User login
ACR reacts to study disclosing industry donations
Institutions receive most research funding
Second only to the American Society of Clinical Oncology, the volunteer leaders of the American College of Rheumatology with ties to industry received the highest median payment amounts, a new cross-sectional study reveals.
Total research payments exceeded $54 million to ASCO leaders and $20 million to ACR leaders, for example. The investigators identified the 10 most common and costly conditions in the United States – including heart disease, trauma-related disorders, mental disorders, and others. They then used a new national database to explore the financial relationships between pharmaceutical and device manufacturers and the leaders of “influential U.S. professional medical associations active across these disease areas.” Steven Echard, executive vice president of the American College of Rheumatology, responded to the study findings, published in the BMJ. “We require our leaders to tell us about any payments they receive as part of their professional and personal activities that may pose actual or potential conflicts, post this information transparently to our website, and adjust what projects and initiatives they can participate in to avoid any undue influence,” he said in an interview.
“Many of the disclosures included in the study were due to individuals participating in research projects to move the needle forward in the care of rheumatology patients,” he added.
There remains an ongoing debate about how close the relationships should be between medical associations and industry, wrote lead author Ray Moynihan, PhD, assistant professor in the Institute for Evidence-Based Healthcare at Bond University, Gold Coast, Australia, and colleagues.
Majority report relationship with industry
Dr. Moynihan and associates conducted the first study to evaluate these relationships in such detail. Using the U.S. government’s Open Payments database, established in 2013, they assessed research payments and general payments for consultancy, royalties, and hospitality. The study included the current year of board membership, as well as the 4 years prior and 1 year after membership.
Overall, out of 293 physician association leaders, 235 or 80% had a financial relationship. None of the associations in the study featured a leadership free of financial ties.
Payments totaled almost $130 million, including nearly $25 million in general payments, almost $105 million for research, and about half a million in other payments. The research payments went primarily to institutions with leaders named as principal investigators, they noted.
“The most common misperception [in the BMJ study] is that all the funding identified represents personal payments to the individuals,” Mr. Echard said. “Because research dollars are included, almost 80% of the payments referenced in the article – sometimes more – go to individuals’ academic institutions.” These funds cover overhead, lab materials, protected time for said research, research assistant salaries, and other expenses.
“We have other board of director members who have no industry relationships at all,” he added.
Part of business model
“Faculty members are expected to bring in funding to support research, so most academic institutions receive industry funding, and it’s considered part of their business model,” Mr. Echard said. “For this reason, we generally do not consider these payments the same as being on advisory boards, speakers bureaus, or going to dinners, etc.”
The median total amounts linked to individual leaders varied between associations. For example, the median amount was $518,000 for ASCO leaders, as previously reported by Medscape Medical News.
Volunteer leaders for the ACR received a median $251,000. Not all ACR board of director members receive funding in this range, Mr. Echard said. “We have board of director members who work at academic institutions that can receive funding that high due to their participation in research studies, particularly when looking over a 4-year period prior to their leadership role, as the study did.”
At the lower end, median total payments were $404 for leaders of the American College of Physicians and $212 for those of the American Psychiatric Association.
In an accompanying editorial, Jake Checketts, DO, and Matt Vassar, PhD, of the Oklahoma State University Center for Health Sciences, Tulsa, proposed five actions that “could mitigate or even eliminate the overwhelming presence of financial conflicts of interest among medical societies and associations. This would protect these groups from producing biased documents or policies, which in turn would protect all physicians and the patients they treat.”
They made five proposals:
- Each association must take the initiative to evaluate its present conflicts using open payments.
- Associations should alter their recruitment processes to yield balanced and diverse groups of physician leaders largely free from financial conflicts of interest.
- The creation of standards for promoting medical associations that are free from financial conflicts of interest, similar to the framework within the Institute of Medicine’s standards for producing clinical practice guidelines we can trust.
- Greater reliance on the Sunshine Act and open payments in the United States (and elsewhere for countries with similar data) could eliminate the need for the traditional “honor system” of financial self-disclosure, which is ineffective and inaccurate at best.
- All medical associations, guidelines groups, and policy makers provide links from their documents and websites to open payments data for each U.S.-based physician contributor. Such links would make it easier for anyone, including patients, to evaluate any risk of bias.
On a final note, the ACR prohibits key college leaders, including the ACR president, ACR president-elect, foundation president, and others from having direct financial ties to commercial entities in their conflict of interest guidelines.
“We agree that actively managing conflict of interest is important to maintaining the integrity and reputation of an association with the medical community and with the public,” Mr. Echard said. “And all actual, potential, and perceived conflicts of interest should be addressed and managed through a disclosure process.”
Several authors of the study reported receiving grants from the Australian National Health and Medical Research Council. Mr. Echard, Dr. Checketts, and Dr. Vassar had no relevant disclosures.
SOURCE: Moynihan R et al. BMJ. 2020;369:m1505.
Institutions receive most research funding
Institutions receive most research funding
Second only to the American Society of Clinical Oncology, the volunteer leaders of the American College of Rheumatology with ties to industry received the highest median payment amounts, a new cross-sectional study reveals.
Total research payments exceeded $54 million to ASCO leaders and $20 million to ACR leaders, for example. The investigators identified the 10 most common and costly conditions in the United States – including heart disease, trauma-related disorders, mental disorders, and others. They then used a new national database to explore the financial relationships between pharmaceutical and device manufacturers and the leaders of “influential U.S. professional medical associations active across these disease areas.” Steven Echard, executive vice president of the American College of Rheumatology, responded to the study findings, published in the BMJ. “We require our leaders to tell us about any payments they receive as part of their professional and personal activities that may pose actual or potential conflicts, post this information transparently to our website, and adjust what projects and initiatives they can participate in to avoid any undue influence,” he said in an interview.
“Many of the disclosures included in the study were due to individuals participating in research projects to move the needle forward in the care of rheumatology patients,” he added.
There remains an ongoing debate about how close the relationships should be between medical associations and industry, wrote lead author Ray Moynihan, PhD, assistant professor in the Institute for Evidence-Based Healthcare at Bond University, Gold Coast, Australia, and colleagues.
Majority report relationship with industry
Dr. Moynihan and associates conducted the first study to evaluate these relationships in such detail. Using the U.S. government’s Open Payments database, established in 2013, they assessed research payments and general payments for consultancy, royalties, and hospitality. The study included the current year of board membership, as well as the 4 years prior and 1 year after membership.
Overall, out of 293 physician association leaders, 235 or 80% had a financial relationship. None of the associations in the study featured a leadership free of financial ties.
Payments totaled almost $130 million, including nearly $25 million in general payments, almost $105 million for research, and about half a million in other payments. The research payments went primarily to institutions with leaders named as principal investigators, they noted.
“The most common misperception [in the BMJ study] is that all the funding identified represents personal payments to the individuals,” Mr. Echard said. “Because research dollars are included, almost 80% of the payments referenced in the article – sometimes more – go to individuals’ academic institutions.” These funds cover overhead, lab materials, protected time for said research, research assistant salaries, and other expenses.
“We have other board of director members who have no industry relationships at all,” he added.
Part of business model
“Faculty members are expected to bring in funding to support research, so most academic institutions receive industry funding, and it’s considered part of their business model,” Mr. Echard said. “For this reason, we generally do not consider these payments the same as being on advisory boards, speakers bureaus, or going to dinners, etc.”
The median total amounts linked to individual leaders varied between associations. For example, the median amount was $518,000 for ASCO leaders, as previously reported by Medscape Medical News.
Volunteer leaders for the ACR received a median $251,000. Not all ACR board of director members receive funding in this range, Mr. Echard said. “We have board of director members who work at academic institutions that can receive funding that high due to their participation in research studies, particularly when looking over a 4-year period prior to their leadership role, as the study did.”
At the lower end, median total payments were $404 for leaders of the American College of Physicians and $212 for those of the American Psychiatric Association.
In an accompanying editorial, Jake Checketts, DO, and Matt Vassar, PhD, of the Oklahoma State University Center for Health Sciences, Tulsa, proposed five actions that “could mitigate or even eliminate the overwhelming presence of financial conflicts of interest among medical societies and associations. This would protect these groups from producing biased documents or policies, which in turn would protect all physicians and the patients they treat.”
They made five proposals:
- Each association must take the initiative to evaluate its present conflicts using open payments.
- Associations should alter their recruitment processes to yield balanced and diverse groups of physician leaders largely free from financial conflicts of interest.
- The creation of standards for promoting medical associations that are free from financial conflicts of interest, similar to the framework within the Institute of Medicine’s standards for producing clinical practice guidelines we can trust.
- Greater reliance on the Sunshine Act and open payments in the United States (and elsewhere for countries with similar data) could eliminate the need for the traditional “honor system” of financial self-disclosure, which is ineffective and inaccurate at best.
- All medical associations, guidelines groups, and policy makers provide links from their documents and websites to open payments data for each U.S.-based physician contributor. Such links would make it easier for anyone, including patients, to evaluate any risk of bias.
On a final note, the ACR prohibits key college leaders, including the ACR president, ACR president-elect, foundation president, and others from having direct financial ties to commercial entities in their conflict of interest guidelines.
“We agree that actively managing conflict of interest is important to maintaining the integrity and reputation of an association with the medical community and with the public,” Mr. Echard said. “And all actual, potential, and perceived conflicts of interest should be addressed and managed through a disclosure process.”
Several authors of the study reported receiving grants from the Australian National Health and Medical Research Council. Mr. Echard, Dr. Checketts, and Dr. Vassar had no relevant disclosures.
SOURCE: Moynihan R et al. BMJ. 2020;369:m1505.
Second only to the American Society of Clinical Oncology, the volunteer leaders of the American College of Rheumatology with ties to industry received the highest median payment amounts, a new cross-sectional study reveals.
Total research payments exceeded $54 million to ASCO leaders and $20 million to ACR leaders, for example. The investigators identified the 10 most common and costly conditions in the United States – including heart disease, trauma-related disorders, mental disorders, and others. They then used a new national database to explore the financial relationships between pharmaceutical and device manufacturers and the leaders of “influential U.S. professional medical associations active across these disease areas.” Steven Echard, executive vice president of the American College of Rheumatology, responded to the study findings, published in the BMJ. “We require our leaders to tell us about any payments they receive as part of their professional and personal activities that may pose actual or potential conflicts, post this information transparently to our website, and adjust what projects and initiatives they can participate in to avoid any undue influence,” he said in an interview.
“Many of the disclosures included in the study were due to individuals participating in research projects to move the needle forward in the care of rheumatology patients,” he added.
There remains an ongoing debate about how close the relationships should be between medical associations and industry, wrote lead author Ray Moynihan, PhD, assistant professor in the Institute for Evidence-Based Healthcare at Bond University, Gold Coast, Australia, and colleagues.
Majority report relationship with industry
Dr. Moynihan and associates conducted the first study to evaluate these relationships in such detail. Using the U.S. government’s Open Payments database, established in 2013, they assessed research payments and general payments for consultancy, royalties, and hospitality. The study included the current year of board membership, as well as the 4 years prior and 1 year after membership.
Overall, out of 293 physician association leaders, 235 or 80% had a financial relationship. None of the associations in the study featured a leadership free of financial ties.
Payments totaled almost $130 million, including nearly $25 million in general payments, almost $105 million for research, and about half a million in other payments. The research payments went primarily to institutions with leaders named as principal investigators, they noted.
“The most common misperception [in the BMJ study] is that all the funding identified represents personal payments to the individuals,” Mr. Echard said. “Because research dollars are included, almost 80% of the payments referenced in the article – sometimes more – go to individuals’ academic institutions.” These funds cover overhead, lab materials, protected time for said research, research assistant salaries, and other expenses.
“We have other board of director members who have no industry relationships at all,” he added.
Part of business model
“Faculty members are expected to bring in funding to support research, so most academic institutions receive industry funding, and it’s considered part of their business model,” Mr. Echard said. “For this reason, we generally do not consider these payments the same as being on advisory boards, speakers bureaus, or going to dinners, etc.”
The median total amounts linked to individual leaders varied between associations. For example, the median amount was $518,000 for ASCO leaders, as previously reported by Medscape Medical News.
Volunteer leaders for the ACR received a median $251,000. Not all ACR board of director members receive funding in this range, Mr. Echard said. “We have board of director members who work at academic institutions that can receive funding that high due to their participation in research studies, particularly when looking over a 4-year period prior to their leadership role, as the study did.”
At the lower end, median total payments were $404 for leaders of the American College of Physicians and $212 for those of the American Psychiatric Association.
In an accompanying editorial, Jake Checketts, DO, and Matt Vassar, PhD, of the Oklahoma State University Center for Health Sciences, Tulsa, proposed five actions that “could mitigate or even eliminate the overwhelming presence of financial conflicts of interest among medical societies and associations. This would protect these groups from producing biased documents or policies, which in turn would protect all physicians and the patients they treat.”
They made five proposals:
- Each association must take the initiative to evaluate its present conflicts using open payments.
- Associations should alter their recruitment processes to yield balanced and diverse groups of physician leaders largely free from financial conflicts of interest.
- The creation of standards for promoting medical associations that are free from financial conflicts of interest, similar to the framework within the Institute of Medicine’s standards for producing clinical practice guidelines we can trust.
- Greater reliance on the Sunshine Act and open payments in the United States (and elsewhere for countries with similar data) could eliminate the need for the traditional “honor system” of financial self-disclosure, which is ineffective and inaccurate at best.
- All medical associations, guidelines groups, and policy makers provide links from their documents and websites to open payments data for each U.S.-based physician contributor. Such links would make it easier for anyone, including patients, to evaluate any risk of bias.
On a final note, the ACR prohibits key college leaders, including the ACR president, ACR president-elect, foundation president, and others from having direct financial ties to commercial entities in their conflict of interest guidelines.
“We agree that actively managing conflict of interest is important to maintaining the integrity and reputation of an association with the medical community and with the public,” Mr. Echard said. “And all actual, potential, and perceived conflicts of interest should be addressed and managed through a disclosure process.”
Several authors of the study reported receiving grants from the Australian National Health and Medical Research Council. Mr. Echard, Dr. Checketts, and Dr. Vassar had no relevant disclosures.
SOURCE: Moynihan R et al. BMJ. 2020;369:m1505.
FROM THE BMJ
The CDI APP adviser
A novel approach to APP documentation engagement
As hospitals and clinicians, we are facing increased scrutiny of the care we provide to our patients. There is increased demand for more transparency of our outcomes and a need for increased efficiency of the care we provide in the setting of already significant documentation burden and its known impact on provider burnout.
Clinical documentation integrity (CDI) is an instrumental department which supports complete and accurate documentation, serves as a bridge between physicians and hospital coders such that hospital reimbursement is appropriate and quality metrics are attributed appropriately to the hospital, service lines, and individual providers. Complete and accurate documentation also leads to the submission of coded/claims-based data reflecting provider true intent and to clinically valid data for research and patient centric purposes. For this reason, the physician adviser role as a liaison between physicians and CDI and coding, in addition to utilization management and case management, has become more commonplace. The physician adviser role has been a mainstay of CDI programs across the United States since as early as 2012.
At the University of Colorado Health (UCHealth), the physician adviser role first began in 2015 at our major academic medical center, the University of Colorado Hospital (UCH). That physician adviser, after the additional physician adviser FTE at UCH, having established relationships with physicians across service lines, began to focus on CDI-related education and communication as it pertained to inpatient documentation.
At our institution we have approximately 500 advance practice providers (APPs). Approximately two-thirds of the APPs care for inpatients on a myriad of different service lines and, along with physician learners from interns to fellows, complete the bulk of the documentation in the electronic health record.
In early 2018, the UCHealth office of advanced practice collaborated with CDI in its mission to optimize documentation with the aim to have a positive impact on reimbursement and quality metrics while highlighting APP value. In the relatively early stages of the collaboration it became evident that an APP adviser could be an innovative and effective approach in engaging our many APPs with CDI as faculty members who are generally service line based and, as such, invested in hospital and service line outcomes.
A business case for a new position of APP adviser for CDI was formulated based on not only the number of APP faculty and learners at our institution, but also on the premise that the level of consistency APPs provide would increase reliability in the adoption and adaptation of documentation practices as medicine and coding rules evolve. In addition, APP documentation can stand alone without physician attestation or signature, unlike physicians in training, further making them ideally suited collaborators. The position was approved by hospital leadership and the first APP adviser for CDI in the country (of whom we are aware) was hired at UCH in July 2019.
A dedicated APP CDI adviser facilitates the success of a CDI/APP collaboration through a better understanding of APP engagement needs largely by creating new and/or fostering existing relationships between the APP adviser and the APPs for each service line. The APP CDI adviser identifies the needs of the team in order to maximally enhance their documentation while illustrating how the work/collaboration can positively contribute to APP clinical and/or academic goals. The APP CDI adviser possesses a deeper knowledge of APP clinical work flow and how that work flow might be impacting the documentation. He or she utilizes information gathered from the APP team to create more efficient note templates, provide lunch and learns with different service line APPs, and offering 1:1 drop-in documentation support, allowing for more feedback flexibility in context of their clinical work flow.
This real time input may be received more positively and be perceived as less intimidating in the peer-to-peer context. The APP adviser also attends various educational forums to which the physician advisers may not have access. For example, the APP adviser attends monthly APP orientation to meet new APPs for the institution, attends APP council, is a member of the APP steering committee, and provides documentation tips for the APP monthly newsletter.
At this point we are in the process of collecting pre- and post data to illustrate the benefit of a CDI APP adviser (and the CDI APP collaboration as a whole) through metrics such as CC/MCC capture rate, case mix index, and mortality and length of stay as influenced by the level of complexity in documentation. We hope to add APPs as advisers across the UCHealth system over time and to continue to highlight and publish the experience and outcomes related to this innovative role as it evolves such that other institutions across the country will consider this type of collaboration.
Dr. Anoff is associate professor of clinical practice in the division of hospital medicine and medical director of clinical documentation integrity at University of Colorado Health, Denver. Ms. Brill is senior instructor in the department of neurosurgery and APP adviser of clinical documentation integrity at UCHealth Denver Metro.
A novel approach to APP documentation engagement
A novel approach to APP documentation engagement
As hospitals and clinicians, we are facing increased scrutiny of the care we provide to our patients. There is increased demand for more transparency of our outcomes and a need for increased efficiency of the care we provide in the setting of already significant documentation burden and its known impact on provider burnout.
Clinical documentation integrity (CDI) is an instrumental department which supports complete and accurate documentation, serves as a bridge between physicians and hospital coders such that hospital reimbursement is appropriate and quality metrics are attributed appropriately to the hospital, service lines, and individual providers. Complete and accurate documentation also leads to the submission of coded/claims-based data reflecting provider true intent and to clinically valid data for research and patient centric purposes. For this reason, the physician adviser role as a liaison between physicians and CDI and coding, in addition to utilization management and case management, has become more commonplace. The physician adviser role has been a mainstay of CDI programs across the United States since as early as 2012.
At the University of Colorado Health (UCHealth), the physician adviser role first began in 2015 at our major academic medical center, the University of Colorado Hospital (UCH). That physician adviser, after the additional physician adviser FTE at UCH, having established relationships with physicians across service lines, began to focus on CDI-related education and communication as it pertained to inpatient documentation.
At our institution we have approximately 500 advance practice providers (APPs). Approximately two-thirds of the APPs care for inpatients on a myriad of different service lines and, along with physician learners from interns to fellows, complete the bulk of the documentation in the electronic health record.
In early 2018, the UCHealth office of advanced practice collaborated with CDI in its mission to optimize documentation with the aim to have a positive impact on reimbursement and quality metrics while highlighting APP value. In the relatively early stages of the collaboration it became evident that an APP adviser could be an innovative and effective approach in engaging our many APPs with CDI as faculty members who are generally service line based and, as such, invested in hospital and service line outcomes.
A business case for a new position of APP adviser for CDI was formulated based on not only the number of APP faculty and learners at our institution, but also on the premise that the level of consistency APPs provide would increase reliability in the adoption and adaptation of documentation practices as medicine and coding rules evolve. In addition, APP documentation can stand alone without physician attestation or signature, unlike physicians in training, further making them ideally suited collaborators. The position was approved by hospital leadership and the first APP adviser for CDI in the country (of whom we are aware) was hired at UCH in July 2019.
A dedicated APP CDI adviser facilitates the success of a CDI/APP collaboration through a better understanding of APP engagement needs largely by creating new and/or fostering existing relationships between the APP adviser and the APPs for each service line. The APP CDI adviser identifies the needs of the team in order to maximally enhance their documentation while illustrating how the work/collaboration can positively contribute to APP clinical and/or academic goals. The APP CDI adviser possesses a deeper knowledge of APP clinical work flow and how that work flow might be impacting the documentation. He or she utilizes information gathered from the APP team to create more efficient note templates, provide lunch and learns with different service line APPs, and offering 1:1 drop-in documentation support, allowing for more feedback flexibility in context of their clinical work flow.
This real time input may be received more positively and be perceived as less intimidating in the peer-to-peer context. The APP adviser also attends various educational forums to which the physician advisers may not have access. For example, the APP adviser attends monthly APP orientation to meet new APPs for the institution, attends APP council, is a member of the APP steering committee, and provides documentation tips for the APP monthly newsletter.
At this point we are in the process of collecting pre- and post data to illustrate the benefit of a CDI APP adviser (and the CDI APP collaboration as a whole) through metrics such as CC/MCC capture rate, case mix index, and mortality and length of stay as influenced by the level of complexity in documentation. We hope to add APPs as advisers across the UCHealth system over time and to continue to highlight and publish the experience and outcomes related to this innovative role as it evolves such that other institutions across the country will consider this type of collaboration.
Dr. Anoff is associate professor of clinical practice in the division of hospital medicine and medical director of clinical documentation integrity at University of Colorado Health, Denver. Ms. Brill is senior instructor in the department of neurosurgery and APP adviser of clinical documentation integrity at UCHealth Denver Metro.
As hospitals and clinicians, we are facing increased scrutiny of the care we provide to our patients. There is increased demand for more transparency of our outcomes and a need for increased efficiency of the care we provide in the setting of already significant documentation burden and its known impact on provider burnout.
Clinical documentation integrity (CDI) is an instrumental department which supports complete and accurate documentation, serves as a bridge between physicians and hospital coders such that hospital reimbursement is appropriate and quality metrics are attributed appropriately to the hospital, service lines, and individual providers. Complete and accurate documentation also leads to the submission of coded/claims-based data reflecting provider true intent and to clinically valid data for research and patient centric purposes. For this reason, the physician adviser role as a liaison between physicians and CDI and coding, in addition to utilization management and case management, has become more commonplace. The physician adviser role has been a mainstay of CDI programs across the United States since as early as 2012.
At the University of Colorado Health (UCHealth), the physician adviser role first began in 2015 at our major academic medical center, the University of Colorado Hospital (UCH). That physician adviser, after the additional physician adviser FTE at UCH, having established relationships with physicians across service lines, began to focus on CDI-related education and communication as it pertained to inpatient documentation.
At our institution we have approximately 500 advance practice providers (APPs). Approximately two-thirds of the APPs care for inpatients on a myriad of different service lines and, along with physician learners from interns to fellows, complete the bulk of the documentation in the electronic health record.
In early 2018, the UCHealth office of advanced practice collaborated with CDI in its mission to optimize documentation with the aim to have a positive impact on reimbursement and quality metrics while highlighting APP value. In the relatively early stages of the collaboration it became evident that an APP adviser could be an innovative and effective approach in engaging our many APPs with CDI as faculty members who are generally service line based and, as such, invested in hospital and service line outcomes.
A business case for a new position of APP adviser for CDI was formulated based on not only the number of APP faculty and learners at our institution, but also on the premise that the level of consistency APPs provide would increase reliability in the adoption and adaptation of documentation practices as medicine and coding rules evolve. In addition, APP documentation can stand alone without physician attestation or signature, unlike physicians in training, further making them ideally suited collaborators. The position was approved by hospital leadership and the first APP adviser for CDI in the country (of whom we are aware) was hired at UCH in July 2019.
A dedicated APP CDI adviser facilitates the success of a CDI/APP collaboration through a better understanding of APP engagement needs largely by creating new and/or fostering existing relationships between the APP adviser and the APPs for each service line. The APP CDI adviser identifies the needs of the team in order to maximally enhance their documentation while illustrating how the work/collaboration can positively contribute to APP clinical and/or academic goals. The APP CDI adviser possesses a deeper knowledge of APP clinical work flow and how that work flow might be impacting the documentation. He or she utilizes information gathered from the APP team to create more efficient note templates, provide lunch and learns with different service line APPs, and offering 1:1 drop-in documentation support, allowing for more feedback flexibility in context of their clinical work flow.
This real time input may be received more positively and be perceived as less intimidating in the peer-to-peer context. The APP adviser also attends various educational forums to which the physician advisers may not have access. For example, the APP adviser attends monthly APP orientation to meet new APPs for the institution, attends APP council, is a member of the APP steering committee, and provides documentation tips for the APP monthly newsletter.
At this point we are in the process of collecting pre- and post data to illustrate the benefit of a CDI APP adviser (and the CDI APP collaboration as a whole) through metrics such as CC/MCC capture rate, case mix index, and mortality and length of stay as influenced by the level of complexity in documentation. We hope to add APPs as advisers across the UCHealth system over time and to continue to highlight and publish the experience and outcomes related to this innovative role as it evolves such that other institutions across the country will consider this type of collaboration.
Dr. Anoff is associate professor of clinical practice in the division of hospital medicine and medical director of clinical documentation integrity at University of Colorado Health, Denver. Ms. Brill is senior instructor in the department of neurosurgery and APP adviser of clinical documentation integrity at UCHealth Denver Metro.
Former smokers using e-cigarettes at risk for cigarette smoking relapse
The use of , results from a large longitudinal cohort study demonstrated.
“For the many clinicians treating former smokers who have successfully quit all nicotine products, the implications are that use of [electronic nicotine delivery systems] should be discouraged, just as use of all other tobacco products is discouraged,” researchers led by Colm D. Everard, PhD, reported in a study published in JAMA Network Open (2020 Jun 5. doi: 10.1001/jamanetworkopen.2020.4813).
Dr. Everard, of the National Institute on Drug Abuse, and colleagues based their comments on results from a survey of adult former smokers who participated in Waves 1-4 of the Population Assessment of Tobacco and Health (PATH) Study (2013-2018). They limited the analysis to 2,273 former cigarette smokers who self-reported reported no tobacco product use at Wave 1, and categorized them as recent former smokers (defined as having last smoked within the past 12 previous months) or as long-term former smokers (defined as having last smoked for longer ago than in the previous 12 months). The main outcome of interest was the self-reported current use of cigarettes at follow-up interviews, which was defined as every day or some days. Electronic nicotine delivery systems (ENDS) comprised e-cigarettes, e-cigars, e-pipes, and e-hookahs. Other tobacco products included cigars, pipe tobacco, hookahs, snus tobacco, other smokeless tobacco, and dissolvable tobacco.
Of the 2,273 adult former smokers, 52% were women, 60% were older than age 50, and 80% were non-Hispanic white. Adjusted hazard ratio (AHR) analysis revealed that the use of ENDS was associated with significant risk of cigarette smoking relapse among recent former smokers (AHR 1.63) and among long-term former smokers (AHR 3.79). The use of other tobacco products was associated with significant risk for cigarette smoking relapse among recent former smokers (AHR 1.97) and among long-term former smokers (AHR 3.82).
The authors acknowledged certain limitations of the study, including the fact that it did not assess different ENDS devices, different e-liquid nicotine levels, or frequency of ENDS use and their associations with cigarette smoking relapse. It also did not explore the mechanism by which ENDS use may lead to reestablishing or reinforcing nicotine-seeking behavior among former cigarette users. “Determining pharmacologic, behavioral, or some other explanation for these findings may require laboratory-based research,” they wrote.
The PATH Study is supported with federal funds from the National Institute on Drug Abuse, the National Institutes of Health, and the Food and Drug Administration and Department of Health and Human Services under a contract to Westat. One of the study authors, Wilson M. Compton, MD, reported having long-term stock holdings in General Electric, 3M, and Pfizer. The other authors reported having no financial disclosures.
The use of , results from a large longitudinal cohort study demonstrated.
“For the many clinicians treating former smokers who have successfully quit all nicotine products, the implications are that use of [electronic nicotine delivery systems] should be discouraged, just as use of all other tobacco products is discouraged,” researchers led by Colm D. Everard, PhD, reported in a study published in JAMA Network Open (2020 Jun 5. doi: 10.1001/jamanetworkopen.2020.4813).
Dr. Everard, of the National Institute on Drug Abuse, and colleagues based their comments on results from a survey of adult former smokers who participated in Waves 1-4 of the Population Assessment of Tobacco and Health (PATH) Study (2013-2018). They limited the analysis to 2,273 former cigarette smokers who self-reported reported no tobacco product use at Wave 1, and categorized them as recent former smokers (defined as having last smoked within the past 12 previous months) or as long-term former smokers (defined as having last smoked for longer ago than in the previous 12 months). The main outcome of interest was the self-reported current use of cigarettes at follow-up interviews, which was defined as every day or some days. Electronic nicotine delivery systems (ENDS) comprised e-cigarettes, e-cigars, e-pipes, and e-hookahs. Other tobacco products included cigars, pipe tobacco, hookahs, snus tobacco, other smokeless tobacco, and dissolvable tobacco.
Of the 2,273 adult former smokers, 52% were women, 60% were older than age 50, and 80% were non-Hispanic white. Adjusted hazard ratio (AHR) analysis revealed that the use of ENDS was associated with significant risk of cigarette smoking relapse among recent former smokers (AHR 1.63) and among long-term former smokers (AHR 3.79). The use of other tobacco products was associated with significant risk for cigarette smoking relapse among recent former smokers (AHR 1.97) and among long-term former smokers (AHR 3.82).
The authors acknowledged certain limitations of the study, including the fact that it did not assess different ENDS devices, different e-liquid nicotine levels, or frequency of ENDS use and their associations with cigarette smoking relapse. It also did not explore the mechanism by which ENDS use may lead to reestablishing or reinforcing nicotine-seeking behavior among former cigarette users. “Determining pharmacologic, behavioral, or some other explanation for these findings may require laboratory-based research,” they wrote.
The PATH Study is supported with federal funds from the National Institute on Drug Abuse, the National Institutes of Health, and the Food and Drug Administration and Department of Health and Human Services under a contract to Westat. One of the study authors, Wilson M. Compton, MD, reported having long-term stock holdings in General Electric, 3M, and Pfizer. The other authors reported having no financial disclosures.
The use of , results from a large longitudinal cohort study demonstrated.
“For the many clinicians treating former smokers who have successfully quit all nicotine products, the implications are that use of [electronic nicotine delivery systems] should be discouraged, just as use of all other tobacco products is discouraged,” researchers led by Colm D. Everard, PhD, reported in a study published in JAMA Network Open (2020 Jun 5. doi: 10.1001/jamanetworkopen.2020.4813).
Dr. Everard, of the National Institute on Drug Abuse, and colleagues based their comments on results from a survey of adult former smokers who participated in Waves 1-4 of the Population Assessment of Tobacco and Health (PATH) Study (2013-2018). They limited the analysis to 2,273 former cigarette smokers who self-reported reported no tobacco product use at Wave 1, and categorized them as recent former smokers (defined as having last smoked within the past 12 previous months) or as long-term former smokers (defined as having last smoked for longer ago than in the previous 12 months). The main outcome of interest was the self-reported current use of cigarettes at follow-up interviews, which was defined as every day or some days. Electronic nicotine delivery systems (ENDS) comprised e-cigarettes, e-cigars, e-pipes, and e-hookahs. Other tobacco products included cigars, pipe tobacco, hookahs, snus tobacco, other smokeless tobacco, and dissolvable tobacco.
Of the 2,273 adult former smokers, 52% were women, 60% were older than age 50, and 80% were non-Hispanic white. Adjusted hazard ratio (AHR) analysis revealed that the use of ENDS was associated with significant risk of cigarette smoking relapse among recent former smokers (AHR 1.63) and among long-term former smokers (AHR 3.79). The use of other tobacco products was associated with significant risk for cigarette smoking relapse among recent former smokers (AHR 1.97) and among long-term former smokers (AHR 3.82).
The authors acknowledged certain limitations of the study, including the fact that it did not assess different ENDS devices, different e-liquid nicotine levels, or frequency of ENDS use and their associations with cigarette smoking relapse. It also did not explore the mechanism by which ENDS use may lead to reestablishing or reinforcing nicotine-seeking behavior among former cigarette users. “Determining pharmacologic, behavioral, or some other explanation for these findings may require laboratory-based research,” they wrote.
The PATH Study is supported with federal funds from the National Institute on Drug Abuse, the National Institutes of Health, and the Food and Drug Administration and Department of Health and Human Services under a contract to Westat. One of the study authors, Wilson M. Compton, MD, reported having long-term stock holdings in General Electric, 3M, and Pfizer. The other authors reported having no financial disclosures.
FROM JAMA NETWORK OPEN
Outcomes Comparison of the Veterans’ Choice Program With the Veterans Affairs Health Care System for Hepatitis C Treatment
Population studies show high prevalence of chronic hepatitis C virus (HCV) infection among veterans, especially Vietnam War era veterans.1,2 The development of safe and efficacious direct-acting antiviral (DAA) medications to treat HCV infection made the majority of those infected eligible for treatment. However, the large number of veterans needing DAA treatment stressed the resources of the US Department of Veterans Affairs (VA) health care system. This occurred while Congress was focused on reducing wait times for veterans receiving care at the VA.
Congress passed the Veterans Access, Choice, and Accountability Act on August 7, 2014, leading to the creation of the Veterans Choice Program. Legislators felt there were inappropriate delays in care at the VA, and the Choice program was meant to address this problem and provide an “apples-to-apples comparison [of the VA] with non-VA hospitals.”3
Congress acknowledged the importance of curing HCV in the veteran population and allocated $1.5 billion for fiscal year (FY) 2016 for DAAs. The VA Central Office (VACO) carefully monitored these resources. The first policy memorandum from VACO for HCV care, issued on May 21, 2015, recommended that the sickest patients who will benefit from the treatment “receive priority over those who are less ill.”4,5 Those who met criteria for advanced liver disease were prioritized for treatment at the VA, while those who did not meet criteria were given the option of receiving treatment through Choice, or waiting for a change in policy.6 Over time, revisions to the guidelines relaxed the criteria for VA treatment eligibility, and on February 24, 2016, all restrictions on HCV treatment at the VA were lifted.7,8
The aim of this study was to provide a comparison of VA and non-VA care, specifically to determine whether care provided through Choice was timelier and more cost effective than care provided by the VA, and whether there was a quality difference. The high prevalence among veterans, wellestablished standards of care, and finite treatment course with clear indicators of success and failure makes HCV treatment an ideal disease with which to make this comparison.
Methods
We retrospectively analyzed the VA electronic health records of all veterans seen in the VA Loma Linda Healthcare System (VALLHCS) Hepatology clinic for chronic HCV infection during FY 2016 who were referred to Choice for HCV treatment. One hundred veterans met these criteria, encompassing the Choice population; 71 were seen at least once by a non-VA (Choice) health care provider (HCP) and 61 completed a treatment regimen through Choice. Treatment completion was defined as cessation of medication after the planned duration of therapy, or early termination of medication without resumption by that HCP. The Choice population was matched to an equal number of veterans who received HCV treatment from VALLHCS HCPs.
Data collected included age, gender, HCV genotype, determinants of liver fibrosis, and treatment success (defined as sustained virologic response at 12 weeks after the last dose of medication [SVR12]). Determinants of liver fibrosis included documented cirrhosis or complications of cirrhosis, Fibrosis-4 score (Fib-4), and platelet count.
Treatment failures were categorized as nonresponse (defined as detectable HCV viral load at the end of treatment), relapse (defined as an undetectable HCV viral load at the end of treatment with a subsequent positive test), and early termination (defined as a failure to complete the planned treatment regimen). Documented patient nonadherence, medical comorbidities that affected the treatment protocol, mental health diagnoses, and active social issues (defined as active or history of heavy alcohol use, active or history of illicit drug use, lack of social support, and homelessness) were noted.
Timeliness of delivery of care was measured in days. For the VA group, the wait time was defined as the date the consult for HCV treatment was placed to the date of the initial appointment with the HCV treatment provider. For the Choice group, the wait time was defined as the date the referral to the Choice program was made to the date of the initial appointment with the Choice HCP. Treatment regimens were evaluated for appropriateness based on guidelines from VACO and the American Association for the Study of Liver Diseases.9-11
Tests performed by Choice providers were evaluated for whether they were relevant to HCV care and whether similar data already were available from VALLHCS. Tests that were not indicated were identified as unnecessary costs incurred by the Choice program, as were tests that had to be repeated at the VA because of a lack of documentation from the Choice provider. All medications given inappropriately were considered added costs. Medicare reimbursement rates for the most applicable Current Procedural Terminology (CPT) code and VA national contract pricing for medications were used for calculations. This study was approved by the VALLHCS institutional review board.
Statistical Analysis
IBM (Armonk, NY) Statistical Package for Social Sciences software was used to evaluate for differences in Fib-4, platelet count, prevalence of cirrhosis, prevalence of medical comorbidities, prevalence of mental health comorbidities, prevalence of the social issues defined in the Methods section, time from referral to time of appointment date, and SVR12 rate between the VA and Choice groups.
Exclusions
There were 15 veterans in the VA group who had a wait time of > 100 days. Of these, 5 (33%) were initially Choice referrals, but due to negative interactions with the Choice provider, the veterans returned to VALLHCS for care. Two of the 15 (13%) did not keep appointments and were lost to follow up. Six of the 15 (40%) had medical comorbidities that required more immediate attention, so HCV treatment initiation was deliberately moved back. The final 2 veterans scheduled their appointments unusually far apart, artificially increasing their wait time. Given that these were unique situations and some of the veterans received care from both Choice and VA providers, a decision was made to exclude these individuals from the study.
It has been shown that platelet count correlates with degree of liver fibrosis, a concept that is the basis for the Fib-4 scoring system.12 Studies have shown that platelet count is a survival predictor in those with cirrhosis, and thrombocytopenia is a negative predictor of HCV treatment success using peginterferon and ribavirin13,14 Therefore, the VA memorandum automatically assigned the sickest individuals to the VA for HCV treatment. The goal of this study was to compare the impact of factors other than stage of fibrosis on HCV treatment success, which is why the 12 veterans with platelet count < 100,000 in the VA group were excluded. There were no veterans with platelet count < 100,000 in the Choice group.
When comparing SVR12 rates between the VA and Choice groups, every veteran treated at VALLHCS in FY 2016 was included, increasing the number in the VA group from 100 to 320 and therefore the power of this comparison.
Results
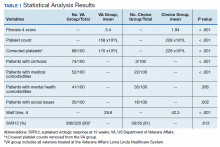
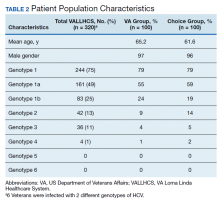
A summary of the statistical analysis can be found in Table 1. The genotype distribution was consistent with epidemiological studies, including those specific to veterans.15,16 There were statistically significant differences (P < .001) in mean Fib-4 and mean platelet count. The VA group had a higher Fib-4 and lower platelet count. Seventy-four percent of the VA population was defined as cirrhotic, while only 3% of the Choice population met similar criteria (P < .001). The VA and Choice groups were similar in terms of age, gender, and genotype distribution (Table 2).
The VA group was found to have a higher prevalence of comorbidities that affected HCV treatment. These conditions included but were not limited to: chronic kidney disease that precluded the use of certain medications, any condition that required medication with a known interaction with DAAs (ie, proton pump inhibitors, statins, and amiodarone), and cirrhosis if it impacted the treatment regimen. The difference in the prevalence of mental health comorbidities was not significant (P = .39), but there was a higher prevalence of social issues among the VA group (P = .002).
The mean wait time from referral to appointment was 28.6 days for the VA group and 42.3 days for the Choice group (P < .001), indicating that a Choice referral took longer to complete than a referral within the VA for HCV treatment. Thirty of the 71 (42%) veterans seen by a Choice provider accrued extraneous cost, with a mean additional cost of $8,561.40 per veteran. In the Choice group, 61 veterans completed a treatment regimen with the Choice HCP. Fifty-five veterans completed treatment and had available SVR12 data (6 were lost to follow up without SVR12 testing) and 50 (91%) had confirmed SVR12. The charts of the 5 treatment failures were reviewed to discern the cause for failure. Two cases involved early termination of therapy, 3 involved relapse and 2 failed to comply with medication instructions. There was 1 case of the Choice HCP not addressing simultaneous use of ledipasvir and a proton pump inhibitor, potentially causing an interaction, and 1 case where both the VA and Choice providers failed to recognize indicators of cirrhosis, which impacted the regimen used.
In the VALLHCS group, records of 320 veterans who completed treatment and had SVR12 testing were reviewed. While the Choice memorandum was active, veterans selected to be treated at VALLHCS had advanced liver fibrosis or cirrhosis, medical and mental health comorbidities that increased the risk of treatment complications or were considered to have difficulty adhering to the medication regimen. For this group, 296 (93%) had confirmed SVR12. Eighteen of the 24 (75%) treatment failures were complicated by nonadherence, including all 13 cases of early termination. One patient died from complications of decompensated cirrhosis before completing treatment, and 1 did not receive HCV medications during a hospital admission due to poor coordination of care between the VA inpatient and outpatient pharmacy services, leading to multiple missed doses.
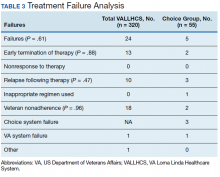
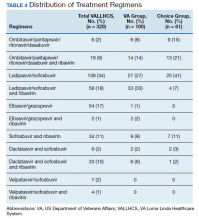
The difference in SVR12 rates (ie, treatment failure rates), between the VA and Choice groups was not statistically significant (P = .61). None of the specific reasons for treatment failure had a statistically significant difference between groups. A treatment failure analysis is shown in Table 3, and Table 4 indicates the breakdown of treatment regimens.
Discussion
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, consisting of 152 medical centers and > 1,700 sites of care. The VA has the potential to meet the health care needs of 21.6 million veterans. About 9 million veterans are enrolled in the VA system and 5.9 million received health care through VHA.17 However, every medical service cannot realistically be made available at every facility, and some veterans have difficulty gaining access to VHA care; distance and wait times have been well-publicized issues that need further exploration.18,19 The Choice program is an attempt to meet gaps in VA coverage using non-VA HCPs.
HCV infection is a specific diagnosis with national treatment guidelines and wellstudied treatments; it can be cured, with an evidence- based definition of cure. The VACO policy memorandum to refer less sick veterans to Choice while treating sicker veterans at the VA provided the opportunity to directly compare the quality of the 2 programs. The SVR12 rates of VALLHCS and Choice providers were comparable to the national average at the time, and while the difference in SVR12 rate was not significant, VALLHCS treated a significantly higher number of patients with cirrhosis because of the referral criteria.20
The significant difference in medical comorbidities between the VA and Choice groups was not surprising, partly because of the referral criteria. Cirrhosis can impact the treatment regimen, especially in regard to use of ribavirin. Since the presence of mental health comorbidities did not affect selection into the Choice group, it makes sense that there was no significant difference in prevalence between the groups.
VACO allowed veterans with HCV treatment plans that VA HCPs felt were too complicated for the Choice program to be treated by VHA HCPs.9 VALLHCS exercised this right for veterans at risk for nonadherence, because in HCV treatment, nonadherence leads to treatment failure and development of drug resistant virus strains. Therefore, veterans who would have difficulty traveling to VALLHCS to pick up medications, those who lacked means of communication (such as those who were homeless), and those who had active substance abuse were treated at the VA, where closer monitoring and immediate access to a wide range of services was possible. Studies have confirmed the impact of these types of issues on HCV treatment adherence and success. 21 This explains the higher prevalence of social issues in the VA group.
For an internal referral for HCV treatment at VALLHCS, the hepatology provider submits a consult request to the HCV treatment provider, who works in the same office, making direct communication simple. The main administrative limiting factor to minimizing wait times is the number of HCPs, which is dependent on hiring allowances.
When a veteran is referred to Choice, the VA provider places a consult for non-VA care, which the VA Office of Community Care processes by compiling relevant documents and sending the package to Triwest Healthcare Alliance, a private insurance processor contracted with the VA. Triwest selects the Choice provider, often without any input from the VA, and arranges the veteran’s initial appointment.22 Geographic distance to the veteran’s address is the main selection criteria for Triwest. Documents sent between the Choice and VA HCPs go through the Office of Community Care and Triwest. This significantly increases the potential for delays and failed communication. Triwest had a comprehensive list of providers deemed to be qualified to treat HCV within the geographic catchment of VALLHCS. This list was reviewed, and all veterans referred to Choice had HCPs near their home address; therefore, availability of Choice HCPs was not an issue.
The VA can provide guidance on management of the veteran in the form of bundle packages containing a list of services for which the Choice provider is authorized to provide, and ones the Choice provider is not authorized to provide. Some Choice HCPs ordered tests that were not authorized for HCV treatment such as esophagogastroduodenoscopy, colonoscopy, and liver biopsy. In all, 17 of 71 (24%) veterans seen by Choice HCPs had tests performed or ordered that VA HCPs would not have obtained for the purpose of HCV treatment (Table 5).
In order to prevent veterans from receiving unnecessary tests, a VALLHCS hepatologist asked to be notified by VA administrators overseeing Choice referrals whenever a secondary authorization request (SAR) was submitted by a Choice HCP. This strategy is not standard VA practice, therefore at many VA sites these requested tests would have been performed by the Choice HCP, which is why SARs were factored into cost analysis.
SVR12 test results that were drawn too early and had to be repeated at VALLHCS were a cost unique to the Choice program. Duplicate tests, particularly imaging studies and blood work, were extraneous costs. The largest extraneous costs were treatment regimens prescribed by Choice HCPs that did not follow standard of care and required VA provider intervention. Thirty of the 71 (42%) veterans seen by a Choice provider accrued a mean $8,561.40 in extra costs. As a result, the Choice program cost VHA $250,000 more to provide care for 30 veterans (enough to pay for a physician’s annual salary).
Some inappropriate treatment regimens were the result of Choice HCP error, such as 1 case in which a veteran was inadvertently switched from ledipasvir/sofosbuvir to ombitasvir/ paritaprevir/ritonavir/dasabuvir after week 4. The veteran had to start therapy over but still achieved SVR12. Other cases saw veterans receive regimens for which they had clear contraindications, such as creatinine clearance < 30 mL/min/1.73m2 for sofosbuvir or a positive resistance panel for specific medications. Eleven of 62 (18%) veterans who were started on HCV treatment by a Choice HCP received a regimen not consistent with VA guidelines—an alarming result.
Follow up for veterans referred to Choice was extremely labor intensive, and assessment of personnel requirements in a Choice-based VA system must take this into consideration. The Choice HCP has no obligation to communicate with the VA HCP. At the time of chart review, 57 of 71 (80%) Choice veterans had inadequate documentation to make a confident assessment of the treatment outcome. Multiple calls to the offices of the Choice HCP were needed to acquire records, and veterans had to be tracked down for additional tests. Veterans often would complete treatment and stop following up with the Choice provider before SVR12 confirmation. The VA hepatology provider reviewing Choice referrals served as clinician, case manager, and clerk in order to get veterans to an appropriate end point in their hepatitis C treatment, with mostly unmeasured hours of work.
Limitations
The study population size was limited by the number of veterans able to complete treatment through Choice. The parameters in the VACO policy memos automatically selected the VA and Choice groups but made them clinically distinct populations. New treatment medications were released during the study period, which impacted management strategy. Occasionally, VA and non- VA HCPs preferred different treatment regimens, leading to variation in the distribution of regimens used despite similar genotype distribution (Tables 2 and 4). In addition, a retrospective study is at risk for recall bias. A prospective study randomizing veterans to the Choice and VA groups is an important future endeavor. Comparing veteran satisfaction for Choice and VA services is also crucial.
Conclusions
This study demonstrates that the VA was able to provide more cost-effective and more timely care for HCV treatment to a relatively sicker population with no reduction in treatment success when compared with non-VA HCPs through the Choice program. While the Choice program can help veterans receive services they may otherwise not have access to and reduce travel time, the current system introduces inefficiencies that delay care and decrease cost-effectiveness. The Choice HCP selection process is based on proximity rather than quality, which may place the veteran at risk for receiving substandard care. Large-scale quality of care studies that compare efficiency measures, clinical outcomes, patient demographics, travel distance, cost efficacy and patient satisfaction for veterans receiving similar services at a VA facility and through Choice should be performed to ensure that veterans receive the best care available.
1. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300. doi:10.7326/M13-1133
2. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88-96. doi:10.1002/hep.20502
3. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
4. Tuchschmidt J. Attachment C: Provision of hepatitis C treatment. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/education /choice-provision-hcv-treatment.asp. Published May 21, 2015. [Nonpublic site.]
5. Tuchschmidt J. Attachment A: Provision of hepatitis C (HCV) treatment through the Choice program. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http:// vaww.hepatitis.va.gov/pdf/choice-attachment-a-FY16 .pdf. Published May 21, 2015. [Nonpublic site.]
6. Tuchschmidt J. Attachment B: Initiation of hepatitis C virus (HCV) treatment: protocol for prioritization. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww .hepatitis.va.gov/pdf/provision-HCV-treatment-attach ment-b.pdf. Published May 21, 2015. [Nonpublic site.]
7. Murphy, JP. Hepatitis C virus funding and prioritization status. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations. http://vaww.hepatitis .va.gov/education/choice-memo-hcv-funding-and -prioritization-status-01272016.asp. Published January 27, 2016. [Nonpublic site.]
8. Lynch TJ, McCarthy MF. Hepatitis C virus funding and prioritization status update. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations and Acting Assistant Deputy Under Secretary for Health for Patient Care Services. http://vaww.hepatitis.va.gov /education/choice-funding-update-feb-2016.asp. Published February 24, 2016. [Nonpublic site.]
9. Morgan TR, Yee H; US Department of Veterans Affairs National Hepatitis C Resource Center Program and the National Viral Hepatitis Program in the Office of Patient Care Services. Chronic hepatitis C virus (HCV) infection: treatment considerations. http://vaww.hepatitis.va.gov /pdf/treatment-considerations-2016-03-28.pdf. Published March 28, 2016. [Nonpublic site.]
10. American Association for the Study of Liver Diseases; Infectious Diseases Society of America. Initial Treatment Box. http://hcvguidelines.org/full-report/initial-treatment -box-summary-recommendations-patients-who-are -initiating-therapy-hcv. Updated November 6, 2019. Accessed May 11, 2020.
11. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3): 932-954. doi:10.1002/hep.27950
12. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006; 43(6):1317-1325. doi:10.1002/hep.21178
13. Realdi G, Fattovich G, Hadziyannis S, et al. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. The Investigators of the European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol. 1994;21(4):656-666. doi:10.1016/s0168 -8278(94)80115-0
14. Kanda T, Kato K, Tsubota A, et al. Platelet count and sustained virological response in hepatitis C treatment. World J Hepatol. 2013;5(4):182-188. doi:10.4254/wjh.v5.i4.182
15. Manos MM, Shvachko VA, Murphy RC, Arduino JM, Shire NJ. Distribution of hepatitis C virus genotypes in a diverse US integrated health care population. J Med Virol. 2012;84(11):1744-1750. doi:10.1002/jmv.23399
16. Cheung RC. Epidemiology of hepatitis C virus infection in American veterans. Am J Gastroenterol. 2000;95(3): 740-747. doi:10.1111/j.1572-0241.2000.01854.x
17. Bagalman E. The number of Veterans that use VA health care services: a fact sheet. Congressional Research Service Report R43579. https://fas.org/sgp/crs/misc/R43579.pdf. Published June 3, 2014. Accessed May 11, 2020.
18. US General Accounting Office. Report to the Ranking Minority Member, Subcommittee on Compensation, Pension, Insurance, and Memorial Affairs, Committee on Veterans’ Affairs, US House of Representatives. How distance from VA facilities affects veterans’ use of VA services. https:// www.gao.gov/assets/230/221992.pdf. Published December 1995. Accessed May 11, 2020.
19. Bronstein S, Griffin D. A fatal wait: Veterans languish and die on a VA hospital’s secret list. http://www.cnn .com/2014/04/23/health/veterans-dying-health-care -delays/index.html. Published April 23, 2014. Accessed May 11, 2020.
20. Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs national health care system. Gastroenterology. 2016;151(3):457- 471. doi:10.1053/j.gastro.2016.05.049
21. Malespin MH, Harris C, Kanar O, et al. Barriers to treatment of chronic hepatitis C with direct acting antivirals in an urban clinic. Ann Hepatol. 2019;18(2):304-309. doi:10.1016/j.aohep.2018.06.001
22. Tuchschmidt J. Attachment D: Hepatitis C virus (HCV) fact sheet for Veterans Choice Program for both VA and Choice providers. US Department of Veterans Affairs Central Office Memorandum from the Deputy Under Secretary for Health for Policy and Services and the Acting Deputy Undersecretary for Health for Operations and Management. http://vaww .hepatitis.va.gov/educatiochoice-provision-HCV-treatment -additional.asp. [Nonpublic site.]
Population studies show high prevalence of chronic hepatitis C virus (HCV) infection among veterans, especially Vietnam War era veterans.1,2 The development of safe and efficacious direct-acting antiviral (DAA) medications to treat HCV infection made the majority of those infected eligible for treatment. However, the large number of veterans needing DAA treatment stressed the resources of the US Department of Veterans Affairs (VA) health care system. This occurred while Congress was focused on reducing wait times for veterans receiving care at the VA.
Congress passed the Veterans Access, Choice, and Accountability Act on August 7, 2014, leading to the creation of the Veterans Choice Program. Legislators felt there were inappropriate delays in care at the VA, and the Choice program was meant to address this problem and provide an “apples-to-apples comparison [of the VA] with non-VA hospitals.”3
Congress acknowledged the importance of curing HCV in the veteran population and allocated $1.5 billion for fiscal year (FY) 2016 for DAAs. The VA Central Office (VACO) carefully monitored these resources. The first policy memorandum from VACO for HCV care, issued on May 21, 2015, recommended that the sickest patients who will benefit from the treatment “receive priority over those who are less ill.”4,5 Those who met criteria for advanced liver disease were prioritized for treatment at the VA, while those who did not meet criteria were given the option of receiving treatment through Choice, or waiting for a change in policy.6 Over time, revisions to the guidelines relaxed the criteria for VA treatment eligibility, and on February 24, 2016, all restrictions on HCV treatment at the VA were lifted.7,8
The aim of this study was to provide a comparison of VA and non-VA care, specifically to determine whether care provided through Choice was timelier and more cost effective than care provided by the VA, and whether there was a quality difference. The high prevalence among veterans, wellestablished standards of care, and finite treatment course with clear indicators of success and failure makes HCV treatment an ideal disease with which to make this comparison.
Methods
We retrospectively analyzed the VA electronic health records of all veterans seen in the VA Loma Linda Healthcare System (VALLHCS) Hepatology clinic for chronic HCV infection during FY 2016 who were referred to Choice for HCV treatment. One hundred veterans met these criteria, encompassing the Choice population; 71 were seen at least once by a non-VA (Choice) health care provider (HCP) and 61 completed a treatment regimen through Choice. Treatment completion was defined as cessation of medication after the planned duration of therapy, or early termination of medication without resumption by that HCP. The Choice population was matched to an equal number of veterans who received HCV treatment from VALLHCS HCPs.
Data collected included age, gender, HCV genotype, determinants of liver fibrosis, and treatment success (defined as sustained virologic response at 12 weeks after the last dose of medication [SVR12]). Determinants of liver fibrosis included documented cirrhosis or complications of cirrhosis, Fibrosis-4 score (Fib-4), and platelet count.
Treatment failures were categorized as nonresponse (defined as detectable HCV viral load at the end of treatment), relapse (defined as an undetectable HCV viral load at the end of treatment with a subsequent positive test), and early termination (defined as a failure to complete the planned treatment regimen). Documented patient nonadherence, medical comorbidities that affected the treatment protocol, mental health diagnoses, and active social issues (defined as active or history of heavy alcohol use, active or history of illicit drug use, lack of social support, and homelessness) were noted.
Timeliness of delivery of care was measured in days. For the VA group, the wait time was defined as the date the consult for HCV treatment was placed to the date of the initial appointment with the HCV treatment provider. For the Choice group, the wait time was defined as the date the referral to the Choice program was made to the date of the initial appointment with the Choice HCP. Treatment regimens were evaluated for appropriateness based on guidelines from VACO and the American Association for the Study of Liver Diseases.9-11
Tests performed by Choice providers were evaluated for whether they were relevant to HCV care and whether similar data already were available from VALLHCS. Tests that were not indicated were identified as unnecessary costs incurred by the Choice program, as were tests that had to be repeated at the VA because of a lack of documentation from the Choice provider. All medications given inappropriately were considered added costs. Medicare reimbursement rates for the most applicable Current Procedural Terminology (CPT) code and VA national contract pricing for medications were used for calculations. This study was approved by the VALLHCS institutional review board.
Statistical Analysis
IBM (Armonk, NY) Statistical Package for Social Sciences software was used to evaluate for differences in Fib-4, platelet count, prevalence of cirrhosis, prevalence of medical comorbidities, prevalence of mental health comorbidities, prevalence of the social issues defined in the Methods section, time from referral to time of appointment date, and SVR12 rate between the VA and Choice groups.
Exclusions
There were 15 veterans in the VA group who had a wait time of > 100 days. Of these, 5 (33%) were initially Choice referrals, but due to negative interactions with the Choice provider, the veterans returned to VALLHCS for care. Two of the 15 (13%) did not keep appointments and were lost to follow up. Six of the 15 (40%) had medical comorbidities that required more immediate attention, so HCV treatment initiation was deliberately moved back. The final 2 veterans scheduled their appointments unusually far apart, artificially increasing their wait time. Given that these were unique situations and some of the veterans received care from both Choice and VA providers, a decision was made to exclude these individuals from the study.
It has been shown that platelet count correlates with degree of liver fibrosis, a concept that is the basis for the Fib-4 scoring system.12 Studies have shown that platelet count is a survival predictor in those with cirrhosis, and thrombocytopenia is a negative predictor of HCV treatment success using peginterferon and ribavirin13,14 Therefore, the VA memorandum automatically assigned the sickest individuals to the VA for HCV treatment. The goal of this study was to compare the impact of factors other than stage of fibrosis on HCV treatment success, which is why the 12 veterans with platelet count < 100,000 in the VA group were excluded. There were no veterans with platelet count < 100,000 in the Choice group.
When comparing SVR12 rates between the VA and Choice groups, every veteran treated at VALLHCS in FY 2016 was included, increasing the number in the VA group from 100 to 320 and therefore the power of this comparison.
Results
A summary of the statistical analysis can be found in Table 1. The genotype distribution was consistent with epidemiological studies, including those specific to veterans.15,16 There were statistically significant differences (P < .001) in mean Fib-4 and mean platelet count. The VA group had a higher Fib-4 and lower platelet count. Seventy-four percent of the VA population was defined as cirrhotic, while only 3% of the Choice population met similar criteria (P < .001). The VA and Choice groups were similar in terms of age, gender, and genotype distribution (Table 2).
The VA group was found to have a higher prevalence of comorbidities that affected HCV treatment. These conditions included but were not limited to: chronic kidney disease that precluded the use of certain medications, any condition that required medication with a known interaction with DAAs (ie, proton pump inhibitors, statins, and amiodarone), and cirrhosis if it impacted the treatment regimen. The difference in the prevalence of mental health comorbidities was not significant (P = .39), but there was a higher prevalence of social issues among the VA group (P = .002).
The mean wait time from referral to appointment was 28.6 days for the VA group and 42.3 days for the Choice group (P < .001), indicating that a Choice referral took longer to complete than a referral within the VA for HCV treatment. Thirty of the 71 (42%) veterans seen by a Choice provider accrued extraneous cost, with a mean additional cost of $8,561.40 per veteran. In the Choice group, 61 veterans completed a treatment regimen with the Choice HCP. Fifty-five veterans completed treatment and had available SVR12 data (6 were lost to follow up without SVR12 testing) and 50 (91%) had confirmed SVR12. The charts of the 5 treatment failures were reviewed to discern the cause for failure. Two cases involved early termination of therapy, 3 involved relapse and 2 failed to comply with medication instructions. There was 1 case of the Choice HCP not addressing simultaneous use of ledipasvir and a proton pump inhibitor, potentially causing an interaction, and 1 case where both the VA and Choice providers failed to recognize indicators of cirrhosis, which impacted the regimen used.
In the VALLHCS group, records of 320 veterans who completed treatment and had SVR12 testing were reviewed. While the Choice memorandum was active, veterans selected to be treated at VALLHCS had advanced liver fibrosis or cirrhosis, medical and mental health comorbidities that increased the risk of treatment complications or were considered to have difficulty adhering to the medication regimen. For this group, 296 (93%) had confirmed SVR12. Eighteen of the 24 (75%) treatment failures were complicated by nonadherence, including all 13 cases of early termination. One patient died from complications of decompensated cirrhosis before completing treatment, and 1 did not receive HCV medications during a hospital admission due to poor coordination of care between the VA inpatient and outpatient pharmacy services, leading to multiple missed doses.
The difference in SVR12 rates (ie, treatment failure rates), between the VA and Choice groups was not statistically significant (P = .61). None of the specific reasons for treatment failure had a statistically significant difference between groups. A treatment failure analysis is shown in Table 3, and Table 4 indicates the breakdown of treatment regimens.
Discussion
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, consisting of 152 medical centers and > 1,700 sites of care. The VA has the potential to meet the health care needs of 21.6 million veterans. About 9 million veterans are enrolled in the VA system and 5.9 million received health care through VHA.17 However, every medical service cannot realistically be made available at every facility, and some veterans have difficulty gaining access to VHA care; distance and wait times have been well-publicized issues that need further exploration.18,19 The Choice program is an attempt to meet gaps in VA coverage using non-VA HCPs.
HCV infection is a specific diagnosis with national treatment guidelines and wellstudied treatments; it can be cured, with an evidence- based definition of cure. The VACO policy memorandum to refer less sick veterans to Choice while treating sicker veterans at the VA provided the opportunity to directly compare the quality of the 2 programs. The SVR12 rates of VALLHCS and Choice providers were comparable to the national average at the time, and while the difference in SVR12 rate was not significant, VALLHCS treated a significantly higher number of patients with cirrhosis because of the referral criteria.20
The significant difference in medical comorbidities between the VA and Choice groups was not surprising, partly because of the referral criteria. Cirrhosis can impact the treatment regimen, especially in regard to use of ribavirin. Since the presence of mental health comorbidities did not affect selection into the Choice group, it makes sense that there was no significant difference in prevalence between the groups.
VACO allowed veterans with HCV treatment plans that VA HCPs felt were too complicated for the Choice program to be treated by VHA HCPs.9 VALLHCS exercised this right for veterans at risk for nonadherence, because in HCV treatment, nonadherence leads to treatment failure and development of drug resistant virus strains. Therefore, veterans who would have difficulty traveling to VALLHCS to pick up medications, those who lacked means of communication (such as those who were homeless), and those who had active substance abuse were treated at the VA, where closer monitoring and immediate access to a wide range of services was possible. Studies have confirmed the impact of these types of issues on HCV treatment adherence and success. 21 This explains the higher prevalence of social issues in the VA group.
For an internal referral for HCV treatment at VALLHCS, the hepatology provider submits a consult request to the HCV treatment provider, who works in the same office, making direct communication simple. The main administrative limiting factor to minimizing wait times is the number of HCPs, which is dependent on hiring allowances.
When a veteran is referred to Choice, the VA provider places a consult for non-VA care, which the VA Office of Community Care processes by compiling relevant documents and sending the package to Triwest Healthcare Alliance, a private insurance processor contracted with the VA. Triwest selects the Choice provider, often without any input from the VA, and arranges the veteran’s initial appointment.22 Geographic distance to the veteran’s address is the main selection criteria for Triwest. Documents sent between the Choice and VA HCPs go through the Office of Community Care and Triwest. This significantly increases the potential for delays and failed communication. Triwest had a comprehensive list of providers deemed to be qualified to treat HCV within the geographic catchment of VALLHCS. This list was reviewed, and all veterans referred to Choice had HCPs near their home address; therefore, availability of Choice HCPs was not an issue.
The VA can provide guidance on management of the veteran in the form of bundle packages containing a list of services for which the Choice provider is authorized to provide, and ones the Choice provider is not authorized to provide. Some Choice HCPs ordered tests that were not authorized for HCV treatment such as esophagogastroduodenoscopy, colonoscopy, and liver biopsy. In all, 17 of 71 (24%) veterans seen by Choice HCPs had tests performed or ordered that VA HCPs would not have obtained for the purpose of HCV treatment (Table 5).
In order to prevent veterans from receiving unnecessary tests, a VALLHCS hepatologist asked to be notified by VA administrators overseeing Choice referrals whenever a secondary authorization request (SAR) was submitted by a Choice HCP. This strategy is not standard VA practice, therefore at many VA sites these requested tests would have been performed by the Choice HCP, which is why SARs were factored into cost analysis.
SVR12 test results that were drawn too early and had to be repeated at VALLHCS were a cost unique to the Choice program. Duplicate tests, particularly imaging studies and blood work, were extraneous costs. The largest extraneous costs were treatment regimens prescribed by Choice HCPs that did not follow standard of care and required VA provider intervention. Thirty of the 71 (42%) veterans seen by a Choice provider accrued a mean $8,561.40 in extra costs. As a result, the Choice program cost VHA $250,000 more to provide care for 30 veterans (enough to pay for a physician’s annual salary).
Some inappropriate treatment regimens were the result of Choice HCP error, such as 1 case in which a veteran was inadvertently switched from ledipasvir/sofosbuvir to ombitasvir/ paritaprevir/ritonavir/dasabuvir after week 4. The veteran had to start therapy over but still achieved SVR12. Other cases saw veterans receive regimens for which they had clear contraindications, such as creatinine clearance < 30 mL/min/1.73m2 for sofosbuvir or a positive resistance panel for specific medications. Eleven of 62 (18%) veterans who were started on HCV treatment by a Choice HCP received a regimen not consistent with VA guidelines—an alarming result.
Follow up for veterans referred to Choice was extremely labor intensive, and assessment of personnel requirements in a Choice-based VA system must take this into consideration. The Choice HCP has no obligation to communicate with the VA HCP. At the time of chart review, 57 of 71 (80%) Choice veterans had inadequate documentation to make a confident assessment of the treatment outcome. Multiple calls to the offices of the Choice HCP were needed to acquire records, and veterans had to be tracked down for additional tests. Veterans often would complete treatment and stop following up with the Choice provider before SVR12 confirmation. The VA hepatology provider reviewing Choice referrals served as clinician, case manager, and clerk in order to get veterans to an appropriate end point in their hepatitis C treatment, with mostly unmeasured hours of work.
Limitations
The study population size was limited by the number of veterans able to complete treatment through Choice. The parameters in the VACO policy memos automatically selected the VA and Choice groups but made them clinically distinct populations. New treatment medications were released during the study period, which impacted management strategy. Occasionally, VA and non- VA HCPs preferred different treatment regimens, leading to variation in the distribution of regimens used despite similar genotype distribution (Tables 2 and 4). In addition, a retrospective study is at risk for recall bias. A prospective study randomizing veterans to the Choice and VA groups is an important future endeavor. Comparing veteran satisfaction for Choice and VA services is also crucial.
Conclusions
This study demonstrates that the VA was able to provide more cost-effective and more timely care for HCV treatment to a relatively sicker population with no reduction in treatment success when compared with non-VA HCPs through the Choice program. While the Choice program can help veterans receive services they may otherwise not have access to and reduce travel time, the current system introduces inefficiencies that delay care and decrease cost-effectiveness. The Choice HCP selection process is based on proximity rather than quality, which may place the veteran at risk for receiving substandard care. Large-scale quality of care studies that compare efficiency measures, clinical outcomes, patient demographics, travel distance, cost efficacy and patient satisfaction for veterans receiving similar services at a VA facility and through Choice should be performed to ensure that veterans receive the best care available.
Population studies show high prevalence of chronic hepatitis C virus (HCV) infection among veterans, especially Vietnam War era veterans.1,2 The development of safe and efficacious direct-acting antiviral (DAA) medications to treat HCV infection made the majority of those infected eligible for treatment. However, the large number of veterans needing DAA treatment stressed the resources of the US Department of Veterans Affairs (VA) health care system. This occurred while Congress was focused on reducing wait times for veterans receiving care at the VA.
Congress passed the Veterans Access, Choice, and Accountability Act on August 7, 2014, leading to the creation of the Veterans Choice Program. Legislators felt there were inappropriate delays in care at the VA, and the Choice program was meant to address this problem and provide an “apples-to-apples comparison [of the VA] with non-VA hospitals.”3
Congress acknowledged the importance of curing HCV in the veteran population and allocated $1.5 billion for fiscal year (FY) 2016 for DAAs. The VA Central Office (VACO) carefully monitored these resources. The first policy memorandum from VACO for HCV care, issued on May 21, 2015, recommended that the sickest patients who will benefit from the treatment “receive priority over those who are less ill.”4,5 Those who met criteria for advanced liver disease were prioritized for treatment at the VA, while those who did not meet criteria were given the option of receiving treatment through Choice, or waiting for a change in policy.6 Over time, revisions to the guidelines relaxed the criteria for VA treatment eligibility, and on February 24, 2016, all restrictions on HCV treatment at the VA were lifted.7,8
The aim of this study was to provide a comparison of VA and non-VA care, specifically to determine whether care provided through Choice was timelier and more cost effective than care provided by the VA, and whether there was a quality difference. The high prevalence among veterans, wellestablished standards of care, and finite treatment course with clear indicators of success and failure makes HCV treatment an ideal disease with which to make this comparison.
Methods
We retrospectively analyzed the VA electronic health records of all veterans seen in the VA Loma Linda Healthcare System (VALLHCS) Hepatology clinic for chronic HCV infection during FY 2016 who were referred to Choice for HCV treatment. One hundred veterans met these criteria, encompassing the Choice population; 71 were seen at least once by a non-VA (Choice) health care provider (HCP) and 61 completed a treatment regimen through Choice. Treatment completion was defined as cessation of medication after the planned duration of therapy, or early termination of medication without resumption by that HCP. The Choice population was matched to an equal number of veterans who received HCV treatment from VALLHCS HCPs.
Data collected included age, gender, HCV genotype, determinants of liver fibrosis, and treatment success (defined as sustained virologic response at 12 weeks after the last dose of medication [SVR12]). Determinants of liver fibrosis included documented cirrhosis or complications of cirrhosis, Fibrosis-4 score (Fib-4), and platelet count.
Treatment failures were categorized as nonresponse (defined as detectable HCV viral load at the end of treatment), relapse (defined as an undetectable HCV viral load at the end of treatment with a subsequent positive test), and early termination (defined as a failure to complete the planned treatment regimen). Documented patient nonadherence, medical comorbidities that affected the treatment protocol, mental health diagnoses, and active social issues (defined as active or history of heavy alcohol use, active or history of illicit drug use, lack of social support, and homelessness) were noted.
Timeliness of delivery of care was measured in days. For the VA group, the wait time was defined as the date the consult for HCV treatment was placed to the date of the initial appointment with the HCV treatment provider. For the Choice group, the wait time was defined as the date the referral to the Choice program was made to the date of the initial appointment with the Choice HCP. Treatment regimens were evaluated for appropriateness based on guidelines from VACO and the American Association for the Study of Liver Diseases.9-11
Tests performed by Choice providers were evaluated for whether they were relevant to HCV care and whether similar data already were available from VALLHCS. Tests that were not indicated were identified as unnecessary costs incurred by the Choice program, as were tests that had to be repeated at the VA because of a lack of documentation from the Choice provider. All medications given inappropriately were considered added costs. Medicare reimbursement rates for the most applicable Current Procedural Terminology (CPT) code and VA national contract pricing for medications were used for calculations. This study was approved by the VALLHCS institutional review board.
Statistical Analysis
IBM (Armonk, NY) Statistical Package for Social Sciences software was used to evaluate for differences in Fib-4, platelet count, prevalence of cirrhosis, prevalence of medical comorbidities, prevalence of mental health comorbidities, prevalence of the social issues defined in the Methods section, time from referral to time of appointment date, and SVR12 rate between the VA and Choice groups.
Exclusions
There were 15 veterans in the VA group who had a wait time of > 100 days. Of these, 5 (33%) were initially Choice referrals, but due to negative interactions with the Choice provider, the veterans returned to VALLHCS for care. Two of the 15 (13%) did not keep appointments and were lost to follow up. Six of the 15 (40%) had medical comorbidities that required more immediate attention, so HCV treatment initiation was deliberately moved back. The final 2 veterans scheduled their appointments unusually far apart, artificially increasing their wait time. Given that these were unique situations and some of the veterans received care from both Choice and VA providers, a decision was made to exclude these individuals from the study.
It has been shown that platelet count correlates with degree of liver fibrosis, a concept that is the basis for the Fib-4 scoring system.12 Studies have shown that platelet count is a survival predictor in those with cirrhosis, and thrombocytopenia is a negative predictor of HCV treatment success using peginterferon and ribavirin13,14 Therefore, the VA memorandum automatically assigned the sickest individuals to the VA for HCV treatment. The goal of this study was to compare the impact of factors other than stage of fibrosis on HCV treatment success, which is why the 12 veterans with platelet count < 100,000 in the VA group were excluded. There were no veterans with platelet count < 100,000 in the Choice group.
When comparing SVR12 rates between the VA and Choice groups, every veteran treated at VALLHCS in FY 2016 was included, increasing the number in the VA group from 100 to 320 and therefore the power of this comparison.
Results
A summary of the statistical analysis can be found in Table 1. The genotype distribution was consistent with epidemiological studies, including those specific to veterans.15,16 There were statistically significant differences (P < .001) in mean Fib-4 and mean platelet count. The VA group had a higher Fib-4 and lower platelet count. Seventy-four percent of the VA population was defined as cirrhotic, while only 3% of the Choice population met similar criteria (P < .001). The VA and Choice groups were similar in terms of age, gender, and genotype distribution (Table 2).
The VA group was found to have a higher prevalence of comorbidities that affected HCV treatment. These conditions included but were not limited to: chronic kidney disease that precluded the use of certain medications, any condition that required medication with a known interaction with DAAs (ie, proton pump inhibitors, statins, and amiodarone), and cirrhosis if it impacted the treatment regimen. The difference in the prevalence of mental health comorbidities was not significant (P = .39), but there was a higher prevalence of social issues among the VA group (P = .002).
The mean wait time from referral to appointment was 28.6 days for the VA group and 42.3 days for the Choice group (P < .001), indicating that a Choice referral took longer to complete than a referral within the VA for HCV treatment. Thirty of the 71 (42%) veterans seen by a Choice provider accrued extraneous cost, with a mean additional cost of $8,561.40 per veteran. In the Choice group, 61 veterans completed a treatment regimen with the Choice HCP. Fifty-five veterans completed treatment and had available SVR12 data (6 were lost to follow up without SVR12 testing) and 50 (91%) had confirmed SVR12. The charts of the 5 treatment failures were reviewed to discern the cause for failure. Two cases involved early termination of therapy, 3 involved relapse and 2 failed to comply with medication instructions. There was 1 case of the Choice HCP not addressing simultaneous use of ledipasvir and a proton pump inhibitor, potentially causing an interaction, and 1 case where both the VA and Choice providers failed to recognize indicators of cirrhosis, which impacted the regimen used.
In the VALLHCS group, records of 320 veterans who completed treatment and had SVR12 testing were reviewed. While the Choice memorandum was active, veterans selected to be treated at VALLHCS had advanced liver fibrosis or cirrhosis, medical and mental health comorbidities that increased the risk of treatment complications or were considered to have difficulty adhering to the medication regimen. For this group, 296 (93%) had confirmed SVR12. Eighteen of the 24 (75%) treatment failures were complicated by nonadherence, including all 13 cases of early termination. One patient died from complications of decompensated cirrhosis before completing treatment, and 1 did not receive HCV medications during a hospital admission due to poor coordination of care between the VA inpatient and outpatient pharmacy services, leading to multiple missed doses.
The difference in SVR12 rates (ie, treatment failure rates), between the VA and Choice groups was not statistically significant (P = .61). None of the specific reasons for treatment failure had a statistically significant difference between groups. A treatment failure analysis is shown in Table 3, and Table 4 indicates the breakdown of treatment regimens.
Discussion
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, consisting of 152 medical centers and > 1,700 sites of care. The VA has the potential to meet the health care needs of 21.6 million veterans. About 9 million veterans are enrolled in the VA system and 5.9 million received health care through VHA.17 However, every medical service cannot realistically be made available at every facility, and some veterans have difficulty gaining access to VHA care; distance and wait times have been well-publicized issues that need further exploration.18,19 The Choice program is an attempt to meet gaps in VA coverage using non-VA HCPs.
HCV infection is a specific diagnosis with national treatment guidelines and wellstudied treatments; it can be cured, with an evidence- based definition of cure. The VACO policy memorandum to refer less sick veterans to Choice while treating sicker veterans at the VA provided the opportunity to directly compare the quality of the 2 programs. The SVR12 rates of VALLHCS and Choice providers were comparable to the national average at the time, and while the difference in SVR12 rate was not significant, VALLHCS treated a significantly higher number of patients with cirrhosis because of the referral criteria.20
The significant difference in medical comorbidities between the VA and Choice groups was not surprising, partly because of the referral criteria. Cirrhosis can impact the treatment regimen, especially in regard to use of ribavirin. Since the presence of mental health comorbidities did not affect selection into the Choice group, it makes sense that there was no significant difference in prevalence between the groups.
VACO allowed veterans with HCV treatment plans that VA HCPs felt were too complicated for the Choice program to be treated by VHA HCPs.9 VALLHCS exercised this right for veterans at risk for nonadherence, because in HCV treatment, nonadherence leads to treatment failure and development of drug resistant virus strains. Therefore, veterans who would have difficulty traveling to VALLHCS to pick up medications, those who lacked means of communication (such as those who were homeless), and those who had active substance abuse were treated at the VA, where closer monitoring and immediate access to a wide range of services was possible. Studies have confirmed the impact of these types of issues on HCV treatment adherence and success. 21 This explains the higher prevalence of social issues in the VA group.
For an internal referral for HCV treatment at VALLHCS, the hepatology provider submits a consult request to the HCV treatment provider, who works in the same office, making direct communication simple. The main administrative limiting factor to minimizing wait times is the number of HCPs, which is dependent on hiring allowances.
When a veteran is referred to Choice, the VA provider places a consult for non-VA care, which the VA Office of Community Care processes by compiling relevant documents and sending the package to Triwest Healthcare Alliance, a private insurance processor contracted with the VA. Triwest selects the Choice provider, often without any input from the VA, and arranges the veteran’s initial appointment.22 Geographic distance to the veteran’s address is the main selection criteria for Triwest. Documents sent between the Choice and VA HCPs go through the Office of Community Care and Triwest. This significantly increases the potential for delays and failed communication. Triwest had a comprehensive list of providers deemed to be qualified to treat HCV within the geographic catchment of VALLHCS. This list was reviewed, and all veterans referred to Choice had HCPs near their home address; therefore, availability of Choice HCPs was not an issue.
The VA can provide guidance on management of the veteran in the form of bundle packages containing a list of services for which the Choice provider is authorized to provide, and ones the Choice provider is not authorized to provide. Some Choice HCPs ordered tests that were not authorized for HCV treatment such as esophagogastroduodenoscopy, colonoscopy, and liver biopsy. In all, 17 of 71 (24%) veterans seen by Choice HCPs had tests performed or ordered that VA HCPs would not have obtained for the purpose of HCV treatment (Table 5).
In order to prevent veterans from receiving unnecessary tests, a VALLHCS hepatologist asked to be notified by VA administrators overseeing Choice referrals whenever a secondary authorization request (SAR) was submitted by a Choice HCP. This strategy is not standard VA practice, therefore at many VA sites these requested tests would have been performed by the Choice HCP, which is why SARs were factored into cost analysis.
SVR12 test results that were drawn too early and had to be repeated at VALLHCS were a cost unique to the Choice program. Duplicate tests, particularly imaging studies and blood work, were extraneous costs. The largest extraneous costs were treatment regimens prescribed by Choice HCPs that did not follow standard of care and required VA provider intervention. Thirty of the 71 (42%) veterans seen by a Choice provider accrued a mean $8,561.40 in extra costs. As a result, the Choice program cost VHA $250,000 more to provide care for 30 veterans (enough to pay for a physician’s annual salary).
Some inappropriate treatment regimens were the result of Choice HCP error, such as 1 case in which a veteran was inadvertently switched from ledipasvir/sofosbuvir to ombitasvir/ paritaprevir/ritonavir/dasabuvir after week 4. The veteran had to start therapy over but still achieved SVR12. Other cases saw veterans receive regimens for which they had clear contraindications, such as creatinine clearance < 30 mL/min/1.73m2 for sofosbuvir or a positive resistance panel for specific medications. Eleven of 62 (18%) veterans who were started on HCV treatment by a Choice HCP received a regimen not consistent with VA guidelines—an alarming result.
Follow up for veterans referred to Choice was extremely labor intensive, and assessment of personnel requirements in a Choice-based VA system must take this into consideration. The Choice HCP has no obligation to communicate with the VA HCP. At the time of chart review, 57 of 71 (80%) Choice veterans had inadequate documentation to make a confident assessment of the treatment outcome. Multiple calls to the offices of the Choice HCP were needed to acquire records, and veterans had to be tracked down for additional tests. Veterans often would complete treatment and stop following up with the Choice provider before SVR12 confirmation. The VA hepatology provider reviewing Choice referrals served as clinician, case manager, and clerk in order to get veterans to an appropriate end point in their hepatitis C treatment, with mostly unmeasured hours of work.
Limitations
The study population size was limited by the number of veterans able to complete treatment through Choice. The parameters in the VACO policy memos automatically selected the VA and Choice groups but made them clinically distinct populations. New treatment medications were released during the study period, which impacted management strategy. Occasionally, VA and non- VA HCPs preferred different treatment regimens, leading to variation in the distribution of regimens used despite similar genotype distribution (Tables 2 and 4). In addition, a retrospective study is at risk for recall bias. A prospective study randomizing veterans to the Choice and VA groups is an important future endeavor. Comparing veteran satisfaction for Choice and VA services is also crucial.
Conclusions
This study demonstrates that the VA was able to provide more cost-effective and more timely care for HCV treatment to a relatively sicker population with no reduction in treatment success when compared with non-VA HCPs through the Choice program. While the Choice program can help veterans receive services they may otherwise not have access to and reduce travel time, the current system introduces inefficiencies that delay care and decrease cost-effectiveness. The Choice HCP selection process is based on proximity rather than quality, which may place the veteran at risk for receiving substandard care. Large-scale quality of care studies that compare efficiency measures, clinical outcomes, patient demographics, travel distance, cost efficacy and patient satisfaction for veterans receiving similar services at a VA facility and through Choice should be performed to ensure that veterans receive the best care available.
1. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300. doi:10.7326/M13-1133
2. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88-96. doi:10.1002/hep.20502
3. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
4. Tuchschmidt J. Attachment C: Provision of hepatitis C treatment. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/education /choice-provision-hcv-treatment.asp. Published May 21, 2015. [Nonpublic site.]
5. Tuchschmidt J. Attachment A: Provision of hepatitis C (HCV) treatment through the Choice program. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http:// vaww.hepatitis.va.gov/pdf/choice-attachment-a-FY16 .pdf. Published May 21, 2015. [Nonpublic site.]
6. Tuchschmidt J. Attachment B: Initiation of hepatitis C virus (HCV) treatment: protocol for prioritization. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww .hepatitis.va.gov/pdf/provision-HCV-treatment-attach ment-b.pdf. Published May 21, 2015. [Nonpublic site.]
7. Murphy, JP. Hepatitis C virus funding and prioritization status. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations. http://vaww.hepatitis .va.gov/education/choice-memo-hcv-funding-and -prioritization-status-01272016.asp. Published January 27, 2016. [Nonpublic site.]
8. Lynch TJ, McCarthy MF. Hepatitis C virus funding and prioritization status update. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations and Acting Assistant Deputy Under Secretary for Health for Patient Care Services. http://vaww.hepatitis.va.gov /education/choice-funding-update-feb-2016.asp. Published February 24, 2016. [Nonpublic site.]
9. Morgan TR, Yee H; US Department of Veterans Affairs National Hepatitis C Resource Center Program and the National Viral Hepatitis Program in the Office of Patient Care Services. Chronic hepatitis C virus (HCV) infection: treatment considerations. http://vaww.hepatitis.va.gov /pdf/treatment-considerations-2016-03-28.pdf. Published March 28, 2016. [Nonpublic site.]
10. American Association for the Study of Liver Diseases; Infectious Diseases Society of America. Initial Treatment Box. http://hcvguidelines.org/full-report/initial-treatment -box-summary-recommendations-patients-who-are -initiating-therapy-hcv. Updated November 6, 2019. Accessed May 11, 2020.
11. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3): 932-954. doi:10.1002/hep.27950
12. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006; 43(6):1317-1325. doi:10.1002/hep.21178
13. Realdi G, Fattovich G, Hadziyannis S, et al. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. The Investigators of the European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol. 1994;21(4):656-666. doi:10.1016/s0168 -8278(94)80115-0
14. Kanda T, Kato K, Tsubota A, et al. Platelet count and sustained virological response in hepatitis C treatment. World J Hepatol. 2013;5(4):182-188. doi:10.4254/wjh.v5.i4.182
15. Manos MM, Shvachko VA, Murphy RC, Arduino JM, Shire NJ. Distribution of hepatitis C virus genotypes in a diverse US integrated health care population. J Med Virol. 2012;84(11):1744-1750. doi:10.1002/jmv.23399
16. Cheung RC. Epidemiology of hepatitis C virus infection in American veterans. Am J Gastroenterol. 2000;95(3): 740-747. doi:10.1111/j.1572-0241.2000.01854.x
17. Bagalman E. The number of Veterans that use VA health care services: a fact sheet. Congressional Research Service Report R43579. https://fas.org/sgp/crs/misc/R43579.pdf. Published June 3, 2014. Accessed May 11, 2020.
18. US General Accounting Office. Report to the Ranking Minority Member, Subcommittee on Compensation, Pension, Insurance, and Memorial Affairs, Committee on Veterans’ Affairs, US House of Representatives. How distance from VA facilities affects veterans’ use of VA services. https:// www.gao.gov/assets/230/221992.pdf. Published December 1995. Accessed May 11, 2020.
19. Bronstein S, Griffin D. A fatal wait: Veterans languish and die on a VA hospital’s secret list. http://www.cnn .com/2014/04/23/health/veterans-dying-health-care -delays/index.html. Published April 23, 2014. Accessed May 11, 2020.
20. Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs national health care system. Gastroenterology. 2016;151(3):457- 471. doi:10.1053/j.gastro.2016.05.049
21. Malespin MH, Harris C, Kanar O, et al. Barriers to treatment of chronic hepatitis C with direct acting antivirals in an urban clinic. Ann Hepatol. 2019;18(2):304-309. doi:10.1016/j.aohep.2018.06.001
22. Tuchschmidt J. Attachment D: Hepatitis C virus (HCV) fact sheet for Veterans Choice Program for both VA and Choice providers. US Department of Veterans Affairs Central Office Memorandum from the Deputy Under Secretary for Health for Policy and Services and the Acting Deputy Undersecretary for Health for Operations and Management. http://vaww .hepatitis.va.gov/educatiochoice-provision-HCV-treatment -additional.asp. [Nonpublic site.]
1. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300. doi:10.7326/M13-1133
2. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88-96. doi:10.1002/hep.20502
3. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
4. Tuchschmidt J. Attachment C: Provision of hepatitis C treatment. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/education /choice-provision-hcv-treatment.asp. Published May 21, 2015. [Nonpublic site.]
5. Tuchschmidt J. Attachment A: Provision of hepatitis C (HCV) treatment through the Choice program. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http:// vaww.hepatitis.va.gov/pdf/choice-attachment-a-FY16 .pdf. Published May 21, 2015. [Nonpublic site.]
6. Tuchschmidt J. Attachment B: Initiation of hepatitis C virus (HCV) treatment: protocol for prioritization. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww .hepatitis.va.gov/pdf/provision-HCV-treatment-attach ment-b.pdf. Published May 21, 2015. [Nonpublic site.]
7. Murphy, JP. Hepatitis C virus funding and prioritization status. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations. http://vaww.hepatitis .va.gov/education/choice-memo-hcv-funding-and -prioritization-status-01272016.asp. Published January 27, 2016. [Nonpublic site.]
8. Lynch TJ, McCarthy MF. Hepatitis C virus funding and prioritization status update. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations and Acting Assistant Deputy Under Secretary for Health for Patient Care Services. http://vaww.hepatitis.va.gov /education/choice-funding-update-feb-2016.asp. Published February 24, 2016. [Nonpublic site.]
9. Morgan TR, Yee H; US Department of Veterans Affairs National Hepatitis C Resource Center Program and the National Viral Hepatitis Program in the Office of Patient Care Services. Chronic hepatitis C virus (HCV) infection: treatment considerations. http://vaww.hepatitis.va.gov /pdf/treatment-considerations-2016-03-28.pdf. Published March 28, 2016. [Nonpublic site.]
10. American Association for the Study of Liver Diseases; Infectious Diseases Society of America. Initial Treatment Box. http://hcvguidelines.org/full-report/initial-treatment -box-summary-recommendations-patients-who-are -initiating-therapy-hcv. Updated November 6, 2019. Accessed May 11, 2020.
11. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3): 932-954. doi:10.1002/hep.27950
12. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006; 43(6):1317-1325. doi:10.1002/hep.21178
13. Realdi G, Fattovich G, Hadziyannis S, et al. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. The Investigators of the European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol. 1994;21(4):656-666. doi:10.1016/s0168 -8278(94)80115-0
14. Kanda T, Kato K, Tsubota A, et al. Platelet count and sustained virological response in hepatitis C treatment. World J Hepatol. 2013;5(4):182-188. doi:10.4254/wjh.v5.i4.182
15. Manos MM, Shvachko VA, Murphy RC, Arduino JM, Shire NJ. Distribution of hepatitis C virus genotypes in a diverse US integrated health care population. J Med Virol. 2012;84(11):1744-1750. doi:10.1002/jmv.23399
16. Cheung RC. Epidemiology of hepatitis C virus infection in American veterans. Am J Gastroenterol. 2000;95(3): 740-747. doi:10.1111/j.1572-0241.2000.01854.x
17. Bagalman E. The number of Veterans that use VA health care services: a fact sheet. Congressional Research Service Report R43579. https://fas.org/sgp/crs/misc/R43579.pdf. Published June 3, 2014. Accessed May 11, 2020.
18. US General Accounting Office. Report to the Ranking Minority Member, Subcommittee on Compensation, Pension, Insurance, and Memorial Affairs, Committee on Veterans’ Affairs, US House of Representatives. How distance from VA facilities affects veterans’ use of VA services. https:// www.gao.gov/assets/230/221992.pdf. Published December 1995. Accessed May 11, 2020.
19. Bronstein S, Griffin D. A fatal wait: Veterans languish and die on a VA hospital’s secret list. http://www.cnn .com/2014/04/23/health/veterans-dying-health-care -delays/index.html. Published April 23, 2014. Accessed May 11, 2020.
20. Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs national health care system. Gastroenterology. 2016;151(3):457- 471. doi:10.1053/j.gastro.2016.05.049
21. Malespin MH, Harris C, Kanar O, et al. Barriers to treatment of chronic hepatitis C with direct acting antivirals in an urban clinic. Ann Hepatol. 2019;18(2):304-309. doi:10.1016/j.aohep.2018.06.001
22. Tuchschmidt J. Attachment D: Hepatitis C virus (HCV) fact sheet for Veterans Choice Program for both VA and Choice providers. US Department of Veterans Affairs Central Office Memorandum from the Deputy Under Secretary for Health for Policy and Services and the Acting Deputy Undersecretary for Health for Operations and Management. http://vaww .hepatitis.va.gov/educatiochoice-provision-HCV-treatment -additional.asp. [Nonpublic site.]
Pulmonary Neuroendocrine Tumor Presenting as a Left Pleural Effusion
Neuroendocrine tumors (NETs) account for about 0.5% of all newly diagnosed malignancies.1 Pulmonary NETs are rare, accounting for 1 to 2% of all invasive lung malignancies and involve about 20 to 25% of primary lung malignancies. 2,3 Their prevalence has increased by an estimated 6% per year over the past 30 years.2 Nonetheless, the time of diagnosis is frequently delayed because of nonspecific symptoms that may imitate other pulmonary conditions.
In the normal pleural space, there is a steady state in which there is a roughly equal rate of fluid formation and absorption. Any disequilibrium may produce a pleural effusion. Pleural fluids can be transudates or exudates. Transudates result from imbalances in hydrostatic and oncotic pressures in the pleural space. Exudates result primarily from pleural and/or lung inflammation or from impaired lymphatic drainage of the pleural space. Clinical manifestations include cough, wheezing, recurrent pneumonia, hemoptysis and pleural effusions. We present a case of a man who developed a large left pleural effusion with a pathology report suggesting a pulmonary NET as the etiology. Being aware of this rare entity may help improve prognosis by making an earlier diagnosis and starting treatment sooner.
Case Presentation
A 90-year-old man with a medical history of arterial hypertension, hyperlipidemia, type 2 diabetes mellitus, coronary artery disease, and vascular dementia presented to the emergency department with hypoactivity, poor appetite, productive cough, and shortness of breath. The patient was a former smoker (unknown pack-years) who quit smoking cigarettes 7 years prior. Vital signs showed sinus tachycardia and peripheral oxygen saturation of 90% at room air. The initial physical examination was remarkable for decreased breath sounds and crackles at the left lung base. Laboratory findings showed leukocytosis with neutrophilia and chronic normocytic anemia. Chest computed tomography (CT) showed a large left-sided pleural effusion occupying most of the left hemithorax with adjacent atelectatic lung, enlarged pretracheal, subcarinal, and left perihilar lymph nodes (Figure 1).
The patient was admitted to the internal medicine ward with the diagnosis of left pneumonic process and started on IV levofloxacin. However, despite 7 days of antibiotic therapy, the patient’s respiratory symptoms worsened. This clinical deterioration prompted pulmonary service consultation. Chest radiography demonstrated an enlarging left pleural effusion (Figure 2). A thoracentesis drained 1.2 L of serosanguineous pleural fluid. Pleural fluid analysis showed a cell count of 947/cm3 with 79% of lymphocytes, total protein 3.8 g/dL, lactic dehydrogenase (LDH) level 607 U/L, and glucose level 109 mg/dL. Serum total protein was 6.62 g/dL, LDH 666 U/L and glucose 92 mg/dL (Tables 1 and 2). Alanine transaminase (ALT) and aspartate aminotransferase (AST) were 11 U/L and 21 U/L, respectively. Using Light criteria, the pleural:serum protein ratio was 0.57, the pleural:serum LDH ratio was 0.91, and the pleural LDH was more than two-thirds of the serum LDH. These calculations were consistent with an exudative effusion. An infectious disease workup, including blood and pleural fluid cultures, was negative.
The pleural fluid concentrated cell block hematoxylin and eosin (H&E) staining showed chromatin, prominent nucleoli, and nuclear molding, which was compatible with high-grade lung NET (Figure 3). The cell block immunohistochemistry (IHC) was positive for synaptophysin, chromogranin A, and neuron specific enolase (NSE) also consistent with a high-grade pulmonary NET (Figure 4). The proliferation marker protein Ki-67 labeling index (LI) showed a proliferation index > 20% (Figure 5). The patient did not have decision-making capacity given vascular dementia. Multiple attempts to contact the next of kin or family members were unsuccessful. Risks vs benefits were evaluated, and given the patient’s advanced age and multiple comorbidities, a conservative management approach under palliative care was chosen. For this reason, further genomic studies were not done.
Discussion
NETs are a group of neoplasms that differ in site, amount of cell propagation, and clinical manifestations.4 These tumors are rare with an estimated incidence of 25 to 50 per 100,000.4 The most commonly affected organ systems are the gastroenteropancreatic and the bronchopulmonary tracts, accounting for 60% and 25% of the tumors, respectively.4 The incidence is increasing over the past years in part because of novel diagnostic techniques.
The average age of diagnosis is between the fourth and sixth decades, affecting more women than men.5 Smoking has been identified as a possible culprit for the development of these neoplasms; nonetheless, the association is still not clear.4 For example, poorly differentiated pulmonary NETs have a strong association with smoking but not well-differentiated pulmonary NETs.2
Patients typically present with cough, wheezing, hemoptysis, and recurrent pneumonias, which are in part a consequence of obstruction caused by the mass.2 Sometimes, obstruction may yield persistent pleural effusions. Hemoptysis may be seen secondary to the vascularity of pulmonary NETs.
The diagnosis is often delayed because patients are frequently treated for infection before being diagnosed with the malignancy, such as in our case. Radiologic image findings include round opacities, central masses, and atelectasis. Pulmonary NETs are frequently found incidentally as solitary lung nodules. The CT scan is the most common diagnostic modality and can provide information about the borders of the tumor, the location and surrounding structures, including the presence of atelectasis.5 Pulmonary NETs are usually centrally located in an accessible region for lung biopsy. In cases where the mass is not easily reachable, thoracentesis may provide the only available specimen.
The 2015 World Health Organization classification has identified 4 histologic types of pulmonary NETs, namely, typical carcinoid (TC), atypical carcinoid (AC), large cell neuroendocrine carcinoma (LCNEC) and small cell lung carcinoma (SCLC).6 The low-grade pulmonary NET, the typical carcinoid, is slow growing and has lower rates of metastasis. The intermediate-grade NET, the atypical carcinoid, is more aggressive. The highgrade NETs, the LCNEC and the SCLC, are aggressive and spread quickly to other places.6 Consequently, LCNEC and SCLC have higher mortalities with a 5-year survival, ranging from 13 to 57% and 5%, respectively.7
Tumors may be histomorphologically classified by H&E staining. The main characteristics that differentiate the low- and high-grade NETs are the presence of necrosis and the mitotic rate. Both categories form neuropeptides and have dense granular cores when seen with an electron microscopy.6 The TC and AC have welldefined, organized histologic patterns, no necrosis, and scarce mitosis. On the other hand, the LCNEC and SCLC are poorly differentiated tumors with necrosis, atypia, and mitosis.6 LCNEC can be separated from SCLC and other tumors by IHC staining, whereas SCLC is primarily distinguished by morphology.
If the biopsy sample size is small, then IHC morphology and markers are helpful for subclassification.8 IHC is used to discern between neuroendocrine (NE) vs non-NE. The evaluation of pleural fluid includes preparation of cell blocks. Cell block staining is deemed better for IHC because it mimics a small biopsy that enables superior stains.9 The need for a pleural biopsy in cases where the cytology is negative depends on treatment aims, the kind of tumor, and the presence of metastasis.10 In almost 80% of cases, pleural biopsy and cytology are the only specimens obtained for analysis.Therefore, identification of these markers is practical for diagnosis.10 For this reason, pleural effusion samples are appropriate options to lung biopsy for molecular studies.10
Ki-67 LI in samples has the highest specificity and sensitivity for low-tointermediate- grade vs high-grade tumors. It is being used for guiding clinical and treatment decisions.6 In SCLC, the Ki-67 LI is not necessary for diagnosis but will be about 80%.11 The tumor cells will show epithelial characteristics with positive cytokeratin AE1/AE3 and monoclonal antibody CAM5.2 and neuroendocrine markers, including NCAM/CD56, chromogranin A, and synaptophysin.11
Thyroid transcription factor-1 (TTF- 1) is positive in most cases. In LCNEC, the Ki-67 LI is between 40% and 80%. NCAM/ CD56, chromogranin A, and synaptophysin are present in 92 to 100%, 80 to 85%, and 50 to 60%, respectively.11 TTF-1 is identified in half of the tumors. All these tumors express pancytokeratin (AE1/AE3), cytokeratin 7 or low-molecular-weight cytokeratin. Likewise, the carcinoids will show markers, such as chromogranin A, synaptophysin, CD56, and epithelial markers like pancytokeratin.11 However, the high-molecular-weight cytokeratin and TTF-1 are negative. Furthermore, NSE is considered a good tumor marker in the diagnosis and prognosis of SCLC. NSE also has been reported in NSCLC. The level of NSE correlates with tumor burden, number of metastatic sites, and response to treatment. 12 A potentially useful marker is the insulinoma-associated protein 1, which is a nuclear determinant of NE differentiation that stains all types of pulmonary NETs irrespective of the histology but does not stain adenocarcinoma or squamous cell carcinoma (SCC).6
Recently, genomic studies have identified gene alterations that have become standard of care for diagnosis and targeted therapies.8 For example, epidermal growth factor receptor (EGFR) and echinoderm microtubule- associated proteinlike 4, and anaplastic lymphoma kinase (EML4-ALK) mutations have been found in about 25% of lung adenocarcinomas. 8 Other abnormalities in LKB1/STK11, NF1, CDKN2A, SMARCA4 and KEAP1, KRAS, MET, ROS1, and RET have also been identified.8 On the other hand, SCC rarely have derangements in EGFR and EML4-ALK, but do show changes in RTKs, DDR2M, FGGRs, among others.8 In TC and AC, observed molecular alterations include MEN1 mutations, mTOR, and SSTRs pathway activation, and GC/ CEACAM1 and CD44/OTP expression.13 LCNEC and SCLC have shown TP53 and RB1 mutations and CDX2/VIL1/BAI3 expression. DLL3 expression and MET mutations may be present in SCLC.13 Last, chromatin remodeling gene mutations have been identified in all these lung NET types.13
Furthermore, neuropeptides and neuroamines may be measured in the blood and urine.14 Pulmonary NETs may be functional and secrete these substances, leading to systemic symptoms based on the released molecules.15 However, pulmonary NETs produce less serotonin than gastrointestinal NETs; therefore, carcinoid syndrome is less frequent in pulmonary NETs.16 Liver metastasis is often present when it occurs.5 Other possible clinical features include Cushing syndrome and acromegaly depending on the secreted hormones.5
In a recent metanalysis, serum LDH has been found to have a prognostic role in Ewing sarcoma, urologic cancers, malignant mesothelioma, among others.17 It demonstrated that a higher LDH concentration is associated with worse survival in patients with lung cancer.17 Serum LDH is an enzyme that catalyzes the reaction between lactic acid and pyruvic acid that typically takes place in anaerobic conditions.17 LDH levels are elevated in malignancies because tumors have an anaerobic environment. Elevated LDH levels correlate with the anaerobic metabolism in the tumor. Other studies also have noted that patients with high metastatic score have higher LDH levels.17 Therefore, LDH may reflect tumor extension.
In addition, other techniques, such as somatostatin- receptor imaging are specifically beneficial in tumors that express the somatostatin receptor.16 For this reason, this type of study is typically indicated in patients with known metastasis, not in patients with low-grade tumors. Abdominal CT scans are done because the liver is a common site for metastasis.
Our case report demonstrates how biomarkers help diagnose these potentially aggressive and life-threatening tumors that may present as a common condition such as a pleural effusion. Using a less invasive and quicker approach with thoracentesis rather than with lung biopsies is a diagnostic tool in this entity. IHC in cell blocks is a reasonable diagnostic method especially in patients in whom performing a lung biopsy is difficult.
Conclusions
The presence of a symptomatic and recurrent unilateral pleural effusion must urge physicians to consider thoracentesis with mindful use of biomarkers not only for therapeutic purposes, but also for diagnosis of a variety of etiologies, both benign and malignant.
1. Oronsky B, Ma PC, Morgensztern D, Carter CA. Nothing but NET: a review of neuroendocrine tumors and carcinomas. Neoplasia. 2017;19(12):991-1002. doi: 10.1016/j.neo.2017.09.002
2. Hendifar AE, Marchevsky AM, Tuli R. Neuroendocrine tumors of the lung: current challenges and advances in the diagnosis and management of well-differentiated disease. J Thorac Oncol. 2017;12(3):425-436. doi: 10.1016/j.jtho.2016.11.2222
3. Fisseler-Eckhoff A, Demes M. Neuroendocrine tumors of the lung. Cancers (Basel). 2012;4(3):777-798. doi: 10.3390/cancers4030777
4. Mandegaran R, David S, Screaton N. Cardiothoracic manifestations of neuroendocrine tumours. Br J Radiol. 2016;89(1060). doi: 10.1259/bjr.20150787
5. Caplin ME, Baudin E, Ferolla P, et al; ENETS consensus conference participants. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol. 2015;26(8):1604-1620. doi: 10.1093/annonc/mdv041
6. Pelosi G, Sonzogni A, Harari S, et al. Classification of pulmonary neuroendocrine tumors: new insights. Transl Lung Cancer Res. 2017;6(5):513-529. doi: 10.21037/tlcr.2017.09.04
7. Rossi G, Bertero L, Marchiò C, Papotti M. Molecular alterations of neuroendocrine tumours of the lung. Histopathology. 2018;72(1):142-152. doi: 10.1111/his.13394.
8. Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): moving from targeted therapy to immunotherapy. Semin Cancer Biol. 2018;52(pt 1):103-109. doi: 10.1016/j.semcancer.2017.11.019
9. Kaur G, Nijhawan R, Gupta N, Singh N, Rajwanshi A. Pleural fluid cytology samples in cases of suspected lung cancer: an experience from a tertiary care centre. Diagn Cytopathol. 2017;45(3):195-201.
10. Porcel JM. Biomarkers in the diagnosis of pleural diseases: a 2018 update. Ther Adv Respir Dis. 2018;12. doi: 10.1177/1753466618808660
11. Kim JY, Hong SM, Ro JY. Recent updates on grading and classification of neuroendocrine tumors. Ann Diagn Pathol. 2017;29:11-16. doi: 10.1016/j.anndiagpath.2017.04.005
12. Isgrò MA, Bottoni P, Scatena R. Neuron-specific enolase as a biomarker: biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:125-143. doi: 10.1007/978-94-017-7215-0_9
13. Rossi G, Bertero L, Marchiò C, Papotti M. Molecular alterations of neuroendocrine tumours of the lung. Histopathology. 2018;72(1):142-152. doi: 10.1111/his.13394
14. Eriksson B, Oberg K, Stridsberg M. Tumor markers in neuroendocrine tumors. Digestion. 2000;62(suppl 1):33-38.
15. Melosky B. Low grade neuroendocrine tumors of the lung. Front Oncol. 2017;7:119. doi: 10.3389/fonc.2017.00119
16. Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2001;113(1):5-21. https://doi.org/10.1002/cncr.23542
17. Deng T, Zhang J, Meng Y, Zhou Y, Li W. Higher pretreatment lactate dehydrogenase concentration predicts worse overall survival in patients with lung cancer. Medicine (Baltimore). 2018;97(38):e12524
Neuroendocrine tumors (NETs) account for about 0.5% of all newly diagnosed malignancies.1 Pulmonary NETs are rare, accounting for 1 to 2% of all invasive lung malignancies and involve about 20 to 25% of primary lung malignancies. 2,3 Their prevalence has increased by an estimated 6% per year over the past 30 years.2 Nonetheless, the time of diagnosis is frequently delayed because of nonspecific symptoms that may imitate other pulmonary conditions.
In the normal pleural space, there is a steady state in which there is a roughly equal rate of fluid formation and absorption. Any disequilibrium may produce a pleural effusion. Pleural fluids can be transudates or exudates. Transudates result from imbalances in hydrostatic and oncotic pressures in the pleural space. Exudates result primarily from pleural and/or lung inflammation or from impaired lymphatic drainage of the pleural space. Clinical manifestations include cough, wheezing, recurrent pneumonia, hemoptysis and pleural effusions. We present a case of a man who developed a large left pleural effusion with a pathology report suggesting a pulmonary NET as the etiology. Being aware of this rare entity may help improve prognosis by making an earlier diagnosis and starting treatment sooner.
Case Presentation
A 90-year-old man with a medical history of arterial hypertension, hyperlipidemia, type 2 diabetes mellitus, coronary artery disease, and vascular dementia presented to the emergency department with hypoactivity, poor appetite, productive cough, and shortness of breath. The patient was a former smoker (unknown pack-years) who quit smoking cigarettes 7 years prior. Vital signs showed sinus tachycardia and peripheral oxygen saturation of 90% at room air. The initial physical examination was remarkable for decreased breath sounds and crackles at the left lung base. Laboratory findings showed leukocytosis with neutrophilia and chronic normocytic anemia. Chest computed tomography (CT) showed a large left-sided pleural effusion occupying most of the left hemithorax with adjacent atelectatic lung, enlarged pretracheal, subcarinal, and left perihilar lymph nodes (Figure 1).
The patient was admitted to the internal medicine ward with the diagnosis of left pneumonic process and started on IV levofloxacin. However, despite 7 days of antibiotic therapy, the patient’s respiratory symptoms worsened. This clinical deterioration prompted pulmonary service consultation. Chest radiography demonstrated an enlarging left pleural effusion (Figure 2). A thoracentesis drained 1.2 L of serosanguineous pleural fluid. Pleural fluid analysis showed a cell count of 947/cm3 with 79% of lymphocytes, total protein 3.8 g/dL, lactic dehydrogenase (LDH) level 607 U/L, and glucose level 109 mg/dL. Serum total protein was 6.62 g/dL, LDH 666 U/L and glucose 92 mg/dL (Tables 1 and 2). Alanine transaminase (ALT) and aspartate aminotransferase (AST) were 11 U/L and 21 U/L, respectively. Using Light criteria, the pleural:serum protein ratio was 0.57, the pleural:serum LDH ratio was 0.91, and the pleural LDH was more than two-thirds of the serum LDH. These calculations were consistent with an exudative effusion. An infectious disease workup, including blood and pleural fluid cultures, was negative.
The pleural fluid concentrated cell block hematoxylin and eosin (H&E) staining showed chromatin, prominent nucleoli, and nuclear molding, which was compatible with high-grade lung NET (Figure 3). The cell block immunohistochemistry (IHC) was positive for synaptophysin, chromogranin A, and neuron specific enolase (NSE) also consistent with a high-grade pulmonary NET (Figure 4). The proliferation marker protein Ki-67 labeling index (LI) showed a proliferation index > 20% (Figure 5). The patient did not have decision-making capacity given vascular dementia. Multiple attempts to contact the next of kin or family members were unsuccessful. Risks vs benefits were evaluated, and given the patient’s advanced age and multiple comorbidities, a conservative management approach under palliative care was chosen. For this reason, further genomic studies were not done.
Discussion
NETs are a group of neoplasms that differ in site, amount of cell propagation, and clinical manifestations.4 These tumors are rare with an estimated incidence of 25 to 50 per 100,000.4 The most commonly affected organ systems are the gastroenteropancreatic and the bronchopulmonary tracts, accounting for 60% and 25% of the tumors, respectively.4 The incidence is increasing over the past years in part because of novel diagnostic techniques.
The average age of diagnosis is between the fourth and sixth decades, affecting more women than men.5 Smoking has been identified as a possible culprit for the development of these neoplasms; nonetheless, the association is still not clear.4 For example, poorly differentiated pulmonary NETs have a strong association with smoking but not well-differentiated pulmonary NETs.2
Patients typically present with cough, wheezing, hemoptysis, and recurrent pneumonias, which are in part a consequence of obstruction caused by the mass.2 Sometimes, obstruction may yield persistent pleural effusions. Hemoptysis may be seen secondary to the vascularity of pulmonary NETs.
The diagnosis is often delayed because patients are frequently treated for infection before being diagnosed with the malignancy, such as in our case. Radiologic image findings include round opacities, central masses, and atelectasis. Pulmonary NETs are frequently found incidentally as solitary lung nodules. The CT scan is the most common diagnostic modality and can provide information about the borders of the tumor, the location and surrounding structures, including the presence of atelectasis.5 Pulmonary NETs are usually centrally located in an accessible region for lung biopsy. In cases where the mass is not easily reachable, thoracentesis may provide the only available specimen.
The 2015 World Health Organization classification has identified 4 histologic types of pulmonary NETs, namely, typical carcinoid (TC), atypical carcinoid (AC), large cell neuroendocrine carcinoma (LCNEC) and small cell lung carcinoma (SCLC).6 The low-grade pulmonary NET, the typical carcinoid, is slow growing and has lower rates of metastasis. The intermediate-grade NET, the atypical carcinoid, is more aggressive. The highgrade NETs, the LCNEC and the SCLC, are aggressive and spread quickly to other places.6 Consequently, LCNEC and SCLC have higher mortalities with a 5-year survival, ranging from 13 to 57% and 5%, respectively.7
Tumors may be histomorphologically classified by H&E staining. The main characteristics that differentiate the low- and high-grade NETs are the presence of necrosis and the mitotic rate. Both categories form neuropeptides and have dense granular cores when seen with an electron microscopy.6 The TC and AC have welldefined, organized histologic patterns, no necrosis, and scarce mitosis. On the other hand, the LCNEC and SCLC are poorly differentiated tumors with necrosis, atypia, and mitosis.6 LCNEC can be separated from SCLC and other tumors by IHC staining, whereas SCLC is primarily distinguished by morphology.
If the biopsy sample size is small, then IHC morphology and markers are helpful for subclassification.8 IHC is used to discern between neuroendocrine (NE) vs non-NE. The evaluation of pleural fluid includes preparation of cell blocks. Cell block staining is deemed better for IHC because it mimics a small biopsy that enables superior stains.9 The need for a pleural biopsy in cases where the cytology is negative depends on treatment aims, the kind of tumor, and the presence of metastasis.10 In almost 80% of cases, pleural biopsy and cytology are the only specimens obtained for analysis.Therefore, identification of these markers is practical for diagnosis.10 For this reason, pleural effusion samples are appropriate options to lung biopsy for molecular studies.10
Ki-67 LI in samples has the highest specificity and sensitivity for low-tointermediate- grade vs high-grade tumors. It is being used for guiding clinical and treatment decisions.6 In SCLC, the Ki-67 LI is not necessary for diagnosis but will be about 80%.11 The tumor cells will show epithelial characteristics with positive cytokeratin AE1/AE3 and monoclonal antibody CAM5.2 and neuroendocrine markers, including NCAM/CD56, chromogranin A, and synaptophysin.11
Thyroid transcription factor-1 (TTF- 1) is positive in most cases. In LCNEC, the Ki-67 LI is between 40% and 80%. NCAM/ CD56, chromogranin A, and synaptophysin are present in 92 to 100%, 80 to 85%, and 50 to 60%, respectively.11 TTF-1 is identified in half of the tumors. All these tumors express pancytokeratin (AE1/AE3), cytokeratin 7 or low-molecular-weight cytokeratin. Likewise, the carcinoids will show markers, such as chromogranin A, synaptophysin, CD56, and epithelial markers like pancytokeratin.11 However, the high-molecular-weight cytokeratin and TTF-1 are negative. Furthermore, NSE is considered a good tumor marker in the diagnosis and prognosis of SCLC. NSE also has been reported in NSCLC. The level of NSE correlates with tumor burden, number of metastatic sites, and response to treatment. 12 A potentially useful marker is the insulinoma-associated protein 1, which is a nuclear determinant of NE differentiation that stains all types of pulmonary NETs irrespective of the histology but does not stain adenocarcinoma or squamous cell carcinoma (SCC).6
Recently, genomic studies have identified gene alterations that have become standard of care for diagnosis and targeted therapies.8 For example, epidermal growth factor receptor (EGFR) and echinoderm microtubule- associated proteinlike 4, and anaplastic lymphoma kinase (EML4-ALK) mutations have been found in about 25% of lung adenocarcinomas. 8 Other abnormalities in LKB1/STK11, NF1, CDKN2A, SMARCA4 and KEAP1, KRAS, MET, ROS1, and RET have also been identified.8 On the other hand, SCC rarely have derangements in EGFR and EML4-ALK, but do show changes in RTKs, DDR2M, FGGRs, among others.8 In TC and AC, observed molecular alterations include MEN1 mutations, mTOR, and SSTRs pathway activation, and GC/ CEACAM1 and CD44/OTP expression.13 LCNEC and SCLC have shown TP53 and RB1 mutations and CDX2/VIL1/BAI3 expression. DLL3 expression and MET mutations may be present in SCLC.13 Last, chromatin remodeling gene mutations have been identified in all these lung NET types.13
Furthermore, neuropeptides and neuroamines may be measured in the blood and urine.14 Pulmonary NETs may be functional and secrete these substances, leading to systemic symptoms based on the released molecules.15 However, pulmonary NETs produce less serotonin than gastrointestinal NETs; therefore, carcinoid syndrome is less frequent in pulmonary NETs.16 Liver metastasis is often present when it occurs.5 Other possible clinical features include Cushing syndrome and acromegaly depending on the secreted hormones.5
In a recent metanalysis, serum LDH has been found to have a prognostic role in Ewing sarcoma, urologic cancers, malignant mesothelioma, among others.17 It demonstrated that a higher LDH concentration is associated with worse survival in patients with lung cancer.17 Serum LDH is an enzyme that catalyzes the reaction between lactic acid and pyruvic acid that typically takes place in anaerobic conditions.17 LDH levels are elevated in malignancies because tumors have an anaerobic environment. Elevated LDH levels correlate with the anaerobic metabolism in the tumor. Other studies also have noted that patients with high metastatic score have higher LDH levels.17 Therefore, LDH may reflect tumor extension.
In addition, other techniques, such as somatostatin- receptor imaging are specifically beneficial in tumors that express the somatostatin receptor.16 For this reason, this type of study is typically indicated in patients with known metastasis, not in patients with low-grade tumors. Abdominal CT scans are done because the liver is a common site for metastasis.
Our case report demonstrates how biomarkers help diagnose these potentially aggressive and life-threatening tumors that may present as a common condition such as a pleural effusion. Using a less invasive and quicker approach with thoracentesis rather than with lung biopsies is a diagnostic tool in this entity. IHC in cell blocks is a reasonable diagnostic method especially in patients in whom performing a lung biopsy is difficult.
Conclusions
The presence of a symptomatic and recurrent unilateral pleural effusion must urge physicians to consider thoracentesis with mindful use of biomarkers not only for therapeutic purposes, but also for diagnosis of a variety of etiologies, both benign and malignant.
Neuroendocrine tumors (NETs) account for about 0.5% of all newly diagnosed malignancies.1 Pulmonary NETs are rare, accounting for 1 to 2% of all invasive lung malignancies and involve about 20 to 25% of primary lung malignancies. 2,3 Their prevalence has increased by an estimated 6% per year over the past 30 years.2 Nonetheless, the time of diagnosis is frequently delayed because of nonspecific symptoms that may imitate other pulmonary conditions.
In the normal pleural space, there is a steady state in which there is a roughly equal rate of fluid formation and absorption. Any disequilibrium may produce a pleural effusion. Pleural fluids can be transudates or exudates. Transudates result from imbalances in hydrostatic and oncotic pressures in the pleural space. Exudates result primarily from pleural and/or lung inflammation or from impaired lymphatic drainage of the pleural space. Clinical manifestations include cough, wheezing, recurrent pneumonia, hemoptysis and pleural effusions. We present a case of a man who developed a large left pleural effusion with a pathology report suggesting a pulmonary NET as the etiology. Being aware of this rare entity may help improve prognosis by making an earlier diagnosis and starting treatment sooner.
Case Presentation
A 90-year-old man with a medical history of arterial hypertension, hyperlipidemia, type 2 diabetes mellitus, coronary artery disease, and vascular dementia presented to the emergency department with hypoactivity, poor appetite, productive cough, and shortness of breath. The patient was a former smoker (unknown pack-years) who quit smoking cigarettes 7 years prior. Vital signs showed sinus tachycardia and peripheral oxygen saturation of 90% at room air. The initial physical examination was remarkable for decreased breath sounds and crackles at the left lung base. Laboratory findings showed leukocytosis with neutrophilia and chronic normocytic anemia. Chest computed tomography (CT) showed a large left-sided pleural effusion occupying most of the left hemithorax with adjacent atelectatic lung, enlarged pretracheal, subcarinal, and left perihilar lymph nodes (Figure 1).
The patient was admitted to the internal medicine ward with the diagnosis of left pneumonic process and started on IV levofloxacin. However, despite 7 days of antibiotic therapy, the patient’s respiratory symptoms worsened. This clinical deterioration prompted pulmonary service consultation. Chest radiography demonstrated an enlarging left pleural effusion (Figure 2). A thoracentesis drained 1.2 L of serosanguineous pleural fluid. Pleural fluid analysis showed a cell count of 947/cm3 with 79% of lymphocytes, total protein 3.8 g/dL, lactic dehydrogenase (LDH) level 607 U/L, and glucose level 109 mg/dL. Serum total protein was 6.62 g/dL, LDH 666 U/L and glucose 92 mg/dL (Tables 1 and 2). Alanine transaminase (ALT) and aspartate aminotransferase (AST) were 11 U/L and 21 U/L, respectively. Using Light criteria, the pleural:serum protein ratio was 0.57, the pleural:serum LDH ratio was 0.91, and the pleural LDH was more than two-thirds of the serum LDH. These calculations were consistent with an exudative effusion. An infectious disease workup, including blood and pleural fluid cultures, was negative.
The pleural fluid concentrated cell block hematoxylin and eosin (H&E) staining showed chromatin, prominent nucleoli, and nuclear molding, which was compatible with high-grade lung NET (Figure 3). The cell block immunohistochemistry (IHC) was positive for synaptophysin, chromogranin A, and neuron specific enolase (NSE) also consistent with a high-grade pulmonary NET (Figure 4). The proliferation marker protein Ki-67 labeling index (LI) showed a proliferation index > 20% (Figure 5). The patient did not have decision-making capacity given vascular dementia. Multiple attempts to contact the next of kin or family members were unsuccessful. Risks vs benefits were evaluated, and given the patient’s advanced age and multiple comorbidities, a conservative management approach under palliative care was chosen. For this reason, further genomic studies were not done.
Discussion
NETs are a group of neoplasms that differ in site, amount of cell propagation, and clinical manifestations.4 These tumors are rare with an estimated incidence of 25 to 50 per 100,000.4 The most commonly affected organ systems are the gastroenteropancreatic and the bronchopulmonary tracts, accounting for 60% and 25% of the tumors, respectively.4 The incidence is increasing over the past years in part because of novel diagnostic techniques.
The average age of diagnosis is between the fourth and sixth decades, affecting more women than men.5 Smoking has been identified as a possible culprit for the development of these neoplasms; nonetheless, the association is still not clear.4 For example, poorly differentiated pulmonary NETs have a strong association with smoking but not well-differentiated pulmonary NETs.2
Patients typically present with cough, wheezing, hemoptysis, and recurrent pneumonias, which are in part a consequence of obstruction caused by the mass.2 Sometimes, obstruction may yield persistent pleural effusions. Hemoptysis may be seen secondary to the vascularity of pulmonary NETs.
The diagnosis is often delayed because patients are frequently treated for infection before being diagnosed with the malignancy, such as in our case. Radiologic image findings include round opacities, central masses, and atelectasis. Pulmonary NETs are frequently found incidentally as solitary lung nodules. The CT scan is the most common diagnostic modality and can provide information about the borders of the tumor, the location and surrounding structures, including the presence of atelectasis.5 Pulmonary NETs are usually centrally located in an accessible region for lung biopsy. In cases where the mass is not easily reachable, thoracentesis may provide the only available specimen.
The 2015 World Health Organization classification has identified 4 histologic types of pulmonary NETs, namely, typical carcinoid (TC), atypical carcinoid (AC), large cell neuroendocrine carcinoma (LCNEC) and small cell lung carcinoma (SCLC).6 The low-grade pulmonary NET, the typical carcinoid, is slow growing and has lower rates of metastasis. The intermediate-grade NET, the atypical carcinoid, is more aggressive. The highgrade NETs, the LCNEC and the SCLC, are aggressive and spread quickly to other places.6 Consequently, LCNEC and SCLC have higher mortalities with a 5-year survival, ranging from 13 to 57% and 5%, respectively.7
Tumors may be histomorphologically classified by H&E staining. The main characteristics that differentiate the low- and high-grade NETs are the presence of necrosis and the mitotic rate. Both categories form neuropeptides and have dense granular cores when seen with an electron microscopy.6 The TC and AC have welldefined, organized histologic patterns, no necrosis, and scarce mitosis. On the other hand, the LCNEC and SCLC are poorly differentiated tumors with necrosis, atypia, and mitosis.6 LCNEC can be separated from SCLC and other tumors by IHC staining, whereas SCLC is primarily distinguished by morphology.
If the biopsy sample size is small, then IHC morphology and markers are helpful for subclassification.8 IHC is used to discern between neuroendocrine (NE) vs non-NE. The evaluation of pleural fluid includes preparation of cell blocks. Cell block staining is deemed better for IHC because it mimics a small biopsy that enables superior stains.9 The need for a pleural biopsy in cases where the cytology is negative depends on treatment aims, the kind of tumor, and the presence of metastasis.10 In almost 80% of cases, pleural biopsy and cytology are the only specimens obtained for analysis.Therefore, identification of these markers is practical for diagnosis.10 For this reason, pleural effusion samples are appropriate options to lung biopsy for molecular studies.10
Ki-67 LI in samples has the highest specificity and sensitivity for low-tointermediate- grade vs high-grade tumors. It is being used for guiding clinical and treatment decisions.6 In SCLC, the Ki-67 LI is not necessary for diagnosis but will be about 80%.11 The tumor cells will show epithelial characteristics with positive cytokeratin AE1/AE3 and monoclonal antibody CAM5.2 and neuroendocrine markers, including NCAM/CD56, chromogranin A, and synaptophysin.11
Thyroid transcription factor-1 (TTF- 1) is positive in most cases. In LCNEC, the Ki-67 LI is between 40% and 80%. NCAM/ CD56, chromogranin A, and synaptophysin are present in 92 to 100%, 80 to 85%, and 50 to 60%, respectively.11 TTF-1 is identified in half of the tumors. All these tumors express pancytokeratin (AE1/AE3), cytokeratin 7 or low-molecular-weight cytokeratin. Likewise, the carcinoids will show markers, such as chromogranin A, synaptophysin, CD56, and epithelial markers like pancytokeratin.11 However, the high-molecular-weight cytokeratin and TTF-1 are negative. Furthermore, NSE is considered a good tumor marker in the diagnosis and prognosis of SCLC. NSE also has been reported in NSCLC. The level of NSE correlates with tumor burden, number of metastatic sites, and response to treatment. 12 A potentially useful marker is the insulinoma-associated protein 1, which is a nuclear determinant of NE differentiation that stains all types of pulmonary NETs irrespective of the histology but does not stain adenocarcinoma or squamous cell carcinoma (SCC).6
Recently, genomic studies have identified gene alterations that have become standard of care for diagnosis and targeted therapies.8 For example, epidermal growth factor receptor (EGFR) and echinoderm microtubule- associated proteinlike 4, and anaplastic lymphoma kinase (EML4-ALK) mutations have been found in about 25% of lung adenocarcinomas. 8 Other abnormalities in LKB1/STK11, NF1, CDKN2A, SMARCA4 and KEAP1, KRAS, MET, ROS1, and RET have also been identified.8 On the other hand, SCC rarely have derangements in EGFR and EML4-ALK, but do show changes in RTKs, DDR2M, FGGRs, among others.8 In TC and AC, observed molecular alterations include MEN1 mutations, mTOR, and SSTRs pathway activation, and GC/ CEACAM1 and CD44/OTP expression.13 LCNEC and SCLC have shown TP53 and RB1 mutations and CDX2/VIL1/BAI3 expression. DLL3 expression and MET mutations may be present in SCLC.13 Last, chromatin remodeling gene mutations have been identified in all these lung NET types.13
Furthermore, neuropeptides and neuroamines may be measured in the blood and urine.14 Pulmonary NETs may be functional and secrete these substances, leading to systemic symptoms based on the released molecules.15 However, pulmonary NETs produce less serotonin than gastrointestinal NETs; therefore, carcinoid syndrome is less frequent in pulmonary NETs.16 Liver metastasis is often present when it occurs.5 Other possible clinical features include Cushing syndrome and acromegaly depending on the secreted hormones.5
In a recent metanalysis, serum LDH has been found to have a prognostic role in Ewing sarcoma, urologic cancers, malignant mesothelioma, among others.17 It demonstrated that a higher LDH concentration is associated with worse survival in patients with lung cancer.17 Serum LDH is an enzyme that catalyzes the reaction between lactic acid and pyruvic acid that typically takes place in anaerobic conditions.17 LDH levels are elevated in malignancies because tumors have an anaerobic environment. Elevated LDH levels correlate with the anaerobic metabolism in the tumor. Other studies also have noted that patients with high metastatic score have higher LDH levels.17 Therefore, LDH may reflect tumor extension.
In addition, other techniques, such as somatostatin- receptor imaging are specifically beneficial in tumors that express the somatostatin receptor.16 For this reason, this type of study is typically indicated in patients with known metastasis, not in patients with low-grade tumors. Abdominal CT scans are done because the liver is a common site for metastasis.
Our case report demonstrates how biomarkers help diagnose these potentially aggressive and life-threatening tumors that may present as a common condition such as a pleural effusion. Using a less invasive and quicker approach with thoracentesis rather than with lung biopsies is a diagnostic tool in this entity. IHC in cell blocks is a reasonable diagnostic method especially in patients in whom performing a lung biopsy is difficult.
Conclusions
The presence of a symptomatic and recurrent unilateral pleural effusion must urge physicians to consider thoracentesis with mindful use of biomarkers not only for therapeutic purposes, but also for diagnosis of a variety of etiologies, both benign and malignant.
1. Oronsky B, Ma PC, Morgensztern D, Carter CA. Nothing but NET: a review of neuroendocrine tumors and carcinomas. Neoplasia. 2017;19(12):991-1002. doi: 10.1016/j.neo.2017.09.002
2. Hendifar AE, Marchevsky AM, Tuli R. Neuroendocrine tumors of the lung: current challenges and advances in the diagnosis and management of well-differentiated disease. J Thorac Oncol. 2017;12(3):425-436. doi: 10.1016/j.jtho.2016.11.2222
3. Fisseler-Eckhoff A, Demes M. Neuroendocrine tumors of the lung. Cancers (Basel). 2012;4(3):777-798. doi: 10.3390/cancers4030777
4. Mandegaran R, David S, Screaton N. Cardiothoracic manifestations of neuroendocrine tumours. Br J Radiol. 2016;89(1060). doi: 10.1259/bjr.20150787
5. Caplin ME, Baudin E, Ferolla P, et al; ENETS consensus conference participants. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol. 2015;26(8):1604-1620. doi: 10.1093/annonc/mdv041
6. Pelosi G, Sonzogni A, Harari S, et al. Classification of pulmonary neuroendocrine tumors: new insights. Transl Lung Cancer Res. 2017;6(5):513-529. doi: 10.21037/tlcr.2017.09.04
7. Rossi G, Bertero L, Marchiò C, Papotti M. Molecular alterations of neuroendocrine tumours of the lung. Histopathology. 2018;72(1):142-152. doi: 10.1111/his.13394.
8. Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): moving from targeted therapy to immunotherapy. Semin Cancer Biol. 2018;52(pt 1):103-109. doi: 10.1016/j.semcancer.2017.11.019
9. Kaur G, Nijhawan R, Gupta N, Singh N, Rajwanshi A. Pleural fluid cytology samples in cases of suspected lung cancer: an experience from a tertiary care centre. Diagn Cytopathol. 2017;45(3):195-201.
10. Porcel JM. Biomarkers in the diagnosis of pleural diseases: a 2018 update. Ther Adv Respir Dis. 2018;12. doi: 10.1177/1753466618808660
11. Kim JY, Hong SM, Ro JY. Recent updates on grading and classification of neuroendocrine tumors. Ann Diagn Pathol. 2017;29:11-16. doi: 10.1016/j.anndiagpath.2017.04.005
12. Isgrò MA, Bottoni P, Scatena R. Neuron-specific enolase as a biomarker: biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:125-143. doi: 10.1007/978-94-017-7215-0_9
13. Rossi G, Bertero L, Marchiò C, Papotti M. Molecular alterations of neuroendocrine tumours of the lung. Histopathology. 2018;72(1):142-152. doi: 10.1111/his.13394
14. Eriksson B, Oberg K, Stridsberg M. Tumor markers in neuroendocrine tumors. Digestion. 2000;62(suppl 1):33-38.
15. Melosky B. Low grade neuroendocrine tumors of the lung. Front Oncol. 2017;7:119. doi: 10.3389/fonc.2017.00119
16. Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2001;113(1):5-21. https://doi.org/10.1002/cncr.23542
17. Deng T, Zhang J, Meng Y, Zhou Y, Li W. Higher pretreatment lactate dehydrogenase concentration predicts worse overall survival in patients with lung cancer. Medicine (Baltimore). 2018;97(38):e12524
1. Oronsky B, Ma PC, Morgensztern D, Carter CA. Nothing but NET: a review of neuroendocrine tumors and carcinomas. Neoplasia. 2017;19(12):991-1002. doi: 10.1016/j.neo.2017.09.002
2. Hendifar AE, Marchevsky AM, Tuli R. Neuroendocrine tumors of the lung: current challenges and advances in the diagnosis and management of well-differentiated disease. J Thorac Oncol. 2017;12(3):425-436. doi: 10.1016/j.jtho.2016.11.2222
3. Fisseler-Eckhoff A, Demes M. Neuroendocrine tumors of the lung. Cancers (Basel). 2012;4(3):777-798. doi: 10.3390/cancers4030777
4. Mandegaran R, David S, Screaton N. Cardiothoracic manifestations of neuroendocrine tumours. Br J Radiol. 2016;89(1060). doi: 10.1259/bjr.20150787
5. Caplin ME, Baudin E, Ferolla P, et al; ENETS consensus conference participants. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol. 2015;26(8):1604-1620. doi: 10.1093/annonc/mdv041
6. Pelosi G, Sonzogni A, Harari S, et al. Classification of pulmonary neuroendocrine tumors: new insights. Transl Lung Cancer Res. 2017;6(5):513-529. doi: 10.21037/tlcr.2017.09.04
7. Rossi G, Bertero L, Marchiò C, Papotti M. Molecular alterations of neuroendocrine tumours of the lung. Histopathology. 2018;72(1):142-152. doi: 10.1111/his.13394.
8. Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): moving from targeted therapy to immunotherapy. Semin Cancer Biol. 2018;52(pt 1):103-109. doi: 10.1016/j.semcancer.2017.11.019
9. Kaur G, Nijhawan R, Gupta N, Singh N, Rajwanshi A. Pleural fluid cytology samples in cases of suspected lung cancer: an experience from a tertiary care centre. Diagn Cytopathol. 2017;45(3):195-201.
10. Porcel JM. Biomarkers in the diagnosis of pleural diseases: a 2018 update. Ther Adv Respir Dis. 2018;12. doi: 10.1177/1753466618808660
11. Kim JY, Hong SM, Ro JY. Recent updates on grading and classification of neuroendocrine tumors. Ann Diagn Pathol. 2017;29:11-16. doi: 10.1016/j.anndiagpath.2017.04.005
12. Isgrò MA, Bottoni P, Scatena R. Neuron-specific enolase as a biomarker: biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:125-143. doi: 10.1007/978-94-017-7215-0_9
13. Rossi G, Bertero L, Marchiò C, Papotti M. Molecular alterations of neuroendocrine tumours of the lung. Histopathology. 2018;72(1):142-152. doi: 10.1111/his.13394
14. Eriksson B, Oberg K, Stridsberg M. Tumor markers in neuroendocrine tumors. Digestion. 2000;62(suppl 1):33-38.
15. Melosky B. Low grade neuroendocrine tumors of the lung. Front Oncol. 2017;7:119. doi: 10.3389/fonc.2017.00119
16. Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2001;113(1):5-21. https://doi.org/10.1002/cncr.23542
17. Deng T, Zhang J, Meng Y, Zhou Y, Li W. Higher pretreatment lactate dehydrogenase concentration predicts worse overall survival in patients with lung cancer. Medicine (Baltimore). 2018;97(38):e12524
WHO clarifies comments on asymptomatic transmission of SARS-CoV-2
A World Health Organization (WHO) official is walking back her comments characterizing the spread of SARS-CoV-2 by asymptomatic individuals as “rare.”
Maria Van Kerkhove, PhD, WHO’s COVID-19 technical lead and an infectious disease epidemiologist, caused a stir June 8 when she said that countries are reporting that many of their asymptomatic cases develop into cases of mild disease. For patients with truly asymptomatic disease, countries are “not finding secondary transmission onward. It’s very rare,” she said.
Suppressing symptomatic cases, on the other hand, would result in a “drastic reduction” in transmission, she noted. “But from the data we have, it still seems to be rare that an asymptomatic person actually transmits onward to a secondary individual,” she said.
But on June 9 – following a day of confusion and criticism – Dr. Van Kerkhove sought to clarify her comments on asymptomatic transmission during a live social media Q&A. She noted that while “the majority of transmission that we know about” is through individuals with symptoms, “there are a subset of people who don’t develop symptoms, and to truly understand how many people don’t have symptoms – we don’t actually have that answer yet.”
Between 6% and 41% of individuals may be asymptomatic based on estimates, she acknowledged.“What we need to better understand is how many of the people in the population don’t have symptoms, and separately, how many of those individuals go on to transmit to others,” she said.
Dr. Van Kerkhove also emphasized that her initial comments were made in response to a question raised at the press conference, and called it a misunderstanding. “I wasn’t stating a policy of WHO or anything like that,” she said. “I was just trying to articulate what we know.”
The phrase “very rare” referred to a subset of studies and reports WHO had received from its member states following asymptomatic individuals with COVID-19. “I was referring to some detailed investigations, cluster investigations, case contact tracing, where we had reports from member states saying that, when we follow asymptomatic cases, it’s very rare – and I used the phrase very rare – that we found a secondary transmission,” she said.
Dr. Van Kerkhove’s initial comments drew criticism from medical and public health professionals, who said the statement was “confusing” and communicated poorly.
Eric J. Topol, MD, tweeted that WHO had “engendered considerable confusion” with the comments about asymptomatic individuals rarely transmitting SARS-CoV-2. Dr. Topol, the author of a recent analysis published in Annals of Internal Medicine that suggested as many as 40%-45% of COVID-19 cases may be asymptomatic, said that it was not possible to determine whether asymptomatic individuals in the cohorts he studied were capable of spread like pre-symptomatic individuals. “We only know the viral loads are similar from multiple reports. And we do know some spread occurs from [asymptomatic] people,” he said.
Andy Slavitt, former acting administrator of the Centers for Medicare and Medicaid Services, said in a tweet that he believed WHO made “an irresponsible statement even though it was based on legitimate observations.” Reports by Member States do not reach a “bar of rigor,” he said.
Natalie E. Dean, PhD, assistant professor of biostatistics at the University of Florida, tweeted that the initial comments by the WHO seemed to be trying to draw a distinction between asymptomatic individuals who never develop symptoms, and presymptomatic individuals who present as asymptomatic, but later develop symptoms. Finding that asymptomatic cases rarely transmit the virus could change how people exposed to those asymptomatic individuals are monitored, but “it seems more of scientific than practical interest,” she noted. “People without current symptoms could be infectious. Act accordingly.”
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, also weighed in on the controversial WHO comments, telling Good Morning America on June 10 that Dr. Van Kerkhove's initial statement that asymptomatic SARS-CoV-2 transmission is a rare event is "not correct."
This article was updated 6/10/20.
A World Health Organization (WHO) official is walking back her comments characterizing the spread of SARS-CoV-2 by asymptomatic individuals as “rare.”
Maria Van Kerkhove, PhD, WHO’s COVID-19 technical lead and an infectious disease epidemiologist, caused a stir June 8 when she said that countries are reporting that many of their asymptomatic cases develop into cases of mild disease. For patients with truly asymptomatic disease, countries are “not finding secondary transmission onward. It’s very rare,” she said.
Suppressing symptomatic cases, on the other hand, would result in a “drastic reduction” in transmission, she noted. “But from the data we have, it still seems to be rare that an asymptomatic person actually transmits onward to a secondary individual,” she said.
But on June 9 – following a day of confusion and criticism – Dr. Van Kerkhove sought to clarify her comments on asymptomatic transmission during a live social media Q&A. She noted that while “the majority of transmission that we know about” is through individuals with symptoms, “there are a subset of people who don’t develop symptoms, and to truly understand how many people don’t have symptoms – we don’t actually have that answer yet.”
Between 6% and 41% of individuals may be asymptomatic based on estimates, she acknowledged.“What we need to better understand is how many of the people in the population don’t have symptoms, and separately, how many of those individuals go on to transmit to others,” she said.
Dr. Van Kerkhove also emphasized that her initial comments were made in response to a question raised at the press conference, and called it a misunderstanding. “I wasn’t stating a policy of WHO or anything like that,” she said. “I was just trying to articulate what we know.”
The phrase “very rare” referred to a subset of studies and reports WHO had received from its member states following asymptomatic individuals with COVID-19. “I was referring to some detailed investigations, cluster investigations, case contact tracing, where we had reports from member states saying that, when we follow asymptomatic cases, it’s very rare – and I used the phrase very rare – that we found a secondary transmission,” she said.
Dr. Van Kerkhove’s initial comments drew criticism from medical and public health professionals, who said the statement was “confusing” and communicated poorly.
Eric J. Topol, MD, tweeted that WHO had “engendered considerable confusion” with the comments about asymptomatic individuals rarely transmitting SARS-CoV-2. Dr. Topol, the author of a recent analysis published in Annals of Internal Medicine that suggested as many as 40%-45% of COVID-19 cases may be asymptomatic, said that it was not possible to determine whether asymptomatic individuals in the cohorts he studied were capable of spread like pre-symptomatic individuals. “We only know the viral loads are similar from multiple reports. And we do know some spread occurs from [asymptomatic] people,” he said.
Andy Slavitt, former acting administrator of the Centers for Medicare and Medicaid Services, said in a tweet that he believed WHO made “an irresponsible statement even though it was based on legitimate observations.” Reports by Member States do not reach a “bar of rigor,” he said.
Natalie E. Dean, PhD, assistant professor of biostatistics at the University of Florida, tweeted that the initial comments by the WHO seemed to be trying to draw a distinction between asymptomatic individuals who never develop symptoms, and presymptomatic individuals who present as asymptomatic, but later develop symptoms. Finding that asymptomatic cases rarely transmit the virus could change how people exposed to those asymptomatic individuals are monitored, but “it seems more of scientific than practical interest,” she noted. “People without current symptoms could be infectious. Act accordingly.”
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, also weighed in on the controversial WHO comments, telling Good Morning America on June 10 that Dr. Van Kerkhove's initial statement that asymptomatic SARS-CoV-2 transmission is a rare event is "not correct."
This article was updated 6/10/20.
A World Health Organization (WHO) official is walking back her comments characterizing the spread of SARS-CoV-2 by asymptomatic individuals as “rare.”
Maria Van Kerkhove, PhD, WHO’s COVID-19 technical lead and an infectious disease epidemiologist, caused a stir June 8 when she said that countries are reporting that many of their asymptomatic cases develop into cases of mild disease. For patients with truly asymptomatic disease, countries are “not finding secondary transmission onward. It’s very rare,” she said.
Suppressing symptomatic cases, on the other hand, would result in a “drastic reduction” in transmission, she noted. “But from the data we have, it still seems to be rare that an asymptomatic person actually transmits onward to a secondary individual,” she said.
But on June 9 – following a day of confusion and criticism – Dr. Van Kerkhove sought to clarify her comments on asymptomatic transmission during a live social media Q&A. She noted that while “the majority of transmission that we know about” is through individuals with symptoms, “there are a subset of people who don’t develop symptoms, and to truly understand how many people don’t have symptoms – we don’t actually have that answer yet.”
Between 6% and 41% of individuals may be asymptomatic based on estimates, she acknowledged.“What we need to better understand is how many of the people in the population don’t have symptoms, and separately, how many of those individuals go on to transmit to others,” she said.
Dr. Van Kerkhove also emphasized that her initial comments were made in response to a question raised at the press conference, and called it a misunderstanding. “I wasn’t stating a policy of WHO or anything like that,” she said. “I was just trying to articulate what we know.”
The phrase “very rare” referred to a subset of studies and reports WHO had received from its member states following asymptomatic individuals with COVID-19. “I was referring to some detailed investigations, cluster investigations, case contact tracing, where we had reports from member states saying that, when we follow asymptomatic cases, it’s very rare – and I used the phrase very rare – that we found a secondary transmission,” she said.
Dr. Van Kerkhove’s initial comments drew criticism from medical and public health professionals, who said the statement was “confusing” and communicated poorly.
Eric J. Topol, MD, tweeted that WHO had “engendered considerable confusion” with the comments about asymptomatic individuals rarely transmitting SARS-CoV-2. Dr. Topol, the author of a recent analysis published in Annals of Internal Medicine that suggested as many as 40%-45% of COVID-19 cases may be asymptomatic, said that it was not possible to determine whether asymptomatic individuals in the cohorts he studied were capable of spread like pre-symptomatic individuals. “We only know the viral loads are similar from multiple reports. And we do know some spread occurs from [asymptomatic] people,” he said.
Andy Slavitt, former acting administrator of the Centers for Medicare and Medicaid Services, said in a tweet that he believed WHO made “an irresponsible statement even though it was based on legitimate observations.” Reports by Member States do not reach a “bar of rigor,” he said.
Natalie E. Dean, PhD, assistant professor of biostatistics at the University of Florida, tweeted that the initial comments by the WHO seemed to be trying to draw a distinction between asymptomatic individuals who never develop symptoms, and presymptomatic individuals who present as asymptomatic, but later develop symptoms. Finding that asymptomatic cases rarely transmit the virus could change how people exposed to those asymptomatic individuals are monitored, but “it seems more of scientific than practical interest,” she noted. “People without current symptoms could be infectious. Act accordingly.”
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, also weighed in on the controversial WHO comments, telling Good Morning America on June 10 that Dr. Van Kerkhove's initial statement that asymptomatic SARS-CoV-2 transmission is a rare event is "not correct."
This article was updated 6/10/20.
Tepotinib elicits responses in METex14 NSCLC
The objective response rate was 46.5% among the 99 patients followed for 9 or more months, as assessed by independent reviewers. There were no complete responders, according to the reviewers.
However, the response rate according to investigator assessment was 55.6%, including two responses that were judged to be complete.
The median duration of response was 11.1 months according to reviewers and 14 months according to investigators.
“The success of this trial, alongside other studies on the same class of drugs, establishes MET exon 14 as an actionable target for non–small cell lung cancer,” said senior author Xiuning Le, MD, PhD, assistant professor in the department of thoracic/head and neck medical oncology at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Le presented results from this trial as part of the American Society of Clinical Oncology virtual scientific program. Results were published simultaneously in the New England Journal of Medicine.
The trial, dubbed VISION, already won tepotinib approval in Japan to treat NSCLC patients with MET exon 14–skipping mutations. The Food and Drug Administration has granted tepotinib breakthrough status, and Merck, the drug’s manufacturer, plans to submit tepotinib for review this year.
The VISION trial enrolled 152 patients with NSCLC – 99 with at least 9 months of follow-up and 53 with shorter follow-up. The patients’ MET exon 14 mutations were confirmed by liquid or tissue biopsy.
The patients’ median age at baseline was 74 years, 54% were men, and almost half had no smoking history. Patients received tepotinib at 500 mg daily until disease progression or intolerable toxicity.
Overall, the VISION results “compare favorably” with those from studies of other MET inhibitors, the investigators wrote.
The 46.5% objective response rate (per independent reviewers) included the 99 patients with follow-up of at least 9 months who were liquid- or tissue-biopsy positive (combined group). The response rate was 48.5% among the 66 patients with positive liquid biopsies and 50% among the 60 patients with positive tissue biopsies.
The median progression-free survival was 8.5 months in the combined group, 8.5 months in the liquid-biopsy group, and 11 months in the tissue-biopsy group. The median overall survival was 17.1 months, 15.8 months, and 22.3 months, respectively.
The 11 patients with brain metastases at baseline had results that were in line with the other patients’ results. Patients with brain metastases had an objective response rate of 54.5%, a median response duration of 9.5 months, and a median progression-free survival of 10.9 months.
Overall, 88.8% of patients reported adverse events related to treatment, including peripheral edema in 63.2%, nausea in 25.7%, and diarrhea in 21.7%.
Grade 3-4 adverse events occurred in 27% of patients. Peripheral edema was the most common of these events, reported in 7.2% of patients.
“Proactive monitoring for peripheral edema is recommended and can be managed with temporary discontinuation of tepotinib or dose reduction,” the investigators wrote.
The death of a 79-year-old patient with respiratory failure and dyspnea, secondary to interstitial lung disease, was the only death considered to be treatment related.
The study was funded by Merck. Dr. Le and other investigators disclosed relationships, including employment, with the company.
SOURCE: Le X et al. ASCO 2020, Abstract 9556.
The objective response rate was 46.5% among the 99 patients followed for 9 or more months, as assessed by independent reviewers. There were no complete responders, according to the reviewers.
However, the response rate according to investigator assessment was 55.6%, including two responses that were judged to be complete.
The median duration of response was 11.1 months according to reviewers and 14 months according to investigators.
“The success of this trial, alongside other studies on the same class of drugs, establishes MET exon 14 as an actionable target for non–small cell lung cancer,” said senior author Xiuning Le, MD, PhD, assistant professor in the department of thoracic/head and neck medical oncology at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Le presented results from this trial as part of the American Society of Clinical Oncology virtual scientific program. Results were published simultaneously in the New England Journal of Medicine.
The trial, dubbed VISION, already won tepotinib approval in Japan to treat NSCLC patients with MET exon 14–skipping mutations. The Food and Drug Administration has granted tepotinib breakthrough status, and Merck, the drug’s manufacturer, plans to submit tepotinib for review this year.
The VISION trial enrolled 152 patients with NSCLC – 99 with at least 9 months of follow-up and 53 with shorter follow-up. The patients’ MET exon 14 mutations were confirmed by liquid or tissue biopsy.
The patients’ median age at baseline was 74 years, 54% were men, and almost half had no smoking history. Patients received tepotinib at 500 mg daily until disease progression or intolerable toxicity.
Overall, the VISION results “compare favorably” with those from studies of other MET inhibitors, the investigators wrote.
The 46.5% objective response rate (per independent reviewers) included the 99 patients with follow-up of at least 9 months who were liquid- or tissue-biopsy positive (combined group). The response rate was 48.5% among the 66 patients with positive liquid biopsies and 50% among the 60 patients with positive tissue biopsies.
The median progression-free survival was 8.5 months in the combined group, 8.5 months in the liquid-biopsy group, and 11 months in the tissue-biopsy group. The median overall survival was 17.1 months, 15.8 months, and 22.3 months, respectively.
The 11 patients with brain metastases at baseline had results that were in line with the other patients’ results. Patients with brain metastases had an objective response rate of 54.5%, a median response duration of 9.5 months, and a median progression-free survival of 10.9 months.
Overall, 88.8% of patients reported adverse events related to treatment, including peripheral edema in 63.2%, nausea in 25.7%, and diarrhea in 21.7%.
Grade 3-4 adverse events occurred in 27% of patients. Peripheral edema was the most common of these events, reported in 7.2% of patients.
“Proactive monitoring for peripheral edema is recommended and can be managed with temporary discontinuation of tepotinib or dose reduction,” the investigators wrote.
The death of a 79-year-old patient with respiratory failure and dyspnea, secondary to interstitial lung disease, was the only death considered to be treatment related.
The study was funded by Merck. Dr. Le and other investigators disclosed relationships, including employment, with the company.
SOURCE: Le X et al. ASCO 2020, Abstract 9556.
The objective response rate was 46.5% among the 99 patients followed for 9 or more months, as assessed by independent reviewers. There were no complete responders, according to the reviewers.
However, the response rate according to investigator assessment was 55.6%, including two responses that were judged to be complete.
The median duration of response was 11.1 months according to reviewers and 14 months according to investigators.
“The success of this trial, alongside other studies on the same class of drugs, establishes MET exon 14 as an actionable target for non–small cell lung cancer,” said senior author Xiuning Le, MD, PhD, assistant professor in the department of thoracic/head and neck medical oncology at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Le presented results from this trial as part of the American Society of Clinical Oncology virtual scientific program. Results were published simultaneously in the New England Journal of Medicine.
The trial, dubbed VISION, already won tepotinib approval in Japan to treat NSCLC patients with MET exon 14–skipping mutations. The Food and Drug Administration has granted tepotinib breakthrough status, and Merck, the drug’s manufacturer, plans to submit tepotinib for review this year.
The VISION trial enrolled 152 patients with NSCLC – 99 with at least 9 months of follow-up and 53 with shorter follow-up. The patients’ MET exon 14 mutations were confirmed by liquid or tissue biopsy.
The patients’ median age at baseline was 74 years, 54% were men, and almost half had no smoking history. Patients received tepotinib at 500 mg daily until disease progression or intolerable toxicity.
Overall, the VISION results “compare favorably” with those from studies of other MET inhibitors, the investigators wrote.
The 46.5% objective response rate (per independent reviewers) included the 99 patients with follow-up of at least 9 months who were liquid- or tissue-biopsy positive (combined group). The response rate was 48.5% among the 66 patients with positive liquid biopsies and 50% among the 60 patients with positive tissue biopsies.
The median progression-free survival was 8.5 months in the combined group, 8.5 months in the liquid-biopsy group, and 11 months in the tissue-biopsy group. The median overall survival was 17.1 months, 15.8 months, and 22.3 months, respectively.
The 11 patients with brain metastases at baseline had results that were in line with the other patients’ results. Patients with brain metastases had an objective response rate of 54.5%, a median response duration of 9.5 months, and a median progression-free survival of 10.9 months.
Overall, 88.8% of patients reported adverse events related to treatment, including peripheral edema in 63.2%, nausea in 25.7%, and diarrhea in 21.7%.
Grade 3-4 adverse events occurred in 27% of patients. Peripheral edema was the most common of these events, reported in 7.2% of patients.
“Proactive monitoring for peripheral edema is recommended and can be managed with temporary discontinuation of tepotinib or dose reduction,” the investigators wrote.
The death of a 79-year-old patient with respiratory failure and dyspnea, secondary to interstitial lung disease, was the only death considered to be treatment related.
The study was funded by Merck. Dr. Le and other investigators disclosed relationships, including employment, with the company.
SOURCE: Le X et al. ASCO 2020, Abstract 9556.
FROM ASCO 2020
In-hospital formula feeding more than doubles odds of early weaning
Breastfed infants who receive formula in the hospital are more than twofold more likely to wean during the first year, compared with infants who are exclusively breastfed, according to research published online in Pediatrics.
The finding is based on an analysis of data from over 8,000 infants in the Minnesota Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). The researchers used propensity scoring methods to match breastfed infants who received in-hospital formula to those who were exclusively breastfed. The researchers adjusted for potential confounders such as maternal age, cultural identity, marital status, education level, smoking, body mass index, diabetes mellitus, previous breastfeeding experience, and infant gestational age and birth weight.
“Our study strengthens the evidence that formula supplementation of breastfed infants negatively affects breastfeeding duration,” said Marcia Burton McCoy, MPH, of the Minnesota Department of Health’s WIC, and Pamela Heggie, MD, of the University of Minnesota in Minneapolis. “This finding has important clinical implications because breastfeeding duration has been shown to have a significant impact on numerous health outcomes, with a dose-response protective effect for sudden infant death syndrome, infection in infancy, and childhood obesity.”
Breastfeeding has various medical and neurodevelopmental benefits, and “even brief exposure to formula alters the infant microbiome long-term and increases the risk of allergy at 2 years of age,” the authors said.
In their study, one analysis that included 5,310 infants assumed that all bias was controlled through matching. A second, more conservative analysis that corrected for medically necessary supplementation included 4,836 infants. The researchers used data about in-hospital feeding which the Minnesota WIC staff collected in 2016 during WIC appointments.
In the first analysis, the hazard ratio of weaning across the first year was 6.1 among breastfed infants exposed to in-hospital formula feeding. In the second analysis, the hazard ratio was 2.5.
In-hospital formula feeding often leads to continued supplementation after discharge and may directly affect milk supply, Ms. McCoy and Dr. Heggie said. In-hospital formula feeding “is seldom medically necessary and, with rare exceptions, not medically indicated when the mother’s own milk or pasteurized donor milk is available.”
The study population was of lower income and more culturally diverse, compared with the general population, which may limit generalizability of the results, the authors noted.
With propensity scoring, the investigators found an association between in-hospital formula feeding and early weaning that “is analogous to previous estimates” that relied on more traditional observational methods, Lori B. Feldman-Winter, MD, MPH, professor of pediatrics at Cooper Medical School of Rowan University in Camden, N.J., and Ann L. Kellams, MD, professor of pediatrics at the University of Virginia in Charlottesville, said in an accompanying editorial.
“Maternal conditions such as obesity ... previous breast surgery, infertility, polycystic ovarian syndrome, and breast anomalies may lead to difficulties in establishing and maintaining sufficient milk supply as well as affect duration of continued breastfeeding,” the editorialists said. “Cultural, racial, and ethnic factors are also potential nonmedical reasons for breastfeeding supplementation.” In addition, implicit biases of health care practitioners may influence breastfeeding outcomes.
“The article by McCoy and Heggie gives us a compelling reason to avoid unnecessary supplementation, but there are also significant consequences of missing suboptimal intake in the newborn,” Dr. Feldman-Winter and Dr. Kellams emphasized. “Future research should be focused on methods of identifying both women and infants at risk for suboptimal intake, biological consequences of early formula supplementation, and best methods to preserve exclusive breastfeeding or human milk feeding.”
The study authors and the editorialists had no relevant financial disclosures.
SOURCES: McCoy MB et al. Pediatrics. 2020 Jun 9. doi: 10.1542/peds.2019-2946; Feldman-Winter LB and Kellams AL. Pediatrics. 2020 Jun 9. doi: 10.1542/peds.2020-1221.
Breastfed infants who receive formula in the hospital are more than twofold more likely to wean during the first year, compared with infants who are exclusively breastfed, according to research published online in Pediatrics.
The finding is based on an analysis of data from over 8,000 infants in the Minnesota Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). The researchers used propensity scoring methods to match breastfed infants who received in-hospital formula to those who were exclusively breastfed. The researchers adjusted for potential confounders such as maternal age, cultural identity, marital status, education level, smoking, body mass index, diabetes mellitus, previous breastfeeding experience, and infant gestational age and birth weight.
“Our study strengthens the evidence that formula supplementation of breastfed infants negatively affects breastfeeding duration,” said Marcia Burton McCoy, MPH, of the Minnesota Department of Health’s WIC, and Pamela Heggie, MD, of the University of Minnesota in Minneapolis. “This finding has important clinical implications because breastfeeding duration has been shown to have a significant impact on numerous health outcomes, with a dose-response protective effect for sudden infant death syndrome, infection in infancy, and childhood obesity.”
Breastfeeding has various medical and neurodevelopmental benefits, and “even brief exposure to formula alters the infant microbiome long-term and increases the risk of allergy at 2 years of age,” the authors said.
In their study, one analysis that included 5,310 infants assumed that all bias was controlled through matching. A second, more conservative analysis that corrected for medically necessary supplementation included 4,836 infants. The researchers used data about in-hospital feeding which the Minnesota WIC staff collected in 2016 during WIC appointments.
In the first analysis, the hazard ratio of weaning across the first year was 6.1 among breastfed infants exposed to in-hospital formula feeding. In the second analysis, the hazard ratio was 2.5.
In-hospital formula feeding often leads to continued supplementation after discharge and may directly affect milk supply, Ms. McCoy and Dr. Heggie said. In-hospital formula feeding “is seldom medically necessary and, with rare exceptions, not medically indicated when the mother’s own milk or pasteurized donor milk is available.”
The study population was of lower income and more culturally diverse, compared with the general population, which may limit generalizability of the results, the authors noted.
With propensity scoring, the investigators found an association between in-hospital formula feeding and early weaning that “is analogous to previous estimates” that relied on more traditional observational methods, Lori B. Feldman-Winter, MD, MPH, professor of pediatrics at Cooper Medical School of Rowan University in Camden, N.J., and Ann L. Kellams, MD, professor of pediatrics at the University of Virginia in Charlottesville, said in an accompanying editorial.
“Maternal conditions such as obesity ... previous breast surgery, infertility, polycystic ovarian syndrome, and breast anomalies may lead to difficulties in establishing and maintaining sufficient milk supply as well as affect duration of continued breastfeeding,” the editorialists said. “Cultural, racial, and ethnic factors are also potential nonmedical reasons for breastfeeding supplementation.” In addition, implicit biases of health care practitioners may influence breastfeeding outcomes.
“The article by McCoy and Heggie gives us a compelling reason to avoid unnecessary supplementation, but there are also significant consequences of missing suboptimal intake in the newborn,” Dr. Feldman-Winter and Dr. Kellams emphasized. “Future research should be focused on methods of identifying both women and infants at risk for suboptimal intake, biological consequences of early formula supplementation, and best methods to preserve exclusive breastfeeding or human milk feeding.”
The study authors and the editorialists had no relevant financial disclosures.
SOURCES: McCoy MB et al. Pediatrics. 2020 Jun 9. doi: 10.1542/peds.2019-2946; Feldman-Winter LB and Kellams AL. Pediatrics. 2020 Jun 9. doi: 10.1542/peds.2020-1221.
Breastfed infants who receive formula in the hospital are more than twofold more likely to wean during the first year, compared with infants who are exclusively breastfed, according to research published online in Pediatrics.
The finding is based on an analysis of data from over 8,000 infants in the Minnesota Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). The researchers used propensity scoring methods to match breastfed infants who received in-hospital formula to those who were exclusively breastfed. The researchers adjusted for potential confounders such as maternal age, cultural identity, marital status, education level, smoking, body mass index, diabetes mellitus, previous breastfeeding experience, and infant gestational age and birth weight.
“Our study strengthens the evidence that formula supplementation of breastfed infants negatively affects breastfeeding duration,” said Marcia Burton McCoy, MPH, of the Minnesota Department of Health’s WIC, and Pamela Heggie, MD, of the University of Minnesota in Minneapolis. “This finding has important clinical implications because breastfeeding duration has been shown to have a significant impact on numerous health outcomes, with a dose-response protective effect for sudden infant death syndrome, infection in infancy, and childhood obesity.”
Breastfeeding has various medical and neurodevelopmental benefits, and “even brief exposure to formula alters the infant microbiome long-term and increases the risk of allergy at 2 years of age,” the authors said.
In their study, one analysis that included 5,310 infants assumed that all bias was controlled through matching. A second, more conservative analysis that corrected for medically necessary supplementation included 4,836 infants. The researchers used data about in-hospital feeding which the Minnesota WIC staff collected in 2016 during WIC appointments.
In the first analysis, the hazard ratio of weaning across the first year was 6.1 among breastfed infants exposed to in-hospital formula feeding. In the second analysis, the hazard ratio was 2.5.
In-hospital formula feeding often leads to continued supplementation after discharge and may directly affect milk supply, Ms. McCoy and Dr. Heggie said. In-hospital formula feeding “is seldom medically necessary and, with rare exceptions, not medically indicated when the mother’s own milk or pasteurized donor milk is available.”
The study population was of lower income and more culturally diverse, compared with the general population, which may limit generalizability of the results, the authors noted.
With propensity scoring, the investigators found an association between in-hospital formula feeding and early weaning that “is analogous to previous estimates” that relied on more traditional observational methods, Lori B. Feldman-Winter, MD, MPH, professor of pediatrics at Cooper Medical School of Rowan University in Camden, N.J., and Ann L. Kellams, MD, professor of pediatrics at the University of Virginia in Charlottesville, said in an accompanying editorial.
“Maternal conditions such as obesity ... previous breast surgery, infertility, polycystic ovarian syndrome, and breast anomalies may lead to difficulties in establishing and maintaining sufficient milk supply as well as affect duration of continued breastfeeding,” the editorialists said. “Cultural, racial, and ethnic factors are also potential nonmedical reasons for breastfeeding supplementation.” In addition, implicit biases of health care practitioners may influence breastfeeding outcomes.
“The article by McCoy and Heggie gives us a compelling reason to avoid unnecessary supplementation, but there are also significant consequences of missing suboptimal intake in the newborn,” Dr. Feldman-Winter and Dr. Kellams emphasized. “Future research should be focused on methods of identifying both women and infants at risk for suboptimal intake, biological consequences of early formula supplementation, and best methods to preserve exclusive breastfeeding or human milk feeding.”
The study authors and the editorialists had no relevant financial disclosures.
SOURCES: McCoy MB et al. Pediatrics. 2020 Jun 9. doi: 10.1542/peds.2019-2946; Feldman-Winter LB and Kellams AL. Pediatrics. 2020 Jun 9. doi: 10.1542/peds.2020-1221.
FROM PEDIATRICS
Difluoroethane Inhalant Abuse, Skeletal Fluorosis, and Withdrawal
Difluoroethane (DFE) is an easily acquired and inexpensive volatile substance that can be inhaled recreationally. 1 It is found in common household items, including compressed air dusters, refrigerants, and propellants. DFE is a central nervous system (CNS) depressant associated with a brief sensation of euphoria when inhaled.2 Prolonged or excessive use is associated with toxicity, and abrupt cessation can induce withdrawal.3-5 We present a case of DFE abuse associated with skeletal fluorosis and withdrawal psychosis.
Case Presentation
A 39-year-old man with a 6-month history of inhaling 20 to 25 cans of DFE per day presented to the emergency department after abruptly stopping use 6 days prior. He described irritability, agitation, auditory hallucinations, and delusions of “demons trying to harm him.”
On presentation, the patient was afebrile with a mild sinus tachycardia. He was calm and cooperative but reported delusions and auditory hallucinations. He denied suicidal or homicidal ideation. His physical examination was remarkable for bony deformities of his hands (Figure 1).
The initial workup included a complete blood count; basic metabolic panel; liver function tests; urine toxicology; and testing for hepatitis B/C and HIV; all unremarkable. Psychiatry and poison control were consulted, and he was admitted.
After 72 hours, the patient's irritability, agitation, and sinus tachycardia resolved; however, his psychosis and hallucinations persisted. He was started on olanzapine and transferred to inpatient psychiatry. Additional laboratory tests revealed a serum fluoride of 0.35 mg/L (normal, 1-47 ug/L), C-telopeptide of 2,663 pg/mL (normal, 70-780 pg/mL), and hand X-rays showing diffuse bilateral periosteal reaction in the phalanges and distal ulnas (Figure 2).6
Discussion
DFE acts as a CNS depressant via glutamate and γ-aminobutyric acid receptors, causing a brief euphoria when inhaled.2 Acute toxicity can cause nausea, vomiting, abdominal pain, and altered mental status. Severe complications include loss of consciousness, mucosal frostbite, angioedema, cardiac arrhythmias, and skeletal fluorosis.2,7
Skeletal fluorosis is a rare ramification of excessive or prolonged DFE inhalation. DFE is metabolized into a fluorinated compound that accumulates and leaches calcium from bone, altering its structure. This can manifest as bony deformities with diffuse periosteal reaction and elevated serum fluoride levels. Furthermore, the elevated C-telopeptide level seen in this case may suggest increased bone turnover.
Approximately 50% of patients report withdrawal symptoms, but the timing, duration, and associated symptoms are not well understood.3 Withdrawal can include tremors, diaphoresis, nausea, vomiting, depression, anxiety, irritability, psychosis, and hallucinations. Symptoms typically start within 24 to 48 hours of cessation and last for 3 to 7 days.5 Psychotic symptoms often abate quickly; however, anxiety and insomnia can persist for weeks.5 There are no formal treatment guidelines, but poison control suggests observation and as-needed benzodiazepines. Although this patient’s irritability and agitation resolved, his psychosis and hallucinations persisted, raising concern for an underlying psychiatric diagnosis and prompting transfer to inpatient psychiatry.
Conslusion
Health care providers should recognize the symptoms of DFE toxicity, its complications, and withdrawal. Collaborating with psychiatry and poison control is beneficial in providing guidelines for supportive care.
1. Arroyo JP, Johnson DC, Lewis JB, et al. Treatment of acute intoxication from inhaled 1,2-difluoroethane. Ann Intern Med. 2018;169(11):820‐822. doi:10.7326/L18-0186
2. National Library of Medicine, PubChem. Hazardous Substance Data Bank (HSDB) 1,1-Difluoroethane. https:// pubchem.ncbi.nlm.nih.gov/source/hsdb/5205. Updated October 25, 2016. Accessed May 20, 2020.
3. Perron BE, Glass JE, Ahmedani BK, Vaughn MG, Roberts DE, Wu LT. The prevalence and clinical significance of inhalant withdrawal symptoms among a national sample. Subst Abuse Rehabil. 2011;2011(2):69‐76. doi:10.2147/SAR.S14937
4. Perron BE, Howard MO, Vaughn MG, Jarman CN. Inhalant withdrawal as a clinically significant feature of inhalant dependence disorder. Med Hypotheses. 2009;73(6):935‐937. doi:10.1016/j.mehy.2009.06.036
5. Addiction Center. Inhalant withdrawal and detox. https://www.addictioncenter.com/drugs/inhalants /withdrawal-detox. Accessed May 18, 2020.
6. Torra M, Rodamilans M, Corbella J. Serum and urine ionic fluoride: normal range in a nonexposed population. Biol Trace Elem Res. 1998;63(1):67‐71. doi:10.1007/BF02785278 7. Cohen E, Hsu RY, Evangelista P, Aaron R, Rubin LE. Rapid-onset diffuse skeletal fluorosis from inhalant abuse: a case report. JBJS Case Connect. 2014;4(4):e108. doi:10.2106/JBJS.CC.N.00085
Difluoroethane (DFE) is an easily acquired and inexpensive volatile substance that can be inhaled recreationally. 1 It is found in common household items, including compressed air dusters, refrigerants, and propellants. DFE is a central nervous system (CNS) depressant associated with a brief sensation of euphoria when inhaled.2 Prolonged or excessive use is associated with toxicity, and abrupt cessation can induce withdrawal.3-5 We present a case of DFE abuse associated with skeletal fluorosis and withdrawal psychosis.
Case Presentation
A 39-year-old man with a 6-month history of inhaling 20 to 25 cans of DFE per day presented to the emergency department after abruptly stopping use 6 days prior. He described irritability, agitation, auditory hallucinations, and delusions of “demons trying to harm him.”
On presentation, the patient was afebrile with a mild sinus tachycardia. He was calm and cooperative but reported delusions and auditory hallucinations. He denied suicidal or homicidal ideation. His physical examination was remarkable for bony deformities of his hands (Figure 1).
The initial workup included a complete blood count; basic metabolic panel; liver function tests; urine toxicology; and testing for hepatitis B/C and HIV; all unremarkable. Psychiatry and poison control were consulted, and he was admitted.
After 72 hours, the patient's irritability, agitation, and sinus tachycardia resolved; however, his psychosis and hallucinations persisted. He was started on olanzapine and transferred to inpatient psychiatry. Additional laboratory tests revealed a serum fluoride of 0.35 mg/L (normal, 1-47 ug/L), C-telopeptide of 2,663 pg/mL (normal, 70-780 pg/mL), and hand X-rays showing diffuse bilateral periosteal reaction in the phalanges and distal ulnas (Figure 2).6
Discussion
DFE acts as a CNS depressant via glutamate and γ-aminobutyric acid receptors, causing a brief euphoria when inhaled.2 Acute toxicity can cause nausea, vomiting, abdominal pain, and altered mental status. Severe complications include loss of consciousness, mucosal frostbite, angioedema, cardiac arrhythmias, and skeletal fluorosis.2,7
Skeletal fluorosis is a rare ramification of excessive or prolonged DFE inhalation. DFE is metabolized into a fluorinated compound that accumulates and leaches calcium from bone, altering its structure. This can manifest as bony deformities with diffuse periosteal reaction and elevated serum fluoride levels. Furthermore, the elevated C-telopeptide level seen in this case may suggest increased bone turnover.
Approximately 50% of patients report withdrawal symptoms, but the timing, duration, and associated symptoms are not well understood.3 Withdrawal can include tremors, diaphoresis, nausea, vomiting, depression, anxiety, irritability, psychosis, and hallucinations. Symptoms typically start within 24 to 48 hours of cessation and last for 3 to 7 days.5 Psychotic symptoms often abate quickly; however, anxiety and insomnia can persist for weeks.5 There are no formal treatment guidelines, but poison control suggests observation and as-needed benzodiazepines. Although this patient’s irritability and agitation resolved, his psychosis and hallucinations persisted, raising concern for an underlying psychiatric diagnosis and prompting transfer to inpatient psychiatry.
Conslusion
Health care providers should recognize the symptoms of DFE toxicity, its complications, and withdrawal. Collaborating with psychiatry and poison control is beneficial in providing guidelines for supportive care.
Difluoroethane (DFE) is an easily acquired and inexpensive volatile substance that can be inhaled recreationally. 1 It is found in common household items, including compressed air dusters, refrigerants, and propellants. DFE is a central nervous system (CNS) depressant associated with a brief sensation of euphoria when inhaled.2 Prolonged or excessive use is associated with toxicity, and abrupt cessation can induce withdrawal.3-5 We present a case of DFE abuse associated with skeletal fluorosis and withdrawal psychosis.
Case Presentation
A 39-year-old man with a 6-month history of inhaling 20 to 25 cans of DFE per day presented to the emergency department after abruptly stopping use 6 days prior. He described irritability, agitation, auditory hallucinations, and delusions of “demons trying to harm him.”
On presentation, the patient was afebrile with a mild sinus tachycardia. He was calm and cooperative but reported delusions and auditory hallucinations. He denied suicidal or homicidal ideation. His physical examination was remarkable for bony deformities of his hands (Figure 1).
The initial workup included a complete blood count; basic metabolic panel; liver function tests; urine toxicology; and testing for hepatitis B/C and HIV; all unremarkable. Psychiatry and poison control were consulted, and he was admitted.
After 72 hours, the patient's irritability, agitation, and sinus tachycardia resolved; however, his psychosis and hallucinations persisted. He was started on olanzapine and transferred to inpatient psychiatry. Additional laboratory tests revealed a serum fluoride of 0.35 mg/L (normal, 1-47 ug/L), C-telopeptide of 2,663 pg/mL (normal, 70-780 pg/mL), and hand X-rays showing diffuse bilateral periosteal reaction in the phalanges and distal ulnas (Figure 2).6
Discussion
DFE acts as a CNS depressant via glutamate and γ-aminobutyric acid receptors, causing a brief euphoria when inhaled.2 Acute toxicity can cause nausea, vomiting, abdominal pain, and altered mental status. Severe complications include loss of consciousness, mucosal frostbite, angioedema, cardiac arrhythmias, and skeletal fluorosis.2,7
Skeletal fluorosis is a rare ramification of excessive or prolonged DFE inhalation. DFE is metabolized into a fluorinated compound that accumulates and leaches calcium from bone, altering its structure. This can manifest as bony deformities with diffuse periosteal reaction and elevated serum fluoride levels. Furthermore, the elevated C-telopeptide level seen in this case may suggest increased bone turnover.
Approximately 50% of patients report withdrawal symptoms, but the timing, duration, and associated symptoms are not well understood.3 Withdrawal can include tremors, diaphoresis, nausea, vomiting, depression, anxiety, irritability, psychosis, and hallucinations. Symptoms typically start within 24 to 48 hours of cessation and last for 3 to 7 days.5 Psychotic symptoms often abate quickly; however, anxiety and insomnia can persist for weeks.5 There are no formal treatment guidelines, but poison control suggests observation and as-needed benzodiazepines. Although this patient’s irritability and agitation resolved, his psychosis and hallucinations persisted, raising concern for an underlying psychiatric diagnosis and prompting transfer to inpatient psychiatry.
Conslusion
Health care providers should recognize the symptoms of DFE toxicity, its complications, and withdrawal. Collaborating with psychiatry and poison control is beneficial in providing guidelines for supportive care.
1. Arroyo JP, Johnson DC, Lewis JB, et al. Treatment of acute intoxication from inhaled 1,2-difluoroethane. Ann Intern Med. 2018;169(11):820‐822. doi:10.7326/L18-0186
2. National Library of Medicine, PubChem. Hazardous Substance Data Bank (HSDB) 1,1-Difluoroethane. https:// pubchem.ncbi.nlm.nih.gov/source/hsdb/5205. Updated October 25, 2016. Accessed May 20, 2020.
3. Perron BE, Glass JE, Ahmedani BK, Vaughn MG, Roberts DE, Wu LT. The prevalence and clinical significance of inhalant withdrawal symptoms among a national sample. Subst Abuse Rehabil. 2011;2011(2):69‐76. doi:10.2147/SAR.S14937
4. Perron BE, Howard MO, Vaughn MG, Jarman CN. Inhalant withdrawal as a clinically significant feature of inhalant dependence disorder. Med Hypotheses. 2009;73(6):935‐937. doi:10.1016/j.mehy.2009.06.036
5. Addiction Center. Inhalant withdrawal and detox. https://www.addictioncenter.com/drugs/inhalants /withdrawal-detox. Accessed May 18, 2020.
6. Torra M, Rodamilans M, Corbella J. Serum and urine ionic fluoride: normal range in a nonexposed population. Biol Trace Elem Res. 1998;63(1):67‐71. doi:10.1007/BF02785278 7. Cohen E, Hsu RY, Evangelista P, Aaron R, Rubin LE. Rapid-onset diffuse skeletal fluorosis from inhalant abuse: a case report. JBJS Case Connect. 2014;4(4):e108. doi:10.2106/JBJS.CC.N.00085
1. Arroyo JP, Johnson DC, Lewis JB, et al. Treatment of acute intoxication from inhaled 1,2-difluoroethane. Ann Intern Med. 2018;169(11):820‐822. doi:10.7326/L18-0186
2. National Library of Medicine, PubChem. Hazardous Substance Data Bank (HSDB) 1,1-Difluoroethane. https:// pubchem.ncbi.nlm.nih.gov/source/hsdb/5205. Updated October 25, 2016. Accessed May 20, 2020.
3. Perron BE, Glass JE, Ahmedani BK, Vaughn MG, Roberts DE, Wu LT. The prevalence and clinical significance of inhalant withdrawal symptoms among a national sample. Subst Abuse Rehabil. 2011;2011(2):69‐76. doi:10.2147/SAR.S14937
4. Perron BE, Howard MO, Vaughn MG, Jarman CN. Inhalant withdrawal as a clinically significant feature of inhalant dependence disorder. Med Hypotheses. 2009;73(6):935‐937. doi:10.1016/j.mehy.2009.06.036
5. Addiction Center. Inhalant withdrawal and detox. https://www.addictioncenter.com/drugs/inhalants /withdrawal-detox. Accessed May 18, 2020.
6. Torra M, Rodamilans M, Corbella J. Serum and urine ionic fluoride: normal range in a nonexposed population. Biol Trace Elem Res. 1998;63(1):67‐71. doi:10.1007/BF02785278 7. Cohen E, Hsu RY, Evangelista P, Aaron R, Rubin LE. Rapid-onset diffuse skeletal fluorosis from inhalant abuse: a case report. JBJS Case Connect. 2014;4(4):e108. doi:10.2106/JBJS.CC.N.00085
Age leads COVID-19 hospitalization risk factors in RMDs
Being aged older than 65 years was associated with the highest risk of people with rheumatic and musculoskeletal diseases (RMDs) needing hospital treatment for COVID-19, according to the first results to be reported from ReCoVery, the German national COVID-19 registry.
Older patients with RMDs were five times more likely than younger patients to be hospitalized if they tested positive for SARS‑CoV‑2 and developed COVID-19 (odds ratio, 5.1; 95% confidence interval, 2.3-11.4).
The likelihood of hospitalization was also significantly increased by the current or prior use of glucocorticoids (OR, 2.59; 95% CI, 1.2-5.4) and by the presence of cardiovascular disease (OR, 2.27; 95% CI, 1.2-5.4).
“The register is a joint initiative of the German Society for Rheumatology and the Justus Liebig University in Giessen,” explained Anne Regierer, MD, during a live session of the annual European Congress of Rheumatology, held online this year due to COVID-19.
“The current pandemic has changed all of our lives. For patients it brought a lot of uncertainty and fears,” said Dr. Regierer, of the German Rheumatism Research Center Berlin.
“The risk of SARS-CoV-2 infection in patients with inflammatory rheumatic diseases [IRD] is still largely unknown. We still don’t know whether they have a high risk of getting the infection or whether they have a higher risk of a severer case ... therefore there’s an urgent need to have data to generate evidence for the management of our patients.”
Launched at the end of March 2020, the German registry now includes data on 251 patients – 194 of whom have recovered – provided by more than 200 registered rheumatologists. The registry data have now been integrated into the EULAR COVID-19 Database, which is itself part of a global effort to better understand and optimally manage RMD patients during the pandemic.
“The data presented by Dr. Regierer looked at similar outcomes and found quite similar results, which is reassuring,” Kimme Hyrich, MD, PhD, professor of epidemiology at the University of Manchester (England) and a consultant rheumatologist in the Kellgren Centre for Rheumatology at Manchester University Hospitals NHS Foundation Trust, said in an interview.
“We are very grateful for this collaboration [with the German society and others]. Our first publication has looked at hospitalization, but with more data we may have the opportunity to look at less-common outcomes [e.g. death, other COVID complications] or within individual diseases or treatments. So far I don’t think we will come to a different conclusion,” observed Dr. Hyrich, who is on the steering committee for the EULAR COVID-19 Database.
“These initial data are reassuring in that the majority of cases of COVID reported to our database have recovered, including those who were hospitalized,” she said.
Current EULAR advice is to continue treatment with glucocorticoids in patients who are being chronically treated, but to use them at the lowest possible dose.
The objectives of this first analysis of the German registry was to provide a description of the patients who did and did not require hospitalization and those who needed ventilation, as well as look at possible risk factors for hospitalization.
Dr. Regierer reported that, of 192 patients they included – all with a positive lab test for SARS-CoV-2 – 128 (67%) did not require hospital admission. Of those that did (n = 64), 43 (22%) did not need ventilation and 21 (11%) did. Fifteen patients died, all of whom had been hospitalized, and all but one of them had needed ventilation.
Concerning the characteristics of the patients, those who needed hospital treatment with and without ventilation were older than those who were not admitted (70 vs. 65 vs. 54 years, respectively).
“Looking at the sexes, the gender distribution is also interesting. We see 69% females in the nonhospitalized patients, 65% of the inpatients without ventilation, but only 43% females in the ventilated patients. So in this group, the male patients are the majority,” Dr. Regierer observed.
Just over half of all patients in the nonhospitalized and the hospitalized without ventilation groups had IRD in remission, but those in the hospitalized with ventilation group less than one-fifth had their IRD under control.
“Of course we have to keep in mind the small sample sizes,” Dr. Regierer said, but the distribution of patients by disease type was “what you’d expect in clinical care.” The majority of patients in each of the three groups had RA (47%, 56%, and 57%), followed by psoriatic arthritis (19%, 7%, and 14%), axial spondyloarthritis (11%, 5%, and 0%), systemic lupus erythematosus (6%, 2%, and 0%), and vasculitis (1%, 5%, and 5%).
Patients who were hospitalized with and without ventilation were more likely to have more than one comorbidity than those who were not hospitalized with COVID-19.
“The most frequent comorbidity was cardiovascular disease with 58% and 76% in the inpatient groups,” Dr. Regierer reported. One-third of the nonhospitalized patients had a cardiovascular comorbidity.
“If we look at pulmonary disease, we see that 38% of the ventilator patients had an underlying pulmonary disease,” she added. This was in comparison with 19% of the hospitalized without ventilation and 13% of the nonhospitalized patients. Diabetes was another common comorbidity in hospitalized patients with (16%) and without (19%) ventilation versus just 2% of nonhospitalized patients. While these and other comorbidities such as chronic renal insufficiency were associated with higher odds ratios in the multivariate risk factor analysis, they did not reach statistical significance.
With regard to RMD treatments, more than 60% of patients in the hospitalized group had received treatment with glucocorticoids versus 37% of those who did not get admitted. No differences were seen for the other treatments.
Interestingly, “female sex, remission, and use of NSAIDs have an odds ratio smaller than 1. So there might be a lower risk of hospitalization associated with these factors,” Dr. Regierer said.
Dr. Regierer has received grant support and is part of speaker’s bureaus for a variety of pharmaceutical companies. Dr. Hyrich disclosed grant income from Bristol-Myers Squibb, UCB, and Pfizer, and receiving speaker fees from AbbVie.
Being aged older than 65 years was associated with the highest risk of people with rheumatic and musculoskeletal diseases (RMDs) needing hospital treatment for COVID-19, according to the first results to be reported from ReCoVery, the German national COVID-19 registry.
Older patients with RMDs were five times more likely than younger patients to be hospitalized if they tested positive for SARS‑CoV‑2 and developed COVID-19 (odds ratio, 5.1; 95% confidence interval, 2.3-11.4).
The likelihood of hospitalization was also significantly increased by the current or prior use of glucocorticoids (OR, 2.59; 95% CI, 1.2-5.4) and by the presence of cardiovascular disease (OR, 2.27; 95% CI, 1.2-5.4).
“The register is a joint initiative of the German Society for Rheumatology and the Justus Liebig University in Giessen,” explained Anne Regierer, MD, during a live session of the annual European Congress of Rheumatology, held online this year due to COVID-19.
“The current pandemic has changed all of our lives. For patients it brought a lot of uncertainty and fears,” said Dr. Regierer, of the German Rheumatism Research Center Berlin.
“The risk of SARS-CoV-2 infection in patients with inflammatory rheumatic diseases [IRD] is still largely unknown. We still don’t know whether they have a high risk of getting the infection or whether they have a higher risk of a severer case ... therefore there’s an urgent need to have data to generate evidence for the management of our patients.”
Launched at the end of March 2020, the German registry now includes data on 251 patients – 194 of whom have recovered – provided by more than 200 registered rheumatologists. The registry data have now been integrated into the EULAR COVID-19 Database, which is itself part of a global effort to better understand and optimally manage RMD patients during the pandemic.
“The data presented by Dr. Regierer looked at similar outcomes and found quite similar results, which is reassuring,” Kimme Hyrich, MD, PhD, professor of epidemiology at the University of Manchester (England) and a consultant rheumatologist in the Kellgren Centre for Rheumatology at Manchester University Hospitals NHS Foundation Trust, said in an interview.
“We are very grateful for this collaboration [with the German society and others]. Our first publication has looked at hospitalization, but with more data we may have the opportunity to look at less-common outcomes [e.g. death, other COVID complications] or within individual diseases or treatments. So far I don’t think we will come to a different conclusion,” observed Dr. Hyrich, who is on the steering committee for the EULAR COVID-19 Database.
“These initial data are reassuring in that the majority of cases of COVID reported to our database have recovered, including those who were hospitalized,” she said.
Current EULAR advice is to continue treatment with glucocorticoids in patients who are being chronically treated, but to use them at the lowest possible dose.
The objectives of this first analysis of the German registry was to provide a description of the patients who did and did not require hospitalization and those who needed ventilation, as well as look at possible risk factors for hospitalization.
Dr. Regierer reported that, of 192 patients they included – all with a positive lab test for SARS-CoV-2 – 128 (67%) did not require hospital admission. Of those that did (n = 64), 43 (22%) did not need ventilation and 21 (11%) did. Fifteen patients died, all of whom had been hospitalized, and all but one of them had needed ventilation.
Concerning the characteristics of the patients, those who needed hospital treatment with and without ventilation were older than those who were not admitted (70 vs. 65 vs. 54 years, respectively).
“Looking at the sexes, the gender distribution is also interesting. We see 69% females in the nonhospitalized patients, 65% of the inpatients without ventilation, but only 43% females in the ventilated patients. So in this group, the male patients are the majority,” Dr. Regierer observed.
Just over half of all patients in the nonhospitalized and the hospitalized without ventilation groups had IRD in remission, but those in the hospitalized with ventilation group less than one-fifth had their IRD under control.
“Of course we have to keep in mind the small sample sizes,” Dr. Regierer said, but the distribution of patients by disease type was “what you’d expect in clinical care.” The majority of patients in each of the three groups had RA (47%, 56%, and 57%), followed by psoriatic arthritis (19%, 7%, and 14%), axial spondyloarthritis (11%, 5%, and 0%), systemic lupus erythematosus (6%, 2%, and 0%), and vasculitis (1%, 5%, and 5%).
Patients who were hospitalized with and without ventilation were more likely to have more than one comorbidity than those who were not hospitalized with COVID-19.
“The most frequent comorbidity was cardiovascular disease with 58% and 76% in the inpatient groups,” Dr. Regierer reported. One-third of the nonhospitalized patients had a cardiovascular comorbidity.
“If we look at pulmonary disease, we see that 38% of the ventilator patients had an underlying pulmonary disease,” she added. This was in comparison with 19% of the hospitalized without ventilation and 13% of the nonhospitalized patients. Diabetes was another common comorbidity in hospitalized patients with (16%) and without (19%) ventilation versus just 2% of nonhospitalized patients. While these and other comorbidities such as chronic renal insufficiency were associated with higher odds ratios in the multivariate risk factor analysis, they did not reach statistical significance.
With regard to RMD treatments, more than 60% of patients in the hospitalized group had received treatment with glucocorticoids versus 37% of those who did not get admitted. No differences were seen for the other treatments.
Interestingly, “female sex, remission, and use of NSAIDs have an odds ratio smaller than 1. So there might be a lower risk of hospitalization associated with these factors,” Dr. Regierer said.
Dr. Regierer has received grant support and is part of speaker’s bureaus for a variety of pharmaceutical companies. Dr. Hyrich disclosed grant income from Bristol-Myers Squibb, UCB, and Pfizer, and receiving speaker fees from AbbVie.
Being aged older than 65 years was associated with the highest risk of people with rheumatic and musculoskeletal diseases (RMDs) needing hospital treatment for COVID-19, according to the first results to be reported from ReCoVery, the German national COVID-19 registry.
Older patients with RMDs were five times more likely than younger patients to be hospitalized if they tested positive for SARS‑CoV‑2 and developed COVID-19 (odds ratio, 5.1; 95% confidence interval, 2.3-11.4).
The likelihood of hospitalization was also significantly increased by the current or prior use of glucocorticoids (OR, 2.59; 95% CI, 1.2-5.4) and by the presence of cardiovascular disease (OR, 2.27; 95% CI, 1.2-5.4).
“The register is a joint initiative of the German Society for Rheumatology and the Justus Liebig University in Giessen,” explained Anne Regierer, MD, during a live session of the annual European Congress of Rheumatology, held online this year due to COVID-19.
“The current pandemic has changed all of our lives. For patients it brought a lot of uncertainty and fears,” said Dr. Regierer, of the German Rheumatism Research Center Berlin.
“The risk of SARS-CoV-2 infection in patients with inflammatory rheumatic diseases [IRD] is still largely unknown. We still don’t know whether they have a high risk of getting the infection or whether they have a higher risk of a severer case ... therefore there’s an urgent need to have data to generate evidence for the management of our patients.”
Launched at the end of March 2020, the German registry now includes data on 251 patients – 194 of whom have recovered – provided by more than 200 registered rheumatologists. The registry data have now been integrated into the EULAR COVID-19 Database, which is itself part of a global effort to better understand and optimally manage RMD patients during the pandemic.
“The data presented by Dr. Regierer looked at similar outcomes and found quite similar results, which is reassuring,” Kimme Hyrich, MD, PhD, professor of epidemiology at the University of Manchester (England) and a consultant rheumatologist in the Kellgren Centre for Rheumatology at Manchester University Hospitals NHS Foundation Trust, said in an interview.
“We are very grateful for this collaboration [with the German society and others]. Our first publication has looked at hospitalization, but with more data we may have the opportunity to look at less-common outcomes [e.g. death, other COVID complications] or within individual diseases or treatments. So far I don’t think we will come to a different conclusion,” observed Dr. Hyrich, who is on the steering committee for the EULAR COVID-19 Database.
“These initial data are reassuring in that the majority of cases of COVID reported to our database have recovered, including those who were hospitalized,” she said.
Current EULAR advice is to continue treatment with glucocorticoids in patients who are being chronically treated, but to use them at the lowest possible dose.
The objectives of this first analysis of the German registry was to provide a description of the patients who did and did not require hospitalization and those who needed ventilation, as well as look at possible risk factors for hospitalization.
Dr. Regierer reported that, of 192 patients they included – all with a positive lab test for SARS-CoV-2 – 128 (67%) did not require hospital admission. Of those that did (n = 64), 43 (22%) did not need ventilation and 21 (11%) did. Fifteen patients died, all of whom had been hospitalized, and all but one of them had needed ventilation.
Concerning the characteristics of the patients, those who needed hospital treatment with and without ventilation were older than those who were not admitted (70 vs. 65 vs. 54 years, respectively).
“Looking at the sexes, the gender distribution is also interesting. We see 69% females in the nonhospitalized patients, 65% of the inpatients without ventilation, but only 43% females in the ventilated patients. So in this group, the male patients are the majority,” Dr. Regierer observed.
Just over half of all patients in the nonhospitalized and the hospitalized without ventilation groups had IRD in remission, but those in the hospitalized with ventilation group less than one-fifth had their IRD under control.
“Of course we have to keep in mind the small sample sizes,” Dr. Regierer said, but the distribution of patients by disease type was “what you’d expect in clinical care.” The majority of patients in each of the three groups had RA (47%, 56%, and 57%), followed by psoriatic arthritis (19%, 7%, and 14%), axial spondyloarthritis (11%, 5%, and 0%), systemic lupus erythematosus (6%, 2%, and 0%), and vasculitis (1%, 5%, and 5%).
Patients who were hospitalized with and without ventilation were more likely to have more than one comorbidity than those who were not hospitalized with COVID-19.
“The most frequent comorbidity was cardiovascular disease with 58% and 76% in the inpatient groups,” Dr. Regierer reported. One-third of the nonhospitalized patients had a cardiovascular comorbidity.
“If we look at pulmonary disease, we see that 38% of the ventilator patients had an underlying pulmonary disease,” she added. This was in comparison with 19% of the hospitalized without ventilation and 13% of the nonhospitalized patients. Diabetes was another common comorbidity in hospitalized patients with (16%) and without (19%) ventilation versus just 2% of nonhospitalized patients. While these and other comorbidities such as chronic renal insufficiency were associated with higher odds ratios in the multivariate risk factor analysis, they did not reach statistical significance.
With regard to RMD treatments, more than 60% of patients in the hospitalized group had received treatment with glucocorticoids versus 37% of those who did not get admitted. No differences were seen for the other treatments.
Interestingly, “female sex, remission, and use of NSAIDs have an odds ratio smaller than 1. So there might be a lower risk of hospitalization associated with these factors,” Dr. Regierer said.
Dr. Regierer has received grant support and is part of speaker’s bureaus for a variety of pharmaceutical companies. Dr. Hyrich disclosed grant income from Bristol-Myers Squibb, UCB, and Pfizer, and receiving speaker fees from AbbVie.
FROM THE EULAR 2020 E-CONGRESS