User login
Money worries during COVID-19? Six tips to keep your finances afloat
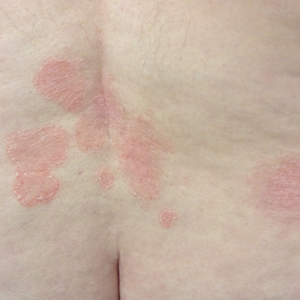
Even before Atlanta had an official shelter-in-place order, patients at the private plastic surgery practice of Nicholas Jones, MD, began canceling and rescheduled planned procedures.
After a few weeks, Dr. Jones, aged 40 years, stopped seeing patients entirely, but as a self-employed independent contractor, that means he’d lost most of his income. Dr. Jones still makes some money via a wound care job at a local nursing home, but he’s concerned that job may also be eliminated.
“I’m not hurting yet,” he said. “But I’m preparing for the worst possible scenario.”
In preparation, he and his fiancé have cut back on extraneous expenses like Uber Eats, magazine subscriptions, and streaming music services. Even though he has a 6-month emergency fund, Jones has reached out to utility companies, mortgage lenders, and student loan servicers to find out about any programs they offer to people who’ve suffered financially from the coronavirus crisis.
He’s also considered traveling to one of the COVID-19 epicenters – he has family in New Orleans and Chicago – to work in a hospital there. Jones has trauma experience and is double-boarded in general and plastic surgery.
“I could provide relief to those in need and also float through this troubled time with some financial relief,” he said.
Whereas much of the world’s attention has been on physicians who are on the front line and working around the clock in hospitals to help COVID-19 patients, thousands of other physicians are experiencing the opposite phenomenon – a slowdown or even stoppage of work (and income) altogether.
Even among those practices that remain open, the number of patients has declined as people avoid going to the office unless they absolutely have to.
At the same time, doctors in two-income households may have a spouse experiencing a job loss or income decline. Nearly 10 million Americans applied for unemployment benefits in the last 2 weeks of March, the largest number on record.
Still, while there’s uncertainty around how long the coronavirus crisis will last, experts agree that at some point America will return to a “new normal” and business operations will begin to reopen. For physicians experiencing a reduction in income who, like Jones, have an emergency fund with a few months’ worth of expenses, now’s the time to tap into it. (Or if you still have income, now’s the time to focus on growing that emergency fund to give yourself an even bigger safety net.)
If you’re among the more than half of Americans with less than 6 months of expenses saved for a rainy day, here’s how to stay afloat in the near term:
Cut back on expenses
Some household spending has naturally tapered off for many families because social distancing restrictions reduce spending on eating out, travel, and other leisure activities. But this is also an opportunity to look for other ways to reduce spending. Look through your credit card bills to see whether there are recurring payments you can cut, such as a payment to a gym that’s temporarily closed or a monthly subscription box that you don’t need.
Some gyms are not allowing membership termination right now, but it pays to ask. If a service you’re not using won’t facilitate the cancellation, call your credit card company to dispute and stop the charges, and report them to the Better Business Bureau.
You should also stop contributing to nonemergency savings accounts such as your retirement fund or your children’s college funds.
“A lot of people are hesitant to stop their automatic savings if they’ve been maxing out their 401(k) contribution or 529 accounts,” says Andrew Musbach, a certified financial planner and cofounder of MD Wealth Management in Chelsea, Mich. “But if you’re thinking long term, the reality is that missing a couple of months won’t make or break a plan. Cutting back on the amount you’re saving in the short term will increase your cash flow and is a good way to make ends meet.”
Take advantage of regulatory changes
Although many physicians won’t qualify for direct payments via the Coronavirus Aid Relief and Economic Security (CARES) Act (the $1,200 payments to individuals start phasing out once income hits $75,000 and disappear entirely for those making more than $99,000), there are other provisions in the stimulus bill that may help physicians. The bill, for example, boosts state unemployment payments by $600 per week for the next 4 months, meaning qualified workers could receive an average of nearly $1,000 per week, depending on their state, and there are new provisions providing unemployment payments to self-employed and contract workers.
The CARES Act also includes a break for federal student loan holders. Under that rule, you can skip your payments through September without incurring additional interest. Physicians in the loan forgiveness program will still get credit for payments skipped during this program.
Separately, the IRS has extended the tax deadline from April 15 to July 15, which means not only do you not have to file your taxes until then, you also don’t have to pay any taxes you owe until mid-July. The deadline for first quarter estimated tax payments has also moved to July 15. (If you’re expecting a refund, however, you should file ASAP, since the IRS will typically issue those within a few weeks of receiving your returns.)
Tap your home equity – if you’re planning to stay put
If you have good credit and still have some income, you might consider refinancing your home mortgage or opening a home equity line of credit. Interest rates have fallen recently amid economic turbulence, so if you haven’t refinanced recently you may be able to shave your monthly payment. If you need cash, a cash-out refinance, home equity line of credit, or a reverse mortgage (available if you’re over age 62) are among the lowest-cost ways to borrow.
“With interest rates so low, there can be a lot of benefit to refinancing and leveraging your house, especially if you’re planning to stay there,” says Jamie Hopkins, a director at the Carson Group. “The challenge is if you’re planning to move in the next few years. There’s a real risk that the housing market could go down in the next couple of years, and if you’re planning to sell, there’s a risk that you might not get back what you borrowed.”
Communicate early with your bank or landlord
If you don’t have the income to refinance, and you think you’re going to run into trouble making your housing payment, you should let your bank or landlord know as soon as possible. The CARES Act allows homeowners with federally backed mortgages to obtain a 180-day postponement of mortgage payments because of COVID-19 financial hardship, with the potential to extend for another 180 days. It also bans eviction by landlords with federal mortgages for 120 days.
Even if you don’t have a federally backed mortgage, you should still get in touch with your lender. Many mortgage servicers have their own forbearance programs for borrowers who can prove a temporary financial hardship. (Some banks are also waiving fees on early withdrawals on CDs and giving cardholders a reprieve on credit card payments.) Commercial landlords are also working with struggling tenants, so you may also be able to get some relief on your office lease as well.
“All of the lenders are setting up helplines for people affected,” says Amy Guerich, a partner with Stepp & Rothwell, a Kansas City–based financial planning firm. “The best thing you can do is contact them right away if you think that you’re going to have a problem vs. just letting the bills go.”
Consider retirement account withdrawals
Standard personal finance advice holds that you should exhaust all other options before pulling money out of your retirement account because of the high penalties for early withdrawals and because money removed from retirement accounts is no longer compounding over time.
Still, the CARES act has provisions making it less financially onerous to pull money from your retirement accounts. Under the new law, you can take a distribution of up to $100,000 from your IRA or 401(k) without having to pay the 10% early withdrawal penalty. You’ll owe ordinary income taxes on the withdrawal, but you have 3 years to pay them or to return the money to your retirement account.
“That’s a great relief provision, especially for higher-income physicians who might have a higher 401(k) balance,” said Jamie Hopkins.
Be smart about credit cards
Although using credit cards that you can’t pay off every month is typically an expensive way to access money, getting a new card with a low or zero percent introductory rate is a short-term strategy to consider when you’ve exhausted other options. If you have good credit, you may be able to qualify for a credit card with a 0% introductory interest rate on new transactions. Pay close attention to the fine print, including the cap on the balance you can carry without interest and whether you’ll be required to make minimum payments.
The average 0% credit card offer is for 11 months, but there are some cards that can extend the offer for up to a year-and-a-half. If you choose to use this strategy, you’ll need a plan to pay off the entire balance before the introductory period ends. If there’s a balance remaining once the rate resets, you may end up owing deferred interest on it.
The financial ramifications of the coronavirus can feel overwhelming, but it’s important not to panic. While it remains unclear how long the current crisis will last, making some smart money moves to preserve your cash in the meantime can help you stay afloat.
A version of this article originally appeared on Medscape.com.
Even before Atlanta had an official shelter-in-place order, patients at the private plastic surgery practice of Nicholas Jones, MD, began canceling and rescheduled planned procedures.
After a few weeks, Dr. Jones, aged 40 years, stopped seeing patients entirely, but as a self-employed independent contractor, that means he’d lost most of his income. Dr. Jones still makes some money via a wound care job at a local nursing home, but he’s concerned that job may also be eliminated.
“I’m not hurting yet,” he said. “But I’m preparing for the worst possible scenario.”
In preparation, he and his fiancé have cut back on extraneous expenses like Uber Eats, magazine subscriptions, and streaming music services. Even though he has a 6-month emergency fund, Jones has reached out to utility companies, mortgage lenders, and student loan servicers to find out about any programs they offer to people who’ve suffered financially from the coronavirus crisis.
He’s also considered traveling to one of the COVID-19 epicenters – he has family in New Orleans and Chicago – to work in a hospital there. Jones has trauma experience and is double-boarded in general and plastic surgery.
“I could provide relief to those in need and also float through this troubled time with some financial relief,” he said.
Whereas much of the world’s attention has been on physicians who are on the front line and working around the clock in hospitals to help COVID-19 patients, thousands of other physicians are experiencing the opposite phenomenon – a slowdown or even stoppage of work (and income) altogether.
Even among those practices that remain open, the number of patients has declined as people avoid going to the office unless they absolutely have to.
At the same time, doctors in two-income households may have a spouse experiencing a job loss or income decline. Nearly 10 million Americans applied for unemployment benefits in the last 2 weeks of March, the largest number on record.
Still, while there’s uncertainty around how long the coronavirus crisis will last, experts agree that at some point America will return to a “new normal” and business operations will begin to reopen. For physicians experiencing a reduction in income who, like Jones, have an emergency fund with a few months’ worth of expenses, now’s the time to tap into it. (Or if you still have income, now’s the time to focus on growing that emergency fund to give yourself an even bigger safety net.)
If you’re among the more than half of Americans with less than 6 months of expenses saved for a rainy day, here’s how to stay afloat in the near term:
Cut back on expenses
Some household spending has naturally tapered off for many families because social distancing restrictions reduce spending on eating out, travel, and other leisure activities. But this is also an opportunity to look for other ways to reduce spending. Look through your credit card bills to see whether there are recurring payments you can cut, such as a payment to a gym that’s temporarily closed or a monthly subscription box that you don’t need.
Some gyms are not allowing membership termination right now, but it pays to ask. If a service you’re not using won’t facilitate the cancellation, call your credit card company to dispute and stop the charges, and report them to the Better Business Bureau.
You should also stop contributing to nonemergency savings accounts such as your retirement fund or your children’s college funds.
“A lot of people are hesitant to stop their automatic savings if they’ve been maxing out their 401(k) contribution or 529 accounts,” says Andrew Musbach, a certified financial planner and cofounder of MD Wealth Management in Chelsea, Mich. “But if you’re thinking long term, the reality is that missing a couple of months won’t make or break a plan. Cutting back on the amount you’re saving in the short term will increase your cash flow and is a good way to make ends meet.”
Take advantage of regulatory changes
Although many physicians won’t qualify for direct payments via the Coronavirus Aid Relief and Economic Security (CARES) Act (the $1,200 payments to individuals start phasing out once income hits $75,000 and disappear entirely for those making more than $99,000), there are other provisions in the stimulus bill that may help physicians. The bill, for example, boosts state unemployment payments by $600 per week for the next 4 months, meaning qualified workers could receive an average of nearly $1,000 per week, depending on their state, and there are new provisions providing unemployment payments to self-employed and contract workers.
The CARES Act also includes a break for federal student loan holders. Under that rule, you can skip your payments through September without incurring additional interest. Physicians in the loan forgiveness program will still get credit for payments skipped during this program.
Separately, the IRS has extended the tax deadline from April 15 to July 15, which means not only do you not have to file your taxes until then, you also don’t have to pay any taxes you owe until mid-July. The deadline for first quarter estimated tax payments has also moved to July 15. (If you’re expecting a refund, however, you should file ASAP, since the IRS will typically issue those within a few weeks of receiving your returns.)
Tap your home equity – if you’re planning to stay put
If you have good credit and still have some income, you might consider refinancing your home mortgage or opening a home equity line of credit. Interest rates have fallen recently amid economic turbulence, so if you haven’t refinanced recently you may be able to shave your monthly payment. If you need cash, a cash-out refinance, home equity line of credit, or a reverse mortgage (available if you’re over age 62) are among the lowest-cost ways to borrow.
“With interest rates so low, there can be a lot of benefit to refinancing and leveraging your house, especially if you’re planning to stay there,” says Jamie Hopkins, a director at the Carson Group. “The challenge is if you’re planning to move in the next few years. There’s a real risk that the housing market could go down in the next couple of years, and if you’re planning to sell, there’s a risk that you might not get back what you borrowed.”
Communicate early with your bank or landlord
If you don’t have the income to refinance, and you think you’re going to run into trouble making your housing payment, you should let your bank or landlord know as soon as possible. The CARES Act allows homeowners with federally backed mortgages to obtain a 180-day postponement of mortgage payments because of COVID-19 financial hardship, with the potential to extend for another 180 days. It also bans eviction by landlords with federal mortgages for 120 days.
Even if you don’t have a federally backed mortgage, you should still get in touch with your lender. Many mortgage servicers have their own forbearance programs for borrowers who can prove a temporary financial hardship. (Some banks are also waiving fees on early withdrawals on CDs and giving cardholders a reprieve on credit card payments.) Commercial landlords are also working with struggling tenants, so you may also be able to get some relief on your office lease as well.
“All of the lenders are setting up helplines for people affected,” says Amy Guerich, a partner with Stepp & Rothwell, a Kansas City–based financial planning firm. “The best thing you can do is contact them right away if you think that you’re going to have a problem vs. just letting the bills go.”
Consider retirement account withdrawals
Standard personal finance advice holds that you should exhaust all other options before pulling money out of your retirement account because of the high penalties for early withdrawals and because money removed from retirement accounts is no longer compounding over time.
Still, the CARES act has provisions making it less financially onerous to pull money from your retirement accounts. Under the new law, you can take a distribution of up to $100,000 from your IRA or 401(k) without having to pay the 10% early withdrawal penalty. You’ll owe ordinary income taxes on the withdrawal, but you have 3 years to pay them or to return the money to your retirement account.
“That’s a great relief provision, especially for higher-income physicians who might have a higher 401(k) balance,” said Jamie Hopkins.
Be smart about credit cards
Although using credit cards that you can’t pay off every month is typically an expensive way to access money, getting a new card with a low or zero percent introductory rate is a short-term strategy to consider when you’ve exhausted other options. If you have good credit, you may be able to qualify for a credit card with a 0% introductory interest rate on new transactions. Pay close attention to the fine print, including the cap on the balance you can carry without interest and whether you’ll be required to make minimum payments.
The average 0% credit card offer is for 11 months, but there are some cards that can extend the offer for up to a year-and-a-half. If you choose to use this strategy, you’ll need a plan to pay off the entire balance before the introductory period ends. If there’s a balance remaining once the rate resets, you may end up owing deferred interest on it.
The financial ramifications of the coronavirus can feel overwhelming, but it’s important not to panic. While it remains unclear how long the current crisis will last, making some smart money moves to preserve your cash in the meantime can help you stay afloat.
A version of this article originally appeared on Medscape.com.
Even before Atlanta had an official shelter-in-place order, patients at the private plastic surgery practice of Nicholas Jones, MD, began canceling and rescheduled planned procedures.
After a few weeks, Dr. Jones, aged 40 years, stopped seeing patients entirely, but as a self-employed independent contractor, that means he’d lost most of his income. Dr. Jones still makes some money via a wound care job at a local nursing home, but he’s concerned that job may also be eliminated.
“I’m not hurting yet,” he said. “But I’m preparing for the worst possible scenario.”
In preparation, he and his fiancé have cut back on extraneous expenses like Uber Eats, magazine subscriptions, and streaming music services. Even though he has a 6-month emergency fund, Jones has reached out to utility companies, mortgage lenders, and student loan servicers to find out about any programs they offer to people who’ve suffered financially from the coronavirus crisis.
He’s also considered traveling to one of the COVID-19 epicenters – he has family in New Orleans and Chicago – to work in a hospital there. Jones has trauma experience and is double-boarded in general and plastic surgery.
“I could provide relief to those in need and also float through this troubled time with some financial relief,” he said.
Whereas much of the world’s attention has been on physicians who are on the front line and working around the clock in hospitals to help COVID-19 patients, thousands of other physicians are experiencing the opposite phenomenon – a slowdown or even stoppage of work (and income) altogether.
Even among those practices that remain open, the number of patients has declined as people avoid going to the office unless they absolutely have to.
At the same time, doctors in two-income households may have a spouse experiencing a job loss or income decline. Nearly 10 million Americans applied for unemployment benefits in the last 2 weeks of March, the largest number on record.
Still, while there’s uncertainty around how long the coronavirus crisis will last, experts agree that at some point America will return to a “new normal” and business operations will begin to reopen. For physicians experiencing a reduction in income who, like Jones, have an emergency fund with a few months’ worth of expenses, now’s the time to tap into it. (Or if you still have income, now’s the time to focus on growing that emergency fund to give yourself an even bigger safety net.)
If you’re among the more than half of Americans with less than 6 months of expenses saved for a rainy day, here’s how to stay afloat in the near term:
Cut back on expenses
Some household spending has naturally tapered off for many families because social distancing restrictions reduce spending on eating out, travel, and other leisure activities. But this is also an opportunity to look for other ways to reduce spending. Look through your credit card bills to see whether there are recurring payments you can cut, such as a payment to a gym that’s temporarily closed or a monthly subscription box that you don’t need.
Some gyms are not allowing membership termination right now, but it pays to ask. If a service you’re not using won’t facilitate the cancellation, call your credit card company to dispute and stop the charges, and report them to the Better Business Bureau.
You should also stop contributing to nonemergency savings accounts such as your retirement fund or your children’s college funds.
“A lot of people are hesitant to stop their automatic savings if they’ve been maxing out their 401(k) contribution or 529 accounts,” says Andrew Musbach, a certified financial planner and cofounder of MD Wealth Management in Chelsea, Mich. “But if you’re thinking long term, the reality is that missing a couple of months won’t make or break a plan. Cutting back on the amount you’re saving in the short term will increase your cash flow and is a good way to make ends meet.”
Take advantage of regulatory changes
Although many physicians won’t qualify for direct payments via the Coronavirus Aid Relief and Economic Security (CARES) Act (the $1,200 payments to individuals start phasing out once income hits $75,000 and disappear entirely for those making more than $99,000), there are other provisions in the stimulus bill that may help physicians. The bill, for example, boosts state unemployment payments by $600 per week for the next 4 months, meaning qualified workers could receive an average of nearly $1,000 per week, depending on their state, and there are new provisions providing unemployment payments to self-employed and contract workers.
The CARES Act also includes a break for federal student loan holders. Under that rule, you can skip your payments through September without incurring additional interest. Physicians in the loan forgiveness program will still get credit for payments skipped during this program.
Separately, the IRS has extended the tax deadline from April 15 to July 15, which means not only do you not have to file your taxes until then, you also don’t have to pay any taxes you owe until mid-July. The deadline for first quarter estimated tax payments has also moved to July 15. (If you’re expecting a refund, however, you should file ASAP, since the IRS will typically issue those within a few weeks of receiving your returns.)
Tap your home equity – if you’re planning to stay put
If you have good credit and still have some income, you might consider refinancing your home mortgage or opening a home equity line of credit. Interest rates have fallen recently amid economic turbulence, so if you haven’t refinanced recently you may be able to shave your monthly payment. If you need cash, a cash-out refinance, home equity line of credit, or a reverse mortgage (available if you’re over age 62) are among the lowest-cost ways to borrow.
“With interest rates so low, there can be a lot of benefit to refinancing and leveraging your house, especially if you’re planning to stay there,” says Jamie Hopkins, a director at the Carson Group. “The challenge is if you’re planning to move in the next few years. There’s a real risk that the housing market could go down in the next couple of years, and if you’re planning to sell, there’s a risk that you might not get back what you borrowed.”
Communicate early with your bank or landlord
If you don’t have the income to refinance, and you think you’re going to run into trouble making your housing payment, you should let your bank or landlord know as soon as possible. The CARES Act allows homeowners with federally backed mortgages to obtain a 180-day postponement of mortgage payments because of COVID-19 financial hardship, with the potential to extend for another 180 days. It also bans eviction by landlords with federal mortgages for 120 days.
Even if you don’t have a federally backed mortgage, you should still get in touch with your lender. Many mortgage servicers have their own forbearance programs for borrowers who can prove a temporary financial hardship. (Some banks are also waiving fees on early withdrawals on CDs and giving cardholders a reprieve on credit card payments.) Commercial landlords are also working with struggling tenants, so you may also be able to get some relief on your office lease as well.
“All of the lenders are setting up helplines for people affected,” says Amy Guerich, a partner with Stepp & Rothwell, a Kansas City–based financial planning firm. “The best thing you can do is contact them right away if you think that you’re going to have a problem vs. just letting the bills go.”
Consider retirement account withdrawals
Standard personal finance advice holds that you should exhaust all other options before pulling money out of your retirement account because of the high penalties for early withdrawals and because money removed from retirement accounts is no longer compounding over time.
Still, the CARES act has provisions making it less financially onerous to pull money from your retirement accounts. Under the new law, you can take a distribution of up to $100,000 from your IRA or 401(k) without having to pay the 10% early withdrawal penalty. You’ll owe ordinary income taxes on the withdrawal, but you have 3 years to pay them or to return the money to your retirement account.
“That’s a great relief provision, especially for higher-income physicians who might have a higher 401(k) balance,” said Jamie Hopkins.
Be smart about credit cards
Although using credit cards that you can’t pay off every month is typically an expensive way to access money, getting a new card with a low or zero percent introductory rate is a short-term strategy to consider when you’ve exhausted other options. If you have good credit, you may be able to qualify for a credit card with a 0% introductory interest rate on new transactions. Pay close attention to the fine print, including the cap on the balance you can carry without interest and whether you’ll be required to make minimum payments.
The average 0% credit card offer is for 11 months, but there are some cards that can extend the offer for up to a year-and-a-half. If you choose to use this strategy, you’ll need a plan to pay off the entire balance before the introductory period ends. If there’s a balance remaining once the rate resets, you may end up owing deferred interest on it.
The financial ramifications of the coronavirus can feel overwhelming, but it’s important not to panic. While it remains unclear how long the current crisis will last, making some smart money moves to preserve your cash in the meantime can help you stay afloat.
A version of this article originally appeared on Medscape.com.
Daily Recap: How to stay afloat financially during COVID-19, more bad news on e-cigs
Here are the stories our MDedge editors across specialties think you need to know about today:
Tips to keep your finances healthy during COVID-19
If you’re among the more than half of Americans with less than 6 months of expenses saved for a rainy day, here are some tips on how to stay afloat in the near term. Cut back on expenses: Look through your credit card bills to see whether there are recurring payments you can cut, such as a payment to a gym that’s temporarily closed or a monthly subscription box that you don’t need. Tap your home equity: If you have good credit and still have some income, you might consider refinancing your home mortgage or opening a home equity line of credit. Consider retirement account withdrawals: the CARES Act has provisions making it less financially onerous to pull money from your retirement accounts. Under the new law, you can take a distribution of up to $100,000 from your IRA or 401(k) without having to pay the 10% early withdrawal penalty. Read more.
Nursing homes overhaul infection control
The toll that COVID-19 has taken on nursing homes and their postacute and long-term care residents has a multilayered backstory involving underresourced organizational structures, inherent susceptibilities, minimally trained infection prevention staff, variable abilities to isolate and quarantine large numbers of patients and residents, and a lack of governmental support. “Nursing homes have been trying their best to combat this pandemic using the best infection control procedures they have, but blindfolded and with their hands tied behind their backs,” said Joseph G. Ouslander, MD, professor of geriatric medicine at Florida Atlantic University, Boca Raton. Experts in both long-term care and infectious disease said in interviews that, through the rest of the pandemic and beyond, nursing homes need the following: “Infection preventionists” to lead improvements in emergency preparedness and infection prevention and control, well-qualified and engaged medical directors, a survey/inspection process that focuses on education, and more resources and attention to structural reform. Read more.
WHO backtracks on asymptomatic SARS-CoV-2 transmission
Maria Van Kerkhove, PhD, WHO’s COVID-19 technical lead and an infectious disease epidemiologist, caused a stir on June 8 when she said that countries are reporting that many of their asymptomatic cases develop into cases of mild disease. For patients with truly asymptomatic disease, countries are “not finding secondary transmission onward. It’s very rare,” she said. But on June 9 – following a day of criticism – Dr. Van Kerkhove sought to clarify her comments on asymptomatic transmission during a live social media Q&A. She noted that while “the majority of transmission that we know about” is through individuals with symptoms, “there are a subset of people who don’t develop symptoms, and to truly understand how many people don’t have symptoms – we don’t actually have that answer yet.” Physicians and public health experts slammed the initial comments, saying that they created confusion. Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, weighed in on the controversial WHO comments, telling Good Morning America on June 10 that Dr. Van Kerkhove’s initial statement that asymptomatic SARS-CoV-2 transmission is a rare event is “not correct.” Read more.
E-cigs linked to smoking relapse
The use of electronic nicotine delivery systems is associated with increased risk of cigarette smoking relapse among former smokers, results from a large longitudinal cohort study demonstrated. The findings come from a survey of adult former smokers who participated in the Population Assessment of Tobacco and Health (PATH) Study (2013-2018). Adjusted hazard ratio (AHR) analysis revealed that the use of electronic nicotine delivery systems was associated with significant risk of cigarette smoking relapse among recent former smokers (AHR 1.63) and among long-term former smokers (AHR 3.79). The use of other tobacco products was similarly associated with a significant risk for cigarette smoking relapse among recent former smokers (AHR 1.97) and among long-term former smokers (AHR 3.82). “For the many clinicians treating former smokers who have successfully quit all nicotine products, the implications are that use of [electronic nicotine delivery systems] should be discouraged, just as use of all other tobacco products is discouraged,” researchers led by Colm D. Everard, PhD, reported in a study published in JAMA Network Open. Read more.
Formula feeding leads to early weaning
Breastfed infants who receive formula in the hospital are more than twofold more likely to wean during the first year, compared with infants who are exclusively breastfed, according to research published online in Pediatrics. The finding is based on an analysis of data from over 8,000 infants in the Minnesota Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). “Our study strengthens the evidence that formula supplementation of breastfed infants negatively affects breastfeeding duration,” said Marcia Burton McCoy, MPH, of the Minnesota Department of Health’s WIC, and Pamela Heggie, MD, of the University of Minnesota in Minneapolis. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Tips to keep your finances healthy during COVID-19
If you’re among the more than half of Americans with less than 6 months of expenses saved for a rainy day, here are some tips on how to stay afloat in the near term. Cut back on expenses: Look through your credit card bills to see whether there are recurring payments you can cut, such as a payment to a gym that’s temporarily closed or a monthly subscription box that you don’t need. Tap your home equity: If you have good credit and still have some income, you might consider refinancing your home mortgage or opening a home equity line of credit. Consider retirement account withdrawals: the CARES Act has provisions making it less financially onerous to pull money from your retirement accounts. Under the new law, you can take a distribution of up to $100,000 from your IRA or 401(k) without having to pay the 10% early withdrawal penalty. Read more.
Nursing homes overhaul infection control
The toll that COVID-19 has taken on nursing homes and their postacute and long-term care residents has a multilayered backstory involving underresourced organizational structures, inherent susceptibilities, minimally trained infection prevention staff, variable abilities to isolate and quarantine large numbers of patients and residents, and a lack of governmental support. “Nursing homes have been trying their best to combat this pandemic using the best infection control procedures they have, but blindfolded and with their hands tied behind their backs,” said Joseph G. Ouslander, MD, professor of geriatric medicine at Florida Atlantic University, Boca Raton. Experts in both long-term care and infectious disease said in interviews that, through the rest of the pandemic and beyond, nursing homes need the following: “Infection preventionists” to lead improvements in emergency preparedness and infection prevention and control, well-qualified and engaged medical directors, a survey/inspection process that focuses on education, and more resources and attention to structural reform. Read more.
WHO backtracks on asymptomatic SARS-CoV-2 transmission
Maria Van Kerkhove, PhD, WHO’s COVID-19 technical lead and an infectious disease epidemiologist, caused a stir on June 8 when she said that countries are reporting that many of their asymptomatic cases develop into cases of mild disease. For patients with truly asymptomatic disease, countries are “not finding secondary transmission onward. It’s very rare,” she said. But on June 9 – following a day of criticism – Dr. Van Kerkhove sought to clarify her comments on asymptomatic transmission during a live social media Q&A. She noted that while “the majority of transmission that we know about” is through individuals with symptoms, “there are a subset of people who don’t develop symptoms, and to truly understand how many people don’t have symptoms – we don’t actually have that answer yet.” Physicians and public health experts slammed the initial comments, saying that they created confusion. Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, weighed in on the controversial WHO comments, telling Good Morning America on June 10 that Dr. Van Kerkhove’s initial statement that asymptomatic SARS-CoV-2 transmission is a rare event is “not correct.” Read more.
E-cigs linked to smoking relapse
The use of electronic nicotine delivery systems is associated with increased risk of cigarette smoking relapse among former smokers, results from a large longitudinal cohort study demonstrated. The findings come from a survey of adult former smokers who participated in the Population Assessment of Tobacco and Health (PATH) Study (2013-2018). Adjusted hazard ratio (AHR) analysis revealed that the use of electronic nicotine delivery systems was associated with significant risk of cigarette smoking relapse among recent former smokers (AHR 1.63) and among long-term former smokers (AHR 3.79). The use of other tobacco products was similarly associated with a significant risk for cigarette smoking relapse among recent former smokers (AHR 1.97) and among long-term former smokers (AHR 3.82). “For the many clinicians treating former smokers who have successfully quit all nicotine products, the implications are that use of [electronic nicotine delivery systems] should be discouraged, just as use of all other tobacco products is discouraged,” researchers led by Colm D. Everard, PhD, reported in a study published in JAMA Network Open. Read more.
Formula feeding leads to early weaning
Breastfed infants who receive formula in the hospital are more than twofold more likely to wean during the first year, compared with infants who are exclusively breastfed, according to research published online in Pediatrics. The finding is based on an analysis of data from over 8,000 infants in the Minnesota Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). “Our study strengthens the evidence that formula supplementation of breastfed infants negatively affects breastfeeding duration,” said Marcia Burton McCoy, MPH, of the Minnesota Department of Health’s WIC, and Pamela Heggie, MD, of the University of Minnesota in Minneapolis. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Tips to keep your finances healthy during COVID-19
If you’re among the more than half of Americans with less than 6 months of expenses saved for a rainy day, here are some tips on how to stay afloat in the near term. Cut back on expenses: Look through your credit card bills to see whether there are recurring payments you can cut, such as a payment to a gym that’s temporarily closed or a monthly subscription box that you don’t need. Tap your home equity: If you have good credit and still have some income, you might consider refinancing your home mortgage or opening a home equity line of credit. Consider retirement account withdrawals: the CARES Act has provisions making it less financially onerous to pull money from your retirement accounts. Under the new law, you can take a distribution of up to $100,000 from your IRA or 401(k) without having to pay the 10% early withdrawal penalty. Read more.
Nursing homes overhaul infection control
The toll that COVID-19 has taken on nursing homes and their postacute and long-term care residents has a multilayered backstory involving underresourced organizational structures, inherent susceptibilities, minimally trained infection prevention staff, variable abilities to isolate and quarantine large numbers of patients and residents, and a lack of governmental support. “Nursing homes have been trying their best to combat this pandemic using the best infection control procedures they have, but blindfolded and with their hands tied behind their backs,” said Joseph G. Ouslander, MD, professor of geriatric medicine at Florida Atlantic University, Boca Raton. Experts in both long-term care and infectious disease said in interviews that, through the rest of the pandemic and beyond, nursing homes need the following: “Infection preventionists” to lead improvements in emergency preparedness and infection prevention and control, well-qualified and engaged medical directors, a survey/inspection process that focuses on education, and more resources and attention to structural reform. Read more.
WHO backtracks on asymptomatic SARS-CoV-2 transmission
Maria Van Kerkhove, PhD, WHO’s COVID-19 technical lead and an infectious disease epidemiologist, caused a stir on June 8 when she said that countries are reporting that many of their asymptomatic cases develop into cases of mild disease. For patients with truly asymptomatic disease, countries are “not finding secondary transmission onward. It’s very rare,” she said. But on June 9 – following a day of criticism – Dr. Van Kerkhove sought to clarify her comments on asymptomatic transmission during a live social media Q&A. She noted that while “the majority of transmission that we know about” is through individuals with symptoms, “there are a subset of people who don’t develop symptoms, and to truly understand how many people don’t have symptoms – we don’t actually have that answer yet.” Physicians and public health experts slammed the initial comments, saying that they created confusion. Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, weighed in on the controversial WHO comments, telling Good Morning America on June 10 that Dr. Van Kerkhove’s initial statement that asymptomatic SARS-CoV-2 transmission is a rare event is “not correct.” Read more.
E-cigs linked to smoking relapse
The use of electronic nicotine delivery systems is associated with increased risk of cigarette smoking relapse among former smokers, results from a large longitudinal cohort study demonstrated. The findings come from a survey of adult former smokers who participated in the Population Assessment of Tobacco and Health (PATH) Study (2013-2018). Adjusted hazard ratio (AHR) analysis revealed that the use of electronic nicotine delivery systems was associated with significant risk of cigarette smoking relapse among recent former smokers (AHR 1.63) and among long-term former smokers (AHR 3.79). The use of other tobacco products was similarly associated with a significant risk for cigarette smoking relapse among recent former smokers (AHR 1.97) and among long-term former smokers (AHR 3.82). “For the many clinicians treating former smokers who have successfully quit all nicotine products, the implications are that use of [electronic nicotine delivery systems] should be discouraged, just as use of all other tobacco products is discouraged,” researchers led by Colm D. Everard, PhD, reported in a study published in JAMA Network Open. Read more.
Formula feeding leads to early weaning
Breastfed infants who receive formula in the hospital are more than twofold more likely to wean during the first year, compared with infants who are exclusively breastfed, according to research published online in Pediatrics. The finding is based on an analysis of data from over 8,000 infants in the Minnesota Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). “Our study strengthens the evidence that formula supplementation of breastfed infants negatively affects breastfeeding duration,” said Marcia Burton McCoy, MPH, of the Minnesota Department of Health’s WIC, and Pamela Heggie, MD, of the University of Minnesota in Minneapolis. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
AGA Clinical practice update: Maintain IBD remission during pandemic
Inflammatory bowel disease (IBD) does not seem to make patients any more likely to contract SARS-COV-2 or develop COVID-19, but a rapid review commissioned by the American Gastroenterological Association acknowledges that determination is based on limited evidence and IBD patients should nonetheless maintain remission to reduce their risk of relapse or hospitalization during the COVID-19 pandemic.
The AGA has published a clinical practice update based on that rapid review online in Gastroenterology (2020. doi: 10.1053/j.gastro.2020.04.012).
Because of the widespread use of immunosuppressive or immune-modifying drugs, “It is understandable why patients with Crohn’s disease and ulcerative colitis have specific concerns and potential for increased risk of infection with SARS-CoV-2,” wrote David T. Rubin, MD, AGAF, of the University of Chicago and colleagues in the expert commentary.
They noted that, while association between GI symptoms and COVID-19 RNA in stool samples isn’t clear, given the reports of GI symptoms in COVID-19 patients – ranging from 10% (JAMA. 2020:323:1061-9) to half of patients (Am J Gastroenterol. 2020;115:766-73) – “the clinical implications of this are quite important.” In IBD patients, changes in digestive symptoms without accompanying fever or respiratory symptoms can be monitored for symptoms that could merit testing for the virus as well as “trigger additional treatment adjustments.”
In accordance with the IOIBD (International Organization for the Study of Inflammatory Bowel Disease) consensus, the update noted that IBD patients should continue going to infusion centers for therapies, provided that the centers use a COVID-19 screening protocol.
Patients with IBD who contract COVID-19 seem more likely to be hospitalized for one or the other disease, but that’s based on data from the international SECURE-IBD registry that had 164 patients as of the writing of the update. The update provides guidance for three scenarios for IBD patients during the pandemic:
- Patients not infected with SARS-CoV-2 should maintain their IBD therapies to sustain remission and avoid relapses. “Aside from the obvious negative consequences of a relapse, relapsing IBD will strain available medical resources, may require steroid therapy or necessitate hospitalization, outcomes that are all much worse than the known risks of existing IBD therapies,” Dr. Rubin and colleagues noted.
- Patients who are infected but have no symptoms of COVID-19 should have their dosing of prednisone adjusted to less than 20 mg/day or switched to budesonide; suspend thiopurines, methotrexate, and tofacitinib; and delay dosing of monoclonal antibodies (anti–tumor necrosis factor [anti-TNF] drugs, ustekinumab, or vedolizumab) for 2 weeks while their symptoms for COVID-19 are monitored. “Restarting therapy after 2 weeks if the patient has not developed manifestations of COVID-19 is reasonable,” wrote Dr. Rubin and colleagues. Emerging serial testing should indicate antibody status, but the effectiveness of stool testing for SARS-CoV-2 in these cases “remains to be seen.”
- In the patient with confirmed COVID-19, adjustment of IBD therapy “is appropriate, based on the understanding of the immune activity of the therapy and whether that therapy may worsen outcomes with COVID-19,” the update stated. Therapy adjustment should focus on reducing immune suppression during the active viral infection. Some studies are evaluating anticytokine-based therapies as COVID-19 treatments, so continued anti-TNF therapies might prevent acute respiratory distress syndrome and multiorgan failure, Dr. Rubin and colleagues wrote. “However, in the absence of those data, guidance is currently based on deciding whether to hold or to continue specific IBD therapies.”
The update considers most IBD therapies, specifically aminosalicylates, topical rectal therapy, dietary management, and antibiotics “safe and may be continued,” and oral budesonide can be continued if it’s needed for ongoing IBD control. However, corticosteroids “should be avoided and discontinued quickly.”
Likewise, during the acute stage of COVID-19, thiopurines, methotrexate, and tofacitinib should be discontinued, and anti-TNF drugs and ustekinumab should be stopped during viral illness. Holding vedolizumab during viral illness is also appropriate, according to the update, although the IOIBD group was uncertain if doing so was necessary.
If the IBD patient has digestive symptoms with COVID-19, ongoing supportive care of the COVID-19 is “reasonable,” but investigating the causes of the digestive symptoms “is critically important.” That should include ruling out enteric infections and confirming active inflammation with nonendoscopic testing. Endoscopy should be relegated to only urgent and emergent cases.
Management of IBD in COVID-19 patients also depends on disease severity. Safer therapies are indicated for mild disease, but withholding IBD therapy in moderate to severe cases may not be practical. “In this setting, the risks and benefits of escalating IBD therapy must be carefully weighed against the severity of the COVID-19,” Dr. Rubin and colleagues noted.
In hospitalized patients with severe COVID-19 and poor prognoses, “IBD therapy will likely take a back seat,” the update stated, although COVID-19 therapies should take the concomitant IBD into account. In patients with milder cases of COVID-19, IBD management should focus on acute manifestations, but intravenous steroid therapy shouldn’t exceed 3 days. The update urged providers to submit cases of IBD and confirmed COVID-19 to the SECURE-IBD registry at COVIDIBD.org.
Dr. Rubin disclosed financial relationships with AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene/Syneos, Gilead Sciences, Takeda, and many other pharmaceutical companies. Coauthors disclosed relationships with those companies and Medimmune, Novus Therapeutics, Osiris Therapeutics, RedHill Biopharma, Sanofi-Aventis, UCB Pharma, and multiple other pharmaceutical companies.
SOURCE: Rubin DT, et al. Gastroenterology. 2020: doi: 10.1053/j.gastro.2020.04.012
Inflammatory bowel disease (IBD) does not seem to make patients any more likely to contract SARS-COV-2 or develop COVID-19, but a rapid review commissioned by the American Gastroenterological Association acknowledges that determination is based on limited evidence and IBD patients should nonetheless maintain remission to reduce their risk of relapse or hospitalization during the COVID-19 pandemic.
The AGA has published a clinical practice update based on that rapid review online in Gastroenterology (2020. doi: 10.1053/j.gastro.2020.04.012).
Because of the widespread use of immunosuppressive or immune-modifying drugs, “It is understandable why patients with Crohn’s disease and ulcerative colitis have specific concerns and potential for increased risk of infection with SARS-CoV-2,” wrote David T. Rubin, MD, AGAF, of the University of Chicago and colleagues in the expert commentary.
They noted that, while association between GI symptoms and COVID-19 RNA in stool samples isn’t clear, given the reports of GI symptoms in COVID-19 patients – ranging from 10% (JAMA. 2020:323:1061-9) to half of patients (Am J Gastroenterol. 2020;115:766-73) – “the clinical implications of this are quite important.” In IBD patients, changes in digestive symptoms without accompanying fever or respiratory symptoms can be monitored for symptoms that could merit testing for the virus as well as “trigger additional treatment adjustments.”
In accordance with the IOIBD (International Organization for the Study of Inflammatory Bowel Disease) consensus, the update noted that IBD patients should continue going to infusion centers for therapies, provided that the centers use a COVID-19 screening protocol.
Patients with IBD who contract COVID-19 seem more likely to be hospitalized for one or the other disease, but that’s based on data from the international SECURE-IBD registry that had 164 patients as of the writing of the update. The update provides guidance for three scenarios for IBD patients during the pandemic:
- Patients not infected with SARS-CoV-2 should maintain their IBD therapies to sustain remission and avoid relapses. “Aside from the obvious negative consequences of a relapse, relapsing IBD will strain available medical resources, may require steroid therapy or necessitate hospitalization, outcomes that are all much worse than the known risks of existing IBD therapies,” Dr. Rubin and colleagues noted.
- Patients who are infected but have no symptoms of COVID-19 should have their dosing of prednisone adjusted to less than 20 mg/day or switched to budesonide; suspend thiopurines, methotrexate, and tofacitinib; and delay dosing of monoclonal antibodies (anti–tumor necrosis factor [anti-TNF] drugs, ustekinumab, or vedolizumab) for 2 weeks while their symptoms for COVID-19 are monitored. “Restarting therapy after 2 weeks if the patient has not developed manifestations of COVID-19 is reasonable,” wrote Dr. Rubin and colleagues. Emerging serial testing should indicate antibody status, but the effectiveness of stool testing for SARS-CoV-2 in these cases “remains to be seen.”
- In the patient with confirmed COVID-19, adjustment of IBD therapy “is appropriate, based on the understanding of the immune activity of the therapy and whether that therapy may worsen outcomes with COVID-19,” the update stated. Therapy adjustment should focus on reducing immune suppression during the active viral infection. Some studies are evaluating anticytokine-based therapies as COVID-19 treatments, so continued anti-TNF therapies might prevent acute respiratory distress syndrome and multiorgan failure, Dr. Rubin and colleagues wrote. “However, in the absence of those data, guidance is currently based on deciding whether to hold or to continue specific IBD therapies.”
The update considers most IBD therapies, specifically aminosalicylates, topical rectal therapy, dietary management, and antibiotics “safe and may be continued,” and oral budesonide can be continued if it’s needed for ongoing IBD control. However, corticosteroids “should be avoided and discontinued quickly.”
Likewise, during the acute stage of COVID-19, thiopurines, methotrexate, and tofacitinib should be discontinued, and anti-TNF drugs and ustekinumab should be stopped during viral illness. Holding vedolizumab during viral illness is also appropriate, according to the update, although the IOIBD group was uncertain if doing so was necessary.
If the IBD patient has digestive symptoms with COVID-19, ongoing supportive care of the COVID-19 is “reasonable,” but investigating the causes of the digestive symptoms “is critically important.” That should include ruling out enteric infections and confirming active inflammation with nonendoscopic testing. Endoscopy should be relegated to only urgent and emergent cases.
Management of IBD in COVID-19 patients also depends on disease severity. Safer therapies are indicated for mild disease, but withholding IBD therapy in moderate to severe cases may not be practical. “In this setting, the risks and benefits of escalating IBD therapy must be carefully weighed against the severity of the COVID-19,” Dr. Rubin and colleagues noted.
In hospitalized patients with severe COVID-19 and poor prognoses, “IBD therapy will likely take a back seat,” the update stated, although COVID-19 therapies should take the concomitant IBD into account. In patients with milder cases of COVID-19, IBD management should focus on acute manifestations, but intravenous steroid therapy shouldn’t exceed 3 days. The update urged providers to submit cases of IBD and confirmed COVID-19 to the SECURE-IBD registry at COVIDIBD.org.
Dr. Rubin disclosed financial relationships with AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene/Syneos, Gilead Sciences, Takeda, and many other pharmaceutical companies. Coauthors disclosed relationships with those companies and Medimmune, Novus Therapeutics, Osiris Therapeutics, RedHill Biopharma, Sanofi-Aventis, UCB Pharma, and multiple other pharmaceutical companies.
SOURCE: Rubin DT, et al. Gastroenterology. 2020: doi: 10.1053/j.gastro.2020.04.012
Inflammatory bowel disease (IBD) does not seem to make patients any more likely to contract SARS-COV-2 or develop COVID-19, but a rapid review commissioned by the American Gastroenterological Association acknowledges that determination is based on limited evidence and IBD patients should nonetheless maintain remission to reduce their risk of relapse or hospitalization during the COVID-19 pandemic.
The AGA has published a clinical practice update based on that rapid review online in Gastroenterology (2020. doi: 10.1053/j.gastro.2020.04.012).
Because of the widespread use of immunosuppressive or immune-modifying drugs, “It is understandable why patients with Crohn’s disease and ulcerative colitis have specific concerns and potential for increased risk of infection with SARS-CoV-2,” wrote David T. Rubin, MD, AGAF, of the University of Chicago and colleagues in the expert commentary.
They noted that, while association between GI symptoms and COVID-19 RNA in stool samples isn’t clear, given the reports of GI symptoms in COVID-19 patients – ranging from 10% (JAMA. 2020:323:1061-9) to half of patients (Am J Gastroenterol. 2020;115:766-73) – “the clinical implications of this are quite important.” In IBD patients, changes in digestive symptoms without accompanying fever or respiratory symptoms can be monitored for symptoms that could merit testing for the virus as well as “trigger additional treatment adjustments.”
In accordance with the IOIBD (International Organization for the Study of Inflammatory Bowel Disease) consensus, the update noted that IBD patients should continue going to infusion centers for therapies, provided that the centers use a COVID-19 screening protocol.
Patients with IBD who contract COVID-19 seem more likely to be hospitalized for one or the other disease, but that’s based on data from the international SECURE-IBD registry that had 164 patients as of the writing of the update. The update provides guidance for three scenarios for IBD patients during the pandemic:
- Patients not infected with SARS-CoV-2 should maintain their IBD therapies to sustain remission and avoid relapses. “Aside from the obvious negative consequences of a relapse, relapsing IBD will strain available medical resources, may require steroid therapy or necessitate hospitalization, outcomes that are all much worse than the known risks of existing IBD therapies,” Dr. Rubin and colleagues noted.
- Patients who are infected but have no symptoms of COVID-19 should have their dosing of prednisone adjusted to less than 20 mg/day or switched to budesonide; suspend thiopurines, methotrexate, and tofacitinib; and delay dosing of monoclonal antibodies (anti–tumor necrosis factor [anti-TNF] drugs, ustekinumab, or vedolizumab) for 2 weeks while their symptoms for COVID-19 are monitored. “Restarting therapy after 2 weeks if the patient has not developed manifestations of COVID-19 is reasonable,” wrote Dr. Rubin and colleagues. Emerging serial testing should indicate antibody status, but the effectiveness of stool testing for SARS-CoV-2 in these cases “remains to be seen.”
- In the patient with confirmed COVID-19, adjustment of IBD therapy “is appropriate, based on the understanding of the immune activity of the therapy and whether that therapy may worsen outcomes with COVID-19,” the update stated. Therapy adjustment should focus on reducing immune suppression during the active viral infection. Some studies are evaluating anticytokine-based therapies as COVID-19 treatments, so continued anti-TNF therapies might prevent acute respiratory distress syndrome and multiorgan failure, Dr. Rubin and colleagues wrote. “However, in the absence of those data, guidance is currently based on deciding whether to hold or to continue specific IBD therapies.”
The update considers most IBD therapies, specifically aminosalicylates, topical rectal therapy, dietary management, and antibiotics “safe and may be continued,” and oral budesonide can be continued if it’s needed for ongoing IBD control. However, corticosteroids “should be avoided and discontinued quickly.”
Likewise, during the acute stage of COVID-19, thiopurines, methotrexate, and tofacitinib should be discontinued, and anti-TNF drugs and ustekinumab should be stopped during viral illness. Holding vedolizumab during viral illness is also appropriate, according to the update, although the IOIBD group was uncertain if doing so was necessary.
If the IBD patient has digestive symptoms with COVID-19, ongoing supportive care of the COVID-19 is “reasonable,” but investigating the causes of the digestive symptoms “is critically important.” That should include ruling out enteric infections and confirming active inflammation with nonendoscopic testing. Endoscopy should be relegated to only urgent and emergent cases.
Management of IBD in COVID-19 patients also depends on disease severity. Safer therapies are indicated for mild disease, but withholding IBD therapy in moderate to severe cases may not be practical. “In this setting, the risks and benefits of escalating IBD therapy must be carefully weighed against the severity of the COVID-19,” Dr. Rubin and colleagues noted.
In hospitalized patients with severe COVID-19 and poor prognoses, “IBD therapy will likely take a back seat,” the update stated, although COVID-19 therapies should take the concomitant IBD into account. In patients with milder cases of COVID-19, IBD management should focus on acute manifestations, but intravenous steroid therapy shouldn’t exceed 3 days. The update urged providers to submit cases of IBD and confirmed COVID-19 to the SECURE-IBD registry at COVIDIBD.org.
Dr. Rubin disclosed financial relationships with AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene/Syneos, Gilead Sciences, Takeda, and many other pharmaceutical companies. Coauthors disclosed relationships with those companies and Medimmune, Novus Therapeutics, Osiris Therapeutics, RedHill Biopharma, Sanofi-Aventis, UCB Pharma, and multiple other pharmaceutical companies.
SOURCE: Rubin DT, et al. Gastroenterology. 2020: doi: 10.1053/j.gastro.2020.04.012
FROM GASTROENTEROLOGY
No OS benefit with gefitinib vs. chemo for EGFR+ NSCLC
The median OS was 75.5 months in patients randomized to adjuvant gefitinib and 62.8 months in patients randomized to vinorelbine plus cisplatin.
Yi-Long Wu, MD, of Guangdong Lung Cancer Institute in Guangzhou, China, reported these results as part of the American Society of Clinical Oncology virtual scientific program.
Prior results from this trial had shown a disease-free survival (DFS) benefit with gefitinib, but this did not translate to an OS benefit at the final analysis, Dr. Wu said.
He noted, however, that the median OS of 75.5 months in the gefitinib arm “was one of the best in resected EGFR-mutant non–small cell lung cancer, compared with historical data.”
The findings also suggest a possible benefit with at least 18 months of gefitinib and show that adjuvant EGFR tyrosine kinase inhibitors (TKIs) should be considered the optimal therapy to improve DFS and achieve potentially better OS in this setting, Dr. Wu said.
Study details and DFS
The ADJUVANT trial (NCT01405079) randomized 222 patients, aged 18-75 years, with EGFR-mutant, stage II-IIIA (N1-N2) NSCLC who had undergone complete resection. Patients were enrolled at 27 sites between September 2011 and April 2014.
The patients were randomized 1:1 to receive 250 mg of gefitinib once daily for 24 months, or 25 mg/m2 of vinorelbine on days 1 and 8 plus 75 mg/m2 of cisplatin on day 1 every 3 weeks for 4 cycles.
The intent-to-treat (ITT) population included 111 patients in each arm. The per-protocol population included 106 patients in the gefitinib arm and 87 patients in the chemotherapy arm.
Primary results from this trial showed a significant improvement in DFS with gefitinib (Lancet Oncol. 2018 Jan;19[1]:139-48). That improvement was maintained in the final analysis.
The median DFS was 30.8 months in the gefitinib arm and 19.8 months in the chemotherapy arm for both the ITT and per-protocol populations. The hazard ratio (HR) was 0.56 (P = .001) in the ITT population and 0.51 (P < .001) in the per-protocol population.
In the ITT population, the 5-year DFS rates were 22.6% in the gefitinib arm and 23.2% in the chemotherapy arm. In the per-protocol population, the 5-year DFS rates were 22.6% and 22.8%, respectively.
OS results
The median OS was 75.5 months in the gefitinib arm and 62.8 months in the chemotherapy arm for both the ITT and per-protocol populations. The HR was 0.92 in both the ITT (P = .674) and per-protocol populations (P = .686).
In the ITT population, the 5-year OS rates were 53.2% in the gefitinib arm and 51.2% in the chemotherapy arm. In the per-protocol population, the 5-year OS rates were 53.2% and 50.7%, respectively.
Subgroup analyses by age, gender, lymph node status, and EGFR mutation showed trends toward improved OS with gefitinib, but the differences were not statistically significant.
The researchers conducted a post hoc analysis to assess the effect of subsequent treatment on patient outcomes. The analysis showed that patients who received gefitinib with subsequent EGFR-TKIs had the best responses and OS.
The median OS was not reached among patients who received gefitinib and subsequent EGFR-TKIs, whereas the median OS ranged from 15.6 months to 62.8 months in other groups. The shortest OS was observed in patients who received adjuvant chemotherapy without subsequent therapy.
The duration of gefitinib treatment also appeared to affect OS. The median OS was 35.7 months in patients who received gefitinib for less than 18 months, and the median OS was not reached in patients who received gefitinib for 18 months or longer (HR, 0.38; P < .001).
Implications and potential next steps
Despite the lack of OS improvement with gefitinib, “all of the patients on this study did much, much better than historical non–small cell lung cancer not specified by the EGFR mutation, with 70 months median survival compared to 35 months median survival for N2-positive disease,” said invited discussant Christopher G. Azzoli, MD, director of thoracic oncology at Lifespan Cancer Institute at Brown University in Providence, R.I.
“But you can’t avoid noticing how the curves come back together in terms of disease-free survival when your effective treatment is limited to 24 months,” he added.
An apparent risk of late brain recurrence in the gefitinib arm is also a concern, Dr. Azzoli said. “So ... longer duration of treatment with a drug that has better control of CNS [central nervous system] disease, such as osimertinib, may improve both DFS and OS,” he added.
Only about 50% of patients in the chemotherapy arm received a TKI at recurrence. The post hoc analysis showing that TKI recipients had the best outcomes raises the question of whether “the survival benefit could be conferred by delivering a superior drug merely at recurrence, or is there benefit to earlier delivery of an effective drug,” Dr. Azzoli said.
Given the high cost of continuous therapy, biomarker refinement could help improve treatment decision-making, he said, noting that “early testing of blood DNA to detect cancer in the body as minimal residual disease is showing promise,” and that many phase 3 studies of EGFR-TKIs are ongoing.
The current trial was sponsored by the Guangdong Association of Clinical Trials. Dr. Wu disclosed relationships with AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb/China, Lilly, MSD Oncology, Pfizer, and Roche. Dr. Azzoli reported having no disclosures.
SOURCE: Wu Y et al. ASCO 2020, Abstract 9005.
The median OS was 75.5 months in patients randomized to adjuvant gefitinib and 62.8 months in patients randomized to vinorelbine plus cisplatin.
Yi-Long Wu, MD, of Guangdong Lung Cancer Institute in Guangzhou, China, reported these results as part of the American Society of Clinical Oncology virtual scientific program.
Prior results from this trial had shown a disease-free survival (DFS) benefit with gefitinib, but this did not translate to an OS benefit at the final analysis, Dr. Wu said.
He noted, however, that the median OS of 75.5 months in the gefitinib arm “was one of the best in resected EGFR-mutant non–small cell lung cancer, compared with historical data.”
The findings also suggest a possible benefit with at least 18 months of gefitinib and show that adjuvant EGFR tyrosine kinase inhibitors (TKIs) should be considered the optimal therapy to improve DFS and achieve potentially better OS in this setting, Dr. Wu said.
Study details and DFS
The ADJUVANT trial (NCT01405079) randomized 222 patients, aged 18-75 years, with EGFR-mutant, stage II-IIIA (N1-N2) NSCLC who had undergone complete resection. Patients were enrolled at 27 sites between September 2011 and April 2014.
The patients were randomized 1:1 to receive 250 mg of gefitinib once daily for 24 months, or 25 mg/m2 of vinorelbine on days 1 and 8 plus 75 mg/m2 of cisplatin on day 1 every 3 weeks for 4 cycles.
The intent-to-treat (ITT) population included 111 patients in each arm. The per-protocol population included 106 patients in the gefitinib arm and 87 patients in the chemotherapy arm.
Primary results from this trial showed a significant improvement in DFS with gefitinib (Lancet Oncol. 2018 Jan;19[1]:139-48). That improvement was maintained in the final analysis.
The median DFS was 30.8 months in the gefitinib arm and 19.8 months in the chemotherapy arm for both the ITT and per-protocol populations. The hazard ratio (HR) was 0.56 (P = .001) in the ITT population and 0.51 (P < .001) in the per-protocol population.
In the ITT population, the 5-year DFS rates were 22.6% in the gefitinib arm and 23.2% in the chemotherapy arm. In the per-protocol population, the 5-year DFS rates were 22.6% and 22.8%, respectively.
OS results
The median OS was 75.5 months in the gefitinib arm and 62.8 months in the chemotherapy arm for both the ITT and per-protocol populations. The HR was 0.92 in both the ITT (P = .674) and per-protocol populations (P = .686).
In the ITT population, the 5-year OS rates were 53.2% in the gefitinib arm and 51.2% in the chemotherapy arm. In the per-protocol population, the 5-year OS rates were 53.2% and 50.7%, respectively.
Subgroup analyses by age, gender, lymph node status, and EGFR mutation showed trends toward improved OS with gefitinib, but the differences were not statistically significant.
The researchers conducted a post hoc analysis to assess the effect of subsequent treatment on patient outcomes. The analysis showed that patients who received gefitinib with subsequent EGFR-TKIs had the best responses and OS.
The median OS was not reached among patients who received gefitinib and subsequent EGFR-TKIs, whereas the median OS ranged from 15.6 months to 62.8 months in other groups. The shortest OS was observed in patients who received adjuvant chemotherapy without subsequent therapy.
The duration of gefitinib treatment also appeared to affect OS. The median OS was 35.7 months in patients who received gefitinib for less than 18 months, and the median OS was not reached in patients who received gefitinib for 18 months or longer (HR, 0.38; P < .001).
Implications and potential next steps
Despite the lack of OS improvement with gefitinib, “all of the patients on this study did much, much better than historical non–small cell lung cancer not specified by the EGFR mutation, with 70 months median survival compared to 35 months median survival for N2-positive disease,” said invited discussant Christopher G. Azzoli, MD, director of thoracic oncology at Lifespan Cancer Institute at Brown University in Providence, R.I.
“But you can’t avoid noticing how the curves come back together in terms of disease-free survival when your effective treatment is limited to 24 months,” he added.
An apparent risk of late brain recurrence in the gefitinib arm is also a concern, Dr. Azzoli said. “So ... longer duration of treatment with a drug that has better control of CNS [central nervous system] disease, such as osimertinib, may improve both DFS and OS,” he added.
Only about 50% of patients in the chemotherapy arm received a TKI at recurrence. The post hoc analysis showing that TKI recipients had the best outcomes raises the question of whether “the survival benefit could be conferred by delivering a superior drug merely at recurrence, or is there benefit to earlier delivery of an effective drug,” Dr. Azzoli said.
Given the high cost of continuous therapy, biomarker refinement could help improve treatment decision-making, he said, noting that “early testing of blood DNA to detect cancer in the body as minimal residual disease is showing promise,” and that many phase 3 studies of EGFR-TKIs are ongoing.
The current trial was sponsored by the Guangdong Association of Clinical Trials. Dr. Wu disclosed relationships with AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb/China, Lilly, MSD Oncology, Pfizer, and Roche. Dr. Azzoli reported having no disclosures.
SOURCE: Wu Y et al. ASCO 2020, Abstract 9005.
The median OS was 75.5 months in patients randomized to adjuvant gefitinib and 62.8 months in patients randomized to vinorelbine plus cisplatin.
Yi-Long Wu, MD, of Guangdong Lung Cancer Institute in Guangzhou, China, reported these results as part of the American Society of Clinical Oncology virtual scientific program.
Prior results from this trial had shown a disease-free survival (DFS) benefit with gefitinib, but this did not translate to an OS benefit at the final analysis, Dr. Wu said.
He noted, however, that the median OS of 75.5 months in the gefitinib arm “was one of the best in resected EGFR-mutant non–small cell lung cancer, compared with historical data.”
The findings also suggest a possible benefit with at least 18 months of gefitinib and show that adjuvant EGFR tyrosine kinase inhibitors (TKIs) should be considered the optimal therapy to improve DFS and achieve potentially better OS in this setting, Dr. Wu said.
Study details and DFS
The ADJUVANT trial (NCT01405079) randomized 222 patients, aged 18-75 years, with EGFR-mutant, stage II-IIIA (N1-N2) NSCLC who had undergone complete resection. Patients were enrolled at 27 sites between September 2011 and April 2014.
The patients were randomized 1:1 to receive 250 mg of gefitinib once daily for 24 months, or 25 mg/m2 of vinorelbine on days 1 and 8 plus 75 mg/m2 of cisplatin on day 1 every 3 weeks for 4 cycles.
The intent-to-treat (ITT) population included 111 patients in each arm. The per-protocol population included 106 patients in the gefitinib arm and 87 patients in the chemotherapy arm.
Primary results from this trial showed a significant improvement in DFS with gefitinib (Lancet Oncol. 2018 Jan;19[1]:139-48). That improvement was maintained in the final analysis.
The median DFS was 30.8 months in the gefitinib arm and 19.8 months in the chemotherapy arm for both the ITT and per-protocol populations. The hazard ratio (HR) was 0.56 (P = .001) in the ITT population and 0.51 (P < .001) in the per-protocol population.
In the ITT population, the 5-year DFS rates were 22.6% in the gefitinib arm and 23.2% in the chemotherapy arm. In the per-protocol population, the 5-year DFS rates were 22.6% and 22.8%, respectively.
OS results
The median OS was 75.5 months in the gefitinib arm and 62.8 months in the chemotherapy arm for both the ITT and per-protocol populations. The HR was 0.92 in both the ITT (P = .674) and per-protocol populations (P = .686).
In the ITT population, the 5-year OS rates were 53.2% in the gefitinib arm and 51.2% in the chemotherapy arm. In the per-protocol population, the 5-year OS rates were 53.2% and 50.7%, respectively.
Subgroup analyses by age, gender, lymph node status, and EGFR mutation showed trends toward improved OS with gefitinib, but the differences were not statistically significant.
The researchers conducted a post hoc analysis to assess the effect of subsequent treatment on patient outcomes. The analysis showed that patients who received gefitinib with subsequent EGFR-TKIs had the best responses and OS.
The median OS was not reached among patients who received gefitinib and subsequent EGFR-TKIs, whereas the median OS ranged from 15.6 months to 62.8 months in other groups. The shortest OS was observed in patients who received adjuvant chemotherapy without subsequent therapy.
The duration of gefitinib treatment also appeared to affect OS. The median OS was 35.7 months in patients who received gefitinib for less than 18 months, and the median OS was not reached in patients who received gefitinib for 18 months or longer (HR, 0.38; P < .001).
Implications and potential next steps
Despite the lack of OS improvement with gefitinib, “all of the patients on this study did much, much better than historical non–small cell lung cancer not specified by the EGFR mutation, with 70 months median survival compared to 35 months median survival for N2-positive disease,” said invited discussant Christopher G. Azzoli, MD, director of thoracic oncology at Lifespan Cancer Institute at Brown University in Providence, R.I.
“But you can’t avoid noticing how the curves come back together in terms of disease-free survival when your effective treatment is limited to 24 months,” he added.
An apparent risk of late brain recurrence in the gefitinib arm is also a concern, Dr. Azzoli said. “So ... longer duration of treatment with a drug that has better control of CNS [central nervous system] disease, such as osimertinib, may improve both DFS and OS,” he added.
Only about 50% of patients in the chemotherapy arm received a TKI at recurrence. The post hoc analysis showing that TKI recipients had the best outcomes raises the question of whether “the survival benefit could be conferred by delivering a superior drug merely at recurrence, or is there benefit to earlier delivery of an effective drug,” Dr. Azzoli said.
Given the high cost of continuous therapy, biomarker refinement could help improve treatment decision-making, he said, noting that “early testing of blood DNA to detect cancer in the body as minimal residual disease is showing promise,” and that many phase 3 studies of EGFR-TKIs are ongoing.
The current trial was sponsored by the Guangdong Association of Clinical Trials. Dr. Wu disclosed relationships with AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb/China, Lilly, MSD Oncology, Pfizer, and Roche. Dr. Azzoli reported having no disclosures.
SOURCE: Wu Y et al. ASCO 2020, Abstract 9005.
FROM ASCO 2020
Kids with food allergies the newest victims of COVID-19?
Food insecurity is not knowing how you will get your next meal. This pandemic has led to a lot of it, especially as a result of massive unemployment. Now imagine being in that situation with a food-allergic child. It would be frightening.
There is always a level of anxiety for parents of food-allergic children, but the Food and Drug Administration–mandated labeling of food allergens has helped to allay some of those concerns. Shopping can feel safer, even if it’s not foolproof.
Now, that fear for the safety of food-allergic children is going to be compounded by the FDA’s latest announcement, made at the behest of the food industry.
Disruptions in the food supply chain caused by the COVID-19 pandemic have created some problems for the food industry. The industry sought – and received – relief from the FDA; they are now allowing some ingredient substitutions without mandating a change in labeling. These changes were made without opportunity for public comment, according to the FDA, because of the exigency of the situation. Furthermore, the changes may stay in effect for an indeterminate period of time after the pandemic is deemed under control.
Labeling of gluten and the major eight allergens (peanuts, tree nuts, milk, eggs, soy, wheat, fish, and crustacean shellfish) cannot change under the new guidelines. The FDA also advised “consideration” of major food allergens recognized in other countries (sesame, celery, lupin, buckwheat, molluscan shellfish, and mustard). Of these, lupin is known to cross-react with peanut, and sesame seed allergy is increasingly prevalent. In fact, the FDA has considered adding it to the list of major allergens.
Meanwhile, according to this temporary FDA policy, substitutions should be limited to no more than 2% of the weight of the final product unless it is a variety of the same ingredient. The example provided is substitution of one type of mushroom for another, but even that could be an issue for the rare patient. And what if this is misinterpreted – as will surely happen somewhere – and one seed is substituted for another?
A friend of mine is a pediatrician and mother of a child who is allergic to sesame, peanuts, tree nuts, and garbanzo beans. Naturally, she had grave concerns about these changes. She also wondered what the liability would be for the food manufacturing company in the current situation despite the FDA notice, which seems like a valid point. It is worth noting that, at the very top of this FDA notice, are the words “contains nonbinding recommendations,” so manufacturers may want to think twice about how they approach this. A minority of companies have pledged to relabel foods if necessary. Meanwhile, without any alert in advance, it is now up to patients and their physicians to sort out the attendant risks.
The FDA should have advised or mandated that food manufacturers give notice to online and physical retailers of ingredient changes. A simple sign in front of a display or alert online would be a very reasonable solution and pose no burden to those involved. It should be self-evident that mistakes always happen, especially under duress, and that the loosening of these regulations will have unintended consequences. To the severe problem of food insecurity, we can add one more concern for the parents of allergic children: food-allergen insecurity.
A version of this article originally appeared on Medscape.com.
Food insecurity is not knowing how you will get your next meal. This pandemic has led to a lot of it, especially as a result of massive unemployment. Now imagine being in that situation with a food-allergic child. It would be frightening.
There is always a level of anxiety for parents of food-allergic children, but the Food and Drug Administration–mandated labeling of food allergens has helped to allay some of those concerns. Shopping can feel safer, even if it’s not foolproof.
Now, that fear for the safety of food-allergic children is going to be compounded by the FDA’s latest announcement, made at the behest of the food industry.
Disruptions in the food supply chain caused by the COVID-19 pandemic have created some problems for the food industry. The industry sought – and received – relief from the FDA; they are now allowing some ingredient substitutions without mandating a change in labeling. These changes were made without opportunity for public comment, according to the FDA, because of the exigency of the situation. Furthermore, the changes may stay in effect for an indeterminate period of time after the pandemic is deemed under control.
Labeling of gluten and the major eight allergens (peanuts, tree nuts, milk, eggs, soy, wheat, fish, and crustacean shellfish) cannot change under the new guidelines. The FDA also advised “consideration” of major food allergens recognized in other countries (sesame, celery, lupin, buckwheat, molluscan shellfish, and mustard). Of these, lupin is known to cross-react with peanut, and sesame seed allergy is increasingly prevalent. In fact, the FDA has considered adding it to the list of major allergens.
Meanwhile, according to this temporary FDA policy, substitutions should be limited to no more than 2% of the weight of the final product unless it is a variety of the same ingredient. The example provided is substitution of one type of mushroom for another, but even that could be an issue for the rare patient. And what if this is misinterpreted – as will surely happen somewhere – and one seed is substituted for another?
A friend of mine is a pediatrician and mother of a child who is allergic to sesame, peanuts, tree nuts, and garbanzo beans. Naturally, she had grave concerns about these changes. She also wondered what the liability would be for the food manufacturing company in the current situation despite the FDA notice, which seems like a valid point. It is worth noting that, at the very top of this FDA notice, are the words “contains nonbinding recommendations,” so manufacturers may want to think twice about how they approach this. A minority of companies have pledged to relabel foods if necessary. Meanwhile, without any alert in advance, it is now up to patients and their physicians to sort out the attendant risks.
The FDA should have advised or mandated that food manufacturers give notice to online and physical retailers of ingredient changes. A simple sign in front of a display or alert online would be a very reasonable solution and pose no burden to those involved. It should be self-evident that mistakes always happen, especially under duress, and that the loosening of these regulations will have unintended consequences. To the severe problem of food insecurity, we can add one more concern for the parents of allergic children: food-allergen insecurity.
A version of this article originally appeared on Medscape.com.
Food insecurity is not knowing how you will get your next meal. This pandemic has led to a lot of it, especially as a result of massive unemployment. Now imagine being in that situation with a food-allergic child. It would be frightening.
There is always a level of anxiety for parents of food-allergic children, but the Food and Drug Administration–mandated labeling of food allergens has helped to allay some of those concerns. Shopping can feel safer, even if it’s not foolproof.
Now, that fear for the safety of food-allergic children is going to be compounded by the FDA’s latest announcement, made at the behest of the food industry.
Disruptions in the food supply chain caused by the COVID-19 pandemic have created some problems for the food industry. The industry sought – and received – relief from the FDA; they are now allowing some ingredient substitutions without mandating a change in labeling. These changes were made without opportunity for public comment, according to the FDA, because of the exigency of the situation. Furthermore, the changes may stay in effect for an indeterminate period of time after the pandemic is deemed under control.
Labeling of gluten and the major eight allergens (peanuts, tree nuts, milk, eggs, soy, wheat, fish, and crustacean shellfish) cannot change under the new guidelines. The FDA also advised “consideration” of major food allergens recognized in other countries (sesame, celery, lupin, buckwheat, molluscan shellfish, and mustard). Of these, lupin is known to cross-react with peanut, and sesame seed allergy is increasingly prevalent. In fact, the FDA has considered adding it to the list of major allergens.
Meanwhile, according to this temporary FDA policy, substitutions should be limited to no more than 2% of the weight of the final product unless it is a variety of the same ingredient. The example provided is substitution of one type of mushroom for another, but even that could be an issue for the rare patient. And what if this is misinterpreted – as will surely happen somewhere – and one seed is substituted for another?
A friend of mine is a pediatrician and mother of a child who is allergic to sesame, peanuts, tree nuts, and garbanzo beans. Naturally, she had grave concerns about these changes. She also wondered what the liability would be for the food manufacturing company in the current situation despite the FDA notice, which seems like a valid point. It is worth noting that, at the very top of this FDA notice, are the words “contains nonbinding recommendations,” so manufacturers may want to think twice about how they approach this. A minority of companies have pledged to relabel foods if necessary. Meanwhile, without any alert in advance, it is now up to patients and their physicians to sort out the attendant risks.
The FDA should have advised or mandated that food manufacturers give notice to online and physical retailers of ingredient changes. A simple sign in front of a display or alert online would be a very reasonable solution and pose no burden to those involved. It should be self-evident that mistakes always happen, especially under duress, and that the loosening of these regulations will have unintended consequences. To the severe problem of food insecurity, we can add one more concern for the parents of allergic children: food-allergen insecurity.
A version of this article originally appeared on Medscape.com.
GI cancer death disproportional to incidence rates
Cancers of the gastrointestinal tract represented about one in four cancer cases in 2018 but more than one in three cancer-related deaths, underscoring the importance of preventive measures, according to an analysis of global cancer datasets that identified trends in five major GI cancer types – esophageal, stomach, colorectal, liver, and pancreatic – published in Gastroenterology.
“Although the incidence of some GI cancer types has decreased, this group of malignancies continues to pose major challenges to public health,” wrote Melina Arnold, PhD, of the International for Research on Cancer in Lyon, France, and colleagues. “Primary and secondary prevention measures are important for controlling these malignancies – most importantly, reducing consumption of tobacco and alcohol, obesity control, immunizing populations against hepatitis B virus infection, and screening for colorectal cancer.”
For example, the study found that the proportion of GI cancer deaths in Asia exceeds that of new cases, with the greatest disparity in China, while the opposite can be said of GI cancer trends in Europe and North America.
The study reported 4.8 million new cases of GI cancer and 3.4 million deaths worldwide in 2018. GI cancers accounted for 26% of the global cancer burden but 35% of cancer-related deaths. Incidence-to-death disparities were greatest for Africa, accounting for 4% of new cases and 5% of deaths; and Asia, with about 63% of new cases but 65% of deaths. The disparity was even wider in China, which accounted for 38% of worldwide cases but 41% of the deaths. Europe and North America, on the other hand, accounted for 26% of global cases but 23% of deaths. High death rates of these cancers are associated with late detection, Dr. Arnold and colleagues noted.
Regarding the five different types of GI cancer, the study reported the following:
- Esophageal cancer accounted for 572,000 new cases and 508,000 deaths in 2018, making it the sixth-most-deadly cancer worldwide. Rates in men are two to three times higher than in women. Eastern Asia has the highest rates, 12.2 per 100,000 person-years, followed by eastern Africa (8.3) and southern Africa (7.4). China alone accounts for 54% of the global burden. A large percentage of these cancers in developing countries are squamous cell carcinoma, the most common form of esophageal cancer globally, which has been linked to tobacco use, heavy alcohol consumption, opium intake, air pollution, and diet.
- Gastric cancer accounted for more than 1 million new cases and nearly 800,000 deaths in 2018. Again, the incidence is twice as high in men as in women, and eastern Asia has the highest rates of 22 per 100,000 vs. < 5 in Africa, North America, and northern Europe. Cardia gastric cancer (CGC), associated with obesity and gastroesophageal reflux disease, is more prevalent in Western countries, while noncardia gastric cancer (CG) is more prevalent in countries with higher rates of Helicobacter pylori infection. In the United States specifically, CGC is more common in non-Hispanic whites than other ethnic groups. Gastric cancer rates have been declining in recent years, although trends of CGC and cancer of the gastric corpus, which the study terms “a noncardia subsite,” among younger people “may lead to a deceleration or a reversal” of declining gastric cancer rates, stated Dr. Arnold and colleagues.
- Colorectal cancer remained the most commonly diagnosed GI cancer in 2018, accounting for 1.8 million cases and 881,000 deaths, which represents 1 in 10 cancer deaths. The highest incidence was found in Australia/New Zealand, the lowest in south and central Asia. Colorectal cancers are in “transition” from infection-related cancers to those related to “rapid societal and economic change.” Dr. Arnold and colleagues attributed these changes to higher dietary intake of fats, sugar, and animal-source foods and increases in sedentary behavior and obesity. Despite advances in cures for colorectal cancer, disparities continue, even in high-income countries, and screening programs have been limited, according to the study. Colorectal cancer will be “one of the main contributors” to the doubling of cancer rates in older adults by 2035.
- Liver cancer comprised 841,000 cases and 782,000 deaths in 2018, making it the sixth-most-diagnosed cancer but the fourth most deadly. “Transitioning countries” in eastern Asia, Micronesia, and northern Africa have the highest rates. In eastern Asia, hepatitis B infections and aflatoxins are the primary risk factors for hepatocellular carcinoma, while in Japan and Europe hepatitis C is the main cause for hepatocellular carcinoma. Decreases in both infections and aflatoxins may explain declines in liver cancer rates in those regions. Whereas in lower-risk areas, increasing liver cancer rates caused by more widespread obesity and diabetes may be offsetting declines in HBV and HCV rates.
- Pancreatic cancer was the 12th-most-common cancer but the seventh leading cause of cancer death, with 432,000 cases and 459,000 deaths in 2018. Wealthy countries have incidence and death rates three to four times higher than do less-developed countries, with rates highest in Europe, North America, and Australia/New Zealand. Because pancreatic cancer isn’t typically diagnosed until it is in the metastatic or locally advanced state, curative surgery isn’t feasible, Dr. Arnold and colleagues stated. Population aging and growth, along with advances in treating other types of cancers, mean that pancreatic cancer “has become or is set out to become one of the leading causes of cancer-related death in many countries,” Dr. Arnold and colleagues stated. They added that, in the European Union, pancreatic cancer is already the third-leading cause of cancer death after lung and colorectal cancer.
The findings, Dr. Arnold and colleagues wrote, underscore the shift of the cancer burden toward transitioning countries, “which are less equipped to manage this increasing burden.” In the United States, the rates of all five GI cancers in young adults (aged 25-49 years) have increased.
GI cancers, with the exception of colorectal cancers, also contribute disproportionately to cancer-related death rates, mostly because all but colorectal cancers are difficult to diagnose.
However, early detection and screening programs for gastric cancer in Japan and Korea, and esophageal cancer in China “have shown promising results,” Dr. Arnold and colleagues said. “Pancreatic cancer, on the other hand, is becoming a more important contributor to cancer-related mortality as a consequence of improved diagnosis and management of the historically most common forms of cancer death,” they wrote.
Prevention remains key, and lifestyle choices like smoking, alcohol intake, and physical activity are all drivers of GI cancer burden. “Primary and secondary prevention measures remain the most important tools to control this group of malignancies, particularly in light of their preventability and often dreadful prognosis,” Dr. Arnold and colleagues wrote.
Dr. Arnold and colleagues have no financial relationships to disclose.
SOURCE: Arnold M et al. Gastroenterology. 2020 Apr 2;S0016-5085(20)30452-2. doi: 10.1053/j.gastro.2020.02.068.
Cancers of the gastrointestinal tract represented about one in four cancer cases in 2018 but more than one in three cancer-related deaths, underscoring the importance of preventive measures, according to an analysis of global cancer datasets that identified trends in five major GI cancer types – esophageal, stomach, colorectal, liver, and pancreatic – published in Gastroenterology.
“Although the incidence of some GI cancer types has decreased, this group of malignancies continues to pose major challenges to public health,” wrote Melina Arnold, PhD, of the International for Research on Cancer in Lyon, France, and colleagues. “Primary and secondary prevention measures are important for controlling these malignancies – most importantly, reducing consumption of tobacco and alcohol, obesity control, immunizing populations against hepatitis B virus infection, and screening for colorectal cancer.”
For example, the study found that the proportion of GI cancer deaths in Asia exceeds that of new cases, with the greatest disparity in China, while the opposite can be said of GI cancer trends in Europe and North America.
The study reported 4.8 million new cases of GI cancer and 3.4 million deaths worldwide in 2018. GI cancers accounted for 26% of the global cancer burden but 35% of cancer-related deaths. Incidence-to-death disparities were greatest for Africa, accounting for 4% of new cases and 5% of deaths; and Asia, with about 63% of new cases but 65% of deaths. The disparity was even wider in China, which accounted for 38% of worldwide cases but 41% of the deaths. Europe and North America, on the other hand, accounted for 26% of global cases but 23% of deaths. High death rates of these cancers are associated with late detection, Dr. Arnold and colleagues noted.
Regarding the five different types of GI cancer, the study reported the following:
- Esophageal cancer accounted for 572,000 new cases and 508,000 deaths in 2018, making it the sixth-most-deadly cancer worldwide. Rates in men are two to three times higher than in women. Eastern Asia has the highest rates, 12.2 per 100,000 person-years, followed by eastern Africa (8.3) and southern Africa (7.4). China alone accounts for 54% of the global burden. A large percentage of these cancers in developing countries are squamous cell carcinoma, the most common form of esophageal cancer globally, which has been linked to tobacco use, heavy alcohol consumption, opium intake, air pollution, and diet.
- Gastric cancer accounted for more than 1 million new cases and nearly 800,000 deaths in 2018. Again, the incidence is twice as high in men as in women, and eastern Asia has the highest rates of 22 per 100,000 vs. < 5 in Africa, North America, and northern Europe. Cardia gastric cancer (CGC), associated with obesity and gastroesophageal reflux disease, is more prevalent in Western countries, while noncardia gastric cancer (CG) is more prevalent in countries with higher rates of Helicobacter pylori infection. In the United States specifically, CGC is more common in non-Hispanic whites than other ethnic groups. Gastric cancer rates have been declining in recent years, although trends of CGC and cancer of the gastric corpus, which the study terms “a noncardia subsite,” among younger people “may lead to a deceleration or a reversal” of declining gastric cancer rates, stated Dr. Arnold and colleagues.
- Colorectal cancer remained the most commonly diagnosed GI cancer in 2018, accounting for 1.8 million cases and 881,000 deaths, which represents 1 in 10 cancer deaths. The highest incidence was found in Australia/New Zealand, the lowest in south and central Asia. Colorectal cancers are in “transition” from infection-related cancers to those related to “rapid societal and economic change.” Dr. Arnold and colleagues attributed these changes to higher dietary intake of fats, sugar, and animal-source foods and increases in sedentary behavior and obesity. Despite advances in cures for colorectal cancer, disparities continue, even in high-income countries, and screening programs have been limited, according to the study. Colorectal cancer will be “one of the main contributors” to the doubling of cancer rates in older adults by 2035.
- Liver cancer comprised 841,000 cases and 782,000 deaths in 2018, making it the sixth-most-diagnosed cancer but the fourth most deadly. “Transitioning countries” in eastern Asia, Micronesia, and northern Africa have the highest rates. In eastern Asia, hepatitis B infections and aflatoxins are the primary risk factors for hepatocellular carcinoma, while in Japan and Europe hepatitis C is the main cause for hepatocellular carcinoma. Decreases in both infections and aflatoxins may explain declines in liver cancer rates in those regions. Whereas in lower-risk areas, increasing liver cancer rates caused by more widespread obesity and diabetes may be offsetting declines in HBV and HCV rates.
- Pancreatic cancer was the 12th-most-common cancer but the seventh leading cause of cancer death, with 432,000 cases and 459,000 deaths in 2018. Wealthy countries have incidence and death rates three to four times higher than do less-developed countries, with rates highest in Europe, North America, and Australia/New Zealand. Because pancreatic cancer isn’t typically diagnosed until it is in the metastatic or locally advanced state, curative surgery isn’t feasible, Dr. Arnold and colleagues stated. Population aging and growth, along with advances in treating other types of cancers, mean that pancreatic cancer “has become or is set out to become one of the leading causes of cancer-related death in many countries,” Dr. Arnold and colleagues stated. They added that, in the European Union, pancreatic cancer is already the third-leading cause of cancer death after lung and colorectal cancer.
The findings, Dr. Arnold and colleagues wrote, underscore the shift of the cancer burden toward transitioning countries, “which are less equipped to manage this increasing burden.” In the United States, the rates of all five GI cancers in young adults (aged 25-49 years) have increased.
GI cancers, with the exception of colorectal cancers, also contribute disproportionately to cancer-related death rates, mostly because all but colorectal cancers are difficult to diagnose.
However, early detection and screening programs for gastric cancer in Japan and Korea, and esophageal cancer in China “have shown promising results,” Dr. Arnold and colleagues said. “Pancreatic cancer, on the other hand, is becoming a more important contributor to cancer-related mortality as a consequence of improved diagnosis and management of the historically most common forms of cancer death,” they wrote.
Prevention remains key, and lifestyle choices like smoking, alcohol intake, and physical activity are all drivers of GI cancer burden. “Primary and secondary prevention measures remain the most important tools to control this group of malignancies, particularly in light of their preventability and often dreadful prognosis,” Dr. Arnold and colleagues wrote.
Dr. Arnold and colleagues have no financial relationships to disclose.
SOURCE: Arnold M et al. Gastroenterology. 2020 Apr 2;S0016-5085(20)30452-2. doi: 10.1053/j.gastro.2020.02.068.
Cancers of the gastrointestinal tract represented about one in four cancer cases in 2018 but more than one in three cancer-related deaths, underscoring the importance of preventive measures, according to an analysis of global cancer datasets that identified trends in five major GI cancer types – esophageal, stomach, colorectal, liver, and pancreatic – published in Gastroenterology.
“Although the incidence of some GI cancer types has decreased, this group of malignancies continues to pose major challenges to public health,” wrote Melina Arnold, PhD, of the International for Research on Cancer in Lyon, France, and colleagues. “Primary and secondary prevention measures are important for controlling these malignancies – most importantly, reducing consumption of tobacco and alcohol, obesity control, immunizing populations against hepatitis B virus infection, and screening for colorectal cancer.”
For example, the study found that the proportion of GI cancer deaths in Asia exceeds that of new cases, with the greatest disparity in China, while the opposite can be said of GI cancer trends in Europe and North America.
The study reported 4.8 million new cases of GI cancer and 3.4 million deaths worldwide in 2018. GI cancers accounted for 26% of the global cancer burden but 35% of cancer-related deaths. Incidence-to-death disparities were greatest for Africa, accounting for 4% of new cases and 5% of deaths; and Asia, with about 63% of new cases but 65% of deaths. The disparity was even wider in China, which accounted for 38% of worldwide cases but 41% of the deaths. Europe and North America, on the other hand, accounted for 26% of global cases but 23% of deaths. High death rates of these cancers are associated with late detection, Dr. Arnold and colleagues noted.
Regarding the five different types of GI cancer, the study reported the following:
- Esophageal cancer accounted for 572,000 new cases and 508,000 deaths in 2018, making it the sixth-most-deadly cancer worldwide. Rates in men are two to three times higher than in women. Eastern Asia has the highest rates, 12.2 per 100,000 person-years, followed by eastern Africa (8.3) and southern Africa (7.4). China alone accounts for 54% of the global burden. A large percentage of these cancers in developing countries are squamous cell carcinoma, the most common form of esophageal cancer globally, which has been linked to tobacco use, heavy alcohol consumption, opium intake, air pollution, and diet.
- Gastric cancer accounted for more than 1 million new cases and nearly 800,000 deaths in 2018. Again, the incidence is twice as high in men as in women, and eastern Asia has the highest rates of 22 per 100,000 vs. < 5 in Africa, North America, and northern Europe. Cardia gastric cancer (CGC), associated with obesity and gastroesophageal reflux disease, is more prevalent in Western countries, while noncardia gastric cancer (CG) is more prevalent in countries with higher rates of Helicobacter pylori infection. In the United States specifically, CGC is more common in non-Hispanic whites than other ethnic groups. Gastric cancer rates have been declining in recent years, although trends of CGC and cancer of the gastric corpus, which the study terms “a noncardia subsite,” among younger people “may lead to a deceleration or a reversal” of declining gastric cancer rates, stated Dr. Arnold and colleagues.
- Colorectal cancer remained the most commonly diagnosed GI cancer in 2018, accounting for 1.8 million cases and 881,000 deaths, which represents 1 in 10 cancer deaths. The highest incidence was found in Australia/New Zealand, the lowest in south and central Asia. Colorectal cancers are in “transition” from infection-related cancers to those related to “rapid societal and economic change.” Dr. Arnold and colleagues attributed these changes to higher dietary intake of fats, sugar, and animal-source foods and increases in sedentary behavior and obesity. Despite advances in cures for colorectal cancer, disparities continue, even in high-income countries, and screening programs have been limited, according to the study. Colorectal cancer will be “one of the main contributors” to the doubling of cancer rates in older adults by 2035.
- Liver cancer comprised 841,000 cases and 782,000 deaths in 2018, making it the sixth-most-diagnosed cancer but the fourth most deadly. “Transitioning countries” in eastern Asia, Micronesia, and northern Africa have the highest rates. In eastern Asia, hepatitis B infections and aflatoxins are the primary risk factors for hepatocellular carcinoma, while in Japan and Europe hepatitis C is the main cause for hepatocellular carcinoma. Decreases in both infections and aflatoxins may explain declines in liver cancer rates in those regions. Whereas in lower-risk areas, increasing liver cancer rates caused by more widespread obesity and diabetes may be offsetting declines in HBV and HCV rates.
- Pancreatic cancer was the 12th-most-common cancer but the seventh leading cause of cancer death, with 432,000 cases and 459,000 deaths in 2018. Wealthy countries have incidence and death rates three to four times higher than do less-developed countries, with rates highest in Europe, North America, and Australia/New Zealand. Because pancreatic cancer isn’t typically diagnosed until it is in the metastatic or locally advanced state, curative surgery isn’t feasible, Dr. Arnold and colleagues stated. Population aging and growth, along with advances in treating other types of cancers, mean that pancreatic cancer “has become or is set out to become one of the leading causes of cancer-related death in many countries,” Dr. Arnold and colleagues stated. They added that, in the European Union, pancreatic cancer is already the third-leading cause of cancer death after lung and colorectal cancer.
The findings, Dr. Arnold and colleagues wrote, underscore the shift of the cancer burden toward transitioning countries, “which are less equipped to manage this increasing burden.” In the United States, the rates of all five GI cancers in young adults (aged 25-49 years) have increased.
GI cancers, with the exception of colorectal cancers, also contribute disproportionately to cancer-related death rates, mostly because all but colorectal cancers are difficult to diagnose.
However, early detection and screening programs for gastric cancer in Japan and Korea, and esophageal cancer in China “have shown promising results,” Dr. Arnold and colleagues said. “Pancreatic cancer, on the other hand, is becoming a more important contributor to cancer-related mortality as a consequence of improved diagnosis and management of the historically most common forms of cancer death,” they wrote.
Prevention remains key, and lifestyle choices like smoking, alcohol intake, and physical activity are all drivers of GI cancer burden. “Primary and secondary prevention measures remain the most important tools to control this group of malignancies, particularly in light of their preventability and often dreadful prognosis,” Dr. Arnold and colleagues wrote.
Dr. Arnold and colleagues have no financial relationships to disclose.
SOURCE: Arnold M et al. Gastroenterology. 2020 Apr 2;S0016-5085(20)30452-2. doi: 10.1053/j.gastro.2020.02.068.
FROM GASTROENTEROLOGY
AGA clinical practice update: Pancreatic cancer screening
Individuals at high risk for pancreatic cancer should at least be considered for screening for the disease, states a new clinical practice update from the American Gastroenterological Association that further defines what constitutes high risk for pancreatic cancer, when and how screenings should occur, and the role of genetic testing and counseling (Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.03.088).
Individuals who have a first-degree relative with two or more genetically related relatives with pancreatic cancer should be considered for screening, as should people with Peutz-Jeghers syndrome, a CDKN2A gene mutation, one or more first-degree relatives with pancreatic cancer with Lynch syndrome and mutations in the BRCA1, BRCA2, PALB2, and ATM genes, the clinical update stated. Screening in high-risk individuals should begin at age 50, but some groups should start having screening earlier: age 40 in carriers of the CKDN2A and PRSS1 mutations with hereditary pancreatitis; and age 35 in those with Peutz-Jeghers syndrome.
“Studies to date have demonstrated variability regarding definitions of high-risk groups and the age at which screening should be initiated,” wrote Harry R. Aslanian, MD, AGAF, of Yale New Haven (Conn.) Hospital, and coauthors. “The genetic basis of much of the inherited susceptibility to pancreas cancer remains unexplained (in approximately 90% of cases) and familial history is important in risk stratification.”
Genetic testing and counseling should be considered for any familial pancreas cancer relative – that is, a person with two or more first-degree relatives with pancreas cancer that’s outside the definition of other hereditary cancers. “A positive germline mutation is associated with an increased risk of neoplastic progression and may also lead to screening for other relevant associated cancers,” wrote Dr. Aslanian and coauthors.
The screening itself should consist of MRI and endoscopic ultrasonography (EUS) in combination, the clinical practice update states. It defines detectable targets as stage 1 pancreatic ductal adenocarcinoma and high-risk neoplasms such as intraductal papillary mucinous neoplasms with high-grade dysplasia and some enlarged pancreatic intraepithelial neoplasms.
High-risk patients having screening should also be enrolled in a registry and referred to a pancreas center of excellence.
The update suggests screening every 12 months when the baseline screening is negative for any suspect lesions, with shorter intervals for EUS when suspected lesions are found: 6-12 months for low-risk lesions; 3-6 months for intermediate lesions; and 3 months for high-risk lesions if the patient hasn’t had surgery to remove the lesions.
Regarding management of positive screening results, a multidisciplinary team should confer with the individual and family to determine therapy. If surgery is indicated, it should be done at a high-volume center.
The update also provides guidance for two scenarios when patients shouldn’t undergo screening: those at average risk; and those at high-risk more likely to die from another cause. Of course, the physician should review the limitations and risk of screening with patients beforehand.
Dr. Aslanian and coauthors noted that a number of areas require further study, including defining the highest-risk groups and refining screening tests with high sensitivity and specificity to detect high-grade precursors, along with more data on risks of precursor lesions themselves.
They also acknowledged the need for more study into the effectiveness of pancreatic cancer screening, although a randomized clinical trial comparing screening vs. no screening “might be challenging to conduct given implementation of clinical screening as standard of care in some practices.” Blood tests for pancreatic cancer screening in high-risk patients also need more study, they stated.
The authors did not report any funding sources or conflicts of interest.
SOURCE: Aslanian HR et al. Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.03.088.
Individuals at high risk for pancreatic cancer should at least be considered for screening for the disease, states a new clinical practice update from the American Gastroenterological Association that further defines what constitutes high risk for pancreatic cancer, when and how screenings should occur, and the role of genetic testing and counseling (Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.03.088).
Individuals who have a first-degree relative with two or more genetically related relatives with pancreatic cancer should be considered for screening, as should people with Peutz-Jeghers syndrome, a CDKN2A gene mutation, one or more first-degree relatives with pancreatic cancer with Lynch syndrome and mutations in the BRCA1, BRCA2, PALB2, and ATM genes, the clinical update stated. Screening in high-risk individuals should begin at age 50, but some groups should start having screening earlier: age 40 in carriers of the CKDN2A and PRSS1 mutations with hereditary pancreatitis; and age 35 in those with Peutz-Jeghers syndrome.
“Studies to date have demonstrated variability regarding definitions of high-risk groups and the age at which screening should be initiated,” wrote Harry R. Aslanian, MD, AGAF, of Yale New Haven (Conn.) Hospital, and coauthors. “The genetic basis of much of the inherited susceptibility to pancreas cancer remains unexplained (in approximately 90% of cases) and familial history is important in risk stratification.”
Genetic testing and counseling should be considered for any familial pancreas cancer relative – that is, a person with two or more first-degree relatives with pancreas cancer that’s outside the definition of other hereditary cancers. “A positive germline mutation is associated with an increased risk of neoplastic progression and may also lead to screening for other relevant associated cancers,” wrote Dr. Aslanian and coauthors.
The screening itself should consist of MRI and endoscopic ultrasonography (EUS) in combination, the clinical practice update states. It defines detectable targets as stage 1 pancreatic ductal adenocarcinoma and high-risk neoplasms such as intraductal papillary mucinous neoplasms with high-grade dysplasia and some enlarged pancreatic intraepithelial neoplasms.
High-risk patients having screening should also be enrolled in a registry and referred to a pancreas center of excellence.
The update suggests screening every 12 months when the baseline screening is negative for any suspect lesions, with shorter intervals for EUS when suspected lesions are found: 6-12 months for low-risk lesions; 3-6 months for intermediate lesions; and 3 months for high-risk lesions if the patient hasn’t had surgery to remove the lesions.
Regarding management of positive screening results, a multidisciplinary team should confer with the individual and family to determine therapy. If surgery is indicated, it should be done at a high-volume center.
The update also provides guidance for two scenarios when patients shouldn’t undergo screening: those at average risk; and those at high-risk more likely to die from another cause. Of course, the physician should review the limitations and risk of screening with patients beforehand.
Dr. Aslanian and coauthors noted that a number of areas require further study, including defining the highest-risk groups and refining screening tests with high sensitivity and specificity to detect high-grade precursors, along with more data on risks of precursor lesions themselves.
They also acknowledged the need for more study into the effectiveness of pancreatic cancer screening, although a randomized clinical trial comparing screening vs. no screening “might be challenging to conduct given implementation of clinical screening as standard of care in some practices.” Blood tests for pancreatic cancer screening in high-risk patients also need more study, they stated.
The authors did not report any funding sources or conflicts of interest.
SOURCE: Aslanian HR et al. Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.03.088.
Individuals at high risk for pancreatic cancer should at least be considered for screening for the disease, states a new clinical practice update from the American Gastroenterological Association that further defines what constitutes high risk for pancreatic cancer, when and how screenings should occur, and the role of genetic testing and counseling (Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.03.088).
Individuals who have a first-degree relative with two or more genetically related relatives with pancreatic cancer should be considered for screening, as should people with Peutz-Jeghers syndrome, a CDKN2A gene mutation, one or more first-degree relatives with pancreatic cancer with Lynch syndrome and mutations in the BRCA1, BRCA2, PALB2, and ATM genes, the clinical update stated. Screening in high-risk individuals should begin at age 50, but some groups should start having screening earlier: age 40 in carriers of the CKDN2A and PRSS1 mutations with hereditary pancreatitis; and age 35 in those with Peutz-Jeghers syndrome.
“Studies to date have demonstrated variability regarding definitions of high-risk groups and the age at which screening should be initiated,” wrote Harry R. Aslanian, MD, AGAF, of Yale New Haven (Conn.) Hospital, and coauthors. “The genetic basis of much of the inherited susceptibility to pancreas cancer remains unexplained (in approximately 90% of cases) and familial history is important in risk stratification.”
Genetic testing and counseling should be considered for any familial pancreas cancer relative – that is, a person with two or more first-degree relatives with pancreas cancer that’s outside the definition of other hereditary cancers. “A positive germline mutation is associated with an increased risk of neoplastic progression and may also lead to screening for other relevant associated cancers,” wrote Dr. Aslanian and coauthors.
The screening itself should consist of MRI and endoscopic ultrasonography (EUS) in combination, the clinical practice update states. It defines detectable targets as stage 1 pancreatic ductal adenocarcinoma and high-risk neoplasms such as intraductal papillary mucinous neoplasms with high-grade dysplasia and some enlarged pancreatic intraepithelial neoplasms.
High-risk patients having screening should also be enrolled in a registry and referred to a pancreas center of excellence.
The update suggests screening every 12 months when the baseline screening is negative for any suspect lesions, with shorter intervals for EUS when suspected lesions are found: 6-12 months for low-risk lesions; 3-6 months for intermediate lesions; and 3 months for high-risk lesions if the patient hasn’t had surgery to remove the lesions.
Regarding management of positive screening results, a multidisciplinary team should confer with the individual and family to determine therapy. If surgery is indicated, it should be done at a high-volume center.
The update also provides guidance for two scenarios when patients shouldn’t undergo screening: those at average risk; and those at high-risk more likely to die from another cause. Of course, the physician should review the limitations and risk of screening with patients beforehand.
Dr. Aslanian and coauthors noted that a number of areas require further study, including defining the highest-risk groups and refining screening tests with high sensitivity and specificity to detect high-grade precursors, along with more data on risks of precursor lesions themselves.
They also acknowledged the need for more study into the effectiveness of pancreatic cancer screening, although a randomized clinical trial comparing screening vs. no screening “might be challenging to conduct given implementation of clinical screening as standard of care in some practices.” Blood tests for pancreatic cancer screening in high-risk patients also need more study, they stated.
The authors did not report any funding sources or conflicts of interest.
SOURCE: Aslanian HR et al. Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.03.088.
Large study finds no link between gluten, IBD risk
Among women without celiac disease, dietary gluten intake was not associated with the risk of developing either Crohn’s disease or ulcerative colitis, investigators reported.
The findings spanned subgroups stratified by age, body mass index, smoking status, and whether individuals primarily consumed refined or whole grains, said Emily Walsh Lopes, MD, gastroenterology clinical and research fellow at Massachusetts General Hospital in Boston. She and associates reported the combined analysis of the prospective Nurses’ Health Study and Nurses’ Health Study II in an abstract released as part of the annual Digestive Disease Week.®
“Avoidance of dietary gluten is common, and many patients attribute gastrointestinal symptoms to gluten intake,” Dr. Lopes said in an interview. “Though our findings warrant further study, the results suggest to patients and providers that eating gluten does not increase a person’s chance of getting diagnosed with inflammatory bowel disease.”
Prior studies have found that many individuals with inflammatory bowel disease avoid gluten and report subsequent improvements in gastrointestinal symptoms, even if they do not have celiac disease. However, it remains unclear whether dietary gluten is a risk factor for new-onset inflammatory bowel disease.
To address this question, Dr. Lopes and associates analyzed data collected from 165,327 women who took part in the Nurses’ Health Study (1986 to 2016) or the Nurses’ Health Study II (1991 through 2017). None of the women had a preexisting diagnosis of celiac disease or inflammatory bowel disease. Dietary gluten intake was estimated based on food frequency questionnaires completed by the women at baseline and every 4 years. The researchers also reviewed medical records to confirm self-reported cases of new-onset ulcerative colitis and Crohn’s disease.
Over 4.02 million person-years of follow-up, 277 women developed Crohn’s disease and 359 developed ulcerative colitis. Gluten intake was not associated with the risk of either type of inflammatory bowel disease, even after the researchers controlled for multiple demographic and clinical risk factors.
After submitting their abstract, Dr. Lopes and coinvestigators expanded the dataset to include a large cohort of men from the prospective Health Professionals Follow-up Study. The final pooled cohort included more than 208,000 women and men followed for more than 20 years. Through the end of follow-up, the researchers documented 337 cases of Crohn’s disease and 446 cases of ulcerative colitis. “Inclusion of the male cohort in the pooled analysis did not materially change our estimates,” Dr. Lopes told MDedge. “That is, no association was seen between gluten intake and risk of either Crohn’s disease or ulcerative colitis in the final cohort.”
She noted that the findings cannot be extrapolated to individuals who are already diagnosed with inflammatory bowel disease. “It is possible that different mechanisms exist to explain how gluten intake impacts those already diagnosed with IBD, and this topic warrants further study,” she said. Also, because the three cohort studies were observational, they are subject to bias. “While we tried to account for this in our analyses, residual bias may still exist.”
Dr. Lopes reported having no conflicts of interest.
SOURCE: Walsh Lopes E et al. DDW 2020, abstract 847.
Among women without celiac disease, dietary gluten intake was not associated with the risk of developing either Crohn’s disease or ulcerative colitis, investigators reported.
The findings spanned subgroups stratified by age, body mass index, smoking status, and whether individuals primarily consumed refined or whole grains, said Emily Walsh Lopes, MD, gastroenterology clinical and research fellow at Massachusetts General Hospital in Boston. She and associates reported the combined analysis of the prospective Nurses’ Health Study and Nurses’ Health Study II in an abstract released as part of the annual Digestive Disease Week.®
“Avoidance of dietary gluten is common, and many patients attribute gastrointestinal symptoms to gluten intake,” Dr. Lopes said in an interview. “Though our findings warrant further study, the results suggest to patients and providers that eating gluten does not increase a person’s chance of getting diagnosed with inflammatory bowel disease.”
Prior studies have found that many individuals with inflammatory bowel disease avoid gluten and report subsequent improvements in gastrointestinal symptoms, even if they do not have celiac disease. However, it remains unclear whether dietary gluten is a risk factor for new-onset inflammatory bowel disease.
To address this question, Dr. Lopes and associates analyzed data collected from 165,327 women who took part in the Nurses’ Health Study (1986 to 2016) or the Nurses’ Health Study II (1991 through 2017). None of the women had a preexisting diagnosis of celiac disease or inflammatory bowel disease. Dietary gluten intake was estimated based on food frequency questionnaires completed by the women at baseline and every 4 years. The researchers also reviewed medical records to confirm self-reported cases of new-onset ulcerative colitis and Crohn’s disease.
Over 4.02 million person-years of follow-up, 277 women developed Crohn’s disease and 359 developed ulcerative colitis. Gluten intake was not associated with the risk of either type of inflammatory bowel disease, even after the researchers controlled for multiple demographic and clinical risk factors.
After submitting their abstract, Dr. Lopes and coinvestigators expanded the dataset to include a large cohort of men from the prospective Health Professionals Follow-up Study. The final pooled cohort included more than 208,000 women and men followed for more than 20 years. Through the end of follow-up, the researchers documented 337 cases of Crohn’s disease and 446 cases of ulcerative colitis. “Inclusion of the male cohort in the pooled analysis did not materially change our estimates,” Dr. Lopes told MDedge. “That is, no association was seen between gluten intake and risk of either Crohn’s disease or ulcerative colitis in the final cohort.”
She noted that the findings cannot be extrapolated to individuals who are already diagnosed with inflammatory bowel disease. “It is possible that different mechanisms exist to explain how gluten intake impacts those already diagnosed with IBD, and this topic warrants further study,” she said. Also, because the three cohort studies were observational, they are subject to bias. “While we tried to account for this in our analyses, residual bias may still exist.”
Dr. Lopes reported having no conflicts of interest.
SOURCE: Walsh Lopes E et al. DDW 2020, abstract 847.
Among women without celiac disease, dietary gluten intake was not associated with the risk of developing either Crohn’s disease or ulcerative colitis, investigators reported.
The findings spanned subgroups stratified by age, body mass index, smoking status, and whether individuals primarily consumed refined or whole grains, said Emily Walsh Lopes, MD, gastroenterology clinical and research fellow at Massachusetts General Hospital in Boston. She and associates reported the combined analysis of the prospective Nurses’ Health Study and Nurses’ Health Study II in an abstract released as part of the annual Digestive Disease Week.®
“Avoidance of dietary gluten is common, and many patients attribute gastrointestinal symptoms to gluten intake,” Dr. Lopes said in an interview. “Though our findings warrant further study, the results suggest to patients and providers that eating gluten does not increase a person’s chance of getting diagnosed with inflammatory bowel disease.”
Prior studies have found that many individuals with inflammatory bowel disease avoid gluten and report subsequent improvements in gastrointestinal symptoms, even if they do not have celiac disease. However, it remains unclear whether dietary gluten is a risk factor for new-onset inflammatory bowel disease.
To address this question, Dr. Lopes and associates analyzed data collected from 165,327 women who took part in the Nurses’ Health Study (1986 to 2016) or the Nurses’ Health Study II (1991 through 2017). None of the women had a preexisting diagnosis of celiac disease or inflammatory bowel disease. Dietary gluten intake was estimated based on food frequency questionnaires completed by the women at baseline and every 4 years. The researchers also reviewed medical records to confirm self-reported cases of new-onset ulcerative colitis and Crohn’s disease.
Over 4.02 million person-years of follow-up, 277 women developed Crohn’s disease and 359 developed ulcerative colitis. Gluten intake was not associated with the risk of either type of inflammatory bowel disease, even after the researchers controlled for multiple demographic and clinical risk factors.
After submitting their abstract, Dr. Lopes and coinvestigators expanded the dataset to include a large cohort of men from the prospective Health Professionals Follow-up Study. The final pooled cohort included more than 208,000 women and men followed for more than 20 years. Through the end of follow-up, the researchers documented 337 cases of Crohn’s disease and 446 cases of ulcerative colitis. “Inclusion of the male cohort in the pooled analysis did not materially change our estimates,” Dr. Lopes told MDedge. “That is, no association was seen between gluten intake and risk of either Crohn’s disease or ulcerative colitis in the final cohort.”
She noted that the findings cannot be extrapolated to individuals who are already diagnosed with inflammatory bowel disease. “It is possible that different mechanisms exist to explain how gluten intake impacts those already diagnosed with IBD, and this topic warrants further study,” she said. Also, because the three cohort studies were observational, they are subject to bias. “While we tried to account for this in our analyses, residual bias may still exist.”
Dr. Lopes reported having no conflicts of interest.
SOURCE: Walsh Lopes E et al. DDW 2020, abstract 847.
FROM DDW 2020
Topical Clobetasol Propionate Treatment and Cutaneous Adverse Effects in Patients With Early-Stage Mycosis Fungoides: An Observational Study
Mycosis fungoides (MF), the most common variant of cutaneous T-cell lymphoma, is a non-Hodgkin lymphoma of T-cell origin that primarily develops in the skin and has a chronic relapsing course. Early-stage MF (stages IA–IIA) is defined as papules, patches, or plaques with limited (if any) lymph node and blood involvement and no visceral involvement.1 Early-stage MF has a favorable prognosis, and first-line treatments are skin-directed therapies including topical corticosteroids (CSs), topical chemotherapy (nitrogen mustard or carmustine), topical retinoids, topical imiquimod, local radiation, or phototherapy.2 Topical CSs are effective in treating early-stage MF and have been widely used for this indication for several decades; however, there are very little data in the literature on topical CS use in MF.3 Superpotent topical CSs have been shown to have a high overall response rate in early-stage MF3; however, cutaneous side effects associated with long-term topical use include cutaneous atrophy, striae formation, skin fragility, and irritation.
The US Food and Drug Administration (FDA) approved bexarotene gel and mechlorethamine gel for topical treatment of cutaneous lesions in patients with stage IA and IB MF in 2000 and 2013, respectively. Although each may be effective in achieving complete or partial response in MF, both agents are associated with cutaneous side effects, mainly irritation and frequent contact hypersensitivity reactions, respectively.4,5 Additionally, their high prices and limited availability are other major drawbacks of treatment.
At our institution, high-potency topical CSs, specifically once or twice daily clobetasol propionate cream 0.05% prescribed as monotherapy for at least several months, remain the mainstay of treatment in patients with limited patches, papules, and plaques covering less than 10% of the skin surface (stage IA). In this study, we aimed to assess the risk of cutaneous side effects in patients with early-stage MF who were treated with long-term, high-potency topical CSs.
Methods
This prospective observational cohort study included patients with early-stage MF who were seen at the Cutaneous Lymphoma Clinic at Memorial Sloan Kettering Cancer Center (MSKCC) in New York, New York, and were started on a superpotent (class I) topical CS (clobetasol propionate cream 0.05%) as monotherapy for MF from July 2016 to July 2017. The diagnosis of MF had to be supported by clinical findings and histopathologic features. All patients were Fitzpatrick skin types I, II, or III. Eligible patients were evaluated for development of CS-induced cutaneous AEs by physical examination and clinical photography of the treated lesions performed at baseline and as part of routine follow-up visits (usually scheduled every 2 to 6 months) at the MSKCC Cutaneous Lymphoma Clinic. Patients’ skin was evaluated clinically for MF activity, atrophy, telangiectasia, purpura, hypopigmentation, and stretch marks (striae). Use of the topical CS was self-reported and also was documented at follow-up visits. Treatment response was defined as follows: complete clinical response (CCR) if the treated lesions resolved completely compared to initial photography; minimal active disease (MAD) if resolution of the vast majority (≥75%) of lesions was seen; and partial response (PR) if some of the lesions resolved (<75%). We analyzed the treatment response rates and adverse effects (AEs). Results were summarized using descriptive statistics.
Results
We identified 13 patients who were started on topical clobetasol propionate as monotherapy for early-stage MF during the study period. Our cohort included 6 males and 7 females aged 36 to 76 years (median age, 61 years). All but 1 participant were diagnosed with stage IA MF (12/13 [92.3%]); of those, 9 (75.0%) had patch-stage disease and 3 (25.0%) presented with plaques. One (7.7%) participant presented with hyperpigmented patches and plaques that involved a little more than 10% of the skin surface (stage IB), and involvement of the hair follicles was noted on histology (folliculotropic MF). All prior treatments were stopped when participants started the superpotent topical CS: 6 (46.2%) participants had been treated with lower-potency topical agents and 1 (7.7%) participant was getting psoralen plus UVA therapy, while the other 6 (46.2%) participants were receiving no therapy for MF prior to starting the study. All participants were prescribed clobetasol propionate cream 0.05% once or twice daily as monotherapy and were instructed to apply it to the MF lesions only, avoiding skin folds and the face. One participant was lost to follow-up, and another stopped using the clobetasol propionate cream after 1.5 months due to local irritation associated with treatment. At their follow-up visits, the other 11 participants were advised to continue with once-daily treatment with clobetasol propionate or were tapered to once every other day, twice weekly, or once weekly depending on their response to treatment and AEs (Table). Participants were advised not to use more than 50 g of clobetasol propionate cream weekly.
All participants responded to the clobetasol propionate cream, and improvement was noted in the treated lesions; however, progression of disease (from stage IA to stage IB) occurred in 1 (8.3%) participant, and phototherapy was added with good response. The participants in our cohort were followed for 4 to 17 months (median, 11.5 months). At the last follow-up visit, all 12 participants showed treatment response: 4 (33.3%) had CCR, 5 (41.7%) had MAD; and 3 (25.0%) had PR. In one participant with a history of partial response to bexarotene gel 1%, daily clobetasol propionate cream 0.05% initially was used alone for 9 months and was later combined with bexarotene gel once weekly, resulting in MAD.
In 7 (58.3%) participants, no AEs to topical clobetasol propionate were recorded. Four (33.3%) participants developed local hypopigmentation at the application site, and 2 (16.7%) developed cutaneous atrophy with local fine wrinkling of the skin (Figure 1); none of the participants developed stretch marks (striae), telangiectases, or skin fragility. One (8.3%) participant developed a petechial rash at the clobetasol propionate application site that resolved once treatment was discontinued and did not recur after restarting clobetasol propionate twice weekly.
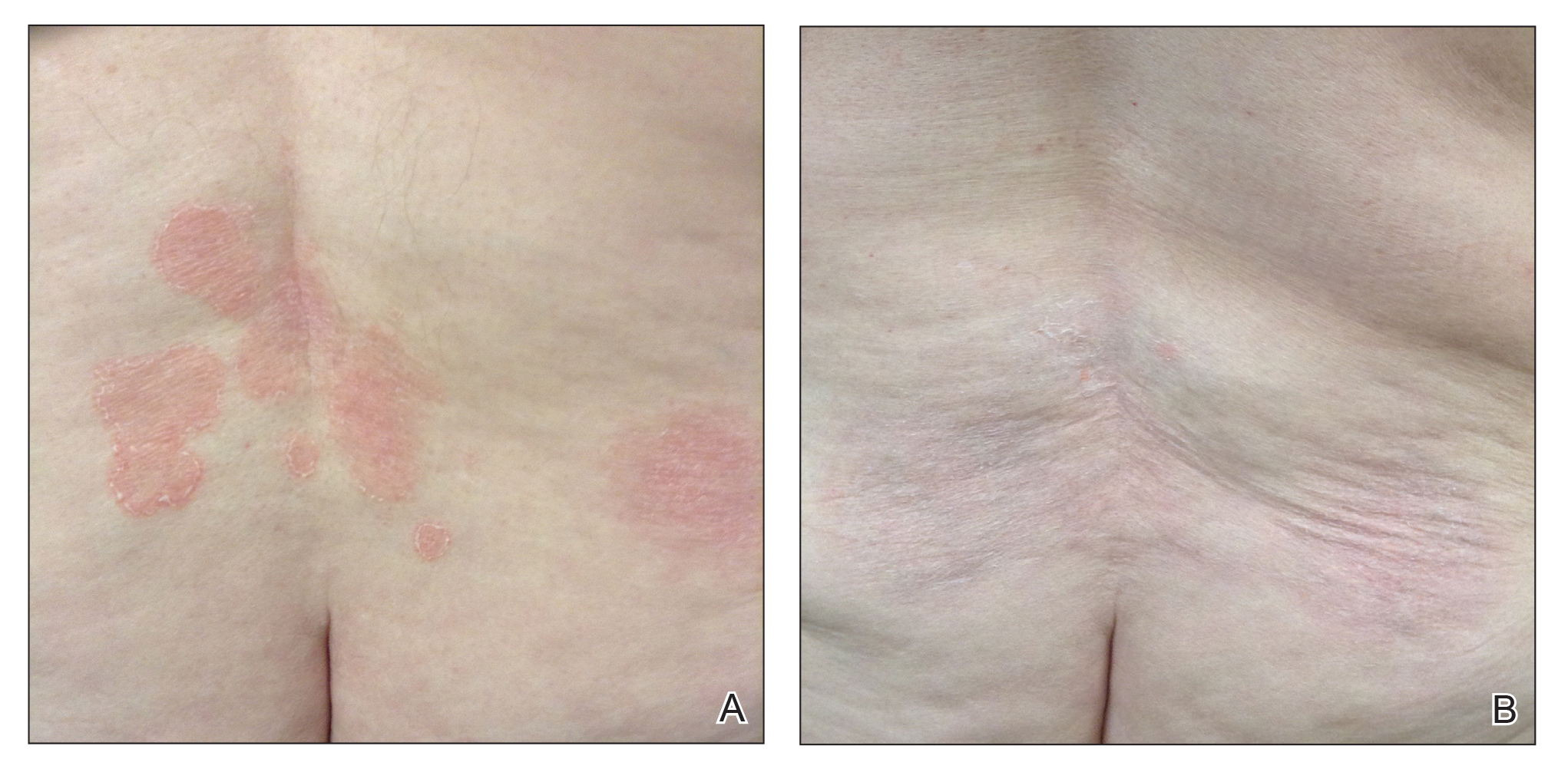
Comment
Topical CSs are the most commonly prescribed agents, either as monotherapy or in combination with other agents, in the treatment of numerous dermatologic conditions, including cutaneous T-cell lymphoma and MF. Cutaneous and systemic AEs have been associated with topical CS use. Local AEs are encountered more frequently and include cutaneous atrophy, striae, telangiectasia, purpura, skin fragility, hypopigmentation, hyperpigmentation, acneform eruptions, and hypertrichosis.6 Factors other than potency of the topical CS agent may affect the development of skin atrophy, including anatomic location, duration of therapy, vehicle, and method and frequency of application.7 The potential for systemic AEs due to percutaneous absorption of high-potency CSs, specifically Cushing syndrome and pathologic adrenal suppression, has been a long-standing concern and led the FDA to recommend limiting the use of superpotent CSs to 50 g weekly for 2 or 4 consecutive weeks.8 However, if using an excess of 50 g weekly is avoided, superpotent topical CSs may be safe to use consecutively for months, perhaps even years, without causing systemic effects.9
The effects of topical CSs in MF include induction of apoptosis; inhibition of lymphocyte binding to the endothelium; and downregulation of transcription factors with decreased cytokines, adhesion molecules, and production of growth factors.2 For patients with limited early-stage MF patches and thin plaques, topical CSs often control the disease for many years and frequently are the only form of therapy required. Intralesional steroids can be effective in treating thicker lesions, such as plaques or tumors.10 In an uncontrolled study, Zackheim et al11 prospectively evaluated the effectiveness and safety of twice-daily use of mainly high-potency topical CSs in 79 patients with MF stages IA to IB and observed an overall response rate of 94%. None of the patients were using systemic agents while being treated with topical CSs. Adverse effects were rare: 2 (2.5%) patients experienced temporary minor irritation from the topical CS, 1 (1.3%) patient developed localized skin atrophy under the breast that resolved several months after she stopped treatment, and 1 (1.3%) patient developed stretch marks on the thighs.11 Zackheim12 later reported treatment of approximately 200 patients with class I topical CSs, and overall response rates were over 90% in stage T1 and over 80% in stage T2 patients. Response to topical CS was reported to be evident within 3 months and often much sooner. Side effects were most likely related to the more prolonged treatment periods. Irritant dermatitis or purpura developed in approximately 10% to 20% of patients, and purpura was seen at the sites of treatment as well as at distant sites. Only a small number of patients developed cutaneous atrophy and striae, which were reversible.12 Successful use of intralesional steroids for treatment-resistant MF was reported in 4 patients who tolerated treatment well without any side effects other than local hypopigmentation in a single patient.13
At MSKCC, the first line of treatment in localized (stage IA) MF in light-skinned individuals most frequently is class I topical CSs, usually clobetasol propionate cream 0.05%. Patients are instructed to apply the cream twice daily on active MF lesions uninterruptedly until completely clear and to avoid using it on the face and in skin folds (axillary, inguinal, and abdominal). Patients are instructed to observe themselves for possible cutaneous AEs related to treatment and to stop or taper treatment if any AEs are noticed. In patients with darker skin, we may recommend other modalities such as narrowband UVB phototherapy for even limited MF disease because of the risk for uneven/hypopigmentation with superpotent CSs.
The current study offers a real-life observation of topical high-potency CSs for treatment of early-stage MF and the associated cutaneous AEs. Local hypopigmentation was identified in 4 participants (33.3%), local skin atrophy was seen in 2 participants (16.7%), and local purpura and irritation were seen in 1 participant each (8.3%). All patients responded to therapy and 75.0% (9/12) achieved CCR or showed only MAD at their last follow-up visit. The limitations of our study were the small number of patients included and the relatively short follow-up period.
In MF patients, patches can present as fine wrinkling of the skin resembling atrophy, which can make it difficult to differentiate active MF from CS-induced atrophy in patients treated with topical CSs (Figure 1) and may have caused us to overestimate the occurrence of this AE. Corticosteroid-induced skin atrophy has been studied mainly in normal skin and to a lesser extent in pathological skin in psoriasis and atopic dermatitis. Some of these studies reported that CS-induced atrophy is reversible, and skin thickness can return to normal after topical application of CS is stopped.7
When hypopigmentation is seen around MF lesions, it is a confirmation that the patient is compliant with the therapy. From our experience, local hypopigmentation due to topical CSs is reversible (Figure 2). In some cases, MF patients have applied topical clobetasol propionate to lesional and surrounding skin, and hypopigmentation can be lessened with more careful limited application. In most cases, after discontinuation or tapering of the therapy, the skin returns to its normal color.
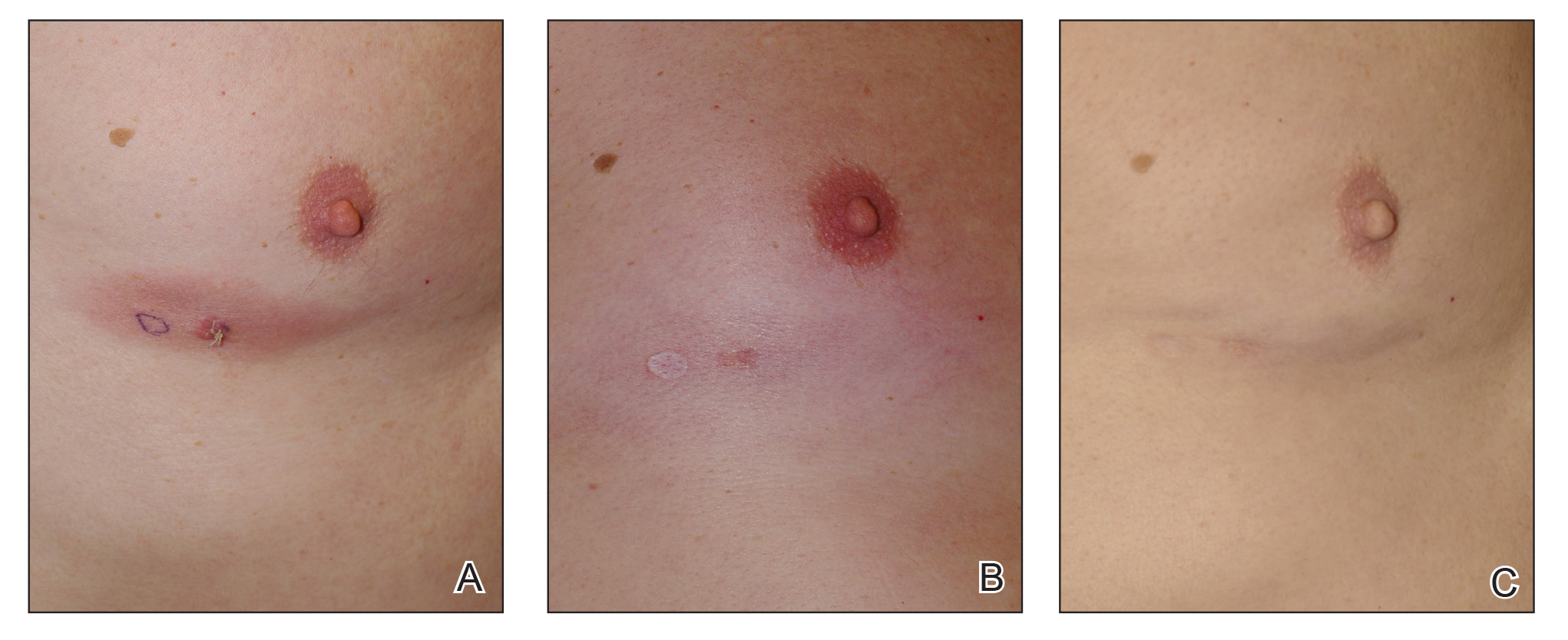
Based on our experience and the results of the current study, we conclude that topical superpotent CSs should remain the first-choice treatment for patients with early-stage MF (stage IA). Although bexarotene gel and mechlorethamine gel are FDA approved for early-stage MF, they are not widely available outside of the United States and are associated with AEs, mainly local skin irritation, rash, and pruritus.4,5 In contrast to bexarotene gel and mechlorethamine gel, topical clobetasol propionate can be used in young children (>12 years) and is classified as pregnancy category C.8
Conclusion
Patients with early-stage MF should be treated with skin-directed therapies, and the choice between different therapeutic options is made based on the physician’s experience with the treatment, patient characteristics, location and morphology of the MF lesions, and the AE profile of the treatment. Based on our experience, superpotent topical CSs are readily available and easily applied, have minor side effects, and remain the mainstay of therapy in patients with stage IA disease. Patients with MF on superpotent topical CS therapy should be monitored periodically and instructed how to identify cutaneous AEs related to treatment.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sezary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e221-217; quiz 240-222.
- Weberschock T, Strametz R, Lorenz M, et al. Interventions for mycosis fungoides [published online September 12, 2012]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD008946.pub2.
- Heald P, Mehlmauer M, Martin AG, et al. Topical bexarotene therapy for patients with refractory or persistent early-stage cutaneous T-cell lymphoma: results of the phase III clinical trial. J Am Acad Dermatol. 2003;49:801-815.
- Lessin SR, Duvic M, Guitart J, et al. Topical chemotherapy in cutaneous T-cell lymphoma: positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. 2013;149:25-32.
- Tadicherla S, Ross K, Shenefelt PD, et al. Topical corticosteroids in dermatology. J Drugs Dermatol. 2009;8:1093-1105.
- Barnes L, Kaya G, Rollason V. Topical corticosteroid-induced skin atrophy: a comprehensive review. Drug Saf. 2015;38:493-509.
- Temovate E (Clobetasol Propionate) Cream, 0.05% [package insert]. Melville, NY: PharmaDerm, a division of Fougera Pharmaceuticals Inc; 2012.
- Nakamura M, Abrouk M, Zhu H, et al. Update on the systemic risks of superpotent topical steroids. J Drugs Dermatol. 2017;16:643-648.
- Prince HM, Whittaker S, Hoppe RT. How I treat mycosis fungoides and Sezary syndrome. Blood. 2009;114:4337-4353.
- Zackheim HS, Kashani-Sabet M, Amin S. Topical corticosteroids for mycosis fungoides. experience in 79 patients. Arch Dermatol. 1998;134:949-954.
- Zackheim HS. Treatment of patch-stage mycosis fungoides with topical corticosteroids. Dermatol Ther. 2003;16:283-287.
- Liu DY, Shaath T, Rajpara AN, et al. Safe and efficacious use of intralesional steroids for the treatment of focally resistant mycosis fungoides. J Drugs Dermatol. 2015;14:466-471.
Mycosis fungoides (MF), the most common variant of cutaneous T-cell lymphoma, is a non-Hodgkin lymphoma of T-cell origin that primarily develops in the skin and has a chronic relapsing course. Early-stage MF (stages IA–IIA) is defined as papules, patches, or plaques with limited (if any) lymph node and blood involvement and no visceral involvement.1 Early-stage MF has a favorable prognosis, and first-line treatments are skin-directed therapies including topical corticosteroids (CSs), topical chemotherapy (nitrogen mustard or carmustine), topical retinoids, topical imiquimod, local radiation, or phototherapy.2 Topical CSs are effective in treating early-stage MF and have been widely used for this indication for several decades; however, there are very little data in the literature on topical CS use in MF.3 Superpotent topical CSs have been shown to have a high overall response rate in early-stage MF3; however, cutaneous side effects associated with long-term topical use include cutaneous atrophy, striae formation, skin fragility, and irritation.
The US Food and Drug Administration (FDA) approved bexarotene gel and mechlorethamine gel for topical treatment of cutaneous lesions in patients with stage IA and IB MF in 2000 and 2013, respectively. Although each may be effective in achieving complete or partial response in MF, both agents are associated with cutaneous side effects, mainly irritation and frequent contact hypersensitivity reactions, respectively.4,5 Additionally, their high prices and limited availability are other major drawbacks of treatment.
At our institution, high-potency topical CSs, specifically once or twice daily clobetasol propionate cream 0.05% prescribed as monotherapy for at least several months, remain the mainstay of treatment in patients with limited patches, papules, and plaques covering less than 10% of the skin surface (stage IA). In this study, we aimed to assess the risk of cutaneous side effects in patients with early-stage MF who were treated with long-term, high-potency topical CSs.
Methods
This prospective observational cohort study included patients with early-stage MF who were seen at the Cutaneous Lymphoma Clinic at Memorial Sloan Kettering Cancer Center (MSKCC) in New York, New York, and were started on a superpotent (class I) topical CS (clobetasol propionate cream 0.05%) as monotherapy for MF from July 2016 to July 2017. The diagnosis of MF had to be supported by clinical findings and histopathologic features. All patients were Fitzpatrick skin types I, II, or III. Eligible patients were evaluated for development of CS-induced cutaneous AEs by physical examination and clinical photography of the treated lesions performed at baseline and as part of routine follow-up visits (usually scheduled every 2 to 6 months) at the MSKCC Cutaneous Lymphoma Clinic. Patients’ skin was evaluated clinically for MF activity, atrophy, telangiectasia, purpura, hypopigmentation, and stretch marks (striae). Use of the topical CS was self-reported and also was documented at follow-up visits. Treatment response was defined as follows: complete clinical response (CCR) if the treated lesions resolved completely compared to initial photography; minimal active disease (MAD) if resolution of the vast majority (≥75%) of lesions was seen; and partial response (PR) if some of the lesions resolved (<75%). We analyzed the treatment response rates and adverse effects (AEs). Results were summarized using descriptive statistics.
Results
We identified 13 patients who were started on topical clobetasol propionate as monotherapy for early-stage MF during the study period. Our cohort included 6 males and 7 females aged 36 to 76 years (median age, 61 years). All but 1 participant were diagnosed with stage IA MF (12/13 [92.3%]); of those, 9 (75.0%) had patch-stage disease and 3 (25.0%) presented with plaques. One (7.7%) participant presented with hyperpigmented patches and plaques that involved a little more than 10% of the skin surface (stage IB), and involvement of the hair follicles was noted on histology (folliculotropic MF). All prior treatments were stopped when participants started the superpotent topical CS: 6 (46.2%) participants had been treated with lower-potency topical agents and 1 (7.7%) participant was getting psoralen plus UVA therapy, while the other 6 (46.2%) participants were receiving no therapy for MF prior to starting the study. All participants were prescribed clobetasol propionate cream 0.05% once or twice daily as monotherapy and were instructed to apply it to the MF lesions only, avoiding skin folds and the face. One participant was lost to follow-up, and another stopped using the clobetasol propionate cream after 1.5 months due to local irritation associated with treatment. At their follow-up visits, the other 11 participants were advised to continue with once-daily treatment with clobetasol propionate or were tapered to once every other day, twice weekly, or once weekly depending on their response to treatment and AEs (Table). Participants were advised not to use more than 50 g of clobetasol propionate cream weekly.

All participants responded to the clobetasol propionate cream, and improvement was noted in the treated lesions; however, progression of disease (from stage IA to stage IB) occurred in 1 (8.3%) participant, and phototherapy was added with good response. The participants in our cohort were followed for 4 to 17 months (median, 11.5 months). At the last follow-up visit, all 12 participants showed treatment response: 4 (33.3%) had CCR, 5 (41.7%) had MAD; and 3 (25.0%) had PR. In one participant with a history of partial response to bexarotene gel 1%, daily clobetasol propionate cream 0.05% initially was used alone for 9 months and was later combined with bexarotene gel once weekly, resulting in MAD.
In 7 (58.3%) participants, no AEs to topical clobetasol propionate were recorded. Four (33.3%) participants developed local hypopigmentation at the application site, and 2 (16.7%) developed cutaneous atrophy with local fine wrinkling of the skin (Figure 1); none of the participants developed stretch marks (striae), telangiectases, or skin fragility. One (8.3%) participant developed a petechial rash at the clobetasol propionate application site that resolved once treatment was discontinued and did not recur after restarting clobetasol propionate twice weekly.

Comment
Topical CSs are the most commonly prescribed agents, either as monotherapy or in combination with other agents, in the treatment of numerous dermatologic conditions, including cutaneous T-cell lymphoma and MF. Cutaneous and systemic AEs have been associated with topical CS use. Local AEs are encountered more frequently and include cutaneous atrophy, striae, telangiectasia, purpura, skin fragility, hypopigmentation, hyperpigmentation, acneform eruptions, and hypertrichosis.6 Factors other than potency of the topical CS agent may affect the development of skin atrophy, including anatomic location, duration of therapy, vehicle, and method and frequency of application.7 The potential for systemic AEs due to percutaneous absorption of high-potency CSs, specifically Cushing syndrome and pathologic adrenal suppression, has been a long-standing concern and led the FDA to recommend limiting the use of superpotent CSs to 50 g weekly for 2 or 4 consecutive weeks.8 However, if using an excess of 50 g weekly is avoided, superpotent topical CSs may be safe to use consecutively for months, perhaps even years, without causing systemic effects.9
The effects of topical CSs in MF include induction of apoptosis; inhibition of lymphocyte binding to the endothelium; and downregulation of transcription factors with decreased cytokines, adhesion molecules, and production of growth factors.2 For patients with limited early-stage MF patches and thin plaques, topical CSs often control the disease for many years and frequently are the only form of therapy required. Intralesional steroids can be effective in treating thicker lesions, such as plaques or tumors.10 In an uncontrolled study, Zackheim et al11 prospectively evaluated the effectiveness and safety of twice-daily use of mainly high-potency topical CSs in 79 patients with MF stages IA to IB and observed an overall response rate of 94%. None of the patients were using systemic agents while being treated with topical CSs. Adverse effects were rare: 2 (2.5%) patients experienced temporary minor irritation from the topical CS, 1 (1.3%) patient developed localized skin atrophy under the breast that resolved several months after she stopped treatment, and 1 (1.3%) patient developed stretch marks on the thighs.11 Zackheim12 later reported treatment of approximately 200 patients with class I topical CSs, and overall response rates were over 90% in stage T1 and over 80% in stage T2 patients. Response to topical CS was reported to be evident within 3 months and often much sooner. Side effects were most likely related to the more prolonged treatment periods. Irritant dermatitis or purpura developed in approximately 10% to 20% of patients, and purpura was seen at the sites of treatment as well as at distant sites. Only a small number of patients developed cutaneous atrophy and striae, which were reversible.12 Successful use of intralesional steroids for treatment-resistant MF was reported in 4 patients who tolerated treatment well without any side effects other than local hypopigmentation in a single patient.13
At MSKCC, the first line of treatment in localized (stage IA) MF in light-skinned individuals most frequently is class I topical CSs, usually clobetasol propionate cream 0.05%. Patients are instructed to apply the cream twice daily on active MF lesions uninterruptedly until completely clear and to avoid using it on the face and in skin folds (axillary, inguinal, and abdominal). Patients are instructed to observe themselves for possible cutaneous AEs related to treatment and to stop or taper treatment if any AEs are noticed. In patients with darker skin, we may recommend other modalities such as narrowband UVB phototherapy for even limited MF disease because of the risk for uneven/hypopigmentation with superpotent CSs.
The current study offers a real-life observation of topical high-potency CSs for treatment of early-stage MF and the associated cutaneous AEs. Local hypopigmentation was identified in 4 participants (33.3%), local skin atrophy was seen in 2 participants (16.7%), and local purpura and irritation were seen in 1 participant each (8.3%). All patients responded to therapy and 75.0% (9/12) achieved CCR or showed only MAD at their last follow-up visit. The limitations of our study were the small number of patients included and the relatively short follow-up period.
In MF patients, patches can present as fine wrinkling of the skin resembling atrophy, which can make it difficult to differentiate active MF from CS-induced atrophy in patients treated with topical CSs (Figure 1) and may have caused us to overestimate the occurrence of this AE. Corticosteroid-induced skin atrophy has been studied mainly in normal skin and to a lesser extent in pathological skin in psoriasis and atopic dermatitis. Some of these studies reported that CS-induced atrophy is reversible, and skin thickness can return to normal after topical application of CS is stopped.7
When hypopigmentation is seen around MF lesions, it is a confirmation that the patient is compliant with the therapy. From our experience, local hypopigmentation due to topical CSs is reversible (Figure 2). In some cases, MF patients have applied topical clobetasol propionate to lesional and surrounding skin, and hypopigmentation can be lessened with more careful limited application. In most cases, after discontinuation or tapering of the therapy, the skin returns to its normal color.

Based on our experience and the results of the current study, we conclude that topical superpotent CSs should remain the first-choice treatment for patients with early-stage MF (stage IA). Although bexarotene gel and mechlorethamine gel are FDA approved for early-stage MF, they are not widely available outside of the United States and are associated with AEs, mainly local skin irritation, rash, and pruritus.4,5 In contrast to bexarotene gel and mechlorethamine gel, topical clobetasol propionate can be used in young children (>12 years) and is classified as pregnancy category C.8
Conclusion
Patients with early-stage MF should be treated with skin-directed therapies, and the choice between different therapeutic options is made based on the physician’s experience with the treatment, patient characteristics, location and morphology of the MF lesions, and the AE profile of the treatment. Based on our experience, superpotent topical CSs are readily available and easily applied, have minor side effects, and remain the mainstay of therapy in patients with stage IA disease. Patients with MF on superpotent topical CS therapy should be monitored periodically and instructed how to identify cutaneous AEs related to treatment.
Mycosis fungoides (MF), the most common variant of cutaneous T-cell lymphoma, is a non-Hodgkin lymphoma of T-cell origin that primarily develops in the skin and has a chronic relapsing course. Early-stage MF (stages IA–IIA) is defined as papules, patches, or plaques with limited (if any) lymph node and blood involvement and no visceral involvement.1 Early-stage MF has a favorable prognosis, and first-line treatments are skin-directed therapies including topical corticosteroids (CSs), topical chemotherapy (nitrogen mustard or carmustine), topical retinoids, topical imiquimod, local radiation, or phototherapy.2 Topical CSs are effective in treating early-stage MF and have been widely used for this indication for several decades; however, there are very little data in the literature on topical CS use in MF.3 Superpotent topical CSs have been shown to have a high overall response rate in early-stage MF3; however, cutaneous side effects associated with long-term topical use include cutaneous atrophy, striae formation, skin fragility, and irritation.
The US Food and Drug Administration (FDA) approved bexarotene gel and mechlorethamine gel for topical treatment of cutaneous lesions in patients with stage IA and IB MF in 2000 and 2013, respectively. Although each may be effective in achieving complete or partial response in MF, both agents are associated with cutaneous side effects, mainly irritation and frequent contact hypersensitivity reactions, respectively.4,5 Additionally, their high prices and limited availability are other major drawbacks of treatment.
At our institution, high-potency topical CSs, specifically once or twice daily clobetasol propionate cream 0.05% prescribed as monotherapy for at least several months, remain the mainstay of treatment in patients with limited patches, papules, and plaques covering less than 10% of the skin surface (stage IA). In this study, we aimed to assess the risk of cutaneous side effects in patients with early-stage MF who were treated with long-term, high-potency topical CSs.
Methods
This prospective observational cohort study included patients with early-stage MF who were seen at the Cutaneous Lymphoma Clinic at Memorial Sloan Kettering Cancer Center (MSKCC) in New York, New York, and were started on a superpotent (class I) topical CS (clobetasol propionate cream 0.05%) as monotherapy for MF from July 2016 to July 2017. The diagnosis of MF had to be supported by clinical findings and histopathologic features. All patients were Fitzpatrick skin types I, II, or III. Eligible patients were evaluated for development of CS-induced cutaneous AEs by physical examination and clinical photography of the treated lesions performed at baseline and as part of routine follow-up visits (usually scheduled every 2 to 6 months) at the MSKCC Cutaneous Lymphoma Clinic. Patients’ skin was evaluated clinically for MF activity, atrophy, telangiectasia, purpura, hypopigmentation, and stretch marks (striae). Use of the topical CS was self-reported and also was documented at follow-up visits. Treatment response was defined as follows: complete clinical response (CCR) if the treated lesions resolved completely compared to initial photography; minimal active disease (MAD) if resolution of the vast majority (≥75%) of lesions was seen; and partial response (PR) if some of the lesions resolved (<75%). We analyzed the treatment response rates and adverse effects (AEs). Results were summarized using descriptive statistics.
Results
We identified 13 patients who were started on topical clobetasol propionate as monotherapy for early-stage MF during the study period. Our cohort included 6 males and 7 females aged 36 to 76 years (median age, 61 years). All but 1 participant were diagnosed with stage IA MF (12/13 [92.3%]); of those, 9 (75.0%) had patch-stage disease and 3 (25.0%) presented with plaques. One (7.7%) participant presented with hyperpigmented patches and plaques that involved a little more than 10% of the skin surface (stage IB), and involvement of the hair follicles was noted on histology (folliculotropic MF). All prior treatments were stopped when participants started the superpotent topical CS: 6 (46.2%) participants had been treated with lower-potency topical agents and 1 (7.7%) participant was getting psoralen plus UVA therapy, while the other 6 (46.2%) participants were receiving no therapy for MF prior to starting the study. All participants were prescribed clobetasol propionate cream 0.05% once or twice daily as monotherapy and were instructed to apply it to the MF lesions only, avoiding skin folds and the face. One participant was lost to follow-up, and another stopped using the clobetasol propionate cream after 1.5 months due to local irritation associated with treatment. At their follow-up visits, the other 11 participants were advised to continue with once-daily treatment with clobetasol propionate or were tapered to once every other day, twice weekly, or once weekly depending on their response to treatment and AEs (Table). Participants were advised not to use more than 50 g of clobetasol propionate cream weekly.

All participants responded to the clobetasol propionate cream, and improvement was noted in the treated lesions; however, progression of disease (from stage IA to stage IB) occurred in 1 (8.3%) participant, and phototherapy was added with good response. The participants in our cohort were followed for 4 to 17 months (median, 11.5 months). At the last follow-up visit, all 12 participants showed treatment response: 4 (33.3%) had CCR, 5 (41.7%) had MAD; and 3 (25.0%) had PR. In one participant with a history of partial response to bexarotene gel 1%, daily clobetasol propionate cream 0.05% initially was used alone for 9 months and was later combined with bexarotene gel once weekly, resulting in MAD.
In 7 (58.3%) participants, no AEs to topical clobetasol propionate were recorded. Four (33.3%) participants developed local hypopigmentation at the application site, and 2 (16.7%) developed cutaneous atrophy with local fine wrinkling of the skin (Figure 1); none of the participants developed stretch marks (striae), telangiectases, or skin fragility. One (8.3%) participant developed a petechial rash at the clobetasol propionate application site that resolved once treatment was discontinued and did not recur after restarting clobetasol propionate twice weekly.

Comment
Topical CSs are the most commonly prescribed agents, either as monotherapy or in combination with other agents, in the treatment of numerous dermatologic conditions, including cutaneous T-cell lymphoma and MF. Cutaneous and systemic AEs have been associated with topical CS use. Local AEs are encountered more frequently and include cutaneous atrophy, striae, telangiectasia, purpura, skin fragility, hypopigmentation, hyperpigmentation, acneform eruptions, and hypertrichosis.6 Factors other than potency of the topical CS agent may affect the development of skin atrophy, including anatomic location, duration of therapy, vehicle, and method and frequency of application.7 The potential for systemic AEs due to percutaneous absorption of high-potency CSs, specifically Cushing syndrome and pathologic adrenal suppression, has been a long-standing concern and led the FDA to recommend limiting the use of superpotent CSs to 50 g weekly for 2 or 4 consecutive weeks.8 However, if using an excess of 50 g weekly is avoided, superpotent topical CSs may be safe to use consecutively for months, perhaps even years, without causing systemic effects.9
The effects of topical CSs in MF include induction of apoptosis; inhibition of lymphocyte binding to the endothelium; and downregulation of transcription factors with decreased cytokines, adhesion molecules, and production of growth factors.2 For patients with limited early-stage MF patches and thin plaques, topical CSs often control the disease for many years and frequently are the only form of therapy required. Intralesional steroids can be effective in treating thicker lesions, such as plaques or tumors.10 In an uncontrolled study, Zackheim et al11 prospectively evaluated the effectiveness and safety of twice-daily use of mainly high-potency topical CSs in 79 patients with MF stages IA to IB and observed an overall response rate of 94%. None of the patients were using systemic agents while being treated with topical CSs. Adverse effects were rare: 2 (2.5%) patients experienced temporary minor irritation from the topical CS, 1 (1.3%) patient developed localized skin atrophy under the breast that resolved several months after she stopped treatment, and 1 (1.3%) patient developed stretch marks on the thighs.11 Zackheim12 later reported treatment of approximately 200 patients with class I topical CSs, and overall response rates were over 90% in stage T1 and over 80% in stage T2 patients. Response to topical CS was reported to be evident within 3 months and often much sooner. Side effects were most likely related to the more prolonged treatment periods. Irritant dermatitis or purpura developed in approximately 10% to 20% of patients, and purpura was seen at the sites of treatment as well as at distant sites. Only a small number of patients developed cutaneous atrophy and striae, which were reversible.12 Successful use of intralesional steroids for treatment-resistant MF was reported in 4 patients who tolerated treatment well without any side effects other than local hypopigmentation in a single patient.13
At MSKCC, the first line of treatment in localized (stage IA) MF in light-skinned individuals most frequently is class I topical CSs, usually clobetasol propionate cream 0.05%. Patients are instructed to apply the cream twice daily on active MF lesions uninterruptedly until completely clear and to avoid using it on the face and in skin folds (axillary, inguinal, and abdominal). Patients are instructed to observe themselves for possible cutaneous AEs related to treatment and to stop or taper treatment if any AEs are noticed. In patients with darker skin, we may recommend other modalities such as narrowband UVB phototherapy for even limited MF disease because of the risk for uneven/hypopigmentation with superpotent CSs.
The current study offers a real-life observation of topical high-potency CSs for treatment of early-stage MF and the associated cutaneous AEs. Local hypopigmentation was identified in 4 participants (33.3%), local skin atrophy was seen in 2 participants (16.7%), and local purpura and irritation were seen in 1 participant each (8.3%). All patients responded to therapy and 75.0% (9/12) achieved CCR or showed only MAD at their last follow-up visit. The limitations of our study were the small number of patients included and the relatively short follow-up period.
In MF patients, patches can present as fine wrinkling of the skin resembling atrophy, which can make it difficult to differentiate active MF from CS-induced atrophy in patients treated with topical CSs (Figure 1) and may have caused us to overestimate the occurrence of this AE. Corticosteroid-induced skin atrophy has been studied mainly in normal skin and to a lesser extent in pathological skin in psoriasis and atopic dermatitis. Some of these studies reported that CS-induced atrophy is reversible, and skin thickness can return to normal after topical application of CS is stopped.7
When hypopigmentation is seen around MF lesions, it is a confirmation that the patient is compliant with the therapy. From our experience, local hypopigmentation due to topical CSs is reversible (Figure 2). In some cases, MF patients have applied topical clobetasol propionate to lesional and surrounding skin, and hypopigmentation can be lessened with more careful limited application. In most cases, after discontinuation or tapering of the therapy, the skin returns to its normal color.

Based on our experience and the results of the current study, we conclude that topical superpotent CSs should remain the first-choice treatment for patients with early-stage MF (stage IA). Although bexarotene gel and mechlorethamine gel are FDA approved for early-stage MF, they are not widely available outside of the United States and are associated with AEs, mainly local skin irritation, rash, and pruritus.4,5 In contrast to bexarotene gel and mechlorethamine gel, topical clobetasol propionate can be used in young children (>12 years) and is classified as pregnancy category C.8
Conclusion
Patients with early-stage MF should be treated with skin-directed therapies, and the choice between different therapeutic options is made based on the physician’s experience with the treatment, patient characteristics, location and morphology of the MF lesions, and the AE profile of the treatment. Based on our experience, superpotent topical CSs are readily available and easily applied, have minor side effects, and remain the mainstay of therapy in patients with stage IA disease. Patients with MF on superpotent topical CS therapy should be monitored periodically and instructed how to identify cutaneous AEs related to treatment.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sezary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e221-217; quiz 240-222.
- Weberschock T, Strametz R, Lorenz M, et al. Interventions for mycosis fungoides [published online September 12, 2012]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD008946.pub2.
- Heald P, Mehlmauer M, Martin AG, et al. Topical bexarotene therapy for patients with refractory or persistent early-stage cutaneous T-cell lymphoma: results of the phase III clinical trial. J Am Acad Dermatol. 2003;49:801-815.
- Lessin SR, Duvic M, Guitart J, et al. Topical chemotherapy in cutaneous T-cell lymphoma: positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. 2013;149:25-32.
- Tadicherla S, Ross K, Shenefelt PD, et al. Topical corticosteroids in dermatology. J Drugs Dermatol. 2009;8:1093-1105.
- Barnes L, Kaya G, Rollason V. Topical corticosteroid-induced skin atrophy: a comprehensive review. Drug Saf. 2015;38:493-509.
- Temovate E (Clobetasol Propionate) Cream, 0.05% [package insert]. Melville, NY: PharmaDerm, a division of Fougera Pharmaceuticals Inc; 2012.
- Nakamura M, Abrouk M, Zhu H, et al. Update on the systemic risks of superpotent topical steroids. J Drugs Dermatol. 2017;16:643-648.
- Prince HM, Whittaker S, Hoppe RT. How I treat mycosis fungoides and Sezary syndrome. Blood. 2009;114:4337-4353.
- Zackheim HS, Kashani-Sabet M, Amin S. Topical corticosteroids for mycosis fungoides. experience in 79 patients. Arch Dermatol. 1998;134:949-954.
- Zackheim HS. Treatment of patch-stage mycosis fungoides with topical corticosteroids. Dermatol Ther. 2003;16:283-287.
- Liu DY, Shaath T, Rajpara AN, et al. Safe and efficacious use of intralesional steroids for the treatment of focally resistant mycosis fungoides. J Drugs Dermatol. 2015;14:466-471.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sezary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e221-217; quiz 240-222.
- Weberschock T, Strametz R, Lorenz M, et al. Interventions for mycosis fungoides [published online September 12, 2012]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD008946.pub2.
- Heald P, Mehlmauer M, Martin AG, et al. Topical bexarotene therapy for patients with refractory or persistent early-stage cutaneous T-cell lymphoma: results of the phase III clinical trial. J Am Acad Dermatol. 2003;49:801-815.
- Lessin SR, Duvic M, Guitart J, et al. Topical chemotherapy in cutaneous T-cell lymphoma: positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. 2013;149:25-32.
- Tadicherla S, Ross K, Shenefelt PD, et al. Topical corticosteroids in dermatology. J Drugs Dermatol. 2009;8:1093-1105.
- Barnes L, Kaya G, Rollason V. Topical corticosteroid-induced skin atrophy: a comprehensive review. Drug Saf. 2015;38:493-509.
- Temovate E (Clobetasol Propionate) Cream, 0.05% [package insert]. Melville, NY: PharmaDerm, a division of Fougera Pharmaceuticals Inc; 2012.
- Nakamura M, Abrouk M, Zhu H, et al. Update on the systemic risks of superpotent topical steroids. J Drugs Dermatol. 2017;16:643-648.
- Prince HM, Whittaker S, Hoppe RT. How I treat mycosis fungoides and Sezary syndrome. Blood. 2009;114:4337-4353.
- Zackheim HS, Kashani-Sabet M, Amin S. Topical corticosteroids for mycosis fungoides. experience in 79 patients. Arch Dermatol. 1998;134:949-954.
- Zackheim HS. Treatment of patch-stage mycosis fungoides with topical corticosteroids. Dermatol Ther. 2003;16:283-287.
- Liu DY, Shaath T, Rajpara AN, et al. Safe and efficacious use of intralesional steroids for the treatment of focally resistant mycosis fungoides. J Drugs Dermatol. 2015;14:466-471.
Practice Points
- Topical corticosteroid (CS) treatment is a safe skin-directed therapy that can effectively obtain complete and long-term response in patients with early-stage mycosis fungoides (MF).
- Despite the availability of optional topical treatments in MF, topical superpotent class I CSs are still considered the first-line treatment in patients with limited disease (stage IA).
- Patients using prolonged topical superpotent CSs should be monitored periodically and instructed on how to identify cutaneous adverse effects related to treatment, mainly local hypopigmentation and skin atrophy.
No link seen between methotrexate, interstitial lung disease in RA
Patients with rheumatoid arthritis (RA) have an elevated risk of interstitial lung disease (ILD), but methotrexate does not accentuate that risk and may in fact be protective, new data show. These were among key findings of a pair of studies reported at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Although a guideline-recommended cornerstone in the management of RA, methotrexate has been associated with both hypersensitivity pneumonitis and diffuse lung disease. However, its involvement in the development of ILD among patients with RA is unclear.
A Danish study of more than 30,000 RA patients reported at the congress found that their risk of ILD was about three to five times that of the general population. However, risk did not differ significantly whether they had filled a methotrexate prescription or not.
In addition, a multinational case-control study of more than 1,000 RA patients also reported at the congress found that, compared with never-users of methotrexate, ever-users actually had a 59% lower likelihood of developing ILD.
However, both studies were limited by their retrospective design, Elizabeth R. Volkmann, MD, codirector of the connective tissue disease–related interstitial lung disease program at the University of California, Los Angeles, cautioned in an interview. Hence, there was likely systematic bias and confounding.
“I would interpret the conclusions of both studies with caution,” she maintained. “To understand how a particular intervention, such as methotrexate use, affects the outcome of ILD development, a prospective design is needed, which adequately adjusts for known ILD risk factors, such as male sex and smoking.”
As to whether the new findings are practice changing and how they might affect patient counseling, “the answers to these questions are not straightforward and depend on other patient-related factors,” according to Dr. Volkmann.
Danish nationwide study
René Cordtz, MD, a clinical assistant at the Center for Rheumatology and Spine Diseases, Rigshospitalet‐Gentofte, Copenhagen, and colleagues conducted a nationwide population-based cohort study using registry data from 1997 to 2015 to assess lung disease among patients with RA by prescriptions filled.
Results based on 30,512 RA patients showed that, compared with peers filling no methotrexate prescriptions, patients filling at least one did not have a significantly elevated risk of ILD at either 1 year of follow-up (hazard ratio, 1.03) or 5 years of follow-up (HR, 1.00). (Findings were similar for sulfasalazine, with respective nonsignificant HRs of 0.88 and 1.14.)
In addition, patients with RA had a similarly sharply elevated 5-year risk of ILD relative to the general population regardless of whether they had filled neither methotrexate nor sulfasalazine prescriptions (standardized incidence ratio, 3.38) or had filled prescriptions for methotrexate only (SIR, 3.63), sulfasalazine only (SIR, 4.12), or both (SIR, 5.45).
“RA patients have an increased risk of ILD, compared to the general population, which was not surprising, but very importantly, that risk was not further exacerbated in those treated with methotrexate,” Dr. Cordtz concluded. “We do acknowledge that purchasing your medicine is different from taking your medicine, which is why we found it extra reassuring that when requiring at least two methotrexate prescriptions to be considered exposed, it did not change our results.”
Multinational study
Pierre-Antoine Juge, MD, a rheumatologist at Bichat-Claude Bernard Hospital, Paris, and colleagues performed a case-control study among 482 RA patients with ILD and 741 RA patients without ILD in three cohorts: a French discovery cohort, a multinational (Brazilian, Italian, Mexican, United Kingdom, and United States) replication cohort, and a combined cohort. Those with methotrexate hypersensitivity pneumonitis were excluded.
Results showed that relative to peers without ILD, patients with ILD had a lower prevalence of ever having used methotrexate and had received a lower cumulative methotrexate dose, findings that were consistent across all three cohorts.
Methotrexate ever-use was associated with a significantly lower adjusted likelihood of ILD in the discovery cohort (odds ratio, 0.46), the replication cohort (OR, 0.38), and the combined cohort (OR, 0.41). Furthermore, ever-users were less commonly represented among patients with ILD regardless of chest high-resolution CT pattern (usual interstitial pneumonia pattern vs. not).
Finally, methotrexate use appeared to delay the adjusted time to onset of ILD by 3.5 years in the discovery cohort (P = .001), by 3.2 years in the replication cohort (P < .0001), and by 3.5 years in the combined cohort (P < .0001).
“Outside of methotrexate hypersensitivity pneumonitis, methotrexate was not a risk factor for RA-associated ILD in our study. We observed an inverse relationship that was similar whatever the high-resolution CT pattern,” Dr. Juge commented. “But this possible protective effect should be confirmed through a dedicated prospective, randomized, controlled trial.”
“Methotrexate should not be considered as a causal factor for RA-associated ILD, and its [discontinuation] should be discussed through a multidisciplinary discussion,” he recommended. In addition, “this study does not investigate the impact of methotrexate use on RA-associated ILD prognosis.”
The Danish study did not receive any specific funding, and none of its authors reported having any financial disclosures. The multinational study did not receive any specific funding. Dr. Juge disclosed that he had no relevant conflicts of interest, but many of his coauthors reported financial relationships with industry. Dr. Volkmann disclosed consulting for Boehringer Ingelheim and Forbius, and receiving grant support from Forbius and Corbus.
SOURCES: Cordtz R et al. Ann Rheum Dis. 2020;79[suppl 1]:147-8, Abstract OP0232; Juge P-A et al. Ann Rheum Dis. 2020;79[suppl 1]:25, Abstract OP0236.
Patients with rheumatoid arthritis (RA) have an elevated risk of interstitial lung disease (ILD), but methotrexate does not accentuate that risk and may in fact be protective, new data show. These were among key findings of a pair of studies reported at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Although a guideline-recommended cornerstone in the management of RA, methotrexate has been associated with both hypersensitivity pneumonitis and diffuse lung disease. However, its involvement in the development of ILD among patients with RA is unclear.
A Danish study of more than 30,000 RA patients reported at the congress found that their risk of ILD was about three to five times that of the general population. However, risk did not differ significantly whether they had filled a methotrexate prescription or not.
In addition, a multinational case-control study of more than 1,000 RA patients also reported at the congress found that, compared with never-users of methotrexate, ever-users actually had a 59% lower likelihood of developing ILD.
However, both studies were limited by their retrospective design, Elizabeth R. Volkmann, MD, codirector of the connective tissue disease–related interstitial lung disease program at the University of California, Los Angeles, cautioned in an interview. Hence, there was likely systematic bias and confounding.
“I would interpret the conclusions of both studies with caution,” she maintained. “To understand how a particular intervention, such as methotrexate use, affects the outcome of ILD development, a prospective design is needed, which adequately adjusts for known ILD risk factors, such as male sex and smoking.”
As to whether the new findings are practice changing and how they might affect patient counseling, “the answers to these questions are not straightforward and depend on other patient-related factors,” according to Dr. Volkmann.
Danish nationwide study
René Cordtz, MD, a clinical assistant at the Center for Rheumatology and Spine Diseases, Rigshospitalet‐Gentofte, Copenhagen, and colleagues conducted a nationwide population-based cohort study using registry data from 1997 to 2015 to assess lung disease among patients with RA by prescriptions filled.
Results based on 30,512 RA patients showed that, compared with peers filling no methotrexate prescriptions, patients filling at least one did not have a significantly elevated risk of ILD at either 1 year of follow-up (hazard ratio, 1.03) or 5 years of follow-up (HR, 1.00). (Findings were similar for sulfasalazine, with respective nonsignificant HRs of 0.88 and 1.14.)
In addition, patients with RA had a similarly sharply elevated 5-year risk of ILD relative to the general population regardless of whether they had filled neither methotrexate nor sulfasalazine prescriptions (standardized incidence ratio, 3.38) or had filled prescriptions for methotrexate only (SIR, 3.63), sulfasalazine only (SIR, 4.12), or both (SIR, 5.45).
“RA patients have an increased risk of ILD, compared to the general population, which was not surprising, but very importantly, that risk was not further exacerbated in those treated with methotrexate,” Dr. Cordtz concluded. “We do acknowledge that purchasing your medicine is different from taking your medicine, which is why we found it extra reassuring that when requiring at least two methotrexate prescriptions to be considered exposed, it did not change our results.”
Multinational study
Pierre-Antoine Juge, MD, a rheumatologist at Bichat-Claude Bernard Hospital, Paris, and colleagues performed a case-control study among 482 RA patients with ILD and 741 RA patients without ILD in three cohorts: a French discovery cohort, a multinational (Brazilian, Italian, Mexican, United Kingdom, and United States) replication cohort, and a combined cohort. Those with methotrexate hypersensitivity pneumonitis were excluded.
Results showed that relative to peers without ILD, patients with ILD had a lower prevalence of ever having used methotrexate and had received a lower cumulative methotrexate dose, findings that were consistent across all three cohorts.
Methotrexate ever-use was associated with a significantly lower adjusted likelihood of ILD in the discovery cohort (odds ratio, 0.46), the replication cohort (OR, 0.38), and the combined cohort (OR, 0.41). Furthermore, ever-users were less commonly represented among patients with ILD regardless of chest high-resolution CT pattern (usual interstitial pneumonia pattern vs. not).
Finally, methotrexate use appeared to delay the adjusted time to onset of ILD by 3.5 years in the discovery cohort (P = .001), by 3.2 years in the replication cohort (P < .0001), and by 3.5 years in the combined cohort (P < .0001).
“Outside of methotrexate hypersensitivity pneumonitis, methotrexate was not a risk factor for RA-associated ILD in our study. We observed an inverse relationship that was similar whatever the high-resolution CT pattern,” Dr. Juge commented. “But this possible protective effect should be confirmed through a dedicated prospective, randomized, controlled trial.”
“Methotrexate should not be considered as a causal factor for RA-associated ILD, and its [discontinuation] should be discussed through a multidisciplinary discussion,” he recommended. In addition, “this study does not investigate the impact of methotrexate use on RA-associated ILD prognosis.”
The Danish study did not receive any specific funding, and none of its authors reported having any financial disclosures. The multinational study did not receive any specific funding. Dr. Juge disclosed that he had no relevant conflicts of interest, but many of his coauthors reported financial relationships with industry. Dr. Volkmann disclosed consulting for Boehringer Ingelheim and Forbius, and receiving grant support from Forbius and Corbus.
SOURCES: Cordtz R et al. Ann Rheum Dis. 2020;79[suppl 1]:147-8, Abstract OP0232; Juge P-A et al. Ann Rheum Dis. 2020;79[suppl 1]:25, Abstract OP0236.
Patients with rheumatoid arthritis (RA) have an elevated risk of interstitial lung disease (ILD), but methotrexate does not accentuate that risk and may in fact be protective, new data show. These were among key findings of a pair of studies reported at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Although a guideline-recommended cornerstone in the management of RA, methotrexate has been associated with both hypersensitivity pneumonitis and diffuse lung disease. However, its involvement in the development of ILD among patients with RA is unclear.
A Danish study of more than 30,000 RA patients reported at the congress found that their risk of ILD was about three to five times that of the general population. However, risk did not differ significantly whether they had filled a methotrexate prescription or not.
In addition, a multinational case-control study of more than 1,000 RA patients also reported at the congress found that, compared with never-users of methotrexate, ever-users actually had a 59% lower likelihood of developing ILD.
However, both studies were limited by their retrospective design, Elizabeth R. Volkmann, MD, codirector of the connective tissue disease–related interstitial lung disease program at the University of California, Los Angeles, cautioned in an interview. Hence, there was likely systematic bias and confounding.
“I would interpret the conclusions of both studies with caution,” she maintained. “To understand how a particular intervention, such as methotrexate use, affects the outcome of ILD development, a prospective design is needed, which adequately adjusts for known ILD risk factors, such as male sex and smoking.”
As to whether the new findings are practice changing and how they might affect patient counseling, “the answers to these questions are not straightforward and depend on other patient-related factors,” according to Dr. Volkmann.
Danish nationwide study
René Cordtz, MD, a clinical assistant at the Center for Rheumatology and Spine Diseases, Rigshospitalet‐Gentofte, Copenhagen, and colleagues conducted a nationwide population-based cohort study using registry data from 1997 to 2015 to assess lung disease among patients with RA by prescriptions filled.
Results based on 30,512 RA patients showed that, compared with peers filling no methotrexate prescriptions, patients filling at least one did not have a significantly elevated risk of ILD at either 1 year of follow-up (hazard ratio, 1.03) or 5 years of follow-up (HR, 1.00). (Findings were similar for sulfasalazine, with respective nonsignificant HRs of 0.88 and 1.14.)
In addition, patients with RA had a similarly sharply elevated 5-year risk of ILD relative to the general population regardless of whether they had filled neither methotrexate nor sulfasalazine prescriptions (standardized incidence ratio, 3.38) or had filled prescriptions for methotrexate only (SIR, 3.63), sulfasalazine only (SIR, 4.12), or both (SIR, 5.45).
“RA patients have an increased risk of ILD, compared to the general population, which was not surprising, but very importantly, that risk was not further exacerbated in those treated with methotrexate,” Dr. Cordtz concluded. “We do acknowledge that purchasing your medicine is different from taking your medicine, which is why we found it extra reassuring that when requiring at least two methotrexate prescriptions to be considered exposed, it did not change our results.”
Multinational study
Pierre-Antoine Juge, MD, a rheumatologist at Bichat-Claude Bernard Hospital, Paris, and colleagues performed a case-control study among 482 RA patients with ILD and 741 RA patients without ILD in three cohorts: a French discovery cohort, a multinational (Brazilian, Italian, Mexican, United Kingdom, and United States) replication cohort, and a combined cohort. Those with methotrexate hypersensitivity pneumonitis were excluded.
Results showed that relative to peers without ILD, patients with ILD had a lower prevalence of ever having used methotrexate and had received a lower cumulative methotrexate dose, findings that were consistent across all three cohorts.
Methotrexate ever-use was associated with a significantly lower adjusted likelihood of ILD in the discovery cohort (odds ratio, 0.46), the replication cohort (OR, 0.38), and the combined cohort (OR, 0.41). Furthermore, ever-users were less commonly represented among patients with ILD regardless of chest high-resolution CT pattern (usual interstitial pneumonia pattern vs. not).
Finally, methotrexate use appeared to delay the adjusted time to onset of ILD by 3.5 years in the discovery cohort (P = .001), by 3.2 years in the replication cohort (P < .0001), and by 3.5 years in the combined cohort (P < .0001).
“Outside of methotrexate hypersensitivity pneumonitis, methotrexate was not a risk factor for RA-associated ILD in our study. We observed an inverse relationship that was similar whatever the high-resolution CT pattern,” Dr. Juge commented. “But this possible protective effect should be confirmed through a dedicated prospective, randomized, controlled trial.”
“Methotrexate should not be considered as a causal factor for RA-associated ILD, and its [discontinuation] should be discussed through a multidisciplinary discussion,” he recommended. In addition, “this study does not investigate the impact of methotrexate use on RA-associated ILD prognosis.”
The Danish study did not receive any specific funding, and none of its authors reported having any financial disclosures. The multinational study did not receive any specific funding. Dr. Juge disclosed that he had no relevant conflicts of interest, but many of his coauthors reported financial relationships with industry. Dr. Volkmann disclosed consulting for Boehringer Ingelheim and Forbius, and receiving grant support from Forbius and Corbus.
SOURCES: Cordtz R et al. Ann Rheum Dis. 2020;79[suppl 1]:147-8, Abstract OP0232; Juge P-A et al. Ann Rheum Dis. 2020;79[suppl 1]:25, Abstract OP0236.
FROM THE EULAR 2020 E-CONGRESS