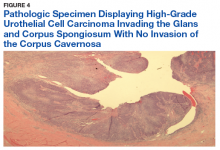
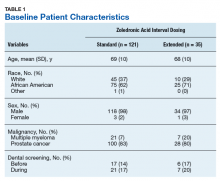
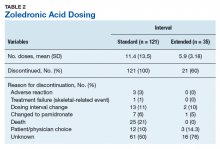
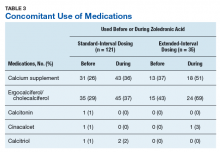
User login
Management of Patients With Treatment-Resistant Metastatic Prostate Cancer (FULL)
Sequencing Therapies
Mark Klein, MD. The last few years, there have been several new trials in prostate cancer for people in a metastatic setting or more advanced local setting, such as the STAMPEDE, LATITUDE, and CHAARTED trials.1-4 In addition, recently a few trials have examined apalutamide and enzalutamide for people who have had PSA (prostate-specific antigen) levels rapidly rising within about 10 months or so. One of the questions that arises is, how do we wrap our heads around sequencing these therapies. Is there a sequence that we should be doing and thinking about upfront and how do the different trials compare?
Julie Graff, MD. It just got more complicated. There was news today (December 20, 2018) that using enzalutamide early on in newly diagnosed metastatic prostate cancer may have positive results. It is not yet approved by the US Food and Drug Administration (FDA), but for patients who present with metastatic prostate cancer, we may have 4 potential treatments. We could have androgen deprivation therapy (ADT) alone, ADT plus docetaxel, enzalutamide, or abiraterone.
When I see patients in this situation, I talk to them about their options, the pros and cons of each option, and try to cover all the trials that look at these combinations. It can be quite a long visit. I talk to the patient about who benefits most, whether it is patients with high-risk factors or high-volume cancers. Also, I talk with the patient about all the adverse effects (AEs), and I look at my patients’ comorbid conditions and come up with a plan.
I encourage any patient who has high-volume or high-risk disease to consider more than just ADT alone. For many patients, I have been using abiraterone plus ADT. I have a wonderful pharmacist. As a medical oncologist, I can’t do it on my own. I need someone to follow patients’ laboratory results and to be available for medication questions and complications.
Elizabeth Hansen, PharmD. With the increasing number of patients on oral antineoplastics, monitoring patients in the outpatient setting has become an increasing priority and one of my major roles as a pharmacist in the clinic at the Chalmers P. Wylie VA Ambulatory Care Center in Columbus, Ohio. This is especially important as some of these treatments require frequent laboratory monitoring, such as abiraterone with liver function tests every 2 weeks for the first 3 months of treatment and monthly thereafter. Without frequent-follow up it’s easy for these patients to get lost in the shuffle.
Abhishek Solanki, MD. You could argue that a fifth option is prostate-directed radiation for patients who have limited metastases based on the STAMPEDE trial, which we’ve started integrating into our practice at the Edward Hines, Jr. Veterans Affairs Hospital in Chicago, Illinois.4
Mark Klein. Do you have a feel for the data and using radiation in oligometastatic (≤ 5 metastatic tumors) disease in prostate cancer and how well that might work?
Abhishek Solanki. The best data we have are from the multi-arm, multistage STAMPEDE trial systemic therapies and local therapy in the setting of high-risk localized disease and metastatic disease.6 The most recent publication looked specifically at the population with newly diagnosed metastatic disease and compared standard ADT (and docetaxel in about 18% of the patients) with or without prostate-directed radiation therapy. There was no survival benefit with radiation in the overall population, but in the subgroup of patients with low metastatic burden, there was an 8% survival benefit at 3 years.
It’s difficult to know what to make of that information because, as we’ve discussed already, there are other systemic therapy options that are being used more and more upfront such as abiraterone. Can you see the same benefit of radiation in that setting? The flip side is that in this study, radiation just targeted the prostate; could survival be improved even more by targeting all sites of disease in patients with oligometastatic disease? These are still open questions in prostate cancer and there are clinical trials attempting to define the clinical benefit of radiation in the metastatic setting for patients with limited metastases.
Mark Klein. How do you select patients for radiation in this particular situation; How do you approach stratification when radiation is started upfront?
Abhishek Solanki. In the STAMPEDE trial, low metastatic burden was defined based on the definition in the CHAARTED trial, which was those patients who did not have ≥ 4 bone metastases with ≥ 1 outside the vertebral bodies or pelvis, and did not have visceral metastases.7 That’s tough, because this definition could be a patient with a solitary bone metastasis but also could include some patients who have involved nodes extending all the way up to the retroperitoneal nodes—that is a fairly heterogeneous population. What we have done at our institution is select patients who have 3 to 5 metastases, administer prostate radiation therapy, and add stereotactic body radiation therapy (SBRT) for the other sites of disease, invoking the oligometastasis approach.
We have been doing this more frequently in the last few months. Typically, we’ll do 3 to 5 fractions of SBRT to metastases. For the primary, if the patient chooses SBRT, we’ll take that approach. If the patient chooses a more standard fractionation, we’ll do 20 treatments, but from a logistic perspective, most patients would rather come in for 5 treatments than 20. We also typically would start these patients on systemic hormonal therapy.
Mark Klein. At that point, are they referred back to medical oncology for surveillance?
Abhishek Solanki. Yes, they are followed by medical oncology and radiation oncology, and typically would continue hormonal therapy.
Mark Klein. Julie, how have you thought about presenting the therapeutic options for those patients who would be either eligible for docetaxel with high-bulk disease or abiraterone? Do you find patients prefer one or the other?
Julie Graff. I try to be very open about all the possibilities, and I present both. I don’t just decide for the patient chemotherapy vs abiraterone, but after we talk about it, most of my patients do opt for the abiraterone. I had a patient referred from the community—we are seeing more and more of this because abiraterone is so expensive—whose ejection fraction was about 38%. I said to that patient, “we could do chemotherapy, but we shouldn’t do abiraterone.” But usually it’s not that clear-cut.
Elizabeth Hansen. There was also an update from the STAMPEDE trial published recently comparing upfront abiraterone and prednisone to docetaxel (18 weeks) in advanced or metastatic prostate cancer. Results from this trial indicated a nearly identical overall survival (OS) (hazard ratio [HR] = 1.16; 95% CI, 0.82-1.65; P = .40). However, the failure-free survival (HR = 0.51; 95% CI, 0.39-0.67; P < .001) and progression-free survival (PFS) (HR= 0.65; 95% CI, 0.0.48-0.88; P = .005) favored abiraterone.8,9 The authors argue that while there was no change in OS, this trial demonstrates an important difference in the pattern of treatment failure.
Julie, do you think there will be any change in the treatment paradigm between docetaxel and abiraterone with this new update?
Julie Graff. I wasn’t that impressed by that study. I do not see it as practice changing, and it makes sense to me that the PFS is different in the 2 arms because we give chemotherapy and take a break vs giving abiraterone indefinitely. For me, there’s not really a shift.
Patients With Rising PSAs
Mark Klein. Let’s discuss the data from the recent studies on enzalutamide and apalutamide for the patients with fast-rising PSAs. In your discussions with other prostate researchers, will this become a standard part of practice or not?
Julie Graff. I was one of the authors on the SPARTAN apalutamide study.10 For a long time, we have had patients without metastatic disease but with a PSA relapse after surgery or radiation; and the PSA levels climb when the cancer becomes resistant to ADT. We haven’t had many options in that setting except to use bicalutamide and some older androgen receptor (AR) antagonists. We used to use estrogen and ketoconazole as well.
But now 2 studies have come out looking at a primary endpoint of metastases-free survival. Patients whose PSA was doubling every 10 months or shorter were randomized to either apalutamide (SPARTAN10) or enzalutamide (PROSPER11), both second-generation AR antagonists. There was a placebo control arm in each of the studies. Both studies found that adding the second-generation AR targeting agent delayed the time to metastatic disease by about 2 years. There is not any signal yet for statistically significant OS benefit, so it is not entirely clear if you could wait for the first metastasis to develop and then give 1 of these treatments and have the same OS benefit.
At the VA Portland Health Care System (VAPORHCS), it took a while to make these drugs available. My fellows were excited to give these drugs right away, but I often counsel patients that we don’t know if the second-generation AR targeting agents will improve survival. They almost certainly will bring down PSAs, which helps with peace of mind, but anything we add to the ADT can cause more AEs.
I have been cautious with second-generation AR antagonists because patients, when they take one of these drugs, are going to be on it for a long time. The FDA has approved those 2 drugs regardless of PSA doubling time, but I would not give it for a PSA doubling time > 10 months. In my practice about a quarter of patients who would qualify for apalutamide or enzalutamide are actually taking one, and the others are monitored closely with computed tomography (CT) and bone scans. When the disease becomes metastatic, then we start those drugs.
Mark Klein. Why 10 months, why not 6 months, a year, or 18 months? Is there reasoning behind that?
Julie Graff. There was a publication by Matthew Smith showing that the PSA doubling time was predictive of the development of metastatic disease and cancer death or prostate cancer death, and that 10 months seemed to be the cutoff between when the prostate cancer was going to become deadly vs not.12 If you actually look at the trial data, I think the PSA doubling time was between 3 and 4 months for the participants, so pretty short.
Adverse Effects
Mark Klein. What are the AEs people are seeing from using apalutamide, enzalutamide, and abiraterone? What are they seeing in their practice vs what is in the studies? When I have had to stop people on abiraterone or drop down the dose, almost always it has been for fatigue. We check liver function tests (LFTs) repeatedly, but I can’t remember ever having to drop down the dose or take it away even for that reason.
Elizabeth Hansen.
Mark Klein. At the Minneapolis VA Health Care System (MVAHCS) when apalutamide first came out, for the PSA rapid doubling, there had already been an abstract presenting the enzalutamide data. We have chosen to recommend enzalutamide as our choice for the people with PSA doubling based on the cost. It’s significantly cheaper for the VA. Between the 2 papers there is very little difference in the efficacy data. I’m wondering what other sites have done with regard to that specific point at their VAs?
Elizabeth Hansen. In Columbus, we prefer to use either abiraterone and enzalutamide because they’re essentially cost neutral. However, this may change with generic abiraterone coming to market. Apalutamide is really cost prohibitive currently.
Julie Graff. I agree.
Patient Education
Mark Klein. At MVAHCS, the navigators handle a lot of upfront education. We have 3 navigators, including Kathleen Nelson who is on this roundtable. She works with patients and provides much of the patient education. How have you handled education for patients?
Kathleen Nelson. For the most part, our pharmacists do the drug-specific education for the oral agents, and the nurse navigators provide more generic education. We did a trial for patients on IV therapies. We learned that patients really don’t report in much detail, but if you call and ask them specific questions, then you can tease out some more detail.
Elizabeth Hansen. It is interesting that every site is different. One of my main roles is oral antineoplastic monitoring, which includes many patients on enzalutamide or abiraterone. At least initially with these patients, I try to follow them closely—abiraterone more so than enzalutamide. I typically call every 2 to 4 weeks, in between clinic visits, to follow up the laboratory tests and manage the AEs. I always try to ask direct and open-ended questions: How often are you checking your blood pressure? What is your current weight? How has your energy level changed since therapy initiation?
The VA telehealth system is amazing. For patients who need to monitor blood pressure regularly, it’s really nice for them to have those numbers come directly back to me in CPRS (Computerized Patient Record System). That has worked wonders for some of our patients to get them through therapy.
Mark Klein. What do you tend to use when the prostate cancer is progressing for a patient? And how do you determine that progression? Some studies will use PSA rise only as a marker for progression. Other studies have not used PSA rise as the only marker for progression and oftentimes require some sort of bone scan criteria or CT imaging criteria for progression.
Julie Graff. We have a limited number of treatment options. Providers typically use enzalutamide or abiraterone as there is a high degree of resistance between the 2. Then there is chemotherapy and then radium, which quite a few people don’t qualify for. We need to be very thoughtful when we change treatments. I look at the 3 factors of biochemical progression or response—PSA, radiographic progression, and clinical progression. If I don’t see 2 out of 3, I typically don’t change treatments. Then after enzalutamide or abiraterone, I wait until there are cancer-related symptoms before I consider chemotherapy and closely monitor my patients.
Imaging Modalities
Abhishek Solanki. Over the last few years the Hines VA Hospital has used fluciclovine positron emission tomography (PET), which is one of the novel imaging modalities for prostate cancer. Really the 2 novel imaging modalities that have gained the most excitement are prostate-specific membrane antigen (PSMA) PET and fluciclovine PET. Fluciclovine PET is based on a synthetic amino acid that’s taken up in multiple tissues, including prostate cancer. It has changed our practice in the localized setting for patients who have developed recurrence after radiation or radical prostatectomy. We have incorporated the scan into our workup of patients with recurrent disease, which can give us some more information at lower PSAs than historically we could get with CT, bone scan, or magnetic resonance imaging.
Our medical oncologists have started using it more and more as well. We are getting a lot of patients who have a negative CT or bone scan but have a positive fluciclovine PET. There are a few different disease settings where that becomes relevant. In patients who develop biochemical recurrence after radiation or salvage radiation after radical, we are finding that a lot of these patients who have no CT or bone scan findings of disease ultimately are found to have a PET-positive lesion. Sometimes it’s difficult to know how best to help patients with PET-only disease. Should you target the disease with an oligometastasis approach or just pursue systemic therapy or surveillance? It is challenging but more and more we are moving toward metastasis-directed therapy. There are multiple randomized trials in progress testing whether metastasis-directed therapy to the PET areas of recurrence can improve outcomes or delay systemic ADT. The STOMP trial randomized surveillance vs SBRT or surgery for patients with oligometastatic disease that showed improvement in biochemical control and ADT-free survival.13 However this was a small trial that tried to identify a signal. More definitive trials are necessary.
The other setting where we have found novel PET imaging to be helpful is in patients who have become castration resistant but don’t have clear metastases on conventional imaging. We’re identifying more patients who have only a few sites of progression, and we’ll pursue metastasis-directed therapy to those areas to try to get more mileage out of the systemic therapy that the patient is currently on and to try to avoid having to switch to the next line with the idea that, potentially, the progression site is just a limited clone that is progressing despite the current systemic therapy.
Mark Klein. I find that to be a very attractive approach. I’m assuming you do that for any systemic therapy where people have maybe 1 or 2 sites and they do not have a big PSA jump. Do you have a number of sites that you’re willing to radiate? And then, when you do that, what radiation fractionation and dosing do you use? Is there any observational data behind that for efficacy?
Abhishek Solanki. It is a patient by patient decision. Some patients, if they have a very rapid pace of progression shortly after starting systemic therapy and metastases have grown in several areas, we think that perhaps this person may benefit less from aggressive local therapy. But if it’s somebody who has been on systemic therapy for a while and has up to 3 sites of disease growth, we consider SBRT for oligoprogressive disease. Typically, we’ll use SBRT, which delivers a high dose of radiation over 3 to 5 treatments. With SBRT you can give a higher biologic dose and use more sophisticated treatment machines and image guidance for treatments to focus the radiation on the tumor area and limit exposure to normal tissue structures.
In prostate cancer to the primary site, we will typically do around 35 to 40 Gy in 5 fractions. For metastases, it depends on the site. If it’s in the lung, typically we will do 3 to 5 treatments, giving approximately 50 to 60 Gy in that course. In the spine, we use lower doses near the spinal cord and the cauda equina, typically about 30 Gy in 3 fractions. In the liver, similar to the lung, we’ll typically do 50-54 Gy in 3-5 fractions. There aren’t a lot of high-level data guiding the optimal dose/fractionation to metastases, but these are the doses we’ll use for various malignancies.
Treatment Options for Patients With Adverse Events
Mark Klein. I was just reviewing the 2004 study that randomized patients to mitoxantrone or docetaxel for up to 10 cycles.14,15 Who are good candidates for docetaxel after they have exhausted abiraterone and enzalutamide? How long do you hold to the 10-cycle rule, or do you go beyond that if they’re doing well? And if they’re not a good candidate, what are some options?
Julie Graff. The best candidates are those who are having a cancer-related AE, particularly pain, because docetaxel only improves survival over mitoxantrone by about 2.5 months. I don’t talk to patients about it as though it is a life extender, but it seems to help control pain—about 70% of patients benefited in terms of pain or some other cancer-related symptom.14
I have a lot of patients who say, “Never will I do chemotherapy.” I refer those patients to hospice, or if they’re appropriate for radium-223, I consider that. I typically give about 6 cycles of chemotherapy and then see how they’re doing. In some patients, the cancer just doesn’t respond to it.
I do tell patients about the papers that you mentioned, the 2 studies of docetaxel vs mitoxantrone where they use about 10 cycles, and some of my patients go all 10.14,15 Sometimes we have to stop because of neuropathy or some other AE. I believe in taking breaks and that you can probably start it later.
Elizabeth Hansen. I agree, our practice is similar. A lot of our patients are not very interested in chemotherapy. You have to take into consideration their ECOG (Eastern Cooperative Oncology Group) status, their goals, and quality of life when talking to them about these medications. And a lot of them tend to choose more of a palliative route. Depending on their AEs and how things are going, we will dose reduce, hold treatment, or give treatment holidays.
Mark Klein. If patients are progressing on docetaxel, what are options that people would use? Radium-223 certainly is available for patients with nonvisceral metastases, as well as cabazitaxel, mitoxantrone, estramustine and other older drugs.
Julie Graff. We have some clinical trials for patients postdocetaxel. We have the TRITON2 and TRITON3 studies open at the VA. (NCT02952534 and NCT02975934, respectively) A lot of patients would get a biopsy, and we’d look for a BRCA 1 or 2 and ATM mutation. For those patients who don’t have those mutations—and maybe 80% of them don’t—we talk about radium-223 for the patients without visceral metastases and bone pain. I have had a fair number of patients go on cabazitaxel, but I have not used mitoxantrone since cabazitaxel came out. It’s not off the table, but it hasn’t shown improvement in survival.
Elizabeth Hansen. One of our challenges, because we’re an ambulatory care center, is that we are unable to give radium-223 in house, and these services have to be sent out to a non-VA facility. It is doable, but it takes more legwork and organization on our part.
Julie Graff. We have not had radium-223, although we’re working to get that online. And we are physically connected to Oregon Health Science University (OHSU), so we send our patients there for radium. It is a pain because the doctors at OHSU don’t have CPRS access. I’m often in the middle of making sure the complete blood counts (CBCs) are sent to OHSU and to get my patients their treatments.
Mark Klein. The Minneapolis VAMC has radium-223 on site, and we have used it for patients whose cancer has progressed while on docetaxel without visceral metastases. Katie, have you had an opportunity to coordinate that care for patients?
Kathleen Nelson. Radium is administered at our facility by one of our nuclear medicine physicians. A complete blood count is checked at least 3 days prior to the infusion date but no sooner than 6 days. Due to the cost of the material, ordering without knowing the patient’s counts are within a safe range to administer is prohibitive. This adds an additional burden of 2 visits (lab with return visit) to the patient. We have treated 12 patients. Four patients stopped treatment prior to completing the 6 planned treatments citing debilitating fatigue and/or nonresolution of symptoms as their reason to stop treatment. One patient died. The 7 remaining patients subjectively reported varying degrees of pain relief.
Elizabeth Hansen. Another thing to mention is the lack of a PSA response from radium-223 as well. Patients are generally very diligent about monitoring their PSA, so this can be a bit distressing.
Mark Klein. Julie, have you noticed a PSA flare with radium-223? I know it has been reported.
Julie Graff. I haven’t. But I put little stock in PSAs in these patients. I spend 20 minutes explaining to patients that the PSA is not helpful in determining whether or not the radium is working. I tell them that the bone marker alkaline phosphatase may decrease. And I think it’s important to note, too, that radium-223 is not a treatment we have on the shelf. We order it from Denver I believe. It is weight based, and it takes 5 days to get.
Clinical Trials
Mark Klein. That leads us into clinical trials. What is the role for precision oncology in prostate cancer right now, specifically looking at particular panels? One would be the DNA repair enzyme-based genes and/or also the AR variants and any other markers.
Elizabeth Hansen. The National Comprehensive Cancer Network came out with a statement recommending germ-line and somatic-mutation testing in all patients with metastatic prostate cancer. This highlights the need to offer patients the availability of clinical trials.
Julie Graff. I agree. We occasionally get to a place in the disease where patients are feeling fine, but we don’t have anything else to offer. The studies by Robinson16 and then Matteo17 showed that (a) these DNA repair defects are present in about a quarter of patients; and (b) that PARP inhibitors can help these patients. At least it has an anticancer effect.
What’s interesting is that we have TRITON2, and TRITON3, which are sponsored by Clovis,for patients with BRCA 1/2 and ATM mutations and using the PARP-inhibitor rucaparib. Based on the data we have available, we thought a quarter of patients would have the mutation in the tumor, but they’re finding that it is more like 10% to 15%. They are screening many patients but not finding it.
I agree that clinical trials are the way to go. I am hopeful that we’ll get more treatments based on molecular markers. The approval for pembrolizumab in any tumor type with microsatellite instability is interesting, but in prostate cancer, I believe that’s about 3%. I haven’t seen anyone qualify for pembrolizumab based on that. Another plug for clinical trials: Let’s learn more and offer our patients potentially beneficial treatments earlier.
Mark Klein. The first interim analysis from the TRITON2 study found about 12% of patients had alterations in BRCA 1/2. But in those that met the RECIST criteria, they were able to have evaluable disease via that standard with about a 44% response rate so far and a 51% PSA response rate. It is promising data, but it’s only 85 patients so far. We’ll know more because the TRITON2 study is of a more pretreated population than the TRITION3 study at this point. Are there any data on precision medicine and radiation in prostate cancer?
Abhishek Solanki. In the prostate cancer setting, there are not a lot of emerging data specifically looking at using precision oncology biomarkers to help guide decisions in radiation therapy. For example, genomic classifiers, like GenomeDx Decipher (Vancouver, BC) and Myriad Genetics Prolaris (Salt Lake City, UT) are increasingly being utilized in patients with localized disease. Decipher can help predict the risk of recurrence after radical prostatectomy. The difficulty is that there are limited data that show that by using these genomic classifiers, one can improve outcomes in patients over traditional clinical characteristics.
There are 2 trials currently ongoing through NRG Oncology that are using Decipher. The GU002 is a trial for patients who had a radical prostatectomy and had a postoperative PSA that never nadired below 0.2. These patients are randomized between salvage radiation with hormone therapy with or without docetaxel. This trial is collecting Decipher results for patients enrolled in the study. The GU006 is a trial for a slightly more favorable group of patients who do nadir but still have biochemical recurrence and relatively low PSAs. This trial randomizes between radiotherapy alone and radiotherapy and 6 months of apalutamide, stratifying patients based on Decipher results, specially differentiating between patients who have a luminal vs basal subtype of prostate cancer. There are data that suggest that patients who have a luminal subtype may benefit more from the combination of radiation and hormone therapy vs patients who have basal subtype.18 However this hasn’t been validated in a prospective setting, and that’s what this trial will hopefully do.
Immunotherapies
Mark Klein. Outside of prostate cancer, there has been a lot of research trying to determine how to improve PD-L1 expression. Where are immunotherapy trials moving? How radiation might play a role in conjunction with immunotherapy.
Julie Graff. Two phase 3 studies did not show statistically improved survival or statistically significant survival improvement on ipilimumab, an immunotherapy agent that targets CTLA4. Some early studies of the PD-1 drugs nivolumab and pembrolizumab did not show much response with monotherapy. Despite the negative phase 3 studies for ipilimumab, we periodically see exceptional responses.
In prostate cancer, enzalutamide is FDA approved. And there’s currently a phase 3 study of the PD-L1 inhibitor atezolizumab plus enzalutamide in patients who have progressed on abiraterone. That trial is fully accrued, bu
I just received a Prostate Cancer Foundation Challenge Award to open a VA-only study looking at fecal microbiota transplant from responders to nonresponders to see how manipulating host factors can increase potential responses to PD-1 inhibition.
Abhishek Solanki. The classic mechanism by which radiation therapy works is direct DNA damage and indirect DNA damage through hydroxyl radicals that leads to cytotoxicity. But preclinical and clinical data suggest that radiation therapy can augment the local and systemic immunotherapy response. The radiation oncologist’s dream is what is called the abscopal effect, which is the idea that when you treat one site of disease with radiation, it can induce a response at other sites that didn’t get radiation therapy through reactivation of the immune system. I like to think of the abscopal effect like bigfoot—it’s elusive. However, it seems that the setting it is most likely to happen in is in combination with immunotherapy.
One of the ways that radiation fails locally is that it can upregulate PD-1 expression, and as a result, you can have progression of the tumor because of local immune suppression. We know that T cells are important for the activity of radiation therapy. If you combine checkpoint inhibition with radiation therapy, you can not only have better local control in the area of the tumor, but perhaps you can release tumor antigens that will then induce a systemic response.
The other potential mechanism by which radiation may work synergistically with immunotherapy is as a debulking agent. There are some data that suggest that the ratio of T-cell reinvigoration to bulk of disease, or the volume of tumor burden, is important. That is, having T-cell reinvigoration may not be sufficient to have a response to immunotherapy in patients with a large burden of disease. By using radiation to debulk disease, perhaps you could help make checkpoint inhibition more effective. Ultimately, in the setting of prostate cancer, there are not a lot of data yet showing meaningful benefits with the combination of immunotherapy and radiotherapy, but there are trials that are ongoing that will educate on potential synergy.
Pharmacy
Julie Graff. Before we end I want to make sure that we applaud the amazing pharmacists and patient care navigation teams in the VA who do such a great job of getting veterans the appropriate treatment expeditiously and keeping them safe. It’s something that is truly unique to the VA. And I want to thank the people on this call who do this every day.
Elizabeth Hansen. Thank you Julie. Compared with working in the community, at the VA I’m honestly amazed by the ease of access to these medications for our patients. Being able to deliver medications sometimes the same day to the patient is just not something that happens in the community. It’s nice to see that our veterans are getting cared for in that manner.
Author disclosures
Dr. Solanki participated in advisory boards for Blue Earth Diagnostics’ fluciclovine PET and was previously paid as a consultant. Dr. Graff is a consultant for Sanofi (docetaxel) and Astellas (enzalutamide), and has received research funding (no personal funding)from Sanofi, Merck (pembrolizumab), Astellas, and Jannsen (abiraterone, apalutamide). The other authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. James ND, de Bono JS, Spears MR, et al; STAMPEDE Investigators. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377(4):338-351.
2. James ND, Sydes MR, Clarke NW, et al; STAMPEDE Investigators. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2017;387(10024):1163-1177.
3. Fizazi K, Tran N, Fein L, et al; LATITUDE Investigators. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352-360.
4. Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized Phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36(11):1080-1087.
5. Tosoian JJ, Gorin MA, Ross AE, Pienta KJ, Tran PT, Schaeffer EM. Oligometastatic prostate cancer: definitions, clinical outcomes, and treatment considerations. Nat Rev Urol. 2017;14(1):15-25.
6. Parker CC, James ND, Brawley CD, et al; Systemic Therapy for Advanced or Metastatic Prostate cancer: Evaluation of Drug Efficacy (STAMPEDE) investigators. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353-2366.
7. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737-746.
8. Feyerabend S, Saad F, Li T, et al. Survival benefit, disease progression and quality-of-life outcomes of abiraterone acetate plus prednisone versus docetaxel in metastatic hormone-sensitive prostate cancer: a network meta-analysis. Eur J Cancer. 2018;103:78-87.
9. Sydes MR, Spears MR, Mason MD, et al; STAMPEDE Investigators. Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. Ann Oncol. 2018;29(5):1235-1248.
10. Smith MR, Saad F, Chowdhury S, et al; SPARTAN Investigators. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408-1418.
11. Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378(26):2465-2474.
12. Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23(13):2918-2925.
13. Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446-453.
14. Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513-1520.
15. Tannock IF, de Wit R, Berry WR, et al; TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502-1512.
16. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215-1228.
17. Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697-1708.
18. Zhao SG, Chang SL, Erho N, et al. Associations of luminal and basal subtyping of prostate cancer with prognosis and response to androgen deprivation therapy. JAMA Oncol. 2017;3(12):1663-1672.
Sequencing Therapies
Mark Klein, MD. The last few years, there have been several new trials in prostate cancer for people in a metastatic setting or more advanced local setting, such as the STAMPEDE, LATITUDE, and CHAARTED trials.1-4 In addition, recently a few trials have examined apalutamide and enzalutamide for people who have had PSA (prostate-specific antigen) levels rapidly rising within about 10 months or so. One of the questions that arises is, how do we wrap our heads around sequencing these therapies. Is there a sequence that we should be doing and thinking about upfront and how do the different trials compare?
Julie Graff, MD. It just got more complicated. There was news today (December 20, 2018) that using enzalutamide early on in newly diagnosed metastatic prostate cancer may have positive results. It is not yet approved by the US Food and Drug Administration (FDA), but for patients who present with metastatic prostate cancer, we may have 4 potential treatments. We could have androgen deprivation therapy (ADT) alone, ADT plus docetaxel, enzalutamide, or abiraterone.
When I see patients in this situation, I talk to them about their options, the pros and cons of each option, and try to cover all the trials that look at these combinations. It can be quite a long visit. I talk to the patient about who benefits most, whether it is patients with high-risk factors or high-volume cancers. Also, I talk with the patient about all the adverse effects (AEs), and I look at my patients’ comorbid conditions and come up with a plan.
I encourage any patient who has high-volume or high-risk disease to consider more than just ADT alone. For many patients, I have been using abiraterone plus ADT. I have a wonderful pharmacist. As a medical oncologist, I can’t do it on my own. I need someone to follow patients’ laboratory results and to be available for medication questions and complications.
Elizabeth Hansen, PharmD. With the increasing number of patients on oral antineoplastics, monitoring patients in the outpatient setting has become an increasing priority and one of my major roles as a pharmacist in the clinic at the Chalmers P. Wylie VA Ambulatory Care Center in Columbus, Ohio. This is especially important as some of these treatments require frequent laboratory monitoring, such as abiraterone with liver function tests every 2 weeks for the first 3 months of treatment and monthly thereafter. Without frequent-follow up it’s easy for these patients to get lost in the shuffle.
Abhishek Solanki, MD. You could argue that a fifth option is prostate-directed radiation for patients who have limited metastases based on the STAMPEDE trial, which we’ve started integrating into our practice at the Edward Hines, Jr. Veterans Affairs Hospital in Chicago, Illinois.4
Mark Klein. Do you have a feel for the data and using radiation in oligometastatic (≤ 5 metastatic tumors) disease in prostate cancer and how well that might work?
Abhishek Solanki. The best data we have are from the multi-arm, multistage STAMPEDE trial systemic therapies and local therapy in the setting of high-risk localized disease and metastatic disease.6 The most recent publication looked specifically at the population with newly diagnosed metastatic disease and compared standard ADT (and docetaxel in about 18% of the patients) with or without prostate-directed radiation therapy. There was no survival benefit with radiation in the overall population, but in the subgroup of patients with low metastatic burden, there was an 8% survival benefit at 3 years.
It’s difficult to know what to make of that information because, as we’ve discussed already, there are other systemic therapy options that are being used more and more upfront such as abiraterone. Can you see the same benefit of radiation in that setting? The flip side is that in this study, radiation just targeted the prostate; could survival be improved even more by targeting all sites of disease in patients with oligometastatic disease? These are still open questions in prostate cancer and there are clinical trials attempting to define the clinical benefit of radiation in the metastatic setting for patients with limited metastases.
Mark Klein. How do you select patients for radiation in this particular situation; How do you approach stratification when radiation is started upfront?
Abhishek Solanki. In the STAMPEDE trial, low metastatic burden was defined based on the definition in the CHAARTED trial, which was those patients who did not have ≥ 4 bone metastases with ≥ 1 outside the vertebral bodies or pelvis, and did not have visceral metastases.7 That’s tough, because this definition could be a patient with a solitary bone metastasis but also could include some patients who have involved nodes extending all the way up to the retroperitoneal nodes—that is a fairly heterogeneous population. What we have done at our institution is select patients who have 3 to 5 metastases, administer prostate radiation therapy, and add stereotactic body radiation therapy (SBRT) for the other sites of disease, invoking the oligometastasis approach.
We have been doing this more frequently in the last few months. Typically, we’ll do 3 to 5 fractions of SBRT to metastases. For the primary, if the patient chooses SBRT, we’ll take that approach. If the patient chooses a more standard fractionation, we’ll do 20 treatments, but from a logistic perspective, most patients would rather come in for 5 treatments than 20. We also typically would start these patients on systemic hormonal therapy.
Mark Klein. At that point, are they referred back to medical oncology for surveillance?
Abhishek Solanki. Yes, they are followed by medical oncology and radiation oncology, and typically would continue hormonal therapy.
Mark Klein. Julie, how have you thought about presenting the therapeutic options for those patients who would be either eligible for docetaxel with high-bulk disease or abiraterone? Do you find patients prefer one or the other?
Julie Graff. I try to be very open about all the possibilities, and I present both. I don’t just decide for the patient chemotherapy vs abiraterone, but after we talk about it, most of my patients do opt for the abiraterone. I had a patient referred from the community—we are seeing more and more of this because abiraterone is so expensive—whose ejection fraction was about 38%. I said to that patient, “we could do chemotherapy, but we shouldn’t do abiraterone.” But usually it’s not that clear-cut.
Elizabeth Hansen. There was also an update from the STAMPEDE trial published recently comparing upfront abiraterone and prednisone to docetaxel (18 weeks) in advanced or metastatic prostate cancer. Results from this trial indicated a nearly identical overall survival (OS) (hazard ratio [HR] = 1.16; 95% CI, 0.82-1.65; P = .40). However, the failure-free survival (HR = 0.51; 95% CI, 0.39-0.67; P < .001) and progression-free survival (PFS) (HR= 0.65; 95% CI, 0.0.48-0.88; P = .005) favored abiraterone.8,9 The authors argue that while there was no change in OS, this trial demonstrates an important difference in the pattern of treatment failure.
Julie, do you think there will be any change in the treatment paradigm between docetaxel and abiraterone with this new update?
Julie Graff. I wasn’t that impressed by that study. I do not see it as practice changing, and it makes sense to me that the PFS is different in the 2 arms because we give chemotherapy and take a break vs giving abiraterone indefinitely. For me, there’s not really a shift.
Patients With Rising PSAs
Mark Klein. Let’s discuss the data from the recent studies on enzalutamide and apalutamide for the patients with fast-rising PSAs. In your discussions with other prostate researchers, will this become a standard part of practice or not?
Julie Graff. I was one of the authors on the SPARTAN apalutamide study.10 For a long time, we have had patients without metastatic disease but with a PSA relapse after surgery or radiation; and the PSA levels climb when the cancer becomes resistant to ADT. We haven’t had many options in that setting except to use bicalutamide and some older androgen receptor (AR) antagonists. We used to use estrogen and ketoconazole as well.
But now 2 studies have come out looking at a primary endpoint of metastases-free survival. Patients whose PSA was doubling every 10 months or shorter were randomized to either apalutamide (SPARTAN10) or enzalutamide (PROSPER11), both second-generation AR antagonists. There was a placebo control arm in each of the studies. Both studies found that adding the second-generation AR targeting agent delayed the time to metastatic disease by about 2 years. There is not any signal yet for statistically significant OS benefit, so it is not entirely clear if you could wait for the first metastasis to develop and then give 1 of these treatments and have the same OS benefit.
At the VA Portland Health Care System (VAPORHCS), it took a while to make these drugs available. My fellows were excited to give these drugs right away, but I often counsel patients that we don’t know if the second-generation AR targeting agents will improve survival. They almost certainly will bring down PSAs, which helps with peace of mind, but anything we add to the ADT can cause more AEs.
I have been cautious with second-generation AR antagonists because patients, when they take one of these drugs, are going to be on it for a long time. The FDA has approved those 2 drugs regardless of PSA doubling time, but I would not give it for a PSA doubling time > 10 months. In my practice about a quarter of patients who would qualify for apalutamide or enzalutamide are actually taking one, and the others are monitored closely with computed tomography (CT) and bone scans. When the disease becomes metastatic, then we start those drugs.
Mark Klein. Why 10 months, why not 6 months, a year, or 18 months? Is there reasoning behind that?
Julie Graff. There was a publication by Matthew Smith showing that the PSA doubling time was predictive of the development of metastatic disease and cancer death or prostate cancer death, and that 10 months seemed to be the cutoff between when the prostate cancer was going to become deadly vs not.12 If you actually look at the trial data, I think the PSA doubling time was between 3 and 4 months for the participants, so pretty short.
Adverse Effects
Mark Klein. What are the AEs people are seeing from using apalutamide, enzalutamide, and abiraterone? What are they seeing in their practice vs what is in the studies? When I have had to stop people on abiraterone or drop down the dose, almost always it has been for fatigue. We check liver function tests (LFTs) repeatedly, but I can’t remember ever having to drop down the dose or take it away even for that reason.
Elizabeth Hansen.
Mark Klein. At the Minneapolis VA Health Care System (MVAHCS) when apalutamide first came out, for the PSA rapid doubling, there had already been an abstract presenting the enzalutamide data. We have chosen to recommend enzalutamide as our choice for the people with PSA doubling based on the cost. It’s significantly cheaper for the VA. Between the 2 papers there is very little difference in the efficacy data. I’m wondering what other sites have done with regard to that specific point at their VAs?
Elizabeth Hansen. In Columbus, we prefer to use either abiraterone and enzalutamide because they’re essentially cost neutral. However, this may change with generic abiraterone coming to market. Apalutamide is really cost prohibitive currently.
Julie Graff. I agree.
Patient Education
Mark Klein. At MVAHCS, the navigators handle a lot of upfront education. We have 3 navigators, including Kathleen Nelson who is on this roundtable. She works with patients and provides much of the patient education. How have you handled education for patients?
Kathleen Nelson. For the most part, our pharmacists do the drug-specific education for the oral agents, and the nurse navigators provide more generic education. We did a trial for patients on IV therapies. We learned that patients really don’t report in much detail, but if you call and ask them specific questions, then you can tease out some more detail.
Elizabeth Hansen. It is interesting that every site is different. One of my main roles is oral antineoplastic monitoring, which includes many patients on enzalutamide or abiraterone. At least initially with these patients, I try to follow them closely—abiraterone more so than enzalutamide. I typically call every 2 to 4 weeks, in between clinic visits, to follow up the laboratory tests and manage the AEs. I always try to ask direct and open-ended questions: How often are you checking your blood pressure? What is your current weight? How has your energy level changed since therapy initiation?
The VA telehealth system is amazing. For patients who need to monitor blood pressure regularly, it’s really nice for them to have those numbers come directly back to me in CPRS (Computerized Patient Record System). That has worked wonders for some of our patients to get them through therapy.
Mark Klein. What do you tend to use when the prostate cancer is progressing for a patient? And how do you determine that progression? Some studies will use PSA rise only as a marker for progression. Other studies have not used PSA rise as the only marker for progression and oftentimes require some sort of bone scan criteria or CT imaging criteria for progression.
Julie Graff. We have a limited number of treatment options. Providers typically use enzalutamide or abiraterone as there is a high degree of resistance between the 2. Then there is chemotherapy and then radium, which quite a few people don’t qualify for. We need to be very thoughtful when we change treatments. I look at the 3 factors of biochemical progression or response—PSA, radiographic progression, and clinical progression. If I don’t see 2 out of 3, I typically don’t change treatments. Then after enzalutamide or abiraterone, I wait until there are cancer-related symptoms before I consider chemotherapy and closely monitor my patients.
Imaging Modalities
Abhishek Solanki. Over the last few years the Hines VA Hospital has used fluciclovine positron emission tomography (PET), which is one of the novel imaging modalities for prostate cancer. Really the 2 novel imaging modalities that have gained the most excitement are prostate-specific membrane antigen (PSMA) PET and fluciclovine PET. Fluciclovine PET is based on a synthetic amino acid that’s taken up in multiple tissues, including prostate cancer. It has changed our practice in the localized setting for patients who have developed recurrence after radiation or radical prostatectomy. We have incorporated the scan into our workup of patients with recurrent disease, which can give us some more information at lower PSAs than historically we could get with CT, bone scan, or magnetic resonance imaging.
Our medical oncologists have started using it more and more as well. We are getting a lot of patients who have a negative CT or bone scan but have a positive fluciclovine PET. There are a few different disease settings where that becomes relevant. In patients who develop biochemical recurrence after radiation or salvage radiation after radical, we are finding that a lot of these patients who have no CT or bone scan findings of disease ultimately are found to have a PET-positive lesion. Sometimes it’s difficult to know how best to help patients with PET-only disease. Should you target the disease with an oligometastasis approach or just pursue systemic therapy or surveillance? It is challenging but more and more we are moving toward metastasis-directed therapy. There are multiple randomized trials in progress testing whether metastasis-directed therapy to the PET areas of recurrence can improve outcomes or delay systemic ADT. The STOMP trial randomized surveillance vs SBRT or surgery for patients with oligometastatic disease that showed improvement in biochemical control and ADT-free survival.13 However this was a small trial that tried to identify a signal. More definitive trials are necessary.
The other setting where we have found novel PET imaging to be helpful is in patients who have become castration resistant but don’t have clear metastases on conventional imaging. We’re identifying more patients who have only a few sites of progression, and we’ll pursue metastasis-directed therapy to those areas to try to get more mileage out of the systemic therapy that the patient is currently on and to try to avoid having to switch to the next line with the idea that, potentially, the progression site is just a limited clone that is progressing despite the current systemic therapy.
Mark Klein. I find that to be a very attractive approach. I’m assuming you do that for any systemic therapy where people have maybe 1 or 2 sites and they do not have a big PSA jump. Do you have a number of sites that you’re willing to radiate? And then, when you do that, what radiation fractionation and dosing do you use? Is there any observational data behind that for efficacy?
Abhishek Solanki. It is a patient by patient decision. Some patients, if they have a very rapid pace of progression shortly after starting systemic therapy and metastases have grown in several areas, we think that perhaps this person may benefit less from aggressive local therapy. But if it’s somebody who has been on systemic therapy for a while and has up to 3 sites of disease growth, we consider SBRT for oligoprogressive disease. Typically, we’ll use SBRT, which delivers a high dose of radiation over 3 to 5 treatments. With SBRT you can give a higher biologic dose and use more sophisticated treatment machines and image guidance for treatments to focus the radiation on the tumor area and limit exposure to normal tissue structures.
In prostate cancer to the primary site, we will typically do around 35 to 40 Gy in 5 fractions. For metastases, it depends on the site. If it’s in the lung, typically we will do 3 to 5 treatments, giving approximately 50 to 60 Gy in that course. In the spine, we use lower doses near the spinal cord and the cauda equina, typically about 30 Gy in 3 fractions. In the liver, similar to the lung, we’ll typically do 50-54 Gy in 3-5 fractions. There aren’t a lot of high-level data guiding the optimal dose/fractionation to metastases, but these are the doses we’ll use for various malignancies.
Treatment Options for Patients With Adverse Events
Mark Klein. I was just reviewing the 2004 study that randomized patients to mitoxantrone or docetaxel for up to 10 cycles.14,15 Who are good candidates for docetaxel after they have exhausted abiraterone and enzalutamide? How long do you hold to the 10-cycle rule, or do you go beyond that if they’re doing well? And if they’re not a good candidate, what are some options?
Julie Graff. The best candidates are those who are having a cancer-related AE, particularly pain, because docetaxel only improves survival over mitoxantrone by about 2.5 months. I don’t talk to patients about it as though it is a life extender, but it seems to help control pain—about 70% of patients benefited in terms of pain or some other cancer-related symptom.14
I have a lot of patients who say, “Never will I do chemotherapy.” I refer those patients to hospice, or if they’re appropriate for radium-223, I consider that. I typically give about 6 cycles of chemotherapy and then see how they’re doing. In some patients, the cancer just doesn’t respond to it.
I do tell patients about the papers that you mentioned, the 2 studies of docetaxel vs mitoxantrone where they use about 10 cycles, and some of my patients go all 10.14,15 Sometimes we have to stop because of neuropathy or some other AE. I believe in taking breaks and that you can probably start it later.
Elizabeth Hansen. I agree, our practice is similar. A lot of our patients are not very interested in chemotherapy. You have to take into consideration their ECOG (Eastern Cooperative Oncology Group) status, their goals, and quality of life when talking to them about these medications. And a lot of them tend to choose more of a palliative route. Depending on their AEs and how things are going, we will dose reduce, hold treatment, or give treatment holidays.
Mark Klein. If patients are progressing on docetaxel, what are options that people would use? Radium-223 certainly is available for patients with nonvisceral metastases, as well as cabazitaxel, mitoxantrone, estramustine and other older drugs.
Julie Graff. We have some clinical trials for patients postdocetaxel. We have the TRITON2 and TRITON3 studies open at the VA. (NCT02952534 and NCT02975934, respectively) A lot of patients would get a biopsy, and we’d look for a BRCA 1 or 2 and ATM mutation. For those patients who don’t have those mutations—and maybe 80% of them don’t—we talk about radium-223 for the patients without visceral metastases and bone pain. I have had a fair number of patients go on cabazitaxel, but I have not used mitoxantrone since cabazitaxel came out. It’s not off the table, but it hasn’t shown improvement in survival.
Elizabeth Hansen. One of our challenges, because we’re an ambulatory care center, is that we are unable to give radium-223 in house, and these services have to be sent out to a non-VA facility. It is doable, but it takes more legwork and organization on our part.
Julie Graff. We have not had radium-223, although we’re working to get that online. And we are physically connected to Oregon Health Science University (OHSU), so we send our patients there for radium. It is a pain because the doctors at OHSU don’t have CPRS access. I’m often in the middle of making sure the complete blood counts (CBCs) are sent to OHSU and to get my patients their treatments.
Mark Klein. The Minneapolis VAMC has radium-223 on site, and we have used it for patients whose cancer has progressed while on docetaxel without visceral metastases. Katie, have you had an opportunity to coordinate that care for patients?
Kathleen Nelson. Radium is administered at our facility by one of our nuclear medicine physicians. A complete blood count is checked at least 3 days prior to the infusion date but no sooner than 6 days. Due to the cost of the material, ordering without knowing the patient’s counts are within a safe range to administer is prohibitive. This adds an additional burden of 2 visits (lab with return visit) to the patient. We have treated 12 patients. Four patients stopped treatment prior to completing the 6 planned treatments citing debilitating fatigue and/or nonresolution of symptoms as their reason to stop treatment. One patient died. The 7 remaining patients subjectively reported varying degrees of pain relief.
Elizabeth Hansen. Another thing to mention is the lack of a PSA response from radium-223 as well. Patients are generally very diligent about monitoring their PSA, so this can be a bit distressing.
Mark Klein. Julie, have you noticed a PSA flare with radium-223? I know it has been reported.
Julie Graff. I haven’t. But I put little stock in PSAs in these patients. I spend 20 minutes explaining to patients that the PSA is not helpful in determining whether or not the radium is working. I tell them that the bone marker alkaline phosphatase may decrease. And I think it’s important to note, too, that radium-223 is not a treatment we have on the shelf. We order it from Denver I believe. It is weight based, and it takes 5 days to get.
Clinical Trials
Mark Klein. That leads us into clinical trials. What is the role for precision oncology in prostate cancer right now, specifically looking at particular panels? One would be the DNA repair enzyme-based genes and/or also the AR variants and any other markers.
Elizabeth Hansen. The National Comprehensive Cancer Network came out with a statement recommending germ-line and somatic-mutation testing in all patients with metastatic prostate cancer. This highlights the need to offer patients the availability of clinical trials.
Julie Graff. I agree. We occasionally get to a place in the disease where patients are feeling fine, but we don’t have anything else to offer. The studies by Robinson16 and then Matteo17 showed that (a) these DNA repair defects are present in about a quarter of patients; and (b) that PARP inhibitors can help these patients. At least it has an anticancer effect.
What’s interesting is that we have TRITON2, and TRITON3, which are sponsored by Clovis,for patients with BRCA 1/2 and ATM mutations and using the PARP-inhibitor rucaparib. Based on the data we have available, we thought a quarter of patients would have the mutation in the tumor, but they’re finding that it is more like 10% to 15%. They are screening many patients but not finding it.
I agree that clinical trials are the way to go. I am hopeful that we’ll get more treatments based on molecular markers. The approval for pembrolizumab in any tumor type with microsatellite instability is interesting, but in prostate cancer, I believe that’s about 3%. I haven’t seen anyone qualify for pembrolizumab based on that. Another plug for clinical trials: Let’s learn more and offer our patients potentially beneficial treatments earlier.
Mark Klein. The first interim analysis from the TRITON2 study found about 12% of patients had alterations in BRCA 1/2. But in those that met the RECIST criteria, they were able to have evaluable disease via that standard with about a 44% response rate so far and a 51% PSA response rate. It is promising data, but it’s only 85 patients so far. We’ll know more because the TRITON2 study is of a more pretreated population than the TRITION3 study at this point. Are there any data on precision medicine and radiation in prostate cancer?
Abhishek Solanki. In the prostate cancer setting, there are not a lot of emerging data specifically looking at using precision oncology biomarkers to help guide decisions in radiation therapy. For example, genomic classifiers, like GenomeDx Decipher (Vancouver, BC) and Myriad Genetics Prolaris (Salt Lake City, UT) are increasingly being utilized in patients with localized disease. Decipher can help predict the risk of recurrence after radical prostatectomy. The difficulty is that there are limited data that show that by using these genomic classifiers, one can improve outcomes in patients over traditional clinical characteristics.
There are 2 trials currently ongoing through NRG Oncology that are using Decipher. The GU002 is a trial for patients who had a radical prostatectomy and had a postoperative PSA that never nadired below 0.2. These patients are randomized between salvage radiation with hormone therapy with or without docetaxel. This trial is collecting Decipher results for patients enrolled in the study. The GU006 is a trial for a slightly more favorable group of patients who do nadir but still have biochemical recurrence and relatively low PSAs. This trial randomizes between radiotherapy alone and radiotherapy and 6 months of apalutamide, stratifying patients based on Decipher results, specially differentiating between patients who have a luminal vs basal subtype of prostate cancer. There are data that suggest that patients who have a luminal subtype may benefit more from the combination of radiation and hormone therapy vs patients who have basal subtype.18 However this hasn’t been validated in a prospective setting, and that’s what this trial will hopefully do.
Immunotherapies
Mark Klein. Outside of prostate cancer, there has been a lot of research trying to determine how to improve PD-L1 expression. Where are immunotherapy trials moving? How radiation might play a role in conjunction with immunotherapy.
Julie Graff. Two phase 3 studies did not show statistically improved survival or statistically significant survival improvement on ipilimumab, an immunotherapy agent that targets CTLA4. Some early studies of the PD-1 drugs nivolumab and pembrolizumab did not show much response with monotherapy. Despite the negative phase 3 studies for ipilimumab, we periodically see exceptional responses.
In prostate cancer, enzalutamide is FDA approved. And there’s currently a phase 3 study of the PD-L1 inhibitor atezolizumab plus enzalutamide in patients who have progressed on abiraterone. That trial is fully accrued, bu
I just received a Prostate Cancer Foundation Challenge Award to open a VA-only study looking at fecal microbiota transplant from responders to nonresponders to see how manipulating host factors can increase potential responses to PD-1 inhibition.
Abhishek Solanki. The classic mechanism by which radiation therapy works is direct DNA damage and indirect DNA damage through hydroxyl radicals that leads to cytotoxicity. But preclinical and clinical data suggest that radiation therapy can augment the local and systemic immunotherapy response. The radiation oncologist’s dream is what is called the abscopal effect, which is the idea that when you treat one site of disease with radiation, it can induce a response at other sites that didn’t get radiation therapy through reactivation of the immune system. I like to think of the abscopal effect like bigfoot—it’s elusive. However, it seems that the setting it is most likely to happen in is in combination with immunotherapy.
One of the ways that radiation fails locally is that it can upregulate PD-1 expression, and as a result, you can have progression of the tumor because of local immune suppression. We know that T cells are important for the activity of radiation therapy. If you combine checkpoint inhibition with radiation therapy, you can not only have better local control in the area of the tumor, but perhaps you can release tumor antigens that will then induce a systemic response.
The other potential mechanism by which radiation may work synergistically with immunotherapy is as a debulking agent. There are some data that suggest that the ratio of T-cell reinvigoration to bulk of disease, or the volume of tumor burden, is important. That is, having T-cell reinvigoration may not be sufficient to have a response to immunotherapy in patients with a large burden of disease. By using radiation to debulk disease, perhaps you could help make checkpoint inhibition more effective. Ultimately, in the setting of prostate cancer, there are not a lot of data yet showing meaningful benefits with the combination of immunotherapy and radiotherapy, but there are trials that are ongoing that will educate on potential synergy.
Pharmacy
Julie Graff. Before we end I want to make sure that we applaud the amazing pharmacists and patient care navigation teams in the VA who do such a great job of getting veterans the appropriate treatment expeditiously and keeping them safe. It’s something that is truly unique to the VA. And I want to thank the people on this call who do this every day.
Elizabeth Hansen. Thank you Julie. Compared with working in the community, at the VA I’m honestly amazed by the ease of access to these medications for our patients. Being able to deliver medications sometimes the same day to the patient is just not something that happens in the community. It’s nice to see that our veterans are getting cared for in that manner.
Author disclosures
Dr. Solanki participated in advisory boards for Blue Earth Diagnostics’ fluciclovine PET and was previously paid as a consultant. Dr. Graff is a consultant for Sanofi (docetaxel) and Astellas (enzalutamide), and has received research funding (no personal funding)from Sanofi, Merck (pembrolizumab), Astellas, and Jannsen (abiraterone, apalutamide). The other authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Sequencing Therapies
Mark Klein, MD. The last few years, there have been several new trials in prostate cancer for people in a metastatic setting or more advanced local setting, such as the STAMPEDE, LATITUDE, and CHAARTED trials.1-4 In addition, recently a few trials have examined apalutamide and enzalutamide for people who have had PSA (prostate-specific antigen) levels rapidly rising within about 10 months or so. One of the questions that arises is, how do we wrap our heads around sequencing these therapies. Is there a sequence that we should be doing and thinking about upfront and how do the different trials compare?
Julie Graff, MD. It just got more complicated. There was news today (December 20, 2018) that using enzalutamide early on in newly diagnosed metastatic prostate cancer may have positive results. It is not yet approved by the US Food and Drug Administration (FDA), but for patients who present with metastatic prostate cancer, we may have 4 potential treatments. We could have androgen deprivation therapy (ADT) alone, ADT plus docetaxel, enzalutamide, or abiraterone.
When I see patients in this situation, I talk to them about their options, the pros and cons of each option, and try to cover all the trials that look at these combinations. It can be quite a long visit. I talk to the patient about who benefits most, whether it is patients with high-risk factors or high-volume cancers. Also, I talk with the patient about all the adverse effects (AEs), and I look at my patients’ comorbid conditions and come up with a plan.
I encourage any patient who has high-volume or high-risk disease to consider more than just ADT alone. For many patients, I have been using abiraterone plus ADT. I have a wonderful pharmacist. As a medical oncologist, I can’t do it on my own. I need someone to follow patients’ laboratory results and to be available for medication questions and complications.
Elizabeth Hansen, PharmD. With the increasing number of patients on oral antineoplastics, monitoring patients in the outpatient setting has become an increasing priority and one of my major roles as a pharmacist in the clinic at the Chalmers P. Wylie VA Ambulatory Care Center in Columbus, Ohio. This is especially important as some of these treatments require frequent laboratory monitoring, such as abiraterone with liver function tests every 2 weeks for the first 3 months of treatment and monthly thereafter. Without frequent-follow up it’s easy for these patients to get lost in the shuffle.
Abhishek Solanki, MD. You could argue that a fifth option is prostate-directed radiation for patients who have limited metastases based on the STAMPEDE trial, which we’ve started integrating into our practice at the Edward Hines, Jr. Veterans Affairs Hospital in Chicago, Illinois.4
Mark Klein. Do you have a feel for the data and using radiation in oligometastatic (≤ 5 metastatic tumors) disease in prostate cancer and how well that might work?
Abhishek Solanki. The best data we have are from the multi-arm, multistage STAMPEDE trial systemic therapies and local therapy in the setting of high-risk localized disease and metastatic disease.6 The most recent publication looked specifically at the population with newly diagnosed metastatic disease and compared standard ADT (and docetaxel in about 18% of the patients) with or without prostate-directed radiation therapy. There was no survival benefit with radiation in the overall population, but in the subgroup of patients with low metastatic burden, there was an 8% survival benefit at 3 years.
It’s difficult to know what to make of that information because, as we’ve discussed already, there are other systemic therapy options that are being used more and more upfront such as abiraterone. Can you see the same benefit of radiation in that setting? The flip side is that in this study, radiation just targeted the prostate; could survival be improved even more by targeting all sites of disease in patients with oligometastatic disease? These are still open questions in prostate cancer and there are clinical trials attempting to define the clinical benefit of radiation in the metastatic setting for patients with limited metastases.
Mark Klein. How do you select patients for radiation in this particular situation; How do you approach stratification when radiation is started upfront?
Abhishek Solanki. In the STAMPEDE trial, low metastatic burden was defined based on the definition in the CHAARTED trial, which was those patients who did not have ≥ 4 bone metastases with ≥ 1 outside the vertebral bodies or pelvis, and did not have visceral metastases.7 That’s tough, because this definition could be a patient with a solitary bone metastasis but also could include some patients who have involved nodes extending all the way up to the retroperitoneal nodes—that is a fairly heterogeneous population. What we have done at our institution is select patients who have 3 to 5 metastases, administer prostate radiation therapy, and add stereotactic body radiation therapy (SBRT) for the other sites of disease, invoking the oligometastasis approach.
We have been doing this more frequently in the last few months. Typically, we’ll do 3 to 5 fractions of SBRT to metastases. For the primary, if the patient chooses SBRT, we’ll take that approach. If the patient chooses a more standard fractionation, we’ll do 20 treatments, but from a logistic perspective, most patients would rather come in for 5 treatments than 20. We also typically would start these patients on systemic hormonal therapy.
Mark Klein. At that point, are they referred back to medical oncology for surveillance?
Abhishek Solanki. Yes, they are followed by medical oncology and radiation oncology, and typically would continue hormonal therapy.
Mark Klein. Julie, how have you thought about presenting the therapeutic options for those patients who would be either eligible for docetaxel with high-bulk disease or abiraterone? Do you find patients prefer one or the other?
Julie Graff. I try to be very open about all the possibilities, and I present both. I don’t just decide for the patient chemotherapy vs abiraterone, but after we talk about it, most of my patients do opt for the abiraterone. I had a patient referred from the community—we are seeing more and more of this because abiraterone is so expensive—whose ejection fraction was about 38%. I said to that patient, “we could do chemotherapy, but we shouldn’t do abiraterone.” But usually it’s not that clear-cut.
Elizabeth Hansen. There was also an update from the STAMPEDE trial published recently comparing upfront abiraterone and prednisone to docetaxel (18 weeks) in advanced or metastatic prostate cancer. Results from this trial indicated a nearly identical overall survival (OS) (hazard ratio [HR] = 1.16; 95% CI, 0.82-1.65; P = .40). However, the failure-free survival (HR = 0.51; 95% CI, 0.39-0.67; P < .001) and progression-free survival (PFS) (HR= 0.65; 95% CI, 0.0.48-0.88; P = .005) favored abiraterone.8,9 The authors argue that while there was no change in OS, this trial demonstrates an important difference in the pattern of treatment failure.
Julie, do you think there will be any change in the treatment paradigm between docetaxel and abiraterone with this new update?
Julie Graff. I wasn’t that impressed by that study. I do not see it as practice changing, and it makes sense to me that the PFS is different in the 2 arms because we give chemotherapy and take a break vs giving abiraterone indefinitely. For me, there’s not really a shift.
Patients With Rising PSAs
Mark Klein. Let’s discuss the data from the recent studies on enzalutamide and apalutamide for the patients with fast-rising PSAs. In your discussions with other prostate researchers, will this become a standard part of practice or not?
Julie Graff. I was one of the authors on the SPARTAN apalutamide study.10 For a long time, we have had patients without metastatic disease but with a PSA relapse after surgery or radiation; and the PSA levels climb when the cancer becomes resistant to ADT. We haven’t had many options in that setting except to use bicalutamide and some older androgen receptor (AR) antagonists. We used to use estrogen and ketoconazole as well.
But now 2 studies have come out looking at a primary endpoint of metastases-free survival. Patients whose PSA was doubling every 10 months or shorter were randomized to either apalutamide (SPARTAN10) or enzalutamide (PROSPER11), both second-generation AR antagonists. There was a placebo control arm in each of the studies. Both studies found that adding the second-generation AR targeting agent delayed the time to metastatic disease by about 2 years. There is not any signal yet for statistically significant OS benefit, so it is not entirely clear if you could wait for the first metastasis to develop and then give 1 of these treatments and have the same OS benefit.
At the VA Portland Health Care System (VAPORHCS), it took a while to make these drugs available. My fellows were excited to give these drugs right away, but I often counsel patients that we don’t know if the second-generation AR targeting agents will improve survival. They almost certainly will bring down PSAs, which helps with peace of mind, but anything we add to the ADT can cause more AEs.
I have been cautious with second-generation AR antagonists because patients, when they take one of these drugs, are going to be on it for a long time. The FDA has approved those 2 drugs regardless of PSA doubling time, but I would not give it for a PSA doubling time > 10 months. In my practice about a quarter of patients who would qualify for apalutamide or enzalutamide are actually taking one, and the others are monitored closely with computed tomography (CT) and bone scans. When the disease becomes metastatic, then we start those drugs.
Mark Klein. Why 10 months, why not 6 months, a year, or 18 months? Is there reasoning behind that?
Julie Graff. There was a publication by Matthew Smith showing that the PSA doubling time was predictive of the development of metastatic disease and cancer death or prostate cancer death, and that 10 months seemed to be the cutoff between when the prostate cancer was going to become deadly vs not.12 If you actually look at the trial data, I think the PSA doubling time was between 3 and 4 months for the participants, so pretty short.
Adverse Effects
Mark Klein. What are the AEs people are seeing from using apalutamide, enzalutamide, and abiraterone? What are they seeing in their practice vs what is in the studies? When I have had to stop people on abiraterone or drop down the dose, almost always it has been for fatigue. We check liver function tests (LFTs) repeatedly, but I can’t remember ever having to drop down the dose or take it away even for that reason.
Elizabeth Hansen.
Mark Klein. At the Minneapolis VA Health Care System (MVAHCS) when apalutamide first came out, for the PSA rapid doubling, there had already been an abstract presenting the enzalutamide data. We have chosen to recommend enzalutamide as our choice for the people with PSA doubling based on the cost. It’s significantly cheaper for the VA. Between the 2 papers there is very little difference in the efficacy data. I’m wondering what other sites have done with regard to that specific point at their VAs?
Elizabeth Hansen. In Columbus, we prefer to use either abiraterone and enzalutamide because they’re essentially cost neutral. However, this may change with generic abiraterone coming to market. Apalutamide is really cost prohibitive currently.
Julie Graff. I agree.
Patient Education
Mark Klein. At MVAHCS, the navigators handle a lot of upfront education. We have 3 navigators, including Kathleen Nelson who is on this roundtable. She works with patients and provides much of the patient education. How have you handled education for patients?
Kathleen Nelson. For the most part, our pharmacists do the drug-specific education for the oral agents, and the nurse navigators provide more generic education. We did a trial for patients on IV therapies. We learned that patients really don’t report in much detail, but if you call and ask them specific questions, then you can tease out some more detail.
Elizabeth Hansen. It is interesting that every site is different. One of my main roles is oral antineoplastic monitoring, which includes many patients on enzalutamide or abiraterone. At least initially with these patients, I try to follow them closely—abiraterone more so than enzalutamide. I typically call every 2 to 4 weeks, in between clinic visits, to follow up the laboratory tests and manage the AEs. I always try to ask direct and open-ended questions: How often are you checking your blood pressure? What is your current weight? How has your energy level changed since therapy initiation?
The VA telehealth system is amazing. For patients who need to monitor blood pressure regularly, it’s really nice for them to have those numbers come directly back to me in CPRS (Computerized Patient Record System). That has worked wonders for some of our patients to get them through therapy.
Mark Klein. What do you tend to use when the prostate cancer is progressing for a patient? And how do you determine that progression? Some studies will use PSA rise only as a marker for progression. Other studies have not used PSA rise as the only marker for progression and oftentimes require some sort of bone scan criteria or CT imaging criteria for progression.
Julie Graff. We have a limited number of treatment options. Providers typically use enzalutamide or abiraterone as there is a high degree of resistance between the 2. Then there is chemotherapy and then radium, which quite a few people don’t qualify for. We need to be very thoughtful when we change treatments. I look at the 3 factors of biochemical progression or response—PSA, radiographic progression, and clinical progression. If I don’t see 2 out of 3, I typically don’t change treatments. Then after enzalutamide or abiraterone, I wait until there are cancer-related symptoms before I consider chemotherapy and closely monitor my patients.
Imaging Modalities
Abhishek Solanki. Over the last few years the Hines VA Hospital has used fluciclovine positron emission tomography (PET), which is one of the novel imaging modalities for prostate cancer. Really the 2 novel imaging modalities that have gained the most excitement are prostate-specific membrane antigen (PSMA) PET and fluciclovine PET. Fluciclovine PET is based on a synthetic amino acid that’s taken up in multiple tissues, including prostate cancer. It has changed our practice in the localized setting for patients who have developed recurrence after radiation or radical prostatectomy. We have incorporated the scan into our workup of patients with recurrent disease, which can give us some more information at lower PSAs than historically we could get with CT, bone scan, or magnetic resonance imaging.
Our medical oncologists have started using it more and more as well. We are getting a lot of patients who have a negative CT or bone scan but have a positive fluciclovine PET. There are a few different disease settings where that becomes relevant. In patients who develop biochemical recurrence after radiation or salvage radiation after radical, we are finding that a lot of these patients who have no CT or bone scan findings of disease ultimately are found to have a PET-positive lesion. Sometimes it’s difficult to know how best to help patients with PET-only disease. Should you target the disease with an oligometastasis approach or just pursue systemic therapy or surveillance? It is challenging but more and more we are moving toward metastasis-directed therapy. There are multiple randomized trials in progress testing whether metastasis-directed therapy to the PET areas of recurrence can improve outcomes or delay systemic ADT. The STOMP trial randomized surveillance vs SBRT or surgery for patients with oligometastatic disease that showed improvement in biochemical control and ADT-free survival.13 However this was a small trial that tried to identify a signal. More definitive trials are necessary.
The other setting where we have found novel PET imaging to be helpful is in patients who have become castration resistant but don’t have clear metastases on conventional imaging. We’re identifying more patients who have only a few sites of progression, and we’ll pursue metastasis-directed therapy to those areas to try to get more mileage out of the systemic therapy that the patient is currently on and to try to avoid having to switch to the next line with the idea that, potentially, the progression site is just a limited clone that is progressing despite the current systemic therapy.
Mark Klein. I find that to be a very attractive approach. I’m assuming you do that for any systemic therapy where people have maybe 1 or 2 sites and they do not have a big PSA jump. Do you have a number of sites that you’re willing to radiate? And then, when you do that, what radiation fractionation and dosing do you use? Is there any observational data behind that for efficacy?
Abhishek Solanki. It is a patient by patient decision. Some patients, if they have a very rapid pace of progression shortly after starting systemic therapy and metastases have grown in several areas, we think that perhaps this person may benefit less from aggressive local therapy. But if it’s somebody who has been on systemic therapy for a while and has up to 3 sites of disease growth, we consider SBRT for oligoprogressive disease. Typically, we’ll use SBRT, which delivers a high dose of radiation over 3 to 5 treatments. With SBRT you can give a higher biologic dose and use more sophisticated treatment machines and image guidance for treatments to focus the radiation on the tumor area and limit exposure to normal tissue structures.
In prostate cancer to the primary site, we will typically do around 35 to 40 Gy in 5 fractions. For metastases, it depends on the site. If it’s in the lung, typically we will do 3 to 5 treatments, giving approximately 50 to 60 Gy in that course. In the spine, we use lower doses near the spinal cord and the cauda equina, typically about 30 Gy in 3 fractions. In the liver, similar to the lung, we’ll typically do 50-54 Gy in 3-5 fractions. There aren’t a lot of high-level data guiding the optimal dose/fractionation to metastases, but these are the doses we’ll use for various malignancies.
Treatment Options for Patients With Adverse Events
Mark Klein. I was just reviewing the 2004 study that randomized patients to mitoxantrone or docetaxel for up to 10 cycles.14,15 Who are good candidates for docetaxel after they have exhausted abiraterone and enzalutamide? How long do you hold to the 10-cycle rule, or do you go beyond that if they’re doing well? And if they’re not a good candidate, what are some options?
Julie Graff. The best candidates are those who are having a cancer-related AE, particularly pain, because docetaxel only improves survival over mitoxantrone by about 2.5 months. I don’t talk to patients about it as though it is a life extender, but it seems to help control pain—about 70% of patients benefited in terms of pain or some other cancer-related symptom.14
I have a lot of patients who say, “Never will I do chemotherapy.” I refer those patients to hospice, or if they’re appropriate for radium-223, I consider that. I typically give about 6 cycles of chemotherapy and then see how they’re doing. In some patients, the cancer just doesn’t respond to it.
I do tell patients about the papers that you mentioned, the 2 studies of docetaxel vs mitoxantrone where they use about 10 cycles, and some of my patients go all 10.14,15 Sometimes we have to stop because of neuropathy or some other AE. I believe in taking breaks and that you can probably start it later.
Elizabeth Hansen. I agree, our practice is similar. A lot of our patients are not very interested in chemotherapy. You have to take into consideration their ECOG (Eastern Cooperative Oncology Group) status, their goals, and quality of life when talking to them about these medications. And a lot of them tend to choose more of a palliative route. Depending on their AEs and how things are going, we will dose reduce, hold treatment, or give treatment holidays.
Mark Klein. If patients are progressing on docetaxel, what are options that people would use? Radium-223 certainly is available for patients with nonvisceral metastases, as well as cabazitaxel, mitoxantrone, estramustine and other older drugs.
Julie Graff. We have some clinical trials for patients postdocetaxel. We have the TRITON2 and TRITON3 studies open at the VA. (NCT02952534 and NCT02975934, respectively) A lot of patients would get a biopsy, and we’d look for a BRCA 1 or 2 and ATM mutation. For those patients who don’t have those mutations—and maybe 80% of them don’t—we talk about radium-223 for the patients without visceral metastases and bone pain. I have had a fair number of patients go on cabazitaxel, but I have not used mitoxantrone since cabazitaxel came out. It’s not off the table, but it hasn’t shown improvement in survival.
Elizabeth Hansen. One of our challenges, because we’re an ambulatory care center, is that we are unable to give radium-223 in house, and these services have to be sent out to a non-VA facility. It is doable, but it takes more legwork and organization on our part.
Julie Graff. We have not had radium-223, although we’re working to get that online. And we are physically connected to Oregon Health Science University (OHSU), so we send our patients there for radium. It is a pain because the doctors at OHSU don’t have CPRS access. I’m often in the middle of making sure the complete blood counts (CBCs) are sent to OHSU and to get my patients their treatments.
Mark Klein. The Minneapolis VAMC has radium-223 on site, and we have used it for patients whose cancer has progressed while on docetaxel without visceral metastases. Katie, have you had an opportunity to coordinate that care for patients?
Kathleen Nelson. Radium is administered at our facility by one of our nuclear medicine physicians. A complete blood count is checked at least 3 days prior to the infusion date but no sooner than 6 days. Due to the cost of the material, ordering without knowing the patient’s counts are within a safe range to administer is prohibitive. This adds an additional burden of 2 visits (lab with return visit) to the patient. We have treated 12 patients. Four patients stopped treatment prior to completing the 6 planned treatments citing debilitating fatigue and/or nonresolution of symptoms as their reason to stop treatment. One patient died. The 7 remaining patients subjectively reported varying degrees of pain relief.
Elizabeth Hansen. Another thing to mention is the lack of a PSA response from radium-223 as well. Patients are generally very diligent about monitoring their PSA, so this can be a bit distressing.
Mark Klein. Julie, have you noticed a PSA flare with radium-223? I know it has been reported.
Julie Graff. I haven’t. But I put little stock in PSAs in these patients. I spend 20 minutes explaining to patients that the PSA is not helpful in determining whether or not the radium is working. I tell them that the bone marker alkaline phosphatase may decrease. And I think it’s important to note, too, that radium-223 is not a treatment we have on the shelf. We order it from Denver I believe. It is weight based, and it takes 5 days to get.
Clinical Trials
Mark Klein. That leads us into clinical trials. What is the role for precision oncology in prostate cancer right now, specifically looking at particular panels? One would be the DNA repair enzyme-based genes and/or also the AR variants and any other markers.
Elizabeth Hansen. The National Comprehensive Cancer Network came out with a statement recommending germ-line and somatic-mutation testing in all patients with metastatic prostate cancer. This highlights the need to offer patients the availability of clinical trials.
Julie Graff. I agree. We occasionally get to a place in the disease where patients are feeling fine, but we don’t have anything else to offer. The studies by Robinson16 and then Matteo17 showed that (a) these DNA repair defects are present in about a quarter of patients; and (b) that PARP inhibitors can help these patients. At least it has an anticancer effect.
What’s interesting is that we have TRITON2, and TRITON3, which are sponsored by Clovis,for patients with BRCA 1/2 and ATM mutations and using the PARP-inhibitor rucaparib. Based on the data we have available, we thought a quarter of patients would have the mutation in the tumor, but they’re finding that it is more like 10% to 15%. They are screening many patients but not finding it.
I agree that clinical trials are the way to go. I am hopeful that we’ll get more treatments based on molecular markers. The approval for pembrolizumab in any tumor type with microsatellite instability is interesting, but in prostate cancer, I believe that’s about 3%. I haven’t seen anyone qualify for pembrolizumab based on that. Another plug for clinical trials: Let’s learn more and offer our patients potentially beneficial treatments earlier.
Mark Klein. The first interim analysis from the TRITON2 study found about 12% of patients had alterations in BRCA 1/2. But in those that met the RECIST criteria, they were able to have evaluable disease via that standard with about a 44% response rate so far and a 51% PSA response rate. It is promising data, but it’s only 85 patients so far. We’ll know more because the TRITON2 study is of a more pretreated population than the TRITION3 study at this point. Are there any data on precision medicine and radiation in prostate cancer?
Abhishek Solanki. In the prostate cancer setting, there are not a lot of emerging data specifically looking at using precision oncology biomarkers to help guide decisions in radiation therapy. For example, genomic classifiers, like GenomeDx Decipher (Vancouver, BC) and Myriad Genetics Prolaris (Salt Lake City, UT) are increasingly being utilized in patients with localized disease. Decipher can help predict the risk of recurrence after radical prostatectomy. The difficulty is that there are limited data that show that by using these genomic classifiers, one can improve outcomes in patients over traditional clinical characteristics.
There are 2 trials currently ongoing through NRG Oncology that are using Decipher. The GU002 is a trial for patients who had a radical prostatectomy and had a postoperative PSA that never nadired below 0.2. These patients are randomized between salvage radiation with hormone therapy with or without docetaxel. This trial is collecting Decipher results for patients enrolled in the study. The GU006 is a trial for a slightly more favorable group of patients who do nadir but still have biochemical recurrence and relatively low PSAs. This trial randomizes between radiotherapy alone and radiotherapy and 6 months of apalutamide, stratifying patients based on Decipher results, specially differentiating between patients who have a luminal vs basal subtype of prostate cancer. There are data that suggest that patients who have a luminal subtype may benefit more from the combination of radiation and hormone therapy vs patients who have basal subtype.18 However this hasn’t been validated in a prospective setting, and that’s what this trial will hopefully do.
Immunotherapies
Mark Klein. Outside of prostate cancer, there has been a lot of research trying to determine how to improve PD-L1 expression. Where are immunotherapy trials moving? How radiation might play a role in conjunction with immunotherapy.
Julie Graff. Two phase 3 studies did not show statistically improved survival or statistically significant survival improvement on ipilimumab, an immunotherapy agent that targets CTLA4. Some early studies of the PD-1 drugs nivolumab and pembrolizumab did not show much response with monotherapy. Despite the negative phase 3 studies for ipilimumab, we periodically see exceptional responses.
In prostate cancer, enzalutamide is FDA approved. And there’s currently a phase 3 study of the PD-L1 inhibitor atezolizumab plus enzalutamide in patients who have progressed on abiraterone. That trial is fully accrued, bu
I just received a Prostate Cancer Foundation Challenge Award to open a VA-only study looking at fecal microbiota transplant from responders to nonresponders to see how manipulating host factors can increase potential responses to PD-1 inhibition.
Abhishek Solanki. The classic mechanism by which radiation therapy works is direct DNA damage and indirect DNA damage through hydroxyl radicals that leads to cytotoxicity. But preclinical and clinical data suggest that radiation therapy can augment the local and systemic immunotherapy response. The radiation oncologist’s dream is what is called the abscopal effect, which is the idea that when you treat one site of disease with radiation, it can induce a response at other sites that didn’t get radiation therapy through reactivation of the immune system. I like to think of the abscopal effect like bigfoot—it’s elusive. However, it seems that the setting it is most likely to happen in is in combination with immunotherapy.
One of the ways that radiation fails locally is that it can upregulate PD-1 expression, and as a result, you can have progression of the tumor because of local immune suppression. We know that T cells are important for the activity of radiation therapy. If you combine checkpoint inhibition with radiation therapy, you can not only have better local control in the area of the tumor, but perhaps you can release tumor antigens that will then induce a systemic response.
The other potential mechanism by which radiation may work synergistically with immunotherapy is as a debulking agent. There are some data that suggest that the ratio of T-cell reinvigoration to bulk of disease, or the volume of tumor burden, is important. That is, having T-cell reinvigoration may not be sufficient to have a response to immunotherapy in patients with a large burden of disease. By using radiation to debulk disease, perhaps you could help make checkpoint inhibition more effective. Ultimately, in the setting of prostate cancer, there are not a lot of data yet showing meaningful benefits with the combination of immunotherapy and radiotherapy, but there are trials that are ongoing that will educate on potential synergy.
Pharmacy
Julie Graff. Before we end I want to make sure that we applaud the amazing pharmacists and patient care navigation teams in the VA who do such a great job of getting veterans the appropriate treatment expeditiously and keeping them safe. It’s something that is truly unique to the VA. And I want to thank the people on this call who do this every day.
Elizabeth Hansen. Thank you Julie. Compared with working in the community, at the VA I’m honestly amazed by the ease of access to these medications for our patients. Being able to deliver medications sometimes the same day to the patient is just not something that happens in the community. It’s nice to see that our veterans are getting cared for in that manner.
Author disclosures
Dr. Solanki participated in advisory boards for Blue Earth Diagnostics’ fluciclovine PET and was previously paid as a consultant. Dr. Graff is a consultant for Sanofi (docetaxel) and Astellas (enzalutamide), and has received research funding (no personal funding)from Sanofi, Merck (pembrolizumab), Astellas, and Jannsen (abiraterone, apalutamide). The other authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. James ND, de Bono JS, Spears MR, et al; STAMPEDE Investigators. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377(4):338-351.
2. James ND, Sydes MR, Clarke NW, et al; STAMPEDE Investigators. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2017;387(10024):1163-1177.
3. Fizazi K, Tran N, Fein L, et al; LATITUDE Investigators. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352-360.
4. Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized Phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36(11):1080-1087.
5. Tosoian JJ, Gorin MA, Ross AE, Pienta KJ, Tran PT, Schaeffer EM. Oligometastatic prostate cancer: definitions, clinical outcomes, and treatment considerations. Nat Rev Urol. 2017;14(1):15-25.
6. Parker CC, James ND, Brawley CD, et al; Systemic Therapy for Advanced or Metastatic Prostate cancer: Evaluation of Drug Efficacy (STAMPEDE) investigators. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353-2366.
7. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737-746.
8. Feyerabend S, Saad F, Li T, et al. Survival benefit, disease progression and quality-of-life outcomes of abiraterone acetate plus prednisone versus docetaxel in metastatic hormone-sensitive prostate cancer: a network meta-analysis. Eur J Cancer. 2018;103:78-87.
9. Sydes MR, Spears MR, Mason MD, et al; STAMPEDE Investigators. Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. Ann Oncol. 2018;29(5):1235-1248.
10. Smith MR, Saad F, Chowdhury S, et al; SPARTAN Investigators. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408-1418.
11. Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378(26):2465-2474.
12. Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23(13):2918-2925.
13. Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446-453.
14. Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513-1520.
15. Tannock IF, de Wit R, Berry WR, et al; TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502-1512.
16. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215-1228.
17. Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697-1708.
18. Zhao SG, Chang SL, Erho N, et al. Associations of luminal and basal subtyping of prostate cancer with prognosis and response to androgen deprivation therapy. JAMA Oncol. 2017;3(12):1663-1672.
1. James ND, de Bono JS, Spears MR, et al; STAMPEDE Investigators. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377(4):338-351.
2. James ND, Sydes MR, Clarke NW, et al; STAMPEDE Investigators. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2017;387(10024):1163-1177.
3. Fizazi K, Tran N, Fein L, et al; LATITUDE Investigators. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352-360.
4. Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized Phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36(11):1080-1087.
5. Tosoian JJ, Gorin MA, Ross AE, Pienta KJ, Tran PT, Schaeffer EM. Oligometastatic prostate cancer: definitions, clinical outcomes, and treatment considerations. Nat Rev Urol. 2017;14(1):15-25.
6. Parker CC, James ND, Brawley CD, et al; Systemic Therapy for Advanced or Metastatic Prostate cancer: Evaluation of Drug Efficacy (STAMPEDE) investigators. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353-2366.
7. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737-746.
8. Feyerabend S, Saad F, Li T, et al. Survival benefit, disease progression and quality-of-life outcomes of abiraterone acetate plus prednisone versus docetaxel in metastatic hormone-sensitive prostate cancer: a network meta-analysis. Eur J Cancer. 2018;103:78-87.
9. Sydes MR, Spears MR, Mason MD, et al; STAMPEDE Investigators. Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. Ann Oncol. 2018;29(5):1235-1248.
10. Smith MR, Saad F, Chowdhury S, et al; SPARTAN Investigators. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408-1418.
11. Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378(26):2465-2474.
12. Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23(13):2918-2925.
13. Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446-453.
14. Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513-1520.
15. Tannock IF, de Wit R, Berry WR, et al; TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502-1512.
16. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215-1228.
17. Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697-1708.
18. Zhao SG, Chang SL, Erho N, et al. Associations of luminal and basal subtyping of prostate cancer with prognosis and response to androgen deprivation therapy. JAMA Oncol. 2017;3(12):1663-1672.
Presentation of a Rare Malignancy: Leiomyosarcoma of the Prostate (FULL)
Prostatic leiomyosarcoma is an aggressive malignancy with a high risk of metastasis and a poor prognosis that poses unique diagnostic and treatment challenges.
Prostatic leiomyosarcoma is a rare tumor.1 This neoplasm is composed of highly aggressive prostatic smooth muscle cells that present with nonspecific signs and symptoms mimicking other forms of prostatic pathology. Of the primary prostatic sarcomas, leiomyosarcoma represents the most common subtype in adults and is found in 38% to 52% of newly diagnosed prostate sarcoma.1,2 The prognosis is poor, and no clear guidelines exist regarding the optimal treatment approach. We report a case of prostate leiomyosarcoma and describe the disease characteristics, diagnostic modalities, and treatment approach regarding these rare malignancies.
Case Presentation
A 72-year-old male presented with 6 months of progressive severe lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction. The patient was refractory to medical management with combination α-blocker and 5-α-reductase inhibitor therapy and continued to require multiple emergent bladder catheterizations. Workup with urinalysis, blood biochemistry, and prostate specific antigen (PSA) levels were persistently normal. He reported no hematuria, weight loss, or perineal pain. The patient reported no history of tobacco use, exposure to hazardous chemicals, and had no family history of genitourinary cancers. On rectal exam, the prostate was firm and nodular, with induration noted along the right upper lobe of the prostate.
The patient was referred for a urology consultation and subsequently underwent transurethral resection of the prostate (TURP) for suspected severe benign prostatic hypertrophy (BPH). A histopathologic examination demonstrated atypical cytology consistent with high- grade leiomyosarcoma. Immunohistochemical analysis revealed positive staining for vimentin, smooth muscle actin, desmin (partial), cytokeratin, smooth muscle myosin, muscle specific actin, and Ki-67 (50%-60% expression).
Fluorodeoxyglucose positron emission tomography (FDG-PET) scan revealed a 5.7 x 5.9 cm tumor with a maximum standardized uptake value (SUVmax) of 12.6 in the right posterior prostate, without evidence of metastatic disease (Figures 1A and 1B).
Discussion
Originating from prostatic interstitial cells, prostatic leiomyosarcoma is a rare tumor that accounts for < 0.1% of all primary prostatic malignancies.1 Since its first description in 1950 by Riba and colleagues, < 200 cases have been reported worldwide.2 Among the sarcomas of the prostate, it is the most common tumor, accounting for around 38% to 52% of prostate sarcoma presentations.1,2
Patients typically present between the ages of 41 and 78 years (mean age 61 years).2,3 Signs and symptoms at presentation may vary; however, the most common symptoms are related to lower urinary tract obstruction (89.4% of patients). These symptoms include urinary frequency, urgency, nocturia, and may mimic the presentation of BPH.
Symptoms commonly associated with other malignancies, including constitutional symptoms such as weight loss, tend to occur less frequently or may be absent. Perineal or rectal pain may only be present in 25.6% of patients. Hematuria, burning on ejaculation, and constitutional symptoms are a less common presentation (< 10% of patients).3,4 PSA levels typically do not rise and are found to be within normal limits. The lack of PSA elevation is related to the tumors nonepithelial origin and may contribute to a delay in diagnosis.2,4,5
Diagnosis
Diagnosis may be further eluded as digital rectal exam (DRE) findings tend to reveal nonspecific enlargement of the prostate, resembling that of BPH. DRE may show a hard and firm prostate with nodular induration at the base or over the lobes of the prostate.6 At this stage a urology consultation is useful, as diagnosis is most commonly achieved using transrectal ultrasound (TRUS) with ultrasound-guided needle biopsy or after a TURP procedure.3
Prostate sarcoma is associated with markedly enlarged prostate volume, irregular margins with invasion, or heterogenous hypoechoic lesions on TRUS.7 Transperineal biopsy, computed tomography (CT)-guided biopsy, or suprapubic prostatectomy have been less frequently employed for diagnosis in previously reported cases.8 Specialized imaging modalities, such as CT scan or bone scan, do not show any specific findings with regards to these tumors; their role is limited to evaluation of the local and distant metastasis and for follow-up assessments.9 Transabdominal ultrasound may assess hydronephrosis or enlarged prostate and its relation to nearby structures, although it has not been shown to be helpful in establishing a specific diagnosis.6
Histologically, prostatic leiomyosarcoma is a distinct subtype of prostatic sarcoma. Other subtypes include stromal tumors such as rhabdomyosarcoma, fibrosarcoma, and spindle cell sarcoma.2 The majority of leiomyosarcomas are high-grade lesions demonstrating neoplastic spindle cells with nuclear atypia, multifocal necrosis, and cystic degeneration. Low-grade leiomyosarcomas are very rare.10 Immunohistochemistry is characteristically positive for vimentin, smooth muscle actin, and desmin expression. Cytokeratin may be positive in up to 25% of cases, whereas S-100, CD34, CD117, and PSA are negative.2,3 These histopathological findings help to differentiate leiomyosarcoma from other prostatic tumors.
Tumor size may vary greatly, and measurements have been reported to range from 3 cm to 21 cm, frequently presenting with invasion of local structures.11 Advanced stage disease is commonly found at initial diagnosis and is thought to be due to the lack of early specific symptoms. Metastatic disease at presentation may be found in up to one-third of patients, with the lungs being the most common site of metastasis followed by the liver. Local extent and distant spread of disease may be determined by CT or magnetic resonance imaging (MRI) scans, which provide clear delineation of neoplastic and nonneoplastic tissues.
Treatment
Treatment regimens may include a multimodal approach of combination surgery, radiation, and chemotherapy. However, there are currently no standardized guidelines for treatment and the optimal therapy remains unknown.2,3,6 Surgery remains the mainstay of treatment, and patients with surgically resectable tumors are treated with curative intent. Surgeries performed include radical retropubic prostatectomy, radical cystoprostatectomy, suprapubic prostatectomy, and pelvic exenteration.2,5,8,12 These operations may be preceded or followed by radiation therapy and/or chemotherapy depending on extent of disease.
It has been reported that neo-adjuvant chemotherapy and/or radiotherapy can aid in decreasing tumor burden to facilitate a complete resection.2,8,13,14 Patients who are determined to not be candidates for surgery or whom have widespread disease may be offered systemic chemotherapy. Chemotherapy regimens vary, but common regimens include anthracyclines (doxorubicin or epirubicin), alkylating agents (cyclophosphamide, ifosfamide, dacarbazine), and/or vinca alkaloids (vinblastine or vincristine). Patients who do not receive surgical intervention rarely achieve a sustained remission.3,5,8
The long-term prognosis of prostatic leiomyosarcoma is poor due to the aggressive nature of the neoplasm and the high chance of disease recurrence or metastasis. Median survival is estimated at 17 months, and from 50% to 75% of patients die within 2 to 5 years of diagnosis.2,3 Prognosis may be improved in patients with localized disease at diagnosis who are candidates for complete surgical resection with negative margins.13 Adverse prognostic factors include metastatic disease at presentation and the presence of positive surgical margins after surgery.
Overall survival is very poor, and it is estimated that the 1-, 3-, and 5-year survival rates are 68%, 34%, and 26%, respectively.3 However, some studies estimate the 5-year survival to be anywhere from 0 to 60%.8,9 Due to the substantially high risk of death, prostatic leiomyosarcoma may be one of the most aggressive and poorly prognostic malignancies involving the prostate.
Conclusion
Prostatic leiomyosarcoma poses a unique diagnostic challenge, as clinical presentation alone may not always be suggestive of underlying malignancy. This challenge is further exacerbated by its aggressive nature, high risk of metastasis, and difficulties with unclear treatment. Proper history and physical examination, differential diagnosis, and a multidisciplinary approach to patient care are the foundation for early detection and promoting improved survival.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Miedler JD, MacLennan GT. Leiomyosarcoma of the prostate. J Urol. 2007;178(2):668.
2. Zazzara M, Divenuto L, Scarcia M, Cardo G, Maselli FP, Ludovico GM. Leiomyosarcoma of prostate: case report and literature review. Urol Case Rep. 2018;17:4-6.
3. Vandoros GP, Manolidis T, Karamouzis MV, et al. Leiomyosarcoma of the prostate: case report and review of 54 previously published cases. Sarcoma. 2008;2008:458709.
4. Talapatra K, Nemade B, Bhutani R, et al. Recurrent episodes of hematuria: a rare presentation of leiomyosarcoma of prostate. J Cancer Res Ther. 2006;2(4):212-214.
5. Cheville JC, Dundore PA, Nascimento AG, et al. Leiomyosarcoma of the prostate. Report of 23 cases. Cancer. 1995;76(8):1422-1427.
6. Venyo AK. A review of the literature on primary leiomyosarcoma of the prostate gland. Adv Urol. 2015;2015:485786.
7. Stilgenbauer R, Benedict M, Bamshad R, Viduetsky A. Sarcoma of the prostate: sonographic findings and pathologic correlation. J Ultrasound Med. 2007;26(12):1789-1793.
8. Sexton WJ, Lance RE, Reyes AO, Pisters PW, Tu SM, Pisters LL. Adult prostate sarcoma: the M.D. Anderson Cancer Center experience. J Urol. 2001;166(2):521-525.
9. Singh JP, Chakraborty D, Bera MK, Pal D. Leiomyosarcoma of prostate: a rare, aggressive tumor. J Cancer Res Ther. 2013;9(4):743-745.
10. Hansel DE, Herawi M, Montgomery E, Epstein JI. Spindle cell lesions of the adult prostate. Mod Pathol. 2007;20(1):148-158.
11. Punt SE, Eary JF, O'Sullivan J, Conrad EU. Fluorodeoxyglucose positron emission tomography in leiomyosarcoma: imaging characteristics. Nucl Med Commun. 2009;30(7):546-549.
12. Dotan ZA, Tal R, Golijanin D, et al. Adult genitourinary sarcoma: the 25-year Memorial Sloan-Kettering experience. J Urol. 2006;176(5):2033-2038.
13. Musser JE, Assel M, Mashni JW, Sjoberg DD, Russo P. Adult prostate sarcoma: the Memorial Sloan Kettering experience. Urology. 2014;84(3):624-628.
14. Janet NL, May AW, Akins RS. Sarcoma of the prostate: a single institutional review. Am J Clin Oncol. 2009;32:27-29
Prostatic leiomyosarcoma is an aggressive malignancy with a high risk of metastasis and a poor prognosis that poses unique diagnostic and treatment challenges.
Prostatic leiomyosarcoma is an aggressive malignancy with a high risk of metastasis and a poor prognosis that poses unique diagnostic and treatment challenges.
Prostatic leiomyosarcoma is a rare tumor.1 This neoplasm is composed of highly aggressive prostatic smooth muscle cells that present with nonspecific signs and symptoms mimicking other forms of prostatic pathology. Of the primary prostatic sarcomas, leiomyosarcoma represents the most common subtype in adults and is found in 38% to 52% of newly diagnosed prostate sarcoma.1,2 The prognosis is poor, and no clear guidelines exist regarding the optimal treatment approach. We report a case of prostate leiomyosarcoma and describe the disease characteristics, diagnostic modalities, and treatment approach regarding these rare malignancies.
Case Presentation
A 72-year-old male presented with 6 months of progressive severe lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction. The patient was refractory to medical management with combination α-blocker and 5-α-reductase inhibitor therapy and continued to require multiple emergent bladder catheterizations. Workup with urinalysis, blood biochemistry, and prostate specific antigen (PSA) levels were persistently normal. He reported no hematuria, weight loss, or perineal pain. The patient reported no history of tobacco use, exposure to hazardous chemicals, and had no family history of genitourinary cancers. On rectal exam, the prostate was firm and nodular, with induration noted along the right upper lobe of the prostate.
The patient was referred for a urology consultation and subsequently underwent transurethral resection of the prostate (TURP) for suspected severe benign prostatic hypertrophy (BPH). A histopathologic examination demonstrated atypical cytology consistent with high- grade leiomyosarcoma. Immunohistochemical analysis revealed positive staining for vimentin, smooth muscle actin, desmin (partial), cytokeratin, smooth muscle myosin, muscle specific actin, and Ki-67 (50%-60% expression).
Fluorodeoxyglucose positron emission tomography (FDG-PET) scan revealed a 5.7 x 5.9 cm tumor with a maximum standardized uptake value (SUVmax) of 12.6 in the right posterior prostate, without evidence of metastatic disease (Figures 1A and 1B).
Discussion
Originating from prostatic interstitial cells, prostatic leiomyosarcoma is a rare tumor that accounts for < 0.1% of all primary prostatic malignancies.1 Since its first description in 1950 by Riba and colleagues, < 200 cases have been reported worldwide.2 Among the sarcomas of the prostate, it is the most common tumor, accounting for around 38% to 52% of prostate sarcoma presentations.1,2
Patients typically present between the ages of 41 and 78 years (mean age 61 years).2,3 Signs and symptoms at presentation may vary; however, the most common symptoms are related to lower urinary tract obstruction (89.4% of patients). These symptoms include urinary frequency, urgency, nocturia, and may mimic the presentation of BPH.
Symptoms commonly associated with other malignancies, including constitutional symptoms such as weight loss, tend to occur less frequently or may be absent. Perineal or rectal pain may only be present in 25.6% of patients. Hematuria, burning on ejaculation, and constitutional symptoms are a less common presentation (< 10% of patients).3,4 PSA levels typically do not rise and are found to be within normal limits. The lack of PSA elevation is related to the tumors nonepithelial origin and may contribute to a delay in diagnosis.2,4,5
Diagnosis
Diagnosis may be further eluded as digital rectal exam (DRE) findings tend to reveal nonspecific enlargement of the prostate, resembling that of BPH. DRE may show a hard and firm prostate with nodular induration at the base or over the lobes of the prostate.6 At this stage a urology consultation is useful, as diagnosis is most commonly achieved using transrectal ultrasound (TRUS) with ultrasound-guided needle biopsy or after a TURP procedure.3
Prostate sarcoma is associated with markedly enlarged prostate volume, irregular margins with invasion, or heterogenous hypoechoic lesions on TRUS.7 Transperineal biopsy, computed tomography (CT)-guided biopsy, or suprapubic prostatectomy have been less frequently employed for diagnosis in previously reported cases.8 Specialized imaging modalities, such as CT scan or bone scan, do not show any specific findings with regards to these tumors; their role is limited to evaluation of the local and distant metastasis and for follow-up assessments.9 Transabdominal ultrasound may assess hydronephrosis or enlarged prostate and its relation to nearby structures, although it has not been shown to be helpful in establishing a specific diagnosis.6
Histologically, prostatic leiomyosarcoma is a distinct subtype of prostatic sarcoma. Other subtypes include stromal tumors such as rhabdomyosarcoma, fibrosarcoma, and spindle cell sarcoma.2 The majority of leiomyosarcomas are high-grade lesions demonstrating neoplastic spindle cells with nuclear atypia, multifocal necrosis, and cystic degeneration. Low-grade leiomyosarcomas are very rare.10 Immunohistochemistry is characteristically positive for vimentin, smooth muscle actin, and desmin expression. Cytokeratin may be positive in up to 25% of cases, whereas S-100, CD34, CD117, and PSA are negative.2,3 These histopathological findings help to differentiate leiomyosarcoma from other prostatic tumors.
Tumor size may vary greatly, and measurements have been reported to range from 3 cm to 21 cm, frequently presenting with invasion of local structures.11 Advanced stage disease is commonly found at initial diagnosis and is thought to be due to the lack of early specific symptoms. Metastatic disease at presentation may be found in up to one-third of patients, with the lungs being the most common site of metastasis followed by the liver. Local extent and distant spread of disease may be determined by CT or magnetic resonance imaging (MRI) scans, which provide clear delineation of neoplastic and nonneoplastic tissues.
Treatment
Treatment regimens may include a multimodal approach of combination surgery, radiation, and chemotherapy. However, there are currently no standardized guidelines for treatment and the optimal therapy remains unknown.2,3,6 Surgery remains the mainstay of treatment, and patients with surgically resectable tumors are treated with curative intent. Surgeries performed include radical retropubic prostatectomy, radical cystoprostatectomy, suprapubic prostatectomy, and pelvic exenteration.2,5,8,12 These operations may be preceded or followed by radiation therapy and/or chemotherapy depending on extent of disease.
It has been reported that neo-adjuvant chemotherapy and/or radiotherapy can aid in decreasing tumor burden to facilitate a complete resection.2,8,13,14 Patients who are determined to not be candidates for surgery or whom have widespread disease may be offered systemic chemotherapy. Chemotherapy regimens vary, but common regimens include anthracyclines (doxorubicin or epirubicin), alkylating agents (cyclophosphamide, ifosfamide, dacarbazine), and/or vinca alkaloids (vinblastine or vincristine). Patients who do not receive surgical intervention rarely achieve a sustained remission.3,5,8
The long-term prognosis of prostatic leiomyosarcoma is poor due to the aggressive nature of the neoplasm and the high chance of disease recurrence or metastasis. Median survival is estimated at 17 months, and from 50% to 75% of patients die within 2 to 5 years of diagnosis.2,3 Prognosis may be improved in patients with localized disease at diagnosis who are candidates for complete surgical resection with negative margins.13 Adverse prognostic factors include metastatic disease at presentation and the presence of positive surgical margins after surgery.
Overall survival is very poor, and it is estimated that the 1-, 3-, and 5-year survival rates are 68%, 34%, and 26%, respectively.3 However, some studies estimate the 5-year survival to be anywhere from 0 to 60%.8,9 Due to the substantially high risk of death, prostatic leiomyosarcoma may be one of the most aggressive and poorly prognostic malignancies involving the prostate.
Conclusion
Prostatic leiomyosarcoma poses a unique diagnostic challenge, as clinical presentation alone may not always be suggestive of underlying malignancy. This challenge is further exacerbated by its aggressive nature, high risk of metastasis, and difficulties with unclear treatment. Proper history and physical examination, differential diagnosis, and a multidisciplinary approach to patient care are the foundation for early detection and promoting improved survival.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Prostatic leiomyosarcoma is a rare tumor.1 This neoplasm is composed of highly aggressive prostatic smooth muscle cells that present with nonspecific signs and symptoms mimicking other forms of prostatic pathology. Of the primary prostatic sarcomas, leiomyosarcoma represents the most common subtype in adults and is found in 38% to 52% of newly diagnosed prostate sarcoma.1,2 The prognosis is poor, and no clear guidelines exist regarding the optimal treatment approach. We report a case of prostate leiomyosarcoma and describe the disease characteristics, diagnostic modalities, and treatment approach regarding these rare malignancies.
Case Presentation
A 72-year-old male presented with 6 months of progressive severe lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction. The patient was refractory to medical management with combination α-blocker and 5-α-reductase inhibitor therapy and continued to require multiple emergent bladder catheterizations. Workup with urinalysis, blood biochemistry, and prostate specific antigen (PSA) levels were persistently normal. He reported no hematuria, weight loss, or perineal pain. The patient reported no history of tobacco use, exposure to hazardous chemicals, and had no family history of genitourinary cancers. On rectal exam, the prostate was firm and nodular, with induration noted along the right upper lobe of the prostate.
The patient was referred for a urology consultation and subsequently underwent transurethral resection of the prostate (TURP) for suspected severe benign prostatic hypertrophy (BPH). A histopathologic examination demonstrated atypical cytology consistent with high- grade leiomyosarcoma. Immunohistochemical analysis revealed positive staining for vimentin, smooth muscle actin, desmin (partial), cytokeratin, smooth muscle myosin, muscle specific actin, and Ki-67 (50%-60% expression).
Fluorodeoxyglucose positron emission tomography (FDG-PET) scan revealed a 5.7 x 5.9 cm tumor with a maximum standardized uptake value (SUVmax) of 12.6 in the right posterior prostate, without evidence of metastatic disease (Figures 1A and 1B).
Discussion
Originating from prostatic interstitial cells, prostatic leiomyosarcoma is a rare tumor that accounts for < 0.1% of all primary prostatic malignancies.1 Since its first description in 1950 by Riba and colleagues, < 200 cases have been reported worldwide.2 Among the sarcomas of the prostate, it is the most common tumor, accounting for around 38% to 52% of prostate sarcoma presentations.1,2
Patients typically present between the ages of 41 and 78 years (mean age 61 years).2,3 Signs and symptoms at presentation may vary; however, the most common symptoms are related to lower urinary tract obstruction (89.4% of patients). These symptoms include urinary frequency, urgency, nocturia, and may mimic the presentation of BPH.
Symptoms commonly associated with other malignancies, including constitutional symptoms such as weight loss, tend to occur less frequently or may be absent. Perineal or rectal pain may only be present in 25.6% of patients. Hematuria, burning on ejaculation, and constitutional symptoms are a less common presentation (< 10% of patients).3,4 PSA levels typically do not rise and are found to be within normal limits. The lack of PSA elevation is related to the tumors nonepithelial origin and may contribute to a delay in diagnosis.2,4,5
Diagnosis
Diagnosis may be further eluded as digital rectal exam (DRE) findings tend to reveal nonspecific enlargement of the prostate, resembling that of BPH. DRE may show a hard and firm prostate with nodular induration at the base or over the lobes of the prostate.6 At this stage a urology consultation is useful, as diagnosis is most commonly achieved using transrectal ultrasound (TRUS) with ultrasound-guided needle biopsy or after a TURP procedure.3
Prostate sarcoma is associated with markedly enlarged prostate volume, irregular margins with invasion, or heterogenous hypoechoic lesions on TRUS.7 Transperineal biopsy, computed tomography (CT)-guided biopsy, or suprapubic prostatectomy have been less frequently employed for diagnosis in previously reported cases.8 Specialized imaging modalities, such as CT scan or bone scan, do not show any specific findings with regards to these tumors; their role is limited to evaluation of the local and distant metastasis and for follow-up assessments.9 Transabdominal ultrasound may assess hydronephrosis or enlarged prostate and its relation to nearby structures, although it has not been shown to be helpful in establishing a specific diagnosis.6
Histologically, prostatic leiomyosarcoma is a distinct subtype of prostatic sarcoma. Other subtypes include stromal tumors such as rhabdomyosarcoma, fibrosarcoma, and spindle cell sarcoma.2 The majority of leiomyosarcomas are high-grade lesions demonstrating neoplastic spindle cells with nuclear atypia, multifocal necrosis, and cystic degeneration. Low-grade leiomyosarcomas are very rare.10 Immunohistochemistry is characteristically positive for vimentin, smooth muscle actin, and desmin expression. Cytokeratin may be positive in up to 25% of cases, whereas S-100, CD34, CD117, and PSA are negative.2,3 These histopathological findings help to differentiate leiomyosarcoma from other prostatic tumors.
Tumor size may vary greatly, and measurements have been reported to range from 3 cm to 21 cm, frequently presenting with invasion of local structures.11 Advanced stage disease is commonly found at initial diagnosis and is thought to be due to the lack of early specific symptoms. Metastatic disease at presentation may be found in up to one-third of patients, with the lungs being the most common site of metastasis followed by the liver. Local extent and distant spread of disease may be determined by CT or magnetic resonance imaging (MRI) scans, which provide clear delineation of neoplastic and nonneoplastic tissues.
Treatment
Treatment regimens may include a multimodal approach of combination surgery, radiation, and chemotherapy. However, there are currently no standardized guidelines for treatment and the optimal therapy remains unknown.2,3,6 Surgery remains the mainstay of treatment, and patients with surgically resectable tumors are treated with curative intent. Surgeries performed include radical retropubic prostatectomy, radical cystoprostatectomy, suprapubic prostatectomy, and pelvic exenteration.2,5,8,12 These operations may be preceded or followed by radiation therapy and/or chemotherapy depending on extent of disease.
It has been reported that neo-adjuvant chemotherapy and/or radiotherapy can aid in decreasing tumor burden to facilitate a complete resection.2,8,13,14 Patients who are determined to not be candidates for surgery or whom have widespread disease may be offered systemic chemotherapy. Chemotherapy regimens vary, but common regimens include anthracyclines (doxorubicin or epirubicin), alkylating agents (cyclophosphamide, ifosfamide, dacarbazine), and/or vinca alkaloids (vinblastine or vincristine). Patients who do not receive surgical intervention rarely achieve a sustained remission.3,5,8
The long-term prognosis of prostatic leiomyosarcoma is poor due to the aggressive nature of the neoplasm and the high chance of disease recurrence or metastasis. Median survival is estimated at 17 months, and from 50% to 75% of patients die within 2 to 5 years of diagnosis.2,3 Prognosis may be improved in patients with localized disease at diagnosis who are candidates for complete surgical resection with negative margins.13 Adverse prognostic factors include metastatic disease at presentation and the presence of positive surgical margins after surgery.
Overall survival is very poor, and it is estimated that the 1-, 3-, and 5-year survival rates are 68%, 34%, and 26%, respectively.3 However, some studies estimate the 5-year survival to be anywhere from 0 to 60%.8,9 Due to the substantially high risk of death, prostatic leiomyosarcoma may be one of the most aggressive and poorly prognostic malignancies involving the prostate.
Conclusion
Prostatic leiomyosarcoma poses a unique diagnostic challenge, as clinical presentation alone may not always be suggestive of underlying malignancy. This challenge is further exacerbated by its aggressive nature, high risk of metastasis, and difficulties with unclear treatment. Proper history and physical examination, differential diagnosis, and a multidisciplinary approach to patient care are the foundation for early detection and promoting improved survival.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Miedler JD, MacLennan GT. Leiomyosarcoma of the prostate. J Urol. 2007;178(2):668.
2. Zazzara M, Divenuto L, Scarcia M, Cardo G, Maselli FP, Ludovico GM. Leiomyosarcoma of prostate: case report and literature review. Urol Case Rep. 2018;17:4-6.
3. Vandoros GP, Manolidis T, Karamouzis MV, et al. Leiomyosarcoma of the prostate: case report and review of 54 previously published cases. Sarcoma. 2008;2008:458709.
4. Talapatra K, Nemade B, Bhutani R, et al. Recurrent episodes of hematuria: a rare presentation of leiomyosarcoma of prostate. J Cancer Res Ther. 2006;2(4):212-214.
5. Cheville JC, Dundore PA, Nascimento AG, et al. Leiomyosarcoma of the prostate. Report of 23 cases. Cancer. 1995;76(8):1422-1427.
6. Venyo AK. A review of the literature on primary leiomyosarcoma of the prostate gland. Adv Urol. 2015;2015:485786.
7. Stilgenbauer R, Benedict M, Bamshad R, Viduetsky A. Sarcoma of the prostate: sonographic findings and pathologic correlation. J Ultrasound Med. 2007;26(12):1789-1793.
8. Sexton WJ, Lance RE, Reyes AO, Pisters PW, Tu SM, Pisters LL. Adult prostate sarcoma: the M.D. Anderson Cancer Center experience. J Urol. 2001;166(2):521-525.
9. Singh JP, Chakraborty D, Bera MK, Pal D. Leiomyosarcoma of prostate: a rare, aggressive tumor. J Cancer Res Ther. 2013;9(4):743-745.
10. Hansel DE, Herawi M, Montgomery E, Epstein JI. Spindle cell lesions of the adult prostate. Mod Pathol. 2007;20(1):148-158.
11. Punt SE, Eary JF, O'Sullivan J, Conrad EU. Fluorodeoxyglucose positron emission tomography in leiomyosarcoma: imaging characteristics. Nucl Med Commun. 2009;30(7):546-549.
12. Dotan ZA, Tal R, Golijanin D, et al. Adult genitourinary sarcoma: the 25-year Memorial Sloan-Kettering experience. J Urol. 2006;176(5):2033-2038.
13. Musser JE, Assel M, Mashni JW, Sjoberg DD, Russo P. Adult prostate sarcoma: the Memorial Sloan Kettering experience. Urology. 2014;84(3):624-628.
14. Janet NL, May AW, Akins RS. Sarcoma of the prostate: a single institutional review. Am J Clin Oncol. 2009;32:27-29
1. Miedler JD, MacLennan GT. Leiomyosarcoma of the prostate. J Urol. 2007;178(2):668.
2. Zazzara M, Divenuto L, Scarcia M, Cardo G, Maselli FP, Ludovico GM. Leiomyosarcoma of prostate: case report and literature review. Urol Case Rep. 2018;17:4-6.
3. Vandoros GP, Manolidis T, Karamouzis MV, et al. Leiomyosarcoma of the prostate: case report and review of 54 previously published cases. Sarcoma. 2008;2008:458709.
4. Talapatra K, Nemade B, Bhutani R, et al. Recurrent episodes of hematuria: a rare presentation of leiomyosarcoma of prostate. J Cancer Res Ther. 2006;2(4):212-214.
5. Cheville JC, Dundore PA, Nascimento AG, et al. Leiomyosarcoma of the prostate. Report of 23 cases. Cancer. 1995;76(8):1422-1427.
6. Venyo AK. A review of the literature on primary leiomyosarcoma of the prostate gland. Adv Urol. 2015;2015:485786.
7. Stilgenbauer R, Benedict M, Bamshad R, Viduetsky A. Sarcoma of the prostate: sonographic findings and pathologic correlation. J Ultrasound Med. 2007;26(12):1789-1793.
8. Sexton WJ, Lance RE, Reyes AO, Pisters PW, Tu SM, Pisters LL. Adult prostate sarcoma: the M.D. Anderson Cancer Center experience. J Urol. 2001;166(2):521-525.
9. Singh JP, Chakraborty D, Bera MK, Pal D. Leiomyosarcoma of prostate: a rare, aggressive tumor. J Cancer Res Ther. 2013;9(4):743-745.
10. Hansel DE, Herawi M, Montgomery E, Epstein JI. Spindle cell lesions of the adult prostate. Mod Pathol. 2007;20(1):148-158.
11. Punt SE, Eary JF, O'Sullivan J, Conrad EU. Fluorodeoxyglucose positron emission tomography in leiomyosarcoma: imaging characteristics. Nucl Med Commun. 2009;30(7):546-549.
12. Dotan ZA, Tal R, Golijanin D, et al. Adult genitourinary sarcoma: the 25-year Memorial Sloan-Kettering experience. J Urol. 2006;176(5):2033-2038.
13. Musser JE, Assel M, Mashni JW, Sjoberg DD, Russo P. Adult prostate sarcoma: the Memorial Sloan Kettering experience. Urology. 2014;84(3):624-628.
14. Janet NL, May AW, Akins RS. Sarcoma of the prostate: a single institutional review. Am J Clin Oncol. 2009;32:27-29
Primary Urethral Carcinoma With Nodal Metastasis (FULL)
The presentation of a fungating penile mass often indicates penile carcinoma, but providers should be aware of urethral carcinoma in the differential diagnosis.
Primary urethral carcinoma (PUC) is a rare but morbid disease, representing < 1% of all urologic malignancies.1 Up to one-third of male patients may present with nodal metastases.2-4 The overall survival (OS) for all male PUC is < 50% at 5 years and is lower still in patients with nodal involvement.4
Although surgical intervention, including radical resection, has been a mainstay in disease management, the presence of high-stage disease may warrant multimodal treatment with chemotherapy, radiation, and surgery. Recent series have described success with neoadjuvant and adjuvant chemoradiation, yet the optimal regimen remains unestablished.5,6 Although nodal disease is commonly encountered with proximal, high-stage tumors, this case exhibits a rare presentation of a distal fungating penile mass with low pathologic stage but rapid progression to nodal disease.
Case Presentation
A male veteran aged 77 years with a history of diabetes mellitus and stroke presented with obstructive urinary symptoms, gross hematuria, and 15-pound weight loss. Examination revealed a distal penile mass with purulent exudate at the meatus but no inguinal lymphadenopathy. Two fragments of this mass detached during office cystoscopy, and pathology revealed high-grade urothelial cell carcinoma (UCC). A magnetic resonance image of the pelvis with and without IV contrast revealed a 2.4-cm tumor in the glans penis with possible extension into the subcutaneous connective tissue of the penis and penile skin, without invasion of the corpora cavernosa/spongiosum or lymphadenopathy (Figure 1).
Prostatic urethral and random bladder biopsies, bilateral retrograde pyelograms, and selective ureteral washings revealed no abnormalities or signs of disease. Percutaneous biopsy of the inguinal node confirmed metastatic UCC. The patient underwent radical penectomy, creation of a perineal urethrostomy, and suprapubic cystostomy tube placement. Negative margins were confirmed on the urethral stump and corpus spongiosum. Final pathology revealed high-grade UCC with squamous differentiation on hematoxylin and eosin staining, arising from the penile urethra, invading the glans and corpus spongiosum, with no invasion of the corpus cavernosa (Figures 3 and 4).
Immunohistochemical stains were performed and strongly positive for cytokeratin 7 and p63. Final pathologic stage was described as pT2N1, with negative margins, indicating an American Joint Committee on Cancer classification of Stage III disease.7 The patient was referred postoperatively for adjuvant chemoradiation.
Discussion
The low incidence of PUC, coupled with a high morbidity/mortality rate, creates a difficult scenario in choosing the best oncologic management for this disease. National guidelines stratify treatment algorithms by stage and location of primary tumor, as these were found to be the 2 most important prognostic factors for men.1 The location of the primary tumor is most often in the bulbomembranous urethra, but up to one-third occur in the pendulous urethra.2
A recent review reported that UCC is the most common histologic subtype.4 When considering the differential diagnosis, a distal penile mass may represent a malignant penile lesion, such as squamous cell carcinoma, Buschke-Lowenstein tumor, Kaposi sarcoma, or precancerous lesions. Additional benign and infectious disorders include epidermoid and retention cysts, leukoplakia, balanitis xerotica obliterans, condyloma acuminatum, chancre/chancroid, lymphogranuloma venereum, granuloma inguinale, and tuberculosis. Clinical workup typically includes physical examination, cystourethroscopy and biopsy, chest X-ray, and pelvic/abdominal cross-sectional imaging.9,10 Magnetic resonance imaging of the abdomen and pelvis is ideal in identifying soft tissue structures and extension of tumor.
In male patients with PUC, nodal metastases are commonly seen at initial presentation in up to one-third of patients, while distant metastases may be present in up to 6% at presentation.2-4 When tumors arise from the anterior urethra, the primary lymphatic drainage is first to the inguinal lymph nodes, whereas posterior tumors drain to the pelvic lymph nodes. A multivariate analysis of men with PUC within the Surveillance, Epidemiology, and End Results database demonstrated an OS across all stages to be 46.2% and 29.3% at 5 and 10 years, respectively. Increased likelihood of death was predicted by advanced age, high grade/stage, systemic metastases, non-UCC histology, and the lack of surgery.4
Surgical intervention, including radical resection via penectomy, has been the mainstay in disease management and was first described by Marshall in 1957 for bulbar urethral cancer.11 In 1998, Gheiler and colleagues demonstrated that surgical resection alone yielded excellent outcomes in patients with low-stage disease with 89% of patients disease free at mean 42 months. This was in stark contrast to patients with advanced stage disease (T3 or N+) who exhibited a disease-free survival rate of 42% at the same follow-up interval and benefited from combined chemoradiation and surgical resection.3
In the presence of high-stage disease, multimodal therapy with chemotherapy, radiation, and/or surgery is warranted. A study in 2008 reviewed chemoradiation in which patients with PUC received a 5-week protocol of external beam radiotherapy to the genitals, inguinal/pelvic lymph nodes, plus an additional radiation bolus to the primary tumor.5 In the 18 patients reported, 15 had complete response to therapy, and only 4 patients required salvage surgical resection. The 7-year survival for the cohort was 72% with chemoradiation alone, with about half the population recurring or progressing at 7 years. However, all patients that avoided surgical resection went on to develop urethral strictures that required surgical therapy, 3 of which required complex reconstructive procedures.
To place this survival into context, the 1999 study by Dalbagni and colleagues reported a 5-year OS of 42% when surgical resection alone was performed in 40/46 men with PUC.2 Last, a large retrospective series of 44 patients reported mostly advanced-stage patients with PUC and analyzed patients treated with chemotherapy based on histologic pathology. The results demonstrated a 72% overall response rate to neoadjuvant chemotherapy, with a median OS of 32 months in patients undergoing chemotherapy vs 46 months in patients who underwent subsequent surgery. This study solidified that for patients with PUC involving the lymph nodes; optimal treatment includes neoadjuvant cisplatin-based chemotherapy followed by surgical resection.6
As medicine and oncologic therapies become more individualized, physicians are looking to new immunologic agents for systemic therapy. Immune checkpoint inhibitors were approved by the US Food and Drug Administration for UCC of the bladder in 2016.12 Unfortunately, due to the rarity of PUC and the recent development of immune checkpoint inhibitors, there have been no published reports of these or other immunotherapies in PUC. However, given the histologic similarity and pathogenesis, checkpoint inhibitors may have a future indication in the systemic management of this disease.
Conclusion
This patient’s PUC represents a rare presentation of a distal urethral carcinoma, T2-staged tumor, with rapid progression to nodal metastases. Additionally, the presentation of a fungating penile mass would usually indicate penile carcinoma, but providers should be aware of urethral carcinoma in the differential diagnosis. Notably, the patient was found to have progression to lymph node involvement during a mere 2-month period.
Recent case series have published encouraging results with neoadjuvant chemotherapy or chemoradiation.5,6 However, radical resection in men with T2 to T4 disease is associated with significantly higher cancer-specific survival. Given our concern of a loss to follow-up, we felt that radical resection of the primary tumor and adjuvant chemoradiation represented the patient’s best oncologic outcomes. Therefore, he underwent radical penectomy and creation of a perineal urethrostomy. As of his 6-month follow-up, he showed no evidence of disease, had returned to his preoperative functional status, and was referred for chemoradiation.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Swartz MA, Porter MP, Lin DW, Weiss NS. Incidence of primary urethral carcinoma in the United States. Urology. 2006;68(6):1164-1168.
2. Dalbagni G, Zhang ZF, Lacombe L, Herr HW. Male urethral carcinoma: analysis of treatment outcome. Urology. 1999;53(6):1126-1132.
3. Gheiler EL, Tefilli MV, Tiguert R, de Oliveira JG, Pontes JE, Wood DP Jr. Management of primary urethral cancer. Urology. 1998;52(3):487-493.
4. Rabbani F. Prognostic factors in male urethral cancer. Cancer. 2011;117(11):2426-2434.
5. Cohen MS, Triaca V, Billmeyer B, et al. Coordinated chemoradiation therapy with genital preservation for the treatment of primary invasive carcinoma of the male urethra. J Urol. 2008;179(2):536-541; discussion 541.
6. Dayyani F, Pettaway CA, Kamat AM, Munsell MF, Sircar K, Pagliaro LC. Retrospective analysis of survival outcomes and the role of cisplatin-based chemotherapy in patients with urethral carcinomas referred to medical oncologists. Urol Oncol. 2013;31(7):1171-1177.
7. American Joint Committee on Cancer. AJCC cancer staging manual. 8th ed. https://cancerstaging.org/references-tools/deskreferences/Documents/AJCC%20Cancer%20Staging%20Form%20Supplement.pdf. Updated June 5, 2018. Accessed January 22, 2019.
8. Gakis G, Witjes JA, Compérat E, et al. European Association of Urology guidelines on primary urethral carcinoma. https://uroweb.org/wp-content/uploads/EAU-Guidelines-Primary-Urethral-Carcinoma-2016-1.pdf. Updated March 2015. Accessed January 22, 2019
9. National Comprehensive Cancer Network. Bladder Cancer. Version 1.2019. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Updated December 20, 2018. Accessed January 17, 2019.
10. Dayyani F, Hoffman K, Eifel P, et al. Management of advanced primary urethral carcinomas. BJU Int. 2014;114(1):25-31.
11. Marshall VF. Radical excision of locally extensive carcinoma of the deep male urethra. J Urol. 1957;78(3):252-264.
12. Hsu FS, Su CH, Huang KH. A comprehensive review of US FDA-approved immune checkpoint inhibitors in urothelial carcinoma. J Immunol Res. 2017;2017:6940546.
The presentation of a fungating penile mass often indicates penile carcinoma, but providers should be aware of urethral carcinoma in the differential diagnosis.
The presentation of a fungating penile mass often indicates penile carcinoma, but providers should be aware of urethral carcinoma in the differential diagnosis.
Primary urethral carcinoma (PUC) is a rare but morbid disease, representing < 1% of all urologic malignancies.1 Up to one-third of male patients may present with nodal metastases.2-4 The overall survival (OS) for all male PUC is < 50% at 5 years and is lower still in patients with nodal involvement.4
Although surgical intervention, including radical resection, has been a mainstay in disease management, the presence of high-stage disease may warrant multimodal treatment with chemotherapy, radiation, and surgery. Recent series have described success with neoadjuvant and adjuvant chemoradiation, yet the optimal regimen remains unestablished.5,6 Although nodal disease is commonly encountered with proximal, high-stage tumors, this case exhibits a rare presentation of a distal fungating penile mass with low pathologic stage but rapid progression to nodal disease.
Case Presentation
A male veteran aged 77 years with a history of diabetes mellitus and stroke presented with obstructive urinary symptoms, gross hematuria, and 15-pound weight loss. Examination revealed a distal penile mass with purulent exudate at the meatus but no inguinal lymphadenopathy. Two fragments of this mass detached during office cystoscopy, and pathology revealed high-grade urothelial cell carcinoma (UCC). A magnetic resonance image of the pelvis with and without IV contrast revealed a 2.4-cm tumor in the glans penis with possible extension into the subcutaneous connective tissue of the penis and penile skin, without invasion of the corpora cavernosa/spongiosum or lymphadenopathy (Figure 1).
Prostatic urethral and random bladder biopsies, bilateral retrograde pyelograms, and selective ureteral washings revealed no abnormalities or signs of disease. Percutaneous biopsy of the inguinal node confirmed metastatic UCC. The patient underwent radical penectomy, creation of a perineal urethrostomy, and suprapubic cystostomy tube placement. Negative margins were confirmed on the urethral stump and corpus spongiosum. Final pathology revealed high-grade UCC with squamous differentiation on hematoxylin and eosin staining, arising from the penile urethra, invading the glans and corpus spongiosum, with no invasion of the corpus cavernosa (Figures 3 and 4).
Immunohistochemical stains were performed and strongly positive for cytokeratin 7 and p63. Final pathologic stage was described as pT2N1, with negative margins, indicating an American Joint Committee on Cancer classification of Stage III disease.7 The patient was referred postoperatively for adjuvant chemoradiation.
Discussion
The low incidence of PUC, coupled with a high morbidity/mortality rate, creates a difficult scenario in choosing the best oncologic management for this disease. National guidelines stratify treatment algorithms by stage and location of primary tumor, as these were found to be the 2 most important prognostic factors for men.1 The location of the primary tumor is most often in the bulbomembranous urethra, but up to one-third occur in the pendulous urethra.2
A recent review reported that UCC is the most common histologic subtype.4 When considering the differential diagnosis, a distal penile mass may represent a malignant penile lesion, such as squamous cell carcinoma, Buschke-Lowenstein tumor, Kaposi sarcoma, or precancerous lesions. Additional benign and infectious disorders include epidermoid and retention cysts, leukoplakia, balanitis xerotica obliterans, condyloma acuminatum, chancre/chancroid, lymphogranuloma venereum, granuloma inguinale, and tuberculosis. Clinical workup typically includes physical examination, cystourethroscopy and biopsy, chest X-ray, and pelvic/abdominal cross-sectional imaging.9,10 Magnetic resonance imaging of the abdomen and pelvis is ideal in identifying soft tissue structures and extension of tumor.
In male patients with PUC, nodal metastases are commonly seen at initial presentation in up to one-third of patients, while distant metastases may be present in up to 6% at presentation.2-4 When tumors arise from the anterior urethra, the primary lymphatic drainage is first to the inguinal lymph nodes, whereas posterior tumors drain to the pelvic lymph nodes. A multivariate analysis of men with PUC within the Surveillance, Epidemiology, and End Results database demonstrated an OS across all stages to be 46.2% and 29.3% at 5 and 10 years, respectively. Increased likelihood of death was predicted by advanced age, high grade/stage, systemic metastases, non-UCC histology, and the lack of surgery.4
Surgical intervention, including radical resection via penectomy, has been the mainstay in disease management and was first described by Marshall in 1957 for bulbar urethral cancer.11 In 1998, Gheiler and colleagues demonstrated that surgical resection alone yielded excellent outcomes in patients with low-stage disease with 89% of patients disease free at mean 42 months. This was in stark contrast to patients with advanced stage disease (T3 or N+) who exhibited a disease-free survival rate of 42% at the same follow-up interval and benefited from combined chemoradiation and surgical resection.3
In the presence of high-stage disease, multimodal therapy with chemotherapy, radiation, and/or surgery is warranted. A study in 2008 reviewed chemoradiation in which patients with PUC received a 5-week protocol of external beam radiotherapy to the genitals, inguinal/pelvic lymph nodes, plus an additional radiation bolus to the primary tumor.5 In the 18 patients reported, 15 had complete response to therapy, and only 4 patients required salvage surgical resection. The 7-year survival for the cohort was 72% with chemoradiation alone, with about half the population recurring or progressing at 7 years. However, all patients that avoided surgical resection went on to develop urethral strictures that required surgical therapy, 3 of which required complex reconstructive procedures.
To place this survival into context, the 1999 study by Dalbagni and colleagues reported a 5-year OS of 42% when surgical resection alone was performed in 40/46 men with PUC.2 Last, a large retrospective series of 44 patients reported mostly advanced-stage patients with PUC and analyzed patients treated with chemotherapy based on histologic pathology. The results demonstrated a 72% overall response rate to neoadjuvant chemotherapy, with a median OS of 32 months in patients undergoing chemotherapy vs 46 months in patients who underwent subsequent surgery. This study solidified that for patients with PUC involving the lymph nodes; optimal treatment includes neoadjuvant cisplatin-based chemotherapy followed by surgical resection.6
As medicine and oncologic therapies become more individualized, physicians are looking to new immunologic agents for systemic therapy. Immune checkpoint inhibitors were approved by the US Food and Drug Administration for UCC of the bladder in 2016.12 Unfortunately, due to the rarity of PUC and the recent development of immune checkpoint inhibitors, there have been no published reports of these or other immunotherapies in PUC. However, given the histologic similarity and pathogenesis, checkpoint inhibitors may have a future indication in the systemic management of this disease.
Conclusion
This patient’s PUC represents a rare presentation of a distal urethral carcinoma, T2-staged tumor, with rapid progression to nodal metastases. Additionally, the presentation of a fungating penile mass would usually indicate penile carcinoma, but providers should be aware of urethral carcinoma in the differential diagnosis. Notably, the patient was found to have progression to lymph node involvement during a mere 2-month period.
Recent case series have published encouraging results with neoadjuvant chemotherapy or chemoradiation.5,6 However, radical resection in men with T2 to T4 disease is associated with significantly higher cancer-specific survival. Given our concern of a loss to follow-up, we felt that radical resection of the primary tumor and adjuvant chemoradiation represented the patient’s best oncologic outcomes. Therefore, he underwent radical penectomy and creation of a perineal urethrostomy. As of his 6-month follow-up, he showed no evidence of disease, had returned to his preoperative functional status, and was referred for chemoradiation.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Primary urethral carcinoma (PUC) is a rare but morbid disease, representing < 1% of all urologic malignancies.1 Up to one-third of male patients may present with nodal metastases.2-4 The overall survival (OS) for all male PUC is < 50% at 5 years and is lower still in patients with nodal involvement.4
Although surgical intervention, including radical resection, has been a mainstay in disease management, the presence of high-stage disease may warrant multimodal treatment with chemotherapy, radiation, and surgery. Recent series have described success with neoadjuvant and adjuvant chemoradiation, yet the optimal regimen remains unestablished.5,6 Although nodal disease is commonly encountered with proximal, high-stage tumors, this case exhibits a rare presentation of a distal fungating penile mass with low pathologic stage but rapid progression to nodal disease.
Case Presentation
A male veteran aged 77 years with a history of diabetes mellitus and stroke presented with obstructive urinary symptoms, gross hematuria, and 15-pound weight loss. Examination revealed a distal penile mass with purulent exudate at the meatus but no inguinal lymphadenopathy. Two fragments of this mass detached during office cystoscopy, and pathology revealed high-grade urothelial cell carcinoma (UCC). A magnetic resonance image of the pelvis with and without IV contrast revealed a 2.4-cm tumor in the glans penis with possible extension into the subcutaneous connective tissue of the penis and penile skin, without invasion of the corpora cavernosa/spongiosum or lymphadenopathy (Figure 1).
Prostatic urethral and random bladder biopsies, bilateral retrograde pyelograms, and selective ureteral washings revealed no abnormalities or signs of disease. Percutaneous biopsy of the inguinal node confirmed metastatic UCC. The patient underwent radical penectomy, creation of a perineal urethrostomy, and suprapubic cystostomy tube placement. Negative margins were confirmed on the urethral stump and corpus spongiosum. Final pathology revealed high-grade UCC with squamous differentiation on hematoxylin and eosin staining, arising from the penile urethra, invading the glans and corpus spongiosum, with no invasion of the corpus cavernosa (Figures 3 and 4).
Immunohistochemical stains were performed and strongly positive for cytokeratin 7 and p63. Final pathologic stage was described as pT2N1, with negative margins, indicating an American Joint Committee on Cancer classification of Stage III disease.7 The patient was referred postoperatively for adjuvant chemoradiation.
Discussion
The low incidence of PUC, coupled with a high morbidity/mortality rate, creates a difficult scenario in choosing the best oncologic management for this disease. National guidelines stratify treatment algorithms by stage and location of primary tumor, as these were found to be the 2 most important prognostic factors for men.1 The location of the primary tumor is most often in the bulbomembranous urethra, but up to one-third occur in the pendulous urethra.2
A recent review reported that UCC is the most common histologic subtype.4 When considering the differential diagnosis, a distal penile mass may represent a malignant penile lesion, such as squamous cell carcinoma, Buschke-Lowenstein tumor, Kaposi sarcoma, or precancerous lesions. Additional benign and infectious disorders include epidermoid and retention cysts, leukoplakia, balanitis xerotica obliterans, condyloma acuminatum, chancre/chancroid, lymphogranuloma venereum, granuloma inguinale, and tuberculosis. Clinical workup typically includes physical examination, cystourethroscopy and biopsy, chest X-ray, and pelvic/abdominal cross-sectional imaging.9,10 Magnetic resonance imaging of the abdomen and pelvis is ideal in identifying soft tissue structures and extension of tumor.
In male patients with PUC, nodal metastases are commonly seen at initial presentation in up to one-third of patients, while distant metastases may be present in up to 6% at presentation.2-4 When tumors arise from the anterior urethra, the primary lymphatic drainage is first to the inguinal lymph nodes, whereas posterior tumors drain to the pelvic lymph nodes. A multivariate analysis of men with PUC within the Surveillance, Epidemiology, and End Results database demonstrated an OS across all stages to be 46.2% and 29.3% at 5 and 10 years, respectively. Increased likelihood of death was predicted by advanced age, high grade/stage, systemic metastases, non-UCC histology, and the lack of surgery.4
Surgical intervention, including radical resection via penectomy, has been the mainstay in disease management and was first described by Marshall in 1957 for bulbar urethral cancer.11 In 1998, Gheiler and colleagues demonstrated that surgical resection alone yielded excellent outcomes in patients with low-stage disease with 89% of patients disease free at mean 42 months. This was in stark contrast to patients with advanced stage disease (T3 or N+) who exhibited a disease-free survival rate of 42% at the same follow-up interval and benefited from combined chemoradiation and surgical resection.3
In the presence of high-stage disease, multimodal therapy with chemotherapy, radiation, and/or surgery is warranted. A study in 2008 reviewed chemoradiation in which patients with PUC received a 5-week protocol of external beam radiotherapy to the genitals, inguinal/pelvic lymph nodes, plus an additional radiation bolus to the primary tumor.5 In the 18 patients reported, 15 had complete response to therapy, and only 4 patients required salvage surgical resection. The 7-year survival for the cohort was 72% with chemoradiation alone, with about half the population recurring or progressing at 7 years. However, all patients that avoided surgical resection went on to develop urethral strictures that required surgical therapy, 3 of which required complex reconstructive procedures.
To place this survival into context, the 1999 study by Dalbagni and colleagues reported a 5-year OS of 42% when surgical resection alone was performed in 40/46 men with PUC.2 Last, a large retrospective series of 44 patients reported mostly advanced-stage patients with PUC and analyzed patients treated with chemotherapy based on histologic pathology. The results demonstrated a 72% overall response rate to neoadjuvant chemotherapy, with a median OS of 32 months in patients undergoing chemotherapy vs 46 months in patients who underwent subsequent surgery. This study solidified that for patients with PUC involving the lymph nodes; optimal treatment includes neoadjuvant cisplatin-based chemotherapy followed by surgical resection.6
As medicine and oncologic therapies become more individualized, physicians are looking to new immunologic agents for systemic therapy. Immune checkpoint inhibitors were approved by the US Food and Drug Administration for UCC of the bladder in 2016.12 Unfortunately, due to the rarity of PUC and the recent development of immune checkpoint inhibitors, there have been no published reports of these or other immunotherapies in PUC. However, given the histologic similarity and pathogenesis, checkpoint inhibitors may have a future indication in the systemic management of this disease.
Conclusion
This patient’s PUC represents a rare presentation of a distal urethral carcinoma, T2-staged tumor, with rapid progression to nodal metastases. Additionally, the presentation of a fungating penile mass would usually indicate penile carcinoma, but providers should be aware of urethral carcinoma in the differential diagnosis. Notably, the patient was found to have progression to lymph node involvement during a mere 2-month period.
Recent case series have published encouraging results with neoadjuvant chemotherapy or chemoradiation.5,6 However, radical resection in men with T2 to T4 disease is associated with significantly higher cancer-specific survival. Given our concern of a loss to follow-up, we felt that radical resection of the primary tumor and adjuvant chemoradiation represented the patient’s best oncologic outcomes. Therefore, he underwent radical penectomy and creation of a perineal urethrostomy. As of his 6-month follow-up, he showed no evidence of disease, had returned to his preoperative functional status, and was referred for chemoradiation.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Swartz MA, Porter MP, Lin DW, Weiss NS. Incidence of primary urethral carcinoma in the United States. Urology. 2006;68(6):1164-1168.
2. Dalbagni G, Zhang ZF, Lacombe L, Herr HW. Male urethral carcinoma: analysis of treatment outcome. Urology. 1999;53(6):1126-1132.
3. Gheiler EL, Tefilli MV, Tiguert R, de Oliveira JG, Pontes JE, Wood DP Jr. Management of primary urethral cancer. Urology. 1998;52(3):487-493.
4. Rabbani F. Prognostic factors in male urethral cancer. Cancer. 2011;117(11):2426-2434.
5. Cohen MS, Triaca V, Billmeyer B, et al. Coordinated chemoradiation therapy with genital preservation for the treatment of primary invasive carcinoma of the male urethra. J Urol. 2008;179(2):536-541; discussion 541.
6. Dayyani F, Pettaway CA, Kamat AM, Munsell MF, Sircar K, Pagliaro LC. Retrospective analysis of survival outcomes and the role of cisplatin-based chemotherapy in patients with urethral carcinomas referred to medical oncologists. Urol Oncol. 2013;31(7):1171-1177.
7. American Joint Committee on Cancer. AJCC cancer staging manual. 8th ed. https://cancerstaging.org/references-tools/deskreferences/Documents/AJCC%20Cancer%20Staging%20Form%20Supplement.pdf. Updated June 5, 2018. Accessed January 22, 2019.
8. Gakis G, Witjes JA, Compérat E, et al. European Association of Urology guidelines on primary urethral carcinoma. https://uroweb.org/wp-content/uploads/EAU-Guidelines-Primary-Urethral-Carcinoma-2016-1.pdf. Updated March 2015. Accessed January 22, 2019
9. National Comprehensive Cancer Network. Bladder Cancer. Version 1.2019. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Updated December 20, 2018. Accessed January 17, 2019.
10. Dayyani F, Hoffman K, Eifel P, et al. Management of advanced primary urethral carcinomas. BJU Int. 2014;114(1):25-31.
11. Marshall VF. Radical excision of locally extensive carcinoma of the deep male urethra. J Urol. 1957;78(3):252-264.
12. Hsu FS, Su CH, Huang KH. A comprehensive review of US FDA-approved immune checkpoint inhibitors in urothelial carcinoma. J Immunol Res. 2017;2017:6940546.
1. Swartz MA, Porter MP, Lin DW, Weiss NS. Incidence of primary urethral carcinoma in the United States. Urology. 2006;68(6):1164-1168.
2. Dalbagni G, Zhang ZF, Lacombe L, Herr HW. Male urethral carcinoma: analysis of treatment outcome. Urology. 1999;53(6):1126-1132.
3. Gheiler EL, Tefilli MV, Tiguert R, de Oliveira JG, Pontes JE, Wood DP Jr. Management of primary urethral cancer. Urology. 1998;52(3):487-493.
4. Rabbani F. Prognostic factors in male urethral cancer. Cancer. 2011;117(11):2426-2434.
5. Cohen MS, Triaca V, Billmeyer B, et al. Coordinated chemoradiation therapy with genital preservation for the treatment of primary invasive carcinoma of the male urethra. J Urol. 2008;179(2):536-541; discussion 541.
6. Dayyani F, Pettaway CA, Kamat AM, Munsell MF, Sircar K, Pagliaro LC. Retrospective analysis of survival outcomes and the role of cisplatin-based chemotherapy in patients with urethral carcinomas referred to medical oncologists. Urol Oncol. 2013;31(7):1171-1177.
7. American Joint Committee on Cancer. AJCC cancer staging manual. 8th ed. https://cancerstaging.org/references-tools/deskreferences/Documents/AJCC%20Cancer%20Staging%20Form%20Supplement.pdf. Updated June 5, 2018. Accessed January 22, 2019.
8. Gakis G, Witjes JA, Compérat E, et al. European Association of Urology guidelines on primary urethral carcinoma. https://uroweb.org/wp-content/uploads/EAU-Guidelines-Primary-Urethral-Carcinoma-2016-1.pdf. Updated March 2015. Accessed January 22, 2019
9. National Comprehensive Cancer Network. Bladder Cancer. Version 1.2019. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Updated December 20, 2018. Accessed January 17, 2019.
10. Dayyani F, Hoffman K, Eifel P, et al. Management of advanced primary urethral carcinomas. BJU Int. 2014;114(1):25-31.
11. Marshall VF. Radical excision of locally extensive carcinoma of the deep male urethra. J Urol. 1957;78(3):252-264.
12. Hsu FS, Su CH, Huang KH. A comprehensive review of US FDA-approved immune checkpoint inhibitors in urothelial carcinoma. J Immunol Res. 2017;2017:6940546.
Skeletal-Related Events in Patients With Multiple Myeloma and Prostate Cancer Who Receive Standard vs Extended-Interval Bisphosphonate Dosing (FULL)
In patients with multiple myeloma and prostate cancer, extending the bisphosphonatedosing interval may help decrease medication-related morbidity without compromising therapeutic benefit.
Bone pain is one of the most common causes of morbidity in multiple myeloma (MM) and metastatic prostate cancer (CaP). This pain originates with the underlying pathologic processes of the cancer and with downstream skeletal-related events (SREs). SREs—fractures, spinal cord compression, and irradiation or surgery performed in ≥ 1 bone sites—represent a significant health care burden, particularly given the incidence of the underlying malignancies. According to American Cancer Society statistics, CaP is the second most common cancer in American men, and MM the second most common hematologic malignancy, despite its relatively low overall lifetime risk.1,2 Regardless of the underlying malignancy, bisphosphonates are the cornerstone of SRE prevention, though the optimal dosing strategy is the subject of clinical debate.
Although similar in SRE incidence, MM and CaP have distinct pathophysiologic processes in the dysregulation of bone resorption. MM is a hematologic malignancy that increases the risk of SREs by osteoclast up-regulation, primarily through the RANK (receptor activator of nuclear factor α-B) signaling pathway.3 CaP is a solid tumor malignancy that metastasizes to bone. Dysregulation of the bone resorption or formation cycle and net bone loss are a result of endogenous osteoclast up-regulation in response to abnormal bone formation in osteoblastic bone metastases.4 Androgen-deprivation therapy, the cornerstone of CaP treatment, further predisposes CaP patients to osteoporosis and SREs.
Prevention of SREs is pharmacologically driven by bisphosphonates, which have antiresorptive effects on bone through promotion of osteoclast apoptosis.5 Two IV formulations, pamidronate and zoledronic acid (ZA), are US Food and Drug Administration approved for use in bone metastases from MM or solid tumors.6-10 Although generally well tolerated, bisphosphonates can cause osteonecrosis of the jaw (ONJ), an avascular death of bone tissue, particularly with prolonged use.11 With its documented incidence of 5% to 6.7% in bone metastasis, ONJ represents a significant morbidity risk in patients with MM and CaP who are treated with IV bisphosphonates.12
Investigators are exploring bisphosphonate dosing intervals to determine which is most appropriate in mitigating the risk of ONJ. Before 2006, bisphosphonates were consistently dosed once monthly in patients with MM or metastatic bone disease—a standard derived empirically rather than from comparative studies or compelling pharmacodynamic data.13-15 In a 2006 consensus statement, the Mayo Clinic issued an expert opinion recommendation for increasing the bisphosphonate dosing interval to every 3 months in patients with MM.16 The first objective evidence for the clinical applicability of extending the ZA dosing interval was reported by Himelstein and colleagues in 2017.17 The randomized clinical trial found no differences in SRE rates when ZA was dosed every 12 weeks,17 prompting a conditional recommendation for dosing interval extension in the American Society of Clinical Oncology MM treatment guidelines (2018).13 Because of the age and racial demographics of the patients in these studies, many questions remain unanswered.
For the US Department of Veterans Affairs (VA) population, the pharmacokinetic and dynamic differences imposed by age and race limit the applicability of the available data. However, in veterans with MM or CaP, extending the bisphosphonate dosing interval may help decrease medication-related morbidity (eg, ONJ, nephrotoxicity) without compromising therapeutic benefit. To this end at the Memphis VA Medical Center (VAMC), we assessed for differences in SRE rates by comparing outcomes of patients who received ZA in standard- vs extended-interval dosing.
Methods
We retrospectively reviewed the Computerized Patient Record System for veterans with MM or metastatic CaP treated with ZA at the Memphis VAMC. Study inclusion criteria were aged > 18 years and care provided by a Memphis VAMC oncologist between January 2003 and January 2018. The study was approved by the Memphis VAMC’s Institutional Review Board, and procedures were followed in accordance with the ethical standards of its committee on human experimentation.
Using Microsoft SQL 2016 (Redmond, WA), we performed a query to identify patients who were prescribed ZA during the study period. Exclusion criteria were ZA prescribed for an indication other than MM or CaP (ie, osteoporosis) and receipt of ≤ 1 dose of ZA. Once a list was compiled, patients were stratified by ZA dosing interval: standard (mean, every month) or extended (mean, every 3 months). Patients whose ZA dosing interval was changed during treatment were included as independent data points in each group.
Skeletal-related events included fractures, spinal compression, irradiation, and surgery. Fractures and spinal compression were pertinent in the presence of radiographic documentation (eg, X-ray, magnetic resonance imaging scan) during the period the patient received ZA or within 1 dosing interval of the last recorded ZA dose. Irradiation was defined as documented application of radiation therapy to ≥ 1 bone sites for palliation of pain or as an intervention in the setting of spinal compression. Surgery was defined as any procedure performed to correct a fracture or spinal compression. Each SRE was counted as a single occurrence.
Osteonecrosis of the jaw was defined as radiographically documented necrosis of the mandible or associated structures with assessment by a VA dentist. Records from non-VA dental practices were not available for assessment. Documentation of dental assessment before the first dose of ZA and any assessments during treatment were recorded.
Medication use was assessed before and during ZA treatment. Number of ZA doses and reasons for any discontinuations were documented, as was concomitant use of calcium supplements, vitamin D supplements, calcitriol, paricalcitol, calcitonin, cinacalcet, and pamidronate.
The primary study outcome was observed difference in incidence of SREs between standard- and extended-interval dosing of ZA. Secondary outcomes included difference in incidence of ONJ as well as incidence of SREs and ONJ by disease subtype (MM, CaP).
Descriptive statistics were used to summarize demographic data and assess prespecified outcomes. Differences in rates of SREs and ONJ between dosing interval groups were analyzed with the Pearson χ2 test. The predetermined a priori level of significance was .05.
Results
Of the 300 patients prescribed ZA at the Memphis VAMC, 177 were excluded (96 for indication,78 for receiving only 1 dose of ZA, 3 for not receiving any doses of ZA). The remaining 123 patients were stratified into a standard-interval dosing group (121) and an extended-interval dosing group (35). Of the 123 patients, 33 received both standard- and extended-interval dosing of ZA over the course of the study period and were included discretely in each group for the duration of each dosing strategy.
Pre-ZA dental screenings were documented in 14% of standard-interval patients and 17% of extended-interval patients, and during-ZA screenings were documented in 17% of standard-interval patients and 20% of extended-interval patients. Chi-square analysis revealed no significant difference in rates of dental screening before or during use of ZA.
Standard-interval patients received a mean (SD) 11.4 (13.5) doses of ZA (range, 2-124). Extended-interval patients received a mean (SD) of 5.9 (3.18) doses (range, 2-14). All standard-interval patients had discontinued treatment at the time of the study, most commonly because of death or for an unknown reason. Sixty percent of extended-interval patients had discontinued treatment, most commonly because of patient/physician choice or for an unknown reason (Table 2).
Skeletal-related events were observed in 31% of standard-interval patients and 23% of extended-interval patients. There were no statistically significant differences in SRE rates between groups (P = .374). The most common SRE in both groups was bone irradiation (42% and 60%, respectively), with no statistically significant difference in proportion between groups (Table 4).
Discussion
This retrospective review of patients with MM and CaP receiving ZA for bone metastasesfound no differences in the rates of SREs when ZA was dosed monthly vs every 3 months.
Earlier studies found that ZA can decrease SRE rates, but a major concern is that frequent, prolonged exposure to IV bisphosphonates may increase the risk of ONJ. No significant differences in ONJ rates existed between dosing groups, but all documented cases of ONJ occurred in the standard-interval group, suggesting a trend toward decreased incidence with an extension of the dosing interval.
Limitations
This study had several limitations. Geriatric African American men comprised the majority of the study population, and patients with MM accounted for only 22% of included regimens, limiting external validity. Patient overlap between groups may have confounded the results. The retrospective design precluded the ability to control for confounding variables, such as concomitant medication use and medication adherence, and significant heterogeneity was noted in rates of adherence with ZA infusion schedules regardless of dosing group. Use of medications associated with increased risk of osteoporosis—including corticosteroids and proton pump inhibitors—was not assessed.
Assessment of ONJ incidence was limited by the lack of access to dental records from providers outside the VA. Many patients in this review were not eligible for VA dental benefits because of requirements involving time and service connection, a reimbursement measurement that reflects health conditions “incurred or aggravated during active military service.”18
The results of this study provide further support for extended-interval dosing of ZA as a potential method of increasing patient adherence and decreasing the possibility of adverse drug reactions without compromising therapeutic benefit. Further randomized controlled trials are needed to define the potential decrease in ONJ incidence.
Conclusion
In comparisons of standard- and extended-interval dosing of ZA, there was no difference in the incidence of skeletal-related events in veteran patients with bone metastases from MM or CaP.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018.
2. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review (CSR), 1975-2014 [based on November 2016 SEER data submission posted to SEER website April 2017]. Bethesda, MD: National Cancer Institute; 2017. https://seer.cancer.gov/archive/csr/1975_2014/. Accessed January 12, 2019.
3. Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23(3):435-441.
4. Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378(7):645-657.
5. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83(9):1032-1045.
6. Zometa [package insert]. East Hanover, NJ: Novartis; 2016.
7. Aredia [package insert]. East Hanover, NJ: Novartis; 2011.
8. Berenson JR, Rosen LS, Howell A, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases: a double-blind, randomized dose-response study [published correction appears in Cancer. 2001;91(10):1956]. Cancer. 2001;91(7):1191-1200.
9. Berenson JR, Lichtenstein A, Porter L, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med. 1996;334(8):488-493.
10. Mhaskar R, Redzepovic J, Wheatley K, et al. Bisphosphonates in multiple myeloma: a network meta-analysis. Cochrane Database Syst Rev. 2012;(5):CD003188.
11. Wu S, Dahut WL, Gulley JL. The use of bisphosphonates in cancer patients. Acta Oncol. 2007;46(5):581-591.
12. Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23(34):8580-8587.
13. Anderson K, Ismaila N, Flynn PJ, et al. Role of bone-modifying agents in multiple myeloma: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(8):812-818.
14. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines). Multiple Myeloma. Version 2.2019. https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf. Accessed January 29, 2019.
15. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines). Prostate Cancer. Version 4.2018. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed January 29, 2019.
16. Lacy MQ, Dispenzieri A, Gertz MA, et al. Mayo Clinic consensus statement for the use of bisphosphonates in multiple myeloma. Mayo Clin Proc. 2006;81(8):1047-1053.
17. Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer-interval vs. standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317(1):48-58.
18. Office of Public and Intergovernmental Affairs, US Department of Veterans Affairs. Service connected disabilities. In: Federal Benefits for Veterans, Dependents, and Survivors. https://www.va.gov/opa/publications/benefits_book/benefits_chap02.asp. Published April 2015. Accessed May 22, 2018.
In patients with multiple myeloma and prostate cancer, extending the bisphosphonatedosing interval may help decrease medication-related morbidity without compromising therapeutic benefit.
In patients with multiple myeloma and prostate cancer, extending the bisphosphonatedosing interval may help decrease medication-related morbidity without compromising therapeutic benefit.
Bone pain is one of the most common causes of morbidity in multiple myeloma (MM) and metastatic prostate cancer (CaP). This pain originates with the underlying pathologic processes of the cancer and with downstream skeletal-related events (SREs). SREs—fractures, spinal cord compression, and irradiation or surgery performed in ≥ 1 bone sites—represent a significant health care burden, particularly given the incidence of the underlying malignancies. According to American Cancer Society statistics, CaP is the second most common cancer in American men, and MM the second most common hematologic malignancy, despite its relatively low overall lifetime risk.1,2 Regardless of the underlying malignancy, bisphosphonates are the cornerstone of SRE prevention, though the optimal dosing strategy is the subject of clinical debate.
Although similar in SRE incidence, MM and CaP have distinct pathophysiologic processes in the dysregulation of bone resorption. MM is a hematologic malignancy that increases the risk of SREs by osteoclast up-regulation, primarily through the RANK (receptor activator of nuclear factor α-B) signaling pathway.3 CaP is a solid tumor malignancy that metastasizes to bone. Dysregulation of the bone resorption or formation cycle and net bone loss are a result of endogenous osteoclast up-regulation in response to abnormal bone formation in osteoblastic bone metastases.4 Androgen-deprivation therapy, the cornerstone of CaP treatment, further predisposes CaP patients to osteoporosis and SREs.
Prevention of SREs is pharmacologically driven by bisphosphonates, which have antiresorptive effects on bone through promotion of osteoclast apoptosis.5 Two IV formulations, pamidronate and zoledronic acid (ZA), are US Food and Drug Administration approved for use in bone metastases from MM or solid tumors.6-10 Although generally well tolerated, bisphosphonates can cause osteonecrosis of the jaw (ONJ), an avascular death of bone tissue, particularly with prolonged use.11 With its documented incidence of 5% to 6.7% in bone metastasis, ONJ represents a significant morbidity risk in patients with MM and CaP who are treated with IV bisphosphonates.12
Investigators are exploring bisphosphonate dosing intervals to determine which is most appropriate in mitigating the risk of ONJ. Before 2006, bisphosphonates were consistently dosed once monthly in patients with MM or metastatic bone disease—a standard derived empirically rather than from comparative studies or compelling pharmacodynamic data.13-15 In a 2006 consensus statement, the Mayo Clinic issued an expert opinion recommendation for increasing the bisphosphonate dosing interval to every 3 months in patients with MM.16 The first objective evidence for the clinical applicability of extending the ZA dosing interval was reported by Himelstein and colleagues in 2017.17 The randomized clinical trial found no differences in SRE rates when ZA was dosed every 12 weeks,17 prompting a conditional recommendation for dosing interval extension in the American Society of Clinical Oncology MM treatment guidelines (2018).13 Because of the age and racial demographics of the patients in these studies, many questions remain unanswered.
For the US Department of Veterans Affairs (VA) population, the pharmacokinetic and dynamic differences imposed by age and race limit the applicability of the available data. However, in veterans with MM or CaP, extending the bisphosphonate dosing interval may help decrease medication-related morbidity (eg, ONJ, nephrotoxicity) without compromising therapeutic benefit. To this end at the Memphis VA Medical Center (VAMC), we assessed for differences in SRE rates by comparing outcomes of patients who received ZA in standard- vs extended-interval dosing.
Methods
We retrospectively reviewed the Computerized Patient Record System for veterans with MM or metastatic CaP treated with ZA at the Memphis VAMC. Study inclusion criteria were aged > 18 years and care provided by a Memphis VAMC oncologist between January 2003 and January 2018. The study was approved by the Memphis VAMC’s Institutional Review Board, and procedures were followed in accordance with the ethical standards of its committee on human experimentation.
Using Microsoft SQL 2016 (Redmond, WA), we performed a query to identify patients who were prescribed ZA during the study period. Exclusion criteria were ZA prescribed for an indication other than MM or CaP (ie, osteoporosis) and receipt of ≤ 1 dose of ZA. Once a list was compiled, patients were stratified by ZA dosing interval: standard (mean, every month) or extended (mean, every 3 months). Patients whose ZA dosing interval was changed during treatment were included as independent data points in each group.
Skeletal-related events included fractures, spinal compression, irradiation, and surgery. Fractures and spinal compression were pertinent in the presence of radiographic documentation (eg, X-ray, magnetic resonance imaging scan) during the period the patient received ZA or within 1 dosing interval of the last recorded ZA dose. Irradiation was defined as documented application of radiation therapy to ≥ 1 bone sites for palliation of pain or as an intervention in the setting of spinal compression. Surgery was defined as any procedure performed to correct a fracture or spinal compression. Each SRE was counted as a single occurrence.
Osteonecrosis of the jaw was defined as radiographically documented necrosis of the mandible or associated structures with assessment by a VA dentist. Records from non-VA dental practices were not available for assessment. Documentation of dental assessment before the first dose of ZA and any assessments during treatment were recorded.
Medication use was assessed before and during ZA treatment. Number of ZA doses and reasons for any discontinuations were documented, as was concomitant use of calcium supplements, vitamin D supplements, calcitriol, paricalcitol, calcitonin, cinacalcet, and pamidronate.
The primary study outcome was observed difference in incidence of SREs between standard- and extended-interval dosing of ZA. Secondary outcomes included difference in incidence of ONJ as well as incidence of SREs and ONJ by disease subtype (MM, CaP).
Descriptive statistics were used to summarize demographic data and assess prespecified outcomes. Differences in rates of SREs and ONJ between dosing interval groups were analyzed with the Pearson χ2 test. The predetermined a priori level of significance was .05.
Results
Of the 300 patients prescribed ZA at the Memphis VAMC, 177 were excluded (96 for indication,78 for receiving only 1 dose of ZA, 3 for not receiving any doses of ZA). The remaining 123 patients were stratified into a standard-interval dosing group (121) and an extended-interval dosing group (35). Of the 123 patients, 33 received both standard- and extended-interval dosing of ZA over the course of the study period and were included discretely in each group for the duration of each dosing strategy.
Pre-ZA dental screenings were documented in 14% of standard-interval patients and 17% of extended-interval patients, and during-ZA screenings were documented in 17% of standard-interval patients and 20% of extended-interval patients. Chi-square analysis revealed no significant difference in rates of dental screening before or during use of ZA.
Standard-interval patients received a mean (SD) 11.4 (13.5) doses of ZA (range, 2-124). Extended-interval patients received a mean (SD) of 5.9 (3.18) doses (range, 2-14). All standard-interval patients had discontinued treatment at the time of the study, most commonly because of death or for an unknown reason. Sixty percent of extended-interval patients had discontinued treatment, most commonly because of patient/physician choice or for an unknown reason (Table 2).
Skeletal-related events were observed in 31% of standard-interval patients and 23% of extended-interval patients. There were no statistically significant differences in SRE rates between groups (P = .374). The most common SRE in both groups was bone irradiation (42% and 60%, respectively), with no statistically significant difference in proportion between groups (Table 4).
Discussion
This retrospective review of patients with MM and CaP receiving ZA for bone metastasesfound no differences in the rates of SREs when ZA was dosed monthly vs every 3 months.
Earlier studies found that ZA can decrease SRE rates, but a major concern is that frequent, prolonged exposure to IV bisphosphonates may increase the risk of ONJ. No significant differences in ONJ rates existed between dosing groups, but all documented cases of ONJ occurred in the standard-interval group, suggesting a trend toward decreased incidence with an extension of the dosing interval.
Limitations
This study had several limitations. Geriatric African American men comprised the majority of the study population, and patients with MM accounted for only 22% of included regimens, limiting external validity. Patient overlap between groups may have confounded the results. The retrospective design precluded the ability to control for confounding variables, such as concomitant medication use and medication adherence, and significant heterogeneity was noted in rates of adherence with ZA infusion schedules regardless of dosing group. Use of medications associated with increased risk of osteoporosis—including corticosteroids and proton pump inhibitors—was not assessed.
Assessment of ONJ incidence was limited by the lack of access to dental records from providers outside the VA. Many patients in this review were not eligible for VA dental benefits because of requirements involving time and service connection, a reimbursement measurement that reflects health conditions “incurred or aggravated during active military service.”18
The results of this study provide further support for extended-interval dosing of ZA as a potential method of increasing patient adherence and decreasing the possibility of adverse drug reactions without compromising therapeutic benefit. Further randomized controlled trials are needed to define the potential decrease in ONJ incidence.
Conclusion
In comparisons of standard- and extended-interval dosing of ZA, there was no difference in the incidence of skeletal-related events in veteran patients with bone metastases from MM or CaP.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Bone pain is one of the most common causes of morbidity in multiple myeloma (MM) and metastatic prostate cancer (CaP). This pain originates with the underlying pathologic processes of the cancer and with downstream skeletal-related events (SREs). SREs—fractures, spinal cord compression, and irradiation or surgery performed in ≥ 1 bone sites—represent a significant health care burden, particularly given the incidence of the underlying malignancies. According to American Cancer Society statistics, CaP is the second most common cancer in American men, and MM the second most common hematologic malignancy, despite its relatively low overall lifetime risk.1,2 Regardless of the underlying malignancy, bisphosphonates are the cornerstone of SRE prevention, though the optimal dosing strategy is the subject of clinical debate.
Although similar in SRE incidence, MM and CaP have distinct pathophysiologic processes in the dysregulation of bone resorption. MM is a hematologic malignancy that increases the risk of SREs by osteoclast up-regulation, primarily through the RANK (receptor activator of nuclear factor α-B) signaling pathway.3 CaP is a solid tumor malignancy that metastasizes to bone. Dysregulation of the bone resorption or formation cycle and net bone loss are a result of endogenous osteoclast up-regulation in response to abnormal bone formation in osteoblastic bone metastases.4 Androgen-deprivation therapy, the cornerstone of CaP treatment, further predisposes CaP patients to osteoporosis and SREs.
Prevention of SREs is pharmacologically driven by bisphosphonates, which have antiresorptive effects on bone through promotion of osteoclast apoptosis.5 Two IV formulations, pamidronate and zoledronic acid (ZA), are US Food and Drug Administration approved for use in bone metastases from MM or solid tumors.6-10 Although generally well tolerated, bisphosphonates can cause osteonecrosis of the jaw (ONJ), an avascular death of bone tissue, particularly with prolonged use.11 With its documented incidence of 5% to 6.7% in bone metastasis, ONJ represents a significant morbidity risk in patients with MM and CaP who are treated with IV bisphosphonates.12
Investigators are exploring bisphosphonate dosing intervals to determine which is most appropriate in mitigating the risk of ONJ. Before 2006, bisphosphonates were consistently dosed once monthly in patients with MM or metastatic bone disease—a standard derived empirically rather than from comparative studies or compelling pharmacodynamic data.13-15 In a 2006 consensus statement, the Mayo Clinic issued an expert opinion recommendation for increasing the bisphosphonate dosing interval to every 3 months in patients with MM.16 The first objective evidence for the clinical applicability of extending the ZA dosing interval was reported by Himelstein and colleagues in 2017.17 The randomized clinical trial found no differences in SRE rates when ZA was dosed every 12 weeks,17 prompting a conditional recommendation for dosing interval extension in the American Society of Clinical Oncology MM treatment guidelines (2018).13 Because of the age and racial demographics of the patients in these studies, many questions remain unanswered.
For the US Department of Veterans Affairs (VA) population, the pharmacokinetic and dynamic differences imposed by age and race limit the applicability of the available data. However, in veterans with MM or CaP, extending the bisphosphonate dosing interval may help decrease medication-related morbidity (eg, ONJ, nephrotoxicity) without compromising therapeutic benefit. To this end at the Memphis VA Medical Center (VAMC), we assessed for differences in SRE rates by comparing outcomes of patients who received ZA in standard- vs extended-interval dosing.
Methods
We retrospectively reviewed the Computerized Patient Record System for veterans with MM or metastatic CaP treated with ZA at the Memphis VAMC. Study inclusion criteria were aged > 18 years and care provided by a Memphis VAMC oncologist between January 2003 and January 2018. The study was approved by the Memphis VAMC’s Institutional Review Board, and procedures were followed in accordance with the ethical standards of its committee on human experimentation.
Using Microsoft SQL 2016 (Redmond, WA), we performed a query to identify patients who were prescribed ZA during the study period. Exclusion criteria were ZA prescribed for an indication other than MM or CaP (ie, osteoporosis) and receipt of ≤ 1 dose of ZA. Once a list was compiled, patients were stratified by ZA dosing interval: standard (mean, every month) or extended (mean, every 3 months). Patients whose ZA dosing interval was changed during treatment were included as independent data points in each group.
Skeletal-related events included fractures, spinal compression, irradiation, and surgery. Fractures and spinal compression were pertinent in the presence of radiographic documentation (eg, X-ray, magnetic resonance imaging scan) during the period the patient received ZA or within 1 dosing interval of the last recorded ZA dose. Irradiation was defined as documented application of radiation therapy to ≥ 1 bone sites for palliation of pain or as an intervention in the setting of spinal compression. Surgery was defined as any procedure performed to correct a fracture or spinal compression. Each SRE was counted as a single occurrence.
Osteonecrosis of the jaw was defined as radiographically documented necrosis of the mandible or associated structures with assessment by a VA dentist. Records from non-VA dental practices were not available for assessment. Documentation of dental assessment before the first dose of ZA and any assessments during treatment were recorded.
Medication use was assessed before and during ZA treatment. Number of ZA doses and reasons for any discontinuations were documented, as was concomitant use of calcium supplements, vitamin D supplements, calcitriol, paricalcitol, calcitonin, cinacalcet, and pamidronate.
The primary study outcome was observed difference in incidence of SREs between standard- and extended-interval dosing of ZA. Secondary outcomes included difference in incidence of ONJ as well as incidence of SREs and ONJ by disease subtype (MM, CaP).
Descriptive statistics were used to summarize demographic data and assess prespecified outcomes. Differences in rates of SREs and ONJ between dosing interval groups were analyzed with the Pearson χ2 test. The predetermined a priori level of significance was .05.
Results
Of the 300 patients prescribed ZA at the Memphis VAMC, 177 were excluded (96 for indication,78 for receiving only 1 dose of ZA, 3 for not receiving any doses of ZA). The remaining 123 patients were stratified into a standard-interval dosing group (121) and an extended-interval dosing group (35). Of the 123 patients, 33 received both standard- and extended-interval dosing of ZA over the course of the study period and were included discretely in each group for the duration of each dosing strategy.
Pre-ZA dental screenings were documented in 14% of standard-interval patients and 17% of extended-interval patients, and during-ZA screenings were documented in 17% of standard-interval patients and 20% of extended-interval patients. Chi-square analysis revealed no significant difference in rates of dental screening before or during use of ZA.
Standard-interval patients received a mean (SD) 11.4 (13.5) doses of ZA (range, 2-124). Extended-interval patients received a mean (SD) of 5.9 (3.18) doses (range, 2-14). All standard-interval patients had discontinued treatment at the time of the study, most commonly because of death or for an unknown reason. Sixty percent of extended-interval patients had discontinued treatment, most commonly because of patient/physician choice or for an unknown reason (Table 2).
Skeletal-related events were observed in 31% of standard-interval patients and 23% of extended-interval patients. There were no statistically significant differences in SRE rates between groups (P = .374). The most common SRE in both groups was bone irradiation (42% and 60%, respectively), with no statistically significant difference in proportion between groups (Table 4).
Discussion
This retrospective review of patients with MM and CaP receiving ZA for bone metastasesfound no differences in the rates of SREs when ZA was dosed monthly vs every 3 months.
Earlier studies found that ZA can decrease SRE rates, but a major concern is that frequent, prolonged exposure to IV bisphosphonates may increase the risk of ONJ. No significant differences in ONJ rates existed between dosing groups, but all documented cases of ONJ occurred in the standard-interval group, suggesting a trend toward decreased incidence with an extension of the dosing interval.
Limitations
This study had several limitations. Geriatric African American men comprised the majority of the study population, and patients with MM accounted for only 22% of included regimens, limiting external validity. Patient overlap between groups may have confounded the results. The retrospective design precluded the ability to control for confounding variables, such as concomitant medication use and medication adherence, and significant heterogeneity was noted in rates of adherence with ZA infusion schedules regardless of dosing group. Use of medications associated with increased risk of osteoporosis—including corticosteroids and proton pump inhibitors—was not assessed.
Assessment of ONJ incidence was limited by the lack of access to dental records from providers outside the VA. Many patients in this review were not eligible for VA dental benefits because of requirements involving time and service connection, a reimbursement measurement that reflects health conditions “incurred or aggravated during active military service.”18
The results of this study provide further support for extended-interval dosing of ZA as a potential method of increasing patient adherence and decreasing the possibility of adverse drug reactions without compromising therapeutic benefit. Further randomized controlled trials are needed to define the potential decrease in ONJ incidence.
Conclusion
In comparisons of standard- and extended-interval dosing of ZA, there was no difference in the incidence of skeletal-related events in veteran patients with bone metastases from MM or CaP.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018.
2. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review (CSR), 1975-2014 [based on November 2016 SEER data submission posted to SEER website April 2017]. Bethesda, MD: National Cancer Institute; 2017. https://seer.cancer.gov/archive/csr/1975_2014/. Accessed January 12, 2019.
3. Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23(3):435-441.
4. Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378(7):645-657.
5. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83(9):1032-1045.
6. Zometa [package insert]. East Hanover, NJ: Novartis; 2016.
7. Aredia [package insert]. East Hanover, NJ: Novartis; 2011.
8. Berenson JR, Rosen LS, Howell A, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases: a double-blind, randomized dose-response study [published correction appears in Cancer. 2001;91(10):1956]. Cancer. 2001;91(7):1191-1200.
9. Berenson JR, Lichtenstein A, Porter L, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med. 1996;334(8):488-493.
10. Mhaskar R, Redzepovic J, Wheatley K, et al. Bisphosphonates in multiple myeloma: a network meta-analysis. Cochrane Database Syst Rev. 2012;(5):CD003188.
11. Wu S, Dahut WL, Gulley JL. The use of bisphosphonates in cancer patients. Acta Oncol. 2007;46(5):581-591.
12. Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23(34):8580-8587.
13. Anderson K, Ismaila N, Flynn PJ, et al. Role of bone-modifying agents in multiple myeloma: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(8):812-818.
14. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines). Multiple Myeloma. Version 2.2019. https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf. Accessed January 29, 2019.
15. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines). Prostate Cancer. Version 4.2018. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed January 29, 2019.
16. Lacy MQ, Dispenzieri A, Gertz MA, et al. Mayo Clinic consensus statement for the use of bisphosphonates in multiple myeloma. Mayo Clin Proc. 2006;81(8):1047-1053.
17. Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer-interval vs. standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317(1):48-58.
18. Office of Public and Intergovernmental Affairs, US Department of Veterans Affairs. Service connected disabilities. In: Federal Benefits for Veterans, Dependents, and Survivors. https://www.va.gov/opa/publications/benefits_book/benefits_chap02.asp. Published April 2015. Accessed May 22, 2018.
1. American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018.
2. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review (CSR), 1975-2014 [based on November 2016 SEER data submission posted to SEER website April 2017]. Bethesda, MD: National Cancer Institute; 2017. https://seer.cancer.gov/archive/csr/1975_2014/. Accessed January 12, 2019.
3. Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23(3):435-441.
4. Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378(7):645-657.
5. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83(9):1032-1045.
6. Zometa [package insert]. East Hanover, NJ: Novartis; 2016.
7. Aredia [package insert]. East Hanover, NJ: Novartis; 2011.
8. Berenson JR, Rosen LS, Howell A, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases: a double-blind, randomized dose-response study [published correction appears in Cancer. 2001;91(10):1956]. Cancer. 2001;91(7):1191-1200.
9. Berenson JR, Lichtenstein A, Porter L, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med. 1996;334(8):488-493.
10. Mhaskar R, Redzepovic J, Wheatley K, et al. Bisphosphonates in multiple myeloma: a network meta-analysis. Cochrane Database Syst Rev. 2012;(5):CD003188.
11. Wu S, Dahut WL, Gulley JL. The use of bisphosphonates in cancer patients. Acta Oncol. 2007;46(5):581-591.
12. Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23(34):8580-8587.
13. Anderson K, Ismaila N, Flynn PJ, et al. Role of bone-modifying agents in multiple myeloma: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(8):812-818.
14. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines). Multiple Myeloma. Version 2.2019. https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf. Accessed January 29, 2019.
15. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines). Prostate Cancer. Version 4.2018. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed January 29, 2019.
16. Lacy MQ, Dispenzieri A, Gertz MA, et al. Mayo Clinic consensus statement for the use of bisphosphonates in multiple myeloma. Mayo Clin Proc. 2006;81(8):1047-1053.
17. Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer-interval vs. standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317(1):48-58.
18. Office of Public and Intergovernmental Affairs, US Department of Veterans Affairs. Service connected disabilities. In: Federal Benefits for Veterans, Dependents, and Survivors. https://www.va.gov/opa/publications/benefits_book/benefits_chap02.asp. Published April 2015. Accessed May 22, 2018.
Opioid use disorder in adolescents: An overview
Ms. L, age 17, seeks treatment because she has an ongoing struggle with multiple substances, including benzodiazepines, heroin, alcohol, cannabis, and prescription opioids.
She reports that she was 13 when she first used a prescription opioid that was not prescribed for her. She also reports engaging in unsafe sexual practices while using these substances, and has been diagnosed and treated for a sexually transmitted disease. She dropped out of school and is estranged from her family. She says that for a long time she has felt depressed and that she uses drugs to “self-medicate my emotions.” She endorses high anxiety and lack of motivation. Ms. L also reports having several criminal charges for theft, assault, and exchanging sex for drugs. She has undergone 3 admissions for detoxification, but promptly resumed using drugs, primarily heroin and oxycodone, immediately after discharge. Ms. L meets DSM-5 criteria for opioid use disorder (OUD).
Ms. L’s case illustrates a disturbing trend in the current opioid epidemic in the United States. Nearly 11.8 million individuals age ≥12 reported misuse of opioids in the last year.1 Adolescents who misuse prescription or illicit opioids are more likely to be involved with the legal system due to truancy, running away from home, physical altercations, prostitution, exchanging sex for drugs, robbery, and gang involvement. Adolescents who use opioids may also struggle with academic decline, drop out of school early, be unable to maintain a job, and have relationship difficulties, especially with family members.
In this article, I describe the scope of OUD among adolescents, including epidemiology, clinical manifestations, screening tools, and treatment approaches.
Scope of the problem
According to the most recent Monitoring the Future survey of more than 42,500 8th, 10th, and 12th grade students, 2.7% of 12th graders reported prescription opioid misuse (reported in the survey as “narcotics other than heroin”) in the past year.2 In addition, 0.4% of 12th graders reported heroin use over the same period.2 Although the prevalence of opioid use among adolescents has been declining over the past 5 years,2 it still represents a serious health crisis.
Part of the issue may relate to easier access to more potent opioids. For example, heroin available today can be >4 times purer than it was in the past. In 2002, t
Between 1997 and 2012, the annual incidence of youth (age 15 to 19) hospitalizations for prescription opioid poisoning increased >170%.5 Approximately 6% to 9% of youth involved in risky opioid use develop OUD 6 to 12 months after s
Continue to: In recent years...
In recent years, deaths from drug overdose have increased for all age groups; however, limited data is available regarding adolescent overdose deaths. According to the Centers for Disease Control and Prevention (CDC), from 2015 to 2016, drug overdose death rates for persons age 15 to 24 increased to 28%.9
How opioids work
Opioids activate specific transmembrane neurotransmitter receptors, including mu, kappa, and delta, in the CNS and peripheral nervous system (PNS). This leads to activation of G protein–mediated intracellular signal transduction. Mainly it is activation of endogenous mu opioid receptors that mediates the reward, withdrawal, and analgesic effects of opioids. These effects depend on the location of mu receptors. In the CNS, activation of mu opioid receptors may cause miosis, respiratory depression, euphoria, and analgesia.10
Different opioids vary in terms of their half-life; for most opioids, the half-life ranges from 2 to 4 hours.10 Heroin has a half-life of 30 minutes, but due to active metabolites its duration of action is 4 to 5 hours. Opioid metabolites can be detected in urine toxicology within approximately 1 to 2 days since last use.10
Chronic opioid use is associated with neurologic effects that change the function of areas of the brain that control pleasure/reward, stress, decision-making, and more. This leads to cravings, continued substance use, and dependence.11 After continued long-term use, patients report decreased euphoria, but typically they continue to use opioids to avoid withdrawal symptoms or worsening mood.
Criteria for opioid use disorder
In DSM-5, substance use disorders (SUDs)are no longer categorized as abuse or dependence.12 For opioids, the diagnosis is OUD. The Table12 outlines the DSM-5 criteria for OUD. Craving opioids is included for the first time in the OUD diagnosis. Having problems with the legal system is no longer considered a diagnostic criterion for OUD.
Continue to: A vulnerable population
A vulnerable population
As defined by Erik Erikson’s psychosocial stages of development, adolescents struggle between establishing their own identity vs role confusion.13 In an attempt to relate to peers or give in to peer pressure, some adolescents start by experimenting with nicotine, alcohol, and/or marijuana; however, some may move on to using other illicit drugs.14 Risk factors for the development of SUDs include early onset of substance use and a rapid progression through stages of substance use from experimentation to regular use, risky use, and dependence.15 In our case study, Ms. L’s substance use followed a similar pattern. Further, the comorbidity of SUDs and other psychiatric disorders may add a layer of complexity when caring for adolescents. Box 116-20 describes the relationship between comorbid psychiatric disorders and SUDs in adolescents.
Box 1
Disruptive behavior disorders are the most common coexisting psychiatric disorders in an adolescent with a substance use disorder (SUD), including opioid use disorder. These individuals typically present with aggression and other conduct disorder symptoms, and have early involvement with the legal system. Conversely, patients with conduct disorder are at high risk of early initiation of illicit substance use, including opioids. Early onset of substance use is a strong risk factor for developing an SUD.16
Mood disorders, particularly depression, can either precede or occur as a result of heavy and prolonged substance use.17 The estimated prevalence of major depressive disorder in individuals with an SUD is 24% to 50%. Among adolescents, an SUD is also a risk factor for suicidal ideation, suicide attempts, and completed suicide.18-20
Anxiety disorders, especially social phobia, and posttraumatic stress disorder are common in individuals with SUD.
Adolescents with SUD should be carefully evaluated for comorbid psychiatric disorders and treated accordingly.
Clinical manifestations
Common clinical manifestations of opioid use vary depending on when the patient is seen. An individual with OUD may appear acutely intoxicated, be in withdrawal, or show no effects. Chronic/prolonged use can lead to tolerance, such that a user needs to ingest larger amounts of the opioid to produce the same effects.
Acute intoxication can cause sedation, slurring of speech, and pinpoint pupils. Fresh injection sites may be visible on physical examination of IV users. The effects of acute intoxication usually depend on the half-life of the specific opioid and the individual’s tolerance.10 Tolerance to heroin can occur in 10 days and withdrawal can manifest in 3 to 7 hours after last use, depending on dose and purity.3 Tolerance can lead to unintentional overdose and death.
Withdrawal. Individuals experiencing withdrawal from opioids present with flu-like physical symptoms, including generalized body ache, rhinorrhea, diarrhea, goose bumps, lacrimation, and vomiting. Individuals also may experience irritability, restlessness, insomnia, anxiety, and depression during withdrawal.
Other manifestations. Excessive and chronic/prolonged opioid use can adversely impact socio-occupational functioning and cause academic decline in adolescents and youth. Personal relationships are significantly affected. Opioid users may have legal difficulties as a result of committing crimes such as theft, prostitution, or robbery in order to obtain opioids.
Continue to: Screening for OUD
Screening for OUD
Several screening tools are available to assess adolescents for SUDs, including OUD.
CRAFFT is a 6-item, clinician-administered screening tool that has been approved by American Academy of Pediatrics’ Committee on Substance Abuse for adolescents and young adults age <21.21-23 This commonly used tool can assess for alcohol, cannabis, and other drug use. A score ≥2 is considered positive for drug use, indicating that the individual would require further evaluation and assessment22,23 (Figure). There is also a self-administered CRAFFT questionnaire that can be completed by the patient.
NIDA-modified ASSIST. The American Psychiatric Association has adapted the National Institute on Drug Abuse (NIDA)-modified ASSIST. One version is designated for parents/guardians to administer to their children (age 6 to 17), and one is designated for adolescents (age 11 to 17) to self-administer.24,25 Each screening tool has 2 levels: Level 1 screens for substance use and other mental health symptoms, and Level 2 is more specific for substance use alone.
Drug Use Screening Inventory (DUSI) is a self-report questionnaire that has 149 items that assess the use of numerous drugs. It is designed to quantify the severity of consequences associated with drug and alcohol use.26,27
Problem-Oriented Screening Instrument for Teenagers (PO
Continue to: Personal Experience Screening Questionnaire (PESQ)...
Personal Experience Screening Questionnaire (PESQ) is a brief, 40-item, cost-effective, self-report questionnaire that can help identify adolescents (age 12 to 18) who should be referred for further evaluation.30
Addressing treatment expectations
For an adolescent with OUD, treatment should begin in the least restrictive environment that is perceived as safe for the patient. An adolescent’s readiness and motivation to achieve and maintain abstinence are crucial. Treatment planning should include the adolescent as well as his/her family to ensure they are able to verbalize their expectations. Start with a definitive treatment plan that addresses an individual’s needs. The plan should provide structure and an understanding of treatment expectations. The treatment team should clarify the realistic plan and goals based on empirical and clinical evidence. Treatment goals should include interventions to strengthen interpersonal relationships and assist with rehabilitation, such as establishing academic and/or vocational goals. Addressing readiness and working on a patient’s motivation is extremely important for most of these interventions.
In order for any intervention to be successful, clinicians need to establish and foster rapport with the adolescent. By law, substance use or behaviors related to substance use are not allowed to be shared outside the patient-clinician relationship, unless the adolescent gives consent or there are concerns that such behaviors might put the patient or others at risk. It is important to prime the adolescent and help them understand that any information pertaining to their safety or the safety of others may need to be shared outside the patient-clinician relationship.
Choosing an intervention
Less than 50% of a nationally representative sample of 345 addiction treatment programs serving adolescents and adults offer medications for treating OUD.31 Even in programs that offer pharmacotherapy, medications are significantly underutilized. Fewer than 30% of patients in addiction treatment programs receive medication, compared with 74% of patients receiving treatment for other mental health disorders.31 A
Psychotherapy may be used to treat OUD in adolescents. Several family therapies have been studied and are considered as critical psychotherapeutic interventions for treating SUDs, including structural family treatment and functional family therapy approaches.34 An integrated behavioral and family therapy model is also recommended for adolescent patients with SUDs. Cognitive distortions and use of self-deprecatory statements are common among adolescents.35 Therefore, using approaches of cognitive-behavioral therapy (CBT), or CBT plus motivational enhancement therapy, also might be effective for this population.36 The adolescent community reinforcement approach (A-CRA) is a behavioral treatment designed to help adolescents and their families learn how to lead a healthy and happy life without the use of drugs or alcohol by increasing access to social, familial, and educational/vocational reinforcers. Support groups and peer and family support should be encouraged as adjuncts to other interventions. In some areas, sober housing options for adolescents are also available.
Continue to: Harm-reduction strategies
Harm-reduction strategies. Although the primary goal of treatment for adolescents with OUD is to achieve and maintain abstinence from opioid use, implicit and explicit goals can be set. Short-term implicit goals may include harm-reduction strategies that emphasize decreasing the duration, frequency, and amount of substance use and limiting the chances of adverse effects, while the long-term explicit goal should be abstinence from opioid use.
Naloxone nasal spray is used as a harm-reduction strategy. It is an FDA-approved formulation that can reverse the effects of unintentional opioid overdoses and potentially prevent death from respiratory depression.37 Other harm-reduction strategies include needle exchange programs, which provide sterile needles to individuals who inject drugs in an effort to prevent or reduce the transmission of human immunodeficiency virus and other bloodborne viruses that can be spread via shared injection equipment. Fentanyl testing strips allow opioid users to test for the presence fentanyl and fentanyl analogs in the unregulated “street” opioid supply.
Pharmacologic interventions. Because there is limited empirical evidence on the efficacy of medication-assisted treatment (MAT) for adolescents with OUD, clinicians need to rely on evidence from research and experience with adults. Unfortunately, MAT is offered to adolescents considerably less often than it is to adults. Feder et al38 reported that only 2.4% of adolescents received MAT for heroin use and only 0.4% of adolescents received MAT for prescription opioid use, compared with 26.3% and 12% of adults, respectively.
Detoxification. Medications available for detoxification from opioids include opiates (such as methadone or buprenorphine) and clonidine (a central sympathomimetic). If the patient has used heroin for a short period (<1 year) and has no history of detoxification, consider a detoxification strategy with a longer-term taper (90 to 180 days) to allow for stabilization.
Maintenance treatment. Consider maintenance treatment for adolescents with a history of long-term opioid use and at least 2 prior short-term detoxification attempts or nonpharmacotherapy-based treatment within 12 months. Be sure to receive consent from a legal guardian and the patient. Maintenance treatment is usually recommended to continue for 1 to 6 years. Maintenance programs with longer durations have shown higher rates of abstinence, improved engagement, and retention in treatment.39
Continue to: According to guidelines from...
According to guidelines from the American Society of Addiction Medicine (ASAM), adolescents age >16 should be offered MAT; the first-line treatment is buprenorphine.40 To avoid risks of abuse and diversion, a combination of buprenorphine/naloxone may be administered.
Maintenance with buprenorphine
In order to prescribe and dispense buprenorphine, clinicians need to obtain a waiver from the Substance Abuse and Mental Health Services Administration. Before initiating buprenorphine, consider the type of opioid the individual used (short- or long-acting), the severity of the OUD, and the last reported use. The 3 phases of buprenorphine treatment are41:
- Induction phase. Buprenorphine can be initiated at 2 to 4 mg/d. Some patients may require up to 8 mg/d on the first day, which can be administered in divided doses.42 Evaluate and monitor patients carefully during the first few hours after the first dose. Patients should be in early withdrawal; otherwise, the buprenorphine might precipitate withdrawal. The induction phase can be completed in 2 to 4 days by titrating the dose so that the signs and symptoms of opioid withdrawal are minimal, and the patient is able to continue treatment. It may be helpful to have the patient’s legal guardian nearby in case the patient does not tolerate the medication or experiences withdrawal. The initial target dose for buprenorphine is approximately 12 to 16 mg/d.
- Stabilization phase. Patients no longer experience withdrawal symptoms and no longer have cravings. This phase can last 6 to 8 weeks. During this phase, patients should be seen weekly and doses should be adjusted if necessary. As a partial mu agonist, buprenorphine does not activate mu receptors fully and reaches a ceiling effect. Hence, doses >24 mg/d have limited added agonist properties.
- Maintenance phase. Because discontinuation of buprenorphine is associated with high relapse rates, patients may need to be maintained long-term on their stabilization dose, and for some patients, the length of time could be indefinite.39 During this phase, patients continue to undergo follow-up, but do so less frequently.
Methadone maintenance is generally not recommended for individuals age <18.
Preventing opioid diversion
Prescription medications that are kept in the home are a substantial source of opioids for adolescents. In 2014, 56% of 12th graders who did not need medications for medical purposes were able to acquire them from their friends or relatives; 36% of 12th graders used their own prescriptions.21 Limiting adolescents’ access to prescription opioids is the first line of prevention. Box 2 describes interventions and strategies to limit adolescents’ access to opioids.
Box 2
Many adolescents obtain opioids for recreational use from medications that were legitimately prescribed to family or friends. Both clinicians and parents/ guardians can take steps to reduce or prevent this type of diversion
Health care facilities. Regulating the number of pills dispensed to patients is crucial. It is highly recommended to prescribe only the minimal number of opioids necessary. In most cases, 3 to 7 days’ worth of opioids at a time might be sufficient, especially after surgical procedures.
Home. Families can limit adolescents’ access to prescription opioids in the home by keeping all medications in a lock box.
Proper disposal. Various entities offer locations for patients to drop off their unused opioids and other medications for safe disposal. These include police or fire departments and retail pharmacies. The US Drug Enforcement Administration sponsors a National Prescription Drug Take Back Day; see https://www.deadiversion.usdoj.gov/drug_disposal/takeback/index.html. The FDA also offers information on where and how to dispose of unused medicines at https://www.fda.gov/consumers/consumer-updates/where-and-how-dispose-unused-medicines.
CASE CONTINUED
Ms. L is initially prescribed, clonidine, 0.1 mg every 6 hours, to address opioid withdrawal. Clonidine is then tapered and maintained at 0.1 mg twice a day for irritability and impulse control. She is also prescribed sertraline, 100 mg/d, for depression and anxiety, and trazodone, 75 mg as needed at night, to assist with sleep.
Continue to: Following inpatient hospitalization...
Following inpatient hospitalization, during 12 weeks of partial hospital treatment, Ms. L participates in individual psychotherapy sessions 5 days/week; family therapy sessions once a week; and experiential therapy along with group sessions with other peers. She undergoes medication evaluations and adjustments on a weekly basis. Ms. L is now working at a store and is pursuing a high school equivalency certificate. She manages to avoid high-risk behaviors, although she reports having occasional cravings. Ms. L is actively involved in Narcotics Anonymous and has a sponsor. She has reconciled with her mother and moved back home, so she can stay away from her former acquaintances who are still using.
Bottom Line
Adolescents with opioid use disorder can benefit from an individualized treatment plan that includes psychosocial interventions, pharmacotherapy, or a combination of the two. Treatment planning should include the adolescent and his/her family to ensure they are able to verbalize their expectations. Treatment should focus on interventions that strengthen interpersonal relationships and assist with rehabilitation. Ongoing follow-up care is necessary for maintaining abstinence.
Related Resource
- Patkar AA, Weisler RH. Opioid abuse and overdose: Keep your patients safe. Current Psychiatry. 2017;16(8):8-12,14-16.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone
Clonidine • Clorpres
Methadone • Methadose
Naloxone • Narcan
Oxycodone • OxyContin
Sertraline • Zoloft
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
1. Davis JP, Prindle JJ, Eddie D, et al. Addressing the opioid epidemic with behavioral interventions for adolescents and young adults: a quasi-experimental design. J Consult Clin Psychol. 2019;87(10):941-951.
2. National Institute on Drug Abuse; National Institutes of Health; U.S. Department of Health and Human Services. Monitoring the Future Survey: High School and Youth Trends. https://www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends. Updated December 2019. Accessed January 13, 2020.
3. Hopfer CJ, Khuri E, Crowley TJ. Treating adolescent heroin use. J Am Acad Child Adolesc Psychiatry. 2003;42(5):609-611.
4. US Department of Justice, Drug Enforcement Agency, Diversion Control Division. https://www.deadiversion.usdoj.gov/. Accessed January 21, 2020.
5. Gaither JR, Leventhal JM, Ryan SA, et al. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997-2012. JAMA Pediatr. 2016;170(12):1195-1201.
6. Parker MA, Anthony JC. Epidemiological evidence on extra-medical use of prescription pain relievers: transitions from newly incident use to dependence among 12-21 year olds in United States using meta-analysis, 2002-13. Peer J. 2015;3:e1340. doi: 10.7717/peerj.1340. eCollection 2015.
7. Subramaniam GA, Fishman MJ, Woody G. Treatment of opioid-dependent adolescents and young adults with buprenorphine. Curr Psychiatry Rep. 2009;11(5):360-363.
8. Borodovsky JT, Levy S, Fishman M. Buprenorphine treatment for adolescents and young adults with opioid use disorders: a narrative review. J Addict Med. 2018;12(3):170-183.
9. Centers for Disease Control and Prevention: National Center for Health Statistics. Drug overdose deaths in the United States, 1999-2016. https://www.cdc.gov/nchs/products/databriefs/db294.htm. Published December 2017. Accessed January 15, 2020.
10. Strain E. Opioid use disorder: epidemiology, pharmacology, clinical manifestation, course, screening, assessment, diagnosis. https://www.uptodate.com/contents/opioid-use-disorder-epidemiology-pharmacology-clinical-manifestations-course-screening-assessment-and-diagnosis. Updated August 15, 2019. Accessed January 21, 2020.
11. American Academy of Pediatrics Committee on Substance Use and Prevention. Policy statement: medication-assisted treatment of adolescents with opioid use disorder. Pediatrics. 2016;138(3):e20161893. doi: https://doi.org/10.1542/peds.2016-1893.
12. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:514.
13. Sadock BJ, Sadock VA. Chapter 6: Theories of personality and psychopathology. In: Sadock BJ, Sadock VA, eds. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:209.
14. Kandel DB. Stages and pathways of drug involvement: examining the gateway hypothesis. Cambridge, United Kingdom: Cambridge University Press; 2002.
15. Robins LN, McEvoy L. Conduct problems as predictors of substance abuse. In: Robins LN, Rutter M, eds. Straight and devious pathways from childhood to adulthood. Cambridge, United Kingdom: Cambridge University Press; 1990;182-204.
16. Hopfer C, Salomonsen-Sautel S, Mikulich-Gilbertson S, et al. Conduct disorder and initiation of substance use: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2013;52(5):511-518.e4.
17. Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol. 2002;70(6):1224-1239.
18. Crumley FE. Substance abuse and adolescent suicidal behavior. JAMA. 1990;263(22):3051-3056.
19. Lewinsohn PM, Rohde P, Seeley JR. Adolescent suicidal ideation and attempts: prevalence, risk factors, and clinical implications. Clinical Psychology: Science and Practice. 1996;3(1):25-46.
20. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorder in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
21. Yule AM, Wilens TE, Rausch PK. The opioid epidemic: what a child psychiatrist is to do? J Am Acad Child Adolesc Psychiatry. 2017;56(7);541-543.
22. CRAFFT. https://crafft.org. Accessed January 21, 2020.
23. Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27(1):67-73.
24. American Psychiatric Association. Online assessment measures. https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures. Accessed January 15, 2020.
25. National Institute of Drug Abuse. American Psychiatric Association adapted NIDA modified ASSIST tools. https://www.drugabuse.gov/nidamed-medical-health-professionals/tool-resources-your-practice/screening-assessment-drug-testing-resources/american-psychiatric-association-adapted-nida. Updated November 15, 2015. Accessed January 21, 2020.
26. Canada’s Mental Health & Addiction Network. Drug Use Screening Inventory (DUSI). https://www.porticonetwork.ca/web/knowledgex-archive/amh-specialists/screening-for-cd-in-youth/screening-both-mh-sud/dusi. Published 2009. Accessed January 21, 2020.
27. Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am J Drug Alcohol Abuse. 1990;16(1-2):1-46.
28. Klitzner M, Gruenwald PJ, Taff GA, et al. The adolescent assessment referral system-final report. National Institute on Drug Abuse; Rockville, MD: 1993. NIDA Contract No. 271-89-8252.
29. Slesnick N, Tonigan JS. Assessment of alcohol and other drug use by runaway youths: a test-retest study of the Form 90. Alcohol Treat Q. 2004;22(2):21-34.
30. Winters KC, Kaminer Y. Screening and assessing adolescent substance use disorders in clinical populations. J Am Acad Child Adolesc Psychiatry. 2008;47(7):740-744.
31. Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011;5(1):21-27.
32. Deas D, Thomas SE. An overview of controlled study of adolescent substance abuse treatment. Am J Addiction. 2001;10(2):178-189.
33. William RJ, Chang, SY. A comprehensive and comparative review of adolescent substance abuse treatment outcome. Clinical Psychology: Science and Practice. 2000;7(2):138-166.
34. Bukstein OG, Work Group on Quality Issues. Practice parameters for the assessment and treatment of children and adolescents with substance use disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(6):609-621.
35. Van Hasselt VB, Null JA, Kempton T, et al. Social skills and depression in adolescent substance abusers. Addict Behav. 1993;18(1):9-18.
36. Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27(3):197-213.
37. US Food and Drug Administration. Information about naloxone. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-about-naloxone. Updated December 19, 2019. Accessed January 21, 2020.
38. Feder KA, Krawcyzk N, Saloner, B. Medication-assisted treatment for adolescents in specialty treatment for opioid use disorder. J Adolesc Health. 2018;60(6):747-750.
39. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003-2011.
40. US Department of Health and Human Services. Substance Abuse and Mental Health Ser-vices Administration. Medication-assisted treatment for opioid addiction in opioid treatment programs: a treatment improvement protocol TIP 43. https://www.asam.org/docs/advocacy/samhsa_tip43_matforopioidaddiction.pdf?sfvrsn=0. Published 2005. Accessed January 15, 2020.
41. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated September 9, 2019. Accessed January 21, 2020.
42. Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(suppl 2):S59-S77.
Ms. L, age 17, seeks treatment because she has an ongoing struggle with multiple substances, including benzodiazepines, heroin, alcohol, cannabis, and prescription opioids.
She reports that she was 13 when she first used a prescription opioid that was not prescribed for her. She also reports engaging in unsafe sexual practices while using these substances, and has been diagnosed and treated for a sexually transmitted disease. She dropped out of school and is estranged from her family. She says that for a long time she has felt depressed and that she uses drugs to “self-medicate my emotions.” She endorses high anxiety and lack of motivation. Ms. L also reports having several criminal charges for theft, assault, and exchanging sex for drugs. She has undergone 3 admissions for detoxification, but promptly resumed using drugs, primarily heroin and oxycodone, immediately after discharge. Ms. L meets DSM-5 criteria for opioid use disorder (OUD).
Ms. L’s case illustrates a disturbing trend in the current opioid epidemic in the United States. Nearly 11.8 million individuals age ≥12 reported misuse of opioids in the last year.1 Adolescents who misuse prescription or illicit opioids are more likely to be involved with the legal system due to truancy, running away from home, physical altercations, prostitution, exchanging sex for drugs, robbery, and gang involvement. Adolescents who use opioids may also struggle with academic decline, drop out of school early, be unable to maintain a job, and have relationship difficulties, especially with family members.
In this article, I describe the scope of OUD among adolescents, including epidemiology, clinical manifestations, screening tools, and treatment approaches.
Scope of the problem
According to the most recent Monitoring the Future survey of more than 42,500 8th, 10th, and 12th grade students, 2.7% of 12th graders reported prescription opioid misuse (reported in the survey as “narcotics other than heroin”) in the past year.2 In addition, 0.4% of 12th graders reported heroin use over the same period.2 Although the prevalence of opioid use among adolescents has been declining over the past 5 years,2 it still represents a serious health crisis.
Part of the issue may relate to easier access to more potent opioids. For example, heroin available today can be >4 times purer than it was in the past. In 2002, t
Between 1997 and 2012, the annual incidence of youth (age 15 to 19) hospitalizations for prescription opioid poisoning increased >170%.5 Approximately 6% to 9% of youth involved in risky opioid use develop OUD 6 to 12 months after s
Continue to: In recent years...
In recent years, deaths from drug overdose have increased for all age groups; however, limited data is available regarding adolescent overdose deaths. According to the Centers for Disease Control and Prevention (CDC), from 2015 to 2016, drug overdose death rates for persons age 15 to 24 increased to 28%.9
How opioids work
Opioids activate specific transmembrane neurotransmitter receptors, including mu, kappa, and delta, in the CNS and peripheral nervous system (PNS). This leads to activation of G protein–mediated intracellular signal transduction. Mainly it is activation of endogenous mu opioid receptors that mediates the reward, withdrawal, and analgesic effects of opioids. These effects depend on the location of mu receptors. In the CNS, activation of mu opioid receptors may cause miosis, respiratory depression, euphoria, and analgesia.10
Different opioids vary in terms of their half-life; for most opioids, the half-life ranges from 2 to 4 hours.10 Heroin has a half-life of 30 minutes, but due to active metabolites its duration of action is 4 to 5 hours. Opioid metabolites can be detected in urine toxicology within approximately 1 to 2 days since last use.10
Chronic opioid use is associated with neurologic effects that change the function of areas of the brain that control pleasure/reward, stress, decision-making, and more. This leads to cravings, continued substance use, and dependence.11 After continued long-term use, patients report decreased euphoria, but typically they continue to use opioids to avoid withdrawal symptoms or worsening mood.
Criteria for opioid use disorder
In DSM-5, substance use disorders (SUDs)are no longer categorized as abuse or dependence.12 For opioids, the diagnosis is OUD. The Table12 outlines the DSM-5 criteria for OUD. Craving opioids is included for the first time in the OUD diagnosis. Having problems with the legal system is no longer considered a diagnostic criterion for OUD.
Continue to: A vulnerable population
A vulnerable population
As defined by Erik Erikson’s psychosocial stages of development, adolescents struggle between establishing their own identity vs role confusion.13 In an attempt to relate to peers or give in to peer pressure, some adolescents start by experimenting with nicotine, alcohol, and/or marijuana; however, some may move on to using other illicit drugs.14 Risk factors for the development of SUDs include early onset of substance use and a rapid progression through stages of substance use from experimentation to regular use, risky use, and dependence.15 In our case study, Ms. L’s substance use followed a similar pattern. Further, the comorbidity of SUDs and other psychiatric disorders may add a layer of complexity when caring for adolescents. Box 116-20 describes the relationship between comorbid psychiatric disorders and SUDs in adolescents.
Box 1
Disruptive behavior disorders are the most common coexisting psychiatric disorders in an adolescent with a substance use disorder (SUD), including opioid use disorder. These individuals typically present with aggression and other conduct disorder symptoms, and have early involvement with the legal system. Conversely, patients with conduct disorder are at high risk of early initiation of illicit substance use, including opioids. Early onset of substance use is a strong risk factor for developing an SUD.16
Mood disorders, particularly depression, can either precede or occur as a result of heavy and prolonged substance use.17 The estimated prevalence of major depressive disorder in individuals with an SUD is 24% to 50%. Among adolescents, an SUD is also a risk factor for suicidal ideation, suicide attempts, and completed suicide.18-20
Anxiety disorders, especially social phobia, and posttraumatic stress disorder are common in individuals with SUD.
Adolescents with SUD should be carefully evaluated for comorbid psychiatric disorders and treated accordingly.
Clinical manifestations
Common clinical manifestations of opioid use vary depending on when the patient is seen. An individual with OUD may appear acutely intoxicated, be in withdrawal, or show no effects. Chronic/prolonged use can lead to tolerance, such that a user needs to ingest larger amounts of the opioid to produce the same effects.
Acute intoxication can cause sedation, slurring of speech, and pinpoint pupils. Fresh injection sites may be visible on physical examination of IV users. The effects of acute intoxication usually depend on the half-life of the specific opioid and the individual’s tolerance.10 Tolerance to heroin can occur in 10 days and withdrawal can manifest in 3 to 7 hours after last use, depending on dose and purity.3 Tolerance can lead to unintentional overdose and death.
Withdrawal. Individuals experiencing withdrawal from opioids present with flu-like physical symptoms, including generalized body ache, rhinorrhea, diarrhea, goose bumps, lacrimation, and vomiting. Individuals also may experience irritability, restlessness, insomnia, anxiety, and depression during withdrawal.
Other manifestations. Excessive and chronic/prolonged opioid use can adversely impact socio-occupational functioning and cause academic decline in adolescents and youth. Personal relationships are significantly affected. Opioid users may have legal difficulties as a result of committing crimes such as theft, prostitution, or robbery in order to obtain opioids.
Continue to: Screening for OUD
Screening for OUD
Several screening tools are available to assess adolescents for SUDs, including OUD.
CRAFFT is a 6-item, clinician-administered screening tool that has been approved by American Academy of Pediatrics’ Committee on Substance Abuse for adolescents and young adults age <21.21-23 This commonly used tool can assess for alcohol, cannabis, and other drug use. A score ≥2 is considered positive for drug use, indicating that the individual would require further evaluation and assessment22,23 (Figure). There is also a self-administered CRAFFT questionnaire that can be completed by the patient.
NIDA-modified ASSIST. The American Psychiatric Association has adapted the National Institute on Drug Abuse (NIDA)-modified ASSIST. One version is designated for parents/guardians to administer to their children (age 6 to 17), and one is designated for adolescents (age 11 to 17) to self-administer.24,25 Each screening tool has 2 levels: Level 1 screens for substance use and other mental health symptoms, and Level 2 is more specific for substance use alone.
Drug Use Screening Inventory (DUSI) is a self-report questionnaire that has 149 items that assess the use of numerous drugs. It is designed to quantify the severity of consequences associated with drug and alcohol use.26,27
Problem-Oriented Screening Instrument for Teenagers (PO
Continue to: Personal Experience Screening Questionnaire (PESQ)...
Personal Experience Screening Questionnaire (PESQ) is a brief, 40-item, cost-effective, self-report questionnaire that can help identify adolescents (age 12 to 18) who should be referred for further evaluation.30
Addressing treatment expectations
For an adolescent with OUD, treatment should begin in the least restrictive environment that is perceived as safe for the patient. An adolescent’s readiness and motivation to achieve and maintain abstinence are crucial. Treatment planning should include the adolescent as well as his/her family to ensure they are able to verbalize their expectations. Start with a definitive treatment plan that addresses an individual’s needs. The plan should provide structure and an understanding of treatment expectations. The treatment team should clarify the realistic plan and goals based on empirical and clinical evidence. Treatment goals should include interventions to strengthen interpersonal relationships and assist with rehabilitation, such as establishing academic and/or vocational goals. Addressing readiness and working on a patient’s motivation is extremely important for most of these interventions.
In order for any intervention to be successful, clinicians need to establish and foster rapport with the adolescent. By law, substance use or behaviors related to substance use are not allowed to be shared outside the patient-clinician relationship, unless the adolescent gives consent or there are concerns that such behaviors might put the patient or others at risk. It is important to prime the adolescent and help them understand that any information pertaining to their safety or the safety of others may need to be shared outside the patient-clinician relationship.
Choosing an intervention
Less than 50% of a nationally representative sample of 345 addiction treatment programs serving adolescents and adults offer medications for treating OUD.31 Even in programs that offer pharmacotherapy, medications are significantly underutilized. Fewer than 30% of patients in addiction treatment programs receive medication, compared with 74% of patients receiving treatment for other mental health disorders.31 A
Psychotherapy may be used to treat OUD in adolescents. Several family therapies have been studied and are considered as critical psychotherapeutic interventions for treating SUDs, including structural family treatment and functional family therapy approaches.34 An integrated behavioral and family therapy model is also recommended for adolescent patients with SUDs. Cognitive distortions and use of self-deprecatory statements are common among adolescents.35 Therefore, using approaches of cognitive-behavioral therapy (CBT), or CBT plus motivational enhancement therapy, also might be effective for this population.36 The adolescent community reinforcement approach (A-CRA) is a behavioral treatment designed to help adolescents and their families learn how to lead a healthy and happy life without the use of drugs or alcohol by increasing access to social, familial, and educational/vocational reinforcers. Support groups and peer and family support should be encouraged as adjuncts to other interventions. In some areas, sober housing options for adolescents are also available.
Continue to: Harm-reduction strategies
Harm-reduction strategies. Although the primary goal of treatment for adolescents with OUD is to achieve and maintain abstinence from opioid use, implicit and explicit goals can be set. Short-term implicit goals may include harm-reduction strategies that emphasize decreasing the duration, frequency, and amount of substance use and limiting the chances of adverse effects, while the long-term explicit goal should be abstinence from opioid use.
Naloxone nasal spray is used as a harm-reduction strategy. It is an FDA-approved formulation that can reverse the effects of unintentional opioid overdoses and potentially prevent death from respiratory depression.37 Other harm-reduction strategies include needle exchange programs, which provide sterile needles to individuals who inject drugs in an effort to prevent or reduce the transmission of human immunodeficiency virus and other bloodborne viruses that can be spread via shared injection equipment. Fentanyl testing strips allow opioid users to test for the presence fentanyl and fentanyl analogs in the unregulated “street” opioid supply.
Pharmacologic interventions. Because there is limited empirical evidence on the efficacy of medication-assisted treatment (MAT) for adolescents with OUD, clinicians need to rely on evidence from research and experience with adults. Unfortunately, MAT is offered to adolescents considerably less often than it is to adults. Feder et al38 reported that only 2.4% of adolescents received MAT for heroin use and only 0.4% of adolescents received MAT for prescription opioid use, compared with 26.3% and 12% of adults, respectively.
Detoxification. Medications available for detoxification from opioids include opiates (such as methadone or buprenorphine) and clonidine (a central sympathomimetic). If the patient has used heroin for a short period (<1 year) and has no history of detoxification, consider a detoxification strategy with a longer-term taper (90 to 180 days) to allow for stabilization.
Maintenance treatment. Consider maintenance treatment for adolescents with a history of long-term opioid use and at least 2 prior short-term detoxification attempts or nonpharmacotherapy-based treatment within 12 months. Be sure to receive consent from a legal guardian and the patient. Maintenance treatment is usually recommended to continue for 1 to 6 years. Maintenance programs with longer durations have shown higher rates of abstinence, improved engagement, and retention in treatment.39
Continue to: According to guidelines from...
According to guidelines from the American Society of Addiction Medicine (ASAM), adolescents age >16 should be offered MAT; the first-line treatment is buprenorphine.40 To avoid risks of abuse and diversion, a combination of buprenorphine/naloxone may be administered.
Maintenance with buprenorphine
In order to prescribe and dispense buprenorphine, clinicians need to obtain a waiver from the Substance Abuse and Mental Health Services Administration. Before initiating buprenorphine, consider the type of opioid the individual used (short- or long-acting), the severity of the OUD, and the last reported use. The 3 phases of buprenorphine treatment are41:
- Induction phase. Buprenorphine can be initiated at 2 to 4 mg/d. Some patients may require up to 8 mg/d on the first day, which can be administered in divided doses.42 Evaluate and monitor patients carefully during the first few hours after the first dose. Patients should be in early withdrawal; otherwise, the buprenorphine might precipitate withdrawal. The induction phase can be completed in 2 to 4 days by titrating the dose so that the signs and symptoms of opioid withdrawal are minimal, and the patient is able to continue treatment. It may be helpful to have the patient’s legal guardian nearby in case the patient does not tolerate the medication or experiences withdrawal. The initial target dose for buprenorphine is approximately 12 to 16 mg/d.
- Stabilization phase. Patients no longer experience withdrawal symptoms and no longer have cravings. This phase can last 6 to 8 weeks. During this phase, patients should be seen weekly and doses should be adjusted if necessary. As a partial mu agonist, buprenorphine does not activate mu receptors fully and reaches a ceiling effect. Hence, doses >24 mg/d have limited added agonist properties.
- Maintenance phase. Because discontinuation of buprenorphine is associated with high relapse rates, patients may need to be maintained long-term on their stabilization dose, and for some patients, the length of time could be indefinite.39 During this phase, patients continue to undergo follow-up, but do so less frequently.
Methadone maintenance is generally not recommended for individuals age <18.
Preventing opioid diversion
Prescription medications that are kept in the home are a substantial source of opioids for adolescents. In 2014, 56% of 12th graders who did not need medications for medical purposes were able to acquire them from their friends or relatives; 36% of 12th graders used their own prescriptions.21 Limiting adolescents’ access to prescription opioids is the first line of prevention. Box 2 describes interventions and strategies to limit adolescents’ access to opioids.
Box 2
Many adolescents obtain opioids for recreational use from medications that were legitimately prescribed to family or friends. Both clinicians and parents/ guardians can take steps to reduce or prevent this type of diversion
Health care facilities. Regulating the number of pills dispensed to patients is crucial. It is highly recommended to prescribe only the minimal number of opioids necessary. In most cases, 3 to 7 days’ worth of opioids at a time might be sufficient, especially after surgical procedures.
Home. Families can limit adolescents’ access to prescription opioids in the home by keeping all medications in a lock box.
Proper disposal. Various entities offer locations for patients to drop off their unused opioids and other medications for safe disposal. These include police or fire departments and retail pharmacies. The US Drug Enforcement Administration sponsors a National Prescription Drug Take Back Day; see https://www.deadiversion.usdoj.gov/drug_disposal/takeback/index.html. The FDA also offers information on where and how to dispose of unused medicines at https://www.fda.gov/consumers/consumer-updates/where-and-how-dispose-unused-medicines.
CASE CONTINUED
Ms. L is initially prescribed, clonidine, 0.1 mg every 6 hours, to address opioid withdrawal. Clonidine is then tapered and maintained at 0.1 mg twice a day for irritability and impulse control. She is also prescribed sertraline, 100 mg/d, for depression and anxiety, and trazodone, 75 mg as needed at night, to assist with sleep.
Continue to: Following inpatient hospitalization...
Following inpatient hospitalization, during 12 weeks of partial hospital treatment, Ms. L participates in individual psychotherapy sessions 5 days/week; family therapy sessions once a week; and experiential therapy along with group sessions with other peers. She undergoes medication evaluations and adjustments on a weekly basis. Ms. L is now working at a store and is pursuing a high school equivalency certificate. She manages to avoid high-risk behaviors, although she reports having occasional cravings. Ms. L is actively involved in Narcotics Anonymous and has a sponsor. She has reconciled with her mother and moved back home, so she can stay away from her former acquaintances who are still using.
Bottom Line
Adolescents with opioid use disorder can benefit from an individualized treatment plan that includes psychosocial interventions, pharmacotherapy, or a combination of the two. Treatment planning should include the adolescent and his/her family to ensure they are able to verbalize their expectations. Treatment should focus on interventions that strengthen interpersonal relationships and assist with rehabilitation. Ongoing follow-up care is necessary for maintaining abstinence.
Related Resource
- Patkar AA, Weisler RH. Opioid abuse and overdose: Keep your patients safe. Current Psychiatry. 2017;16(8):8-12,14-16.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone
Clonidine • Clorpres
Methadone • Methadose
Naloxone • Narcan
Oxycodone • OxyContin
Sertraline • Zoloft
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
Ms. L, age 17, seeks treatment because she has an ongoing struggle with multiple substances, including benzodiazepines, heroin, alcohol, cannabis, and prescription opioids.
She reports that she was 13 when she first used a prescription opioid that was not prescribed for her. She also reports engaging in unsafe sexual practices while using these substances, and has been diagnosed and treated for a sexually transmitted disease. She dropped out of school and is estranged from her family. She says that for a long time she has felt depressed and that she uses drugs to “self-medicate my emotions.” She endorses high anxiety and lack of motivation. Ms. L also reports having several criminal charges for theft, assault, and exchanging sex for drugs. She has undergone 3 admissions for detoxification, but promptly resumed using drugs, primarily heroin and oxycodone, immediately after discharge. Ms. L meets DSM-5 criteria for opioid use disorder (OUD).
Ms. L’s case illustrates a disturbing trend in the current opioid epidemic in the United States. Nearly 11.8 million individuals age ≥12 reported misuse of opioids in the last year.1 Adolescents who misuse prescription or illicit opioids are more likely to be involved with the legal system due to truancy, running away from home, physical altercations, prostitution, exchanging sex for drugs, robbery, and gang involvement. Adolescents who use opioids may also struggle with academic decline, drop out of school early, be unable to maintain a job, and have relationship difficulties, especially with family members.
In this article, I describe the scope of OUD among adolescents, including epidemiology, clinical manifestations, screening tools, and treatment approaches.
Scope of the problem
According to the most recent Monitoring the Future survey of more than 42,500 8th, 10th, and 12th grade students, 2.7% of 12th graders reported prescription opioid misuse (reported in the survey as “narcotics other than heroin”) in the past year.2 In addition, 0.4% of 12th graders reported heroin use over the same period.2 Although the prevalence of opioid use among adolescents has been declining over the past 5 years,2 it still represents a serious health crisis.
Part of the issue may relate to easier access to more potent opioids. For example, heroin available today can be >4 times purer than it was in the past. In 2002, t
Between 1997 and 2012, the annual incidence of youth (age 15 to 19) hospitalizations for prescription opioid poisoning increased >170%.5 Approximately 6% to 9% of youth involved in risky opioid use develop OUD 6 to 12 months after s
Continue to: In recent years...
In recent years, deaths from drug overdose have increased for all age groups; however, limited data is available regarding adolescent overdose deaths. According to the Centers for Disease Control and Prevention (CDC), from 2015 to 2016, drug overdose death rates for persons age 15 to 24 increased to 28%.9
How opioids work
Opioids activate specific transmembrane neurotransmitter receptors, including mu, kappa, and delta, in the CNS and peripheral nervous system (PNS). This leads to activation of G protein–mediated intracellular signal transduction. Mainly it is activation of endogenous mu opioid receptors that mediates the reward, withdrawal, and analgesic effects of opioids. These effects depend on the location of mu receptors. In the CNS, activation of mu opioid receptors may cause miosis, respiratory depression, euphoria, and analgesia.10
Different opioids vary in terms of their half-life; for most opioids, the half-life ranges from 2 to 4 hours.10 Heroin has a half-life of 30 minutes, but due to active metabolites its duration of action is 4 to 5 hours. Opioid metabolites can be detected in urine toxicology within approximately 1 to 2 days since last use.10
Chronic opioid use is associated with neurologic effects that change the function of areas of the brain that control pleasure/reward, stress, decision-making, and more. This leads to cravings, continued substance use, and dependence.11 After continued long-term use, patients report decreased euphoria, but typically they continue to use opioids to avoid withdrawal symptoms or worsening mood.
Criteria for opioid use disorder
In DSM-5, substance use disorders (SUDs)are no longer categorized as abuse or dependence.12 For opioids, the diagnosis is OUD. The Table12 outlines the DSM-5 criteria for OUD. Craving opioids is included for the first time in the OUD diagnosis. Having problems with the legal system is no longer considered a diagnostic criterion for OUD.
Continue to: A vulnerable population
A vulnerable population
As defined by Erik Erikson’s psychosocial stages of development, adolescents struggle between establishing their own identity vs role confusion.13 In an attempt to relate to peers or give in to peer pressure, some adolescents start by experimenting with nicotine, alcohol, and/or marijuana; however, some may move on to using other illicit drugs.14 Risk factors for the development of SUDs include early onset of substance use and a rapid progression through stages of substance use from experimentation to regular use, risky use, and dependence.15 In our case study, Ms. L’s substance use followed a similar pattern. Further, the comorbidity of SUDs and other psychiatric disorders may add a layer of complexity when caring for adolescents. Box 116-20 describes the relationship between comorbid psychiatric disorders and SUDs in adolescents.
Box 1
Disruptive behavior disorders are the most common coexisting psychiatric disorders in an adolescent with a substance use disorder (SUD), including opioid use disorder. These individuals typically present with aggression and other conduct disorder symptoms, and have early involvement with the legal system. Conversely, patients with conduct disorder are at high risk of early initiation of illicit substance use, including opioids. Early onset of substance use is a strong risk factor for developing an SUD.16
Mood disorders, particularly depression, can either precede or occur as a result of heavy and prolonged substance use.17 The estimated prevalence of major depressive disorder in individuals with an SUD is 24% to 50%. Among adolescents, an SUD is also a risk factor for suicidal ideation, suicide attempts, and completed suicide.18-20
Anxiety disorders, especially social phobia, and posttraumatic stress disorder are common in individuals with SUD.
Adolescents with SUD should be carefully evaluated for comorbid psychiatric disorders and treated accordingly.
Clinical manifestations
Common clinical manifestations of opioid use vary depending on when the patient is seen. An individual with OUD may appear acutely intoxicated, be in withdrawal, or show no effects. Chronic/prolonged use can lead to tolerance, such that a user needs to ingest larger amounts of the opioid to produce the same effects.
Acute intoxication can cause sedation, slurring of speech, and pinpoint pupils. Fresh injection sites may be visible on physical examination of IV users. The effects of acute intoxication usually depend on the half-life of the specific opioid and the individual’s tolerance.10 Tolerance to heroin can occur in 10 days and withdrawal can manifest in 3 to 7 hours after last use, depending on dose and purity.3 Tolerance can lead to unintentional overdose and death.
Withdrawal. Individuals experiencing withdrawal from opioids present with flu-like physical symptoms, including generalized body ache, rhinorrhea, diarrhea, goose bumps, lacrimation, and vomiting. Individuals also may experience irritability, restlessness, insomnia, anxiety, and depression during withdrawal.
Other manifestations. Excessive and chronic/prolonged opioid use can adversely impact socio-occupational functioning and cause academic decline in adolescents and youth. Personal relationships are significantly affected. Opioid users may have legal difficulties as a result of committing crimes such as theft, prostitution, or robbery in order to obtain opioids.
Continue to: Screening for OUD
Screening for OUD
Several screening tools are available to assess adolescents for SUDs, including OUD.
CRAFFT is a 6-item, clinician-administered screening tool that has been approved by American Academy of Pediatrics’ Committee on Substance Abuse for adolescents and young adults age <21.21-23 This commonly used tool can assess for alcohol, cannabis, and other drug use. A score ≥2 is considered positive for drug use, indicating that the individual would require further evaluation and assessment22,23 (Figure). There is also a self-administered CRAFFT questionnaire that can be completed by the patient.
NIDA-modified ASSIST. The American Psychiatric Association has adapted the National Institute on Drug Abuse (NIDA)-modified ASSIST. One version is designated for parents/guardians to administer to their children (age 6 to 17), and one is designated for adolescents (age 11 to 17) to self-administer.24,25 Each screening tool has 2 levels: Level 1 screens for substance use and other mental health symptoms, and Level 2 is more specific for substance use alone.
Drug Use Screening Inventory (DUSI) is a self-report questionnaire that has 149 items that assess the use of numerous drugs. It is designed to quantify the severity of consequences associated with drug and alcohol use.26,27
Problem-Oriented Screening Instrument for Teenagers (PO
Continue to: Personal Experience Screening Questionnaire (PESQ)...
Personal Experience Screening Questionnaire (PESQ) is a brief, 40-item, cost-effective, self-report questionnaire that can help identify adolescents (age 12 to 18) who should be referred for further evaluation.30
Addressing treatment expectations
For an adolescent with OUD, treatment should begin in the least restrictive environment that is perceived as safe for the patient. An adolescent’s readiness and motivation to achieve and maintain abstinence are crucial. Treatment planning should include the adolescent as well as his/her family to ensure they are able to verbalize their expectations. Start with a definitive treatment plan that addresses an individual’s needs. The plan should provide structure and an understanding of treatment expectations. The treatment team should clarify the realistic plan and goals based on empirical and clinical evidence. Treatment goals should include interventions to strengthen interpersonal relationships and assist with rehabilitation, such as establishing academic and/or vocational goals. Addressing readiness and working on a patient’s motivation is extremely important for most of these interventions.
In order for any intervention to be successful, clinicians need to establish and foster rapport with the adolescent. By law, substance use or behaviors related to substance use are not allowed to be shared outside the patient-clinician relationship, unless the adolescent gives consent or there are concerns that such behaviors might put the patient or others at risk. It is important to prime the adolescent and help them understand that any information pertaining to their safety or the safety of others may need to be shared outside the patient-clinician relationship.
Choosing an intervention
Less than 50% of a nationally representative sample of 345 addiction treatment programs serving adolescents and adults offer medications for treating OUD.31 Even in programs that offer pharmacotherapy, medications are significantly underutilized. Fewer than 30% of patients in addiction treatment programs receive medication, compared with 74% of patients receiving treatment for other mental health disorders.31 A
Psychotherapy may be used to treat OUD in adolescents. Several family therapies have been studied and are considered as critical psychotherapeutic interventions for treating SUDs, including structural family treatment and functional family therapy approaches.34 An integrated behavioral and family therapy model is also recommended for adolescent patients with SUDs. Cognitive distortions and use of self-deprecatory statements are common among adolescents.35 Therefore, using approaches of cognitive-behavioral therapy (CBT), or CBT plus motivational enhancement therapy, also might be effective for this population.36 The adolescent community reinforcement approach (A-CRA) is a behavioral treatment designed to help adolescents and their families learn how to lead a healthy and happy life without the use of drugs or alcohol by increasing access to social, familial, and educational/vocational reinforcers. Support groups and peer and family support should be encouraged as adjuncts to other interventions. In some areas, sober housing options for adolescents are also available.
Continue to: Harm-reduction strategies
Harm-reduction strategies. Although the primary goal of treatment for adolescents with OUD is to achieve and maintain abstinence from opioid use, implicit and explicit goals can be set. Short-term implicit goals may include harm-reduction strategies that emphasize decreasing the duration, frequency, and amount of substance use and limiting the chances of adverse effects, while the long-term explicit goal should be abstinence from opioid use.
Naloxone nasal spray is used as a harm-reduction strategy. It is an FDA-approved formulation that can reverse the effects of unintentional opioid overdoses and potentially prevent death from respiratory depression.37 Other harm-reduction strategies include needle exchange programs, which provide sterile needles to individuals who inject drugs in an effort to prevent or reduce the transmission of human immunodeficiency virus and other bloodborne viruses that can be spread via shared injection equipment. Fentanyl testing strips allow opioid users to test for the presence fentanyl and fentanyl analogs in the unregulated “street” opioid supply.
Pharmacologic interventions. Because there is limited empirical evidence on the efficacy of medication-assisted treatment (MAT) for adolescents with OUD, clinicians need to rely on evidence from research and experience with adults. Unfortunately, MAT is offered to adolescents considerably less often than it is to adults. Feder et al38 reported that only 2.4% of adolescents received MAT for heroin use and only 0.4% of adolescents received MAT for prescription opioid use, compared with 26.3% and 12% of adults, respectively.
Detoxification. Medications available for detoxification from opioids include opiates (such as methadone or buprenorphine) and clonidine (a central sympathomimetic). If the patient has used heroin for a short period (<1 year) and has no history of detoxification, consider a detoxification strategy with a longer-term taper (90 to 180 days) to allow for stabilization.
Maintenance treatment. Consider maintenance treatment for adolescents with a history of long-term opioid use and at least 2 prior short-term detoxification attempts or nonpharmacotherapy-based treatment within 12 months. Be sure to receive consent from a legal guardian and the patient. Maintenance treatment is usually recommended to continue for 1 to 6 years. Maintenance programs with longer durations have shown higher rates of abstinence, improved engagement, and retention in treatment.39
Continue to: According to guidelines from...
According to guidelines from the American Society of Addiction Medicine (ASAM), adolescents age >16 should be offered MAT; the first-line treatment is buprenorphine.40 To avoid risks of abuse and diversion, a combination of buprenorphine/naloxone may be administered.
Maintenance with buprenorphine
In order to prescribe and dispense buprenorphine, clinicians need to obtain a waiver from the Substance Abuse and Mental Health Services Administration. Before initiating buprenorphine, consider the type of opioid the individual used (short- or long-acting), the severity of the OUD, and the last reported use. The 3 phases of buprenorphine treatment are41:
- Induction phase. Buprenorphine can be initiated at 2 to 4 mg/d. Some patients may require up to 8 mg/d on the first day, which can be administered in divided doses.42 Evaluate and monitor patients carefully during the first few hours after the first dose. Patients should be in early withdrawal; otherwise, the buprenorphine might precipitate withdrawal. The induction phase can be completed in 2 to 4 days by titrating the dose so that the signs and symptoms of opioid withdrawal are minimal, and the patient is able to continue treatment. It may be helpful to have the patient’s legal guardian nearby in case the patient does not tolerate the medication or experiences withdrawal. The initial target dose for buprenorphine is approximately 12 to 16 mg/d.
- Stabilization phase. Patients no longer experience withdrawal symptoms and no longer have cravings. This phase can last 6 to 8 weeks. During this phase, patients should be seen weekly and doses should be adjusted if necessary. As a partial mu agonist, buprenorphine does not activate mu receptors fully and reaches a ceiling effect. Hence, doses >24 mg/d have limited added agonist properties.
- Maintenance phase. Because discontinuation of buprenorphine is associated with high relapse rates, patients may need to be maintained long-term on their stabilization dose, and for some patients, the length of time could be indefinite.39 During this phase, patients continue to undergo follow-up, but do so less frequently.
Methadone maintenance is generally not recommended for individuals age <18.
Preventing opioid diversion
Prescription medications that are kept in the home are a substantial source of opioids for adolescents. In 2014, 56% of 12th graders who did not need medications for medical purposes were able to acquire them from their friends or relatives; 36% of 12th graders used their own prescriptions.21 Limiting adolescents’ access to prescription opioids is the first line of prevention. Box 2 describes interventions and strategies to limit adolescents’ access to opioids.
Box 2
Many adolescents obtain opioids for recreational use from medications that were legitimately prescribed to family or friends. Both clinicians and parents/ guardians can take steps to reduce or prevent this type of diversion
Health care facilities. Regulating the number of pills dispensed to patients is crucial. It is highly recommended to prescribe only the minimal number of opioids necessary. In most cases, 3 to 7 days’ worth of opioids at a time might be sufficient, especially after surgical procedures.
Home. Families can limit adolescents’ access to prescription opioids in the home by keeping all medications in a lock box.
Proper disposal. Various entities offer locations for patients to drop off their unused opioids and other medications for safe disposal. These include police or fire departments and retail pharmacies. The US Drug Enforcement Administration sponsors a National Prescription Drug Take Back Day; see https://www.deadiversion.usdoj.gov/drug_disposal/takeback/index.html. The FDA also offers information on where and how to dispose of unused medicines at https://www.fda.gov/consumers/consumer-updates/where-and-how-dispose-unused-medicines.
CASE CONTINUED
Ms. L is initially prescribed, clonidine, 0.1 mg every 6 hours, to address opioid withdrawal. Clonidine is then tapered and maintained at 0.1 mg twice a day for irritability and impulse control. She is also prescribed sertraline, 100 mg/d, for depression and anxiety, and trazodone, 75 mg as needed at night, to assist with sleep.
Continue to: Following inpatient hospitalization...
Following inpatient hospitalization, during 12 weeks of partial hospital treatment, Ms. L participates in individual psychotherapy sessions 5 days/week; family therapy sessions once a week; and experiential therapy along with group sessions with other peers. She undergoes medication evaluations and adjustments on a weekly basis. Ms. L is now working at a store and is pursuing a high school equivalency certificate. She manages to avoid high-risk behaviors, although she reports having occasional cravings. Ms. L is actively involved in Narcotics Anonymous and has a sponsor. She has reconciled with her mother and moved back home, so she can stay away from her former acquaintances who are still using.
Bottom Line
Adolescents with opioid use disorder can benefit from an individualized treatment plan that includes psychosocial interventions, pharmacotherapy, or a combination of the two. Treatment planning should include the adolescent and his/her family to ensure they are able to verbalize their expectations. Treatment should focus on interventions that strengthen interpersonal relationships and assist with rehabilitation. Ongoing follow-up care is necessary for maintaining abstinence.
Related Resource
- Patkar AA, Weisler RH. Opioid abuse and overdose: Keep your patients safe. Current Psychiatry. 2017;16(8):8-12,14-16.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone
Clonidine • Clorpres
Methadone • Methadose
Naloxone • Narcan
Oxycodone • OxyContin
Sertraline • Zoloft
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
1. Davis JP, Prindle JJ, Eddie D, et al. Addressing the opioid epidemic with behavioral interventions for adolescents and young adults: a quasi-experimental design. J Consult Clin Psychol. 2019;87(10):941-951.
2. National Institute on Drug Abuse; National Institutes of Health; U.S. Department of Health and Human Services. Monitoring the Future Survey: High School and Youth Trends. https://www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends. Updated December 2019. Accessed January 13, 2020.
3. Hopfer CJ, Khuri E, Crowley TJ. Treating adolescent heroin use. J Am Acad Child Adolesc Psychiatry. 2003;42(5):609-611.
4. US Department of Justice, Drug Enforcement Agency, Diversion Control Division. https://www.deadiversion.usdoj.gov/. Accessed January 21, 2020.
5. Gaither JR, Leventhal JM, Ryan SA, et al. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997-2012. JAMA Pediatr. 2016;170(12):1195-1201.
6. Parker MA, Anthony JC. Epidemiological evidence on extra-medical use of prescription pain relievers: transitions from newly incident use to dependence among 12-21 year olds in United States using meta-analysis, 2002-13. Peer J. 2015;3:e1340. doi: 10.7717/peerj.1340. eCollection 2015.
7. Subramaniam GA, Fishman MJ, Woody G. Treatment of opioid-dependent adolescents and young adults with buprenorphine. Curr Psychiatry Rep. 2009;11(5):360-363.
8. Borodovsky JT, Levy S, Fishman M. Buprenorphine treatment for adolescents and young adults with opioid use disorders: a narrative review. J Addict Med. 2018;12(3):170-183.
9. Centers for Disease Control and Prevention: National Center for Health Statistics. Drug overdose deaths in the United States, 1999-2016. https://www.cdc.gov/nchs/products/databriefs/db294.htm. Published December 2017. Accessed January 15, 2020.
10. Strain E. Opioid use disorder: epidemiology, pharmacology, clinical manifestation, course, screening, assessment, diagnosis. https://www.uptodate.com/contents/opioid-use-disorder-epidemiology-pharmacology-clinical-manifestations-course-screening-assessment-and-diagnosis. Updated August 15, 2019. Accessed January 21, 2020.
11. American Academy of Pediatrics Committee on Substance Use and Prevention. Policy statement: medication-assisted treatment of adolescents with opioid use disorder. Pediatrics. 2016;138(3):e20161893. doi: https://doi.org/10.1542/peds.2016-1893.
12. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:514.
13. Sadock BJ, Sadock VA. Chapter 6: Theories of personality and psychopathology. In: Sadock BJ, Sadock VA, eds. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:209.
14. Kandel DB. Stages and pathways of drug involvement: examining the gateway hypothesis. Cambridge, United Kingdom: Cambridge University Press; 2002.
15. Robins LN, McEvoy L. Conduct problems as predictors of substance abuse. In: Robins LN, Rutter M, eds. Straight and devious pathways from childhood to adulthood. Cambridge, United Kingdom: Cambridge University Press; 1990;182-204.
16. Hopfer C, Salomonsen-Sautel S, Mikulich-Gilbertson S, et al. Conduct disorder and initiation of substance use: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2013;52(5):511-518.e4.
17. Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol. 2002;70(6):1224-1239.
18. Crumley FE. Substance abuse and adolescent suicidal behavior. JAMA. 1990;263(22):3051-3056.
19. Lewinsohn PM, Rohde P, Seeley JR. Adolescent suicidal ideation and attempts: prevalence, risk factors, and clinical implications. Clinical Psychology: Science and Practice. 1996;3(1):25-46.
20. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorder in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
21. Yule AM, Wilens TE, Rausch PK. The opioid epidemic: what a child psychiatrist is to do? J Am Acad Child Adolesc Psychiatry. 2017;56(7);541-543.
22. CRAFFT. https://crafft.org. Accessed January 21, 2020.
23. Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27(1):67-73.
24. American Psychiatric Association. Online assessment measures. https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures. Accessed January 15, 2020.
25. National Institute of Drug Abuse. American Psychiatric Association adapted NIDA modified ASSIST tools. https://www.drugabuse.gov/nidamed-medical-health-professionals/tool-resources-your-practice/screening-assessment-drug-testing-resources/american-psychiatric-association-adapted-nida. Updated November 15, 2015. Accessed January 21, 2020.
26. Canada’s Mental Health & Addiction Network. Drug Use Screening Inventory (DUSI). https://www.porticonetwork.ca/web/knowledgex-archive/amh-specialists/screening-for-cd-in-youth/screening-both-mh-sud/dusi. Published 2009. Accessed January 21, 2020.
27. Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am J Drug Alcohol Abuse. 1990;16(1-2):1-46.
28. Klitzner M, Gruenwald PJ, Taff GA, et al. The adolescent assessment referral system-final report. National Institute on Drug Abuse; Rockville, MD: 1993. NIDA Contract No. 271-89-8252.
29. Slesnick N, Tonigan JS. Assessment of alcohol and other drug use by runaway youths: a test-retest study of the Form 90. Alcohol Treat Q. 2004;22(2):21-34.
30. Winters KC, Kaminer Y. Screening and assessing adolescent substance use disorders in clinical populations. J Am Acad Child Adolesc Psychiatry. 2008;47(7):740-744.
31. Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011;5(1):21-27.
32. Deas D, Thomas SE. An overview of controlled study of adolescent substance abuse treatment. Am J Addiction. 2001;10(2):178-189.
33. William RJ, Chang, SY. A comprehensive and comparative review of adolescent substance abuse treatment outcome. Clinical Psychology: Science and Practice. 2000;7(2):138-166.
34. Bukstein OG, Work Group on Quality Issues. Practice parameters for the assessment and treatment of children and adolescents with substance use disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(6):609-621.
35. Van Hasselt VB, Null JA, Kempton T, et al. Social skills and depression in adolescent substance abusers. Addict Behav. 1993;18(1):9-18.
36. Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27(3):197-213.
37. US Food and Drug Administration. Information about naloxone. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-about-naloxone. Updated December 19, 2019. Accessed January 21, 2020.
38. Feder KA, Krawcyzk N, Saloner, B. Medication-assisted treatment for adolescents in specialty treatment for opioid use disorder. J Adolesc Health. 2018;60(6):747-750.
39. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003-2011.
40. US Department of Health and Human Services. Substance Abuse and Mental Health Ser-vices Administration. Medication-assisted treatment for opioid addiction in opioid treatment programs: a treatment improvement protocol TIP 43. https://www.asam.org/docs/advocacy/samhsa_tip43_matforopioidaddiction.pdf?sfvrsn=0. Published 2005. Accessed January 15, 2020.
41. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated September 9, 2019. Accessed January 21, 2020.
42. Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(suppl 2):S59-S77.
1. Davis JP, Prindle JJ, Eddie D, et al. Addressing the opioid epidemic with behavioral interventions for adolescents and young adults: a quasi-experimental design. J Consult Clin Psychol. 2019;87(10):941-951.
2. National Institute on Drug Abuse; National Institutes of Health; U.S. Department of Health and Human Services. Monitoring the Future Survey: High School and Youth Trends. https://www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends. Updated December 2019. Accessed January 13, 2020.
3. Hopfer CJ, Khuri E, Crowley TJ. Treating adolescent heroin use. J Am Acad Child Adolesc Psychiatry. 2003;42(5):609-611.
4. US Department of Justice, Drug Enforcement Agency, Diversion Control Division. https://www.deadiversion.usdoj.gov/. Accessed January 21, 2020.
5. Gaither JR, Leventhal JM, Ryan SA, et al. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997-2012. JAMA Pediatr. 2016;170(12):1195-1201.
6. Parker MA, Anthony JC. Epidemiological evidence on extra-medical use of prescription pain relievers: transitions from newly incident use to dependence among 12-21 year olds in United States using meta-analysis, 2002-13. Peer J. 2015;3:e1340. doi: 10.7717/peerj.1340. eCollection 2015.
7. Subramaniam GA, Fishman MJ, Woody G. Treatment of opioid-dependent adolescents and young adults with buprenorphine. Curr Psychiatry Rep. 2009;11(5):360-363.
8. Borodovsky JT, Levy S, Fishman M. Buprenorphine treatment for adolescents and young adults with opioid use disorders: a narrative review. J Addict Med. 2018;12(3):170-183.
9. Centers for Disease Control and Prevention: National Center for Health Statistics. Drug overdose deaths in the United States, 1999-2016. https://www.cdc.gov/nchs/products/databriefs/db294.htm. Published December 2017. Accessed January 15, 2020.
10. Strain E. Opioid use disorder: epidemiology, pharmacology, clinical manifestation, course, screening, assessment, diagnosis. https://www.uptodate.com/contents/opioid-use-disorder-epidemiology-pharmacology-clinical-manifestations-course-screening-assessment-and-diagnosis. Updated August 15, 2019. Accessed January 21, 2020.
11. American Academy of Pediatrics Committee on Substance Use and Prevention. Policy statement: medication-assisted treatment of adolescents with opioid use disorder. Pediatrics. 2016;138(3):e20161893. doi: https://doi.org/10.1542/peds.2016-1893.
12. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:514.
13. Sadock BJ, Sadock VA. Chapter 6: Theories of personality and psychopathology. In: Sadock BJ, Sadock VA, eds. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:209.
14. Kandel DB. Stages and pathways of drug involvement: examining the gateway hypothesis. Cambridge, United Kingdom: Cambridge University Press; 2002.
15. Robins LN, McEvoy L. Conduct problems as predictors of substance abuse. In: Robins LN, Rutter M, eds. Straight and devious pathways from childhood to adulthood. Cambridge, United Kingdom: Cambridge University Press; 1990;182-204.
16. Hopfer C, Salomonsen-Sautel S, Mikulich-Gilbertson S, et al. Conduct disorder and initiation of substance use: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2013;52(5):511-518.e4.
17. Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol. 2002;70(6):1224-1239.
18. Crumley FE. Substance abuse and adolescent suicidal behavior. JAMA. 1990;263(22):3051-3056.
19. Lewinsohn PM, Rohde P, Seeley JR. Adolescent suicidal ideation and attempts: prevalence, risk factors, and clinical implications. Clinical Psychology: Science and Practice. 1996;3(1):25-46.
20. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorder in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
21. Yule AM, Wilens TE, Rausch PK. The opioid epidemic: what a child psychiatrist is to do? J Am Acad Child Adolesc Psychiatry. 2017;56(7);541-543.
22. CRAFFT. https://crafft.org. Accessed January 21, 2020.
23. Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27(1):67-73.
24. American Psychiatric Association. Online assessment measures. https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures. Accessed January 15, 2020.
25. National Institute of Drug Abuse. American Psychiatric Association adapted NIDA modified ASSIST tools. https://www.drugabuse.gov/nidamed-medical-health-professionals/tool-resources-your-practice/screening-assessment-drug-testing-resources/american-psychiatric-association-adapted-nida. Updated November 15, 2015. Accessed January 21, 2020.
26. Canada’s Mental Health & Addiction Network. Drug Use Screening Inventory (DUSI). https://www.porticonetwork.ca/web/knowledgex-archive/amh-specialists/screening-for-cd-in-youth/screening-both-mh-sud/dusi. Published 2009. Accessed January 21, 2020.
27. Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am J Drug Alcohol Abuse. 1990;16(1-2):1-46.
28. Klitzner M, Gruenwald PJ, Taff GA, et al. The adolescent assessment referral system-final report. National Institute on Drug Abuse; Rockville, MD: 1993. NIDA Contract No. 271-89-8252.
29. Slesnick N, Tonigan JS. Assessment of alcohol and other drug use by runaway youths: a test-retest study of the Form 90. Alcohol Treat Q. 2004;22(2):21-34.
30. Winters KC, Kaminer Y. Screening and assessing adolescent substance use disorders in clinical populations. J Am Acad Child Adolesc Psychiatry. 2008;47(7):740-744.
31. Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011;5(1):21-27.
32. Deas D, Thomas SE. An overview of controlled study of adolescent substance abuse treatment. Am J Addiction. 2001;10(2):178-189.
33. William RJ, Chang, SY. A comprehensive and comparative review of adolescent substance abuse treatment outcome. Clinical Psychology: Science and Practice. 2000;7(2):138-166.
34. Bukstein OG, Work Group on Quality Issues. Practice parameters for the assessment and treatment of children and adolescents with substance use disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(6):609-621.
35. Van Hasselt VB, Null JA, Kempton T, et al. Social skills and depression in adolescent substance abusers. Addict Behav. 1993;18(1):9-18.
36. Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27(3):197-213.
37. US Food and Drug Administration. Information about naloxone. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-about-naloxone. Updated December 19, 2019. Accessed January 21, 2020.
38. Feder KA, Krawcyzk N, Saloner, B. Medication-assisted treatment for adolescents in specialty treatment for opioid use disorder. J Adolesc Health. 2018;60(6):747-750.
39. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003-2011.
40. US Department of Health and Human Services. Substance Abuse and Mental Health Ser-vices Administration. Medication-assisted treatment for opioid addiction in opioid treatment programs: a treatment improvement protocol TIP 43. https://www.asam.org/docs/advocacy/samhsa_tip43_matforopioidaddiction.pdf?sfvrsn=0. Published 2005. Accessed January 15, 2020.
41. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated September 9, 2019. Accessed January 21, 2020.
42. Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(suppl 2):S59-S77.
Top research findings of 2018-2019 for clinical practice
In Part 1 of this article, published in

1. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
In light of the association of major depressive disorder (MDD) with an increased risk of aging-related diseases, Han et al2 examined whether MDD was associated with higher epigenetic aging in blood as measured by DNA methylation patterns. They also studied whether clinical characteristics of MDD had a further impact on these patterns, and whether the findings replicated in brain tissue. Many differentially methylated regions of our DNA tend to change as we age. Han et al2 used these age-sensitive differentially methylated regions to estimate chronological age, using DNA extracted from various tissues, including blood and brain.
Study design
- As a part of the Netherlands Study of Depression and Anxiety (NESDA), this study included 811 patients with MDD and 319 control participants with no lifetime psychiatric disorders and low depressive symptoms (Inventory of Depressive Symptomatology score <14).
- Diagnosis of MDD and clinical characteristics were assessed by questionnaires and psychiatric interviews. Childhood trauma was assessed using the NEMESIS childhood trauma interview, which included a structured inventory of trauma exposure during childhood.
- DNA methylation age was estimated using all methylation sites in the blood of 811 patients with MDD and 319 control participants. The residuals of the DNA methylation age estimates regressed on chronological age were calculated to indicate epigenetic aging.
- Analyses were adjusted for sociodemographic characteristics, lifestyle, and health status.
- Postmortem brain samples of 74 patients with MDD and 64 control participants were used for replication.
Outcomes
- Significantly higher epigenetic aging was observed in patients with MDD compared with control participants (Cohen’s d = 0.18), which suggests that patients with MDD are biologically older than their corresponding chronological age. There was a significant dose effect with increasing symptom severity in the overall sample.
- In the MDD group, epigenetic aging was positively and significantly associated with childhood trauma.
- The case-control difference was replicated in an independent analysis of postmortem brain samples.
Conclusion
- These findings suggest that patients with MDD and people with a history of childhood trauma may biologically age relatively faster than those without MDD or childhood trauma. These findings may represent a biomarker of aging and might help identify patients who may benefit from early and intensive interventions to reduce the physical comorbidities of MDD.
- This study raises the possibility that MDD may be causally related to epigenetic age acceleration. However, it only points out the associations; there are other possible explanations for this correlation, including the possibility that a shared risk factor accounts for the observed association.
2. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
Delirium is common and often goes underdiagnosed. It is particularly prevalent among hospitalized geriatric patients. Several medications have been suggested to have a role in treating or preventing delirium. However, it remains uncertain which medications provide the best response rate, the lowest rate of delirium occurrence, and the best tolerability. In an attempt to find answers to these questions, Wu et al3 reviewed studies that evaluated the use of various medications used for delirium.
Study design
- Researchers conducted a systematic review and network meta-analysis of randomized controlled trials (RCTs) that investigated various pharmacologic agents used to treat or prevent delirium.
- Fifty-eight RCTs were included in the analyses. Of these, 20 RCTs with a total of 1,435 participants compared the outcomes of treatments of delirium, and 38 RCTs with a total of 8,168 participants examined prevention.
- A network meta-analysis was performed to determine if an agent or combinations of agents were superior to placebo or widely used medications.
Continue to: Outcomes
Outcomes
- Haloperidol plus lorazepam provided the best response rate for treating delirium compared with placebo/control.
- For delirium prevention, patients who received ramelteon, olanzapine, risperidone, or dexmedetomidine had significantly lower delirium occurrence rates than those receiving placebo/control.
- None of the pharmacologic treatments were significantly associated with a higher risk of all-cause mortality compared with placebo/control.
Conclusion
- Haloperidol plus lorazepam might be the best treatment and ramelteon the best preventive medicine for delirium. None of the pharmacologic interventions for treatment or prophylaxis increased all-cause mortality.
- However, network meta-analyses involve extrapolating treatment comparisons that are not made directly. As Blazer8 pointed out, both findings in this study (that haloperidol plus lorazepam is a unique intervention among the treatment trials and ramelteon is a unique intervention for prevention) seemed to be driven by 2 of the 58 studies that Wu et al3 examined.Wu et al3 also cautioned that both of these interventions needed to be further researched for efficacy.
3. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
While some evidence suggests that elevated brain noradrenergic activity is involved in the initiation and maintenance of alcohol use disorder,9 current medications used to treat alcohol use disorder do not target brain noradrenergic pathways. In an RCT, Simpson et al4 tested prazosin, an alpha-1 adrenergic receptor antagonist, for the treatment of alcohol use disorder.
Study design
- In this 12-week double-blind study, 92 participants with alcohol use disorder were randomly assigned to receive prazosin or placebo. Individuals with posttraumatic stress disorder were excluded.
- Prazosin was titrated to a target dosing schedule of 4 mg in the morning, 4 mg in the afternoon, and 8 mg at bedtime by the end of Week 2. The behavioral platform was medical management. Participants provided daily data on their alcohol consumption.
- Generalized linear mixed-effects models were used to examine the impact of prazosin compared with placebo on number of drinks per week, number of drinking days per week, and number of heavy drinking days per week.
Outcomes
- Among the 80 participants who completed the titration period and were included in the primary analyses, prazosin was associated with self-reported fewer heavy drinking days, and fewer drinks per week (Palatino LT Std−8 vs Palatino LT Std−1.5 with placebo). Drinking days per week and craving showed no group differences.
- The rate of drinking and the probability of heavy drinking showed a greater decrease over time for participants receiving prazosin compared with those receiving placebo.
Continue to: Conclusion
Conclusion
- These findings of moderate reductions in heavy drinking days and drinks per week with prazosin suggest that prazosin may be a promising harm-reduction treatment for alcohol use disorder.
4. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
Postpartum depression is among the most common complications of childbirth. It can result in considerable suffering for mothers, children, and families. Gamma-aminobutyric acid (GABA) signaling has previously been reported to be involved in the pathophysiology of postpartum depression. Meltzer-Brody et al5 conducted 2 double-blind, randomized, placebo-controlled, phase 3 trials comparing brexanolone with placebo in women with postpartum depression at 30 clinical research centers and specialized psychiatric units in the United States.
Study design
- Participants were women age 18 to 45, Palatino LT Std≤6 months postpartum at screening, with postpartum depression as indicated by a qualifying 17-item Hamilton Depression Rating Scale (HAM-D) score of ≥26 for Study 1 or 20 to 25 for Study 2.
- Of the 375 women who were screened simultaneously across both studies, 138 were randomly assigned (1:1:1) to receive a single IV injection of brexanolone, 90 μg/kg per hour (BRX90) (n = 45), brexanolone, 60 μg/kg per hour (BRX60) (n = 47), or placebo (n = 46) for 60 hours in Study 1, and 108 were randomly assigned (1:1) to receive BRX90 (n = 54) or placebo (n = 54) for 60 hours in Study 2.
- The primary efficacy endpoint was change in total score on the HAM-D from baseline to 60 hours. Patients were followed until Day 30.
Outcomes
- In Study 1, at 60 hours, the least-squares (LS) mean reduction in HAM-D total score from baseline was 19.5 points (standard error [SE] 1.2) in the BRX60 group and 17.7 points (SE 1.2) in the BRX90 group, compared with 14.0 points (SE 1.1) in the placebo group.
- In Study 2, at 60 hours, the LS mean reduction in HAM-D total score from baseline was 14.6 points (SE 0.8) in the BRX90 group compared with 12.1 points (SE 0.8) for the placebo group.
- In Study 1, one patient in the BRX60 group had 2 serious adverse events (suicidal ideation and intentional overdose attempt during follow-up). In Study 2, one patient in the BRX90 group had 2 serious adverse events (altered state of consciousness and syncope), which were considered treatment-related.
Conclusion
- Administration of brexanolone injection for postpartum depression resulted in significant, clinically meaningful reductions in HAM-D total score at 60 hours compared with placebo, with a rapid onset of action and durable treatment response during the study period. These results suggest that brexanolone injection has the potential to improve treatment options for women with this disorder.
Continue to: #5
5. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
In clinical practice, the use of multiple antipsychotic agents for the maintenance treatment of schizophrenia is common but generally not recommended. The effectiveness of antipsychotic polypharmacy in preventing relapse of schizophrenia has not been established, and whether specific antipsychotic combinations are superior to monotherapies for maintenance treatment of schizophrenia is unknown. Tiihonen et al6 investigated the association of specific antipsychotic combinations with psychiatric rehospitalization, which was used as a marker for relapse.
Study design
- This study included 62,250 patients with schizophrenia, treated between January 1, 1996 and December 31, 2015, in a comprehensive, nationwide cohort in Finland. Overall, 31,257 individuals (50.2%) were men, and the median age was 45.6 (interquartile range, 34.6 to 57.9).
- Patients were receiving 29 different antipsychotic monotherapy or polypharmacy regimens.
- Researchers analyzed data from April 24 to June 15, 2018 using psychiatric rehospitalization as a marker for relapse. To minimize selection bias, rehospitalization risks were investigated using within-individual analyses.
- The main outcome was the hazard ratio (HR) for psychiatric rehospitalization during use of polypharmacy vs monotherapy by the same patient.
Outcomes
- Clozapine plus aripiprazole was associated with the lowest risk of psychiatric rehospitalization, with a difference of 14% (HR, .86; CI, .79 to .94) compared with clozapine monotherapy in the analysis that included all polypharmacy periods, and 18% (HR, .82; CI, .75 to .89) in the conservatively defined polypharmacy analysis that excluded periods <90 days.
- Among patients experiencing their first episode of schizophrenia, the differences between clozapine plus aripiprazole vs clozapine monotherapy were greater, with a difference of 22% in the analysis that included all polypharmacy periods, and 23% in the conservatively defined polypharmacy analysis.
- At the aggregate level, any antipsychotic polypharmacy was associated with a 7% to 13% lower risk of psychiatric rehospitalization compared with any monotherapy.
- Clozapine was the only monotherapy among the 10 best treatments.
- Results on all-cause and somatic hospitalization, mortality, and other sensitivity analyses were in line with the primary outcomes.
Conclusion
- This study suggests that certain types of antipsychotic polypharmacy may reduce the risk of rehospitalization in patients with schizophrenia. Current treatment guidelines state that clinicians should prefer antipsychotic monotherapy and avoid polypharmacy. Tiihonen et al6 raise the question whether current treatment guidelines should continue to discourage antipsychotic polypharmacy in the maintenance treatment of schizophrenia.
- Despite the large administrative databases and sophisticated statistical methods used in this study, this approach has important limitations. As Goff10 points out, despite efforts to minimize bias, these results should be considered preliminary until confirmed by RCTs.
6. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
In routine clinical practice, patients with schizophrenia are often treated with combinations of antipsychotics and other psychotropic medications. However, there is little evidence about the comparative effectiveness of these adjunctive treatment strategies. Stroup et al7 investigated the comparative real-world effectiveness of adjunctive psychotropic treatments for patients with schizophrenia.
Continue to: Study design
Study design
- This comparative effectiveness study used US Medicaid data from January 1, 2001, to December 31, 2010. Data analysis was performed from January 1, 2017, to June 30, 2018.
- The study cohort included 81,921 adult outpatients diagnosed with schizophrenia with a mean age of 40.7 (range: 18 to 64), including 37,515 women (45.8%). All patients were stably treated with a single antipsychotic and then started on an adjunctive antidepressant (n = 31,117), benzodiazepine (n = 11,941), mood stabilizer (n = 12,849), or another antipsychotic (n = 26,014).
- Researchers used multinomial logistic regression models to estimate propensity scores to balance covariates across the 4 medication groups. Weighted Cox proportional hazards regression models were used to compare treatment outcomes during 365 days on an intention-to-treat basis.
- The main outcomes and measures included risk of hospitalization for a mental disorder (primary), emergency department (ED) visits for a mental disorder, and all-cause mortality.
Outcomes
- Compared with starting another antipsychotic, initiating use of an antidepressant was associated with a lower risk of psychiatric hospitalization, and initiating use of a benzodiazepine was associated with a higher risk. Initiating use of a mood stabilizer was not significantly different from initiating use of another antipsychotic.
- A similar pattern of associations was observed in psychiatric ED visits for initiating use of an antidepressant, benzodiazepine, or mood stabilizer.
- Initiating use of a mood stabilizer was associated with an increased risk of mortality.
Conclusion
- Compared with the addition of a second antipsychotic, adding an antidepressant was associated with substantially reduced rates of hospitalization, whereas adding a benzodiazepine was associated with a modest increase in the risk of hospitalization. While the addition of a mood stabilizer was not associated with a significant difference in the risk of hospitalization, it was associated with higher mortality.
- Despite the limitations associated with this study, the associations of benzodiazepines and mood stabilizers with poorer outcomes warrant clinical caution and further investigation.
Bottom Line
Significantly higher epigenetic aging has been observed in patients with major depressive disorder. Haloperidol plus lorazepam might be an effective treatment for delirium; and ramelteon may be effective for preventing delirium. Prazosin reduces heavy drinking in patients with alcohol use disorder. A 60-hour infusion of brexanolone can help alleviate postpartum depression. Clozapine plus aripiprazole reduces the risk of rehospitalization among patients with schizophrenia. Adding an antidepressant to an antipsychotic also can reduce the risk of rehospitalization among patients with schizophrenia.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Aripiprazole • Abilify
Brexanolone • Zulresso
Clozapine • Clozaril
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Ramelteon • Rozerem
Risperidone • Risperdal
1. Saeed SA, Stanley JB. Top research findings of 2018-2019. First of 2 parts. Current Psychiatry. 2020;19(1):13-18.
2. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
3. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
4. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
5. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
6. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
7. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
8. Blazer DG. Pharmacologic intervention for the treatment and prevention of delirium: looking beneath the modeling. JAMA Psychiatry. 2019;76(5):472-473.
9. Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61-75.
10. Goff DC. Can adjunctive pharmacotherapy reduce hospitalization in schizophrenia? Insights from administrative databases. JAMA Psychiatry. 2019;76(5):468-469.
In Part 1 of this article, published in

1. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
In light of the association of major depressive disorder (MDD) with an increased risk of aging-related diseases, Han et al2 examined whether MDD was associated with higher epigenetic aging in blood as measured by DNA methylation patterns. They also studied whether clinical characteristics of MDD had a further impact on these patterns, and whether the findings replicated in brain tissue. Many differentially methylated regions of our DNA tend to change as we age. Han et al2 used these age-sensitive differentially methylated regions to estimate chronological age, using DNA extracted from various tissues, including blood and brain.
Study design
- As a part of the Netherlands Study of Depression and Anxiety (NESDA), this study included 811 patients with MDD and 319 control participants with no lifetime psychiatric disorders and low depressive symptoms (Inventory of Depressive Symptomatology score <14).
- Diagnosis of MDD and clinical characteristics were assessed by questionnaires and psychiatric interviews. Childhood trauma was assessed using the NEMESIS childhood trauma interview, which included a structured inventory of trauma exposure during childhood.
- DNA methylation age was estimated using all methylation sites in the blood of 811 patients with MDD and 319 control participants. The residuals of the DNA methylation age estimates regressed on chronological age were calculated to indicate epigenetic aging.
- Analyses were adjusted for sociodemographic characteristics, lifestyle, and health status.
- Postmortem brain samples of 74 patients with MDD and 64 control participants were used for replication.
Outcomes
- Significantly higher epigenetic aging was observed in patients with MDD compared with control participants (Cohen’s d = 0.18), which suggests that patients with MDD are biologically older than their corresponding chronological age. There was a significant dose effect with increasing symptom severity in the overall sample.
- In the MDD group, epigenetic aging was positively and significantly associated with childhood trauma.
- The case-control difference was replicated in an independent analysis of postmortem brain samples.
Conclusion
- These findings suggest that patients with MDD and people with a history of childhood trauma may biologically age relatively faster than those without MDD or childhood trauma. These findings may represent a biomarker of aging and might help identify patients who may benefit from early and intensive interventions to reduce the physical comorbidities of MDD.
- This study raises the possibility that MDD may be causally related to epigenetic age acceleration. However, it only points out the associations; there are other possible explanations for this correlation, including the possibility that a shared risk factor accounts for the observed association.
2. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
Delirium is common and often goes underdiagnosed. It is particularly prevalent among hospitalized geriatric patients. Several medications have been suggested to have a role in treating or preventing delirium. However, it remains uncertain which medications provide the best response rate, the lowest rate of delirium occurrence, and the best tolerability. In an attempt to find answers to these questions, Wu et al3 reviewed studies that evaluated the use of various medications used for delirium.
Study design
- Researchers conducted a systematic review and network meta-analysis of randomized controlled trials (RCTs) that investigated various pharmacologic agents used to treat or prevent delirium.
- Fifty-eight RCTs were included in the analyses. Of these, 20 RCTs with a total of 1,435 participants compared the outcomes of treatments of delirium, and 38 RCTs with a total of 8,168 participants examined prevention.
- A network meta-analysis was performed to determine if an agent or combinations of agents were superior to placebo or widely used medications.
Continue to: Outcomes
Outcomes
- Haloperidol plus lorazepam provided the best response rate for treating delirium compared with placebo/control.
- For delirium prevention, patients who received ramelteon, olanzapine, risperidone, or dexmedetomidine had significantly lower delirium occurrence rates than those receiving placebo/control.
- None of the pharmacologic treatments were significantly associated with a higher risk of all-cause mortality compared with placebo/control.
Conclusion
- Haloperidol plus lorazepam might be the best treatment and ramelteon the best preventive medicine for delirium. None of the pharmacologic interventions for treatment or prophylaxis increased all-cause mortality.
- However, network meta-analyses involve extrapolating treatment comparisons that are not made directly. As Blazer8 pointed out, both findings in this study (that haloperidol plus lorazepam is a unique intervention among the treatment trials and ramelteon is a unique intervention for prevention) seemed to be driven by 2 of the 58 studies that Wu et al3 examined.Wu et al3 also cautioned that both of these interventions needed to be further researched for efficacy.
3. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
While some evidence suggests that elevated brain noradrenergic activity is involved in the initiation and maintenance of alcohol use disorder,9 current medications used to treat alcohol use disorder do not target brain noradrenergic pathways. In an RCT, Simpson et al4 tested prazosin, an alpha-1 adrenergic receptor antagonist, for the treatment of alcohol use disorder.
Study design
- In this 12-week double-blind study, 92 participants with alcohol use disorder were randomly assigned to receive prazosin or placebo. Individuals with posttraumatic stress disorder were excluded.
- Prazosin was titrated to a target dosing schedule of 4 mg in the morning, 4 mg in the afternoon, and 8 mg at bedtime by the end of Week 2. The behavioral platform was medical management. Participants provided daily data on their alcohol consumption.
- Generalized linear mixed-effects models were used to examine the impact of prazosin compared with placebo on number of drinks per week, number of drinking days per week, and number of heavy drinking days per week.
Outcomes
- Among the 80 participants who completed the titration period and were included in the primary analyses, prazosin was associated with self-reported fewer heavy drinking days, and fewer drinks per week (Palatino LT Std−8 vs Palatino LT Std−1.5 with placebo). Drinking days per week and craving showed no group differences.
- The rate of drinking and the probability of heavy drinking showed a greater decrease over time for participants receiving prazosin compared with those receiving placebo.
Continue to: Conclusion
Conclusion
- These findings of moderate reductions in heavy drinking days and drinks per week with prazosin suggest that prazosin may be a promising harm-reduction treatment for alcohol use disorder.
4. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
Postpartum depression is among the most common complications of childbirth. It can result in considerable suffering for mothers, children, and families. Gamma-aminobutyric acid (GABA) signaling has previously been reported to be involved in the pathophysiology of postpartum depression. Meltzer-Brody et al5 conducted 2 double-blind, randomized, placebo-controlled, phase 3 trials comparing brexanolone with placebo in women with postpartum depression at 30 clinical research centers and specialized psychiatric units in the United States.
Study design
- Participants were women age 18 to 45, Palatino LT Std≤6 months postpartum at screening, with postpartum depression as indicated by a qualifying 17-item Hamilton Depression Rating Scale (HAM-D) score of ≥26 for Study 1 or 20 to 25 for Study 2.
- Of the 375 women who were screened simultaneously across both studies, 138 were randomly assigned (1:1:1) to receive a single IV injection of brexanolone, 90 μg/kg per hour (BRX90) (n = 45), brexanolone, 60 μg/kg per hour (BRX60) (n = 47), or placebo (n = 46) for 60 hours in Study 1, and 108 were randomly assigned (1:1) to receive BRX90 (n = 54) or placebo (n = 54) for 60 hours in Study 2.
- The primary efficacy endpoint was change in total score on the HAM-D from baseline to 60 hours. Patients were followed until Day 30.
Outcomes
- In Study 1, at 60 hours, the least-squares (LS) mean reduction in HAM-D total score from baseline was 19.5 points (standard error [SE] 1.2) in the BRX60 group and 17.7 points (SE 1.2) in the BRX90 group, compared with 14.0 points (SE 1.1) in the placebo group.
- In Study 2, at 60 hours, the LS mean reduction in HAM-D total score from baseline was 14.6 points (SE 0.8) in the BRX90 group compared with 12.1 points (SE 0.8) for the placebo group.
- In Study 1, one patient in the BRX60 group had 2 serious adverse events (suicidal ideation and intentional overdose attempt during follow-up). In Study 2, one patient in the BRX90 group had 2 serious adverse events (altered state of consciousness and syncope), which were considered treatment-related.
Conclusion
- Administration of brexanolone injection for postpartum depression resulted in significant, clinically meaningful reductions in HAM-D total score at 60 hours compared with placebo, with a rapid onset of action and durable treatment response during the study period. These results suggest that brexanolone injection has the potential to improve treatment options for women with this disorder.
Continue to: #5
5. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
In clinical practice, the use of multiple antipsychotic agents for the maintenance treatment of schizophrenia is common but generally not recommended. The effectiveness of antipsychotic polypharmacy in preventing relapse of schizophrenia has not been established, and whether specific antipsychotic combinations are superior to monotherapies for maintenance treatment of schizophrenia is unknown. Tiihonen et al6 investigated the association of specific antipsychotic combinations with psychiatric rehospitalization, which was used as a marker for relapse.
Study design
- This study included 62,250 patients with schizophrenia, treated between January 1, 1996 and December 31, 2015, in a comprehensive, nationwide cohort in Finland. Overall, 31,257 individuals (50.2%) were men, and the median age was 45.6 (interquartile range, 34.6 to 57.9).
- Patients were receiving 29 different antipsychotic monotherapy or polypharmacy regimens.
- Researchers analyzed data from April 24 to June 15, 2018 using psychiatric rehospitalization as a marker for relapse. To minimize selection bias, rehospitalization risks were investigated using within-individual analyses.
- The main outcome was the hazard ratio (HR) for psychiatric rehospitalization during use of polypharmacy vs monotherapy by the same patient.
Outcomes
- Clozapine plus aripiprazole was associated with the lowest risk of psychiatric rehospitalization, with a difference of 14% (HR, .86; CI, .79 to .94) compared with clozapine monotherapy in the analysis that included all polypharmacy periods, and 18% (HR, .82; CI, .75 to .89) in the conservatively defined polypharmacy analysis that excluded periods <90 days.
- Among patients experiencing their first episode of schizophrenia, the differences between clozapine plus aripiprazole vs clozapine monotherapy were greater, with a difference of 22% in the analysis that included all polypharmacy periods, and 23% in the conservatively defined polypharmacy analysis.
- At the aggregate level, any antipsychotic polypharmacy was associated with a 7% to 13% lower risk of psychiatric rehospitalization compared with any monotherapy.
- Clozapine was the only monotherapy among the 10 best treatments.
- Results on all-cause and somatic hospitalization, mortality, and other sensitivity analyses were in line with the primary outcomes.
Conclusion
- This study suggests that certain types of antipsychotic polypharmacy may reduce the risk of rehospitalization in patients with schizophrenia. Current treatment guidelines state that clinicians should prefer antipsychotic monotherapy and avoid polypharmacy. Tiihonen et al6 raise the question whether current treatment guidelines should continue to discourage antipsychotic polypharmacy in the maintenance treatment of schizophrenia.
- Despite the large administrative databases and sophisticated statistical methods used in this study, this approach has important limitations. As Goff10 points out, despite efforts to minimize bias, these results should be considered preliminary until confirmed by RCTs.
6. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
In routine clinical practice, patients with schizophrenia are often treated with combinations of antipsychotics and other psychotropic medications. However, there is little evidence about the comparative effectiveness of these adjunctive treatment strategies. Stroup et al7 investigated the comparative real-world effectiveness of adjunctive psychotropic treatments for patients with schizophrenia.
Continue to: Study design
Study design
- This comparative effectiveness study used US Medicaid data from January 1, 2001, to December 31, 2010. Data analysis was performed from January 1, 2017, to June 30, 2018.
- The study cohort included 81,921 adult outpatients diagnosed with schizophrenia with a mean age of 40.7 (range: 18 to 64), including 37,515 women (45.8%). All patients were stably treated with a single antipsychotic and then started on an adjunctive antidepressant (n = 31,117), benzodiazepine (n = 11,941), mood stabilizer (n = 12,849), or another antipsychotic (n = 26,014).
- Researchers used multinomial logistic regression models to estimate propensity scores to balance covariates across the 4 medication groups. Weighted Cox proportional hazards regression models were used to compare treatment outcomes during 365 days on an intention-to-treat basis.
- The main outcomes and measures included risk of hospitalization for a mental disorder (primary), emergency department (ED) visits for a mental disorder, and all-cause mortality.
Outcomes
- Compared with starting another antipsychotic, initiating use of an antidepressant was associated with a lower risk of psychiatric hospitalization, and initiating use of a benzodiazepine was associated with a higher risk. Initiating use of a mood stabilizer was not significantly different from initiating use of another antipsychotic.
- A similar pattern of associations was observed in psychiatric ED visits for initiating use of an antidepressant, benzodiazepine, or mood stabilizer.
- Initiating use of a mood stabilizer was associated with an increased risk of mortality.
Conclusion
- Compared with the addition of a second antipsychotic, adding an antidepressant was associated with substantially reduced rates of hospitalization, whereas adding a benzodiazepine was associated with a modest increase in the risk of hospitalization. While the addition of a mood stabilizer was not associated with a significant difference in the risk of hospitalization, it was associated with higher mortality.
- Despite the limitations associated with this study, the associations of benzodiazepines and mood stabilizers with poorer outcomes warrant clinical caution and further investigation.
Bottom Line
Significantly higher epigenetic aging has been observed in patients with major depressive disorder. Haloperidol plus lorazepam might be an effective treatment for delirium; and ramelteon may be effective for preventing delirium. Prazosin reduces heavy drinking in patients with alcohol use disorder. A 60-hour infusion of brexanolone can help alleviate postpartum depression. Clozapine plus aripiprazole reduces the risk of rehospitalization among patients with schizophrenia. Adding an antidepressant to an antipsychotic also can reduce the risk of rehospitalization among patients with schizophrenia.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Aripiprazole • Abilify
Brexanolone • Zulresso
Clozapine • Clozaril
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Ramelteon • Rozerem
Risperidone • Risperdal
In Part 1 of this article, published in

1. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
In light of the association of major depressive disorder (MDD) with an increased risk of aging-related diseases, Han et al2 examined whether MDD was associated with higher epigenetic aging in blood as measured by DNA methylation patterns. They also studied whether clinical characteristics of MDD had a further impact on these patterns, and whether the findings replicated in brain tissue. Many differentially methylated regions of our DNA tend to change as we age. Han et al2 used these age-sensitive differentially methylated regions to estimate chronological age, using DNA extracted from various tissues, including blood and brain.
Study design
- As a part of the Netherlands Study of Depression and Anxiety (NESDA), this study included 811 patients with MDD and 319 control participants with no lifetime psychiatric disorders and low depressive symptoms (Inventory of Depressive Symptomatology score <14).
- Diagnosis of MDD and clinical characteristics were assessed by questionnaires and psychiatric interviews. Childhood trauma was assessed using the NEMESIS childhood trauma interview, which included a structured inventory of trauma exposure during childhood.
- DNA methylation age was estimated using all methylation sites in the blood of 811 patients with MDD and 319 control participants. The residuals of the DNA methylation age estimates regressed on chronological age were calculated to indicate epigenetic aging.
- Analyses were adjusted for sociodemographic characteristics, lifestyle, and health status.
- Postmortem brain samples of 74 patients with MDD and 64 control participants were used for replication.
Outcomes
- Significantly higher epigenetic aging was observed in patients with MDD compared with control participants (Cohen’s d = 0.18), which suggests that patients with MDD are biologically older than their corresponding chronological age. There was a significant dose effect with increasing symptom severity in the overall sample.
- In the MDD group, epigenetic aging was positively and significantly associated with childhood trauma.
- The case-control difference was replicated in an independent analysis of postmortem brain samples.
Conclusion
- These findings suggest that patients with MDD and people with a history of childhood trauma may biologically age relatively faster than those without MDD or childhood trauma. These findings may represent a biomarker of aging and might help identify patients who may benefit from early and intensive interventions to reduce the physical comorbidities of MDD.
- This study raises the possibility that MDD may be causally related to epigenetic age acceleration. However, it only points out the associations; there are other possible explanations for this correlation, including the possibility that a shared risk factor accounts for the observed association.
2. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
Delirium is common and often goes underdiagnosed. It is particularly prevalent among hospitalized geriatric patients. Several medications have been suggested to have a role in treating or preventing delirium. However, it remains uncertain which medications provide the best response rate, the lowest rate of delirium occurrence, and the best tolerability. In an attempt to find answers to these questions, Wu et al3 reviewed studies that evaluated the use of various medications used for delirium.
Study design
- Researchers conducted a systematic review and network meta-analysis of randomized controlled trials (RCTs) that investigated various pharmacologic agents used to treat or prevent delirium.
- Fifty-eight RCTs were included in the analyses. Of these, 20 RCTs with a total of 1,435 participants compared the outcomes of treatments of delirium, and 38 RCTs with a total of 8,168 participants examined prevention.
- A network meta-analysis was performed to determine if an agent or combinations of agents were superior to placebo or widely used medications.
Continue to: Outcomes
Outcomes
- Haloperidol plus lorazepam provided the best response rate for treating delirium compared with placebo/control.
- For delirium prevention, patients who received ramelteon, olanzapine, risperidone, or dexmedetomidine had significantly lower delirium occurrence rates than those receiving placebo/control.
- None of the pharmacologic treatments were significantly associated with a higher risk of all-cause mortality compared with placebo/control.
Conclusion
- Haloperidol plus lorazepam might be the best treatment and ramelteon the best preventive medicine for delirium. None of the pharmacologic interventions for treatment or prophylaxis increased all-cause mortality.
- However, network meta-analyses involve extrapolating treatment comparisons that are not made directly. As Blazer8 pointed out, both findings in this study (that haloperidol plus lorazepam is a unique intervention among the treatment trials and ramelteon is a unique intervention for prevention) seemed to be driven by 2 of the 58 studies that Wu et al3 examined.Wu et al3 also cautioned that both of these interventions needed to be further researched for efficacy.
3. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
While some evidence suggests that elevated brain noradrenergic activity is involved in the initiation and maintenance of alcohol use disorder,9 current medications used to treat alcohol use disorder do not target brain noradrenergic pathways. In an RCT, Simpson et al4 tested prazosin, an alpha-1 adrenergic receptor antagonist, for the treatment of alcohol use disorder.
Study design
- In this 12-week double-blind study, 92 participants with alcohol use disorder were randomly assigned to receive prazosin or placebo. Individuals with posttraumatic stress disorder were excluded.
- Prazosin was titrated to a target dosing schedule of 4 mg in the morning, 4 mg in the afternoon, and 8 mg at bedtime by the end of Week 2. The behavioral platform was medical management. Participants provided daily data on their alcohol consumption.
- Generalized linear mixed-effects models were used to examine the impact of prazosin compared with placebo on number of drinks per week, number of drinking days per week, and number of heavy drinking days per week.
Outcomes
- Among the 80 participants who completed the titration period and were included in the primary analyses, prazosin was associated with self-reported fewer heavy drinking days, and fewer drinks per week (Palatino LT Std−8 vs Palatino LT Std−1.5 with placebo). Drinking days per week and craving showed no group differences.
- The rate of drinking and the probability of heavy drinking showed a greater decrease over time for participants receiving prazosin compared with those receiving placebo.
Continue to: Conclusion
Conclusion
- These findings of moderate reductions in heavy drinking days and drinks per week with prazosin suggest that prazosin may be a promising harm-reduction treatment for alcohol use disorder.
4. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
Postpartum depression is among the most common complications of childbirth. It can result in considerable suffering for mothers, children, and families. Gamma-aminobutyric acid (GABA) signaling has previously been reported to be involved in the pathophysiology of postpartum depression. Meltzer-Brody et al5 conducted 2 double-blind, randomized, placebo-controlled, phase 3 trials comparing brexanolone with placebo in women with postpartum depression at 30 clinical research centers and specialized psychiatric units in the United States.
Study design
- Participants were women age 18 to 45, Palatino LT Std≤6 months postpartum at screening, with postpartum depression as indicated by a qualifying 17-item Hamilton Depression Rating Scale (HAM-D) score of ≥26 for Study 1 or 20 to 25 for Study 2.
- Of the 375 women who were screened simultaneously across both studies, 138 were randomly assigned (1:1:1) to receive a single IV injection of brexanolone, 90 μg/kg per hour (BRX90) (n = 45), brexanolone, 60 μg/kg per hour (BRX60) (n = 47), or placebo (n = 46) for 60 hours in Study 1, and 108 were randomly assigned (1:1) to receive BRX90 (n = 54) or placebo (n = 54) for 60 hours in Study 2.
- The primary efficacy endpoint was change in total score on the HAM-D from baseline to 60 hours. Patients were followed until Day 30.
Outcomes
- In Study 1, at 60 hours, the least-squares (LS) mean reduction in HAM-D total score from baseline was 19.5 points (standard error [SE] 1.2) in the BRX60 group and 17.7 points (SE 1.2) in the BRX90 group, compared with 14.0 points (SE 1.1) in the placebo group.
- In Study 2, at 60 hours, the LS mean reduction in HAM-D total score from baseline was 14.6 points (SE 0.8) in the BRX90 group compared with 12.1 points (SE 0.8) for the placebo group.
- In Study 1, one patient in the BRX60 group had 2 serious adverse events (suicidal ideation and intentional overdose attempt during follow-up). In Study 2, one patient in the BRX90 group had 2 serious adverse events (altered state of consciousness and syncope), which were considered treatment-related.
Conclusion
- Administration of brexanolone injection for postpartum depression resulted in significant, clinically meaningful reductions in HAM-D total score at 60 hours compared with placebo, with a rapid onset of action and durable treatment response during the study period. These results suggest that brexanolone injection has the potential to improve treatment options for women with this disorder.
Continue to: #5
5. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
In clinical practice, the use of multiple antipsychotic agents for the maintenance treatment of schizophrenia is common but generally not recommended. The effectiveness of antipsychotic polypharmacy in preventing relapse of schizophrenia has not been established, and whether specific antipsychotic combinations are superior to monotherapies for maintenance treatment of schizophrenia is unknown. Tiihonen et al6 investigated the association of specific antipsychotic combinations with psychiatric rehospitalization, which was used as a marker for relapse.
Study design
- This study included 62,250 patients with schizophrenia, treated between January 1, 1996 and December 31, 2015, in a comprehensive, nationwide cohort in Finland. Overall, 31,257 individuals (50.2%) were men, and the median age was 45.6 (interquartile range, 34.6 to 57.9).
- Patients were receiving 29 different antipsychotic monotherapy or polypharmacy regimens.
- Researchers analyzed data from April 24 to June 15, 2018 using psychiatric rehospitalization as a marker for relapse. To minimize selection bias, rehospitalization risks were investigated using within-individual analyses.
- The main outcome was the hazard ratio (HR) for psychiatric rehospitalization during use of polypharmacy vs monotherapy by the same patient.
Outcomes
- Clozapine plus aripiprazole was associated with the lowest risk of psychiatric rehospitalization, with a difference of 14% (HR, .86; CI, .79 to .94) compared with clozapine monotherapy in the analysis that included all polypharmacy periods, and 18% (HR, .82; CI, .75 to .89) in the conservatively defined polypharmacy analysis that excluded periods <90 days.
- Among patients experiencing their first episode of schizophrenia, the differences between clozapine plus aripiprazole vs clozapine monotherapy were greater, with a difference of 22% in the analysis that included all polypharmacy periods, and 23% in the conservatively defined polypharmacy analysis.
- At the aggregate level, any antipsychotic polypharmacy was associated with a 7% to 13% lower risk of psychiatric rehospitalization compared with any monotherapy.
- Clozapine was the only monotherapy among the 10 best treatments.
- Results on all-cause and somatic hospitalization, mortality, and other sensitivity analyses were in line with the primary outcomes.
Conclusion
- This study suggests that certain types of antipsychotic polypharmacy may reduce the risk of rehospitalization in patients with schizophrenia. Current treatment guidelines state that clinicians should prefer antipsychotic monotherapy and avoid polypharmacy. Tiihonen et al6 raise the question whether current treatment guidelines should continue to discourage antipsychotic polypharmacy in the maintenance treatment of schizophrenia.
- Despite the large administrative databases and sophisticated statistical methods used in this study, this approach has important limitations. As Goff10 points out, despite efforts to minimize bias, these results should be considered preliminary until confirmed by RCTs.
6. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
In routine clinical practice, patients with schizophrenia are often treated with combinations of antipsychotics and other psychotropic medications. However, there is little evidence about the comparative effectiveness of these adjunctive treatment strategies. Stroup et al7 investigated the comparative real-world effectiveness of adjunctive psychotropic treatments for patients with schizophrenia.
Continue to: Study design
Study design
- This comparative effectiveness study used US Medicaid data from January 1, 2001, to December 31, 2010. Data analysis was performed from January 1, 2017, to June 30, 2018.
- The study cohort included 81,921 adult outpatients diagnosed with schizophrenia with a mean age of 40.7 (range: 18 to 64), including 37,515 women (45.8%). All patients were stably treated with a single antipsychotic and then started on an adjunctive antidepressant (n = 31,117), benzodiazepine (n = 11,941), mood stabilizer (n = 12,849), or another antipsychotic (n = 26,014).
- Researchers used multinomial logistic regression models to estimate propensity scores to balance covariates across the 4 medication groups. Weighted Cox proportional hazards regression models were used to compare treatment outcomes during 365 days on an intention-to-treat basis.
- The main outcomes and measures included risk of hospitalization for a mental disorder (primary), emergency department (ED) visits for a mental disorder, and all-cause mortality.
Outcomes
- Compared with starting another antipsychotic, initiating use of an antidepressant was associated with a lower risk of psychiatric hospitalization, and initiating use of a benzodiazepine was associated with a higher risk. Initiating use of a mood stabilizer was not significantly different from initiating use of another antipsychotic.
- A similar pattern of associations was observed in psychiatric ED visits for initiating use of an antidepressant, benzodiazepine, or mood stabilizer.
- Initiating use of a mood stabilizer was associated with an increased risk of mortality.
Conclusion
- Compared with the addition of a second antipsychotic, adding an antidepressant was associated with substantially reduced rates of hospitalization, whereas adding a benzodiazepine was associated with a modest increase in the risk of hospitalization. While the addition of a mood stabilizer was not associated with a significant difference in the risk of hospitalization, it was associated with higher mortality.
- Despite the limitations associated with this study, the associations of benzodiazepines and mood stabilizers with poorer outcomes warrant clinical caution and further investigation.
Bottom Line
Significantly higher epigenetic aging has been observed in patients with major depressive disorder. Haloperidol plus lorazepam might be an effective treatment for delirium; and ramelteon may be effective for preventing delirium. Prazosin reduces heavy drinking in patients with alcohol use disorder. A 60-hour infusion of brexanolone can help alleviate postpartum depression. Clozapine plus aripiprazole reduces the risk of rehospitalization among patients with schizophrenia. Adding an antidepressant to an antipsychotic also can reduce the risk of rehospitalization among patients with schizophrenia.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Aripiprazole • Abilify
Brexanolone • Zulresso
Clozapine • Clozaril
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Ramelteon • Rozerem
Risperidone • Risperdal
1. Saeed SA, Stanley JB. Top research findings of 2018-2019. First of 2 parts. Current Psychiatry. 2020;19(1):13-18.
2. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
3. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
4. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
5. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
6. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
7. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
8. Blazer DG. Pharmacologic intervention for the treatment and prevention of delirium: looking beneath the modeling. JAMA Psychiatry. 2019;76(5):472-473.
9. Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61-75.
10. Goff DC. Can adjunctive pharmacotherapy reduce hospitalization in schizophrenia? Insights from administrative databases. JAMA Psychiatry. 2019;76(5):468-469.
1. Saeed SA, Stanley JB. Top research findings of 2018-2019. First of 2 parts. Current Psychiatry. 2020;19(1):13-18.
2. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
3. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
4. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
5. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
6. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
7. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
8. Blazer DG. Pharmacologic intervention for the treatment and prevention of delirium: looking beneath the modeling. JAMA Psychiatry. 2019;76(5):472-473.
9. Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61-75.
10. Goff DC. Can adjunctive pharmacotherapy reduce hospitalization in schizophrenia? Insights from administrative databases. JAMA Psychiatry. 2019;76(5):468-469.
Legalization of marijuana and youths’ attitudes toward its use
The legal status of marijuana has changed a great deal during the last 4 decades. In the United States, several states have legalized the use of marijuana to treat several medical conditions. Some states have decriminalized marijuana possession, and several have legalized marijuana for recreational use by adults. These changes have contributed to a growing misperception among young people that marijuana is harmless or not as risky as other illicit
In this article, I explore the effect the legalization of marijuana has had on young peoples’ attitudes toward its use.
Marijuana use among adolescents
Among adolescents, marijuana is the most commonly used illicit substance, after alcohol.1 According to data from the 2019 Monitoring the Future Survey, while past month, past year, and lifetime marijuana use among 8th and 10th graders remained steady from 2018 to 2019, daily marijuana use among these adolescents increased.2 This survey also reported increases in adolescent marijuana vaping from 2018 to 2019.2 Further, the percentage of adolescents who think that the regular use of marijuana is risky has been trending down since the mid-2000s.2
Youth substance use rates depend on numerous factors, including legal status, availability, ease of access to the substance, and perception of harm.3 Although the legalization of marijuana for recreational use has been for adults only, based on rates of tobacco and alcohol use in adolescents (both of which are legal for adults), the legalization of marijuana is likely to have implications for adolescents.4
Adverse effects among adolescents
During adolescence, the brain is still developing, and marijuana use during this time could cause decreased cognitive functioning, especially executive fun
- an increased risk of mental health disorders, including depression, anxiety, and psychosis, particularly among adolescents at higher risk, such as those with a family history of psychiatric illness
- a decline in school performance
- an increased school dropout rate
- an increased risk of marijuana dependence
- an elevated rate of engaging in risky behaviors.
Factors by which the legalization of marijuana might increase its use among adolescents include4:
- perceived decreased risk of marijuana use
- increased availability
- lower cost
- decreased fear of legal consequences of marijuana use.
Increased parental use is an indirect way in which legalization of marijuana for adult recreational use might increase use in youth.
Continue to: What the evidence says
What the evidence says
Colorado legalized marijuana for medical use in 2000, and for adult recreational use in 2014. A 2012 study of adolescents receiving substance abuse treatment in Colorado found diversion of medical marijuana to these adolescents was common.6 This study also reported that compared with those who did not use medical marijuana, adolescents who used medical marijuana had an earlier age of regular marijuana use, more marijuana use disorder symptoms, and more symptoms of conduct disorder.6 However, data from the US Substance Abuse and Mental Health Services Administration7 and from the Colorado Department of Public Health & Environment8 suggest that marijuana use among adolescents has not increased since legalization in Colorado.
In 2012, voters in Washington state legalized marijuana for recreational use. In 2013, Moreno et al9 interviewed college students in Washington, where marijuana had just been legalized, and Wisconsin, where it had not. In both states, most participants indicated that legalization would not change their attitude towards use. A small proportion of students felt that legalization would signify an endorsement of marijuana, and they were likely to perceive it as safe to use.
In an analysis of data on more than 250,000 students in 8th, 10th, and 12th grade, Cerdá et al10 found that after legalization in Washington, the perceived harmfulness of marijuana decreased and marijuana use increased among 8th and 10th graders in Washington; however, there were no significant differences noted among adolescents in Colorado.
In 2010, voters in California passed legislation to decriminalize marijuana. In an analysis of data from 8th, 10th, and 12th graders in California, Miech et al11 found a positive correlation between decriminalization and increases in youth future marijuana use. They also found that compared with their peers in other states, 12thgraders in California were more likely to have used marijuana in the last 30 days, less likely to perceive marijuana use as a health risk, and less likely to disapprove of its use.11
Although some studies have suggested that legalization of marijuana might increase use among adolescents, limitations of these studies include that they relied on self-reported use by adolescents, and they did not evaluate adolescent populations outside of school settings.
Continue to: Addressing adolescents' marijuana use
Addressing adolescents’ marijuana use
Strategies for preventing or reducing marijuana use among adolescents might include imposing restrictions and passing stricter laws on the sale of marijuana to individuals age <21, regulating marijuana advertising, increasing adolescent substance use prevention program initiatives, and educating youth about the negative effects of marijuana. Further research is needed to clearly establish if the legalization of marijuana for adult recreational use will increase its use among adolescents.
1. US Department of Health & Human Services. Marijuana use in adolescence. https://www.hhs.gov/ash/oah/adolescent-development/substance-use/marijuana/index.html. Updated April 19, 2019. Accessed January 15, 2020.
2. University of Michigan Institute for Social Research. National adolescent drug trends in 2019: Findings released. http://monitoringthefuture.org//pressreleases/19drugpr.pdf. Updated December 18, 2019. Accessed January 13, 2020.
3. Ammerman S, Ryan S, William P; Committee on Substance Abuse, the Committee on Adolescence. The impact of marijuana policies on youth: clinical, research, and legal update. Pediatrics. 2015;135(3):584-587.
4. Hopfer C. Implications of marijuana legalization for adolescent substance use. Subst Abus. 2014;35(4):331-335.
5. Silins E, Horwood LJ, Patton GC, et al. Young adult sequelae of adolescent cannabis use: an integrative analysis. Lancet Psychiatry. 2014;1(4):286-293.
6. Salomonsen-Sautel S, Sakai JT, Thurstone C, et al. Medical marijuana use among adolescents in substance abuse treatment. J Am Acad Child Adolesc Psychiatry. 2012;51(7):694-702.
7. US Department of Health & Human Services. Substance Abuse and Mental Health Services Administration. National Survey on Drug Use and Health: Comparison of 2014-2015 and 2015-2016 Population Percentages (50 States and the District of Columbia). https://www.samhsa.gov/data/sites/default/files/NSDUHsaeShortTermCHG2016/NSDUHsaeShortTermCHG2016.htm. Accessed January 15, 2020.
8. Colorado Department of Public Health & Environment. Data Brief: Colorado youth marijuana use 2017. https://drive.google.com/file/d/1AX_2RWWgygGXtGpAGoOMTe84Crzsv62T/view. Accessed January 15, 2020.
9. Moreno MA, Whitehill JM, Quach V, et al. Marijuana experiences, voting behaviors, and early perspectives regarding marijuana legalization among college students from 2 states. J Am Coll Health. 2016;64(1):9-18.
10. Cerdá M, Wall M, Feng T, et al. Association of state recreational marijuana laws with adolescent marijuana use. JAMA Pediatrics. 2017;171(2):142-149.
11. Miech RA, Johnston L, O’Malley PM, et al. Trends in use of marijuana and attitudes toward marijuana among youth before and after decriminalization: the case of California 2007-2013. Int J Drug Policy. 2015;26(4):336-344.
The legal status of marijuana has changed a great deal during the last 4 decades. In the United States, several states have legalized the use of marijuana to treat several medical conditions. Some states have decriminalized marijuana possession, and several have legalized marijuana for recreational use by adults. These changes have contributed to a growing misperception among young people that marijuana is harmless or not as risky as other illicit
In this article, I explore the effect the legalization of marijuana has had on young peoples’ attitudes toward its use.
Marijuana use among adolescents
Among adolescents, marijuana is the most commonly used illicit substance, after alcohol.1 According to data from the 2019 Monitoring the Future Survey, while past month, past year, and lifetime marijuana use among 8th and 10th graders remained steady from 2018 to 2019, daily marijuana use among these adolescents increased.2 This survey also reported increases in adolescent marijuana vaping from 2018 to 2019.2 Further, the percentage of adolescents who think that the regular use of marijuana is risky has been trending down since the mid-2000s.2
Youth substance use rates depend on numerous factors, including legal status, availability, ease of access to the substance, and perception of harm.3 Although the legalization of marijuana for recreational use has been for adults only, based on rates of tobacco and alcohol use in adolescents (both of which are legal for adults), the legalization of marijuana is likely to have implications for adolescents.4
Adverse effects among adolescents
During adolescence, the brain is still developing, and marijuana use during this time could cause decreased cognitive functioning, especially executive fun
- an increased risk of mental health disorders, including depression, anxiety, and psychosis, particularly among adolescents at higher risk, such as those with a family history of psychiatric illness
- a decline in school performance
- an increased school dropout rate
- an increased risk of marijuana dependence
- an elevated rate of engaging in risky behaviors.
Factors by which the legalization of marijuana might increase its use among adolescents include4:
- perceived decreased risk of marijuana use
- increased availability
- lower cost
- decreased fear of legal consequences of marijuana use.
Increased parental use is an indirect way in which legalization of marijuana for adult recreational use might increase use in youth.
Continue to: What the evidence says
What the evidence says
Colorado legalized marijuana for medical use in 2000, and for adult recreational use in 2014. A 2012 study of adolescents receiving substance abuse treatment in Colorado found diversion of medical marijuana to these adolescents was common.6 This study also reported that compared with those who did not use medical marijuana, adolescents who used medical marijuana had an earlier age of regular marijuana use, more marijuana use disorder symptoms, and more symptoms of conduct disorder.6 However, data from the US Substance Abuse and Mental Health Services Administration7 and from the Colorado Department of Public Health & Environment8 suggest that marijuana use among adolescents has not increased since legalization in Colorado.
In 2012, voters in Washington state legalized marijuana for recreational use. In 2013, Moreno et al9 interviewed college students in Washington, where marijuana had just been legalized, and Wisconsin, where it had not. In both states, most participants indicated that legalization would not change their attitude towards use. A small proportion of students felt that legalization would signify an endorsement of marijuana, and they were likely to perceive it as safe to use.
In an analysis of data on more than 250,000 students in 8th, 10th, and 12th grade, Cerdá et al10 found that after legalization in Washington, the perceived harmfulness of marijuana decreased and marijuana use increased among 8th and 10th graders in Washington; however, there were no significant differences noted among adolescents in Colorado.
In 2010, voters in California passed legislation to decriminalize marijuana. In an analysis of data from 8th, 10th, and 12th graders in California, Miech et al11 found a positive correlation between decriminalization and increases in youth future marijuana use. They also found that compared with their peers in other states, 12thgraders in California were more likely to have used marijuana in the last 30 days, less likely to perceive marijuana use as a health risk, and less likely to disapprove of its use.11
Although some studies have suggested that legalization of marijuana might increase use among adolescents, limitations of these studies include that they relied on self-reported use by adolescents, and they did not evaluate adolescent populations outside of school settings.
Continue to: Addressing adolescents' marijuana use
Addressing adolescents’ marijuana use
Strategies for preventing or reducing marijuana use among adolescents might include imposing restrictions and passing stricter laws on the sale of marijuana to individuals age <21, regulating marijuana advertising, increasing adolescent substance use prevention program initiatives, and educating youth about the negative effects of marijuana. Further research is needed to clearly establish if the legalization of marijuana for adult recreational use will increase its use among adolescents.
The legal status of marijuana has changed a great deal during the last 4 decades. In the United States, several states have legalized the use of marijuana to treat several medical conditions. Some states have decriminalized marijuana possession, and several have legalized marijuana for recreational use by adults. These changes have contributed to a growing misperception among young people that marijuana is harmless or not as risky as other illicit
In this article, I explore the effect the legalization of marijuana has had on young peoples’ attitudes toward its use.
Marijuana use among adolescents
Among adolescents, marijuana is the most commonly used illicit substance, after alcohol.1 According to data from the 2019 Monitoring the Future Survey, while past month, past year, and lifetime marijuana use among 8th and 10th graders remained steady from 2018 to 2019, daily marijuana use among these adolescents increased.2 This survey also reported increases in adolescent marijuana vaping from 2018 to 2019.2 Further, the percentage of adolescents who think that the regular use of marijuana is risky has been trending down since the mid-2000s.2
Youth substance use rates depend on numerous factors, including legal status, availability, ease of access to the substance, and perception of harm.3 Although the legalization of marijuana for recreational use has been for adults only, based on rates of tobacco and alcohol use in adolescents (both of which are legal for adults), the legalization of marijuana is likely to have implications for adolescents.4
Adverse effects among adolescents
During adolescence, the brain is still developing, and marijuana use during this time could cause decreased cognitive functioning, especially executive fun
- an increased risk of mental health disorders, including depression, anxiety, and psychosis, particularly among adolescents at higher risk, such as those with a family history of psychiatric illness
- a decline in school performance
- an increased school dropout rate
- an increased risk of marijuana dependence
- an elevated rate of engaging in risky behaviors.
Factors by which the legalization of marijuana might increase its use among adolescents include4:
- perceived decreased risk of marijuana use
- increased availability
- lower cost
- decreased fear of legal consequences of marijuana use.
Increased parental use is an indirect way in which legalization of marijuana for adult recreational use might increase use in youth.
Continue to: What the evidence says
What the evidence says
Colorado legalized marijuana for medical use in 2000, and for adult recreational use in 2014. A 2012 study of adolescents receiving substance abuse treatment in Colorado found diversion of medical marijuana to these adolescents was common.6 This study also reported that compared with those who did not use medical marijuana, adolescents who used medical marijuana had an earlier age of regular marijuana use, more marijuana use disorder symptoms, and more symptoms of conduct disorder.6 However, data from the US Substance Abuse and Mental Health Services Administration7 and from the Colorado Department of Public Health & Environment8 suggest that marijuana use among adolescents has not increased since legalization in Colorado.
In 2012, voters in Washington state legalized marijuana for recreational use. In 2013, Moreno et al9 interviewed college students in Washington, where marijuana had just been legalized, and Wisconsin, where it had not. In both states, most participants indicated that legalization would not change their attitude towards use. A small proportion of students felt that legalization would signify an endorsement of marijuana, and they were likely to perceive it as safe to use.
In an analysis of data on more than 250,000 students in 8th, 10th, and 12th grade, Cerdá et al10 found that after legalization in Washington, the perceived harmfulness of marijuana decreased and marijuana use increased among 8th and 10th graders in Washington; however, there were no significant differences noted among adolescents in Colorado.
In 2010, voters in California passed legislation to decriminalize marijuana. In an analysis of data from 8th, 10th, and 12th graders in California, Miech et al11 found a positive correlation between decriminalization and increases in youth future marijuana use. They also found that compared with their peers in other states, 12thgraders in California were more likely to have used marijuana in the last 30 days, less likely to perceive marijuana use as a health risk, and less likely to disapprove of its use.11
Although some studies have suggested that legalization of marijuana might increase use among adolescents, limitations of these studies include that they relied on self-reported use by adolescents, and they did not evaluate adolescent populations outside of school settings.
Continue to: Addressing adolescents' marijuana use
Addressing adolescents’ marijuana use
Strategies for preventing or reducing marijuana use among adolescents might include imposing restrictions and passing stricter laws on the sale of marijuana to individuals age <21, regulating marijuana advertising, increasing adolescent substance use prevention program initiatives, and educating youth about the negative effects of marijuana. Further research is needed to clearly establish if the legalization of marijuana for adult recreational use will increase its use among adolescents.
1. US Department of Health & Human Services. Marijuana use in adolescence. https://www.hhs.gov/ash/oah/adolescent-development/substance-use/marijuana/index.html. Updated April 19, 2019. Accessed January 15, 2020.
2. University of Michigan Institute for Social Research. National adolescent drug trends in 2019: Findings released. http://monitoringthefuture.org//pressreleases/19drugpr.pdf. Updated December 18, 2019. Accessed January 13, 2020.
3. Ammerman S, Ryan S, William P; Committee on Substance Abuse, the Committee on Adolescence. The impact of marijuana policies on youth: clinical, research, and legal update. Pediatrics. 2015;135(3):584-587.
4. Hopfer C. Implications of marijuana legalization for adolescent substance use. Subst Abus. 2014;35(4):331-335.
5. Silins E, Horwood LJ, Patton GC, et al. Young adult sequelae of adolescent cannabis use: an integrative analysis. Lancet Psychiatry. 2014;1(4):286-293.
6. Salomonsen-Sautel S, Sakai JT, Thurstone C, et al. Medical marijuana use among adolescents in substance abuse treatment. J Am Acad Child Adolesc Psychiatry. 2012;51(7):694-702.
7. US Department of Health & Human Services. Substance Abuse and Mental Health Services Administration. National Survey on Drug Use and Health: Comparison of 2014-2015 and 2015-2016 Population Percentages (50 States and the District of Columbia). https://www.samhsa.gov/data/sites/default/files/NSDUHsaeShortTermCHG2016/NSDUHsaeShortTermCHG2016.htm. Accessed January 15, 2020.
8. Colorado Department of Public Health & Environment. Data Brief: Colorado youth marijuana use 2017. https://drive.google.com/file/d/1AX_2RWWgygGXtGpAGoOMTe84Crzsv62T/view. Accessed January 15, 2020.
9. Moreno MA, Whitehill JM, Quach V, et al. Marijuana experiences, voting behaviors, and early perspectives regarding marijuana legalization among college students from 2 states. J Am Coll Health. 2016;64(1):9-18.
10. Cerdá M, Wall M, Feng T, et al. Association of state recreational marijuana laws with adolescent marijuana use. JAMA Pediatrics. 2017;171(2):142-149.
11. Miech RA, Johnston L, O’Malley PM, et al. Trends in use of marijuana and attitudes toward marijuana among youth before and after decriminalization: the case of California 2007-2013. Int J Drug Policy. 2015;26(4):336-344.
1. US Department of Health & Human Services. Marijuana use in adolescence. https://www.hhs.gov/ash/oah/adolescent-development/substance-use/marijuana/index.html. Updated April 19, 2019. Accessed January 15, 2020.
2. University of Michigan Institute for Social Research. National adolescent drug trends in 2019: Findings released. http://monitoringthefuture.org//pressreleases/19drugpr.pdf. Updated December 18, 2019. Accessed January 13, 2020.
3. Ammerman S, Ryan S, William P; Committee on Substance Abuse, the Committee on Adolescence. The impact of marijuana policies on youth: clinical, research, and legal update. Pediatrics. 2015;135(3):584-587.
4. Hopfer C. Implications of marijuana legalization for adolescent substance use. Subst Abus. 2014;35(4):331-335.
5. Silins E, Horwood LJ, Patton GC, et al. Young adult sequelae of adolescent cannabis use: an integrative analysis. Lancet Psychiatry. 2014;1(4):286-293.
6. Salomonsen-Sautel S, Sakai JT, Thurstone C, et al. Medical marijuana use among adolescents in substance abuse treatment. J Am Acad Child Adolesc Psychiatry. 2012;51(7):694-702.
7. US Department of Health & Human Services. Substance Abuse and Mental Health Services Administration. National Survey on Drug Use and Health: Comparison of 2014-2015 and 2015-2016 Population Percentages (50 States and the District of Columbia). https://www.samhsa.gov/data/sites/default/files/NSDUHsaeShortTermCHG2016/NSDUHsaeShortTermCHG2016.htm. Accessed January 15, 2020.
8. Colorado Department of Public Health & Environment. Data Brief: Colorado youth marijuana use 2017. https://drive.google.com/file/d/1AX_2RWWgygGXtGpAGoOMTe84Crzsv62T/view. Accessed January 15, 2020.
9. Moreno MA, Whitehill JM, Quach V, et al. Marijuana experiences, voting behaviors, and early perspectives regarding marijuana legalization among college students from 2 states. J Am Coll Health. 2016;64(1):9-18.
10. Cerdá M, Wall M, Feng T, et al. Association of state recreational marijuana laws with adolescent marijuana use. JAMA Pediatrics. 2017;171(2):142-149.
11. Miech RA, Johnston L, O’Malley PM, et al. Trends in use of marijuana and attitudes toward marijuana among youth before and after decriminalization: the case of California 2007-2013. Int J Drug Policy. 2015;26(4):336-344.
Caution on pharmacogenetic testing
The general public may have been led to believe that by decoding genes into their constituent parts, clinicians can prevent or predict serious illnesses and personalize treatment. While this may be true in some areas of medicine, such as oncology, using a pharmacogenetic testing-based “lookup table” to prescribe psychiatric medications is disturbing. This practice could lead to incorrect prescriptions, as well as a lack of follow-up or appropriate dosage titration or medication switching. These problems could put a patient’s life at risk and, consequently, bring the field of psychiatry into disrepute.
In the last few years, using pharmacogenetics to predict or prevent illness and personalize treatment has become very attractive. A 2019 meta-analysis of 5 randomized controlled trials examined the use of pharmacogenetic-guided decision support tools for major depressive disorder (MDD). Researchers randomized 1,737 participants with MDD to either pharmacogenetic-guided decision support tools or treatment as usual.1 Patients were assessed using the Hamilton Depression Rating Scale–17 three times over 8 weeks. Compared with those who received treatment as usual, those who were managed using pharmacogenetic-guided decision support tools were more likely to achieve remission from depressive symptoms (relative risk = 1.71; 95% CI, 1.17 to 2.48; P = .005). However, these results are controversial because the included studies were industry-funded, and proprietary algorithms were used to interpret the results. (Editor's note: For more information about this study and pharmacogenetic testing, see “Pharmacogenomics testing: What the FDA says,” Savvy Psychopharmacology,
In a policy statement on the use of pharmacogenetic testing in psychiatry, the International Society of Psychiatric Genetics (ISPG) explained that such testing should be viewed as a decision support tool to assist in implementing good clinical care, rather than as an alternative to standard protocols.2 Furthermore, the ISPG stated that “common genetic variants are not sufficient to cause psychiatric disorders such as depression, bipolar disorder, substance dependence, or schizophrenia.”2
Some manufacturers have claimed that their pharmacogenetic tests can provide information on how a patient will respond to medications for treating depression and other conditions, and when a clinician can or should change a patient’s medication. However, the relationship between DNA variations and the effectiveness of antidepressant medications has not been established, and basing clinical decisions on the results of these tests may lead to inappropriate medication changes.
Pharmacogenetic tests are being advertised to both clinicians and patients, but the FDA has not approved the use of any test for providing information on a patient’s ability to respond to any specific medication.3 Therefore, psychiatrists should discuss the use of pharmacogenetic testing with their patients, and advise patients to avoid stopping or changing their medications based on the results of any pharmacogenetic test. Clinicians should not change a patient’s medication regimen solely based on the results of pharmacogenetic testing. These tests are not supported by scientific or clinical evidence, and using these tests for clinical decisions may put the patient at risk for potentially serious health consequences.
Aneela Jafri, MD, MS
Research Volunteer
Ocean Medical Center
Nutley, New Jersey
Ramon Solhkhah, MD
Founding Chair and Professor
Department of Psychiatry and Behavioral Health
Hackensack Meridian School of Medicine at Seton Hall University
Nutley, New Jersey
Chair
Department of Psychiatry
Jersey Shore University Medical Center
Neptune, New Jersey
Residency Training Director
General Psychiatry
Ocean Medical Center
Brick, New Jersey
Stacy Doumas, MD
Vice Chair
Associate Professor
Department of Psychiatry and Behavioral Health
Hackensack Meridian School of Medicine at Seton Hall University
Nutley, New Jersey
Vice Chair for Education & Research
Residency Training Director
General Psychiatry
Jersey Shore University Medical Center Neptune, New Jersey
Saba Afzal, MD
Assistant Professor
Department of Psychiatry and Behavioral Health
Hackensack Meridian School of Medicine at Seton Hall University
Nutley, New Jersey
Associate Residency Training Director General Psychiatry
Ocean Medical Center
Brick, New Jersey
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
1. Bousman CA, Arandjelovic K, Mancuso SG, et al. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenetics. 2019;20(1):37-47.
2. International Society for Psychiatric Genetics. Genetics testing statement: genetic testing and psychiatric disorders. https://ispg.net/genetic-testing-statement. Updated March 11, 2019. Accessed January 9, 2020.
3. Zubenko GS, Sommer BR, Cohen BM. Pharmacogenetics in psychiatry; a companion, rather than competitor, to protocol-based care-reply. JAMA Psychiatry. 2018;75(10):1090-1091.
The general public may have been led to believe that by decoding genes into their constituent parts, clinicians can prevent or predict serious illnesses and personalize treatment. While this may be true in some areas of medicine, such as oncology, using a pharmacogenetic testing-based “lookup table” to prescribe psychiatric medications is disturbing. This practice could lead to incorrect prescriptions, as well as a lack of follow-up or appropriate dosage titration or medication switching. These problems could put a patient’s life at risk and, consequently, bring the field of psychiatry into disrepute.
In the last few years, using pharmacogenetics to predict or prevent illness and personalize treatment has become very attractive. A 2019 meta-analysis of 5 randomized controlled trials examined the use of pharmacogenetic-guided decision support tools for major depressive disorder (MDD). Researchers randomized 1,737 participants with MDD to either pharmacogenetic-guided decision support tools or treatment as usual.1 Patients were assessed using the Hamilton Depression Rating Scale–17 three times over 8 weeks. Compared with those who received treatment as usual, those who were managed using pharmacogenetic-guided decision support tools were more likely to achieve remission from depressive symptoms (relative risk = 1.71; 95% CI, 1.17 to 2.48; P = .005). However, these results are controversial because the included studies were industry-funded, and proprietary algorithms were used to interpret the results. (Editor's note: For more information about this study and pharmacogenetic testing, see “Pharmacogenomics testing: What the FDA says,” Savvy Psychopharmacology,
In a policy statement on the use of pharmacogenetic testing in psychiatry, the International Society of Psychiatric Genetics (ISPG) explained that such testing should be viewed as a decision support tool to assist in implementing good clinical care, rather than as an alternative to standard protocols.2 Furthermore, the ISPG stated that “common genetic variants are not sufficient to cause psychiatric disorders such as depression, bipolar disorder, substance dependence, or schizophrenia.”2
Some manufacturers have claimed that their pharmacogenetic tests can provide information on how a patient will respond to medications for treating depression and other conditions, and when a clinician can or should change a patient’s medication. However, the relationship between DNA variations and the effectiveness of antidepressant medications has not been established, and basing clinical decisions on the results of these tests may lead to inappropriate medication changes.
Pharmacogenetic tests are being advertised to both clinicians and patients, but the FDA has not approved the use of any test for providing information on a patient’s ability to respond to any specific medication.3 Therefore, psychiatrists should discuss the use of pharmacogenetic testing with their patients, and advise patients to avoid stopping or changing their medications based on the results of any pharmacogenetic test. Clinicians should not change a patient’s medication regimen solely based on the results of pharmacogenetic testing. These tests are not supported by scientific or clinical evidence, and using these tests for clinical decisions may put the patient at risk for potentially serious health consequences.
Aneela Jafri, MD, MS
Research Volunteer
Ocean Medical Center
Nutley, New Jersey
Ramon Solhkhah, MD
Founding Chair and Professor
Department of Psychiatry and Behavioral Health
Hackensack Meridian School of Medicine at Seton Hall University
Nutley, New Jersey
Chair
Department of Psychiatry
Jersey Shore University Medical Center
Neptune, New Jersey
Residency Training Director
General Psychiatry
Ocean Medical Center
Brick, New Jersey
Stacy Doumas, MD
Vice Chair
Associate Professor
Department of Psychiatry and Behavioral Health
Hackensack Meridian School of Medicine at Seton Hall University
Nutley, New Jersey
Vice Chair for Education & Research
Residency Training Director
General Psychiatry
Jersey Shore University Medical Center Neptune, New Jersey
Saba Afzal, MD
Assistant Professor
Department of Psychiatry and Behavioral Health
Hackensack Meridian School of Medicine at Seton Hall University
Nutley, New Jersey
Associate Residency Training Director General Psychiatry
Ocean Medical Center
Brick, New Jersey
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
The general public may have been led to believe that by decoding genes into their constituent parts, clinicians can prevent or predict serious illnesses and personalize treatment. While this may be true in some areas of medicine, such as oncology, using a pharmacogenetic testing-based “lookup table” to prescribe psychiatric medications is disturbing. This practice could lead to incorrect prescriptions, as well as a lack of follow-up or appropriate dosage titration or medication switching. These problems could put a patient’s life at risk and, consequently, bring the field of psychiatry into disrepute.
In the last few years, using pharmacogenetics to predict or prevent illness and personalize treatment has become very attractive. A 2019 meta-analysis of 5 randomized controlled trials examined the use of pharmacogenetic-guided decision support tools for major depressive disorder (MDD). Researchers randomized 1,737 participants with MDD to either pharmacogenetic-guided decision support tools or treatment as usual.1 Patients were assessed using the Hamilton Depression Rating Scale–17 three times over 8 weeks. Compared with those who received treatment as usual, those who were managed using pharmacogenetic-guided decision support tools were more likely to achieve remission from depressive symptoms (relative risk = 1.71; 95% CI, 1.17 to 2.48; P = .005). However, these results are controversial because the included studies were industry-funded, and proprietary algorithms were used to interpret the results. (Editor's note: For more information about this study and pharmacogenetic testing, see “Pharmacogenomics testing: What the FDA says,” Savvy Psychopharmacology,
In a policy statement on the use of pharmacogenetic testing in psychiatry, the International Society of Psychiatric Genetics (ISPG) explained that such testing should be viewed as a decision support tool to assist in implementing good clinical care, rather than as an alternative to standard protocols.2 Furthermore, the ISPG stated that “common genetic variants are not sufficient to cause psychiatric disorders such as depression, bipolar disorder, substance dependence, or schizophrenia.”2
Some manufacturers have claimed that their pharmacogenetic tests can provide information on how a patient will respond to medications for treating depression and other conditions, and when a clinician can or should change a patient’s medication. However, the relationship between DNA variations and the effectiveness of antidepressant medications has not been established, and basing clinical decisions on the results of these tests may lead to inappropriate medication changes.
Pharmacogenetic tests are being advertised to both clinicians and patients, but the FDA has not approved the use of any test for providing information on a patient’s ability to respond to any specific medication.3 Therefore, psychiatrists should discuss the use of pharmacogenetic testing with their patients, and advise patients to avoid stopping or changing their medications based on the results of any pharmacogenetic test. Clinicians should not change a patient’s medication regimen solely based on the results of pharmacogenetic testing. These tests are not supported by scientific or clinical evidence, and using these tests for clinical decisions may put the patient at risk for potentially serious health consequences.
Aneela Jafri, MD, MS
Research Volunteer
Ocean Medical Center
Nutley, New Jersey
Ramon Solhkhah, MD
Founding Chair and Professor
Department of Psychiatry and Behavioral Health
Hackensack Meridian School of Medicine at Seton Hall University
Nutley, New Jersey
Chair
Department of Psychiatry
Jersey Shore University Medical Center
Neptune, New Jersey
Residency Training Director
General Psychiatry
Ocean Medical Center
Brick, New Jersey
Stacy Doumas, MD
Vice Chair
Associate Professor
Department of Psychiatry and Behavioral Health
Hackensack Meridian School of Medicine at Seton Hall University
Nutley, New Jersey
Vice Chair for Education & Research
Residency Training Director
General Psychiatry
Jersey Shore University Medical Center Neptune, New Jersey
Saba Afzal, MD
Assistant Professor
Department of Psychiatry and Behavioral Health
Hackensack Meridian School of Medicine at Seton Hall University
Nutley, New Jersey
Associate Residency Training Director General Psychiatry
Ocean Medical Center
Brick, New Jersey
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
1. Bousman CA, Arandjelovic K, Mancuso SG, et al. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenetics. 2019;20(1):37-47.
2. International Society for Psychiatric Genetics. Genetics testing statement: genetic testing and psychiatric disorders. https://ispg.net/genetic-testing-statement. Updated March 11, 2019. Accessed January 9, 2020.
3. Zubenko GS, Sommer BR, Cohen BM. Pharmacogenetics in psychiatry; a companion, rather than competitor, to protocol-based care-reply. JAMA Psychiatry. 2018;75(10):1090-1091.
1. Bousman CA, Arandjelovic K, Mancuso SG, et al. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenetics. 2019;20(1):37-47.
2. International Society for Psychiatric Genetics. Genetics testing statement: genetic testing and psychiatric disorders. https://ispg.net/genetic-testing-statement. Updated March 11, 2019. Accessed January 9, 2020.
3. Zubenko GS, Sommer BR, Cohen BM. Pharmacogenetics in psychiatry; a companion, rather than competitor, to protocol-based care-reply. JAMA Psychiatry. 2018;75(10):1090-1091.
We are physicians, not providers, and we treat patients, not clients!
One of the most malignant threats that is adversely impacting physicians is the insidious metastasis of the term “provider” within the national health care system over the past 2 to 3 decades.
This demeaning adjective is outrageously inappropriate and beneath the stature of medical doctors (MDs) who sacrificed 12 to 15 years of their lives in college, medical schools, residency programs, and post-residency fellowships to become physicians, specialists, and subspecialists. It is distressing to see hospitals, clinics, pharmacies, insurance corporations, and managed care companies refer to psychiatrists and other physicians as “providers.” It is time to fight back and restore our noble medical identity, which society has always respected and appreciated.
Our unique professional identify is at stake. We do not want to be lumped with nonphysicians as if we are interchangeable parts of a health care system or cogs in a wheel. No other mental health professional has the extensive training, scientific knowledge, clinical expertise, research accomplishments, and teaching/supervisory abilities that physicians have. We strongly uphold the sacred tenet of the physician-patient relationship, and adamantly reject its corruption into a provider-consumer transaction.
Even plumbers and electricians are not referred to as “providers.” Lawyers are not called legal aid providers. Teachers are not called knowledge providers, and administrators and CEOs are not called management providers. So why should physicians in any specialty, including psychiatry, obsequiously accept the denigration of their esteemed medical identify into the vague, amorphous ipseity of a “provider”? Family physicians, internists, and pediatricians used to be called primary care physicians, but have been reduced to primary care providers, which is insulting and degrading to these highly trained MD specialists.
The corruption and debasement of the professional identify of physicians and the propagation of the usage of the belittling term “provider” can be traced back to 3 entities:
1. The Nazi Third Reich. This is the most evil origin of the term “provider,” inflicted on Jewish physicians as part of the despicable persecution of German Jews in the 1930s. The Nazis decided to deprive pediatricians of being called physicians (“Arzt” in German) and forcefully relabeled them as “behandlers” or “providers,” thus erasing their noble medical identity.1 In 1933, all Jewish pediatricians were expelled or forced to resign from the German Society of Pediatrics and were no longer allowed to be called doctors. This deliberate and systematic humiliation of pediatric clinicians and scientists was followed by deporting the lowly “providers” to concentration camps. So why perpetuate this pernicious Nazi terminology?
2. The Federal Government. The term “provider” was introduced and propagated in Public Law 93-641 titled “The National Health Planning and Resource Development Act of 1974.” In that document, patients were referred to as “consumers” and physicians as “providers” (this term was used 19 times in that law). At that time, the civil service employees who drafted the law that marginalized physicians by using generic, nonmedical nomenclature may not have realized the dire consequences of relabeling physicians as “providers.”
Continue to: Insurance companies, managed care companies, and consolidated health systems...
3. Insurance companies, managed care companies, and consolidated health systems have jubilantly adopted the term “provider” because they can equate physicians with less expensive, nonphysician clinicians (physician assistants, nurse practitioners, and certified registered nurse anesthetists), especially when physicians across several specialties (particularly psychiatry) are in short supply. None of these clinicians deserve to be labeled “providers,” either.
To understand why the term “provider” was used instead of “clinicians” or “clinical practitioner,” one must recognize the “businessification” of medicine and the commoditization of clinical care in our country. In some ways, health care has adopted a model similar to a fast-food joint, where workers provide customers with a hamburger. The question here is why did the 1.1 million physicians in the United States not halt this terminology shift before it spread and permeated the national health care system? Physicians who graduate from medical schools (not “provider” schools!) must vigorously and loudly fight back and put this wicked genie back in its bottle. This is feasible only if the American Medical Association (which would never conceive of itself as the “American Provider Association”), along with all 48 specialty organizations (Table), including the American Psychiatric Association (APA), unite and demand that physicians be called medical doctors or physicians, or by a term that reflects their specialty (orthopedists, psychiatrists, oncologists, gastroenterologists, anesthesiologists, cardiologists, etc.). This is an urgent issue to prevent the dissolution of our professional identity and its highly regarded societal image. It is a travesty that we physicians have allowed it to go on unopposed and to become entrenched in the dumbed-down jargon of health care. Physicians tend to avoid confrontation and adversarial stances, but we must unite and demand a return to the traditional nomenclature of medicine.
Much debate has emerged lately about an epidemic of “burnout” among physicians. Proposed causes include the savage increase in the amount of paperwork at the expense of patient care, the sense of helplessness that pre-authorization has inflicted on physicians’ decision-making, and the tyranny of relative value units (RVUs) as a benchmark for physician performance, as if healing patients is like manufacturing widgets. However, the blow to the self-esteem of physicians by being called “providers” daily is certainly another major factor contributing to burnout. It is perfectly legitimate for physicians to expect recognition for their long, rigorous, and uniquely advanced medical training, instead of being lumped together with less qualified professionals as anonymous “providers” in the name of politically correct pseudo-equality of all clinical practitioners. Let the administrators stop and contemplate whether tertiary or quaternary care for the most complex and severely ill patients in medical, surgical, or psychiatric intensive care units can operate without highly specialized physicians.
I urge APA leadership to take a visible and strong stand to rid psychiatrists of this assault on our medical identity. As I mentioned in my January 2020 editorial,2 it is vital that the name of our national psychiatric organization (APA) be modified to the American Psychiatric Physicians Association, to remind all health care systems, as well as patients, the public, and the media, of our medical identity as physicians before we specialized in psychiatry.
Continue to: Patients, not clients
Patients, not clients
We should also emphasize that our suffering and medically ill patients with serious neuropsychiatric disorders such as schizophrenia, bipolar disorder, depression, panic disorder, or obsessive-compulsive disorder are patients, not clients. The terminology used in community mental health centers around the country almost universally includes “providers” and “clients.” This de-medicalization of psychiatrists and our patients must be corrected and reversed so that the public understands that treating mental illness is not a business transaction between a “provider” and a “client.” Using the correct terminology may help generate sympathy and compassion towards patients with serious psychiatric illnesses, just as it does for patients with cancer, heart disease, or stroke. The term “client” will never evoke the public sympathy and support that our patients truly deserve.
Let’s keep this issue alive and translate our demands into actions, both locally and nationally. Psychiatrists and physicians of all other specialties must stand up for their rights and inform their systems of care that they must be called by their legitimate and lawful name: physicians or medical doctors (never “providers”). This is an issue that unites all 1.1 million of us. The US health care system would collapse without us, and asking that we be called exactly what our medical license displays is our right and our professional identity.
1. Saenger P. Jewish pediatricians in Nazi Germany: victims of persecution. Isr Med Assoc J. 2006;8(5):324-328.
2. Nasrallah HA. 20 Reasons to celebrate our APA membership in 2020. Current Psychiatry. 2020;19(1):6-9.
One of the most malignant threats that is adversely impacting physicians is the insidious metastasis of the term “provider” within the national health care system over the past 2 to 3 decades.
This demeaning adjective is outrageously inappropriate and beneath the stature of medical doctors (MDs) who sacrificed 12 to 15 years of their lives in college, medical schools, residency programs, and post-residency fellowships to become physicians, specialists, and subspecialists. It is distressing to see hospitals, clinics, pharmacies, insurance corporations, and managed care companies refer to psychiatrists and other physicians as “providers.” It is time to fight back and restore our noble medical identity, which society has always respected and appreciated.
Our unique professional identify is at stake. We do not want to be lumped with nonphysicians as if we are interchangeable parts of a health care system or cogs in a wheel. No other mental health professional has the extensive training, scientific knowledge, clinical expertise, research accomplishments, and teaching/supervisory abilities that physicians have. We strongly uphold the sacred tenet of the physician-patient relationship, and adamantly reject its corruption into a provider-consumer transaction.
Even plumbers and electricians are not referred to as “providers.” Lawyers are not called legal aid providers. Teachers are not called knowledge providers, and administrators and CEOs are not called management providers. So why should physicians in any specialty, including psychiatry, obsequiously accept the denigration of their esteemed medical identify into the vague, amorphous ipseity of a “provider”? Family physicians, internists, and pediatricians used to be called primary care physicians, but have been reduced to primary care providers, which is insulting and degrading to these highly trained MD specialists.
The corruption and debasement of the professional identify of physicians and the propagation of the usage of the belittling term “provider” can be traced back to 3 entities:
1. The Nazi Third Reich. This is the most evil origin of the term “provider,” inflicted on Jewish physicians as part of the despicable persecution of German Jews in the 1930s. The Nazis decided to deprive pediatricians of being called physicians (“Arzt” in German) and forcefully relabeled them as “behandlers” or “providers,” thus erasing their noble medical identity.1 In 1933, all Jewish pediatricians were expelled or forced to resign from the German Society of Pediatrics and were no longer allowed to be called doctors. This deliberate and systematic humiliation of pediatric clinicians and scientists was followed by deporting the lowly “providers” to concentration camps. So why perpetuate this pernicious Nazi terminology?
2. The Federal Government. The term “provider” was introduced and propagated in Public Law 93-641 titled “The National Health Planning and Resource Development Act of 1974.” In that document, patients were referred to as “consumers” and physicians as “providers” (this term was used 19 times in that law). At that time, the civil service employees who drafted the law that marginalized physicians by using generic, nonmedical nomenclature may not have realized the dire consequences of relabeling physicians as “providers.”
Continue to: Insurance companies, managed care companies, and consolidated health systems...
3. Insurance companies, managed care companies, and consolidated health systems have jubilantly adopted the term “provider” because they can equate physicians with less expensive, nonphysician clinicians (physician assistants, nurse practitioners, and certified registered nurse anesthetists), especially when physicians across several specialties (particularly psychiatry) are in short supply. None of these clinicians deserve to be labeled “providers,” either.
To understand why the term “provider” was used instead of “clinicians” or “clinical practitioner,” one must recognize the “businessification” of medicine and the commoditization of clinical care in our country. In some ways, health care has adopted a model similar to a fast-food joint, where workers provide customers with a hamburger. The question here is why did the 1.1 million physicians in the United States not halt this terminology shift before it spread and permeated the national health care system? Physicians who graduate from medical schools (not “provider” schools!) must vigorously and loudly fight back and put this wicked genie back in its bottle. This is feasible only if the American Medical Association (which would never conceive of itself as the “American Provider Association”), along with all 48 specialty organizations (Table), including the American Psychiatric Association (APA), unite and demand that physicians be called medical doctors or physicians, or by a term that reflects their specialty (orthopedists, psychiatrists, oncologists, gastroenterologists, anesthesiologists, cardiologists, etc.). This is an urgent issue to prevent the dissolution of our professional identity and its highly regarded societal image. It is a travesty that we physicians have allowed it to go on unopposed and to become entrenched in the dumbed-down jargon of health care. Physicians tend to avoid confrontation and adversarial stances, but we must unite and demand a return to the traditional nomenclature of medicine.
Much debate has emerged lately about an epidemic of “burnout” among physicians. Proposed causes include the savage increase in the amount of paperwork at the expense of patient care, the sense of helplessness that pre-authorization has inflicted on physicians’ decision-making, and the tyranny of relative value units (RVUs) as a benchmark for physician performance, as if healing patients is like manufacturing widgets. However, the blow to the self-esteem of physicians by being called “providers” daily is certainly another major factor contributing to burnout. It is perfectly legitimate for physicians to expect recognition for their long, rigorous, and uniquely advanced medical training, instead of being lumped together with less qualified professionals as anonymous “providers” in the name of politically correct pseudo-equality of all clinical practitioners. Let the administrators stop and contemplate whether tertiary or quaternary care for the most complex and severely ill patients in medical, surgical, or psychiatric intensive care units can operate without highly specialized physicians.
I urge APA leadership to take a visible and strong stand to rid psychiatrists of this assault on our medical identity. As I mentioned in my January 2020 editorial,2 it is vital that the name of our national psychiatric organization (APA) be modified to the American Psychiatric Physicians Association, to remind all health care systems, as well as patients, the public, and the media, of our medical identity as physicians before we specialized in psychiatry.
Continue to: Patients, not clients
Patients, not clients
We should also emphasize that our suffering and medically ill patients with serious neuropsychiatric disorders such as schizophrenia, bipolar disorder, depression, panic disorder, or obsessive-compulsive disorder are patients, not clients. The terminology used in community mental health centers around the country almost universally includes “providers” and “clients.” This de-medicalization of psychiatrists and our patients must be corrected and reversed so that the public understands that treating mental illness is not a business transaction between a “provider” and a “client.” Using the correct terminology may help generate sympathy and compassion towards patients with serious psychiatric illnesses, just as it does for patients with cancer, heart disease, or stroke. The term “client” will never evoke the public sympathy and support that our patients truly deserve.
Let’s keep this issue alive and translate our demands into actions, both locally and nationally. Psychiatrists and physicians of all other specialties must stand up for their rights and inform their systems of care that they must be called by their legitimate and lawful name: physicians or medical doctors (never “providers”). This is an issue that unites all 1.1 million of us. The US health care system would collapse without us, and asking that we be called exactly what our medical license displays is our right and our professional identity.
One of the most malignant threats that is adversely impacting physicians is the insidious metastasis of the term “provider” within the national health care system over the past 2 to 3 decades.
This demeaning adjective is outrageously inappropriate and beneath the stature of medical doctors (MDs) who sacrificed 12 to 15 years of their lives in college, medical schools, residency programs, and post-residency fellowships to become physicians, specialists, and subspecialists. It is distressing to see hospitals, clinics, pharmacies, insurance corporations, and managed care companies refer to psychiatrists and other physicians as “providers.” It is time to fight back and restore our noble medical identity, which society has always respected and appreciated.
Our unique professional identify is at stake. We do not want to be lumped with nonphysicians as if we are interchangeable parts of a health care system or cogs in a wheel. No other mental health professional has the extensive training, scientific knowledge, clinical expertise, research accomplishments, and teaching/supervisory abilities that physicians have. We strongly uphold the sacred tenet of the physician-patient relationship, and adamantly reject its corruption into a provider-consumer transaction.
Even plumbers and electricians are not referred to as “providers.” Lawyers are not called legal aid providers. Teachers are not called knowledge providers, and administrators and CEOs are not called management providers. So why should physicians in any specialty, including psychiatry, obsequiously accept the denigration of their esteemed medical identify into the vague, amorphous ipseity of a “provider”? Family physicians, internists, and pediatricians used to be called primary care physicians, but have been reduced to primary care providers, which is insulting and degrading to these highly trained MD specialists.
The corruption and debasement of the professional identify of physicians and the propagation of the usage of the belittling term “provider” can be traced back to 3 entities:
1. The Nazi Third Reich. This is the most evil origin of the term “provider,” inflicted on Jewish physicians as part of the despicable persecution of German Jews in the 1930s. The Nazis decided to deprive pediatricians of being called physicians (“Arzt” in German) and forcefully relabeled them as “behandlers” or “providers,” thus erasing their noble medical identity.1 In 1933, all Jewish pediatricians were expelled or forced to resign from the German Society of Pediatrics and were no longer allowed to be called doctors. This deliberate and systematic humiliation of pediatric clinicians and scientists was followed by deporting the lowly “providers” to concentration camps. So why perpetuate this pernicious Nazi terminology?
2. The Federal Government. The term “provider” was introduced and propagated in Public Law 93-641 titled “The National Health Planning and Resource Development Act of 1974.” In that document, patients were referred to as “consumers” and physicians as “providers” (this term was used 19 times in that law). At that time, the civil service employees who drafted the law that marginalized physicians by using generic, nonmedical nomenclature may not have realized the dire consequences of relabeling physicians as “providers.”
Continue to: Insurance companies, managed care companies, and consolidated health systems...
3. Insurance companies, managed care companies, and consolidated health systems have jubilantly adopted the term “provider” because they can equate physicians with less expensive, nonphysician clinicians (physician assistants, nurse practitioners, and certified registered nurse anesthetists), especially when physicians across several specialties (particularly psychiatry) are in short supply. None of these clinicians deserve to be labeled “providers,” either.
To understand why the term “provider” was used instead of “clinicians” or “clinical practitioner,” one must recognize the “businessification” of medicine and the commoditization of clinical care in our country. In some ways, health care has adopted a model similar to a fast-food joint, where workers provide customers with a hamburger. The question here is why did the 1.1 million physicians in the United States not halt this terminology shift before it spread and permeated the national health care system? Physicians who graduate from medical schools (not “provider” schools!) must vigorously and loudly fight back and put this wicked genie back in its bottle. This is feasible only if the American Medical Association (which would never conceive of itself as the “American Provider Association”), along with all 48 specialty organizations (Table), including the American Psychiatric Association (APA), unite and demand that physicians be called medical doctors or physicians, or by a term that reflects their specialty (orthopedists, psychiatrists, oncologists, gastroenterologists, anesthesiologists, cardiologists, etc.). This is an urgent issue to prevent the dissolution of our professional identity and its highly regarded societal image. It is a travesty that we physicians have allowed it to go on unopposed and to become entrenched in the dumbed-down jargon of health care. Physicians tend to avoid confrontation and adversarial stances, but we must unite and demand a return to the traditional nomenclature of medicine.
Much debate has emerged lately about an epidemic of “burnout” among physicians. Proposed causes include the savage increase in the amount of paperwork at the expense of patient care, the sense of helplessness that pre-authorization has inflicted on physicians’ decision-making, and the tyranny of relative value units (RVUs) as a benchmark for physician performance, as if healing patients is like manufacturing widgets. However, the blow to the self-esteem of physicians by being called “providers” daily is certainly another major factor contributing to burnout. It is perfectly legitimate for physicians to expect recognition for their long, rigorous, and uniquely advanced medical training, instead of being lumped together with less qualified professionals as anonymous “providers” in the name of politically correct pseudo-equality of all clinical practitioners. Let the administrators stop and contemplate whether tertiary or quaternary care for the most complex and severely ill patients in medical, surgical, or psychiatric intensive care units can operate without highly specialized physicians.
I urge APA leadership to take a visible and strong stand to rid psychiatrists of this assault on our medical identity. As I mentioned in my January 2020 editorial,2 it is vital that the name of our national psychiatric organization (APA) be modified to the American Psychiatric Physicians Association, to remind all health care systems, as well as patients, the public, and the media, of our medical identity as physicians before we specialized in psychiatry.
Continue to: Patients, not clients
Patients, not clients
We should also emphasize that our suffering and medically ill patients with serious neuropsychiatric disorders such as schizophrenia, bipolar disorder, depression, panic disorder, or obsessive-compulsive disorder are patients, not clients. The terminology used in community mental health centers around the country almost universally includes “providers” and “clients.” This de-medicalization of psychiatrists and our patients must be corrected and reversed so that the public understands that treating mental illness is not a business transaction between a “provider” and a “client.” Using the correct terminology may help generate sympathy and compassion towards patients with serious psychiatric illnesses, just as it does for patients with cancer, heart disease, or stroke. The term “client” will never evoke the public sympathy and support that our patients truly deserve.
Let’s keep this issue alive and translate our demands into actions, both locally and nationally. Psychiatrists and physicians of all other specialties must stand up for their rights and inform their systems of care that they must be called by their legitimate and lawful name: physicians or medical doctors (never “providers”). This is an issue that unites all 1.1 million of us. The US health care system would collapse without us, and asking that we be called exactly what our medical license displays is our right and our professional identity.
1. Saenger P. Jewish pediatricians in Nazi Germany: victims of persecution. Isr Med Assoc J. 2006;8(5):324-328.
2. Nasrallah HA. 20 Reasons to celebrate our APA membership in 2020. Current Psychiatry. 2020;19(1):6-9.
1. Saenger P. Jewish pediatricians in Nazi Germany: victims of persecution. Isr Med Assoc J. 2006;8(5):324-328.
2. Nasrallah HA. 20 Reasons to celebrate our APA membership in 2020. Current Psychiatry. 2020;19(1):6-9.
Lumateperone for schizophrenia
Antipsychotic nonadherence is a known contributor to relapse risk among patients with schizophrenia.1 Because relapse episodes may be associated with antipsychotic treatment resistance, this must be avoided as much as possible by appropriate medication selection.2 Adverse effect burden is an important factor leading to oral antipsychotic nonadherence, with patient-derived data indicating that extrapyramidal symptoms (EPS) (odds ratio [OR] 0.57, P = .0007), sedation/cognitive adverse effects (OR 0.70, P = .033), prolactin/endocrine effects (OR 0.69, P = .0342), and metabolic adverse effects (OR 0.64, P = .0079) are all significantly related to lower rates of adherence.3 With this in mind, successive generations of antipsychotics have been released, with fewer tolerability issues present than seen with earlier compounds.1,4 Although these newer second-generation antipsychotics (SGAs) have not proven more effective for schizophrenia than those first marketed in the 1990s, they generally possess lower rates of EPS, hyperprolactinemia, anticholinergic and antihistaminic properties, metabolic adverse effects, and orthostasis.5 This improved adverse effect profile will hopefully increase the chances of antipsychotic acceptance in patients with schizophrenia, and thereby promote improved adherence.
Lumateperone (Caplyta) is a novel oral antipsychotic approved for the treatment of adult patients with schizophrenia (Table 1). It possesses some properties seen with other SGAs, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM) and lower affinity for dopamine D2 receptors (Ki 32 nM), along with low affinity for alpha1-adrenergic receptors (Ki 73 nM), and muscarinic and histaminergic receptors (Ki > 100 nM).6,7 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that of other SGAs such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18)8; at steady state, the D2 occupancy remains <40% (Figure) and the corresponding rates of EPS/akathisia were only 6.7% for lumateperone vs 6.3% for placebo in short-term clinical trials.7,9
How it works
A unique aspect of lumateperone’s pharmacology may relate to differential actions at presynaptic and postsynaptic dopamine D2 receptors. Other antipsychotics possess comparable antagonist (or partial agonist) properties at postsynaptic D2 receptors (the D2 long isoform) and the presynaptic autoreceptor (the D2 short isoform). By blocking the presynaptic autoreceptor, feedback inhibition on dopamine release is removed; therefore, the required higher levels of postsynaptic D2 receptor occupancy needed for effective antipsychotic action (eg, 60% to 80% for antagonists, and 83% to 100% for partial agonists) may be a product of the need to oppose this increased presynaptic release of dopamine. In vitro assays show that lumateperone does not increase presynaptic dopamine release, indicating that it possesses agonist properties at the presynaptic D2 short receptor.10 That property may explain how lumateperone functions as an antipsychotic despite low levels of D2 receptor occupancy.10
Another hypothesis is based on our understanding of pimavanserin’s pharmacology. Pimavanserin is a selective 5HT2A antagonist FDA-approved for the treatment of Parkinson’s disease psychosis (PDP), with extremely high receptor affinity (Ki 0.087 nM) and no appreciable binding at dopamine receptors.5 Pimavanserin not only treats PDP, but is being evaluated in clinical trials for dementia-related psychosis, and has positive data for augmenting antipsychotics when there is a low level of D2 blockade.11,12 In a controlled trial, pimavanserin added to risperidone, 2 mg/d, was as effective as risperidone, 6 mg/d, illustrating the point that near-saturation of the 5HT2A receptor can increase antipsychotic efficacy when dopamine blockade is relatively low. For risperidone, 2 mg/d, the expected D2 occupancy is only 60%.13
Lumateperone also has moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM). Serotonin transporter occupancy at the dose approved for schizophrenia (42 mg/d) is approximately 30%,14 below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitor (SSRI) antidepressants; nevertheless, there is evidence for antidepressant effects seen in preclinical assays, schizophrenia studies, and phase III trials for bipolar depression.8,15,16 It is hypothesized that near-saturation of the 5HT2A receptor might act synergistically with the modest extent of 5HT reuptake inhibition to promote downstream effects associated with effective antidepressant treatments.8 In vivo data also showed phosphorylation of N-methyl-
Clinical implications
Nonadherence with oral antipsychotics among patients with schizophrenia is often related to adverse effects.17 The SGAs marketed since 2000 generally have lower rates of sedation and metabolic and/or endocrine adverse events than earlier compounds, yet each has limitations:
- asenapine: sedation and weight gain
- the partial agonists (aripiprazole, brexpiprazole, cariprazine): akathisia
- lurasidone: dose-dependent EPS and akathisia
- iloperidone: orthostasis.18
Ziprasidone is an exception, because it had low rates of most adverse effects in schizophrenia trials, but the need to take it twice daily with a 500 kcal meal hampers its use. A meta-analysis of 32 oral antipsychotics, including first-generation agents, noted that the efficacy differences between medications are slight for patients without treatment-resistant schizophrenia, but “differences in side-effects are more marked.”18
Continue to: Until novel mechanisms are discovered...
Until novel mechanisms are discovered that increase schizophrenia response rates, the availability of newer antipsychotics with more favorable tolerability profiles presents clinicians and patients with added options when adverse effects interfere with prior treatment. In all phases of the adult schizophrenia trial program for lumateperone, 811 patients received short-term (4- to 6-week) exposure (dose range: 14 to 84 mg/d), while 329 had ≥6 months exposure and 108 had ≥1 year of exposure to the 42-mg/d dose. In these studies, there was no single adverse reaction leading to discontinuation that occurred at a rate >2%. The only adverse events that occurred at rates ≥5% and more than twice the rate of placebo were somnolence/sedation (lumateperone 24%, placebo 10%), and dry mouth (lumateperone 6%, placebo 2%). Nausea was present in 9% of the lumateperone group compared with 5% for placebo.7 In the short-term studies, the combined rate of EPS and akathisia was 6.7% for lumateperone and 6.3% for placebo.7 This difference translates to a number needed to harm of 250 for these neurologic adverse effects. The functional impact of lumateperone’s glutamatergic mechanisms is not well characterized within the schizophrenia population, but the antidepressant potential has been studied for patients with bipolar depression, with 1 positive phase III trial.19
Efficacy in adults with schizophrenia. The efficacy of lumateperone has been established in 2 pivotal, double-blind, placebo-controlled trials. The first was a 4-week, phase II trial (Study 005) in which 335 adults age 18 to 55 with an acute exacerbation of schizophrenia were randomized in a 1:1:1:1 manner to lumateperone, 42 mg/d (60 mg of lumateperone tosylate), lumateperone, 84 mg/d (120 mg of lumateperone tosylate), risperidone, 4 mg/d, or placebo, all taken once daily.20 For the 4 treatment arms, the least squares mean changes from baseline to the Day 28 endpoint on the primary outcome measure, Positive and Negative Syndrome Scale (PANSS) total score, were: lumateperone, 42 mg/d: −13.2 points; lumateperone, 84 mg/d: −8.3 points; risperidone, 4 mg/d: −13.4 points; and placebo: −7.4 points. Both lumateperone, 42 mg/d, and risperidone, 4 mg/d, were significantly different than placebo, and with identical moderate effect sizes of 0.4.20 Lumateperone, 84 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that a similar proportion of patients (40%) randomized to lumateperone, 42 mg/d, or risperidone, 4 mg/d, improved by ≥30% on PANSS total score.
The second pivotal trial (Study 301) was a phase III, double-blind, placebo-controlled trial of 450 adults, age 18 to 60, with an acute exacerbation of schizophrenia who were randomized in 1:1:1 manner to receive lumateperone, 42 mg/d (lumateperone tosylate 60 mg), lumateperone, 28 mg/d (lumateperone tosylate 40 mg), or placebo once daily for 4 weeks.21 For the 3 treatment arms, the least squares mean changes on PANSS total score from baseline to the Day 28 endpoint were: lumateperone, 42 mg/d: −14.5 points; lumateperone, 28 mg/d: −12.9 points; and placebo: −10.3 points. Lumateperone, 28 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that 36.5% of those receiving lumateperone, 42 mg/d, and 36.3% of those receiving lumateperone, 28 mg/d, improved by ≥30% on PANSS total score, compared with 25.5% of patients treated with placebo.
Unlike the 2 positive trials in which placebo change in total PANSS scores were −7.4 and −10.3 points, respectively, in a phase III trial (Study 302) with 696 participants, placebo showed a 15.1-point decrease from baseline PANSS total score.19 Among the 3 treatment arms of this study (lumateperone, 14 mg/d, lumateperone, 42 mg/d, and risperidone, 4 mg/d), only risperidone was superior to placebo.
Adverse events
In the phase II pivotal study, completion rates among the 4 arms were comparable: lumateperone, 42 mg/d: 71%; lumateperone, 84 mg/d: 76%; risperidone, 4 mg/d: 77%; and placebo: 72%.20 There were no serious adverse events (SAEs) associated with lumateperone; the 2 SAEs that occurred involved worsening of schizophrenia/psychotic disorder for risperidone (n = 1) and for placebo (n = 1). Five participants discontinued the study due to an adverse event: 2 who were receiving lumateperone (1 due to dry mouth, and 1 due to worsening of schizophrenia) and 3 who were receiving risperidone (2 due to akathisia, and 1 due to blood creatine phosphokinase increase).20 The most frequent adverse event was somnolence/sedation (placebo: 13%; lumateperone, 42 mg/d: 17%; risperidone, 4 mg/d: 21%; and lumateperone, 84 mg/d: 32.5%). Neither dose of lumateperone was associated with increased rates of EPS. Median weight gain to Day 28 was 1 kg for placebo and for each dose of lumateperone, and 2.5 kg for risperidone. Compared with risperidone, lumateperone showed statistically significantly lower prolactin levels (lumateperone, 42 mg/d and 84 mg/d: P < .001), and metabolic parameters, including fasting glucose (lumateperone 42 mg/d: P = .007; lumateperone, 84 mg/d: P = .023), total cholesterol (lumateperone, 42 mg/d: P = .012; lumateperone, 84 mg/d: P = .004), and triglycerides (lumateperone, 42 mg/d: P = .074; lumateperone, 84 mg/d: P = .002).20 There was no significant impact on the corrected QT interval.
Continue to: In the phase III trial...
In the phase III trial, completion rates among the 3 arms were lumateperone, 42 mg/d: 85%; lumateperone, 28 mg/d: 80%; and placebo: 74%. There was 1 SAE in a patient receiving lumateperone, 28 mg/d. This individual had preexisting risk factors and a history of seizures, and experienced a seizure during the study. Adverse events that occurred in either lumateperone group at a rate ≥5% and more than twice the rate of placebo were somnolence (lumateperone, 42 mg/d: 17.3%; lumateperone, 28 mg/d: 11.3%; and placebo: 4.0%); sedation (lumateperone, 42 mg/d: 12.7%; lumateperone, 28 mg/d: 9.3%; and placebo: 5.4%); fatigue (lumateperone, 42 mg/d: 5.3%; lumateperone, 28 mg/d: 4.7%; and placebo: 1.3%); and constipation (lumateperone, 42 mg/d: 6.7%; lumateperone, 28 mg/d: 4.0%; and placebo: 2.7%).21 No EPS-related adverse events occurred in ≥5% patients in any treatment arm. Median change in weight from baseline to Day 28 was 0.9 kg for lumateperone, 42 mg/d, 0.6 kg for lumateperone, 28 mg/d, and 0.7 kg for placebo. There were no significant mean changes in metabolic parameters for any treatment arm, and none of the patients had a corrected QT interval (QTc) >500 ms or a change in QTc >60 ms from baseline.21
Pharmacologic profile
Lumateperone’s in vitro binding profile includes high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), lower affinity for dopamine D2 receptors (Ki 32 nM), moderate binding affinity for SERT (Ki 33 nM), and lower affinity for alpha 1-adrenergic receptors (Ki 73 nM) and muscarinic and histaminergic receptors (Ki >100 nM).6,7 As noted above, this 60-fold ratio of 5HT2A to D2 affinity is extremely high; moreover, imaging data reveal low D2 receptor occupancy, consistent with the lack of clinically significant EPS seen in the trials. In vitro assays also reveal impact on glutamate pathways, and pathways associated with antidepressant response.8 The clinical benefits of the glutamatergic properties remain theoretical, but the antidepressant benefit has been seen in a positive phase III trial for bipolar depression.19
Clinical considerations
Effect sizes in the 2 positive pivotal trials were 0.3 and 0.4, comparable with those for other antipsychotics approved within the last decade: brexpiprazole, 0.26; cariprazine, 0.34; and lurasidone, 0.36.21 The absence of clinically significant EPS, lack of impact on metabolic or endocrine parameters, and lack of titration are all appealing properties. That only 42 mg/d proved effective may reflect the fact that the other doses studied to date in randomized, fixed-dose studies were 14 mg/d (Study 302) and 84 mg/d (Study 005), evaluated in one study each. While those 2 doses might indeed be outside the therapeutic window, given the heterogeneity of schizophrenia, future studies might help further refine the therapeutic range for schizophrenia, especially for doses closer to 42 mg/d (eg, 28 mg/d, 63 mg/d). Should 42 mg/d not prove effective, there is no data for now to suggest whether a dose increase may be helpful. As there is only 1 marketed dose of lumateperone (42-mg capsules), and no easy way to modify this dose, lumateperone’s package insert includes cautionary language regarding situations where there will be less-than-expected drug exposure (use of cytochrome P450 [CYP] 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant EPS and metabolic or endocrine adverse effects. In vitro data indicate glutamatergic effects, and human data indicate antidepressant effects in bipolar patients. Despite the absence of significant histamine H1 or muscarinic affinity, the rate of somnolence/sedation was twice that of placebo (lumateperone 24%, placebo 10%).7
Why Rx? Reasons to prescribe lumateperone for adult patients with schizophrenia include:
- Favorable tolerability profile, including no significant signal for EPS or endocrine or metabolic adverse effects, and no QT prolongation
- No need for titration.
Dosing. There is only 1 dose available for lumateperone, 42-mg capsules (Table 2). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less-than-expected drug exposure (use of CYP 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh criteria (Child-Pugh B or C). These are not contraindications.
Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Continue to: Bottom Line
Bottom Line
Lumateperone is a novel oral antipsychotic indicated for treating adults with schizophrenia. Its unique properties include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant extrapyramidal symptoms and metabolic or endocrine adverse effects. In clinical trials, the most frequent adverse event was somnolence/sedation.
Related Resource
- Fulton D. FDA approves Caplyta to treat schizophrenenia in adults. https://www.mdedge.com/psychiatry/article/214733/schizophrenia-other-psychotic-disorders/fda-approves-caplyta-treat.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Pimavanserin • Nuplazid
Risperidone • Risperdal
Ziprasidone • Geodon
1. Dufort A, Zipursky RB. Understanding and managing treatment adherence in schizophrenia [published online January 3, 2019]. Clin Schizophr Relat Psychoses. 2019. doi: 10.3371/CSRP.ADRZ.121218.
2. Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? Antipsychotic response in first- vs. second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036-1042.
3. Dibonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12:20.
4. Kurokawa S, Kishimoto T, Su K-P, et al. Psychiatrists’ perceptions of medication adherence among patients with schizophrenia: an international survey. Schizophr Res. 2019;211:105-107.
5. Meyer JM. Pharmacotherapy of psychosis and mania. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. Chicago, Illinois: McGraw-Hill; 2018:279-302.
6. Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614.
7. Caplyta [package Insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
8. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
9. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
10. Zhang L, Hendrick JP. The presynaptic D2 partial agonist lumateperone acts as a postsynaptic D2 antagonist. Matters. 2018. doi: 10.19185/matters.201712000006.
11. Meltzer HY, Elkis H, Vanover K, et al. Pimavanserin, a selective serotonin (5-HT)2A-inverse agonist, enhances the efficacy and safety of risperidone, 2mg/day, but does not enhance efficacy of haloperidol, 2mg/day: comparison with reference dose risperidone, 6mg/day. Schizophr Res. 2012;141(2-3):144-152.
12. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
13. Remington G, Mamo D, Labelle A, et al. A PET study evaluating dopamine D2 receptor occupancy for long-acting injectable risperidone. Am J Psychiatry. 2006;163(3):396-401.
14. Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl). 2015;232(15):2863-2872.
15. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018;54(12):713-719.
16. Vanover K, Glass S, Kozauer S, et al. 30 lumateperone (ITI-007) for the treatment of schizophrenia: overview of placebo-controlled clinical trials and an open-label safety switching study. CNS Spectr. 2019;24(1):190-191.
17. Young SL, Taylor M, Lawrie SM. “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. 2015;29(4):353-362.
18. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939-951.
19. Vyas P, Hwang BJ, Brašic ´ JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2019;1-7.
20. Lieberman JA, Davis RE, Correll CU, et al. ITI-007 for the treatment of schizophrenia: a 4-week randomized, double-blind, controlled trial. Biol Psychiatry. 2016;79(12):952-961.
21. Correll CU, Davis RE, Weingart M, et al. Efficacy and safety of lumateperone for treatment of schizophrenia [published online January 8, 2020]. JAMA Psychiatry. 2020;E1-E10.
Antipsychotic nonadherence is a known contributor to relapse risk among patients with schizophrenia.1 Because relapse episodes may be associated with antipsychotic treatment resistance, this must be avoided as much as possible by appropriate medication selection.2 Adverse effect burden is an important factor leading to oral antipsychotic nonadherence, with patient-derived data indicating that extrapyramidal symptoms (EPS) (odds ratio [OR] 0.57, P = .0007), sedation/cognitive adverse effects (OR 0.70, P = .033), prolactin/endocrine effects (OR 0.69, P = .0342), and metabolic adverse effects (OR 0.64, P = .0079) are all significantly related to lower rates of adherence.3 With this in mind, successive generations of antipsychotics have been released, with fewer tolerability issues present than seen with earlier compounds.1,4 Although these newer second-generation antipsychotics (SGAs) have not proven more effective for schizophrenia than those first marketed in the 1990s, they generally possess lower rates of EPS, hyperprolactinemia, anticholinergic and antihistaminic properties, metabolic adverse effects, and orthostasis.5 This improved adverse effect profile will hopefully increase the chances of antipsychotic acceptance in patients with schizophrenia, and thereby promote improved adherence.
Lumateperone (Caplyta) is a novel oral antipsychotic approved for the treatment of adult patients with schizophrenia (Table 1). It possesses some properties seen with other SGAs, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM) and lower affinity for dopamine D2 receptors (Ki 32 nM), along with low affinity for alpha1-adrenergic receptors (Ki 73 nM), and muscarinic and histaminergic receptors (Ki > 100 nM).6,7 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that of other SGAs such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18)8; at steady state, the D2 occupancy remains <40% (Figure) and the corresponding rates of EPS/akathisia were only 6.7% for lumateperone vs 6.3% for placebo in short-term clinical trials.7,9
How it works
A unique aspect of lumateperone’s pharmacology may relate to differential actions at presynaptic and postsynaptic dopamine D2 receptors. Other antipsychotics possess comparable antagonist (or partial agonist) properties at postsynaptic D2 receptors (the D2 long isoform) and the presynaptic autoreceptor (the D2 short isoform). By blocking the presynaptic autoreceptor, feedback inhibition on dopamine release is removed; therefore, the required higher levels of postsynaptic D2 receptor occupancy needed for effective antipsychotic action (eg, 60% to 80% for antagonists, and 83% to 100% for partial agonists) may be a product of the need to oppose this increased presynaptic release of dopamine. In vitro assays show that lumateperone does not increase presynaptic dopamine release, indicating that it possesses agonist properties at the presynaptic D2 short receptor.10 That property may explain how lumateperone functions as an antipsychotic despite low levels of D2 receptor occupancy.10
Another hypothesis is based on our understanding of pimavanserin’s pharmacology. Pimavanserin is a selective 5HT2A antagonist FDA-approved for the treatment of Parkinson’s disease psychosis (PDP), with extremely high receptor affinity (Ki 0.087 nM) and no appreciable binding at dopamine receptors.5 Pimavanserin not only treats PDP, but is being evaluated in clinical trials for dementia-related psychosis, and has positive data for augmenting antipsychotics when there is a low level of D2 blockade.11,12 In a controlled trial, pimavanserin added to risperidone, 2 mg/d, was as effective as risperidone, 6 mg/d, illustrating the point that near-saturation of the 5HT2A receptor can increase antipsychotic efficacy when dopamine blockade is relatively low. For risperidone, 2 mg/d, the expected D2 occupancy is only 60%.13
Lumateperone also has moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM). Serotonin transporter occupancy at the dose approved for schizophrenia (42 mg/d) is approximately 30%,14 below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitor (SSRI) antidepressants; nevertheless, there is evidence for antidepressant effects seen in preclinical assays, schizophrenia studies, and phase III trials for bipolar depression.8,15,16 It is hypothesized that near-saturation of the 5HT2A receptor might act synergistically with the modest extent of 5HT reuptake inhibition to promote downstream effects associated with effective antidepressant treatments.8 In vivo data also showed phosphorylation of N-methyl-
Clinical implications
Nonadherence with oral antipsychotics among patients with schizophrenia is often related to adverse effects.17 The SGAs marketed since 2000 generally have lower rates of sedation and metabolic and/or endocrine adverse events than earlier compounds, yet each has limitations:
- asenapine: sedation and weight gain
- the partial agonists (aripiprazole, brexpiprazole, cariprazine): akathisia
- lurasidone: dose-dependent EPS and akathisia
- iloperidone: orthostasis.18
Ziprasidone is an exception, because it had low rates of most adverse effects in schizophrenia trials, but the need to take it twice daily with a 500 kcal meal hampers its use. A meta-analysis of 32 oral antipsychotics, including first-generation agents, noted that the efficacy differences between medications are slight for patients without treatment-resistant schizophrenia, but “differences in side-effects are more marked.”18
Continue to: Until novel mechanisms are discovered...
Until novel mechanisms are discovered that increase schizophrenia response rates, the availability of newer antipsychotics with more favorable tolerability profiles presents clinicians and patients with added options when adverse effects interfere with prior treatment. In all phases of the adult schizophrenia trial program for lumateperone, 811 patients received short-term (4- to 6-week) exposure (dose range: 14 to 84 mg/d), while 329 had ≥6 months exposure and 108 had ≥1 year of exposure to the 42-mg/d dose. In these studies, there was no single adverse reaction leading to discontinuation that occurred at a rate >2%. The only adverse events that occurred at rates ≥5% and more than twice the rate of placebo were somnolence/sedation (lumateperone 24%, placebo 10%), and dry mouth (lumateperone 6%, placebo 2%). Nausea was present in 9% of the lumateperone group compared with 5% for placebo.7 In the short-term studies, the combined rate of EPS and akathisia was 6.7% for lumateperone and 6.3% for placebo.7 This difference translates to a number needed to harm of 250 for these neurologic adverse effects. The functional impact of lumateperone’s glutamatergic mechanisms is not well characterized within the schizophrenia population, but the antidepressant potential has been studied for patients with bipolar depression, with 1 positive phase III trial.19
Efficacy in adults with schizophrenia. The efficacy of lumateperone has been established in 2 pivotal, double-blind, placebo-controlled trials. The first was a 4-week, phase II trial (Study 005) in which 335 adults age 18 to 55 with an acute exacerbation of schizophrenia were randomized in a 1:1:1:1 manner to lumateperone, 42 mg/d (60 mg of lumateperone tosylate), lumateperone, 84 mg/d (120 mg of lumateperone tosylate), risperidone, 4 mg/d, or placebo, all taken once daily.20 For the 4 treatment arms, the least squares mean changes from baseline to the Day 28 endpoint on the primary outcome measure, Positive and Negative Syndrome Scale (PANSS) total score, were: lumateperone, 42 mg/d: −13.2 points; lumateperone, 84 mg/d: −8.3 points; risperidone, 4 mg/d: −13.4 points; and placebo: −7.4 points. Both lumateperone, 42 mg/d, and risperidone, 4 mg/d, were significantly different than placebo, and with identical moderate effect sizes of 0.4.20 Lumateperone, 84 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that a similar proportion of patients (40%) randomized to lumateperone, 42 mg/d, or risperidone, 4 mg/d, improved by ≥30% on PANSS total score.
The second pivotal trial (Study 301) was a phase III, double-blind, placebo-controlled trial of 450 adults, age 18 to 60, with an acute exacerbation of schizophrenia who were randomized in 1:1:1 manner to receive lumateperone, 42 mg/d (lumateperone tosylate 60 mg), lumateperone, 28 mg/d (lumateperone tosylate 40 mg), or placebo once daily for 4 weeks.21 For the 3 treatment arms, the least squares mean changes on PANSS total score from baseline to the Day 28 endpoint were: lumateperone, 42 mg/d: −14.5 points; lumateperone, 28 mg/d: −12.9 points; and placebo: −10.3 points. Lumateperone, 28 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that 36.5% of those receiving lumateperone, 42 mg/d, and 36.3% of those receiving lumateperone, 28 mg/d, improved by ≥30% on PANSS total score, compared with 25.5% of patients treated with placebo.
Unlike the 2 positive trials in which placebo change in total PANSS scores were −7.4 and −10.3 points, respectively, in a phase III trial (Study 302) with 696 participants, placebo showed a 15.1-point decrease from baseline PANSS total score.19 Among the 3 treatment arms of this study (lumateperone, 14 mg/d, lumateperone, 42 mg/d, and risperidone, 4 mg/d), only risperidone was superior to placebo.
Adverse events
In the phase II pivotal study, completion rates among the 4 arms were comparable: lumateperone, 42 mg/d: 71%; lumateperone, 84 mg/d: 76%; risperidone, 4 mg/d: 77%; and placebo: 72%.20 There were no serious adverse events (SAEs) associated with lumateperone; the 2 SAEs that occurred involved worsening of schizophrenia/psychotic disorder for risperidone (n = 1) and for placebo (n = 1). Five participants discontinued the study due to an adverse event: 2 who were receiving lumateperone (1 due to dry mouth, and 1 due to worsening of schizophrenia) and 3 who were receiving risperidone (2 due to akathisia, and 1 due to blood creatine phosphokinase increase).20 The most frequent adverse event was somnolence/sedation (placebo: 13%; lumateperone, 42 mg/d: 17%; risperidone, 4 mg/d: 21%; and lumateperone, 84 mg/d: 32.5%). Neither dose of lumateperone was associated with increased rates of EPS. Median weight gain to Day 28 was 1 kg for placebo and for each dose of lumateperone, and 2.5 kg for risperidone. Compared with risperidone, lumateperone showed statistically significantly lower prolactin levels (lumateperone, 42 mg/d and 84 mg/d: P < .001), and metabolic parameters, including fasting glucose (lumateperone 42 mg/d: P = .007; lumateperone, 84 mg/d: P = .023), total cholesterol (lumateperone, 42 mg/d: P = .012; lumateperone, 84 mg/d: P = .004), and triglycerides (lumateperone, 42 mg/d: P = .074; lumateperone, 84 mg/d: P = .002).20 There was no significant impact on the corrected QT interval.
Continue to: In the phase III trial...
In the phase III trial, completion rates among the 3 arms were lumateperone, 42 mg/d: 85%; lumateperone, 28 mg/d: 80%; and placebo: 74%. There was 1 SAE in a patient receiving lumateperone, 28 mg/d. This individual had preexisting risk factors and a history of seizures, and experienced a seizure during the study. Adverse events that occurred in either lumateperone group at a rate ≥5% and more than twice the rate of placebo were somnolence (lumateperone, 42 mg/d: 17.3%; lumateperone, 28 mg/d: 11.3%; and placebo: 4.0%); sedation (lumateperone, 42 mg/d: 12.7%; lumateperone, 28 mg/d: 9.3%; and placebo: 5.4%); fatigue (lumateperone, 42 mg/d: 5.3%; lumateperone, 28 mg/d: 4.7%; and placebo: 1.3%); and constipation (lumateperone, 42 mg/d: 6.7%; lumateperone, 28 mg/d: 4.0%; and placebo: 2.7%).21 No EPS-related adverse events occurred in ≥5% patients in any treatment arm. Median change in weight from baseline to Day 28 was 0.9 kg for lumateperone, 42 mg/d, 0.6 kg for lumateperone, 28 mg/d, and 0.7 kg for placebo. There were no significant mean changes in metabolic parameters for any treatment arm, and none of the patients had a corrected QT interval (QTc) >500 ms or a change in QTc >60 ms from baseline.21
Pharmacologic profile
Lumateperone’s in vitro binding profile includes high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), lower affinity for dopamine D2 receptors (Ki 32 nM), moderate binding affinity for SERT (Ki 33 nM), and lower affinity for alpha 1-adrenergic receptors (Ki 73 nM) and muscarinic and histaminergic receptors (Ki >100 nM).6,7 As noted above, this 60-fold ratio of 5HT2A to D2 affinity is extremely high; moreover, imaging data reveal low D2 receptor occupancy, consistent with the lack of clinically significant EPS seen in the trials. In vitro assays also reveal impact on glutamate pathways, and pathways associated with antidepressant response.8 The clinical benefits of the glutamatergic properties remain theoretical, but the antidepressant benefit has been seen in a positive phase III trial for bipolar depression.19
Clinical considerations
Effect sizes in the 2 positive pivotal trials were 0.3 and 0.4, comparable with those for other antipsychotics approved within the last decade: brexpiprazole, 0.26; cariprazine, 0.34; and lurasidone, 0.36.21 The absence of clinically significant EPS, lack of impact on metabolic or endocrine parameters, and lack of titration are all appealing properties. That only 42 mg/d proved effective may reflect the fact that the other doses studied to date in randomized, fixed-dose studies were 14 mg/d (Study 302) and 84 mg/d (Study 005), evaluated in one study each. While those 2 doses might indeed be outside the therapeutic window, given the heterogeneity of schizophrenia, future studies might help further refine the therapeutic range for schizophrenia, especially for doses closer to 42 mg/d (eg, 28 mg/d, 63 mg/d). Should 42 mg/d not prove effective, there is no data for now to suggest whether a dose increase may be helpful. As there is only 1 marketed dose of lumateperone (42-mg capsules), and no easy way to modify this dose, lumateperone’s package insert includes cautionary language regarding situations where there will be less-than-expected drug exposure (use of cytochrome P450 [CYP] 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant EPS and metabolic or endocrine adverse effects. In vitro data indicate glutamatergic effects, and human data indicate antidepressant effects in bipolar patients. Despite the absence of significant histamine H1 or muscarinic affinity, the rate of somnolence/sedation was twice that of placebo (lumateperone 24%, placebo 10%).7
Why Rx? Reasons to prescribe lumateperone for adult patients with schizophrenia include:
- Favorable tolerability profile, including no significant signal for EPS or endocrine or metabolic adverse effects, and no QT prolongation
- No need for titration.
Dosing. There is only 1 dose available for lumateperone, 42-mg capsules (Table 2). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less-than-expected drug exposure (use of CYP 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh criteria (Child-Pugh B or C). These are not contraindications.
Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Continue to: Bottom Line
Bottom Line
Lumateperone is a novel oral antipsychotic indicated for treating adults with schizophrenia. Its unique properties include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant extrapyramidal symptoms and metabolic or endocrine adverse effects. In clinical trials, the most frequent adverse event was somnolence/sedation.
Related Resource
- Fulton D. FDA approves Caplyta to treat schizophrenenia in adults. https://www.mdedge.com/psychiatry/article/214733/schizophrenia-other-psychotic-disorders/fda-approves-caplyta-treat.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Pimavanserin • Nuplazid
Risperidone • Risperdal
Ziprasidone • Geodon
Antipsychotic nonadherence is a known contributor to relapse risk among patients with schizophrenia.1 Because relapse episodes may be associated with antipsychotic treatment resistance, this must be avoided as much as possible by appropriate medication selection.2 Adverse effect burden is an important factor leading to oral antipsychotic nonadherence, with patient-derived data indicating that extrapyramidal symptoms (EPS) (odds ratio [OR] 0.57, P = .0007), sedation/cognitive adverse effects (OR 0.70, P = .033), prolactin/endocrine effects (OR 0.69, P = .0342), and metabolic adverse effects (OR 0.64, P = .0079) are all significantly related to lower rates of adherence.3 With this in mind, successive generations of antipsychotics have been released, with fewer tolerability issues present than seen with earlier compounds.1,4 Although these newer second-generation antipsychotics (SGAs) have not proven more effective for schizophrenia than those first marketed in the 1990s, they generally possess lower rates of EPS, hyperprolactinemia, anticholinergic and antihistaminic properties, metabolic adverse effects, and orthostasis.5 This improved adverse effect profile will hopefully increase the chances of antipsychotic acceptance in patients with schizophrenia, and thereby promote improved adherence.
Lumateperone (Caplyta) is a novel oral antipsychotic approved for the treatment of adult patients with schizophrenia (Table 1). It possesses some properties seen with other SGAs, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM) and lower affinity for dopamine D2 receptors (Ki 32 nM), along with low affinity for alpha1-adrenergic receptors (Ki 73 nM), and muscarinic and histaminergic receptors (Ki > 100 nM).6,7 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that of other SGAs such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18)8; at steady state, the D2 occupancy remains <40% (Figure) and the corresponding rates of EPS/akathisia were only 6.7% for lumateperone vs 6.3% for placebo in short-term clinical trials.7,9
How it works
A unique aspect of lumateperone’s pharmacology may relate to differential actions at presynaptic and postsynaptic dopamine D2 receptors. Other antipsychotics possess comparable antagonist (or partial agonist) properties at postsynaptic D2 receptors (the D2 long isoform) and the presynaptic autoreceptor (the D2 short isoform). By blocking the presynaptic autoreceptor, feedback inhibition on dopamine release is removed; therefore, the required higher levels of postsynaptic D2 receptor occupancy needed for effective antipsychotic action (eg, 60% to 80% for antagonists, and 83% to 100% for partial agonists) may be a product of the need to oppose this increased presynaptic release of dopamine. In vitro assays show that lumateperone does not increase presynaptic dopamine release, indicating that it possesses agonist properties at the presynaptic D2 short receptor.10 That property may explain how lumateperone functions as an antipsychotic despite low levels of D2 receptor occupancy.10
Another hypothesis is based on our understanding of pimavanserin’s pharmacology. Pimavanserin is a selective 5HT2A antagonist FDA-approved for the treatment of Parkinson’s disease psychosis (PDP), with extremely high receptor affinity (Ki 0.087 nM) and no appreciable binding at dopamine receptors.5 Pimavanserin not only treats PDP, but is being evaluated in clinical trials for dementia-related psychosis, and has positive data for augmenting antipsychotics when there is a low level of D2 blockade.11,12 In a controlled trial, pimavanserin added to risperidone, 2 mg/d, was as effective as risperidone, 6 mg/d, illustrating the point that near-saturation of the 5HT2A receptor can increase antipsychotic efficacy when dopamine blockade is relatively low. For risperidone, 2 mg/d, the expected D2 occupancy is only 60%.13
Lumateperone also has moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM). Serotonin transporter occupancy at the dose approved for schizophrenia (42 mg/d) is approximately 30%,14 below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitor (SSRI) antidepressants; nevertheless, there is evidence for antidepressant effects seen in preclinical assays, schizophrenia studies, and phase III trials for bipolar depression.8,15,16 It is hypothesized that near-saturation of the 5HT2A receptor might act synergistically with the modest extent of 5HT reuptake inhibition to promote downstream effects associated with effective antidepressant treatments.8 In vivo data also showed phosphorylation of N-methyl-
Clinical implications
Nonadherence with oral antipsychotics among patients with schizophrenia is often related to adverse effects.17 The SGAs marketed since 2000 generally have lower rates of sedation and metabolic and/or endocrine adverse events than earlier compounds, yet each has limitations:
- asenapine: sedation and weight gain
- the partial agonists (aripiprazole, brexpiprazole, cariprazine): akathisia
- lurasidone: dose-dependent EPS and akathisia
- iloperidone: orthostasis.18
Ziprasidone is an exception, because it had low rates of most adverse effects in schizophrenia trials, but the need to take it twice daily with a 500 kcal meal hampers its use. A meta-analysis of 32 oral antipsychotics, including first-generation agents, noted that the efficacy differences between medications are slight for patients without treatment-resistant schizophrenia, but “differences in side-effects are more marked.”18
Continue to: Until novel mechanisms are discovered...
Until novel mechanisms are discovered that increase schizophrenia response rates, the availability of newer antipsychotics with more favorable tolerability profiles presents clinicians and patients with added options when adverse effects interfere with prior treatment. In all phases of the adult schizophrenia trial program for lumateperone, 811 patients received short-term (4- to 6-week) exposure (dose range: 14 to 84 mg/d), while 329 had ≥6 months exposure and 108 had ≥1 year of exposure to the 42-mg/d dose. In these studies, there was no single adverse reaction leading to discontinuation that occurred at a rate >2%. The only adverse events that occurred at rates ≥5% and more than twice the rate of placebo were somnolence/sedation (lumateperone 24%, placebo 10%), and dry mouth (lumateperone 6%, placebo 2%). Nausea was present in 9% of the lumateperone group compared with 5% for placebo.7 In the short-term studies, the combined rate of EPS and akathisia was 6.7% for lumateperone and 6.3% for placebo.7 This difference translates to a number needed to harm of 250 for these neurologic adverse effects. The functional impact of lumateperone’s glutamatergic mechanisms is not well characterized within the schizophrenia population, but the antidepressant potential has been studied for patients with bipolar depression, with 1 positive phase III trial.19
Efficacy in adults with schizophrenia. The efficacy of lumateperone has been established in 2 pivotal, double-blind, placebo-controlled trials. The first was a 4-week, phase II trial (Study 005) in which 335 adults age 18 to 55 with an acute exacerbation of schizophrenia were randomized in a 1:1:1:1 manner to lumateperone, 42 mg/d (60 mg of lumateperone tosylate), lumateperone, 84 mg/d (120 mg of lumateperone tosylate), risperidone, 4 mg/d, or placebo, all taken once daily.20 For the 4 treatment arms, the least squares mean changes from baseline to the Day 28 endpoint on the primary outcome measure, Positive and Negative Syndrome Scale (PANSS) total score, were: lumateperone, 42 mg/d: −13.2 points; lumateperone, 84 mg/d: −8.3 points; risperidone, 4 mg/d: −13.4 points; and placebo: −7.4 points. Both lumateperone, 42 mg/d, and risperidone, 4 mg/d, were significantly different than placebo, and with identical moderate effect sizes of 0.4.20 Lumateperone, 84 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that a similar proportion of patients (40%) randomized to lumateperone, 42 mg/d, or risperidone, 4 mg/d, improved by ≥30% on PANSS total score.
The second pivotal trial (Study 301) was a phase III, double-blind, placebo-controlled trial of 450 adults, age 18 to 60, with an acute exacerbation of schizophrenia who were randomized in 1:1:1 manner to receive lumateperone, 42 mg/d (lumateperone tosylate 60 mg), lumateperone, 28 mg/d (lumateperone tosylate 40 mg), or placebo once daily for 4 weeks.21 For the 3 treatment arms, the least squares mean changes on PANSS total score from baseline to the Day 28 endpoint were: lumateperone, 42 mg/d: −14.5 points; lumateperone, 28 mg/d: −12.9 points; and placebo: −10.3 points. Lumateperone, 28 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that 36.5% of those receiving lumateperone, 42 mg/d, and 36.3% of those receiving lumateperone, 28 mg/d, improved by ≥30% on PANSS total score, compared with 25.5% of patients treated with placebo.
Unlike the 2 positive trials in which placebo change in total PANSS scores were −7.4 and −10.3 points, respectively, in a phase III trial (Study 302) with 696 participants, placebo showed a 15.1-point decrease from baseline PANSS total score.19 Among the 3 treatment arms of this study (lumateperone, 14 mg/d, lumateperone, 42 mg/d, and risperidone, 4 mg/d), only risperidone was superior to placebo.
Adverse events
In the phase II pivotal study, completion rates among the 4 arms were comparable: lumateperone, 42 mg/d: 71%; lumateperone, 84 mg/d: 76%; risperidone, 4 mg/d: 77%; and placebo: 72%.20 There were no serious adverse events (SAEs) associated with lumateperone; the 2 SAEs that occurred involved worsening of schizophrenia/psychotic disorder for risperidone (n = 1) and for placebo (n = 1). Five participants discontinued the study due to an adverse event: 2 who were receiving lumateperone (1 due to dry mouth, and 1 due to worsening of schizophrenia) and 3 who were receiving risperidone (2 due to akathisia, and 1 due to blood creatine phosphokinase increase).20 The most frequent adverse event was somnolence/sedation (placebo: 13%; lumateperone, 42 mg/d: 17%; risperidone, 4 mg/d: 21%; and lumateperone, 84 mg/d: 32.5%). Neither dose of lumateperone was associated with increased rates of EPS. Median weight gain to Day 28 was 1 kg for placebo and for each dose of lumateperone, and 2.5 kg for risperidone. Compared with risperidone, lumateperone showed statistically significantly lower prolactin levels (lumateperone, 42 mg/d and 84 mg/d: P < .001), and metabolic parameters, including fasting glucose (lumateperone 42 mg/d: P = .007; lumateperone, 84 mg/d: P = .023), total cholesterol (lumateperone, 42 mg/d: P = .012; lumateperone, 84 mg/d: P = .004), and triglycerides (lumateperone, 42 mg/d: P = .074; lumateperone, 84 mg/d: P = .002).20 There was no significant impact on the corrected QT interval.
Continue to: In the phase III trial...
In the phase III trial, completion rates among the 3 arms were lumateperone, 42 mg/d: 85%; lumateperone, 28 mg/d: 80%; and placebo: 74%. There was 1 SAE in a patient receiving lumateperone, 28 mg/d. This individual had preexisting risk factors and a history of seizures, and experienced a seizure during the study. Adverse events that occurred in either lumateperone group at a rate ≥5% and more than twice the rate of placebo were somnolence (lumateperone, 42 mg/d: 17.3%; lumateperone, 28 mg/d: 11.3%; and placebo: 4.0%); sedation (lumateperone, 42 mg/d: 12.7%; lumateperone, 28 mg/d: 9.3%; and placebo: 5.4%); fatigue (lumateperone, 42 mg/d: 5.3%; lumateperone, 28 mg/d: 4.7%; and placebo: 1.3%); and constipation (lumateperone, 42 mg/d: 6.7%; lumateperone, 28 mg/d: 4.0%; and placebo: 2.7%).21 No EPS-related adverse events occurred in ≥5% patients in any treatment arm. Median change in weight from baseline to Day 28 was 0.9 kg for lumateperone, 42 mg/d, 0.6 kg for lumateperone, 28 mg/d, and 0.7 kg for placebo. There were no significant mean changes in metabolic parameters for any treatment arm, and none of the patients had a corrected QT interval (QTc) >500 ms or a change in QTc >60 ms from baseline.21
Pharmacologic profile
Lumateperone’s in vitro binding profile includes high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), lower affinity for dopamine D2 receptors (Ki 32 nM), moderate binding affinity for SERT (Ki 33 nM), and lower affinity for alpha 1-adrenergic receptors (Ki 73 nM) and muscarinic and histaminergic receptors (Ki >100 nM).6,7 As noted above, this 60-fold ratio of 5HT2A to D2 affinity is extremely high; moreover, imaging data reveal low D2 receptor occupancy, consistent with the lack of clinically significant EPS seen in the trials. In vitro assays also reveal impact on glutamate pathways, and pathways associated with antidepressant response.8 The clinical benefits of the glutamatergic properties remain theoretical, but the antidepressant benefit has been seen in a positive phase III trial for bipolar depression.19
Clinical considerations
Effect sizes in the 2 positive pivotal trials were 0.3 and 0.4, comparable with those for other antipsychotics approved within the last decade: brexpiprazole, 0.26; cariprazine, 0.34; and lurasidone, 0.36.21 The absence of clinically significant EPS, lack of impact on metabolic or endocrine parameters, and lack of titration are all appealing properties. That only 42 mg/d proved effective may reflect the fact that the other doses studied to date in randomized, fixed-dose studies were 14 mg/d (Study 302) and 84 mg/d (Study 005), evaluated in one study each. While those 2 doses might indeed be outside the therapeutic window, given the heterogeneity of schizophrenia, future studies might help further refine the therapeutic range for schizophrenia, especially for doses closer to 42 mg/d (eg, 28 mg/d, 63 mg/d). Should 42 mg/d not prove effective, there is no data for now to suggest whether a dose increase may be helpful. As there is only 1 marketed dose of lumateperone (42-mg capsules), and no easy way to modify this dose, lumateperone’s package insert includes cautionary language regarding situations where there will be less-than-expected drug exposure (use of cytochrome P450 [CYP] 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant EPS and metabolic or endocrine adverse effects. In vitro data indicate glutamatergic effects, and human data indicate antidepressant effects in bipolar patients. Despite the absence of significant histamine H1 or muscarinic affinity, the rate of somnolence/sedation was twice that of placebo (lumateperone 24%, placebo 10%).7
Why Rx? Reasons to prescribe lumateperone for adult patients with schizophrenia include:
- Favorable tolerability profile, including no significant signal for EPS or endocrine or metabolic adverse effects, and no QT prolongation
- No need for titration.
Dosing. There is only 1 dose available for lumateperone, 42-mg capsules (Table 2). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less-than-expected drug exposure (use of CYP 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh criteria (Child-Pugh B or C). These are not contraindications.
Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Continue to: Bottom Line
Bottom Line
Lumateperone is a novel oral antipsychotic indicated for treating adults with schizophrenia. Its unique properties include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant extrapyramidal symptoms and metabolic or endocrine adverse effects. In clinical trials, the most frequent adverse event was somnolence/sedation.
Related Resource
- Fulton D. FDA approves Caplyta to treat schizophrenenia in adults. https://www.mdedge.com/psychiatry/article/214733/schizophrenia-other-psychotic-disorders/fda-approves-caplyta-treat.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Pimavanserin • Nuplazid
Risperidone • Risperdal
Ziprasidone • Geodon
1. Dufort A, Zipursky RB. Understanding and managing treatment adherence in schizophrenia [published online January 3, 2019]. Clin Schizophr Relat Psychoses. 2019. doi: 10.3371/CSRP.ADRZ.121218.
2. Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? Antipsychotic response in first- vs. second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036-1042.
3. Dibonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12:20.
4. Kurokawa S, Kishimoto T, Su K-P, et al. Psychiatrists’ perceptions of medication adherence among patients with schizophrenia: an international survey. Schizophr Res. 2019;211:105-107.
5. Meyer JM. Pharmacotherapy of psychosis and mania. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. Chicago, Illinois: McGraw-Hill; 2018:279-302.
6. Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614.
7. Caplyta [package Insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
8. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
9. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
10. Zhang L, Hendrick JP. The presynaptic D2 partial agonist lumateperone acts as a postsynaptic D2 antagonist. Matters. 2018. doi: 10.19185/matters.201712000006.
11. Meltzer HY, Elkis H, Vanover K, et al. Pimavanserin, a selective serotonin (5-HT)2A-inverse agonist, enhances the efficacy and safety of risperidone, 2mg/day, but does not enhance efficacy of haloperidol, 2mg/day: comparison with reference dose risperidone, 6mg/day. Schizophr Res. 2012;141(2-3):144-152.
12. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
13. Remington G, Mamo D, Labelle A, et al. A PET study evaluating dopamine D2 receptor occupancy for long-acting injectable risperidone. Am J Psychiatry. 2006;163(3):396-401.
14. Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl). 2015;232(15):2863-2872.
15. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018;54(12):713-719.
16. Vanover K, Glass S, Kozauer S, et al. 30 lumateperone (ITI-007) for the treatment of schizophrenia: overview of placebo-controlled clinical trials and an open-label safety switching study. CNS Spectr. 2019;24(1):190-191.
17. Young SL, Taylor M, Lawrie SM. “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. 2015;29(4):353-362.
18. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939-951.
19. Vyas P, Hwang BJ, Brašic ´ JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2019;1-7.
20. Lieberman JA, Davis RE, Correll CU, et al. ITI-007 for the treatment of schizophrenia: a 4-week randomized, double-blind, controlled trial. Biol Psychiatry. 2016;79(12):952-961.
21. Correll CU, Davis RE, Weingart M, et al. Efficacy and safety of lumateperone for treatment of schizophrenia [published online January 8, 2020]. JAMA Psychiatry. 2020;E1-E10.
1. Dufort A, Zipursky RB. Understanding and managing treatment adherence in schizophrenia [published online January 3, 2019]. Clin Schizophr Relat Psychoses. 2019. doi: 10.3371/CSRP.ADRZ.121218.
2. Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? Antipsychotic response in first- vs. second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036-1042.
3. Dibonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12:20.
4. Kurokawa S, Kishimoto T, Su K-P, et al. Psychiatrists’ perceptions of medication adherence among patients with schizophrenia: an international survey. Schizophr Res. 2019;211:105-107.
5. Meyer JM. Pharmacotherapy of psychosis and mania. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. Chicago, Illinois: McGraw-Hill; 2018:279-302.
6. Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614.
7. Caplyta [package Insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
8. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
9. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
10. Zhang L, Hendrick JP. The presynaptic D2 partial agonist lumateperone acts as a postsynaptic D2 antagonist. Matters. 2018. doi: 10.19185/matters.201712000006.
11. Meltzer HY, Elkis H, Vanover K, et al. Pimavanserin, a selective serotonin (5-HT)2A-inverse agonist, enhances the efficacy and safety of risperidone, 2mg/day, but does not enhance efficacy of haloperidol, 2mg/day: comparison with reference dose risperidone, 6mg/day. Schizophr Res. 2012;141(2-3):144-152.
12. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
13. Remington G, Mamo D, Labelle A, et al. A PET study evaluating dopamine D2 receptor occupancy for long-acting injectable risperidone. Am J Psychiatry. 2006;163(3):396-401.
14. Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl). 2015;232(15):2863-2872.
15. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018;54(12):713-719.
16. Vanover K, Glass S, Kozauer S, et al. 30 lumateperone (ITI-007) for the treatment of schizophrenia: overview of placebo-controlled clinical trials and an open-label safety switching study. CNS Spectr. 2019;24(1):190-191.
17. Young SL, Taylor M, Lawrie SM. “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. 2015;29(4):353-362.
18. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939-951.
19. Vyas P, Hwang BJ, Brašic ´ JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2019;1-7.
20. Lieberman JA, Davis RE, Correll CU, et al. ITI-007 for the treatment of schizophrenia: a 4-week randomized, double-blind, controlled trial. Biol Psychiatry. 2016;79(12):952-961.
21. Correll CU, Davis RE, Weingart M, et al. Efficacy and safety of lumateperone for treatment of schizophrenia [published online January 8, 2020]. JAMA Psychiatry. 2020;E1-E10.