User login
Nearly 25% of U.S. adults take an obesogenic prescription drug
LAS VEGAS – based on national U.S. data collected during 2013-2016.
The Endocrine Society, the STOP Obesity Alliance, and other medical societies have recommended that clinicians try to minimize use of obesogenic drugs and focus on prescribing agents that are weight neutral or that trigger weight loss when those options are available and appropriate, and the new findings add further evidence that clinicians need to be more mindful of this issue, Craig M. Hales, MD, said at a meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
Among the American adults interviewed for the survey, 40% of those on at least one prescription medication were on at least one drug that is considered obesogenic, said Dr. Hales, a medical epidemiologist at the Centers for Disease Control and Prevention in Hyattsville, Md.
According to practice guidelines published by the Endocrine Society, all drugs in the classes of glucocorticoids, beta-blockers, and antihistamines are obesogenic, as well as selected agents in the classes of antidepressant drugs, antipsychotics, antidepressants, antidiabetics, and contraceptives that are progestin only, said Dr. Hales (J Clin Endocrinol Metab. 2015 Feb;100[2]:342-62).
The data he reported came from the National Health and Nutrition Examination Survey (NHANES) run by the CDC during 2013-2016 that included 11,055 adults who were at least 20 years old. The findings showed that 23% of those adults had taken at least one drug that was considered obesogenic during the 30 days preceding the survey date. By comparison, 35% of the same adults had taken any type of prescription drug during the previous 30 days. That meant that overall, 40% of surveyed adults who had recently used any prescription medication had taken an obesogenic drug.
The 23% prevalence of recent obesogenic drug use was fairly stable at that level during several preceding NHANES surveys going back to 2001, suggesting that the increasing use of obesogenic drugs during the period since 2001 was not a factor in the recent increased prevalence of obesity among U.S. residents, added Dr. Hales.
The 2013-2016 analysis also showed a strong link between obesogenic drug use and increasing obesity severity. Among survey participants with a body mass index (BMI) in the normal range (18.5-24 kg/m2), 16% had recent use of an obesogenic drug. This prevalence increased to 22% among those who were overweight (BMI, 25-29 kg/m2), 29% among those with class 1 or 2 obesity (BMI, 30-39 kg/m2), and 33% among those with class 3 obesity (BMI, 40 kg/m2 or greater).
In contrast, recent use of prescription medications that do not contribute to obesity showed no significant relationship with BMI, with rates that ranged from 34% among those with a normal BMI, to 37% among those with class 3 obesity.
As an example of this relationship for a specific obesogenic drug class, the prevalence of beta-blocker use was about 7% among people with a normal BMI, about 10% among those who were overweight, about 14% among people with class 1 or 2 obesity, and about 17% among people with class 3 obesity, a statistically significant link suggesting that the relationship between use of obesogenic drugs and obesity is “bidirectional,” Dr. Hales said, in that increasing obesogenic drug use likely contributes to obesity, while simultaneously, the more obese people become, the more likely they are to take additional prescription drugs, particularly those that are obesogenic.
NHANES is run by the CDC and receives no commercial funding. The authors reported no conflicts of interest.
SOURCE: Hales CM et al. Obesity Week 2019, Abstract T-OR-2037.
LAS VEGAS – based on national U.S. data collected during 2013-2016.
The Endocrine Society, the STOP Obesity Alliance, and other medical societies have recommended that clinicians try to minimize use of obesogenic drugs and focus on prescribing agents that are weight neutral or that trigger weight loss when those options are available and appropriate, and the new findings add further evidence that clinicians need to be more mindful of this issue, Craig M. Hales, MD, said at a meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
Among the American adults interviewed for the survey, 40% of those on at least one prescription medication were on at least one drug that is considered obesogenic, said Dr. Hales, a medical epidemiologist at the Centers for Disease Control and Prevention in Hyattsville, Md.
According to practice guidelines published by the Endocrine Society, all drugs in the classes of glucocorticoids, beta-blockers, and antihistamines are obesogenic, as well as selected agents in the classes of antidepressant drugs, antipsychotics, antidepressants, antidiabetics, and contraceptives that are progestin only, said Dr. Hales (J Clin Endocrinol Metab. 2015 Feb;100[2]:342-62).
The data he reported came from the National Health and Nutrition Examination Survey (NHANES) run by the CDC during 2013-2016 that included 11,055 adults who were at least 20 years old. The findings showed that 23% of those adults had taken at least one drug that was considered obesogenic during the 30 days preceding the survey date. By comparison, 35% of the same adults had taken any type of prescription drug during the previous 30 days. That meant that overall, 40% of surveyed adults who had recently used any prescription medication had taken an obesogenic drug.
The 23% prevalence of recent obesogenic drug use was fairly stable at that level during several preceding NHANES surveys going back to 2001, suggesting that the increasing use of obesogenic drugs during the period since 2001 was not a factor in the recent increased prevalence of obesity among U.S. residents, added Dr. Hales.
The 2013-2016 analysis also showed a strong link between obesogenic drug use and increasing obesity severity. Among survey participants with a body mass index (BMI) in the normal range (18.5-24 kg/m2), 16% had recent use of an obesogenic drug. This prevalence increased to 22% among those who were overweight (BMI, 25-29 kg/m2), 29% among those with class 1 or 2 obesity (BMI, 30-39 kg/m2), and 33% among those with class 3 obesity (BMI, 40 kg/m2 or greater).
In contrast, recent use of prescription medications that do not contribute to obesity showed no significant relationship with BMI, with rates that ranged from 34% among those with a normal BMI, to 37% among those with class 3 obesity.
As an example of this relationship for a specific obesogenic drug class, the prevalence of beta-blocker use was about 7% among people with a normal BMI, about 10% among those who were overweight, about 14% among people with class 1 or 2 obesity, and about 17% among people with class 3 obesity, a statistically significant link suggesting that the relationship between use of obesogenic drugs and obesity is “bidirectional,” Dr. Hales said, in that increasing obesogenic drug use likely contributes to obesity, while simultaneously, the more obese people become, the more likely they are to take additional prescription drugs, particularly those that are obesogenic.
NHANES is run by the CDC and receives no commercial funding. The authors reported no conflicts of interest.
SOURCE: Hales CM et al. Obesity Week 2019, Abstract T-OR-2037.
LAS VEGAS – based on national U.S. data collected during 2013-2016.
The Endocrine Society, the STOP Obesity Alliance, and other medical societies have recommended that clinicians try to minimize use of obesogenic drugs and focus on prescribing agents that are weight neutral or that trigger weight loss when those options are available and appropriate, and the new findings add further evidence that clinicians need to be more mindful of this issue, Craig M. Hales, MD, said at a meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
Among the American adults interviewed for the survey, 40% of those on at least one prescription medication were on at least one drug that is considered obesogenic, said Dr. Hales, a medical epidemiologist at the Centers for Disease Control and Prevention in Hyattsville, Md.
According to practice guidelines published by the Endocrine Society, all drugs in the classes of glucocorticoids, beta-blockers, and antihistamines are obesogenic, as well as selected agents in the classes of antidepressant drugs, antipsychotics, antidepressants, antidiabetics, and contraceptives that are progestin only, said Dr. Hales (J Clin Endocrinol Metab. 2015 Feb;100[2]:342-62).
The data he reported came from the National Health and Nutrition Examination Survey (NHANES) run by the CDC during 2013-2016 that included 11,055 adults who were at least 20 years old. The findings showed that 23% of those adults had taken at least one drug that was considered obesogenic during the 30 days preceding the survey date. By comparison, 35% of the same adults had taken any type of prescription drug during the previous 30 days. That meant that overall, 40% of surveyed adults who had recently used any prescription medication had taken an obesogenic drug.
The 23% prevalence of recent obesogenic drug use was fairly stable at that level during several preceding NHANES surveys going back to 2001, suggesting that the increasing use of obesogenic drugs during the period since 2001 was not a factor in the recent increased prevalence of obesity among U.S. residents, added Dr. Hales.
The 2013-2016 analysis also showed a strong link between obesogenic drug use and increasing obesity severity. Among survey participants with a body mass index (BMI) in the normal range (18.5-24 kg/m2), 16% had recent use of an obesogenic drug. This prevalence increased to 22% among those who were overweight (BMI, 25-29 kg/m2), 29% among those with class 1 or 2 obesity (BMI, 30-39 kg/m2), and 33% among those with class 3 obesity (BMI, 40 kg/m2 or greater).
In contrast, recent use of prescription medications that do not contribute to obesity showed no significant relationship with BMI, with rates that ranged from 34% among those with a normal BMI, to 37% among those with class 3 obesity.
As an example of this relationship for a specific obesogenic drug class, the prevalence of beta-blocker use was about 7% among people with a normal BMI, about 10% among those who were overweight, about 14% among people with class 1 or 2 obesity, and about 17% among people with class 3 obesity, a statistically significant link suggesting that the relationship between use of obesogenic drugs and obesity is “bidirectional,” Dr. Hales said, in that increasing obesogenic drug use likely contributes to obesity, while simultaneously, the more obese people become, the more likely they are to take additional prescription drugs, particularly those that are obesogenic.
NHANES is run by the CDC and receives no commercial funding. The authors reported no conflicts of interest.
SOURCE: Hales CM et al. Obesity Week 2019, Abstract T-OR-2037.
REPORTING FROM OBESITY WEEK 2019
Flu records most active December since 2003
The 2019-2020 flu season took a big jump in severity during the last full week of 2019, according to the Centers for Disease Control and Prevention.
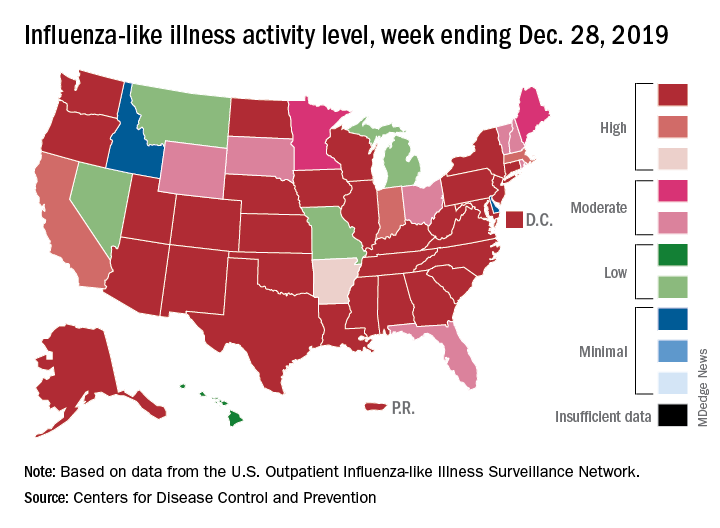
For the week ending Dec. 28, 6.9% of all outpatient visits to health care providers were for influenza-like illness (ILI), the CDC’s influenza division reported Jan. 3. That is up from 5.1% the previous week and is the highest rate recorded in December since 2003. During the flu pandemic season of 2009-2010, the rate peaked in October and dropped to relatively normal levels by the end of November, CDC data show.
This marks the eighth consecutive week that the outpatient visit rate has been at or above the nation’s baseline level of 2.4%, but the data for this week “may in part be influenced by changes in healthcare-seeking behavior that can occur during the holidays,” the CDC suggested.
All those outpatient visits mean that the ILI activity map is getting quite red. Thirty states, as well as the District of Columbia and Puerto Rico, were at the highest level on the CDC’s 1-10 activity scale during the week ending Dec. 28, compared with 20 the week before. Four states were categorized in the “high” range with activity levels of 8 and 9.
There have been approximately 6.4 million flu illnesses so far this season, the CDC estimated, along with 55,000 hospitalizations, although the ILI admission rate of 9.2 per 100,000 population is fairly typical for this time of year.
The week of Dec. 28 also brought reports of five more ILI-related pediatric deaths, which all occurred in the two previous weeks. A total of 27 children have died from the flu so far during the 2019-2020 season, the CDC said.
The 2019-2020 flu season took a big jump in severity during the last full week of 2019, according to the Centers for Disease Control and Prevention.

For the week ending Dec. 28, 6.9% of all outpatient visits to health care providers were for influenza-like illness (ILI), the CDC’s influenza division reported Jan. 3. That is up from 5.1% the previous week and is the highest rate recorded in December since 2003. During the flu pandemic season of 2009-2010, the rate peaked in October and dropped to relatively normal levels by the end of November, CDC data show.
This marks the eighth consecutive week that the outpatient visit rate has been at or above the nation’s baseline level of 2.4%, but the data for this week “may in part be influenced by changes in healthcare-seeking behavior that can occur during the holidays,” the CDC suggested.
All those outpatient visits mean that the ILI activity map is getting quite red. Thirty states, as well as the District of Columbia and Puerto Rico, were at the highest level on the CDC’s 1-10 activity scale during the week ending Dec. 28, compared with 20 the week before. Four states were categorized in the “high” range with activity levels of 8 and 9.
There have been approximately 6.4 million flu illnesses so far this season, the CDC estimated, along with 55,000 hospitalizations, although the ILI admission rate of 9.2 per 100,000 population is fairly typical for this time of year.
The week of Dec. 28 also brought reports of five more ILI-related pediatric deaths, which all occurred in the two previous weeks. A total of 27 children have died from the flu so far during the 2019-2020 season, the CDC said.
The 2019-2020 flu season took a big jump in severity during the last full week of 2019, according to the Centers for Disease Control and Prevention.

For the week ending Dec. 28, 6.9% of all outpatient visits to health care providers were for influenza-like illness (ILI), the CDC’s influenza division reported Jan. 3. That is up from 5.1% the previous week and is the highest rate recorded in December since 2003. During the flu pandemic season of 2009-2010, the rate peaked in October and dropped to relatively normal levels by the end of November, CDC data show.
This marks the eighth consecutive week that the outpatient visit rate has been at or above the nation’s baseline level of 2.4%, but the data for this week “may in part be influenced by changes in healthcare-seeking behavior that can occur during the holidays,” the CDC suggested.
All those outpatient visits mean that the ILI activity map is getting quite red. Thirty states, as well as the District of Columbia and Puerto Rico, were at the highest level on the CDC’s 1-10 activity scale during the week ending Dec. 28, compared with 20 the week before. Four states were categorized in the “high” range with activity levels of 8 and 9.
There have been approximately 6.4 million flu illnesses so far this season, the CDC estimated, along with 55,000 hospitalizations, although the ILI admission rate of 9.2 per 100,000 population is fairly typical for this time of year.
The week of Dec. 28 also brought reports of five more ILI-related pediatric deaths, which all occurred in the two previous weeks. A total of 27 children have died from the flu so far during the 2019-2020 season, the CDC said.
Despite PCV, pediatric asthma patients face pneumococcal risks
Even on-time pneumococcal vaccines don’t completely protect children with asthma from developing invasive pneumococcal disease, a meta-analysis has determined.
Despite receiving pneumococcal valent 7, 10, or 13, children with asthma were still almost twice as likely to develop the disease as were children without asthma, Jose A. Castro-Rodriguez, MD, PhD, and colleagues reported in Pediatrics (2020 Jan. doi: 10.1542/peds.2019-1200). None of the studies included rates for those who received the pneumococcal polysaccharide vaccine (PPSV23).
“For the first time, this meta-analysis reveals 90% increased odds of invasive pneumococcal disease (IPD) among [vaccinated] children with asthma,” said Dr. Castro-Rodriguez, of Pontificia Universidad Católica de Chile, Santiago, and colleagues. “If confirmed, these findings will bear clinical and public health importance,” they noted, because guidelines now recommend PPSV23 after age 2 in children with asthma only if they’re treated with prolonged high-dose oral corticosteroids.
However, because the analysis comprised only four studies, the authors cautioned that the results aren’t enough to justify changes to practice recommendations.
Asthma treatment with inhaled corticosteroids (ICS) may be driving the increased risk, Dr. Castro-Rodriguez and his coauthors suggested. ICS deposition in the oropharynx could boost oropharyngeal candidiasis risk by weakening the mucosal immune response, the researchers noted. And that same process may be at work with Streptococcus pneumoniae.
A prior study found that children with asthma who received ICS for at least 1 month were almost four times more likely to have oropharyngeal colonization by S. pneumoniae as were those who didn’t get the drugs. Thus, a higher carrier rate of S. pneumoniae in the oropharynx, along with asthma’s impaired airway clearance, might increase the risk of pneumococcal diseases, the investigators explained.
Dr. Castro-Rodriguez and colleagues analyzed four studies with more than 4,000 cases and controls, and about 26 million person-years of follow-up.
Rates and risks of IPD in the four studies were as follows:
- Among those with IPD, 27% had asthma, with 18% of those without, an adjusted odds ratio (aOR) of 1.8.
- In a European of patients who received at least 3 doses of PCV7, IPD rates per 100,000 person-years for 5-year-olds were 11.6 for children with asthma and 7.3 for those without. For 5- to 17-year-olds with and without asthma, the rates were 2.3 and 1.6, respectively.
- In 2001, a Korean found an aOR of 2.08 for IPD in children with asthma, compared with those without. In 2010, the aOR was 3.26. No vaccine types were reported in the study.
- of IPD were 3.7 per 100,000 person-years for children with asthma, compared with 2.5 for healthy controls – an adjusted relative risk of 1.5.
The pooled estimate of the four studies revealed an aOR of 1.9 for IPD among children with asthma, compared with those without, Dr. Castro-Rodriguez and his team concluded.
None of the studies reported hospital admissions, mortality, length of hospital stay, intensive care admission, invasive respiratory support, or additional medication use.
One, however, did find asthma severity was significantly associated with increasing IPD treatment costs per 100,000 person-years: $72,581 for healthy controls, compared with $100,020 for children with mild asthma, $172,002 for moderate asthma, and $638,452 for severe asthma.
In addition, treating all-cause pneumonia was more expensive in children with asthma. For all-cause pneumonia, the researchers found that estimated costs per 100,000 person-years for mild, moderate, and severe asthma were $7.5 million, $14.6 million, and $46.8 million, respectively, compared with $1.7 million for healthy controls.
The authors had no relevant financial disclosures.
SOURCE: Castro-Rodriguez J et al. Pediatrics. 2020 Jan. doi: 10.1542/peds.2019-1200.
The meta-analysis contains some important lessons for pediatricians, Tina Q. Tan, MD, wrote in an accompanying editorial.
“First, asthma remains a risk factor for invasive pneumococcal disease and pneumococcal pneumonia, even in the era of widespread use of PCV,” Dr. Tan noted. “Second, it is important that all patients, especially those with asthma, are receiving their vaccinations on time and, most notably, are up to date on their pneumococcal vaccinations. This will provide the best protection against pneumococcal infections and their complications for pediatric patients with asthma.”
Pneumococcal conjugate vaccines (PCV) have impressively decreased rates of invasive pneumococcal disease (IPD) and pneumonia in children in the United States, Dr. Tan explained. Overall, incidence dropped from 95 cases per 100,000 person-years in 1998 to only 9 cases per 100,000 in 2016.
In addition, the incidence of IPD caused by 13-valent PCV serotypes fell, from 88 cases per 100,000 in 1998 to 2 cases per 100,000 in 2016.
The threat is not over, however.
“IPD still remains a leading cause of morbidity and mortality in the United States and worldwide,” Dr. Tan cautioned. “In 2017, the CDC’s Active Bacterial Core surveillance network reported that there were 31,000 cases of IPD (meningitis, bacteremia, and bacteremic pneumonia) and 3,590 deaths, of which 147 cases and 9 deaths occurred in children younger than 5 years of age.”
Dr. Tan is a professor of pediatrics at Northwestern University, Chicago. Her comments appear in Pediatrics 2020 Jan. doi: 10.1542/peds.2019-3360 .
The meta-analysis contains some important lessons for pediatricians, Tina Q. Tan, MD, wrote in an accompanying editorial.
“First, asthma remains a risk factor for invasive pneumococcal disease and pneumococcal pneumonia, even in the era of widespread use of PCV,” Dr. Tan noted. “Second, it is important that all patients, especially those with asthma, are receiving their vaccinations on time and, most notably, are up to date on their pneumococcal vaccinations. This will provide the best protection against pneumococcal infections and their complications for pediatric patients with asthma.”
Pneumococcal conjugate vaccines (PCV) have impressively decreased rates of invasive pneumococcal disease (IPD) and pneumonia in children in the United States, Dr. Tan explained. Overall, incidence dropped from 95 cases per 100,000 person-years in 1998 to only 9 cases per 100,000 in 2016.
In addition, the incidence of IPD caused by 13-valent PCV serotypes fell, from 88 cases per 100,000 in 1998 to 2 cases per 100,000 in 2016.
The threat is not over, however.
“IPD still remains a leading cause of morbidity and mortality in the United States and worldwide,” Dr. Tan cautioned. “In 2017, the CDC’s Active Bacterial Core surveillance network reported that there were 31,000 cases of IPD (meningitis, bacteremia, and bacteremic pneumonia) and 3,590 deaths, of which 147 cases and 9 deaths occurred in children younger than 5 years of age.”
Dr. Tan is a professor of pediatrics at Northwestern University, Chicago. Her comments appear in Pediatrics 2020 Jan. doi: 10.1542/peds.2019-3360 .
The meta-analysis contains some important lessons for pediatricians, Tina Q. Tan, MD, wrote in an accompanying editorial.
“First, asthma remains a risk factor for invasive pneumococcal disease and pneumococcal pneumonia, even in the era of widespread use of PCV,” Dr. Tan noted. “Second, it is important that all patients, especially those with asthma, are receiving their vaccinations on time and, most notably, are up to date on their pneumococcal vaccinations. This will provide the best protection against pneumococcal infections and their complications for pediatric patients with asthma.”
Pneumococcal conjugate vaccines (PCV) have impressively decreased rates of invasive pneumococcal disease (IPD) and pneumonia in children in the United States, Dr. Tan explained. Overall, incidence dropped from 95 cases per 100,000 person-years in 1998 to only 9 cases per 100,000 in 2016.
In addition, the incidence of IPD caused by 13-valent PCV serotypes fell, from 88 cases per 100,000 in 1998 to 2 cases per 100,000 in 2016.
The threat is not over, however.
“IPD still remains a leading cause of morbidity and mortality in the United States and worldwide,” Dr. Tan cautioned. “In 2017, the CDC’s Active Bacterial Core surveillance network reported that there were 31,000 cases of IPD (meningitis, bacteremia, and bacteremic pneumonia) and 3,590 deaths, of which 147 cases and 9 deaths occurred in children younger than 5 years of age.”
Dr. Tan is a professor of pediatrics at Northwestern University, Chicago. Her comments appear in Pediatrics 2020 Jan. doi: 10.1542/peds.2019-3360 .
Even on-time pneumococcal vaccines don’t completely protect children with asthma from developing invasive pneumococcal disease, a meta-analysis has determined.
Despite receiving pneumococcal valent 7, 10, or 13, children with asthma were still almost twice as likely to develop the disease as were children without asthma, Jose A. Castro-Rodriguez, MD, PhD, and colleagues reported in Pediatrics (2020 Jan. doi: 10.1542/peds.2019-1200). None of the studies included rates for those who received the pneumococcal polysaccharide vaccine (PPSV23).
“For the first time, this meta-analysis reveals 90% increased odds of invasive pneumococcal disease (IPD) among [vaccinated] children with asthma,” said Dr. Castro-Rodriguez, of Pontificia Universidad Católica de Chile, Santiago, and colleagues. “If confirmed, these findings will bear clinical and public health importance,” they noted, because guidelines now recommend PPSV23 after age 2 in children with asthma only if they’re treated with prolonged high-dose oral corticosteroids.
However, because the analysis comprised only four studies, the authors cautioned that the results aren’t enough to justify changes to practice recommendations.
Asthma treatment with inhaled corticosteroids (ICS) may be driving the increased risk, Dr. Castro-Rodriguez and his coauthors suggested. ICS deposition in the oropharynx could boost oropharyngeal candidiasis risk by weakening the mucosal immune response, the researchers noted. And that same process may be at work with Streptococcus pneumoniae.
A prior study found that children with asthma who received ICS for at least 1 month were almost four times more likely to have oropharyngeal colonization by S. pneumoniae as were those who didn’t get the drugs. Thus, a higher carrier rate of S. pneumoniae in the oropharynx, along with asthma’s impaired airway clearance, might increase the risk of pneumococcal diseases, the investigators explained.
Dr. Castro-Rodriguez and colleagues analyzed four studies with more than 4,000 cases and controls, and about 26 million person-years of follow-up.
Rates and risks of IPD in the four studies were as follows:
- Among those with IPD, 27% had asthma, with 18% of those without, an adjusted odds ratio (aOR) of 1.8.
- In a European of patients who received at least 3 doses of PCV7, IPD rates per 100,000 person-years for 5-year-olds were 11.6 for children with asthma and 7.3 for those without. For 5- to 17-year-olds with and without asthma, the rates were 2.3 and 1.6, respectively.
- In 2001, a Korean found an aOR of 2.08 for IPD in children with asthma, compared with those without. In 2010, the aOR was 3.26. No vaccine types were reported in the study.
- of IPD were 3.7 per 100,000 person-years for children with asthma, compared with 2.5 for healthy controls – an adjusted relative risk of 1.5.
The pooled estimate of the four studies revealed an aOR of 1.9 for IPD among children with asthma, compared with those without, Dr. Castro-Rodriguez and his team concluded.
None of the studies reported hospital admissions, mortality, length of hospital stay, intensive care admission, invasive respiratory support, or additional medication use.
One, however, did find asthma severity was significantly associated with increasing IPD treatment costs per 100,000 person-years: $72,581 for healthy controls, compared with $100,020 for children with mild asthma, $172,002 for moderate asthma, and $638,452 for severe asthma.
In addition, treating all-cause pneumonia was more expensive in children with asthma. For all-cause pneumonia, the researchers found that estimated costs per 100,000 person-years for mild, moderate, and severe asthma were $7.5 million, $14.6 million, and $46.8 million, respectively, compared with $1.7 million for healthy controls.
The authors had no relevant financial disclosures.
SOURCE: Castro-Rodriguez J et al. Pediatrics. 2020 Jan. doi: 10.1542/peds.2019-1200.
Even on-time pneumococcal vaccines don’t completely protect children with asthma from developing invasive pneumococcal disease, a meta-analysis has determined.
Despite receiving pneumococcal valent 7, 10, or 13, children with asthma were still almost twice as likely to develop the disease as were children without asthma, Jose A. Castro-Rodriguez, MD, PhD, and colleagues reported in Pediatrics (2020 Jan. doi: 10.1542/peds.2019-1200). None of the studies included rates for those who received the pneumococcal polysaccharide vaccine (PPSV23).
“For the first time, this meta-analysis reveals 90% increased odds of invasive pneumococcal disease (IPD) among [vaccinated] children with asthma,” said Dr. Castro-Rodriguez, of Pontificia Universidad Católica de Chile, Santiago, and colleagues. “If confirmed, these findings will bear clinical and public health importance,” they noted, because guidelines now recommend PPSV23 after age 2 in children with asthma only if they’re treated with prolonged high-dose oral corticosteroids.
However, because the analysis comprised only four studies, the authors cautioned that the results aren’t enough to justify changes to practice recommendations.
Asthma treatment with inhaled corticosteroids (ICS) may be driving the increased risk, Dr. Castro-Rodriguez and his coauthors suggested. ICS deposition in the oropharynx could boost oropharyngeal candidiasis risk by weakening the mucosal immune response, the researchers noted. And that same process may be at work with Streptococcus pneumoniae.
A prior study found that children with asthma who received ICS for at least 1 month were almost four times more likely to have oropharyngeal colonization by S. pneumoniae as were those who didn’t get the drugs. Thus, a higher carrier rate of S. pneumoniae in the oropharynx, along with asthma’s impaired airway clearance, might increase the risk of pneumococcal diseases, the investigators explained.
Dr. Castro-Rodriguez and colleagues analyzed four studies with more than 4,000 cases and controls, and about 26 million person-years of follow-up.
Rates and risks of IPD in the four studies were as follows:
- Among those with IPD, 27% had asthma, with 18% of those without, an adjusted odds ratio (aOR) of 1.8.
- In a European of patients who received at least 3 doses of PCV7, IPD rates per 100,000 person-years for 5-year-olds were 11.6 for children with asthma and 7.3 for those without. For 5- to 17-year-olds with and without asthma, the rates were 2.3 and 1.6, respectively.
- In 2001, a Korean found an aOR of 2.08 for IPD in children with asthma, compared with those without. In 2010, the aOR was 3.26. No vaccine types were reported in the study.
- of IPD were 3.7 per 100,000 person-years for children with asthma, compared with 2.5 for healthy controls – an adjusted relative risk of 1.5.
The pooled estimate of the four studies revealed an aOR of 1.9 for IPD among children with asthma, compared with those without, Dr. Castro-Rodriguez and his team concluded.
None of the studies reported hospital admissions, mortality, length of hospital stay, intensive care admission, invasive respiratory support, or additional medication use.
One, however, did find asthma severity was significantly associated with increasing IPD treatment costs per 100,000 person-years: $72,581 for healthy controls, compared with $100,020 for children with mild asthma, $172,002 for moderate asthma, and $638,452 for severe asthma.
In addition, treating all-cause pneumonia was more expensive in children with asthma. For all-cause pneumonia, the researchers found that estimated costs per 100,000 person-years for mild, moderate, and severe asthma were $7.5 million, $14.6 million, and $46.8 million, respectively, compared with $1.7 million for healthy controls.
The authors had no relevant financial disclosures.
SOURCE: Castro-Rodriguez J et al. Pediatrics. 2020 Jan. doi: 10.1542/peds.2019-1200.
FROM PEDIATRICS
EEG surveillance, preseizure treatment prevents TSC epilepsy, cognitive loss
BALTIMORE – Monitoring children who have tuberous sclerosis with EEG and treating them with vigabatrin (Sabril) at the first sign of preseizure abnormalities, rather than the usual practice of no surveillance and waiting until they have seizures, prevents epilepsy and cognitive decline, according to European investigators.
Early surveillance is recommended and standard practice in Europe. That’s not the case in the United States, but might be someday pending the results of the PREVENT trial (Preventing Epilepsy Using Vigabatrin In Infants With Tuberous Sclerosis Complex), an ongoing, National Institute of Neurological Disorders and Stroke–funded study to confirm the European findings.
“We are trying to convince doctors” in the United States and other “countries to do this. If you are not convinced to do early treatment,” at least “do surveillance with EEG. You will diagnose epilepsy earlier, and treat earlier, and children will do much better,” said Sergiusz Jozwiak, MD, PhD, head of pediatric neurology at Warsaw Medical University and recipient of an award from the U.S. Tuberous Sclerosis Alliance for his pioneering work.
Some U.S. physicians are already doing preventive treatment, but it’s hit and miss. “We are talking about monitoring children below the age of 2 years,” when seizures are associated with cognitive decline, he noted at the annual meeting of the American Epilepsy Society.
Dr. Jozwiak presented a follow-up at the meeting to his 2011 investigation, the first prevention study in tuberous sclerosis. Fourteen infants diagnosed within 2 months of birth underwent video-EEG monitoring every 4-6 weeks until age 2 years and were treated with vigabatrin 100-150 mg/kg per day when multifocal epileptiform discharges – a sign of impending seizures – were detected. Outcomes were compared with infants treated traditionally, with no EEG monitoring and vigabatrin only after they seized.
The children are about 9 years old now; the median IQ in the prevention arm is 94 versus 46 in the control group (P less than .03). Seven of the 14 prevention children (50%) never had a clinical seizure, while all but 1 of 25 (96%) in the control arm did (P = .001). Six of 11 prevention children (55%) versus 4 of 24 in the control group (17%), were able to come off antiepileptic drugs altogether, with no seizures (P less than .03). The work was published shortly before the epilepsy meeting.
The original 2011 report, which had similarly favorable outcomes when the children were 2 years old, led directly to the EpiStop trial, conducted at 16 mostly European centers and also reported at the meeting. Dr. Jozwiak was the senior investigator.
The design was different; all of the infants had EEG monitoring every 4 weeks until month 6, then every 6 weeks until age 12 months, then every 2 months until age 2 years. At the first detection of multifocal epileptiform discharges, infants were randomized 1:1 to vigabatrin or to the control group, with further monitoring followed by vigabatrin at the first seizure on EEG or first clinical seizure. An additional group of children – the open-label arm – also had EEG monitoring, but when to start vigabatrin was left up to the study site.
Only 50 of the original 94 children completed the trial to the full 2 years; tuberous sclerosis comorbidities drove many of them out, said lead investigator Katarzyna Kotulska-Jozwiak, MD, PhD, head of neurology at Children’s Memorial Health Institute, Warsaw.
Even so, the 25 children treated preventively in the randomized and open-label cohorts were more than three times as likely to be seizure free at 2 years (P = .01), and 74% less likely to develop drug-resistant epilepsy (P = .013). None of the prevention children developed infantile spasms versus 10 controls (40%) treated at first clinical or EEG seizure.
The incidence of neurodevelopmental delay was 34%, and autism 33%, at 24 months, and did not differ between prevention and control subjects. It’s probably because even children in the control group benefited from EEG surveillance and early treatment, the investigators said.
Historically, the rate of intellectual disability with usual treatment is around 60%, Dr. Kotulska-Jozwiak noted.
Overall, Dr. Jozwiak said that European physicians are more comfortable using vigabatrin than U.S. doctors, where the drug hasn’t been on the market as long and carries a Food and Drug Administration boxed warning of visual impairment. Its indications in the United States include infantile spasms in children 1-24 months old.
Levetiracetam (Keppra) is another option, but it’s not as effective in tuberous sclerosis. The PREVENT trial is using vigabatrin, and some U.S. doctors “are changing their minds, but it takes time,” Dr. Jozwiak said.
He noted that TSC is increasingly being diagnosed in utero, which gives a leg up on early diagnosis and prevention. The giveaways are heart tumors on ECG and cortical tubers on fetal MRI.
Dr. Jozwiak thinks the prevention approach might also help in other early seizure disorders, such as Sturge-Weber syndrome.
The work was funded by the European Commission and Polish government. Dr. Jozwiak and Dr. Kotulska-Jozwiak didn’t have any disclosures.
SOURCES: Jozwiak S et al. AES 2019, Abstract 1.218; Kotulska-Jozwiak K et al. AES 2019, Abstract 2.121.
BALTIMORE – Monitoring children who have tuberous sclerosis with EEG and treating them with vigabatrin (Sabril) at the first sign of preseizure abnormalities, rather than the usual practice of no surveillance and waiting until they have seizures, prevents epilepsy and cognitive decline, according to European investigators.
Early surveillance is recommended and standard practice in Europe. That’s not the case in the United States, but might be someday pending the results of the PREVENT trial (Preventing Epilepsy Using Vigabatrin In Infants With Tuberous Sclerosis Complex), an ongoing, National Institute of Neurological Disorders and Stroke–funded study to confirm the European findings.
“We are trying to convince doctors” in the United States and other “countries to do this. If you are not convinced to do early treatment,” at least “do surveillance with EEG. You will diagnose epilepsy earlier, and treat earlier, and children will do much better,” said Sergiusz Jozwiak, MD, PhD, head of pediatric neurology at Warsaw Medical University and recipient of an award from the U.S. Tuberous Sclerosis Alliance for his pioneering work.
Some U.S. physicians are already doing preventive treatment, but it’s hit and miss. “We are talking about monitoring children below the age of 2 years,” when seizures are associated with cognitive decline, he noted at the annual meeting of the American Epilepsy Society.
Dr. Jozwiak presented a follow-up at the meeting to his 2011 investigation, the first prevention study in tuberous sclerosis. Fourteen infants diagnosed within 2 months of birth underwent video-EEG monitoring every 4-6 weeks until age 2 years and were treated with vigabatrin 100-150 mg/kg per day when multifocal epileptiform discharges – a sign of impending seizures – were detected. Outcomes were compared with infants treated traditionally, with no EEG monitoring and vigabatrin only after they seized.
The children are about 9 years old now; the median IQ in the prevention arm is 94 versus 46 in the control group (P less than .03). Seven of the 14 prevention children (50%) never had a clinical seizure, while all but 1 of 25 (96%) in the control arm did (P = .001). Six of 11 prevention children (55%) versus 4 of 24 in the control group (17%), were able to come off antiepileptic drugs altogether, with no seizures (P less than .03). The work was published shortly before the epilepsy meeting.
The original 2011 report, which had similarly favorable outcomes when the children were 2 years old, led directly to the EpiStop trial, conducted at 16 mostly European centers and also reported at the meeting. Dr. Jozwiak was the senior investigator.
The design was different; all of the infants had EEG monitoring every 4 weeks until month 6, then every 6 weeks until age 12 months, then every 2 months until age 2 years. At the first detection of multifocal epileptiform discharges, infants were randomized 1:1 to vigabatrin or to the control group, with further monitoring followed by vigabatrin at the first seizure on EEG or first clinical seizure. An additional group of children – the open-label arm – also had EEG monitoring, but when to start vigabatrin was left up to the study site.
Only 50 of the original 94 children completed the trial to the full 2 years; tuberous sclerosis comorbidities drove many of them out, said lead investigator Katarzyna Kotulska-Jozwiak, MD, PhD, head of neurology at Children’s Memorial Health Institute, Warsaw.
Even so, the 25 children treated preventively in the randomized and open-label cohorts were more than three times as likely to be seizure free at 2 years (P = .01), and 74% less likely to develop drug-resistant epilepsy (P = .013). None of the prevention children developed infantile spasms versus 10 controls (40%) treated at first clinical or EEG seizure.
The incidence of neurodevelopmental delay was 34%, and autism 33%, at 24 months, and did not differ between prevention and control subjects. It’s probably because even children in the control group benefited from EEG surveillance and early treatment, the investigators said.
Historically, the rate of intellectual disability with usual treatment is around 60%, Dr. Kotulska-Jozwiak noted.
Overall, Dr. Jozwiak said that European physicians are more comfortable using vigabatrin than U.S. doctors, where the drug hasn’t been on the market as long and carries a Food and Drug Administration boxed warning of visual impairment. Its indications in the United States include infantile spasms in children 1-24 months old.
Levetiracetam (Keppra) is another option, but it’s not as effective in tuberous sclerosis. The PREVENT trial is using vigabatrin, and some U.S. doctors “are changing their minds, but it takes time,” Dr. Jozwiak said.
He noted that TSC is increasingly being diagnosed in utero, which gives a leg up on early diagnosis and prevention. The giveaways are heart tumors on ECG and cortical tubers on fetal MRI.
Dr. Jozwiak thinks the prevention approach might also help in other early seizure disorders, such as Sturge-Weber syndrome.
The work was funded by the European Commission and Polish government. Dr. Jozwiak and Dr. Kotulska-Jozwiak didn’t have any disclosures.
SOURCES: Jozwiak S et al. AES 2019, Abstract 1.218; Kotulska-Jozwiak K et al. AES 2019, Abstract 2.121.
BALTIMORE – Monitoring children who have tuberous sclerosis with EEG and treating them with vigabatrin (Sabril) at the first sign of preseizure abnormalities, rather than the usual practice of no surveillance and waiting until they have seizures, prevents epilepsy and cognitive decline, according to European investigators.
Early surveillance is recommended and standard practice in Europe. That’s not the case in the United States, but might be someday pending the results of the PREVENT trial (Preventing Epilepsy Using Vigabatrin In Infants With Tuberous Sclerosis Complex), an ongoing, National Institute of Neurological Disorders and Stroke–funded study to confirm the European findings.
“We are trying to convince doctors” in the United States and other “countries to do this. If you are not convinced to do early treatment,” at least “do surveillance with EEG. You will diagnose epilepsy earlier, and treat earlier, and children will do much better,” said Sergiusz Jozwiak, MD, PhD, head of pediatric neurology at Warsaw Medical University and recipient of an award from the U.S. Tuberous Sclerosis Alliance for his pioneering work.
Some U.S. physicians are already doing preventive treatment, but it’s hit and miss. “We are talking about monitoring children below the age of 2 years,” when seizures are associated with cognitive decline, he noted at the annual meeting of the American Epilepsy Society.
Dr. Jozwiak presented a follow-up at the meeting to his 2011 investigation, the first prevention study in tuberous sclerosis. Fourteen infants diagnosed within 2 months of birth underwent video-EEG monitoring every 4-6 weeks until age 2 years and were treated with vigabatrin 100-150 mg/kg per day when multifocal epileptiform discharges – a sign of impending seizures – were detected. Outcomes were compared with infants treated traditionally, with no EEG monitoring and vigabatrin only after they seized.
The children are about 9 years old now; the median IQ in the prevention arm is 94 versus 46 in the control group (P less than .03). Seven of the 14 prevention children (50%) never had a clinical seizure, while all but 1 of 25 (96%) in the control arm did (P = .001). Six of 11 prevention children (55%) versus 4 of 24 in the control group (17%), were able to come off antiepileptic drugs altogether, with no seizures (P less than .03). The work was published shortly before the epilepsy meeting.
The original 2011 report, which had similarly favorable outcomes when the children were 2 years old, led directly to the EpiStop trial, conducted at 16 mostly European centers and also reported at the meeting. Dr. Jozwiak was the senior investigator.
The design was different; all of the infants had EEG monitoring every 4 weeks until month 6, then every 6 weeks until age 12 months, then every 2 months until age 2 years. At the first detection of multifocal epileptiform discharges, infants were randomized 1:1 to vigabatrin or to the control group, with further monitoring followed by vigabatrin at the first seizure on EEG or first clinical seizure. An additional group of children – the open-label arm – also had EEG monitoring, but when to start vigabatrin was left up to the study site.
Only 50 of the original 94 children completed the trial to the full 2 years; tuberous sclerosis comorbidities drove many of them out, said lead investigator Katarzyna Kotulska-Jozwiak, MD, PhD, head of neurology at Children’s Memorial Health Institute, Warsaw.
Even so, the 25 children treated preventively in the randomized and open-label cohorts were more than three times as likely to be seizure free at 2 years (P = .01), and 74% less likely to develop drug-resistant epilepsy (P = .013). None of the prevention children developed infantile spasms versus 10 controls (40%) treated at first clinical or EEG seizure.
The incidence of neurodevelopmental delay was 34%, and autism 33%, at 24 months, and did not differ between prevention and control subjects. It’s probably because even children in the control group benefited from EEG surveillance and early treatment, the investigators said.
Historically, the rate of intellectual disability with usual treatment is around 60%, Dr. Kotulska-Jozwiak noted.
Overall, Dr. Jozwiak said that European physicians are more comfortable using vigabatrin than U.S. doctors, where the drug hasn’t been on the market as long and carries a Food and Drug Administration boxed warning of visual impairment. Its indications in the United States include infantile spasms in children 1-24 months old.
Levetiracetam (Keppra) is another option, but it’s not as effective in tuberous sclerosis. The PREVENT trial is using vigabatrin, and some U.S. doctors “are changing their minds, but it takes time,” Dr. Jozwiak said.
He noted that TSC is increasingly being diagnosed in utero, which gives a leg up on early diagnosis and prevention. The giveaways are heart tumors on ECG and cortical tubers on fetal MRI.
Dr. Jozwiak thinks the prevention approach might also help in other early seizure disorders, such as Sturge-Weber syndrome.
The work was funded by the European Commission and Polish government. Dr. Jozwiak and Dr. Kotulska-Jozwiak didn’t have any disclosures.
SOURCES: Jozwiak S et al. AES 2019, Abstract 1.218; Kotulska-Jozwiak K et al. AES 2019, Abstract 2.121.
REPORTING FROM AES 2019
Cultivating patient activation through technology
Tech alone is not enough
Patient activation refers to an individual’s knowledge, skill, and confidence in managing their health and health care, according to a recent BMJ editorial. It’s recognized as a critical aspect of high-quality, patient-centered health care – patient activation has the potential to improve patient outcomes while reducing costs.
Total knee replacement offers a great opportunity to study patient activation, said editorial lead author Jesse I. Wolfstadt, MD, MS, FRCSC, of the University of Toronto. “It may help address the one in five patients who are unsatisfied with their knee replacement despite an otherwise technically sound procedure.”
The authors considered some patient activation studies that have shown positive results for cultivating activation through technology. In one, patients engaging with a bedside multimedia intervention on a tablet after undergoing knee replacement reported better pain scores, length of stay, knee function, and satisfaction with care. Another study showed patients who received automated text messages after joint replacement improved time spent on home exercises, decreased their use of narcotics, and had fewer calls to the surgeon’s office.
But “negative mobile app studies seem to suggest that when technologies are used as a passive educational intervention, patient activation may suffer,” according to the editorial. “One possible key ingredient to successful patient activation is the engagement of the health care team that is facilitated through mobile technology. ... Mobile apps and other technological interventions also must have clear goals if they are to be used successfully; and these goals are likely to differ for different patient populations and disease processes.”
Technology alone is not enough to affect patient activation, Dr. Wolfstadt said. “The key to success will likely involve tailoring interventions to individual patients and facilitating increased engagement with the health care team. You can’t just give a patient an app or other form of technology and expect it to replace the function of patient-clinician communication/interaction.”
Reference
1. Wolfstadt JI et ak. Improving patient outcomes following total joint arthroplasty: Is there an app for that? BMJ Qual Saf. 2019 May 2019. doi: 10.1136/bmjqs-2019-009571.
Tech alone is not enough
Tech alone is not enough
Patient activation refers to an individual’s knowledge, skill, and confidence in managing their health and health care, according to a recent BMJ editorial. It’s recognized as a critical aspect of high-quality, patient-centered health care – patient activation has the potential to improve patient outcomes while reducing costs.
Total knee replacement offers a great opportunity to study patient activation, said editorial lead author Jesse I. Wolfstadt, MD, MS, FRCSC, of the University of Toronto. “It may help address the one in five patients who are unsatisfied with their knee replacement despite an otherwise technically sound procedure.”
The authors considered some patient activation studies that have shown positive results for cultivating activation through technology. In one, patients engaging with a bedside multimedia intervention on a tablet after undergoing knee replacement reported better pain scores, length of stay, knee function, and satisfaction with care. Another study showed patients who received automated text messages after joint replacement improved time spent on home exercises, decreased their use of narcotics, and had fewer calls to the surgeon’s office.
But “negative mobile app studies seem to suggest that when technologies are used as a passive educational intervention, patient activation may suffer,” according to the editorial. “One possible key ingredient to successful patient activation is the engagement of the health care team that is facilitated through mobile technology. ... Mobile apps and other technological interventions also must have clear goals if they are to be used successfully; and these goals are likely to differ for different patient populations and disease processes.”
Technology alone is not enough to affect patient activation, Dr. Wolfstadt said. “The key to success will likely involve tailoring interventions to individual patients and facilitating increased engagement with the health care team. You can’t just give a patient an app or other form of technology and expect it to replace the function of patient-clinician communication/interaction.”
Reference
1. Wolfstadt JI et ak. Improving patient outcomes following total joint arthroplasty: Is there an app for that? BMJ Qual Saf. 2019 May 2019. doi: 10.1136/bmjqs-2019-009571.
Patient activation refers to an individual’s knowledge, skill, and confidence in managing their health and health care, according to a recent BMJ editorial. It’s recognized as a critical aspect of high-quality, patient-centered health care – patient activation has the potential to improve patient outcomes while reducing costs.
Total knee replacement offers a great opportunity to study patient activation, said editorial lead author Jesse I. Wolfstadt, MD, MS, FRCSC, of the University of Toronto. “It may help address the one in five patients who are unsatisfied with their knee replacement despite an otherwise technically sound procedure.”
The authors considered some patient activation studies that have shown positive results for cultivating activation through technology. In one, patients engaging with a bedside multimedia intervention on a tablet after undergoing knee replacement reported better pain scores, length of stay, knee function, and satisfaction with care. Another study showed patients who received automated text messages after joint replacement improved time spent on home exercises, decreased their use of narcotics, and had fewer calls to the surgeon’s office.
But “negative mobile app studies seem to suggest that when technologies are used as a passive educational intervention, patient activation may suffer,” according to the editorial. “One possible key ingredient to successful patient activation is the engagement of the health care team that is facilitated through mobile technology. ... Mobile apps and other technological interventions also must have clear goals if they are to be used successfully; and these goals are likely to differ for different patient populations and disease processes.”
Technology alone is not enough to affect patient activation, Dr. Wolfstadt said. “The key to success will likely involve tailoring interventions to individual patients and facilitating increased engagement with the health care team. You can’t just give a patient an app or other form of technology and expect it to replace the function of patient-clinician communication/interaction.”
Reference
1. Wolfstadt JI et ak. Improving patient outcomes following total joint arthroplasty: Is there an app for that? BMJ Qual Saf. 2019 May 2019. doi: 10.1136/bmjqs-2019-009571.
AGA publishes clinical practice guidelines for gastric intestinal metaplasia
The American Gastroenterological Association (AGA) recently published clinical practice guidelines for managing gastric intestinal metaplasia (GIM).
The guidelines are the first of their kind to be published in the United States, according to lead author Samir Gupta, MD, of the University of California San Diego, and colleagues. The panelists suggested that the guidelines may help standardize decision making in a common clinical scenario.
“GIM has been considered as one specific marker to identify patients who might benefit from surveillance because it has been associated with increased risk for gastric cancer and is routinely encountered in clinical practice,” the panelists wrote in Gastroenterology.
The guideline panel was composed of three gastroenterologists, two guideline methodologist trainees, and three GRADE experts. Recommendations were based on the AGA guideline development process, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology, best practices set forth by the Academy of Medicine, and a technical review.
“Given the paucity of robust direct data on GIM in the U.S., evidence from all regions of the world was considered relevant in the evidence-gathering phase,” the panelists wrote (Gastroenterology. 2019 Dec 6. doi: 10.1053/j.gastro.2019.12.003).
Based on available evidence, the expert panel developed three clinical recommendations.
First, the panelists recommended that clinicians test all patients with GIM for Helicobacter pylori, followed by eradication, over no testing and eradication. This recommendation was strong and based on moderate quality evidence from 22 studies, including 7 randomized controlled trials. These studies showed that, compared with placebo, eradication of H. pylori was associated with a 32% pooled relative risk reduction in gastric cancer and a 33% pooled relative risk reduction in gastric cancer mortality among patients with or without GIM. The pooled relative risk reduction rate was similar in analyses solely composed of individuals with GIM, the panelists noted, whereas mortality data restricted to individuals with GIM were lacking.
“Overall, the known strong association of H. pylori with risk for incident gastric cancer and the technical review’s findings, which reinforce the evidence of reduced risk for incident gastric cancer after H. pylori eradication, supports the AGA recommendation to test for and eradicate H. pylori,” the panelists wrote.
The second recommendation, which was conditional and based on very low quality evidence, advised against routine use of endoscopic surveillance for patients with GIM. Still, surveillance may be considered for patients with higher risk of gastric cancer, including those with incomplete and/or extensive GIM, a family history of gastric cancer, racial/ethnic minorities, and immigrants from high incidence regions, the panelists wrote.
“Although the technical review did not find evidence supporting increased risk for gastric cancer among racial/ethnic minorities or immigrants with documented GIM, an overall increased risk for gastric cancer (irrespective of presence/absence of GIM) has been established among these groups, and may be considered as part of decision making regarding surveillance,” the panelists wrote.
The third and final recommendation was also conditional and based on very weak evidence; the panelists recommended against routine short-interval repeat endoscopy for the purpose of risk stratification.
“The technical review found no direct evidence to support the impact of short-interval (less than 12 months) repeat upper endoscopy among patients with incidental GIM on patient-important outcomes,” the panelists wrote.
However, the guidelines note that patients with potentially elevated risk profiles, such as patients with a family history of gastric cancer, “may reasonably elect for repeat endoscopy within 1 year for risk stratification.”
Comparing these guidelines with those from other organizations, such as the European Society of Gastrointestinal Endoscopy (ESGE), the panelists concluded that recommendations across organizations are “generally similar.”
Finally, the panelists outlined relevant knowledge gaps and pointed to future research topics. For instance, data are scarce comparing outcomes in relation to surveillance versus no surveillance among patients with GIM; and biomarkers such as pepsinogen levels, which are used in Asian countries for risk stratification of gastric cancer, have been studied minimally in the United States.
Guideline development was funded by the AGA. The panelists disclosed no conflicts of interest.
The American Gastroenterological Association (AGA) recently published clinical practice guidelines for managing gastric intestinal metaplasia (GIM).
The guidelines are the first of their kind to be published in the United States, according to lead author Samir Gupta, MD, of the University of California San Diego, and colleagues. The panelists suggested that the guidelines may help standardize decision making in a common clinical scenario.
“GIM has been considered as one specific marker to identify patients who might benefit from surveillance because it has been associated with increased risk for gastric cancer and is routinely encountered in clinical practice,” the panelists wrote in Gastroenterology.
The guideline panel was composed of three gastroenterologists, two guideline methodologist trainees, and three GRADE experts. Recommendations were based on the AGA guideline development process, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology, best practices set forth by the Academy of Medicine, and a technical review.
“Given the paucity of robust direct data on GIM in the U.S., evidence from all regions of the world was considered relevant in the evidence-gathering phase,” the panelists wrote (Gastroenterology. 2019 Dec 6. doi: 10.1053/j.gastro.2019.12.003).
Based on available evidence, the expert panel developed three clinical recommendations.
First, the panelists recommended that clinicians test all patients with GIM for Helicobacter pylori, followed by eradication, over no testing and eradication. This recommendation was strong and based on moderate quality evidence from 22 studies, including 7 randomized controlled trials. These studies showed that, compared with placebo, eradication of H. pylori was associated with a 32% pooled relative risk reduction in gastric cancer and a 33% pooled relative risk reduction in gastric cancer mortality among patients with or without GIM. The pooled relative risk reduction rate was similar in analyses solely composed of individuals with GIM, the panelists noted, whereas mortality data restricted to individuals with GIM were lacking.
“Overall, the known strong association of H. pylori with risk for incident gastric cancer and the technical review’s findings, which reinforce the evidence of reduced risk for incident gastric cancer after H. pylori eradication, supports the AGA recommendation to test for and eradicate H. pylori,” the panelists wrote.
The second recommendation, which was conditional and based on very low quality evidence, advised against routine use of endoscopic surveillance for patients with GIM. Still, surveillance may be considered for patients with higher risk of gastric cancer, including those with incomplete and/or extensive GIM, a family history of gastric cancer, racial/ethnic minorities, and immigrants from high incidence regions, the panelists wrote.
“Although the technical review did not find evidence supporting increased risk for gastric cancer among racial/ethnic minorities or immigrants with documented GIM, an overall increased risk for gastric cancer (irrespective of presence/absence of GIM) has been established among these groups, and may be considered as part of decision making regarding surveillance,” the panelists wrote.
The third and final recommendation was also conditional and based on very weak evidence; the panelists recommended against routine short-interval repeat endoscopy for the purpose of risk stratification.
“The technical review found no direct evidence to support the impact of short-interval (less than 12 months) repeat upper endoscopy among patients with incidental GIM on patient-important outcomes,” the panelists wrote.
However, the guidelines note that patients with potentially elevated risk profiles, such as patients with a family history of gastric cancer, “may reasonably elect for repeat endoscopy within 1 year for risk stratification.”
Comparing these guidelines with those from other organizations, such as the European Society of Gastrointestinal Endoscopy (ESGE), the panelists concluded that recommendations across organizations are “generally similar.”
Finally, the panelists outlined relevant knowledge gaps and pointed to future research topics. For instance, data are scarce comparing outcomes in relation to surveillance versus no surveillance among patients with GIM; and biomarkers such as pepsinogen levels, which are used in Asian countries for risk stratification of gastric cancer, have been studied minimally in the United States.
Guideline development was funded by the AGA. The panelists disclosed no conflicts of interest.
The American Gastroenterological Association (AGA) recently published clinical practice guidelines for managing gastric intestinal metaplasia (GIM).
The guidelines are the first of their kind to be published in the United States, according to lead author Samir Gupta, MD, of the University of California San Diego, and colleagues. The panelists suggested that the guidelines may help standardize decision making in a common clinical scenario.
“GIM has been considered as one specific marker to identify patients who might benefit from surveillance because it has been associated with increased risk for gastric cancer and is routinely encountered in clinical practice,” the panelists wrote in Gastroenterology.
The guideline panel was composed of three gastroenterologists, two guideline methodologist trainees, and three GRADE experts. Recommendations were based on the AGA guideline development process, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology, best practices set forth by the Academy of Medicine, and a technical review.
“Given the paucity of robust direct data on GIM in the U.S., evidence from all regions of the world was considered relevant in the evidence-gathering phase,” the panelists wrote (Gastroenterology. 2019 Dec 6. doi: 10.1053/j.gastro.2019.12.003).
Based on available evidence, the expert panel developed three clinical recommendations.
First, the panelists recommended that clinicians test all patients with GIM for Helicobacter pylori, followed by eradication, over no testing and eradication. This recommendation was strong and based on moderate quality evidence from 22 studies, including 7 randomized controlled trials. These studies showed that, compared with placebo, eradication of H. pylori was associated with a 32% pooled relative risk reduction in gastric cancer and a 33% pooled relative risk reduction in gastric cancer mortality among patients with or without GIM. The pooled relative risk reduction rate was similar in analyses solely composed of individuals with GIM, the panelists noted, whereas mortality data restricted to individuals with GIM were lacking.
“Overall, the known strong association of H. pylori with risk for incident gastric cancer and the technical review’s findings, which reinforce the evidence of reduced risk for incident gastric cancer after H. pylori eradication, supports the AGA recommendation to test for and eradicate H. pylori,” the panelists wrote.
The second recommendation, which was conditional and based on very low quality evidence, advised against routine use of endoscopic surveillance for patients with GIM. Still, surveillance may be considered for patients with higher risk of gastric cancer, including those with incomplete and/or extensive GIM, a family history of gastric cancer, racial/ethnic minorities, and immigrants from high incidence regions, the panelists wrote.
“Although the technical review did not find evidence supporting increased risk for gastric cancer among racial/ethnic minorities or immigrants with documented GIM, an overall increased risk for gastric cancer (irrespective of presence/absence of GIM) has been established among these groups, and may be considered as part of decision making regarding surveillance,” the panelists wrote.
The third and final recommendation was also conditional and based on very weak evidence; the panelists recommended against routine short-interval repeat endoscopy for the purpose of risk stratification.
“The technical review found no direct evidence to support the impact of short-interval (less than 12 months) repeat upper endoscopy among patients with incidental GIM on patient-important outcomes,” the panelists wrote.
However, the guidelines note that patients with potentially elevated risk profiles, such as patients with a family history of gastric cancer, “may reasonably elect for repeat endoscopy within 1 year for risk stratification.”
Comparing these guidelines with those from other organizations, such as the European Society of Gastrointestinal Endoscopy (ESGE), the panelists concluded that recommendations across organizations are “generally similar.”
Finally, the panelists outlined relevant knowledge gaps and pointed to future research topics. For instance, data are scarce comparing outcomes in relation to surveillance versus no surveillance among patients with GIM; and biomarkers such as pepsinogen levels, which are used in Asian countries for risk stratification of gastric cancer, have been studied minimally in the United States.
Guideline development was funded by the AGA. The panelists disclosed no conflicts of interest.
FROM GASTROENTEROLOGY
Pelvic insufficiency fractures are common after chemoradiotherapy for cervical cancer
CHICAGO – Radiation therapy for cervical cancer resulted in pelvic insufficiency fractures more frequently than previously thought, and many fractures were slow to heal, according to research presented at the annual meeting of the Radiological Society of North America.
“Pelvic insufficiency fractures had a prevalence of 38% on MRI follow-up” after chemoradiotherapy for locally advanced cervical cancer, said Alina Dragan, MD. This figure is more than double the previously reported prevalence of about 14%.
Dr. Dragan, a radiology resident at London North West Healthcare, National Health Service Trust, and coinvestigators also tracked the natural history of these fractures over time, to fill a knowledge gap about whether, and at what rate, these pelvic insufficiency fractures healed.
In the single-center retrospective study, the investigators found that just 14% of sacral fractures healed during the period of observation. For acetabular and pubic fractures, roughly one in three fractures had healed by the last MRI scan. About a third of all fractures remained stable across scans, while just over 10% of fractures were either fluctuant or worsened.
The study included 115 women with locally advanced cervical cancer who were treated with radical or adjuvant concurrent chemoradiotherapy over a 5-year period, and had MRI scans performed in-house; the follow-up protocol had patients receiving scans at 3, 12, and 24 months post treatment. From an initial pool of 197 patients, those who had previously had pelvic radiation or were receiving palliative treatment, as well as those with incomplete imaging follow-up and those with metal implants or prostheses that could affect radiation therapy delivery or imaging quality were excluded.
The chemoradiotherapy protocol involved five doses of weekly cisplatin at 400 mg/m2 of body surface area, as well as high–dose rate cervix brachytherapy. In practice, all but six participants received these treatments. Patients also received external beam radiotherapy with or without a simultaneous integrated boost to target affected lymph nodes, as clinically indicated.
The fractures were graded as mild, moderate, or severe by the interpreting radiologist according to the course of the fracture line and corresponding bone edema.
Patients were aged a median of 54 years, and 64 (56%) were postmenopausal. Most patients (n = 84; 73%) had never used tobacco. Participants’ median body mass index was 26 kg/m2.
Most patients (n = 73; 64%) were International Federation of Gynecology and Obstetrics stage 2b, and almost half (n = 55; 48%) had pelvic nodal involvement.
Patients were followed for a median of 12 months, with patients receiving a median of two MRIs curing that period. In all, 105 fractures were identified in 44 patients. A median of two fractures were identified among the group of patients who had pelvic insufficiency fractures.
The fractures were graded as mild, moderate, or severe by the interpreting radiologist according to the course of the fracture line and corresponding bone edema. In this schema, 41% of identified fractures were considered mild, while 32% were moderate and 12% were severe.
Although just over two-thirds of fractures (70%) were identified within 6 months of beginning surveillance, a quarter were not identified until 9-13 months post therapy, and 5% were found after more than 13 months.
Sacral fractures accounted for 72% of those identified, in keeping with previous findings, said Dr. Dragan. Acetabular and pubic fractures made up 16% and 10% of fractures, respectively. One fracture was seen at the ilium and one at the ischium.
Dr. Dragan and colleagues turned to multivariable analysis to look for risk factors for pelvic insufficiency fractures in this cohort of cervical cancer patients. Younger patients had a hazard ratio of 0.30 for fracture, compared with those over the age of 50 years (P less than .01). Similarly, being menopausal carried a hazard ratio of 2.25 for fracture. Higher radiation doses to the sacrum also boosted fracture risk (HR, 2.00; P = .03). Neither sacral volume and slope nor the receipt of simultaneous integrated boost were associated with increased fracture risk.
Dr. Dragan reported that she had no relevant conflicts of interest. She reported no outside sources of funding.
SOURCE: Dragan A et al. RSNA 2019, Presentation SSE25-03.
CHICAGO – Radiation therapy for cervical cancer resulted in pelvic insufficiency fractures more frequently than previously thought, and many fractures were slow to heal, according to research presented at the annual meeting of the Radiological Society of North America.
“Pelvic insufficiency fractures had a prevalence of 38% on MRI follow-up” after chemoradiotherapy for locally advanced cervical cancer, said Alina Dragan, MD. This figure is more than double the previously reported prevalence of about 14%.
Dr. Dragan, a radiology resident at London North West Healthcare, National Health Service Trust, and coinvestigators also tracked the natural history of these fractures over time, to fill a knowledge gap about whether, and at what rate, these pelvic insufficiency fractures healed.
In the single-center retrospective study, the investigators found that just 14% of sacral fractures healed during the period of observation. For acetabular and pubic fractures, roughly one in three fractures had healed by the last MRI scan. About a third of all fractures remained stable across scans, while just over 10% of fractures were either fluctuant or worsened.
The study included 115 women with locally advanced cervical cancer who were treated with radical or adjuvant concurrent chemoradiotherapy over a 5-year period, and had MRI scans performed in-house; the follow-up protocol had patients receiving scans at 3, 12, and 24 months post treatment. From an initial pool of 197 patients, those who had previously had pelvic radiation or were receiving palliative treatment, as well as those with incomplete imaging follow-up and those with metal implants or prostheses that could affect radiation therapy delivery or imaging quality were excluded.
The chemoradiotherapy protocol involved five doses of weekly cisplatin at 400 mg/m2 of body surface area, as well as high–dose rate cervix brachytherapy. In practice, all but six participants received these treatments. Patients also received external beam radiotherapy with or without a simultaneous integrated boost to target affected lymph nodes, as clinically indicated.
The fractures were graded as mild, moderate, or severe by the interpreting radiologist according to the course of the fracture line and corresponding bone edema.
Patients were aged a median of 54 years, and 64 (56%) were postmenopausal. Most patients (n = 84; 73%) had never used tobacco. Participants’ median body mass index was 26 kg/m2.
Most patients (n = 73; 64%) were International Federation of Gynecology and Obstetrics stage 2b, and almost half (n = 55; 48%) had pelvic nodal involvement.
Patients were followed for a median of 12 months, with patients receiving a median of two MRIs curing that period. In all, 105 fractures were identified in 44 patients. A median of two fractures were identified among the group of patients who had pelvic insufficiency fractures.
The fractures were graded as mild, moderate, or severe by the interpreting radiologist according to the course of the fracture line and corresponding bone edema. In this schema, 41% of identified fractures were considered mild, while 32% were moderate and 12% were severe.
Although just over two-thirds of fractures (70%) were identified within 6 months of beginning surveillance, a quarter were not identified until 9-13 months post therapy, and 5% were found after more than 13 months.
Sacral fractures accounted for 72% of those identified, in keeping with previous findings, said Dr. Dragan. Acetabular and pubic fractures made up 16% and 10% of fractures, respectively. One fracture was seen at the ilium and one at the ischium.
Dr. Dragan and colleagues turned to multivariable analysis to look for risk factors for pelvic insufficiency fractures in this cohort of cervical cancer patients. Younger patients had a hazard ratio of 0.30 for fracture, compared with those over the age of 50 years (P less than .01). Similarly, being menopausal carried a hazard ratio of 2.25 for fracture. Higher radiation doses to the sacrum also boosted fracture risk (HR, 2.00; P = .03). Neither sacral volume and slope nor the receipt of simultaneous integrated boost were associated with increased fracture risk.
Dr. Dragan reported that she had no relevant conflicts of interest. She reported no outside sources of funding.
SOURCE: Dragan A et al. RSNA 2019, Presentation SSE25-03.
CHICAGO – Radiation therapy for cervical cancer resulted in pelvic insufficiency fractures more frequently than previously thought, and many fractures were slow to heal, according to research presented at the annual meeting of the Radiological Society of North America.
“Pelvic insufficiency fractures had a prevalence of 38% on MRI follow-up” after chemoradiotherapy for locally advanced cervical cancer, said Alina Dragan, MD. This figure is more than double the previously reported prevalence of about 14%.
Dr. Dragan, a radiology resident at London North West Healthcare, National Health Service Trust, and coinvestigators also tracked the natural history of these fractures over time, to fill a knowledge gap about whether, and at what rate, these pelvic insufficiency fractures healed.
In the single-center retrospective study, the investigators found that just 14% of sacral fractures healed during the period of observation. For acetabular and pubic fractures, roughly one in three fractures had healed by the last MRI scan. About a third of all fractures remained stable across scans, while just over 10% of fractures were either fluctuant or worsened.
The study included 115 women with locally advanced cervical cancer who were treated with radical or adjuvant concurrent chemoradiotherapy over a 5-year period, and had MRI scans performed in-house; the follow-up protocol had patients receiving scans at 3, 12, and 24 months post treatment. From an initial pool of 197 patients, those who had previously had pelvic radiation or were receiving palliative treatment, as well as those with incomplete imaging follow-up and those with metal implants or prostheses that could affect radiation therapy delivery or imaging quality were excluded.
The chemoradiotherapy protocol involved five doses of weekly cisplatin at 400 mg/m2 of body surface area, as well as high–dose rate cervix brachytherapy. In practice, all but six participants received these treatments. Patients also received external beam radiotherapy with or without a simultaneous integrated boost to target affected lymph nodes, as clinically indicated.
The fractures were graded as mild, moderate, or severe by the interpreting radiologist according to the course of the fracture line and corresponding bone edema.
Patients were aged a median of 54 years, and 64 (56%) were postmenopausal. Most patients (n = 84; 73%) had never used tobacco. Participants’ median body mass index was 26 kg/m2.
Most patients (n = 73; 64%) were International Federation of Gynecology and Obstetrics stage 2b, and almost half (n = 55; 48%) had pelvic nodal involvement.
Patients were followed for a median of 12 months, with patients receiving a median of two MRIs curing that period. In all, 105 fractures were identified in 44 patients. A median of two fractures were identified among the group of patients who had pelvic insufficiency fractures.
The fractures were graded as mild, moderate, or severe by the interpreting radiologist according to the course of the fracture line and corresponding bone edema. In this schema, 41% of identified fractures were considered mild, while 32% were moderate and 12% were severe.
Although just over two-thirds of fractures (70%) were identified within 6 months of beginning surveillance, a quarter were not identified until 9-13 months post therapy, and 5% were found after more than 13 months.
Sacral fractures accounted for 72% of those identified, in keeping with previous findings, said Dr. Dragan. Acetabular and pubic fractures made up 16% and 10% of fractures, respectively. One fracture was seen at the ilium and one at the ischium.
Dr. Dragan and colleagues turned to multivariable analysis to look for risk factors for pelvic insufficiency fractures in this cohort of cervical cancer patients. Younger patients had a hazard ratio of 0.30 for fracture, compared with those over the age of 50 years (P less than .01). Similarly, being menopausal carried a hazard ratio of 2.25 for fracture. Higher radiation doses to the sacrum also boosted fracture risk (HR, 2.00; P = .03). Neither sacral volume and slope nor the receipt of simultaneous integrated boost were associated with increased fracture risk.
Dr. Dragan reported that she had no relevant conflicts of interest. She reported no outside sources of funding.
SOURCE: Dragan A et al. RSNA 2019, Presentation SSE25-03.
REPORTING FROM RSNA 2019
Appeals court rules ACA’s individual mandate is unconstitutional
A federal appeals court ruled Dec. 18 that the individual mandate of the Affordable Care Act (ACA) is unconstitutional, but the panel sent the case back to a lower court to decide how much of the remainder of the law could topple along with it.
The three-judge panel of the New Orleans-based U.S. Fifth Circuit Court of Appeals said, “The individual mandate is unconstitutional because, under [a previous ruling, National Federation of Independent Business v Sebelius], it finds no constitutional footing in either the Interstate Commerce Clause or the Necessary and Proper Clause.”
The ruling upholds a December 2018 US District Court decision in which Judge Reed O’Connor found that the individual mandate that most Americans must have health insurance or pay a fine was unconstitutional and that without it the ACA itself was invalid.
In sending the case back to a Texas district court, however, the federal panel is asking for a central question to be resolved: Whether the individual mandate is “severable” from the rest of the law, while the rest of the law can be left intact.
If the district court eventually decides that the individual mandate cannot be severed from the rest of the ACA, the entire law will likely be ruled invalid, and some 24 million Americans could lose health coverage.
“Today’s ruling is the result of the Trump administration and congressional Republicans attempting to make dangerous health policy using the courts since they failed to succeed in Congress,” House Ways and Means Committee Chairman Richard E. Neal (D-Mass.) said in a statement. “This is a blow to our nation’s health care system and the millions of Americans who have gained coverage and protections under the Affordable Care Act. Democrats will continue to fight to protect Americans’ access to quality, affordable care.”
Some groups are applauding the decision, though. The Citizens’ Council for Health Freedom (CCHF), which filed an amicus brief with the Fifth Circuit arguing against the ACA, said it wants more.
“We are pleased with the Fifth Circuit Court of Appeals ruling, but it didn’t go far enough,” said Twila Brase, president and cofounder of CCHF, in a statement. “The individual mandate cannot be severed from the rest of the 2,700-page Affordable Care Act, thus the court should have ruled that the entire law is invalid, as the lower district court found.
“As the Court notes in the first paragraph of the ruling, we argued in our Amicus Brief, filed jointly with the Association of American Physicians and Surgeons, that the Act ‘has deprived patients nationwide of a competitive market for affordable high-deductible health insurance,’ leaving ‘patients with no alternative to ... skyrocketing premiums,’ “ Ms. Brase added. “Sending it back to the lower court, which already ruled the right way, continues to deprive citizens and patients of the affordable coverage that freedom from Obamacare would bring.”
Future uncertain
The ruling in Texas v Azar is not a surprise because, during oral arguments in July, as reported by Medscape Medical News, at least two of the three judges – Jennifer Walker Elrod, appointed by President George W. Bush in 2007, and Kurt Engelhardt, appointed by President Donald J. Trump in 2018 – appeared to be more receptive to the arguments of a group of 18 Republican states and two individuals seeking to invalidate the ACA.
Judge Carolyn Dineen King, appointed by President Jimmy Carter in 1979, did not comment during the hearing.
The Trump administration chose not to defend the ACA, but it does not seem entirely prepared for what might happen if the law is overturned. In a briefing before the Fifth Circuit hearing, the administration argued that, if ultimately the law is ruled unconstitutional, it should be struck down only in the states seeking to overturn the law.
“A lot of this has to get sorted out – it’s complicated,” said August E. Flentje, a U.S. Department of Justice lawyer, at the oral arguments in July.
For now, though, the ACA remains.
“In 2012, the Supreme Court upheld Obamacare, despite serious constitutional issues with the federal government forcing Americans to purchase a product from a private company. Until an ultimate decision is made by the Supreme Court or Congress decides otherwise, the Affordable Care Act will remain the law of the land,” Senate Finance Committee Chairman Chuck Grassley (R-Iowa), said in a statement.
And those who have led the court battle to keep the ACA intact plan to keep fighting. “For now, the President got the gift he wanted – uncertainty in the health care system and a pathway to repeal – so that the health care that seniors, workers, and families secured under the Affordable Care Act can be yanked from under them. This decision could take us to a dangerous and irresponsible place, not just for the 133 million Americans with pre-existing conditions, but for our seniors who use Medicare, our children under the age of 26, and the 20 million additional Americans covered directly through the ACA marketplace. California will move swiftly to challenge this decision because this could mean the difference between life and death for so many Americans and their families,” California Attorney General Xavier Becerra said in a statement.
Learn more about AGA’s position on patient protections and access to care at https://www.gastro.org/
A version of this story first appeared on Medscape.com.
A federal appeals court ruled Dec. 18 that the individual mandate of the Affordable Care Act (ACA) is unconstitutional, but the panel sent the case back to a lower court to decide how much of the remainder of the law could topple along with it.
The three-judge panel of the New Orleans-based U.S. Fifth Circuit Court of Appeals said, “The individual mandate is unconstitutional because, under [a previous ruling, National Federation of Independent Business v Sebelius], it finds no constitutional footing in either the Interstate Commerce Clause or the Necessary and Proper Clause.”
The ruling upholds a December 2018 US District Court decision in which Judge Reed O’Connor found that the individual mandate that most Americans must have health insurance or pay a fine was unconstitutional and that without it the ACA itself was invalid.
In sending the case back to a Texas district court, however, the federal panel is asking for a central question to be resolved: Whether the individual mandate is “severable” from the rest of the law, while the rest of the law can be left intact.
If the district court eventually decides that the individual mandate cannot be severed from the rest of the ACA, the entire law will likely be ruled invalid, and some 24 million Americans could lose health coverage.
“Today’s ruling is the result of the Trump administration and congressional Republicans attempting to make dangerous health policy using the courts since they failed to succeed in Congress,” House Ways and Means Committee Chairman Richard E. Neal (D-Mass.) said in a statement. “This is a blow to our nation’s health care system and the millions of Americans who have gained coverage and protections under the Affordable Care Act. Democrats will continue to fight to protect Americans’ access to quality, affordable care.”
Some groups are applauding the decision, though. The Citizens’ Council for Health Freedom (CCHF), which filed an amicus brief with the Fifth Circuit arguing against the ACA, said it wants more.
“We are pleased with the Fifth Circuit Court of Appeals ruling, but it didn’t go far enough,” said Twila Brase, president and cofounder of CCHF, in a statement. “The individual mandate cannot be severed from the rest of the 2,700-page Affordable Care Act, thus the court should have ruled that the entire law is invalid, as the lower district court found.
“As the Court notes in the first paragraph of the ruling, we argued in our Amicus Brief, filed jointly with the Association of American Physicians and Surgeons, that the Act ‘has deprived patients nationwide of a competitive market for affordable high-deductible health insurance,’ leaving ‘patients with no alternative to ... skyrocketing premiums,’ “ Ms. Brase added. “Sending it back to the lower court, which already ruled the right way, continues to deprive citizens and patients of the affordable coverage that freedom from Obamacare would bring.”
Future uncertain
The ruling in Texas v Azar is not a surprise because, during oral arguments in July, as reported by Medscape Medical News, at least two of the three judges – Jennifer Walker Elrod, appointed by President George W. Bush in 2007, and Kurt Engelhardt, appointed by President Donald J. Trump in 2018 – appeared to be more receptive to the arguments of a group of 18 Republican states and two individuals seeking to invalidate the ACA.
Judge Carolyn Dineen King, appointed by President Jimmy Carter in 1979, did not comment during the hearing.
The Trump administration chose not to defend the ACA, but it does not seem entirely prepared for what might happen if the law is overturned. In a briefing before the Fifth Circuit hearing, the administration argued that, if ultimately the law is ruled unconstitutional, it should be struck down only in the states seeking to overturn the law.
“A lot of this has to get sorted out – it’s complicated,” said August E. Flentje, a U.S. Department of Justice lawyer, at the oral arguments in July.
For now, though, the ACA remains.
“In 2012, the Supreme Court upheld Obamacare, despite serious constitutional issues with the federal government forcing Americans to purchase a product from a private company. Until an ultimate decision is made by the Supreme Court or Congress decides otherwise, the Affordable Care Act will remain the law of the land,” Senate Finance Committee Chairman Chuck Grassley (R-Iowa), said in a statement.
And those who have led the court battle to keep the ACA intact plan to keep fighting. “For now, the President got the gift he wanted – uncertainty in the health care system and a pathway to repeal – so that the health care that seniors, workers, and families secured under the Affordable Care Act can be yanked from under them. This decision could take us to a dangerous and irresponsible place, not just for the 133 million Americans with pre-existing conditions, but for our seniors who use Medicare, our children under the age of 26, and the 20 million additional Americans covered directly through the ACA marketplace. California will move swiftly to challenge this decision because this could mean the difference between life and death for so many Americans and their families,” California Attorney General Xavier Becerra said in a statement.
Learn more about AGA’s position on patient protections and access to care at https://www.gastro.org/
A version of this story first appeared on Medscape.com.
A federal appeals court ruled Dec. 18 that the individual mandate of the Affordable Care Act (ACA) is unconstitutional, but the panel sent the case back to a lower court to decide how much of the remainder of the law could topple along with it.
The three-judge panel of the New Orleans-based U.S. Fifth Circuit Court of Appeals said, “The individual mandate is unconstitutional because, under [a previous ruling, National Federation of Independent Business v Sebelius], it finds no constitutional footing in either the Interstate Commerce Clause or the Necessary and Proper Clause.”
The ruling upholds a December 2018 US District Court decision in which Judge Reed O’Connor found that the individual mandate that most Americans must have health insurance or pay a fine was unconstitutional and that without it the ACA itself was invalid.
In sending the case back to a Texas district court, however, the federal panel is asking for a central question to be resolved: Whether the individual mandate is “severable” from the rest of the law, while the rest of the law can be left intact.
If the district court eventually decides that the individual mandate cannot be severed from the rest of the ACA, the entire law will likely be ruled invalid, and some 24 million Americans could lose health coverage.
“Today’s ruling is the result of the Trump administration and congressional Republicans attempting to make dangerous health policy using the courts since they failed to succeed in Congress,” House Ways and Means Committee Chairman Richard E. Neal (D-Mass.) said in a statement. “This is a blow to our nation’s health care system and the millions of Americans who have gained coverage and protections under the Affordable Care Act. Democrats will continue to fight to protect Americans’ access to quality, affordable care.”
Some groups are applauding the decision, though. The Citizens’ Council for Health Freedom (CCHF), which filed an amicus brief with the Fifth Circuit arguing against the ACA, said it wants more.
“We are pleased with the Fifth Circuit Court of Appeals ruling, but it didn’t go far enough,” said Twila Brase, president and cofounder of CCHF, in a statement. “The individual mandate cannot be severed from the rest of the 2,700-page Affordable Care Act, thus the court should have ruled that the entire law is invalid, as the lower district court found.
“As the Court notes in the first paragraph of the ruling, we argued in our Amicus Brief, filed jointly with the Association of American Physicians and Surgeons, that the Act ‘has deprived patients nationwide of a competitive market for affordable high-deductible health insurance,’ leaving ‘patients with no alternative to ... skyrocketing premiums,’ “ Ms. Brase added. “Sending it back to the lower court, which already ruled the right way, continues to deprive citizens and patients of the affordable coverage that freedom from Obamacare would bring.”
Future uncertain
The ruling in Texas v Azar is not a surprise because, during oral arguments in July, as reported by Medscape Medical News, at least two of the three judges – Jennifer Walker Elrod, appointed by President George W. Bush in 2007, and Kurt Engelhardt, appointed by President Donald J. Trump in 2018 – appeared to be more receptive to the arguments of a group of 18 Republican states and two individuals seeking to invalidate the ACA.
Judge Carolyn Dineen King, appointed by President Jimmy Carter in 1979, did not comment during the hearing.
The Trump administration chose not to defend the ACA, but it does not seem entirely prepared for what might happen if the law is overturned. In a briefing before the Fifth Circuit hearing, the administration argued that, if ultimately the law is ruled unconstitutional, it should be struck down only in the states seeking to overturn the law.
“A lot of this has to get sorted out – it’s complicated,” said August E. Flentje, a U.S. Department of Justice lawyer, at the oral arguments in July.
For now, though, the ACA remains.
“In 2012, the Supreme Court upheld Obamacare, despite serious constitutional issues with the federal government forcing Americans to purchase a product from a private company. Until an ultimate decision is made by the Supreme Court or Congress decides otherwise, the Affordable Care Act will remain the law of the land,” Senate Finance Committee Chairman Chuck Grassley (R-Iowa), said in a statement.
And those who have led the court battle to keep the ACA intact plan to keep fighting. “For now, the President got the gift he wanted – uncertainty in the health care system and a pathway to repeal – so that the health care that seniors, workers, and families secured under the Affordable Care Act can be yanked from under them. This decision could take us to a dangerous and irresponsible place, not just for the 133 million Americans with pre-existing conditions, but for our seniors who use Medicare, our children under the age of 26, and the 20 million additional Americans covered directly through the ACA marketplace. California will move swiftly to challenge this decision because this could mean the difference between life and death for so many Americans and their families,” California Attorney General Xavier Becerra said in a statement.
Learn more about AGA’s position on patient protections and access to care at https://www.gastro.org/
A version of this story first appeared on Medscape.com.
New ustekinumab response predictor in Crohn’s called ‘brilliant’
SAN ANTONIO – The probability of achieving clinical remission of Crohn’s disease in response to ustekinumab can now be readily estimated by using a clinical prediction tool, Parambir S. Dulai, MBBS, announced at the annual meeting of the American College of Gastroenterology.
This new clinical decision support tool also provides individualized stratification of the rapidity with which symptoms will be reduced in response to the anti-interleukin-12/23 biologic, added Dr. Dulai, a gastroenterologist at the University of California, San Diego.
He and his coinvestigators developed the prediction tool through analysis of detailed data on 781 patients with active Crohn’s disease treated with ustekinumab (Stelara) during both the induction and maintenance portions of the phase 3 UNITI randomized trials conducted in the biologic’s development program. The researchers identified a series of baseline features associated with clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 by week 16 of treatment. Through statistical manipulation, they transformed the data into a predictive model and then went one step further by turning the model into a decision support tool with points given for the individual predictive variables (see graphic).
Patients with 5 or more total points were categorized as having a high probability of week-16 clinical remission. Patients with 0 or 1 point were deemed low probability, and a score of 2-4 indicated an intermediate likelihood of clinical remission.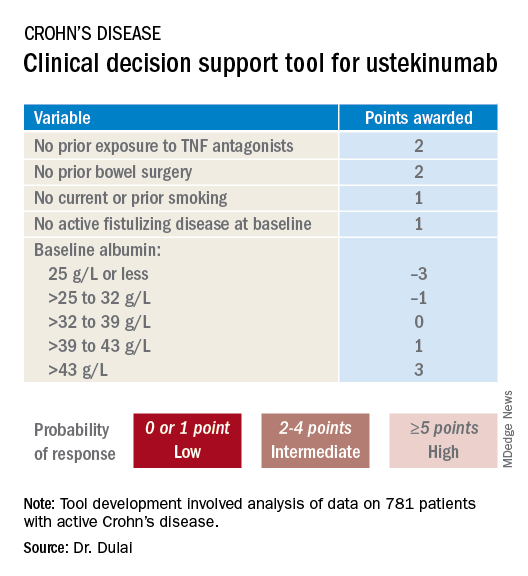
Next, the investigators applied their new clinical decision support tool to the 781 ustekinumab-treated patients included in the derivation analysis. The tool performed well: The high-probability group had a 57% clinical remission rate, significantly better than the 34% rate in the intermediate-probability group, which in turn was significantly better than the 21% rate of clinical remission in the group with a baseline score of 0 or 1.
In addition, onset of treatment benefit was significantly faster in the group having a score of 5 or more. They had a significantly higher clinical remission rate than the intermediate- and low-probability groups at all scheduled assessments, which were conducted at weeks 3, 6, 8, and 16. Indeed, by week 3 the high-probability group experienced a mean 69-point drop from baseline in CDAI and a 94-point drop by week 8, as compared with week-8 reductions of 54 and 40 points in the intermediate- and low-probability groups, respectively.
In an exploratory analysis involving the 122 patients who underwent week-8 endoscopy, endoscopic remission was documented in 12% of patients whose baseline scores placed them in the high-probability group, 10% in the intermediate group, and 8% of those in the low-probability group.
The high-probability group had significantly higher ustekinumab trough concentrations than did the intermediate- and low-probability groups when measured at weeks 3, 6, 8, and 16.
An external validation study conducted in a large cohort of Crohn’s disease patients seen in routine clinical practice has recently been completed, with the results now being analyzed, according to Dr. Dulai.
Miguel Requeiro, MD, chairman of gastroenterology and hepatology at the Cleveland Clinic, rose from the audience to declare the creation of the decision support tool to be “brilliant work.” He asked if it has changed clinical practice for Dr. Dulai and his coworkers.
“We’ve begun doing two things differently,” Dr. Dulai replied. “First, we’ve built a similar model for vedolizumab and Crohn’s. That means we can use both tools together to discriminate between a patient who should get vedolizumab versus ustekinumab because the variables and their weighting differ between the two. And the other thing we’ve been able to do is argue with payers for positioning of the treatments when we have evidence to support that we can use them earlier in the treatment course to optimize outcomes.”
Another audience member, David T. Rubin, MD, also praised the decision support tool as “brilliant” and “definitely needed.”
“Thank you for hitting the sweet spot of what we’ve all been waiting for,” added Dr. Rubin, professor of medicine and codirector of the Digestive Diseases Center at the University of Chicago.
Dr. Dulai reported receiving a research grant for the project from Janssen, which markets ustekinumab.
Help your patients better understand their Crohn’s disease treatment options by sharing AGA patient education at https://www.gastro.org/
SAN ANTONIO – The probability of achieving clinical remission of Crohn’s disease in response to ustekinumab can now be readily estimated by using a clinical prediction tool, Parambir S. Dulai, MBBS, announced at the annual meeting of the American College of Gastroenterology.
This new clinical decision support tool also provides individualized stratification of the rapidity with which symptoms will be reduced in response to the anti-interleukin-12/23 biologic, added Dr. Dulai, a gastroenterologist at the University of California, San Diego.
He and his coinvestigators developed the prediction tool through analysis of detailed data on 781 patients with active Crohn’s disease treated with ustekinumab (Stelara) during both the induction and maintenance portions of the phase 3 UNITI randomized trials conducted in the biologic’s development program. The researchers identified a series of baseline features associated with clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 by week 16 of treatment. Through statistical manipulation, they transformed the data into a predictive model and then went one step further by turning the model into a decision support tool with points given for the individual predictive variables (see graphic).
Patients with 5 or more total points were categorized as having a high probability of week-16 clinical remission. Patients with 0 or 1 point were deemed low probability, and a score of 2-4 indicated an intermediate likelihood of clinical remission.
Next, the investigators applied their new clinical decision support tool to the 781 ustekinumab-treated patients included in the derivation analysis. The tool performed well: The high-probability group had a 57% clinical remission rate, significantly better than the 34% rate in the intermediate-probability group, which in turn was significantly better than the 21% rate of clinical remission in the group with a baseline score of 0 or 1.
In addition, onset of treatment benefit was significantly faster in the group having a score of 5 or more. They had a significantly higher clinical remission rate than the intermediate- and low-probability groups at all scheduled assessments, which were conducted at weeks 3, 6, 8, and 16. Indeed, by week 3 the high-probability group experienced a mean 69-point drop from baseline in CDAI and a 94-point drop by week 8, as compared with week-8 reductions of 54 and 40 points in the intermediate- and low-probability groups, respectively.
In an exploratory analysis involving the 122 patients who underwent week-8 endoscopy, endoscopic remission was documented in 12% of patients whose baseline scores placed them in the high-probability group, 10% in the intermediate group, and 8% of those in the low-probability group.
The high-probability group had significantly higher ustekinumab trough concentrations than did the intermediate- and low-probability groups when measured at weeks 3, 6, 8, and 16.
An external validation study conducted in a large cohort of Crohn’s disease patients seen in routine clinical practice has recently been completed, with the results now being analyzed, according to Dr. Dulai.
Miguel Requeiro, MD, chairman of gastroenterology and hepatology at the Cleveland Clinic, rose from the audience to declare the creation of the decision support tool to be “brilliant work.” He asked if it has changed clinical practice for Dr. Dulai and his coworkers.
“We’ve begun doing two things differently,” Dr. Dulai replied. “First, we’ve built a similar model for vedolizumab and Crohn’s. That means we can use both tools together to discriminate between a patient who should get vedolizumab versus ustekinumab because the variables and their weighting differ between the two. And the other thing we’ve been able to do is argue with payers for positioning of the treatments when we have evidence to support that we can use them earlier in the treatment course to optimize outcomes.”
Another audience member, David T. Rubin, MD, also praised the decision support tool as “brilliant” and “definitely needed.”
“Thank you for hitting the sweet spot of what we’ve all been waiting for,” added Dr. Rubin, professor of medicine and codirector of the Digestive Diseases Center at the University of Chicago.
Dr. Dulai reported receiving a research grant for the project from Janssen, which markets ustekinumab.
Help your patients better understand their Crohn’s disease treatment options by sharing AGA patient education at https://www.gastro.org/
SAN ANTONIO – The probability of achieving clinical remission of Crohn’s disease in response to ustekinumab can now be readily estimated by using a clinical prediction tool, Parambir S. Dulai, MBBS, announced at the annual meeting of the American College of Gastroenterology.
This new clinical decision support tool also provides individualized stratification of the rapidity with which symptoms will be reduced in response to the anti-interleukin-12/23 biologic, added Dr. Dulai, a gastroenterologist at the University of California, San Diego.
He and his coinvestigators developed the prediction tool through analysis of detailed data on 781 patients with active Crohn’s disease treated with ustekinumab (Stelara) during both the induction and maintenance portions of the phase 3 UNITI randomized trials conducted in the biologic’s development program. The researchers identified a series of baseline features associated with clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 by week 16 of treatment. Through statistical manipulation, they transformed the data into a predictive model and then went one step further by turning the model into a decision support tool with points given for the individual predictive variables (see graphic).
Patients with 5 or more total points were categorized as having a high probability of week-16 clinical remission. Patients with 0 or 1 point were deemed low probability, and a score of 2-4 indicated an intermediate likelihood of clinical remission.
Next, the investigators applied their new clinical decision support tool to the 781 ustekinumab-treated patients included in the derivation analysis. The tool performed well: The high-probability group had a 57% clinical remission rate, significantly better than the 34% rate in the intermediate-probability group, which in turn was significantly better than the 21% rate of clinical remission in the group with a baseline score of 0 or 1.
In addition, onset of treatment benefit was significantly faster in the group having a score of 5 or more. They had a significantly higher clinical remission rate than the intermediate- and low-probability groups at all scheduled assessments, which were conducted at weeks 3, 6, 8, and 16. Indeed, by week 3 the high-probability group experienced a mean 69-point drop from baseline in CDAI and a 94-point drop by week 8, as compared with week-8 reductions of 54 and 40 points in the intermediate- and low-probability groups, respectively.
In an exploratory analysis involving the 122 patients who underwent week-8 endoscopy, endoscopic remission was documented in 12% of patients whose baseline scores placed them in the high-probability group, 10% in the intermediate group, and 8% of those in the low-probability group.
The high-probability group had significantly higher ustekinumab trough concentrations than did the intermediate- and low-probability groups when measured at weeks 3, 6, 8, and 16.
An external validation study conducted in a large cohort of Crohn’s disease patients seen in routine clinical practice has recently been completed, with the results now being analyzed, according to Dr. Dulai.
Miguel Requeiro, MD, chairman of gastroenterology and hepatology at the Cleveland Clinic, rose from the audience to declare the creation of the decision support tool to be “brilliant work.” He asked if it has changed clinical practice for Dr. Dulai and his coworkers.
“We’ve begun doing two things differently,” Dr. Dulai replied. “First, we’ve built a similar model for vedolizumab and Crohn’s. That means we can use both tools together to discriminate between a patient who should get vedolizumab versus ustekinumab because the variables and their weighting differ between the two. And the other thing we’ve been able to do is argue with payers for positioning of the treatments when we have evidence to support that we can use them earlier in the treatment course to optimize outcomes.”
Another audience member, David T. Rubin, MD, also praised the decision support tool as “brilliant” and “definitely needed.”
“Thank you for hitting the sweet spot of what we’ve all been waiting for,” added Dr. Rubin, professor of medicine and codirector of the Digestive Diseases Center at the University of Chicago.
Dr. Dulai reported receiving a research grant for the project from Janssen, which markets ustekinumab.
Help your patients better understand their Crohn’s disease treatment options by sharing AGA patient education at https://www.gastro.org/
REPORTING FROM ACG 2019
Experts call to revise the Uniform Determination of Death Act
, according to an editorial published online Dec. 24, 2019, in Annals of Internal Medicine. Proposed revisions would identify the standards for determining death by neurologic criteria and address the question of whether consent is required to make this determination. If accepted, the revisions would enhance public trust in the determination of death by neurologic criteria, the authors said.
“There is a disconnect between the medical and legal standards for brain death,” said Ariane K. Lewis, MD, associate professor of neurology and neurosurgery at New York University and lead author of the editorial. The discrepancy must be remedied because it has led to lawsuits and has proved to be problematic from a societal standpoint, she added.
“We defend changing the law to match medical practice, rather than changing medical practice to match the law,” said Thaddeus Mason Pope, JD, PhD, director of the Health Law Institute at Mitchell Hamline School of Law in Saint Paul, Minnesota, and an author of the editorial.
Accepted medical standards are unclear
The UDDA was drafted in 1981 to establish a uniform legal standard for death by neurologic criteria. A person with “irreversible cessation of all functions of the entire brain, including the brainstem,” is dead, according to the statute. A determination of death, it adds, “must be made in accordance with accepted medical standards.”
But the medical standards used to determine death by neurologic cause have not been uniform. In 2015, the Supreme Court of Nevada ruled that it was not clear that the standard published by the American Academy of Neurology (AAN), which had been used in the case at issue, was the “accepted medical standard.” An AAN summit later affirmed that the accepted medical standards for determination of death by neurologic cause are the 2010 AAN standard for determination of brain death in adults and the 2011 Society of Critical Care Medicine (SCCM), American Academy of Pediatrics (AAP), and Child Neurology Society (CNS) standard for determination of brain death in children. The Nevada legislature amended the state UDDA to identify these standards as the accepted standards. A revised UDDA also should identify these standards and grant an administrative agency (i.e., the board of medicine) the power to review and update the accepted medical standards as needed, according to the editorial.
To the extent that hospitals are not following the AAN or SCCM/AAP/CNS standards for determining death by neurologic cause, “enshrining” these standards in a revised UDDA “should increase uniformity and consistency” in hospitals’ policies on brain death, Dr. Pope said.
The question of hormonal function
Lawsuits in California and Nevada raised the question of whether the pituitary gland and hypothalamus are parts of the brain. If so, then the accepted medical standards for death by neurologic cause are not consistent with the statutory requirements for the determination of death, since the former do not test for cessation of hormonal function.
The current edition of the adult standards for determining death by neurologic cause were published in 2010. “Whenever we measure brain death, we’re not measuring the cessation of all functions of the entire brain,” Dr. Pope said. “That’s not a new thing; that’s been the case for a long time.”
To address the discrepancy between medical practice and the legal statute, Dr. Lewis and colleagues proposed that the UDDA’s reference to “irreversible cessation of functions of the entire brain” be followed by the following clause: “including the brainstem, leading to unresponsive coma with loss of capacity for consciousness, brainstem areflexia, and the inability to breathe spontaneously.” An alternative revision would be to add the briefer phrase “... with the exception of hormonal function.”
Authors say consent is not required for testing
Other complications have arisen from the UDDA’s failure to specify whether consent is required for a determination of death by neurologic cause. Court rulings on this question have not been consistent. Dr. Lewis and colleagues propose adding the following text to the UDDA: “Reasonable efforts should be made to notify a patient’s legally authorized decision-maker before performing a determination of death by neurologic criteria, but consent is not required to initiate such an evaluation.”
The proposed revisions to the UDDA “might give [clinicians] more confidence to proceed with brain death testing, because it would clarify that they don’t need the parents’ [or the patient’s legally authorized decision-maker] consent to do the tests,” said Dr. Pope. “If anything, they might even have a duty to do the tests.”
The final problem with the UDDA that Dr. Lewis and colleagues cited is that it does not provide clear guidance about how to respond to religious objections to discontinuation of organ support after a determination of death by neurologic cause. “Because the issue is rather complicated, we have not advocated for a singular position related to this [question] in our revised UDDA,” Dr. Lewis said. “Rather, we recommended the need for a multidisciplinary group to come together to determine what is the best approach. In an ideal world, this [approach] would be universal throughout the country.”
Although a revised UDDA would provide greater clarity to physicians and promote uniformity of practice, it would not resolve ongoing theological and philosophical debates about whether brain death is biological death, Dr. Pope said. “The key thing is that it would give clinicians a green light or certainty and clarity that they may proceed to do the test in the first place. If the tests are positive and the patient really is dead, then they could proceed to organ procurement or to move to the morgue.”
Dr. Lewis is a member of various AAN committees and working groups but receives no compensation for her role. A coauthor received personal fees from the AAN that were unrelated to the editorial.
SOURCE: Lewis A et al. Ann Intern Med. 2019 Dec 24. doi: 10.7326/M19-2731.
, according to an editorial published online Dec. 24, 2019, in Annals of Internal Medicine. Proposed revisions would identify the standards for determining death by neurologic criteria and address the question of whether consent is required to make this determination. If accepted, the revisions would enhance public trust in the determination of death by neurologic criteria, the authors said.
“There is a disconnect between the medical and legal standards for brain death,” said Ariane K. Lewis, MD, associate professor of neurology and neurosurgery at New York University and lead author of the editorial. The discrepancy must be remedied because it has led to lawsuits and has proved to be problematic from a societal standpoint, she added.
“We defend changing the law to match medical practice, rather than changing medical practice to match the law,” said Thaddeus Mason Pope, JD, PhD, director of the Health Law Institute at Mitchell Hamline School of Law in Saint Paul, Minnesota, and an author of the editorial.
Accepted medical standards are unclear
The UDDA was drafted in 1981 to establish a uniform legal standard for death by neurologic criteria. A person with “irreversible cessation of all functions of the entire brain, including the brainstem,” is dead, according to the statute. A determination of death, it adds, “must be made in accordance with accepted medical standards.”
But the medical standards used to determine death by neurologic cause have not been uniform. In 2015, the Supreme Court of Nevada ruled that it was not clear that the standard published by the American Academy of Neurology (AAN), which had been used in the case at issue, was the “accepted medical standard.” An AAN summit later affirmed that the accepted medical standards for determination of death by neurologic cause are the 2010 AAN standard for determination of brain death in adults and the 2011 Society of Critical Care Medicine (SCCM), American Academy of Pediatrics (AAP), and Child Neurology Society (CNS) standard for determination of brain death in children. The Nevada legislature amended the state UDDA to identify these standards as the accepted standards. A revised UDDA also should identify these standards and grant an administrative agency (i.e., the board of medicine) the power to review and update the accepted medical standards as needed, according to the editorial.
To the extent that hospitals are not following the AAN or SCCM/AAP/CNS standards for determining death by neurologic cause, “enshrining” these standards in a revised UDDA “should increase uniformity and consistency” in hospitals’ policies on brain death, Dr. Pope said.
The question of hormonal function
Lawsuits in California and Nevada raised the question of whether the pituitary gland and hypothalamus are parts of the brain. If so, then the accepted medical standards for death by neurologic cause are not consistent with the statutory requirements for the determination of death, since the former do not test for cessation of hormonal function.
The current edition of the adult standards for determining death by neurologic cause were published in 2010. “Whenever we measure brain death, we’re not measuring the cessation of all functions of the entire brain,” Dr. Pope said. “That’s not a new thing; that’s been the case for a long time.”
To address the discrepancy between medical practice and the legal statute, Dr. Lewis and colleagues proposed that the UDDA’s reference to “irreversible cessation of functions of the entire brain” be followed by the following clause: “including the brainstem, leading to unresponsive coma with loss of capacity for consciousness, brainstem areflexia, and the inability to breathe spontaneously.” An alternative revision would be to add the briefer phrase “... with the exception of hormonal function.”
Authors say consent is not required for testing
Other complications have arisen from the UDDA’s failure to specify whether consent is required for a determination of death by neurologic cause. Court rulings on this question have not been consistent. Dr. Lewis and colleagues propose adding the following text to the UDDA: “Reasonable efforts should be made to notify a patient’s legally authorized decision-maker before performing a determination of death by neurologic criteria, but consent is not required to initiate such an evaluation.”
The proposed revisions to the UDDA “might give [clinicians] more confidence to proceed with brain death testing, because it would clarify that they don’t need the parents’ [or the patient’s legally authorized decision-maker] consent to do the tests,” said Dr. Pope. “If anything, they might even have a duty to do the tests.”
The final problem with the UDDA that Dr. Lewis and colleagues cited is that it does not provide clear guidance about how to respond to religious objections to discontinuation of organ support after a determination of death by neurologic cause. “Because the issue is rather complicated, we have not advocated for a singular position related to this [question] in our revised UDDA,” Dr. Lewis said. “Rather, we recommended the need for a multidisciplinary group to come together to determine what is the best approach. In an ideal world, this [approach] would be universal throughout the country.”
Although a revised UDDA would provide greater clarity to physicians and promote uniformity of practice, it would not resolve ongoing theological and philosophical debates about whether brain death is biological death, Dr. Pope said. “The key thing is that it would give clinicians a green light or certainty and clarity that they may proceed to do the test in the first place. If the tests are positive and the patient really is dead, then they could proceed to organ procurement or to move to the morgue.”
Dr. Lewis is a member of various AAN committees and working groups but receives no compensation for her role. A coauthor received personal fees from the AAN that were unrelated to the editorial.
SOURCE: Lewis A et al. Ann Intern Med. 2019 Dec 24. doi: 10.7326/M19-2731.
, according to an editorial published online Dec. 24, 2019, in Annals of Internal Medicine. Proposed revisions would identify the standards for determining death by neurologic criteria and address the question of whether consent is required to make this determination. If accepted, the revisions would enhance public trust in the determination of death by neurologic criteria, the authors said.
“There is a disconnect between the medical and legal standards for brain death,” said Ariane K. Lewis, MD, associate professor of neurology and neurosurgery at New York University and lead author of the editorial. The discrepancy must be remedied because it has led to lawsuits and has proved to be problematic from a societal standpoint, she added.
“We defend changing the law to match medical practice, rather than changing medical practice to match the law,” said Thaddeus Mason Pope, JD, PhD, director of the Health Law Institute at Mitchell Hamline School of Law in Saint Paul, Minnesota, and an author of the editorial.
Accepted medical standards are unclear
The UDDA was drafted in 1981 to establish a uniform legal standard for death by neurologic criteria. A person with “irreversible cessation of all functions of the entire brain, including the brainstem,” is dead, according to the statute. A determination of death, it adds, “must be made in accordance with accepted medical standards.”
But the medical standards used to determine death by neurologic cause have not been uniform. In 2015, the Supreme Court of Nevada ruled that it was not clear that the standard published by the American Academy of Neurology (AAN), which had been used in the case at issue, was the “accepted medical standard.” An AAN summit later affirmed that the accepted medical standards for determination of death by neurologic cause are the 2010 AAN standard for determination of brain death in adults and the 2011 Society of Critical Care Medicine (SCCM), American Academy of Pediatrics (AAP), and Child Neurology Society (CNS) standard for determination of brain death in children. The Nevada legislature amended the state UDDA to identify these standards as the accepted standards. A revised UDDA also should identify these standards and grant an administrative agency (i.e., the board of medicine) the power to review and update the accepted medical standards as needed, according to the editorial.
To the extent that hospitals are not following the AAN or SCCM/AAP/CNS standards for determining death by neurologic cause, “enshrining” these standards in a revised UDDA “should increase uniformity and consistency” in hospitals’ policies on brain death, Dr. Pope said.
The question of hormonal function
Lawsuits in California and Nevada raised the question of whether the pituitary gland and hypothalamus are parts of the brain. If so, then the accepted medical standards for death by neurologic cause are not consistent with the statutory requirements for the determination of death, since the former do not test for cessation of hormonal function.
The current edition of the adult standards for determining death by neurologic cause were published in 2010. “Whenever we measure brain death, we’re not measuring the cessation of all functions of the entire brain,” Dr. Pope said. “That’s not a new thing; that’s been the case for a long time.”
To address the discrepancy between medical practice and the legal statute, Dr. Lewis and colleagues proposed that the UDDA’s reference to “irreversible cessation of functions of the entire brain” be followed by the following clause: “including the brainstem, leading to unresponsive coma with loss of capacity for consciousness, brainstem areflexia, and the inability to breathe spontaneously.” An alternative revision would be to add the briefer phrase “... with the exception of hormonal function.”
Authors say consent is not required for testing
Other complications have arisen from the UDDA’s failure to specify whether consent is required for a determination of death by neurologic cause. Court rulings on this question have not been consistent. Dr. Lewis and colleagues propose adding the following text to the UDDA: “Reasonable efforts should be made to notify a patient’s legally authorized decision-maker before performing a determination of death by neurologic criteria, but consent is not required to initiate such an evaluation.”
The proposed revisions to the UDDA “might give [clinicians] more confidence to proceed with brain death testing, because it would clarify that they don’t need the parents’ [or the patient’s legally authorized decision-maker] consent to do the tests,” said Dr. Pope. “If anything, they might even have a duty to do the tests.”
The final problem with the UDDA that Dr. Lewis and colleagues cited is that it does not provide clear guidance about how to respond to religious objections to discontinuation of organ support after a determination of death by neurologic cause. “Because the issue is rather complicated, we have not advocated for a singular position related to this [question] in our revised UDDA,” Dr. Lewis said. “Rather, we recommended the need for a multidisciplinary group to come together to determine what is the best approach. In an ideal world, this [approach] would be universal throughout the country.”
Although a revised UDDA would provide greater clarity to physicians and promote uniformity of practice, it would not resolve ongoing theological and philosophical debates about whether brain death is biological death, Dr. Pope said. “The key thing is that it would give clinicians a green light or certainty and clarity that they may proceed to do the test in the first place. If the tests are positive and the patient really is dead, then they could proceed to organ procurement or to move to the morgue.”
Dr. Lewis is a member of various AAN committees and working groups but receives no compensation for her role. A coauthor received personal fees from the AAN that were unrelated to the editorial.
SOURCE: Lewis A et al. Ann Intern Med. 2019 Dec 24. doi: 10.7326/M19-2731.
FROM ANNALS OF INTERNAL MEDICINE