User login
Patients’ perceptions and high hospital use
Background: A small proportion of patients accounts for a large proportion of hospital use and readmissions. As hospitals and hospitalists focus efforts to improve transitions of care, there is a paucity of data that incorporates patients’ perspectives into the design of these programs.
Study design: Qualitative research study.
Setting: Northwestern Memorial Hospital, a single urban academic medical center in Chicago.
Synopsis: Eligible patients had two unplanned 30-day readmissions within the prior 12 months in addition to one or more of the following: at least one readmission in the last 6 months; a referral from a patient’s medical provider; or at least three observation visits.
A research coordinator conducted one-on-one semistructured interviews. Each interview was recorded, transcribed, and then coded using a team-based approach; 26 patients completed the interview process. From the analysis, four major themes emerged: Major medical problems were universal but high hospital use onset varied; participants noted that fluctuations in their course were often related to social, economic, and psychological stressors; onset and progression of episodes seemed uncontrollable and unpredictable; participants preferred to avoid hospitalization and sought care when attempts at self-management failed. The major limitation of this study was the small sample size located at one medical center, creating a data pool that is potentially not generalizable to other medical centers. These findings, however, are an important reminder to focus our interventions with patients’ needs and perceptions in mind.
Bottom line: Frequently hospitalized patients have insights into factors contributing to their high hospital use. Engaging patients in this discussion can enable us to create sustainable patient-centered programs that avoid rehospitalization.
Citation: O’Leary KJ et al. Frequently hospitalized patients’ perceptions of factors contributing to high hospital use. J Hosp Med. 2019 Mar 20;14:e1-6.
Dr. Richardson is a hospitalist at Duke University Health System.
Background: A small proportion of patients accounts for a large proportion of hospital use and readmissions. As hospitals and hospitalists focus efforts to improve transitions of care, there is a paucity of data that incorporates patients’ perspectives into the design of these programs.
Study design: Qualitative research study.
Setting: Northwestern Memorial Hospital, a single urban academic medical center in Chicago.
Synopsis: Eligible patients had two unplanned 30-day readmissions within the prior 12 months in addition to one or more of the following: at least one readmission in the last 6 months; a referral from a patient’s medical provider; or at least three observation visits.
A research coordinator conducted one-on-one semistructured interviews. Each interview was recorded, transcribed, and then coded using a team-based approach; 26 patients completed the interview process. From the analysis, four major themes emerged: Major medical problems were universal but high hospital use onset varied; participants noted that fluctuations in their course were often related to social, economic, and psychological stressors; onset and progression of episodes seemed uncontrollable and unpredictable; participants preferred to avoid hospitalization and sought care when attempts at self-management failed. The major limitation of this study was the small sample size located at one medical center, creating a data pool that is potentially not generalizable to other medical centers. These findings, however, are an important reminder to focus our interventions with patients’ needs and perceptions in mind.
Bottom line: Frequently hospitalized patients have insights into factors contributing to their high hospital use. Engaging patients in this discussion can enable us to create sustainable patient-centered programs that avoid rehospitalization.
Citation: O’Leary KJ et al. Frequently hospitalized patients’ perceptions of factors contributing to high hospital use. J Hosp Med. 2019 Mar 20;14:e1-6.
Dr. Richardson is a hospitalist at Duke University Health System.
Background: A small proportion of patients accounts for a large proportion of hospital use and readmissions. As hospitals and hospitalists focus efforts to improve transitions of care, there is a paucity of data that incorporates patients’ perspectives into the design of these programs.
Study design: Qualitative research study.
Setting: Northwestern Memorial Hospital, a single urban academic medical center in Chicago.
Synopsis: Eligible patients had two unplanned 30-day readmissions within the prior 12 months in addition to one or more of the following: at least one readmission in the last 6 months; a referral from a patient’s medical provider; or at least three observation visits.
A research coordinator conducted one-on-one semistructured interviews. Each interview was recorded, transcribed, and then coded using a team-based approach; 26 patients completed the interview process. From the analysis, four major themes emerged: Major medical problems were universal but high hospital use onset varied; participants noted that fluctuations in their course were often related to social, economic, and psychological stressors; onset and progression of episodes seemed uncontrollable and unpredictable; participants preferred to avoid hospitalization and sought care when attempts at self-management failed. The major limitation of this study was the small sample size located at one medical center, creating a data pool that is potentially not generalizable to other medical centers. These findings, however, are an important reminder to focus our interventions with patients’ needs and perceptions in mind.
Bottom line: Frequently hospitalized patients have insights into factors contributing to their high hospital use. Engaging patients in this discussion can enable us to create sustainable patient-centered programs that avoid rehospitalization.
Citation: O’Leary KJ et al. Frequently hospitalized patients’ perceptions of factors contributing to high hospital use. J Hosp Med. 2019 Mar 20;14:e1-6.
Dr. Richardson is a hospitalist at Duke University Health System.
More fatalities in heart transplant patients with COVID-19
COVID-19 infection is associated with a high risk for mortality in heart transplant (HT) recipients, a new case series suggests.
Investigators looked at data on 28 patients with a confirmed diagnosis of COVID-19 who received a HT between March 1, 2020, and April 24, 2020 and found a case-fatality rate of 25%.
“The high case fatality in our case series should alert physicians to the vulnerability of heart transplant recipients during the COVID-19 pandemic,” senior author Nir Uriel, MD, MSc, professor of medicine at Columbia University, New York, said in an interview.
“These patients require extra precautions to prevent the development of infection,” said Dr. Uriel, who is also a cardiologist at New York Presbyterian/Columbia University Irving Medical Center.
The study was published online May 13 in JAMA Cardiology.
Similar presentation
HT recipients can have several comorbidities after the procedure, including hypertension, diabetes, cardiac allograft vasculopathy, and ongoing immunosuppression, all of which can place them at risk for infection and adverse outcomes with COVID-19 infection, the authors wrote.
The researchers therefore embarked on a case series looking at 28 HT recipients with COVID-19 infection (median age, 64.0 years; interquartile range, 53.5-70.5; 79% male) to “describe the outcomes of recipients of HT who are chronically immunosuppressed and develop COVID-19 and raise important questions about the role of the immune system in the process.”
The median time from HT to study period was 8.6 (IQR, 4.2-14.5) years. Most patients had numerous comorbidities.
“The presentation of COVID-19 was similar to nontransplant patients with fever, dyspnea, cough, and GI symptoms,” Dr. Uriel reported.
No protective effect
Twenty-two patients (79%) required admission to the hospital, seven of whom (25%) required admission to the ICU and mechanical ventilation.
Despite the presence of immunosuppressive therapy, all patients had significant elevation of inflammatory biomarkers (median peak high-sensitivity C-reactive protein [hs-CRP], 11.83 mg/dL; IQR, 7.44-19.26; median peak interleukin [IL]-6, 105 pg/mL; IQR, 38-296).
Three-quarters had myocardial injury, with a median high-sensitivity troponin T of 0.055 (0.0205 - 0.1345) ng/mL.
Treatments of COVID-19 included hydroxychloroquine (18 patients; 78%), high-dose corticosteroids (eight patients; 47%), and IL-6 receptor antagonists (six patients; 26%).
Moreover, during hospitalization, mycophenolate mofetil was discontinued in most (70%) patients, and one-quarter had a reduction in their calcineurin inhibitor dose.
“Heart transplant recipients generally require more intense immunosuppressive therapy than most other solid organ transplant recipients, and this high baseline immunosuppression increases their propensity to develop infections and their likelihood of experiencing severe manifestations of infections,” Dr. Uriel commented.
“With COVID-19, in which the body’s inflammatory reaction appears to play a role in disease severity, there has been a question of whether immunosuppression may offer a protective effect,” he continued.
“This case series suggests that this is not the case, although this would need to be confirmed in larger studies,” he said.
Low threshold
Among the 22 patients who were admitted to the hospital, half were discharged home and four (18%) were still hospitalized at the end of the study.
Of the seven patients who died, two died at the study center, and five died in an outside institution.
“In the HT population, social distancing (or isolation), strict use of masks when in public, proper handwashing, and sanitization of surfaces are of paramount importance in the prevention of COVID-19 infection,” Dr. Uriel stated.
“In addition, we have restricted these patients’ contact with the hospital as much as possible during the pandemic,” he said.
However, “there should be a low threshold to hospitalize heart transplant patients who develop infection with COVID-19. Furthermore, in our series, outcomes were better for patients hospitalized at the transplant center; therefore, strong consideration should be given to transferring HT patients when hospitalized at another hospital,” he added.
The authors emphasized that COVID-19 patients “will require ongoing monitoring in the recovery phase, as an immunosuppression regimen is reintroduced and the consequences to the allograft itself become apparent.”
Vulnerable population
Commenting on the study, Mandeep R. Mehra, MD, MSc, William Harvey Distinguished Chair in Advanced Cardiovascular Medicine at Brigham and Women’s Hospital, Boston, suggested that “in epidemiological terms, [the findings] might not look as bad as the way they are reflected in the paper.”
Given that Columbia is “one of the larger heart transplant centers in the U.S., following probably 1,000 patients, having only 22 out of perhaps thousands whom they transplanted or are actively following would actually represent a low serious infection rate,” said Dr. Mehra, who is also the executive director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, also in Boston.
“We must not forget to emphasize that, when assessing these case fatality rates, we must look at the entire population at risk, not only the handful that we were able to observe,” explained Dr. Mehra, who was not involved with the study.
Moreover, the patients were “older and had comorbidities, with poor underlying kidney function and other complications, and underlying coronary artery disease in the transplanted heart,” so “it would not surprise me that they had such a high fatality rate, since they had a high degree of vulnerability,” he said.
Dr. Mehra, who is also the editor-in-chief of the Journal of Heart and Lung Transplantation, said that the journal has received manuscripts still in the review process that suggest different fatality rates than those found in the current case series.
However, he acknowledged that, because these are patients with serious vulnerability due to underlying heart disease, “you can’t be lackadaisical and need to do everything to decrease this vulnerability.”
The authors noted that, although their study did not show a protective effect from immunosuppression against COVID-19, further studies are needed to assess each individual immunosuppressive agent and provide a definitive answer.
The study was supported by a grant to one of the investigators from the National Heart, Lung, and Blood Institute. Dr. Uriel reports no relevant financial relationships. The other authors’ disclosures are listed in the publication. Dr. Mehra reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
COVID-19 infection is associated with a high risk for mortality in heart transplant (HT) recipients, a new case series suggests.
Investigators looked at data on 28 patients with a confirmed diagnosis of COVID-19 who received a HT between March 1, 2020, and April 24, 2020 and found a case-fatality rate of 25%.
“The high case fatality in our case series should alert physicians to the vulnerability of heart transplant recipients during the COVID-19 pandemic,” senior author Nir Uriel, MD, MSc, professor of medicine at Columbia University, New York, said in an interview.
“These patients require extra precautions to prevent the development of infection,” said Dr. Uriel, who is also a cardiologist at New York Presbyterian/Columbia University Irving Medical Center.
The study was published online May 13 in JAMA Cardiology.
Similar presentation
HT recipients can have several comorbidities after the procedure, including hypertension, diabetes, cardiac allograft vasculopathy, and ongoing immunosuppression, all of which can place them at risk for infection and adverse outcomes with COVID-19 infection, the authors wrote.
The researchers therefore embarked on a case series looking at 28 HT recipients with COVID-19 infection (median age, 64.0 years; interquartile range, 53.5-70.5; 79% male) to “describe the outcomes of recipients of HT who are chronically immunosuppressed and develop COVID-19 and raise important questions about the role of the immune system in the process.”
The median time from HT to study period was 8.6 (IQR, 4.2-14.5) years. Most patients had numerous comorbidities.
“The presentation of COVID-19 was similar to nontransplant patients with fever, dyspnea, cough, and GI symptoms,” Dr. Uriel reported.
No protective effect
Twenty-two patients (79%) required admission to the hospital, seven of whom (25%) required admission to the ICU and mechanical ventilation.
Despite the presence of immunosuppressive therapy, all patients had significant elevation of inflammatory biomarkers (median peak high-sensitivity C-reactive protein [hs-CRP], 11.83 mg/dL; IQR, 7.44-19.26; median peak interleukin [IL]-6, 105 pg/mL; IQR, 38-296).
Three-quarters had myocardial injury, with a median high-sensitivity troponin T of 0.055 (0.0205 - 0.1345) ng/mL.
Treatments of COVID-19 included hydroxychloroquine (18 patients; 78%), high-dose corticosteroids (eight patients; 47%), and IL-6 receptor antagonists (six patients; 26%).
Moreover, during hospitalization, mycophenolate mofetil was discontinued in most (70%) patients, and one-quarter had a reduction in their calcineurin inhibitor dose.
“Heart transplant recipients generally require more intense immunosuppressive therapy than most other solid organ transplant recipients, and this high baseline immunosuppression increases their propensity to develop infections and their likelihood of experiencing severe manifestations of infections,” Dr. Uriel commented.
“With COVID-19, in which the body’s inflammatory reaction appears to play a role in disease severity, there has been a question of whether immunosuppression may offer a protective effect,” he continued.
“This case series suggests that this is not the case, although this would need to be confirmed in larger studies,” he said.
Low threshold
Among the 22 patients who were admitted to the hospital, half were discharged home and four (18%) were still hospitalized at the end of the study.
Of the seven patients who died, two died at the study center, and five died in an outside institution.
“In the HT population, social distancing (or isolation), strict use of masks when in public, proper handwashing, and sanitization of surfaces are of paramount importance in the prevention of COVID-19 infection,” Dr. Uriel stated.
“In addition, we have restricted these patients’ contact with the hospital as much as possible during the pandemic,” he said.
However, “there should be a low threshold to hospitalize heart transplant patients who develop infection with COVID-19. Furthermore, in our series, outcomes were better for patients hospitalized at the transplant center; therefore, strong consideration should be given to transferring HT patients when hospitalized at another hospital,” he added.
The authors emphasized that COVID-19 patients “will require ongoing monitoring in the recovery phase, as an immunosuppression regimen is reintroduced and the consequences to the allograft itself become apparent.”
Vulnerable population
Commenting on the study, Mandeep R. Mehra, MD, MSc, William Harvey Distinguished Chair in Advanced Cardiovascular Medicine at Brigham and Women’s Hospital, Boston, suggested that “in epidemiological terms, [the findings] might not look as bad as the way they are reflected in the paper.”
Given that Columbia is “one of the larger heart transplant centers in the U.S., following probably 1,000 patients, having only 22 out of perhaps thousands whom they transplanted or are actively following would actually represent a low serious infection rate,” said Dr. Mehra, who is also the executive director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, also in Boston.
“We must not forget to emphasize that, when assessing these case fatality rates, we must look at the entire population at risk, not only the handful that we were able to observe,” explained Dr. Mehra, who was not involved with the study.
Moreover, the patients were “older and had comorbidities, with poor underlying kidney function and other complications, and underlying coronary artery disease in the transplanted heart,” so “it would not surprise me that they had such a high fatality rate, since they had a high degree of vulnerability,” he said.
Dr. Mehra, who is also the editor-in-chief of the Journal of Heart and Lung Transplantation, said that the journal has received manuscripts still in the review process that suggest different fatality rates than those found in the current case series.
However, he acknowledged that, because these are patients with serious vulnerability due to underlying heart disease, “you can’t be lackadaisical and need to do everything to decrease this vulnerability.”
The authors noted that, although their study did not show a protective effect from immunosuppression against COVID-19, further studies are needed to assess each individual immunosuppressive agent and provide a definitive answer.
The study was supported by a grant to one of the investigators from the National Heart, Lung, and Blood Institute. Dr. Uriel reports no relevant financial relationships. The other authors’ disclosures are listed in the publication. Dr. Mehra reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
COVID-19 infection is associated with a high risk for mortality in heart transplant (HT) recipients, a new case series suggests.
Investigators looked at data on 28 patients with a confirmed diagnosis of COVID-19 who received a HT between March 1, 2020, and April 24, 2020 and found a case-fatality rate of 25%.
“The high case fatality in our case series should alert physicians to the vulnerability of heart transplant recipients during the COVID-19 pandemic,” senior author Nir Uriel, MD, MSc, professor of medicine at Columbia University, New York, said in an interview.
“These patients require extra precautions to prevent the development of infection,” said Dr. Uriel, who is also a cardiologist at New York Presbyterian/Columbia University Irving Medical Center.
The study was published online May 13 in JAMA Cardiology.
Similar presentation
HT recipients can have several comorbidities after the procedure, including hypertension, diabetes, cardiac allograft vasculopathy, and ongoing immunosuppression, all of which can place them at risk for infection and adverse outcomes with COVID-19 infection, the authors wrote.
The researchers therefore embarked on a case series looking at 28 HT recipients with COVID-19 infection (median age, 64.0 years; interquartile range, 53.5-70.5; 79% male) to “describe the outcomes of recipients of HT who are chronically immunosuppressed and develop COVID-19 and raise important questions about the role of the immune system in the process.”
The median time from HT to study period was 8.6 (IQR, 4.2-14.5) years. Most patients had numerous comorbidities.
“The presentation of COVID-19 was similar to nontransplant patients with fever, dyspnea, cough, and GI symptoms,” Dr. Uriel reported.
No protective effect
Twenty-two patients (79%) required admission to the hospital, seven of whom (25%) required admission to the ICU and mechanical ventilation.
Despite the presence of immunosuppressive therapy, all patients had significant elevation of inflammatory biomarkers (median peak high-sensitivity C-reactive protein [hs-CRP], 11.83 mg/dL; IQR, 7.44-19.26; median peak interleukin [IL]-6, 105 pg/mL; IQR, 38-296).
Three-quarters had myocardial injury, with a median high-sensitivity troponin T of 0.055 (0.0205 - 0.1345) ng/mL.
Treatments of COVID-19 included hydroxychloroquine (18 patients; 78%), high-dose corticosteroids (eight patients; 47%), and IL-6 receptor antagonists (six patients; 26%).
Moreover, during hospitalization, mycophenolate mofetil was discontinued in most (70%) patients, and one-quarter had a reduction in their calcineurin inhibitor dose.
“Heart transplant recipients generally require more intense immunosuppressive therapy than most other solid organ transplant recipients, and this high baseline immunosuppression increases their propensity to develop infections and their likelihood of experiencing severe manifestations of infections,” Dr. Uriel commented.
“With COVID-19, in which the body’s inflammatory reaction appears to play a role in disease severity, there has been a question of whether immunosuppression may offer a protective effect,” he continued.
“This case series suggests that this is not the case, although this would need to be confirmed in larger studies,” he said.
Low threshold
Among the 22 patients who were admitted to the hospital, half were discharged home and four (18%) were still hospitalized at the end of the study.
Of the seven patients who died, two died at the study center, and five died in an outside institution.
“In the HT population, social distancing (or isolation), strict use of masks when in public, proper handwashing, and sanitization of surfaces are of paramount importance in the prevention of COVID-19 infection,” Dr. Uriel stated.
“In addition, we have restricted these patients’ contact with the hospital as much as possible during the pandemic,” he said.
However, “there should be a low threshold to hospitalize heart transplant patients who develop infection with COVID-19. Furthermore, in our series, outcomes were better for patients hospitalized at the transplant center; therefore, strong consideration should be given to transferring HT patients when hospitalized at another hospital,” he added.
The authors emphasized that COVID-19 patients “will require ongoing monitoring in the recovery phase, as an immunosuppression regimen is reintroduced and the consequences to the allograft itself become apparent.”
Vulnerable population
Commenting on the study, Mandeep R. Mehra, MD, MSc, William Harvey Distinguished Chair in Advanced Cardiovascular Medicine at Brigham and Women’s Hospital, Boston, suggested that “in epidemiological terms, [the findings] might not look as bad as the way they are reflected in the paper.”
Given that Columbia is “one of the larger heart transplant centers in the U.S., following probably 1,000 patients, having only 22 out of perhaps thousands whom they transplanted or are actively following would actually represent a low serious infection rate,” said Dr. Mehra, who is also the executive director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, also in Boston.
“We must not forget to emphasize that, when assessing these case fatality rates, we must look at the entire population at risk, not only the handful that we were able to observe,” explained Dr. Mehra, who was not involved with the study.
Moreover, the patients were “older and had comorbidities, with poor underlying kidney function and other complications, and underlying coronary artery disease in the transplanted heart,” so “it would not surprise me that they had such a high fatality rate, since they had a high degree of vulnerability,” he said.
Dr. Mehra, who is also the editor-in-chief of the Journal of Heart and Lung Transplantation, said that the journal has received manuscripts still in the review process that suggest different fatality rates than those found in the current case series.
However, he acknowledged that, because these are patients with serious vulnerability due to underlying heart disease, “you can’t be lackadaisical and need to do everything to decrease this vulnerability.”
The authors noted that, although their study did not show a protective effect from immunosuppression against COVID-19, further studies are needed to assess each individual immunosuppressive agent and provide a definitive answer.
The study was supported by a grant to one of the investigators from the National Heart, Lung, and Blood Institute. Dr. Uriel reports no relevant financial relationships. The other authors’ disclosures are listed in the publication. Dr. Mehra reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
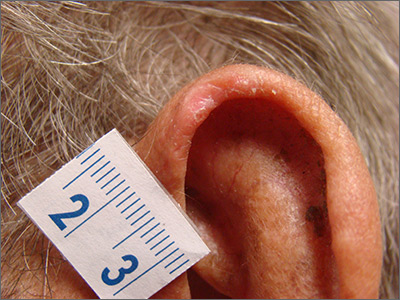
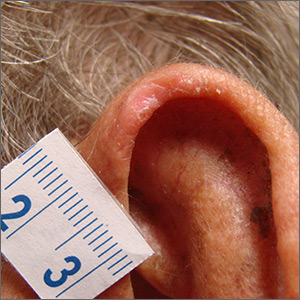
Painful ear lesion
The patient was given a diagnosis of chondrodermatitis nodularis helicis (CNH), an inflammation of the cartilage and overlying skin causing a painful nodule of the helix. These lesions typically have a more prominent nodular component and a central ulceration or firm scale. They are thought to be due to chronic pressure on the ear.
There is a slight male predominance of CNH and onset is usually gradual. Patients often experience pain when sleeping on the affected side. The tenderness usually can be reproduced clinically by pressing on the lesion. The ears also are a high-risk area for actinic keratoses (AK) and nonmelanoma skin cancer (NMSC); if there is doubt about the diagnosis, a biopsy may be warranted to rule out AK or NMSC. In this patient, a shave biopsy was performed.
Various treatment regimens are available for CNH. The least invasive treatment approach is to use a “cut out” foam or a special donut-shaped pillow to protect the area from further pressure. By protecting from pressure and irritation, the lesion resolves in 57% to 92% of cases in clinical studies. Intralesional injection with 0.2 mL of 10 mg/mL triamcinolone acetonide is a simple in-office procedure that frequently helps the pain and may be curative; although, repeat injections may be necessary. Excision of the overlying skin and the affected cartilage is a more aggressive treatment with high success rates. More recently, treatment with topical nitroglycerin patches or photodynamic therapy have been described in small trials.
After confirming by a shave biopsy that the lesion was not cancerous, the patient returned for an elliptical excision of the lesion. A dermal curette was used to remove the rough abnormal inflammation of the underlying cartilage, and the elliptical wound was sutured with a linear closure.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Juul Nielsen L, Holkmann Olsen C, Lock-Andersen J. Therapeutic options of chondrodermatitis nodularis helicis [published online January 27, 2016]. Plast Surg Int. 2016;2016:4340168.
The patient was given a diagnosis of chondrodermatitis nodularis helicis (CNH), an inflammation of the cartilage and overlying skin causing a painful nodule of the helix. These lesions typically have a more prominent nodular component and a central ulceration or firm scale. They are thought to be due to chronic pressure on the ear.
There is a slight male predominance of CNH and onset is usually gradual. Patients often experience pain when sleeping on the affected side. The tenderness usually can be reproduced clinically by pressing on the lesion. The ears also are a high-risk area for actinic keratoses (AK) and nonmelanoma skin cancer (NMSC); if there is doubt about the diagnosis, a biopsy may be warranted to rule out AK or NMSC. In this patient, a shave biopsy was performed.
Various treatment regimens are available for CNH. The least invasive treatment approach is to use a “cut out” foam or a special donut-shaped pillow to protect the area from further pressure. By protecting from pressure and irritation, the lesion resolves in 57% to 92% of cases in clinical studies. Intralesional injection with 0.2 mL of 10 mg/mL triamcinolone acetonide is a simple in-office procedure that frequently helps the pain and may be curative; although, repeat injections may be necessary. Excision of the overlying skin and the affected cartilage is a more aggressive treatment with high success rates. More recently, treatment with topical nitroglycerin patches or photodynamic therapy have been described in small trials.
After confirming by a shave biopsy that the lesion was not cancerous, the patient returned for an elliptical excision of the lesion. A dermal curette was used to remove the rough abnormal inflammation of the underlying cartilage, and the elliptical wound was sutured with a linear closure.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
The patient was given a diagnosis of chondrodermatitis nodularis helicis (CNH), an inflammation of the cartilage and overlying skin causing a painful nodule of the helix. These lesions typically have a more prominent nodular component and a central ulceration or firm scale. They are thought to be due to chronic pressure on the ear.
There is a slight male predominance of CNH and onset is usually gradual. Patients often experience pain when sleeping on the affected side. The tenderness usually can be reproduced clinically by pressing on the lesion. The ears also are a high-risk area for actinic keratoses (AK) and nonmelanoma skin cancer (NMSC); if there is doubt about the diagnosis, a biopsy may be warranted to rule out AK or NMSC. In this patient, a shave biopsy was performed.
Various treatment regimens are available for CNH. The least invasive treatment approach is to use a “cut out” foam or a special donut-shaped pillow to protect the area from further pressure. By protecting from pressure and irritation, the lesion resolves in 57% to 92% of cases in clinical studies. Intralesional injection with 0.2 mL of 10 mg/mL triamcinolone acetonide is a simple in-office procedure that frequently helps the pain and may be curative; although, repeat injections may be necessary. Excision of the overlying skin and the affected cartilage is a more aggressive treatment with high success rates. More recently, treatment with topical nitroglycerin patches or photodynamic therapy have been described in small trials.
After confirming by a shave biopsy that the lesion was not cancerous, the patient returned for an elliptical excision of the lesion. A dermal curette was used to remove the rough abnormal inflammation of the underlying cartilage, and the elliptical wound was sutured with a linear closure.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Juul Nielsen L, Holkmann Olsen C, Lock-Andersen J. Therapeutic options of chondrodermatitis nodularis helicis [published online January 27, 2016]. Plast Surg Int. 2016;2016:4340168.
Juul Nielsen L, Holkmann Olsen C, Lock-Andersen J. Therapeutic options of chondrodermatitis nodularis helicis [published online January 27, 2016]. Plast Surg Int. 2016;2016:4340168.
‘Promising’ durvalumab results spark phase 3 trial in mesothelioma
Adding durvalumab to first-line pemetrexed and cisplatin improved survival in patients with unresectable malignant pleural mesothelioma (MPM) in a phase 2 trial, compared with historical controls who received only pemetrexed and cisplatin.
The median overall survival was 20.4 months in patients who received durvalumab plus pemetrexed-cisplatin. This is significantly longer than the median overall survival of 12.1 months (P = .0014) observed with pemetrexed-cisplatin in a prior phase 3 study (J Clin Oncol. 2003 Jul 15;21[14]:2636-44).
The new phase 2 results are “promising,” said lead investigator Patrick Forde, MBBCh, director of the thoracic cancer clinical research program at Johns Hopkins University in Baltimore.
He presented the results as part of the American Society of Clinical Oncology virtual scientific program.
Dr. Forde noted that a phase 3 trial directly comparing pemetrexed-cisplatin plus durvalumab to pemetrexed-cisplatin will begin recruiting this year. The trial is a collaboration between U.S. investigators and Australian researchers who reported their own phase 2 results with durvalumab plus pemetrexed-cisplatin in 2018 (J Thorac Oncol. 2018 Oct;13[10]:S338-339).
Study details
Dr. Forde’s phase 2 study enrolled 55 patients with treatment-naive, unresectable MPM. Their median age was 68 years (range, 35-83 years), and 45 (82%) were men. All had an Eastern Cooperative Oncology Group performance status of 0-1.
Epithelioid mesothelioma was the histologic subtype in three-quarters of patients. “It was a fairly typical mesothelioma population,” Dr. Forde said.
The patients received durvalumab at 1,120 mg plus pemetrexed at 500 mg/m2 and cisplatin at 75 mg/m2 every 3 weeks for up to six cycles. Carboplatin was substituted when cisplatin was contraindicated or patients developed toxicities.
All but one patient had stable or responding disease on radiography and went on to durvalumab maintenance, also given at 1,120 mg every 3 weeks, for up to 1 year from study entry.
Results
Dr. Forde said this study had 90% power to detect a 58% improvement in median overall survival, from the 12.1 months seen in historical controls to 19 months, which was the goal of this study.
It was a positive study, he said, as the median overall survival was 20.4 months (P = .0014).
The overall survival rate was 87.2% at 6 months, 70.4% at 12 months, and 44.2% at 24 months. The progression-free survival rate was 69.1% at 6 months, 16.4% at 12 months, and 10.9% at 24 months.
The overall response rate was 56.4%, which comprised 31 partial responses. Forty percent of patients (n = 22) had stable disease. One patient had progressive disease, and one was not evaluable (1.8% each).
To help with future patient selection, the researchers looked for baseline biomarkers that predicted response. Tumor PD-L1 expression, tumor mutation burden, and other potential candidates haven’t worked out so far, but the work continues, Dr. Forde said.
He noted that many of the adverse events in this trial are those typically seen with platinum-based chemotherapy.
Grade 3/4 treatment-emergent adverse events included anemia (n = 14), fatigue (n = 4), decreased appetite (n = 1), and hypomagnesemia (n = 1).
The most common grade 1/2 adverse events of special interest were hypothyroidism (n = 7), rash (n = 5), pruritus (n = 3), AST elevation (n = 3), and hyperthyroidism (n = 3).
Putting the results in context
Given the role of inflammation in MPM, durvalumab is among several immunotherapies under investigation for the disease.
A phase 3 French trial showed MPM patients had a median overall survival of 18.8 months with pemetrexed-cisplatin plus bevacizumab versus 16.1 months with pemetrexed-cisplatin only (Lancet. 2016 Apr 2;387[10026]:1405-1414).
The higher overall survival in the French study’s pemetrexed-cisplatin arm, compared with the 2003 trial results, is likely due to the use of modern second-line options, said Marjorie Zauderer, MD, codirector of the mesothelioma program at Memorial Sloan Kettering Cancer Center in New York, who was the discussant for Dr. Forde’s presentation.
“I think the improvement in overall survival presented by Dr. Forde is potentially clinically meaningful,” she said, but it was “well within the 95% confidence interval” of the bevacizumab trial. Even so, “I look forward” to the phase 3 results, she said.
Dr. Zauderer also pointed out an April press release from Bristol Myers Squibb that reported improved survival over pemetrexed-cisplatin with two of the company’s immunotherapies, nivolumab and ipilimumab, not as additions but as replacement first-line therapy. However, the randomized trial data haven’t been released yet. “We are all eager to evaluate this option further,” she said.
AstraZeneca, maker of durvalumab, funded the current study. Dr. Forde is an adviser for the company and reported research funding. Dr. Zauderer reported a relationship with Roche, which markets bevacizumab through its subsidiary, Genentech. She also disclosed research funding from Bristol Myers Squibb.
SOURCE: Forde PM et al. ASCO 2020, Abstract 9003.
Adding durvalumab to first-line pemetrexed and cisplatin improved survival in patients with unresectable malignant pleural mesothelioma (MPM) in a phase 2 trial, compared with historical controls who received only pemetrexed and cisplatin.
The median overall survival was 20.4 months in patients who received durvalumab plus pemetrexed-cisplatin. This is significantly longer than the median overall survival of 12.1 months (P = .0014) observed with pemetrexed-cisplatin in a prior phase 3 study (J Clin Oncol. 2003 Jul 15;21[14]:2636-44).
The new phase 2 results are “promising,” said lead investigator Patrick Forde, MBBCh, director of the thoracic cancer clinical research program at Johns Hopkins University in Baltimore.
He presented the results as part of the American Society of Clinical Oncology virtual scientific program.
Dr. Forde noted that a phase 3 trial directly comparing pemetrexed-cisplatin plus durvalumab to pemetrexed-cisplatin will begin recruiting this year. The trial is a collaboration between U.S. investigators and Australian researchers who reported their own phase 2 results with durvalumab plus pemetrexed-cisplatin in 2018 (J Thorac Oncol. 2018 Oct;13[10]:S338-339).
Study details
Dr. Forde’s phase 2 study enrolled 55 patients with treatment-naive, unresectable MPM. Their median age was 68 years (range, 35-83 years), and 45 (82%) were men. All had an Eastern Cooperative Oncology Group performance status of 0-1.
Epithelioid mesothelioma was the histologic subtype in three-quarters of patients. “It was a fairly typical mesothelioma population,” Dr. Forde said.
The patients received durvalumab at 1,120 mg plus pemetrexed at 500 mg/m2 and cisplatin at 75 mg/m2 every 3 weeks for up to six cycles. Carboplatin was substituted when cisplatin was contraindicated or patients developed toxicities.
All but one patient had stable or responding disease on radiography and went on to durvalumab maintenance, also given at 1,120 mg every 3 weeks, for up to 1 year from study entry.
Results
Dr. Forde said this study had 90% power to detect a 58% improvement in median overall survival, from the 12.1 months seen in historical controls to 19 months, which was the goal of this study.
It was a positive study, he said, as the median overall survival was 20.4 months (P = .0014).
The overall survival rate was 87.2% at 6 months, 70.4% at 12 months, and 44.2% at 24 months. The progression-free survival rate was 69.1% at 6 months, 16.4% at 12 months, and 10.9% at 24 months.
The overall response rate was 56.4%, which comprised 31 partial responses. Forty percent of patients (n = 22) had stable disease. One patient had progressive disease, and one was not evaluable (1.8% each).
To help with future patient selection, the researchers looked for baseline biomarkers that predicted response. Tumor PD-L1 expression, tumor mutation burden, and other potential candidates haven’t worked out so far, but the work continues, Dr. Forde said.
He noted that many of the adverse events in this trial are those typically seen with platinum-based chemotherapy.
Grade 3/4 treatment-emergent adverse events included anemia (n = 14), fatigue (n = 4), decreased appetite (n = 1), and hypomagnesemia (n = 1).
The most common grade 1/2 adverse events of special interest were hypothyroidism (n = 7), rash (n = 5), pruritus (n = 3), AST elevation (n = 3), and hyperthyroidism (n = 3).
Putting the results in context
Given the role of inflammation in MPM, durvalumab is among several immunotherapies under investigation for the disease.
A phase 3 French trial showed MPM patients had a median overall survival of 18.8 months with pemetrexed-cisplatin plus bevacizumab versus 16.1 months with pemetrexed-cisplatin only (Lancet. 2016 Apr 2;387[10026]:1405-1414).
The higher overall survival in the French study’s pemetrexed-cisplatin arm, compared with the 2003 trial results, is likely due to the use of modern second-line options, said Marjorie Zauderer, MD, codirector of the mesothelioma program at Memorial Sloan Kettering Cancer Center in New York, who was the discussant for Dr. Forde’s presentation.
“I think the improvement in overall survival presented by Dr. Forde is potentially clinically meaningful,” she said, but it was “well within the 95% confidence interval” of the bevacizumab trial. Even so, “I look forward” to the phase 3 results, she said.
Dr. Zauderer also pointed out an April press release from Bristol Myers Squibb that reported improved survival over pemetrexed-cisplatin with two of the company’s immunotherapies, nivolumab and ipilimumab, not as additions but as replacement first-line therapy. However, the randomized trial data haven’t been released yet. “We are all eager to evaluate this option further,” she said.
AstraZeneca, maker of durvalumab, funded the current study. Dr. Forde is an adviser for the company and reported research funding. Dr. Zauderer reported a relationship with Roche, which markets bevacizumab through its subsidiary, Genentech. She also disclosed research funding from Bristol Myers Squibb.
SOURCE: Forde PM et al. ASCO 2020, Abstract 9003.
Adding durvalumab to first-line pemetrexed and cisplatin improved survival in patients with unresectable malignant pleural mesothelioma (MPM) in a phase 2 trial, compared with historical controls who received only pemetrexed and cisplatin.
The median overall survival was 20.4 months in patients who received durvalumab plus pemetrexed-cisplatin. This is significantly longer than the median overall survival of 12.1 months (P = .0014) observed with pemetrexed-cisplatin in a prior phase 3 study (J Clin Oncol. 2003 Jul 15;21[14]:2636-44).
The new phase 2 results are “promising,” said lead investigator Patrick Forde, MBBCh, director of the thoracic cancer clinical research program at Johns Hopkins University in Baltimore.
He presented the results as part of the American Society of Clinical Oncology virtual scientific program.
Dr. Forde noted that a phase 3 trial directly comparing pemetrexed-cisplatin plus durvalumab to pemetrexed-cisplatin will begin recruiting this year. The trial is a collaboration between U.S. investigators and Australian researchers who reported their own phase 2 results with durvalumab plus pemetrexed-cisplatin in 2018 (J Thorac Oncol. 2018 Oct;13[10]:S338-339).
Study details
Dr. Forde’s phase 2 study enrolled 55 patients with treatment-naive, unresectable MPM. Their median age was 68 years (range, 35-83 years), and 45 (82%) were men. All had an Eastern Cooperative Oncology Group performance status of 0-1.
Epithelioid mesothelioma was the histologic subtype in three-quarters of patients. “It was a fairly typical mesothelioma population,” Dr. Forde said.
The patients received durvalumab at 1,120 mg plus pemetrexed at 500 mg/m2 and cisplatin at 75 mg/m2 every 3 weeks for up to six cycles. Carboplatin was substituted when cisplatin was contraindicated or patients developed toxicities.
All but one patient had stable or responding disease on radiography and went on to durvalumab maintenance, also given at 1,120 mg every 3 weeks, for up to 1 year from study entry.
Results
Dr. Forde said this study had 90% power to detect a 58% improvement in median overall survival, from the 12.1 months seen in historical controls to 19 months, which was the goal of this study.
It was a positive study, he said, as the median overall survival was 20.4 months (P = .0014).
The overall survival rate was 87.2% at 6 months, 70.4% at 12 months, and 44.2% at 24 months. The progression-free survival rate was 69.1% at 6 months, 16.4% at 12 months, and 10.9% at 24 months.
The overall response rate was 56.4%, which comprised 31 partial responses. Forty percent of patients (n = 22) had stable disease. One patient had progressive disease, and one was not evaluable (1.8% each).
To help with future patient selection, the researchers looked for baseline biomarkers that predicted response. Tumor PD-L1 expression, tumor mutation burden, and other potential candidates haven’t worked out so far, but the work continues, Dr. Forde said.
He noted that many of the adverse events in this trial are those typically seen with platinum-based chemotherapy.
Grade 3/4 treatment-emergent adverse events included anemia (n = 14), fatigue (n = 4), decreased appetite (n = 1), and hypomagnesemia (n = 1).
The most common grade 1/2 adverse events of special interest were hypothyroidism (n = 7), rash (n = 5), pruritus (n = 3), AST elevation (n = 3), and hyperthyroidism (n = 3).
Putting the results in context
Given the role of inflammation in MPM, durvalumab is among several immunotherapies under investigation for the disease.
A phase 3 French trial showed MPM patients had a median overall survival of 18.8 months with pemetrexed-cisplatin plus bevacizumab versus 16.1 months with pemetrexed-cisplatin only (Lancet. 2016 Apr 2;387[10026]:1405-1414).
The higher overall survival in the French study’s pemetrexed-cisplatin arm, compared with the 2003 trial results, is likely due to the use of modern second-line options, said Marjorie Zauderer, MD, codirector of the mesothelioma program at Memorial Sloan Kettering Cancer Center in New York, who was the discussant for Dr. Forde’s presentation.
“I think the improvement in overall survival presented by Dr. Forde is potentially clinically meaningful,” she said, but it was “well within the 95% confidence interval” of the bevacizumab trial. Even so, “I look forward” to the phase 3 results, she said.
Dr. Zauderer also pointed out an April press release from Bristol Myers Squibb that reported improved survival over pemetrexed-cisplatin with two of the company’s immunotherapies, nivolumab and ipilimumab, not as additions but as replacement first-line therapy. However, the randomized trial data haven’t been released yet. “We are all eager to evaluate this option further,” she said.
AstraZeneca, maker of durvalumab, funded the current study. Dr. Forde is an adviser for the company and reported research funding. Dr. Zauderer reported a relationship with Roche, which markets bevacizumab through its subsidiary, Genentech. She also disclosed research funding from Bristol Myers Squibb.
SOURCE: Forde PM et al. ASCO 2020, Abstract 9003.
FROM ASCO 2020
Celecoxib ‘should not be used’ as adjuvant therapy for stage III colon cancer
results of the phase 3 CALGB/SWOG 80702 trial showed.
The trial included 2,526 patients randomized to either 6 or 12 cycles of adjuvant FOLFOX with either celecoxib or placebo. The 3-year disease-free survival (DFS) rate was 76.3% for patients on celecoxib and 73.4% for those on placebo. The 5-year overall survival (OS) was 84% and 81.8%, respectively.
“The addition of celecoxib to FOLFOX adjuvant therapy in stage III colon cancer did not significantly improve disease-free or overall survival,” Jeffrey A. Meyerhardt, MD, of the Dana-Farber Cancer Institute in Boston, said while presenting the results as part of the American Society of Clinical Oncology virtual scientific program.
“We did not detect a significant interaction between celecoxib and duration of FOLFOX therapy for disease-free survival. Similarly, we did not detect a significant interaction between celecoxib and duration of FOLFOX therapy for overall survival,” Dr. Meyerhardt said.
“Simply put, celecoxib should not be used for the secondary prevention of colon cancer,” said invited discussant Christopher Lieu, MD, of the University of Colorado Cancer Center, Aurora.
Trial details
The 80702 trial was designed to test whether the COX-2 inhibitor celecoxib could help further reduce the risk of recurrence when added to adjuvant chemotherapy with 3 or 6 months of FOLFOX. Data on the FOLFOX portion of the trial were previously reported as part of the International Duration Evaluation of Adjuvant Therapy collaboration (N Engl J Med. 2018;378:1177-88).
The trial enrolled 2,526 patients with resected stage III colon cancer. They were randomized to receive 3 or 6 months (6 or 12 cycles) of FOLFOX with either concurrent celecoxib at 400 mg daily (n = 1,265) or placebo (n = 1,261) for 3 years from the start of the trial.
The primary endpoint of 3-year DFS did not differ between the groups (hazard ratio, 0.90; P = .16). Likewise, 5-year OS rates did not differ significantly (HR, 0.89; P = .22).
As Dr. Meyerhardt said, there were no significant interactions detected among any subgroups for DFS or OS, including by age, N or T stage, risk group, concurrent low-dose aspirin use, sex, race/ethnicity, baseline performance status, FOLFOX duration, body mass index, or tumor location.
Rates of toxicities were similar between the groups, except for a higher incidence of hypertension of any grade with celecoxib during FOLFOX therapy (14.6% vs. 10.9%, P = .01) and a grade 2 or greater creatinine increase with celecoxib after FOLFOX (1.7% vs. 0.5%, P = .01).
About 40% of patients completed all 3 years of celecoxib or placebo. Reasons for discontinuation included recurrent disease, adverse events, patient withdrawal from the study, physician decision, or other complicating disease.
Why didn’t it work?
Previous studies have indicated that aspirin and cyclooxygenase (COX)-2 inhibitors are associated with a reduced risk of colorectal polyps and cancer, Dr. Lieu said. So why didn’t celecoxib improve survival in the current trial?
“There are obviously COX-1 and COX-independent targets that aspirin hit that celecoxib does not, and its survival impact of affecting these targets is still largely unclear,” Dr. Lieu said.
He added that ongoing trials – including the Add-Aspirin and ASCOLT studies – should provide more insight.
The current trial was sponsored by the Alliance for Clinical Trials in Oncology in collaboration with the National Cancer Institute. Pfizer provided the celecoxib and placebo. Dr. Meyerhardt disclosed relationships with Cota Healthcare, Taiho Pharmaceutical, and Array BioPharma. Dr. Lieu disclosed relationships with Foundation Medicine, Ipsen, Merck, and Immune Design.
SOURCE: Meyerhardt JA et al. ASCO 2020, Abstract 4003.
results of the phase 3 CALGB/SWOG 80702 trial showed.
The trial included 2,526 patients randomized to either 6 or 12 cycles of adjuvant FOLFOX with either celecoxib or placebo. The 3-year disease-free survival (DFS) rate was 76.3% for patients on celecoxib and 73.4% for those on placebo. The 5-year overall survival (OS) was 84% and 81.8%, respectively.
“The addition of celecoxib to FOLFOX adjuvant therapy in stage III colon cancer did not significantly improve disease-free or overall survival,” Jeffrey A. Meyerhardt, MD, of the Dana-Farber Cancer Institute in Boston, said while presenting the results as part of the American Society of Clinical Oncology virtual scientific program.
“We did not detect a significant interaction between celecoxib and duration of FOLFOX therapy for disease-free survival. Similarly, we did not detect a significant interaction between celecoxib and duration of FOLFOX therapy for overall survival,” Dr. Meyerhardt said.
“Simply put, celecoxib should not be used for the secondary prevention of colon cancer,” said invited discussant Christopher Lieu, MD, of the University of Colorado Cancer Center, Aurora.
Trial details
The 80702 trial was designed to test whether the COX-2 inhibitor celecoxib could help further reduce the risk of recurrence when added to adjuvant chemotherapy with 3 or 6 months of FOLFOX. Data on the FOLFOX portion of the trial were previously reported as part of the International Duration Evaluation of Adjuvant Therapy collaboration (N Engl J Med. 2018;378:1177-88).
The trial enrolled 2,526 patients with resected stage III colon cancer. They were randomized to receive 3 or 6 months (6 or 12 cycles) of FOLFOX with either concurrent celecoxib at 400 mg daily (n = 1,265) or placebo (n = 1,261) for 3 years from the start of the trial.
The primary endpoint of 3-year DFS did not differ between the groups (hazard ratio, 0.90; P = .16). Likewise, 5-year OS rates did not differ significantly (HR, 0.89; P = .22).
As Dr. Meyerhardt said, there were no significant interactions detected among any subgroups for DFS or OS, including by age, N or T stage, risk group, concurrent low-dose aspirin use, sex, race/ethnicity, baseline performance status, FOLFOX duration, body mass index, or tumor location.
Rates of toxicities were similar between the groups, except for a higher incidence of hypertension of any grade with celecoxib during FOLFOX therapy (14.6% vs. 10.9%, P = .01) and a grade 2 or greater creatinine increase with celecoxib after FOLFOX (1.7% vs. 0.5%, P = .01).
About 40% of patients completed all 3 years of celecoxib or placebo. Reasons for discontinuation included recurrent disease, adverse events, patient withdrawal from the study, physician decision, or other complicating disease.
Why didn’t it work?
Previous studies have indicated that aspirin and cyclooxygenase (COX)-2 inhibitors are associated with a reduced risk of colorectal polyps and cancer, Dr. Lieu said. So why didn’t celecoxib improve survival in the current trial?
“There are obviously COX-1 and COX-independent targets that aspirin hit that celecoxib does not, and its survival impact of affecting these targets is still largely unclear,” Dr. Lieu said.
He added that ongoing trials – including the Add-Aspirin and ASCOLT studies – should provide more insight.
The current trial was sponsored by the Alliance for Clinical Trials in Oncology in collaboration with the National Cancer Institute. Pfizer provided the celecoxib and placebo. Dr. Meyerhardt disclosed relationships with Cota Healthcare, Taiho Pharmaceutical, and Array BioPharma. Dr. Lieu disclosed relationships with Foundation Medicine, Ipsen, Merck, and Immune Design.
SOURCE: Meyerhardt JA et al. ASCO 2020, Abstract 4003.
results of the phase 3 CALGB/SWOG 80702 trial showed.
The trial included 2,526 patients randomized to either 6 or 12 cycles of adjuvant FOLFOX with either celecoxib or placebo. The 3-year disease-free survival (DFS) rate was 76.3% for patients on celecoxib and 73.4% for those on placebo. The 5-year overall survival (OS) was 84% and 81.8%, respectively.
“The addition of celecoxib to FOLFOX adjuvant therapy in stage III colon cancer did not significantly improve disease-free or overall survival,” Jeffrey A. Meyerhardt, MD, of the Dana-Farber Cancer Institute in Boston, said while presenting the results as part of the American Society of Clinical Oncology virtual scientific program.
“We did not detect a significant interaction between celecoxib and duration of FOLFOX therapy for disease-free survival. Similarly, we did not detect a significant interaction between celecoxib and duration of FOLFOX therapy for overall survival,” Dr. Meyerhardt said.
“Simply put, celecoxib should not be used for the secondary prevention of colon cancer,” said invited discussant Christopher Lieu, MD, of the University of Colorado Cancer Center, Aurora.
Trial details
The 80702 trial was designed to test whether the COX-2 inhibitor celecoxib could help further reduce the risk of recurrence when added to adjuvant chemotherapy with 3 or 6 months of FOLFOX. Data on the FOLFOX portion of the trial were previously reported as part of the International Duration Evaluation of Adjuvant Therapy collaboration (N Engl J Med. 2018;378:1177-88).
The trial enrolled 2,526 patients with resected stage III colon cancer. They were randomized to receive 3 or 6 months (6 or 12 cycles) of FOLFOX with either concurrent celecoxib at 400 mg daily (n = 1,265) or placebo (n = 1,261) for 3 years from the start of the trial.
The primary endpoint of 3-year DFS did not differ between the groups (hazard ratio, 0.90; P = .16). Likewise, 5-year OS rates did not differ significantly (HR, 0.89; P = .22).
As Dr. Meyerhardt said, there were no significant interactions detected among any subgroups for DFS or OS, including by age, N or T stage, risk group, concurrent low-dose aspirin use, sex, race/ethnicity, baseline performance status, FOLFOX duration, body mass index, or tumor location.
Rates of toxicities were similar between the groups, except for a higher incidence of hypertension of any grade with celecoxib during FOLFOX therapy (14.6% vs. 10.9%, P = .01) and a grade 2 or greater creatinine increase with celecoxib after FOLFOX (1.7% vs. 0.5%, P = .01).
About 40% of patients completed all 3 years of celecoxib or placebo. Reasons for discontinuation included recurrent disease, adverse events, patient withdrawal from the study, physician decision, or other complicating disease.
Why didn’t it work?
Previous studies have indicated that aspirin and cyclooxygenase (COX)-2 inhibitors are associated with a reduced risk of colorectal polyps and cancer, Dr. Lieu said. So why didn’t celecoxib improve survival in the current trial?
“There are obviously COX-1 and COX-independent targets that aspirin hit that celecoxib does not, and its survival impact of affecting these targets is still largely unclear,” Dr. Lieu said.
He added that ongoing trials – including the Add-Aspirin and ASCOLT studies – should provide more insight.
The current trial was sponsored by the Alliance for Clinical Trials in Oncology in collaboration with the National Cancer Institute. Pfizer provided the celecoxib and placebo. Dr. Meyerhardt disclosed relationships with Cota Healthcare, Taiho Pharmaceutical, and Array BioPharma. Dr. Lieu disclosed relationships with Foundation Medicine, Ipsen, Merck, and Immune Design.
SOURCE: Meyerhardt JA et al. ASCO 2020, Abstract 4003.
FROM ASCO 2020
High out-of-pocket costs for type 1 diabetes patients: It’s not just insulin
For privately insured individuals with type 1 diabetes in the United States, out-of-pocket costs for insulin are typically lower than for other diabetes-related supplies. But overall out-of-pocket expenditure – taking into account everything that is needed to manage diabetes – is still very high.
Indeed, insulin costs have remained relatively stable over time in such private insurance plans, according to another analysis that looked at all types of diabetes.
Those are the findings of two separate research letters published June 1 in JAMA Internal Medicine.
The first research letter examined all costs for privately insured patients with type 1 diabetes, finding a mean out-of-pocket spend of approximately $2,500 a year.
“Insulin is the difference between life and death for patients with type 1 diabetes, and efforts to make it more affordable are critical,” said lead author of the first letter, Kao-Ping Chua, MD, PhD, of the department of pediatrics, University of Michigan, Ann Arbor.
“However, our study shows that even if insulin were free, families would still have substantial out-of-pocket costs for other health care,” he noted in a press release from his institution.
The other research letter examined trends in insulin out-of-pocket costs in 2006-2017 among U.S. patients with any type of diabetes who had different types of private health insurance plans. The study was by Amir Meiri, MD, of Harvard Pilgrim Health Care Institute, Harvard Medical School, Boston, and colleagues.
Although the study showed relatively stable costs associated with insulin for many privately insured patients with diabetes over the time period examined, “monthly out-of-pocket payments” may still “be burdensome for low-income individuals,” the authors said.
Writing in an accompanying editorial, Laura M. Nally, MD, and Kasia J. Lipska, MD, both of Yale University, New Haven, Conn., agreed that “insulin is only one component of diabetes management.”
Yet they stressed: “Diabetes does not selectively occur among individuals who can afford insulin and who have health insurance; it affects people regardless of their socioeconomic status.”
“The federal health care system should urgently act to make insulin, diabetes-related supplies, and other health care services affordable and available to everyone who needs them.”
Out-of-pocket costs for supplies higher than for insulin
Dr. Chua and colleagues compared out-of-pocket costs for insulin with those for other diabetes-related items, including insulin pump supplies, and glucose meters/continuous monitors, for privately insured patients with type 1 diabetes during 2018.
They included data for 65,192 patients aged 1-64 years with type 1 diabetes who had employer-sponsored coverage from medium to large firms.
The population included children of employees (12%), and 22.5% of patients had enrolled in high-deductible ($1,350 individual/$2,700 family) private plans. Overall, 56.8% used insulin pumps and/or continuous glucose monitors (CGMs).
Annual out-of-pocket spending was lower for insulin ($435) than other diabetes-related supplies ($490), including insulin pump supplies, continuous and fingerstick glucose monitoring equipment, urine testing strips, pen needles, and syringes.
Mean annual overall out-of-pocket spending was $2,414, but this varied widely.
For 8% of the population spending exceeded $5,000. Insulin accounted for just 18% of overall out-of-pocket spending.
Not surprisingly, out-of-pocket spending increased with the sophistication of the diabetes technology used, ranging from just $79 for those using injections and fingerstick monitoring to $1,037 for those using both insulin pumps and CGMs.
In general, for children, out-of-pocket costs of diabetes-related supplies were considerably higher than for insulin ($823 vs. $497), while for adults the two were similar ($445 vs. $427).
“These technologies can improve quality of life and improve diabetes control for all patients, but can be especially important to the families of children with type 1 diabetes,” Dr. Chua said.
Also not surprisingly, those with high-deductible plans had greater out-of-pocket costs in each category ($3,132 vs. $2,205 overall).
Dr. Chua said the study’s findings are particularly timely given recent efforts by states and insurers to cap out-of-pocket costs for insulin, calling these “important first steps.”
But there is still a long way to go, he said.
“Policymakers should improve the affordability of all care for type 1 diabetes,” Dr. Chua noted.
Dr. Nally and Dr. Lipska agreed.
“Although capping insulin copayments is a step in the right direction, such a state law does not protect many individuals with federally regulated insurance plans, with Medicare, or without any insurance,” they noted.
“In addition, insulin copayment caps do little to ease the financial burden of paying for diabetes-related supplies or other healthcare services,” they pointed out.
Private plans shield members from out-of-pocket insulin costs
The other study examined out-of-pocket spending for 10,954,436 insulin claims for 612,071 unique patients with diabetes (either type) in different types of private commercial health plans during 2006-2017:
- High-deductible health plans (HDHP) with a health savings account (HSA), which have high medication costs because they require payment of the full reimbursement price until the annual deductible is reached (7% of claims).
- Plans with health reimbursement arrangement (HRA), which typically have tiered drug copayments and members can use their reimbursement accounts to pay for medical expenses (4% of claims).
- No-account plans (without an HSA) that also typically have tiered drug copayments (80% of claims).
The price of insulin per patient per month rose from $143 in 2006 to $280 in 2012 to $394 in 2017.
However, the share of the insulin price per member per month paid by the patient actually declined from 24% in 2006 to 16% in 2012 to just 10% in 2017.
Because of the increase in insulin price, those corresponding costs still rose from $52 in 2006 to $72 in 2012, but then dropped to $64 in 2017.
By plan type, out-of-pocket costs per member per month were lowest for those no-account plans (from $52 in 2006 to $48 in 2017) and highest for those with HDHP HSA plans ($93 in 2006 to $141 in 2017).
“The data suggest that privately insured patients have been relatively shielded from insulin price increases and that commercial health insurers have accommodated higher insulin prices by increasing premiums or deductibles for all members,” Dr. Meiri and colleagues write.
Most vulnerable missing from study: COVID-19 will strike further blow
Although generally agreeing with this conclusion, Dr. Nally and Dr. Lipska nevertheless faulted the data from Dr. Meiri and colleagues on several counts.
First, they reiterated that the population was limited to those with private insurance plans, and therefore “the groups most vulnerable to high insulin costs may be missing from the study.”
Also, the data do not capture all sources of out-of-pocket insulin spending for people with high copayments, such as the federal 340B Drug Pricing Program, GoodRx, or drug manufacturer discounts.
Moreover, the editorialists noted, the study assessed only mean out-of-pocket costs without assessing differences in spending across individuals.
And, Dr. Nally and Dr. Lipska pointed out, the data do not account for rebates and discounts negotiated between pharmacy benefit managers and drug manufacturers. “As a result, these data on health plan spending on insulin may overestimate the net health plan expenditures,” they wrote.
Dr. Chua also warned that the COVID-19 pandemic has had a major adverse impact on the diabetes community.
“Many people with private insurance have lost their jobs and insurance coverage ... This may put health care like insulin and diabetes-related supplies out of reach,” he said.
Dr. Chua has reported receiving support from the National Institute on Drug Abuse. Dr. Meiri has reported receiving grants from the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases for the study. Dr. Nally has reported receiving a grant from Novo Nordisk outside the submitted work. Dr. Lipska has reported receiving support from the Centers for Medicare & Medicaid Services and the National Institutes of Health.
A version of this article originally appeared on Medscape.com.
For privately insured individuals with type 1 diabetes in the United States, out-of-pocket costs for insulin are typically lower than for other diabetes-related supplies. But overall out-of-pocket expenditure – taking into account everything that is needed to manage diabetes – is still very high.
Indeed, insulin costs have remained relatively stable over time in such private insurance plans, according to another analysis that looked at all types of diabetes.
Those are the findings of two separate research letters published June 1 in JAMA Internal Medicine.
The first research letter examined all costs for privately insured patients with type 1 diabetes, finding a mean out-of-pocket spend of approximately $2,500 a year.
“Insulin is the difference between life and death for patients with type 1 diabetes, and efforts to make it more affordable are critical,” said lead author of the first letter, Kao-Ping Chua, MD, PhD, of the department of pediatrics, University of Michigan, Ann Arbor.
“However, our study shows that even if insulin were free, families would still have substantial out-of-pocket costs for other health care,” he noted in a press release from his institution.
The other research letter examined trends in insulin out-of-pocket costs in 2006-2017 among U.S. patients with any type of diabetes who had different types of private health insurance plans. The study was by Amir Meiri, MD, of Harvard Pilgrim Health Care Institute, Harvard Medical School, Boston, and colleagues.
Although the study showed relatively stable costs associated with insulin for many privately insured patients with diabetes over the time period examined, “monthly out-of-pocket payments” may still “be burdensome for low-income individuals,” the authors said.
Writing in an accompanying editorial, Laura M. Nally, MD, and Kasia J. Lipska, MD, both of Yale University, New Haven, Conn., agreed that “insulin is only one component of diabetes management.”
Yet they stressed: “Diabetes does not selectively occur among individuals who can afford insulin and who have health insurance; it affects people regardless of their socioeconomic status.”
“The federal health care system should urgently act to make insulin, diabetes-related supplies, and other health care services affordable and available to everyone who needs them.”
Out-of-pocket costs for supplies higher than for insulin
Dr. Chua and colleagues compared out-of-pocket costs for insulin with those for other diabetes-related items, including insulin pump supplies, and glucose meters/continuous monitors, for privately insured patients with type 1 diabetes during 2018.
They included data for 65,192 patients aged 1-64 years with type 1 diabetes who had employer-sponsored coverage from medium to large firms.
The population included children of employees (12%), and 22.5% of patients had enrolled in high-deductible ($1,350 individual/$2,700 family) private plans. Overall, 56.8% used insulin pumps and/or continuous glucose monitors (CGMs).
Annual out-of-pocket spending was lower for insulin ($435) than other diabetes-related supplies ($490), including insulin pump supplies, continuous and fingerstick glucose monitoring equipment, urine testing strips, pen needles, and syringes.
Mean annual overall out-of-pocket spending was $2,414, but this varied widely.
For 8% of the population spending exceeded $5,000. Insulin accounted for just 18% of overall out-of-pocket spending.
Not surprisingly, out-of-pocket spending increased with the sophistication of the diabetes technology used, ranging from just $79 for those using injections and fingerstick monitoring to $1,037 for those using both insulin pumps and CGMs.
In general, for children, out-of-pocket costs of diabetes-related supplies were considerably higher than for insulin ($823 vs. $497), while for adults the two were similar ($445 vs. $427).
“These technologies can improve quality of life and improve diabetes control for all patients, but can be especially important to the families of children with type 1 diabetes,” Dr. Chua said.
Also not surprisingly, those with high-deductible plans had greater out-of-pocket costs in each category ($3,132 vs. $2,205 overall).
Dr. Chua said the study’s findings are particularly timely given recent efforts by states and insurers to cap out-of-pocket costs for insulin, calling these “important first steps.”
But there is still a long way to go, he said.
“Policymakers should improve the affordability of all care for type 1 diabetes,” Dr. Chua noted.
Dr. Nally and Dr. Lipska agreed.
“Although capping insulin copayments is a step in the right direction, such a state law does not protect many individuals with federally regulated insurance plans, with Medicare, or without any insurance,” they noted.
“In addition, insulin copayment caps do little to ease the financial burden of paying for diabetes-related supplies or other healthcare services,” they pointed out.
Private plans shield members from out-of-pocket insulin costs
The other study examined out-of-pocket spending for 10,954,436 insulin claims for 612,071 unique patients with diabetes (either type) in different types of private commercial health plans during 2006-2017:
- High-deductible health plans (HDHP) with a health savings account (HSA), which have high medication costs because they require payment of the full reimbursement price until the annual deductible is reached (7% of claims).
- Plans with health reimbursement arrangement (HRA), which typically have tiered drug copayments and members can use their reimbursement accounts to pay for medical expenses (4% of claims).
- No-account plans (without an HSA) that also typically have tiered drug copayments (80% of claims).
The price of insulin per patient per month rose from $143 in 2006 to $280 in 2012 to $394 in 2017.
However, the share of the insulin price per member per month paid by the patient actually declined from 24% in 2006 to 16% in 2012 to just 10% in 2017.
Because of the increase in insulin price, those corresponding costs still rose from $52 in 2006 to $72 in 2012, but then dropped to $64 in 2017.
By plan type, out-of-pocket costs per member per month were lowest for those no-account plans (from $52 in 2006 to $48 in 2017) and highest for those with HDHP HSA plans ($93 in 2006 to $141 in 2017).
“The data suggest that privately insured patients have been relatively shielded from insulin price increases and that commercial health insurers have accommodated higher insulin prices by increasing premiums or deductibles for all members,” Dr. Meiri and colleagues write.
Most vulnerable missing from study: COVID-19 will strike further blow
Although generally agreeing with this conclusion, Dr. Nally and Dr. Lipska nevertheless faulted the data from Dr. Meiri and colleagues on several counts.
First, they reiterated that the population was limited to those with private insurance plans, and therefore “the groups most vulnerable to high insulin costs may be missing from the study.”
Also, the data do not capture all sources of out-of-pocket insulin spending for people with high copayments, such as the federal 340B Drug Pricing Program, GoodRx, or drug manufacturer discounts.
Moreover, the editorialists noted, the study assessed only mean out-of-pocket costs without assessing differences in spending across individuals.
And, Dr. Nally and Dr. Lipska pointed out, the data do not account for rebates and discounts negotiated between pharmacy benefit managers and drug manufacturers. “As a result, these data on health plan spending on insulin may overestimate the net health plan expenditures,” they wrote.
Dr. Chua also warned that the COVID-19 pandemic has had a major adverse impact on the diabetes community.
“Many people with private insurance have lost their jobs and insurance coverage ... This may put health care like insulin and diabetes-related supplies out of reach,” he said.
Dr. Chua has reported receiving support from the National Institute on Drug Abuse. Dr. Meiri has reported receiving grants from the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases for the study. Dr. Nally has reported receiving a grant from Novo Nordisk outside the submitted work. Dr. Lipska has reported receiving support from the Centers for Medicare & Medicaid Services and the National Institutes of Health.
A version of this article originally appeared on Medscape.com.
For privately insured individuals with type 1 diabetes in the United States, out-of-pocket costs for insulin are typically lower than for other diabetes-related supplies. But overall out-of-pocket expenditure – taking into account everything that is needed to manage diabetes – is still very high.
Indeed, insulin costs have remained relatively stable over time in such private insurance plans, according to another analysis that looked at all types of diabetes.
Those are the findings of two separate research letters published June 1 in JAMA Internal Medicine.
The first research letter examined all costs for privately insured patients with type 1 diabetes, finding a mean out-of-pocket spend of approximately $2,500 a year.
“Insulin is the difference between life and death for patients with type 1 diabetes, and efforts to make it more affordable are critical,” said lead author of the first letter, Kao-Ping Chua, MD, PhD, of the department of pediatrics, University of Michigan, Ann Arbor.
“However, our study shows that even if insulin were free, families would still have substantial out-of-pocket costs for other health care,” he noted in a press release from his institution.
The other research letter examined trends in insulin out-of-pocket costs in 2006-2017 among U.S. patients with any type of diabetes who had different types of private health insurance plans. The study was by Amir Meiri, MD, of Harvard Pilgrim Health Care Institute, Harvard Medical School, Boston, and colleagues.
Although the study showed relatively stable costs associated with insulin for many privately insured patients with diabetes over the time period examined, “monthly out-of-pocket payments” may still “be burdensome for low-income individuals,” the authors said.
Writing in an accompanying editorial, Laura M. Nally, MD, and Kasia J. Lipska, MD, both of Yale University, New Haven, Conn., agreed that “insulin is only one component of diabetes management.”
Yet they stressed: “Diabetes does not selectively occur among individuals who can afford insulin and who have health insurance; it affects people regardless of their socioeconomic status.”
“The federal health care system should urgently act to make insulin, diabetes-related supplies, and other health care services affordable and available to everyone who needs them.”
Out-of-pocket costs for supplies higher than for insulin
Dr. Chua and colleagues compared out-of-pocket costs for insulin with those for other diabetes-related items, including insulin pump supplies, and glucose meters/continuous monitors, for privately insured patients with type 1 diabetes during 2018.
They included data for 65,192 patients aged 1-64 years with type 1 diabetes who had employer-sponsored coverage from medium to large firms.
The population included children of employees (12%), and 22.5% of patients had enrolled in high-deductible ($1,350 individual/$2,700 family) private plans. Overall, 56.8% used insulin pumps and/or continuous glucose monitors (CGMs).
Annual out-of-pocket spending was lower for insulin ($435) than other diabetes-related supplies ($490), including insulin pump supplies, continuous and fingerstick glucose monitoring equipment, urine testing strips, pen needles, and syringes.
Mean annual overall out-of-pocket spending was $2,414, but this varied widely.
For 8% of the population spending exceeded $5,000. Insulin accounted for just 18% of overall out-of-pocket spending.
Not surprisingly, out-of-pocket spending increased with the sophistication of the diabetes technology used, ranging from just $79 for those using injections and fingerstick monitoring to $1,037 for those using both insulin pumps and CGMs.
In general, for children, out-of-pocket costs of diabetes-related supplies were considerably higher than for insulin ($823 vs. $497), while for adults the two were similar ($445 vs. $427).
“These technologies can improve quality of life and improve diabetes control for all patients, but can be especially important to the families of children with type 1 diabetes,” Dr. Chua said.
Also not surprisingly, those with high-deductible plans had greater out-of-pocket costs in each category ($3,132 vs. $2,205 overall).
Dr. Chua said the study’s findings are particularly timely given recent efforts by states and insurers to cap out-of-pocket costs for insulin, calling these “important first steps.”
But there is still a long way to go, he said.
“Policymakers should improve the affordability of all care for type 1 diabetes,” Dr. Chua noted.
Dr. Nally and Dr. Lipska agreed.
“Although capping insulin copayments is a step in the right direction, such a state law does not protect many individuals with federally regulated insurance plans, with Medicare, or without any insurance,” they noted.
“In addition, insulin copayment caps do little to ease the financial burden of paying for diabetes-related supplies or other healthcare services,” they pointed out.
Private plans shield members from out-of-pocket insulin costs
The other study examined out-of-pocket spending for 10,954,436 insulin claims for 612,071 unique patients with diabetes (either type) in different types of private commercial health plans during 2006-2017:
- High-deductible health plans (HDHP) with a health savings account (HSA), which have high medication costs because they require payment of the full reimbursement price until the annual deductible is reached (7% of claims).
- Plans with health reimbursement arrangement (HRA), which typically have tiered drug copayments and members can use their reimbursement accounts to pay for medical expenses (4% of claims).
- No-account plans (without an HSA) that also typically have tiered drug copayments (80% of claims).
The price of insulin per patient per month rose from $143 in 2006 to $280 in 2012 to $394 in 2017.
However, the share of the insulin price per member per month paid by the patient actually declined from 24% in 2006 to 16% in 2012 to just 10% in 2017.
Because of the increase in insulin price, those corresponding costs still rose from $52 in 2006 to $72 in 2012, but then dropped to $64 in 2017.
By plan type, out-of-pocket costs per member per month were lowest for those no-account plans (from $52 in 2006 to $48 in 2017) and highest for those with HDHP HSA plans ($93 in 2006 to $141 in 2017).
“The data suggest that privately insured patients have been relatively shielded from insulin price increases and that commercial health insurers have accommodated higher insulin prices by increasing premiums or deductibles for all members,” Dr. Meiri and colleagues write.
Most vulnerable missing from study: COVID-19 will strike further blow
Although generally agreeing with this conclusion, Dr. Nally and Dr. Lipska nevertheless faulted the data from Dr. Meiri and colleagues on several counts.
First, they reiterated that the population was limited to those with private insurance plans, and therefore “the groups most vulnerable to high insulin costs may be missing from the study.”
Also, the data do not capture all sources of out-of-pocket insulin spending for people with high copayments, such as the federal 340B Drug Pricing Program, GoodRx, or drug manufacturer discounts.
Moreover, the editorialists noted, the study assessed only mean out-of-pocket costs without assessing differences in spending across individuals.
And, Dr. Nally and Dr. Lipska pointed out, the data do not account for rebates and discounts negotiated between pharmacy benefit managers and drug manufacturers. “As a result, these data on health plan spending on insulin may overestimate the net health plan expenditures,” they wrote.
Dr. Chua also warned that the COVID-19 pandemic has had a major adverse impact on the diabetes community.
“Many people with private insurance have lost their jobs and insurance coverage ... This may put health care like insulin and diabetes-related supplies out of reach,” he said.
Dr. Chua has reported receiving support from the National Institute on Drug Abuse. Dr. Meiri has reported receiving grants from the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases for the study. Dr. Nally has reported receiving a grant from Novo Nordisk outside the submitted work. Dr. Lipska has reported receiving support from the Centers for Medicare & Medicaid Services and the National Institutes of Health.
A version of this article originally appeared on Medscape.com.
Avacopan notches a win in ANCA-associated vasculitis
Avacopan, an investigational oral inhibitor of complement activation, is efficacious and safe for treating antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis, based on the results of the pivotal phase 3 ADVOCATE trial.
The trial results were reported in the opening plenary abstract session at the annual European Congress of Rheumatology, held online this year because of COVID-19.
“Standard of care for induction of remission includes high-dose glucocorticoids with either cyclophosphamide or rituximab. However, glucocorticoids are the major cause of treatment-related harm,” noted lead investigator Peter A. Merkel, MD, MPH, chief of the division of rheumatology at the University of Pennsylvania, Philadelphia.
The 331 patients in the trial had active ANCA-associated vasculitis (granulomatosis with polyangiitis or microscopic polyangiitis), either new onset or relapsed, with positivity for either proteinase 3 or myeloperoxidase antibodies and moderate to high disease activity.
They were randomized evenly to double-blind avacopan 30 mg or tapering prednisone from 60 mg/day to zero over 20 weeks, each combined either with rituximab (Rituxan) or with cyclophosphamide followed by azathioprine. Avacopan (formerly called CCX168) is a selective antagonist of the complement C5a receptor that has orphan-drug designation from the Food and Drug Administration for this disease.
Trial results showed that avacopan was noninferior to prednisone with respect to the week 26 rate of remission on the Birmingham Vasculitis Activity Score, with an estimate of common difference of 3.4%. And it was superior to prednisone with respect to the week 52 rate of sustained remission, which required remission from week 26 onward, with an estimate of common difference of 12.5%.
The avacopan group also had less glucocorticoid-related toxicity and, among patients with preexisting renal disease, greater improvement in renal function.
“This large, randomized trial met both of its primary endpoints. Important secondary endpoints were also achieved, with a very acceptable safety profile,” Dr. Merkel summarized.
Making sense of the results
The optimal duration of avacopan therapy is unclear, he noted. “We are still going to be learning how to use this drug, if it’s approved, in routine practice. But the data from the second 6 months – from week 26 to week 52 – implies that there is ongoing benefit to being on avacopan after remission is achieved.”
Avacopan worked similarly well regardless of disease status in ADVOCATE, according to Dr. Merkel. “We have not seen significant differences in efficacy of other drugs in our trials [by disease status], in the trials of ANCA-associated vasculitis. So I think we would treat moderate to serious disease similarly, whether it is new onset or recurrence, in terms of efficacy of the drug.”
“The topline phase 3 data from ADVOCATE sort of even exceeded my expectations in terms of the ability to show not just noninferiority, but superiority of avacopan at week 52 in maintaining sustained remission,” Lindsay S. Lally, MD, assistant professor of medicine at the Hospital for Special Surgery in New York, commented in an interview. “It’s spectacular to treat patients with this serious vasculitis without any steroids or with very minimal steroids, and see superiority at a year. That is really game changing.”
The ADVOCATE findings will likely pass muster with the FDA, according to Dr. Lally. “The bar that was set in terms of the coprimary endpoints was very stringent and in line with other registration trials, particularly the RAVE trial that led to the approval of rituximab,” she elaborated. “I don’t think there is any significant safety signal in the data related to avacopan.
“This study is going to move forward our ability to treat this disease effectively, as we have been able to do in some of our other vasculitis syndromes, by finding drugs that have significant steroid-sparing effects,” Dr. Lally predicted.
Study details
ADVOCATE results reported at the congress showed that the week 26 rate of disease remission was 72.3% with avacopan versus 70.1% with prednisone, with the difference falling within the 20% boundary for noninferiority (P < .0001) but missing the mark for superiority (P = .2387).
However, the week 52 rate of sustained disease remission was 65.7% versus 54.9%, respectively, yielding a difference in favor of avacopan that was statistically both noninferior (P < .0001) and superior (P = .0066).
At week 26, patients in the avacopan group had more favorable Glucocorticoid Toxicity Index scores for cumulative worsening (39.7 vs. 56.6; P = .0002) and for aggregate improvement (11.2 vs. 23.4; P = .008).
Among patients who had renal disease at baseline, those in the avacopan group had a greater increase in estimated glomerular filtration rate at week 52 (7.3 vs. 4.1 mL/min per 1.73 m2; P = .029).
“Particularly interesting is the fact that, even after week 26, when the patients were in remission, there was continued improvement in renal function,” Dr. Merkel noted.
Overall, avacopan had a good safety profile. “This was a sick population with many complications, but there were no important safety signals of the study medication,” he reported.
The avacopan and prednisone groups had a similar rate of severe adverse events (23.5% vs. 25.0%). But the former had lower rates of life-threatening adverse events (4.8% vs. 8.5%), adverse events potentially related to glucocorticoids (66.3% vs. 80.5%), deaths (1.2% vs. 2.4%), and deaths specifically caused by infection (0.6% vs. 1.2%).
The trial was sponsored by ChemoCentryx. Dr. Merkel disclosed receiving grant/research support from and consulting fees from ChemoCentryx, among other disclosures. Dr. Lally disclosed that she was an investigator in the trial.
SOURCE: Merkel PA et al. Ann Rheum Dis. 2020;79[suppl 1]:8, Abstract OP0011.
Avacopan, an investigational oral inhibitor of complement activation, is efficacious and safe for treating antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis, based on the results of the pivotal phase 3 ADVOCATE trial.
The trial results were reported in the opening plenary abstract session at the annual European Congress of Rheumatology, held online this year because of COVID-19.
“Standard of care for induction of remission includes high-dose glucocorticoids with either cyclophosphamide or rituximab. However, glucocorticoids are the major cause of treatment-related harm,” noted lead investigator Peter A. Merkel, MD, MPH, chief of the division of rheumatology at the University of Pennsylvania, Philadelphia.
The 331 patients in the trial had active ANCA-associated vasculitis (granulomatosis with polyangiitis or microscopic polyangiitis), either new onset or relapsed, with positivity for either proteinase 3 or myeloperoxidase antibodies and moderate to high disease activity.
They were randomized evenly to double-blind avacopan 30 mg or tapering prednisone from 60 mg/day to zero over 20 weeks, each combined either with rituximab (Rituxan) or with cyclophosphamide followed by azathioprine. Avacopan (formerly called CCX168) is a selective antagonist of the complement C5a receptor that has orphan-drug designation from the Food and Drug Administration for this disease.
Trial results showed that avacopan was noninferior to prednisone with respect to the week 26 rate of remission on the Birmingham Vasculitis Activity Score, with an estimate of common difference of 3.4%. And it was superior to prednisone with respect to the week 52 rate of sustained remission, which required remission from week 26 onward, with an estimate of common difference of 12.5%.
The avacopan group also had less glucocorticoid-related toxicity and, among patients with preexisting renal disease, greater improvement in renal function.
“This large, randomized trial met both of its primary endpoints. Important secondary endpoints were also achieved, with a very acceptable safety profile,” Dr. Merkel summarized.
Making sense of the results
The optimal duration of avacopan therapy is unclear, he noted. “We are still going to be learning how to use this drug, if it’s approved, in routine practice. But the data from the second 6 months – from week 26 to week 52 – implies that there is ongoing benefit to being on avacopan after remission is achieved.”
Avacopan worked similarly well regardless of disease status in ADVOCATE, according to Dr. Merkel. “We have not seen significant differences in efficacy of other drugs in our trials [by disease status], in the trials of ANCA-associated vasculitis. So I think we would treat moderate to serious disease similarly, whether it is new onset or recurrence, in terms of efficacy of the drug.”
“The topline phase 3 data from ADVOCATE sort of even exceeded my expectations in terms of the ability to show not just noninferiority, but superiority of avacopan at week 52 in maintaining sustained remission,” Lindsay S. Lally, MD, assistant professor of medicine at the Hospital for Special Surgery in New York, commented in an interview. “It’s spectacular to treat patients with this serious vasculitis without any steroids or with very minimal steroids, and see superiority at a year. That is really game changing.”
The ADVOCATE findings will likely pass muster with the FDA, according to Dr. Lally. “The bar that was set in terms of the coprimary endpoints was very stringent and in line with other registration trials, particularly the RAVE trial that led to the approval of rituximab,” she elaborated. “I don’t think there is any significant safety signal in the data related to avacopan.
“This study is going to move forward our ability to treat this disease effectively, as we have been able to do in some of our other vasculitis syndromes, by finding drugs that have significant steroid-sparing effects,” Dr. Lally predicted.
Study details
ADVOCATE results reported at the congress showed that the week 26 rate of disease remission was 72.3% with avacopan versus 70.1% with prednisone, with the difference falling within the 20% boundary for noninferiority (P < .0001) but missing the mark for superiority (P = .2387).
However, the week 52 rate of sustained disease remission was 65.7% versus 54.9%, respectively, yielding a difference in favor of avacopan that was statistically both noninferior (P < .0001) and superior (P = .0066).
At week 26, patients in the avacopan group had more favorable Glucocorticoid Toxicity Index scores for cumulative worsening (39.7 vs. 56.6; P = .0002) and for aggregate improvement (11.2 vs. 23.4; P = .008).
Among patients who had renal disease at baseline, those in the avacopan group had a greater increase in estimated glomerular filtration rate at week 52 (7.3 vs. 4.1 mL/min per 1.73 m2; P = .029).
“Particularly interesting is the fact that, even after week 26, when the patients were in remission, there was continued improvement in renal function,” Dr. Merkel noted.
Overall, avacopan had a good safety profile. “This was a sick population with many complications, but there were no important safety signals of the study medication,” he reported.
The avacopan and prednisone groups had a similar rate of severe adverse events (23.5% vs. 25.0%). But the former had lower rates of life-threatening adverse events (4.8% vs. 8.5%), adverse events potentially related to glucocorticoids (66.3% vs. 80.5%), deaths (1.2% vs. 2.4%), and deaths specifically caused by infection (0.6% vs. 1.2%).
The trial was sponsored by ChemoCentryx. Dr. Merkel disclosed receiving grant/research support from and consulting fees from ChemoCentryx, among other disclosures. Dr. Lally disclosed that she was an investigator in the trial.
SOURCE: Merkel PA et al. Ann Rheum Dis. 2020;79[suppl 1]:8, Abstract OP0011.
Avacopan, an investigational oral inhibitor of complement activation, is efficacious and safe for treating antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis, based on the results of the pivotal phase 3 ADVOCATE trial.
The trial results were reported in the opening plenary abstract session at the annual European Congress of Rheumatology, held online this year because of COVID-19.
“Standard of care for induction of remission includes high-dose glucocorticoids with either cyclophosphamide or rituximab. However, glucocorticoids are the major cause of treatment-related harm,” noted lead investigator Peter A. Merkel, MD, MPH, chief of the division of rheumatology at the University of Pennsylvania, Philadelphia.
The 331 patients in the trial had active ANCA-associated vasculitis (granulomatosis with polyangiitis or microscopic polyangiitis), either new onset or relapsed, with positivity for either proteinase 3 or myeloperoxidase antibodies and moderate to high disease activity.
They were randomized evenly to double-blind avacopan 30 mg or tapering prednisone from 60 mg/day to zero over 20 weeks, each combined either with rituximab (Rituxan) or with cyclophosphamide followed by azathioprine. Avacopan (formerly called CCX168) is a selective antagonist of the complement C5a receptor that has orphan-drug designation from the Food and Drug Administration for this disease.
Trial results showed that avacopan was noninferior to prednisone with respect to the week 26 rate of remission on the Birmingham Vasculitis Activity Score, with an estimate of common difference of 3.4%. And it was superior to prednisone with respect to the week 52 rate of sustained remission, which required remission from week 26 onward, with an estimate of common difference of 12.5%.
The avacopan group also had less glucocorticoid-related toxicity and, among patients with preexisting renal disease, greater improvement in renal function.
“This large, randomized trial met both of its primary endpoints. Important secondary endpoints were also achieved, with a very acceptable safety profile,” Dr. Merkel summarized.
Making sense of the results
The optimal duration of avacopan therapy is unclear, he noted. “We are still going to be learning how to use this drug, if it’s approved, in routine practice. But the data from the second 6 months – from week 26 to week 52 – implies that there is ongoing benefit to being on avacopan after remission is achieved.”
Avacopan worked similarly well regardless of disease status in ADVOCATE, according to Dr. Merkel. “We have not seen significant differences in efficacy of other drugs in our trials [by disease status], in the trials of ANCA-associated vasculitis. So I think we would treat moderate to serious disease similarly, whether it is new onset or recurrence, in terms of efficacy of the drug.”
“The topline phase 3 data from ADVOCATE sort of even exceeded my expectations in terms of the ability to show not just noninferiority, but superiority of avacopan at week 52 in maintaining sustained remission,” Lindsay S. Lally, MD, assistant professor of medicine at the Hospital for Special Surgery in New York, commented in an interview. “It’s spectacular to treat patients with this serious vasculitis without any steroids or with very minimal steroids, and see superiority at a year. That is really game changing.”
The ADVOCATE findings will likely pass muster with the FDA, according to Dr. Lally. “The bar that was set in terms of the coprimary endpoints was very stringent and in line with other registration trials, particularly the RAVE trial that led to the approval of rituximab,” she elaborated. “I don’t think there is any significant safety signal in the data related to avacopan.
“This study is going to move forward our ability to treat this disease effectively, as we have been able to do in some of our other vasculitis syndromes, by finding drugs that have significant steroid-sparing effects,” Dr. Lally predicted.
Study details
ADVOCATE results reported at the congress showed that the week 26 rate of disease remission was 72.3% with avacopan versus 70.1% with prednisone, with the difference falling within the 20% boundary for noninferiority (P < .0001) but missing the mark for superiority (P = .2387).
However, the week 52 rate of sustained disease remission was 65.7% versus 54.9%, respectively, yielding a difference in favor of avacopan that was statistically both noninferior (P < .0001) and superior (P = .0066).
At week 26, patients in the avacopan group had more favorable Glucocorticoid Toxicity Index scores for cumulative worsening (39.7 vs. 56.6; P = .0002) and for aggregate improvement (11.2 vs. 23.4; P = .008).
Among patients who had renal disease at baseline, those in the avacopan group had a greater increase in estimated glomerular filtration rate at week 52 (7.3 vs. 4.1 mL/min per 1.73 m2; P = .029).
“Particularly interesting is the fact that, even after week 26, when the patients were in remission, there was continued improvement in renal function,” Dr. Merkel noted.
Overall, avacopan had a good safety profile. “This was a sick population with many complications, but there were no important safety signals of the study medication,” he reported.
The avacopan and prednisone groups had a similar rate of severe adverse events (23.5% vs. 25.0%). But the former had lower rates of life-threatening adverse events (4.8% vs. 8.5%), adverse events potentially related to glucocorticoids (66.3% vs. 80.5%), deaths (1.2% vs. 2.4%), and deaths specifically caused by infection (0.6% vs. 1.2%).
The trial was sponsored by ChemoCentryx. Dr. Merkel disclosed receiving grant/research support from and consulting fees from ChemoCentryx, among other disclosures. Dr. Lally disclosed that she was an investigator in the trial.
SOURCE: Merkel PA et al. Ann Rheum Dis. 2020;79[suppl 1]:8, Abstract OP0011.
FROM EULAR 2020 E-CONGRESS
Study tests a simpler low disease activity measure for lupus
An alternative disease activity index for patients with systemic lupus erythematosus called the SLE-DAS (Disease Activity Score) has shown similar results to the Lupus Low Disease Activity State (LLDAS) in classifying low disease activity but may be easier to potentially apply in daily clinical practice in treat-to-target strategies, according to research presented at the annual European Congress of Rheumatology, held online this year because of COVID-19.
A treat-to-target approach, in which therapies are adjusted and the patient monitored to achieve the desired endpoint, has been proposed for patients with SLE. Clinical remission is the ideal goal, followed by achieving low disease activity (LDA) when clinical remission is unattainable, the first author of the SLE-DAS study, Helena Assunção, MD, of the department of rheumatology at Centro Hospitalar e Universitário de Coimbra (Portugal), said in an interview prior to the presentation of the study at the e-congress.
But to conduct a treat-to-target approach in the clinical setting, clinicians must have reliable, user-friendly targets to assess a patient’s progress, she said. But that’s not available right now. Proposed definitions of LDA, such as the LLDAS, are based on the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K). This index doesn’t address some important manifestations of SLE and it is scored dichotomously – for example, giving a similar score for thrombocytopenia when platelet count is reduced to 100,000 or to 10,000.
To compensate for these limitations, the current LLDAS definition also requires the Physician Global Assessment and other steps, including a review of medication and changes to treatment or clinical status since the previous visit.
“It is not easy to apply,” Dr. Assunção said.
The SLE-DAS is a continuous index involving 17 parameters (4 continuous: arthritis, proteinuria, thrombocytopenia, and leukopenia), assigning higher scores when a manifestation is more severe, and has manifestation information that SLEDAI-2K lacks (cardiopulmonary involvement, lupus enteritis, and hemolytic anemia).
In contrast, the LLDAS is defined as:
- A SLEDAI-2k score of 4 or less with no major organ involvement
- No new disease activity
- A physician global assessment of the patient of 1 or less on a 0-3 scale
- Maintenance on a prednisolone dosage of 7.5 mg/day or less
- Maintenance on a standard immunosuppressive regimen
A previous study validated the SLE-DAS (Ann Rheum Dis. 2019 Mar;78[3]:365-71), and another exploratory study identified a cutoff SLE-DAS value of 3.77 or lower for LDA with SLE-DAS (Ann Rheum Dis. 2019;78:411-2).
Her group compared LDA status as measured with LLDAS versus the SLE-DAS in a cross-sectional study of 292 consecutive patients at their hospital. LDA on the SLE-DAS was defined as a score 3.77 or lower and a prednisolone dose of 7.5 mg/day or less. A total of 85% of patients were in LDA with SLE-DAS and 83.9% with LLDAS, and the agreement between LLDAS and SLE-DAS LDA was very high (Cohen’s kappa coefficient test; kappa = 0.831; P < .01). Out of 292 patients, only 13 were classified differently by the two definitions, 8 of which were classified as LDA by SLE-DAS, and 5 by LLDAS. Overall, 87% of patients were women and had a mean age of nearly 49 years, with a mean disease duration of about 14 years.
Dr. Assunção feels that the SLE-DAS LDA should be sufficient to monitor disease activity without adding the Physician Global Assessment and other steps, which would make it easier to apply than LLDAS. The fact that it is based on a continuous index is also an important difference. “Especially for low disease activity, it’s very good to be able to define it with a continuous index, because you are not that bad, but not that good, you’re in the middle,” she said.
The study should be regarded as exploratory, she said, but the results were encouraging. “We got similar results, and it’s definitely easier to apply.” She can also personally attest that the new model is easier to use, since she personally collected data for LLDAS assignment. “I had to check this, and this, and this … [SLE-DAS] is easier.”
Future work from her group will aim at deriving and validating a more robust definition of LDA, which will again be compared with the current LLDAS definition.
Her colleagues have already developed and validated a definition for clinical remission using SLE-DAS, although those results have not yet been published. They hope to define activity states using SLE-DAS, including mild, moderate, and high disease activity.
The team has produced an online SLE-DAS calculator (http://sle-das.eu/) where clinicians can score the 17 parameters. “You just input the values and it gives a number reflecting disease activity. Using this definition of SLE-DAS LDA you only need that number and to verify that the prednisolone dose is equal to or inferior to 7.5 mg/day,” said Dr. Assunção.
The study received no funding. Dr. Assunção has no financial disclosures, but one coauthor reported receiving grant/research support from Pfizer and AbbVie and serving as a consultant to Pfizer, AbbVie, Roche, Lilly, and Novartis.
SOURCE: Assunção H et al. Ann Rheum Dis 2020;79[suppl 1]:60, Abstract OP0092.
An alternative disease activity index for patients with systemic lupus erythematosus called the SLE-DAS (Disease Activity Score) has shown similar results to the Lupus Low Disease Activity State (LLDAS) in classifying low disease activity but may be easier to potentially apply in daily clinical practice in treat-to-target strategies, according to research presented at the annual European Congress of Rheumatology, held online this year because of COVID-19.
A treat-to-target approach, in which therapies are adjusted and the patient monitored to achieve the desired endpoint, has been proposed for patients with SLE. Clinical remission is the ideal goal, followed by achieving low disease activity (LDA) when clinical remission is unattainable, the first author of the SLE-DAS study, Helena Assunção, MD, of the department of rheumatology at Centro Hospitalar e Universitário de Coimbra (Portugal), said in an interview prior to the presentation of the study at the e-congress.
But to conduct a treat-to-target approach in the clinical setting, clinicians must have reliable, user-friendly targets to assess a patient’s progress, she said. But that’s not available right now. Proposed definitions of LDA, such as the LLDAS, are based on the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K). This index doesn’t address some important manifestations of SLE and it is scored dichotomously – for example, giving a similar score for thrombocytopenia when platelet count is reduced to 100,000 or to 10,000.
To compensate for these limitations, the current LLDAS definition also requires the Physician Global Assessment and other steps, including a review of medication and changes to treatment or clinical status since the previous visit.
“It is not easy to apply,” Dr. Assunção said.
The SLE-DAS is a continuous index involving 17 parameters (4 continuous: arthritis, proteinuria, thrombocytopenia, and leukopenia), assigning higher scores when a manifestation is more severe, and has manifestation information that SLEDAI-2K lacks (cardiopulmonary involvement, lupus enteritis, and hemolytic anemia).
In contrast, the LLDAS is defined as:
- A SLEDAI-2k score of 4 or less with no major organ involvement
- No new disease activity
- A physician global assessment of the patient of 1 or less on a 0-3 scale
- Maintenance on a prednisolone dosage of 7.5 mg/day or less
- Maintenance on a standard immunosuppressive regimen
A previous study validated the SLE-DAS (Ann Rheum Dis. 2019 Mar;78[3]:365-71), and another exploratory study identified a cutoff SLE-DAS value of 3.77 or lower for LDA with SLE-DAS (Ann Rheum Dis. 2019;78:411-2).
Her group compared LDA status as measured with LLDAS versus the SLE-DAS in a cross-sectional study of 292 consecutive patients at their hospital. LDA on the SLE-DAS was defined as a score 3.77 or lower and a prednisolone dose of 7.5 mg/day or less. A total of 85% of patients were in LDA with SLE-DAS and 83.9% with LLDAS, and the agreement between LLDAS and SLE-DAS LDA was very high (Cohen’s kappa coefficient test; kappa = 0.831; P < .01). Out of 292 patients, only 13 were classified differently by the two definitions, 8 of which were classified as LDA by SLE-DAS, and 5 by LLDAS. Overall, 87% of patients were women and had a mean age of nearly 49 years, with a mean disease duration of about 14 years.
Dr. Assunção feels that the SLE-DAS LDA should be sufficient to monitor disease activity without adding the Physician Global Assessment and other steps, which would make it easier to apply than LLDAS. The fact that it is based on a continuous index is also an important difference. “Especially for low disease activity, it’s very good to be able to define it with a continuous index, because you are not that bad, but not that good, you’re in the middle,” she said.
The study should be regarded as exploratory, she said, but the results were encouraging. “We got similar results, and it’s definitely easier to apply.” She can also personally attest that the new model is easier to use, since she personally collected data for LLDAS assignment. “I had to check this, and this, and this … [SLE-DAS] is easier.”
Future work from her group will aim at deriving and validating a more robust definition of LDA, which will again be compared with the current LLDAS definition.
Her colleagues have already developed and validated a definition for clinical remission using SLE-DAS, although those results have not yet been published. They hope to define activity states using SLE-DAS, including mild, moderate, and high disease activity.
The team has produced an online SLE-DAS calculator (http://sle-das.eu/) where clinicians can score the 17 parameters. “You just input the values and it gives a number reflecting disease activity. Using this definition of SLE-DAS LDA you only need that number and to verify that the prednisolone dose is equal to or inferior to 7.5 mg/day,” said Dr. Assunção.
The study received no funding. Dr. Assunção has no financial disclosures, but one coauthor reported receiving grant/research support from Pfizer and AbbVie and serving as a consultant to Pfizer, AbbVie, Roche, Lilly, and Novartis.
SOURCE: Assunção H et al. Ann Rheum Dis 2020;79[suppl 1]:60, Abstract OP0092.
An alternative disease activity index for patients with systemic lupus erythematosus called the SLE-DAS (Disease Activity Score) has shown similar results to the Lupus Low Disease Activity State (LLDAS) in classifying low disease activity but may be easier to potentially apply in daily clinical practice in treat-to-target strategies, according to research presented at the annual European Congress of Rheumatology, held online this year because of COVID-19.
A treat-to-target approach, in which therapies are adjusted and the patient monitored to achieve the desired endpoint, has been proposed for patients with SLE. Clinical remission is the ideal goal, followed by achieving low disease activity (LDA) when clinical remission is unattainable, the first author of the SLE-DAS study, Helena Assunção, MD, of the department of rheumatology at Centro Hospitalar e Universitário de Coimbra (Portugal), said in an interview prior to the presentation of the study at the e-congress.
But to conduct a treat-to-target approach in the clinical setting, clinicians must have reliable, user-friendly targets to assess a patient’s progress, she said. But that’s not available right now. Proposed definitions of LDA, such as the LLDAS, are based on the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K). This index doesn’t address some important manifestations of SLE and it is scored dichotomously – for example, giving a similar score for thrombocytopenia when platelet count is reduced to 100,000 or to 10,000.
To compensate for these limitations, the current LLDAS definition also requires the Physician Global Assessment and other steps, including a review of medication and changes to treatment or clinical status since the previous visit.
“It is not easy to apply,” Dr. Assunção said.
The SLE-DAS is a continuous index involving 17 parameters (4 continuous: arthritis, proteinuria, thrombocytopenia, and leukopenia), assigning higher scores when a manifestation is more severe, and has manifestation information that SLEDAI-2K lacks (cardiopulmonary involvement, lupus enteritis, and hemolytic anemia).
In contrast, the LLDAS is defined as:
- A SLEDAI-2k score of 4 or less with no major organ involvement
- No new disease activity
- A physician global assessment of the patient of 1 or less on a 0-3 scale
- Maintenance on a prednisolone dosage of 7.5 mg/day or less
- Maintenance on a standard immunosuppressive regimen
A previous study validated the SLE-DAS (Ann Rheum Dis. 2019 Mar;78[3]:365-71), and another exploratory study identified a cutoff SLE-DAS value of 3.77 or lower for LDA with SLE-DAS (Ann Rheum Dis. 2019;78:411-2).
Her group compared LDA status as measured with LLDAS versus the SLE-DAS in a cross-sectional study of 292 consecutive patients at their hospital. LDA on the SLE-DAS was defined as a score 3.77 or lower and a prednisolone dose of 7.5 mg/day or less. A total of 85% of patients were in LDA with SLE-DAS and 83.9% with LLDAS, and the agreement between LLDAS and SLE-DAS LDA was very high (Cohen’s kappa coefficient test; kappa = 0.831; P < .01). Out of 292 patients, only 13 were classified differently by the two definitions, 8 of which were classified as LDA by SLE-DAS, and 5 by LLDAS. Overall, 87% of patients were women and had a mean age of nearly 49 years, with a mean disease duration of about 14 years.
Dr. Assunção feels that the SLE-DAS LDA should be sufficient to monitor disease activity without adding the Physician Global Assessment and other steps, which would make it easier to apply than LLDAS. The fact that it is based on a continuous index is also an important difference. “Especially for low disease activity, it’s very good to be able to define it with a continuous index, because you are not that bad, but not that good, you’re in the middle,” she said.
The study should be regarded as exploratory, she said, but the results were encouraging. “We got similar results, and it’s definitely easier to apply.” She can also personally attest that the new model is easier to use, since she personally collected data for LLDAS assignment. “I had to check this, and this, and this … [SLE-DAS] is easier.”
Future work from her group will aim at deriving and validating a more robust definition of LDA, which will again be compared with the current LLDAS definition.
Her colleagues have already developed and validated a definition for clinical remission using SLE-DAS, although those results have not yet been published. They hope to define activity states using SLE-DAS, including mild, moderate, and high disease activity.
The team has produced an online SLE-DAS calculator (http://sle-das.eu/) where clinicians can score the 17 parameters. “You just input the values and it gives a number reflecting disease activity. Using this definition of SLE-DAS LDA you only need that number and to verify that the prednisolone dose is equal to or inferior to 7.5 mg/day,” said Dr. Assunção.
The study received no funding. Dr. Assunção has no financial disclosures, but one coauthor reported receiving grant/research support from Pfizer and AbbVie and serving as a consultant to Pfizer, AbbVie, Roche, Lilly, and Novartis.
SOURCE: Assunção H et al. Ann Rheum Dis 2020;79[suppl 1]:60, Abstract OP0092.
FROM EULAR 2020 E-CONGRESS
Sex-based disparities in liver allocation driven by organ size mismatch, MELD score
Addressing local supply constraints may be insufficient to improve poorer outcomes among women who need a liver transplant, based on a large retrospective analysis.
Sex-based disparities in liver allocation were more strongly associated with liver size mismatch and MELD (Model for End-stage Liver Disease) score than geographic factors, reported lead author Jayme E. Locke, MD, of the University of Alabama at Birmingham, and colleagues.
“Currently, the transplant community is considering geographic redistribution ... to redefine local organ supply by replacing donor service areas with fixed concentric circles around donor hospitals,” the investigators wrote in JAMA Surgery. “However, newly proposed geographic models rely on the same metric for medical urgency, the MELD score, and offer no solution for candidates with small body stature who may appear at the top of the match run yet are routinely skipped secondary to discrepancies in donor-recipient size.”
To further investigate the driving forces behind sex-based disparities, the investigators conducted the first national study of its kind, involving 81,357 adults who were wait-listed for liver transplant. Primary outcomes included deceased donor liver transplant and wait list mortality. Using multivariate regression models and inverse odds ratio weighting, the investigators determined proportions of disparity shared across MELD score, candidate anthropometric and liver measurements, and geographic location.
Compared with men, women were 14.4% less likely to receive a transplant, and 8.6% more likely to die on the wait list.
The only geographic factor significantly associated with the increased disparity between female sex and wait list mortality was organ procurement organization, which was associated with a 22% increase. The disparity between rates of transplant receipt was not linked with any geographic factors.
In contrast, MELD score accounted for increases in disparity of 10.3% and 50.1% for organ receipt and wait list mortality, respectively. Candidate anthropometric and liver measurements played an even greater role, raising disparity by 49.0% for organ receipt and 125.8% for wait list mortality.
“Size mismatch between the donor and intended recipient and incorrect assessments of liver disease severity were more strongly associated with the observed sex disparity in wait list mortality than local supply of organs,” the investigators wrote.
Dr. Locke and colleagues noted that ongoing debates about geographic disparity hinge upon the assumption that the MELD score accurately measures disease severity, despite known shortcomings, including reliance upon serum creatinine level, which is influenced by muscle mass and therefore overestimates kidney function in women, and sex-based differences in size, which the MELD score does not incorporate whatsoever.
As such, the investigators suggested that addressing issues with the MELD score and organ size mismatch should be part of a more comprehensive approach to fixing sex-based disparities among candidates for liver transplant.
“Although geographic factors matter, examining geographic access alone may be insufficient,” they concluded.
James F. Markmann, MD, PhD, chief of the division of transplantation at Massachusetts General Hospital, Boston, who has previously published research in support of geographic redistribution, said in an interview that the study by Dr. Locke and colleagues “highlights a well-known problem in the liver transplant field.”
“The cause of this disparity is nicely illustrated by Dr. Locke’s work, which shows multiple contributing factors,” Dr. Markmann said.
While Dr. Markmann agreed with Dr. Locke and colleagues’ proposal that estimated glomerular filtration rate, instead of creatinine, could be used to more accurately measure renal function across sexes, he suggested that the disparities uncovered by their analysis are more likely driven by body size than sex.
“A more impactful factor and one obvious to those performing transplants is that on average the smaller body habitus of females makes more organs unsuitable due to size mismatch,” Dr. Markmann said. “In general, it is technically much less of a barrier to put a small liver into a large patient, than a large liver in a small patient. But, the same disparity in access almost certainly applies to small males; unfortunately, the authors did not examine this point. If allocation changes are envisioned to gain greater fairness in organ access, at least for the recipient size issue, it should be a size issue and not a sex issue.”
Dr. Markmann went on to explain that steps are currently being taken to make liver access more equitable.
“As of February 4th of this year, a broader sharing program for deceased donor livers was implemented,” he said. “This will make more organs available to those in greatest need. It will also potentially increase the number of liver offers to sick patients with a small body habitus and will hopefully reduce the excess morbidity and mortality they suffer.”
According to Willscott E. Naugler, MD and Susan L. Orloff, MD, of Oregon Health & Science University, Portland, novel clinical strategies need to be reinforced with a broader mindset in order to close the gap between men and women.
“A change in the MELD score is unlikely to fix this problem,” they wrote in an accompanying JAMA Surgery editorial, “but it is not hard to think of solutions; one could imagine, for example, allowing women of small stature to access pediatric livers while ramping up liver splits to increase contributions to the pediatric pool.”
Dr. Naugler and Dr. Orloff went on to suggest that barriers to equity may be culturally insidious.
“It is likely that the same unconscious biases that lead us to pay women surgeons less account for the lack of will to make these simple changes,” they wrote. “Not mentioned are multiple sociocultural elements that favor men over women in organ transplant. ... These realities cannot be fixed with changes to the MELD score, and we must be mindful not to let such notions distract from the essential hard work of creating long-lasting cultural changes that underpin a true path forward.”
The investigators disclosed relationships with Sanofi, Hansa Medical, Natera, and others.
SOURCE: Locke JE et al. JAMA Surg. 2020 May 20. doi: 10.1001/jamasurg.2020.1129.
Addressing local supply constraints may be insufficient to improve poorer outcomes among women who need a liver transplant, based on a large retrospective analysis.
Sex-based disparities in liver allocation were more strongly associated with liver size mismatch and MELD (Model for End-stage Liver Disease) score than geographic factors, reported lead author Jayme E. Locke, MD, of the University of Alabama at Birmingham, and colleagues.
“Currently, the transplant community is considering geographic redistribution ... to redefine local organ supply by replacing donor service areas with fixed concentric circles around donor hospitals,” the investigators wrote in JAMA Surgery. “However, newly proposed geographic models rely on the same metric for medical urgency, the MELD score, and offer no solution for candidates with small body stature who may appear at the top of the match run yet are routinely skipped secondary to discrepancies in donor-recipient size.”
To further investigate the driving forces behind sex-based disparities, the investigators conducted the first national study of its kind, involving 81,357 adults who were wait-listed for liver transplant. Primary outcomes included deceased donor liver transplant and wait list mortality. Using multivariate regression models and inverse odds ratio weighting, the investigators determined proportions of disparity shared across MELD score, candidate anthropometric and liver measurements, and geographic location.
Compared with men, women were 14.4% less likely to receive a transplant, and 8.6% more likely to die on the wait list.
The only geographic factor significantly associated with the increased disparity between female sex and wait list mortality was organ procurement organization, which was associated with a 22% increase. The disparity between rates of transplant receipt was not linked with any geographic factors.
In contrast, MELD score accounted for increases in disparity of 10.3% and 50.1% for organ receipt and wait list mortality, respectively. Candidate anthropometric and liver measurements played an even greater role, raising disparity by 49.0% for organ receipt and 125.8% for wait list mortality.
“Size mismatch between the donor and intended recipient and incorrect assessments of liver disease severity were more strongly associated with the observed sex disparity in wait list mortality than local supply of organs,” the investigators wrote.
Dr. Locke and colleagues noted that ongoing debates about geographic disparity hinge upon the assumption that the MELD score accurately measures disease severity, despite known shortcomings, including reliance upon serum creatinine level, which is influenced by muscle mass and therefore overestimates kidney function in women, and sex-based differences in size, which the MELD score does not incorporate whatsoever.
As such, the investigators suggested that addressing issues with the MELD score and organ size mismatch should be part of a more comprehensive approach to fixing sex-based disparities among candidates for liver transplant.
“Although geographic factors matter, examining geographic access alone may be insufficient,” they concluded.
James F. Markmann, MD, PhD, chief of the division of transplantation at Massachusetts General Hospital, Boston, who has previously published research in support of geographic redistribution, said in an interview that the study by Dr. Locke and colleagues “highlights a well-known problem in the liver transplant field.”
“The cause of this disparity is nicely illustrated by Dr. Locke’s work, which shows multiple contributing factors,” Dr. Markmann said.
While Dr. Markmann agreed with Dr. Locke and colleagues’ proposal that estimated glomerular filtration rate, instead of creatinine, could be used to more accurately measure renal function across sexes, he suggested that the disparities uncovered by their analysis are more likely driven by body size than sex.
“A more impactful factor and one obvious to those performing transplants is that on average the smaller body habitus of females makes more organs unsuitable due to size mismatch,” Dr. Markmann said. “In general, it is technically much less of a barrier to put a small liver into a large patient, than a large liver in a small patient. But, the same disparity in access almost certainly applies to small males; unfortunately, the authors did not examine this point. If allocation changes are envisioned to gain greater fairness in organ access, at least for the recipient size issue, it should be a size issue and not a sex issue.”
Dr. Markmann went on to explain that steps are currently being taken to make liver access more equitable.
“As of February 4th of this year, a broader sharing program for deceased donor livers was implemented,” he said. “This will make more organs available to those in greatest need. It will also potentially increase the number of liver offers to sick patients with a small body habitus and will hopefully reduce the excess morbidity and mortality they suffer.”
According to Willscott E. Naugler, MD and Susan L. Orloff, MD, of Oregon Health & Science University, Portland, novel clinical strategies need to be reinforced with a broader mindset in order to close the gap between men and women.
“A change in the MELD score is unlikely to fix this problem,” they wrote in an accompanying JAMA Surgery editorial, “but it is not hard to think of solutions; one could imagine, for example, allowing women of small stature to access pediatric livers while ramping up liver splits to increase contributions to the pediatric pool.”
Dr. Naugler and Dr. Orloff went on to suggest that barriers to equity may be culturally insidious.
“It is likely that the same unconscious biases that lead us to pay women surgeons less account for the lack of will to make these simple changes,” they wrote. “Not mentioned are multiple sociocultural elements that favor men over women in organ transplant. ... These realities cannot be fixed with changes to the MELD score, and we must be mindful not to let such notions distract from the essential hard work of creating long-lasting cultural changes that underpin a true path forward.”
The investigators disclosed relationships with Sanofi, Hansa Medical, Natera, and others.
SOURCE: Locke JE et al. JAMA Surg. 2020 May 20. doi: 10.1001/jamasurg.2020.1129.
Addressing local supply constraints may be insufficient to improve poorer outcomes among women who need a liver transplant, based on a large retrospective analysis.
Sex-based disparities in liver allocation were more strongly associated with liver size mismatch and MELD (Model for End-stage Liver Disease) score than geographic factors, reported lead author Jayme E. Locke, MD, of the University of Alabama at Birmingham, and colleagues.
“Currently, the transplant community is considering geographic redistribution ... to redefine local organ supply by replacing donor service areas with fixed concentric circles around donor hospitals,” the investigators wrote in JAMA Surgery. “However, newly proposed geographic models rely on the same metric for medical urgency, the MELD score, and offer no solution for candidates with small body stature who may appear at the top of the match run yet are routinely skipped secondary to discrepancies in donor-recipient size.”
To further investigate the driving forces behind sex-based disparities, the investigators conducted the first national study of its kind, involving 81,357 adults who were wait-listed for liver transplant. Primary outcomes included deceased donor liver transplant and wait list mortality. Using multivariate regression models and inverse odds ratio weighting, the investigators determined proportions of disparity shared across MELD score, candidate anthropometric and liver measurements, and geographic location.
Compared with men, women were 14.4% less likely to receive a transplant, and 8.6% more likely to die on the wait list.
The only geographic factor significantly associated with the increased disparity between female sex and wait list mortality was organ procurement organization, which was associated with a 22% increase. The disparity between rates of transplant receipt was not linked with any geographic factors.
In contrast, MELD score accounted for increases in disparity of 10.3% and 50.1% for organ receipt and wait list mortality, respectively. Candidate anthropometric and liver measurements played an even greater role, raising disparity by 49.0% for organ receipt and 125.8% for wait list mortality.
“Size mismatch between the donor and intended recipient and incorrect assessments of liver disease severity were more strongly associated with the observed sex disparity in wait list mortality than local supply of organs,” the investigators wrote.
Dr. Locke and colleagues noted that ongoing debates about geographic disparity hinge upon the assumption that the MELD score accurately measures disease severity, despite known shortcomings, including reliance upon serum creatinine level, which is influenced by muscle mass and therefore overestimates kidney function in women, and sex-based differences in size, which the MELD score does not incorporate whatsoever.
As such, the investigators suggested that addressing issues with the MELD score and organ size mismatch should be part of a more comprehensive approach to fixing sex-based disparities among candidates for liver transplant.
“Although geographic factors matter, examining geographic access alone may be insufficient,” they concluded.
James F. Markmann, MD, PhD, chief of the division of transplantation at Massachusetts General Hospital, Boston, who has previously published research in support of geographic redistribution, said in an interview that the study by Dr. Locke and colleagues “highlights a well-known problem in the liver transplant field.”
“The cause of this disparity is nicely illustrated by Dr. Locke’s work, which shows multiple contributing factors,” Dr. Markmann said.
While Dr. Markmann agreed with Dr. Locke and colleagues’ proposal that estimated glomerular filtration rate, instead of creatinine, could be used to more accurately measure renal function across sexes, he suggested that the disparities uncovered by their analysis are more likely driven by body size than sex.
“A more impactful factor and one obvious to those performing transplants is that on average the smaller body habitus of females makes more organs unsuitable due to size mismatch,” Dr. Markmann said. “In general, it is technically much less of a barrier to put a small liver into a large patient, than a large liver in a small patient. But, the same disparity in access almost certainly applies to small males; unfortunately, the authors did not examine this point. If allocation changes are envisioned to gain greater fairness in organ access, at least for the recipient size issue, it should be a size issue and not a sex issue.”
Dr. Markmann went on to explain that steps are currently being taken to make liver access more equitable.
“As of February 4th of this year, a broader sharing program for deceased donor livers was implemented,” he said. “This will make more organs available to those in greatest need. It will also potentially increase the number of liver offers to sick patients with a small body habitus and will hopefully reduce the excess morbidity and mortality they suffer.”
According to Willscott E. Naugler, MD and Susan L. Orloff, MD, of Oregon Health & Science University, Portland, novel clinical strategies need to be reinforced with a broader mindset in order to close the gap between men and women.
“A change in the MELD score is unlikely to fix this problem,” they wrote in an accompanying JAMA Surgery editorial, “but it is not hard to think of solutions; one could imagine, for example, allowing women of small stature to access pediatric livers while ramping up liver splits to increase contributions to the pediatric pool.”
Dr. Naugler and Dr. Orloff went on to suggest that barriers to equity may be culturally insidious.
“It is likely that the same unconscious biases that lead us to pay women surgeons less account for the lack of will to make these simple changes,” they wrote. “Not mentioned are multiple sociocultural elements that favor men over women in organ transplant. ... These realities cannot be fixed with changes to the MELD score, and we must be mindful not to let such notions distract from the essential hard work of creating long-lasting cultural changes that underpin a true path forward.”
The investigators disclosed relationships with Sanofi, Hansa Medical, Natera, and others.
SOURCE: Locke JE et al. JAMA Surg. 2020 May 20. doi: 10.1001/jamasurg.2020.1129.
FROM JAMA SURGERY
Fracture risk higher for children with anxiety on benzodiazepines
a new study found, which offers further argument for caution with this class of drugs in young patients.
In research published in Pediatrics, Greta A. Bushnell, PhD, of Columbia University in New York and colleagues, looked at private insurance claims data including prescription records from 120,715 children aged 6-17 years diagnosed with an anxiety disorder and from 179,768 young adults aged 18-24 years also diagnosed with anxiety.
The investigators compared fracture incidence within 3 months of treatment initiation between the group prescribed benzodiazepines for anxiety and the group prescribed SSRIs. Subjects prescribed both classes of drugs were excluded from the analysis.
Of patients aged 6-17 years, 11% were prescribed benzodiazepines, with the remainder receiving SSRIs. Children on benzodiazepines saw 33 fractures per 1,000 person-years, compared with 25 of those on SSRIs, with an adjusted incidence rate ratio of 1.53. These were fractures in the upper and lower limbs.
Similar differences in fracture risk were not seen among the young adults in the study, of whom 32% were prescribed benzodiazepines and among whom fracture rates were low overall, 9 per 1,000 person-years in both medication groups.
Several SSRIs have been approved by the Food and Drug Administration to treat anxiety disorders in children, but benzodiazepines are used off label in youth. The drugs most commonly prescribed in the study were alprazolam and lorazepam, and 82% of the group in this study aged 6-17 years did not fill their prescriptions beyond 1 month.
In adults, benzodiazepine treatment has been shown to cause drowsiness, dizziness, and weakness, which can result in injury, and it also is associated with increased risk of car accidents, falls, and fractures. The higher fracture rate among children on benzodiazepine treatment seen in this study is similar to rates reported in studies of older adults, Dr. Bushnell and colleagues noted.
The researchers could not explain why the young adults in the study did not see a higher risk of fractures on benzodiazepines, compared with that among those taking SSRIs. They hypothesized that young adults are less active than children, with fewer opportunities for falls, and there were few fractures among the 18- to 24-year-old cohort in general.
David C. Rettew, MD, from the University of Vermont in Burlington, commented in an interview that, while there are plenty of reasons to be cautious about using benzodiazepines in youth, “fracture risk isn’t usually very prominent among them, so it is a nice reminder to have this on the radar screen.” Most clinicians, he said, already are quite wary of using benzodiazepines in children, which is suggested by the small proportion of children treated with them in this study.
“It seems quite possible that children and adolescents prescribed benzodiazepines are quite different clinically than the group prescribed SSRIs, despite the strong measures the study authors took to control for other variables between the two groups,” Dr. Rettew added. “I’d have to wonder if those clinical differences may be behind some of the fracture rate differences” seen in the study.
Dr. Bushnell and her colleagues acknowledged this among the study’s several limitations. “It is unclear how much unmeasured differences in psychiatric condition severity exist between youth initiating a benzodiazepine versus SSRI and how anxiety severity impacts fracture risk.” The researchers also noted that they could not measure use of the drugs beyond whether and when prescriptions were filled.
Dr. Bushnell and colleagues’ study was funded by the National Institute of Mental Health and by grants from the Agency for Healthcare Research and Quality, the Patient-Centered Outcomes Research Institute, and the National Institutes of Health. One of its coauthors disclosed financial relationships with several pharmaceutical manufacturers. Dr. Rettew said he had no relevant financial disclosures
SOURCE: Bushnell GA et al. Pediatrics. 2020 Jun. doi: 10.1542/peds.2019-3478.
a new study found, which offers further argument for caution with this class of drugs in young patients.
In research published in Pediatrics, Greta A. Bushnell, PhD, of Columbia University in New York and colleagues, looked at private insurance claims data including prescription records from 120,715 children aged 6-17 years diagnosed with an anxiety disorder and from 179,768 young adults aged 18-24 years also diagnosed with anxiety.
The investigators compared fracture incidence within 3 months of treatment initiation between the group prescribed benzodiazepines for anxiety and the group prescribed SSRIs. Subjects prescribed both classes of drugs were excluded from the analysis.
Of patients aged 6-17 years, 11% were prescribed benzodiazepines, with the remainder receiving SSRIs. Children on benzodiazepines saw 33 fractures per 1,000 person-years, compared with 25 of those on SSRIs, with an adjusted incidence rate ratio of 1.53. These were fractures in the upper and lower limbs.
Similar differences in fracture risk were not seen among the young adults in the study, of whom 32% were prescribed benzodiazepines and among whom fracture rates were low overall, 9 per 1,000 person-years in both medication groups.
Several SSRIs have been approved by the Food and Drug Administration to treat anxiety disorders in children, but benzodiazepines are used off label in youth. The drugs most commonly prescribed in the study were alprazolam and lorazepam, and 82% of the group in this study aged 6-17 years did not fill their prescriptions beyond 1 month.
In adults, benzodiazepine treatment has been shown to cause drowsiness, dizziness, and weakness, which can result in injury, and it also is associated with increased risk of car accidents, falls, and fractures. The higher fracture rate among children on benzodiazepine treatment seen in this study is similar to rates reported in studies of older adults, Dr. Bushnell and colleagues noted.
The researchers could not explain why the young adults in the study did not see a higher risk of fractures on benzodiazepines, compared with that among those taking SSRIs. They hypothesized that young adults are less active than children, with fewer opportunities for falls, and there were few fractures among the 18- to 24-year-old cohort in general.
David C. Rettew, MD, from the University of Vermont in Burlington, commented in an interview that, while there are plenty of reasons to be cautious about using benzodiazepines in youth, “fracture risk isn’t usually very prominent among them, so it is a nice reminder to have this on the radar screen.” Most clinicians, he said, already are quite wary of using benzodiazepines in children, which is suggested by the small proportion of children treated with them in this study.
“It seems quite possible that children and adolescents prescribed benzodiazepines are quite different clinically than the group prescribed SSRIs, despite the strong measures the study authors took to control for other variables between the two groups,” Dr. Rettew added. “I’d have to wonder if those clinical differences may be behind some of the fracture rate differences” seen in the study.
Dr. Bushnell and her colleagues acknowledged this among the study’s several limitations. “It is unclear how much unmeasured differences in psychiatric condition severity exist between youth initiating a benzodiazepine versus SSRI and how anxiety severity impacts fracture risk.” The researchers also noted that they could not measure use of the drugs beyond whether and when prescriptions were filled.
Dr. Bushnell and colleagues’ study was funded by the National Institute of Mental Health and by grants from the Agency for Healthcare Research and Quality, the Patient-Centered Outcomes Research Institute, and the National Institutes of Health. One of its coauthors disclosed financial relationships with several pharmaceutical manufacturers. Dr. Rettew said he had no relevant financial disclosures
SOURCE: Bushnell GA et al. Pediatrics. 2020 Jun. doi: 10.1542/peds.2019-3478.
a new study found, which offers further argument for caution with this class of drugs in young patients.
In research published in Pediatrics, Greta A. Bushnell, PhD, of Columbia University in New York and colleagues, looked at private insurance claims data including prescription records from 120,715 children aged 6-17 years diagnosed with an anxiety disorder and from 179,768 young adults aged 18-24 years also diagnosed with anxiety.
The investigators compared fracture incidence within 3 months of treatment initiation between the group prescribed benzodiazepines for anxiety and the group prescribed SSRIs. Subjects prescribed both classes of drugs were excluded from the analysis.
Of patients aged 6-17 years, 11% were prescribed benzodiazepines, with the remainder receiving SSRIs. Children on benzodiazepines saw 33 fractures per 1,000 person-years, compared with 25 of those on SSRIs, with an adjusted incidence rate ratio of 1.53. These were fractures in the upper and lower limbs.
Similar differences in fracture risk were not seen among the young adults in the study, of whom 32% were prescribed benzodiazepines and among whom fracture rates were low overall, 9 per 1,000 person-years in both medication groups.
Several SSRIs have been approved by the Food and Drug Administration to treat anxiety disorders in children, but benzodiazepines are used off label in youth. The drugs most commonly prescribed in the study were alprazolam and lorazepam, and 82% of the group in this study aged 6-17 years did not fill their prescriptions beyond 1 month.
In adults, benzodiazepine treatment has been shown to cause drowsiness, dizziness, and weakness, which can result in injury, and it also is associated with increased risk of car accidents, falls, and fractures. The higher fracture rate among children on benzodiazepine treatment seen in this study is similar to rates reported in studies of older adults, Dr. Bushnell and colleagues noted.
The researchers could not explain why the young adults in the study did not see a higher risk of fractures on benzodiazepines, compared with that among those taking SSRIs. They hypothesized that young adults are less active than children, with fewer opportunities for falls, and there were few fractures among the 18- to 24-year-old cohort in general.
David C. Rettew, MD, from the University of Vermont in Burlington, commented in an interview that, while there are plenty of reasons to be cautious about using benzodiazepines in youth, “fracture risk isn’t usually very prominent among them, so it is a nice reminder to have this on the radar screen.” Most clinicians, he said, already are quite wary of using benzodiazepines in children, which is suggested by the small proportion of children treated with them in this study.
“It seems quite possible that children and adolescents prescribed benzodiazepines are quite different clinically than the group prescribed SSRIs, despite the strong measures the study authors took to control for other variables between the two groups,” Dr. Rettew added. “I’d have to wonder if those clinical differences may be behind some of the fracture rate differences” seen in the study.
Dr. Bushnell and her colleagues acknowledged this among the study’s several limitations. “It is unclear how much unmeasured differences in psychiatric condition severity exist between youth initiating a benzodiazepine versus SSRI and how anxiety severity impacts fracture risk.” The researchers also noted that they could not measure use of the drugs beyond whether and when prescriptions were filled.
Dr. Bushnell and colleagues’ study was funded by the National Institute of Mental Health and by grants from the Agency for Healthcare Research and Quality, the Patient-Centered Outcomes Research Institute, and the National Institutes of Health. One of its coauthors disclosed financial relationships with several pharmaceutical manufacturers. Dr. Rettew said he had no relevant financial disclosures
SOURCE: Bushnell GA et al. Pediatrics. 2020 Jun. doi: 10.1542/peds.2019-3478.
FROM PEDIATRICS
Key clinical point: Children aged 6-17 years prescribed sedatives for anxiety saw a higher risk of fractures, compared with those on SSRIs.
Major finding: Children prescribed benzodiazepines for anxiety had 33 fractures per 1,000 person-years versus 25 among children prescribed SSRIs (adjusted incidence rate ratio, 1.53).
Study details: A retrospective cohort study using commercial insurance claims data from 120,715 children aged 6-17 years and 179,768 young adults ages 18-24 years from 2007 through 2016, all with anxiety diagnoses and prescribed either benzodiazepines or SSRIs.
Disclosures: Dr. Bushnell and colleagues’ study was funded by the National Institute of Mental Health, and grants from the Agency for Healthcare Research and Quality, the Patient-Centered Outcomes Research Institute, and the National Institutes of Health. One of its coauthors disclosed financial relationships with several pharmaceutical manufacturers.
Source: Bushnell GA et al. Pediatrics. 2020 Jun. doi: 10.1542/peds.2019-3478.