User login
‘Doc, can I get a mask exemption?’
As more jurisdictions mandate facial coverings in public, questions have arisen about whether it’s safe for everyone – including those with lung disease – to wear masks.
To address these issues, Medscape spoke with the chief medical officer of the American Lung Association, Dr. Albert Rizzo.
The CDC recommendations on mask wearing say, “Cloth face coverings should not be placed on young children under age 2, anyone who has trouble breathing, or is unconscious, incapacitated, or otherwise unable to remove the mask without assistance.” Does this language suggest that there indeed is a subset of the adult population with lung disease who shouldn’t wear masks?
It makes sense to say that if it makes you uncomfortable to wear a mask because it affects your breathing, you should think twice about getting in a situation where you would have to wear a mask.
I’ve told many of my high-risk patients, “The best way to avoid getting COVID-19 is to stay home and stay away from sick people, especially if you feel that you are not going to be able to wear a mask or facial covering of some sort.”
The reason that some people have trouble with a mask is that they haven’t tried the right style of mask – by that I mean how tightly it fits and the material it’s made out of. Sometimes it really is just that people with lung disease don’t like to have anything covering their faces. Many of these patients feel better where there is air blowing across their faces – they will have a fan blowing even in the middle of winter because they feel more comfortable.
I won’t say it’s all in their heads, but sometimes it’s a matter of desensitizing themselves to wearing a mask. I liken it to people who have sleep apnea. We often have to desensitize them to wearing a mask for sleeping. We tell them to put it on while they are watching TV — don’t hook it up to anything yet, just get used to having something on your face.
I’ve told my patients the same thing about masks for COVID-19. Put on the mask, see how it feels. If you become uncomfortable breathing with it on, take it off, but maybe you can handle it for a half hour or 45 minutes. Find out how much time you have for a trip to the grocery store based on how comfortable you are wearing it at home.
It’s a matter of training the patient, giving them options of how to get comfortable with it, and then making them realize that they have to weigh the benefits and risks of wearing the mask and feeling out of breath versus going out in public and being potentially exposed to coronavirus. And the bottom line is, anybody who is wearing a mask and starts to feel uncomfortable, they can take the mask off.
You mentioned different types of masks. Is there a type of mask that is typically more breathable that clinicians can recommend to patients with lung disease?
First, I remind patients who think they will have trouble breathing with a mask on that they are choosing a mask not so much to protect themselves – that would take an N95 mask to filter out the virus. The mask is worn so that when they cough or drink or speak, they aren’t sending respiratory droplets out into the environment. Even when we speak, respiratory droplets can easily go out as far as 6 feet, or further with coughing or sneezing. With facial coverings, we try to keep those respiratory droplets from getting out and infecting others.
So when choosing a mask, you don’t have to worry as much about a tight-fitting mask. I recommend a loose-fitting mask that covers the nose and mouth and isn’t going to fall off but isn’t so tight around the ears and neck to make them feel uncomfortable. Even though it doesn’t really protect the wearer, it is cutting down on the ability to breathe in droplets – maybe not microscopic particles, but it’s better than nothing.
Is a face shield a reasonable alternative for someone who feels they can’t breathe with a mask on?
Yes. I’m surprised that face shields don’t get more attention. I’ve tried them out, and they are actually more comfortable than masks. They do impede the spilling out of droplets into the public, but they are not as close fitting to the face as a mask. If you want to protect others, the face shield should be adequate. It is not as good at preventing you from breathing in viral particles.
Some people have claimed that wearing a mask makes them hyperventilate and feel like they are going to pass out, or the mask causes them to become hypoxic. Are these valid concerns?
We get two questions about masks from patients who feel that they are short of breath or are worried about wearing a mask. One is whether their oxygen level is dropping. It’s usually not that. It’s usually because they feel that the mask is an impediment to getting air in. Their oxygen levels are stable.
The other question is whether the mask causes CO2 retention. For the mask to trap enough exhaled CO2 and for us to breathe enough of that CO2 back in to raise our CO2 level, it has to be a pretty tight-fitting mask. With the type of masks we are suggesting that people wear, that’s very unlikely to occur.
What can clinicians do to reassure patients with some type of lung disease that they can safely wear masks?
There are a few things they can do right in the office. Have them put the mask on for a few minutes and make sure they feel comfortable with it. With an oximeter, patients can see that their oxygen levels don’t change when they are breathing through the mask for a period of time.
You can’t really measure CO2 retention that easily, but most patients with chronic obstructive pulmonary disease or pulmonary fibrosis don’t have an elevated CO2 at baseline. A little more education is helpful in those situations. In most cases, they aren’t going to retain enough CO2 to have problems wearing a mask.
Only a small percentage of patients with lung disease are CO2 retainers, and many of those patients are being seen by pulmonary specialists. Those are the patients you might want to be more cautious with, to make sure they aren’t wearing anything that is tight fitting or that makes them work harder to breathe. It’s not that the mask is causing CO2 retention, but the increased work of breathing may make it harder to exhale the CO2.
Does a mask interfere with supplemental oxygen in any way?
Supplemental oxygen is typically supplied through a nasal cannula, so 100% oxygen is still getting to the nasal passages and entrained down into the airway, so it shouldn’t be a problem.
Some of the resistance to wearing masks has come from people with asthma. Is it safe for patients with asthma to wear masks, or should these patients be exempt from wearing masks?
In general, the breathing of people with mild asthma, both young and old, should not be impeded by the wearing of facial coverings. The concerns about oxygen and carbon dioxide among patients with more severe lung disease should not play a role in asthma.
Since younger adults with COVID-19 seem to have fewer or no symptoms and may actually be carrying the virus unknowingly, this should be the main population who should wear masks to prevent transmission to others.
Exemptions for mask wearing for mild asthma should be discouraged and dealt with on a case-by-case basis if there is a particular concern for that individual.
How do you respond if a patient asks you for a formal medical exemption to wearing a mask?
We’ve been asked to do a lot of letter writing for patients around going back to work, as well as the issue of wearing masks. The discussion usually revolves around trying to avoid going somewhere where you would have to wear a mask if it makes you feel uncomfortable.
I do not recommend automatically exempting individuals from wearing masks, even many of my pulmonary patients. There needs to be an understanding by the patient regarding the purpose of the mask and the overall advice to stay out of situations where social distancing is not being practiced. If you can take the time to discuss options as mentioned above – mask styles, desensitization, etc – the patient usually understands and will try wearing a mask.
On a case-by-case basis, some individuals may need to be exempted, but I feel this is a small number. I prefer my high-risk (older, chronic disease, etc) patients do everything they can to avoid infection – handwashing, mask wearing, and socially distancing.
They should also realize that even with a note, it is not going to help if they are in the middle of the grocery store and someone confronts them about not wearing a mask. It may help as they enter a store that says “masks required” and they can show it to someone monitoring the door. But I’m not really sure in what situations having that note is going to be helpful if confrontations occur.
Patients are also asking how safe is it for them to go back to work and be out in public. I tell them, nothing is going to be 100% safe. Until we have an effective vaccine, we are all going to have to weigh the potential risks of going to an area where social distancing isn’t maintained, people aren’t wearing face masks, and you can’t wash your hands as much as you’d like to. That’s going to be a struggle for all of us to get back out into situations where people interact socially.
Albert A. Rizzo, MD, is chief medical officer for the American Lung Association, chief of the Section of Pulmonary and Critical Care Medicine at the Christiana Care Health System in Newark, Delaware, and a member of Christiana Care Pulmonary Associates. He is board certified in internal medicine, pulmonary medicine, critical care medicine, and sleep medicine and is a clinical assistant professor of medicine at Thomas Jefferson University Medical School, Philadelphia.
This article first appeared on Medscape.com.
As more jurisdictions mandate facial coverings in public, questions have arisen about whether it’s safe for everyone – including those with lung disease – to wear masks.
To address these issues, Medscape spoke with the chief medical officer of the American Lung Association, Dr. Albert Rizzo.
The CDC recommendations on mask wearing say, “Cloth face coverings should not be placed on young children under age 2, anyone who has trouble breathing, or is unconscious, incapacitated, or otherwise unable to remove the mask without assistance.” Does this language suggest that there indeed is a subset of the adult population with lung disease who shouldn’t wear masks?
It makes sense to say that if it makes you uncomfortable to wear a mask because it affects your breathing, you should think twice about getting in a situation where you would have to wear a mask.
I’ve told many of my high-risk patients, “The best way to avoid getting COVID-19 is to stay home and stay away from sick people, especially if you feel that you are not going to be able to wear a mask or facial covering of some sort.”
The reason that some people have trouble with a mask is that they haven’t tried the right style of mask – by that I mean how tightly it fits and the material it’s made out of. Sometimes it really is just that people with lung disease don’t like to have anything covering their faces. Many of these patients feel better where there is air blowing across their faces – they will have a fan blowing even in the middle of winter because they feel more comfortable.
I won’t say it’s all in their heads, but sometimes it’s a matter of desensitizing themselves to wearing a mask. I liken it to people who have sleep apnea. We often have to desensitize them to wearing a mask for sleeping. We tell them to put it on while they are watching TV — don’t hook it up to anything yet, just get used to having something on your face.
I’ve told my patients the same thing about masks for COVID-19. Put on the mask, see how it feels. If you become uncomfortable breathing with it on, take it off, but maybe you can handle it for a half hour or 45 minutes. Find out how much time you have for a trip to the grocery store based on how comfortable you are wearing it at home.
It’s a matter of training the patient, giving them options of how to get comfortable with it, and then making them realize that they have to weigh the benefits and risks of wearing the mask and feeling out of breath versus going out in public and being potentially exposed to coronavirus. And the bottom line is, anybody who is wearing a mask and starts to feel uncomfortable, they can take the mask off.
You mentioned different types of masks. Is there a type of mask that is typically more breathable that clinicians can recommend to patients with lung disease?
First, I remind patients who think they will have trouble breathing with a mask on that they are choosing a mask not so much to protect themselves – that would take an N95 mask to filter out the virus. The mask is worn so that when they cough or drink or speak, they aren’t sending respiratory droplets out into the environment. Even when we speak, respiratory droplets can easily go out as far as 6 feet, or further with coughing or sneezing. With facial coverings, we try to keep those respiratory droplets from getting out and infecting others.
So when choosing a mask, you don’t have to worry as much about a tight-fitting mask. I recommend a loose-fitting mask that covers the nose and mouth and isn’t going to fall off but isn’t so tight around the ears and neck to make them feel uncomfortable. Even though it doesn’t really protect the wearer, it is cutting down on the ability to breathe in droplets – maybe not microscopic particles, but it’s better than nothing.
Is a face shield a reasonable alternative for someone who feels they can’t breathe with a mask on?
Yes. I’m surprised that face shields don’t get more attention. I’ve tried them out, and they are actually more comfortable than masks. They do impede the spilling out of droplets into the public, but they are not as close fitting to the face as a mask. If you want to protect others, the face shield should be adequate. It is not as good at preventing you from breathing in viral particles.
Some people have claimed that wearing a mask makes them hyperventilate and feel like they are going to pass out, or the mask causes them to become hypoxic. Are these valid concerns?
We get two questions about masks from patients who feel that they are short of breath or are worried about wearing a mask. One is whether their oxygen level is dropping. It’s usually not that. It’s usually because they feel that the mask is an impediment to getting air in. Their oxygen levels are stable.
The other question is whether the mask causes CO2 retention. For the mask to trap enough exhaled CO2 and for us to breathe enough of that CO2 back in to raise our CO2 level, it has to be a pretty tight-fitting mask. With the type of masks we are suggesting that people wear, that’s very unlikely to occur.
What can clinicians do to reassure patients with some type of lung disease that they can safely wear masks?
There are a few things they can do right in the office. Have them put the mask on for a few minutes and make sure they feel comfortable with it. With an oximeter, patients can see that their oxygen levels don’t change when they are breathing through the mask for a period of time.
You can’t really measure CO2 retention that easily, but most patients with chronic obstructive pulmonary disease or pulmonary fibrosis don’t have an elevated CO2 at baseline. A little more education is helpful in those situations. In most cases, they aren’t going to retain enough CO2 to have problems wearing a mask.
Only a small percentage of patients with lung disease are CO2 retainers, and many of those patients are being seen by pulmonary specialists. Those are the patients you might want to be more cautious with, to make sure they aren’t wearing anything that is tight fitting or that makes them work harder to breathe. It’s not that the mask is causing CO2 retention, but the increased work of breathing may make it harder to exhale the CO2.
Does a mask interfere with supplemental oxygen in any way?
Supplemental oxygen is typically supplied through a nasal cannula, so 100% oxygen is still getting to the nasal passages and entrained down into the airway, so it shouldn’t be a problem.
Some of the resistance to wearing masks has come from people with asthma. Is it safe for patients with asthma to wear masks, or should these patients be exempt from wearing masks?
In general, the breathing of people with mild asthma, both young and old, should not be impeded by the wearing of facial coverings. The concerns about oxygen and carbon dioxide among patients with more severe lung disease should not play a role in asthma.
Since younger adults with COVID-19 seem to have fewer or no symptoms and may actually be carrying the virus unknowingly, this should be the main population who should wear masks to prevent transmission to others.
Exemptions for mask wearing for mild asthma should be discouraged and dealt with on a case-by-case basis if there is a particular concern for that individual.
How do you respond if a patient asks you for a formal medical exemption to wearing a mask?
We’ve been asked to do a lot of letter writing for patients around going back to work, as well as the issue of wearing masks. The discussion usually revolves around trying to avoid going somewhere where you would have to wear a mask if it makes you feel uncomfortable.
I do not recommend automatically exempting individuals from wearing masks, even many of my pulmonary patients. There needs to be an understanding by the patient regarding the purpose of the mask and the overall advice to stay out of situations where social distancing is not being practiced. If you can take the time to discuss options as mentioned above – mask styles, desensitization, etc – the patient usually understands and will try wearing a mask.
On a case-by-case basis, some individuals may need to be exempted, but I feel this is a small number. I prefer my high-risk (older, chronic disease, etc) patients do everything they can to avoid infection – handwashing, mask wearing, and socially distancing.
They should also realize that even with a note, it is not going to help if they are in the middle of the grocery store and someone confronts them about not wearing a mask. It may help as they enter a store that says “masks required” and they can show it to someone monitoring the door. But I’m not really sure in what situations having that note is going to be helpful if confrontations occur.
Patients are also asking how safe is it for them to go back to work and be out in public. I tell them, nothing is going to be 100% safe. Until we have an effective vaccine, we are all going to have to weigh the potential risks of going to an area where social distancing isn’t maintained, people aren’t wearing face masks, and you can’t wash your hands as much as you’d like to. That’s going to be a struggle for all of us to get back out into situations where people interact socially.
Albert A. Rizzo, MD, is chief medical officer for the American Lung Association, chief of the Section of Pulmonary and Critical Care Medicine at the Christiana Care Health System in Newark, Delaware, and a member of Christiana Care Pulmonary Associates. He is board certified in internal medicine, pulmonary medicine, critical care medicine, and sleep medicine and is a clinical assistant professor of medicine at Thomas Jefferson University Medical School, Philadelphia.
This article first appeared on Medscape.com.
As more jurisdictions mandate facial coverings in public, questions have arisen about whether it’s safe for everyone – including those with lung disease – to wear masks.
To address these issues, Medscape spoke with the chief medical officer of the American Lung Association, Dr. Albert Rizzo.
The CDC recommendations on mask wearing say, “Cloth face coverings should not be placed on young children under age 2, anyone who has trouble breathing, or is unconscious, incapacitated, or otherwise unable to remove the mask without assistance.” Does this language suggest that there indeed is a subset of the adult population with lung disease who shouldn’t wear masks?
It makes sense to say that if it makes you uncomfortable to wear a mask because it affects your breathing, you should think twice about getting in a situation where you would have to wear a mask.
I’ve told many of my high-risk patients, “The best way to avoid getting COVID-19 is to stay home and stay away from sick people, especially if you feel that you are not going to be able to wear a mask or facial covering of some sort.”
The reason that some people have trouble with a mask is that they haven’t tried the right style of mask – by that I mean how tightly it fits and the material it’s made out of. Sometimes it really is just that people with lung disease don’t like to have anything covering their faces. Many of these patients feel better where there is air blowing across their faces – they will have a fan blowing even in the middle of winter because they feel more comfortable.
I won’t say it’s all in their heads, but sometimes it’s a matter of desensitizing themselves to wearing a mask. I liken it to people who have sleep apnea. We often have to desensitize them to wearing a mask for sleeping. We tell them to put it on while they are watching TV — don’t hook it up to anything yet, just get used to having something on your face.
I’ve told my patients the same thing about masks for COVID-19. Put on the mask, see how it feels. If you become uncomfortable breathing with it on, take it off, but maybe you can handle it for a half hour or 45 minutes. Find out how much time you have for a trip to the grocery store based on how comfortable you are wearing it at home.
It’s a matter of training the patient, giving them options of how to get comfortable with it, and then making them realize that they have to weigh the benefits and risks of wearing the mask and feeling out of breath versus going out in public and being potentially exposed to coronavirus. And the bottom line is, anybody who is wearing a mask and starts to feel uncomfortable, they can take the mask off.
You mentioned different types of masks. Is there a type of mask that is typically more breathable that clinicians can recommend to patients with lung disease?
First, I remind patients who think they will have trouble breathing with a mask on that they are choosing a mask not so much to protect themselves – that would take an N95 mask to filter out the virus. The mask is worn so that when they cough or drink or speak, they aren’t sending respiratory droplets out into the environment. Even when we speak, respiratory droplets can easily go out as far as 6 feet, or further with coughing or sneezing. With facial coverings, we try to keep those respiratory droplets from getting out and infecting others.
So when choosing a mask, you don’t have to worry as much about a tight-fitting mask. I recommend a loose-fitting mask that covers the nose and mouth and isn’t going to fall off but isn’t so tight around the ears and neck to make them feel uncomfortable. Even though it doesn’t really protect the wearer, it is cutting down on the ability to breathe in droplets – maybe not microscopic particles, but it’s better than nothing.
Is a face shield a reasonable alternative for someone who feels they can’t breathe with a mask on?
Yes. I’m surprised that face shields don’t get more attention. I’ve tried them out, and they are actually more comfortable than masks. They do impede the spilling out of droplets into the public, but they are not as close fitting to the face as a mask. If you want to protect others, the face shield should be adequate. It is not as good at preventing you from breathing in viral particles.
Some people have claimed that wearing a mask makes them hyperventilate and feel like they are going to pass out, or the mask causes them to become hypoxic. Are these valid concerns?
We get two questions about masks from patients who feel that they are short of breath or are worried about wearing a mask. One is whether their oxygen level is dropping. It’s usually not that. It’s usually because they feel that the mask is an impediment to getting air in. Their oxygen levels are stable.
The other question is whether the mask causes CO2 retention. For the mask to trap enough exhaled CO2 and for us to breathe enough of that CO2 back in to raise our CO2 level, it has to be a pretty tight-fitting mask. With the type of masks we are suggesting that people wear, that’s very unlikely to occur.
What can clinicians do to reassure patients with some type of lung disease that they can safely wear masks?
There are a few things they can do right in the office. Have them put the mask on for a few minutes and make sure they feel comfortable with it. With an oximeter, patients can see that their oxygen levels don’t change when they are breathing through the mask for a period of time.
You can’t really measure CO2 retention that easily, but most patients with chronic obstructive pulmonary disease or pulmonary fibrosis don’t have an elevated CO2 at baseline. A little more education is helpful in those situations. In most cases, they aren’t going to retain enough CO2 to have problems wearing a mask.
Only a small percentage of patients with lung disease are CO2 retainers, and many of those patients are being seen by pulmonary specialists. Those are the patients you might want to be more cautious with, to make sure they aren’t wearing anything that is tight fitting or that makes them work harder to breathe. It’s not that the mask is causing CO2 retention, but the increased work of breathing may make it harder to exhale the CO2.
Does a mask interfere with supplemental oxygen in any way?
Supplemental oxygen is typically supplied through a nasal cannula, so 100% oxygen is still getting to the nasal passages and entrained down into the airway, so it shouldn’t be a problem.
Some of the resistance to wearing masks has come from people with asthma. Is it safe for patients with asthma to wear masks, or should these patients be exempt from wearing masks?
In general, the breathing of people with mild asthma, both young and old, should not be impeded by the wearing of facial coverings. The concerns about oxygen and carbon dioxide among patients with more severe lung disease should not play a role in asthma.
Since younger adults with COVID-19 seem to have fewer or no symptoms and may actually be carrying the virus unknowingly, this should be the main population who should wear masks to prevent transmission to others.
Exemptions for mask wearing for mild asthma should be discouraged and dealt with on a case-by-case basis if there is a particular concern for that individual.
How do you respond if a patient asks you for a formal medical exemption to wearing a mask?
We’ve been asked to do a lot of letter writing for patients around going back to work, as well as the issue of wearing masks. The discussion usually revolves around trying to avoid going somewhere where you would have to wear a mask if it makes you feel uncomfortable.
I do not recommend automatically exempting individuals from wearing masks, even many of my pulmonary patients. There needs to be an understanding by the patient regarding the purpose of the mask and the overall advice to stay out of situations where social distancing is not being practiced. If you can take the time to discuss options as mentioned above – mask styles, desensitization, etc – the patient usually understands and will try wearing a mask.
On a case-by-case basis, some individuals may need to be exempted, but I feel this is a small number. I prefer my high-risk (older, chronic disease, etc) patients do everything they can to avoid infection – handwashing, mask wearing, and socially distancing.
They should also realize that even with a note, it is not going to help if they are in the middle of the grocery store and someone confronts them about not wearing a mask. It may help as they enter a store that says “masks required” and they can show it to someone monitoring the door. But I’m not really sure in what situations having that note is going to be helpful if confrontations occur.
Patients are also asking how safe is it for them to go back to work and be out in public. I tell them, nothing is going to be 100% safe. Until we have an effective vaccine, we are all going to have to weigh the potential risks of going to an area where social distancing isn’t maintained, people aren’t wearing face masks, and you can’t wash your hands as much as you’d like to. That’s going to be a struggle for all of us to get back out into situations where people interact socially.
Albert A. Rizzo, MD, is chief medical officer for the American Lung Association, chief of the Section of Pulmonary and Critical Care Medicine at the Christiana Care Health System in Newark, Delaware, and a member of Christiana Care Pulmonary Associates. He is board certified in internal medicine, pulmonary medicine, critical care medicine, and sleep medicine and is a clinical assistant professor of medicine at Thomas Jefferson University Medical School, Philadelphia.
This article first appeared on Medscape.com.
Hypnosis may relieve pain, cut reliance on morphine at atrial flutter ablation
used as a control, in a small randomized trial.
Ablation is typically performed using conscious sedation and “requires sometimes very high dosages of morphine, and there are sometimes some complications, blood pressure drop, or oxygen desaturation,” Rodrigue Garcia, MD, Poitiers (France) University Hospital, said in an interview.
But patients in the study assigned to undergo hypnosis during the AFlut ablation, performed by practitioners hailing from the French Hypnosis Association, consistently perceived significantly less pain throughout the procedure than those in the active-control group.
They also used almost two-thirds less morphine, which was available to both groups on demand, reported Dr. Garcia, who presented the results of the PAINLESS study at the European Heart Rhythm Association 2020 Virtual Congress. The annual meeting was conducted online this year because of the COVID-19 pandemic.
Hypnotism for pain control may not be widely available in hospitals, “but it’s becoming more and more frequent in the different centers, especially in France,” he said.
The technique is probably also suitable for catheter ablation of ventricular tachycardia, Dr. Garcia said, and “we already use it for atrial fibrillation ablation, because it’s a very common procedure and because, in France, for example, there is a lack of anesthesiologists.” One limitation of hypnosis for such procedures, he said, is that it requires a practitioner with a lot of training and experience.
The current study, “I think, is one of the few, if not the first, randomized trial on this topic, at least for flutter,” Elena Arbelo, MD, PhD, MSc, Hospital Clinic of Barcelona and the University of Barcelona, said in an interview.
“I thought it was very interesting. Many centers have the issue of not having anesthesiology support for their procedures. We have the option of having anesthesiology with us only a few days a week,” said Dr. Arbelo, who was not an investigator with the study.
“If it’s validated in larger cohorts and in different cultures, it may be an interesting way of reducing the need for anesthesiology support, which is a main issue. I know for sure in Europe,” she said, that “some centers do struggle to have anesthesiology support for their EP procedures.”
The single-center trial randomized adults slated to undergo cavotricuspid isthmus ablation (n = 116) for AFlut to receive hypnosis or a control procedure, consisting of nonhypnotic relaxation suggestions and white noise delivered through earphones – 56 and 57 patients, respectively, after exclusion of several who ultimately did not undergo ablation. Any patient could receive 1 mg of morphine if self-reported pain was 5 or greater on a 10-point visual analog scale, or simply on demand.
The hypnosis and control groups were predominantly male and well matched for age (mean, about 69 years in both groups), prevalence of atrial fibrillation, and left ventricular ejection fraction (about 55% for both). Also, in both groups, the procedure duration was approximately 36 minutes.
Asked if all patients in the hypnosis group were actually hypnotized, Dr. Garcia said: “That’s a tricky question” because there was no prespecified definition for successful hypnosis. Between 70% and 80% achieved a hypnotized state, he estimated.
Hypnosis was superior to the control intervention for the primary outcome of pain self-assessment during the ablation procedure, as recorded 45 minutes after ablation. Also, using a 10-point visual analog scale, the hypnosis group rated the average pain intensity as 4.0, whereas the control group rated it as 5.5 (P < .001).
Similarly, instantaneous pain intensity, rated on a 10-point scale every 5 minutes, was lower throughout the procedure for the hypnosis patients than for the control patients (P < .05 at all assessments). Maximum pain intensities, which occurred at the 15- to 25-minute points, were no greater than 3 for hypnosis patients and peaked at approximately 5 for the control patients.
Two of three secondary end points favored the hypnosis group. Morphine consumption averaged 1.3 mg, compared with 3.6 mg for the control group (P < .001). Observer-assessed degrees of sedation were 8.3 and 5.4, respectively, on a 10-point scale (P < .001). And patient self-assessment of anxiety during the procedure was 1.5 in the hypnosis group and 2.5 in the control group on a similar scale.
Regarding morphine use in the two groups, Dr. Garcia said, “It was more than 2 mg of difference, and this can be very important, especially in certain types of patients,” such as those with compromised lung function.
All six complications (11%) observed during the study occurred in the control group. There were four severe hypotensive episodes, one case of oxygen desaturation, and one case of pericardial effusion (P = .03 vs the hypnosis group).
After pointing out the substantial risk for adverse events associated with deep analgesia, particularly from the use of opiates, Paulus Kirchhof, MD, PhD, said, “I think it’s a clinically relevant topic, in the context of reducing the risk of ablation procedures, to try to minimize the use of opiates or other strong anesthetics.”
A multicenter trial could be the next step, said Dr. Kirchhof, from the University Heart and Vascular Center UKE Hamburg (Germany). That would potentially provide “the first evidence for me that this is not sort of something that works in one specific setting, but that it is transferable to other centers, other countries, where practices and complication rates of analgosedation may be different.”
Dr. Kirchhof praised the study design for comparing hypnosis with an active standard-of-care control group. “That is one of the strengths of the study; they tried to design it in a way that didn’t disadvantage the control group.”
The study was funded by the University Hospital of Poitiers. Dr. Garcia and Dr. Arbelo reported no conflicts of interest. Dr. Kirchhof reported support for basic, translational, and clinical research projects from the European Union, the British Heart Foundation, the Leducq Foundation, the Medical Research Council, and the German Centre for Cardiovascular Research, and from several drug and device companies active in atrial fibrillation, from which he received honoraria more than 3 years ago; he is listed as inventor on two patents held by the University of Birmingham (England).
A version of this article originally appeared on Medscape.com.
used as a control, in a small randomized trial.
Ablation is typically performed using conscious sedation and “requires sometimes very high dosages of morphine, and there are sometimes some complications, blood pressure drop, or oxygen desaturation,” Rodrigue Garcia, MD, Poitiers (France) University Hospital, said in an interview.
But patients in the study assigned to undergo hypnosis during the AFlut ablation, performed by practitioners hailing from the French Hypnosis Association, consistently perceived significantly less pain throughout the procedure than those in the active-control group.
They also used almost two-thirds less morphine, which was available to both groups on demand, reported Dr. Garcia, who presented the results of the PAINLESS study at the European Heart Rhythm Association 2020 Virtual Congress. The annual meeting was conducted online this year because of the COVID-19 pandemic.
Hypnotism for pain control may not be widely available in hospitals, “but it’s becoming more and more frequent in the different centers, especially in France,” he said.
The technique is probably also suitable for catheter ablation of ventricular tachycardia, Dr. Garcia said, and “we already use it for atrial fibrillation ablation, because it’s a very common procedure and because, in France, for example, there is a lack of anesthesiologists.” One limitation of hypnosis for such procedures, he said, is that it requires a practitioner with a lot of training and experience.
The current study, “I think, is one of the few, if not the first, randomized trial on this topic, at least for flutter,” Elena Arbelo, MD, PhD, MSc, Hospital Clinic of Barcelona and the University of Barcelona, said in an interview.
“I thought it was very interesting. Many centers have the issue of not having anesthesiology support for their procedures. We have the option of having anesthesiology with us only a few days a week,” said Dr. Arbelo, who was not an investigator with the study.
“If it’s validated in larger cohorts and in different cultures, it may be an interesting way of reducing the need for anesthesiology support, which is a main issue. I know for sure in Europe,” she said, that “some centers do struggle to have anesthesiology support for their EP procedures.”
The single-center trial randomized adults slated to undergo cavotricuspid isthmus ablation (n = 116) for AFlut to receive hypnosis or a control procedure, consisting of nonhypnotic relaxation suggestions and white noise delivered through earphones – 56 and 57 patients, respectively, after exclusion of several who ultimately did not undergo ablation. Any patient could receive 1 mg of morphine if self-reported pain was 5 or greater on a 10-point visual analog scale, or simply on demand.
The hypnosis and control groups were predominantly male and well matched for age (mean, about 69 years in both groups), prevalence of atrial fibrillation, and left ventricular ejection fraction (about 55% for both). Also, in both groups, the procedure duration was approximately 36 minutes.
Asked if all patients in the hypnosis group were actually hypnotized, Dr. Garcia said: “That’s a tricky question” because there was no prespecified definition for successful hypnosis. Between 70% and 80% achieved a hypnotized state, he estimated.
Hypnosis was superior to the control intervention for the primary outcome of pain self-assessment during the ablation procedure, as recorded 45 minutes after ablation. Also, using a 10-point visual analog scale, the hypnosis group rated the average pain intensity as 4.0, whereas the control group rated it as 5.5 (P < .001).
Similarly, instantaneous pain intensity, rated on a 10-point scale every 5 minutes, was lower throughout the procedure for the hypnosis patients than for the control patients (P < .05 at all assessments). Maximum pain intensities, which occurred at the 15- to 25-minute points, were no greater than 3 for hypnosis patients and peaked at approximately 5 for the control patients.
Two of three secondary end points favored the hypnosis group. Morphine consumption averaged 1.3 mg, compared with 3.6 mg for the control group (P < .001). Observer-assessed degrees of sedation were 8.3 and 5.4, respectively, on a 10-point scale (P < .001). And patient self-assessment of anxiety during the procedure was 1.5 in the hypnosis group and 2.5 in the control group on a similar scale.
Regarding morphine use in the two groups, Dr. Garcia said, “It was more than 2 mg of difference, and this can be very important, especially in certain types of patients,” such as those with compromised lung function.
All six complications (11%) observed during the study occurred in the control group. There were four severe hypotensive episodes, one case of oxygen desaturation, and one case of pericardial effusion (P = .03 vs the hypnosis group).
After pointing out the substantial risk for adverse events associated with deep analgesia, particularly from the use of opiates, Paulus Kirchhof, MD, PhD, said, “I think it’s a clinically relevant topic, in the context of reducing the risk of ablation procedures, to try to minimize the use of opiates or other strong anesthetics.”
A multicenter trial could be the next step, said Dr. Kirchhof, from the University Heart and Vascular Center UKE Hamburg (Germany). That would potentially provide “the first evidence for me that this is not sort of something that works in one specific setting, but that it is transferable to other centers, other countries, where practices and complication rates of analgosedation may be different.”
Dr. Kirchhof praised the study design for comparing hypnosis with an active standard-of-care control group. “That is one of the strengths of the study; they tried to design it in a way that didn’t disadvantage the control group.”
The study was funded by the University Hospital of Poitiers. Dr. Garcia and Dr. Arbelo reported no conflicts of interest. Dr. Kirchhof reported support for basic, translational, and clinical research projects from the European Union, the British Heart Foundation, the Leducq Foundation, the Medical Research Council, and the German Centre for Cardiovascular Research, and from several drug and device companies active in atrial fibrillation, from which he received honoraria more than 3 years ago; he is listed as inventor on two patents held by the University of Birmingham (England).
A version of this article originally appeared on Medscape.com.
used as a control, in a small randomized trial.
Ablation is typically performed using conscious sedation and “requires sometimes very high dosages of morphine, and there are sometimes some complications, blood pressure drop, or oxygen desaturation,” Rodrigue Garcia, MD, Poitiers (France) University Hospital, said in an interview.
But patients in the study assigned to undergo hypnosis during the AFlut ablation, performed by practitioners hailing from the French Hypnosis Association, consistently perceived significantly less pain throughout the procedure than those in the active-control group.
They also used almost two-thirds less morphine, which was available to both groups on demand, reported Dr. Garcia, who presented the results of the PAINLESS study at the European Heart Rhythm Association 2020 Virtual Congress. The annual meeting was conducted online this year because of the COVID-19 pandemic.
Hypnotism for pain control may not be widely available in hospitals, “but it’s becoming more and more frequent in the different centers, especially in France,” he said.
The technique is probably also suitable for catheter ablation of ventricular tachycardia, Dr. Garcia said, and “we already use it for atrial fibrillation ablation, because it’s a very common procedure and because, in France, for example, there is a lack of anesthesiologists.” One limitation of hypnosis for such procedures, he said, is that it requires a practitioner with a lot of training and experience.
The current study, “I think, is one of the few, if not the first, randomized trial on this topic, at least for flutter,” Elena Arbelo, MD, PhD, MSc, Hospital Clinic of Barcelona and the University of Barcelona, said in an interview.
“I thought it was very interesting. Many centers have the issue of not having anesthesiology support for their procedures. We have the option of having anesthesiology with us only a few days a week,” said Dr. Arbelo, who was not an investigator with the study.
“If it’s validated in larger cohorts and in different cultures, it may be an interesting way of reducing the need for anesthesiology support, which is a main issue. I know for sure in Europe,” she said, that “some centers do struggle to have anesthesiology support for their EP procedures.”
The single-center trial randomized adults slated to undergo cavotricuspid isthmus ablation (n = 116) for AFlut to receive hypnosis or a control procedure, consisting of nonhypnotic relaxation suggestions and white noise delivered through earphones – 56 and 57 patients, respectively, after exclusion of several who ultimately did not undergo ablation. Any patient could receive 1 mg of morphine if self-reported pain was 5 or greater on a 10-point visual analog scale, or simply on demand.
The hypnosis and control groups were predominantly male and well matched for age (mean, about 69 years in both groups), prevalence of atrial fibrillation, and left ventricular ejection fraction (about 55% for both). Also, in both groups, the procedure duration was approximately 36 minutes.
Asked if all patients in the hypnosis group were actually hypnotized, Dr. Garcia said: “That’s a tricky question” because there was no prespecified definition for successful hypnosis. Between 70% and 80% achieved a hypnotized state, he estimated.
Hypnosis was superior to the control intervention for the primary outcome of pain self-assessment during the ablation procedure, as recorded 45 minutes after ablation. Also, using a 10-point visual analog scale, the hypnosis group rated the average pain intensity as 4.0, whereas the control group rated it as 5.5 (P < .001).
Similarly, instantaneous pain intensity, rated on a 10-point scale every 5 minutes, was lower throughout the procedure for the hypnosis patients than for the control patients (P < .05 at all assessments). Maximum pain intensities, which occurred at the 15- to 25-minute points, were no greater than 3 for hypnosis patients and peaked at approximately 5 for the control patients.
Two of three secondary end points favored the hypnosis group. Morphine consumption averaged 1.3 mg, compared with 3.6 mg for the control group (P < .001). Observer-assessed degrees of sedation were 8.3 and 5.4, respectively, on a 10-point scale (P < .001). And patient self-assessment of anxiety during the procedure was 1.5 in the hypnosis group and 2.5 in the control group on a similar scale.
Regarding morphine use in the two groups, Dr. Garcia said, “It was more than 2 mg of difference, and this can be very important, especially in certain types of patients,” such as those with compromised lung function.
All six complications (11%) observed during the study occurred in the control group. There were four severe hypotensive episodes, one case of oxygen desaturation, and one case of pericardial effusion (P = .03 vs the hypnosis group).
After pointing out the substantial risk for adverse events associated with deep analgesia, particularly from the use of opiates, Paulus Kirchhof, MD, PhD, said, “I think it’s a clinically relevant topic, in the context of reducing the risk of ablation procedures, to try to minimize the use of opiates or other strong anesthetics.”
A multicenter trial could be the next step, said Dr. Kirchhof, from the University Heart and Vascular Center UKE Hamburg (Germany). That would potentially provide “the first evidence for me that this is not sort of something that works in one specific setting, but that it is transferable to other centers, other countries, where practices and complication rates of analgosedation may be different.”
Dr. Kirchhof praised the study design for comparing hypnosis with an active standard-of-care control group. “That is one of the strengths of the study; they tried to design it in a way that didn’t disadvantage the control group.”
The study was funded by the University Hospital of Poitiers. Dr. Garcia and Dr. Arbelo reported no conflicts of interest. Dr. Kirchhof reported support for basic, translational, and clinical research projects from the European Union, the British Heart Foundation, the Leducq Foundation, the Medical Research Council, and the German Centre for Cardiovascular Research, and from several drug and device companies active in atrial fibrillation, from which he received honoraria more than 3 years ago; he is listed as inventor on two patents held by the University of Birmingham (England).
A version of this article originally appeared on Medscape.com.
Does moderate drinking slow cognitive decline?
new research suggests. However, at least one expert urges caution in interpreting the findings.
Investigators found that consuming 10-14 alcoholic drinks per week had the strongest cognitive benefit. The findings “add more weight” to the growing body of research identifying beneficial cognitive effects of moderate alcohol consumption, said lead author, Ruiyuan Zhang, MD, of the department of epidemiology and biostatistics at the University of Georgia, Athens. However, Dr. Zhang emphasized that nondrinkers should not take up drinking to protect brain function, as alcohol can have negative effects.
The study was published online in JAMA Network Open.
Slower cognitive decline
The observational study was a secondary analysis of data from the Health and Retirement Study, a nationally representative U.S. survey of middle-aged and older adults. The survey, which began in 1992, is conducted every 2 years and collects health and economic data.
The current analysis used data from 1996 to 2008 and included information from individuals who participated in at least three surveys. The study included 19,887 participants, with a mean age 61.8 years. Most (60.1%) were women and white (85.2%). Mean follow-up was 9.1 years.
Researchers measured cognitive domains of mental status, word recall, and vocabulary. They also calculated a total cognition score, with higher scores indicating better cognitive abilities.
For each cognitive function measure, researchers categorized participants into a consistently low–trajectory group in which cognitive test scores from baseline through follow-up were consistently low or a consistently high–trajectory group, where cognitive test scores from baseline through follow-up were consistently high.
Based on self-reports, the investigators categorized participants as never drinkers (41.8%), former drinkers (39.5%), or current drinkers (18.7%). For current drinkers, researchers determined the number of drinking days per week and number of drinks per day. They further categorized these participants as low to moderate drinkers or heavy drinkers.
One drink was defined as a 12-ounce bottle of beer, a 5-ounce glass of wine, or a 1.5-ounce shot of spirits, said Dr. Zhang.
Women who consumed 8 or more drinks per week and men who drank 15 or more drinks per week were considered heavy drinkers. Other current drinkers were deemed low to moderate drinkers. Most current drinkers (85.2%) were low to moderate drinkers.
Other covariates included age, sex, race/ethnicity, years of education, marital status, tobacco smoking status, and body mass index.
Results showed moderate drinking was associated with relatively high cognitive test scores. After controlling for all covariates, compared with never drinkers, current low to moderate drinkers were significantly less likely to have consistently low trajectories for total cognitive score (odds ratio, 0.66; 95% confidence interval, 0.59-0.74), mental status (OR, 0.71; 95% CI, 0.63-0.81), word recall (OR, 0.74; 95% CI, 0.69-0.80), and vocabulary (OR, 0.64; 95% CI, 0.56-0.74) (all P < .001).
Former drinkers also had better cognitive outcomes for all cognitive domains. Heavy drinkers had lower odds of being in the consistently low trajectory group only for the vocabulary test.
Heavy drinking ‘risky’
Because few participants were deemed to be heavy drinkers, the power to identify an association between heavy drinking and cognitive function was limited. Dr. Zhang acknowledged, though he noted that heavy drinking is “risky.”
“We found that, after the drinking dosage passes the moderate level, the risk of low cognitive function increases very fast, which indicates that heavy drinking may harm cognitive function.” Limiting alcohol consumption “is still very important,” he said.
The associations of alcohol and cognitive functions differed by race/ethnicity. Low to moderate drinking was significantly associated with a lower odds of having a consistently low trajectory for all four cognitive function measures only among white participants.
A possible reason for this is that the study had so few African Americans (who made up only 14.8% of the sample), which limited the ability to identify relationships between alcohol intake and cognitive function, said Dr. Zhang. “Another reason is that the sensitivity to alcohol may be different between white and African American subjects.”
There was a significant U-shaped association between weekly amounts of alcohol and the odds of being in the consistently low–trajectory group for all cognitive functions. Depending on the function tested, the optimal number of weekly drinks ranged from 10-14.
Dr. Zhang noted that, when women were examined separately, alcohol consumption had a significant U-shaped relationship only with word recall, with the optimal dosage being around eight drinks.
U-shaped relationship an ‘important finding’
The U-shaped relationship is “an important finding,” said Dr. Zhang. “It shows that the human body may act differently to low and high doses of alcohol. Knowing why and how this happens is very important as it would help us understand how alcohol affects the function of the human body.”
Sensitivity analyses among participants with no chronic diseases showed the U-shaped association was still significant for scores of total word recall and vocabulary, but not for mental status or total cognition score.
The authors noted that 77.2% of participants had at least one chronic disease. They maintained that the association between alcohol consumption and cognitive function may be applicable both to healthy people and to those with a chronic disease.
The study also found that low to moderate drinkers had slower rates of cognitive decline over time for all cognition domains.
Although the mechanisms underlying the cognitive benefits of alcohol consumption are unclear, the authors believe it may be via cerebrovascular and cardiovascular pathways.
Alcohol may increase levels of brain-derived neurotrophic factor, a key regulator of neuronal plasticity and development in the dorsal striatum, they noted.
Balancing act
However, there’s also evidence that drinking, especially heavy drinking, increases the risk of hypertension, stroke, liver damage, and some cancers. “We think the role of alcohol drinking in cognitive function may be a balance of its beneficial and harmful effects on the cardiovascular system,” said Dr. Zhang.
“For the low to moderate drinker, the beneficial effects may outweigh the harmful effects on the small blood vessels in the brain. In this way, it could preserve cognition,” he added.
Dr. Zhang also noted that the study focused on middle-aged and older adults. “We can’t say whether or not moderate alcohol could benefit younger people” because they may have different characteristics, he said.
The findings of other studies examining the effects of alcohol on cognitive function are mixed. While studies have identified a beneficial effect, others have uncovered no, minimal, or adverse effects. This could be due to the use of different tests of cognitive function or different study populations, said Dr. Zhang.
A limitation of the current study was that assessment of alcohol consumption was based on self-report, which might have introduced recall bias. In addition, because individuals tend to underestimate their alcohol consumption, heavy drinkers could be misclassified as low to moderate drinkers, and low to moderate drinkers as former drinkers.
“This may make our study underestimate the association between low to moderate drinking and cognitive function,” said Dr. Zhang. In addition, alcohol consumption tended to change with time, and this change may be associated with other factors that led to changes in cognitive function, the authors noted.
Interpret with caution
Commenting on the study, Brent P. Forester, MD, chief of the Center of Excellence in Geriatric Psychiatry at McLean Hospital in Belmont, Mass., associate professor of psychiatry at Harvard Medical School, Boston, and a member of the American Psychiatric Association Council on Geriatric Psychiatry, said he views the study with some trepidation.
“As a clinician taking care of older adults, I would be very cautious about overinterpreting the beneficial effects of alcohol before we understand the mechanism better,” he said.
He noted that all of the risk factors associated with heart attack and stroke are also risk factors for Alzheimer’s disease and cognitive decline more broadly. “One of the issues here is how in the world does alcohol reduce cardiovascular and cerebrovascular risks, if you know it increases the risk of hypertension and stroke, regardless of dose.”
With regard to the possible impact of alcohol on brain-derived neurotrophic factor, Dr. Forester said, “it’s an interesting idea” but the actual mechanism is still unclear.
Even with dietary studies, such as those on the Mediterranean diet that include red wine, showing cognitive benefit, Dr. Forester said he’s still concerned about the adverse effects of alcohol on older people. These can include falls and sleep disturbances in addition to cognitive issues, and these effects can increase with age.
He was somewhat surprised at the level of alcohol that the study determined was beneficial. “Essentially, what they’re saying here is that, for men, it’s two drinks a day.” This could be “problematic” as two drinks per day can quickly escalate as individuals build tolerance.
He also pointed out that the study does not determine cause and effect, noting that it’s only an association.
Dr. Forester said the study raises a number of questions, including the type of alcohol study participants consumed and whether this has any impact on cognitive benefit. He also questioned whether the mediating effects of alcohol were associated with something that wasn’t measured, such as socioeconomic status.
Another question, he said, is what factors in individuals’ medical or psychiatric history determine whether they are more or less likely to benefit from low to moderate alcohol intake.
Perhaps alcohol should be recommended only for “select subpopulations” – for example, those who are healthy and have a family history of cognitive decline –but not for those with a history of substance abuse, including alcohol abuse, said Dr. Forester.
“For this population, the last thing you want to do is recommend alcohol to reduce risk of cognitive decline,” he cautioned.
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health. The investigators and Dr. Forester have reported no relevant financial disclosures.
A version of this story originally appeared on Medscape.com.
new research suggests. However, at least one expert urges caution in interpreting the findings.
Investigators found that consuming 10-14 alcoholic drinks per week had the strongest cognitive benefit. The findings “add more weight” to the growing body of research identifying beneficial cognitive effects of moderate alcohol consumption, said lead author, Ruiyuan Zhang, MD, of the department of epidemiology and biostatistics at the University of Georgia, Athens. However, Dr. Zhang emphasized that nondrinkers should not take up drinking to protect brain function, as alcohol can have negative effects.
The study was published online in JAMA Network Open.
Slower cognitive decline
The observational study was a secondary analysis of data from the Health and Retirement Study, a nationally representative U.S. survey of middle-aged and older adults. The survey, which began in 1992, is conducted every 2 years and collects health and economic data.
The current analysis used data from 1996 to 2008 and included information from individuals who participated in at least three surveys. The study included 19,887 participants, with a mean age 61.8 years. Most (60.1%) were women and white (85.2%). Mean follow-up was 9.1 years.
Researchers measured cognitive domains of mental status, word recall, and vocabulary. They also calculated a total cognition score, with higher scores indicating better cognitive abilities.
For each cognitive function measure, researchers categorized participants into a consistently low–trajectory group in which cognitive test scores from baseline through follow-up were consistently low or a consistently high–trajectory group, where cognitive test scores from baseline through follow-up were consistently high.
Based on self-reports, the investigators categorized participants as never drinkers (41.8%), former drinkers (39.5%), or current drinkers (18.7%). For current drinkers, researchers determined the number of drinking days per week and number of drinks per day. They further categorized these participants as low to moderate drinkers or heavy drinkers.
One drink was defined as a 12-ounce bottle of beer, a 5-ounce glass of wine, or a 1.5-ounce shot of spirits, said Dr. Zhang.
Women who consumed 8 or more drinks per week and men who drank 15 or more drinks per week were considered heavy drinkers. Other current drinkers were deemed low to moderate drinkers. Most current drinkers (85.2%) were low to moderate drinkers.
Other covariates included age, sex, race/ethnicity, years of education, marital status, tobacco smoking status, and body mass index.
Results showed moderate drinking was associated with relatively high cognitive test scores. After controlling for all covariates, compared with never drinkers, current low to moderate drinkers were significantly less likely to have consistently low trajectories for total cognitive score (odds ratio, 0.66; 95% confidence interval, 0.59-0.74), mental status (OR, 0.71; 95% CI, 0.63-0.81), word recall (OR, 0.74; 95% CI, 0.69-0.80), and vocabulary (OR, 0.64; 95% CI, 0.56-0.74) (all P < .001).
Former drinkers also had better cognitive outcomes for all cognitive domains. Heavy drinkers had lower odds of being in the consistently low trajectory group only for the vocabulary test.
Heavy drinking ‘risky’
Because few participants were deemed to be heavy drinkers, the power to identify an association between heavy drinking and cognitive function was limited. Dr. Zhang acknowledged, though he noted that heavy drinking is “risky.”
“We found that, after the drinking dosage passes the moderate level, the risk of low cognitive function increases very fast, which indicates that heavy drinking may harm cognitive function.” Limiting alcohol consumption “is still very important,” he said.
The associations of alcohol and cognitive functions differed by race/ethnicity. Low to moderate drinking was significantly associated with a lower odds of having a consistently low trajectory for all four cognitive function measures only among white participants.
A possible reason for this is that the study had so few African Americans (who made up only 14.8% of the sample), which limited the ability to identify relationships between alcohol intake and cognitive function, said Dr. Zhang. “Another reason is that the sensitivity to alcohol may be different between white and African American subjects.”
There was a significant U-shaped association between weekly amounts of alcohol and the odds of being in the consistently low–trajectory group for all cognitive functions. Depending on the function tested, the optimal number of weekly drinks ranged from 10-14.
Dr. Zhang noted that, when women were examined separately, alcohol consumption had a significant U-shaped relationship only with word recall, with the optimal dosage being around eight drinks.
U-shaped relationship an ‘important finding’
The U-shaped relationship is “an important finding,” said Dr. Zhang. “It shows that the human body may act differently to low and high doses of alcohol. Knowing why and how this happens is very important as it would help us understand how alcohol affects the function of the human body.”
Sensitivity analyses among participants with no chronic diseases showed the U-shaped association was still significant for scores of total word recall and vocabulary, but not for mental status or total cognition score.
The authors noted that 77.2% of participants had at least one chronic disease. They maintained that the association between alcohol consumption and cognitive function may be applicable both to healthy people and to those with a chronic disease.
The study also found that low to moderate drinkers had slower rates of cognitive decline over time for all cognition domains.
Although the mechanisms underlying the cognitive benefits of alcohol consumption are unclear, the authors believe it may be via cerebrovascular and cardiovascular pathways.
Alcohol may increase levels of brain-derived neurotrophic factor, a key regulator of neuronal plasticity and development in the dorsal striatum, they noted.
Balancing act
However, there’s also evidence that drinking, especially heavy drinking, increases the risk of hypertension, stroke, liver damage, and some cancers. “We think the role of alcohol drinking in cognitive function may be a balance of its beneficial and harmful effects on the cardiovascular system,” said Dr. Zhang.
“For the low to moderate drinker, the beneficial effects may outweigh the harmful effects on the small blood vessels in the brain. In this way, it could preserve cognition,” he added.
Dr. Zhang also noted that the study focused on middle-aged and older adults. “We can’t say whether or not moderate alcohol could benefit younger people” because they may have different characteristics, he said.
The findings of other studies examining the effects of alcohol on cognitive function are mixed. While studies have identified a beneficial effect, others have uncovered no, minimal, or adverse effects. This could be due to the use of different tests of cognitive function or different study populations, said Dr. Zhang.
A limitation of the current study was that assessment of alcohol consumption was based on self-report, which might have introduced recall bias. In addition, because individuals tend to underestimate their alcohol consumption, heavy drinkers could be misclassified as low to moderate drinkers, and low to moderate drinkers as former drinkers.
“This may make our study underestimate the association between low to moderate drinking and cognitive function,” said Dr. Zhang. In addition, alcohol consumption tended to change with time, and this change may be associated with other factors that led to changes in cognitive function, the authors noted.
Interpret with caution
Commenting on the study, Brent P. Forester, MD, chief of the Center of Excellence in Geriatric Psychiatry at McLean Hospital in Belmont, Mass., associate professor of psychiatry at Harvard Medical School, Boston, and a member of the American Psychiatric Association Council on Geriatric Psychiatry, said he views the study with some trepidation.
“As a clinician taking care of older adults, I would be very cautious about overinterpreting the beneficial effects of alcohol before we understand the mechanism better,” he said.
He noted that all of the risk factors associated with heart attack and stroke are also risk factors for Alzheimer’s disease and cognitive decline more broadly. “One of the issues here is how in the world does alcohol reduce cardiovascular and cerebrovascular risks, if you know it increases the risk of hypertension and stroke, regardless of dose.”
With regard to the possible impact of alcohol on brain-derived neurotrophic factor, Dr. Forester said, “it’s an interesting idea” but the actual mechanism is still unclear.
Even with dietary studies, such as those on the Mediterranean diet that include red wine, showing cognitive benefit, Dr. Forester said he’s still concerned about the adverse effects of alcohol on older people. These can include falls and sleep disturbances in addition to cognitive issues, and these effects can increase with age.
He was somewhat surprised at the level of alcohol that the study determined was beneficial. “Essentially, what they’re saying here is that, for men, it’s two drinks a day.” This could be “problematic” as two drinks per day can quickly escalate as individuals build tolerance.
He also pointed out that the study does not determine cause and effect, noting that it’s only an association.
Dr. Forester said the study raises a number of questions, including the type of alcohol study participants consumed and whether this has any impact on cognitive benefit. He also questioned whether the mediating effects of alcohol were associated with something that wasn’t measured, such as socioeconomic status.
Another question, he said, is what factors in individuals’ medical or psychiatric history determine whether they are more or less likely to benefit from low to moderate alcohol intake.
Perhaps alcohol should be recommended only for “select subpopulations” – for example, those who are healthy and have a family history of cognitive decline –but not for those with a history of substance abuse, including alcohol abuse, said Dr. Forester.
“For this population, the last thing you want to do is recommend alcohol to reduce risk of cognitive decline,” he cautioned.
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health. The investigators and Dr. Forester have reported no relevant financial disclosures.
A version of this story originally appeared on Medscape.com.
new research suggests. However, at least one expert urges caution in interpreting the findings.
Investigators found that consuming 10-14 alcoholic drinks per week had the strongest cognitive benefit. The findings “add more weight” to the growing body of research identifying beneficial cognitive effects of moderate alcohol consumption, said lead author, Ruiyuan Zhang, MD, of the department of epidemiology and biostatistics at the University of Georgia, Athens. However, Dr. Zhang emphasized that nondrinkers should not take up drinking to protect brain function, as alcohol can have negative effects.
The study was published online in JAMA Network Open.
Slower cognitive decline
The observational study was a secondary analysis of data from the Health and Retirement Study, a nationally representative U.S. survey of middle-aged and older adults. The survey, which began in 1992, is conducted every 2 years and collects health and economic data.
The current analysis used data from 1996 to 2008 and included information from individuals who participated in at least three surveys. The study included 19,887 participants, with a mean age 61.8 years. Most (60.1%) were women and white (85.2%). Mean follow-up was 9.1 years.
Researchers measured cognitive domains of mental status, word recall, and vocabulary. They also calculated a total cognition score, with higher scores indicating better cognitive abilities.
For each cognitive function measure, researchers categorized participants into a consistently low–trajectory group in which cognitive test scores from baseline through follow-up were consistently low or a consistently high–trajectory group, where cognitive test scores from baseline through follow-up were consistently high.
Based on self-reports, the investigators categorized participants as never drinkers (41.8%), former drinkers (39.5%), or current drinkers (18.7%). For current drinkers, researchers determined the number of drinking days per week and number of drinks per day. They further categorized these participants as low to moderate drinkers or heavy drinkers.
One drink was defined as a 12-ounce bottle of beer, a 5-ounce glass of wine, or a 1.5-ounce shot of spirits, said Dr. Zhang.
Women who consumed 8 or more drinks per week and men who drank 15 or more drinks per week were considered heavy drinkers. Other current drinkers were deemed low to moderate drinkers. Most current drinkers (85.2%) were low to moderate drinkers.
Other covariates included age, sex, race/ethnicity, years of education, marital status, tobacco smoking status, and body mass index.
Results showed moderate drinking was associated with relatively high cognitive test scores. After controlling for all covariates, compared with never drinkers, current low to moderate drinkers were significantly less likely to have consistently low trajectories for total cognitive score (odds ratio, 0.66; 95% confidence interval, 0.59-0.74), mental status (OR, 0.71; 95% CI, 0.63-0.81), word recall (OR, 0.74; 95% CI, 0.69-0.80), and vocabulary (OR, 0.64; 95% CI, 0.56-0.74) (all P < .001).
Former drinkers also had better cognitive outcomes for all cognitive domains. Heavy drinkers had lower odds of being in the consistently low trajectory group only for the vocabulary test.
Heavy drinking ‘risky’
Because few participants were deemed to be heavy drinkers, the power to identify an association between heavy drinking and cognitive function was limited. Dr. Zhang acknowledged, though he noted that heavy drinking is “risky.”
“We found that, after the drinking dosage passes the moderate level, the risk of low cognitive function increases very fast, which indicates that heavy drinking may harm cognitive function.” Limiting alcohol consumption “is still very important,” he said.
The associations of alcohol and cognitive functions differed by race/ethnicity. Low to moderate drinking was significantly associated with a lower odds of having a consistently low trajectory for all four cognitive function measures only among white participants.
A possible reason for this is that the study had so few African Americans (who made up only 14.8% of the sample), which limited the ability to identify relationships between alcohol intake and cognitive function, said Dr. Zhang. “Another reason is that the sensitivity to alcohol may be different between white and African American subjects.”
There was a significant U-shaped association between weekly amounts of alcohol and the odds of being in the consistently low–trajectory group for all cognitive functions. Depending on the function tested, the optimal number of weekly drinks ranged from 10-14.
Dr. Zhang noted that, when women were examined separately, alcohol consumption had a significant U-shaped relationship only with word recall, with the optimal dosage being around eight drinks.
U-shaped relationship an ‘important finding’
The U-shaped relationship is “an important finding,” said Dr. Zhang. “It shows that the human body may act differently to low and high doses of alcohol. Knowing why and how this happens is very important as it would help us understand how alcohol affects the function of the human body.”
Sensitivity analyses among participants with no chronic diseases showed the U-shaped association was still significant for scores of total word recall and vocabulary, but not for mental status or total cognition score.
The authors noted that 77.2% of participants had at least one chronic disease. They maintained that the association between alcohol consumption and cognitive function may be applicable both to healthy people and to those with a chronic disease.
The study also found that low to moderate drinkers had slower rates of cognitive decline over time for all cognition domains.
Although the mechanisms underlying the cognitive benefits of alcohol consumption are unclear, the authors believe it may be via cerebrovascular and cardiovascular pathways.
Alcohol may increase levels of brain-derived neurotrophic factor, a key regulator of neuronal plasticity and development in the dorsal striatum, they noted.
Balancing act
However, there’s also evidence that drinking, especially heavy drinking, increases the risk of hypertension, stroke, liver damage, and some cancers. “We think the role of alcohol drinking in cognitive function may be a balance of its beneficial and harmful effects on the cardiovascular system,” said Dr. Zhang.
“For the low to moderate drinker, the beneficial effects may outweigh the harmful effects on the small blood vessels in the brain. In this way, it could preserve cognition,” he added.
Dr. Zhang also noted that the study focused on middle-aged and older adults. “We can’t say whether or not moderate alcohol could benefit younger people” because they may have different characteristics, he said.
The findings of other studies examining the effects of alcohol on cognitive function are mixed. While studies have identified a beneficial effect, others have uncovered no, minimal, or adverse effects. This could be due to the use of different tests of cognitive function or different study populations, said Dr. Zhang.
A limitation of the current study was that assessment of alcohol consumption was based on self-report, which might have introduced recall bias. In addition, because individuals tend to underestimate their alcohol consumption, heavy drinkers could be misclassified as low to moderate drinkers, and low to moderate drinkers as former drinkers.
“This may make our study underestimate the association between low to moderate drinking and cognitive function,” said Dr. Zhang. In addition, alcohol consumption tended to change with time, and this change may be associated with other factors that led to changes in cognitive function, the authors noted.
Interpret with caution
Commenting on the study, Brent P. Forester, MD, chief of the Center of Excellence in Geriatric Psychiatry at McLean Hospital in Belmont, Mass., associate professor of psychiatry at Harvard Medical School, Boston, and a member of the American Psychiatric Association Council on Geriatric Psychiatry, said he views the study with some trepidation.
“As a clinician taking care of older adults, I would be very cautious about overinterpreting the beneficial effects of alcohol before we understand the mechanism better,” he said.
He noted that all of the risk factors associated with heart attack and stroke are also risk factors for Alzheimer’s disease and cognitive decline more broadly. “One of the issues here is how in the world does alcohol reduce cardiovascular and cerebrovascular risks, if you know it increases the risk of hypertension and stroke, regardless of dose.”
With regard to the possible impact of alcohol on brain-derived neurotrophic factor, Dr. Forester said, “it’s an interesting idea” but the actual mechanism is still unclear.
Even with dietary studies, such as those on the Mediterranean diet that include red wine, showing cognitive benefit, Dr. Forester said he’s still concerned about the adverse effects of alcohol on older people. These can include falls and sleep disturbances in addition to cognitive issues, and these effects can increase with age.
He was somewhat surprised at the level of alcohol that the study determined was beneficial. “Essentially, what they’re saying here is that, for men, it’s two drinks a day.” This could be “problematic” as two drinks per day can quickly escalate as individuals build tolerance.
He also pointed out that the study does not determine cause and effect, noting that it’s only an association.
Dr. Forester said the study raises a number of questions, including the type of alcohol study participants consumed and whether this has any impact on cognitive benefit. He also questioned whether the mediating effects of alcohol were associated with something that wasn’t measured, such as socioeconomic status.
Another question, he said, is what factors in individuals’ medical or psychiatric history determine whether they are more or less likely to benefit from low to moderate alcohol intake.
Perhaps alcohol should be recommended only for “select subpopulations” – for example, those who are healthy and have a family history of cognitive decline –but not for those with a history of substance abuse, including alcohol abuse, said Dr. Forester.
“For this population, the last thing you want to do is recommend alcohol to reduce risk of cognitive decline,” he cautioned.
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health. The investigators and Dr. Forester have reported no relevant financial disclosures.
A version of this story originally appeared on Medscape.com.
Dropping race-based eGFR adjustment gains traction in U.S.
a measure of renal function.
The move aims to correct a race-based health access inequity that’s been in place for more than 2 decades, say advocates, while others voice concern that the change threatens over-diagnosis of both chronic and end-stage kidney disease in some patients.
In late June, the Boston-based Massachusetts General Brigham health system stopped noting the race-based modifier when its laboratories reported eGFR, and the leadership sent its staff a message discouraging them from applying the modifier. A similar change in eGFR reporting started on June 1 at the University of Washington health system, UW Medicine, Seattle.
These steps followed what is widely regarded as the first institutional change away from race-based adjustment of eGFR, which began in March 2017 at Beth Israel Deaconess Medical Center in Boston, and they have come amid a growing movement by some individual U.S. physicians to drop the modifier from their practice.
“Momentum is clearly building,” said Nwamaka D. Eneanya, MD, a nephrologist at the University of Pennsylvania in Philadelphia and lead author of a commentary published a little more than a year ago that laid out the case for reconsidering how to calculate eGFR in African Americans.
“Many discussions are happening at other [US] academic medical centers,” Dr. Eneanya added, including the system where she works.
Why was the decision taken to modify eGFR in African Americans?
The concept is that the formula used to calculate eGFR systematically underestimates the value in African Americans. Hence, it requires a small but meaningful up-adjustment, which can be traced back to the introduction of the Modification of Diet in Renal Disease (MDRD) study equation in 1999.
The idea was perpetuated in an improved calculation formula, the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI), that came out a decade later.
These are the most widely used U.S. approaches to eGFR calculation, with the newer CKD-EPI formula predominating.
The rationale for including a modifier for Blacks in the 2009 formula was for improved accuracy relative to the standard reference measure based on iothalamate clearance.
The data used to develop the CKD-EPI formula showed that Black individuals in the dataset had, on average, GFR levels that were 16% higher than people of other races with the same age, sex, and serum creatinine level, according to a recent commentary. The first author, Andrew S. Levey, MD, was also lead author of the reports that introduced both the MDRD and CKD-EPI equations.
But the argument withers in the light of both its flimsy underpinning – race assessment – and the medical and social consequences of its application, say those who have sought change.
Reporting eGFR by race might do more harm than good
“Race is a social, not a biological, construct and the kidney-function race multiplier ignores the substantial genetic diversity within self-identified Black patients,” said Thomas D. Sequist, MD, professor of medicine at Harvard Medical School in Boston and chief patient experience and equity officer for Mass General Brigham, who spearheaded the policy change for that system.
“Do we really believe that the population breaks down into just ‘Black’ and ‘not Black,’ as the CKD-EPI equation asks us to believe?” he said in an interview. “The equation was developed from a few thousand patients, and we now apply it to millions of people using a very imprecise measure – race.”
“Reporting eGFR by race perpetuates a notion that race is a biologic construct when it’s not,” agreed Rajnish Mehrotra, MD, a professor and chief of nephrology at the University of Washington in Seattle and leader of the eGFR change within his medical system.
Equally compelling, said Dr. Mehrotra, Dr. Sequist, and others, are the health inequities that have resulted from routinely raising the eGFR in African Americans.
This has led to “withholding treatment from people longer than needed. We arrived at the conclusion that reporting eGFR by race does more harm than good,” Dr. Mehrotra said in an interview.
Dr. Sequist added: “Researchers across Mass General Brigham have demonstrated that use of these race multipliers can lead to important delays in care for Black patients, such as timely evaluation for kidney transplantation.”
“Our main concern is that race correction is creating harm.”
Dr. Eneanya concurs: “It was never designed to oppress patients, but that’s where we are. No one ever thought about the repercussions of using race.”
And while the movement to eliminate the race modifier is clearly gaining steam, it’s also receiving pushback from those who see benefit from the modification and have concern that its abolition could lead to overestimates of kidney disease severity.
Some clinicians “have a hard time letting the race modifier go,” Dr. Eneanya noted.
“In the nephrology community, it’s pretty controversial”
In their 2020 commentary, Levey and coauthors wrote: “We propose a more cautious approach that maintains and improves accuracy of GFR estimates and avoids disadvantaging any racial group.”
Their suggested remedies included full disclosure of use of race, accommodation of people who decline to self-identify themselves that way, shared decision-making, and “mindful” use of cystatin C, an alternative to serum creatinine for calculating eGFR.
The latter is regarded as more precise and accurate than serum creatinine across populations but is often not as readily available to many clinicians. Their article also supported looking for even better and more accessible ways to calculate eGFR.
“In the nephrology community, it’s pretty controversial,” said Mallika L. Mendu, MD, a nephrologist at Brigham and Women’s Hospital in Boston, Massachusetts, who has studied the effects of using the modifier on patient assessment.
Her recent review of Mass General Brigham patients found that close to a third of African Americans would have been reclassified with a more severe form of kidney disease if their eGFR had remained unmodified.
“That raised concerns that, by using race adjustment we’re potentially leading to less equitable outcomes for African American patients,” she said. “I’d rather over diagnose than not diagnose in a timely way.”
The research that led to development of the MDRD and CKD-EPI equations “are gold-standard studies” that “saw a real difference,” Dr. Mendu acknowledged in an interview. “But the way those studies were run and the way they defined the patients was problematic.” Despite that, “many nephrologists” agree with the position taken by Dr. Levey and coauthors in their recent commentary, she said.
She added that she stopped using the modifier about a year ago in her own practice, well before the system where she works adopted the same approach.
Consensus takes time
In one sign of the controversy, a quartet of clinicians affiliated with San Francisco General Hospital (SFGH) recently posted an online petition in which they noted that the race modifier had been eliminated in eGFR reports from the hospital’s laboratory in October 2019, but more recently had been slated for reinstitution. “We were deeply distressed to recently discover the intended plan to revert back to race-based eGFR reporting at SFGH,” they noted.
The same four clinicians also wrote an opinion piece calling for elimination of the modifier in November 2019 in the San Francisco Examiner.
Controversy will likely linger as the movement to withdraw the race modifier spreads without clear agreement on what to do instead.
Dr. Mehrotra said he’s received inquiries about his system’s experience from clinicians at several U.S. medical centers and systems, and he remains comfortable applying the unadjusted CKD-EPI formula to all adults, an approach he called “sufficient.”
Other physicians, like nephrologist Vanessa Grubbs, MD, call for a rapid shift to a cystatin C–based, fully race-neutral method for calculating eGFR, a position she detailed in a recent editorial.
And at the University of Pennsylvania, where the health system continues to issue eGFR reports with the race modifier, Dr. Eneanya says that she stopped using the modifier “some time” ago.
“People have a hard time letting it go because it is so important in clinical care. Getting everyone to come to a consensus takes time,” she said.
Dr. Eneanya, Dr. Sequist, and Dr. Mendu have reported no relevant financial relationships. Dr. Mehrotra has been a consultant for Baxter Healthcare.
A version of this article originally appeared on Medscape.com.
a measure of renal function.
The move aims to correct a race-based health access inequity that’s been in place for more than 2 decades, say advocates, while others voice concern that the change threatens over-diagnosis of both chronic and end-stage kidney disease in some patients.
In late June, the Boston-based Massachusetts General Brigham health system stopped noting the race-based modifier when its laboratories reported eGFR, and the leadership sent its staff a message discouraging them from applying the modifier. A similar change in eGFR reporting started on June 1 at the University of Washington health system, UW Medicine, Seattle.
These steps followed what is widely regarded as the first institutional change away from race-based adjustment of eGFR, which began in March 2017 at Beth Israel Deaconess Medical Center in Boston, and they have come amid a growing movement by some individual U.S. physicians to drop the modifier from their practice.
“Momentum is clearly building,” said Nwamaka D. Eneanya, MD, a nephrologist at the University of Pennsylvania in Philadelphia and lead author of a commentary published a little more than a year ago that laid out the case for reconsidering how to calculate eGFR in African Americans.
“Many discussions are happening at other [US] academic medical centers,” Dr. Eneanya added, including the system where she works.
Why was the decision taken to modify eGFR in African Americans?
The concept is that the formula used to calculate eGFR systematically underestimates the value in African Americans. Hence, it requires a small but meaningful up-adjustment, which can be traced back to the introduction of the Modification of Diet in Renal Disease (MDRD) study equation in 1999.
The idea was perpetuated in an improved calculation formula, the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI), that came out a decade later.
These are the most widely used U.S. approaches to eGFR calculation, with the newer CKD-EPI formula predominating.
The rationale for including a modifier for Blacks in the 2009 formula was for improved accuracy relative to the standard reference measure based on iothalamate clearance.
The data used to develop the CKD-EPI formula showed that Black individuals in the dataset had, on average, GFR levels that were 16% higher than people of other races with the same age, sex, and serum creatinine level, according to a recent commentary. The first author, Andrew S. Levey, MD, was also lead author of the reports that introduced both the MDRD and CKD-EPI equations.
But the argument withers in the light of both its flimsy underpinning – race assessment – and the medical and social consequences of its application, say those who have sought change.
Reporting eGFR by race might do more harm than good
“Race is a social, not a biological, construct and the kidney-function race multiplier ignores the substantial genetic diversity within self-identified Black patients,” said Thomas D. Sequist, MD, professor of medicine at Harvard Medical School in Boston and chief patient experience and equity officer for Mass General Brigham, who spearheaded the policy change for that system.
“Do we really believe that the population breaks down into just ‘Black’ and ‘not Black,’ as the CKD-EPI equation asks us to believe?” he said in an interview. “The equation was developed from a few thousand patients, and we now apply it to millions of people using a very imprecise measure – race.”
“Reporting eGFR by race perpetuates a notion that race is a biologic construct when it’s not,” agreed Rajnish Mehrotra, MD, a professor and chief of nephrology at the University of Washington in Seattle and leader of the eGFR change within his medical system.
Equally compelling, said Dr. Mehrotra, Dr. Sequist, and others, are the health inequities that have resulted from routinely raising the eGFR in African Americans.
This has led to “withholding treatment from people longer than needed. We arrived at the conclusion that reporting eGFR by race does more harm than good,” Dr. Mehrotra said in an interview.
Dr. Sequist added: “Researchers across Mass General Brigham have demonstrated that use of these race multipliers can lead to important delays in care for Black patients, such as timely evaluation for kidney transplantation.”
“Our main concern is that race correction is creating harm.”
Dr. Eneanya concurs: “It was never designed to oppress patients, but that’s where we are. No one ever thought about the repercussions of using race.”
And while the movement to eliminate the race modifier is clearly gaining steam, it’s also receiving pushback from those who see benefit from the modification and have concern that its abolition could lead to overestimates of kidney disease severity.
Some clinicians “have a hard time letting the race modifier go,” Dr. Eneanya noted.
“In the nephrology community, it’s pretty controversial”
In their 2020 commentary, Levey and coauthors wrote: “We propose a more cautious approach that maintains and improves accuracy of GFR estimates and avoids disadvantaging any racial group.”
Their suggested remedies included full disclosure of use of race, accommodation of people who decline to self-identify themselves that way, shared decision-making, and “mindful” use of cystatin C, an alternative to serum creatinine for calculating eGFR.
The latter is regarded as more precise and accurate than serum creatinine across populations but is often not as readily available to many clinicians. Their article also supported looking for even better and more accessible ways to calculate eGFR.
“In the nephrology community, it’s pretty controversial,” said Mallika L. Mendu, MD, a nephrologist at Brigham and Women’s Hospital in Boston, Massachusetts, who has studied the effects of using the modifier on patient assessment.
Her recent review of Mass General Brigham patients found that close to a third of African Americans would have been reclassified with a more severe form of kidney disease if their eGFR had remained unmodified.
“That raised concerns that, by using race adjustment we’re potentially leading to less equitable outcomes for African American patients,” she said. “I’d rather over diagnose than not diagnose in a timely way.”
The research that led to development of the MDRD and CKD-EPI equations “are gold-standard studies” that “saw a real difference,” Dr. Mendu acknowledged in an interview. “But the way those studies were run and the way they defined the patients was problematic.” Despite that, “many nephrologists” agree with the position taken by Dr. Levey and coauthors in their recent commentary, she said.
She added that she stopped using the modifier about a year ago in her own practice, well before the system where she works adopted the same approach.
Consensus takes time
In one sign of the controversy, a quartet of clinicians affiliated with San Francisco General Hospital (SFGH) recently posted an online petition in which they noted that the race modifier had been eliminated in eGFR reports from the hospital’s laboratory in October 2019, but more recently had been slated for reinstitution. “We were deeply distressed to recently discover the intended plan to revert back to race-based eGFR reporting at SFGH,” they noted.
The same four clinicians also wrote an opinion piece calling for elimination of the modifier in November 2019 in the San Francisco Examiner.
Controversy will likely linger as the movement to withdraw the race modifier spreads without clear agreement on what to do instead.
Dr. Mehrotra said he’s received inquiries about his system’s experience from clinicians at several U.S. medical centers and systems, and he remains comfortable applying the unadjusted CKD-EPI formula to all adults, an approach he called “sufficient.”
Other physicians, like nephrologist Vanessa Grubbs, MD, call for a rapid shift to a cystatin C–based, fully race-neutral method for calculating eGFR, a position she detailed in a recent editorial.
And at the University of Pennsylvania, where the health system continues to issue eGFR reports with the race modifier, Dr. Eneanya says that she stopped using the modifier “some time” ago.
“People have a hard time letting it go because it is so important in clinical care. Getting everyone to come to a consensus takes time,” she said.
Dr. Eneanya, Dr. Sequist, and Dr. Mendu have reported no relevant financial relationships. Dr. Mehrotra has been a consultant for Baxter Healthcare.
A version of this article originally appeared on Medscape.com.
a measure of renal function.
The move aims to correct a race-based health access inequity that’s been in place for more than 2 decades, say advocates, while others voice concern that the change threatens over-diagnosis of both chronic and end-stage kidney disease in some patients.
In late June, the Boston-based Massachusetts General Brigham health system stopped noting the race-based modifier when its laboratories reported eGFR, and the leadership sent its staff a message discouraging them from applying the modifier. A similar change in eGFR reporting started on June 1 at the University of Washington health system, UW Medicine, Seattle.
These steps followed what is widely regarded as the first institutional change away from race-based adjustment of eGFR, which began in March 2017 at Beth Israel Deaconess Medical Center in Boston, and they have come amid a growing movement by some individual U.S. physicians to drop the modifier from their practice.
“Momentum is clearly building,” said Nwamaka D. Eneanya, MD, a nephrologist at the University of Pennsylvania in Philadelphia and lead author of a commentary published a little more than a year ago that laid out the case for reconsidering how to calculate eGFR in African Americans.
“Many discussions are happening at other [US] academic medical centers,” Dr. Eneanya added, including the system where she works.
Why was the decision taken to modify eGFR in African Americans?
The concept is that the formula used to calculate eGFR systematically underestimates the value in African Americans. Hence, it requires a small but meaningful up-adjustment, which can be traced back to the introduction of the Modification of Diet in Renal Disease (MDRD) study equation in 1999.
The idea was perpetuated in an improved calculation formula, the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI), that came out a decade later.
These are the most widely used U.S. approaches to eGFR calculation, with the newer CKD-EPI formula predominating.
The rationale for including a modifier for Blacks in the 2009 formula was for improved accuracy relative to the standard reference measure based on iothalamate clearance.
The data used to develop the CKD-EPI formula showed that Black individuals in the dataset had, on average, GFR levels that were 16% higher than people of other races with the same age, sex, and serum creatinine level, according to a recent commentary. The first author, Andrew S. Levey, MD, was also lead author of the reports that introduced both the MDRD and CKD-EPI equations.
But the argument withers in the light of both its flimsy underpinning – race assessment – and the medical and social consequences of its application, say those who have sought change.
Reporting eGFR by race might do more harm than good
“Race is a social, not a biological, construct and the kidney-function race multiplier ignores the substantial genetic diversity within self-identified Black patients,” said Thomas D. Sequist, MD, professor of medicine at Harvard Medical School in Boston and chief patient experience and equity officer for Mass General Brigham, who spearheaded the policy change for that system.
“Do we really believe that the population breaks down into just ‘Black’ and ‘not Black,’ as the CKD-EPI equation asks us to believe?” he said in an interview. “The equation was developed from a few thousand patients, and we now apply it to millions of people using a very imprecise measure – race.”
“Reporting eGFR by race perpetuates a notion that race is a biologic construct when it’s not,” agreed Rajnish Mehrotra, MD, a professor and chief of nephrology at the University of Washington in Seattle and leader of the eGFR change within his medical system.
Equally compelling, said Dr. Mehrotra, Dr. Sequist, and others, are the health inequities that have resulted from routinely raising the eGFR in African Americans.
This has led to “withholding treatment from people longer than needed. We arrived at the conclusion that reporting eGFR by race does more harm than good,” Dr. Mehrotra said in an interview.
Dr. Sequist added: “Researchers across Mass General Brigham have demonstrated that use of these race multipliers can lead to important delays in care for Black patients, such as timely evaluation for kidney transplantation.”
“Our main concern is that race correction is creating harm.”
Dr. Eneanya concurs: “It was never designed to oppress patients, but that’s where we are. No one ever thought about the repercussions of using race.”
And while the movement to eliminate the race modifier is clearly gaining steam, it’s also receiving pushback from those who see benefit from the modification and have concern that its abolition could lead to overestimates of kidney disease severity.
Some clinicians “have a hard time letting the race modifier go,” Dr. Eneanya noted.
“In the nephrology community, it’s pretty controversial”
In their 2020 commentary, Levey and coauthors wrote: “We propose a more cautious approach that maintains and improves accuracy of GFR estimates and avoids disadvantaging any racial group.”
Their suggested remedies included full disclosure of use of race, accommodation of people who decline to self-identify themselves that way, shared decision-making, and “mindful” use of cystatin C, an alternative to serum creatinine for calculating eGFR.
The latter is regarded as more precise and accurate than serum creatinine across populations but is often not as readily available to many clinicians. Their article also supported looking for even better and more accessible ways to calculate eGFR.
“In the nephrology community, it’s pretty controversial,” said Mallika L. Mendu, MD, a nephrologist at Brigham and Women’s Hospital in Boston, Massachusetts, who has studied the effects of using the modifier on patient assessment.
Her recent review of Mass General Brigham patients found that close to a third of African Americans would have been reclassified with a more severe form of kidney disease if their eGFR had remained unmodified.
“That raised concerns that, by using race adjustment we’re potentially leading to less equitable outcomes for African American patients,” she said. “I’d rather over diagnose than not diagnose in a timely way.”
The research that led to development of the MDRD and CKD-EPI equations “are gold-standard studies” that “saw a real difference,” Dr. Mendu acknowledged in an interview. “But the way those studies were run and the way they defined the patients was problematic.” Despite that, “many nephrologists” agree with the position taken by Dr. Levey and coauthors in their recent commentary, she said.
She added that she stopped using the modifier about a year ago in her own practice, well before the system where she works adopted the same approach.
Consensus takes time
In one sign of the controversy, a quartet of clinicians affiliated with San Francisco General Hospital (SFGH) recently posted an online petition in which they noted that the race modifier had been eliminated in eGFR reports from the hospital’s laboratory in October 2019, but more recently had been slated for reinstitution. “We were deeply distressed to recently discover the intended plan to revert back to race-based eGFR reporting at SFGH,” they noted.
The same four clinicians also wrote an opinion piece calling for elimination of the modifier in November 2019 in the San Francisco Examiner.
Controversy will likely linger as the movement to withdraw the race modifier spreads without clear agreement on what to do instead.
Dr. Mehrotra said he’s received inquiries about his system’s experience from clinicians at several U.S. medical centers and systems, and he remains comfortable applying the unadjusted CKD-EPI formula to all adults, an approach he called “sufficient.”
Other physicians, like nephrologist Vanessa Grubbs, MD, call for a rapid shift to a cystatin C–based, fully race-neutral method for calculating eGFR, a position she detailed in a recent editorial.
And at the University of Pennsylvania, where the health system continues to issue eGFR reports with the race modifier, Dr. Eneanya says that she stopped using the modifier “some time” ago.
“People have a hard time letting it go because it is so important in clinical care. Getting everyone to come to a consensus takes time,” she said.
Dr. Eneanya, Dr. Sequist, and Dr. Mendu have reported no relevant financial relationships. Dr. Mehrotra has been a consultant for Baxter Healthcare.
A version of this article originally appeared on Medscape.com.
Can DBS in early Parkinson’s disease reduce disease progression?
According to the investigators, a larger trial is needed to confirm these findings, which were published online ahead of print June 29 in Neurology.
Adverse events were similar between patients who underwent DBS and drug therapy and those who underwent drug therapy alone. This result is a preliminary indication of the safety of long-term DBS therapy, according to the researchers. Furthermore, patients who received DBS required a significantly lower levodopa equivalent daily dose (LEDD) and were less likely to need polypharmacy than were patients who received medical treatment alone.
“While we can be really excited about these findings, we can’t change our practice, what we recommend to patients, based on this [study],” said David Charles, MD, professor and vice chair of neurology at Vanderbilt University, Nashville, Tenn. “We have to do the next trial to get that class of evidence.”
An extension of a pilot trial
Previous research has indicated that treatment with DBS and optimal medical therapy provides benefits beyond those of medical therapy alone in patients with mid-stage or advanced Parkinson’s disease. Dr. Charles and colleagues conducted a randomized, single-blind pilot study to examine the safety and tolerability of STN DBS in 30 patients with early Parkinson’s disease. Eligible participants had Hoehn and Yahr stage II off medication, were between 50 and 75 years of age, had taken medication for 6 months to 4 years, and had no dyskinesia or other motor fluctuations.
Patients were randomly assigned in equal groups to optimal drug therapy plus STN DBS or to drug therapy alone. Investigators evaluated patients every 6 months for 2 years. The results suggested that STN DBS was safe and slowed the progression of rest tremor in this population.
Apart from research that included patients with advanced Parkinson’s disease, data relating to long-term follow-up of patients undergoing DBS for Parkinson’s disease have been limited. Prospective studies have found that DBS provides motor benefits in patients with advanced Parkinson’s disease after 5-10 years, but they have not included control groups of patients randomly assigned to medication alone. Understanding the durability of effect of DBS is particularly important in patients with early Parkinson’s disease, because they could be exposed to stimulation for a longer time than other patients.
DBS may slow progression of rest tremor
Dr. Charles and colleagues invited patients who completed their pilot study to participate in an observational follow-up study. All 29 patients who completed the pilot study consented to participate in the follow-up. The investigators conducted annual outpatient examinations at 3, 4, and 5 years after baseline. These examinations were similar to those conducted at baseline in the pilot trial. Patients’ scores on the Unified Parkinson’s Disease Rating Scale (UPDRS) Part III were obtained through blinded video assessment. Rigidity was not assessed. The investigators calculated patients’ levodopa equivalent daily dose (LEDD) and total electrical energy delivered (TEED). Adverse events were classified as mild, moderate, or severe.
Because of a problem with study funding, the investigators examined only eight patients in the optimal therapy group and nine patients in the DBS group at 3 years. The final analysis included 28 patients, because one patient was found not to have met inclusion criteria after the trial was completed.
At 5 years, participants’ mean age was 66.1 years. Participants had been taking medications for Parkinson’s disease for a mean duration of 7.2 years. No deaths occurred during the study. Four participants who had been assigned randomly to optimal drug therapy chose to receive STN DBS during the study. The investigators evaluated these participants in the treatment group to which they had been assigned at randomization using an intention-to-treat analysis that compared early STN DBS plus drug therapy with drug therapy alone.
Among patients with early DBS, the odds ratio (OR) of worse UPDRS III scores during 5 years was 0.42, compared with the medical therapy group. The difference in mean UPDRS III score between groups due to randomization was 3.70, which was a clinically important difference, according to the investigators.
In the early DBS group, the OR of worse rest tremor was 0.21, compared with the drug therapy group. The between-group difference in mean rest tremor score favored the DBS group. Excluding rest tremor from participants’ UPDRS III scores eliminated between-group differences in the odds of having worse motor symptoms and in the magnitude of difference of motor symptom score.
In the early DBS group, the OR of requiring a greater LEDD was 0.26, compared with the drug therapy group. The between group difference in mean LEDD significantly favored the DBS group. In addition, at 5 years, the proportion of patients requiring polypharmacy was 93% in the drug therapy group and 43% in the DBS group.
The investigators found no difference between groups in the prevalence of dyskinesia at baseline. At 5 years, the prevalence of dyskinesia was 50% in the drug therapy group and 21% in the DBS group. The difference was not statistically significant, however.
The study groups had similar adverse event profiles. Five adverse events during follow-up were related to surgery or the DBS device. The most common of the 13 study-related adverse events was nausea.
The study’s most significant finding is that “DBS implanted in early Parkinson’s disease decreases the risk of disease progression,” said Dr. Charles. No therapy, including DBS, has been proven to decrease this risk. “This is class II evidence. We have to get class I evidence before we change practice.”
Dr. Charles and colleagues have received Food and Drug Administration approval for a multicenter phase 3 trial to obtain this evidence. The new trial may extend findings regarding DBS in mid-stage and advanced Parkinson’s disease to early-stage Parkinson’s disease. That is, it may show that DBS plus drug therapy in early stage Parkinson’s disease is safe, efficacious, and superior to standard medical therapy alone. “But the reason to do the trial is to determine if it changes or slows the progression of the disease,” said Dr. Charles.
Effect on dyskinesia is unclear
“If a patient does go on to develop problems that need DBS management, and only a small fraction of patients with Parkinson’s disease evolve to this need, then this procedure can be performed at that time,” said Peter A. LeWitt, MD, Sastry Foundation Endowed Chair in Neurology at Wayne State University in Detroit.
“One confound of the study is that DBS provides symptomatic relief of dyskinesias if a patient has developed this problem after a few years of levodopa treatment,” Dr. LeWitt added. “To demonstrate that early use of DBS prevented the development of dyskinesias, the study design should have included a period of turning off the stimulators to determine whether the generation of dyskinesias was prevented, rather than merely suppressed by DBS, as any patient would experience.
“Finally, the goal of reducing use of levodopa dose medications or polypharmacy doesn’t justify subjecting a patient to a brain operation that is not without risks and great expense,” Dr. LeWitt continued. “The results of this underpowered study add to my opinion that the ‘premature’ use of DBS is not a good idea for the management of Parkinson’s disease.”
Medtronic, which manufactures the DBS device that the investigators used, provided part of the study’s funding. Vanderbilt University receives income for research or educational programs that Dr. Charles leads. Dr. LeWitt had no pertinent disclosures.
SOURCE: Hacker ML et al. Neurology. 2020 Jun 29. doi: 10.1212/WNL.0000000000009946.
According to the investigators, a larger trial is needed to confirm these findings, which were published online ahead of print June 29 in Neurology.
Adverse events were similar between patients who underwent DBS and drug therapy and those who underwent drug therapy alone. This result is a preliminary indication of the safety of long-term DBS therapy, according to the researchers. Furthermore, patients who received DBS required a significantly lower levodopa equivalent daily dose (LEDD) and were less likely to need polypharmacy than were patients who received medical treatment alone.
“While we can be really excited about these findings, we can’t change our practice, what we recommend to patients, based on this [study],” said David Charles, MD, professor and vice chair of neurology at Vanderbilt University, Nashville, Tenn. “We have to do the next trial to get that class of evidence.”
An extension of a pilot trial
Previous research has indicated that treatment with DBS and optimal medical therapy provides benefits beyond those of medical therapy alone in patients with mid-stage or advanced Parkinson’s disease. Dr. Charles and colleagues conducted a randomized, single-blind pilot study to examine the safety and tolerability of STN DBS in 30 patients with early Parkinson’s disease. Eligible participants had Hoehn and Yahr stage II off medication, were between 50 and 75 years of age, had taken medication for 6 months to 4 years, and had no dyskinesia or other motor fluctuations.
Patients were randomly assigned in equal groups to optimal drug therapy plus STN DBS or to drug therapy alone. Investigators evaluated patients every 6 months for 2 years. The results suggested that STN DBS was safe and slowed the progression of rest tremor in this population.
Apart from research that included patients with advanced Parkinson’s disease, data relating to long-term follow-up of patients undergoing DBS for Parkinson’s disease have been limited. Prospective studies have found that DBS provides motor benefits in patients with advanced Parkinson’s disease after 5-10 years, but they have not included control groups of patients randomly assigned to medication alone. Understanding the durability of effect of DBS is particularly important in patients with early Parkinson’s disease, because they could be exposed to stimulation for a longer time than other patients.
DBS may slow progression of rest tremor
Dr. Charles and colleagues invited patients who completed their pilot study to participate in an observational follow-up study. All 29 patients who completed the pilot study consented to participate in the follow-up. The investigators conducted annual outpatient examinations at 3, 4, and 5 years after baseline. These examinations were similar to those conducted at baseline in the pilot trial. Patients’ scores on the Unified Parkinson’s Disease Rating Scale (UPDRS) Part III were obtained through blinded video assessment. Rigidity was not assessed. The investigators calculated patients’ levodopa equivalent daily dose (LEDD) and total electrical energy delivered (TEED). Adverse events were classified as mild, moderate, or severe.
Because of a problem with study funding, the investigators examined only eight patients in the optimal therapy group and nine patients in the DBS group at 3 years. The final analysis included 28 patients, because one patient was found not to have met inclusion criteria after the trial was completed.
At 5 years, participants’ mean age was 66.1 years. Participants had been taking medications for Parkinson’s disease for a mean duration of 7.2 years. No deaths occurred during the study. Four participants who had been assigned randomly to optimal drug therapy chose to receive STN DBS during the study. The investigators evaluated these participants in the treatment group to which they had been assigned at randomization using an intention-to-treat analysis that compared early STN DBS plus drug therapy with drug therapy alone.
Among patients with early DBS, the odds ratio (OR) of worse UPDRS III scores during 5 years was 0.42, compared with the medical therapy group. The difference in mean UPDRS III score between groups due to randomization was 3.70, which was a clinically important difference, according to the investigators.
In the early DBS group, the OR of worse rest tremor was 0.21, compared with the drug therapy group. The between-group difference in mean rest tremor score favored the DBS group. Excluding rest tremor from participants’ UPDRS III scores eliminated between-group differences in the odds of having worse motor symptoms and in the magnitude of difference of motor symptom score.
In the early DBS group, the OR of requiring a greater LEDD was 0.26, compared with the drug therapy group. The between group difference in mean LEDD significantly favored the DBS group. In addition, at 5 years, the proportion of patients requiring polypharmacy was 93% in the drug therapy group and 43% in the DBS group.
The investigators found no difference between groups in the prevalence of dyskinesia at baseline. At 5 years, the prevalence of dyskinesia was 50% in the drug therapy group and 21% in the DBS group. The difference was not statistically significant, however.
The study groups had similar adverse event profiles. Five adverse events during follow-up were related to surgery or the DBS device. The most common of the 13 study-related adverse events was nausea.
The study’s most significant finding is that “DBS implanted in early Parkinson’s disease decreases the risk of disease progression,” said Dr. Charles. No therapy, including DBS, has been proven to decrease this risk. “This is class II evidence. We have to get class I evidence before we change practice.”
Dr. Charles and colleagues have received Food and Drug Administration approval for a multicenter phase 3 trial to obtain this evidence. The new trial may extend findings regarding DBS in mid-stage and advanced Parkinson’s disease to early-stage Parkinson’s disease. That is, it may show that DBS plus drug therapy in early stage Parkinson’s disease is safe, efficacious, and superior to standard medical therapy alone. “But the reason to do the trial is to determine if it changes or slows the progression of the disease,” said Dr. Charles.
Effect on dyskinesia is unclear
“If a patient does go on to develop problems that need DBS management, and only a small fraction of patients with Parkinson’s disease evolve to this need, then this procedure can be performed at that time,” said Peter A. LeWitt, MD, Sastry Foundation Endowed Chair in Neurology at Wayne State University in Detroit.
“One confound of the study is that DBS provides symptomatic relief of dyskinesias if a patient has developed this problem after a few years of levodopa treatment,” Dr. LeWitt added. “To demonstrate that early use of DBS prevented the development of dyskinesias, the study design should have included a period of turning off the stimulators to determine whether the generation of dyskinesias was prevented, rather than merely suppressed by DBS, as any patient would experience.
“Finally, the goal of reducing use of levodopa dose medications or polypharmacy doesn’t justify subjecting a patient to a brain operation that is not without risks and great expense,” Dr. LeWitt continued. “The results of this underpowered study add to my opinion that the ‘premature’ use of DBS is not a good idea for the management of Parkinson’s disease.”
Medtronic, which manufactures the DBS device that the investigators used, provided part of the study’s funding. Vanderbilt University receives income for research or educational programs that Dr. Charles leads. Dr. LeWitt had no pertinent disclosures.
SOURCE: Hacker ML et al. Neurology. 2020 Jun 29. doi: 10.1212/WNL.0000000000009946.
According to the investigators, a larger trial is needed to confirm these findings, which were published online ahead of print June 29 in Neurology.
Adverse events were similar between patients who underwent DBS and drug therapy and those who underwent drug therapy alone. This result is a preliminary indication of the safety of long-term DBS therapy, according to the researchers. Furthermore, patients who received DBS required a significantly lower levodopa equivalent daily dose (LEDD) and were less likely to need polypharmacy than were patients who received medical treatment alone.
“While we can be really excited about these findings, we can’t change our practice, what we recommend to patients, based on this [study],” said David Charles, MD, professor and vice chair of neurology at Vanderbilt University, Nashville, Tenn. “We have to do the next trial to get that class of evidence.”
An extension of a pilot trial
Previous research has indicated that treatment with DBS and optimal medical therapy provides benefits beyond those of medical therapy alone in patients with mid-stage or advanced Parkinson’s disease. Dr. Charles and colleagues conducted a randomized, single-blind pilot study to examine the safety and tolerability of STN DBS in 30 patients with early Parkinson’s disease. Eligible participants had Hoehn and Yahr stage II off medication, were between 50 and 75 years of age, had taken medication for 6 months to 4 years, and had no dyskinesia or other motor fluctuations.
Patients were randomly assigned in equal groups to optimal drug therapy plus STN DBS or to drug therapy alone. Investigators evaluated patients every 6 months for 2 years. The results suggested that STN DBS was safe and slowed the progression of rest tremor in this population.
Apart from research that included patients with advanced Parkinson’s disease, data relating to long-term follow-up of patients undergoing DBS for Parkinson’s disease have been limited. Prospective studies have found that DBS provides motor benefits in patients with advanced Parkinson’s disease after 5-10 years, but they have not included control groups of patients randomly assigned to medication alone. Understanding the durability of effect of DBS is particularly important in patients with early Parkinson’s disease, because they could be exposed to stimulation for a longer time than other patients.
DBS may slow progression of rest tremor
Dr. Charles and colleagues invited patients who completed their pilot study to participate in an observational follow-up study. All 29 patients who completed the pilot study consented to participate in the follow-up. The investigators conducted annual outpatient examinations at 3, 4, and 5 years after baseline. These examinations were similar to those conducted at baseline in the pilot trial. Patients’ scores on the Unified Parkinson’s Disease Rating Scale (UPDRS) Part III were obtained through blinded video assessment. Rigidity was not assessed. The investigators calculated patients’ levodopa equivalent daily dose (LEDD) and total electrical energy delivered (TEED). Adverse events were classified as mild, moderate, or severe.
Because of a problem with study funding, the investigators examined only eight patients in the optimal therapy group and nine patients in the DBS group at 3 years. The final analysis included 28 patients, because one patient was found not to have met inclusion criteria after the trial was completed.
At 5 years, participants’ mean age was 66.1 years. Participants had been taking medications for Parkinson’s disease for a mean duration of 7.2 years. No deaths occurred during the study. Four participants who had been assigned randomly to optimal drug therapy chose to receive STN DBS during the study. The investigators evaluated these participants in the treatment group to which they had been assigned at randomization using an intention-to-treat analysis that compared early STN DBS plus drug therapy with drug therapy alone.
Among patients with early DBS, the odds ratio (OR) of worse UPDRS III scores during 5 years was 0.42, compared with the medical therapy group. The difference in mean UPDRS III score between groups due to randomization was 3.70, which was a clinically important difference, according to the investigators.
In the early DBS group, the OR of worse rest tremor was 0.21, compared with the drug therapy group. The between-group difference in mean rest tremor score favored the DBS group. Excluding rest tremor from participants’ UPDRS III scores eliminated between-group differences in the odds of having worse motor symptoms and in the magnitude of difference of motor symptom score.
In the early DBS group, the OR of requiring a greater LEDD was 0.26, compared with the drug therapy group. The between group difference in mean LEDD significantly favored the DBS group. In addition, at 5 years, the proportion of patients requiring polypharmacy was 93% in the drug therapy group and 43% in the DBS group.
The investigators found no difference between groups in the prevalence of dyskinesia at baseline. At 5 years, the prevalence of dyskinesia was 50% in the drug therapy group and 21% in the DBS group. The difference was not statistically significant, however.
The study groups had similar adverse event profiles. Five adverse events during follow-up were related to surgery or the DBS device. The most common of the 13 study-related adverse events was nausea.
The study’s most significant finding is that “DBS implanted in early Parkinson’s disease decreases the risk of disease progression,” said Dr. Charles. No therapy, including DBS, has been proven to decrease this risk. “This is class II evidence. We have to get class I evidence before we change practice.”
Dr. Charles and colleagues have received Food and Drug Administration approval for a multicenter phase 3 trial to obtain this evidence. The new trial may extend findings regarding DBS in mid-stage and advanced Parkinson’s disease to early-stage Parkinson’s disease. That is, it may show that DBS plus drug therapy in early stage Parkinson’s disease is safe, efficacious, and superior to standard medical therapy alone. “But the reason to do the trial is to determine if it changes or slows the progression of the disease,” said Dr. Charles.
Effect on dyskinesia is unclear
“If a patient does go on to develop problems that need DBS management, and only a small fraction of patients with Parkinson’s disease evolve to this need, then this procedure can be performed at that time,” said Peter A. LeWitt, MD, Sastry Foundation Endowed Chair in Neurology at Wayne State University in Detroit.
“One confound of the study is that DBS provides symptomatic relief of dyskinesias if a patient has developed this problem after a few years of levodopa treatment,” Dr. LeWitt added. “To demonstrate that early use of DBS prevented the development of dyskinesias, the study design should have included a period of turning off the stimulators to determine whether the generation of dyskinesias was prevented, rather than merely suppressed by DBS, as any patient would experience.
“Finally, the goal of reducing use of levodopa dose medications or polypharmacy doesn’t justify subjecting a patient to a brain operation that is not without risks and great expense,” Dr. LeWitt continued. “The results of this underpowered study add to my opinion that the ‘premature’ use of DBS is not a good idea for the management of Parkinson’s disease.”
Medtronic, which manufactures the DBS device that the investigators used, provided part of the study’s funding. Vanderbilt University receives income for research or educational programs that Dr. Charles leads. Dr. LeWitt had no pertinent disclosures.
SOURCE: Hacker ML et al. Neurology. 2020 Jun 29. doi: 10.1212/WNL.0000000000009946.
FROM NEUROLOGY
Granular Parakeratosis
To the Editor:
A 46-year-old overweight woman presented with a rash in the axillae of 2 months’ duration. She did not report any additional symptoms such as pruritus or pain. She reported changing her deodorant recently from Secret Original to Secret Clinical Strength (both Procter & Gamble). Her medical history was remarkable for asthma and gastroesophageal reflux disease. Clinical examination revealed erythematous-brown, stuccolike, hyperkeratotic papules coalescing into plaques in recently shaved axillae, affecting the left axilla more than the right axilla (Figure 1). The clinical differential diagnosis included granular parakeratosis, intertrigo, Hailey-Hailey disease, Darier disease, pemphigus vegetans, confluent and reticulated papillomatosis, acanthosis nigricans, seborrheic keratoses, and irritant or allergic contact dermatitis. A punch biopsy revealed a marked compact parakeratotic horn with retention of keratohyalin granules (Figure 2). The subjacent epidermis showed some acanthosis and spongiosis with mild chronic inflammation of the dermal rim. Based on histopathology, granular parakeratosis was diagnosed.
At a subsequent visit 2 weeks later, we prescribed glycolic acid lotion 10% applied to the axillae twice daily, plus tretinoin gel 0.05% applied to the axillae each evening. She reported clearing after 1 week of therapy. She also had changed her deodorant from Secret Clinical Strength back to the usual Secret Original. The patient discontinued topical treatment after clearing of the lesions. Three weeks later, clinical examination revealed postinflammatory hyperpigmentation in the axillae, and the prior lesions had resolved (Figure 3).
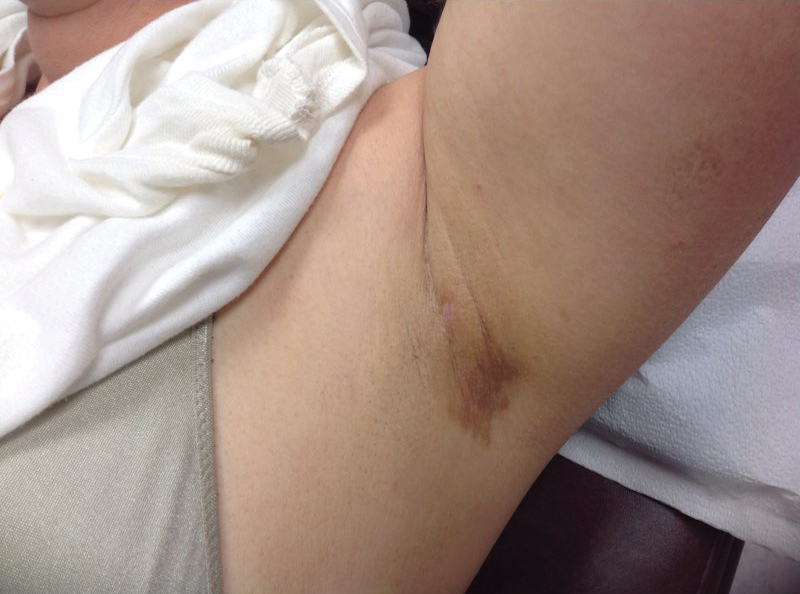
Granular parakeratosis is an unusual condition most commonly presenting in middle-aged women in the axillae, with a clinical presentation of erythematous to brownish hyperkeratotic papules coalescing into plaques. Although few cases have been reported, granular parakeratosis likely is more common than has been reported. There have been reports involving the scalp, cheeks, abdomen, thighs, and other intertriginous areas including inguinal folds and the submammary region.1-4 There also is an infantile form related to diapers and zinc oxide paste.5 Although uncommon, granular parakeratosis can occur as a single papule or plaque and is termed granular parakeratotic acanthoma.6 Lesions may persist for months, spontaneously resolve and recur, and occasionally evolve into fissures and erosions due to irritation. Pruritus is a common concern. Histology of granular parakeratosis reveals hyperkeratosis with eosinophilic staining, compact parakeratosis with retention of basophilic keratohyalin granules, and vascular proliferation and ectasia.5
The cause is unknown but possibly related to irritation from rubbing, occlusion, sweating, or deodorants.5,7 Cases indicate a link to obesity. Hypotheses as to the etiology include the disruption of cornification. Normally, filaggrin maintains the keratohyaline granules in the stratum corneum during cornification. Therefore, the retention of keratohyaline granules in granular parakeratosis may be due to a defect in processing profilaggrin to filaggrin, which has been proposed based on ultrastructural and immunohistochemical studies.8
The differential diagnosis includes granular parakeratosis, intertrigo (caused by seborrheic dermatitis, candidiasis, inverse psoriasis, or erythrasma), Hailey-Hailey disease, Darier disease, pemphigus vegetans, confluent and reticulated papillomatosis, and irritant or allergic contact dermatitis. The papules may resemble seborrheic keratoses, while the plaques can be mistaken for acanthosis nigricans.
Therapeutic success has been reported with topical corticosteroids, vitamin D analogues, topical or oral retinoids, ammonium lactate, calcineurin inhibitors, topical or oral antifungals, cryotherapy, and botulinum toxin injections.3,9-11 In addition, parakeratosis has decreased in biopsies from psoriatic patients after acitretin, methotrexate, and phototherapy, which may be alternative treatments for unusually difficult or recalcitrant cases of granular parakeratosis. To minimize side effects and resolve the papules quickly, we combined 2 synergistic agents—glycolic acid and tretinoin—each with different mechanisms of action, and we observed excellent clinical response.
Granular parakeratosis is possibly related to a combination of topical products that potentiate irritation, rubbing, and occlusion of sweat. Multiple treatment modalities likely contribute to clearing, the most important being removal of any triggering topical products. Our patient’s change in deodorant may have been the inciting factor for the disease. Withdrawal of the Secret Clinical Strength deodorant prompted clearing, though topical retinoid and glycolic acid acted as facilitating therapies for timely results. A thorough history, as highlighted by this case, may help pinpoint etiologic factors. By identifying a seemingly innocuous change in hygienic routine, we were able to minimize the need for ongoing therapy.
- Graham R. Intertriginous granular parakeratosis: a case report and review of the literature. J Am Acad Dermatol. 2011;64:AB45-AB45.
- Compton AK, Jackson JM. Isotretinoin as a treatment for axillary granular parakeratosis. Cutis. 2007;80:55-56.
- Channual J, Fife DJ, Wu JJ. Axillary granular parakeratosis. Cutis. 2013;92;61, 65-66.
- Streams S, Gottwald L, Zaher A, et al. Granular parakeratosis of the scalp: a case report. J Am Acad Dermatol. 2007;56:AB81-AB81.
- James WD, Berger T, Elston D. Andrews’ Diseases of the Skin. 12th ed. Philadelphia, PA: Elsevier, Inc; 2015.
- Resnik KS, Kantor GR, DiLeonardo M. Granular parakeratotic acanthoma. Am J Dermatopathol. 2005;27:393-396.
- Naylor E, Wartman D, Telang G, et al. Granular parakeratosis secondary to postsurgical occlusion. J Am Acad Dermatol. 2008;58:AB126.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier, Inc; 2012.
- Baum B, Skopit S. Granular parakeratosis treatment with tacrolimus 0.1% ointment: a case presentation and discussion. J Am Osteo Coll Dermatol. 2013;26:40-41.
- Brown SK, Heilman ER. Granular parakeratosis: resolution with topical tretinoin. J Am Acad Dermatol. 2002;47:S279-S280.
- Webster CG, Resnik KS, Webster GF. Axillary granular parakeratosis: response to isotretinoin. J Am Acad Dermatol. 1997;37:789790.
To the Editor:
A 46-year-old overweight woman presented with a rash in the axillae of 2 months’ duration. She did not report any additional symptoms such as pruritus or pain. She reported changing her deodorant recently from Secret Original to Secret Clinical Strength (both Procter & Gamble). Her medical history was remarkable for asthma and gastroesophageal reflux disease. Clinical examination revealed erythematous-brown, stuccolike, hyperkeratotic papules coalescing into plaques in recently shaved axillae, affecting the left axilla more than the right axilla (Figure 1). The clinical differential diagnosis included granular parakeratosis, intertrigo, Hailey-Hailey disease, Darier disease, pemphigus vegetans, confluent and reticulated papillomatosis, acanthosis nigricans, seborrheic keratoses, and irritant or allergic contact dermatitis. A punch biopsy revealed a marked compact parakeratotic horn with retention of keratohyalin granules (Figure 2). The subjacent epidermis showed some acanthosis and spongiosis with mild chronic inflammation of the dermal rim. Based on histopathology, granular parakeratosis was diagnosed.


At a subsequent visit 2 weeks later, we prescribed glycolic acid lotion 10% applied to the axillae twice daily, plus tretinoin gel 0.05% applied to the axillae each evening. She reported clearing after 1 week of therapy. She also had changed her deodorant from Secret Clinical Strength back to the usual Secret Original. The patient discontinued topical treatment after clearing of the lesions. Three weeks later, clinical examination revealed postinflammatory hyperpigmentation in the axillae, and the prior lesions had resolved (Figure 3).

Granular parakeratosis is an unusual condition most commonly presenting in middle-aged women in the axillae, with a clinical presentation of erythematous to brownish hyperkeratotic papules coalescing into plaques. Although few cases have been reported, granular parakeratosis likely is more common than has been reported. There have been reports involving the scalp, cheeks, abdomen, thighs, and other intertriginous areas including inguinal folds and the submammary region.1-4 There also is an infantile form related to diapers and zinc oxide paste.5 Although uncommon, granular parakeratosis can occur as a single papule or plaque and is termed granular parakeratotic acanthoma.6 Lesions may persist for months, spontaneously resolve and recur, and occasionally evolve into fissures and erosions due to irritation. Pruritus is a common concern. Histology of granular parakeratosis reveals hyperkeratosis with eosinophilic staining, compact parakeratosis with retention of basophilic keratohyalin granules, and vascular proliferation and ectasia.5
The cause is unknown but possibly related to irritation from rubbing, occlusion, sweating, or deodorants.5,7 Cases indicate a link to obesity. Hypotheses as to the etiology include the disruption of cornification. Normally, filaggrin maintains the keratohyaline granules in the stratum corneum during cornification. Therefore, the retention of keratohyaline granules in granular parakeratosis may be due to a defect in processing profilaggrin to filaggrin, which has been proposed based on ultrastructural and immunohistochemical studies.8
The differential diagnosis includes granular parakeratosis, intertrigo (caused by seborrheic dermatitis, candidiasis, inverse psoriasis, or erythrasma), Hailey-Hailey disease, Darier disease, pemphigus vegetans, confluent and reticulated papillomatosis, and irritant or allergic contact dermatitis. The papules may resemble seborrheic keratoses, while the plaques can be mistaken for acanthosis nigricans.
Therapeutic success has been reported with topical corticosteroids, vitamin D analogues, topical or oral retinoids, ammonium lactate, calcineurin inhibitors, topical or oral antifungals, cryotherapy, and botulinum toxin injections.3,9-11 In addition, parakeratosis has decreased in biopsies from psoriatic patients after acitretin, methotrexate, and phototherapy, which may be alternative treatments for unusually difficult or recalcitrant cases of granular parakeratosis. To minimize side effects and resolve the papules quickly, we combined 2 synergistic agents—glycolic acid and tretinoin—each with different mechanisms of action, and we observed excellent clinical response.
Granular parakeratosis is possibly related to a combination of topical products that potentiate irritation, rubbing, and occlusion of sweat. Multiple treatment modalities likely contribute to clearing, the most important being removal of any triggering topical products. Our patient’s change in deodorant may have been the inciting factor for the disease. Withdrawal of the Secret Clinical Strength deodorant prompted clearing, though topical retinoid and glycolic acid acted as facilitating therapies for timely results. A thorough history, as highlighted by this case, may help pinpoint etiologic factors. By identifying a seemingly innocuous change in hygienic routine, we were able to minimize the need for ongoing therapy.
To the Editor:
A 46-year-old overweight woman presented with a rash in the axillae of 2 months’ duration. She did not report any additional symptoms such as pruritus or pain. She reported changing her deodorant recently from Secret Original to Secret Clinical Strength (both Procter & Gamble). Her medical history was remarkable for asthma and gastroesophageal reflux disease. Clinical examination revealed erythematous-brown, stuccolike, hyperkeratotic papules coalescing into plaques in recently shaved axillae, affecting the left axilla more than the right axilla (Figure 1). The clinical differential diagnosis included granular parakeratosis, intertrigo, Hailey-Hailey disease, Darier disease, pemphigus vegetans, confluent and reticulated papillomatosis, acanthosis nigricans, seborrheic keratoses, and irritant or allergic contact dermatitis. A punch biopsy revealed a marked compact parakeratotic horn with retention of keratohyalin granules (Figure 2). The subjacent epidermis showed some acanthosis and spongiosis with mild chronic inflammation of the dermal rim. Based on histopathology, granular parakeratosis was diagnosed.


At a subsequent visit 2 weeks later, we prescribed glycolic acid lotion 10% applied to the axillae twice daily, plus tretinoin gel 0.05% applied to the axillae each evening. She reported clearing after 1 week of therapy. She also had changed her deodorant from Secret Clinical Strength back to the usual Secret Original. The patient discontinued topical treatment after clearing of the lesions. Three weeks later, clinical examination revealed postinflammatory hyperpigmentation in the axillae, and the prior lesions had resolved (Figure 3).

Granular parakeratosis is an unusual condition most commonly presenting in middle-aged women in the axillae, with a clinical presentation of erythematous to brownish hyperkeratotic papules coalescing into plaques. Although few cases have been reported, granular parakeratosis likely is more common than has been reported. There have been reports involving the scalp, cheeks, abdomen, thighs, and other intertriginous areas including inguinal folds and the submammary region.1-4 There also is an infantile form related to diapers and zinc oxide paste.5 Although uncommon, granular parakeratosis can occur as a single papule or plaque and is termed granular parakeratotic acanthoma.6 Lesions may persist for months, spontaneously resolve and recur, and occasionally evolve into fissures and erosions due to irritation. Pruritus is a common concern. Histology of granular parakeratosis reveals hyperkeratosis with eosinophilic staining, compact parakeratosis with retention of basophilic keratohyalin granules, and vascular proliferation and ectasia.5
The cause is unknown but possibly related to irritation from rubbing, occlusion, sweating, or deodorants.5,7 Cases indicate a link to obesity. Hypotheses as to the etiology include the disruption of cornification. Normally, filaggrin maintains the keratohyaline granules in the stratum corneum during cornification. Therefore, the retention of keratohyaline granules in granular parakeratosis may be due to a defect in processing profilaggrin to filaggrin, which has been proposed based on ultrastructural and immunohistochemical studies.8
The differential diagnosis includes granular parakeratosis, intertrigo (caused by seborrheic dermatitis, candidiasis, inverse psoriasis, or erythrasma), Hailey-Hailey disease, Darier disease, pemphigus vegetans, confluent and reticulated papillomatosis, and irritant or allergic contact dermatitis. The papules may resemble seborrheic keratoses, while the plaques can be mistaken for acanthosis nigricans.
Therapeutic success has been reported with topical corticosteroids, vitamin D analogues, topical or oral retinoids, ammonium lactate, calcineurin inhibitors, topical or oral antifungals, cryotherapy, and botulinum toxin injections.3,9-11 In addition, parakeratosis has decreased in biopsies from psoriatic patients after acitretin, methotrexate, and phototherapy, which may be alternative treatments for unusually difficult or recalcitrant cases of granular parakeratosis. To minimize side effects and resolve the papules quickly, we combined 2 synergistic agents—glycolic acid and tretinoin—each with different mechanisms of action, and we observed excellent clinical response.
Granular parakeratosis is possibly related to a combination of topical products that potentiate irritation, rubbing, and occlusion of sweat. Multiple treatment modalities likely contribute to clearing, the most important being removal of any triggering topical products. Our patient’s change in deodorant may have been the inciting factor for the disease. Withdrawal of the Secret Clinical Strength deodorant prompted clearing, though topical retinoid and glycolic acid acted as facilitating therapies for timely results. A thorough history, as highlighted by this case, may help pinpoint etiologic factors. By identifying a seemingly innocuous change in hygienic routine, we were able to minimize the need for ongoing therapy.
- Graham R. Intertriginous granular parakeratosis: a case report and review of the literature. J Am Acad Dermatol. 2011;64:AB45-AB45.
- Compton AK, Jackson JM. Isotretinoin as a treatment for axillary granular parakeratosis. Cutis. 2007;80:55-56.
- Channual J, Fife DJ, Wu JJ. Axillary granular parakeratosis. Cutis. 2013;92;61, 65-66.
- Streams S, Gottwald L, Zaher A, et al. Granular parakeratosis of the scalp: a case report. J Am Acad Dermatol. 2007;56:AB81-AB81.
- James WD, Berger T, Elston D. Andrews’ Diseases of the Skin. 12th ed. Philadelphia, PA: Elsevier, Inc; 2015.
- Resnik KS, Kantor GR, DiLeonardo M. Granular parakeratotic acanthoma. Am J Dermatopathol. 2005;27:393-396.
- Naylor E, Wartman D, Telang G, et al. Granular parakeratosis secondary to postsurgical occlusion. J Am Acad Dermatol. 2008;58:AB126.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier, Inc; 2012.
- Baum B, Skopit S. Granular parakeratosis treatment with tacrolimus 0.1% ointment: a case presentation and discussion. J Am Osteo Coll Dermatol. 2013;26:40-41.
- Brown SK, Heilman ER. Granular parakeratosis: resolution with topical tretinoin. J Am Acad Dermatol. 2002;47:S279-S280.
- Webster CG, Resnik KS, Webster GF. Axillary granular parakeratosis: response to isotretinoin. J Am Acad Dermatol. 1997;37:789790.
- Graham R. Intertriginous granular parakeratosis: a case report and review of the literature. J Am Acad Dermatol. 2011;64:AB45-AB45.
- Compton AK, Jackson JM. Isotretinoin as a treatment for axillary granular parakeratosis. Cutis. 2007;80:55-56.
- Channual J, Fife DJ, Wu JJ. Axillary granular parakeratosis. Cutis. 2013;92;61, 65-66.
- Streams S, Gottwald L, Zaher A, et al. Granular parakeratosis of the scalp: a case report. J Am Acad Dermatol. 2007;56:AB81-AB81.
- James WD, Berger T, Elston D. Andrews’ Diseases of the Skin. 12th ed. Philadelphia, PA: Elsevier, Inc; 2015.
- Resnik KS, Kantor GR, DiLeonardo M. Granular parakeratotic acanthoma. Am J Dermatopathol. 2005;27:393-396.
- Naylor E, Wartman D, Telang G, et al. Granular parakeratosis secondary to postsurgical occlusion. J Am Acad Dermatol. 2008;58:AB126.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier, Inc; 2012.
- Baum B, Skopit S. Granular parakeratosis treatment with tacrolimus 0.1% ointment: a case presentation and discussion. J Am Osteo Coll Dermatol. 2013;26:40-41.
- Brown SK, Heilman ER. Granular parakeratosis: resolution with topical tretinoin. J Am Acad Dermatol. 2002;47:S279-S280.
- Webster CG, Resnik KS, Webster GF. Axillary granular parakeratosis: response to isotretinoin. J Am Acad Dermatol. 1997;37:789790.
Practice Points
- Granular parakeratosis most commonly presents in middle-aged women in the axillae.
- The cause is unknown but possibly related to irritation from rubbing, occlusion, sweating, or deodorants.
- Multiple treatment modalities likely contribute to clearing, the most important being removal of any triggering topical products.
Eczema Herpeticum in a Patient With Hailey-Hailey Disease Confounded by Coexistent Psoriasis
To the Editor:
Hailey-Hailey disease (HHD), also known as benign familial pemphigus, is an uncommon autosomal-dominant skin disease.1 Defects in the ATPase type 2C member 1 gene, ATP2C1, result in abnormal intracellular epidermal adherence, and patients experience recurring blisters in skin folds. Longitudinal white streaks of the fingernails also may be present.1 The illness does not appear until puberty and is heightened by the second or third decade of life. Family history often suggests the presence of disease.2 Misdiagnosis of HHD occurs because of a wide spectrum of presentations. The presence of superimposed infections and carcinomas may both obscure and exacerbate this disease.2
Herpes simplex viruse types 1 and 2 (HSV-1 and HSV-2) are DNA viruses that cause common recurrent diseases. Usually, HSV-1 is associated with infection of the mouth and HSV-2 is associated with infection of the genitalia.3 Longitudinal cutaneous lesions manifest as grouped vesicles on an erythematous base. Tzanck smear of herpetic vesicles will reveal the presence of multinucleated giant cells. A direct fluorescent antibody technique also may be used to confirm the diagnosis.3
Erythrodermic HHD disease is a rare condition; moreover, there are only a few reported cases with coexistence of HHD and HSV in the literature.3-6 We report a rare presentation of erythrodermic HHD and coexistent psoriasis with HSV superinfection.
A 69-year-old man presented to an outpatient dermatology clinic for evaluation and treatment of a rash on the scalp, face, back, and lower legs. The patient confirmed a dandruff diagnosis on the scalp and face as well as psoriasis on the trunk and extremities for the last 45 years. He described a history of successful treatment with topical agents and UV light therapy. A family history revealed that the patient’s father and 1 of 2 siblings had a similar rash and “skin problems.” The patient had a medical history of thyroid cancer treated with radiation treatment and a partial thyroidectomy 35 years prior to the current presentation as well as incompletely treated chronic hepatitis C.
A search of medical records revealed a punch biopsy from the posterior neck that demonstrated an acantholytic dyskeratosis with suprabasal acantholysis. Clinicians were unable to differentiate if it was Darier disease (DAR) or HHD. Treatment of the patient’s seborrheic dermatitis and acantholytic disorder was successful at that time with ketoconazole shampoo, ketoconazole cream, desonide cream, and triamcinolone cream. The patient remained stable for 5 years before presenting again to the dermatology clinic for worsening rash despite topical therapies.
At the current presentation, physical examination at the outpatient dermatology clinic revealed few scaly, erythematous, eroded papules distributed on the mid-back; erythematous greasy scaling on the scalp, face, and chest; and pink scaly plaques with white-silvery scale on the anterior lower legs. Histopathology of a specimen from the right mid-back demonstrated acantholysis with suprabasal clefting, hyperkeratosis, and parakeratosis with no dyskeratotic cells identified. The pathologic differential diagnosis included primary acantholytic processes including Grover disease, DAR, HHD, and pemphigus. Pathology from the right shin demonstrated acanthosis, confluent parakeratosis with associated decreased granular cell layer and collections of neutrophils within the stratum corneum, spongiosis, and superficial dermal perivascular chronic inflammation with focal exocytosis and dilated blood vessels in the papillary dermis. The clinical and pathological diagnosis on the lower legs was consistent with psoriasis. Diagnoses of seborrheic dermatitis, psoriasis on the lower legs, and HHD vs DAR on the back and chest were made. The patient was instructed to continue ketoconazole shampoo, ketoconazole cream, and desonide for seborrheic dermatitis; fluocinonide ointment 0.05% to the lower legs for psoriasis; and triamcinolone cream and a bland moisturizer to the back and chest for HHD.
Over the ensuing months, the rash worsened with erythema and scaling affecting more than half of the body surface area. Topical corticosteroids and bland emollients resulted in minimal success. Biologics and acitretin were considered for the psoriasiform dermatitis but avoided due to the patient’s medical history of thyroid cancer and chronic hepatitis C infection. Because the patient described prior success with UV light therapy for psoriasis, he requested light therapy. A subsequent trial of narrowband UVB light therapy initially improved some of the psoriasiform dermatitis on the trunk and extremities; however, after 4 weeks of treatment, the patient described pain in some of the skin and felt he was burned by minimal exposure to light therapy on one particular visit, which caused him to stop light therapy.
Approximately 2 weeks later, the patient presented to the emergency department stating his psoriasis was infected; he was diagnosed with psoriasis with secondary cellulitis and received intravenous vancomycin and piperacillin-tazobactam, with bacterial cultures demonstrating Corynebacterium and methicillin-resistant Staphylococcus aureus. Some improvement was noted in the patient’s skin after antibiotics were initiated, but he continued to describe worsening “burning and pain” throughout the psoriasis lesions. The patient’s care was transferred to the Veterans Affairs hospital where a dermatology inpatient consultation was placed.
Our initial dermatologic examination revealed generalized scaly erythroderma on the neck, trunk, and extremities, sparing the face, palms, and soles (Figure 1). Multiple crusted and intact vesicles also were present overlying the erythematous plaques on the chest, back, and proximal extremities, most grouped in clusters. The patient endorsed new symptoms of pain and burning. Tzanck smear from the abdomen along with shave biopsies from the left flank and right abdomen were performed, and intravenous acyclovir was initiated immediately after these procedures.
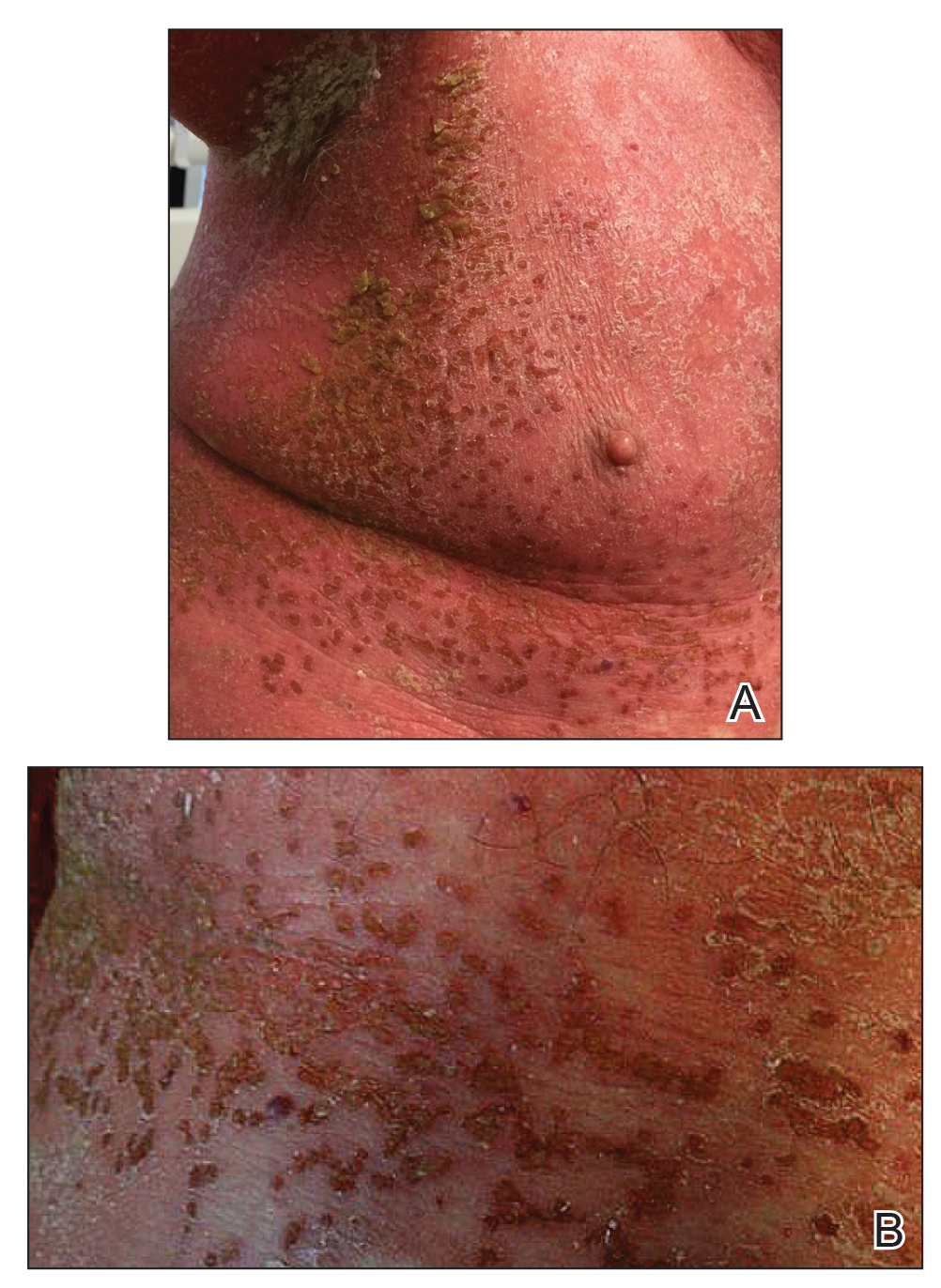
Viral cultures were taken but were incorrectly processed by the laboratory. Tzanck smear showed severe acute inflammation with numerous neutrophils, multinucleated giant cells with viral nuclear changes, and positive immunostaining for HSV and negative immunostaining for herpes zoster. Both pathology specimens revealed an intense acute mixed, mainly neutrophilic, inflammatory infiltrate extending into the deeper dermis as well as distorted and necrotic hair follicles, some of which displayed multinucleated epithelial cells with margination of chromatin that were positive for both HSV-1 and HSV-2 and negative for herpes zoster (Figure 2). The positivity of both HSV strains might represent co-infection or could be a cross-reaction of antibodies used in immunohistochemistry to the HSV antigens. There was acantholysis surrounding the ulceration and extending through the full thickness of the epidermis with a dilapidated brick wall pattern (Figure 3) as well as negative immunohistochemical staining for HSV-1 and HSV-2 antigens. The clinical and histological picture together, along with prior clinical and pathological reports, confirmed the diagnoses of acute erythrodermic HHD with HSV superinfection.
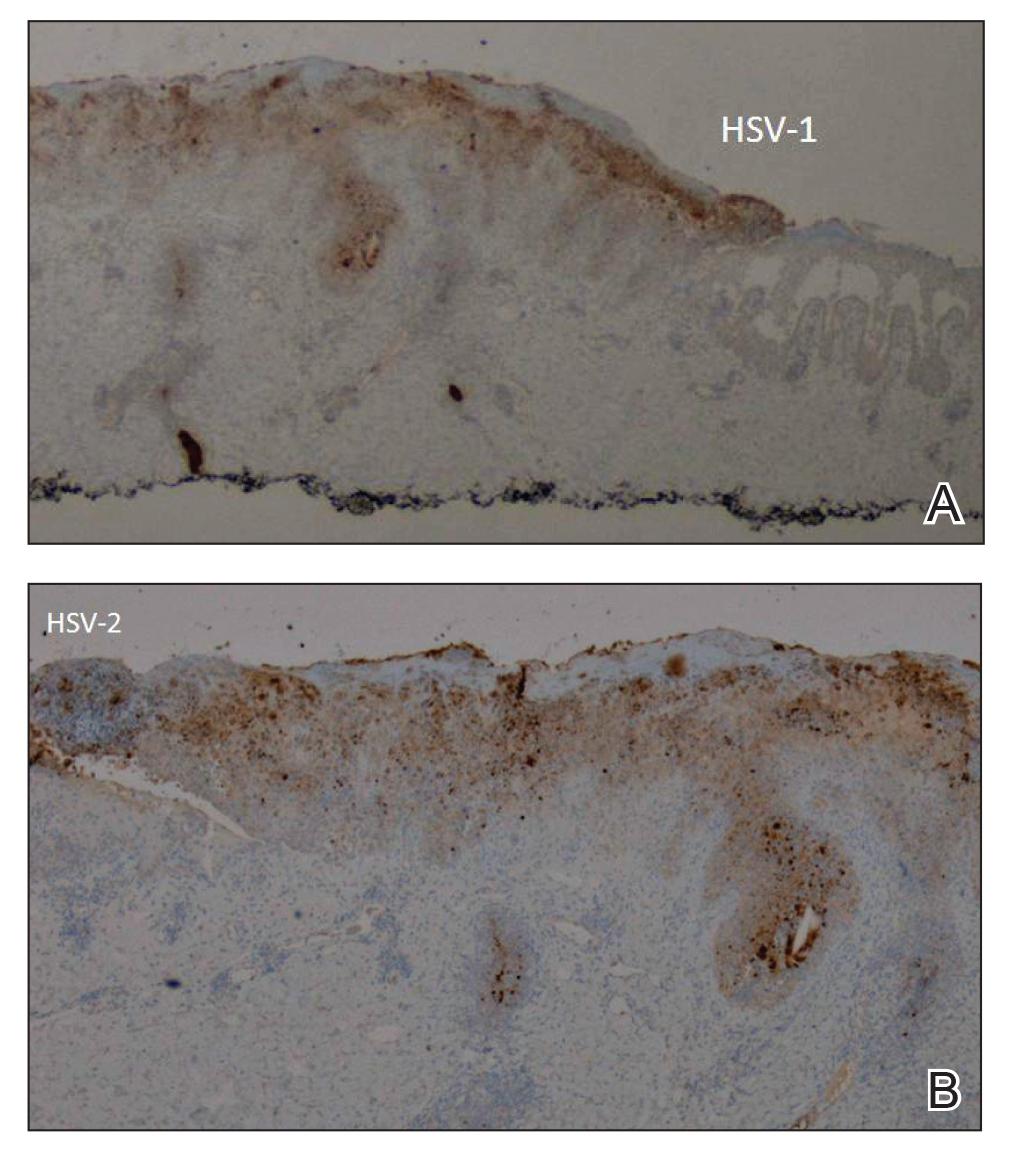
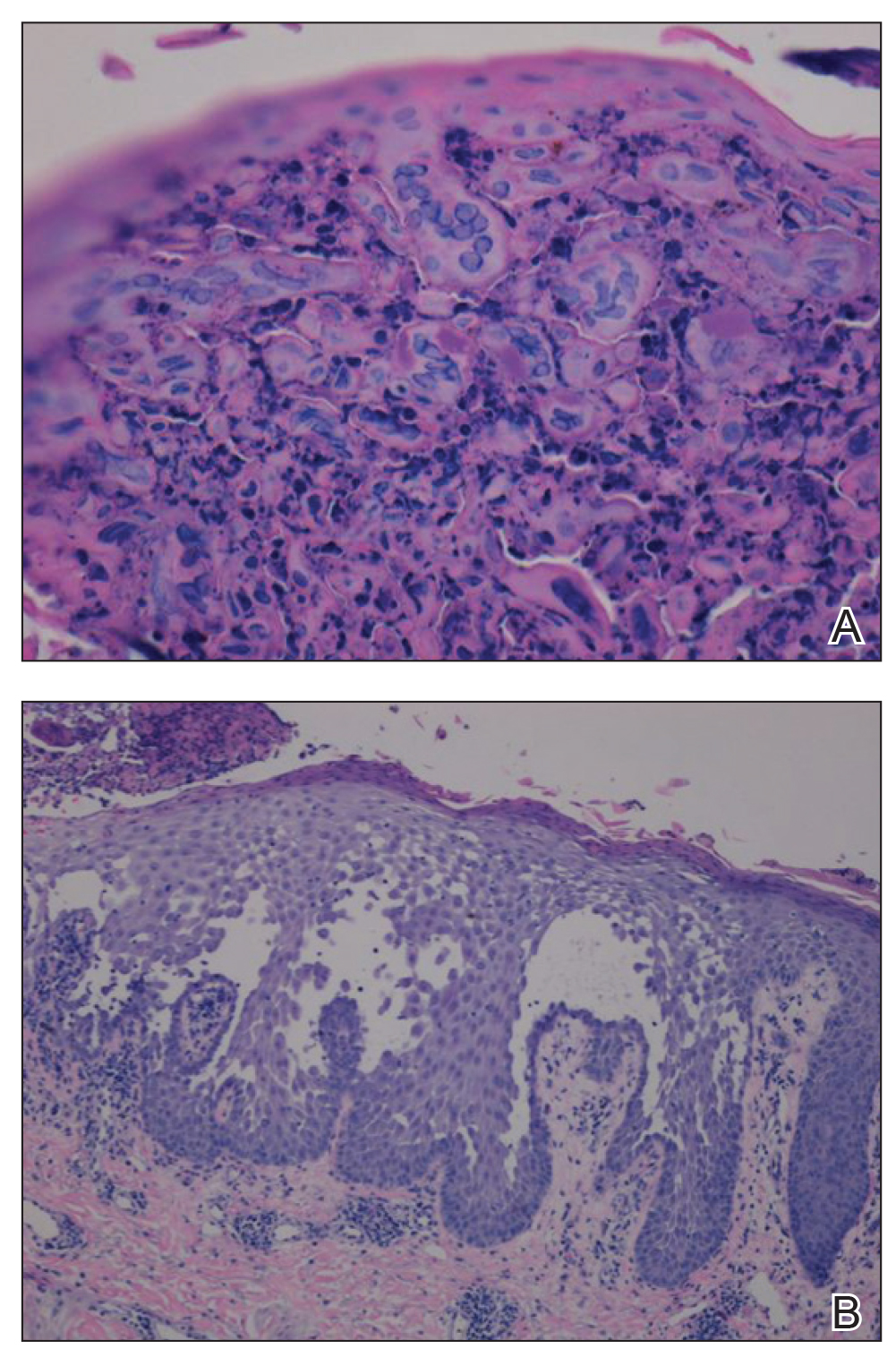
The patient’s condition and pain improved within 24 hours on intravenous acyclovir. On the third day, his lesions were resolving and symptoms improved, so he was transitioned to oral acyclovir and discharged from the hospital. Follow-up in the dermatology outpatient clinic 1 week later revealed that all vesicles and papules had cleared, but the patient was still erythrodermic. Because HHD cannot always be distinguished histologically from other forms of pemphigus but yields a negative immunofluorescence, direct immunofluorescence and indirect immunofluorescence were obtained upon patient follow-up in the clinic and were both negative. Hepatitis C viral loads were undetectable. Consultations to gastroenterology and oncology teams were placed for consideration of systemic agents, and the patient was initiated on oral acitretin 25 mg daily, along with clobetasol as adjuvant therapy for any residual skin plaques. The laboratory results were closely monitored. Within 4 weeks after starting acitretin, the patient’s erythroderma had completely resolved. The patient has remained stable since then, except for one episode of secondary Staphylococcus infection that cleared on oral antibiotics. The patient remains stable and clear on oral acitretin 25 mg daily, with concomitant desonide cream and fluocinonide ointment as needed.
Hailey-Hailey disease is characterized by recurrent episodes of erythema, blisters, and plaques localized to intertriginous and perianal areas.1,2 Patients display a spectrum of lesions that vary in severity.8 Typical histologic examination reveals a dilapidated brick wall appearance. Pathology of well-developed lesions will show suprabasal acantholysis with minimal dyskeratosis.2
The generalized form of HHD is an extremely rare variant of the disease.10 Generalized HHD may resemble acute hypersensitivity reaction, erythema multiforme, and toxic epidermal necrolysis.1 Chronic diseases, such as psoriasis (as in this patient), also may contribute to a clinically confusing picture.8 Hailey-Hailey disease and psoriasis are thought to occasionally koebnerize (isomorphic response) to areas of trauma.16 Our patient experienced widespread erythematous papules and plaques not restricted to skin folds. His skin lesions continued to worsen over several months progressing to erythroderma. The presence of suprabasal acantholysis in a dilapidated brick wall pattern, along with the patient’s history, prior pathology reports, clinical picture, and negative direct immunofluorescence and indirect immunofluorescence studies helped to confirm the diagnosis of erythrodermic HHD.
Hailey-Hailey disease is caused by heterozygous mutations in the ATP2C1 gene on chromosome 3q21-24 coding for a Golgi ATPase called SPCA1 (secretory pathway calcium/manganese-ATPase).9 Subsequent disturbances in cytosolic-Golgi calcium concentrations interfere with epidermal keratinocyte adherence resulting in acantholytic disease. Studies of interfamilial and intrafamilial mutations fail to pinpoint a common mutation pattern among patients with generalized phenotypes,9 which further supports theories that intrinsic or extrinsic factors such as friction, heat, radiation, contact allergens, and infection affect the severity of HHD disease and not the type of mutation.3,9
Generalization of HHD is likely caused by nonspecific triggers in an already genetically disturbed epidermis.10 Interrupted epithelial function exposes skin to infections that exacerbate the underlying disease. Superimposing bacterial infections are commonly reported in HHD. Staphylococcus, Streptococcus, and Candida species colonize the skin and aggravate the disease.11 Much less commonly, HSV superinfection can complicate HHD.3-7 No data are currently available about the frequency or incidence of Herpesviridae in HHD.7 Some studies suggest that UVB light therapy can be an exacerbating factor in DAR and some but not all HHD patients,12,13 while other case reports14,15 document clinically improved responses using phototherapy for patients with HHD. Clinicians should remain suspicious and evaluate for HSV infection in refractory or sudden exacerbation of HHD.7 Furthermore, coexistent psoriasis and HHD also is a rare entity but has been described,8 which illustrates the importance of not attributing all skin manifestations to a previously diagnosed disorder but instead keeping an open mind in case new dermatologic conditions present themselves at a later time.
We present a rare case of erythrodermic HHD and coexistent psoriasis with HSV superinfection. We hope to draw awareness to this association of generalized HHD with both HSV and psoriasis to help clinicians make the correct diagnosis promptly in similar cases in the future.
- Chave TA, Milligan A. Acute generalized Hailey-Hailey disease. Clin Exp Dermatol. 2002;27:290-292.
- Mohr MR, Erdag G, Shada Al, et al. Two patients with Hailey-Hailey disease, multiple primary melanomas, and other cancers. Arch Dermatol. 2011;147:211-215.
- Lee GM, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey Disease. Ann Dermatol. 2009;21:311-314.
- Zaim MT, Bickers DR. Herpes simplex associated with Hailey-Hailey disease. J Am Acad Dermatol. 1987;17:701-702.
- Peppiatt T, Keefe M, White JE. Hailey-Hailey disease-exacerbation by herpes simplex virus and patch tests. Clin Exp Dermatol. 2006;17:201-202.
- Almeida L, Grossman ME. Benign familial pemphigus complicated by herpes simplex virus. Cutis. 1989;44:261-262.
- Nikkels AF, Delvenne P, Herfs M, et al. Occult herpes simplex virus colonization of bullous dermatitides. Am J Clin Dermatol. 2008;9:163-168.
- Chao SC, Lee JY, Wu MC, et al. A novel splice mutation in the ATP2C1 gene in a woman with concomitant psoriasis vulgaris and disseminated Hailey-Hailey disease. Int J Dermatol. 2012;51:947-951.
- Ikeda S, Shigihara T, Mayuzumi N, et al. Mutations of ATP2C1 in Japanese patients with Hailey-Hailey disease: intrafamilial and interfamilial phenotype variations and lack of correlation with mutation patterns. J Invest Dermatol. 2001;117:1654-1656.
- Marsch W, Stuttgen G. Generalized Hailey-Hailey disease. Br J Dermatol. 1978;99:553-559.
- Friedman-Birnbaum R, Haim S, Marcus S. Generalized familial benign chronic pemphigus. Dermatologica. 1980;161:112-115.
- Richard G, Linse R, Harth W. Hailey-Hailey disease. early detection of heterozygotes by an ultraviolet provocation tests—clinical relevance of the method. Hautarzt. 1993;44:376-379.
- Mayuzumi N, Ikeda S, Kawada H, et al. Effects of ultraviolet B irradiation, proinflammatory cytokines and raised extracellular calcium concentration on the expression of ATP2A2 and ATP2C1. Br J Dermatol. 2005;152:697-701.
- Vanderbeck KA, Giroux L, Murugan NJ, et al. Combined therapeutic use of oral alitretinoin and narrowband ultraviolet-B therapy in the treatment of Hailey-Hailey disease. Dermatol Rep. 2014;6:5604.
- Mizuno K, Hamada T, Hasimoto T, et al. Successful treatment with narrow-band UVB therapy for a case of generalized Hailey-Hailey disease with a novel splice-site mutation in ATP2C1 gene. Dermatol Ther. 2014;27:233-235.
- Thappa DM. The isomorphic phenomenon of Koebner. Indian J Dermatol Venereol Leprol. 2004;70:187-189.
To the Editor:
Hailey-Hailey disease (HHD), also known as benign familial pemphigus, is an uncommon autosomal-dominant skin disease.1 Defects in the ATPase type 2C member 1 gene, ATP2C1, result in abnormal intracellular epidermal adherence, and patients experience recurring blisters in skin folds. Longitudinal white streaks of the fingernails also may be present.1 The illness does not appear until puberty and is heightened by the second or third decade of life. Family history often suggests the presence of disease.2 Misdiagnosis of HHD occurs because of a wide spectrum of presentations. The presence of superimposed infections and carcinomas may both obscure and exacerbate this disease.2
Herpes simplex viruse types 1 and 2 (HSV-1 and HSV-2) are DNA viruses that cause common recurrent diseases. Usually, HSV-1 is associated with infection of the mouth and HSV-2 is associated with infection of the genitalia.3 Longitudinal cutaneous lesions manifest as grouped vesicles on an erythematous base. Tzanck smear of herpetic vesicles will reveal the presence of multinucleated giant cells. A direct fluorescent antibody technique also may be used to confirm the diagnosis.3
Erythrodermic HHD disease is a rare condition; moreover, there are only a few reported cases with coexistence of HHD and HSV in the literature.3-6 We report a rare presentation of erythrodermic HHD and coexistent psoriasis with HSV superinfection.
A 69-year-old man presented to an outpatient dermatology clinic for evaluation and treatment of a rash on the scalp, face, back, and lower legs. The patient confirmed a dandruff diagnosis on the scalp and face as well as psoriasis on the trunk and extremities for the last 45 years. He described a history of successful treatment with topical agents and UV light therapy. A family history revealed that the patient’s father and 1 of 2 siblings had a similar rash and “skin problems.” The patient had a medical history of thyroid cancer treated with radiation treatment and a partial thyroidectomy 35 years prior to the current presentation as well as incompletely treated chronic hepatitis C.
A search of medical records revealed a punch biopsy from the posterior neck that demonstrated an acantholytic dyskeratosis with suprabasal acantholysis. Clinicians were unable to differentiate if it was Darier disease (DAR) or HHD. Treatment of the patient’s seborrheic dermatitis and acantholytic disorder was successful at that time with ketoconazole shampoo, ketoconazole cream, desonide cream, and triamcinolone cream. The patient remained stable for 5 years before presenting again to the dermatology clinic for worsening rash despite topical therapies.
At the current presentation, physical examination at the outpatient dermatology clinic revealed few scaly, erythematous, eroded papules distributed on the mid-back; erythematous greasy scaling on the scalp, face, and chest; and pink scaly plaques with white-silvery scale on the anterior lower legs. Histopathology of a specimen from the right mid-back demonstrated acantholysis with suprabasal clefting, hyperkeratosis, and parakeratosis with no dyskeratotic cells identified. The pathologic differential diagnosis included primary acantholytic processes including Grover disease, DAR, HHD, and pemphigus. Pathology from the right shin demonstrated acanthosis, confluent parakeratosis with associated decreased granular cell layer and collections of neutrophils within the stratum corneum, spongiosis, and superficial dermal perivascular chronic inflammation with focal exocytosis and dilated blood vessels in the papillary dermis. The clinical and pathological diagnosis on the lower legs was consistent with psoriasis. Diagnoses of seborrheic dermatitis, psoriasis on the lower legs, and HHD vs DAR on the back and chest were made. The patient was instructed to continue ketoconazole shampoo, ketoconazole cream, and desonide for seborrheic dermatitis; fluocinonide ointment 0.05% to the lower legs for psoriasis; and triamcinolone cream and a bland moisturizer to the back and chest for HHD.
Over the ensuing months, the rash worsened with erythema and scaling affecting more than half of the body surface area. Topical corticosteroids and bland emollients resulted in minimal success. Biologics and acitretin were considered for the psoriasiform dermatitis but avoided due to the patient’s medical history of thyroid cancer and chronic hepatitis C infection. Because the patient described prior success with UV light therapy for psoriasis, he requested light therapy. A subsequent trial of narrowband UVB light therapy initially improved some of the psoriasiform dermatitis on the trunk and extremities; however, after 4 weeks of treatment, the patient described pain in some of the skin and felt he was burned by minimal exposure to light therapy on one particular visit, which caused him to stop light therapy.
Approximately 2 weeks later, the patient presented to the emergency department stating his psoriasis was infected; he was diagnosed with psoriasis with secondary cellulitis and received intravenous vancomycin and piperacillin-tazobactam, with bacterial cultures demonstrating Corynebacterium and methicillin-resistant Staphylococcus aureus. Some improvement was noted in the patient’s skin after antibiotics were initiated, but he continued to describe worsening “burning and pain” throughout the psoriasis lesions. The patient’s care was transferred to the Veterans Affairs hospital where a dermatology inpatient consultation was placed.
Our initial dermatologic examination revealed generalized scaly erythroderma on the neck, trunk, and extremities, sparing the face, palms, and soles (Figure 1). Multiple crusted and intact vesicles also were present overlying the erythematous plaques on the chest, back, and proximal extremities, most grouped in clusters. The patient endorsed new symptoms of pain and burning. Tzanck smear from the abdomen along with shave biopsies from the left flank and right abdomen were performed, and intravenous acyclovir was initiated immediately after these procedures.

Viral cultures were taken but were incorrectly processed by the laboratory. Tzanck smear showed severe acute inflammation with numerous neutrophils, multinucleated giant cells with viral nuclear changes, and positive immunostaining for HSV and negative immunostaining for herpes zoster. Both pathology specimens revealed an intense acute mixed, mainly neutrophilic, inflammatory infiltrate extending into the deeper dermis as well as distorted and necrotic hair follicles, some of which displayed multinucleated epithelial cells with margination of chromatin that were positive for both HSV-1 and HSV-2 and negative for herpes zoster (Figure 2). The positivity of both HSV strains might represent co-infection or could be a cross-reaction of antibodies used in immunohistochemistry to the HSV antigens. There was acantholysis surrounding the ulceration and extending through the full thickness of the epidermis with a dilapidated brick wall pattern (Figure 3) as well as negative immunohistochemical staining for HSV-1 and HSV-2 antigens. The clinical and histological picture together, along with prior clinical and pathological reports, confirmed the diagnoses of acute erythrodermic HHD with HSV superinfection.


The patient’s condition and pain improved within 24 hours on intravenous acyclovir. On the third day, his lesions were resolving and symptoms improved, so he was transitioned to oral acyclovir and discharged from the hospital. Follow-up in the dermatology outpatient clinic 1 week later revealed that all vesicles and papules had cleared, but the patient was still erythrodermic. Because HHD cannot always be distinguished histologically from other forms of pemphigus but yields a negative immunofluorescence, direct immunofluorescence and indirect immunofluorescence were obtained upon patient follow-up in the clinic and were both negative. Hepatitis C viral loads were undetectable. Consultations to gastroenterology and oncology teams were placed for consideration of systemic agents, and the patient was initiated on oral acitretin 25 mg daily, along with clobetasol as adjuvant therapy for any residual skin plaques. The laboratory results were closely monitored. Within 4 weeks after starting acitretin, the patient’s erythroderma had completely resolved. The patient has remained stable since then, except for one episode of secondary Staphylococcus infection that cleared on oral antibiotics. The patient remains stable and clear on oral acitretin 25 mg daily, with concomitant desonide cream and fluocinonide ointment as needed.
Hailey-Hailey disease is characterized by recurrent episodes of erythema, blisters, and plaques localized to intertriginous and perianal areas.1,2 Patients display a spectrum of lesions that vary in severity.8 Typical histologic examination reveals a dilapidated brick wall appearance. Pathology of well-developed lesions will show suprabasal acantholysis with minimal dyskeratosis.2
The generalized form of HHD is an extremely rare variant of the disease.10 Generalized HHD may resemble acute hypersensitivity reaction, erythema multiforme, and toxic epidermal necrolysis.1 Chronic diseases, such as psoriasis (as in this patient), also may contribute to a clinically confusing picture.8 Hailey-Hailey disease and psoriasis are thought to occasionally koebnerize (isomorphic response) to areas of trauma.16 Our patient experienced widespread erythematous papules and plaques not restricted to skin folds. His skin lesions continued to worsen over several months progressing to erythroderma. The presence of suprabasal acantholysis in a dilapidated brick wall pattern, along with the patient’s history, prior pathology reports, clinical picture, and negative direct immunofluorescence and indirect immunofluorescence studies helped to confirm the diagnosis of erythrodermic HHD.
Hailey-Hailey disease is caused by heterozygous mutations in the ATP2C1 gene on chromosome 3q21-24 coding for a Golgi ATPase called SPCA1 (secretory pathway calcium/manganese-ATPase).9 Subsequent disturbances in cytosolic-Golgi calcium concentrations interfere with epidermal keratinocyte adherence resulting in acantholytic disease. Studies of interfamilial and intrafamilial mutations fail to pinpoint a common mutation pattern among patients with generalized phenotypes,9 which further supports theories that intrinsic or extrinsic factors such as friction, heat, radiation, contact allergens, and infection affect the severity of HHD disease and not the type of mutation.3,9
Generalization of HHD is likely caused by nonspecific triggers in an already genetically disturbed epidermis.10 Interrupted epithelial function exposes skin to infections that exacerbate the underlying disease. Superimposing bacterial infections are commonly reported in HHD. Staphylococcus, Streptococcus, and Candida species colonize the skin and aggravate the disease.11 Much less commonly, HSV superinfection can complicate HHD.3-7 No data are currently available about the frequency or incidence of Herpesviridae in HHD.7 Some studies suggest that UVB light therapy can be an exacerbating factor in DAR and some but not all HHD patients,12,13 while other case reports14,15 document clinically improved responses using phototherapy for patients with HHD. Clinicians should remain suspicious and evaluate for HSV infection in refractory or sudden exacerbation of HHD.7 Furthermore, coexistent psoriasis and HHD also is a rare entity but has been described,8 which illustrates the importance of not attributing all skin manifestations to a previously diagnosed disorder but instead keeping an open mind in case new dermatologic conditions present themselves at a later time.
We present a rare case of erythrodermic HHD and coexistent psoriasis with HSV superinfection. We hope to draw awareness to this association of generalized HHD with both HSV and psoriasis to help clinicians make the correct diagnosis promptly in similar cases in the future.
To the Editor:
Hailey-Hailey disease (HHD), also known as benign familial pemphigus, is an uncommon autosomal-dominant skin disease.1 Defects in the ATPase type 2C member 1 gene, ATP2C1, result in abnormal intracellular epidermal adherence, and patients experience recurring blisters in skin folds. Longitudinal white streaks of the fingernails also may be present.1 The illness does not appear until puberty and is heightened by the second or third decade of life. Family history often suggests the presence of disease.2 Misdiagnosis of HHD occurs because of a wide spectrum of presentations. The presence of superimposed infections and carcinomas may both obscure and exacerbate this disease.2
Herpes simplex viruse types 1 and 2 (HSV-1 and HSV-2) are DNA viruses that cause common recurrent diseases. Usually, HSV-1 is associated with infection of the mouth and HSV-2 is associated with infection of the genitalia.3 Longitudinal cutaneous lesions manifest as grouped vesicles on an erythematous base. Tzanck smear of herpetic vesicles will reveal the presence of multinucleated giant cells. A direct fluorescent antibody technique also may be used to confirm the diagnosis.3
Erythrodermic HHD disease is a rare condition; moreover, there are only a few reported cases with coexistence of HHD and HSV in the literature.3-6 We report a rare presentation of erythrodermic HHD and coexistent psoriasis with HSV superinfection.
A 69-year-old man presented to an outpatient dermatology clinic for evaluation and treatment of a rash on the scalp, face, back, and lower legs. The patient confirmed a dandruff diagnosis on the scalp and face as well as psoriasis on the trunk and extremities for the last 45 years. He described a history of successful treatment with topical agents and UV light therapy. A family history revealed that the patient’s father and 1 of 2 siblings had a similar rash and “skin problems.” The patient had a medical history of thyroid cancer treated with radiation treatment and a partial thyroidectomy 35 years prior to the current presentation as well as incompletely treated chronic hepatitis C.
A search of medical records revealed a punch biopsy from the posterior neck that demonstrated an acantholytic dyskeratosis with suprabasal acantholysis. Clinicians were unable to differentiate if it was Darier disease (DAR) or HHD. Treatment of the patient’s seborrheic dermatitis and acantholytic disorder was successful at that time with ketoconazole shampoo, ketoconazole cream, desonide cream, and triamcinolone cream. The patient remained stable for 5 years before presenting again to the dermatology clinic for worsening rash despite topical therapies.
At the current presentation, physical examination at the outpatient dermatology clinic revealed few scaly, erythematous, eroded papules distributed on the mid-back; erythematous greasy scaling on the scalp, face, and chest; and pink scaly plaques with white-silvery scale on the anterior lower legs. Histopathology of a specimen from the right mid-back demonstrated acantholysis with suprabasal clefting, hyperkeratosis, and parakeratosis with no dyskeratotic cells identified. The pathologic differential diagnosis included primary acantholytic processes including Grover disease, DAR, HHD, and pemphigus. Pathology from the right shin demonstrated acanthosis, confluent parakeratosis with associated decreased granular cell layer and collections of neutrophils within the stratum corneum, spongiosis, and superficial dermal perivascular chronic inflammation with focal exocytosis and dilated blood vessels in the papillary dermis. The clinical and pathological diagnosis on the lower legs was consistent with psoriasis. Diagnoses of seborrheic dermatitis, psoriasis on the lower legs, and HHD vs DAR on the back and chest were made. The patient was instructed to continue ketoconazole shampoo, ketoconazole cream, and desonide for seborrheic dermatitis; fluocinonide ointment 0.05% to the lower legs for psoriasis; and triamcinolone cream and a bland moisturizer to the back and chest for HHD.
Over the ensuing months, the rash worsened with erythema and scaling affecting more than half of the body surface area. Topical corticosteroids and bland emollients resulted in minimal success. Biologics and acitretin were considered for the psoriasiform dermatitis but avoided due to the patient’s medical history of thyroid cancer and chronic hepatitis C infection. Because the patient described prior success with UV light therapy for psoriasis, he requested light therapy. A subsequent trial of narrowband UVB light therapy initially improved some of the psoriasiform dermatitis on the trunk and extremities; however, after 4 weeks of treatment, the patient described pain in some of the skin and felt he was burned by minimal exposure to light therapy on one particular visit, which caused him to stop light therapy.
Approximately 2 weeks later, the patient presented to the emergency department stating his psoriasis was infected; he was diagnosed with psoriasis with secondary cellulitis and received intravenous vancomycin and piperacillin-tazobactam, with bacterial cultures demonstrating Corynebacterium and methicillin-resistant Staphylococcus aureus. Some improvement was noted in the patient’s skin after antibiotics were initiated, but he continued to describe worsening “burning and pain” throughout the psoriasis lesions. The patient’s care was transferred to the Veterans Affairs hospital where a dermatology inpatient consultation was placed.
Our initial dermatologic examination revealed generalized scaly erythroderma on the neck, trunk, and extremities, sparing the face, palms, and soles (Figure 1). Multiple crusted and intact vesicles also were present overlying the erythematous plaques on the chest, back, and proximal extremities, most grouped in clusters. The patient endorsed new symptoms of pain and burning. Tzanck smear from the abdomen along with shave biopsies from the left flank and right abdomen were performed, and intravenous acyclovir was initiated immediately after these procedures.

Viral cultures were taken but were incorrectly processed by the laboratory. Tzanck smear showed severe acute inflammation with numerous neutrophils, multinucleated giant cells with viral nuclear changes, and positive immunostaining for HSV and negative immunostaining for herpes zoster. Both pathology specimens revealed an intense acute mixed, mainly neutrophilic, inflammatory infiltrate extending into the deeper dermis as well as distorted and necrotic hair follicles, some of which displayed multinucleated epithelial cells with margination of chromatin that were positive for both HSV-1 and HSV-2 and negative for herpes zoster (Figure 2). The positivity of both HSV strains might represent co-infection or could be a cross-reaction of antibodies used in immunohistochemistry to the HSV antigens. There was acantholysis surrounding the ulceration and extending through the full thickness of the epidermis with a dilapidated brick wall pattern (Figure 3) as well as negative immunohistochemical staining for HSV-1 and HSV-2 antigens. The clinical and histological picture together, along with prior clinical and pathological reports, confirmed the diagnoses of acute erythrodermic HHD with HSV superinfection.


The patient’s condition and pain improved within 24 hours on intravenous acyclovir. On the third day, his lesions were resolving and symptoms improved, so he was transitioned to oral acyclovir and discharged from the hospital. Follow-up in the dermatology outpatient clinic 1 week later revealed that all vesicles and papules had cleared, but the patient was still erythrodermic. Because HHD cannot always be distinguished histologically from other forms of pemphigus but yields a negative immunofluorescence, direct immunofluorescence and indirect immunofluorescence were obtained upon patient follow-up in the clinic and were both negative. Hepatitis C viral loads were undetectable. Consultations to gastroenterology and oncology teams were placed for consideration of systemic agents, and the patient was initiated on oral acitretin 25 mg daily, along with clobetasol as adjuvant therapy for any residual skin plaques. The laboratory results were closely monitored. Within 4 weeks after starting acitretin, the patient’s erythroderma had completely resolved. The patient has remained stable since then, except for one episode of secondary Staphylococcus infection that cleared on oral antibiotics. The patient remains stable and clear on oral acitretin 25 mg daily, with concomitant desonide cream and fluocinonide ointment as needed.
Hailey-Hailey disease is characterized by recurrent episodes of erythema, blisters, and plaques localized to intertriginous and perianal areas.1,2 Patients display a spectrum of lesions that vary in severity.8 Typical histologic examination reveals a dilapidated brick wall appearance. Pathology of well-developed lesions will show suprabasal acantholysis with minimal dyskeratosis.2
The generalized form of HHD is an extremely rare variant of the disease.10 Generalized HHD may resemble acute hypersensitivity reaction, erythema multiforme, and toxic epidermal necrolysis.1 Chronic diseases, such as psoriasis (as in this patient), also may contribute to a clinically confusing picture.8 Hailey-Hailey disease and psoriasis are thought to occasionally koebnerize (isomorphic response) to areas of trauma.16 Our patient experienced widespread erythematous papules and plaques not restricted to skin folds. His skin lesions continued to worsen over several months progressing to erythroderma. The presence of suprabasal acantholysis in a dilapidated brick wall pattern, along with the patient’s history, prior pathology reports, clinical picture, and negative direct immunofluorescence and indirect immunofluorescence studies helped to confirm the diagnosis of erythrodermic HHD.
Hailey-Hailey disease is caused by heterozygous mutations in the ATP2C1 gene on chromosome 3q21-24 coding for a Golgi ATPase called SPCA1 (secretory pathway calcium/manganese-ATPase).9 Subsequent disturbances in cytosolic-Golgi calcium concentrations interfere with epidermal keratinocyte adherence resulting in acantholytic disease. Studies of interfamilial and intrafamilial mutations fail to pinpoint a common mutation pattern among patients with generalized phenotypes,9 which further supports theories that intrinsic or extrinsic factors such as friction, heat, radiation, contact allergens, and infection affect the severity of HHD disease and not the type of mutation.3,9
Generalization of HHD is likely caused by nonspecific triggers in an already genetically disturbed epidermis.10 Interrupted epithelial function exposes skin to infections that exacerbate the underlying disease. Superimposing bacterial infections are commonly reported in HHD. Staphylococcus, Streptococcus, and Candida species colonize the skin and aggravate the disease.11 Much less commonly, HSV superinfection can complicate HHD.3-7 No data are currently available about the frequency or incidence of Herpesviridae in HHD.7 Some studies suggest that UVB light therapy can be an exacerbating factor in DAR and some but not all HHD patients,12,13 while other case reports14,15 document clinically improved responses using phototherapy for patients with HHD. Clinicians should remain suspicious and evaluate for HSV infection in refractory or sudden exacerbation of HHD.7 Furthermore, coexistent psoriasis and HHD also is a rare entity but has been described,8 which illustrates the importance of not attributing all skin manifestations to a previously diagnosed disorder but instead keeping an open mind in case new dermatologic conditions present themselves at a later time.
We present a rare case of erythrodermic HHD and coexistent psoriasis with HSV superinfection. We hope to draw awareness to this association of generalized HHD with both HSV and psoriasis to help clinicians make the correct diagnosis promptly in similar cases in the future.
- Chave TA, Milligan A. Acute generalized Hailey-Hailey disease. Clin Exp Dermatol. 2002;27:290-292.
- Mohr MR, Erdag G, Shada Al, et al. Two patients with Hailey-Hailey disease, multiple primary melanomas, and other cancers. Arch Dermatol. 2011;147:211-215.
- Lee GM, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey Disease. Ann Dermatol. 2009;21:311-314.
- Zaim MT, Bickers DR. Herpes simplex associated with Hailey-Hailey disease. J Am Acad Dermatol. 1987;17:701-702.
- Peppiatt T, Keefe M, White JE. Hailey-Hailey disease-exacerbation by herpes simplex virus and patch tests. Clin Exp Dermatol. 2006;17:201-202.
- Almeida L, Grossman ME. Benign familial pemphigus complicated by herpes simplex virus. Cutis. 1989;44:261-262.
- Nikkels AF, Delvenne P, Herfs M, et al. Occult herpes simplex virus colonization of bullous dermatitides. Am J Clin Dermatol. 2008;9:163-168.
- Chao SC, Lee JY, Wu MC, et al. A novel splice mutation in the ATP2C1 gene in a woman with concomitant psoriasis vulgaris and disseminated Hailey-Hailey disease. Int J Dermatol. 2012;51:947-951.
- Ikeda S, Shigihara T, Mayuzumi N, et al. Mutations of ATP2C1 in Japanese patients with Hailey-Hailey disease: intrafamilial and interfamilial phenotype variations and lack of correlation with mutation patterns. J Invest Dermatol. 2001;117:1654-1656.
- Marsch W, Stuttgen G. Generalized Hailey-Hailey disease. Br J Dermatol. 1978;99:553-559.
- Friedman-Birnbaum R, Haim S, Marcus S. Generalized familial benign chronic pemphigus. Dermatologica. 1980;161:112-115.
- Richard G, Linse R, Harth W. Hailey-Hailey disease. early detection of heterozygotes by an ultraviolet provocation tests—clinical relevance of the method. Hautarzt. 1993;44:376-379.
- Mayuzumi N, Ikeda S, Kawada H, et al. Effects of ultraviolet B irradiation, proinflammatory cytokines and raised extracellular calcium concentration on the expression of ATP2A2 and ATP2C1. Br J Dermatol. 2005;152:697-701.
- Vanderbeck KA, Giroux L, Murugan NJ, et al. Combined therapeutic use of oral alitretinoin and narrowband ultraviolet-B therapy in the treatment of Hailey-Hailey disease. Dermatol Rep. 2014;6:5604.
- Mizuno K, Hamada T, Hasimoto T, et al. Successful treatment with narrow-band UVB therapy for a case of generalized Hailey-Hailey disease with a novel splice-site mutation in ATP2C1 gene. Dermatol Ther. 2014;27:233-235.
- Thappa DM. The isomorphic phenomenon of Koebner. Indian J Dermatol Venereol Leprol. 2004;70:187-189.
- Chave TA, Milligan A. Acute generalized Hailey-Hailey disease. Clin Exp Dermatol. 2002;27:290-292.
- Mohr MR, Erdag G, Shada Al, et al. Two patients with Hailey-Hailey disease, multiple primary melanomas, and other cancers. Arch Dermatol. 2011;147:211-215.
- Lee GM, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey Disease. Ann Dermatol. 2009;21:311-314.
- Zaim MT, Bickers DR. Herpes simplex associated with Hailey-Hailey disease. J Am Acad Dermatol. 1987;17:701-702.
- Peppiatt T, Keefe M, White JE. Hailey-Hailey disease-exacerbation by herpes simplex virus and patch tests. Clin Exp Dermatol. 2006;17:201-202.
- Almeida L, Grossman ME. Benign familial pemphigus complicated by herpes simplex virus. Cutis. 1989;44:261-262.
- Nikkels AF, Delvenne P, Herfs M, et al. Occult herpes simplex virus colonization of bullous dermatitides. Am J Clin Dermatol. 2008;9:163-168.
- Chao SC, Lee JY, Wu MC, et al. A novel splice mutation in the ATP2C1 gene in a woman with concomitant psoriasis vulgaris and disseminated Hailey-Hailey disease. Int J Dermatol. 2012;51:947-951.
- Ikeda S, Shigihara T, Mayuzumi N, et al. Mutations of ATP2C1 in Japanese patients with Hailey-Hailey disease: intrafamilial and interfamilial phenotype variations and lack of correlation with mutation patterns. J Invest Dermatol. 2001;117:1654-1656.
- Marsch W, Stuttgen G. Generalized Hailey-Hailey disease. Br J Dermatol. 1978;99:553-559.
- Friedman-Birnbaum R, Haim S, Marcus S. Generalized familial benign chronic pemphigus. Dermatologica. 1980;161:112-115.
- Richard G, Linse R, Harth W. Hailey-Hailey disease. early detection of heterozygotes by an ultraviolet provocation tests—clinical relevance of the method. Hautarzt. 1993;44:376-379.
- Mayuzumi N, Ikeda S, Kawada H, et al. Effects of ultraviolet B irradiation, proinflammatory cytokines and raised extracellular calcium concentration on the expression of ATP2A2 and ATP2C1. Br J Dermatol. 2005;152:697-701.
- Vanderbeck KA, Giroux L, Murugan NJ, et al. Combined therapeutic use of oral alitretinoin and narrowband ultraviolet-B therapy in the treatment of Hailey-Hailey disease. Dermatol Rep. 2014;6:5604.
- Mizuno K, Hamada T, Hasimoto T, et al. Successful treatment with narrow-band UVB therapy for a case of generalized Hailey-Hailey disease with a novel splice-site mutation in ATP2C1 gene. Dermatol Ther. 2014;27:233-235.
- Thappa DM. The isomorphic phenomenon of Koebner. Indian J Dermatol Venereol Leprol. 2004;70:187-189.
Practice Points
- Misdiagnosis of Hailey-Hailey disease (HHD) occurs because of a wide spectrum of presentations.
- Hailey-Hailey disease and psoriasis are thought to occasionally koebnerize (isomorphic response) to areas of trauma.
- Clinicians should remain suspicious and evaluate for herpes simplex virus infection in refractory or sudden exacerbation of HHD.
Variations in Preference for Topical Vehicles Among Demographic Groups
Topical medication is a mainstay in the treatment of dermatologic conditions. Adherence to medication regimens can be challenging in patients requiring long-term topical treatment, and nonadherence is multifactorial. A major modifiable contributing factor is patient dissatisfaction with the vehicle used. Medications often have options for different topical preparations. Therefore, it is important to consider patient preference when prescribing topical treatments to maximize adherence, ensure patient satisfaction, and optimize outcomes.
We hypothesized that notable differences exist among demographic groups regarding preference for topical vehicles. Little research has been conducted to delineate trends. This study aimed to identify variations in preference for creams, lotions, and ointments by age, gender, and ethnicity.
Methods
Data were collected through surveys distributed to all patients seen at the Truman Medical Center University Health Dermatology Clinic in Kansas City, Missouri, between September 2018 and June 2019. The study was approved by the University of Missouri Kansas City institutional review board. An estimated response rate of 95% was achieved. Each patient was informed that the survey was voluntary and anonymous, and declining to complete the survey had no effect on the care provided. Each patient completed only 1 survey and returned it to a collection box before departing from clinic.
In the survey, patients provided demographic information, including age, gender, and ethnicity. Age groups included patients younger than 40 years, 40 to 60 years, and older than 60 years. Gender groups included male and female. Ethnicity included white, black, Hispanic/Latino, and Asian/Pacific Islander or other. Patients then chose 1 of 3 options for topical vehicle preference: cream, lotion, or ointment. Each of these options was accompanied by a brief description of the vehicle, a photograph, and examples of common commercial products to aid in decision-making. The expected values were calculated based on a probability distribution under the assumption that variables have no association. Therefore, the discrepancy between the expected value and the observed value was used to describe the significance of the association between variables.
Data were analyzed using χ2 tests with the aid of a statistician. P<.05 was considered statistically significant.
Results
A total of 404 surveys were collected and recorded. Data showed statistically significant trends in each demographic parameter.
Age
First, we analyzed differences in preference based on age (Table 1). Of 404 patients, 163 were younger than 40 years, 171 were aged 40 to 60 years, and 70 were older than 60 years. Patients younger than 40 years preferred lotion (68 vs 46.0 expected). Patients aged 40 to 60 years showed preference for cream (83 vs 76.6 expected) and ointment (56 vs 46.1 expected). Patients older than 60 years preferred cream (41 vs 31.4 expected). These findings were statistically significant (P<.0001).
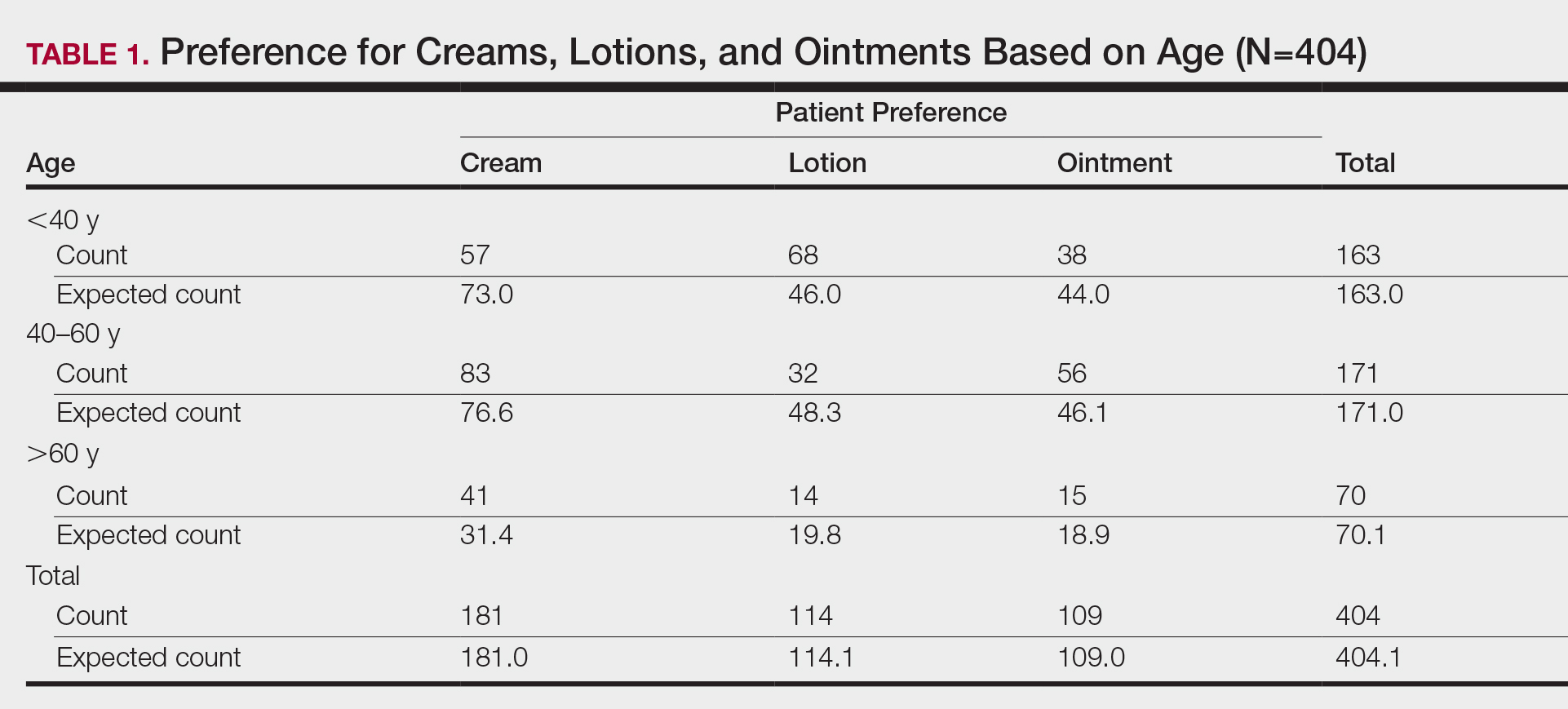
Gender
Next, we evaluated variations based on gender (Table 2). Of 404 patients, 254 were female and 150 were male. Females preferred cream (127 vs 113.8 expected). Males exhibited preference for lotion (50 vs 42.3 expected) and ointment (46 vs 40.5 expected). Differences between genders were statistically significant (P=.023).

Ethnicity
We then analyzed preferences based on ethnicity (Table 3). Of 404 patients, 30 were Hispanic/Latino, 26 were Asian/Pacific Islander or other, 227 were white, and 121 were black. Hispanic/Latino patients showed equivocal findings, aligning with expected counts. Asian/Pacific Islander or other patients exhibited slight preferences for cream (14 vs 11.6 expected) and lotion (10 vs 7.3 expected). White patients preferred cream (119 vs 101.7 expected) and lotion (82 vs 64.1 expected). Black patients showed strong preference for ointment (72 vs 32.6 expected). Differences in preferences based on ethnicity were statistically significant (P<.0001).

Comment
Topical medication is a mainstay of dermatologic therapy. Many topical preparations (or vehicles) exist, including ointments, creams, lotions, gels, solutions, and foams. Vehicle type not only influences bioavailability of the prepared medication but also has a notable impact on adherence and subsequent efficacy of the topical therapy.
Medication adherence is especially challenging in dermatology, as topical medications play a central role in treatment. Compliance with the medication regimen is paramount in treatment efficacy.1 In dermatology, adherence with oral medications is higher than it is for topical medications2; various factors contribute to this difference. Compliance may decline with topical treatment due to time-consuming application, misunderstanding about the disease or the treatment regimen, frequency of administration, dissatisfaction with efficacy or appearance, and other variables.3
Other factors have been found to be important to topical medication adherence; younger age, female gender, marriage, employment, nonsmoking, nondrinking, and higher cognitive ability were associated with higher topical medication adherence.4 Our study focused on one factor: identification of demographic-specific preferences that might have implications on adherence within the studied demographic groups.
It is known that individual preferences exist when patients are choosing a topical preparation. However, a PubMed search of articles indexed for MEDLINE using the terms topical, vehicle, preparation, adherence, and preference revealed few studies that examined the preference for topical vehicle by age, gender, or ethnicity.
Existing studies have examined preferences for topical preparations based on specific disease states; this literature, albeit limited, demonstrates that preferences for topical product formulations vary among acne, atopic dermatitis, and plaque psoriasis patients.5 Other studies focus on specific patient populations or medications. For example, one study found that preference for corticosteroid vehicles among psoriasis patients was highly variable and choice of vehicle was critical to adherence.6 Another study highlighted differences in vehicle choice between younger and older age groups with psoriasis.7
Given the limited data overall, it was our goal to determine if any patterns of preference existed by age, gender, or ethnicity, regardless of disease state or indication for topical product. Importantly, over-the-counter products—cosmetic or otherwise—were not differentiated from prescribed topical medications. Our survey elucidated significant differences in preference by age, gender, and ethnicity.
Notable Findings
Regarding age, patients younger than 40 years preferred lotion, patients aged 40 to 60 years preferred cream, and patients older than 60 years preferred cream. Analysis based on gender showed that females preferred cream, and males preferred lotion and ointment. Analysis based on ethnicity most notably demonstrated a strong preference for ointment in black patients while showing preference for cream in white patients.
Potential Biases and Pitfalls
Limitations of this study included the small Hispanic/Latino and Asian/Pacific Islander populations surveyed, possible misunderstanding of the survey by respondents, and the potential for surveys being filled out twice by the same patient. Future surveys could be conducted over a longer period to increase the total sample size and to better characterize less-represented populations, such as Hispanic and Asian patients. To avoid repeat participation, the first question of the survey asked patients to indicate if they had previously completed the survey and instructed patients who had to return the repeat survey to the front desk.
To limit other errors, our survey included concise accessible descriptions of each preparation along with clear representative photographs and examples of common brands. Still, it is possible that some mistakes could have been made while patients filled out the survey based on comprehension deficits, oversight, or other reasons. It also is possible that preference might vary individually depending on the indication of the topical product—cosmetic or therapeutic—or even by anatomic site of application. Neither of these considerations was assessed specifically in our survey.
Conclusion
Our hope is that this study helps practitioners better anticipate topical preferences among patients with the ultimate goal of increasing medication adherence and patient outcomes. Nevertheless, although these general trends can provide helpful guidance, we acknowledge that individual preferences vary, and care should always be patient centered.
Acknowledgment
We thank An-Lin Cheng, PhD (Kansas City, Missouri), for assistance with the statistical analysis.
- Kircik LH. Vehicles always matter. J Drugs Dermatol. 2019;18:s99.
- Furue M, Onozuka D, Takeuchi S, et al. Poor adherence to oral andtopical medication in 3096 dermatological patients as assessed by the Morisky Medication Adherence Scale-8. Br J Dermatol. 2015;172:272-275.
- Tan X, Feldman SR, Chang, J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Ahn CS, Culp L, Huang WW, et al. Adherence in dermatology. J Dermatolog Treat. 2017;28:94-103.
- Eastman WJ, Malahias S, Delconte J, et al. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94:46-53.
- Felix K, Unrue E, Inyang M, et al. Patients preferences for different corticosteroid vehicles are highly variable. J Dermatolog Treat. 2019;31:147-151.
- Hong C-H, Papp KA, Lophaven KW, et al. Patients with psoriasis have different preferences for topical therapy, highlighting the importance of individualized treatment approaches: randomized phase IIIb PSO-INSIGHTFUL study. J Eur Acad Dermatol Venereol. 2017;31:1876-1883.
Topical medication is a mainstay in the treatment of dermatologic conditions. Adherence to medication regimens can be challenging in patients requiring long-term topical treatment, and nonadherence is multifactorial. A major modifiable contributing factor is patient dissatisfaction with the vehicle used. Medications often have options for different topical preparations. Therefore, it is important to consider patient preference when prescribing topical treatments to maximize adherence, ensure patient satisfaction, and optimize outcomes.
We hypothesized that notable differences exist among demographic groups regarding preference for topical vehicles. Little research has been conducted to delineate trends. This study aimed to identify variations in preference for creams, lotions, and ointments by age, gender, and ethnicity.
Methods
Data were collected through surveys distributed to all patients seen at the Truman Medical Center University Health Dermatology Clinic in Kansas City, Missouri, between September 2018 and June 2019. The study was approved by the University of Missouri Kansas City institutional review board. An estimated response rate of 95% was achieved. Each patient was informed that the survey was voluntary and anonymous, and declining to complete the survey had no effect on the care provided. Each patient completed only 1 survey and returned it to a collection box before departing from clinic.
In the survey, patients provided demographic information, including age, gender, and ethnicity. Age groups included patients younger than 40 years, 40 to 60 years, and older than 60 years. Gender groups included male and female. Ethnicity included white, black, Hispanic/Latino, and Asian/Pacific Islander or other. Patients then chose 1 of 3 options for topical vehicle preference: cream, lotion, or ointment. Each of these options was accompanied by a brief description of the vehicle, a photograph, and examples of common commercial products to aid in decision-making. The expected values were calculated based on a probability distribution under the assumption that variables have no association. Therefore, the discrepancy between the expected value and the observed value was used to describe the significance of the association between variables.
Data were analyzed using χ2 tests with the aid of a statistician. P<.05 was considered statistically significant.
Results
A total of 404 surveys were collected and recorded. Data showed statistically significant trends in each demographic parameter.
Age
First, we analyzed differences in preference based on age (Table 1). Of 404 patients, 163 were younger than 40 years, 171 were aged 40 to 60 years, and 70 were older than 60 years. Patients younger than 40 years preferred lotion (68 vs 46.0 expected). Patients aged 40 to 60 years showed preference for cream (83 vs 76.6 expected) and ointment (56 vs 46.1 expected). Patients older than 60 years preferred cream (41 vs 31.4 expected). These findings were statistically significant (P<.0001).

Gender
Next, we evaluated variations based on gender (Table 2). Of 404 patients, 254 were female and 150 were male. Females preferred cream (127 vs 113.8 expected). Males exhibited preference for lotion (50 vs 42.3 expected) and ointment (46 vs 40.5 expected). Differences between genders were statistically significant (P=.023).

Ethnicity
We then analyzed preferences based on ethnicity (Table 3). Of 404 patients, 30 were Hispanic/Latino, 26 were Asian/Pacific Islander or other, 227 were white, and 121 were black. Hispanic/Latino patients showed equivocal findings, aligning with expected counts. Asian/Pacific Islander or other patients exhibited slight preferences for cream (14 vs 11.6 expected) and lotion (10 vs 7.3 expected). White patients preferred cream (119 vs 101.7 expected) and lotion (82 vs 64.1 expected). Black patients showed strong preference for ointment (72 vs 32.6 expected). Differences in preferences based on ethnicity were statistically significant (P<.0001).

Comment
Topical medication is a mainstay of dermatologic therapy. Many topical preparations (or vehicles) exist, including ointments, creams, lotions, gels, solutions, and foams. Vehicle type not only influences bioavailability of the prepared medication but also has a notable impact on adherence and subsequent efficacy of the topical therapy.
Medication adherence is especially challenging in dermatology, as topical medications play a central role in treatment. Compliance with the medication regimen is paramount in treatment efficacy.1 In dermatology, adherence with oral medications is higher than it is for topical medications2; various factors contribute to this difference. Compliance may decline with topical treatment due to time-consuming application, misunderstanding about the disease or the treatment regimen, frequency of administration, dissatisfaction with efficacy or appearance, and other variables.3
Other factors have been found to be important to topical medication adherence; younger age, female gender, marriage, employment, nonsmoking, nondrinking, and higher cognitive ability were associated with higher topical medication adherence.4 Our study focused on one factor: identification of demographic-specific preferences that might have implications on adherence within the studied demographic groups.
It is known that individual preferences exist when patients are choosing a topical preparation. However, a PubMed search of articles indexed for MEDLINE using the terms topical, vehicle, preparation, adherence, and preference revealed few studies that examined the preference for topical vehicle by age, gender, or ethnicity.
Existing studies have examined preferences for topical preparations based on specific disease states; this literature, albeit limited, demonstrates that preferences for topical product formulations vary among acne, atopic dermatitis, and plaque psoriasis patients.5 Other studies focus on specific patient populations or medications. For example, one study found that preference for corticosteroid vehicles among psoriasis patients was highly variable and choice of vehicle was critical to adherence.6 Another study highlighted differences in vehicle choice between younger and older age groups with psoriasis.7
Given the limited data overall, it was our goal to determine if any patterns of preference existed by age, gender, or ethnicity, regardless of disease state or indication for topical product. Importantly, over-the-counter products—cosmetic or otherwise—were not differentiated from prescribed topical medications. Our survey elucidated significant differences in preference by age, gender, and ethnicity.
Notable Findings
Regarding age, patients younger than 40 years preferred lotion, patients aged 40 to 60 years preferred cream, and patients older than 60 years preferred cream. Analysis based on gender showed that females preferred cream, and males preferred lotion and ointment. Analysis based on ethnicity most notably demonstrated a strong preference for ointment in black patients while showing preference for cream in white patients.
Potential Biases and Pitfalls
Limitations of this study included the small Hispanic/Latino and Asian/Pacific Islander populations surveyed, possible misunderstanding of the survey by respondents, and the potential for surveys being filled out twice by the same patient. Future surveys could be conducted over a longer period to increase the total sample size and to better characterize less-represented populations, such as Hispanic and Asian patients. To avoid repeat participation, the first question of the survey asked patients to indicate if they had previously completed the survey and instructed patients who had to return the repeat survey to the front desk.
To limit other errors, our survey included concise accessible descriptions of each preparation along with clear representative photographs and examples of common brands. Still, it is possible that some mistakes could have been made while patients filled out the survey based on comprehension deficits, oversight, or other reasons. It also is possible that preference might vary individually depending on the indication of the topical product—cosmetic or therapeutic—or even by anatomic site of application. Neither of these considerations was assessed specifically in our survey.
Conclusion
Our hope is that this study helps practitioners better anticipate topical preferences among patients with the ultimate goal of increasing medication adherence and patient outcomes. Nevertheless, although these general trends can provide helpful guidance, we acknowledge that individual preferences vary, and care should always be patient centered.
Acknowledgment
We thank An-Lin Cheng, PhD (Kansas City, Missouri), for assistance with the statistical analysis.
Topical medication is a mainstay in the treatment of dermatologic conditions. Adherence to medication regimens can be challenging in patients requiring long-term topical treatment, and nonadherence is multifactorial. A major modifiable contributing factor is patient dissatisfaction with the vehicle used. Medications often have options for different topical preparations. Therefore, it is important to consider patient preference when prescribing topical treatments to maximize adherence, ensure patient satisfaction, and optimize outcomes.
We hypothesized that notable differences exist among demographic groups regarding preference for topical vehicles. Little research has been conducted to delineate trends. This study aimed to identify variations in preference for creams, lotions, and ointments by age, gender, and ethnicity.
Methods
Data were collected through surveys distributed to all patients seen at the Truman Medical Center University Health Dermatology Clinic in Kansas City, Missouri, between September 2018 and June 2019. The study was approved by the University of Missouri Kansas City institutional review board. An estimated response rate of 95% was achieved. Each patient was informed that the survey was voluntary and anonymous, and declining to complete the survey had no effect on the care provided. Each patient completed only 1 survey and returned it to a collection box before departing from clinic.
In the survey, patients provided demographic information, including age, gender, and ethnicity. Age groups included patients younger than 40 years, 40 to 60 years, and older than 60 years. Gender groups included male and female. Ethnicity included white, black, Hispanic/Latino, and Asian/Pacific Islander or other. Patients then chose 1 of 3 options for topical vehicle preference: cream, lotion, or ointment. Each of these options was accompanied by a brief description of the vehicle, a photograph, and examples of common commercial products to aid in decision-making. The expected values were calculated based on a probability distribution under the assumption that variables have no association. Therefore, the discrepancy between the expected value and the observed value was used to describe the significance of the association between variables.
Data were analyzed using χ2 tests with the aid of a statistician. P<.05 was considered statistically significant.
Results
A total of 404 surveys were collected and recorded. Data showed statistically significant trends in each demographic parameter.
Age
First, we analyzed differences in preference based on age (Table 1). Of 404 patients, 163 were younger than 40 years, 171 were aged 40 to 60 years, and 70 were older than 60 years. Patients younger than 40 years preferred lotion (68 vs 46.0 expected). Patients aged 40 to 60 years showed preference for cream (83 vs 76.6 expected) and ointment (56 vs 46.1 expected). Patients older than 60 years preferred cream (41 vs 31.4 expected). These findings were statistically significant (P<.0001).

Gender
Next, we evaluated variations based on gender (Table 2). Of 404 patients, 254 were female and 150 were male. Females preferred cream (127 vs 113.8 expected). Males exhibited preference for lotion (50 vs 42.3 expected) and ointment (46 vs 40.5 expected). Differences between genders were statistically significant (P=.023).

Ethnicity
We then analyzed preferences based on ethnicity (Table 3). Of 404 patients, 30 were Hispanic/Latino, 26 were Asian/Pacific Islander or other, 227 were white, and 121 were black. Hispanic/Latino patients showed equivocal findings, aligning with expected counts. Asian/Pacific Islander or other patients exhibited slight preferences for cream (14 vs 11.6 expected) and lotion (10 vs 7.3 expected). White patients preferred cream (119 vs 101.7 expected) and lotion (82 vs 64.1 expected). Black patients showed strong preference for ointment (72 vs 32.6 expected). Differences in preferences based on ethnicity were statistically significant (P<.0001).

Comment
Topical medication is a mainstay of dermatologic therapy. Many topical preparations (or vehicles) exist, including ointments, creams, lotions, gels, solutions, and foams. Vehicle type not only influences bioavailability of the prepared medication but also has a notable impact on adherence and subsequent efficacy of the topical therapy.
Medication adherence is especially challenging in dermatology, as topical medications play a central role in treatment. Compliance with the medication regimen is paramount in treatment efficacy.1 In dermatology, adherence with oral medications is higher than it is for topical medications2; various factors contribute to this difference. Compliance may decline with topical treatment due to time-consuming application, misunderstanding about the disease or the treatment regimen, frequency of administration, dissatisfaction with efficacy or appearance, and other variables.3
Other factors have been found to be important to topical medication adherence; younger age, female gender, marriage, employment, nonsmoking, nondrinking, and higher cognitive ability were associated with higher topical medication adherence.4 Our study focused on one factor: identification of demographic-specific preferences that might have implications on adherence within the studied demographic groups.
It is known that individual preferences exist when patients are choosing a topical preparation. However, a PubMed search of articles indexed for MEDLINE using the terms topical, vehicle, preparation, adherence, and preference revealed few studies that examined the preference for topical vehicle by age, gender, or ethnicity.
Existing studies have examined preferences for topical preparations based on specific disease states; this literature, albeit limited, demonstrates that preferences for topical product formulations vary among acne, atopic dermatitis, and plaque psoriasis patients.5 Other studies focus on specific patient populations or medications. For example, one study found that preference for corticosteroid vehicles among psoriasis patients was highly variable and choice of vehicle was critical to adherence.6 Another study highlighted differences in vehicle choice between younger and older age groups with psoriasis.7
Given the limited data overall, it was our goal to determine if any patterns of preference existed by age, gender, or ethnicity, regardless of disease state or indication for topical product. Importantly, over-the-counter products—cosmetic or otherwise—were not differentiated from prescribed topical medications. Our survey elucidated significant differences in preference by age, gender, and ethnicity.
Notable Findings
Regarding age, patients younger than 40 years preferred lotion, patients aged 40 to 60 years preferred cream, and patients older than 60 years preferred cream. Analysis based on gender showed that females preferred cream, and males preferred lotion and ointment. Analysis based on ethnicity most notably demonstrated a strong preference for ointment in black patients while showing preference for cream in white patients.
Potential Biases and Pitfalls
Limitations of this study included the small Hispanic/Latino and Asian/Pacific Islander populations surveyed, possible misunderstanding of the survey by respondents, and the potential for surveys being filled out twice by the same patient. Future surveys could be conducted over a longer period to increase the total sample size and to better characterize less-represented populations, such as Hispanic and Asian patients. To avoid repeat participation, the first question of the survey asked patients to indicate if they had previously completed the survey and instructed patients who had to return the repeat survey to the front desk.
To limit other errors, our survey included concise accessible descriptions of each preparation along with clear representative photographs and examples of common brands. Still, it is possible that some mistakes could have been made while patients filled out the survey based on comprehension deficits, oversight, or other reasons. It also is possible that preference might vary individually depending on the indication of the topical product—cosmetic or therapeutic—or even by anatomic site of application. Neither of these considerations was assessed specifically in our survey.
Conclusion
Our hope is that this study helps practitioners better anticipate topical preferences among patients with the ultimate goal of increasing medication adherence and patient outcomes. Nevertheless, although these general trends can provide helpful guidance, we acknowledge that individual preferences vary, and care should always be patient centered.
Acknowledgment
We thank An-Lin Cheng, PhD (Kansas City, Missouri), for assistance with the statistical analysis.
- Kircik LH. Vehicles always matter. J Drugs Dermatol. 2019;18:s99.
- Furue M, Onozuka D, Takeuchi S, et al. Poor adherence to oral andtopical medication in 3096 dermatological patients as assessed by the Morisky Medication Adherence Scale-8. Br J Dermatol. 2015;172:272-275.
- Tan X, Feldman SR, Chang, J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Ahn CS, Culp L, Huang WW, et al. Adherence in dermatology. J Dermatolog Treat. 2017;28:94-103.
- Eastman WJ, Malahias S, Delconte J, et al. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94:46-53.
- Felix K, Unrue E, Inyang M, et al. Patients preferences for different corticosteroid vehicles are highly variable. J Dermatolog Treat. 2019;31:147-151.
- Hong C-H, Papp KA, Lophaven KW, et al. Patients with psoriasis have different preferences for topical therapy, highlighting the importance of individualized treatment approaches: randomized phase IIIb PSO-INSIGHTFUL study. J Eur Acad Dermatol Venereol. 2017;31:1876-1883.
- Kircik LH. Vehicles always matter. J Drugs Dermatol. 2019;18:s99.
- Furue M, Onozuka D, Takeuchi S, et al. Poor adherence to oral andtopical medication in 3096 dermatological patients as assessed by the Morisky Medication Adherence Scale-8. Br J Dermatol. 2015;172:272-275.
- Tan X, Feldman SR, Chang, J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Ahn CS, Culp L, Huang WW, et al. Adherence in dermatology. J Dermatolog Treat. 2017;28:94-103.
- Eastman WJ, Malahias S, Delconte J, et al. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94:46-53.
- Felix K, Unrue E, Inyang M, et al. Patients preferences for different corticosteroid vehicles are highly variable. J Dermatolog Treat. 2019;31:147-151.
- Hong C-H, Papp KA, Lophaven KW, et al. Patients with psoriasis have different preferences for topical therapy, highlighting the importance of individualized treatment approaches: randomized phase IIIb PSO-INSIGHTFUL study. J Eur Acad Dermatol Venereol. 2017;31:1876-1883.
Practice Points
- Variations exist in preference for topical vehicles by age group, gender, and ethnicity.
- Identifying and utilizing preferred treatment options can help maximize patient outcomes.
How to Obtain a Dermatology Residency: A Guide Targeted to Underrepresented in Medicine Medical Students
There has been increasing attention focused on the lack of diversity within dermatology academic and residency programs.1-6 Several factors have been identified as contributing to this narrow pipeline of qualified applicants, including lack of mentorship, delayed exposure to the field, implicit bias, and lack of an overall holistic review of applications with overemphasis on board scores.1,5 In an effort to provide guidance to underrepresented in medicine (UIM) students who are interested in dermatology, the Skin of Color Society (SOCS) has created a detailed, step-by-step guide on how to obtain a position in a dermatology residency program,7 which was modeled after a similar resource created by the American Academy of Orthopaedic Surgeons.8 Here, we highlight the main SOCS recommendations to help guide medical students through a systematic approach to becoming successful applicants for dermatology residency.
Start Early
Competitive fields such as dermatology require intentional efforts starting at the beginning of medical school. Regardless of what specialty is right for you, begin by constructing a well-rounded application for residency immediately. Start by shadowing dermatologists and attending Grand Rounds held in your institution’s dermatology department to ensure that this field is right for you. Students are encouraged to meet with academic advisors and upperclassmen to seek guidance on gaining early exposure to dermatology at their home institutions (or nearby programs) during their first year. As a platform for learning about community-based dermatology activities, join your school’s Dermatology Interest Group, keeping in mind that an executive position in such a group can help foster relationships with faculty and residents of the dermatology department. A long-term commitment to community service also contributes to your depth as an applicant. Getting involved early helps students uncover health disparities in medicine and allows time to formulate ideas to implement change. Forming a well-rounded application mandates maintaining good academic standing, and students should prioritize mastering the curriculum, excelling in clinical rotations, and studying for the US Medical Licensing Examination (USMLE).
Choose a Mentor
The summer between your first and second years of medical school is an opportune time to explore research opportunities. Students successfully complete research by taking ownership of a project, efficiently meeting deadlines, maintaining contact with research mentors by quickly responding to emails, and producing quality work. Research outside of dermatology also is valued. Research mentors often provide future letters of recommendation, so commit to doing an outstanding job. For those finding it difficult to locate a mentor, consider searching the American Academy of Dermatology (AAD)(https://www.aad.org/mentorship/) or SOCS (https://skinofcolorsociety.org/) websites. The AAD has an established Diversity Mentorship Program (https://www.aad.org/member/career/volunteer/diversity-mentorship) that provides members with direct guidance from dermatologists for 4 weeks. Students use this time to conduct research, learn more about the specialty, and foster a relationship with their mentor. Students can apply any year of medical school; however, the typical awardee usually is a third-year or fourth-year student. The AAD may provide a stipend to help offset expenses.
Prepare for Boards
Second year is a continuation of the agenda set forth in first year, now with the focus shifting toward board preparation and excelling in clinical core didactics and rotations. According to data from the 2018 National Resident Matching Program,9 the mean USMLE Step 1 score for US allopathic senior medical students who matched into dermatology was 249 compared to 241 who did not match into dermatology. However, the mean score is just that—a mean—and people have matched with lower scores. Do not be intimidated by this number; instead, be driven to commit the time and resources to master the content and do your personal best on the USMLE Step examinations. Given the shift in some programs for earlier clinical exposure and postponement of boards until the third year, the recommendations in this timeline can be catered to fit a medical student’s specific situation.
Build Your Application
The third year of medical school is a busy year. Prepare for third-year clinical rotations by speaking with upperclassmen and clinical preceptors as you progress through your rotations. Evaluations and recommendations are weighed heavily by residency program directors, as this information is used to ascertain your clinical abilities. Seek feedback from your preceptors early and often with a sincere attempt to integrate suggested improvements. Schedule a dermatology rotation at your home institution after completing the core rotations. Although they are not required, applicants may complete away rotations early in their fourth year; the application period for visiting student learning opportunities typically opens April 1 of the third year, if not earlier. Free resources are available to help prepare for your dermatology rotations. Start by reviewing the Basic Dermatology Curriculum on the AAD website (https://www.aad.org/member/education/residents/bdc). Make contributions to
Interviewing for Residency
During your fourth year of medical school, you will be completing dermatology rotations, submitting your applications through the Electronic Residency Application Service, and interviewing with residency programs. When deciding which programs to apply to, consider referencing the American Medical Association Residency and Fellowship Database (https://freida.ama-assn.org/Freida/#/). Also keep in mind that, depending on your competitiveness, you should expect to receive 1 interview for every 10 programs you apply to, thus the application process can be quite costly. It is highly encouraged that you ask for letters of recommendation prior to August 15 and that you submit your applications by September 15. Complete mock interviews with a mentor and research commonly asked questions. Prior to your interview day, you want to spend time researching the program, browsing faculty publications, and reviewing your application. Dress in a comfortable suit, shoes, and minimal accessories; arrive early knowing that your interview begins even before you meet your interviewer, so treat everyone you meet with respect. Refrain from speaking to anyone in a casual way and have questions prepared to ask each interviewer. After your interviews, be sure to write thank you notes or emails if a program does not specifically discourage postinterview communication. Continuous efforts will improve your success in obtaining a dermatology residency position.
Final Thoughts
Recent articles have underscored and emphasized the importance of diversity in our field, with a call to action to find meaningful and overdue solutions.2,6 We acknowledge the important role that mentors play in providing timely, honest, and encouraging guidance to UIM students interested in careers in dermatology. We hope to provide readily available and detailed guidance to these students on how they can present themselves as excellent and qualified applicants through this summary and other platforms.
Acknowledgment
The authors would like to thank the members of the SOCS Diversity Task Force for their assistance in creating the original guide.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Taylor SC. Meeting the unique dermatologic needs of black patients [published online August 21, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1963.
- Skin of Color Society. How to obtain a position in a dermatology residency program. https://skinofcolorsociety.org/wp-content/uploads/2019/10/How-to-Obtain-a-Position-in-a-Dermatology-Residency-Program-10-08-2019.pdf. Accessed June 24, 2020.
- American Academy of Orthopaedic Surgeons. How to obtain an orthopedic residency by the American Academy of Orthopaedic Surgeons. https://www.aaos.org/globalassets/about/diversity/how-to-obtain-an-orthopaedic-residency.pdf. Accessed June 24, 2020.
- Results and Data—2018 Main Residency Match. Washington, DC: National Resident Matching Program; 2018. Published April 2018. Accessed June 24, 2020.
There has been increasing attention focused on the lack of diversity within dermatology academic and residency programs.1-6 Several factors have been identified as contributing to this narrow pipeline of qualified applicants, including lack of mentorship, delayed exposure to the field, implicit bias, and lack of an overall holistic review of applications with overemphasis on board scores.1,5 In an effort to provide guidance to underrepresented in medicine (UIM) students who are interested in dermatology, the Skin of Color Society (SOCS) has created a detailed, step-by-step guide on how to obtain a position in a dermatology residency program,7 which was modeled after a similar resource created by the American Academy of Orthopaedic Surgeons.8 Here, we highlight the main SOCS recommendations to help guide medical students through a systematic approach to becoming successful applicants for dermatology residency.
Start Early
Competitive fields such as dermatology require intentional efforts starting at the beginning of medical school. Regardless of what specialty is right for you, begin by constructing a well-rounded application for residency immediately. Start by shadowing dermatologists and attending Grand Rounds held in your institution’s dermatology department to ensure that this field is right for you. Students are encouraged to meet with academic advisors and upperclassmen to seek guidance on gaining early exposure to dermatology at their home institutions (or nearby programs) during their first year. As a platform for learning about community-based dermatology activities, join your school’s Dermatology Interest Group, keeping in mind that an executive position in such a group can help foster relationships with faculty and residents of the dermatology department. A long-term commitment to community service also contributes to your depth as an applicant. Getting involved early helps students uncover health disparities in medicine and allows time to formulate ideas to implement change. Forming a well-rounded application mandates maintaining good academic standing, and students should prioritize mastering the curriculum, excelling in clinical rotations, and studying for the US Medical Licensing Examination (USMLE).
Choose a Mentor
The summer between your first and second years of medical school is an opportune time to explore research opportunities. Students successfully complete research by taking ownership of a project, efficiently meeting deadlines, maintaining contact with research mentors by quickly responding to emails, and producing quality work. Research outside of dermatology also is valued. Research mentors often provide future letters of recommendation, so commit to doing an outstanding job. For those finding it difficult to locate a mentor, consider searching the American Academy of Dermatology (AAD)(https://www.aad.org/mentorship/) or SOCS (https://skinofcolorsociety.org/) websites. The AAD has an established Diversity Mentorship Program (https://www.aad.org/member/career/volunteer/diversity-mentorship) that provides members with direct guidance from dermatologists for 4 weeks. Students use this time to conduct research, learn more about the specialty, and foster a relationship with their mentor. Students can apply any year of medical school; however, the typical awardee usually is a third-year or fourth-year student. The AAD may provide a stipend to help offset expenses.
Prepare for Boards
Second year is a continuation of the agenda set forth in first year, now with the focus shifting toward board preparation and excelling in clinical core didactics and rotations. According to data from the 2018 National Resident Matching Program,9 the mean USMLE Step 1 score for US allopathic senior medical students who matched into dermatology was 249 compared to 241 who did not match into dermatology. However, the mean score is just that—a mean—and people have matched with lower scores. Do not be intimidated by this number; instead, be driven to commit the time and resources to master the content and do your personal best on the USMLE Step examinations. Given the shift in some programs for earlier clinical exposure and postponement of boards until the third year, the recommendations in this timeline can be catered to fit a medical student’s specific situation.
Build Your Application
The third year of medical school is a busy year. Prepare for third-year clinical rotations by speaking with upperclassmen and clinical preceptors as you progress through your rotations. Evaluations and recommendations are weighed heavily by residency program directors, as this information is used to ascertain your clinical abilities. Seek feedback from your preceptors early and often with a sincere attempt to integrate suggested improvements. Schedule a dermatology rotation at your home institution after completing the core rotations. Although they are not required, applicants may complete away rotations early in their fourth year; the application period for visiting student learning opportunities typically opens April 1 of the third year, if not earlier. Free resources are available to help prepare for your dermatology rotations. Start by reviewing the Basic Dermatology Curriculum on the AAD website (https://www.aad.org/member/education/residents/bdc). Make contributions to
Interviewing for Residency
During your fourth year of medical school, you will be completing dermatology rotations, submitting your applications through the Electronic Residency Application Service, and interviewing with residency programs. When deciding which programs to apply to, consider referencing the American Medical Association Residency and Fellowship Database (https://freida.ama-assn.org/Freida/#/). Also keep in mind that, depending on your competitiveness, you should expect to receive 1 interview for every 10 programs you apply to, thus the application process can be quite costly. It is highly encouraged that you ask for letters of recommendation prior to August 15 and that you submit your applications by September 15. Complete mock interviews with a mentor and research commonly asked questions. Prior to your interview day, you want to spend time researching the program, browsing faculty publications, and reviewing your application. Dress in a comfortable suit, shoes, and minimal accessories; arrive early knowing that your interview begins even before you meet your interviewer, so treat everyone you meet with respect. Refrain from speaking to anyone in a casual way and have questions prepared to ask each interviewer. After your interviews, be sure to write thank you notes or emails if a program does not specifically discourage postinterview communication. Continuous efforts will improve your success in obtaining a dermatology residency position.
Final Thoughts
Recent articles have underscored and emphasized the importance of diversity in our field, with a call to action to find meaningful and overdue solutions.2,6 We acknowledge the important role that mentors play in providing timely, honest, and encouraging guidance to UIM students interested in careers in dermatology. We hope to provide readily available and detailed guidance to these students on how they can present themselves as excellent and qualified applicants through this summary and other platforms.
Acknowledgment
The authors would like to thank the members of the SOCS Diversity Task Force for their assistance in creating the original guide.
There has been increasing attention focused on the lack of diversity within dermatology academic and residency programs.1-6 Several factors have been identified as contributing to this narrow pipeline of qualified applicants, including lack of mentorship, delayed exposure to the field, implicit bias, and lack of an overall holistic review of applications with overemphasis on board scores.1,5 In an effort to provide guidance to underrepresented in medicine (UIM) students who are interested in dermatology, the Skin of Color Society (SOCS) has created a detailed, step-by-step guide on how to obtain a position in a dermatology residency program,7 which was modeled after a similar resource created by the American Academy of Orthopaedic Surgeons.8 Here, we highlight the main SOCS recommendations to help guide medical students through a systematic approach to becoming successful applicants for dermatology residency.
Start Early
Competitive fields such as dermatology require intentional efforts starting at the beginning of medical school. Regardless of what specialty is right for you, begin by constructing a well-rounded application for residency immediately. Start by shadowing dermatologists and attending Grand Rounds held in your institution’s dermatology department to ensure that this field is right for you. Students are encouraged to meet with academic advisors and upperclassmen to seek guidance on gaining early exposure to dermatology at their home institutions (or nearby programs) during their first year. As a platform for learning about community-based dermatology activities, join your school’s Dermatology Interest Group, keeping in mind that an executive position in such a group can help foster relationships with faculty and residents of the dermatology department. A long-term commitment to community service also contributes to your depth as an applicant. Getting involved early helps students uncover health disparities in medicine and allows time to formulate ideas to implement change. Forming a well-rounded application mandates maintaining good academic standing, and students should prioritize mastering the curriculum, excelling in clinical rotations, and studying for the US Medical Licensing Examination (USMLE).
Choose a Mentor
The summer between your first and second years of medical school is an opportune time to explore research opportunities. Students successfully complete research by taking ownership of a project, efficiently meeting deadlines, maintaining contact with research mentors by quickly responding to emails, and producing quality work. Research outside of dermatology also is valued. Research mentors often provide future letters of recommendation, so commit to doing an outstanding job. For those finding it difficult to locate a mentor, consider searching the American Academy of Dermatology (AAD)(https://www.aad.org/mentorship/) or SOCS (https://skinofcolorsociety.org/) websites. The AAD has an established Diversity Mentorship Program (https://www.aad.org/member/career/volunteer/diversity-mentorship) that provides members with direct guidance from dermatologists for 4 weeks. Students use this time to conduct research, learn more about the specialty, and foster a relationship with their mentor. Students can apply any year of medical school; however, the typical awardee usually is a third-year or fourth-year student. The AAD may provide a stipend to help offset expenses.
Prepare for Boards
Second year is a continuation of the agenda set forth in first year, now with the focus shifting toward board preparation and excelling in clinical core didactics and rotations. According to data from the 2018 National Resident Matching Program,9 the mean USMLE Step 1 score for US allopathic senior medical students who matched into dermatology was 249 compared to 241 who did not match into dermatology. However, the mean score is just that—a mean—and people have matched with lower scores. Do not be intimidated by this number; instead, be driven to commit the time and resources to master the content and do your personal best on the USMLE Step examinations. Given the shift in some programs for earlier clinical exposure and postponement of boards until the third year, the recommendations in this timeline can be catered to fit a medical student’s specific situation.
Build Your Application
The third year of medical school is a busy year. Prepare for third-year clinical rotations by speaking with upperclassmen and clinical preceptors as you progress through your rotations. Evaluations and recommendations are weighed heavily by residency program directors, as this information is used to ascertain your clinical abilities. Seek feedback from your preceptors early and often with a sincere attempt to integrate suggested improvements. Schedule a dermatology rotation at your home institution after completing the core rotations. Although they are not required, applicants may complete away rotations early in their fourth year; the application period for visiting student learning opportunities typically opens April 1 of the third year, if not earlier. Free resources are available to help prepare for your dermatology rotations. Start by reviewing the Basic Dermatology Curriculum on the AAD website (https://www.aad.org/member/education/residents/bdc). Make contributions to
Interviewing for Residency
During your fourth year of medical school, you will be completing dermatology rotations, submitting your applications through the Electronic Residency Application Service, and interviewing with residency programs. When deciding which programs to apply to, consider referencing the American Medical Association Residency and Fellowship Database (https://freida.ama-assn.org/Freida/#/). Also keep in mind that, depending on your competitiveness, you should expect to receive 1 interview for every 10 programs you apply to, thus the application process can be quite costly. It is highly encouraged that you ask for letters of recommendation prior to August 15 and that you submit your applications by September 15. Complete mock interviews with a mentor and research commonly asked questions. Prior to your interview day, you want to spend time researching the program, browsing faculty publications, and reviewing your application. Dress in a comfortable suit, shoes, and minimal accessories; arrive early knowing that your interview begins even before you meet your interviewer, so treat everyone you meet with respect. Refrain from speaking to anyone in a casual way and have questions prepared to ask each interviewer. After your interviews, be sure to write thank you notes or emails if a program does not specifically discourage postinterview communication. Continuous efforts will improve your success in obtaining a dermatology residency position.
Final Thoughts
Recent articles have underscored and emphasized the importance of diversity in our field, with a call to action to find meaningful and overdue solutions.2,6 We acknowledge the important role that mentors play in providing timely, honest, and encouraging guidance to UIM students interested in careers in dermatology. We hope to provide readily available and detailed guidance to these students on how they can present themselves as excellent and qualified applicants through this summary and other platforms.
Acknowledgment
The authors would like to thank the members of the SOCS Diversity Task Force for their assistance in creating the original guide.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Taylor SC. Meeting the unique dermatologic needs of black patients [published online August 21, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1963.
- Skin of Color Society. How to obtain a position in a dermatology residency program. https://skinofcolorsociety.org/wp-content/uploads/2019/10/How-to-Obtain-a-Position-in-a-Dermatology-Residency-Program-10-08-2019.pdf. Accessed June 24, 2020.
- American Academy of Orthopaedic Surgeons. How to obtain an orthopedic residency by the American Academy of Orthopaedic Surgeons. https://www.aaos.org/globalassets/about/diversity/how-to-obtain-an-orthopaedic-residency.pdf. Accessed June 24, 2020.
- Results and Data—2018 Main Residency Match. Washington, DC: National Resident Matching Program; 2018. Published April 2018. Accessed June 24, 2020.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Taylor SC. Meeting the unique dermatologic needs of black patients [published online August 21, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1963.
- Skin of Color Society. How to obtain a position in a dermatology residency program. https://skinofcolorsociety.org/wp-content/uploads/2019/10/How-to-Obtain-a-Position-in-a-Dermatology-Residency-Program-10-08-2019.pdf. Accessed June 24, 2020.
- American Academy of Orthopaedic Surgeons. How to obtain an orthopedic residency by the American Academy of Orthopaedic Surgeons. https://www.aaos.org/globalassets/about/diversity/how-to-obtain-an-orthopaedic-residency.pdf. Accessed June 24, 2020.
- Results and Data—2018 Main Residency Match. Washington, DC: National Resident Matching Program; 2018. Published April 2018. Accessed June 24, 2020.
Practice Points
- Students interested in dermatology are encouraged to seek mentorship, strive for their academic best, and maintain their unique personal interests that make them a well-rounded applicant.
- Increasing diversity in dermatology requires initiative from students as well as dermatologists who are willing to mentor and sponsor.
Product News July 2020
Arazlo Now Available in the United States
Ortho Dermatologics launches Arazlo (tazarotene) Lotion 0.045% to health care professionals in the United States. Arazlo was approved by the US Food and Drug Administration in December 2019 for the treatment of acne in patients 9 years and older. Clinical trials indicated that Arazlo Lotion offers a tolerable formulation without sacrificing efficacy. For more information, visit arazlo.com/hcp/.
FDA Approves Updated Label for Seysara Tablets
Almirall, LLC, receives US Food and Drug Administration (FDA) approval for an update to the Seysara (sarecycline) Tablets label stating that Propionibacterium acnes strains displayed a low propensity for the development of resistance to sarecycline, which is important when considering the appropriate use of antibiotics. The FDA approved Seysara in 2018 for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients 9 years and older. For more information, visit seysara.com.
Jublia Labeling Extends to Pediatric Patients
Ortho Dermatologics announces the US Food and Drug Administration has approved a supplemental New Drug Application for Jublia (efinaconazole) Solution 10% for the treatment of onychomycosis in patients 6 years and older. Jublia was initially approved in 2014 for patients 18 years and older. With its safety and efficacy profile demonstrated in real-world use over the last 6 years, Jublia can now be a valuable treatment option for children with toenail fungal infections. For more information, visit jubliarx.com.
Revance and Mylan to Develop Biosimilar to Botox
Revance Therapeutics, Inc, and Mylan N.V. announce they are moving forward with a 351(k) development plan for a proposed biosimilar to Botox and Botox Cosmetic (onabotulinumtoxinA), which would allow Revance to financially participate in the short-acting neurotoxin space while focusing commercial efforts in the long-acting neuromodulator category. Mylan will handle the commercialization of the new biosimilar in the United States, Europe, and other markets worldwide. For more information, visit revance.com and mylan.com.
The Skin Cancer Foundation Awards $125,000 in Research Grants
The Skin Cancer Foundation awards $125,000 in grants to 3 separate researchers who submitted proposals to further prevention, early detection, and treatment of skin cancer. Matthew Hangauer, PhD (La Jolla, California), received $50,000 for “Targeting Immunotherapy-Tolerant Melanoma Persister Cells”; Lee E. Wheless, MD, PhD (Nashville, Tennessee), received $50,000 for “Using Bioinformatics to Stratify Skin Cancer Risk in Organ Transplant Recipients”; and Vishal Patel, MD (Washington, DC), received $25,000 for “Delphi Consensus Determination of a Tumor Stage Based Approach to High-Risk Cutaneous Squamous Cell Carcinoma.” The Skin Cancer Foundation’s Research Grants program helps provide funding for studies that have led to lifesaving breakthroughs. The 2021 research grants application period will open soon. For more information, visit SkinCancer.org/research.
Arazlo Now Available in the United States
Ortho Dermatologics launches Arazlo (tazarotene) Lotion 0.045% to health care professionals in the United States. Arazlo was approved by the US Food and Drug Administration in December 2019 for the treatment of acne in patients 9 years and older. Clinical trials indicated that Arazlo Lotion offers a tolerable formulation without sacrificing efficacy. For more information, visit arazlo.com/hcp/.
FDA Approves Updated Label for Seysara Tablets
Almirall, LLC, receives US Food and Drug Administration (FDA) approval for an update to the Seysara (sarecycline) Tablets label stating that Propionibacterium acnes strains displayed a low propensity for the development of resistance to sarecycline, which is important when considering the appropriate use of antibiotics. The FDA approved Seysara in 2018 for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients 9 years and older. For more information, visit seysara.com.
Jublia Labeling Extends to Pediatric Patients
Ortho Dermatologics announces the US Food and Drug Administration has approved a supplemental New Drug Application for Jublia (efinaconazole) Solution 10% for the treatment of onychomycosis in patients 6 years and older. Jublia was initially approved in 2014 for patients 18 years and older. With its safety and efficacy profile demonstrated in real-world use over the last 6 years, Jublia can now be a valuable treatment option for children with toenail fungal infections. For more information, visit jubliarx.com.
Revance and Mylan to Develop Biosimilar to Botox
Revance Therapeutics, Inc, and Mylan N.V. announce they are moving forward with a 351(k) development plan for a proposed biosimilar to Botox and Botox Cosmetic (onabotulinumtoxinA), which would allow Revance to financially participate in the short-acting neurotoxin space while focusing commercial efforts in the long-acting neuromodulator category. Mylan will handle the commercialization of the new biosimilar in the United States, Europe, and other markets worldwide. For more information, visit revance.com and mylan.com.
The Skin Cancer Foundation Awards $125,000 in Research Grants
The Skin Cancer Foundation awards $125,000 in grants to 3 separate researchers who submitted proposals to further prevention, early detection, and treatment of skin cancer. Matthew Hangauer, PhD (La Jolla, California), received $50,000 for “Targeting Immunotherapy-Tolerant Melanoma Persister Cells”; Lee E. Wheless, MD, PhD (Nashville, Tennessee), received $50,000 for “Using Bioinformatics to Stratify Skin Cancer Risk in Organ Transplant Recipients”; and Vishal Patel, MD (Washington, DC), received $25,000 for “Delphi Consensus Determination of a Tumor Stage Based Approach to High-Risk Cutaneous Squamous Cell Carcinoma.” The Skin Cancer Foundation’s Research Grants program helps provide funding for studies that have led to lifesaving breakthroughs. The 2021 research grants application period will open soon. For more information, visit SkinCancer.org/research.
Arazlo Now Available in the United States
Ortho Dermatologics launches Arazlo (tazarotene) Lotion 0.045% to health care professionals in the United States. Arazlo was approved by the US Food and Drug Administration in December 2019 for the treatment of acne in patients 9 years and older. Clinical trials indicated that Arazlo Lotion offers a tolerable formulation without sacrificing efficacy. For more information, visit arazlo.com/hcp/.
FDA Approves Updated Label for Seysara Tablets
Almirall, LLC, receives US Food and Drug Administration (FDA) approval for an update to the Seysara (sarecycline) Tablets label stating that Propionibacterium acnes strains displayed a low propensity for the development of resistance to sarecycline, which is important when considering the appropriate use of antibiotics. The FDA approved Seysara in 2018 for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients 9 years and older. For more information, visit seysara.com.
Jublia Labeling Extends to Pediatric Patients
Ortho Dermatologics announces the US Food and Drug Administration has approved a supplemental New Drug Application for Jublia (efinaconazole) Solution 10% for the treatment of onychomycosis in patients 6 years and older. Jublia was initially approved in 2014 for patients 18 years and older. With its safety and efficacy profile demonstrated in real-world use over the last 6 years, Jublia can now be a valuable treatment option for children with toenail fungal infections. For more information, visit jubliarx.com.
Revance and Mylan to Develop Biosimilar to Botox
Revance Therapeutics, Inc, and Mylan N.V. announce they are moving forward with a 351(k) development plan for a proposed biosimilar to Botox and Botox Cosmetic (onabotulinumtoxinA), which would allow Revance to financially participate in the short-acting neurotoxin space while focusing commercial efforts in the long-acting neuromodulator category. Mylan will handle the commercialization of the new biosimilar in the United States, Europe, and other markets worldwide. For more information, visit revance.com and mylan.com.
The Skin Cancer Foundation Awards $125,000 in Research Grants
The Skin Cancer Foundation awards $125,000 in grants to 3 separate researchers who submitted proposals to further prevention, early detection, and treatment of skin cancer. Matthew Hangauer, PhD (La Jolla, California), received $50,000 for “Targeting Immunotherapy-Tolerant Melanoma Persister Cells”; Lee E. Wheless, MD, PhD (Nashville, Tennessee), received $50,000 for “Using Bioinformatics to Stratify Skin Cancer Risk in Organ Transplant Recipients”; and Vishal Patel, MD (Washington, DC), received $25,000 for “Delphi Consensus Determination of a Tumor Stage Based Approach to High-Risk Cutaneous Squamous Cell Carcinoma.” The Skin Cancer Foundation’s Research Grants program helps provide funding for studies that have led to lifesaving breakthroughs. The 2021 research grants application period will open soon. For more information, visit SkinCancer.org/research.