User login
High-dose tafamidis boosts survival in transthyretin amyloidosis cardiomyopathy
Treatment with oral tafamidis at 80 mg/day provided a significantly greater survival benefit than dosing at 20 mg/day in patients with transthyretin amyloid cardiomyopathy in the long-term extension of the landmark ATTR-ACT trial, Thibaud Damy, MD, PhD, reported at the European Society of Cardiology Heart Failure Discoveries virtual meeting.
Moreover, the superior survival benefit achieved by taking four 20-mg capsules of tafamidis (Vyndaqel) once daily – or its more convenient once-daily, single-capsule, 61-mg bioequivalent formulation marketed as Vyndamax – came at no cost in terms of side effects and toxicity, compared with low-dose therapy for this progressive multisystem disease, according to Dr. Damy, professor of cardiology at the University of Paris and head of the French National Referral Center for Cardiac Amyloidosis at Henri Mondor University Hospital, Créteil, France.
“There are no side effects with tafamidis,” he said. “It doesn’t act on any receptors, it just acts on the formation of amyloid fibrils, so there are no side effects at whatever dosage is used. And in ATTR-ACT there was actually a trend towards increased side effects in the placebo group because the amyloidosis is everywhere, so by decreasing the amyloidosis process you improve not only the heart but all the organs, and the patient has a better quality of life.”
ATTR-ACT (Transthyretin Amyloidosis Cardiomyopathy Clinical Trial) was a phase 3, double-blind study in which 441 patients with transthyretin amyloidosis cardiomyopathy (TAC) in 13 countries were randomized to tafamidis at either 80 mg or 20 mg per day or placebo and followed prospectively for 30 months. At 30 months, all-cause mortality was 29.5% in patients who received tafamidis, compared with 42.9% in controls, for a statistically significant and clinically important 30% relative risk reduction, establishing tafamidis as the first disease-modifying therapy for this disease (N Engl J Med. 2018 Sep 13;379[11]:1007-16).
Patients in the 80-mg group had a 20% reduction in the risk of death, compared with the 20-mg group, at 30 months in an analysis adjusted for baseline age, 6-minute walk distance, and N-terminal pro-B-type natriuretic peptide, all of which are known to impact survival in TAC. This between-group survival difference wasn’t statistically significant, providing one impetus for the subsequent long-term extension study, in which patients remained on their original dose of tafamidis, and the controls who’d been on placebo for 30 months were randomized 2:1 to tafamidis at 80 mg or 20 mg per day.
The primary endpoint in the long-term extension was a composite of all-cause mortality, heart transplantation, or implantation of a ventricular assist device. At a median follow-up of 39 months since ATTR-ACT began, the high-dose tafamidis group had an adjusted 33% reduction in the risk of this endpoint, compared with patients on 20 mg per day, a difference that barely missed statistical significance. At that point, everyone in the long-term extension was switched to the once-daily 61-mg formulation of tafamidis free acid, which is bioequivalent to four 20-mg capsules of tafamidis.
Dr. Damy’s key message: At a median of 51 months of follow-up, the group originally on 80 mg of tafamidis displayed a highly significant adjusted 43% reduction in risk of the composite endpoint, compared with those who had been on 20 mg per day.
Session chair Petar M. Seferovic, MD, PhD, pronounced the ATTR-ACT trial and its long-term extension “a breakthrough advancement.”
“This is the first time in human medical history that we have a drug which improves the long-term outcome, including survival, in patients with this form of hypertrophic cardiomyopathy. So this is extremely important. It’s one of the major steps forward in the treatment of patients with myocardial disease,” said Dr. Seferovic, president of the European Society of Cardiology Heart Failure Association and professor of internal medicine at the University of Belgrade, Serbia.
Discussant Loreena Hill, PhD, of Queen’s University in Belfast, Northern Ireland, observed that TAC is a devastating disease with a formidable symptom burden and an average survival of just 2-5 years after diagnosis.
“It is often underdiagnosed, and yet it is estimated to account for up to 13% of patients with heart failure and preserved ejection fraction,” she said, adding that she considers the long-term extension results “extremely positive.”
Nailing down the prevalence of hereditary TAC: the DISCOVERY study
TAC occurs when transthyretin, a transport protein, becomes destabilized and misfolds, promoting deposition of amyloid fibrils in the myocardium and elsewhere. In the heart, the result is progressive ventricular wall thickening and stiffness, manifest as restrictive cardiomyopathy and progressive nonischemic heart failure. The cause of transthyretin destabilization can be either autosomal dominant inheritance of any of more than 100 pathogenic mutations in the transthyretin gene identified to date or a spontaneous wild-type protein.
Dr. Damy was a coinvestigator in the recently published multicenter DISCOVERY study, in which 1,001 patients with clinically suspected cardiac amyloidosis, the great majority of them from the United States, were screened for pathogenic transthyretin genetic mutations. The overall prevalence of such mutations was 8% in the American patients, with the Val122Ile mutation being identified in 11% of African Americans (Amyloid. 2020 May 26;1-8).
The prevalence of wild-type amyloidosis causing TAC hasn’t yet been studied with anything approaching the rigor of DISCOVERY, but the available evidence suggests the wild-type version is roughly as common as the hereditary forms.
Although DISCOVERY and other studies indicate that TAC is far more common than generally realized, Pfizer has priced Vyndaqel and Vyndamax as though TAC is a rare disease, with a U.S. list price of around $225,000 per year.
“Obviously, the cost will go down over time,” Dr. Seferovic predicted.
Diagnosing TAC
Audience members mostly wanted to know how to identify individuals with TAC who are buried within the huge population of patients with heart failure with preserved ejection fraction. Dr. Damy said it’s actually a simple matter using a screening framework developed by an 11-member TAC expert panel on which he served. A definitive diagnosis can usually be achieved noninvasively at a low cost using bone scintigraphy, he added.
The panel recommended screening via bone scintigraphy in patients with an increased left ventricular wall thickness of 14 mm or more in men over age 65 and women older than 70 who either have heart failure or red flag symptoms.
These red flags for TAC include an echocardiographic finding of reduced longitudinal strain with relative apical sparing, a discrepancy between left ventricular wall thickness on imaging and normal or low-normal voltages on a standard 12-lead ECG, diffuse gadolinium enhancement or marked extracellular volume expansion on cardiac magnetic resonance imaging, a history of bilateral carpal tunnel syndrome, symptoms of polyneuropathy, and mildly increased serum troponin levels on multiple occasions (JACC Heart Fail. 2019 Aug;7[8]:709-16).
Dr. Damy reported receiving institutional research grant support from Pfizer, the study sponsor, and serving on a scientific advisory board for the company.
Treatment with oral tafamidis at 80 mg/day provided a significantly greater survival benefit than dosing at 20 mg/day in patients with transthyretin amyloid cardiomyopathy in the long-term extension of the landmark ATTR-ACT trial, Thibaud Damy, MD, PhD, reported at the European Society of Cardiology Heart Failure Discoveries virtual meeting.
Moreover, the superior survival benefit achieved by taking four 20-mg capsules of tafamidis (Vyndaqel) once daily – or its more convenient once-daily, single-capsule, 61-mg bioequivalent formulation marketed as Vyndamax – came at no cost in terms of side effects and toxicity, compared with low-dose therapy for this progressive multisystem disease, according to Dr. Damy, professor of cardiology at the University of Paris and head of the French National Referral Center for Cardiac Amyloidosis at Henri Mondor University Hospital, Créteil, France.
“There are no side effects with tafamidis,” he said. “It doesn’t act on any receptors, it just acts on the formation of amyloid fibrils, so there are no side effects at whatever dosage is used. And in ATTR-ACT there was actually a trend towards increased side effects in the placebo group because the amyloidosis is everywhere, so by decreasing the amyloidosis process you improve not only the heart but all the organs, and the patient has a better quality of life.”
ATTR-ACT (Transthyretin Amyloidosis Cardiomyopathy Clinical Trial) was a phase 3, double-blind study in which 441 patients with transthyretin amyloidosis cardiomyopathy (TAC) in 13 countries were randomized to tafamidis at either 80 mg or 20 mg per day or placebo and followed prospectively for 30 months. At 30 months, all-cause mortality was 29.5% in patients who received tafamidis, compared with 42.9% in controls, for a statistically significant and clinically important 30% relative risk reduction, establishing tafamidis as the first disease-modifying therapy for this disease (N Engl J Med. 2018 Sep 13;379[11]:1007-16).
Patients in the 80-mg group had a 20% reduction in the risk of death, compared with the 20-mg group, at 30 months in an analysis adjusted for baseline age, 6-minute walk distance, and N-terminal pro-B-type natriuretic peptide, all of which are known to impact survival in TAC. This between-group survival difference wasn’t statistically significant, providing one impetus for the subsequent long-term extension study, in which patients remained on their original dose of tafamidis, and the controls who’d been on placebo for 30 months were randomized 2:1 to tafamidis at 80 mg or 20 mg per day.
The primary endpoint in the long-term extension was a composite of all-cause mortality, heart transplantation, or implantation of a ventricular assist device. At a median follow-up of 39 months since ATTR-ACT began, the high-dose tafamidis group had an adjusted 33% reduction in the risk of this endpoint, compared with patients on 20 mg per day, a difference that barely missed statistical significance. At that point, everyone in the long-term extension was switched to the once-daily 61-mg formulation of tafamidis free acid, which is bioequivalent to four 20-mg capsules of tafamidis.
Dr. Damy’s key message: At a median of 51 months of follow-up, the group originally on 80 mg of tafamidis displayed a highly significant adjusted 43% reduction in risk of the composite endpoint, compared with those who had been on 20 mg per day.
Session chair Petar M. Seferovic, MD, PhD, pronounced the ATTR-ACT trial and its long-term extension “a breakthrough advancement.”
“This is the first time in human medical history that we have a drug which improves the long-term outcome, including survival, in patients with this form of hypertrophic cardiomyopathy. So this is extremely important. It’s one of the major steps forward in the treatment of patients with myocardial disease,” said Dr. Seferovic, president of the European Society of Cardiology Heart Failure Association and professor of internal medicine at the University of Belgrade, Serbia.
Discussant Loreena Hill, PhD, of Queen’s University in Belfast, Northern Ireland, observed that TAC is a devastating disease with a formidable symptom burden and an average survival of just 2-5 years after diagnosis.
“It is often underdiagnosed, and yet it is estimated to account for up to 13% of patients with heart failure and preserved ejection fraction,” she said, adding that she considers the long-term extension results “extremely positive.”
Nailing down the prevalence of hereditary TAC: the DISCOVERY study
TAC occurs when transthyretin, a transport protein, becomes destabilized and misfolds, promoting deposition of amyloid fibrils in the myocardium and elsewhere. In the heart, the result is progressive ventricular wall thickening and stiffness, manifest as restrictive cardiomyopathy and progressive nonischemic heart failure. The cause of transthyretin destabilization can be either autosomal dominant inheritance of any of more than 100 pathogenic mutations in the transthyretin gene identified to date or a spontaneous wild-type protein.
Dr. Damy was a coinvestigator in the recently published multicenter DISCOVERY study, in which 1,001 patients with clinically suspected cardiac amyloidosis, the great majority of them from the United States, were screened for pathogenic transthyretin genetic mutations. The overall prevalence of such mutations was 8% in the American patients, with the Val122Ile mutation being identified in 11% of African Americans (Amyloid. 2020 May 26;1-8).
The prevalence of wild-type amyloidosis causing TAC hasn’t yet been studied with anything approaching the rigor of DISCOVERY, but the available evidence suggests the wild-type version is roughly as common as the hereditary forms.
Although DISCOVERY and other studies indicate that TAC is far more common than generally realized, Pfizer has priced Vyndaqel and Vyndamax as though TAC is a rare disease, with a U.S. list price of around $225,000 per year.
“Obviously, the cost will go down over time,” Dr. Seferovic predicted.
Diagnosing TAC
Audience members mostly wanted to know how to identify individuals with TAC who are buried within the huge population of patients with heart failure with preserved ejection fraction. Dr. Damy said it’s actually a simple matter using a screening framework developed by an 11-member TAC expert panel on which he served. A definitive diagnosis can usually be achieved noninvasively at a low cost using bone scintigraphy, he added.
The panel recommended screening via bone scintigraphy in patients with an increased left ventricular wall thickness of 14 mm or more in men over age 65 and women older than 70 who either have heart failure or red flag symptoms.
These red flags for TAC include an echocardiographic finding of reduced longitudinal strain with relative apical sparing, a discrepancy between left ventricular wall thickness on imaging and normal or low-normal voltages on a standard 12-lead ECG, diffuse gadolinium enhancement or marked extracellular volume expansion on cardiac magnetic resonance imaging, a history of bilateral carpal tunnel syndrome, symptoms of polyneuropathy, and mildly increased serum troponin levels on multiple occasions (JACC Heart Fail. 2019 Aug;7[8]:709-16).
Dr. Damy reported receiving institutional research grant support from Pfizer, the study sponsor, and serving on a scientific advisory board for the company.
Treatment with oral tafamidis at 80 mg/day provided a significantly greater survival benefit than dosing at 20 mg/day in patients with transthyretin amyloid cardiomyopathy in the long-term extension of the landmark ATTR-ACT trial, Thibaud Damy, MD, PhD, reported at the European Society of Cardiology Heart Failure Discoveries virtual meeting.
Moreover, the superior survival benefit achieved by taking four 20-mg capsules of tafamidis (Vyndaqel) once daily – or its more convenient once-daily, single-capsule, 61-mg bioequivalent formulation marketed as Vyndamax – came at no cost in terms of side effects and toxicity, compared with low-dose therapy for this progressive multisystem disease, according to Dr. Damy, professor of cardiology at the University of Paris and head of the French National Referral Center for Cardiac Amyloidosis at Henri Mondor University Hospital, Créteil, France.
“There are no side effects with tafamidis,” he said. “It doesn’t act on any receptors, it just acts on the formation of amyloid fibrils, so there are no side effects at whatever dosage is used. And in ATTR-ACT there was actually a trend towards increased side effects in the placebo group because the amyloidosis is everywhere, so by decreasing the amyloidosis process you improve not only the heart but all the organs, and the patient has a better quality of life.”
ATTR-ACT (Transthyretin Amyloidosis Cardiomyopathy Clinical Trial) was a phase 3, double-blind study in which 441 patients with transthyretin amyloidosis cardiomyopathy (TAC) in 13 countries were randomized to tafamidis at either 80 mg or 20 mg per day or placebo and followed prospectively for 30 months. At 30 months, all-cause mortality was 29.5% in patients who received tafamidis, compared with 42.9% in controls, for a statistically significant and clinically important 30% relative risk reduction, establishing tafamidis as the first disease-modifying therapy for this disease (N Engl J Med. 2018 Sep 13;379[11]:1007-16).
Patients in the 80-mg group had a 20% reduction in the risk of death, compared with the 20-mg group, at 30 months in an analysis adjusted for baseline age, 6-minute walk distance, and N-terminal pro-B-type natriuretic peptide, all of which are known to impact survival in TAC. This between-group survival difference wasn’t statistically significant, providing one impetus for the subsequent long-term extension study, in which patients remained on their original dose of tafamidis, and the controls who’d been on placebo for 30 months were randomized 2:1 to tafamidis at 80 mg or 20 mg per day.
The primary endpoint in the long-term extension was a composite of all-cause mortality, heart transplantation, or implantation of a ventricular assist device. At a median follow-up of 39 months since ATTR-ACT began, the high-dose tafamidis group had an adjusted 33% reduction in the risk of this endpoint, compared with patients on 20 mg per day, a difference that barely missed statistical significance. At that point, everyone in the long-term extension was switched to the once-daily 61-mg formulation of tafamidis free acid, which is bioequivalent to four 20-mg capsules of tafamidis.
Dr. Damy’s key message: At a median of 51 months of follow-up, the group originally on 80 mg of tafamidis displayed a highly significant adjusted 43% reduction in risk of the composite endpoint, compared with those who had been on 20 mg per day.
Session chair Petar M. Seferovic, MD, PhD, pronounced the ATTR-ACT trial and its long-term extension “a breakthrough advancement.”
“This is the first time in human medical history that we have a drug which improves the long-term outcome, including survival, in patients with this form of hypertrophic cardiomyopathy. So this is extremely important. It’s one of the major steps forward in the treatment of patients with myocardial disease,” said Dr. Seferovic, president of the European Society of Cardiology Heart Failure Association and professor of internal medicine at the University of Belgrade, Serbia.
Discussant Loreena Hill, PhD, of Queen’s University in Belfast, Northern Ireland, observed that TAC is a devastating disease with a formidable symptom burden and an average survival of just 2-5 years after diagnosis.
“It is often underdiagnosed, and yet it is estimated to account for up to 13% of patients with heart failure and preserved ejection fraction,” she said, adding that she considers the long-term extension results “extremely positive.”
Nailing down the prevalence of hereditary TAC: the DISCOVERY study
TAC occurs when transthyretin, a transport protein, becomes destabilized and misfolds, promoting deposition of amyloid fibrils in the myocardium and elsewhere. In the heart, the result is progressive ventricular wall thickening and stiffness, manifest as restrictive cardiomyopathy and progressive nonischemic heart failure. The cause of transthyretin destabilization can be either autosomal dominant inheritance of any of more than 100 pathogenic mutations in the transthyretin gene identified to date or a spontaneous wild-type protein.
Dr. Damy was a coinvestigator in the recently published multicenter DISCOVERY study, in which 1,001 patients with clinically suspected cardiac amyloidosis, the great majority of them from the United States, were screened for pathogenic transthyretin genetic mutations. The overall prevalence of such mutations was 8% in the American patients, with the Val122Ile mutation being identified in 11% of African Americans (Amyloid. 2020 May 26;1-8).
The prevalence of wild-type amyloidosis causing TAC hasn’t yet been studied with anything approaching the rigor of DISCOVERY, but the available evidence suggests the wild-type version is roughly as common as the hereditary forms.
Although DISCOVERY and other studies indicate that TAC is far more common than generally realized, Pfizer has priced Vyndaqel and Vyndamax as though TAC is a rare disease, with a U.S. list price of around $225,000 per year.
“Obviously, the cost will go down over time,” Dr. Seferovic predicted.
Diagnosing TAC
Audience members mostly wanted to know how to identify individuals with TAC who are buried within the huge population of patients with heart failure with preserved ejection fraction. Dr. Damy said it’s actually a simple matter using a screening framework developed by an 11-member TAC expert panel on which he served. A definitive diagnosis can usually be achieved noninvasively at a low cost using bone scintigraphy, he added.
The panel recommended screening via bone scintigraphy in patients with an increased left ventricular wall thickness of 14 mm or more in men over age 65 and women older than 70 who either have heart failure or red flag symptoms.
These red flags for TAC include an echocardiographic finding of reduced longitudinal strain with relative apical sparing, a discrepancy between left ventricular wall thickness on imaging and normal or low-normal voltages on a standard 12-lead ECG, diffuse gadolinium enhancement or marked extracellular volume expansion on cardiac magnetic resonance imaging, a history of bilateral carpal tunnel syndrome, symptoms of polyneuropathy, and mildly increased serum troponin levels on multiple occasions (JACC Heart Fail. 2019 Aug;7[8]:709-16).
Dr. Damy reported receiving institutional research grant support from Pfizer, the study sponsor, and serving on a scientific advisory board for the company.
FROM ESC HEART FAILURE 2020
Painful foot or ankle? Don't overlook these 5 injuries
Foot and ankle injuries are among the most common conditions evaluated at primary care visits; the differential diagnosis of such injury is broad.1 Although many of these injuries are easily identified on imaging studies, a number of subtle, yet important, conditions can be easily missed, especially if you do not routinely encounter them. Given that broad differential, a high degree of suspicion is required to make an accurate diagnosis, which allows appropriate treatment within a reasonable time frame and minimizes the risk of long-term morbidity.
This article outlines the diagnosis and initial management of 5 important, yet often elusive, types of foot and ankle conditions: Achilles tendon rupture, injury to the syndesmosis, ankle fracture, Lisfranc injury, and proximal fracture of the fifth metatarsal.
Achilles tendon rupture
The Achilles tendon is the most frequently ruptured tendon in the body (approximately 20% of all large-tendon injuries)2; as many as 25% of cases are initially misdiagnosed.3
Presentation. Patients frequently present with pain at the Achilles tendon—2 to 6 cm above the insertion into the calcaneus—and an inability to fully bear weight.4,5 A small percentage of patients are able to ambulate on the affected side, albeit with minor pain, which likely contributes to the rate of missed diagnosis. Absence of difficulty bearing weight is due to the presence of secondary plantar flexors, which can compensate for loss of chief plantar flexor function by the Achilles tendon.2
Examination of a patient with an Achilles tendon rupture typically reveals edema, bruising, and a palpable gap within the tendon, 2 to 6 cm proximal to insertion.3,4 The Thompson test—squeezing the calf with the patient prone and the knee on the affected side flexed—can aid in diagnosis. When the Achilles tendon is intact, plantar flexion occurs at the ankle; when the tendon is ruptured, plantar flexion is absent.5 The test can be modified when examining a patient who is unable to lie prone by having them rest the flexed knee on a chair while standing on the unaffected leg.
A diagnosis of Achilles tendon rupture is supported when at least 2 of the following conditions are met4,5:
- positive Thompson test
- decreased strength during plantar flexion of the ankle
- palpable gap or pain at the typical location (2-6 cm above insertion)
- increased passive ankle dorsiflexion upon gentle ranging of the ankle joint.
Imaging has a limited role in the diagnosis of Achilles tendon rupture; because the findings of the physical examination are reliable, reserve x-rays for cases in which the diagnosis remains uncertain after examination.2 Consider ordering plain x-rays to rule out an avulsion fracture at the insertion of the Achilles tendon; ultrasonography or magnetic resonance imaging (MRI) might assist you in detecting the rupture proper, along with the location of the tear for surgical planning, if surgery is deemed necessary by an orthopedic surgeon.3-5
Continue to: Management
Management. Some degree of controversy surrounds preferred treatment of Achilles tendon rupture, although available evidence demonstrates that these injuries can be effectively managed by surgical repair or nonoperative treatment, as outcomes are comparable.3,5 Operative management tends to reduce the risk of repeat rupture, compared to nonoperative treatment; however, the potential for surgical complications, including wound infection, sensory disturbance, and adhesions favors nonoperative treatment.3,4,6
Nonoperative treatment consists of referral to a functional rehabilitation program, without which outcomes are, on the whole, less favorable than with surgery.3,6 Surgery is preferred if functional rehabilitation is unavailable, 6 months of conservative management fails, or there is avulsion injury.3,4,6
Injury to the syndesmosis
A complex of ligaments that provide dynamic stability to the ankle joint, the tibiofibular syndesmosis comprises:
- the anterior inferior tibiofibular ligament
- the posterior inferior tibiofibular ligament
- the inferior transverse tibiofibular ligament
- the interosseous membrane.
These structures are further supported by the deltoid ligament.7,8
Commonly referred to as a “high ankle sprain,” a syndesmotic injury is present in as many as 20% of ankle fractures and 5% to 10% of ankle sprains. Injury typically results from external rotation with hyperdorsiflexion of the ankle. Recovery is typically prolonged (ie, twice as long as recovery from a lateral ankle sprain). The diagnosis is missed in as many as 20% of patients; failure to recognize and treat syndesmotic instability appropriately can lead to posttraumatic arthritis.7,9
Continue to: Presentation
Presentation. Patients generally present with ankle pain, swelling, instability, pain when walking on uneven terrain, and pain upon push-off.9
Examination reveals reduced passive ankle dorsiflexion and tenderness upon palpation of individual ligaments. Several clinical tests have been described to aid in detecting this often-elusive diagnosis7,9,10,11:
- Squeeze test. The patient sits with the knee on the affected side bent at a 90° degree angle while the examiner applies compression, with one or both hands, to the tibia and fibula at midcalf. The test is positive when pain is elicited at the level of the syndesmosis just above the ankle joint.9,11
- External rotation test. External rotation of the foot and ankle relative to the tibia reproduces pain.
- Crossed leg test. The affected ankle is crossed over the opposite knee in a figure-4 position. The test is positive when pain is elicited at the syndesmosis.10
- Cotton test. The proximal lower leg is steadied with 1 hand and the plantar heel grasped with the other hand. Pain when the heel is externally rotated (and radiographic widening of the syndesmosis under fluoroscopy) signal syndesmotic instability.
- Fibular translation test. When anterior or posterior drawer force is applied to the fibula, pain and increased translation of the fibula (compared to the contralateral side) suggest instability.
With the Cotton and fibular translation tests, interexaminer technique is more variable and findings are less reproducible.8 Taken alone, none of the above-listed tests are diagnostic; they can, however, assist in making a diagnosis of an injury to the syndesmosis.11
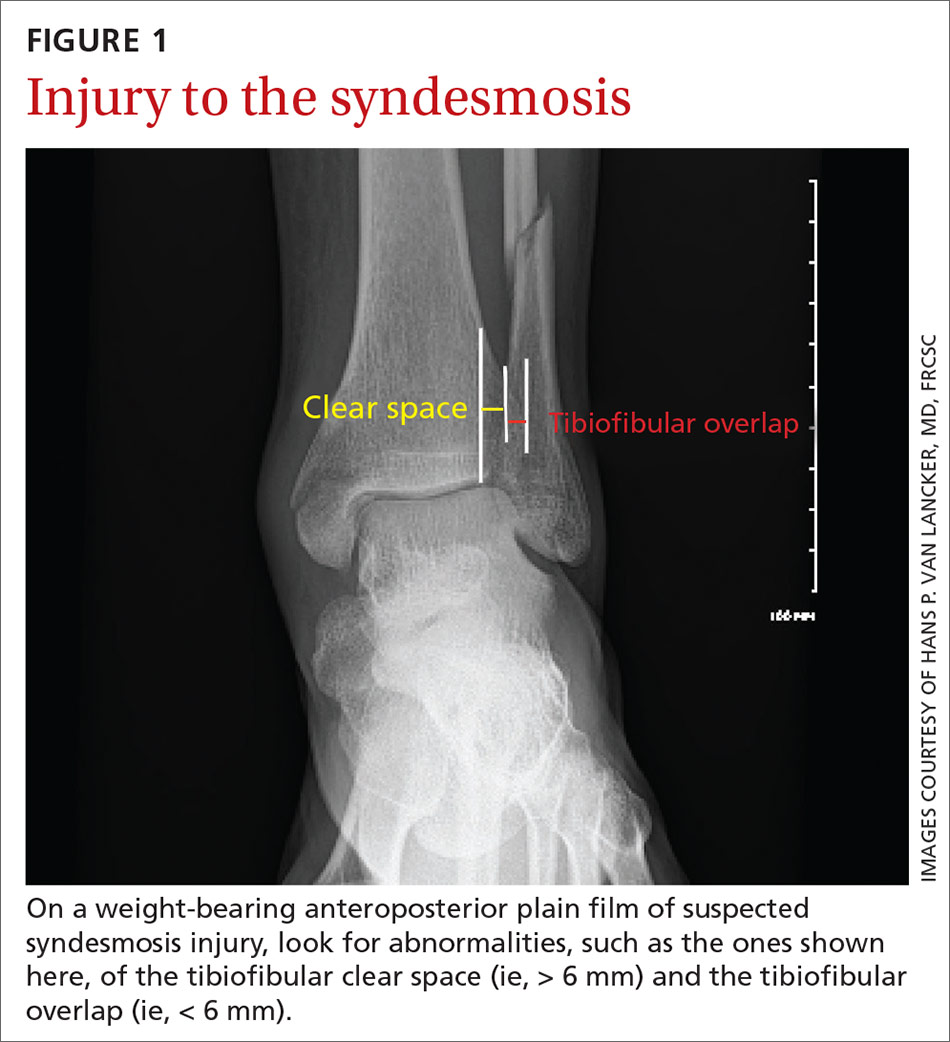
Imaging typically involves anteroposterior [AP], lateral, and mortise plain films of the ankle and weight-bearing AP and lateral views of the tibia and fibula.9 Important measures on weight-bearing AP x-rays are the tibiofibular clear space (abnormal, > 6 mm) and the tibiofibular overlap (abnormal, < 6 mm) (both abnormalities shown in FIGURE 1). Comparing films of the affected ankle with views of the contralateral ankle is often useful.
Management of syndesmotic injuries depends on degree of disruption:
- Grade 1 injury is a sprain without diastasis on imaging. Management is conservative, with immobilization in a splint or boot for 1 to 3 weeks, followed by functional rehabilitation over 3 to 6 weeks.10
- Grade 2 injury is demonstrated by diastasis on a stress radiograph. Although evidence to guide successful identification of a grade 2 injury is lacking, it is clinically important to make that identification because these injuries might require surgical intervention, due to instability. Because the diagnosis of this injury can be challenging in primary care, high clinical suspicion of a grade 2 injury makes it appropriate to defer further evaluation to an orthopedic surgeon. On the other hand, if suspicion of a grade 2 injury is low, a trial of conservative management, with weekly clinical assessment, can be considered. A diagnosis of grade 2 injury can be inferred when a patient is unable to perform a single-leg hop after 3 weeks of immobilization; referral to an orthopedic surgeon is then indicated.12
- Grade 3 injury is frank separation at the distal tibiofibular joint that is detectable on a routine plain film. Management—surgical intervention to address instability—is often provided concurrently with the treatment for a Danis-Weber B or C fracture, which tends to coexist with grade 3 syndesmotic injury. (The Danis-Weber A–B–C classification of lateral ankle fracture will be discussed in a bit.)
Continue to: Ankle fracture
Ankle fracture
Fracture of the ankle joint is among the more common fractures in adults, comprising 10% of all fractures.13,14 The ankle joint is defined as the junction of 3 bony structures: (1) the distal ends of the tibia and fibula and (2) the trochlea of the talus, all stabilized by (3) the collateral ligament complex. Appropriate diagnosis and timely intervention are needed to prevent long-term posttraumatic joint degeneration.
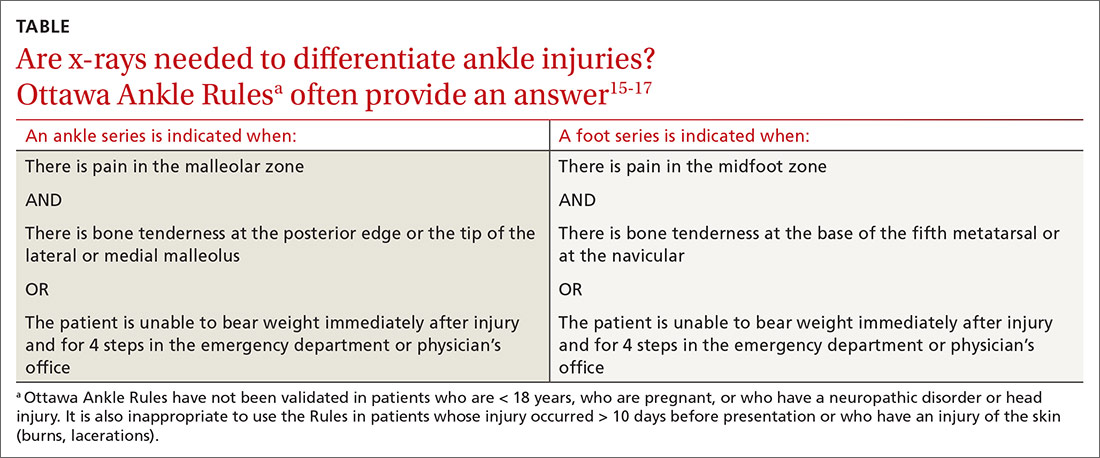
Presentation, examination, and imaging. In addition to difficulty bearing (or inability to bear) weight, patients with suspected ankle fracture can present with tenderness or pain, swelling (generally, the more severe the injury, the more severe the swelling, although this finding is time-dependent), and ecchymosis. However, distinguishing fracture from a ligamentous injury is often difficult by physical examination alone; the evidence-based Ottawa Ankle Rules can guide determination of the need for radiographic imaging, although this tool is less reliable in certain patient populations (TABLE15-17).13,15-17
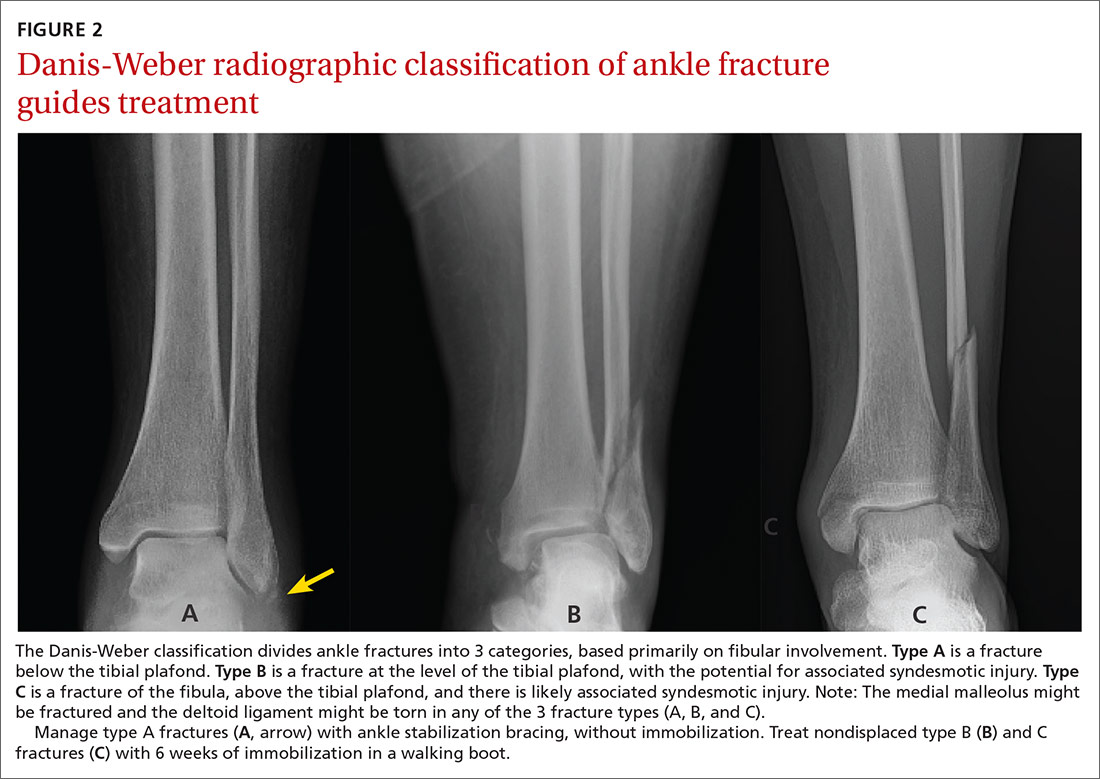
Management. A widely used classification system for guiding ankle fracture management is the Danis-Weber classification (FIGURE 2). In this scheme, type A fractures (distal to the level of the tibial plafond) are managed with ankle stabilization bracing without immobilization. Nondisplaced type B and C fractures (at the level of the tibial plafond and proximal to it, respectively) should be treated with 6 weeks of immobilization in a walking boot; close follow-up within 1 week of injury is recommended to ensure that no displacement of fragments has occurred. Type B and C fractures need to be followed until bony union is achieved. If there is radiologic evidence of a fracture line after 3 months, referral to an orthopedic surgeon is indicated for management of delayed union.
Common indications for referral to Orthopedics for surgical intervention of ankle fracture include open fracture, bimalleolar and trimalleolar fracture, posterior malleolar fracture, medial malleolar displacement > 2 mm, and lateral malleolar displacement > 3 mm.18
Special concern: Talar fracture. Although talar fracture is rare, the injury is important to detect because a limited blood supply places fragments at risk of avascular necrosis.19 Talus fracture is frequently confused with ankle sprain because initial x-rays are not always revelatory.20 A high index of suspicion is required to make the diagnosis, which should be suspected in high-energy injuries that result in pain and swelling of the ankle accompanied by difficulty weight-bearing, severely reduced range of motion, and tenderness to palpation at different areas of the talus.1 Computed tomography (CT) or MRI might be necessary to detect a talar fracture if initial x-rays are negative. A low threshold for surgical management of talar fracture means that referral to Orthopedics is indicated once this injury is diagnosed.21
Continue to: Other frequently missed types of ankle fracture
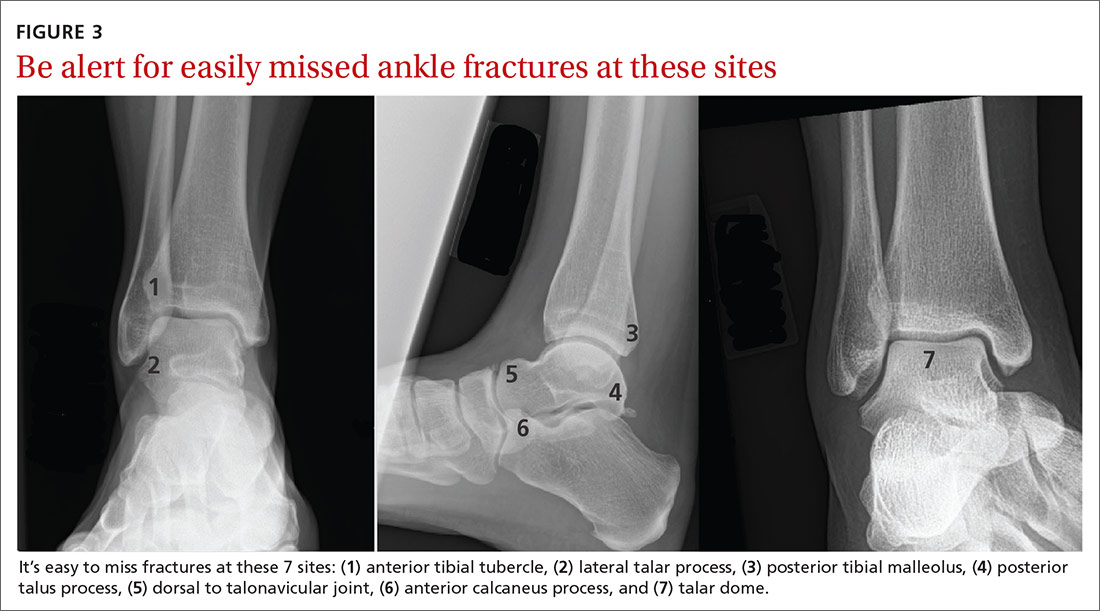
Other frequently missed types of ankle fracture are shown in FIGURE 3.22 These are relatively uncommon injuries that can be missed for a number of reasons, alone or in combination, including their subtlety on radiography, their often vague clinical presentation, and providers’ lack of awareness of these types. Identification or strong suspicion of fracture at any of these sites (ie, in a patient who is persistently unable to bear weight) should prompt orthopedic referral.
Lisfranc injury
The tarsometatarsal joint comprises 3 cuneiforms, the cuboid, and 5 metatarsals. Stability is maintained by an intricate ligamentous complex. Lisfranc injury comprises a spectrum of midfoot injuries in which 1 or more metatarsals are displaced from the tarsus. These injuries are both rare and notoriously difficult to diagnose: As many as 20% of cases are missed on initial assessment. Without proper treatment, long-term disability and deformity, such as pes planus, can result.22-24 Lisfranc injuries typically result from a direct blow to the midfoot or excessive pronation or supination in a plantarflexed foot.23
Presentation. A historical clue to Lisfranc injury is a report of pain while walking down stairs. Patients can present with pain, swelling, and tenderness to palpation over the dorsal aspect of the Lisfranc joint. Weight-bearing on the injured foot frequently cannot be tolerated but is occasionally possible in some patients, especially those who have diabetes or other baseline neuropathy.23
Examination. Physical examination can also reveal plantar ecchymosis, which is considered pathognomonic. Another highly supportive maneuver is passive abduction and pronation of the forefoot, which can elicit pain.25,26
Imaging. Lisfranc injury can be diagnosed on weight-bearing x-rays; as many as one-half of cases are missed when only non-weight-bearing films are obtained. If initial weight-bearing cannot be tolerated by the patient, another attempt at imaging can be made after 1 week of rest.24
Continue to: Distance > 2 mm between the base...
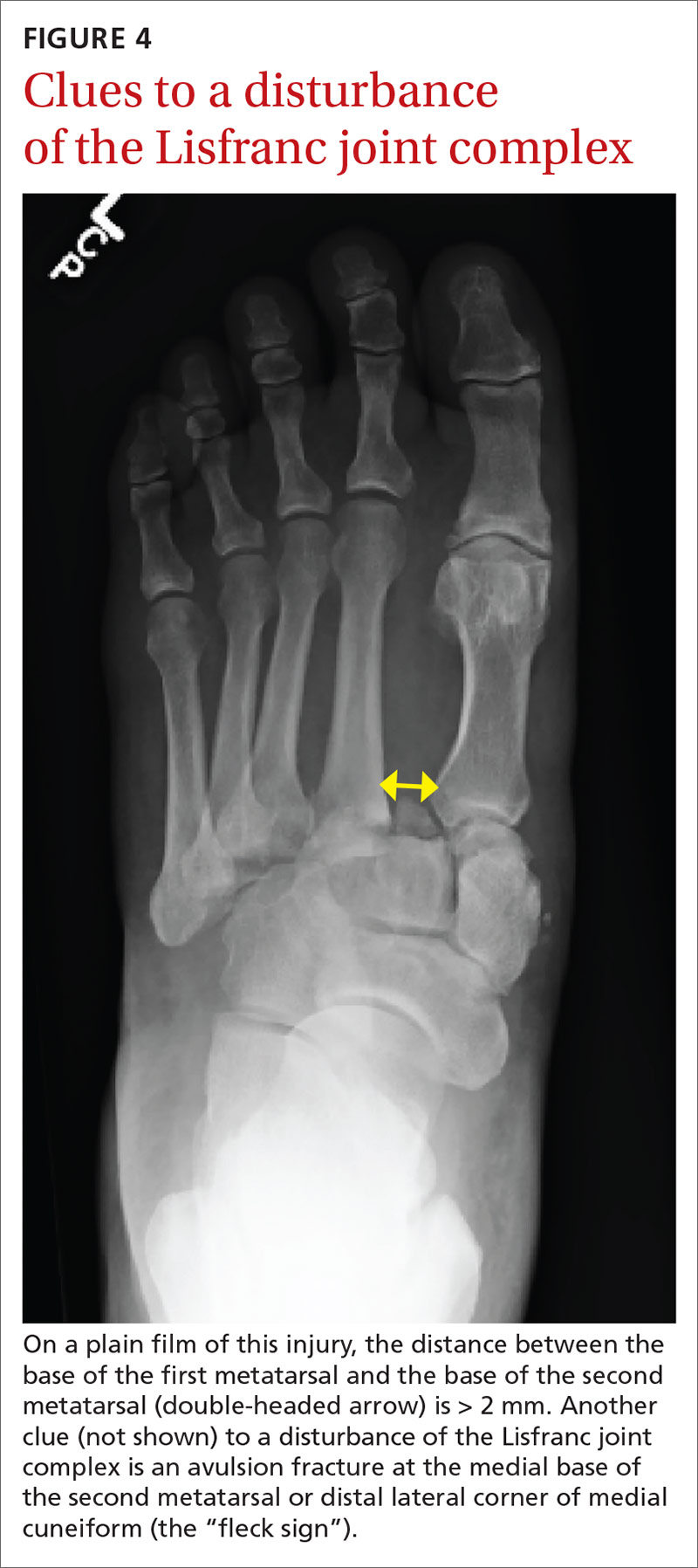
Distance > 2 mm between the base of the first and second metatarsals (FIGURE 4) or an avulsion fracture at the medial base of the second metatarsal or distal lateral corner of the medial cuneiform (the “fleck sign”) supports a disturbance of the Lisfranc joint complex.24 Imaging of the contralateral foot might highlight the injury in subtle cases, followed by CT when diagnostic uncertainty persists.24,25
Management of Lisfranc injury depends on the stability of the joint complex. Stable injury without diastasis can be managed conservatively with immobilization in a short walker boot and limited weight-bearing for 2 weeks, followed by weight-bearing as tolerated in the boot if tenderness has improved.24 After 6 to 8 weeks, if the patient is pain-free with abduction stress, weight-bearing without the boot (but with a rigid-sole shoe) is permissible for an additional 6 months. Sport-specific rehabilitation for an athlete can begin once the patient can walk down multiple flights of stairs without pain.24
Orthopedic referral for surgical evaluation is recommended for all patients who have any radiographic evidence of dynamic instability, indicated by the fleck sign; displacement; or obvious diastasis between the metatarsals on imaging. A delay of 1 to 2 weeks from injury to fixation has not been associated with a negative outcome; delay as long as 6 weeks is permissible in some cases. Longer delay in surgical treatment (≥ 6 months) can be associated with posttraumatic arthritis and the need for Lisfranc fusion.24-26
Proximal fifth-metatarsal fractures
These common fractures are classified in 3 broad categories: tuberosity avulsion fracture, proximal diaphyseal (Jones) fracture, and stress fractures of the diaphysis (immediately distal to the site of the Jones fracture zone).27-29 Differentiating an acute Jones fracture and other fracture types is clinically important because the watershed area at the metaphysis–diaphysis junction results in a higher risk of delayed union and nonunion of Jones fractures, compared to other fractures in this region (FIGURE 5).28,29
Presentation. Proximal fifth-metatarsal fractures generally present with lateral foot pain and tenderness at the base of the fifth metatarsal, made worse by inversion of the foot, and inability to bear weight on the lateral aspect of the foot. Acute pain can follow a more insidious course of lateral foot pain in stress fracture.
Continue to: Examination
Examination. On exam, there might be swelling and ecchymosis over the lateral foot, with sharp tenderness to palpation at the base of the fifth metatarsal.
Imaging. Most fractures are revealed on standing AP, oblique, and lateral x-rays. Plain films are often falsely negative early in stress fracture; MRI is the gold standard of diagnosis.27,30
Management. Preferred treatment for a nondisplaced tuberosity avulsion fracture is typically 2-pronged: compressive dressings or casting for pain control and weight-bearing and range-of-motion exercises as tolerated.1 Follow-up every 2 to 3 weeks is recommended to ensure appropriate healing—ie, pain nearly resolved by 3 weeks post-injury and radiographic union evident at 8 weeks. If displacement is > 3 mm, > 60% of the metatarsal–cuboid joint surface is affected, or there is a 1 to 2 mm step-off on the cuboid articular surface, consider referral to an orthopedist.1,29
Jones fractures can be managed initially with posterior splinting, non-weight-bearing, and close follow-up. When radiographic healing has not been achieved by 6 to 8 weeks, non-weight-bearing status can be extended by another 4 weeks. When displacement is > 2 mm, or there is no healing after 12 weeks of immobilization and delayed union on x-rays, referral for surgical management is indicated.1 In select cases, when earlier return to activity is desired, referral for early surgical fixation is appropriate.27
Surgical referral is indicated in all cases of diaphysial stress fracture because of the high rate of nonunion and refracture. Conservative management, based on the orthopedic surgeon’s assessment, might be an option in a minority of patients.29
CORRESPONDENCE
Aileen Roman, MD, Boston University Medical School, Department of Family Medicine, 11 Melnea Cass Boulevard, Boston MA, 02119; aileen.roman@bmc.org
1. Bica D, Sprouse RA, Armen J. Diagnosis and management of common foot fractures. Am Fam Physician. 2016;93:183-191.
2. Gross CE, Nunley JA 2nd. Acute Achilles tendon ruptures. Foot Ankle Int. 2016;37:233-239.
3. Cooper MT. Acute Achilles tendon ruptures: does surgery offer superior results (and other confusing issues)? Clin Sports Med. 2015;34:595-606.
4. Maffulli N, Via AG, Oliva F. Chronic Achilles tendon disorders: tendinopathy and chronic rupture. Clin Sports Med. 2015;34:607-624.
5. Hutchison A-M, Evans R, Bodger O, et al. What is the best clinical test for Achilles tendinopathy? Foot Ankle Surg. 2013;19:112-117.
6. Kadakia AR, Dekker RG 2nd, Ho BS. Acute Achilles tendon ruptures: an update on treatment. Am Acad Orthop Surg. 2017;25:23-31.
7. van Zuuren WJ, Schepers T, Beumer A, et al. Acute syndesmotic instability in ankle fractures: a review. Foot Ankle Surg. 2017;23:135-141.
8. van Dijk CN, Longo UG, Loppini M, et al. Classification and diagnosis of acute isolated syndesmotic injuries: ESSKA–AFAS consensus and guidelines. Knee Surg Sports Traumatol Arthrosc. 2016;24:1200-1216.
9. Fort NM, Aiyer AA, Kaplan JR, et al. Management of acute injuries of the tibiofibular syndesmosis. Eur J Orthop Surg Traumatol. 2017;27:449-459.
10. Miller TL, Skalak T. Evaluation and treatment recommendations for acute injuries to the ankle syndesmosis without associated fracture. Sports Med. 2014;44:179-188.
11. Hunt KJ, Phisitkul P, Pirolo J, et al. High ankle sprains and syndesmotic injuries in athletes. J Am Acad Orthop Surg. 2015;23:661-673.
12. DeWeber K. Syndesmotic ankle injury (high ankle sprain). UpToDate. September 17, 2019. www.uptodate.com/contents/syndesmotic-ankle-injury-high-ankle-sprain. Accessed May 26, 2020.
13. Goost H, Wimmer MD, Barg A, et al. Fractures of the ankle joint: investigation and treatment options. Dtsch Arztebl Int. 2014;111:377-388.
14. Qin C, Dekker RG, Helfrich MM, et al. Outpatient management of ankle fractures. Orthop Clin North Am. 2018;49:103-108.
15. Stiell IG, Greenberg GH, McKnight RD, et al. Decision rules for the use of radiography in acute ankle injuries. Refinement and prospective validation. JAMA. 1993;269:1127-1132.
16. Jenkin M, Sitler MR, Kelly JD. Clinical usefulness of the Ottawa Ankle Rules for detecting fractures of the ankle and midfoot. J Athl Train. 2010;45:480-482.
17. Glas AS, Pijnenburg BACM, Lijmer JG, et al. Comparison of diagnostic decision rules and structured data collection in assessment of acute ankle injury. CMAJ. 2002;166:727-733.
18. Leduc S, Nault M-L, Rouleau DM, et al. My experience as a foot and ankle trauma surgeon in Montreal, Canada: what’s not in the books. Foot Ankle Clin. 2016;21:297-334.
19. Ibrahim MS, Jordan R, Lotfi N, et al. Talar head fracture: a case report, systematic review and suggested algorithm of treatment. Foot (Edinb). 2015;25:258-264.
20. Shank JR, Benirschke SK, Swords MP. Treatment of peripheral talus fractures. Foot Ankle Clin. 2017;22:181-192.
21. Kwaadu KY. Management of talar fractures. Clin Podiatr Med Sur. 2018;35:161-173.
22. Yu JS. Easily missed fractures in the lower extremity. Radiol Clin North Am. 2015;53:737-755.
23. Welck MJ, Zinchenko R, Rudge B. Lisfranc injuries. Injury. 2015;46:536-541.
24. Seybold JD, Coetzee JC. Lisfranc injuries: when to observe, fix, or fuse. Clin Sports Med. 2015;34:705-723.
25. Puna RA, Tomlinson MPW. The role of percutaneous reduction and fixation of lisfranc injuries. Foot Ankle Clin. 2017;22:15-34.
26. Weatherford BM, Bohay DR, Anderson JG. Open reduction and internal fixation versus primary arthrodesis for Lisfranc injuries. Foot Ankle Clin. 2017;22:1-14.
27. Porter DA. Fifth metatarsal Jones fractures in the athlete. Foot Ankle Int. 2018;39:250-258.
28. Cheung CN, Lui TH. Proximal fifth metatarsal fractures: anatomy, classification, treatment and complications. Arch Trauma Res. 2016;5:e32298.
29. Alsobrook J, Hatch RL. Proximal fifth metatarsal fractures. UpToDate. January 31, 2020. www.uptodate.com/contents/proximal-fifth-metatarsal-fractures. Accessed May 26, 2020.
30. Welck MJ, Hayes T, Pastides P, et al. Stress fractures of the foot and ankle. Injury. 2017;48:1722-1726.
Foot and ankle injuries are among the most common conditions evaluated at primary care visits; the differential diagnosis of such injury is broad.1 Although many of these injuries are easily identified on imaging studies, a number of subtle, yet important, conditions can be easily missed, especially if you do not routinely encounter them. Given that broad differential, a high degree of suspicion is required to make an accurate diagnosis, which allows appropriate treatment within a reasonable time frame and minimizes the risk of long-term morbidity.
This article outlines the diagnosis and initial management of 5 important, yet often elusive, types of foot and ankle conditions: Achilles tendon rupture, injury to the syndesmosis, ankle fracture, Lisfranc injury, and proximal fracture of the fifth metatarsal.
Achilles tendon rupture
The Achilles tendon is the most frequently ruptured tendon in the body (approximately 20% of all large-tendon injuries)2; as many as 25% of cases are initially misdiagnosed.3
Presentation. Patients frequently present with pain at the Achilles tendon—2 to 6 cm above the insertion into the calcaneus—and an inability to fully bear weight.4,5 A small percentage of patients are able to ambulate on the affected side, albeit with minor pain, which likely contributes to the rate of missed diagnosis. Absence of difficulty bearing weight is due to the presence of secondary plantar flexors, which can compensate for loss of chief plantar flexor function by the Achilles tendon.2
Examination of a patient with an Achilles tendon rupture typically reveals edema, bruising, and a palpable gap within the tendon, 2 to 6 cm proximal to insertion.3,4 The Thompson test—squeezing the calf with the patient prone and the knee on the affected side flexed—can aid in diagnosis. When the Achilles tendon is intact, plantar flexion occurs at the ankle; when the tendon is ruptured, plantar flexion is absent.5 The test can be modified when examining a patient who is unable to lie prone by having them rest the flexed knee on a chair while standing on the unaffected leg.
A diagnosis of Achilles tendon rupture is supported when at least 2 of the following conditions are met4,5:
- positive Thompson test
- decreased strength during plantar flexion of the ankle
- palpable gap or pain at the typical location (2-6 cm above insertion)
- increased passive ankle dorsiflexion upon gentle ranging of the ankle joint.
Imaging has a limited role in the diagnosis of Achilles tendon rupture; because the findings of the physical examination are reliable, reserve x-rays for cases in which the diagnosis remains uncertain after examination.2 Consider ordering plain x-rays to rule out an avulsion fracture at the insertion of the Achilles tendon; ultrasonography or magnetic resonance imaging (MRI) might assist you in detecting the rupture proper, along with the location of the tear for surgical planning, if surgery is deemed necessary by an orthopedic surgeon.3-5
Continue to: Management
Management. Some degree of controversy surrounds preferred treatment of Achilles tendon rupture, although available evidence demonstrates that these injuries can be effectively managed by surgical repair or nonoperative treatment, as outcomes are comparable.3,5 Operative management tends to reduce the risk of repeat rupture, compared to nonoperative treatment; however, the potential for surgical complications, including wound infection, sensory disturbance, and adhesions favors nonoperative treatment.3,4,6
Nonoperative treatment consists of referral to a functional rehabilitation program, without which outcomes are, on the whole, less favorable than with surgery.3,6 Surgery is preferred if functional rehabilitation is unavailable, 6 months of conservative management fails, or there is avulsion injury.3,4,6
Injury to the syndesmosis
A complex of ligaments that provide dynamic stability to the ankle joint, the tibiofibular syndesmosis comprises:
- the anterior inferior tibiofibular ligament
- the posterior inferior tibiofibular ligament
- the inferior transverse tibiofibular ligament
- the interosseous membrane.
These structures are further supported by the deltoid ligament.7,8
Commonly referred to as a “high ankle sprain,” a syndesmotic injury is present in as many as 20% of ankle fractures and 5% to 10% of ankle sprains. Injury typically results from external rotation with hyperdorsiflexion of the ankle. Recovery is typically prolonged (ie, twice as long as recovery from a lateral ankle sprain). The diagnosis is missed in as many as 20% of patients; failure to recognize and treat syndesmotic instability appropriately can lead to posttraumatic arthritis.7,9
Continue to: Presentation
Presentation. Patients generally present with ankle pain, swelling, instability, pain when walking on uneven terrain, and pain upon push-off.9
Examination reveals reduced passive ankle dorsiflexion and tenderness upon palpation of individual ligaments. Several clinical tests have been described to aid in detecting this often-elusive diagnosis7,9,10,11:
- Squeeze test. The patient sits with the knee on the affected side bent at a 90° degree angle while the examiner applies compression, with one or both hands, to the tibia and fibula at midcalf. The test is positive when pain is elicited at the level of the syndesmosis just above the ankle joint.9,11
- External rotation test. External rotation of the foot and ankle relative to the tibia reproduces pain.
- Crossed leg test. The affected ankle is crossed over the opposite knee in a figure-4 position. The test is positive when pain is elicited at the syndesmosis.10
- Cotton test. The proximal lower leg is steadied with 1 hand and the plantar heel grasped with the other hand. Pain when the heel is externally rotated (and radiographic widening of the syndesmosis under fluoroscopy) signal syndesmotic instability.
- Fibular translation test. When anterior or posterior drawer force is applied to the fibula, pain and increased translation of the fibula (compared to the contralateral side) suggest instability.
With the Cotton and fibular translation tests, interexaminer technique is more variable and findings are less reproducible.8 Taken alone, none of the above-listed tests are diagnostic; they can, however, assist in making a diagnosis of an injury to the syndesmosis.11
Imaging typically involves anteroposterior [AP], lateral, and mortise plain films of the ankle and weight-bearing AP and lateral views of the tibia and fibula.9 Important measures on weight-bearing AP x-rays are the tibiofibular clear space (abnormal, > 6 mm) and the tibiofibular overlap (abnormal, < 6 mm) (both abnormalities shown in FIGURE 1). Comparing films of the affected ankle with views of the contralateral ankle is often useful.
Management of syndesmotic injuries depends on degree of disruption:
- Grade 1 injury is a sprain without diastasis on imaging. Management is conservative, with immobilization in a splint or boot for 1 to 3 weeks, followed by functional rehabilitation over 3 to 6 weeks.10
- Grade 2 injury is demonstrated by diastasis on a stress radiograph. Although evidence to guide successful identification of a grade 2 injury is lacking, it is clinically important to make that identification because these injuries might require surgical intervention, due to instability. Because the diagnosis of this injury can be challenging in primary care, high clinical suspicion of a grade 2 injury makes it appropriate to defer further evaluation to an orthopedic surgeon. On the other hand, if suspicion of a grade 2 injury is low, a trial of conservative management, with weekly clinical assessment, can be considered. A diagnosis of grade 2 injury can be inferred when a patient is unable to perform a single-leg hop after 3 weeks of immobilization; referral to an orthopedic surgeon is then indicated.12
- Grade 3 injury is frank separation at the distal tibiofibular joint that is detectable on a routine plain film. Management—surgical intervention to address instability—is often provided concurrently with the treatment for a Danis-Weber B or C fracture, which tends to coexist with grade 3 syndesmotic injury. (The Danis-Weber A–B–C classification of lateral ankle fracture will be discussed in a bit.)
Continue to: Ankle fracture
Ankle fracture
Fracture of the ankle joint is among the more common fractures in adults, comprising 10% of all fractures.13,14 The ankle joint is defined as the junction of 3 bony structures: (1) the distal ends of the tibia and fibula and (2) the trochlea of the talus, all stabilized by (3) the collateral ligament complex. Appropriate diagnosis and timely intervention are needed to prevent long-term posttraumatic joint degeneration.
Presentation, examination, and imaging. In addition to difficulty bearing (or inability to bear) weight, patients with suspected ankle fracture can present with tenderness or pain, swelling (generally, the more severe the injury, the more severe the swelling, although this finding is time-dependent), and ecchymosis. However, distinguishing fracture from a ligamentous injury is often difficult by physical examination alone; the evidence-based Ottawa Ankle Rules can guide determination of the need for radiographic imaging, although this tool is less reliable in certain patient populations (TABLE15-17).13,15-17
Management. A widely used classification system for guiding ankle fracture management is the Danis-Weber classification (FIGURE 2). In this scheme, type A fractures (distal to the level of the tibial plafond) are managed with ankle stabilization bracing without immobilization. Nondisplaced type B and C fractures (at the level of the tibial plafond and proximal to it, respectively) should be treated with 6 weeks of immobilization in a walking boot; close follow-up within 1 week of injury is recommended to ensure that no displacement of fragments has occurred. Type B and C fractures need to be followed until bony union is achieved. If there is radiologic evidence of a fracture line after 3 months, referral to an orthopedic surgeon is indicated for management of delayed union.
Common indications for referral to Orthopedics for surgical intervention of ankle fracture include open fracture, bimalleolar and trimalleolar fracture, posterior malleolar fracture, medial malleolar displacement > 2 mm, and lateral malleolar displacement > 3 mm.18
Special concern: Talar fracture. Although talar fracture is rare, the injury is important to detect because a limited blood supply places fragments at risk of avascular necrosis.19 Talus fracture is frequently confused with ankle sprain because initial x-rays are not always revelatory.20 A high index of suspicion is required to make the diagnosis, which should be suspected in high-energy injuries that result in pain and swelling of the ankle accompanied by difficulty weight-bearing, severely reduced range of motion, and tenderness to palpation at different areas of the talus.1 Computed tomography (CT) or MRI might be necessary to detect a talar fracture if initial x-rays are negative. A low threshold for surgical management of talar fracture means that referral to Orthopedics is indicated once this injury is diagnosed.21
Continue to: Other frequently missed types of ankle fracture
Other frequently missed types of ankle fracture are shown in FIGURE 3.22 These are relatively uncommon injuries that can be missed for a number of reasons, alone or in combination, including their subtlety on radiography, their often vague clinical presentation, and providers’ lack of awareness of these types. Identification or strong suspicion of fracture at any of these sites (ie, in a patient who is persistently unable to bear weight) should prompt orthopedic referral.
Lisfranc injury
The tarsometatarsal joint comprises 3 cuneiforms, the cuboid, and 5 metatarsals. Stability is maintained by an intricate ligamentous complex. Lisfranc injury comprises a spectrum of midfoot injuries in which 1 or more metatarsals are displaced from the tarsus. These injuries are both rare and notoriously difficult to diagnose: As many as 20% of cases are missed on initial assessment. Without proper treatment, long-term disability and deformity, such as pes planus, can result.22-24 Lisfranc injuries typically result from a direct blow to the midfoot or excessive pronation or supination in a plantarflexed foot.23
Presentation. A historical clue to Lisfranc injury is a report of pain while walking down stairs. Patients can present with pain, swelling, and tenderness to palpation over the dorsal aspect of the Lisfranc joint. Weight-bearing on the injured foot frequently cannot be tolerated but is occasionally possible in some patients, especially those who have diabetes or other baseline neuropathy.23
Examination. Physical examination can also reveal plantar ecchymosis, which is considered pathognomonic. Another highly supportive maneuver is passive abduction and pronation of the forefoot, which can elicit pain.25,26
Imaging. Lisfranc injury can be diagnosed on weight-bearing x-rays; as many as one-half of cases are missed when only non-weight-bearing films are obtained. If initial weight-bearing cannot be tolerated by the patient, another attempt at imaging can be made after 1 week of rest.24
Continue to: Distance > 2 mm between the base...
Distance > 2 mm between the base of the first and second metatarsals (FIGURE 4) or an avulsion fracture at the medial base of the second metatarsal or distal lateral corner of the medial cuneiform (the “fleck sign”) supports a disturbance of the Lisfranc joint complex.24 Imaging of the contralateral foot might highlight the injury in subtle cases, followed by CT when diagnostic uncertainty persists.24,25
Management of Lisfranc injury depends on the stability of the joint complex. Stable injury without diastasis can be managed conservatively with immobilization in a short walker boot and limited weight-bearing for 2 weeks, followed by weight-bearing as tolerated in the boot if tenderness has improved.24 After 6 to 8 weeks, if the patient is pain-free with abduction stress, weight-bearing without the boot (but with a rigid-sole shoe) is permissible for an additional 6 months. Sport-specific rehabilitation for an athlete can begin once the patient can walk down multiple flights of stairs without pain.24
Orthopedic referral for surgical evaluation is recommended for all patients who have any radiographic evidence of dynamic instability, indicated by the fleck sign; displacement; or obvious diastasis between the metatarsals on imaging. A delay of 1 to 2 weeks from injury to fixation has not been associated with a negative outcome; delay as long as 6 weeks is permissible in some cases. Longer delay in surgical treatment (≥ 6 months) can be associated with posttraumatic arthritis and the need for Lisfranc fusion.24-26
Proximal fifth-metatarsal fractures
These common fractures are classified in 3 broad categories: tuberosity avulsion fracture, proximal diaphyseal (Jones) fracture, and stress fractures of the diaphysis (immediately distal to the site of the Jones fracture zone).27-29 Differentiating an acute Jones fracture and other fracture types is clinically important because the watershed area at the metaphysis–diaphysis junction results in a higher risk of delayed union and nonunion of Jones fractures, compared to other fractures in this region (FIGURE 5).28,29
Presentation. Proximal fifth-metatarsal fractures generally present with lateral foot pain and tenderness at the base of the fifth metatarsal, made worse by inversion of the foot, and inability to bear weight on the lateral aspect of the foot. Acute pain can follow a more insidious course of lateral foot pain in stress fracture.
Continue to: Examination
Examination. On exam, there might be swelling and ecchymosis over the lateral foot, with sharp tenderness to palpation at the base of the fifth metatarsal.
Imaging. Most fractures are revealed on standing AP, oblique, and lateral x-rays. Plain films are often falsely negative early in stress fracture; MRI is the gold standard of diagnosis.27,30
Management. Preferred treatment for a nondisplaced tuberosity avulsion fracture is typically 2-pronged: compressive dressings or casting for pain control and weight-bearing and range-of-motion exercises as tolerated.1 Follow-up every 2 to 3 weeks is recommended to ensure appropriate healing—ie, pain nearly resolved by 3 weeks post-injury and radiographic union evident at 8 weeks. If displacement is > 3 mm, > 60% of the metatarsal–cuboid joint surface is affected, or there is a 1 to 2 mm step-off on the cuboid articular surface, consider referral to an orthopedist.1,29
Jones fractures can be managed initially with posterior splinting, non-weight-bearing, and close follow-up. When radiographic healing has not been achieved by 6 to 8 weeks, non-weight-bearing status can be extended by another 4 weeks. When displacement is > 2 mm, or there is no healing after 12 weeks of immobilization and delayed union on x-rays, referral for surgical management is indicated.1 In select cases, when earlier return to activity is desired, referral for early surgical fixation is appropriate.27
Surgical referral is indicated in all cases of diaphysial stress fracture because of the high rate of nonunion and refracture. Conservative management, based on the orthopedic surgeon’s assessment, might be an option in a minority of patients.29
CORRESPONDENCE
Aileen Roman, MD, Boston University Medical School, Department of Family Medicine, 11 Melnea Cass Boulevard, Boston MA, 02119; aileen.roman@bmc.org
Foot and ankle injuries are among the most common conditions evaluated at primary care visits; the differential diagnosis of such injury is broad.1 Although many of these injuries are easily identified on imaging studies, a number of subtle, yet important, conditions can be easily missed, especially if you do not routinely encounter them. Given that broad differential, a high degree of suspicion is required to make an accurate diagnosis, which allows appropriate treatment within a reasonable time frame and minimizes the risk of long-term morbidity.
This article outlines the diagnosis and initial management of 5 important, yet often elusive, types of foot and ankle conditions: Achilles tendon rupture, injury to the syndesmosis, ankle fracture, Lisfranc injury, and proximal fracture of the fifth metatarsal.
Achilles tendon rupture
The Achilles tendon is the most frequently ruptured tendon in the body (approximately 20% of all large-tendon injuries)2; as many as 25% of cases are initially misdiagnosed.3
Presentation. Patients frequently present with pain at the Achilles tendon—2 to 6 cm above the insertion into the calcaneus—and an inability to fully bear weight.4,5 A small percentage of patients are able to ambulate on the affected side, albeit with minor pain, which likely contributes to the rate of missed diagnosis. Absence of difficulty bearing weight is due to the presence of secondary plantar flexors, which can compensate for loss of chief plantar flexor function by the Achilles tendon.2
Examination of a patient with an Achilles tendon rupture typically reveals edema, bruising, and a palpable gap within the tendon, 2 to 6 cm proximal to insertion.3,4 The Thompson test—squeezing the calf with the patient prone and the knee on the affected side flexed—can aid in diagnosis. When the Achilles tendon is intact, plantar flexion occurs at the ankle; when the tendon is ruptured, plantar flexion is absent.5 The test can be modified when examining a patient who is unable to lie prone by having them rest the flexed knee on a chair while standing on the unaffected leg.
A diagnosis of Achilles tendon rupture is supported when at least 2 of the following conditions are met4,5:
- positive Thompson test
- decreased strength during plantar flexion of the ankle
- palpable gap or pain at the typical location (2-6 cm above insertion)
- increased passive ankle dorsiflexion upon gentle ranging of the ankle joint.
Imaging has a limited role in the diagnosis of Achilles tendon rupture; because the findings of the physical examination are reliable, reserve x-rays for cases in which the diagnosis remains uncertain after examination.2 Consider ordering plain x-rays to rule out an avulsion fracture at the insertion of the Achilles tendon; ultrasonography or magnetic resonance imaging (MRI) might assist you in detecting the rupture proper, along with the location of the tear for surgical planning, if surgery is deemed necessary by an orthopedic surgeon.3-5
Continue to: Management
Management. Some degree of controversy surrounds preferred treatment of Achilles tendon rupture, although available evidence demonstrates that these injuries can be effectively managed by surgical repair or nonoperative treatment, as outcomes are comparable.3,5 Operative management tends to reduce the risk of repeat rupture, compared to nonoperative treatment; however, the potential for surgical complications, including wound infection, sensory disturbance, and adhesions favors nonoperative treatment.3,4,6
Nonoperative treatment consists of referral to a functional rehabilitation program, without which outcomes are, on the whole, less favorable than with surgery.3,6 Surgery is preferred if functional rehabilitation is unavailable, 6 months of conservative management fails, or there is avulsion injury.3,4,6
Injury to the syndesmosis
A complex of ligaments that provide dynamic stability to the ankle joint, the tibiofibular syndesmosis comprises:
- the anterior inferior tibiofibular ligament
- the posterior inferior tibiofibular ligament
- the inferior transverse tibiofibular ligament
- the interosseous membrane.
These structures are further supported by the deltoid ligament.7,8
Commonly referred to as a “high ankle sprain,” a syndesmotic injury is present in as many as 20% of ankle fractures and 5% to 10% of ankle sprains. Injury typically results from external rotation with hyperdorsiflexion of the ankle. Recovery is typically prolonged (ie, twice as long as recovery from a lateral ankle sprain). The diagnosis is missed in as many as 20% of patients; failure to recognize and treat syndesmotic instability appropriately can lead to posttraumatic arthritis.7,9
Continue to: Presentation
Presentation. Patients generally present with ankle pain, swelling, instability, pain when walking on uneven terrain, and pain upon push-off.9
Examination reveals reduced passive ankle dorsiflexion and tenderness upon palpation of individual ligaments. Several clinical tests have been described to aid in detecting this often-elusive diagnosis7,9,10,11:
- Squeeze test. The patient sits with the knee on the affected side bent at a 90° degree angle while the examiner applies compression, with one or both hands, to the tibia and fibula at midcalf. The test is positive when pain is elicited at the level of the syndesmosis just above the ankle joint.9,11
- External rotation test. External rotation of the foot and ankle relative to the tibia reproduces pain.
- Crossed leg test. The affected ankle is crossed over the opposite knee in a figure-4 position. The test is positive when pain is elicited at the syndesmosis.10
- Cotton test. The proximal lower leg is steadied with 1 hand and the plantar heel grasped with the other hand. Pain when the heel is externally rotated (and radiographic widening of the syndesmosis under fluoroscopy) signal syndesmotic instability.
- Fibular translation test. When anterior or posterior drawer force is applied to the fibula, pain and increased translation of the fibula (compared to the contralateral side) suggest instability.
With the Cotton and fibular translation tests, interexaminer technique is more variable and findings are less reproducible.8 Taken alone, none of the above-listed tests are diagnostic; they can, however, assist in making a diagnosis of an injury to the syndesmosis.11
Imaging typically involves anteroposterior [AP], lateral, and mortise plain films of the ankle and weight-bearing AP and lateral views of the tibia and fibula.9 Important measures on weight-bearing AP x-rays are the tibiofibular clear space (abnormal, > 6 mm) and the tibiofibular overlap (abnormal, < 6 mm) (both abnormalities shown in FIGURE 1). Comparing films of the affected ankle with views of the contralateral ankle is often useful.
Management of syndesmotic injuries depends on degree of disruption:
- Grade 1 injury is a sprain without diastasis on imaging. Management is conservative, with immobilization in a splint or boot for 1 to 3 weeks, followed by functional rehabilitation over 3 to 6 weeks.10
- Grade 2 injury is demonstrated by diastasis on a stress radiograph. Although evidence to guide successful identification of a grade 2 injury is lacking, it is clinically important to make that identification because these injuries might require surgical intervention, due to instability. Because the diagnosis of this injury can be challenging in primary care, high clinical suspicion of a grade 2 injury makes it appropriate to defer further evaluation to an orthopedic surgeon. On the other hand, if suspicion of a grade 2 injury is low, a trial of conservative management, with weekly clinical assessment, can be considered. A diagnosis of grade 2 injury can be inferred when a patient is unable to perform a single-leg hop after 3 weeks of immobilization; referral to an orthopedic surgeon is then indicated.12
- Grade 3 injury is frank separation at the distal tibiofibular joint that is detectable on a routine plain film. Management—surgical intervention to address instability—is often provided concurrently with the treatment for a Danis-Weber B or C fracture, which tends to coexist with grade 3 syndesmotic injury. (The Danis-Weber A–B–C classification of lateral ankle fracture will be discussed in a bit.)
Continue to: Ankle fracture
Ankle fracture
Fracture of the ankle joint is among the more common fractures in adults, comprising 10% of all fractures.13,14 The ankle joint is defined as the junction of 3 bony structures: (1) the distal ends of the tibia and fibula and (2) the trochlea of the talus, all stabilized by (3) the collateral ligament complex. Appropriate diagnosis and timely intervention are needed to prevent long-term posttraumatic joint degeneration.
Presentation, examination, and imaging. In addition to difficulty bearing (or inability to bear) weight, patients with suspected ankle fracture can present with tenderness or pain, swelling (generally, the more severe the injury, the more severe the swelling, although this finding is time-dependent), and ecchymosis. However, distinguishing fracture from a ligamentous injury is often difficult by physical examination alone; the evidence-based Ottawa Ankle Rules can guide determination of the need for radiographic imaging, although this tool is less reliable in certain patient populations (TABLE15-17).13,15-17
Management. A widely used classification system for guiding ankle fracture management is the Danis-Weber classification (FIGURE 2). In this scheme, type A fractures (distal to the level of the tibial plafond) are managed with ankle stabilization bracing without immobilization. Nondisplaced type B and C fractures (at the level of the tibial plafond and proximal to it, respectively) should be treated with 6 weeks of immobilization in a walking boot; close follow-up within 1 week of injury is recommended to ensure that no displacement of fragments has occurred. Type B and C fractures need to be followed until bony union is achieved. If there is radiologic evidence of a fracture line after 3 months, referral to an orthopedic surgeon is indicated for management of delayed union.
Common indications for referral to Orthopedics for surgical intervention of ankle fracture include open fracture, bimalleolar and trimalleolar fracture, posterior malleolar fracture, medial malleolar displacement > 2 mm, and lateral malleolar displacement > 3 mm.18
Special concern: Talar fracture. Although talar fracture is rare, the injury is important to detect because a limited blood supply places fragments at risk of avascular necrosis.19 Talus fracture is frequently confused with ankle sprain because initial x-rays are not always revelatory.20 A high index of suspicion is required to make the diagnosis, which should be suspected in high-energy injuries that result in pain and swelling of the ankle accompanied by difficulty weight-bearing, severely reduced range of motion, and tenderness to palpation at different areas of the talus.1 Computed tomography (CT) or MRI might be necessary to detect a talar fracture if initial x-rays are negative. A low threshold for surgical management of talar fracture means that referral to Orthopedics is indicated once this injury is diagnosed.21
Continue to: Other frequently missed types of ankle fracture
Other frequently missed types of ankle fracture are shown in FIGURE 3.22 These are relatively uncommon injuries that can be missed for a number of reasons, alone or in combination, including their subtlety on radiography, their often vague clinical presentation, and providers’ lack of awareness of these types. Identification or strong suspicion of fracture at any of these sites (ie, in a patient who is persistently unable to bear weight) should prompt orthopedic referral.
Lisfranc injury
The tarsometatarsal joint comprises 3 cuneiforms, the cuboid, and 5 metatarsals. Stability is maintained by an intricate ligamentous complex. Lisfranc injury comprises a spectrum of midfoot injuries in which 1 or more metatarsals are displaced from the tarsus. These injuries are both rare and notoriously difficult to diagnose: As many as 20% of cases are missed on initial assessment. Without proper treatment, long-term disability and deformity, such as pes planus, can result.22-24 Lisfranc injuries typically result from a direct blow to the midfoot or excessive pronation or supination in a plantarflexed foot.23
Presentation. A historical clue to Lisfranc injury is a report of pain while walking down stairs. Patients can present with pain, swelling, and tenderness to palpation over the dorsal aspect of the Lisfranc joint. Weight-bearing on the injured foot frequently cannot be tolerated but is occasionally possible in some patients, especially those who have diabetes or other baseline neuropathy.23
Examination. Physical examination can also reveal plantar ecchymosis, which is considered pathognomonic. Another highly supportive maneuver is passive abduction and pronation of the forefoot, which can elicit pain.25,26
Imaging. Lisfranc injury can be diagnosed on weight-bearing x-rays; as many as one-half of cases are missed when only non-weight-bearing films are obtained. If initial weight-bearing cannot be tolerated by the patient, another attempt at imaging can be made after 1 week of rest.24
Continue to: Distance > 2 mm between the base...
Distance > 2 mm between the base of the first and second metatarsals (FIGURE 4) or an avulsion fracture at the medial base of the second metatarsal or distal lateral corner of the medial cuneiform (the “fleck sign”) supports a disturbance of the Lisfranc joint complex.24 Imaging of the contralateral foot might highlight the injury in subtle cases, followed by CT when diagnostic uncertainty persists.24,25
Management of Lisfranc injury depends on the stability of the joint complex. Stable injury without diastasis can be managed conservatively with immobilization in a short walker boot and limited weight-bearing for 2 weeks, followed by weight-bearing as tolerated in the boot if tenderness has improved.24 After 6 to 8 weeks, if the patient is pain-free with abduction stress, weight-bearing without the boot (but with a rigid-sole shoe) is permissible for an additional 6 months. Sport-specific rehabilitation for an athlete can begin once the patient can walk down multiple flights of stairs without pain.24
Orthopedic referral for surgical evaluation is recommended for all patients who have any radiographic evidence of dynamic instability, indicated by the fleck sign; displacement; or obvious diastasis between the metatarsals on imaging. A delay of 1 to 2 weeks from injury to fixation has not been associated with a negative outcome; delay as long as 6 weeks is permissible in some cases. Longer delay in surgical treatment (≥ 6 months) can be associated with posttraumatic arthritis and the need for Lisfranc fusion.24-26
Proximal fifth-metatarsal fractures
These common fractures are classified in 3 broad categories: tuberosity avulsion fracture, proximal diaphyseal (Jones) fracture, and stress fractures of the diaphysis (immediately distal to the site of the Jones fracture zone).27-29 Differentiating an acute Jones fracture and other fracture types is clinically important because the watershed area at the metaphysis–diaphysis junction results in a higher risk of delayed union and nonunion of Jones fractures, compared to other fractures in this region (FIGURE 5).28,29
Presentation. Proximal fifth-metatarsal fractures generally present with lateral foot pain and tenderness at the base of the fifth metatarsal, made worse by inversion of the foot, and inability to bear weight on the lateral aspect of the foot. Acute pain can follow a more insidious course of lateral foot pain in stress fracture.
Continue to: Examination
Examination. On exam, there might be swelling and ecchymosis over the lateral foot, with sharp tenderness to palpation at the base of the fifth metatarsal.
Imaging. Most fractures are revealed on standing AP, oblique, and lateral x-rays. Plain films are often falsely negative early in stress fracture; MRI is the gold standard of diagnosis.27,30
Management. Preferred treatment for a nondisplaced tuberosity avulsion fracture is typically 2-pronged: compressive dressings or casting for pain control and weight-bearing and range-of-motion exercises as tolerated.1 Follow-up every 2 to 3 weeks is recommended to ensure appropriate healing—ie, pain nearly resolved by 3 weeks post-injury and radiographic union evident at 8 weeks. If displacement is > 3 mm, > 60% of the metatarsal–cuboid joint surface is affected, or there is a 1 to 2 mm step-off on the cuboid articular surface, consider referral to an orthopedist.1,29
Jones fractures can be managed initially with posterior splinting, non-weight-bearing, and close follow-up. When radiographic healing has not been achieved by 6 to 8 weeks, non-weight-bearing status can be extended by another 4 weeks. When displacement is > 2 mm, or there is no healing after 12 weeks of immobilization and delayed union on x-rays, referral for surgical management is indicated.1 In select cases, when earlier return to activity is desired, referral for early surgical fixation is appropriate.27
Surgical referral is indicated in all cases of diaphysial stress fracture because of the high rate of nonunion and refracture. Conservative management, based on the orthopedic surgeon’s assessment, might be an option in a minority of patients.29
CORRESPONDENCE
Aileen Roman, MD, Boston University Medical School, Department of Family Medicine, 11 Melnea Cass Boulevard, Boston MA, 02119; aileen.roman@bmc.org
1. Bica D, Sprouse RA, Armen J. Diagnosis and management of common foot fractures. Am Fam Physician. 2016;93:183-191.
2. Gross CE, Nunley JA 2nd. Acute Achilles tendon ruptures. Foot Ankle Int. 2016;37:233-239.
3. Cooper MT. Acute Achilles tendon ruptures: does surgery offer superior results (and other confusing issues)? Clin Sports Med. 2015;34:595-606.
4. Maffulli N, Via AG, Oliva F. Chronic Achilles tendon disorders: tendinopathy and chronic rupture. Clin Sports Med. 2015;34:607-624.
5. Hutchison A-M, Evans R, Bodger O, et al. What is the best clinical test for Achilles tendinopathy? Foot Ankle Surg. 2013;19:112-117.
6. Kadakia AR, Dekker RG 2nd, Ho BS. Acute Achilles tendon ruptures: an update on treatment. Am Acad Orthop Surg. 2017;25:23-31.
7. van Zuuren WJ, Schepers T, Beumer A, et al. Acute syndesmotic instability in ankle fractures: a review. Foot Ankle Surg. 2017;23:135-141.
8. van Dijk CN, Longo UG, Loppini M, et al. Classification and diagnosis of acute isolated syndesmotic injuries: ESSKA–AFAS consensus and guidelines. Knee Surg Sports Traumatol Arthrosc. 2016;24:1200-1216.
9. Fort NM, Aiyer AA, Kaplan JR, et al. Management of acute injuries of the tibiofibular syndesmosis. Eur J Orthop Surg Traumatol. 2017;27:449-459.
10. Miller TL, Skalak T. Evaluation and treatment recommendations for acute injuries to the ankle syndesmosis without associated fracture. Sports Med. 2014;44:179-188.
11. Hunt KJ, Phisitkul P, Pirolo J, et al. High ankle sprains and syndesmotic injuries in athletes. J Am Acad Orthop Surg. 2015;23:661-673.
12. DeWeber K. Syndesmotic ankle injury (high ankle sprain). UpToDate. September 17, 2019. www.uptodate.com/contents/syndesmotic-ankle-injury-high-ankle-sprain. Accessed May 26, 2020.
13. Goost H, Wimmer MD, Barg A, et al. Fractures of the ankle joint: investigation and treatment options. Dtsch Arztebl Int. 2014;111:377-388.
14. Qin C, Dekker RG, Helfrich MM, et al. Outpatient management of ankle fractures. Orthop Clin North Am. 2018;49:103-108.
15. Stiell IG, Greenberg GH, McKnight RD, et al. Decision rules for the use of radiography in acute ankle injuries. Refinement and prospective validation. JAMA. 1993;269:1127-1132.
16. Jenkin M, Sitler MR, Kelly JD. Clinical usefulness of the Ottawa Ankle Rules for detecting fractures of the ankle and midfoot. J Athl Train. 2010;45:480-482.
17. Glas AS, Pijnenburg BACM, Lijmer JG, et al. Comparison of diagnostic decision rules and structured data collection in assessment of acute ankle injury. CMAJ. 2002;166:727-733.
18. Leduc S, Nault M-L, Rouleau DM, et al. My experience as a foot and ankle trauma surgeon in Montreal, Canada: what’s not in the books. Foot Ankle Clin. 2016;21:297-334.
19. Ibrahim MS, Jordan R, Lotfi N, et al. Talar head fracture: a case report, systematic review and suggested algorithm of treatment. Foot (Edinb). 2015;25:258-264.
20. Shank JR, Benirschke SK, Swords MP. Treatment of peripheral talus fractures. Foot Ankle Clin. 2017;22:181-192.
21. Kwaadu KY. Management of talar fractures. Clin Podiatr Med Sur. 2018;35:161-173.
22. Yu JS. Easily missed fractures in the lower extremity. Radiol Clin North Am. 2015;53:737-755.
23. Welck MJ, Zinchenko R, Rudge B. Lisfranc injuries. Injury. 2015;46:536-541.
24. Seybold JD, Coetzee JC. Lisfranc injuries: when to observe, fix, or fuse. Clin Sports Med. 2015;34:705-723.
25. Puna RA, Tomlinson MPW. The role of percutaneous reduction and fixation of lisfranc injuries. Foot Ankle Clin. 2017;22:15-34.
26. Weatherford BM, Bohay DR, Anderson JG. Open reduction and internal fixation versus primary arthrodesis for Lisfranc injuries. Foot Ankle Clin. 2017;22:1-14.
27. Porter DA. Fifth metatarsal Jones fractures in the athlete. Foot Ankle Int. 2018;39:250-258.
28. Cheung CN, Lui TH. Proximal fifth metatarsal fractures: anatomy, classification, treatment and complications. Arch Trauma Res. 2016;5:e32298.
29. Alsobrook J, Hatch RL. Proximal fifth metatarsal fractures. UpToDate. January 31, 2020. www.uptodate.com/contents/proximal-fifth-metatarsal-fractures. Accessed May 26, 2020.
30. Welck MJ, Hayes T, Pastides P, et al. Stress fractures of the foot and ankle. Injury. 2017;48:1722-1726.
1. Bica D, Sprouse RA, Armen J. Diagnosis and management of common foot fractures. Am Fam Physician. 2016;93:183-191.
2. Gross CE, Nunley JA 2nd. Acute Achilles tendon ruptures. Foot Ankle Int. 2016;37:233-239.
3. Cooper MT. Acute Achilles tendon ruptures: does surgery offer superior results (and other confusing issues)? Clin Sports Med. 2015;34:595-606.
4. Maffulli N, Via AG, Oliva F. Chronic Achilles tendon disorders: tendinopathy and chronic rupture. Clin Sports Med. 2015;34:607-624.
5. Hutchison A-M, Evans R, Bodger O, et al. What is the best clinical test for Achilles tendinopathy? Foot Ankle Surg. 2013;19:112-117.
6. Kadakia AR, Dekker RG 2nd, Ho BS. Acute Achilles tendon ruptures: an update on treatment. Am Acad Orthop Surg. 2017;25:23-31.
7. van Zuuren WJ, Schepers T, Beumer A, et al. Acute syndesmotic instability in ankle fractures: a review. Foot Ankle Surg. 2017;23:135-141.
8. van Dijk CN, Longo UG, Loppini M, et al. Classification and diagnosis of acute isolated syndesmotic injuries: ESSKA–AFAS consensus and guidelines. Knee Surg Sports Traumatol Arthrosc. 2016;24:1200-1216.
9. Fort NM, Aiyer AA, Kaplan JR, et al. Management of acute injuries of the tibiofibular syndesmosis. Eur J Orthop Surg Traumatol. 2017;27:449-459.
10. Miller TL, Skalak T. Evaluation and treatment recommendations for acute injuries to the ankle syndesmosis without associated fracture. Sports Med. 2014;44:179-188.
11. Hunt KJ, Phisitkul P, Pirolo J, et al. High ankle sprains and syndesmotic injuries in athletes. J Am Acad Orthop Surg. 2015;23:661-673.
12. DeWeber K. Syndesmotic ankle injury (high ankle sprain). UpToDate. September 17, 2019. www.uptodate.com/contents/syndesmotic-ankle-injury-high-ankle-sprain. Accessed May 26, 2020.
13. Goost H, Wimmer MD, Barg A, et al. Fractures of the ankle joint: investigation and treatment options. Dtsch Arztebl Int. 2014;111:377-388.
14. Qin C, Dekker RG, Helfrich MM, et al. Outpatient management of ankle fractures. Orthop Clin North Am. 2018;49:103-108.
15. Stiell IG, Greenberg GH, McKnight RD, et al. Decision rules for the use of radiography in acute ankle injuries. Refinement and prospective validation. JAMA. 1993;269:1127-1132.
16. Jenkin M, Sitler MR, Kelly JD. Clinical usefulness of the Ottawa Ankle Rules for detecting fractures of the ankle and midfoot. J Athl Train. 2010;45:480-482.
17. Glas AS, Pijnenburg BACM, Lijmer JG, et al. Comparison of diagnostic decision rules and structured data collection in assessment of acute ankle injury. CMAJ. 2002;166:727-733.
18. Leduc S, Nault M-L, Rouleau DM, et al. My experience as a foot and ankle trauma surgeon in Montreal, Canada: what’s not in the books. Foot Ankle Clin. 2016;21:297-334.
19. Ibrahim MS, Jordan R, Lotfi N, et al. Talar head fracture: a case report, systematic review and suggested algorithm of treatment. Foot (Edinb). 2015;25:258-264.
20. Shank JR, Benirschke SK, Swords MP. Treatment of peripheral talus fractures. Foot Ankle Clin. 2017;22:181-192.
21. Kwaadu KY. Management of talar fractures. Clin Podiatr Med Sur. 2018;35:161-173.
22. Yu JS. Easily missed fractures in the lower extremity. Radiol Clin North Am. 2015;53:737-755.
23. Welck MJ, Zinchenko R, Rudge B. Lisfranc injuries. Injury. 2015;46:536-541.
24. Seybold JD, Coetzee JC. Lisfranc injuries: when to observe, fix, or fuse. Clin Sports Med. 2015;34:705-723.
25. Puna RA, Tomlinson MPW. The role of percutaneous reduction and fixation of lisfranc injuries. Foot Ankle Clin. 2017;22:15-34.
26. Weatherford BM, Bohay DR, Anderson JG. Open reduction and internal fixation versus primary arthrodesis for Lisfranc injuries. Foot Ankle Clin. 2017;22:1-14.
27. Porter DA. Fifth metatarsal Jones fractures in the athlete. Foot Ankle Int. 2018;39:250-258.
28. Cheung CN, Lui TH. Proximal fifth metatarsal fractures: anatomy, classification, treatment and complications. Arch Trauma Res. 2016;5:e32298.
29. Alsobrook J, Hatch RL. Proximal fifth metatarsal fractures. UpToDate. January 31, 2020. www.uptodate.com/contents/proximal-fifth-metatarsal-fractures. Accessed May 26, 2020.
30. Welck MJ, Hayes T, Pastides P, et al. Stress fractures of the foot and ankle. Injury. 2017;48:1722-1726.
PRACTICE RECOMMENDATIONS
› Suspect higher-grade syndesmotic disruption (which typically requires surgical intervention) in patients whose ankle pain persists after 3 weeks of immobilization or who have a tibial or fibular diastasis on a plain film. C
› Order weight-bearing x-rays to make an accurate diagnosis of Lisfranc injury. Refer for potential surgical intervention if diastasis is evident at the base between the first and second metatarsals. C
› Distinguish between proximal diaphysial (Jones) fracture of the fifth metatarsal, diaphysial stress fracture, and avulsion fracture—essential because avulsion fracture can be treated nonoperatively but the other 2 require surgical intervention. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Earning the trust of families
In a difficult field like medicine, it’s always nice when people appreciate what you’re trying to do. Even if things are good or bad in a case, it means a lot when they know you’re trying your best and are grateful for it.
I’m not saying I expect it (I don’t), but it’s still nice when it happens.
Most of the time someone will say thank you. Occasionally I’ll get a card, or rarely a small gift or box of candy at the holidays. I’m not asking for them, but it’s thoughtful when they do that.
But perhaps Or two. Or three.
Last week I had a nice college kid in to see me. I’d seen his mother in the past. And both of her parents.
When you have a third generation of a family coming in ... you must be doing something right.
I got curious, began looking through my charts, and was surprised by how many different families had two to three generations seeing me. In several cases the original patient had passed on, but obviously the family had felt good enough about me to come here when the need arose.
That really means a lot when you think about it. In a world in which many see doctors as interchangeable with each other and physician extenders, and where insurance plans seem to drop and sign practices at random, people have a lot of doctors to choose from. The fact that a family thinks highly enough of me to keep returning is flattering.
Medicine is never an easy job, even outside the endless paperwork and other, often pointless, things it requires. In spite of this, we all work hard to care for patients to the best of our ability. It’s nice when they feel we are, too, and trust us enough to share that sentiment with loved ones.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In a difficult field like medicine, it’s always nice when people appreciate what you’re trying to do. Even if things are good or bad in a case, it means a lot when they know you’re trying your best and are grateful for it.
I’m not saying I expect it (I don’t), but it’s still nice when it happens.
Most of the time someone will say thank you. Occasionally I’ll get a card, or rarely a small gift or box of candy at the holidays. I’m not asking for them, but it’s thoughtful when they do that.
But perhaps Or two. Or three.
Last week I had a nice college kid in to see me. I’d seen his mother in the past. And both of her parents.
When you have a third generation of a family coming in ... you must be doing something right.
I got curious, began looking through my charts, and was surprised by how many different families had two to three generations seeing me. In several cases the original patient had passed on, but obviously the family had felt good enough about me to come here when the need arose.
That really means a lot when you think about it. In a world in which many see doctors as interchangeable with each other and physician extenders, and where insurance plans seem to drop and sign practices at random, people have a lot of doctors to choose from. The fact that a family thinks highly enough of me to keep returning is flattering.
Medicine is never an easy job, even outside the endless paperwork and other, often pointless, things it requires. In spite of this, we all work hard to care for patients to the best of our ability. It’s nice when they feel we are, too, and trust us enough to share that sentiment with loved ones.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In a difficult field like medicine, it’s always nice when people appreciate what you’re trying to do. Even if things are good or bad in a case, it means a lot when they know you’re trying your best and are grateful for it.
I’m not saying I expect it (I don’t), but it’s still nice when it happens.
Most of the time someone will say thank you. Occasionally I’ll get a card, or rarely a small gift or box of candy at the holidays. I’m not asking for them, but it’s thoughtful when they do that.
But perhaps Or two. Or three.
Last week I had a nice college kid in to see me. I’d seen his mother in the past. And both of her parents.
When you have a third generation of a family coming in ... you must be doing something right.
I got curious, began looking through my charts, and was surprised by how many different families had two to three generations seeing me. In several cases the original patient had passed on, but obviously the family had felt good enough about me to come here when the need arose.
That really means a lot when you think about it. In a world in which many see doctors as interchangeable with each other and physician extenders, and where insurance plans seem to drop and sign practices at random, people have a lot of doctors to choose from. The fact that a family thinks highly enough of me to keep returning is flattering.
Medicine is never an easy job, even outside the endless paperwork and other, often pointless, things it requires. In spite of this, we all work hard to care for patients to the best of our ability. It’s nice when they feel we are, too, and trust us enough to share that sentiment with loved ones.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Anorexia nervosa and COVID-19
Recent concerns surrounding coronavirus disease 2019 (COVID-19) make it timely to reexamine the complex findings related to eating disorders and the immune system, and the risks for and detection of infection in patients with anorexia nervosa (AN) and similar disorders. To date, there are no published studies evaluating patients with eating disorders and COVID-19. However, it may be helpful to review the data on the infectious process in this patient population to improve patient communication, enhance surveillance and detection, and possibly reduce morbidity and mortality.
The Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) issued warnings that individuals who are older, have underlying medical conditions, and/or are immunocompromised face the greatest risk of serious complications and death as a result of COVID-19, the disease process caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Due to malnutrition, patients with eating disorders, especially AN, may be perceived to have an increased risk of medical conditions and infection. Despite many studies on specific changes and differences in the immune system of patients with eating disorders, the consequences of these changes remain controversial and inconclusive.
This article reviews research on eating disorders, focusing on published data regarding the effects of AN on the immune system, susceptibility to infections, infectious detection, and morbidity. We also discuss clinical considerations related to COVID-19 and patients with AN.
Infection risks: Conflicting data
In a 1981 study that included 9 participants, Golla et al1 concluded that patients with AN may have “resistance” to infections based on a suggested protective factor within the immune system of these patients. Because this study has been cited repeatedly in multiple articles about AN and cell-mediated immunity,2-7 some clinicians have accepted this evidence of resistance to infection in patients with AN, which may lower their suspicion for and detection of infections in patients with AN.
However, studies published both before and after Golla et al1 have shown statistically significant results that contradict those researchers’ conclusion. A study that compared the medical records of 68 patients with AN with those who did not have AN found no significant difference, and concluded that the rate of infection among patients with AN is the same as among controls.8 These researchers noted that infection rates may be higher among patients with later-stage, more severe AN. In a 1986 study of 12 patients with AN, Cason et al9 concluded that while cellular immunity function is abnormal in patients with AN, their results were not compatible with prior studies that suggested AN patients were more resistant to infection.1,2,8
More recently, researchers compared 1,592 patients with eating disorders with 6,368 matched controls; they reviewed prescriptions of antibacterial, antifungal, and antiviral medications as a measure of infection rates.10 Compared with controls, patients with binge eating disorder (BED), patients with bulimia nervosa (BN), and males with AN more often received prescriptions for antimicrobial medications. There was no statistically significant difference between controls and females with AN, which is consistent with other reports of no increased or decreased risk of infection among females with AN. In terms of antiviral use, this study showed an increased prescription of antivirals only in the BN group.
Several other studies examining the rate of infection in patients with AN concluded that there is neither an increased nor decreased rate of infection in patients with AN, and that the rate of infection in this population is similar to that of the general population.8,10-12 Because studies that have included patients with AN have evaluated only symptomatic viral infections, some researchers have proposed that patients with AN may show lower rates of symptomatic viral infection but higher rates of asymptomatic infection, as evidenced by higher viral titers.6 Further research is required. Despite controversy regarding infection rates, studies have found that patients with AN have increased rates of morbidity and mortality from infections.6,12-16
Continue to: Obstacles to detecting infections
Obstacles to detecting infections
Several factors can complicate the surveillance and detection of infections in patients with eating disorders, especially those with AN. These include:
- an accepted predisposition to infection secondary to malnutrition
- a lack of visual or reported infectious symptoms
- misrepresentation and assumptions from published research.
Clinicians who report fewer observed cases of infections among patients with AN may be overlooking comorbid disease processes due to a bias from the literature and/or a lack of awareness of symptom parameters in patients with AN.
Features of AN include a loss of adipose tissue responsible for pro-inflammatory cytokines, and excessive exercise, which stimulates anti-inflammatory myokines. This can modulate the experience of illness that impacts the core features of disease,17 possibly reducing symptomatic presentation of infections.
Fever. The presence and intensity of fever may be altered in patients with eating disorders, especially those with AN. In a study of 311 inpatients with AN, researchers found that patients with AN had a significant delay in fever response in AN.12 Of 23 patients with an active bacterial infection, all but 5 had a fever <37°C, with some as low as 35.5°C. A detectable fever response and unexplained fevers were found in 2 of the 6 patients with a viral infection. A series of case studies found that patients with AN with bacterial infections also had a delayed fever response.18
For patients with infections that commonly present with fever, such as COVID-19, a delayed fever response can delay or evade the detection of infection, thus increasing potential complications as well viral exposure to others. Thus, clinicians should use caution when ruling out COVID-19 or other infections because of a lack of significant fever.
Continue to: Overlapping symptoms
Overlapping symptoms. The symptoms of viral infection can mimic the symptoms of AN, which further complicates screening and diagnosis of infection in these patients. Although up to 80% of individuals infected with COVID-19 may be asymptomatic or have a mild presentation, the most common reported symptoms are fever (92.6%), shortness of breath (50.8%), expectoration (41.4%), fatigue (46.4%), dry cough (33.3%), and myalgia (21.4%).19-21 Gastrointestinal (GI) symptoms have been reported in patients with COVID-19, as well as a loss of taste and smell.
Commonly reported physical symptoms of AN include an intolerance to cold, general fatigue, muscle aches and pains, restlessness, emesis, and a multitude of GI complaints. Patients with AN also have been reported to experience shortness of breath due to conditions such as respiratory muscle weakness,22 nutritional emphysema,23 and anxiety and panic attack.24 These conditions could lead to an increased susceptibility to COVID-19 and increased complications during treatment. Cardiac abnormalities, which are common in patients with AN and BN, may increase the risk of adverse events. While these symptoms may be an important part of screening for diseases such as COVID-19, suspicion of infection also may be lower because of the overlap of AN symptomology, underlying conditions, and a delayed fever response.
Laboratory findings. Laboratory testing results for patients with COVID-19 include lower lymphocyte counts, higher leukocyte counts, elevated levels of infection-related biomarkers and inflammatory cytokines, and significantly decreased T-cell counts.19 Similar values are also found in patients with AN.
The similar clinical presentations and laboratory values of AN and COVID-19 could lead to delayed diagnosis, increased disease transmission, cross-contamination of facilities, and higher incidences of medical complications and mortality.
The immunology of AN and correlations with COVID-19
Many studies examining the immune system of patients with eating disorders, especially those with AN, have discovered changes and differences in both cell-mediated and humoral response to infections.1,3,5,7,9,11,16,21,25-27 Whether these differences represent a dysfunctional immune system, an immunocompromised state, or even a protective factor remains unclear.
Continue to: While some studies have reported...
While some studies have reported that AN represents an immunocompromised state, others describe the immune system of patients with AN as dysfunctional or simply altered.9,11,22,28 Some studies have found that patients with AN had delayed reactions to pathogen skin exposures compared with healthy controls, which provides evidence of an impaired cell-mediated immune system.9,27,29
Some studies have considered the consequences of infection and immunologic findings as markers of or contributing to the onset of AN.2,30,31 Numerous studies have noted abnormalities in AN with regards to cell-mediated immunity, the humoral system, the lymphoreticular system, and the innate immune system, and potential contributions from increased oxidative stress, a chronically activated sympathetic nervous system and hypothalamic-pituitary-adrenal axis, altered intestinal microbiota, and an abnormal bone marrow microenvironment.2
Box 1
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a new beta-coronavirus that is still being studied for its effects on the immune system. It may take years to fully understand the nature of the pathogen and the response of the human immune system. To better understand COVID19, researchers have been turning to what they learned from the past outbreaks of severe acute respiratory syndrome (SARS) in 2003- 2004 and Middle East respiratory syndrome (MERS) in 2011, both caused by betacoronaviruses with a zoonotic origin.25,32
The proposed pathogenesis for infection of SARS-CoV-2 is similar to SARS and occurs when aerosolized droplets containing the virus enter the host.32 While currently there is only initial data on the host innate immune status of patients infected with SARS-CoV-2, initial findings of a report on 99 cases in Wuhan, China included increased total neutrophils (38%), reduced total lymphocytes (35%), increased serum interleukin-6 (52%), and increased C-reactive protein (84%).33 Additional findings were decreased percentages of monocytes, eosinophils, and basophils, as well as significantly decreased levels of cytokines and T-cells in more severe cases.19 Past research with SARS reported similar T-cell findings, with a more frequent CD8+ response and a greater magnitude of CD4+.34
Box 119,25,32-34 describes some of the initial immunologic findings reported in patients with COVID-19. In Box 2,5,8,11,13,14,19,26,28,35-40 we discuss reports that describe the immunologic overlay of COVID-19 and AN.
Box 2
Leukopenia (low leukocyte levels) is a common finding in patients with anorexia nervosa (AN),8 and often leads clinicians to lower their suspicion for infection. A 2008 Hungarian study that evaluated lymphocyte activation parameters and clinical status in 11 adolescents (10 girls and 1 boy) with AN, 12 obese adolescents, and 10 healthy controls did not find any association between the variables.35 While many studies have focused on adults, it is important to note that leukopenia is a common finding in adolescents (age 12 to 17) with AN.36
Leukocyte counts are elevated in coronavirus disease 2019 (COVID-19), possibly offsetting AN’s leukopenia. In addition, neutrophil counts are elevated and monocyte, eosinophil, basophil, and especially lymphocyte counts are significantly decreased. A meta-analysis that included 22 studies and 924 participants (512 with AN and 412 controls) examined common inflammatory cytokine findings in patients with AN.11 Compared with healthy controls, patients with AN had significantly elevated levels of tumor necrosis factor alpha (TNF-alpha), interleukin (IL)-1, IL-6, and TNF-receptor II, and significantly decreased levels of C-reactive protein and IL-6 receptor. Elevated levels of TNF-alpha and IL-6 also have been reported in patients with COVID-19.19 These findings may mask suspicion for infection in patients with AN.19
In patients with AN and those with bulimia nervosa, CD4+-to-CD8+ ratios also have been found to be low as a result of normal-tohigher levels of CD4+ cells and lower levels of CD8+ cells.36-39 Researchers have also proposed that the lymphocytosis observed in AN is a result of increased naïve CD4+.36 In AN, total lymphocyte counts have been found to correlate positively with a patient’s body mass index (BMI), while the CD4+ T-lymphocyte correlated negatively with BMI and were critically low in patients with severe malnutrition.26,40 In patients with COVID-19, CD4+ levels have reported to be within normal range, naïve CD4+ cells were elevated, and CD8+ cells were slightly decreased,19 which is similar to the findings in AN.
Fewer studies have evaluated humoral immune response in AN, and results have varied. One study (N = 46) found elevated B-cell counts in adolescents with AN-restricting type,36 while another (N = 40) reported normal levels of B-cells.5 Specific decreases in immunoglobulin (Ig) G and IgM have also been reported in AN, while IgA, IgG, and IgM usually are normal in COVID-19.19
Despite differences in immune system function, cellular immunity appears to remain relatively intact in patients with AN, but can become compromised with severe malnutrition or with advanced weight loss.28,40 This compromised immunity related to severe AN with a very low BMI likely leads to the increased morbidity and mortality.8,13,14
Malnutrition and the immune system
Differences in the type of malnutrition observed in low-weight patients with AN may help explain why patients with AN can maintain a relatively intact cell-mediated immune system.1 Protein-energy malnutrition (PEM), which is found in typical states of starvation, consists of deficiencies in multiple vitamins, protein, and energy (caloric content), whereas the dietary habits of patients with AN usually result in a deficiency of carbohydrates and fats.41 Studies that examined the impact of PEM on immunity to influenza infection have suggested that balanced protein energy replenishment may be a strategy for boosting immunity against influenza viral infections.42 However, carbohydrates are the primary nutrients for human bone marrow fat cells, which play a crucial role in the maturation of white blood cells. This may account for the leukopenia that is common in patients with AN.6,43 The protein-sparing aspect of the typical AN diet may account for the immune system changes observed in patients with AN.44
Although some studies have proposed that immune deficiencies observed in patients with AN are secondary to malnutrition and return to normal with refeeding,5,40,45 others have concluded that immune function is not compromised by factors such as nutritional status or body weight in AN.26,43,46
Continue to: Clinical considerations
Clinical considerations
Neither the CDC nor the WHO have issued a specific protocol for monitoring for and treating COVID-19 in patients with eating disorders; however, the guidelines offered by these organizations for the general population should be followed for patients with eating disorders.
When screening a patient with an eating disorder, keep in mind that the symptoms of eating disorders, such as AN, may mimic an infectious process. Mood symptoms, such as depression or anxiety, could represent physiological responses to infection. Patients with GI symptoms that typically are considered part of the pathology of an eating disorder should be more carefully considered for COVID-19. Monitor a patient’s basal body temperature, and be mindful that a patient with AN may exhibit a delayed fever response. Be vigilant for a recent loss of taste or smell, which should raise suspicion for COVID-19. When monitoring vital signs, pay careful attention for any decompensation in a patient’s pulse oximetry. Whenever possible, order COVID-19 testing for any patient you suspect may be infected.
Outpatient clinicians should work closely in a collaborative manner with a patient’s eating disorder treatment team. Psychiatrists, primary care physicians, psychotherapists, nutritionists, and other clinicians should all follow CDC/WHO guidelines regarding COVID-19, provide surveillance, and communicate any suspicions to the medical team. Eating disorder treatment programs, including residential centers, partial hospital programs (PHP), and intensive outpatient programs (IOP), must enhance monitoring for COVID-19, and exercise caution by practicing social distancing and providing adequate personal protective equipment for patients and staff. To reduce the spread of COVID-19, many IOPs and PHPs have transitioned to virtual treatment. Residential centers must carefully screen patients before admission to weigh the risks and benefits of inpatient vs outpatient care.
Bottom Line
Differences in the immune system of patients with an eating disorder do not necessarily confer a higher or lower risk of infection. Symptoms of some infections can mimic the symptoms of anorexia nervosa. Recognizing infections in patients with eating disorders is critical because compared with the general population, they have higher rates of infection-related morbidity and mortality.
Related Resources
- Congress J, Madaan V. 6 ‘M’s to keep in mind when you next see a patient with anorexia nervosa. Current Psychiatry. 2014;13(5):58-59.
- Westmoreland P. Eating disorders: Masterclass lecture part I. Psychcast (podcast). https://www.mdedge.com/podcasts/psychcast/eating-disorders-masterclass-lecture-part-i.
1. Golla JA, Larson LA, Anderson CF, et al. An immunological assessment of patients with anorexia nervosa. Am J Clin Nutr. 1981;34(12):2756-2762.
2. Gibson D, Mehler PS. Anorexia nervosa and the immune system—a narrative review. J Clin Med. 2019;8(11):1915. doi: 10.3390/jcm8111915.
3. Słotwin
4. Nova E, Samartín S, Gómez S, et al. The adaptive response of the immune system to the particular malnutrition of eating disorders. Eur J Clin Nutr. 2002;56(suppl 3):S34-S37.
5. Allende LM, Corell A, Manzanares J, et al. Immunodeficiency associated with anorexia nervosa is secondary and improves after refeeding. Immunology. 1998;94(4):543-551.
6. Brown RF, Bartrop R, Birmingham CL. Immunological disturbance and infectious disease in anorexia nervosa: a review. Acta Neuropsychiatr. 2008;20(3):117-128.
7. Polack E, Nahmod VE, Emeric-Sauval E, et al. Low lymphocyte interferon-gamma production and variable proliferative response in anorexia nervosa patients. J Clin Immunol. 1993;13(6):445-451.
8. Bowers TK, Eckert E. Leukopenia in anorexia nervosa. Lack of increased risk of infection. Arch Intern Med. 1978;138(10):1520-1523.
9. Cason J, Ainley CC, Wolstencroft RA, et al. Cell-mediated immunity in anorexia nervosa. Clin Exp Immunol. 1986;64(2):370-375.
10. Raevuori A, Lukkariniemi L, Suokas JT, et al. Increased use of antimicrobial medication in bulimia nervosa and binge-eating disorder prior to the eating disorder treatment. Int J Eat Disord. 2016;49(6):542-552.
11. Solmi M, Veronese N, Favaro A, et al. Inflammatory cytokines and anorexia nervosa: a meta-analysis of cross-sectional and longitudinal studies. Psychoneuroendocrinology. 2015;51:237-252.
12. Brown RF, Bartrop R, Beumont P, et al. Bacterial infections in anorexia nervosa: delayed recognition increases complications. Int J Eat Disord. 2005;37(3):261-265.
13. Theander S. Anorexia nervosa. A psychiatric investigation of 94 female patients. Acta Psychiatr Scand Suppl. 1970;214:1-194.
14. Warren MP, Vande Wiele RL. Clinical and metabolic features of anorexia nervosa. Am J Obstet Gynecol. 1973;117(3):435-449.
15. Copeland PM, Herzog DB. Hypoglycemia and death in anorexia nervosa. Psychother Psychosom. 1987;48(1-4):146-150.
16. Devuyst O, Lambert M, Rodhain J, et al. Haematological changes and infectious complications in anorexia nervosa: a case-control study. Q J Med. 1993;86(12):791-799.
17. Pisetsky DS, Trace SE, Brownley KA, et al. The expression of cytokines and chemokines in the blood of patients with severe weight loss from anorexia nervosa: an exploratory study. Cytokine. 2014;69(1):110-115.
18. Birmingham CL, Hodgson DM, Fung J, et al. Reduced febrile response to bacterial infection in anorexia nervosa patients. Int J Eat Disord. 2003;34(2):269-272.
19. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China [published online March 12, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa248.
20. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
21. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514-523.
22. Birmingham CL, Tan AO. Respiratory muscle weakness and anorexia nervosa. Int J Eat Disord. 2003;33(2):230-233.
23. Cook VJ, Coxson HO, Mason AG, et al. Bullae, bronchiectasis and nutritional emphysema in severe anorexia nervosa. Can Respir J. 2001;8(5):361-365.
24. Khalsa SS, Hassanpour MS, Strober M, et al. Interoceptive anxiety and body representation in anorexia nervosa [published online September 21, 2018]. Front Psychiatry. 2018;9:444. doi: 10.3389/fpsyt.2018.00444.
25. van West D, Maes M. Cytokines in de obsessief compulsieve stoornis en in anorexia nervosa: een overzicht. Acta Neuropsychiatr. 1999;11(4):125-129.
26. Komorowska-Pietrzykowska R, Rajewski A, Wiktorowicz K, et al. Czynnos
27. Marcos A, Varela P, Toro O, et al. Interactions between nutrition and immunity in anorexia nervosa: a 1-y follow-up study. Am J Clin Nutr. 1997;66(2):485S-490S.
28. Pertschuk MJ, Crosby LO, Barot L, et al. Immunocompetency in anorexia nervosa. Am J Clin Nutr. 1982;35(5):968-972.
29. Varela P, Marcos A, Navarro MP. Zinc status in anorexia nervosa. Ann Nutr Metab. 1992;36(4):197-202.
30. Breithaupt L, Köhler-Forsberg O, Larsen JT, et al. Association of exposure to infections in childhood with risk of eating disorders in adolescent girls. JAMA Psychiatry. 2019;76(8):800-809.
31. Brambilla F, Monti D, Franceschi C. Plasma concentrations of interleukin-1-beta, interleukin-6 and tumor necrosis factor-alpha, and of their soluble receptors and receptor antagonist in anorexia nervosa. Psychiatry Res. 2001;103(2-3):107-114.
32. Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic [published online February 27, 2020]. Asian Pac J Allergy Immunol. doi: 10.12932/AP-200220-0772.
33. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270-273.
34. Li CK, Wu H, Yan H, et al. T cell responses to whole SARS coronavirus in humans. J Immunol. 2008;181(8):5490-5500.
35. Páli AA, Pászthy B. Az immunrendszer muködésének megváltozása a táplálkozási magatartás zavarai esetén [Changes of the immune functions in patients with eating disorders]. Ideggyogy Sz. 2008;61(11-12):381‐384.
36. Elegido A, Graell M, Andrés P, et al. Increased naive CD4+ and B lymphocyte subsets are associated with body mass loss and drive relative lymphocytosis in anorexia nervosa patients. Nutr Res. 2017;39:43-50.
37. Marcos A, Varela P, Santacruz I, et al. Nutritional status and immunocompetence in eating disorders. A comparative study. Eur J Clin Nutr. 1993;47(11):787-793.
38. Mustafa A, Ward A, Treasure J, et al. T lymphocyte subpopulations in anorexia nervosa and refeeding. Clin Immunol Immunopathol. 1997;82(3):282-289.
39. Nagata T, Kiriike N, Tobitani W, et al. Lymphocyte subset, lymphocyte proliferative response, and soluble interleukin-2 receptor in anorexic patients. Biol Psychiatry. 1999;45(4):471-474.
40. Saito H, Nomura K, Hotta M, et al. Malnutrition induces dissociated changes in lymphocyte count and subset proportion in patients with anorexia nervosa. Int J Eat Disord. 2007;40(6):575-579.
41. Nova E, Varela P, López-Vidriero I, et al. A one-year follow-up study in anorexia nervosa. Dietary pattern and anthropometrical evolution. Eur J Clin Nutr. 2001;55(7):547-554.
42. Taylor AK, Cao W, Vora KP, et al. Protein energy malnutrition decreases immunity and increases susceptibility to influenza infection in mice. J Infect Dis. 2013;207(3):501-510.
43. Mant MJ, Faragher BS. The hematology of anorexia nervosa. Br J Haematol. 1972;23(6):737-749.
44. Marcos A. The immune system in eating disorders: an overview. Nutrition. 1997;13(10):853-862.
45. Schattner A, Tepper R, Steinbock M, et al. TNF, interferon-gamma and cell-mediated cytotoxicity in anorexia nervosa; effect of refeeding. J Clin Lab Immunol. 1990;32(4):183-184.
46. Nagata T, Tobitani W, Kiriike N, et al. Capacity to produce cytokines during weight restoration in patients with anorexia nervosa. Psychosom Med. 1999;61(3):371-377.
Recent concerns surrounding coronavirus disease 2019 (COVID-19) make it timely to reexamine the complex findings related to eating disorders and the immune system, and the risks for and detection of infection in patients with anorexia nervosa (AN) and similar disorders. To date, there are no published studies evaluating patients with eating disorders and COVID-19. However, it may be helpful to review the data on the infectious process in this patient population to improve patient communication, enhance surveillance and detection, and possibly reduce morbidity and mortality.
The Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) issued warnings that individuals who are older, have underlying medical conditions, and/or are immunocompromised face the greatest risk of serious complications and death as a result of COVID-19, the disease process caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Due to malnutrition, patients with eating disorders, especially AN, may be perceived to have an increased risk of medical conditions and infection. Despite many studies on specific changes and differences in the immune system of patients with eating disorders, the consequences of these changes remain controversial and inconclusive.
This article reviews research on eating disorders, focusing on published data regarding the effects of AN on the immune system, susceptibility to infections, infectious detection, and morbidity. We also discuss clinical considerations related to COVID-19 and patients with AN.
Infection risks: Conflicting data
In a 1981 study that included 9 participants, Golla et al1 concluded that patients with AN may have “resistance” to infections based on a suggested protective factor within the immune system of these patients. Because this study has been cited repeatedly in multiple articles about AN and cell-mediated immunity,2-7 some clinicians have accepted this evidence of resistance to infection in patients with AN, which may lower their suspicion for and detection of infections in patients with AN.
However, studies published both before and after Golla et al1 have shown statistically significant results that contradict those researchers’ conclusion. A study that compared the medical records of 68 patients with AN with those who did not have AN found no significant difference, and concluded that the rate of infection among patients with AN is the same as among controls.8 These researchers noted that infection rates may be higher among patients with later-stage, more severe AN. In a 1986 study of 12 patients with AN, Cason et al9 concluded that while cellular immunity function is abnormal in patients with AN, their results were not compatible with prior studies that suggested AN patients were more resistant to infection.1,2,8
More recently, researchers compared 1,592 patients with eating disorders with 6,368 matched controls; they reviewed prescriptions of antibacterial, antifungal, and antiviral medications as a measure of infection rates.10 Compared with controls, patients with binge eating disorder (BED), patients with bulimia nervosa (BN), and males with AN more often received prescriptions for antimicrobial medications. There was no statistically significant difference between controls and females with AN, which is consistent with other reports of no increased or decreased risk of infection among females with AN. In terms of antiviral use, this study showed an increased prescription of antivirals only in the BN group.
Several other studies examining the rate of infection in patients with AN concluded that there is neither an increased nor decreased rate of infection in patients with AN, and that the rate of infection in this population is similar to that of the general population.8,10-12 Because studies that have included patients with AN have evaluated only symptomatic viral infections, some researchers have proposed that patients with AN may show lower rates of symptomatic viral infection but higher rates of asymptomatic infection, as evidenced by higher viral titers.6 Further research is required. Despite controversy regarding infection rates, studies have found that patients with AN have increased rates of morbidity and mortality from infections.6,12-16
Continue to: Obstacles to detecting infections
Obstacles to detecting infections
Several factors can complicate the surveillance and detection of infections in patients with eating disorders, especially those with AN. These include:
- an accepted predisposition to infection secondary to malnutrition
- a lack of visual or reported infectious symptoms
- misrepresentation and assumptions from published research.
Clinicians who report fewer observed cases of infections among patients with AN may be overlooking comorbid disease processes due to a bias from the literature and/or a lack of awareness of symptom parameters in patients with AN.
Features of AN include a loss of adipose tissue responsible for pro-inflammatory cytokines, and excessive exercise, which stimulates anti-inflammatory myokines. This can modulate the experience of illness that impacts the core features of disease,17 possibly reducing symptomatic presentation of infections.
Fever. The presence and intensity of fever may be altered in patients with eating disorders, especially those with AN. In a study of 311 inpatients with AN, researchers found that patients with AN had a significant delay in fever response in AN.12 Of 23 patients with an active bacterial infection, all but 5 had a fever <37°C, with some as low as 35.5°C. A detectable fever response and unexplained fevers were found in 2 of the 6 patients with a viral infection. A series of case studies found that patients with AN with bacterial infections also had a delayed fever response.18
For patients with infections that commonly present with fever, such as COVID-19, a delayed fever response can delay or evade the detection of infection, thus increasing potential complications as well viral exposure to others. Thus, clinicians should use caution when ruling out COVID-19 or other infections because of a lack of significant fever.
Continue to: Overlapping symptoms
Overlapping symptoms. The symptoms of viral infection can mimic the symptoms of AN, which further complicates screening and diagnosis of infection in these patients. Although up to 80% of individuals infected with COVID-19 may be asymptomatic or have a mild presentation, the most common reported symptoms are fever (92.6%), shortness of breath (50.8%), expectoration (41.4%), fatigue (46.4%), dry cough (33.3%), and myalgia (21.4%).19-21 Gastrointestinal (GI) symptoms have been reported in patients with COVID-19, as well as a loss of taste and smell.
Commonly reported physical symptoms of AN include an intolerance to cold, general fatigue, muscle aches and pains, restlessness, emesis, and a multitude of GI complaints. Patients with AN also have been reported to experience shortness of breath due to conditions such as respiratory muscle weakness,22 nutritional emphysema,23 and anxiety and panic attack.24 These conditions could lead to an increased susceptibility to COVID-19 and increased complications during treatment. Cardiac abnormalities, which are common in patients with AN and BN, may increase the risk of adverse events. While these symptoms may be an important part of screening for diseases such as COVID-19, suspicion of infection also may be lower because of the overlap of AN symptomology, underlying conditions, and a delayed fever response.
Laboratory findings. Laboratory testing results for patients with COVID-19 include lower lymphocyte counts, higher leukocyte counts, elevated levels of infection-related biomarkers and inflammatory cytokines, and significantly decreased T-cell counts.19 Similar values are also found in patients with AN.
The similar clinical presentations and laboratory values of AN and COVID-19 could lead to delayed diagnosis, increased disease transmission, cross-contamination of facilities, and higher incidences of medical complications and mortality.
The immunology of AN and correlations with COVID-19
Many studies examining the immune system of patients with eating disorders, especially those with AN, have discovered changes and differences in both cell-mediated and humoral response to infections.1,3,5,7,9,11,16,21,25-27 Whether these differences represent a dysfunctional immune system, an immunocompromised state, or even a protective factor remains unclear.
Continue to: While some studies have reported...
While some studies have reported that AN represents an immunocompromised state, others describe the immune system of patients with AN as dysfunctional or simply altered.9,11,22,28 Some studies have found that patients with AN had delayed reactions to pathogen skin exposures compared with healthy controls, which provides evidence of an impaired cell-mediated immune system.9,27,29
Some studies have considered the consequences of infection and immunologic findings as markers of or contributing to the onset of AN.2,30,31 Numerous studies have noted abnormalities in AN with regards to cell-mediated immunity, the humoral system, the lymphoreticular system, and the innate immune system, and potential contributions from increased oxidative stress, a chronically activated sympathetic nervous system and hypothalamic-pituitary-adrenal axis, altered intestinal microbiota, and an abnormal bone marrow microenvironment.2
Box 1
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a new beta-coronavirus that is still being studied for its effects on the immune system. It may take years to fully understand the nature of the pathogen and the response of the human immune system. To better understand COVID19, researchers have been turning to what they learned from the past outbreaks of severe acute respiratory syndrome (SARS) in 2003- 2004 and Middle East respiratory syndrome (MERS) in 2011, both caused by betacoronaviruses with a zoonotic origin.25,32
The proposed pathogenesis for infection of SARS-CoV-2 is similar to SARS and occurs when aerosolized droplets containing the virus enter the host.32 While currently there is only initial data on the host innate immune status of patients infected with SARS-CoV-2, initial findings of a report on 99 cases in Wuhan, China included increased total neutrophils (38%), reduced total lymphocytes (35%), increased serum interleukin-6 (52%), and increased C-reactive protein (84%).33 Additional findings were decreased percentages of monocytes, eosinophils, and basophils, as well as significantly decreased levels of cytokines and T-cells in more severe cases.19 Past research with SARS reported similar T-cell findings, with a more frequent CD8+ response and a greater magnitude of CD4+.34
Box 119,25,32-34 describes some of the initial immunologic findings reported in patients with COVID-19. In Box 2,5,8,11,13,14,19,26,28,35-40 we discuss reports that describe the immunologic overlay of COVID-19 and AN.
Box 2
Leukopenia (low leukocyte levels) is a common finding in patients with anorexia nervosa (AN),8 and often leads clinicians to lower their suspicion for infection. A 2008 Hungarian study that evaluated lymphocyte activation parameters and clinical status in 11 adolescents (10 girls and 1 boy) with AN, 12 obese adolescents, and 10 healthy controls did not find any association between the variables.35 While many studies have focused on adults, it is important to note that leukopenia is a common finding in adolescents (age 12 to 17) with AN.36
Leukocyte counts are elevated in coronavirus disease 2019 (COVID-19), possibly offsetting AN’s leukopenia. In addition, neutrophil counts are elevated and monocyte, eosinophil, basophil, and especially lymphocyte counts are significantly decreased. A meta-analysis that included 22 studies and 924 participants (512 with AN and 412 controls) examined common inflammatory cytokine findings in patients with AN.11 Compared with healthy controls, patients with AN had significantly elevated levels of tumor necrosis factor alpha (TNF-alpha), interleukin (IL)-1, IL-6, and TNF-receptor II, and significantly decreased levels of C-reactive protein and IL-6 receptor. Elevated levels of TNF-alpha and IL-6 also have been reported in patients with COVID-19.19 These findings may mask suspicion for infection in patients with AN.19
In patients with AN and those with bulimia nervosa, CD4+-to-CD8+ ratios also have been found to be low as a result of normal-tohigher levels of CD4+ cells and lower levels of CD8+ cells.36-39 Researchers have also proposed that the lymphocytosis observed in AN is a result of increased naïve CD4+.36 In AN, total lymphocyte counts have been found to correlate positively with a patient’s body mass index (BMI), while the CD4+ T-lymphocyte correlated negatively with BMI and were critically low in patients with severe malnutrition.26,40 In patients with COVID-19, CD4+ levels have reported to be within normal range, naïve CD4+ cells were elevated, and CD8+ cells were slightly decreased,19 which is similar to the findings in AN.
Fewer studies have evaluated humoral immune response in AN, and results have varied. One study (N = 46) found elevated B-cell counts in adolescents with AN-restricting type,36 while another (N = 40) reported normal levels of B-cells.5 Specific decreases in immunoglobulin (Ig) G and IgM have also been reported in AN, while IgA, IgG, and IgM usually are normal in COVID-19.19
Despite differences in immune system function, cellular immunity appears to remain relatively intact in patients with AN, but can become compromised with severe malnutrition or with advanced weight loss.28,40 This compromised immunity related to severe AN with a very low BMI likely leads to the increased morbidity and mortality.8,13,14
Malnutrition and the immune system
Differences in the type of malnutrition observed in low-weight patients with AN may help explain why patients with AN can maintain a relatively intact cell-mediated immune system.1 Protein-energy malnutrition (PEM), which is found in typical states of starvation, consists of deficiencies in multiple vitamins, protein, and energy (caloric content), whereas the dietary habits of patients with AN usually result in a deficiency of carbohydrates and fats.41 Studies that examined the impact of PEM on immunity to influenza infection have suggested that balanced protein energy replenishment may be a strategy for boosting immunity against influenza viral infections.42 However, carbohydrates are the primary nutrients for human bone marrow fat cells, which play a crucial role in the maturation of white blood cells. This may account for the leukopenia that is common in patients with AN.6,43 The protein-sparing aspect of the typical AN diet may account for the immune system changes observed in patients with AN.44
Although some studies have proposed that immune deficiencies observed in patients with AN are secondary to malnutrition and return to normal with refeeding,5,40,45 others have concluded that immune function is not compromised by factors such as nutritional status or body weight in AN.26,43,46
Continue to: Clinical considerations
Clinical considerations
Neither the CDC nor the WHO have issued a specific protocol for monitoring for and treating COVID-19 in patients with eating disorders; however, the guidelines offered by these organizations for the general population should be followed for patients with eating disorders.
When screening a patient with an eating disorder, keep in mind that the symptoms of eating disorders, such as AN, may mimic an infectious process. Mood symptoms, such as depression or anxiety, could represent physiological responses to infection. Patients with GI symptoms that typically are considered part of the pathology of an eating disorder should be more carefully considered for COVID-19. Monitor a patient’s basal body temperature, and be mindful that a patient with AN may exhibit a delayed fever response. Be vigilant for a recent loss of taste or smell, which should raise suspicion for COVID-19. When monitoring vital signs, pay careful attention for any decompensation in a patient’s pulse oximetry. Whenever possible, order COVID-19 testing for any patient you suspect may be infected.
Outpatient clinicians should work closely in a collaborative manner with a patient’s eating disorder treatment team. Psychiatrists, primary care physicians, psychotherapists, nutritionists, and other clinicians should all follow CDC/WHO guidelines regarding COVID-19, provide surveillance, and communicate any suspicions to the medical team. Eating disorder treatment programs, including residential centers, partial hospital programs (PHP), and intensive outpatient programs (IOP), must enhance monitoring for COVID-19, and exercise caution by practicing social distancing and providing adequate personal protective equipment for patients and staff. To reduce the spread of COVID-19, many IOPs and PHPs have transitioned to virtual treatment. Residential centers must carefully screen patients before admission to weigh the risks and benefits of inpatient vs outpatient care.
Bottom Line
Differences in the immune system of patients with an eating disorder do not necessarily confer a higher or lower risk of infection. Symptoms of some infections can mimic the symptoms of anorexia nervosa. Recognizing infections in patients with eating disorders is critical because compared with the general population, they have higher rates of infection-related morbidity and mortality.
Related Resources
- Congress J, Madaan V. 6 ‘M’s to keep in mind when you next see a patient with anorexia nervosa. Current Psychiatry. 2014;13(5):58-59.
- Westmoreland P. Eating disorders: Masterclass lecture part I. Psychcast (podcast). https://www.mdedge.com/podcasts/psychcast/eating-disorders-masterclass-lecture-part-i.
Recent concerns surrounding coronavirus disease 2019 (COVID-19) make it timely to reexamine the complex findings related to eating disorders and the immune system, and the risks for and detection of infection in patients with anorexia nervosa (AN) and similar disorders. To date, there are no published studies evaluating patients with eating disorders and COVID-19. However, it may be helpful to review the data on the infectious process in this patient population to improve patient communication, enhance surveillance and detection, and possibly reduce morbidity and mortality.
The Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) issued warnings that individuals who are older, have underlying medical conditions, and/or are immunocompromised face the greatest risk of serious complications and death as a result of COVID-19, the disease process caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Due to malnutrition, patients with eating disorders, especially AN, may be perceived to have an increased risk of medical conditions and infection. Despite many studies on specific changes and differences in the immune system of patients with eating disorders, the consequences of these changes remain controversial and inconclusive.
This article reviews research on eating disorders, focusing on published data regarding the effects of AN on the immune system, susceptibility to infections, infectious detection, and morbidity. We also discuss clinical considerations related to COVID-19 and patients with AN.
Infection risks: Conflicting data
In a 1981 study that included 9 participants, Golla et al1 concluded that patients with AN may have “resistance” to infections based on a suggested protective factor within the immune system of these patients. Because this study has been cited repeatedly in multiple articles about AN and cell-mediated immunity,2-7 some clinicians have accepted this evidence of resistance to infection in patients with AN, which may lower their suspicion for and detection of infections in patients with AN.
However, studies published both before and after Golla et al1 have shown statistically significant results that contradict those researchers’ conclusion. A study that compared the medical records of 68 patients with AN with those who did not have AN found no significant difference, and concluded that the rate of infection among patients with AN is the same as among controls.8 These researchers noted that infection rates may be higher among patients with later-stage, more severe AN. In a 1986 study of 12 patients with AN, Cason et al9 concluded that while cellular immunity function is abnormal in patients with AN, their results were not compatible with prior studies that suggested AN patients were more resistant to infection.1,2,8
More recently, researchers compared 1,592 patients with eating disorders with 6,368 matched controls; they reviewed prescriptions of antibacterial, antifungal, and antiviral medications as a measure of infection rates.10 Compared with controls, patients with binge eating disorder (BED), patients with bulimia nervosa (BN), and males with AN more often received prescriptions for antimicrobial medications. There was no statistically significant difference between controls and females with AN, which is consistent with other reports of no increased or decreased risk of infection among females with AN. In terms of antiviral use, this study showed an increased prescription of antivirals only in the BN group.
Several other studies examining the rate of infection in patients with AN concluded that there is neither an increased nor decreased rate of infection in patients with AN, and that the rate of infection in this population is similar to that of the general population.8,10-12 Because studies that have included patients with AN have evaluated only symptomatic viral infections, some researchers have proposed that patients with AN may show lower rates of symptomatic viral infection but higher rates of asymptomatic infection, as evidenced by higher viral titers.6 Further research is required. Despite controversy regarding infection rates, studies have found that patients with AN have increased rates of morbidity and mortality from infections.6,12-16
Continue to: Obstacles to detecting infections
Obstacles to detecting infections
Several factors can complicate the surveillance and detection of infections in patients with eating disorders, especially those with AN. These include:
- an accepted predisposition to infection secondary to malnutrition
- a lack of visual or reported infectious symptoms
- misrepresentation and assumptions from published research.
Clinicians who report fewer observed cases of infections among patients with AN may be overlooking comorbid disease processes due to a bias from the literature and/or a lack of awareness of symptom parameters in patients with AN.
Features of AN include a loss of adipose tissue responsible for pro-inflammatory cytokines, and excessive exercise, which stimulates anti-inflammatory myokines. This can modulate the experience of illness that impacts the core features of disease,17 possibly reducing symptomatic presentation of infections.
Fever. The presence and intensity of fever may be altered in patients with eating disorders, especially those with AN. In a study of 311 inpatients with AN, researchers found that patients with AN had a significant delay in fever response in AN.12 Of 23 patients with an active bacterial infection, all but 5 had a fever <37°C, with some as low as 35.5°C. A detectable fever response and unexplained fevers were found in 2 of the 6 patients with a viral infection. A series of case studies found that patients with AN with bacterial infections also had a delayed fever response.18
For patients with infections that commonly present with fever, such as COVID-19, a delayed fever response can delay or evade the detection of infection, thus increasing potential complications as well viral exposure to others. Thus, clinicians should use caution when ruling out COVID-19 or other infections because of a lack of significant fever.
Continue to: Overlapping symptoms
Overlapping symptoms. The symptoms of viral infection can mimic the symptoms of AN, which further complicates screening and diagnosis of infection in these patients. Although up to 80% of individuals infected with COVID-19 may be asymptomatic or have a mild presentation, the most common reported symptoms are fever (92.6%), shortness of breath (50.8%), expectoration (41.4%), fatigue (46.4%), dry cough (33.3%), and myalgia (21.4%).19-21 Gastrointestinal (GI) symptoms have been reported in patients with COVID-19, as well as a loss of taste and smell.
Commonly reported physical symptoms of AN include an intolerance to cold, general fatigue, muscle aches and pains, restlessness, emesis, and a multitude of GI complaints. Patients with AN also have been reported to experience shortness of breath due to conditions such as respiratory muscle weakness,22 nutritional emphysema,23 and anxiety and panic attack.24 These conditions could lead to an increased susceptibility to COVID-19 and increased complications during treatment. Cardiac abnormalities, which are common in patients with AN and BN, may increase the risk of adverse events. While these symptoms may be an important part of screening for diseases such as COVID-19, suspicion of infection also may be lower because of the overlap of AN symptomology, underlying conditions, and a delayed fever response.
Laboratory findings. Laboratory testing results for patients with COVID-19 include lower lymphocyte counts, higher leukocyte counts, elevated levels of infection-related biomarkers and inflammatory cytokines, and significantly decreased T-cell counts.19 Similar values are also found in patients with AN.
The similar clinical presentations and laboratory values of AN and COVID-19 could lead to delayed diagnosis, increased disease transmission, cross-contamination of facilities, and higher incidences of medical complications and mortality.
The immunology of AN and correlations with COVID-19
Many studies examining the immune system of patients with eating disorders, especially those with AN, have discovered changes and differences in both cell-mediated and humoral response to infections.1,3,5,7,9,11,16,21,25-27 Whether these differences represent a dysfunctional immune system, an immunocompromised state, or even a protective factor remains unclear.
Continue to: While some studies have reported...
While some studies have reported that AN represents an immunocompromised state, others describe the immune system of patients with AN as dysfunctional or simply altered.9,11,22,28 Some studies have found that patients with AN had delayed reactions to pathogen skin exposures compared with healthy controls, which provides evidence of an impaired cell-mediated immune system.9,27,29
Some studies have considered the consequences of infection and immunologic findings as markers of or contributing to the onset of AN.2,30,31 Numerous studies have noted abnormalities in AN with regards to cell-mediated immunity, the humoral system, the lymphoreticular system, and the innate immune system, and potential contributions from increased oxidative stress, a chronically activated sympathetic nervous system and hypothalamic-pituitary-adrenal axis, altered intestinal microbiota, and an abnormal bone marrow microenvironment.2
Box 1
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a new beta-coronavirus that is still being studied for its effects on the immune system. It may take years to fully understand the nature of the pathogen and the response of the human immune system. To better understand COVID19, researchers have been turning to what they learned from the past outbreaks of severe acute respiratory syndrome (SARS) in 2003- 2004 and Middle East respiratory syndrome (MERS) in 2011, both caused by betacoronaviruses with a zoonotic origin.25,32
The proposed pathogenesis for infection of SARS-CoV-2 is similar to SARS and occurs when aerosolized droplets containing the virus enter the host.32 While currently there is only initial data on the host innate immune status of patients infected with SARS-CoV-2, initial findings of a report on 99 cases in Wuhan, China included increased total neutrophils (38%), reduced total lymphocytes (35%), increased serum interleukin-6 (52%), and increased C-reactive protein (84%).33 Additional findings were decreased percentages of monocytes, eosinophils, and basophils, as well as significantly decreased levels of cytokines and T-cells in more severe cases.19 Past research with SARS reported similar T-cell findings, with a more frequent CD8+ response and a greater magnitude of CD4+.34
Box 119,25,32-34 describes some of the initial immunologic findings reported in patients with COVID-19. In Box 2,5,8,11,13,14,19,26,28,35-40 we discuss reports that describe the immunologic overlay of COVID-19 and AN.
Box 2
Leukopenia (low leukocyte levels) is a common finding in patients with anorexia nervosa (AN),8 and often leads clinicians to lower their suspicion for infection. A 2008 Hungarian study that evaluated lymphocyte activation parameters and clinical status in 11 adolescents (10 girls and 1 boy) with AN, 12 obese adolescents, and 10 healthy controls did not find any association between the variables.35 While many studies have focused on adults, it is important to note that leukopenia is a common finding in adolescents (age 12 to 17) with AN.36
Leukocyte counts are elevated in coronavirus disease 2019 (COVID-19), possibly offsetting AN’s leukopenia. In addition, neutrophil counts are elevated and monocyte, eosinophil, basophil, and especially lymphocyte counts are significantly decreased. A meta-analysis that included 22 studies and 924 participants (512 with AN and 412 controls) examined common inflammatory cytokine findings in patients with AN.11 Compared with healthy controls, patients with AN had significantly elevated levels of tumor necrosis factor alpha (TNF-alpha), interleukin (IL)-1, IL-6, and TNF-receptor II, and significantly decreased levels of C-reactive protein and IL-6 receptor. Elevated levels of TNF-alpha and IL-6 also have been reported in patients with COVID-19.19 These findings may mask suspicion for infection in patients with AN.19
In patients with AN and those with bulimia nervosa, CD4+-to-CD8+ ratios also have been found to be low as a result of normal-tohigher levels of CD4+ cells and lower levels of CD8+ cells.36-39 Researchers have also proposed that the lymphocytosis observed in AN is a result of increased naïve CD4+.36 In AN, total lymphocyte counts have been found to correlate positively with a patient’s body mass index (BMI), while the CD4+ T-lymphocyte correlated negatively with BMI and were critically low in patients with severe malnutrition.26,40 In patients with COVID-19, CD4+ levels have reported to be within normal range, naïve CD4+ cells were elevated, and CD8+ cells were slightly decreased,19 which is similar to the findings in AN.
Fewer studies have evaluated humoral immune response in AN, and results have varied. One study (N = 46) found elevated B-cell counts in adolescents with AN-restricting type,36 while another (N = 40) reported normal levels of B-cells.5 Specific decreases in immunoglobulin (Ig) G and IgM have also been reported in AN, while IgA, IgG, and IgM usually are normal in COVID-19.19
Despite differences in immune system function, cellular immunity appears to remain relatively intact in patients with AN, but can become compromised with severe malnutrition or with advanced weight loss.28,40 This compromised immunity related to severe AN with a very low BMI likely leads to the increased morbidity and mortality.8,13,14
Malnutrition and the immune system
Differences in the type of malnutrition observed in low-weight patients with AN may help explain why patients with AN can maintain a relatively intact cell-mediated immune system.1 Protein-energy malnutrition (PEM), which is found in typical states of starvation, consists of deficiencies in multiple vitamins, protein, and energy (caloric content), whereas the dietary habits of patients with AN usually result in a deficiency of carbohydrates and fats.41 Studies that examined the impact of PEM on immunity to influenza infection have suggested that balanced protein energy replenishment may be a strategy for boosting immunity against influenza viral infections.42 However, carbohydrates are the primary nutrients for human bone marrow fat cells, which play a crucial role in the maturation of white blood cells. This may account for the leukopenia that is common in patients with AN.6,43 The protein-sparing aspect of the typical AN diet may account for the immune system changes observed in patients with AN.44
Although some studies have proposed that immune deficiencies observed in patients with AN are secondary to malnutrition and return to normal with refeeding,5,40,45 others have concluded that immune function is not compromised by factors such as nutritional status or body weight in AN.26,43,46
Continue to: Clinical considerations
Clinical considerations
Neither the CDC nor the WHO have issued a specific protocol for monitoring for and treating COVID-19 in patients with eating disorders; however, the guidelines offered by these organizations for the general population should be followed for patients with eating disorders.
When screening a patient with an eating disorder, keep in mind that the symptoms of eating disorders, such as AN, may mimic an infectious process. Mood symptoms, such as depression or anxiety, could represent physiological responses to infection. Patients with GI symptoms that typically are considered part of the pathology of an eating disorder should be more carefully considered for COVID-19. Monitor a patient’s basal body temperature, and be mindful that a patient with AN may exhibit a delayed fever response. Be vigilant for a recent loss of taste or smell, which should raise suspicion for COVID-19. When monitoring vital signs, pay careful attention for any decompensation in a patient’s pulse oximetry. Whenever possible, order COVID-19 testing for any patient you suspect may be infected.
Outpatient clinicians should work closely in a collaborative manner with a patient’s eating disorder treatment team. Psychiatrists, primary care physicians, psychotherapists, nutritionists, and other clinicians should all follow CDC/WHO guidelines regarding COVID-19, provide surveillance, and communicate any suspicions to the medical team. Eating disorder treatment programs, including residential centers, partial hospital programs (PHP), and intensive outpatient programs (IOP), must enhance monitoring for COVID-19, and exercise caution by practicing social distancing and providing adequate personal protective equipment for patients and staff. To reduce the spread of COVID-19, many IOPs and PHPs have transitioned to virtual treatment. Residential centers must carefully screen patients before admission to weigh the risks and benefits of inpatient vs outpatient care.
Bottom Line
Differences in the immune system of patients with an eating disorder do not necessarily confer a higher or lower risk of infection. Symptoms of some infections can mimic the symptoms of anorexia nervosa. Recognizing infections in patients with eating disorders is critical because compared with the general population, they have higher rates of infection-related morbidity and mortality.
Related Resources
- Congress J, Madaan V. 6 ‘M’s to keep in mind when you next see a patient with anorexia nervosa. Current Psychiatry. 2014;13(5):58-59.
- Westmoreland P. Eating disorders: Masterclass lecture part I. Psychcast (podcast). https://www.mdedge.com/podcasts/psychcast/eating-disorders-masterclass-lecture-part-i.
1. Golla JA, Larson LA, Anderson CF, et al. An immunological assessment of patients with anorexia nervosa. Am J Clin Nutr. 1981;34(12):2756-2762.
2. Gibson D, Mehler PS. Anorexia nervosa and the immune system—a narrative review. J Clin Med. 2019;8(11):1915. doi: 10.3390/jcm8111915.
3. Słotwin
4. Nova E, Samartín S, Gómez S, et al. The adaptive response of the immune system to the particular malnutrition of eating disorders. Eur J Clin Nutr. 2002;56(suppl 3):S34-S37.
5. Allende LM, Corell A, Manzanares J, et al. Immunodeficiency associated with anorexia nervosa is secondary and improves after refeeding. Immunology. 1998;94(4):543-551.
6. Brown RF, Bartrop R, Birmingham CL. Immunological disturbance and infectious disease in anorexia nervosa: a review. Acta Neuropsychiatr. 2008;20(3):117-128.
7. Polack E, Nahmod VE, Emeric-Sauval E, et al. Low lymphocyte interferon-gamma production and variable proliferative response in anorexia nervosa patients. J Clin Immunol. 1993;13(6):445-451.
8. Bowers TK, Eckert E. Leukopenia in anorexia nervosa. Lack of increased risk of infection. Arch Intern Med. 1978;138(10):1520-1523.
9. Cason J, Ainley CC, Wolstencroft RA, et al. Cell-mediated immunity in anorexia nervosa. Clin Exp Immunol. 1986;64(2):370-375.
10. Raevuori A, Lukkariniemi L, Suokas JT, et al. Increased use of antimicrobial medication in bulimia nervosa and binge-eating disorder prior to the eating disorder treatment. Int J Eat Disord. 2016;49(6):542-552.
11. Solmi M, Veronese N, Favaro A, et al. Inflammatory cytokines and anorexia nervosa: a meta-analysis of cross-sectional and longitudinal studies. Psychoneuroendocrinology. 2015;51:237-252.
12. Brown RF, Bartrop R, Beumont P, et al. Bacterial infections in anorexia nervosa: delayed recognition increases complications. Int J Eat Disord. 2005;37(3):261-265.
13. Theander S. Anorexia nervosa. A psychiatric investigation of 94 female patients. Acta Psychiatr Scand Suppl. 1970;214:1-194.
14. Warren MP, Vande Wiele RL. Clinical and metabolic features of anorexia nervosa. Am J Obstet Gynecol. 1973;117(3):435-449.
15. Copeland PM, Herzog DB. Hypoglycemia and death in anorexia nervosa. Psychother Psychosom. 1987;48(1-4):146-150.
16. Devuyst O, Lambert M, Rodhain J, et al. Haematological changes and infectious complications in anorexia nervosa: a case-control study. Q J Med. 1993;86(12):791-799.
17. Pisetsky DS, Trace SE, Brownley KA, et al. The expression of cytokines and chemokines in the blood of patients with severe weight loss from anorexia nervosa: an exploratory study. Cytokine. 2014;69(1):110-115.
18. Birmingham CL, Hodgson DM, Fung J, et al. Reduced febrile response to bacterial infection in anorexia nervosa patients. Int J Eat Disord. 2003;34(2):269-272.
19. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China [published online March 12, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa248.
20. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
21. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514-523.
22. Birmingham CL, Tan AO. Respiratory muscle weakness and anorexia nervosa. Int J Eat Disord. 2003;33(2):230-233.
23. Cook VJ, Coxson HO, Mason AG, et al. Bullae, bronchiectasis and nutritional emphysema in severe anorexia nervosa. Can Respir J. 2001;8(5):361-365.
24. Khalsa SS, Hassanpour MS, Strober M, et al. Interoceptive anxiety and body representation in anorexia nervosa [published online September 21, 2018]. Front Psychiatry. 2018;9:444. doi: 10.3389/fpsyt.2018.00444.
25. van West D, Maes M. Cytokines in de obsessief compulsieve stoornis en in anorexia nervosa: een overzicht. Acta Neuropsychiatr. 1999;11(4):125-129.
26. Komorowska-Pietrzykowska R, Rajewski A, Wiktorowicz K, et al. Czynnos
27. Marcos A, Varela P, Toro O, et al. Interactions between nutrition and immunity in anorexia nervosa: a 1-y follow-up study. Am J Clin Nutr. 1997;66(2):485S-490S.
28. Pertschuk MJ, Crosby LO, Barot L, et al. Immunocompetency in anorexia nervosa. Am J Clin Nutr. 1982;35(5):968-972.
29. Varela P, Marcos A, Navarro MP. Zinc status in anorexia nervosa. Ann Nutr Metab. 1992;36(4):197-202.
30. Breithaupt L, Köhler-Forsberg O, Larsen JT, et al. Association of exposure to infections in childhood with risk of eating disorders in adolescent girls. JAMA Psychiatry. 2019;76(8):800-809.
31. Brambilla F, Monti D, Franceschi C. Plasma concentrations of interleukin-1-beta, interleukin-6 and tumor necrosis factor-alpha, and of their soluble receptors and receptor antagonist in anorexia nervosa. Psychiatry Res. 2001;103(2-3):107-114.
32. Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic [published online February 27, 2020]. Asian Pac J Allergy Immunol. doi: 10.12932/AP-200220-0772.
33. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270-273.
34. Li CK, Wu H, Yan H, et al. T cell responses to whole SARS coronavirus in humans. J Immunol. 2008;181(8):5490-5500.
35. Páli AA, Pászthy B. Az immunrendszer muködésének megváltozása a táplálkozási magatartás zavarai esetén [Changes of the immune functions in patients with eating disorders]. Ideggyogy Sz. 2008;61(11-12):381‐384.
36. Elegido A, Graell M, Andrés P, et al. Increased naive CD4+ and B lymphocyte subsets are associated with body mass loss and drive relative lymphocytosis in anorexia nervosa patients. Nutr Res. 2017;39:43-50.
37. Marcos A, Varela P, Santacruz I, et al. Nutritional status and immunocompetence in eating disorders. A comparative study. Eur J Clin Nutr. 1993;47(11):787-793.
38. Mustafa A, Ward A, Treasure J, et al. T lymphocyte subpopulations in anorexia nervosa and refeeding. Clin Immunol Immunopathol. 1997;82(3):282-289.
39. Nagata T, Kiriike N, Tobitani W, et al. Lymphocyte subset, lymphocyte proliferative response, and soluble interleukin-2 receptor in anorexic patients. Biol Psychiatry. 1999;45(4):471-474.
40. Saito H, Nomura K, Hotta M, et al. Malnutrition induces dissociated changes in lymphocyte count and subset proportion in patients with anorexia nervosa. Int J Eat Disord. 2007;40(6):575-579.
41. Nova E, Varela P, López-Vidriero I, et al. A one-year follow-up study in anorexia nervosa. Dietary pattern and anthropometrical evolution. Eur J Clin Nutr. 2001;55(7):547-554.
42. Taylor AK, Cao W, Vora KP, et al. Protein energy malnutrition decreases immunity and increases susceptibility to influenza infection in mice. J Infect Dis. 2013;207(3):501-510.
43. Mant MJ, Faragher BS. The hematology of anorexia nervosa. Br J Haematol. 1972;23(6):737-749.
44. Marcos A. The immune system in eating disorders: an overview. Nutrition. 1997;13(10):853-862.
45. Schattner A, Tepper R, Steinbock M, et al. TNF, interferon-gamma and cell-mediated cytotoxicity in anorexia nervosa; effect of refeeding. J Clin Lab Immunol. 1990;32(4):183-184.
46. Nagata T, Tobitani W, Kiriike N, et al. Capacity to produce cytokines during weight restoration in patients with anorexia nervosa. Psychosom Med. 1999;61(3):371-377.
1. Golla JA, Larson LA, Anderson CF, et al. An immunological assessment of patients with anorexia nervosa. Am J Clin Nutr. 1981;34(12):2756-2762.
2. Gibson D, Mehler PS. Anorexia nervosa and the immune system—a narrative review. J Clin Med. 2019;8(11):1915. doi: 10.3390/jcm8111915.
3. Słotwin
4. Nova E, Samartín S, Gómez S, et al. The adaptive response of the immune system to the particular malnutrition of eating disorders. Eur J Clin Nutr. 2002;56(suppl 3):S34-S37.
5. Allende LM, Corell A, Manzanares J, et al. Immunodeficiency associated with anorexia nervosa is secondary and improves after refeeding. Immunology. 1998;94(4):543-551.
6. Brown RF, Bartrop R, Birmingham CL. Immunological disturbance and infectious disease in anorexia nervosa: a review. Acta Neuropsychiatr. 2008;20(3):117-128.
7. Polack E, Nahmod VE, Emeric-Sauval E, et al. Low lymphocyte interferon-gamma production and variable proliferative response in anorexia nervosa patients. J Clin Immunol. 1993;13(6):445-451.
8. Bowers TK, Eckert E. Leukopenia in anorexia nervosa. Lack of increased risk of infection. Arch Intern Med. 1978;138(10):1520-1523.
9. Cason J, Ainley CC, Wolstencroft RA, et al. Cell-mediated immunity in anorexia nervosa. Clin Exp Immunol. 1986;64(2):370-375.
10. Raevuori A, Lukkariniemi L, Suokas JT, et al. Increased use of antimicrobial medication in bulimia nervosa and binge-eating disorder prior to the eating disorder treatment. Int J Eat Disord. 2016;49(6):542-552.
11. Solmi M, Veronese N, Favaro A, et al. Inflammatory cytokines and anorexia nervosa: a meta-analysis of cross-sectional and longitudinal studies. Psychoneuroendocrinology. 2015;51:237-252.
12. Brown RF, Bartrop R, Beumont P, et al. Bacterial infections in anorexia nervosa: delayed recognition increases complications. Int J Eat Disord. 2005;37(3):261-265.
13. Theander S. Anorexia nervosa. A psychiatric investigation of 94 female patients. Acta Psychiatr Scand Suppl. 1970;214:1-194.
14. Warren MP, Vande Wiele RL. Clinical and metabolic features of anorexia nervosa. Am J Obstet Gynecol. 1973;117(3):435-449.
15. Copeland PM, Herzog DB. Hypoglycemia and death in anorexia nervosa. Psychother Psychosom. 1987;48(1-4):146-150.
16. Devuyst O, Lambert M, Rodhain J, et al. Haematological changes and infectious complications in anorexia nervosa: a case-control study. Q J Med. 1993;86(12):791-799.
17. Pisetsky DS, Trace SE, Brownley KA, et al. The expression of cytokines and chemokines in the blood of patients with severe weight loss from anorexia nervosa: an exploratory study. Cytokine. 2014;69(1):110-115.
18. Birmingham CL, Hodgson DM, Fung J, et al. Reduced febrile response to bacterial infection in anorexia nervosa patients. Int J Eat Disord. 2003;34(2):269-272.
19. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China [published online March 12, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa248.
20. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
21. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514-523.
22. Birmingham CL, Tan AO. Respiratory muscle weakness and anorexia nervosa. Int J Eat Disord. 2003;33(2):230-233.
23. Cook VJ, Coxson HO, Mason AG, et al. Bullae, bronchiectasis and nutritional emphysema in severe anorexia nervosa. Can Respir J. 2001;8(5):361-365.
24. Khalsa SS, Hassanpour MS, Strober M, et al. Interoceptive anxiety and body representation in anorexia nervosa [published online September 21, 2018]. Front Psychiatry. 2018;9:444. doi: 10.3389/fpsyt.2018.00444.
25. van West D, Maes M. Cytokines in de obsessief compulsieve stoornis en in anorexia nervosa: een overzicht. Acta Neuropsychiatr. 1999;11(4):125-129.
26. Komorowska-Pietrzykowska R, Rajewski A, Wiktorowicz K, et al. Czynnos
27. Marcos A, Varela P, Toro O, et al. Interactions between nutrition and immunity in anorexia nervosa: a 1-y follow-up study. Am J Clin Nutr. 1997;66(2):485S-490S.
28. Pertschuk MJ, Crosby LO, Barot L, et al. Immunocompetency in anorexia nervosa. Am J Clin Nutr. 1982;35(5):968-972.
29. Varela P, Marcos A, Navarro MP. Zinc status in anorexia nervosa. Ann Nutr Metab. 1992;36(4):197-202.
30. Breithaupt L, Köhler-Forsberg O, Larsen JT, et al. Association of exposure to infections in childhood with risk of eating disorders in adolescent girls. JAMA Psychiatry. 2019;76(8):800-809.
31. Brambilla F, Monti D, Franceschi C. Plasma concentrations of interleukin-1-beta, interleukin-6 and tumor necrosis factor-alpha, and of their soluble receptors and receptor antagonist in anorexia nervosa. Psychiatry Res. 2001;103(2-3):107-114.
32. Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic [published online February 27, 2020]. Asian Pac J Allergy Immunol. doi: 10.12932/AP-200220-0772.
33. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270-273.
34. Li CK, Wu H, Yan H, et al. T cell responses to whole SARS coronavirus in humans. J Immunol. 2008;181(8):5490-5500.
35. Páli AA, Pászthy B. Az immunrendszer muködésének megváltozása a táplálkozási magatartás zavarai esetén [Changes of the immune functions in patients with eating disorders]. Ideggyogy Sz. 2008;61(11-12):381‐384.
36. Elegido A, Graell M, Andrés P, et al. Increased naive CD4+ and B lymphocyte subsets are associated with body mass loss and drive relative lymphocytosis in anorexia nervosa patients. Nutr Res. 2017;39:43-50.
37. Marcos A, Varela P, Santacruz I, et al. Nutritional status and immunocompetence in eating disorders. A comparative study. Eur J Clin Nutr. 1993;47(11):787-793.
38. Mustafa A, Ward A, Treasure J, et al. T lymphocyte subpopulations in anorexia nervosa and refeeding. Clin Immunol Immunopathol. 1997;82(3):282-289.
39. Nagata T, Kiriike N, Tobitani W, et al. Lymphocyte subset, lymphocyte proliferative response, and soluble interleukin-2 receptor in anorexic patients. Biol Psychiatry. 1999;45(4):471-474.
40. Saito H, Nomura K, Hotta M, et al. Malnutrition induces dissociated changes in lymphocyte count and subset proportion in patients with anorexia nervosa. Int J Eat Disord. 2007;40(6):575-579.
41. Nova E, Varela P, López-Vidriero I, et al. A one-year follow-up study in anorexia nervosa. Dietary pattern and anthropometrical evolution. Eur J Clin Nutr. 2001;55(7):547-554.
42. Taylor AK, Cao W, Vora KP, et al. Protein energy malnutrition decreases immunity and increases susceptibility to influenza infection in mice. J Infect Dis. 2013;207(3):501-510.
43. Mant MJ, Faragher BS. The hematology of anorexia nervosa. Br J Haematol. 1972;23(6):737-749.
44. Marcos A. The immune system in eating disorders: an overview. Nutrition. 1997;13(10):853-862.
45. Schattner A, Tepper R, Steinbock M, et al. TNF, interferon-gamma and cell-mediated cytotoxicity in anorexia nervosa; effect of refeeding. J Clin Lab Immunol. 1990;32(4):183-184.
46. Nagata T, Tobitani W, Kiriike N, et al. Capacity to produce cytokines during weight restoration in patients with anorexia nervosa. Psychosom Med. 1999;61(3):371-377.
A Clinical Program to Implement Repetitive Transcranial Magnetic Stimulation for Depression in the Department of Veterans Affairs
Repetitive transcranial magnetic stimulation (rTMS) is an emerging therapy approved by the US Food and Drug Administration (FDA) for mental health indications but not widely available in the US Department of Veterans Affairs (VA). rTMS uses a device to create magnetic fields that cause electrical current to flow into targeted neurons in the brain.1 The area of the brain targeted depends on the shape of the magnetic coil and dose of stimulation (Figures 1 and 2). The most common coil shape is the figure-8 coil, which is believed to stimulate about a 2- to 3-cm2 area of the brain at a depth of about 2 cm from the coil surface. The stimulus is thought to activate certain nerve growth factors and ultimately relevant neurotransmitters in the stimulated areas and parts of the brain connected to where the stimulus occurs.2
The most common clinical use of rTMS is for the treatment of major depressive disorder (MDD). The FDA has approved rTMS for the treatment of MDD and for at least 4 device manufacturers. The treatment has been studied in multiple clinical trials.3 An overview of these trials, additional rTMS training and educational materials, and device information can be accessed at www.mirecc.va.gov/visn21/education/tms_education.asp. rTMS for MDD administers a personalized dose with stimulation delivered over the dorsolateral prefrontal cortex. A typical clinical course runs for 40 minutes a day for 20 to 30 sessions. In addition to studies of depression,1,4-7 rTMS has been studied for the following diseases and conditions:
- Headache (especially migraine)8
- Alzheimer disease9
- Obsessive compulsive disorder (OCD)10
- Obesity11
- Schizophrenia12
- Posttraumatic stress disorder (PTSD)13
- Alcohol and nicotine dependence14
The FDA also has approved the use of rTMS for OCD. In addition, some health care providers (HCPs) are treating depression with rTMS in conjunction with electroconvulsive therapy (ECT).
Treatment for Veterans
MDD is one of the most significant risk factors for suicide. Therefore, treating depression with rTMS would likely diminish suicide risk. The annual suicide rate among veterans has been higher than the national average.15 However, most of these veterans are not getting their care at the Veterans Health Administration (VHA). Major efforts at the VA have been made to address this problem, including modification and promotion of the Veterans Crisis Line, increased mental health clinic hours, mental health same-day appointment availability for veterans, as well as raising awareness of suicide and suicidal ideation.16 George and colleagues showed that it is safe and feasible to treat acutely suicidal inpatients at a VA or US Department of Defense hospital over an intensive 3 day, 3 treatments per day regimen. This regimen would be potentially useful in a suicidal inpatient population, a technically and ethically difficult group to study.17
MDD in many patients can be chronic and reoccurring with medication and psychotherapy providing inadequate relief.17 There clearly is a need for additional treatment options. MDD and OCD are the only indications that have received FDA approval for rTMS use. The initial FDA approval for MDD was based on a 2007 study of medication-free patients who had failed previous therapy and found a significant effect of rTMS compared with a sham procedure.7 MDD remains a common problem among veterans who have failed one or more antidepressant medications. Such patients might benefit from rTMS.6,18
rTMS has several advantages over ECT, another significant FDA-approved, nonpharmacologic treatment alternative for medication-refractory MDD. rTMS is less invasive, requires fewer resources, does not require anesthesia or restrict activities, and does not cause memory loss. After an rTMS treatment, the patient can drive home.
Nationwide Pilot Program
The VA pilot program was created to supply rTMS machines nationwide to VHA sites and to offer a framework for establishing a clinical program. Preliminary program evaluation data suggest patients experienced a reduction in depression and suicidal ideation.
There were many challenges to implementation; for example, one VA site was eager to start using the device but could not secure space or personnel. An interdisciplinary team consisting of physicians, nurses, psychologists, suicide prevention coordinators, and others in the VA Palo Alto Health Care System (VAPAHCS) Precision Neurostimulation Clinic (PNC) has been instrumental in overcoming these challenges. VAPAHCS oversees the pilot and employs the national director.
Thirty-five sites nationwide were initially selected due to their ability to provide space for a rTMS machine and appropriate staffing to set up and run a Clinic (Figure 3). The pilot started with tertiary care VA medical centers then expanded to include community-based outpatient clinics as resources permitted. Sites that were unable to meet these standards were not included. Of these 35 original sites, 26 are treating patients and collecting data. Some early delays were due to unassigned relative value units (RVUs) to rTMS, which since have been revised as imputed RVU values. The American Medical Association established and defined RVUs to compare the value of different health care roles.19 The clinics have been established with smooth operations as the pilot program has provided the infrastructure.
REDCap (www.project-redcap.org), a data collection tool used primarily in academic research settings, was selected to gather program evaluation data through patient questionnaires informed by the VHA measurement-based care initiative. Standard psychometrics were readily available in the VHA application and REDCap Mental Health Assistant includes the Patient Health Questionnaire 9 (PHQ-9) Brief Symptom Inventory 18, Posttraumatic Checklist 5, Beck Scale for Suicidal Ideation, and Quality of Life Inventory. The Timberlawn Couple and Family Evaluation Scale (TCFES), which can be completed in 30 to 35 minutes and is a measure of overall function of relevant relationships, also may be added. Future studies are needed to confirm psychometrics of this scale in this setting, but the TCFES metric is widely used for similar purposes.
Nationwide, more than 950 patients have started treatment (ie, including active, completed, and discontinued treatment) and 412 veterans have completed the rTMS treatment. The goal of the program evaluation is to examine large scale rTMS efficacy in a large veteran population as well as determine predictors of individual patient response. Nationwide, PHQ-9 depression scores declined from a pretreatment average (SD) of 18.2 (5.5; range, 5-27) to a posttreatment average (SD) of 11.0 (7.1; range, 0-27). Patients also have indicated a high level of satisfaction with the treatment (Figure 4). Collecting data on a national level is a powerful way to examine rTMS efficacy and predictors of response that might be lost in a smaller subset of cases.
Implementation
It took 11 months for the VA contracting department to determine which machine to buy. However, the lengthy process assured that the equipment selected met all standards for clinical safety and efficacy. Furthermore, provision was made to allow for additional orders as new sites came online as well as upgrading the equipment for advances in technology.
The PNC set up several training programs to ensure proper use of this novel treatment. The education is ongoing and available as new sites are identified and initiated. The education includes, but is not limited to, in-person onsite and offsite training programs, online training modules that are available in the VA Electronic Educational Services (EES), and video telehealth consultations. Participants can view online lectures and then receive hands-on training as part of the educational program. Up to 3 HCPs for each site can receive funding to attend. Online programs also are available for new material to support continuing medical education. However, hands-on training is essential to understand how to obtain the motor threshold, which is used to determine the strength of the rTMS stimulus dose. Furthermore, hands-on training is essential for the proper localization of the stimulus, which is determined by certain anatomical landmarks. A phantom mannequin (ERIK [Evaluating Resting motor threshold and Insuring Kappa]) has been developed to assist in the hands-on learning.20
Relative Value Units
The VHA uses RVUs to properly account for workload and clinician activities. As a result, RVUs play an essential role as a currency that denotes the relative value of one type of clinical activity when compared with other activities. Depending on the treating specialty, clinicians generally use procedure codes outlined in the Current Procedural Terminology (CPT) code set or the Healthcare Common Procedure Coding System (HCPCS) for medical billing. Most insurance carriers use RVUs set by the Centers for Medicare and Medicaid Services (CMS) system as a standard system to determine HCP reimbursement for medical procedures.
The CPT codes associated with rTMS currently are 90867 to 90869. CMS had initially assigned a zero RVU to these CPT codes due to wide variations in the cost of performing rTMS. When we began implementing rTMS in the VHA, the lack of RVUs for rTMS rendered it impossible to show clinical workload for this activity using established VHA clinical accounting methods. The lack of RVUs assigned to rTMS CPT codes made justification for this treatment to clinical management difficult, which limited its clinical use in the VHA. In addition, HCPs who were using rTMS to treat severely ill veterans appeared artificially unproductive despite a significant patient workload. As we and VHA leadership became aware the program could not be staffed locally without getting workload credit for work done, the value was raised to 1.37 for treatment (90868) and 2.12 and 1.93 for evaluations (90867) and reevaluations (90869), respectively, thus reducing a potential roadblock to implementation.
Challenges as the Program Expands
Future challenges include upgrading machines to do intermittent θ burst stimulation (iTBS), which decreases the standard treatment time from 37.5 minutes to 3 minutes. Both patients and HCPs find iTBS to have similar tolerability to standard rTMS but in much less time. iTBS mimics endogenous θ rhythms and has been shown to be noninferior to rTMS for depression.21,22 Several devices have received FDA approval to treat MDD, including the Magstim and MagVenture TMS devices used in this program.
A major challenge for the VHA with rTMS will be to maintain a consistent level of competence and training. There is a need for continued maintenance of staff competence with ongoing training and training for new staff. Novel ways of training operators have been developed including ERIK.
Determining treatment interaction with other psychotherapies and pharmacotherapies is another challenge. Currently, rTMS is considered an adjunctive treatment added to the current patient treatment plan. We do not know yet how best to incorporate this somatic treatment with other approaches, and further research is necessary. A key issue is to determine which approach provides the best long-term results for a patient at risk for recurrence of depression. In addition, more research into maintaining healthy relationships for veterans with both MDD and PTSD is needed.
Many misconceptions exist about rTMS and HCPs need to be educated about the benefits of this modality. In addition, patients should understand the differences between rTMS and ECT. Even with newer approaches that streamline rTMS, the therapy remains costly in terms of direct costs as well as patient and HCP time.
Streamlining rTMS treatment remains an important concern. Compressing treatment schedules (ie, many treatments delivered to a patient in a single day) would allow the entire process to be delivered in days, not weeks. This would be especially advantageous to patients who live far from a treatment site. Performing multiple rTMS daily treatments is especially feasible with iTBS with its short treatment time.
Conclusions
rTMS is an emerging modality with both established and novel applications. The best studied application is treatment resistant MDD. Currently, rTMS has only been approved by the FDA for treatment of MDD. A pilot program was established by the VHA to distribute 30 rTMS machines sites nationwide. Results from data collected by these sites have shown patients improving on standard psychometric scales. Future changes include upgrading the machines to provide θ bursts, which has been shown to be faster and noninferior. Integrating rTMS with other pharmacotherapies and psychotherapies remains poorly understood and needs more research.
1. George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6(14):1853‐1856. doi:10.1097/00001756-199510020-00008
2. Tik M, Hoffmann A, Sladky R, et al. Towards understanding rTMS mechanism of action: stimulation of the DLPFC causes network-specific increase in functional connectivity. Neuroimage. 2017;162:289‐296. doi:10.1016/j.neuroimage.2017.09.022
3. Perera T, George MS, Grammer G, Janicak PG, Pascual-Leone A, Wirecki TS. The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9(3):336‐346. doi:10.1016/j.brs.2016.03.010
4. George MS, Taylor JJ, Short EB. The expanding evidence base for rTMS treatment of depression. Curr Opin Psychiatry. 2013;26(1):13‐18. doi:10.1097/YCO.0b013e32835ab46d
5. Lisanby SH, Husain MM, Rosenquist PB, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34(2):522‐534. doi:10.1038/npp.2008.118
6. Yesavage JA, Fairchild JK, Mi Z, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75(9):884‐893. doi:10.1001/jamapsychiatry.2018.1483
7. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208‐1216. doi:10.1016/j.biopsych.2007.01.018
8. Stilling JM, Monchi O, Amoozegar F, Debert CT. Transcranial magnetic and direct current stimulation (TMS/tDCS) for the treatment of headache: a systematic review. Headache. 2019;59(3):339‐357. doi:10.1111/head.13479
9. Lin Y, Jiang WJ, Shan PY, et al. The role of repetitive transcranial magnetic stimulation (rTMS) in the treatment of cognitive impairment in patients with Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Sci. 2019;398:184‐191. doi:10.1016/j.jns.2019.01.038
10. Carmi L, Tendler A, Bystritsky A, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. 2019;176(11):931‐938. doi:10.1176/appi.ajp.2019.18101180
11. Song S, Zilverstand A, Gui W, Li HJ, Zhou X. Effects of single-session versus multi-session non-invasive brain stimulation on craving and consumption in individuals with drug addiction, eating disorders or obesity: a meta-analysis. Brain Stimul. 2019;12(3):606‐618. doi:10.1016/j.brs.2018.12.975
12. Wagner E, Wobrock T, Kunze B, et al. Efficacy of high-frequency repetitive transcranial magnetic stimulation in schizophrenia patients with treatment-resistant negative symptoms treated with clozapine. Schizophr Res. 2019;208:370‐376. doi:10.1016/j.schres.2019.01.021
13. Kozel FA, Van Trees K, Larson V, et al. One hertz versus ten hertz repetitive TMS treatment of PTSD: a randomized clinical trial. Psychiatry Res. 2019;273:153‐162. doi:10.1016/j.psychres.2019.01.004
14. Coles AS, Kozak K, George TP. A review of brain stimulation methods to treat substance use disorders. Am J Addict. 2018;27(2):71‐91. doi:10.1111/ajad.12674
15. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. 2019 National veteran suicide prevention annual report. https://www.mentalhealth.va.gov/docs/data-sheets/2019/2019_National_Veteran_Suicide_Prevention_Annual_Report_508.pdf. Published September 19, 2019. Accessed May 18, 2020.
16. Ritchie EC. Improving Veteran engagement with mental health care. Fed Pract. 2017;34(8):55‐56.
17. Rush AJ, Trivedi MH, Wisniewski SR, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231‐1242. doi:10.1056/NEJMoa052963
18. Kozel FA, Hernandez M, Van Trees K, et al. Clinical repetitive transcranial magnetic stimulation for veterans with major depressive disorder. Ann Clin Psychiatry. 2017;29(4):242‐248.
19. National Health Policy Forum. The basics: relative value units (RVUs). https://collections.nlm.nih.gov/master/borndig/101513853/Relative%20Value%20Units.pdf. Published January 12, 2015. Accessed May 18, 2020.
20. Finetto C, Glusman C, Doolittle J, George MS. Presenting ERIK, the TMS phantom: a novel device for training and testing operators. Brain Stimul. 2019;12(4):1095‐1097. doi:10.1016/j.brs.2019.04.01521. Trevizol AP, Vigod SN, Daskalakis ZJ, Vila-Rodriguez F, Downar J, Blumberger DM. Intermittent theta burst stimulation for major depression during pregnancy. Brain Stimul. 2019;12(3):772‐774. doi:10.1016/j.brs.2019.01.003
22. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial [published correction appears in Lancet. 2018 Jun 23;391(10139):e24]. Lancet. 2018;391(10131):1683‐1692. doi:10.1016/S0140-6736(18)30295-2
Repetitive transcranial magnetic stimulation (rTMS) is an emerging therapy approved by the US Food and Drug Administration (FDA) for mental health indications but not widely available in the US Department of Veterans Affairs (VA). rTMS uses a device to create magnetic fields that cause electrical current to flow into targeted neurons in the brain.1 The area of the brain targeted depends on the shape of the magnetic coil and dose of stimulation (Figures 1 and 2). The most common coil shape is the figure-8 coil, which is believed to stimulate about a 2- to 3-cm2 area of the brain at a depth of about 2 cm from the coil surface. The stimulus is thought to activate certain nerve growth factors and ultimately relevant neurotransmitters in the stimulated areas and parts of the brain connected to where the stimulus occurs.2
The most common clinical use of rTMS is for the treatment of major depressive disorder (MDD). The FDA has approved rTMS for the treatment of MDD and for at least 4 device manufacturers. The treatment has been studied in multiple clinical trials.3 An overview of these trials, additional rTMS training and educational materials, and device information can be accessed at www.mirecc.va.gov/visn21/education/tms_education.asp. rTMS for MDD administers a personalized dose with stimulation delivered over the dorsolateral prefrontal cortex. A typical clinical course runs for 40 minutes a day for 20 to 30 sessions. In addition to studies of depression,1,4-7 rTMS has been studied for the following diseases and conditions:
- Headache (especially migraine)8
- Alzheimer disease9
- Obsessive compulsive disorder (OCD)10
- Obesity11
- Schizophrenia12
- Posttraumatic stress disorder (PTSD)13
- Alcohol and nicotine dependence14
The FDA also has approved the use of rTMS for OCD. In addition, some health care providers (HCPs) are treating depression with rTMS in conjunction with electroconvulsive therapy (ECT).
Treatment for Veterans
MDD is one of the most significant risk factors for suicide. Therefore, treating depression with rTMS would likely diminish suicide risk. The annual suicide rate among veterans has been higher than the national average.15 However, most of these veterans are not getting their care at the Veterans Health Administration (VHA). Major efforts at the VA have been made to address this problem, including modification and promotion of the Veterans Crisis Line, increased mental health clinic hours, mental health same-day appointment availability for veterans, as well as raising awareness of suicide and suicidal ideation.16 George and colleagues showed that it is safe and feasible to treat acutely suicidal inpatients at a VA or US Department of Defense hospital over an intensive 3 day, 3 treatments per day regimen. This regimen would be potentially useful in a suicidal inpatient population, a technically and ethically difficult group to study.17
MDD in many patients can be chronic and reoccurring with medication and psychotherapy providing inadequate relief.17 There clearly is a need for additional treatment options. MDD and OCD are the only indications that have received FDA approval for rTMS use. The initial FDA approval for MDD was based on a 2007 study of medication-free patients who had failed previous therapy and found a significant effect of rTMS compared with a sham procedure.7 MDD remains a common problem among veterans who have failed one or more antidepressant medications. Such patients might benefit from rTMS.6,18
rTMS has several advantages over ECT, another significant FDA-approved, nonpharmacologic treatment alternative for medication-refractory MDD. rTMS is less invasive, requires fewer resources, does not require anesthesia or restrict activities, and does not cause memory loss. After an rTMS treatment, the patient can drive home.
Nationwide Pilot Program
The VA pilot program was created to supply rTMS machines nationwide to VHA sites and to offer a framework for establishing a clinical program. Preliminary program evaluation data suggest patients experienced a reduction in depression and suicidal ideation.
There were many challenges to implementation; for example, one VA site was eager to start using the device but could not secure space or personnel. An interdisciplinary team consisting of physicians, nurses, psychologists, suicide prevention coordinators, and others in the VA Palo Alto Health Care System (VAPAHCS) Precision Neurostimulation Clinic (PNC) has been instrumental in overcoming these challenges. VAPAHCS oversees the pilot and employs the national director.
Thirty-five sites nationwide were initially selected due to their ability to provide space for a rTMS machine and appropriate staffing to set up and run a Clinic (Figure 3). The pilot started with tertiary care VA medical centers then expanded to include community-based outpatient clinics as resources permitted. Sites that were unable to meet these standards were not included. Of these 35 original sites, 26 are treating patients and collecting data. Some early delays were due to unassigned relative value units (RVUs) to rTMS, which since have been revised as imputed RVU values. The American Medical Association established and defined RVUs to compare the value of different health care roles.19 The clinics have been established with smooth operations as the pilot program has provided the infrastructure.
REDCap (www.project-redcap.org), a data collection tool used primarily in academic research settings, was selected to gather program evaluation data through patient questionnaires informed by the VHA measurement-based care initiative. Standard psychometrics were readily available in the VHA application and REDCap Mental Health Assistant includes the Patient Health Questionnaire 9 (PHQ-9) Brief Symptom Inventory 18, Posttraumatic Checklist 5, Beck Scale for Suicidal Ideation, and Quality of Life Inventory. The Timberlawn Couple and Family Evaluation Scale (TCFES), which can be completed in 30 to 35 minutes and is a measure of overall function of relevant relationships, also may be added. Future studies are needed to confirm psychometrics of this scale in this setting, but the TCFES metric is widely used for similar purposes.
Nationwide, more than 950 patients have started treatment (ie, including active, completed, and discontinued treatment) and 412 veterans have completed the rTMS treatment. The goal of the program evaluation is to examine large scale rTMS efficacy in a large veteran population as well as determine predictors of individual patient response. Nationwide, PHQ-9 depression scores declined from a pretreatment average (SD) of 18.2 (5.5; range, 5-27) to a posttreatment average (SD) of 11.0 (7.1; range, 0-27). Patients also have indicated a high level of satisfaction with the treatment (Figure 4). Collecting data on a national level is a powerful way to examine rTMS efficacy and predictors of response that might be lost in a smaller subset of cases.
Implementation
It took 11 months for the VA contracting department to determine which machine to buy. However, the lengthy process assured that the equipment selected met all standards for clinical safety and efficacy. Furthermore, provision was made to allow for additional orders as new sites came online as well as upgrading the equipment for advances in technology.
The PNC set up several training programs to ensure proper use of this novel treatment. The education is ongoing and available as new sites are identified and initiated. The education includes, but is not limited to, in-person onsite and offsite training programs, online training modules that are available in the VA Electronic Educational Services (EES), and video telehealth consultations. Participants can view online lectures and then receive hands-on training as part of the educational program. Up to 3 HCPs for each site can receive funding to attend. Online programs also are available for new material to support continuing medical education. However, hands-on training is essential to understand how to obtain the motor threshold, which is used to determine the strength of the rTMS stimulus dose. Furthermore, hands-on training is essential for the proper localization of the stimulus, which is determined by certain anatomical landmarks. A phantom mannequin (ERIK [Evaluating Resting motor threshold and Insuring Kappa]) has been developed to assist in the hands-on learning.20
Relative Value Units
The VHA uses RVUs to properly account for workload and clinician activities. As a result, RVUs play an essential role as a currency that denotes the relative value of one type of clinical activity when compared with other activities. Depending on the treating specialty, clinicians generally use procedure codes outlined in the Current Procedural Terminology (CPT) code set or the Healthcare Common Procedure Coding System (HCPCS) for medical billing. Most insurance carriers use RVUs set by the Centers for Medicare and Medicaid Services (CMS) system as a standard system to determine HCP reimbursement for medical procedures.
The CPT codes associated with rTMS currently are 90867 to 90869. CMS had initially assigned a zero RVU to these CPT codes due to wide variations in the cost of performing rTMS. When we began implementing rTMS in the VHA, the lack of RVUs for rTMS rendered it impossible to show clinical workload for this activity using established VHA clinical accounting methods. The lack of RVUs assigned to rTMS CPT codes made justification for this treatment to clinical management difficult, which limited its clinical use in the VHA. In addition, HCPs who were using rTMS to treat severely ill veterans appeared artificially unproductive despite a significant patient workload. As we and VHA leadership became aware the program could not be staffed locally without getting workload credit for work done, the value was raised to 1.37 for treatment (90868) and 2.12 and 1.93 for evaluations (90867) and reevaluations (90869), respectively, thus reducing a potential roadblock to implementation.
Challenges as the Program Expands
Future challenges include upgrading machines to do intermittent θ burst stimulation (iTBS), which decreases the standard treatment time from 37.5 minutes to 3 minutes. Both patients and HCPs find iTBS to have similar tolerability to standard rTMS but in much less time. iTBS mimics endogenous θ rhythms and has been shown to be noninferior to rTMS for depression.21,22 Several devices have received FDA approval to treat MDD, including the Magstim and MagVenture TMS devices used in this program.
A major challenge for the VHA with rTMS will be to maintain a consistent level of competence and training. There is a need for continued maintenance of staff competence with ongoing training and training for new staff. Novel ways of training operators have been developed including ERIK.
Determining treatment interaction with other psychotherapies and pharmacotherapies is another challenge. Currently, rTMS is considered an adjunctive treatment added to the current patient treatment plan. We do not know yet how best to incorporate this somatic treatment with other approaches, and further research is necessary. A key issue is to determine which approach provides the best long-term results for a patient at risk for recurrence of depression. In addition, more research into maintaining healthy relationships for veterans with both MDD and PTSD is needed.
Many misconceptions exist about rTMS and HCPs need to be educated about the benefits of this modality. In addition, patients should understand the differences between rTMS and ECT. Even with newer approaches that streamline rTMS, the therapy remains costly in terms of direct costs as well as patient and HCP time.
Streamlining rTMS treatment remains an important concern. Compressing treatment schedules (ie, many treatments delivered to a patient in a single day) would allow the entire process to be delivered in days, not weeks. This would be especially advantageous to patients who live far from a treatment site. Performing multiple rTMS daily treatments is especially feasible with iTBS with its short treatment time.
Conclusions
rTMS is an emerging modality with both established and novel applications. The best studied application is treatment resistant MDD. Currently, rTMS has only been approved by the FDA for treatment of MDD. A pilot program was established by the VHA to distribute 30 rTMS machines sites nationwide. Results from data collected by these sites have shown patients improving on standard psychometric scales. Future changes include upgrading the machines to provide θ bursts, which has been shown to be faster and noninferior. Integrating rTMS with other pharmacotherapies and psychotherapies remains poorly understood and needs more research.
Repetitive transcranial magnetic stimulation (rTMS) is an emerging therapy approved by the US Food and Drug Administration (FDA) for mental health indications but not widely available in the US Department of Veterans Affairs (VA). rTMS uses a device to create magnetic fields that cause electrical current to flow into targeted neurons in the brain.1 The area of the brain targeted depends on the shape of the magnetic coil and dose of stimulation (Figures 1 and 2). The most common coil shape is the figure-8 coil, which is believed to stimulate about a 2- to 3-cm2 area of the brain at a depth of about 2 cm from the coil surface. The stimulus is thought to activate certain nerve growth factors and ultimately relevant neurotransmitters in the stimulated areas and parts of the brain connected to where the stimulus occurs.2
The most common clinical use of rTMS is for the treatment of major depressive disorder (MDD). The FDA has approved rTMS for the treatment of MDD and for at least 4 device manufacturers. The treatment has been studied in multiple clinical trials.3 An overview of these trials, additional rTMS training and educational materials, and device information can be accessed at www.mirecc.va.gov/visn21/education/tms_education.asp. rTMS for MDD administers a personalized dose with stimulation delivered over the dorsolateral prefrontal cortex. A typical clinical course runs for 40 minutes a day for 20 to 30 sessions. In addition to studies of depression,1,4-7 rTMS has been studied for the following diseases and conditions:
- Headache (especially migraine)8
- Alzheimer disease9
- Obsessive compulsive disorder (OCD)10
- Obesity11
- Schizophrenia12
- Posttraumatic stress disorder (PTSD)13
- Alcohol and nicotine dependence14
The FDA also has approved the use of rTMS for OCD. In addition, some health care providers (HCPs) are treating depression with rTMS in conjunction with electroconvulsive therapy (ECT).
Treatment for Veterans
MDD is one of the most significant risk factors for suicide. Therefore, treating depression with rTMS would likely diminish suicide risk. The annual suicide rate among veterans has been higher than the national average.15 However, most of these veterans are not getting their care at the Veterans Health Administration (VHA). Major efforts at the VA have been made to address this problem, including modification and promotion of the Veterans Crisis Line, increased mental health clinic hours, mental health same-day appointment availability for veterans, as well as raising awareness of suicide and suicidal ideation.16 George and colleagues showed that it is safe and feasible to treat acutely suicidal inpatients at a VA or US Department of Defense hospital over an intensive 3 day, 3 treatments per day regimen. This regimen would be potentially useful in a suicidal inpatient population, a technically and ethically difficult group to study.17
MDD in many patients can be chronic and reoccurring with medication and psychotherapy providing inadequate relief.17 There clearly is a need for additional treatment options. MDD and OCD are the only indications that have received FDA approval for rTMS use. The initial FDA approval for MDD was based on a 2007 study of medication-free patients who had failed previous therapy and found a significant effect of rTMS compared with a sham procedure.7 MDD remains a common problem among veterans who have failed one or more antidepressant medications. Such patients might benefit from rTMS.6,18
rTMS has several advantages over ECT, another significant FDA-approved, nonpharmacologic treatment alternative for medication-refractory MDD. rTMS is less invasive, requires fewer resources, does not require anesthesia or restrict activities, and does not cause memory loss. After an rTMS treatment, the patient can drive home.
Nationwide Pilot Program
The VA pilot program was created to supply rTMS machines nationwide to VHA sites and to offer a framework for establishing a clinical program. Preliminary program evaluation data suggest patients experienced a reduction in depression and suicidal ideation.
There were many challenges to implementation; for example, one VA site was eager to start using the device but could not secure space or personnel. An interdisciplinary team consisting of physicians, nurses, psychologists, suicide prevention coordinators, and others in the VA Palo Alto Health Care System (VAPAHCS) Precision Neurostimulation Clinic (PNC) has been instrumental in overcoming these challenges. VAPAHCS oversees the pilot and employs the national director.
Thirty-five sites nationwide were initially selected due to their ability to provide space for a rTMS machine and appropriate staffing to set up and run a Clinic (Figure 3). The pilot started with tertiary care VA medical centers then expanded to include community-based outpatient clinics as resources permitted. Sites that were unable to meet these standards were not included. Of these 35 original sites, 26 are treating patients and collecting data. Some early delays were due to unassigned relative value units (RVUs) to rTMS, which since have been revised as imputed RVU values. The American Medical Association established and defined RVUs to compare the value of different health care roles.19 The clinics have been established with smooth operations as the pilot program has provided the infrastructure.
REDCap (www.project-redcap.org), a data collection tool used primarily in academic research settings, was selected to gather program evaluation data through patient questionnaires informed by the VHA measurement-based care initiative. Standard psychometrics were readily available in the VHA application and REDCap Mental Health Assistant includes the Patient Health Questionnaire 9 (PHQ-9) Brief Symptom Inventory 18, Posttraumatic Checklist 5, Beck Scale for Suicidal Ideation, and Quality of Life Inventory. The Timberlawn Couple and Family Evaluation Scale (TCFES), which can be completed in 30 to 35 minutes and is a measure of overall function of relevant relationships, also may be added. Future studies are needed to confirm psychometrics of this scale in this setting, but the TCFES metric is widely used for similar purposes.
Nationwide, more than 950 patients have started treatment (ie, including active, completed, and discontinued treatment) and 412 veterans have completed the rTMS treatment. The goal of the program evaluation is to examine large scale rTMS efficacy in a large veteran population as well as determine predictors of individual patient response. Nationwide, PHQ-9 depression scores declined from a pretreatment average (SD) of 18.2 (5.5; range, 5-27) to a posttreatment average (SD) of 11.0 (7.1; range, 0-27). Patients also have indicated a high level of satisfaction with the treatment (Figure 4). Collecting data on a national level is a powerful way to examine rTMS efficacy and predictors of response that might be lost in a smaller subset of cases.
Implementation
It took 11 months for the VA contracting department to determine which machine to buy. However, the lengthy process assured that the equipment selected met all standards for clinical safety and efficacy. Furthermore, provision was made to allow for additional orders as new sites came online as well as upgrading the equipment for advances in technology.
The PNC set up several training programs to ensure proper use of this novel treatment. The education is ongoing and available as new sites are identified and initiated. The education includes, but is not limited to, in-person onsite and offsite training programs, online training modules that are available in the VA Electronic Educational Services (EES), and video telehealth consultations. Participants can view online lectures and then receive hands-on training as part of the educational program. Up to 3 HCPs for each site can receive funding to attend. Online programs also are available for new material to support continuing medical education. However, hands-on training is essential to understand how to obtain the motor threshold, which is used to determine the strength of the rTMS stimulus dose. Furthermore, hands-on training is essential for the proper localization of the stimulus, which is determined by certain anatomical landmarks. A phantom mannequin (ERIK [Evaluating Resting motor threshold and Insuring Kappa]) has been developed to assist in the hands-on learning.20
Relative Value Units
The VHA uses RVUs to properly account for workload and clinician activities. As a result, RVUs play an essential role as a currency that denotes the relative value of one type of clinical activity when compared with other activities. Depending on the treating specialty, clinicians generally use procedure codes outlined in the Current Procedural Terminology (CPT) code set or the Healthcare Common Procedure Coding System (HCPCS) for medical billing. Most insurance carriers use RVUs set by the Centers for Medicare and Medicaid Services (CMS) system as a standard system to determine HCP reimbursement for medical procedures.
The CPT codes associated with rTMS currently are 90867 to 90869. CMS had initially assigned a zero RVU to these CPT codes due to wide variations in the cost of performing rTMS. When we began implementing rTMS in the VHA, the lack of RVUs for rTMS rendered it impossible to show clinical workload for this activity using established VHA clinical accounting methods. The lack of RVUs assigned to rTMS CPT codes made justification for this treatment to clinical management difficult, which limited its clinical use in the VHA. In addition, HCPs who were using rTMS to treat severely ill veterans appeared artificially unproductive despite a significant patient workload. As we and VHA leadership became aware the program could not be staffed locally without getting workload credit for work done, the value was raised to 1.37 for treatment (90868) and 2.12 and 1.93 for evaluations (90867) and reevaluations (90869), respectively, thus reducing a potential roadblock to implementation.
Challenges as the Program Expands
Future challenges include upgrading machines to do intermittent θ burst stimulation (iTBS), which decreases the standard treatment time from 37.5 minutes to 3 minutes. Both patients and HCPs find iTBS to have similar tolerability to standard rTMS but in much less time. iTBS mimics endogenous θ rhythms and has been shown to be noninferior to rTMS for depression.21,22 Several devices have received FDA approval to treat MDD, including the Magstim and MagVenture TMS devices used in this program.
A major challenge for the VHA with rTMS will be to maintain a consistent level of competence and training. There is a need for continued maintenance of staff competence with ongoing training and training for new staff. Novel ways of training operators have been developed including ERIK.
Determining treatment interaction with other psychotherapies and pharmacotherapies is another challenge. Currently, rTMS is considered an adjunctive treatment added to the current patient treatment plan. We do not know yet how best to incorporate this somatic treatment with other approaches, and further research is necessary. A key issue is to determine which approach provides the best long-term results for a patient at risk for recurrence of depression. In addition, more research into maintaining healthy relationships for veterans with both MDD and PTSD is needed.
Many misconceptions exist about rTMS and HCPs need to be educated about the benefits of this modality. In addition, patients should understand the differences between rTMS and ECT. Even with newer approaches that streamline rTMS, the therapy remains costly in terms of direct costs as well as patient and HCP time.
Streamlining rTMS treatment remains an important concern. Compressing treatment schedules (ie, many treatments delivered to a patient in a single day) would allow the entire process to be delivered in days, not weeks. This would be especially advantageous to patients who live far from a treatment site. Performing multiple rTMS daily treatments is especially feasible with iTBS with its short treatment time.
Conclusions
rTMS is an emerging modality with both established and novel applications. The best studied application is treatment resistant MDD. Currently, rTMS has only been approved by the FDA for treatment of MDD. A pilot program was established by the VHA to distribute 30 rTMS machines sites nationwide. Results from data collected by these sites have shown patients improving on standard psychometric scales. Future changes include upgrading the machines to provide θ bursts, which has been shown to be faster and noninferior. Integrating rTMS with other pharmacotherapies and psychotherapies remains poorly understood and needs more research.
1. George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6(14):1853‐1856. doi:10.1097/00001756-199510020-00008
2. Tik M, Hoffmann A, Sladky R, et al. Towards understanding rTMS mechanism of action: stimulation of the DLPFC causes network-specific increase in functional connectivity. Neuroimage. 2017;162:289‐296. doi:10.1016/j.neuroimage.2017.09.022
3. Perera T, George MS, Grammer G, Janicak PG, Pascual-Leone A, Wirecki TS. The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9(3):336‐346. doi:10.1016/j.brs.2016.03.010
4. George MS, Taylor JJ, Short EB. The expanding evidence base for rTMS treatment of depression. Curr Opin Psychiatry. 2013;26(1):13‐18. doi:10.1097/YCO.0b013e32835ab46d
5. Lisanby SH, Husain MM, Rosenquist PB, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34(2):522‐534. doi:10.1038/npp.2008.118
6. Yesavage JA, Fairchild JK, Mi Z, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75(9):884‐893. doi:10.1001/jamapsychiatry.2018.1483
7. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208‐1216. doi:10.1016/j.biopsych.2007.01.018
8. Stilling JM, Monchi O, Amoozegar F, Debert CT. Transcranial magnetic and direct current stimulation (TMS/tDCS) for the treatment of headache: a systematic review. Headache. 2019;59(3):339‐357. doi:10.1111/head.13479
9. Lin Y, Jiang WJ, Shan PY, et al. The role of repetitive transcranial magnetic stimulation (rTMS) in the treatment of cognitive impairment in patients with Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Sci. 2019;398:184‐191. doi:10.1016/j.jns.2019.01.038
10. Carmi L, Tendler A, Bystritsky A, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. 2019;176(11):931‐938. doi:10.1176/appi.ajp.2019.18101180
11. Song S, Zilverstand A, Gui W, Li HJ, Zhou X. Effects of single-session versus multi-session non-invasive brain stimulation on craving and consumption in individuals with drug addiction, eating disorders or obesity: a meta-analysis. Brain Stimul. 2019;12(3):606‐618. doi:10.1016/j.brs.2018.12.975
12. Wagner E, Wobrock T, Kunze B, et al. Efficacy of high-frequency repetitive transcranial magnetic stimulation in schizophrenia patients with treatment-resistant negative symptoms treated with clozapine. Schizophr Res. 2019;208:370‐376. doi:10.1016/j.schres.2019.01.021
13. Kozel FA, Van Trees K, Larson V, et al. One hertz versus ten hertz repetitive TMS treatment of PTSD: a randomized clinical trial. Psychiatry Res. 2019;273:153‐162. doi:10.1016/j.psychres.2019.01.004
14. Coles AS, Kozak K, George TP. A review of brain stimulation methods to treat substance use disorders. Am J Addict. 2018;27(2):71‐91. doi:10.1111/ajad.12674
15. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. 2019 National veteran suicide prevention annual report. https://www.mentalhealth.va.gov/docs/data-sheets/2019/2019_National_Veteran_Suicide_Prevention_Annual_Report_508.pdf. Published September 19, 2019. Accessed May 18, 2020.
16. Ritchie EC. Improving Veteran engagement with mental health care. Fed Pract. 2017;34(8):55‐56.
17. Rush AJ, Trivedi MH, Wisniewski SR, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231‐1242. doi:10.1056/NEJMoa052963
18. Kozel FA, Hernandez M, Van Trees K, et al. Clinical repetitive transcranial magnetic stimulation for veterans with major depressive disorder. Ann Clin Psychiatry. 2017;29(4):242‐248.
19. National Health Policy Forum. The basics: relative value units (RVUs). https://collections.nlm.nih.gov/master/borndig/101513853/Relative%20Value%20Units.pdf. Published January 12, 2015. Accessed May 18, 2020.
20. Finetto C, Glusman C, Doolittle J, George MS. Presenting ERIK, the TMS phantom: a novel device for training and testing operators. Brain Stimul. 2019;12(4):1095‐1097. doi:10.1016/j.brs.2019.04.01521. Trevizol AP, Vigod SN, Daskalakis ZJ, Vila-Rodriguez F, Downar J, Blumberger DM. Intermittent theta burst stimulation for major depression during pregnancy. Brain Stimul. 2019;12(3):772‐774. doi:10.1016/j.brs.2019.01.003
22. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial [published correction appears in Lancet. 2018 Jun 23;391(10139):e24]. Lancet. 2018;391(10131):1683‐1692. doi:10.1016/S0140-6736(18)30295-2
1. George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6(14):1853‐1856. doi:10.1097/00001756-199510020-00008
2. Tik M, Hoffmann A, Sladky R, et al. Towards understanding rTMS mechanism of action: stimulation of the DLPFC causes network-specific increase in functional connectivity. Neuroimage. 2017;162:289‐296. doi:10.1016/j.neuroimage.2017.09.022
3. Perera T, George MS, Grammer G, Janicak PG, Pascual-Leone A, Wirecki TS. The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9(3):336‐346. doi:10.1016/j.brs.2016.03.010
4. George MS, Taylor JJ, Short EB. The expanding evidence base for rTMS treatment of depression. Curr Opin Psychiatry. 2013;26(1):13‐18. doi:10.1097/YCO.0b013e32835ab46d
5. Lisanby SH, Husain MM, Rosenquist PB, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34(2):522‐534. doi:10.1038/npp.2008.118
6. Yesavage JA, Fairchild JK, Mi Z, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75(9):884‐893. doi:10.1001/jamapsychiatry.2018.1483
7. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208‐1216. doi:10.1016/j.biopsych.2007.01.018
8. Stilling JM, Monchi O, Amoozegar F, Debert CT. Transcranial magnetic and direct current stimulation (TMS/tDCS) for the treatment of headache: a systematic review. Headache. 2019;59(3):339‐357. doi:10.1111/head.13479
9. Lin Y, Jiang WJ, Shan PY, et al. The role of repetitive transcranial magnetic stimulation (rTMS) in the treatment of cognitive impairment in patients with Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Sci. 2019;398:184‐191. doi:10.1016/j.jns.2019.01.038
10. Carmi L, Tendler A, Bystritsky A, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. 2019;176(11):931‐938. doi:10.1176/appi.ajp.2019.18101180
11. Song S, Zilverstand A, Gui W, Li HJ, Zhou X. Effects of single-session versus multi-session non-invasive brain stimulation on craving and consumption in individuals with drug addiction, eating disorders or obesity: a meta-analysis. Brain Stimul. 2019;12(3):606‐618. doi:10.1016/j.brs.2018.12.975
12. Wagner E, Wobrock T, Kunze B, et al. Efficacy of high-frequency repetitive transcranial magnetic stimulation in schizophrenia patients with treatment-resistant negative symptoms treated with clozapine. Schizophr Res. 2019;208:370‐376. doi:10.1016/j.schres.2019.01.021
13. Kozel FA, Van Trees K, Larson V, et al. One hertz versus ten hertz repetitive TMS treatment of PTSD: a randomized clinical trial. Psychiatry Res. 2019;273:153‐162. doi:10.1016/j.psychres.2019.01.004
14. Coles AS, Kozak K, George TP. A review of brain stimulation methods to treat substance use disorders. Am J Addict. 2018;27(2):71‐91. doi:10.1111/ajad.12674
15. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. 2019 National veteran suicide prevention annual report. https://www.mentalhealth.va.gov/docs/data-sheets/2019/2019_National_Veteran_Suicide_Prevention_Annual_Report_508.pdf. Published September 19, 2019. Accessed May 18, 2020.
16. Ritchie EC. Improving Veteran engagement with mental health care. Fed Pract. 2017;34(8):55‐56.
17. Rush AJ, Trivedi MH, Wisniewski SR, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231‐1242. doi:10.1056/NEJMoa052963
18. Kozel FA, Hernandez M, Van Trees K, et al. Clinical repetitive transcranial magnetic stimulation for veterans with major depressive disorder. Ann Clin Psychiatry. 2017;29(4):242‐248.
19. National Health Policy Forum. The basics: relative value units (RVUs). https://collections.nlm.nih.gov/master/borndig/101513853/Relative%20Value%20Units.pdf. Published January 12, 2015. Accessed May 18, 2020.
20. Finetto C, Glusman C, Doolittle J, George MS. Presenting ERIK, the TMS phantom: a novel device for training and testing operators. Brain Stimul. 2019;12(4):1095‐1097. doi:10.1016/j.brs.2019.04.01521. Trevizol AP, Vigod SN, Daskalakis ZJ, Vila-Rodriguez F, Downar J, Blumberger DM. Intermittent theta burst stimulation for major depression during pregnancy. Brain Stimul. 2019;12(3):772‐774. doi:10.1016/j.brs.2019.01.003
22. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial [published correction appears in Lancet. 2018 Jun 23;391(10139):e24]. Lancet. 2018;391(10131):1683‐1692. doi:10.1016/S0140-6736(18)30295-2
Steroid-Induced Sleep Disturbance and Delirium: A Focused Review for Critically Ill Patients
Sleep disturbance in the critically ill has received much attention over recent years as this is a common result of intensive care unit (ICU) admission. Disruptions in sleep not only can, at a minimum, cause distress and lower patient satisfaction, but also inhibit recovery from illness and increase morbidity.1,2 Several studies have been conducted highlighting the altered sleep patterns of critically ill patients; although total sleep time may seem normal (7-9 hours), patients can experience multiple awakenings per hour, more time in light sleep (stages 1 and 2), and less time in restorative sleep (stages 3 and 4, [REM]rapid eye movement).2-5
There are several hypothesized physiologic detriments that contribute to slower ICU recovery with sleep deprivation. Research in noncritically ill subjects suggests that sleep deprivation contributes to hypoventilation and potentially prolonged time on the ventilator.6-9 Cardiovascular morbidity may be adversely affected by inflammatory cytokine release seen in sleep disruption.10,11 Studies of noncritically ill patients also suggest that immune response is impaired, potentially protracting infection recovery.12,13 Finally, although not directly investigated, sleep deprivation may contribute to ICU delirium, an independent adverse effect (AE) associated with increased mortality and worse long-term outcomes.14-16
The Society of Critical Care Medicine (SCCM) recently updated its consensus guidelines for the management of pain, agitation/sedation, delirium, immobility, and sleep disruption (PADIS) in adult patients.17 These guidelines offer limited interventions to promote sleep in ICU patients based on available evidence and steer the clinician toward minimizing exacerbating factors. Although factors that affect sleep patterns are multifactorial, such as noise levels, pain, mechanical ventilation, and inflammatory mediators, medication therapy is a known modifiable risk factor for sleep disturbance in critically ill patients.2 This focused review will specifically evaluate the effects of steroids on sleep deprivation, psychosis, delirium, and what is known about these effects in a critically ill population.
To include articles relevant to a critically ill population, a systematic search of MEDLINE and PubMed from 1966 to 2019 was performed using the following Medical Subject Headings (MeSH) terms: delirium/etiology, psychoses, substance-induced/etiology, sleep-wake disorders/chemically induced, neurocognitive disorders/chemically induced, dyssomnias/drug effects plus glucocorticoids/adverse effects, adrenal cortex hormones/adverse effects, prednisone/adverse effects, methylprednisolone/adverse effects, and hydrocortisone/adverse effects. The initial search produced 285 articles. Case reports, reviews, letters, and articles pertaining to primary care or palliative populations were excluded, leaving 8 relevant articles for inclusion (Table 1).18-25
ICU Steroid Use
Steroids are commonly used in the ICU and affect nearly every critically ill population. Common indications for steroids in the ICU include anaphylaxis, airway edema, septic shock, asthma and COPD exacerbations, pneumocystis pneumonia, adrenal crisis, antiemetic treatment, elevated intracranial pressure from tumors, autoimmune disorders, and stress doses needed for chronic steroid users before invasive procedures.26 Whether divided into glucocorticoid or mineralocorticoid subgroups, corticosteroids offer therapeutic benefit from their pharmacologic similarity to endogenously produced cortisol, which includes anti-inflammatory, immunosuppressive, antiproliferative, and vasoconstrictive effects.
Steroid receptors are present in most human tissue, and in varying degrees of binding affinity produce a wide variety of effects. After passive diffusion across cell membranes, steroid-receptor activation binds to various DNA sites, called glucocorticoid regulatory elements, which either stimulates or inhibits transcription of multiple nearby genes.
At the cellular level, corticosteroids inhibit the release of arachidonic acid through upstream production of lipocortin peptides and antagonism of phospholipase A2. This action decreases subsequent inflammatory mediators, including kinins, histamine, liposomal enzymes, and prostaglandins. Steroids also inhibit NF-κB, which further decreases expression of proinflammatory genes while promoting interleukin-10 and its anti-inflammatory properties. Antiproliferative effects of steroids are seen by triggering cell apoptosis and inhibition of fibroblast proliferation.27,28
By binding to mineralocorticoid receptors, steroids cause sodium retention coupled with hydrogen and potassium excretion in the distal renal tubule. Steroids also promote vasoconstriction by upregulating the production and sensitivity of β receptors in the endothelium while suppressing the production of vasodilators. Although rarely used for these physiologic effects, steroids also are involved in a number of metabolic pathways, including calcium regulation, gluconeogenesis, protein metabolism, and fat distribution. Given the similar structure to cortisol, exogenous steroids depress the hypothalamic-pituitary axis (HPA) and decrease the release of adrenocorticotropic hormone (ACTH). Tapering doses of steroid regimens is often required to allow natural androgen and cortisol synthesis and prevent steroid withdrawal.27,28
The potency of various exogenous steroids closely parallels their ability to retain sodium (Table 2). Prolonged activation of steroid receptors can have numerous systemic AEs, including unwanted neurocognitive effects (Table 3). Insomnia and psychosis are commonly described in corticosteroid clinical trials, and in one meta-analysis, both are associated with high costs per episode per year.29
Steroid-Induced Sleep Disruption and Psychosis
Sleep disruption caused by exogenous administration of steroids is thought to trigger other psychostimulant effects, such as mood swings, nervousness, psychoses, and delirium.30 Similarly, the SCCM PADIS guidelines included an ungraded statement: “although an association between sleep quality and delirium occurrence exists in critically ill adults, a cause-effect relationship has not been established.”17 For this review, these AEs will be discussed as related events.
The medical literature proposes 3 pathways primarily responsible for neurocognitive AEs of steroids: behavior changes through modification of the HPA axis, changes in natural sleep-wake cycles, and hyperarousal caused by modification in neuroinhibitory pathways (Figure).
HPA Axis Modification
Under either physical or psychological stress, neural circuits in the brain release corticotropin-releasing hormone (CRH), dehydroepiandrosterone (DHEA), and arginine vasopressin, which go on to activate the sympathetic nervous system and the HPA axis. CRH from the hypothalamus goes on to stimulate ACTH release from the pituitary. ACTH then stimulates cortisol secretion from the adrenal glands. Circulating cortisol feeds into several structures of the brain, including the pituitary, hippocampus, and amygdala. Steroid-receptor complexes alter gene transcription in the central nervous system (CNS), affecting the production of neurotransmitters (eg, dopamine, serotonin) and neuropeptides (eg, somatostatin, β-endorphin). Feedback inhibition ensues, with downregulation of the HPA axis, which prevents depletion of endogenous production of steroids.31 DHEA has protective effects against excessive cortisol activity, but DHEA secretion declines with prolonged cortisol exposure. Exogenous steroids may have different effects than endogenous steroids, and neurocognitive sequelae stem from disruption and imbalance of these physiologic mechanisms.32,33
Steroid receptors are densely located in behavior centers in the brain: the amygdala, septum, and hippocampus. Pharmacologic changes in gene expression alter norepinephrine and serotonin levels in the brain as well as their receptors.32 Prolonged exposure to exogenous steroids has been shown to decrease amygdala and hippocampal volumes.34,35 Furthermore, prolonged corticosteroid exposure has been shown to decrease the number of steroid receptors in the hippocampus, pituitary gland, and amygdala.36 In a somewhat paradoxical finding, the production of CNS proinflammatory cytokines like interleuken-1β and tumor necrosis factor α has been seen after steroid administration, suggesting alternate gene signaling in the CNS.37 Although not proven conclusively, it is felt that these physiologic changes and hyperactivity of the HPA axis are predominantly responsible for changes in behavior, mood, memory, and eventually psychosis in steroid-treated patients.33,38
Finally, alterations in cognition and behavior may be related to steroid-induced changes in CNS carbohydrate, protein, and lipid metabolism with subsequent cellular neurotoxicity.32,38 Glucose uptake into the hippocampus is decreased with steroid exposure. Additionally, breakdown of metabolic compounds to produce energy can be destructive if left unchecked for prolonged periods. DHEA, growth hormone, and testosterone work to repair catabolic damage produced by cortisol, known as anabolic balance. A low anabolic balance (low DHEA levels to high cortisol levels) leads to a cascade of dysregulation in brain activity.39
Changes in Natural Sleep-Wake Cycles
Natural sleep pathways are also affected by steroids. The sleep-wake cycle is primarily regulated in the hypothalamus with circadian release of melatonin from the pineal gland. Melatonin release is highest at night, where it promotes sleep onset and continuity. Upstream, tryptophan is an amino acid that serves as a precursor to serotonin and melatonin.40 Both endogenous and exogenous corticosteroids decrease serum melatonin levels with a markedly diminished circadian rhythm secretion.41,42Demish and colleagues found a significant decrease in mean (SD) nocturnal melatonin plasma levels after the evening administration of oral dexamethasone 1 mg in 11 healthy volunteers: 127 (42) pg/mL before vs 73 (38) pg/mL after; P < .01.42 This result is likely due to decreased cellular metabolism and melatonin synthesis in the pineal gland. Of note, melatonin has neuroprotective affects, and the administration of melatonin has been shown to reverse some steroid-induced neurotoxicities in animal models.43
Steroids also reduce the uptake of tryptophan into the brain.33 Additionally, in animal models, dexamethasone administration caused a significant decrease in the gene expression of tryptophan hydroxylase, which is part of the multistep pathway in synthesizing serotonin from L-tryptophan. These effects upstream could inhibit the biosynthetic capacity of both melatonin and serotonin.44
A third pathway investigated in sleep regulation are the orexin neuropeptides. Orexins are produced in the hypothalamus and stimulate daytime wake activity in monoaminergic and cholinergic neurons. Subsequently, orexin receptor antagonists are a newer class of drugs aimed at mitigating nighttime hyperarousal and sleep disruption. Orexin overexpression may be a causal factor in steroid-induced sleep disturbance. However, this effect was specifically evaluated in a recent study in children with acute lymphoblastic leukemia, which showed that cerebral spinal fluid orexin levels (SD) were not significantly different from baseline after dexamethasone administration: 574 (26.6) pg/mL vs 580 (126.1) pg/mL; P = .8.45
Hyperarousal State
Finally, a hyperarousal state is thought to be produced by nongenomic changes to natural neuroinhibitory regulation seen with nonclassical steroid production called neurosteroids. Animal studies revealed that high levels of steroids were found in the CNS long after adrenalectomy, suggesting CNS de novo synthesis.46 In addition to altering gene expression at classic intercellular steroid receptors, neurosteroids can alter neurotransmission by direct interaction on ion-gated membranes and other receptors on the cell surface. Restlessness and insomnia could be due to γ-aminobutyric acid type A (GABAA) receptor modulation in the CNS where neuroactive steroids slow the rate of recovery of GABAA and potentially inhibit postsynaptic GABAergic transmission. It also is hypothesized that neuroactive steroids have excitatory action at nicotinic acetylcholine, 5HT3 receptors, and through increasing the fractional open time of the N-methyl-D-aspartate -activated channels.47 Allopregnanolone and DHEA are neurosteroids that act as GABAA agonists and have neuroprotective effects with anxiolytic, antidepressant, and antiaggressive properties.
Neurosteroids are synthesized from cholesterol in the hippocampus. Neurosteroids are upregulated in response to stress by CNS cortisol effects on various enzyme expressions.47 Whether exogenous steroid administration affects this biosynthesis vs the stress response in the HPA axis itself is not fully elucidated. Monteleone and colleagues found that dexamethasone 1 mg given orally significantly reduced cortisol and DHEA and allopregnanolone levels in both healthy volunteers and anorexia nervosa patients.48 Similarly, Genazzani and colleagues demonstrated that oral dexamethasone administration (0.5 mg every 6 hours) caused significant reductions in both serum allopregnanolone and DHEA levels.49
Outcomes Studies
The majority of reported data in steroid-induced insomnia and psychosis is in noncritically ill populations. In a randomized, prospective crossover study of healthy volunteers, dexamethasone administration (3 mg every 8 hours for 48 hours) resulted in significant changes in sleep patterns measured with polysomnography. Compared with placebo, steroid treatment showed significantly longer percentage (SD) of stage 0/awake times (11.7% [11.4] vs 2.9% [1.8]; P < .05); longer percentage (SD) of REM sleep latency (363.8 [74.5] minutes vs 202.8 [79.6] minutes; P < .01), and a reduced number (SD) of REM periods (3.8 [2.6] vs 9.7 [3.6]; P < .01).50 Insomnia was one of the most commonly self-reported AEs (> 60%) in a survey of 2,446 chronic steroid users, and the incidence increased as steroid doses increased.51
A prospective, open-label study of 240 patients with cancer demonstrated significant sleep disruptions using the Pittsburgh Sleep Quality Index with the use of high-dose steroids in chemotherapy.52 Naber and colleagues evaluated 50 previously healthy patients taking methylprednisolone 119 mg (41 mg/d) for retinitis and uveitis.53 They reported 26% to 34% of subjects experienced hypomanic syndrome based on a semistructured interview examination. Symptoms developed within 3 days and persisted for the 8-day course of therapy. Brown and colleagues prospectively evaluated 32 asthmatic patients prescribed bursts of prednisone > 40 mg daily. They observed significantly increased scores in the Young Mania Rating Scale within 3 to 7 days of starting therapy, which dissipated to baseline after stopping therapy.54
Despite a high reported incidence of neurologic AEs, outcomes in critically ill populations are mixed. Study methods are varied, and many were largely observational. No prospective, randomized studies exist to date specifically aimed and powered to evaluate the effects of steroids on sleep disturbances or delirium in a critically ill population. Furthermore, sleep quality is difficult to measure in this population, and self-reporting often is not an option. In critical care trials, if AEs such as insomnia, delirium, or psychosis are recorded at all, there is heterogeneity in the definitions, and these AEs are generally poorly defined (eg, psychiatric or neurologic disorder not otherwise specified), making pooled analysis of this outcome difficult.55
One of the largest observational studies in hospitalized patients was through the Boston Collaborative Drug Surveillance Program. A total of 718 consecutively enrolled inpatients who received prednisone were monitored for acute reactions. Psychiatric AEs were rare (1.3%) with low doses (< 40 mg/d), more prevalent (4.6%) with higher doses (41-80 mg/d), and most prevalent (18.4%) with the highest doses (> 80 mg/d), suggesting CNS AEs are dose dependent.18 A single-center, retrospective review of 755 psychiatric consults in hospitalized patients revealed that 54% of manic patients were due to corticosteroid administration.19 In a prospective observational study of 206 consecutive ICU admissions, steroid administration was an independent risk factor for development of ICU delirium, using the Confusion Assessment Method-ICU (CAM-ICU) at a single center (odds ratio [OR], 2.8; 95% CI, 1.05-7.28).25
Two studies in hospitalized oncology patients found conflicting results using the Nursing Delirium Screening Scale (Nu-DESC). One did not find a significant association between delirium and dexamethasone equivalent doses > 15 mg, while the second found an increased hazard ratio (HR) for a positive Nu-DESC score (HR, 2.67; 95% CI, 1.18-6.03).20,21 Similarly, conflicting results were found in 2 studies using first-order Markov models. In one prospective cohort study, 520 consecutive mechanically ventilated patients in 13 ICUs were monitored for the transition to delirium (CAM-ICU positive) from nondelirium states. Steroid administration was significantly associated with transitioning to delirium (OR, 1.52; 95% CI, 1.05-2.21).22 This conflicts with a similar study by Wolters and colleagues, which monitored 1,112 ICU patients who were given a median prednisone equivalent of 50 mg (interquartile range, 25-75 mg). Steroid administration was not significantly associated with the transition to delirium from an awake without delirium state (OR, 1.08; 95% CI, 0.89-1.32; adjusted OR, 1.00; 95% CI, 0.99-1.01 per 10-mg increase in prednisone equivalent).23
Mitigating Effects
Although steroid therapy often cannot be altered in the critically ill population, research showed that steroid overuse is common in ICUs.56,57 Minimizing dosage and duration are important ways clinicians can mitigate unwanted effects. CNS AEs seen with steroids often can be reversed once therapy is discontinued. Avoiding split-dose administration has been proposed given the natural diurnal production of cortisol.58 A review by Flaherty discusses the importance of avoiding pharmacologic agents in hospitalized older patients if possible due to known risks (falls, dependency, hip fractures, rebound insomnia, and risk of delirium) and provides a HELP ME SLEEP nomogram for nonpharmacologic interventions in hospitalized patients (Table 4).59
Historically, lithium has been recommended for steroid-induced mania with chronic steroid use; however, given the large volume and electrolyte shifts seen in critically ill patients, this may not be a viable option. Antidepressants, especially tricyclics, should generally be avoided in steroid-induced psychosis as these may exacerbate symptoms. If symptoms are severe, either typical (haloperidol) or atypical (olanzapine, quetiapine, risperidone) antipsychotics have been used with success.60 Given the known depletion of serum melatonin levels, melatonin supplements are an attractive and relatively safe option for steroid-induced insomnia; however, there are no robust studies specifically aimed at this intervention for this population.
Conclusions
With known, multimodal foci driving sleep impairment in ICU patients, PADIS guidelines recommend myriad interventions for improvement. Recommendations include noise and light reduction with earplugs and/or eyeshades to improve sleep quality. Nocturnal assist-control ventilation may improve sleep quality in ventilated patients. Finally, the development of institutional protocols for promoting sleep quality in ICU patients is recommended.17
1. Simini B. Patients’ perceptions of intensive care. Lancet. 1999;354(9178):571-572. doi: 10.1016/S0140-6736(99)02728-2
2. Delaney LJ, Van Haren F, Lopez V. Sleeping on a problem: the impact of sleep disturbance on intensive care patients—a clinical review. Ann Intensive Care. 2015;15:3. doi: 10.1186/s13613-015-0043-2
3. Friese RS, Diaz-Arrastia R, McBride D, Frankel H, Gentilello LM. Quality and quantity of sleep in the surgical intensive care unit; are our patients sleeping? J Trauma. 2007;63(6):1210-1214. doi: 10.1097/TA.0b013e31815b83d7
4. Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care 2013;17(2):R46.
5. Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in patients receiving postoperative care. BJM (Clin Res Ed). 1985;290(6474)1029-1032. doi: 10.1136/bmj.290.6474.1029
6. White DP, Douglas NJ, Pickett CK, Zwillich CW, Weil JV. Sleep deprivation and the control of ventilation. Am Rev Respir Dis. 1983;128(6):984-986. doi: 10.1164/arrd.1983.128.6.984
7. Series F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150(2):481-485. doi: 10.1164/ajrccm.150.2.8049833
8. Tadjalli A, Peever J. Sleep loss reduces respiratory motor plasticity. Adv Exp Med Biol. 2010;669:289-292.
doi: 10.1007/978-1-4419-5692-7_59
9. Roche Campo F, Drouot X, Thille AW, et al. Poor sleep quality is associated with late noninvasive ventilation failure in patients with acute hypercapnic respiratory failure. Crit Care Med. 2010;38(2):447-485. doi: 10.1097/CCM.0b013e3181bc8243
10. Sauvet F, Leftheriotis G, Gomez-Merino D, et al. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol (1985). 2010;108(1):68-75. doi: 10.1152/japplphysiol.00851.2009
11. Frey DJ, Fleshner M, Wright KP Jr. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21(8):1050-1057. doi: 10.1016/j.bbi.2007.04.003
12. Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA 2002;288(12):1471-1472. doi: 10.1001/jama.288.12.1471-a
13. Dinges DF, Douglas SD, Zuagg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93(5):1930-1939. doi: 10.1172/JCI117184
14. Weinhouse GL, Schwab RJ, Watson PL, et al. Bench-to-bedside review: delirium in ICU patients— importance of sleep deprivation. Crit Care. 2009;13(6):234. doi: 10.1186/cc8131
15. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. doi: 10.1001/jama.291.14.1753
16. Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513-1520. doi: 10.1097/CCM.0b013e3181e47be1
17. Devlin JW, Skrobik Y, Gelinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873
18. The Boston Collaborative Drug Surveillance Program. Acute adverse reactions to prednisone in relation to dosage. Clin Pharmacol Ther. 1972;13(5):694-698. doi: 10.1002/cpt1972135part1694
19. Rundell JR, Wise MG. Causes of organic mood disorder. J Neuropsychiatry Clin Neurosci. 1989;1(4):398-400. doi: 10.1176/jnp.1.4.398
20. Gaudreau JD, Gagnon P, Harel F, Roy MA, Tremblay A. Psychoactive medications and risk of delirium in hospitalized cancer patients. J Clin Oncol. 2005;23(27):6712-6718. doi: 10.1200/JCO.2005.05.140
21. Gaudreau JD, Gagnon P, Roy MA, Harel F, Tremblay A. Opioid medications and longitudinal risk of delirium in hospitalized cancer patients. Cancer. 2007;109(11):2365-2373.
doi: 10.1002/cncr.22665
22. Schreiber MP, Colantuoni E, Bienvenu OJ, et al. Corticosteroids and transition to delirium in patients with acute lung injury. Crit Care Med. 2014;42(6):1480-1486. doi: 10.1097/CCM.0000000000000247
23. Wolters AE, Veldhuijzen DS, Zaal IJ, et al. Systemic corticosteroids and transition to delirium in critically ill patients. Crit Care Med. 2015;43(12):e585-e588. doi: 10.1097/CCM.0000000000001302
24. Matschke J, Muller-Beissenhirtz H, Novotny J, et al. A randomized trial of daily prednisone versus pulsed dexamethasone in treatment-naïve adult patients with immune thrombocytopenia: EIS 2002 study. Acta Haematol. 2016;136(2):101-107. doi: 10.1159/000445420
25. Tilouche N, Hassen M, Ali HBS, Jaoued AHO, Gharbi R, Atrous SS. Delirium in the intensive care unit: incidence, risk factors, and impact on outcome. Indian J Crit Care Med. 2018;22:144-149. doi: 10.4103/ijccm.IJCCM_244_17
26. Young A, Marsh S. Steroid use in critical care. BJA Education. 2018;18(5):129-134. doi: 10.1016/j.bjae.2018.01.005
27. DiPiro J, Talbert R, Yee G, Matzke GR, Wells BG, Posey M. Pharmacotherapy: A Pathophysiologic Approach. 4th ed. New York: McGraw-Hill; 1999:1277-1278.
28. Schimmer
29. Sarnes E, Crofford L, Watson M, Dennis G, Kan H, Bass D. Incidence of US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther. 2011;33(10):1413-1432.
30. Idzikowsi C, Shapiro CM. ABC of sleep disorders, non-psychotropic drugs and sleep. BMJ. 1993;306(6885):1118-1120. doi: 10.1136/bmj.306.6885.1118

31. Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14(4):398-406.
doi: 10.3109/10253890.2011.586446
32. Wolkowitz OM, Reus VI, Weingartner H, et al. Cognitive effects of corticosteroids. Am J Psychiatry 1990;147(10):1297-1303. doi: 10.1176/ajp.147.10.1297
33. McEwen BS, Davis PG, Parsons B, Pfaff DW. The brain as a target for steroid hormone action. Ann Rev Neurosci. 1979;2:65-112. doi: 10.1146/annurev.ne.02.030179.000433
34. Brown ES, Woolston DJ, Frol AM. Amygdala volume in patients receiving chronic corticosteroid therapy. Biol Psychiatry. 2008;63(7):705-709.
doi: 10.1016/j.biopsych.2007.09.014
35. Brown ES, Woolston D, Frol A, et al. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid. Biol Psychiatry. 2004;55(5):538-545.
36. Sapolsky RM, McEwen BS. Down-regulation of neural corticosterone receptors by corticosterone and dexamethasone. Brain Res. 1985;339(1):161-165.
doi: 10.1016/0006-8993(85)90638-9
37. Sorrells SF, Caso JR, Munhoz CD, Spolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64(1):33-39.
doi: 10.1016/j.neuron.2009.09.032
38. Wolkowitz OM, Burke H, Epel ES, Reus VI. Glucocorticoids: mood, memory, and mechanisms. Ann NY Acad Sci. 2009;1179:19-40. doi: 10.1111/j.1749-6632.2009.04980.x
39. Wolkowitz OM, Epel ES, Reus VI. Stress hormone-related psychopathology: pathophysiological and treatment implications. World J Biol Psychiatry. 2001;2(3):115-143. doi: 10.3109/15622970109026799
40. Paredes S, Barriga C, Reiter R, Rodrigues A. Assessment of the potential role of tryptophan as the precursor of serotonin and melatonin for the aged sleep-wake cycle and immune function: Streptopelia Risoria as a model. Int J Tryptophan Res. 2009;2:23-36. doi: 10.4137/ijtr.s1129
41. Soszyński P, Stowińska-Srzednicka J, Kasperlik-Zatuska A, Zgliczyński S. Decreased melatonin concentration in Cushing’s Syndrome. Horm Metab Res. 1989;21(12):673-674. doi: 10.1055/s-2007-1009317
42. Demish L, Demish K, Neckelsen T. Influence of dexamethasone on nocturnal melatonin production in healthy adult subjects. J Pineal Res. 1988;5(3):317-321. doi: 10.1111/j.1600-079x.1988.tb00657.x
43. Assaf N, Shalby AB, Khalil WK, Ahmed HH. Biochemical and genetic alterations of oxidant/antioxidant status of the brain in rats treated with dexamethasone: protective roles of melatonin and acetyl-L-carnitine. J Physiol Biochem. 2012;68(1):77-90. doi: 10.1007/s13105-011-0121-3
44. Clark MS, Russo AF. Tissue-specific glucocorticoid regulation of tryptophan hydroxylase mRNA levels. Brain Res Mol Brain Res. 1997;48(2):346-54. doi: 10.1016/s0169-328x(97)00106-x
45. Kram DE, Krasnow SM, Levasseur PR, Zhu X, Stork LC, Marks DL. Dexamethasone chemotherapy does not disrupt orexin signaling. PLoS One. 2016;11(12):e0168731. doi: 10.1371/journal.pone.0168731
46. Mellon S. Neurosteroids: biochemistry, modes of action, and clinical relevance. J Clin Endocrinol Metab. 1994;78(5):1003-1008. doi: 10.1210/jcem.78.5.8175951
47. Zorumski C, Paul SM, Izumi Y, Covey DF, Mennerick S . Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci Biobehav Rev. 2013;37(1):109-122. doi: 10.1016/j.neubiorev.2012.10.005
48. Monteleone P, Luisi M, Martiadis V, et al. Impaired reduction of enhanced levels of dehydroepiandrosterone by oral dexamethasone in anorexia nervosa. Psychoneuroendocrinology. 2006;31(4):537-542. doi: 10.1016/j.psyneuen.2005.08.015
49. Genazzani AR, Petraglia F, Bernardi F, et al. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998;83(6):2099-3103. doi: 10.1210/jcem.83.6.4905
50. Moser NJ, Phillips BA, Guthrie G, Barnett G. Effects of dexamethasone on sleep. Pharmacol Toxicol. 1996;79(2):100-102. doi: 10.1111/j.1600-0773.1996.tb00249.x
51. Curtis J, Westfall A, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55(3):420-426. doi: 10.1002/art.21984
52. Zhao J, Dai YH, Xi QS, Yu SY. A clinical study on insomnia in patients with cancer during chemotherapy containing high-dose glucocorticoids. Pharmazie. 2013;68(6):421-427
53. Naber D, Sand P, Heigl B. Psychopathological and neuropsychological effects of 8-days corticosteroid treatment. A prospective study. Psychoneuroendocrinology. 1996;21(1):25-31. doi: 10.1016/0306-4530(95)00031-3
54. Brown ES, Suppes T, Khan DA, Carmody TJ 3rd. Mood changes during prednisone bursts in outpatients with asthma. J Clin Psychopharmacol. 2002;22(1):55-61.
doi: 10.1097/00004714-200202000-00009
55. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367. doi: 10.4065/81.10.1361
56. Britt RC, Devine A, Swallen KC et al. Corticosteroid use in the intensive care unit: at what cost? Arch Surg. 2006;141(2):145-159. doi:10.1001/archsurg.141.2.145
57. Kiser TH, Allen RR, Valuck RJ, Moss M, Vanivier RW. Outcomes associated with corticosteroid dosage in critically ill patients in acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):1052-1064. doi: 10.1164/rccm.201401-0058OC
58. Bourne RS, Mills GH. Sleep disruption in critically ill patients—pharmacological considerations. Anaesthesia. 2004;59(4):374-384. doi: 10.1111/j. 1365-2044.2004.03664.x
59. Flaherty JH. Insomnia among hospitalized older persons. Clin Geriatr Med. 2008;24(1):51-67. doi: 10.1016/j.cger.2007.08.012
60. Sirios F. Steroid psychosis: a review. Gen Hosp Psychiatry. 2003;25(1):27-33. doi: 10.1016/s0163-8343(02)00241-4
Sleep disturbance in the critically ill has received much attention over recent years as this is a common result of intensive care unit (ICU) admission. Disruptions in sleep not only can, at a minimum, cause distress and lower patient satisfaction, but also inhibit recovery from illness and increase morbidity.1,2 Several studies have been conducted highlighting the altered sleep patterns of critically ill patients; although total sleep time may seem normal (7-9 hours), patients can experience multiple awakenings per hour, more time in light sleep (stages 1 and 2), and less time in restorative sleep (stages 3 and 4, [REM]rapid eye movement).2-5
There are several hypothesized physiologic detriments that contribute to slower ICU recovery with sleep deprivation. Research in noncritically ill subjects suggests that sleep deprivation contributes to hypoventilation and potentially prolonged time on the ventilator.6-9 Cardiovascular morbidity may be adversely affected by inflammatory cytokine release seen in sleep disruption.10,11 Studies of noncritically ill patients also suggest that immune response is impaired, potentially protracting infection recovery.12,13 Finally, although not directly investigated, sleep deprivation may contribute to ICU delirium, an independent adverse effect (AE) associated with increased mortality and worse long-term outcomes.14-16
The Society of Critical Care Medicine (SCCM) recently updated its consensus guidelines for the management of pain, agitation/sedation, delirium, immobility, and sleep disruption (PADIS) in adult patients.17 These guidelines offer limited interventions to promote sleep in ICU patients based on available evidence and steer the clinician toward minimizing exacerbating factors. Although factors that affect sleep patterns are multifactorial, such as noise levels, pain, mechanical ventilation, and inflammatory mediators, medication therapy is a known modifiable risk factor for sleep disturbance in critically ill patients.2 This focused review will specifically evaluate the effects of steroids on sleep deprivation, psychosis, delirium, and what is known about these effects in a critically ill population.
To include articles relevant to a critically ill population, a systematic search of MEDLINE and PubMed from 1966 to 2019 was performed using the following Medical Subject Headings (MeSH) terms: delirium/etiology, psychoses, substance-induced/etiology, sleep-wake disorders/chemically induced, neurocognitive disorders/chemically induced, dyssomnias/drug effects plus glucocorticoids/adverse effects, adrenal cortex hormones/adverse effects, prednisone/adverse effects, methylprednisolone/adverse effects, and hydrocortisone/adverse effects. The initial search produced 285 articles. Case reports, reviews, letters, and articles pertaining to primary care or palliative populations were excluded, leaving 8 relevant articles for inclusion (Table 1).18-25
ICU Steroid Use
Steroids are commonly used in the ICU and affect nearly every critically ill population. Common indications for steroids in the ICU include anaphylaxis, airway edema, septic shock, asthma and COPD exacerbations, pneumocystis pneumonia, adrenal crisis, antiemetic treatment, elevated intracranial pressure from tumors, autoimmune disorders, and stress doses needed for chronic steroid users before invasive procedures.26 Whether divided into glucocorticoid or mineralocorticoid subgroups, corticosteroids offer therapeutic benefit from their pharmacologic similarity to endogenously produced cortisol, which includes anti-inflammatory, immunosuppressive, antiproliferative, and vasoconstrictive effects.
Steroid receptors are present in most human tissue, and in varying degrees of binding affinity produce a wide variety of effects. After passive diffusion across cell membranes, steroid-receptor activation binds to various DNA sites, called glucocorticoid regulatory elements, which either stimulates or inhibits transcription of multiple nearby genes.
At the cellular level, corticosteroids inhibit the release of arachidonic acid through upstream production of lipocortin peptides and antagonism of phospholipase A2. This action decreases subsequent inflammatory mediators, including kinins, histamine, liposomal enzymes, and prostaglandins. Steroids also inhibit NF-κB, which further decreases expression of proinflammatory genes while promoting interleukin-10 and its anti-inflammatory properties. Antiproliferative effects of steroids are seen by triggering cell apoptosis and inhibition of fibroblast proliferation.27,28
By binding to mineralocorticoid receptors, steroids cause sodium retention coupled with hydrogen and potassium excretion in the distal renal tubule. Steroids also promote vasoconstriction by upregulating the production and sensitivity of β receptors in the endothelium while suppressing the production of vasodilators. Although rarely used for these physiologic effects, steroids also are involved in a number of metabolic pathways, including calcium regulation, gluconeogenesis, protein metabolism, and fat distribution. Given the similar structure to cortisol, exogenous steroids depress the hypothalamic-pituitary axis (HPA) and decrease the release of adrenocorticotropic hormone (ACTH). Tapering doses of steroid regimens is often required to allow natural androgen and cortisol synthesis and prevent steroid withdrawal.27,28
The potency of various exogenous steroids closely parallels their ability to retain sodium (Table 2). Prolonged activation of steroid receptors can have numerous systemic AEs, including unwanted neurocognitive effects (Table 3). Insomnia and psychosis are commonly described in corticosteroid clinical trials, and in one meta-analysis, both are associated with high costs per episode per year.29
Steroid-Induced Sleep Disruption and Psychosis
Sleep disruption caused by exogenous administration of steroids is thought to trigger other psychostimulant effects, such as mood swings, nervousness, psychoses, and delirium.30 Similarly, the SCCM PADIS guidelines included an ungraded statement: “although an association between sleep quality and delirium occurrence exists in critically ill adults, a cause-effect relationship has not been established.”17 For this review, these AEs will be discussed as related events.
The medical literature proposes 3 pathways primarily responsible for neurocognitive AEs of steroids: behavior changes through modification of the HPA axis, changes in natural sleep-wake cycles, and hyperarousal caused by modification in neuroinhibitory pathways (Figure).
HPA Axis Modification
Under either physical or psychological stress, neural circuits in the brain release corticotropin-releasing hormone (CRH), dehydroepiandrosterone (DHEA), and arginine vasopressin, which go on to activate the sympathetic nervous system and the HPA axis. CRH from the hypothalamus goes on to stimulate ACTH release from the pituitary. ACTH then stimulates cortisol secretion from the adrenal glands. Circulating cortisol feeds into several structures of the brain, including the pituitary, hippocampus, and amygdala. Steroid-receptor complexes alter gene transcription in the central nervous system (CNS), affecting the production of neurotransmitters (eg, dopamine, serotonin) and neuropeptides (eg, somatostatin, β-endorphin). Feedback inhibition ensues, with downregulation of the HPA axis, which prevents depletion of endogenous production of steroids.31 DHEA has protective effects against excessive cortisol activity, but DHEA secretion declines with prolonged cortisol exposure. Exogenous steroids may have different effects than endogenous steroids, and neurocognitive sequelae stem from disruption and imbalance of these physiologic mechanisms.32,33
Steroid receptors are densely located in behavior centers in the brain: the amygdala, septum, and hippocampus. Pharmacologic changes in gene expression alter norepinephrine and serotonin levels in the brain as well as their receptors.32 Prolonged exposure to exogenous steroids has been shown to decrease amygdala and hippocampal volumes.34,35 Furthermore, prolonged corticosteroid exposure has been shown to decrease the number of steroid receptors in the hippocampus, pituitary gland, and amygdala.36 In a somewhat paradoxical finding, the production of CNS proinflammatory cytokines like interleuken-1β and tumor necrosis factor α has been seen after steroid administration, suggesting alternate gene signaling in the CNS.37 Although not proven conclusively, it is felt that these physiologic changes and hyperactivity of the HPA axis are predominantly responsible for changes in behavior, mood, memory, and eventually psychosis in steroid-treated patients.33,38
Finally, alterations in cognition and behavior may be related to steroid-induced changes in CNS carbohydrate, protein, and lipid metabolism with subsequent cellular neurotoxicity.32,38 Glucose uptake into the hippocampus is decreased with steroid exposure. Additionally, breakdown of metabolic compounds to produce energy can be destructive if left unchecked for prolonged periods. DHEA, growth hormone, and testosterone work to repair catabolic damage produced by cortisol, known as anabolic balance. A low anabolic balance (low DHEA levels to high cortisol levels) leads to a cascade of dysregulation in brain activity.39
Changes in Natural Sleep-Wake Cycles
Natural sleep pathways are also affected by steroids. The sleep-wake cycle is primarily regulated in the hypothalamus with circadian release of melatonin from the pineal gland. Melatonin release is highest at night, where it promotes sleep onset and continuity. Upstream, tryptophan is an amino acid that serves as a precursor to serotonin and melatonin.40 Both endogenous and exogenous corticosteroids decrease serum melatonin levels with a markedly diminished circadian rhythm secretion.41,42Demish and colleagues found a significant decrease in mean (SD) nocturnal melatonin plasma levels after the evening administration of oral dexamethasone 1 mg in 11 healthy volunteers: 127 (42) pg/mL before vs 73 (38) pg/mL after; P < .01.42 This result is likely due to decreased cellular metabolism and melatonin synthesis in the pineal gland. Of note, melatonin has neuroprotective affects, and the administration of melatonin has been shown to reverse some steroid-induced neurotoxicities in animal models.43
Steroids also reduce the uptake of tryptophan into the brain.33 Additionally, in animal models, dexamethasone administration caused a significant decrease in the gene expression of tryptophan hydroxylase, which is part of the multistep pathway in synthesizing serotonin from L-tryptophan. These effects upstream could inhibit the biosynthetic capacity of both melatonin and serotonin.44
A third pathway investigated in sleep regulation are the orexin neuropeptides. Orexins are produced in the hypothalamus and stimulate daytime wake activity in monoaminergic and cholinergic neurons. Subsequently, orexin receptor antagonists are a newer class of drugs aimed at mitigating nighttime hyperarousal and sleep disruption. Orexin overexpression may be a causal factor in steroid-induced sleep disturbance. However, this effect was specifically evaluated in a recent study in children with acute lymphoblastic leukemia, which showed that cerebral spinal fluid orexin levels (SD) were not significantly different from baseline after dexamethasone administration: 574 (26.6) pg/mL vs 580 (126.1) pg/mL; P = .8.45
Hyperarousal State
Finally, a hyperarousal state is thought to be produced by nongenomic changes to natural neuroinhibitory regulation seen with nonclassical steroid production called neurosteroids. Animal studies revealed that high levels of steroids were found in the CNS long after adrenalectomy, suggesting CNS de novo synthesis.46 In addition to altering gene expression at classic intercellular steroid receptors, neurosteroids can alter neurotransmission by direct interaction on ion-gated membranes and other receptors on the cell surface. Restlessness and insomnia could be due to γ-aminobutyric acid type A (GABAA) receptor modulation in the CNS where neuroactive steroids slow the rate of recovery of GABAA and potentially inhibit postsynaptic GABAergic transmission. It also is hypothesized that neuroactive steroids have excitatory action at nicotinic acetylcholine, 5HT3 receptors, and through increasing the fractional open time of the N-methyl-D-aspartate -activated channels.47 Allopregnanolone and DHEA are neurosteroids that act as GABAA agonists and have neuroprotective effects with anxiolytic, antidepressant, and antiaggressive properties.
Neurosteroids are synthesized from cholesterol in the hippocampus. Neurosteroids are upregulated in response to stress by CNS cortisol effects on various enzyme expressions.47 Whether exogenous steroid administration affects this biosynthesis vs the stress response in the HPA axis itself is not fully elucidated. Monteleone and colleagues found that dexamethasone 1 mg given orally significantly reduced cortisol and DHEA and allopregnanolone levels in both healthy volunteers and anorexia nervosa patients.48 Similarly, Genazzani and colleagues demonstrated that oral dexamethasone administration (0.5 mg every 6 hours) caused significant reductions in both serum allopregnanolone and DHEA levels.49
Outcomes Studies
The majority of reported data in steroid-induced insomnia and psychosis is in noncritically ill populations. In a randomized, prospective crossover study of healthy volunteers, dexamethasone administration (3 mg every 8 hours for 48 hours) resulted in significant changes in sleep patterns measured with polysomnography. Compared with placebo, steroid treatment showed significantly longer percentage (SD) of stage 0/awake times (11.7% [11.4] vs 2.9% [1.8]; P < .05); longer percentage (SD) of REM sleep latency (363.8 [74.5] minutes vs 202.8 [79.6] minutes; P < .01), and a reduced number (SD) of REM periods (3.8 [2.6] vs 9.7 [3.6]; P < .01).50 Insomnia was one of the most commonly self-reported AEs (> 60%) in a survey of 2,446 chronic steroid users, and the incidence increased as steroid doses increased.51
A prospective, open-label study of 240 patients with cancer demonstrated significant sleep disruptions using the Pittsburgh Sleep Quality Index with the use of high-dose steroids in chemotherapy.52 Naber and colleagues evaluated 50 previously healthy patients taking methylprednisolone 119 mg (41 mg/d) for retinitis and uveitis.53 They reported 26% to 34% of subjects experienced hypomanic syndrome based on a semistructured interview examination. Symptoms developed within 3 days and persisted for the 8-day course of therapy. Brown and colleagues prospectively evaluated 32 asthmatic patients prescribed bursts of prednisone > 40 mg daily. They observed significantly increased scores in the Young Mania Rating Scale within 3 to 7 days of starting therapy, which dissipated to baseline after stopping therapy.54
Despite a high reported incidence of neurologic AEs, outcomes in critically ill populations are mixed. Study methods are varied, and many were largely observational. No prospective, randomized studies exist to date specifically aimed and powered to evaluate the effects of steroids on sleep disturbances or delirium in a critically ill population. Furthermore, sleep quality is difficult to measure in this population, and self-reporting often is not an option. In critical care trials, if AEs such as insomnia, delirium, or psychosis are recorded at all, there is heterogeneity in the definitions, and these AEs are generally poorly defined (eg, psychiatric or neurologic disorder not otherwise specified), making pooled analysis of this outcome difficult.55
One of the largest observational studies in hospitalized patients was through the Boston Collaborative Drug Surveillance Program. A total of 718 consecutively enrolled inpatients who received prednisone were monitored for acute reactions. Psychiatric AEs were rare (1.3%) with low doses (< 40 mg/d), more prevalent (4.6%) with higher doses (41-80 mg/d), and most prevalent (18.4%) with the highest doses (> 80 mg/d), suggesting CNS AEs are dose dependent.18 A single-center, retrospective review of 755 psychiatric consults in hospitalized patients revealed that 54% of manic patients were due to corticosteroid administration.19 In a prospective observational study of 206 consecutive ICU admissions, steroid administration was an independent risk factor for development of ICU delirium, using the Confusion Assessment Method-ICU (CAM-ICU) at a single center (odds ratio [OR], 2.8; 95% CI, 1.05-7.28).25
Two studies in hospitalized oncology patients found conflicting results using the Nursing Delirium Screening Scale (Nu-DESC). One did not find a significant association between delirium and dexamethasone equivalent doses > 15 mg, while the second found an increased hazard ratio (HR) for a positive Nu-DESC score (HR, 2.67; 95% CI, 1.18-6.03).20,21 Similarly, conflicting results were found in 2 studies using first-order Markov models. In one prospective cohort study, 520 consecutive mechanically ventilated patients in 13 ICUs were monitored for the transition to delirium (CAM-ICU positive) from nondelirium states. Steroid administration was significantly associated with transitioning to delirium (OR, 1.52; 95% CI, 1.05-2.21).22 This conflicts with a similar study by Wolters and colleagues, which monitored 1,112 ICU patients who were given a median prednisone equivalent of 50 mg (interquartile range, 25-75 mg). Steroid administration was not significantly associated with the transition to delirium from an awake without delirium state (OR, 1.08; 95% CI, 0.89-1.32; adjusted OR, 1.00; 95% CI, 0.99-1.01 per 10-mg increase in prednisone equivalent).23
Mitigating Effects
Although steroid therapy often cannot be altered in the critically ill population, research showed that steroid overuse is common in ICUs.56,57 Minimizing dosage and duration are important ways clinicians can mitigate unwanted effects. CNS AEs seen with steroids often can be reversed once therapy is discontinued. Avoiding split-dose administration has been proposed given the natural diurnal production of cortisol.58 A review by Flaherty discusses the importance of avoiding pharmacologic agents in hospitalized older patients if possible due to known risks (falls, dependency, hip fractures, rebound insomnia, and risk of delirium) and provides a HELP ME SLEEP nomogram for nonpharmacologic interventions in hospitalized patients (Table 4).59
Historically, lithium has been recommended for steroid-induced mania with chronic steroid use; however, given the large volume and electrolyte shifts seen in critically ill patients, this may not be a viable option. Antidepressants, especially tricyclics, should generally be avoided in steroid-induced psychosis as these may exacerbate symptoms. If symptoms are severe, either typical (haloperidol) or atypical (olanzapine, quetiapine, risperidone) antipsychotics have been used with success.60 Given the known depletion of serum melatonin levels, melatonin supplements are an attractive and relatively safe option for steroid-induced insomnia; however, there are no robust studies specifically aimed at this intervention for this population.
Conclusions
With known, multimodal foci driving sleep impairment in ICU patients, PADIS guidelines recommend myriad interventions for improvement. Recommendations include noise and light reduction with earplugs and/or eyeshades to improve sleep quality. Nocturnal assist-control ventilation may improve sleep quality in ventilated patients. Finally, the development of institutional protocols for promoting sleep quality in ICU patients is recommended.17
Sleep disturbance in the critically ill has received much attention over recent years as this is a common result of intensive care unit (ICU) admission. Disruptions in sleep not only can, at a minimum, cause distress and lower patient satisfaction, but also inhibit recovery from illness and increase morbidity.1,2 Several studies have been conducted highlighting the altered sleep patterns of critically ill patients; although total sleep time may seem normal (7-9 hours), patients can experience multiple awakenings per hour, more time in light sleep (stages 1 and 2), and less time in restorative sleep (stages 3 and 4, [REM]rapid eye movement).2-5
There are several hypothesized physiologic detriments that contribute to slower ICU recovery with sleep deprivation. Research in noncritically ill subjects suggests that sleep deprivation contributes to hypoventilation and potentially prolonged time on the ventilator.6-9 Cardiovascular morbidity may be adversely affected by inflammatory cytokine release seen in sleep disruption.10,11 Studies of noncritically ill patients also suggest that immune response is impaired, potentially protracting infection recovery.12,13 Finally, although not directly investigated, sleep deprivation may contribute to ICU delirium, an independent adverse effect (AE) associated with increased mortality and worse long-term outcomes.14-16
The Society of Critical Care Medicine (SCCM) recently updated its consensus guidelines for the management of pain, agitation/sedation, delirium, immobility, and sleep disruption (PADIS) in adult patients.17 These guidelines offer limited interventions to promote sleep in ICU patients based on available evidence and steer the clinician toward minimizing exacerbating factors. Although factors that affect sleep patterns are multifactorial, such as noise levels, pain, mechanical ventilation, and inflammatory mediators, medication therapy is a known modifiable risk factor for sleep disturbance in critically ill patients.2 This focused review will specifically evaluate the effects of steroids on sleep deprivation, psychosis, delirium, and what is known about these effects in a critically ill population.
To include articles relevant to a critically ill population, a systematic search of MEDLINE and PubMed from 1966 to 2019 was performed using the following Medical Subject Headings (MeSH) terms: delirium/etiology, psychoses, substance-induced/etiology, sleep-wake disorders/chemically induced, neurocognitive disorders/chemically induced, dyssomnias/drug effects plus glucocorticoids/adverse effects, adrenal cortex hormones/adverse effects, prednisone/adverse effects, methylprednisolone/adverse effects, and hydrocortisone/adverse effects. The initial search produced 285 articles. Case reports, reviews, letters, and articles pertaining to primary care or palliative populations were excluded, leaving 8 relevant articles for inclusion (Table 1).18-25
ICU Steroid Use
Steroids are commonly used in the ICU and affect nearly every critically ill population. Common indications for steroids in the ICU include anaphylaxis, airway edema, septic shock, asthma and COPD exacerbations, pneumocystis pneumonia, adrenal crisis, antiemetic treatment, elevated intracranial pressure from tumors, autoimmune disorders, and stress doses needed for chronic steroid users before invasive procedures.26 Whether divided into glucocorticoid or mineralocorticoid subgroups, corticosteroids offer therapeutic benefit from their pharmacologic similarity to endogenously produced cortisol, which includes anti-inflammatory, immunosuppressive, antiproliferative, and vasoconstrictive effects.
Steroid receptors are present in most human tissue, and in varying degrees of binding affinity produce a wide variety of effects. After passive diffusion across cell membranes, steroid-receptor activation binds to various DNA sites, called glucocorticoid regulatory elements, which either stimulates or inhibits transcription of multiple nearby genes.
At the cellular level, corticosteroids inhibit the release of arachidonic acid through upstream production of lipocortin peptides and antagonism of phospholipase A2. This action decreases subsequent inflammatory mediators, including kinins, histamine, liposomal enzymes, and prostaglandins. Steroids also inhibit NF-κB, which further decreases expression of proinflammatory genes while promoting interleukin-10 and its anti-inflammatory properties. Antiproliferative effects of steroids are seen by triggering cell apoptosis and inhibition of fibroblast proliferation.27,28
By binding to mineralocorticoid receptors, steroids cause sodium retention coupled with hydrogen and potassium excretion in the distal renal tubule. Steroids also promote vasoconstriction by upregulating the production and sensitivity of β receptors in the endothelium while suppressing the production of vasodilators. Although rarely used for these physiologic effects, steroids also are involved in a number of metabolic pathways, including calcium regulation, gluconeogenesis, protein metabolism, and fat distribution. Given the similar structure to cortisol, exogenous steroids depress the hypothalamic-pituitary axis (HPA) and decrease the release of adrenocorticotropic hormone (ACTH). Tapering doses of steroid regimens is often required to allow natural androgen and cortisol synthesis and prevent steroid withdrawal.27,28
The potency of various exogenous steroids closely parallels their ability to retain sodium (Table 2). Prolonged activation of steroid receptors can have numerous systemic AEs, including unwanted neurocognitive effects (Table 3). Insomnia and psychosis are commonly described in corticosteroid clinical trials, and in one meta-analysis, both are associated with high costs per episode per year.29
Steroid-Induced Sleep Disruption and Psychosis
Sleep disruption caused by exogenous administration of steroids is thought to trigger other psychostimulant effects, such as mood swings, nervousness, psychoses, and delirium.30 Similarly, the SCCM PADIS guidelines included an ungraded statement: “although an association between sleep quality and delirium occurrence exists in critically ill adults, a cause-effect relationship has not been established.”17 For this review, these AEs will be discussed as related events.
The medical literature proposes 3 pathways primarily responsible for neurocognitive AEs of steroids: behavior changes through modification of the HPA axis, changes in natural sleep-wake cycles, and hyperarousal caused by modification in neuroinhibitory pathways (Figure).
HPA Axis Modification
Under either physical or psychological stress, neural circuits in the brain release corticotropin-releasing hormone (CRH), dehydroepiandrosterone (DHEA), and arginine vasopressin, which go on to activate the sympathetic nervous system and the HPA axis. CRH from the hypothalamus goes on to stimulate ACTH release from the pituitary. ACTH then stimulates cortisol secretion from the adrenal glands. Circulating cortisol feeds into several structures of the brain, including the pituitary, hippocampus, and amygdala. Steroid-receptor complexes alter gene transcription in the central nervous system (CNS), affecting the production of neurotransmitters (eg, dopamine, serotonin) and neuropeptides (eg, somatostatin, β-endorphin). Feedback inhibition ensues, with downregulation of the HPA axis, which prevents depletion of endogenous production of steroids.31 DHEA has protective effects against excessive cortisol activity, but DHEA secretion declines with prolonged cortisol exposure. Exogenous steroids may have different effects than endogenous steroids, and neurocognitive sequelae stem from disruption and imbalance of these physiologic mechanisms.32,33
Steroid receptors are densely located in behavior centers in the brain: the amygdala, septum, and hippocampus. Pharmacologic changes in gene expression alter norepinephrine and serotonin levels in the brain as well as their receptors.32 Prolonged exposure to exogenous steroids has been shown to decrease amygdala and hippocampal volumes.34,35 Furthermore, prolonged corticosteroid exposure has been shown to decrease the number of steroid receptors in the hippocampus, pituitary gland, and amygdala.36 In a somewhat paradoxical finding, the production of CNS proinflammatory cytokines like interleuken-1β and tumor necrosis factor α has been seen after steroid administration, suggesting alternate gene signaling in the CNS.37 Although not proven conclusively, it is felt that these physiologic changes and hyperactivity of the HPA axis are predominantly responsible for changes in behavior, mood, memory, and eventually psychosis in steroid-treated patients.33,38
Finally, alterations in cognition and behavior may be related to steroid-induced changes in CNS carbohydrate, protein, and lipid metabolism with subsequent cellular neurotoxicity.32,38 Glucose uptake into the hippocampus is decreased with steroid exposure. Additionally, breakdown of metabolic compounds to produce energy can be destructive if left unchecked for prolonged periods. DHEA, growth hormone, and testosterone work to repair catabolic damage produced by cortisol, known as anabolic balance. A low anabolic balance (low DHEA levels to high cortisol levels) leads to a cascade of dysregulation in brain activity.39
Changes in Natural Sleep-Wake Cycles
Natural sleep pathways are also affected by steroids. The sleep-wake cycle is primarily regulated in the hypothalamus with circadian release of melatonin from the pineal gland. Melatonin release is highest at night, where it promotes sleep onset and continuity. Upstream, tryptophan is an amino acid that serves as a precursor to serotonin and melatonin.40 Both endogenous and exogenous corticosteroids decrease serum melatonin levels with a markedly diminished circadian rhythm secretion.41,42Demish and colleagues found a significant decrease in mean (SD) nocturnal melatonin plasma levels after the evening administration of oral dexamethasone 1 mg in 11 healthy volunteers: 127 (42) pg/mL before vs 73 (38) pg/mL after; P < .01.42 This result is likely due to decreased cellular metabolism and melatonin synthesis in the pineal gland. Of note, melatonin has neuroprotective affects, and the administration of melatonin has been shown to reverse some steroid-induced neurotoxicities in animal models.43
Steroids also reduce the uptake of tryptophan into the brain.33 Additionally, in animal models, dexamethasone administration caused a significant decrease in the gene expression of tryptophan hydroxylase, which is part of the multistep pathway in synthesizing serotonin from L-tryptophan. These effects upstream could inhibit the biosynthetic capacity of both melatonin and serotonin.44
A third pathway investigated in sleep regulation are the orexin neuropeptides. Orexins are produced in the hypothalamus and stimulate daytime wake activity in monoaminergic and cholinergic neurons. Subsequently, orexin receptor antagonists are a newer class of drugs aimed at mitigating nighttime hyperarousal and sleep disruption. Orexin overexpression may be a causal factor in steroid-induced sleep disturbance. However, this effect was specifically evaluated in a recent study in children with acute lymphoblastic leukemia, which showed that cerebral spinal fluid orexin levels (SD) were not significantly different from baseline after dexamethasone administration: 574 (26.6) pg/mL vs 580 (126.1) pg/mL; P = .8.45
Hyperarousal State
Finally, a hyperarousal state is thought to be produced by nongenomic changes to natural neuroinhibitory regulation seen with nonclassical steroid production called neurosteroids. Animal studies revealed that high levels of steroids were found in the CNS long after adrenalectomy, suggesting CNS de novo synthesis.46 In addition to altering gene expression at classic intercellular steroid receptors, neurosteroids can alter neurotransmission by direct interaction on ion-gated membranes and other receptors on the cell surface. Restlessness and insomnia could be due to γ-aminobutyric acid type A (GABAA) receptor modulation in the CNS where neuroactive steroids slow the rate of recovery of GABAA and potentially inhibit postsynaptic GABAergic transmission. It also is hypothesized that neuroactive steroids have excitatory action at nicotinic acetylcholine, 5HT3 receptors, and through increasing the fractional open time of the N-methyl-D-aspartate -activated channels.47 Allopregnanolone and DHEA are neurosteroids that act as GABAA agonists and have neuroprotective effects with anxiolytic, antidepressant, and antiaggressive properties.
Neurosteroids are synthesized from cholesterol in the hippocampus. Neurosteroids are upregulated in response to stress by CNS cortisol effects on various enzyme expressions.47 Whether exogenous steroid administration affects this biosynthesis vs the stress response in the HPA axis itself is not fully elucidated. Monteleone and colleagues found that dexamethasone 1 mg given orally significantly reduced cortisol and DHEA and allopregnanolone levels in both healthy volunteers and anorexia nervosa patients.48 Similarly, Genazzani and colleagues demonstrated that oral dexamethasone administration (0.5 mg every 6 hours) caused significant reductions in both serum allopregnanolone and DHEA levels.49
Outcomes Studies
The majority of reported data in steroid-induced insomnia and psychosis is in noncritically ill populations. In a randomized, prospective crossover study of healthy volunteers, dexamethasone administration (3 mg every 8 hours for 48 hours) resulted in significant changes in sleep patterns measured with polysomnography. Compared with placebo, steroid treatment showed significantly longer percentage (SD) of stage 0/awake times (11.7% [11.4] vs 2.9% [1.8]; P < .05); longer percentage (SD) of REM sleep latency (363.8 [74.5] minutes vs 202.8 [79.6] minutes; P < .01), and a reduced number (SD) of REM periods (3.8 [2.6] vs 9.7 [3.6]; P < .01).50 Insomnia was one of the most commonly self-reported AEs (> 60%) in a survey of 2,446 chronic steroid users, and the incidence increased as steroid doses increased.51
A prospective, open-label study of 240 patients with cancer demonstrated significant sleep disruptions using the Pittsburgh Sleep Quality Index with the use of high-dose steroids in chemotherapy.52 Naber and colleagues evaluated 50 previously healthy patients taking methylprednisolone 119 mg (41 mg/d) for retinitis and uveitis.53 They reported 26% to 34% of subjects experienced hypomanic syndrome based on a semistructured interview examination. Symptoms developed within 3 days and persisted for the 8-day course of therapy. Brown and colleagues prospectively evaluated 32 asthmatic patients prescribed bursts of prednisone > 40 mg daily. They observed significantly increased scores in the Young Mania Rating Scale within 3 to 7 days of starting therapy, which dissipated to baseline after stopping therapy.54
Despite a high reported incidence of neurologic AEs, outcomes in critically ill populations are mixed. Study methods are varied, and many were largely observational. No prospective, randomized studies exist to date specifically aimed and powered to evaluate the effects of steroids on sleep disturbances or delirium in a critically ill population. Furthermore, sleep quality is difficult to measure in this population, and self-reporting often is not an option. In critical care trials, if AEs such as insomnia, delirium, or psychosis are recorded at all, there is heterogeneity in the definitions, and these AEs are generally poorly defined (eg, psychiatric or neurologic disorder not otherwise specified), making pooled analysis of this outcome difficult.55
One of the largest observational studies in hospitalized patients was through the Boston Collaborative Drug Surveillance Program. A total of 718 consecutively enrolled inpatients who received prednisone were monitored for acute reactions. Psychiatric AEs were rare (1.3%) with low doses (< 40 mg/d), more prevalent (4.6%) with higher doses (41-80 mg/d), and most prevalent (18.4%) with the highest doses (> 80 mg/d), suggesting CNS AEs are dose dependent.18 A single-center, retrospective review of 755 psychiatric consults in hospitalized patients revealed that 54% of manic patients were due to corticosteroid administration.19 In a prospective observational study of 206 consecutive ICU admissions, steroid administration was an independent risk factor for development of ICU delirium, using the Confusion Assessment Method-ICU (CAM-ICU) at a single center (odds ratio [OR], 2.8; 95% CI, 1.05-7.28).25
Two studies in hospitalized oncology patients found conflicting results using the Nursing Delirium Screening Scale (Nu-DESC). One did not find a significant association between delirium and dexamethasone equivalent doses > 15 mg, while the second found an increased hazard ratio (HR) for a positive Nu-DESC score (HR, 2.67; 95% CI, 1.18-6.03).20,21 Similarly, conflicting results were found in 2 studies using first-order Markov models. In one prospective cohort study, 520 consecutive mechanically ventilated patients in 13 ICUs were monitored for the transition to delirium (CAM-ICU positive) from nondelirium states. Steroid administration was significantly associated with transitioning to delirium (OR, 1.52; 95% CI, 1.05-2.21).22 This conflicts with a similar study by Wolters and colleagues, which monitored 1,112 ICU patients who were given a median prednisone equivalent of 50 mg (interquartile range, 25-75 mg). Steroid administration was not significantly associated with the transition to delirium from an awake without delirium state (OR, 1.08; 95% CI, 0.89-1.32; adjusted OR, 1.00; 95% CI, 0.99-1.01 per 10-mg increase in prednisone equivalent).23
Mitigating Effects
Although steroid therapy often cannot be altered in the critically ill population, research showed that steroid overuse is common in ICUs.56,57 Minimizing dosage and duration are important ways clinicians can mitigate unwanted effects. CNS AEs seen with steroids often can be reversed once therapy is discontinued. Avoiding split-dose administration has been proposed given the natural diurnal production of cortisol.58 A review by Flaherty discusses the importance of avoiding pharmacologic agents in hospitalized older patients if possible due to known risks (falls, dependency, hip fractures, rebound insomnia, and risk of delirium) and provides a HELP ME SLEEP nomogram for nonpharmacologic interventions in hospitalized patients (Table 4).59
Historically, lithium has been recommended for steroid-induced mania with chronic steroid use; however, given the large volume and electrolyte shifts seen in critically ill patients, this may not be a viable option. Antidepressants, especially tricyclics, should generally be avoided in steroid-induced psychosis as these may exacerbate symptoms. If symptoms are severe, either typical (haloperidol) or atypical (olanzapine, quetiapine, risperidone) antipsychotics have been used with success.60 Given the known depletion of serum melatonin levels, melatonin supplements are an attractive and relatively safe option for steroid-induced insomnia; however, there are no robust studies specifically aimed at this intervention for this population.
Conclusions
With known, multimodal foci driving sleep impairment in ICU patients, PADIS guidelines recommend myriad interventions for improvement. Recommendations include noise and light reduction with earplugs and/or eyeshades to improve sleep quality. Nocturnal assist-control ventilation may improve sleep quality in ventilated patients. Finally, the development of institutional protocols for promoting sleep quality in ICU patients is recommended.17
1. Simini B. Patients’ perceptions of intensive care. Lancet. 1999;354(9178):571-572. doi: 10.1016/S0140-6736(99)02728-2
2. Delaney LJ, Van Haren F, Lopez V. Sleeping on a problem: the impact of sleep disturbance on intensive care patients—a clinical review. Ann Intensive Care. 2015;15:3. doi: 10.1186/s13613-015-0043-2
3. Friese RS, Diaz-Arrastia R, McBride D, Frankel H, Gentilello LM. Quality and quantity of sleep in the surgical intensive care unit; are our patients sleeping? J Trauma. 2007;63(6):1210-1214. doi: 10.1097/TA.0b013e31815b83d7
4. Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care 2013;17(2):R46.
5. Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in patients receiving postoperative care. BJM (Clin Res Ed). 1985;290(6474)1029-1032. doi: 10.1136/bmj.290.6474.1029
6. White DP, Douglas NJ, Pickett CK, Zwillich CW, Weil JV. Sleep deprivation and the control of ventilation. Am Rev Respir Dis. 1983;128(6):984-986. doi: 10.1164/arrd.1983.128.6.984
7. Series F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150(2):481-485. doi: 10.1164/ajrccm.150.2.8049833
8. Tadjalli A, Peever J. Sleep loss reduces respiratory motor plasticity. Adv Exp Med Biol. 2010;669:289-292.
doi: 10.1007/978-1-4419-5692-7_59
9. Roche Campo F, Drouot X, Thille AW, et al. Poor sleep quality is associated with late noninvasive ventilation failure in patients with acute hypercapnic respiratory failure. Crit Care Med. 2010;38(2):447-485. doi: 10.1097/CCM.0b013e3181bc8243
10. Sauvet F, Leftheriotis G, Gomez-Merino D, et al. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol (1985). 2010;108(1):68-75. doi: 10.1152/japplphysiol.00851.2009
11. Frey DJ, Fleshner M, Wright KP Jr. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21(8):1050-1057. doi: 10.1016/j.bbi.2007.04.003
12. Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA 2002;288(12):1471-1472. doi: 10.1001/jama.288.12.1471-a
13. Dinges DF, Douglas SD, Zuagg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93(5):1930-1939. doi: 10.1172/JCI117184
14. Weinhouse GL, Schwab RJ, Watson PL, et al. Bench-to-bedside review: delirium in ICU patients— importance of sleep deprivation. Crit Care. 2009;13(6):234. doi: 10.1186/cc8131
15. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. doi: 10.1001/jama.291.14.1753
16. Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513-1520. doi: 10.1097/CCM.0b013e3181e47be1
17. Devlin JW, Skrobik Y, Gelinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873
18. The Boston Collaborative Drug Surveillance Program. Acute adverse reactions to prednisone in relation to dosage. Clin Pharmacol Ther. 1972;13(5):694-698. doi: 10.1002/cpt1972135part1694
19. Rundell JR, Wise MG. Causes of organic mood disorder. J Neuropsychiatry Clin Neurosci. 1989;1(4):398-400. doi: 10.1176/jnp.1.4.398
20. Gaudreau JD, Gagnon P, Harel F, Roy MA, Tremblay A. Psychoactive medications and risk of delirium in hospitalized cancer patients. J Clin Oncol. 2005;23(27):6712-6718. doi: 10.1200/JCO.2005.05.140
21. Gaudreau JD, Gagnon P, Roy MA, Harel F, Tremblay A. Opioid medications and longitudinal risk of delirium in hospitalized cancer patients. Cancer. 2007;109(11):2365-2373.
doi: 10.1002/cncr.22665
22. Schreiber MP, Colantuoni E, Bienvenu OJ, et al. Corticosteroids and transition to delirium in patients with acute lung injury. Crit Care Med. 2014;42(6):1480-1486. doi: 10.1097/CCM.0000000000000247
23. Wolters AE, Veldhuijzen DS, Zaal IJ, et al. Systemic corticosteroids and transition to delirium in critically ill patients. Crit Care Med. 2015;43(12):e585-e588. doi: 10.1097/CCM.0000000000001302
24. Matschke J, Muller-Beissenhirtz H, Novotny J, et al. A randomized trial of daily prednisone versus pulsed dexamethasone in treatment-naïve adult patients with immune thrombocytopenia: EIS 2002 study. Acta Haematol. 2016;136(2):101-107. doi: 10.1159/000445420
25. Tilouche N, Hassen M, Ali HBS, Jaoued AHO, Gharbi R, Atrous SS. Delirium in the intensive care unit: incidence, risk factors, and impact on outcome. Indian J Crit Care Med. 2018;22:144-149. doi: 10.4103/ijccm.IJCCM_244_17
26. Young A, Marsh S. Steroid use in critical care. BJA Education. 2018;18(5):129-134. doi: 10.1016/j.bjae.2018.01.005
27. DiPiro J, Talbert R, Yee G, Matzke GR, Wells BG, Posey M. Pharmacotherapy: A Pathophysiologic Approach. 4th ed. New York: McGraw-Hill; 1999:1277-1278.
28. Schimmer
29. Sarnes E, Crofford L, Watson M, Dennis G, Kan H, Bass D. Incidence of US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther. 2011;33(10):1413-1432.
30. Idzikowsi C, Shapiro CM. ABC of sleep disorders, non-psychotropic drugs and sleep. BMJ. 1993;306(6885):1118-1120. doi: 10.1136/bmj.306.6885.1118

31. Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14(4):398-406.
doi: 10.3109/10253890.2011.586446
32. Wolkowitz OM, Reus VI, Weingartner H, et al. Cognitive effects of corticosteroids. Am J Psychiatry 1990;147(10):1297-1303. doi: 10.1176/ajp.147.10.1297
33. McEwen BS, Davis PG, Parsons B, Pfaff DW. The brain as a target for steroid hormone action. Ann Rev Neurosci. 1979;2:65-112. doi: 10.1146/annurev.ne.02.030179.000433
34. Brown ES, Woolston DJ, Frol AM. Amygdala volume in patients receiving chronic corticosteroid therapy. Biol Psychiatry. 2008;63(7):705-709.
doi: 10.1016/j.biopsych.2007.09.014
35. Brown ES, Woolston D, Frol A, et al. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid. Biol Psychiatry. 2004;55(5):538-545.
36. Sapolsky RM, McEwen BS. Down-regulation of neural corticosterone receptors by corticosterone and dexamethasone. Brain Res. 1985;339(1):161-165.
doi: 10.1016/0006-8993(85)90638-9
37. Sorrells SF, Caso JR, Munhoz CD, Spolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64(1):33-39.
doi: 10.1016/j.neuron.2009.09.032
38. Wolkowitz OM, Burke H, Epel ES, Reus VI. Glucocorticoids: mood, memory, and mechanisms. Ann NY Acad Sci. 2009;1179:19-40. doi: 10.1111/j.1749-6632.2009.04980.x
39. Wolkowitz OM, Epel ES, Reus VI. Stress hormone-related psychopathology: pathophysiological and treatment implications. World J Biol Psychiatry. 2001;2(3):115-143. doi: 10.3109/15622970109026799
40. Paredes S, Barriga C, Reiter R, Rodrigues A. Assessment of the potential role of tryptophan as the precursor of serotonin and melatonin for the aged sleep-wake cycle and immune function: Streptopelia Risoria as a model. Int J Tryptophan Res. 2009;2:23-36. doi: 10.4137/ijtr.s1129
41. Soszyński P, Stowińska-Srzednicka J, Kasperlik-Zatuska A, Zgliczyński S. Decreased melatonin concentration in Cushing’s Syndrome. Horm Metab Res. 1989;21(12):673-674. doi: 10.1055/s-2007-1009317
42. Demish L, Demish K, Neckelsen T. Influence of dexamethasone on nocturnal melatonin production in healthy adult subjects. J Pineal Res. 1988;5(3):317-321. doi: 10.1111/j.1600-079x.1988.tb00657.x
43. Assaf N, Shalby AB, Khalil WK, Ahmed HH. Biochemical and genetic alterations of oxidant/antioxidant status of the brain in rats treated with dexamethasone: protective roles of melatonin and acetyl-L-carnitine. J Physiol Biochem. 2012;68(1):77-90. doi: 10.1007/s13105-011-0121-3
44. Clark MS, Russo AF. Tissue-specific glucocorticoid regulation of tryptophan hydroxylase mRNA levels. Brain Res Mol Brain Res. 1997;48(2):346-54. doi: 10.1016/s0169-328x(97)00106-x
45. Kram DE, Krasnow SM, Levasseur PR, Zhu X, Stork LC, Marks DL. Dexamethasone chemotherapy does not disrupt orexin signaling. PLoS One. 2016;11(12):e0168731. doi: 10.1371/journal.pone.0168731
46. Mellon S. Neurosteroids: biochemistry, modes of action, and clinical relevance. J Clin Endocrinol Metab. 1994;78(5):1003-1008. doi: 10.1210/jcem.78.5.8175951
47. Zorumski C, Paul SM, Izumi Y, Covey DF, Mennerick S . Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci Biobehav Rev. 2013;37(1):109-122. doi: 10.1016/j.neubiorev.2012.10.005
48. Monteleone P, Luisi M, Martiadis V, et al. Impaired reduction of enhanced levels of dehydroepiandrosterone by oral dexamethasone in anorexia nervosa. Psychoneuroendocrinology. 2006;31(4):537-542. doi: 10.1016/j.psyneuen.2005.08.015
49. Genazzani AR, Petraglia F, Bernardi F, et al. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998;83(6):2099-3103. doi: 10.1210/jcem.83.6.4905
50. Moser NJ, Phillips BA, Guthrie G, Barnett G. Effects of dexamethasone on sleep. Pharmacol Toxicol. 1996;79(2):100-102. doi: 10.1111/j.1600-0773.1996.tb00249.x
51. Curtis J, Westfall A, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55(3):420-426. doi: 10.1002/art.21984
52. Zhao J, Dai YH, Xi QS, Yu SY. A clinical study on insomnia in patients with cancer during chemotherapy containing high-dose glucocorticoids. Pharmazie. 2013;68(6):421-427
53. Naber D, Sand P, Heigl B. Psychopathological and neuropsychological effects of 8-days corticosteroid treatment. A prospective study. Psychoneuroendocrinology. 1996;21(1):25-31. doi: 10.1016/0306-4530(95)00031-3
54. Brown ES, Suppes T, Khan DA, Carmody TJ 3rd. Mood changes during prednisone bursts in outpatients with asthma. J Clin Psychopharmacol. 2002;22(1):55-61.
doi: 10.1097/00004714-200202000-00009
55. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367. doi: 10.4065/81.10.1361
56. Britt RC, Devine A, Swallen KC et al. Corticosteroid use in the intensive care unit: at what cost? Arch Surg. 2006;141(2):145-159. doi:10.1001/archsurg.141.2.145
57. Kiser TH, Allen RR, Valuck RJ, Moss M, Vanivier RW. Outcomes associated with corticosteroid dosage in critically ill patients in acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):1052-1064. doi: 10.1164/rccm.201401-0058OC
58. Bourne RS, Mills GH. Sleep disruption in critically ill patients—pharmacological considerations. Anaesthesia. 2004;59(4):374-384. doi: 10.1111/j. 1365-2044.2004.03664.x
59. Flaherty JH. Insomnia among hospitalized older persons. Clin Geriatr Med. 2008;24(1):51-67. doi: 10.1016/j.cger.2007.08.012
60. Sirios F. Steroid psychosis: a review. Gen Hosp Psychiatry. 2003;25(1):27-33. doi: 10.1016/s0163-8343(02)00241-4
1. Simini B. Patients’ perceptions of intensive care. Lancet. 1999;354(9178):571-572. doi: 10.1016/S0140-6736(99)02728-2
2. Delaney LJ, Van Haren F, Lopez V. Sleeping on a problem: the impact of sleep disturbance on intensive care patients—a clinical review. Ann Intensive Care. 2015;15:3. doi: 10.1186/s13613-015-0043-2
3. Friese RS, Diaz-Arrastia R, McBride D, Frankel H, Gentilello LM. Quality and quantity of sleep in the surgical intensive care unit; are our patients sleeping? J Trauma. 2007;63(6):1210-1214. doi: 10.1097/TA.0b013e31815b83d7
4. Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care 2013;17(2):R46.
5. Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in patients receiving postoperative care. BJM (Clin Res Ed). 1985;290(6474)1029-1032. doi: 10.1136/bmj.290.6474.1029
6. White DP, Douglas NJ, Pickett CK, Zwillich CW, Weil JV. Sleep deprivation and the control of ventilation. Am Rev Respir Dis. 1983;128(6):984-986. doi: 10.1164/arrd.1983.128.6.984
7. Series F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150(2):481-485. doi: 10.1164/ajrccm.150.2.8049833
8. Tadjalli A, Peever J. Sleep loss reduces respiratory motor plasticity. Adv Exp Med Biol. 2010;669:289-292.
doi: 10.1007/978-1-4419-5692-7_59
9. Roche Campo F, Drouot X, Thille AW, et al. Poor sleep quality is associated with late noninvasive ventilation failure in patients with acute hypercapnic respiratory failure. Crit Care Med. 2010;38(2):447-485. doi: 10.1097/CCM.0b013e3181bc8243
10. Sauvet F, Leftheriotis G, Gomez-Merino D, et al. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol (1985). 2010;108(1):68-75. doi: 10.1152/japplphysiol.00851.2009
11. Frey DJ, Fleshner M, Wright KP Jr. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21(8):1050-1057. doi: 10.1016/j.bbi.2007.04.003
12. Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA 2002;288(12):1471-1472. doi: 10.1001/jama.288.12.1471-a
13. Dinges DF, Douglas SD, Zuagg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93(5):1930-1939. doi: 10.1172/JCI117184
14. Weinhouse GL, Schwab RJ, Watson PL, et al. Bench-to-bedside review: delirium in ICU patients— importance of sleep deprivation. Crit Care. 2009;13(6):234. doi: 10.1186/cc8131
15. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. doi: 10.1001/jama.291.14.1753
16. Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513-1520. doi: 10.1097/CCM.0b013e3181e47be1
17. Devlin JW, Skrobik Y, Gelinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873
18. The Boston Collaborative Drug Surveillance Program. Acute adverse reactions to prednisone in relation to dosage. Clin Pharmacol Ther. 1972;13(5):694-698. doi: 10.1002/cpt1972135part1694
19. Rundell JR, Wise MG. Causes of organic mood disorder. J Neuropsychiatry Clin Neurosci. 1989;1(4):398-400. doi: 10.1176/jnp.1.4.398
20. Gaudreau JD, Gagnon P, Harel F, Roy MA, Tremblay A. Psychoactive medications and risk of delirium in hospitalized cancer patients. J Clin Oncol. 2005;23(27):6712-6718. doi: 10.1200/JCO.2005.05.140
21. Gaudreau JD, Gagnon P, Roy MA, Harel F, Tremblay A. Opioid medications and longitudinal risk of delirium in hospitalized cancer patients. Cancer. 2007;109(11):2365-2373.
doi: 10.1002/cncr.22665
22. Schreiber MP, Colantuoni E, Bienvenu OJ, et al. Corticosteroids and transition to delirium in patients with acute lung injury. Crit Care Med. 2014;42(6):1480-1486. doi: 10.1097/CCM.0000000000000247
23. Wolters AE, Veldhuijzen DS, Zaal IJ, et al. Systemic corticosteroids and transition to delirium in critically ill patients. Crit Care Med. 2015;43(12):e585-e588. doi: 10.1097/CCM.0000000000001302
24. Matschke J, Muller-Beissenhirtz H, Novotny J, et al. A randomized trial of daily prednisone versus pulsed dexamethasone in treatment-naïve adult patients with immune thrombocytopenia: EIS 2002 study. Acta Haematol. 2016;136(2):101-107. doi: 10.1159/000445420
25. Tilouche N, Hassen M, Ali HBS, Jaoued AHO, Gharbi R, Atrous SS. Delirium in the intensive care unit: incidence, risk factors, and impact on outcome. Indian J Crit Care Med. 2018;22:144-149. doi: 10.4103/ijccm.IJCCM_244_17
26. Young A, Marsh S. Steroid use in critical care. BJA Education. 2018;18(5):129-134. doi: 10.1016/j.bjae.2018.01.005
27. DiPiro J, Talbert R, Yee G, Matzke GR, Wells BG, Posey M. Pharmacotherapy: A Pathophysiologic Approach. 4th ed. New York: McGraw-Hill; 1999:1277-1278.
28. Schimmer
29. Sarnes E, Crofford L, Watson M, Dennis G, Kan H, Bass D. Incidence of US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther. 2011;33(10):1413-1432.
30. Idzikowsi C, Shapiro CM. ABC of sleep disorders, non-psychotropic drugs and sleep. BMJ. 1993;306(6885):1118-1120. doi: 10.1136/bmj.306.6885.1118

31. Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14(4):398-406.
doi: 10.3109/10253890.2011.586446
32. Wolkowitz OM, Reus VI, Weingartner H, et al. Cognitive effects of corticosteroids. Am J Psychiatry 1990;147(10):1297-1303. doi: 10.1176/ajp.147.10.1297
33. McEwen BS, Davis PG, Parsons B, Pfaff DW. The brain as a target for steroid hormone action. Ann Rev Neurosci. 1979;2:65-112. doi: 10.1146/annurev.ne.02.030179.000433
34. Brown ES, Woolston DJ, Frol AM. Amygdala volume in patients receiving chronic corticosteroid therapy. Biol Psychiatry. 2008;63(7):705-709.
doi: 10.1016/j.biopsych.2007.09.014
35. Brown ES, Woolston D, Frol A, et al. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid. Biol Psychiatry. 2004;55(5):538-545.
36. Sapolsky RM, McEwen BS. Down-regulation of neural corticosterone receptors by corticosterone and dexamethasone. Brain Res. 1985;339(1):161-165.
doi: 10.1016/0006-8993(85)90638-9
37. Sorrells SF, Caso JR, Munhoz CD, Spolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64(1):33-39.
doi: 10.1016/j.neuron.2009.09.032
38. Wolkowitz OM, Burke H, Epel ES, Reus VI. Glucocorticoids: mood, memory, and mechanisms. Ann NY Acad Sci. 2009;1179:19-40. doi: 10.1111/j.1749-6632.2009.04980.x
39. Wolkowitz OM, Epel ES, Reus VI. Stress hormone-related psychopathology: pathophysiological and treatment implications. World J Biol Psychiatry. 2001;2(3):115-143. doi: 10.3109/15622970109026799
40. Paredes S, Barriga C, Reiter R, Rodrigues A. Assessment of the potential role of tryptophan as the precursor of serotonin and melatonin for the aged sleep-wake cycle and immune function: Streptopelia Risoria as a model. Int J Tryptophan Res. 2009;2:23-36. doi: 10.4137/ijtr.s1129
41. Soszyński P, Stowińska-Srzednicka J, Kasperlik-Zatuska A, Zgliczyński S. Decreased melatonin concentration in Cushing’s Syndrome. Horm Metab Res. 1989;21(12):673-674. doi: 10.1055/s-2007-1009317
42. Demish L, Demish K, Neckelsen T. Influence of dexamethasone on nocturnal melatonin production in healthy adult subjects. J Pineal Res. 1988;5(3):317-321. doi: 10.1111/j.1600-079x.1988.tb00657.x
43. Assaf N, Shalby AB, Khalil WK, Ahmed HH. Biochemical and genetic alterations of oxidant/antioxidant status of the brain in rats treated with dexamethasone: protective roles of melatonin and acetyl-L-carnitine. J Physiol Biochem. 2012;68(1):77-90. doi: 10.1007/s13105-011-0121-3
44. Clark MS, Russo AF. Tissue-specific glucocorticoid regulation of tryptophan hydroxylase mRNA levels. Brain Res Mol Brain Res. 1997;48(2):346-54. doi: 10.1016/s0169-328x(97)00106-x
45. Kram DE, Krasnow SM, Levasseur PR, Zhu X, Stork LC, Marks DL. Dexamethasone chemotherapy does not disrupt orexin signaling. PLoS One. 2016;11(12):e0168731. doi: 10.1371/journal.pone.0168731
46. Mellon S. Neurosteroids: biochemistry, modes of action, and clinical relevance. J Clin Endocrinol Metab. 1994;78(5):1003-1008. doi: 10.1210/jcem.78.5.8175951
47. Zorumski C, Paul SM, Izumi Y, Covey DF, Mennerick S . Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci Biobehav Rev. 2013;37(1):109-122. doi: 10.1016/j.neubiorev.2012.10.005
48. Monteleone P, Luisi M, Martiadis V, et al. Impaired reduction of enhanced levels of dehydroepiandrosterone by oral dexamethasone in anorexia nervosa. Psychoneuroendocrinology. 2006;31(4):537-542. doi: 10.1016/j.psyneuen.2005.08.015
49. Genazzani AR, Petraglia F, Bernardi F, et al. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998;83(6):2099-3103. doi: 10.1210/jcem.83.6.4905
50. Moser NJ, Phillips BA, Guthrie G, Barnett G. Effects of dexamethasone on sleep. Pharmacol Toxicol. 1996;79(2):100-102. doi: 10.1111/j.1600-0773.1996.tb00249.x
51. Curtis J, Westfall A, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55(3):420-426. doi: 10.1002/art.21984
52. Zhao J, Dai YH, Xi QS, Yu SY. A clinical study on insomnia in patients with cancer during chemotherapy containing high-dose glucocorticoids. Pharmazie. 2013;68(6):421-427
53. Naber D, Sand P, Heigl B. Psychopathological and neuropsychological effects of 8-days corticosteroid treatment. A prospective study. Psychoneuroendocrinology. 1996;21(1):25-31. doi: 10.1016/0306-4530(95)00031-3
54. Brown ES, Suppes T, Khan DA, Carmody TJ 3rd. Mood changes during prednisone bursts in outpatients with asthma. J Clin Psychopharmacol. 2002;22(1):55-61.
doi: 10.1097/00004714-200202000-00009
55. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367. doi: 10.4065/81.10.1361
56. Britt RC, Devine A, Swallen KC et al. Corticosteroid use in the intensive care unit: at what cost? Arch Surg. 2006;141(2):145-159. doi:10.1001/archsurg.141.2.145
57. Kiser TH, Allen RR, Valuck RJ, Moss M, Vanivier RW. Outcomes associated with corticosteroid dosage in critically ill patients in acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):1052-1064. doi: 10.1164/rccm.201401-0058OC
58. Bourne RS, Mills GH. Sleep disruption in critically ill patients—pharmacological considerations. Anaesthesia. 2004;59(4):374-384. doi: 10.1111/j. 1365-2044.2004.03664.x
59. Flaherty JH. Insomnia among hospitalized older persons. Clin Geriatr Med. 2008;24(1):51-67. doi: 10.1016/j.cger.2007.08.012
60. Sirios F. Steroid psychosis: a review. Gen Hosp Psychiatry. 2003;25(1):27-33. doi: 10.1016/s0163-8343(02)00241-4
Microthrombotic Complications of COVID-19 Are Likely Due to Embolism of Circulating Endothelial Derived Ultralarge von Willebrand Factor (eULVWF) Decorated-Platelet Strings
To the Editor: COVID-19 is a pandemic caused by the virus SARS-CoV-2. Serious complications of COVID-19 are characterized by acute respiratory distress syndrome (ARDS), pneumonia and rapidly progressing to multiorgan dysfunction syndrome (MODS).
The pathophysiology of COVID-19 is not fully understood yet and neither vaccine nor clearly effective antiviral treatment is available at this time. Based on the endothelial pathogenesis of viral sepsis, which includes ARDS as seen in severe acute respiratory syndrome (SARS) due to SARS-CoV and Middle East respiratory syndrome due to MERS-CoV,1,2 we believe COVID-19-associated ARDS is also caused by endotheliopathy-associated vascular microthrombotic disease (EA-VMTD), which also involves multiorgan dysfunction syndrome (MODS) that has been reported as the cause of death.3 We suspect these complications are secondary to disequilibrium state (for various reasons4,5) between insufficient ADAMTS13 and excessive exocytosis of ultra large von Willebrand factor multimers (ULVWF) from Weibel-Palade bodies present in endothelial cells due to COVID-19-induced endotheliopathy.
Endothelial-derived ULVWF multimers anchored to the endothelial surface of the vascular wall recruit platelets and initiate microthrombogenesis within the microvasculature, leading to large microthrombi strings composed of platelet and eULVWF complexes like “beads-on-a-string structures”6 where platelets firmly adhere to eULVWF, instead of roll on eULVWF strings.4 Platelets, once adhered to eULVWF strings, are rapidly activated causing platelet aggregation and also recruit leukocytes in a P-selectin dependent manner.4 These aggregates grow until they become sufficiently large and can no longer be held onto the eULVWF strings against the force of blood flow and released from endothelial cells into the circulation.4 It appears to us that in COVID-19 microthrombotic disease, large amounts of circulating complexes of endothelial-derived ULVWF decorated-platelet microthrombi strings are filtered in the microvasculature (embolism) or develops in the microvasculature in situ causing microthrombotic occlusion. During our data search, we have come across several articles published by Chang, including on endotheliopathy causing vascular microthrombotic disease based on a novel concept of “TTP-like syndrome”7
The genesis of EA-VMTD in TTP like syndrome is suspected to be triggered by complement activation and terminal complement complex (C5b-9, membrane attack complex, MAC) may play a key role in producing endotheliopathy.7 Magro and colleagues reported that COVID-19 patients have demonstrated generalized thrombotic microvascular injury involving the lungs and skin showing intense complement activation and C5b-9 deposition in the tissue.8 Also, recent pathology reports of COVID-19 diseased lungs showed extensive platelet-rich clotting with adherent mononuclear cells and extensive fibrin clotting,9 which appear consistent with involvement of NETosis.10 In another case report from Switzerland, a patient with severe COVID-19 had massive elevation of VWF antigen and activity (555% and 520%, respectively) and increased Factor VIII clotting activity (369%).11 These findings support vascular endotheliopathy causing exocytosis of ULVWF and associated increase in Factor VIII causing microthrombotic disease/embolism.
COVID-19 clinical syndrome appears very much consistent with EA-VMTD presenting with ARDS and MODS as well as micro-macro-thrombotic complications, including peripheral ischemia/gangrene involving fingers and toes and skin necrosis.8,12
We believe that an appropriate therapy may not be anticoagulation but should include antimicrothrombotic therapy targeting endotheliopathy and primary hemostasis in the early stages of the disease (platelet adhesion, activation, and aggregation; especially eULVWF) like recombinant CD59 (membrane attack complex inhibition factor [MACIF]), recombinant ADAMTS13, glycoprotein IIb/IIIa receptor blocker, therapeutic plasma exchange, and perhaps anticomplement therapy (in selected cases) and others; these need to be validated in clinical trials prior to clinical application.
Of note, ADAMTS13 is a zinc containing protease. We noted that zinc and calcium concentrations play a significant role (in vitro) in ADAMTS13 activity in citrated plasma and recombinant ADAMTS13 activity with no added chelators (recombinant ADAMTS13 activity can enhance up to 200-fold); whereas in high zinc concentrations, ADAMTS13 gets deactivated.13 We suggest this finding merits an urgent clinical trial since it appears to us as the best possible cost-effective treatment for COVID-19 microthrombotic complications.
In this view of clinical pathophysiology of sepsis in COVID-19, we would like to enlighten the relationship between endothelial pathogenesis of coronaviral sepsis and vascular microthrombotic disease and would urge the medical community to immediately explore appropriate therapeutic options.
N. Varatharajah, MD
Suganthi Rajah, MD
Virginia, US
1. Chang JC. Sepsis and septic shock: endothelial molecular pathogenesis associated with vascular microthrombotic disease. Thromb J. 2019;17:10. Published 2019 May 30. doi:10.1186/s12959-019-0198-4
2. Chang JC. Acute respiratory distress syndrome as an organ phenotype of vascular microthrombotic disease: based on hemostatic theory and endothelial molecular pathogenesis. Clin Appl Thromb Hemost. 2019;25:1076029619887437. doi:10.1177/1076029619887437
3. Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and multi-organ response. Curr Probl Cardiol. 2020;100618. In press. doi:10.1016/j.cpcardiol.2020.100618
4. Bernardo A, Ball C, Nolasco L, Choi H, Moake JL, Dong JF. Platelets adhered to endothelial cell-bound ultra-large von Willebrand factor strings support leukocyte tethering and rolling under high shear stress. J Thromb Haemost. 2005;3(3):562‐570. doi:10.1111/j.1538-7836.2005.01122.x https://doi.org/10.1111/j.1538-7836.2005.01122.x
5. Mannucci PM, Canciani MT, Forza I, Lussana F, Lattuada A, Rossi E. Changes in health and disease of the metalloprotease that cleaves von Willebrand factor. Blood. 2001;98(9):2730‐2735. doi:10.1182/blood.v98.9.2730
6. Dong JF, Moake JL, Nolasco L, et al. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100(12):4033‐4039. doi:10.1182/blood-2002-05-1401
7. Chang JC. TTP-like syndrome: novel concept and molecular pathogenesis of endotheliopathy-associated vascular microthrombotic disease. Thromb J. 2018;16:20. Published 2018 Aug 11. doi:10.1186/s12959-018-0174-4
8. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. [Published online ahead of print, 2020 Apr 15.] Transl Res. 2020;S1931-5244(20)30070-0. doi:10.1016/j.trsl.2020.04.007
9. Guang Li, Sharon E. Fox, Brian Summa, et al. Multiscale 3-dimensional pathology findings of COVID-19 diseased lung using high-resolution cleared tissue microscopy. https://www.biorxiv.org/content/10.1101/2020.04.11.037473v1.full.pdf. Posted April 20, 2020. Accessed May 14, 2020. doi: 10.1101/2020.04.11.037473
10. de Bont CM, Boelens WC, Pruijn GJM. NETosis, complement, and coagulation: a triangular relationship. Cell Mol Immunol. 2019;16(1):19‐27. doi:10.1038/s41423-018-0024-0
11. Escher R, Breakey N, Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb Res. 2020;190:62. doi:10.1016/j.thromres.2020.04.014 https://doi.org/10.1016/j.thromres.2020.04.014
12. Landa N, Mendieta-Eckert M, Fonda-Pascual P, Aguirre T. Chilblain-like lesions on feet and hands during the COVID-19 Pandemic. Int J Dermatol. 2020;59(6):739‐743. doi:10.1111/ijd.14937
13. Anderson PJ, Kokame K, Sadler JE. Zinc and calcium ions cooperatively modulate ADAMTS13 activity. J Biol Chem. 2006;281(2):850‐857. doi:10.1074/jbc.M504540200
To the Editor: COVID-19 is a pandemic caused by the virus SARS-CoV-2. Serious complications of COVID-19 are characterized by acute respiratory distress syndrome (ARDS), pneumonia and rapidly progressing to multiorgan dysfunction syndrome (MODS).
The pathophysiology of COVID-19 is not fully understood yet and neither vaccine nor clearly effective antiviral treatment is available at this time. Based on the endothelial pathogenesis of viral sepsis, which includes ARDS as seen in severe acute respiratory syndrome (SARS) due to SARS-CoV and Middle East respiratory syndrome due to MERS-CoV,1,2 we believe COVID-19-associated ARDS is also caused by endotheliopathy-associated vascular microthrombotic disease (EA-VMTD), which also involves multiorgan dysfunction syndrome (MODS) that has been reported as the cause of death.3 We suspect these complications are secondary to disequilibrium state (for various reasons4,5) between insufficient ADAMTS13 and excessive exocytosis of ultra large von Willebrand factor multimers (ULVWF) from Weibel-Palade bodies present in endothelial cells due to COVID-19-induced endotheliopathy.
Endothelial-derived ULVWF multimers anchored to the endothelial surface of the vascular wall recruit platelets and initiate microthrombogenesis within the microvasculature, leading to large microthrombi strings composed of platelet and eULVWF complexes like “beads-on-a-string structures”6 where platelets firmly adhere to eULVWF, instead of roll on eULVWF strings.4 Platelets, once adhered to eULVWF strings, are rapidly activated causing platelet aggregation and also recruit leukocytes in a P-selectin dependent manner.4 These aggregates grow until they become sufficiently large and can no longer be held onto the eULVWF strings against the force of blood flow and released from endothelial cells into the circulation.4 It appears to us that in COVID-19 microthrombotic disease, large amounts of circulating complexes of endothelial-derived ULVWF decorated-platelet microthrombi strings are filtered in the microvasculature (embolism) or develops in the microvasculature in situ causing microthrombotic occlusion. During our data search, we have come across several articles published by Chang, including on endotheliopathy causing vascular microthrombotic disease based on a novel concept of “TTP-like syndrome”7
The genesis of EA-VMTD in TTP like syndrome is suspected to be triggered by complement activation and terminal complement complex (C5b-9, membrane attack complex, MAC) may play a key role in producing endotheliopathy.7 Magro and colleagues reported that COVID-19 patients have demonstrated generalized thrombotic microvascular injury involving the lungs and skin showing intense complement activation and C5b-9 deposition in the tissue.8 Also, recent pathology reports of COVID-19 diseased lungs showed extensive platelet-rich clotting with adherent mononuclear cells and extensive fibrin clotting,9 which appear consistent with involvement of NETosis.10 In another case report from Switzerland, a patient with severe COVID-19 had massive elevation of VWF antigen and activity (555% and 520%, respectively) and increased Factor VIII clotting activity (369%).11 These findings support vascular endotheliopathy causing exocytosis of ULVWF and associated increase in Factor VIII causing microthrombotic disease/embolism.
COVID-19 clinical syndrome appears very much consistent with EA-VMTD presenting with ARDS and MODS as well as micro-macro-thrombotic complications, including peripheral ischemia/gangrene involving fingers and toes and skin necrosis.8,12
We believe that an appropriate therapy may not be anticoagulation but should include antimicrothrombotic therapy targeting endotheliopathy and primary hemostasis in the early stages of the disease (platelet adhesion, activation, and aggregation; especially eULVWF) like recombinant CD59 (membrane attack complex inhibition factor [MACIF]), recombinant ADAMTS13, glycoprotein IIb/IIIa receptor blocker, therapeutic plasma exchange, and perhaps anticomplement therapy (in selected cases) and others; these need to be validated in clinical trials prior to clinical application.
Of note, ADAMTS13 is a zinc containing protease. We noted that zinc and calcium concentrations play a significant role (in vitro) in ADAMTS13 activity in citrated plasma and recombinant ADAMTS13 activity with no added chelators (recombinant ADAMTS13 activity can enhance up to 200-fold); whereas in high zinc concentrations, ADAMTS13 gets deactivated.13 We suggest this finding merits an urgent clinical trial since it appears to us as the best possible cost-effective treatment for COVID-19 microthrombotic complications.
In this view of clinical pathophysiology of sepsis in COVID-19, we would like to enlighten the relationship between endothelial pathogenesis of coronaviral sepsis and vascular microthrombotic disease and would urge the medical community to immediately explore appropriate therapeutic options.
N. Varatharajah, MD
Suganthi Rajah, MD
Virginia, US
To the Editor: COVID-19 is a pandemic caused by the virus SARS-CoV-2. Serious complications of COVID-19 are characterized by acute respiratory distress syndrome (ARDS), pneumonia and rapidly progressing to multiorgan dysfunction syndrome (MODS).
The pathophysiology of COVID-19 is not fully understood yet and neither vaccine nor clearly effective antiviral treatment is available at this time. Based on the endothelial pathogenesis of viral sepsis, which includes ARDS as seen in severe acute respiratory syndrome (SARS) due to SARS-CoV and Middle East respiratory syndrome due to MERS-CoV,1,2 we believe COVID-19-associated ARDS is also caused by endotheliopathy-associated vascular microthrombotic disease (EA-VMTD), which also involves multiorgan dysfunction syndrome (MODS) that has been reported as the cause of death.3 We suspect these complications are secondary to disequilibrium state (for various reasons4,5) between insufficient ADAMTS13 and excessive exocytosis of ultra large von Willebrand factor multimers (ULVWF) from Weibel-Palade bodies present in endothelial cells due to COVID-19-induced endotheliopathy.
Endothelial-derived ULVWF multimers anchored to the endothelial surface of the vascular wall recruit platelets and initiate microthrombogenesis within the microvasculature, leading to large microthrombi strings composed of platelet and eULVWF complexes like “beads-on-a-string structures”6 where platelets firmly adhere to eULVWF, instead of roll on eULVWF strings.4 Platelets, once adhered to eULVWF strings, are rapidly activated causing platelet aggregation and also recruit leukocytes in a P-selectin dependent manner.4 These aggregates grow until they become sufficiently large and can no longer be held onto the eULVWF strings against the force of blood flow and released from endothelial cells into the circulation.4 It appears to us that in COVID-19 microthrombotic disease, large amounts of circulating complexes of endothelial-derived ULVWF decorated-platelet microthrombi strings are filtered in the microvasculature (embolism) or develops in the microvasculature in situ causing microthrombotic occlusion. During our data search, we have come across several articles published by Chang, including on endotheliopathy causing vascular microthrombotic disease based on a novel concept of “TTP-like syndrome”7
The genesis of EA-VMTD in TTP like syndrome is suspected to be triggered by complement activation and terminal complement complex (C5b-9, membrane attack complex, MAC) may play a key role in producing endotheliopathy.7 Magro and colleagues reported that COVID-19 patients have demonstrated generalized thrombotic microvascular injury involving the lungs and skin showing intense complement activation and C5b-9 deposition in the tissue.8 Also, recent pathology reports of COVID-19 diseased lungs showed extensive platelet-rich clotting with adherent mononuclear cells and extensive fibrin clotting,9 which appear consistent with involvement of NETosis.10 In another case report from Switzerland, a patient with severe COVID-19 had massive elevation of VWF antigen and activity (555% and 520%, respectively) and increased Factor VIII clotting activity (369%).11 These findings support vascular endotheliopathy causing exocytosis of ULVWF and associated increase in Factor VIII causing microthrombotic disease/embolism.
COVID-19 clinical syndrome appears very much consistent with EA-VMTD presenting with ARDS and MODS as well as micro-macro-thrombotic complications, including peripheral ischemia/gangrene involving fingers and toes and skin necrosis.8,12
We believe that an appropriate therapy may not be anticoagulation but should include antimicrothrombotic therapy targeting endotheliopathy and primary hemostasis in the early stages of the disease (platelet adhesion, activation, and aggregation; especially eULVWF) like recombinant CD59 (membrane attack complex inhibition factor [MACIF]), recombinant ADAMTS13, glycoprotein IIb/IIIa receptor blocker, therapeutic plasma exchange, and perhaps anticomplement therapy (in selected cases) and others; these need to be validated in clinical trials prior to clinical application.
Of note, ADAMTS13 is a zinc containing protease. We noted that zinc and calcium concentrations play a significant role (in vitro) in ADAMTS13 activity in citrated plasma and recombinant ADAMTS13 activity with no added chelators (recombinant ADAMTS13 activity can enhance up to 200-fold); whereas in high zinc concentrations, ADAMTS13 gets deactivated.13 We suggest this finding merits an urgent clinical trial since it appears to us as the best possible cost-effective treatment for COVID-19 microthrombotic complications.
In this view of clinical pathophysiology of sepsis in COVID-19, we would like to enlighten the relationship between endothelial pathogenesis of coronaviral sepsis and vascular microthrombotic disease and would urge the medical community to immediately explore appropriate therapeutic options.
N. Varatharajah, MD
Suganthi Rajah, MD
Virginia, US
1. Chang JC. Sepsis and septic shock: endothelial molecular pathogenesis associated with vascular microthrombotic disease. Thromb J. 2019;17:10. Published 2019 May 30. doi:10.1186/s12959-019-0198-4
2. Chang JC. Acute respiratory distress syndrome as an organ phenotype of vascular microthrombotic disease: based on hemostatic theory and endothelial molecular pathogenesis. Clin Appl Thromb Hemost. 2019;25:1076029619887437. doi:10.1177/1076029619887437
3. Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and multi-organ response. Curr Probl Cardiol. 2020;100618. In press. doi:10.1016/j.cpcardiol.2020.100618
4. Bernardo A, Ball C, Nolasco L, Choi H, Moake JL, Dong JF. Platelets adhered to endothelial cell-bound ultra-large von Willebrand factor strings support leukocyte tethering and rolling under high shear stress. J Thromb Haemost. 2005;3(3):562‐570. doi:10.1111/j.1538-7836.2005.01122.x https://doi.org/10.1111/j.1538-7836.2005.01122.x
5. Mannucci PM, Canciani MT, Forza I, Lussana F, Lattuada A, Rossi E. Changes in health and disease of the metalloprotease that cleaves von Willebrand factor. Blood. 2001;98(9):2730‐2735. doi:10.1182/blood.v98.9.2730
6. Dong JF, Moake JL, Nolasco L, et al. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100(12):4033‐4039. doi:10.1182/blood-2002-05-1401
7. Chang JC. TTP-like syndrome: novel concept and molecular pathogenesis of endotheliopathy-associated vascular microthrombotic disease. Thromb J. 2018;16:20. Published 2018 Aug 11. doi:10.1186/s12959-018-0174-4
8. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. [Published online ahead of print, 2020 Apr 15.] Transl Res. 2020;S1931-5244(20)30070-0. doi:10.1016/j.trsl.2020.04.007
9. Guang Li, Sharon E. Fox, Brian Summa, et al. Multiscale 3-dimensional pathology findings of COVID-19 diseased lung using high-resolution cleared tissue microscopy. https://www.biorxiv.org/content/10.1101/2020.04.11.037473v1.full.pdf. Posted April 20, 2020. Accessed May 14, 2020. doi: 10.1101/2020.04.11.037473
10. de Bont CM, Boelens WC, Pruijn GJM. NETosis, complement, and coagulation: a triangular relationship. Cell Mol Immunol. 2019;16(1):19‐27. doi:10.1038/s41423-018-0024-0
11. Escher R, Breakey N, Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb Res. 2020;190:62. doi:10.1016/j.thromres.2020.04.014 https://doi.org/10.1016/j.thromres.2020.04.014
12. Landa N, Mendieta-Eckert M, Fonda-Pascual P, Aguirre T. Chilblain-like lesions on feet and hands during the COVID-19 Pandemic. Int J Dermatol. 2020;59(6):739‐743. doi:10.1111/ijd.14937
13. Anderson PJ, Kokame K, Sadler JE. Zinc and calcium ions cooperatively modulate ADAMTS13 activity. J Biol Chem. 2006;281(2):850‐857. doi:10.1074/jbc.M504540200
1. Chang JC. Sepsis and septic shock: endothelial molecular pathogenesis associated with vascular microthrombotic disease. Thromb J. 2019;17:10. Published 2019 May 30. doi:10.1186/s12959-019-0198-4
2. Chang JC. Acute respiratory distress syndrome as an organ phenotype of vascular microthrombotic disease: based on hemostatic theory and endothelial molecular pathogenesis. Clin Appl Thromb Hemost. 2019;25:1076029619887437. doi:10.1177/1076029619887437
3. Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and multi-organ response. Curr Probl Cardiol. 2020;100618. In press. doi:10.1016/j.cpcardiol.2020.100618
4. Bernardo A, Ball C, Nolasco L, Choi H, Moake JL, Dong JF. Platelets adhered to endothelial cell-bound ultra-large von Willebrand factor strings support leukocyte tethering and rolling under high shear stress. J Thromb Haemost. 2005;3(3):562‐570. doi:10.1111/j.1538-7836.2005.01122.x https://doi.org/10.1111/j.1538-7836.2005.01122.x
5. Mannucci PM, Canciani MT, Forza I, Lussana F, Lattuada A, Rossi E. Changes in health and disease of the metalloprotease that cleaves von Willebrand factor. Blood. 2001;98(9):2730‐2735. doi:10.1182/blood.v98.9.2730
6. Dong JF, Moake JL, Nolasco L, et al. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100(12):4033‐4039. doi:10.1182/blood-2002-05-1401
7. Chang JC. TTP-like syndrome: novel concept and molecular pathogenesis of endotheliopathy-associated vascular microthrombotic disease. Thromb J. 2018;16:20. Published 2018 Aug 11. doi:10.1186/s12959-018-0174-4
8. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. [Published online ahead of print, 2020 Apr 15.] Transl Res. 2020;S1931-5244(20)30070-0. doi:10.1016/j.trsl.2020.04.007
9. Guang Li, Sharon E. Fox, Brian Summa, et al. Multiscale 3-dimensional pathology findings of COVID-19 diseased lung using high-resolution cleared tissue microscopy. https://www.biorxiv.org/content/10.1101/2020.04.11.037473v1.full.pdf. Posted April 20, 2020. Accessed May 14, 2020. doi: 10.1101/2020.04.11.037473
10. de Bont CM, Boelens WC, Pruijn GJM. NETosis, complement, and coagulation: a triangular relationship. Cell Mol Immunol. 2019;16(1):19‐27. doi:10.1038/s41423-018-0024-0
11. Escher R, Breakey N, Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb Res. 2020;190:62. doi:10.1016/j.thromres.2020.04.014 https://doi.org/10.1016/j.thromres.2020.04.014
12. Landa N, Mendieta-Eckert M, Fonda-Pascual P, Aguirre T. Chilblain-like lesions on feet and hands during the COVID-19 Pandemic. Int J Dermatol. 2020;59(6):739‐743. doi:10.1111/ijd.14937
13. Anderson PJ, Kokame K, Sadler JE. Zinc and calcium ions cooperatively modulate ADAMTS13 activity. J Biol Chem. 2006;281(2):850‐857. doi:10.1074/jbc.M504540200
A Tale of 2 Medications: A Desperate Race for Hope
For health care professionals, especially those in the epicenters of the pandemic, among the most distressing aspects of this first wave of COVID-19 has been the absence of any drug to treat the virus. The practitioners on the frontlines have confronted repeated surges of critically ill and dying patients without any effective treatment to offer, resulting in feelings of hopelessness, guilt, moral distress, depression, and in some tragic cases, suicide.2
On May 12th, the Centers of Disease Control and Prevention (CDC) released additional guidance on the antiviral medications that are the subject of this essay. The CDC may have updated its treatment guidelines in part to try and bring a measure of clinical reasoning and scientific order into the impassioned and politicized chaos that surrounded hydrocloroquine and remdesivir in the media.3
In this fourth installment of my series on pandemic ethics, we examine the desperate race for hope in the form of drug treatments for COVID-19. The race has been run faster than any in history thanks to biotechnology, genetic engineering, and artificial intelligence, although many experts believe it will still be a marathon rather than a sprint to a vaccine.4
The first editorial in this series provided a primer of the key differences between public health ethics and clinical ethics. Another crucial distinction is the far more pervasive and powerful influence of nonmedical factors in decision making, including political agendas, economic motives, journalistic hyperbole, and cultural biases and orientations. These competing interests make it even more challenging for scientists of integrity and health care institutions that are trying to uphold core values to make principled judgments about what is best for critically ill patients and the demoralized staff caring for them. In the remainder of this column, I will trace the dynamics of these forces as they impact the use of 2 drugs in federal practice: hydroxychloroquine and remdesivir.
The trajectory of hydroxychloroquine has been a political and medical roller-coaster since the pandemic hit, as is evident in its US Department of Veterans Affairs (VA) ride. Various media outlets have reported that beginning about March 26, 2020, VA placed orders for up to $400,000 of the antimalarial drug hydroxychloroquine to be given to veterans hospitalized with COVID-19.5 The same day the VA Office of Inspector General (OIG) issued a report critical of VA pandemic readiness and its availability of hydroxychloroquine.6
The VA strongly refuted the report, objecting to the premise of the OIG investigation, which was to determine whether VA facilities had on hand a 14-day supply of chloroquine or hydroxychloroquine. “This is both inaccurate and irresponsible.” Noting that the drugs were still under investigation, the VA insisted that “No conclusions have been made on their effectiveness. To insist that a 14 days’ supply of these drugs is appropriate or not appropriate displays this dangerous lack of expertise on COVID-19 and Pandemic response.”6
In April, National Institutes of Health-sponsored researchers released data that hydroxychloroquine actually increased mortality among VA patients with COVID-19,7 leading veterans’ groups and the Senate minority leader to demand that VA cease to use hydroxychloroquine for COVID-19.8 As recently as May 15, the Associated Press reported that top VA officials have defended their use of the medication and stated they will not stop administering the medication for this indication.9 And VA is not alone, many other health care institutions are still prescribing hydroxychloroquine even amid scientific controversy about its putative benefits. In response to the growing awareness of the potential harms of the drug, the World Health Organization on May 25 announced it was halting all hydroxychloroquine trials.10 Why then do some physicians and health care providers continue to prescribe it? Because when nothing else stands between the patient and certain death even if there are known risks and uncertain benefits, some in health care feel morally obliged to use their best clinical judgment to help a patient.
Remdesivir’s fortunes both scientific and monetary also rose and fell on the tide of mixed results from studies. Military Times reported on March 10, 2020, that the US Army Medical Research and Development Command had made an agreement with Gilead Sciences, the manufacturer of remdesivir, to provide the medication to COVID-19-positive service members.11 The antiviral had failed against Ebola and hepatitis but showed some efficacy for Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). On April 15, the Secretary of the Army announced that 2 COVID-19-positive soldiers had recovered after being given remdesivir.12 In late April, the National Institute of Allergy and Infectious Diseases reported that in the scientific gold standard randomized placebo-controlled trial, remdesivir did speed the recovery of patients with advanced COVID-19. With the publication of the study in the prestigious New England Journal of Medicine on May 22, 2020, clearly the Army had bet on the right horse.13
This column has not been about quack cures and patent medicines that greed and ignorance breed in almost every American public health crisis—although these are by no means absent in this pandemic. This is about the serious endeavor of the top scientists and physicians in the country and, indeed, the world to discover a new medication or to repurpose an older pharmaceutical that is effective in the battle against COVID-19. The pressure on scientists and physicians to find a magic bullet in the battle against such an implacable enemy is unprecedented and unimaginable and can easily lead to sloppy science and ethical erosion.
In a utopia, pharmaceutical and vaccine research would be a matter of the discoveries of basic science trialed in the proof of concept of clinical care on a methodical, deliberate, and exacting timetable that balanced burdens and benefits.
In our current dystopia, science and medicine are only one of the many considerations affecting drug and vaccine development. As scientists and health care practitioners, we all experience a therapeutic imperative that we must heed with both caution and courage. Without caution we risk causing more harm than the disease we are fighting. Without courage we lose hope, the most potent antidote of all.
1. de Kruif P. Microbe Hunters. San Diego, CA: Harcourt Brace Jovanavick; 1926.
2. Watkins A, Rothfeld M, Rashbaum WK, Rosenthal BM. Top ER doctor who treated patients dies by suicide. New York Times . April 27, 2020. https://www.nytimes.com/2020/04/27/nyregion/new-york-city-doctor-suicide-coronavirus.html. Updated April 29, 2020. Accessed May 26, 2020.
3. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/whats-new. Updated May 12, 2020. Accessed June 5, 2020.
4. Doheny K. Finish line unpredictable for COVID-19 vaccine race. https://www.webmd.com/lung/news/20200424/finish-line-unpredictable-for-covid-vaccine-race. Published April 29, 2020. Accessed May 26, 2020.
5. Horton A. What VA isn’t saying about hydroxychloroquine—and everything else related to coronavirus. Washington Post . May 1, 2020. https://www.washingtonpost.com/national-security/2020/05/01/hydroxychloroquine-veterans-trump. Accessed May 27, 2020.
6. US Department of Veterans Affairs, Veterans Health Administration, Office of the Inspector General, Office of Healthcare Inspections. OIG inspection of Veterans Health Administration COVID-19 screening processes and pandemic readiness. https://www.va.gov/oig/pubs/VAOIG-20-02221-120.pdf. Published March 19-24, 2020. Accessed May 26, 2020.
7. Maganoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19 [preprint]. doi.org/10.1101/2020.04.16.20065920.
8. Yen H, Balsamo M. Schumer calls on VA to explain use of unproven drug on vets. Associated Press. May10, 2020. https://apnews.com/a2830445e55c6ea324e9a23e4c38f7c3. Accessed May 27, 2020.
9. Yen H. VA says it won’t stop use of unproven drug on vets for now. Associated Press, May 15, 2020. https://apnews.com/2edd19decf58ed921d9b7ba9f6a2b44e. Accessed May 27, 2020.
10. World Health Organization. Coronavirus: WHO halts trials of hydroxychloroquine over safety fears. http://www.bbc.com/news/health-52799120. Accessed May 29, 2020.
11. Kime P. Army signs agreement with drug giant Gilead on experimental COVID-19 treatment. Military Times . March 10, 2020. https://www.militarytimes.com/news/your-military/2020/03/10/army-signs-agreement-with-drug-giant-gilead-on-experimental-covid-19-treatment. Accessed May 27, 2020.
12. Cox M. Two U.S. soldiers with Covid-19 ‘up and walking around’ after taking Ebola drug. https://www.military.com/daily-news/2020/04/15/two-us-soldiers-covid-19-and-walking-around-after-taking-ebola-drug.html. Published April 15, 2020. Accessed May 27, 2020.
13. Beigel JH, Tomashek KM, Dodd LE, et al; ACTT-1 Study Group Members. Remdesivir for the treatment of COVID-19—preliminary report. N Engl J Med. May 22, 2020. doi: 10.1056/NEJMoa2007764
For health care professionals, especially those in the epicenters of the pandemic, among the most distressing aspects of this first wave of COVID-19 has been the absence of any drug to treat the virus. The practitioners on the frontlines have confronted repeated surges of critically ill and dying patients without any effective treatment to offer, resulting in feelings of hopelessness, guilt, moral distress, depression, and in some tragic cases, suicide.2
On May 12th, the Centers of Disease Control and Prevention (CDC) released additional guidance on the antiviral medications that are the subject of this essay. The CDC may have updated its treatment guidelines in part to try and bring a measure of clinical reasoning and scientific order into the impassioned and politicized chaos that surrounded hydrocloroquine and remdesivir in the media.3
In this fourth installment of my series on pandemic ethics, we examine the desperate race for hope in the form of drug treatments for COVID-19. The race has been run faster than any in history thanks to biotechnology, genetic engineering, and artificial intelligence, although many experts believe it will still be a marathon rather than a sprint to a vaccine.4
The first editorial in this series provided a primer of the key differences between public health ethics and clinical ethics. Another crucial distinction is the far more pervasive and powerful influence of nonmedical factors in decision making, including political agendas, economic motives, journalistic hyperbole, and cultural biases and orientations. These competing interests make it even more challenging for scientists of integrity and health care institutions that are trying to uphold core values to make principled judgments about what is best for critically ill patients and the demoralized staff caring for them. In the remainder of this column, I will trace the dynamics of these forces as they impact the use of 2 drugs in federal practice: hydroxychloroquine and remdesivir.
The trajectory of hydroxychloroquine has been a political and medical roller-coaster since the pandemic hit, as is evident in its US Department of Veterans Affairs (VA) ride. Various media outlets have reported that beginning about March 26, 2020, VA placed orders for up to $400,000 of the antimalarial drug hydroxychloroquine to be given to veterans hospitalized with COVID-19.5 The same day the VA Office of Inspector General (OIG) issued a report critical of VA pandemic readiness and its availability of hydroxychloroquine.6
The VA strongly refuted the report, objecting to the premise of the OIG investigation, which was to determine whether VA facilities had on hand a 14-day supply of chloroquine or hydroxychloroquine. “This is both inaccurate and irresponsible.” Noting that the drugs were still under investigation, the VA insisted that “No conclusions have been made on their effectiveness. To insist that a 14 days’ supply of these drugs is appropriate or not appropriate displays this dangerous lack of expertise on COVID-19 and Pandemic response.”6
In April, National Institutes of Health-sponsored researchers released data that hydroxychloroquine actually increased mortality among VA patients with COVID-19,7 leading veterans’ groups and the Senate minority leader to demand that VA cease to use hydroxychloroquine for COVID-19.8 As recently as May 15, the Associated Press reported that top VA officials have defended their use of the medication and stated they will not stop administering the medication for this indication.9 And VA is not alone, many other health care institutions are still prescribing hydroxychloroquine even amid scientific controversy about its putative benefits. In response to the growing awareness of the potential harms of the drug, the World Health Organization on May 25 announced it was halting all hydroxychloroquine trials.10 Why then do some physicians and health care providers continue to prescribe it? Because when nothing else stands between the patient and certain death even if there are known risks and uncertain benefits, some in health care feel morally obliged to use their best clinical judgment to help a patient.
Remdesivir’s fortunes both scientific and monetary also rose and fell on the tide of mixed results from studies. Military Times reported on March 10, 2020, that the US Army Medical Research and Development Command had made an agreement with Gilead Sciences, the manufacturer of remdesivir, to provide the medication to COVID-19-positive service members.11 The antiviral had failed against Ebola and hepatitis but showed some efficacy for Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). On April 15, the Secretary of the Army announced that 2 COVID-19-positive soldiers had recovered after being given remdesivir.12 In late April, the National Institute of Allergy and Infectious Diseases reported that in the scientific gold standard randomized placebo-controlled trial, remdesivir did speed the recovery of patients with advanced COVID-19. With the publication of the study in the prestigious New England Journal of Medicine on May 22, 2020, clearly the Army had bet on the right horse.13
This column has not been about quack cures and patent medicines that greed and ignorance breed in almost every American public health crisis—although these are by no means absent in this pandemic. This is about the serious endeavor of the top scientists and physicians in the country and, indeed, the world to discover a new medication or to repurpose an older pharmaceutical that is effective in the battle against COVID-19. The pressure on scientists and physicians to find a magic bullet in the battle against such an implacable enemy is unprecedented and unimaginable and can easily lead to sloppy science and ethical erosion.
In a utopia, pharmaceutical and vaccine research would be a matter of the discoveries of basic science trialed in the proof of concept of clinical care on a methodical, deliberate, and exacting timetable that balanced burdens and benefits.
In our current dystopia, science and medicine are only one of the many considerations affecting drug and vaccine development. As scientists and health care practitioners, we all experience a therapeutic imperative that we must heed with both caution and courage. Without caution we risk causing more harm than the disease we are fighting. Without courage we lose hope, the most potent antidote of all.
For health care professionals, especially those in the epicenters of the pandemic, among the most distressing aspects of this first wave of COVID-19 has been the absence of any drug to treat the virus. The practitioners on the frontlines have confronted repeated surges of critically ill and dying patients without any effective treatment to offer, resulting in feelings of hopelessness, guilt, moral distress, depression, and in some tragic cases, suicide.2
On May 12th, the Centers of Disease Control and Prevention (CDC) released additional guidance on the antiviral medications that are the subject of this essay. The CDC may have updated its treatment guidelines in part to try and bring a measure of clinical reasoning and scientific order into the impassioned and politicized chaos that surrounded hydrocloroquine and remdesivir in the media.3
In this fourth installment of my series on pandemic ethics, we examine the desperate race for hope in the form of drug treatments for COVID-19. The race has been run faster than any in history thanks to biotechnology, genetic engineering, and artificial intelligence, although many experts believe it will still be a marathon rather than a sprint to a vaccine.4
The first editorial in this series provided a primer of the key differences between public health ethics and clinical ethics. Another crucial distinction is the far more pervasive and powerful influence of nonmedical factors in decision making, including political agendas, economic motives, journalistic hyperbole, and cultural biases and orientations. These competing interests make it even more challenging for scientists of integrity and health care institutions that are trying to uphold core values to make principled judgments about what is best for critically ill patients and the demoralized staff caring for them. In the remainder of this column, I will trace the dynamics of these forces as they impact the use of 2 drugs in federal practice: hydroxychloroquine and remdesivir.
The trajectory of hydroxychloroquine has been a political and medical roller-coaster since the pandemic hit, as is evident in its US Department of Veterans Affairs (VA) ride. Various media outlets have reported that beginning about March 26, 2020, VA placed orders for up to $400,000 of the antimalarial drug hydroxychloroquine to be given to veterans hospitalized with COVID-19.5 The same day the VA Office of Inspector General (OIG) issued a report critical of VA pandemic readiness and its availability of hydroxychloroquine.6
The VA strongly refuted the report, objecting to the premise of the OIG investigation, which was to determine whether VA facilities had on hand a 14-day supply of chloroquine or hydroxychloroquine. “This is both inaccurate and irresponsible.” Noting that the drugs were still under investigation, the VA insisted that “No conclusions have been made on their effectiveness. To insist that a 14 days’ supply of these drugs is appropriate or not appropriate displays this dangerous lack of expertise on COVID-19 and Pandemic response.”6
In April, National Institutes of Health-sponsored researchers released data that hydroxychloroquine actually increased mortality among VA patients with COVID-19,7 leading veterans’ groups and the Senate minority leader to demand that VA cease to use hydroxychloroquine for COVID-19.8 As recently as May 15, the Associated Press reported that top VA officials have defended their use of the medication and stated they will not stop administering the medication for this indication.9 And VA is not alone, many other health care institutions are still prescribing hydroxychloroquine even amid scientific controversy about its putative benefits. In response to the growing awareness of the potential harms of the drug, the World Health Organization on May 25 announced it was halting all hydroxychloroquine trials.10 Why then do some physicians and health care providers continue to prescribe it? Because when nothing else stands between the patient and certain death even if there are known risks and uncertain benefits, some in health care feel morally obliged to use their best clinical judgment to help a patient.
Remdesivir’s fortunes both scientific and monetary also rose and fell on the tide of mixed results from studies. Military Times reported on March 10, 2020, that the US Army Medical Research and Development Command had made an agreement with Gilead Sciences, the manufacturer of remdesivir, to provide the medication to COVID-19-positive service members.11 The antiviral had failed against Ebola and hepatitis but showed some efficacy for Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). On April 15, the Secretary of the Army announced that 2 COVID-19-positive soldiers had recovered after being given remdesivir.12 In late April, the National Institute of Allergy and Infectious Diseases reported that in the scientific gold standard randomized placebo-controlled trial, remdesivir did speed the recovery of patients with advanced COVID-19. With the publication of the study in the prestigious New England Journal of Medicine on May 22, 2020, clearly the Army had bet on the right horse.13
This column has not been about quack cures and patent medicines that greed and ignorance breed in almost every American public health crisis—although these are by no means absent in this pandemic. This is about the serious endeavor of the top scientists and physicians in the country and, indeed, the world to discover a new medication or to repurpose an older pharmaceutical that is effective in the battle against COVID-19. The pressure on scientists and physicians to find a magic bullet in the battle against such an implacable enemy is unprecedented and unimaginable and can easily lead to sloppy science and ethical erosion.
In a utopia, pharmaceutical and vaccine research would be a matter of the discoveries of basic science trialed in the proof of concept of clinical care on a methodical, deliberate, and exacting timetable that balanced burdens and benefits.
In our current dystopia, science and medicine are only one of the many considerations affecting drug and vaccine development. As scientists and health care practitioners, we all experience a therapeutic imperative that we must heed with both caution and courage. Without caution we risk causing more harm than the disease we are fighting. Without courage we lose hope, the most potent antidote of all.
1. de Kruif P. Microbe Hunters. San Diego, CA: Harcourt Brace Jovanavick; 1926.
2. Watkins A, Rothfeld M, Rashbaum WK, Rosenthal BM. Top ER doctor who treated patients dies by suicide. New York Times . April 27, 2020. https://www.nytimes.com/2020/04/27/nyregion/new-york-city-doctor-suicide-coronavirus.html. Updated April 29, 2020. Accessed May 26, 2020.
3. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/whats-new. Updated May 12, 2020. Accessed June 5, 2020.
4. Doheny K. Finish line unpredictable for COVID-19 vaccine race. https://www.webmd.com/lung/news/20200424/finish-line-unpredictable-for-covid-vaccine-race. Published April 29, 2020. Accessed May 26, 2020.
5. Horton A. What VA isn’t saying about hydroxychloroquine—and everything else related to coronavirus. Washington Post . May 1, 2020. https://www.washingtonpost.com/national-security/2020/05/01/hydroxychloroquine-veterans-trump. Accessed May 27, 2020.
6. US Department of Veterans Affairs, Veterans Health Administration, Office of the Inspector General, Office of Healthcare Inspections. OIG inspection of Veterans Health Administration COVID-19 screening processes and pandemic readiness. https://www.va.gov/oig/pubs/VAOIG-20-02221-120.pdf. Published March 19-24, 2020. Accessed May 26, 2020.
7. Maganoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19 [preprint]. doi.org/10.1101/2020.04.16.20065920.
8. Yen H, Balsamo M. Schumer calls on VA to explain use of unproven drug on vets. Associated Press. May10, 2020. https://apnews.com/a2830445e55c6ea324e9a23e4c38f7c3. Accessed May 27, 2020.
9. Yen H. VA says it won’t stop use of unproven drug on vets for now. Associated Press, May 15, 2020. https://apnews.com/2edd19decf58ed921d9b7ba9f6a2b44e. Accessed May 27, 2020.
10. World Health Organization. Coronavirus: WHO halts trials of hydroxychloroquine over safety fears. http://www.bbc.com/news/health-52799120. Accessed May 29, 2020.
11. Kime P. Army signs agreement with drug giant Gilead on experimental COVID-19 treatment. Military Times . March 10, 2020. https://www.militarytimes.com/news/your-military/2020/03/10/army-signs-agreement-with-drug-giant-gilead-on-experimental-covid-19-treatment. Accessed May 27, 2020.
12. Cox M. Two U.S. soldiers with Covid-19 ‘up and walking around’ after taking Ebola drug. https://www.military.com/daily-news/2020/04/15/two-us-soldiers-covid-19-and-walking-around-after-taking-ebola-drug.html. Published April 15, 2020. Accessed May 27, 2020.
13. Beigel JH, Tomashek KM, Dodd LE, et al; ACTT-1 Study Group Members. Remdesivir for the treatment of COVID-19—preliminary report. N Engl J Med. May 22, 2020. doi: 10.1056/NEJMoa2007764
1. de Kruif P. Microbe Hunters. San Diego, CA: Harcourt Brace Jovanavick; 1926.
2. Watkins A, Rothfeld M, Rashbaum WK, Rosenthal BM. Top ER doctor who treated patients dies by suicide. New York Times . April 27, 2020. https://www.nytimes.com/2020/04/27/nyregion/new-york-city-doctor-suicide-coronavirus.html. Updated April 29, 2020. Accessed May 26, 2020.
3. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/whats-new. Updated May 12, 2020. Accessed June 5, 2020.
4. Doheny K. Finish line unpredictable for COVID-19 vaccine race. https://www.webmd.com/lung/news/20200424/finish-line-unpredictable-for-covid-vaccine-race. Published April 29, 2020. Accessed May 26, 2020.
5. Horton A. What VA isn’t saying about hydroxychloroquine—and everything else related to coronavirus. Washington Post . May 1, 2020. https://www.washingtonpost.com/national-security/2020/05/01/hydroxychloroquine-veterans-trump. Accessed May 27, 2020.
6. US Department of Veterans Affairs, Veterans Health Administration, Office of the Inspector General, Office of Healthcare Inspections. OIG inspection of Veterans Health Administration COVID-19 screening processes and pandemic readiness. https://www.va.gov/oig/pubs/VAOIG-20-02221-120.pdf. Published March 19-24, 2020. Accessed May 26, 2020.
7. Maganoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19 [preprint]. doi.org/10.1101/2020.04.16.20065920.
8. Yen H, Balsamo M. Schumer calls on VA to explain use of unproven drug on vets. Associated Press. May10, 2020. https://apnews.com/a2830445e55c6ea324e9a23e4c38f7c3. Accessed May 27, 2020.
9. Yen H. VA says it won’t stop use of unproven drug on vets for now. Associated Press, May 15, 2020. https://apnews.com/2edd19decf58ed921d9b7ba9f6a2b44e. Accessed May 27, 2020.
10. World Health Organization. Coronavirus: WHO halts trials of hydroxychloroquine over safety fears. http://www.bbc.com/news/health-52799120. Accessed May 29, 2020.
11. Kime P. Army signs agreement with drug giant Gilead on experimental COVID-19 treatment. Military Times . March 10, 2020. https://www.militarytimes.com/news/your-military/2020/03/10/army-signs-agreement-with-drug-giant-gilead-on-experimental-covid-19-treatment. Accessed May 27, 2020.
12. Cox M. Two U.S. soldiers with Covid-19 ‘up and walking around’ after taking Ebola drug. https://www.military.com/daily-news/2020/04/15/two-us-soldiers-covid-19-and-walking-around-after-taking-ebola-drug.html. Published April 15, 2020. Accessed May 27, 2020.
13. Beigel JH, Tomashek KM, Dodd LE, et al; ACTT-1 Study Group Members. Remdesivir for the treatment of COVID-19—preliminary report. N Engl J Med. May 22, 2020. doi: 10.1056/NEJMoa2007764
Purpuric Bullae on the Lower Extremities
The Diagnosis: Bullous Leukocytoclastic Vasculitis
Histopathology with hematoxylin and eosin (H&E) stain showed a perivascular neutrophilic infiltrate, karyorrhexis, red blood cell extravasation, and fibrin deposition in the vessel wall (quiz images). Direct immunofluorescence (DIF) showed fibrin surrounding the vasculature, consistent with vasculitis. The clinical and histopathological evaluation supported the diagnosis of bullous leukocytoclastic vasculitis (LCV). The patient had a full LCV workup including antinuclear antibody, rheumatoid factor, hepatitis B and hepatitis C screening, erythrocyte sedimentation rate, C-reactive protein, and C3/C4/total complement level, which were all within reference range. The patient denied that she had taken any medications prior to the onset of the rash. She was started on a 12-day prednisone taper starting at 60 mg, and the rash resolved in 1 week.
Although the incidence of LCV is estimated to be 30 cases per million individuals per year,1 bullous LCV is a rarer entity with only a few cases reported in the literature.2,3 As in our patient's case, up to 50% of LCV cases are idiopathic or the etiology cannot be determined despite laboratory workup and medication review. Other cases can be secondary to medication, infection, collagen vascular disease, or malignancy.3 Despite the exact pathogenesis of bullous LCV being unknown,4 it likely is related to a type III hypersensitivity reaction with immune complex deposition in postcapillary venules leading to endothelial injury, activation of the complement cascade, and development of intraepidermal or subepidermal blister formation depending on location of inflammation and edema.2 Clinically, an intraepidermal split would be more flaccid, similar to pemphigus vulgaris, while a subepidermal split, as in our patient, would be taut bullae. The subepidermal split more commonly is seen in bullous LCV.2
Leukocytoclastic vasculitis on H&E staining characteristically has a perivascular inflammatory infiltrate, neutrophilic fragments called leukocytoclasis, and blood extravasation.3 Extravasated blood presents clinically as petechiae. In this case, the petechiae helped distinguish this entity from the differential diagnosis. Furthermore, DIF would be helpful in distinguishing bullous diseases such as bullous pemphigoid (BP) and pemphigus vulgaris from LCV.2 Direct immunofluorescence in bullous LCV would have fibrinogen surrounding the vasculature without C3 and IgG deposition (intraepidermal or subepidermal).
Mild cases of LCV often resolve with supportive measures including elevation of the legs, ice packs applied to the affected area, and removal of the inciting drug or event.4 In the few cases reported in the literature, bullous LCV presented more diffusely than classic LCV with bullous lesions on the forearms and the lower extremities. Oral steroids are efficacious for extensive bullous LCV.4
The differential diagnosis of bullous LCV includes bullous diseases with subepidermal split including BP and linear IgA bullous dermatosis (LABD). Bullous pemphigoid is an autoimmune subepidermal blistering disease typically affecting patients older than 60 years.5 The pathogenesis of BP is related to development of autoantibodies directed against hemidesmosome components, bullous pemphigoid antigen (BPAG) 1 or BPAG2.5 Bullous pemphigoid presents clinically as widespread, generally pruritic, erythematous, urticarial plaques with bullae. Histologically, BP characteristically has a subepidermal split with superficial dermal edema and eosinophils at the dermoepidermal junction (Figure 1). Direct immunofluorescence confirms the diagnosis with IgG and C3 deposition in an n-serrated pattern at the dermoepidermal junction.6 Bullous pemphigoid can be distinguished from bullous LCV by the older age of presentation, DIF findings, and the absence of purpura.
Linear IgA bullous dermatosis represents a rare subepidermal vesiculobullous disease occurring in patients in their 60s.7 Clinically, this entity presents as tense bullae often located on the periphery of an urticarial plaque, classically called the "string of pearls sign." Histologically, LABD also presents with subepidermal split; however, neutrophils are the predominant cell type vs eosinophils in BP (Figure 2).7 Direct immunofluorescence is specific with a linear deposition of IgA at the dermoepidermal junction. Linear IgA bullous dermatosis most commonly is induced by vancomycin. Unlike bullous LCV, the bullae of LABD have an annular peripheral pattern on an erythematous base and lack purpura.
Stasis dermatitis is inflammation of the dermis due to venous insufficiency that often is present in the bilateral lower extremities. The disorder affects approximately 7% of adults older than 50 years, but it also can occur in younger patients.8 The pathophysiology of stasis dermatitis is caused by edema, which leads to extracellular fluid, plasma proteins, macrophages, and erythrocytes passing into the interstitial space. Patients with stasis dermatitis present with scaly erythematous papules and plaques or edematous blisters on the lower extremities. Diagnosis usually can be made clinically; however, a skin biopsy also can be helpful. Hematoxylin and eosin shows a pauci-inflammatory subepidermal bulla with fibrin (Figure 3).8 The overlying epidermis is intact. The dermis has cannon ball angiomatosis, red blood cell extravasation, and fibrosis typical of stasis dermatitis. Stasis dermatitis with bullae is cell poor and lacks the perivascular inflammatory infiltrate and neutrophilic fragments that often are present in LCV, making the 2 entities distinguishable.

Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) lies on a spectrum of severe cutaneous drug reactions involving the skin and mucous membranes. Cutaneous involvement typically begins on the trunk and face and later can involve the palms and soles.9 Similar drugs have been implicated in bullous LCV and SJS/TEN, including nonsteroidal anti-inflammatory drugs and antibiotics. Histologically, SJS/TEN has full-thickness epidermal necrolysis, vacuolar interface, and keratinocyte apoptosis (Figure 4).9 The clinical presentation of sloughing of skin with positive Nikolsky sign, oral involvement, and H&E and DIF findings can help differentiate this entity from bullous LCV.
- Einhorn J, Levis JT. Dermatologic diagnosis: leukocytoclastic vasculitis. Perm J. 2015;19:77-78.
- Davidson KA, Ringpfeil F, Lee JB. Ibuprofen-induced bullous leukocytoclastic vasculitis. Cutis. 2001;67:303-307.
- Lazic T, Fonder M, Robinson-Bostom L, et al. Orlistat-induced bullous leukocytoclastic vasculitis. Cutis. 2013;91:148-149.
- Mericliler M, Shnawa A, Al-Qaysi D, et al. Oxacillin-induced leukocytoclastic vasculitis. IDCases. 2019;17:E00539.
- Bernard P, Antonicelli F. Bullous pemphigoid: a review of its diagnosis, associations and treatment. Am J Clin Dermatol. 2017;18:513-528.
- High WA. Blistering disorders. In: Elston DM, Ferringer T, Ko C, et al, eds. Dermatopathology. 3rd ed. Philadelphia, PA: Elsevier; 2019:161-171.
- Visentainer L, Massuda JY, Cintra ML, et al. Vancomycin-induced linear IgA bullous dermatosis (LABD)--an atypical presentation. Clin Case Rep. 2019;7:1091-1093.
- Hyman DA, Cohen PR. Stasis dermatitis as a complication of recurrent levofloxacin-associated bilateral leg edema. Dermatol Online J. 2013;19:20399.
- Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39.
The Diagnosis: Bullous Leukocytoclastic Vasculitis
Histopathology with hematoxylin and eosin (H&E) stain showed a perivascular neutrophilic infiltrate, karyorrhexis, red blood cell extravasation, and fibrin deposition in the vessel wall (quiz images). Direct immunofluorescence (DIF) showed fibrin surrounding the vasculature, consistent with vasculitis. The clinical and histopathological evaluation supported the diagnosis of bullous leukocytoclastic vasculitis (LCV). The patient had a full LCV workup including antinuclear antibody, rheumatoid factor, hepatitis B and hepatitis C screening, erythrocyte sedimentation rate, C-reactive protein, and C3/C4/total complement level, which were all within reference range. The patient denied that she had taken any medications prior to the onset of the rash. She was started on a 12-day prednisone taper starting at 60 mg, and the rash resolved in 1 week.
Although the incidence of LCV is estimated to be 30 cases per million individuals per year,1 bullous LCV is a rarer entity with only a few cases reported in the literature.2,3 As in our patient's case, up to 50% of LCV cases are idiopathic or the etiology cannot be determined despite laboratory workup and medication review. Other cases can be secondary to medication, infection, collagen vascular disease, or malignancy.3 Despite the exact pathogenesis of bullous LCV being unknown,4 it likely is related to a type III hypersensitivity reaction with immune complex deposition in postcapillary venules leading to endothelial injury, activation of the complement cascade, and development of intraepidermal or subepidermal blister formation depending on location of inflammation and edema.2 Clinically, an intraepidermal split would be more flaccid, similar to pemphigus vulgaris, while a subepidermal split, as in our patient, would be taut bullae. The subepidermal split more commonly is seen in bullous LCV.2
Leukocytoclastic vasculitis on H&E staining characteristically has a perivascular inflammatory infiltrate, neutrophilic fragments called leukocytoclasis, and blood extravasation.3 Extravasated blood presents clinically as petechiae. In this case, the petechiae helped distinguish this entity from the differential diagnosis. Furthermore, DIF would be helpful in distinguishing bullous diseases such as bullous pemphigoid (BP) and pemphigus vulgaris from LCV.2 Direct immunofluorescence in bullous LCV would have fibrinogen surrounding the vasculature without C3 and IgG deposition (intraepidermal or subepidermal).
Mild cases of LCV often resolve with supportive measures including elevation of the legs, ice packs applied to the affected area, and removal of the inciting drug or event.4 In the few cases reported in the literature, bullous LCV presented more diffusely than classic LCV with bullous lesions on the forearms and the lower extremities. Oral steroids are efficacious for extensive bullous LCV.4
The differential diagnosis of bullous LCV includes bullous diseases with subepidermal split including BP and linear IgA bullous dermatosis (LABD). Bullous pemphigoid is an autoimmune subepidermal blistering disease typically affecting patients older than 60 years.5 The pathogenesis of BP is related to development of autoantibodies directed against hemidesmosome components, bullous pemphigoid antigen (BPAG) 1 or BPAG2.5 Bullous pemphigoid presents clinically as widespread, generally pruritic, erythematous, urticarial plaques with bullae. Histologically, BP characteristically has a subepidermal split with superficial dermal edema and eosinophils at the dermoepidermal junction (Figure 1). Direct immunofluorescence confirms the diagnosis with IgG and C3 deposition in an n-serrated pattern at the dermoepidermal junction.6 Bullous pemphigoid can be distinguished from bullous LCV by the older age of presentation, DIF findings, and the absence of purpura.

Linear IgA bullous dermatosis represents a rare subepidermal vesiculobullous disease occurring in patients in their 60s.7 Clinically, this entity presents as tense bullae often located on the periphery of an urticarial plaque, classically called the "string of pearls sign." Histologically, LABD also presents with subepidermal split; however, neutrophils are the predominant cell type vs eosinophils in BP (Figure 2).7 Direct immunofluorescence is specific with a linear deposition of IgA at the dermoepidermal junction. Linear IgA bullous dermatosis most commonly is induced by vancomycin. Unlike bullous LCV, the bullae of LABD have an annular peripheral pattern on an erythematous base and lack purpura.

Stasis dermatitis is inflammation of the dermis due to venous insufficiency that often is present in the bilateral lower extremities. The disorder affects approximately 7% of adults older than 50 years, but it also can occur in younger patients.8 The pathophysiology of stasis dermatitis is caused by edema, which leads to extracellular fluid, plasma proteins, macrophages, and erythrocytes passing into the interstitial space. Patients with stasis dermatitis present with scaly erythematous papules and plaques or edematous blisters on the lower extremities. Diagnosis usually can be made clinically; however, a skin biopsy also can be helpful. Hematoxylin and eosin shows a pauci-inflammatory subepidermal bulla with fibrin (Figure 3).8 The overlying epidermis is intact. The dermis has cannon ball angiomatosis, red blood cell extravasation, and fibrosis typical of stasis dermatitis. Stasis dermatitis with bullae is cell poor and lacks the perivascular inflammatory infiltrate and neutrophilic fragments that often are present in LCV, making the 2 entities distinguishable.

Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) lies on a spectrum of severe cutaneous drug reactions involving the skin and mucous membranes. Cutaneous involvement typically begins on the trunk and face and later can involve the palms and soles.9 Similar drugs have been implicated in bullous LCV and SJS/TEN, including nonsteroidal anti-inflammatory drugs and antibiotics. Histologically, SJS/TEN has full-thickness epidermal necrolysis, vacuolar interface, and keratinocyte apoptosis (Figure 4).9 The clinical presentation of sloughing of skin with positive Nikolsky sign, oral involvement, and H&E and DIF findings can help differentiate this entity from bullous LCV.

The Diagnosis: Bullous Leukocytoclastic Vasculitis
Histopathology with hematoxylin and eosin (H&E) stain showed a perivascular neutrophilic infiltrate, karyorrhexis, red blood cell extravasation, and fibrin deposition in the vessel wall (quiz images). Direct immunofluorescence (DIF) showed fibrin surrounding the vasculature, consistent with vasculitis. The clinical and histopathological evaluation supported the diagnosis of bullous leukocytoclastic vasculitis (LCV). The patient had a full LCV workup including antinuclear antibody, rheumatoid factor, hepatitis B and hepatitis C screening, erythrocyte sedimentation rate, C-reactive protein, and C3/C4/total complement level, which were all within reference range. The patient denied that she had taken any medications prior to the onset of the rash. She was started on a 12-day prednisone taper starting at 60 mg, and the rash resolved in 1 week.
Although the incidence of LCV is estimated to be 30 cases per million individuals per year,1 bullous LCV is a rarer entity with only a few cases reported in the literature.2,3 As in our patient's case, up to 50% of LCV cases are idiopathic or the etiology cannot be determined despite laboratory workup and medication review. Other cases can be secondary to medication, infection, collagen vascular disease, or malignancy.3 Despite the exact pathogenesis of bullous LCV being unknown,4 it likely is related to a type III hypersensitivity reaction with immune complex deposition in postcapillary venules leading to endothelial injury, activation of the complement cascade, and development of intraepidermal or subepidermal blister formation depending on location of inflammation and edema.2 Clinically, an intraepidermal split would be more flaccid, similar to pemphigus vulgaris, while a subepidermal split, as in our patient, would be taut bullae. The subepidermal split more commonly is seen in bullous LCV.2
Leukocytoclastic vasculitis on H&E staining characteristically has a perivascular inflammatory infiltrate, neutrophilic fragments called leukocytoclasis, and blood extravasation.3 Extravasated blood presents clinically as petechiae. In this case, the petechiae helped distinguish this entity from the differential diagnosis. Furthermore, DIF would be helpful in distinguishing bullous diseases such as bullous pemphigoid (BP) and pemphigus vulgaris from LCV.2 Direct immunofluorescence in bullous LCV would have fibrinogen surrounding the vasculature without C3 and IgG deposition (intraepidermal or subepidermal).
Mild cases of LCV often resolve with supportive measures including elevation of the legs, ice packs applied to the affected area, and removal of the inciting drug or event.4 In the few cases reported in the literature, bullous LCV presented more diffusely than classic LCV with bullous lesions on the forearms and the lower extremities. Oral steroids are efficacious for extensive bullous LCV.4
The differential diagnosis of bullous LCV includes bullous diseases with subepidermal split including BP and linear IgA bullous dermatosis (LABD). Bullous pemphigoid is an autoimmune subepidermal blistering disease typically affecting patients older than 60 years.5 The pathogenesis of BP is related to development of autoantibodies directed against hemidesmosome components, bullous pemphigoid antigen (BPAG) 1 or BPAG2.5 Bullous pemphigoid presents clinically as widespread, generally pruritic, erythematous, urticarial plaques with bullae. Histologically, BP characteristically has a subepidermal split with superficial dermal edema and eosinophils at the dermoepidermal junction (Figure 1). Direct immunofluorescence confirms the diagnosis with IgG and C3 deposition in an n-serrated pattern at the dermoepidermal junction.6 Bullous pemphigoid can be distinguished from bullous LCV by the older age of presentation, DIF findings, and the absence of purpura.

Linear IgA bullous dermatosis represents a rare subepidermal vesiculobullous disease occurring in patients in their 60s.7 Clinically, this entity presents as tense bullae often located on the periphery of an urticarial plaque, classically called the "string of pearls sign." Histologically, LABD also presents with subepidermal split; however, neutrophils are the predominant cell type vs eosinophils in BP (Figure 2).7 Direct immunofluorescence is specific with a linear deposition of IgA at the dermoepidermal junction. Linear IgA bullous dermatosis most commonly is induced by vancomycin. Unlike bullous LCV, the bullae of LABD have an annular peripheral pattern on an erythematous base and lack purpura.

Stasis dermatitis is inflammation of the dermis due to venous insufficiency that often is present in the bilateral lower extremities. The disorder affects approximately 7% of adults older than 50 years, but it also can occur in younger patients.8 The pathophysiology of stasis dermatitis is caused by edema, which leads to extracellular fluid, plasma proteins, macrophages, and erythrocytes passing into the interstitial space. Patients with stasis dermatitis present with scaly erythematous papules and plaques or edematous blisters on the lower extremities. Diagnosis usually can be made clinically; however, a skin biopsy also can be helpful. Hematoxylin and eosin shows a pauci-inflammatory subepidermal bulla with fibrin (Figure 3).8 The overlying epidermis is intact. The dermis has cannon ball angiomatosis, red blood cell extravasation, and fibrosis typical of stasis dermatitis. Stasis dermatitis with bullae is cell poor and lacks the perivascular inflammatory infiltrate and neutrophilic fragments that often are present in LCV, making the 2 entities distinguishable.

Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) lies on a spectrum of severe cutaneous drug reactions involving the skin and mucous membranes. Cutaneous involvement typically begins on the trunk and face and later can involve the palms and soles.9 Similar drugs have been implicated in bullous LCV and SJS/TEN, including nonsteroidal anti-inflammatory drugs and antibiotics. Histologically, SJS/TEN has full-thickness epidermal necrolysis, vacuolar interface, and keratinocyte apoptosis (Figure 4).9 The clinical presentation of sloughing of skin with positive Nikolsky sign, oral involvement, and H&E and DIF findings can help differentiate this entity from bullous LCV.

- Einhorn J, Levis JT. Dermatologic diagnosis: leukocytoclastic vasculitis. Perm J. 2015;19:77-78.
- Davidson KA, Ringpfeil F, Lee JB. Ibuprofen-induced bullous leukocytoclastic vasculitis. Cutis. 2001;67:303-307.
- Lazic T, Fonder M, Robinson-Bostom L, et al. Orlistat-induced bullous leukocytoclastic vasculitis. Cutis. 2013;91:148-149.
- Mericliler M, Shnawa A, Al-Qaysi D, et al. Oxacillin-induced leukocytoclastic vasculitis. IDCases. 2019;17:E00539.
- Bernard P, Antonicelli F. Bullous pemphigoid: a review of its diagnosis, associations and treatment. Am J Clin Dermatol. 2017;18:513-528.
- High WA. Blistering disorders. In: Elston DM, Ferringer T, Ko C, et al, eds. Dermatopathology. 3rd ed. Philadelphia, PA: Elsevier; 2019:161-171.
- Visentainer L, Massuda JY, Cintra ML, et al. Vancomycin-induced linear IgA bullous dermatosis (LABD)--an atypical presentation. Clin Case Rep. 2019;7:1091-1093.
- Hyman DA, Cohen PR. Stasis dermatitis as a complication of recurrent levofloxacin-associated bilateral leg edema. Dermatol Online J. 2013;19:20399.
- Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39.
- Einhorn J, Levis JT. Dermatologic diagnosis: leukocytoclastic vasculitis. Perm J. 2015;19:77-78.
- Davidson KA, Ringpfeil F, Lee JB. Ibuprofen-induced bullous leukocytoclastic vasculitis. Cutis. 2001;67:303-307.
- Lazic T, Fonder M, Robinson-Bostom L, et al. Orlistat-induced bullous leukocytoclastic vasculitis. Cutis. 2013;91:148-149.
- Mericliler M, Shnawa A, Al-Qaysi D, et al. Oxacillin-induced leukocytoclastic vasculitis. IDCases. 2019;17:E00539.
- Bernard P, Antonicelli F. Bullous pemphigoid: a review of its diagnosis, associations and treatment. Am J Clin Dermatol. 2017;18:513-528.
- High WA. Blistering disorders. In: Elston DM, Ferringer T, Ko C, et al, eds. Dermatopathology. 3rd ed. Philadelphia, PA: Elsevier; 2019:161-171.
- Visentainer L, Massuda JY, Cintra ML, et al. Vancomycin-induced linear IgA bullous dermatosis (LABD)--an atypical presentation. Clin Case Rep. 2019;7:1091-1093.
- Hyman DA, Cohen PR. Stasis dermatitis as a complication of recurrent levofloxacin-associated bilateral leg edema. Dermatol Online J. 2013;19:20399.
- Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39.
A 30-year-old woman with a medical history of uncontrolled type 2 diabetes mellitus and morbid obesity presented to the dermatology clinic with a painful blistering rash on the lower extremities with scattered red-purple papules of 1 week's duration. The rash began on the left dorsal foot. Physical examination showed nonblanching, 2- to 4-mm, violaceous papules with numerous vesiculopustular bullae on the lower extremities from the dorsal feet to the proximal knee. A shave biopsy with hematoxylin and eosin stain and a punch biopsy for direct immunofluorescence were performed.
Restriction of Foley catheters in older trauma patients improved outcomes
and led to earlier discharge, findings from a study revealed. The results of the study were reported in an abstract scheduled for release at the annual meeting of the American Academy of Orthopaedic Surgeons. The meeting was canceled because of COVID-19.
“We reduced the use of Foley catheters in our target population by more than 50%, which led to a decrease in the rate of hospital-acquired UTI and positively affected other perioperative outcomes,” reported Sanjit R. Konda, MD, an orthopedic surgeon with New York University Langone Health.
The quality initiative was introduced about 2 years ago specifically to reduce the risk of UTI in older patients admitted for femur or hip fractures. Previously at the level 1 trauma center where this quality initiative was introduced, placement of Foley catheters in these types of patients had been routine.
After the policy change, Foley catheters were only offered to these trauma patients 55 years of age or older when more than three episodes or urinary retention had been documented with a bladder scan. Urinary retention was defined as a volume of at least 600 mL.
When outcomes in 184 patients treated in the 15 months after the policy change were compared with 393 treated in the prior 38 months, Foley catheter use was substantially and significantly reduced (43.5% vs. 95.5%; P < .001), Dr. Konda said in an interview.
Although the lower rate of UTI following the policy change fell short of statistical significance (10.33% vs. 14.5%; P = .167), the policy change was associated with a decreased time to surgery (33.27 vs. 38.54 hours; P = .001), shorter length of stay (6.89 vs. 8.34 days; P < .001), and higher rate of home discharge (22.8% vs. 15.6%; P = .038).
When those who avoided a Foley catheter were compared with those who did not after the policy change, there was a significant reduction in UTI (4.81% vs. 17.4%; P = .014). In addition, patients who avoided a Foley catheter had a decreased time to surgery (P = .014), shorter length of stay (P < .001) and an almost 900% greater likelihood of home discharge (odds ratio, 9.9; P < .001).
“This quality initiative does increase the number of bladder scans required, meaning more work for nurses, but the program was developed in collaboration with our nursing staff, who were supportive of the goals,” Dr. Konda reported.
Reducing the incidence of UTI is an important initiative because the Centers for Medicare & Medicaid Services and other third-party payers employ this as a quality metric, according to Dr. Konda. This explains why hospital administrators generally embrace effective strategies to reduce UTI rates.
The improvement in outcomes, including the reduction in UTIs and length of stay, has cost implications, which will be evaluated in a future analysis, according to Dr. Konda.
Although this quality initiative was undertaken in a level 1 trauma center, Dr. Konda believes the same principles can be applied to other settings.
Jennifer A. Meddings, MD, an associate professor of medicine at the University of Michigan, Ann Arbor, agreed. Active in the evaluation of strategies to reduce hospital-acquired complications, Dr. Meddings published a study of procedural appropriateness ratings to guide strategies for improving the likelihood that catheters are employed only when needed (BMJ Qual Saf. 2019;28:56-66).
“In addition to avoiding UTI, reducing unnecessary placement of Foley catheters also eliminates the risk of trauma to the urinary tract,” Dr. Meddings said. This is a complication that is not well appreciated because the trauma is not always documented, according to Dr. Meddings, who believes increased risk of both UTI and urinary tract trauma should discourage use of Foley catheters when there is not a specific indication.
Although there are criteria other than excess bladder volume to determine when to consider a Foley catheter, Dr. Meddings encourages any systematic approach that increases the likelihood that catheters are not placed unnecessarily. She emphasized that a hip fracture by itself “is not a criterion for catheterization.”
Dr. Konda reported a financial relationship with Stryker.
and led to earlier discharge, findings from a study revealed. The results of the study were reported in an abstract scheduled for release at the annual meeting of the American Academy of Orthopaedic Surgeons. The meeting was canceled because of COVID-19.
“We reduced the use of Foley catheters in our target population by more than 50%, which led to a decrease in the rate of hospital-acquired UTI and positively affected other perioperative outcomes,” reported Sanjit R. Konda, MD, an orthopedic surgeon with New York University Langone Health.
The quality initiative was introduced about 2 years ago specifically to reduce the risk of UTI in older patients admitted for femur or hip fractures. Previously at the level 1 trauma center where this quality initiative was introduced, placement of Foley catheters in these types of patients had been routine.
After the policy change, Foley catheters were only offered to these trauma patients 55 years of age or older when more than three episodes or urinary retention had been documented with a bladder scan. Urinary retention was defined as a volume of at least 600 mL.
When outcomes in 184 patients treated in the 15 months after the policy change were compared with 393 treated in the prior 38 months, Foley catheter use was substantially and significantly reduced (43.5% vs. 95.5%; P < .001), Dr. Konda said in an interview.
Although the lower rate of UTI following the policy change fell short of statistical significance (10.33% vs. 14.5%; P = .167), the policy change was associated with a decreased time to surgery (33.27 vs. 38.54 hours; P = .001), shorter length of stay (6.89 vs. 8.34 days; P < .001), and higher rate of home discharge (22.8% vs. 15.6%; P = .038).
When those who avoided a Foley catheter were compared with those who did not after the policy change, there was a significant reduction in UTI (4.81% vs. 17.4%; P = .014). In addition, patients who avoided a Foley catheter had a decreased time to surgery (P = .014), shorter length of stay (P < .001) and an almost 900% greater likelihood of home discharge (odds ratio, 9.9; P < .001).
“This quality initiative does increase the number of bladder scans required, meaning more work for nurses, but the program was developed in collaboration with our nursing staff, who were supportive of the goals,” Dr. Konda reported.
Reducing the incidence of UTI is an important initiative because the Centers for Medicare & Medicaid Services and other third-party payers employ this as a quality metric, according to Dr. Konda. This explains why hospital administrators generally embrace effective strategies to reduce UTI rates.
The improvement in outcomes, including the reduction in UTIs and length of stay, has cost implications, which will be evaluated in a future analysis, according to Dr. Konda.
Although this quality initiative was undertaken in a level 1 trauma center, Dr. Konda believes the same principles can be applied to other settings.
Jennifer A. Meddings, MD, an associate professor of medicine at the University of Michigan, Ann Arbor, agreed. Active in the evaluation of strategies to reduce hospital-acquired complications, Dr. Meddings published a study of procedural appropriateness ratings to guide strategies for improving the likelihood that catheters are employed only when needed (BMJ Qual Saf. 2019;28:56-66).
“In addition to avoiding UTI, reducing unnecessary placement of Foley catheters also eliminates the risk of trauma to the urinary tract,” Dr. Meddings said. This is a complication that is not well appreciated because the trauma is not always documented, according to Dr. Meddings, who believes increased risk of both UTI and urinary tract trauma should discourage use of Foley catheters when there is not a specific indication.
Although there are criteria other than excess bladder volume to determine when to consider a Foley catheter, Dr. Meddings encourages any systematic approach that increases the likelihood that catheters are not placed unnecessarily. She emphasized that a hip fracture by itself “is not a criterion for catheterization.”
Dr. Konda reported a financial relationship with Stryker.
and led to earlier discharge, findings from a study revealed. The results of the study were reported in an abstract scheduled for release at the annual meeting of the American Academy of Orthopaedic Surgeons. The meeting was canceled because of COVID-19.
“We reduced the use of Foley catheters in our target population by more than 50%, which led to a decrease in the rate of hospital-acquired UTI and positively affected other perioperative outcomes,” reported Sanjit R. Konda, MD, an orthopedic surgeon with New York University Langone Health.
The quality initiative was introduced about 2 years ago specifically to reduce the risk of UTI in older patients admitted for femur or hip fractures. Previously at the level 1 trauma center where this quality initiative was introduced, placement of Foley catheters in these types of patients had been routine.
After the policy change, Foley catheters were only offered to these trauma patients 55 years of age or older when more than three episodes or urinary retention had been documented with a bladder scan. Urinary retention was defined as a volume of at least 600 mL.
When outcomes in 184 patients treated in the 15 months after the policy change were compared with 393 treated in the prior 38 months, Foley catheter use was substantially and significantly reduced (43.5% vs. 95.5%; P < .001), Dr. Konda said in an interview.
Although the lower rate of UTI following the policy change fell short of statistical significance (10.33% vs. 14.5%; P = .167), the policy change was associated with a decreased time to surgery (33.27 vs. 38.54 hours; P = .001), shorter length of stay (6.89 vs. 8.34 days; P < .001), and higher rate of home discharge (22.8% vs. 15.6%; P = .038).
When those who avoided a Foley catheter were compared with those who did not after the policy change, there was a significant reduction in UTI (4.81% vs. 17.4%; P = .014). In addition, patients who avoided a Foley catheter had a decreased time to surgery (P = .014), shorter length of stay (P < .001) and an almost 900% greater likelihood of home discharge (odds ratio, 9.9; P < .001).
“This quality initiative does increase the number of bladder scans required, meaning more work for nurses, but the program was developed in collaboration with our nursing staff, who were supportive of the goals,” Dr. Konda reported.
Reducing the incidence of UTI is an important initiative because the Centers for Medicare & Medicaid Services and other third-party payers employ this as a quality metric, according to Dr. Konda. This explains why hospital administrators generally embrace effective strategies to reduce UTI rates.
The improvement in outcomes, including the reduction in UTIs and length of stay, has cost implications, which will be evaluated in a future analysis, according to Dr. Konda.
Although this quality initiative was undertaken in a level 1 trauma center, Dr. Konda believes the same principles can be applied to other settings.
Jennifer A. Meddings, MD, an associate professor of medicine at the University of Michigan, Ann Arbor, agreed. Active in the evaluation of strategies to reduce hospital-acquired complications, Dr. Meddings published a study of procedural appropriateness ratings to guide strategies for improving the likelihood that catheters are employed only when needed (BMJ Qual Saf. 2019;28:56-66).
“In addition to avoiding UTI, reducing unnecessary placement of Foley catheters also eliminates the risk of trauma to the urinary tract,” Dr. Meddings said. This is a complication that is not well appreciated because the trauma is not always documented, according to Dr. Meddings, who believes increased risk of both UTI and urinary tract trauma should discourage use of Foley catheters when there is not a specific indication.
Although there are criteria other than excess bladder volume to determine when to consider a Foley catheter, Dr. Meddings encourages any systematic approach that increases the likelihood that catheters are not placed unnecessarily. She emphasized that a hip fracture by itself “is not a criterion for catheterization.”
Dr. Konda reported a financial relationship with Stryker.
FROM AAOS 2020