User login
Polygenic risk score helps target AAA screening
A polygenic risk score based on analysis of 29 discrete genetic variants linked with abdominal aortic aneurysms appeared better than the current criteria that clinicians use to identify people to screen for this disorder, potentially paving the way for more efficient use of screening resources.
Future screening guidelines for abdominal aortic aneurysms (AAA) “should consider including individuals with high polygenic risk for screening ultrasonography,” Derek Klarin, MD, said at the virtual Vascular Discovery Scientific Sessions 2020, organized by the American Heart Association.
The data he reported showed that when researchers applied the polygenic risk score (PRS) to men aged older than 50 years in three independent validation cohorts of people with primarily European ancestry, those with scores in the top 5 percentile within each cohort had a collective AAA prevalence rate of 8.6% (95% CI 7.3%-9.8%).
This 8.6% pick-up rate using the PRS to help identify screening candidates for this male demographic subgroup compared favorably with previously reported prevalence rates of AAA detected by ultrasonography (defined as aneurysms of at least 3.0 cm in diameter) in men aged 65 years or older with a history of ever smoking. Last year, the U.S. Preventive Services Task Force issued an updated recommendation to perform a one-time ultrasound screening of 65- to 75-year-old men who ever smoked and cited five reported screening studies that found prevalence rates in these people of 3.3%-7.6% (JAMA. 2019 Dec 10;322[22]:2211-8). An earlier review of the topic by the task force cited an average estimated prevalence of 6%-7% in men at least 65 years old and with a smoking history (Ann Intern Med. 2014 Aug 19;161[4]: 281-90).
“You can use [the PRS] with other risk factors to increase the yield of identifying those at high risk,” Dr. Klarin said during a discussion of his report. He noted the possibility of using it to identify people at-risk early on, at birth, “prior to other risk factors being present,” as well as using the PRS as an add-on to known risk factors when assessing adults. He stressed that validations he has run so far still leave the PRS a step away from routine use, although it is “quite close,” said Dr. Klarin, a vascular surgeon at the University of Florida in Gainesville.
For use in routine practice, the PRS needs “further validation,” including further assessment of its performance in other age groups, in a wider range of ethnic groups, and in women, said Chris Semsarian, MBBS, professor of medicine at the University of Sydney and head of the Molecular Cardiology Program at its Centenary Institute. However, Dr. Semsarian also said that he saw great promise for the potential of the PRS, and its development so far had been solid.
“The study was meticulously undertaken, with a large number of AAA cases and controls. Both the derivation and validation are robust. There is great potential to use such a genetic risk score in the clinical setting, along with other risk factors such as smoking, high blood pressure, and lipid levels. The PRS adds another piece in the puzzle of risk of AAA by adding in genetic or inherited risk. An additional 1%-2% in pick-up rate would still lead to many thousands more AAA detected and lives saved. The PRS doesn’t replace environmental factors that contribute to AAA risk but adds a genetic component” when estimating a person’s overall risk and the appropriateness of screening ultrasound, Dr. Semsarian said in an interview.
The derivation analysis that Dr. Klarin and associates ran used data from the Million Veteran Program that included 7,642 people with AAA and matched them with more than 172,000 controls from the same database. This generated three alternative PRSs that involved testing for 29, 301, or 3,699 different mutations or polymorphisms that discriminated cases from controls. They compared these three scoring formulas in 1,000 AAA cases and 7,700 matched controls from the Mayo Clinic’s patient database, which showed that the 29-item PRS performed best, boosting identification of cases with a statistically significant odds ratio of 1.26.
They then ran the 29-item PRS in four additional large data banks, three that included mostly people of European ancestry and one that included mostly people with an African heritage. In the three data banks with people of mostly European background, the 29-item PRS performed even better than it did using the Mayo Clinic data, but this PRS was less informative in people with African ancestry. The analyses also suggested that the PRS identified elevated AAA risk independently of information on a family history of AAA, Dr. Klarin said.
The study had no commercial funding. Dr. Klarin has been a consultant to Regeneron.
SOURCE: Klarin D et al. Vascular Discovery 2020, abstract 170.
A polygenic risk score based on analysis of 29 discrete genetic variants linked with abdominal aortic aneurysms appeared better than the current criteria that clinicians use to identify people to screen for this disorder, potentially paving the way for more efficient use of screening resources.
Future screening guidelines for abdominal aortic aneurysms (AAA) “should consider including individuals with high polygenic risk for screening ultrasonography,” Derek Klarin, MD, said at the virtual Vascular Discovery Scientific Sessions 2020, organized by the American Heart Association.
The data he reported showed that when researchers applied the polygenic risk score (PRS) to men aged older than 50 years in three independent validation cohorts of people with primarily European ancestry, those with scores in the top 5 percentile within each cohort had a collective AAA prevalence rate of 8.6% (95% CI 7.3%-9.8%).
This 8.6% pick-up rate using the PRS to help identify screening candidates for this male demographic subgroup compared favorably with previously reported prevalence rates of AAA detected by ultrasonography (defined as aneurysms of at least 3.0 cm in diameter) in men aged 65 years or older with a history of ever smoking. Last year, the U.S. Preventive Services Task Force issued an updated recommendation to perform a one-time ultrasound screening of 65- to 75-year-old men who ever smoked and cited five reported screening studies that found prevalence rates in these people of 3.3%-7.6% (JAMA. 2019 Dec 10;322[22]:2211-8). An earlier review of the topic by the task force cited an average estimated prevalence of 6%-7% in men at least 65 years old and with a smoking history (Ann Intern Med. 2014 Aug 19;161[4]: 281-90).
“You can use [the PRS] with other risk factors to increase the yield of identifying those at high risk,” Dr. Klarin said during a discussion of his report. He noted the possibility of using it to identify people at-risk early on, at birth, “prior to other risk factors being present,” as well as using the PRS as an add-on to known risk factors when assessing adults. He stressed that validations he has run so far still leave the PRS a step away from routine use, although it is “quite close,” said Dr. Klarin, a vascular surgeon at the University of Florida in Gainesville.
For use in routine practice, the PRS needs “further validation,” including further assessment of its performance in other age groups, in a wider range of ethnic groups, and in women, said Chris Semsarian, MBBS, professor of medicine at the University of Sydney and head of the Molecular Cardiology Program at its Centenary Institute. However, Dr. Semsarian also said that he saw great promise for the potential of the PRS, and its development so far had been solid.
“The study was meticulously undertaken, with a large number of AAA cases and controls. Both the derivation and validation are robust. There is great potential to use such a genetic risk score in the clinical setting, along with other risk factors such as smoking, high blood pressure, and lipid levels. The PRS adds another piece in the puzzle of risk of AAA by adding in genetic or inherited risk. An additional 1%-2% in pick-up rate would still lead to many thousands more AAA detected and lives saved. The PRS doesn’t replace environmental factors that contribute to AAA risk but adds a genetic component” when estimating a person’s overall risk and the appropriateness of screening ultrasound, Dr. Semsarian said in an interview.
The derivation analysis that Dr. Klarin and associates ran used data from the Million Veteran Program that included 7,642 people with AAA and matched them with more than 172,000 controls from the same database. This generated three alternative PRSs that involved testing for 29, 301, or 3,699 different mutations or polymorphisms that discriminated cases from controls. They compared these three scoring formulas in 1,000 AAA cases and 7,700 matched controls from the Mayo Clinic’s patient database, which showed that the 29-item PRS performed best, boosting identification of cases with a statistically significant odds ratio of 1.26.
They then ran the 29-item PRS in four additional large data banks, three that included mostly people of European ancestry and one that included mostly people with an African heritage. In the three data banks with people of mostly European background, the 29-item PRS performed even better than it did using the Mayo Clinic data, but this PRS was less informative in people with African ancestry. The analyses also suggested that the PRS identified elevated AAA risk independently of information on a family history of AAA, Dr. Klarin said.
The study had no commercial funding. Dr. Klarin has been a consultant to Regeneron.
SOURCE: Klarin D et al. Vascular Discovery 2020, abstract 170.
A polygenic risk score based on analysis of 29 discrete genetic variants linked with abdominal aortic aneurysms appeared better than the current criteria that clinicians use to identify people to screen for this disorder, potentially paving the way for more efficient use of screening resources.
Future screening guidelines for abdominal aortic aneurysms (AAA) “should consider including individuals with high polygenic risk for screening ultrasonography,” Derek Klarin, MD, said at the virtual Vascular Discovery Scientific Sessions 2020, organized by the American Heart Association.
The data he reported showed that when researchers applied the polygenic risk score (PRS) to men aged older than 50 years in three independent validation cohorts of people with primarily European ancestry, those with scores in the top 5 percentile within each cohort had a collective AAA prevalence rate of 8.6% (95% CI 7.3%-9.8%).
This 8.6% pick-up rate using the PRS to help identify screening candidates for this male demographic subgroup compared favorably with previously reported prevalence rates of AAA detected by ultrasonography (defined as aneurysms of at least 3.0 cm in diameter) in men aged 65 years or older with a history of ever smoking. Last year, the U.S. Preventive Services Task Force issued an updated recommendation to perform a one-time ultrasound screening of 65- to 75-year-old men who ever smoked and cited five reported screening studies that found prevalence rates in these people of 3.3%-7.6% (JAMA. 2019 Dec 10;322[22]:2211-8). An earlier review of the topic by the task force cited an average estimated prevalence of 6%-7% in men at least 65 years old and with a smoking history (Ann Intern Med. 2014 Aug 19;161[4]: 281-90).
“You can use [the PRS] with other risk factors to increase the yield of identifying those at high risk,” Dr. Klarin said during a discussion of his report. He noted the possibility of using it to identify people at-risk early on, at birth, “prior to other risk factors being present,” as well as using the PRS as an add-on to known risk factors when assessing adults. He stressed that validations he has run so far still leave the PRS a step away from routine use, although it is “quite close,” said Dr. Klarin, a vascular surgeon at the University of Florida in Gainesville.
For use in routine practice, the PRS needs “further validation,” including further assessment of its performance in other age groups, in a wider range of ethnic groups, and in women, said Chris Semsarian, MBBS, professor of medicine at the University of Sydney and head of the Molecular Cardiology Program at its Centenary Institute. However, Dr. Semsarian also said that he saw great promise for the potential of the PRS, and its development so far had been solid.
“The study was meticulously undertaken, with a large number of AAA cases and controls. Both the derivation and validation are robust. There is great potential to use such a genetic risk score in the clinical setting, along with other risk factors such as smoking, high blood pressure, and lipid levels. The PRS adds another piece in the puzzle of risk of AAA by adding in genetic or inherited risk. An additional 1%-2% in pick-up rate would still lead to many thousands more AAA detected and lives saved. The PRS doesn’t replace environmental factors that contribute to AAA risk but adds a genetic component” when estimating a person’s overall risk and the appropriateness of screening ultrasound, Dr. Semsarian said in an interview.
The derivation analysis that Dr. Klarin and associates ran used data from the Million Veteran Program that included 7,642 people with AAA and matched them with more than 172,000 controls from the same database. This generated three alternative PRSs that involved testing for 29, 301, or 3,699 different mutations or polymorphisms that discriminated cases from controls. They compared these three scoring formulas in 1,000 AAA cases and 7,700 matched controls from the Mayo Clinic’s patient database, which showed that the 29-item PRS performed best, boosting identification of cases with a statistically significant odds ratio of 1.26.
They then ran the 29-item PRS in four additional large data banks, three that included mostly people of European ancestry and one that included mostly people with an African heritage. In the three data banks with people of mostly European background, the 29-item PRS performed even better than it did using the Mayo Clinic data, but this PRS was less informative in people with African ancestry. The analyses also suggested that the PRS identified elevated AAA risk independently of information on a family history of AAA, Dr. Klarin said.
The study had no commercial funding. Dr. Klarin has been a consultant to Regeneron.
SOURCE: Klarin D et al. Vascular Discovery 2020, abstract 170.
FROM AHA VASCULAR DISCOVERY 2020
Masks, fear, and loss of connection in the era of COVID-19
Over the din of the negative pressure machine, I shouted goodbye to my patient and zipped my way out of one of the little plastic enclosures in our ED and carefully shed my gloves, gown, and face shield, leaving on my precious mask. I discarded the rest with disgust and a bit of fear. I thought, “This is a whole new world, and I hate it.”
I feel as if I am constantly battling the fear of dying from COVID-19 but am doing the best I can, given the circumstances at hand. I have the proper equipment and use it well. My work still brings meaning: I serve those in need without hesitation. The problem is that deep feeling of connection with patients, which is such an important part of this work, feels like fraying threads moving further apart because of the havoc this virus has wrought. A few weeks ago, the intricate fabric of what it is to be human connected me to patients through the basics: touch, facial expressions, a physical proximity, and openhearted, honest dialogue. Much of that’s gone, and while I can carry on, I will surely burn out if I can’t figure out how to get at least some of that connection back.
Overwhelmed by the amount of information I need to process daily, I had not been thinking about the interpersonal side of the pandemic for the first weeks. I felt it leaving the ED that morning and later that day, and I felt it again with Ms. Z, who was not even suspected of having COVID. She is a 62-year-old I interviewed with the help of a translator phone. At the end of our encounter, she said “But doctor, will you make my tumor go away?” From across the room, I said, “I will try.” I saw her eyes dampen as I made a hasty exit, following protocol to limit time in the room of all patients.
Typically, leaving a patient’s room, I would feel a fullness associated with a sense of meaning. How did I feel after that? In that moment, mostly ashamed at my lack of compassion during my time with Ms. Z. Then, with further reflection, tense from all things COVID-19! Having an amped-up sympathetic nervous system is understandable, but it’s not where we want to be for our compassion to flow.
We connect best when our parasympathetic nervous system is predominant. So much of the stimuli we need to activate that part of the nervous system is gone. There is a virtuous cycle, much of it unconscious, where something positive leads to more positivity, which is crucial to meaningful patient encounters. We read each other’s facial expressions, hear the tone of voice, and as we pick up subtle cues from our patient, our nervous system is further engaged and our hearts opened.
The specter of COVID-19 has us battling a negative spiral of stress and fear. For the most part, I try to keep that from consuming me, but it clearly saps my energy during encounters. In the same way we need to marshal our resources to battle both the stress and the disease itself, we need to actively engage pro-social elements of providing care to maintain our compassion. Clearly, I needed a more concerted effort to kick start this virtuous cycle of compassion.
My next patient was Ms. J., a 55-year-old with advanced chronic obstructive pulmonary disease (COPD) who came in the night before with shortness of breath. Her slight frame shook from coughing as I entered the room. I did not think she had COVID-19, but we were ruling it out.
We reviewed how she felt since admission, and I performed a hasty exam and stepped back across the room. She coughed again and said, “I feel so weak, and the world feels so crazy; tell it to me straight.” Then looking in my eyes, “I am going to make it, doc?”
I took my cue from her; I walked back to the bedside, placed a gloved hand on her shoulder and with the other, I took her hand. I bent forward just a little. Making eye contact and attempting a comforting tone of voice, I said, “Everyone is a little scared, including me. We need each other more than ever these days. We will do our best for you. That means thoughtful medical care and a whole lot of love! And, truly, I don’t think you are dying; this is just one of your COPD flares.”
“God bless you!” she said, squeezing my hand as a tear rolled down her cheek.
“Bless you, too. We all need blessing with this madness going on,” I replied. Despite the mask, I am sure she saw the smile in my eyes. “Thanks for being the beautiful person you are and opening up to me. That’s the way we will make it through this. I will see you tomorrow.” Backing away, hands together in prayer, I gave a little bow and left the room.
With Ms. J.’s help, I began to figure it out. To tackle the stress of COVID, we need to be very direct – almost to the point of exaggeration – to make sure our words and actions convey what we need to express. William James, the father of psychology, believed that if you force a smile, your emotions would follow. The neural pathways could work backward in that way. He said, “If you want a quality, act as if you have it.” The modern translation would be, “Fake it ’til you make it.’ ” You may be feeling stressed, but with a deep breath and a moment’s reflection on the suffering of that patient you are about to see, you can turn the tide on anxiety and give those under your care what they need.
These are unprecedented times; anxiety abounds. While we can aspire to positivity, there are times when we simply can’t muster showing it. Alternatively, as I experienced with Ms. J., honesty and vulnerability can open the door to meaningful connection. This can be quite powerful when we, as physicians, open up to our patients.
People are yearning for deep connection, and we should attempt to deliver it with:
- Touch (as we can) to convey connection.
- Body language that adds emphasis to our message and our emotions that may go above and beyond what we are used to.
- Tone of voice that enhances our words.
- Talk that emphasizes the big stuff, such as love, fear, connection and community
With gloves, masks, distance, and fear between and us and our patients, we need to actively engage our pro-social tools to turn the negative spiral of fear into the virtuous cycle of positive emotions that promotes healing of our patients and emotional engagement for those providing their care.
Dr. Hass was trained in family medicine at University of California, San Francisco, after receiving his medical degree from the McGill University faculty of medicine, Montreal. He works as a hospitalist with Sutter Health in Oakland, Calif. He is an adviser on health and health care for the Greater Good Science Center at UC Berkeley and clinical faculty at UCSF School of Medicine. This article appeared initially at The Hospital Leader, the official blog of SHM.
Over the din of the negative pressure machine, I shouted goodbye to my patient and zipped my way out of one of the little plastic enclosures in our ED and carefully shed my gloves, gown, and face shield, leaving on my precious mask. I discarded the rest with disgust and a bit of fear. I thought, “This is a whole new world, and I hate it.”
I feel as if I am constantly battling the fear of dying from COVID-19 but am doing the best I can, given the circumstances at hand. I have the proper equipment and use it well. My work still brings meaning: I serve those in need without hesitation. The problem is that deep feeling of connection with patients, which is such an important part of this work, feels like fraying threads moving further apart because of the havoc this virus has wrought. A few weeks ago, the intricate fabric of what it is to be human connected me to patients through the basics: touch, facial expressions, a physical proximity, and openhearted, honest dialogue. Much of that’s gone, and while I can carry on, I will surely burn out if I can’t figure out how to get at least some of that connection back.
Overwhelmed by the amount of information I need to process daily, I had not been thinking about the interpersonal side of the pandemic for the first weeks. I felt it leaving the ED that morning and later that day, and I felt it again with Ms. Z, who was not even suspected of having COVID. She is a 62-year-old I interviewed with the help of a translator phone. At the end of our encounter, she said “But doctor, will you make my tumor go away?” From across the room, I said, “I will try.” I saw her eyes dampen as I made a hasty exit, following protocol to limit time in the room of all patients.
Typically, leaving a patient’s room, I would feel a fullness associated with a sense of meaning. How did I feel after that? In that moment, mostly ashamed at my lack of compassion during my time with Ms. Z. Then, with further reflection, tense from all things COVID-19! Having an amped-up sympathetic nervous system is understandable, but it’s not where we want to be for our compassion to flow.
We connect best when our parasympathetic nervous system is predominant. So much of the stimuli we need to activate that part of the nervous system is gone. There is a virtuous cycle, much of it unconscious, where something positive leads to more positivity, which is crucial to meaningful patient encounters. We read each other’s facial expressions, hear the tone of voice, and as we pick up subtle cues from our patient, our nervous system is further engaged and our hearts opened.
The specter of COVID-19 has us battling a negative spiral of stress and fear. For the most part, I try to keep that from consuming me, but it clearly saps my energy during encounters. In the same way we need to marshal our resources to battle both the stress and the disease itself, we need to actively engage pro-social elements of providing care to maintain our compassion. Clearly, I needed a more concerted effort to kick start this virtuous cycle of compassion.
My next patient was Ms. J., a 55-year-old with advanced chronic obstructive pulmonary disease (COPD) who came in the night before with shortness of breath. Her slight frame shook from coughing as I entered the room. I did not think she had COVID-19, but we were ruling it out.
We reviewed how she felt since admission, and I performed a hasty exam and stepped back across the room. She coughed again and said, “I feel so weak, and the world feels so crazy; tell it to me straight.” Then looking in my eyes, “I am going to make it, doc?”
I took my cue from her; I walked back to the bedside, placed a gloved hand on her shoulder and with the other, I took her hand. I bent forward just a little. Making eye contact and attempting a comforting tone of voice, I said, “Everyone is a little scared, including me. We need each other more than ever these days. We will do our best for you. That means thoughtful medical care and a whole lot of love! And, truly, I don’t think you are dying; this is just one of your COPD flares.”
“God bless you!” she said, squeezing my hand as a tear rolled down her cheek.
“Bless you, too. We all need blessing with this madness going on,” I replied. Despite the mask, I am sure she saw the smile in my eyes. “Thanks for being the beautiful person you are and opening up to me. That’s the way we will make it through this. I will see you tomorrow.” Backing away, hands together in prayer, I gave a little bow and left the room.
With Ms. J.’s help, I began to figure it out. To tackle the stress of COVID, we need to be very direct – almost to the point of exaggeration – to make sure our words and actions convey what we need to express. William James, the father of psychology, believed that if you force a smile, your emotions would follow. The neural pathways could work backward in that way. He said, “If you want a quality, act as if you have it.” The modern translation would be, “Fake it ’til you make it.’ ” You may be feeling stressed, but with a deep breath and a moment’s reflection on the suffering of that patient you are about to see, you can turn the tide on anxiety and give those under your care what they need.
These are unprecedented times; anxiety abounds. While we can aspire to positivity, there are times when we simply can’t muster showing it. Alternatively, as I experienced with Ms. J., honesty and vulnerability can open the door to meaningful connection. This can be quite powerful when we, as physicians, open up to our patients.
People are yearning for deep connection, and we should attempt to deliver it with:
- Touch (as we can) to convey connection.
- Body language that adds emphasis to our message and our emotions that may go above and beyond what we are used to.
- Tone of voice that enhances our words.
- Talk that emphasizes the big stuff, such as love, fear, connection and community
With gloves, masks, distance, and fear between and us and our patients, we need to actively engage our pro-social tools to turn the negative spiral of fear into the virtuous cycle of positive emotions that promotes healing of our patients and emotional engagement for those providing their care.
Dr. Hass was trained in family medicine at University of California, San Francisco, after receiving his medical degree from the McGill University faculty of medicine, Montreal. He works as a hospitalist with Sutter Health in Oakland, Calif. He is an adviser on health and health care for the Greater Good Science Center at UC Berkeley and clinical faculty at UCSF School of Medicine. This article appeared initially at The Hospital Leader, the official blog of SHM.
Over the din of the negative pressure machine, I shouted goodbye to my patient and zipped my way out of one of the little plastic enclosures in our ED and carefully shed my gloves, gown, and face shield, leaving on my precious mask. I discarded the rest with disgust and a bit of fear. I thought, “This is a whole new world, and I hate it.”
I feel as if I am constantly battling the fear of dying from COVID-19 but am doing the best I can, given the circumstances at hand. I have the proper equipment and use it well. My work still brings meaning: I serve those in need without hesitation. The problem is that deep feeling of connection with patients, which is such an important part of this work, feels like fraying threads moving further apart because of the havoc this virus has wrought. A few weeks ago, the intricate fabric of what it is to be human connected me to patients through the basics: touch, facial expressions, a physical proximity, and openhearted, honest dialogue. Much of that’s gone, and while I can carry on, I will surely burn out if I can’t figure out how to get at least some of that connection back.
Overwhelmed by the amount of information I need to process daily, I had not been thinking about the interpersonal side of the pandemic for the first weeks. I felt it leaving the ED that morning and later that day, and I felt it again with Ms. Z, who was not even suspected of having COVID. She is a 62-year-old I interviewed with the help of a translator phone. At the end of our encounter, she said “But doctor, will you make my tumor go away?” From across the room, I said, “I will try.” I saw her eyes dampen as I made a hasty exit, following protocol to limit time in the room of all patients.
Typically, leaving a patient’s room, I would feel a fullness associated with a sense of meaning. How did I feel after that? In that moment, mostly ashamed at my lack of compassion during my time with Ms. Z. Then, with further reflection, tense from all things COVID-19! Having an amped-up sympathetic nervous system is understandable, but it’s not where we want to be for our compassion to flow.
We connect best when our parasympathetic nervous system is predominant. So much of the stimuli we need to activate that part of the nervous system is gone. There is a virtuous cycle, much of it unconscious, where something positive leads to more positivity, which is crucial to meaningful patient encounters. We read each other’s facial expressions, hear the tone of voice, and as we pick up subtle cues from our patient, our nervous system is further engaged and our hearts opened.
The specter of COVID-19 has us battling a negative spiral of stress and fear. For the most part, I try to keep that from consuming me, but it clearly saps my energy during encounters. In the same way we need to marshal our resources to battle both the stress and the disease itself, we need to actively engage pro-social elements of providing care to maintain our compassion. Clearly, I needed a more concerted effort to kick start this virtuous cycle of compassion.
My next patient was Ms. J., a 55-year-old with advanced chronic obstructive pulmonary disease (COPD) who came in the night before with shortness of breath. Her slight frame shook from coughing as I entered the room. I did not think she had COVID-19, but we were ruling it out.
We reviewed how she felt since admission, and I performed a hasty exam and stepped back across the room. She coughed again and said, “I feel so weak, and the world feels so crazy; tell it to me straight.” Then looking in my eyes, “I am going to make it, doc?”
I took my cue from her; I walked back to the bedside, placed a gloved hand on her shoulder and with the other, I took her hand. I bent forward just a little. Making eye contact and attempting a comforting tone of voice, I said, “Everyone is a little scared, including me. We need each other more than ever these days. We will do our best for you. That means thoughtful medical care and a whole lot of love! And, truly, I don’t think you are dying; this is just one of your COPD flares.”
“God bless you!” she said, squeezing my hand as a tear rolled down her cheek.
“Bless you, too. We all need blessing with this madness going on,” I replied. Despite the mask, I am sure she saw the smile in my eyes. “Thanks for being the beautiful person you are and opening up to me. That’s the way we will make it through this. I will see you tomorrow.” Backing away, hands together in prayer, I gave a little bow and left the room.
With Ms. J.’s help, I began to figure it out. To tackle the stress of COVID, we need to be very direct – almost to the point of exaggeration – to make sure our words and actions convey what we need to express. William James, the father of psychology, believed that if you force a smile, your emotions would follow. The neural pathways could work backward in that way. He said, “If you want a quality, act as if you have it.” The modern translation would be, “Fake it ’til you make it.’ ” You may be feeling stressed, but with a deep breath and a moment’s reflection on the suffering of that patient you are about to see, you can turn the tide on anxiety and give those under your care what they need.
These are unprecedented times; anxiety abounds. While we can aspire to positivity, there are times when we simply can’t muster showing it. Alternatively, as I experienced with Ms. J., honesty and vulnerability can open the door to meaningful connection. This can be quite powerful when we, as physicians, open up to our patients.
People are yearning for deep connection, and we should attempt to deliver it with:
- Touch (as we can) to convey connection.
- Body language that adds emphasis to our message and our emotions that may go above and beyond what we are used to.
- Tone of voice that enhances our words.
- Talk that emphasizes the big stuff, such as love, fear, connection and community
With gloves, masks, distance, and fear between and us and our patients, we need to actively engage our pro-social tools to turn the negative spiral of fear into the virtuous cycle of positive emotions that promotes healing of our patients and emotional engagement for those providing their care.
Dr. Hass was trained in family medicine at University of California, San Francisco, after receiving his medical degree from the McGill University faculty of medicine, Montreal. He works as a hospitalist with Sutter Health in Oakland, Calif. He is an adviser on health and health care for the Greater Good Science Center at UC Berkeley and clinical faculty at UCSF School of Medicine. This article appeared initially at The Hospital Leader, the official blog of SHM.
Natalizumab bests fingolimod for relapsing-remitting MS
(RRMS). Use of natalizumab was associated with fewer new T2 lesions (0.7 vs 1.4 with fingolimod) and gadolinium-enhancing lesions (0.03 vs. 0.5, respectively) at 12 months, for example.
“The take-home message is that natalizumab showed significant superiority compared to fingolimod on the primary outcome, which was the proportion of patients reaching NEDA [no evidence of disease activity] at 12 months,” lead author Mikael Cohen, MD, said.
“The difference between both drugs was prominent on MRI parameters, especially regarding the number of gadolinium-enhancing lesions,” added Dr. Cohen, of the Department of Neurology at University Hospital Center in Nice, France.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Twelve-month results
The design of the Best Escalation Strategy in MS (BEST MS) study makes it unique, Dr. Cohen said. “It was a prospective and standardized study, unlike most other publications comparing efficacy of those two drugs that were based on retrospective analysis of data registries,” he said. Although BEST MS was an open-label, real-life analysis, the neuroradiologist who analyzed MRI images was blinded to treatment arms, he added.
The multicenter study began in France in 2013, when natalizumab and fingolimod were the two most commonly used agents for active RRMS.
Dr. Cohen and colleagues assessed 230 patients with the condition. The mean age was 38 years, and 75% were women. At the discretion of the treating physician, 113 participants received natalizumab, and 117 were treated with fingolimod.
A multivariate analysis confirmed that fingolimod was associated with a lower likelihood of achieving NEDA at 12 months.
Most relapses occurred early, and the annual relapse rate favored natalizumab, the researchers noted. In addition, the number of discontinuations due to adverse events was higher in the fingolimod group.
“We are working to submit the paper for publication,” Dr. Cohen said. It has also been submitted to the ECTRIMS/ACTRIMS Joint Congress in Washington, DC, for presentation in September 2020.
More tesearch warranted
Commenting on the study, Michelle H. Cameron, MD, said the findings are difficult to interpret because “this was not a randomized controlled trial. Treatment choice was at the discretion of the providers.
“It is hard to know what biases this approach introduced – although it is reassuring that the baseline clinical and radiographic characteristics are described as similar,” said Cameron, codirector of the MS Center of Excellence West at the VA Portland Health Care System, Oregon.
In addition, the superior MRI outcomes at 12 months with natalizumab need to be backed up by clinical outcomes, she said, preferably spanning at least 2 years.
“Overall, these results seem to be consistent with the randomized controlled trials of these individual agents,” Dr. Cameron concluded.
BEST MS was an institutional study and was not funded by any pharmaceutical firm. Dr. Cohen has disclosed no relevant financial relationships. Dr. Cameron is a consultant for Greenwich Biosciences and Adamas Pharmaceuticals.
This article first appeared on Medscape.com.
(RRMS). Use of natalizumab was associated with fewer new T2 lesions (0.7 vs 1.4 with fingolimod) and gadolinium-enhancing lesions (0.03 vs. 0.5, respectively) at 12 months, for example.
“The take-home message is that natalizumab showed significant superiority compared to fingolimod on the primary outcome, which was the proportion of patients reaching NEDA [no evidence of disease activity] at 12 months,” lead author Mikael Cohen, MD, said.
“The difference between both drugs was prominent on MRI parameters, especially regarding the number of gadolinium-enhancing lesions,” added Dr. Cohen, of the Department of Neurology at University Hospital Center in Nice, France.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Twelve-month results
The design of the Best Escalation Strategy in MS (BEST MS) study makes it unique, Dr. Cohen said. “It was a prospective and standardized study, unlike most other publications comparing efficacy of those two drugs that were based on retrospective analysis of data registries,” he said. Although BEST MS was an open-label, real-life analysis, the neuroradiologist who analyzed MRI images was blinded to treatment arms, he added.
The multicenter study began in France in 2013, when natalizumab and fingolimod were the two most commonly used agents for active RRMS.
Dr. Cohen and colleagues assessed 230 patients with the condition. The mean age was 38 years, and 75% were women. At the discretion of the treating physician, 113 participants received natalizumab, and 117 were treated with fingolimod.
A multivariate analysis confirmed that fingolimod was associated with a lower likelihood of achieving NEDA at 12 months.
Most relapses occurred early, and the annual relapse rate favored natalizumab, the researchers noted. In addition, the number of discontinuations due to adverse events was higher in the fingolimod group.
“We are working to submit the paper for publication,” Dr. Cohen said. It has also been submitted to the ECTRIMS/ACTRIMS Joint Congress in Washington, DC, for presentation in September 2020.
More tesearch warranted
Commenting on the study, Michelle H. Cameron, MD, said the findings are difficult to interpret because “this was not a randomized controlled trial. Treatment choice was at the discretion of the providers.
“It is hard to know what biases this approach introduced – although it is reassuring that the baseline clinical and radiographic characteristics are described as similar,” said Cameron, codirector of the MS Center of Excellence West at the VA Portland Health Care System, Oregon.
In addition, the superior MRI outcomes at 12 months with natalizumab need to be backed up by clinical outcomes, she said, preferably spanning at least 2 years.
“Overall, these results seem to be consistent with the randomized controlled trials of these individual agents,” Dr. Cameron concluded.
BEST MS was an institutional study and was not funded by any pharmaceutical firm. Dr. Cohen has disclosed no relevant financial relationships. Dr. Cameron is a consultant for Greenwich Biosciences and Adamas Pharmaceuticals.
This article first appeared on Medscape.com.
(RRMS). Use of natalizumab was associated with fewer new T2 lesions (0.7 vs 1.4 with fingolimod) and gadolinium-enhancing lesions (0.03 vs. 0.5, respectively) at 12 months, for example.
“The take-home message is that natalizumab showed significant superiority compared to fingolimod on the primary outcome, which was the proportion of patients reaching NEDA [no evidence of disease activity] at 12 months,” lead author Mikael Cohen, MD, said.
“The difference between both drugs was prominent on MRI parameters, especially regarding the number of gadolinium-enhancing lesions,” added Dr. Cohen, of the Department of Neurology at University Hospital Center in Nice, France.
This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Twelve-month results
The design of the Best Escalation Strategy in MS (BEST MS) study makes it unique, Dr. Cohen said. “It was a prospective and standardized study, unlike most other publications comparing efficacy of those two drugs that were based on retrospective analysis of data registries,” he said. Although BEST MS was an open-label, real-life analysis, the neuroradiologist who analyzed MRI images was blinded to treatment arms, he added.
The multicenter study began in France in 2013, when natalizumab and fingolimod were the two most commonly used agents for active RRMS.
Dr. Cohen and colleagues assessed 230 patients with the condition. The mean age was 38 years, and 75% were women. At the discretion of the treating physician, 113 participants received natalizumab, and 117 were treated with fingolimod.
A multivariate analysis confirmed that fingolimod was associated with a lower likelihood of achieving NEDA at 12 months.
Most relapses occurred early, and the annual relapse rate favored natalizumab, the researchers noted. In addition, the number of discontinuations due to adverse events was higher in the fingolimod group.
“We are working to submit the paper for publication,” Dr. Cohen said. It has also been submitted to the ECTRIMS/ACTRIMS Joint Congress in Washington, DC, for presentation in September 2020.
More tesearch warranted
Commenting on the study, Michelle H. Cameron, MD, said the findings are difficult to interpret because “this was not a randomized controlled trial. Treatment choice was at the discretion of the providers.
“It is hard to know what biases this approach introduced – although it is reassuring that the baseline clinical and radiographic characteristics are described as similar,” said Cameron, codirector of the MS Center of Excellence West at the VA Portland Health Care System, Oregon.
In addition, the superior MRI outcomes at 12 months with natalizumab need to be backed up by clinical outcomes, she said, preferably spanning at least 2 years.
“Overall, these results seem to be consistent with the randomized controlled trials of these individual agents,” Dr. Cameron concluded.
BEST MS was an institutional study and was not funded by any pharmaceutical firm. Dr. Cohen has disclosed no relevant financial relationships. Dr. Cameron is a consultant for Greenwich Biosciences and Adamas Pharmaceuticals.
This article first appeared on Medscape.com.
First-Line Treatment of IDA in NDD-CKD
In this supplement to Internal Medicine News, Kamyar Kalantar-Zadeh, MD, MPH, PhD, discusses a first-line treatment option for iron deficiency anemia (IDA) in patients with non-dialysis-dependent chronic kidney disease (NDD-CKD). Topics include:
- The interplay between IDA and CKD
- Recognizing IDA in patients with CKD
- First-line treatment efficacy and safety information
In this supplement to Internal Medicine News, Kamyar Kalantar-Zadeh, MD, MPH, PhD, discusses a first-line treatment option for iron deficiency anemia (IDA) in patients with non-dialysis-dependent chronic kidney disease (NDD-CKD). Topics include:
- The interplay between IDA and CKD
- Recognizing IDA in patients with CKD
- First-line treatment efficacy and safety information
In this supplement to Internal Medicine News, Kamyar Kalantar-Zadeh, MD, MPH, PhD, discusses a first-line treatment option for iron deficiency anemia (IDA) in patients with non-dialysis-dependent chronic kidney disease (NDD-CKD). Topics include:
- The interplay between IDA and CKD
- Recognizing IDA in patients with CKD
- First-line treatment efficacy and safety information
A toddler with a fever and desquamating perineal rash
Kawasaki disease
Given (KD). An echocardiogram revealed diffuse dilation of the left anterior descending artery without evidence of an aneurysm. The patient was promptly started on 2 g/kg IVIG and high-dose aspirin. She was later transitioned to low-dose aspirin. Long-term follow-up thus far has revealed no cardiac sequelae.
KD, or mucocutaneous lymph node syndrome, is a multisystem vasculitis with predilection for the coronary arteries that most commonly affects children between 6 months and 5 years of age.1 While the etiology remains unclear, the pathogenesis is thought to be the result of an immune response to an infection in the setting of genetic susceptibility.1 Approximately 90% of patients have mucocutaneous manifestations, highlighting the important role dermatologists play in the diagnosis and early intervention to prevent cardiovascular morbidity.
The diagnostic criteria include fever for at least 5 days accompanied by at least four of the following:
- Bilateral bulbar conjunctival injection without exudate that is classically limbal sparing.
- Oral mucosal changes with cracked fissured lips, “strawberry tongue,” or erythema of the lips and mucosa.
- Changes in the extremities: erythema, swelling, or periungual peeling.
- Polymorphous exanthem.
- Cervical lymphadenopathy, often unilateral (greater than 1.5 cm).
Although nonspecific for diagnosis, laboratory abnormalities are common, including anemia, thrombocytosis, leukocytosis, elevated inflammatory markers, elevated alanine aminotransferase (ALT), hypoalbuminemia, and sterile pyuria on urine analysis.1
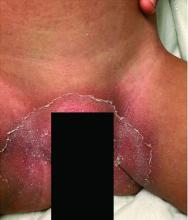
Notably, a classic finding of KD is perineal dermatitis with desquamation occurring in the acute phase of disease in 80%-90% of patients.2-5 In a retrospective review, up to 67% of patients with KD developed a perineal rash in the first week, most often beginning in the diaper area.2 The perineal rash classically desquamates early during the acute phase of the disease.1
While most individuals with KD follow a benign disease course, it is the most common cause of acquired heart disease in the United States.1 Treatment is aimed at decreasing the risk of developing coronary abnormalities through the prompt administration of IVIG and high-dose aspirin initiated early in the acute phase.6 A second dose of IVIG may be given to patients who remain febrile within 24-48 hours after treatment.6 Infliximab has been used safely and effectively in patients with refractory KD.7 Long-term cardiac follow-up of KD patients is recommended.
Recently, there has been an emerging association between COVID-19 and pediatric multi-system inflammatory syndrome, which shares features with KD. Patients with pediatric multi-system inflammatory syndrome who meet clinical criteria for KD should be promptly treated with IVIG and aspirin to avoid long-term cardiac sequelae.
This case and the photos were submitted by Dr. Elizabeth H. Cusick and Dr. Molly E. Plovanich, both with the department of dermatology at the University of Rochester (N.Y.). Dr. Donna Bilu Martin edited the case.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Bayers S et al. (2013). J Am Acad Dermatol. 2013 Oct;69(4):501.e1-11.
2. Friter BS and Lucky AW. Arch Dermatol. 1988 Dec;124(12):1805-10.
3. Urbach AH et al. Am J Dis Child. 1988 Nov;142(11):1174-6.
4. Fink CW. Pediatr Infect Dis. 1983 Mar-Apr; 2(2):140-1.
5. Aballi A J and Bisken LC. Pediatr Infect Dis. 1984 Mar-Apr;3(2):187.
6. McCrindle BW et al. Circulation. 2017 Apr 25;135(17):e927-e99.
7.Sauvaget E et al. J Pediatr. 2012 May; 160(5),875-6.
Kawasaki disease
Given (KD). An echocardiogram revealed diffuse dilation of the left anterior descending artery without evidence of an aneurysm. The patient was promptly started on 2 g/kg IVIG and high-dose aspirin. She was later transitioned to low-dose aspirin. Long-term follow-up thus far has revealed no cardiac sequelae.
KD, or mucocutaneous lymph node syndrome, is a multisystem vasculitis with predilection for the coronary arteries that most commonly affects children between 6 months and 5 years of age.1 While the etiology remains unclear, the pathogenesis is thought to be the result of an immune response to an infection in the setting of genetic susceptibility.1 Approximately 90% of patients have mucocutaneous manifestations, highlighting the important role dermatologists play in the diagnosis and early intervention to prevent cardiovascular morbidity.
The diagnostic criteria include fever for at least 5 days accompanied by at least four of the following:
- Bilateral bulbar conjunctival injection without exudate that is classically limbal sparing.
- Oral mucosal changes with cracked fissured lips, “strawberry tongue,” or erythema of the lips and mucosa.
- Changes in the extremities: erythema, swelling, or periungual peeling.
- Polymorphous exanthem.
- Cervical lymphadenopathy, often unilateral (greater than 1.5 cm).
Although nonspecific for diagnosis, laboratory abnormalities are common, including anemia, thrombocytosis, leukocytosis, elevated inflammatory markers, elevated alanine aminotransferase (ALT), hypoalbuminemia, and sterile pyuria on urine analysis.1
Notably, a classic finding of KD is perineal dermatitis with desquamation occurring in the acute phase of disease in 80%-90% of patients.2-5 In a retrospective review, up to 67% of patients with KD developed a perineal rash in the first week, most often beginning in the diaper area.2 The perineal rash classically desquamates early during the acute phase of the disease.1
While most individuals with KD follow a benign disease course, it is the most common cause of acquired heart disease in the United States.1 Treatment is aimed at decreasing the risk of developing coronary abnormalities through the prompt administration of IVIG and high-dose aspirin initiated early in the acute phase.6 A second dose of IVIG may be given to patients who remain febrile within 24-48 hours after treatment.6 Infliximab has been used safely and effectively in patients with refractory KD.7 Long-term cardiac follow-up of KD patients is recommended.
Recently, there has been an emerging association between COVID-19 and pediatric multi-system inflammatory syndrome, which shares features with KD. Patients with pediatric multi-system inflammatory syndrome who meet clinical criteria for KD should be promptly treated with IVIG and aspirin to avoid long-term cardiac sequelae.
This case and the photos were submitted by Dr. Elizabeth H. Cusick and Dr. Molly E. Plovanich, both with the department of dermatology at the University of Rochester (N.Y.). Dr. Donna Bilu Martin edited the case.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Bayers S et al. (2013). J Am Acad Dermatol. 2013 Oct;69(4):501.e1-11.
2. Friter BS and Lucky AW. Arch Dermatol. 1988 Dec;124(12):1805-10.
3. Urbach AH et al. Am J Dis Child. 1988 Nov;142(11):1174-6.
4. Fink CW. Pediatr Infect Dis. 1983 Mar-Apr; 2(2):140-1.
5. Aballi A J and Bisken LC. Pediatr Infect Dis. 1984 Mar-Apr;3(2):187.
6. McCrindle BW et al. Circulation. 2017 Apr 25;135(17):e927-e99.
7.Sauvaget E et al. J Pediatr. 2012 May; 160(5),875-6.
Kawasaki disease
Given (KD). An echocardiogram revealed diffuse dilation of the left anterior descending artery without evidence of an aneurysm. The patient was promptly started on 2 g/kg IVIG and high-dose aspirin. She was later transitioned to low-dose aspirin. Long-term follow-up thus far has revealed no cardiac sequelae.
KD, or mucocutaneous lymph node syndrome, is a multisystem vasculitis with predilection for the coronary arteries that most commonly affects children between 6 months and 5 years of age.1 While the etiology remains unclear, the pathogenesis is thought to be the result of an immune response to an infection in the setting of genetic susceptibility.1 Approximately 90% of patients have mucocutaneous manifestations, highlighting the important role dermatologists play in the diagnosis and early intervention to prevent cardiovascular morbidity.
The diagnostic criteria include fever for at least 5 days accompanied by at least four of the following:
- Bilateral bulbar conjunctival injection without exudate that is classically limbal sparing.
- Oral mucosal changes with cracked fissured lips, “strawberry tongue,” or erythema of the lips and mucosa.
- Changes in the extremities: erythema, swelling, or periungual peeling.
- Polymorphous exanthem.
- Cervical lymphadenopathy, often unilateral (greater than 1.5 cm).
Although nonspecific for diagnosis, laboratory abnormalities are common, including anemia, thrombocytosis, leukocytosis, elevated inflammatory markers, elevated alanine aminotransferase (ALT), hypoalbuminemia, and sterile pyuria on urine analysis.1
Notably, a classic finding of KD is perineal dermatitis with desquamation occurring in the acute phase of disease in 80%-90% of patients.2-5 In a retrospective review, up to 67% of patients with KD developed a perineal rash in the first week, most often beginning in the diaper area.2 The perineal rash classically desquamates early during the acute phase of the disease.1
While most individuals with KD follow a benign disease course, it is the most common cause of acquired heart disease in the United States.1 Treatment is aimed at decreasing the risk of developing coronary abnormalities through the prompt administration of IVIG and high-dose aspirin initiated early in the acute phase.6 A second dose of IVIG may be given to patients who remain febrile within 24-48 hours after treatment.6 Infliximab has been used safely and effectively in patients with refractory KD.7 Long-term cardiac follow-up of KD patients is recommended.
Recently, there has been an emerging association between COVID-19 and pediatric multi-system inflammatory syndrome, which shares features with KD. Patients with pediatric multi-system inflammatory syndrome who meet clinical criteria for KD should be promptly treated with IVIG and aspirin to avoid long-term cardiac sequelae.
This case and the photos were submitted by Dr. Elizabeth H. Cusick and Dr. Molly E. Plovanich, both with the department of dermatology at the University of Rochester (N.Y.). Dr. Donna Bilu Martin edited the case.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Bayers S et al. (2013). J Am Acad Dermatol. 2013 Oct;69(4):501.e1-11.
2. Friter BS and Lucky AW. Arch Dermatol. 1988 Dec;124(12):1805-10.
3. Urbach AH et al. Am J Dis Child. 1988 Nov;142(11):1174-6.
4. Fink CW. Pediatr Infect Dis. 1983 Mar-Apr; 2(2):140-1.
5. Aballi A J and Bisken LC. Pediatr Infect Dis. 1984 Mar-Apr;3(2):187.
6. McCrindle BW et al. Circulation. 2017 Apr 25;135(17):e927-e99.
7.Sauvaget E et al. J Pediatr. 2012 May; 160(5),875-6.
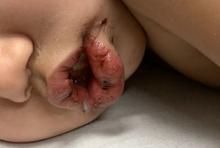
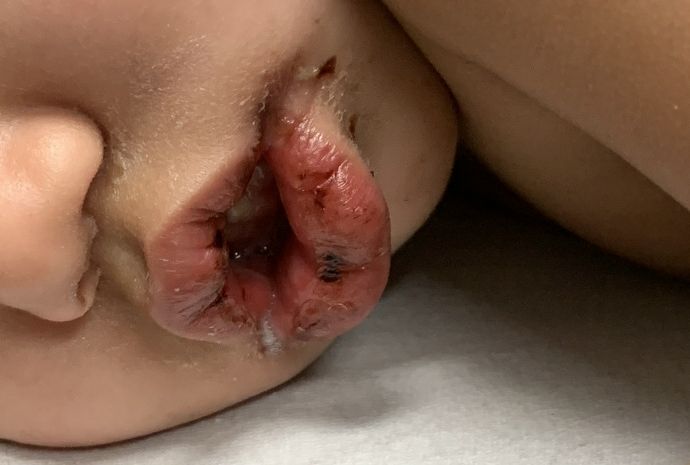
An otherwise healthy 18-month-old female presented to the emergency department with 5 days of fever, erythema, fissuring of the lips, conjunctival injection, and a desquamating perineal rash. In addition, she had nasal congestion and cough for which she was started on amoxicillin 2 days prior to presentation given concern for pneumonia.
On exam, she was also noted to have several palpable cervical lymph nodes and edematous hands with overlying erythema. Laboratory evaluation was notable for respiratory syncytial virus positivity by polymerase chain reaction assay, leukocytosis, and elevated inflammatory markers (erythrocyte sedimentation rate and C-reactive protein).
Yoga is a good adjunct to migraine therapy
in Neurology.
The structured yoga program resulted in “remarkably improved” outcomes at 3 months of follow-up in CONTAIN, with both headache frequency and use of medications cut in half, compared with baseline, according to the investigators.
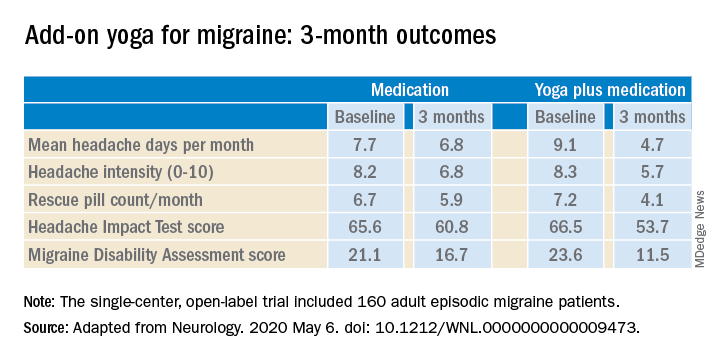
Compared with the control group on standard antimigraine medications alone, the yoga group demonstrated significantly greater reductions in pain intensity, headache frequency, pill counts, and validated measures of disability and headache impact on daily life (see graphic).
“The good news is that practicing something as simple and accessible as yoga may help much more than medications alone. And all you need is a mat,” observed Dr. Bhatia, professor of neurology at the All India Institute of Medical Sciences in New Delhi.
The single-center, open-label, blinded-assessment CONTAIN trial included 160 adult episodic migraine patients ages 18-50 years experiencing 4-14 headaches per month. They were randomized to prophylactic and acute rescue medications alone or in combination with yoga instruction by a qualified yoga therapist in a class that met at the medical center 3 days per week for 1 month. This was followed by practice of the hour-long yoga program at home 5 days per week for the next 2 months, with twice-monthly telephone calls from the yoga center to encourage adherence and encouragement to call if questions arose. Both groups received counseling about the importance of lifestyle changes that may help with migraine, including diet, physical activity, adequate sleep, and stress reduction. Outcomes were assessed in an intent-to-treat analysis.
The yoga program included specific relaxation exercises, breathing techniques, meditation, and yoga postures, or asanas. The migraine-tailored program was vetted by yoga experts at five renowned Indian yoga centers.
No safety issues arose with the yoga program.
The investigators noted that the 47% reduction in migraine medication pill count and 49% decrease in headache frequency over the course of 3 months in the adjunctive yoga group have important implications, not only in a limited-resource country such as India, but also in the United States, where Americans spend an estimated $3.2 billion annually on prescription and over the counter headache medications, and the indirect cost associated with lost productivity due to migraine has been put at $13 billion per year.
Dr. Bhatia and colleagues speculated that the observed benefits of add-on yoga in migraineurs may involve previously described improved vagal tone and parasympathetic drive coupled with decreased sympathetic tone, increased nitric oxide levels, and loosening of stiff muscles, which can trigger headaches.
Real-life goals
Commenting on the research, neurologist Holly Yancy, DO, a headache specialist at the Banner Health - University Medicine Neuroscience Institute in Phoenix, said she was impressed by the high quality of this well-designed, adequately powered study of a complementary and alternative therapy.
“The primary and secondary endpoints were real-life goals of migraine treatment that we strive to achieve in clinical practice – and they were met in the study,” she observed. “To start with a month of in-house yoga classes to instill a baseline competence in yoga prior to transitioning to home practice and to provide resources for ongoing assistance for questions were nice touches.”
She noted that the control group also experienced reductions in migraine frequency, severity, and disability scores, albeit of significantly lesser magnitude than in the yoga group. This underscores how important it is in clinical practice to spend time counseling migraine patients on lifestyle choices.
“A trial such as this provides neurologists and other health care providers with an accessible, evidence-based treatment for migraines that can be used with other preventive treatments to decrease the frequency and the amount of medication their patients are taking. In addition, it is a behavioral therapy that can decrease triggers and potentially help patients cope with pain,” Dr. Yancy said.
“I suspect I’ll not hesitate to recommend yoga as an adjunctive treatment for patients in my clinic that are physically capable. I think it would be logical to try to extrapolate the concept to a chronic migraine population as well, though it would be ideal to base that recommendation on another study conducted with a chronic migraine population.”
Dr. Bhatia and his coinvestigators reported having no financial conflicts regarding their study, funded by the Government of India and the All India Institute of Medical Sciences.
SOURCE: Kumar A et al. Neurology. 2020 May 6. doi: 10.1212/WNL.0000000000009473.
in Neurology.

The structured yoga program resulted in “remarkably improved” outcomes at 3 months of follow-up in CONTAIN, with both headache frequency and use of medications cut in half, compared with baseline, according to the investigators.
Compared with the control group on standard antimigraine medications alone, the yoga group demonstrated significantly greater reductions in pain intensity, headache frequency, pill counts, and validated measures of disability and headache impact on daily life (see graphic).
“The good news is that practicing something as simple and accessible as yoga may help much more than medications alone. And all you need is a mat,” observed Dr. Bhatia, professor of neurology at the All India Institute of Medical Sciences in New Delhi.
The single-center, open-label, blinded-assessment CONTAIN trial included 160 adult episodic migraine patients ages 18-50 years experiencing 4-14 headaches per month. They were randomized to prophylactic and acute rescue medications alone or in combination with yoga instruction by a qualified yoga therapist in a class that met at the medical center 3 days per week for 1 month. This was followed by practice of the hour-long yoga program at home 5 days per week for the next 2 months, with twice-monthly telephone calls from the yoga center to encourage adherence and encouragement to call if questions arose. Both groups received counseling about the importance of lifestyle changes that may help with migraine, including diet, physical activity, adequate sleep, and stress reduction. Outcomes were assessed in an intent-to-treat analysis.
The yoga program included specific relaxation exercises, breathing techniques, meditation, and yoga postures, or asanas. The migraine-tailored program was vetted by yoga experts at five renowned Indian yoga centers.
No safety issues arose with the yoga program.
The investigators noted that the 47% reduction in migraine medication pill count and 49% decrease in headache frequency over the course of 3 months in the adjunctive yoga group have important implications, not only in a limited-resource country such as India, but also in the United States, where Americans spend an estimated $3.2 billion annually on prescription and over the counter headache medications, and the indirect cost associated with lost productivity due to migraine has been put at $13 billion per year.
Dr. Bhatia and colleagues speculated that the observed benefits of add-on yoga in migraineurs may involve previously described improved vagal tone and parasympathetic drive coupled with decreased sympathetic tone, increased nitric oxide levels, and loosening of stiff muscles, which can trigger headaches.
Real-life goals
Commenting on the research, neurologist Holly Yancy, DO, a headache specialist at the Banner Health - University Medicine Neuroscience Institute in Phoenix, said she was impressed by the high quality of this well-designed, adequately powered study of a complementary and alternative therapy.
“The primary and secondary endpoints were real-life goals of migraine treatment that we strive to achieve in clinical practice – and they were met in the study,” she observed. “To start with a month of in-house yoga classes to instill a baseline competence in yoga prior to transitioning to home practice and to provide resources for ongoing assistance for questions were nice touches.”
She noted that the control group also experienced reductions in migraine frequency, severity, and disability scores, albeit of significantly lesser magnitude than in the yoga group. This underscores how important it is in clinical practice to spend time counseling migraine patients on lifestyle choices.
“A trial such as this provides neurologists and other health care providers with an accessible, evidence-based treatment for migraines that can be used with other preventive treatments to decrease the frequency and the amount of medication their patients are taking. In addition, it is a behavioral therapy that can decrease triggers and potentially help patients cope with pain,” Dr. Yancy said.
“I suspect I’ll not hesitate to recommend yoga as an adjunctive treatment for patients in my clinic that are physically capable. I think it would be logical to try to extrapolate the concept to a chronic migraine population as well, though it would be ideal to base that recommendation on another study conducted with a chronic migraine population.”
Dr. Bhatia and his coinvestigators reported having no financial conflicts regarding their study, funded by the Government of India and the All India Institute of Medical Sciences.
SOURCE: Kumar A et al. Neurology. 2020 May 6. doi: 10.1212/WNL.0000000000009473.
in Neurology.

The structured yoga program resulted in “remarkably improved” outcomes at 3 months of follow-up in CONTAIN, with both headache frequency and use of medications cut in half, compared with baseline, according to the investigators.
Compared with the control group on standard antimigraine medications alone, the yoga group demonstrated significantly greater reductions in pain intensity, headache frequency, pill counts, and validated measures of disability and headache impact on daily life (see graphic).
“The good news is that practicing something as simple and accessible as yoga may help much more than medications alone. And all you need is a mat,” observed Dr. Bhatia, professor of neurology at the All India Institute of Medical Sciences in New Delhi.
The single-center, open-label, blinded-assessment CONTAIN trial included 160 adult episodic migraine patients ages 18-50 years experiencing 4-14 headaches per month. They were randomized to prophylactic and acute rescue medications alone or in combination with yoga instruction by a qualified yoga therapist in a class that met at the medical center 3 days per week for 1 month. This was followed by practice of the hour-long yoga program at home 5 days per week for the next 2 months, with twice-monthly telephone calls from the yoga center to encourage adherence and encouragement to call if questions arose. Both groups received counseling about the importance of lifestyle changes that may help with migraine, including diet, physical activity, adequate sleep, and stress reduction. Outcomes were assessed in an intent-to-treat analysis.
The yoga program included specific relaxation exercises, breathing techniques, meditation, and yoga postures, or asanas. The migraine-tailored program was vetted by yoga experts at five renowned Indian yoga centers.
No safety issues arose with the yoga program.
The investigators noted that the 47% reduction in migraine medication pill count and 49% decrease in headache frequency over the course of 3 months in the adjunctive yoga group have important implications, not only in a limited-resource country such as India, but also in the United States, where Americans spend an estimated $3.2 billion annually on prescription and over the counter headache medications, and the indirect cost associated with lost productivity due to migraine has been put at $13 billion per year.
Dr. Bhatia and colleagues speculated that the observed benefits of add-on yoga in migraineurs may involve previously described improved vagal tone and parasympathetic drive coupled with decreased sympathetic tone, increased nitric oxide levels, and loosening of stiff muscles, which can trigger headaches.
Real-life goals
Commenting on the research, neurologist Holly Yancy, DO, a headache specialist at the Banner Health - University Medicine Neuroscience Institute in Phoenix, said she was impressed by the high quality of this well-designed, adequately powered study of a complementary and alternative therapy.
“The primary and secondary endpoints were real-life goals of migraine treatment that we strive to achieve in clinical practice – and they were met in the study,” she observed. “To start with a month of in-house yoga classes to instill a baseline competence in yoga prior to transitioning to home practice and to provide resources for ongoing assistance for questions were nice touches.”
She noted that the control group also experienced reductions in migraine frequency, severity, and disability scores, albeit of significantly lesser magnitude than in the yoga group. This underscores how important it is in clinical practice to spend time counseling migraine patients on lifestyle choices.
“A trial such as this provides neurologists and other health care providers with an accessible, evidence-based treatment for migraines that can be used with other preventive treatments to decrease the frequency and the amount of medication their patients are taking. In addition, it is a behavioral therapy that can decrease triggers and potentially help patients cope with pain,” Dr. Yancy said.
“I suspect I’ll not hesitate to recommend yoga as an adjunctive treatment for patients in my clinic that are physically capable. I think it would be logical to try to extrapolate the concept to a chronic migraine population as well, though it would be ideal to base that recommendation on another study conducted with a chronic migraine population.”
Dr. Bhatia and his coinvestigators reported having no financial conflicts regarding their study, funded by the Government of India and the All India Institute of Medical Sciences.
SOURCE: Kumar A et al. Neurology. 2020 May 6. doi: 10.1212/WNL.0000000000009473.
FROM NEUROLOGY
‘Momentous’ data for first-line combo in liver cancer
New clinical data are set to change the treatment landscape for advanced liver cancer.
The data showed that atezolizumab plus bevacizumab improved survival, when compared with sorafenib, in patients with unresectable hepatocellular carcinoma (HCC).
The advanced liver cancer space has been dominated for a more than a decade by sorafenib (Nexavar, Bayer), which was the first systemic therapy to confer “a meaningful survival benefit in the treatment of advanced hepatocellular carcinoma,” notes Robin K. Kelley, MD, from the University of California, San Francisco.
“Since then, no treatment had surpassed the effect of sorafenib in the first line until the regimen of atezolizumab and bevacizumab” that is now being reported, she notes.
The new data come from the IMbrave150 study, published on May 14 in the New England Journal of Medicine.
“The combination of atezolizumab plus bevacizumab has become the new benchmark for first-line therapy in advanced hepatocellular carcinoma,” Kelley writes in an accompanying editorial.
“These data are momentous, since they identify not only the first therapy to improve survival beyond sorafenib, but also the first successful combination regimen and the first positive randomized, phase 3 trial of immune checkpoint inhibition in this challenging cancer,” she added.
The IMbrave 150 study was sponsored by Roche, manufacturer of both the checkpoint inhibitor atezolizumab (Tecentriq, Genentech/Roche) and the antiangiogenic agent bevacizumab (Avastin, Genentech/Roche); the company has submitted an FDA approval application for use of this combination for inoperable liver cancer.
'Results represent a breakthrough'
“These results represent a breakthrough in the management of advanced HCC,” said Josep M. Llovet, MD, PhD, director of the Mount Sinai Liver Cancer Program, Icahn School of Medicine at Mount Sinai, New York, and professor of medicine in hepatic oncology at the University of Barcelona, Spain.
The combination has already been acknowledged as a milestone in the management of HCC, he said.
Llovet was approached for comment by Medscape Medical News. He was not involved with IMbrave150 but was the lead author on the SHARP study, which led to the first-line approval of sorafenib.
He explained that, since the approval of sorafenib in 2008, lenvatinib (Lenvima, Eisai) has been the only other agent approved for the front-line treatment of HCC after hitting the noninferiority endpoint for survival in comparison with sorafenib. “Up to now, there was no agent superior to sorafenib, the standard of care,” he said.
Now, the combination of atezolizumab-bevacizumab has shown superior efficacy compared with sorafenib, Llovet noted. It is not only the first combination to show efficacy but is also the first checkpoint inhibitor that has demonstrated efficacy in HCC. “Previous studies of checkpoint inhibitors used as single agents in the front-line or second-line setting of advanced liver cancer were negative,” he said.
'Game-changer' in liver cancer
“The atezolizumab-bevacizumab combination is a game-changer in liver cancer,” the lead author of the IMbrave 150 trial, Richard S. Finn, MD, of the David Geffen School of Medicine at the University of California, Los Angeles, told Medscape Medical News.
“The combination has established a new standard of care that is predicated on the gold standard of overall survival [OS] and is underscored by prolonged progression-free survival [PFS] and high response rates that are durable,” Finn said.
In the IMbrave150 trial, treatment-naive patients who had unresectable liver cancer received either atezolizumab-bevacizumab (n = 336) or sorafenib (n = 165).
After a median follow-up of 8.6 months, median survival was significantly longer for the patients who received atezolizumab-bevacizumab: 13.2 months. For the patients who did not receive the combination, median survival was not reached (hazard ratio [HR], 0.58; P < .001). Six-month OS was 84.8% with the combination versus 72.2% with sorafenib.
Median PFS was also significantly longer for patients who received combination therapy: 6.8 months with the combination versus 4.3 months with sorafenib (HR, 0.59; P < .001). Six-month PFS was 54.4% with the combination versus 37.3% with sorafenib.
The objective response rate was 27.3% (complete response, 5.5%) with the combination versus 11.9% (complete response, 0%) with sorafenib
Median time to deterioration of quality of life was also longer for patients who received combination therapy: 11.2 months with atezolizumab-bevacizumab and 3.6 months for sorafenib.
Incidence of grade 3 or 4 adverse events was similar in both arms of the study: 56.5% for the combination versus 55.1% with sorafenib. Adverse events leading to withdrawal from any study drug was not significantly different: 15.5% versus 10.3% for sorafenib.
The percentage of patients who experienced bleeding of any grade (attributed to bevacizumab) was 25.2% with the combination versus 17.3% with sorafenib. In addition, six incidents of fatal bleeding or perforated ulcer were recorded in the combination group, compared with one for the sorafenib group.
Appropriate for all patients?
Llovet told Medscape Medical News that the combination of atezolizumab and bevacizumab, although still awaiting approval for use in liver cancer, will be adopted by guidelines in the management of HCC as first-line therapy.
It has been accepted as the new front-line standard of care in a soon-to-be-published consensus on trial design and endpoints in HCC that he has authored.
Llovet said that the intravenous (IV) dosing of the combination was not likely to be a problem (sorafenib is administered orally). For patients with untreated advanced HCC, median survival is 8 months; it is 11-13 months with sorafenib. With this combination, the median was not reached, but it is expected to be beyond 17 months. “In this scenario, IV versus oral dosing will only have implications if the treatments had similar efficacy but not significantly better performance,” he said.
In her editorial, Kelley suggests caution when considering use of the combination in a patient population broader than that defined by the IMbrave150 study.
She points out that patients in IMbrave150 were required to have well-compensated liver disease (Child-Pugh class A liver function), and patients with untreated or incompletely treated esophageal or gastric varices with bleeding or those who were at high risk of bleeding were excluded from the study.
“Safety has not been established for persons in the Childs-Pugh class B population, and alternative therapies should be considered for patients at high risk for bleeding,” Kelley writes in her editorial.
Bleeding events, including fatal bleeding and perforated ulcers, “underscore important considerations for the application of the atezolizumab-bevacizumab regimen to a broader population beyond the clinical trial setting,” Kelley noted.
She recommends that all patients at risk for varices undergo “appropriate endoscopic evaluation and management before treatment is initiated.”
Llovet agreed and noted that upper endoscopy is not currently practiced in the management of advanced HCC. “One important issue in the clinical practice will be that all patients require an upper endoscopy to rule out esophageal varices, which are at risk of bleeding with bevacizumab,” he said.
Trial investigator Finn explained that IMbrave 150 is not unique and that every phase 3 study has included patients with Child-Pugh class A liver function.
“Patients with Child-Pugh class B are a heterogeneous group of patients,” Finn said. Physicians should use their judgment in providing this combination to patients with Child-Pugh scores of 7–9, he added. “All patients with advanced liver cancer need an endoscopy,” Finn said.
Kelley and Llovet also observed that several ongoing trials, some of which have been completed, are evaluating combinations of immunotherapy and other antiangiogenic agents or combinations of immunotherapies. The results from these trials will inform how such combinations will be used in the future.
Kelley predicted that, if some of these trials are positive, in the absence of a predictive biomarker, physicians will be guided by “the safety profiles of the combinations as well as the inference of synergy on the basis of depth and durability of responses.”
“Results of these trials will be known within the next 12-18 months and might further improve the current standards for patients with inoperable HCC,” Llovet said.
IMbrave150 was sponsored by F. Hoffmann–La Roche/Genentech. Finn has consulted for AtraZeneca, Bayer, Bristol-Myers Squibb, CStone, Eisai, Eli Lilly, Exelixis, Roche, Genentech, Merck & Co, Novartis, and Pfizer. Finn’s coauthors also report relationships with pharmaceutical companies. Kelley reports institutional research support from many pharmaceutical companies and served on the independent data monitoring committee for the IMbrave150 trial. Llovet has received research support from Bayer HealthCare Pharmaceuticals, Eisai, Bristol-Myers Squibb, Boehringer Ingelheim, and Ipsen and consulting fees from Bayer HealthCare Pharmaceuticals, Merck, Eisai Inc, Bristol-Myers Squibb, Celsion Corporation, Eli Lilly, Roche, Genentech, Glycotest, Nucleix, Can-Fite Biopharma, AstraZeneca, and Exelixis.
This article first appeared on Medscape.com.
New clinical data are set to change the treatment landscape for advanced liver cancer.
The data showed that atezolizumab plus bevacizumab improved survival, when compared with sorafenib, in patients with unresectable hepatocellular carcinoma (HCC).
The advanced liver cancer space has been dominated for a more than a decade by sorafenib (Nexavar, Bayer), which was the first systemic therapy to confer “a meaningful survival benefit in the treatment of advanced hepatocellular carcinoma,” notes Robin K. Kelley, MD, from the University of California, San Francisco.
“Since then, no treatment had surpassed the effect of sorafenib in the first line until the regimen of atezolizumab and bevacizumab” that is now being reported, she notes.
The new data come from the IMbrave150 study, published on May 14 in the New England Journal of Medicine.
“The combination of atezolizumab plus bevacizumab has become the new benchmark for first-line therapy in advanced hepatocellular carcinoma,” Kelley writes in an accompanying editorial.
“These data are momentous, since they identify not only the first therapy to improve survival beyond sorafenib, but also the first successful combination regimen and the first positive randomized, phase 3 trial of immune checkpoint inhibition in this challenging cancer,” she added.
The IMbrave 150 study was sponsored by Roche, manufacturer of both the checkpoint inhibitor atezolizumab (Tecentriq, Genentech/Roche) and the antiangiogenic agent bevacizumab (Avastin, Genentech/Roche); the company has submitted an FDA approval application for use of this combination for inoperable liver cancer.
'Results represent a breakthrough'
“These results represent a breakthrough in the management of advanced HCC,” said Josep M. Llovet, MD, PhD, director of the Mount Sinai Liver Cancer Program, Icahn School of Medicine at Mount Sinai, New York, and professor of medicine in hepatic oncology at the University of Barcelona, Spain.
The combination has already been acknowledged as a milestone in the management of HCC, he said.
Llovet was approached for comment by Medscape Medical News. He was not involved with IMbrave150 but was the lead author on the SHARP study, which led to the first-line approval of sorafenib.
He explained that, since the approval of sorafenib in 2008, lenvatinib (Lenvima, Eisai) has been the only other agent approved for the front-line treatment of HCC after hitting the noninferiority endpoint for survival in comparison with sorafenib. “Up to now, there was no agent superior to sorafenib, the standard of care,” he said.
Now, the combination of atezolizumab-bevacizumab has shown superior efficacy compared with sorafenib, Llovet noted. It is not only the first combination to show efficacy but is also the first checkpoint inhibitor that has demonstrated efficacy in HCC. “Previous studies of checkpoint inhibitors used as single agents in the front-line or second-line setting of advanced liver cancer were negative,” he said.
'Game-changer' in liver cancer
“The atezolizumab-bevacizumab combination is a game-changer in liver cancer,” the lead author of the IMbrave 150 trial, Richard S. Finn, MD, of the David Geffen School of Medicine at the University of California, Los Angeles, told Medscape Medical News.
“The combination has established a new standard of care that is predicated on the gold standard of overall survival [OS] and is underscored by prolonged progression-free survival [PFS] and high response rates that are durable,” Finn said.
In the IMbrave150 trial, treatment-naive patients who had unresectable liver cancer received either atezolizumab-bevacizumab (n = 336) or sorafenib (n = 165).
After a median follow-up of 8.6 months, median survival was significantly longer for the patients who received atezolizumab-bevacizumab: 13.2 months. For the patients who did not receive the combination, median survival was not reached (hazard ratio [HR], 0.58; P < .001). Six-month OS was 84.8% with the combination versus 72.2% with sorafenib.
Median PFS was also significantly longer for patients who received combination therapy: 6.8 months with the combination versus 4.3 months with sorafenib (HR, 0.59; P < .001). Six-month PFS was 54.4% with the combination versus 37.3% with sorafenib.
The objective response rate was 27.3% (complete response, 5.5%) with the combination versus 11.9% (complete response, 0%) with sorafenib
Median time to deterioration of quality of life was also longer for patients who received combination therapy: 11.2 months with atezolizumab-bevacizumab and 3.6 months for sorafenib.
Incidence of grade 3 or 4 adverse events was similar in both arms of the study: 56.5% for the combination versus 55.1% with sorafenib. Adverse events leading to withdrawal from any study drug was not significantly different: 15.5% versus 10.3% for sorafenib.
The percentage of patients who experienced bleeding of any grade (attributed to bevacizumab) was 25.2% with the combination versus 17.3% with sorafenib. In addition, six incidents of fatal bleeding or perforated ulcer were recorded in the combination group, compared with one for the sorafenib group.
Appropriate for all patients?
Llovet told Medscape Medical News that the combination of atezolizumab and bevacizumab, although still awaiting approval for use in liver cancer, will be adopted by guidelines in the management of HCC as first-line therapy.
It has been accepted as the new front-line standard of care in a soon-to-be-published consensus on trial design and endpoints in HCC that he has authored.
Llovet said that the intravenous (IV) dosing of the combination was not likely to be a problem (sorafenib is administered orally). For patients with untreated advanced HCC, median survival is 8 months; it is 11-13 months with sorafenib. With this combination, the median was not reached, but it is expected to be beyond 17 months. “In this scenario, IV versus oral dosing will only have implications if the treatments had similar efficacy but not significantly better performance,” he said.
In her editorial, Kelley suggests caution when considering use of the combination in a patient population broader than that defined by the IMbrave150 study.
She points out that patients in IMbrave150 were required to have well-compensated liver disease (Child-Pugh class A liver function), and patients with untreated or incompletely treated esophageal or gastric varices with bleeding or those who were at high risk of bleeding were excluded from the study.
“Safety has not been established for persons in the Childs-Pugh class B population, and alternative therapies should be considered for patients at high risk for bleeding,” Kelley writes in her editorial.
Bleeding events, including fatal bleeding and perforated ulcers, “underscore important considerations for the application of the atezolizumab-bevacizumab regimen to a broader population beyond the clinical trial setting,” Kelley noted.
She recommends that all patients at risk for varices undergo “appropriate endoscopic evaluation and management before treatment is initiated.”
Llovet agreed and noted that upper endoscopy is not currently practiced in the management of advanced HCC. “One important issue in the clinical practice will be that all patients require an upper endoscopy to rule out esophageal varices, which are at risk of bleeding with bevacizumab,” he said.
Trial investigator Finn explained that IMbrave 150 is not unique and that every phase 3 study has included patients with Child-Pugh class A liver function.
“Patients with Child-Pugh class B are a heterogeneous group of patients,” Finn said. Physicians should use their judgment in providing this combination to patients with Child-Pugh scores of 7–9, he added. “All patients with advanced liver cancer need an endoscopy,” Finn said.
Kelley and Llovet also observed that several ongoing trials, some of which have been completed, are evaluating combinations of immunotherapy and other antiangiogenic agents or combinations of immunotherapies. The results from these trials will inform how such combinations will be used in the future.
Kelley predicted that, if some of these trials are positive, in the absence of a predictive biomarker, physicians will be guided by “the safety profiles of the combinations as well as the inference of synergy on the basis of depth and durability of responses.”
“Results of these trials will be known within the next 12-18 months and might further improve the current standards for patients with inoperable HCC,” Llovet said.
IMbrave150 was sponsored by F. Hoffmann–La Roche/Genentech. Finn has consulted for AtraZeneca, Bayer, Bristol-Myers Squibb, CStone, Eisai, Eli Lilly, Exelixis, Roche, Genentech, Merck & Co, Novartis, and Pfizer. Finn’s coauthors also report relationships with pharmaceutical companies. Kelley reports institutional research support from many pharmaceutical companies and served on the independent data monitoring committee for the IMbrave150 trial. Llovet has received research support from Bayer HealthCare Pharmaceuticals, Eisai, Bristol-Myers Squibb, Boehringer Ingelheim, and Ipsen and consulting fees from Bayer HealthCare Pharmaceuticals, Merck, Eisai Inc, Bristol-Myers Squibb, Celsion Corporation, Eli Lilly, Roche, Genentech, Glycotest, Nucleix, Can-Fite Biopharma, AstraZeneca, and Exelixis.
This article first appeared on Medscape.com.
New clinical data are set to change the treatment landscape for advanced liver cancer.
The data showed that atezolizumab plus bevacizumab improved survival, when compared with sorafenib, in patients with unresectable hepatocellular carcinoma (HCC).
The advanced liver cancer space has been dominated for a more than a decade by sorafenib (Nexavar, Bayer), which was the first systemic therapy to confer “a meaningful survival benefit in the treatment of advanced hepatocellular carcinoma,” notes Robin K. Kelley, MD, from the University of California, San Francisco.
“Since then, no treatment had surpassed the effect of sorafenib in the first line until the regimen of atezolizumab and bevacizumab” that is now being reported, she notes.
The new data come from the IMbrave150 study, published on May 14 in the New England Journal of Medicine.
“The combination of atezolizumab plus bevacizumab has become the new benchmark for first-line therapy in advanced hepatocellular carcinoma,” Kelley writes in an accompanying editorial.
“These data are momentous, since they identify not only the first therapy to improve survival beyond sorafenib, but also the first successful combination regimen and the first positive randomized, phase 3 trial of immune checkpoint inhibition in this challenging cancer,” she added.
The IMbrave 150 study was sponsored by Roche, manufacturer of both the checkpoint inhibitor atezolizumab (Tecentriq, Genentech/Roche) and the antiangiogenic agent bevacizumab (Avastin, Genentech/Roche); the company has submitted an FDA approval application for use of this combination for inoperable liver cancer.
'Results represent a breakthrough'
“These results represent a breakthrough in the management of advanced HCC,” said Josep M. Llovet, MD, PhD, director of the Mount Sinai Liver Cancer Program, Icahn School of Medicine at Mount Sinai, New York, and professor of medicine in hepatic oncology at the University of Barcelona, Spain.
The combination has already been acknowledged as a milestone in the management of HCC, he said.
Llovet was approached for comment by Medscape Medical News. He was not involved with IMbrave150 but was the lead author on the SHARP study, which led to the first-line approval of sorafenib.
He explained that, since the approval of sorafenib in 2008, lenvatinib (Lenvima, Eisai) has been the only other agent approved for the front-line treatment of HCC after hitting the noninferiority endpoint for survival in comparison with sorafenib. “Up to now, there was no agent superior to sorafenib, the standard of care,” he said.
Now, the combination of atezolizumab-bevacizumab has shown superior efficacy compared with sorafenib, Llovet noted. It is not only the first combination to show efficacy but is also the first checkpoint inhibitor that has demonstrated efficacy in HCC. “Previous studies of checkpoint inhibitors used as single agents in the front-line or second-line setting of advanced liver cancer were negative,” he said.
'Game-changer' in liver cancer
“The atezolizumab-bevacizumab combination is a game-changer in liver cancer,” the lead author of the IMbrave 150 trial, Richard S. Finn, MD, of the David Geffen School of Medicine at the University of California, Los Angeles, told Medscape Medical News.
“The combination has established a new standard of care that is predicated on the gold standard of overall survival [OS] and is underscored by prolonged progression-free survival [PFS] and high response rates that are durable,” Finn said.
In the IMbrave150 trial, treatment-naive patients who had unresectable liver cancer received either atezolizumab-bevacizumab (n = 336) or sorafenib (n = 165).
After a median follow-up of 8.6 months, median survival was significantly longer for the patients who received atezolizumab-bevacizumab: 13.2 months. For the patients who did not receive the combination, median survival was not reached (hazard ratio [HR], 0.58; P < .001). Six-month OS was 84.8% with the combination versus 72.2% with sorafenib.
Median PFS was also significantly longer for patients who received combination therapy: 6.8 months with the combination versus 4.3 months with sorafenib (HR, 0.59; P < .001). Six-month PFS was 54.4% with the combination versus 37.3% with sorafenib.
The objective response rate was 27.3% (complete response, 5.5%) with the combination versus 11.9% (complete response, 0%) with sorafenib
Median time to deterioration of quality of life was also longer for patients who received combination therapy: 11.2 months with atezolizumab-bevacizumab and 3.6 months for sorafenib.
Incidence of grade 3 or 4 adverse events was similar in both arms of the study: 56.5% for the combination versus 55.1% with sorafenib. Adverse events leading to withdrawal from any study drug was not significantly different: 15.5% versus 10.3% for sorafenib.
The percentage of patients who experienced bleeding of any grade (attributed to bevacizumab) was 25.2% with the combination versus 17.3% with sorafenib. In addition, six incidents of fatal bleeding or perforated ulcer were recorded in the combination group, compared with one for the sorafenib group.
Appropriate for all patients?
Llovet told Medscape Medical News that the combination of atezolizumab and bevacizumab, although still awaiting approval for use in liver cancer, will be adopted by guidelines in the management of HCC as first-line therapy.
It has been accepted as the new front-line standard of care in a soon-to-be-published consensus on trial design and endpoints in HCC that he has authored.
Llovet said that the intravenous (IV) dosing of the combination was not likely to be a problem (sorafenib is administered orally). For patients with untreated advanced HCC, median survival is 8 months; it is 11-13 months with sorafenib. With this combination, the median was not reached, but it is expected to be beyond 17 months. “In this scenario, IV versus oral dosing will only have implications if the treatments had similar efficacy but not significantly better performance,” he said.
In her editorial, Kelley suggests caution when considering use of the combination in a patient population broader than that defined by the IMbrave150 study.
She points out that patients in IMbrave150 were required to have well-compensated liver disease (Child-Pugh class A liver function), and patients with untreated or incompletely treated esophageal or gastric varices with bleeding or those who were at high risk of bleeding were excluded from the study.
“Safety has not been established for persons in the Childs-Pugh class B population, and alternative therapies should be considered for patients at high risk for bleeding,” Kelley writes in her editorial.
Bleeding events, including fatal bleeding and perforated ulcers, “underscore important considerations for the application of the atezolizumab-bevacizumab regimen to a broader population beyond the clinical trial setting,” Kelley noted.
She recommends that all patients at risk for varices undergo “appropriate endoscopic evaluation and management before treatment is initiated.”
Llovet agreed and noted that upper endoscopy is not currently practiced in the management of advanced HCC. “One important issue in the clinical practice will be that all patients require an upper endoscopy to rule out esophageal varices, which are at risk of bleeding with bevacizumab,” he said.
Trial investigator Finn explained that IMbrave 150 is not unique and that every phase 3 study has included patients with Child-Pugh class A liver function.
“Patients with Child-Pugh class B are a heterogeneous group of patients,” Finn said. Physicians should use their judgment in providing this combination to patients with Child-Pugh scores of 7–9, he added. “All patients with advanced liver cancer need an endoscopy,” Finn said.
Kelley and Llovet also observed that several ongoing trials, some of which have been completed, are evaluating combinations of immunotherapy and other antiangiogenic agents or combinations of immunotherapies. The results from these trials will inform how such combinations will be used in the future.
Kelley predicted that, if some of these trials are positive, in the absence of a predictive biomarker, physicians will be guided by “the safety profiles of the combinations as well as the inference of synergy on the basis of depth and durability of responses.”
“Results of these trials will be known within the next 12-18 months and might further improve the current standards for patients with inoperable HCC,” Llovet said.
IMbrave150 was sponsored by F. Hoffmann–La Roche/Genentech. Finn has consulted for AtraZeneca, Bayer, Bristol-Myers Squibb, CStone, Eisai, Eli Lilly, Exelixis, Roche, Genentech, Merck & Co, Novartis, and Pfizer. Finn’s coauthors also report relationships with pharmaceutical companies. Kelley reports institutional research support from many pharmaceutical companies and served on the independent data monitoring committee for the IMbrave150 trial. Llovet has received research support from Bayer HealthCare Pharmaceuticals, Eisai, Bristol-Myers Squibb, Boehringer Ingelheim, and Ipsen and consulting fees from Bayer HealthCare Pharmaceuticals, Merck, Eisai Inc, Bristol-Myers Squibb, Celsion Corporation, Eli Lilly, Roche, Genentech, Glycotest, Nucleix, Can-Fite Biopharma, AstraZeneca, and Exelixis.
This article first appeared on Medscape.com.
New OS data with olaparib support ‘new era’ for ovarian cancer
Women with platinum-sensitive relapsed ovarian cancer and a BRCA mutation could see their survival extended by over a year by maintenance therapy with the PARP inhibitor olaparib (Lynparza, AstraZeneca).
The new overall survival (OS) data come from the SOLO2 study and were described as “a significant advance” in a cancer “that has a historically poor prognosis” by Richard Schilsky, MD, senior vice president and chief medical officer of the American Society of Clinical Oncology.
The results were highlighted at a presscast prior to being presented during the virtual scientific program of the 2020 ASCO annual meeting (abstract 6002).
The SOLO2 study randomly assigned almost 300 women with relapsed BRCA-related ovarian cancer that was responding to platinum-based chemotherapy to maintenance therapy with either olaparib or placebo.
Earlier results from this study showed that olaparib was associated with an investigator-assessed progression-free survival (PFS) of 19.1 months, versus just 5.5 months with placebo, as previously reported by Medscape Medical News.
New data from this trial, presented by Andrés Poveda, MD, Initia Oncology, Hospital Quironsalud, in Valencia, Spain, show that olaparib improved median OS by 12.9 months compared to placebo (51.7 months with olaparib vs. 38.8 months with placebo; hazard ratio, 0.74; P = .054).
At 5 years’ follow-up, 42.1% of women taking olaparib were alive, versus 33.2% taking placebo.
In an ASCO press release, Poveda described the improvement in median OS with olaparib as “impressive” and that it offers a “substantial benefit to our patients.
“This study helps usher in a new era of personalized medicine for women with this difficult-to-treat cancer,” he added.
Poveda told reporters that this study is “the first randomized phase 3 trial to provide overall survival data for maintenance PARP inhibitors.
“The finding that 22% of patient in the olaparib group received the study treatment for more than 5 years is unprecedented in the setting of relapsed ovarian cancer,” he added.
The new OS data were welcomed by Konstantin Zakashansky, MD, director of gynecologic oncology at Mount Sinai West, New York, who was not involved in the study.
“PARP inhibitor trials have revolutionized therapy for ovarian cancer in the front line, as well as in the recurrent setting, [with] all of the recently presented trials showing significant improvement in progression free survival,” he said.
“Overall survival data, however, which is considered the most clinically relevant endpoint in oncology trials and remains the ‘gold standard’ because of its relevance and objectivity, have been limited,” he continued.
Zakashansky recalled that when the earlier PFS data from SOLO2 were presented, “questions were raised regarding the clinical uncertainty of the benefit associated with olaparib maintenance, primarily whether the PFS benefit...would translate to a long-term overall survival benefit.”
For him, the current results “answer that question” and offer the “largest improvement in overall survival of any recurrent ovarian cancer patient trial reported to date.”
Schilsky added that the new data confirm that olaparib “should be the standard maintenance therapy for patients with BRCA-related relapsed ovarian cancer responding to platinum-based chemotherapy.”
The drug is already approved for this indication, but the new data showing a significant survival benefit are “comforting” and “good news,” he said.
Adverse events with olaparib
Treatment-emergent adverse events seen in the study were “consistent with the known tolerability profile of olaparib,” Poveda commented.
The most common events of any grade were nausea, fatigue/asthenia, and anemia. The most common event of grade ≥3 was anemia.
Adverse events leading to dose interruptions occurred in 50% of patients who received olaparib and 19% of patients who took placebo. Adverse events leading to dose reductions occurred in 28% and 3%, respectively.
Treatment discontinuation because of adverse events was reported in 17% of patients given olaparib and 3% of those in the placebo arm.
The study was funded by AstraZeneca and Merck Sharp & Dohme. Poveda reports a consulting or advisory role with AstraZeneca, Clovis Oncology, PharmaMar, Roche, and Tesaro and receiving expenses from PharmaMar. Many coauthors also report relationships with pharmaceutical companies.
This article first appeared on Medscape.com.
Women with platinum-sensitive relapsed ovarian cancer and a BRCA mutation could see their survival extended by over a year by maintenance therapy with the PARP inhibitor olaparib (Lynparza, AstraZeneca).
The new overall survival (OS) data come from the SOLO2 study and were described as “a significant advance” in a cancer “that has a historically poor prognosis” by Richard Schilsky, MD, senior vice president and chief medical officer of the American Society of Clinical Oncology.
The results were highlighted at a presscast prior to being presented during the virtual scientific program of the 2020 ASCO annual meeting (abstract 6002).
The SOLO2 study randomly assigned almost 300 women with relapsed BRCA-related ovarian cancer that was responding to platinum-based chemotherapy to maintenance therapy with either olaparib or placebo.
Earlier results from this study showed that olaparib was associated with an investigator-assessed progression-free survival (PFS) of 19.1 months, versus just 5.5 months with placebo, as previously reported by Medscape Medical News.
New data from this trial, presented by Andrés Poveda, MD, Initia Oncology, Hospital Quironsalud, in Valencia, Spain, show that olaparib improved median OS by 12.9 months compared to placebo (51.7 months with olaparib vs. 38.8 months with placebo; hazard ratio, 0.74; P = .054).
At 5 years’ follow-up, 42.1% of women taking olaparib were alive, versus 33.2% taking placebo.
In an ASCO press release, Poveda described the improvement in median OS with olaparib as “impressive” and that it offers a “substantial benefit to our patients.
“This study helps usher in a new era of personalized medicine for women with this difficult-to-treat cancer,” he added.
Poveda told reporters that this study is “the first randomized phase 3 trial to provide overall survival data for maintenance PARP inhibitors.
“The finding that 22% of patient in the olaparib group received the study treatment for more than 5 years is unprecedented in the setting of relapsed ovarian cancer,” he added.
The new OS data were welcomed by Konstantin Zakashansky, MD, director of gynecologic oncology at Mount Sinai West, New York, who was not involved in the study.
“PARP inhibitor trials have revolutionized therapy for ovarian cancer in the front line, as well as in the recurrent setting, [with] all of the recently presented trials showing significant improvement in progression free survival,” he said.
“Overall survival data, however, which is considered the most clinically relevant endpoint in oncology trials and remains the ‘gold standard’ because of its relevance and objectivity, have been limited,” he continued.
Zakashansky recalled that when the earlier PFS data from SOLO2 were presented, “questions were raised regarding the clinical uncertainty of the benefit associated with olaparib maintenance, primarily whether the PFS benefit...would translate to a long-term overall survival benefit.”
For him, the current results “answer that question” and offer the “largest improvement in overall survival of any recurrent ovarian cancer patient trial reported to date.”
Schilsky added that the new data confirm that olaparib “should be the standard maintenance therapy for patients with BRCA-related relapsed ovarian cancer responding to platinum-based chemotherapy.”
The drug is already approved for this indication, but the new data showing a significant survival benefit are “comforting” and “good news,” he said.
Adverse events with olaparib
Treatment-emergent adverse events seen in the study were “consistent with the known tolerability profile of olaparib,” Poveda commented.
The most common events of any grade were nausea, fatigue/asthenia, and anemia. The most common event of grade ≥3 was anemia.
Adverse events leading to dose interruptions occurred in 50% of patients who received olaparib and 19% of patients who took placebo. Adverse events leading to dose reductions occurred in 28% and 3%, respectively.
Treatment discontinuation because of adverse events was reported in 17% of patients given olaparib and 3% of those in the placebo arm.
The study was funded by AstraZeneca and Merck Sharp & Dohme. Poveda reports a consulting or advisory role with AstraZeneca, Clovis Oncology, PharmaMar, Roche, and Tesaro and receiving expenses from PharmaMar. Many coauthors also report relationships with pharmaceutical companies.
This article first appeared on Medscape.com.
Women with platinum-sensitive relapsed ovarian cancer and a BRCA mutation could see their survival extended by over a year by maintenance therapy with the PARP inhibitor olaparib (Lynparza, AstraZeneca).
The new overall survival (OS) data come from the SOLO2 study and were described as “a significant advance” in a cancer “that has a historically poor prognosis” by Richard Schilsky, MD, senior vice president and chief medical officer of the American Society of Clinical Oncology.
The results were highlighted at a presscast prior to being presented during the virtual scientific program of the 2020 ASCO annual meeting (abstract 6002).
The SOLO2 study randomly assigned almost 300 women with relapsed BRCA-related ovarian cancer that was responding to platinum-based chemotherapy to maintenance therapy with either olaparib or placebo.
Earlier results from this study showed that olaparib was associated with an investigator-assessed progression-free survival (PFS) of 19.1 months, versus just 5.5 months with placebo, as previously reported by Medscape Medical News.
New data from this trial, presented by Andrés Poveda, MD, Initia Oncology, Hospital Quironsalud, in Valencia, Spain, show that olaparib improved median OS by 12.9 months compared to placebo (51.7 months with olaparib vs. 38.8 months with placebo; hazard ratio, 0.74; P = .054).
At 5 years’ follow-up, 42.1% of women taking olaparib were alive, versus 33.2% taking placebo.
In an ASCO press release, Poveda described the improvement in median OS with olaparib as “impressive” and that it offers a “substantial benefit to our patients.
“This study helps usher in a new era of personalized medicine for women with this difficult-to-treat cancer,” he added.
Poveda told reporters that this study is “the first randomized phase 3 trial to provide overall survival data for maintenance PARP inhibitors.
“The finding that 22% of patient in the olaparib group received the study treatment for more than 5 years is unprecedented in the setting of relapsed ovarian cancer,” he added.
The new OS data were welcomed by Konstantin Zakashansky, MD, director of gynecologic oncology at Mount Sinai West, New York, who was not involved in the study.
“PARP inhibitor trials have revolutionized therapy for ovarian cancer in the front line, as well as in the recurrent setting, [with] all of the recently presented trials showing significant improvement in progression free survival,” he said.
“Overall survival data, however, which is considered the most clinically relevant endpoint in oncology trials and remains the ‘gold standard’ because of its relevance and objectivity, have been limited,” he continued.
Zakashansky recalled that when the earlier PFS data from SOLO2 were presented, “questions were raised regarding the clinical uncertainty of the benefit associated with olaparib maintenance, primarily whether the PFS benefit...would translate to a long-term overall survival benefit.”
For him, the current results “answer that question” and offer the “largest improvement in overall survival of any recurrent ovarian cancer patient trial reported to date.”
Schilsky added that the new data confirm that olaparib “should be the standard maintenance therapy for patients with BRCA-related relapsed ovarian cancer responding to platinum-based chemotherapy.”
The drug is already approved for this indication, but the new data showing a significant survival benefit are “comforting” and “good news,” he said.
Adverse events with olaparib
Treatment-emergent adverse events seen in the study were “consistent with the known tolerability profile of olaparib,” Poveda commented.
The most common events of any grade were nausea, fatigue/asthenia, and anemia. The most common event of grade ≥3 was anemia.
Adverse events leading to dose interruptions occurred in 50% of patients who received olaparib and 19% of patients who took placebo. Adverse events leading to dose reductions occurred in 28% and 3%, respectively.
Treatment discontinuation because of adverse events was reported in 17% of patients given olaparib and 3% of those in the placebo arm.
The study was funded by AstraZeneca and Merck Sharp & Dohme. Poveda reports a consulting or advisory role with AstraZeneca, Clovis Oncology, PharmaMar, Roche, and Tesaro and receiving expenses from PharmaMar. Many coauthors also report relationships with pharmaceutical companies.
This article first appeared on Medscape.com.
FROM ASCO 2020
Satralizumab monotherapy reduces NMOSD relapse rate
according to trial results published in the Lancet Neurology.
The findings help confirm the role of interleukin-6 in the pathobiology of aquaporin-4 autoantibody (AQP4-IgG)–seropositive disease. For patients who are AQP4-IgG seronegative, however, “there is insufficient evidence to indicate a risk reduction” with this drug, the investigators wrote. In addition, satralizumab did not significantly affect pain or fatigue.
“The limitations of the study include the relatively small group sizes and low number of relapses. Despite these limitations, a significant treatment benefit was observed with satralizumab, compared with placebo in the study population, with efficacy and safety comparable to satralizumab used in combination with immunosuppressants,” reported lead author Anthony Traboulsee, MD, professor of neurology at the University of British Columbia, Vancouver, and colleagues.
Satralizumab is a humanized monoclonal antibody targeting the IL-6 receptor. A prior phase 3 study, SakuraSky, found that the drug reduces the risk of NMOSD relapse when added to immunosuppressant therapy. To assess the safety and efficacy of satralizumab monotherapy, Dr. Traboulsee and colleagues conducted SakuraStar, a randomized, double-blind, placebo-controlled trial.
Evaluating drug as monotherapy
The phase 3 trial enrolled 95 patients aged 18-74 years at 44 sites in 13 countries. The investigators included patients with AQP4-IgG–seropositive or –seronegative neuromyelitis optica using the 2006 Wingerchuk criteria or with AQP4-IgG–seropositive NMOSD with at least one event of longitudinally extensive myelitis or optic neuritis. The researchers limited the number of AQP4-IgG–seronegative patients to about 30% of the study population. Eligible participants had at least one NMOSD attack or relapse in the past 12 months and a score of 6.5 or less on the Expanded Disability Status Scale (EDSS). The investigators excluded patients with a clinical relapse in the 30 days before study baseline. Participants were randomly assigned 2:1 to receive satralizumab 120 mg or placebo subcutaneously at weeks 0, 2, 4, and every 4 weeks thereafter. Concomitant immunosuppressant use was prohibited, although corticosteroids and intravenous immunoglobulin were permitted as rescue therapy for the treatment of relapse.
The primary endpoint was time to first relapse, and the trial was designed to continue until 44 relapses occurred or for 1.5 years after the last patient entered the trial, whichever occurred first.
“Because even one NMOSD attack can have serious neurological consequences, this design allowed patients who had an attack on placebo to enter the open-label phase and receive the active drug,” the researchers wrote. “The trial design used unequal randomization to minimize exposure to placebo; because patients were not permitted to receive concomitant immunosuppressant treatment in this trial, the design limited the number of patients not receiving any treatment for the disorder. Placebo was selected with the consideration that no drugs were approved for the treatment of NMOSD when the trial was designed.” Recent trials of eculizumab, inebilizumab, and satralizumab have found that the agents are effective treatments for NMOSD. In 2019, the Food and Drug Administration approved eculizumab, a complement inhibitor, for the treatment of AQP4-IgG–seropositive NMOSD.
For the primary endpoint of SakuraStar, the researchers defined relapses as new or worsening objective neurologic symptoms with at least one of the following:
- Increase of 1 or more EDSS points from a baseline EDSS score of more than 0, or increase of 2 or more EDSS points from a baseline EDSS score of 0
- Increase of 2 or more points on at least one appropriate symptom-specific functional system score for pyramidal, cerebellar, brain stem, sensory, bowel or bladder, or a single eye
- Increase of 1 or more points on more than one symptom-specific functional system score with a baseline of at least 1
- Increase of 1 or more points on a single-eye symptom-specific functional system score with a baseline score of at least 1
In addition, symptoms had to be attributable to NMOSD; persist for more than 24 hours; and not be attributable to confounding clinical factors such as fever, infection, injury, change in mood, or adverse reactions to medications. Researchers assessed EDSS and functional system scores within 7 days of a patient reporting symptoms.
The double-blind treatment period ended 1.5 years after the last enrolled patient was assigned to satralizumab or placebo. More than 80% of the participants were women, including 73% of the satralizumab group and 97% of the placebo group. In all, 95 participants were assigned to a treatment between 2014 and 2017 – 63 to satralizumab and 32 to placebo. Relapses occurred in 19 patients receiving satralizumab (30%) and 16 receiving placebo (50%). The hazard ratio was 0.45.
“Patients treated with placebo showed a shorter time to relapse and a higher withdrawal rate than did patients treated with satralizumab,” wrote Dr. Traboulsee and colleagues. The Kaplan-Meier method suggested that 76% of patients on satralizumab had not relapsed at 48 weeks, compared with 62% of patients on placebo. And at 96 weeks, 72% of patients on satralizumab had not relapsed, compared with 51% of patients on placebo.
Among patients who were AQP4-IgG seropositive, the proportion with protocol-defined relapse was 22% in the satralizumab group versus 57% in the placebo group. Among patients who were AQP4-IgG seronegative, the proportion with a protocol-defined relapse was 46% in the satralizumab group versus 33% in the placebo group.
The most common adverse events were urinary tract infection and upper respiratory tract infection, and most adverse events were mild to moderate. A higher rate of severe adverse events was reported in the satralizumab group than in the placebo group (32.1 vs. 9.9 events per 100 patient-years). The investigators considered most of the severe adverse events unrelated to the study treatment. “None of the severe adverse events led to discontinuation of the study drug except one severe event of pneumonia in the satralizumab group,” the researchers wrote.
Data confirm efficacy of IL-6 blockade
“Satralizumab was well tolerated and no meaningful adverse effects from the drug were reported and no deaths occurred,” said Michael Levy, MD, PhD, director of the NMO clinic and research laboratory at Massachusetts General Hospital, Boston, in an accompanying editorial. “This trial of satralizumab was done shortly after the completion of a parallel trial of satralizumab in patients with NMOSD in which the same dose of satralizumab reduced the risk of relapse by 62%, compared with placebo. The main difference between these two trials is that, in the first published study, participants were permitted to use background immunotherapy; otherwise, the trial designs were nearly identical, and the enrolled participants are comparable. Together, the findings from these studies suggest that background therapy seems to provide only a small additional benefit to satralizumab alone.”
Dr. Levy also discussed findings from a phase 2 study of tocilizumab for the prevention of relapse in patients with NMOSD published in the same issue of the Lancet Neurology. The satralizumab and tocilizumab data have “confirmed that IL-6 blockade reduces the risk of relapse in patients with NMOSD,” Dr. Levy said. “IL-6 is a crucial component of the immune system, but when IL-6 production is altered during autoimmune attacks and sepsis, there can be severe consequences.”
The phase 2 trial of tocilizumab, which was described at the 2019 annual congress of the European Committee for Treatment and Research in Multiple Sclerosis, included 118 patients, 87% of whom were AQP4-IgG seropositive. Patients received intravenous tocilizumab or oral azathioprine for up to 60 weeks. Overall, 14% of patients in the tocilizumab group relapsed, compared with 47% of patients in the azathioprine group.
“The main differences between this trial of tocilizumab and the two satralizumab trials are that the tocilizumab was administered intravenously, rather than subcutaneously, the study duration was approximately 1 year, and the investigators were not masked to the treatment allocation,” Dr. Levy said. “Similar to satralizumab, adverse effects with tocilizumab were mild, including asymptomatic elevations in liver enzymes and an increased incidence of respiratory and urinary infections, with no significant differences identified between the tocilizumab and azathioprine groups.”
Various immunopathologic mechanisms may influence outcomes in NMOSD. While satralizumab and tocilizumab target IL-6, eculizumab is a C5 complement inhibitor and inebilizumab is a CD19 B-cell depleting monoclonal antibody, Dr. Levy said. “The safety concerns regarding these approaches are all substantially outweighed by the benefit of preventing NMOSD relapses.”
A need to understand AQP4-IgG–seronegative disease
SakuraStar “provides convincing data for the efficacy of satralizumab monotherapy in NMOSD with subgroup analysis showing that the benefit was seen in AQP4-IgG seropositive patients,” commented Dean M. Wingerchuk, MD, director of the division of multiple sclerosis and autoimmune neurology at the Mayo Clinic in Phoenix. “The results help confirm the key role of IL-6 in the pathobiology of AQP4-IgG–seropositive disease.”
Questions about AQP4-IgG seronegative disease remain. “The results are quite similar to the SakuraSky trial, in which satralizumab was used in conjunction with other background immunosuppressive therapies, suggesting that the primary benefit may be derived primarily from satralizumab. Both trials also showed that satralizumab did not benefit the NMOSD without AQP4-IgG subgroup though the subject numbers are rather small. We need to know more about the clinical and laboratory characteristics of the seronegative patients as they likely comprise a heterogeneous group. For example, did any of them have other autoantibodies such as MOG-IgG? Depending on the results, those details may help us understand the relative role of IL-6 in AQP4-IgG–seronegative subgroups, an important area of further study.”
SakuraStar was funded by Chugai Pharmaceutical, a member of the Roche group. Dr. Traboulsee reported grants, personal fees, and nonfinancial support from Chugai Pharmaceutical during the study, and several coauthors were employees of Chugai Pharmaceutical. Additional coauthors reported personal fees from Chugai, Roche, and other companies. Dr. Levy has received consulting fees from Alexion, Viela Bio, Chugai Pharmaceutical, Quest Diagnostics, and UCB Pharmaceuticals.
SOURCE: Traboulsee A et al. Lancet Neurol. 2020;19(5):402-12.
according to trial results published in the Lancet Neurology.
The findings help confirm the role of interleukin-6 in the pathobiology of aquaporin-4 autoantibody (AQP4-IgG)–seropositive disease. For patients who are AQP4-IgG seronegative, however, “there is insufficient evidence to indicate a risk reduction” with this drug, the investigators wrote. In addition, satralizumab did not significantly affect pain or fatigue.
“The limitations of the study include the relatively small group sizes and low number of relapses. Despite these limitations, a significant treatment benefit was observed with satralizumab, compared with placebo in the study population, with efficacy and safety comparable to satralizumab used in combination with immunosuppressants,” reported lead author Anthony Traboulsee, MD, professor of neurology at the University of British Columbia, Vancouver, and colleagues.
Satralizumab is a humanized monoclonal antibody targeting the IL-6 receptor. A prior phase 3 study, SakuraSky, found that the drug reduces the risk of NMOSD relapse when added to immunosuppressant therapy. To assess the safety and efficacy of satralizumab monotherapy, Dr. Traboulsee and colleagues conducted SakuraStar, a randomized, double-blind, placebo-controlled trial.
Evaluating drug as monotherapy
The phase 3 trial enrolled 95 patients aged 18-74 years at 44 sites in 13 countries. The investigators included patients with AQP4-IgG–seropositive or –seronegative neuromyelitis optica using the 2006 Wingerchuk criteria or with AQP4-IgG–seropositive NMOSD with at least one event of longitudinally extensive myelitis or optic neuritis. The researchers limited the number of AQP4-IgG–seronegative patients to about 30% of the study population. Eligible participants had at least one NMOSD attack or relapse in the past 12 months and a score of 6.5 or less on the Expanded Disability Status Scale (EDSS). The investigators excluded patients with a clinical relapse in the 30 days before study baseline. Participants were randomly assigned 2:1 to receive satralizumab 120 mg or placebo subcutaneously at weeks 0, 2, 4, and every 4 weeks thereafter. Concomitant immunosuppressant use was prohibited, although corticosteroids and intravenous immunoglobulin were permitted as rescue therapy for the treatment of relapse.
The primary endpoint was time to first relapse, and the trial was designed to continue until 44 relapses occurred or for 1.5 years after the last patient entered the trial, whichever occurred first.
“Because even one NMOSD attack can have serious neurological consequences, this design allowed patients who had an attack on placebo to enter the open-label phase and receive the active drug,” the researchers wrote. “The trial design used unequal randomization to minimize exposure to placebo; because patients were not permitted to receive concomitant immunosuppressant treatment in this trial, the design limited the number of patients not receiving any treatment for the disorder. Placebo was selected with the consideration that no drugs were approved for the treatment of NMOSD when the trial was designed.” Recent trials of eculizumab, inebilizumab, and satralizumab have found that the agents are effective treatments for NMOSD. In 2019, the Food and Drug Administration approved eculizumab, a complement inhibitor, for the treatment of AQP4-IgG–seropositive NMOSD.
For the primary endpoint of SakuraStar, the researchers defined relapses as new or worsening objective neurologic symptoms with at least one of the following:
- Increase of 1 or more EDSS points from a baseline EDSS score of more than 0, or increase of 2 or more EDSS points from a baseline EDSS score of 0
- Increase of 2 or more points on at least one appropriate symptom-specific functional system score for pyramidal, cerebellar, brain stem, sensory, bowel or bladder, or a single eye
- Increase of 1 or more points on more than one symptom-specific functional system score with a baseline of at least 1
- Increase of 1 or more points on a single-eye symptom-specific functional system score with a baseline score of at least 1
In addition, symptoms had to be attributable to NMOSD; persist for more than 24 hours; and not be attributable to confounding clinical factors such as fever, infection, injury, change in mood, or adverse reactions to medications. Researchers assessed EDSS and functional system scores within 7 days of a patient reporting symptoms.
The double-blind treatment period ended 1.5 years after the last enrolled patient was assigned to satralizumab or placebo. More than 80% of the participants were women, including 73% of the satralizumab group and 97% of the placebo group. In all, 95 participants were assigned to a treatment between 2014 and 2017 – 63 to satralizumab and 32 to placebo. Relapses occurred in 19 patients receiving satralizumab (30%) and 16 receiving placebo (50%). The hazard ratio was 0.45.
“Patients treated with placebo showed a shorter time to relapse and a higher withdrawal rate than did patients treated with satralizumab,” wrote Dr. Traboulsee and colleagues. The Kaplan-Meier method suggested that 76% of patients on satralizumab had not relapsed at 48 weeks, compared with 62% of patients on placebo. And at 96 weeks, 72% of patients on satralizumab had not relapsed, compared with 51% of patients on placebo.
Among patients who were AQP4-IgG seropositive, the proportion with protocol-defined relapse was 22% in the satralizumab group versus 57% in the placebo group. Among patients who were AQP4-IgG seronegative, the proportion with a protocol-defined relapse was 46% in the satralizumab group versus 33% in the placebo group.
The most common adverse events were urinary tract infection and upper respiratory tract infection, and most adverse events were mild to moderate. A higher rate of severe adverse events was reported in the satralizumab group than in the placebo group (32.1 vs. 9.9 events per 100 patient-years). The investigators considered most of the severe adverse events unrelated to the study treatment. “None of the severe adverse events led to discontinuation of the study drug except one severe event of pneumonia in the satralizumab group,” the researchers wrote.
Data confirm efficacy of IL-6 blockade
“Satralizumab was well tolerated and no meaningful adverse effects from the drug were reported and no deaths occurred,” said Michael Levy, MD, PhD, director of the NMO clinic and research laboratory at Massachusetts General Hospital, Boston, in an accompanying editorial. “This trial of satralizumab was done shortly after the completion of a parallel trial of satralizumab in patients with NMOSD in which the same dose of satralizumab reduced the risk of relapse by 62%, compared with placebo. The main difference between these two trials is that, in the first published study, participants were permitted to use background immunotherapy; otherwise, the trial designs were nearly identical, and the enrolled participants are comparable. Together, the findings from these studies suggest that background therapy seems to provide only a small additional benefit to satralizumab alone.”
Dr. Levy also discussed findings from a phase 2 study of tocilizumab for the prevention of relapse in patients with NMOSD published in the same issue of the Lancet Neurology. The satralizumab and tocilizumab data have “confirmed that IL-6 blockade reduces the risk of relapse in patients with NMOSD,” Dr. Levy said. “IL-6 is a crucial component of the immune system, but when IL-6 production is altered during autoimmune attacks and sepsis, there can be severe consequences.”
The phase 2 trial of tocilizumab, which was described at the 2019 annual congress of the European Committee for Treatment and Research in Multiple Sclerosis, included 118 patients, 87% of whom were AQP4-IgG seropositive. Patients received intravenous tocilizumab or oral azathioprine for up to 60 weeks. Overall, 14% of patients in the tocilizumab group relapsed, compared with 47% of patients in the azathioprine group.
“The main differences between this trial of tocilizumab and the two satralizumab trials are that the tocilizumab was administered intravenously, rather than subcutaneously, the study duration was approximately 1 year, and the investigators were not masked to the treatment allocation,” Dr. Levy said. “Similar to satralizumab, adverse effects with tocilizumab were mild, including asymptomatic elevations in liver enzymes and an increased incidence of respiratory and urinary infections, with no significant differences identified between the tocilizumab and azathioprine groups.”
Various immunopathologic mechanisms may influence outcomes in NMOSD. While satralizumab and tocilizumab target IL-6, eculizumab is a C5 complement inhibitor and inebilizumab is a CD19 B-cell depleting monoclonal antibody, Dr. Levy said. “The safety concerns regarding these approaches are all substantially outweighed by the benefit of preventing NMOSD relapses.”
A need to understand AQP4-IgG–seronegative disease
SakuraStar “provides convincing data for the efficacy of satralizumab monotherapy in NMOSD with subgroup analysis showing that the benefit was seen in AQP4-IgG seropositive patients,” commented Dean M. Wingerchuk, MD, director of the division of multiple sclerosis and autoimmune neurology at the Mayo Clinic in Phoenix. “The results help confirm the key role of IL-6 in the pathobiology of AQP4-IgG–seropositive disease.”
Questions about AQP4-IgG seronegative disease remain. “The results are quite similar to the SakuraSky trial, in which satralizumab was used in conjunction with other background immunosuppressive therapies, suggesting that the primary benefit may be derived primarily from satralizumab. Both trials also showed that satralizumab did not benefit the NMOSD without AQP4-IgG subgroup though the subject numbers are rather small. We need to know more about the clinical and laboratory characteristics of the seronegative patients as they likely comprise a heterogeneous group. For example, did any of them have other autoantibodies such as MOG-IgG? Depending on the results, those details may help us understand the relative role of IL-6 in AQP4-IgG–seronegative subgroups, an important area of further study.”
SakuraStar was funded by Chugai Pharmaceutical, a member of the Roche group. Dr. Traboulsee reported grants, personal fees, and nonfinancial support from Chugai Pharmaceutical during the study, and several coauthors were employees of Chugai Pharmaceutical. Additional coauthors reported personal fees from Chugai, Roche, and other companies. Dr. Levy has received consulting fees from Alexion, Viela Bio, Chugai Pharmaceutical, Quest Diagnostics, and UCB Pharmaceuticals.
SOURCE: Traboulsee A et al. Lancet Neurol. 2020;19(5):402-12.
according to trial results published in the Lancet Neurology.
The findings help confirm the role of interleukin-6 in the pathobiology of aquaporin-4 autoantibody (AQP4-IgG)–seropositive disease. For patients who are AQP4-IgG seronegative, however, “there is insufficient evidence to indicate a risk reduction” with this drug, the investigators wrote. In addition, satralizumab did not significantly affect pain or fatigue.
“The limitations of the study include the relatively small group sizes and low number of relapses. Despite these limitations, a significant treatment benefit was observed with satralizumab, compared with placebo in the study population, with efficacy and safety comparable to satralizumab used in combination with immunosuppressants,” reported lead author Anthony Traboulsee, MD, professor of neurology at the University of British Columbia, Vancouver, and colleagues.
Satralizumab is a humanized monoclonal antibody targeting the IL-6 receptor. A prior phase 3 study, SakuraSky, found that the drug reduces the risk of NMOSD relapse when added to immunosuppressant therapy. To assess the safety and efficacy of satralizumab monotherapy, Dr. Traboulsee and colleagues conducted SakuraStar, a randomized, double-blind, placebo-controlled trial.
Evaluating drug as monotherapy
The phase 3 trial enrolled 95 patients aged 18-74 years at 44 sites in 13 countries. The investigators included patients with AQP4-IgG–seropositive or –seronegative neuromyelitis optica using the 2006 Wingerchuk criteria or with AQP4-IgG–seropositive NMOSD with at least one event of longitudinally extensive myelitis or optic neuritis. The researchers limited the number of AQP4-IgG–seronegative patients to about 30% of the study population. Eligible participants had at least one NMOSD attack or relapse in the past 12 months and a score of 6.5 or less on the Expanded Disability Status Scale (EDSS). The investigators excluded patients with a clinical relapse in the 30 days before study baseline. Participants were randomly assigned 2:1 to receive satralizumab 120 mg or placebo subcutaneously at weeks 0, 2, 4, and every 4 weeks thereafter. Concomitant immunosuppressant use was prohibited, although corticosteroids and intravenous immunoglobulin were permitted as rescue therapy for the treatment of relapse.
The primary endpoint was time to first relapse, and the trial was designed to continue until 44 relapses occurred or for 1.5 years after the last patient entered the trial, whichever occurred first.
“Because even one NMOSD attack can have serious neurological consequences, this design allowed patients who had an attack on placebo to enter the open-label phase and receive the active drug,” the researchers wrote. “The trial design used unequal randomization to minimize exposure to placebo; because patients were not permitted to receive concomitant immunosuppressant treatment in this trial, the design limited the number of patients not receiving any treatment for the disorder. Placebo was selected with the consideration that no drugs were approved for the treatment of NMOSD when the trial was designed.” Recent trials of eculizumab, inebilizumab, and satralizumab have found that the agents are effective treatments for NMOSD. In 2019, the Food and Drug Administration approved eculizumab, a complement inhibitor, for the treatment of AQP4-IgG–seropositive NMOSD.
For the primary endpoint of SakuraStar, the researchers defined relapses as new or worsening objective neurologic symptoms with at least one of the following:
- Increase of 1 or more EDSS points from a baseline EDSS score of more than 0, or increase of 2 or more EDSS points from a baseline EDSS score of 0
- Increase of 2 or more points on at least one appropriate symptom-specific functional system score for pyramidal, cerebellar, brain stem, sensory, bowel or bladder, or a single eye
- Increase of 1 or more points on more than one symptom-specific functional system score with a baseline of at least 1
- Increase of 1 or more points on a single-eye symptom-specific functional system score with a baseline score of at least 1
In addition, symptoms had to be attributable to NMOSD; persist for more than 24 hours; and not be attributable to confounding clinical factors such as fever, infection, injury, change in mood, or adverse reactions to medications. Researchers assessed EDSS and functional system scores within 7 days of a patient reporting symptoms.
The double-blind treatment period ended 1.5 years after the last enrolled patient was assigned to satralizumab or placebo. More than 80% of the participants were women, including 73% of the satralizumab group and 97% of the placebo group. In all, 95 participants were assigned to a treatment between 2014 and 2017 – 63 to satralizumab and 32 to placebo. Relapses occurred in 19 patients receiving satralizumab (30%) and 16 receiving placebo (50%). The hazard ratio was 0.45.
“Patients treated with placebo showed a shorter time to relapse and a higher withdrawal rate than did patients treated with satralizumab,” wrote Dr. Traboulsee and colleagues. The Kaplan-Meier method suggested that 76% of patients on satralizumab had not relapsed at 48 weeks, compared with 62% of patients on placebo. And at 96 weeks, 72% of patients on satralizumab had not relapsed, compared with 51% of patients on placebo.
Among patients who were AQP4-IgG seropositive, the proportion with protocol-defined relapse was 22% in the satralizumab group versus 57% in the placebo group. Among patients who were AQP4-IgG seronegative, the proportion with a protocol-defined relapse was 46% in the satralizumab group versus 33% in the placebo group.
The most common adverse events were urinary tract infection and upper respiratory tract infection, and most adverse events were mild to moderate. A higher rate of severe adverse events was reported in the satralizumab group than in the placebo group (32.1 vs. 9.9 events per 100 patient-years). The investigators considered most of the severe adverse events unrelated to the study treatment. “None of the severe adverse events led to discontinuation of the study drug except one severe event of pneumonia in the satralizumab group,” the researchers wrote.
Data confirm efficacy of IL-6 blockade
“Satralizumab was well tolerated and no meaningful adverse effects from the drug were reported and no deaths occurred,” said Michael Levy, MD, PhD, director of the NMO clinic and research laboratory at Massachusetts General Hospital, Boston, in an accompanying editorial. “This trial of satralizumab was done shortly after the completion of a parallel trial of satralizumab in patients with NMOSD in which the same dose of satralizumab reduced the risk of relapse by 62%, compared with placebo. The main difference between these two trials is that, in the first published study, participants were permitted to use background immunotherapy; otherwise, the trial designs were nearly identical, and the enrolled participants are comparable. Together, the findings from these studies suggest that background therapy seems to provide only a small additional benefit to satralizumab alone.”
Dr. Levy also discussed findings from a phase 2 study of tocilizumab for the prevention of relapse in patients with NMOSD published in the same issue of the Lancet Neurology. The satralizumab and tocilizumab data have “confirmed that IL-6 blockade reduces the risk of relapse in patients with NMOSD,” Dr. Levy said. “IL-6 is a crucial component of the immune system, but when IL-6 production is altered during autoimmune attacks and sepsis, there can be severe consequences.”
The phase 2 trial of tocilizumab, which was described at the 2019 annual congress of the European Committee for Treatment and Research in Multiple Sclerosis, included 118 patients, 87% of whom were AQP4-IgG seropositive. Patients received intravenous tocilizumab or oral azathioprine for up to 60 weeks. Overall, 14% of patients in the tocilizumab group relapsed, compared with 47% of patients in the azathioprine group.
“The main differences between this trial of tocilizumab and the two satralizumab trials are that the tocilizumab was administered intravenously, rather than subcutaneously, the study duration was approximately 1 year, and the investigators were not masked to the treatment allocation,” Dr. Levy said. “Similar to satralizumab, adverse effects with tocilizumab were mild, including asymptomatic elevations in liver enzymes and an increased incidence of respiratory and urinary infections, with no significant differences identified between the tocilizumab and azathioprine groups.”
Various immunopathologic mechanisms may influence outcomes in NMOSD. While satralizumab and tocilizumab target IL-6, eculizumab is a C5 complement inhibitor and inebilizumab is a CD19 B-cell depleting monoclonal antibody, Dr. Levy said. “The safety concerns regarding these approaches are all substantially outweighed by the benefit of preventing NMOSD relapses.”
A need to understand AQP4-IgG–seronegative disease
SakuraStar “provides convincing data for the efficacy of satralizumab monotherapy in NMOSD with subgroup analysis showing that the benefit was seen in AQP4-IgG seropositive patients,” commented Dean M. Wingerchuk, MD, director of the division of multiple sclerosis and autoimmune neurology at the Mayo Clinic in Phoenix. “The results help confirm the key role of IL-6 in the pathobiology of AQP4-IgG–seropositive disease.”
Questions about AQP4-IgG seronegative disease remain. “The results are quite similar to the SakuraSky trial, in which satralizumab was used in conjunction with other background immunosuppressive therapies, suggesting that the primary benefit may be derived primarily from satralizumab. Both trials also showed that satralizumab did not benefit the NMOSD without AQP4-IgG subgroup though the subject numbers are rather small. We need to know more about the clinical and laboratory characteristics of the seronegative patients as they likely comprise a heterogeneous group. For example, did any of them have other autoantibodies such as MOG-IgG? Depending on the results, those details may help us understand the relative role of IL-6 in AQP4-IgG–seronegative subgroups, an important area of further study.”
SakuraStar was funded by Chugai Pharmaceutical, a member of the Roche group. Dr. Traboulsee reported grants, personal fees, and nonfinancial support from Chugai Pharmaceutical during the study, and several coauthors were employees of Chugai Pharmaceutical. Additional coauthors reported personal fees from Chugai, Roche, and other companies. Dr. Levy has received consulting fees from Alexion, Viela Bio, Chugai Pharmaceutical, Quest Diagnostics, and UCB Pharmaceuticals.
SOURCE: Traboulsee A et al. Lancet Neurol. 2020;19(5):402-12.
FROM THE LANCET NEUROLOGY
Letters: Disappointment in the Wrapping
To the Editor: I am very disappointed to continue receiving my copies of the Federal Practitioner in nonrecyclable plastic. As a health care professional, I am on the mailing list for numerous other medical publications, all of which seem to be able to utilize recyclable plastic wrappers. I hope you can rectify this problem, which is an embarrassment to me, a serviceconnected veteran.
C. William Kaiser, MD
To the Editor: I am very disappointed to continue receiving my copies of the Federal Practitioner in nonrecyclable plastic. As a health care professional, I am on the mailing list for numerous other medical publications, all of which seem to be able to utilize recyclable plastic wrappers. I hope you can rectify this problem, which is an embarrassment to me, a serviceconnected veteran.
C. William Kaiser, MD
To the Editor: I am very disappointed to continue receiving my copies of the Federal Practitioner in nonrecyclable plastic. As a health care professional, I am on the mailing list for numerous other medical publications, all of which seem to be able to utilize recyclable plastic wrappers. I hope you can rectify this problem, which is an embarrassment to me, a serviceconnected veteran.
C. William Kaiser, MD