/10.1016/s0140-6736(18)31139-5
5. Kassirer JR. Clinical problem-solving — a new feature in the journal. N Engl J Med. 1992;326(1):60-61. https://doi.org/10.1056/nejm199201023260112
6. Henderson M, Keenan C, Kohlwes J, Dhaliwal G. Introducing exercises in clinical reasoning. J Gen Intern Med. 2010;25(1):9. https://doi.org/10.1007/s11606-009-1185-4
7. Lead Ratings; 2019. Gender Guesser, Python 3. Accessed July 7, 2019. https://github.com/lead-ratings/gender-guesser
8. Michael J. genderReader. 2007. Accessed July 7, 2019. https://github.com/cstuder/genderReader/blob/master/gender.c/gender.c
9. Association of American Medical Colleges. Active Physicians by Sex and Specialty, 2017. Physician Specialty Data Report. Accessed April 15, 2020. https://www.aamc.org/data-reports/workforce/interactive-data/active-physicians-sex-and-specialty-2017
10. Association of American Medical Colleges. More Women Than Men Enrolled in U.S. Medical Schools in 2017. AAMC Press Releases. December 17, 2017. Accessed April 15, 2020. https://www.aamc.org/news-insights/press-releases/more-women-men-enrolled-us-medical-schools-2017
11. Yedidia MJ, Bickel J. Why aren’t there more women leaders in academic medicine? the views of clinical department chairs. Acad Med. 2001;76(5):453-465. https://doi.org/10.1097/00001888-200105000-00017
12. Thomas EG, Jayabalasingham B, Collins T, Geertzen J, Bui C, Dominici F. Gender disparities in invited commentary authorship in 2459 medical journals. JAMA Netw Open. 2019;2(10):e1913682. https://doi.org/10.1001/jamanetworkopen.2019.13682
13. Mullangi S, Jagsi R. Imposter syndrome: treat the cause, not the symptom. JAMA. 2019;322(5):403-404. https://doi.org/10.1001/jama.2019.9788
14. Shah SS, Shaughnessy EE, Spector ND. Leading by example: how medical journals can improve representation in academic medicine. J Hosp Med. 2019;14(7):393. https://doi.org/10.12788/jhm.3247
A large body of evidence has demonstrated significant gender disparities in academic medicine. Women are less likely than men to reach the rank of full professor, be speakers at Grand Rounds, and author studies in medical journals.1-4 Gender-based differences in these achievements reduce the visibility of women role models in all academic medicine domains, including research, education, health systems leadership, and clinical excellence. Clinical problem-solving exercises are an opportunity to highlight the skills of women physicians as master clinicians and to establish women as clinician role models.
Clinical problem-solving exercises are highly visible demonstrations of clinical excellence in the medical literature. These exercises follow a specific format in which a clinician analyzes a diagnostic dilemma in a step-by-step manner in response to sequential segments of clinical data. The clinical problem-solving format was introduced in 1992 in the New England Journal of Medicine and has been adopted by other journals.5 (The clinical problem-solving format differs from the clinical pathologic conference format, in which an entire case is presented followed by an extended analysis). Clinical problem-solving publications are forums for learners of all levels to witness an expert clinician reason through a case.
Authorship teams on clinical reasoning exercises typically include the patient’s physician(s), specialists relevant to the final diagnosis, and the invited discussant who analyzes the clinical dilemma. Journals stipulate in the author instructions, series introductions, or standardized manuscript text of the series that the discussant be a skilled and experienced clinician.5,6 The patient’s physicians who initiate the clinical reasoning manuscript typically select the discussant; in some journals, the series editors may provide input on discussant choice. To our knowledge, this is the only author role in the medical literature in which authors are invited specifically for their diagnostic reasoning ability.
While women have been authors on fewer original research articles and guest editorials than men have,3 the proportion of women among authors of published clinical reasoning exercises is unknown. This represents a gap in our understanding of the landscape of gender inequity in academic medicine. We sought to determine the proportion of women authors in major clinical problem-solving series and examine the change in women authorship over time.
METHODS
We selected published clinical problem-solving series targeting a general medicine audience. We excluded general medicine journals in which authors were restricted to one institution or those in which the clinical problem-solving format was not a regular series. Series which met these criteria were the Clinical Problem-Solving series in the New England Journal of Medicine (NEJM), the Clinical Care Conundrums series in the Journal of Hospital Medicine (JHM), and the Exercises in Clinical Reasoning series in the Journal of General Internal Medicine (JGIM). We analyzed the proportion of women authors in each clinical reasoning series from the inaugural articles (1992 for NEJM, 2006 for JHM, and 2010 for JGIM) until July 2019. We also analyzed the change in proportion of women authors from year to year by using data up to 2018 to avoid including a partial year.
We used the gender-guesser python library7 to categorize the gender of first, last, and all authors based on their first names. The library uses a database of approximately 40,000 names8 and maps first names to the genders they are associated with across languages, classifying each name as “man,” “woman,” “mostly man,” ”mostly woman,” “androgynous,” or “unknown.” When a name is commonly associated with multiple genders, or is associated with different genders in different languages, it is classified either as mostly man, mostly woman, or androgynous. When a name is not found in the database, it is classified as unknown. For all names classified by the database as unknown, androgynous, or mostly man/mostly woman, we determined gender identities by finding the authors’ institutional webpages and consulting their listed gender pronouns. We used gender based on first name to best approximate what a reader would interpret as the author’s gender. We used gender rather than biological sex because authors may have changed their names to better express their gender identity, which may differ from sex assigned at birth.
To test for the statistical significance of changes in the proportion of women authors over time, we performed the Cochran-Armitage trend test. A P value less than .05 was considered significant.
RESULTS
We analyzed 402 articles: 280 from NEJM, 83 from JHM, and 39 from JGIM. There were 1,026 authors of clinical reasoning articles from NEJM, 362 from JHM, and 168 from JGIM. The Table shows the number of total articles, total authors, and women among first, last, and all authors by journal and by year (inaugural year and 2018). Data for all years are shown in the Appendix Table.

Over the entire time period studied, the percentage of women across the three journals was lowest for last authors (28/280 [10.0%] for NEJM, 6/83 [7.2%] for JHM, and 9/39 [23.1%] for JGIM) and highest for first authors (80/280 [28.6%] for NEJM, 36/83 [43.4%] for JHM, and 13/39 [33.3%] for JGIM). The percentage of women among all authors was similar for all three journals: 224/1,026 (21.8%) for NEJM, 83/362 (22.9%) for JHM, and 36/168 (21.4%) for JGIM.
The Figure shows the change in percentage of women authors from year to year through 2018. There was a significant increase in the proportion of women first authors in NEJM (from 0/12 [0.0%] in 1992 to 4/12 [33.3%] in 2018; P < .0001) and JHM (from 2/5 [40.0%] in 2006 to 7/9 [77.8%] in 2018 P = .01). There was also a significant increase in the proportion of women among all authors in NEJM (from 0/17 [0.0%] in 1992 to 17/59 [28.8%] in 2018; P < .0001) and JHM (from 3/19 [15.8%] in 2006 to 14/37 [37.8%] in 2018; P = .005). There was no significant change in the proportion of women last authors in any of the three journals. There were no statistically significant changes in JGIM authorship over time.

DISCUSSION
Clinical problem-solving exercises provide a forum for physicians to demonstrate diagnostic reasoning skills and clinical acumen. In this study, we focused on three prominent clinical problem-solving series in general medicine journals. We found that women authors were underrepresented in each series. The percentage of women authors has increased over time, especially among first and all authors; however, there was no change in the last author position. In all three series women still constituted less than 40% of all authors and less than 25% of last authors. In comparison, women currently constitute about 40% of general internal medicine physicians, and this proportion has been rapidly growing over time; women now represent over half of all medical school graduates as opposed to 6% in 1960.9,10 Our findings are consistent with the large body of evidence that describes gender-based differences in opportunities within academic medicine.
Prior studies have shown that gender inequities in academic medicine stem from a longstanding culture of sexism; these inequities are perpetuated in part by having too few visible women role models and mentors.11 These factors may lead to editorial practices that favor articles written by men. In addition, women may be less likely to be invited as expert discussants if other authors have a bias of associating clinical expertise with men physicians. This is consistent with data showing that women are less likely to be invited to write commentaries in peer-reviewed journals.12
Gender-based differences in authorship of clinical problem-solving publications also have important implications for women in medicine. In order to address the gender gap in academic achievement, women need visible role models and mentors.13 Including more women authors of clinical reasoning publications has the potential to establish more women as master clinicians and role models.
There are a number of actions that can help establish more women clinical problem-solving authors. Editorial boards and editors in chief should track their review and publication practices to hold themselves accountable to author diversity. For example, JHM has announced plans to analyze author representation of women and racial and ethnic minorities, including those among first and senior authors.14 Clinicians who are assembling author teams for clinical problem-solving manuscripts should also strongly consider if an equal number of men and women have been invited to serve as specialty consultants and case discussants.
Our study has limitations. We used a python library to classify author gender based on first name (supplemented by internet searches), which may have misclassified authors and did not take into account nonbinary gender identities. Because there is no convention for assigning the expert discussant to a specific author position, we could not determine the gender distribution of the discussants. However, given that women were underrepresented among first, last, and all authors in all three journals, they are likely a minority of discussants as well.
CONCLUSION
A preponderance of male voices in clinical reasoning exercises, in which learners see clinical role models, may perpetuate a culture in which women are not seen—and do not see themselves—as having the potential to be master clinicians. Including more women in clinical reasoning exercises is an opportunity to amplify the voices of women as master clinicians and combat gender discrimination in medicine.
A large body of evidence has demonstrated significant gender disparities in academic medicine. Women are less likely than men to reach the rank of full professor, be speakers at Grand Rounds, and author studies in medical journals.1-4 Gender-based differences in these achievements reduce the visibility of women role models in all academic medicine domains, including research, education, health systems leadership, and clinical excellence. Clinical problem-solving exercises are an opportunity to highlight the skills of women physicians as master clinicians and to establish women as clinician role models.
Clinical problem-solving exercises are highly visible demonstrations of clinical excellence in the medical literature. These exercises follow a specific format in which a clinician analyzes a diagnostic dilemma in a step-by-step manner in response to sequential segments of clinical data. The clinical problem-solving format was introduced in 1992 in the New England Journal of Medicine and has been adopted by other journals.5 (The clinical problem-solving format differs from the clinical pathologic conference format, in which an entire case is presented followed by an extended analysis). Clinical problem-solving publications are forums for learners of all levels to witness an expert clinician reason through a case.
Authorship teams on clinical reasoning exercises typically include the patient’s physician(s), specialists relevant to the final diagnosis, and the invited discussant who analyzes the clinical dilemma. Journals stipulate in the author instructions, series introductions, or standardized manuscript text of the series that the discussant be a skilled and experienced clinician.5,6 The patient’s physicians who initiate the clinical reasoning manuscript typically select the discussant; in some journals, the series editors may provide input on discussant choice. To our knowledge, this is the only author role in the medical literature in which authors are invited specifically for their diagnostic reasoning ability.
While women have been authors on fewer original research articles and guest editorials than men have,3 the proportion of women among authors of published clinical reasoning exercises is unknown. This represents a gap in our understanding of the landscape of gender inequity in academic medicine. We sought to determine the proportion of women authors in major clinical problem-solving series and examine the change in women authorship over time.
METHODS
We selected published clinical problem-solving series targeting a general medicine audience. We excluded general medicine journals in which authors were restricted to one institution or those in which the clinical problem-solving format was not a regular series. Series which met these criteria were the Clinical Problem-Solving series in the New England Journal of Medicine (NEJM), the Clinical Care Conundrums series in the Journal of Hospital Medicine (JHM), and the Exercises in Clinical Reasoning series in the Journal of General Internal Medicine (JGIM). We analyzed the proportion of women authors in each clinical reasoning series from the inaugural articles (1992 for NEJM, 2006 for JHM, and 2010 for JGIM) until July 2019. We also analyzed the change in proportion of women authors from year to year by using data up to 2018 to avoid including a partial year.
We used the gender-guesser python library7 to categorize the gender of first, last, and all authors based on their first names. The library uses a database of approximately 40,000 names8 and maps first names to the genders they are associated with across languages, classifying each name as “man,” “woman,” “mostly man,” ”mostly woman,” “androgynous,” or “unknown.” When a name is commonly associated with multiple genders, or is associated with different genders in different languages, it is classified either as mostly man, mostly woman, or androgynous. When a name is not found in the database, it is classified as unknown. For all names classified by the database as unknown, androgynous, or mostly man/mostly woman, we determined gender identities by finding the authors’ institutional webpages and consulting their listed gender pronouns. We used gender based on first name to best approximate what a reader would interpret as the author’s gender. We used gender rather than biological sex because authors may have changed their names to better express their gender identity, which may differ from sex assigned at birth.
To test for the statistical significance of changes in the proportion of women authors over time, we performed the Cochran-Armitage trend test. A P value less than .05 was considered significant.
RESULTS
We analyzed 402 articles: 280 from NEJM, 83 from JHM, and 39 from JGIM. There were 1,026 authors of clinical reasoning articles from NEJM, 362 from JHM, and 168 from JGIM. The Table shows the number of total articles, total authors, and women among first, last, and all authors by journal and by year (inaugural year and 2018). Data for all years are shown in the Appendix Table.

Over the entire time period studied, the percentage of women across the three journals was lowest for last authors (28/280 [10.0%] for NEJM, 6/83 [7.2%] for JHM, and 9/39 [23.1%] for JGIM) and highest for first authors (80/280 [28.6%] for NEJM, 36/83 [43.4%] for JHM, and 13/39 [33.3%] for JGIM). The percentage of women among all authors was similar for all three journals: 224/1,026 (21.8%) for NEJM, 83/362 (22.9%) for JHM, and 36/168 (21.4%) for JGIM.
The Figure shows the change in percentage of women authors from year to year through 2018. There was a significant increase in the proportion of women first authors in NEJM (from 0/12 [0.0%] in 1992 to 4/12 [33.3%] in 2018; P < .0001) and JHM (from 2/5 [40.0%] in 2006 to 7/9 [77.8%] in 2018 P = .01). There was also a significant increase in the proportion of women among all authors in NEJM (from 0/17 [0.0%] in 1992 to 17/59 [28.8%] in 2018; P < .0001) and JHM (from 3/19 [15.8%] in 2006 to 14/37 [37.8%] in 2018; P = .005). There was no significant change in the proportion of women last authors in any of the three journals. There were no statistically significant changes in JGIM authorship over time.

DISCUSSION
Clinical problem-solving exercises provide a forum for physicians to demonstrate diagnostic reasoning skills and clinical acumen. In this study, we focused on three prominent clinical problem-solving series in general medicine journals. We found that women authors were underrepresented in each series. The percentage of women authors has increased over time, especially among first and all authors; however, there was no change in the last author position. In all three series women still constituted less than 40% of all authors and less than 25% of last authors. In comparison, women currently constitute about 40% of general internal medicine physicians, and this proportion has been rapidly growing over time; women now represent over half of all medical school graduates as opposed to 6% in 1960.9,10 Our findings are consistent with the large body of evidence that describes gender-based differences in opportunities within academic medicine.
Prior studies have shown that gender inequities in academic medicine stem from a longstanding culture of sexism; these inequities are perpetuated in part by having too few visible women role models and mentors.11 These factors may lead to editorial practices that favor articles written by men. In addition, women may be less likely to be invited as expert discussants if other authors have a bias of associating clinical expertise with men physicians. This is consistent with data showing that women are less likely to be invited to write commentaries in peer-reviewed journals.12
Gender-based differences in authorship of clinical problem-solving publications also have important implications for women in medicine. In order to address the gender gap in academic achievement, women need visible role models and mentors.13 Including more women authors of clinical reasoning publications has the potential to establish more women as master clinicians and role models.
There are a number of actions that can help establish more women clinical problem-solving authors. Editorial boards and editors in chief should track their review and publication practices to hold themselves accountable to author diversity. For example, JHM has announced plans to analyze author representation of women and racial and ethnic minorities, including those among first and senior authors.14 Clinicians who are assembling author teams for clinical problem-solving manuscripts should also strongly consider if an equal number of men and women have been invited to serve as specialty consultants and case discussants.
Our study has limitations. We used a python library to classify author gender based on first name (supplemented by internet searches), which may have misclassified authors and did not take into account nonbinary gender identities. Because there is no convention for assigning the expert discussant to a specific author position, we could not determine the gender distribution of the discussants. However, given that women were underrepresented among first, last, and all authors in all three journals, they are likely a minority of discussants as well.
CONCLUSION
A preponderance of male voices in clinical reasoning exercises, in which learners see clinical role models, may perpetuate a culture in which women are not seen—and do not see themselves—as having the potential to be master clinicians. Including more women in clinical reasoning exercises is an opportunity to amplify the voices of women as master clinicians and combat gender discrimination in medicine.
1. Jena AB, Khullar D, Ho O, Olenski AR, Blumenthal DM. Sex differences in academic rank in US medical schools in 2014. JAMA. 2015;314(11):1149-1158. https://doi.org/10.1001/jama.2015.10680
2. Boiko JR, Anderson AJM, Gordon RA. Representation of women among academic grand rounds speakers. JAMA Intern Med. 2017;177(5):722-724. https://doi.org/10.1001/jamainternmed.2016.9646
3. Jagsi R, Guancial EA, Worobey CC, et al. The “gender gap” in authorship of academic medical literature--a 35-year perspective. N Engl J Med. 2006;355(3):281-287. https://doi.org/10.1056/nejmsa053910
4. González-Alvarez J. Author gender in The Lancet journals. Lancet. 2018;391(10140):2601. https://doi.org/10.1016/s0140-6736(18)31139-5
5. Kassirer JR. Clinical problem-solving — a new feature in the journal. N Engl J Med. 1992;326(1):60-61. https://doi.org/10.1056/nejm199201023260112
6. Henderson M, Keenan C, Kohlwes J, Dhaliwal G. Introducing exercises in clinical reasoning. J Gen Intern Med. 2010;25(1):9. https://doi.org/10.1007/s11606-009-1185-4
7. Lead Ratings; 2019. Gender Guesser, Python 3. Accessed July 7, 2019. https://github.com/lead-ratings/gender-guesser
8. Michael J. genderReader. 2007. Accessed July 7, 2019. https://github.com/cstuder/genderReader/blob/master/gender.c/gender.c
9. Association of American Medical Colleges. Active Physicians by Sex and Specialty, 2017. Physician Specialty Data Report. Accessed April 15, 2020. https://www.aamc.org/data-reports/workforce/interactive-data/active-physicians-sex-and-specialty-2017
10. Association of American Medical Colleges. More Women Than Men Enrolled in U.S. Medical Schools in 2017. AAMC Press Releases. December 17, 2017. Accessed April 15, 2020. https://www.aamc.org/news-insights/press-releases/more-women-men-enrolled-us-medical-schools-2017
11. Yedidia MJ, Bickel J. Why aren’t there more women leaders in academic medicine? the views of clinical department chairs. Acad Med. 2001;76(5):453-465. https://doi.org/10.1097/00001888-200105000-00017
12. Thomas EG, Jayabalasingham B, Collins T, Geertzen J, Bui C, Dominici F. Gender disparities in invited commentary authorship in 2459 medical journals. JAMA Netw Open. 2019;2(10):e1913682. https://doi.org/10.1001/jamanetworkopen.2019.13682
13. Mullangi S, Jagsi R. Imposter syndrome: treat the cause, not the symptom. JAMA. 2019;322(5):403-404. https://doi.org/10.1001/jama.2019.9788
14. Shah SS, Shaughnessy EE, Spector ND. Leading by example: how medical journals can improve representation in academic medicine. J Hosp Med. 2019;14(7):393. https://doi.org/10.12788/jhm.3247
1. Jena AB, Khullar D, Ho O, Olenski AR, Blumenthal DM. Sex differences in academic rank in US medical schools in 2014. JAMA. 2015;314(11):1149-1158. https://doi.org/10.1001/jama.2015.10680
2. Boiko JR, Anderson AJM, Gordon RA. Representation of women among academic grand rounds speakers. JAMA Intern Med. 2017;177(5):722-724. https://doi.org/10.1001/jamainternmed.2016.9646
3. Jagsi R, Guancial EA, Worobey CC, et al. The “gender gap” in authorship of academic medical literature--a 35-year perspective. N Engl J Med. 2006;355(3):281-287. https://doi.org/10.1056/nejmsa053910
4. González-Alvarez J. Author gender in The Lancet journals. Lancet. 2018;391(10140):2601. https://doi.org/10.1016/s0140-6736(18)31139-5
5. Kassirer JR. Clinical problem-solving — a new feature in the journal. N Engl J Med. 1992;326(1):60-61. https://doi.org/10.1056/nejm199201023260112
6. Henderson M, Keenan C, Kohlwes J, Dhaliwal G. Introducing exercises in clinical reasoning. J Gen Intern Med. 2010;25(1):9. https://doi.org/10.1007/s11606-009-1185-4
7. Lead Ratings; 2019. Gender Guesser, Python 3. Accessed July 7, 2019. https://github.com/lead-ratings/gender-guesser
8. Michael J. genderReader. 2007. Accessed July 7, 2019. https://github.com/cstuder/genderReader/blob/master/gender.c/gender.c
9. Association of American Medical Colleges. Active Physicians by Sex and Specialty, 2017. Physician Specialty Data Report. Accessed April 15, 2020. https://www.aamc.org/data-reports/workforce/interactive-data/active-physicians-sex-and-specialty-2017
10. Association of American Medical Colleges. More Women Than Men Enrolled in U.S. Medical Schools in 2017. AAMC Press Releases. December 17, 2017. Accessed April 15, 2020. https://www.aamc.org/news-insights/press-releases/more-women-men-enrolled-us-medical-schools-2017
11. Yedidia MJ, Bickel J. Why aren’t there more women leaders in academic medicine? the views of clinical department chairs. Acad Med. 2001;76(5):453-465. https://doi.org/10.1097/00001888-200105000-00017
12. Thomas EG, Jayabalasingham B, Collins T, Geertzen J, Bui C, Dominici F. Gender disparities in invited commentary authorship in 2459 medical journals. JAMA Netw Open. 2019;2(10):e1913682. https://doi.org/10.1001/jamanetworkopen.2019.13682
13. Mullangi S, Jagsi R. Imposter syndrome: treat the cause, not the symptom. JAMA. 2019;322(5):403-404. https://doi.org/10.1001/jama.2019.9788
14. Shah SS, Shaughnessy EE, Spector ND. Leading by example: how medical journals can improve representation in academic medicine. J Hosp Med. 2019;14(7):393. https://doi.org/10.12788/jhm.3247
© 2020 Society of Hospital Medicine
Establishing an Orthopedic Excess Hospital Days in Acute Care Program
Total joint arthroplasty (TJA) procedures currently account for more Medicare expenses than any other inpatient procedure.1 In 2015, Centers for Medicare & Medicaid Services (CMS) announced the Comprehensive Care for Joint Replacement (CJR) model in which hospitals are paid one bundled payment for all related items and services utilized within a 90-day episode of care.2 Recent studies have suggested that the best opportunity to lower episode costs appears to be in the post-acute care setting and reducing readmissions.1,3
Surgical comanagement, which provides shared management of surgical patients between surgeons and hospitalists, is typically used in orthopedic surgery, neurosurgery, vascular surgery, and general surgery.4 Among patients with at least one medical comorbidity, surgical comanagement decreases length of stay (LOS), 30-day readmission rate for medical causes, and the proportion of patients with at least two medical consultants.5,6 Not all studies have shown that comanagement is beneficial. Maxwell et al found no significant differences in mortality or morbidity among hip fracture patients who did or did not receive comanagement7; however, comanaged patients were older and had more significant comorbidities, and there was no standard definition of comanagement among the participating institutions.
Comanagement after patients are discharged is a concept that has not been previously published but may become important with the Bundled Payments for Care Improvement initiative and high costs of excess days in acute care (EDAC). Hospitalists may be able to continue their work after discharge as part of the 90-day episode of care.8 TJA patients often have comorbidities, and surgical site infections and cardiovascular events are the most common causes of 30-day TJA readmissions.9
At our institution, 25% of TJA patients who presented to the Emergency Department (ED) within 90 days of surgery required a stay of less than 48 hours for conditions that did not require inpatient level of care. In addition, 50% of readmissions were secondary to medical complications. We also found significant variation in the management of common postoperative complications, such as postoperative fever, dislocation, anemia, and shortness of breath, especially among the different service lines caring for these patients. Therefore, we developed an Orthopedic EDAC program to reduce readmissions and to implement standardized admission algorithms and evidenced-based treatment protocols for common postoperative problems.
METHODS
Setting/Participants
We included patients who underwent total knee arthroplasty (TKA), total hip arthroplasty (THA), revision TKA, or revision THA from April 1, 2017, to September 30, 2018, at an urban teaching hospital. Patients were followed for 90 days after discharge. Factors such as age, sex, race, primary payer, Medicare Severity-Diagnosis Related Group (MS-DRG), discharge destination (home, home with home health, skilled nursing facility [SNF], acute rehab, other), and EDAC LOS were compared. An interdisciplinary committee comprising representatives from orthopedic surgery, hospital medicine, emergency medicine, and case management formulated observation criteria for the Orthopedic EDAC program. To be eligible for inclusion, observation patients had to have re-presented within 90 days from their initial surgery, could not be safely discharged home immediately from the ED, and did not require inpatient level of care. Patients qualifying for orthopedic observation were assigned rooms on the orthopedic wards to maintain continuity with nursing, physical therapy/occupational therapy, and case management staff. The University of Pennsylvania institutional review board reviewed this study and determined the project to be exempt.
Study Design
The Figure shows the admitting algorithm for TJA patients re-presenting within 90 days of their surgery. The ED evaluated the eligible patients; if they were not able to discharge the patient home, they notified the orthopedic resident on call for evaluation. Eligible diagnoses for the orthopedic observation in which orthopedics was the primary service included the need for postoperative pain control, fever (without signs or symptoms of sepsis), deep venous thrombosis or pulmonary embolism without hemodynamic instability, hemodynamically stable hypovolemia, symptomatic anemia secondary to acute blood loss anemia following surgery, and postoperative nausea, vomiting, constipation, ileus, and cellulitis. Eligible diagnoses for medical observation on the Medicine service included mild exacerbations of chronic obstructive pulmonary disease (COPD), syncope, upper respiratory tract infections, chest pain, delirium, and other exacerbation of medical problems. Full admission to Orthopedics included patients with wound infections requiring surgical washout, periprosthetic fractures/hematoma requiring operative management, and wound dehiscence requiring repair. All other readmissions requiring a stay of 48 or more hours were admitted to the medical or subspecialty medical service lines (eg, internal medicine, family medicine, geriatrics, cardiology, or pulmonary critical care).
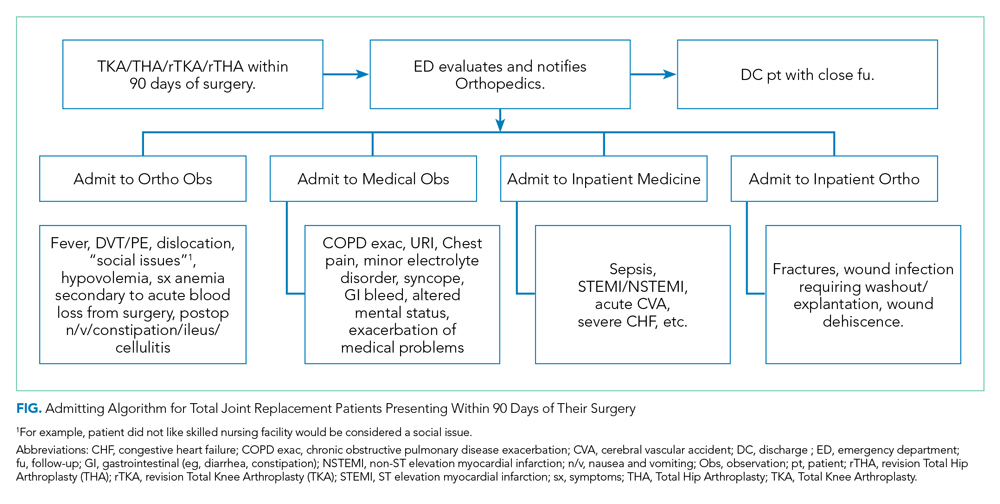
Development of Evidence-Based Algorithms
Patients who re-presented to acute care (for either observation stays or readmissions) were treated based on standardized algorithms. The interdisciplinary work group developed evidence-based evaluation and treatment plans for common postoperative problems, including postoperative fever, postoperative shortness of breath, and postoperative septic joints. This was based on a comprehensive literature review and consensus among emergency medicine, hospital medicine, and orthopedic surgery. Appendix 1 illustrates an example of a standardized algorithm for the workup of hypoxia.
Definition of Readmissions and EDAC
Readmission and observation stays were flagged on re-presentation, and reasons for readmission or observation status were analyzed. Observation cutoffs of “successful” (<48 hours) vs “unsuccessful” (≥48 hours and/or conversion to inpatient status) were based on the CMS Two-Midnight Rule in accordance with past studies.10 Readmissions were defined as patients who required an acute stay of 48 or more hours within 90 days of discharge from their original surgical stay. Patients admitted under observation status who required a stay of less than 48 hours did not count as a readmission but did count toward EDAC.
We acknowledge that our definition of Orthopedic EDAC is not the same as CMS’s definition of EDAC for other conditions such as congestive heart failure, which includes hours in observation, readmissions, and ED visits. We focused on studying and reducing days in the hospital (observation status and readmissions), and our intervention was not intended to prevent issues that would cause patients to present to the ED. Therefore, including ED visits in our operational definition of EDAC would add an unnecessary source of confounding that would bias our results toward the null hypothesis.
Data Collection and Data Analysis
The Orthopedic EDAC program was implemented on October 1, 2017, based on the above triage and treatment plans. We analyzed demographic and outcome data (readmissions, LOS, time in observation status, reason for readmission/observation status) for 6 months prior (April 1, 2017, to September 30, 2017) and 1 year after (October 1, 2017, to September 30, 2018). Microsoft Excel (Jones, 2013) was used for data analysis. Paired t-test with P < .05 was predefined as significant.
Eligible patients were identified from previous admission diagnoses obtained through Vizient, which is a collaboration of academic medical centers that maintains a hospital discharge data set (the Clinical Data Base/Resource Manager CDB/RM). It included patient demographics, discharge diagnoses, procedures, and outcomes.11 The Vizient database is a respected source of data and has been used for several scholarly studies.10-12 We queried the Vizient Clinical Data Base/Resource Manager v. 8.12.0.11 (Vizient Inc., Irvine, TX) for the following data from both before and after the program’s implementation: disposition, LOS, insurance information, gender, type of surgery, MS-DRG, and race.
The five included MS-DRGs represented major hip and knee joint replacements with and without major comorbid conditions (MCCs; MS-DRG 469 and MS-DRG 470, respectively) and revision hip or knee replacement with MCCs, with comorbid conditions (CCs), and without MCCs or CCs (MS-DRG 466, MS-DRG 467, and MS-DRG 468, respectively). MCCs included but were not limited to decubitus ulcer, severe malnutrition, quadriplegia, and end-stage renal disease. Examples of CCs included transplant patients, lymphoma, leukemia, and malignancies (except breast or prostate), based on CMS definitions.13
RESULTS
Table 1 compares the demographics of the pre-implementation and post-implementation periods. There were a total of 2,662 admissions (799 before program implementation and 1,863 after). TKA and THA patients without MCCs (MS-DRG 470) accounted for 80% of patients during both periods. In both periods, approximately 60% of patients were female, 50% of patients were White, 40% were Black, and 10% were another race. The mean age was 63.6 years old. Most patients had Medicare or commercial insurance. Discharge destinations were similar during both periods.
Table 2 illustrates how the patients who re-presented to acute care were triaged based on the algorithm described in the Figure. Among the 64 patients who re-presented during the pre-implementation period, there were no observation stays; there were 38 patients who were placed under medicine inpatient services. During post-implementation, there were 48 patients (29 on orthopedics, 17 on medicine, and 2 on other service lines) who were admitted under observation status. Twenty-three patients were discharged on observation status. Of those patients, 20 were admitted to orthopedic observation and 3 patients to medicine observation. Among the 71 patients who re-presented during the post-implementation period, 40.8% (29 patients) were admitted to inpatient orthopedic services, and 17 patients were readmitted to medicine services (24.9%). Among re-presenting patients, 70% were admitted to orthopedics inpatient and observation combined, in contrast to just 35% during the pre-implementation period.
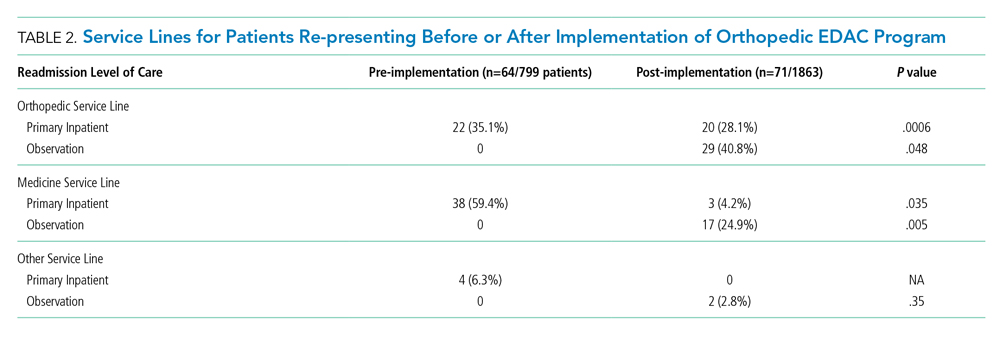
Readmissions decreased from 6.1% during pre-implementation to 2% during post-implementation (P = .004). In addition, the LOS for patients re-presenting during post-implementation was significantly lower than it was during pre-implementation. Table 3 details the associated LOS based on study period and readmission diagnosis. The aggregate LOS for all readmissions decreased from 7.75 days to 4.73 days (P = .005). The LOS decreased across all realms of readmission diagnoses. An outlier with an LOS greater than 100 days was removed from the pre-implementation group.
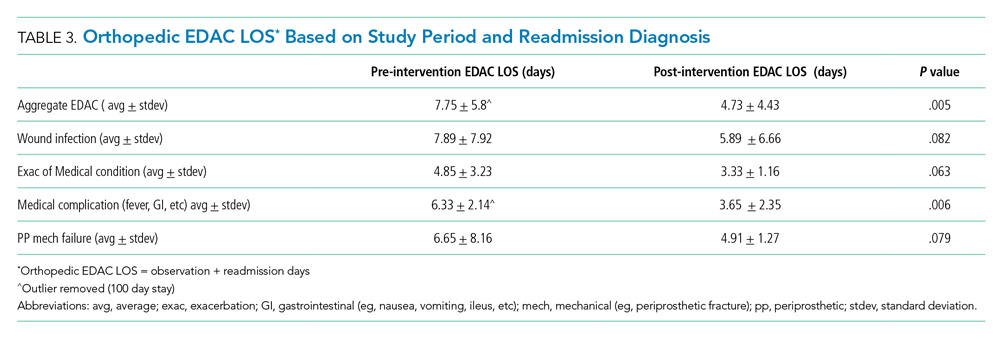
Appendix 2 further looked at patients who had observation orders, reasons for observation stay, and which patients were able to be discharged on observation status. Patients with medical complications such as fever and urinary tract infection were more likely to be discharged on observation status than were patients with wound drainage or redness that was concerning for a periprosthetic joint infection.
DISCUSSION
To our knowledge, this is the first description of a published Orthopedic EDAC program using orthopedic observation, standardized admitting and treatment algorithms, and comanagement of patients who re-presented after their original surgery. The development of an Orthopedic EDAC program at our hospital with comanagement was successful in reducing readmissions, decreasing LOS for readmitted patients, and increasing continuity of care. A number of points require more elaboration.
The Orthopedic EDAC program’s improvement in both reducing readmissions and decreasing LOS for EDAC (including days for observation and readmissions) was not caused by simply shifting patients with shorter LOS from inpatient to observation because the inpatients did not have a longer LOS. We had lower Orthopedic EDAC during the post-implementation vs pre-implementation even when considering EDAC in terms of both observation and readmissions. The decrease in readmissions is not only from the patients that were discharged on observation status, but also a result of other concurrent interventions, such as encouraging discharge to home rather than to rehabilitation facilities and more rigorous preoperative optimization.
The national rates of 30- and 90-day readmissions after primary TKA were 4% (95% CI, 3.8%-4.0%) and 7% (95% CI, 6.8%-7.2%), respectively,10 and the average cost of readmission for medical causes was $22,775 for THA and $11,682 for TKA.12 If one considers the 23 “saved readmissions” with 12 surgical complications and 11 medical complications, we “saved” roughly $591,105. Also, with the decrease in LOS for each readmission for any cause from 7.75 days to 4.73 days, the 48 readmissions had a 150 day lower LOS overall. With the average hospital day costing $2,289/day at nonprofit hospitals,13 there are additional cost savings of $343,350 overall. Therefore, the grand total estimated savings during this pilot was $934,455.
The decrease in post-implementation LOS vs pre-implementation LOS was likely multifactorial. The Orthopedic EDAC program improved continuity of care with orthopedic surgery and support staff (registered nurses, social workers, physical therapists) and utilized standardized protocols for work-up of common postoperative problems. These evidence-based protocols reduced waste that resulted in less testing with fewer incidental findings and side effects. The clinical history and patient circumstance did not need to be reestablished and tests did not need to be duplicated, which led to decreased LOS. Observation status allowed us to return patients to SNFs without the tedious procedure of insurance reauthorization and reevaluation by physical therapy and occupational therapy. Other factors such as “discharge before noon” and early physical therapy services ongoing during post-implementation also contributed to the decreased LOS.
Our Orthopedic EDAC program did not deliberately place patients on observation status who met full inpatient criteria solely to decrease the readmission rate. Our average LOS on observation status was 26 hours. In contrast, a study of observation stays at another tertiary academic medical center showed longer LOS: The average observation LOS was 33.3 hours with 44.4% of stays less than 24 hours and 16.5% greater than 48 hours.11 The use of EDAC hours in our study, which included both observation hours and readmission hours, made our impact more than simply a shifting of readmissions to observation stays.
It is important to utilize observation stays as they were intended—ie, stays requiring less than 48 hours. Over the past 10 years, the incidence and duration of observation stays has increased significantly while readmissions have decreased.14,15 Observation status has serious financial implications, and it is estimated that 10% of observation stays end up costing the patient more than an inpatient stay would and patients must pay 20% of services after the Part B deductible.16,17 In addition, Medicare beneficiaries have no cap on costs for an observation stay.16 Therefore, it is important to determine which patients and diagnoses are best suited for observation status. We found that younger patients without comorbidities who came from home and presented with complications such as fever and syncope were most likely to be successfully discharged on observation status with the Orthopedic EDAC program. SNF patients on observation status in particular may have large hospital bills because they often require 3 midnight stays but do not meet inpatient level of care and are thus not covered as inpatients.18
The Orthopedic EDAC program emphasized continuity of care with the primary orthopedic surgery team. Prior to implementation, orthopedics was often not even notified when their patients were in the ED or readmitted because the prevailing practice was that once surgery was completed, the surgeon’s job was done. Post-implementation, orthopedics was called for every bundled patient re-presenting within 90 days after a TJA. The triage protocol (Figure) was agreed upon prior to implementation by orthopedics, hospital medicine, and emergency medicine. Orthopedic attendings wanted to play a larger role and more strongly influence care of their patients on re-presentation because these attendings had become frustrated with the great disparities in work-up when patients went to various other services instead. Pre-implementation, many patients admitted to the primary orthopedic service had lower acuity, and they tended to be younger and have less medical complexity. Post-implementation, primary orthopedic services took care of more patients under observation status and those with “mechanical” complications that required surgery.
It is important to note that, while comanagement is common preoperatively and immediately postoperatively, studies of comanaged patients on re-presentation have apparently not been previously published. In addition, a recent study by Maxwell et al found that patients who were comanaged perioperatively had higher mortality and morbidity than did patients who were not comanaged.7 These findings reflect the need for more studies to be done to best optimize the use of comanagement. Comanagement as part of the Orthopedic EDAC program at our institution was successful in keeping patients who re-presented on the orthopedic service, decreasing LOS, and decreasing readmissions.
The study has some limitations. First, this was a retrospective study, so confounding variables may not be completely eliminated. Second, our study was conducted at a single center for total joint arthroplasty and did not consider other orthopedic conditions; however, our readmission numbers and demographics are similar to past studies. Third, we had small numbers of readmissions and observation patients, which resulted in a small effect size; however, our intervention demonstrated significant changes in LOS and readmissions. Fourth, our data is based on prior billing and coding, which may not always be accurate or inclusive. Fifth, we did not have THA or TKA patients on overnight recovery status or same day surgeries during either period studied; however, we are developing infrastructure to implement this in the future. Finally, ED visit data was not readily available to us, so we were not able to calculate the traditional EDAC. Despite these limitations, this study provides an important look at how an Orthopedic EDAC program can decrease readmissions, decrease LOS, and improve continuity of care in patients undergoing TJA.
CONCLUSION
An Orthopedic EDAC program with comanagement may decrease readmissions, improve continuity of care on re-presentation, and decrease LOS for total joint arthroplasty patients who presented after initial surgery and lead to substantial cost savings.
Disclosures
The authors have no potential conflicts to disclose. Dr Greysen was supported by a career development award from the National Institute on Aging (K23AG045338).
1. Hawker GA, Badley EM, Croxford R, et al. A population based nested case-control study of the costs of hip and knee replacement surgery. Med Care. 2009;47(7):732-741. https://doi.org/10.1097/MLR.0b013e3181934553
2. Kilgore M, Patel HK, Kielhorn A, Maya JF, Sharma P. Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Manag Healthc Policy. 2017;10:63-70. https://doi.org/10.2147/RMHP.S130341
3. McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskeletal Med. 2017;10(3):370-377. https://doi.org/10.1007/s12178-017-9423-6
4. The Society of Hospital Medicine. The Evolution of Co-Management. 2017. Accessed October 30, 2019. https://www.hospitalmedicine.org/globalassets/practice-management/practice-management-pdf/pm-19-0004-co-management-white-paper_minor-update-m.pdf
5. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: a propensity score analysis. Ann Surg. 2016;264(2):275-282. https://doi.org/10.1097/SLA.0000000000001629
6. Fitzgerald SJ, Palmer TC, Kraay MJ. Improved perioperative care of elective joint replacement patients: the impact of an orthopedic perioperative hospitalist. J Arthroplasty. 2018;33(8):2387-2391. https://doi,org/10.1016/j.arth.2018.03.029
7. Maxwell BG, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: a propensity score-matched retrospective cohort analysis of the National Surgical Quality Improvement Project. J Hosp Med. 2019;14:E1-E7. https://doi.org/10.12788/jhm.3343
8. Centers for Medicare & Medicaid Services. Medicare Program; Comprehensive Care for Joint Replacement Payment Model for Acute Care Hospitals Furnishing Lower Extremity Joint Replacement Services; Final Rule. November 24, 2015. https://www.govinfo.gov/content/pkg/FR-2015-11-24/pdf/2015-29438.pdf
9. Avram V, Petruccelli D, Winemaker M, de Beer J. Total joint arthroplasty readmission rates and reasons for 30-day hospital readmission. J Arthroplasty. 2014;29(3):465-468. https://doi.org/10.1016/j.arth.2013.07.039
10. ICD-10-CM/PCS MS-DRG v37.0 Definitions Manual. Accessed April 27, 2020. https://www.cms.gov/icd10m/version37-fullcode-cms/fullcode_cms/P0031.html
11. Chaudhary NS, Donnelly JP, Wang HE. Racial differences in sepsis mortality at United States academic medical center-affiliated hospitals. Crit Care Med. 2018;46(6):878-883. https://doi.org/10.1097/CCM.0000000000003020
12. Clair AJ, Evangelista PJ, Lajam CM, Slover JD, Bosco JA, Iorio R. Cost analysis of total joint arthroplasty readmissions in a Bundled Payment Care Improvement Initiative. J Arthroplasty. 2016;31(9):1862-1865.
13. Kaiser Family Foundation. Hospital Adjusted Expenses per Inpatient Day by Ownership. Kaiser Family Foundation. Accessed April 27, 2020. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D
14. Goldstein JN, Zhang Z, Schwartz JS, Hicks LS. Observation status, poverty, and high financial liability among Medicare beneficiaries. Am J Med. 2018;131(1):101.e9-101.e15. https://doi.org/10.1016/j.amjmed.2017.07.013
15. Lind KD, Noel-Miller CM, Sangaralingham LR, et al. Increasing trends in the use of hospital observation services for older Medicare Advantage and privately insured patients. Med Care Res Rev. 2019;76(2):229-239. https://doi.org/10.1177/1077558717718026
16. Sabbatini AK, Wright B. Excluding observation stays from readmission rates - what quality measures are missing. N Engl J Med. 2018;378(22):2062-2065. https://doi.org/10.1056/NEJMp1800732
17. Gabayan GZ, Doyle B, Liang, L, Donkor K, Huang, D, Sarkisian CA. Who has an unsuccessful observation care stay? Healthcare (Basel). 2018;6(4):138. https://doi.org/10.3390/healthcare6040138
18. Fang M, Hume E, Ibrahim S. Race, Bundled payment policy, and discharge destination after TKA: the experience of an urban academic hospital. Geriatr Orthop Surg Rehabil. 2018. https://doi.org/10.1177/2151459318803222
Total joint arthroplasty (TJA) procedures currently account for more Medicare expenses than any other inpatient procedure.1 In 2015, Centers for Medicare & Medicaid Services (CMS) announced the Comprehensive Care for Joint Replacement (CJR) model in which hospitals are paid one bundled payment for all related items and services utilized within a 90-day episode of care.2 Recent studies have suggested that the best opportunity to lower episode costs appears to be in the post-acute care setting and reducing readmissions.1,3
Surgical comanagement, which provides shared management of surgical patients between surgeons and hospitalists, is typically used in orthopedic surgery, neurosurgery, vascular surgery, and general surgery.4 Among patients with at least one medical comorbidity, surgical comanagement decreases length of stay (LOS), 30-day readmission rate for medical causes, and the proportion of patients with at least two medical consultants.5,6 Not all studies have shown that comanagement is beneficial. Maxwell et al found no significant differences in mortality or morbidity among hip fracture patients who did or did not receive comanagement7; however, comanaged patients were older and had more significant comorbidities, and there was no standard definition of comanagement among the participating institutions.
Comanagement after patients are discharged is a concept that has not been previously published but may become important with the Bundled Payments for Care Improvement initiative and high costs of excess days in acute care (EDAC). Hospitalists may be able to continue their work after discharge as part of the 90-day episode of care.8 TJA patients often have comorbidities, and surgical site infections and cardiovascular events are the most common causes of 30-day TJA readmissions.9
At our institution, 25% of TJA patients who presented to the Emergency Department (ED) within 90 days of surgery required a stay of less than 48 hours for conditions that did not require inpatient level of care. In addition, 50% of readmissions were secondary to medical complications. We also found significant variation in the management of common postoperative complications, such as postoperative fever, dislocation, anemia, and shortness of breath, especially among the different service lines caring for these patients. Therefore, we developed an Orthopedic EDAC program to reduce readmissions and to implement standardized admission algorithms and evidenced-based treatment protocols for common postoperative problems.
METHODS
Setting/Participants
We included patients who underwent total knee arthroplasty (TKA), total hip arthroplasty (THA), revision TKA, or revision THA from April 1, 2017, to September 30, 2018, at an urban teaching hospital. Patients were followed for 90 days after discharge. Factors such as age, sex, race, primary payer, Medicare Severity-Diagnosis Related Group (MS-DRG), discharge destination (home, home with home health, skilled nursing facility [SNF], acute rehab, other), and EDAC LOS were compared. An interdisciplinary committee comprising representatives from orthopedic surgery, hospital medicine, emergency medicine, and case management formulated observation criteria for the Orthopedic EDAC program. To be eligible for inclusion, observation patients had to have re-presented within 90 days from their initial surgery, could not be safely discharged home immediately from the ED, and did not require inpatient level of care. Patients qualifying for orthopedic observation were assigned rooms on the orthopedic wards to maintain continuity with nursing, physical therapy/occupational therapy, and case management staff. The University of Pennsylvania institutional review board reviewed this study and determined the project to be exempt.
Study Design
The Figure shows the admitting algorithm for TJA patients re-presenting within 90 days of their surgery. The ED evaluated the eligible patients; if they were not able to discharge the patient home, they notified the orthopedic resident on call for evaluation. Eligible diagnoses for the orthopedic observation in which orthopedics was the primary service included the need for postoperative pain control, fever (without signs or symptoms of sepsis), deep venous thrombosis or pulmonary embolism without hemodynamic instability, hemodynamically stable hypovolemia, symptomatic anemia secondary to acute blood loss anemia following surgery, and postoperative nausea, vomiting, constipation, ileus, and cellulitis. Eligible diagnoses for medical observation on the Medicine service included mild exacerbations of chronic obstructive pulmonary disease (COPD), syncope, upper respiratory tract infections, chest pain, delirium, and other exacerbation of medical problems. Full admission to Orthopedics included patients with wound infections requiring surgical washout, periprosthetic fractures/hematoma requiring operative management, and wound dehiscence requiring repair. All other readmissions requiring a stay of 48 or more hours were admitted to the medical or subspecialty medical service lines (eg, internal medicine, family medicine, geriatrics, cardiology, or pulmonary critical care).

Development of Evidence-Based Algorithms
Patients who re-presented to acute care (for either observation stays or readmissions) were treated based on standardized algorithms. The interdisciplinary work group developed evidence-based evaluation and treatment plans for common postoperative problems, including postoperative fever, postoperative shortness of breath, and postoperative septic joints. This was based on a comprehensive literature review and consensus among emergency medicine, hospital medicine, and orthopedic surgery. Appendix 1 illustrates an example of a standardized algorithm for the workup of hypoxia.
Definition of Readmissions and EDAC
Readmission and observation stays were flagged on re-presentation, and reasons for readmission or observation status were analyzed. Observation cutoffs of “successful” (<48 hours) vs “unsuccessful” (≥48 hours and/or conversion to inpatient status) were based on the CMS Two-Midnight Rule in accordance with past studies.10 Readmissions were defined as patients who required an acute stay of 48 or more hours within 90 days of discharge from their original surgical stay. Patients admitted under observation status who required a stay of less than 48 hours did not count as a readmission but did count toward EDAC.
We acknowledge that our definition of Orthopedic EDAC is not the same as CMS’s definition of EDAC for other conditions such as congestive heart failure, which includes hours in observation, readmissions, and ED visits. We focused on studying and reducing days in the hospital (observation status and readmissions), and our intervention was not intended to prevent issues that would cause patients to present to the ED. Therefore, including ED visits in our operational definition of EDAC would add an unnecessary source of confounding that would bias our results toward the null hypothesis.
Data Collection and Data Analysis
The Orthopedic EDAC program was implemented on October 1, 2017, based on the above triage and treatment plans. We analyzed demographic and outcome data (readmissions, LOS, time in observation status, reason for readmission/observation status) for 6 months prior (April 1, 2017, to September 30, 2017) and 1 year after (October 1, 2017, to September 30, 2018). Microsoft Excel (Jones, 2013) was used for data analysis. Paired t-test with P < .05 was predefined as significant.
Eligible patients were identified from previous admission diagnoses obtained through Vizient, which is a collaboration of academic medical centers that maintains a hospital discharge data set (the Clinical Data Base/Resource Manager CDB/RM). It included patient demographics, discharge diagnoses, procedures, and outcomes.11 The Vizient database is a respected source of data and has been used for several scholarly studies.10-12 We queried the Vizient Clinical Data Base/Resource Manager v. 8.12.0.11 (Vizient Inc., Irvine, TX) for the following data from both before and after the program’s implementation: disposition, LOS, insurance information, gender, type of surgery, MS-DRG, and race.
The five included MS-DRGs represented major hip and knee joint replacements with and without major comorbid conditions (MCCs; MS-DRG 469 and MS-DRG 470, respectively) and revision hip or knee replacement with MCCs, with comorbid conditions (CCs), and without MCCs or CCs (MS-DRG 466, MS-DRG 467, and MS-DRG 468, respectively). MCCs included but were not limited to decubitus ulcer, severe malnutrition, quadriplegia, and end-stage renal disease. Examples of CCs included transplant patients, lymphoma, leukemia, and malignancies (except breast or prostate), based on CMS definitions.13
RESULTS
Table 1 compares the demographics of the pre-implementation and post-implementation periods. There were a total of 2,662 admissions (799 before program implementation and 1,863 after). TKA and THA patients without MCCs (MS-DRG 470) accounted for 80% of patients during both periods. In both periods, approximately 60% of patients were female, 50% of patients were White, 40% were Black, and 10% were another race. The mean age was 63.6 years old. Most patients had Medicare or commercial insurance. Discharge destinations were similar during both periods.
Table 2 illustrates how the patients who re-presented to acute care were triaged based on the algorithm described in the Figure. Among the 64 patients who re-presented during the pre-implementation period, there were no observation stays; there were 38 patients who were placed under medicine inpatient services. During post-implementation, there were 48 patients (29 on orthopedics, 17 on medicine, and 2 on other service lines) who were admitted under observation status. Twenty-three patients were discharged on observation status. Of those patients, 20 were admitted to orthopedic observation and 3 patients to medicine observation. Among the 71 patients who re-presented during the post-implementation period, 40.8% (29 patients) were admitted to inpatient orthopedic services, and 17 patients were readmitted to medicine services (24.9%). Among re-presenting patients, 70% were admitted to orthopedics inpatient and observation combined, in contrast to just 35% during the pre-implementation period.

Readmissions decreased from 6.1% during pre-implementation to 2% during post-implementation (P = .004). In addition, the LOS for patients re-presenting during post-implementation was significantly lower than it was during pre-implementation. Table 3 details the associated LOS based on study period and readmission diagnosis. The aggregate LOS for all readmissions decreased from 7.75 days to 4.73 days (P = .005). The LOS decreased across all realms of readmission diagnoses. An outlier with an LOS greater than 100 days was removed from the pre-implementation group.

Appendix 2 further looked at patients who had observation orders, reasons for observation stay, and which patients were able to be discharged on observation status. Patients with medical complications such as fever and urinary tract infection were more likely to be discharged on observation status than were patients with wound drainage or redness that was concerning for a periprosthetic joint infection.
DISCUSSION
To our knowledge, this is the first description of a published Orthopedic EDAC program using orthopedic observation, standardized admitting and treatment algorithms, and comanagement of patients who re-presented after their original surgery. The development of an Orthopedic EDAC program at our hospital with comanagement was successful in reducing readmissions, decreasing LOS for readmitted patients, and increasing continuity of care. A number of points require more elaboration.
The Orthopedic EDAC program’s improvement in both reducing readmissions and decreasing LOS for EDAC (including days for observation and readmissions) was not caused by simply shifting patients with shorter LOS from inpatient to observation because the inpatients did not have a longer LOS. We had lower Orthopedic EDAC during the post-implementation vs pre-implementation even when considering EDAC in terms of both observation and readmissions. The decrease in readmissions is not only from the patients that were discharged on observation status, but also a result of other concurrent interventions, such as encouraging discharge to home rather than to rehabilitation facilities and more rigorous preoperative optimization.
The national rates of 30- and 90-day readmissions after primary TKA were 4% (95% CI, 3.8%-4.0%) and 7% (95% CI, 6.8%-7.2%), respectively,10 and the average cost of readmission for medical causes was $22,775 for THA and $11,682 for TKA.12 If one considers the 23 “saved readmissions” with 12 surgical complications and 11 medical complications, we “saved” roughly $591,105. Also, with the decrease in LOS for each readmission for any cause from 7.75 days to 4.73 days, the 48 readmissions had a 150 day lower LOS overall. With the average hospital day costing $2,289/day at nonprofit hospitals,13 there are additional cost savings of $343,350 overall. Therefore, the grand total estimated savings during this pilot was $934,455.
The decrease in post-implementation LOS vs pre-implementation LOS was likely multifactorial. The Orthopedic EDAC program improved continuity of care with orthopedic surgery and support staff (registered nurses, social workers, physical therapists) and utilized standardized protocols for work-up of common postoperative problems. These evidence-based protocols reduced waste that resulted in less testing with fewer incidental findings and side effects. The clinical history and patient circumstance did not need to be reestablished and tests did not need to be duplicated, which led to decreased LOS. Observation status allowed us to return patients to SNFs without the tedious procedure of insurance reauthorization and reevaluation by physical therapy and occupational therapy. Other factors such as “discharge before noon” and early physical therapy services ongoing during post-implementation also contributed to the decreased LOS.
Our Orthopedic EDAC program did not deliberately place patients on observation status who met full inpatient criteria solely to decrease the readmission rate. Our average LOS on observation status was 26 hours. In contrast, a study of observation stays at another tertiary academic medical center showed longer LOS: The average observation LOS was 33.3 hours with 44.4% of stays less than 24 hours and 16.5% greater than 48 hours.11 The use of EDAC hours in our study, which included both observation hours and readmission hours, made our impact more than simply a shifting of readmissions to observation stays.
It is important to utilize observation stays as they were intended—ie, stays requiring less than 48 hours. Over the past 10 years, the incidence and duration of observation stays has increased significantly while readmissions have decreased.14,15 Observation status has serious financial implications, and it is estimated that 10% of observation stays end up costing the patient more than an inpatient stay would and patients must pay 20% of services after the Part B deductible.16,17 In addition, Medicare beneficiaries have no cap on costs for an observation stay.16 Therefore, it is important to determine which patients and diagnoses are best suited for observation status. We found that younger patients without comorbidities who came from home and presented with complications such as fever and syncope were most likely to be successfully discharged on observation status with the Orthopedic EDAC program. SNF patients on observation status in particular may have large hospital bills because they often require 3 midnight stays but do not meet inpatient level of care and are thus not covered as inpatients.18
The Orthopedic EDAC program emphasized continuity of care with the primary orthopedic surgery team. Prior to implementation, orthopedics was often not even notified when their patients were in the ED or readmitted because the prevailing practice was that once surgery was completed, the surgeon’s job was done. Post-implementation, orthopedics was called for every bundled patient re-presenting within 90 days after a TJA. The triage protocol (Figure) was agreed upon prior to implementation by orthopedics, hospital medicine, and emergency medicine. Orthopedic attendings wanted to play a larger role and more strongly influence care of their patients on re-presentation because these attendings had become frustrated with the great disparities in work-up when patients went to various other services instead. Pre-implementation, many patients admitted to the primary orthopedic service had lower acuity, and they tended to be younger and have less medical complexity. Post-implementation, primary orthopedic services took care of more patients under observation status and those with “mechanical” complications that required surgery.
It is important to note that, while comanagement is common preoperatively and immediately postoperatively, studies of comanaged patients on re-presentation have apparently not been previously published. In addition, a recent study by Maxwell et al found that patients who were comanaged perioperatively had higher mortality and morbidity than did patients who were not comanaged.7 These findings reflect the need for more studies to be done to best optimize the use of comanagement. Comanagement as part of the Orthopedic EDAC program at our institution was successful in keeping patients who re-presented on the orthopedic service, decreasing LOS, and decreasing readmissions.
The study has some limitations. First, this was a retrospective study, so confounding variables may not be completely eliminated. Second, our study was conducted at a single center for total joint arthroplasty and did not consider other orthopedic conditions; however, our readmission numbers and demographics are similar to past studies. Third, we had small numbers of readmissions and observation patients, which resulted in a small effect size; however, our intervention demonstrated significant changes in LOS and readmissions. Fourth, our data is based on prior billing and coding, which may not always be accurate or inclusive. Fifth, we did not have THA or TKA patients on overnight recovery status or same day surgeries during either period studied; however, we are developing infrastructure to implement this in the future. Finally, ED visit data was not readily available to us, so we were not able to calculate the traditional EDAC. Despite these limitations, this study provides an important look at how an Orthopedic EDAC program can decrease readmissions, decrease LOS, and improve continuity of care in patients undergoing TJA.
CONCLUSION
An Orthopedic EDAC program with comanagement may decrease readmissions, improve continuity of care on re-presentation, and decrease LOS for total joint arthroplasty patients who presented after initial surgery and lead to substantial cost savings.
Disclosures
The authors have no potential conflicts to disclose. Dr Greysen was supported by a career development award from the National Institute on Aging (K23AG045338).
Total joint arthroplasty (TJA) procedures currently account for more Medicare expenses than any other inpatient procedure.1 In 2015, Centers for Medicare & Medicaid Services (CMS) announced the Comprehensive Care for Joint Replacement (CJR) model in which hospitals are paid one bundled payment for all related items and services utilized within a 90-day episode of care.2 Recent studies have suggested that the best opportunity to lower episode costs appears to be in the post-acute care setting and reducing readmissions.1,3
Surgical comanagement, which provides shared management of surgical patients between surgeons and hospitalists, is typically used in orthopedic surgery, neurosurgery, vascular surgery, and general surgery.4 Among patients with at least one medical comorbidity, surgical comanagement decreases length of stay (LOS), 30-day readmission rate for medical causes, and the proportion of patients with at least two medical consultants.5,6 Not all studies have shown that comanagement is beneficial. Maxwell et al found no significant differences in mortality or morbidity among hip fracture patients who did or did not receive comanagement7; however, comanaged patients were older and had more significant comorbidities, and there was no standard definition of comanagement among the participating institutions.
Comanagement after patients are discharged is a concept that has not been previously published but may become important with the Bundled Payments for Care Improvement initiative and high costs of excess days in acute care (EDAC). Hospitalists may be able to continue their work after discharge as part of the 90-day episode of care.8 TJA patients often have comorbidities, and surgical site infections and cardiovascular events are the most common causes of 30-day TJA readmissions.9
At our institution, 25% of TJA patients who presented to the Emergency Department (ED) within 90 days of surgery required a stay of less than 48 hours for conditions that did not require inpatient level of care. In addition, 50% of readmissions were secondary to medical complications. We also found significant variation in the management of common postoperative complications, such as postoperative fever, dislocation, anemia, and shortness of breath, especially among the different service lines caring for these patients. Therefore, we developed an Orthopedic EDAC program to reduce readmissions and to implement standardized admission algorithms and evidenced-based treatment protocols for common postoperative problems.
METHODS
Setting/Participants
We included patients who underwent total knee arthroplasty (TKA), total hip arthroplasty (THA), revision TKA, or revision THA from April 1, 2017, to September 30, 2018, at an urban teaching hospital. Patients were followed for 90 days after discharge. Factors such as age, sex, race, primary payer, Medicare Severity-Diagnosis Related Group (MS-DRG), discharge destination (home, home with home health, skilled nursing facility [SNF], acute rehab, other), and EDAC LOS were compared. An interdisciplinary committee comprising representatives from orthopedic surgery, hospital medicine, emergency medicine, and case management formulated observation criteria for the Orthopedic EDAC program. To be eligible for inclusion, observation patients had to have re-presented within 90 days from their initial surgery, could not be safely discharged home immediately from the ED, and did not require inpatient level of care. Patients qualifying for orthopedic observation were assigned rooms on the orthopedic wards to maintain continuity with nursing, physical therapy/occupational therapy, and case management staff. The University of Pennsylvania institutional review board reviewed this study and determined the project to be exempt.
Study Design
The Figure shows the admitting algorithm for TJA patients re-presenting within 90 days of their surgery. The ED evaluated the eligible patients; if they were not able to discharge the patient home, they notified the orthopedic resident on call for evaluation. Eligible diagnoses for the orthopedic observation in which orthopedics was the primary service included the need for postoperative pain control, fever (without signs or symptoms of sepsis), deep venous thrombosis or pulmonary embolism without hemodynamic instability, hemodynamically stable hypovolemia, symptomatic anemia secondary to acute blood loss anemia following surgery, and postoperative nausea, vomiting, constipation, ileus, and cellulitis. Eligible diagnoses for medical observation on the Medicine service included mild exacerbations of chronic obstructive pulmonary disease (COPD), syncope, upper respiratory tract infections, chest pain, delirium, and other exacerbation of medical problems. Full admission to Orthopedics included patients with wound infections requiring surgical washout, periprosthetic fractures/hematoma requiring operative management, and wound dehiscence requiring repair. All other readmissions requiring a stay of 48 or more hours were admitted to the medical or subspecialty medical service lines (eg, internal medicine, family medicine, geriatrics, cardiology, or pulmonary critical care).

Development of Evidence-Based Algorithms
Patients who re-presented to acute care (for either observation stays or readmissions) were treated based on standardized algorithms. The interdisciplinary work group developed evidence-based evaluation and treatment plans for common postoperative problems, including postoperative fever, postoperative shortness of breath, and postoperative septic joints. This was based on a comprehensive literature review and consensus among emergency medicine, hospital medicine, and orthopedic surgery. Appendix 1 illustrates an example of a standardized algorithm for the workup of hypoxia.
Definition of Readmissions and EDAC
Readmission and observation stays were flagged on re-presentation, and reasons for readmission or observation status were analyzed. Observation cutoffs of “successful” (<48 hours) vs “unsuccessful” (≥48 hours and/or conversion to inpatient status) were based on the CMS Two-Midnight Rule in accordance with past studies.10 Readmissions were defined as patients who required an acute stay of 48 or more hours within 90 days of discharge from their original surgical stay. Patients admitted under observation status who required a stay of less than 48 hours did not count as a readmission but did count toward EDAC.
We acknowledge that our definition of Orthopedic EDAC is not the same as CMS’s definition of EDAC for other conditions such as congestive heart failure, which includes hours in observation, readmissions, and ED visits. We focused on studying and reducing days in the hospital (observation status and readmissions), and our intervention was not intended to prevent issues that would cause patients to present to the ED. Therefore, including ED visits in our operational definition of EDAC would add an unnecessary source of confounding that would bias our results toward the null hypothesis.
Data Collection and Data Analysis
The Orthopedic EDAC program was implemented on October 1, 2017, based on the above triage and treatment plans. We analyzed demographic and outcome data (readmissions, LOS, time in observation status, reason for readmission/observation status) for 6 months prior (April 1, 2017, to September 30, 2017) and 1 year after (October 1, 2017, to September 30, 2018). Microsoft Excel (Jones, 2013) was used for data analysis. Paired t-test with P < .05 was predefined as significant.
Eligible patients were identified from previous admission diagnoses obtained through Vizient, which is a collaboration of academic medical centers that maintains a hospital discharge data set (the Clinical Data Base/Resource Manager CDB/RM). It included patient demographics, discharge diagnoses, procedures, and outcomes.11 The Vizient database is a respected source of data and has been used for several scholarly studies.10-12 We queried the Vizient Clinical Data Base/Resource Manager v. 8.12.0.11 (Vizient Inc., Irvine, TX) for the following data from both before and after the program’s implementation: disposition, LOS, insurance information, gender, type of surgery, MS-DRG, and race.
The five included MS-DRGs represented major hip and knee joint replacements with and without major comorbid conditions (MCCs; MS-DRG 469 and MS-DRG 470, respectively) and revision hip or knee replacement with MCCs, with comorbid conditions (CCs), and without MCCs or CCs (MS-DRG 466, MS-DRG 467, and MS-DRG 468, respectively). MCCs included but were not limited to decubitus ulcer, severe malnutrition, quadriplegia, and end-stage renal disease. Examples of CCs included transplant patients, lymphoma, leukemia, and malignancies (except breast or prostate), based on CMS definitions.13
RESULTS
Table 1 compares the demographics of the pre-implementation and post-implementation periods. There were a total of 2,662 admissions (799 before program implementation and 1,863 after). TKA and THA patients without MCCs (MS-DRG 470) accounted for 80% of patients during both periods. In both periods, approximately 60% of patients were female, 50% of patients were White, 40% were Black, and 10% were another race. The mean age was 63.6 years old. Most patients had Medicare or commercial insurance. Discharge destinations were similar during both periods.
Table 2 illustrates how the patients who re-presented to acute care were triaged based on the algorithm described in the Figure. Among the 64 patients who re-presented during the pre-implementation period, there were no observation stays; there were 38 patients who were placed under medicine inpatient services. During post-implementation, there were 48 patients (29 on orthopedics, 17 on medicine, and 2 on other service lines) who were admitted under observation status. Twenty-three patients were discharged on observation status. Of those patients, 20 were admitted to orthopedic observation and 3 patients to medicine observation. Among the 71 patients who re-presented during the post-implementation period, 40.8% (29 patients) were admitted to inpatient orthopedic services, and 17 patients were readmitted to medicine services (24.9%). Among re-presenting patients, 70% were admitted to orthopedics inpatient and observation combined, in contrast to just 35% during the pre-implementation period.

Readmissions decreased from 6.1% during pre-implementation to 2% during post-implementation (P = .004). In addition, the LOS for patients re-presenting during post-implementation was significantly lower than it was during pre-implementation. Table 3 details the associated LOS based on study period and readmission diagnosis. The aggregate LOS for all readmissions decreased from 7.75 days to 4.73 days (P = .005). The LOS decreased across all realms of readmission diagnoses. An outlier with an LOS greater than 100 days was removed from the pre-implementation group.

Appendix 2 further looked at patients who had observation orders, reasons for observation stay, and which patients were able to be discharged on observation status. Patients with medical complications such as fever and urinary tract infection were more likely to be discharged on observation status than were patients with wound drainage or redness that was concerning for a periprosthetic joint infection.
DISCUSSION
To our knowledge, this is the first description of a published Orthopedic EDAC program using orthopedic observation, standardized admitting and treatment algorithms, and comanagement of patients who re-presented after their original surgery. The development of an Orthopedic EDAC program at our hospital with comanagement was successful in reducing readmissions, decreasing LOS for readmitted patients, and increasing continuity of care. A number of points require more elaboration.
The Orthopedic EDAC program’s improvement in both reducing readmissions and decreasing LOS for EDAC (including days for observation and readmissions) was not caused by simply shifting patients with shorter LOS from inpatient to observation because the inpatients did not have a longer LOS. We had lower Orthopedic EDAC during the post-implementation vs pre-implementation even when considering EDAC in terms of both observation and readmissions. The decrease in readmissions is not only from the patients that were discharged on observation status, but also a result of other concurrent interventions, such as encouraging discharge to home rather than to rehabilitation facilities and more rigorous preoperative optimization.
The national rates of 30- and 90-day readmissions after primary TKA were 4% (95% CI, 3.8%-4.0%) and 7% (95% CI, 6.8%-7.2%), respectively,10 and the average cost of readmission for medical causes was $22,775 for THA and $11,682 for TKA.12 If one considers the 23 “saved readmissions” with 12 surgical complications and 11 medical complications, we “saved” roughly $591,105. Also, with the decrease in LOS for each readmission for any cause from 7.75 days to 4.73 days, the 48 readmissions had a 150 day lower LOS overall. With the average hospital day costing $2,289/day at nonprofit hospitals,13 there are additional cost savings of $343,350 overall. Therefore, the grand total estimated savings during this pilot was $934,455.
The decrease in post-implementation LOS vs pre-implementation LOS was likely multifactorial. The Orthopedic EDAC program improved continuity of care with orthopedic surgery and support staff (registered nurses, social workers, physical therapists) and utilized standardized protocols for work-up of common postoperative problems. These evidence-based protocols reduced waste that resulted in less testing with fewer incidental findings and side effects. The clinical history and patient circumstance did not need to be reestablished and tests did not need to be duplicated, which led to decreased LOS. Observation status allowed us to return patients to SNFs without the tedious procedure of insurance reauthorization and reevaluation by physical therapy and occupational therapy. Other factors such as “discharge before noon” and early physical therapy services ongoing during post-implementation also contributed to the decreased LOS.
Our Orthopedic EDAC program did not deliberately place patients on observation status who met full inpatient criteria solely to decrease the readmission rate. Our average LOS on observation status was 26 hours. In contrast, a study of observation stays at another tertiary academic medical center showed longer LOS: The average observation LOS was 33.3 hours with 44.4% of stays less than 24 hours and 16.5% greater than 48 hours.11 The use of EDAC hours in our study, which included both observation hours and readmission hours, made our impact more than simply a shifting of readmissions to observation stays.
It is important to utilize observation stays as they were intended—ie, stays requiring less than 48 hours. Over the past 10 years, the incidence and duration of observation stays has increased significantly while readmissions have decreased.14,15 Observation status has serious financial implications, and it is estimated that 10% of observation stays end up costing the patient more than an inpatient stay would and patients must pay 20% of services after the Part B deductible.16,17 In addition, Medicare beneficiaries have no cap on costs for an observation stay.16 Therefore, it is important to determine which patients and diagnoses are best suited for observation status. We found that younger patients without comorbidities who came from home and presented with complications such as fever and syncope were most likely to be successfully discharged on observation status with the Orthopedic EDAC program. SNF patients on observation status in particular may have large hospital bills because they often require 3 midnight stays but do not meet inpatient level of care and are thus not covered as inpatients.18
The Orthopedic EDAC program emphasized continuity of care with the primary orthopedic surgery team. Prior to implementation, orthopedics was often not even notified when their patients were in the ED or readmitted because the prevailing practice was that once surgery was completed, the surgeon’s job was done. Post-implementation, orthopedics was called for every bundled patient re-presenting within 90 days after a TJA. The triage protocol (Figure) was agreed upon prior to implementation by orthopedics, hospital medicine, and emergency medicine. Orthopedic attendings wanted to play a larger role and more strongly influence care of their patients on re-presentation because these attendings had become frustrated with the great disparities in work-up when patients went to various other services instead. Pre-implementation, many patients admitted to the primary orthopedic service had lower acuity, and they tended to be younger and have less medical complexity. Post-implementation, primary orthopedic services took care of more patients under observation status and those with “mechanical” complications that required surgery.
It is important to note that, while comanagement is common preoperatively and immediately postoperatively, studies of comanaged patients on re-presentation have apparently not been previously published. In addition, a recent study by Maxwell et al found that patients who were comanaged perioperatively had higher mortality and morbidity than did patients who were not comanaged.7 These findings reflect the need for more studies to be done to best optimize the use of comanagement. Comanagement as part of the Orthopedic EDAC program at our institution was successful in keeping patients who re-presented on the orthopedic service, decreasing LOS, and decreasing readmissions.
The study has some limitations. First, this was a retrospective study, so confounding variables may not be completely eliminated. Second, our study was conducted at a single center for total joint arthroplasty and did not consider other orthopedic conditions; however, our readmission numbers and demographics are similar to past studies. Third, we had small numbers of readmissions and observation patients, which resulted in a small effect size; however, our intervention demonstrated significant changes in LOS and readmissions. Fourth, our data is based on prior billing and coding, which may not always be accurate or inclusive. Fifth, we did not have THA or TKA patients on overnight recovery status or same day surgeries during either period studied; however, we are developing infrastructure to implement this in the future. Finally, ED visit data was not readily available to us, so we were not able to calculate the traditional EDAC. Despite these limitations, this study provides an important look at how an Orthopedic EDAC program can decrease readmissions, decrease LOS, and improve continuity of care in patients undergoing TJA.
CONCLUSION
An Orthopedic EDAC program with comanagement may decrease readmissions, improve continuity of care on re-presentation, and decrease LOS for total joint arthroplasty patients who presented after initial surgery and lead to substantial cost savings.
Disclosures
The authors have no potential conflicts to disclose. Dr Greysen was supported by a career development award from the National Institute on Aging (K23AG045338).
1. Hawker GA, Badley EM, Croxford R, et al. A population based nested case-control study of the costs of hip and knee replacement surgery. Med Care. 2009;47(7):732-741. https://doi.org/10.1097/MLR.0b013e3181934553
2. Kilgore M, Patel HK, Kielhorn A, Maya JF, Sharma P. Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Manag Healthc Policy. 2017;10:63-70. https://doi.org/10.2147/RMHP.S130341
3. McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskeletal Med. 2017;10(3):370-377. https://doi.org/10.1007/s12178-017-9423-6
4. The Society of Hospital Medicine. The Evolution of Co-Management. 2017. Accessed October 30, 2019. https://www.hospitalmedicine.org/globalassets/practice-management/practice-management-pdf/pm-19-0004-co-management-white-paper_minor-update-m.pdf
5. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: a propensity score analysis. Ann Surg. 2016;264(2):275-282. https://doi.org/10.1097/SLA.0000000000001629
6. Fitzgerald SJ, Palmer TC, Kraay MJ. Improved perioperative care of elective joint replacement patients: the impact of an orthopedic perioperative hospitalist. J Arthroplasty. 2018;33(8):2387-2391. https://doi,org/10.1016/j.arth.2018.03.029
7. Maxwell BG, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: a propensity score-matched retrospective cohort analysis of the National Surgical Quality Improvement Project. J Hosp Med. 2019;14:E1-E7. https://doi.org/10.12788/jhm.3343
8. Centers for Medicare & Medicaid Services. Medicare Program; Comprehensive Care for Joint Replacement Payment Model for Acute Care Hospitals Furnishing Lower Extremity Joint Replacement Services; Final Rule. November 24, 2015. https://www.govinfo.gov/content/pkg/FR-2015-11-24/pdf/2015-29438.pdf
9. Avram V, Petruccelli D, Winemaker M, de Beer J. Total joint arthroplasty readmission rates and reasons for 30-day hospital readmission. J Arthroplasty. 2014;29(3):465-468. https://doi.org/10.1016/j.arth.2013.07.039
10. ICD-10-CM/PCS MS-DRG v37.0 Definitions Manual. Accessed April 27, 2020. https://www.cms.gov/icd10m/version37-fullcode-cms/fullcode_cms/P0031.html
11. Chaudhary NS, Donnelly JP, Wang HE. Racial differences in sepsis mortality at United States academic medical center-affiliated hospitals. Crit Care Med. 2018;46(6):878-883. https://doi.org/10.1097/CCM.0000000000003020
12. Clair AJ, Evangelista PJ, Lajam CM, Slover JD, Bosco JA, Iorio R. Cost analysis of total joint arthroplasty readmissions in a Bundled Payment Care Improvement Initiative. J Arthroplasty. 2016;31(9):1862-1865.
13. Kaiser Family Foundation. Hospital Adjusted Expenses per Inpatient Day by Ownership. Kaiser Family Foundation. Accessed April 27, 2020. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D
14. Goldstein JN, Zhang Z, Schwartz JS, Hicks LS. Observation status, poverty, and high financial liability among Medicare beneficiaries. Am J Med. 2018;131(1):101.e9-101.e15. https://doi.org/10.1016/j.amjmed.2017.07.013
15. Lind KD, Noel-Miller CM, Sangaralingham LR, et al. Increasing trends in the use of hospital observation services for older Medicare Advantage and privately insured patients. Med Care Res Rev. 2019;76(2):229-239. https://doi.org/10.1177/1077558717718026
16. Sabbatini AK, Wright B. Excluding observation stays from readmission rates - what quality measures are missing. N Engl J Med. 2018;378(22):2062-2065. https://doi.org/10.1056/NEJMp1800732
17. Gabayan GZ, Doyle B, Liang, L, Donkor K, Huang, D, Sarkisian CA. Who has an unsuccessful observation care stay? Healthcare (Basel). 2018;6(4):138. https://doi.org/10.3390/healthcare6040138
18. Fang M, Hume E, Ibrahim S. Race, Bundled payment policy, and discharge destination after TKA: the experience of an urban academic hospital. Geriatr Orthop Surg Rehabil. 2018. https://doi.org/10.1177/2151459318803222
1. Hawker GA, Badley EM, Croxford R, et al. A population based nested case-control study of the costs of hip and knee replacement surgery. Med Care. 2009;47(7):732-741. https://doi.org/10.1097/MLR.0b013e3181934553
2. Kilgore M, Patel HK, Kielhorn A, Maya JF, Sharma P. Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Manag Healthc Policy. 2017;10:63-70. https://doi.org/10.2147/RMHP.S130341
3. McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskeletal Med. 2017;10(3):370-377. https://doi.org/10.1007/s12178-017-9423-6
4. The Society of Hospital Medicine. The Evolution of Co-Management. 2017. Accessed October 30, 2019. https://www.hospitalmedicine.org/globalassets/practice-management/practice-management-pdf/pm-19-0004-co-management-white-paper_minor-update-m.pdf
5. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: a propensity score analysis. Ann Surg. 2016;264(2):275-282. https://doi.org/10.1097/SLA.0000000000001629
6. Fitzgerald SJ, Palmer TC, Kraay MJ. Improved perioperative care of elective joint replacement patients: the impact of an orthopedic perioperative hospitalist. J Arthroplasty. 2018;33(8):2387-2391. https://doi,org/10.1016/j.arth.2018.03.029
7. Maxwell BG, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: a propensity score-matched retrospective cohort analysis of the National Surgical Quality Improvement Project. J Hosp Med. 2019;14:E1-E7. https://doi.org/10.12788/jhm.3343
8. Centers for Medicare & Medicaid Services. Medicare Program; Comprehensive Care for Joint Replacement Payment Model for Acute Care Hospitals Furnishing Lower Extremity Joint Replacement Services; Final Rule. November 24, 2015. https://www.govinfo.gov/content/pkg/FR-2015-11-24/pdf/2015-29438.pdf
9. Avram V, Petruccelli D, Winemaker M, de Beer J. Total joint arthroplasty readmission rates and reasons for 30-day hospital readmission. J Arthroplasty. 2014;29(3):465-468. https://doi.org/10.1016/j.arth.2013.07.039
10. ICD-10-CM/PCS MS-DRG v37.0 Definitions Manual. Accessed April 27, 2020. https://www.cms.gov/icd10m/version37-fullcode-cms/fullcode_cms/P0031.html
11. Chaudhary NS, Donnelly JP, Wang HE. Racial differences in sepsis mortality at United States academic medical center-affiliated hospitals. Crit Care Med. 2018;46(6):878-883. https://doi.org/10.1097/CCM.0000000000003020
12. Clair AJ, Evangelista PJ, Lajam CM, Slover JD, Bosco JA, Iorio R. Cost analysis of total joint arthroplasty readmissions in a Bundled Payment Care Improvement Initiative. J Arthroplasty. 2016;31(9):1862-1865.
13. Kaiser Family Foundation. Hospital Adjusted Expenses per Inpatient Day by Ownership. Kaiser Family Foundation. Accessed April 27, 2020. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D
14. Goldstein JN, Zhang Z, Schwartz JS, Hicks LS. Observation status, poverty, and high financial liability among Medicare beneficiaries. Am J Med. 2018;131(1):101.e9-101.e15. https://doi.org/10.1016/j.amjmed.2017.07.013
15. Lind KD, Noel-Miller CM, Sangaralingham LR, et al. Increasing trends in the use of hospital observation services for older Medicare Advantage and privately insured patients. Med Care Res Rev. 2019;76(2):229-239. https://doi.org/10.1177/1077558717718026
16. Sabbatini AK, Wright B. Excluding observation stays from readmission rates - what quality measures are missing. N Engl J Med. 2018;378(22):2062-2065. https://doi.org/10.1056/NEJMp1800732
17. Gabayan GZ, Doyle B, Liang, L, Donkor K, Huang, D, Sarkisian CA. Who has an unsuccessful observation care stay? Healthcare (Basel). 2018;6(4):138. https://doi.org/10.3390/healthcare6040138
18. Fang M, Hume E, Ibrahim S. Race, Bundled payment policy, and discharge destination after TKA: the experience of an urban academic hospital. Geriatr Orthop Surg Rehabil. 2018. https://doi.org/10.1177/2151459318803222
© 2020 Society of Hospital Medicine
Strategies of Female Teaching Attending Physicians to Navigate Gender-Based Challenges: An Exploratory Qualitative Study
The demographic composition of physicians has shifted dramatically in the last five decades. The number of women matriculating into medical school rose from 6% in the 1960s1 to 52% in 20192; women accounted for 39% of full-time faculty in 2015.3 Despite this evolution of the physician gender array, many challenges remain.4 Women represented only 35% of all associate professors and 22% of full professors in 2015.3 Women experience gender-based discrimination, hostility, and unconscious bias as medical trainees5-9 and as attending physicians10-13 with significant deleterious effects including burnout and suicidal thoughts.14 While types of gender-based challenges are well described in the literature, strategies to navigate and respond to these challenges are less understood.
The approaches and techniques of exemplary teaching attending physicians (hereafter referred to as “attendings”) have previously been reported from groups of predominantly male attendings.15-18 Because of gender-based challenges female physicians face that lead them to reduce their effort or leave the medical field,19 there is concern that prior scholarship in effective teaching may not adequately capture the approaches and techniques of female attendings. To our knowledge, no studies have specifically examined female attendings. Therefore, we sought to explore the lived experiences of six female attendings with particular emphasis on how they navigate and respond to gender-based challenges in clinical environments.
METHODS
Study Design and Sampling
This was a multisite study using an exploratory qualitative approach to inquiry. We aimed to examine techniques, approaches, and attitudes of outstanding general medicine teaching attendings among groups previously not well represented (ie, women and self-identified underrepresented minorities [URMs] in medicine). URM was defined by the Association of American Medical Colleges as “those racial and ethnic populations that are underrepresented in the medical profession relative to their numbers in the general population.”20 A modified snowball sampling approach21 was employed to identify attendings as delineated below.
To maintain quality while guaranteeing diversity in geography and population, potential institutions in which to observe attendings were determined by first creating the following lists: The top 20 hospitals in the U.S. News & World Report’s 2017-2018 Best Hospitals Honor Roll,22 top-rated institutions by Doximity in each geographic region and among rural training sites,23 and four historically Black colleges and universities (HBCUs) with medical schools. Institutions visited during a previous similar study16 were excluded. Next, the list was narrowed to 25 by randomly selecting five in each main geographic region and five rural institutions. These were combined with all four HBCUs to create a final list of 29 institutions.
Next, division of hospital medicine chiefs (and/or general medicine chiefs) and internal medicine residency directors at each of these 29 institutions were asked to nominate exemplary attendings, particularly those who identified as women and URMs. Twelve attendings who were themselves observed in a previous study16 were also asked for nominations. Finally, recommendations were sought from leaders of relevant American Medical Association member groups.24
Using this sampling method, 43 physicians were identified. An internet search was conducted to identify individual characteristics including medical education, training, clinical and research interests, and educational awards. These characteristics were considered and discussed by the research team. Preference was given to those attendings nominated by more than one individual (n = 3), those who had received teaching awards, and those with interests involving women in medicine. Research team members narrowed the list to seven attendings who were contacted via email and invited to participate. One did not respond, while six agreed to participate. The six attendings identified current team members who would be rounding on the visit date. Attendings were asked to recommend 6-10 former learners; we contacted these former learners and invited them to participate. Former learners were included to understand lasting effects from their attendings.
Data Collection
Observations
All 1-day site visits were conducted by two research team members, a physician (NH) and a qualitative research specialist (MQ). In four visits, an additional author accompanied the research team. In order to ensure consistency and diversity in perspectives, all authors attended at least one visit. These occurred between April 16 and August 28, 2018. Each visit began with direct observation of attendings (n = 6) and current learners (n = 24) during inpatient general medicine teaching rounds. Each researcher unobtrusively recorded their observations via handwritten, open field notes, paying particular attention to group interactions, teaching approach, conversations within and peripheral to the team, and patient–team interactions. After each visit, researchers met to compare and combine field notes.
Interviews and Focus Groups
Researchers then conducted individual, semistructured interviews with attendings and focus groups with current (n = 21) and former (n = 17) learners. Focus groups with learners varied in size from two to five participants. Former learners were occasionally not available for on-site focus groups and were interviewed separately by telephone after the visit. The interview guide for attendings (Appendix 1) was adapted from the prior study16 but expanded with questions related to experiences, challenges, and approaches of female and URM physicians. A separate guide was used to facilitate focus groups with learners (Appendix 1
This study was determined to be exempt by the University of Michigan Institutional Review Board. All participants were informed that their participation was completely voluntary and that they could terminate their involvement at any time.
Data Analysis
Data were analyzed using a content analysis approach.25 Inductive coding was used to identify codes derived from the data. Two team members (MQ and MH) independently coded the first transcript to develop a codebook, then met to compare and discuss codes. Codes and definitions were entered into the codebook. These team members continued coding five additional transcripts, meeting to compare codes, discussing any discrepancies until agreement was reached, adding new codes identified, and ensuring consistent code application. They reviewed prior transcripts and recoded if necessary. Once no new codes were identified, one team member coded the remaining transcripts. The same codebook was used to code field note documents using the same iterative process. After all qualitative data were coded and verified, they were entered into NVivo 10. Code reports were generated and reviewed by three team members to identify themes and check for coding consistency.
Role of the Funding Source
This study received no external funding.
RESULTS
We examined six exemplary attendings through direct observation of rounds and individual interviews. We also discussed these attendings with 21 current learners and 17 former learners (Appendix 2). All attendings self-identified as female. The group was diverse in terms of race/ethnicity, with three identifying as Black or African American, two as Asian, and one as White or Caucasian. Levels of experience as an attending ranged from 8 to 20 years (mean, 15.3 years). At the time of observation, two were professors and four were associate professors. The group included all three attendings who had been nominated by more than one individual, and all six had won multiple teaching awards. The observation sites represented several areas of the United States (Table 1).
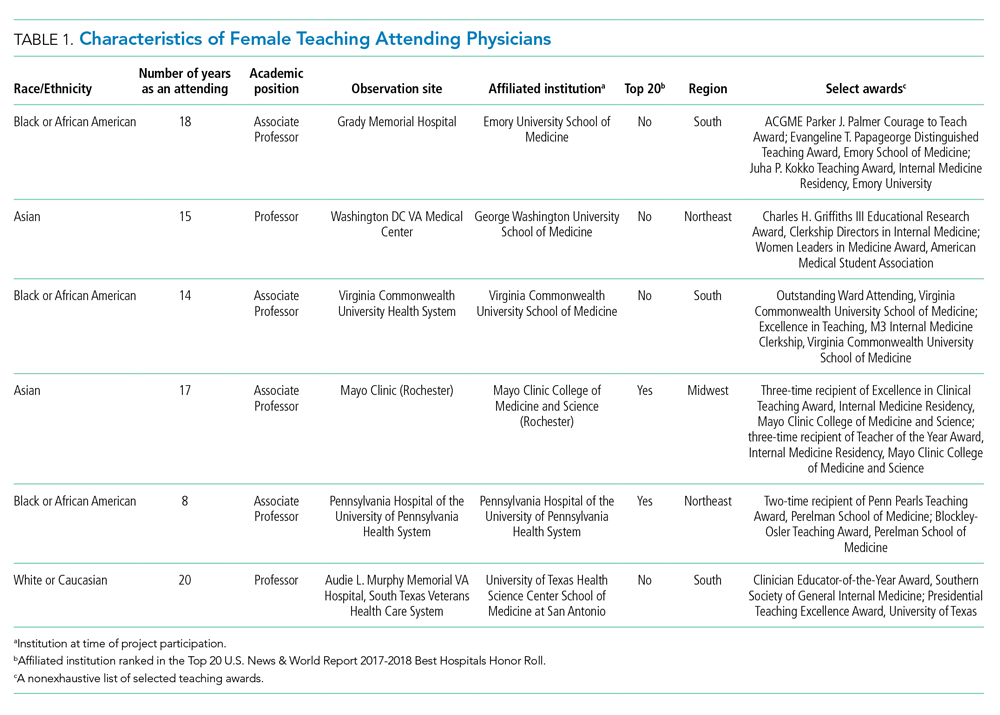
The coded interview data and field notes were categorized into three broad overlapping themes based on strategies our attendings used to respond to gender-based challenges. The following sections describe types of challenges faced by female attendings along with specific strategies they employed to actively position themselves as physician team leaders, manage gender-based stereotypes and perceptions, and identify and embrace their unique qualities. Illustrative quotations or observations that further elucidate meaning are provided.
Female Attendings Actively Position Themselves as Physician Team Leaders
Our attendings frequently stated that they were assumed to be other healthcare provider types, such as nurses or physical therapists, and that these assumptions originated from patients, faculty, and staff (Table 2). Attending 3 commented, “I think every woman in this role has been mistaken for a different caretaker role, so lots of requests for nursing help. I’m sure I have taken more patients off of bed pans and brought more cups of water than maybe some of my male counterparts.” Some attendings responded to this challenge with the strategy of routinely wearing a white coat during rounds and patient encounters. This external visual cue was seen as a necessary reminder of the female attending role.
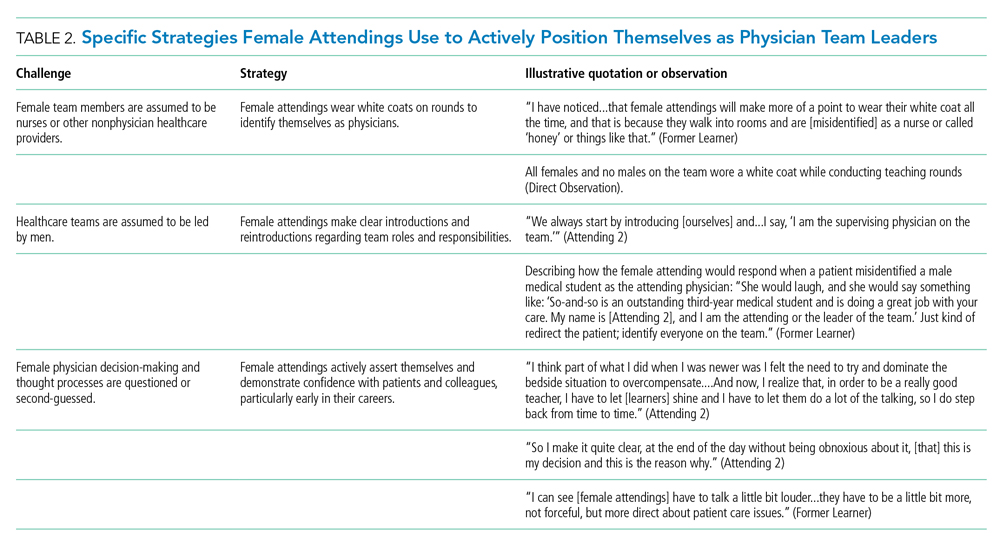
We found that patients and healthcare providers often believe teams are led by men, leading to a feeling of invisibility for female attendings. One current learner remarked, “If it was a new patient, more than likely, if we had a female attending, the patient’s eyes would always divert to the male physician.” This was not limited to patients. Attending 6 remembered comments from her consultants including, “‘Who is your attending? Let me talk with them,’ kind of assuming that I’m not the person making the decisions.” Female attendings would respond to this challenge by clearly introducing team members, including themselves, with roles and responsibilities. At times, this would require reintroductions and redirection if individuals still misidentified female team members.
Female attendings’ decision-making and thought processes were frequently second-guessed. This would often lead to power struggles with consultants, nurses, and learners. Attending 5 commented, “Even in residency, I felt this sometimes adversarial relationship with...female nurses where they would treat [female attendings] differently...questioning our decisions.” Female attendings would respond to this challenge by asserting themselves and demonstrating confidence with colleagues and at the bedside. This was an active process for women, as one former learner described: “[Female] attendings have to be a little bit more ‘on’—whatever ‘on’ is—more forceful, more direct....There is more slack given to a male attending.”
Female Attendings Consciously Work to Manage Gender-Based Stereotypes and Perceptions
Our attendings navigated gender-based stereotypes and perceptions, ranging from subtle microaggressions to overt sexual harassment (Table 3). This required balance between extremes of being perceived as “too nice” and “too aggressive,” each of which was associated with negativity. Attending 1 remarked, “I know that other [female] faculty struggle with that a bit, with being...assertive. They are assertive, and it’s interpreted [negatively].” Attending 6 described insidiously sexist comments from patients: “‘You are too young to be a physician, you are too pretty to be a physician.’ ‘Oh, the woman doctor...rather than just ‘doctor.’” During one observation of rounds, a patient remarked to the attending, “You have cold hands. You know, I’m going to have to warm those up.” Our attendings responded to these challenges by proactively avoiding characteristics and behaviors considered to be stereotypically feminine in order to draw attention to their qualities as physicians rather than as women. During interviews, some attendings directed conversation away from themselves and instead placed emphasis on coaching female learners to navigate their own demeanors, behaviors, and responses to gender bias and harassment. This would include intentional planning of how to carry oneself, as well as feedback and debrief sessions after instances of harassment.
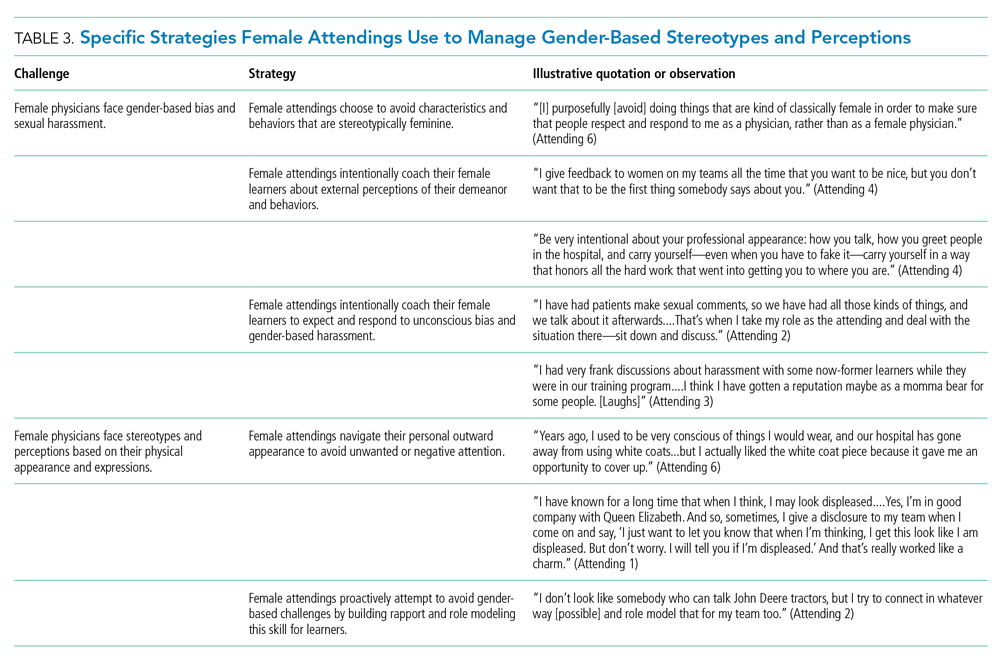
Our attendings grappled with how to physically portray themselves to avoid gender-based stereotypes. Attending 6 said, “Sometimes you might be taken less seriously if you pay more attention to your makeup or jewelry.” The same attending recalled “times where people would say inappropriate things based on what I was wearing—and I know that doesn’t happen with my male colleagues.” Our attendings responded to this challenge through purposeful choices of attire, personal appearance, and even external facial expressions that would avoid drawing unwanted or negative personal attention outside of the attending role.
Female Attendings Intentionally Identify and Embrace Their Unique Qualities
Our attendings identified societal gender norms and “traditional” masculine expectations in medicine (Table 4). Attending 4 drew attention to her institution’s healthcare leaders by remarking, “I think that women in medicine have similar challenges as women in other professional fields....Well, I guess it is different in that the pictures on the wall behind me are all White men.” Female attendings responded to this challenge by eschewing stereotypical qualities and intentionally finding and exhibiting their own unique strengths (eg, teaching approaches, areas of expertise, communication styles). By embracing their unique strengths, attendings gained confidence and felt more comfortable as physicians and educators. Advice from Attending 3 for other female physicians encapsulated this strategy: “But if [medicine] is what you love doing, then find a style that works for you, even if it’s different....Embrace being different.”
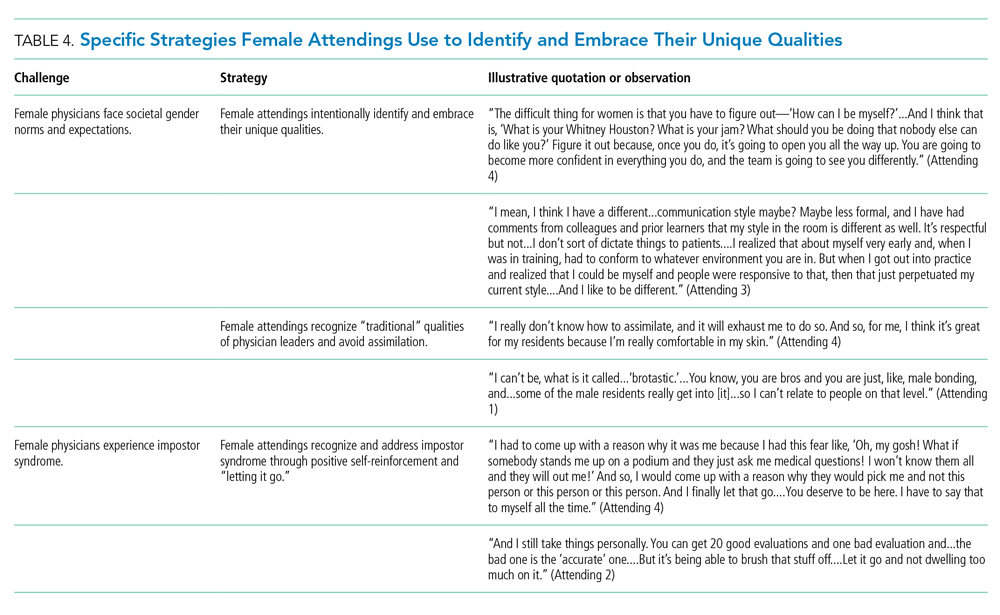
Several attendings identified patterns of thought in themselves that caused them to doubt their accomplishments and have a persistent fear of being exposed as a fraud, commonly known as impostor syndrome. Attending 2 summarized this with, “I know it’s irrational a little bit, but part of me [asks], ‘Am I getting all these opportunities because I’m female, because I’m a minority?’” Our attendings responded by recognizing impostor syndrome and addressing it through repeated positive self-reinforcing thoughts and language and by “letting go” of the doubt. Attending 4 recalled her feelings after being announced as a teaching award recipient for the fourth year in a row: “It was just like something changed in me....Maybe you are a good attending. Maybe you are doing something that is resonating with a unique class of medical students year after year.”
Our interviews also revealed strategies used by female attendings to support and advance their own careers, as well as those of other female faculty, to address the effects of impostor syndrome. Our participants noted the important role of female mentors and sponsors. One former learner mentioned, “I think some of the administration, there are definitely females that are helping promote [the attending].” During an observation, Attending 1 indicated that she was part of a network of women and junior faculty forged to promote each other’s work since “some people are good at self-promotion and some are not.” This group shares accomplishments by distributing and publicizing their accolades.
DISCUSSION
This multisite, qualitative study informs the complex ways in which exemplary female teaching attendings must navigate being women in medicine. We identified myriad challenges female attendings face originating from patients, from healthcare workers, and within themselves. Our attendings relied upon the following key strategies to mitigate such challenges: (1) they actively position themselves as physician team leaders, (2) they consciously work to manage gender-based stereotypes and perceptions, and (3) they intentionally identify and embrace their unique qualities.
Prior scholarship surrounding gender-based challenges has focused primarily on strategies to improve healthcare systems for women. Much scrutiny has been placed on elevating institutional culture,26-29 enacting clear policy surrounding sexual harassment,30 ensuring women are actively recruited and retained,31 providing resources to assist in work-life balance,26,32 and cultivating effective mentorship and social networks.11,33,34
While our findings support the importance of improving healthcare systems, they are more congruent with recent scholarship on explicit personal tactics to mitigate gender-based challenges. Researchers have suggested physicians use algorithmic responses to patient-initiated sexual harassment,35 advocate for those who experience harassment in real time,36 and engage in dedicated practice responding to harassment.37,38 Our results build on these studies by outlining strategies intended to navigate complex gender dynamics and role model approaches for learners. Interestingly, it was more common for attendings to discuss how they guide their learners and debrief after difficult situations than to discuss how they personally respond to gender-based harassment. While we are not certain why this occurred, three factors may have contributed. First, attendings mentioned that these conversations are often uncomfortable. Second, attendings appeared to accept a higher level of gender-based challenges than they would have tolerated for their learners. Lastly, although we did not gather demographic data from learners, several attendings voiced a strong desire to advocate for and equip female learners with strategies to address and navigate these challenges for themselves.
Gender stereotypes are ubiquitous and firmly rooted in long-standing belief patterns. Certain characteristics are considered masculine (eg, aggressiveness, confidence) and others feminine (eg, kindness, cooperation).10 Role congruity theory purports that stereotypes lead women to demonstrate behaviors that reflect socially accepted gender norms39 and that social approval is at risk if they behave in ways discordant with these norms.10,40 Our study provides perspectives from female physicians who walk the tightrope of forcefully asserting themselves more than their male counterparts while not being overly aggressive, since both approaches may have negative connotations.
This study has several limitations. First, it was conducted with a limited number of site visits, attendings, and learners. Likewise, attendings were internists with relatively advanced academic rank. This may reduce the study’s generalizability since attendings in other fields and at earlier career stages may utilize different strategies. However, we believe that if more senior-level female attendings experienced difficulties being recognized and legitimized in their roles, then one can assume that junior-level female faculty would experience these challenges even more so. Likewise, data saturation was not the goal of this exploratory study. Through intensive qualitative data collection, we sought to obtain an in-depth understanding of challenges and strategies. Second, many exemplary female attendings were overlooked by our selection methodology, particularly since women are often underrepresented in the factors we chose. The multisite design, modified snowball sampling, and purposeful randomized selection methodology were used to ensure quality and diversity. Third, attendings provided lists of their former learners, and thus, selection and recall biases may have been introduced since attendings may have more readily identified learners with whom they formed positive relationships. Finally, we cannot eliminate a potential Hawthorne effect on data collection. Researchers attempted to lessen this by standing apart from teams and remaining unobtrusive.
CONCLUSION
We identified strategies employed by exemplary female attendings to navigate gender-based challenges in their workplaces. We found that female attendings face unconscious bias, labels, power struggles, and harassment, simply because of their gender. They consciously and constantly navigate these challenges by positioning themselves to be seen and heard as team leaders, balancing aspects of their outward appearance and demeanor, embracing their differences and avoiding assimilation to masculine stereotypes of physician leaders, working to manage self-doubt, and coaching their female learners in these areas.
Acknowledgment
The authors are indebted to Suzanne Winter, MS, for assisting with coordination of study participants and site visits.
1. More ES. Restoring the Balance: Women Physicians and the Profession of Medicine, 1850-1995. Harvard University Press; 1999.
2. Table A-7.2: Applicants, first-time applicants, acceptees, and matriculants to U.S. medical schools by sex, 2010-2011 through 2019-2020. Association of American Medical Colleges. Published October 4, 2019. Accessed December 13, 2019. https://www.aamc.org/system/files/2019-10/2019_FACTS_Table_A-7.2.pdf
3. Table 3: Distribution of full-time faculty by department, rank, and gender, 2015. Association of American Medical Colleges. Published December 31, 2015. Accessed September 14, 2019. https://www.aamc.org/download/481182/data/2015table3.pdf
4. Shrier DK, Zucker AN, Mercurio AE, Landry LJ, Rich M, Shrier LA. Generation to generation: discrimination and harassment experiences of physician mothers and their physician daughters. J Womens Health (Larchmt). 2007;16(6):883-894. https://doi.org/10.1089/jwh.2006.0127
5. Osborn EH, Ernster VL, Martin JB. Women’s attitudes toward careers in academic medicine at the University of California, San Francisco. Acad Med. 1992;67(1):59-62. https://doi.org/10.1097/00001888-199201000-00012
6. Komaromy M, Bindman AB, Haber RJ, Sande MA. Sexual harassment in medical training. N Engl J Med. 1993;328(5):322-326. https://doi.org/10.1056/nejm199302043280507
7. Bickel J, Ruffin A. Gender-associated differences in matriculating and graduating medical students. Acad Med. 1995;70(6):552-529. https://doi.org/10.1097/00001888-199506000-00021
8. Larsson C, Hensing G, Allebeck P. Sexual and gender-related harassment in medical education and research training: results from a Swedish survey. Med Educ. 2003;37(1):39-50. https://doi.org/10.1046/j.1365-2923.2003.01404.x
9. Cochran A, Hauschild T, Elder WB, Neumayer LA, Brasel KJ, Crandall ML. Perceived gender-based barriers to careers in academic surgery. Am J Surg. 2013;206(2):263-268. https://doi.org/10.1016/j.amjsurg.2012.07.044
10. Heilman ME. Description and prescription: how gender stereotypes prevent women’s ascent up the organizational ladder. J Soc Issues. 2002;57(4):657-674. https://doi.org/10.1111/0022-4537.00234
11. Amon MJ. Looking through the glass ceiling: a qualitative study of STEM women’s career narratives. Front Psychol. 2017;8:236. https://doi.org/10.3389/fpsyg.2017.00236
12. Choo EK, van Dis J, Kass D. Time’s up for medicine? only time will tell. N Engl J Med. 2018;379(17):1592-1593. https://doi.org/10.1056/nejmp1809351
13. Adesoye T, Mangurian C, Choo EK, et al. Perceived discrimination experienced by physician mothers and desired workplace changes: a cross-sectional survey. JAMA Intern Med. 2017;177(7):1033-1036. https://doi.org/10.1001/jamainternmed.2017.1394
14. Hu YY, Ellis RJ, Hewitt DB, et al. Discrimination, abuse, harassment, and burnout in surgical residency training. N Engl J Med. 2019;381(18):1741-1752. https://doi.org/10.1056/nejmsa1903759
15. Irby DM. How attending physicians make instructional decisions when conducting teaching rounds. Acad Med. 1992;67(10):630-638. https://doi.org/10.1097/00001888-199210000-00002
16. Houchens N, Harrod M, Moody S, Fowler K, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. https://doi.org/10.12788/jhm.2763
17. Houchens N, Harrod M, Fowler KE, Moody S, Saint S. How exemplary inpatient teaching physicians foster clinical reasoning. Am J Med. 2017;130(9):1113.e1‐1113.e8. https://doi.org/10.1016/j.amjmed.2017.03.050
18. Saint S, Harrod M, Fowler KE, Houchens N. How exemplary teaching physicians interact with hospitalized patients. J Hosp Med. 2017;12(12):974-978. https://doi.org/10.12788/jhm.2844
19. Beckett L, Nettiksimmons J, Howell LP, Villablanca AC. Do family responsibilities and a clinical versus research faculty position affect satisfaction with career and work-life balance for medical school faculty? J Womens Health (Larchmt). 2015;24(6):471-480. https://doi.org/10.1089/jwh.2014.4858
20. Underrepresented in Medicine Definition. Association of American Medical Colleges. Accessed February 2, 2019. https://www.aamc.org/what-we-do/mission-areas/diversity-inclusion/underrepresented-in-medicine
21. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Sage Publications; 2002.
22. Harder B. 2019-20 Best Hospitals Honor Roll and Medical Specialties Rankings. U.S. News and World Report - Health. Accessed January 6, 2018. https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
23. Internal Medicine Residency Programs. Doximity. Accessed January 6, 2018. https://residency.doximity.com/programs?residency_specialty_id=39&sort_by=reputation&location_type=region
24. Member Groups Sections. American Medical Association. Accessed January 6, 2018. https://www.ama-assn.org/member-groups-sections
25. Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115. https://doi.org/10.1111/j.1365-2648.2007.04569.x
26. Edmunds LD, Ovseiko PV, Shepperd S, et al. Why do women choose or reject careers in academic medicine? A narrative review of empirical evidence. Lancet. 2016;388(10062):2948-2958. https://doi.org/10.1016/s0140-6736(15)01091-0
27. Magrane D, Helitzer D, Morahan P, et al. Systems of career influences: a conceptual model for evaluating the professional development of women in academic medicine. J Womens Health (Larchmt). 2012;21(12):1244-1251. https://doi.org/10.1089/jwh.2012.3638
28. Pololi LH, Civian JT, Brennan RT, Dottolo AL, Krupat E. Experiencing the culture of academic medicine: gender matters, a national study. J Gen Intern Med. 2013;28(2):201-207. https://doi.org/10.1007/s11606-012-2207-1
29. Krupat E, Pololi L, Schnell ER, Kern DE. Changing the culture of academic medicine: the C-Change learning action network and its impact at participating medical schools. Acad Med. 2013;88(9):1252-1258. https://doi.org/10.1097/acm.0b013e31829e84e0
30. Viglianti EM, Oliverio AL, Cascino TM, et al. The policy gap: a survey of patient-perpetrated sexual harassment policies for residents and fellows in prominent US hospitals. J Gen Intern Med. 2019;34(11):2326-2328. https://doi.org/10.1007/s11606-019-05229-7
31. Hoff T, Scott S. The gendered realities and talent management imperatives of women physicians. Health Care Manage Rev. 2016;41(3):189-199. https://doi.org/10.1097/hmr.0000000000000069
32. Seemann NM, Webster F, Holden HA, et al. Women in academic surgery: why is the playing field still not level? Am J Surg. 2016;211(2):343-349. https://doi.org/10.1016/j.amjsurg.2015.08.036
33. Ahmadiyeh N, Cho NL, Kellogg KC, et al. Career satisfaction of women in surgery: perceptions, factors, and strategies. J Am Coll Surg. 2010;210(1):23-28. https://doi.org/10.1016/j.jamcollsurg.2009.08.011
34. Coleman VH, Power ML, Williams S, Carpentieri A, Schulkin J. Continuing professional development: racial and gender differences in obstetrics and gynecology residents’ perceptions of mentoring. J Contin Educ Health Prof. 2005;25(4):268-277. https://doi.org/10.1002/chp.40
35. Viglianti EM, Oliverio AL, Meeks LM. Sexual harassment and abuse: when the patient is the perpetrator. Lancet. 2018;392(10145):368-370. https://doi.org/10.1016/s0140-6736(18)31502-2
36. Killeen OJ, Bridges L. Solving the silence. JAMA. 2018;320(19):1979-1980. https://doi.org/10.1001/jama.2018.15686
37. Cowan AN. Inappropriate behavior by patients and their families-call it out. JAMA Intern Med. 2018;178(11):1441. https://doi.org/10.1001/jamainternmed.2018.4348
38. Shankar M, Albert T, Yee N, et al. Approaches for residents to address problematic patient behavior: before, during, and after the clinical encounter. J Grad Med Educ. 2019;11(4):371-374. https://doi.org/10.4300/jgme-d-19-00075.1
39. Eagly AH, Karau SJ. Role congruity theory of prejudice toward female leaders. Psychol Rev. 2002;109(3):573. https://doi.org/10.1037/0033-295x.109.3.573
40. Ellinas EH, Fouad N, Byars-Winston A. Women and the decision to leave, linger, or lean in: predictors of intent to leave and aspirations to leadership and advancement in academic medicine. J Womens Health (Larchmt). 2018;27(3):324-332. https://doi.org/10.1089/jwh.2017.6457
The demographic composition of physicians has shifted dramatically in the last five decades. The number of women matriculating into medical school rose from 6% in the 1960s1 to 52% in 20192; women accounted for 39% of full-time faculty in 2015.3 Despite this evolution of the physician gender array, many challenges remain.4 Women represented only 35% of all associate professors and 22% of full professors in 2015.3 Women experience gender-based discrimination, hostility, and unconscious bias as medical trainees5-9 and as attending physicians10-13 with significant deleterious effects including burnout and suicidal thoughts.14 While types of gender-based challenges are well described in the literature, strategies to navigate and respond to these challenges are less understood.
The approaches and techniques of exemplary teaching attending physicians (hereafter referred to as “attendings”) have previously been reported from groups of predominantly male attendings.15-18 Because of gender-based challenges female physicians face that lead them to reduce their effort or leave the medical field,19 there is concern that prior scholarship in effective teaching may not adequately capture the approaches and techniques of female attendings. To our knowledge, no studies have specifically examined female attendings. Therefore, we sought to explore the lived experiences of six female attendings with particular emphasis on how they navigate and respond to gender-based challenges in clinical environments.
METHODS
Study Design and Sampling
This was a multisite study using an exploratory qualitative approach to inquiry. We aimed to examine techniques, approaches, and attitudes of outstanding general medicine teaching attendings among groups previously not well represented (ie, women and self-identified underrepresented minorities [URMs] in medicine). URM was defined by the Association of American Medical Colleges as “those racial and ethnic populations that are underrepresented in the medical profession relative to their numbers in the general population.”20 A modified snowball sampling approach21 was employed to identify attendings as delineated below.
To maintain quality while guaranteeing diversity in geography and population, potential institutions in which to observe attendings were determined by first creating the following lists: The top 20 hospitals in the U.S. News & World Report’s 2017-2018 Best Hospitals Honor Roll,22 top-rated institutions by Doximity in each geographic region and among rural training sites,23 and four historically Black colleges and universities (HBCUs) with medical schools. Institutions visited during a previous similar study16 were excluded. Next, the list was narrowed to 25 by randomly selecting five in each main geographic region and five rural institutions. These were combined with all four HBCUs to create a final list of 29 institutions.
Next, division of hospital medicine chiefs (and/or general medicine chiefs) and internal medicine residency directors at each of these 29 institutions were asked to nominate exemplary attendings, particularly those who identified as women and URMs. Twelve attendings who were themselves observed in a previous study16 were also asked for nominations. Finally, recommendations were sought from leaders of relevant American Medical Association member groups.24
Using this sampling method, 43 physicians were identified. An internet search was conducted to identify individual characteristics including medical education, training, clinical and research interests, and educational awards. These characteristics were considered and discussed by the research team. Preference was given to those attendings nominated by more than one individual (n = 3), those who had received teaching awards, and those with interests involving women in medicine. Research team members narrowed the list to seven attendings who were contacted via email and invited to participate. One did not respond, while six agreed to participate. The six attendings identified current team members who would be rounding on the visit date. Attendings were asked to recommend 6-10 former learners; we contacted these former learners and invited them to participate. Former learners were included to understand lasting effects from their attendings.
Data Collection
Observations
All 1-day site visits were conducted by two research team members, a physician (NH) and a qualitative research specialist (MQ). In four visits, an additional author accompanied the research team. In order to ensure consistency and diversity in perspectives, all authors attended at least one visit. These occurred between April 16 and August 28, 2018. Each visit began with direct observation of attendings (n = 6) and current learners (n = 24) during inpatient general medicine teaching rounds. Each researcher unobtrusively recorded their observations via handwritten, open field notes, paying particular attention to group interactions, teaching approach, conversations within and peripheral to the team, and patient–team interactions. After each visit, researchers met to compare and combine field notes.
Interviews and Focus Groups
Researchers then conducted individual, semistructured interviews with attendings and focus groups with current (n = 21) and former (n = 17) learners. Focus groups with learners varied in size from two to five participants. Former learners were occasionally not available for on-site focus groups and were interviewed separately by telephone after the visit. The interview guide for attendings (Appendix 1) was adapted from the prior study16 but expanded with questions related to experiences, challenges, and approaches of female and URM physicians. A separate guide was used to facilitate focus groups with learners (Appendix 1
This study was determined to be exempt by the University of Michigan Institutional Review Board. All participants were informed that their participation was completely voluntary and that they could terminate their involvement at any time.
Data Analysis
Data were analyzed using a content analysis approach.25 Inductive coding was used to identify codes derived from the data. Two team members (MQ and MH) independently coded the first transcript to develop a codebook, then met to compare and discuss codes. Codes and definitions were entered into the codebook. These team members continued coding five additional transcripts, meeting to compare codes, discussing any discrepancies until agreement was reached, adding new codes identified, and ensuring consistent code application. They reviewed prior transcripts and recoded if necessary. Once no new codes were identified, one team member coded the remaining transcripts. The same codebook was used to code field note documents using the same iterative process. After all qualitative data were coded and verified, they were entered into NVivo 10. Code reports were generated and reviewed by three team members to identify themes and check for coding consistency.
Role of the Funding Source
This study received no external funding.
RESULTS
We examined six exemplary attendings through direct observation of rounds and individual interviews. We also discussed these attendings with 21 current learners and 17 former learners (Appendix 2). All attendings self-identified as female. The group was diverse in terms of race/ethnicity, with three identifying as Black or African American, two as Asian, and one as White or Caucasian. Levels of experience as an attending ranged from 8 to 20 years (mean, 15.3 years). At the time of observation, two were professors and four were associate professors. The group included all three attendings who had been nominated by more than one individual, and all six had won multiple teaching awards. The observation sites represented several areas of the United States (Table 1).

The coded interview data and field notes were categorized into three broad overlapping themes based on strategies our attendings used to respond to gender-based challenges. The following sections describe types of challenges faced by female attendings along with specific strategies they employed to actively position themselves as physician team leaders, manage gender-based stereotypes and perceptions, and identify and embrace their unique qualities. Illustrative quotations or observations that further elucidate meaning are provided.
Female Attendings Actively Position Themselves as Physician Team Leaders
Our attendings frequently stated that they were assumed to be other healthcare provider types, such as nurses or physical therapists, and that these assumptions originated from patients, faculty, and staff (Table 2). Attending 3 commented, “I think every woman in this role has been mistaken for a different caretaker role, so lots of requests for nursing help. I’m sure I have taken more patients off of bed pans and brought more cups of water than maybe some of my male counterparts.” Some attendings responded to this challenge with the strategy of routinely wearing a white coat during rounds and patient encounters. This external visual cue was seen as a necessary reminder of the female attending role.

We found that patients and healthcare providers often believe teams are led by men, leading to a feeling of invisibility for female attendings. One current learner remarked, “If it was a new patient, more than likely, if we had a female attending, the patient’s eyes would always divert to the male physician.” This was not limited to patients. Attending 6 remembered comments from her consultants including, “‘Who is your attending? Let me talk with them,’ kind of assuming that I’m not the person making the decisions.” Female attendings would respond to this challenge by clearly introducing team members, including themselves, with roles and responsibilities. At times, this would require reintroductions and redirection if individuals still misidentified female team members.
Female attendings’ decision-making and thought processes were frequently second-guessed. This would often lead to power struggles with consultants, nurses, and learners. Attending 5 commented, “Even in residency, I felt this sometimes adversarial relationship with...female nurses where they would treat [female attendings] differently...questioning our decisions.” Female attendings would respond to this challenge by asserting themselves and demonstrating confidence with colleagues and at the bedside. This was an active process for women, as one former learner described: “[Female] attendings have to be a little bit more ‘on’—whatever ‘on’ is—more forceful, more direct....There is more slack given to a male attending.”
Female Attendings Consciously Work to Manage Gender-Based Stereotypes and Perceptions
Our attendings navigated gender-based stereotypes and perceptions, ranging from subtle microaggressions to overt sexual harassment (Table 3). This required balance between extremes of being perceived as “too nice” and “too aggressive,” each of which was associated with negativity. Attending 1 remarked, “I know that other [female] faculty struggle with that a bit, with being...assertive. They are assertive, and it’s interpreted [negatively].” Attending 6 described insidiously sexist comments from patients: “‘You are too young to be a physician, you are too pretty to be a physician.’ ‘Oh, the woman doctor...rather than just ‘doctor.’” During one observation of rounds, a patient remarked to the attending, “You have cold hands. You know, I’m going to have to warm those up.” Our attendings responded to these challenges by proactively avoiding characteristics and behaviors considered to be stereotypically feminine in order to draw attention to their qualities as physicians rather than as women. During interviews, some attendings directed conversation away from themselves and instead placed emphasis on coaching female learners to navigate their own demeanors, behaviors, and responses to gender bias and harassment. This would include intentional planning of how to carry oneself, as well as feedback and debrief sessions after instances of harassment.

Our attendings grappled with how to physically portray themselves to avoid gender-based stereotypes. Attending 6 said, “Sometimes you might be taken less seriously if you pay more attention to your makeup or jewelry.” The same attending recalled “times where people would say inappropriate things based on what I was wearing—and I know that doesn’t happen with my male colleagues.” Our attendings responded to this challenge through purposeful choices of attire, personal appearance, and even external facial expressions that would avoid drawing unwanted or negative personal attention outside of the attending role.
Female Attendings Intentionally Identify and Embrace Their Unique Qualities
Our attendings identified societal gender norms and “traditional” masculine expectations in medicine (Table 4). Attending 4 drew attention to her institution’s healthcare leaders by remarking, “I think that women in medicine have similar challenges as women in other professional fields....Well, I guess it is different in that the pictures on the wall behind me are all White men.” Female attendings responded to this challenge by eschewing stereotypical qualities and intentionally finding and exhibiting their own unique strengths (eg, teaching approaches, areas of expertise, communication styles). By embracing their unique strengths, attendings gained confidence and felt more comfortable as physicians and educators. Advice from Attending 3 for other female physicians encapsulated this strategy: “But if [medicine] is what you love doing, then find a style that works for you, even if it’s different....Embrace being different.”

Several attendings identified patterns of thought in themselves that caused them to doubt their accomplishments and have a persistent fear of being exposed as a fraud, commonly known as impostor syndrome. Attending 2 summarized this with, “I know it’s irrational a little bit, but part of me [asks], ‘Am I getting all these opportunities because I’m female, because I’m a minority?’” Our attendings responded by recognizing impostor syndrome and addressing it through repeated positive self-reinforcing thoughts and language and by “letting go” of the doubt. Attending 4 recalled her feelings after being announced as a teaching award recipient for the fourth year in a row: “It was just like something changed in me....Maybe you are a good attending. Maybe you are doing something that is resonating with a unique class of medical students year after year.”
Our interviews also revealed strategies used by female attendings to support and advance their own careers, as well as those of other female faculty, to address the effects of impostor syndrome. Our participants noted the important role of female mentors and sponsors. One former learner mentioned, “I think some of the administration, there are definitely females that are helping promote [the attending].” During an observation, Attending 1 indicated that she was part of a network of women and junior faculty forged to promote each other’s work since “some people are good at self-promotion and some are not.” This group shares accomplishments by distributing and publicizing their accolades.
DISCUSSION
This multisite, qualitative study informs the complex ways in which exemplary female teaching attendings must navigate being women in medicine. We identified myriad challenges female attendings face originating from patients, from healthcare workers, and within themselves. Our attendings relied upon the following key strategies to mitigate such challenges: (1) they actively position themselves as physician team leaders, (2) they consciously work to manage gender-based stereotypes and perceptions, and (3) they intentionally identify and embrace their unique qualities.
Prior scholarship surrounding gender-based challenges has focused primarily on strategies to improve healthcare systems for women. Much scrutiny has been placed on elevating institutional culture,26-29 enacting clear policy surrounding sexual harassment,30 ensuring women are actively recruited and retained,31 providing resources to assist in work-life balance,26,32 and cultivating effective mentorship and social networks.11,33,34
While our findings support the importance of improving healthcare systems, they are more congruent with recent scholarship on explicit personal tactics to mitigate gender-based challenges. Researchers have suggested physicians use algorithmic responses to patient-initiated sexual harassment,35 advocate for those who experience harassment in real time,36 and engage in dedicated practice responding to harassment.37,38 Our results build on these studies by outlining strategies intended to navigate complex gender dynamics and role model approaches for learners. Interestingly, it was more common for attendings to discuss how they guide their learners and debrief after difficult situations than to discuss how they personally respond to gender-based harassment. While we are not certain why this occurred, three factors may have contributed. First, attendings mentioned that these conversations are often uncomfortable. Second, attendings appeared to accept a higher level of gender-based challenges than they would have tolerated for their learners. Lastly, although we did not gather demographic data from learners, several attendings voiced a strong desire to advocate for and equip female learners with strategies to address and navigate these challenges for themselves.
Gender stereotypes are ubiquitous and firmly rooted in long-standing belief patterns. Certain characteristics are considered masculine (eg, aggressiveness, confidence) and others feminine (eg, kindness, cooperation).10 Role congruity theory purports that stereotypes lead women to demonstrate behaviors that reflect socially accepted gender norms39 and that social approval is at risk if they behave in ways discordant with these norms.10,40 Our study provides perspectives from female physicians who walk the tightrope of forcefully asserting themselves more than their male counterparts while not being overly aggressive, since both approaches may have negative connotations.
This study has several limitations. First, it was conducted with a limited number of site visits, attendings, and learners. Likewise, attendings were internists with relatively advanced academic rank. This may reduce the study’s generalizability since attendings in other fields and at earlier career stages may utilize different strategies. However, we believe that if more senior-level female attendings experienced difficulties being recognized and legitimized in their roles, then one can assume that junior-level female faculty would experience these challenges even more so. Likewise, data saturation was not the goal of this exploratory study. Through intensive qualitative data collection, we sought to obtain an in-depth understanding of challenges and strategies. Second, many exemplary female attendings were overlooked by our selection methodology, particularly since women are often underrepresented in the factors we chose. The multisite design, modified snowball sampling, and purposeful randomized selection methodology were used to ensure quality and diversity. Third, attendings provided lists of their former learners, and thus, selection and recall biases may have been introduced since attendings may have more readily identified learners with whom they formed positive relationships. Finally, we cannot eliminate a potential Hawthorne effect on data collection. Researchers attempted to lessen this by standing apart from teams and remaining unobtrusive.
CONCLUSION
We identified strategies employed by exemplary female attendings to navigate gender-based challenges in their workplaces. We found that female attendings face unconscious bias, labels, power struggles, and harassment, simply because of their gender. They consciously and constantly navigate these challenges by positioning themselves to be seen and heard as team leaders, balancing aspects of their outward appearance and demeanor, embracing their differences and avoiding assimilation to masculine stereotypes of physician leaders, working to manage self-doubt, and coaching their female learners in these areas.
Acknowledgment
The authors are indebted to Suzanne Winter, MS, for assisting with coordination of study participants and site visits.
The demographic composition of physicians has shifted dramatically in the last five decades. The number of women matriculating into medical school rose from 6% in the 1960s1 to 52% in 20192; women accounted for 39% of full-time faculty in 2015.3 Despite this evolution of the physician gender array, many challenges remain.4 Women represented only 35% of all associate professors and 22% of full professors in 2015.3 Women experience gender-based discrimination, hostility, and unconscious bias as medical trainees5-9 and as attending physicians10-13 with significant deleterious effects including burnout and suicidal thoughts.14 While types of gender-based challenges are well described in the literature, strategies to navigate and respond to these challenges are less understood.
The approaches and techniques of exemplary teaching attending physicians (hereafter referred to as “attendings”) have previously been reported from groups of predominantly male attendings.15-18 Because of gender-based challenges female physicians face that lead them to reduce their effort or leave the medical field,19 there is concern that prior scholarship in effective teaching may not adequately capture the approaches and techniques of female attendings. To our knowledge, no studies have specifically examined female attendings. Therefore, we sought to explore the lived experiences of six female attendings with particular emphasis on how they navigate and respond to gender-based challenges in clinical environments.
METHODS
Study Design and Sampling
This was a multisite study using an exploratory qualitative approach to inquiry. We aimed to examine techniques, approaches, and attitudes of outstanding general medicine teaching attendings among groups previously not well represented (ie, women and self-identified underrepresented minorities [URMs] in medicine). URM was defined by the Association of American Medical Colleges as “those racial and ethnic populations that are underrepresented in the medical profession relative to their numbers in the general population.”20 A modified snowball sampling approach21 was employed to identify attendings as delineated below.
To maintain quality while guaranteeing diversity in geography and population, potential institutions in which to observe attendings were determined by first creating the following lists: The top 20 hospitals in the U.S. News & World Report’s 2017-2018 Best Hospitals Honor Roll,22 top-rated institutions by Doximity in each geographic region and among rural training sites,23 and four historically Black colleges and universities (HBCUs) with medical schools. Institutions visited during a previous similar study16 were excluded. Next, the list was narrowed to 25 by randomly selecting five in each main geographic region and five rural institutions. These were combined with all four HBCUs to create a final list of 29 institutions.
Next, division of hospital medicine chiefs (and/or general medicine chiefs) and internal medicine residency directors at each of these 29 institutions were asked to nominate exemplary attendings, particularly those who identified as women and URMs. Twelve attendings who were themselves observed in a previous study16 were also asked for nominations. Finally, recommendations were sought from leaders of relevant American Medical Association member groups.24
Using this sampling method, 43 physicians were identified. An internet search was conducted to identify individual characteristics including medical education, training, clinical and research interests, and educational awards. These characteristics were considered and discussed by the research team. Preference was given to those attendings nominated by more than one individual (n = 3), those who had received teaching awards, and those with interests involving women in medicine. Research team members narrowed the list to seven attendings who were contacted via email and invited to participate. One did not respond, while six agreed to participate. The six attendings identified current team members who would be rounding on the visit date. Attendings were asked to recommend 6-10 former learners; we contacted these former learners and invited them to participate. Former learners were included to understand lasting effects from their attendings.
Data Collection
Observations
All 1-day site visits were conducted by two research team members, a physician (NH) and a qualitative research specialist (MQ). In four visits, an additional author accompanied the research team. In order to ensure consistency and diversity in perspectives, all authors attended at least one visit. These occurred between April 16 and August 28, 2018. Each visit began with direct observation of attendings (n = 6) and current learners (n = 24) during inpatient general medicine teaching rounds. Each researcher unobtrusively recorded their observations via handwritten, open field notes, paying particular attention to group interactions, teaching approach, conversations within and peripheral to the team, and patient–team interactions. After each visit, researchers met to compare and combine field notes.
Interviews and Focus Groups
Researchers then conducted individual, semistructured interviews with attendings and focus groups with current (n = 21) and former (n = 17) learners. Focus groups with learners varied in size from two to five participants. Former learners were occasionally not available for on-site focus groups and were interviewed separately by telephone after the visit. The interview guide for attendings (Appendix 1) was adapted from the prior study16 but expanded with questions related to experiences, challenges, and approaches of female and URM physicians. A separate guide was used to facilitate focus groups with learners (Appendix 1
This study was determined to be exempt by the University of Michigan Institutional Review Board. All participants were informed that their participation was completely voluntary and that they could terminate their involvement at any time.
Data Analysis
Data were analyzed using a content analysis approach.25 Inductive coding was used to identify codes derived from the data. Two team members (MQ and MH) independently coded the first transcript to develop a codebook, then met to compare and discuss codes. Codes and definitions were entered into the codebook. These team members continued coding five additional transcripts, meeting to compare codes, discussing any discrepancies until agreement was reached, adding new codes identified, and ensuring consistent code application. They reviewed prior transcripts and recoded if necessary. Once no new codes were identified, one team member coded the remaining transcripts. The same codebook was used to code field note documents using the same iterative process. After all qualitative data were coded and verified, they were entered into NVivo 10. Code reports were generated and reviewed by three team members to identify themes and check for coding consistency.
Role of the Funding Source
This study received no external funding.
RESULTS
We examined six exemplary attendings through direct observation of rounds and individual interviews. We also discussed these attendings with 21 current learners and 17 former learners (Appendix 2). All attendings self-identified as female. The group was diverse in terms of race/ethnicity, with three identifying as Black or African American, two as Asian, and one as White or Caucasian. Levels of experience as an attending ranged from 8 to 20 years (mean, 15.3 years). At the time of observation, two were professors and four were associate professors. The group included all three attendings who had been nominated by more than one individual, and all six had won multiple teaching awards. The observation sites represented several areas of the United States (Table 1).

The coded interview data and field notes were categorized into three broad overlapping themes based on strategies our attendings used to respond to gender-based challenges. The following sections describe types of challenges faced by female attendings along with specific strategies they employed to actively position themselves as physician team leaders, manage gender-based stereotypes and perceptions, and identify and embrace their unique qualities. Illustrative quotations or observations that further elucidate meaning are provided.
Female Attendings Actively Position Themselves as Physician Team Leaders
Our attendings frequently stated that they were assumed to be other healthcare provider types, such as nurses or physical therapists, and that these assumptions originated from patients, faculty, and staff (Table 2). Attending 3 commented, “I think every woman in this role has been mistaken for a different caretaker role, so lots of requests for nursing help. I’m sure I have taken more patients off of bed pans and brought more cups of water than maybe some of my male counterparts.” Some attendings responded to this challenge with the strategy of routinely wearing a white coat during rounds and patient encounters. This external visual cue was seen as a necessary reminder of the female attending role.

We found that patients and healthcare providers often believe teams are led by men, leading to a feeling of invisibility for female attendings. One current learner remarked, “If it was a new patient, more than likely, if we had a female attending, the patient’s eyes would always divert to the male physician.” This was not limited to patients. Attending 6 remembered comments from her consultants including, “‘Who is your attending? Let me talk with them,’ kind of assuming that I’m not the person making the decisions.” Female attendings would respond to this challenge by clearly introducing team members, including themselves, with roles and responsibilities. At times, this would require reintroductions and redirection if individuals still misidentified female team members.
Female attendings’ decision-making and thought processes were frequently second-guessed. This would often lead to power struggles with consultants, nurses, and learners. Attending 5 commented, “Even in residency, I felt this sometimes adversarial relationship with...female nurses where they would treat [female attendings] differently...questioning our decisions.” Female attendings would respond to this challenge by asserting themselves and demonstrating confidence with colleagues and at the bedside. This was an active process for women, as one former learner described: “[Female] attendings have to be a little bit more ‘on’—whatever ‘on’ is—more forceful, more direct....There is more slack given to a male attending.”
Female Attendings Consciously Work to Manage Gender-Based Stereotypes and Perceptions
Our attendings navigated gender-based stereotypes and perceptions, ranging from subtle microaggressions to overt sexual harassment (Table 3). This required balance between extremes of being perceived as “too nice” and “too aggressive,” each of which was associated with negativity. Attending 1 remarked, “I know that other [female] faculty struggle with that a bit, with being...assertive. They are assertive, and it’s interpreted [negatively].” Attending 6 described insidiously sexist comments from patients: “‘You are too young to be a physician, you are too pretty to be a physician.’ ‘Oh, the woman doctor...rather than just ‘doctor.’” During one observation of rounds, a patient remarked to the attending, “You have cold hands. You know, I’m going to have to warm those up.” Our attendings responded to these challenges by proactively avoiding characteristics and behaviors considered to be stereotypically feminine in order to draw attention to their qualities as physicians rather than as women. During interviews, some attendings directed conversation away from themselves and instead placed emphasis on coaching female learners to navigate their own demeanors, behaviors, and responses to gender bias and harassment. This would include intentional planning of how to carry oneself, as well as feedback and debrief sessions after instances of harassment.

Our attendings grappled with how to physically portray themselves to avoid gender-based stereotypes. Attending 6 said, “Sometimes you might be taken less seriously if you pay more attention to your makeup or jewelry.” The same attending recalled “times where people would say inappropriate things based on what I was wearing—and I know that doesn’t happen with my male colleagues.” Our attendings responded to this challenge through purposeful choices of attire, personal appearance, and even external facial expressions that would avoid drawing unwanted or negative personal attention outside of the attending role.
Female Attendings Intentionally Identify and Embrace Their Unique Qualities
Our attendings identified societal gender norms and “traditional” masculine expectations in medicine (Table 4). Attending 4 drew attention to her institution’s healthcare leaders by remarking, “I think that women in medicine have similar challenges as women in other professional fields....Well, I guess it is different in that the pictures on the wall behind me are all White men.” Female attendings responded to this challenge by eschewing stereotypical qualities and intentionally finding and exhibiting their own unique strengths (eg, teaching approaches, areas of expertise, communication styles). By embracing their unique strengths, attendings gained confidence and felt more comfortable as physicians and educators. Advice from Attending 3 for other female physicians encapsulated this strategy: “But if [medicine] is what you love doing, then find a style that works for you, even if it’s different....Embrace being different.”

Several attendings identified patterns of thought in themselves that caused them to doubt their accomplishments and have a persistent fear of being exposed as a fraud, commonly known as impostor syndrome. Attending 2 summarized this with, “I know it’s irrational a little bit, but part of me [asks], ‘Am I getting all these opportunities because I’m female, because I’m a minority?’” Our attendings responded by recognizing impostor syndrome and addressing it through repeated positive self-reinforcing thoughts and language and by “letting go” of the doubt. Attending 4 recalled her feelings after being announced as a teaching award recipient for the fourth year in a row: “It was just like something changed in me....Maybe you are a good attending. Maybe you are doing something that is resonating with a unique class of medical students year after year.”
Our interviews also revealed strategies used by female attendings to support and advance their own careers, as well as those of other female faculty, to address the effects of impostor syndrome. Our participants noted the important role of female mentors and sponsors. One former learner mentioned, “I think some of the administration, there are definitely females that are helping promote [the attending].” During an observation, Attending 1 indicated that she was part of a network of women and junior faculty forged to promote each other’s work since “some people are good at self-promotion and some are not.” This group shares accomplishments by distributing and publicizing their accolades.
DISCUSSION
This multisite, qualitative study informs the complex ways in which exemplary female teaching attendings must navigate being women in medicine. We identified myriad challenges female attendings face originating from patients, from healthcare workers, and within themselves. Our attendings relied upon the following key strategies to mitigate such challenges: (1) they actively position themselves as physician team leaders, (2) they consciously work to manage gender-based stereotypes and perceptions, and (3) they intentionally identify and embrace their unique qualities.
Prior scholarship surrounding gender-based challenges has focused primarily on strategies to improve healthcare systems for women. Much scrutiny has been placed on elevating institutional culture,26-29 enacting clear policy surrounding sexual harassment,30 ensuring women are actively recruited and retained,31 providing resources to assist in work-life balance,26,32 and cultivating effective mentorship and social networks.11,33,34
While our findings support the importance of improving healthcare systems, they are more congruent with recent scholarship on explicit personal tactics to mitigate gender-based challenges. Researchers have suggested physicians use algorithmic responses to patient-initiated sexual harassment,35 advocate for those who experience harassment in real time,36 and engage in dedicated practice responding to harassment.37,38 Our results build on these studies by outlining strategies intended to navigate complex gender dynamics and role model approaches for learners. Interestingly, it was more common for attendings to discuss how they guide their learners and debrief after difficult situations than to discuss how they personally respond to gender-based harassment. While we are not certain why this occurred, three factors may have contributed. First, attendings mentioned that these conversations are often uncomfortable. Second, attendings appeared to accept a higher level of gender-based challenges than they would have tolerated for their learners. Lastly, although we did not gather demographic data from learners, several attendings voiced a strong desire to advocate for and equip female learners with strategies to address and navigate these challenges for themselves.
Gender stereotypes are ubiquitous and firmly rooted in long-standing belief patterns. Certain characteristics are considered masculine (eg, aggressiveness, confidence) and others feminine (eg, kindness, cooperation).10 Role congruity theory purports that stereotypes lead women to demonstrate behaviors that reflect socially accepted gender norms39 and that social approval is at risk if they behave in ways discordant with these norms.10,40 Our study provides perspectives from female physicians who walk the tightrope of forcefully asserting themselves more than their male counterparts while not being overly aggressive, since both approaches may have negative connotations.
This study has several limitations. First, it was conducted with a limited number of site visits, attendings, and learners. Likewise, attendings were internists with relatively advanced academic rank. This may reduce the study’s generalizability since attendings in other fields and at earlier career stages may utilize different strategies. However, we believe that if more senior-level female attendings experienced difficulties being recognized and legitimized in their roles, then one can assume that junior-level female faculty would experience these challenges even more so. Likewise, data saturation was not the goal of this exploratory study. Through intensive qualitative data collection, we sought to obtain an in-depth understanding of challenges and strategies. Second, many exemplary female attendings were overlooked by our selection methodology, particularly since women are often underrepresented in the factors we chose. The multisite design, modified snowball sampling, and purposeful randomized selection methodology were used to ensure quality and diversity. Third, attendings provided lists of their former learners, and thus, selection and recall biases may have been introduced since attendings may have more readily identified learners with whom they formed positive relationships. Finally, we cannot eliminate a potential Hawthorne effect on data collection. Researchers attempted to lessen this by standing apart from teams and remaining unobtrusive.
CONCLUSION
We identified strategies employed by exemplary female attendings to navigate gender-based challenges in their workplaces. We found that female attendings face unconscious bias, labels, power struggles, and harassment, simply because of their gender. They consciously and constantly navigate these challenges by positioning themselves to be seen and heard as team leaders, balancing aspects of their outward appearance and demeanor, embracing their differences and avoiding assimilation to masculine stereotypes of physician leaders, working to manage self-doubt, and coaching their female learners in these areas.
Acknowledgment
The authors are indebted to Suzanne Winter, MS, for assisting with coordination of study participants and site visits.
1. More ES. Restoring the Balance: Women Physicians and the Profession of Medicine, 1850-1995. Harvard University Press; 1999.
2. Table A-7.2: Applicants, first-time applicants, acceptees, and matriculants to U.S. medical schools by sex, 2010-2011 through 2019-2020. Association of American Medical Colleges. Published October 4, 2019. Accessed December 13, 2019. https://www.aamc.org/system/files/2019-10/2019_FACTS_Table_A-7.2.pdf
3. Table 3: Distribution of full-time faculty by department, rank, and gender, 2015. Association of American Medical Colleges. Published December 31, 2015. Accessed September 14, 2019. https://www.aamc.org/download/481182/data/2015table3.pdf
4. Shrier DK, Zucker AN, Mercurio AE, Landry LJ, Rich M, Shrier LA. Generation to generation: discrimination and harassment experiences of physician mothers and their physician daughters. J Womens Health (Larchmt). 2007;16(6):883-894. https://doi.org/10.1089/jwh.2006.0127
5. Osborn EH, Ernster VL, Martin JB. Women’s attitudes toward careers in academic medicine at the University of California, San Francisco. Acad Med. 1992;67(1):59-62. https://doi.org/10.1097/00001888-199201000-00012
6. Komaromy M, Bindman AB, Haber RJ, Sande MA. Sexual harassment in medical training. N Engl J Med. 1993;328(5):322-326. https://doi.org/10.1056/nejm199302043280507
7. Bickel J, Ruffin A. Gender-associated differences in matriculating and graduating medical students. Acad Med. 1995;70(6):552-529. https://doi.org/10.1097/00001888-199506000-00021
8. Larsson C, Hensing G, Allebeck P. Sexual and gender-related harassment in medical education and research training: results from a Swedish survey. Med Educ. 2003;37(1):39-50. https://doi.org/10.1046/j.1365-2923.2003.01404.x
9. Cochran A, Hauschild T, Elder WB, Neumayer LA, Brasel KJ, Crandall ML. Perceived gender-based barriers to careers in academic surgery. Am J Surg. 2013;206(2):263-268. https://doi.org/10.1016/j.amjsurg.2012.07.044
10. Heilman ME. Description and prescription: how gender stereotypes prevent women’s ascent up the organizational ladder. J Soc Issues. 2002;57(4):657-674. https://doi.org/10.1111/0022-4537.00234
11. Amon MJ. Looking through the glass ceiling: a qualitative study of STEM women’s career narratives. Front Psychol. 2017;8:236. https://doi.org/10.3389/fpsyg.2017.00236
12. Choo EK, van Dis J, Kass D. Time’s up for medicine? only time will tell. N Engl J Med. 2018;379(17):1592-1593. https://doi.org/10.1056/nejmp1809351
13. Adesoye T, Mangurian C, Choo EK, et al. Perceived discrimination experienced by physician mothers and desired workplace changes: a cross-sectional survey. JAMA Intern Med. 2017;177(7):1033-1036. https://doi.org/10.1001/jamainternmed.2017.1394
14. Hu YY, Ellis RJ, Hewitt DB, et al. Discrimination, abuse, harassment, and burnout in surgical residency training. N Engl J Med. 2019;381(18):1741-1752. https://doi.org/10.1056/nejmsa1903759
15. Irby DM. How attending physicians make instructional decisions when conducting teaching rounds. Acad Med. 1992;67(10):630-638. https://doi.org/10.1097/00001888-199210000-00002
16. Houchens N, Harrod M, Moody S, Fowler K, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. https://doi.org/10.12788/jhm.2763
17. Houchens N, Harrod M, Fowler KE, Moody S, Saint S. How exemplary inpatient teaching physicians foster clinical reasoning. Am J Med. 2017;130(9):1113.e1‐1113.e8. https://doi.org/10.1016/j.amjmed.2017.03.050
18. Saint S, Harrod M, Fowler KE, Houchens N. How exemplary teaching physicians interact with hospitalized patients. J Hosp Med. 2017;12(12):974-978. https://doi.org/10.12788/jhm.2844
19. Beckett L, Nettiksimmons J, Howell LP, Villablanca AC. Do family responsibilities and a clinical versus research faculty position affect satisfaction with career and work-life balance for medical school faculty? J Womens Health (Larchmt). 2015;24(6):471-480. https://doi.org/10.1089/jwh.2014.4858
20. Underrepresented in Medicine Definition. Association of American Medical Colleges. Accessed February 2, 2019. https://www.aamc.org/what-we-do/mission-areas/diversity-inclusion/underrepresented-in-medicine
21. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Sage Publications; 2002.
22. Harder B. 2019-20 Best Hospitals Honor Roll and Medical Specialties Rankings. U.S. News and World Report - Health. Accessed January 6, 2018. https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
23. Internal Medicine Residency Programs. Doximity. Accessed January 6, 2018. https://residency.doximity.com/programs?residency_specialty_id=39&sort_by=reputation&location_type=region
24. Member Groups Sections. American Medical Association. Accessed January 6, 2018. https://www.ama-assn.org/member-groups-sections
25. Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115. https://doi.org/10.1111/j.1365-2648.2007.04569.x
26. Edmunds LD, Ovseiko PV, Shepperd S, et al. Why do women choose or reject careers in academic medicine? A narrative review of empirical evidence. Lancet. 2016;388(10062):2948-2958. https://doi.org/10.1016/s0140-6736(15)01091-0
27. Magrane D, Helitzer D, Morahan P, et al. Systems of career influences: a conceptual model for evaluating the professional development of women in academic medicine. J Womens Health (Larchmt). 2012;21(12):1244-1251. https://doi.org/10.1089/jwh.2012.3638
28. Pololi LH, Civian JT, Brennan RT, Dottolo AL, Krupat E. Experiencing the culture of academic medicine: gender matters, a national study. J Gen Intern Med. 2013;28(2):201-207. https://doi.org/10.1007/s11606-012-2207-1
29. Krupat E, Pololi L, Schnell ER, Kern DE. Changing the culture of academic medicine: the C-Change learning action network and its impact at participating medical schools. Acad Med. 2013;88(9):1252-1258. https://doi.org/10.1097/acm.0b013e31829e84e0
30. Viglianti EM, Oliverio AL, Cascino TM, et al. The policy gap: a survey of patient-perpetrated sexual harassment policies for residents and fellows in prominent US hospitals. J Gen Intern Med. 2019;34(11):2326-2328. https://doi.org/10.1007/s11606-019-05229-7
31. Hoff T, Scott S. The gendered realities and talent management imperatives of women physicians. Health Care Manage Rev. 2016;41(3):189-199. https://doi.org/10.1097/hmr.0000000000000069
32. Seemann NM, Webster F, Holden HA, et al. Women in academic surgery: why is the playing field still not level? Am J Surg. 2016;211(2):343-349. https://doi.org/10.1016/j.amjsurg.2015.08.036
33. Ahmadiyeh N, Cho NL, Kellogg KC, et al. Career satisfaction of women in surgery: perceptions, factors, and strategies. J Am Coll Surg. 2010;210(1):23-28. https://doi.org/10.1016/j.jamcollsurg.2009.08.011
34. Coleman VH, Power ML, Williams S, Carpentieri A, Schulkin J. Continuing professional development: racial and gender differences in obstetrics and gynecology residents’ perceptions of mentoring. J Contin Educ Health Prof. 2005;25(4):268-277. https://doi.org/10.1002/chp.40
35. Viglianti EM, Oliverio AL, Meeks LM. Sexual harassment and abuse: when the patient is the perpetrator. Lancet. 2018;392(10145):368-370. https://doi.org/10.1016/s0140-6736(18)31502-2
36. Killeen OJ, Bridges L. Solving the silence. JAMA. 2018;320(19):1979-1980. https://doi.org/10.1001/jama.2018.15686
37. Cowan AN. Inappropriate behavior by patients and their families-call it out. JAMA Intern Med. 2018;178(11):1441. https://doi.org/10.1001/jamainternmed.2018.4348
38. Shankar M, Albert T, Yee N, et al. Approaches for residents to address problematic patient behavior: before, during, and after the clinical encounter. J Grad Med Educ. 2019;11(4):371-374. https://doi.org/10.4300/jgme-d-19-00075.1
39. Eagly AH, Karau SJ. Role congruity theory of prejudice toward female leaders. Psychol Rev. 2002;109(3):573. https://doi.org/10.1037/0033-295x.109.3.573
40. Ellinas EH, Fouad N, Byars-Winston A. Women and the decision to leave, linger, or lean in: predictors of intent to leave and aspirations to leadership and advancement in academic medicine. J Womens Health (Larchmt). 2018;27(3):324-332. https://doi.org/10.1089/jwh.2017.6457
1. More ES. Restoring the Balance: Women Physicians and the Profession of Medicine, 1850-1995. Harvard University Press; 1999.
2. Table A-7.2: Applicants, first-time applicants, acceptees, and matriculants to U.S. medical schools by sex, 2010-2011 through 2019-2020. Association of American Medical Colleges. Published October 4, 2019. Accessed December 13, 2019. https://www.aamc.org/system/files/2019-10/2019_FACTS_Table_A-7.2.pdf
3. Table 3: Distribution of full-time faculty by department, rank, and gender, 2015. Association of American Medical Colleges. Published December 31, 2015. Accessed September 14, 2019. https://www.aamc.org/download/481182/data/2015table3.pdf
4. Shrier DK, Zucker AN, Mercurio AE, Landry LJ, Rich M, Shrier LA. Generation to generation: discrimination and harassment experiences of physician mothers and their physician daughters. J Womens Health (Larchmt). 2007;16(6):883-894. https://doi.org/10.1089/jwh.2006.0127
5. Osborn EH, Ernster VL, Martin JB. Women’s attitudes toward careers in academic medicine at the University of California, San Francisco. Acad Med. 1992;67(1):59-62. https://doi.org/10.1097/00001888-199201000-00012
6. Komaromy M, Bindman AB, Haber RJ, Sande MA. Sexual harassment in medical training. N Engl J Med. 1993;328(5):322-326. https://doi.org/10.1056/nejm199302043280507
7. Bickel J, Ruffin A. Gender-associated differences in matriculating and graduating medical students. Acad Med. 1995;70(6):552-529. https://doi.org/10.1097/00001888-199506000-00021
8. Larsson C, Hensing G, Allebeck P. Sexual and gender-related harassment in medical education and research training: results from a Swedish survey. Med Educ. 2003;37(1):39-50. https://doi.org/10.1046/j.1365-2923.2003.01404.x
9. Cochran A, Hauschild T, Elder WB, Neumayer LA, Brasel KJ, Crandall ML. Perceived gender-based barriers to careers in academic surgery. Am J Surg. 2013;206(2):263-268. https://doi.org/10.1016/j.amjsurg.2012.07.044
10. Heilman ME. Description and prescription: how gender stereotypes prevent women’s ascent up the organizational ladder. J Soc Issues. 2002;57(4):657-674. https://doi.org/10.1111/0022-4537.00234
11. Amon MJ. Looking through the glass ceiling: a qualitative study of STEM women’s career narratives. Front Psychol. 2017;8:236. https://doi.org/10.3389/fpsyg.2017.00236
12. Choo EK, van Dis J, Kass D. Time’s up for medicine? only time will tell. N Engl J Med. 2018;379(17):1592-1593. https://doi.org/10.1056/nejmp1809351
13. Adesoye T, Mangurian C, Choo EK, et al. Perceived discrimination experienced by physician mothers and desired workplace changes: a cross-sectional survey. JAMA Intern Med. 2017;177(7):1033-1036. https://doi.org/10.1001/jamainternmed.2017.1394
14. Hu YY, Ellis RJ, Hewitt DB, et al. Discrimination, abuse, harassment, and burnout in surgical residency training. N Engl J Med. 2019;381(18):1741-1752. https://doi.org/10.1056/nejmsa1903759
15. Irby DM. How attending physicians make instructional decisions when conducting teaching rounds. Acad Med. 1992;67(10):630-638. https://doi.org/10.1097/00001888-199210000-00002
16. Houchens N, Harrod M, Moody S, Fowler K, Saint S. Techniques and behaviors associated with exemplary inpatient general medicine teaching: an exploratory qualitative study. J Hosp Med. 2017;12(7):503-509. https://doi.org/10.12788/jhm.2763
17. Houchens N, Harrod M, Fowler KE, Moody S, Saint S. How exemplary inpatient teaching physicians foster clinical reasoning. Am J Med. 2017;130(9):1113.e1‐1113.e8. https://doi.org/10.1016/j.amjmed.2017.03.050
18. Saint S, Harrod M, Fowler KE, Houchens N. How exemplary teaching physicians interact with hospitalized patients. J Hosp Med. 2017;12(12):974-978. https://doi.org/10.12788/jhm.2844
19. Beckett L, Nettiksimmons J, Howell LP, Villablanca AC. Do family responsibilities and a clinical versus research faculty position affect satisfaction with career and work-life balance for medical school faculty? J Womens Health (Larchmt). 2015;24(6):471-480. https://doi.org/10.1089/jwh.2014.4858
20. Underrepresented in Medicine Definition. Association of American Medical Colleges. Accessed February 2, 2019. https://www.aamc.org/what-we-do/mission-areas/diversity-inclusion/underrepresented-in-medicine
21. Patton MQ. Qualitative Research and Evaluation Methods. 3rd ed. Sage Publications; 2002.
22. Harder B. 2019-20 Best Hospitals Honor Roll and Medical Specialties Rankings. U.S. News and World Report - Health. Accessed January 6, 2018. https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
23. Internal Medicine Residency Programs. Doximity. Accessed January 6, 2018. https://residency.doximity.com/programs?residency_specialty_id=39&sort_by=reputation&location_type=region
24. Member Groups Sections. American Medical Association. Accessed January 6, 2018. https://www.ama-assn.org/member-groups-sections
25. Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115. https://doi.org/10.1111/j.1365-2648.2007.04569.x
26. Edmunds LD, Ovseiko PV, Shepperd S, et al. Why do women choose or reject careers in academic medicine? A narrative review of empirical evidence. Lancet. 2016;388(10062):2948-2958. https://doi.org/10.1016/s0140-6736(15)01091-0
27. Magrane D, Helitzer D, Morahan P, et al. Systems of career influences: a conceptual model for evaluating the professional development of women in academic medicine. J Womens Health (Larchmt). 2012;21(12):1244-1251. https://doi.org/10.1089/jwh.2012.3638
28. Pololi LH, Civian JT, Brennan RT, Dottolo AL, Krupat E. Experiencing the culture of academic medicine: gender matters, a national study. J Gen Intern Med. 2013;28(2):201-207. https://doi.org/10.1007/s11606-012-2207-1
29. Krupat E, Pololi L, Schnell ER, Kern DE. Changing the culture of academic medicine: the C-Change learning action network and its impact at participating medical schools. Acad Med. 2013;88(9):1252-1258. https://doi.org/10.1097/acm.0b013e31829e84e0
30. Viglianti EM, Oliverio AL, Cascino TM, et al. The policy gap: a survey of patient-perpetrated sexual harassment policies for residents and fellows in prominent US hospitals. J Gen Intern Med. 2019;34(11):2326-2328. https://doi.org/10.1007/s11606-019-05229-7
31. Hoff T, Scott S. The gendered realities and talent management imperatives of women physicians. Health Care Manage Rev. 2016;41(3):189-199. https://doi.org/10.1097/hmr.0000000000000069
32. Seemann NM, Webster F, Holden HA, et al. Women in academic surgery: why is the playing field still not level? Am J Surg. 2016;211(2):343-349. https://doi.org/10.1016/j.amjsurg.2015.08.036
33. Ahmadiyeh N, Cho NL, Kellogg KC, et al. Career satisfaction of women in surgery: perceptions, factors, and strategies. J Am Coll Surg. 2010;210(1):23-28. https://doi.org/10.1016/j.jamcollsurg.2009.08.011
34. Coleman VH, Power ML, Williams S, Carpentieri A, Schulkin J. Continuing professional development: racial and gender differences in obstetrics and gynecology residents’ perceptions of mentoring. J Contin Educ Health Prof. 2005;25(4):268-277. https://doi.org/10.1002/chp.40
35. Viglianti EM, Oliverio AL, Meeks LM. Sexual harassment and abuse: when the patient is the perpetrator. Lancet. 2018;392(10145):368-370. https://doi.org/10.1016/s0140-6736(18)31502-2
36. Killeen OJ, Bridges L. Solving the silence. JAMA. 2018;320(19):1979-1980. https://doi.org/10.1001/jama.2018.15686
37. Cowan AN. Inappropriate behavior by patients and their families-call it out. JAMA Intern Med. 2018;178(11):1441. https://doi.org/10.1001/jamainternmed.2018.4348
38. Shankar M, Albert T, Yee N, et al. Approaches for residents to address problematic patient behavior: before, during, and after the clinical encounter. J Grad Med Educ. 2019;11(4):371-374. https://doi.org/10.4300/jgme-d-19-00075.1
39. Eagly AH, Karau SJ. Role congruity theory of prejudice toward female leaders. Psychol Rev. 2002;109(3):573. https://doi.org/10.1037/0033-295x.109.3.573
40. Ellinas EH, Fouad N, Byars-Winston A. Women and the decision to leave, linger, or lean in: predictors of intent to leave and aspirations to leadership and advancement in academic medicine. J Womens Health (Larchmt). 2018;27(3):324-332. https://doi.org/10.1089/jwh.2017.6457
© 2020 Society of Hospital Medicine
‘Knowledge is power’: Knowing BRCA1/2 status tied to survival
The study, conducted among Ashkenazi Jewish women in Israel, showed that among women who knew their carrier status before they developed breast cancer, diagnoses were made at an earlier disease stage and 5-year survival was improved compared to women who learned their carrier status only after their disease had been diagnosed.
The study was published online on July 9 in JAMA Oncology.
“I don’t want to belittle the complexities of knowing that you’re a carrier. But I think these results really show that knowledge is power,” first author Ephrat Levy-Lahad, MD, director of the medical genetics unit at Shaare Zedek Medical Center in Jerusalem, Israel, told Medscape Medical News.
Carrying a BRCA1/2 pathogenic mutation is associated with a 70% to 80% lifetime risk for breast cancer and about a 10% to 50% lifetime risk for ovarian cancer, depending on the specific mutation. Only about 10% of carriers will not develop either cancer during their lifetime.
The study provides support for genetic screening for pathogenic BRCA1/2 mutations, especially in high-risk populations, according to Levy-Lahad.
“For me, the results are part of a bigger picture.... I think we should be moving towards general population screening, certainly in high-risk populations like Ashkenazi Jews,” she said.
In Israel, that decision has already been made: a new policy, introduced in January 2020, offers testing for common BRCA1/2 mutations for all Ashkenazi Jewish women.
However, women in other countries may also benefit from testing, she argues. About half of BRCA1/2 carriers in a general population like that of the United States do not have a family history that would indicate a need for testing. That means many women who carry these mutations may not be taking advantage of recommended surveillance and prevention measures, she said.
But screening for BRCA1/2 mutations becomes more complicated when applied to more general populations, she acknowledged.
About 2.5% of women of Ashkenazi Jewish descent carry pathogenic mutations for BRCA1/2, compared to 0.5% in the general White population.
Also, screening in the Ashkenazi Jewish population is probably simpler than in the general population. Just three mutations are definitely known to cause disease and need to be tested for among Ashkenazi Jews. Screening in a larger population would require full sequencing of the gene. That increases the likelihood of finding variants of unknown significance (VUSs), which muddies the water. Knowledge is incomplete about whether some of these VUSs increase cancer risk, and physicians do not always know how to manage them in women who test positive.
Moreover, Israel has a national health system. Screening in a country without universal health insurance such as the United States raises questions about whether follow-up would be covered by insurance carriers for women who test positive.
Mehmet Copur, MD, an oncologist at Morrison Cancer Center in Hastings, Nebraska, questions how general population screening could be done in “real life.”
“These findings should be taken into consideration in the context of the patient population who would agree to genetic testing, who would agree to comply with the recommended guidelines for risk reduction, and who would have insurance coverage or resources to comply with the recommendations,” Copur told Medscape Medical News.
“If BRCA-positive patients did not or could not follow these recommendations, the results would different,” he added.
The most crucial component of screening for these mutations is genetic counselors, who are in short supply in the United States, according to Copur.
Another issue is that of cost. Genetic counseling is not always covered by insurance, especially for individuals who do not have a family history of BRCA-related cancers. Genetic testing is not cheap, and the costs of monitoring women who test positive could be prohibitive, especially in a healthcare system burdened by COVID-19.
“Whether our current healthcare system could bear the cost of such a change is up for debate. The screening itself may be feasible, but offering lifelong surveillance to every woman identified with mutations could present huge capacity issues,” Copur said. “Maybe in the future, the healthcare system can be ready for such an undertaking, but I don’t think we are there yet.”
Although she acknowledges the differences in risk between Ashkenazi Jews and the general population, Levy-Lahad thinks not having screening is like “throwing the baby out with the bath water.”
“Maybe we’re not ready for total general population screening, but I think we have to start thinking along those lines,” she said. “We have this incredible tool for cancer prevention, and we should really be using it, certainly in populations like Ashkenazi Jews.”
Researchers conducted a retrospective analysis that included 105 women diagnosed with breast cancer at Shaare Zedek Medical Center in Jerusalem between 2005 and 2016. Forty-two women knew they were carriers before their breast cancer diagnosis, and 63 learned of their carrier status only after diagnosis. Of the participants, 83% were Ashkenazi Jews. For both prediagnosis and postdiagnosis groups, the age at diagnosis was the same (50.4 years). For both groups, distributions of pathogenic mutations were similar. There were no significant differences in hormone receptor or ERBB2 status.
Among women who knew they were carriers before diagnosis, 80.9% (34/42) were diagnosed either with ductal carcinoma in situ or stage 1 disease. Only 9.5% (4/42) of these women were diagnosed with disease of stage 2 or higher.
In comparison, among women who learned their carrier status after diagnosis, 30% (19/63) had early-stage disease at diagnosis, and 52.4% (33/63) were diagnosed at stage 2 or higher (P < .001).
Compared to women who knew their carrier status before diagnosis, women who found out after diagnosis had 12 times higher odds of being diagnosed with disease of advanced clinical stage (P = .001) and eight times higher odds of being diagnosed with disease of advanced pathologic stage (P = .002).
A sentinel node biopsy was sufficient in 85.7% (36/42) of women who knew their carrier status before diagnosis; 7.2% (3/42) of these women needed a full lymph node dissection. In contrast, 3.2% (2/63) of women who learned their carrier status after diagnosis underwent sentinel node biopsy, and 34.9% (25/105) needed a full lymph node dissection (P < .001).
Among women who knew their carrier status before diagnosis, 54.8% (23/42) did not need chemotherapy at all, and none needed neoadjuvant chemotherapy. Only 4.8% (3/63) of women who learned their mutation status after diagnosis were able to forgo chemotherapy (P < .001); 22.2% (14/63) needed neoadjuvant therapy (P = .001).
These findings appeared to translate into better outcomes. Overall 5-year survival was significantly higher among women who knew their carrier status before diagnosis compared to women who found out afterward (94% [SE 4%] vs 78% [SE 5%]; P = .03). Only two of 42 women (4.8%) in the prediagnosis group died, compared to 16 of 63 (25.4%) in the postdiagnosis group.
Analyses that controlled for year at diagnosis showed that women who learned their carrier status before diagnosis had significantly lower risk for overall mortality compared with those who found out after diagnosis (hazard ratio [HR], 0.20; 95% CI, 0.04 – 0.93; P = .04). However, these results lost significance when controlled for age, socioeconomic index, family history, and gene variant (HR, 0.16; 95% CI, 0.02 – 1.4; P = .10).
Higher socioeconomic status (HR, 0.76; 95% CI, 0.6 – 0.97; P = .03), gene variant (BRCA2 vs BRCA1: HR, 0.15; 95% CI, 0.03 – 0.75; P = .02), and age at diagnosis (HR, 1.047; 95% CI, 1.003 – 1.093; P = .04) were all associated with overall mortality.
“I can’t infer causation, but we suspect that the reason for these results is the difference in follow-up,” Levy-Lahad said.
Most of the women (95.2%, 40/42) who knew their carrier status before diagnosis received their follow-up at the medical center’s high-risk carrier clinic. Twenty-seven of 42 (64.3%) of these women were diagnosed with breast MRI. By contrast, only 1.6% (1/63) of women who found out their carrier status after diagnosis were diagnosed with breast MRI. Breast MRI is not routinely used for breast cancer screening but can be more sensitive than mammography for detecting breast cancer.
The study was funded by the Breast Cancer Research Foundation and by a gift from Ellie and David Werber to ShaareZedek Medical Center.
Levy-Lahad received grants from the Breast Cancer Research Foundation and from the Israel Cancer Association during the conduct of the study and personal fees from AstraZeneca outside the submitted work. Copur has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The study, conducted among Ashkenazi Jewish women in Israel, showed that among women who knew their carrier status before they developed breast cancer, diagnoses were made at an earlier disease stage and 5-year survival was improved compared to women who learned their carrier status only after their disease had been diagnosed.
The study was published online on July 9 in JAMA Oncology.
“I don’t want to belittle the complexities of knowing that you’re a carrier. But I think these results really show that knowledge is power,” first author Ephrat Levy-Lahad, MD, director of the medical genetics unit at Shaare Zedek Medical Center in Jerusalem, Israel, told Medscape Medical News.
Carrying a BRCA1/2 pathogenic mutation is associated with a 70% to 80% lifetime risk for breast cancer and about a 10% to 50% lifetime risk for ovarian cancer, depending on the specific mutation. Only about 10% of carriers will not develop either cancer during their lifetime.
The study provides support for genetic screening for pathogenic BRCA1/2 mutations, especially in high-risk populations, according to Levy-Lahad.
“For me, the results are part of a bigger picture.... I think we should be moving towards general population screening, certainly in high-risk populations like Ashkenazi Jews,” she said.
In Israel, that decision has already been made: a new policy, introduced in January 2020, offers testing for common BRCA1/2 mutations for all Ashkenazi Jewish women.
However, women in other countries may also benefit from testing, she argues. About half of BRCA1/2 carriers in a general population like that of the United States do not have a family history that would indicate a need for testing. That means many women who carry these mutations may not be taking advantage of recommended surveillance and prevention measures, she said.
But screening for BRCA1/2 mutations becomes more complicated when applied to more general populations, she acknowledged.
About 2.5% of women of Ashkenazi Jewish descent carry pathogenic mutations for BRCA1/2, compared to 0.5% in the general White population.
Also, screening in the Ashkenazi Jewish population is probably simpler than in the general population. Just three mutations are definitely known to cause disease and need to be tested for among Ashkenazi Jews. Screening in a larger population would require full sequencing of the gene. That increases the likelihood of finding variants of unknown significance (VUSs), which muddies the water. Knowledge is incomplete about whether some of these VUSs increase cancer risk, and physicians do not always know how to manage them in women who test positive.
Moreover, Israel has a national health system. Screening in a country without universal health insurance such as the United States raises questions about whether follow-up would be covered by insurance carriers for women who test positive.
Mehmet Copur, MD, an oncologist at Morrison Cancer Center in Hastings, Nebraska, questions how general population screening could be done in “real life.”
“These findings should be taken into consideration in the context of the patient population who would agree to genetic testing, who would agree to comply with the recommended guidelines for risk reduction, and who would have insurance coverage or resources to comply with the recommendations,” Copur told Medscape Medical News.
“If BRCA-positive patients did not or could not follow these recommendations, the results would different,” he added.
The most crucial component of screening for these mutations is genetic counselors, who are in short supply in the United States, according to Copur.
Another issue is that of cost. Genetic counseling is not always covered by insurance, especially for individuals who do not have a family history of BRCA-related cancers. Genetic testing is not cheap, and the costs of monitoring women who test positive could be prohibitive, especially in a healthcare system burdened by COVID-19.
“Whether our current healthcare system could bear the cost of such a change is up for debate. The screening itself may be feasible, but offering lifelong surveillance to every woman identified with mutations could present huge capacity issues,” Copur said. “Maybe in the future, the healthcare system can be ready for such an undertaking, but I don’t think we are there yet.”
Although she acknowledges the differences in risk between Ashkenazi Jews and the general population, Levy-Lahad thinks not having screening is like “throwing the baby out with the bath water.”
“Maybe we’re not ready for total general population screening, but I think we have to start thinking along those lines,” she said. “We have this incredible tool for cancer prevention, and we should really be using it, certainly in populations like Ashkenazi Jews.”
Researchers conducted a retrospective analysis that included 105 women diagnosed with breast cancer at Shaare Zedek Medical Center in Jerusalem between 2005 and 2016. Forty-two women knew they were carriers before their breast cancer diagnosis, and 63 learned of their carrier status only after diagnosis. Of the participants, 83% were Ashkenazi Jews. For both prediagnosis and postdiagnosis groups, the age at diagnosis was the same (50.4 years). For both groups, distributions of pathogenic mutations were similar. There were no significant differences in hormone receptor or ERBB2 status.
Among women who knew they were carriers before diagnosis, 80.9% (34/42) were diagnosed either with ductal carcinoma in situ or stage 1 disease. Only 9.5% (4/42) of these women were diagnosed with disease of stage 2 or higher.
In comparison, among women who learned their carrier status after diagnosis, 30% (19/63) had early-stage disease at diagnosis, and 52.4% (33/63) were diagnosed at stage 2 or higher (P < .001).
Compared to women who knew their carrier status before diagnosis, women who found out after diagnosis had 12 times higher odds of being diagnosed with disease of advanced clinical stage (P = .001) and eight times higher odds of being diagnosed with disease of advanced pathologic stage (P = .002).
A sentinel node biopsy was sufficient in 85.7% (36/42) of women who knew their carrier status before diagnosis; 7.2% (3/42) of these women needed a full lymph node dissection. In contrast, 3.2% (2/63) of women who learned their carrier status after diagnosis underwent sentinel node biopsy, and 34.9% (25/105) needed a full lymph node dissection (P < .001).
Among women who knew their carrier status before diagnosis, 54.8% (23/42) did not need chemotherapy at all, and none needed neoadjuvant chemotherapy. Only 4.8% (3/63) of women who learned their mutation status after diagnosis were able to forgo chemotherapy (P < .001); 22.2% (14/63) needed neoadjuvant therapy (P = .001).
These findings appeared to translate into better outcomes. Overall 5-year survival was significantly higher among women who knew their carrier status before diagnosis compared to women who found out afterward (94% [SE 4%] vs 78% [SE 5%]; P = .03). Only two of 42 women (4.8%) in the prediagnosis group died, compared to 16 of 63 (25.4%) in the postdiagnosis group.
Analyses that controlled for year at diagnosis showed that women who learned their carrier status before diagnosis had significantly lower risk for overall mortality compared with those who found out after diagnosis (hazard ratio [HR], 0.20; 95% CI, 0.04 – 0.93; P = .04). However, these results lost significance when controlled for age, socioeconomic index, family history, and gene variant (HR, 0.16; 95% CI, 0.02 – 1.4; P = .10).
Higher socioeconomic status (HR, 0.76; 95% CI, 0.6 – 0.97; P = .03), gene variant (BRCA2 vs BRCA1: HR, 0.15; 95% CI, 0.03 – 0.75; P = .02), and age at diagnosis (HR, 1.047; 95% CI, 1.003 – 1.093; P = .04) were all associated with overall mortality.
“I can’t infer causation, but we suspect that the reason for these results is the difference in follow-up,” Levy-Lahad said.
Most of the women (95.2%, 40/42) who knew their carrier status before diagnosis received their follow-up at the medical center’s high-risk carrier clinic. Twenty-seven of 42 (64.3%) of these women were diagnosed with breast MRI. By contrast, only 1.6% (1/63) of women who found out their carrier status after diagnosis were diagnosed with breast MRI. Breast MRI is not routinely used for breast cancer screening but can be more sensitive than mammography for detecting breast cancer.
The study was funded by the Breast Cancer Research Foundation and by a gift from Ellie and David Werber to ShaareZedek Medical Center.
Levy-Lahad received grants from the Breast Cancer Research Foundation and from the Israel Cancer Association during the conduct of the study and personal fees from AstraZeneca outside the submitted work. Copur has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The study, conducted among Ashkenazi Jewish women in Israel, showed that among women who knew their carrier status before they developed breast cancer, diagnoses were made at an earlier disease stage and 5-year survival was improved compared to women who learned their carrier status only after their disease had been diagnosed.
The study was published online on July 9 in JAMA Oncology.
“I don’t want to belittle the complexities of knowing that you’re a carrier. But I think these results really show that knowledge is power,” first author Ephrat Levy-Lahad, MD, director of the medical genetics unit at Shaare Zedek Medical Center in Jerusalem, Israel, told Medscape Medical News.
Carrying a BRCA1/2 pathogenic mutation is associated with a 70% to 80% lifetime risk for breast cancer and about a 10% to 50% lifetime risk for ovarian cancer, depending on the specific mutation. Only about 10% of carriers will not develop either cancer during their lifetime.
The study provides support for genetic screening for pathogenic BRCA1/2 mutations, especially in high-risk populations, according to Levy-Lahad.
“For me, the results are part of a bigger picture.... I think we should be moving towards general population screening, certainly in high-risk populations like Ashkenazi Jews,” she said.
In Israel, that decision has already been made: a new policy, introduced in January 2020, offers testing for common BRCA1/2 mutations for all Ashkenazi Jewish women.
However, women in other countries may also benefit from testing, she argues. About half of BRCA1/2 carriers in a general population like that of the United States do not have a family history that would indicate a need for testing. That means many women who carry these mutations may not be taking advantage of recommended surveillance and prevention measures, she said.
But screening for BRCA1/2 mutations becomes more complicated when applied to more general populations, she acknowledged.
About 2.5% of women of Ashkenazi Jewish descent carry pathogenic mutations for BRCA1/2, compared to 0.5% in the general White population.
Also, screening in the Ashkenazi Jewish population is probably simpler than in the general population. Just three mutations are definitely known to cause disease and need to be tested for among Ashkenazi Jews. Screening in a larger population would require full sequencing of the gene. That increases the likelihood of finding variants of unknown significance (VUSs), which muddies the water. Knowledge is incomplete about whether some of these VUSs increase cancer risk, and physicians do not always know how to manage them in women who test positive.
Moreover, Israel has a national health system. Screening in a country without universal health insurance such as the United States raises questions about whether follow-up would be covered by insurance carriers for women who test positive.
Mehmet Copur, MD, an oncologist at Morrison Cancer Center in Hastings, Nebraska, questions how general population screening could be done in “real life.”
“These findings should be taken into consideration in the context of the patient population who would agree to genetic testing, who would agree to comply with the recommended guidelines for risk reduction, and who would have insurance coverage or resources to comply with the recommendations,” Copur told Medscape Medical News.
“If BRCA-positive patients did not or could not follow these recommendations, the results would different,” he added.
The most crucial component of screening for these mutations is genetic counselors, who are in short supply in the United States, according to Copur.
Another issue is that of cost. Genetic counseling is not always covered by insurance, especially for individuals who do not have a family history of BRCA-related cancers. Genetic testing is not cheap, and the costs of monitoring women who test positive could be prohibitive, especially in a healthcare system burdened by COVID-19.
“Whether our current healthcare system could bear the cost of such a change is up for debate. The screening itself may be feasible, but offering lifelong surveillance to every woman identified with mutations could present huge capacity issues,” Copur said. “Maybe in the future, the healthcare system can be ready for such an undertaking, but I don’t think we are there yet.”
Although she acknowledges the differences in risk between Ashkenazi Jews and the general population, Levy-Lahad thinks not having screening is like “throwing the baby out with the bath water.”
“Maybe we’re not ready for total general population screening, but I think we have to start thinking along those lines,” she said. “We have this incredible tool for cancer prevention, and we should really be using it, certainly in populations like Ashkenazi Jews.”
Researchers conducted a retrospective analysis that included 105 women diagnosed with breast cancer at Shaare Zedek Medical Center in Jerusalem between 2005 and 2016. Forty-two women knew they were carriers before their breast cancer diagnosis, and 63 learned of their carrier status only after diagnosis. Of the participants, 83% were Ashkenazi Jews. For both prediagnosis and postdiagnosis groups, the age at diagnosis was the same (50.4 years). For both groups, distributions of pathogenic mutations were similar. There were no significant differences in hormone receptor or ERBB2 status.
Among women who knew they were carriers before diagnosis, 80.9% (34/42) were diagnosed either with ductal carcinoma in situ or stage 1 disease. Only 9.5% (4/42) of these women were diagnosed with disease of stage 2 or higher.
In comparison, among women who learned their carrier status after diagnosis, 30% (19/63) had early-stage disease at diagnosis, and 52.4% (33/63) were diagnosed at stage 2 or higher (P < .001).
Compared to women who knew their carrier status before diagnosis, women who found out after diagnosis had 12 times higher odds of being diagnosed with disease of advanced clinical stage (P = .001) and eight times higher odds of being diagnosed with disease of advanced pathologic stage (P = .002).
A sentinel node biopsy was sufficient in 85.7% (36/42) of women who knew their carrier status before diagnosis; 7.2% (3/42) of these women needed a full lymph node dissection. In contrast, 3.2% (2/63) of women who learned their carrier status after diagnosis underwent sentinel node biopsy, and 34.9% (25/105) needed a full lymph node dissection (P < .001).
Among women who knew their carrier status before diagnosis, 54.8% (23/42) did not need chemotherapy at all, and none needed neoadjuvant chemotherapy. Only 4.8% (3/63) of women who learned their mutation status after diagnosis were able to forgo chemotherapy (P < .001); 22.2% (14/63) needed neoadjuvant therapy (P = .001).
These findings appeared to translate into better outcomes. Overall 5-year survival was significantly higher among women who knew their carrier status before diagnosis compared to women who found out afterward (94% [SE 4%] vs 78% [SE 5%]; P = .03). Only two of 42 women (4.8%) in the prediagnosis group died, compared to 16 of 63 (25.4%) in the postdiagnosis group.
Analyses that controlled for year at diagnosis showed that women who learned their carrier status before diagnosis had significantly lower risk for overall mortality compared with those who found out after diagnosis (hazard ratio [HR], 0.20; 95% CI, 0.04 – 0.93; P = .04). However, these results lost significance when controlled for age, socioeconomic index, family history, and gene variant (HR, 0.16; 95% CI, 0.02 – 1.4; P = .10).
Higher socioeconomic status (HR, 0.76; 95% CI, 0.6 – 0.97; P = .03), gene variant (BRCA2 vs BRCA1: HR, 0.15; 95% CI, 0.03 – 0.75; P = .02), and age at diagnosis (HR, 1.047; 95% CI, 1.003 – 1.093; P = .04) were all associated with overall mortality.
“I can’t infer causation, but we suspect that the reason for these results is the difference in follow-up,” Levy-Lahad said.
Most of the women (95.2%, 40/42) who knew their carrier status before diagnosis received their follow-up at the medical center’s high-risk carrier clinic. Twenty-seven of 42 (64.3%) of these women were diagnosed with breast MRI. By contrast, only 1.6% (1/63) of women who found out their carrier status after diagnosis were diagnosed with breast MRI. Breast MRI is not routinely used for breast cancer screening but can be more sensitive than mammography for detecting breast cancer.
The study was funded by the Breast Cancer Research Foundation and by a gift from Ellie and David Werber to ShaareZedek Medical Center.
Levy-Lahad received grants from the Breast Cancer Research Foundation and from the Israel Cancer Association during the conduct of the study and personal fees from AstraZeneca outside the submitted work. Copur has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.




