User login
WHO: Asymptomatic COVID-19 spread deemed ‘rare’
An official with the World Health Organization (WHO) has stated that it appears to be “rare” that an asymptomatic individual can pass SARS-CoV-2 to someone else.
“From the data we have, it still seems to be rare that an asymptomatic person actually transmits onward to a secondary individual,” Maria Van Kerkhove, PhD, WHO’s COVID-19 technical lead and an infectious disease epidemiologist, said June 8 at a news briefing from the agency’s Geneva headquarters.
This announcement came on the heels of the publication of an analysis in the Annals of Internal Medicine, which suggested that as many as 40-45% of COVID-19 cases may be asymptomatic. In this paper, the authors, Daniel P. Oran, AM, and Eric J. Topol, MD, of the Scripps Research Translational Institute in La Jolla, Calif stated: “The likelihood that approximately 40%-45% of those infected with SARS-CoV-2 will remain asymptomatic suggests that the virus might have greater potential than previously estimated to spread silently and deeply through human populations.”
"The early data that we have assembled on the prevalence of asymptomatic SARS-CoV-2 infection suggest that this is a significant factor in the rapid progression of the COVID-19 pandemic," the authors concluded.
Dr. Van Kerkhove also made comments suggesting otherwise on Twitter, citing a new summary by WHO: “@WHO recently published a summary of transmission of #COVID19, incl. symptomatic, pre-symptomatic and asymptomatic transmission.”
She also tweeted the following lines from the WHO summary: “Comprehensive studies on transmission from asymptomatic individuals are difficult to conduct, but the available evidence from contact tracing reported by Member States suggests that asymptomatically-infected individuals are much less likely to transmit the virus than those who develop symptoms.”
In an additional post, Dr. Van Kerkhove added: “In these data, it is important to breakdown truly asymptomatic vs pre-symptomatic vs mildly symptomatic... also to note that the [percentage] reported or estimated to be ‘asymptomatic’ is not the same as the [percentage] that are asymptomatic that actually transmit.”
In the paper published in the Annals of Internal Medicine, Mr. Oran and Dr. Topol analyzed data of asymptomatic individuals from 16 cohorts between April 19 and May 26, 2020 – a wide-ranging group consisting of residents of cities, health care workers, individuals in homeless shelters, obstetric patients, residents of a nursing home, crew members of aircraft carriers, passengers on cruise ships, and inmates in correctional facilities. Each cohort had varying rates of asymptomatic or presymptomatic cases..
When residents of Iceland were tested, 43 of 100 individuals who tested positive for SARS-CoV-2 did not show symptoms. In Vo’, Italy, 30 of 73 people (41.1%) with positive SARS-CoV-2 test results did not have symptoms in a first round of testing, and 13 of 29 (44.8%) had no symptoms in a second round of testing. Over half of residents of San Francisco’s Mission District who received testing (39 of 74; 52.7%) did not have symptoms, while slightly less than half of Indiana residents tested showed no symptoms (35 of 78; 44.8%).
A majority of 41 individuals (65.9%) who were mostly health care workers at Rutgers University reported no symptoms of COVID-19 at the time of testing. Data from homeless shelters in Boston (129 of 147; 87.7%) and Los Angeles (27 of 43; 62.7%) also showed a high rate of individuals without symptoms. Among 33 obstetric patients in New York City who tested positive for SARS-CoV-2, 29 women (87.9%) were asymptomatic during a median 2-day length of stay. In a Washington state nursing facility, 12 of 23 individuals (52.1%) were positive for SARS-CoV-2 without showing symptoms in a first round of testing, with another 15 of 24 residents (62.5%) not showing symptoms in a second round of testing. Of these residents, 24 individuals (88.9%) later went on to show symptoms of COVID-19.
Most of the 783 Greek citizens who tested positive for SARS-CoV-2 after being evacuated from Spain, Turkey, and the United Kingdom showed no symptoms of COVID-19 (35 of 40; 87.5%). A group of 565 Japanese citizens evacuated from Wuhan, China, had a lower number of cases without initial symptoms – 13 people were positive for SARS-CoV-2, and 4 of 13 (30.8%) had no symptoms.
In closed cohorts, there appeared to also be a high rate of COVID-19 cases without initial symptoms. Of 3,277 inmates from correctional facilities in Arkansas, North Carolina, Ohio, and Virginia, 3,146 individuals (96%) had no symptoms at the time of testing. There was also a large percentage of passengers and crew of the Diamond Princess cruise ship (331 of 712; 46.5%) and an Argentine cruise ship (104 of 128; 81.3%) who were positive for SARS-CoV-2 without symptoms. On the aircraft carrier U.S.S. Theodore Roosevelt, 60% of 856 individuals, while on the French aircraft carrier Charles de Gaulle, nearly 50% of individuals were asymptomatic.
It is difficult to tell the difference between people who are presymptomatic and will later go on to develop symptoms of COVID-19 and those who will remain asymptomatic. “The simple solution to this conundrum is longitudinal testing – that is, repeated observations of the individual over time,” but only 5 of 16 cohorts studied had longitudinal data on individuals, Mr. Oran and Dr. Topol said.
Seth Trueger, MD, an emergency physician and assistant professor of emergency medicine at Northwestern University, Chicago, who was not involved in the study, said it was important to see this information all in one place, even if the data isn’t new.
“I think we’ve certainly kind of seen from the beginning there’s some level of asymptomatic and presymptomatic spread,” Dr. Trueger said. “In health care, we’ve been lucky to get those lessons early on and start to think of things like universal masking in hospitals, and unfortunate things like limiting visitors.”
A more nuanced understanding of how SARS-CoV-2 spreads has been difficult to capture, in part because of operating under a shortened time frame and handicapped testing capacity, he noted. “[Even] in the best of possible circumstances, trying to figure out epidemiology in people who don’t have symptoms is really tough,” Dr. Truegar said.
“Even the best studies are still relatively decent samples, and not totally representative,” he added.
Another limitation to capturing accurate data is method of testing. Real-time reverse transcriptase polymerase chain reaction using nasopharyngeal swabs can detect RNA fragments from SARS-CoV-2, which could potentially affect the results. “It’s really hard to know what is actually infected virus versus just fragments of RNA that make the test positive,” Dr. Trueger said.
If the rate of asymptomatic cases is higher than previously thought, it’s a “double-edged sword,” he noted. It may mean the infection fatality rate is lower than predicted, but “even at high levels of what we think community levels might be, we’re far from herd immunity.”
The study authors and Dr. Trueger reported no relevant conflicts of interest.
SOURCE: Oran DP, Topol EJ. Ann Intern Med. 2020 Jun 3. doi: 10.7326/M20-3012.
This article was updated 6/8/20.
An official with the World Health Organization (WHO) has stated that it appears to be “rare” that an asymptomatic individual can pass SARS-CoV-2 to someone else.
“From the data we have, it still seems to be rare that an asymptomatic person actually transmits onward to a secondary individual,” Maria Van Kerkhove, PhD, WHO’s COVID-19 technical lead and an infectious disease epidemiologist, said June 8 at a news briefing from the agency’s Geneva headquarters.
This announcement came on the heels of the publication of an analysis in the Annals of Internal Medicine, which suggested that as many as 40-45% of COVID-19 cases may be asymptomatic. In this paper, the authors, Daniel P. Oran, AM, and Eric J. Topol, MD, of the Scripps Research Translational Institute in La Jolla, Calif stated: “The likelihood that approximately 40%-45% of those infected with SARS-CoV-2 will remain asymptomatic suggests that the virus might have greater potential than previously estimated to spread silently and deeply through human populations.”
"The early data that we have assembled on the prevalence of asymptomatic SARS-CoV-2 infection suggest that this is a significant factor in the rapid progression of the COVID-19 pandemic," the authors concluded.
Dr. Van Kerkhove also made comments suggesting otherwise on Twitter, citing a new summary by WHO: “@WHO recently published a summary of transmission of #COVID19, incl. symptomatic, pre-symptomatic and asymptomatic transmission.”
She also tweeted the following lines from the WHO summary: “Comprehensive studies on transmission from asymptomatic individuals are difficult to conduct, but the available evidence from contact tracing reported by Member States suggests that asymptomatically-infected individuals are much less likely to transmit the virus than those who develop symptoms.”
In an additional post, Dr. Van Kerkhove added: “In these data, it is important to breakdown truly asymptomatic vs pre-symptomatic vs mildly symptomatic... also to note that the [percentage] reported or estimated to be ‘asymptomatic’ is not the same as the [percentage] that are asymptomatic that actually transmit.”
In the paper published in the Annals of Internal Medicine, Mr. Oran and Dr. Topol analyzed data of asymptomatic individuals from 16 cohorts between April 19 and May 26, 2020 – a wide-ranging group consisting of residents of cities, health care workers, individuals in homeless shelters, obstetric patients, residents of a nursing home, crew members of aircraft carriers, passengers on cruise ships, and inmates in correctional facilities. Each cohort had varying rates of asymptomatic or presymptomatic cases..
When residents of Iceland were tested, 43 of 100 individuals who tested positive for SARS-CoV-2 did not show symptoms. In Vo’, Italy, 30 of 73 people (41.1%) with positive SARS-CoV-2 test results did not have symptoms in a first round of testing, and 13 of 29 (44.8%) had no symptoms in a second round of testing. Over half of residents of San Francisco’s Mission District who received testing (39 of 74; 52.7%) did not have symptoms, while slightly less than half of Indiana residents tested showed no symptoms (35 of 78; 44.8%).
A majority of 41 individuals (65.9%) who were mostly health care workers at Rutgers University reported no symptoms of COVID-19 at the time of testing. Data from homeless shelters in Boston (129 of 147; 87.7%) and Los Angeles (27 of 43; 62.7%) also showed a high rate of individuals without symptoms. Among 33 obstetric patients in New York City who tested positive for SARS-CoV-2, 29 women (87.9%) were asymptomatic during a median 2-day length of stay. In a Washington state nursing facility, 12 of 23 individuals (52.1%) were positive for SARS-CoV-2 without showing symptoms in a first round of testing, with another 15 of 24 residents (62.5%) not showing symptoms in a second round of testing. Of these residents, 24 individuals (88.9%) later went on to show symptoms of COVID-19.
Most of the 783 Greek citizens who tested positive for SARS-CoV-2 after being evacuated from Spain, Turkey, and the United Kingdom showed no symptoms of COVID-19 (35 of 40; 87.5%). A group of 565 Japanese citizens evacuated from Wuhan, China, had a lower number of cases without initial symptoms – 13 people were positive for SARS-CoV-2, and 4 of 13 (30.8%) had no symptoms.
In closed cohorts, there appeared to also be a high rate of COVID-19 cases without initial symptoms. Of 3,277 inmates from correctional facilities in Arkansas, North Carolina, Ohio, and Virginia, 3,146 individuals (96%) had no symptoms at the time of testing. There was also a large percentage of passengers and crew of the Diamond Princess cruise ship (331 of 712; 46.5%) and an Argentine cruise ship (104 of 128; 81.3%) who were positive for SARS-CoV-2 without symptoms. On the aircraft carrier U.S.S. Theodore Roosevelt, 60% of 856 individuals, while on the French aircraft carrier Charles de Gaulle, nearly 50% of individuals were asymptomatic.
It is difficult to tell the difference between people who are presymptomatic and will later go on to develop symptoms of COVID-19 and those who will remain asymptomatic. “The simple solution to this conundrum is longitudinal testing – that is, repeated observations of the individual over time,” but only 5 of 16 cohorts studied had longitudinal data on individuals, Mr. Oran and Dr. Topol said.
Seth Trueger, MD, an emergency physician and assistant professor of emergency medicine at Northwestern University, Chicago, who was not involved in the study, said it was important to see this information all in one place, even if the data isn’t new.
“I think we’ve certainly kind of seen from the beginning there’s some level of asymptomatic and presymptomatic spread,” Dr. Trueger said. “In health care, we’ve been lucky to get those lessons early on and start to think of things like universal masking in hospitals, and unfortunate things like limiting visitors.”
A more nuanced understanding of how SARS-CoV-2 spreads has been difficult to capture, in part because of operating under a shortened time frame and handicapped testing capacity, he noted. “[Even] in the best of possible circumstances, trying to figure out epidemiology in people who don’t have symptoms is really tough,” Dr. Truegar said.
“Even the best studies are still relatively decent samples, and not totally representative,” he added.
Another limitation to capturing accurate data is method of testing. Real-time reverse transcriptase polymerase chain reaction using nasopharyngeal swabs can detect RNA fragments from SARS-CoV-2, which could potentially affect the results. “It’s really hard to know what is actually infected virus versus just fragments of RNA that make the test positive,” Dr. Trueger said.
If the rate of asymptomatic cases is higher than previously thought, it’s a “double-edged sword,” he noted. It may mean the infection fatality rate is lower than predicted, but “even at high levels of what we think community levels might be, we’re far from herd immunity.”
The study authors and Dr. Trueger reported no relevant conflicts of interest.
SOURCE: Oran DP, Topol EJ. Ann Intern Med. 2020 Jun 3. doi: 10.7326/M20-3012.
This article was updated 6/8/20.
An official with the World Health Organization (WHO) has stated that it appears to be “rare” that an asymptomatic individual can pass SARS-CoV-2 to someone else.
“From the data we have, it still seems to be rare that an asymptomatic person actually transmits onward to a secondary individual,” Maria Van Kerkhove, PhD, WHO’s COVID-19 technical lead and an infectious disease epidemiologist, said June 8 at a news briefing from the agency’s Geneva headquarters.
This announcement came on the heels of the publication of an analysis in the Annals of Internal Medicine, which suggested that as many as 40-45% of COVID-19 cases may be asymptomatic. In this paper, the authors, Daniel P. Oran, AM, and Eric J. Topol, MD, of the Scripps Research Translational Institute in La Jolla, Calif stated: “The likelihood that approximately 40%-45% of those infected with SARS-CoV-2 will remain asymptomatic suggests that the virus might have greater potential than previously estimated to spread silently and deeply through human populations.”
"The early data that we have assembled on the prevalence of asymptomatic SARS-CoV-2 infection suggest that this is a significant factor in the rapid progression of the COVID-19 pandemic," the authors concluded.
Dr. Van Kerkhove also made comments suggesting otherwise on Twitter, citing a new summary by WHO: “@WHO recently published a summary of transmission of #COVID19, incl. symptomatic, pre-symptomatic and asymptomatic transmission.”
She also tweeted the following lines from the WHO summary: “Comprehensive studies on transmission from asymptomatic individuals are difficult to conduct, but the available evidence from contact tracing reported by Member States suggests that asymptomatically-infected individuals are much less likely to transmit the virus than those who develop symptoms.”
In an additional post, Dr. Van Kerkhove added: “In these data, it is important to breakdown truly asymptomatic vs pre-symptomatic vs mildly symptomatic... also to note that the [percentage] reported or estimated to be ‘asymptomatic’ is not the same as the [percentage] that are asymptomatic that actually transmit.”
In the paper published in the Annals of Internal Medicine, Mr. Oran and Dr. Topol analyzed data of asymptomatic individuals from 16 cohorts between April 19 and May 26, 2020 – a wide-ranging group consisting of residents of cities, health care workers, individuals in homeless shelters, obstetric patients, residents of a nursing home, crew members of aircraft carriers, passengers on cruise ships, and inmates in correctional facilities. Each cohort had varying rates of asymptomatic or presymptomatic cases..
When residents of Iceland were tested, 43 of 100 individuals who tested positive for SARS-CoV-2 did not show symptoms. In Vo’, Italy, 30 of 73 people (41.1%) with positive SARS-CoV-2 test results did not have symptoms in a first round of testing, and 13 of 29 (44.8%) had no symptoms in a second round of testing. Over half of residents of San Francisco’s Mission District who received testing (39 of 74; 52.7%) did not have symptoms, while slightly less than half of Indiana residents tested showed no symptoms (35 of 78; 44.8%).
A majority of 41 individuals (65.9%) who were mostly health care workers at Rutgers University reported no symptoms of COVID-19 at the time of testing. Data from homeless shelters in Boston (129 of 147; 87.7%) and Los Angeles (27 of 43; 62.7%) also showed a high rate of individuals without symptoms. Among 33 obstetric patients in New York City who tested positive for SARS-CoV-2, 29 women (87.9%) were asymptomatic during a median 2-day length of stay. In a Washington state nursing facility, 12 of 23 individuals (52.1%) were positive for SARS-CoV-2 without showing symptoms in a first round of testing, with another 15 of 24 residents (62.5%) not showing symptoms in a second round of testing. Of these residents, 24 individuals (88.9%) later went on to show symptoms of COVID-19.
Most of the 783 Greek citizens who tested positive for SARS-CoV-2 after being evacuated from Spain, Turkey, and the United Kingdom showed no symptoms of COVID-19 (35 of 40; 87.5%). A group of 565 Japanese citizens evacuated from Wuhan, China, had a lower number of cases without initial symptoms – 13 people were positive for SARS-CoV-2, and 4 of 13 (30.8%) had no symptoms.
In closed cohorts, there appeared to also be a high rate of COVID-19 cases without initial symptoms. Of 3,277 inmates from correctional facilities in Arkansas, North Carolina, Ohio, and Virginia, 3,146 individuals (96%) had no symptoms at the time of testing. There was also a large percentage of passengers and crew of the Diamond Princess cruise ship (331 of 712; 46.5%) and an Argentine cruise ship (104 of 128; 81.3%) who were positive for SARS-CoV-2 without symptoms. On the aircraft carrier U.S.S. Theodore Roosevelt, 60% of 856 individuals, while on the French aircraft carrier Charles de Gaulle, nearly 50% of individuals were asymptomatic.
It is difficult to tell the difference between people who are presymptomatic and will later go on to develop symptoms of COVID-19 and those who will remain asymptomatic. “The simple solution to this conundrum is longitudinal testing – that is, repeated observations of the individual over time,” but only 5 of 16 cohorts studied had longitudinal data on individuals, Mr. Oran and Dr. Topol said.
Seth Trueger, MD, an emergency physician and assistant professor of emergency medicine at Northwestern University, Chicago, who was not involved in the study, said it was important to see this information all in one place, even if the data isn’t new.
“I think we’ve certainly kind of seen from the beginning there’s some level of asymptomatic and presymptomatic spread,” Dr. Trueger said. “In health care, we’ve been lucky to get those lessons early on and start to think of things like universal masking in hospitals, and unfortunate things like limiting visitors.”
A more nuanced understanding of how SARS-CoV-2 spreads has been difficult to capture, in part because of operating under a shortened time frame and handicapped testing capacity, he noted. “[Even] in the best of possible circumstances, trying to figure out epidemiology in people who don’t have symptoms is really tough,” Dr. Truegar said.
“Even the best studies are still relatively decent samples, and not totally representative,” he added.
Another limitation to capturing accurate data is method of testing. Real-time reverse transcriptase polymerase chain reaction using nasopharyngeal swabs can detect RNA fragments from SARS-CoV-2, which could potentially affect the results. “It’s really hard to know what is actually infected virus versus just fragments of RNA that make the test positive,” Dr. Trueger said.
If the rate of asymptomatic cases is higher than previously thought, it’s a “double-edged sword,” he noted. It may mean the infection fatality rate is lower than predicted, but “even at high levels of what we think community levels might be, we’re far from herd immunity.”
The study authors and Dr. Trueger reported no relevant conflicts of interest.
SOURCE: Oran DP, Topol EJ. Ann Intern Med. 2020 Jun 3. doi: 10.7326/M20-3012.
This article was updated 6/8/20.
FROM ANNALS OF INTERNAL MEDICINE
Analysis of Pharmacist Interventions Used to Resolve Safety Target of Polypharmacy (STOP) Drug Interactions
Statins are one of the most common medications dispensed in the US and are associated with clinically significant drug interactions.1,2 The most common adverse drug reaction (ADR) of statin drug interactions is muscle-related toxicities.2 Despite technology advances to alert clinicians to drug interactions, updated statin manufacturer labeling, and guideline recommendations, inappropriate prescribing and dispensing of statin drug interactions continues to occur in health care systems.2-10
The medical literature has demonstrated many opportunities for pharmacists to prevent and mitigate drug interactions. At the points of prescribing and dispensing, pharmacists can reduce the number of potential drug interactions for the patient.11-13 Pharmacists also have identified and resolved drug interactions through quality assurance review after dispensing to a patient.7,8
Regardless of the time point of an intervention, the most common method pharmacists used to resolve drug interactions was through recommendations to a prescriber. The recommendations were generated through academic detailing, clinical decision support algorithms, drug conversions, or the pharmacist’s expertise. Regardless of the method the pharmacist used, the prescriber had the final authority to accept or decline the recommendation.7,8,11-13 Although these interventions were effective, pharmacists could further streamline the process by autonomously resolving drug interactions. However, these types of interventions are not well described in the medical literature.
Background
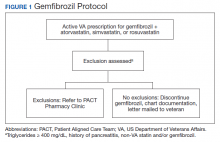
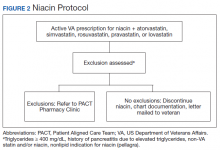
The US Department of Veterans Affairs (VA) Veterans Integrated Service Network (VISN), established the Safety Target of Polypharmacy (STOP) report in 2015. At each facility in the network, the report identified patients who were dispensed medications known to have drug interactions. The interactions were chosen by the VISN, and the severity of the interactions was based on coding parameters within the VA computerized order entry system, which uses a severity score based on First Databank data. At the Harry S. Truman Memorial Veterans’ Hospital (Truman VA) in Columbia, Missouri, > 500 drug interactions were initially active on the STOP report. The most common drug interactions were statins with gemfibrozil and statins with niacin.14-18 The Truman VA Pharmacy Service was charged with resolving the interactions for the facility.
The Truman VA employs 3 Patient Aligned Care Team (PACT) Clinical Pharmacy Specialists (CPS) practicing within primary care clinics. PACT is the patientcentered medical home model used by the VA. PACT CPS are ambulatory care pharmacists who assist providers in managing diseases using a scope of practice. Having a scope of practice would have allowed the PACT CPS to manage drug interactions with independent prescribing authority. However, due to the high volume of STOP report interactions and limited PACT CPS resources, the Pharmacy Service needed to develop an efficient, patient-centered method to resolve them. The intervention also needed to allow pharmacists, both with and without a scope of practice, to address the interactions.
Methods
The Truman VA Pharmacy Service developed protocols, approved by the Pharmacy and Therapeutics (P&T) Committee, to manage the specific gemfibrozil-statin and niacinstatin interactions chosen for the VISN 15 STOP report (Figures 1 and 2). The protocols were designed to identify patients who did not have a clear indication for gemfibrozil or niacin, were likely to maintain triglycerides (TGs) < 500 mg/dL without these medications, and would not likely require close monitoring after discontinuation.19 The protocols allowed pharmacists to autonomously discontinue gemfibrozil or niacin if patients did not have a history of pancreatitis, TGs ≥ 400 mg/dL or a nonlipid indication for niacin (eg, pellagra) after establishing care at Truman VA. Additionally, both interacting medications had to be dispensed by the VA. When pharmacists discontinued a medication, it was documented in a note in the patient electronic health record. The prescriber was notified through the note and the patient received a notification letter. Follow-up laboratory monitoring was not required as part of the protocol.
If patients met any of the exclusion criteria for discontinuation, the primary care provider (PCP) was notified to place a consult to the PACT Pharmacy Clinic for individualized interventions and close monitoring. Patients prescribed niacin for nonlipid indications were allowed to continue with their current drug regimen. At each encounter, the PACT CPS assessed for ADRs, made individualized medication changes, and arranged follow-up appointments. Once the interaction was resolved and treatment goals met, the PCP resumed monitoring of the patient’s lipid therapy.
Following all pharmacist interventions, a retrospective quality improvement analysis was conducted. The primary outcome was to evaluate the impact of discontinuing gemfibrozil and niacin by protocol on patients’ laboratory results. The coprimary endpoints were to describe the change in TG levels and the percentage of patients with TGs ≥ 500 mg/dL at least 5 weeks following the pharmacist-directed discontinuation by protocol. Secondary outcomes included the time required to resolve the interactions and a description of the PACT CPS pharmacologic interventions. Additionally, a quality assurance peer review was used to ensure the pharmacists appropriately utilized the protocols.
Data were collected from August 2016 to September 2017 for patients prescribed gemfibrozil and from May 2017 to January 2018 for patients prescribed niacin. The time spent resolving interactions was quantified based on encounter data. Descriptive statistics were used to analyze demographic information and the endpoints associated with each outcome. The project was reviewed by the University of Missouri Institutional Review Board, Truman VA privacy and information security officers, and was determined to meet guidelines for quality improvement.
Results
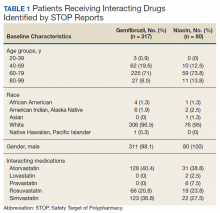
The original STOP report included 397 drug interactions involving statins with gemfibrozil or niacin (Table 1). The majority of patients were white and male aged 60 to 79 years. Gemfibrozil was the most common drug involved in all interactions (79.8%). The most common statins were atorvastatin (40%) and simvastatin (36.5%).
Gemfibrozil-Statin Interactions
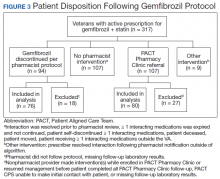
Pharmacists discontinued gemfibrozil by protocol for 94 patients (29.6%), and 107 patients (33.8%) were referred to the PACT Pharmacy Clinic (Figure 3). For the remaining 116 patients (36.6%), the drug interaction was addressed outside of the protocol for the following reasons: the drug interaction was resolved prior to pharmacist review; an interacting prescription was expired and not to be continued; the patient self-discontinued ≥ 1 interacting medications; the patient was deceased; the patient moved; the patient was receiving ≥ 1 interacting medications outside of the VA; or the prescriber resolved the interaction following notification by the pharmacist.
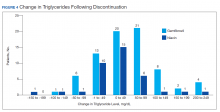
Ultimately, the interaction was resolved for all patients with a gemfibrozil-statin interaction on the STOP report. Following gemfibrozil discontinuation by protocol, 76 patients (80.9%) had TG laboratory results available and were included in the analysis. Sixty-two patients’ (82%) TG levels decreased or increased by < 100 mg/dL (Figure 4), and the TG levels of 1 patient (1.3%) increased above the threshold of 500 mg/dL. The mean (SD) time to the first laboratory result after the pharmacists mailed the notification letter was 6.5 (3.6) months (range, 1-17). The pharmacists spent a mean of 16 minutes per patient resolving each interaction.
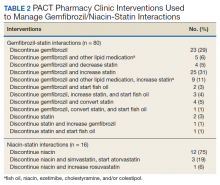
Of the 107 patients referred to the PACT Pharmacy Clinic, 80 (74.8%) had TG laboratory results available and were included in the analysis. These patients were followed by the PACT CPS until the drug interaction was resolved and confirmed to have TG levels at goal (< 500 mg/dL). Gemfibrozil doses ranged from 300 mg daily to 600 mg twice daily, with 70% (n = 56) of patients taking 600 mg twice daily. The PACT CPS made 148 interventions (Table 2). Twenty-three (29%) patients required only gemfibrozil discontinuation. The remaining 57 patients (71%) required at least 2 medication interventions. The PACT CPS generated 213 encounters for resolving drug interactions with a median of 2 encounters per patient.
Quality assurance review identified 5 patients (5.3%) who underwent gemfibrozil discontinuation by protocol, despite having criteria that would have recommended against discontinuation. In accordance with the protocol criteria, these patients were later referred to the PACT Pharmacy Clinic. None of these patients experienced a TG increase at or above the threshold of 500 mg/dL after gemfibrozil was initially discontinued but were excluded from the earlier analysis.
Niacin-Statin Interactions
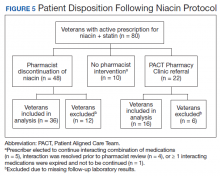
Pharmacists discontinued niacin by protocol for 48 patients (60.0%), and 22 patients (27.5%) were referred to the PACT Pharmacy Clinic (Figure 5). For the remaining 5 patients (6.3%), the interaction was either addressed outside the protocol prior to pharmacist review, or an interacting prescription was expired and not to be continued. Additionally, niacin was continued per prescriber preference in 5 patients (6.3%).
Thirty-six patients (75%) had TG laboratory results available following niacin discontinuation by protocol and were included in the analysis. Most patients’ (n = 33, 91.7%) TG levels decreased or increased by < 100 mg/dL. No patient had a TG level that increased higher than the threshold of 500 mg/dL. The mean (SD) time to the first laboratory result after the pharmacists mailed the notification letter, was 5.3 (2.5) months (range, 1.2-9.8). The pharmacists spent a mean of 15 minutes per patient resolving each interaction. The quality assurance review found no discrepancies in the pharmacists’ application of the protocol.
Of the 22 patients referred to the PACT Pharmacy Clinic, 16 (72.7%) patients had TG laboratory results available and were included in the analysis. As with the gemfibrozil interactions, these patients were followed by the PACT Pharmacy Clinic until the drug interaction was resolved and confirmed to have TGs at goal (< 500 mg/dL). Niacin doses ranged from 500 mg daily to 2,000 mg daily, with the majority of patients taking 1,000 mg daily. The PACT CPS made 23 interventions. The PACT CPS generated 46 encounters for resolving drug interactions with a median of 2 encounters per patient.
Discussion
Following gemfibrozil or niacin discontinuation by protocol, most patients with available laboratory results experienced either a decrease or modest TG elevation. The proportion of patients experiencing a decrease in TGs was unexpected but potentially multifactorial. Individual causes for the decrease in TGs were beyond the scope of this analysis. The retrospective design limited the ability to identify variables that could have impacted TG levels when gemfibrozil or niacin were started and discontinued. Although the treatment of TG levels is not indicated until it is ≥ 500 mg/dL, due to an increased risk of pancreatitis, both protocols excluded patients with a history of TGs ≥ 400 mg/dL.19 The lower threshold was set to compensate for anticipated increase in TG levels, following gemfibrozil or niacin discontinuation, and to minimize the number of patients with TG levels ≥ 500 mg/dL. The actual impact on patients’ TG levels supports the use of this lower threshold in the protocol.
When TG levels increased by 200 to 249 mg/dL after gemfibrozil or niacin discontinuation, patients were evaluated for possible underlying causes, which occurred for 4 gemfibrozil and 1 niacin patient. One patient started a β-blocker after gemfibrozil was initiated, and 3 patients were taking gemfibrozil prior to establishing care at the VA. The TG levels of the patient taking niacin correlated with an increased hemoglobin A1c. The TG level for only 1 patient taking gemfibrozil increased above the 500 mg/dL threshold. The patient had several comorbidities known to increase TG levels, but the comorbidities were previously well controlled. No additional medication changes were made at that time, and the TG levels on the next fasting lipid panel decreased to goal. The patient did not experience any negative clinical sequelae from the elevated TG levels.
Thirty-five patients (36%) who were referred to the PACT Pharmacy Clinic required only either gemfibrozil or niacin discontinuation. These patients were evaluated to identify whether adjustments to the protocols would have allowed for pharmacist discontinuation without referral to the PACT Pharmacy Clinic. Twenty-four of these patients (69%) had repeated TG levels ≥ 400 mg/dL prior to referral to the PACT Pharmacy Clinic. Additionally, there was no correlation between the gemfibrozil or niacin doses and the change in TG levels following discontinuation. These data indicate the protocols appropriately identified patients who did not have an indication for gemfibrozil or niacin.
In addition to drug interactions identified on the STOP report, the PACT CPS resolved 12 additional interactions involving simvastatin and gemfibrozil. Additionally, unnecessary lipid medications were deprescribed. The PACT CPS identified 13 patients who experienced myalgias, an ADR attributed to the gemfibrozil- statin interaction. Of those, 9 patients’ ADRs resolved after discontinuing gemfibrozil alone. For the remaining 4 patients, additional interventions to convert the patient to another statin were required to resolve the ADR.
Using pharmacists to address the drug interactions shifted workload from the prescribers and other primary care team members. The mean time spent to resolve both gemfibrozil and niacin interactions by protocol was 15.5 minutes. One hundred fortytwo patients (35.8%) had drug interactions resolved by protocol, saving the PACT CPS’ expertise for patients requiring individualized interventions. Drug interactions were resolved within 4 PACT CPS encounters for 93.8% of the patients taking gemfibrozil and within 3 PACT CPS encounters for 93.8% of the patients taking niacin.
The protocols allowed 12 additional pharmacists who did not have an ambulatory care scope of practice to assist the PACT CPS in mitigating the STOP drug interactions. These pharmacists otherwise would have been limited to making consultative recommendations. Simultaneously, the design allowed for the PACT pharmacists’ expertise to be allocated for patients most likely to require interventions beyond the protocols. This type of intraprofessional referral process is not well described in the medical literature. To the authors’ knowledge, the only studies described referrals from hospital pharmacists to community pharmacists during transitions of care on hospital discharge.20,21
Limitations
The results of this study are derived from a retrospective chart review at a single VA facility. The autonomous nature of PACT CPS interventions may be difficult to replicate in other settings that do not permit pharmacists the same prescriptive authority. This analysis was designed to demonstrate the impact of the pharmacist in resolving major drug interactions. Patients referred to the PACT Pharmacy Clinic who also had their lipid medications adjusted by a nonpharmacist provider were excluded. However, this may have minimized the impact of the PACT CPS on the patient care provided. As postintervention laboratory results were not available for all patients, some patients’ TG levels could have increased above the 500 mg/dL threshold but were not identified. The time investment was extensive and likely underestimates the true cost of implementing the interventions.
Because notification letters were used to instruct patients to stop gemfibrozil or niacin, several considerations need to be addressed when interpreting the follow-up laboratory results. First, we cannot confirm whether the patients received the letter or the exact date the letter was received. Additionally, we cannot confirm whether the patients followed the instructions to stop the interacting medications or the date the medications were stopped. It is possible some patients were still taking the interacting medication when the first laboratory was drawn. Should a patient have continued the interacting medication, most would have run out and been unable to obtain a refill within 90 days of receiving the letter, as this is the maximum amount dispensed at one time. The mean time to the first laboratory result for both gemfibrozil and niacin was 6.5 and 5.3 months, respectively. Approximately 85% of patients completed the first laboratory test at least 3 months after the letter was mailed.
The protocols were designed to assess whether gemfibrozil or niacin was indicated and did not assess whether the statin was indicated. Therefore, discontinuing the statin also could have resolved the interaction appropriately. However, due to characteristics of the patient population and recommendations in current lipid guidelines, it was more likely the statin would be indicated.22,23 The protocols also assumed that patients eligible for gemfibrozil or niacin discontinuation would not need additional changes to their lipid medications. The medication changes made by the PACT CPS may have gone beyond those minimally necessary to resolve the drug interaction and maintain TG goals. Patients who had gemfibrozil or niacin discontinued by protocol also may have benefited from additional optimization of their lipid medications.
Conclusions
This quality improvement analysis supports further evaluation of the complementary use of protocols and PACT CPS prescriptive authority to resolve statin drug interactions. The gemfibrozil and niacin protocols appropriately identified patients who were less likely to experience an adverse change in TG laboratory results. Patients more likely to require additional medication interventions were appropriately referred to the PACT Pharmacy Clinics for individualized care. These data support expanded roles for pharmacists, across various settings, to mitigate select drug interactions at the Truman VA.
Acknowledgments
This quality improvement project is the result of work supported with resources and use of the Harry S. Truman Memorial Veterans’ Hospital in Columbia, Missouri.
1. The top 200 drugs of 2020 Provided by the ClinCalc DrugStats Database. http://clincalc.com/DrugStats /Top200Drugs.aspx. Updated February 11, 2017. Accessed May 12, 2020.
2. Wiggins BS, Saseen JJ, Page RL 2nd, et al; American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology; Council on Hypertension; Council on Quality of Care and Outcomes Research; and Council on Functional Genomics and Translational Biology. Recommendations for management of clinically significant drug-drug interactions with statins and select agents used in patients with cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2016;134(21):e468‐e495. doi:10.1161/CIR.0000000000000456
3. Smithburger PL, Buckley MS, Bejian S, Burenheide K, Kane-Gill SL. A critical evaluation of clinical decision support for the detection of drug-drug interactions. Expert Opin Drug Saf. 2011;10(6):871‐882. doi:10.1517/14740338.2011.583916
4. US Food and Drug Administration. FDA drug safety communication: new restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury. https://www.fda.gov/Drugs/DrugSafety /ucm256581.htm. Updated December 15, 2017. Accessed May 12, 2020.
5. US Food and Drug Administration. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. https://www.fda.gov /Drugs/DrugSafety/ucm293101.htm. Updated January 19, 2016. Accessed May 12, 2020.
6. US Food and Drug Administration Federal Register. AbbVie Inc. et al; withdrawal of approval of indications related to the coadministration with statins in applications for niacin extended-release tablets and fenofibric acid delayed-release capsules. https://www.federalregister .gov/documents/2016/04/18/2016-08887/abbvie-inc -et-al-withdrawal-of-approval-of-indications-related -to-the-coadministration-with-statins. Published April 18, 2016. Accessed May 12, 2020.
7. Lamprecht DG Jr, Todd BA, Denham AM, Ruppe LK, Stadler SL. Clinical pharmacist patient-safety initiative to reduce against-label prescribing of statins with cyclosporine. Ann Pharmacother. 2017;51(2):140‐145. doi:10.1177/1060028016675352
8. Roblek T, Deticek A, Leskovar B, et al. Clinical-pharmacist intervention reduces clinically relevant drugdrug interactions in patients with heart failure: A randomized, double-blind, controlled trial. Int J Cardiol. 2016;203:647‐652. doi:10.1016/j.ijcard.2015.10.206
9. Tuchscherer RM, Nair K, Ghushchyan V, Saseen JJ. Simvastatin prescribing patterns before and after FDA dosing restrictions: a retrospective analysis of a large healthcare claims database. Am J Cardiovasc Drugs. 2015;15(1):27‐34. doi:10.1007/s40256-014-0096-x
10. Alford JC, Saseen JJ, Allen RR, Nair KV. Persistent use of against-label statin-fibrate combinations from 2003-2009 despite United States Food and Drug Administration dose restrictions. Pharmacotherapy. 2012;32(7):623‐630. doi:10.1002/j.1875-9114.2011.01090.x
11. Leape LL, Cullen DJ, Clapp MD, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit [published correction appears in JAMA 2000 Mar 8;283(10):1293]. JAMA. 1999;282(3):267‐270. doi:10.1001/jama.282.3.267
12. Kucukarslan SN, Peters M, Mlynarek M, Nafziger DA. Pharmacists on rounding teams reduce preventable adverse drug events in hospital general medicine units. Arch Intern Med. 2003;163(17):2014‐2018. doi:10.1001/archinte.163.17.2014
13. Humphries TL, Carroll N, Chester EA, Magid D, Rocho B. Evaluation of an electronic critical drug interaction program coupled with active pharmacist intervention. Ann Pharmacother. 2007;41(12):1979‐1985. doi:10.1345/aph.1K349
14. Zocor [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2018.
15. Lipitor [package insert]. New York, NY: Pfizer; 2017.
16. Crestor [package insert]. Wilmington, DE: AstraZeneca; 2018.
17. Mevacor [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2012.
18. Wolters Kluwer Health, Lexi-Drugs, Lexicomp. Pravastatin. www.online.lexi.com. [Source not verified.]
19. Miller M, Stone NJ, Ballantyne C, et al; American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292-2333. doi: 10.1161/CIR.0b013e3182160726
20. Ferguson J, Seston L, Ashcroft DM. Refer-to-pharmacy: a qualitative study exploring the implementation of an electronic transfer of care initiative to improve medicines optimisation following hospital discharge. BMC Health Serv Res. 2018;18(1):424. doi:10.1186/s12913-018-3262-z
21. Ensing HT, Koster ES, Dubero DJ, van Dooren AA, Bouvy ML. Collaboration between hospital and community pharmacists to address drug-related problems: the HomeCoMe-program. Res Social Adm Pharm. 2019;15(3):267‐278. doi:10.1016/j.sapharm.2018.05.001
22. US Department of Defense, US Department of Veterans Affairs. VA/DoD clinical practice guideline for the management of dyslipidemia for cardiovascular risk reduction guideline summary. https://www.healthquality.va.gov /guidelines/CD/lipids/LipidSumOptSinglePg31Aug15.pdf. Published 2014. Accessed May 14, 2020.
23. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014 Jun 24;129(25) (suppl 2):S46-48] [published correction appears in Circulation. 2015 Dec 22;132(25):e396]. Circulation. 2014;129(25)(suppl 2): S1‐S45. doi:10.1161/01.cir.0000437738.63853.7a
Statins are one of the most common medications dispensed in the US and are associated with clinically significant drug interactions.1,2 The most common adverse drug reaction (ADR) of statin drug interactions is muscle-related toxicities.2 Despite technology advances to alert clinicians to drug interactions, updated statin manufacturer labeling, and guideline recommendations, inappropriate prescribing and dispensing of statin drug interactions continues to occur in health care systems.2-10
The medical literature has demonstrated many opportunities for pharmacists to prevent and mitigate drug interactions. At the points of prescribing and dispensing, pharmacists can reduce the number of potential drug interactions for the patient.11-13 Pharmacists also have identified and resolved drug interactions through quality assurance review after dispensing to a patient.7,8
Regardless of the time point of an intervention, the most common method pharmacists used to resolve drug interactions was through recommendations to a prescriber. The recommendations were generated through academic detailing, clinical decision support algorithms, drug conversions, or the pharmacist’s expertise. Regardless of the method the pharmacist used, the prescriber had the final authority to accept or decline the recommendation.7,8,11-13 Although these interventions were effective, pharmacists could further streamline the process by autonomously resolving drug interactions. However, these types of interventions are not well described in the medical literature.
Background
The US Department of Veterans Affairs (VA) Veterans Integrated Service Network (VISN), established the Safety Target of Polypharmacy (STOP) report in 2015. At each facility in the network, the report identified patients who were dispensed medications known to have drug interactions. The interactions were chosen by the VISN, and the severity of the interactions was based on coding parameters within the VA computerized order entry system, which uses a severity score based on First Databank data. At the Harry S. Truman Memorial Veterans’ Hospital (Truman VA) in Columbia, Missouri, > 500 drug interactions were initially active on the STOP report. The most common drug interactions were statins with gemfibrozil and statins with niacin.14-18 The Truman VA Pharmacy Service was charged with resolving the interactions for the facility.
The Truman VA employs 3 Patient Aligned Care Team (PACT) Clinical Pharmacy Specialists (CPS) practicing within primary care clinics. PACT is the patientcentered medical home model used by the VA. PACT CPS are ambulatory care pharmacists who assist providers in managing diseases using a scope of practice. Having a scope of practice would have allowed the PACT CPS to manage drug interactions with independent prescribing authority. However, due to the high volume of STOP report interactions and limited PACT CPS resources, the Pharmacy Service needed to develop an efficient, patient-centered method to resolve them. The intervention also needed to allow pharmacists, both with and without a scope of practice, to address the interactions.
Methods
The Truman VA Pharmacy Service developed protocols, approved by the Pharmacy and Therapeutics (P&T) Committee, to manage the specific gemfibrozil-statin and niacinstatin interactions chosen for the VISN 15 STOP report (Figures 1 and 2). The protocols were designed to identify patients who did not have a clear indication for gemfibrozil or niacin, were likely to maintain triglycerides (TGs) < 500 mg/dL without these medications, and would not likely require close monitoring after discontinuation.19 The protocols allowed pharmacists to autonomously discontinue gemfibrozil or niacin if patients did not have a history of pancreatitis, TGs ≥ 400 mg/dL or a nonlipid indication for niacin (eg, pellagra) after establishing care at Truman VA. Additionally, both interacting medications had to be dispensed by the VA. When pharmacists discontinued a medication, it was documented in a note in the patient electronic health record. The prescriber was notified through the note and the patient received a notification letter. Follow-up laboratory monitoring was not required as part of the protocol.
If patients met any of the exclusion criteria for discontinuation, the primary care provider (PCP) was notified to place a consult to the PACT Pharmacy Clinic for individualized interventions and close monitoring. Patients prescribed niacin for nonlipid indications were allowed to continue with their current drug regimen. At each encounter, the PACT CPS assessed for ADRs, made individualized medication changes, and arranged follow-up appointments. Once the interaction was resolved and treatment goals met, the PCP resumed monitoring of the patient’s lipid therapy.
Following all pharmacist interventions, a retrospective quality improvement analysis was conducted. The primary outcome was to evaluate the impact of discontinuing gemfibrozil and niacin by protocol on patients’ laboratory results. The coprimary endpoints were to describe the change in TG levels and the percentage of patients with TGs ≥ 500 mg/dL at least 5 weeks following the pharmacist-directed discontinuation by protocol. Secondary outcomes included the time required to resolve the interactions and a description of the PACT CPS pharmacologic interventions. Additionally, a quality assurance peer review was used to ensure the pharmacists appropriately utilized the protocols.
Data were collected from August 2016 to September 2017 for patients prescribed gemfibrozil and from May 2017 to January 2018 for patients prescribed niacin. The time spent resolving interactions was quantified based on encounter data. Descriptive statistics were used to analyze demographic information and the endpoints associated with each outcome. The project was reviewed by the University of Missouri Institutional Review Board, Truman VA privacy and information security officers, and was determined to meet guidelines for quality improvement.
Results
The original STOP report included 397 drug interactions involving statins with gemfibrozil or niacin (Table 1). The majority of patients were white and male aged 60 to 79 years. Gemfibrozil was the most common drug involved in all interactions (79.8%). The most common statins were atorvastatin (40%) and simvastatin (36.5%).
Gemfibrozil-Statin Interactions
Pharmacists discontinued gemfibrozil by protocol for 94 patients (29.6%), and 107 patients (33.8%) were referred to the PACT Pharmacy Clinic (Figure 3). For the remaining 116 patients (36.6%), the drug interaction was addressed outside of the protocol for the following reasons: the drug interaction was resolved prior to pharmacist review; an interacting prescription was expired and not to be continued; the patient self-discontinued ≥ 1 interacting medications; the patient was deceased; the patient moved; the patient was receiving ≥ 1 interacting medications outside of the VA; or the prescriber resolved the interaction following notification by the pharmacist.
Ultimately, the interaction was resolved for all patients with a gemfibrozil-statin interaction on the STOP report. Following gemfibrozil discontinuation by protocol, 76 patients (80.9%) had TG laboratory results available and were included in the analysis. Sixty-two patients’ (82%) TG levels decreased or increased by < 100 mg/dL (Figure 4), and the TG levels of 1 patient (1.3%) increased above the threshold of 500 mg/dL. The mean (SD) time to the first laboratory result after the pharmacists mailed the notification letter was 6.5 (3.6) months (range, 1-17). The pharmacists spent a mean of 16 minutes per patient resolving each interaction.
Of the 107 patients referred to the PACT Pharmacy Clinic, 80 (74.8%) had TG laboratory results available and were included in the analysis. These patients were followed by the PACT CPS until the drug interaction was resolved and confirmed to have TG levels at goal (< 500 mg/dL). Gemfibrozil doses ranged from 300 mg daily to 600 mg twice daily, with 70% (n = 56) of patients taking 600 mg twice daily. The PACT CPS made 148 interventions (Table 2). Twenty-three (29%) patients required only gemfibrozil discontinuation. The remaining 57 patients (71%) required at least 2 medication interventions. The PACT CPS generated 213 encounters for resolving drug interactions with a median of 2 encounters per patient.
Quality assurance review identified 5 patients (5.3%) who underwent gemfibrozil discontinuation by protocol, despite having criteria that would have recommended against discontinuation. In accordance with the protocol criteria, these patients were later referred to the PACT Pharmacy Clinic. None of these patients experienced a TG increase at or above the threshold of 500 mg/dL after gemfibrozil was initially discontinued but were excluded from the earlier analysis.
Niacin-Statin Interactions
Pharmacists discontinued niacin by protocol for 48 patients (60.0%), and 22 patients (27.5%) were referred to the PACT Pharmacy Clinic (Figure 5). For the remaining 5 patients (6.3%), the interaction was either addressed outside the protocol prior to pharmacist review, or an interacting prescription was expired and not to be continued. Additionally, niacin was continued per prescriber preference in 5 patients (6.3%).
Thirty-six patients (75%) had TG laboratory results available following niacin discontinuation by protocol and were included in the analysis. Most patients’ (n = 33, 91.7%) TG levels decreased or increased by < 100 mg/dL. No patient had a TG level that increased higher than the threshold of 500 mg/dL. The mean (SD) time to the first laboratory result after the pharmacists mailed the notification letter, was 5.3 (2.5) months (range, 1.2-9.8). The pharmacists spent a mean of 15 minutes per patient resolving each interaction. The quality assurance review found no discrepancies in the pharmacists’ application of the protocol.
Of the 22 patients referred to the PACT Pharmacy Clinic, 16 (72.7%) patients had TG laboratory results available and were included in the analysis. As with the gemfibrozil interactions, these patients were followed by the PACT Pharmacy Clinic until the drug interaction was resolved and confirmed to have TGs at goal (< 500 mg/dL). Niacin doses ranged from 500 mg daily to 2,000 mg daily, with the majority of patients taking 1,000 mg daily. The PACT CPS made 23 interventions. The PACT CPS generated 46 encounters for resolving drug interactions with a median of 2 encounters per patient.
Discussion
Following gemfibrozil or niacin discontinuation by protocol, most patients with available laboratory results experienced either a decrease or modest TG elevation. The proportion of patients experiencing a decrease in TGs was unexpected but potentially multifactorial. Individual causes for the decrease in TGs were beyond the scope of this analysis. The retrospective design limited the ability to identify variables that could have impacted TG levels when gemfibrozil or niacin were started and discontinued. Although the treatment of TG levels is not indicated until it is ≥ 500 mg/dL, due to an increased risk of pancreatitis, both protocols excluded patients with a history of TGs ≥ 400 mg/dL.19 The lower threshold was set to compensate for anticipated increase in TG levels, following gemfibrozil or niacin discontinuation, and to minimize the number of patients with TG levels ≥ 500 mg/dL. The actual impact on patients’ TG levels supports the use of this lower threshold in the protocol.
When TG levels increased by 200 to 249 mg/dL after gemfibrozil or niacin discontinuation, patients were evaluated for possible underlying causes, which occurred for 4 gemfibrozil and 1 niacin patient. One patient started a β-blocker after gemfibrozil was initiated, and 3 patients were taking gemfibrozil prior to establishing care at the VA. The TG levels of the patient taking niacin correlated with an increased hemoglobin A1c. The TG level for only 1 patient taking gemfibrozil increased above the 500 mg/dL threshold. The patient had several comorbidities known to increase TG levels, but the comorbidities were previously well controlled. No additional medication changes were made at that time, and the TG levels on the next fasting lipid panel decreased to goal. The patient did not experience any negative clinical sequelae from the elevated TG levels.
Thirty-five patients (36%) who were referred to the PACT Pharmacy Clinic required only either gemfibrozil or niacin discontinuation. These patients were evaluated to identify whether adjustments to the protocols would have allowed for pharmacist discontinuation without referral to the PACT Pharmacy Clinic. Twenty-four of these patients (69%) had repeated TG levels ≥ 400 mg/dL prior to referral to the PACT Pharmacy Clinic. Additionally, there was no correlation between the gemfibrozil or niacin doses and the change in TG levels following discontinuation. These data indicate the protocols appropriately identified patients who did not have an indication for gemfibrozil or niacin.
In addition to drug interactions identified on the STOP report, the PACT CPS resolved 12 additional interactions involving simvastatin and gemfibrozil. Additionally, unnecessary lipid medications were deprescribed. The PACT CPS identified 13 patients who experienced myalgias, an ADR attributed to the gemfibrozil- statin interaction. Of those, 9 patients’ ADRs resolved after discontinuing gemfibrozil alone. For the remaining 4 patients, additional interventions to convert the patient to another statin were required to resolve the ADR.
Using pharmacists to address the drug interactions shifted workload from the prescribers and other primary care team members. The mean time spent to resolve both gemfibrozil and niacin interactions by protocol was 15.5 minutes. One hundred fortytwo patients (35.8%) had drug interactions resolved by protocol, saving the PACT CPS’ expertise for patients requiring individualized interventions. Drug interactions were resolved within 4 PACT CPS encounters for 93.8% of the patients taking gemfibrozil and within 3 PACT CPS encounters for 93.8% of the patients taking niacin.
The protocols allowed 12 additional pharmacists who did not have an ambulatory care scope of practice to assist the PACT CPS in mitigating the STOP drug interactions. These pharmacists otherwise would have been limited to making consultative recommendations. Simultaneously, the design allowed for the PACT pharmacists’ expertise to be allocated for patients most likely to require interventions beyond the protocols. This type of intraprofessional referral process is not well described in the medical literature. To the authors’ knowledge, the only studies described referrals from hospital pharmacists to community pharmacists during transitions of care on hospital discharge.20,21
Limitations
The results of this study are derived from a retrospective chart review at a single VA facility. The autonomous nature of PACT CPS interventions may be difficult to replicate in other settings that do not permit pharmacists the same prescriptive authority. This analysis was designed to demonstrate the impact of the pharmacist in resolving major drug interactions. Patients referred to the PACT Pharmacy Clinic who also had their lipid medications adjusted by a nonpharmacist provider were excluded. However, this may have minimized the impact of the PACT CPS on the patient care provided. As postintervention laboratory results were not available for all patients, some patients’ TG levels could have increased above the 500 mg/dL threshold but were not identified. The time investment was extensive and likely underestimates the true cost of implementing the interventions.
Because notification letters were used to instruct patients to stop gemfibrozil or niacin, several considerations need to be addressed when interpreting the follow-up laboratory results. First, we cannot confirm whether the patients received the letter or the exact date the letter was received. Additionally, we cannot confirm whether the patients followed the instructions to stop the interacting medications or the date the medications were stopped. It is possible some patients were still taking the interacting medication when the first laboratory was drawn. Should a patient have continued the interacting medication, most would have run out and been unable to obtain a refill within 90 days of receiving the letter, as this is the maximum amount dispensed at one time. The mean time to the first laboratory result for both gemfibrozil and niacin was 6.5 and 5.3 months, respectively. Approximately 85% of patients completed the first laboratory test at least 3 months after the letter was mailed.
The protocols were designed to assess whether gemfibrozil or niacin was indicated and did not assess whether the statin was indicated. Therefore, discontinuing the statin also could have resolved the interaction appropriately. However, due to characteristics of the patient population and recommendations in current lipid guidelines, it was more likely the statin would be indicated.22,23 The protocols also assumed that patients eligible for gemfibrozil or niacin discontinuation would not need additional changes to their lipid medications. The medication changes made by the PACT CPS may have gone beyond those minimally necessary to resolve the drug interaction and maintain TG goals. Patients who had gemfibrozil or niacin discontinued by protocol also may have benefited from additional optimization of their lipid medications.
Conclusions
This quality improvement analysis supports further evaluation of the complementary use of protocols and PACT CPS prescriptive authority to resolve statin drug interactions. The gemfibrozil and niacin protocols appropriately identified patients who were less likely to experience an adverse change in TG laboratory results. Patients more likely to require additional medication interventions were appropriately referred to the PACT Pharmacy Clinics for individualized care. These data support expanded roles for pharmacists, across various settings, to mitigate select drug interactions at the Truman VA.
Acknowledgments
This quality improvement project is the result of work supported with resources and use of the Harry S. Truman Memorial Veterans’ Hospital in Columbia, Missouri.
Statins are one of the most common medications dispensed in the US and are associated with clinically significant drug interactions.1,2 The most common adverse drug reaction (ADR) of statin drug interactions is muscle-related toxicities.2 Despite technology advances to alert clinicians to drug interactions, updated statin manufacturer labeling, and guideline recommendations, inappropriate prescribing and dispensing of statin drug interactions continues to occur in health care systems.2-10
The medical literature has demonstrated many opportunities for pharmacists to prevent and mitigate drug interactions. At the points of prescribing and dispensing, pharmacists can reduce the number of potential drug interactions for the patient.11-13 Pharmacists also have identified and resolved drug interactions through quality assurance review after dispensing to a patient.7,8
Regardless of the time point of an intervention, the most common method pharmacists used to resolve drug interactions was through recommendations to a prescriber. The recommendations were generated through academic detailing, clinical decision support algorithms, drug conversions, or the pharmacist’s expertise. Regardless of the method the pharmacist used, the prescriber had the final authority to accept or decline the recommendation.7,8,11-13 Although these interventions were effective, pharmacists could further streamline the process by autonomously resolving drug interactions. However, these types of interventions are not well described in the medical literature.
Background
The US Department of Veterans Affairs (VA) Veterans Integrated Service Network (VISN), established the Safety Target of Polypharmacy (STOP) report in 2015. At each facility in the network, the report identified patients who were dispensed medications known to have drug interactions. The interactions were chosen by the VISN, and the severity of the interactions was based on coding parameters within the VA computerized order entry system, which uses a severity score based on First Databank data. At the Harry S. Truman Memorial Veterans’ Hospital (Truman VA) in Columbia, Missouri, > 500 drug interactions were initially active on the STOP report. The most common drug interactions were statins with gemfibrozil and statins with niacin.14-18 The Truman VA Pharmacy Service was charged with resolving the interactions for the facility.
The Truman VA employs 3 Patient Aligned Care Team (PACT) Clinical Pharmacy Specialists (CPS) practicing within primary care clinics. PACT is the patientcentered medical home model used by the VA. PACT CPS are ambulatory care pharmacists who assist providers in managing diseases using a scope of practice. Having a scope of practice would have allowed the PACT CPS to manage drug interactions with independent prescribing authority. However, due to the high volume of STOP report interactions and limited PACT CPS resources, the Pharmacy Service needed to develop an efficient, patient-centered method to resolve them. The intervention also needed to allow pharmacists, both with and without a scope of practice, to address the interactions.
Methods
The Truman VA Pharmacy Service developed protocols, approved by the Pharmacy and Therapeutics (P&T) Committee, to manage the specific gemfibrozil-statin and niacinstatin interactions chosen for the VISN 15 STOP report (Figures 1 and 2). The protocols were designed to identify patients who did not have a clear indication for gemfibrozil or niacin, were likely to maintain triglycerides (TGs) < 500 mg/dL without these medications, and would not likely require close monitoring after discontinuation.19 The protocols allowed pharmacists to autonomously discontinue gemfibrozil or niacin if patients did not have a history of pancreatitis, TGs ≥ 400 mg/dL or a nonlipid indication for niacin (eg, pellagra) after establishing care at Truman VA. Additionally, both interacting medications had to be dispensed by the VA. When pharmacists discontinued a medication, it was documented in a note in the patient electronic health record. The prescriber was notified through the note and the patient received a notification letter. Follow-up laboratory monitoring was not required as part of the protocol.
If patients met any of the exclusion criteria for discontinuation, the primary care provider (PCP) was notified to place a consult to the PACT Pharmacy Clinic for individualized interventions and close monitoring. Patients prescribed niacin for nonlipid indications were allowed to continue with their current drug regimen. At each encounter, the PACT CPS assessed for ADRs, made individualized medication changes, and arranged follow-up appointments. Once the interaction was resolved and treatment goals met, the PCP resumed monitoring of the patient’s lipid therapy.
Following all pharmacist interventions, a retrospective quality improvement analysis was conducted. The primary outcome was to evaluate the impact of discontinuing gemfibrozil and niacin by protocol on patients’ laboratory results. The coprimary endpoints were to describe the change in TG levels and the percentage of patients with TGs ≥ 500 mg/dL at least 5 weeks following the pharmacist-directed discontinuation by protocol. Secondary outcomes included the time required to resolve the interactions and a description of the PACT CPS pharmacologic interventions. Additionally, a quality assurance peer review was used to ensure the pharmacists appropriately utilized the protocols.
Data were collected from August 2016 to September 2017 for patients prescribed gemfibrozil and from May 2017 to January 2018 for patients prescribed niacin. The time spent resolving interactions was quantified based on encounter data. Descriptive statistics were used to analyze demographic information and the endpoints associated with each outcome. The project was reviewed by the University of Missouri Institutional Review Board, Truman VA privacy and information security officers, and was determined to meet guidelines for quality improvement.
Results
The original STOP report included 397 drug interactions involving statins with gemfibrozil or niacin (Table 1). The majority of patients were white and male aged 60 to 79 years. Gemfibrozil was the most common drug involved in all interactions (79.8%). The most common statins were atorvastatin (40%) and simvastatin (36.5%).
Gemfibrozil-Statin Interactions
Pharmacists discontinued gemfibrozil by protocol for 94 patients (29.6%), and 107 patients (33.8%) were referred to the PACT Pharmacy Clinic (Figure 3). For the remaining 116 patients (36.6%), the drug interaction was addressed outside of the protocol for the following reasons: the drug interaction was resolved prior to pharmacist review; an interacting prescription was expired and not to be continued; the patient self-discontinued ≥ 1 interacting medications; the patient was deceased; the patient moved; the patient was receiving ≥ 1 interacting medications outside of the VA; or the prescriber resolved the interaction following notification by the pharmacist.
Ultimately, the interaction was resolved for all patients with a gemfibrozil-statin interaction on the STOP report. Following gemfibrozil discontinuation by protocol, 76 patients (80.9%) had TG laboratory results available and were included in the analysis. Sixty-two patients’ (82%) TG levels decreased or increased by < 100 mg/dL (Figure 4), and the TG levels of 1 patient (1.3%) increased above the threshold of 500 mg/dL. The mean (SD) time to the first laboratory result after the pharmacists mailed the notification letter was 6.5 (3.6) months (range, 1-17). The pharmacists spent a mean of 16 minutes per patient resolving each interaction.
Of the 107 patients referred to the PACT Pharmacy Clinic, 80 (74.8%) had TG laboratory results available and were included in the analysis. These patients were followed by the PACT CPS until the drug interaction was resolved and confirmed to have TG levels at goal (< 500 mg/dL). Gemfibrozil doses ranged from 300 mg daily to 600 mg twice daily, with 70% (n = 56) of patients taking 600 mg twice daily. The PACT CPS made 148 interventions (Table 2). Twenty-three (29%) patients required only gemfibrozil discontinuation. The remaining 57 patients (71%) required at least 2 medication interventions. The PACT CPS generated 213 encounters for resolving drug interactions with a median of 2 encounters per patient.
Quality assurance review identified 5 patients (5.3%) who underwent gemfibrozil discontinuation by protocol, despite having criteria that would have recommended against discontinuation. In accordance with the protocol criteria, these patients were later referred to the PACT Pharmacy Clinic. None of these patients experienced a TG increase at or above the threshold of 500 mg/dL after gemfibrozil was initially discontinued but were excluded from the earlier analysis.
Niacin-Statin Interactions
Pharmacists discontinued niacin by protocol for 48 patients (60.0%), and 22 patients (27.5%) were referred to the PACT Pharmacy Clinic (Figure 5). For the remaining 5 patients (6.3%), the interaction was either addressed outside the protocol prior to pharmacist review, or an interacting prescription was expired and not to be continued. Additionally, niacin was continued per prescriber preference in 5 patients (6.3%).
Thirty-six patients (75%) had TG laboratory results available following niacin discontinuation by protocol and were included in the analysis. Most patients’ (n = 33, 91.7%) TG levels decreased or increased by < 100 mg/dL. No patient had a TG level that increased higher than the threshold of 500 mg/dL. The mean (SD) time to the first laboratory result after the pharmacists mailed the notification letter, was 5.3 (2.5) months (range, 1.2-9.8). The pharmacists spent a mean of 15 minutes per patient resolving each interaction. The quality assurance review found no discrepancies in the pharmacists’ application of the protocol.
Of the 22 patients referred to the PACT Pharmacy Clinic, 16 (72.7%) patients had TG laboratory results available and were included in the analysis. As with the gemfibrozil interactions, these patients were followed by the PACT Pharmacy Clinic until the drug interaction was resolved and confirmed to have TGs at goal (< 500 mg/dL). Niacin doses ranged from 500 mg daily to 2,000 mg daily, with the majority of patients taking 1,000 mg daily. The PACT CPS made 23 interventions. The PACT CPS generated 46 encounters for resolving drug interactions with a median of 2 encounters per patient.
Discussion
Following gemfibrozil or niacin discontinuation by protocol, most patients with available laboratory results experienced either a decrease or modest TG elevation. The proportion of patients experiencing a decrease in TGs was unexpected but potentially multifactorial. Individual causes for the decrease in TGs were beyond the scope of this analysis. The retrospective design limited the ability to identify variables that could have impacted TG levels when gemfibrozil or niacin were started and discontinued. Although the treatment of TG levels is not indicated until it is ≥ 500 mg/dL, due to an increased risk of pancreatitis, both protocols excluded patients with a history of TGs ≥ 400 mg/dL.19 The lower threshold was set to compensate for anticipated increase in TG levels, following gemfibrozil or niacin discontinuation, and to minimize the number of patients with TG levels ≥ 500 mg/dL. The actual impact on patients’ TG levels supports the use of this lower threshold in the protocol.
When TG levels increased by 200 to 249 mg/dL after gemfibrozil or niacin discontinuation, patients were evaluated for possible underlying causes, which occurred for 4 gemfibrozil and 1 niacin patient. One patient started a β-blocker after gemfibrozil was initiated, and 3 patients were taking gemfibrozil prior to establishing care at the VA. The TG levels of the patient taking niacin correlated with an increased hemoglobin A1c. The TG level for only 1 patient taking gemfibrozil increased above the 500 mg/dL threshold. The patient had several comorbidities known to increase TG levels, but the comorbidities were previously well controlled. No additional medication changes were made at that time, and the TG levels on the next fasting lipid panel decreased to goal. The patient did not experience any negative clinical sequelae from the elevated TG levels.
Thirty-five patients (36%) who were referred to the PACT Pharmacy Clinic required only either gemfibrozil or niacin discontinuation. These patients were evaluated to identify whether adjustments to the protocols would have allowed for pharmacist discontinuation without referral to the PACT Pharmacy Clinic. Twenty-four of these patients (69%) had repeated TG levels ≥ 400 mg/dL prior to referral to the PACT Pharmacy Clinic. Additionally, there was no correlation between the gemfibrozil or niacin doses and the change in TG levels following discontinuation. These data indicate the protocols appropriately identified patients who did not have an indication for gemfibrozil or niacin.
In addition to drug interactions identified on the STOP report, the PACT CPS resolved 12 additional interactions involving simvastatin and gemfibrozil. Additionally, unnecessary lipid medications were deprescribed. The PACT CPS identified 13 patients who experienced myalgias, an ADR attributed to the gemfibrozil- statin interaction. Of those, 9 patients’ ADRs resolved after discontinuing gemfibrozil alone. For the remaining 4 patients, additional interventions to convert the patient to another statin were required to resolve the ADR.
Using pharmacists to address the drug interactions shifted workload from the prescribers and other primary care team members. The mean time spent to resolve both gemfibrozil and niacin interactions by protocol was 15.5 minutes. One hundred fortytwo patients (35.8%) had drug interactions resolved by protocol, saving the PACT CPS’ expertise for patients requiring individualized interventions. Drug interactions were resolved within 4 PACT CPS encounters for 93.8% of the patients taking gemfibrozil and within 3 PACT CPS encounters for 93.8% of the patients taking niacin.
The protocols allowed 12 additional pharmacists who did not have an ambulatory care scope of practice to assist the PACT CPS in mitigating the STOP drug interactions. These pharmacists otherwise would have been limited to making consultative recommendations. Simultaneously, the design allowed for the PACT pharmacists’ expertise to be allocated for patients most likely to require interventions beyond the protocols. This type of intraprofessional referral process is not well described in the medical literature. To the authors’ knowledge, the only studies described referrals from hospital pharmacists to community pharmacists during transitions of care on hospital discharge.20,21
Limitations
The results of this study are derived from a retrospective chart review at a single VA facility. The autonomous nature of PACT CPS interventions may be difficult to replicate in other settings that do not permit pharmacists the same prescriptive authority. This analysis was designed to demonstrate the impact of the pharmacist in resolving major drug interactions. Patients referred to the PACT Pharmacy Clinic who also had their lipid medications adjusted by a nonpharmacist provider were excluded. However, this may have minimized the impact of the PACT CPS on the patient care provided. As postintervention laboratory results were not available for all patients, some patients’ TG levels could have increased above the 500 mg/dL threshold but were not identified. The time investment was extensive and likely underestimates the true cost of implementing the interventions.
Because notification letters were used to instruct patients to stop gemfibrozil or niacin, several considerations need to be addressed when interpreting the follow-up laboratory results. First, we cannot confirm whether the patients received the letter or the exact date the letter was received. Additionally, we cannot confirm whether the patients followed the instructions to stop the interacting medications or the date the medications were stopped. It is possible some patients were still taking the interacting medication when the first laboratory was drawn. Should a patient have continued the interacting medication, most would have run out and been unable to obtain a refill within 90 days of receiving the letter, as this is the maximum amount dispensed at one time. The mean time to the first laboratory result for both gemfibrozil and niacin was 6.5 and 5.3 months, respectively. Approximately 85% of patients completed the first laboratory test at least 3 months after the letter was mailed.
The protocols were designed to assess whether gemfibrozil or niacin was indicated and did not assess whether the statin was indicated. Therefore, discontinuing the statin also could have resolved the interaction appropriately. However, due to characteristics of the patient population and recommendations in current lipid guidelines, it was more likely the statin would be indicated.22,23 The protocols also assumed that patients eligible for gemfibrozil or niacin discontinuation would not need additional changes to their lipid medications. The medication changes made by the PACT CPS may have gone beyond those minimally necessary to resolve the drug interaction and maintain TG goals. Patients who had gemfibrozil or niacin discontinued by protocol also may have benefited from additional optimization of their lipid medications.
Conclusions
This quality improvement analysis supports further evaluation of the complementary use of protocols and PACT CPS prescriptive authority to resolve statin drug interactions. The gemfibrozil and niacin protocols appropriately identified patients who were less likely to experience an adverse change in TG laboratory results. Patients more likely to require additional medication interventions were appropriately referred to the PACT Pharmacy Clinics for individualized care. These data support expanded roles for pharmacists, across various settings, to mitigate select drug interactions at the Truman VA.
Acknowledgments
This quality improvement project is the result of work supported with resources and use of the Harry S. Truman Memorial Veterans’ Hospital in Columbia, Missouri.
1. The top 200 drugs of 2020 Provided by the ClinCalc DrugStats Database. http://clincalc.com/DrugStats /Top200Drugs.aspx. Updated February 11, 2017. Accessed May 12, 2020.
2. Wiggins BS, Saseen JJ, Page RL 2nd, et al; American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology; Council on Hypertension; Council on Quality of Care and Outcomes Research; and Council on Functional Genomics and Translational Biology. Recommendations for management of clinically significant drug-drug interactions with statins and select agents used in patients with cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2016;134(21):e468‐e495. doi:10.1161/CIR.0000000000000456
3. Smithburger PL, Buckley MS, Bejian S, Burenheide K, Kane-Gill SL. A critical evaluation of clinical decision support for the detection of drug-drug interactions. Expert Opin Drug Saf. 2011;10(6):871‐882. doi:10.1517/14740338.2011.583916
4. US Food and Drug Administration. FDA drug safety communication: new restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury. https://www.fda.gov/Drugs/DrugSafety /ucm256581.htm. Updated December 15, 2017. Accessed May 12, 2020.
5. US Food and Drug Administration. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. https://www.fda.gov /Drugs/DrugSafety/ucm293101.htm. Updated January 19, 2016. Accessed May 12, 2020.
6. US Food and Drug Administration Federal Register. AbbVie Inc. et al; withdrawal of approval of indications related to the coadministration with statins in applications for niacin extended-release tablets and fenofibric acid delayed-release capsules. https://www.federalregister .gov/documents/2016/04/18/2016-08887/abbvie-inc -et-al-withdrawal-of-approval-of-indications-related -to-the-coadministration-with-statins. Published April 18, 2016. Accessed May 12, 2020.
7. Lamprecht DG Jr, Todd BA, Denham AM, Ruppe LK, Stadler SL. Clinical pharmacist patient-safety initiative to reduce against-label prescribing of statins with cyclosporine. Ann Pharmacother. 2017;51(2):140‐145. doi:10.1177/1060028016675352
8. Roblek T, Deticek A, Leskovar B, et al. Clinical-pharmacist intervention reduces clinically relevant drugdrug interactions in patients with heart failure: A randomized, double-blind, controlled trial. Int J Cardiol. 2016;203:647‐652. doi:10.1016/j.ijcard.2015.10.206
9. Tuchscherer RM, Nair K, Ghushchyan V, Saseen JJ. Simvastatin prescribing patterns before and after FDA dosing restrictions: a retrospective analysis of a large healthcare claims database. Am J Cardiovasc Drugs. 2015;15(1):27‐34. doi:10.1007/s40256-014-0096-x
10. Alford JC, Saseen JJ, Allen RR, Nair KV. Persistent use of against-label statin-fibrate combinations from 2003-2009 despite United States Food and Drug Administration dose restrictions. Pharmacotherapy. 2012;32(7):623‐630. doi:10.1002/j.1875-9114.2011.01090.x
11. Leape LL, Cullen DJ, Clapp MD, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit [published correction appears in JAMA 2000 Mar 8;283(10):1293]. JAMA. 1999;282(3):267‐270. doi:10.1001/jama.282.3.267
12. Kucukarslan SN, Peters M, Mlynarek M, Nafziger DA. Pharmacists on rounding teams reduce preventable adverse drug events in hospital general medicine units. Arch Intern Med. 2003;163(17):2014‐2018. doi:10.1001/archinte.163.17.2014
13. Humphries TL, Carroll N, Chester EA, Magid D, Rocho B. Evaluation of an electronic critical drug interaction program coupled with active pharmacist intervention. Ann Pharmacother. 2007;41(12):1979‐1985. doi:10.1345/aph.1K349
14. Zocor [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2018.
15. Lipitor [package insert]. New York, NY: Pfizer; 2017.
16. Crestor [package insert]. Wilmington, DE: AstraZeneca; 2018.
17. Mevacor [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2012.
18. Wolters Kluwer Health, Lexi-Drugs, Lexicomp. Pravastatin. www.online.lexi.com. [Source not verified.]
19. Miller M, Stone NJ, Ballantyne C, et al; American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292-2333. doi: 10.1161/CIR.0b013e3182160726
20. Ferguson J, Seston L, Ashcroft DM. Refer-to-pharmacy: a qualitative study exploring the implementation of an electronic transfer of care initiative to improve medicines optimisation following hospital discharge. BMC Health Serv Res. 2018;18(1):424. doi:10.1186/s12913-018-3262-z
21. Ensing HT, Koster ES, Dubero DJ, van Dooren AA, Bouvy ML. Collaboration between hospital and community pharmacists to address drug-related problems: the HomeCoMe-program. Res Social Adm Pharm. 2019;15(3):267‐278. doi:10.1016/j.sapharm.2018.05.001
22. US Department of Defense, US Department of Veterans Affairs. VA/DoD clinical practice guideline for the management of dyslipidemia for cardiovascular risk reduction guideline summary. https://www.healthquality.va.gov /guidelines/CD/lipids/LipidSumOptSinglePg31Aug15.pdf. Published 2014. Accessed May 14, 2020.
23. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014 Jun 24;129(25) (suppl 2):S46-48] [published correction appears in Circulation. 2015 Dec 22;132(25):e396]. Circulation. 2014;129(25)(suppl 2): S1‐S45. doi:10.1161/01.cir.0000437738.63853.7a
1. The top 200 drugs of 2020 Provided by the ClinCalc DrugStats Database. http://clincalc.com/DrugStats /Top200Drugs.aspx. Updated February 11, 2017. Accessed May 12, 2020.
2. Wiggins BS, Saseen JJ, Page RL 2nd, et al; American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology; Council on Hypertension; Council on Quality of Care and Outcomes Research; and Council on Functional Genomics and Translational Biology. Recommendations for management of clinically significant drug-drug interactions with statins and select agents used in patients with cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2016;134(21):e468‐e495. doi:10.1161/CIR.0000000000000456
3. Smithburger PL, Buckley MS, Bejian S, Burenheide K, Kane-Gill SL. A critical evaluation of clinical decision support for the detection of drug-drug interactions. Expert Opin Drug Saf. 2011;10(6):871‐882. doi:10.1517/14740338.2011.583916
4. US Food and Drug Administration. FDA drug safety communication: new restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury. https://www.fda.gov/Drugs/DrugSafety /ucm256581.htm. Updated December 15, 2017. Accessed May 12, 2020.
5. US Food and Drug Administration. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. https://www.fda.gov /Drugs/DrugSafety/ucm293101.htm. Updated January 19, 2016. Accessed May 12, 2020.
6. US Food and Drug Administration Federal Register. AbbVie Inc. et al; withdrawal of approval of indications related to the coadministration with statins in applications for niacin extended-release tablets and fenofibric acid delayed-release capsules. https://www.federalregister .gov/documents/2016/04/18/2016-08887/abbvie-inc -et-al-withdrawal-of-approval-of-indications-related -to-the-coadministration-with-statins. Published April 18, 2016. Accessed May 12, 2020.
7. Lamprecht DG Jr, Todd BA, Denham AM, Ruppe LK, Stadler SL. Clinical pharmacist patient-safety initiative to reduce against-label prescribing of statins with cyclosporine. Ann Pharmacother. 2017;51(2):140‐145. doi:10.1177/1060028016675352
8. Roblek T, Deticek A, Leskovar B, et al. Clinical-pharmacist intervention reduces clinically relevant drugdrug interactions in patients with heart failure: A randomized, double-blind, controlled trial. Int J Cardiol. 2016;203:647‐652. doi:10.1016/j.ijcard.2015.10.206
9. Tuchscherer RM, Nair K, Ghushchyan V, Saseen JJ. Simvastatin prescribing patterns before and after FDA dosing restrictions: a retrospective analysis of a large healthcare claims database. Am J Cardiovasc Drugs. 2015;15(1):27‐34. doi:10.1007/s40256-014-0096-x
10. Alford JC, Saseen JJ, Allen RR, Nair KV. Persistent use of against-label statin-fibrate combinations from 2003-2009 despite United States Food and Drug Administration dose restrictions. Pharmacotherapy. 2012;32(7):623‐630. doi:10.1002/j.1875-9114.2011.01090.x
11. Leape LL, Cullen DJ, Clapp MD, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit [published correction appears in JAMA 2000 Mar 8;283(10):1293]. JAMA. 1999;282(3):267‐270. doi:10.1001/jama.282.3.267
12. Kucukarslan SN, Peters M, Mlynarek M, Nafziger DA. Pharmacists on rounding teams reduce preventable adverse drug events in hospital general medicine units. Arch Intern Med. 2003;163(17):2014‐2018. doi:10.1001/archinte.163.17.2014
13. Humphries TL, Carroll N, Chester EA, Magid D, Rocho B. Evaluation of an electronic critical drug interaction program coupled with active pharmacist intervention. Ann Pharmacother. 2007;41(12):1979‐1985. doi:10.1345/aph.1K349
14. Zocor [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2018.
15. Lipitor [package insert]. New York, NY: Pfizer; 2017.
16. Crestor [package insert]. Wilmington, DE: AstraZeneca; 2018.
17. Mevacor [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2012.
18. Wolters Kluwer Health, Lexi-Drugs, Lexicomp. Pravastatin. www.online.lexi.com. [Source not verified.]
19. Miller M, Stone NJ, Ballantyne C, et al; American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292-2333. doi: 10.1161/CIR.0b013e3182160726
20. Ferguson J, Seston L, Ashcroft DM. Refer-to-pharmacy: a qualitative study exploring the implementation of an electronic transfer of care initiative to improve medicines optimisation following hospital discharge. BMC Health Serv Res. 2018;18(1):424. doi:10.1186/s12913-018-3262-z
21. Ensing HT, Koster ES, Dubero DJ, van Dooren AA, Bouvy ML. Collaboration between hospital and community pharmacists to address drug-related problems: the HomeCoMe-program. Res Social Adm Pharm. 2019;15(3):267‐278. doi:10.1016/j.sapharm.2018.05.001
22. US Department of Defense, US Department of Veterans Affairs. VA/DoD clinical practice guideline for the management of dyslipidemia for cardiovascular risk reduction guideline summary. https://www.healthquality.va.gov /guidelines/CD/lipids/LipidSumOptSinglePg31Aug15.pdf. Published 2014. Accessed May 14, 2020.
23. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014 Jun 24;129(25) (suppl 2):S46-48] [published correction appears in Circulation. 2015 Dec 22;132(25):e396]. Circulation. 2014;129(25)(suppl 2): S1‐S45. doi:10.1161/01.cir.0000437738.63853.7a
Catheter ablation of AFib improves quality of life more than medications do
Background: Catheter ablation of AFib (primarily pulmonary vein isolation) has been shown to result in better maintenance of sinus rhythm than medications. Small studies of QOL have shown mixed results. Larger trials were needed.
Study design: Open-label randomized multisite clinical trial of catheter ablation (pulmonary vein isolation with additional ablation procedure at the treating physician discretion) versus standard rate and/or rhythm control medications (chosen by clinician discretion). Patients were included for paroxysmal or persistent AFib and either age 65 years or older or age younger than 65 years with one additional stroke risk factor. Quality of life surveys – the Atrial Fibrillation Effect on Quality of Life (AFEQT) questionnaire and the Mayo AF-Specific Symptom Inventory (MAFSI) – were completed at baseline, and at 3, 12, 24, 36, 48, and 60 months.
Setting: 126 centers in 10 countries.
Synopsis: The study included 2,204 patients with median age of 68 years, diagnosed with AFib a median of 1.1 years prior, who were followed for a median of 48 months. The median CHA2DS2-VASc score was 3.0.
Self-reported AFib dropped from 86.0% to 21.1% in the ablation group and from 83.7% to 39.8% in the medication group at 12 months. The AFEQT score (range 0-100, higher score indicating better QOL) increased from 62.9 to 86.4 in the ablation group and increased from 63.1 to 80.9 in the medication group (for a mean difference of 5.3 points [95% confidence interval, 3.7-6.9; P less than .001] favoring ablation). MAFSI symptom frequency score and symptom severity score also showed improvement in symptoms favoring ablation. Post hoc subgroup analysis showed that those with the most severe symptoms had the largest benefit from ablation.
The primary limitation is the lack of patient blinding (may bias self-reported symptoms).
While the CABANA trial efficacy study (published separately) showed that catheter ablation results in no significant difference in the combined outcome of death, disabling stroke, serious bleeding, or cardiac arrest, the CABANA QOL study, reviewed here, shows that ablation does result in improved QOL and reduced symptoms, compared with medical therapy.
Bottom line: Catheter ablation of AFib can be done safely and successfully at experienced centers. In patients with AFib-related symptoms, ablation reduces symptoms and improves QOL somewhat more than medications do. The most severely symptomatic patients appear to obtain the most benefit.
Citation: Packer DL et al. Effect of catheter ablation vs. antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: The CABANA Randomized Clinical Trial. JAMA. 2019 Mar 15. doi: 10.1001/jama.2019.0693.
Dr. Stafford is a hospitalist at Duke University Health System.
Background: Catheter ablation of AFib (primarily pulmonary vein isolation) has been shown to result in better maintenance of sinus rhythm than medications. Small studies of QOL have shown mixed results. Larger trials were needed.
Study design: Open-label randomized multisite clinical trial of catheter ablation (pulmonary vein isolation with additional ablation procedure at the treating physician discretion) versus standard rate and/or rhythm control medications (chosen by clinician discretion). Patients were included for paroxysmal or persistent AFib and either age 65 years or older or age younger than 65 years with one additional stroke risk factor. Quality of life surveys – the Atrial Fibrillation Effect on Quality of Life (AFEQT) questionnaire and the Mayo AF-Specific Symptom Inventory (MAFSI) – were completed at baseline, and at 3, 12, 24, 36, 48, and 60 months.
Setting: 126 centers in 10 countries.
Synopsis: The study included 2,204 patients with median age of 68 years, diagnosed with AFib a median of 1.1 years prior, who were followed for a median of 48 months. The median CHA2DS2-VASc score was 3.0.
Self-reported AFib dropped from 86.0% to 21.1% in the ablation group and from 83.7% to 39.8% in the medication group at 12 months. The AFEQT score (range 0-100, higher score indicating better QOL) increased from 62.9 to 86.4 in the ablation group and increased from 63.1 to 80.9 in the medication group (for a mean difference of 5.3 points [95% confidence interval, 3.7-6.9; P less than .001] favoring ablation). MAFSI symptom frequency score and symptom severity score also showed improvement in symptoms favoring ablation. Post hoc subgroup analysis showed that those with the most severe symptoms had the largest benefit from ablation.
The primary limitation is the lack of patient blinding (may bias self-reported symptoms).
While the CABANA trial efficacy study (published separately) showed that catheter ablation results in no significant difference in the combined outcome of death, disabling stroke, serious bleeding, or cardiac arrest, the CABANA QOL study, reviewed here, shows that ablation does result in improved QOL and reduced symptoms, compared with medical therapy.
Bottom line: Catheter ablation of AFib can be done safely and successfully at experienced centers. In patients with AFib-related symptoms, ablation reduces symptoms and improves QOL somewhat more than medications do. The most severely symptomatic patients appear to obtain the most benefit.
Citation: Packer DL et al. Effect of catheter ablation vs. antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: The CABANA Randomized Clinical Trial. JAMA. 2019 Mar 15. doi: 10.1001/jama.2019.0693.
Dr. Stafford is a hospitalist at Duke University Health System.
Background: Catheter ablation of AFib (primarily pulmonary vein isolation) has been shown to result in better maintenance of sinus rhythm than medications. Small studies of QOL have shown mixed results. Larger trials were needed.
Study design: Open-label randomized multisite clinical trial of catheter ablation (pulmonary vein isolation with additional ablation procedure at the treating physician discretion) versus standard rate and/or rhythm control medications (chosen by clinician discretion). Patients were included for paroxysmal or persistent AFib and either age 65 years or older or age younger than 65 years with one additional stroke risk factor. Quality of life surveys – the Atrial Fibrillation Effect on Quality of Life (AFEQT) questionnaire and the Mayo AF-Specific Symptom Inventory (MAFSI) – were completed at baseline, and at 3, 12, 24, 36, 48, and 60 months.
Setting: 126 centers in 10 countries.
Synopsis: The study included 2,204 patients with median age of 68 years, diagnosed with AFib a median of 1.1 years prior, who were followed for a median of 48 months. The median CHA2DS2-VASc score was 3.0.
Self-reported AFib dropped from 86.0% to 21.1% in the ablation group and from 83.7% to 39.8% in the medication group at 12 months. The AFEQT score (range 0-100, higher score indicating better QOL) increased from 62.9 to 86.4 in the ablation group and increased from 63.1 to 80.9 in the medication group (for a mean difference of 5.3 points [95% confidence interval, 3.7-6.9; P less than .001] favoring ablation). MAFSI symptom frequency score and symptom severity score also showed improvement in symptoms favoring ablation. Post hoc subgroup analysis showed that those with the most severe symptoms had the largest benefit from ablation.
The primary limitation is the lack of patient blinding (may bias self-reported symptoms).
While the CABANA trial efficacy study (published separately) showed that catheter ablation results in no significant difference in the combined outcome of death, disabling stroke, serious bleeding, or cardiac arrest, the CABANA QOL study, reviewed here, shows that ablation does result in improved QOL and reduced symptoms, compared with medical therapy.
Bottom line: Catheter ablation of AFib can be done safely and successfully at experienced centers. In patients with AFib-related symptoms, ablation reduces symptoms and improves QOL somewhat more than medications do. The most severely symptomatic patients appear to obtain the most benefit.
Citation: Packer DL et al. Effect of catheter ablation vs. antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: The CABANA Randomized Clinical Trial. JAMA. 2019 Mar 15. doi: 10.1001/jama.2019.0693.
Dr. Stafford is a hospitalist at Duke University Health System.
High ‘forever chemicals’ in blood linked to earlier menopause
In a national sample of U.S. women in their mid-40s to mid-50s, those with high serum levels of per- and polyfluoroalkyl substances (PFAS) were likely to enter menopause 2 years earlier than those with low levels of these chemicals.
That is, the median age of natural menopause was 52.8 years versus 50.8 years in women with high versus low serum levels of these chemicals in an analysis of data from more than 1,100 women in the Study of Women’s Health Across the Nation (SWAN) Multi-Pollutant Study, which excluded women with premature menopause (before age 40) or early menopause (before age 45).
“This study suggests that select PFAS serum concentrations are associated with earlier natural menopause, a risk factor for adverse health outcomes in later life,” Ning Ding, PhD, MPH, University of Michigan, Ann Arbor, and colleagues concluded in their article, published online June 3 in the Journal of Clinical Endocrinology & Metabolism.
“Even menopause a few years earlier than usual could have a significant impact on cardiovascular and bone health, quality of life, and overall health in general among women,” senior author Sung Kyun Park, ScD, MPH, from the same institution, added in a statement.
PFAS don’t break down in the body, build up with time
PFAS have been widely used in many consumer and industrial products such as nonstick cookware, stain-repellent carpets, waterproof rain gear, microwave popcorn bags, and firefighting foam, the authors explained.
These have been dubbed “forever chemicals” because they do not degrade. Household water for an estimated 110 million Americans (one in three) may be contaminated with these chemicals, according to an Endocrine Society press release.
“PFAS are everywhere. Once they enter the body, they don’t break down and [they] build up over time,” said Dr. Ding. “Because of their persistence in humans and potentially detrimental effects on ovarian function, it is important to raise awareness of this issue and reduce exposure to these chemicals.”
Environmental exposure and accelerated ovarian aging
Earlier menopause has been associated with an increased risk of cardiovascular disease, osteoporosis, and earlier cardiovascular and overall mortality, and environmental exposure may accelerate ovarian aging, the authors wrote.
PFAS, especially the most studied types – perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) – are plausible endocrine-disrupting chemicals, but findings so far have been inconsistent.
A study of people in Ohio exposed to contaminated water found that women with earlier natural menopause had higher serum PFOA and PFOS levels (J Clin Endocriniol Metab. 2011;96:1747-53).
But in research based on National Health and Nutrition Survey Examination data, higher PFOA, PFOS, or perfluorononanoic acid (PFNA) levels were not linked to earlier menopause, although higher levels of perfluorohexane sulfonic acid (PFHxS) were (Environ Health Perspect. 2014;122:145-50).
There may have been reverse causation, where postmenopausal women had higher PFAS levels because they were not excreting these chemicals in menstrual blood.
In a third study, PFOA exposure was not linked with age at menopause onset, but this was based on recall from 10 years earlier (Environ Res. 2016;146:323-30).
The current analysis examined data from 1,120 premenopausal women who were aged 45-56 years from 1999 to 2000.
The women were seen at five sites (Boston; Detroit; Los Angeles; Oakland, Calif.; and Pittsburgh) and were ethnically diverse (577 white, 235 black, 142 Chinese, and 166 Japanese).
Baseline serum PFAS levels were measured using high performance liquid chromatography-mass spectrometry. The women were followed up to 2017 and incident menopause (12 consecutive months with no menstruation) was determined from annual interviews.
Of the 1,120 women and 5,466 person-years of follow-up, 578 women had a known date of natural incident menopause and were included in the analysis. The remaining 542 women were excluded mainly because their date of final menstruation was unknown because of hormone therapy (451) or they had a hysterectomy, or did not enter menopause during the study.
Compared with women in the lowest tertile of PFOS levels, women in the highest tertile had a significant 26%-27% greater risk of incident menopause – after adjusting for age, body mass index, and prior hormone use, race/ethnicity, study site, education, physical activity, smoking status, and parity.
Higher PFOA and PFNA levels but not higher PFHxS levels were also associated with increased risk.
Compared with women with a low overall PFAS level, those with a high level had a 63% increased risk of incident menopause (hazard ratio, 1.63; 95% confidence interval, 1.08-2.45), equivalent to having menopause a median of 2 years earlier.
Although production and use of some types of PFAS in the United States are declining, Dr. Ding and colleagues wrote, exposure continues, along with associated potential hazards to human reproductive health.
“Due to PFAS widespread use and environmental persistence, their potential adverse effects remain a public health concern,” they concluded.
SWAN was supported by the National Institutes of Health, Department of Health & Human Services through the National Institute on Aging, National Institute of Nursing Research, NIH Office of Research on Women’s Health, and the SWAN repository. The current article was supported by the National Center for Research Resources and National Center for Advancing Translational Sciences, NIH, National Institute of Environmental Health Sciences, and Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In a national sample of U.S. women in their mid-40s to mid-50s, those with high serum levels of per- and polyfluoroalkyl substances (PFAS) were likely to enter menopause 2 years earlier than those with low levels of these chemicals.
That is, the median age of natural menopause was 52.8 years versus 50.8 years in women with high versus low serum levels of these chemicals in an analysis of data from more than 1,100 women in the Study of Women’s Health Across the Nation (SWAN) Multi-Pollutant Study, which excluded women with premature menopause (before age 40) or early menopause (before age 45).
“This study suggests that select PFAS serum concentrations are associated with earlier natural menopause, a risk factor for adverse health outcomes in later life,” Ning Ding, PhD, MPH, University of Michigan, Ann Arbor, and colleagues concluded in their article, published online June 3 in the Journal of Clinical Endocrinology & Metabolism.
“Even menopause a few years earlier than usual could have a significant impact on cardiovascular and bone health, quality of life, and overall health in general among women,” senior author Sung Kyun Park, ScD, MPH, from the same institution, added in a statement.
PFAS don’t break down in the body, build up with time
PFAS have been widely used in many consumer and industrial products such as nonstick cookware, stain-repellent carpets, waterproof rain gear, microwave popcorn bags, and firefighting foam, the authors explained.
These have been dubbed “forever chemicals” because they do not degrade. Household water for an estimated 110 million Americans (one in three) may be contaminated with these chemicals, according to an Endocrine Society press release.
“PFAS are everywhere. Once they enter the body, they don’t break down and [they] build up over time,” said Dr. Ding. “Because of their persistence in humans and potentially detrimental effects on ovarian function, it is important to raise awareness of this issue and reduce exposure to these chemicals.”
Environmental exposure and accelerated ovarian aging
Earlier menopause has been associated with an increased risk of cardiovascular disease, osteoporosis, and earlier cardiovascular and overall mortality, and environmental exposure may accelerate ovarian aging, the authors wrote.
PFAS, especially the most studied types – perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) – are plausible endocrine-disrupting chemicals, but findings so far have been inconsistent.
A study of people in Ohio exposed to contaminated water found that women with earlier natural menopause had higher serum PFOA and PFOS levels (J Clin Endocriniol Metab. 2011;96:1747-53).
But in research based on National Health and Nutrition Survey Examination data, higher PFOA, PFOS, or perfluorononanoic acid (PFNA) levels were not linked to earlier menopause, although higher levels of perfluorohexane sulfonic acid (PFHxS) were (Environ Health Perspect. 2014;122:145-50).
There may have been reverse causation, where postmenopausal women had higher PFAS levels because they were not excreting these chemicals in menstrual blood.
In a third study, PFOA exposure was not linked with age at menopause onset, but this was based on recall from 10 years earlier (Environ Res. 2016;146:323-30).
The current analysis examined data from 1,120 premenopausal women who were aged 45-56 years from 1999 to 2000.
The women were seen at five sites (Boston; Detroit; Los Angeles; Oakland, Calif.; and Pittsburgh) and were ethnically diverse (577 white, 235 black, 142 Chinese, and 166 Japanese).
Baseline serum PFAS levels were measured using high performance liquid chromatography-mass spectrometry. The women were followed up to 2017 and incident menopause (12 consecutive months with no menstruation) was determined from annual interviews.
Of the 1,120 women and 5,466 person-years of follow-up, 578 women had a known date of natural incident menopause and were included in the analysis. The remaining 542 women were excluded mainly because their date of final menstruation was unknown because of hormone therapy (451) or they had a hysterectomy, or did not enter menopause during the study.
Compared with women in the lowest tertile of PFOS levels, women in the highest tertile had a significant 26%-27% greater risk of incident menopause – after adjusting for age, body mass index, and prior hormone use, race/ethnicity, study site, education, physical activity, smoking status, and parity.
Higher PFOA and PFNA levels but not higher PFHxS levels were also associated with increased risk.
Compared with women with a low overall PFAS level, those with a high level had a 63% increased risk of incident menopause (hazard ratio, 1.63; 95% confidence interval, 1.08-2.45), equivalent to having menopause a median of 2 years earlier.
Although production and use of some types of PFAS in the United States are declining, Dr. Ding and colleagues wrote, exposure continues, along with associated potential hazards to human reproductive health.
“Due to PFAS widespread use and environmental persistence, their potential adverse effects remain a public health concern,” they concluded.
SWAN was supported by the National Institutes of Health, Department of Health & Human Services through the National Institute on Aging, National Institute of Nursing Research, NIH Office of Research on Women’s Health, and the SWAN repository. The current article was supported by the National Center for Research Resources and National Center for Advancing Translational Sciences, NIH, National Institute of Environmental Health Sciences, and Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In a national sample of U.S. women in their mid-40s to mid-50s, those with high serum levels of per- and polyfluoroalkyl substances (PFAS) were likely to enter menopause 2 years earlier than those with low levels of these chemicals.
That is, the median age of natural menopause was 52.8 years versus 50.8 years in women with high versus low serum levels of these chemicals in an analysis of data from more than 1,100 women in the Study of Women’s Health Across the Nation (SWAN) Multi-Pollutant Study, which excluded women with premature menopause (before age 40) or early menopause (before age 45).
“This study suggests that select PFAS serum concentrations are associated with earlier natural menopause, a risk factor for adverse health outcomes in later life,” Ning Ding, PhD, MPH, University of Michigan, Ann Arbor, and colleagues concluded in their article, published online June 3 in the Journal of Clinical Endocrinology & Metabolism.
“Even menopause a few years earlier than usual could have a significant impact on cardiovascular and bone health, quality of life, and overall health in general among women,” senior author Sung Kyun Park, ScD, MPH, from the same institution, added in a statement.
PFAS don’t break down in the body, build up with time
PFAS have been widely used in many consumer and industrial products such as nonstick cookware, stain-repellent carpets, waterproof rain gear, microwave popcorn bags, and firefighting foam, the authors explained.
These have been dubbed “forever chemicals” because they do not degrade. Household water for an estimated 110 million Americans (one in three) may be contaminated with these chemicals, according to an Endocrine Society press release.
“PFAS are everywhere. Once they enter the body, they don’t break down and [they] build up over time,” said Dr. Ding. “Because of their persistence in humans and potentially detrimental effects on ovarian function, it is important to raise awareness of this issue and reduce exposure to these chemicals.”
Environmental exposure and accelerated ovarian aging
Earlier menopause has been associated with an increased risk of cardiovascular disease, osteoporosis, and earlier cardiovascular and overall mortality, and environmental exposure may accelerate ovarian aging, the authors wrote.
PFAS, especially the most studied types – perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) – are plausible endocrine-disrupting chemicals, but findings so far have been inconsistent.
A study of people in Ohio exposed to contaminated water found that women with earlier natural menopause had higher serum PFOA and PFOS levels (J Clin Endocriniol Metab. 2011;96:1747-53).
But in research based on National Health and Nutrition Survey Examination data, higher PFOA, PFOS, or perfluorononanoic acid (PFNA) levels were not linked to earlier menopause, although higher levels of perfluorohexane sulfonic acid (PFHxS) were (Environ Health Perspect. 2014;122:145-50).
There may have been reverse causation, where postmenopausal women had higher PFAS levels because they were not excreting these chemicals in menstrual blood.
In a third study, PFOA exposure was not linked with age at menopause onset, but this was based on recall from 10 years earlier (Environ Res. 2016;146:323-30).
The current analysis examined data from 1,120 premenopausal women who were aged 45-56 years from 1999 to 2000.
The women were seen at five sites (Boston; Detroit; Los Angeles; Oakland, Calif.; and Pittsburgh) and were ethnically diverse (577 white, 235 black, 142 Chinese, and 166 Japanese).
Baseline serum PFAS levels were measured using high performance liquid chromatography-mass spectrometry. The women were followed up to 2017 and incident menopause (12 consecutive months with no menstruation) was determined from annual interviews.
Of the 1,120 women and 5,466 person-years of follow-up, 578 women had a known date of natural incident menopause and were included in the analysis. The remaining 542 women were excluded mainly because their date of final menstruation was unknown because of hormone therapy (451) or they had a hysterectomy, or did not enter menopause during the study.
Compared with women in the lowest tertile of PFOS levels, women in the highest tertile had a significant 26%-27% greater risk of incident menopause – after adjusting for age, body mass index, and prior hormone use, race/ethnicity, study site, education, physical activity, smoking status, and parity.
Higher PFOA and PFNA levels but not higher PFHxS levels were also associated with increased risk.
Compared with women with a low overall PFAS level, those with a high level had a 63% increased risk of incident menopause (hazard ratio, 1.63; 95% confidence interval, 1.08-2.45), equivalent to having menopause a median of 2 years earlier.
Although production and use of some types of PFAS in the United States are declining, Dr. Ding and colleagues wrote, exposure continues, along with associated potential hazards to human reproductive health.
“Due to PFAS widespread use and environmental persistence, their potential adverse effects remain a public health concern,” they concluded.
SWAN was supported by the National Institutes of Health, Department of Health & Human Services through the National Institute on Aging, National Institute of Nursing Research, NIH Office of Research on Women’s Health, and the SWAN repository. The current article was supported by the National Center for Research Resources and National Center for Advancing Translational Sciences, NIH, National Institute of Environmental Health Sciences, and Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Erectile dysfunction: How to help patients & partners
THE CASE
Eric M,* a 36-year-old new patient, visits a primary care clinic for a check-up accompanied by his wife. A thorough history and physical exam reveal no concerns. He is active and a nonsmoker, drinks only socially, takes no medications, and reports no concerning symptoms. At the end of the visit, though, he says he has been experiencing erectile dysfunction for the past 6 months. What began as intermittent difficulty maintaining erections now “happens a lot.” He is distressed and says, “It just came out of the blue.” The patient’s wife then says she believes men cannot achieve erections if they are having an affair. When the chagrined patient simply asks for “those pills,” his wife says in a raised voice, “He’s a liar!”
●
*The patient’s name has been changed to protect his identity.
Some family physicians may feel ill-equipped to talk about sexual and relational problems and lack the skills to effectively counsel on these matters.1 Despite the fact that more than 70% of adult patients want to discuss sexual topics with their family physician, sexual problems are documented in as few as 2% of patient notes.2 One of the most commonly noted sexual health concerns is erectile dysfunction (ED), estimated to occur in 35% of men ages 40 to 70.3 Many ED cases have psychological antecedents including stress, depression, performance anxiety, pornography addiction, and relationship concerns.4,5
Assessing ED. The inability to achieve or maintain an erection needed for satisfactory sexual activity is typically diagnosed through symptom self-report and with thorough history taking and physical examination.6 However, more objective scales can be used. In particular, the International Index of Erectile Function, a 15-question scale, is useful for both diagnosis and treatment monitoring (www.baus.org.uk/_userfiles/pages/files/Patients/Leaflets/iief.pdf).7 Common contributors to ED can be vascular (eg, hypertension), neurologic (eg, multiple sclerosis), psychological (noted earlier), or hormonal (eg, thyroid imbalances).6 In this article, we focus on the relationship context in which ED exists. A review of medical evaluation and management can be found elsewhere.8
Key relational questions
Identify norms that are specific to the couple. Patients from a variety of cultures prefer that their clinicians initiate the conversation about ED.11,12 We specifically recommend that clinicians, using relationally focused questions, inquire about sexual norms and desires that may be situated in culture, family of origin, or gender (TABLE 1).
Continue to: Treating ED within a relationship
Treating ED within a relationship
Once a couple’s sexual relationship has been fully assessed, you may confidently develop a treatment plan for managing sexual dysfunction relationally as well as medically, an approach to ED advised by the American Urological Association.13 We propose that primary care treatment for ED involve collaboration between the physician, the patient/couple (if the patient is partnered), and, as needed, a behavioral health specialist.
The physician’s role ...
Managing ED relationally is important on many fronts. If, for instance, a type-5 phosphodiesterase (PDE-5) inhibitor is needed, both the patient and partner should learn about best practices for optimizing success, such as avoiding excessive alcohol intake or high-fat meals immediately before and after taking a PDE-5.14
Sex ed. Regardless of the couple’s age, be prepared to offer high-quality sexual education. Either partner may have faulty knowledge (or even a lack of knowledge) of basic sexual functioning. Physicians have an opportunity to explain healthy erectile functioning, the sexual response cycle, and ways in which PDE-5 medications work (and do not work). (For a list of resources to facilitate these discussions, see TABLE 2.)
Avoid avoidance. Physicians can intervene on patterns of shame that may surround ED simply by discussing sexual functioning openly and honestly. ED often persists due to avoidance—ie, anxiety about sexual performance can lead couples to avoid sex, which perpetuates more anxiety and avoidance. Normalizing typical sexual functioning, encouraging couples to “avoid avoidance,” and providing referrals as needed are core elements of relational intervention for ED.
Setting the tone. Family physicians are not routinely trained in couples therapy. However, you can employ communication skills that allow each partner to be heard by using empathic/reflective listening, de-escalation, and reframing. Asking “What effect are the sexual concerns having on both of you?” and “What were the circumstances of the last sexual encounter that were pleasing to both of you?” can help promote intimacy and mutual satisfaction.
Continue to: The behavioral specialist's role
The behavioral specialist’s role
Behavioral health specialists may treat ED using methods such as cognitive behavioral therapy or evidence-based couple interventions.4 Cognitive methods for the treatment of ED include examination of maladaptive thoughts around pressure to perform and achieving sexual pleasure. Behavioral methods for treatment of ED are typically aimed at the de-coupling of anxiety and sexual activity. These treatments can include relaxation and desensitization, specifically sensate focus therapy.15
Sensate focus therapy involves a specific set of prescriptive rules for sexual activity, initially restricting touch to non-demand pleasurable touch (eg, holding hands) that allows couples to connect in a low-anxiety context focused on relaxation and connection. As couples are able to control anxiety while engaging in these activities, they engage in increasingly more intimate activities. Additionally, behavioral health specialists trained in couples therapy are vital to helping increase communication regarding sexual activity, sexual scripts, and the relationship in general.4
Identifying a treatment team
In coordinating couples care in the treatment of ED, enlist the help of a therapist who has specific knowledge and skills in the treatment of sexual disorders. While the number of qualified or certified sex therapists is limited, referring providers can visit the American Association of Sexuality Educators, Counselors, and Therapists Web site (www.aasect.org) for possible referral sources. Another option is the American Association for Marriage and Family Therapy Web site (www.aamft.org) under “Find a therapist.” Lastly, the Society for Sex Therapy and Research (www.sstarnet.org) is another professional association that provides information and local referral sources. For patients and partners located in rural areas where access is limited, telehealth options may need to be explored.
THE CASE
Mr. M and his wife were seen for a follow-up appointment by his primary care provider, who ruled out any additional causes of ED (eg, hormonal, vascular), discussed with both the patient and his wife basic sexual health and sexual functioning, dispelled several commonly held myths (ie, individuals cannot obtain an erection because of infidelity or lying), validated sexual concerns as a significant health issue, and prescribed a PDE-5 inhibitor.
Mr. M and his wife were referred to a behavioral health specialist in the clinic who had expertise in couples therapy. At several subsequent visits, the patient and his wife worked on improving the quality and quantity of communication regarding their sexual goals, mutual de-escalation of anxiety, increased emotional intimacy, and sensate focus techniques.
Continue to: As the result of the interventions...
As the result of these interventions, both the patient and his wife were able to engage in sex with less anxiety, and the patient increasingly was able to achieve more satisfactory erections without the use of the PDE-5 inhibitor. At the conclusion of therapy, the patient and his wife reported an increase in sexual satisfaction.
CORRESPONDENCE
Katherine Buck, PhD, Family Health Center, John Peter Smith Health Network, 1500 S. Main Street, Fort Worth, TX 76104; kbuck@jpshealth.org.
1. Macdowall W, Parker R, Nanchahal K, et al. ‘Talking of Sex’: developing and piloting a sexual health communication tool for use in primary care. Patient Educ Couns. 2010;81:332-337.
2. Sadovsky R. Asking the questions and offering solutions: the ongoing dialogue between the primary care physician and the patient with erectile dysfunction. Rev Urol. 2003;5(suppl 7):S35-S48.
3. Boston University School of Medicine. Sexual Medicine. Epidemiology of ED. 2019. www.bumc.bu.edu/sexualmedicine/physicianinformation/epidemiology-of-ed/. Accessed May 27, 2020.
4. Weeks GR, Gambescia N, Hertlein KM, eds. A Clinician’s Guide to Systemic Sex Therapy. 2nd ed. London, England: Routledge; 2016.
5. Colson MH, Cuzin A, Faix A, et al. Current epidemiology of erectile dysfunction, an update. Sexologies. 2018;27:e7-e13.
6. Rew KT, Heidelbaugh JJ. Erectile dysfunction. Am Fam Physician. 2016;94820-94827.
7. Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319-326.
8. Rew KT, Heidelbaugh JJ. Erectile dysfunction. Am Fam Physician. 2016;94:820-827.
9. Dean J, Rubio-Aurioles E, McCabe M, et al. Integrating partners into erectile dysfunction treatment: improving the sexual experience for the couple. Int J Clin Pract. 2008;62:127-133.
10. Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013; 381:153-165.
11. Lo WH, Fu SN, Wong SN, et al. Prevalence, correlates, attitude and treatment seeking of erectile dysfunction among type 2 diabetic Chinese men attending primary care outpatient clinics. Asian J Androl. 2014;16:755-760.
12. Zweifler J, Padilla A, Schafer S. Barriers to recognition of erectile dysfunction among diabetic Mexican-American men. J Am Board Fam Pract. 1998;11:259-263.
13. American Urological Society. Erectile dysfunction: AUA guideline (2018). www.auanet.org/guidelines/erectile-dysfunction-(ed)-guideline. Accessed May 27, 2020.
14. Huang S, Lie J. Phosphodiesterase-5 (PDE5) inhibitors in the management of erectile dysfunction. P T. 2013;38;414-419.
15. Masters WH, Johnson VE. Human Sexual Inadequacy. Boston: Little Brown; 1970.
THE CASE
Eric M,* a 36-year-old new patient, visits a primary care clinic for a check-up accompanied by his wife. A thorough history and physical exam reveal no concerns. He is active and a nonsmoker, drinks only socially, takes no medications, and reports no concerning symptoms. At the end of the visit, though, he says he has been experiencing erectile dysfunction for the past 6 months. What began as intermittent difficulty maintaining erections now “happens a lot.” He is distressed and says, “It just came out of the blue.” The patient’s wife then says she believes men cannot achieve erections if they are having an affair. When the chagrined patient simply asks for “those pills,” his wife says in a raised voice, “He’s a liar!”
●
*The patient’s name has been changed to protect his identity.
Some family physicians may feel ill-equipped to talk about sexual and relational problems and lack the skills to effectively counsel on these matters.1 Despite the fact that more than 70% of adult patients want to discuss sexual topics with their family physician, sexual problems are documented in as few as 2% of patient notes.2 One of the most commonly noted sexual health concerns is erectile dysfunction (ED), estimated to occur in 35% of men ages 40 to 70.3 Many ED cases have psychological antecedents including stress, depression, performance anxiety, pornography addiction, and relationship concerns.4,5
Assessing ED. The inability to achieve or maintain an erection needed for satisfactory sexual activity is typically diagnosed through symptom self-report and with thorough history taking and physical examination.6 However, more objective scales can be used. In particular, the International Index of Erectile Function, a 15-question scale, is useful for both diagnosis and treatment monitoring (www.baus.org.uk/_userfiles/pages/files/Patients/Leaflets/iief.pdf).7 Common contributors to ED can be vascular (eg, hypertension), neurologic (eg, multiple sclerosis), psychological (noted earlier), or hormonal (eg, thyroid imbalances).6 In this article, we focus on the relationship context in which ED exists. A review of medical evaluation and management can be found elsewhere.8
Key relational questions
Identify norms that are specific to the couple. Patients from a variety of cultures prefer that their clinicians initiate the conversation about ED.11,12 We specifically recommend that clinicians, using relationally focused questions, inquire about sexual norms and desires that may be situated in culture, family of origin, or gender (TABLE 1).
Continue to: Treating ED within a relationship
Treating ED within a relationship
Once a couple’s sexual relationship has been fully assessed, you may confidently develop a treatment plan for managing sexual dysfunction relationally as well as medically, an approach to ED advised by the American Urological Association.13 We propose that primary care treatment for ED involve collaboration between the physician, the patient/couple (if the patient is partnered), and, as needed, a behavioral health specialist.
The physician’s role ...
Managing ED relationally is important on many fronts. If, for instance, a type-5 phosphodiesterase (PDE-5) inhibitor is needed, both the patient and partner should learn about best practices for optimizing success, such as avoiding excessive alcohol intake or high-fat meals immediately before and after taking a PDE-5.14
Sex ed. Regardless of the couple’s age, be prepared to offer high-quality sexual education. Either partner may have faulty knowledge (or even a lack of knowledge) of basic sexual functioning. Physicians have an opportunity to explain healthy erectile functioning, the sexual response cycle, and ways in which PDE-5 medications work (and do not work). (For a list of resources to facilitate these discussions, see TABLE 2.)
Avoid avoidance. Physicians can intervene on patterns of shame that may surround ED simply by discussing sexual functioning openly and honestly. ED often persists due to avoidance—ie, anxiety about sexual performance can lead couples to avoid sex, which perpetuates more anxiety and avoidance. Normalizing typical sexual functioning, encouraging couples to “avoid avoidance,” and providing referrals as needed are core elements of relational intervention for ED.
Setting the tone. Family physicians are not routinely trained in couples therapy. However, you can employ communication skills that allow each partner to be heard by using empathic/reflective listening, de-escalation, and reframing. Asking “What effect are the sexual concerns having on both of you?” and “What were the circumstances of the last sexual encounter that were pleasing to both of you?” can help promote intimacy and mutual satisfaction.
Continue to: The behavioral specialist's role
The behavioral specialist’s role
Behavioral health specialists may treat ED using methods such as cognitive behavioral therapy or evidence-based couple interventions.4 Cognitive methods for the treatment of ED include examination of maladaptive thoughts around pressure to perform and achieving sexual pleasure. Behavioral methods for treatment of ED are typically aimed at the de-coupling of anxiety and sexual activity. These treatments can include relaxation and desensitization, specifically sensate focus therapy.15
Sensate focus therapy involves a specific set of prescriptive rules for sexual activity, initially restricting touch to non-demand pleasurable touch (eg, holding hands) that allows couples to connect in a low-anxiety context focused on relaxation and connection. As couples are able to control anxiety while engaging in these activities, they engage in increasingly more intimate activities. Additionally, behavioral health specialists trained in couples therapy are vital to helping increase communication regarding sexual activity, sexual scripts, and the relationship in general.4
Identifying a treatment team
In coordinating couples care in the treatment of ED, enlist the help of a therapist who has specific knowledge and skills in the treatment of sexual disorders. While the number of qualified or certified sex therapists is limited, referring providers can visit the American Association of Sexuality Educators, Counselors, and Therapists Web site (www.aasect.org) for possible referral sources. Another option is the American Association for Marriage and Family Therapy Web site (www.aamft.org) under “Find a therapist.” Lastly, the Society for Sex Therapy and Research (www.sstarnet.org) is another professional association that provides information and local referral sources. For patients and partners located in rural areas where access is limited, telehealth options may need to be explored.
THE CASE
Mr. M and his wife were seen for a follow-up appointment by his primary care provider, who ruled out any additional causes of ED (eg, hormonal, vascular), discussed with both the patient and his wife basic sexual health and sexual functioning, dispelled several commonly held myths (ie, individuals cannot obtain an erection because of infidelity or lying), validated sexual concerns as a significant health issue, and prescribed a PDE-5 inhibitor.
Mr. M and his wife were referred to a behavioral health specialist in the clinic who had expertise in couples therapy. At several subsequent visits, the patient and his wife worked on improving the quality and quantity of communication regarding their sexual goals, mutual de-escalation of anxiety, increased emotional intimacy, and sensate focus techniques.
Continue to: As the result of the interventions...
As the result of these interventions, both the patient and his wife were able to engage in sex with less anxiety, and the patient increasingly was able to achieve more satisfactory erections without the use of the PDE-5 inhibitor. At the conclusion of therapy, the patient and his wife reported an increase in sexual satisfaction.
CORRESPONDENCE
Katherine Buck, PhD, Family Health Center, John Peter Smith Health Network, 1500 S. Main Street, Fort Worth, TX 76104; kbuck@jpshealth.org.
THE CASE
Eric M,* a 36-year-old new patient, visits a primary care clinic for a check-up accompanied by his wife. A thorough history and physical exam reveal no concerns. He is active and a nonsmoker, drinks only socially, takes no medications, and reports no concerning symptoms. At the end of the visit, though, he says he has been experiencing erectile dysfunction for the past 6 months. What began as intermittent difficulty maintaining erections now “happens a lot.” He is distressed and says, “It just came out of the blue.” The patient’s wife then says she believes men cannot achieve erections if they are having an affair. When the chagrined patient simply asks for “those pills,” his wife says in a raised voice, “He’s a liar!”
●
*The patient’s name has been changed to protect his identity.
Some family physicians may feel ill-equipped to talk about sexual and relational problems and lack the skills to effectively counsel on these matters.1 Despite the fact that more than 70% of adult patients want to discuss sexual topics with their family physician, sexual problems are documented in as few as 2% of patient notes.2 One of the most commonly noted sexual health concerns is erectile dysfunction (ED), estimated to occur in 35% of men ages 40 to 70.3 Many ED cases have psychological antecedents including stress, depression, performance anxiety, pornography addiction, and relationship concerns.4,5
Assessing ED. The inability to achieve or maintain an erection needed for satisfactory sexual activity is typically diagnosed through symptom self-report and with thorough history taking and physical examination.6 However, more objective scales can be used. In particular, the International Index of Erectile Function, a 15-question scale, is useful for both diagnosis and treatment monitoring (www.baus.org.uk/_userfiles/pages/files/Patients/Leaflets/iief.pdf).7 Common contributors to ED can be vascular (eg, hypertension), neurologic (eg, multiple sclerosis), psychological (noted earlier), or hormonal (eg, thyroid imbalances).6 In this article, we focus on the relationship context in which ED exists. A review of medical evaluation and management can be found elsewhere.8
Key relational questions
Identify norms that are specific to the couple. Patients from a variety of cultures prefer that their clinicians initiate the conversation about ED.11,12 We specifically recommend that clinicians, using relationally focused questions, inquire about sexual norms and desires that may be situated in culture, family of origin, or gender (TABLE 1).
Continue to: Treating ED within a relationship
Treating ED within a relationship
Once a couple’s sexual relationship has been fully assessed, you may confidently develop a treatment plan for managing sexual dysfunction relationally as well as medically, an approach to ED advised by the American Urological Association.13 We propose that primary care treatment for ED involve collaboration between the physician, the patient/couple (if the patient is partnered), and, as needed, a behavioral health specialist.
The physician’s role ...
Managing ED relationally is important on many fronts. If, for instance, a type-5 phosphodiesterase (PDE-5) inhibitor is needed, both the patient and partner should learn about best practices for optimizing success, such as avoiding excessive alcohol intake or high-fat meals immediately before and after taking a PDE-5.14
Sex ed. Regardless of the couple’s age, be prepared to offer high-quality sexual education. Either partner may have faulty knowledge (or even a lack of knowledge) of basic sexual functioning. Physicians have an opportunity to explain healthy erectile functioning, the sexual response cycle, and ways in which PDE-5 medications work (and do not work). (For a list of resources to facilitate these discussions, see TABLE 2.)
Avoid avoidance. Physicians can intervene on patterns of shame that may surround ED simply by discussing sexual functioning openly and honestly. ED often persists due to avoidance—ie, anxiety about sexual performance can lead couples to avoid sex, which perpetuates more anxiety and avoidance. Normalizing typical sexual functioning, encouraging couples to “avoid avoidance,” and providing referrals as needed are core elements of relational intervention for ED.
Setting the tone. Family physicians are not routinely trained in couples therapy. However, you can employ communication skills that allow each partner to be heard by using empathic/reflective listening, de-escalation, and reframing. Asking “What effect are the sexual concerns having on both of you?” and “What were the circumstances of the last sexual encounter that were pleasing to both of you?” can help promote intimacy and mutual satisfaction.
Continue to: The behavioral specialist's role
The behavioral specialist’s role
Behavioral health specialists may treat ED using methods such as cognitive behavioral therapy or evidence-based couple interventions.4 Cognitive methods for the treatment of ED include examination of maladaptive thoughts around pressure to perform and achieving sexual pleasure. Behavioral methods for treatment of ED are typically aimed at the de-coupling of anxiety and sexual activity. These treatments can include relaxation and desensitization, specifically sensate focus therapy.15
Sensate focus therapy involves a specific set of prescriptive rules for sexual activity, initially restricting touch to non-demand pleasurable touch (eg, holding hands) that allows couples to connect in a low-anxiety context focused on relaxation and connection. As couples are able to control anxiety while engaging in these activities, they engage in increasingly more intimate activities. Additionally, behavioral health specialists trained in couples therapy are vital to helping increase communication regarding sexual activity, sexual scripts, and the relationship in general.4
Identifying a treatment team
In coordinating couples care in the treatment of ED, enlist the help of a therapist who has specific knowledge and skills in the treatment of sexual disorders. While the number of qualified or certified sex therapists is limited, referring providers can visit the American Association of Sexuality Educators, Counselors, and Therapists Web site (www.aasect.org) for possible referral sources. Another option is the American Association for Marriage and Family Therapy Web site (www.aamft.org) under “Find a therapist.” Lastly, the Society for Sex Therapy and Research (www.sstarnet.org) is another professional association that provides information and local referral sources. For patients and partners located in rural areas where access is limited, telehealth options may need to be explored.
THE CASE
Mr. M and his wife were seen for a follow-up appointment by his primary care provider, who ruled out any additional causes of ED (eg, hormonal, vascular), discussed with both the patient and his wife basic sexual health and sexual functioning, dispelled several commonly held myths (ie, individuals cannot obtain an erection because of infidelity or lying), validated sexual concerns as a significant health issue, and prescribed a PDE-5 inhibitor.
Mr. M and his wife were referred to a behavioral health specialist in the clinic who had expertise in couples therapy. At several subsequent visits, the patient and his wife worked on improving the quality and quantity of communication regarding their sexual goals, mutual de-escalation of anxiety, increased emotional intimacy, and sensate focus techniques.
Continue to: As the result of the interventions...
As the result of these interventions, both the patient and his wife were able to engage in sex with less anxiety, and the patient increasingly was able to achieve more satisfactory erections without the use of the PDE-5 inhibitor. At the conclusion of therapy, the patient and his wife reported an increase in sexual satisfaction.
CORRESPONDENCE
Katherine Buck, PhD, Family Health Center, John Peter Smith Health Network, 1500 S. Main Street, Fort Worth, TX 76104; kbuck@jpshealth.org.
1. Macdowall W, Parker R, Nanchahal K, et al. ‘Talking of Sex’: developing and piloting a sexual health communication tool for use in primary care. Patient Educ Couns. 2010;81:332-337.
2. Sadovsky R. Asking the questions and offering solutions: the ongoing dialogue between the primary care physician and the patient with erectile dysfunction. Rev Urol. 2003;5(suppl 7):S35-S48.
3. Boston University School of Medicine. Sexual Medicine. Epidemiology of ED. 2019. www.bumc.bu.edu/sexualmedicine/physicianinformation/epidemiology-of-ed/. Accessed May 27, 2020.
4. Weeks GR, Gambescia N, Hertlein KM, eds. A Clinician’s Guide to Systemic Sex Therapy. 2nd ed. London, England: Routledge; 2016.
5. Colson MH, Cuzin A, Faix A, et al. Current epidemiology of erectile dysfunction, an update. Sexologies. 2018;27:e7-e13.
6. Rew KT, Heidelbaugh JJ. Erectile dysfunction. Am Fam Physician. 2016;94820-94827.
7. Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319-326.
8. Rew KT, Heidelbaugh JJ. Erectile dysfunction. Am Fam Physician. 2016;94:820-827.
9. Dean J, Rubio-Aurioles E, McCabe M, et al. Integrating partners into erectile dysfunction treatment: improving the sexual experience for the couple. Int J Clin Pract. 2008;62:127-133.
10. Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013; 381:153-165.
11. Lo WH, Fu SN, Wong SN, et al. Prevalence, correlates, attitude and treatment seeking of erectile dysfunction among type 2 diabetic Chinese men attending primary care outpatient clinics. Asian J Androl. 2014;16:755-760.
12. Zweifler J, Padilla A, Schafer S. Barriers to recognition of erectile dysfunction among diabetic Mexican-American men. J Am Board Fam Pract. 1998;11:259-263.
13. American Urological Society. Erectile dysfunction: AUA guideline (2018). www.auanet.org/guidelines/erectile-dysfunction-(ed)-guideline. Accessed May 27, 2020.
14. Huang S, Lie J. Phosphodiesterase-5 (PDE5) inhibitors in the management of erectile dysfunction. P T. 2013;38;414-419.
15. Masters WH, Johnson VE. Human Sexual Inadequacy. Boston: Little Brown; 1970.
1. Macdowall W, Parker R, Nanchahal K, et al. ‘Talking of Sex’: developing and piloting a sexual health communication tool for use in primary care. Patient Educ Couns. 2010;81:332-337.
2. Sadovsky R. Asking the questions and offering solutions: the ongoing dialogue between the primary care physician and the patient with erectile dysfunction. Rev Urol. 2003;5(suppl 7):S35-S48.
3. Boston University School of Medicine. Sexual Medicine. Epidemiology of ED. 2019. www.bumc.bu.edu/sexualmedicine/physicianinformation/epidemiology-of-ed/. Accessed May 27, 2020.
4. Weeks GR, Gambescia N, Hertlein KM, eds. A Clinician’s Guide to Systemic Sex Therapy. 2nd ed. London, England: Routledge; 2016.
5. Colson MH, Cuzin A, Faix A, et al. Current epidemiology of erectile dysfunction, an update. Sexologies. 2018;27:e7-e13.
6. Rew KT, Heidelbaugh JJ. Erectile dysfunction. Am Fam Physician. 2016;94820-94827.
7. Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319-326.
8. Rew KT, Heidelbaugh JJ. Erectile dysfunction. Am Fam Physician. 2016;94:820-827.
9. Dean J, Rubio-Aurioles E, McCabe M, et al. Integrating partners into erectile dysfunction treatment: improving the sexual experience for the couple. Int J Clin Pract. 2008;62:127-133.
10. Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013; 381:153-165.
11. Lo WH, Fu SN, Wong SN, et al. Prevalence, correlates, attitude and treatment seeking of erectile dysfunction among type 2 diabetic Chinese men attending primary care outpatient clinics. Asian J Androl. 2014;16:755-760.
12. Zweifler J, Padilla A, Schafer S. Barriers to recognition of erectile dysfunction among diabetic Mexican-American men. J Am Board Fam Pract. 1998;11:259-263.
13. American Urological Society. Erectile dysfunction: AUA guideline (2018). www.auanet.org/guidelines/erectile-dysfunction-(ed)-guideline. Accessed May 27, 2020.
14. Huang S, Lie J. Phosphodiesterase-5 (PDE5) inhibitors in the management of erectile dysfunction. P T. 2013;38;414-419.
15. Masters WH, Johnson VE. Human Sexual Inadequacy. Boston: Little Brown; 1970.
Biologics may carry melanoma risk for patients with immune-mediated inflammatory diseases
The in a systematic review and meta-analysis published in JAMA Dermatology.
The studies included in the analysis, however, had limitations, including a lack of those comparing biologic and conventional systemic therapy in psoriasis and inflammatory bowel disease (IBD), according to Shamarke Esse, MRes, of the division of musculoskeletal and dermatological sciences at the University of Manchester (England) and colleagues. “We advocate for more large, well-designed studies of this issue to be performed to help improve certainty” regarding this association, they wrote.
Previous studies that have found an increased risk of melanoma in patients on biologics for psoriasis, rheumatoid arthritis, and IBD have “typically used the general population as the comparator,” they noted. There is a large amount of evidence that has established short-term efficacy and safety of biologics, compared with conventional systemic treatments, but concerns about longer-term cancer risk associated with biologics remains a concern. Moreover, they added, “melanoma is a highly immunogenic skin cancer and therefore of concern to patients treated with TNFIs [tumor necrosis factor inhibitors] because melanoma risk increases with suppression of the immune system and TNF-alpha plays an important role in the immune surveillance of tumors.12,13
In their review, the researchers identified seven cohort studies from MEDLINE, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) databases published between January 1995 and February 2019 that evaluated melanoma risk in about 34,000 patients receiving biologics and 135,370 patients who had never been treated with biologics, and were receiving conventional systemic therapy for psoriasis, RA, or IBD. Of these, four studies were in patients with RA, two studies were in patients with IBD, and a single study was in patients with psoriasis. Six studies examined patients taking TNF inhibitors, but only one of six studies had information on specific TNF inhibitors (adalimumab, etanercept, and infliximab) in patients with RA. One study evaluated abatacept and rituximab in RA patients.
The researchers analyzed the pooled relative risk across all studies. Compared with patients who received conventional systemic therapy, there was a nonsignificant association with risk of melanoma in patients with psoriasis (hazard ratio, 1.57; 95% confidence interval, 0.61-4.09), RA (pooled relative risk, 1.20; 95% CI, 0.83-1.74), and IBD (pRR, 1.20; 95% CI, 0.60-2.40).
Among RA patients who received TNF inhibitors only, there was a slightly elevated nonsignificant risk of melanoma (pRR, 1.08; 95% CI, 0.81-1.43). Patients receiving rituximab had a pRR of 0.73 (95% CI, 0.38-1.39), and patients taking abatacept had a pRR of 1.43 (95% CI, 0.66-3.09), compared with RA patients receiving conventional systemic therapy. When excluding two major studies in the RA subgroup of patients in a sensitivity analysis, pooled risk estimates varied from 0.91 (95% CI, 0.69-1.18) to 1.95 (95% CI, 1.16- 3.30). There were no significant between-study heterogeneity or publication bias among the IBD and RA studies.
Mr. Esse and colleagues acknowledged the small number of IBD and psoriasis studies in the meta-analysis, which could affect pooled risk estimates. “Any future update of our study through the inclusion of newly published studies may produce significantly different pooled risk estimates than those reported in our meta-analysis,” they said. In addition, the use of health insurance databases, lack of risk factors for melanoma, and inconsistent information about treatment duration for patients receiving conventional systemic therapy were also limitations.
“Prospective cohort studies using an active comparator, new-user study design providing detailed information on treatment history, concomitant treatments, biologic and conventional systemic treatment duration, recreational and treatment-related UV exposure, skin color, and date of melanoma diagnosis are required to help improve certainty. These studies would also need to account for key risk factors and the latency period of melanoma,” the researchers said.
Mr. Esse disclosed being funded by a PhD studentship from the Psoriasis Association. One author disclosed receiving personal fees from Janssen, LEO Pharma, Lilly, and Novartis outside the study; another disclosed receiving grants and personal fees from those and several other pharmaceutical companies during the study, and personal fees from several pharmaceutical companies outside of the submitted work; the fourth author had no disclosures.
SOURCE: Esse S et al. JAMA Dermatol. 2020 May 20;e201300.
The in a systematic review and meta-analysis published in JAMA Dermatology.
The studies included in the analysis, however, had limitations, including a lack of those comparing biologic and conventional systemic therapy in psoriasis and inflammatory bowel disease (IBD), according to Shamarke Esse, MRes, of the division of musculoskeletal and dermatological sciences at the University of Manchester (England) and colleagues. “We advocate for more large, well-designed studies of this issue to be performed to help improve certainty” regarding this association, they wrote.
Previous studies that have found an increased risk of melanoma in patients on biologics for psoriasis, rheumatoid arthritis, and IBD have “typically used the general population as the comparator,” they noted. There is a large amount of evidence that has established short-term efficacy and safety of biologics, compared with conventional systemic treatments, but concerns about longer-term cancer risk associated with biologics remains a concern. Moreover, they added, “melanoma is a highly immunogenic skin cancer and therefore of concern to patients treated with TNFIs [tumor necrosis factor inhibitors] because melanoma risk increases with suppression of the immune system and TNF-alpha plays an important role in the immune surveillance of tumors.12,13
In their review, the researchers identified seven cohort studies from MEDLINE, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) databases published between January 1995 and February 2019 that evaluated melanoma risk in about 34,000 patients receiving biologics and 135,370 patients who had never been treated with biologics, and were receiving conventional systemic therapy for psoriasis, RA, or IBD. Of these, four studies were in patients with RA, two studies were in patients with IBD, and a single study was in patients with psoriasis. Six studies examined patients taking TNF inhibitors, but only one of six studies had information on specific TNF inhibitors (adalimumab, etanercept, and infliximab) in patients with RA. One study evaluated abatacept and rituximab in RA patients.
The researchers analyzed the pooled relative risk across all studies. Compared with patients who received conventional systemic therapy, there was a nonsignificant association with risk of melanoma in patients with psoriasis (hazard ratio, 1.57; 95% confidence interval, 0.61-4.09), RA (pooled relative risk, 1.20; 95% CI, 0.83-1.74), and IBD (pRR, 1.20; 95% CI, 0.60-2.40).
Among RA patients who received TNF inhibitors only, there was a slightly elevated nonsignificant risk of melanoma (pRR, 1.08; 95% CI, 0.81-1.43). Patients receiving rituximab had a pRR of 0.73 (95% CI, 0.38-1.39), and patients taking abatacept had a pRR of 1.43 (95% CI, 0.66-3.09), compared with RA patients receiving conventional systemic therapy. When excluding two major studies in the RA subgroup of patients in a sensitivity analysis, pooled risk estimates varied from 0.91 (95% CI, 0.69-1.18) to 1.95 (95% CI, 1.16- 3.30). There were no significant between-study heterogeneity or publication bias among the IBD and RA studies.
Mr. Esse and colleagues acknowledged the small number of IBD and psoriasis studies in the meta-analysis, which could affect pooled risk estimates. “Any future update of our study through the inclusion of newly published studies may produce significantly different pooled risk estimates than those reported in our meta-analysis,” they said. In addition, the use of health insurance databases, lack of risk factors for melanoma, and inconsistent information about treatment duration for patients receiving conventional systemic therapy were also limitations.
“Prospective cohort studies using an active comparator, new-user study design providing detailed information on treatment history, concomitant treatments, biologic and conventional systemic treatment duration, recreational and treatment-related UV exposure, skin color, and date of melanoma diagnosis are required to help improve certainty. These studies would also need to account for key risk factors and the latency period of melanoma,” the researchers said.
Mr. Esse disclosed being funded by a PhD studentship from the Psoriasis Association. One author disclosed receiving personal fees from Janssen, LEO Pharma, Lilly, and Novartis outside the study; another disclosed receiving grants and personal fees from those and several other pharmaceutical companies during the study, and personal fees from several pharmaceutical companies outside of the submitted work; the fourth author had no disclosures.
SOURCE: Esse S et al. JAMA Dermatol. 2020 May 20;e201300.
The in a systematic review and meta-analysis published in JAMA Dermatology.
The studies included in the analysis, however, had limitations, including a lack of those comparing biologic and conventional systemic therapy in psoriasis and inflammatory bowel disease (IBD), according to Shamarke Esse, MRes, of the division of musculoskeletal and dermatological sciences at the University of Manchester (England) and colleagues. “We advocate for more large, well-designed studies of this issue to be performed to help improve certainty” regarding this association, they wrote.
Previous studies that have found an increased risk of melanoma in patients on biologics for psoriasis, rheumatoid arthritis, and IBD have “typically used the general population as the comparator,” they noted. There is a large amount of evidence that has established short-term efficacy and safety of biologics, compared with conventional systemic treatments, but concerns about longer-term cancer risk associated with biologics remains a concern. Moreover, they added, “melanoma is a highly immunogenic skin cancer and therefore of concern to patients treated with TNFIs [tumor necrosis factor inhibitors] because melanoma risk increases with suppression of the immune system and TNF-alpha plays an important role in the immune surveillance of tumors.12,13
In their review, the researchers identified seven cohort studies from MEDLINE, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) databases published between January 1995 and February 2019 that evaluated melanoma risk in about 34,000 patients receiving biologics and 135,370 patients who had never been treated with biologics, and were receiving conventional systemic therapy for psoriasis, RA, or IBD. Of these, four studies were in patients with RA, two studies were in patients with IBD, and a single study was in patients with psoriasis. Six studies examined patients taking TNF inhibitors, but only one of six studies had information on specific TNF inhibitors (adalimumab, etanercept, and infliximab) in patients with RA. One study evaluated abatacept and rituximab in RA patients.
The researchers analyzed the pooled relative risk across all studies. Compared with patients who received conventional systemic therapy, there was a nonsignificant association with risk of melanoma in patients with psoriasis (hazard ratio, 1.57; 95% confidence interval, 0.61-4.09), RA (pooled relative risk, 1.20; 95% CI, 0.83-1.74), and IBD (pRR, 1.20; 95% CI, 0.60-2.40).
Among RA patients who received TNF inhibitors only, there was a slightly elevated nonsignificant risk of melanoma (pRR, 1.08; 95% CI, 0.81-1.43). Patients receiving rituximab had a pRR of 0.73 (95% CI, 0.38-1.39), and patients taking abatacept had a pRR of 1.43 (95% CI, 0.66-3.09), compared with RA patients receiving conventional systemic therapy. When excluding two major studies in the RA subgroup of patients in a sensitivity analysis, pooled risk estimates varied from 0.91 (95% CI, 0.69-1.18) to 1.95 (95% CI, 1.16- 3.30). There were no significant between-study heterogeneity or publication bias among the IBD and RA studies.
Mr. Esse and colleagues acknowledged the small number of IBD and psoriasis studies in the meta-analysis, which could affect pooled risk estimates. “Any future update of our study through the inclusion of newly published studies may produce significantly different pooled risk estimates than those reported in our meta-analysis,” they said. In addition, the use of health insurance databases, lack of risk factors for melanoma, and inconsistent information about treatment duration for patients receiving conventional systemic therapy were also limitations.
“Prospective cohort studies using an active comparator, new-user study design providing detailed information on treatment history, concomitant treatments, biologic and conventional systemic treatment duration, recreational and treatment-related UV exposure, skin color, and date of melanoma diagnosis are required to help improve certainty. These studies would also need to account for key risk factors and the latency period of melanoma,” the researchers said.
Mr. Esse disclosed being funded by a PhD studentship from the Psoriasis Association. One author disclosed receiving personal fees from Janssen, LEO Pharma, Lilly, and Novartis outside the study; another disclosed receiving grants and personal fees from those and several other pharmaceutical companies during the study, and personal fees from several pharmaceutical companies outside of the submitted work; the fourth author had no disclosures.
SOURCE: Esse S et al. JAMA Dermatol. 2020 May 20;e201300.
FROM JAMA DERMATOLOGY
Acute rhinosinusitis: When to prescribe an antibiotic
An estimated 30 million cases of acute rhinosinusitis (ARS) occur every year in the United States.1 More than 80% of people with ARS are prescribed antibiotics in North America, accounting for 15% to 20% of all antibiotic prescriptions in the adult outpatient setting.2,3 Many of these prescriptions are unnecessary, as the most common cause of ARS is a virus.4,5 Evidence consistently shows that symptoms of ARS will resolve spontaneously in most patients and that only those patients with severe or prolonged symptoms require consideration of antibiotic therapy.1,2,4,6 Nearly half of all patients will improve within 1 week and two-thirds of patients will improve within 2 weeks without the use of antibiotics.7 In children, only about 6% to 7% presenting with upper respiratory symptoms meet the criteria for acute bacterial rhinosinusitis (ABRS),8 which we’ll detail in a bit. For most patients, treatment should consist of symptom management.5
But what about the minority who require antibiotic therapy? This article reviews how to evaluate patients with ARS, identify those who require antibiotics, and prescribe the most appropriate antibiotic treatment regimens.
Diagnosis: Distinguishing viral from bacterial disease
ARS is defined as the sudden onset of purulent nasal discharge plus either nasal blockage or facial pressure/pain lasting < 4 weeks.3,9 Additional signs and symptoms may include postnasal drip, a reduced sense of smell, sinus tenderness to palpation, and maxillary toothaches.10,11
ARS may be viral or bacterial in etiology, with the most common bacterial organisms being Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis.1,3,5 The most common viral causes are influenza, parainfluenza, and rhinovirus. Approximately 90% to 98% of cases of ARS are viral6,11; only about 0.5% to 2% of viral rhinosinusitis episodes are complicated by bacterial infection.1,10-12
Diagnose ABRS when symptoms of ARS fail to improve after 10 days or symptoms of ARS worsen within 10 days after initial improvement (“double sickening”).1,11 Symptoms that are significantly associated with ABRS are unilateral sinus pain and reported maxillary pain. The presence of facial or dental pain correlates with ABRS but does not identify the specific sinus involved.1
There isn’t good correlation between patients saying they have sinusitis and actually having it.13 A 2019 meta-analysis by Ebell et al14 reported that based on limited data, the overall clinical impression, fetid odor on the breath, and pain in the teeth are the best individual clinical predictors of ABRS.
As recommended by the Infectious Disease Society of America (IDSA), a diagnosis of ABRS is also reasonable in patients who present with severe symptoms at the onset.6 Although there is no consensus about what constitutes “severe symptoms,” they are often described as a temperature ≥ 102°F (39°C) plus 3 to 4 days of purulent nasal drainage.1,4,6
Continue to: Additional symptoms of ABRS may include...
Additional symptoms of ABRS may include cough, fatigue, decreased or lack of sense of smell (hyposmia or anosmia), and ear pressure.10 Another sign of “double sickening” is the development of a fever after several days of symptoms.1,9,15 Viral sinusitis typically lasts 5 to 7 days with a peak at days 2 to 3.1,15 If symptoms continue for 10 days, there is a 60% chance of bacterial sinusitis, although some viral rhinosinusitis symptoms persist for > 14 days.1,5 Beyond 4 to 12 weeks, sinusitis is classified as subacute or chronic.3
Physical exam findings and the limited roles of imaging and labs
Common physical exam findings associated with the diagnosis of ABRS include altered speech indicating nasal obstruction; edema or erythema of the skin indicating congested capillaries; tenderness to palpation over the cheeks or upper teeth; odorous breath; and purulent drainage from the nose or in the posterior pharynx.
In a study by Hansen et al13 (N = 174), the only sign that showed significant association with ABRS (diagnosed by sinus aspiration or lavage) was unilateral tenderness of the maxillary sinuses. The presence of purulent drainage in the nose or posterior pharynx also has significant diagnostic value, as it predicts the presence of bacteria on antral aspiration.1 Purulent discharge in the pharynx is associated with a higher likelihood of benefit from antibiotic therapy compared to placebo (number needed to treat [NNT] = 8).16 However, colored nasal discharge indicates the presence of neutrophils—not bacteria—and does not predict the likelihood of bacterial sinus infection.14,17 Therefore, the history and physical exam should focus on location of pain (sinus and/or teeth), duration of symptoms, presence of fever, change in symptom severity, attempted home therapies, sinus tenderness on exam, breath odor, and purulent drainage seen in the nasal cavity or posterior pharynx.13,14
Radiographic imaging has no role in the diagnosis or treatment of uncomplicated ABRS because viral and bacterial etiologies have similar radiographic appearances. Additionally, employing radiologic imaging would increase health care costs by at least 4-fold.5,6,8,17 The American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) clinical practice guidelines recommend against radiographic imaging for patients who meet the diagnostic criteria for ABRS unless concern exists for a complication or an alternate diagnosis is suspected.1 Computed tomography (CT) imaging of the sinuses may be warranted in patients with severe headaches, facial swelling, cranial nerve palsies, or bulging of the eye (proptosis), all of which indicate a potential complication of ABRS.1
Laboratory evaluations. ABRS is a clinical diagnosis; therefore, routine lab work, such as a white blood cell count, C-reactive protein (CRP) level, and/or erythrocyte sedimentation rate (ESR), are not indicated unless an alternate diagnosis is suspected.1,5,13,18,19
Continue to: In one study...
In one study, CRP > 10 mg/L and ESR > 10 mm/h were the strongest individual predictors of purulent antral puncture aspirate or positive bacterial culture of aspirate, which is considered diagnostic for ABRS. 20 However, CRP and ESR by themselves are not adequate to diagnose ABRS.20 This study developed a clinical decision rule that used symptoms, signs, and laboratory values to rate the likelihood of ABRS as being either low, moderate, or high. However, this clinical decision rule has not been prospectively validated.
Thus, CRP and ESR elevations can support the diagnosis of ABRS, but the low sensitivity of these tests precludes their use as a screening tool for ABRS.14,18 Studies by Ebell19 and Huang21 have shown some benefit to dipstick assay of nasal secretions for the diagnosis of ABRS, but this method is not validated or widely used.19,21
Treatment: From managing symptoms to prescribing antibiotics
Overprescribing antibiotics for ARS is a prominent health care issue. In fact, 5 of 9 placebo-controlled studies showed that most people improve within 2 weeks regardless of antibiotic use (N = 1058).3 Therefore, weigh the decision to treat ABRS with antibiotics against the risk for potential adverse reactions and within the context of antibiotic stewardship.2,9,12,22-24 Consider antibiotics only if patients meet the diagnostic criteria for ABRS (TABLE 11,6) or, occasionally, for patients with severe symptoms upon presentation, such as a temperature ≥ 102°F (39°C) plus purulent nasal discharge for 3 to 4 days.1 The most commonly reported adverse effects of antibiotics are gastrointestinal in nature and include nausea, vomiting, and diarrhea.2,9
Symptomatic management for both ARS and ABRS is recommended as first-line therapy; it should be offered to patients before making a diagnosis of ABRS.1,5,9,25 Consider using analgesics, topical intranasal steroids, and/or nasal saline irrigation to alleviate symptoms and improve quality of life.1,5,25 Interventions with questionable or unproven efficacy include the use of antihistamines, systemic steroids, decongestants, and mucolytics, but they may be considered on an individual basis.1 A systematic review found that topical nasal steroids relieved facial pain and nasal congestion in patients with rhinitis and acute sinusitis (NNT = 14).1,26
Even after diagnosing ABRS, clinicians should offer watchful waiting and symptomatic therapies as long as patients have adequate access to follow-up (TABLE 2,1,15FIGURE1,6). Antibiotic therapy can then be initiated if symptoms do not improve after an additional 7 days of watchful waiting or if symptoms worsen at any time. It is reasonable to give patients a prescription to keep on hand to be used if symptoms worsen, with instructions to notify the provider if antibiotics are started.1
Continue to: Antibiotic therapy
Antibiotic therapy. The rationale for treating ABRS with antibiotics is to expedite recovery and prevent complications such as periorbital or orbital cellulitis, meningitis, frontal osteomyelitis, cavernous sinus thrombosis, and other serious illness.27 Antibiotic treatment is associated with a shorter duration of symptoms (NNT = 19) but an increased risk of adverse events (NNH = 8).7,19
Amoxicillin with or without clavulanate for 5 to 10 days is first-line antibiotic therapy for most adults with ABRS.1,3,5,8,9,11 Per AAO-HNS, the “justification for amoxicillin as first-line treatment relates to its safety, efficacy, low cost, and narrow microbiologic spectrum.”1 Amoxicillin may be dosed 500 mg tid for 5 to 10 days. Amoxicillin/clavulanate (Augmentin) is recommended for patients with comorbid conditions or with increased risk of bacterial resistance. Dosing for amoxicillin/clavulanate is 500/125 mg tid or 875/125 mg bid for 5 to 10 days. Duration of therapy should be determined by the severity of symptoms.5
For penicillin-allergic patients, doxycycline or a respiratory fluoroquinolone (levofloxacin or moxifloxacin) is considered first-line treatment.1,6 Doxycycline is preferred because of its narrower spectrum and fewer adverse effects than the fluoroquinolones. Fluoroquinolones should be reserved for patients who fail first-line treatment and are penicillin allergic.1 Because of the high rates of resistance among S pneumoniae and H influenzae, macrolides, trimethoprim/sulfamethoxazole (TMP/SMX), and cephalosporins are not recommended as first-line therapy.1,5
How antibiotic options compare. A Cochrane review of 54 studies comparing different antibiotics showed no antibiotic was superior.3 Of the 54 studies, 6 studies (N = 1887) were pooled to compare cephalosporins to amoxicillin/clavulanate at 7 to 15 days. The findings indicated a statistically significant difference for amoxicillin/clavulanate with a relative risk (RR) of 1.37 (confidence interval [CI], 1.04-1.8).3 However, none of these 6 studies were graded as having a low risk of bias; therefore, confidence in this finding was deemed limited due to the quality of included studies. The failure rate for cephalosporins was 12% vs 8% for amoxicillin/clavulanate.3
Treatment failure is considered when a patient has not improved by Day 7 after ABRS diagnosis (with or without medication) or when symptoms worsen at any time. If watchful waiting was chosen and a safety net prescription was provided, the antibiotics should be filled and started. If no antibiotic was prescribed at the time watchful waiting commenced, the patient should return for further evaluation and be started on antibiotics. If antibiotics were prescribed initially for severe symptoms, a change in antibiotic therapy is indicated, and a broader-spectrum antibiotic should be chosen. If amoxicillin was prescribed, the patient should be switched to amoxicillin/clavulanate, doxycycline, a respiratory fluoroquinolone, or a combination of clindamycin plus a third-generation cephalosporin.1
Continue to: Diagnosis and management of pediatric patients
Diagnosis and management of pediatric patients
Diagnosis of ABRS in children is defined as an acute upper respiratory infection (URI) accompanied by persistent nasal discharge, daytime cough for ≥ 10 days without improvement, an episode of “double sickening,” or severe onset with a temperature ≥ 102°F and purulent nasal discharge for 3 days.15
Initial presentations of viral URIs and ABRS are almost identical; thus, persistence of symptoms is key to diagnosis.6 Nasal discharge tends to appear several days after initial symptoms manifest for viral infections including influenza. In children < 5 years of age, the most common complication involves the orbit.15 Orbital complications generally manifest with eye pain and/or periorbital swelling and may be accompanied by proptosis or decreased functioning of extraocular musculature. The differential diagnosis for orbital complications includes cavernous sinus thrombosis, orbital cellulitis/abscess, subperiosteal abscess, and inflammatory edema.27,28 Intracranial complications are also possible with severe ABRS.12
Radiology studies are not recommended for the initial diagnosis of ABRS in children, as again, imaging does not differentiate between viral and bacterial etiologies. However, in children with complications such as orbital or cerebral involvement, a contrast-enhanced CT scan of the paranasal sinuses is indicated.15
Antibiotic therapy is indicated in children with a diagnosis of severe ABRS or in cases of “double sickening.” Clinicians may consider watchful waiting for 3 additional days before initiating antibiotics in patients meeting criteria for ABRS.Amoxicillin with or without clavulanate is the antibiotic of choice.15
For penicillin-allergic children without a history of anaphylactoid reaction, treatment with cefpodoxime, cefdinir, or cefuroxime is appropriate. For children with a history of anaphylaxis, treatment with a combination of clindamycin (or linezolid) and cefixime is indicated. Alternatively, a fluoroquinolone such as levofloxacin may be used, but adverse effects and emerging resistance limit its use.15
Continue to: Specialist referral
Specialist referral
Referral to Otolaryngology is indicated for patients with > 3 episodes of clinically diagnosed bacterial sinusitis in 1 year, evidence of fungal disease (which is outside the scope of this article), immunocompromised status, or a persistent temperature ≥ 102°F despite antibiotic therapy. Also consider otolaryngology referral for patients with a history of sinus surgery.2,5,6
CORRESPONDENCE
Pamela R. Hughes, Family Medicine Residency Clinic, Mike O’Callaghan Military Medical Center, 4700 Las Vegas Boulevard North, Nellis AFB, NV 89191; pamela.r.hughes4.mil@mail.mil.
1. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 suppl):S1-S39.
2. Fokkens WJ, Hoffmans R, Thomas M. Avoid prescribing antibiotics in acute rhinosinusitis. BMJ. 2014;349:g5703.
3. Ahovuo-Saloranta A, Rautakorpi UM, Borisenko OV, et al. Antibiotics for acute maxillary sinusitis in adults. Cochrane Database Syst Rev. 2014:CD000243.
4. Burgstaller, JM, Steurer J, Holzmann D, et al. Antibiotic efficacy in patients with a moderate probability of acute rhinosinusitis: a systematic review. Eur Arch Otorhinolaryngol. 2016;273:1067-1077.
5. Aring AM, Chan MM. Current concepts in adult acute rhinosinusitis. Am Fam Physician. 2016;94:97-105.
6. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e72-e112.
7. Lemiengre MB, van Driel ML, Merenstein D, et al. Antibiotics for acute rhinosinusitis in adults. Cochrane Database Syst Rev. 2018:CD006089.
8. Harris AM, Hicks LA, Qaseem A. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164:425-434.
9. Sng WJ, Wang DY. Efficacy and side effects of antibiotics in the treatment of acute rhinosinusitis: a systematic review. Rhinology. 2015;53:3-9.
10. Benninger M, Segreti J. Is it bacterial or viral? Criteria for distinguishing bacterial and viral infections. J Fam Pract. 2008;57(2 suppl):S5-S11.
11. Sharma P, Finley R, Weese S, et al. Antibiotic prescriptions for outpatient acute rhinosinusitis in Canada, 2007-2013. PLoS One. 2017;12:e0181957.
12. Pynnonen MA, Lynn S, Kern HE, et al. Diagnosis and treatment of acute sinusitis in the primary care setting: a retrospective cohort. Laryngoscope. 2015;125:2266-2272.
13. Hansen JG, Schmidt H, Rosborg J, et al. Predicting acute maxillary sinusitis in a general practice population. BMJ 1995;311:233-236.
14. Ebell MH, McKay B, Dale, A, et al. Accuracy of signs and symptoms for the diagnosis of acute rhinosinusitis and acute bacterial rhinosinusitis. Ann Fam Med. 2019;17:164-172.
15. Wald ER, Applegate KE, Bordley C, et al. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics. 2013;132:e262-e280.
16. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
17. Smith SS, Ference EH, Evan CT, et al. The prevalence of bacterial infection in acute rhinosinusitis: a systematic review and meta-analysis. Laryngoscope. 2015;125:57-69.
18. Autio TJ, Koskenkorva T, Koivunen P, et al. Inflammatory biomarkers during bacterial acute rhinosinusitis. Curr Allergy Asthma Rep. 2018;18:13.

19. Ebell MH, McKay B, Guilbault R, et al. Diagnosis of acute rhinosinusitis in primary care: a systematic review of test accuracy. Br J Gen Pract. 2016;66:e612-e632.
20. Ebell MH, Hansen JG. Proposed clinical decision rules to diagnose acute rhinosinusitis among adults in primary care. Ann Fam Med. 2017;15:347-354.
21. Huang SW, Small PA. Rapid diagnosis of bacterial sinusitis in patients using a simple test of nasal secretions. Allergy Asthma Proc. 2008;29:640-643.
22. Smith SS, Evans CT, Tan BK, et al. National burden of antibiotic use for adult rhinosinusitis. J Allergy Clin Immunol. 2013;132.
23. Barlam TF, Soria-Saucedo R, Cabral HJ, et al. Unnecessary antibiotics for acute respiratory tract infections: association with care setting and patient demographics. Open Forum Infect Dis. 2016;3:1-7.
24. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
25. Garbutt JM, Banister C, Spitznagel E, et al. Amoxicillin for acute rhinosinusitis: a randomized controlled trial. JAMA. 2012;307:685-692.
26. Zalmanovici Trestioreanu A, Yaphe J. Intranasal steroids for acute sinusitis. Cochrane Database Syst Rev. 2013:CD005149.
27. Abzug MJ. Acute sinusitis in children: do antibiotics have any role? J Infect. 2014;68 (suppl 1):S33-S37.
28. Williams JW Jr, Simel DL, Roberts L, et al. Clinical evaluation for sinusitis. Making the diagnosis by history and physical examination. Ann Intern Med. 1992;117:705-710.
An estimated 30 million cases of acute rhinosinusitis (ARS) occur every year in the United States.1 More than 80% of people with ARS are prescribed antibiotics in North America, accounting for 15% to 20% of all antibiotic prescriptions in the adult outpatient setting.2,3 Many of these prescriptions are unnecessary, as the most common cause of ARS is a virus.4,5 Evidence consistently shows that symptoms of ARS will resolve spontaneously in most patients and that only those patients with severe or prolonged symptoms require consideration of antibiotic therapy.1,2,4,6 Nearly half of all patients will improve within 1 week and two-thirds of patients will improve within 2 weeks without the use of antibiotics.7 In children, only about 6% to 7% presenting with upper respiratory symptoms meet the criteria for acute bacterial rhinosinusitis (ABRS),8 which we’ll detail in a bit. For most patients, treatment should consist of symptom management.5
But what about the minority who require antibiotic therapy? This article reviews how to evaluate patients with ARS, identify those who require antibiotics, and prescribe the most appropriate antibiotic treatment regimens.
Diagnosis: Distinguishing viral from bacterial disease
ARS is defined as the sudden onset of purulent nasal discharge plus either nasal blockage or facial pressure/pain lasting < 4 weeks.3,9 Additional signs and symptoms may include postnasal drip, a reduced sense of smell, sinus tenderness to palpation, and maxillary toothaches.10,11
ARS may be viral or bacterial in etiology, with the most common bacterial organisms being Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis.1,3,5 The most common viral causes are influenza, parainfluenza, and rhinovirus. Approximately 90% to 98% of cases of ARS are viral6,11; only about 0.5% to 2% of viral rhinosinusitis episodes are complicated by bacterial infection.1,10-12
Diagnose ABRS when symptoms of ARS fail to improve after 10 days or symptoms of ARS worsen within 10 days after initial improvement (“double sickening”).1,11 Symptoms that are significantly associated with ABRS are unilateral sinus pain and reported maxillary pain. The presence of facial or dental pain correlates with ABRS but does not identify the specific sinus involved.1
There isn’t good correlation between patients saying they have sinusitis and actually having it.13 A 2019 meta-analysis by Ebell et al14 reported that based on limited data, the overall clinical impression, fetid odor on the breath, and pain in the teeth are the best individual clinical predictors of ABRS.
As recommended by the Infectious Disease Society of America (IDSA), a diagnosis of ABRS is also reasonable in patients who present with severe symptoms at the onset.6 Although there is no consensus about what constitutes “severe symptoms,” they are often described as a temperature ≥ 102°F (39°C) plus 3 to 4 days of purulent nasal drainage.1,4,6
Continue to: Additional symptoms of ABRS may include...
Additional symptoms of ABRS may include cough, fatigue, decreased or lack of sense of smell (hyposmia or anosmia), and ear pressure.10 Another sign of “double sickening” is the development of a fever after several days of symptoms.1,9,15 Viral sinusitis typically lasts 5 to 7 days with a peak at days 2 to 3.1,15 If symptoms continue for 10 days, there is a 60% chance of bacterial sinusitis, although some viral rhinosinusitis symptoms persist for > 14 days.1,5 Beyond 4 to 12 weeks, sinusitis is classified as subacute or chronic.3
Physical exam findings and the limited roles of imaging and labs
Common physical exam findings associated with the diagnosis of ABRS include altered speech indicating nasal obstruction; edema or erythema of the skin indicating congested capillaries; tenderness to palpation over the cheeks or upper teeth; odorous breath; and purulent drainage from the nose or in the posterior pharynx.
In a study by Hansen et al13 (N = 174), the only sign that showed significant association with ABRS (diagnosed by sinus aspiration or lavage) was unilateral tenderness of the maxillary sinuses. The presence of purulent drainage in the nose or posterior pharynx also has significant diagnostic value, as it predicts the presence of bacteria on antral aspiration.1 Purulent discharge in the pharynx is associated with a higher likelihood of benefit from antibiotic therapy compared to placebo (number needed to treat [NNT] = 8).16 However, colored nasal discharge indicates the presence of neutrophils—not bacteria—and does not predict the likelihood of bacterial sinus infection.14,17 Therefore, the history and physical exam should focus on location of pain (sinus and/or teeth), duration of symptoms, presence of fever, change in symptom severity, attempted home therapies, sinus tenderness on exam, breath odor, and purulent drainage seen in the nasal cavity or posterior pharynx.13,14
Radiographic imaging has no role in the diagnosis or treatment of uncomplicated ABRS because viral and bacterial etiologies have similar radiographic appearances. Additionally, employing radiologic imaging would increase health care costs by at least 4-fold.5,6,8,17 The American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) clinical practice guidelines recommend against radiographic imaging for patients who meet the diagnostic criteria for ABRS unless concern exists for a complication or an alternate diagnosis is suspected.1 Computed tomography (CT) imaging of the sinuses may be warranted in patients with severe headaches, facial swelling, cranial nerve palsies, or bulging of the eye (proptosis), all of which indicate a potential complication of ABRS.1
Laboratory evaluations. ABRS is a clinical diagnosis; therefore, routine lab work, such as a white blood cell count, C-reactive protein (CRP) level, and/or erythrocyte sedimentation rate (ESR), are not indicated unless an alternate diagnosis is suspected.1,5,13,18,19
Continue to: In one study...
In one study, CRP > 10 mg/L and ESR > 10 mm/h were the strongest individual predictors of purulent antral puncture aspirate or positive bacterial culture of aspirate, which is considered diagnostic for ABRS. 20 However, CRP and ESR by themselves are not adequate to diagnose ABRS.20 This study developed a clinical decision rule that used symptoms, signs, and laboratory values to rate the likelihood of ABRS as being either low, moderate, or high. However, this clinical decision rule has not been prospectively validated.
Thus, CRP and ESR elevations can support the diagnosis of ABRS, but the low sensitivity of these tests precludes their use as a screening tool for ABRS.14,18 Studies by Ebell19 and Huang21 have shown some benefit to dipstick assay of nasal secretions for the diagnosis of ABRS, but this method is not validated or widely used.19,21
Treatment: From managing symptoms to prescribing antibiotics
Overprescribing antibiotics for ARS is a prominent health care issue. In fact, 5 of 9 placebo-controlled studies showed that most people improve within 2 weeks regardless of antibiotic use (N = 1058).3 Therefore, weigh the decision to treat ABRS with antibiotics against the risk for potential adverse reactions and within the context of antibiotic stewardship.2,9,12,22-24 Consider antibiotics only if patients meet the diagnostic criteria for ABRS (TABLE 11,6) or, occasionally, for patients with severe symptoms upon presentation, such as a temperature ≥ 102°F (39°C) plus purulent nasal discharge for 3 to 4 days.1 The most commonly reported adverse effects of antibiotics are gastrointestinal in nature and include nausea, vomiting, and diarrhea.2,9
Symptomatic management for both ARS and ABRS is recommended as first-line therapy; it should be offered to patients before making a diagnosis of ABRS.1,5,9,25 Consider using analgesics, topical intranasal steroids, and/or nasal saline irrigation to alleviate symptoms and improve quality of life.1,5,25 Interventions with questionable or unproven efficacy include the use of antihistamines, systemic steroids, decongestants, and mucolytics, but they may be considered on an individual basis.1 A systematic review found that topical nasal steroids relieved facial pain and nasal congestion in patients with rhinitis and acute sinusitis (NNT = 14).1,26
Even after diagnosing ABRS, clinicians should offer watchful waiting and symptomatic therapies as long as patients have adequate access to follow-up (TABLE 2,1,15FIGURE1,6). Antibiotic therapy can then be initiated if symptoms do not improve after an additional 7 days of watchful waiting or if symptoms worsen at any time. It is reasonable to give patients a prescription to keep on hand to be used if symptoms worsen, with instructions to notify the provider if antibiotics are started.1
Continue to: Antibiotic therapy
Antibiotic therapy. The rationale for treating ABRS with antibiotics is to expedite recovery and prevent complications such as periorbital or orbital cellulitis, meningitis, frontal osteomyelitis, cavernous sinus thrombosis, and other serious illness.27 Antibiotic treatment is associated with a shorter duration of symptoms (NNT = 19) but an increased risk of adverse events (NNH = 8).7,19
Amoxicillin with or without clavulanate for 5 to 10 days is first-line antibiotic therapy for most adults with ABRS.1,3,5,8,9,11 Per AAO-HNS, the “justification for amoxicillin as first-line treatment relates to its safety, efficacy, low cost, and narrow microbiologic spectrum.”1 Amoxicillin may be dosed 500 mg tid for 5 to 10 days. Amoxicillin/clavulanate (Augmentin) is recommended for patients with comorbid conditions or with increased risk of bacterial resistance. Dosing for amoxicillin/clavulanate is 500/125 mg tid or 875/125 mg bid for 5 to 10 days. Duration of therapy should be determined by the severity of symptoms.5
For penicillin-allergic patients, doxycycline or a respiratory fluoroquinolone (levofloxacin or moxifloxacin) is considered first-line treatment.1,6 Doxycycline is preferred because of its narrower spectrum and fewer adverse effects than the fluoroquinolones. Fluoroquinolones should be reserved for patients who fail first-line treatment and are penicillin allergic.1 Because of the high rates of resistance among S pneumoniae and H influenzae, macrolides, trimethoprim/sulfamethoxazole (TMP/SMX), and cephalosporins are not recommended as first-line therapy.1,5
How antibiotic options compare. A Cochrane review of 54 studies comparing different antibiotics showed no antibiotic was superior.3 Of the 54 studies, 6 studies (N = 1887) were pooled to compare cephalosporins to amoxicillin/clavulanate at 7 to 15 days. The findings indicated a statistically significant difference for amoxicillin/clavulanate with a relative risk (RR) of 1.37 (confidence interval [CI], 1.04-1.8).3 However, none of these 6 studies were graded as having a low risk of bias; therefore, confidence in this finding was deemed limited due to the quality of included studies. The failure rate for cephalosporins was 12% vs 8% for amoxicillin/clavulanate.3
Treatment failure is considered when a patient has not improved by Day 7 after ABRS diagnosis (with or without medication) or when symptoms worsen at any time. If watchful waiting was chosen and a safety net prescription was provided, the antibiotics should be filled and started. If no antibiotic was prescribed at the time watchful waiting commenced, the patient should return for further evaluation and be started on antibiotics. If antibiotics were prescribed initially for severe symptoms, a change in antibiotic therapy is indicated, and a broader-spectrum antibiotic should be chosen. If amoxicillin was prescribed, the patient should be switched to amoxicillin/clavulanate, doxycycline, a respiratory fluoroquinolone, or a combination of clindamycin plus a third-generation cephalosporin.1
Continue to: Diagnosis and management of pediatric patients
Diagnosis and management of pediatric patients
Diagnosis of ABRS in children is defined as an acute upper respiratory infection (URI) accompanied by persistent nasal discharge, daytime cough for ≥ 10 days without improvement, an episode of “double sickening,” or severe onset with a temperature ≥ 102°F and purulent nasal discharge for 3 days.15
Initial presentations of viral URIs and ABRS are almost identical; thus, persistence of symptoms is key to diagnosis.6 Nasal discharge tends to appear several days after initial symptoms manifest for viral infections including influenza. In children < 5 years of age, the most common complication involves the orbit.15 Orbital complications generally manifest with eye pain and/or periorbital swelling and may be accompanied by proptosis or decreased functioning of extraocular musculature. The differential diagnosis for orbital complications includes cavernous sinus thrombosis, orbital cellulitis/abscess, subperiosteal abscess, and inflammatory edema.27,28 Intracranial complications are also possible with severe ABRS.12
Radiology studies are not recommended for the initial diagnosis of ABRS in children, as again, imaging does not differentiate between viral and bacterial etiologies. However, in children with complications such as orbital or cerebral involvement, a contrast-enhanced CT scan of the paranasal sinuses is indicated.15
Antibiotic therapy is indicated in children with a diagnosis of severe ABRS or in cases of “double sickening.” Clinicians may consider watchful waiting for 3 additional days before initiating antibiotics in patients meeting criteria for ABRS.Amoxicillin with or without clavulanate is the antibiotic of choice.15
For penicillin-allergic children without a history of anaphylactoid reaction, treatment with cefpodoxime, cefdinir, or cefuroxime is appropriate. For children with a history of anaphylaxis, treatment with a combination of clindamycin (or linezolid) and cefixime is indicated. Alternatively, a fluoroquinolone such as levofloxacin may be used, but adverse effects and emerging resistance limit its use.15
Continue to: Specialist referral
Specialist referral
Referral to Otolaryngology is indicated for patients with > 3 episodes of clinically diagnosed bacterial sinusitis in 1 year, evidence of fungal disease (which is outside the scope of this article), immunocompromised status, or a persistent temperature ≥ 102°F despite antibiotic therapy. Also consider otolaryngology referral for patients with a history of sinus surgery.2,5,6
CORRESPONDENCE
Pamela R. Hughes, Family Medicine Residency Clinic, Mike O’Callaghan Military Medical Center, 4700 Las Vegas Boulevard North, Nellis AFB, NV 89191; pamela.r.hughes4.mil@mail.mil.
An estimated 30 million cases of acute rhinosinusitis (ARS) occur every year in the United States.1 More than 80% of people with ARS are prescribed antibiotics in North America, accounting for 15% to 20% of all antibiotic prescriptions in the adult outpatient setting.2,3 Many of these prescriptions are unnecessary, as the most common cause of ARS is a virus.4,5 Evidence consistently shows that symptoms of ARS will resolve spontaneously in most patients and that only those patients with severe or prolonged symptoms require consideration of antibiotic therapy.1,2,4,6 Nearly half of all patients will improve within 1 week and two-thirds of patients will improve within 2 weeks without the use of antibiotics.7 In children, only about 6% to 7% presenting with upper respiratory symptoms meet the criteria for acute bacterial rhinosinusitis (ABRS),8 which we’ll detail in a bit. For most patients, treatment should consist of symptom management.5
But what about the minority who require antibiotic therapy? This article reviews how to evaluate patients with ARS, identify those who require antibiotics, and prescribe the most appropriate antibiotic treatment regimens.
Diagnosis: Distinguishing viral from bacterial disease
ARS is defined as the sudden onset of purulent nasal discharge plus either nasal blockage or facial pressure/pain lasting < 4 weeks.3,9 Additional signs and symptoms may include postnasal drip, a reduced sense of smell, sinus tenderness to palpation, and maxillary toothaches.10,11
ARS may be viral or bacterial in etiology, with the most common bacterial organisms being Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis.1,3,5 The most common viral causes are influenza, parainfluenza, and rhinovirus. Approximately 90% to 98% of cases of ARS are viral6,11; only about 0.5% to 2% of viral rhinosinusitis episodes are complicated by bacterial infection.1,10-12
Diagnose ABRS when symptoms of ARS fail to improve after 10 days or symptoms of ARS worsen within 10 days after initial improvement (“double sickening”).1,11 Symptoms that are significantly associated with ABRS are unilateral sinus pain and reported maxillary pain. The presence of facial or dental pain correlates with ABRS but does not identify the specific sinus involved.1
There isn’t good correlation between patients saying they have sinusitis and actually having it.13 A 2019 meta-analysis by Ebell et al14 reported that based on limited data, the overall clinical impression, fetid odor on the breath, and pain in the teeth are the best individual clinical predictors of ABRS.
As recommended by the Infectious Disease Society of America (IDSA), a diagnosis of ABRS is also reasonable in patients who present with severe symptoms at the onset.6 Although there is no consensus about what constitutes “severe symptoms,” they are often described as a temperature ≥ 102°F (39°C) plus 3 to 4 days of purulent nasal drainage.1,4,6
Continue to: Additional symptoms of ABRS may include...
Additional symptoms of ABRS may include cough, fatigue, decreased or lack of sense of smell (hyposmia or anosmia), and ear pressure.10 Another sign of “double sickening” is the development of a fever after several days of symptoms.1,9,15 Viral sinusitis typically lasts 5 to 7 days with a peak at days 2 to 3.1,15 If symptoms continue for 10 days, there is a 60% chance of bacterial sinusitis, although some viral rhinosinusitis symptoms persist for > 14 days.1,5 Beyond 4 to 12 weeks, sinusitis is classified as subacute or chronic.3
Physical exam findings and the limited roles of imaging and labs
Common physical exam findings associated with the diagnosis of ABRS include altered speech indicating nasal obstruction; edema or erythema of the skin indicating congested capillaries; tenderness to palpation over the cheeks or upper teeth; odorous breath; and purulent drainage from the nose or in the posterior pharynx.
In a study by Hansen et al13 (N = 174), the only sign that showed significant association with ABRS (diagnosed by sinus aspiration or lavage) was unilateral tenderness of the maxillary sinuses. The presence of purulent drainage in the nose or posterior pharynx also has significant diagnostic value, as it predicts the presence of bacteria on antral aspiration.1 Purulent discharge in the pharynx is associated with a higher likelihood of benefit from antibiotic therapy compared to placebo (number needed to treat [NNT] = 8).16 However, colored nasal discharge indicates the presence of neutrophils—not bacteria—and does not predict the likelihood of bacterial sinus infection.14,17 Therefore, the history and physical exam should focus on location of pain (sinus and/or teeth), duration of symptoms, presence of fever, change in symptom severity, attempted home therapies, sinus tenderness on exam, breath odor, and purulent drainage seen in the nasal cavity or posterior pharynx.13,14
Radiographic imaging has no role in the diagnosis or treatment of uncomplicated ABRS because viral and bacterial etiologies have similar radiographic appearances. Additionally, employing radiologic imaging would increase health care costs by at least 4-fold.5,6,8,17 The American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) clinical practice guidelines recommend against radiographic imaging for patients who meet the diagnostic criteria for ABRS unless concern exists for a complication or an alternate diagnosis is suspected.1 Computed tomography (CT) imaging of the sinuses may be warranted in patients with severe headaches, facial swelling, cranial nerve palsies, or bulging of the eye (proptosis), all of which indicate a potential complication of ABRS.1
Laboratory evaluations. ABRS is a clinical diagnosis; therefore, routine lab work, such as a white blood cell count, C-reactive protein (CRP) level, and/or erythrocyte sedimentation rate (ESR), are not indicated unless an alternate diagnosis is suspected.1,5,13,18,19
Continue to: In one study...
In one study, CRP > 10 mg/L and ESR > 10 mm/h were the strongest individual predictors of purulent antral puncture aspirate or positive bacterial culture of aspirate, which is considered diagnostic for ABRS. 20 However, CRP and ESR by themselves are not adequate to diagnose ABRS.20 This study developed a clinical decision rule that used symptoms, signs, and laboratory values to rate the likelihood of ABRS as being either low, moderate, or high. However, this clinical decision rule has not been prospectively validated.
Thus, CRP and ESR elevations can support the diagnosis of ABRS, but the low sensitivity of these tests precludes their use as a screening tool for ABRS.14,18 Studies by Ebell19 and Huang21 have shown some benefit to dipstick assay of nasal secretions for the diagnosis of ABRS, but this method is not validated or widely used.19,21
Treatment: From managing symptoms to prescribing antibiotics
Overprescribing antibiotics for ARS is a prominent health care issue. In fact, 5 of 9 placebo-controlled studies showed that most people improve within 2 weeks regardless of antibiotic use (N = 1058).3 Therefore, weigh the decision to treat ABRS with antibiotics against the risk for potential adverse reactions and within the context of antibiotic stewardship.2,9,12,22-24 Consider antibiotics only if patients meet the diagnostic criteria for ABRS (TABLE 11,6) or, occasionally, for patients with severe symptoms upon presentation, such as a temperature ≥ 102°F (39°C) plus purulent nasal discharge for 3 to 4 days.1 The most commonly reported adverse effects of antibiotics are gastrointestinal in nature and include nausea, vomiting, and diarrhea.2,9
Symptomatic management for both ARS and ABRS is recommended as first-line therapy; it should be offered to patients before making a diagnosis of ABRS.1,5,9,25 Consider using analgesics, topical intranasal steroids, and/or nasal saline irrigation to alleviate symptoms and improve quality of life.1,5,25 Interventions with questionable or unproven efficacy include the use of antihistamines, systemic steroids, decongestants, and mucolytics, but they may be considered on an individual basis.1 A systematic review found that topical nasal steroids relieved facial pain and nasal congestion in patients with rhinitis and acute sinusitis (NNT = 14).1,26
Even after diagnosing ABRS, clinicians should offer watchful waiting and symptomatic therapies as long as patients have adequate access to follow-up (TABLE 2,1,15FIGURE1,6). Antibiotic therapy can then be initiated if symptoms do not improve after an additional 7 days of watchful waiting or if symptoms worsen at any time. It is reasonable to give patients a prescription to keep on hand to be used if symptoms worsen, with instructions to notify the provider if antibiotics are started.1
Continue to: Antibiotic therapy
Antibiotic therapy. The rationale for treating ABRS with antibiotics is to expedite recovery and prevent complications such as periorbital or orbital cellulitis, meningitis, frontal osteomyelitis, cavernous sinus thrombosis, and other serious illness.27 Antibiotic treatment is associated with a shorter duration of symptoms (NNT = 19) but an increased risk of adverse events (NNH = 8).7,19
Amoxicillin with or without clavulanate for 5 to 10 days is first-line antibiotic therapy for most adults with ABRS.1,3,5,8,9,11 Per AAO-HNS, the “justification for amoxicillin as first-line treatment relates to its safety, efficacy, low cost, and narrow microbiologic spectrum.”1 Amoxicillin may be dosed 500 mg tid for 5 to 10 days. Amoxicillin/clavulanate (Augmentin) is recommended for patients with comorbid conditions or with increased risk of bacterial resistance. Dosing for amoxicillin/clavulanate is 500/125 mg tid or 875/125 mg bid for 5 to 10 days. Duration of therapy should be determined by the severity of symptoms.5
For penicillin-allergic patients, doxycycline or a respiratory fluoroquinolone (levofloxacin or moxifloxacin) is considered first-line treatment.1,6 Doxycycline is preferred because of its narrower spectrum and fewer adverse effects than the fluoroquinolones. Fluoroquinolones should be reserved for patients who fail first-line treatment and are penicillin allergic.1 Because of the high rates of resistance among S pneumoniae and H influenzae, macrolides, trimethoprim/sulfamethoxazole (TMP/SMX), and cephalosporins are not recommended as first-line therapy.1,5
How antibiotic options compare. A Cochrane review of 54 studies comparing different antibiotics showed no antibiotic was superior.3 Of the 54 studies, 6 studies (N = 1887) were pooled to compare cephalosporins to amoxicillin/clavulanate at 7 to 15 days. The findings indicated a statistically significant difference for amoxicillin/clavulanate with a relative risk (RR) of 1.37 (confidence interval [CI], 1.04-1.8).3 However, none of these 6 studies were graded as having a low risk of bias; therefore, confidence in this finding was deemed limited due to the quality of included studies. The failure rate for cephalosporins was 12% vs 8% for amoxicillin/clavulanate.3
Treatment failure is considered when a patient has not improved by Day 7 after ABRS diagnosis (with or without medication) or when symptoms worsen at any time. If watchful waiting was chosen and a safety net prescription was provided, the antibiotics should be filled and started. If no antibiotic was prescribed at the time watchful waiting commenced, the patient should return for further evaluation and be started on antibiotics. If antibiotics were prescribed initially for severe symptoms, a change in antibiotic therapy is indicated, and a broader-spectrum antibiotic should be chosen. If amoxicillin was prescribed, the patient should be switched to amoxicillin/clavulanate, doxycycline, a respiratory fluoroquinolone, or a combination of clindamycin plus a third-generation cephalosporin.1
Continue to: Diagnosis and management of pediatric patients
Diagnosis and management of pediatric patients
Diagnosis of ABRS in children is defined as an acute upper respiratory infection (URI) accompanied by persistent nasal discharge, daytime cough for ≥ 10 days without improvement, an episode of “double sickening,” or severe onset with a temperature ≥ 102°F and purulent nasal discharge for 3 days.15
Initial presentations of viral URIs and ABRS are almost identical; thus, persistence of symptoms is key to diagnosis.6 Nasal discharge tends to appear several days after initial symptoms manifest for viral infections including influenza. In children < 5 years of age, the most common complication involves the orbit.15 Orbital complications generally manifest with eye pain and/or periorbital swelling and may be accompanied by proptosis or decreased functioning of extraocular musculature. The differential diagnosis for orbital complications includes cavernous sinus thrombosis, orbital cellulitis/abscess, subperiosteal abscess, and inflammatory edema.27,28 Intracranial complications are also possible with severe ABRS.12
Radiology studies are not recommended for the initial diagnosis of ABRS in children, as again, imaging does not differentiate between viral and bacterial etiologies. However, in children with complications such as orbital or cerebral involvement, a contrast-enhanced CT scan of the paranasal sinuses is indicated.15
Antibiotic therapy is indicated in children with a diagnosis of severe ABRS or in cases of “double sickening.” Clinicians may consider watchful waiting for 3 additional days before initiating antibiotics in patients meeting criteria for ABRS.Amoxicillin with or without clavulanate is the antibiotic of choice.15
For penicillin-allergic children without a history of anaphylactoid reaction, treatment with cefpodoxime, cefdinir, or cefuroxime is appropriate. For children with a history of anaphylaxis, treatment with a combination of clindamycin (or linezolid) and cefixime is indicated. Alternatively, a fluoroquinolone such as levofloxacin may be used, but adverse effects and emerging resistance limit its use.15
Continue to: Specialist referral
Specialist referral
Referral to Otolaryngology is indicated for patients with > 3 episodes of clinically diagnosed bacterial sinusitis in 1 year, evidence of fungal disease (which is outside the scope of this article), immunocompromised status, or a persistent temperature ≥ 102°F despite antibiotic therapy. Also consider otolaryngology referral for patients with a history of sinus surgery.2,5,6
CORRESPONDENCE
Pamela R. Hughes, Family Medicine Residency Clinic, Mike O’Callaghan Military Medical Center, 4700 Las Vegas Boulevard North, Nellis AFB, NV 89191; pamela.r.hughes4.mil@mail.mil.
1. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 suppl):S1-S39.
2. Fokkens WJ, Hoffmans R, Thomas M. Avoid prescribing antibiotics in acute rhinosinusitis. BMJ. 2014;349:g5703.
3. Ahovuo-Saloranta A, Rautakorpi UM, Borisenko OV, et al. Antibiotics for acute maxillary sinusitis in adults. Cochrane Database Syst Rev. 2014:CD000243.
4. Burgstaller, JM, Steurer J, Holzmann D, et al. Antibiotic efficacy in patients with a moderate probability of acute rhinosinusitis: a systematic review. Eur Arch Otorhinolaryngol. 2016;273:1067-1077.
5. Aring AM, Chan MM. Current concepts in adult acute rhinosinusitis. Am Fam Physician. 2016;94:97-105.
6. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e72-e112.
7. Lemiengre MB, van Driel ML, Merenstein D, et al. Antibiotics for acute rhinosinusitis in adults. Cochrane Database Syst Rev. 2018:CD006089.
8. Harris AM, Hicks LA, Qaseem A. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164:425-434.
9. Sng WJ, Wang DY. Efficacy and side effects of antibiotics in the treatment of acute rhinosinusitis: a systematic review. Rhinology. 2015;53:3-9.
10. Benninger M, Segreti J. Is it bacterial or viral? Criteria for distinguishing bacterial and viral infections. J Fam Pract. 2008;57(2 suppl):S5-S11.
11. Sharma P, Finley R, Weese S, et al. Antibiotic prescriptions for outpatient acute rhinosinusitis in Canada, 2007-2013. PLoS One. 2017;12:e0181957.
12. Pynnonen MA, Lynn S, Kern HE, et al. Diagnosis and treatment of acute sinusitis in the primary care setting: a retrospective cohort. Laryngoscope. 2015;125:2266-2272.
13. Hansen JG, Schmidt H, Rosborg J, et al. Predicting acute maxillary sinusitis in a general practice population. BMJ 1995;311:233-236.
14. Ebell MH, McKay B, Dale, A, et al. Accuracy of signs and symptoms for the diagnosis of acute rhinosinusitis and acute bacterial rhinosinusitis. Ann Fam Med. 2019;17:164-172.
15. Wald ER, Applegate KE, Bordley C, et al. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics. 2013;132:e262-e280.
16. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
17. Smith SS, Ference EH, Evan CT, et al. The prevalence of bacterial infection in acute rhinosinusitis: a systematic review and meta-analysis. Laryngoscope. 2015;125:57-69.
18. Autio TJ, Koskenkorva T, Koivunen P, et al. Inflammatory biomarkers during bacterial acute rhinosinusitis. Curr Allergy Asthma Rep. 2018;18:13.

19. Ebell MH, McKay B, Guilbault R, et al. Diagnosis of acute rhinosinusitis in primary care: a systematic review of test accuracy. Br J Gen Pract. 2016;66:e612-e632.
20. Ebell MH, Hansen JG. Proposed clinical decision rules to diagnose acute rhinosinusitis among adults in primary care. Ann Fam Med. 2017;15:347-354.
21. Huang SW, Small PA. Rapid diagnosis of bacterial sinusitis in patients using a simple test of nasal secretions. Allergy Asthma Proc. 2008;29:640-643.
22. Smith SS, Evans CT, Tan BK, et al. National burden of antibiotic use for adult rhinosinusitis. J Allergy Clin Immunol. 2013;132.
23. Barlam TF, Soria-Saucedo R, Cabral HJ, et al. Unnecessary antibiotics for acute respiratory tract infections: association with care setting and patient demographics. Open Forum Infect Dis. 2016;3:1-7.
24. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
25. Garbutt JM, Banister C, Spitznagel E, et al. Amoxicillin for acute rhinosinusitis: a randomized controlled trial. JAMA. 2012;307:685-692.
26. Zalmanovici Trestioreanu A, Yaphe J. Intranasal steroids for acute sinusitis. Cochrane Database Syst Rev. 2013:CD005149.
27. Abzug MJ. Acute sinusitis in children: do antibiotics have any role? J Infect. 2014;68 (suppl 1):S33-S37.
28. Williams JW Jr, Simel DL, Roberts L, et al. Clinical evaluation for sinusitis. Making the diagnosis by history and physical examination. Ann Intern Med. 1992;117:705-710.
1. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 suppl):S1-S39.
2. Fokkens WJ, Hoffmans R, Thomas M. Avoid prescribing antibiotics in acute rhinosinusitis. BMJ. 2014;349:g5703.
3. Ahovuo-Saloranta A, Rautakorpi UM, Borisenko OV, et al. Antibiotics for acute maxillary sinusitis in adults. Cochrane Database Syst Rev. 2014:CD000243.
4. Burgstaller, JM, Steurer J, Holzmann D, et al. Antibiotic efficacy in patients with a moderate probability of acute rhinosinusitis: a systematic review. Eur Arch Otorhinolaryngol. 2016;273:1067-1077.
5. Aring AM, Chan MM. Current concepts in adult acute rhinosinusitis. Am Fam Physician. 2016;94:97-105.
6. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e72-e112.
7. Lemiengre MB, van Driel ML, Merenstein D, et al. Antibiotics for acute rhinosinusitis in adults. Cochrane Database Syst Rev. 2018:CD006089.
8. Harris AM, Hicks LA, Qaseem A. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164:425-434.
9. Sng WJ, Wang DY. Efficacy and side effects of antibiotics in the treatment of acute rhinosinusitis: a systematic review. Rhinology. 2015;53:3-9.
10. Benninger M, Segreti J. Is it bacterial or viral? Criteria for distinguishing bacterial and viral infections. J Fam Pract. 2008;57(2 suppl):S5-S11.
11. Sharma P, Finley R, Weese S, et al. Antibiotic prescriptions for outpatient acute rhinosinusitis in Canada, 2007-2013. PLoS One. 2017;12:e0181957.
12. Pynnonen MA, Lynn S, Kern HE, et al. Diagnosis and treatment of acute sinusitis in the primary care setting: a retrospective cohort. Laryngoscope. 2015;125:2266-2272.
13. Hansen JG, Schmidt H, Rosborg J, et al. Predicting acute maxillary sinusitis in a general practice population. BMJ 1995;311:233-236.
14. Ebell MH, McKay B, Dale, A, et al. Accuracy of signs and symptoms for the diagnosis of acute rhinosinusitis and acute bacterial rhinosinusitis. Ann Fam Med. 2019;17:164-172.
15. Wald ER, Applegate KE, Bordley C, et al. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics. 2013;132:e262-e280.
16. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
17. Smith SS, Ference EH, Evan CT, et al. The prevalence of bacterial infection in acute rhinosinusitis: a systematic review and meta-analysis. Laryngoscope. 2015;125:57-69.
18. Autio TJ, Koskenkorva T, Koivunen P, et al. Inflammatory biomarkers during bacterial acute rhinosinusitis. Curr Allergy Asthma Rep. 2018;18:13.

19. Ebell MH, McKay B, Guilbault R, et al. Diagnosis of acute rhinosinusitis in primary care: a systematic review of test accuracy. Br J Gen Pract. 2016;66:e612-e632.
20. Ebell MH, Hansen JG. Proposed clinical decision rules to diagnose acute rhinosinusitis among adults in primary care. Ann Fam Med. 2017;15:347-354.
21. Huang SW, Small PA. Rapid diagnosis of bacterial sinusitis in patients using a simple test of nasal secretions. Allergy Asthma Proc. 2008;29:640-643.
22. Smith SS, Evans CT, Tan BK, et al. National burden of antibiotic use for adult rhinosinusitis. J Allergy Clin Immunol. 2013;132.
23. Barlam TF, Soria-Saucedo R, Cabral HJ, et al. Unnecessary antibiotics for acute respiratory tract infections: association with care setting and patient demographics. Open Forum Infect Dis. 2016;3:1-7.
24. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
25. Garbutt JM, Banister C, Spitznagel E, et al. Amoxicillin for acute rhinosinusitis: a randomized controlled trial. JAMA. 2012;307:685-692.
26. Zalmanovici Trestioreanu A, Yaphe J. Intranasal steroids for acute sinusitis. Cochrane Database Syst Rev. 2013:CD005149.
27. Abzug MJ. Acute sinusitis in children: do antibiotics have any role? J Infect. 2014;68 (suppl 1):S33-S37.
28. Williams JW Jr, Simel DL, Roberts L, et al. Clinical evaluation for sinusitis. Making the diagnosis by history and physical examination. Ann Intern Med. 1992;117:705-710.
PRACTICE RECOMMENDATIONS
› Reserve antibiotics for patients who meet diagnostic criteria for acute bacterial rhinosinusitis (ABRS). Patients must have purulent nasal drainage that is accompanied by either nasal obstruction or facial pain/pressure/fullness and EITHER symptoms that persist without improvement for at least 10 days OR symptoms that worsen within 10 days of initial improvement (“double sickening”). A
› Offer watchful waiting and delay antibiotics for up to 7 days after diagnosing ABRS in a patient if adequate access to follow-up is available; otherwise, treat with amoxicillin (with or without clavulanate) for 5 to 10 days. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Passing on the “FastPass”
As part of the COVID-19 pandemic, I see signs everywhere saying they have perks for health care workers. I can go to the front of the line at Costco, or for takeout at a restaurant, or to checkout at the grocery store. Certainly it would be easy, I always have my hospital ID in my car.
I have no interest in doing so. None.
As I’ve previously written, I’m in the back seat right now. For me to take out my hospital ID and grandstand to get in front of the line is not only a lie, but takes away from someone – a nurse, a paramedic, another doctor, whatever – who actually is on the front line of the pandemic and may be in a hurry to get home or back to work.
Me? I may be a doctor, but certainly not part of fighting the pandemic (unless you count wearing a mask and washing my hands frequently as such). I’m here for anyone who needs a neurologist, and my office is open, but that’s always been my normal day at work. I’m not at the hospital, or a screening center, or urgent care.
To me it seems pretty hypocritical, or at least inappropriate, for me to take advantage of a “FastPass” (as Disneyland calls it) when I’m really not one of the people it is intended for.
Perhaps it’s a minor point, but I have three kids, and part of raising them is leading by example. Don’t take something that isn’t yours.
Which is what it would feel like to me.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
As part of the COVID-19 pandemic, I see signs everywhere saying they have perks for health care workers. I can go to the front of the line at Costco, or for takeout at a restaurant, or to checkout at the grocery store. Certainly it would be easy, I always have my hospital ID in my car.
I have no interest in doing so. None.
As I’ve previously written, I’m in the back seat right now. For me to take out my hospital ID and grandstand to get in front of the line is not only a lie, but takes away from someone – a nurse, a paramedic, another doctor, whatever – who actually is on the front line of the pandemic and may be in a hurry to get home or back to work.
Me? I may be a doctor, but certainly not part of fighting the pandemic (unless you count wearing a mask and washing my hands frequently as such). I’m here for anyone who needs a neurologist, and my office is open, but that’s always been my normal day at work. I’m not at the hospital, or a screening center, or urgent care.
To me it seems pretty hypocritical, or at least inappropriate, for me to take advantage of a “FastPass” (as Disneyland calls it) when I’m really not one of the people it is intended for.
Perhaps it’s a minor point, but I have three kids, and part of raising them is leading by example. Don’t take something that isn’t yours.
Which is what it would feel like to me.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
As part of the COVID-19 pandemic, I see signs everywhere saying they have perks for health care workers. I can go to the front of the line at Costco, or for takeout at a restaurant, or to checkout at the grocery store. Certainly it would be easy, I always have my hospital ID in my car.
I have no interest in doing so. None.
As I’ve previously written, I’m in the back seat right now. For me to take out my hospital ID and grandstand to get in front of the line is not only a lie, but takes away from someone – a nurse, a paramedic, another doctor, whatever – who actually is on the front line of the pandemic and may be in a hurry to get home or back to work.
Me? I may be a doctor, but certainly not part of fighting the pandemic (unless you count wearing a mask and washing my hands frequently as such). I’m here for anyone who needs a neurologist, and my office is open, but that’s always been my normal day at work. I’m not at the hospital, or a screening center, or urgent care.
To me it seems pretty hypocritical, or at least inappropriate, for me to take advantage of a “FastPass” (as Disneyland calls it) when I’m really not one of the people it is intended for.
Perhaps it’s a minor point, but I have three kids, and part of raising them is leading by example. Don’t take something that isn’t yours.
Which is what it would feel like to me.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Managing a woman with BRCA mutations? Shared decision-making is key
CASE
Sara T* recently moved back to the area to be closer to her family. The 34-year-old patient visited our office to discuss the benefits and potential risks of genetic counseling. She explained that her aunt had just died at age 64 of ovarian cancer. Also, her maternal cousin had been diagnosed at age 42 with breast cancer, and her maternal grandmother had died at age 45 of an unknown “female cancer.” She was scared to find out if she had high-risk genes because she felt it would change her life forever. However, if she ignored the issue, she thought she might worry too much.
We discussed the implications of a positive result, such as having to live with the knowledge and to make decisions about potential screening and risk-reducing surgery. On the other hand, not knowing could allow for the undetected growth of cancer that might otherwise be mitigated to some degree if she knew her risk status and pursued an aggressive screening program.
We worked with Ms. T to map out her next steps.
*The patient’s name has been changed to protect her identity.
Breast cancer is the most commonly diagnosed cancer in women worldwide, representing nearly one-quarter of all female cancer diagnoses in 2018.1 It is the second-leading cause of cancer death in women in developed nations and the leading cause of cancer death in women in developing nations.1 In the United States, 1 in 8 women will develop breast cancer in her lifetime.2 By comparison, the rate of ovarian cancer is much lower, with a lifetime prevalence of 1 in 70 to 80 women.3,4 Although ovarian cancer is less common than breast cancer, its associated mortality is high, and most cases are discovered at advanced stages.
The outsized threat of BRCA mutations. It is estimated that 5% to 10% of all breast cancers are hereditary, with 80% of these attributable to BRCA1 (45%) and BRCA2 (35%).5 These autosomal dominant mutations occur at the germline level, within the egg or sperm, and are therefore incorporated into the DNA of every cell and passed from one generation to the next. Families with BRCA mutations have much higher lifetime rates of cancer. The lifetime risk of breast cancer due to BRCA mutations is estimated at > 80% (BRCA1) and 45% (BRCA2).5BRCA mutations account for between
Male BRCA carriers have a lifetime breast cancer risk of 1% to 5% with BRCA1 and 5% to 10% with BRCA2,7,8 compared with about 1:1000 lifetime incidence in the unselected male population. Male carriers are also at risk for more aggressive prostate cancers.7,8
Continue to: Certain populatiosn carry undue burden of BRCA-related disease
Certain populations carry undue burden of BRCA-related disease due to specific founder mutations. While the estimated global prevalence of BRCA mutations is 0.2% to 1%, for those of Ashkenazi Jewish descent the range is 2% to 3%, representing a relative risk up to 15 times that of the general population.9 Hispanic Americans also appear to have higher rates of BRCA-related cancers.10 Ongoing genetics research continues to identify founder mutations worldwide,10 which may inform future screening guidelines.
In addition to BRCA mutations, there are other, less common mutations (TABLE 15) known to cause hereditary breast and ovarian cancer.
Identifying BRCA genes enables treatment planning. Compared with sporadic cancers, BRCA-related breast cancers
Similarly, BRCA-related ovarian cancer is more likely to be high-grade and endometrioid or serous subtype.14
Shared decision-making helps give clarity to the way forward
Shared decision-making is a process of communication whereby the clinician and the patient identify a decision to be made, review data relevant to clinical options, discuss patient perspectives and preferences regarding each option, and arrive at the decision together.17 Shared decision-making is important when treating women with BRCA mutations because there is no single correct plan. Individual values and competing medical issues may strongly guide each woman’s decisions about screening and cancer prevention treatment decisions.
Continue to: Shared decision-making in this situation...
Shared decision-making in this situation is a strategy to use the evidence of risk along with patient preferences around fertility issues to help come to a decision that is the right one for the patient. Primary care clinicians aware of the general risks and benefits of each available option can refer women at high risk for breast or ovarian cancer to a specialist multidisciplinary clinic that can provide tailored risk assessment and risk reduction counseling as needed.18-20
Genetic screening recommendations
Screening is recommended for women who have any 1 of several family risk factors (TABLE 221). A number of risk assessment tools are available for primary care clinicians to determine which patients are at high enough risk for a hereditary breast or ovarian cancer to warrant referral to a genetic counselor.22-25 If screening suggests high risk, the US Preventive Services Task Force (USPSTF) recommends (Grade B) referral for genetic counseling.21
Explain to patients who are candidates for further investigation that a genetic counselor will review their family history and recommend testing for the specific mutations that increase cancer risk. Discuss potential benefits and harms of genetic testing. A benefit of genetic testing is that aggressive screening may suggest preventive procedures to reduce the risk of future cancer. Most tests come back definitively positive or negative, but an indeterminate result may cause harm. A small minority of results may indicate a genetic variant of unknown significance. The ramifications of this variant may not be known. Some women will experience anxiety about nonspecific test results and will be afraid to share them with family members. There is also some concern about privacy issues, potential insurance bias, and coverage of any preventive strategies.26
CASE
Based on Ms. T’s family history and her desire to know more, we referred her to a genetic counselor and she decided to undergo genetic testing. She screened positive for BRCA1. Ms. T was in a serious relationship and thought she would like to have children at some point. She returned to our office after receiving the positive genetic test results, wondering about screening for breast and ovarian cancer.
Breast cancer screening and risk-reduction strategies
Screening. Because the risk of breast cancer is high in women with BRCA mutations, and because cancer in these women is more likely to be advanced at diagnosis, starting a screening program at an early age is prudent. Observational studies suggest that breastfeeding reduces the risk of breast and ovarian cancer in women with BRCA mutations, as it does for women in the general population.27 Women should return for a clinical breast exam every 6 to 12 months starting at age 25; they should start radiologic screening with magnetic resonance imaging at age 25 and mammography at age 30 (TABLE 327,28).
Continue to: Risk-reduction strategies
Risk-reduction strategies. There is weak evidence to support the use of tamoxifen or other synthetic estrogen reuptake modulators (SERMs) to reduce breast cancer risk in women with BRCA mutations. Many of these cancers do not express estrogen receptors, which may explain the lack of efficacy in certain cases. Several observational studies have shown that tamoxifen can reduce the risk of contralateral breast cancer in women with BRCA mutations who have already been diagnosed with cancer in the other breast.29-31 However, tamoxifen does not reduce a patient’s risk of ovarian cancer, and it may increase her risk of uterine cancer.
Prophylactic bilateral mastectomy is the mainstay of breast cancer prevention in this population. Data from a systematic review suggest that this surgery may prevent the incidence of breast cancer in women with BRCA mutations by 90% to 95%.32 However, this review did not demonstrate a reduction in mortality from breast cancer, likely due to poor data quality.32 The National Comprehensive Cancer Network (NCCN) recommends discussing prophylactic mastectomy with all women who have BRCA mutations.28 Further conversations are important to review the risk of tissue left behind and quality-of-life issues, including the inability to breastfeed if the woman wants more children and the cosmetic changes with reconstruction.
Ovarian cancer screening and risk-reduction strategies
Screening. No effective screening strategy has been endorsed for ovarian cancer, as most previous studies have shown screening to be ineffective.26,33 Recently, studies both in the United Kingdom and the United States have investigated a screening strategy using the risk-of-ovarian-cancer algorithm (ROCA), which calculates an individual’s risk based on serum levels of cancer antigen 125 (CA-125).34,35 These studies measured CA-125 levels every 3 to 4 months followed by transvaginal ultrasound if CA-125 increased substantially (as determined by ROCA). Absent an abnormal increase in CA-125, transvaginal ultrasound was performed annually. These screening strategies showed improved specificity over annual screening programs, and the cancers detected were more likely to be diagnosed at an early stage (stage II vs stage III) and had higher rates of zero residual disease after surgery compared with those detected 1 year after screening ended.34,35 However, survival data are not yet available. More research is needed to determine if more frequent screening approaches could improve survival in high-risk women.
NCCN and the American College of Obstetricians and Gynecologists (ACOG) do not endorse routine screening with transvaginal ultrasound and serum CA-125 for high-risk women, as the benefits are uncertain. However, they do advise that these screens may be considered as a short-term strategy for women ages 30 to 35 who defer risk-reducing surgery.26,36 The USPSTF does not make a recommendation regarding ovarian cancer screening in high-risk women.37
Risk-reduction strategies. Risk-reducing bilateral salpingo-oophorectomy (RRSO) is the only recommended technique for reducing the risk of ovarian cancer in women at high risk.26,33,36 Meta-analyses have shown an 80% reduction in ovarian cancer risk16 and 68% reduction in all-cause mortality with this approach.38 The NCCN recommends RRSO for women with a known BRCA1 mutation between the ages of 35 and 40 who have completed childbearing.36 Since the onset of ovarian cancer tends to be later in women with BRCA2 mutations, it is reasonable to delay RRSO until age 40 to 45 in this population if they have taken other steps to maximize breast cancer prevention (ie, bilateral mastectomy).36
Continue to: Adverse effects of RRSO...
Adverse effects of RRSO include surgery complications (wound infection, small bowel obstruction, bladder perforation) and effects of early menopause (vasomotor symptoms, decreased sexual functioning, and increased risk of osteoporosis, cardiovascular disease, and all-cause mortality).39-41 In the absence of contraindications, ACOG recommends using hormone therapy in women undergoing RRSO until the natural age of menopause,42 particularly if their breast tissue has been removed.
Salpingectomy as an alternative. In an attempt to reduce these adverse effects of early menopause, and because a large proportion of high-grade serous tumors originate in the fallopian tube,43 interest has increased in the use of risk-reducing salpingectomy (removal of fallopian tubes) and delayed oophorectomy in women at high risk of ovarian cancer.42 Studies have shown this may be a cost-effective approach and an acceptable alternative in BRCA mutation carriers who are unwilling to undergo RRSO.44,45 A clinical trial investigating this approach in women with BRCA mutations is currently underway in the United States.46 Many centers offer salpingectomy to high-risk patients < 40 years old, understanding that ovary removal is an eventuality for these patients.
When oral contraceptive pills might be beneficial. In younger women with BRCA mutations, there may also be a role for oral contraceptive pills (OCPs) as a risk-reducing strategy. Meta-analyses have shown an approximately 50% reduction in the risk of ovarian cancer among women with BRCA mutations who use OCPs.47-49
ACOG advises that it is appropriate for women with BRCA mutations to use oral contraceptives if indicated (for pregnancy prevention or menstrual cycle regulation), and that it is reasonable to use them for cancer prevention.26 NCCN does not make a formal recommendation, although it does state OCPs may reduce the risk of ovarian cancer in women with a BRCA mutation.36 Case-control studies have produced conflicting data on the association between OCP use and breast cancer risk in BRCA mutation carriers,50-53 although 2 meta-analyses found no significant association in this population.47,48
Decision aids for women with BRCA mutations
Decision aids are visual displays of risk that help patients work through complex decisions. Most decision aids are in print or digital format and include information about the decision to be made as well as pictorial examples of possible outcomes. Pictographs are especially helpful in communicating information. Some decision aids for women with BRCA mutations can be complicated with multiple outcomes (ie, breast cancer and ovarian cancer) and multiple potential interventions (risk-reducing surgery, enhanced screening options).54
Continue to: A Cochrane review...
A Cochrane review found that decision aids increased patients’ knowledge, helped patients clarify their values, and may improve value-concordant decisions.55 Two papers describing the use of decision aids for women with BRCA mutations56,57 documented decreased decisional conflict and increased satisfaction.
CASE
Ms. T underwent the recommended mammogram and MRI screening for breast cancer, as well as testing with serum CA-125 and ultrasound examinations for ovarian cancer. Her initial mammogram and MRI revealed early stage, triple-negative right breast cancer. She chose to undergo bilateral mastectomy and reconstruction. She has now completed treatment and continues to work closely with her oncology team for appropriate breast follow-up.
One year after her initial diagnosis, at the age of 35, she returned to discuss fertility. She was recently married, and she and her husband wanted to start having children. She was concerned about a safe timeline for her to pursue pregnancy, saying she felt “like a ticking time-bomb” given her prior cancer and carrier status. She wanted to discuss the risks and benefits of pregnancy and when she should consider prophylactic oophorectomy. She had a few options. She could have a baby and then undergo an RRSO, or she could talk to her gynecologist about having a salpingectomy to reduce her risk now and use assisted reproductive technology to get pregnant. She could also freeze eggs or embryos, have an RRSO, and then use a surrogate to get pregnant. We informed her that pregnancy would not affect her risk of ovarian cancer and discussed the options for pre-implantation genetic testing to assure that her children would not carry the genetic mutation.58
We provided Ms. T and her husband with a decision aid to help them navigate the decision. They are currently evaluating the options and said they would let us know when they made a decision.
CORRESPONDENCE
Sarina Schrager, MD, MS, Northeast Family Medicine Center, 3209 Dryden Drive, Madison, WI, 53704; sbschrag@wisc.edu.
1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953.
2. SEER Cancer Statistics Review, 1975-2016. Cancer of the female breast. [Table 4.1] National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_04_breast.pdf. Accessed May 27, 2020.
3. SEER Cancer Statistics Review, 1975-2016. Cancer of the ovary. [Table 21.10] National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_21_ovary.pdf. Accessed May 22, 2020.
4. Torre LA, Trabert B, DeSantis C, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284-296.
5. Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4:665-676.
6. Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807-2816.
7. Tai YC, Domchek S, Parmigiani G, et al. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:1811-1814.
8. Evans DG, Susnerwala I, Dawson J, et al. Risk of breast cancer in male BRCA2 carriers. J Med Genet. 2010;47:710-711.
9. CDC. Jewish women and BRCA gene mutations. www.cdc.gov/cancer/breast/young_women/bringyourbrave/hereditary_breast_cancer/jewish_women_brca.htm. Accessed May 22, 2020.
10. Rebbeck TR, Friebel TM, Friedman E, et al. Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum Mutat. 2018;39:593-620.
11. Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330.
12. Wang YA, Jian JW, Hung CF, et al. Germline breast cancer susceptibility gene mutations and breast cancer outcomes. BMC Cancer. 2018;18:315.
13. Baretta Z, Mocellin S, Goldin E, et al. Effect of BRCA germline mutations on breast cancer prognosis: a systematic review and meta-analysis. Medicine. 2016;95:e4975.
14. Lakhani SR, Manek S, Penault-Llorca F, et al. Pathology of ovarian cancers in BRCA1 and BRCA2 carriers. Clin Cancer Res. 2004;10:2473-2481.
15. Kurian AW. BRCA1 and BRCA2 mutations across race and ethnicity: distribution and clinical implications. Curr Opin Obstet Gynecol. 2010;22:72-78.
16. Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80-87.
17. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Int Med. 2012;27:1361-1367.
18. Ardern-Jones A, Eeles R. Developments in clinical practice: follow up clinic for BRCA mutation carriers: a case study highlighting the “virtual clinic.” Hered Cancer Clin Pract. 2004;2:77-79.
19. Yerushalmi R, Rizel S, Zoref D, et al. A dedicated follow-up clinic for BRCA mutation carriers. Isr Med Assoc J. 2016;18:549-552.
20. Pichert G, Jacobs C, Jacobs I, et al. Novel one-stop multidisciplinary follow-up clinic significantly improves cancer risk management in BRCA1/2 carriers. Fam Cancer. 2010;9:313-319.
21. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:652-665.
22. Evans D, Eccles D, Rahman N, et al. A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J Med Genet. 2004;41:474-480.
23. Bellcross CA, Lemke AA, Pape LS, et al. Evaluation of a breast/ovarian cancer genetics referral screening tool in a mammography population. Genet Med. 2009;11:783-789.
24. Hoskins KF, Zwaagstra A, Ranz M. Validation of a tool for identifying women at high risk for hereditary breast cancer in population based screening. Cancer. 2006;107:1769-1776.
25. Gilpin CA, Carson N, Hunter AG. A preliminary validation of a family history assessment form to select women at risk for breast or ovarian cancer for referral to a genetics center. Clin Genet. 2000;58:299-308.
26. Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No 182: Hereditary Breast and Ovarian Cancer Syndrome. Obstet Gynecol. 2017;130:e110-e126.
27. Paluch-Shimon S, Cardoso F, Sessa C, et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol. 2016;27(suppl 5):v103-v110.
28. National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian. 2019. NCCN Clinical Practice Guidelines in Oncology. www2.tri-kobe.org/nccn/guideline/gynecological/english/genetic_familial.pdf. Accessed May 22, 2020.
29. Phillips KA, Milne RL, Rookus MA, et al. Tamoxifen and risk of contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2013;31:3091-3099.
30. Foulkes WD, Goffin J, Brunet JS, et al. Tamoxifen may be an effective adjuvant treatment for BRCA1-related breast cancer irrespective of estrogen receptor status. J Natl Cancer Inst. 2002;94:1504-1506.
31. Gronwald J, Tung N, Foulkes WD, et al. Tamoxifen and contralateral breast cancer in BRCA1 and BRCA2 carriers: an update. Int J Cancer. 2006;118:2281-2284.
32. Ludwig KK, Neuner J, Butler A, et al. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am J Surgery. 2016;212:660-669.
33. Bougie O, Weberpals JI. Clinical considerations of BRCA1- and BRCA2-mutation carriers: a review. Int J Surg Oncol. 2011;2011:374012.
34. Rosenthal AN, Fraser LSM, Philpott S, et al. Evidence of stage shift in women diagnosed with ovarian cancer during phase II of the United Kingdom Familial Ovarian Cancer Screening Study. J Clin Oncol. 2017;35:1411-1420.
35. Skates SJ, Greene MH, Buys SS, et al. Early detection of ovarian cancer using the Risk of Ovarian Cancer Algorithm with frequent CA125 testing in women at increased familial risk—combined results from two screening trials. Clin Cancer Res. 2017;23:3628-3637.
36. Daly MB, Pilarski R, Berry M, et al. NCCN guidelines insights: genetic/familial high-risk assessment: breast and ovarian, version 2.2017. J Natl Compr Canc Netw. 2017;15:9-20.
37. Grossman DC, Curry SJ, Owens DK, et al. Screening for ovarian cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:588-594.
38. Marchetti C, De Felice F, Palaia I, et al. Risk-reducing salpingo-oophorectomy: a meta-analysis on impact on ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2 mutation carriers. BMC Womens Health. 2014;14:150.
39. Nelson HD, Pappas M, Zakher B, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: a systematic review to update the US Preventive Services Task Force recommendation. Ann Intern Med. 2014;160:255-266.
40. Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet Gynecol. 2013;121:709-716.
41. Faubion SS, Kuhle CL, Shuster LT, et al. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18:483-491.
42. Menon U, Karpinskyj C, Gentry-Maharaj A. Ovarian cancer prevention and screening. Obstet Gynecol. 2018;131:909-927.
43. Crum CP, Drapkin R, Miron A, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3-9.
44. Kwon JS, Tinker A, Pansegrau G, et al. Prophylactic salpingectomy and delayed oophorectomy as an alternative for BRCA mutation carriers. Obstet Gynecol. 2013;121:14-24.
45. Holman LL, Friedman S, Daniels MS, et al. Acceptability of prophylactic salpingectomy with delayed oophorectomy as risk-reducing surgery among BRCA mutation carriers. Gynecol Oncol. 2014;133:283-286.
46. MD Anderson Cancer Center. Prophylactic salpingectomy with delayed oophorectomy, risk-reducing salpingo-oophorectomy, and ovarian cancer screening among BRCA mutation carriers: a proof-of-concept study. www.mdanderson.org/patients-family/diagnosis-treatment/clinical-trials/clinical-trials-index/clinical-trials-detail.ID2013-0340.html. Accessed May 22, 2020.
47. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275-2284.
48. Moorman PG, Havrilesky LJ, Gierisch JM, et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J Clin Oncol. 2013;31:4188-4198.
49. Friebel TM, Domchek SM, Rebbeck TR. Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju091.
50. Haile RW, Thomas DC, McGuire V, et al. BRCA1 and BRCA2 mutation carriers, oral contraceptive use, and breast cancer before age 50. Cancer Epidemiol Biomarkers Prev. 2006;15:1863-1870.
51. Lee E, Ma H, McKean-Cowdin R, et al. Effect of reproductive factors and oral contraceptives on breast cancer risk in BRCA1/2 mutation carriers and noncarriers: results from a population-based study. Cancer Epidemiol Biomarkers Prev. 2008;17:3170-3178.
52. Narod SA, Dubé MP, Klijn J, et al. Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2002;94:1773-1779.
53. Milne RL, Knight JA, John EM, et al. Oral contraceptive use and risk of early-onset breast cancer in carriers and noncarriers of BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev. 2005;14:350-356.
54. Culver JO, MacDonald DJ, Thornton AA, et al. Development and evaluation of a decision aid for BRCA carriers with breast cancer. J Genet Couns. 2011;20:294-307.
55. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Syst Rev. 2017;4:CD001431.
56. Schwartz MD, Valdimarsdottir HB, DeMarco TA, et al. Randomized trial of a decision aid for BRCA1/BRCA2 mutation carriers: impact on measures of decision making and satisfaction. Health Psychol. 2009;28:11-19.
57. Metcalfe KA, Dennis CL, Poll A, et al. Effect of decision aid for breast cancer prevention on decisional conflict in women with a BRCA1 or BRCA2 mutation: a multisite, randomized, controlled trial. Gen Med. 2017;19:330-336.
58. Friedman LC, Kramer RM. Reproductive issues for women with BRCA mutations. J Natl Cancer Inst Monogr. 2005;34:83-86.
CASE
Sara T* recently moved back to the area to be closer to her family. The 34-year-old patient visited our office to discuss the benefits and potential risks of genetic counseling. She explained that her aunt had just died at age 64 of ovarian cancer. Also, her maternal cousin had been diagnosed at age 42 with breast cancer, and her maternal grandmother had died at age 45 of an unknown “female cancer.” She was scared to find out if she had high-risk genes because she felt it would change her life forever. However, if she ignored the issue, she thought she might worry too much.
We discussed the implications of a positive result, such as having to live with the knowledge and to make decisions about potential screening and risk-reducing surgery. On the other hand, not knowing could allow for the undetected growth of cancer that might otherwise be mitigated to some degree if she knew her risk status and pursued an aggressive screening program.
We worked with Ms. T to map out her next steps.
*The patient’s name has been changed to protect her identity.
Breast cancer is the most commonly diagnosed cancer in women worldwide, representing nearly one-quarter of all female cancer diagnoses in 2018.1 It is the second-leading cause of cancer death in women in developed nations and the leading cause of cancer death in women in developing nations.1 In the United States, 1 in 8 women will develop breast cancer in her lifetime.2 By comparison, the rate of ovarian cancer is much lower, with a lifetime prevalence of 1 in 70 to 80 women.3,4 Although ovarian cancer is less common than breast cancer, its associated mortality is high, and most cases are discovered at advanced stages.
The outsized threat of BRCA mutations. It is estimated that 5% to 10% of all breast cancers are hereditary, with 80% of these attributable to BRCA1 (45%) and BRCA2 (35%).5 These autosomal dominant mutations occur at the germline level, within the egg or sperm, and are therefore incorporated into the DNA of every cell and passed from one generation to the next. Families with BRCA mutations have much higher lifetime rates of cancer. The lifetime risk of breast cancer due to BRCA mutations is estimated at > 80% (BRCA1) and 45% (BRCA2).5BRCA mutations account for between
Male BRCA carriers have a lifetime breast cancer risk of 1% to 5% with BRCA1 and 5% to 10% with BRCA2,7,8 compared with about 1:1000 lifetime incidence in the unselected male population. Male carriers are also at risk for more aggressive prostate cancers.7,8
Continue to: Certain populatiosn carry undue burden of BRCA-related disease
Certain populations carry undue burden of BRCA-related disease due to specific founder mutations. While the estimated global prevalence of BRCA mutations is 0.2% to 1%, for those of Ashkenazi Jewish descent the range is 2% to 3%, representing a relative risk up to 15 times that of the general population.9 Hispanic Americans also appear to have higher rates of BRCA-related cancers.10 Ongoing genetics research continues to identify founder mutations worldwide,10 which may inform future screening guidelines.
In addition to BRCA mutations, there are other, less common mutations (TABLE 15) known to cause hereditary breast and ovarian cancer.
Identifying BRCA genes enables treatment planning. Compared with sporadic cancers, BRCA-related breast cancers
Similarly, BRCA-related ovarian cancer is more likely to be high-grade and endometrioid or serous subtype.14
Shared decision-making helps give clarity to the way forward
Shared decision-making is a process of communication whereby the clinician and the patient identify a decision to be made, review data relevant to clinical options, discuss patient perspectives and preferences regarding each option, and arrive at the decision together.17 Shared decision-making is important when treating women with BRCA mutations because there is no single correct plan. Individual values and competing medical issues may strongly guide each woman’s decisions about screening and cancer prevention treatment decisions.
Continue to: Shared decision-making in this situation...
Shared decision-making in this situation is a strategy to use the evidence of risk along with patient preferences around fertility issues to help come to a decision that is the right one for the patient. Primary care clinicians aware of the general risks and benefits of each available option can refer women at high risk for breast or ovarian cancer to a specialist multidisciplinary clinic that can provide tailored risk assessment and risk reduction counseling as needed.18-20
Genetic screening recommendations
Screening is recommended for women who have any 1 of several family risk factors (TABLE 221). A number of risk assessment tools are available for primary care clinicians to determine which patients are at high enough risk for a hereditary breast or ovarian cancer to warrant referral to a genetic counselor.22-25 If screening suggests high risk, the US Preventive Services Task Force (USPSTF) recommends (Grade B) referral for genetic counseling.21
Explain to patients who are candidates for further investigation that a genetic counselor will review their family history and recommend testing for the specific mutations that increase cancer risk. Discuss potential benefits and harms of genetic testing. A benefit of genetic testing is that aggressive screening may suggest preventive procedures to reduce the risk of future cancer. Most tests come back definitively positive or negative, but an indeterminate result may cause harm. A small minority of results may indicate a genetic variant of unknown significance. The ramifications of this variant may not be known. Some women will experience anxiety about nonspecific test results and will be afraid to share them with family members. There is also some concern about privacy issues, potential insurance bias, and coverage of any preventive strategies.26
CASE
Based on Ms. T’s family history and her desire to know more, we referred her to a genetic counselor and she decided to undergo genetic testing. She screened positive for BRCA1. Ms. T was in a serious relationship and thought she would like to have children at some point. She returned to our office after receiving the positive genetic test results, wondering about screening for breast and ovarian cancer.
Breast cancer screening and risk-reduction strategies
Screening. Because the risk of breast cancer is high in women with BRCA mutations, and because cancer in these women is more likely to be advanced at diagnosis, starting a screening program at an early age is prudent. Observational studies suggest that breastfeeding reduces the risk of breast and ovarian cancer in women with BRCA mutations, as it does for women in the general population.27 Women should return for a clinical breast exam every 6 to 12 months starting at age 25; they should start radiologic screening with magnetic resonance imaging at age 25 and mammography at age 30 (TABLE 327,28).
Continue to: Risk-reduction strategies
Risk-reduction strategies. There is weak evidence to support the use of tamoxifen or other synthetic estrogen reuptake modulators (SERMs) to reduce breast cancer risk in women with BRCA mutations. Many of these cancers do not express estrogen receptors, which may explain the lack of efficacy in certain cases. Several observational studies have shown that tamoxifen can reduce the risk of contralateral breast cancer in women with BRCA mutations who have already been diagnosed with cancer in the other breast.29-31 However, tamoxifen does not reduce a patient’s risk of ovarian cancer, and it may increase her risk of uterine cancer.
Prophylactic bilateral mastectomy is the mainstay of breast cancer prevention in this population. Data from a systematic review suggest that this surgery may prevent the incidence of breast cancer in women with BRCA mutations by 90% to 95%.32 However, this review did not demonstrate a reduction in mortality from breast cancer, likely due to poor data quality.32 The National Comprehensive Cancer Network (NCCN) recommends discussing prophylactic mastectomy with all women who have BRCA mutations.28 Further conversations are important to review the risk of tissue left behind and quality-of-life issues, including the inability to breastfeed if the woman wants more children and the cosmetic changes with reconstruction.
Ovarian cancer screening and risk-reduction strategies
Screening. No effective screening strategy has been endorsed for ovarian cancer, as most previous studies have shown screening to be ineffective.26,33 Recently, studies both in the United Kingdom and the United States have investigated a screening strategy using the risk-of-ovarian-cancer algorithm (ROCA), which calculates an individual’s risk based on serum levels of cancer antigen 125 (CA-125).34,35 These studies measured CA-125 levels every 3 to 4 months followed by transvaginal ultrasound if CA-125 increased substantially (as determined by ROCA). Absent an abnormal increase in CA-125, transvaginal ultrasound was performed annually. These screening strategies showed improved specificity over annual screening programs, and the cancers detected were more likely to be diagnosed at an early stage (stage II vs stage III) and had higher rates of zero residual disease after surgery compared with those detected 1 year after screening ended.34,35 However, survival data are not yet available. More research is needed to determine if more frequent screening approaches could improve survival in high-risk women.
NCCN and the American College of Obstetricians and Gynecologists (ACOG) do not endorse routine screening with transvaginal ultrasound and serum CA-125 for high-risk women, as the benefits are uncertain. However, they do advise that these screens may be considered as a short-term strategy for women ages 30 to 35 who defer risk-reducing surgery.26,36 The USPSTF does not make a recommendation regarding ovarian cancer screening in high-risk women.37
Risk-reduction strategies. Risk-reducing bilateral salpingo-oophorectomy (RRSO) is the only recommended technique for reducing the risk of ovarian cancer in women at high risk.26,33,36 Meta-analyses have shown an 80% reduction in ovarian cancer risk16 and 68% reduction in all-cause mortality with this approach.38 The NCCN recommends RRSO for women with a known BRCA1 mutation between the ages of 35 and 40 who have completed childbearing.36 Since the onset of ovarian cancer tends to be later in women with BRCA2 mutations, it is reasonable to delay RRSO until age 40 to 45 in this population if they have taken other steps to maximize breast cancer prevention (ie, bilateral mastectomy).36
Continue to: Adverse effects of RRSO...
Adverse effects of RRSO include surgery complications (wound infection, small bowel obstruction, bladder perforation) and effects of early menopause (vasomotor symptoms, decreased sexual functioning, and increased risk of osteoporosis, cardiovascular disease, and all-cause mortality).39-41 In the absence of contraindications, ACOG recommends using hormone therapy in women undergoing RRSO until the natural age of menopause,42 particularly if their breast tissue has been removed.
Salpingectomy as an alternative. In an attempt to reduce these adverse effects of early menopause, and because a large proportion of high-grade serous tumors originate in the fallopian tube,43 interest has increased in the use of risk-reducing salpingectomy (removal of fallopian tubes) and delayed oophorectomy in women at high risk of ovarian cancer.42 Studies have shown this may be a cost-effective approach and an acceptable alternative in BRCA mutation carriers who are unwilling to undergo RRSO.44,45 A clinical trial investigating this approach in women with BRCA mutations is currently underway in the United States.46 Many centers offer salpingectomy to high-risk patients < 40 years old, understanding that ovary removal is an eventuality for these patients.
When oral contraceptive pills might be beneficial. In younger women with BRCA mutations, there may also be a role for oral contraceptive pills (OCPs) as a risk-reducing strategy. Meta-analyses have shown an approximately 50% reduction in the risk of ovarian cancer among women with BRCA mutations who use OCPs.47-49
ACOG advises that it is appropriate for women with BRCA mutations to use oral contraceptives if indicated (for pregnancy prevention or menstrual cycle regulation), and that it is reasonable to use them for cancer prevention.26 NCCN does not make a formal recommendation, although it does state OCPs may reduce the risk of ovarian cancer in women with a BRCA mutation.36 Case-control studies have produced conflicting data on the association between OCP use and breast cancer risk in BRCA mutation carriers,50-53 although 2 meta-analyses found no significant association in this population.47,48
Decision aids for women with BRCA mutations
Decision aids are visual displays of risk that help patients work through complex decisions. Most decision aids are in print or digital format and include information about the decision to be made as well as pictorial examples of possible outcomes. Pictographs are especially helpful in communicating information. Some decision aids for women with BRCA mutations can be complicated with multiple outcomes (ie, breast cancer and ovarian cancer) and multiple potential interventions (risk-reducing surgery, enhanced screening options).54
Continue to: A Cochrane review...
A Cochrane review found that decision aids increased patients’ knowledge, helped patients clarify their values, and may improve value-concordant decisions.55 Two papers describing the use of decision aids for women with BRCA mutations56,57 documented decreased decisional conflict and increased satisfaction.
CASE
Ms. T underwent the recommended mammogram and MRI screening for breast cancer, as well as testing with serum CA-125 and ultrasound examinations for ovarian cancer. Her initial mammogram and MRI revealed early stage, triple-negative right breast cancer. She chose to undergo bilateral mastectomy and reconstruction. She has now completed treatment and continues to work closely with her oncology team for appropriate breast follow-up.
One year after her initial diagnosis, at the age of 35, she returned to discuss fertility. She was recently married, and she and her husband wanted to start having children. She was concerned about a safe timeline for her to pursue pregnancy, saying she felt “like a ticking time-bomb” given her prior cancer and carrier status. She wanted to discuss the risks and benefits of pregnancy and when she should consider prophylactic oophorectomy. She had a few options. She could have a baby and then undergo an RRSO, or she could talk to her gynecologist about having a salpingectomy to reduce her risk now and use assisted reproductive technology to get pregnant. She could also freeze eggs or embryos, have an RRSO, and then use a surrogate to get pregnant. We informed her that pregnancy would not affect her risk of ovarian cancer and discussed the options for pre-implantation genetic testing to assure that her children would not carry the genetic mutation.58
We provided Ms. T and her husband with a decision aid to help them navigate the decision. They are currently evaluating the options and said they would let us know when they made a decision.
CORRESPONDENCE
Sarina Schrager, MD, MS, Northeast Family Medicine Center, 3209 Dryden Drive, Madison, WI, 53704; sbschrag@wisc.edu.
CASE
Sara T* recently moved back to the area to be closer to her family. The 34-year-old patient visited our office to discuss the benefits and potential risks of genetic counseling. She explained that her aunt had just died at age 64 of ovarian cancer. Also, her maternal cousin had been diagnosed at age 42 with breast cancer, and her maternal grandmother had died at age 45 of an unknown “female cancer.” She was scared to find out if she had high-risk genes because she felt it would change her life forever. However, if she ignored the issue, she thought she might worry too much.
We discussed the implications of a positive result, such as having to live with the knowledge and to make decisions about potential screening and risk-reducing surgery. On the other hand, not knowing could allow for the undetected growth of cancer that might otherwise be mitigated to some degree if she knew her risk status and pursued an aggressive screening program.
We worked with Ms. T to map out her next steps.
*The patient’s name has been changed to protect her identity.
Breast cancer is the most commonly diagnosed cancer in women worldwide, representing nearly one-quarter of all female cancer diagnoses in 2018.1 It is the second-leading cause of cancer death in women in developed nations and the leading cause of cancer death in women in developing nations.1 In the United States, 1 in 8 women will develop breast cancer in her lifetime.2 By comparison, the rate of ovarian cancer is much lower, with a lifetime prevalence of 1 in 70 to 80 women.3,4 Although ovarian cancer is less common than breast cancer, its associated mortality is high, and most cases are discovered at advanced stages.
The outsized threat of BRCA mutations. It is estimated that 5% to 10% of all breast cancers are hereditary, with 80% of these attributable to BRCA1 (45%) and BRCA2 (35%).5 These autosomal dominant mutations occur at the germline level, within the egg or sperm, and are therefore incorporated into the DNA of every cell and passed from one generation to the next. Families with BRCA mutations have much higher lifetime rates of cancer. The lifetime risk of breast cancer due to BRCA mutations is estimated at > 80% (BRCA1) and 45% (BRCA2).5BRCA mutations account for between
Male BRCA carriers have a lifetime breast cancer risk of 1% to 5% with BRCA1 and 5% to 10% with BRCA2,7,8 compared with about 1:1000 lifetime incidence in the unselected male population. Male carriers are also at risk for more aggressive prostate cancers.7,8
Continue to: Certain populatiosn carry undue burden of BRCA-related disease
Certain populations carry undue burden of BRCA-related disease due to specific founder mutations. While the estimated global prevalence of BRCA mutations is 0.2% to 1%, for those of Ashkenazi Jewish descent the range is 2% to 3%, representing a relative risk up to 15 times that of the general population.9 Hispanic Americans also appear to have higher rates of BRCA-related cancers.10 Ongoing genetics research continues to identify founder mutations worldwide,10 which may inform future screening guidelines.
In addition to BRCA mutations, there are other, less common mutations (TABLE 15) known to cause hereditary breast and ovarian cancer.
Identifying BRCA genes enables treatment planning. Compared with sporadic cancers, BRCA-related breast cancers
Similarly, BRCA-related ovarian cancer is more likely to be high-grade and endometrioid or serous subtype.14
Shared decision-making helps give clarity to the way forward
Shared decision-making is a process of communication whereby the clinician and the patient identify a decision to be made, review data relevant to clinical options, discuss patient perspectives and preferences regarding each option, and arrive at the decision together.17 Shared decision-making is important when treating women with BRCA mutations because there is no single correct plan. Individual values and competing medical issues may strongly guide each woman’s decisions about screening and cancer prevention treatment decisions.
Continue to: Shared decision-making in this situation...
Shared decision-making in this situation is a strategy to use the evidence of risk along with patient preferences around fertility issues to help come to a decision that is the right one for the patient. Primary care clinicians aware of the general risks and benefits of each available option can refer women at high risk for breast or ovarian cancer to a specialist multidisciplinary clinic that can provide tailored risk assessment and risk reduction counseling as needed.18-20
Genetic screening recommendations
Screening is recommended for women who have any 1 of several family risk factors (TABLE 221). A number of risk assessment tools are available for primary care clinicians to determine which patients are at high enough risk for a hereditary breast or ovarian cancer to warrant referral to a genetic counselor.22-25 If screening suggests high risk, the US Preventive Services Task Force (USPSTF) recommends (Grade B) referral for genetic counseling.21
Explain to patients who are candidates for further investigation that a genetic counselor will review their family history and recommend testing for the specific mutations that increase cancer risk. Discuss potential benefits and harms of genetic testing. A benefit of genetic testing is that aggressive screening may suggest preventive procedures to reduce the risk of future cancer. Most tests come back definitively positive or negative, but an indeterminate result may cause harm. A small minority of results may indicate a genetic variant of unknown significance. The ramifications of this variant may not be known. Some women will experience anxiety about nonspecific test results and will be afraid to share them with family members. There is also some concern about privacy issues, potential insurance bias, and coverage of any preventive strategies.26
CASE
Based on Ms. T’s family history and her desire to know more, we referred her to a genetic counselor and she decided to undergo genetic testing. She screened positive for BRCA1. Ms. T was in a serious relationship and thought she would like to have children at some point. She returned to our office after receiving the positive genetic test results, wondering about screening for breast and ovarian cancer.
Breast cancer screening and risk-reduction strategies
Screening. Because the risk of breast cancer is high in women with BRCA mutations, and because cancer in these women is more likely to be advanced at diagnosis, starting a screening program at an early age is prudent. Observational studies suggest that breastfeeding reduces the risk of breast and ovarian cancer in women with BRCA mutations, as it does for women in the general population.27 Women should return for a clinical breast exam every 6 to 12 months starting at age 25; they should start radiologic screening with magnetic resonance imaging at age 25 and mammography at age 30 (TABLE 327,28).
Continue to: Risk-reduction strategies
Risk-reduction strategies. There is weak evidence to support the use of tamoxifen or other synthetic estrogen reuptake modulators (SERMs) to reduce breast cancer risk in women with BRCA mutations. Many of these cancers do not express estrogen receptors, which may explain the lack of efficacy in certain cases. Several observational studies have shown that tamoxifen can reduce the risk of contralateral breast cancer in women with BRCA mutations who have already been diagnosed with cancer in the other breast.29-31 However, tamoxifen does not reduce a patient’s risk of ovarian cancer, and it may increase her risk of uterine cancer.
Prophylactic bilateral mastectomy is the mainstay of breast cancer prevention in this population. Data from a systematic review suggest that this surgery may prevent the incidence of breast cancer in women with BRCA mutations by 90% to 95%.32 However, this review did not demonstrate a reduction in mortality from breast cancer, likely due to poor data quality.32 The National Comprehensive Cancer Network (NCCN) recommends discussing prophylactic mastectomy with all women who have BRCA mutations.28 Further conversations are important to review the risk of tissue left behind and quality-of-life issues, including the inability to breastfeed if the woman wants more children and the cosmetic changes with reconstruction.
Ovarian cancer screening and risk-reduction strategies
Screening. No effective screening strategy has been endorsed for ovarian cancer, as most previous studies have shown screening to be ineffective.26,33 Recently, studies both in the United Kingdom and the United States have investigated a screening strategy using the risk-of-ovarian-cancer algorithm (ROCA), which calculates an individual’s risk based on serum levels of cancer antigen 125 (CA-125).34,35 These studies measured CA-125 levels every 3 to 4 months followed by transvaginal ultrasound if CA-125 increased substantially (as determined by ROCA). Absent an abnormal increase in CA-125, transvaginal ultrasound was performed annually. These screening strategies showed improved specificity over annual screening programs, and the cancers detected were more likely to be diagnosed at an early stage (stage II vs stage III) and had higher rates of zero residual disease after surgery compared with those detected 1 year after screening ended.34,35 However, survival data are not yet available. More research is needed to determine if more frequent screening approaches could improve survival in high-risk women.
NCCN and the American College of Obstetricians and Gynecologists (ACOG) do not endorse routine screening with transvaginal ultrasound and serum CA-125 for high-risk women, as the benefits are uncertain. However, they do advise that these screens may be considered as a short-term strategy for women ages 30 to 35 who defer risk-reducing surgery.26,36 The USPSTF does not make a recommendation regarding ovarian cancer screening in high-risk women.37
Risk-reduction strategies. Risk-reducing bilateral salpingo-oophorectomy (RRSO) is the only recommended technique for reducing the risk of ovarian cancer in women at high risk.26,33,36 Meta-analyses have shown an 80% reduction in ovarian cancer risk16 and 68% reduction in all-cause mortality with this approach.38 The NCCN recommends RRSO for women with a known BRCA1 mutation between the ages of 35 and 40 who have completed childbearing.36 Since the onset of ovarian cancer tends to be later in women with BRCA2 mutations, it is reasonable to delay RRSO until age 40 to 45 in this population if they have taken other steps to maximize breast cancer prevention (ie, bilateral mastectomy).36
Continue to: Adverse effects of RRSO...
Adverse effects of RRSO include surgery complications (wound infection, small bowel obstruction, bladder perforation) and effects of early menopause (vasomotor symptoms, decreased sexual functioning, and increased risk of osteoporosis, cardiovascular disease, and all-cause mortality).39-41 In the absence of contraindications, ACOG recommends using hormone therapy in women undergoing RRSO until the natural age of menopause,42 particularly if their breast tissue has been removed.
Salpingectomy as an alternative. In an attempt to reduce these adverse effects of early menopause, and because a large proportion of high-grade serous tumors originate in the fallopian tube,43 interest has increased in the use of risk-reducing salpingectomy (removal of fallopian tubes) and delayed oophorectomy in women at high risk of ovarian cancer.42 Studies have shown this may be a cost-effective approach and an acceptable alternative in BRCA mutation carriers who are unwilling to undergo RRSO.44,45 A clinical trial investigating this approach in women with BRCA mutations is currently underway in the United States.46 Many centers offer salpingectomy to high-risk patients < 40 years old, understanding that ovary removal is an eventuality for these patients.
When oral contraceptive pills might be beneficial. In younger women with BRCA mutations, there may also be a role for oral contraceptive pills (OCPs) as a risk-reducing strategy. Meta-analyses have shown an approximately 50% reduction in the risk of ovarian cancer among women with BRCA mutations who use OCPs.47-49
ACOG advises that it is appropriate for women with BRCA mutations to use oral contraceptives if indicated (for pregnancy prevention or menstrual cycle regulation), and that it is reasonable to use them for cancer prevention.26 NCCN does not make a formal recommendation, although it does state OCPs may reduce the risk of ovarian cancer in women with a BRCA mutation.36 Case-control studies have produced conflicting data on the association between OCP use and breast cancer risk in BRCA mutation carriers,50-53 although 2 meta-analyses found no significant association in this population.47,48
Decision aids for women with BRCA mutations
Decision aids are visual displays of risk that help patients work through complex decisions. Most decision aids are in print or digital format and include information about the decision to be made as well as pictorial examples of possible outcomes. Pictographs are especially helpful in communicating information. Some decision aids for women with BRCA mutations can be complicated with multiple outcomes (ie, breast cancer and ovarian cancer) and multiple potential interventions (risk-reducing surgery, enhanced screening options).54
Continue to: A Cochrane review...
A Cochrane review found that decision aids increased patients’ knowledge, helped patients clarify their values, and may improve value-concordant decisions.55 Two papers describing the use of decision aids for women with BRCA mutations56,57 documented decreased decisional conflict and increased satisfaction.
CASE
Ms. T underwent the recommended mammogram and MRI screening for breast cancer, as well as testing with serum CA-125 and ultrasound examinations for ovarian cancer. Her initial mammogram and MRI revealed early stage, triple-negative right breast cancer. She chose to undergo bilateral mastectomy and reconstruction. She has now completed treatment and continues to work closely with her oncology team for appropriate breast follow-up.
One year after her initial diagnosis, at the age of 35, she returned to discuss fertility. She was recently married, and she and her husband wanted to start having children. She was concerned about a safe timeline for her to pursue pregnancy, saying she felt “like a ticking time-bomb” given her prior cancer and carrier status. She wanted to discuss the risks and benefits of pregnancy and when she should consider prophylactic oophorectomy. She had a few options. She could have a baby and then undergo an RRSO, or she could talk to her gynecologist about having a salpingectomy to reduce her risk now and use assisted reproductive technology to get pregnant. She could also freeze eggs or embryos, have an RRSO, and then use a surrogate to get pregnant. We informed her that pregnancy would not affect her risk of ovarian cancer and discussed the options for pre-implantation genetic testing to assure that her children would not carry the genetic mutation.58
We provided Ms. T and her husband with a decision aid to help them navigate the decision. They are currently evaluating the options and said they would let us know when they made a decision.
CORRESPONDENCE
Sarina Schrager, MD, MS, Northeast Family Medicine Center, 3209 Dryden Drive, Madison, WI, 53704; sbschrag@wisc.edu.
1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953.
2. SEER Cancer Statistics Review, 1975-2016. Cancer of the female breast. [Table 4.1] National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_04_breast.pdf. Accessed May 27, 2020.
3. SEER Cancer Statistics Review, 1975-2016. Cancer of the ovary. [Table 21.10] National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_21_ovary.pdf. Accessed May 22, 2020.
4. Torre LA, Trabert B, DeSantis C, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284-296.
5. Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4:665-676.
6. Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807-2816.
7. Tai YC, Domchek S, Parmigiani G, et al. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:1811-1814.
8. Evans DG, Susnerwala I, Dawson J, et al. Risk of breast cancer in male BRCA2 carriers. J Med Genet. 2010;47:710-711.
9. CDC. Jewish women and BRCA gene mutations. www.cdc.gov/cancer/breast/young_women/bringyourbrave/hereditary_breast_cancer/jewish_women_brca.htm. Accessed May 22, 2020.
10. Rebbeck TR, Friebel TM, Friedman E, et al. Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum Mutat. 2018;39:593-620.
11. Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330.
12. Wang YA, Jian JW, Hung CF, et al. Germline breast cancer susceptibility gene mutations and breast cancer outcomes. BMC Cancer. 2018;18:315.
13. Baretta Z, Mocellin S, Goldin E, et al. Effect of BRCA germline mutations on breast cancer prognosis: a systematic review and meta-analysis. Medicine. 2016;95:e4975.
14. Lakhani SR, Manek S, Penault-Llorca F, et al. Pathology of ovarian cancers in BRCA1 and BRCA2 carriers. Clin Cancer Res. 2004;10:2473-2481.
15. Kurian AW. BRCA1 and BRCA2 mutations across race and ethnicity: distribution and clinical implications. Curr Opin Obstet Gynecol. 2010;22:72-78.
16. Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80-87.
17. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Int Med. 2012;27:1361-1367.
18. Ardern-Jones A, Eeles R. Developments in clinical practice: follow up clinic for BRCA mutation carriers: a case study highlighting the “virtual clinic.” Hered Cancer Clin Pract. 2004;2:77-79.
19. Yerushalmi R, Rizel S, Zoref D, et al. A dedicated follow-up clinic for BRCA mutation carriers. Isr Med Assoc J. 2016;18:549-552.
20. Pichert G, Jacobs C, Jacobs I, et al. Novel one-stop multidisciplinary follow-up clinic significantly improves cancer risk management in BRCA1/2 carriers. Fam Cancer. 2010;9:313-319.
21. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:652-665.
22. Evans D, Eccles D, Rahman N, et al. A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J Med Genet. 2004;41:474-480.
23. Bellcross CA, Lemke AA, Pape LS, et al. Evaluation of a breast/ovarian cancer genetics referral screening tool in a mammography population. Genet Med. 2009;11:783-789.
24. Hoskins KF, Zwaagstra A, Ranz M. Validation of a tool for identifying women at high risk for hereditary breast cancer in population based screening. Cancer. 2006;107:1769-1776.
25. Gilpin CA, Carson N, Hunter AG. A preliminary validation of a family history assessment form to select women at risk for breast or ovarian cancer for referral to a genetics center. Clin Genet. 2000;58:299-308.
26. Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No 182: Hereditary Breast and Ovarian Cancer Syndrome. Obstet Gynecol. 2017;130:e110-e126.
27. Paluch-Shimon S, Cardoso F, Sessa C, et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol. 2016;27(suppl 5):v103-v110.
28. National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian. 2019. NCCN Clinical Practice Guidelines in Oncology. www2.tri-kobe.org/nccn/guideline/gynecological/english/genetic_familial.pdf. Accessed May 22, 2020.
29. Phillips KA, Milne RL, Rookus MA, et al. Tamoxifen and risk of contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2013;31:3091-3099.
30. Foulkes WD, Goffin J, Brunet JS, et al. Tamoxifen may be an effective adjuvant treatment for BRCA1-related breast cancer irrespective of estrogen receptor status. J Natl Cancer Inst. 2002;94:1504-1506.
31. Gronwald J, Tung N, Foulkes WD, et al. Tamoxifen and contralateral breast cancer in BRCA1 and BRCA2 carriers: an update. Int J Cancer. 2006;118:2281-2284.
32. Ludwig KK, Neuner J, Butler A, et al. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am J Surgery. 2016;212:660-669.
33. Bougie O, Weberpals JI. Clinical considerations of BRCA1- and BRCA2-mutation carriers: a review. Int J Surg Oncol. 2011;2011:374012.
34. Rosenthal AN, Fraser LSM, Philpott S, et al. Evidence of stage shift in women diagnosed with ovarian cancer during phase II of the United Kingdom Familial Ovarian Cancer Screening Study. J Clin Oncol. 2017;35:1411-1420.
35. Skates SJ, Greene MH, Buys SS, et al. Early detection of ovarian cancer using the Risk of Ovarian Cancer Algorithm with frequent CA125 testing in women at increased familial risk—combined results from two screening trials. Clin Cancer Res. 2017;23:3628-3637.
36. Daly MB, Pilarski R, Berry M, et al. NCCN guidelines insights: genetic/familial high-risk assessment: breast and ovarian, version 2.2017. J Natl Compr Canc Netw. 2017;15:9-20.
37. Grossman DC, Curry SJ, Owens DK, et al. Screening for ovarian cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:588-594.
38. Marchetti C, De Felice F, Palaia I, et al. Risk-reducing salpingo-oophorectomy: a meta-analysis on impact on ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2 mutation carriers. BMC Womens Health. 2014;14:150.
39. Nelson HD, Pappas M, Zakher B, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: a systematic review to update the US Preventive Services Task Force recommendation. Ann Intern Med. 2014;160:255-266.
40. Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet Gynecol. 2013;121:709-716.
41. Faubion SS, Kuhle CL, Shuster LT, et al. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18:483-491.
42. Menon U, Karpinskyj C, Gentry-Maharaj A. Ovarian cancer prevention and screening. Obstet Gynecol. 2018;131:909-927.
43. Crum CP, Drapkin R, Miron A, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3-9.
44. Kwon JS, Tinker A, Pansegrau G, et al. Prophylactic salpingectomy and delayed oophorectomy as an alternative for BRCA mutation carriers. Obstet Gynecol. 2013;121:14-24.
45. Holman LL, Friedman S, Daniels MS, et al. Acceptability of prophylactic salpingectomy with delayed oophorectomy as risk-reducing surgery among BRCA mutation carriers. Gynecol Oncol. 2014;133:283-286.
46. MD Anderson Cancer Center. Prophylactic salpingectomy with delayed oophorectomy, risk-reducing salpingo-oophorectomy, and ovarian cancer screening among BRCA mutation carriers: a proof-of-concept study. www.mdanderson.org/patients-family/diagnosis-treatment/clinical-trials/clinical-trials-index/clinical-trials-detail.ID2013-0340.html. Accessed May 22, 2020.
47. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275-2284.
48. Moorman PG, Havrilesky LJ, Gierisch JM, et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J Clin Oncol. 2013;31:4188-4198.
49. Friebel TM, Domchek SM, Rebbeck TR. Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju091.
50. Haile RW, Thomas DC, McGuire V, et al. BRCA1 and BRCA2 mutation carriers, oral contraceptive use, and breast cancer before age 50. Cancer Epidemiol Biomarkers Prev. 2006;15:1863-1870.
51. Lee E, Ma H, McKean-Cowdin R, et al. Effect of reproductive factors and oral contraceptives on breast cancer risk in BRCA1/2 mutation carriers and noncarriers: results from a population-based study. Cancer Epidemiol Biomarkers Prev. 2008;17:3170-3178.
52. Narod SA, Dubé MP, Klijn J, et al. Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2002;94:1773-1779.
53. Milne RL, Knight JA, John EM, et al. Oral contraceptive use and risk of early-onset breast cancer in carriers and noncarriers of BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev. 2005;14:350-356.
54. Culver JO, MacDonald DJ, Thornton AA, et al. Development and evaluation of a decision aid for BRCA carriers with breast cancer. J Genet Couns. 2011;20:294-307.
55. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Syst Rev. 2017;4:CD001431.
56. Schwartz MD, Valdimarsdottir HB, DeMarco TA, et al. Randomized trial of a decision aid for BRCA1/BRCA2 mutation carriers: impact on measures of decision making and satisfaction. Health Psychol. 2009;28:11-19.
57. Metcalfe KA, Dennis CL, Poll A, et al. Effect of decision aid for breast cancer prevention on decisional conflict in women with a BRCA1 or BRCA2 mutation: a multisite, randomized, controlled trial. Gen Med. 2017;19:330-336.
58. Friedman LC, Kramer RM. Reproductive issues for women with BRCA mutations. J Natl Cancer Inst Monogr. 2005;34:83-86.
1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953.
2. SEER Cancer Statistics Review, 1975-2016. Cancer of the female breast. [Table 4.1] National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_04_breast.pdf. Accessed May 27, 2020.
3. SEER Cancer Statistics Review, 1975-2016. Cancer of the ovary. [Table 21.10] National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_21_ovary.pdf. Accessed May 22, 2020.
4. Torre LA, Trabert B, DeSantis C, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284-296.
5. Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4:665-676.
6. Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807-2816.
7. Tai YC, Domchek S, Parmigiani G, et al. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:1811-1814.
8. Evans DG, Susnerwala I, Dawson J, et al. Risk of breast cancer in male BRCA2 carriers. J Med Genet. 2010;47:710-711.
9. CDC. Jewish women and BRCA gene mutations. www.cdc.gov/cancer/breast/young_women/bringyourbrave/hereditary_breast_cancer/jewish_women_brca.htm. Accessed May 22, 2020.
10. Rebbeck TR, Friebel TM, Friedman E, et al. Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum Mutat. 2018;39:593-620.
11. Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330.
12. Wang YA, Jian JW, Hung CF, et al. Germline breast cancer susceptibility gene mutations and breast cancer outcomes. BMC Cancer. 2018;18:315.
13. Baretta Z, Mocellin S, Goldin E, et al. Effect of BRCA germline mutations on breast cancer prognosis: a systematic review and meta-analysis. Medicine. 2016;95:e4975.
14. Lakhani SR, Manek S, Penault-Llorca F, et al. Pathology of ovarian cancers in BRCA1 and BRCA2 carriers. Clin Cancer Res. 2004;10:2473-2481.
15. Kurian AW. BRCA1 and BRCA2 mutations across race and ethnicity: distribution and clinical implications. Curr Opin Obstet Gynecol. 2010;22:72-78.
16. Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80-87.
17. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Int Med. 2012;27:1361-1367.
18. Ardern-Jones A, Eeles R. Developments in clinical practice: follow up clinic for BRCA mutation carriers: a case study highlighting the “virtual clinic.” Hered Cancer Clin Pract. 2004;2:77-79.
19. Yerushalmi R, Rizel S, Zoref D, et al. A dedicated follow-up clinic for BRCA mutation carriers. Isr Med Assoc J. 2016;18:549-552.
20. Pichert G, Jacobs C, Jacobs I, et al. Novel one-stop multidisciplinary follow-up clinic significantly improves cancer risk management in BRCA1/2 carriers. Fam Cancer. 2010;9:313-319.
21. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:652-665.
22. Evans D, Eccles D, Rahman N, et al. A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J Med Genet. 2004;41:474-480.
23. Bellcross CA, Lemke AA, Pape LS, et al. Evaluation of a breast/ovarian cancer genetics referral screening tool in a mammography population. Genet Med. 2009;11:783-789.
24. Hoskins KF, Zwaagstra A, Ranz M. Validation of a tool for identifying women at high risk for hereditary breast cancer in population based screening. Cancer. 2006;107:1769-1776.
25. Gilpin CA, Carson N, Hunter AG. A preliminary validation of a family history assessment form to select women at risk for breast or ovarian cancer for referral to a genetics center. Clin Genet. 2000;58:299-308.
26. Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No 182: Hereditary Breast and Ovarian Cancer Syndrome. Obstet Gynecol. 2017;130:e110-e126.
27. Paluch-Shimon S, Cardoso F, Sessa C, et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol. 2016;27(suppl 5):v103-v110.
28. National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian. 2019. NCCN Clinical Practice Guidelines in Oncology. www2.tri-kobe.org/nccn/guideline/gynecological/english/genetic_familial.pdf. Accessed May 22, 2020.
29. Phillips KA, Milne RL, Rookus MA, et al. Tamoxifen and risk of contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2013;31:3091-3099.
30. Foulkes WD, Goffin J, Brunet JS, et al. Tamoxifen may be an effective adjuvant treatment for BRCA1-related breast cancer irrespective of estrogen receptor status. J Natl Cancer Inst. 2002;94:1504-1506.
31. Gronwald J, Tung N, Foulkes WD, et al. Tamoxifen and contralateral breast cancer in BRCA1 and BRCA2 carriers: an update. Int J Cancer. 2006;118:2281-2284.
32. Ludwig KK, Neuner J, Butler A, et al. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am J Surgery. 2016;212:660-669.
33. Bougie O, Weberpals JI. Clinical considerations of BRCA1- and BRCA2-mutation carriers: a review. Int J Surg Oncol. 2011;2011:374012.
34. Rosenthal AN, Fraser LSM, Philpott S, et al. Evidence of stage shift in women diagnosed with ovarian cancer during phase II of the United Kingdom Familial Ovarian Cancer Screening Study. J Clin Oncol. 2017;35:1411-1420.
35. Skates SJ, Greene MH, Buys SS, et al. Early detection of ovarian cancer using the Risk of Ovarian Cancer Algorithm with frequent CA125 testing in women at increased familial risk—combined results from two screening trials. Clin Cancer Res. 2017;23:3628-3637.
36. Daly MB, Pilarski R, Berry M, et al. NCCN guidelines insights: genetic/familial high-risk assessment: breast and ovarian, version 2.2017. J Natl Compr Canc Netw. 2017;15:9-20.
37. Grossman DC, Curry SJ, Owens DK, et al. Screening for ovarian cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:588-594.
38. Marchetti C, De Felice F, Palaia I, et al. Risk-reducing salpingo-oophorectomy: a meta-analysis on impact on ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2 mutation carriers. BMC Womens Health. 2014;14:150.
39. Nelson HD, Pappas M, Zakher B, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: a systematic review to update the US Preventive Services Task Force recommendation. Ann Intern Med. 2014;160:255-266.
40. Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet Gynecol. 2013;121:709-716.
41. Faubion SS, Kuhle CL, Shuster LT, et al. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18:483-491.
42. Menon U, Karpinskyj C, Gentry-Maharaj A. Ovarian cancer prevention and screening. Obstet Gynecol. 2018;131:909-927.
43. Crum CP, Drapkin R, Miron A, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3-9.
44. Kwon JS, Tinker A, Pansegrau G, et al. Prophylactic salpingectomy and delayed oophorectomy as an alternative for BRCA mutation carriers. Obstet Gynecol. 2013;121:14-24.
45. Holman LL, Friedman S, Daniels MS, et al. Acceptability of prophylactic salpingectomy with delayed oophorectomy as risk-reducing surgery among BRCA mutation carriers. Gynecol Oncol. 2014;133:283-286.
46. MD Anderson Cancer Center. Prophylactic salpingectomy with delayed oophorectomy, risk-reducing salpingo-oophorectomy, and ovarian cancer screening among BRCA mutation carriers: a proof-of-concept study. www.mdanderson.org/patients-family/diagnosis-treatment/clinical-trials/clinical-trials-index/clinical-trials-detail.ID2013-0340.html. Accessed May 22, 2020.
47. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275-2284.
48. Moorman PG, Havrilesky LJ, Gierisch JM, et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J Clin Oncol. 2013;31:4188-4198.
49. Friebel TM, Domchek SM, Rebbeck TR. Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju091.
50. Haile RW, Thomas DC, McGuire V, et al. BRCA1 and BRCA2 mutation carriers, oral contraceptive use, and breast cancer before age 50. Cancer Epidemiol Biomarkers Prev. 2006;15:1863-1870.
51. Lee E, Ma H, McKean-Cowdin R, et al. Effect of reproductive factors and oral contraceptives on breast cancer risk in BRCA1/2 mutation carriers and noncarriers: results from a population-based study. Cancer Epidemiol Biomarkers Prev. 2008;17:3170-3178.
52. Narod SA, Dubé MP, Klijn J, et al. Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2002;94:1773-1779.
53. Milne RL, Knight JA, John EM, et al. Oral contraceptive use and risk of early-onset breast cancer in carriers and noncarriers of BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev. 2005;14:350-356.
54. Culver JO, MacDonald DJ, Thornton AA, et al. Development and evaluation of a decision aid for BRCA carriers with breast cancer. J Genet Couns. 2011;20:294-307.
55. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Syst Rev. 2017;4:CD001431.
56. Schwartz MD, Valdimarsdottir HB, DeMarco TA, et al. Randomized trial of a decision aid for BRCA1/BRCA2 mutation carriers: impact on measures of decision making and satisfaction. Health Psychol. 2009;28:11-19.
57. Metcalfe KA, Dennis CL, Poll A, et al. Effect of decision aid for breast cancer prevention on decisional conflict in women with a BRCA1 or BRCA2 mutation: a multisite, randomized, controlled trial. Gen Med. 2017;19:330-336.
58. Friedman LC, Kramer RM. Reproductive issues for women with BRCA mutations. J Natl Cancer Inst Monogr. 2005;34:83-86.
PRACTICE RECOMMENDATIONS
› Recommend genetic screening for the BRCA mutation if a patient’s family history includes a breast cancer diagnosis before age 50, occurrences of both breast and ovarian cancers, or other suggestive features. C
› Advise women with the BRCA gene to return for a clinical breast exam every 6 to 12 months starting at age 25, and to start radiologic screening at age 30. C
› Consider recommending bilateral salpingo-oophorectomy to prevent ovarian cancer in women 35 to 40 years of age with a BRCA1 mutation who have completed childbearing. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Leveraging CAM to treat depression and anxiety
Almost 8% of Americans ages ≥ 12 years have depression and 19.1% of Americans ages ≥ 18 years have experienced an anxiety disorder in the past year.1,2 Furthermore, suicide, which can result from depression and anxiety, is the 10th leading cause of death in the United States, claiming about 40,000 to 49,000 lives per year since 2012, with increasing yearly rates.3 While multiple conventional medication and therapy treatments are available, patients remain interested in complementary and alternative medicine (CAM) options. According to the National Center for Complementary and Integrative Health, more than 30% of American adults use CAM treatments.4
This article provides an overview of the evidence for commonly used CAM treatments for unipolar depression and anxiety in adults. It is designed to serve as a useful resource when patients are interested in looking beyond conventional medications.
St. John’s wort: ‘Yes’ for depression; ‘no’ for anxiety
Hypericum perforatum, more commonly known as St. John’s wort, is a widely used antidepressant, especially in Europe where it is prescribed, rather than offered over the counter as it is here. Its mechanism of action is not completely understood because its various constituents have different neuropharmacologic activities.5,6
A 2008 Cochrane review evaluated 29 randomized, double-blind studies (N = 5489) that compared St. John’s wort with placebo or standard antidepressants in the treatment of depression.7 St. John’s wort was found to be superior to placebo and comparable to standard antidepressants. More recently, a 2017 meta-analysis of 27 studies (N = 3808) had similar findings.8 In patients with mild-to-moderate depression, St. John’s wart produced rates of remission that were comparable to those produced by selective serotonin reuptake inhibitors (SSRIs) but with a lower discontinuation rate.
Mood disorders other than depression. Studies do not support a role for St. John’s wort in the treatment of anxiety disorders. There are no trials that assess the efficacy of St. John’s wort for the reduction of symptoms of general anxiety disorder as a primary outcome of treatment. Some small clinical trials have investigated the efficacy of St. John’s wort in obsessive-compulsive disorder and social anxiety disorder. In those studies, St. John’s wort performed no better than placebo.9,10
A few words of caution. Preparations of St. John’s wort in the United States are not standardized, so St. John’s wort should be used in America with caution. Furthermore, long-term use of St. John’s wort for depression is questionable given that most studies have evaluated only up to 12 weeks of use.8
If used, studies indicate that the St. John’s wort extract that should be used is 0.3% hypericin or 5% hyperforin administered in a dosage of 300 to 400 mg tid.7,8,11 Physicians and patients can use www.consumerlabs.com to find St. John’s wort brands that have met specified quality criteria based on independent laboratory studies. This Web site can also be used to investigate the quality of the brands available for the other supplements discussed in this article.
Continue to: Adverse effects
Adverse effects. St. John’s wort and an SSRI can lead to serotonin syndrome, which is a constellation of symptoms involving mental status changes and autonomic and neuromuscular hyperactivity caused by serotonin overactivity.12 Furthermore, treatment failures with anticoagulants, digoxin, hormonal contraceptives, immunosuppressants, and narcotics due to concomitant use with St. John’s wort have been reported.13
The most common adverse reactions to St. John’s wort include gastrointestinal symptoms, dizziness, sedation, photosensitivity, dry mouth, urinary frequency, anorgasmia, and swelling.14 However, multiple studies have supported St. John’s wort to be equally or better tolerated than conventional antidepressants.7,8
Certain forms of folate can be adjunctive treatment for depression
Methylfolate is the form of folate that crosses the blood–brain barrier. A prospective observational study evaluated the cerebral spinal fluid of 33 patients with refractory depression. The authors found metabolic abnormalities in the cerebrospinal fluid of most of those patients, the most common of which was folate deficiency in 12 patients despite normal serum folate levels.15
Additionally, current understanding of the role of the Methylenetetrahydrofolate reductase (MTHFR) gene and the folate cycle in depression supports a potential role of methylfolate in depression treatment.16 The MTHFR gene encodes for an enzyme called MTHFR. The MTHFR enzyme converts 5,10-MTHF to 5-MTHF, which then crosses the blood–brain barrier and donates a methyl group for the conversion of homocysteine to methionine. Methionine is a precursor to monoamine neurotransmitters. Thus, decreased expression of the MTHFR gene leads to decreased methylfolate levels, which, in turn, potentially leads to insufficient neurotransmitter synthesis and homocysteine excess.
MTHFR gene polymorphisms and increased homocysteine levels have been found to be associated with the occurrence of depression. One thought is that methylfolate supplementation compensates for an underlying MTHFR enzyme deficiency in patients with depression. Further studies are needed to determine if screening depressed patients for MTHFR gene polymorphisms is of benefit.16
Continue to: A 2012 randomized controlled trial...
A 2012 randomized controlled trial (RCT) (N = 75) compared L-methylfolate 15 mg/d plus an SSRI with placebo plus an SSRI in patients with SSRI-resistant major depression.17 The trial found that a reduction of baseline symptoms by ≥ 50% occurred in more patients who received adjunctive L-methylfolate than placebo (32% vs 15%) and tolerability was comparable. These findings were again supported in 2016 with a 12-month study showing L-methylfolate to have long-term tolerability comparable to placebo,18 and in 2017 with a randomized trial (N = 260) that found escitalopram 10 mg/d plus L-methylfolate 15 mg/d to be significantly more effective at treating depression than escitalopram 10 mg/d alone.19 Thus, methylfolate may be an effective adjunctive treatment for depression at a dosage of 15 mg/d.
S-adenosyl methionine (SAMe) is a metabolite of folate derived from methionine that facilitates the synthesis of neurotransmitters including dopamine, norepinephrine, and serotonin. In Europe, as is the case with St. John’s wort, it is a prescription medication.
A randomized trial (N = 73) compared adjunctive SAMe 800 mg bid with placebo in the treatment of patients with unipolar major depression who did not experience improvement with SSRI treatment alone.20 The investigators found that more patients who received SAMe than who received placebo had improvement in their depression (36.1% vs 17.6%), and more patients who received SAMe compared to placebo went into depression remission (25.8% vs 11.7%).
Adverse effects were comparable in both groups. Thus, SAMe at a dosage of 400 to 1600 mg/d may be effective in the treatment of depression.20,21 The findings of 1 study (N = 65) suggest that patients could experience further improvement in their depression symptoms with SAMe at doses of as much as 3200 mg/d; however, 3200 mg/d increased the occurrence of gastrointestinal adverse effects (31.3% in the SAMe arm vs 3.8% in the placebo group).21
Folate. With regard to folate itself, randomized trials have not supported its efficacy in the treatment of depression in the general population.22
Continue to: VItamin D may improve anxiety/depression in those with low levels
Vitamin D may improve anxiety/depression in those with low levels
Vitamin D supplementation is also being used more frequently in the treatment of depression. Case-control, cross-sectional, and cohort studies have linked low vitamin D levels to the occurrence of depression. A 2013 systematic review of 14 such studies (N = 31,424) found lower vitamin D levels in people with depression compared with controls.23 Further studies are needed to determine if this relationship is causal, and quality RCTs investigating the effect of vitamin D supplementation on depression are lacking.
A 2019 study (N = 30) evaluated the impact of vitamin D supplementation on generalized anxiety disorder in patients with co-occurring vitamin D deficiency. Half received standard-of-care general anxiety disorder treatment plus 50,000 IU of vitamin D weekly for 3 months, while the other half received standard of care alone. Significant improvements in anxiety scores, increases in serum serotonin, and decreases in serum neopterin (an inflammatory marker) were observed in the vitamin D–treated group compared to the group that did not receive vitamin D.24
It is not currently standard of care to check vitamin D levels in all patients presenting with mood disorders. However, if screening is indicated for another reason and low levels are confirmed, vitamin D replacement may improve anxiety and/or depressive symptoms. Despite this, no evidence exists to support vitamin D supplementation for depression or anxiety in patients with normal vitamin D levels.24,25
The effects of omega-3 fatty acids are largely unclear
Research has shown that omega-3 polyunsaturated fatty acids (n-3 PUFA), which are found in fish oil, protect glutamatergic neurotransmission from glucocorticoids, which are released in the body during a stress response.26 Small clinical trials have found n-3 PUFAs to reduce the symptoms of anxiety compared with placebo, but the acids have not been studied directly for anxiety disorders.27,28
With regard to depression, evidence is conflicting as to whether n-3 PUFAs are of any benefit in treatment.29 Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are hypothesized to be the components in omega-3 fatty acid preparations that could lead to a reduction in depressive symptoms. However, randomized trials have shown that EPA-predominant omega-3 fatty acid formulations have only a moderate or no clinically significant effect on depression over placebo, and that DHA-predominant omega-3 fatty acid formulations are only comparable or inferior to placebo.30
Continue to: Response to EPA...
Response to EPA may be greater in patients with depression who have high levels of inflammatory biomarkers, such as interleuken (IL)-1 receptor antagonist (1Ra), IL-6, high-sensitivity C-reactive protein (hs-CRP), leptin, and adiponectin, than in patients with low levels. An 8-week trial randomly assigned patients with unipolar major depression (N = 155) to receive either EPA, DHA, or placebo and found that improvement for the 3 groups was comparable; however, in the subgroup of patients with high levels of inflammatory markers, improvement in depressive symptoms was significantly greater with EPA than with either DHA or placebo.31
It is unclear whether different sources of n-3 PUFA, such as whole fish vs fish oil vs prescription omega-3 acid ethyl esters (Lovaza), are more or less efficacious in the treatment of anxiety or depression. Furthermore, there is no standard dosing for n-3 PUFA in the treatment of mood disorders. Given that the US Food and Drug Administration recommends no more than 2 g/d of combined EPA and DHA supplementation, we recommend using 2 g/d if one decides to treat depression/anxiety with n-3 PUFA.32
N-3 PUFA supplementation is fairly benign. There have been previous concerns about n-3 PUFA supplementation increasing patients’ risk for gastrointestinal bleeding, but a 2006 systematic review that included 9 trials (N = 2612) that looked at clinically significant bleeding episodes found that even patients at high risk for bleeding (ie, those taking aspirin or warfarin) had no increased bleeding risk from taking n-3 PUFA supplementation at up to 4 g/d.33
Don’t underestimate exercise and meditation; consider acupuncture
Exercise. Multiple practice guidelines, including the American Psychiatric Association’s “Practice Guideline for the Treatment of Patients with Major Depressive Disorder,” and meta-analyses have supported the use of exercise to treat unipolar major depression and anxiety.34-37 However, only about 26% of American men and 19% of American women met the US Department of Health and Human Services’ “Federal Physical Activity Guidelines for Americans” in 2016.38
Exercise alone is a reasonable monotherapy, as long as patients are monitored closely for worsening symptoms. Additionally, exercise as an add-on treatment can be helpful for more severe depression or anxiety.39 The best type, duration, and frequency of exercise specifically for the treatment of depression or anxiety has yet to be determined, but physicians may base their exercise recommendations on the “Federal Physical Activity Guidelines for Americans” for general good health (TABLE).38
Continue to: Meditation
Meditation, especially mindfulness meditation, is another strategy that has gained popularity in the treatment of anxiety and depression. Mindfulness has been defined as “the practice of maintaining a nonjudgmental state of heightened or complete awareness of one’s thoughts, emotions, or experiences on a moment-to-moment basis.”40 A 2014 systematic review and meta-analysis of 47 trials with 3515 participants found that mindfulness meditation programs led to clinically significant moderate reductions in anxiety.41 Smaller effects were found for depression.
Nevertheless, meditation may be beneficial as an adjunctive treatment for depression. A small randomized trial (N = 25) compared an adjunctive breathing-based meditation intervention with a waitlist control (delayed yoga) in patients with unipolar major depression who failed to respond to at least 8 weeks of antidepressant treatment.42 The meditation intervention consisted of a group program with sitting meditation, breathing exercises, and yoga postures. Participants engaged in the meditation intervention for 2 to 3.5 hours per day for 8 weeks and demonstrated significant improvement in depression symptoms compared with the control group.42
Acupuncture. A 2018 meta-analysis of 64 studies (N = 7104) suggests that acupuncture results in a small-to-moderate reduction in depressive symptoms when compared to no treatment, control/sham acupuncture, or medication.43 Furthermore, acupuncture plus medication compared to medication alone results in a higher reduction in depressive symptoms without an increase in adverse events.43
Additionally, a 2019 analysis of 10 systematic reviews found acupuncture to be more effective than control/sham acupuncture in the treatment of general anxiety.44 It should be noted, however, that a lot of heterogeneity and potential for bias existed across all of the studies. The studies analyzed were very low to low in quality. Thus, the evidence is insufficient to strongly recommend the use of acupuncture for depression or anxiety, although acupuncture is a safe intervention with low rates of adverse events.
Emotional support animals: Beneficial, but evidence is weak
Emotional support animals are gaining in popularity with Americans who have mood disorders. An important distinction must be made, however, between service animals and emotional support animals. A service animal is one “that is individually trained to do work or perform tasks for the benefit of an individual with a disability, including a physical, sensory, psychiatric, intellectual, or other mental disability.”45 Under the Americans with Disabilities Act (ADA), service animals are limited to dogs, and, in some cases, specially trained miniature horses. Psychiatric service dogs can be trained to do anything from reminding their owner to take medicine to stopping self-mutilation activities.
Continue to: Emotional support animals...
Emotional support animals are not specially trained to perform tasks to help with disabilities. It’s their companionship that helps relieve symptoms of depression and/or anxiety.45 Thus, emotional support animals are not covered under federal laws that apply to service animals. However, the Air Carrier Access Act does require airlines to allow emotional support animals to fly in the cabin for free. Furthermore, the Fair Housing Act allows emotional support animals to circumvent no-pet rules in housing and dorms. Airplanes and housing are the only places legally required to allow the unrestricted presence of emotional support animals.46
Also, there are important distinctions between emotional support animals and pets. While anyone can own a pet, an emotional support animal is prescribed by a licensed mental health professional as a treatment for a mood disorder. Housing facilities and airlines will usually require an emotional support animal “prescription” or letter from a physician to recognize animals as such.
A 2018 systematic review evaluated the evidence behind emotional support animals, which included 17 peer-reviewed journal articles, conference papers, and research dissertations (N = 1727) mostly containing qualitative evidence.47 Unfortunately, there are no RCTs, and there are limited case-control and cohort studies evaluating the effect of an emotional support animal on mood disorders. Based on the available evidence, there does seem to be a psychological benefit to owning an animal for both those with a diagnosable mental health disorder and the general population. This benefit seems to stem from a perceived reduction in social isolation and an increase in emotional support. Factors that determine the psychological benefit of emotional support animals include the type of pet, the number of pets, the attachment to the pet, and the perceived friendliness of the pet.47
Animal-assisted therapy. A 2014 systematic review evaluated higher-level evidence behind animal-assisted therapy (AAT).48 Although participating in therapy that involves interaction with animals is not the same as owning an emotional support animal, the concept—using an animal to improve mental health—is the same. The systematic review looked at 11 RCTs (N = 411) that studied the effect of AAT on mental health. Animals studied included dogs, cats, dolphins, birds, cows, rabbits, ferrets, and guinea pigs. Mental health disorders studied included schizophrenia, depression, anxiety, alcohol/drug abuse, and other addictive behaviors.
Therapeutic animal exposure led to reported improvements in mood, quality of life, and social behavior. These improvements were attributed to the animals buffering people’s reactions to mental stressors. The animals provided a sense of comfort and safety and diverted attention away from immediate stressors. Furthermore, the memory of the animals brought participants a sense of comfort/happiness when they were later without the animal. However, the majority of participants were people who liked animals at baseline.48
CORRESPONDENCE
Amanda E. Olagunju, DO, Operational Medicine Clinic, Langley AFB Hospital, 77 Nealy Avenue, Langley AFB, VA 23665; amanda.olagunju@gmail.com.
1. National Center for Health Statistics, Centers for Disease Control and Prevention. FastStats: Depression. Last reviewed October 7, 2015. www.cdc.gov/nchs/fastats/depression.htm. Accessed May 26, 2020.
2. National Institute of Mental Health. Mental health information—statistics: any anxiety disorder. Last updated November 2017. www.nimh.nih.gov/health/statistics/any-anxiety-disorder.shtml. Accessed May 26, 2020.
3. Hedegaard H, Curtin SC, Warner M. Increase in suicide mortality in the United States, 1999–2018. NCHS Data Brief, no 362. Hyattsville, MD: National Center for Health Statistics; 2020.
4. National Center for Complementary and Integrative Health. Complementary, alternative, or integrative health: what’s in a name? Last updated July 2018. www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name. Accessed May 26, 2020.
5. Bennett DA Jr, Phun L, Polk JF, et al. Neuropharmacology of St. John’s wort (Hypericum). Ann Pharmacother. 1998;32:1201-1208.
6. Müller WE, Singer A, Wonnemann M, et al. Hyperforin represents the neurotransmitter reuptake inhibiting constituent of hypericum extract. Pharmacopsychiatry. 1998;31(suppl 1):16-21.
7. Linde K, Berner MM, Kriston L. St John’s wort for major depression. Cochrane Database Syst Rev. 2008;CD000448.
8.
9. Kobak KA, Taylor LV, Bystritsky A, et al. St John’s wort versus placebo in obsessive-compulsive disorder: results from a double-blind study. Int Clin Psychopharmacol. 2005;20:299-304.
10. Kobak KA, Taylor LV, Warner G, et al. St. John’s wort versus placebo in social phobia: results from a placebo-controlled pilot study. J Clin Psychopharmacol. 2005;25:51-58.
11. Product reviews: St. John’s wort supplements review. ConsumerLab.com. September 23, 2016. www.consumerlab.com/reviews/St_Johns_Wort/stjohnswort/. Accessed May 26, 2020.
12. Simhan S. Serotonin syndrome. In: Abd-Elsayed A. (ed) Pain: A Review Guide. New York, NY: Springer; 2019.
13. Chrubasik-Hausmann S, Vlachojannis J, McLachlan A. Understanding drug interactions with St. John’s wort (Hypericum perforatum L.): impact of hyperforin content. J Pharm Pharmacol. 2019;71:129-138.
14. Knüppel L, Linde K. Adverse effects of St. John’s wort: a systematic review. J Clin Psychiatry. 2004;65:1470-1479.
15. Pan LA, Martin P, Zimmer T, et al. Neurometabolic disorders: potentially treatable abnormalities in patients with treatment-refractory depression and suicidal behavior. Am J Psychiatry. 2017;174:42-50.
16. Kandler C, Lam S. Methylenetetrahydrofolate reductase screening in treatment-resistant depression. Fed Pract. 2019;36:207-208.
17. Papakostas GI, Shelton RC, Zajecka JM, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012;169:1267-1274.
18. Zajecka J, Fava M, Shelton R, et al. Long-term efficacy, safety, and tolerability of L-methylfolate calcium 15 mg as adjunctive therapy with selective serotonin reuptake inhibitors: a 12-month, open-label study following a placebo-controlled acute study. J Clin Psychiatry. 2016;77:654-660.
19. Kakar MS, Jehangir S, Mustafa M, et al. Therapeutic efficacy of combination therapy of L-methylfolate and escitalopram in depression. Pakistan Armed Forces Med J. 2017;67:976-981.
20. Papakostas GI, Mischoulon D, Shyu I, et al. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: a double-blind, randomized clinical trial. Am J Psychiatry. 2010;167:942-948.
21.
22. Sarris J, Murphy J, Mischoulon D, et al. Adjunctive nutraceuticals for depression: a systematic review and meta-analyses. Am J Psychiatry. 2016;173:575-587.
23. Anglin RE, Samaan Z, Walter SD, et al. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100-107.
24. Eid A, Khoja S, AlGhamdi S, et al. Vitamin D supplementation ameliorates severity of generalized anxiety disorder (GAD). Metab Brain Dis. 2019;34:1781-1786.
25. Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab. 2014;99:757-767.
26. Hennebelle M, Champeil-Potokar G, Lavialle M, et al. Omega-3 polyunsaturated fatty acids and chronic stress-induced modulations of glutamatergic neurotransmission in the hippocampus. Nutr Rev. 2014;72:99-112.
27. Kiecolt-Glaser JK, Belury MA, Andridge R, et al. Omega-3 supplementation lowers inflammation and anxiety in medical students: a randomized controlled trial. Brain Behav Immun. 2011;25:1725-1734.
28. Buydens-Branchey L, Branchey M, Hibbeln JR. Associations between increases in plasma n-3 polyunsaturated fatty acids following supplementation and decreases in anger and anxiety in substance abusers. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:568-575.
29. Lin PY, Mischoulon D, Freeman MP, et al. Are omega-3 fatty acids antidepressants or just mood-improving agents? The effect depends upon diagnosis, supplement preparation, and severity of depression. Mol Psychiatry 2012;17:1161-1163.
30. Hallahan B, Ryan T, Hibbeln JR, et al. Efficacy of omega-3 highly unsaturated fatty acids in the treatment of depression. Br J Psychiatry. 2016;209:192-201.
31. Rapaport MH, Nierenberg AA, Schettler PJ, et al. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol Psychiatry. 2016;21:71-79.
32. National Institutes of Health Office of Dietary Supplements. Omega-3 fatty acids. Updated October 17, 2019. https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional. Accessed May 26, 2020.
33. Wang C, Harris WS, Chung M, et al. n-3 fatty acids from fish or fish-oil supplements, but not alpha-linoleic acid, benefit cardiovascular disease outcomes in primary- and secondary- prevention studies: a systematic review. Am J Clin Nutr. 2006;84:5-17.
34. American Psychiatric Association Practice. Guideline for the Treatment of Patients with Major Depressive Disorder. 3rd Edition. 2010. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Accessed May 26, 2020.
35. Gordon B, McDowell C, Lyons M, et al. The effects of resistance exercise training on anxiety: a meta-analysis and meta-regression analysis of randomized controlled trials. Sports Med. 2017;47:2521-2532.
36. Stubbs B, Vancampfort D, Rosenbaum S, et al. An examination of the anxiolytic effects of exercise for people with anxiety and stress-related disorders: a meta-analysis. Psychiatry Res. 2017;249:102-108.
37. Rethorst CD, Trivedi MH. Evidence-based recommendations for the prescription of exercise for major depressive disorder. J Psychiatr Pract. 2013;19:204-212.
38. US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd edition. Washington, DC: US Department of Health and Human Services; 2018.
39. Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;CD004366.
40. Merriam-Webster Dictionary. "Mindfulness." www.merriam-webster.com/dictionary/mindfulness. Accessed May 26, 2020.
41. Goyal M, Singh S, Sibinga EM, et al. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med. 2014;174:357-368.
42. Sharma A, Barrett MS, Cucchiara AJ, et al. A breathing-based meditation intervention for patients with major depressive disorder following inadequate response to antidepressants: a randomized pilot study. J Clin Psychiatry. 2017;78:e59-e63.
43. Smith CA, Armour M, Soo Lee M, et al. Acupuncture for depression. Cochrane Database Syst Rev. 2018;CD004046.
44. Li M
45. Brennan J. Service animals and emotional support animals. ADA National Network Information Guidance and Training on the Americans with Disabilities Act. Last updated April 2020. adata.org/publication/service-animals-booklet. Accessed May 26, 2020.
46. Clay RA. Is that a pet or therapeutic aid? Monitor on Psychology. 2016;47:38.
47. Brooks HL, Rushton K, Lovell K, et al. The power of support from companion animals for people living with mental health problems: a systematic review and narrative synthesis of the evidence. BMC Psychiatry. 2018;18:31.
48. Kamioka H, Okada S, Tsutani K, et al. Effectiveness of animal-assisted therapy: a systematic review of randomized controlled trials. Complement Ther Med. 2014;22:371-390.
Almost 8% of Americans ages ≥ 12 years have depression and 19.1% of Americans ages ≥ 18 years have experienced an anxiety disorder in the past year.1,2 Furthermore, suicide, which can result from depression and anxiety, is the 10th leading cause of death in the United States, claiming about 40,000 to 49,000 lives per year since 2012, with increasing yearly rates.3 While multiple conventional medication and therapy treatments are available, patients remain interested in complementary and alternative medicine (CAM) options. According to the National Center for Complementary and Integrative Health, more than 30% of American adults use CAM treatments.4
This article provides an overview of the evidence for commonly used CAM treatments for unipolar depression and anxiety in adults. It is designed to serve as a useful resource when patients are interested in looking beyond conventional medications.
St. John’s wort: ‘Yes’ for depression; ‘no’ for anxiety
Hypericum perforatum, more commonly known as St. John’s wort, is a widely used antidepressant, especially in Europe where it is prescribed, rather than offered over the counter as it is here. Its mechanism of action is not completely understood because its various constituents have different neuropharmacologic activities.5,6
A 2008 Cochrane review evaluated 29 randomized, double-blind studies (N = 5489) that compared St. John’s wort with placebo or standard antidepressants in the treatment of depression.7 St. John’s wort was found to be superior to placebo and comparable to standard antidepressants. More recently, a 2017 meta-analysis of 27 studies (N = 3808) had similar findings.8 In patients with mild-to-moderate depression, St. John’s wart produced rates of remission that were comparable to those produced by selective serotonin reuptake inhibitors (SSRIs) but with a lower discontinuation rate.
Mood disorders other than depression. Studies do not support a role for St. John’s wort in the treatment of anxiety disorders. There are no trials that assess the efficacy of St. John’s wort for the reduction of symptoms of general anxiety disorder as a primary outcome of treatment. Some small clinical trials have investigated the efficacy of St. John’s wort in obsessive-compulsive disorder and social anxiety disorder. In those studies, St. John’s wort performed no better than placebo.9,10
A few words of caution. Preparations of St. John’s wort in the United States are not standardized, so St. John’s wort should be used in America with caution. Furthermore, long-term use of St. John’s wort for depression is questionable given that most studies have evaluated only up to 12 weeks of use.8
If used, studies indicate that the St. John’s wort extract that should be used is 0.3% hypericin or 5% hyperforin administered in a dosage of 300 to 400 mg tid.7,8,11 Physicians and patients can use www.consumerlabs.com to find St. John’s wort brands that have met specified quality criteria based on independent laboratory studies. This Web site can also be used to investigate the quality of the brands available for the other supplements discussed in this article.
Continue to: Adverse effects
Adverse effects. St. John’s wort and an SSRI can lead to serotonin syndrome, which is a constellation of symptoms involving mental status changes and autonomic and neuromuscular hyperactivity caused by serotonin overactivity.12 Furthermore, treatment failures with anticoagulants, digoxin, hormonal contraceptives, immunosuppressants, and narcotics due to concomitant use with St. John’s wort have been reported.13
The most common adverse reactions to St. John’s wort include gastrointestinal symptoms, dizziness, sedation, photosensitivity, dry mouth, urinary frequency, anorgasmia, and swelling.14 However, multiple studies have supported St. John’s wort to be equally or better tolerated than conventional antidepressants.7,8
Certain forms of folate can be adjunctive treatment for depression
Methylfolate is the form of folate that crosses the blood–brain barrier. A prospective observational study evaluated the cerebral spinal fluid of 33 patients with refractory depression. The authors found metabolic abnormalities in the cerebrospinal fluid of most of those patients, the most common of which was folate deficiency in 12 patients despite normal serum folate levels.15
Additionally, current understanding of the role of the Methylenetetrahydrofolate reductase (MTHFR) gene and the folate cycle in depression supports a potential role of methylfolate in depression treatment.16 The MTHFR gene encodes for an enzyme called MTHFR. The MTHFR enzyme converts 5,10-MTHF to 5-MTHF, which then crosses the blood–brain barrier and donates a methyl group for the conversion of homocysteine to methionine. Methionine is a precursor to monoamine neurotransmitters. Thus, decreased expression of the MTHFR gene leads to decreased methylfolate levels, which, in turn, potentially leads to insufficient neurotransmitter synthesis and homocysteine excess.
MTHFR gene polymorphisms and increased homocysteine levels have been found to be associated with the occurrence of depression. One thought is that methylfolate supplementation compensates for an underlying MTHFR enzyme deficiency in patients with depression. Further studies are needed to determine if screening depressed patients for MTHFR gene polymorphisms is of benefit.16
Continue to: A 2012 randomized controlled trial...
A 2012 randomized controlled trial (RCT) (N = 75) compared L-methylfolate 15 mg/d plus an SSRI with placebo plus an SSRI in patients with SSRI-resistant major depression.17 The trial found that a reduction of baseline symptoms by ≥ 50% occurred in more patients who received adjunctive L-methylfolate than placebo (32% vs 15%) and tolerability was comparable. These findings were again supported in 2016 with a 12-month study showing L-methylfolate to have long-term tolerability comparable to placebo,18 and in 2017 with a randomized trial (N = 260) that found escitalopram 10 mg/d plus L-methylfolate 15 mg/d to be significantly more effective at treating depression than escitalopram 10 mg/d alone.19 Thus, methylfolate may be an effective adjunctive treatment for depression at a dosage of 15 mg/d.
S-adenosyl methionine (SAMe) is a metabolite of folate derived from methionine that facilitates the synthesis of neurotransmitters including dopamine, norepinephrine, and serotonin. In Europe, as is the case with St. John’s wort, it is a prescription medication.
A randomized trial (N = 73) compared adjunctive SAMe 800 mg bid with placebo in the treatment of patients with unipolar major depression who did not experience improvement with SSRI treatment alone.20 The investigators found that more patients who received SAMe than who received placebo had improvement in their depression (36.1% vs 17.6%), and more patients who received SAMe compared to placebo went into depression remission (25.8% vs 11.7%).
Adverse effects were comparable in both groups. Thus, SAMe at a dosage of 400 to 1600 mg/d may be effective in the treatment of depression.20,21 The findings of 1 study (N = 65) suggest that patients could experience further improvement in their depression symptoms with SAMe at doses of as much as 3200 mg/d; however, 3200 mg/d increased the occurrence of gastrointestinal adverse effects (31.3% in the SAMe arm vs 3.8% in the placebo group).21
Folate. With regard to folate itself, randomized trials have not supported its efficacy in the treatment of depression in the general population.22
Continue to: VItamin D may improve anxiety/depression in those with low levels
Vitamin D may improve anxiety/depression in those with low levels
Vitamin D supplementation is also being used more frequently in the treatment of depression. Case-control, cross-sectional, and cohort studies have linked low vitamin D levels to the occurrence of depression. A 2013 systematic review of 14 such studies (N = 31,424) found lower vitamin D levels in people with depression compared with controls.23 Further studies are needed to determine if this relationship is causal, and quality RCTs investigating the effect of vitamin D supplementation on depression are lacking.
A 2019 study (N = 30) evaluated the impact of vitamin D supplementation on generalized anxiety disorder in patients with co-occurring vitamin D deficiency. Half received standard-of-care general anxiety disorder treatment plus 50,000 IU of vitamin D weekly for 3 months, while the other half received standard of care alone. Significant improvements in anxiety scores, increases in serum serotonin, and decreases in serum neopterin (an inflammatory marker) were observed in the vitamin D–treated group compared to the group that did not receive vitamin D.24
It is not currently standard of care to check vitamin D levels in all patients presenting with mood disorders. However, if screening is indicated for another reason and low levels are confirmed, vitamin D replacement may improve anxiety and/or depressive symptoms. Despite this, no evidence exists to support vitamin D supplementation for depression or anxiety in patients with normal vitamin D levels.24,25
The effects of omega-3 fatty acids are largely unclear
Research has shown that omega-3 polyunsaturated fatty acids (n-3 PUFA), which are found in fish oil, protect glutamatergic neurotransmission from glucocorticoids, which are released in the body during a stress response.26 Small clinical trials have found n-3 PUFAs to reduce the symptoms of anxiety compared with placebo, but the acids have not been studied directly for anxiety disorders.27,28
With regard to depression, evidence is conflicting as to whether n-3 PUFAs are of any benefit in treatment.29 Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are hypothesized to be the components in omega-3 fatty acid preparations that could lead to a reduction in depressive symptoms. However, randomized trials have shown that EPA-predominant omega-3 fatty acid formulations have only a moderate or no clinically significant effect on depression over placebo, and that DHA-predominant omega-3 fatty acid formulations are only comparable or inferior to placebo.30
Continue to: Response to EPA...
Response to EPA may be greater in patients with depression who have high levels of inflammatory biomarkers, such as interleuken (IL)-1 receptor antagonist (1Ra), IL-6, high-sensitivity C-reactive protein (hs-CRP), leptin, and adiponectin, than in patients with low levels. An 8-week trial randomly assigned patients with unipolar major depression (N = 155) to receive either EPA, DHA, or placebo and found that improvement for the 3 groups was comparable; however, in the subgroup of patients with high levels of inflammatory markers, improvement in depressive symptoms was significantly greater with EPA than with either DHA or placebo.31
It is unclear whether different sources of n-3 PUFA, such as whole fish vs fish oil vs prescription omega-3 acid ethyl esters (Lovaza), are more or less efficacious in the treatment of anxiety or depression. Furthermore, there is no standard dosing for n-3 PUFA in the treatment of mood disorders. Given that the US Food and Drug Administration recommends no more than 2 g/d of combined EPA and DHA supplementation, we recommend using 2 g/d if one decides to treat depression/anxiety with n-3 PUFA.32
N-3 PUFA supplementation is fairly benign. There have been previous concerns about n-3 PUFA supplementation increasing patients’ risk for gastrointestinal bleeding, but a 2006 systematic review that included 9 trials (N = 2612) that looked at clinically significant bleeding episodes found that even patients at high risk for bleeding (ie, those taking aspirin or warfarin) had no increased bleeding risk from taking n-3 PUFA supplementation at up to 4 g/d.33
Don’t underestimate exercise and meditation; consider acupuncture
Exercise. Multiple practice guidelines, including the American Psychiatric Association’s “Practice Guideline for the Treatment of Patients with Major Depressive Disorder,” and meta-analyses have supported the use of exercise to treat unipolar major depression and anxiety.34-37 However, only about 26% of American men and 19% of American women met the US Department of Health and Human Services’ “Federal Physical Activity Guidelines for Americans” in 2016.38
Exercise alone is a reasonable monotherapy, as long as patients are monitored closely for worsening symptoms. Additionally, exercise as an add-on treatment can be helpful for more severe depression or anxiety.39 The best type, duration, and frequency of exercise specifically for the treatment of depression or anxiety has yet to be determined, but physicians may base their exercise recommendations on the “Federal Physical Activity Guidelines for Americans” for general good health (TABLE).38
Continue to: Meditation
Meditation, especially mindfulness meditation, is another strategy that has gained popularity in the treatment of anxiety and depression. Mindfulness has been defined as “the practice of maintaining a nonjudgmental state of heightened or complete awareness of one’s thoughts, emotions, or experiences on a moment-to-moment basis.”40 A 2014 systematic review and meta-analysis of 47 trials with 3515 participants found that mindfulness meditation programs led to clinically significant moderate reductions in anxiety.41 Smaller effects were found for depression.
Nevertheless, meditation may be beneficial as an adjunctive treatment for depression. A small randomized trial (N = 25) compared an adjunctive breathing-based meditation intervention with a waitlist control (delayed yoga) in patients with unipolar major depression who failed to respond to at least 8 weeks of antidepressant treatment.42 The meditation intervention consisted of a group program with sitting meditation, breathing exercises, and yoga postures. Participants engaged in the meditation intervention for 2 to 3.5 hours per day for 8 weeks and demonstrated significant improvement in depression symptoms compared with the control group.42
Acupuncture. A 2018 meta-analysis of 64 studies (N = 7104) suggests that acupuncture results in a small-to-moderate reduction in depressive symptoms when compared to no treatment, control/sham acupuncture, or medication.43 Furthermore, acupuncture plus medication compared to medication alone results in a higher reduction in depressive symptoms without an increase in adverse events.43
Additionally, a 2019 analysis of 10 systematic reviews found acupuncture to be more effective than control/sham acupuncture in the treatment of general anxiety.44 It should be noted, however, that a lot of heterogeneity and potential for bias existed across all of the studies. The studies analyzed were very low to low in quality. Thus, the evidence is insufficient to strongly recommend the use of acupuncture for depression or anxiety, although acupuncture is a safe intervention with low rates of adverse events.
Emotional support animals: Beneficial, but evidence is weak
Emotional support animals are gaining in popularity with Americans who have mood disorders. An important distinction must be made, however, between service animals and emotional support animals. A service animal is one “that is individually trained to do work or perform tasks for the benefit of an individual with a disability, including a physical, sensory, psychiatric, intellectual, or other mental disability.”45 Under the Americans with Disabilities Act (ADA), service animals are limited to dogs, and, in some cases, specially trained miniature horses. Psychiatric service dogs can be trained to do anything from reminding their owner to take medicine to stopping self-mutilation activities.
Continue to: Emotional support animals...
Emotional support animals are not specially trained to perform tasks to help with disabilities. It’s their companionship that helps relieve symptoms of depression and/or anxiety.45 Thus, emotional support animals are not covered under federal laws that apply to service animals. However, the Air Carrier Access Act does require airlines to allow emotional support animals to fly in the cabin for free. Furthermore, the Fair Housing Act allows emotional support animals to circumvent no-pet rules in housing and dorms. Airplanes and housing are the only places legally required to allow the unrestricted presence of emotional support animals.46
Also, there are important distinctions between emotional support animals and pets. While anyone can own a pet, an emotional support animal is prescribed by a licensed mental health professional as a treatment for a mood disorder. Housing facilities and airlines will usually require an emotional support animal “prescription” or letter from a physician to recognize animals as such.
A 2018 systematic review evaluated the evidence behind emotional support animals, which included 17 peer-reviewed journal articles, conference papers, and research dissertations (N = 1727) mostly containing qualitative evidence.47 Unfortunately, there are no RCTs, and there are limited case-control and cohort studies evaluating the effect of an emotional support animal on mood disorders. Based on the available evidence, there does seem to be a psychological benefit to owning an animal for both those with a diagnosable mental health disorder and the general population. This benefit seems to stem from a perceived reduction in social isolation and an increase in emotional support. Factors that determine the psychological benefit of emotional support animals include the type of pet, the number of pets, the attachment to the pet, and the perceived friendliness of the pet.47
Animal-assisted therapy. A 2014 systematic review evaluated higher-level evidence behind animal-assisted therapy (AAT).48 Although participating in therapy that involves interaction with animals is not the same as owning an emotional support animal, the concept—using an animal to improve mental health—is the same. The systematic review looked at 11 RCTs (N = 411) that studied the effect of AAT on mental health. Animals studied included dogs, cats, dolphins, birds, cows, rabbits, ferrets, and guinea pigs. Mental health disorders studied included schizophrenia, depression, anxiety, alcohol/drug abuse, and other addictive behaviors.
Therapeutic animal exposure led to reported improvements in mood, quality of life, and social behavior. These improvements were attributed to the animals buffering people’s reactions to mental stressors. The animals provided a sense of comfort and safety and diverted attention away from immediate stressors. Furthermore, the memory of the animals brought participants a sense of comfort/happiness when they were later without the animal. However, the majority of participants were people who liked animals at baseline.48
CORRESPONDENCE
Amanda E. Olagunju, DO, Operational Medicine Clinic, Langley AFB Hospital, 77 Nealy Avenue, Langley AFB, VA 23665; amanda.olagunju@gmail.com.
Almost 8% of Americans ages ≥ 12 years have depression and 19.1% of Americans ages ≥ 18 years have experienced an anxiety disorder in the past year.1,2 Furthermore, suicide, which can result from depression and anxiety, is the 10th leading cause of death in the United States, claiming about 40,000 to 49,000 lives per year since 2012, with increasing yearly rates.3 While multiple conventional medication and therapy treatments are available, patients remain interested in complementary and alternative medicine (CAM) options. According to the National Center for Complementary and Integrative Health, more than 30% of American adults use CAM treatments.4
This article provides an overview of the evidence for commonly used CAM treatments for unipolar depression and anxiety in adults. It is designed to serve as a useful resource when patients are interested in looking beyond conventional medications.
St. John’s wort: ‘Yes’ for depression; ‘no’ for anxiety
Hypericum perforatum, more commonly known as St. John’s wort, is a widely used antidepressant, especially in Europe where it is prescribed, rather than offered over the counter as it is here. Its mechanism of action is not completely understood because its various constituents have different neuropharmacologic activities.5,6
A 2008 Cochrane review evaluated 29 randomized, double-blind studies (N = 5489) that compared St. John’s wort with placebo or standard antidepressants in the treatment of depression.7 St. John’s wort was found to be superior to placebo and comparable to standard antidepressants. More recently, a 2017 meta-analysis of 27 studies (N = 3808) had similar findings.8 In patients with mild-to-moderate depression, St. John’s wart produced rates of remission that were comparable to those produced by selective serotonin reuptake inhibitors (SSRIs) but with a lower discontinuation rate.
Mood disorders other than depression. Studies do not support a role for St. John’s wort in the treatment of anxiety disorders. There are no trials that assess the efficacy of St. John’s wort for the reduction of symptoms of general anxiety disorder as a primary outcome of treatment. Some small clinical trials have investigated the efficacy of St. John’s wort in obsessive-compulsive disorder and social anxiety disorder. In those studies, St. John’s wort performed no better than placebo.9,10
A few words of caution. Preparations of St. John’s wort in the United States are not standardized, so St. John’s wort should be used in America with caution. Furthermore, long-term use of St. John’s wort for depression is questionable given that most studies have evaluated only up to 12 weeks of use.8
If used, studies indicate that the St. John’s wort extract that should be used is 0.3% hypericin or 5% hyperforin administered in a dosage of 300 to 400 mg tid.7,8,11 Physicians and patients can use www.consumerlabs.com to find St. John’s wort brands that have met specified quality criteria based on independent laboratory studies. This Web site can also be used to investigate the quality of the brands available for the other supplements discussed in this article.
Continue to: Adverse effects
Adverse effects. St. John’s wort and an SSRI can lead to serotonin syndrome, which is a constellation of symptoms involving mental status changes and autonomic and neuromuscular hyperactivity caused by serotonin overactivity.12 Furthermore, treatment failures with anticoagulants, digoxin, hormonal contraceptives, immunosuppressants, and narcotics due to concomitant use with St. John’s wort have been reported.13
The most common adverse reactions to St. John’s wort include gastrointestinal symptoms, dizziness, sedation, photosensitivity, dry mouth, urinary frequency, anorgasmia, and swelling.14 However, multiple studies have supported St. John’s wort to be equally or better tolerated than conventional antidepressants.7,8
Certain forms of folate can be adjunctive treatment for depression
Methylfolate is the form of folate that crosses the blood–brain barrier. A prospective observational study evaluated the cerebral spinal fluid of 33 patients with refractory depression. The authors found metabolic abnormalities in the cerebrospinal fluid of most of those patients, the most common of which was folate deficiency in 12 patients despite normal serum folate levels.15
Additionally, current understanding of the role of the Methylenetetrahydrofolate reductase (MTHFR) gene and the folate cycle in depression supports a potential role of methylfolate in depression treatment.16 The MTHFR gene encodes for an enzyme called MTHFR. The MTHFR enzyme converts 5,10-MTHF to 5-MTHF, which then crosses the blood–brain barrier and donates a methyl group for the conversion of homocysteine to methionine. Methionine is a precursor to monoamine neurotransmitters. Thus, decreased expression of the MTHFR gene leads to decreased methylfolate levels, which, in turn, potentially leads to insufficient neurotransmitter synthesis and homocysteine excess.
MTHFR gene polymorphisms and increased homocysteine levels have been found to be associated with the occurrence of depression. One thought is that methylfolate supplementation compensates for an underlying MTHFR enzyme deficiency in patients with depression. Further studies are needed to determine if screening depressed patients for MTHFR gene polymorphisms is of benefit.16
Continue to: A 2012 randomized controlled trial...
A 2012 randomized controlled trial (RCT) (N = 75) compared L-methylfolate 15 mg/d plus an SSRI with placebo plus an SSRI in patients with SSRI-resistant major depression.17 The trial found that a reduction of baseline symptoms by ≥ 50% occurred in more patients who received adjunctive L-methylfolate than placebo (32% vs 15%) and tolerability was comparable. These findings were again supported in 2016 with a 12-month study showing L-methylfolate to have long-term tolerability comparable to placebo,18 and in 2017 with a randomized trial (N = 260) that found escitalopram 10 mg/d plus L-methylfolate 15 mg/d to be significantly more effective at treating depression than escitalopram 10 mg/d alone.19 Thus, methylfolate may be an effective adjunctive treatment for depression at a dosage of 15 mg/d.
S-adenosyl methionine (SAMe) is a metabolite of folate derived from methionine that facilitates the synthesis of neurotransmitters including dopamine, norepinephrine, and serotonin. In Europe, as is the case with St. John’s wort, it is a prescription medication.
A randomized trial (N = 73) compared adjunctive SAMe 800 mg bid with placebo in the treatment of patients with unipolar major depression who did not experience improvement with SSRI treatment alone.20 The investigators found that more patients who received SAMe than who received placebo had improvement in their depression (36.1% vs 17.6%), and more patients who received SAMe compared to placebo went into depression remission (25.8% vs 11.7%).
Adverse effects were comparable in both groups. Thus, SAMe at a dosage of 400 to 1600 mg/d may be effective in the treatment of depression.20,21 The findings of 1 study (N = 65) suggest that patients could experience further improvement in their depression symptoms with SAMe at doses of as much as 3200 mg/d; however, 3200 mg/d increased the occurrence of gastrointestinal adverse effects (31.3% in the SAMe arm vs 3.8% in the placebo group).21
Folate. With regard to folate itself, randomized trials have not supported its efficacy in the treatment of depression in the general population.22
Continue to: VItamin D may improve anxiety/depression in those with low levels
Vitamin D may improve anxiety/depression in those with low levels
Vitamin D supplementation is also being used more frequently in the treatment of depression. Case-control, cross-sectional, and cohort studies have linked low vitamin D levels to the occurrence of depression. A 2013 systematic review of 14 such studies (N = 31,424) found lower vitamin D levels in people with depression compared with controls.23 Further studies are needed to determine if this relationship is causal, and quality RCTs investigating the effect of vitamin D supplementation on depression are lacking.
A 2019 study (N = 30) evaluated the impact of vitamin D supplementation on generalized anxiety disorder in patients with co-occurring vitamin D deficiency. Half received standard-of-care general anxiety disorder treatment plus 50,000 IU of vitamin D weekly for 3 months, while the other half received standard of care alone. Significant improvements in anxiety scores, increases in serum serotonin, and decreases in serum neopterin (an inflammatory marker) were observed in the vitamin D–treated group compared to the group that did not receive vitamin D.24
It is not currently standard of care to check vitamin D levels in all patients presenting with mood disorders. However, if screening is indicated for another reason and low levels are confirmed, vitamin D replacement may improve anxiety and/or depressive symptoms. Despite this, no evidence exists to support vitamin D supplementation for depression or anxiety in patients with normal vitamin D levels.24,25
The effects of omega-3 fatty acids are largely unclear
Research has shown that omega-3 polyunsaturated fatty acids (n-3 PUFA), which are found in fish oil, protect glutamatergic neurotransmission from glucocorticoids, which are released in the body during a stress response.26 Small clinical trials have found n-3 PUFAs to reduce the symptoms of anxiety compared with placebo, but the acids have not been studied directly for anxiety disorders.27,28
With regard to depression, evidence is conflicting as to whether n-3 PUFAs are of any benefit in treatment.29 Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are hypothesized to be the components in omega-3 fatty acid preparations that could lead to a reduction in depressive symptoms. However, randomized trials have shown that EPA-predominant omega-3 fatty acid formulations have only a moderate or no clinically significant effect on depression over placebo, and that DHA-predominant omega-3 fatty acid formulations are only comparable or inferior to placebo.30
Continue to: Response to EPA...
Response to EPA may be greater in patients with depression who have high levels of inflammatory biomarkers, such as interleuken (IL)-1 receptor antagonist (1Ra), IL-6, high-sensitivity C-reactive protein (hs-CRP), leptin, and adiponectin, than in patients with low levels. An 8-week trial randomly assigned patients with unipolar major depression (N = 155) to receive either EPA, DHA, or placebo and found that improvement for the 3 groups was comparable; however, in the subgroup of patients with high levels of inflammatory markers, improvement in depressive symptoms was significantly greater with EPA than with either DHA or placebo.31
It is unclear whether different sources of n-3 PUFA, such as whole fish vs fish oil vs prescription omega-3 acid ethyl esters (Lovaza), are more or less efficacious in the treatment of anxiety or depression. Furthermore, there is no standard dosing for n-3 PUFA in the treatment of mood disorders. Given that the US Food and Drug Administration recommends no more than 2 g/d of combined EPA and DHA supplementation, we recommend using 2 g/d if one decides to treat depression/anxiety with n-3 PUFA.32
N-3 PUFA supplementation is fairly benign. There have been previous concerns about n-3 PUFA supplementation increasing patients’ risk for gastrointestinal bleeding, but a 2006 systematic review that included 9 trials (N = 2612) that looked at clinically significant bleeding episodes found that even patients at high risk for bleeding (ie, those taking aspirin or warfarin) had no increased bleeding risk from taking n-3 PUFA supplementation at up to 4 g/d.33
Don’t underestimate exercise and meditation; consider acupuncture
Exercise. Multiple practice guidelines, including the American Psychiatric Association’s “Practice Guideline for the Treatment of Patients with Major Depressive Disorder,” and meta-analyses have supported the use of exercise to treat unipolar major depression and anxiety.34-37 However, only about 26% of American men and 19% of American women met the US Department of Health and Human Services’ “Federal Physical Activity Guidelines for Americans” in 2016.38
Exercise alone is a reasonable monotherapy, as long as patients are monitored closely for worsening symptoms. Additionally, exercise as an add-on treatment can be helpful for more severe depression or anxiety.39 The best type, duration, and frequency of exercise specifically for the treatment of depression or anxiety has yet to be determined, but physicians may base their exercise recommendations on the “Federal Physical Activity Guidelines for Americans” for general good health (TABLE).38
Continue to: Meditation
Meditation, especially mindfulness meditation, is another strategy that has gained popularity in the treatment of anxiety and depression. Mindfulness has been defined as “the practice of maintaining a nonjudgmental state of heightened or complete awareness of one’s thoughts, emotions, or experiences on a moment-to-moment basis.”40 A 2014 systematic review and meta-analysis of 47 trials with 3515 participants found that mindfulness meditation programs led to clinically significant moderate reductions in anxiety.41 Smaller effects were found for depression.
Nevertheless, meditation may be beneficial as an adjunctive treatment for depression. A small randomized trial (N = 25) compared an adjunctive breathing-based meditation intervention with a waitlist control (delayed yoga) in patients with unipolar major depression who failed to respond to at least 8 weeks of antidepressant treatment.42 The meditation intervention consisted of a group program with sitting meditation, breathing exercises, and yoga postures. Participants engaged in the meditation intervention for 2 to 3.5 hours per day for 8 weeks and demonstrated significant improvement in depression symptoms compared with the control group.42
Acupuncture. A 2018 meta-analysis of 64 studies (N = 7104) suggests that acupuncture results in a small-to-moderate reduction in depressive symptoms when compared to no treatment, control/sham acupuncture, or medication.43 Furthermore, acupuncture plus medication compared to medication alone results in a higher reduction in depressive symptoms without an increase in adverse events.43
Additionally, a 2019 analysis of 10 systematic reviews found acupuncture to be more effective than control/sham acupuncture in the treatment of general anxiety.44 It should be noted, however, that a lot of heterogeneity and potential for bias existed across all of the studies. The studies analyzed were very low to low in quality. Thus, the evidence is insufficient to strongly recommend the use of acupuncture for depression or anxiety, although acupuncture is a safe intervention with low rates of adverse events.
Emotional support animals: Beneficial, but evidence is weak
Emotional support animals are gaining in popularity with Americans who have mood disorders. An important distinction must be made, however, between service animals and emotional support animals. A service animal is one “that is individually trained to do work or perform tasks for the benefit of an individual with a disability, including a physical, sensory, psychiatric, intellectual, or other mental disability.”45 Under the Americans with Disabilities Act (ADA), service animals are limited to dogs, and, in some cases, specially trained miniature horses. Psychiatric service dogs can be trained to do anything from reminding their owner to take medicine to stopping self-mutilation activities.
Continue to: Emotional support animals...
Emotional support animals are not specially trained to perform tasks to help with disabilities. It’s their companionship that helps relieve symptoms of depression and/or anxiety.45 Thus, emotional support animals are not covered under federal laws that apply to service animals. However, the Air Carrier Access Act does require airlines to allow emotional support animals to fly in the cabin for free. Furthermore, the Fair Housing Act allows emotional support animals to circumvent no-pet rules in housing and dorms. Airplanes and housing are the only places legally required to allow the unrestricted presence of emotional support animals.46
Also, there are important distinctions between emotional support animals and pets. While anyone can own a pet, an emotional support animal is prescribed by a licensed mental health professional as a treatment for a mood disorder. Housing facilities and airlines will usually require an emotional support animal “prescription” or letter from a physician to recognize animals as such.
A 2018 systematic review evaluated the evidence behind emotional support animals, which included 17 peer-reviewed journal articles, conference papers, and research dissertations (N = 1727) mostly containing qualitative evidence.47 Unfortunately, there are no RCTs, and there are limited case-control and cohort studies evaluating the effect of an emotional support animal on mood disorders. Based on the available evidence, there does seem to be a psychological benefit to owning an animal for both those with a diagnosable mental health disorder and the general population. This benefit seems to stem from a perceived reduction in social isolation and an increase in emotional support. Factors that determine the psychological benefit of emotional support animals include the type of pet, the number of pets, the attachment to the pet, and the perceived friendliness of the pet.47
Animal-assisted therapy. A 2014 systematic review evaluated higher-level evidence behind animal-assisted therapy (AAT).48 Although participating in therapy that involves interaction with animals is not the same as owning an emotional support animal, the concept—using an animal to improve mental health—is the same. The systematic review looked at 11 RCTs (N = 411) that studied the effect of AAT on mental health. Animals studied included dogs, cats, dolphins, birds, cows, rabbits, ferrets, and guinea pigs. Mental health disorders studied included schizophrenia, depression, anxiety, alcohol/drug abuse, and other addictive behaviors.
Therapeutic animal exposure led to reported improvements in mood, quality of life, and social behavior. These improvements were attributed to the animals buffering people’s reactions to mental stressors. The animals provided a sense of comfort and safety and diverted attention away from immediate stressors. Furthermore, the memory of the animals brought participants a sense of comfort/happiness when they were later without the animal. However, the majority of participants were people who liked animals at baseline.48
CORRESPONDENCE
Amanda E. Olagunju, DO, Operational Medicine Clinic, Langley AFB Hospital, 77 Nealy Avenue, Langley AFB, VA 23665; amanda.olagunju@gmail.com.
1. National Center for Health Statistics, Centers for Disease Control and Prevention. FastStats: Depression. Last reviewed October 7, 2015. www.cdc.gov/nchs/fastats/depression.htm. Accessed May 26, 2020.
2. National Institute of Mental Health. Mental health information—statistics: any anxiety disorder. Last updated November 2017. www.nimh.nih.gov/health/statistics/any-anxiety-disorder.shtml. Accessed May 26, 2020.
3. Hedegaard H, Curtin SC, Warner M. Increase in suicide mortality in the United States, 1999–2018. NCHS Data Brief, no 362. Hyattsville, MD: National Center for Health Statistics; 2020.
4. National Center for Complementary and Integrative Health. Complementary, alternative, or integrative health: what’s in a name? Last updated July 2018. www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name. Accessed May 26, 2020.
5. Bennett DA Jr, Phun L, Polk JF, et al. Neuropharmacology of St. John’s wort (Hypericum). Ann Pharmacother. 1998;32:1201-1208.
6. Müller WE, Singer A, Wonnemann M, et al. Hyperforin represents the neurotransmitter reuptake inhibiting constituent of hypericum extract. Pharmacopsychiatry. 1998;31(suppl 1):16-21.
7. Linde K, Berner MM, Kriston L. St John’s wort for major depression. Cochrane Database Syst Rev. 2008;CD000448.
8.
9. Kobak KA, Taylor LV, Bystritsky A, et al. St John’s wort versus placebo in obsessive-compulsive disorder: results from a double-blind study. Int Clin Psychopharmacol. 2005;20:299-304.
10. Kobak KA, Taylor LV, Warner G, et al. St. John’s wort versus placebo in social phobia: results from a placebo-controlled pilot study. J Clin Psychopharmacol. 2005;25:51-58.
11. Product reviews: St. John’s wort supplements review. ConsumerLab.com. September 23, 2016. www.consumerlab.com/reviews/St_Johns_Wort/stjohnswort/. Accessed May 26, 2020.
12. Simhan S. Serotonin syndrome. In: Abd-Elsayed A. (ed) Pain: A Review Guide. New York, NY: Springer; 2019.
13. Chrubasik-Hausmann S, Vlachojannis J, McLachlan A. Understanding drug interactions with St. John’s wort (Hypericum perforatum L.): impact of hyperforin content. J Pharm Pharmacol. 2019;71:129-138.
14. Knüppel L, Linde K. Adverse effects of St. John’s wort: a systematic review. J Clin Psychiatry. 2004;65:1470-1479.
15. Pan LA, Martin P, Zimmer T, et al. Neurometabolic disorders: potentially treatable abnormalities in patients with treatment-refractory depression and suicidal behavior. Am J Psychiatry. 2017;174:42-50.
16. Kandler C, Lam S. Methylenetetrahydrofolate reductase screening in treatment-resistant depression. Fed Pract. 2019;36:207-208.
17. Papakostas GI, Shelton RC, Zajecka JM, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012;169:1267-1274.
18. Zajecka J, Fava M, Shelton R, et al. Long-term efficacy, safety, and tolerability of L-methylfolate calcium 15 mg as adjunctive therapy with selective serotonin reuptake inhibitors: a 12-month, open-label study following a placebo-controlled acute study. J Clin Psychiatry. 2016;77:654-660.
19. Kakar MS, Jehangir S, Mustafa M, et al. Therapeutic efficacy of combination therapy of L-methylfolate and escitalopram in depression. Pakistan Armed Forces Med J. 2017;67:976-981.
20. Papakostas GI, Mischoulon D, Shyu I, et al. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: a double-blind, randomized clinical trial. Am J Psychiatry. 2010;167:942-948.
21.
22. Sarris J, Murphy J, Mischoulon D, et al. Adjunctive nutraceuticals for depression: a systematic review and meta-analyses. Am J Psychiatry. 2016;173:575-587.
23. Anglin RE, Samaan Z, Walter SD, et al. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100-107.
24. Eid A, Khoja S, AlGhamdi S, et al. Vitamin D supplementation ameliorates severity of generalized anxiety disorder (GAD). Metab Brain Dis. 2019;34:1781-1786.
25. Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab. 2014;99:757-767.
26. Hennebelle M, Champeil-Potokar G, Lavialle M, et al. Omega-3 polyunsaturated fatty acids and chronic stress-induced modulations of glutamatergic neurotransmission in the hippocampus. Nutr Rev. 2014;72:99-112.
27. Kiecolt-Glaser JK, Belury MA, Andridge R, et al. Omega-3 supplementation lowers inflammation and anxiety in medical students: a randomized controlled trial. Brain Behav Immun. 2011;25:1725-1734.
28. Buydens-Branchey L, Branchey M, Hibbeln JR. Associations between increases in plasma n-3 polyunsaturated fatty acids following supplementation and decreases in anger and anxiety in substance abusers. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:568-575.
29. Lin PY, Mischoulon D, Freeman MP, et al. Are omega-3 fatty acids antidepressants or just mood-improving agents? The effect depends upon diagnosis, supplement preparation, and severity of depression. Mol Psychiatry 2012;17:1161-1163.
30. Hallahan B, Ryan T, Hibbeln JR, et al. Efficacy of omega-3 highly unsaturated fatty acids in the treatment of depression. Br J Psychiatry. 2016;209:192-201.
31. Rapaport MH, Nierenberg AA, Schettler PJ, et al. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol Psychiatry. 2016;21:71-79.
32. National Institutes of Health Office of Dietary Supplements. Omega-3 fatty acids. Updated October 17, 2019. https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional. Accessed May 26, 2020.
33. Wang C, Harris WS, Chung M, et al. n-3 fatty acids from fish or fish-oil supplements, but not alpha-linoleic acid, benefit cardiovascular disease outcomes in primary- and secondary- prevention studies: a systematic review. Am J Clin Nutr. 2006;84:5-17.
34. American Psychiatric Association Practice. Guideline for the Treatment of Patients with Major Depressive Disorder. 3rd Edition. 2010. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Accessed May 26, 2020.
35. Gordon B, McDowell C, Lyons M, et al. The effects of resistance exercise training on anxiety: a meta-analysis and meta-regression analysis of randomized controlled trials. Sports Med. 2017;47:2521-2532.
36. Stubbs B, Vancampfort D, Rosenbaum S, et al. An examination of the anxiolytic effects of exercise for people with anxiety and stress-related disorders: a meta-analysis. Psychiatry Res. 2017;249:102-108.
37. Rethorst CD, Trivedi MH. Evidence-based recommendations for the prescription of exercise for major depressive disorder. J Psychiatr Pract. 2013;19:204-212.
38. US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd edition. Washington, DC: US Department of Health and Human Services; 2018.
39. Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;CD004366.
40. Merriam-Webster Dictionary. "Mindfulness." www.merriam-webster.com/dictionary/mindfulness. Accessed May 26, 2020.
41. Goyal M, Singh S, Sibinga EM, et al. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med. 2014;174:357-368.
42. Sharma A, Barrett MS, Cucchiara AJ, et al. A breathing-based meditation intervention for patients with major depressive disorder following inadequate response to antidepressants: a randomized pilot study. J Clin Psychiatry. 2017;78:e59-e63.
43. Smith CA, Armour M, Soo Lee M, et al. Acupuncture for depression. Cochrane Database Syst Rev. 2018;CD004046.
44. Li M
45. Brennan J. Service animals and emotional support animals. ADA National Network Information Guidance and Training on the Americans with Disabilities Act. Last updated April 2020. adata.org/publication/service-animals-booklet. Accessed May 26, 2020.
46. Clay RA. Is that a pet or therapeutic aid? Monitor on Psychology. 2016;47:38.
47. Brooks HL, Rushton K, Lovell K, et al. The power of support from companion animals for people living with mental health problems: a systematic review and narrative synthesis of the evidence. BMC Psychiatry. 2018;18:31.
48. Kamioka H, Okada S, Tsutani K, et al. Effectiveness of animal-assisted therapy: a systematic review of randomized controlled trials. Complement Ther Med. 2014;22:371-390.
1. National Center for Health Statistics, Centers for Disease Control and Prevention. FastStats: Depression. Last reviewed October 7, 2015. www.cdc.gov/nchs/fastats/depression.htm. Accessed May 26, 2020.
2. National Institute of Mental Health. Mental health information—statistics: any anxiety disorder. Last updated November 2017. www.nimh.nih.gov/health/statistics/any-anxiety-disorder.shtml. Accessed May 26, 2020.
3. Hedegaard H, Curtin SC, Warner M. Increase in suicide mortality in the United States, 1999–2018. NCHS Data Brief, no 362. Hyattsville, MD: National Center for Health Statistics; 2020.
4. National Center for Complementary and Integrative Health. Complementary, alternative, or integrative health: what’s in a name? Last updated July 2018. www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name. Accessed May 26, 2020.
5. Bennett DA Jr, Phun L, Polk JF, et al. Neuropharmacology of St. John’s wort (Hypericum). Ann Pharmacother. 1998;32:1201-1208.
6. Müller WE, Singer A, Wonnemann M, et al. Hyperforin represents the neurotransmitter reuptake inhibiting constituent of hypericum extract. Pharmacopsychiatry. 1998;31(suppl 1):16-21.
7. Linde K, Berner MM, Kriston L. St John’s wort for major depression. Cochrane Database Syst Rev. 2008;CD000448.
8.
9. Kobak KA, Taylor LV, Bystritsky A, et al. St John’s wort versus placebo in obsessive-compulsive disorder: results from a double-blind study. Int Clin Psychopharmacol. 2005;20:299-304.
10. Kobak KA, Taylor LV, Warner G, et al. St. John’s wort versus placebo in social phobia: results from a placebo-controlled pilot study. J Clin Psychopharmacol. 2005;25:51-58.
11. Product reviews: St. John’s wort supplements review. ConsumerLab.com. September 23, 2016. www.consumerlab.com/reviews/St_Johns_Wort/stjohnswort/. Accessed May 26, 2020.
12. Simhan S. Serotonin syndrome. In: Abd-Elsayed A. (ed) Pain: A Review Guide. New York, NY: Springer; 2019.
13. Chrubasik-Hausmann S, Vlachojannis J, McLachlan A. Understanding drug interactions with St. John’s wort (Hypericum perforatum L.): impact of hyperforin content. J Pharm Pharmacol. 2019;71:129-138.
14. Knüppel L, Linde K. Adverse effects of St. John’s wort: a systematic review. J Clin Psychiatry. 2004;65:1470-1479.
15. Pan LA, Martin P, Zimmer T, et al. Neurometabolic disorders: potentially treatable abnormalities in patients with treatment-refractory depression and suicidal behavior. Am J Psychiatry. 2017;174:42-50.
16. Kandler C, Lam S. Methylenetetrahydrofolate reductase screening in treatment-resistant depression. Fed Pract. 2019;36:207-208.
17. Papakostas GI, Shelton RC, Zajecka JM, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012;169:1267-1274.
18. Zajecka J, Fava M, Shelton R, et al. Long-term efficacy, safety, and tolerability of L-methylfolate calcium 15 mg as adjunctive therapy with selective serotonin reuptake inhibitors: a 12-month, open-label study following a placebo-controlled acute study. J Clin Psychiatry. 2016;77:654-660.
19. Kakar MS, Jehangir S, Mustafa M, et al. Therapeutic efficacy of combination therapy of L-methylfolate and escitalopram in depression. Pakistan Armed Forces Med J. 2017;67:976-981.
20. Papakostas GI, Mischoulon D, Shyu I, et al. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: a double-blind, randomized clinical trial. Am J Psychiatry. 2010;167:942-948.
21.
22. Sarris J, Murphy J, Mischoulon D, et al. Adjunctive nutraceuticals for depression: a systematic review and meta-analyses. Am J Psychiatry. 2016;173:575-587.
23. Anglin RE, Samaan Z, Walter SD, et al. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100-107.
24. Eid A, Khoja S, AlGhamdi S, et al. Vitamin D supplementation ameliorates severity of generalized anxiety disorder (GAD). Metab Brain Dis. 2019;34:1781-1786.
25. Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab. 2014;99:757-767.
26. Hennebelle M, Champeil-Potokar G, Lavialle M, et al. Omega-3 polyunsaturated fatty acids and chronic stress-induced modulations of glutamatergic neurotransmission in the hippocampus. Nutr Rev. 2014;72:99-112.
27. Kiecolt-Glaser JK, Belury MA, Andridge R, et al. Omega-3 supplementation lowers inflammation and anxiety in medical students: a randomized controlled trial. Brain Behav Immun. 2011;25:1725-1734.
28. Buydens-Branchey L, Branchey M, Hibbeln JR. Associations between increases in plasma n-3 polyunsaturated fatty acids following supplementation and decreases in anger and anxiety in substance abusers. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:568-575.
29. Lin PY, Mischoulon D, Freeman MP, et al. Are omega-3 fatty acids antidepressants or just mood-improving agents? The effect depends upon diagnosis, supplement preparation, and severity of depression. Mol Psychiatry 2012;17:1161-1163.
30. Hallahan B, Ryan T, Hibbeln JR, et al. Efficacy of omega-3 highly unsaturated fatty acids in the treatment of depression. Br J Psychiatry. 2016;209:192-201.
31. Rapaport MH, Nierenberg AA, Schettler PJ, et al. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol Psychiatry. 2016;21:71-79.
32. National Institutes of Health Office of Dietary Supplements. Omega-3 fatty acids. Updated October 17, 2019. https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional. Accessed May 26, 2020.
33. Wang C, Harris WS, Chung M, et al. n-3 fatty acids from fish or fish-oil supplements, but not alpha-linoleic acid, benefit cardiovascular disease outcomes in primary- and secondary- prevention studies: a systematic review. Am J Clin Nutr. 2006;84:5-17.
34. American Psychiatric Association Practice. Guideline for the Treatment of Patients with Major Depressive Disorder. 3rd Edition. 2010. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Accessed May 26, 2020.
35. Gordon B, McDowell C, Lyons M, et al. The effects of resistance exercise training on anxiety: a meta-analysis and meta-regression analysis of randomized controlled trials. Sports Med. 2017;47:2521-2532.
36. Stubbs B, Vancampfort D, Rosenbaum S, et al. An examination of the anxiolytic effects of exercise for people with anxiety and stress-related disorders: a meta-analysis. Psychiatry Res. 2017;249:102-108.
37. Rethorst CD, Trivedi MH. Evidence-based recommendations for the prescription of exercise for major depressive disorder. J Psychiatr Pract. 2013;19:204-212.
38. US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd edition. Washington, DC: US Department of Health and Human Services; 2018.
39. Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;CD004366.
40. Merriam-Webster Dictionary. "Mindfulness." www.merriam-webster.com/dictionary/mindfulness. Accessed May 26, 2020.
41. Goyal M, Singh S, Sibinga EM, et al. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med. 2014;174:357-368.
42. Sharma A, Barrett MS, Cucchiara AJ, et al. A breathing-based meditation intervention for patients with major depressive disorder following inadequate response to antidepressants: a randomized pilot study. J Clin Psychiatry. 2017;78:e59-e63.
43. Smith CA, Armour M, Soo Lee M, et al. Acupuncture for depression. Cochrane Database Syst Rev. 2018;CD004046.
44. Li M
45. Brennan J. Service animals and emotional support animals. ADA National Network Information Guidance and Training on the Americans with Disabilities Act. Last updated April 2020. adata.org/publication/service-animals-booklet. Accessed May 26, 2020.
46. Clay RA. Is that a pet or therapeutic aid? Monitor on Psychology. 2016;47:38.
47. Brooks HL, Rushton K, Lovell K, et al. The power of support from companion animals for people living with mental health problems: a systematic review and narrative synthesis of the evidence. BMC Psychiatry. 2018;18:31.
48. Kamioka H, Okada S, Tsutani K, et al. Effectiveness of animal-assisted therapy: a systematic review of randomized controlled trials. Complement Ther Med. 2014;22:371-390.
PRACTICE RECOMMENDATIONS
› Consider standardized preparations of St. John’s wort for the treatment of mild to moderate depression in certain patients. A
› Encourage patients with depression or anxiety to engage in exercise and meditation to help with symptom management. A
› Consider methylfolate and S-adenosyl methionine as adjunctive treatments to improve depression. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series