User login
With massive reach, telemedicine transforms STEMI care in Latin America
A novel telemedicine approach to remotely guide ST-segment elevation myocardial infarction treatment in four Latin American countries screened more than 780,000 patients and resulted in a mortality rate of 5.2%, results from a 1-year, prospective, observational study showed.
“We have created a modality where the care of acute MI can be remotely guided,” lead investigator Sameer Mehta, MD, MBA, said during a press briefing at the Society for Cardiovascular Angiography & Interventions virtual annual scientific sessions. “This flattens the disparity between the developed and the developing countries, particularly in the poorer parts of Africa, the Middle East, and Southeast Asia.”
Dr. Mehta, chairman of the Lumen Foundation in Miami, and colleagues developed a “hub and spoke” platform to expand STEMI access to more than 100 million people in Brazil, Colombia, Mexico, and Argentina. For the effort, known as the Latin America Telemedicine Infarct Network (LATIN), “spokes” consisted of small clinics and primary health care centers in remote locations, while the “hubs” were medical centers that provided percutaneous coronary intervention (PCI) and/or coronary artery bypass graft (CABG) surgery. There were 313 spokes, 47 hubs, and more than 2,000 health care professionals who participated in the endeavor, including about 600 physicians.
The study, which is the largest of its kind, implemented a 3T strategy: telemedicine, triage, and transport, “which was the hardest part,” Dr. Mehta said. “In some cases, the spokes were located up to 300 miles away from the hubs. Up to 11% of these spokes in the remote areas did not even have a physician. Some had nurses who were triaging the patients.”
Patients who presented at spoke sites were enrolled into LATIN and data were collected through a form that included patient demographics, previous medical history, and an ECG. This information was sent through an app to one of three telemedicine diagnosis centers with 24/7 access to a cardiologist: one in Colombia, one in Brazil, one in Argentina. Once STEMI was identified by ECG, the STEMI protocol was activated, sending alerts to both designated hub and spoke sites and triggering ambulance dispatch. At the spoke sites, thrombolysis, a pharmaco-invasive strategy, or a primary PCI was performed, depending on case and treatment availability. Patients with successful thrombolysis were stabilized for up to 24 hours before transferral to a hub. Patients for whom reperfusion failed were transferred immediately to a hub for rescue PCI.
Dr. Mehta reported findings from 780,234 telemedicine encounters that occurred in the LATIN network in 2018. Telemedicine experts diagnosed 8,395 patients (1%) with STEMI, of which 3,872 (46%) were urgently treated at 47 hubs. A total of 3,015 (78%) were reperfused with PCI. Time-to-telemedicine diagnosis averaged 3.5 minutes. “It used to take us 11 minutes of time to make a diagnosis by telemedicine,” Dr. Mehta said. “By the time we were done with the trial, the time to diagnosis was brought down to 3.5 minutes.” Average door-to-balloon time was 48 minutes and the STEMI mortality was 5.2%. This represents a 55% reduction in STEMI mortality from when LATIN began as a pilot project in 2013, Dr. Mehta said.
Hypertension was the most prevalent underlying disease (59%), followed by smoking (30%) and diabetes (29%), and the male to female STEMI diagnosis ratio was 1.71. The chief reason for nontreatment was coverage denial from insurance carriers (71%). “Getting payers onboard is extremely difficult, because being located here in Miami, is it very hard for me to convince them about the importance of supporting these people,” Dr. Mehta said. “However, as time has passed [and with] coverage of LATIN by the media, the program has become better known. We have been able to work mainly through the health secretaries [in these four countries], but is difficult from there onward.”
LATIN investigators faced other hurdles, which were unique in each of the four countries. “In Colombia, we were facing all sorts of geographical challenges; Brazil was challenging because of its size of the country and [difficulty establishing relationships with] some of the inner-city hospitals,” he said. “Mexico and Argentina were unique from the telemedicine point of view.” The fact that the care of LATIN patients was navigated from one of three telemedicine diagnosis centers “demonstrates the ability of telemedicine,” he said. “If I am able to guide a patient in Mexico from Bogotá, Colombia, it should be easy to guide a patient from Miami who’s presenting in Zambia.”
Dealing with the lack of ambulance services in Brazil, Colombia, Mexico, and Argentina has also been a hitch to the effort. “There is either a complete lack of ambulances or there is no central ambulance system,” he said. “In one of the earlier cities where we started the program in Colombia, 84% of patients used to self-transport. At the moment, 79% are being transported by ambulance. So, the halo effect of how LATIN has helped MI management has been impressive.”
Despite the lack of a comparator study as robust as LATIN, the program was estimated to reach between $39.6 million and $119 million USD total savings during the study period. This includes the cost of tele-emergency encounters, avoided transfers, and the cost of transportation. The investigators project that by the year 2026, 5 million patients could be triaged by this telemedicine pathway, saving $249 million. “As we are getting excited about the developments and the possibilities of telemedicine in the COVID-19 era, I think the work of LATIN becomes all the more relevant,” Dr. Mehta said during his main presentation.
During the press briefing, Timothy D. Henry, MD, praised the success of LATIN in reaching an underserved population. “The majority of these patients 10 years ago were not being treated with any reperfusion therapy at all,” said Dr. Henry, medical director of The Carl and Edyth Lindner Center for Research and Education at The Christ Hospital in Cincinnati. “With rapid diagnosis and the process of putting [LATIN] in place, that has increased to the point where 78% are now getting primary PCI. That is remarkable.”
LATIN was supported by an educational grant from the Medtronic Foundation. Dr. Mehta and Dr. Henry both reported having no financial disclosures.
A novel telemedicine approach to remotely guide ST-segment elevation myocardial infarction treatment in four Latin American countries screened more than 780,000 patients and resulted in a mortality rate of 5.2%, results from a 1-year, prospective, observational study showed.
“We have created a modality where the care of acute MI can be remotely guided,” lead investigator Sameer Mehta, MD, MBA, said during a press briefing at the Society for Cardiovascular Angiography & Interventions virtual annual scientific sessions. “This flattens the disparity between the developed and the developing countries, particularly in the poorer parts of Africa, the Middle East, and Southeast Asia.”
Dr. Mehta, chairman of the Lumen Foundation in Miami, and colleagues developed a “hub and spoke” platform to expand STEMI access to more than 100 million people in Brazil, Colombia, Mexico, and Argentina. For the effort, known as the Latin America Telemedicine Infarct Network (LATIN), “spokes” consisted of small clinics and primary health care centers in remote locations, while the “hubs” were medical centers that provided percutaneous coronary intervention (PCI) and/or coronary artery bypass graft (CABG) surgery. There were 313 spokes, 47 hubs, and more than 2,000 health care professionals who participated in the endeavor, including about 600 physicians.
The study, which is the largest of its kind, implemented a 3T strategy: telemedicine, triage, and transport, “which was the hardest part,” Dr. Mehta said. “In some cases, the spokes were located up to 300 miles away from the hubs. Up to 11% of these spokes in the remote areas did not even have a physician. Some had nurses who were triaging the patients.”
Patients who presented at spoke sites were enrolled into LATIN and data were collected through a form that included patient demographics, previous medical history, and an ECG. This information was sent through an app to one of three telemedicine diagnosis centers with 24/7 access to a cardiologist: one in Colombia, one in Brazil, one in Argentina. Once STEMI was identified by ECG, the STEMI protocol was activated, sending alerts to both designated hub and spoke sites and triggering ambulance dispatch. At the spoke sites, thrombolysis, a pharmaco-invasive strategy, or a primary PCI was performed, depending on case and treatment availability. Patients with successful thrombolysis were stabilized for up to 24 hours before transferral to a hub. Patients for whom reperfusion failed were transferred immediately to a hub for rescue PCI.
Dr. Mehta reported findings from 780,234 telemedicine encounters that occurred in the LATIN network in 2018. Telemedicine experts diagnosed 8,395 patients (1%) with STEMI, of which 3,872 (46%) were urgently treated at 47 hubs. A total of 3,015 (78%) were reperfused with PCI. Time-to-telemedicine diagnosis averaged 3.5 minutes. “It used to take us 11 minutes of time to make a diagnosis by telemedicine,” Dr. Mehta said. “By the time we were done with the trial, the time to diagnosis was brought down to 3.5 minutes.” Average door-to-balloon time was 48 minutes and the STEMI mortality was 5.2%. This represents a 55% reduction in STEMI mortality from when LATIN began as a pilot project in 2013, Dr. Mehta said.
Hypertension was the most prevalent underlying disease (59%), followed by smoking (30%) and diabetes (29%), and the male to female STEMI diagnosis ratio was 1.71. The chief reason for nontreatment was coverage denial from insurance carriers (71%). “Getting payers onboard is extremely difficult, because being located here in Miami, is it very hard for me to convince them about the importance of supporting these people,” Dr. Mehta said. “However, as time has passed [and with] coverage of LATIN by the media, the program has become better known. We have been able to work mainly through the health secretaries [in these four countries], but is difficult from there onward.”
LATIN investigators faced other hurdles, which were unique in each of the four countries. “In Colombia, we were facing all sorts of geographical challenges; Brazil was challenging because of its size of the country and [difficulty establishing relationships with] some of the inner-city hospitals,” he said. “Mexico and Argentina were unique from the telemedicine point of view.” The fact that the care of LATIN patients was navigated from one of three telemedicine diagnosis centers “demonstrates the ability of telemedicine,” he said. “If I am able to guide a patient in Mexico from Bogotá, Colombia, it should be easy to guide a patient from Miami who’s presenting in Zambia.”
Dealing with the lack of ambulance services in Brazil, Colombia, Mexico, and Argentina has also been a hitch to the effort. “There is either a complete lack of ambulances or there is no central ambulance system,” he said. “In one of the earlier cities where we started the program in Colombia, 84% of patients used to self-transport. At the moment, 79% are being transported by ambulance. So, the halo effect of how LATIN has helped MI management has been impressive.”
Despite the lack of a comparator study as robust as LATIN, the program was estimated to reach between $39.6 million and $119 million USD total savings during the study period. This includes the cost of tele-emergency encounters, avoided transfers, and the cost of transportation. The investigators project that by the year 2026, 5 million patients could be triaged by this telemedicine pathway, saving $249 million. “As we are getting excited about the developments and the possibilities of telemedicine in the COVID-19 era, I think the work of LATIN becomes all the more relevant,” Dr. Mehta said during his main presentation.
During the press briefing, Timothy D. Henry, MD, praised the success of LATIN in reaching an underserved population. “The majority of these patients 10 years ago were not being treated with any reperfusion therapy at all,” said Dr. Henry, medical director of The Carl and Edyth Lindner Center for Research and Education at The Christ Hospital in Cincinnati. “With rapid diagnosis and the process of putting [LATIN] in place, that has increased to the point where 78% are now getting primary PCI. That is remarkable.”
LATIN was supported by an educational grant from the Medtronic Foundation. Dr. Mehta and Dr. Henry both reported having no financial disclosures.
A novel telemedicine approach to remotely guide ST-segment elevation myocardial infarction treatment in four Latin American countries screened more than 780,000 patients and resulted in a mortality rate of 5.2%, results from a 1-year, prospective, observational study showed.
“We have created a modality where the care of acute MI can be remotely guided,” lead investigator Sameer Mehta, MD, MBA, said during a press briefing at the Society for Cardiovascular Angiography & Interventions virtual annual scientific sessions. “This flattens the disparity between the developed and the developing countries, particularly in the poorer parts of Africa, the Middle East, and Southeast Asia.”
Dr. Mehta, chairman of the Lumen Foundation in Miami, and colleagues developed a “hub and spoke” platform to expand STEMI access to more than 100 million people in Brazil, Colombia, Mexico, and Argentina. For the effort, known as the Latin America Telemedicine Infarct Network (LATIN), “spokes” consisted of small clinics and primary health care centers in remote locations, while the “hubs” were medical centers that provided percutaneous coronary intervention (PCI) and/or coronary artery bypass graft (CABG) surgery. There were 313 spokes, 47 hubs, and more than 2,000 health care professionals who participated in the endeavor, including about 600 physicians.
The study, which is the largest of its kind, implemented a 3T strategy: telemedicine, triage, and transport, “which was the hardest part,” Dr. Mehta said. “In some cases, the spokes were located up to 300 miles away from the hubs. Up to 11% of these spokes in the remote areas did not even have a physician. Some had nurses who were triaging the patients.”
Patients who presented at spoke sites were enrolled into LATIN and data were collected through a form that included patient demographics, previous medical history, and an ECG. This information was sent through an app to one of three telemedicine diagnosis centers with 24/7 access to a cardiologist: one in Colombia, one in Brazil, one in Argentina. Once STEMI was identified by ECG, the STEMI protocol was activated, sending alerts to both designated hub and spoke sites and triggering ambulance dispatch. At the spoke sites, thrombolysis, a pharmaco-invasive strategy, or a primary PCI was performed, depending on case and treatment availability. Patients with successful thrombolysis were stabilized for up to 24 hours before transferral to a hub. Patients for whom reperfusion failed were transferred immediately to a hub for rescue PCI.
Dr. Mehta reported findings from 780,234 telemedicine encounters that occurred in the LATIN network in 2018. Telemedicine experts diagnosed 8,395 patients (1%) with STEMI, of which 3,872 (46%) were urgently treated at 47 hubs. A total of 3,015 (78%) were reperfused with PCI. Time-to-telemedicine diagnosis averaged 3.5 minutes. “It used to take us 11 minutes of time to make a diagnosis by telemedicine,” Dr. Mehta said. “By the time we were done with the trial, the time to diagnosis was brought down to 3.5 minutes.” Average door-to-balloon time was 48 minutes and the STEMI mortality was 5.2%. This represents a 55% reduction in STEMI mortality from when LATIN began as a pilot project in 2013, Dr. Mehta said.
Hypertension was the most prevalent underlying disease (59%), followed by smoking (30%) and diabetes (29%), and the male to female STEMI diagnosis ratio was 1.71. The chief reason for nontreatment was coverage denial from insurance carriers (71%). “Getting payers onboard is extremely difficult, because being located here in Miami, is it very hard for me to convince them about the importance of supporting these people,” Dr. Mehta said. “However, as time has passed [and with] coverage of LATIN by the media, the program has become better known. We have been able to work mainly through the health secretaries [in these four countries], but is difficult from there onward.”
LATIN investigators faced other hurdles, which were unique in each of the four countries. “In Colombia, we were facing all sorts of geographical challenges; Brazil was challenging because of its size of the country and [difficulty establishing relationships with] some of the inner-city hospitals,” he said. “Mexico and Argentina were unique from the telemedicine point of view.” The fact that the care of LATIN patients was navigated from one of three telemedicine diagnosis centers “demonstrates the ability of telemedicine,” he said. “If I am able to guide a patient in Mexico from Bogotá, Colombia, it should be easy to guide a patient from Miami who’s presenting in Zambia.”
Dealing with the lack of ambulance services in Brazil, Colombia, Mexico, and Argentina has also been a hitch to the effort. “There is either a complete lack of ambulances or there is no central ambulance system,” he said. “In one of the earlier cities where we started the program in Colombia, 84% of patients used to self-transport. At the moment, 79% are being transported by ambulance. So, the halo effect of how LATIN has helped MI management has been impressive.”
Despite the lack of a comparator study as robust as LATIN, the program was estimated to reach between $39.6 million and $119 million USD total savings during the study period. This includes the cost of tele-emergency encounters, avoided transfers, and the cost of transportation. The investigators project that by the year 2026, 5 million patients could be triaged by this telemedicine pathway, saving $249 million. “As we are getting excited about the developments and the possibilities of telemedicine in the COVID-19 era, I think the work of LATIN becomes all the more relevant,” Dr. Mehta said during his main presentation.
During the press briefing, Timothy D. Henry, MD, praised the success of LATIN in reaching an underserved population. “The majority of these patients 10 years ago were not being treated with any reperfusion therapy at all,” said Dr. Henry, medical director of The Carl and Edyth Lindner Center for Research and Education at The Christ Hospital in Cincinnati. “With rapid diagnosis and the process of putting [LATIN] in place, that has increased to the point where 78% are now getting primary PCI. That is remarkable.”
LATIN was supported by an educational grant from the Medtronic Foundation. Dr. Mehta and Dr. Henry both reported having no financial disclosures.
REPORTING FROM SCAI 2020
Bariatric surgery in advanced heart failure wins transplant eligibility
Bariatric surgery is a safe and effective means for obese patients with advanced heart failure supported by a left ventricular assist device to qualify for heart transplantation, Praneet Wander, MD, reported in an abstract released as part of the annual Digestive Disease Week®.
She presented a systematic review and meta-analysis of nine retrospective or cross-sectional cohort studies totaling
Of the 86 patients, 50 (58%) were able to drop their BMI below 35, a requirement for inclusion on the heart transplant waiting list, noted Dr. Wander, a gastroenterology fellow at Hofstra University, Hempstead, N.Y., and North Shore LIJ Hospital in Manhasset, N.Y.
“A lot of bariatric surgeons don’t feel comfortable operating on patients who have a low ejection fraction,” she explained in an interview. “This study should encourage bariatric surgeons to do procedures even in patients with advanced heart failure so they can meet the BMI requirement for heart transplantation.”
Even if patients don’t actually undergo heart transplantation because of the perpetual donor organ shortage or inability to meet non–BMI-related eligibility criteria, they gain other major benefits from bariatric surgery: Their blood pressure goes down, their diabetes improves, and they become better able to engage in physical activity, she added.
Of the 86 patients in the meta-analysis, 84 underwent laparoscopic sleeve gastrectomy. That’s the preferred bariatric operation in patients with advanced heart failure at the Mayo Clinic as well, according to Andres J. Acosta, MD, PhD, a gastroenterologist at the medical center in Rochester, Minn.
There’s less weight loss achieved than with an open Roux-en-Y gastric bypass, but it’s a simpler operation in these high-risk patients, who typically have multiple comorbid conditions, he explained.
He predicted that Dr. Wander’s study will indeed influence bariatric surgeons at tertiary medical centers around the country to become more willing to consider weight-loss surgery in patients with advanced heart failure, while those in community practice will likely continue to be most comfortable operating on more stable patients with minimal comorbidities aside from their obesity.
“Data such as [these] will be reassuring to bariatric surgery programs such as ours, where we’re able to say: ‘Yes, there are risks, but these patients will benefit in the long term if we assume those risks,’ ” Dr. Acosta said.
He’s confident that, in the near future, the preferred form of bariatric surgery in patients with advanced heart failure will be a minimally invasive procedure performed endoscopically by gastroenterologists. He and his Mayo Clinic colleagues have already established a track record of success with endoscopic sleeve gastrectomy in patients with advanced kidney, liver, or lung disease in order to make them eligible for transplantation, as well as for the ancillary benefits provided by massive weight loss.
“There’s a little less weight loss than with laparoscopic sleeve gastrectomy, but it’s a significantly less risky operation. Shorter operative time, shorter hospital length of stay, less risk of infections and leaks,” he said in an interview. “We haven’t done it yet in heart disease, but I think based on this study this should be the next step at Mayo.”
Radha Gopalan, MD, director of heart failure and transplantation at Banner–University Medical Center in Phoenix, pronounced Dr. Wander’s meta-analysis “a positive study that’s very supportive of what we’re doing at our center.
“At a busy heart transplant center like ours, we are comfortable managing these patients, so the bariatric surgeons are reassured that the heart failure team is behind them. The risk of the procedure is mitigated by the availability of the multidisciplinary team to get the patient with obesity and heart failure through the surgery,” he explained.
Dr. Gopalan heads a novel bariatric heart failure program at Banner. While Dr. Wander’s meta-analysis focused on bariatric surgery in heart failure patients on LVAD circulatory support, Dr. Gopalan and colleagues are moving the intervention upstream. Roughly roughly 80% of patients in his bariatric heart failure program who meet criteria for LVAD implantation are now offered bariatric surgery before an LVAD is put in.
“I am moving away from putting the LVAD in first and then doing bariatric surgery. We have gotten comfortable taking these patients for bariatric surgery with inotropic support before going to the LVAD, which has the potential to even eliminate the requirement for an LVAD. Some patients get so much better that they become transplant ineligible,” Dr. Gopalan said.
Dr. Wander reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Wander P. DDW 2020 Abstract, #Mo2010.
Bariatric surgery is a safe and effective means for obese patients with advanced heart failure supported by a left ventricular assist device to qualify for heart transplantation, Praneet Wander, MD, reported in an abstract released as part of the annual Digestive Disease Week®.
She presented a systematic review and meta-analysis of nine retrospective or cross-sectional cohort studies totaling
Of the 86 patients, 50 (58%) were able to drop their BMI below 35, a requirement for inclusion on the heart transplant waiting list, noted Dr. Wander, a gastroenterology fellow at Hofstra University, Hempstead, N.Y., and North Shore LIJ Hospital in Manhasset, N.Y.
“A lot of bariatric surgeons don’t feel comfortable operating on patients who have a low ejection fraction,” she explained in an interview. “This study should encourage bariatric surgeons to do procedures even in patients with advanced heart failure so they can meet the BMI requirement for heart transplantation.”
Even if patients don’t actually undergo heart transplantation because of the perpetual donor organ shortage or inability to meet non–BMI-related eligibility criteria, they gain other major benefits from bariatric surgery: Their blood pressure goes down, their diabetes improves, and they become better able to engage in physical activity, she added.
Of the 86 patients in the meta-analysis, 84 underwent laparoscopic sleeve gastrectomy. That’s the preferred bariatric operation in patients with advanced heart failure at the Mayo Clinic as well, according to Andres J. Acosta, MD, PhD, a gastroenterologist at the medical center in Rochester, Minn.
There’s less weight loss achieved than with an open Roux-en-Y gastric bypass, but it’s a simpler operation in these high-risk patients, who typically have multiple comorbid conditions, he explained.
He predicted that Dr. Wander’s study will indeed influence bariatric surgeons at tertiary medical centers around the country to become more willing to consider weight-loss surgery in patients with advanced heart failure, while those in community practice will likely continue to be most comfortable operating on more stable patients with minimal comorbidities aside from their obesity.
“Data such as [these] will be reassuring to bariatric surgery programs such as ours, where we’re able to say: ‘Yes, there are risks, but these patients will benefit in the long term if we assume those risks,’ ” Dr. Acosta said.
He’s confident that, in the near future, the preferred form of bariatric surgery in patients with advanced heart failure will be a minimally invasive procedure performed endoscopically by gastroenterologists. He and his Mayo Clinic colleagues have already established a track record of success with endoscopic sleeve gastrectomy in patients with advanced kidney, liver, or lung disease in order to make them eligible for transplantation, as well as for the ancillary benefits provided by massive weight loss.
“There’s a little less weight loss than with laparoscopic sleeve gastrectomy, but it’s a significantly less risky operation. Shorter operative time, shorter hospital length of stay, less risk of infections and leaks,” he said in an interview. “We haven’t done it yet in heart disease, but I think based on this study this should be the next step at Mayo.”
Radha Gopalan, MD, director of heart failure and transplantation at Banner–University Medical Center in Phoenix, pronounced Dr. Wander’s meta-analysis “a positive study that’s very supportive of what we’re doing at our center.
“At a busy heart transplant center like ours, we are comfortable managing these patients, so the bariatric surgeons are reassured that the heart failure team is behind them. The risk of the procedure is mitigated by the availability of the multidisciplinary team to get the patient with obesity and heart failure through the surgery,” he explained.
Dr. Gopalan heads a novel bariatric heart failure program at Banner. While Dr. Wander’s meta-analysis focused on bariatric surgery in heart failure patients on LVAD circulatory support, Dr. Gopalan and colleagues are moving the intervention upstream. Roughly roughly 80% of patients in his bariatric heart failure program who meet criteria for LVAD implantation are now offered bariatric surgery before an LVAD is put in.
“I am moving away from putting the LVAD in first and then doing bariatric surgery. We have gotten comfortable taking these patients for bariatric surgery with inotropic support before going to the LVAD, which has the potential to even eliminate the requirement for an LVAD. Some patients get so much better that they become transplant ineligible,” Dr. Gopalan said.
Dr. Wander reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Wander P. DDW 2020 Abstract, #Mo2010.
Bariatric surgery is a safe and effective means for obese patients with advanced heart failure supported by a left ventricular assist device to qualify for heart transplantation, Praneet Wander, MD, reported in an abstract released as part of the annual Digestive Disease Week®.
She presented a systematic review and meta-analysis of nine retrospective or cross-sectional cohort studies totaling
Of the 86 patients, 50 (58%) were able to drop their BMI below 35, a requirement for inclusion on the heart transplant waiting list, noted Dr. Wander, a gastroenterology fellow at Hofstra University, Hempstead, N.Y., and North Shore LIJ Hospital in Manhasset, N.Y.
“A lot of bariatric surgeons don’t feel comfortable operating on patients who have a low ejection fraction,” she explained in an interview. “This study should encourage bariatric surgeons to do procedures even in patients with advanced heart failure so they can meet the BMI requirement for heart transplantation.”
Even if patients don’t actually undergo heart transplantation because of the perpetual donor organ shortage or inability to meet non–BMI-related eligibility criteria, they gain other major benefits from bariatric surgery: Their blood pressure goes down, their diabetes improves, and they become better able to engage in physical activity, she added.
Of the 86 patients in the meta-analysis, 84 underwent laparoscopic sleeve gastrectomy. That’s the preferred bariatric operation in patients with advanced heart failure at the Mayo Clinic as well, according to Andres J. Acosta, MD, PhD, a gastroenterologist at the medical center in Rochester, Minn.
There’s less weight loss achieved than with an open Roux-en-Y gastric bypass, but it’s a simpler operation in these high-risk patients, who typically have multiple comorbid conditions, he explained.
He predicted that Dr. Wander’s study will indeed influence bariatric surgeons at tertiary medical centers around the country to become more willing to consider weight-loss surgery in patients with advanced heart failure, while those in community practice will likely continue to be most comfortable operating on more stable patients with minimal comorbidities aside from their obesity.
“Data such as [these] will be reassuring to bariatric surgery programs such as ours, where we’re able to say: ‘Yes, there are risks, but these patients will benefit in the long term if we assume those risks,’ ” Dr. Acosta said.
He’s confident that, in the near future, the preferred form of bariatric surgery in patients with advanced heart failure will be a minimally invasive procedure performed endoscopically by gastroenterologists. He and his Mayo Clinic colleagues have already established a track record of success with endoscopic sleeve gastrectomy in patients with advanced kidney, liver, or lung disease in order to make them eligible for transplantation, as well as for the ancillary benefits provided by massive weight loss.
“There’s a little less weight loss than with laparoscopic sleeve gastrectomy, but it’s a significantly less risky operation. Shorter operative time, shorter hospital length of stay, less risk of infections and leaks,” he said in an interview. “We haven’t done it yet in heart disease, but I think based on this study this should be the next step at Mayo.”
Radha Gopalan, MD, director of heart failure and transplantation at Banner–University Medical Center in Phoenix, pronounced Dr. Wander’s meta-analysis “a positive study that’s very supportive of what we’re doing at our center.
“At a busy heart transplant center like ours, we are comfortable managing these patients, so the bariatric surgeons are reassured that the heart failure team is behind them. The risk of the procedure is mitigated by the availability of the multidisciplinary team to get the patient with obesity and heart failure through the surgery,” he explained.
Dr. Gopalan heads a novel bariatric heart failure program at Banner. While Dr. Wander’s meta-analysis focused on bariatric surgery in heart failure patients on LVAD circulatory support, Dr. Gopalan and colleagues are moving the intervention upstream. Roughly roughly 80% of patients in his bariatric heart failure program who meet criteria for LVAD implantation are now offered bariatric surgery before an LVAD is put in.
“I am moving away from putting the LVAD in first and then doing bariatric surgery. We have gotten comfortable taking these patients for bariatric surgery with inotropic support before going to the LVAD, which has the potential to even eliminate the requirement for an LVAD. Some patients get so much better that they become transplant ineligible,” Dr. Gopalan said.
Dr. Wander reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Wander P. DDW 2020 Abstract, #Mo2010.
FROM DDW 2020
Sericin, a versatile silk protein, has multiple potential roles in dermatology
Inexpensively obtained as a silk industry by-product, sericin is a glycoprotein found to confer various biologic effects.1 The globular protein sericin has also long been known to exhibit antityrosinase and immunomodulatory activities.2,3 This column focuses on the wide range of emerging and potential applications of sericin in cutaneous treatments.
Protection against solar radiation and photoaging
Studies in mice to evaluate the potential antioxidant and skin-protective effects of sericin by Zhaorigetu et al. in 2003 revealed that, by diminishing oxidative stress, cyclooxygenase-2 protein, and cell proliferation, sericin exerted a photoprotective effect against acute harm and tumor promotion elicited by UVB.4
Using mouse skin models, Dash et al. showed in 2008 that the silk protein sericin derived from the tropical tasar silkworm is a robust antioxidant and photoprotective agent, displaying a capacity to block UVB-induced apoptosis in irradiated (30 mJ/cm2 UVB) human keratinocytes and, as compared with the mulberry silkworm, yielding protection against oxidative stress.5,6
In 2015, Berardesca et al. conducted a randomized, double-blind, vehicle-controlled, split-face study over 8 weeks in 40 women (ages 40-70 years) to assess the antiaging effects of topically applied combination therapy including gold silk sericin, niacinamide, and signaline. The investigators observed significant improvements in stratum corneum hydration, barrier function, skin elasticity, and roughness as compared with skin treated with the control formulation. They concluded that this combination formulation featuring gold silk sericin warrants attention in the arsenal for ameliorating signs of aging female facial skin.7
A year earlier, Aramwit and Bang introduced a bacterial nanocellulose gel shown to effectively release silk sericin for facial treatment. Formulated at a pH of 4.5, the bioactive mask exhibited an ultrafine and pure fiber network structure. The authors noted that the gel was less adhesive than the commercially available paper mask, while the silk sericin product displayed greater moisture absorption capacity. In vitro cytotoxicity assessments also revealed that the product is safe for facial treatments.8
Cosmeceutical antioxidant for hyperpigmentation
In 2019, Kumar et al. demonstrated the inhibitory effect of topically applied silk sericin derived from Antheraea assamensis against UV-induced melanogenesis in mouse melanoma. They suggested that the formulation shows promise as a cosmeceutical antioxidant agent designed to address hyperpigmentation.3
The previous year, Aramwit et al. demonstrated using an in vitro model that urea-extracted sericin displays a capacity to inhibit melanogenesis by hindering tyrosinase activity, attenuating inflammation and allergic reactions, and reducing the expression of microphthalmia-associated transcription factor, a marker of melanogenesis regulation, in melanocytes and keratinocytes.2
Potential use as an adjunct psoriasis treatment
A combination of naringin (from Citrus maxima) and sericin (from Bombyx mori) was evaluated in 2019 by Deenonpoe et al. for the treatment of psoriasis. They isolated human peripheral blood mononuclear cells from 10 healthy subjects and 10 patients with psoriasis. The combination formulation was much more effective than either compound alone in significantly reducing mRNA expression and the synthesis of proinflammatory cytokines in samples from psoriasis patients. The investigators concluded that the down-regulation of proinflammatory cytokines imparted by the naringin/sericin product points toward its possible clinical use as a complementary treatment for psoriasis and other inflammation-mediated conditions.9
Uremic pruritus and burn wounds
A randomized, double-blind, placebo-controlled 6-week study in 2012 conducted by Aramwit et al. assessed the use of sericin cream versus a cream base placebo in the treatment of uremic pruritus in 50 hemodialysis patients, 47 of whom completed the study. Significant differences in the creams were identified, with hydration vastly improved in patients using the sericin cream. Significant reductions in pruritus and dyspigmentation were also observed in the treatment group, with an overall quality of life improvement noted in relation to pain score.10
The ensuing year, Aramwit et al. showed that silk sericin promoted wound healing in vitro and, when added to silver sulfadiazine cream and evaluated in a randomized, double-blind, standard-controlled study, demonstrated clinical efficacy in healing burn wounds.11
Wound healing
An expanding body of research suggests the role of sericin in wound healing. In 2007, Aramwit et al. found that sericin, which boasts notable hydrophilic qualities, was effective as a wound-healing agent in rats. The tested sericin cream successfully reduced wound size and wound healing time was substantially shorter than in animals treated with control formula. Treatment for 15 days yielded complete healing, no ulceration, and higher collagen levels, as determined by histologic examination, in comparison with control.12 Other studies using sericin hydrogel as well as a sericin-based nanofibrous matrix with chitosan have demonstrated success in wound healing in mice.13,14
Human studies
In 2018, Napavichayanun et al. reported on the clinical efficacy and safety of bacterial cellulose wound dressings including silk sericin and PHMB as compared with Bactigras (an antiseptic dressing) as a control in split-thickness skin graft donor-site wound treatment. In this single-blinded, randomized, controlled study of 21 patients, pain scores were significantly lower and wound quality higher in the skin treated with the sericin product. The test formulation was protected against infection without inducing adverse effects.15
Previously, a silk sericin–releasing wound dressing introduced in 2014 was found to significantly diminish pain and promote more rapid healing in patients with split-thickness skin graft donor sites as compared with treatment with the Bactigras wound dressing.16
Sericin in tissue repair and as a drug delivery carrier
Sericin is associated with antioxidant and moisturizing properties as well as a mitogenic influence on mammalian cells, with a particular impact on keratinocytes and fibroblasts that render it useful in biomaterials designed for skin tissue repair.17
Wang et al. have cross-linked dialdehyde carboxymethyl cellulose with silk sericin derived from the B. mori cocoon to develop a film with impressive blood compatibility and cytocompatibility that shows potential for use as a wound dressing, artificial skin, and in tissue engineering.18
Similarly, Liang et al. have been successful in preparing a medical tissue glue incorporating a gelatin, sericin, and carboxymethyl chitosan blend solution, cross-linked with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide. The tissue glue has been found to offer notable biocompatibility and structural traits at low cost.19
Sericin protein also evinces potential as a biocompatible, bioviable carrier for drug delivery. Suktham et al. showed that resveratrol-loaded sericin nanoparticles robustly hindered growth of colorectal adenocarcinoma cells while cytotoxic to skin fibroblasts, suggesting the viability or potential of sericin nanoparticles as bionanocarriers in a drug delivery system.20 In addition, Tao et al. found silk sericin to be effective when blended with poly(vinyl alcohol) in a hydrogel with antibacterial properties as a drug delivery carrier with potential for use as wound dressing.21
Conclusion
Much more research is necessary, though, to explore how the antioxidant and moisturizing activities of the protein may be harnessed to confer skin-protective effects, especially against UV damage.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers,“The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com
References
1. Lamboni L et al. Biotechnol Adv. 2015 Dec;33(8):1855-67.
2. Aramwit P et al. Biol Res. 2018 Nov 29;51(1):54.
3. Kumar JP, Mandal BB. Photochem Photobiol Sci. 2019 Oct 9:18(10):2497-508.
4. Zhaorigetu S et al. J Photochem Photobiol B. 2003 Oct 15;71(1-3):11-7.
5. Dash R et al. Mol Cell Biochem. 2008 Apr;311(1-2):111-9.
6. Dash R et al. BMB Rep. 2008 Mar 31;41(3):236-41.
7. Berardesca E et al. Int J Cosmet Sci. 2015 Dec;37(6):606-12.
8. Aramwit P, Bang N. BMC Biotechnol. 2014 Dec 9;14:104.
9. Deenonpoe R et al. BMC Complement Altern Med. 2019 Jul 10;19(1):168.
10. Aramwit P et al. BMC Nephrol. 2012 Sep 24;13:119.
11. Aramwit P et al. Arch Dermatol Res. 2013 Sep;305(7):585-94.
12. Aramwit P, Sangcakul A. Biosci Biotechnol Biochem. 2007 Oct;71(10):2473-7.
13. Qi C et al. Biomater Sci. 2018 Nov 1;6(11):2859-70.
14. Sapru S et al. Acta Biomater. 2018 Sep 15;78:137-50.
15. Napavichayanun S et al. Arch Dermatol Res. 2018 Dec;310(10):795-805.
16. Siritientong T et al. Pharm Res. 2014 Jan;31(1):104-16.
17. Lamboni L et al. Biotechnol Adv. 2015 Dec;33(8):1855-67.
18. Wang P et al. Carbohydr Polym. 2019 May 15;212:403-11.
19. Liang M et al. J Appl Biomater Funct Mater. 2018 Apr;16(2):97-106.
20. Suktham K et al. Int J Pharm. 2018 Feb 15;537(1-2):48-56.
21. Tao G et al. Mater Sci Eng C Mater Biol Appl. 2019 Aug;101:341-51.
Inexpensively obtained as a silk industry by-product, sericin is a glycoprotein found to confer various biologic effects.1 The globular protein sericin has also long been known to exhibit antityrosinase and immunomodulatory activities.2,3 This column focuses on the wide range of emerging and potential applications of sericin in cutaneous treatments.
Protection against solar radiation and photoaging
Studies in mice to evaluate the potential antioxidant and skin-protective effects of sericin by Zhaorigetu et al. in 2003 revealed that, by diminishing oxidative stress, cyclooxygenase-2 protein, and cell proliferation, sericin exerted a photoprotective effect against acute harm and tumor promotion elicited by UVB.4
Using mouse skin models, Dash et al. showed in 2008 that the silk protein sericin derived from the tropical tasar silkworm is a robust antioxidant and photoprotective agent, displaying a capacity to block UVB-induced apoptosis in irradiated (30 mJ/cm2 UVB) human keratinocytes and, as compared with the mulberry silkworm, yielding protection against oxidative stress.5,6
In 2015, Berardesca et al. conducted a randomized, double-blind, vehicle-controlled, split-face study over 8 weeks in 40 women (ages 40-70 years) to assess the antiaging effects of topically applied combination therapy including gold silk sericin, niacinamide, and signaline. The investigators observed significant improvements in stratum corneum hydration, barrier function, skin elasticity, and roughness as compared with skin treated with the control formulation. They concluded that this combination formulation featuring gold silk sericin warrants attention in the arsenal for ameliorating signs of aging female facial skin.7
A year earlier, Aramwit and Bang introduced a bacterial nanocellulose gel shown to effectively release silk sericin for facial treatment. Formulated at a pH of 4.5, the bioactive mask exhibited an ultrafine and pure fiber network structure. The authors noted that the gel was less adhesive than the commercially available paper mask, while the silk sericin product displayed greater moisture absorption capacity. In vitro cytotoxicity assessments also revealed that the product is safe for facial treatments.8
Cosmeceutical antioxidant for hyperpigmentation
In 2019, Kumar et al. demonstrated the inhibitory effect of topically applied silk sericin derived from Antheraea assamensis against UV-induced melanogenesis in mouse melanoma. They suggested that the formulation shows promise as a cosmeceutical antioxidant agent designed to address hyperpigmentation.3
The previous year, Aramwit et al. demonstrated using an in vitro model that urea-extracted sericin displays a capacity to inhibit melanogenesis by hindering tyrosinase activity, attenuating inflammation and allergic reactions, and reducing the expression of microphthalmia-associated transcription factor, a marker of melanogenesis regulation, in melanocytes and keratinocytes.2
Potential use as an adjunct psoriasis treatment
A combination of naringin (from Citrus maxima) and sericin (from Bombyx mori) was evaluated in 2019 by Deenonpoe et al. for the treatment of psoriasis. They isolated human peripheral blood mononuclear cells from 10 healthy subjects and 10 patients with psoriasis. The combination formulation was much more effective than either compound alone in significantly reducing mRNA expression and the synthesis of proinflammatory cytokines in samples from psoriasis patients. The investigators concluded that the down-regulation of proinflammatory cytokines imparted by the naringin/sericin product points toward its possible clinical use as a complementary treatment for psoriasis and other inflammation-mediated conditions.9
Uremic pruritus and burn wounds
A randomized, double-blind, placebo-controlled 6-week study in 2012 conducted by Aramwit et al. assessed the use of sericin cream versus a cream base placebo in the treatment of uremic pruritus in 50 hemodialysis patients, 47 of whom completed the study. Significant differences in the creams were identified, with hydration vastly improved in patients using the sericin cream. Significant reductions in pruritus and dyspigmentation were also observed in the treatment group, with an overall quality of life improvement noted in relation to pain score.10
The ensuing year, Aramwit et al. showed that silk sericin promoted wound healing in vitro and, when added to silver sulfadiazine cream and evaluated in a randomized, double-blind, standard-controlled study, demonstrated clinical efficacy in healing burn wounds.11
Wound healing
An expanding body of research suggests the role of sericin in wound healing. In 2007, Aramwit et al. found that sericin, which boasts notable hydrophilic qualities, was effective as a wound-healing agent in rats. The tested sericin cream successfully reduced wound size and wound healing time was substantially shorter than in animals treated with control formula. Treatment for 15 days yielded complete healing, no ulceration, and higher collagen levels, as determined by histologic examination, in comparison with control.12 Other studies using sericin hydrogel as well as a sericin-based nanofibrous matrix with chitosan have demonstrated success in wound healing in mice.13,14
Human studies
In 2018, Napavichayanun et al. reported on the clinical efficacy and safety of bacterial cellulose wound dressings including silk sericin and PHMB as compared with Bactigras (an antiseptic dressing) as a control in split-thickness skin graft donor-site wound treatment. In this single-blinded, randomized, controlled study of 21 patients, pain scores were significantly lower and wound quality higher in the skin treated with the sericin product. The test formulation was protected against infection without inducing adverse effects.15
Previously, a silk sericin–releasing wound dressing introduced in 2014 was found to significantly diminish pain and promote more rapid healing in patients with split-thickness skin graft donor sites as compared with treatment with the Bactigras wound dressing.16
Sericin in tissue repair and as a drug delivery carrier
Sericin is associated with antioxidant and moisturizing properties as well as a mitogenic influence on mammalian cells, with a particular impact on keratinocytes and fibroblasts that render it useful in biomaterials designed for skin tissue repair.17
Wang et al. have cross-linked dialdehyde carboxymethyl cellulose with silk sericin derived from the B. mori cocoon to develop a film with impressive blood compatibility and cytocompatibility that shows potential for use as a wound dressing, artificial skin, and in tissue engineering.18
Similarly, Liang et al. have been successful in preparing a medical tissue glue incorporating a gelatin, sericin, and carboxymethyl chitosan blend solution, cross-linked with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide. The tissue glue has been found to offer notable biocompatibility and structural traits at low cost.19
Sericin protein also evinces potential as a biocompatible, bioviable carrier for drug delivery. Suktham et al. showed that resveratrol-loaded sericin nanoparticles robustly hindered growth of colorectal adenocarcinoma cells while cytotoxic to skin fibroblasts, suggesting the viability or potential of sericin nanoparticles as bionanocarriers in a drug delivery system.20 In addition, Tao et al. found silk sericin to be effective when blended with poly(vinyl alcohol) in a hydrogel with antibacterial properties as a drug delivery carrier with potential for use as wound dressing.21
Conclusion
Much more research is necessary, though, to explore how the antioxidant and moisturizing activities of the protein may be harnessed to confer skin-protective effects, especially against UV damage.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers,“The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com
References
1. Lamboni L et al. Biotechnol Adv. 2015 Dec;33(8):1855-67.
2. Aramwit P et al. Biol Res. 2018 Nov 29;51(1):54.
3. Kumar JP, Mandal BB. Photochem Photobiol Sci. 2019 Oct 9:18(10):2497-508.
4. Zhaorigetu S et al. J Photochem Photobiol B. 2003 Oct 15;71(1-3):11-7.
5. Dash R et al. Mol Cell Biochem. 2008 Apr;311(1-2):111-9.
6. Dash R et al. BMB Rep. 2008 Mar 31;41(3):236-41.
7. Berardesca E et al. Int J Cosmet Sci. 2015 Dec;37(6):606-12.
8. Aramwit P, Bang N. BMC Biotechnol. 2014 Dec 9;14:104.
9. Deenonpoe R et al. BMC Complement Altern Med. 2019 Jul 10;19(1):168.
10. Aramwit P et al. BMC Nephrol. 2012 Sep 24;13:119.
11. Aramwit P et al. Arch Dermatol Res. 2013 Sep;305(7):585-94.
12. Aramwit P, Sangcakul A. Biosci Biotechnol Biochem. 2007 Oct;71(10):2473-7.
13. Qi C et al. Biomater Sci. 2018 Nov 1;6(11):2859-70.
14. Sapru S et al. Acta Biomater. 2018 Sep 15;78:137-50.
15. Napavichayanun S et al. Arch Dermatol Res. 2018 Dec;310(10):795-805.
16. Siritientong T et al. Pharm Res. 2014 Jan;31(1):104-16.
17. Lamboni L et al. Biotechnol Adv. 2015 Dec;33(8):1855-67.
18. Wang P et al. Carbohydr Polym. 2019 May 15;212:403-11.
19. Liang M et al. J Appl Biomater Funct Mater. 2018 Apr;16(2):97-106.
20. Suktham K et al. Int J Pharm. 2018 Feb 15;537(1-2):48-56.
21. Tao G et al. Mater Sci Eng C Mater Biol Appl. 2019 Aug;101:341-51.
Inexpensively obtained as a silk industry by-product, sericin is a glycoprotein found to confer various biologic effects.1 The globular protein sericin has also long been known to exhibit antityrosinase and immunomodulatory activities.2,3 This column focuses on the wide range of emerging and potential applications of sericin in cutaneous treatments.
Protection against solar radiation and photoaging
Studies in mice to evaluate the potential antioxidant and skin-protective effects of sericin by Zhaorigetu et al. in 2003 revealed that, by diminishing oxidative stress, cyclooxygenase-2 protein, and cell proliferation, sericin exerted a photoprotective effect against acute harm and tumor promotion elicited by UVB.4
Using mouse skin models, Dash et al. showed in 2008 that the silk protein sericin derived from the tropical tasar silkworm is a robust antioxidant and photoprotective agent, displaying a capacity to block UVB-induced apoptosis in irradiated (30 mJ/cm2 UVB) human keratinocytes and, as compared with the mulberry silkworm, yielding protection against oxidative stress.5,6
In 2015, Berardesca et al. conducted a randomized, double-blind, vehicle-controlled, split-face study over 8 weeks in 40 women (ages 40-70 years) to assess the antiaging effects of topically applied combination therapy including gold silk sericin, niacinamide, and signaline. The investigators observed significant improvements in stratum corneum hydration, barrier function, skin elasticity, and roughness as compared with skin treated with the control formulation. They concluded that this combination formulation featuring gold silk sericin warrants attention in the arsenal for ameliorating signs of aging female facial skin.7
A year earlier, Aramwit and Bang introduced a bacterial nanocellulose gel shown to effectively release silk sericin for facial treatment. Formulated at a pH of 4.5, the bioactive mask exhibited an ultrafine and pure fiber network structure. The authors noted that the gel was less adhesive than the commercially available paper mask, while the silk sericin product displayed greater moisture absorption capacity. In vitro cytotoxicity assessments also revealed that the product is safe for facial treatments.8
Cosmeceutical antioxidant for hyperpigmentation
In 2019, Kumar et al. demonstrated the inhibitory effect of topically applied silk sericin derived from Antheraea assamensis against UV-induced melanogenesis in mouse melanoma. They suggested that the formulation shows promise as a cosmeceutical antioxidant agent designed to address hyperpigmentation.3
The previous year, Aramwit et al. demonstrated using an in vitro model that urea-extracted sericin displays a capacity to inhibit melanogenesis by hindering tyrosinase activity, attenuating inflammation and allergic reactions, and reducing the expression of microphthalmia-associated transcription factor, a marker of melanogenesis regulation, in melanocytes and keratinocytes.2
Potential use as an adjunct psoriasis treatment
A combination of naringin (from Citrus maxima) and sericin (from Bombyx mori) was evaluated in 2019 by Deenonpoe et al. for the treatment of psoriasis. They isolated human peripheral blood mononuclear cells from 10 healthy subjects and 10 patients with psoriasis. The combination formulation was much more effective than either compound alone in significantly reducing mRNA expression and the synthesis of proinflammatory cytokines in samples from psoriasis patients. The investigators concluded that the down-regulation of proinflammatory cytokines imparted by the naringin/sericin product points toward its possible clinical use as a complementary treatment for psoriasis and other inflammation-mediated conditions.9
Uremic pruritus and burn wounds
A randomized, double-blind, placebo-controlled 6-week study in 2012 conducted by Aramwit et al. assessed the use of sericin cream versus a cream base placebo in the treatment of uremic pruritus in 50 hemodialysis patients, 47 of whom completed the study. Significant differences in the creams were identified, with hydration vastly improved in patients using the sericin cream. Significant reductions in pruritus and dyspigmentation were also observed in the treatment group, with an overall quality of life improvement noted in relation to pain score.10
The ensuing year, Aramwit et al. showed that silk sericin promoted wound healing in vitro and, when added to silver sulfadiazine cream and evaluated in a randomized, double-blind, standard-controlled study, demonstrated clinical efficacy in healing burn wounds.11
Wound healing
An expanding body of research suggests the role of sericin in wound healing. In 2007, Aramwit et al. found that sericin, which boasts notable hydrophilic qualities, was effective as a wound-healing agent in rats. The tested sericin cream successfully reduced wound size and wound healing time was substantially shorter than in animals treated with control formula. Treatment for 15 days yielded complete healing, no ulceration, and higher collagen levels, as determined by histologic examination, in comparison with control.12 Other studies using sericin hydrogel as well as a sericin-based nanofibrous matrix with chitosan have demonstrated success in wound healing in mice.13,14
Human studies
In 2018, Napavichayanun et al. reported on the clinical efficacy and safety of bacterial cellulose wound dressings including silk sericin and PHMB as compared with Bactigras (an antiseptic dressing) as a control in split-thickness skin graft donor-site wound treatment. In this single-blinded, randomized, controlled study of 21 patients, pain scores were significantly lower and wound quality higher in the skin treated with the sericin product. The test formulation was protected against infection without inducing adverse effects.15
Previously, a silk sericin–releasing wound dressing introduced in 2014 was found to significantly diminish pain and promote more rapid healing in patients with split-thickness skin graft donor sites as compared with treatment with the Bactigras wound dressing.16
Sericin in tissue repair and as a drug delivery carrier
Sericin is associated with antioxidant and moisturizing properties as well as a mitogenic influence on mammalian cells, with a particular impact on keratinocytes and fibroblasts that render it useful in biomaterials designed for skin tissue repair.17
Wang et al. have cross-linked dialdehyde carboxymethyl cellulose with silk sericin derived from the B. mori cocoon to develop a film with impressive blood compatibility and cytocompatibility that shows potential for use as a wound dressing, artificial skin, and in tissue engineering.18
Similarly, Liang et al. have been successful in preparing a medical tissue glue incorporating a gelatin, sericin, and carboxymethyl chitosan blend solution, cross-linked with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide. The tissue glue has been found to offer notable biocompatibility and structural traits at low cost.19
Sericin protein also evinces potential as a biocompatible, bioviable carrier for drug delivery. Suktham et al. showed that resveratrol-loaded sericin nanoparticles robustly hindered growth of colorectal adenocarcinoma cells while cytotoxic to skin fibroblasts, suggesting the viability or potential of sericin nanoparticles as bionanocarriers in a drug delivery system.20 In addition, Tao et al. found silk sericin to be effective when blended with poly(vinyl alcohol) in a hydrogel with antibacterial properties as a drug delivery carrier with potential for use as wound dressing.21
Conclusion
Much more research is necessary, though, to explore how the antioxidant and moisturizing activities of the protein may be harnessed to confer skin-protective effects, especially against UV damage.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers,“The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com
References
1. Lamboni L et al. Biotechnol Adv. 2015 Dec;33(8):1855-67.
2. Aramwit P et al. Biol Res. 2018 Nov 29;51(1):54.
3. Kumar JP, Mandal BB. Photochem Photobiol Sci. 2019 Oct 9:18(10):2497-508.
4. Zhaorigetu S et al. J Photochem Photobiol B. 2003 Oct 15;71(1-3):11-7.
5. Dash R et al. Mol Cell Biochem. 2008 Apr;311(1-2):111-9.
6. Dash R et al. BMB Rep. 2008 Mar 31;41(3):236-41.
7. Berardesca E et al. Int J Cosmet Sci. 2015 Dec;37(6):606-12.
8. Aramwit P, Bang N. BMC Biotechnol. 2014 Dec 9;14:104.
9. Deenonpoe R et al. BMC Complement Altern Med. 2019 Jul 10;19(1):168.
10. Aramwit P et al. BMC Nephrol. 2012 Sep 24;13:119.
11. Aramwit P et al. Arch Dermatol Res. 2013 Sep;305(7):585-94.
12. Aramwit P, Sangcakul A. Biosci Biotechnol Biochem. 2007 Oct;71(10):2473-7.
13. Qi C et al. Biomater Sci. 2018 Nov 1;6(11):2859-70.
14. Sapru S et al. Acta Biomater. 2018 Sep 15;78:137-50.
15. Napavichayanun S et al. Arch Dermatol Res. 2018 Dec;310(10):795-805.
16. Siritientong T et al. Pharm Res. 2014 Jan;31(1):104-16.
17. Lamboni L et al. Biotechnol Adv. 2015 Dec;33(8):1855-67.
18. Wang P et al. Carbohydr Polym. 2019 May 15;212:403-11.
19. Liang M et al. J Appl Biomater Funct Mater. 2018 Apr;16(2):97-106.
20. Suktham K et al. Int J Pharm. 2018 Feb 15;537(1-2):48-56.
21. Tao G et al. Mater Sci Eng C Mater Biol Appl. 2019 Aug;101:341-51.
What's your diagnosis?
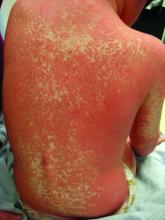
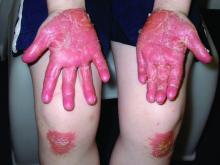
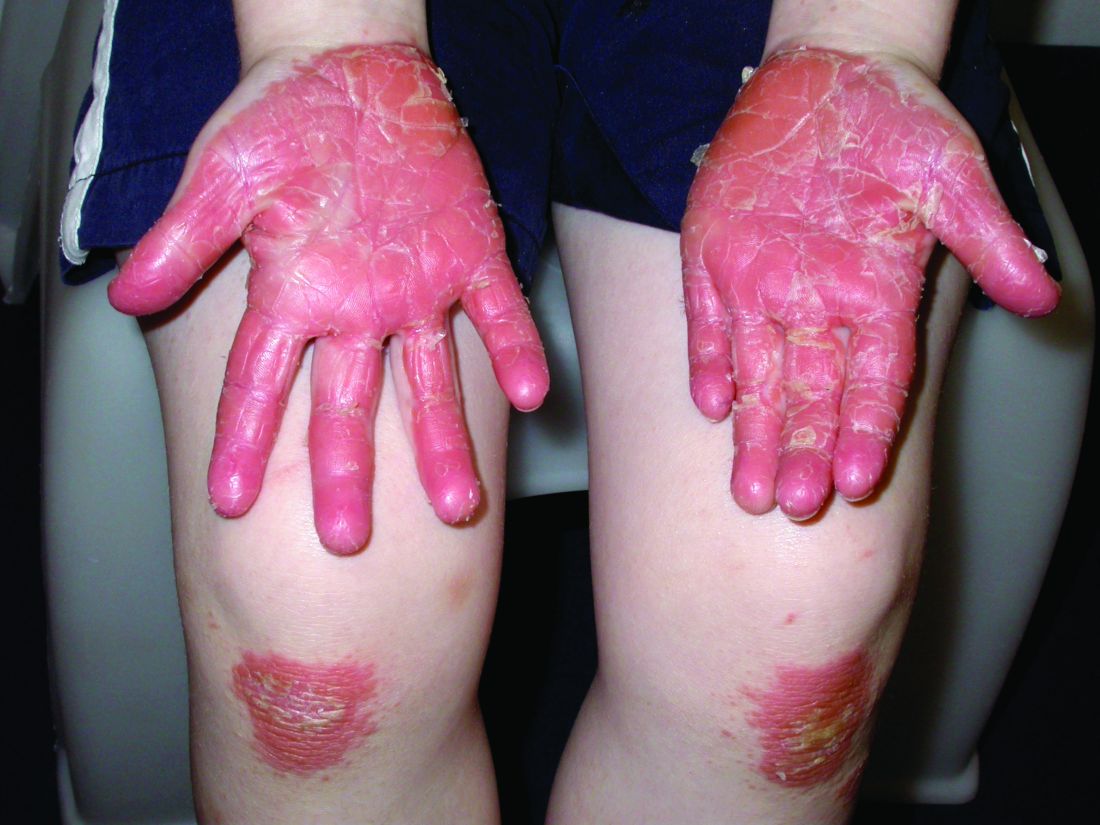
Pityriasis rubra pilaris (PRP) is the name given to a heterogeneous group of rare inflammatory papulosquamous dermatoses. There are six sub-types that can present with various skin findings, however, the cardinal features across sub-types include well-defined, red-orange hued plaques with varying scale, palmoplantar keratoderma, and follicular keratosis. In the more generalized subtypes, there is a characteristic feature of intervening areas of unaffected skin often referred to as “islands of sparing.” The plaques may cover the entire body or just parts of the body such as the elbows and knees, palms and soles. Lesions are generally asymptomatic; occasionally patients complain of mild pruritus.
The etiology and pathophysiology of this group of disorders is not well understood. However, there are several hypotheses including dysfunction in vitamin A metabolism, autoimmune dysregulation, as well as environmental and immunologic triggers such as infection and ultraviolet exposure. Although most cases are sporadic, genetics do seem to play a role in the development of some cases. Caspase recruitment domain-containing protein 14 (CARD14) mutations are seen in familial PRP, and occasionally in patients with sporadic PRP, with gain of function mutations. Interestingly, CARD14 mutations are also associated with psoriasis in some individuals.1 The type-VI PRP variant has been associated with HIV, although this is incredibly rare in pediatrics.2
PRP shows significant clinical diversity, with six subtypes defined by age of onset, distribution, and appearance of lesions, and presence of HIV. This includes type I (classical adult onset), type II (atypical adult onset), type III (classical juvenile onset), type IV (circumscribed juvenile onset), type V (atypical juvenile onset), and type VI (HIV-associated). As mentioned earlier, shared features that appear across subtypes in variable degrees include red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis.
Of the six subtypes, type III, IV, and V occur in the pediatric population. Type III, classic juvenile PRP, typically occurs within the first 2 years of life or in adolescence. Only 10% of cases fall into this category. It shares similar features to type I PRP including red-orange plaques; islands of sparing, perifollicular hyperkeratotic papules; waxy palmoplantar keratoderma; and the distribution of affected skin is more diffuse overall. While some children clear within a few years, more recent studies stress a more prolonged course similar to the type IV variant.2
Type-IV PRP, also known as circumscribed juvenile PRP, is a focal variant, usually seen in prepubertal children and making up 25% of total cases. Clinically, these patients tend to have sharply demarcated grouped erythematous, follicular papules on the elbows, knees and over bony prominences.2
Type-V PRP is an atypical generalized juvenile variant which affects 5% of patients. It is a non-remitting hereditary condition with classic characteristics similar to type III with additional scleroderma-like changes involving the palms and soles.2
Diagnosis of PRP is based on clinical recognition and biopsy can be important to secure a diagnosis.
PRP, in many cases is self-limited and asymptomatic, and therefore does not necessarily require treatment. In other patients treatment can be challenging, and referral to a pediatric dermatology specialist is reasonable. Most practitioners recommend combination therapy with topical agents (emollients, topical corticosteroids, tazarotene, topical calcineurin inhibitors, and keratolytic agents such as urea, salicylic acid, or alpha-hydroxy acids) for symptomatic management and systemic therapies (methotrexate, isotretinoin) aimed at reducing inflammation. There is some data that CARD14-associated PRP can respond well to targeted biologic therapies.1
The subtypes of PRP can present in a myriad of ways and often the disease is misdiagnosed. Depending on the particular subtype and findings present, the differential can vary considerably. Commonly, physicians need to consider: psoriasis, seborrheic dermatitis, atopic dermatitis, ichthyoses, and other conditions which can cause erythroderma.3 The characteristic red-orange color and variable associated edema helps to distinguish keratoderma of PRP from psoriasis, atopic dermatitis, ichthyosis, and hereditary palmoplantar keratoderma. Scalp involvement of PRP should be differentiated from the waxy scale of seborrheic dermatitis and the well demarcated silvery scale of psoriasis. History alone may assist in distinguishing PRP from other major causes of generalized erythroderma, although biopsy is warranted in these cases.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital-San Diego and the University of California, San Diego. They have no relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. J Am Acad Dermatol. 2018 Sep;79(3):487-94.
2. “Pityriasis Rubra Pilaris” (Treasure Island, Fla.: StatPearls Publishing, July 20, 2019). 3. JAMA Dermatol. 2016 Jun 1;152(6):670-5.
Pityriasis rubra pilaris (PRP) is the name given to a heterogeneous group of rare inflammatory papulosquamous dermatoses. There are six sub-types that can present with various skin findings, however, the cardinal features across sub-types include well-defined, red-orange hued plaques with varying scale, palmoplantar keratoderma, and follicular keratosis. In the more generalized subtypes, there is a characteristic feature of intervening areas of unaffected skin often referred to as “islands of sparing.” The plaques may cover the entire body or just parts of the body such as the elbows and knees, palms and soles. Lesions are generally asymptomatic; occasionally patients complain of mild pruritus.
The etiology and pathophysiology of this group of disorders is not well understood. However, there are several hypotheses including dysfunction in vitamin A metabolism, autoimmune dysregulation, as well as environmental and immunologic triggers such as infection and ultraviolet exposure. Although most cases are sporadic, genetics do seem to play a role in the development of some cases. Caspase recruitment domain-containing protein 14 (CARD14) mutations are seen in familial PRP, and occasionally in patients with sporadic PRP, with gain of function mutations. Interestingly, CARD14 mutations are also associated with psoriasis in some individuals.1 The type-VI PRP variant has been associated with HIV, although this is incredibly rare in pediatrics.2
PRP shows significant clinical diversity, with six subtypes defined by age of onset, distribution, and appearance of lesions, and presence of HIV. This includes type I (classical adult onset), type II (atypical adult onset), type III (classical juvenile onset), type IV (circumscribed juvenile onset), type V (atypical juvenile onset), and type VI (HIV-associated). As mentioned earlier, shared features that appear across subtypes in variable degrees include red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis.
Of the six subtypes, type III, IV, and V occur in the pediatric population. Type III, classic juvenile PRP, typically occurs within the first 2 years of life or in adolescence. Only 10% of cases fall into this category. It shares similar features to type I PRP including red-orange plaques; islands of sparing, perifollicular hyperkeratotic papules; waxy palmoplantar keratoderma; and the distribution of affected skin is more diffuse overall. While some children clear within a few years, more recent studies stress a more prolonged course similar to the type IV variant.2
Type-IV PRP, also known as circumscribed juvenile PRP, is a focal variant, usually seen in prepubertal children and making up 25% of total cases. Clinically, these patients tend to have sharply demarcated grouped erythematous, follicular papules on the elbows, knees and over bony prominences.2
Type-V PRP is an atypical generalized juvenile variant which affects 5% of patients. It is a non-remitting hereditary condition with classic characteristics similar to type III with additional scleroderma-like changes involving the palms and soles.2
Diagnosis of PRP is based on clinical recognition and biopsy can be important to secure a diagnosis.
PRP, in many cases is self-limited and asymptomatic, and therefore does not necessarily require treatment. In other patients treatment can be challenging, and referral to a pediatric dermatology specialist is reasonable. Most practitioners recommend combination therapy with topical agents (emollients, topical corticosteroids, tazarotene, topical calcineurin inhibitors, and keratolytic agents such as urea, salicylic acid, or alpha-hydroxy acids) for symptomatic management and systemic therapies (methotrexate, isotretinoin) aimed at reducing inflammation. There is some data that CARD14-associated PRP can respond well to targeted biologic therapies.1
The subtypes of PRP can present in a myriad of ways and often the disease is misdiagnosed. Depending on the particular subtype and findings present, the differential can vary considerably. Commonly, physicians need to consider: psoriasis, seborrheic dermatitis, atopic dermatitis, ichthyoses, and other conditions which can cause erythroderma.3 The characteristic red-orange color and variable associated edema helps to distinguish keratoderma of PRP from psoriasis, atopic dermatitis, ichthyosis, and hereditary palmoplantar keratoderma. Scalp involvement of PRP should be differentiated from the waxy scale of seborrheic dermatitis and the well demarcated silvery scale of psoriasis. History alone may assist in distinguishing PRP from other major causes of generalized erythroderma, although biopsy is warranted in these cases.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital-San Diego and the University of California, San Diego. They have no relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. J Am Acad Dermatol. 2018 Sep;79(3):487-94.
2. “Pityriasis Rubra Pilaris” (Treasure Island, Fla.: StatPearls Publishing, July 20, 2019). 3. JAMA Dermatol. 2016 Jun 1;152(6):670-5.
Pityriasis rubra pilaris (PRP) is the name given to a heterogeneous group of rare inflammatory papulosquamous dermatoses. There are six sub-types that can present with various skin findings, however, the cardinal features across sub-types include well-defined, red-orange hued plaques with varying scale, palmoplantar keratoderma, and follicular keratosis. In the more generalized subtypes, there is a characteristic feature of intervening areas of unaffected skin often referred to as “islands of sparing.” The plaques may cover the entire body or just parts of the body such as the elbows and knees, palms and soles. Lesions are generally asymptomatic; occasionally patients complain of mild pruritus.
The etiology and pathophysiology of this group of disorders is not well understood. However, there are several hypotheses including dysfunction in vitamin A metabolism, autoimmune dysregulation, as well as environmental and immunologic triggers such as infection and ultraviolet exposure. Although most cases are sporadic, genetics do seem to play a role in the development of some cases. Caspase recruitment domain-containing protein 14 (CARD14) mutations are seen in familial PRP, and occasionally in patients with sporadic PRP, with gain of function mutations. Interestingly, CARD14 mutations are also associated with psoriasis in some individuals.1 The type-VI PRP variant has been associated with HIV, although this is incredibly rare in pediatrics.2
PRP shows significant clinical diversity, with six subtypes defined by age of onset, distribution, and appearance of lesions, and presence of HIV. This includes type I (classical adult onset), type II (atypical adult onset), type III (classical juvenile onset), type IV (circumscribed juvenile onset), type V (atypical juvenile onset), and type VI (HIV-associated). As mentioned earlier, shared features that appear across subtypes in variable degrees include red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis.
Of the six subtypes, type III, IV, and V occur in the pediatric population. Type III, classic juvenile PRP, typically occurs within the first 2 years of life or in adolescence. Only 10% of cases fall into this category. It shares similar features to type I PRP including red-orange plaques; islands of sparing, perifollicular hyperkeratotic papules; waxy palmoplantar keratoderma; and the distribution of affected skin is more diffuse overall. While some children clear within a few years, more recent studies stress a more prolonged course similar to the type IV variant.2
Type-IV PRP, also known as circumscribed juvenile PRP, is a focal variant, usually seen in prepubertal children and making up 25% of total cases. Clinically, these patients tend to have sharply demarcated grouped erythematous, follicular papules on the elbows, knees and over bony prominences.2
Type-V PRP is an atypical generalized juvenile variant which affects 5% of patients. It is a non-remitting hereditary condition with classic characteristics similar to type III with additional scleroderma-like changes involving the palms and soles.2
Diagnosis of PRP is based on clinical recognition and biopsy can be important to secure a diagnosis.
PRP, in many cases is self-limited and asymptomatic, and therefore does not necessarily require treatment. In other patients treatment can be challenging, and referral to a pediatric dermatology specialist is reasonable. Most practitioners recommend combination therapy with topical agents (emollients, topical corticosteroids, tazarotene, topical calcineurin inhibitors, and keratolytic agents such as urea, salicylic acid, or alpha-hydroxy acids) for symptomatic management and systemic therapies (methotrexate, isotretinoin) aimed at reducing inflammation. There is some data that CARD14-associated PRP can respond well to targeted biologic therapies.1
The subtypes of PRP can present in a myriad of ways and often the disease is misdiagnosed. Depending on the particular subtype and findings present, the differential can vary considerably. Commonly, physicians need to consider: psoriasis, seborrheic dermatitis, atopic dermatitis, ichthyoses, and other conditions which can cause erythroderma.3 The characteristic red-orange color and variable associated edema helps to distinguish keratoderma of PRP from psoriasis, atopic dermatitis, ichthyosis, and hereditary palmoplantar keratoderma. Scalp involvement of PRP should be differentiated from the waxy scale of seborrheic dermatitis and the well demarcated silvery scale of psoriasis. History alone may assist in distinguishing PRP from other major causes of generalized erythroderma, although biopsy is warranted in these cases.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital-San Diego and the University of California, San Diego. They have no relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. J Am Acad Dermatol. 2018 Sep;79(3):487-94.
2. “Pityriasis Rubra Pilaris” (Treasure Island, Fla.: StatPearls Publishing, July 20, 2019). 3. JAMA Dermatol. 2016 Jun 1;152(6):670-5.
A 10-year-old, otherwise healthy female with no prior significant medical history is brought into clinic for evaluation of orange-red scaly papules and plaques that first started on the face, neck, and fingers and began spreading to the trunk, arms, and knees. The mother of the patient also had noticed thickening of the skin on her palms and soles. The rash has been present for 2 months. Patient does not appear to be itchy, and otherwise is in normal state without pain, fever, drainage from sites, or known exposures. She was initially treated with topical triamcinolone with minimal improvement.
On physical exam, she is noted to have reddish-orange hyperkeratotic scaling papules coalescing into large plaques with follicular prominence diffusely on the face, neck, trunk, and upper extremities with smaller islands of skin that are normal-appearing. There is diffuse fine scale throughout the scalp and thickening of the skin on the palms and soles with a yellowish waxy appearance.
Hazard pay included in new COVID-19 relief bill
Hazard pay for frontline health care workers – an idea that has been championed by President Donald J. Trump and Senate Minority Leader Chuck Schumer, among others – is included in a just-released COVID-19 relief package assembled by Democrats in the House of Representatives.
according to a report in the Washington Post.
But it is far from a done deal. “The Democrats’ spending bill is a Pelosi-led pipe dream written in private,” said House Republican Leader Kevin McCarthy (Calif.) in a Fox News interview posted May 12 on Facebook.
Senate Majority Leader Mitch McConnell condemned the package. “This is exactly the wrong approach,” he said in a prepared statement that instead laid out a variety of liability protections, which he said should be the first priority.
“We are not going to let health care heroes emerge from this crisis facing a tidal wave of medical malpractice lawsuits so that trial lawyers can line their pockets,” said Sen. McConnell, adding that his plan would “raise the liability threshold for COVID-related malpractice lawsuits.”
Ingrida Lusis, vice president of government affairs and health policy at the American Nurses Association, said in an interview that the ANA had lobbied for hazard pay and was told it would be in the next relief package.
“Though there is an inherent risk in the nursing profession, we think that this is really critical to ensuring that we have a workforce to meet the intense demands of this pandemic,” said Ms. Lusis.
“If health care workers are not treated and compensated appropriately for what they’re going through right now, then we may not have a next generation that will want to enter the field,” she said.
Various nursing organizations, nurses’ unions, and health care unions, such as the American Federation of State, County and Municipal Employees (AFSCME) and the Service Employees International Union, have advocated for hazard pay.
Physicians’ organizations have not been vocal on the issue, however. The American Medical Association, for instance, pushed for hazard pay for residents but has not made any further public statements. An AMA spokesman said that the group was monitoring the situation but declined further comment.
Multiple online petitions seeking hazard pay for health care workers have been circulated, including one seeking the same $600 bump for essential workers that was given out as part of unemployment benefits in the first COVID-19 relief package. More than 1.2 million had signed the petition as of May 12.
‘Heroes fund’
The president first suggested hazard pay for health care workers on March 30 Fox News broadcast. “These are really brave people,” he said, adding that the administration was considering different ways of boosting pay, primarily through hospitals.
“We are asking the hospitals to do it and to consider something, including bonuses,” said Trump. “If anybody’s entitled to it, they are.”
On April 7, Sen. Schumer proposed a “Heroes Fund.” It would give public, private, and tribal frontline employees – including doctors, nurses, first responders, and transit, grocery, and postal workers – a $13 per hour raise up to $25,000 in additional pay through Dec. 31 for workers earning up to $200,000 and $5,000 in additional pay for those earning more than $200,000. It would also provide a $15,000 signing bonus to those who agree to take on such a position.
Rep. Matt Cartwright (D-Pa.) introduced a bill in mid-April, the Coronavirus Frontline Workers Fair Pay Act (HR 6709), that would provide similar pay increases. Health care workers would receive an additional $13 per hour. It would be retroactive to Jan. 31, 2020, and would be available through the end of 2020.
Molly Kinder of the Brookings Institution, a self-described nonpartisan Washington policy institute, estimates that Sen. Schumer’s proposal would represent the equivalent of double-time pay for the average low-wage worker, a 50% pay increase for a mail carrier, a 20% boost for a pharmacist, and less than a 15% increase for a surgeon, as determined from median 2018 wages.
Before the House Democrats unveiled their bill, Isabel Soto of the center-right group American Action Forum estimated that a $13 per hour wage increase could cost $398.9 billion just from the end of March to the end of September. A great proportion of that amount – $264 billion – would go to some 10 million health care workers, Ms. Soto calculated.
Some already offering pay boost
A few states and hospital systems are already offering hazard pay.
On April 12, Massachusetts agreed to give about 6,500 AFSCME union members who work at state human services facilities and group homes a $5 or a $10 per hour pay increase, depending on duties. It was to stay in effect until at least May 30.
Maine Governor Janet Mills (D) also agreed to increase pay by $3-$5 an hour for AFSCME workers in state correctional and mental health facilities beginning March 29.
In New York City, the biggest hospital network, Northwell Health, in late April gave 45,000 workers – including nurses, physicians, respiratory therapists, environmental services workers, housekeepers, and people in outpatient and corporate roles – a lump sum bonus payment of up to $2,500 and 1 week of paid time off. The money came out of the system’s general fund.
“As an organization, we want to continue to support, motivate and inspire our team members,” said Northwell President and CEO Michael Dowling in a statement at the time.
On April 2, New York–Presbyterian Hospital’s chair of the department of surgery, Craig Smith, MD, announced that the facility was “providing a $1,250 bonus for everyone who has worked in or supported the COVID-19 front lines, for at least 1 week.”
Advocate Aurora, with 15 hospitals and 32,000 employees in Wisconsin, said in early April that it was giving increases of $6.25-$15.00 an hour at least through the end of May.
A version of this article originally appeared on Medscape.com.
Hazard pay for frontline health care workers – an idea that has been championed by President Donald J. Trump and Senate Minority Leader Chuck Schumer, among others – is included in a just-released COVID-19 relief package assembled by Democrats in the House of Representatives.
according to a report in the Washington Post.
But it is far from a done deal. “The Democrats’ spending bill is a Pelosi-led pipe dream written in private,” said House Republican Leader Kevin McCarthy (Calif.) in a Fox News interview posted May 12 on Facebook.
Senate Majority Leader Mitch McConnell condemned the package. “This is exactly the wrong approach,” he said in a prepared statement that instead laid out a variety of liability protections, which he said should be the first priority.
“We are not going to let health care heroes emerge from this crisis facing a tidal wave of medical malpractice lawsuits so that trial lawyers can line their pockets,” said Sen. McConnell, adding that his plan would “raise the liability threshold for COVID-related malpractice lawsuits.”
Ingrida Lusis, vice president of government affairs and health policy at the American Nurses Association, said in an interview that the ANA had lobbied for hazard pay and was told it would be in the next relief package.
“Though there is an inherent risk in the nursing profession, we think that this is really critical to ensuring that we have a workforce to meet the intense demands of this pandemic,” said Ms. Lusis.
“If health care workers are not treated and compensated appropriately for what they’re going through right now, then we may not have a next generation that will want to enter the field,” she said.
Various nursing organizations, nurses’ unions, and health care unions, such as the American Federation of State, County and Municipal Employees (AFSCME) and the Service Employees International Union, have advocated for hazard pay.
Physicians’ organizations have not been vocal on the issue, however. The American Medical Association, for instance, pushed for hazard pay for residents but has not made any further public statements. An AMA spokesman said that the group was monitoring the situation but declined further comment.
Multiple online petitions seeking hazard pay for health care workers have been circulated, including one seeking the same $600 bump for essential workers that was given out as part of unemployment benefits in the first COVID-19 relief package. More than 1.2 million had signed the petition as of May 12.
‘Heroes fund’
The president first suggested hazard pay for health care workers on March 30 Fox News broadcast. “These are really brave people,” he said, adding that the administration was considering different ways of boosting pay, primarily through hospitals.
“We are asking the hospitals to do it and to consider something, including bonuses,” said Trump. “If anybody’s entitled to it, they are.”
On April 7, Sen. Schumer proposed a “Heroes Fund.” It would give public, private, and tribal frontline employees – including doctors, nurses, first responders, and transit, grocery, and postal workers – a $13 per hour raise up to $25,000 in additional pay through Dec. 31 for workers earning up to $200,000 and $5,000 in additional pay for those earning more than $200,000. It would also provide a $15,000 signing bonus to those who agree to take on such a position.
Rep. Matt Cartwright (D-Pa.) introduced a bill in mid-April, the Coronavirus Frontline Workers Fair Pay Act (HR 6709), that would provide similar pay increases. Health care workers would receive an additional $13 per hour. It would be retroactive to Jan. 31, 2020, and would be available through the end of 2020.
Molly Kinder of the Brookings Institution, a self-described nonpartisan Washington policy institute, estimates that Sen. Schumer’s proposal would represent the equivalent of double-time pay for the average low-wage worker, a 50% pay increase for a mail carrier, a 20% boost for a pharmacist, and less than a 15% increase for a surgeon, as determined from median 2018 wages.
Before the House Democrats unveiled their bill, Isabel Soto of the center-right group American Action Forum estimated that a $13 per hour wage increase could cost $398.9 billion just from the end of March to the end of September. A great proportion of that amount – $264 billion – would go to some 10 million health care workers, Ms. Soto calculated.
Some already offering pay boost
A few states and hospital systems are already offering hazard pay.
On April 12, Massachusetts agreed to give about 6,500 AFSCME union members who work at state human services facilities and group homes a $5 or a $10 per hour pay increase, depending on duties. It was to stay in effect until at least May 30.
Maine Governor Janet Mills (D) also agreed to increase pay by $3-$5 an hour for AFSCME workers in state correctional and mental health facilities beginning March 29.
In New York City, the biggest hospital network, Northwell Health, in late April gave 45,000 workers – including nurses, physicians, respiratory therapists, environmental services workers, housekeepers, and people in outpatient and corporate roles – a lump sum bonus payment of up to $2,500 and 1 week of paid time off. The money came out of the system’s general fund.
“As an organization, we want to continue to support, motivate and inspire our team members,” said Northwell President and CEO Michael Dowling in a statement at the time.
On April 2, New York–Presbyterian Hospital’s chair of the department of surgery, Craig Smith, MD, announced that the facility was “providing a $1,250 bonus for everyone who has worked in or supported the COVID-19 front lines, for at least 1 week.”
Advocate Aurora, with 15 hospitals and 32,000 employees in Wisconsin, said in early April that it was giving increases of $6.25-$15.00 an hour at least through the end of May.
A version of this article originally appeared on Medscape.com.
Hazard pay for frontline health care workers – an idea that has been championed by President Donald J. Trump and Senate Minority Leader Chuck Schumer, among others – is included in a just-released COVID-19 relief package assembled by Democrats in the House of Representatives.
according to a report in the Washington Post.
But it is far from a done deal. “The Democrats’ spending bill is a Pelosi-led pipe dream written in private,” said House Republican Leader Kevin McCarthy (Calif.) in a Fox News interview posted May 12 on Facebook.
Senate Majority Leader Mitch McConnell condemned the package. “This is exactly the wrong approach,” he said in a prepared statement that instead laid out a variety of liability protections, which he said should be the first priority.
“We are not going to let health care heroes emerge from this crisis facing a tidal wave of medical malpractice lawsuits so that trial lawyers can line their pockets,” said Sen. McConnell, adding that his plan would “raise the liability threshold for COVID-related malpractice lawsuits.”
Ingrida Lusis, vice president of government affairs and health policy at the American Nurses Association, said in an interview that the ANA had lobbied for hazard pay and was told it would be in the next relief package.
“Though there is an inherent risk in the nursing profession, we think that this is really critical to ensuring that we have a workforce to meet the intense demands of this pandemic,” said Ms. Lusis.
“If health care workers are not treated and compensated appropriately for what they’re going through right now, then we may not have a next generation that will want to enter the field,” she said.
Various nursing organizations, nurses’ unions, and health care unions, such as the American Federation of State, County and Municipal Employees (AFSCME) and the Service Employees International Union, have advocated for hazard pay.
Physicians’ organizations have not been vocal on the issue, however. The American Medical Association, for instance, pushed for hazard pay for residents but has not made any further public statements. An AMA spokesman said that the group was monitoring the situation but declined further comment.
Multiple online petitions seeking hazard pay for health care workers have been circulated, including one seeking the same $600 bump for essential workers that was given out as part of unemployment benefits in the first COVID-19 relief package. More than 1.2 million had signed the petition as of May 12.
‘Heroes fund’
The president first suggested hazard pay for health care workers on March 30 Fox News broadcast. “These are really brave people,” he said, adding that the administration was considering different ways of boosting pay, primarily through hospitals.
“We are asking the hospitals to do it and to consider something, including bonuses,” said Trump. “If anybody’s entitled to it, they are.”
On April 7, Sen. Schumer proposed a “Heroes Fund.” It would give public, private, and tribal frontline employees – including doctors, nurses, first responders, and transit, grocery, and postal workers – a $13 per hour raise up to $25,000 in additional pay through Dec. 31 for workers earning up to $200,000 and $5,000 in additional pay for those earning more than $200,000. It would also provide a $15,000 signing bonus to those who agree to take on such a position.
Rep. Matt Cartwright (D-Pa.) introduced a bill in mid-April, the Coronavirus Frontline Workers Fair Pay Act (HR 6709), that would provide similar pay increases. Health care workers would receive an additional $13 per hour. It would be retroactive to Jan. 31, 2020, and would be available through the end of 2020.
Molly Kinder of the Brookings Institution, a self-described nonpartisan Washington policy institute, estimates that Sen. Schumer’s proposal would represent the equivalent of double-time pay for the average low-wage worker, a 50% pay increase for a mail carrier, a 20% boost for a pharmacist, and less than a 15% increase for a surgeon, as determined from median 2018 wages.
Before the House Democrats unveiled their bill, Isabel Soto of the center-right group American Action Forum estimated that a $13 per hour wage increase could cost $398.9 billion just from the end of March to the end of September. A great proportion of that amount – $264 billion – would go to some 10 million health care workers, Ms. Soto calculated.
Some already offering pay boost
A few states and hospital systems are already offering hazard pay.
On April 12, Massachusetts agreed to give about 6,500 AFSCME union members who work at state human services facilities and group homes a $5 or a $10 per hour pay increase, depending on duties. It was to stay in effect until at least May 30.
Maine Governor Janet Mills (D) also agreed to increase pay by $3-$5 an hour for AFSCME workers in state correctional and mental health facilities beginning March 29.
In New York City, the biggest hospital network, Northwell Health, in late April gave 45,000 workers – including nurses, physicians, respiratory therapists, environmental services workers, housekeepers, and people in outpatient and corporate roles – a lump sum bonus payment of up to $2,500 and 1 week of paid time off. The money came out of the system’s general fund.
“As an organization, we want to continue to support, motivate and inspire our team members,” said Northwell President and CEO Michael Dowling in a statement at the time.
On April 2, New York–Presbyterian Hospital’s chair of the department of surgery, Craig Smith, MD, announced that the facility was “providing a $1,250 bonus for everyone who has worked in or supported the COVID-19 front lines, for at least 1 week.”
Advocate Aurora, with 15 hospitals and 32,000 employees in Wisconsin, said in early April that it was giving increases of $6.25-$15.00 an hour at least through the end of May.
A version of this article originally appeared on Medscape.com.
Summary of the IDSA guidelines on the diagnosis of COVID-19
These guidelines were developed using a rigorous evidence-based approach, the GRADE framework, which involved identifying the important questions that need to be addressed ahead of time and, later, integrating the best available evidence into the recommendations.
The Food and Drug Administration’s Emergency Use Authorization is useful for understanding any recommendations related to COVID-19 testing. Under usual FDA approval, a manufacturer has to submit data on the performance of a test in human subjects. Under the Emergency Use Authorization for development and approval of SARS-CoV-2 testing, approval is based on “acceptable analytical accuracy,” meaning that a test is assessed using manufactured reagents. The approved test is not tested in real-world clinical situations prior to FDA approval, and the test’s sensitivity and specificity are not well described.
IDSA formulated 15 recommendations, of which the most relevant to primary care clinicians are described and discussed below. The complete set of recommendations can be viewed on the IDSA website:
Recommendation 1
The IDSA panel recommends a SARS-CoV-2 nucleic acid amplification test in symptomatic individuals in the community suspected of having COVID-19, even when the clinical suspicion is low (strong recommendation, very low certainty of evidence). The panel placed a high value on accurate assessment of COVID-19 with the intent of minimizing overdiagnosis of COVID-19 using clinical diagnosis alone. Without testing, the rate of overdiagnosis ranges from 62% to 98%.
If patients are misdiagnosed as having COVID-19, they may spend unnecessary time in quarantine and then may stop taking appropriate safety precautions to protect themselves from infection.
Recommendation 2
The IDSA panel suggests collecting nasopharyngeal, or mid-turbinate or nasal swabs, rather than oropharyngeal swabs or saliva alone for SARS-CoV-2 RNA testing in symptomatic individuals with upper respiratory tract infection or influenza-like illness suspected of having COVID-19 (conditional recommendation, very low certainty of evidence).
The rationale for this recommendation is that comparative data showed a much lower sensitivity for oral sampling, compared with nasopharyngeal, mid-turbinate, or nasal sampling.
The average sensitivity of oral swabs is 56%, compared with nasopharyngeal at 97%, mid-turbinate at 100%, and nasal sampling at 95%. Given these test characteristics, there are far less false-negative tests with nasopharyngeal, mid-turbinate, and nasal swabs. Fewer false negatives means fewer instances of incorrectly telling COVID-19–positive patients that they do not have the illness. An exciting new area of testing that is being evaluated is saliva, which appears to have a sensitivity of 85%.
Recommendation 3
The IDSA panel suggests that nasal and mid-turbinate swab specimens may be collected for SARS-CoV-2 RNA testing by either patients or health care providers in symptomatic individuals with upper respiratory tract infection or influenza-like illness suspected of having COVID-19 (conditional recommendation, low certainty of evidence).
This recommendation is particularly exciting because patient self-collection provides the potential for health care personnel to avoid exposure to infection, as can occur when health care personnel are swabbing a patient; this is ow testing has been done at most testing centers.
While the data are limited, it appears that patient self-collection of nasal or mid-turbinate swabs results in similar detection rates as occurs with health care personnel–collected nasopharyngeal swabs.
Recommendation 6
The IDSA panel suggests repeating viral RNA testing when the initial test is negative (versus performing a single test) in symptomatic individuals with an intermediate or high clinical suspicion of COVID-19 (conditional recommendation, low certainty of evidence).
Since none of the tests are perfect and any can have false negatives, the panel places a high value on detecting infection when present. If there is a low clinical likelihood of disease, the panel recommends not retesting. When the clinical likelihood of COVID-19 is moderate to high, in the event that the initial test is negative, the panel recommends retesting for COVID-19 1-2 days after the initial test.
Recommendation 8
The IDSA panel suggests SARS-CoV-2 RNA testing in asymptomatic individuals who are either known or suspected to have been exposed to COVID-19 (conditional recommendation, very low certainty of evidence).
For this recommendation, a known contact is defined as someone who has had direct contact with a confirmed case.
A suspected exposure occurs when someone is working or living in a congregate setting such as long-term care, a correctional facility, or a cruise ship in which there is an outbreak. The time frame during which to do post-exposure testing is five to seven days after the exposure.
Recommendation 10
The IDSA panel recommends direct SARS-CoV-2 RNA testing in asymptomatic individuals with no known contact with COVID-19 who are being hospitalized in areas with a high prevalence of COVID-19 in the community (conditional recommendation, very low certainty of evidence).
The idea is to do rapid testing to identify individuals entering the hospital either for other illnesses or for procedures, in order to be able to institute appropriate precautions and decrease the likelihood of nosocomial transmission and/or transmission to health care personnel. It is worth noting that the recommendations do not address testing in areas with a low or intermediate prevalence of COVID-19. In the absence of an official guideline-based-recommendation, the decision about testing needs to made by the local hospital system.
Recommendations 11, 12, and 13
The IDSA panel recommends SARS-CoV-2 RNA testing in immunocompromised asymptomatic individuals who are being admitted to the hospital and in asymptomatic individuals prior to receiving immunosuppressive therapy regardless of exposure to COVID-19. It is also recommended to test asymptomatic individuals planning to undergo major surgery.
The rationale for this recommendation is that patients who are to receive chemotherapy, other immunosuppressive procedures, or surgery are at high risk if they have COVID-19 and may be better off delaying the procedure.
Some additional issues were addressed, though not in the form of additional recommendations. It was clarified that some individuals remain nucleic acid positive after their symptoms resolve, and sometimes even after seroconversion. It is not clear if those individuals remain infectious to others. The recommendations did not address serologic testing for public health surveillance.
Dr. Skolnik is professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
SOURCE: Hanson KE et al. Infectious Diseases Society of America guidelines on the diagnosis of COVID-19.
These guidelines were developed using a rigorous evidence-based approach, the GRADE framework, which involved identifying the important questions that need to be addressed ahead of time and, later, integrating the best available evidence into the recommendations.
The Food and Drug Administration’s Emergency Use Authorization is useful for understanding any recommendations related to COVID-19 testing. Under usual FDA approval, a manufacturer has to submit data on the performance of a test in human subjects. Under the Emergency Use Authorization for development and approval of SARS-CoV-2 testing, approval is based on “acceptable analytical accuracy,” meaning that a test is assessed using manufactured reagents. The approved test is not tested in real-world clinical situations prior to FDA approval, and the test’s sensitivity and specificity are not well described.
IDSA formulated 15 recommendations, of which the most relevant to primary care clinicians are described and discussed below. The complete set of recommendations can be viewed on the IDSA website:
Recommendation 1
The IDSA panel recommends a SARS-CoV-2 nucleic acid amplification test in symptomatic individuals in the community suspected of having COVID-19, even when the clinical suspicion is low (strong recommendation, very low certainty of evidence). The panel placed a high value on accurate assessment of COVID-19 with the intent of minimizing overdiagnosis of COVID-19 using clinical diagnosis alone. Without testing, the rate of overdiagnosis ranges from 62% to 98%.
If patients are misdiagnosed as having COVID-19, they may spend unnecessary time in quarantine and then may stop taking appropriate safety precautions to protect themselves from infection.
Recommendation 2
The IDSA panel suggests collecting nasopharyngeal, or mid-turbinate or nasal swabs, rather than oropharyngeal swabs or saliva alone for SARS-CoV-2 RNA testing in symptomatic individuals with upper respiratory tract infection or influenza-like illness suspected of having COVID-19 (conditional recommendation, very low certainty of evidence).
The rationale for this recommendation is that comparative data showed a much lower sensitivity for oral sampling, compared with nasopharyngeal, mid-turbinate, or nasal sampling.
The average sensitivity of oral swabs is 56%, compared with nasopharyngeal at 97%, mid-turbinate at 100%, and nasal sampling at 95%. Given these test characteristics, there are far less false-negative tests with nasopharyngeal, mid-turbinate, and nasal swabs. Fewer false negatives means fewer instances of incorrectly telling COVID-19–positive patients that they do not have the illness. An exciting new area of testing that is being evaluated is saliva, which appears to have a sensitivity of 85%.
Recommendation 3
The IDSA panel suggests that nasal and mid-turbinate swab specimens may be collected for SARS-CoV-2 RNA testing by either patients or health care providers in symptomatic individuals with upper respiratory tract infection or influenza-like illness suspected of having COVID-19 (conditional recommendation, low certainty of evidence).
This recommendation is particularly exciting because patient self-collection provides the potential for health care personnel to avoid exposure to infection, as can occur when health care personnel are swabbing a patient; this is ow testing has been done at most testing centers.
While the data are limited, it appears that patient self-collection of nasal or mid-turbinate swabs results in similar detection rates as occurs with health care personnel–collected nasopharyngeal swabs.
Recommendation 6
The IDSA panel suggests repeating viral RNA testing when the initial test is negative (versus performing a single test) in symptomatic individuals with an intermediate or high clinical suspicion of COVID-19 (conditional recommendation, low certainty of evidence).
Since none of the tests are perfect and any can have false negatives, the panel places a high value on detecting infection when present. If there is a low clinical likelihood of disease, the panel recommends not retesting. When the clinical likelihood of COVID-19 is moderate to high, in the event that the initial test is negative, the panel recommends retesting for COVID-19 1-2 days after the initial test.
Recommendation 8
The IDSA panel suggests SARS-CoV-2 RNA testing in asymptomatic individuals who are either known or suspected to have been exposed to COVID-19 (conditional recommendation, very low certainty of evidence).
For this recommendation, a known contact is defined as someone who has had direct contact with a confirmed case.
A suspected exposure occurs when someone is working or living in a congregate setting such as long-term care, a correctional facility, or a cruise ship in which there is an outbreak. The time frame during which to do post-exposure testing is five to seven days after the exposure.
Recommendation 10
The IDSA panel recommends direct SARS-CoV-2 RNA testing in asymptomatic individuals with no known contact with COVID-19 who are being hospitalized in areas with a high prevalence of COVID-19 in the community (conditional recommendation, very low certainty of evidence).
The idea is to do rapid testing to identify individuals entering the hospital either for other illnesses or for procedures, in order to be able to institute appropriate precautions and decrease the likelihood of nosocomial transmission and/or transmission to health care personnel. It is worth noting that the recommendations do not address testing in areas with a low or intermediate prevalence of COVID-19. In the absence of an official guideline-based-recommendation, the decision about testing needs to made by the local hospital system.
Recommendations 11, 12, and 13
The IDSA panel recommends SARS-CoV-2 RNA testing in immunocompromised asymptomatic individuals who are being admitted to the hospital and in asymptomatic individuals prior to receiving immunosuppressive therapy regardless of exposure to COVID-19. It is also recommended to test asymptomatic individuals planning to undergo major surgery.
The rationale for this recommendation is that patients who are to receive chemotherapy, other immunosuppressive procedures, or surgery are at high risk if they have COVID-19 and may be better off delaying the procedure.
Some additional issues were addressed, though not in the form of additional recommendations. It was clarified that some individuals remain nucleic acid positive after their symptoms resolve, and sometimes even after seroconversion. It is not clear if those individuals remain infectious to others. The recommendations did not address serologic testing for public health surveillance.
Dr. Skolnik is professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
SOURCE: Hanson KE et al. Infectious Diseases Society of America guidelines on the diagnosis of COVID-19.
These guidelines were developed using a rigorous evidence-based approach, the GRADE framework, which involved identifying the important questions that need to be addressed ahead of time and, later, integrating the best available evidence into the recommendations.
The Food and Drug Administration’s Emergency Use Authorization is useful for understanding any recommendations related to COVID-19 testing. Under usual FDA approval, a manufacturer has to submit data on the performance of a test in human subjects. Under the Emergency Use Authorization for development and approval of SARS-CoV-2 testing, approval is based on “acceptable analytical accuracy,” meaning that a test is assessed using manufactured reagents. The approved test is not tested in real-world clinical situations prior to FDA approval, and the test’s sensitivity and specificity are not well described.
IDSA formulated 15 recommendations, of which the most relevant to primary care clinicians are described and discussed below. The complete set of recommendations can be viewed on the IDSA website:
Recommendation 1
The IDSA panel recommends a SARS-CoV-2 nucleic acid amplification test in symptomatic individuals in the community suspected of having COVID-19, even when the clinical suspicion is low (strong recommendation, very low certainty of evidence). The panel placed a high value on accurate assessment of COVID-19 with the intent of minimizing overdiagnosis of COVID-19 using clinical diagnosis alone. Without testing, the rate of overdiagnosis ranges from 62% to 98%.
If patients are misdiagnosed as having COVID-19, they may spend unnecessary time in quarantine and then may stop taking appropriate safety precautions to protect themselves from infection.
Recommendation 2
The IDSA panel suggests collecting nasopharyngeal, or mid-turbinate or nasal swabs, rather than oropharyngeal swabs or saliva alone for SARS-CoV-2 RNA testing in symptomatic individuals with upper respiratory tract infection or influenza-like illness suspected of having COVID-19 (conditional recommendation, very low certainty of evidence).
The rationale for this recommendation is that comparative data showed a much lower sensitivity for oral sampling, compared with nasopharyngeal, mid-turbinate, or nasal sampling.
The average sensitivity of oral swabs is 56%, compared with nasopharyngeal at 97%, mid-turbinate at 100%, and nasal sampling at 95%. Given these test characteristics, there are far less false-negative tests with nasopharyngeal, mid-turbinate, and nasal swabs. Fewer false negatives means fewer instances of incorrectly telling COVID-19–positive patients that they do not have the illness. An exciting new area of testing that is being evaluated is saliva, which appears to have a sensitivity of 85%.
Recommendation 3
The IDSA panel suggests that nasal and mid-turbinate swab specimens may be collected for SARS-CoV-2 RNA testing by either patients or health care providers in symptomatic individuals with upper respiratory tract infection or influenza-like illness suspected of having COVID-19 (conditional recommendation, low certainty of evidence).
This recommendation is particularly exciting because patient self-collection provides the potential for health care personnel to avoid exposure to infection, as can occur when health care personnel are swabbing a patient; this is ow testing has been done at most testing centers.
While the data are limited, it appears that patient self-collection of nasal or mid-turbinate swabs results in similar detection rates as occurs with health care personnel–collected nasopharyngeal swabs.
Recommendation 6
The IDSA panel suggests repeating viral RNA testing when the initial test is negative (versus performing a single test) in symptomatic individuals with an intermediate or high clinical suspicion of COVID-19 (conditional recommendation, low certainty of evidence).
Since none of the tests are perfect and any can have false negatives, the panel places a high value on detecting infection when present. If there is a low clinical likelihood of disease, the panel recommends not retesting. When the clinical likelihood of COVID-19 is moderate to high, in the event that the initial test is negative, the panel recommends retesting for COVID-19 1-2 days after the initial test.
Recommendation 8
The IDSA panel suggests SARS-CoV-2 RNA testing in asymptomatic individuals who are either known or suspected to have been exposed to COVID-19 (conditional recommendation, very low certainty of evidence).
For this recommendation, a known contact is defined as someone who has had direct contact with a confirmed case.
A suspected exposure occurs when someone is working or living in a congregate setting such as long-term care, a correctional facility, or a cruise ship in which there is an outbreak. The time frame during which to do post-exposure testing is five to seven days after the exposure.
Recommendation 10
The IDSA panel recommends direct SARS-CoV-2 RNA testing in asymptomatic individuals with no known contact with COVID-19 who are being hospitalized in areas with a high prevalence of COVID-19 in the community (conditional recommendation, very low certainty of evidence).
The idea is to do rapid testing to identify individuals entering the hospital either for other illnesses or for procedures, in order to be able to institute appropriate precautions and decrease the likelihood of nosocomial transmission and/or transmission to health care personnel. It is worth noting that the recommendations do not address testing in areas with a low or intermediate prevalence of COVID-19. In the absence of an official guideline-based-recommendation, the decision about testing needs to made by the local hospital system.
Recommendations 11, 12, and 13
The IDSA panel recommends SARS-CoV-2 RNA testing in immunocompromised asymptomatic individuals who are being admitted to the hospital and in asymptomatic individuals prior to receiving immunosuppressive therapy regardless of exposure to COVID-19. It is also recommended to test asymptomatic individuals planning to undergo major surgery.
The rationale for this recommendation is that patients who are to receive chemotherapy, other immunosuppressive procedures, or surgery are at high risk if they have COVID-19 and may be better off delaying the procedure.
Some additional issues were addressed, though not in the form of additional recommendations. It was clarified that some individuals remain nucleic acid positive after their symptoms resolve, and sometimes even after seroconversion. It is not clear if those individuals remain infectious to others. The recommendations did not address serologic testing for public health surveillance.
Dr. Skolnik is professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
SOURCE: Hanson KE et al. Infectious Diseases Society of America guidelines on the diagnosis of COVID-19.
ER docs ask, “Where are our patients?”
according to an expert panel on unanticipated consequences of pandemic care hosted by the presidents of the Society of Critical Care Medicine and the American College of Emergency Physicians.*
“At the peak of exposure to COVID-19 illness or infection, ED volumes in my system, which are really not much different from others across the country, were cut in half, if not more. And those changes happened across virtually every form of ED presentation, from the highest acuity to the lowest. We’re now beyond our highest level of exposure to COVID-19 clinically symptomatic patients in western Pennsylvania, but that recovery in volume hasn’t occurred yet, although there are some embers,” explained Donald M. Yealy, MD, professor and chair of the department of emergency medicine at the University of Pittsburgh.
He and other panelists also addressed some of the other unanticipated developments in the COVID-19 pandemic, including a recently recognized childhood manifestation called for now COVID-associated pediatric multisystem inflammatory syndrome, an anticipated massive second wave of non-COVID patients expected to present late to EDs and primary care clinics after having avoided needed medical care out of fear of infection, and the pandemic’s negative impact upon medical education.
Who’s not showing up in the ED
Dr. Yealy said that across the country, the number of patients arriving in EDs with acute ST-elevation MI, stroke, trauma, and other highest-acuity presentations is down substantially. But the volume of patients with more routine, bread-and-butter conditions typically seen in EDs is down even more.
“You might say, if I was designing from the insurance side, this is exactly what I’d hope for. I’ve heard that some people on the insurance-only side of the business really are experiencing a pretty good deal right now: They’re collecting premiums and not having to pay out on the ED or hospital side,” he said.
Tweaking the public health message on seeking medical care
“One of the unanticipated casualties of the pandemic are the patients who don’t have it. It will take a whole lot of work and coordinated effort to re-engage with those patients,” predicted SCCM President Lewis J. Kaplan, MD, professor of surgery at the University of Pennsylvania, Philadelphia.
Evie G. Marcolini, MD, described what she believes is necessary now: “We need to have a big focus on getting the word out to the public that acute MI, stroke, and other acute injuries are still a time-sensitive problem and they warrant at least a call to their physician or consideration of coming in to the ED.
“I think when we started out, we were telling people, ‘Don’t come in.’ Now we’re trying to dial it back a little bit and say, ‘Listen, there are things you really do need to come in for. And we will keep you safe,’” said Dr. Marcolini, an emergency medicine and neurocritical care specialist at Dartmouth-Hitchcock Medical Center, Hanover, N.H.
“It is safe,” Dr. Yealy agreed. “The safest place in the world to be right now is the ED. Everybody’s cordoned off. There’s way more PPE [personal protective equipment]. There’s a level of precision now that should have existed but never did in our previous influenza seasons. So we have something very unique to offer, and we can put people’s minds at rest.”
He spoke of a coming “tsunami of untreated illness.”
“My concern is there is a significant subset of people who are not only eschewing ED care but staying away from their primary care provider. My fear is that we’re not as well aware of this,” he said. “Together with our primary care partners, we have to figure out ways to reach the people who are ignoring illnesses and injuries that they’re making long-term decisions about without realizing it. We have to find a way to reach those people and say it’s okay to reach for care.”
SCCM Immediate Past President Heatherlee Bailey, MD, also sees a problematic looming wave.
“I’m quite concerned about the coming second wave of non-COVID patients who’ve sat home with their worsening renal failure that’s gone from 2 to 5 because they’ve been taking a lot of NSAIDs, or the individual who’s had several TIAs that self-resolved, and we’ve missed an opportunity to prevent some significant disease. At some point they’re going to come back, and we need to figure out how to get these individuals hooked up with care, either through the ED or with their primary care provider, to prevent these potential bad outcomes,” said Dr. Bailey of the Durham (N.C.) Veterans Affairs Medical Center.
Interim guidance for pediatricians on an alarming new syndrome
Edward E. Conway Jr., MD, recalled that early in the U.S. pandemic, pediatricians felt a sense of relief that children appeared to be spared from severe COVID-19 disease. But, in just the past few weeks, a new syndrome has emerged. New York City has recorded more than 100 cases of what’s provisionally being called COVID-associated pediatric multisystem inflammatory syndrome. Dr. Conway and others are working with the Centers for Disease Control and Prevention to develop a case definition for the syndrome, first reported by pediatricians in Italy and the United Kingdom.
“We’re trying to get the word out to general pediatricians as to the common signs and symptoms that should prompt parents to bring their children in for medical care,” according to Dr. Conway, chief of pediatric critical care medicine and vice-chair of pediatrics at Jacobi Medical Center in New York.
Ninety percent of affected children have abdominal symptoms early on, including abdominal pain, diarrhea, emesis, or enteritis upon imaging. A nondescript rash, headache, conjunctivitis, and irritability are common, cough much less so – under 25%.
“The thought is that if any one of these is associated with a fever lasting more than 4 days, we suggest these children be brought in and seen by a pediatrician. We don’t have a formal guideline – we’re working on that – but basically the current recommendation is to screen them initially with a CBC with differential, a chem 10, and liver function tests, but also to look for inflammatory markers that we see in our COVID patients. We’ve been quite surprised: These patients have C-reactive proteins of about 240 mg/L on average, ferritin is quite high at around 1,200 ng/mL, and d-dimers of 2,300 ng/mL. We’ve also found very high brain natriuretic peptides and troponins in these patients,” according to Dr. Conway.
Analogies have been made between this COVID-19 pediatric syndrome and Kawasaki disease. Dr. Conway is unconvinced.
“This is quite different from Kawasaki in that these children are usually thrombocytopenic and usually present with DIC [disseminated intravascular coagulation], and the d-dimers are extraordinarily high, compared to what we’re used to seeing in pediatric patients,” he said.
Symptomatic children with laboratory red flags should be hospitalized. Most of the affected New York City children have recovered after 5 or 6 days in the pediatric ICU with empiric treatment using intravenous immunoglobulin (IVIG), corticosteroids, and/or interleukin-6 inhibitors. However, five recent deaths are now under study.
Dr. Yealy commented that this new pediatric syndrome is “really interesting,” but to date, it affects only a very small percentage of children, and children overall have been much less affected by the pandemic than are adults.
“The populations being disproportionately impacted are the elderly, the elderly, the elderly, and then other vulnerable populations, particularly congregants and the poor,” he said. “At my site, three-quarters of the patients coming in are either patients at assisted-living facilities or work at one of those congregant facilities.”
The pandemic’s impact on medical education
In many hospitals, grand rounds are being done virtually via videoconferencing, often with attendant challenges in asking and answering questions. Hospital patient volumes are diminished. Medical students aren’t coming in to do clinical rotations. Medical students and residents can’t travel to interview for future residencies or jobs.
“It’s affecting education across all of the components of medicine. It’s hard to say how long this pandemic is going to last. We’re all trying to be innovative in using online tools, but I believe it’s going to have a long-lasting effect on our education system,” Dr. Marcolini predicted.
Remote interface while working from home has become frustrating, especially during peak Internet use hours.
“It’s staggering how slow my home system has become in comparison to what’s wired at work. Now many times when you try to get into your work system from home, you time out while you’re waiting for the next piece of information to come across,” Dr. Kaplan commented.
All panel participants reported having no financial conflicts of interest.
*Correction, 5/15/20: An earlier version of this article misstated the name of the American College of Emergency Physicians.)
according to an expert panel on unanticipated consequences of pandemic care hosted by the presidents of the Society of Critical Care Medicine and the American College of Emergency Physicians.*
“At the peak of exposure to COVID-19 illness or infection, ED volumes in my system, which are really not much different from others across the country, were cut in half, if not more. And those changes happened across virtually every form of ED presentation, from the highest acuity to the lowest. We’re now beyond our highest level of exposure to COVID-19 clinically symptomatic patients in western Pennsylvania, but that recovery in volume hasn’t occurred yet, although there are some embers,” explained Donald M. Yealy, MD, professor and chair of the department of emergency medicine at the University of Pittsburgh.
He and other panelists also addressed some of the other unanticipated developments in the COVID-19 pandemic, including a recently recognized childhood manifestation called for now COVID-associated pediatric multisystem inflammatory syndrome, an anticipated massive second wave of non-COVID patients expected to present late to EDs and primary care clinics after having avoided needed medical care out of fear of infection, and the pandemic’s negative impact upon medical education.
Who’s not showing up in the ED
Dr. Yealy said that across the country, the number of patients arriving in EDs with acute ST-elevation MI, stroke, trauma, and other highest-acuity presentations is down substantially. But the volume of patients with more routine, bread-and-butter conditions typically seen in EDs is down even more.
“You might say, if I was designing from the insurance side, this is exactly what I’d hope for. I’ve heard that some people on the insurance-only side of the business really are experiencing a pretty good deal right now: They’re collecting premiums and not having to pay out on the ED or hospital side,” he said.
Tweaking the public health message on seeking medical care
“One of the unanticipated casualties of the pandemic are the patients who don’t have it. It will take a whole lot of work and coordinated effort to re-engage with those patients,” predicted SCCM President Lewis J. Kaplan, MD, professor of surgery at the University of Pennsylvania, Philadelphia.
Evie G. Marcolini, MD, described what she believes is necessary now: “We need to have a big focus on getting the word out to the public that acute MI, stroke, and other acute injuries are still a time-sensitive problem and they warrant at least a call to their physician or consideration of coming in to the ED.
“I think when we started out, we were telling people, ‘Don’t come in.’ Now we’re trying to dial it back a little bit and say, ‘Listen, there are things you really do need to come in for. And we will keep you safe,’” said Dr. Marcolini, an emergency medicine and neurocritical care specialist at Dartmouth-Hitchcock Medical Center, Hanover, N.H.
“It is safe,” Dr. Yealy agreed. “The safest place in the world to be right now is the ED. Everybody’s cordoned off. There’s way more PPE [personal protective equipment]. There’s a level of precision now that should have existed but never did in our previous influenza seasons. So we have something very unique to offer, and we can put people’s minds at rest.”
He spoke of a coming “tsunami of untreated illness.”
“My concern is there is a significant subset of people who are not only eschewing ED care but staying away from their primary care provider. My fear is that we’re not as well aware of this,” he said. “Together with our primary care partners, we have to figure out ways to reach the people who are ignoring illnesses and injuries that they’re making long-term decisions about without realizing it. We have to find a way to reach those people and say it’s okay to reach for care.”
SCCM Immediate Past President Heatherlee Bailey, MD, also sees a problematic looming wave.
“I’m quite concerned about the coming second wave of non-COVID patients who’ve sat home with their worsening renal failure that’s gone from 2 to 5 because they’ve been taking a lot of NSAIDs, or the individual who’s had several TIAs that self-resolved, and we’ve missed an opportunity to prevent some significant disease. At some point they’re going to come back, and we need to figure out how to get these individuals hooked up with care, either through the ED or with their primary care provider, to prevent these potential bad outcomes,” said Dr. Bailey of the Durham (N.C.) Veterans Affairs Medical Center.
Interim guidance for pediatricians on an alarming new syndrome
Edward E. Conway Jr., MD, recalled that early in the U.S. pandemic, pediatricians felt a sense of relief that children appeared to be spared from severe COVID-19 disease. But, in just the past few weeks, a new syndrome has emerged. New York City has recorded more than 100 cases of what’s provisionally being called COVID-associated pediatric multisystem inflammatory syndrome. Dr. Conway and others are working with the Centers for Disease Control and Prevention to develop a case definition for the syndrome, first reported by pediatricians in Italy and the United Kingdom.
“We’re trying to get the word out to general pediatricians as to the common signs and symptoms that should prompt parents to bring their children in for medical care,” according to Dr. Conway, chief of pediatric critical care medicine and vice-chair of pediatrics at Jacobi Medical Center in New York.
Ninety percent of affected children have abdominal symptoms early on, including abdominal pain, diarrhea, emesis, or enteritis upon imaging. A nondescript rash, headache, conjunctivitis, and irritability are common, cough much less so – under 25%.
“The thought is that if any one of these is associated with a fever lasting more than 4 days, we suggest these children be brought in and seen by a pediatrician. We don’t have a formal guideline – we’re working on that – but basically the current recommendation is to screen them initially with a CBC with differential, a chem 10, and liver function tests, but also to look for inflammatory markers that we see in our COVID patients. We’ve been quite surprised: These patients have C-reactive proteins of about 240 mg/L on average, ferritin is quite high at around 1,200 ng/mL, and d-dimers of 2,300 ng/mL. We’ve also found very high brain natriuretic peptides and troponins in these patients,” according to Dr. Conway.
Analogies have been made between this COVID-19 pediatric syndrome and Kawasaki disease. Dr. Conway is unconvinced.
“This is quite different from Kawasaki in that these children are usually thrombocytopenic and usually present with DIC [disseminated intravascular coagulation], and the d-dimers are extraordinarily high, compared to what we’re used to seeing in pediatric patients,” he said.
Symptomatic children with laboratory red flags should be hospitalized. Most of the affected New York City children have recovered after 5 or 6 days in the pediatric ICU with empiric treatment using intravenous immunoglobulin (IVIG), corticosteroids, and/or interleukin-6 inhibitors. However, five recent deaths are now under study.
Dr. Yealy commented that this new pediatric syndrome is “really interesting,” but to date, it affects only a very small percentage of children, and children overall have been much less affected by the pandemic than are adults.
“The populations being disproportionately impacted are the elderly, the elderly, the elderly, and then other vulnerable populations, particularly congregants and the poor,” he said. “At my site, three-quarters of the patients coming in are either patients at assisted-living facilities or work at one of those congregant facilities.”
The pandemic’s impact on medical education
In many hospitals, grand rounds are being done virtually via videoconferencing, often with attendant challenges in asking and answering questions. Hospital patient volumes are diminished. Medical students aren’t coming in to do clinical rotations. Medical students and residents can’t travel to interview for future residencies or jobs.
“It’s affecting education across all of the components of medicine. It’s hard to say how long this pandemic is going to last. We’re all trying to be innovative in using online tools, but I believe it’s going to have a long-lasting effect on our education system,” Dr. Marcolini predicted.
Remote interface while working from home has become frustrating, especially during peak Internet use hours.
“It’s staggering how slow my home system has become in comparison to what’s wired at work. Now many times when you try to get into your work system from home, you time out while you’re waiting for the next piece of information to come across,” Dr. Kaplan commented.
All panel participants reported having no financial conflicts of interest.
*Correction, 5/15/20: An earlier version of this article misstated the name of the American College of Emergency Physicians.)
according to an expert panel on unanticipated consequences of pandemic care hosted by the presidents of the Society of Critical Care Medicine and the American College of Emergency Physicians.*
“At the peak of exposure to COVID-19 illness or infection, ED volumes in my system, which are really not much different from others across the country, were cut in half, if not more. And those changes happened across virtually every form of ED presentation, from the highest acuity to the lowest. We’re now beyond our highest level of exposure to COVID-19 clinically symptomatic patients in western Pennsylvania, but that recovery in volume hasn’t occurred yet, although there are some embers,” explained Donald M. Yealy, MD, professor and chair of the department of emergency medicine at the University of Pittsburgh.
He and other panelists also addressed some of the other unanticipated developments in the COVID-19 pandemic, including a recently recognized childhood manifestation called for now COVID-associated pediatric multisystem inflammatory syndrome, an anticipated massive second wave of non-COVID patients expected to present late to EDs and primary care clinics after having avoided needed medical care out of fear of infection, and the pandemic’s negative impact upon medical education.
Who’s not showing up in the ED
Dr. Yealy said that across the country, the number of patients arriving in EDs with acute ST-elevation MI, stroke, trauma, and other highest-acuity presentations is down substantially. But the volume of patients with more routine, bread-and-butter conditions typically seen in EDs is down even more.
“You might say, if I was designing from the insurance side, this is exactly what I’d hope for. I’ve heard that some people on the insurance-only side of the business really are experiencing a pretty good deal right now: They’re collecting premiums and not having to pay out on the ED or hospital side,” he said.
Tweaking the public health message on seeking medical care
“One of the unanticipated casualties of the pandemic are the patients who don’t have it. It will take a whole lot of work and coordinated effort to re-engage with those patients,” predicted SCCM President Lewis J. Kaplan, MD, professor of surgery at the University of Pennsylvania, Philadelphia.
Evie G. Marcolini, MD, described what she believes is necessary now: “We need to have a big focus on getting the word out to the public that acute MI, stroke, and other acute injuries are still a time-sensitive problem and they warrant at least a call to their physician or consideration of coming in to the ED.
“I think when we started out, we were telling people, ‘Don’t come in.’ Now we’re trying to dial it back a little bit and say, ‘Listen, there are things you really do need to come in for. And we will keep you safe,’” said Dr. Marcolini, an emergency medicine and neurocritical care specialist at Dartmouth-Hitchcock Medical Center, Hanover, N.H.
“It is safe,” Dr. Yealy agreed. “The safest place in the world to be right now is the ED. Everybody’s cordoned off. There’s way more PPE [personal protective equipment]. There’s a level of precision now that should have existed but never did in our previous influenza seasons. So we have something very unique to offer, and we can put people’s minds at rest.”
He spoke of a coming “tsunami of untreated illness.”
“My concern is there is a significant subset of people who are not only eschewing ED care but staying away from their primary care provider. My fear is that we’re not as well aware of this,” he said. “Together with our primary care partners, we have to figure out ways to reach the people who are ignoring illnesses and injuries that they’re making long-term decisions about without realizing it. We have to find a way to reach those people and say it’s okay to reach for care.”
SCCM Immediate Past President Heatherlee Bailey, MD, also sees a problematic looming wave.
“I’m quite concerned about the coming second wave of non-COVID patients who’ve sat home with their worsening renal failure that’s gone from 2 to 5 because they’ve been taking a lot of NSAIDs, or the individual who’s had several TIAs that self-resolved, and we’ve missed an opportunity to prevent some significant disease. At some point they’re going to come back, and we need to figure out how to get these individuals hooked up with care, either through the ED or with their primary care provider, to prevent these potential bad outcomes,” said Dr. Bailey of the Durham (N.C.) Veterans Affairs Medical Center.
Interim guidance for pediatricians on an alarming new syndrome
Edward E. Conway Jr., MD, recalled that early in the U.S. pandemic, pediatricians felt a sense of relief that children appeared to be spared from severe COVID-19 disease. But, in just the past few weeks, a new syndrome has emerged. New York City has recorded more than 100 cases of what’s provisionally being called COVID-associated pediatric multisystem inflammatory syndrome. Dr. Conway and others are working with the Centers for Disease Control and Prevention to develop a case definition for the syndrome, first reported by pediatricians in Italy and the United Kingdom.
“We’re trying to get the word out to general pediatricians as to the common signs and symptoms that should prompt parents to bring their children in for medical care,” according to Dr. Conway, chief of pediatric critical care medicine and vice-chair of pediatrics at Jacobi Medical Center in New York.
Ninety percent of affected children have abdominal symptoms early on, including abdominal pain, diarrhea, emesis, or enteritis upon imaging. A nondescript rash, headache, conjunctivitis, and irritability are common, cough much less so – under 25%.
“The thought is that if any one of these is associated with a fever lasting more than 4 days, we suggest these children be brought in and seen by a pediatrician. We don’t have a formal guideline – we’re working on that – but basically the current recommendation is to screen them initially with a CBC with differential, a chem 10, and liver function tests, but also to look for inflammatory markers that we see in our COVID patients. We’ve been quite surprised: These patients have C-reactive proteins of about 240 mg/L on average, ferritin is quite high at around 1,200 ng/mL, and d-dimers of 2,300 ng/mL. We’ve also found very high brain natriuretic peptides and troponins in these patients,” according to Dr. Conway.
Analogies have been made between this COVID-19 pediatric syndrome and Kawasaki disease. Dr. Conway is unconvinced.
“This is quite different from Kawasaki in that these children are usually thrombocytopenic and usually present with DIC [disseminated intravascular coagulation], and the d-dimers are extraordinarily high, compared to what we’re used to seeing in pediatric patients,” he said.
Symptomatic children with laboratory red flags should be hospitalized. Most of the affected New York City children have recovered after 5 or 6 days in the pediatric ICU with empiric treatment using intravenous immunoglobulin (IVIG), corticosteroids, and/or interleukin-6 inhibitors. However, five recent deaths are now under study.
Dr. Yealy commented that this new pediatric syndrome is “really interesting,” but to date, it affects only a very small percentage of children, and children overall have been much less affected by the pandemic than are adults.
“The populations being disproportionately impacted are the elderly, the elderly, the elderly, and then other vulnerable populations, particularly congregants and the poor,” he said. “At my site, three-quarters of the patients coming in are either patients at assisted-living facilities or work at one of those congregant facilities.”
The pandemic’s impact on medical education
In many hospitals, grand rounds are being done virtually via videoconferencing, often with attendant challenges in asking and answering questions. Hospital patient volumes are diminished. Medical students aren’t coming in to do clinical rotations. Medical students and residents can’t travel to interview for future residencies or jobs.
“It’s affecting education across all of the components of medicine. It’s hard to say how long this pandemic is going to last. We’re all trying to be innovative in using online tools, but I believe it’s going to have a long-lasting effect on our education system,” Dr. Marcolini predicted.
Remote interface while working from home has become frustrating, especially during peak Internet use hours.
“It’s staggering how slow my home system has become in comparison to what’s wired at work. Now many times when you try to get into your work system from home, you time out while you’re waiting for the next piece of information to come across,” Dr. Kaplan commented.
All panel participants reported having no financial conflicts of interest.
*Correction, 5/15/20: An earlier version of this article misstated the name of the American College of Emergency Physicians.)
Quitting smoking just 2 years before lung cancer diagnosis may improve survival
Quitting smoking prior to a lung cancer diagnosis is associated with a survival benefit, even among patients who recently stopped smoking, according to results of a pooled analysis.
The overall survival advantage was significant regardless of how long ago patients had last smoked, including among those who quit within 2 years prior to their diagnosis.
These findings create a “teachable moment” for health care providers in scenarios when patients might be more receptive to a stop-smoking message, according to investigator Aline F. Fares, MD, a clinical research fellow at Princess Margaret Cancer Centre in Toronto.
“Our study can be summarized to patients as, ‘it’s never too late to quit,’ ” Dr. Fares said.
She presented results from this study at the American Society of Clinical Oncology virtual scientific program during a press briefing in advance of the meeting. This year, ASCO’s annual meeting is split into two parts. The virtual scientific program will be presented online May 29-31. The virtual education program will be available Aug. 8-10.
Results
Dr. Fares presented data on 35,481 patients with a diagnosis of lung cancer who had been enrolled in 17 studies conducted by the International Lung Cancer Consortium. (Data in the presentation were updated from the abstract.)
At diagnosis, 47.5% of the patients were current smokers, 30% were former smokers, and 22.5% were never smokers.
The risk of death from any cause was cut by 20% among former smokers who quit more than 5 years before their lung cancer diagnosis (P < .001). Patients who quit smoking 2-5 years before diagnosis had a 16% reduction in the risk of death, while those who quit within 2 years of diagnosis had a 12% reduced risk (P < .001 for both comparisons).
The overall survival advantage was evident in this pooled analysis regardless of patient sex, disease stage, histology, or amount of smoking as measured in pack-years, according to Dr. Fares. That said, the overall survival advantage appeared to be even greater among heavier smokers (i.e., greater than 30 pack-years) as compared with lighter smokers.
Lung cancer–specific survival was improved by 15% for patients who quit smoking more than 5 years prior to their diagnosis. For those who had quit more recently, there was a nonsignificant trend toward improvement in this outcome.
Overall survival was higher in never smokers in comparison with current smokers, a finding that was expected based on previous studies, according to Dr. Fares.
Implications
These findings could be important to share with individuals who are current smokers at the time of lung cancer screening, according to Maher A. Karam-Hage, MD, medical director of the tobacco treatment program at the University of Texas MD Anderson Cancer Center, Houston.
“The power of this data is that it shows quitting makes a difference, and that it can be more impactful the longer you quit before you get diagnosed,” Dr. Karam-Hage said in an interview.
Negative lung cancer screening results sometimes give individuals the false impression that they are “one of the lucky ones” who won’t get lung cancer and don’t have to quit smoking, according to Dr. Karam-Hage, who is studying the comparative effectiveness of different smoking cessation strategies.
“Now, as part of shared decision making, we can provide people with specific numbers before the scan that [suggest] no matter what the scan comes out with, the earlier they quit, the better off they will be,” he said.
In her presentation, Dr. Fares said that lung cancer screening may be an “interesting time” to address smoking cessation, particularly among patients with a heavier smoking history.
“After a lifetime of smoking, patients often feel it’s too late to quit smoking and that the damage has already been done,” she added.
The International Lung Cancer Consortium studies had multiple supporters. Dr. Fares reported having no disclosures related to the research. One researcher reported relationships with AbbVie, AstraZeneca, MedImmune, Bayer, Bristol-Myers Squibb, Merck, Novartis, Pfizer, Roche Canada, and Takeda. Dr. Karam-Hage reported having no relevant disclosures.
SOURCE: Fares AF et al. ASCO 2020, Abstract 1512.
This article was updated 5/15/20.
Quitting smoking prior to a lung cancer diagnosis is associated with a survival benefit, even among patients who recently stopped smoking, according to results of a pooled analysis.
The overall survival advantage was significant regardless of how long ago patients had last smoked, including among those who quit within 2 years prior to their diagnosis.
These findings create a “teachable moment” for health care providers in scenarios when patients might be more receptive to a stop-smoking message, according to investigator Aline F. Fares, MD, a clinical research fellow at Princess Margaret Cancer Centre in Toronto.
“Our study can be summarized to patients as, ‘it’s never too late to quit,’ ” Dr. Fares said.
She presented results from this study at the American Society of Clinical Oncology virtual scientific program during a press briefing in advance of the meeting. This year, ASCO’s annual meeting is split into two parts. The virtual scientific program will be presented online May 29-31. The virtual education program will be available Aug. 8-10.
Results
Dr. Fares presented data on 35,481 patients with a diagnosis of lung cancer who had been enrolled in 17 studies conducted by the International Lung Cancer Consortium. (Data in the presentation were updated from the abstract.)
At diagnosis, 47.5% of the patients were current smokers, 30% were former smokers, and 22.5% were never smokers.
The risk of death from any cause was cut by 20% among former smokers who quit more than 5 years before their lung cancer diagnosis (P < .001). Patients who quit smoking 2-5 years before diagnosis had a 16% reduction in the risk of death, while those who quit within 2 years of diagnosis had a 12% reduced risk (P < .001 for both comparisons).
The overall survival advantage was evident in this pooled analysis regardless of patient sex, disease stage, histology, or amount of smoking as measured in pack-years, according to Dr. Fares. That said, the overall survival advantage appeared to be even greater among heavier smokers (i.e., greater than 30 pack-years) as compared with lighter smokers.
Lung cancer–specific survival was improved by 15% for patients who quit smoking more than 5 years prior to their diagnosis. For those who had quit more recently, there was a nonsignificant trend toward improvement in this outcome.
Overall survival was higher in never smokers in comparison with current smokers, a finding that was expected based on previous studies, according to Dr. Fares.
Implications
These findings could be important to share with individuals who are current smokers at the time of lung cancer screening, according to Maher A. Karam-Hage, MD, medical director of the tobacco treatment program at the University of Texas MD Anderson Cancer Center, Houston.
“The power of this data is that it shows quitting makes a difference, and that it can be more impactful the longer you quit before you get diagnosed,” Dr. Karam-Hage said in an interview.
Negative lung cancer screening results sometimes give individuals the false impression that they are “one of the lucky ones” who won’t get lung cancer and don’t have to quit smoking, according to Dr. Karam-Hage, who is studying the comparative effectiveness of different smoking cessation strategies.
“Now, as part of shared decision making, we can provide people with specific numbers before the scan that [suggest] no matter what the scan comes out with, the earlier they quit, the better off they will be,” he said.
In her presentation, Dr. Fares said that lung cancer screening may be an “interesting time” to address smoking cessation, particularly among patients with a heavier smoking history.
“After a lifetime of smoking, patients often feel it’s too late to quit smoking and that the damage has already been done,” she added.
The International Lung Cancer Consortium studies had multiple supporters. Dr. Fares reported having no disclosures related to the research. One researcher reported relationships with AbbVie, AstraZeneca, MedImmune, Bayer, Bristol-Myers Squibb, Merck, Novartis, Pfizer, Roche Canada, and Takeda. Dr. Karam-Hage reported having no relevant disclosures.
SOURCE: Fares AF et al. ASCO 2020, Abstract 1512.
This article was updated 5/15/20.
Quitting smoking prior to a lung cancer diagnosis is associated with a survival benefit, even among patients who recently stopped smoking, according to results of a pooled analysis.
The overall survival advantage was significant regardless of how long ago patients had last smoked, including among those who quit within 2 years prior to their diagnosis.
These findings create a “teachable moment” for health care providers in scenarios when patients might be more receptive to a stop-smoking message, according to investigator Aline F. Fares, MD, a clinical research fellow at Princess Margaret Cancer Centre in Toronto.
“Our study can be summarized to patients as, ‘it’s never too late to quit,’ ” Dr. Fares said.
She presented results from this study at the American Society of Clinical Oncology virtual scientific program during a press briefing in advance of the meeting. This year, ASCO’s annual meeting is split into two parts. The virtual scientific program will be presented online May 29-31. The virtual education program will be available Aug. 8-10.
Results
Dr. Fares presented data on 35,481 patients with a diagnosis of lung cancer who had been enrolled in 17 studies conducted by the International Lung Cancer Consortium. (Data in the presentation were updated from the abstract.)
At diagnosis, 47.5% of the patients were current smokers, 30% were former smokers, and 22.5% were never smokers.
The risk of death from any cause was cut by 20% among former smokers who quit more than 5 years before their lung cancer diagnosis (P < .001). Patients who quit smoking 2-5 years before diagnosis had a 16% reduction in the risk of death, while those who quit within 2 years of diagnosis had a 12% reduced risk (P < .001 for both comparisons).
The overall survival advantage was evident in this pooled analysis regardless of patient sex, disease stage, histology, or amount of smoking as measured in pack-years, according to Dr. Fares. That said, the overall survival advantage appeared to be even greater among heavier smokers (i.e., greater than 30 pack-years) as compared with lighter smokers.
Lung cancer–specific survival was improved by 15% for patients who quit smoking more than 5 years prior to their diagnosis. For those who had quit more recently, there was a nonsignificant trend toward improvement in this outcome.
Overall survival was higher in never smokers in comparison with current smokers, a finding that was expected based on previous studies, according to Dr. Fares.
Implications
These findings could be important to share with individuals who are current smokers at the time of lung cancer screening, according to Maher A. Karam-Hage, MD, medical director of the tobacco treatment program at the University of Texas MD Anderson Cancer Center, Houston.
“The power of this data is that it shows quitting makes a difference, and that it can be more impactful the longer you quit before you get diagnosed,” Dr. Karam-Hage said in an interview.
Negative lung cancer screening results sometimes give individuals the false impression that they are “one of the lucky ones” who won’t get lung cancer and don’t have to quit smoking, according to Dr. Karam-Hage, who is studying the comparative effectiveness of different smoking cessation strategies.
“Now, as part of shared decision making, we can provide people with specific numbers before the scan that [suggest] no matter what the scan comes out with, the earlier they quit, the better off they will be,” he said.
In her presentation, Dr. Fares said that lung cancer screening may be an “interesting time” to address smoking cessation, particularly among patients with a heavier smoking history.
“After a lifetime of smoking, patients often feel it’s too late to quit smoking and that the damage has already been done,” she added.
The International Lung Cancer Consortium studies had multiple supporters. Dr. Fares reported having no disclosures related to the research. One researcher reported relationships with AbbVie, AstraZeneca, MedImmune, Bayer, Bristol-Myers Squibb, Merck, Novartis, Pfizer, Roche Canada, and Takeda. Dr. Karam-Hage reported having no relevant disclosures.
SOURCE: Fares AF et al. ASCO 2020, Abstract 1512.
This article was updated 5/15/20.
FROM ASCO 2020
Even with mild COVID-19, athletes need cardiac testing before returning to play
Potential risks of cardiac injury posed by coronavirus disease 2019 (COVID-19) infection warrant a cautious return-to-play for highly active people and competitive athletes who test positive, according to leading sports cardiologists.
To prevent cardiac injury, athletes should rest for at least 2 weeks after symptoms resolve, then undergo cardiac testing before returning high-level competitive sports, reported lead author Dermot Phelan, MD, PhD, of Atrium Health in Charlotte, N.C., and colleagues.
These recommendations, which were published in JAMA Cardiology, are part of a clinical algorithm that sorts athletes based on coronavirus test status and symptom severity. The algorithm offers a clear timeline for resumption of activity, with management decisions for symptomatic individuals based on additional diagnostics, such as high-sensitivity troponin testing and electrocardiogram.
Despite a scarcity of relevant clinical data, Dr. Phelan said that he and his colleagues wanted to offer their best recommendations to the athletic community, who had been reaching out for help.
“We were getting calls and messages from amateur and professional sporting organizations from around the country asking for guidance about what to do,” Dr. Phelan said. “So a number of us from the American College of Cardiology Sports and Exercise Council decided that we really should provide some guidance even in the absence of good, strong data, for what we feel is a reasonable approach.”
The recommendations were based on what is known of other viral infections, as well as risks posed by COVID-19 that may be worsened by athletic activity.
“We know that, when people have an active infection, vigorous exercise can lower immunity, and that can make the infection worse,” Dr. Phelan said. “That really applies very strongly in people who have had myocarditis. If you exercise when you have myocarditis, it actually increases viral replication and results in increased necrosis of the heart muscle. We really want to avoid exercising during that active infection phase.”
Myocarditis is one of the top causes of sudden cardiac death among young athletes, Dr. Phelan said, “so that’s a major concern for us.”
According to Dr. Phelan, existing data suggest a wide range of incidence of 7%-33% for cardiac injury among patients hospitalized for COVID-19. Even the low end of this range, at 7%, is significantly higher than the incidence rate of 1% found in patients with non–COVID-19 acute viral infections.
“This particular virus appears to cause more cardiac insults than other viruses,” Dr. Phelan said.
The incidence of cardiac injury among nonhospitalized patients remains unknown, leaving a wide knowledge gap that shaped the conservative nature of the present recommendations.
With more information, however, the guidance may “change dramatically,” Dr. Phelan said.
“If the data come back and show that no nonhospitalized patients got cardiac injury, then we would be much more comfortable allowing return to play without the need for cardiac testing,” he said.
Conversely, if cardiac injury is more common than anticipated, then more extensive testing may be needed, he added.
As the algorithm stands, high-sensitivity troponin testing and/or cardiac studies are recommended for all symptomatic athletes; if troponin levels are greater than the 99th percentile or a cardiac study is abnormal, then clinicians should follow return-to-play guidelines for myocarditis. For athletes with normal tests, slow resumption of activity is recommended, including close monitoring for clinical deterioration.
As Dr. Phelan discussed these recommendations in a broader context, he emphasized the need for caution, both preventively, and for cardiologists working with recovering athletes.
“For the early stage of this reentry into normal life while this is still an active pandemic, we need to be cautious,” Dr. Phelan said. “We need to follow the regular CDC guidelines, in terms of social distancing and handwashing, but we also need to consider that those people who have suffered from COVID-19 may have had cardiac injury. We don’t know that yet. But we need to be cautious with these individuals and test them before they return to high-level competitive sports.”
One author disclosed a relationship with the Atlanta Falcons.
SOURCE: Phelan D et al. JAMA Cardiology. 2020 Apr 13. doi: 10.1001/jamacardio.2020.2136.
Potential risks of cardiac injury posed by coronavirus disease 2019 (COVID-19) infection warrant a cautious return-to-play for highly active people and competitive athletes who test positive, according to leading sports cardiologists.
To prevent cardiac injury, athletes should rest for at least 2 weeks after symptoms resolve, then undergo cardiac testing before returning high-level competitive sports, reported lead author Dermot Phelan, MD, PhD, of Atrium Health in Charlotte, N.C., and colleagues.
These recommendations, which were published in JAMA Cardiology, are part of a clinical algorithm that sorts athletes based on coronavirus test status and symptom severity. The algorithm offers a clear timeline for resumption of activity, with management decisions for symptomatic individuals based on additional diagnostics, such as high-sensitivity troponin testing and electrocardiogram.
Despite a scarcity of relevant clinical data, Dr. Phelan said that he and his colleagues wanted to offer their best recommendations to the athletic community, who had been reaching out for help.
“We were getting calls and messages from amateur and professional sporting organizations from around the country asking for guidance about what to do,” Dr. Phelan said. “So a number of us from the American College of Cardiology Sports and Exercise Council decided that we really should provide some guidance even in the absence of good, strong data, for what we feel is a reasonable approach.”
The recommendations were based on what is known of other viral infections, as well as risks posed by COVID-19 that may be worsened by athletic activity.
“We know that, when people have an active infection, vigorous exercise can lower immunity, and that can make the infection worse,” Dr. Phelan said. “That really applies very strongly in people who have had myocarditis. If you exercise when you have myocarditis, it actually increases viral replication and results in increased necrosis of the heart muscle. We really want to avoid exercising during that active infection phase.”
Myocarditis is one of the top causes of sudden cardiac death among young athletes, Dr. Phelan said, “so that’s a major concern for us.”
According to Dr. Phelan, existing data suggest a wide range of incidence of 7%-33% for cardiac injury among patients hospitalized for COVID-19. Even the low end of this range, at 7%, is significantly higher than the incidence rate of 1% found in patients with non–COVID-19 acute viral infections.
“This particular virus appears to cause more cardiac insults than other viruses,” Dr. Phelan said.
The incidence of cardiac injury among nonhospitalized patients remains unknown, leaving a wide knowledge gap that shaped the conservative nature of the present recommendations.
With more information, however, the guidance may “change dramatically,” Dr. Phelan said.
“If the data come back and show that no nonhospitalized patients got cardiac injury, then we would be much more comfortable allowing return to play without the need for cardiac testing,” he said.
Conversely, if cardiac injury is more common than anticipated, then more extensive testing may be needed, he added.
As the algorithm stands, high-sensitivity troponin testing and/or cardiac studies are recommended for all symptomatic athletes; if troponin levels are greater than the 99th percentile or a cardiac study is abnormal, then clinicians should follow return-to-play guidelines for myocarditis. For athletes with normal tests, slow resumption of activity is recommended, including close monitoring for clinical deterioration.
As Dr. Phelan discussed these recommendations in a broader context, he emphasized the need for caution, both preventively, and for cardiologists working with recovering athletes.
“For the early stage of this reentry into normal life while this is still an active pandemic, we need to be cautious,” Dr. Phelan said. “We need to follow the regular CDC guidelines, in terms of social distancing and handwashing, but we also need to consider that those people who have suffered from COVID-19 may have had cardiac injury. We don’t know that yet. But we need to be cautious with these individuals and test them before they return to high-level competitive sports.”
One author disclosed a relationship with the Atlanta Falcons.
SOURCE: Phelan D et al. JAMA Cardiology. 2020 Apr 13. doi: 10.1001/jamacardio.2020.2136.
Potential risks of cardiac injury posed by coronavirus disease 2019 (COVID-19) infection warrant a cautious return-to-play for highly active people and competitive athletes who test positive, according to leading sports cardiologists.
To prevent cardiac injury, athletes should rest for at least 2 weeks after symptoms resolve, then undergo cardiac testing before returning high-level competitive sports, reported lead author Dermot Phelan, MD, PhD, of Atrium Health in Charlotte, N.C., and colleagues.
These recommendations, which were published in JAMA Cardiology, are part of a clinical algorithm that sorts athletes based on coronavirus test status and symptom severity. The algorithm offers a clear timeline for resumption of activity, with management decisions for symptomatic individuals based on additional diagnostics, such as high-sensitivity troponin testing and electrocardiogram.
Despite a scarcity of relevant clinical data, Dr. Phelan said that he and his colleagues wanted to offer their best recommendations to the athletic community, who had been reaching out for help.
“We were getting calls and messages from amateur and professional sporting organizations from around the country asking for guidance about what to do,” Dr. Phelan said. “So a number of us from the American College of Cardiology Sports and Exercise Council decided that we really should provide some guidance even in the absence of good, strong data, for what we feel is a reasonable approach.”
The recommendations were based on what is known of other viral infections, as well as risks posed by COVID-19 that may be worsened by athletic activity.
“We know that, when people have an active infection, vigorous exercise can lower immunity, and that can make the infection worse,” Dr. Phelan said. “That really applies very strongly in people who have had myocarditis. If you exercise when you have myocarditis, it actually increases viral replication and results in increased necrosis of the heart muscle. We really want to avoid exercising during that active infection phase.”
Myocarditis is one of the top causes of sudden cardiac death among young athletes, Dr. Phelan said, “so that’s a major concern for us.”
According to Dr. Phelan, existing data suggest a wide range of incidence of 7%-33% for cardiac injury among patients hospitalized for COVID-19. Even the low end of this range, at 7%, is significantly higher than the incidence rate of 1% found in patients with non–COVID-19 acute viral infections.
“This particular virus appears to cause more cardiac insults than other viruses,” Dr. Phelan said.
The incidence of cardiac injury among nonhospitalized patients remains unknown, leaving a wide knowledge gap that shaped the conservative nature of the present recommendations.
With more information, however, the guidance may “change dramatically,” Dr. Phelan said.
“If the data come back and show that no nonhospitalized patients got cardiac injury, then we would be much more comfortable allowing return to play without the need for cardiac testing,” he said.
Conversely, if cardiac injury is more common than anticipated, then more extensive testing may be needed, he added.
As the algorithm stands, high-sensitivity troponin testing and/or cardiac studies are recommended for all symptomatic athletes; if troponin levels are greater than the 99th percentile or a cardiac study is abnormal, then clinicians should follow return-to-play guidelines for myocarditis. For athletes with normal tests, slow resumption of activity is recommended, including close monitoring for clinical deterioration.
As Dr. Phelan discussed these recommendations in a broader context, he emphasized the need for caution, both preventively, and for cardiologists working with recovering athletes.
“For the early stage of this reentry into normal life while this is still an active pandemic, we need to be cautious,” Dr. Phelan said. “We need to follow the regular CDC guidelines, in terms of social distancing and handwashing, but we also need to consider that those people who have suffered from COVID-19 may have had cardiac injury. We don’t know that yet. But we need to be cautious with these individuals and test them before they return to high-level competitive sports.”
One author disclosed a relationship with the Atlanta Falcons.
SOURCE: Phelan D et al. JAMA Cardiology. 2020 Apr 13. doi: 10.1001/jamacardio.2020.2136.
FROM JAMA CARDIOLOGY
Comparing COVID-19, flu death tolls ‘extremely dangerous’
The number of COVID-19 deaths cannot be directly compared to the number of seasonal influenza deaths because they are calculated differently, researchers say in a report released today.
Whereas COVID-19 death rates are determined from actual counts of people who have died, seasonal influenza death rates are estimated by the Centers for Disease Control and Prevention (CDC) using population modeling algorithms, explains Jeremy Samuel Faust, MD, with Harvard Medical School and Brigham and Women’s Hospital, Division of Health Policy and Public Health in Boston, Massachusetts.
The CDC estimates that between 24,000 and 62,000 people died from influenza during the 2019-2020 season (through April 4). At the time of the analysis (as of April 28), COVID-19 deaths had reached 65,000 in the United States.
But making that comparison “is extremely dangerous,” Faust told Medscape Medical News.
“COVID-19 is far more dangerous and is wreaking far more havoc than seasonal influenza ever has,” he said.
Faust coauthored the perspective article, published online in JAMA Internal Medicine, with Carlos del Rio, MD, Division of Infectious Diseases at Emory University School of Medicine in Atlanta, Georgia.
The message and methodology of Faust’s and del Rio’s article are on target, according to Jonathan L. Temte, MD, PhD, who has been working in influenza surveillance for almost 25 years.
Current flu data draw on limited information from primary care practices and hospitals, said Dr. Temte, associate dean for public health and community engagement at the University of Wisconsin School of Medicine and Public Health in Madison. The estimates help bridge the gaps, he said, but the system is inherently vulnerable to error.
“Comparing them – as so many people in this country have done – to try to diminish the impact of SARS-CoV2 is not fair,” he said.
Estimated versus actual influenza deaths
The authors illustrate the difference in the way rates of death from influenza are calculated: “Between 2013-2014 and 2018-2019, the reported yearly estimated influenza deaths ranged from 23,000 to 61,000. Over that same time period, however, the number of counted influenza deaths was between 3,448 and 15,620 yearly.”
“It’s apparent [the CDC has] been overestimating,” Faust said. “If you publish a number on the higher end of the estimate, people might take your public health messages more seriously, such as, it’s important to get your yearly flu shot.”
He added that until influenza death rates started to be compared with COVID-19 rates, “there was never really a downside” to reporting estimates.
Dr. Temte said he doesn’t regard overestimating flu deaths as intentional but rather the result of a longstanding “bias against the elderly in this country” that the estimates are meant to account for.
For example, he says, reporting influenza deaths is mandatory when such deaths involve persons younger than 18 years but not when they involve adults.
Also, traditionally, influenza has been seen “as a cause of death in people with multiple comorbidities that was just part and parcel of wintertime,” Dr. Temte said.
“The likelihood of being tested for influenza goes down greatly when you’re older,” he said. “This is slowly changing.”
The CDC acknowledges on its website that it “does not know the exact number of people who have been sick and affected by influenza because influenza is not a reportable disease in most areas of the US.”
It adds that the burden is estimated through the US Influenza Surveillance System, which covers approximately 8.5% of the US population.
Comparing recorded deaths
It’s more accurate and meaningful to compare actual numbers of deaths for the diseases, Dr. Faust and Dr. del Rio say in their article.
When the authors made that comparison, they drew a stark contrast.
There were 15,455 recorded COVID-19 deaths in the week that ended April 21. The week before, the number of recorded deaths was 14,478, they found. (Those were the two most recent weeks before they submitted their article for publication.)
In comparison, counted deaths ranged from 351 to 1,626 during the peak week of the seven influenza seasons between 2013-2014 and 2019-2020. The average counted deaths for the peak week of the seven seasons was 752.4 (95% confidence interval, 558.8-946.1).
“These statistics on counted deaths suggest that the number of COVID-19 deaths for the week ending April 21 was 9.5-fold to 44.1-fold greater than the peak week of counted influenza deaths during the past seven influenza seasons in the US, with a 20.5-fold mean increase (95% CI, 16.3-27.7),” the authors write.
However, Natasha Chida, MD, MSPH, an infectious disease physician and assistant professor at the Johns Hopkins University School of Medicine in Baltimore, Maryland, said in an interview that the actual number of deaths doesn’t tell the complete flu story either. That count would miss people who later died from secondary complications associated with influenza, she said.
“There’s just no way to reliably count influenza deaths,” she said. “I think if we required it as a reported illness, that would be the ideal situation, but there’s so much flu every year that that probably would not be practical.”
She said she agrees that rates of influenza deaths and rates of COVID-19 deaths cannot be fairly compared.
What the authors don’t touch on, she said, is that flu season lasts 4 to 6 months a year, and just 3 months into the coronavirus pandemic, US deaths due to COVID-19 are already higher than those for seasonal influenza.
“Even if we look at it in the way that people who think we can compare flu and coronavirus do, it’s still not going to work out in their favor from a numbers standpoint,” she said.
The article clarifies the differences for “people who don’t live in the flu world,” she said.
“It is not accurate to compare the two for the reasons the authors described and also because they are very different diseases,” she added.
Real-life validation
Dr. Faust said in an interview that real-life experiences add external validity to their analysis.
Differences in the way deaths are calculated does not reflect frontline clinical conditions during the COVID-19 crisis, with hospitals stretched past their limits, ventilator shortages, and bodies stacking up in some overwhelmed facilities, the authors say.
Dr. Temte said the external validation of the numbers also rings true in light of his own experience.
He said that, in the past 2 months, he has known two people who have had family members who died of COVID-19.
Conversely, “I would have to search long and hard to come up with people I have known or have been one degree of separation from” who have died from influenza, Dr. Temte said.
The authors, Dr. Temte, and Dr. Chida report no relevant financial relationships.
This article first appeared on Medscape.com.
The number of COVID-19 deaths cannot be directly compared to the number of seasonal influenza deaths because they are calculated differently, researchers say in a report released today.
Whereas COVID-19 death rates are determined from actual counts of people who have died, seasonal influenza death rates are estimated by the Centers for Disease Control and Prevention (CDC) using population modeling algorithms, explains Jeremy Samuel Faust, MD, with Harvard Medical School and Brigham and Women’s Hospital, Division of Health Policy and Public Health in Boston, Massachusetts.
The CDC estimates that between 24,000 and 62,000 people died from influenza during the 2019-2020 season (through April 4). At the time of the analysis (as of April 28), COVID-19 deaths had reached 65,000 in the United States.
But making that comparison “is extremely dangerous,” Faust told Medscape Medical News.
“COVID-19 is far more dangerous and is wreaking far more havoc than seasonal influenza ever has,” he said.
Faust coauthored the perspective article, published online in JAMA Internal Medicine, with Carlos del Rio, MD, Division of Infectious Diseases at Emory University School of Medicine in Atlanta, Georgia.
The message and methodology of Faust’s and del Rio’s article are on target, according to Jonathan L. Temte, MD, PhD, who has been working in influenza surveillance for almost 25 years.
Current flu data draw on limited information from primary care practices and hospitals, said Dr. Temte, associate dean for public health and community engagement at the University of Wisconsin School of Medicine and Public Health in Madison. The estimates help bridge the gaps, he said, but the system is inherently vulnerable to error.
“Comparing them – as so many people in this country have done – to try to diminish the impact of SARS-CoV2 is not fair,” he said.
Estimated versus actual influenza deaths
The authors illustrate the difference in the way rates of death from influenza are calculated: “Between 2013-2014 and 2018-2019, the reported yearly estimated influenza deaths ranged from 23,000 to 61,000. Over that same time period, however, the number of counted influenza deaths was between 3,448 and 15,620 yearly.”
“It’s apparent [the CDC has] been overestimating,” Faust said. “If you publish a number on the higher end of the estimate, people might take your public health messages more seriously, such as, it’s important to get your yearly flu shot.”
He added that until influenza death rates started to be compared with COVID-19 rates, “there was never really a downside” to reporting estimates.
Dr. Temte said he doesn’t regard overestimating flu deaths as intentional but rather the result of a longstanding “bias against the elderly in this country” that the estimates are meant to account for.
For example, he says, reporting influenza deaths is mandatory when such deaths involve persons younger than 18 years but not when they involve adults.
Also, traditionally, influenza has been seen “as a cause of death in people with multiple comorbidities that was just part and parcel of wintertime,” Dr. Temte said.
“The likelihood of being tested for influenza goes down greatly when you’re older,” he said. “This is slowly changing.”
The CDC acknowledges on its website that it “does not know the exact number of people who have been sick and affected by influenza because influenza is not a reportable disease in most areas of the US.”
It adds that the burden is estimated through the US Influenza Surveillance System, which covers approximately 8.5% of the US population.
Comparing recorded deaths
It’s more accurate and meaningful to compare actual numbers of deaths for the diseases, Dr. Faust and Dr. del Rio say in their article.
When the authors made that comparison, they drew a stark contrast.
There were 15,455 recorded COVID-19 deaths in the week that ended April 21. The week before, the number of recorded deaths was 14,478, they found. (Those were the two most recent weeks before they submitted their article for publication.)
In comparison, counted deaths ranged from 351 to 1,626 during the peak week of the seven influenza seasons between 2013-2014 and 2019-2020. The average counted deaths for the peak week of the seven seasons was 752.4 (95% confidence interval, 558.8-946.1).
“These statistics on counted deaths suggest that the number of COVID-19 deaths for the week ending April 21 was 9.5-fold to 44.1-fold greater than the peak week of counted influenza deaths during the past seven influenza seasons in the US, with a 20.5-fold mean increase (95% CI, 16.3-27.7),” the authors write.
However, Natasha Chida, MD, MSPH, an infectious disease physician and assistant professor at the Johns Hopkins University School of Medicine in Baltimore, Maryland, said in an interview that the actual number of deaths doesn’t tell the complete flu story either. That count would miss people who later died from secondary complications associated with influenza, she said.
“There’s just no way to reliably count influenza deaths,” she said. “I think if we required it as a reported illness, that would be the ideal situation, but there’s so much flu every year that that probably would not be practical.”
She said she agrees that rates of influenza deaths and rates of COVID-19 deaths cannot be fairly compared.
What the authors don’t touch on, she said, is that flu season lasts 4 to 6 months a year, and just 3 months into the coronavirus pandemic, US deaths due to COVID-19 are already higher than those for seasonal influenza.
“Even if we look at it in the way that people who think we can compare flu and coronavirus do, it’s still not going to work out in their favor from a numbers standpoint,” she said.
The article clarifies the differences for “people who don’t live in the flu world,” she said.
“It is not accurate to compare the two for the reasons the authors described and also because they are very different diseases,” she added.
Real-life validation
Dr. Faust said in an interview that real-life experiences add external validity to their analysis.
Differences in the way deaths are calculated does not reflect frontline clinical conditions during the COVID-19 crisis, with hospitals stretched past their limits, ventilator shortages, and bodies stacking up in some overwhelmed facilities, the authors say.
Dr. Temte said the external validation of the numbers also rings true in light of his own experience.
He said that, in the past 2 months, he has known two people who have had family members who died of COVID-19.
Conversely, “I would have to search long and hard to come up with people I have known or have been one degree of separation from” who have died from influenza, Dr. Temte said.
The authors, Dr. Temte, and Dr. Chida report no relevant financial relationships.
This article first appeared on Medscape.com.
The number of COVID-19 deaths cannot be directly compared to the number of seasonal influenza deaths because they are calculated differently, researchers say in a report released today.
Whereas COVID-19 death rates are determined from actual counts of people who have died, seasonal influenza death rates are estimated by the Centers for Disease Control and Prevention (CDC) using population modeling algorithms, explains Jeremy Samuel Faust, MD, with Harvard Medical School and Brigham and Women’s Hospital, Division of Health Policy and Public Health in Boston, Massachusetts.
The CDC estimates that between 24,000 and 62,000 people died from influenza during the 2019-2020 season (through April 4). At the time of the analysis (as of April 28), COVID-19 deaths had reached 65,000 in the United States.
But making that comparison “is extremely dangerous,” Faust told Medscape Medical News.
“COVID-19 is far more dangerous and is wreaking far more havoc than seasonal influenza ever has,” he said.
Faust coauthored the perspective article, published online in JAMA Internal Medicine, with Carlos del Rio, MD, Division of Infectious Diseases at Emory University School of Medicine in Atlanta, Georgia.
The message and methodology of Faust’s and del Rio’s article are on target, according to Jonathan L. Temte, MD, PhD, who has been working in influenza surveillance for almost 25 years.
Current flu data draw on limited information from primary care practices and hospitals, said Dr. Temte, associate dean for public health and community engagement at the University of Wisconsin School of Medicine and Public Health in Madison. The estimates help bridge the gaps, he said, but the system is inherently vulnerable to error.
“Comparing them – as so many people in this country have done – to try to diminish the impact of SARS-CoV2 is not fair,” he said.
Estimated versus actual influenza deaths
The authors illustrate the difference in the way rates of death from influenza are calculated: “Between 2013-2014 and 2018-2019, the reported yearly estimated influenza deaths ranged from 23,000 to 61,000. Over that same time period, however, the number of counted influenza deaths was between 3,448 and 15,620 yearly.”
“It’s apparent [the CDC has] been overestimating,” Faust said. “If you publish a number on the higher end of the estimate, people might take your public health messages more seriously, such as, it’s important to get your yearly flu shot.”
He added that until influenza death rates started to be compared with COVID-19 rates, “there was never really a downside” to reporting estimates.
Dr. Temte said he doesn’t regard overestimating flu deaths as intentional but rather the result of a longstanding “bias against the elderly in this country” that the estimates are meant to account for.
For example, he says, reporting influenza deaths is mandatory when such deaths involve persons younger than 18 years but not when they involve adults.
Also, traditionally, influenza has been seen “as a cause of death in people with multiple comorbidities that was just part and parcel of wintertime,” Dr. Temte said.
“The likelihood of being tested for influenza goes down greatly when you’re older,” he said. “This is slowly changing.”
The CDC acknowledges on its website that it “does not know the exact number of people who have been sick and affected by influenza because influenza is not a reportable disease in most areas of the US.”
It adds that the burden is estimated through the US Influenza Surveillance System, which covers approximately 8.5% of the US population.
Comparing recorded deaths
It’s more accurate and meaningful to compare actual numbers of deaths for the diseases, Dr. Faust and Dr. del Rio say in their article.
When the authors made that comparison, they drew a stark contrast.
There were 15,455 recorded COVID-19 deaths in the week that ended April 21. The week before, the number of recorded deaths was 14,478, they found. (Those were the two most recent weeks before they submitted their article for publication.)
In comparison, counted deaths ranged from 351 to 1,626 during the peak week of the seven influenza seasons between 2013-2014 and 2019-2020. The average counted deaths for the peak week of the seven seasons was 752.4 (95% confidence interval, 558.8-946.1).
“These statistics on counted deaths suggest that the number of COVID-19 deaths for the week ending April 21 was 9.5-fold to 44.1-fold greater than the peak week of counted influenza deaths during the past seven influenza seasons in the US, with a 20.5-fold mean increase (95% CI, 16.3-27.7),” the authors write.
However, Natasha Chida, MD, MSPH, an infectious disease physician and assistant professor at the Johns Hopkins University School of Medicine in Baltimore, Maryland, said in an interview that the actual number of deaths doesn’t tell the complete flu story either. That count would miss people who later died from secondary complications associated with influenza, she said.
“There’s just no way to reliably count influenza deaths,” she said. “I think if we required it as a reported illness, that would be the ideal situation, but there’s so much flu every year that that probably would not be practical.”
She said she agrees that rates of influenza deaths and rates of COVID-19 deaths cannot be fairly compared.
What the authors don’t touch on, she said, is that flu season lasts 4 to 6 months a year, and just 3 months into the coronavirus pandemic, US deaths due to COVID-19 are already higher than those for seasonal influenza.
“Even if we look at it in the way that people who think we can compare flu and coronavirus do, it’s still not going to work out in their favor from a numbers standpoint,” she said.
The article clarifies the differences for “people who don’t live in the flu world,” she said.
“It is not accurate to compare the two for the reasons the authors described and also because they are very different diseases,” she added.
Real-life validation
Dr. Faust said in an interview that real-life experiences add external validity to their analysis.
Differences in the way deaths are calculated does not reflect frontline clinical conditions during the COVID-19 crisis, with hospitals stretched past their limits, ventilator shortages, and bodies stacking up in some overwhelmed facilities, the authors say.
Dr. Temte said the external validation of the numbers also rings true in light of his own experience.
He said that, in the past 2 months, he has known two people who have had family members who died of COVID-19.
Conversely, “I would have to search long and hard to come up with people I have known or have been one degree of separation from” who have died from influenza, Dr. Temte said.
The authors, Dr. Temte, and Dr. Chida report no relevant financial relationships.
This article first appeared on Medscape.com.