User login
Parenting special needs children: An unlikely model
COVID-19 can give physicians a window into lives of families
The last few months have tested the stamina of most families. Many people are struggling to keep some semblance of normalcy amid a radical transformation of everyday life. It seems as if everything changed overnight.
In a similar way, when a child with many needs is born into a family, adjustments also have to take place to receive the new baby. Families are, in most cases, not prepared for what is to come. Their expectations usually are not in sync with how their lives end up. They are crunched for time. They need to adjust, and at the same time, they mourn the loss of their previous less demanding lifestyle. More importantly, these parents learn that this might be an adjustment that they might need to make for a long time – in some instances, for a lifetime.
Stress load over time can correlate with a sense of burnout, and mental health professionals need to be prepared to address these issues in our patients.
Here is a list of some chronic struggles with which many special needs parents must contend. These strongly resemble the challenges parents in the general population have been facing with their families during this pandemic:
- Bypassing breaks to unwind and having to be always “on” while at home: These parents take care of children who need to be chronically tube fed, can’t sleep well at night because they are often sick, have recurrent seizures or maladaptive behaviors that affect the caretakers and the rest of the family. For parents of children who are on the autism spectrum, these challenges can be a constant struggle. Almost 60% of children with autism spectrum disorder (ASD) experience bodily difficulties, such as trouble breathing. However, nearly 100% of children with ASD experienced difficulties with their abilities and activities, such as self-care tasks like eating and dressing, and emotional or behavioral health, according to a 2016 report on child and adolescent health by the Johns Hopkins Bloomberg School of Public Health.
- Taking on roles for which they are not trained: Parents may take on active roles supplementing their developmentally delayed children with educational experiences or therapeutic modalities in their own homes given that the needs might be too great to just rely on the school or therapy time. There are about 1.17 million children in the United States living with ASD and more than 12% of children with ASD have severe cases, the Hopkins report said. Parents frequently are forced to take on the role of “therapist” to meet the needs of their child.
- Staying home often: Some parents are unable to have a “regular sitter” to provide respite, because the needs of the child require a higher level of care, training, and consideration. Caring for a special child means parents often don’t have the option of leaving their older child alone. As a result, they may end up spending more time at home than their counterpart parents with children who are the same age.
- Struggling to meet everyone’s demands for attention while at home: The child might require full-time attention or prolonged hospitalizations, and the needs of other siblings are sometimes put on hold until time or energy are available for all.
- Not traveling unless absolutely necessary: Families have a hard time leaving home for vacations or for other reasons. They may have to travel with medical supplies and equipment. They need to make sure that their destination is ready to welcome their child with all needs taken into consideration (special diets, activities, and facilities). Will the vacation set them back because it might take more effort to go than to stay home?
- Avoiding unnecessary exposures: Trying to avoid infections (even the ones that may be innocuous to others) if their child is immunocompromised. These children may readily decompensate and end up hospitalized with a more serious medical complication.
- Being very aware of remaining physically distant from others: Parents must go to great lengths not to impinge on other people’s space if the child is being loud or moving in a disruptive way, or if other people negatively affect how the child responds. Some families are apprehensive because they have felt judged by others when they are in the community, restaurants, or other places of gathering.
- Feeling concerned about having the right food, medicines, and supplements in the house: Parents are constantly trying to fulfill special dietary requirements and have the reserve to make sure that all meals and treatments are accounted for in the near future. They might need oxygen or specialized formulas that are hard to find in local stores. Some treatments, when withdrawn or unavailable, can prove life threatening.
- Restricting social circles: Some families with children with severe autism may self-isolate when they feel it is hard to be around them and be friends with them, since they can’t readily participate in “usual family activities,” and the regular norms of socialization can’t apply to their family’s set of behaviors. Their child might seem to be disruptive, or loud, nonverbal, mute, or unable to easily relate to others.
- Experiencing a pervasive sense of uncertainty about the future: A child might continue to miss milestones, or might have a rare condition that hasn’t been diagnosed. When thinking of the future, parents can’t predict what level of care they need to plan and budget for.
- Being concerned about dying early and not being able to provide for their child: Parents worry about who would take care of their child for life. Who would take care of their aging adult “child” after parents are gone? They might have concerns about having a will in place early on.
- Facing financial stress secondary to losing a job or the cost of treatments: Absenteeism might be the end result of having to care for their child’s ongoing needs, appointments, and medical emergencies. Sometimes, they might depend on a caretaker who might be very difficult to replace. It might take extensive training once a candidate is found. Direct costs include medical care, hospitalizations, special education, special therapies (occupational, speech, and physical therapy), and paid caregivers. Indirect costs include lost productivity for family caregivers because of the inability to maintain employment while caring for affected individuals, as well as lost wages and benefits, the Hopkins report said.
- Struggling to coordinate daily schedules: Parents face this challenge not only with young children but with those who are chronically ill and might need ongoing 24/7 care. The schedule might include educational and therapeutic (physical, occupational, speech, language therapy, recreational) interventions regularly or daily. This schedule is to be superimposed on all the other necessary responsibilities parents already have to contend with. Forty-eight percent of school-aged children with ASD use three or more services. In addition, children with moderate or severe cases of ASD used three or more services at almost twice the rate of children with mild cases of ASD (60% vs. 35%).
- Longing for a cure or a medicine that will improve the outcome: Often, parents search for treatments so that their child could live a more comfortable or healthier life. For children who have a rare condition, there may not be sufficient research dedicated to their cause or diagnostic pursuits. Currently, it is estimated that 1 in 10 Americans has a rare disease – about 80% of which are genetically based. Of the nearly 7,000 rare diseases known to exist, less than 500 – roughly 5% – have a known treatment approved by the U.S. Food and Drug Administration, reports the National Center for Advancing Translational Diseases and the Genetic and Rare Diseases Information Center.
- Hoping for better times to come: It is difficult at times to appreciate the present when it happens to be so chronically challenging and exhausting for everyone.
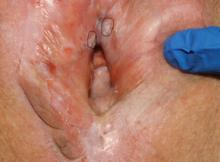
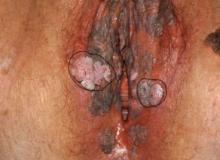
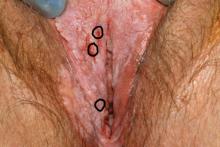
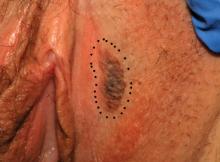
Parents of children with significant special needs experience many hurdles that they learn to endure, overcome, and master. This pandemic can provide physicians with a window into the lives of these families.
Dr. Sotir is a psychiatrist in private practice in Wheaton, Ill. As a parent of three children, one with special needs, she has extensive experience helping parents challenged by having special needs children find balance, support, direction, and joy in all dimensions of individual and family life. This area is the focus of her practice and public speaking. In Part 2, she will explore how psychiatrists as a specialty can support these families. She has no disclosures.
COVID-19 can give physicians a window into lives of families
COVID-19 can give physicians a window into lives of families
The last few months have tested the stamina of most families. Many people are struggling to keep some semblance of normalcy amid a radical transformation of everyday life. It seems as if everything changed overnight.
In a similar way, when a child with many needs is born into a family, adjustments also have to take place to receive the new baby. Families are, in most cases, not prepared for what is to come. Their expectations usually are not in sync with how their lives end up. They are crunched for time. They need to adjust, and at the same time, they mourn the loss of their previous less demanding lifestyle. More importantly, these parents learn that this might be an adjustment that they might need to make for a long time – in some instances, for a lifetime.
Stress load over time can correlate with a sense of burnout, and mental health professionals need to be prepared to address these issues in our patients.
Here is a list of some chronic struggles with which many special needs parents must contend. These strongly resemble the challenges parents in the general population have been facing with their families during this pandemic:
- Bypassing breaks to unwind and having to be always “on” while at home: These parents take care of children who need to be chronically tube fed, can’t sleep well at night because they are often sick, have recurrent seizures or maladaptive behaviors that affect the caretakers and the rest of the family. For parents of children who are on the autism spectrum, these challenges can be a constant struggle. Almost 60% of children with autism spectrum disorder (ASD) experience bodily difficulties, such as trouble breathing. However, nearly 100% of children with ASD experienced difficulties with their abilities and activities, such as self-care tasks like eating and dressing, and emotional or behavioral health, according to a 2016 report on child and adolescent health by the Johns Hopkins Bloomberg School of Public Health.
- Taking on roles for which they are not trained: Parents may take on active roles supplementing their developmentally delayed children with educational experiences or therapeutic modalities in their own homes given that the needs might be too great to just rely on the school or therapy time. There are about 1.17 million children in the United States living with ASD and more than 12% of children with ASD have severe cases, the Hopkins report said. Parents frequently are forced to take on the role of “therapist” to meet the needs of their child.
- Staying home often: Some parents are unable to have a “regular sitter” to provide respite, because the needs of the child require a higher level of care, training, and consideration. Caring for a special child means parents often don’t have the option of leaving their older child alone. As a result, they may end up spending more time at home than their counterpart parents with children who are the same age.
- Struggling to meet everyone’s demands for attention while at home: The child might require full-time attention or prolonged hospitalizations, and the needs of other siblings are sometimes put on hold until time or energy are available for all.
- Not traveling unless absolutely necessary: Families have a hard time leaving home for vacations or for other reasons. They may have to travel with medical supplies and equipment. They need to make sure that their destination is ready to welcome their child with all needs taken into consideration (special diets, activities, and facilities). Will the vacation set them back because it might take more effort to go than to stay home?
- Avoiding unnecessary exposures: Trying to avoid infections (even the ones that may be innocuous to others) if their child is immunocompromised. These children may readily decompensate and end up hospitalized with a more serious medical complication.
- Being very aware of remaining physically distant from others: Parents must go to great lengths not to impinge on other people’s space if the child is being loud or moving in a disruptive way, or if other people negatively affect how the child responds. Some families are apprehensive because they have felt judged by others when they are in the community, restaurants, or other places of gathering.
- Feeling concerned about having the right food, medicines, and supplements in the house: Parents are constantly trying to fulfill special dietary requirements and have the reserve to make sure that all meals and treatments are accounted for in the near future. They might need oxygen or specialized formulas that are hard to find in local stores. Some treatments, when withdrawn or unavailable, can prove life threatening.
- Restricting social circles: Some families with children with severe autism may self-isolate when they feel it is hard to be around them and be friends with them, since they can’t readily participate in “usual family activities,” and the regular norms of socialization can’t apply to their family’s set of behaviors. Their child might seem to be disruptive, or loud, nonverbal, mute, or unable to easily relate to others.
- Experiencing a pervasive sense of uncertainty about the future: A child might continue to miss milestones, or might have a rare condition that hasn’t been diagnosed. When thinking of the future, parents can’t predict what level of care they need to plan and budget for.
- Being concerned about dying early and not being able to provide for their child: Parents worry about who would take care of their child for life. Who would take care of their aging adult “child” after parents are gone? They might have concerns about having a will in place early on.
- Facing financial stress secondary to losing a job or the cost of treatments: Absenteeism might be the end result of having to care for their child’s ongoing needs, appointments, and medical emergencies. Sometimes, they might depend on a caretaker who might be very difficult to replace. It might take extensive training once a candidate is found. Direct costs include medical care, hospitalizations, special education, special therapies (occupational, speech, and physical therapy), and paid caregivers. Indirect costs include lost productivity for family caregivers because of the inability to maintain employment while caring for affected individuals, as well as lost wages and benefits, the Hopkins report said.
- Struggling to coordinate daily schedules: Parents face this challenge not only with young children but with those who are chronically ill and might need ongoing 24/7 care. The schedule might include educational and therapeutic (physical, occupational, speech, language therapy, recreational) interventions regularly or daily. This schedule is to be superimposed on all the other necessary responsibilities parents already have to contend with. Forty-eight percent of school-aged children with ASD use three or more services. In addition, children with moderate or severe cases of ASD used three or more services at almost twice the rate of children with mild cases of ASD (60% vs. 35%).
- Longing for a cure or a medicine that will improve the outcome: Often, parents search for treatments so that their child could live a more comfortable or healthier life. For children who have a rare condition, there may not be sufficient research dedicated to their cause or diagnostic pursuits. Currently, it is estimated that 1 in 10 Americans has a rare disease – about 80% of which are genetically based. Of the nearly 7,000 rare diseases known to exist, less than 500 – roughly 5% – have a known treatment approved by the U.S. Food and Drug Administration, reports the National Center for Advancing Translational Diseases and the Genetic and Rare Diseases Information Center.
- Hoping for better times to come: It is difficult at times to appreciate the present when it happens to be so chronically challenging and exhausting for everyone.
Parents of children with significant special needs experience many hurdles that they learn to endure, overcome, and master. This pandemic can provide physicians with a window into the lives of these families.
Dr. Sotir is a psychiatrist in private practice in Wheaton, Ill. As a parent of three children, one with special needs, she has extensive experience helping parents challenged by having special needs children find balance, support, direction, and joy in all dimensions of individual and family life. This area is the focus of her practice and public speaking. In Part 2, she will explore how psychiatrists as a specialty can support these families. She has no disclosures.
The last few months have tested the stamina of most families. Many people are struggling to keep some semblance of normalcy amid a radical transformation of everyday life. It seems as if everything changed overnight.
In a similar way, when a child with many needs is born into a family, adjustments also have to take place to receive the new baby. Families are, in most cases, not prepared for what is to come. Their expectations usually are not in sync with how their lives end up. They are crunched for time. They need to adjust, and at the same time, they mourn the loss of their previous less demanding lifestyle. More importantly, these parents learn that this might be an adjustment that they might need to make for a long time – in some instances, for a lifetime.
Stress load over time can correlate with a sense of burnout, and mental health professionals need to be prepared to address these issues in our patients.
Here is a list of some chronic struggles with which many special needs parents must contend. These strongly resemble the challenges parents in the general population have been facing with their families during this pandemic:
- Bypassing breaks to unwind and having to be always “on” while at home: These parents take care of children who need to be chronically tube fed, can’t sleep well at night because they are often sick, have recurrent seizures or maladaptive behaviors that affect the caretakers and the rest of the family. For parents of children who are on the autism spectrum, these challenges can be a constant struggle. Almost 60% of children with autism spectrum disorder (ASD) experience bodily difficulties, such as trouble breathing. However, nearly 100% of children with ASD experienced difficulties with their abilities and activities, such as self-care tasks like eating and dressing, and emotional or behavioral health, according to a 2016 report on child and adolescent health by the Johns Hopkins Bloomberg School of Public Health.
- Taking on roles for which they are not trained: Parents may take on active roles supplementing their developmentally delayed children with educational experiences or therapeutic modalities in their own homes given that the needs might be too great to just rely on the school or therapy time. There are about 1.17 million children in the United States living with ASD and more than 12% of children with ASD have severe cases, the Hopkins report said. Parents frequently are forced to take on the role of “therapist” to meet the needs of their child.
- Staying home often: Some parents are unable to have a “regular sitter” to provide respite, because the needs of the child require a higher level of care, training, and consideration. Caring for a special child means parents often don’t have the option of leaving their older child alone. As a result, they may end up spending more time at home than their counterpart parents with children who are the same age.
- Struggling to meet everyone’s demands for attention while at home: The child might require full-time attention or prolonged hospitalizations, and the needs of other siblings are sometimes put on hold until time or energy are available for all.
- Not traveling unless absolutely necessary: Families have a hard time leaving home for vacations or for other reasons. They may have to travel with medical supplies and equipment. They need to make sure that their destination is ready to welcome their child with all needs taken into consideration (special diets, activities, and facilities). Will the vacation set them back because it might take more effort to go than to stay home?
- Avoiding unnecessary exposures: Trying to avoid infections (even the ones that may be innocuous to others) if their child is immunocompromised. These children may readily decompensate and end up hospitalized with a more serious medical complication.
- Being very aware of remaining physically distant from others: Parents must go to great lengths not to impinge on other people’s space if the child is being loud or moving in a disruptive way, or if other people negatively affect how the child responds. Some families are apprehensive because they have felt judged by others when they are in the community, restaurants, or other places of gathering.
- Feeling concerned about having the right food, medicines, and supplements in the house: Parents are constantly trying to fulfill special dietary requirements and have the reserve to make sure that all meals and treatments are accounted for in the near future. They might need oxygen or specialized formulas that are hard to find in local stores. Some treatments, when withdrawn or unavailable, can prove life threatening.
- Restricting social circles: Some families with children with severe autism may self-isolate when they feel it is hard to be around them and be friends with them, since they can’t readily participate in “usual family activities,” and the regular norms of socialization can’t apply to their family’s set of behaviors. Their child might seem to be disruptive, or loud, nonverbal, mute, or unable to easily relate to others.
- Experiencing a pervasive sense of uncertainty about the future: A child might continue to miss milestones, or might have a rare condition that hasn’t been diagnosed. When thinking of the future, parents can’t predict what level of care they need to plan and budget for.
- Being concerned about dying early and not being able to provide for their child: Parents worry about who would take care of their child for life. Who would take care of their aging adult “child” after parents are gone? They might have concerns about having a will in place early on.
- Facing financial stress secondary to losing a job or the cost of treatments: Absenteeism might be the end result of having to care for their child’s ongoing needs, appointments, and medical emergencies. Sometimes, they might depend on a caretaker who might be very difficult to replace. It might take extensive training once a candidate is found. Direct costs include medical care, hospitalizations, special education, special therapies (occupational, speech, and physical therapy), and paid caregivers. Indirect costs include lost productivity for family caregivers because of the inability to maintain employment while caring for affected individuals, as well as lost wages and benefits, the Hopkins report said.
- Struggling to coordinate daily schedules: Parents face this challenge not only with young children but with those who are chronically ill and might need ongoing 24/7 care. The schedule might include educational and therapeutic (physical, occupational, speech, language therapy, recreational) interventions regularly or daily. This schedule is to be superimposed on all the other necessary responsibilities parents already have to contend with. Forty-eight percent of school-aged children with ASD use three or more services. In addition, children with moderate or severe cases of ASD used three or more services at almost twice the rate of children with mild cases of ASD (60% vs. 35%).
- Longing for a cure or a medicine that will improve the outcome: Often, parents search for treatments so that their child could live a more comfortable or healthier life. For children who have a rare condition, there may not be sufficient research dedicated to their cause or diagnostic pursuits. Currently, it is estimated that 1 in 10 Americans has a rare disease – about 80% of which are genetically based. Of the nearly 7,000 rare diseases known to exist, less than 500 – roughly 5% – have a known treatment approved by the U.S. Food and Drug Administration, reports the National Center for Advancing Translational Diseases and the Genetic and Rare Diseases Information Center.
- Hoping for better times to come: It is difficult at times to appreciate the present when it happens to be so chronically challenging and exhausting for everyone.
Parents of children with significant special needs experience many hurdles that they learn to endure, overcome, and master. This pandemic can provide physicians with a window into the lives of these families.
Dr. Sotir is a psychiatrist in private practice in Wheaton, Ill. As a parent of three children, one with special needs, she has extensive experience helping parents challenged by having special needs children find balance, support, direction, and joy in all dimensions of individual and family life. This area is the focus of her practice and public speaking. In Part 2, she will explore how psychiatrists as a specialty can support these families. She has no disclosures.
Telemedicine: Common hurdles and proper coding for ObGyns
Since the COVID-19 pandemic began, many significant changes have occurred that have made the implementation of telemedicine easier and more attractive for gynecologic practices. In the first article in this series, we discussed the benefits of telemedicine to physicians and patients, how to get started using telemedicine, and implementing a workflow. This article will discuss the common hurdles in the process and the proper coding to use to insure reimbursement for services rendered.
Barriers to implementing telemedicine
Incorrect assumptions
Latecomers to telemedicine often assume that patients prefer face-to-face visits when, in fact, many may prefer the convenience of virtual visits. More than 50% of patients who are surveyed about their experience with telemedicine say that online tools have helped improve their relationship with their providers.1 Telemedicine has grown astronomically during the COVID-19 pandemic to the point where many patients now expect their health care providers to be able to conduct virtual visits. Practices that do not offer telemedicine may find their patients seeking services elsewhere. Nearly two-thirds of health care professionals expect their commitment to telemedicine to increase significantly in the next 3 years.2 Of those providers who have not yet adopted the practice, nearly 85% expect to implement telemedicine in the near future.3 COVID-19 has motivated the increased use of telemedicine to enhance the communication with patients, making it possible for patients to have enhanced access to health care during this pandemic while minimizing infectious transmission of COVID-19 to physicians and their staff.4
Admittedly, telemedicine is not appropriate for all patients. In general, situations that do not lend themselves to telemedicine are those for which an in-person visit is required to evaluate the patient via a physical examination, to perform a protocol-driven procedure, or provide an aggressive intervention. Additional patients for whom telemedicine may be inappropriate include those with cognitive disorders, those with language barriers, those with emergency situations that warrant an office visit or a visit to the emergency department, and patients who do not have access to the technology to conduct a virtual visit.
Cost and complexity
The process of implementing electronic health records (EHRs) left a bitter taste in the mouths of many health care professionals. But EHRs are complicated and expensive. Implementation often resulted in lost productivity. Because the learning curve was so steep, many physicians had to decrease the number of patients they saw before becoming comfortable with the conversion from paper charts to an EHR.
Telemedicine implementation is much less onerous and expensive. Telemedicine is available as a cloud-based platform, which requires less information technology (IT) support and less hardware and software. The technology required for patients to participate in telemedicine is nearly ubiquitous. According to the Pew Research Center, 96% of Americans own a cell phone (81% have a smart phone), and more than half (52%) own a tablet, so the basic equipment to connect patients to providers is already in place.5
On the provider side, the basic equipment required for a telemedicine program is a computer with video and audio capabilities and a broadband connection that is fast enough to show video in real time and to provide high-quality viewing of any images to be reviewed.
The growth in telemedicine means that telemedicine options are now more diverse, with many more affordable solutions. However, most telemedicine programs do require the purchase and set-up of new technology and equipment and the training of staff—some of which may be outside the budgets of health care providers in smaller independent practices. Many gynecologists have technology budgets that are already stretched thin. And for patients who do not have access to a smartphone or computer with Internet access, real-time telemedicine may be out of reach.
But with new guidelines put forth by the Centers for Medicare and Medicaid Services (CMS) in March 2020, connectivity can take place inexpensively using free platforms such as Google Hangouts, Skype, Facetime, and Facebook Messenger. If a non‒HIPAA-compliant platform is used initially, conversion to a HIPAA-compliant platform is recommended.6 These platforms do not require the purchase of, or subscription to, any expensive hardware or software. The disadvantages of these programs are the lack of documentation, the failure to be Health Insurance Portability and Accountability Act (HIPAA)-compliant, and the lack of encryption; however, these disadvantages are no longer an issue after the new CMS guidelines.
Depending on the magnitude of the program, IT assistance may be needed to get started. It is imperative that the telemedicine program is interoperable with the EHR and the billing program. Otherwise, double and triple entry will erase the efficiency provided by conducting a virtual visit.
Continue to: Licensing...
Licensing
Another concern or barrier is a license to participate in telemedicine. The March 15, 2020, approval of telemedicine states that physicians who are licensed in the state where the patient is located do not require any additional license or permission to conduct virtual visits.7 CMS has temporarily waived the requirement that out-of-state providers be licensed in the state where they are providing services when they are licensed in another state. For questions regarding licensure, contact your State Board of Medicine or Department of Health for information on requirements for licenses across state lines (see “Resources,” at the end of the article).
Informed consent
Just like with any other aspect of providing care for patients, obtaining informed consent is paramount. Not only is getting informed patient consent a recommended best practice of the American Telemedicine Association (ATA), but it is actually a legal requirement in many states and could be a condition of getting paid, depending on the payer. To check the requirements regarding patient consent in your state, look at The National Telehealth Policy Resource Center’s state map (see “Resources.")
Some states do not have any requirements regarding consent for a virtual visit. Others require verbal consent. Even if it is not a legal requirement in your state, consider making it a part of your practice’s policy to obtain written or verbal consent and to document in the patient’s record that consent was obtained prior to the virtual visit so that you are protected when using this new technology.
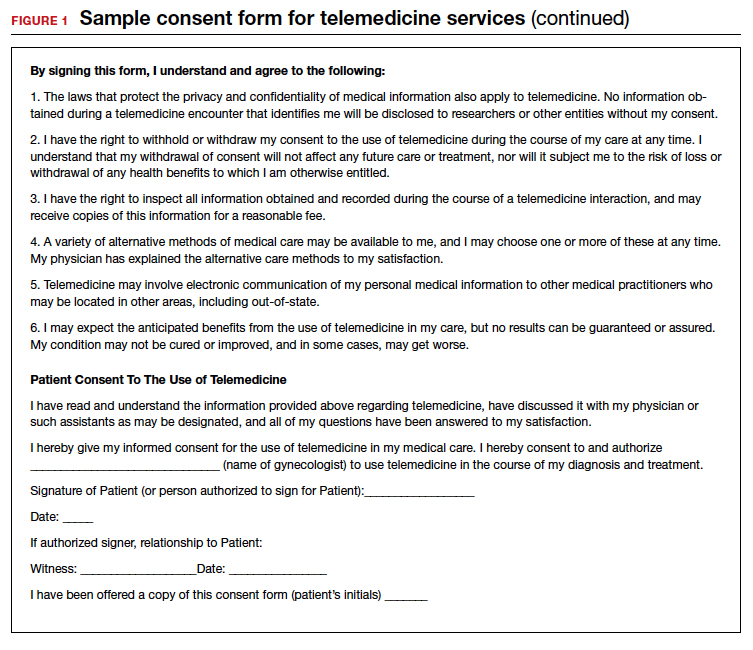
Because telemedicine is a new way of receiving care for many patients, it is important to let them know how it works including how patient confidentiality and privacy are handled, what technical equipment is required, and what they should expect in terms of scheduling, cancellations, and billing policies. A sample consent form for telemedicine use is shown in FIGURE 1.

Liability insurance
Another hurdle that must be considered is liability insurance for conducting virtual visits with patients. Gynecologists who are going to offer telemedicine care to patients should request proof in writing that their liability insurance policy covers telemedicine malpractice and that the coverage extends to other states should the patient be in another state from the state in which the gynecologist holds a license. Additionally, gynecologists who provide telemedicine care should check with liability insurers regarding any requirements or limitations to conducting a virtual visit with their patients and should document them. For example, the policy may require that the physician keep a written or recorded record of the visit in the EHR. If that is the case, then using Skype, Facebook, or Google for the virtual visit, which do not include documentation, would be less desirable.
Privacy
Certainly, there is concern about privacy, and HIPAA compliance is critical to telemedicine success. Because of the COVID-19 emergency, as of March 1, 2020, physicians may now communicate with patients, and provide telehealth services, through remote communications without penalties.8 With these changes in the HIPAA requirements, physicians may use applications that allow for video chats, including Apple FaceTime, Facebook Messenger video chat, Google Hangouts video, and Skype, to provide telehealth without risk that the Office for Civil Rights will impose a penalty for noncompliance with HIPAA rules. The consent for patients should mention that these “public” applications potentially introduce privacy risks. This is a motivation for gynecologists to consider one of the programs that promises encryption, privacy, and HIPAA compliance, such as Updox, Doxy.me, and Amazon Chime. It is also important to recognize that a virtual visit could result in colleagues (if the patient is in an office setting) or family members (if the patient is in the home environment) overhearing conversations between the health care professional and the patient. Therefore, we suggest that patients conduct virtual visits in locations in which they feel assured of some semblance of privacy.
Continue to: Compensation for telemedicine...
Compensation for telemedicine
Perhaps the biggest barrier to virtual health adoption has been compensation for telemedicine visits. Both commercial payers and CMS have been slow to enact formal policies for telemedicine reimbursement. Because of this, the common misconceptions (that providers cannot be reimbursed for telemedicine appointments or that compensation occurs at a reduced rate) have persisted, making telemedicine economically unappealing.
The good news is that this is changing; legislation in most states is quickly embracing virtual health visits as a result of the COVID-19 pandemic.9 In fact, as of January 1, 2020, telemedicine services are no longer considered “optional” coverage in Medicare Advantage plans.10 Nor are they required to have an additional fee. Instead, CMS now allows telemedicine as a standard, covered benefit in all plans, enabling beneficiaries to seek care from their homes rather than requiring them to go to a health care facility.11 In the past, telemedicine was restricted for use in rural areas or when patients resided a great distance from their health care providers. Starting March 6, 2020, and for the duration of the COVID-19 public health emergency, Medicare will make payment for professional services furnished to beneficiaries in all areas of the country in all settings regardless of location or distance between the patient and the health care provider.12
In addition, since March 15, 2020, CMS has expanded access to telemedicine services for all Medicare beneficiaries—not just those who have been diagnosed with COVID-19.13 The expanded access also applies to pre-COVID-19 coverage from physician offices, skilled nursing facilities, and hospitals. This means that Medicare will now make payments to physicians for telemedicine services provided in any health care facility or in a patient’s home, so that patients do not need to go to the physician’s office.
The facts are that there are parity laws and that commercial payers and CMS are required by state law to reimburse for telemedicine—often at the same rate as that for a comparable in-person visit. On the commercial side, there has been an increase in commercial parity legislation that requires health plans to cover virtual visits in the same way they cover face-to-face services. With the new guidelines for reimbursement, every state and Washington DC has parity laws in place. (To stay abreast of state-by-state changes in virtual health reimbursement, the Center for Connected Health Policy and the Advisory Board Primer are valuable resources. See “Resources.”) As long as the provider performs and documents the elements of history and decision-making, including the time spent counseling, and documents the visit as if a face-to-face visit occurred, then clinicians have a billable evaluation and management (E&M) visit.
Continue to: Virtual services for Medicare patients...
Virtual services for Medicare patients
There are 3 main types of virtual services gynecologists can provide to Medicare patients: Medicare telehealth visits, virtual check-ins, and e-visits.
Medicare telehealth visits. Largely because of the COVID-19 pandemic, Medicare patients may now use telecommunication technology for any services that previously occurred in an in-person communication. The gynecologist must use an interactive audio and video telecommunications system that permits real-time communication between the physician and the patient, and the patient should have a prior established relationship with the gynecologist with whom the telemedicine visit is taking place. The new guidelines indicate that the US Department of Health and Human Services (HHS) will not conduct audits to ensure that such a prior relationship exists for claims submitted during this public health emergency.14
The Current Procedural Terminology (CPT) codes for virtual visits using synchronous audio/visual communication are:
- 99201-99295, Office visit for a new patient
- 99211-99215, Office visit for an established patient.
Important modifiers for telemedicine visits include:
- modifier 02 for POS (place of service) for telehealth Medicare
- modifier 95 for commercial payers.
(A list of all available CPT codes for telehealth services from CMS can be found in “Resources.”)
Virtual check-ins. Established Medicare patients may have a brief communication with gynecologists the traditional way using a telephone or via live video. These brief virtual services, usually 5 to 10 minutes in duration, are initiated by the patient. The purpose of the virtual check-in is to determine if an office visit or a test or procedure is indicated.
Medicare pays for these “virtual check-ins” (or brief communication technology-based services) for patients to communicate with their physicians and avoid unnecessary trips to the office. These brief virtual check-ins are only for established patients. If an existing patient contacts the gynecologist’s office to ask a question or determine if an office visit is necessary, the gynecologist may bill for it using code G2012.
E-visits. Established Medicare patients may have non–face-to-face patient-initiated communications with their gynecologists without going to the physician’s office. These services can be billed only when the physician has an established relationship with the patient. The services may be billed using CPT codes 99421 to 99423. Coding for these visits is determined by the length of time the gynecologist spends online with the patient:
- 99421: Online digital evaluation and management service, for an established patient 5 to 10 minutes spent on the virtual visit
- 99422: 11 to 20 minutes
- 99423: ≥ 21 minutes.
Many clinicians want to immediately start the communication process with their patients. Many will avail themselves of the free video communication offered by Google Hangouts, Skype, Facetime, and Facebook Messenger. Since the March 15, 2020, relaxation of the HIPAA restrictions for telemedicine, it is now possible to have a virtual visit with a patient using one of the free, non–HIPAA-compliant connections. This type of visit is no different than a telephone call but with an added video component. Using these free technologies, a gynecologist can have an asynchronous visit with a patient (referred to as the store and forward method of sending information or medical images), which means that the service takes place in one direction with no opportunity for interaction with the patient. Asynchronous visits are akin to video text messages left for the patient. By contrast, a synchronous or real-time video visit with a patient is a 2-way communication that provides medical care without examining the patient.
Using triangulation
There are some downsides to telemedicine visits. First, virtual visits on Skype, FaceTime, and other non–HIPAA-compliant methods are not conducted on an encrypted website. Second, no documentation is created for the doctor-patient encounter. Finally, unless the physician keeps a record of these virtual visits and submits the interactions to the practice coders, there will be no billing and no reimbursement for the visits. In this scenario, physicians are legally responsible for their decision-making, prescription writing, and medical advice, but do not receive compensation for their efforts.
This can be remedied by using “triangulation,” which involves: 1. the physician, 2. the patient, and 3. a scribe or medical assistant who will record the visit. Before initiating the virtual visit using triangulation, it is imperative to ask the patient for permission if your medical assistant (or any other person in the office who functions as a scribe) will be listening to the conversation. It is important to explain that the person is there to take accurate notes and ascertain that the notes are entered into the EHR. Also, the scribe or assistant will record the time, date, and duration of the visit, which is a requirement for billing purposes. The scribe may also ascertain that the visit is properly coded and entered into the practice management system, and that a bill is submitted to the insurance company. By using triangulation, you have documentation that consent was obtained, that the visit took place, that notes were taken, and that the patient’s insurance company will be billed for the visit (see FIGURE 2 for a sample documentation form).

Continue to: Which CPT codes should I use?...
Which CPT codes should I use?
The answer depends on a number of factors, but a good rule of thumb is to use the same codes that you would use for an in-person appointment (CPT codes 99211-99215 for an established patient visit and 99201-99205 for a new patient visit). These are the most common CPT codes for outpatient gynecologic office visits whether they take place face-to-face or as a synchronous virtual visit (via a real-time interactive audio and video telecommunications system).
For example, the reimbursement for code 99213 has a range from $73 to $100. You may wonder how you can achieve the complexity requirements for a level-3 office visit without a physical examination. Whether as a face-to-face or virtual visit, documentation for these encounters requires 2 of 3 of the following components:
- expanded problem-focused history
- expanded problem-focused exam (not accomplished with telemedicine)
- low-complexity medical decision-making OR
- at least 15 minutes spent face to face with the patient if coding is based on time.
If a gynecologist reviews the results of a recent lab test for an estrogen-deficient patient and adjusts the estrogen dosage, writes a prescription, and spends 15 minutes communicating with the patient, he/she has met the complexity requirements for a code 99213. Because Level 3 and 4 visits (99214 and 99215) require a comprehensive physical examination, it is necessary to document the time spent with the patient (code 99214 requires 25 to 39 minutes of consultation and code 99215 requires ≥ 40 minutes).
Some final billing and coding advice
Always confirm telemedicine billing guidelines before beginning to conduct telemedicine visits. Consider starting a phone call to a payer armed with the fact that the payer is required by law to offer parity between telemedicine and face-to-face visits. Then ask which specific billing codes should be used.
Until you and your practice become comfortable with the process of, and the coding and billing for, telemedicine, consider using a telemedicine platform that has a built-in rules engine that offers recommendations for each telemedicine visit based on past claims data. These systems help gynecologists determine which CPT code to use and which modifiers are appropriate for the various insurance companies. In other words, the rules engine helps you submit a clean claim that is less likely to be denied and very likely to be paid. There are some vendors who are so confident that their rules engine will match the service with the proper CPT code and modifier that they guarantee full private payer reimbursement for telemedicine visits, or the vendor will reimburse the claim.
Watch for the third and final installment in this series, which was written with the assistance of 2 attorneys. It will review the legal guidelines for implementing telemedicine in a gynecologic practice and discuss the future of the technology. ●
- COVID-19 and Telehealth Coding Options as of March 20, 2020. https://www.ismanet.org/pdf/COVID-19andTelehealthcodes3-20-2020Updates.pdf.
- Federation of State Medical Boards. US States and Territories Modifying Licensure Requirements for Physicians in Response to COVID-19. Last updated May 26, 2020. https://www.fsmb.org/siteassets/advocacy/pdf/state-emergency-declarations-licensures-requirementscovid-19.pdf.
- Center for Connected Health Policy. Current State Laws and Reimbursement Policies https://www.cchpca.org/telehealth-policy/current-state-laws-and-reimbursement-policies.
- Centers for Medicare and Medicaid Services. List of Telehealth Services. Updated April 30, 2020. https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/Telehealth-Codes.
- American Medical Association. AMA quick guide to telemedicinein practice. Updated May 22, 2020. https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice.
- Eddy N. Patients increasingly trusting of remote care technology. Healthcare IT News. October 22, 2019. https://www.healthcareitnews.com/news/patients-increasingly-trusting-remote-care-technology-says-new-report. Accessed May 26, 2020.
- Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
- Tsai JM, Cheng MJ, Tsai HH, et al. Acceptance and resistance of telehealth: the perspective of dual-factor concepts in technology adoption. Int J Inform Manag. 2019;49:34-44.
- Hollander J, Carr BG. Virtually perfect? Telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
- Pew Research Center. Internet and Technology. Mobile Fact Sheet. June 12, 2019. https://www.pewresearch.org /internet/fact-sheet/mobile/. Accessed May 18, 2020.
- American Medical Association. AMA quick guide to telemedicine in practice. https://www.ama-assn.org/ practice-management/digital/ama-quick-guide-telemedicine- practice. Accessed March 20, 2020.
- Center for Connected Health Policy. Federal and state regulation updates. https://www.cchpca.org. Accessed March 20, 2020.
- The White House. Proclamation on declaring a national emergency concerning the novel coronavirus disease (Covid-19) outbreak. March 13, 2020. https://www.whitehouse.gov/presidential-actions/proclamation-declaring-national-emergency-concerning-novel-coronavirus-disease-covid-19-outbreak/. Accessed May 18, 2020.
- Center for Connected Health Policy. Quick glance state telehealth actions in response to COVID-19. https://www.cchpca.org/sites/default/files/2020-05/STATE%20TELEHEALTH%20ACTIONS%20IN%20RESPONSE%20TO%20COVID%20
OVERVIEW%205.5.2020_0.pdf. AccessedMay 13, 2020. - Medicare.gov. https://www.medicare.gov/sign-up-change -plans/types-of-medicare-health-plans/medicare-advantage-plans/how-do-medicare-advantage-plans-work. Accessed May 13, 2020.
- Centers for Medicare and Medicaid Services. CMS finalizes policies to bring innovative telehealth benefit to Medicare Advantage. April 5, 2019. https://www.cms.gov/newsroom /press-releases/cms-finalizes-policies-bring-innovative-telehealth-benefit-medicare-advantage. Accessed May 18,2020.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Accessed May 30, 2020.
- Centers for Medicare & Medicaid Services. Medicare telehealth frequently asked questions. https://www.cms.gov/files/document/medicare-telehealth-frequently-asked-questions-faqs-31720.pdf.
- American Hospital Association. Coronavirus update: CMS broadens access to telehealth during Covid-19 public health emergency. https://www.aha.org/advisory/2020-03-17-coronavirus-update-cms-broadens-access-telehealth-during-covid-19-public-health. Accessed May 18, 2020.
Since the COVID-19 pandemic began, many significant changes have occurred that have made the implementation of telemedicine easier and more attractive for gynecologic practices. In the first article in this series, we discussed the benefits of telemedicine to physicians and patients, how to get started using telemedicine, and implementing a workflow. This article will discuss the common hurdles in the process and the proper coding to use to insure reimbursement for services rendered.
Barriers to implementing telemedicine
Incorrect assumptions
Latecomers to telemedicine often assume that patients prefer face-to-face visits when, in fact, many may prefer the convenience of virtual visits. More than 50% of patients who are surveyed about their experience with telemedicine say that online tools have helped improve their relationship with their providers.1 Telemedicine has grown astronomically during the COVID-19 pandemic to the point where many patients now expect their health care providers to be able to conduct virtual visits. Practices that do not offer telemedicine may find their patients seeking services elsewhere. Nearly two-thirds of health care professionals expect their commitment to telemedicine to increase significantly in the next 3 years.2 Of those providers who have not yet adopted the practice, nearly 85% expect to implement telemedicine in the near future.3 COVID-19 has motivated the increased use of telemedicine to enhance the communication with patients, making it possible for patients to have enhanced access to health care during this pandemic while minimizing infectious transmission of COVID-19 to physicians and their staff.4
Admittedly, telemedicine is not appropriate for all patients. In general, situations that do not lend themselves to telemedicine are those for which an in-person visit is required to evaluate the patient via a physical examination, to perform a protocol-driven procedure, or provide an aggressive intervention. Additional patients for whom telemedicine may be inappropriate include those with cognitive disorders, those with language barriers, those with emergency situations that warrant an office visit or a visit to the emergency department, and patients who do not have access to the technology to conduct a virtual visit.
Cost and complexity
The process of implementing electronic health records (EHRs) left a bitter taste in the mouths of many health care professionals. But EHRs are complicated and expensive. Implementation often resulted in lost productivity. Because the learning curve was so steep, many physicians had to decrease the number of patients they saw before becoming comfortable with the conversion from paper charts to an EHR.
Telemedicine implementation is much less onerous and expensive. Telemedicine is available as a cloud-based platform, which requires less information technology (IT) support and less hardware and software. The technology required for patients to participate in telemedicine is nearly ubiquitous. According to the Pew Research Center, 96% of Americans own a cell phone (81% have a smart phone), and more than half (52%) own a tablet, so the basic equipment to connect patients to providers is already in place.5
On the provider side, the basic equipment required for a telemedicine program is a computer with video and audio capabilities and a broadband connection that is fast enough to show video in real time and to provide high-quality viewing of any images to be reviewed.
The growth in telemedicine means that telemedicine options are now more diverse, with many more affordable solutions. However, most telemedicine programs do require the purchase and set-up of new technology and equipment and the training of staff—some of which may be outside the budgets of health care providers in smaller independent practices. Many gynecologists have technology budgets that are already stretched thin. And for patients who do not have access to a smartphone or computer with Internet access, real-time telemedicine may be out of reach.
But with new guidelines put forth by the Centers for Medicare and Medicaid Services (CMS) in March 2020, connectivity can take place inexpensively using free platforms such as Google Hangouts, Skype, Facetime, and Facebook Messenger. If a non‒HIPAA-compliant platform is used initially, conversion to a HIPAA-compliant platform is recommended.6 These platforms do not require the purchase of, or subscription to, any expensive hardware or software. The disadvantages of these programs are the lack of documentation, the failure to be Health Insurance Portability and Accountability Act (HIPAA)-compliant, and the lack of encryption; however, these disadvantages are no longer an issue after the new CMS guidelines.
Depending on the magnitude of the program, IT assistance may be needed to get started. It is imperative that the telemedicine program is interoperable with the EHR and the billing program. Otherwise, double and triple entry will erase the efficiency provided by conducting a virtual visit.
Continue to: Licensing...
Licensing
Another concern or barrier is a license to participate in telemedicine. The March 15, 2020, approval of telemedicine states that physicians who are licensed in the state where the patient is located do not require any additional license or permission to conduct virtual visits.7 CMS has temporarily waived the requirement that out-of-state providers be licensed in the state where they are providing services when they are licensed in another state. For questions regarding licensure, contact your State Board of Medicine or Department of Health for information on requirements for licenses across state lines (see “Resources,” at the end of the article).
Informed consent
Just like with any other aspect of providing care for patients, obtaining informed consent is paramount. Not only is getting informed patient consent a recommended best practice of the American Telemedicine Association (ATA), but it is actually a legal requirement in many states and could be a condition of getting paid, depending on the payer. To check the requirements regarding patient consent in your state, look at The National Telehealth Policy Resource Center’s state map (see “Resources.")
Some states do not have any requirements regarding consent for a virtual visit. Others require verbal consent. Even if it is not a legal requirement in your state, consider making it a part of your practice’s policy to obtain written or verbal consent and to document in the patient’s record that consent was obtained prior to the virtual visit so that you are protected when using this new technology.
Because telemedicine is a new way of receiving care for many patients, it is important to let them know how it works including how patient confidentiality and privacy are handled, what technical equipment is required, and what they should expect in terms of scheduling, cancellations, and billing policies. A sample consent form for telemedicine use is shown in FIGURE 1.


Liability insurance
Another hurdle that must be considered is liability insurance for conducting virtual visits with patients. Gynecologists who are going to offer telemedicine care to patients should request proof in writing that their liability insurance policy covers telemedicine malpractice and that the coverage extends to other states should the patient be in another state from the state in which the gynecologist holds a license. Additionally, gynecologists who provide telemedicine care should check with liability insurers regarding any requirements or limitations to conducting a virtual visit with their patients and should document them. For example, the policy may require that the physician keep a written or recorded record of the visit in the EHR. If that is the case, then using Skype, Facebook, or Google for the virtual visit, which do not include documentation, would be less desirable.
Privacy
Certainly, there is concern about privacy, and HIPAA compliance is critical to telemedicine success. Because of the COVID-19 emergency, as of March 1, 2020, physicians may now communicate with patients, and provide telehealth services, through remote communications without penalties.8 With these changes in the HIPAA requirements, physicians may use applications that allow for video chats, including Apple FaceTime, Facebook Messenger video chat, Google Hangouts video, and Skype, to provide telehealth without risk that the Office for Civil Rights will impose a penalty for noncompliance with HIPAA rules. The consent for patients should mention that these “public” applications potentially introduce privacy risks. This is a motivation for gynecologists to consider one of the programs that promises encryption, privacy, and HIPAA compliance, such as Updox, Doxy.me, and Amazon Chime. It is also important to recognize that a virtual visit could result in colleagues (if the patient is in an office setting) or family members (if the patient is in the home environment) overhearing conversations between the health care professional and the patient. Therefore, we suggest that patients conduct virtual visits in locations in which they feel assured of some semblance of privacy.
Continue to: Compensation for telemedicine...
Compensation for telemedicine
Perhaps the biggest barrier to virtual health adoption has been compensation for telemedicine visits. Both commercial payers and CMS have been slow to enact formal policies for telemedicine reimbursement. Because of this, the common misconceptions (that providers cannot be reimbursed for telemedicine appointments or that compensation occurs at a reduced rate) have persisted, making telemedicine economically unappealing.
The good news is that this is changing; legislation in most states is quickly embracing virtual health visits as a result of the COVID-19 pandemic.9 In fact, as of January 1, 2020, telemedicine services are no longer considered “optional” coverage in Medicare Advantage plans.10 Nor are they required to have an additional fee. Instead, CMS now allows telemedicine as a standard, covered benefit in all plans, enabling beneficiaries to seek care from their homes rather than requiring them to go to a health care facility.11 In the past, telemedicine was restricted for use in rural areas or when patients resided a great distance from their health care providers. Starting March 6, 2020, and for the duration of the COVID-19 public health emergency, Medicare will make payment for professional services furnished to beneficiaries in all areas of the country in all settings regardless of location or distance between the patient and the health care provider.12
In addition, since March 15, 2020, CMS has expanded access to telemedicine services for all Medicare beneficiaries—not just those who have been diagnosed with COVID-19.13 The expanded access also applies to pre-COVID-19 coverage from physician offices, skilled nursing facilities, and hospitals. This means that Medicare will now make payments to physicians for telemedicine services provided in any health care facility or in a patient’s home, so that patients do not need to go to the physician’s office.
The facts are that there are parity laws and that commercial payers and CMS are required by state law to reimburse for telemedicine—often at the same rate as that for a comparable in-person visit. On the commercial side, there has been an increase in commercial parity legislation that requires health plans to cover virtual visits in the same way they cover face-to-face services. With the new guidelines for reimbursement, every state and Washington DC has parity laws in place. (To stay abreast of state-by-state changes in virtual health reimbursement, the Center for Connected Health Policy and the Advisory Board Primer are valuable resources. See “Resources.”) As long as the provider performs and documents the elements of history and decision-making, including the time spent counseling, and documents the visit as if a face-to-face visit occurred, then clinicians have a billable evaluation and management (E&M) visit.
Continue to: Virtual services for Medicare patients...
Virtual services for Medicare patients
There are 3 main types of virtual services gynecologists can provide to Medicare patients: Medicare telehealth visits, virtual check-ins, and e-visits.
Medicare telehealth visits. Largely because of the COVID-19 pandemic, Medicare patients may now use telecommunication technology for any services that previously occurred in an in-person communication. The gynecologist must use an interactive audio and video telecommunications system that permits real-time communication between the physician and the patient, and the patient should have a prior established relationship with the gynecologist with whom the telemedicine visit is taking place. The new guidelines indicate that the US Department of Health and Human Services (HHS) will not conduct audits to ensure that such a prior relationship exists for claims submitted during this public health emergency.14
The Current Procedural Terminology (CPT) codes for virtual visits using synchronous audio/visual communication are:
- 99201-99295, Office visit for a new patient
- 99211-99215, Office visit for an established patient.
Important modifiers for telemedicine visits include:
- modifier 02 for POS (place of service) for telehealth Medicare
- modifier 95 for commercial payers.
(A list of all available CPT codes for telehealth services from CMS can be found in “Resources.”)
Virtual check-ins. Established Medicare patients may have a brief communication with gynecologists the traditional way using a telephone or via live video. These brief virtual services, usually 5 to 10 minutes in duration, are initiated by the patient. The purpose of the virtual check-in is to determine if an office visit or a test or procedure is indicated.
Medicare pays for these “virtual check-ins” (or brief communication technology-based services) for patients to communicate with their physicians and avoid unnecessary trips to the office. These brief virtual check-ins are only for established patients. If an existing patient contacts the gynecologist’s office to ask a question or determine if an office visit is necessary, the gynecologist may bill for it using code G2012.
E-visits. Established Medicare patients may have non–face-to-face patient-initiated communications with their gynecologists without going to the physician’s office. These services can be billed only when the physician has an established relationship with the patient. The services may be billed using CPT codes 99421 to 99423. Coding for these visits is determined by the length of time the gynecologist spends online with the patient:
- 99421: Online digital evaluation and management service, for an established patient 5 to 10 minutes spent on the virtual visit
- 99422: 11 to 20 minutes
- 99423: ≥ 21 minutes.
Many clinicians want to immediately start the communication process with their patients. Many will avail themselves of the free video communication offered by Google Hangouts, Skype, Facetime, and Facebook Messenger. Since the March 15, 2020, relaxation of the HIPAA restrictions for telemedicine, it is now possible to have a virtual visit with a patient using one of the free, non–HIPAA-compliant connections. This type of visit is no different than a telephone call but with an added video component. Using these free technologies, a gynecologist can have an asynchronous visit with a patient (referred to as the store and forward method of sending information or medical images), which means that the service takes place in one direction with no opportunity for interaction with the patient. Asynchronous visits are akin to video text messages left for the patient. By contrast, a synchronous or real-time video visit with a patient is a 2-way communication that provides medical care without examining the patient.
Using triangulation
There are some downsides to telemedicine visits. First, virtual visits on Skype, FaceTime, and other non–HIPAA-compliant methods are not conducted on an encrypted website. Second, no documentation is created for the doctor-patient encounter. Finally, unless the physician keeps a record of these virtual visits and submits the interactions to the practice coders, there will be no billing and no reimbursement for the visits. In this scenario, physicians are legally responsible for their decision-making, prescription writing, and medical advice, but do not receive compensation for their efforts.
This can be remedied by using “triangulation,” which involves: 1. the physician, 2. the patient, and 3. a scribe or medical assistant who will record the visit. Before initiating the virtual visit using triangulation, it is imperative to ask the patient for permission if your medical assistant (or any other person in the office who functions as a scribe) will be listening to the conversation. It is important to explain that the person is there to take accurate notes and ascertain that the notes are entered into the EHR. Also, the scribe or assistant will record the time, date, and duration of the visit, which is a requirement for billing purposes. The scribe may also ascertain that the visit is properly coded and entered into the practice management system, and that a bill is submitted to the insurance company. By using triangulation, you have documentation that consent was obtained, that the visit took place, that notes were taken, and that the patient’s insurance company will be billed for the visit (see FIGURE 2 for a sample documentation form).

Continue to: Which CPT codes should I use?...
Which CPT codes should I use?
The answer depends on a number of factors, but a good rule of thumb is to use the same codes that you would use for an in-person appointment (CPT codes 99211-99215 for an established patient visit and 99201-99205 for a new patient visit). These are the most common CPT codes for outpatient gynecologic office visits whether they take place face-to-face or as a synchronous virtual visit (via a real-time interactive audio and video telecommunications system).
For example, the reimbursement for code 99213 has a range from $73 to $100. You may wonder how you can achieve the complexity requirements for a level-3 office visit without a physical examination. Whether as a face-to-face or virtual visit, documentation for these encounters requires 2 of 3 of the following components:
- expanded problem-focused history
- expanded problem-focused exam (not accomplished with telemedicine)
- low-complexity medical decision-making OR
- at least 15 minutes spent face to face with the patient if coding is based on time.
If a gynecologist reviews the results of a recent lab test for an estrogen-deficient patient and adjusts the estrogen dosage, writes a prescription, and spends 15 minutes communicating with the patient, he/she has met the complexity requirements for a code 99213. Because Level 3 and 4 visits (99214 and 99215) require a comprehensive physical examination, it is necessary to document the time spent with the patient (code 99214 requires 25 to 39 minutes of consultation and code 99215 requires ≥ 40 minutes).
Some final billing and coding advice
Always confirm telemedicine billing guidelines before beginning to conduct telemedicine visits. Consider starting a phone call to a payer armed with the fact that the payer is required by law to offer parity between telemedicine and face-to-face visits. Then ask which specific billing codes should be used.
Until you and your practice become comfortable with the process of, and the coding and billing for, telemedicine, consider using a telemedicine platform that has a built-in rules engine that offers recommendations for each telemedicine visit based on past claims data. These systems help gynecologists determine which CPT code to use and which modifiers are appropriate for the various insurance companies. In other words, the rules engine helps you submit a clean claim that is less likely to be denied and very likely to be paid. There are some vendors who are so confident that their rules engine will match the service with the proper CPT code and modifier that they guarantee full private payer reimbursement for telemedicine visits, or the vendor will reimburse the claim.
Watch for the third and final installment in this series, which was written with the assistance of 2 attorneys. It will review the legal guidelines for implementing telemedicine in a gynecologic practice and discuss the future of the technology. ●
- COVID-19 and Telehealth Coding Options as of March 20, 2020. https://www.ismanet.org/pdf/COVID-19andTelehealthcodes3-20-2020Updates.pdf.
- Federation of State Medical Boards. US States and Territories Modifying Licensure Requirements for Physicians in Response to COVID-19. Last updated May 26, 2020. https://www.fsmb.org/siteassets/advocacy/pdf/state-emergency-declarations-licensures-requirementscovid-19.pdf.
- Center for Connected Health Policy. Current State Laws and Reimbursement Policies https://www.cchpca.org/telehealth-policy/current-state-laws-and-reimbursement-policies.
- Centers for Medicare and Medicaid Services. List of Telehealth Services. Updated April 30, 2020. https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/Telehealth-Codes.
- American Medical Association. AMA quick guide to telemedicinein practice. Updated May 22, 2020. https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice.
Since the COVID-19 pandemic began, many significant changes have occurred that have made the implementation of telemedicine easier and more attractive for gynecologic practices. In the first article in this series, we discussed the benefits of telemedicine to physicians and patients, how to get started using telemedicine, and implementing a workflow. This article will discuss the common hurdles in the process and the proper coding to use to insure reimbursement for services rendered.
Barriers to implementing telemedicine
Incorrect assumptions
Latecomers to telemedicine often assume that patients prefer face-to-face visits when, in fact, many may prefer the convenience of virtual visits. More than 50% of patients who are surveyed about their experience with telemedicine say that online tools have helped improve their relationship with their providers.1 Telemedicine has grown astronomically during the COVID-19 pandemic to the point where many patients now expect their health care providers to be able to conduct virtual visits. Practices that do not offer telemedicine may find their patients seeking services elsewhere. Nearly two-thirds of health care professionals expect their commitment to telemedicine to increase significantly in the next 3 years.2 Of those providers who have not yet adopted the practice, nearly 85% expect to implement telemedicine in the near future.3 COVID-19 has motivated the increased use of telemedicine to enhance the communication with patients, making it possible for patients to have enhanced access to health care during this pandemic while minimizing infectious transmission of COVID-19 to physicians and their staff.4
Admittedly, telemedicine is not appropriate for all patients. In general, situations that do not lend themselves to telemedicine are those for which an in-person visit is required to evaluate the patient via a physical examination, to perform a protocol-driven procedure, or provide an aggressive intervention. Additional patients for whom telemedicine may be inappropriate include those with cognitive disorders, those with language barriers, those with emergency situations that warrant an office visit or a visit to the emergency department, and patients who do not have access to the technology to conduct a virtual visit.
Cost and complexity
The process of implementing electronic health records (EHRs) left a bitter taste in the mouths of many health care professionals. But EHRs are complicated and expensive. Implementation often resulted in lost productivity. Because the learning curve was so steep, many physicians had to decrease the number of patients they saw before becoming comfortable with the conversion from paper charts to an EHR.
Telemedicine implementation is much less onerous and expensive. Telemedicine is available as a cloud-based platform, which requires less information technology (IT) support and less hardware and software. The technology required for patients to participate in telemedicine is nearly ubiquitous. According to the Pew Research Center, 96% of Americans own a cell phone (81% have a smart phone), and more than half (52%) own a tablet, so the basic equipment to connect patients to providers is already in place.5
On the provider side, the basic equipment required for a telemedicine program is a computer with video and audio capabilities and a broadband connection that is fast enough to show video in real time and to provide high-quality viewing of any images to be reviewed.
The growth in telemedicine means that telemedicine options are now more diverse, with many more affordable solutions. However, most telemedicine programs do require the purchase and set-up of new technology and equipment and the training of staff—some of which may be outside the budgets of health care providers in smaller independent practices. Many gynecologists have technology budgets that are already stretched thin. And for patients who do not have access to a smartphone or computer with Internet access, real-time telemedicine may be out of reach.
But with new guidelines put forth by the Centers for Medicare and Medicaid Services (CMS) in March 2020, connectivity can take place inexpensively using free platforms such as Google Hangouts, Skype, Facetime, and Facebook Messenger. If a non‒HIPAA-compliant platform is used initially, conversion to a HIPAA-compliant platform is recommended.6 These platforms do not require the purchase of, or subscription to, any expensive hardware or software. The disadvantages of these programs are the lack of documentation, the failure to be Health Insurance Portability and Accountability Act (HIPAA)-compliant, and the lack of encryption; however, these disadvantages are no longer an issue after the new CMS guidelines.
Depending on the magnitude of the program, IT assistance may be needed to get started. It is imperative that the telemedicine program is interoperable with the EHR and the billing program. Otherwise, double and triple entry will erase the efficiency provided by conducting a virtual visit.
Continue to: Licensing...
Licensing
Another concern or barrier is a license to participate in telemedicine. The March 15, 2020, approval of telemedicine states that physicians who are licensed in the state where the patient is located do not require any additional license or permission to conduct virtual visits.7 CMS has temporarily waived the requirement that out-of-state providers be licensed in the state where they are providing services when they are licensed in another state. For questions regarding licensure, contact your State Board of Medicine or Department of Health for information on requirements for licenses across state lines (see “Resources,” at the end of the article).
Informed consent
Just like with any other aspect of providing care for patients, obtaining informed consent is paramount. Not only is getting informed patient consent a recommended best practice of the American Telemedicine Association (ATA), but it is actually a legal requirement in many states and could be a condition of getting paid, depending on the payer. To check the requirements regarding patient consent in your state, look at The National Telehealth Policy Resource Center’s state map (see “Resources.")
Some states do not have any requirements regarding consent for a virtual visit. Others require verbal consent. Even if it is not a legal requirement in your state, consider making it a part of your practice’s policy to obtain written or verbal consent and to document in the patient’s record that consent was obtained prior to the virtual visit so that you are protected when using this new technology.
Because telemedicine is a new way of receiving care for many patients, it is important to let them know how it works including how patient confidentiality and privacy are handled, what technical equipment is required, and what they should expect in terms of scheduling, cancellations, and billing policies. A sample consent form for telemedicine use is shown in FIGURE 1.


Liability insurance
Another hurdle that must be considered is liability insurance for conducting virtual visits with patients. Gynecologists who are going to offer telemedicine care to patients should request proof in writing that their liability insurance policy covers telemedicine malpractice and that the coverage extends to other states should the patient be in another state from the state in which the gynecologist holds a license. Additionally, gynecologists who provide telemedicine care should check with liability insurers regarding any requirements or limitations to conducting a virtual visit with their patients and should document them. For example, the policy may require that the physician keep a written or recorded record of the visit in the EHR. If that is the case, then using Skype, Facebook, or Google for the virtual visit, which do not include documentation, would be less desirable.
Privacy
Certainly, there is concern about privacy, and HIPAA compliance is critical to telemedicine success. Because of the COVID-19 emergency, as of March 1, 2020, physicians may now communicate with patients, and provide telehealth services, through remote communications without penalties.8 With these changes in the HIPAA requirements, physicians may use applications that allow for video chats, including Apple FaceTime, Facebook Messenger video chat, Google Hangouts video, and Skype, to provide telehealth without risk that the Office for Civil Rights will impose a penalty for noncompliance with HIPAA rules. The consent for patients should mention that these “public” applications potentially introduce privacy risks. This is a motivation for gynecologists to consider one of the programs that promises encryption, privacy, and HIPAA compliance, such as Updox, Doxy.me, and Amazon Chime. It is also important to recognize that a virtual visit could result in colleagues (if the patient is in an office setting) or family members (if the patient is in the home environment) overhearing conversations between the health care professional and the patient. Therefore, we suggest that patients conduct virtual visits in locations in which they feel assured of some semblance of privacy.
Continue to: Compensation for telemedicine...
Compensation for telemedicine
Perhaps the biggest barrier to virtual health adoption has been compensation for telemedicine visits. Both commercial payers and CMS have been slow to enact formal policies for telemedicine reimbursement. Because of this, the common misconceptions (that providers cannot be reimbursed for telemedicine appointments or that compensation occurs at a reduced rate) have persisted, making telemedicine economically unappealing.
The good news is that this is changing; legislation in most states is quickly embracing virtual health visits as a result of the COVID-19 pandemic.9 In fact, as of January 1, 2020, telemedicine services are no longer considered “optional” coverage in Medicare Advantage plans.10 Nor are they required to have an additional fee. Instead, CMS now allows telemedicine as a standard, covered benefit in all plans, enabling beneficiaries to seek care from their homes rather than requiring them to go to a health care facility.11 In the past, telemedicine was restricted for use in rural areas or when patients resided a great distance from their health care providers. Starting March 6, 2020, and for the duration of the COVID-19 public health emergency, Medicare will make payment for professional services furnished to beneficiaries in all areas of the country in all settings regardless of location or distance between the patient and the health care provider.12
In addition, since March 15, 2020, CMS has expanded access to telemedicine services for all Medicare beneficiaries—not just those who have been diagnosed with COVID-19.13 The expanded access also applies to pre-COVID-19 coverage from physician offices, skilled nursing facilities, and hospitals. This means that Medicare will now make payments to physicians for telemedicine services provided in any health care facility or in a patient’s home, so that patients do not need to go to the physician’s office.
The facts are that there are parity laws and that commercial payers and CMS are required by state law to reimburse for telemedicine—often at the same rate as that for a comparable in-person visit. On the commercial side, there has been an increase in commercial parity legislation that requires health plans to cover virtual visits in the same way they cover face-to-face services. With the new guidelines for reimbursement, every state and Washington DC has parity laws in place. (To stay abreast of state-by-state changes in virtual health reimbursement, the Center for Connected Health Policy and the Advisory Board Primer are valuable resources. See “Resources.”) As long as the provider performs and documents the elements of history and decision-making, including the time spent counseling, and documents the visit as if a face-to-face visit occurred, then clinicians have a billable evaluation and management (E&M) visit.
Continue to: Virtual services for Medicare patients...
Virtual services for Medicare patients
There are 3 main types of virtual services gynecologists can provide to Medicare patients: Medicare telehealth visits, virtual check-ins, and e-visits.
Medicare telehealth visits. Largely because of the COVID-19 pandemic, Medicare patients may now use telecommunication technology for any services that previously occurred in an in-person communication. The gynecologist must use an interactive audio and video telecommunications system that permits real-time communication between the physician and the patient, and the patient should have a prior established relationship with the gynecologist with whom the telemedicine visit is taking place. The new guidelines indicate that the US Department of Health and Human Services (HHS) will not conduct audits to ensure that such a prior relationship exists for claims submitted during this public health emergency.14
The Current Procedural Terminology (CPT) codes for virtual visits using synchronous audio/visual communication are:
- 99201-99295, Office visit for a new patient
- 99211-99215, Office visit for an established patient.
Important modifiers for telemedicine visits include:
- modifier 02 for POS (place of service) for telehealth Medicare
- modifier 95 for commercial payers.
(A list of all available CPT codes for telehealth services from CMS can be found in “Resources.”)
Virtual check-ins. Established Medicare patients may have a brief communication with gynecologists the traditional way using a telephone or via live video. These brief virtual services, usually 5 to 10 minutes in duration, are initiated by the patient. The purpose of the virtual check-in is to determine if an office visit or a test or procedure is indicated.
Medicare pays for these “virtual check-ins” (or brief communication technology-based services) for patients to communicate with their physicians and avoid unnecessary trips to the office. These brief virtual check-ins are only for established patients. If an existing patient contacts the gynecologist’s office to ask a question or determine if an office visit is necessary, the gynecologist may bill for it using code G2012.
E-visits. Established Medicare patients may have non–face-to-face patient-initiated communications with their gynecologists without going to the physician’s office. These services can be billed only when the physician has an established relationship with the patient. The services may be billed using CPT codes 99421 to 99423. Coding for these visits is determined by the length of time the gynecologist spends online with the patient:
- 99421: Online digital evaluation and management service, for an established patient 5 to 10 minutes spent on the virtual visit
- 99422: 11 to 20 minutes
- 99423: ≥ 21 minutes.
Many clinicians want to immediately start the communication process with their patients. Many will avail themselves of the free video communication offered by Google Hangouts, Skype, Facetime, and Facebook Messenger. Since the March 15, 2020, relaxation of the HIPAA restrictions for telemedicine, it is now possible to have a virtual visit with a patient using one of the free, non–HIPAA-compliant connections. This type of visit is no different than a telephone call but with an added video component. Using these free technologies, a gynecologist can have an asynchronous visit with a patient (referred to as the store and forward method of sending information or medical images), which means that the service takes place in one direction with no opportunity for interaction with the patient. Asynchronous visits are akin to video text messages left for the patient. By contrast, a synchronous or real-time video visit with a patient is a 2-way communication that provides medical care without examining the patient.
Using triangulation
There are some downsides to telemedicine visits. First, virtual visits on Skype, FaceTime, and other non–HIPAA-compliant methods are not conducted on an encrypted website. Second, no documentation is created for the doctor-patient encounter. Finally, unless the physician keeps a record of these virtual visits and submits the interactions to the practice coders, there will be no billing and no reimbursement for the visits. In this scenario, physicians are legally responsible for their decision-making, prescription writing, and medical advice, but do not receive compensation for their efforts.
This can be remedied by using “triangulation,” which involves: 1. the physician, 2. the patient, and 3. a scribe or medical assistant who will record the visit. Before initiating the virtual visit using triangulation, it is imperative to ask the patient for permission if your medical assistant (or any other person in the office who functions as a scribe) will be listening to the conversation. It is important to explain that the person is there to take accurate notes and ascertain that the notes are entered into the EHR. Also, the scribe or assistant will record the time, date, and duration of the visit, which is a requirement for billing purposes. The scribe may also ascertain that the visit is properly coded and entered into the practice management system, and that a bill is submitted to the insurance company. By using triangulation, you have documentation that consent was obtained, that the visit took place, that notes were taken, and that the patient’s insurance company will be billed for the visit (see FIGURE 2 for a sample documentation form).

Continue to: Which CPT codes should I use?...
Which CPT codes should I use?
The answer depends on a number of factors, but a good rule of thumb is to use the same codes that you would use for an in-person appointment (CPT codes 99211-99215 for an established patient visit and 99201-99205 for a new patient visit). These are the most common CPT codes for outpatient gynecologic office visits whether they take place face-to-face or as a synchronous virtual visit (via a real-time interactive audio and video telecommunications system).
For example, the reimbursement for code 99213 has a range from $73 to $100. You may wonder how you can achieve the complexity requirements for a level-3 office visit without a physical examination. Whether as a face-to-face or virtual visit, documentation for these encounters requires 2 of 3 of the following components:
- expanded problem-focused history
- expanded problem-focused exam (not accomplished with telemedicine)
- low-complexity medical decision-making OR
- at least 15 minutes spent face to face with the patient if coding is based on time.
If a gynecologist reviews the results of a recent lab test for an estrogen-deficient patient and adjusts the estrogen dosage, writes a prescription, and spends 15 minutes communicating with the patient, he/she has met the complexity requirements for a code 99213. Because Level 3 and 4 visits (99214 and 99215) require a comprehensive physical examination, it is necessary to document the time spent with the patient (code 99214 requires 25 to 39 minutes of consultation and code 99215 requires ≥ 40 minutes).
Some final billing and coding advice
Always confirm telemedicine billing guidelines before beginning to conduct telemedicine visits. Consider starting a phone call to a payer armed with the fact that the payer is required by law to offer parity between telemedicine and face-to-face visits. Then ask which specific billing codes should be used.
Until you and your practice become comfortable with the process of, and the coding and billing for, telemedicine, consider using a telemedicine platform that has a built-in rules engine that offers recommendations for each telemedicine visit based on past claims data. These systems help gynecologists determine which CPT code to use and which modifiers are appropriate for the various insurance companies. In other words, the rules engine helps you submit a clean claim that is less likely to be denied and very likely to be paid. There are some vendors who are so confident that their rules engine will match the service with the proper CPT code and modifier that they guarantee full private payer reimbursement for telemedicine visits, or the vendor will reimburse the claim.
Watch for the third and final installment in this series, which was written with the assistance of 2 attorneys. It will review the legal guidelines for implementing telemedicine in a gynecologic practice and discuss the future of the technology. ●
- COVID-19 and Telehealth Coding Options as of March 20, 2020. https://www.ismanet.org/pdf/COVID-19andTelehealthcodes3-20-2020Updates.pdf.
- Federation of State Medical Boards. US States and Territories Modifying Licensure Requirements for Physicians in Response to COVID-19. Last updated May 26, 2020. https://www.fsmb.org/siteassets/advocacy/pdf/state-emergency-declarations-licensures-requirementscovid-19.pdf.
- Center for Connected Health Policy. Current State Laws and Reimbursement Policies https://www.cchpca.org/telehealth-policy/current-state-laws-and-reimbursement-policies.
- Centers for Medicare and Medicaid Services. List of Telehealth Services. Updated April 30, 2020. https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/Telehealth-Codes.
- American Medical Association. AMA quick guide to telemedicinein practice. Updated May 22, 2020. https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice.
- Eddy N. Patients increasingly trusting of remote care technology. Healthcare IT News. October 22, 2019. https://www.healthcareitnews.com/news/patients-increasingly-trusting-remote-care-technology-says-new-report. Accessed May 26, 2020.
- Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
- Tsai JM, Cheng MJ, Tsai HH, et al. Acceptance and resistance of telehealth: the perspective of dual-factor concepts in technology adoption. Int J Inform Manag. 2019;49:34-44.
- Hollander J, Carr BG. Virtually perfect? Telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
- Pew Research Center. Internet and Technology. Mobile Fact Sheet. June 12, 2019. https://www.pewresearch.org /internet/fact-sheet/mobile/. Accessed May 18, 2020.
- American Medical Association. AMA quick guide to telemedicine in practice. https://www.ama-assn.org/ practice-management/digital/ama-quick-guide-telemedicine- practice. Accessed March 20, 2020.
- Center for Connected Health Policy. Federal and state regulation updates. https://www.cchpca.org. Accessed March 20, 2020.
- The White House. Proclamation on declaring a national emergency concerning the novel coronavirus disease (Covid-19) outbreak. March 13, 2020. https://www.whitehouse.gov/presidential-actions/proclamation-declaring-national-emergency-concerning-novel-coronavirus-disease-covid-19-outbreak/. Accessed May 18, 2020.
- Center for Connected Health Policy. Quick glance state telehealth actions in response to COVID-19. https://www.cchpca.org/sites/default/files/2020-05/STATE%20TELEHEALTH%20ACTIONS%20IN%20RESPONSE%20TO%20COVID%20
OVERVIEW%205.5.2020_0.pdf. AccessedMay 13, 2020. - Medicare.gov. https://www.medicare.gov/sign-up-change -plans/types-of-medicare-health-plans/medicare-advantage-plans/how-do-medicare-advantage-plans-work. Accessed May 13, 2020.
- Centers for Medicare and Medicaid Services. CMS finalizes policies to bring innovative telehealth benefit to Medicare Advantage. April 5, 2019. https://www.cms.gov/newsroom /press-releases/cms-finalizes-policies-bring-innovative-telehealth-benefit-medicare-advantage. Accessed May 18,2020.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Accessed May 30, 2020.
- Centers for Medicare & Medicaid Services. Medicare telehealth frequently asked questions. https://www.cms.gov/files/document/medicare-telehealth-frequently-asked-questions-faqs-31720.pdf.
- American Hospital Association. Coronavirus update: CMS broadens access to telehealth during Covid-19 public health emergency. https://www.aha.org/advisory/2020-03-17-coronavirus-update-cms-broadens-access-telehealth-during-covid-19-public-health. Accessed May 18, 2020.
- Eddy N. Patients increasingly trusting of remote care technology. Healthcare IT News. October 22, 2019. https://www.healthcareitnews.com/news/patients-increasingly-trusting-remote-care-technology-says-new-report. Accessed May 26, 2020.
- Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
- Tsai JM, Cheng MJ, Tsai HH, et al. Acceptance and resistance of telehealth: the perspective of dual-factor concepts in technology adoption. Int J Inform Manag. 2019;49:34-44.
- Hollander J, Carr BG. Virtually perfect? Telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
- Pew Research Center. Internet and Technology. Mobile Fact Sheet. June 12, 2019. https://www.pewresearch.org /internet/fact-sheet/mobile/. Accessed May 18, 2020.
- American Medical Association. AMA quick guide to telemedicine in practice. https://www.ama-assn.org/ practice-management/digital/ama-quick-guide-telemedicine- practice. Accessed March 20, 2020.
- Center for Connected Health Policy. Federal and state regulation updates. https://www.cchpca.org. Accessed March 20, 2020.
- The White House. Proclamation on declaring a national emergency concerning the novel coronavirus disease (Covid-19) outbreak. March 13, 2020. https://www.whitehouse.gov/presidential-actions/proclamation-declaring-national-emergency-concerning-novel-coronavirus-disease-covid-19-outbreak/. Accessed May 18, 2020.
- Center for Connected Health Policy. Quick glance state telehealth actions in response to COVID-19. https://www.cchpca.org/sites/default/files/2020-05/STATE%20TELEHEALTH%20ACTIONS%20IN%20RESPONSE%20TO%20COVID%20
OVERVIEW%205.5.2020_0.pdf. AccessedMay 13, 2020. - Medicare.gov. https://www.medicare.gov/sign-up-change -plans/types-of-medicare-health-plans/medicare-advantage-plans/how-do-medicare-advantage-plans-work. Accessed May 13, 2020.
- Centers for Medicare and Medicaid Services. CMS finalizes policies to bring innovative telehealth benefit to Medicare Advantage. April 5, 2019. https://www.cms.gov/newsroom /press-releases/cms-finalizes-policies-bring-innovative-telehealth-benefit-medicare-advantage. Accessed May 18,2020.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Accessed May 30, 2020.
- Centers for Medicare & Medicaid Services. Medicare telehealth frequently asked questions. https://www.cms.gov/files/document/medicare-telehealth-frequently-asked-questions-faqs-31720.pdf.
- American Hospital Association. Coronavirus update: CMS broadens access to telehealth during Covid-19 public health emergency. https://www.aha.org/advisory/2020-03-17-coronavirus-update-cms-broadens-access-telehealth-during-covid-19-public-health. Accessed May 18, 2020.
How to perform a vulvar biopsy
Many benign, premalignant, and malignant lesions can occur on the vulva. These can be challenging to differentiate by examination alone. A vulvar biopsy often is needed to appropriately diagnose—and ultimately treat—these various conditions.
In this article, we review vulvar biopsy procedures, describe how to prepare tissue specimens for the pathologist, and provide some brief case examples in which biopsy established the diagnosis.
Ask questions first
Prior to examining a patient with a vulvar lesion, obtain a detailed history. Asking specific questions may aid in making the correct diagnosis, such as:
- How long has the lesion been present? Has it changed? What color is it?
- Was any trigger, or trauma, associated with onset of the lesion?
- Does the lesion itch, burn, or cause pain? Is there any associated bleeding or discharge?
- Are other lesions present in the vagina, anus, or mouth, or are other skin lesions present?
- Are any systemic symptoms present, such as fever, lymphadenopathy, weight loss, or joint pain?
- What is the patient’s previous treatment history, including over-the-counter medications and prescribed medications?
- Has there been any incontinence of urine or stool? Does the patient use a pad?
- Is the patient scratching? Is there any nighttime scratching? It also can be useful to ask her partner, if she has one, about nighttime scratching.
- Is there a family history of vulvar conditions?
- Has there been any change in her use of products like soap, lotions, cleansing wipes, sprays, lubricants, or laundry detergent?
- Has the patient had any new partners or significant travel history?
Preprocedure counseling points
Prior to proceeding with a vulvar biopsy, review with the patient the risks, benefits, and alternatives and obtain patient consent for the procedure. Vulvar biopsy risks include pain, bleeding, infection, injury to surrounding tissue, and the need for further surgery. Make patients aware that some biopsies are nondiagnostic. We recommend that clinicians perform a time-out verification to ensure that the patient’s identity and planned procedure are correct.
Assess the biopsy site
A wide variety of lesions may require a biopsy for diagnosis. While it can be challenging to know where to biopsy, taking the time to determine the proper biopsy site may enhance pathology results.
When considering colored lesions, depth is the important factor, and a punch biopsy often is sufficient. A tumor should be biopsied in the thickest area. Lesions that are concerning for malignancy may require multiple biopsies. An erosion or ulcer is best biopsied on the edge, including a small amount of surrounding tissue. For most patients, biopsy of normal-appearing tissue is of low diagnostic yield. Lastly, we try to avoid biopsies directly on the midline to facilitate better healing.1
A photograph of the vulva prior to biopsy may be helpful for the pathologist to see the tissue. Some electronic medical records have the capability to include photographs. Due to the sensitive nature of these photographs, we prefer that a separate written patient consent be obtained prior to taking photographs. We find also that photos are a useful reference for progression of disease at follow-up in a shared care team.
Continue to: Anesthesia procedure and instrument kit...
Anesthesia procedure and instrument kit
Some patients may benefit from the application of topical lidocaine 4% cream (L.M.X.4) prior to the injection of a local anesthetic for tissue biopsy. Ideally, topical lidocaine should be placed on the vulva and covered with a dressing such as Tegaderm or cellophane up to 30 minutes before the anticipated biopsy procedure. The anesthetic effect generally lasts for about 60 minutes. Many patients report stinging for several seconds upon application. Due to clinic time restrictions, we tend to reserve this method for a limited subset of patients. If planning a return visit for a biopsy, the patient can place the topical anesthetic herself.
For the anesthetic injection, we recommend lidocaine 1% or 2% with epinephrine in all areas of the vulva except for the glans clitoris. For a punch biopsy, we draw up 1 to 3 mL in a 3-mL syringe and inject with a 21- to 30-gauge needle, using a lower gauge for thicker tissue. We have not found buffering the anesthetic with sodium bicarbonate to be of particular use. For the glans clitoris, lidocaine without epinephrine should be utilized.
Equipment. Depending on your office setting, having a premade instrument kit may be preferred to peel-pack equipment. We prefer a premade tray that contains sterile gauze, a hemostat, iris scissors, a needle driver, a scalpel handle, and Adson forceps (FIGURE 1).
Types of biopsy procedures
Punch biopsy. We recommend a 4-mm Keyes biopsy punch. As mentioned, we use a biopsy kit to facilitate the procedure. After the tissue is properly anesthetized and prepped, we test the area via gentle touch to the skin with the hemostat or Adson forceps. To perform the punch biopsy, gentle, consistent pressure in a clockwise-counterclockwise fashion yields the best results. The goal is to obtain a 5-mm depth for hair-bearing skin and a 3-mm depth for all other tissue.2 The tissue should then be excised at the base with scissors, taking care not to crush the specimen with forceps.
Punch biopsy permits sampling of the epidermis, dermis, and subcutaneous tissue. Hemostasis is maintained with either silver nitrate, Monsel’s solution (ferric sulfate), or a dissolvable suture such as 4-0 Monocryl (poliglecaprone 25) or Vicryl Rapide (polyglactin 910).
Stitch biopsy. We find the stitch biopsy to be very useful given the architecture of the vulva. A modification of the shave biopsy, the stitch biopsy is depicted in FIGURE 2. A 3-0 or 4-0 dissolvable suture is placed through the intended area of biopsy. Iris scissors are used to undermine the tissue while the suture is held on tension. The goal is to remove the suture with the specimen. Separate sutures are used for hemostasis. The stitch does not cause the crushing artifacts on prepared specimens. Depending on the proceduralist’s comfort, a relatively large sample can be obtained in this fashion. If the suture held on tension is inadvertently cut, a second pass can be made with suture; alternatively, care can be used to remove remaining tissue with forceps and scissors, again avoiding crush injury to the tissue.

Excisional biopsy. Often, a larger area or margins are desired. We find that with adequate preparation, patients tolerate excisions in the office quite well. The planned area for excision can be marked with ink to ensure margins. Adequate anesthesia is instilled. A No. 15 blade scalpel is often the best size used to excise vulvar tissue in an elliptical fashion. Depending on depth of incision, the tissue may need to be approximated in layers for cosmesis and healing.
When planning an excisional biopsy, place a stitch on the excised tissue to mark orientation or pin out the entire specimen to a foam board to help your pathologist interpret tissue orientation.
The box "Vulvar biopsy established the diagnosis" at the end of this discussion provides 6 case examples of vulvar lesions and the respective diagnoses confirmed by biopsy.
Continue to: Preparing tissue for the pathologist...
Preparing tissue for the pathologist
Here are 5 tips for preparing the biopsied specimen for pathology:
- Include a question for the pathologist, such as “rule out lichen sclerosus or lichen simplex chronicus.” The majority of specimens should be sent in formalin. At times, frozen sections are done in the operating room.
- Double-check that the proper paperwork is included with every specimen and be very specific regarding the exact location of the lesion on the vulva. Include photographs whenever possible.
- Request that a dermatopathologist or a gynecologic pathologist with a special interest in vulvar dermatology, when feasible, review the tissue.
- Check your laboratory’s protocol for sending biopsies from areas around ulcerated tissue. Often, special medium is required for immunohistochemistry stains.
- Call your pathologist with questions about results; he or she often is happy to clarify, and together you may be able to arrive at a diagnosis to better serve your patient.3
Complications and how to avoid them
Bleeding. Any procedure has bleeding risks. To avoid bleeding, review the patient’s medication list and medical history prior to biopsy, as certain medications, such as blood thinners, increase risk for bleeding. Counseling a patient on applying direct pressure to the biopsy site for 2 minutes is generally sufficient for any bleeding that may occur once she is discharged from the clinic.
Infection. With aseptic technique, infection of a biopsy site is rare. We use nonsterile gloves for biopsy procedures. This does not increase the risk of infection.4 If a patient has iodine allergy, dilute chlorhexidine is a reasonable alternative for skin cleansing. Instruct the patient to keep the site clean and dry; if the biopsy proximity is close to the urethra or anus, use of a peri-bottle may be preferred after toileting. Instruct patients not to pull sutures. While instructions are specific for each patient, we generally advise that patients wait 4 to 7 days before resuming use of topical medications.
Scarring or tattooing. Avoid using dyed suture on skin surfaces and counsel the patient that silver nitrate can permanently stain tissue. Usually, small biopsies heal well but a small scar is possible.
Key points to keep in mind
- Counsel patients on biopsy risks, benefits, and alternatives. Counsel regarding possible inconclusive results.
- Take time in choosing the biopsy site and consider multiple biopsies.
- Have all anticipated equipment available; consider using premade biopsy kits.
- Consider performing a stitch biopsy to avoid crush injury.
- Take photographs of the area to be biopsied and communicate with your pathologist to facilitate diagnosis.
Case 1
Biopsies were obtained of the areas highlighted in the photo. Pathology shows dVIN.
Image courtesy of Hope Haefner, MD.
Case 2
The examination is consistent with condylomata acuminata and biopsy is recommended with a 4-mm punch. Biopsy results are consistent with condylomata acuminata.
Image courtesy of Hope Haefner, MD.
Case 3
The final pathology shows high-grade squamous intraepithelial lesions (HSIL) of the vulva.
Image courtesy of Hope Haefner, MD.
Case 4
This presentation is an excellent opportunity for an excisional biopsy of the vulva. A marking pen is used to draw margins. A No. 15 blade is used to outline and then undermine the lesion, removing it in its entirety.
Final pathology shows a compound nevus of the vulva.
Image courtesy of Hope Haefner, MD.
Case 5
A 4-mm punch biopsy result is consistent with a diagnosis of lichen sclerosus.
Image courtesy of Hope Haefner, MD.
Case 6
A 4-mm punch biopsy result reveals that the pathology is significant for squamous cell carcinoma.
Image courtesy of Hope Haefner, MD.
- Edwards L, Lynch PJ. Genital Dermatology Atlas and Manual. 3rd ed. Philadelphia, PA: Wolters Kluwer; 2018.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 93: Diagnosis and management of vulvar skin disorders. Obstet Gynecol. 2008;111:1243-1253.
- Heller DS. Areas of confusion in pathologist-clinician communication as it relates to understanding the vulvar pathology report. J Low Genit Tract Dis. 2017;21:327-328.
- Rietz A, Barzin A, Jones K, et al. Sterile or non-sterile gloves for minor skin excisions? J Fam Pract. 2015;64:723-727.
Many benign, premalignant, and malignant lesions can occur on the vulva. These can be challenging to differentiate by examination alone. A vulvar biopsy often is needed to appropriately diagnose—and ultimately treat—these various conditions.
In this article, we review vulvar biopsy procedures, describe how to prepare tissue specimens for the pathologist, and provide some brief case examples in which biopsy established the diagnosis.
Ask questions first
Prior to examining a patient with a vulvar lesion, obtain a detailed history. Asking specific questions may aid in making the correct diagnosis, such as:
- How long has the lesion been present? Has it changed? What color is it?
- Was any trigger, or trauma, associated with onset of the lesion?
- Does the lesion itch, burn, or cause pain? Is there any associated bleeding or discharge?
- Are other lesions present in the vagina, anus, or mouth, or are other skin lesions present?
- Are any systemic symptoms present, such as fever, lymphadenopathy, weight loss, or joint pain?
- What is the patient’s previous treatment history, including over-the-counter medications and prescribed medications?
- Has there been any incontinence of urine or stool? Does the patient use a pad?
- Is the patient scratching? Is there any nighttime scratching? It also can be useful to ask her partner, if she has one, about nighttime scratching.
- Is there a family history of vulvar conditions?
- Has there been any change in her use of products like soap, lotions, cleansing wipes, sprays, lubricants, or laundry detergent?
- Has the patient had any new partners or significant travel history?
Preprocedure counseling points
Prior to proceeding with a vulvar biopsy, review with the patient the risks, benefits, and alternatives and obtain patient consent for the procedure. Vulvar biopsy risks include pain, bleeding, infection, injury to surrounding tissue, and the need for further surgery. Make patients aware that some biopsies are nondiagnostic. We recommend that clinicians perform a time-out verification to ensure that the patient’s identity and planned procedure are correct.
Assess the biopsy site
A wide variety of lesions may require a biopsy for diagnosis. While it can be challenging to know where to biopsy, taking the time to determine the proper biopsy site may enhance pathology results.
When considering colored lesions, depth is the important factor, and a punch biopsy often is sufficient. A tumor should be biopsied in the thickest area. Lesions that are concerning for malignancy may require multiple biopsies. An erosion or ulcer is best biopsied on the edge, including a small amount of surrounding tissue. For most patients, biopsy of normal-appearing tissue is of low diagnostic yield. Lastly, we try to avoid biopsies directly on the midline to facilitate better healing.1
A photograph of the vulva prior to biopsy may be helpful for the pathologist to see the tissue. Some electronic medical records have the capability to include photographs. Due to the sensitive nature of these photographs, we prefer that a separate written patient consent be obtained prior to taking photographs. We find also that photos are a useful reference for progression of disease at follow-up in a shared care team.
Continue to: Anesthesia procedure and instrument kit...
Anesthesia procedure and instrument kit
Some patients may benefit from the application of topical lidocaine 4% cream (L.M.X.4) prior to the injection of a local anesthetic for tissue biopsy. Ideally, topical lidocaine should be placed on the vulva and covered with a dressing such as Tegaderm or cellophane up to 30 minutes before the anticipated biopsy procedure. The anesthetic effect generally lasts for about 60 minutes. Many patients report stinging for several seconds upon application. Due to clinic time restrictions, we tend to reserve this method for a limited subset of patients. If planning a return visit for a biopsy, the patient can place the topical anesthetic herself.
For the anesthetic injection, we recommend lidocaine 1% or 2% with epinephrine in all areas of the vulva except for the glans clitoris. For a punch biopsy, we draw up 1 to 3 mL in a 3-mL syringe and inject with a 21- to 30-gauge needle, using a lower gauge for thicker tissue. We have not found buffering the anesthetic with sodium bicarbonate to be of particular use. For the glans clitoris, lidocaine without epinephrine should be utilized.
Equipment. Depending on your office setting, having a premade instrument kit may be preferred to peel-pack equipment. We prefer a premade tray that contains sterile gauze, a hemostat, iris scissors, a needle driver, a scalpel handle, and Adson forceps (FIGURE 1).

Types of biopsy procedures
Punch biopsy. We recommend a 4-mm Keyes biopsy punch. As mentioned, we use a biopsy kit to facilitate the procedure. After the tissue is properly anesthetized and prepped, we test the area via gentle touch to the skin with the hemostat or Adson forceps. To perform the punch biopsy, gentle, consistent pressure in a clockwise-counterclockwise fashion yields the best results. The goal is to obtain a 5-mm depth for hair-bearing skin and a 3-mm depth for all other tissue.2 The tissue should then be excised at the base with scissors, taking care not to crush the specimen with forceps.
Punch biopsy permits sampling of the epidermis, dermis, and subcutaneous tissue. Hemostasis is maintained with either silver nitrate, Monsel’s solution (ferric sulfate), or a dissolvable suture such as 4-0 Monocryl (poliglecaprone 25) or Vicryl Rapide (polyglactin 910).
Stitch biopsy. We find the stitch biopsy to be very useful given the architecture of the vulva. A modification of the shave biopsy, the stitch biopsy is depicted in FIGURE 2. A 3-0 or 4-0 dissolvable suture is placed through the intended area of biopsy. Iris scissors are used to undermine the tissue while the suture is held on tension. The goal is to remove the suture with the specimen. Separate sutures are used for hemostasis. The stitch does not cause the crushing artifacts on prepared specimens. Depending on the proceduralist’s comfort, a relatively large sample can be obtained in this fashion. If the suture held on tension is inadvertently cut, a second pass can be made with suture; alternatively, care can be used to remove remaining tissue with forceps and scissors, again avoiding crush injury to the tissue.

Excisional biopsy. Often, a larger area or margins are desired. We find that with adequate preparation, patients tolerate excisions in the office quite well. The planned area for excision can be marked with ink to ensure margins. Adequate anesthesia is instilled. A No. 15 blade scalpel is often the best size used to excise vulvar tissue in an elliptical fashion. Depending on depth of incision, the tissue may need to be approximated in layers for cosmesis and healing.
When planning an excisional biopsy, place a stitch on the excised tissue to mark orientation or pin out the entire specimen to a foam board to help your pathologist interpret tissue orientation.
The box "Vulvar biopsy established the diagnosis" at the end of this discussion provides 6 case examples of vulvar lesions and the respective diagnoses confirmed by biopsy.
Continue to: Preparing tissue for the pathologist...
Preparing tissue for the pathologist
Here are 5 tips for preparing the biopsied specimen for pathology:
- Include a question for the pathologist, such as “rule out lichen sclerosus or lichen simplex chronicus.” The majority of specimens should be sent in formalin. At times, frozen sections are done in the operating room.
- Double-check that the proper paperwork is included with every specimen and be very specific regarding the exact location of the lesion on the vulva. Include photographs whenever possible.
- Request that a dermatopathologist or a gynecologic pathologist with a special interest in vulvar dermatology, when feasible, review the tissue.
- Check your laboratory’s protocol for sending biopsies from areas around ulcerated tissue. Often, special medium is required for immunohistochemistry stains.
- Call your pathologist with questions about results; he or she often is happy to clarify, and together you may be able to arrive at a diagnosis to better serve your patient.3
Complications and how to avoid them
Bleeding. Any procedure has bleeding risks. To avoid bleeding, review the patient’s medication list and medical history prior to biopsy, as certain medications, such as blood thinners, increase risk for bleeding. Counseling a patient on applying direct pressure to the biopsy site for 2 minutes is generally sufficient for any bleeding that may occur once she is discharged from the clinic.
Infection. With aseptic technique, infection of a biopsy site is rare. We use nonsterile gloves for biopsy procedures. This does not increase the risk of infection.4 If a patient has iodine allergy, dilute chlorhexidine is a reasonable alternative for skin cleansing. Instruct the patient to keep the site clean and dry; if the biopsy proximity is close to the urethra or anus, use of a peri-bottle may be preferred after toileting. Instruct patients not to pull sutures. While instructions are specific for each patient, we generally advise that patients wait 4 to 7 days before resuming use of topical medications.
Scarring or tattooing. Avoid using dyed suture on skin surfaces and counsel the patient that silver nitrate can permanently stain tissue. Usually, small biopsies heal well but a small scar is possible.
Key points to keep in mind
- Counsel patients on biopsy risks, benefits, and alternatives. Counsel regarding possible inconclusive results.
- Take time in choosing the biopsy site and consider multiple biopsies.
- Have all anticipated equipment available; consider using premade biopsy kits.
- Consider performing a stitch biopsy to avoid crush injury.
- Take photographs of the area to be biopsied and communicate with your pathologist to facilitate diagnosis.
Case 1
Biopsies were obtained of the areas highlighted in the photo. Pathology shows dVIN.
Image courtesy of Hope Haefner, MD.
Case 2
The examination is consistent with condylomata acuminata and biopsy is recommended with a 4-mm punch. Biopsy results are consistent with condylomata acuminata.
Image courtesy of Hope Haefner, MD.
Case 3
The final pathology shows high-grade squamous intraepithelial lesions (HSIL) of the vulva.
Image courtesy of Hope Haefner, MD.
Case 4
This presentation is an excellent opportunity for an excisional biopsy of the vulva. A marking pen is used to draw margins. A No. 15 blade is used to outline and then undermine the lesion, removing it in its entirety.
Final pathology shows a compound nevus of the vulva.
Image courtesy of Hope Haefner, MD.
Case 5
A 4-mm punch biopsy result is consistent with a diagnosis of lichen sclerosus.
Image courtesy of Hope Haefner, MD.
Case 6
A 4-mm punch biopsy result reveals that the pathology is significant for squamous cell carcinoma.
Image courtesy of Hope Haefner, MD.
Many benign, premalignant, and malignant lesions can occur on the vulva. These can be challenging to differentiate by examination alone. A vulvar biopsy often is needed to appropriately diagnose—and ultimately treat—these various conditions.
In this article, we review vulvar biopsy procedures, describe how to prepare tissue specimens for the pathologist, and provide some brief case examples in which biopsy established the diagnosis.
Ask questions first
Prior to examining a patient with a vulvar lesion, obtain a detailed history. Asking specific questions may aid in making the correct diagnosis, such as:
- How long has the lesion been present? Has it changed? What color is it?
- Was any trigger, or trauma, associated with onset of the lesion?
- Does the lesion itch, burn, or cause pain? Is there any associated bleeding or discharge?
- Are other lesions present in the vagina, anus, or mouth, or are other skin lesions present?
- Are any systemic symptoms present, such as fever, lymphadenopathy, weight loss, or joint pain?
- What is the patient’s previous treatment history, including over-the-counter medications and prescribed medications?
- Has there been any incontinence of urine or stool? Does the patient use a pad?
- Is the patient scratching? Is there any nighttime scratching? It also can be useful to ask her partner, if she has one, about nighttime scratching.
- Is there a family history of vulvar conditions?
- Has there been any change in her use of products like soap, lotions, cleansing wipes, sprays, lubricants, or laundry detergent?
- Has the patient had any new partners or significant travel history?
Preprocedure counseling points
Prior to proceeding with a vulvar biopsy, review with the patient the risks, benefits, and alternatives and obtain patient consent for the procedure. Vulvar biopsy risks include pain, bleeding, infection, injury to surrounding tissue, and the need for further surgery. Make patients aware that some biopsies are nondiagnostic. We recommend that clinicians perform a time-out verification to ensure that the patient’s identity and planned procedure are correct.
Assess the biopsy site
A wide variety of lesions may require a biopsy for diagnosis. While it can be challenging to know where to biopsy, taking the time to determine the proper biopsy site may enhance pathology results.
When considering colored lesions, depth is the important factor, and a punch biopsy often is sufficient. A tumor should be biopsied in the thickest area. Lesions that are concerning for malignancy may require multiple biopsies. An erosion or ulcer is best biopsied on the edge, including a small amount of surrounding tissue. For most patients, biopsy of normal-appearing tissue is of low diagnostic yield. Lastly, we try to avoid biopsies directly on the midline to facilitate better healing.1
A photograph of the vulva prior to biopsy may be helpful for the pathologist to see the tissue. Some electronic medical records have the capability to include photographs. Due to the sensitive nature of these photographs, we prefer that a separate written patient consent be obtained prior to taking photographs. We find also that photos are a useful reference for progression of disease at follow-up in a shared care team.
Continue to: Anesthesia procedure and instrument kit...
Anesthesia procedure and instrument kit
Some patients may benefit from the application of topical lidocaine 4% cream (L.M.X.4) prior to the injection of a local anesthetic for tissue biopsy. Ideally, topical lidocaine should be placed on the vulva and covered with a dressing such as Tegaderm or cellophane up to 30 minutes before the anticipated biopsy procedure. The anesthetic effect generally lasts for about 60 minutes. Many patients report stinging for several seconds upon application. Due to clinic time restrictions, we tend to reserve this method for a limited subset of patients. If planning a return visit for a biopsy, the patient can place the topical anesthetic herself.
For the anesthetic injection, we recommend lidocaine 1% or 2% with epinephrine in all areas of the vulva except for the glans clitoris. For a punch biopsy, we draw up 1 to 3 mL in a 3-mL syringe and inject with a 21- to 30-gauge needle, using a lower gauge for thicker tissue. We have not found buffering the anesthetic with sodium bicarbonate to be of particular use. For the glans clitoris, lidocaine without epinephrine should be utilized.
Equipment. Depending on your office setting, having a premade instrument kit may be preferred to peel-pack equipment. We prefer a premade tray that contains sterile gauze, a hemostat, iris scissors, a needle driver, a scalpel handle, and Adson forceps (FIGURE 1).

Types of biopsy procedures
Punch biopsy. We recommend a 4-mm Keyes biopsy punch. As mentioned, we use a biopsy kit to facilitate the procedure. After the tissue is properly anesthetized and prepped, we test the area via gentle touch to the skin with the hemostat or Adson forceps. To perform the punch biopsy, gentle, consistent pressure in a clockwise-counterclockwise fashion yields the best results. The goal is to obtain a 5-mm depth for hair-bearing skin and a 3-mm depth for all other tissue.2 The tissue should then be excised at the base with scissors, taking care not to crush the specimen with forceps.
Punch biopsy permits sampling of the epidermis, dermis, and subcutaneous tissue. Hemostasis is maintained with either silver nitrate, Monsel’s solution (ferric sulfate), or a dissolvable suture such as 4-0 Monocryl (poliglecaprone 25) or Vicryl Rapide (polyglactin 910).
Stitch biopsy. We find the stitch biopsy to be very useful given the architecture of the vulva. A modification of the shave biopsy, the stitch biopsy is depicted in FIGURE 2. A 3-0 or 4-0 dissolvable suture is placed through the intended area of biopsy. Iris scissors are used to undermine the tissue while the suture is held on tension. The goal is to remove the suture with the specimen. Separate sutures are used for hemostasis. The stitch does not cause the crushing artifacts on prepared specimens. Depending on the proceduralist’s comfort, a relatively large sample can be obtained in this fashion. If the suture held on tension is inadvertently cut, a second pass can be made with suture; alternatively, care can be used to remove remaining tissue with forceps and scissors, again avoiding crush injury to the tissue.

Excisional biopsy. Often, a larger area or margins are desired. We find that with adequate preparation, patients tolerate excisions in the office quite well. The planned area for excision can be marked with ink to ensure margins. Adequate anesthesia is instilled. A No. 15 blade scalpel is often the best size used to excise vulvar tissue in an elliptical fashion. Depending on depth of incision, the tissue may need to be approximated in layers for cosmesis and healing.
When planning an excisional biopsy, place a stitch on the excised tissue to mark orientation or pin out the entire specimen to a foam board to help your pathologist interpret tissue orientation.
The box "Vulvar biopsy established the diagnosis" at the end of this discussion provides 6 case examples of vulvar lesions and the respective diagnoses confirmed by biopsy.
Continue to: Preparing tissue for the pathologist...
Preparing tissue for the pathologist
Here are 5 tips for preparing the biopsied specimen for pathology:
- Include a question for the pathologist, such as “rule out lichen sclerosus or lichen simplex chronicus.” The majority of specimens should be sent in formalin. At times, frozen sections are done in the operating room.
- Double-check that the proper paperwork is included with every specimen and be very specific regarding the exact location of the lesion on the vulva. Include photographs whenever possible.
- Request that a dermatopathologist or a gynecologic pathologist with a special interest in vulvar dermatology, when feasible, review the tissue.
- Check your laboratory’s protocol for sending biopsies from areas around ulcerated tissue. Often, special medium is required for immunohistochemistry stains.
- Call your pathologist with questions about results; he or she often is happy to clarify, and together you may be able to arrive at a diagnosis to better serve your patient.3
Complications and how to avoid them
Bleeding. Any procedure has bleeding risks. To avoid bleeding, review the patient’s medication list and medical history prior to biopsy, as certain medications, such as blood thinners, increase risk for bleeding. Counseling a patient on applying direct pressure to the biopsy site for 2 minutes is generally sufficient for any bleeding that may occur once she is discharged from the clinic.
Infection. With aseptic technique, infection of a biopsy site is rare. We use nonsterile gloves for biopsy procedures. This does not increase the risk of infection.4 If a patient has iodine allergy, dilute chlorhexidine is a reasonable alternative for skin cleansing. Instruct the patient to keep the site clean and dry; if the biopsy proximity is close to the urethra or anus, use of a peri-bottle may be preferred after toileting. Instruct patients not to pull sutures. While instructions are specific for each patient, we generally advise that patients wait 4 to 7 days before resuming use of topical medications.
Scarring or tattooing. Avoid using dyed suture on skin surfaces and counsel the patient that silver nitrate can permanently stain tissue. Usually, small biopsies heal well but a small scar is possible.
Key points to keep in mind
- Counsel patients on biopsy risks, benefits, and alternatives. Counsel regarding possible inconclusive results.
- Take time in choosing the biopsy site and consider multiple biopsies.
- Have all anticipated equipment available; consider using premade biopsy kits.
- Consider performing a stitch biopsy to avoid crush injury.
- Take photographs of the area to be biopsied and communicate with your pathologist to facilitate diagnosis.
Case 1
Biopsies were obtained of the areas highlighted in the photo. Pathology shows dVIN.
Image courtesy of Hope Haefner, MD.
Case 2
The examination is consistent with condylomata acuminata and biopsy is recommended with a 4-mm punch. Biopsy results are consistent with condylomata acuminata.
Image courtesy of Hope Haefner, MD.
Case 3
The final pathology shows high-grade squamous intraepithelial lesions (HSIL) of the vulva.
Image courtesy of Hope Haefner, MD.
Case 4
This presentation is an excellent opportunity for an excisional biopsy of the vulva. A marking pen is used to draw margins. A No. 15 blade is used to outline and then undermine the lesion, removing it in its entirety.
Final pathology shows a compound nevus of the vulva.
Image courtesy of Hope Haefner, MD.
Case 5
A 4-mm punch biopsy result is consistent with a diagnosis of lichen sclerosus.
Image courtesy of Hope Haefner, MD.
Case 6
A 4-mm punch biopsy result reveals that the pathology is significant for squamous cell carcinoma.
Image courtesy of Hope Haefner, MD.
- Edwards L, Lynch PJ. Genital Dermatology Atlas and Manual. 3rd ed. Philadelphia, PA: Wolters Kluwer; 2018.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 93: Diagnosis and management of vulvar skin disorders. Obstet Gynecol. 2008;111:1243-1253.
- Heller DS. Areas of confusion in pathologist-clinician communication as it relates to understanding the vulvar pathology report. J Low Genit Tract Dis. 2017;21:327-328.
- Rietz A, Barzin A, Jones K, et al. Sterile or non-sterile gloves for minor skin excisions? J Fam Pract. 2015;64:723-727.
- Edwards L, Lynch PJ. Genital Dermatology Atlas and Manual. 3rd ed. Philadelphia, PA: Wolters Kluwer; 2018.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 93: Diagnosis and management of vulvar skin disorders. Obstet Gynecol. 2008;111:1243-1253.
- Heller DS. Areas of confusion in pathologist-clinician communication as it relates to understanding the vulvar pathology report. J Low Genit Tract Dis. 2017;21:327-328.
- Rietz A, Barzin A, Jones K, et al. Sterile or non-sterile gloves for minor skin excisions? J Fam Pract. 2015;64:723-727.
2020 Update on Menopause
The term genitourinary syndrome of menopause (GSM) refers to the bothersome symptoms and physical findings associated with estrogen deficiency that involve the labia, vestibular tissue, clitoris, vagina, urethra, and bladder.1 GSM is associated with genital irritation, dryness, and burning; urinary symptoms including urgency, dysuria, and recurrent urinary tract infections; and sexual symptoms including vaginal dryness and pain. Vulvovaginal atrophy (VVA) represents a component of GSM.
GSM is highly prevalent, affecting more than three-quarters of menopausal women. In contrast to menopausal vasomotor symptoms, which often are most severe and frequent in recently menopausal women, GSM commonly presents years following menopause. Unfortunately, VVA symptoms may have a substantial negative impact on women’s quality of life.
In this 2020 Menopause Update, I review a large observational study that provides reassurance to clinicians and patients regarding the safety of the best-studied prescription treatment for GSM—vaginal estrogen. Because some women should not use vaginal estrogen and others choose not to use it, nonhormonal management of GSM is important. Dr. JoAnn Pinkerton provides details on a randomized clinical trial that compared the use of fractionated CO2 laser therapy with vaginal estrogen for the treatment of GSM. In addition, Dr. JoAnn Manson discusses recent studies that found lower health risks with vaginal estrogen use compared with systemic estrogen therapy.
Diagnosing GSM
GSM can be diagnosed presumptively based on a characteristic history in a menopausal patient. Performing a pelvic examination, however, allows clinicians to exclude other conditions that may present with similar symptoms, such as lichen sclerosus, Candida infection, and malignancy.
During inspection of the external genitalia, the clinician may note loss of the fat pad in the labia majora and mons as well as a reduction in labia minora pigmentation and tissue. The urethral meatus often becomes erythematous and prominent. If vaginal or introital narrowing is present, use of a pediatric (ultrathin) speculum reduces patient discomfort. The vaginal mucosa may appear smooth due to loss of rugation; it also may appear shiny and dry. Bleeding (friability) on contact with a spatula or cotton-tipped swab may occur. In addition, the vaginal fornices may become attenuated, leaving the cervix flush with the vaginal apex.
GSM can be diagnosed without laboratory assessment. However, vaginal pH, if measured, is characteristically higher than 5.0; microscopic wet prep often reveals many white blood cells, immature epithelial cells (large nuclei), and reduced or absent lactobacilli.2
Nonhormonal management of GSM
Water, silicone-based, and oil-based lubricants reduce the friction and discomfort associated with sexual activity. By contrast, vaginal moisturizers act longer than lubricants and can be applied several times weekly or daily. Natural oils, including olive and coconut oil, may be useful both as lubricants and as moisturizers. Aqueous lidocaine 4%, applied to vestibular tissue with cotton balls prior to penetration, reduces dyspareunia in women with GSM.3
Vaginal estrogen therapy
When nonhormonal management does not sufficiently reduce GSM symptoms, use of low-dose vaginal estrogen enhances thickness and elasticity of genital tissue and improves vaginal blood flow. Vaginal estrogen creams, tablets, an insert, and a ring are marketed in the United States. Although clinical improvement may be apparent within several weeks of initiating vaginal estrogen, the full benefit of treatment becomes apparent after 2 to 3 months.3
Despite the availability and effectiveness of low-dose vaginal estrogen, fears regarding the safety of menopausal hormone therapy have resulted in the underutilization of vaginal estrogen.4,5 Unfortunately, the package labeling for low-dose vaginal estrogen can exacerbate these fears.
Continue to: Nurses’ Health Study report...
Nurses’ Health Study report provides reassurance on long-term safety of vaginal estrogen
Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603-610
Bhupathiraju and colleagues published a report from the long-running Nurses’ Health prospective cohort study on the health outcomes associated with the use of vaginal estrogen.
Recap of the study
Starting in 1982, participants in the Nurses’Health Study were asked to report their use of vaginal estrogen via a validated questionnaire. For the years 1982 to 2012, investigators analyzed data from 896 and 52,901 women who had and had not used vaginal estrogen, respectively. The mean duration of vaginal estrogen use was 36 months.
In an analysis adjusted for numerous factors, the investigators observed no statistically significant differences in risk for cardiovascular outcomes (myocardial infarction, stroke, deep vein thrombosis, and pulmonary embolism) or invasive cancers (colorectal, endometrial, ovarian, or breast).
Findings uphold safety of vaginal estrogen
This landmark study provides reassurance that 3 years of use of vaginal estrogen does not increase the risk of cardiovascular events or invasive breast cancer, findings that hopefully will allow clinicians and women to feel comfortable regarding the safety of vaginal estrogen. A study of vaginal estrogen from the Women’s Health Initiative provided similar reassurance. Recent research supports guidance from The North American Menopause Society and the American College of Obstetricians and Gynecologists that vaginal estrogen can be used indefinitely, if indicated, and that use of concomitant progestin is not recommended in women who use vaginal estrogen and have an intact uterus.6,7
I agree with the authors, who point out that since treatment of GSM may need to be continued long term (even indefinitely), it would be helpful to have data that assessed the safety of longer-duration use of vaginal estrogen.
Results from Bhupathiraju and colleagues’ analysis of data from the Nurses’ Health Study on the 3-year safety of vaginal estrogen use encourage clinicians to recommend and women to use this safe and effective treatment for GSM.
How CO2 fractionated vaginal laser therapy compares with vaginal estrogen for relief of GSM symptoms
Paraiso MF, Ferrando CA, Sokol ER, et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET trial. Menopause. 2020;27:50-56.
Up to 50% to 60% of postmenopausal women experience GSM symptoms. However, many fewer receive treatment, either because they do not understand that the symptoms are related to menopause or they are not aware that safe and effective treatment is available. Sadly, many women are not asked about their symptoms or are embarrassed to tell providers.
GSM affects relationships and quality of life. Vaginal lubricants or moisturizers may provide relief. US Food and Drug Administration (FDA)–approved therapies include low-dose vaginal estrogen, available as a vaginal tablet, cream, suppository, and ring; intravaginal dehydroepiandrosterone (DHEA); and oral ospemifene, a selective estrogen replacement modulator. If women have an estrogen-sensitive breast or uterine cancer, an oncologist should be involved in decisions about vaginal hormonal therapy.
Energy-based devices such as vaginal lasers appear to induce wound healing; stimulate collagen and elastin fiber formation through increased storage of glycogen; and activate fibroblasts, which leads to increased extracellular matrix and restoration of vaginal pH.
These lasers are FDA approved for use in gynecology but not specifically for the treatment of GSM. In July 2016, the FDA issued a safety alert that energy-based devices, while approved for use in gynecology, have not been approved or adequately tested for menopausal vaginal conditions, and safety concerns include reports of vaginal burns.8 Lacking are publications of adequately powered randomized, sham-con-trolled trials to determine if laser therapy works better for women with GSM than placebos, moisturizers, or vaginal hormone therapies.
Recently, investigators conducted a multicenter, randomized, single-blinded trial of vaginal laser therapy and estrogen cream for treatment of GSM.
Continue to: Details of the study...
Details of the study
Paraiso and colleagues aimed to compare the 6-month efficacy and safety of fractionated CO2 vaginal laser therapy with that of estrogen vaginal cream for the treatment of vaginal dryness/GSM.
Participants randomly assigned to the estrogen therapy arm applied conjugated estrogen cream 0.5 g vaginally daily for 14 days, followed by twice weekly application for 24 weeks (a low-dose vaginal estrogen therapy). Participants randomly assigned to laser therapy underwent 3 vaginal treatments at a minimum of 6 weeks apart.
Sixty-nine women were enrolled in the trial before enrollment was closed because the FDA required that the sponsor obtain and maintain an investigational device exemption. Of 62 women who completed 6 months’ treatment, 30 received 3 laser treatments and 32 received estrogen cream.
The primary outcome compared subjective improvement in vaginal dryness using the visual analog scale (VAS) between the 2 groups at 6 months. Secondary outcomes included comparisons of the vaginal health index (VHI) and vaginal maturation index (VMI), the effect of GSM on quality of life, the effect of treatment on sexual function and urinary symptoms, and patient satisfaction.
Study findings
Efficacy. Laser therapy and estrogen therapy were found to be similarly effective except on the VMI, which favored estrogen. On patient global impression, 85.8% of laser-treated women rated their improvement as ‘‘better or much better’’ and 78.5% reported being either ‘‘satisfied or very satisfied,’’ compared with 70% and 73.3%, respectively, in the estrogen group, a statistically nonsignificant difference.
On linear regression, the investigators found a nonsignificant mean difference in female sexual function index scores. While VMI scores remained higher in the estrogen-treated group (adjusted P = .02), baseline and 6-month follow-up VMI data were available for only 34 participants (16 laser treated, 18 estrogen treated).
Regarding long-term effectiveness, 20% to 25% of the women in the laser-treated group needed further treatment after 1 year while the estrogen cream continued to work as long as it was used as prescribed.
Adverse effects. The incidence of vaginal bleeding was similar in the 2 groups: 6.7% in the laser group and 6.3% in the estrogen group. In the laser therapy group, 3% expe-rienced vaginal pain, discharge, and bladder infections, while in the estrogen cream group, 3% reported breast tenderness, migraine headaches, and abdominal cramping.
Takeaways. This small randomized, open-label (not blinded) trial provides pilot data on the effectiveness of vaginal CO2 laser compared with vaginal estrogen in treating vaginal atrophy, quality-of-life symptoms, sexual function, and urinary symptoms. Adverse events were minimal. Patient global impression of improvement and satisfaction improved for both vaginal laser and vaginal estrogen therapy.
Continue to: Study strengths and limitations...
Study strengths and limitations
To show noninferiority of vaginal laser therapy to vaginal estrogen, 196 study participants were needed. However, after 38% had been enrolled, the FDA sent a warning letter to the Foundation for Female Health Awareness, which required obtainment of an investigational device exemption for the laser and addition of a sham treatment arm.9 Instead of redesigning the trial and reconsenting the participants, the investigators closed the study, and analysis was performed only on the 62 participants who completed the study; vaginal maturation was assessed only in 34 participants.
The study lacked a placebo or sham control, which increases the risk of bias, while small numbers limit the strength of the findings. Longer-term evaluation of the effects of laser therapy beyond 6 months is needed to allow assessment of the effects of scarring on vaginal health, sexual function, and urinary issues.
Discussing therapy with patients
Despite this study’s preliminary findings, and until more robust data are available, providers should discuss the benefits and risks of all available treatment options for vaginal symptoms, including over-the-counter lubricants, vaginal moisturizers, FDA-approved vaginal hormone therapies (such as vaginal estrogen and intravaginal dehydroepiandrosterone), and systemic therapies, such as hormone therapy and ospemifene, to determine the best treatment for the individual woman with GSM.
In a healthy postmenopausal woman with bothersome GSM symptoms not responsive to lubricants and moisturizers, I recommend FDA-approved vaginal therapies as first-line treatment if there are no contraindications. For women with breast cancer, I involve their oncologist. If a patient asks about vaginal laser treatment, I share that vaginal energy-based therapies, such as the vaginal laser, have not been approved for menopausal vaginal concerns. In addition to the possibility of adverse events or unsuccessful treatment, there are significant out-of-pocket costs and the potential need for ongoing therapy after the initial 3 laser treatments.
For GSM that does not respond to lubricants and moisturizers, many FDA-approved vaginal and systemic therapies are available to treat vaginal symptoms. Vaginal laser treatment is a promising therapy for vaginal symptoms of GSM that needs further testing to determine its efficacy, safety, and long-term effects. If discussing vaginal energy-based therapies with patients, include the current lack of FDA approval for specific vaginal indications, potential adverse effects, the need for ongoing retreatment, and out-of-pocket costs.
JoAnn E. Manson, MD, DrPH, NCMP
Having more appropriate, evidence-based labeling of low-dose vaginal estrogen continues to be a high priority for The North American Menopause Society (NAMS), the International Society for the Study of Women’s Sexual Health (ISSWSH), and other professional societies.
NAMS and the Working Group on Women’s Health and Well-Being in Menopause had submitted a citizen’s petition to the US Food and Drug Administration (FDA) in 2016 requesting modification of the label—including removal of the “black box warning”—for low-dose vaginal estrogen products. The petition was, disappointingly, denied in 2018.1
Currently, the class labeling, which was based on the results of randomized trials with systemic hormone therapy, is not applicable to low-dose vaginal estrogen, and the inclusion of the black box warning has led to serious underutilization of an effective and safe treatment for a very common and life-altering condition, the genitourinary syndrome of menopause (GSM). This condition affects nearly half of postmenopausal women. It tends to be chronic and progressive and, unlike hot flashes and vasomotor symptoms, it does not remit or decline over time, and it affects women’s health and quality of life.
While removal of the black box warning would be appropriate, labeling should include emphatic reminders for women that if they have any bleeding or spotting they should seek medical attention immediately, and if they have a history of breast cancer or other estrogen-sensitive cancers they should talk with their oncologist prior to starting treatment with low-dose vaginal estrogen. Although the text would still inform women of research results on systemic hormone therapy, it would explain the differences between low-dose vaginal estrogen and systemic therapy.
Studies show vaginal estrogen has good safety profile
In the last several years, large, observational studies of low-dose vaginal estrogen have suggested that this treatment is not associated with an increase in cardiovascular disease, pulmonary embolism, venous thrombosis, cancer, or dementia—conditions listed in the black box warning that were linked to systemic estrogen therapy plus synthetic progestin. Recent data from the Nurses’ Health Study, for example, demonstrated that 3 years of vaginal estrogen use did not increase the risk of cardiovascular events or invasive breast cancer.
Women’s Health Initiative. In a prospective observational cohort study, Crandall and colleagues used data from participants in the Women’s Health Initiative Observational Study to determine the association between use of vaginal estrogen and risk of a global index event (GIE), defined as time to first occurrence of coronary heart disease, invasive breast cancer, stroke, pulmonary embolism, hip fracture, colorectal cancer, endometrial cancer, or death from any cause.2
Women were recruited from multiple clinical centers, were aged 50 to 79 years at baseline, and did not use systemic estrogen therapy during follow-up. The study included 45,663 women and median follow-up was 7.2 years. The investigators collected data on women’s self-reported use of vaginal estrogen as well as the development of the conditions defined above.
In women with a uterus, there was no significant difference between vaginal estrogen users and nonusers in the risk of stroke, invasive breast cancer, colorectal cancer, endometrial cancer, pulmonary embolism, or deep vein thrombosis. The risks of coronary heart disease, fracture, all-cause mortality, and GIE were lower in vaginal estrogen users than in nonusers (GIE adjusted hazard ratio [HR], 0.68; 95% confidence interval [CI], 0.55–0.86).
In women who had undergone hysterectomy, the risks of the individual GIE components and the overall GIE were not significantly different in users of vaginal estrogen compared with nonusers (GIE adjusted HR, 0.94; 95% CI, 0.70–1.26).
The investigators concluded that the risks of cardiovascular disease and cancer were not increased in postmenopausal women who used vaginal estrogen. Thus, this study offers reassurance on the treatment’s safety.2
Meta-analysis on menopausal hormone therapy and breast cancer risk. Further evidence now indicates that low-dose vaginal estrogen is not linked to chronic health conditions. In a large meta-analysis published in 2019, investigators looked at different types of hormone therapies—oral estrogen plus progestin, transdermal estrogen and progestin, estrogen alone, low-dose vaginal estrogen—and their relationship to breast cancer risk.3
Information on individual participants was obtained from 58 studies, 24 prospective and 34 retrospective. Breast cancer relative risks (RR) during years 5 to 14 of current hormone use were assessed according to the main hormonal contituents, doses, and modes of delivery of the last-used menopausal hormone therapy. For all systemic estrogen-only preparations, the RR was 1.33 (95% CI, 1.28–1.38), while for all estrogen-progestogen preparations, the RR was 2.08 (95% CI, 2.02–2.15). For transdermal estrogen, the RR was 1.35 (95% CI, 1.25–1.46). In contrast, for vaginal estrogen, the RR was 1.09 (95% CI, 0.97–1.23).3
Thus, the analysis found that in all the studies that had been done to date, there was no evidence of increased risk of breast cancer with vaginal estrogen therapy.
The evidence is growing that low-dose vaginal estrogen is different from systemic estrogen in terms of its safety profile and benefit-risk pattern. It is important for the FDA to consider these data and revise the vaginal estrogen label.
On the horizon: New estradiol reference ranges
It would be useful if we could accurately compare estradiol levels in women treated with vaginal estrogen against those of women treated with systemic estrogen therapy. In September 2019, NAMS held a workshop with the goal of establishing reference ranges for estradiol in postmenopausal women.4 It is very important to have good, reliable laboratory assays for estradiol and estrone, and to have a clear understanding of what is a reference range, that is, the range of estradiol levels in postmenopausal women who are not treated with estrogen. That way, you can observe what the estradiol blood levels are in women treated with low-dose vaginal estrogen or those treated with systemic estrogen versus the levels observed among postmenopausal women not receiving any estrogen product.
With the reference range information, we could look at data on the blood levels of estradiol with low-dose vaginal estrogen from the various studies available, as well as the increasing evidence from observational studies of the safety of low-dose vaginal estrogen to better understand its relationship with health. If these studies demonstrate that, with certain doses and formulations of low-dose vaginal estrogen, blood estradiol levels stay within the reference range of postmenopausal estradiol levels, it would inform the labeling modifications of these products. We need this information for future discussions with the FDA.
The laboratory assay technology used for such an investigation is primarily liquid chromatography with tandem mass spectrometry, the so-called LC-MS/MS assay. With use of this technology, the reference range for estradiol may be less than 10 picograms per milliliter. Previously, a very wide and inconsistent range—about 5 to 30 picograms per milliliter—was considered a “normal” range.
NAMS is championing the efforts to define a true evidence-based reference range that would represent the range of levels seen in postmenopausal women.5 This effort has been spearheaded by Dr. Richard Santen and colleagues. Using the more sensitive and specific LC-MS/MS assay will enable researchers and clinicians to better understand how levels on low-dose vaginal estrogen relate to the reference range for postmenopausal women. We are hoping to work together with researchers to establish these reference ranges, and to use that information to look at how low-dose vaginal estrogen compares to levels in untreated postmenopausal women, as well as to levels in women on systemic estrogen.
Hopefully, establishing the reference range can be done in an expeditious and timely way, with discussions with the FDA resuming shortly thereafter.
References
1.NAMS Citizen’s Petition and FDA Response, June 7, 2018. http://www.menopause.org/docs/
2. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. 2018;25:11-20.
3. Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019;394:1159-1168.
4. Santen RJ, Pinkerton JV, Liu JH, et al. Workshop on normal reference ranges for estradiol in postmenopausal women, September 2019, Chicago, Illinois. Menopause. May 4, 2020. doi:10.1097/GME.0000000000001556.
5. Pinkerton JV, Liu JH, Santoro NF, et al. Workshop on normal reference ranges for estradiol in postmenopausal women: commentary from The North American Menopause Society on low-dose vaginal estrogen therapy. Menopause. 2020;27:611-613.
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and The North American Menopause Society. Maturitas. 2014;79:349-354.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126:859-876.
- Shifren JL. Genitourinary syndrome of menopause. Clin Obstet Gynecol. 2018;61:508-516.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803-806.
- Kingsberg SA, Krychman M, Graham S, et al. The women’s EMPOWER survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 Hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- US Food and Drug Administration website. FDA warns against the use of energy-based devices to perform vaginal ‘rejuvenation’ or vaginal cosmetic procedures: FDA safety communication. https://www.fda.gov/medical-devices/safety-communications/fda-warns-against-use-energy-based-devices-perform-vaginal-rejuvenation-or-vaginal-cosmetic. Updated November 20, 2018. Accessed May 21, 2020.
- US Food and Drug Administration website. Letters to industry. https://www.fda.gov/medical-devices/industry-medical-devices/letters-industry. July 24, 2018. Accessed May 21, 2020
The term genitourinary syndrome of menopause (GSM) refers to the bothersome symptoms and physical findings associated with estrogen deficiency that involve the labia, vestibular tissue, clitoris, vagina, urethra, and bladder.1 GSM is associated with genital irritation, dryness, and burning; urinary symptoms including urgency, dysuria, and recurrent urinary tract infections; and sexual symptoms including vaginal dryness and pain. Vulvovaginal atrophy (VVA) represents a component of GSM.
GSM is highly prevalent, affecting more than three-quarters of menopausal women. In contrast to menopausal vasomotor symptoms, which often are most severe and frequent in recently menopausal women, GSM commonly presents years following menopause. Unfortunately, VVA symptoms may have a substantial negative impact on women’s quality of life.
In this 2020 Menopause Update, I review a large observational study that provides reassurance to clinicians and patients regarding the safety of the best-studied prescription treatment for GSM—vaginal estrogen. Because some women should not use vaginal estrogen and others choose not to use it, nonhormonal management of GSM is important. Dr. JoAnn Pinkerton provides details on a randomized clinical trial that compared the use of fractionated CO2 laser therapy with vaginal estrogen for the treatment of GSM. In addition, Dr. JoAnn Manson discusses recent studies that found lower health risks with vaginal estrogen use compared with systemic estrogen therapy.
Diagnosing GSM
GSM can be diagnosed presumptively based on a characteristic history in a menopausal patient. Performing a pelvic examination, however, allows clinicians to exclude other conditions that may present with similar symptoms, such as lichen sclerosus, Candida infection, and malignancy.
During inspection of the external genitalia, the clinician may note loss of the fat pad in the labia majora and mons as well as a reduction in labia minora pigmentation and tissue. The urethral meatus often becomes erythematous and prominent. If vaginal or introital narrowing is present, use of a pediatric (ultrathin) speculum reduces patient discomfort. The vaginal mucosa may appear smooth due to loss of rugation; it also may appear shiny and dry. Bleeding (friability) on contact with a spatula or cotton-tipped swab may occur. In addition, the vaginal fornices may become attenuated, leaving the cervix flush with the vaginal apex.
GSM can be diagnosed without laboratory assessment. However, vaginal pH, if measured, is characteristically higher than 5.0; microscopic wet prep often reveals many white blood cells, immature epithelial cells (large nuclei), and reduced or absent lactobacilli.2

Nonhormonal management of GSM
Water, silicone-based, and oil-based lubricants reduce the friction and discomfort associated with sexual activity. By contrast, vaginal moisturizers act longer than lubricants and can be applied several times weekly or daily. Natural oils, including olive and coconut oil, may be useful both as lubricants and as moisturizers. Aqueous lidocaine 4%, applied to vestibular tissue with cotton balls prior to penetration, reduces dyspareunia in women with GSM.3
Vaginal estrogen therapy
When nonhormonal management does not sufficiently reduce GSM symptoms, use of low-dose vaginal estrogen enhances thickness and elasticity of genital tissue and improves vaginal blood flow. Vaginal estrogen creams, tablets, an insert, and a ring are marketed in the United States. Although clinical improvement may be apparent within several weeks of initiating vaginal estrogen, the full benefit of treatment becomes apparent after 2 to 3 months.3
Despite the availability and effectiveness of low-dose vaginal estrogen, fears regarding the safety of menopausal hormone therapy have resulted in the underutilization of vaginal estrogen.4,5 Unfortunately, the package labeling for low-dose vaginal estrogen can exacerbate these fears.
Continue to: Nurses’ Health Study report...
Nurses’ Health Study report provides reassurance on long-term safety of vaginal estrogen
Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603-610
Bhupathiraju and colleagues published a report from the long-running Nurses’ Health prospective cohort study on the health outcomes associated with the use of vaginal estrogen.
Recap of the study
Starting in 1982, participants in the Nurses’Health Study were asked to report their use of vaginal estrogen via a validated questionnaire. For the years 1982 to 2012, investigators analyzed data from 896 and 52,901 women who had and had not used vaginal estrogen, respectively. The mean duration of vaginal estrogen use was 36 months.
In an analysis adjusted for numerous factors, the investigators observed no statistically significant differences in risk for cardiovascular outcomes (myocardial infarction, stroke, deep vein thrombosis, and pulmonary embolism) or invasive cancers (colorectal, endometrial, ovarian, or breast).
Findings uphold safety of vaginal estrogen
This landmark study provides reassurance that 3 years of use of vaginal estrogen does not increase the risk of cardiovascular events or invasive breast cancer, findings that hopefully will allow clinicians and women to feel comfortable regarding the safety of vaginal estrogen. A study of vaginal estrogen from the Women’s Health Initiative provided similar reassurance. Recent research supports guidance from The North American Menopause Society and the American College of Obstetricians and Gynecologists that vaginal estrogen can be used indefinitely, if indicated, and that use of concomitant progestin is not recommended in women who use vaginal estrogen and have an intact uterus.6,7
I agree with the authors, who point out that since treatment of GSM may need to be continued long term (even indefinitely), it would be helpful to have data that assessed the safety of longer-duration use of vaginal estrogen.
Results from Bhupathiraju and colleagues’ analysis of data from the Nurses’ Health Study on the 3-year safety of vaginal estrogen use encourage clinicians to recommend and women to use this safe and effective treatment for GSM.
How CO2 fractionated vaginal laser therapy compares with vaginal estrogen for relief of GSM symptoms
Paraiso MF, Ferrando CA, Sokol ER, et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET trial. Menopause. 2020;27:50-56.
Up to 50% to 60% of postmenopausal women experience GSM symptoms. However, many fewer receive treatment, either because they do not understand that the symptoms are related to menopause or they are not aware that safe and effective treatment is available. Sadly, many women are not asked about their symptoms or are embarrassed to tell providers.
GSM affects relationships and quality of life. Vaginal lubricants or moisturizers may provide relief. US Food and Drug Administration (FDA)–approved therapies include low-dose vaginal estrogen, available as a vaginal tablet, cream, suppository, and ring; intravaginal dehydroepiandrosterone (DHEA); and oral ospemifene, a selective estrogen replacement modulator. If women have an estrogen-sensitive breast or uterine cancer, an oncologist should be involved in decisions about vaginal hormonal therapy.
Energy-based devices such as vaginal lasers appear to induce wound healing; stimulate collagen and elastin fiber formation through increased storage of glycogen; and activate fibroblasts, which leads to increased extracellular matrix and restoration of vaginal pH.
These lasers are FDA approved for use in gynecology but not specifically for the treatment of GSM. In July 2016, the FDA issued a safety alert that energy-based devices, while approved for use in gynecology, have not been approved or adequately tested for menopausal vaginal conditions, and safety concerns include reports of vaginal burns.8 Lacking are publications of adequately powered randomized, sham-con-trolled trials to determine if laser therapy works better for women with GSM than placebos, moisturizers, or vaginal hormone therapies.
Recently, investigators conducted a multicenter, randomized, single-blinded trial of vaginal laser therapy and estrogen cream for treatment of GSM.
Continue to: Details of the study...
Details of the study
Paraiso and colleagues aimed to compare the 6-month efficacy and safety of fractionated CO2 vaginal laser therapy with that of estrogen vaginal cream for the treatment of vaginal dryness/GSM.
Participants randomly assigned to the estrogen therapy arm applied conjugated estrogen cream 0.5 g vaginally daily for 14 days, followed by twice weekly application for 24 weeks (a low-dose vaginal estrogen therapy). Participants randomly assigned to laser therapy underwent 3 vaginal treatments at a minimum of 6 weeks apart.
Sixty-nine women were enrolled in the trial before enrollment was closed because the FDA required that the sponsor obtain and maintain an investigational device exemption. Of 62 women who completed 6 months’ treatment, 30 received 3 laser treatments and 32 received estrogen cream.
The primary outcome compared subjective improvement in vaginal dryness using the visual analog scale (VAS) between the 2 groups at 6 months. Secondary outcomes included comparisons of the vaginal health index (VHI) and vaginal maturation index (VMI), the effect of GSM on quality of life, the effect of treatment on sexual function and urinary symptoms, and patient satisfaction.
Study findings
Efficacy. Laser therapy and estrogen therapy were found to be similarly effective except on the VMI, which favored estrogen. On patient global impression, 85.8% of laser-treated women rated their improvement as ‘‘better or much better’’ and 78.5% reported being either ‘‘satisfied or very satisfied,’’ compared with 70% and 73.3%, respectively, in the estrogen group, a statistically nonsignificant difference.
On linear regression, the investigators found a nonsignificant mean difference in female sexual function index scores. While VMI scores remained higher in the estrogen-treated group (adjusted P = .02), baseline and 6-month follow-up VMI data were available for only 34 participants (16 laser treated, 18 estrogen treated).
Regarding long-term effectiveness, 20% to 25% of the women in the laser-treated group needed further treatment after 1 year while the estrogen cream continued to work as long as it was used as prescribed.
Adverse effects. The incidence of vaginal bleeding was similar in the 2 groups: 6.7% in the laser group and 6.3% in the estrogen group. In the laser therapy group, 3% expe-rienced vaginal pain, discharge, and bladder infections, while in the estrogen cream group, 3% reported breast tenderness, migraine headaches, and abdominal cramping.
Takeaways. This small randomized, open-label (not blinded) trial provides pilot data on the effectiveness of vaginal CO2 laser compared with vaginal estrogen in treating vaginal atrophy, quality-of-life symptoms, sexual function, and urinary symptoms. Adverse events were minimal. Patient global impression of improvement and satisfaction improved for both vaginal laser and vaginal estrogen therapy.
Continue to: Study strengths and limitations...
Study strengths and limitations
To show noninferiority of vaginal laser therapy to vaginal estrogen, 196 study participants were needed. However, after 38% had been enrolled, the FDA sent a warning letter to the Foundation for Female Health Awareness, which required obtainment of an investigational device exemption for the laser and addition of a sham treatment arm.9 Instead of redesigning the trial and reconsenting the participants, the investigators closed the study, and analysis was performed only on the 62 participants who completed the study; vaginal maturation was assessed only in 34 participants.
The study lacked a placebo or sham control, which increases the risk of bias, while small numbers limit the strength of the findings. Longer-term evaluation of the effects of laser therapy beyond 6 months is needed to allow assessment of the effects of scarring on vaginal health, sexual function, and urinary issues.
Discussing therapy with patients
Despite this study’s preliminary findings, and until more robust data are available, providers should discuss the benefits and risks of all available treatment options for vaginal symptoms, including over-the-counter lubricants, vaginal moisturizers, FDA-approved vaginal hormone therapies (such as vaginal estrogen and intravaginal dehydroepiandrosterone), and systemic therapies, such as hormone therapy and ospemifene, to determine the best treatment for the individual woman with GSM.
In a healthy postmenopausal woman with bothersome GSM symptoms not responsive to lubricants and moisturizers, I recommend FDA-approved vaginal therapies as first-line treatment if there are no contraindications. For women with breast cancer, I involve their oncologist. If a patient asks about vaginal laser treatment, I share that vaginal energy-based therapies, such as the vaginal laser, have not been approved for menopausal vaginal concerns. In addition to the possibility of adverse events or unsuccessful treatment, there are significant out-of-pocket costs and the potential need for ongoing therapy after the initial 3 laser treatments.
For GSM that does not respond to lubricants and moisturizers, many FDA-approved vaginal and systemic therapies are available to treat vaginal symptoms. Vaginal laser treatment is a promising therapy for vaginal symptoms of GSM that needs further testing to determine its efficacy, safety, and long-term effects. If discussing vaginal energy-based therapies with patients, include the current lack of FDA approval for specific vaginal indications, potential adverse effects, the need for ongoing retreatment, and out-of-pocket costs.
JoAnn E. Manson, MD, DrPH, NCMP
Having more appropriate, evidence-based labeling of low-dose vaginal estrogen continues to be a high priority for The North American Menopause Society (NAMS), the International Society for the Study of Women’s Sexual Health (ISSWSH), and other professional societies.
NAMS and the Working Group on Women’s Health and Well-Being in Menopause had submitted a citizen’s petition to the US Food and Drug Administration (FDA) in 2016 requesting modification of the label—including removal of the “black box warning”—for low-dose vaginal estrogen products. The petition was, disappointingly, denied in 2018.1
Currently, the class labeling, which was based on the results of randomized trials with systemic hormone therapy, is not applicable to low-dose vaginal estrogen, and the inclusion of the black box warning has led to serious underutilization of an effective and safe treatment for a very common and life-altering condition, the genitourinary syndrome of menopause (GSM). This condition affects nearly half of postmenopausal women. It tends to be chronic and progressive and, unlike hot flashes and vasomotor symptoms, it does not remit or decline over time, and it affects women’s health and quality of life.
While removal of the black box warning would be appropriate, labeling should include emphatic reminders for women that if they have any bleeding or spotting they should seek medical attention immediately, and if they have a history of breast cancer or other estrogen-sensitive cancers they should talk with their oncologist prior to starting treatment with low-dose vaginal estrogen. Although the text would still inform women of research results on systemic hormone therapy, it would explain the differences between low-dose vaginal estrogen and systemic therapy.
Studies show vaginal estrogen has good safety profile
In the last several years, large, observational studies of low-dose vaginal estrogen have suggested that this treatment is not associated with an increase in cardiovascular disease, pulmonary embolism, venous thrombosis, cancer, or dementia—conditions listed in the black box warning that were linked to systemic estrogen therapy plus synthetic progestin. Recent data from the Nurses’ Health Study, for example, demonstrated that 3 years of vaginal estrogen use did not increase the risk of cardiovascular events or invasive breast cancer.
Women’s Health Initiative. In a prospective observational cohort study, Crandall and colleagues used data from participants in the Women’s Health Initiative Observational Study to determine the association between use of vaginal estrogen and risk of a global index event (GIE), defined as time to first occurrence of coronary heart disease, invasive breast cancer, stroke, pulmonary embolism, hip fracture, colorectal cancer, endometrial cancer, or death from any cause.2
Women were recruited from multiple clinical centers, were aged 50 to 79 years at baseline, and did not use systemic estrogen therapy during follow-up. The study included 45,663 women and median follow-up was 7.2 years. The investigators collected data on women’s self-reported use of vaginal estrogen as well as the development of the conditions defined above.
In women with a uterus, there was no significant difference between vaginal estrogen users and nonusers in the risk of stroke, invasive breast cancer, colorectal cancer, endometrial cancer, pulmonary embolism, or deep vein thrombosis. The risks of coronary heart disease, fracture, all-cause mortality, and GIE were lower in vaginal estrogen users than in nonusers (GIE adjusted hazard ratio [HR], 0.68; 95% confidence interval [CI], 0.55–0.86).
In women who had undergone hysterectomy, the risks of the individual GIE components and the overall GIE were not significantly different in users of vaginal estrogen compared with nonusers (GIE adjusted HR, 0.94; 95% CI, 0.70–1.26).
The investigators concluded that the risks of cardiovascular disease and cancer were not increased in postmenopausal women who used vaginal estrogen. Thus, this study offers reassurance on the treatment’s safety.2
Meta-analysis on menopausal hormone therapy and breast cancer risk. Further evidence now indicates that low-dose vaginal estrogen is not linked to chronic health conditions. In a large meta-analysis published in 2019, investigators looked at different types of hormone therapies—oral estrogen plus progestin, transdermal estrogen and progestin, estrogen alone, low-dose vaginal estrogen—and their relationship to breast cancer risk.3
Information on individual participants was obtained from 58 studies, 24 prospective and 34 retrospective. Breast cancer relative risks (RR) during years 5 to 14 of current hormone use were assessed according to the main hormonal contituents, doses, and modes of delivery of the last-used menopausal hormone therapy. For all systemic estrogen-only preparations, the RR was 1.33 (95% CI, 1.28–1.38), while for all estrogen-progestogen preparations, the RR was 2.08 (95% CI, 2.02–2.15). For transdermal estrogen, the RR was 1.35 (95% CI, 1.25–1.46). In contrast, for vaginal estrogen, the RR was 1.09 (95% CI, 0.97–1.23).3
Thus, the analysis found that in all the studies that had been done to date, there was no evidence of increased risk of breast cancer with vaginal estrogen therapy.
The evidence is growing that low-dose vaginal estrogen is different from systemic estrogen in terms of its safety profile and benefit-risk pattern. It is important for the FDA to consider these data and revise the vaginal estrogen label.
On the horizon: New estradiol reference ranges
It would be useful if we could accurately compare estradiol levels in women treated with vaginal estrogen against those of women treated with systemic estrogen therapy. In September 2019, NAMS held a workshop with the goal of establishing reference ranges for estradiol in postmenopausal women.4 It is very important to have good, reliable laboratory assays for estradiol and estrone, and to have a clear understanding of what is a reference range, that is, the range of estradiol levels in postmenopausal women who are not treated with estrogen. That way, you can observe what the estradiol blood levels are in women treated with low-dose vaginal estrogen or those treated with systemic estrogen versus the levels observed among postmenopausal women not receiving any estrogen product.
With the reference range information, we could look at data on the blood levels of estradiol with low-dose vaginal estrogen from the various studies available, as well as the increasing evidence from observational studies of the safety of low-dose vaginal estrogen to better understand its relationship with health. If these studies demonstrate that, with certain doses and formulations of low-dose vaginal estrogen, blood estradiol levels stay within the reference range of postmenopausal estradiol levels, it would inform the labeling modifications of these products. We need this information for future discussions with the FDA.
The laboratory assay technology used for such an investigation is primarily liquid chromatography with tandem mass spectrometry, the so-called LC-MS/MS assay. With use of this technology, the reference range for estradiol may be less than 10 picograms per milliliter. Previously, a very wide and inconsistent range—about 5 to 30 picograms per milliliter—was considered a “normal” range.
NAMS is championing the efforts to define a true evidence-based reference range that would represent the range of levels seen in postmenopausal women.5 This effort has been spearheaded by Dr. Richard Santen and colleagues. Using the more sensitive and specific LC-MS/MS assay will enable researchers and clinicians to better understand how levels on low-dose vaginal estrogen relate to the reference range for postmenopausal women. We are hoping to work together with researchers to establish these reference ranges, and to use that information to look at how low-dose vaginal estrogen compares to levels in untreated postmenopausal women, as well as to levels in women on systemic estrogen.
Hopefully, establishing the reference range can be done in an expeditious and timely way, with discussions with the FDA resuming shortly thereafter.
References
1.NAMS Citizen’s Petition and FDA Response, June 7, 2018. http://www.menopause.org/docs/
2. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. 2018;25:11-20.
3. Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019;394:1159-1168.
4. Santen RJ, Pinkerton JV, Liu JH, et al. Workshop on normal reference ranges for estradiol in postmenopausal women, September 2019, Chicago, Illinois. Menopause. May 4, 2020. doi:10.1097/GME.0000000000001556.
5. Pinkerton JV, Liu JH, Santoro NF, et al. Workshop on normal reference ranges for estradiol in postmenopausal women: commentary from The North American Menopause Society on low-dose vaginal estrogen therapy. Menopause. 2020;27:611-613.
The term genitourinary syndrome of menopause (GSM) refers to the bothersome symptoms and physical findings associated with estrogen deficiency that involve the labia, vestibular tissue, clitoris, vagina, urethra, and bladder.1 GSM is associated with genital irritation, dryness, and burning; urinary symptoms including urgency, dysuria, and recurrent urinary tract infections; and sexual symptoms including vaginal dryness and pain. Vulvovaginal atrophy (VVA) represents a component of GSM.
GSM is highly prevalent, affecting more than three-quarters of menopausal women. In contrast to menopausal vasomotor symptoms, which often are most severe and frequent in recently menopausal women, GSM commonly presents years following menopause. Unfortunately, VVA symptoms may have a substantial negative impact on women’s quality of life.
In this 2020 Menopause Update, I review a large observational study that provides reassurance to clinicians and patients regarding the safety of the best-studied prescription treatment for GSM—vaginal estrogen. Because some women should not use vaginal estrogen and others choose not to use it, nonhormonal management of GSM is important. Dr. JoAnn Pinkerton provides details on a randomized clinical trial that compared the use of fractionated CO2 laser therapy with vaginal estrogen for the treatment of GSM. In addition, Dr. JoAnn Manson discusses recent studies that found lower health risks with vaginal estrogen use compared with systemic estrogen therapy.
Diagnosing GSM
GSM can be diagnosed presumptively based on a characteristic history in a menopausal patient. Performing a pelvic examination, however, allows clinicians to exclude other conditions that may present with similar symptoms, such as lichen sclerosus, Candida infection, and malignancy.
During inspection of the external genitalia, the clinician may note loss of the fat pad in the labia majora and mons as well as a reduction in labia minora pigmentation and tissue. The urethral meatus often becomes erythematous and prominent. If vaginal or introital narrowing is present, use of a pediatric (ultrathin) speculum reduces patient discomfort. The vaginal mucosa may appear smooth due to loss of rugation; it also may appear shiny and dry. Bleeding (friability) on contact with a spatula or cotton-tipped swab may occur. In addition, the vaginal fornices may become attenuated, leaving the cervix flush with the vaginal apex.
GSM can be diagnosed without laboratory assessment. However, vaginal pH, if measured, is characteristically higher than 5.0; microscopic wet prep often reveals many white blood cells, immature epithelial cells (large nuclei), and reduced or absent lactobacilli.2

Nonhormonal management of GSM
Water, silicone-based, and oil-based lubricants reduce the friction and discomfort associated with sexual activity. By contrast, vaginal moisturizers act longer than lubricants and can be applied several times weekly or daily. Natural oils, including olive and coconut oil, may be useful both as lubricants and as moisturizers. Aqueous lidocaine 4%, applied to vestibular tissue with cotton balls prior to penetration, reduces dyspareunia in women with GSM.3
Vaginal estrogen therapy
When nonhormonal management does not sufficiently reduce GSM symptoms, use of low-dose vaginal estrogen enhances thickness and elasticity of genital tissue and improves vaginal blood flow. Vaginal estrogen creams, tablets, an insert, and a ring are marketed in the United States. Although clinical improvement may be apparent within several weeks of initiating vaginal estrogen, the full benefit of treatment becomes apparent after 2 to 3 months.3
Despite the availability and effectiveness of low-dose vaginal estrogen, fears regarding the safety of menopausal hormone therapy have resulted in the underutilization of vaginal estrogen.4,5 Unfortunately, the package labeling for low-dose vaginal estrogen can exacerbate these fears.
Continue to: Nurses’ Health Study report...
Nurses’ Health Study report provides reassurance on long-term safety of vaginal estrogen
Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603-610
Bhupathiraju and colleagues published a report from the long-running Nurses’ Health prospective cohort study on the health outcomes associated with the use of vaginal estrogen.
Recap of the study
Starting in 1982, participants in the Nurses’Health Study were asked to report their use of vaginal estrogen via a validated questionnaire. For the years 1982 to 2012, investigators analyzed data from 896 and 52,901 women who had and had not used vaginal estrogen, respectively. The mean duration of vaginal estrogen use was 36 months.
In an analysis adjusted for numerous factors, the investigators observed no statistically significant differences in risk for cardiovascular outcomes (myocardial infarction, stroke, deep vein thrombosis, and pulmonary embolism) or invasive cancers (colorectal, endometrial, ovarian, or breast).
Findings uphold safety of vaginal estrogen
This landmark study provides reassurance that 3 years of use of vaginal estrogen does not increase the risk of cardiovascular events or invasive breast cancer, findings that hopefully will allow clinicians and women to feel comfortable regarding the safety of vaginal estrogen. A study of vaginal estrogen from the Women’s Health Initiative provided similar reassurance. Recent research supports guidance from The North American Menopause Society and the American College of Obstetricians and Gynecologists that vaginal estrogen can be used indefinitely, if indicated, and that use of concomitant progestin is not recommended in women who use vaginal estrogen and have an intact uterus.6,7
I agree with the authors, who point out that since treatment of GSM may need to be continued long term (even indefinitely), it would be helpful to have data that assessed the safety of longer-duration use of vaginal estrogen.
Results from Bhupathiraju and colleagues’ analysis of data from the Nurses’ Health Study on the 3-year safety of vaginal estrogen use encourage clinicians to recommend and women to use this safe and effective treatment for GSM.
How CO2 fractionated vaginal laser therapy compares with vaginal estrogen for relief of GSM symptoms
Paraiso MF, Ferrando CA, Sokol ER, et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET trial. Menopause. 2020;27:50-56.
Up to 50% to 60% of postmenopausal women experience GSM symptoms. However, many fewer receive treatment, either because they do not understand that the symptoms are related to menopause or they are not aware that safe and effective treatment is available. Sadly, many women are not asked about their symptoms or are embarrassed to tell providers.
GSM affects relationships and quality of life. Vaginal lubricants or moisturizers may provide relief. US Food and Drug Administration (FDA)–approved therapies include low-dose vaginal estrogen, available as a vaginal tablet, cream, suppository, and ring; intravaginal dehydroepiandrosterone (DHEA); and oral ospemifene, a selective estrogen replacement modulator. If women have an estrogen-sensitive breast or uterine cancer, an oncologist should be involved in decisions about vaginal hormonal therapy.
Energy-based devices such as vaginal lasers appear to induce wound healing; stimulate collagen and elastin fiber formation through increased storage of glycogen; and activate fibroblasts, which leads to increased extracellular matrix and restoration of vaginal pH.
These lasers are FDA approved for use in gynecology but not specifically for the treatment of GSM. In July 2016, the FDA issued a safety alert that energy-based devices, while approved for use in gynecology, have not been approved or adequately tested for menopausal vaginal conditions, and safety concerns include reports of vaginal burns.8 Lacking are publications of adequately powered randomized, sham-con-trolled trials to determine if laser therapy works better for women with GSM than placebos, moisturizers, or vaginal hormone therapies.
Recently, investigators conducted a multicenter, randomized, single-blinded trial of vaginal laser therapy and estrogen cream for treatment of GSM.
Continue to: Details of the study...
Details of the study
Paraiso and colleagues aimed to compare the 6-month efficacy and safety of fractionated CO2 vaginal laser therapy with that of estrogen vaginal cream for the treatment of vaginal dryness/GSM.
Participants randomly assigned to the estrogen therapy arm applied conjugated estrogen cream 0.5 g vaginally daily for 14 days, followed by twice weekly application for 24 weeks (a low-dose vaginal estrogen therapy). Participants randomly assigned to laser therapy underwent 3 vaginal treatments at a minimum of 6 weeks apart.
Sixty-nine women were enrolled in the trial before enrollment was closed because the FDA required that the sponsor obtain and maintain an investigational device exemption. Of 62 women who completed 6 months’ treatment, 30 received 3 laser treatments and 32 received estrogen cream.
The primary outcome compared subjective improvement in vaginal dryness using the visual analog scale (VAS) between the 2 groups at 6 months. Secondary outcomes included comparisons of the vaginal health index (VHI) and vaginal maturation index (VMI), the effect of GSM on quality of life, the effect of treatment on sexual function and urinary symptoms, and patient satisfaction.
Study findings
Efficacy. Laser therapy and estrogen therapy were found to be similarly effective except on the VMI, which favored estrogen. On patient global impression, 85.8% of laser-treated women rated their improvement as ‘‘better or much better’’ and 78.5% reported being either ‘‘satisfied or very satisfied,’’ compared with 70% and 73.3%, respectively, in the estrogen group, a statistically nonsignificant difference.
On linear regression, the investigators found a nonsignificant mean difference in female sexual function index scores. While VMI scores remained higher in the estrogen-treated group (adjusted P = .02), baseline and 6-month follow-up VMI data were available for only 34 participants (16 laser treated, 18 estrogen treated).
Regarding long-term effectiveness, 20% to 25% of the women in the laser-treated group needed further treatment after 1 year while the estrogen cream continued to work as long as it was used as prescribed.
Adverse effects. The incidence of vaginal bleeding was similar in the 2 groups: 6.7% in the laser group and 6.3% in the estrogen group. In the laser therapy group, 3% expe-rienced vaginal pain, discharge, and bladder infections, while in the estrogen cream group, 3% reported breast tenderness, migraine headaches, and abdominal cramping.
Takeaways. This small randomized, open-label (not blinded) trial provides pilot data on the effectiveness of vaginal CO2 laser compared with vaginal estrogen in treating vaginal atrophy, quality-of-life symptoms, sexual function, and urinary symptoms. Adverse events were minimal. Patient global impression of improvement and satisfaction improved for both vaginal laser and vaginal estrogen therapy.
Continue to: Study strengths and limitations...
Study strengths and limitations
To show noninferiority of vaginal laser therapy to vaginal estrogen, 196 study participants were needed. However, after 38% had been enrolled, the FDA sent a warning letter to the Foundation for Female Health Awareness, which required obtainment of an investigational device exemption for the laser and addition of a sham treatment arm.9 Instead of redesigning the trial and reconsenting the participants, the investigators closed the study, and analysis was performed only on the 62 participants who completed the study; vaginal maturation was assessed only in 34 participants.
The study lacked a placebo or sham control, which increases the risk of bias, while small numbers limit the strength of the findings. Longer-term evaluation of the effects of laser therapy beyond 6 months is needed to allow assessment of the effects of scarring on vaginal health, sexual function, and urinary issues.
Discussing therapy with patients
Despite this study’s preliminary findings, and until more robust data are available, providers should discuss the benefits and risks of all available treatment options for vaginal symptoms, including over-the-counter lubricants, vaginal moisturizers, FDA-approved vaginal hormone therapies (such as vaginal estrogen and intravaginal dehydroepiandrosterone), and systemic therapies, such as hormone therapy and ospemifene, to determine the best treatment for the individual woman with GSM.
In a healthy postmenopausal woman with bothersome GSM symptoms not responsive to lubricants and moisturizers, I recommend FDA-approved vaginal therapies as first-line treatment if there are no contraindications. For women with breast cancer, I involve their oncologist. If a patient asks about vaginal laser treatment, I share that vaginal energy-based therapies, such as the vaginal laser, have not been approved for menopausal vaginal concerns. In addition to the possibility of adverse events or unsuccessful treatment, there are significant out-of-pocket costs and the potential need for ongoing therapy after the initial 3 laser treatments.
For GSM that does not respond to lubricants and moisturizers, many FDA-approved vaginal and systemic therapies are available to treat vaginal symptoms. Vaginal laser treatment is a promising therapy for vaginal symptoms of GSM that needs further testing to determine its efficacy, safety, and long-term effects. If discussing vaginal energy-based therapies with patients, include the current lack of FDA approval for specific vaginal indications, potential adverse effects, the need for ongoing retreatment, and out-of-pocket costs.
JoAnn E. Manson, MD, DrPH, NCMP
Having more appropriate, evidence-based labeling of low-dose vaginal estrogen continues to be a high priority for The North American Menopause Society (NAMS), the International Society for the Study of Women’s Sexual Health (ISSWSH), and other professional societies.
NAMS and the Working Group on Women’s Health and Well-Being in Menopause had submitted a citizen’s petition to the US Food and Drug Administration (FDA) in 2016 requesting modification of the label—including removal of the “black box warning”—for low-dose vaginal estrogen products. The petition was, disappointingly, denied in 2018.1
Currently, the class labeling, which was based on the results of randomized trials with systemic hormone therapy, is not applicable to low-dose vaginal estrogen, and the inclusion of the black box warning has led to serious underutilization of an effective and safe treatment for a very common and life-altering condition, the genitourinary syndrome of menopause (GSM). This condition affects nearly half of postmenopausal women. It tends to be chronic and progressive and, unlike hot flashes and vasomotor symptoms, it does not remit or decline over time, and it affects women’s health and quality of life.
While removal of the black box warning would be appropriate, labeling should include emphatic reminders for women that if they have any bleeding or spotting they should seek medical attention immediately, and if they have a history of breast cancer or other estrogen-sensitive cancers they should talk with their oncologist prior to starting treatment with low-dose vaginal estrogen. Although the text would still inform women of research results on systemic hormone therapy, it would explain the differences between low-dose vaginal estrogen and systemic therapy.
Studies show vaginal estrogen has good safety profile
In the last several years, large, observational studies of low-dose vaginal estrogen have suggested that this treatment is not associated with an increase in cardiovascular disease, pulmonary embolism, venous thrombosis, cancer, or dementia—conditions listed in the black box warning that were linked to systemic estrogen therapy plus synthetic progestin. Recent data from the Nurses’ Health Study, for example, demonstrated that 3 years of vaginal estrogen use did not increase the risk of cardiovascular events or invasive breast cancer.
Women’s Health Initiative. In a prospective observational cohort study, Crandall and colleagues used data from participants in the Women’s Health Initiative Observational Study to determine the association between use of vaginal estrogen and risk of a global index event (GIE), defined as time to first occurrence of coronary heart disease, invasive breast cancer, stroke, pulmonary embolism, hip fracture, colorectal cancer, endometrial cancer, or death from any cause.2
Women were recruited from multiple clinical centers, were aged 50 to 79 years at baseline, and did not use systemic estrogen therapy during follow-up. The study included 45,663 women and median follow-up was 7.2 years. The investigators collected data on women’s self-reported use of vaginal estrogen as well as the development of the conditions defined above.
In women with a uterus, there was no significant difference between vaginal estrogen users and nonusers in the risk of stroke, invasive breast cancer, colorectal cancer, endometrial cancer, pulmonary embolism, or deep vein thrombosis. The risks of coronary heart disease, fracture, all-cause mortality, and GIE were lower in vaginal estrogen users than in nonusers (GIE adjusted hazard ratio [HR], 0.68; 95% confidence interval [CI], 0.55–0.86).
In women who had undergone hysterectomy, the risks of the individual GIE components and the overall GIE were not significantly different in users of vaginal estrogen compared with nonusers (GIE adjusted HR, 0.94; 95% CI, 0.70–1.26).
The investigators concluded that the risks of cardiovascular disease and cancer were not increased in postmenopausal women who used vaginal estrogen. Thus, this study offers reassurance on the treatment’s safety.2
Meta-analysis on menopausal hormone therapy and breast cancer risk. Further evidence now indicates that low-dose vaginal estrogen is not linked to chronic health conditions. In a large meta-analysis published in 2019, investigators looked at different types of hormone therapies—oral estrogen plus progestin, transdermal estrogen and progestin, estrogen alone, low-dose vaginal estrogen—and their relationship to breast cancer risk.3
Information on individual participants was obtained from 58 studies, 24 prospective and 34 retrospective. Breast cancer relative risks (RR) during years 5 to 14 of current hormone use were assessed according to the main hormonal contituents, doses, and modes of delivery of the last-used menopausal hormone therapy. For all systemic estrogen-only preparations, the RR was 1.33 (95% CI, 1.28–1.38), while for all estrogen-progestogen preparations, the RR was 2.08 (95% CI, 2.02–2.15). For transdermal estrogen, the RR was 1.35 (95% CI, 1.25–1.46). In contrast, for vaginal estrogen, the RR was 1.09 (95% CI, 0.97–1.23).3
Thus, the analysis found that in all the studies that had been done to date, there was no evidence of increased risk of breast cancer with vaginal estrogen therapy.
The evidence is growing that low-dose vaginal estrogen is different from systemic estrogen in terms of its safety profile and benefit-risk pattern. It is important for the FDA to consider these data and revise the vaginal estrogen label.
On the horizon: New estradiol reference ranges
It would be useful if we could accurately compare estradiol levels in women treated with vaginal estrogen against those of women treated with systemic estrogen therapy. In September 2019, NAMS held a workshop with the goal of establishing reference ranges for estradiol in postmenopausal women.4 It is very important to have good, reliable laboratory assays for estradiol and estrone, and to have a clear understanding of what is a reference range, that is, the range of estradiol levels in postmenopausal women who are not treated with estrogen. That way, you can observe what the estradiol blood levels are in women treated with low-dose vaginal estrogen or those treated with systemic estrogen versus the levels observed among postmenopausal women not receiving any estrogen product.
With the reference range information, we could look at data on the blood levels of estradiol with low-dose vaginal estrogen from the various studies available, as well as the increasing evidence from observational studies of the safety of low-dose vaginal estrogen to better understand its relationship with health. If these studies demonstrate that, with certain doses and formulations of low-dose vaginal estrogen, blood estradiol levels stay within the reference range of postmenopausal estradiol levels, it would inform the labeling modifications of these products. We need this information for future discussions with the FDA.
The laboratory assay technology used for such an investigation is primarily liquid chromatography with tandem mass spectrometry, the so-called LC-MS/MS assay. With use of this technology, the reference range for estradiol may be less than 10 picograms per milliliter. Previously, a very wide and inconsistent range—about 5 to 30 picograms per milliliter—was considered a “normal” range.
NAMS is championing the efforts to define a true evidence-based reference range that would represent the range of levels seen in postmenopausal women.5 This effort has been spearheaded by Dr. Richard Santen and colleagues. Using the more sensitive and specific LC-MS/MS assay will enable researchers and clinicians to better understand how levels on low-dose vaginal estrogen relate to the reference range for postmenopausal women. We are hoping to work together with researchers to establish these reference ranges, and to use that information to look at how low-dose vaginal estrogen compares to levels in untreated postmenopausal women, as well as to levels in women on systemic estrogen.
Hopefully, establishing the reference range can be done in an expeditious and timely way, with discussions with the FDA resuming shortly thereafter.
References
1.NAMS Citizen’s Petition and FDA Response, June 7, 2018. http://www.menopause.org/docs/
2. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. 2018;25:11-20.
3. Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019;394:1159-1168.
4. Santen RJ, Pinkerton JV, Liu JH, et al. Workshop on normal reference ranges for estradiol in postmenopausal women, September 2019, Chicago, Illinois. Menopause. May 4, 2020. doi:10.1097/GME.0000000000001556.
5. Pinkerton JV, Liu JH, Santoro NF, et al. Workshop on normal reference ranges for estradiol in postmenopausal women: commentary from The North American Menopause Society on low-dose vaginal estrogen therapy. Menopause. 2020;27:611-613.
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and The North American Menopause Society. Maturitas. 2014;79:349-354.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126:859-876.
- Shifren JL. Genitourinary syndrome of menopause. Clin Obstet Gynecol. 2018;61:508-516.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803-806.
- Kingsberg SA, Krychman M, Graham S, et al. The women’s EMPOWER survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 Hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- US Food and Drug Administration website. FDA warns against the use of energy-based devices to perform vaginal ‘rejuvenation’ or vaginal cosmetic procedures: FDA safety communication. https://www.fda.gov/medical-devices/safety-communications/fda-warns-against-use-energy-based-devices-perform-vaginal-rejuvenation-or-vaginal-cosmetic. Updated November 20, 2018. Accessed May 21, 2020.
- US Food and Drug Administration website. Letters to industry. https://www.fda.gov/medical-devices/industry-medical-devices/letters-industry. July 24, 2018. Accessed May 21, 2020
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and The North American Menopause Society. Maturitas. 2014;79:349-354.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126:859-876.
- Shifren JL. Genitourinary syndrome of menopause. Clin Obstet Gynecol. 2018;61:508-516.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803-806.
- Kingsberg SA, Krychman M, Graham S, et al. The women’s EMPOWER survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 Hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- US Food and Drug Administration website. FDA warns against the use of energy-based devices to perform vaginal ‘rejuvenation’ or vaginal cosmetic procedures: FDA safety communication. https://www.fda.gov/medical-devices/safety-communications/fda-warns-against-use-energy-based-devices-perform-vaginal-rejuvenation-or-vaginal-cosmetic. Updated November 20, 2018. Accessed May 21, 2020.
- US Food and Drug Administration website. Letters to industry. https://www.fda.gov/medical-devices/industry-medical-devices/letters-industry. July 24, 2018. Accessed May 21, 2020
ASCCP guidelines for managing abnormal cervical cancer tests: What’s new?
The 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors Consensus Guidelines, which represent a consensus of nearly 20 professional organizations and patient advocates, are a culmination of almost 10 years of research.1 With the last version issued in 2012,2 these latest guidelines offer the most recent recommendations regarding safely triaging women with abnormal cervical cancer screening results.
According to the consensus, research has shown that risk-based management allows clinicians to better discriminate women who will likely develop precancer from those who can safely continue with routine screening. As you will hear from guidelines coauthor Dr. Warner Huh, one of the most important differences between these guidelines and the 2012 version is a new emphasis on the principle of “equal management for equal risk.” Essentially, this insures that all women who have the same amount of risk for progression to precancer or cancer are managed the same.
The guidelines were once again published in the Journal of Lower Genital Tract Disease, and the tables they reference are publicly available. Additionally, ASCCP is developing a new management guidelines app to facilitate the use of the guidelines on smartphones and computers. With the publicly available risk tables, and the ASCCP navigation app, the guidelines will more easily accommodate updates as new information and technology become available.
OBG Management : The latest ASCCP guidelines, published in April, represent a “paradigm shift” from results to risk-based guidelines. Can you explain what this means and why the shift was undertaken?
Warner K. Huh, MD: Yes, the shift occurred because we needed to focus less on algorithms and more on risk. We started promulgating a concept of “equal management for equal risk” back in 2012. What this means is that if we have a method to look up a risk score based on relevant test results and other pieces of information, then all patients with that score should be managed in the same manner. We wanted that to be the underlying principle.
Focusing on risk tables also makes it easier to incorporate any future technologies used for risk estimation without having to rebuild algorithms from scratch. ASCCP is developing a new management guidelines app to streamline navigation of the guidelines. This app makes them easier for clinicians to use; they simply plug in certain variables from the patient’s history and receive 1 of 5 outputs: treatment, colposcopy, or surveillance at 1, 3, or 5 years.
The only drawbacks, if you view them as such, are that the clinician must plug in all the variables, and then must sit back and trust in what we have done. Clinicians have to trust that the system works and will simplify the clinical decision making.
We spent a lot of time determining what the risk thresholds should be. Some may argue they are arbitrary, but the decisions were data-driven, and carefully, thoughtfully vetted; we deliberated about whether the cut points actually made sense clinically to a practicing clinician base. The clinical action thresholds refer to a specific percentage below which a woman falls into one bucket and above which she falls into another bucket.
The other element that is unique about the guidelines is that instead of looking at the patient’s current screening result in isolation, the user sees it along with the prior one because prior history dictates subsequent risk.
It’s important that clinicians understand why this system is so markedly different from what we have done previously, and why risk-based guidelines make infinitely more sense than algorithmic ones. It’s because: 1) they can be easier to use; 2) they incorporate new data more efficiently and effectively than algorithm-based guidelines; and 3) they can incorporate future technologies seamlessly rather than having to create yet another algorithm.
Continue to: OBG Management : What do clinicians need in order to execute the guidelines?...
OBG Management : What do clinicians need in order to execute the guidelines?
Dr. Huh: Nothing. All of the information needed—the guidelines article and risk tables—are publicly available. However, to make navigation of the guidelines easier, the plan is for the app that I mentioned. I have the app on my phone and am actively beta testing it now. We’re planning on creating a web-based application as well, that will allow users to access the Internet and their electronic health record system so that they can plug in information directly from patient charts. The web-based app will be similar to the web-based Breast Cancer Surveillance Consortium’s Risk Calculator (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm). You will pull it up, plug in the requested information, including the patient’s age; their Pap smear and genotyping results; and their previous screening history.
OBG Management : When will the app be available for users?
Dr. Huh: It will be available for release on June 8.
OBG Management : Were HPV vaccination levels incorporated into the new guidelines?
OBG Management : Have recommendations regarding colposcopy changed?
Dr. Huh: Not really. About 3 years ago, we created basic colposcopy guidelines—the ASCCP Colposcopy Standards—so everything about colposcopy references back to those guidelines. Those colposcopy standards covered terminology and risk-based colposcopy, which actually aligns beautifully with these guidelines.
OBG Management : To narrow in on some changes from the prior guidelines, can colposcopy be deferred in certain patients?
Dr. Huh: Yes. Not everyone who has an abnormal screening test needs to come back for colposcopy.
OBG Management : How has guidance for expedited treatment or treatment without colposcopic biopsy changed?
Dr. Huh: This was heavily debated within not only the treatment group that I co-chaired with Richard Guido, MD, but also within the entire steering committee. The recommendation is that if the patient has an immediate risk of CIN 3 that is >60%, the patient should go straight to treatment without a colposcopic biopsy. The main reason for this is that you do not want to biopsy a patient and then lose them to follow-up.
When a woman has >60% immediate risk of CIN 3, we are fairly certain that colposcopy is not going to change management ultimately, so we recommend that patients receive treatment right away. We have already been doing this for 15 to 20 years, so this is not a new concept. It is just more formally codified here by assigning a percentage to the risk. Those who have between 25% and 60% immediate risk of CIN 3 should receive immediate colposcopy. We realize that not all clinicians have the ability to do this, so if clinicians can’t treat immediately, we recommend they do whatever they can to prevent losing the patient to follow-up.
OBG Management : How should a positive primary HPV screening test be managed?
Dr. Huh: If a woman has a positive primary HPV screening test, genotyping should be performed. If genotyping reveals HPV 16 or 18, then the patient should proceed to colposcopy. If genotyping reveals other forms of HPV, reflex cytology or a Pap smear should follow. ●
- Perkins RB, Guido RS, Castle PE, et al, for the 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2020;24(2):102-131.
- Massad LS, Einstein MH, Huh WK, et al, for the 2012 ASCCP Consensus Guidelines Conference. 2012 Updated Consensus Guidelines for the Management of Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2013;17(5):S1-S27.
The 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors Consensus Guidelines, which represent a consensus of nearly 20 professional organizations and patient advocates, are a culmination of almost 10 years of research.1 With the last version issued in 2012,2 these latest guidelines offer the most recent recommendations regarding safely triaging women with abnormal cervical cancer screening results.
According to the consensus, research has shown that risk-based management allows clinicians to better discriminate women who will likely develop precancer from those who can safely continue with routine screening. As you will hear from guidelines coauthor Dr. Warner Huh, one of the most important differences between these guidelines and the 2012 version is a new emphasis on the principle of “equal management for equal risk.” Essentially, this insures that all women who have the same amount of risk for progression to precancer or cancer are managed the same.
The guidelines were once again published in the Journal of Lower Genital Tract Disease, and the tables they reference are publicly available. Additionally, ASCCP is developing a new management guidelines app to facilitate the use of the guidelines on smartphones and computers. With the publicly available risk tables, and the ASCCP navigation app, the guidelines will more easily accommodate updates as new information and technology become available.
OBG Management : The latest ASCCP guidelines, published in April, represent a “paradigm shift” from results to risk-based guidelines. Can you explain what this means and why the shift was undertaken?
Warner K. Huh, MD: Yes, the shift occurred because we needed to focus less on algorithms and more on risk. We started promulgating a concept of “equal management for equal risk” back in 2012. What this means is that if we have a method to look up a risk score based on relevant test results and other pieces of information, then all patients with that score should be managed in the same manner. We wanted that to be the underlying principle.
Focusing on risk tables also makes it easier to incorporate any future technologies used for risk estimation without having to rebuild algorithms from scratch. ASCCP is developing a new management guidelines app to streamline navigation of the guidelines. This app makes them easier for clinicians to use; they simply plug in certain variables from the patient’s history and receive 1 of 5 outputs: treatment, colposcopy, or surveillance at 1, 3, or 5 years.
The only drawbacks, if you view them as such, are that the clinician must plug in all the variables, and then must sit back and trust in what we have done. Clinicians have to trust that the system works and will simplify the clinical decision making.
We spent a lot of time determining what the risk thresholds should be. Some may argue they are arbitrary, but the decisions were data-driven, and carefully, thoughtfully vetted; we deliberated about whether the cut points actually made sense clinically to a practicing clinician base. The clinical action thresholds refer to a specific percentage below which a woman falls into one bucket and above which she falls into another bucket.
The other element that is unique about the guidelines is that instead of looking at the patient’s current screening result in isolation, the user sees it along with the prior one because prior history dictates subsequent risk.
It’s important that clinicians understand why this system is so markedly different from what we have done previously, and why risk-based guidelines make infinitely more sense than algorithmic ones. It’s because: 1) they can be easier to use; 2) they incorporate new data more efficiently and effectively than algorithm-based guidelines; and 3) they can incorporate future technologies seamlessly rather than having to create yet another algorithm.
Continue to: OBG Management : What do clinicians need in order to execute the guidelines?...
OBG Management : What do clinicians need in order to execute the guidelines?
Dr. Huh: Nothing. All of the information needed—the guidelines article and risk tables—are publicly available. However, to make navigation of the guidelines easier, the plan is for the app that I mentioned. I have the app on my phone and am actively beta testing it now. We’re planning on creating a web-based application as well, that will allow users to access the Internet and their electronic health record system so that they can plug in information directly from patient charts. The web-based app will be similar to the web-based Breast Cancer Surveillance Consortium’s Risk Calculator (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm). You will pull it up, plug in the requested information, including the patient’s age; their Pap smear and genotyping results; and their previous screening history.
OBG Management : When will the app be available for users?
Dr. Huh: It will be available for release on June 8.
OBG Management : Were HPV vaccination levels incorporated into the new guidelines?
OBG Management : Have recommendations regarding colposcopy changed?
Dr. Huh: Not really. About 3 years ago, we created basic colposcopy guidelines—the ASCCP Colposcopy Standards—so everything about colposcopy references back to those guidelines. Those colposcopy standards covered terminology and risk-based colposcopy, which actually aligns beautifully with these guidelines.
OBG Management : To narrow in on some changes from the prior guidelines, can colposcopy be deferred in certain patients?
Dr. Huh: Yes. Not everyone who has an abnormal screening test needs to come back for colposcopy.
OBG Management : How has guidance for expedited treatment or treatment without colposcopic biopsy changed?
Dr. Huh: This was heavily debated within not only the treatment group that I co-chaired with Richard Guido, MD, but also within the entire steering committee. The recommendation is that if the patient has an immediate risk of CIN 3 that is >60%, the patient should go straight to treatment without a colposcopic biopsy. The main reason for this is that you do not want to biopsy a patient and then lose them to follow-up.
When a woman has >60% immediate risk of CIN 3, we are fairly certain that colposcopy is not going to change management ultimately, so we recommend that patients receive treatment right away. We have already been doing this for 15 to 20 years, so this is not a new concept. It is just more formally codified here by assigning a percentage to the risk. Those who have between 25% and 60% immediate risk of CIN 3 should receive immediate colposcopy. We realize that not all clinicians have the ability to do this, so if clinicians can’t treat immediately, we recommend they do whatever they can to prevent losing the patient to follow-up.
OBG Management : How should a positive primary HPV screening test be managed?
Dr. Huh: If a woman has a positive primary HPV screening test, genotyping should be performed. If genotyping reveals HPV 16 or 18, then the patient should proceed to colposcopy. If genotyping reveals other forms of HPV, reflex cytology or a Pap smear should follow. ●
The 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors Consensus Guidelines, which represent a consensus of nearly 20 professional organizations and patient advocates, are a culmination of almost 10 years of research.1 With the last version issued in 2012,2 these latest guidelines offer the most recent recommendations regarding safely triaging women with abnormal cervical cancer screening results.
According to the consensus, research has shown that risk-based management allows clinicians to better discriminate women who will likely develop precancer from those who can safely continue with routine screening. As you will hear from guidelines coauthor Dr. Warner Huh, one of the most important differences between these guidelines and the 2012 version is a new emphasis on the principle of “equal management for equal risk.” Essentially, this insures that all women who have the same amount of risk for progression to precancer or cancer are managed the same.
The guidelines were once again published in the Journal of Lower Genital Tract Disease, and the tables they reference are publicly available. Additionally, ASCCP is developing a new management guidelines app to facilitate the use of the guidelines on smartphones and computers. With the publicly available risk tables, and the ASCCP navigation app, the guidelines will more easily accommodate updates as new information and technology become available.
OBG Management : The latest ASCCP guidelines, published in April, represent a “paradigm shift” from results to risk-based guidelines. Can you explain what this means and why the shift was undertaken?
Warner K. Huh, MD: Yes, the shift occurred because we needed to focus less on algorithms and more on risk. We started promulgating a concept of “equal management for equal risk” back in 2012. What this means is that if we have a method to look up a risk score based on relevant test results and other pieces of information, then all patients with that score should be managed in the same manner. We wanted that to be the underlying principle.
Focusing on risk tables also makes it easier to incorporate any future technologies used for risk estimation without having to rebuild algorithms from scratch. ASCCP is developing a new management guidelines app to streamline navigation of the guidelines. This app makes them easier for clinicians to use; they simply plug in certain variables from the patient’s history and receive 1 of 5 outputs: treatment, colposcopy, or surveillance at 1, 3, or 5 years.
The only drawbacks, if you view them as such, are that the clinician must plug in all the variables, and then must sit back and trust in what we have done. Clinicians have to trust that the system works and will simplify the clinical decision making.
We spent a lot of time determining what the risk thresholds should be. Some may argue they are arbitrary, but the decisions were data-driven, and carefully, thoughtfully vetted; we deliberated about whether the cut points actually made sense clinically to a practicing clinician base. The clinical action thresholds refer to a specific percentage below which a woman falls into one bucket and above which she falls into another bucket.
The other element that is unique about the guidelines is that instead of looking at the patient’s current screening result in isolation, the user sees it along with the prior one because prior history dictates subsequent risk.
It’s important that clinicians understand why this system is so markedly different from what we have done previously, and why risk-based guidelines make infinitely more sense than algorithmic ones. It’s because: 1) they can be easier to use; 2) they incorporate new data more efficiently and effectively than algorithm-based guidelines; and 3) they can incorporate future technologies seamlessly rather than having to create yet another algorithm.
Continue to: OBG Management : What do clinicians need in order to execute the guidelines?...
OBG Management : What do clinicians need in order to execute the guidelines?
Dr. Huh: Nothing. All of the information needed—the guidelines article and risk tables—are publicly available. However, to make navigation of the guidelines easier, the plan is for the app that I mentioned. I have the app on my phone and am actively beta testing it now. We’re planning on creating a web-based application as well, that will allow users to access the Internet and their electronic health record system so that they can plug in information directly from patient charts. The web-based app will be similar to the web-based Breast Cancer Surveillance Consortium’s Risk Calculator (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm). You will pull it up, plug in the requested information, including the patient’s age; their Pap smear and genotyping results; and their previous screening history.
OBG Management : When will the app be available for users?
Dr. Huh: It will be available for release on June 8.
OBG Management : Were HPV vaccination levels incorporated into the new guidelines?
OBG Management : Have recommendations regarding colposcopy changed?
Dr. Huh: Not really. About 3 years ago, we created basic colposcopy guidelines—the ASCCP Colposcopy Standards—so everything about colposcopy references back to those guidelines. Those colposcopy standards covered terminology and risk-based colposcopy, which actually aligns beautifully with these guidelines.
OBG Management : To narrow in on some changes from the prior guidelines, can colposcopy be deferred in certain patients?
Dr. Huh: Yes. Not everyone who has an abnormal screening test needs to come back for colposcopy.
OBG Management : How has guidance for expedited treatment or treatment without colposcopic biopsy changed?
Dr. Huh: This was heavily debated within not only the treatment group that I co-chaired with Richard Guido, MD, but also within the entire steering committee. The recommendation is that if the patient has an immediate risk of CIN 3 that is >60%, the patient should go straight to treatment without a colposcopic biopsy. The main reason for this is that you do not want to biopsy a patient and then lose them to follow-up.
When a woman has >60% immediate risk of CIN 3, we are fairly certain that colposcopy is not going to change management ultimately, so we recommend that patients receive treatment right away. We have already been doing this for 15 to 20 years, so this is not a new concept. It is just more formally codified here by assigning a percentage to the risk. Those who have between 25% and 60% immediate risk of CIN 3 should receive immediate colposcopy. We realize that not all clinicians have the ability to do this, so if clinicians can’t treat immediately, we recommend they do whatever they can to prevent losing the patient to follow-up.
OBG Management : How should a positive primary HPV screening test be managed?
Dr. Huh: If a woman has a positive primary HPV screening test, genotyping should be performed. If genotyping reveals HPV 16 or 18, then the patient should proceed to colposcopy. If genotyping reveals other forms of HPV, reflex cytology or a Pap smear should follow. ●
- Perkins RB, Guido RS, Castle PE, et al, for the 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2020;24(2):102-131.
- Massad LS, Einstein MH, Huh WK, et al, for the 2012 ASCCP Consensus Guidelines Conference. 2012 Updated Consensus Guidelines for the Management of Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2013;17(5):S1-S27.
- Perkins RB, Guido RS, Castle PE, et al, for the 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2020;24(2):102-131.
- Massad LS, Einstein MH, Huh WK, et al, for the 2012 ASCCP Consensus Guidelines Conference. 2012 Updated Consensus Guidelines for the Management of Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2013;17(5):S1-S27.
In your practice, are you planning to have a chaperone present for all intimate examinations?
Although pelvic examinations may only last a few minutes, the examination is scary and uncomfortable for many patients. To help minimize fear and discomfort, the exam should take place in a comfortable and professional environment. The clinician should provide appropriate gowns, private facilities for undressing, sensitively use draping, and clearly explain the components of the examination. Trained professional chaperones play an important role in intimate physical examinations, including:
- providing reassurance to the patient of the professional integrity of the intimate examination
- supporting and educating the patient during the examination
- increasing the efficiency of the clinician during a procedure
- acting as a witness should a misunderstanding with the patient arise.
Major medical professional societies have issued guidance to clinicians on the use of a chaperone during intimate physical examinations. Professional society guidance ranges from endorsing joint decision-making between physician and patient on the presence of a chaperone to more proscriptive guidance that emphasizes the importance of a chaperone at every intimate physical examination.
Examples of professional societies’ guidance that supports joint decision-making between physician and patient about the presence of a chaperone include:
- American Medical Association: “Adopt a policy that patients are free to request a chaperone and ensure that the policy is communicated to patients. Always honor a patient’s request to have a chaperone.”1
- Society of Obstetricians and Gynaecologists of Canada: “It is a reasonable and acceptable practice to perform a physical examination, including breast and pelvic examination without the presence of a third person in the room unless the woman or health care provider indicates a desire for a third party to be present.” “If the health care provider chooses to have a third person present during all examinations, the health care provider should explain this policy to the woman.”2
- American College of Physicians: “Care and respect should guide the performance of the physical examination. The location and degree of privacy should be appropriate for the examination being performed, with chaperone services as an option. An appropriate setting and sufficient time should be allocated to encourage exploration of aspects of the patient’s life pertinent to health, including habits, relationships, sexuality, vocation, culture, religion, and spirituality.”3
By contrast, the following professional society guidance strongly recommends the presence of a chaperone for every intimate physical examination:
- United States Veterans Administration: “A female chaperone must be in the examination room during breast and pelvic exams…this includes procedures such as urodynamic testing or treatments such as pelvic floor physical therapy.”4
- Royal College of Obstetricians and Gynaecologists: “The presence of a chaperone is considered essential for every pelvic examination. Verbal consent should be obtained in the presence of the chaperone who is to be present during the examination and recorded in the notes. If the patient declines the presence of a chaperone, the doctor should explain that a chaperone is also required to help in many cases and then attempt to arrange for the chaperone to be standing nearby within earshot. The reasons for declining a chaperone and alternative arrangements offered should be documented. Consent should also be specific to whether the intended examination is vaginal, rectal or both. Communication skills are essential in conducting intimate examinations.”5
- American College Health Association (ACHA): “It is ACHA’s recommendation that, as part of institutional policy, a chaperone be provided for every sensitive medical examination and procedure.”6
Continue to: New guidance from ACOG on trained chaperones...
New guidance from ACOG on trained chaperones
The American College of Obstetricians and Gynecologists (ACOG) recently issued a committee opinion recommending “that a chaperone be present for all breast, genital, and rectal examinations. The need for a chaperone is irrespective of the sex or gender of the person performing the examination and applies to examinations performed in the outpatient and inpatient settings, including labor and delivery, as well as during diagnostic studies such as transvaginal ultrasonography and urodynamic testing.”7
This new proscriptive guidance will significantly change practice for the many obstetrician-gynecologists who do not routinely have a chaperone present during intimate examinations. The policy provides exceptions to the presence of a chaperone in cases of medical emergencies and if the patient declines a chaperone. ACOG recommends that when a patient declines a chaperone the clinician should educate the patient that a “chaperone is an integral part of the clinical team whose role includes assisting with the examination and protecting the patient and the physician. Any concerns the patient has regarding the presence of a chaperone should be elicited and addressed if feasible. If, after counseling, the patient refuses the chaperone, this decision should be respected and documented in the medical record.”7 ACOG discourages the use of family members, medical students, and residents as chaperones.
Sexual trauma is common and may cause lasting adverse effects, including poor health.1 When sexual trauma is reported, the experience may not be believed or taken seriously, compounding the injury. Sometimes sexual trauma contributes to risky behaviors including smoking cigarettes, excessive alcohol consumption, drug misuse, and risky sex as a means to cope with the mental distress of the trauma.
Trauma-informed medical care has four pillars:
1. Recognize that many people have experienced significant trauma(s), which adversely impacts their health.
2. Be aware of the signs and symptoms of trauma.
3. Integrate knowledge about trauma into medical encounters.
4. Avoid re-traumatizing the person.
Symptoms of psychological distress caused by past trauma include anxiety, fear, anger, irritability, mood swings, feeling disconnected, numbness, sadness, or hopelessness. Clinical actions that help to reduce distress among trauma survivors include:
• sensitively ask patients to share their traumatic experiences
• empower the patient by explicitly giving her control over all aspects of the examination, indicating that the exam will stop if the patient feels uncomfortable
• explain the steps in the exam and educate about the purpose of each step
• keep the patient’s body covered as much as possible
• use the smallest speculum that permits an adequate exam
• utilize a chaperone to help support the patient.
Clinicians can strengthen their empathic skills by reflecting on how their own personal experiences, traumas, cultural-biases, and gender influence their ap-proach to the care of patients.
Reference
1. Hall KS, Moreau C, Trussell J. Young women’s perceived health and lifetime sexual experience: results from the national survey of family growth. J Sex Med. 2012;9:1382-1391. doi: 10.1111/j.1743-6109.2012.02686.x.
Training of chaperones
Chaperones are health care professionals who should be trained for their specific role. Chaperones need to protect patient privacy and the confidentiality of health information. Chaperones should be trained to recognize the components of a professional intimate examination and to identify variances from standard practice. In many ambulatory practices, medical assistants perform the role of chaperone. The American Association of Medical Assistants (AAMA) offers national certification for medical assistants through an examination developed by the National Board of Medical Examiners. To be eligible for AAMA certification an individual must complete at least two semesters of medical assisting education that includes courses in anatomy, physiology, pharmacology, and relevant mathematics.
Reporting variances that occur during an intimate examination
Best practices are evolving on how to deal with the rare event of a chaperone witnessing a physician perform an intimate examination that is outside of standard professional practice. Chaperones may be reluctant to report a variance because physicians are in a powerful position, and the accuracy of their report will be challenged, threatening the chaperone’s employment. Processes for encouraging all team members to report concerns must be clearly explained to the chaperone and other members of the health care team. Clinicians should be aware that deviations from standard practice will be reported and investigated. Medical practices must develop a reporting system that ensures the reporting individual will be protected from retaliation.
In addition, the chaperone needs to know to whom they should report a variance. In large multispecialty medical practices, chaperones often can report concerns to nursing leaders or human resources. In small ambulatory practices, chaperones may be advised to report concerns about a physician to the practice manager or medical director. Regardless, every practice should have the best process for reporting a concern. In turn, the practice leaders who are responsible for investigating reports of concerning behavior should have a defined process for confidentially interviewing the chaperone, clinician, and patient.
Even when a chaperone is present for intimate examinations, problems can arise if the chaperone is not trained to recognize variances from standard practice or does not have a clear means for reporting variances and when the practice does not have a process for investigating reported variances.
Sadly, misconduct has been documented among priests, ministers, sports coaches, professors, scout masters, and clinicians. Trusted professionals are in positions of power in relation to their clients, patients, and students. Physicians and nurses are held in high esteem and trust by patients. To preserve the trust of the public we must treat all people with dignity and respect their autonomy. The presence of a chaperone during intimate examinations may help us fulfill Hippocrates’ edict, “First, do no harm.” ●
Ronee A. Skornik, MSW, MD
As a female obstetrician-gynecologist trained in psychiatric social work, I have found that some of my patients who have known me over a long period of time find the presence of a chaperone not only unnecessary but also uncomfortable both in terms of physical exposure and in what they may want to tell me during the examination. Personally, I strongly favor a chaperone for all intimate examinations, to safeguard both the patient and the clinician. However, I do understand why some patients prefer to see me without the presence of a chaperone, and I want to honor their wishes. If a chaperone is responsive to the patient’s requests, including where the chaperone stands and his or her role during the exam, the reluctant patient may be more willing to have a chaperone. A chaperone who develops a relationship with the patient and honors the patient’s preferences is a valuable member of the care team.
- American Medical Association. Code of Medical Ethics Opinion 1.2.4. https://www.ama-assn.org/delivering-care/ethics/use-chaperones. Accessed May 26, 2020.
- Society of Obstetricians and Gynaecologists of Canada. No. 266—The presence of a third party during breast and pelvic examinations. J Obstet Gynaecol Can. 2017;39:e496-e497. doi: 10.1016/j.jogc.2017.09.005.
- American College of Physicians. ACP Policy Com-pendium Summer 2016. https://www.acponline.org/system/files/documents/advocacy/acp_policy_compendium_summer_2016.pdf. Accessed May 26, 2020.
- Department of Veterans Affairs. VHA Directive 1330.01(2). Healthcare Services for Women Veterans. February 15, 2017. Amended July 24, 2018. http://www.va.gov/ vhapublications/ viewpublication.asp?pub_id=5332. Accessed May 26, 2020.
- Royal College of Obstetricians and Gynaecologists. Obtaining valid consent: clinical governance advice no. 6. January 2015. https://www.rcog.org.uk/globalassets/documents/guidelines/clinical-governance-advice/cga6.pdf. Accessed May 26, 2020.
- American College Health Association Guidelines. Best practices for sensitive exams. October 2019. https://www.acha.org/documents/resources/guidelines/ACHA_Best_Practices_for_Sensitive_Exams_October2019.pdf. Accessed May 26, 2020.
- American College of Obstetricians and Gynecologists Committee on Ethics. Sexual misconduct: ACOG Committee Opinion No. 796. Obstet Gynecol. 2020;135:e43-e50
Although pelvic examinations may only last a few minutes, the examination is scary and uncomfortable for many patients. To help minimize fear and discomfort, the exam should take place in a comfortable and professional environment. The clinician should provide appropriate gowns, private facilities for undressing, sensitively use draping, and clearly explain the components of the examination. Trained professional chaperones play an important role in intimate physical examinations, including:
- providing reassurance to the patient of the professional integrity of the intimate examination
- supporting and educating the patient during the examination
- increasing the efficiency of the clinician during a procedure
- acting as a witness should a misunderstanding with the patient arise.
Major medical professional societies have issued guidance to clinicians on the use of a chaperone during intimate physical examinations. Professional society guidance ranges from endorsing joint decision-making between physician and patient on the presence of a chaperone to more proscriptive guidance that emphasizes the importance of a chaperone at every intimate physical examination.
Examples of professional societies’ guidance that supports joint decision-making between physician and patient about the presence of a chaperone include:
- American Medical Association: “Adopt a policy that patients are free to request a chaperone and ensure that the policy is communicated to patients. Always honor a patient’s request to have a chaperone.”1
- Society of Obstetricians and Gynaecologists of Canada: “It is a reasonable and acceptable practice to perform a physical examination, including breast and pelvic examination without the presence of a third person in the room unless the woman or health care provider indicates a desire for a third party to be present.” “If the health care provider chooses to have a third person present during all examinations, the health care provider should explain this policy to the woman.”2
- American College of Physicians: “Care and respect should guide the performance of the physical examination. The location and degree of privacy should be appropriate for the examination being performed, with chaperone services as an option. An appropriate setting and sufficient time should be allocated to encourage exploration of aspects of the patient’s life pertinent to health, including habits, relationships, sexuality, vocation, culture, religion, and spirituality.”3
By contrast, the following professional society guidance strongly recommends the presence of a chaperone for every intimate physical examination:
- United States Veterans Administration: “A female chaperone must be in the examination room during breast and pelvic exams…this includes procedures such as urodynamic testing or treatments such as pelvic floor physical therapy.”4
- Royal College of Obstetricians and Gynaecologists: “The presence of a chaperone is considered essential for every pelvic examination. Verbal consent should be obtained in the presence of the chaperone who is to be present during the examination and recorded in the notes. If the patient declines the presence of a chaperone, the doctor should explain that a chaperone is also required to help in many cases and then attempt to arrange for the chaperone to be standing nearby within earshot. The reasons for declining a chaperone and alternative arrangements offered should be documented. Consent should also be specific to whether the intended examination is vaginal, rectal or both. Communication skills are essential in conducting intimate examinations.”5
- American College Health Association (ACHA): “It is ACHA’s recommendation that, as part of institutional policy, a chaperone be provided for every sensitive medical examination and procedure.”6
Continue to: New guidance from ACOG on trained chaperones...
New guidance from ACOG on trained chaperones
The American College of Obstetricians and Gynecologists (ACOG) recently issued a committee opinion recommending “that a chaperone be present for all breast, genital, and rectal examinations. The need for a chaperone is irrespective of the sex or gender of the person performing the examination and applies to examinations performed in the outpatient and inpatient settings, including labor and delivery, as well as during diagnostic studies such as transvaginal ultrasonography and urodynamic testing.”7
This new proscriptive guidance will significantly change practice for the many obstetrician-gynecologists who do not routinely have a chaperone present during intimate examinations. The policy provides exceptions to the presence of a chaperone in cases of medical emergencies and if the patient declines a chaperone. ACOG recommends that when a patient declines a chaperone the clinician should educate the patient that a “chaperone is an integral part of the clinical team whose role includes assisting with the examination and protecting the patient and the physician. Any concerns the patient has regarding the presence of a chaperone should be elicited and addressed if feasible. If, after counseling, the patient refuses the chaperone, this decision should be respected and documented in the medical record.”7 ACOG discourages the use of family members, medical students, and residents as chaperones.
Sexual trauma is common and may cause lasting adverse effects, including poor health.1 When sexual trauma is reported, the experience may not be believed or taken seriously, compounding the injury. Sometimes sexual trauma contributes to risky behaviors including smoking cigarettes, excessive alcohol consumption, drug misuse, and risky sex as a means to cope with the mental distress of the trauma.
Trauma-informed medical care has four pillars:
1. Recognize that many people have experienced significant trauma(s), which adversely impacts their health.
2. Be aware of the signs and symptoms of trauma.
3. Integrate knowledge about trauma into medical encounters.
4. Avoid re-traumatizing the person.
Symptoms of psychological distress caused by past trauma include anxiety, fear, anger, irritability, mood swings, feeling disconnected, numbness, sadness, or hopelessness. Clinical actions that help to reduce distress among trauma survivors include:
• sensitively ask patients to share their traumatic experiences
• empower the patient by explicitly giving her control over all aspects of the examination, indicating that the exam will stop if the patient feels uncomfortable
• explain the steps in the exam and educate about the purpose of each step
• keep the patient’s body covered as much as possible
• use the smallest speculum that permits an adequate exam
• utilize a chaperone to help support the patient.
Clinicians can strengthen their empathic skills by reflecting on how their own personal experiences, traumas, cultural-biases, and gender influence their ap-proach to the care of patients.
Reference
1. Hall KS, Moreau C, Trussell J. Young women’s perceived health and lifetime sexual experience: results from the national survey of family growth. J Sex Med. 2012;9:1382-1391. doi: 10.1111/j.1743-6109.2012.02686.x.
Training of chaperones
Chaperones are health care professionals who should be trained for their specific role. Chaperones need to protect patient privacy and the confidentiality of health information. Chaperones should be trained to recognize the components of a professional intimate examination and to identify variances from standard practice. In many ambulatory practices, medical assistants perform the role of chaperone. The American Association of Medical Assistants (AAMA) offers national certification for medical assistants through an examination developed by the National Board of Medical Examiners. To be eligible for AAMA certification an individual must complete at least two semesters of medical assisting education that includes courses in anatomy, physiology, pharmacology, and relevant mathematics.
Reporting variances that occur during an intimate examination
Best practices are evolving on how to deal with the rare event of a chaperone witnessing a physician perform an intimate examination that is outside of standard professional practice. Chaperones may be reluctant to report a variance because physicians are in a powerful position, and the accuracy of their report will be challenged, threatening the chaperone’s employment. Processes for encouraging all team members to report concerns must be clearly explained to the chaperone and other members of the health care team. Clinicians should be aware that deviations from standard practice will be reported and investigated. Medical practices must develop a reporting system that ensures the reporting individual will be protected from retaliation.
In addition, the chaperone needs to know to whom they should report a variance. In large multispecialty medical practices, chaperones often can report concerns to nursing leaders or human resources. In small ambulatory practices, chaperones may be advised to report concerns about a physician to the practice manager or medical director. Regardless, every practice should have the best process for reporting a concern. In turn, the practice leaders who are responsible for investigating reports of concerning behavior should have a defined process for confidentially interviewing the chaperone, clinician, and patient.
Even when a chaperone is present for intimate examinations, problems can arise if the chaperone is not trained to recognize variances from standard practice or does not have a clear means for reporting variances and when the practice does not have a process for investigating reported variances.
Sadly, misconduct has been documented among priests, ministers, sports coaches, professors, scout masters, and clinicians. Trusted professionals are in positions of power in relation to their clients, patients, and students. Physicians and nurses are held in high esteem and trust by patients. To preserve the trust of the public we must treat all people with dignity and respect their autonomy. The presence of a chaperone during intimate examinations may help us fulfill Hippocrates’ edict, “First, do no harm.” ●
Ronee A. Skornik, MSW, MD
As a female obstetrician-gynecologist trained in psychiatric social work, I have found that some of my patients who have known me over a long period of time find the presence of a chaperone not only unnecessary but also uncomfortable both in terms of physical exposure and in what they may want to tell me during the examination. Personally, I strongly favor a chaperone for all intimate examinations, to safeguard both the patient and the clinician. However, I do understand why some patients prefer to see me without the presence of a chaperone, and I want to honor their wishes. If a chaperone is responsive to the patient’s requests, including where the chaperone stands and his or her role during the exam, the reluctant patient may be more willing to have a chaperone. A chaperone who develops a relationship with the patient and honors the patient’s preferences is a valuable member of the care team.
Although pelvic examinations may only last a few minutes, the examination is scary and uncomfortable for many patients. To help minimize fear and discomfort, the exam should take place in a comfortable and professional environment. The clinician should provide appropriate gowns, private facilities for undressing, sensitively use draping, and clearly explain the components of the examination. Trained professional chaperones play an important role in intimate physical examinations, including:
- providing reassurance to the patient of the professional integrity of the intimate examination
- supporting and educating the patient during the examination
- increasing the efficiency of the clinician during a procedure
- acting as a witness should a misunderstanding with the patient arise.
Major medical professional societies have issued guidance to clinicians on the use of a chaperone during intimate physical examinations. Professional society guidance ranges from endorsing joint decision-making between physician and patient on the presence of a chaperone to more proscriptive guidance that emphasizes the importance of a chaperone at every intimate physical examination.
Examples of professional societies’ guidance that supports joint decision-making between physician and patient about the presence of a chaperone include:
- American Medical Association: “Adopt a policy that patients are free to request a chaperone and ensure that the policy is communicated to patients. Always honor a patient’s request to have a chaperone.”1
- Society of Obstetricians and Gynaecologists of Canada: “It is a reasonable and acceptable practice to perform a physical examination, including breast and pelvic examination without the presence of a third person in the room unless the woman or health care provider indicates a desire for a third party to be present.” “If the health care provider chooses to have a third person present during all examinations, the health care provider should explain this policy to the woman.”2
- American College of Physicians: “Care and respect should guide the performance of the physical examination. The location and degree of privacy should be appropriate for the examination being performed, with chaperone services as an option. An appropriate setting and sufficient time should be allocated to encourage exploration of aspects of the patient’s life pertinent to health, including habits, relationships, sexuality, vocation, culture, religion, and spirituality.”3
By contrast, the following professional society guidance strongly recommends the presence of a chaperone for every intimate physical examination:
- United States Veterans Administration: “A female chaperone must be in the examination room during breast and pelvic exams…this includes procedures such as urodynamic testing or treatments such as pelvic floor physical therapy.”4
- Royal College of Obstetricians and Gynaecologists: “The presence of a chaperone is considered essential for every pelvic examination. Verbal consent should be obtained in the presence of the chaperone who is to be present during the examination and recorded in the notes. If the patient declines the presence of a chaperone, the doctor should explain that a chaperone is also required to help in many cases and then attempt to arrange for the chaperone to be standing nearby within earshot. The reasons for declining a chaperone and alternative arrangements offered should be documented. Consent should also be specific to whether the intended examination is vaginal, rectal or both. Communication skills are essential in conducting intimate examinations.”5
- American College Health Association (ACHA): “It is ACHA’s recommendation that, as part of institutional policy, a chaperone be provided for every sensitive medical examination and procedure.”6
Continue to: New guidance from ACOG on trained chaperones...
New guidance from ACOG on trained chaperones
The American College of Obstetricians and Gynecologists (ACOG) recently issued a committee opinion recommending “that a chaperone be present for all breast, genital, and rectal examinations. The need for a chaperone is irrespective of the sex or gender of the person performing the examination and applies to examinations performed in the outpatient and inpatient settings, including labor and delivery, as well as during diagnostic studies such as transvaginal ultrasonography and urodynamic testing.”7
This new proscriptive guidance will significantly change practice for the many obstetrician-gynecologists who do not routinely have a chaperone present during intimate examinations. The policy provides exceptions to the presence of a chaperone in cases of medical emergencies and if the patient declines a chaperone. ACOG recommends that when a patient declines a chaperone the clinician should educate the patient that a “chaperone is an integral part of the clinical team whose role includes assisting with the examination and protecting the patient and the physician. Any concerns the patient has regarding the presence of a chaperone should be elicited and addressed if feasible. If, after counseling, the patient refuses the chaperone, this decision should be respected and documented in the medical record.”7 ACOG discourages the use of family members, medical students, and residents as chaperones.
Sexual trauma is common and may cause lasting adverse effects, including poor health.1 When sexual trauma is reported, the experience may not be believed or taken seriously, compounding the injury. Sometimes sexual trauma contributes to risky behaviors including smoking cigarettes, excessive alcohol consumption, drug misuse, and risky sex as a means to cope with the mental distress of the trauma.
Trauma-informed medical care has four pillars:
1. Recognize that many people have experienced significant trauma(s), which adversely impacts their health.
2. Be aware of the signs and symptoms of trauma.
3. Integrate knowledge about trauma into medical encounters.
4. Avoid re-traumatizing the person.
Symptoms of psychological distress caused by past trauma include anxiety, fear, anger, irritability, mood swings, feeling disconnected, numbness, sadness, or hopelessness. Clinical actions that help to reduce distress among trauma survivors include:
• sensitively ask patients to share their traumatic experiences
• empower the patient by explicitly giving her control over all aspects of the examination, indicating that the exam will stop if the patient feels uncomfortable
• explain the steps in the exam and educate about the purpose of each step
• keep the patient’s body covered as much as possible
• use the smallest speculum that permits an adequate exam
• utilize a chaperone to help support the patient.
Clinicians can strengthen their empathic skills by reflecting on how their own personal experiences, traumas, cultural-biases, and gender influence their ap-proach to the care of patients.
Reference
1. Hall KS, Moreau C, Trussell J. Young women’s perceived health and lifetime sexual experience: results from the national survey of family growth. J Sex Med. 2012;9:1382-1391. doi: 10.1111/j.1743-6109.2012.02686.x.
Training of chaperones
Chaperones are health care professionals who should be trained for their specific role. Chaperones need to protect patient privacy and the confidentiality of health information. Chaperones should be trained to recognize the components of a professional intimate examination and to identify variances from standard practice. In many ambulatory practices, medical assistants perform the role of chaperone. The American Association of Medical Assistants (AAMA) offers national certification for medical assistants through an examination developed by the National Board of Medical Examiners. To be eligible for AAMA certification an individual must complete at least two semesters of medical assisting education that includes courses in anatomy, physiology, pharmacology, and relevant mathematics.
Reporting variances that occur during an intimate examination
Best practices are evolving on how to deal with the rare event of a chaperone witnessing a physician perform an intimate examination that is outside of standard professional practice. Chaperones may be reluctant to report a variance because physicians are in a powerful position, and the accuracy of their report will be challenged, threatening the chaperone’s employment. Processes for encouraging all team members to report concerns must be clearly explained to the chaperone and other members of the health care team. Clinicians should be aware that deviations from standard practice will be reported and investigated. Medical practices must develop a reporting system that ensures the reporting individual will be protected from retaliation.
In addition, the chaperone needs to know to whom they should report a variance. In large multispecialty medical practices, chaperones often can report concerns to nursing leaders or human resources. In small ambulatory practices, chaperones may be advised to report concerns about a physician to the practice manager or medical director. Regardless, every practice should have the best process for reporting a concern. In turn, the practice leaders who are responsible for investigating reports of concerning behavior should have a defined process for confidentially interviewing the chaperone, clinician, and patient.
Even when a chaperone is present for intimate examinations, problems can arise if the chaperone is not trained to recognize variances from standard practice or does not have a clear means for reporting variances and when the practice does not have a process for investigating reported variances.
Sadly, misconduct has been documented among priests, ministers, sports coaches, professors, scout masters, and clinicians. Trusted professionals are in positions of power in relation to their clients, patients, and students. Physicians and nurses are held in high esteem and trust by patients. To preserve the trust of the public we must treat all people with dignity and respect their autonomy. The presence of a chaperone during intimate examinations may help us fulfill Hippocrates’ edict, “First, do no harm.” ●
Ronee A. Skornik, MSW, MD
As a female obstetrician-gynecologist trained in psychiatric social work, I have found that some of my patients who have known me over a long period of time find the presence of a chaperone not only unnecessary but also uncomfortable both in terms of physical exposure and in what they may want to tell me during the examination. Personally, I strongly favor a chaperone for all intimate examinations, to safeguard both the patient and the clinician. However, I do understand why some patients prefer to see me without the presence of a chaperone, and I want to honor their wishes. If a chaperone is responsive to the patient’s requests, including where the chaperone stands and his or her role during the exam, the reluctant patient may be more willing to have a chaperone. A chaperone who develops a relationship with the patient and honors the patient’s preferences is a valuable member of the care team.
- American Medical Association. Code of Medical Ethics Opinion 1.2.4. https://www.ama-assn.org/delivering-care/ethics/use-chaperones. Accessed May 26, 2020.
- Society of Obstetricians and Gynaecologists of Canada. No. 266—The presence of a third party during breast and pelvic examinations. J Obstet Gynaecol Can. 2017;39:e496-e497. doi: 10.1016/j.jogc.2017.09.005.
- American College of Physicians. ACP Policy Com-pendium Summer 2016. https://www.acponline.org/system/files/documents/advocacy/acp_policy_compendium_summer_2016.pdf. Accessed May 26, 2020.
- Department of Veterans Affairs. VHA Directive 1330.01(2). Healthcare Services for Women Veterans. February 15, 2017. Amended July 24, 2018. http://www.va.gov/ vhapublications/ viewpublication.asp?pub_id=5332. Accessed May 26, 2020.
- Royal College of Obstetricians and Gynaecologists. Obtaining valid consent: clinical governance advice no. 6. January 2015. https://www.rcog.org.uk/globalassets/documents/guidelines/clinical-governance-advice/cga6.pdf. Accessed May 26, 2020.
- American College Health Association Guidelines. Best practices for sensitive exams. October 2019. https://www.acha.org/documents/resources/guidelines/ACHA_Best_Practices_for_Sensitive_Exams_October2019.pdf. Accessed May 26, 2020.
- American College of Obstetricians and Gynecologists Committee on Ethics. Sexual misconduct: ACOG Committee Opinion No. 796. Obstet Gynecol. 2020;135:e43-e50
- American Medical Association. Code of Medical Ethics Opinion 1.2.4. https://www.ama-assn.org/delivering-care/ethics/use-chaperones. Accessed May 26, 2020.
- Society of Obstetricians and Gynaecologists of Canada. No. 266—The presence of a third party during breast and pelvic examinations. J Obstet Gynaecol Can. 2017;39:e496-e497. doi: 10.1016/j.jogc.2017.09.005.
- American College of Physicians. ACP Policy Com-pendium Summer 2016. https://www.acponline.org/system/files/documents/advocacy/acp_policy_compendium_summer_2016.pdf. Accessed May 26, 2020.
- Department of Veterans Affairs. VHA Directive 1330.01(2). Healthcare Services for Women Veterans. February 15, 2017. Amended July 24, 2018. http://www.va.gov/ vhapublications/ viewpublication.asp?pub_id=5332. Accessed May 26, 2020.
- Royal College of Obstetricians and Gynaecologists. Obtaining valid consent: clinical governance advice no. 6. January 2015. https://www.rcog.org.uk/globalassets/documents/guidelines/clinical-governance-advice/cga6.pdf. Accessed May 26, 2020.
- American College Health Association Guidelines. Best practices for sensitive exams. October 2019. https://www.acha.org/documents/resources/guidelines/ACHA_Best_Practices_for_Sensitive_Exams_October2019.pdf. Accessed May 26, 2020.
- American College of Obstetricians and Gynecologists Committee on Ethics. Sexual misconduct: ACOG Committee Opinion No. 796. Obstet Gynecol. 2020;135:e43-e50
‘I didn’t train for this’: Take cues from elite athletes to maintain stamina during the COVID-19 crisis
I am not an elite athlete. Never have been, never will be. But as I contemplated how to support my colleagues at my hospital during the COVID-19 pandemic—knowing that we face more weeks of social distancing, probable virus outbreaks as business opens up, and myriad changes in how we will practice medicine—I wondered how Olympic athletes focus their mental and physical stamina to respond to the challenges of competition.
In this article, I offer some pointers, gleaned from techniques used by exceptional athletes, on how we can maintain our stamina during the COVID-19 marathon.

Train your mind
Elite athletes understand that what will take them to the top of their sport is less about their physical gifts (all elite athletes have superior athletic talent) and more about how they train their minds to achieve their goals. As basketball great Kareem Abdul-Jabbar said, “Your mind is what makes everything else work.” Not surprisingly, athletes who train to be mentally “tough” have a better chance to reach the podium.
One definition of mental toughness is the “ability to perform toward the upper range of your talent and skill” regardless of external circumstances.1 Mental toughness involves an unshakable self-belief, resilience, motivation, focus, and ability to perform under pressure and manage physical and emotional pain.
Like me, most of you probably are not elite athletes, but you can train your mind to be tougher and increase your stamina using some of the following techniques.
Think positively
Do you find yourself dealing with an inner monologue of fear, self-doubt, and feelings of worthlessness? That is the Obnoxious Roommate in your head as described in a HuffPost article and based on the pioneering work of psychiatrist Aaron Beck, MD, the father of cognitive-behavioral therapy.2,3 Such thoughts negatively affect one’s sense of self-efficacy, which is the mental state of believing that you can meet the challenges you face. Elite athletes pay particular attention to their self-talk and replace negative thoughts with positive ones. These may include affirmations of your strengths or cue words that pump you up or help you manage your nerves.
To begin this practice, first observe what you are telling yourself. You may be saying, “I can’t do this. This is too hard.” This is an important step because you may have lived with the Obnoxious Roommate for so long that these thoughts are automatic and seem like a part of you. If, however, you first observe the thought and acknowledge it, you can begin to replace negative thoughts with supportive ones: “This is hard, but I can do this. I am intelligent, strong, capable, and ready.” While practice and repetition are required, thinking positively eventually will become a habit of self-support. Elite athletes know that to reach the top, they need to become their own best friend.4
Continue to: Use visualization...
Use visualization
Elite athletes visualize their events in such meticulous detail that they can actually feel their feet on the track, their muscles tensing, the starter pistol going off, the roar of the crowd. Research shows that the part of the brain that is activated in a race is also activated when one visualizes the race. It is as though the race is happening in real time.5 Physicians could use this approach to visualize the day ahead, picturing the procedures in detail and the clinical encounters going well.
In the current crisis, we frequently need a way to calm down quickly. One suggestion is to find a quiet space and sit for a few minutes, allowing the chair to support you. Then imagine a beautiful, calm, soothing place. Relax there and take in that feeling. Or, imagine a past achievement when you felt good about yourself. See yourself in that moment, remember how you felt, and take it in.
Plan for setbacks
All elite athletes have setbacks: Marathon runners hit a wall, golfers hit into the rough. But they don’t spin out of control at setbacks. They expect them, and they have practiced skills to restore their confidence and re-center themselves.6 For example, some athletes calm themselves by performing a ritualized series of movements. Others use a specific phrase (that positive self-talk again!) that reminds them of their goals or skills, while others play specific songs on their media player, or in their head, to return to their center.
Another technique is to use deep, rhythmic, diaphragmatic breathing for about 30 seconds to bring yourself back to your body.
The point is to have a plan in place to respond to setbacks in a positive manner and get back on track. Don’t waste time on self-criticism.
Continue to: Manage stress...
Manage stress
Not all stress is negative. Everyone has a “sweet spot,” a level of activation from which we operate best.1 If we go too far over that level, though, we can panic. If we aren’t stressed enough, it is hard to get going at all.
If you need to be pumped up to function at your best, try playing music that energizes you. If you work best when you are calm, take deep breaths and attend to the exhalation while you engage in positive self-talk.
Another way to manage stress levels during the COVID-19 pandemic is to employ the ESCAPE mnemonic:
- Exercise. Research shows that regular aerobic exercise is a potent stress reliever; it raises endorphin levels, provides a general sense of well-being, and improves immune function.7 If you exercise regularly, do everything possible to keep doing so. If you do not have an exercise routine, try taking walks. Get out into the fresh air, pay attention to your surroundings, and work to be present in your body as you move.
- Sleep. Getting adequate sleep, 7 to 9 hours a night for most people, is important for maintaining physical and emotional health.8 Elite athletes pay particular attention to sleep because they know that their bodies need to recharge. The COVID-19 crisis has affected sleep for many, and we need to consciously attend to sleep hygiene. This includes limiting use of screen time to no more than 2 hours before bed. As well, limit your intake of the news and definitely avoid it right before bedtime. Take a warm shower, drink a cup of herbal tea, or read a less-than-stimulating journal article. Make sure your bedroom is cool and dark. Keep a regular bedtime and wake up at a regular time.
- Connect. Despite the current need for physical distancing, do not neglect your need to connect to those you love and care about. Whatever modality works for you—telephone, e-mail, video conferencing—if you feel lonely, reach out. Friends and families are concerned about us, and it makes them feel useful to support us. Allow that.
- Appreciate/cultivate gratitude. Look for causes for celebration in your life, whether that is your family, friends, or that you have a job and a home. Perhaps it is the cleaner air or the beauty of nature. Whatever you are grateful for, acknowledge it, perhaps by writing it down in a journal. Remembering these things can serve as an antidote to the stress created by the COVID-19 crisis.
- Play. Seek out things that make you laugh.9 Find your inner goofiness. Get creative and paint, sing, garden, golf, play an instrument, dance, do puzzles, or play games. We need to remember the pleasures in life when times are dark. Find whatever feels like joy.
- Exhale. If you have a meditation practice continue to do it. If not, consider starting. Try yoga as a way to center yourself in your body. Elite athletes use meditation and yoga to harness their minds and bodies to feel more in control.10 When so many things feel out of our control and unpredictable, it can be immensely soothing to connect to yourself in this way and realize you can reliably relax.
Self-care is a necessity
While we are in a marathon not of our choosing, we can use the techniques of elite athletes to think positively, visualize scenarios to achieve our goals or to simply relax, plan for setbacks so that we can bounce back, and manage stress productively. Self-care is paramount as we continue to care for others. Let’s not neglect ourselves, and we will cross the finish line together. ●
- Jones G. What is this thing called mental toughness? An investigation of elite sport performers. J Applied Sport Psych. 2002;14:205-218.
- Gregoire C. The brain-training secrets of Olympic athletes. HuffPost. February 11, 2014. https://www.huffpost.com/entry/mind-hacks-from-olympic-a_n_4747755. Accessed May 27, 2020.
- Beck AT. A 60-year evolution of cognitive theory and therapy. Perspect Psychol Sci. 2019;14:16-20.
- Afremow J. The Champion’s Mind: How Great Athletes Think, Train, and Thrive. New York, NY: Rodale Inc; 2014.
- Ranganathan VK, Siemionow V, Liu JZ, et al. From mental power to muscle power—gaining strength by using the mind. Neuropsychologia. 2004;42:944-956.
- Morgan Griffin R. 5 tips for building mental stamina. July 8, 2013. WebMD. https://www.webmd.com/fitness-exercise/features/mental-stamina#1. Accessed May 27, 2020.
- Hackney AC. Stress and the neuroendocrine system: the role of exercise as a stressor and modifier of stress. Expert Rev Endocrinol Metab. 2006;1:783-792.
- Hirschkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1:40-43.
- Saper B. The therapeutic use of humor for psychiatric disturbances of adolescents and adults. Psychiatr Q. 1990;61:261-272.
- Zeidan F, Johnson SK, Diamond BJ, et al. Mindfulness meditation improves cognition: evidence of brief mental training. Conscious Cogn. 2012;19:597-605.
I am not an elite athlete. Never have been, never will be. But as I contemplated how to support my colleagues at my hospital during the COVID-19 pandemic—knowing that we face more weeks of social distancing, probable virus outbreaks as business opens up, and myriad changes in how we will practice medicine—I wondered how Olympic athletes focus their mental and physical stamina to respond to the challenges of competition.
In this article, I offer some pointers, gleaned from techniques used by exceptional athletes, on how we can maintain our stamina during the COVID-19 marathon.

Train your mind
Elite athletes understand that what will take them to the top of their sport is less about their physical gifts (all elite athletes have superior athletic talent) and more about how they train their minds to achieve their goals. As basketball great Kareem Abdul-Jabbar said, “Your mind is what makes everything else work.” Not surprisingly, athletes who train to be mentally “tough” have a better chance to reach the podium.
One definition of mental toughness is the “ability to perform toward the upper range of your talent and skill” regardless of external circumstances.1 Mental toughness involves an unshakable self-belief, resilience, motivation, focus, and ability to perform under pressure and manage physical and emotional pain.
Like me, most of you probably are not elite athletes, but you can train your mind to be tougher and increase your stamina using some of the following techniques.
Think positively
Do you find yourself dealing with an inner monologue of fear, self-doubt, and feelings of worthlessness? That is the Obnoxious Roommate in your head as described in a HuffPost article and based on the pioneering work of psychiatrist Aaron Beck, MD, the father of cognitive-behavioral therapy.2,3 Such thoughts negatively affect one’s sense of self-efficacy, which is the mental state of believing that you can meet the challenges you face. Elite athletes pay particular attention to their self-talk and replace negative thoughts with positive ones. These may include affirmations of your strengths or cue words that pump you up or help you manage your nerves.
To begin this practice, first observe what you are telling yourself. You may be saying, “I can’t do this. This is too hard.” This is an important step because you may have lived with the Obnoxious Roommate for so long that these thoughts are automatic and seem like a part of you. If, however, you first observe the thought and acknowledge it, you can begin to replace negative thoughts with supportive ones: “This is hard, but I can do this. I am intelligent, strong, capable, and ready.” While practice and repetition are required, thinking positively eventually will become a habit of self-support. Elite athletes know that to reach the top, they need to become their own best friend.4
Continue to: Use visualization...
Use visualization
Elite athletes visualize their events in such meticulous detail that they can actually feel their feet on the track, their muscles tensing, the starter pistol going off, the roar of the crowd. Research shows that the part of the brain that is activated in a race is also activated when one visualizes the race. It is as though the race is happening in real time.5 Physicians could use this approach to visualize the day ahead, picturing the procedures in detail and the clinical encounters going well.
In the current crisis, we frequently need a way to calm down quickly. One suggestion is to find a quiet space and sit for a few minutes, allowing the chair to support you. Then imagine a beautiful, calm, soothing place. Relax there and take in that feeling. Or, imagine a past achievement when you felt good about yourself. See yourself in that moment, remember how you felt, and take it in.
Plan for setbacks
All elite athletes have setbacks: Marathon runners hit a wall, golfers hit into the rough. But they don’t spin out of control at setbacks. They expect them, and they have practiced skills to restore their confidence and re-center themselves.6 For example, some athletes calm themselves by performing a ritualized series of movements. Others use a specific phrase (that positive self-talk again!) that reminds them of their goals or skills, while others play specific songs on their media player, or in their head, to return to their center.
Another technique is to use deep, rhythmic, diaphragmatic breathing for about 30 seconds to bring yourself back to your body.
The point is to have a plan in place to respond to setbacks in a positive manner and get back on track. Don’t waste time on self-criticism.
Continue to: Manage stress...
Manage stress
Not all stress is negative. Everyone has a “sweet spot,” a level of activation from which we operate best.1 If we go too far over that level, though, we can panic. If we aren’t stressed enough, it is hard to get going at all.
If you need to be pumped up to function at your best, try playing music that energizes you. If you work best when you are calm, take deep breaths and attend to the exhalation while you engage in positive self-talk.
Another way to manage stress levels during the COVID-19 pandemic is to employ the ESCAPE mnemonic:
- Exercise. Research shows that regular aerobic exercise is a potent stress reliever; it raises endorphin levels, provides a general sense of well-being, and improves immune function.7 If you exercise regularly, do everything possible to keep doing so. If you do not have an exercise routine, try taking walks. Get out into the fresh air, pay attention to your surroundings, and work to be present in your body as you move.
- Sleep. Getting adequate sleep, 7 to 9 hours a night for most people, is important for maintaining physical and emotional health.8 Elite athletes pay particular attention to sleep because they know that their bodies need to recharge. The COVID-19 crisis has affected sleep for many, and we need to consciously attend to sleep hygiene. This includes limiting use of screen time to no more than 2 hours before bed. As well, limit your intake of the news and definitely avoid it right before bedtime. Take a warm shower, drink a cup of herbal tea, or read a less-than-stimulating journal article. Make sure your bedroom is cool and dark. Keep a regular bedtime and wake up at a regular time.
- Connect. Despite the current need for physical distancing, do not neglect your need to connect to those you love and care about. Whatever modality works for you—telephone, e-mail, video conferencing—if you feel lonely, reach out. Friends and families are concerned about us, and it makes them feel useful to support us. Allow that.
- Appreciate/cultivate gratitude. Look for causes for celebration in your life, whether that is your family, friends, or that you have a job and a home. Perhaps it is the cleaner air or the beauty of nature. Whatever you are grateful for, acknowledge it, perhaps by writing it down in a journal. Remembering these things can serve as an antidote to the stress created by the COVID-19 crisis.
- Play. Seek out things that make you laugh.9 Find your inner goofiness. Get creative and paint, sing, garden, golf, play an instrument, dance, do puzzles, or play games. We need to remember the pleasures in life when times are dark. Find whatever feels like joy.
- Exhale. If you have a meditation practice continue to do it. If not, consider starting. Try yoga as a way to center yourself in your body. Elite athletes use meditation and yoga to harness their minds and bodies to feel more in control.10 When so many things feel out of our control and unpredictable, it can be immensely soothing to connect to yourself in this way and realize you can reliably relax.
Self-care is a necessity
While we are in a marathon not of our choosing, we can use the techniques of elite athletes to think positively, visualize scenarios to achieve our goals or to simply relax, plan for setbacks so that we can bounce back, and manage stress productively. Self-care is paramount as we continue to care for others. Let’s not neglect ourselves, and we will cross the finish line together. ●
I am not an elite athlete. Never have been, never will be. But as I contemplated how to support my colleagues at my hospital during the COVID-19 pandemic—knowing that we face more weeks of social distancing, probable virus outbreaks as business opens up, and myriad changes in how we will practice medicine—I wondered how Olympic athletes focus their mental and physical stamina to respond to the challenges of competition.
In this article, I offer some pointers, gleaned from techniques used by exceptional athletes, on how we can maintain our stamina during the COVID-19 marathon.

Train your mind
Elite athletes understand that what will take them to the top of their sport is less about their physical gifts (all elite athletes have superior athletic talent) and more about how they train their minds to achieve their goals. As basketball great Kareem Abdul-Jabbar said, “Your mind is what makes everything else work.” Not surprisingly, athletes who train to be mentally “tough” have a better chance to reach the podium.
One definition of mental toughness is the “ability to perform toward the upper range of your talent and skill” regardless of external circumstances.1 Mental toughness involves an unshakable self-belief, resilience, motivation, focus, and ability to perform under pressure and manage physical and emotional pain.
Like me, most of you probably are not elite athletes, but you can train your mind to be tougher and increase your stamina using some of the following techniques.
Think positively
Do you find yourself dealing with an inner monologue of fear, self-doubt, and feelings of worthlessness? That is the Obnoxious Roommate in your head as described in a HuffPost article and based on the pioneering work of psychiatrist Aaron Beck, MD, the father of cognitive-behavioral therapy.2,3 Such thoughts negatively affect one’s sense of self-efficacy, which is the mental state of believing that you can meet the challenges you face. Elite athletes pay particular attention to their self-talk and replace negative thoughts with positive ones. These may include affirmations of your strengths or cue words that pump you up or help you manage your nerves.
To begin this practice, first observe what you are telling yourself. You may be saying, “I can’t do this. This is too hard.” This is an important step because you may have lived with the Obnoxious Roommate for so long that these thoughts are automatic and seem like a part of you. If, however, you first observe the thought and acknowledge it, you can begin to replace negative thoughts with supportive ones: “This is hard, but I can do this. I am intelligent, strong, capable, and ready.” While practice and repetition are required, thinking positively eventually will become a habit of self-support. Elite athletes know that to reach the top, they need to become their own best friend.4
Continue to: Use visualization...
Use visualization
Elite athletes visualize their events in such meticulous detail that they can actually feel their feet on the track, their muscles tensing, the starter pistol going off, the roar of the crowd. Research shows that the part of the brain that is activated in a race is also activated when one visualizes the race. It is as though the race is happening in real time.5 Physicians could use this approach to visualize the day ahead, picturing the procedures in detail and the clinical encounters going well.
In the current crisis, we frequently need a way to calm down quickly. One suggestion is to find a quiet space and sit for a few minutes, allowing the chair to support you. Then imagine a beautiful, calm, soothing place. Relax there and take in that feeling. Or, imagine a past achievement when you felt good about yourself. See yourself in that moment, remember how you felt, and take it in.
Plan for setbacks
All elite athletes have setbacks: Marathon runners hit a wall, golfers hit into the rough. But they don’t spin out of control at setbacks. They expect them, and they have practiced skills to restore their confidence and re-center themselves.6 For example, some athletes calm themselves by performing a ritualized series of movements. Others use a specific phrase (that positive self-talk again!) that reminds them of their goals or skills, while others play specific songs on their media player, or in their head, to return to their center.
Another technique is to use deep, rhythmic, diaphragmatic breathing for about 30 seconds to bring yourself back to your body.
The point is to have a plan in place to respond to setbacks in a positive manner and get back on track. Don’t waste time on self-criticism.
Continue to: Manage stress...
Manage stress
Not all stress is negative. Everyone has a “sweet spot,” a level of activation from which we operate best.1 If we go too far over that level, though, we can panic. If we aren’t stressed enough, it is hard to get going at all.
If you need to be pumped up to function at your best, try playing music that energizes you. If you work best when you are calm, take deep breaths and attend to the exhalation while you engage in positive self-talk.
Another way to manage stress levels during the COVID-19 pandemic is to employ the ESCAPE mnemonic:
- Exercise. Research shows that regular aerobic exercise is a potent stress reliever; it raises endorphin levels, provides a general sense of well-being, and improves immune function.7 If you exercise regularly, do everything possible to keep doing so. If you do not have an exercise routine, try taking walks. Get out into the fresh air, pay attention to your surroundings, and work to be present in your body as you move.
- Sleep. Getting adequate sleep, 7 to 9 hours a night for most people, is important for maintaining physical and emotional health.8 Elite athletes pay particular attention to sleep because they know that their bodies need to recharge. The COVID-19 crisis has affected sleep for many, and we need to consciously attend to sleep hygiene. This includes limiting use of screen time to no more than 2 hours before bed. As well, limit your intake of the news and definitely avoid it right before bedtime. Take a warm shower, drink a cup of herbal tea, or read a less-than-stimulating journal article. Make sure your bedroom is cool and dark. Keep a regular bedtime and wake up at a regular time.
- Connect. Despite the current need for physical distancing, do not neglect your need to connect to those you love and care about. Whatever modality works for you—telephone, e-mail, video conferencing—if you feel lonely, reach out. Friends and families are concerned about us, and it makes them feel useful to support us. Allow that.
- Appreciate/cultivate gratitude. Look for causes for celebration in your life, whether that is your family, friends, or that you have a job and a home. Perhaps it is the cleaner air or the beauty of nature. Whatever you are grateful for, acknowledge it, perhaps by writing it down in a journal. Remembering these things can serve as an antidote to the stress created by the COVID-19 crisis.
- Play. Seek out things that make you laugh.9 Find your inner goofiness. Get creative and paint, sing, garden, golf, play an instrument, dance, do puzzles, or play games. We need to remember the pleasures in life when times are dark. Find whatever feels like joy.
- Exhale. If you have a meditation practice continue to do it. If not, consider starting. Try yoga as a way to center yourself in your body. Elite athletes use meditation and yoga to harness their minds and bodies to feel more in control.10 When so many things feel out of our control and unpredictable, it can be immensely soothing to connect to yourself in this way and realize you can reliably relax.
Self-care is a necessity
While we are in a marathon not of our choosing, we can use the techniques of elite athletes to think positively, visualize scenarios to achieve our goals or to simply relax, plan for setbacks so that we can bounce back, and manage stress productively. Self-care is paramount as we continue to care for others. Let’s not neglect ourselves, and we will cross the finish line together. ●
- Jones G. What is this thing called mental toughness? An investigation of elite sport performers. J Applied Sport Psych. 2002;14:205-218.
- Gregoire C. The brain-training secrets of Olympic athletes. HuffPost. February 11, 2014. https://www.huffpost.com/entry/mind-hacks-from-olympic-a_n_4747755. Accessed May 27, 2020.
- Beck AT. A 60-year evolution of cognitive theory and therapy. Perspect Psychol Sci. 2019;14:16-20.
- Afremow J. The Champion’s Mind: How Great Athletes Think, Train, and Thrive. New York, NY: Rodale Inc; 2014.
- Ranganathan VK, Siemionow V, Liu JZ, et al. From mental power to muscle power—gaining strength by using the mind. Neuropsychologia. 2004;42:944-956.
- Morgan Griffin R. 5 tips for building mental stamina. July 8, 2013. WebMD. https://www.webmd.com/fitness-exercise/features/mental-stamina#1. Accessed May 27, 2020.
- Hackney AC. Stress and the neuroendocrine system: the role of exercise as a stressor and modifier of stress. Expert Rev Endocrinol Metab. 2006;1:783-792.
- Hirschkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1:40-43.
- Saper B. The therapeutic use of humor for psychiatric disturbances of adolescents and adults. Psychiatr Q. 1990;61:261-272.
- Zeidan F, Johnson SK, Diamond BJ, et al. Mindfulness meditation improves cognition: evidence of brief mental training. Conscious Cogn. 2012;19:597-605.
- Jones G. What is this thing called mental toughness? An investigation of elite sport performers. J Applied Sport Psych. 2002;14:205-218.
- Gregoire C. The brain-training secrets of Olympic athletes. HuffPost. February 11, 2014. https://www.huffpost.com/entry/mind-hacks-from-olympic-a_n_4747755. Accessed May 27, 2020.
- Beck AT. A 60-year evolution of cognitive theory and therapy. Perspect Psychol Sci. 2019;14:16-20.
- Afremow J. The Champion’s Mind: How Great Athletes Think, Train, and Thrive. New York, NY: Rodale Inc; 2014.
- Ranganathan VK, Siemionow V, Liu JZ, et al. From mental power to muscle power—gaining strength by using the mind. Neuropsychologia. 2004;42:944-956.
- Morgan Griffin R. 5 tips for building mental stamina. July 8, 2013. WebMD. https://www.webmd.com/fitness-exercise/features/mental-stamina#1. Accessed May 27, 2020.
- Hackney AC. Stress and the neuroendocrine system: the role of exercise as a stressor and modifier of stress. Expert Rev Endocrinol Metab. 2006;1:783-792.
- Hirschkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1:40-43.
- Saper B. The therapeutic use of humor for psychiatric disturbances of adolescents and adults. Psychiatr Q. 1990;61:261-272.
- Zeidan F, Johnson SK, Diamond BJ, et al. Mindfulness meditation improves cognition: evidence of brief mental training. Conscious Cogn. 2012;19:597-605.
Patients’ perceptions and high hospital use
Background: A small proportion of patients accounts for a large proportion of hospital use and readmissions. As hospitals and hospitalists focus efforts to improve transitions of care, there is a paucity of data that incorporates patients’ perspectives into the design of these programs.
Study design: Qualitative research study.
Setting: Northwestern Memorial Hospital, a single urban academic medical center in Chicago.
Synopsis: Eligible patients had two unplanned 30-day readmissions within the prior 12 months in addition to one or more of the following: at least one readmission in the last 6 months; a referral from a patient’s medical provider; or at least three observation visits.
A research coordinator conducted one-on-one semistructured interviews. Each interview was recorded, transcribed, and then coded using a team-based approach; 26 patients completed the interview process. From the analysis, four major themes emerged: Major medical problems were universal but high hospital use onset varied; participants noted that fluctuations in their course were often related to social, economic, and psychological stressors; onset and progression of episodes seemed uncontrollable and unpredictable; participants preferred to avoid hospitalization and sought care when attempts at self-management failed. The major limitation of this study was the small sample size located at one medical center, creating a data pool that is potentially not generalizable to other medical centers. These findings, however, are an important reminder to focus our interventions with patients’ needs and perceptions in mind.
Bottom line: Frequently hospitalized patients have insights into factors contributing to their high hospital use. Engaging patients in this discussion can enable us to create sustainable patient-centered programs that avoid rehospitalization.
Citation: O’Leary KJ et al. Frequently hospitalized patients’ perceptions of factors contributing to high hospital use. J Hosp Med. 2019 Mar 20;14:e1-6.
Dr. Richardson is a hospitalist at Duke University Health System.
Background: A small proportion of patients accounts for a large proportion of hospital use and readmissions. As hospitals and hospitalists focus efforts to improve transitions of care, there is a paucity of data that incorporates patients’ perspectives into the design of these programs.
Study design: Qualitative research study.
Setting: Northwestern Memorial Hospital, a single urban academic medical center in Chicago.
Synopsis: Eligible patients had two unplanned 30-day readmissions within the prior 12 months in addition to one or more of the following: at least one readmission in the last 6 months; a referral from a patient’s medical provider; or at least three observation visits.
A research coordinator conducted one-on-one semistructured interviews. Each interview was recorded, transcribed, and then coded using a team-based approach; 26 patients completed the interview process. From the analysis, four major themes emerged: Major medical problems were universal but high hospital use onset varied; participants noted that fluctuations in their course were often related to social, economic, and psychological stressors; onset and progression of episodes seemed uncontrollable and unpredictable; participants preferred to avoid hospitalization and sought care when attempts at self-management failed. The major limitation of this study was the small sample size located at one medical center, creating a data pool that is potentially not generalizable to other medical centers. These findings, however, are an important reminder to focus our interventions with patients’ needs and perceptions in mind.
Bottom line: Frequently hospitalized patients have insights into factors contributing to their high hospital use. Engaging patients in this discussion can enable us to create sustainable patient-centered programs that avoid rehospitalization.
Citation: O’Leary KJ et al. Frequently hospitalized patients’ perceptions of factors contributing to high hospital use. J Hosp Med. 2019 Mar 20;14:e1-6.
Dr. Richardson is a hospitalist at Duke University Health System.
Background: A small proportion of patients accounts for a large proportion of hospital use and readmissions. As hospitals and hospitalists focus efforts to improve transitions of care, there is a paucity of data that incorporates patients’ perspectives into the design of these programs.
Study design: Qualitative research study.
Setting: Northwestern Memorial Hospital, a single urban academic medical center in Chicago.
Synopsis: Eligible patients had two unplanned 30-day readmissions within the prior 12 months in addition to one or more of the following: at least one readmission in the last 6 months; a referral from a patient’s medical provider; or at least three observation visits.
A research coordinator conducted one-on-one semistructured interviews. Each interview was recorded, transcribed, and then coded using a team-based approach; 26 patients completed the interview process. From the analysis, four major themes emerged: Major medical problems were universal but high hospital use onset varied; participants noted that fluctuations in their course were often related to social, economic, and psychological stressors; onset and progression of episodes seemed uncontrollable and unpredictable; participants preferred to avoid hospitalization and sought care when attempts at self-management failed. The major limitation of this study was the small sample size located at one medical center, creating a data pool that is potentially not generalizable to other medical centers. These findings, however, are an important reminder to focus our interventions with patients’ needs and perceptions in mind.
Bottom line: Frequently hospitalized patients have insights into factors contributing to their high hospital use. Engaging patients in this discussion can enable us to create sustainable patient-centered programs that avoid rehospitalization.
Citation: O’Leary KJ et al. Frequently hospitalized patients’ perceptions of factors contributing to high hospital use. J Hosp Med. 2019 Mar 20;14:e1-6.
Dr. Richardson is a hospitalist at Duke University Health System.
More fatalities in heart transplant patients with COVID-19
COVID-19 infection is associated with a high risk for mortality in heart transplant (HT) recipients, a new case series suggests.
Investigators looked at data on 28 patients with a confirmed diagnosis of COVID-19 who received a HT between March 1, 2020, and April 24, 2020 and found a case-fatality rate of 25%.
“The high case fatality in our case series should alert physicians to the vulnerability of heart transplant recipients during the COVID-19 pandemic,” senior author Nir Uriel, MD, MSc, professor of medicine at Columbia University, New York, said in an interview.
“These patients require extra precautions to prevent the development of infection,” said Dr. Uriel, who is also a cardiologist at New York Presbyterian/Columbia University Irving Medical Center.
The study was published online May 13 in JAMA Cardiology.
Similar presentation
HT recipients can have several comorbidities after the procedure, including hypertension, diabetes, cardiac allograft vasculopathy, and ongoing immunosuppression, all of which can place them at risk for infection and adverse outcomes with COVID-19 infection, the authors wrote.
The researchers therefore embarked on a case series looking at 28 HT recipients with COVID-19 infection (median age, 64.0 years; interquartile range, 53.5-70.5; 79% male) to “describe the outcomes of recipients of HT who are chronically immunosuppressed and develop COVID-19 and raise important questions about the role of the immune system in the process.”
The median time from HT to study period was 8.6 (IQR, 4.2-14.5) years. Most patients had numerous comorbidities.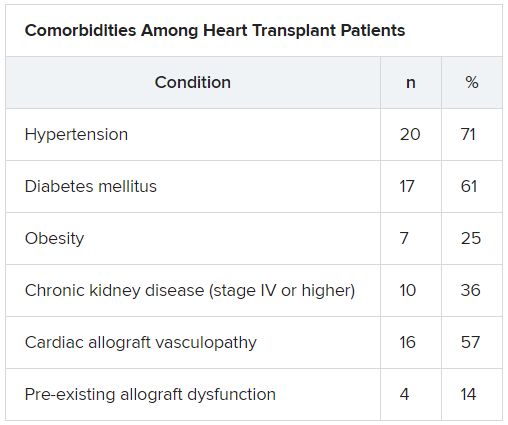
“The presentation of COVID-19 was similar to nontransplant patients with fever, dyspnea, cough, and GI symptoms,” Dr. Uriel reported.
No protective effect
Twenty-two patients (79%) required admission to the hospital, seven of whom (25%) required admission to the ICU and mechanical ventilation.
Despite the presence of immunosuppressive therapy, all patients had significant elevation of inflammatory biomarkers (median peak high-sensitivity C-reactive protein [hs-CRP], 11.83 mg/dL; IQR, 7.44-19.26; median peak interleukin [IL]-6, 105 pg/mL; IQR, 38-296).
Three-quarters had myocardial injury, with a median high-sensitivity troponin T of 0.055 (0.0205 - 0.1345) ng/mL.
Treatments of COVID-19 included hydroxychloroquine (18 patients; 78%), high-dose corticosteroids (eight patients; 47%), and IL-6 receptor antagonists (six patients; 26%).
Moreover, during hospitalization, mycophenolate mofetil was discontinued in most (70%) patients, and one-quarter had a reduction in their calcineurin inhibitor dose.
“Heart transplant recipients generally require more intense immunosuppressive therapy than most other solid organ transplant recipients, and this high baseline immunosuppression increases their propensity to develop infections and their likelihood of experiencing severe manifestations of infections,” Dr. Uriel commented.
“With COVID-19, in which the body’s inflammatory reaction appears to play a role in disease severity, there has been a question of whether immunosuppression may offer a protective effect,” he continued.
“This case series suggests that this is not the case, although this would need to be confirmed in larger studies,” he said.
Low threshold
Among the 22 patients who were admitted to the hospital, half were discharged home and four (18%) were still hospitalized at the end of the study.
Of the seven patients who died, two died at the study center, and five died in an outside institution.
“In the HT population, social distancing (or isolation), strict use of masks when in public, proper handwashing, and sanitization of surfaces are of paramount importance in the prevention of COVID-19 infection,” Dr. Uriel stated.
“In addition, we have restricted these patients’ contact with the hospital as much as possible during the pandemic,” he said.
However, “there should be a low threshold to hospitalize heart transplant patients who develop infection with COVID-19. Furthermore, in our series, outcomes were better for patients hospitalized at the transplant center; therefore, strong consideration should be given to transferring HT patients when hospitalized at another hospital,” he added.
The authors emphasized that COVID-19 patients “will require ongoing monitoring in the recovery phase, as an immunosuppression regimen is reintroduced and the consequences to the allograft itself become apparent.”
Vulnerable population
Commenting on the study, Mandeep R. Mehra, MD, MSc, William Harvey Distinguished Chair in Advanced Cardiovascular Medicine at Brigham and Women’s Hospital, Boston, suggested that “in epidemiological terms, [the findings] might not look as bad as the way they are reflected in the paper.”
Given that Columbia is “one of the larger heart transplant centers in the U.S., following probably 1,000 patients, having only 22 out of perhaps thousands whom they transplanted or are actively following would actually represent a low serious infection rate,” said Dr. Mehra, who is also the executive director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, also in Boston.
“We must not forget to emphasize that, when assessing these case fatality rates, we must look at the entire population at risk, not only the handful that we were able to observe,” explained Dr. Mehra, who was not involved with the study.
Moreover, the patients were “older and had comorbidities, with poor underlying kidney function and other complications, and underlying coronary artery disease in the transplanted heart,” so “it would not surprise me that they had such a high fatality rate, since they had a high degree of vulnerability,” he said.
Dr. Mehra, who is also the editor-in-chief of the Journal of Heart and Lung Transplantation, said that the journal has received manuscripts still in the review process that suggest different fatality rates than those found in the current case series.
However, he acknowledged that, because these are patients with serious vulnerability due to underlying heart disease, “you can’t be lackadaisical and need to do everything to decrease this vulnerability.”
The authors noted that, although their study did not show a protective effect from immunosuppression against COVID-19, further studies are needed to assess each individual immunosuppressive agent and provide a definitive answer.
The study was supported by a grant to one of the investigators from the National Heart, Lung, and Blood Institute. Dr. Uriel reports no relevant financial relationships. The other authors’ disclosures are listed in the publication. Dr. Mehra reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
COVID-19 infection is associated with a high risk for mortality in heart transplant (HT) recipients, a new case series suggests.
Investigators looked at data on 28 patients with a confirmed diagnosis of COVID-19 who received a HT between March 1, 2020, and April 24, 2020 and found a case-fatality rate of 25%.
“The high case fatality in our case series should alert physicians to the vulnerability of heart transplant recipients during the COVID-19 pandemic,” senior author Nir Uriel, MD, MSc, professor of medicine at Columbia University, New York, said in an interview.
“These patients require extra precautions to prevent the development of infection,” said Dr. Uriel, who is also a cardiologist at New York Presbyterian/Columbia University Irving Medical Center.
The study was published online May 13 in JAMA Cardiology.
Similar presentation
HT recipients can have several comorbidities after the procedure, including hypertension, diabetes, cardiac allograft vasculopathy, and ongoing immunosuppression, all of which can place them at risk for infection and adverse outcomes with COVID-19 infection, the authors wrote.
The researchers therefore embarked on a case series looking at 28 HT recipients with COVID-19 infection (median age, 64.0 years; interquartile range, 53.5-70.5; 79% male) to “describe the outcomes of recipients of HT who are chronically immunosuppressed and develop COVID-19 and raise important questions about the role of the immune system in the process.”
The median time from HT to study period was 8.6 (IQR, 4.2-14.5) years. Most patients had numerous comorbidities.
“The presentation of COVID-19 was similar to nontransplant patients with fever, dyspnea, cough, and GI symptoms,” Dr. Uriel reported.
No protective effect
Twenty-two patients (79%) required admission to the hospital, seven of whom (25%) required admission to the ICU and mechanical ventilation.
Despite the presence of immunosuppressive therapy, all patients had significant elevation of inflammatory biomarkers (median peak high-sensitivity C-reactive protein [hs-CRP], 11.83 mg/dL; IQR, 7.44-19.26; median peak interleukin [IL]-6, 105 pg/mL; IQR, 38-296).
Three-quarters had myocardial injury, with a median high-sensitivity troponin T of 0.055 (0.0205 - 0.1345) ng/mL.
Treatments of COVID-19 included hydroxychloroquine (18 patients; 78%), high-dose corticosteroids (eight patients; 47%), and IL-6 receptor antagonists (six patients; 26%).
Moreover, during hospitalization, mycophenolate mofetil was discontinued in most (70%) patients, and one-quarter had a reduction in their calcineurin inhibitor dose.
“Heart transplant recipients generally require more intense immunosuppressive therapy than most other solid organ transplant recipients, and this high baseline immunosuppression increases their propensity to develop infections and their likelihood of experiencing severe manifestations of infections,” Dr. Uriel commented.
“With COVID-19, in which the body’s inflammatory reaction appears to play a role in disease severity, there has been a question of whether immunosuppression may offer a protective effect,” he continued.
“This case series suggests that this is not the case, although this would need to be confirmed in larger studies,” he said.
Low threshold
Among the 22 patients who were admitted to the hospital, half were discharged home and four (18%) were still hospitalized at the end of the study.
Of the seven patients who died, two died at the study center, and five died in an outside institution.
“In the HT population, social distancing (or isolation), strict use of masks when in public, proper handwashing, and sanitization of surfaces are of paramount importance in the prevention of COVID-19 infection,” Dr. Uriel stated.
“In addition, we have restricted these patients’ contact with the hospital as much as possible during the pandemic,” he said.
However, “there should be a low threshold to hospitalize heart transplant patients who develop infection with COVID-19. Furthermore, in our series, outcomes were better for patients hospitalized at the transplant center; therefore, strong consideration should be given to transferring HT patients when hospitalized at another hospital,” he added.
The authors emphasized that COVID-19 patients “will require ongoing monitoring in the recovery phase, as an immunosuppression regimen is reintroduced and the consequences to the allograft itself become apparent.”
Vulnerable population
Commenting on the study, Mandeep R. Mehra, MD, MSc, William Harvey Distinguished Chair in Advanced Cardiovascular Medicine at Brigham and Women’s Hospital, Boston, suggested that “in epidemiological terms, [the findings] might not look as bad as the way they are reflected in the paper.”
Given that Columbia is “one of the larger heart transplant centers in the U.S., following probably 1,000 patients, having only 22 out of perhaps thousands whom they transplanted or are actively following would actually represent a low serious infection rate,” said Dr. Mehra, who is also the executive director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, also in Boston.
“We must not forget to emphasize that, when assessing these case fatality rates, we must look at the entire population at risk, not only the handful that we were able to observe,” explained Dr. Mehra, who was not involved with the study.
Moreover, the patients were “older and had comorbidities, with poor underlying kidney function and other complications, and underlying coronary artery disease in the transplanted heart,” so “it would not surprise me that they had such a high fatality rate, since they had a high degree of vulnerability,” he said.
Dr. Mehra, who is also the editor-in-chief of the Journal of Heart and Lung Transplantation, said that the journal has received manuscripts still in the review process that suggest different fatality rates than those found in the current case series.
However, he acknowledged that, because these are patients with serious vulnerability due to underlying heart disease, “you can’t be lackadaisical and need to do everything to decrease this vulnerability.”
The authors noted that, although their study did not show a protective effect from immunosuppression against COVID-19, further studies are needed to assess each individual immunosuppressive agent and provide a definitive answer.
The study was supported by a grant to one of the investigators from the National Heart, Lung, and Blood Institute. Dr. Uriel reports no relevant financial relationships. The other authors’ disclosures are listed in the publication. Dr. Mehra reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
COVID-19 infection is associated with a high risk for mortality in heart transplant (HT) recipients, a new case series suggests.
Investigators looked at data on 28 patients with a confirmed diagnosis of COVID-19 who received a HT between March 1, 2020, and April 24, 2020 and found a case-fatality rate of 25%.
“The high case fatality in our case series should alert physicians to the vulnerability of heart transplant recipients during the COVID-19 pandemic,” senior author Nir Uriel, MD, MSc, professor of medicine at Columbia University, New York, said in an interview.
“These patients require extra precautions to prevent the development of infection,” said Dr. Uriel, who is also a cardiologist at New York Presbyterian/Columbia University Irving Medical Center.
The study was published online May 13 in JAMA Cardiology.
Similar presentation
HT recipients can have several comorbidities after the procedure, including hypertension, diabetes, cardiac allograft vasculopathy, and ongoing immunosuppression, all of which can place them at risk for infection and adverse outcomes with COVID-19 infection, the authors wrote.
The researchers therefore embarked on a case series looking at 28 HT recipients with COVID-19 infection (median age, 64.0 years; interquartile range, 53.5-70.5; 79% male) to “describe the outcomes of recipients of HT who are chronically immunosuppressed and develop COVID-19 and raise important questions about the role of the immune system in the process.”
The median time from HT to study period was 8.6 (IQR, 4.2-14.5) years. Most patients had numerous comorbidities.
“The presentation of COVID-19 was similar to nontransplant patients with fever, dyspnea, cough, and GI symptoms,” Dr. Uriel reported.
No protective effect
Twenty-two patients (79%) required admission to the hospital, seven of whom (25%) required admission to the ICU and mechanical ventilation.
Despite the presence of immunosuppressive therapy, all patients had significant elevation of inflammatory biomarkers (median peak high-sensitivity C-reactive protein [hs-CRP], 11.83 mg/dL; IQR, 7.44-19.26; median peak interleukin [IL]-6, 105 pg/mL; IQR, 38-296).
Three-quarters had myocardial injury, with a median high-sensitivity troponin T of 0.055 (0.0205 - 0.1345) ng/mL.
Treatments of COVID-19 included hydroxychloroquine (18 patients; 78%), high-dose corticosteroids (eight patients; 47%), and IL-6 receptor antagonists (six patients; 26%).
Moreover, during hospitalization, mycophenolate mofetil was discontinued in most (70%) patients, and one-quarter had a reduction in their calcineurin inhibitor dose.
“Heart transplant recipients generally require more intense immunosuppressive therapy than most other solid organ transplant recipients, and this high baseline immunosuppression increases their propensity to develop infections and their likelihood of experiencing severe manifestations of infections,” Dr. Uriel commented.
“With COVID-19, in which the body’s inflammatory reaction appears to play a role in disease severity, there has been a question of whether immunosuppression may offer a protective effect,” he continued.
“This case series suggests that this is not the case, although this would need to be confirmed in larger studies,” he said.
Low threshold
Among the 22 patients who were admitted to the hospital, half were discharged home and four (18%) were still hospitalized at the end of the study.
Of the seven patients who died, two died at the study center, and five died in an outside institution.
“In the HT population, social distancing (or isolation), strict use of masks when in public, proper handwashing, and sanitization of surfaces are of paramount importance in the prevention of COVID-19 infection,” Dr. Uriel stated.
“In addition, we have restricted these patients’ contact with the hospital as much as possible during the pandemic,” he said.
However, “there should be a low threshold to hospitalize heart transplant patients who develop infection with COVID-19. Furthermore, in our series, outcomes were better for patients hospitalized at the transplant center; therefore, strong consideration should be given to transferring HT patients when hospitalized at another hospital,” he added.
The authors emphasized that COVID-19 patients “will require ongoing monitoring in the recovery phase, as an immunosuppression regimen is reintroduced and the consequences to the allograft itself become apparent.”
Vulnerable population
Commenting on the study, Mandeep R. Mehra, MD, MSc, William Harvey Distinguished Chair in Advanced Cardiovascular Medicine at Brigham and Women’s Hospital, Boston, suggested that “in epidemiological terms, [the findings] might not look as bad as the way they are reflected in the paper.”
Given that Columbia is “one of the larger heart transplant centers in the U.S., following probably 1,000 patients, having only 22 out of perhaps thousands whom they transplanted or are actively following would actually represent a low serious infection rate,” said Dr. Mehra, who is also the executive director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, also in Boston.
“We must not forget to emphasize that, when assessing these case fatality rates, we must look at the entire population at risk, not only the handful that we were able to observe,” explained Dr. Mehra, who was not involved with the study.
Moreover, the patients were “older and had comorbidities, with poor underlying kidney function and other complications, and underlying coronary artery disease in the transplanted heart,” so “it would not surprise me that they had such a high fatality rate, since they had a high degree of vulnerability,” he said.
Dr. Mehra, who is also the editor-in-chief of the Journal of Heart and Lung Transplantation, said that the journal has received manuscripts still in the review process that suggest different fatality rates than those found in the current case series.
However, he acknowledged that, because these are patients with serious vulnerability due to underlying heart disease, “you can’t be lackadaisical and need to do everything to decrease this vulnerability.”
The authors noted that, although their study did not show a protective effect from immunosuppression against COVID-19, further studies are needed to assess each individual immunosuppressive agent and provide a definitive answer.
The study was supported by a grant to one of the investigators from the National Heart, Lung, and Blood Institute. Dr. Uriel reports no relevant financial relationships. The other authors’ disclosures are listed in the publication. Dr. Mehra reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Painful ear lesion
The patient was given a diagnosis of chondrodermatitis nodularis helicis (CNH), an inflammation of the cartilage and overlying skin causing a painful nodule of the helix. These lesions typically have a more prominent nodular component and a central ulceration or firm scale. They are thought to be due to chronic pressure on the ear.
There is a slight male predominance of CNH and onset is usually gradual. Patients often experience pain when sleeping on the affected side. The tenderness usually can be reproduced clinically by pressing on the lesion. The ears also are a high-risk area for actinic keratoses (AK) and nonmelanoma skin cancer (NMSC); if there is doubt about the diagnosis, a biopsy may be warranted to rule out AK or NMSC. In this patient, a shave biopsy was performed.
Various treatment regimens are available for CNH. The least invasive treatment approach is to use a “cut out” foam or a special donut-shaped pillow to protect the area from further pressure. By protecting from pressure and irritation, the lesion resolves in 57% to 92% of cases in clinical studies. Intralesional injection with 0.2 mL of 10 mg/mL triamcinolone acetonide is a simple in-office procedure that frequently helps the pain and may be curative; although, repeat injections may be necessary. Excision of the overlying skin and the affected cartilage is a more aggressive treatment with high success rates. More recently, treatment with topical nitroglycerin patches or photodynamic therapy have been described in small trials.
After confirming by a shave biopsy that the lesion was not cancerous, the patient returned for an elliptical excision of the lesion. A dermal curette was used to remove the rough abnormal inflammation of the underlying cartilage, and the elliptical wound was sutured with a linear closure.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Juul Nielsen L, Holkmann Olsen C, Lock-Andersen J. Therapeutic options of chondrodermatitis nodularis helicis [published online January 27, 2016]. Plast Surg Int. 2016;2016:4340168.
The patient was given a diagnosis of chondrodermatitis nodularis helicis (CNH), an inflammation of the cartilage and overlying skin causing a painful nodule of the helix. These lesions typically have a more prominent nodular component and a central ulceration or firm scale. They are thought to be due to chronic pressure on the ear.
There is a slight male predominance of CNH and onset is usually gradual. Patients often experience pain when sleeping on the affected side. The tenderness usually can be reproduced clinically by pressing on the lesion. The ears also are a high-risk area for actinic keratoses (AK) and nonmelanoma skin cancer (NMSC); if there is doubt about the diagnosis, a biopsy may be warranted to rule out AK or NMSC. In this patient, a shave biopsy was performed.
Various treatment regimens are available for CNH. The least invasive treatment approach is to use a “cut out” foam or a special donut-shaped pillow to protect the area from further pressure. By protecting from pressure and irritation, the lesion resolves in 57% to 92% of cases in clinical studies. Intralesional injection with 0.2 mL of 10 mg/mL triamcinolone acetonide is a simple in-office procedure that frequently helps the pain and may be curative; although, repeat injections may be necessary. Excision of the overlying skin and the affected cartilage is a more aggressive treatment with high success rates. More recently, treatment with topical nitroglycerin patches or photodynamic therapy have been described in small trials.
After confirming by a shave biopsy that the lesion was not cancerous, the patient returned for an elliptical excision of the lesion. A dermal curette was used to remove the rough abnormal inflammation of the underlying cartilage, and the elliptical wound was sutured with a linear closure.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
The patient was given a diagnosis of chondrodermatitis nodularis helicis (CNH), an inflammation of the cartilage and overlying skin causing a painful nodule of the helix. These lesions typically have a more prominent nodular component and a central ulceration or firm scale. They are thought to be due to chronic pressure on the ear.
There is a slight male predominance of CNH and onset is usually gradual. Patients often experience pain when sleeping on the affected side. The tenderness usually can be reproduced clinically by pressing on the lesion. The ears also are a high-risk area for actinic keratoses (AK) and nonmelanoma skin cancer (NMSC); if there is doubt about the diagnosis, a biopsy may be warranted to rule out AK or NMSC. In this patient, a shave biopsy was performed.
Various treatment regimens are available for CNH. The least invasive treatment approach is to use a “cut out” foam or a special donut-shaped pillow to protect the area from further pressure. By protecting from pressure and irritation, the lesion resolves in 57% to 92% of cases in clinical studies. Intralesional injection with 0.2 mL of 10 mg/mL triamcinolone acetonide is a simple in-office procedure that frequently helps the pain and may be curative; although, repeat injections may be necessary. Excision of the overlying skin and the affected cartilage is a more aggressive treatment with high success rates. More recently, treatment with topical nitroglycerin patches or photodynamic therapy have been described in small trials.
After confirming by a shave biopsy that the lesion was not cancerous, the patient returned for an elliptical excision of the lesion. A dermal curette was used to remove the rough abnormal inflammation of the underlying cartilage, and the elliptical wound was sutured with a linear closure.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Juul Nielsen L, Holkmann Olsen C, Lock-Andersen J. Therapeutic options of chondrodermatitis nodularis helicis [published online January 27, 2016]. Plast Surg Int. 2016;2016:4340168.
Juul Nielsen L, Holkmann Olsen C, Lock-Andersen J. Therapeutic options of chondrodermatitis nodularis helicis [published online January 27, 2016]. Plast Surg Int. 2016;2016:4340168.