User login
Treatment guided by remote readings works when used as intended
PHILADELPHIA – A heart failure management strategy guided by home lung fluid measurements from the remote dielectric sensing (ReDS) system can significantly reduce recurrent hospitalizations, as long as the technology is used as intended.
A ReDS-directed management strategy did not significantly reduce recurrent hospitalizations for acute decompensated heart failure (ADHF) in the traditional intent-to-treat analysis of the randomized, controlled SMILE trial, William T. Abraham, MD, of the Ohio State University, Columbus, said at the annual scientific meeting of the Heart Failure Society of America.
However, use of the Food and Drug Administration–cleared thoracic fluid status monitoring system to guide treatment changes did cut such hospitalizations by 58% in a modified intent-to-treat population that excluded patients who failed to take daily measurements at home and who didn’t receive modified treatment despite actionable readings, Dr. Abraham reported in an oral presentation.
“These observations may support an adherence-based approach to the utilization and reimbursement of ReDS-guided management in recently discharged ADHF patients,” he said.
The ReDS system consists of a device that, within about 90 seconds, provides a measurement of lung fluid via a focused electromagnetic radar beam that passes through the right lung, Dr. Abraham said. Clinicians access data from measurements patients initiate at home through a secure, cloud-based system, and then initiate changes to heart failure management as warranted based on a ReDS-specific treatment algorithm, Dr. Abraham said.
The postmarketing SMILE study of the ReDS system was stopped early because of an administrative decision by the sponsor, according to Dr. Abraham. However, 268 patients enrolled at 43 U.S. sites continued to the end of the study, with an average follow-up of about 6 months, while readmissions were collected and adjudicated by an independent clinical events committee.
A total of 135 patients were randomized to a ReDS-based management strategy, while 133 were randomized to standard of care, the investigator said.
Most of the medication changes made in response to ReDS measurements were increased diuretics because of high lung fluid volume measurements, or decreased diuretics in response to low measurements, though some changes in vasodilator medications were also made, Dr. Abraham said.
The ReDS-directed management approach yielded a “highly nonsignificant” 19% reduction in recurrent or cumulative heart failure readmissions (P = .36); by contrast, after removing nonadherent, noncompliant cases, there were 11 hospitalizations in 91 treatment patients, compared with 43 hospitalizations in 133 control patients, yielding a hazard ratio of 0.42 (95% CI, 0.22-0.82; P = .01).
“This comes back to the adage that, if you don’t use it, you can’t improve clinical outcomes,” he said, explaining that this study’s modified intent-to-treat population was defined by excluding patients who took no ReDS measurements for more than 20 consecutive days; or by clinicians who received at least eight notifications of out-of-range ReDS values yet didn’t implement the ReDS treatment algorithm.
There were no adverse events reported as being definitely related to the use of the device, and five adverse events reported as possibly related to the device, Dr. Abraham said.
SMILE was sponsored by Sensible Medical Innovations. Dr. Abraham reported disclosures (consultant/advisory board) related to Sensible Medical Innovations, Abbott, Boehringer Ingelheim, Victorious Medical, V-Wave Medical, and others.
PHILADELPHIA – A heart failure management strategy guided by home lung fluid measurements from the remote dielectric sensing (ReDS) system can significantly reduce recurrent hospitalizations, as long as the technology is used as intended.
A ReDS-directed management strategy did not significantly reduce recurrent hospitalizations for acute decompensated heart failure (ADHF) in the traditional intent-to-treat analysis of the randomized, controlled SMILE trial, William T. Abraham, MD, of the Ohio State University, Columbus, said at the annual scientific meeting of the Heart Failure Society of America.
However, use of the Food and Drug Administration–cleared thoracic fluid status monitoring system to guide treatment changes did cut such hospitalizations by 58% in a modified intent-to-treat population that excluded patients who failed to take daily measurements at home and who didn’t receive modified treatment despite actionable readings, Dr. Abraham reported in an oral presentation.
“These observations may support an adherence-based approach to the utilization and reimbursement of ReDS-guided management in recently discharged ADHF patients,” he said.
The ReDS system consists of a device that, within about 90 seconds, provides a measurement of lung fluid via a focused electromagnetic radar beam that passes through the right lung, Dr. Abraham said. Clinicians access data from measurements patients initiate at home through a secure, cloud-based system, and then initiate changes to heart failure management as warranted based on a ReDS-specific treatment algorithm, Dr. Abraham said.
The postmarketing SMILE study of the ReDS system was stopped early because of an administrative decision by the sponsor, according to Dr. Abraham. However, 268 patients enrolled at 43 U.S. sites continued to the end of the study, with an average follow-up of about 6 months, while readmissions were collected and adjudicated by an independent clinical events committee.
A total of 135 patients were randomized to a ReDS-based management strategy, while 133 were randomized to standard of care, the investigator said.
Most of the medication changes made in response to ReDS measurements were increased diuretics because of high lung fluid volume measurements, or decreased diuretics in response to low measurements, though some changes in vasodilator medications were also made, Dr. Abraham said.
The ReDS-directed management approach yielded a “highly nonsignificant” 19% reduction in recurrent or cumulative heart failure readmissions (P = .36); by contrast, after removing nonadherent, noncompliant cases, there were 11 hospitalizations in 91 treatment patients, compared with 43 hospitalizations in 133 control patients, yielding a hazard ratio of 0.42 (95% CI, 0.22-0.82; P = .01).
“This comes back to the adage that, if you don’t use it, you can’t improve clinical outcomes,” he said, explaining that this study’s modified intent-to-treat population was defined by excluding patients who took no ReDS measurements for more than 20 consecutive days; or by clinicians who received at least eight notifications of out-of-range ReDS values yet didn’t implement the ReDS treatment algorithm.
There were no adverse events reported as being definitely related to the use of the device, and five adverse events reported as possibly related to the device, Dr. Abraham said.
SMILE was sponsored by Sensible Medical Innovations. Dr. Abraham reported disclosures (consultant/advisory board) related to Sensible Medical Innovations, Abbott, Boehringer Ingelheim, Victorious Medical, V-Wave Medical, and others.
PHILADELPHIA – A heart failure management strategy guided by home lung fluid measurements from the remote dielectric sensing (ReDS) system can significantly reduce recurrent hospitalizations, as long as the technology is used as intended.
A ReDS-directed management strategy did not significantly reduce recurrent hospitalizations for acute decompensated heart failure (ADHF) in the traditional intent-to-treat analysis of the randomized, controlled SMILE trial, William T. Abraham, MD, of the Ohio State University, Columbus, said at the annual scientific meeting of the Heart Failure Society of America.
However, use of the Food and Drug Administration–cleared thoracic fluid status monitoring system to guide treatment changes did cut such hospitalizations by 58% in a modified intent-to-treat population that excluded patients who failed to take daily measurements at home and who didn’t receive modified treatment despite actionable readings, Dr. Abraham reported in an oral presentation.
“These observations may support an adherence-based approach to the utilization and reimbursement of ReDS-guided management in recently discharged ADHF patients,” he said.
The ReDS system consists of a device that, within about 90 seconds, provides a measurement of lung fluid via a focused electromagnetic radar beam that passes through the right lung, Dr. Abraham said. Clinicians access data from measurements patients initiate at home through a secure, cloud-based system, and then initiate changes to heart failure management as warranted based on a ReDS-specific treatment algorithm, Dr. Abraham said.
The postmarketing SMILE study of the ReDS system was stopped early because of an administrative decision by the sponsor, according to Dr. Abraham. However, 268 patients enrolled at 43 U.S. sites continued to the end of the study, with an average follow-up of about 6 months, while readmissions were collected and adjudicated by an independent clinical events committee.
A total of 135 patients were randomized to a ReDS-based management strategy, while 133 were randomized to standard of care, the investigator said.
Most of the medication changes made in response to ReDS measurements were increased diuretics because of high lung fluid volume measurements, or decreased diuretics in response to low measurements, though some changes in vasodilator medications were also made, Dr. Abraham said.
The ReDS-directed management approach yielded a “highly nonsignificant” 19% reduction in recurrent or cumulative heart failure readmissions (P = .36); by contrast, after removing nonadherent, noncompliant cases, there were 11 hospitalizations in 91 treatment patients, compared with 43 hospitalizations in 133 control patients, yielding a hazard ratio of 0.42 (95% CI, 0.22-0.82; P = .01).
“This comes back to the adage that, if you don’t use it, you can’t improve clinical outcomes,” he said, explaining that this study’s modified intent-to-treat population was defined by excluding patients who took no ReDS measurements for more than 20 consecutive days; or by clinicians who received at least eight notifications of out-of-range ReDS values yet didn’t implement the ReDS treatment algorithm.
There were no adverse events reported as being definitely related to the use of the device, and five adverse events reported as possibly related to the device, Dr. Abraham said.
SMILE was sponsored by Sensible Medical Innovations. Dr. Abraham reported disclosures (consultant/advisory board) related to Sensible Medical Innovations, Abbott, Boehringer Ingelheim, Victorious Medical, V-Wave Medical, and others.
REPORTING FROM HFSA 2019
Immunotherapies under investigation in newly diagnosed B-ALL
SAN FRANCISCO – Positive results with blinatumomab and inotuzumab ozogamicin in the relapsed/refractory setting have prompted trials of these immunotherapies in newly diagnosed B-cell acute lymphoblastic leukemia (B-ALL).
Blinatumomab and inotuzumab have been shown to improve overall survival, compared with chemotherapy, in patients with relapsed/refractory B-ALL. However, most adults with relapsed/refractory B-ALL still die, so the initial therapy patients receive is “critical,” according to Jae Park, MD, of Memorial Sloan Kettering Cancer Center in New York.
“Ideally, we do not want to deal with the relapse,” Dr. Park said. “It’s better to cure the disease the first time ... which is the reason clinical trials are incorporating these agents earlier.”
Dr. Park discussed these points at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress.
Blinatumomab
Dr. Park cited the phase 3 TOWER trial, which showed that blinatumomab produced better response rates and overall survival compared with standard chemotherapy. The trial enrolled 405 patients with Ph-negative relapsed/refractory B-ALL who were randomized to blinatumomab (n = 271) or chemotherapy (n = 134).
The rate of complete response (CR) with full, partial, or incomplete hematologic recovery was 44% with blinatumomab and 25% with chemotherapy (P less than .001). The median overall survival was 7.7 months and 4.0 months, respectively (P = .01; N Engl J Med 2017; 376:836-47).
Based on these data, researchers decided to test blinatumomab in newly diagnosed, elderly patients (65 years and older) with Ph-negative B-ALL in the phase 2 SWOG 1318 study. The study enrolled 31 patients, and 29 were eligible. Their median age at baseline was 75 years (range 66‐84 years).
The patients received blinatumomab for two to five cycles, followed by 18 months of maintenance with prednisone, vincristine, 6-mercaptopurine, and methotrexate. One patient went on to transplant.
In all, 66% of patients achieved a CR or CR with incomplete count recovery. The estimated overall survival was 79% at 6 months and 65% at 1 year. These results were presented at the 2018 annual meeting of the American Society of Hematology (Blood. 2018;132:33).
Another study of blinatumomab as frontline treatment is the ECOG-E1910 trial. In this phase 3 study, researchers are testing chemotherapy, with or without blinatumomab, in adults (aged 30-70 years) with newly diagnosed, BCR-ABL-negative B-ALL. Results from this study are not yet available.
Inotuzumab ozogamicin
Dr. Park also discussed the INOVATE trial, in which inotuzumab ozogamicin bested standard chemotherapy. The trial enrolled patients with Ph-positive or negative, relapsed/refractory B-ALL.
The patients were randomized to inotuzumab (n = 141) or investigator’s choice of chemotherapy (n = 138). Some patients, 41% in the inotuzumab arm and 11% in the chemotherapy arm, went on to transplant.
The CR rate was 80.7% in the inotuzumab arm and 29.4% in the chemotherapy arm (P less than .001). The median progression-free survival was 5 months and 1.8 months, respectively (P less than .001). The median overall survival was 7.7 months and 6.7 months, respectively (P = .04; N Engl J Med 2016; 375:740-53).
Based on these results, researchers are testing inotuzumab as frontline therapy in young adults (aged 18-39 years) with CD22-positive, Ph-negative B-ALL. In the phase 3 A041501 trial, patients are receiving inotuzumab after the first and second courses of treatment with the CALGB 10403 chemotherapy regimen. Results from this trial are not yet available.
Dr. Park reported relationships with Allogene Therapeutics, Amgen, AstraZeneca, Incyte, Kite Pharma, Novartis, and Takeda.
SAN FRANCISCO – Positive results with blinatumomab and inotuzumab ozogamicin in the relapsed/refractory setting have prompted trials of these immunotherapies in newly diagnosed B-cell acute lymphoblastic leukemia (B-ALL).
Blinatumomab and inotuzumab have been shown to improve overall survival, compared with chemotherapy, in patients with relapsed/refractory B-ALL. However, most adults with relapsed/refractory B-ALL still die, so the initial therapy patients receive is “critical,” according to Jae Park, MD, of Memorial Sloan Kettering Cancer Center in New York.
“Ideally, we do not want to deal with the relapse,” Dr. Park said. “It’s better to cure the disease the first time ... which is the reason clinical trials are incorporating these agents earlier.”
Dr. Park discussed these points at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress.
Blinatumomab
Dr. Park cited the phase 3 TOWER trial, which showed that blinatumomab produced better response rates and overall survival compared with standard chemotherapy. The trial enrolled 405 patients with Ph-negative relapsed/refractory B-ALL who were randomized to blinatumomab (n = 271) or chemotherapy (n = 134).
The rate of complete response (CR) with full, partial, or incomplete hematologic recovery was 44% with blinatumomab and 25% with chemotherapy (P less than .001). The median overall survival was 7.7 months and 4.0 months, respectively (P = .01; N Engl J Med 2017; 376:836-47).
Based on these data, researchers decided to test blinatumomab in newly diagnosed, elderly patients (65 years and older) with Ph-negative B-ALL in the phase 2 SWOG 1318 study. The study enrolled 31 patients, and 29 were eligible. Their median age at baseline was 75 years (range 66‐84 years).
The patients received blinatumomab for two to five cycles, followed by 18 months of maintenance with prednisone, vincristine, 6-mercaptopurine, and methotrexate. One patient went on to transplant.
In all, 66% of patients achieved a CR or CR with incomplete count recovery. The estimated overall survival was 79% at 6 months and 65% at 1 year. These results were presented at the 2018 annual meeting of the American Society of Hematology (Blood. 2018;132:33).
Another study of blinatumomab as frontline treatment is the ECOG-E1910 trial. In this phase 3 study, researchers are testing chemotherapy, with or without blinatumomab, in adults (aged 30-70 years) with newly diagnosed, BCR-ABL-negative B-ALL. Results from this study are not yet available.
Inotuzumab ozogamicin
Dr. Park also discussed the INOVATE trial, in which inotuzumab ozogamicin bested standard chemotherapy. The trial enrolled patients with Ph-positive or negative, relapsed/refractory B-ALL.
The patients were randomized to inotuzumab (n = 141) or investigator’s choice of chemotherapy (n = 138). Some patients, 41% in the inotuzumab arm and 11% in the chemotherapy arm, went on to transplant.
The CR rate was 80.7% in the inotuzumab arm and 29.4% in the chemotherapy arm (P less than .001). The median progression-free survival was 5 months and 1.8 months, respectively (P less than .001). The median overall survival was 7.7 months and 6.7 months, respectively (P = .04; N Engl J Med 2016; 375:740-53).
Based on these results, researchers are testing inotuzumab as frontline therapy in young adults (aged 18-39 years) with CD22-positive, Ph-negative B-ALL. In the phase 3 A041501 trial, patients are receiving inotuzumab after the first and second courses of treatment with the CALGB 10403 chemotherapy regimen. Results from this trial are not yet available.
Dr. Park reported relationships with Allogene Therapeutics, Amgen, AstraZeneca, Incyte, Kite Pharma, Novartis, and Takeda.
SAN FRANCISCO – Positive results with blinatumomab and inotuzumab ozogamicin in the relapsed/refractory setting have prompted trials of these immunotherapies in newly diagnosed B-cell acute lymphoblastic leukemia (B-ALL).
Blinatumomab and inotuzumab have been shown to improve overall survival, compared with chemotherapy, in patients with relapsed/refractory B-ALL. However, most adults with relapsed/refractory B-ALL still die, so the initial therapy patients receive is “critical,” according to Jae Park, MD, of Memorial Sloan Kettering Cancer Center in New York.
“Ideally, we do not want to deal with the relapse,” Dr. Park said. “It’s better to cure the disease the first time ... which is the reason clinical trials are incorporating these agents earlier.”
Dr. Park discussed these points at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress.
Blinatumomab
Dr. Park cited the phase 3 TOWER trial, which showed that blinatumomab produced better response rates and overall survival compared with standard chemotherapy. The trial enrolled 405 patients with Ph-negative relapsed/refractory B-ALL who were randomized to blinatumomab (n = 271) or chemotherapy (n = 134).
The rate of complete response (CR) with full, partial, or incomplete hematologic recovery was 44% with blinatumomab and 25% with chemotherapy (P less than .001). The median overall survival was 7.7 months and 4.0 months, respectively (P = .01; N Engl J Med 2017; 376:836-47).
Based on these data, researchers decided to test blinatumomab in newly diagnosed, elderly patients (65 years and older) with Ph-negative B-ALL in the phase 2 SWOG 1318 study. The study enrolled 31 patients, and 29 were eligible. Their median age at baseline was 75 years (range 66‐84 years).
The patients received blinatumomab for two to five cycles, followed by 18 months of maintenance with prednisone, vincristine, 6-mercaptopurine, and methotrexate. One patient went on to transplant.
In all, 66% of patients achieved a CR or CR with incomplete count recovery. The estimated overall survival was 79% at 6 months and 65% at 1 year. These results were presented at the 2018 annual meeting of the American Society of Hematology (Blood. 2018;132:33).
Another study of blinatumomab as frontline treatment is the ECOG-E1910 trial. In this phase 3 study, researchers are testing chemotherapy, with or without blinatumomab, in adults (aged 30-70 years) with newly diagnosed, BCR-ABL-negative B-ALL. Results from this study are not yet available.
Inotuzumab ozogamicin
Dr. Park also discussed the INOVATE trial, in which inotuzumab ozogamicin bested standard chemotherapy. The trial enrolled patients with Ph-positive or negative, relapsed/refractory B-ALL.
The patients were randomized to inotuzumab (n = 141) or investigator’s choice of chemotherapy (n = 138). Some patients, 41% in the inotuzumab arm and 11% in the chemotherapy arm, went on to transplant.
The CR rate was 80.7% in the inotuzumab arm and 29.4% in the chemotherapy arm (P less than .001). The median progression-free survival was 5 months and 1.8 months, respectively (P less than .001). The median overall survival was 7.7 months and 6.7 months, respectively (P = .04; N Engl J Med 2016; 375:740-53).
Based on these results, researchers are testing inotuzumab as frontline therapy in young adults (aged 18-39 years) with CD22-positive, Ph-negative B-ALL. In the phase 3 A041501 trial, patients are receiving inotuzumab after the first and second courses of treatment with the CALGB 10403 chemotherapy regimen. Results from this trial are not yet available.
Dr. Park reported relationships with Allogene Therapeutics, Amgen, AstraZeneca, Incyte, Kite Pharma, Novartis, and Takeda.
EXPERT ANALYSIS FROM NCCN HEMATOLOGIC MALIGNANCIES
Novel research aims to improve ED care in sickle cell disease
Several initiatives are in the works to improve the management of patients with sickle cell disease in the ED, experts said at a recent webinar held by the National Heart, Lung, and Blood Institute.
In 2014, the NHLBI released evidence-based guidelines for the management of patients with sickle cell disease. The expert panel provided recommendations on the treatment of acute complications of sickle cell disease, many of which are common reasons for ED visits.
Optimizing the treatment of acute complications, namely vasoocclusive crisis, is essential to ensure improved long-term outcomes, explained Paula Tanabe, PhD, of Duke University, Durham, N.C.
Pain management
While the majority of pain-related ED visits in sickle cell are the result of vasoocclusive crisis, other causes, such as acute chest syndrome, abdominal catastrophes, and splenic sequestration, are also important.
The hallmark of pain management in this population is rapid and aggressive treatment with intravenous opioids. The use of individualized doses is also important, but if not available, an sickle cell disease–specific pain protocol can be used, she explained.
Recent evidence has confirmed the benefit of using an individualized (patient-specific) dosing protocol. Dr. Tanabe reported the results of a randomized pilot study that compared two pain protocols for patients undergoing a vasoocclusive episode in the ED.
“The reason we pursued this project is to generate additional evidence beyond the expert panel,” she said.
The primary outcome of the study was the difference in pain scores from arrival to discharge between patients receiving an individualized or weight-based dosing protocol. Secondary outcomes included safety, pain experience, and side effects, among others.
The researchers found that patients who received an individualized protocol had significantly lower pain scores, compared with a standard weight-based protocol (between-protocol pain score difference, 15.6 plus or minus 5.0; P = .002).
Additionally, patients in the individualized dosing arm were admitted less often than those in the weight-based arm (P = .03), Dr. Tanabe reported.
The findings from the previous study formed the basis for an ongoing study that is further examining the impact of patient-specific dosing in patients who present with a vasoocclusive episode. The COMPARE VOE study is currently enrolling patients and is being funded by NHLBI.
The NHLBI also provides funding to eight Sickle Cell Disease Implementation Consortium sites throughout the United States. The objective of this grant funding is to help implement NHLBI recommendations in the emergency setting.
Quality improvement
“One area [that] we want to improve is how quickly we administer [analgesic therapy] to patients when they are experiencing a vasoocclusive episode,” said Caroline Freiermuth, MD, of the University of Cincinnati.
Some common barriers to delivering rapid analgesia in this setting include difficulties in obtaining intravenous access, high patient volumes, lack of education, and provider biases, she explained.
With respect to high patient volumes, one strategy that may help overcome this barrier is to triage patients as Emergency Severity Index level 2, allowing for accelerated room placement.
Sickle cell patients undergoing vasoocclusive crisis meet the criteria for level 2 based on morbidity, degree of pain, and the level of resources often required.
Another important strategy is improving education related to sickle cell disease, particularly the high morbidity and mortality seen in these patients, Dr. Freiermuth said.
“The median lifespan for patients with HbSS disease is in the 40s, basically half of the lifespan of a typical American,” she said.
At present, acute chest syndrome is the principal cause of death in patients with sickle cell disease, and most frequently occurs during a vasoocclusive episode. As a result, screening for this complication is essential to reduce mortality in the emergency setting.
Dr. Freiermuth explained that one of the best ways to prevent acute chest syndrome is to encourage the use of incentive spirometry in patients undergoing a vasoocclusive episode.
In order to increase the likelihood of obtaining intravenous access, the use of ultrasound may help guide placement. Educating nurses on the proper use of ultrasound-guided placement of intravenous catheters is one practical approach, she said.
Alternatively, opioid analgesia can be administered subcutaneously. Benefits of subcutaneous delivery include comparable pharmacokinetics, less pain, and a reduced likelihood of sterile abscesses that are often seen with intramuscular administration.
Dr. Freiermuth outlined the quality-improvement initiative being tested at her institution, which involves the administration of parenteral opioid therapy during triage for sickle cell patients undergoing a suspected vasoocclusive crisis. The initiative was developed with input from both the emergency and hematology departments at the site.
Early results have shown no significant changes using this approach, but the data is still preliminary. Initial feedback has revealed that time to room placement has been the greatest barrier, she reported.
Dr. Tanabe reported grant/research support from the National Institutes of Health and the Agency for Healthcare Research and Quality. Dr. Freiermuth reported research support from Pfizer.
Several initiatives are in the works to improve the management of patients with sickle cell disease in the ED, experts said at a recent webinar held by the National Heart, Lung, and Blood Institute.
In 2014, the NHLBI released evidence-based guidelines for the management of patients with sickle cell disease. The expert panel provided recommendations on the treatment of acute complications of sickle cell disease, many of which are common reasons for ED visits.
Optimizing the treatment of acute complications, namely vasoocclusive crisis, is essential to ensure improved long-term outcomes, explained Paula Tanabe, PhD, of Duke University, Durham, N.C.
Pain management
While the majority of pain-related ED visits in sickle cell are the result of vasoocclusive crisis, other causes, such as acute chest syndrome, abdominal catastrophes, and splenic sequestration, are also important.
The hallmark of pain management in this population is rapid and aggressive treatment with intravenous opioids. The use of individualized doses is also important, but if not available, an sickle cell disease–specific pain protocol can be used, she explained.
Recent evidence has confirmed the benefit of using an individualized (patient-specific) dosing protocol. Dr. Tanabe reported the results of a randomized pilot study that compared two pain protocols for patients undergoing a vasoocclusive episode in the ED.
“The reason we pursued this project is to generate additional evidence beyond the expert panel,” she said.
The primary outcome of the study was the difference in pain scores from arrival to discharge between patients receiving an individualized or weight-based dosing protocol. Secondary outcomes included safety, pain experience, and side effects, among others.
The researchers found that patients who received an individualized protocol had significantly lower pain scores, compared with a standard weight-based protocol (between-protocol pain score difference, 15.6 plus or minus 5.0; P = .002).
Additionally, patients in the individualized dosing arm were admitted less often than those in the weight-based arm (P = .03), Dr. Tanabe reported.
The findings from the previous study formed the basis for an ongoing study that is further examining the impact of patient-specific dosing in patients who present with a vasoocclusive episode. The COMPARE VOE study is currently enrolling patients and is being funded by NHLBI.
The NHLBI also provides funding to eight Sickle Cell Disease Implementation Consortium sites throughout the United States. The objective of this grant funding is to help implement NHLBI recommendations in the emergency setting.
Quality improvement
“One area [that] we want to improve is how quickly we administer [analgesic therapy] to patients when they are experiencing a vasoocclusive episode,” said Caroline Freiermuth, MD, of the University of Cincinnati.
Some common barriers to delivering rapid analgesia in this setting include difficulties in obtaining intravenous access, high patient volumes, lack of education, and provider biases, she explained.
With respect to high patient volumes, one strategy that may help overcome this barrier is to triage patients as Emergency Severity Index level 2, allowing for accelerated room placement.
Sickle cell patients undergoing vasoocclusive crisis meet the criteria for level 2 based on morbidity, degree of pain, and the level of resources often required.
Another important strategy is improving education related to sickle cell disease, particularly the high morbidity and mortality seen in these patients, Dr. Freiermuth said.
“The median lifespan for patients with HbSS disease is in the 40s, basically half of the lifespan of a typical American,” she said.
At present, acute chest syndrome is the principal cause of death in patients with sickle cell disease, and most frequently occurs during a vasoocclusive episode. As a result, screening for this complication is essential to reduce mortality in the emergency setting.
Dr. Freiermuth explained that one of the best ways to prevent acute chest syndrome is to encourage the use of incentive spirometry in patients undergoing a vasoocclusive episode.
In order to increase the likelihood of obtaining intravenous access, the use of ultrasound may help guide placement. Educating nurses on the proper use of ultrasound-guided placement of intravenous catheters is one practical approach, she said.
Alternatively, opioid analgesia can be administered subcutaneously. Benefits of subcutaneous delivery include comparable pharmacokinetics, less pain, and a reduced likelihood of sterile abscesses that are often seen with intramuscular administration.
Dr. Freiermuth outlined the quality-improvement initiative being tested at her institution, which involves the administration of parenteral opioid therapy during triage for sickle cell patients undergoing a suspected vasoocclusive crisis. The initiative was developed with input from both the emergency and hematology departments at the site.
Early results have shown no significant changes using this approach, but the data is still preliminary. Initial feedback has revealed that time to room placement has been the greatest barrier, she reported.
Dr. Tanabe reported grant/research support from the National Institutes of Health and the Agency for Healthcare Research and Quality. Dr. Freiermuth reported research support from Pfizer.
Several initiatives are in the works to improve the management of patients with sickle cell disease in the ED, experts said at a recent webinar held by the National Heart, Lung, and Blood Institute.
In 2014, the NHLBI released evidence-based guidelines for the management of patients with sickle cell disease. The expert panel provided recommendations on the treatment of acute complications of sickle cell disease, many of which are common reasons for ED visits.
Optimizing the treatment of acute complications, namely vasoocclusive crisis, is essential to ensure improved long-term outcomes, explained Paula Tanabe, PhD, of Duke University, Durham, N.C.
Pain management
While the majority of pain-related ED visits in sickle cell are the result of vasoocclusive crisis, other causes, such as acute chest syndrome, abdominal catastrophes, and splenic sequestration, are also important.
The hallmark of pain management in this population is rapid and aggressive treatment with intravenous opioids. The use of individualized doses is also important, but if not available, an sickle cell disease–specific pain protocol can be used, she explained.
Recent evidence has confirmed the benefit of using an individualized (patient-specific) dosing protocol. Dr. Tanabe reported the results of a randomized pilot study that compared two pain protocols for patients undergoing a vasoocclusive episode in the ED.
“The reason we pursued this project is to generate additional evidence beyond the expert panel,” she said.
The primary outcome of the study was the difference in pain scores from arrival to discharge between patients receiving an individualized or weight-based dosing protocol. Secondary outcomes included safety, pain experience, and side effects, among others.
The researchers found that patients who received an individualized protocol had significantly lower pain scores, compared with a standard weight-based protocol (between-protocol pain score difference, 15.6 plus or minus 5.0; P = .002).
Additionally, patients in the individualized dosing arm were admitted less often than those in the weight-based arm (P = .03), Dr. Tanabe reported.
The findings from the previous study formed the basis for an ongoing study that is further examining the impact of patient-specific dosing in patients who present with a vasoocclusive episode. The COMPARE VOE study is currently enrolling patients and is being funded by NHLBI.
The NHLBI also provides funding to eight Sickle Cell Disease Implementation Consortium sites throughout the United States. The objective of this grant funding is to help implement NHLBI recommendations in the emergency setting.
Quality improvement
“One area [that] we want to improve is how quickly we administer [analgesic therapy] to patients when they are experiencing a vasoocclusive episode,” said Caroline Freiermuth, MD, of the University of Cincinnati.
Some common barriers to delivering rapid analgesia in this setting include difficulties in obtaining intravenous access, high patient volumes, lack of education, and provider biases, she explained.
With respect to high patient volumes, one strategy that may help overcome this barrier is to triage patients as Emergency Severity Index level 2, allowing for accelerated room placement.
Sickle cell patients undergoing vasoocclusive crisis meet the criteria for level 2 based on morbidity, degree of pain, and the level of resources often required.
Another important strategy is improving education related to sickle cell disease, particularly the high morbidity and mortality seen in these patients, Dr. Freiermuth said.
“The median lifespan for patients with HbSS disease is in the 40s, basically half of the lifespan of a typical American,” she said.
At present, acute chest syndrome is the principal cause of death in patients with sickle cell disease, and most frequently occurs during a vasoocclusive episode. As a result, screening for this complication is essential to reduce mortality in the emergency setting.
Dr. Freiermuth explained that one of the best ways to prevent acute chest syndrome is to encourage the use of incentive spirometry in patients undergoing a vasoocclusive episode.
In order to increase the likelihood of obtaining intravenous access, the use of ultrasound may help guide placement. Educating nurses on the proper use of ultrasound-guided placement of intravenous catheters is one practical approach, she said.
Alternatively, opioid analgesia can be administered subcutaneously. Benefits of subcutaneous delivery include comparable pharmacokinetics, less pain, and a reduced likelihood of sterile abscesses that are often seen with intramuscular administration.
Dr. Freiermuth outlined the quality-improvement initiative being tested at her institution, which involves the administration of parenteral opioid therapy during triage for sickle cell patients undergoing a suspected vasoocclusive crisis. The initiative was developed with input from both the emergency and hematology departments at the site.
Early results have shown no significant changes using this approach, but the data is still preliminary. Initial feedback has revealed that time to room placement has been the greatest barrier, she reported.
Dr. Tanabe reported grant/research support from the National Institutes of Health and the Agency for Healthcare Research and Quality. Dr. Freiermuth reported research support from Pfizer.
REPORTING FROM AN NIH WEBINAR
Cardiovascular complications of systemic sclerosis: What to look for
Autoimmune rheumatic diseases increase the risk of cardiovascular disease. In rheumatoid arthritis and systemic lupus erythematosus, the risk is driven primarily by the inflammatory milieu, leading to accelerated coronary and cerebrovascular atherosclerosis independent of traditional atherosclerotic risk factors.1–3 The extent of cardiovascular involvement in other rheumatologic diseases has been less well characterized but is an area of growing interest.
In this review, we focus on the cardiovascular complications of systemic sclerosis and review recommendations for monitoring these patients in clinical practice.
SYSTEMIC SCLEROSIS, AN AUTOIMMUNE RHEUMATIC DISEASE
Systemic sclerosis is an autoimmune rheumatic disease characterized by excessive extracellular matrix deposition leading to diffuse fibrosis, endothelial dysfunction, and microvascular injury. It is most common in North America, Southern Europe, and Australia,4,5 and it affects women more than men in ratios ranging from 3:1 to 14:1.6 The mean age at diagnosis is around 50.
The disease can affect the lungs (interstitial lung disease and pulmonary hypertension), the heart, the kidneys, and the gastrointestinal tract.
Systemic sclerosis has 2 main subtypes: limited cutaneous systemic sclerosis, formerly called CREST syndrome) and diffuse cutaneous systemic sclerosis. The limited cutaneous subtype is characterized by tightening of the skin of the distal extremities (below the elbows and knees) and face, while diffuse cutaneous systemic sclerosis can manifest as more extensive skin tightening also involving proximal extremities and the trunk. Both subtypes can have an effect on the cardiovascular system.
Some cardiovascular risk factors such as dyslipidemia, diabetes mellitus, and high body mass index are less common in patients with systemic sclerosis than in patients with rheumatoid arthritis, while the rates of arterial hypertension, smoking, chronic obstructive pulmonary disease, osteoporosis, and neoplasms are similar between the 2 groups.7
HEART INVOLVEMENT HAS SERIOUS CONSEQUENCES
Overt cardiac involvement in systemic sclerosis is associated with a mortality rate of up to 70% over 5 years,8,9 and about one-fourth of deaths in patients with systemic sclerosis are from cardiac causes.10,11 Studies in Europe10,12 showed that many patients with systemic sclerosis have cardiac involvement detectable by magnetic resonance imaging even if they do not have clinical disease. Pulmonary arterial hypertension (PAH) is a complication of both subtypes of systemic sclerosis and portends a higher risk of death.8
Thus, it is critical for clinicians to understand the potential comorbid conditions associated with systemic sclerosis, particularly the cardiovascular ones, and to work closely with cardiologists to help optimize the evaluation and management.
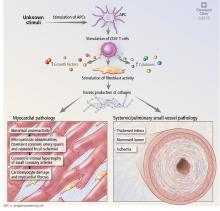
MECHANISMS OF CARDIAC DISEASE IN SYSTEMIC SCLEROSIS
Abnormal vasoreactivity, a consequence of an imbalance between endothelium-derived vasoconstrictors and vasodilators, defective angiogenesis, and endothelial injury, leads to tissue ischemia and vascular endothelial growth factor expression, which initiates injury and fibrosis in the myocardium and in other organs.14–17 Fibrosis involves the myocardium, pericardium, and conduction system.13,18
Myocardial involvement in systemic sclerosis is thought to be due mainly to abnormal vasoreactivity and microvascular abnormalities such as transient coronary artery spasm leading to repeated focal ischemia.19,20 Abnormal vasoreactivity has been demonstrated during cardiac catheterization21: while mean coronary sinus blood flow in systemic sclerosis patients was normal at rest, vasodilator reserve was significantly reduced in patients with diffuse cutaneous systemic sclerosis after maximal vasodilation with dipyridamole. Additionally, endomyocardial biopsy showed fibrosis and concentric intimal hypertrophy with normal epicardial coronary arteries.21
More research into other mechanisms of cardiovascular disease in systemic sclerosis is needed to allow for better preventive care for these patients.
PULMONARY ARTERIAL HYPERTENSION
Systemic sclerosis can be associated with World Health Organization (WHO) groups 1, 2, 3, and 4 pulmonary hypertension. WHO group 1, called pulmonary arterial hypertension or PAH, is one of the most common cardiac complications of systemic sclerosis, with a reported prevalence as high as 12%.22 Systemic sclerosis-associated PAH carries a high mortality rate, with a mean survival of only 3 years.23
With advances in treatments for other complications of systemic sclerosis, the percentage of systemic sclerosis patients who die of PAH has increased from 6% to 33%.24
Compared with patients with idiopathic PAH, those with systemic sclerosis get less of a response from therapy and have poorer outcomes despite lower mean pulmonary artery pressures and similar reductions in cardiac index. However, recent studies have suggested that with aggressive treatment, patients with systemic sclerosis-related PAH can achieve outcomes similar to those with idiopathic PAH.25 Thus, recognizing this condition early is imperative.
Pulmonary arterial hypertension defined
PAH is defined as the combination of all of the following26:
- Mean pulmonary artery pressure > 20 mm Hg at rest
- Normal pulmonary capillary wedge pressure (≤ 15 mm Hg)
- Pulmonary vascular resistance ≥ 3 Wood units on right heart catheterization.
Other causes of pulmonary hypertension such as interstitial lung disease, chronic pulmonary thromboembolic disease, and left heart disease must be excluded.24,27
Remodeling in the pulmonary arteries
The events that lead to PAH in systemic sclerosis remain unclear but are believed to involve initial inflammation or endothelial injury that leads to a dysequilibrium between proliferative mediators and antiproliferative vasodilators. This dysequilibrium, along with endothelial dysfunction, causes an obliterative vasculopathy in the pulmonary artery branches and arterioles. Sympathetic overactivity, hypoxemia, and ischemia-reperfusion injury additionally promote vascular proliferation, fibrosis, and remodeling, leading to increased pulmonary vascular resistance, PAH, and increased right ventricular pressures.23,27
The subtype of systemic sclerosis is an important factor in the development and progression of PAH. PAH appears to be the major cause of death in limited cutaneous systemic sclerosis, while interstitial lung disease is the major cause of death in diffuse cutaneous systemic sclerosis.28
Pulmonary arterial hypertension is a late complication of systemic sclerosis
Data from the South Australian Scleroderma Registry29 revealed that PAH tends to be a late complication of systemic sclerosis, occurring around 20 years after disease onset. In this study of 608 patients, no patient with diffuse cutaneous systemic sclerosis developed PAH.
Systemic sclerosis-related PAH initially follows an indolent course with few symptoms until right ventricular function deteriorates. Early in the disease, patients may experience nonspecific symptoms of fatigue, lightheadedness, and dyspnea on exertion.23 As it progresses, they tend to have worsening dyspnea and may experience exertional syncope, palpitations, and chest pain.
Physical findings may suggest elevated right ventricular pressure and right ventricular failure; these include a loud P2, a prominent jugular a wave, a tricuspid regurgitant murmur, jugular venous distention, and lower-extremity edema.27
Screening for pulmonary arterial hypertension in systemic sclerosis
Significant signs and symptoms usually occur late in the disease; thus, it is important to appropriately screen patients who are at risk so that they can begin aggressive treatment.
Doppler echocardiography is recommended by European and American guidelines to screen for PAH in patients who have systemic sclerosis, and most agree that screening is appropriate even if the patient has no symptoms.30 European consensus documents recommend that transthoracic echocardiography be done annually for the first 5 years of disease and be continued every year in patients at high risk, ie, those with anticentromere antibodies, anti-Th/To antibodies, or interstitial lung disease. Patients not at high risk of developing pulmonary hypertension should also have regular transthoracic echocardiography, though the exact timing is not defined.31 While American societies have not issued corresponding recommendations, many experts follow the European recommendations.
Worrisome features on echocardiography in asymptomatic patients should be followed up with right heart catheterization to assess mean right ventricular pressure. These include:
- Estimated right ventricular systolic pressure ≥ 40 mm Hg
- Tricuspid regurgitant jet velocity > 2.8 m/s
- Right atrial enlargement > 53 mm
- Right ventricular enlargement (mid-cavity dimension > 35 mm).32
Although echocardiography is the most common form of screening, it gives only an estimate of right ventricular systolic pressure, which is imprecise. Other noninvasive markers are helpful and necessary to appropriately screen this population.
Diffusion capacity. The Itinerair study33 found that a diffusing capacity for carbon monoxide (DLCO) of 60% or higher has a high specificity in excluding PAH.
Uric acid has been found to be elevated in patients with systemic sclerosis-related PAH, and levels inversely correlate with 6-minute walking distance.34
Other predictors. N-terminal pro-B-type natriuretic peptide (NT-proBNP), left atrial volume, and the right ventricular myocardial performance index have also been shown to be independent predictors of PAH in patients with systemic sclerosis.35
An algorithm. The DETECT study36 enrolled patients at increased risk who had had systemic sclerosis longer than 3 years and a DLCO less than 60%. The investigators developed a 2-step algorithm to determine which patients should be referred for right heart catheterization to try to detect PAH earlier while minimizing the number of missed diagnoses and optimizing the use of invasive diagnostic right heart catheterization.
The first step was to assess serum values of anticentromere antibodies, NT-proBNP, and urate, and clinical features (telangiectasias), forced vital capacity, and electrocardiographic changes of right axis deviation to derive a prediction score. The second step was to assess surface echocardiographic features of the right atrial area and tricuspid regurgitation velocity.
This approach led to right heart catheterization in 62% of patients and was associated with a false-negative rate of 4%. Importantly, of the patients with PAH, 1 in 5 had no symptoms, and 33% had tricuspid regurgitation velocity less than 2.8 m/s. No single measurement performed well in isolation in this study.37
Thus, we recommend that, in addition to routine surface echocardiography, a multimodal approach be used that includes laboratory testing, clinical features, and electrocardiographic findings when screening this high-risk patient population.
ATHEROSCLEROTIC DISEASES
Although macrovascular disease has not typically been regarded as a significant systemic feature in systemic sclerosis, myocardial infarction and stroke are more common in patients with systemic sclerosis than in controls.38,39
Coronary artery disease in systemic sclerosis
Man et al38 reported that the incidence of myocardial infarction in patients with systemic sclerosis was 4.4 per 1,000 persons per year, and the incidence of stroke was 4.8 per 1,000 persons per year, compared with 2.5 per 1,000 persons per year for both myocardial infarction and stroke in healthy controls matched for age, sex, and time of entry.
The Australian Scleroderma Cohort Study39 found a 3-fold higher prevalence of coronary artery disease in systemic sclerosis patients than in controls after factoring in traditional risk factors.
Aviña-Zubieta et al,40 in a cohort of 1,239 systemic sclerosis patients, estimated a hazard ratio (HR) of 3.49 for myocardial infarction and 2.35 for stroke compared with age- and sex-matched controls. Not all of these events were related to macrovascular atherosclerosis—vasospasm and microvascular ischemia may have played significant roles in the etiology of clinical manifestations.
Studies of coronary atherosclerosis in systemic sclerosis are limited. An autopsy study41 of 58 patients with systemic sclerosis and 58 controls matched for age, sex, and ethnicity found that the prevalence of atherosclerosis of small coronary arteries and arterioles was significantly higher in systemic sclerosis patients than in controls (17% vs 2%, P < .01). However, the prevalence of medium-vessel coronary atherosclerosis was similar (48% vs 43%).
Why patients with systemic sclerosis develop atherosclerosis has not yet been determined. Traditional risk factors such as hypertension, dyslipidemia, diabetes mellitus, and obesity are typically no more prevalent in systemic sclerosis patients than in controls,38,42 and thus do not explain the increased risk of atherosclerotic cardiovascular disease. There is some evidence that novel markers of atherosclerotic risk such as homocysteine,43 lipoprotein[a],44 and oxidized low-density lipoprotein45 are more prevalent in systemic sclerosis, but these results have not been substantiated in more extensive studies.
Peripheral artery disease
It remains unclear whether peripheral artery disease is more prevalent in systemic sclerosis patients than in controls.
Individual studies have shown mixed results in comparing carotid artery stenosis between systemic sclerosis patients and controls using carotid duplex ultrasonography,46 the ankle-brachial index,46–48 carotid intima-media thickness,49–54 and brachial flow-mediated dilation.51,53,55–58 A meta-analysis found that the carotid intima and media are significantly thicker in systemic sclerosis patients than in controls,59 and the magnitude of difference is similar to that in other groups at increased cardiovascular risk, such as those with rheumatoid arthritis, diabetes, and familial hypercholesterolemia.60–63
A meta-analysis of brachial artery findings showed significantly lower flow-mediated dilation in systemic sclerosis patients than in controls.64
Overall, given the inconsistency of study results, systemic sclerosis patients should be screened and managed as in other patients with peripheral artery disease, but the clinician should be aware that there may be a higher risk of peripheral artery disease in these patients.
RIGHT AND LEFT VENTRICULAR DYSFUNCTION
Many patients with systemic sclerosis have right ventricular dysfunction as a consequence of PAH.65 It is important to detect diastolic dysfunction in this population, as it may be an even stronger predictor of death than pulmonary hypertension on right heart catheterization (HR 3.7 vs 2.0).66
Fewer patients have left ventricular dysfunction. In a multicenter study of 570 systemic sclerosis patients, only 1.4% had left ventricular systolic dysfunction on echocardiography, though 22.6% had left ventricular hypertrophy and 17.7% had left ventricular diastolic dysfunction.67 In the European League Against Rheumatism (EULAR) database, the prevalence of reduced left ventricular ejection fraction was 5.4%.68
Though traditional echocardiographic screening suggests the prevalence of left ventricular dysfunction in systemic sclerosis patients is low, cardiac magnetic resonance imaging (MRI) may be more sensitive than echocardiography for detecting subclinical myocardial involvement. Cardiac MRI has been shown to detect evidence of myocardial pathology (increased T2 signal, left ventricular thinning, pericardial effusion, reduced left ventricular and right ventricular ejection fraction, left ventricular diastolic dysfunction, and delayed myocardial contrast enhancement) in up to 75% of systemic sclerosis cases studied.69
Patients with systemic sclerosis should already be undergoing echocardiography every year to screen for PAH, and screening should also include tissue Doppler imaging to detect various forms of left and right ventricular systolic and diastolic dysfunction that may not be clinically apparent.
Though cardiac MRI can provide useful additional information, it is not currently recommended for routine screening in patients with systemic sclerosis.
ARRHYTHMIAS AND CONDUCTION DEFECTS
Patients with systemic sclerosis are prone to arrhythmias due to both conduction system fibrosis and myocardial damage.
Arrhythmias accounted for 6% of the deaths in the EULAR Scleroderma Trials and Research (EUSTAR) database.11
In the Genetics Versus Environment in Scleroderma Outcome Study (GENISOS),70 250 patients who had had systemic sclerosis for at least 3 years were studied during a period of approximately 6 years, during which there were 52 deaths, 29 of which were directly attributable to systemic sclerosis. Multivariable Cox modeling showed that 7 variables predicted mortality:
- Body mass index < 18.5 kg/m2
- Age ≥ 65
- Forced vital capacity < 50% predicted
- Systolic blood pressure ≥ 140 or diastolic blood pressure ≥ 90 mm Hg
- Pulmonary fibrosis
- Positive anticentromere antibodies
- Cardiac arrhythmias.
The hazard ratio for death in patients with arrhythmias in this model was 2.18 (95% CI 1.05–4.50, P = .035). Thus, finding arrhythmias in systemic sclerosis patients can provide important prognostic information.
While resting electrocardiography in patients with systemic sclerosis most commonly shows sinus rhythm, 24-hour electrocardiographic monitoring has revealed nonsustained supraventricular and ventricular arrhythmias in a significant percentage.71,72 Although difficult to quantify in routine practice, parameters controlled by the autonomic nervous system including heart rate variability and heart rate turbulence have been shown to be impaired in systemic sclerosis, and these measures are associated with an increased risk of malignant arrhythmias and sudden cardiac death.73,74
Conduction abnormalities
Conduction abnormalities occur in one-fifth to one-third of patients with systemic sclerosis.75,76 The most common abnormal conduction finding is left bundle branch block, followed by first-degree atrioventricular block. High-degree atrioventricular block is uncommon,76 though a few case reports of complete heart block thought to be related to systemic sclerosis have been published.77–79 An autopsy study showed that the conduction system is relatively spared from myocardial changes seen in systemic sclerosis patients, and thus it is speculated that the conduction disturbances are a consequence of damaged myocardium rather than damage to conduction tissue.80
Given the array of electrophysiologic abnormalities that systemic sclerosis patients can have, it is critical to monitor all patients with routine (annual or biannual) electrocardiography; to take possible arrhythmia-related symptoms seriously; and to evaluate them with further workup such as Holter monitoring for 24 hours or even longer, event monitoring, exercise testing, or tilt-table testing.
PERICARDIAL DISEASE
Pericardial disease is clinically apparent in 5% to 16% of patients with systemic sclerosis81; patients with limited cutaneous systemic sclerosis have more pericardial disease than those with diffuse cutaneous systemic sclerosis (30% vs 16%).82 Forty-one percent of systemic sclerosis patients have been shown to have pericardial effusion by echocardiography,81 but the effusions are typically small and rarely cause tamponade, though tamponade is associated with a poor prognosis.
Large pericardial effusions can develop before skin thickening and diagnosis of systemic sclerosis.81,83,84 Thus, systemic sclerosis should be considered in patients with pericardial effusions of unknown etiology.
In a small study,85 the pericardial fluid in systemic sclerosis was typically exudative, with lactate dehydrogenase greater than 200 U/L, a fluid-serum lactate dehydrogenase ratio greater than 0.6, and a fluid-serum total protein ratio greater than 0.5.
Pericardial effusion can be a sign of impending scleroderma renal crisis,86 and thus renal function should be carefully monitored in systemic sclerosis patients with pericardial effusion. Constrictive pericarditis and restrictive cardiomyopathy can rarely occur in systemic sclerosis and may more commonly present with symptoms.
Pericardial disease in systemic sclerosis should be treated in a standard fashion with nonsteroidal anti-inflammatory drugs. Corticosteroids are generally of limited benefit and should be avoided, especially in the setting of scleroderma renal crisis.81
VALVULAR HEART DISEASE
Based on limited studies, the prevalence of significant valvular heart disease in systemic sclerosis patients does not seem to be higher than that in the general population. While patients with systemic sclerosis and CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) have been shown to have a higher frequency of mitral valve prolapse and mild mitral regurgitation,87,88 these abnormalities do not often progress in severity, and thus their clinical significance is limited.
RECOMMENDATIONS FOR CARE OF SYSTEMIC SCLEROSIS PATIENTS
It is important for physicians caring for patients with systemic sclerosis to be aware of its most common cardiac manifestations, including left and right ventricular systolic and diastolic dysfunction, pulmonary hypertension, conduction abnormalities, arrhythmias, and cardiomyopathy.
Look for volume overload
On clinical examination, assess for clinical markers of volume overload such as distended neck veins, peripheral edema, or an abnormal blood pressure response to the Valsalva maneuver. These findings should prompt measurement of NT-proBNP,89 and may warrant prescription of a diuretic.
Electrocardiography to investigate arrhythmias
Electrocardiography should be done if patients describe symptoms of palpitations, and should also include continuous rhythm monitoring with Holter or event monitoring, depending on the frequency of symptoms. Otherwise, patients should routinely undergo electrocardiography once or twice a year.
Q waves are common in systemic sclerosis patients (especially those with diffuse cutaneous systemic sclerosis), notably in the precordial leads, and can occur without coronary artery disease.90 Symptoms such as presyncope should be further investigated with Holter monitoring and tilt-table testing.
Assess, modify traditional risk factors
Subclinical atherosclerosis as detected by carotid intima-media thickness is as common in systemic sclerosis as in rheumatoid arthritis.61 However, traditional risk indices such as SCORE (Systematic Coronary Risk Evaluation), QRISK2, and the American College of Cardiology/American Heart Association indices may underestimate risk in patients who have systemic sclerosis.
Strict hypertension control should be the goal for all systemic sclerosis patients. Though there are no specific guidelines on which antihypertensive medications are preferred, calcium channel blockers or angiotensin II receptor blockers, which are typically used to treat systemic sclerosis-related Raynaud phenomenon, may be appropriate.
Statins reduce vascular complications and are generally well tolerated in patients with systemic sclerosis.91,92
Aspirin is not recommended for routine primary prevention in view of data suggesting that its benefits in diabetic patients are counterbalanced by increased bleeding risk.93
Echocardiography to detect pulmonary arterial hypertension
At this time, guidelines for monitoring for cardiovascular manifestations in systemic sclerosis patients are limited. The only well-defined ones are European consensus guidelines, which suggest annual transthoracic echocardiography for the first 5 years after systemic sclerosis is diagnosed and continued annual screening in patients at risk of developing PAH.31
We support this strategy, with annual screening for the first 5 years followed by surveillance echocardiography every 2 to 3 years unless there is a high risk of PAH. Specific attention should be paid to right ventricular diastolic function, right atrial volume, and right ventricular myocardial performance index.
Emerging data suggest that the addition of global longitudinal strain of ventricles to routine echocardiography can help detect subclinical cardiac risk.94 Although further study is needed into the predictive value of global longitudinal strain, it is a low-cost and noninvasive addition to standard echocardiography that can help guide risk stratification, and thus we recommend that it be part of the echocardiographic examination for all systemic sclerosis patients.
Pulmonary function testing. In addition to screening for PAH with echocardiography, we recommend obtaining baseline pulmonary function tests, including DLCO, at the time systemic sclerosis is diagnosed, with repeat testing annually.
Magnetic resonance imaging
While echocardiography is the gold standard for monitoring systemic sclerosis patients, cardiovascular MRI may have a role in identifying those at higher risk of dangerous arrhythmias such as ventricular tachycardia and ventricular fibrillation. In addition to assessing ventricular function, MRI can detect myocardial inflammation, ischemia, and fibrosis that may predispose a patient to develop ventricular tachycardia or fibrillation.95 Variables such as T1/T2 mapping, extracellular volume fraction, T2 signal ratio, and early vs late gadolinium enhancement can help identify patients who had past ventricular tachycardia or fibrillation.96
Finding an increased risk of arrhythmias may prompt a conversation between the patient and the physician about the need for an implantable cardiac defibrillator.
If cardiac MRI is available and is reimbursed by the patient’s insurance carrier, physicians should strongly consider obtaining at least one baseline scan in systemic sclerosis patients to identify those at risk of highly fatal arrhythmias.
Teamwork is needed
Systemic sclerosis has not traditionally been associated with cardiovascular disease to the extent of other rheumatic conditions, but the cardiovascular system can be affected in various ways that can ultimately lead to an early death. These manifestations may be asymptomatic for long periods, and overt clinical disease portends a poorer prognosis.
Primary care physicians managing these patients should be aware of the cardiovascular complications of systemic sclerosis and should implement appropriate screening tests in conjunction with rheumatologists and cardiologists. It is also essential for general and subspecialty cardiologists to understand the broad spectrum of organ system involvement that can affect systemic sclerosis patients and to tailor their investigation and management recommendations accordingly. By designing a multidisciplinary approach to the treatment of systemic sclerosis patients, physicians can help to optimize cardiovascular risk modification in this vulnerable population.
- Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 2005; 52(2):402–411. doi:10.1002/art.20853
- Naranjo A, Sokka T, Descalzo MA, et al; QUEST-RA Group. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther 2008; 10(2):R30. doi:10.1186/ar2383
- Innala L, Möller B, Ljung L, et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther 2011; 13(4):R131. doi:10.1186/ar3442
- Barnes J, Mayes MD. Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Curr Opin Rheumatol 2012; 24(2):165–170. doi:10.1097/BOR.0b013e32834ff2e8
- Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum 2008; 37(4):223–235 doi:10.1016/j.semarthrit.2007.05.003
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med 2009; 360(19):1989–2003. doi:10.1056/NEJMra0806188
- Panopoulos S, Tektonidou M, Drosos AA, et al. Prevalence of comorbidities in systemic sclerosis versus rheumatoid arthritis: a comparative, multicenter, matched-cohort study. Arthritis Res Ther 2018; 20(1):267. doi:10.1186/s13075-018-1771-0
- Ferri C, Valentini G, Cozzi F, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002; 81(8):139–153. doi:10.1097/00005792-200203000-00004
- Steen VD, Medsger TA Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum 2000; 43(11):2437–2444. doi:10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010; 69(10):1809–1815. doi:10.1136/ard.2009.114264
- Nassenstein K, Breuckmann F, Huger M, et al. Detection of myocardial fibrosis in systemic sclerosis by contrast-enhanced magnetic resonance imaging. Rofo 2008; 180(12):1054–1060. doi:10.1055/s-2008-1027864
- Psarras A, Soulaidopoulos S, Garyfallos A, Kitas G, Dimitroulas T. A critical view on cardiovascular risk in systemic sclerosis. Rheumatol Int 2017; 37(1):85–95. doi:10.1007/s00296-016-3530-3
- Lekakis J, Mavrikakis M, Emmanuel M, et al. Cold-induced coronary Raynaud’s phenomenon in patients with systemic sclerosis. Clin Exp Rheumatol 1998; 16(2):135–140. pmid:9536388
- Altorok N, Wang Y, Kahaleh B. Endothelial dysfunction in systemic sclerosis. Curr Opin Rheumatol 2014; 26(6):615–620. doi:10.1097/BOR.0000000000000112
- Fleming JN, Nash RA, Mahoney WM Jr, Schwartz SM. Is scleroderma a vasculopathy? Curr Rheumatol Rep 2009; 11(2):103–110. pmid:19296882
- Maurer B, Distler A, Suliman YA, et al. Vascular endothelial growth factor aggravates fibrosis and vasculopathy in experimental models of systemic sclerosis. Ann Rheum Dis 2014; 73(10):1880–1887. doi:10.1136/annrheumdis-2013-203535
- Meune C, Vignaux O, Kahan A, Allanore Y. Heart involvement in systemic sclerosis: evolving concept and diagnostic methodologies. Arch Cardiovasc Dis 2010; 103(1):46–52. doi:10.1016/j.acvd.2009.06.009
- Dimitroulas T, Giannakoulas G, Karvounis H, Garyfallos A, Settas L, Kitas GD. Micro- and macrovascular treatment targets in scleroderma heart disease. Curr Pharm Des 2014; 20(4):536–544. pmid:23565639
- Allanore Y, Meune C. Primary myocardial involvement in systemic sclerosis: evidence for a microvascular origin. Clin Exp Rheumatol 2010; 28(5 suppl 62):S48–S53. pmid:21050545
- Kahan A, Nitenberg A, Foult JM, et al. Decreased coronary reserve in primary scleroderma myocardial disease. Arthritis Rheum 1985; 28(6):637–646. pmid:4004974
- Morrisroe K, Stevens W, Sahhar J, et al. Epidemiology and disease characteristics of systemic sclerosis-related pulmonary arterial hypertension: results from a real-life screening program. Arthritis Res Ther 2017; 19(1):42. doi:10.1186/s13075-017-1250-z
- Chaisson NF, Hassoun PM. Systemic sclerosis-associated pulmonary arterial hypertension. Chest 2013; 144(4):1346–1356. doi:10.1378/chest.12-2396
- Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 2007; 66(7):940–944. doi:10.1136/ard.2006.066068
- Coghlan JG, Galiè N, Barberà JA, et al; AMBITION investigators. Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): subgroup analysis from the AMBITION trial. Ann Rheum Dis 2017; 76(7):1219–1227. doi:10.1136/annrheumdis-2016-210236
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53(1):1801913. doi:10.1183/13993003.01913-2018
- Chatterjee S. Pulmonary hypertension in systemic sclerosis. Semin Arthritis Rheum 2011; 41(1):19–37. doi:10.1016/j.semarthrit.2010.08.004
- Sweiss NJ, Hushaw L, Thenappan T, et al. Diagnosis and management of pulmonary hypertension in systemic sclerosis. Curr Rheumatol Rep 2010; 12(1):8–18. doi:10.1007/s11926-009-0078-1
- Cox SR, Walker JG, Coleman M, et al. Isolated pulmonary hypertension in scleroderma. Intern Med J 2005; 35(1):28–33. doi:10.1111/j.1445-5994.2004.00646.x
- Sánchez-Román J, Opitz CF, Kowal-Bielecka O, García-Hernández FJ, Castillo-Palma MJ, Pittrow D; EPOSS-OMERACT Group. Screening for PAH in patients with systemic sclerosis: focus on Doppler echocardiography. Rheumatology (Oxford) 2008; 47(suppl 5):v33–v35. doi:10.1093/rheumatology/ken306
- Walker KM, Pope J; Scleroderma Clinical Trials Consortium; Canadian Scleroderma Research Group. Expert agreement on EULAR/EUSTAR recommendations for the management of systemic sclerosis. J Rheumatol 2011; 38(7):1326–1328. doi:10.3899/jrheum.101262
- Khanna D, Gladue H, Channick R, et al; Scleroderma Foundation and Pulmonary Hypertension Association. Recommendations for screening and detection of connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheum 2013; 65(12):3194–3201. doi:10.1002/art.38172
- Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 2005; 52(12):3792–3800. doi:10.1002/art.21433
- Dimitroulas T, Giannakoulas G, Dimitroula H, et al. Significance of serum uric acid in pulmonary hypertension due to systemic sclerosis: a pilot study. Rheumatol Int 2011; 31(2):263–267. doi:10.1007/s00296-010-1557-4
- Dimitroulas T, Giannakoulas G, Papadopoulou K, et al. Left atrial volume and N-terminal pro-B type natriuretic peptide are associated with elevated pulmonary artery pressure in patients with systemic sclerosis. Clin Rheumatol 2010; 29(9):957–964. doi:10.1007/s10067-010-1494-3
- Coghlan JG, Denton CP, Grünig E, et al; DETECT study group. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014; 73(7):1340–1349. doi:10.1136/annrheumdis-2013-203301
- Schwaiger JP, Khanna D, Gerry Coghlan J. Screening patients with scleroderma for pulmonary arterial hypertension and implications for other at-risk populations. Eur Respir Rev 2013; 22(130):515–525. doi:10.1183/09059180.00006013
- Man A, Zhu Y, Zhang Y, et al. The risk of cardiovascular disease in systemic sclerosis: a population-based cohort study. Ann Rheum Dis 2013; 72(7):1188–1193. doi:10.1136/annrheumdis-2012-202007
- Ngian G-S, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Aviña-Zubieta JA, Man A, Yurkovich M, Huang K, Sayre EC, Choi HK. Early cardiovascular disease after the diagnosis of systemic sclerosis. Am J Med 2016; 29(3):324–331. doi:10.1016/j.amjmed.2015.10.037
- D’Angelo WA, Fries JF, Masi AT, Shulman LE. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 1969; 46(3):428–440. doi:10.1016/0002-9343(69)90044-8
- Ngian GS, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Khurma V, Meyer C, Park GS, et al. A pilot study of subclinical coronary atherosclerosis in systemic sclerosis: coronary artery calcification in cases and controls. Arthritis Rheum 2008; 59(4):591–597. doi:10.1002/art.23540
- Lippi G, Caramaschi P, Montagnana M, Salvagno GL, Volpe A, Guidi G. Lipoprotein[a] and the lipid profile in patients with systemic sclerosis. Clin Chim Acta 2006; 364(1–2):345–348. doi:10.1016/j.cca.2005.07.015
- Palinski W, Hörkkö S, Miller E, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest 1996; 98(3):800–814. doi:10.1172/JCI118853
- Ho M, Veale D, Eastmond C, Nuki G, Belch J. Macrovascular disease and systemic sclerosis. Ann Rheum Dis 2000; 59(1):39–43. doi:10.1136/ard.59.1.39
- Kaloudi O, Basta G, Perfetto F, et al. Circulating levels of Ne-(carboxymethyl)lysine are increased in systemic sclerosis. Rheumatology (Oxford) 2007; 46(3):412–416. doi:10.1093/rheumatology/kel076
- Muro Y, Sugiura K, Morita Y, Tomita Y. An evaluation of the efficacy of the toe brachial index measuring vascular involvement in systemic sclerosis and other connective tissue diseases. Clin Exp Rheumatol 2009; 27(3 suppl 54):26–31. pmid:19796558
- Cheng K-S, Tiwari A, Boutin A, et al. Differentiation of primary and secondary Raynaud’s disease by carotid arterial stiffness. Eur J Vasc Endovasc Surg 2003; 25(4):336–341. doi:10.1053/ejvs.2002.1845
- Kawasaki M, Ito Y, Yokoyama H, et al. Assessment of arterial medial characteristics in human carotid arteries using integrated backscatter ultrasound and its histological implications. Atherosclerosis 2005; 180(1):145–154. doi:10.1016/j.atherosclerosis.2004.11.018
- Szucs G, Tímár O, Szekanecz Z, et al. Endothelial dysfunction precedes atherosclerosis in systemic sclerosis—relevance for prevention of vascular complications. Rheumatology (Oxford) 2007; 46(5):759–762. doi:10.1093/rheumatology/kel426
- Hettema ME, Zhang D, de Leeuw K, et al. Early atherosclerosis in systemic sclerosis and its relation to disease or traditional risk factors. Arthritis Res Ther 2008;10(2):R49. doi:10.1186/ar2408
- Roustit M, Simmons GH, Baguet JP, Carpentier P, Cracowski JL. Discrepancy between simultaneous digital skin microvascular and brachial artery macrovascular post-occlusive hyperemia in systemic sclerosis. J Rheumatol 2008; 35(8):1576–1583. pmid:18597404
- Vettori S, Maresca L, Cuomo G, Abbadessa S, Leonardo G, Valentini G. Clinical and subclinical atherosclerosis in systemic sclerosis: consequences of previous corticosteroid treatment. Scand J Rheumatol 2010; 39(6):485–489. doi:10.3109/03009741003781985
- Lekakis J, Mavrikakis M, Papamichael C, et al. Short-term estrogen administration improves abnormal endothelial function in women with systemic sclerosis and Raynaud’s phenomenon. Am Heart J 1998; 136(5):905–912. doi:10.1016/s0002-8703(98)70137-1
- Bartoli F, Blagojevic J, Bacci M, et al. Flow-mediated vasodilation and carotid intima-media thickness in systemic sclerosis. Ann N Y Acad Sci 2007; 1108:283–290. doi:10.1196/annals.1422.030
- Rollando D, Bezante GP, Sulli A, et al. Brachial artery endothelial-dependent flow-mediated dilation identifies early-stage endothelial dysfunction in systemic sclerosis and correlates with nailfold microvascular impairment. J Rheumatol 2010; 37(6):1168–1173. doi:10.3899/jrheum.091116
- Andersen GN, Mincheva-Nilsson L, Kazzam E, et al. Assessment of vascular function in systemic sclerosis: indications of the development of nitrate tolerance as a result of enhanced endothelial nitric oxide production. Arthritis Rheum 2002; 46(5):1324–1332. doi:10.1002/art.10191
- Au K, Singh MK, Bodukam V, et al. Atherosclerosis in systemic sclerosis: a systematic review and meta-analysis. Arthritis Rheum 2011; 63(7):2078–2090. doi:10.1002/art.30380
- van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: a meta-analysis. Semin Arthritis Rheum 2011; 40(5):389–397. doi:10.1016/j.semarthrit.2010.06.006
- Brohall G, Odén A, Fagerberg B. Carotid artery intima-media thickness in patients with type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med 2006; 23(6):609–616. doi:10.1111/j.1464-5491.2005.01725.x
- Masoura C, Pitsavos C, Aznaouridis K, Skoumas I, Vlachopoulos C, Stefanadis C. Arterial endothelial function and wall thickness in familial hypercholesterolemia and familial combined hyperlipidemia and the effect of statins. A systematic review and meta-analysis. Atherosclerosis 2011; 214(1):129–138. doi:10.1016/j.atherosclerosis.2010.10.008
- Ozen G, Inanc N, Unal AU, et al. Subclinical atherosclerosis in systemic sclerosis: not less frequent than rheumatoid arthritis and not detected with cardiovascular risk indices. Arthritis Care Res (Hoboken) 2016; 68(10):1538–1546. doi:10.1002/acr.22852
- Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 2010; 26(6):631–640. doi:10.1007/s10554-010-9616-1
- Meune C, Avouac J, Wahbi K, et al. Cardiac involvement in systemic sclerosis assessed by tissue-doppler echocardiography during routine care: a controlled study of 100 consecutive patients. Arthritis Rheum 2008; 58(6):1803–1809. doi:10.1002/art.23463
- Tennøe AH, Murbræch K, Andreassen JC, et al. Left ventricular diastolic dysfunction predicts mortality in patients with systemic sclerosis. J Am Coll Cardiol 2018; 72(15):1804–1813. doi:10.1016/j.jacc.2018.07.068
- de Groote P, Gressin V, Hachulla E, et al; ItinerAIR-Scleroderma Investigators. Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann Rheum Dis 2008; 67(1):31–36. doi:10.1136/ard.2006.057760
- Allanore Y, Meune C, Vonk MC, et al; EUSTAR co-authors. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis 2010; 69(1):218–221. doi:10.1136/ard.2008.103382
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Assassi S, Del Junco D, Sutter K, et al. Clinical and genetic factors predictive of mortality in early systemic sclerosis. Arthritis Rheum 2009; 61(10):1403–1411. doi:10.1002/art.24734
- Rokas S, Mavrikakis M, Agrios N, Mylonas D, Antoniadou L, Moulopoulos S. Electrophysiologic abnormalities of cardiac function in progressive systemic sclerosis. J Electrocardiol 1996; 29(1):17–25. pmid:8808521
- Kostis JB, Seibold JR, Turkevich D, et al. Prognostic importance of cardiac arrhythmias in systemic sclerosis. Am J Med 1988; 84(6):1007–1015. doi:10.1016/0002-9343(88)90305-1
- Biełous-Wilk A, Poreba M, Staniszewska-Marszałek E, et al. Electrocardiographic evaluation in patients with systemic scleroderma and without clinically evident heart disease. Ann Noninvasive Electrocardiol 2009; 14(3):251–257. doi:10.1111/j.1542-474X.2009.00306.x
- Bienias P, Ciurzynski M, Glinska-Wielochowska M, et al. Heart rate turbulence assessment in systemic sclerosis: the role for the detection of cardiac autonomic nervous system dysfunction. Rheumatology (Oxford) 2010; 49(2):355–360. doi:10.1093/rheumatology/kep394
- Ferri C, Bernini L, Bongiorni MG, et al. Noninvasive evaluation of cardiac dysrhythmias, and their relationship with multisystemic symptoms, in progressive systemic sclerosis patients. Arthritis Rheum 1985; 28(11):1259–1266. pmid:4063000
- Roberts NK, Cabeen WR, Moss J, Clements PJ, Furst DE. The prevalence of conduction defects and cardiac arrhythmias in progressive systemic sclerosis. Ann Intern Med 1981; 94(1):38–40. doi:10.7326/0003-4819-94-1-38
- Wang Q, Shang Y, Li S, Wu Y, Wang C, Yan X. Complete heart block in systemic sclerosis: a case report and literature review. Medicine (Baltimore) 2018; 97(46):e13226. doi:10.1097/MD.0000000000013226
- Summerfield BJ. Progressive systemic sclerosis with complete heart block. Br Heart J 1975; 37(12):1308–1310. doi:10.1136/hrt.37.12.1308
- Moyssakis I, Papadopoulos DP, Tzioufas AG, Votteas V. Complete heart block in a patient with systemic sclerosis. Clin Rheumatol 2006; 25(4):551–552. doi:10.1007/s10067-005-0068-2
- Ridolfi RL, Bulkley BH, Hutchins GM. The cardiac conduction system in progressive systemic sclerosis. Clinical and pathologic features of 35 patients. Am J Med 1976; 61(3):361–366. doi:10.1016/0002-9343(76)90373-9
- Champion HC. The heart in scleroderma. Rheum Dis Clin North Am 2008; 34(1):181–190. doi:10.1016/j.rdc.2007.12.002
- Gowda RM, Khan IA, Sacchi TJ, Vasavada BC. Scleroderma pericardial disease presented with a large pericardial effusion—a case report. Angiology 2001; 52(1):59–62. doi:10.1177/000331970105200108
- Meier FMP, Frommer KW, Dinser R, et al; EUSTAR Co-authors. Update on the profile of the EUSTAR cohort: an analysis of the EULAR scleroderma trials and research group database. Ann Rheum Dis 2012; 71(8):1355–1360. doi:10.1136/annrheumdis-2011-200742
- Subramanian SR, Akram R, Velayati A, Chadow H. New development of cardiac tamponade on underlying effusive-constrictive pericarditis: an uncommon initial presentation of scleroderma. BMJ Case Rep 2013; 2013. doi:10.1136/bcr-2013-010254
- Kitchongcharoenying P, Foocharoen C, Mahakkanukrauh A, Suwannaroj S, Nanagara R. Pericardial fluid profiles of pericardial effusion in systemic sclerosis patients. Asian Pac J Allergy Immunol 2013; 31(4):314–319. doi:10.12932/AP0305.31.4.2013
- McWhorter JE, LeRoy EC. Pericardial disease in scleroderma (systemic sclerosis). Am J Med 1974; 57(4):566–575. doi:10.1016/0002-9343(74)90008-4
- Comens SM, Alpert MA, Sharp GC, et al. Frequency of mitral valve prolapse in systemic lupus erythematosus, progressive systemic sclerosis and mixed connective tissue disease. Am J Cardiol 1989; 63(5):369–370. doi:10.1016/0002-9149(89)90351-2
- Candell-Riera J, Armadans-Gil L, Simeón CP, et al. Comprehensive noninvasive assessment of cardiac involvement in limited systemic sclerosis. Arthritis Rheum 1996; 39(7):1138–1145. pmid:8670322
- Caforio ALP, Adler Y, Agostini C, et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J 2017; 38(35):2649–2662. doi:10.1093/eurheartj/ehx321
- Mavrogeni S, Karabela G, Koutsogeorgopoulou L, et al. Pseudo-infarction pattern in diffuse systemic sclerosis. Evaluation using cardiovascular magnetic resonance. Int J Cardiol 2016; 214:465–468. doi:10.1016/j.ijcard.2016.03.235
- Ladak K, Pope JE. A review of the effects of statins in systemic sclerosis. Semin Arthritis Rheum 2016; 45(6):698–705. doi:10.1016/j.semarthrit.2015.10.013
- Abou-Raya A, Abou-Raya S, Helmii M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J Rheumatol 2008; 35(9):1801–1808. pmid:18709692
- ASCEND Study Collaborative Group; Bowman L, Mafham M, Wallendszus K, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018; 379(16):1529–1539. doi:10.1056/NEJMoa1804988
- Guerra F, Stronati G, Fischietti C, et al. Global longitudinal strain measured by speckle tracking identifies subclinical heart involvement in patients with systemic sclerosis. Eur J Prev Cardiol 2018; 25(15):1598–1606. doi:10.1177/2047487318786315
- Mavrogeni SI, Sfikakis PP, Dimitroulas T, et al. Prospects of using cardiovascular magnetic resonance in the identification of arrhythmogenic substrate in autoimmune rheumatic diseases. Rheumatol Int 2018; 38(9):1615–1621. doi:10.1007/s00296-018-4110-5
- Mavrogeni SI, Sfikakis PP, Markousis-Mavrogenis G, et al. Cardiovascular magnetic resonance imaging pattern in patients with autoimmune rheumatic diseases and ventricular tachycardia with preserved ejection fraction. Int J Cardiol 2019; 284:105–109. doi:10.1016/j.ijcard.2018.10.067
Autoimmune rheumatic diseases increase the risk of cardiovascular disease. In rheumatoid arthritis and systemic lupus erythematosus, the risk is driven primarily by the inflammatory milieu, leading to accelerated coronary and cerebrovascular atherosclerosis independent of traditional atherosclerotic risk factors.1–3 The extent of cardiovascular involvement in other rheumatologic diseases has been less well characterized but is an area of growing interest.
In this review, we focus on the cardiovascular complications of systemic sclerosis and review recommendations for monitoring these patients in clinical practice.
SYSTEMIC SCLEROSIS, AN AUTOIMMUNE RHEUMATIC DISEASE
Systemic sclerosis is an autoimmune rheumatic disease characterized by excessive extracellular matrix deposition leading to diffuse fibrosis, endothelial dysfunction, and microvascular injury. It is most common in North America, Southern Europe, and Australia,4,5 and it affects women more than men in ratios ranging from 3:1 to 14:1.6 The mean age at diagnosis is around 50.
The disease can affect the lungs (interstitial lung disease and pulmonary hypertension), the heart, the kidneys, and the gastrointestinal tract.
Systemic sclerosis has 2 main subtypes: limited cutaneous systemic sclerosis, formerly called CREST syndrome) and diffuse cutaneous systemic sclerosis. The limited cutaneous subtype is characterized by tightening of the skin of the distal extremities (below the elbows and knees) and face, while diffuse cutaneous systemic sclerosis can manifest as more extensive skin tightening also involving proximal extremities and the trunk. Both subtypes can have an effect on the cardiovascular system.
Some cardiovascular risk factors such as dyslipidemia, diabetes mellitus, and high body mass index are less common in patients with systemic sclerosis than in patients with rheumatoid arthritis, while the rates of arterial hypertension, smoking, chronic obstructive pulmonary disease, osteoporosis, and neoplasms are similar between the 2 groups.7
HEART INVOLVEMENT HAS SERIOUS CONSEQUENCES
Overt cardiac involvement in systemic sclerosis is associated with a mortality rate of up to 70% over 5 years,8,9 and about one-fourth of deaths in patients with systemic sclerosis are from cardiac causes.10,11 Studies in Europe10,12 showed that many patients with systemic sclerosis have cardiac involvement detectable by magnetic resonance imaging even if they do not have clinical disease. Pulmonary arterial hypertension (PAH) is a complication of both subtypes of systemic sclerosis and portends a higher risk of death.8
Thus, it is critical for clinicians to understand the potential comorbid conditions associated with systemic sclerosis, particularly the cardiovascular ones, and to work closely with cardiologists to help optimize the evaluation and management.
MECHANISMS OF CARDIAC DISEASE IN SYSTEMIC SCLEROSIS
Abnormal vasoreactivity, a consequence of an imbalance between endothelium-derived vasoconstrictors and vasodilators, defective angiogenesis, and endothelial injury, leads to tissue ischemia and vascular endothelial growth factor expression, which initiates injury and fibrosis in the myocardium and in other organs.14–17 Fibrosis involves the myocardium, pericardium, and conduction system.13,18
Myocardial involvement in systemic sclerosis is thought to be due mainly to abnormal vasoreactivity and microvascular abnormalities such as transient coronary artery spasm leading to repeated focal ischemia.19,20 Abnormal vasoreactivity has been demonstrated during cardiac catheterization21: while mean coronary sinus blood flow in systemic sclerosis patients was normal at rest, vasodilator reserve was significantly reduced in patients with diffuse cutaneous systemic sclerosis after maximal vasodilation with dipyridamole. Additionally, endomyocardial biopsy showed fibrosis and concentric intimal hypertrophy with normal epicardial coronary arteries.21
More research into other mechanisms of cardiovascular disease in systemic sclerosis is needed to allow for better preventive care for these patients.
PULMONARY ARTERIAL HYPERTENSION
Systemic sclerosis can be associated with World Health Organization (WHO) groups 1, 2, 3, and 4 pulmonary hypertension. WHO group 1, called pulmonary arterial hypertension or PAH, is one of the most common cardiac complications of systemic sclerosis, with a reported prevalence as high as 12%.22 Systemic sclerosis-associated PAH carries a high mortality rate, with a mean survival of only 3 years.23
With advances in treatments for other complications of systemic sclerosis, the percentage of systemic sclerosis patients who die of PAH has increased from 6% to 33%.24
Compared with patients with idiopathic PAH, those with systemic sclerosis get less of a response from therapy and have poorer outcomes despite lower mean pulmonary artery pressures and similar reductions in cardiac index. However, recent studies have suggested that with aggressive treatment, patients with systemic sclerosis-related PAH can achieve outcomes similar to those with idiopathic PAH.25 Thus, recognizing this condition early is imperative.
Pulmonary arterial hypertension defined
PAH is defined as the combination of all of the following26:
- Mean pulmonary artery pressure > 20 mm Hg at rest
- Normal pulmonary capillary wedge pressure (≤ 15 mm Hg)
- Pulmonary vascular resistance ≥ 3 Wood units on right heart catheterization.
Other causes of pulmonary hypertension such as interstitial lung disease, chronic pulmonary thromboembolic disease, and left heart disease must be excluded.24,27
Remodeling in the pulmonary arteries
The events that lead to PAH in systemic sclerosis remain unclear but are believed to involve initial inflammation or endothelial injury that leads to a dysequilibrium between proliferative mediators and antiproliferative vasodilators. This dysequilibrium, along with endothelial dysfunction, causes an obliterative vasculopathy in the pulmonary artery branches and arterioles. Sympathetic overactivity, hypoxemia, and ischemia-reperfusion injury additionally promote vascular proliferation, fibrosis, and remodeling, leading to increased pulmonary vascular resistance, PAH, and increased right ventricular pressures.23,27
The subtype of systemic sclerosis is an important factor in the development and progression of PAH. PAH appears to be the major cause of death in limited cutaneous systemic sclerosis, while interstitial lung disease is the major cause of death in diffuse cutaneous systemic sclerosis.28
Pulmonary arterial hypertension is a late complication of systemic sclerosis
Data from the South Australian Scleroderma Registry29 revealed that PAH tends to be a late complication of systemic sclerosis, occurring around 20 years after disease onset. In this study of 608 patients, no patient with diffuse cutaneous systemic sclerosis developed PAH.
Systemic sclerosis-related PAH initially follows an indolent course with few symptoms until right ventricular function deteriorates. Early in the disease, patients may experience nonspecific symptoms of fatigue, lightheadedness, and dyspnea on exertion.23 As it progresses, they tend to have worsening dyspnea and may experience exertional syncope, palpitations, and chest pain.
Physical findings may suggest elevated right ventricular pressure and right ventricular failure; these include a loud P2, a prominent jugular a wave, a tricuspid regurgitant murmur, jugular venous distention, and lower-extremity edema.27
Screening for pulmonary arterial hypertension in systemic sclerosis
Significant signs and symptoms usually occur late in the disease; thus, it is important to appropriately screen patients who are at risk so that they can begin aggressive treatment.
Doppler echocardiography is recommended by European and American guidelines to screen for PAH in patients who have systemic sclerosis, and most agree that screening is appropriate even if the patient has no symptoms.30 European consensus documents recommend that transthoracic echocardiography be done annually for the first 5 years of disease and be continued every year in patients at high risk, ie, those with anticentromere antibodies, anti-Th/To antibodies, or interstitial lung disease. Patients not at high risk of developing pulmonary hypertension should also have regular transthoracic echocardiography, though the exact timing is not defined.31 While American societies have not issued corresponding recommendations, many experts follow the European recommendations.
Worrisome features on echocardiography in asymptomatic patients should be followed up with right heart catheterization to assess mean right ventricular pressure. These include:
- Estimated right ventricular systolic pressure ≥ 40 mm Hg
- Tricuspid regurgitant jet velocity > 2.8 m/s
- Right atrial enlargement > 53 mm
- Right ventricular enlargement (mid-cavity dimension > 35 mm).32
Although echocardiography is the most common form of screening, it gives only an estimate of right ventricular systolic pressure, which is imprecise. Other noninvasive markers are helpful and necessary to appropriately screen this population.
Diffusion capacity. The Itinerair study33 found that a diffusing capacity for carbon monoxide (DLCO) of 60% or higher has a high specificity in excluding PAH.
Uric acid has been found to be elevated in patients with systemic sclerosis-related PAH, and levels inversely correlate with 6-minute walking distance.34
Other predictors. N-terminal pro-B-type natriuretic peptide (NT-proBNP), left atrial volume, and the right ventricular myocardial performance index have also been shown to be independent predictors of PAH in patients with systemic sclerosis.35
An algorithm. The DETECT study36 enrolled patients at increased risk who had had systemic sclerosis longer than 3 years and a DLCO less than 60%. The investigators developed a 2-step algorithm to determine which patients should be referred for right heart catheterization to try to detect PAH earlier while minimizing the number of missed diagnoses and optimizing the use of invasive diagnostic right heart catheterization.
The first step was to assess serum values of anticentromere antibodies, NT-proBNP, and urate, and clinical features (telangiectasias), forced vital capacity, and electrocardiographic changes of right axis deviation to derive a prediction score. The second step was to assess surface echocardiographic features of the right atrial area and tricuspid regurgitation velocity.
This approach led to right heart catheterization in 62% of patients and was associated with a false-negative rate of 4%. Importantly, of the patients with PAH, 1 in 5 had no symptoms, and 33% had tricuspid regurgitation velocity less than 2.8 m/s. No single measurement performed well in isolation in this study.37
Thus, we recommend that, in addition to routine surface echocardiography, a multimodal approach be used that includes laboratory testing, clinical features, and electrocardiographic findings when screening this high-risk patient population.
ATHEROSCLEROTIC DISEASES
Although macrovascular disease has not typically been regarded as a significant systemic feature in systemic sclerosis, myocardial infarction and stroke are more common in patients with systemic sclerosis than in controls.38,39
Coronary artery disease in systemic sclerosis
Man et al38 reported that the incidence of myocardial infarction in patients with systemic sclerosis was 4.4 per 1,000 persons per year, and the incidence of stroke was 4.8 per 1,000 persons per year, compared with 2.5 per 1,000 persons per year for both myocardial infarction and stroke in healthy controls matched for age, sex, and time of entry.
The Australian Scleroderma Cohort Study39 found a 3-fold higher prevalence of coronary artery disease in systemic sclerosis patients than in controls after factoring in traditional risk factors.
Aviña-Zubieta et al,40 in a cohort of 1,239 systemic sclerosis patients, estimated a hazard ratio (HR) of 3.49 for myocardial infarction and 2.35 for stroke compared with age- and sex-matched controls. Not all of these events were related to macrovascular atherosclerosis—vasospasm and microvascular ischemia may have played significant roles in the etiology of clinical manifestations.
Studies of coronary atherosclerosis in systemic sclerosis are limited. An autopsy study41 of 58 patients with systemic sclerosis and 58 controls matched for age, sex, and ethnicity found that the prevalence of atherosclerosis of small coronary arteries and arterioles was significantly higher in systemic sclerosis patients than in controls (17% vs 2%, P < .01). However, the prevalence of medium-vessel coronary atherosclerosis was similar (48% vs 43%).
Why patients with systemic sclerosis develop atherosclerosis has not yet been determined. Traditional risk factors such as hypertension, dyslipidemia, diabetes mellitus, and obesity are typically no more prevalent in systemic sclerosis patients than in controls,38,42 and thus do not explain the increased risk of atherosclerotic cardiovascular disease. There is some evidence that novel markers of atherosclerotic risk such as homocysteine,43 lipoprotein[a],44 and oxidized low-density lipoprotein45 are more prevalent in systemic sclerosis, but these results have not been substantiated in more extensive studies.
Peripheral artery disease
It remains unclear whether peripheral artery disease is more prevalent in systemic sclerosis patients than in controls.
Individual studies have shown mixed results in comparing carotid artery stenosis between systemic sclerosis patients and controls using carotid duplex ultrasonography,46 the ankle-brachial index,46–48 carotid intima-media thickness,49–54 and brachial flow-mediated dilation.51,53,55–58 A meta-analysis found that the carotid intima and media are significantly thicker in systemic sclerosis patients than in controls,59 and the magnitude of difference is similar to that in other groups at increased cardiovascular risk, such as those with rheumatoid arthritis, diabetes, and familial hypercholesterolemia.60–63
A meta-analysis of brachial artery findings showed significantly lower flow-mediated dilation in systemic sclerosis patients than in controls.64
Overall, given the inconsistency of study results, systemic sclerosis patients should be screened and managed as in other patients with peripheral artery disease, but the clinician should be aware that there may be a higher risk of peripheral artery disease in these patients.
RIGHT AND LEFT VENTRICULAR DYSFUNCTION
Many patients with systemic sclerosis have right ventricular dysfunction as a consequence of PAH.65 It is important to detect diastolic dysfunction in this population, as it may be an even stronger predictor of death than pulmonary hypertension on right heart catheterization (HR 3.7 vs 2.0).66
Fewer patients have left ventricular dysfunction. In a multicenter study of 570 systemic sclerosis patients, only 1.4% had left ventricular systolic dysfunction on echocardiography, though 22.6% had left ventricular hypertrophy and 17.7% had left ventricular diastolic dysfunction.67 In the European League Against Rheumatism (EULAR) database, the prevalence of reduced left ventricular ejection fraction was 5.4%.68
Though traditional echocardiographic screening suggests the prevalence of left ventricular dysfunction in systemic sclerosis patients is low, cardiac magnetic resonance imaging (MRI) may be more sensitive than echocardiography for detecting subclinical myocardial involvement. Cardiac MRI has been shown to detect evidence of myocardial pathology (increased T2 signal, left ventricular thinning, pericardial effusion, reduced left ventricular and right ventricular ejection fraction, left ventricular diastolic dysfunction, and delayed myocardial contrast enhancement) in up to 75% of systemic sclerosis cases studied.69
Patients with systemic sclerosis should already be undergoing echocardiography every year to screen for PAH, and screening should also include tissue Doppler imaging to detect various forms of left and right ventricular systolic and diastolic dysfunction that may not be clinically apparent.
Though cardiac MRI can provide useful additional information, it is not currently recommended for routine screening in patients with systemic sclerosis.
ARRHYTHMIAS AND CONDUCTION DEFECTS
Patients with systemic sclerosis are prone to arrhythmias due to both conduction system fibrosis and myocardial damage.
Arrhythmias accounted for 6% of the deaths in the EULAR Scleroderma Trials and Research (EUSTAR) database.11
In the Genetics Versus Environment in Scleroderma Outcome Study (GENISOS),70 250 patients who had had systemic sclerosis for at least 3 years were studied during a period of approximately 6 years, during which there were 52 deaths, 29 of which were directly attributable to systemic sclerosis. Multivariable Cox modeling showed that 7 variables predicted mortality:
- Body mass index < 18.5 kg/m2
- Age ≥ 65
- Forced vital capacity < 50% predicted
- Systolic blood pressure ≥ 140 or diastolic blood pressure ≥ 90 mm Hg
- Pulmonary fibrosis
- Positive anticentromere antibodies
- Cardiac arrhythmias.
The hazard ratio for death in patients with arrhythmias in this model was 2.18 (95% CI 1.05–4.50, P = .035). Thus, finding arrhythmias in systemic sclerosis patients can provide important prognostic information.
While resting electrocardiography in patients with systemic sclerosis most commonly shows sinus rhythm, 24-hour electrocardiographic monitoring has revealed nonsustained supraventricular and ventricular arrhythmias in a significant percentage.71,72 Although difficult to quantify in routine practice, parameters controlled by the autonomic nervous system including heart rate variability and heart rate turbulence have been shown to be impaired in systemic sclerosis, and these measures are associated with an increased risk of malignant arrhythmias and sudden cardiac death.73,74
Conduction abnormalities
Conduction abnormalities occur in one-fifth to one-third of patients with systemic sclerosis.75,76 The most common abnormal conduction finding is left bundle branch block, followed by first-degree atrioventricular block. High-degree atrioventricular block is uncommon,76 though a few case reports of complete heart block thought to be related to systemic sclerosis have been published.77–79 An autopsy study showed that the conduction system is relatively spared from myocardial changes seen in systemic sclerosis patients, and thus it is speculated that the conduction disturbances are a consequence of damaged myocardium rather than damage to conduction tissue.80
Given the array of electrophysiologic abnormalities that systemic sclerosis patients can have, it is critical to monitor all patients with routine (annual or biannual) electrocardiography; to take possible arrhythmia-related symptoms seriously; and to evaluate them with further workup such as Holter monitoring for 24 hours or even longer, event monitoring, exercise testing, or tilt-table testing.
PERICARDIAL DISEASE
Pericardial disease is clinically apparent in 5% to 16% of patients with systemic sclerosis81; patients with limited cutaneous systemic sclerosis have more pericardial disease than those with diffuse cutaneous systemic sclerosis (30% vs 16%).82 Forty-one percent of systemic sclerosis patients have been shown to have pericardial effusion by echocardiography,81 but the effusions are typically small and rarely cause tamponade, though tamponade is associated with a poor prognosis.
Large pericardial effusions can develop before skin thickening and diagnosis of systemic sclerosis.81,83,84 Thus, systemic sclerosis should be considered in patients with pericardial effusions of unknown etiology.
In a small study,85 the pericardial fluid in systemic sclerosis was typically exudative, with lactate dehydrogenase greater than 200 U/L, a fluid-serum lactate dehydrogenase ratio greater than 0.6, and a fluid-serum total protein ratio greater than 0.5.
Pericardial effusion can be a sign of impending scleroderma renal crisis,86 and thus renal function should be carefully monitored in systemic sclerosis patients with pericardial effusion. Constrictive pericarditis and restrictive cardiomyopathy can rarely occur in systemic sclerosis and may more commonly present with symptoms.
Pericardial disease in systemic sclerosis should be treated in a standard fashion with nonsteroidal anti-inflammatory drugs. Corticosteroids are generally of limited benefit and should be avoided, especially in the setting of scleroderma renal crisis.81
VALVULAR HEART DISEASE
Based on limited studies, the prevalence of significant valvular heart disease in systemic sclerosis patients does not seem to be higher than that in the general population. While patients with systemic sclerosis and CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) have been shown to have a higher frequency of mitral valve prolapse and mild mitral regurgitation,87,88 these abnormalities do not often progress in severity, and thus their clinical significance is limited.
RECOMMENDATIONS FOR CARE OF SYSTEMIC SCLEROSIS PATIENTS
It is important for physicians caring for patients with systemic sclerosis to be aware of its most common cardiac manifestations, including left and right ventricular systolic and diastolic dysfunction, pulmonary hypertension, conduction abnormalities, arrhythmias, and cardiomyopathy.
Look for volume overload
On clinical examination, assess for clinical markers of volume overload such as distended neck veins, peripheral edema, or an abnormal blood pressure response to the Valsalva maneuver. These findings should prompt measurement of NT-proBNP,89 and may warrant prescription of a diuretic.
Electrocardiography to investigate arrhythmias
Electrocardiography should be done if patients describe symptoms of palpitations, and should also include continuous rhythm monitoring with Holter or event monitoring, depending on the frequency of symptoms. Otherwise, patients should routinely undergo electrocardiography once or twice a year.
Q waves are common in systemic sclerosis patients (especially those with diffuse cutaneous systemic sclerosis), notably in the precordial leads, and can occur without coronary artery disease.90 Symptoms such as presyncope should be further investigated with Holter monitoring and tilt-table testing.
Assess, modify traditional risk factors
Subclinical atherosclerosis as detected by carotid intima-media thickness is as common in systemic sclerosis as in rheumatoid arthritis.61 However, traditional risk indices such as SCORE (Systematic Coronary Risk Evaluation), QRISK2, and the American College of Cardiology/American Heart Association indices may underestimate risk in patients who have systemic sclerosis.
Strict hypertension control should be the goal for all systemic sclerosis patients. Though there are no specific guidelines on which antihypertensive medications are preferred, calcium channel blockers or angiotensin II receptor blockers, which are typically used to treat systemic sclerosis-related Raynaud phenomenon, may be appropriate.
Statins reduce vascular complications and are generally well tolerated in patients with systemic sclerosis.91,92
Aspirin is not recommended for routine primary prevention in view of data suggesting that its benefits in diabetic patients are counterbalanced by increased bleeding risk.93
Echocardiography to detect pulmonary arterial hypertension
At this time, guidelines for monitoring for cardiovascular manifestations in systemic sclerosis patients are limited. The only well-defined ones are European consensus guidelines, which suggest annual transthoracic echocardiography for the first 5 years after systemic sclerosis is diagnosed and continued annual screening in patients at risk of developing PAH.31
We support this strategy, with annual screening for the first 5 years followed by surveillance echocardiography every 2 to 3 years unless there is a high risk of PAH. Specific attention should be paid to right ventricular diastolic function, right atrial volume, and right ventricular myocardial performance index.
Emerging data suggest that the addition of global longitudinal strain of ventricles to routine echocardiography can help detect subclinical cardiac risk.94 Although further study is needed into the predictive value of global longitudinal strain, it is a low-cost and noninvasive addition to standard echocardiography that can help guide risk stratification, and thus we recommend that it be part of the echocardiographic examination for all systemic sclerosis patients.
Pulmonary function testing. In addition to screening for PAH with echocardiography, we recommend obtaining baseline pulmonary function tests, including DLCO, at the time systemic sclerosis is diagnosed, with repeat testing annually.
Magnetic resonance imaging
While echocardiography is the gold standard for monitoring systemic sclerosis patients, cardiovascular MRI may have a role in identifying those at higher risk of dangerous arrhythmias such as ventricular tachycardia and ventricular fibrillation. In addition to assessing ventricular function, MRI can detect myocardial inflammation, ischemia, and fibrosis that may predispose a patient to develop ventricular tachycardia or fibrillation.95 Variables such as T1/T2 mapping, extracellular volume fraction, T2 signal ratio, and early vs late gadolinium enhancement can help identify patients who had past ventricular tachycardia or fibrillation.96
Finding an increased risk of arrhythmias may prompt a conversation between the patient and the physician about the need for an implantable cardiac defibrillator.
If cardiac MRI is available and is reimbursed by the patient’s insurance carrier, physicians should strongly consider obtaining at least one baseline scan in systemic sclerosis patients to identify those at risk of highly fatal arrhythmias.
Teamwork is needed
Systemic sclerosis has not traditionally been associated with cardiovascular disease to the extent of other rheumatic conditions, but the cardiovascular system can be affected in various ways that can ultimately lead to an early death. These manifestations may be asymptomatic for long periods, and overt clinical disease portends a poorer prognosis.
Primary care physicians managing these patients should be aware of the cardiovascular complications of systemic sclerosis and should implement appropriate screening tests in conjunction with rheumatologists and cardiologists. It is also essential for general and subspecialty cardiologists to understand the broad spectrum of organ system involvement that can affect systemic sclerosis patients and to tailor their investigation and management recommendations accordingly. By designing a multidisciplinary approach to the treatment of systemic sclerosis patients, physicians can help to optimize cardiovascular risk modification in this vulnerable population.
Autoimmune rheumatic diseases increase the risk of cardiovascular disease. In rheumatoid arthritis and systemic lupus erythematosus, the risk is driven primarily by the inflammatory milieu, leading to accelerated coronary and cerebrovascular atherosclerosis independent of traditional atherosclerotic risk factors.1–3 The extent of cardiovascular involvement in other rheumatologic diseases has been less well characterized but is an area of growing interest.
In this review, we focus on the cardiovascular complications of systemic sclerosis and review recommendations for monitoring these patients in clinical practice.
SYSTEMIC SCLEROSIS, AN AUTOIMMUNE RHEUMATIC DISEASE
Systemic sclerosis is an autoimmune rheumatic disease characterized by excessive extracellular matrix deposition leading to diffuse fibrosis, endothelial dysfunction, and microvascular injury. It is most common in North America, Southern Europe, and Australia,4,5 and it affects women more than men in ratios ranging from 3:1 to 14:1.6 The mean age at diagnosis is around 50.
The disease can affect the lungs (interstitial lung disease and pulmonary hypertension), the heart, the kidneys, and the gastrointestinal tract.
Systemic sclerosis has 2 main subtypes: limited cutaneous systemic sclerosis, formerly called CREST syndrome) and diffuse cutaneous systemic sclerosis. The limited cutaneous subtype is characterized by tightening of the skin of the distal extremities (below the elbows and knees) and face, while diffuse cutaneous systemic sclerosis can manifest as more extensive skin tightening also involving proximal extremities and the trunk. Both subtypes can have an effect on the cardiovascular system.
Some cardiovascular risk factors such as dyslipidemia, diabetes mellitus, and high body mass index are less common in patients with systemic sclerosis than in patients with rheumatoid arthritis, while the rates of arterial hypertension, smoking, chronic obstructive pulmonary disease, osteoporosis, and neoplasms are similar between the 2 groups.7
HEART INVOLVEMENT HAS SERIOUS CONSEQUENCES
Overt cardiac involvement in systemic sclerosis is associated with a mortality rate of up to 70% over 5 years,8,9 and about one-fourth of deaths in patients with systemic sclerosis are from cardiac causes.10,11 Studies in Europe10,12 showed that many patients with systemic sclerosis have cardiac involvement detectable by magnetic resonance imaging even if they do not have clinical disease. Pulmonary arterial hypertension (PAH) is a complication of both subtypes of systemic sclerosis and portends a higher risk of death.8
Thus, it is critical for clinicians to understand the potential comorbid conditions associated with systemic sclerosis, particularly the cardiovascular ones, and to work closely with cardiologists to help optimize the evaluation and management.
MECHANISMS OF CARDIAC DISEASE IN SYSTEMIC SCLEROSIS
Abnormal vasoreactivity, a consequence of an imbalance between endothelium-derived vasoconstrictors and vasodilators, defective angiogenesis, and endothelial injury, leads to tissue ischemia and vascular endothelial growth factor expression, which initiates injury and fibrosis in the myocardium and in other organs.14–17 Fibrosis involves the myocardium, pericardium, and conduction system.13,18
Myocardial involvement in systemic sclerosis is thought to be due mainly to abnormal vasoreactivity and microvascular abnormalities such as transient coronary artery spasm leading to repeated focal ischemia.19,20 Abnormal vasoreactivity has been demonstrated during cardiac catheterization21: while mean coronary sinus blood flow in systemic sclerosis patients was normal at rest, vasodilator reserve was significantly reduced in patients with diffuse cutaneous systemic sclerosis after maximal vasodilation with dipyridamole. Additionally, endomyocardial biopsy showed fibrosis and concentric intimal hypertrophy with normal epicardial coronary arteries.21
More research into other mechanisms of cardiovascular disease in systemic sclerosis is needed to allow for better preventive care for these patients.
PULMONARY ARTERIAL HYPERTENSION
Systemic sclerosis can be associated with World Health Organization (WHO) groups 1, 2, 3, and 4 pulmonary hypertension. WHO group 1, called pulmonary arterial hypertension or PAH, is one of the most common cardiac complications of systemic sclerosis, with a reported prevalence as high as 12%.22 Systemic sclerosis-associated PAH carries a high mortality rate, with a mean survival of only 3 years.23
With advances in treatments for other complications of systemic sclerosis, the percentage of systemic sclerosis patients who die of PAH has increased from 6% to 33%.24
Compared with patients with idiopathic PAH, those with systemic sclerosis get less of a response from therapy and have poorer outcomes despite lower mean pulmonary artery pressures and similar reductions in cardiac index. However, recent studies have suggested that with aggressive treatment, patients with systemic sclerosis-related PAH can achieve outcomes similar to those with idiopathic PAH.25 Thus, recognizing this condition early is imperative.
Pulmonary arterial hypertension defined
PAH is defined as the combination of all of the following26:
- Mean pulmonary artery pressure > 20 mm Hg at rest
- Normal pulmonary capillary wedge pressure (≤ 15 mm Hg)
- Pulmonary vascular resistance ≥ 3 Wood units on right heart catheterization.
Other causes of pulmonary hypertension such as interstitial lung disease, chronic pulmonary thromboembolic disease, and left heart disease must be excluded.24,27
Remodeling in the pulmonary arteries
The events that lead to PAH in systemic sclerosis remain unclear but are believed to involve initial inflammation or endothelial injury that leads to a dysequilibrium between proliferative mediators and antiproliferative vasodilators. This dysequilibrium, along with endothelial dysfunction, causes an obliterative vasculopathy in the pulmonary artery branches and arterioles. Sympathetic overactivity, hypoxemia, and ischemia-reperfusion injury additionally promote vascular proliferation, fibrosis, and remodeling, leading to increased pulmonary vascular resistance, PAH, and increased right ventricular pressures.23,27
The subtype of systemic sclerosis is an important factor in the development and progression of PAH. PAH appears to be the major cause of death in limited cutaneous systemic sclerosis, while interstitial lung disease is the major cause of death in diffuse cutaneous systemic sclerosis.28
Pulmonary arterial hypertension is a late complication of systemic sclerosis
Data from the South Australian Scleroderma Registry29 revealed that PAH tends to be a late complication of systemic sclerosis, occurring around 20 years after disease onset. In this study of 608 patients, no patient with diffuse cutaneous systemic sclerosis developed PAH.
Systemic sclerosis-related PAH initially follows an indolent course with few symptoms until right ventricular function deteriorates. Early in the disease, patients may experience nonspecific symptoms of fatigue, lightheadedness, and dyspnea on exertion.23 As it progresses, they tend to have worsening dyspnea and may experience exertional syncope, palpitations, and chest pain.
Physical findings may suggest elevated right ventricular pressure and right ventricular failure; these include a loud P2, a prominent jugular a wave, a tricuspid regurgitant murmur, jugular venous distention, and lower-extremity edema.27
Screening for pulmonary arterial hypertension in systemic sclerosis
Significant signs and symptoms usually occur late in the disease; thus, it is important to appropriately screen patients who are at risk so that they can begin aggressive treatment.
Doppler echocardiography is recommended by European and American guidelines to screen for PAH in patients who have systemic sclerosis, and most agree that screening is appropriate even if the patient has no symptoms.30 European consensus documents recommend that transthoracic echocardiography be done annually for the first 5 years of disease and be continued every year in patients at high risk, ie, those with anticentromere antibodies, anti-Th/To antibodies, or interstitial lung disease. Patients not at high risk of developing pulmonary hypertension should also have regular transthoracic echocardiography, though the exact timing is not defined.31 While American societies have not issued corresponding recommendations, many experts follow the European recommendations.
Worrisome features on echocardiography in asymptomatic patients should be followed up with right heart catheterization to assess mean right ventricular pressure. These include:
- Estimated right ventricular systolic pressure ≥ 40 mm Hg
- Tricuspid regurgitant jet velocity > 2.8 m/s
- Right atrial enlargement > 53 mm
- Right ventricular enlargement (mid-cavity dimension > 35 mm).32
Although echocardiography is the most common form of screening, it gives only an estimate of right ventricular systolic pressure, which is imprecise. Other noninvasive markers are helpful and necessary to appropriately screen this population.
Diffusion capacity. The Itinerair study33 found that a diffusing capacity for carbon monoxide (DLCO) of 60% or higher has a high specificity in excluding PAH.
Uric acid has been found to be elevated in patients with systemic sclerosis-related PAH, and levels inversely correlate with 6-minute walking distance.34
Other predictors. N-terminal pro-B-type natriuretic peptide (NT-proBNP), left atrial volume, and the right ventricular myocardial performance index have also been shown to be independent predictors of PAH in patients with systemic sclerosis.35
An algorithm. The DETECT study36 enrolled patients at increased risk who had had systemic sclerosis longer than 3 years and a DLCO less than 60%. The investigators developed a 2-step algorithm to determine which patients should be referred for right heart catheterization to try to detect PAH earlier while minimizing the number of missed diagnoses and optimizing the use of invasive diagnostic right heart catheterization.
The first step was to assess serum values of anticentromere antibodies, NT-proBNP, and urate, and clinical features (telangiectasias), forced vital capacity, and electrocardiographic changes of right axis deviation to derive a prediction score. The second step was to assess surface echocardiographic features of the right atrial area and tricuspid regurgitation velocity.
This approach led to right heart catheterization in 62% of patients and was associated with a false-negative rate of 4%. Importantly, of the patients with PAH, 1 in 5 had no symptoms, and 33% had tricuspid regurgitation velocity less than 2.8 m/s. No single measurement performed well in isolation in this study.37
Thus, we recommend that, in addition to routine surface echocardiography, a multimodal approach be used that includes laboratory testing, clinical features, and electrocardiographic findings when screening this high-risk patient population.
ATHEROSCLEROTIC DISEASES
Although macrovascular disease has not typically been regarded as a significant systemic feature in systemic sclerosis, myocardial infarction and stroke are more common in patients with systemic sclerosis than in controls.38,39
Coronary artery disease in systemic sclerosis
Man et al38 reported that the incidence of myocardial infarction in patients with systemic sclerosis was 4.4 per 1,000 persons per year, and the incidence of stroke was 4.8 per 1,000 persons per year, compared with 2.5 per 1,000 persons per year for both myocardial infarction and stroke in healthy controls matched for age, sex, and time of entry.
The Australian Scleroderma Cohort Study39 found a 3-fold higher prevalence of coronary artery disease in systemic sclerosis patients than in controls after factoring in traditional risk factors.
Aviña-Zubieta et al,40 in a cohort of 1,239 systemic sclerosis patients, estimated a hazard ratio (HR) of 3.49 for myocardial infarction and 2.35 for stroke compared with age- and sex-matched controls. Not all of these events were related to macrovascular atherosclerosis—vasospasm and microvascular ischemia may have played significant roles in the etiology of clinical manifestations.
Studies of coronary atherosclerosis in systemic sclerosis are limited. An autopsy study41 of 58 patients with systemic sclerosis and 58 controls matched for age, sex, and ethnicity found that the prevalence of atherosclerosis of small coronary arteries and arterioles was significantly higher in systemic sclerosis patients than in controls (17% vs 2%, P < .01). However, the prevalence of medium-vessel coronary atherosclerosis was similar (48% vs 43%).
Why patients with systemic sclerosis develop atherosclerosis has not yet been determined. Traditional risk factors such as hypertension, dyslipidemia, diabetes mellitus, and obesity are typically no more prevalent in systemic sclerosis patients than in controls,38,42 and thus do not explain the increased risk of atherosclerotic cardiovascular disease. There is some evidence that novel markers of atherosclerotic risk such as homocysteine,43 lipoprotein[a],44 and oxidized low-density lipoprotein45 are more prevalent in systemic sclerosis, but these results have not been substantiated in more extensive studies.
Peripheral artery disease
It remains unclear whether peripheral artery disease is more prevalent in systemic sclerosis patients than in controls.
Individual studies have shown mixed results in comparing carotid artery stenosis between systemic sclerosis patients and controls using carotid duplex ultrasonography,46 the ankle-brachial index,46–48 carotid intima-media thickness,49–54 and brachial flow-mediated dilation.51,53,55–58 A meta-analysis found that the carotid intima and media are significantly thicker in systemic sclerosis patients than in controls,59 and the magnitude of difference is similar to that in other groups at increased cardiovascular risk, such as those with rheumatoid arthritis, diabetes, and familial hypercholesterolemia.60–63
A meta-analysis of brachial artery findings showed significantly lower flow-mediated dilation in systemic sclerosis patients than in controls.64
Overall, given the inconsistency of study results, systemic sclerosis patients should be screened and managed as in other patients with peripheral artery disease, but the clinician should be aware that there may be a higher risk of peripheral artery disease in these patients.
RIGHT AND LEFT VENTRICULAR DYSFUNCTION
Many patients with systemic sclerosis have right ventricular dysfunction as a consequence of PAH.65 It is important to detect diastolic dysfunction in this population, as it may be an even stronger predictor of death than pulmonary hypertension on right heart catheterization (HR 3.7 vs 2.0).66
Fewer patients have left ventricular dysfunction. In a multicenter study of 570 systemic sclerosis patients, only 1.4% had left ventricular systolic dysfunction on echocardiography, though 22.6% had left ventricular hypertrophy and 17.7% had left ventricular diastolic dysfunction.67 In the European League Against Rheumatism (EULAR) database, the prevalence of reduced left ventricular ejection fraction was 5.4%.68
Though traditional echocardiographic screening suggests the prevalence of left ventricular dysfunction in systemic sclerosis patients is low, cardiac magnetic resonance imaging (MRI) may be more sensitive than echocardiography for detecting subclinical myocardial involvement. Cardiac MRI has been shown to detect evidence of myocardial pathology (increased T2 signal, left ventricular thinning, pericardial effusion, reduced left ventricular and right ventricular ejection fraction, left ventricular diastolic dysfunction, and delayed myocardial contrast enhancement) in up to 75% of systemic sclerosis cases studied.69
Patients with systemic sclerosis should already be undergoing echocardiography every year to screen for PAH, and screening should also include tissue Doppler imaging to detect various forms of left and right ventricular systolic and diastolic dysfunction that may not be clinically apparent.
Though cardiac MRI can provide useful additional information, it is not currently recommended for routine screening in patients with systemic sclerosis.
ARRHYTHMIAS AND CONDUCTION DEFECTS
Patients with systemic sclerosis are prone to arrhythmias due to both conduction system fibrosis and myocardial damage.
Arrhythmias accounted for 6% of the deaths in the EULAR Scleroderma Trials and Research (EUSTAR) database.11
In the Genetics Versus Environment in Scleroderma Outcome Study (GENISOS),70 250 patients who had had systemic sclerosis for at least 3 years were studied during a period of approximately 6 years, during which there were 52 deaths, 29 of which were directly attributable to systemic sclerosis. Multivariable Cox modeling showed that 7 variables predicted mortality:
- Body mass index < 18.5 kg/m2
- Age ≥ 65
- Forced vital capacity < 50% predicted
- Systolic blood pressure ≥ 140 or diastolic blood pressure ≥ 90 mm Hg
- Pulmonary fibrosis
- Positive anticentromere antibodies
- Cardiac arrhythmias.
The hazard ratio for death in patients with arrhythmias in this model was 2.18 (95% CI 1.05–4.50, P = .035). Thus, finding arrhythmias in systemic sclerosis patients can provide important prognostic information.
While resting electrocardiography in patients with systemic sclerosis most commonly shows sinus rhythm, 24-hour electrocardiographic monitoring has revealed nonsustained supraventricular and ventricular arrhythmias in a significant percentage.71,72 Although difficult to quantify in routine practice, parameters controlled by the autonomic nervous system including heart rate variability and heart rate turbulence have been shown to be impaired in systemic sclerosis, and these measures are associated with an increased risk of malignant arrhythmias and sudden cardiac death.73,74
Conduction abnormalities
Conduction abnormalities occur in one-fifth to one-third of patients with systemic sclerosis.75,76 The most common abnormal conduction finding is left bundle branch block, followed by first-degree atrioventricular block. High-degree atrioventricular block is uncommon,76 though a few case reports of complete heart block thought to be related to systemic sclerosis have been published.77–79 An autopsy study showed that the conduction system is relatively spared from myocardial changes seen in systemic sclerosis patients, and thus it is speculated that the conduction disturbances are a consequence of damaged myocardium rather than damage to conduction tissue.80
Given the array of electrophysiologic abnormalities that systemic sclerosis patients can have, it is critical to monitor all patients with routine (annual or biannual) electrocardiography; to take possible arrhythmia-related symptoms seriously; and to evaluate them with further workup such as Holter monitoring for 24 hours or even longer, event monitoring, exercise testing, or tilt-table testing.
PERICARDIAL DISEASE
Pericardial disease is clinically apparent in 5% to 16% of patients with systemic sclerosis81; patients with limited cutaneous systemic sclerosis have more pericardial disease than those with diffuse cutaneous systemic sclerosis (30% vs 16%).82 Forty-one percent of systemic sclerosis patients have been shown to have pericardial effusion by echocardiography,81 but the effusions are typically small and rarely cause tamponade, though tamponade is associated with a poor prognosis.
Large pericardial effusions can develop before skin thickening and diagnosis of systemic sclerosis.81,83,84 Thus, systemic sclerosis should be considered in patients with pericardial effusions of unknown etiology.
In a small study,85 the pericardial fluid in systemic sclerosis was typically exudative, with lactate dehydrogenase greater than 200 U/L, a fluid-serum lactate dehydrogenase ratio greater than 0.6, and a fluid-serum total protein ratio greater than 0.5.
Pericardial effusion can be a sign of impending scleroderma renal crisis,86 and thus renal function should be carefully monitored in systemic sclerosis patients with pericardial effusion. Constrictive pericarditis and restrictive cardiomyopathy can rarely occur in systemic sclerosis and may more commonly present with symptoms.
Pericardial disease in systemic sclerosis should be treated in a standard fashion with nonsteroidal anti-inflammatory drugs. Corticosteroids are generally of limited benefit and should be avoided, especially in the setting of scleroderma renal crisis.81
VALVULAR HEART DISEASE
Based on limited studies, the prevalence of significant valvular heart disease in systemic sclerosis patients does not seem to be higher than that in the general population. While patients with systemic sclerosis and CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) have been shown to have a higher frequency of mitral valve prolapse and mild mitral regurgitation,87,88 these abnormalities do not often progress in severity, and thus their clinical significance is limited.
RECOMMENDATIONS FOR CARE OF SYSTEMIC SCLEROSIS PATIENTS
It is important for physicians caring for patients with systemic sclerosis to be aware of its most common cardiac manifestations, including left and right ventricular systolic and diastolic dysfunction, pulmonary hypertension, conduction abnormalities, arrhythmias, and cardiomyopathy.
Look for volume overload
On clinical examination, assess for clinical markers of volume overload such as distended neck veins, peripheral edema, or an abnormal blood pressure response to the Valsalva maneuver. These findings should prompt measurement of NT-proBNP,89 and may warrant prescription of a diuretic.
Electrocardiography to investigate arrhythmias
Electrocardiography should be done if patients describe symptoms of palpitations, and should also include continuous rhythm monitoring with Holter or event monitoring, depending on the frequency of symptoms. Otherwise, patients should routinely undergo electrocardiography once or twice a year.
Q waves are common in systemic sclerosis patients (especially those with diffuse cutaneous systemic sclerosis), notably in the precordial leads, and can occur without coronary artery disease.90 Symptoms such as presyncope should be further investigated with Holter monitoring and tilt-table testing.
Assess, modify traditional risk factors
Subclinical atherosclerosis as detected by carotid intima-media thickness is as common in systemic sclerosis as in rheumatoid arthritis.61 However, traditional risk indices such as SCORE (Systematic Coronary Risk Evaluation), QRISK2, and the American College of Cardiology/American Heart Association indices may underestimate risk in patients who have systemic sclerosis.
Strict hypertension control should be the goal for all systemic sclerosis patients. Though there are no specific guidelines on which antihypertensive medications are preferred, calcium channel blockers or angiotensin II receptor blockers, which are typically used to treat systemic sclerosis-related Raynaud phenomenon, may be appropriate.
Statins reduce vascular complications and are generally well tolerated in patients with systemic sclerosis.91,92
Aspirin is not recommended for routine primary prevention in view of data suggesting that its benefits in diabetic patients are counterbalanced by increased bleeding risk.93
Echocardiography to detect pulmonary arterial hypertension
At this time, guidelines for monitoring for cardiovascular manifestations in systemic sclerosis patients are limited. The only well-defined ones are European consensus guidelines, which suggest annual transthoracic echocardiography for the first 5 years after systemic sclerosis is diagnosed and continued annual screening in patients at risk of developing PAH.31
We support this strategy, with annual screening for the first 5 years followed by surveillance echocardiography every 2 to 3 years unless there is a high risk of PAH. Specific attention should be paid to right ventricular diastolic function, right atrial volume, and right ventricular myocardial performance index.
Emerging data suggest that the addition of global longitudinal strain of ventricles to routine echocardiography can help detect subclinical cardiac risk.94 Although further study is needed into the predictive value of global longitudinal strain, it is a low-cost and noninvasive addition to standard echocardiography that can help guide risk stratification, and thus we recommend that it be part of the echocardiographic examination for all systemic sclerosis patients.
Pulmonary function testing. In addition to screening for PAH with echocardiography, we recommend obtaining baseline pulmonary function tests, including DLCO, at the time systemic sclerosis is diagnosed, with repeat testing annually.
Magnetic resonance imaging
While echocardiography is the gold standard for monitoring systemic sclerosis patients, cardiovascular MRI may have a role in identifying those at higher risk of dangerous arrhythmias such as ventricular tachycardia and ventricular fibrillation. In addition to assessing ventricular function, MRI can detect myocardial inflammation, ischemia, and fibrosis that may predispose a patient to develop ventricular tachycardia or fibrillation.95 Variables such as T1/T2 mapping, extracellular volume fraction, T2 signal ratio, and early vs late gadolinium enhancement can help identify patients who had past ventricular tachycardia or fibrillation.96
Finding an increased risk of arrhythmias may prompt a conversation between the patient and the physician about the need for an implantable cardiac defibrillator.
If cardiac MRI is available and is reimbursed by the patient’s insurance carrier, physicians should strongly consider obtaining at least one baseline scan in systemic sclerosis patients to identify those at risk of highly fatal arrhythmias.
Teamwork is needed
Systemic sclerosis has not traditionally been associated with cardiovascular disease to the extent of other rheumatic conditions, but the cardiovascular system can be affected in various ways that can ultimately lead to an early death. These manifestations may be asymptomatic for long periods, and overt clinical disease portends a poorer prognosis.
Primary care physicians managing these patients should be aware of the cardiovascular complications of systemic sclerosis and should implement appropriate screening tests in conjunction with rheumatologists and cardiologists. It is also essential for general and subspecialty cardiologists to understand the broad spectrum of organ system involvement that can affect systemic sclerosis patients and to tailor their investigation and management recommendations accordingly. By designing a multidisciplinary approach to the treatment of systemic sclerosis patients, physicians can help to optimize cardiovascular risk modification in this vulnerable population.
- Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 2005; 52(2):402–411. doi:10.1002/art.20853
- Naranjo A, Sokka T, Descalzo MA, et al; QUEST-RA Group. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther 2008; 10(2):R30. doi:10.1186/ar2383
- Innala L, Möller B, Ljung L, et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther 2011; 13(4):R131. doi:10.1186/ar3442
- Barnes J, Mayes MD. Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Curr Opin Rheumatol 2012; 24(2):165–170. doi:10.1097/BOR.0b013e32834ff2e8
- Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum 2008; 37(4):223–235 doi:10.1016/j.semarthrit.2007.05.003
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med 2009; 360(19):1989–2003. doi:10.1056/NEJMra0806188
- Panopoulos S, Tektonidou M, Drosos AA, et al. Prevalence of comorbidities in systemic sclerosis versus rheumatoid arthritis: a comparative, multicenter, matched-cohort study. Arthritis Res Ther 2018; 20(1):267. doi:10.1186/s13075-018-1771-0
- Ferri C, Valentini G, Cozzi F, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002; 81(8):139–153. doi:10.1097/00005792-200203000-00004
- Steen VD, Medsger TA Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum 2000; 43(11):2437–2444. doi:10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010; 69(10):1809–1815. doi:10.1136/ard.2009.114264
- Nassenstein K, Breuckmann F, Huger M, et al. Detection of myocardial fibrosis in systemic sclerosis by contrast-enhanced magnetic resonance imaging. Rofo 2008; 180(12):1054–1060. doi:10.1055/s-2008-1027864
- Psarras A, Soulaidopoulos S, Garyfallos A, Kitas G, Dimitroulas T. A critical view on cardiovascular risk in systemic sclerosis. Rheumatol Int 2017; 37(1):85–95. doi:10.1007/s00296-016-3530-3
- Lekakis J, Mavrikakis M, Emmanuel M, et al. Cold-induced coronary Raynaud’s phenomenon in patients with systemic sclerosis. Clin Exp Rheumatol 1998; 16(2):135–140. pmid:9536388
- Altorok N, Wang Y, Kahaleh B. Endothelial dysfunction in systemic sclerosis. Curr Opin Rheumatol 2014; 26(6):615–620. doi:10.1097/BOR.0000000000000112
- Fleming JN, Nash RA, Mahoney WM Jr, Schwartz SM. Is scleroderma a vasculopathy? Curr Rheumatol Rep 2009; 11(2):103–110. pmid:19296882
- Maurer B, Distler A, Suliman YA, et al. Vascular endothelial growth factor aggravates fibrosis and vasculopathy in experimental models of systemic sclerosis. Ann Rheum Dis 2014; 73(10):1880–1887. doi:10.1136/annrheumdis-2013-203535
- Meune C, Vignaux O, Kahan A, Allanore Y. Heart involvement in systemic sclerosis: evolving concept and diagnostic methodologies. Arch Cardiovasc Dis 2010; 103(1):46–52. doi:10.1016/j.acvd.2009.06.009
- Dimitroulas T, Giannakoulas G, Karvounis H, Garyfallos A, Settas L, Kitas GD. Micro- and macrovascular treatment targets in scleroderma heart disease. Curr Pharm Des 2014; 20(4):536–544. pmid:23565639
- Allanore Y, Meune C. Primary myocardial involvement in systemic sclerosis: evidence for a microvascular origin. Clin Exp Rheumatol 2010; 28(5 suppl 62):S48–S53. pmid:21050545
- Kahan A, Nitenberg A, Foult JM, et al. Decreased coronary reserve in primary scleroderma myocardial disease. Arthritis Rheum 1985; 28(6):637–646. pmid:4004974
- Morrisroe K, Stevens W, Sahhar J, et al. Epidemiology and disease characteristics of systemic sclerosis-related pulmonary arterial hypertension: results from a real-life screening program. Arthritis Res Ther 2017; 19(1):42. doi:10.1186/s13075-017-1250-z
- Chaisson NF, Hassoun PM. Systemic sclerosis-associated pulmonary arterial hypertension. Chest 2013; 144(4):1346–1356. doi:10.1378/chest.12-2396
- Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 2007; 66(7):940–944. doi:10.1136/ard.2006.066068
- Coghlan JG, Galiè N, Barberà JA, et al; AMBITION investigators. Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): subgroup analysis from the AMBITION trial. Ann Rheum Dis 2017; 76(7):1219–1227. doi:10.1136/annrheumdis-2016-210236
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53(1):1801913. doi:10.1183/13993003.01913-2018
- Chatterjee S. Pulmonary hypertension in systemic sclerosis. Semin Arthritis Rheum 2011; 41(1):19–37. doi:10.1016/j.semarthrit.2010.08.004
- Sweiss NJ, Hushaw L, Thenappan T, et al. Diagnosis and management of pulmonary hypertension in systemic sclerosis. Curr Rheumatol Rep 2010; 12(1):8–18. doi:10.1007/s11926-009-0078-1
- Cox SR, Walker JG, Coleman M, et al. Isolated pulmonary hypertension in scleroderma. Intern Med J 2005; 35(1):28–33. doi:10.1111/j.1445-5994.2004.00646.x
- Sánchez-Román J, Opitz CF, Kowal-Bielecka O, García-Hernández FJ, Castillo-Palma MJ, Pittrow D; EPOSS-OMERACT Group. Screening for PAH in patients with systemic sclerosis: focus on Doppler echocardiography. Rheumatology (Oxford) 2008; 47(suppl 5):v33–v35. doi:10.1093/rheumatology/ken306
- Walker KM, Pope J; Scleroderma Clinical Trials Consortium; Canadian Scleroderma Research Group. Expert agreement on EULAR/EUSTAR recommendations for the management of systemic sclerosis. J Rheumatol 2011; 38(7):1326–1328. doi:10.3899/jrheum.101262
- Khanna D, Gladue H, Channick R, et al; Scleroderma Foundation and Pulmonary Hypertension Association. Recommendations for screening and detection of connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheum 2013; 65(12):3194–3201. doi:10.1002/art.38172
- Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 2005; 52(12):3792–3800. doi:10.1002/art.21433
- Dimitroulas T, Giannakoulas G, Dimitroula H, et al. Significance of serum uric acid in pulmonary hypertension due to systemic sclerosis: a pilot study. Rheumatol Int 2011; 31(2):263–267. doi:10.1007/s00296-010-1557-4
- Dimitroulas T, Giannakoulas G, Papadopoulou K, et al. Left atrial volume and N-terminal pro-B type natriuretic peptide are associated with elevated pulmonary artery pressure in patients with systemic sclerosis. Clin Rheumatol 2010; 29(9):957–964. doi:10.1007/s10067-010-1494-3
- Coghlan JG, Denton CP, Grünig E, et al; DETECT study group. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014; 73(7):1340–1349. doi:10.1136/annrheumdis-2013-203301
- Schwaiger JP, Khanna D, Gerry Coghlan J. Screening patients with scleroderma for pulmonary arterial hypertension and implications for other at-risk populations. Eur Respir Rev 2013; 22(130):515–525. doi:10.1183/09059180.00006013
- Man A, Zhu Y, Zhang Y, et al. The risk of cardiovascular disease in systemic sclerosis: a population-based cohort study. Ann Rheum Dis 2013; 72(7):1188–1193. doi:10.1136/annrheumdis-2012-202007
- Ngian G-S, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Aviña-Zubieta JA, Man A, Yurkovich M, Huang K, Sayre EC, Choi HK. Early cardiovascular disease after the diagnosis of systemic sclerosis. Am J Med 2016; 29(3):324–331. doi:10.1016/j.amjmed.2015.10.037
- D’Angelo WA, Fries JF, Masi AT, Shulman LE. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 1969; 46(3):428–440. doi:10.1016/0002-9343(69)90044-8
- Ngian GS, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Khurma V, Meyer C, Park GS, et al. A pilot study of subclinical coronary atherosclerosis in systemic sclerosis: coronary artery calcification in cases and controls. Arthritis Rheum 2008; 59(4):591–597. doi:10.1002/art.23540
- Lippi G, Caramaschi P, Montagnana M, Salvagno GL, Volpe A, Guidi G. Lipoprotein[a] and the lipid profile in patients with systemic sclerosis. Clin Chim Acta 2006; 364(1–2):345–348. doi:10.1016/j.cca.2005.07.015
- Palinski W, Hörkkö S, Miller E, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest 1996; 98(3):800–814. doi:10.1172/JCI118853
- Ho M, Veale D, Eastmond C, Nuki G, Belch J. Macrovascular disease and systemic sclerosis. Ann Rheum Dis 2000; 59(1):39–43. doi:10.1136/ard.59.1.39
- Kaloudi O, Basta G, Perfetto F, et al. Circulating levels of Ne-(carboxymethyl)lysine are increased in systemic sclerosis. Rheumatology (Oxford) 2007; 46(3):412–416. doi:10.1093/rheumatology/kel076
- Muro Y, Sugiura K, Morita Y, Tomita Y. An evaluation of the efficacy of the toe brachial index measuring vascular involvement in systemic sclerosis and other connective tissue diseases. Clin Exp Rheumatol 2009; 27(3 suppl 54):26–31. pmid:19796558
- Cheng K-S, Tiwari A, Boutin A, et al. Differentiation of primary and secondary Raynaud’s disease by carotid arterial stiffness. Eur J Vasc Endovasc Surg 2003; 25(4):336–341. doi:10.1053/ejvs.2002.1845
- Kawasaki M, Ito Y, Yokoyama H, et al. Assessment of arterial medial characteristics in human carotid arteries using integrated backscatter ultrasound and its histological implications. Atherosclerosis 2005; 180(1):145–154. doi:10.1016/j.atherosclerosis.2004.11.018
- Szucs G, Tímár O, Szekanecz Z, et al. Endothelial dysfunction precedes atherosclerosis in systemic sclerosis—relevance for prevention of vascular complications. Rheumatology (Oxford) 2007; 46(5):759–762. doi:10.1093/rheumatology/kel426
- Hettema ME, Zhang D, de Leeuw K, et al. Early atherosclerosis in systemic sclerosis and its relation to disease or traditional risk factors. Arthritis Res Ther 2008;10(2):R49. doi:10.1186/ar2408
- Roustit M, Simmons GH, Baguet JP, Carpentier P, Cracowski JL. Discrepancy between simultaneous digital skin microvascular and brachial artery macrovascular post-occlusive hyperemia in systemic sclerosis. J Rheumatol 2008; 35(8):1576–1583. pmid:18597404
- Vettori S, Maresca L, Cuomo G, Abbadessa S, Leonardo G, Valentini G. Clinical and subclinical atherosclerosis in systemic sclerosis: consequences of previous corticosteroid treatment. Scand J Rheumatol 2010; 39(6):485–489. doi:10.3109/03009741003781985
- Lekakis J, Mavrikakis M, Papamichael C, et al. Short-term estrogen administration improves abnormal endothelial function in women with systemic sclerosis and Raynaud’s phenomenon. Am Heart J 1998; 136(5):905–912. doi:10.1016/s0002-8703(98)70137-1
- Bartoli F, Blagojevic J, Bacci M, et al. Flow-mediated vasodilation and carotid intima-media thickness in systemic sclerosis. Ann N Y Acad Sci 2007; 1108:283–290. doi:10.1196/annals.1422.030
- Rollando D, Bezante GP, Sulli A, et al. Brachial artery endothelial-dependent flow-mediated dilation identifies early-stage endothelial dysfunction in systemic sclerosis and correlates with nailfold microvascular impairment. J Rheumatol 2010; 37(6):1168–1173. doi:10.3899/jrheum.091116
- Andersen GN, Mincheva-Nilsson L, Kazzam E, et al. Assessment of vascular function in systemic sclerosis: indications of the development of nitrate tolerance as a result of enhanced endothelial nitric oxide production. Arthritis Rheum 2002; 46(5):1324–1332. doi:10.1002/art.10191
- Au K, Singh MK, Bodukam V, et al. Atherosclerosis in systemic sclerosis: a systematic review and meta-analysis. Arthritis Rheum 2011; 63(7):2078–2090. doi:10.1002/art.30380
- van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: a meta-analysis. Semin Arthritis Rheum 2011; 40(5):389–397. doi:10.1016/j.semarthrit.2010.06.006
- Brohall G, Odén A, Fagerberg B. Carotid artery intima-media thickness in patients with type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med 2006; 23(6):609–616. doi:10.1111/j.1464-5491.2005.01725.x
- Masoura C, Pitsavos C, Aznaouridis K, Skoumas I, Vlachopoulos C, Stefanadis C. Arterial endothelial function and wall thickness in familial hypercholesterolemia and familial combined hyperlipidemia and the effect of statins. A systematic review and meta-analysis. Atherosclerosis 2011; 214(1):129–138. doi:10.1016/j.atherosclerosis.2010.10.008
- Ozen G, Inanc N, Unal AU, et al. Subclinical atherosclerosis in systemic sclerosis: not less frequent than rheumatoid arthritis and not detected with cardiovascular risk indices. Arthritis Care Res (Hoboken) 2016; 68(10):1538–1546. doi:10.1002/acr.22852
- Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 2010; 26(6):631–640. doi:10.1007/s10554-010-9616-1
- Meune C, Avouac J, Wahbi K, et al. Cardiac involvement in systemic sclerosis assessed by tissue-doppler echocardiography during routine care: a controlled study of 100 consecutive patients. Arthritis Rheum 2008; 58(6):1803–1809. doi:10.1002/art.23463
- Tennøe AH, Murbræch K, Andreassen JC, et al. Left ventricular diastolic dysfunction predicts mortality in patients with systemic sclerosis. J Am Coll Cardiol 2018; 72(15):1804–1813. doi:10.1016/j.jacc.2018.07.068
- de Groote P, Gressin V, Hachulla E, et al; ItinerAIR-Scleroderma Investigators. Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann Rheum Dis 2008; 67(1):31–36. doi:10.1136/ard.2006.057760
- Allanore Y, Meune C, Vonk MC, et al; EUSTAR co-authors. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis 2010; 69(1):218–221. doi:10.1136/ard.2008.103382
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Assassi S, Del Junco D, Sutter K, et al. Clinical and genetic factors predictive of mortality in early systemic sclerosis. Arthritis Rheum 2009; 61(10):1403–1411. doi:10.1002/art.24734
- Rokas S, Mavrikakis M, Agrios N, Mylonas D, Antoniadou L, Moulopoulos S. Electrophysiologic abnormalities of cardiac function in progressive systemic sclerosis. J Electrocardiol 1996; 29(1):17–25. pmid:8808521
- Kostis JB, Seibold JR, Turkevich D, et al. Prognostic importance of cardiac arrhythmias in systemic sclerosis. Am J Med 1988; 84(6):1007–1015. doi:10.1016/0002-9343(88)90305-1
- Biełous-Wilk A, Poreba M, Staniszewska-Marszałek E, et al. Electrocardiographic evaluation in patients with systemic scleroderma and without clinically evident heart disease. Ann Noninvasive Electrocardiol 2009; 14(3):251–257. doi:10.1111/j.1542-474X.2009.00306.x
- Bienias P, Ciurzynski M, Glinska-Wielochowska M, et al. Heart rate turbulence assessment in systemic sclerosis: the role for the detection of cardiac autonomic nervous system dysfunction. Rheumatology (Oxford) 2010; 49(2):355–360. doi:10.1093/rheumatology/kep394
- Ferri C, Bernini L, Bongiorni MG, et al. Noninvasive evaluation of cardiac dysrhythmias, and their relationship with multisystemic symptoms, in progressive systemic sclerosis patients. Arthritis Rheum 1985; 28(11):1259–1266. pmid:4063000
- Roberts NK, Cabeen WR, Moss J, Clements PJ, Furst DE. The prevalence of conduction defects and cardiac arrhythmias in progressive systemic sclerosis. Ann Intern Med 1981; 94(1):38–40. doi:10.7326/0003-4819-94-1-38
- Wang Q, Shang Y, Li S, Wu Y, Wang C, Yan X. Complete heart block in systemic sclerosis: a case report and literature review. Medicine (Baltimore) 2018; 97(46):e13226. doi:10.1097/MD.0000000000013226
- Summerfield BJ. Progressive systemic sclerosis with complete heart block. Br Heart J 1975; 37(12):1308–1310. doi:10.1136/hrt.37.12.1308
- Moyssakis I, Papadopoulos DP, Tzioufas AG, Votteas V. Complete heart block in a patient with systemic sclerosis. Clin Rheumatol 2006; 25(4):551–552. doi:10.1007/s10067-005-0068-2
- Ridolfi RL, Bulkley BH, Hutchins GM. The cardiac conduction system in progressive systemic sclerosis. Clinical and pathologic features of 35 patients. Am J Med 1976; 61(3):361–366. doi:10.1016/0002-9343(76)90373-9
- Champion HC. The heart in scleroderma. Rheum Dis Clin North Am 2008; 34(1):181–190. doi:10.1016/j.rdc.2007.12.002
- Gowda RM, Khan IA, Sacchi TJ, Vasavada BC. Scleroderma pericardial disease presented with a large pericardial effusion—a case report. Angiology 2001; 52(1):59–62. doi:10.1177/000331970105200108
- Meier FMP, Frommer KW, Dinser R, et al; EUSTAR Co-authors. Update on the profile of the EUSTAR cohort: an analysis of the EULAR scleroderma trials and research group database. Ann Rheum Dis 2012; 71(8):1355–1360. doi:10.1136/annrheumdis-2011-200742
- Subramanian SR, Akram R, Velayati A, Chadow H. New development of cardiac tamponade on underlying effusive-constrictive pericarditis: an uncommon initial presentation of scleroderma. BMJ Case Rep 2013; 2013. doi:10.1136/bcr-2013-010254
- Kitchongcharoenying P, Foocharoen C, Mahakkanukrauh A, Suwannaroj S, Nanagara R. Pericardial fluid profiles of pericardial effusion in systemic sclerosis patients. Asian Pac J Allergy Immunol 2013; 31(4):314–319. doi:10.12932/AP0305.31.4.2013
- McWhorter JE, LeRoy EC. Pericardial disease in scleroderma (systemic sclerosis). Am J Med 1974; 57(4):566–575. doi:10.1016/0002-9343(74)90008-4
- Comens SM, Alpert MA, Sharp GC, et al. Frequency of mitral valve prolapse in systemic lupus erythematosus, progressive systemic sclerosis and mixed connective tissue disease. Am J Cardiol 1989; 63(5):369–370. doi:10.1016/0002-9149(89)90351-2
- Candell-Riera J, Armadans-Gil L, Simeón CP, et al. Comprehensive noninvasive assessment of cardiac involvement in limited systemic sclerosis. Arthritis Rheum 1996; 39(7):1138–1145. pmid:8670322
- Caforio ALP, Adler Y, Agostini C, et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J 2017; 38(35):2649–2662. doi:10.1093/eurheartj/ehx321
- Mavrogeni S, Karabela G, Koutsogeorgopoulou L, et al. Pseudo-infarction pattern in diffuse systemic sclerosis. Evaluation using cardiovascular magnetic resonance. Int J Cardiol 2016; 214:465–468. doi:10.1016/j.ijcard.2016.03.235
- Ladak K, Pope JE. A review of the effects of statins in systemic sclerosis. Semin Arthritis Rheum 2016; 45(6):698–705. doi:10.1016/j.semarthrit.2015.10.013
- Abou-Raya A, Abou-Raya S, Helmii M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J Rheumatol 2008; 35(9):1801–1808. pmid:18709692
- ASCEND Study Collaborative Group; Bowman L, Mafham M, Wallendszus K, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018; 379(16):1529–1539. doi:10.1056/NEJMoa1804988
- Guerra F, Stronati G, Fischietti C, et al. Global longitudinal strain measured by speckle tracking identifies subclinical heart involvement in patients with systemic sclerosis. Eur J Prev Cardiol 2018; 25(15):1598–1606. doi:10.1177/2047487318786315
- Mavrogeni SI, Sfikakis PP, Dimitroulas T, et al. Prospects of using cardiovascular magnetic resonance in the identification of arrhythmogenic substrate in autoimmune rheumatic diseases. Rheumatol Int 2018; 38(9):1615–1621. doi:10.1007/s00296-018-4110-5
- Mavrogeni SI, Sfikakis PP, Markousis-Mavrogenis G, et al. Cardiovascular magnetic resonance imaging pattern in patients with autoimmune rheumatic diseases and ventricular tachycardia with preserved ejection fraction. Int J Cardiol 2019; 284:105–109. doi:10.1016/j.ijcard.2018.10.067
- Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 2005; 52(2):402–411. doi:10.1002/art.20853
- Naranjo A, Sokka T, Descalzo MA, et al; QUEST-RA Group. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther 2008; 10(2):R30. doi:10.1186/ar2383
- Innala L, Möller B, Ljung L, et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther 2011; 13(4):R131. doi:10.1186/ar3442
- Barnes J, Mayes MD. Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Curr Opin Rheumatol 2012; 24(2):165–170. doi:10.1097/BOR.0b013e32834ff2e8
- Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum 2008; 37(4):223–235 doi:10.1016/j.semarthrit.2007.05.003
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med 2009; 360(19):1989–2003. doi:10.1056/NEJMra0806188
- Panopoulos S, Tektonidou M, Drosos AA, et al. Prevalence of comorbidities in systemic sclerosis versus rheumatoid arthritis: a comparative, multicenter, matched-cohort study. Arthritis Res Ther 2018; 20(1):267. doi:10.1186/s13075-018-1771-0
- Ferri C, Valentini G, Cozzi F, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002; 81(8):139–153. doi:10.1097/00005792-200203000-00004
- Steen VD, Medsger TA Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum 2000; 43(11):2437–2444. doi:10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010; 69(10):1809–1815. doi:10.1136/ard.2009.114264
- Nassenstein K, Breuckmann F, Huger M, et al. Detection of myocardial fibrosis in systemic sclerosis by contrast-enhanced magnetic resonance imaging. Rofo 2008; 180(12):1054–1060. doi:10.1055/s-2008-1027864
- Psarras A, Soulaidopoulos S, Garyfallos A, Kitas G, Dimitroulas T. A critical view on cardiovascular risk in systemic sclerosis. Rheumatol Int 2017; 37(1):85–95. doi:10.1007/s00296-016-3530-3
- Lekakis J, Mavrikakis M, Emmanuel M, et al. Cold-induced coronary Raynaud’s phenomenon in patients with systemic sclerosis. Clin Exp Rheumatol 1998; 16(2):135–140. pmid:9536388
- Altorok N, Wang Y, Kahaleh B. Endothelial dysfunction in systemic sclerosis. Curr Opin Rheumatol 2014; 26(6):615–620. doi:10.1097/BOR.0000000000000112
- Fleming JN, Nash RA, Mahoney WM Jr, Schwartz SM. Is scleroderma a vasculopathy? Curr Rheumatol Rep 2009; 11(2):103–110. pmid:19296882
- Maurer B, Distler A, Suliman YA, et al. Vascular endothelial growth factor aggravates fibrosis and vasculopathy in experimental models of systemic sclerosis. Ann Rheum Dis 2014; 73(10):1880–1887. doi:10.1136/annrheumdis-2013-203535
- Meune C, Vignaux O, Kahan A, Allanore Y. Heart involvement in systemic sclerosis: evolving concept and diagnostic methodologies. Arch Cardiovasc Dis 2010; 103(1):46–52. doi:10.1016/j.acvd.2009.06.009
- Dimitroulas T, Giannakoulas G, Karvounis H, Garyfallos A, Settas L, Kitas GD. Micro- and macrovascular treatment targets in scleroderma heart disease. Curr Pharm Des 2014; 20(4):536–544. pmid:23565639
- Allanore Y, Meune C. Primary myocardial involvement in systemic sclerosis: evidence for a microvascular origin. Clin Exp Rheumatol 2010; 28(5 suppl 62):S48–S53. pmid:21050545
- Kahan A, Nitenberg A, Foult JM, et al. Decreased coronary reserve in primary scleroderma myocardial disease. Arthritis Rheum 1985; 28(6):637–646. pmid:4004974
- Morrisroe K, Stevens W, Sahhar J, et al. Epidemiology and disease characteristics of systemic sclerosis-related pulmonary arterial hypertension: results from a real-life screening program. Arthritis Res Ther 2017; 19(1):42. doi:10.1186/s13075-017-1250-z
- Chaisson NF, Hassoun PM. Systemic sclerosis-associated pulmonary arterial hypertension. Chest 2013; 144(4):1346–1356. doi:10.1378/chest.12-2396
- Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 2007; 66(7):940–944. doi:10.1136/ard.2006.066068
- Coghlan JG, Galiè N, Barberà JA, et al; AMBITION investigators. Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): subgroup analysis from the AMBITION trial. Ann Rheum Dis 2017; 76(7):1219–1227. doi:10.1136/annrheumdis-2016-210236
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53(1):1801913. doi:10.1183/13993003.01913-2018
- Chatterjee S. Pulmonary hypertension in systemic sclerosis. Semin Arthritis Rheum 2011; 41(1):19–37. doi:10.1016/j.semarthrit.2010.08.004
- Sweiss NJ, Hushaw L, Thenappan T, et al. Diagnosis and management of pulmonary hypertension in systemic sclerosis. Curr Rheumatol Rep 2010; 12(1):8–18. doi:10.1007/s11926-009-0078-1
- Cox SR, Walker JG, Coleman M, et al. Isolated pulmonary hypertension in scleroderma. Intern Med J 2005; 35(1):28–33. doi:10.1111/j.1445-5994.2004.00646.x
- Sánchez-Román J, Opitz CF, Kowal-Bielecka O, García-Hernández FJ, Castillo-Palma MJ, Pittrow D; EPOSS-OMERACT Group. Screening for PAH in patients with systemic sclerosis: focus on Doppler echocardiography. Rheumatology (Oxford) 2008; 47(suppl 5):v33–v35. doi:10.1093/rheumatology/ken306
- Walker KM, Pope J; Scleroderma Clinical Trials Consortium; Canadian Scleroderma Research Group. Expert agreement on EULAR/EUSTAR recommendations for the management of systemic sclerosis. J Rheumatol 2011; 38(7):1326–1328. doi:10.3899/jrheum.101262
- Khanna D, Gladue H, Channick R, et al; Scleroderma Foundation and Pulmonary Hypertension Association. Recommendations for screening and detection of connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheum 2013; 65(12):3194–3201. doi:10.1002/art.38172
- Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 2005; 52(12):3792–3800. doi:10.1002/art.21433
- Dimitroulas T, Giannakoulas G, Dimitroula H, et al. Significance of serum uric acid in pulmonary hypertension due to systemic sclerosis: a pilot study. Rheumatol Int 2011; 31(2):263–267. doi:10.1007/s00296-010-1557-4
- Dimitroulas T, Giannakoulas G, Papadopoulou K, et al. Left atrial volume and N-terminal pro-B type natriuretic peptide are associated with elevated pulmonary artery pressure in patients with systemic sclerosis. Clin Rheumatol 2010; 29(9):957–964. doi:10.1007/s10067-010-1494-3
- Coghlan JG, Denton CP, Grünig E, et al; DETECT study group. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014; 73(7):1340–1349. doi:10.1136/annrheumdis-2013-203301
- Schwaiger JP, Khanna D, Gerry Coghlan J. Screening patients with scleroderma for pulmonary arterial hypertension and implications for other at-risk populations. Eur Respir Rev 2013; 22(130):515–525. doi:10.1183/09059180.00006013
- Man A, Zhu Y, Zhang Y, et al. The risk of cardiovascular disease in systemic sclerosis: a population-based cohort study. Ann Rheum Dis 2013; 72(7):1188–1193. doi:10.1136/annrheumdis-2012-202007
- Ngian G-S, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Aviña-Zubieta JA, Man A, Yurkovich M, Huang K, Sayre EC, Choi HK. Early cardiovascular disease after the diagnosis of systemic sclerosis. Am J Med 2016; 29(3):324–331. doi:10.1016/j.amjmed.2015.10.037
- D’Angelo WA, Fries JF, Masi AT, Shulman LE. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 1969; 46(3):428–440. doi:10.1016/0002-9343(69)90044-8
- Ngian GS, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 2012; 71(12):1980–1983. doi:10.1136/annrheumdis-2011-201176
- Khurma V, Meyer C, Park GS, et al. A pilot study of subclinical coronary atherosclerosis in systemic sclerosis: coronary artery calcification in cases and controls. Arthritis Rheum 2008; 59(4):591–597. doi:10.1002/art.23540
- Lippi G, Caramaschi P, Montagnana M, Salvagno GL, Volpe A, Guidi G. Lipoprotein[a] and the lipid profile in patients with systemic sclerosis. Clin Chim Acta 2006; 364(1–2):345–348. doi:10.1016/j.cca.2005.07.015
- Palinski W, Hörkkö S, Miller E, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest 1996; 98(3):800–814. doi:10.1172/JCI118853
- Ho M, Veale D, Eastmond C, Nuki G, Belch J. Macrovascular disease and systemic sclerosis. Ann Rheum Dis 2000; 59(1):39–43. doi:10.1136/ard.59.1.39
- Kaloudi O, Basta G, Perfetto F, et al. Circulating levels of Ne-(carboxymethyl)lysine are increased in systemic sclerosis. Rheumatology (Oxford) 2007; 46(3):412–416. doi:10.1093/rheumatology/kel076
- Muro Y, Sugiura K, Morita Y, Tomita Y. An evaluation of the efficacy of the toe brachial index measuring vascular involvement in systemic sclerosis and other connective tissue diseases. Clin Exp Rheumatol 2009; 27(3 suppl 54):26–31. pmid:19796558
- Cheng K-S, Tiwari A, Boutin A, et al. Differentiation of primary and secondary Raynaud’s disease by carotid arterial stiffness. Eur J Vasc Endovasc Surg 2003; 25(4):336–341. doi:10.1053/ejvs.2002.1845
- Kawasaki M, Ito Y, Yokoyama H, et al. Assessment of arterial medial characteristics in human carotid arteries using integrated backscatter ultrasound and its histological implications. Atherosclerosis 2005; 180(1):145–154. doi:10.1016/j.atherosclerosis.2004.11.018
- Szucs G, Tímár O, Szekanecz Z, et al. Endothelial dysfunction precedes atherosclerosis in systemic sclerosis—relevance for prevention of vascular complications. Rheumatology (Oxford) 2007; 46(5):759–762. doi:10.1093/rheumatology/kel426
- Hettema ME, Zhang D, de Leeuw K, et al. Early atherosclerosis in systemic sclerosis and its relation to disease or traditional risk factors. Arthritis Res Ther 2008;10(2):R49. doi:10.1186/ar2408
- Roustit M, Simmons GH, Baguet JP, Carpentier P, Cracowski JL. Discrepancy between simultaneous digital skin microvascular and brachial artery macrovascular post-occlusive hyperemia in systemic sclerosis. J Rheumatol 2008; 35(8):1576–1583. pmid:18597404
- Vettori S, Maresca L, Cuomo G, Abbadessa S, Leonardo G, Valentini G. Clinical and subclinical atherosclerosis in systemic sclerosis: consequences of previous corticosteroid treatment. Scand J Rheumatol 2010; 39(6):485–489. doi:10.3109/03009741003781985
- Lekakis J, Mavrikakis M, Papamichael C, et al. Short-term estrogen administration improves abnormal endothelial function in women with systemic sclerosis and Raynaud’s phenomenon. Am Heart J 1998; 136(5):905–912. doi:10.1016/s0002-8703(98)70137-1
- Bartoli F, Blagojevic J, Bacci M, et al. Flow-mediated vasodilation and carotid intima-media thickness in systemic sclerosis. Ann N Y Acad Sci 2007; 1108:283–290. doi:10.1196/annals.1422.030
- Rollando D, Bezante GP, Sulli A, et al. Brachial artery endothelial-dependent flow-mediated dilation identifies early-stage endothelial dysfunction in systemic sclerosis and correlates with nailfold microvascular impairment. J Rheumatol 2010; 37(6):1168–1173. doi:10.3899/jrheum.091116
- Andersen GN, Mincheva-Nilsson L, Kazzam E, et al. Assessment of vascular function in systemic sclerosis: indications of the development of nitrate tolerance as a result of enhanced endothelial nitric oxide production. Arthritis Rheum 2002; 46(5):1324–1332. doi:10.1002/art.10191
- Au K, Singh MK, Bodukam V, et al. Atherosclerosis in systemic sclerosis: a systematic review and meta-analysis. Arthritis Rheum 2011; 63(7):2078–2090. doi:10.1002/art.30380
- van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: a meta-analysis. Semin Arthritis Rheum 2011; 40(5):389–397. doi:10.1016/j.semarthrit.2010.06.006
- Brohall G, Odén A, Fagerberg B. Carotid artery intima-media thickness in patients with type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med 2006; 23(6):609–616. doi:10.1111/j.1464-5491.2005.01725.x
- Masoura C, Pitsavos C, Aznaouridis K, Skoumas I, Vlachopoulos C, Stefanadis C. Arterial endothelial function and wall thickness in familial hypercholesterolemia and familial combined hyperlipidemia and the effect of statins. A systematic review and meta-analysis. Atherosclerosis 2011; 214(1):129–138. doi:10.1016/j.atherosclerosis.2010.10.008
- Ozen G, Inanc N, Unal AU, et al. Subclinical atherosclerosis in systemic sclerosis: not less frequent than rheumatoid arthritis and not detected with cardiovascular risk indices. Arthritis Care Res (Hoboken) 2016; 68(10):1538–1546. doi:10.1002/acr.22852
- Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 2010; 26(6):631–640. doi:10.1007/s10554-010-9616-1
- Meune C, Avouac J, Wahbi K, et al. Cardiac involvement in systemic sclerosis assessed by tissue-doppler echocardiography during routine care: a controlled study of 100 consecutive patients. Arthritis Rheum 2008; 58(6):1803–1809. doi:10.1002/art.23463
- Tennøe AH, Murbræch K, Andreassen JC, et al. Left ventricular diastolic dysfunction predicts mortality in patients with systemic sclerosis. J Am Coll Cardiol 2018; 72(15):1804–1813. doi:10.1016/j.jacc.2018.07.068
- de Groote P, Gressin V, Hachulla E, et al; ItinerAIR-Scleroderma Investigators. Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann Rheum Dis 2008; 67(1):31–36. doi:10.1136/ard.2006.057760
- Allanore Y, Meune C, Vonk MC, et al; EUSTAR co-authors. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis 2010; 69(1):218–221. doi:10.1136/ard.2008.103382
- Hachulla AL, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009; 68(12):1878–1884. doi:10.1136/ard.2008.095836
- Assassi S, Del Junco D, Sutter K, et al. Clinical and genetic factors predictive of mortality in early systemic sclerosis. Arthritis Rheum 2009; 61(10):1403–1411. doi:10.1002/art.24734
- Rokas S, Mavrikakis M, Agrios N, Mylonas D, Antoniadou L, Moulopoulos S. Electrophysiologic abnormalities of cardiac function in progressive systemic sclerosis. J Electrocardiol 1996; 29(1):17–25. pmid:8808521
- Kostis JB, Seibold JR, Turkevich D, et al. Prognostic importance of cardiac arrhythmias in systemic sclerosis. Am J Med 1988; 84(6):1007–1015. doi:10.1016/0002-9343(88)90305-1
- Biełous-Wilk A, Poreba M, Staniszewska-Marszałek E, et al. Electrocardiographic evaluation in patients with systemic scleroderma and without clinically evident heart disease. Ann Noninvasive Electrocardiol 2009; 14(3):251–257. doi:10.1111/j.1542-474X.2009.00306.x
- Bienias P, Ciurzynski M, Glinska-Wielochowska M, et al. Heart rate turbulence assessment in systemic sclerosis: the role for the detection of cardiac autonomic nervous system dysfunction. Rheumatology (Oxford) 2010; 49(2):355–360. doi:10.1093/rheumatology/kep394
- Ferri C, Bernini L, Bongiorni MG, et al. Noninvasive evaluation of cardiac dysrhythmias, and their relationship with multisystemic symptoms, in progressive systemic sclerosis patients. Arthritis Rheum 1985; 28(11):1259–1266. pmid:4063000
- Roberts NK, Cabeen WR, Moss J, Clements PJ, Furst DE. The prevalence of conduction defects and cardiac arrhythmias in progressive systemic sclerosis. Ann Intern Med 1981; 94(1):38–40. doi:10.7326/0003-4819-94-1-38
- Wang Q, Shang Y, Li S, Wu Y, Wang C, Yan X. Complete heart block in systemic sclerosis: a case report and literature review. Medicine (Baltimore) 2018; 97(46):e13226. doi:10.1097/MD.0000000000013226
- Summerfield BJ. Progressive systemic sclerosis with complete heart block. Br Heart J 1975; 37(12):1308–1310. doi:10.1136/hrt.37.12.1308
- Moyssakis I, Papadopoulos DP, Tzioufas AG, Votteas V. Complete heart block in a patient with systemic sclerosis. Clin Rheumatol 2006; 25(4):551–552. doi:10.1007/s10067-005-0068-2
- Ridolfi RL, Bulkley BH, Hutchins GM. The cardiac conduction system in progressive systemic sclerosis. Clinical and pathologic features of 35 patients. Am J Med 1976; 61(3):361–366. doi:10.1016/0002-9343(76)90373-9
- Champion HC. The heart in scleroderma. Rheum Dis Clin North Am 2008; 34(1):181–190. doi:10.1016/j.rdc.2007.12.002
- Gowda RM, Khan IA, Sacchi TJ, Vasavada BC. Scleroderma pericardial disease presented with a large pericardial effusion—a case report. Angiology 2001; 52(1):59–62. doi:10.1177/000331970105200108
- Meier FMP, Frommer KW, Dinser R, et al; EUSTAR Co-authors. Update on the profile of the EUSTAR cohort: an analysis of the EULAR scleroderma trials and research group database. Ann Rheum Dis 2012; 71(8):1355–1360. doi:10.1136/annrheumdis-2011-200742
- Subramanian SR, Akram R, Velayati A, Chadow H. New development of cardiac tamponade on underlying effusive-constrictive pericarditis: an uncommon initial presentation of scleroderma. BMJ Case Rep 2013; 2013. doi:10.1136/bcr-2013-010254
- Kitchongcharoenying P, Foocharoen C, Mahakkanukrauh A, Suwannaroj S, Nanagara R. Pericardial fluid profiles of pericardial effusion in systemic sclerosis patients. Asian Pac J Allergy Immunol 2013; 31(4):314–319. doi:10.12932/AP0305.31.4.2013
- McWhorter JE, LeRoy EC. Pericardial disease in scleroderma (systemic sclerosis). Am J Med 1974; 57(4):566–575. doi:10.1016/0002-9343(74)90008-4
- Comens SM, Alpert MA, Sharp GC, et al. Frequency of mitral valve prolapse in systemic lupus erythematosus, progressive systemic sclerosis and mixed connective tissue disease. Am J Cardiol 1989; 63(5):369–370. doi:10.1016/0002-9149(89)90351-2
- Candell-Riera J, Armadans-Gil L, Simeón CP, et al. Comprehensive noninvasive assessment of cardiac involvement in limited systemic sclerosis. Arthritis Rheum 1996; 39(7):1138–1145. pmid:8670322
- Caforio ALP, Adler Y, Agostini C, et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J 2017; 38(35):2649–2662. doi:10.1093/eurheartj/ehx321
- Mavrogeni S, Karabela G, Koutsogeorgopoulou L, et al. Pseudo-infarction pattern in diffuse systemic sclerosis. Evaluation using cardiovascular magnetic resonance. Int J Cardiol 2016; 214:465–468. doi:10.1016/j.ijcard.2016.03.235
- Ladak K, Pope JE. A review of the effects of statins in systemic sclerosis. Semin Arthritis Rheum 2016; 45(6):698–705. doi:10.1016/j.semarthrit.2015.10.013
- Abou-Raya A, Abou-Raya S, Helmii M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J Rheumatol 2008; 35(9):1801–1808. pmid:18709692
- ASCEND Study Collaborative Group; Bowman L, Mafham M, Wallendszus K, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018; 379(16):1529–1539. doi:10.1056/NEJMoa1804988
- Guerra F, Stronati G, Fischietti C, et al. Global longitudinal strain measured by speckle tracking identifies subclinical heart involvement in patients with systemic sclerosis. Eur J Prev Cardiol 2018; 25(15):1598–1606. doi:10.1177/2047487318786315
- Mavrogeni SI, Sfikakis PP, Dimitroulas T, et al. Prospects of using cardiovascular magnetic resonance in the identification of arrhythmogenic substrate in autoimmune rheumatic diseases. Rheumatol Int 2018; 38(9):1615–1621. doi:10.1007/s00296-018-4110-5
- Mavrogeni SI, Sfikakis PP, Markousis-Mavrogenis G, et al. Cardiovascular magnetic resonance imaging pattern in patients with autoimmune rheumatic diseases and ventricular tachycardia with preserved ejection fraction. Int J Cardiol 2019; 284:105–109. doi:10.1016/j.ijcard.2018.10.067
KEY POINTS
- Pulmonary hypertension is common in systemic sclerosis and carries a poor prognosis. Patients with systemic sclerosis should be screened regularly with echocardiography, followed, when necessary, by right heart catheterization to detect it early.
- Myocardial infarction and stroke are more common in patients with systemic sclerosis, and preventive measures are the same as for the general population.
- Right ventricular dysfunction secondary to pulmonary hypertension is common in systemic sclerosis; left ventricular dysfunction is less so. Routine echocardiography should include assessment of right and left ventricular function.
- Electrocardiography should be performed periodically, and urgently when indicated, to look for potentially dangerous arrhythmias.
2019 Update in perioperative cardiovascular medicine
Perioperative medicine is an evolving field with a rapidly growing body of literature, particularly in cardiology.
In this update, we review 6 articles to answer questions related to preoperative cardiac risk assessment, perioperative medication management, and postoperative cardiac complications. We surveyed perioperative literature from February 2018 through January 2019 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
These summaries are derived from “Updates in Perioperative Medicine” presented at the 14th Annual Perioperative Medicine Summit (Orlando, FL, February 13–16, 2019) and the 2019 Society of Hospital Medicine Annual Meeting (National Harbor, MD, March 24–27, 2019).
PREOPERATIVE CARDIAC EVALUATION
How well do measures of functional capacity predict perioperative complications and mortality in noncardiac surgical patients?
Functional capacity is commonly assessed in preoperative evaluations to estimate patients’ risks of perioperative complications and death. The American College of Cardiology/American Heart Association1 and the European Society of Cardiology2 guidelines both include estimation of cardiopulmonary fitness as a step in preoperative assessment before major noncardiac surgery.
“Subjective assessment” is one way to estimate functional capacity. Simply put, clinicians try to form a rough idea about the fitness of patients by asking questions about routine activities such as walking or climbing stairs. Although commonly used, subjective assessment of functional capacity lacks strong evidence that it predicts adverse perioperative events.
Cardiopulmonary exercise testing is a third option. It measures peak oxygen consumption and anaerobic threshold during exercise. It is probably the best objective measurement of functional capacity, but not necessarily for predicting postoperative cardiac complications, and it is performed relatively infrequently.
[Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0]
In a multicenter, prospective cohort study, Wijeysundera et al4 compared subjective functional capacity assessment, the Duke Activity Status Index, cardiopulmonary exercise testing, and the preoperative N-terminal pro-B-type natriuretic peptide (NT-proBNP) level in their ability to predict complications and death in 1,401 noncardiac surgery patients older than 40 with at least 1 cardiovascular risk factor. After surgery, patients had daily electrocardiograms and troponin measurements until postoperative day 3 or discharge.
The primary outcome was the 30-day incidence of death or myocardial infarction (MI). Additional outcomes included the 30-day incidence of death or myocardial injury after noncardiac surgery (MINS), the 1-year mortality rate, and moderate to severe in-hospital perioperative complications.
Findings. Two percent of patients died or had an MI within 30 days of surgery.4
Subjective assessment had only a 19.2% sensitivity (95% confidence interval [CI] 14.2–25) but a 94.7% specificity (95% CI 93.2–95.9) for predicting inability to attain 4 metabolic equivalents during exercise.4
A lower Duke Activity Status Index predicted the primary outcome of death or MI within 30 days (adjusted odds ratio [OR] 0.96, 95% CI 0.83–0.99, P = .03), and it was the only measure that did so. Additionally, the Duke index and NT-proBNP level predicted the risk of death or MINS within 30 days.4
Only elevated NT-proBNP was associated with death at 1 year.4
On exercise testing, low peak oxygen consumption was significantly associated with perioperative complications.
Limitations. The number of primary outcome events (death and MI) was low, potentially affecting the statistical power of the study.
Conclusions. Subjective assessment of functional capacity misclassifies too many patients as being at low risk of perioperative complications and should not be used for preoperative risk stratification. Other tools, such as the Duke Activity Status Index and NT-proBNP levels, are better predictors of adverse perioperative cardiovascular outcomes and should be considered for use in preoperative cardiac risk assessment.
Although the Duke Activity Status Index is a better predictor of adverse outcomes than subjective functional capacity assessment, a specific perioperative threshold for risk classification has not been established. Its correlate for metabolic equivalents should be considered for use in clinical practice at this point.
PERIOPERATIVE MEDICATION MANAGEMENT
Is perioperative aspirin beneficial in patients undergoing vascular surgery?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial,5 a 2-by-2 factorial randomized controlled trial in which patients received perioperative aspirin, clonidine, both, or neither, demonstrated that perioperative aspirin did not reduce cardiovascular events and increased major bleeding. Patients with recently placed coronary stents and those undergoing carotid endarterectomy were excluded because aspirin is known to have a beneficial effect in these patients.
A subsequent substudy6 found perioperative aspirin to be beneficial in patients with coronary stents placed more than a year before noncardiac surgery. Whether perioperative aspirin is beneficial in other subgroups was unknown.
[Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925]
Biccard et al7 investigated the effect of perioperative aspirin in the subgroup of patients from the POISE-2 trial who underwent vascular surgery. The primary outcome was death or MI within 30 days. Secondary outcomes in this substudy included vascular occlusive complications (amputation and peripheral arterial thrombosis) and major or life-threatening bleeding.
Limitations. There were few adverse events, and this substudy was underpowered for the primary and secondary outcomes.
Conclusion. Starting or continuing aspirin did not improve outcomes, and withdrawing it did not increase cardiovascular or occlusive complications.
Do ACE inhibitors affect risk in noncardiac nonvascular surgery?
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are some of the most commonly used medications for treating hypertension. But whether patients should continue receiving them on the day of surgery or whether they should be held remains unclear.
Although current recommendations are inconsistent, the most recent American College of Cardiology/American Heart Association1 perioperative practice guidelines say that continuing ACE inhibitors or ARBs is reasonable perioperatively. This recommendation, however, acknowledges that published evidence is limited. There is general agreement that preoperative exposure to ACE inhibitors and ARBs is associated with intraoperative hypotension, but whether this increases the risk of adverse clinical outcomes remains unclear. Needed was a study to determine the effect on perioperative morbidity and mortality of continuing vs withholding ACE inhibitors and ARBs before surgery.
[Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036]
Shiffermiller et al8 performed a randomized controlled trial comparing the effect of 2 preoperative ACE inhibitor management protocols in patients undergoing noncardiac nonvascular surgery. Patients were randomized to either receive or not receive their final preoperative ACE inhibitor dose, whether scheduled on the morning of surgery or the night before.
Exclusion criteria included hypotension or hypertension at their preoperative clinic appointment (defined as systolic blood pressure < 90 or ≥ 160 mm Hg, and diastolic blood pressure < 60 or ≥ 95 mm Hg), moderate to severe heart failure, and end-stage renal disease requiring dialysis. Excluded surgery types were cardiac, vascular, organ transplant, oncologic, and all outpatient procedures. Patients taking ARBs were also excluded.
The primary outcome was intraoperative hypotension defined as any systolic blood pressure less than 80 mm Hg from the time of anesthesia induction until transfer to the postanesthesia care unit. Secondary outcomes were measured until hospital discharge and included postoperative acute kidney injury, postoperative hypotension (systolic pressure < 90 mm Hg) and hypertension (systolic pressure > 180 mm Hg), major cardiac events (composite of acute coronary syndrome, acute heart failure, or new-onset arrhythmia), and death.
Findings. A total of 453 patients were screened for eligibility, and of these, 291 were included for randomization. Their average age was 64, 48% were men, and 87% were white. About 50% underwent general anesthesia, 25% spinal, and 25% regional. Over half of the surgeries were orthopedic, and 20% were spine surgeries.
The primary outcome of intraoperative hypotension occurred significantly less often in patients randomized to ACE inhibitor omission than in the continuation group (55% vs 69%, relative risk [RR] 0.81, 95% CI 0.67–0.97, P = .03). This translates to 1 case of intraoperative hypotension for every 7.5 patients continuing an ACE inhibitor perioperatively (number needed to harm 7.5). Intraoperative hypotension associated with vasopressor administration also occurred significantly less frequently in the ACE inhibitor omission group.
Patients in the ACE inhibitor omission group were also less likely to experience postoperative hypotension, but on the other hand, they were more likely to experience severe postoperative hypertension (defined as any systolic blood pressure > 180 mm Hg). The two groups fared the same in terms of rates of acute kidney injury and major adverse cardiac events (MACE) and hospital length of stay, and no patients died in either group.
Limitations. Several factors limit the generalizability of this single-center study, including the many exclusion criteria, the predominance of orthopedic and spine surgeries, and the low-risk patient population (the average Revised Cardiac Risk Index score was 0, range 0–3). Other limitations include not controlling for the specific ACE inhibitor used and not including the precise timing of the final dose in relation to surgery. Lastly, this study lacked power to measure postoperative outcomes.
Conclusions. Continuing ACE inhibitor treatment before noncardiac nonvascular surgery is associated with a greater frequency and duration of intraoperative hypotension, but it did not increase the incidences of acute kidney injury, MACE, or death nor the hospital length of stay.
[Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837]
Hollmann et al9 performed a meta-analysis to determine whether it is better to continue or withhold ACE inhibitors and ARBs before surgery. The patients were adults undergoing noncardiac surgery and receiving an ACE inhibitor or ARB, which was either withheld or continued on the morning of surgery.
Primary outcomes were all-cause mortality and MACE, while secondary outcomes included the incidence of acute kidney injury, heart failure, stroke, intraoperative and postoperative hypotension, and length of hospital stay. Randomized controlled trials and observational studies were included, while case reports and case-control studies were excluded.
Findings. This meta-analysis included 5 randomized controlled trials and 4 cohort studies, with a total of 6,022 patients; 1,816 had their ACE inhibitor or ARB withheld before surgery, while 4,206 continued therapy. It found no difference between the 2 groups in the incidence of death or MACE, and there were not enough data to determine a difference in heart failure, stroke, acute kidney injury, or hospital length of stay.
Seven studies, with 5,414 patients, examined intraoperative hypotension. The overall incidence was 30%, but was significantly lower if the ACE inhibitor or ARB was withheld (OR 0.63, 95% CI 0.47–0.85, P = .002). Findings were similar in an analysis of only the randomized controlled trials. No difference was observed in postoperative hypotension.
Limitations. There was no standard definition of the morbidity outcomes, including hypotension and MACE. The assessment of MACE included data only for MI and not MINS. The specific duration of hypotension was not reported, and this meta-analysis did not take into account different anesthetic techniques. The duration of follow-up varied widely among studies, ranging from the day of hospital discharge to 30 days after surgery. And the randomized controlled trial performed by Shiffermiller et al8 was not included.
Conclusions. While continuing ACE inhibitors or ARBs before noncardiac surgery was associated with intraoperative hypotension, it did not seem to affect other outcomes, including death and MACE. The authors propose that a large randomized controlled trial is needed to determine whether continuing or withholding ACE inhibitor or ARB therapy before surgery is safer.
POSTOPERATIVE CARDIAC COMPLICATIONS
How should we treat MINS?
MINS is associated with an increased risk of cardiovascular events and death in both the short term and long term. MINS is defined as an elevated postoperative troponin level related to an ischemic etiology. However, whether to routinely measure troponin after surgery is unclear, as most patients do not present with ischemic symptoms, and there is no standard of care for treatment of this entity. Limited observational data suggest that starting or intensifying cardiac medications, particularly aspirin and statins, may be beneficial in terms of reducing 30-day mortality rates in patients with MI or cardiac events at 1 year in vascular surgery patients with MINS.
The Management of Myocardial Injury After Noncardiac Surgery (MANAGE) trial was designed to evaluate the potential of the anticoagulant dabigatran to prevent major vascular complications in patients with MINS.
[Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8]
Devereaux et al10 randomized patients who were at least 45 years old and had developed MINS within the previous 35 days to receive dabigatran 110 mg orally twice daily or placebo for up to 2 years. Patients not already taking a proton pump inhibitor were also randomized to take either omeprazole 20 mg once daily or placebo.
The primary efficacy outcome initially was major vascular complications, which included vascular mortality, nonfatal MI, nonhemorrhagic stroke, and peripheral arterial thrombosis. However, amputation and symptomatic venous thromboembolism were subsequently added during the study.
The primary safety outcome was a composite of life-threatening, major, and critical organ bleeding. Major bleeding required a decrease in hemoglobin of at least 4 g/dL, transfusion of at least 3 units of red blood cells within a 24-hour period, or a procedure to stop the bleeding.
Findings. The original goal was to recruit 3,200 patients, but due to slow enrollment and loss of funding, the sample was reduced to 1,754 patients (877 in each group). Approximately 45% of each group stopped taking the study drug prematurely.
The primary efficacy outcome occurred in significantly fewer patients receiving dabigatran (97, 11%) than placebo (133, 15%, HR 0.72, 95% CI 0.55–0.93, P = .0115). The incidence of the primary safety outcome was similar in both groups: 3% with dabigatran and 4% with placebo (HR 0.92, 95% CI 0.55–1.53, P = .76). The only individual efficacy outcome meeting statistical significance was a lower rate of nonhemorrhagic stroke in the dabigatran group. Subgroup analyses showed a trend benefiting patients randomized within 5 days of MINS or with a diagnosis of MI, although it was not statistically significant.
Limitations. The efficacy outcomes were expanded to include venous thromboembolism and others not directly related to MINS, raising questions about the conclusions. Further, as defined by the protocol, bleeding had to be fairly severe to be deemed major. The high number of patients who discontinued the study drug is another limitation of this study.
Conclusion. Dabigatran lowered the risk of major vascular complications with no significant increase in major bleeding in patients with MINS.
What is the risk of thromboembolism in postoperative atrial fibrillation, and what are the benefits of anticoagulation?
Although nonvalvular atrial fibrillation is associated with increased risks of ischemic stroke and systemic embolic events in nonsurgical patients, the association of new-onset postoperative atrial fibrillation with long-term thromboembolic events in the noncardiac surgical population is not well established.
[Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088]
In this retrospective cohort study using a nationwide registry in Denmark, Butt et al11 assessed the long-term risk of thromboembolic events in noncardiac surgical patients with new postoperative atrial fibrillation. Patients were identified who had no previous history of atrial fibrillation and developed it after noncardiac, nonobstetric surgeries, and were matched in a 1:4 ratio with patients who developed nonvalvular atrial fibrillation during nonsurgical hospitalizations. Matching was based on age, sex, heart failure, hypertension, diabetes, known history of thromboembolic events, ischemic heart disease, and the year patients presented with new atrial fibrillation.
Patients were excluded if they received antiarrhythmic drugs or oral anticoagulants before hospitalization or surgery, had cancer in the year prior, or died in the hospital.
The primary outcome of the study was thromboembolic events—a composite of ischemic stroke, transient cerebral ischemia, and peripheral arterial thrombosis or embolism. Secondary outcomes included rehospitalization for atrial fibrillation and all-cause mortality.
Findings. Overall, 0.4% of patients developed new postoperative atrial fibrillation, of whom 3,380 were matched with 15,320 patients with nonvalvular atrial fibrillation. Over a median follow-up of 3.2 years, the risk of thromboembolic events was similar in both groups (31.7 and 29.9 per 1,000 person-years, HR 0.95, 95% CI 0.85–1.07). The groups did not differ in their CHA2DS2-VASc risk scores, HAS-BLED risk scores, or year in which patients were diagnosed.
Anticoagulation lowered the risk of thromboembolic events to a similar extent in both groups compared with no anticoagulation:
- In postoperative atrial fibrillation—HR 0.57, 95% CI 0.40–0.67
- In nonvalvular atrial fibrillation—HR 0.56, 95% CI 0.51–0.62.
Despite the similar reduction in thromboembolic events, only 24.4% of the postoperative atrial fibrillation patients were started on anticoagulation therapy within 30 days of discharge, compared with 41.5% of those with nonvalvular atrial fibrillation.
Limitations. Although this was a large study with excellent follow-up data, it was observational. It may have underestimated the number of patients who developed postoperative atrial fibrillation because episodes that were judged not to be clinically significant may not have been charted. Many patients are not monitored with continuous telemetry postoperatively, which also may have led to underestimation of the number of atrial fibrillation events.
The study also did not examine the number of atrial fibrillation episodes per patient, the heart rhythm at discharge or long-term, or indication for and duration of anticoagulation. There were no data regarding international normalized ratio levels.
Conclusions. Postoperative atrial fibrillation is associated with outcomes similar to those of nonsurgical nonvalvular atrial fibrillation. Anticoagulation decreases the risks of stroke and death. However, substantially fewer patients with postoperative atrial fibrillation receive anticoagulation. Anticoagulation should be considered in these patients, while noting bleeding risk.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–137. doi:10.1016/j.jacc.2014.07.944
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35(35):2383–2431. doi:10.1093/eurheartj/ehu282
- Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (The Duke Activity Status Index). Am J Cardiol 1989; 64(10):651–654. doi:10.1016/0002-9149(89)90496-7
- Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018;168(4):237–244. pmid:29132159
- Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925
- Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036
- Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088
Perioperative medicine is an evolving field with a rapidly growing body of literature, particularly in cardiology.
In this update, we review 6 articles to answer questions related to preoperative cardiac risk assessment, perioperative medication management, and postoperative cardiac complications. We surveyed perioperative literature from February 2018 through January 2019 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
These summaries are derived from “Updates in Perioperative Medicine” presented at the 14th Annual Perioperative Medicine Summit (Orlando, FL, February 13–16, 2019) and the 2019 Society of Hospital Medicine Annual Meeting (National Harbor, MD, March 24–27, 2019).
PREOPERATIVE CARDIAC EVALUATION
How well do measures of functional capacity predict perioperative complications and mortality in noncardiac surgical patients?
Functional capacity is commonly assessed in preoperative evaluations to estimate patients’ risks of perioperative complications and death. The American College of Cardiology/American Heart Association1 and the European Society of Cardiology2 guidelines both include estimation of cardiopulmonary fitness as a step in preoperative assessment before major noncardiac surgery.
“Subjective assessment” is one way to estimate functional capacity. Simply put, clinicians try to form a rough idea about the fitness of patients by asking questions about routine activities such as walking or climbing stairs. Although commonly used, subjective assessment of functional capacity lacks strong evidence that it predicts adverse perioperative events.
Cardiopulmonary exercise testing is a third option. It measures peak oxygen consumption and anaerobic threshold during exercise. It is probably the best objective measurement of functional capacity, but not necessarily for predicting postoperative cardiac complications, and it is performed relatively infrequently.
[Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0]
In a multicenter, prospective cohort study, Wijeysundera et al4 compared subjective functional capacity assessment, the Duke Activity Status Index, cardiopulmonary exercise testing, and the preoperative N-terminal pro-B-type natriuretic peptide (NT-proBNP) level in their ability to predict complications and death in 1,401 noncardiac surgery patients older than 40 with at least 1 cardiovascular risk factor. After surgery, patients had daily electrocardiograms and troponin measurements until postoperative day 3 or discharge.
The primary outcome was the 30-day incidence of death or myocardial infarction (MI). Additional outcomes included the 30-day incidence of death or myocardial injury after noncardiac surgery (MINS), the 1-year mortality rate, and moderate to severe in-hospital perioperative complications.
Findings. Two percent of patients died or had an MI within 30 days of surgery.4
Subjective assessment had only a 19.2% sensitivity (95% confidence interval [CI] 14.2–25) but a 94.7% specificity (95% CI 93.2–95.9) for predicting inability to attain 4 metabolic equivalents during exercise.4
A lower Duke Activity Status Index predicted the primary outcome of death or MI within 30 days (adjusted odds ratio [OR] 0.96, 95% CI 0.83–0.99, P = .03), and it was the only measure that did so. Additionally, the Duke index and NT-proBNP level predicted the risk of death or MINS within 30 days.4
Only elevated NT-proBNP was associated with death at 1 year.4
On exercise testing, low peak oxygen consumption was significantly associated with perioperative complications.
Limitations. The number of primary outcome events (death and MI) was low, potentially affecting the statistical power of the study.
Conclusions. Subjective assessment of functional capacity misclassifies too many patients as being at low risk of perioperative complications and should not be used for preoperative risk stratification. Other tools, such as the Duke Activity Status Index and NT-proBNP levels, are better predictors of adverse perioperative cardiovascular outcomes and should be considered for use in preoperative cardiac risk assessment.
Although the Duke Activity Status Index is a better predictor of adverse outcomes than subjective functional capacity assessment, a specific perioperative threshold for risk classification has not been established. Its correlate for metabolic equivalents should be considered for use in clinical practice at this point.
PERIOPERATIVE MEDICATION MANAGEMENT
Is perioperative aspirin beneficial in patients undergoing vascular surgery?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial,5 a 2-by-2 factorial randomized controlled trial in which patients received perioperative aspirin, clonidine, both, or neither, demonstrated that perioperative aspirin did not reduce cardiovascular events and increased major bleeding. Patients with recently placed coronary stents and those undergoing carotid endarterectomy were excluded because aspirin is known to have a beneficial effect in these patients.
A subsequent substudy6 found perioperative aspirin to be beneficial in patients with coronary stents placed more than a year before noncardiac surgery. Whether perioperative aspirin is beneficial in other subgroups was unknown.
[Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925]
Biccard et al7 investigated the effect of perioperative aspirin in the subgroup of patients from the POISE-2 trial who underwent vascular surgery. The primary outcome was death or MI within 30 days. Secondary outcomes in this substudy included vascular occlusive complications (amputation and peripheral arterial thrombosis) and major or life-threatening bleeding.
Limitations. There were few adverse events, and this substudy was underpowered for the primary and secondary outcomes.
Conclusion. Starting or continuing aspirin did not improve outcomes, and withdrawing it did not increase cardiovascular or occlusive complications.
Do ACE inhibitors affect risk in noncardiac nonvascular surgery?
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are some of the most commonly used medications for treating hypertension. But whether patients should continue receiving them on the day of surgery or whether they should be held remains unclear.
Although current recommendations are inconsistent, the most recent American College of Cardiology/American Heart Association1 perioperative practice guidelines say that continuing ACE inhibitors or ARBs is reasonable perioperatively. This recommendation, however, acknowledges that published evidence is limited. There is general agreement that preoperative exposure to ACE inhibitors and ARBs is associated with intraoperative hypotension, but whether this increases the risk of adverse clinical outcomes remains unclear. Needed was a study to determine the effect on perioperative morbidity and mortality of continuing vs withholding ACE inhibitors and ARBs before surgery.
[Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036]
Shiffermiller et al8 performed a randomized controlled trial comparing the effect of 2 preoperative ACE inhibitor management protocols in patients undergoing noncardiac nonvascular surgery. Patients were randomized to either receive or not receive their final preoperative ACE inhibitor dose, whether scheduled on the morning of surgery or the night before.
Exclusion criteria included hypotension or hypertension at their preoperative clinic appointment (defined as systolic blood pressure < 90 or ≥ 160 mm Hg, and diastolic blood pressure < 60 or ≥ 95 mm Hg), moderate to severe heart failure, and end-stage renal disease requiring dialysis. Excluded surgery types were cardiac, vascular, organ transplant, oncologic, and all outpatient procedures. Patients taking ARBs were also excluded.
The primary outcome was intraoperative hypotension defined as any systolic blood pressure less than 80 mm Hg from the time of anesthesia induction until transfer to the postanesthesia care unit. Secondary outcomes were measured until hospital discharge and included postoperative acute kidney injury, postoperative hypotension (systolic pressure < 90 mm Hg) and hypertension (systolic pressure > 180 mm Hg), major cardiac events (composite of acute coronary syndrome, acute heart failure, or new-onset arrhythmia), and death.
Findings. A total of 453 patients were screened for eligibility, and of these, 291 were included for randomization. Their average age was 64, 48% were men, and 87% were white. About 50% underwent general anesthesia, 25% spinal, and 25% regional. Over half of the surgeries were orthopedic, and 20% were spine surgeries.
The primary outcome of intraoperative hypotension occurred significantly less often in patients randomized to ACE inhibitor omission than in the continuation group (55% vs 69%, relative risk [RR] 0.81, 95% CI 0.67–0.97, P = .03). This translates to 1 case of intraoperative hypotension for every 7.5 patients continuing an ACE inhibitor perioperatively (number needed to harm 7.5). Intraoperative hypotension associated with vasopressor administration also occurred significantly less frequently in the ACE inhibitor omission group.
Patients in the ACE inhibitor omission group were also less likely to experience postoperative hypotension, but on the other hand, they were more likely to experience severe postoperative hypertension (defined as any systolic blood pressure > 180 mm Hg). The two groups fared the same in terms of rates of acute kidney injury and major adverse cardiac events (MACE) and hospital length of stay, and no patients died in either group.
Limitations. Several factors limit the generalizability of this single-center study, including the many exclusion criteria, the predominance of orthopedic and spine surgeries, and the low-risk patient population (the average Revised Cardiac Risk Index score was 0, range 0–3). Other limitations include not controlling for the specific ACE inhibitor used and not including the precise timing of the final dose in relation to surgery. Lastly, this study lacked power to measure postoperative outcomes.
Conclusions. Continuing ACE inhibitor treatment before noncardiac nonvascular surgery is associated with a greater frequency and duration of intraoperative hypotension, but it did not increase the incidences of acute kidney injury, MACE, or death nor the hospital length of stay.
[Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837]
Hollmann et al9 performed a meta-analysis to determine whether it is better to continue or withhold ACE inhibitors and ARBs before surgery. The patients were adults undergoing noncardiac surgery and receiving an ACE inhibitor or ARB, which was either withheld or continued on the morning of surgery.
Primary outcomes were all-cause mortality and MACE, while secondary outcomes included the incidence of acute kidney injury, heart failure, stroke, intraoperative and postoperative hypotension, and length of hospital stay. Randomized controlled trials and observational studies were included, while case reports and case-control studies were excluded.
Findings. This meta-analysis included 5 randomized controlled trials and 4 cohort studies, with a total of 6,022 patients; 1,816 had their ACE inhibitor or ARB withheld before surgery, while 4,206 continued therapy. It found no difference between the 2 groups in the incidence of death or MACE, and there were not enough data to determine a difference in heart failure, stroke, acute kidney injury, or hospital length of stay.
Seven studies, with 5,414 patients, examined intraoperative hypotension. The overall incidence was 30%, but was significantly lower if the ACE inhibitor or ARB was withheld (OR 0.63, 95% CI 0.47–0.85, P = .002). Findings were similar in an analysis of only the randomized controlled trials. No difference was observed in postoperative hypotension.
Limitations. There was no standard definition of the morbidity outcomes, including hypotension and MACE. The assessment of MACE included data only for MI and not MINS. The specific duration of hypotension was not reported, and this meta-analysis did not take into account different anesthetic techniques. The duration of follow-up varied widely among studies, ranging from the day of hospital discharge to 30 days after surgery. And the randomized controlled trial performed by Shiffermiller et al8 was not included.
Conclusions. While continuing ACE inhibitors or ARBs before noncardiac surgery was associated with intraoperative hypotension, it did not seem to affect other outcomes, including death and MACE. The authors propose that a large randomized controlled trial is needed to determine whether continuing or withholding ACE inhibitor or ARB therapy before surgery is safer.
POSTOPERATIVE CARDIAC COMPLICATIONS
How should we treat MINS?
MINS is associated with an increased risk of cardiovascular events and death in both the short term and long term. MINS is defined as an elevated postoperative troponin level related to an ischemic etiology. However, whether to routinely measure troponin after surgery is unclear, as most patients do not present with ischemic symptoms, and there is no standard of care for treatment of this entity. Limited observational data suggest that starting or intensifying cardiac medications, particularly aspirin and statins, may be beneficial in terms of reducing 30-day mortality rates in patients with MI or cardiac events at 1 year in vascular surgery patients with MINS.
The Management of Myocardial Injury After Noncardiac Surgery (MANAGE) trial was designed to evaluate the potential of the anticoagulant dabigatran to prevent major vascular complications in patients with MINS.
[Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8]
Devereaux et al10 randomized patients who were at least 45 years old and had developed MINS within the previous 35 days to receive dabigatran 110 mg orally twice daily or placebo for up to 2 years. Patients not already taking a proton pump inhibitor were also randomized to take either omeprazole 20 mg once daily or placebo.
The primary efficacy outcome initially was major vascular complications, which included vascular mortality, nonfatal MI, nonhemorrhagic stroke, and peripheral arterial thrombosis. However, amputation and symptomatic venous thromboembolism were subsequently added during the study.
The primary safety outcome was a composite of life-threatening, major, and critical organ bleeding. Major bleeding required a decrease in hemoglobin of at least 4 g/dL, transfusion of at least 3 units of red blood cells within a 24-hour period, or a procedure to stop the bleeding.
Findings. The original goal was to recruit 3,200 patients, but due to slow enrollment and loss of funding, the sample was reduced to 1,754 patients (877 in each group). Approximately 45% of each group stopped taking the study drug prematurely.
The primary efficacy outcome occurred in significantly fewer patients receiving dabigatran (97, 11%) than placebo (133, 15%, HR 0.72, 95% CI 0.55–0.93, P = .0115). The incidence of the primary safety outcome was similar in both groups: 3% with dabigatran and 4% with placebo (HR 0.92, 95% CI 0.55–1.53, P = .76). The only individual efficacy outcome meeting statistical significance was a lower rate of nonhemorrhagic stroke in the dabigatran group. Subgroup analyses showed a trend benefiting patients randomized within 5 days of MINS or with a diagnosis of MI, although it was not statistically significant.
Limitations. The efficacy outcomes were expanded to include venous thromboembolism and others not directly related to MINS, raising questions about the conclusions. Further, as defined by the protocol, bleeding had to be fairly severe to be deemed major. The high number of patients who discontinued the study drug is another limitation of this study.
Conclusion. Dabigatran lowered the risk of major vascular complications with no significant increase in major bleeding in patients with MINS.
What is the risk of thromboembolism in postoperative atrial fibrillation, and what are the benefits of anticoagulation?
Although nonvalvular atrial fibrillation is associated with increased risks of ischemic stroke and systemic embolic events in nonsurgical patients, the association of new-onset postoperative atrial fibrillation with long-term thromboembolic events in the noncardiac surgical population is not well established.
[Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088]
In this retrospective cohort study using a nationwide registry in Denmark, Butt et al11 assessed the long-term risk of thromboembolic events in noncardiac surgical patients with new postoperative atrial fibrillation. Patients were identified who had no previous history of atrial fibrillation and developed it after noncardiac, nonobstetric surgeries, and were matched in a 1:4 ratio with patients who developed nonvalvular atrial fibrillation during nonsurgical hospitalizations. Matching was based on age, sex, heart failure, hypertension, diabetes, known history of thromboembolic events, ischemic heart disease, and the year patients presented with new atrial fibrillation.
Patients were excluded if they received antiarrhythmic drugs or oral anticoagulants before hospitalization or surgery, had cancer in the year prior, or died in the hospital.
The primary outcome of the study was thromboembolic events—a composite of ischemic stroke, transient cerebral ischemia, and peripheral arterial thrombosis or embolism. Secondary outcomes included rehospitalization for atrial fibrillation and all-cause mortality.
Findings. Overall, 0.4% of patients developed new postoperative atrial fibrillation, of whom 3,380 were matched with 15,320 patients with nonvalvular atrial fibrillation. Over a median follow-up of 3.2 years, the risk of thromboembolic events was similar in both groups (31.7 and 29.9 per 1,000 person-years, HR 0.95, 95% CI 0.85–1.07). The groups did not differ in their CHA2DS2-VASc risk scores, HAS-BLED risk scores, or year in which patients were diagnosed.
Anticoagulation lowered the risk of thromboembolic events to a similar extent in both groups compared with no anticoagulation:
- In postoperative atrial fibrillation—HR 0.57, 95% CI 0.40–0.67
- In nonvalvular atrial fibrillation—HR 0.56, 95% CI 0.51–0.62.
Despite the similar reduction in thromboembolic events, only 24.4% of the postoperative atrial fibrillation patients were started on anticoagulation therapy within 30 days of discharge, compared with 41.5% of those with nonvalvular atrial fibrillation.
Limitations. Although this was a large study with excellent follow-up data, it was observational. It may have underestimated the number of patients who developed postoperative atrial fibrillation because episodes that were judged not to be clinically significant may not have been charted. Many patients are not monitored with continuous telemetry postoperatively, which also may have led to underestimation of the number of atrial fibrillation events.
The study also did not examine the number of atrial fibrillation episodes per patient, the heart rhythm at discharge or long-term, or indication for and duration of anticoagulation. There were no data regarding international normalized ratio levels.
Conclusions. Postoperative atrial fibrillation is associated with outcomes similar to those of nonsurgical nonvalvular atrial fibrillation. Anticoagulation decreases the risks of stroke and death. However, substantially fewer patients with postoperative atrial fibrillation receive anticoagulation. Anticoagulation should be considered in these patients, while noting bleeding risk.
Perioperative medicine is an evolving field with a rapidly growing body of literature, particularly in cardiology.
In this update, we review 6 articles to answer questions related to preoperative cardiac risk assessment, perioperative medication management, and postoperative cardiac complications. We surveyed perioperative literature from February 2018 through January 2019 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
These summaries are derived from “Updates in Perioperative Medicine” presented at the 14th Annual Perioperative Medicine Summit (Orlando, FL, February 13–16, 2019) and the 2019 Society of Hospital Medicine Annual Meeting (National Harbor, MD, March 24–27, 2019).
PREOPERATIVE CARDIAC EVALUATION
How well do measures of functional capacity predict perioperative complications and mortality in noncardiac surgical patients?
Functional capacity is commonly assessed in preoperative evaluations to estimate patients’ risks of perioperative complications and death. The American College of Cardiology/American Heart Association1 and the European Society of Cardiology2 guidelines both include estimation of cardiopulmonary fitness as a step in preoperative assessment before major noncardiac surgery.
“Subjective assessment” is one way to estimate functional capacity. Simply put, clinicians try to form a rough idea about the fitness of patients by asking questions about routine activities such as walking or climbing stairs. Although commonly used, subjective assessment of functional capacity lacks strong evidence that it predicts adverse perioperative events.
Cardiopulmonary exercise testing is a third option. It measures peak oxygen consumption and anaerobic threshold during exercise. It is probably the best objective measurement of functional capacity, but not necessarily for predicting postoperative cardiac complications, and it is performed relatively infrequently.
[Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0]
In a multicenter, prospective cohort study, Wijeysundera et al4 compared subjective functional capacity assessment, the Duke Activity Status Index, cardiopulmonary exercise testing, and the preoperative N-terminal pro-B-type natriuretic peptide (NT-proBNP) level in their ability to predict complications and death in 1,401 noncardiac surgery patients older than 40 with at least 1 cardiovascular risk factor. After surgery, patients had daily electrocardiograms and troponin measurements until postoperative day 3 or discharge.
The primary outcome was the 30-day incidence of death or myocardial infarction (MI). Additional outcomes included the 30-day incidence of death or myocardial injury after noncardiac surgery (MINS), the 1-year mortality rate, and moderate to severe in-hospital perioperative complications.
Findings. Two percent of patients died or had an MI within 30 days of surgery.4
Subjective assessment had only a 19.2% sensitivity (95% confidence interval [CI] 14.2–25) but a 94.7% specificity (95% CI 93.2–95.9) for predicting inability to attain 4 metabolic equivalents during exercise.4
A lower Duke Activity Status Index predicted the primary outcome of death or MI within 30 days (adjusted odds ratio [OR] 0.96, 95% CI 0.83–0.99, P = .03), and it was the only measure that did so. Additionally, the Duke index and NT-proBNP level predicted the risk of death or MINS within 30 days.4
Only elevated NT-proBNP was associated with death at 1 year.4
On exercise testing, low peak oxygen consumption was significantly associated with perioperative complications.
Limitations. The number of primary outcome events (death and MI) was low, potentially affecting the statistical power of the study.
Conclusions. Subjective assessment of functional capacity misclassifies too many patients as being at low risk of perioperative complications and should not be used for preoperative risk stratification. Other tools, such as the Duke Activity Status Index and NT-proBNP levels, are better predictors of adverse perioperative cardiovascular outcomes and should be considered for use in preoperative cardiac risk assessment.
Although the Duke Activity Status Index is a better predictor of adverse outcomes than subjective functional capacity assessment, a specific perioperative threshold for risk classification has not been established. Its correlate for metabolic equivalents should be considered for use in clinical practice at this point.
PERIOPERATIVE MEDICATION MANAGEMENT
Is perioperative aspirin beneficial in patients undergoing vascular surgery?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial,5 a 2-by-2 factorial randomized controlled trial in which patients received perioperative aspirin, clonidine, both, or neither, demonstrated that perioperative aspirin did not reduce cardiovascular events and increased major bleeding. Patients with recently placed coronary stents and those undergoing carotid endarterectomy were excluded because aspirin is known to have a beneficial effect in these patients.
A subsequent substudy6 found perioperative aspirin to be beneficial in patients with coronary stents placed more than a year before noncardiac surgery. Whether perioperative aspirin is beneficial in other subgroups was unknown.
[Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925]
Biccard et al7 investigated the effect of perioperative aspirin in the subgroup of patients from the POISE-2 trial who underwent vascular surgery. The primary outcome was death or MI within 30 days. Secondary outcomes in this substudy included vascular occlusive complications (amputation and peripheral arterial thrombosis) and major or life-threatening bleeding.
Limitations. There were few adverse events, and this substudy was underpowered for the primary and secondary outcomes.
Conclusion. Starting or continuing aspirin did not improve outcomes, and withdrawing it did not increase cardiovascular or occlusive complications.
Do ACE inhibitors affect risk in noncardiac nonvascular surgery?
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are some of the most commonly used medications for treating hypertension. But whether patients should continue receiving them on the day of surgery or whether they should be held remains unclear.
Although current recommendations are inconsistent, the most recent American College of Cardiology/American Heart Association1 perioperative practice guidelines say that continuing ACE inhibitors or ARBs is reasonable perioperatively. This recommendation, however, acknowledges that published evidence is limited. There is general agreement that preoperative exposure to ACE inhibitors and ARBs is associated with intraoperative hypotension, but whether this increases the risk of adverse clinical outcomes remains unclear. Needed was a study to determine the effect on perioperative morbidity and mortality of continuing vs withholding ACE inhibitors and ARBs before surgery.
[Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036]
Shiffermiller et al8 performed a randomized controlled trial comparing the effect of 2 preoperative ACE inhibitor management protocols in patients undergoing noncardiac nonvascular surgery. Patients were randomized to either receive or not receive their final preoperative ACE inhibitor dose, whether scheduled on the morning of surgery or the night before.
Exclusion criteria included hypotension or hypertension at their preoperative clinic appointment (defined as systolic blood pressure < 90 or ≥ 160 mm Hg, and diastolic blood pressure < 60 or ≥ 95 mm Hg), moderate to severe heart failure, and end-stage renal disease requiring dialysis. Excluded surgery types were cardiac, vascular, organ transplant, oncologic, and all outpatient procedures. Patients taking ARBs were also excluded.
The primary outcome was intraoperative hypotension defined as any systolic blood pressure less than 80 mm Hg from the time of anesthesia induction until transfer to the postanesthesia care unit. Secondary outcomes were measured until hospital discharge and included postoperative acute kidney injury, postoperative hypotension (systolic pressure < 90 mm Hg) and hypertension (systolic pressure > 180 mm Hg), major cardiac events (composite of acute coronary syndrome, acute heart failure, or new-onset arrhythmia), and death.
Findings. A total of 453 patients were screened for eligibility, and of these, 291 were included for randomization. Their average age was 64, 48% were men, and 87% were white. About 50% underwent general anesthesia, 25% spinal, and 25% regional. Over half of the surgeries were orthopedic, and 20% were spine surgeries.
The primary outcome of intraoperative hypotension occurred significantly less often in patients randomized to ACE inhibitor omission than in the continuation group (55% vs 69%, relative risk [RR] 0.81, 95% CI 0.67–0.97, P = .03). This translates to 1 case of intraoperative hypotension for every 7.5 patients continuing an ACE inhibitor perioperatively (number needed to harm 7.5). Intraoperative hypotension associated with vasopressor administration also occurred significantly less frequently in the ACE inhibitor omission group.
Patients in the ACE inhibitor omission group were also less likely to experience postoperative hypotension, but on the other hand, they were more likely to experience severe postoperative hypertension (defined as any systolic blood pressure > 180 mm Hg). The two groups fared the same in terms of rates of acute kidney injury and major adverse cardiac events (MACE) and hospital length of stay, and no patients died in either group.
Limitations. Several factors limit the generalizability of this single-center study, including the many exclusion criteria, the predominance of orthopedic and spine surgeries, and the low-risk patient population (the average Revised Cardiac Risk Index score was 0, range 0–3). Other limitations include not controlling for the specific ACE inhibitor used and not including the precise timing of the final dose in relation to surgery. Lastly, this study lacked power to measure postoperative outcomes.
Conclusions. Continuing ACE inhibitor treatment before noncardiac nonvascular surgery is associated with a greater frequency and duration of intraoperative hypotension, but it did not increase the incidences of acute kidney injury, MACE, or death nor the hospital length of stay.
[Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837]
Hollmann et al9 performed a meta-analysis to determine whether it is better to continue or withhold ACE inhibitors and ARBs before surgery. The patients were adults undergoing noncardiac surgery and receiving an ACE inhibitor or ARB, which was either withheld or continued on the morning of surgery.
Primary outcomes were all-cause mortality and MACE, while secondary outcomes included the incidence of acute kidney injury, heart failure, stroke, intraoperative and postoperative hypotension, and length of hospital stay. Randomized controlled trials and observational studies were included, while case reports and case-control studies were excluded.
Findings. This meta-analysis included 5 randomized controlled trials and 4 cohort studies, with a total of 6,022 patients; 1,816 had their ACE inhibitor or ARB withheld before surgery, while 4,206 continued therapy. It found no difference between the 2 groups in the incidence of death or MACE, and there were not enough data to determine a difference in heart failure, stroke, acute kidney injury, or hospital length of stay.
Seven studies, with 5,414 patients, examined intraoperative hypotension. The overall incidence was 30%, but was significantly lower if the ACE inhibitor or ARB was withheld (OR 0.63, 95% CI 0.47–0.85, P = .002). Findings were similar in an analysis of only the randomized controlled trials. No difference was observed in postoperative hypotension.
Limitations. There was no standard definition of the morbidity outcomes, including hypotension and MACE. The assessment of MACE included data only for MI and not MINS. The specific duration of hypotension was not reported, and this meta-analysis did not take into account different anesthetic techniques. The duration of follow-up varied widely among studies, ranging from the day of hospital discharge to 30 days after surgery. And the randomized controlled trial performed by Shiffermiller et al8 was not included.
Conclusions. While continuing ACE inhibitors or ARBs before noncardiac surgery was associated with intraoperative hypotension, it did not seem to affect other outcomes, including death and MACE. The authors propose that a large randomized controlled trial is needed to determine whether continuing or withholding ACE inhibitor or ARB therapy before surgery is safer.
POSTOPERATIVE CARDIAC COMPLICATIONS
How should we treat MINS?
MINS is associated with an increased risk of cardiovascular events and death in both the short term and long term. MINS is defined as an elevated postoperative troponin level related to an ischemic etiology. However, whether to routinely measure troponin after surgery is unclear, as most patients do not present with ischemic symptoms, and there is no standard of care for treatment of this entity. Limited observational data suggest that starting or intensifying cardiac medications, particularly aspirin and statins, may be beneficial in terms of reducing 30-day mortality rates in patients with MI or cardiac events at 1 year in vascular surgery patients with MINS.
The Management of Myocardial Injury After Noncardiac Surgery (MANAGE) trial was designed to evaluate the potential of the anticoagulant dabigatran to prevent major vascular complications in patients with MINS.
[Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8]
Devereaux et al10 randomized patients who were at least 45 years old and had developed MINS within the previous 35 days to receive dabigatran 110 mg orally twice daily or placebo for up to 2 years. Patients not already taking a proton pump inhibitor were also randomized to take either omeprazole 20 mg once daily or placebo.
The primary efficacy outcome initially was major vascular complications, which included vascular mortality, nonfatal MI, nonhemorrhagic stroke, and peripheral arterial thrombosis. However, amputation and symptomatic venous thromboembolism were subsequently added during the study.
The primary safety outcome was a composite of life-threatening, major, and critical organ bleeding. Major bleeding required a decrease in hemoglobin of at least 4 g/dL, transfusion of at least 3 units of red blood cells within a 24-hour period, or a procedure to stop the bleeding.
Findings. The original goal was to recruit 3,200 patients, but due to slow enrollment and loss of funding, the sample was reduced to 1,754 patients (877 in each group). Approximately 45% of each group stopped taking the study drug prematurely.
The primary efficacy outcome occurred in significantly fewer patients receiving dabigatran (97, 11%) than placebo (133, 15%, HR 0.72, 95% CI 0.55–0.93, P = .0115). The incidence of the primary safety outcome was similar in both groups: 3% with dabigatran and 4% with placebo (HR 0.92, 95% CI 0.55–1.53, P = .76). The only individual efficacy outcome meeting statistical significance was a lower rate of nonhemorrhagic stroke in the dabigatran group. Subgroup analyses showed a trend benefiting patients randomized within 5 days of MINS or with a diagnosis of MI, although it was not statistically significant.
Limitations. The efficacy outcomes were expanded to include venous thromboembolism and others not directly related to MINS, raising questions about the conclusions. Further, as defined by the protocol, bleeding had to be fairly severe to be deemed major. The high number of patients who discontinued the study drug is another limitation of this study.
Conclusion. Dabigatran lowered the risk of major vascular complications with no significant increase in major bleeding in patients with MINS.
What is the risk of thromboembolism in postoperative atrial fibrillation, and what are the benefits of anticoagulation?
Although nonvalvular atrial fibrillation is associated with increased risks of ischemic stroke and systemic embolic events in nonsurgical patients, the association of new-onset postoperative atrial fibrillation with long-term thromboembolic events in the noncardiac surgical population is not well established.
[Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088]
In this retrospective cohort study using a nationwide registry in Denmark, Butt et al11 assessed the long-term risk of thromboembolic events in noncardiac surgical patients with new postoperative atrial fibrillation. Patients were identified who had no previous history of atrial fibrillation and developed it after noncardiac, nonobstetric surgeries, and were matched in a 1:4 ratio with patients who developed nonvalvular atrial fibrillation during nonsurgical hospitalizations. Matching was based on age, sex, heart failure, hypertension, diabetes, known history of thromboembolic events, ischemic heart disease, and the year patients presented with new atrial fibrillation.
Patients were excluded if they received antiarrhythmic drugs or oral anticoagulants before hospitalization or surgery, had cancer in the year prior, or died in the hospital.
The primary outcome of the study was thromboembolic events—a composite of ischemic stroke, transient cerebral ischemia, and peripheral arterial thrombosis or embolism. Secondary outcomes included rehospitalization for atrial fibrillation and all-cause mortality.
Findings. Overall, 0.4% of patients developed new postoperative atrial fibrillation, of whom 3,380 were matched with 15,320 patients with nonvalvular atrial fibrillation. Over a median follow-up of 3.2 years, the risk of thromboembolic events was similar in both groups (31.7 and 29.9 per 1,000 person-years, HR 0.95, 95% CI 0.85–1.07). The groups did not differ in their CHA2DS2-VASc risk scores, HAS-BLED risk scores, or year in which patients were diagnosed.
Anticoagulation lowered the risk of thromboembolic events to a similar extent in both groups compared with no anticoagulation:
- In postoperative atrial fibrillation—HR 0.57, 95% CI 0.40–0.67
- In nonvalvular atrial fibrillation—HR 0.56, 95% CI 0.51–0.62.
Despite the similar reduction in thromboembolic events, only 24.4% of the postoperative atrial fibrillation patients were started on anticoagulation therapy within 30 days of discharge, compared with 41.5% of those with nonvalvular atrial fibrillation.
Limitations. Although this was a large study with excellent follow-up data, it was observational. It may have underestimated the number of patients who developed postoperative atrial fibrillation because episodes that were judged not to be clinically significant may not have been charted. Many patients are not monitored with continuous telemetry postoperatively, which also may have led to underestimation of the number of atrial fibrillation events.
The study also did not examine the number of atrial fibrillation episodes per patient, the heart rhythm at discharge or long-term, or indication for and duration of anticoagulation. There were no data regarding international normalized ratio levels.
Conclusions. Postoperative atrial fibrillation is associated with outcomes similar to those of nonsurgical nonvalvular atrial fibrillation. Anticoagulation decreases the risks of stroke and death. However, substantially fewer patients with postoperative atrial fibrillation receive anticoagulation. Anticoagulation should be considered in these patients, while noting bleeding risk.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–137. doi:10.1016/j.jacc.2014.07.944
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35(35):2383–2431. doi:10.1093/eurheartj/ehu282
- Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (The Duke Activity Status Index). Am J Cardiol 1989; 64(10):651–654. doi:10.1016/0002-9149(89)90496-7
- Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018;168(4):237–244. pmid:29132159
- Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925
- Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036
- Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–137. doi:10.1016/j.jacc.2014.07.944
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35(35):2383–2431. doi:10.1093/eurheartj/ehu282
- Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (The Duke Activity Status Index). Am J Cardiol 1989; 64(10):651–654. doi:10.1016/0002-9149(89)90496-7
- Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018;168(4):237–244. pmid:29132159
- Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925
- Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036
- Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088
KEY POINTS
- The Duke Activity Status Index is a better tool for assessing cardiopulmonary fitness than subjective assessment, and it should be considered for use in guideline algorithms.
- Aspirin should not be given perioperatively in patients undergoing vascular surgery other than carotid endarterectomy.
- Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are associated with intraoperative hypotension if given before surgery. Further study is needed to determined how best to manage ACE inhibitors and ARBs perioperatively.
- In a study, dabigatran given to patients with myocardial injury after noncardiac surgery lowered the risk of major vascular complications, with no significant increase in major bleeding. But the study had major limitations.
- Postoperative atrial fibrillation is associated with outcomes similar to those of nonsurgical nonvalvular atrial fibrillation. Anticoagulation decreases its stroke and mortality risk.
An overview of endoscopy in neurologic surgery
Over the last 3 decades, the endoscope has become a highly valued visualization tool in neurosurgery, applicable to a broad range of neurosurgical procedures. Following the pace of technological innovations, the quality of the instrumentation has greatly improved along with the status of endoscopy in the neurosurgical field. The use of the endoscope in interdisciplinary extended transnasal approaches revolutionized skull-base surgery.1 Transcranial neurosurgery took advantage of the endoscope for inspection, endoscope-assisted, and endoscope-controlled procedures, although the main visualization tool during these interventions remains the operating microscope.
At present, endoscopy has applications in a variety of neurosurgical procedures including transnasal approaches for pituitary and other skull-base tumors, third ventriculostomy, and resection of intraventricular tumors. The range of application is expanding to include extracranial procedures such as peripheral nerve and spine surgery.
optics and instruments are passed through a rigid, multiport chamber. This technique is ideal when performing surgery within the ventricular system using only a standard bur hole craniotomy.
CURRENT CONCEPTS
Hopf and Perneczky2 defined the terminology regarding endoscopic procedures and divided them into 3 categories:
Pure endoscopic neurosurgery, ie, procedures performed through working channels under complete endoscopic visualization and with endoscopic instrumentation (Figure 1).3
Endoscope-controlled microsurgery, ie, operations performed with standard microsurgical instruments under endoscopic visualization—the microscope is not used (Figure 2).
Endoscope-assisted neurosurgery, ie, the use of both microscope and endoscope during the same intervention. In endoscopic inspection the endoscope is solely used as an adjunctive tool for visualization and not for surgical manipulations.
Enhanced area and surgical dissection
Technical innovations are probably the major reason for the growing role of endoscopy in neurosurgery over the last 3 decades.4 High-definition imaging, neuronavigation, new instruments, an interdisciplinary approach mostly with ear, nose, and throat (ENT) surgeons, and detailed anatomic studies led to the breakthrough of endoscopic endonasal extended approaches in skull-base surgery.5
These endoscopic techniques allow the neurosurgeon to optimize tumor resection, increasing the area of surgical dissection without increasing the size of the surgical approach, thereby limiting perioperative morbidity due to surgical manipulation of eloquent brain structures. Endoscopy offers direct illumination of the operative field, magnification, and the ability to look around corners with angled optics.
However, while angled endoscopic optics provide various visual perspectives, the surgical issue is not only to see but also to work on and around remote structures. Microsurgical endoscope-assisted manipulations require optimal working angles that are guaranteed only by a sufficiently large craniotomy. As an example, a dissection study by Chaynes et al6 highlights that a craniotomy that is too narrow often hinders a sufficient exploration of the entire cerebellopontine angle. Most neurosurgeons are familiar with the operating microscope. The microscopic field of inspection is 3-dimensional (3D) and of high quality. However, the light stream is straight and thus limited in the narrow and angled corridor of the cerebellopontine angle or in the perimesencephalic cisterns. In these situations, the angled optic of the endoscope offers the advantage of being able to look around the corner with the appropriate amount of direct illumination.7
Peripheral nerve surgery
Minimally invasive endoscopic approaches are also being used in peripheral nerve surgery, especially carpal tunnel decompression. The first carpal tunnel release treated endoscopically was performed by Okutsu et al in the late 1980s.8 Since that time, endoscopic carpal tunnel decompression has become very common and is the preferred method for many surgeons, using either single-portal or dual-portal techniques. Although the superiority of endoscopic over conventional minimally invasive microsurgical peripheral nerve surgeries has not been proven, large series of endoscopic carpal tunnel decompressions have reported low complication rates and excellent success rates with high patient satisfaction scores.8,9
Visualization of the spinal canal
Expanding the use of the endoscope to spine surgery, endoscopic explorations of the interlaminar spaces after having completed open surgical laminectomies have been reported since the early 1980s,10 while endoscope-assisted interlaminar procedures started in the late 1990s.11–13 The development of fully endoscopic transforaminal or interlaminar approaches for lumbar stenosis or lumbar disk herniation has been ongoing in the last 2 decades. The rationale for direct endoscopic visualization of the spinal canal is to reduce scarring of the epidural space, which might affect the outcome of possible revision surgeries (recurrent disk herniation), and to reduce injury to the paraspinal muscles, which may reduce postoperative incisional pain and length of hospital stay. Major limiting factors for fully endoscopic spine surgeries such as the narrow working channels (which are limited by the osseous perimeter of the neuroforamina, as well as the pelvis and abdominal structures) and the learning curve for the surgeons are, however, still matters of debate and restrict the use of endoscopy to very carefully selected cases.14,15
Pediatric craniosynostosis
Recently, the use of the endoscope has extended to treatment of craniosynostosis in pediatric patients, historically treated with large and occasionally staged craniotomic approaches. A meta-analysis of the literature showed statistically significant reductions in blood loss and rates of perioperative complications, reoperation, and transfusion compared with open approaches.16
Technical limitations
While neurosurgeons increasingly advocate the use of the endoscope in their practice, the development of instruments for endoscopic surgery does not always follow the same pace. There are technical problems with current rigid endoscopes and ergonomic limitations of the endoscope-assisted techniques in transcranial neurosurgery. The endoscope itself occupies space in an already limited surgical corridor like the posterior fossa, the parasellar space, or the intraventricular region. The ideal endoscope is thin and sturdy, does not generate heat, and provides high-resolution images. In addition, a self-irrigating feature could minimize the need to remove and reinsert the endoscope for cleaning. Finally, most intracranial surgery is extremely delicate and requires bimanual dissection. The ideal endoscope should also be easily integrated with a holder that allows the surgeon to easily transition between static and dynamic endoscope movements.
Newer flexible fiberscopes with even smaller diameters are likely to be launched on the market in the near future. When working in a surgical corridor less than 10 mm wide, this difference could be substantial.
In addition, surgical instruments specifically designed for endoscopic endonasal procedures are needed for microdissection in these regions, which were previously only visible but not reachable endoscopically. These include tools such as malleable suctions and curettes, rotatable back-biting microscissors, and malleable bipolar instruments (Figure 3).
IMPACT OF NEUROENDOSCOPY IN CURRENT CLINICAL PRACTICE
The introduction of endoscopy in neurosurgery changed many treatment paradigms and had an important impact on morbidity and outcomes. In this section, we discuss the specific indications, contraindications, and expected benefit of endoscopic vs open surgical approaches applied to neurosurgical pathology at the present time.
Skull-base tumors and CSF leaks
The use of the endoscope in skull-base surgery was originally applied to purely midline intrasellar tumors without suprasellar or lateral extension beyond the carotid cave. Ideal cases were intrasellar pituitary microadenomas not responding to medical treatment or Rathke cleft cysts.
These pathologies were traditionally addressed via microscopic craniotomic approaches and later through sublabial or transnasal transsphenoidal approaches. Traditional transsphenoidal approaches were highly invasive for the oral mucosa, causing delayed healing, oral dysesthesia, and, in some cases, loss of the superior dental arch (sublabial) or limited visualization and surgical maneuverability (microscopic endonasal).
The endoscope offered better visualization and surgical freedom, thus allowing higher resection rates to be achieved. Resection of purely intrasellar pathology with preservation of the diaphragma sellae as a barrier to the subarachnoid cysterns and third ventricle guaranteed a lower incidence of cerebrospinal fluid (CSF) leaks.
New endoscope optics with varied angles, together with dedicated long surgical instruments with low steric volume, offered a large variety of new endonasal surgical corridors, so-called expanded endonasal approaches on the sagittal and coronal planes, as discussed in detail by Kassam et al.17–19 These allowed endoscopic treatment of invasive tumors extending on the coronary plane into the suprasellar region or invading the cavernous sinuses (pituitary macroadenomas, craniopharyngiomas).
Highly specialized centers with expertise in endoscopic skull-base surgery can now also offer pure endoscopic treatment for some selected cases of lesions located far laterally to the cavernous sinus, such as trigeminal schwannomas, or along the sagittal plane like olfactory groove or tuberculum sellae meningiomas and clival lesions (chordomas, chondrosarcomas).
As one might expect, the increase in surgical complexity corresponded to an increase in complication rates. For example, the incidence of CSF leaks varied from 5% for standard midline transsphenoidal approaches to 11% for expanded endonasal approaches.20,21 The consolidation of the use of the endoscope and the cooperation with ENT surgeons led to the development of surgical strategies to prevent and reduce the incidence of CSF leaks, such as the use of “rescue flaps,” nasoseptal flaps, or temporoparietal fascia flaps.21–23
The development of such techniques allowed endoscopic endonasal approaches to be used in treatment of other pathologies, such as spontaneous CSF leaks, treated in the past with large transcranial repairs that carried high morbidity rates due to the surgical frontal lobe retraction and injury to the olfactory mucosa.24,25 Progress in the field of neuroendoscopy therefore led to the creation of specialized endoscopic skull-base surgery centers, including neurosurgery, ENT, ophthalmology, and endocrinology services.
In clinical practice, when evaluating a patient with intracranial skull-base pathology amenable to endoscopic resection, one should consider referring the patient not only to a neurosurgeon, but also to an ENT surgeon for preoperative assessment of the sinonasal cavities. The same concept applies to postsurgical follow-up, which is mostly performed by the ENT physician to assess nasal mucosa healing and nasal hygiene.
Ventricular neuroendoscopy
The introduction of endoscopic third ventriculostomy created the opportunity to offer a more physiologic treatment in selected patients with obstructive hydrocephalus by creating an internal CSF diversion through the basal cisterns. Two advantages of this procedure are that it does not create dependence on a CSF shunt, and it eliminates the related risks of shunt infection and malfunction. Its drawback is the recurrence rate of hydrocephalus (around 58% at 2 years of follow-up) due to formation of scarring in the perforated Liliequist membrane, which may require repeat surgery or conversion to CSF shunting.26,27
Neuroendoscopic approaches are also used in cases of purely intraventricular pathology such as colloid cyst or choroid plexus papillomas. The concept behind neuroendoscopy is to achieve maximal resection in a minimally invasive way, using the natural cavity of the cerebral ventricles and reducing the need for brain retraction and, in particular, the risk of injury of the fornix (therefore causing memory deficits) of open transventricular approaches and of the corpus callosum necessary in interhemispheric approaches. Large tumor size and inability to tolerate a longer surgical procedure can be relative contraindications to a pure endoscopic approach to these lesions.
Degenerative spine disease
In recent years there has been a growing interest in the use of endoscopy for selected cases of degenerative lumbar spondylosis (generally, lateral disk herniation above the L5-S1 level or spinal canal stenosis). This approach has been shown to reduce postoperative incisional pain, scarring of the epidural space affecting the outcome of possible revision surgeries (recurrent disc herniation), and length of hospital stay.14,15 Information on surgical nuances should be provided when consulting on selected patients with lumbar degenerative disease resistant to conservative treatment.
Carpal tunnel syndrome
Although endoscopic carpal tunnel release is controversial, its supporters report smaller incision size and lower recurrence rates due to better visualization of the entire carpal ligament compared with open surgery, with high patient satisfaction scores.8,9,28
Craniosynostosis
Increasing data from specialized centers show that early endoscopic suturectomy is an effective treatment option alone or when combined with open surgeries for patients with syndromic and nonsyndromic craniosynostosis. The aesthetic advantage of small incisions (which can also be achieved with some open techniques) is accompanied by significant reductions in blood loss (median 162.4 mL), operative time (median 112.38 minutes), length of stay (median 2.56 days), and rates of perioperative complications (odds ratio 0.58), reoperation (odds ratio 0.37), and transfusion (odds ratio 0.09) compared with open approaches.16
SURGICAL TRAINING
Today’s patients expect high-quality healthcare, and they approach their surgeons with an enormous amount of information collected through unlimited Web-based access or peer-group blogs. In this respect, the pressure on young surgeons to achieve excellent results is high and growing from the very beginning of their careers.
Residency training programs differ in each country, and surgical standards usually focus on open microscopic procedures rather than newly developed endoscopic techniques. Endoscopic pituitary adenoma surgery, the most frequent neuroendoscopic procedure, is still performed mostly by experienced neurosurgeons, not trainees. Moreover, many training institutions might not offer pediatric neurosurgery care, limiting exposure to endoscopic third ventriculostomy procedures. The European Union of Medical Specialists, responsible for harmonizing and improving the quality of training of medical specialists in Europe, set low neuroendoscopic surgical requirements for trainees to complete their residency programs (minimum of 0 to optimum of 5 total transcranial or transsphenoidal pituitary adenoma resections as first operator, 10 procedures as assistant, and a minimum of 2 to an optimum of 4 endoscopic third ventriculostomies as first operator).29
The need to develop training programs in neuroendoscopy is especially urgent because endoscopic surgery has a steeper learning curve than conventional microneurosurgery. In particular, endoscopy requires a good deal of dexterity and hand-eye coordination, which surgeons consider the main pitfall of neuroendoscopy. For such reasons, many accredited clinical fellowship programs have been developed inside and outside North America that offer intensive training in endoscopic skull-base surgery and pediatric neurosurgery after residency.
Some clinical studies have shown that the complication rate of neuroendoscopy is 15% to 18%.27,30 In view of this statistic, it is ethically questionable to perform a randomized study to prospectively compare microscopic and endoscopic procedures. Surgeons specialize in one technique or the other, experience their own learning curve, and do not randomly decide which tool to use. Furthermore, every intracranial surgical exploration is unique and somewhat difficult to compare with each other without the risk of bias.
FURTHER DEVELOPMENTS
Multivariable rigid endoscopes like the EndoCAMeleon (Karl Storz, Tuttlingen, Germany) or the EndActive (Karl Storz, Tuttlingen, Germany) for cerebellopontine angle surgery represent a starting point to overcome some of the aforementioned limitations.31,32 They are inserted in the surgical field with a direct 0° angulation view into the operative site beyond neurovascular structures that need to be preserved and that obstruct the microscopic view. Once the final position is reached, the field of view is directed toward the region of interest without moving the endoscope tip.
The EndoCAMeleon is a rigid rod-lens endoscope, steerable in one plane from –10° to +120° by a fine optomechanical mechanism. Anatomic laboratory testing found it to be superior in terms of usability and visualization compared with rigid fixed-angle endoscopes.31 The first clinical experiences have been promising; however, ergonomics and the limited perspective of a single plane of rotation leave room for improvement.
The EndActive endoscope might overcome such limitations.33 This device is a rigid videoendoscope connected to a laptop (video data) and USB port (control and power supply); thus, it weighs less and can be held in one hand like a microsurgical instrument. The endoscopic imaging system allows the operator to simultaneously see a 160° wide-angle view of the site and an inset of a specific region of interest. The surgeon can hold the device like a microsurgical instrument in one hand and control movements precisely due to its reduced weight and ergonomic shape.
The multiplanar variable-view rigid endoscope has proven to be useful for working on diverse anatomic structures such as intracranial vessels and cranial nerves. The device is effective in narrow working spaces where even small movements can jeopardize the delicate surrounding structures. The multiplanar variable-viewing mechanism in a compact device offers advantages in terms of safety and ergonomics. Improving the usability will probably optimize the applicability of those endoscopic devices in neurosurgery. A major drawback of the current prototype is poor image resolution, which will probably soon be overcome with the ongoing progress in electronic microchip technology.
The addition of laser technology to endoscopic techniques offers a huge potential to neurosurgery but has achieved little acceptance to date. The reasons include concern regarding heat production, uncontrollable and distant penetration, and tissue interaction. Experiences with a 2-micron continuous- wave laser (RevoLix Jr, LISA Laser Products, Katlenburg-Lindau, Germany) for neuroendoscopic intraventricular procedures proved this laser to be a valuable and useful tool with safe applicability for endoscopic intracranial procedures in patients of all ages.34
Parallel to the launch of video screens for other uses with higher image definition, the image quality on the 2D endoscope cameras has been constantly improving over the last years. At the same time, the introduction of modern 3D endoscopic monitors is promising. However, 3D endoscopes have some disadvantages compared with the 2D endoscopes. First, the smallest 3D endoscopes are 4 mm in diameter, compared with 2.7 mm for 2D endoscopes. Moreover, the field of view with the 3D endoscope is less than half of that with conventional 2D endoscopes.34 When working in and around a region with critical neurovascular structures in close proximity, this loss of field of view can result in an increase in iatrogenic injury from the endoscope. In addition, 3D endoscopes require special glasses, generating a potential obstacle to the seamless integration of visual information from the microscope and endoscope. Finally, some surgeons experience vertigo when looking at the 3D picture through the glasses, which limits its universal applicability.
CONCLUSIONS
Using the endoscope and microscope as complementary and not competing tools allows surgeons to benefit from both technologies at the same time.35,36 The intraoperative combination of these 2 powerful visualization tools expands the effectiveness of microsurgical procedures and has the potential to further improve surgical results and reduce surgical risks. With endoscope-assisted microsurgery, visualization is often far superior to surgical maneuverability.
Endoscopic neurosurgery will likely be influenced by further innovations in optical physics, electronics, and robotics. Specific implementations in endoscopic systems are likely to pave the way for remarkable progress in minimally invasive surgery, such as robotic surgical technology, further miniaturization of devices, improvements in 3D endoscopy, multiport endoscopy, and new designs for surgical instruments. Future progress in flexible endoscopes and wireless capsule or camera technology may reduce our dependence on rigid rod lens systems. Rigid variable-view endoscopes will bring endoscopes closer to ideal attributes utilizing newer instrumentation that is tailored to specific indications and techniques.37,38 Extension of the visual field by the feature of a movable optic lens may allow the neurosurgeon to use tailored keyhole approaches to treat pathologies in smaller surgical corridors with less trauma and greater efficacy.
- Kassam AB, Gardner P, Snyderman C, Mintz A, Carrau R. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus 2005; 19(1):E6. pmid:16078820
- Hopf NJ, Perneczky A. Endoscopic neurosurgery and endoscope-assisted microneurosurgery for the treatment of intracranial cysts. Neurosurgery 1998; 43(6):1330–1336. doi:10.1097/00006123-199812000-00037
- Li KW, Nelson C, Suk I, Jallo GI. Neuroendoscopy: past, present, and future. Neurosurg Focus 2005; 19(6):E1. doi:10.3171/foc.2005.19.6.2
- Prevedello DM, Doglietto F, Jane JA Jr, Jagannathan J, Han J, Laws ER Jr. History of endoscopic skull base surgery: its evolution and current reality. J Neurosurg 2007; 107(1):206–213. doi:10.3171/JNS-07/07/0206
- Schroeder HW, Nehlsen M. Value of high-definition imaging in neuroendoscopy. Neurosurg Rev 2009; 32(3):303–308. doi:10.1007/s10143-009-0200-x
- Chaynes P, Deguine O, Moscovici J, Fraysse B, Becue J, Lazorthes Y. Endoscopic anatomy of the cerebellopontine angle: a study in cadaver brains. Neurosurg Focus 1998; 5(3):e8.
- Setty P, Volkov AA, D'Andrea KP, Pieper DR. Endoscopic vascular decompression for the treatment of trigeminal neuralgia: clinical outcomes and technical note. World Neurosurg 2014; 81(3–4):603–608. doi:10.1016/j.wneu.2013.10.036
- Okutsu I, Hamanaka I, Yoshida A. Retrospective analysis of five-year and longer clinical and electrophysiological results of the world's first endoscopic management for carpal tunnel syndrome. Hand Surg 2013; 18(3):317–323. doi:10.1142/S0218810413500330
- Zuo D, Zhou Z, Wang H, et al. Endoscopic versus open carpal tunnel release for idiopathic carpal tunnel syndrome: a meta-analysis of randomized controlled trials. J Orthop Surg Res 2015; 10:12. doi:10.1186/s13018-014-0148-6
- Forst R, Hausmann B. Nucleoscopy—a new examination technique. Arch Orthop Trauma Surg 1983; 101(3):219–221. pmid:6870510
- Brayda-Bruno M, Cinnella P. Posterior endoscopic discectomy (and other procedures). Eur Spine J 2000; 9(suppl 1):S24–S29. pmid:10766054
- Destandau J. A special device for endoscopic surgery of lumbar disc herniation. Neurol Res 1999; 21(1):39–42. pmid:10048052
- Perez-Cruet MJ, Foley KT, Isaacs RE, et al. Microendoscopic lumbar discectomy: technical note. Neurosurgery 2002; 51(5 suppl):S129–S136. pmid:12234440
- Ruetten S, Komp M, Merk H, Godolias G. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine (Phila Pa 1976) 2008; 33(9):931–939. doi:10.1097/BRS.0b013e31816c8af7
- Komp M, Hahn P, Merk H, Godolias G, Ruetten S. Bilateral operation of lumbar degenerative central spinal stenosis in full-endoscopic interlaminar technique with unilateral approach: prospective 2-year results of 74 patients. J Spinal Disord Tech 2011; 24(5):281–287. doi:10.1097/BSD.0b013e3181f9f55e
- Goyal A, Lu VM, Yolcu YU, Elminawy M, Daniels DJ. Endoscopic versus open approach in craniosynostosis repair: a systematic review and meta-analysis of perioperative outcomes. Childs Nerv Syst 2018; 34(9):1627–1637. doi:10.1007/s00381-018-3852-4
- Kassam AB, Gardner P, Snyderman C, Mintz A, Carrau R. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus 2005; 19(1):E6. pmid:16078820
- Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL. Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg Focus 2005; 19(1):E4. pmid:16078818
- Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus 2005; 19(1):E3. pmid:16078817
- Kassam A, Carrau RL, Snyderman CH, Gardner P, Mintz A. Evolution of reconstructive techniques following endoscopic expanded endonasal approaches. Neurosurg Focus 2005; 19(1):E8. pmid:16078822
- Kassam AB, Thomas A, Carrau RL, et al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery 2008; 63(1 suppl 1):ONS44–ONS52. doi:10.1227/01.NEU.0000297074.13423.F5
- Hadad G, Bassagasteguy L, Carrau RL, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope 2006; 116(10):1882–1886. doi:10.1097/01.mlg.0000234933.37779.e4
- Fortes FS, Carrau RL, Snyderman CH, et al. Transpterygoid transposition of a temporoparietal fascia flap: a new method for skull base reconstruction after endoscopic expanded endonasal approaches. Laryngoscope 2007; 117(6):970–976. doi:10.1097/MLG.0b013e3180471482
- Carrau RL, Snyderman CH, Kassam AB. The management of cerebrospinal fluid leaks in patients at risk for high-pressure hydrocephalus. Laryngoscope 2005; 115(2):205–212. doi:10.1097/01.mlg.0000154719.62668.70
- Zweig JL, Carrau RL, Celin SE, et al. Endoscopic repair of cerebrospinal fluid leaks to the sinonasal tract: predictors of success. Otolaryngol Head Neck Surg 2000; 123(3):195–201. doi:10.1067/mhn.2000.107452
- Kulkarni AV, Riva-Cambrin J, Holubkov R, et al. Endoscopic third ventriculostomy in children: prospective, multicenter results from the Hydrocephalus Clinical Research Network. J Neurosurg Pediatr 2016; 18(4):423–429. doi:10.3171/2016.4.PEDS163
- Ersahin Y, Arslan D. Complications of endoscopic third ventriculostomy. Childs Nerv Syst 2008; 24(8):943–948. doi:10.1007/s00381-008-0589-5
- Martínez-Catasús A, Lobo-Escolar L, García-Bonet J, Corrales-Rodríguez M, Pasarín-Martínez A, Berlanga-de-Mingo D. Comparison between single portal endoscopic, 1-cm open carpal tunnel release. Hand Surg Rehabil 2019. pii:S2468-1229(19)30027-1. doi:10.1016/j.hansur.2019.02.003
- Steers J, Reulen HJ, Lindsay K; European Union of Medical Specialists; Joint Residency Advisory and Accreditation Committee. UEMS charter on training of medical specialists in the EU—the new neurosurgical training charter. Acta Neurochir Suppl 2004; 90:3–11. pmid:15553111
- Mori H, Nishiyama K, Yoshimura J, Tanaka R. Current status of neuroendoscopic surgery in Japan and discussion on the training system. Childs Nerv Syst 2007; 23(6):673–676. doi:10.1007/s00381-007-0329-2
- Aryan HE, Hoeg HD, Marshall LF, Levy ML. Multidirectional projectional rigid neuro-endoscopy: prototype and initial experience. Minim Invasive Neurosurg 2005; 48(5):293–296. doi:10.1055/s-2005-915602
- Ebner FH, Marquardt JS, Hirt B, Tatagiba M, Schuhmann MU. Visualization of the anterior cerebral artery complex with a continuously variable-view rigid endoscope: new options in aneurysm surgery. Neurosurgery 2010; 67(2 suppl operative):321–324. doi:10.1227/NEU.0b013e3181f74548
- Ebner FH, Hirt B, Marquardt JS, Herlan S, Tatagiba M, Schuhmann MU. Actual state of EndActive ventricular endoscopy. Childs Nerv Syst 2012; 28(1):87–91. doi:10.1007/s00381-011-1537-3
- Ebner FH, Nagel C, Tatagiba M, Schuhmann MU. Efficacy and versatility of the 2-micron continuous wave laser in neuroendoscopic procedures. Acta Neurochir Suppl 2012; 113:143–147. doi:10.1007/978-3-7091-0923-6_29
- Van Gompel JJ, Tabor MH, Youssef AS, et al. Field of view comparison between two-dimensional and three-dimensional endoscopy. Laryngoscope 2014; 124(2):387–390. doi:10.1002/lary.24222
- Ebner FH, Roser F, Thaher F, Schittenhelm J, Tatagiba M. Balancing the shortcomings of microscope and endoscope: endoscope-assisted technique in microsurgical removal of recurrent epidermoid cysts in the posterior fossa. Minim Invasive Neurosurg 2010 ;53(5–6):218–222. doi:10.1055/s-0030-1267973
- Perneczky A, Fries G. Endoscope-assisted brain surgery: part 1—evolution, basic concept, and current technique. Neurosurgery 1998; 42(2):219–224. doi:10.1097/00006123-199802000-00001
- Ebner FH, Marquardt JS, Hirt B, Feigl GC, Tatagiba M, Schuhmann MU. Broadening horizons of neuroendoscopy with a variable-view rigid endoscope: an anatomical study. Eur J Surg Oncol 2010; 36(2):195–200. doi:10.1016/j.ejso.2009.07.185
meningioma, peripheral nerve, spinal canal, minimally invasive, carpal tunnel, ventricular neuroendoscopy, craniosynostosis, degenerative spine disease, Luigi Rigante, Hamid Borghei-Razavi, Pablo Recinos, Florian Roser
Over the last 3 decades, the endoscope has become a highly valued visualization tool in neurosurgery, applicable to a broad range of neurosurgical procedures. Following the pace of technological innovations, the quality of the instrumentation has greatly improved along with the status of endoscopy in the neurosurgical field. The use of the endoscope in interdisciplinary extended transnasal approaches revolutionized skull-base surgery.1 Transcranial neurosurgery took advantage of the endoscope for inspection, endoscope-assisted, and endoscope-controlled procedures, although the main visualization tool during these interventions remains the operating microscope.
At present, endoscopy has applications in a variety of neurosurgical procedures including transnasal approaches for pituitary and other skull-base tumors, third ventriculostomy, and resection of intraventricular tumors. The range of application is expanding to include extracranial procedures such as peripheral nerve and spine surgery.
optics and instruments are passed through a rigid, multiport chamber. This technique is ideal when performing surgery within the ventricular system using only a standard bur hole craniotomy.
CURRENT CONCEPTS
Hopf and Perneczky2 defined the terminology regarding endoscopic procedures and divided them into 3 categories:
Pure endoscopic neurosurgery, ie, procedures performed through working channels under complete endoscopic visualization and with endoscopic instrumentation (Figure 1).3
Endoscope-controlled microsurgery, ie, operations performed with standard microsurgical instruments under endoscopic visualization—the microscope is not used (Figure 2).
Endoscope-assisted neurosurgery, ie, the use of both microscope and endoscope during the same intervention. In endoscopic inspection the endoscope is solely used as an adjunctive tool for visualization and not for surgical manipulations.
Enhanced area and surgical dissection
Technical innovations are probably the major reason for the growing role of endoscopy in neurosurgery over the last 3 decades.4 High-definition imaging, neuronavigation, new instruments, an interdisciplinary approach mostly with ear, nose, and throat (ENT) surgeons, and detailed anatomic studies led to the breakthrough of endoscopic endonasal extended approaches in skull-base surgery.5
These endoscopic techniques allow the neurosurgeon to optimize tumor resection, increasing the area of surgical dissection without increasing the size of the surgical approach, thereby limiting perioperative morbidity due to surgical manipulation of eloquent brain structures. Endoscopy offers direct illumination of the operative field, magnification, and the ability to look around corners with angled optics.
However, while angled endoscopic optics provide various visual perspectives, the surgical issue is not only to see but also to work on and around remote structures. Microsurgical endoscope-assisted manipulations require optimal working angles that are guaranteed only by a sufficiently large craniotomy. As an example, a dissection study by Chaynes et al6 highlights that a craniotomy that is too narrow often hinders a sufficient exploration of the entire cerebellopontine angle. Most neurosurgeons are familiar with the operating microscope. The microscopic field of inspection is 3-dimensional (3D) and of high quality. However, the light stream is straight and thus limited in the narrow and angled corridor of the cerebellopontine angle or in the perimesencephalic cisterns. In these situations, the angled optic of the endoscope offers the advantage of being able to look around the corner with the appropriate amount of direct illumination.7
Peripheral nerve surgery
Minimally invasive endoscopic approaches are also being used in peripheral nerve surgery, especially carpal tunnel decompression. The first carpal tunnel release treated endoscopically was performed by Okutsu et al in the late 1980s.8 Since that time, endoscopic carpal tunnel decompression has become very common and is the preferred method for many surgeons, using either single-portal or dual-portal techniques. Although the superiority of endoscopic over conventional minimally invasive microsurgical peripheral nerve surgeries has not been proven, large series of endoscopic carpal tunnel decompressions have reported low complication rates and excellent success rates with high patient satisfaction scores.8,9
Visualization of the spinal canal
Expanding the use of the endoscope to spine surgery, endoscopic explorations of the interlaminar spaces after having completed open surgical laminectomies have been reported since the early 1980s,10 while endoscope-assisted interlaminar procedures started in the late 1990s.11–13 The development of fully endoscopic transforaminal or interlaminar approaches for lumbar stenosis or lumbar disk herniation has been ongoing in the last 2 decades. The rationale for direct endoscopic visualization of the spinal canal is to reduce scarring of the epidural space, which might affect the outcome of possible revision surgeries (recurrent disk herniation), and to reduce injury to the paraspinal muscles, which may reduce postoperative incisional pain and length of hospital stay. Major limiting factors for fully endoscopic spine surgeries such as the narrow working channels (which are limited by the osseous perimeter of the neuroforamina, as well as the pelvis and abdominal structures) and the learning curve for the surgeons are, however, still matters of debate and restrict the use of endoscopy to very carefully selected cases.14,15
Pediatric craniosynostosis
Recently, the use of the endoscope has extended to treatment of craniosynostosis in pediatric patients, historically treated with large and occasionally staged craniotomic approaches. A meta-analysis of the literature showed statistically significant reductions in blood loss and rates of perioperative complications, reoperation, and transfusion compared with open approaches.16
Technical limitations
While neurosurgeons increasingly advocate the use of the endoscope in their practice, the development of instruments for endoscopic surgery does not always follow the same pace. There are technical problems with current rigid endoscopes and ergonomic limitations of the endoscope-assisted techniques in transcranial neurosurgery. The endoscope itself occupies space in an already limited surgical corridor like the posterior fossa, the parasellar space, or the intraventricular region. The ideal endoscope is thin and sturdy, does not generate heat, and provides high-resolution images. In addition, a self-irrigating feature could minimize the need to remove and reinsert the endoscope for cleaning. Finally, most intracranial surgery is extremely delicate and requires bimanual dissection. The ideal endoscope should also be easily integrated with a holder that allows the surgeon to easily transition between static and dynamic endoscope movements.
Newer flexible fiberscopes with even smaller diameters are likely to be launched on the market in the near future. When working in a surgical corridor less than 10 mm wide, this difference could be substantial.
In addition, surgical instruments specifically designed for endoscopic endonasal procedures are needed for microdissection in these regions, which were previously only visible but not reachable endoscopically. These include tools such as malleable suctions and curettes, rotatable back-biting microscissors, and malleable bipolar instruments (Figure 3).
IMPACT OF NEUROENDOSCOPY IN CURRENT CLINICAL PRACTICE
The introduction of endoscopy in neurosurgery changed many treatment paradigms and had an important impact on morbidity and outcomes. In this section, we discuss the specific indications, contraindications, and expected benefit of endoscopic vs open surgical approaches applied to neurosurgical pathology at the present time.
Skull-base tumors and CSF leaks
The use of the endoscope in skull-base surgery was originally applied to purely midline intrasellar tumors without suprasellar or lateral extension beyond the carotid cave. Ideal cases were intrasellar pituitary microadenomas not responding to medical treatment or Rathke cleft cysts.
These pathologies were traditionally addressed via microscopic craniotomic approaches and later through sublabial or transnasal transsphenoidal approaches. Traditional transsphenoidal approaches were highly invasive for the oral mucosa, causing delayed healing, oral dysesthesia, and, in some cases, loss of the superior dental arch (sublabial) or limited visualization and surgical maneuverability (microscopic endonasal).
The endoscope offered better visualization and surgical freedom, thus allowing higher resection rates to be achieved. Resection of purely intrasellar pathology with preservation of the diaphragma sellae as a barrier to the subarachnoid cysterns and third ventricle guaranteed a lower incidence of cerebrospinal fluid (CSF) leaks.
New endoscope optics with varied angles, together with dedicated long surgical instruments with low steric volume, offered a large variety of new endonasal surgical corridors, so-called expanded endonasal approaches on the sagittal and coronal planes, as discussed in detail by Kassam et al.17–19 These allowed endoscopic treatment of invasive tumors extending on the coronary plane into the suprasellar region or invading the cavernous sinuses (pituitary macroadenomas, craniopharyngiomas).
Highly specialized centers with expertise in endoscopic skull-base surgery can now also offer pure endoscopic treatment for some selected cases of lesions located far laterally to the cavernous sinus, such as trigeminal schwannomas, or along the sagittal plane like olfactory groove or tuberculum sellae meningiomas and clival lesions (chordomas, chondrosarcomas).
As one might expect, the increase in surgical complexity corresponded to an increase in complication rates. For example, the incidence of CSF leaks varied from 5% for standard midline transsphenoidal approaches to 11% for expanded endonasal approaches.20,21 The consolidation of the use of the endoscope and the cooperation with ENT surgeons led to the development of surgical strategies to prevent and reduce the incidence of CSF leaks, such as the use of “rescue flaps,” nasoseptal flaps, or temporoparietal fascia flaps.21–23
The development of such techniques allowed endoscopic endonasal approaches to be used in treatment of other pathologies, such as spontaneous CSF leaks, treated in the past with large transcranial repairs that carried high morbidity rates due to the surgical frontal lobe retraction and injury to the olfactory mucosa.24,25 Progress in the field of neuroendoscopy therefore led to the creation of specialized endoscopic skull-base surgery centers, including neurosurgery, ENT, ophthalmology, and endocrinology services.
In clinical practice, when evaluating a patient with intracranial skull-base pathology amenable to endoscopic resection, one should consider referring the patient not only to a neurosurgeon, but also to an ENT surgeon for preoperative assessment of the sinonasal cavities. The same concept applies to postsurgical follow-up, which is mostly performed by the ENT physician to assess nasal mucosa healing and nasal hygiene.
Ventricular neuroendoscopy
The introduction of endoscopic third ventriculostomy created the opportunity to offer a more physiologic treatment in selected patients with obstructive hydrocephalus by creating an internal CSF diversion through the basal cisterns. Two advantages of this procedure are that it does not create dependence on a CSF shunt, and it eliminates the related risks of shunt infection and malfunction. Its drawback is the recurrence rate of hydrocephalus (around 58% at 2 years of follow-up) due to formation of scarring in the perforated Liliequist membrane, which may require repeat surgery or conversion to CSF shunting.26,27
Neuroendoscopic approaches are also used in cases of purely intraventricular pathology such as colloid cyst or choroid plexus papillomas. The concept behind neuroendoscopy is to achieve maximal resection in a minimally invasive way, using the natural cavity of the cerebral ventricles and reducing the need for brain retraction and, in particular, the risk of injury of the fornix (therefore causing memory deficits) of open transventricular approaches and of the corpus callosum necessary in interhemispheric approaches. Large tumor size and inability to tolerate a longer surgical procedure can be relative contraindications to a pure endoscopic approach to these lesions.
Degenerative spine disease
In recent years there has been a growing interest in the use of endoscopy for selected cases of degenerative lumbar spondylosis (generally, lateral disk herniation above the L5-S1 level or spinal canal stenosis). This approach has been shown to reduce postoperative incisional pain, scarring of the epidural space affecting the outcome of possible revision surgeries (recurrent disc herniation), and length of hospital stay.14,15 Information on surgical nuances should be provided when consulting on selected patients with lumbar degenerative disease resistant to conservative treatment.
Carpal tunnel syndrome
Although endoscopic carpal tunnel release is controversial, its supporters report smaller incision size and lower recurrence rates due to better visualization of the entire carpal ligament compared with open surgery, with high patient satisfaction scores.8,9,28
Craniosynostosis
Increasing data from specialized centers show that early endoscopic suturectomy is an effective treatment option alone or when combined with open surgeries for patients with syndromic and nonsyndromic craniosynostosis. The aesthetic advantage of small incisions (which can also be achieved with some open techniques) is accompanied by significant reductions in blood loss (median 162.4 mL), operative time (median 112.38 minutes), length of stay (median 2.56 days), and rates of perioperative complications (odds ratio 0.58), reoperation (odds ratio 0.37), and transfusion (odds ratio 0.09) compared with open approaches.16
SURGICAL TRAINING
Today’s patients expect high-quality healthcare, and they approach their surgeons with an enormous amount of information collected through unlimited Web-based access or peer-group blogs. In this respect, the pressure on young surgeons to achieve excellent results is high and growing from the very beginning of their careers.
Residency training programs differ in each country, and surgical standards usually focus on open microscopic procedures rather than newly developed endoscopic techniques. Endoscopic pituitary adenoma surgery, the most frequent neuroendoscopic procedure, is still performed mostly by experienced neurosurgeons, not trainees. Moreover, many training institutions might not offer pediatric neurosurgery care, limiting exposure to endoscopic third ventriculostomy procedures. The European Union of Medical Specialists, responsible for harmonizing and improving the quality of training of medical specialists in Europe, set low neuroendoscopic surgical requirements for trainees to complete their residency programs (minimum of 0 to optimum of 5 total transcranial or transsphenoidal pituitary adenoma resections as first operator, 10 procedures as assistant, and a minimum of 2 to an optimum of 4 endoscopic third ventriculostomies as first operator).29
The need to develop training programs in neuroendoscopy is especially urgent because endoscopic surgery has a steeper learning curve than conventional microneurosurgery. In particular, endoscopy requires a good deal of dexterity and hand-eye coordination, which surgeons consider the main pitfall of neuroendoscopy. For such reasons, many accredited clinical fellowship programs have been developed inside and outside North America that offer intensive training in endoscopic skull-base surgery and pediatric neurosurgery after residency.
Some clinical studies have shown that the complication rate of neuroendoscopy is 15% to 18%.27,30 In view of this statistic, it is ethically questionable to perform a randomized study to prospectively compare microscopic and endoscopic procedures. Surgeons specialize in one technique or the other, experience their own learning curve, and do not randomly decide which tool to use. Furthermore, every intracranial surgical exploration is unique and somewhat difficult to compare with each other without the risk of bias.
FURTHER DEVELOPMENTS
Multivariable rigid endoscopes like the EndoCAMeleon (Karl Storz, Tuttlingen, Germany) or the EndActive (Karl Storz, Tuttlingen, Germany) for cerebellopontine angle surgery represent a starting point to overcome some of the aforementioned limitations.31,32 They are inserted in the surgical field with a direct 0° angulation view into the operative site beyond neurovascular structures that need to be preserved and that obstruct the microscopic view. Once the final position is reached, the field of view is directed toward the region of interest without moving the endoscope tip.
The EndoCAMeleon is a rigid rod-lens endoscope, steerable in one plane from –10° to +120° by a fine optomechanical mechanism. Anatomic laboratory testing found it to be superior in terms of usability and visualization compared with rigid fixed-angle endoscopes.31 The first clinical experiences have been promising; however, ergonomics and the limited perspective of a single plane of rotation leave room for improvement.
The EndActive endoscope might overcome such limitations.33 This device is a rigid videoendoscope connected to a laptop (video data) and USB port (control and power supply); thus, it weighs less and can be held in one hand like a microsurgical instrument. The endoscopic imaging system allows the operator to simultaneously see a 160° wide-angle view of the site and an inset of a specific region of interest. The surgeon can hold the device like a microsurgical instrument in one hand and control movements precisely due to its reduced weight and ergonomic shape.
The multiplanar variable-view rigid endoscope has proven to be useful for working on diverse anatomic structures such as intracranial vessels and cranial nerves. The device is effective in narrow working spaces where even small movements can jeopardize the delicate surrounding structures. The multiplanar variable-viewing mechanism in a compact device offers advantages in terms of safety and ergonomics. Improving the usability will probably optimize the applicability of those endoscopic devices in neurosurgery. A major drawback of the current prototype is poor image resolution, which will probably soon be overcome with the ongoing progress in electronic microchip technology.
The addition of laser technology to endoscopic techniques offers a huge potential to neurosurgery but has achieved little acceptance to date. The reasons include concern regarding heat production, uncontrollable and distant penetration, and tissue interaction. Experiences with a 2-micron continuous- wave laser (RevoLix Jr, LISA Laser Products, Katlenburg-Lindau, Germany) for neuroendoscopic intraventricular procedures proved this laser to be a valuable and useful tool with safe applicability for endoscopic intracranial procedures in patients of all ages.34
Parallel to the launch of video screens for other uses with higher image definition, the image quality on the 2D endoscope cameras has been constantly improving over the last years. At the same time, the introduction of modern 3D endoscopic monitors is promising. However, 3D endoscopes have some disadvantages compared with the 2D endoscopes. First, the smallest 3D endoscopes are 4 mm in diameter, compared with 2.7 mm for 2D endoscopes. Moreover, the field of view with the 3D endoscope is less than half of that with conventional 2D endoscopes.34 When working in and around a region with critical neurovascular structures in close proximity, this loss of field of view can result in an increase in iatrogenic injury from the endoscope. In addition, 3D endoscopes require special glasses, generating a potential obstacle to the seamless integration of visual information from the microscope and endoscope. Finally, some surgeons experience vertigo when looking at the 3D picture through the glasses, which limits its universal applicability.
CONCLUSIONS
Using the endoscope and microscope as complementary and not competing tools allows surgeons to benefit from both technologies at the same time.35,36 The intraoperative combination of these 2 powerful visualization tools expands the effectiveness of microsurgical procedures and has the potential to further improve surgical results and reduce surgical risks. With endoscope-assisted microsurgery, visualization is often far superior to surgical maneuverability.
Endoscopic neurosurgery will likely be influenced by further innovations in optical physics, electronics, and robotics. Specific implementations in endoscopic systems are likely to pave the way for remarkable progress in minimally invasive surgery, such as robotic surgical technology, further miniaturization of devices, improvements in 3D endoscopy, multiport endoscopy, and new designs for surgical instruments. Future progress in flexible endoscopes and wireless capsule or camera technology may reduce our dependence on rigid rod lens systems. Rigid variable-view endoscopes will bring endoscopes closer to ideal attributes utilizing newer instrumentation that is tailored to specific indications and techniques.37,38 Extension of the visual field by the feature of a movable optic lens may allow the neurosurgeon to use tailored keyhole approaches to treat pathologies in smaller surgical corridors with less trauma and greater efficacy.
Over the last 3 decades, the endoscope has become a highly valued visualization tool in neurosurgery, applicable to a broad range of neurosurgical procedures. Following the pace of technological innovations, the quality of the instrumentation has greatly improved along with the status of endoscopy in the neurosurgical field. The use of the endoscope in interdisciplinary extended transnasal approaches revolutionized skull-base surgery.1 Transcranial neurosurgery took advantage of the endoscope for inspection, endoscope-assisted, and endoscope-controlled procedures, although the main visualization tool during these interventions remains the operating microscope.
At present, endoscopy has applications in a variety of neurosurgical procedures including transnasal approaches for pituitary and other skull-base tumors, third ventriculostomy, and resection of intraventricular tumors. The range of application is expanding to include extracranial procedures such as peripheral nerve and spine surgery.
optics and instruments are passed through a rigid, multiport chamber. This technique is ideal when performing surgery within the ventricular system using only a standard bur hole craniotomy.
CURRENT CONCEPTS
Hopf and Perneczky2 defined the terminology regarding endoscopic procedures and divided them into 3 categories:
Pure endoscopic neurosurgery, ie, procedures performed through working channels under complete endoscopic visualization and with endoscopic instrumentation (Figure 1).3
Endoscope-controlled microsurgery, ie, operations performed with standard microsurgical instruments under endoscopic visualization—the microscope is not used (Figure 2).
Endoscope-assisted neurosurgery, ie, the use of both microscope and endoscope during the same intervention. In endoscopic inspection the endoscope is solely used as an adjunctive tool for visualization and not for surgical manipulations.
Enhanced area and surgical dissection
Technical innovations are probably the major reason for the growing role of endoscopy in neurosurgery over the last 3 decades.4 High-definition imaging, neuronavigation, new instruments, an interdisciplinary approach mostly with ear, nose, and throat (ENT) surgeons, and detailed anatomic studies led to the breakthrough of endoscopic endonasal extended approaches in skull-base surgery.5
These endoscopic techniques allow the neurosurgeon to optimize tumor resection, increasing the area of surgical dissection without increasing the size of the surgical approach, thereby limiting perioperative morbidity due to surgical manipulation of eloquent brain structures. Endoscopy offers direct illumination of the operative field, magnification, and the ability to look around corners with angled optics.
However, while angled endoscopic optics provide various visual perspectives, the surgical issue is not only to see but also to work on and around remote structures. Microsurgical endoscope-assisted manipulations require optimal working angles that are guaranteed only by a sufficiently large craniotomy. As an example, a dissection study by Chaynes et al6 highlights that a craniotomy that is too narrow often hinders a sufficient exploration of the entire cerebellopontine angle. Most neurosurgeons are familiar with the operating microscope. The microscopic field of inspection is 3-dimensional (3D) and of high quality. However, the light stream is straight and thus limited in the narrow and angled corridor of the cerebellopontine angle or in the perimesencephalic cisterns. In these situations, the angled optic of the endoscope offers the advantage of being able to look around the corner with the appropriate amount of direct illumination.7
Peripheral nerve surgery
Minimally invasive endoscopic approaches are also being used in peripheral nerve surgery, especially carpal tunnel decompression. The first carpal tunnel release treated endoscopically was performed by Okutsu et al in the late 1980s.8 Since that time, endoscopic carpal tunnel decompression has become very common and is the preferred method for many surgeons, using either single-portal or dual-portal techniques. Although the superiority of endoscopic over conventional minimally invasive microsurgical peripheral nerve surgeries has not been proven, large series of endoscopic carpal tunnel decompressions have reported low complication rates and excellent success rates with high patient satisfaction scores.8,9
Visualization of the spinal canal
Expanding the use of the endoscope to spine surgery, endoscopic explorations of the interlaminar spaces after having completed open surgical laminectomies have been reported since the early 1980s,10 while endoscope-assisted interlaminar procedures started in the late 1990s.11–13 The development of fully endoscopic transforaminal or interlaminar approaches for lumbar stenosis or lumbar disk herniation has been ongoing in the last 2 decades. The rationale for direct endoscopic visualization of the spinal canal is to reduce scarring of the epidural space, which might affect the outcome of possible revision surgeries (recurrent disk herniation), and to reduce injury to the paraspinal muscles, which may reduce postoperative incisional pain and length of hospital stay. Major limiting factors for fully endoscopic spine surgeries such as the narrow working channels (which are limited by the osseous perimeter of the neuroforamina, as well as the pelvis and abdominal structures) and the learning curve for the surgeons are, however, still matters of debate and restrict the use of endoscopy to very carefully selected cases.14,15
Pediatric craniosynostosis
Recently, the use of the endoscope has extended to treatment of craniosynostosis in pediatric patients, historically treated with large and occasionally staged craniotomic approaches. A meta-analysis of the literature showed statistically significant reductions in blood loss and rates of perioperative complications, reoperation, and transfusion compared with open approaches.16
Technical limitations
While neurosurgeons increasingly advocate the use of the endoscope in their practice, the development of instruments for endoscopic surgery does not always follow the same pace. There are technical problems with current rigid endoscopes and ergonomic limitations of the endoscope-assisted techniques in transcranial neurosurgery. The endoscope itself occupies space in an already limited surgical corridor like the posterior fossa, the parasellar space, or the intraventricular region. The ideal endoscope is thin and sturdy, does not generate heat, and provides high-resolution images. In addition, a self-irrigating feature could minimize the need to remove and reinsert the endoscope for cleaning. Finally, most intracranial surgery is extremely delicate and requires bimanual dissection. The ideal endoscope should also be easily integrated with a holder that allows the surgeon to easily transition between static and dynamic endoscope movements.
Newer flexible fiberscopes with even smaller diameters are likely to be launched on the market in the near future. When working in a surgical corridor less than 10 mm wide, this difference could be substantial.
In addition, surgical instruments specifically designed for endoscopic endonasal procedures are needed for microdissection in these regions, which were previously only visible but not reachable endoscopically. These include tools such as malleable suctions and curettes, rotatable back-biting microscissors, and malleable bipolar instruments (Figure 3).
IMPACT OF NEUROENDOSCOPY IN CURRENT CLINICAL PRACTICE
The introduction of endoscopy in neurosurgery changed many treatment paradigms and had an important impact on morbidity and outcomes. In this section, we discuss the specific indications, contraindications, and expected benefit of endoscopic vs open surgical approaches applied to neurosurgical pathology at the present time.
Skull-base tumors and CSF leaks
The use of the endoscope in skull-base surgery was originally applied to purely midline intrasellar tumors without suprasellar or lateral extension beyond the carotid cave. Ideal cases were intrasellar pituitary microadenomas not responding to medical treatment or Rathke cleft cysts.
These pathologies were traditionally addressed via microscopic craniotomic approaches and later through sublabial or transnasal transsphenoidal approaches. Traditional transsphenoidal approaches were highly invasive for the oral mucosa, causing delayed healing, oral dysesthesia, and, in some cases, loss of the superior dental arch (sublabial) or limited visualization and surgical maneuverability (microscopic endonasal).
The endoscope offered better visualization and surgical freedom, thus allowing higher resection rates to be achieved. Resection of purely intrasellar pathology with preservation of the diaphragma sellae as a barrier to the subarachnoid cysterns and third ventricle guaranteed a lower incidence of cerebrospinal fluid (CSF) leaks.
New endoscope optics with varied angles, together with dedicated long surgical instruments with low steric volume, offered a large variety of new endonasal surgical corridors, so-called expanded endonasal approaches on the sagittal and coronal planes, as discussed in detail by Kassam et al.17–19 These allowed endoscopic treatment of invasive tumors extending on the coronary plane into the suprasellar region or invading the cavernous sinuses (pituitary macroadenomas, craniopharyngiomas).
Highly specialized centers with expertise in endoscopic skull-base surgery can now also offer pure endoscopic treatment for some selected cases of lesions located far laterally to the cavernous sinus, such as trigeminal schwannomas, or along the sagittal plane like olfactory groove or tuberculum sellae meningiomas and clival lesions (chordomas, chondrosarcomas).
As one might expect, the increase in surgical complexity corresponded to an increase in complication rates. For example, the incidence of CSF leaks varied from 5% for standard midline transsphenoidal approaches to 11% for expanded endonasal approaches.20,21 The consolidation of the use of the endoscope and the cooperation with ENT surgeons led to the development of surgical strategies to prevent and reduce the incidence of CSF leaks, such as the use of “rescue flaps,” nasoseptal flaps, or temporoparietal fascia flaps.21–23
The development of such techniques allowed endoscopic endonasal approaches to be used in treatment of other pathologies, such as spontaneous CSF leaks, treated in the past with large transcranial repairs that carried high morbidity rates due to the surgical frontal lobe retraction and injury to the olfactory mucosa.24,25 Progress in the field of neuroendoscopy therefore led to the creation of specialized endoscopic skull-base surgery centers, including neurosurgery, ENT, ophthalmology, and endocrinology services.
In clinical practice, when evaluating a patient with intracranial skull-base pathology amenable to endoscopic resection, one should consider referring the patient not only to a neurosurgeon, but also to an ENT surgeon for preoperative assessment of the sinonasal cavities. The same concept applies to postsurgical follow-up, which is mostly performed by the ENT physician to assess nasal mucosa healing and nasal hygiene.
Ventricular neuroendoscopy
The introduction of endoscopic third ventriculostomy created the opportunity to offer a more physiologic treatment in selected patients with obstructive hydrocephalus by creating an internal CSF diversion through the basal cisterns. Two advantages of this procedure are that it does not create dependence on a CSF shunt, and it eliminates the related risks of shunt infection and malfunction. Its drawback is the recurrence rate of hydrocephalus (around 58% at 2 years of follow-up) due to formation of scarring in the perforated Liliequist membrane, which may require repeat surgery or conversion to CSF shunting.26,27
Neuroendoscopic approaches are also used in cases of purely intraventricular pathology such as colloid cyst or choroid plexus papillomas. The concept behind neuroendoscopy is to achieve maximal resection in a minimally invasive way, using the natural cavity of the cerebral ventricles and reducing the need for brain retraction and, in particular, the risk of injury of the fornix (therefore causing memory deficits) of open transventricular approaches and of the corpus callosum necessary in interhemispheric approaches. Large tumor size and inability to tolerate a longer surgical procedure can be relative contraindications to a pure endoscopic approach to these lesions.
Degenerative spine disease
In recent years there has been a growing interest in the use of endoscopy for selected cases of degenerative lumbar spondylosis (generally, lateral disk herniation above the L5-S1 level or spinal canal stenosis). This approach has been shown to reduce postoperative incisional pain, scarring of the epidural space affecting the outcome of possible revision surgeries (recurrent disc herniation), and length of hospital stay.14,15 Information on surgical nuances should be provided when consulting on selected patients with lumbar degenerative disease resistant to conservative treatment.
Carpal tunnel syndrome
Although endoscopic carpal tunnel release is controversial, its supporters report smaller incision size and lower recurrence rates due to better visualization of the entire carpal ligament compared with open surgery, with high patient satisfaction scores.8,9,28
Craniosynostosis
Increasing data from specialized centers show that early endoscopic suturectomy is an effective treatment option alone or when combined with open surgeries for patients with syndromic and nonsyndromic craniosynostosis. The aesthetic advantage of small incisions (which can also be achieved with some open techniques) is accompanied by significant reductions in blood loss (median 162.4 mL), operative time (median 112.38 minutes), length of stay (median 2.56 days), and rates of perioperative complications (odds ratio 0.58), reoperation (odds ratio 0.37), and transfusion (odds ratio 0.09) compared with open approaches.16
SURGICAL TRAINING
Today’s patients expect high-quality healthcare, and they approach their surgeons with an enormous amount of information collected through unlimited Web-based access or peer-group blogs. In this respect, the pressure on young surgeons to achieve excellent results is high and growing from the very beginning of their careers.
Residency training programs differ in each country, and surgical standards usually focus on open microscopic procedures rather than newly developed endoscopic techniques. Endoscopic pituitary adenoma surgery, the most frequent neuroendoscopic procedure, is still performed mostly by experienced neurosurgeons, not trainees. Moreover, many training institutions might not offer pediatric neurosurgery care, limiting exposure to endoscopic third ventriculostomy procedures. The European Union of Medical Specialists, responsible for harmonizing and improving the quality of training of medical specialists in Europe, set low neuroendoscopic surgical requirements for trainees to complete their residency programs (minimum of 0 to optimum of 5 total transcranial or transsphenoidal pituitary adenoma resections as first operator, 10 procedures as assistant, and a minimum of 2 to an optimum of 4 endoscopic third ventriculostomies as first operator).29
The need to develop training programs in neuroendoscopy is especially urgent because endoscopic surgery has a steeper learning curve than conventional microneurosurgery. In particular, endoscopy requires a good deal of dexterity and hand-eye coordination, which surgeons consider the main pitfall of neuroendoscopy. For such reasons, many accredited clinical fellowship programs have been developed inside and outside North America that offer intensive training in endoscopic skull-base surgery and pediatric neurosurgery after residency.
Some clinical studies have shown that the complication rate of neuroendoscopy is 15% to 18%.27,30 In view of this statistic, it is ethically questionable to perform a randomized study to prospectively compare microscopic and endoscopic procedures. Surgeons specialize in one technique or the other, experience their own learning curve, and do not randomly decide which tool to use. Furthermore, every intracranial surgical exploration is unique and somewhat difficult to compare with each other without the risk of bias.
FURTHER DEVELOPMENTS
Multivariable rigid endoscopes like the EndoCAMeleon (Karl Storz, Tuttlingen, Germany) or the EndActive (Karl Storz, Tuttlingen, Germany) for cerebellopontine angle surgery represent a starting point to overcome some of the aforementioned limitations.31,32 They are inserted in the surgical field with a direct 0° angulation view into the operative site beyond neurovascular structures that need to be preserved and that obstruct the microscopic view. Once the final position is reached, the field of view is directed toward the region of interest without moving the endoscope tip.
The EndoCAMeleon is a rigid rod-lens endoscope, steerable in one plane from –10° to +120° by a fine optomechanical mechanism. Anatomic laboratory testing found it to be superior in terms of usability and visualization compared with rigid fixed-angle endoscopes.31 The first clinical experiences have been promising; however, ergonomics and the limited perspective of a single plane of rotation leave room for improvement.
The EndActive endoscope might overcome such limitations.33 This device is a rigid videoendoscope connected to a laptop (video data) and USB port (control and power supply); thus, it weighs less and can be held in one hand like a microsurgical instrument. The endoscopic imaging system allows the operator to simultaneously see a 160° wide-angle view of the site and an inset of a specific region of interest. The surgeon can hold the device like a microsurgical instrument in one hand and control movements precisely due to its reduced weight and ergonomic shape.
The multiplanar variable-view rigid endoscope has proven to be useful for working on diverse anatomic structures such as intracranial vessels and cranial nerves. The device is effective in narrow working spaces where even small movements can jeopardize the delicate surrounding structures. The multiplanar variable-viewing mechanism in a compact device offers advantages in terms of safety and ergonomics. Improving the usability will probably optimize the applicability of those endoscopic devices in neurosurgery. A major drawback of the current prototype is poor image resolution, which will probably soon be overcome with the ongoing progress in electronic microchip technology.
The addition of laser technology to endoscopic techniques offers a huge potential to neurosurgery but has achieved little acceptance to date. The reasons include concern regarding heat production, uncontrollable and distant penetration, and tissue interaction. Experiences with a 2-micron continuous- wave laser (RevoLix Jr, LISA Laser Products, Katlenburg-Lindau, Germany) for neuroendoscopic intraventricular procedures proved this laser to be a valuable and useful tool with safe applicability for endoscopic intracranial procedures in patients of all ages.34
Parallel to the launch of video screens for other uses with higher image definition, the image quality on the 2D endoscope cameras has been constantly improving over the last years. At the same time, the introduction of modern 3D endoscopic monitors is promising. However, 3D endoscopes have some disadvantages compared with the 2D endoscopes. First, the smallest 3D endoscopes are 4 mm in diameter, compared with 2.7 mm for 2D endoscopes. Moreover, the field of view with the 3D endoscope is less than half of that with conventional 2D endoscopes.34 When working in and around a region with critical neurovascular structures in close proximity, this loss of field of view can result in an increase in iatrogenic injury from the endoscope. In addition, 3D endoscopes require special glasses, generating a potential obstacle to the seamless integration of visual information from the microscope and endoscope. Finally, some surgeons experience vertigo when looking at the 3D picture through the glasses, which limits its universal applicability.
CONCLUSIONS
Using the endoscope and microscope as complementary and not competing tools allows surgeons to benefit from both technologies at the same time.35,36 The intraoperative combination of these 2 powerful visualization tools expands the effectiveness of microsurgical procedures and has the potential to further improve surgical results and reduce surgical risks. With endoscope-assisted microsurgery, visualization is often far superior to surgical maneuverability.
Endoscopic neurosurgery will likely be influenced by further innovations in optical physics, electronics, and robotics. Specific implementations in endoscopic systems are likely to pave the way for remarkable progress in minimally invasive surgery, such as robotic surgical technology, further miniaturization of devices, improvements in 3D endoscopy, multiport endoscopy, and new designs for surgical instruments. Future progress in flexible endoscopes and wireless capsule or camera technology may reduce our dependence on rigid rod lens systems. Rigid variable-view endoscopes will bring endoscopes closer to ideal attributes utilizing newer instrumentation that is tailored to specific indications and techniques.37,38 Extension of the visual field by the feature of a movable optic lens may allow the neurosurgeon to use tailored keyhole approaches to treat pathologies in smaller surgical corridors with less trauma and greater efficacy.
- Kassam AB, Gardner P, Snyderman C, Mintz A, Carrau R. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus 2005; 19(1):E6. pmid:16078820
- Hopf NJ, Perneczky A. Endoscopic neurosurgery and endoscope-assisted microneurosurgery for the treatment of intracranial cysts. Neurosurgery 1998; 43(6):1330–1336. doi:10.1097/00006123-199812000-00037
- Li KW, Nelson C, Suk I, Jallo GI. Neuroendoscopy: past, present, and future. Neurosurg Focus 2005; 19(6):E1. doi:10.3171/foc.2005.19.6.2
- Prevedello DM, Doglietto F, Jane JA Jr, Jagannathan J, Han J, Laws ER Jr. History of endoscopic skull base surgery: its evolution and current reality. J Neurosurg 2007; 107(1):206–213. doi:10.3171/JNS-07/07/0206
- Schroeder HW, Nehlsen M. Value of high-definition imaging in neuroendoscopy. Neurosurg Rev 2009; 32(3):303–308. doi:10.1007/s10143-009-0200-x
- Chaynes P, Deguine O, Moscovici J, Fraysse B, Becue J, Lazorthes Y. Endoscopic anatomy of the cerebellopontine angle: a study in cadaver brains. Neurosurg Focus 1998; 5(3):e8.
- Setty P, Volkov AA, D'Andrea KP, Pieper DR. Endoscopic vascular decompression for the treatment of trigeminal neuralgia: clinical outcomes and technical note. World Neurosurg 2014; 81(3–4):603–608. doi:10.1016/j.wneu.2013.10.036
- Okutsu I, Hamanaka I, Yoshida A. Retrospective analysis of five-year and longer clinical and electrophysiological results of the world's first endoscopic management for carpal tunnel syndrome. Hand Surg 2013; 18(3):317–323. doi:10.1142/S0218810413500330
- Zuo D, Zhou Z, Wang H, et al. Endoscopic versus open carpal tunnel release for idiopathic carpal tunnel syndrome: a meta-analysis of randomized controlled trials. J Orthop Surg Res 2015; 10:12. doi:10.1186/s13018-014-0148-6
- Forst R, Hausmann B. Nucleoscopy—a new examination technique. Arch Orthop Trauma Surg 1983; 101(3):219–221. pmid:6870510
- Brayda-Bruno M, Cinnella P. Posterior endoscopic discectomy (and other procedures). Eur Spine J 2000; 9(suppl 1):S24–S29. pmid:10766054
- Destandau J. A special device for endoscopic surgery of lumbar disc herniation. Neurol Res 1999; 21(1):39–42. pmid:10048052
- Perez-Cruet MJ, Foley KT, Isaacs RE, et al. Microendoscopic lumbar discectomy: technical note. Neurosurgery 2002; 51(5 suppl):S129–S136. pmid:12234440
- Ruetten S, Komp M, Merk H, Godolias G. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine (Phila Pa 1976) 2008; 33(9):931–939. doi:10.1097/BRS.0b013e31816c8af7
- Komp M, Hahn P, Merk H, Godolias G, Ruetten S. Bilateral operation of lumbar degenerative central spinal stenosis in full-endoscopic interlaminar technique with unilateral approach: prospective 2-year results of 74 patients. J Spinal Disord Tech 2011; 24(5):281–287. doi:10.1097/BSD.0b013e3181f9f55e
- Goyal A, Lu VM, Yolcu YU, Elminawy M, Daniels DJ. Endoscopic versus open approach in craniosynostosis repair: a systematic review and meta-analysis of perioperative outcomes. Childs Nerv Syst 2018; 34(9):1627–1637. doi:10.1007/s00381-018-3852-4
- Kassam AB, Gardner P, Snyderman C, Mintz A, Carrau R. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus 2005; 19(1):E6. pmid:16078820
- Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL. Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg Focus 2005; 19(1):E4. pmid:16078818
- Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus 2005; 19(1):E3. pmid:16078817
- Kassam A, Carrau RL, Snyderman CH, Gardner P, Mintz A. Evolution of reconstructive techniques following endoscopic expanded endonasal approaches. Neurosurg Focus 2005; 19(1):E8. pmid:16078822
- Kassam AB, Thomas A, Carrau RL, et al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery 2008; 63(1 suppl 1):ONS44–ONS52. doi:10.1227/01.NEU.0000297074.13423.F5
- Hadad G, Bassagasteguy L, Carrau RL, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope 2006; 116(10):1882–1886. doi:10.1097/01.mlg.0000234933.37779.e4
- Fortes FS, Carrau RL, Snyderman CH, et al. Transpterygoid transposition of a temporoparietal fascia flap: a new method for skull base reconstruction after endoscopic expanded endonasal approaches. Laryngoscope 2007; 117(6):970–976. doi:10.1097/MLG.0b013e3180471482
- Carrau RL, Snyderman CH, Kassam AB. The management of cerebrospinal fluid leaks in patients at risk for high-pressure hydrocephalus. Laryngoscope 2005; 115(2):205–212. doi:10.1097/01.mlg.0000154719.62668.70
- Zweig JL, Carrau RL, Celin SE, et al. Endoscopic repair of cerebrospinal fluid leaks to the sinonasal tract: predictors of success. Otolaryngol Head Neck Surg 2000; 123(3):195–201. doi:10.1067/mhn.2000.107452
- Kulkarni AV, Riva-Cambrin J, Holubkov R, et al. Endoscopic third ventriculostomy in children: prospective, multicenter results from the Hydrocephalus Clinical Research Network. J Neurosurg Pediatr 2016; 18(4):423–429. doi:10.3171/2016.4.PEDS163
- Ersahin Y, Arslan D. Complications of endoscopic third ventriculostomy. Childs Nerv Syst 2008; 24(8):943–948. doi:10.1007/s00381-008-0589-5
- Martínez-Catasús A, Lobo-Escolar L, García-Bonet J, Corrales-Rodríguez M, Pasarín-Martínez A, Berlanga-de-Mingo D. Comparison between single portal endoscopic, 1-cm open carpal tunnel release. Hand Surg Rehabil 2019. pii:S2468-1229(19)30027-1. doi:10.1016/j.hansur.2019.02.003
- Steers J, Reulen HJ, Lindsay K; European Union of Medical Specialists; Joint Residency Advisory and Accreditation Committee. UEMS charter on training of medical specialists in the EU—the new neurosurgical training charter. Acta Neurochir Suppl 2004; 90:3–11. pmid:15553111
- Mori H, Nishiyama K, Yoshimura J, Tanaka R. Current status of neuroendoscopic surgery in Japan and discussion on the training system. Childs Nerv Syst 2007; 23(6):673–676. doi:10.1007/s00381-007-0329-2
- Aryan HE, Hoeg HD, Marshall LF, Levy ML. Multidirectional projectional rigid neuro-endoscopy: prototype and initial experience. Minim Invasive Neurosurg 2005; 48(5):293–296. doi:10.1055/s-2005-915602
- Ebner FH, Marquardt JS, Hirt B, Tatagiba M, Schuhmann MU. Visualization of the anterior cerebral artery complex with a continuously variable-view rigid endoscope: new options in aneurysm surgery. Neurosurgery 2010; 67(2 suppl operative):321–324. doi:10.1227/NEU.0b013e3181f74548
- Ebner FH, Hirt B, Marquardt JS, Herlan S, Tatagiba M, Schuhmann MU. Actual state of EndActive ventricular endoscopy. Childs Nerv Syst 2012; 28(1):87–91. doi:10.1007/s00381-011-1537-3
- Ebner FH, Nagel C, Tatagiba M, Schuhmann MU. Efficacy and versatility of the 2-micron continuous wave laser in neuroendoscopic procedures. Acta Neurochir Suppl 2012; 113:143–147. doi:10.1007/978-3-7091-0923-6_29
- Van Gompel JJ, Tabor MH, Youssef AS, et al. Field of view comparison between two-dimensional and three-dimensional endoscopy. Laryngoscope 2014; 124(2):387–390. doi:10.1002/lary.24222
- Ebner FH, Roser F, Thaher F, Schittenhelm J, Tatagiba M. Balancing the shortcomings of microscope and endoscope: endoscope-assisted technique in microsurgical removal of recurrent epidermoid cysts in the posterior fossa. Minim Invasive Neurosurg 2010 ;53(5–6):218–222. doi:10.1055/s-0030-1267973
- Perneczky A, Fries G. Endoscope-assisted brain surgery: part 1—evolution, basic concept, and current technique. Neurosurgery 1998; 42(2):219–224. doi:10.1097/00006123-199802000-00001
- Ebner FH, Marquardt JS, Hirt B, Feigl GC, Tatagiba M, Schuhmann MU. Broadening horizons of neuroendoscopy with a variable-view rigid endoscope: an anatomical study. Eur J Surg Oncol 2010; 36(2):195–200. doi:10.1016/j.ejso.2009.07.185
- Kassam AB, Gardner P, Snyderman C, Mintz A, Carrau R. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus 2005; 19(1):E6. pmid:16078820
- Hopf NJ, Perneczky A. Endoscopic neurosurgery and endoscope-assisted microneurosurgery for the treatment of intracranial cysts. Neurosurgery 1998; 43(6):1330–1336. doi:10.1097/00006123-199812000-00037
- Li KW, Nelson C, Suk I, Jallo GI. Neuroendoscopy: past, present, and future. Neurosurg Focus 2005; 19(6):E1. doi:10.3171/foc.2005.19.6.2
- Prevedello DM, Doglietto F, Jane JA Jr, Jagannathan J, Han J, Laws ER Jr. History of endoscopic skull base surgery: its evolution and current reality. J Neurosurg 2007; 107(1):206–213. doi:10.3171/JNS-07/07/0206
- Schroeder HW, Nehlsen M. Value of high-definition imaging in neuroendoscopy. Neurosurg Rev 2009; 32(3):303–308. doi:10.1007/s10143-009-0200-x
- Chaynes P, Deguine O, Moscovici J, Fraysse B, Becue J, Lazorthes Y. Endoscopic anatomy of the cerebellopontine angle: a study in cadaver brains. Neurosurg Focus 1998; 5(3):e8.
- Setty P, Volkov AA, D'Andrea KP, Pieper DR. Endoscopic vascular decompression for the treatment of trigeminal neuralgia: clinical outcomes and technical note. World Neurosurg 2014; 81(3–4):603–608. doi:10.1016/j.wneu.2013.10.036
- Okutsu I, Hamanaka I, Yoshida A. Retrospective analysis of five-year and longer clinical and electrophysiological results of the world's first endoscopic management for carpal tunnel syndrome. Hand Surg 2013; 18(3):317–323. doi:10.1142/S0218810413500330
- Zuo D, Zhou Z, Wang H, et al. Endoscopic versus open carpal tunnel release for idiopathic carpal tunnel syndrome: a meta-analysis of randomized controlled trials. J Orthop Surg Res 2015; 10:12. doi:10.1186/s13018-014-0148-6
- Forst R, Hausmann B. Nucleoscopy—a new examination technique. Arch Orthop Trauma Surg 1983; 101(3):219–221. pmid:6870510
- Brayda-Bruno M, Cinnella P. Posterior endoscopic discectomy (and other procedures). Eur Spine J 2000; 9(suppl 1):S24–S29. pmid:10766054
- Destandau J. A special device for endoscopic surgery of lumbar disc herniation. Neurol Res 1999; 21(1):39–42. pmid:10048052
- Perez-Cruet MJ, Foley KT, Isaacs RE, et al. Microendoscopic lumbar discectomy: technical note. Neurosurgery 2002; 51(5 suppl):S129–S136. pmid:12234440
- Ruetten S, Komp M, Merk H, Godolias G. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine (Phila Pa 1976) 2008; 33(9):931–939. doi:10.1097/BRS.0b013e31816c8af7
- Komp M, Hahn P, Merk H, Godolias G, Ruetten S. Bilateral operation of lumbar degenerative central spinal stenosis in full-endoscopic interlaminar technique with unilateral approach: prospective 2-year results of 74 patients. J Spinal Disord Tech 2011; 24(5):281–287. doi:10.1097/BSD.0b013e3181f9f55e
- Goyal A, Lu VM, Yolcu YU, Elminawy M, Daniels DJ. Endoscopic versus open approach in craniosynostosis repair: a systematic review and meta-analysis of perioperative outcomes. Childs Nerv Syst 2018; 34(9):1627–1637. doi:10.1007/s00381-018-3852-4
- Kassam AB, Gardner P, Snyderman C, Mintz A, Carrau R. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus 2005; 19(1):E6. pmid:16078820
- Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL. Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg Focus 2005; 19(1):E4. pmid:16078818
- Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus 2005; 19(1):E3. pmid:16078817
- Kassam A, Carrau RL, Snyderman CH, Gardner P, Mintz A. Evolution of reconstructive techniques following endoscopic expanded endonasal approaches. Neurosurg Focus 2005; 19(1):E8. pmid:16078822
- Kassam AB, Thomas A, Carrau RL, et al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery 2008; 63(1 suppl 1):ONS44–ONS52. doi:10.1227/01.NEU.0000297074.13423.F5
- Hadad G, Bassagasteguy L, Carrau RL, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope 2006; 116(10):1882–1886. doi:10.1097/01.mlg.0000234933.37779.e4
- Fortes FS, Carrau RL, Snyderman CH, et al. Transpterygoid transposition of a temporoparietal fascia flap: a new method for skull base reconstruction after endoscopic expanded endonasal approaches. Laryngoscope 2007; 117(6):970–976. doi:10.1097/MLG.0b013e3180471482
- Carrau RL, Snyderman CH, Kassam AB. The management of cerebrospinal fluid leaks in patients at risk for high-pressure hydrocephalus. Laryngoscope 2005; 115(2):205–212. doi:10.1097/01.mlg.0000154719.62668.70
- Zweig JL, Carrau RL, Celin SE, et al. Endoscopic repair of cerebrospinal fluid leaks to the sinonasal tract: predictors of success. Otolaryngol Head Neck Surg 2000; 123(3):195–201. doi:10.1067/mhn.2000.107452
- Kulkarni AV, Riva-Cambrin J, Holubkov R, et al. Endoscopic third ventriculostomy in children: prospective, multicenter results from the Hydrocephalus Clinical Research Network. J Neurosurg Pediatr 2016; 18(4):423–429. doi:10.3171/2016.4.PEDS163
- Ersahin Y, Arslan D. Complications of endoscopic third ventriculostomy. Childs Nerv Syst 2008; 24(8):943–948. doi:10.1007/s00381-008-0589-5
- Martínez-Catasús A, Lobo-Escolar L, García-Bonet J, Corrales-Rodríguez M, Pasarín-Martínez A, Berlanga-de-Mingo D. Comparison between single portal endoscopic, 1-cm open carpal tunnel release. Hand Surg Rehabil 2019. pii:S2468-1229(19)30027-1. doi:10.1016/j.hansur.2019.02.003
- Steers J, Reulen HJ, Lindsay K; European Union of Medical Specialists; Joint Residency Advisory and Accreditation Committee. UEMS charter on training of medical specialists in the EU—the new neurosurgical training charter. Acta Neurochir Suppl 2004; 90:3–11. pmid:15553111
- Mori H, Nishiyama K, Yoshimura J, Tanaka R. Current status of neuroendoscopic surgery in Japan and discussion on the training system. Childs Nerv Syst 2007; 23(6):673–676. doi:10.1007/s00381-007-0329-2
- Aryan HE, Hoeg HD, Marshall LF, Levy ML. Multidirectional projectional rigid neuro-endoscopy: prototype and initial experience. Minim Invasive Neurosurg 2005; 48(5):293–296. doi:10.1055/s-2005-915602
- Ebner FH, Marquardt JS, Hirt B, Tatagiba M, Schuhmann MU. Visualization of the anterior cerebral artery complex with a continuously variable-view rigid endoscope: new options in aneurysm surgery. Neurosurgery 2010; 67(2 suppl operative):321–324. doi:10.1227/NEU.0b013e3181f74548
- Ebner FH, Hirt B, Marquardt JS, Herlan S, Tatagiba M, Schuhmann MU. Actual state of EndActive ventricular endoscopy. Childs Nerv Syst 2012; 28(1):87–91. doi:10.1007/s00381-011-1537-3
- Ebner FH, Nagel C, Tatagiba M, Schuhmann MU. Efficacy and versatility of the 2-micron continuous wave laser in neuroendoscopic procedures. Acta Neurochir Suppl 2012; 113:143–147. doi:10.1007/978-3-7091-0923-6_29
- Van Gompel JJ, Tabor MH, Youssef AS, et al. Field of view comparison between two-dimensional and three-dimensional endoscopy. Laryngoscope 2014; 124(2):387–390. doi:10.1002/lary.24222
- Ebner FH, Roser F, Thaher F, Schittenhelm J, Tatagiba M. Balancing the shortcomings of microscope and endoscope: endoscope-assisted technique in microsurgical removal of recurrent epidermoid cysts in the posterior fossa. Minim Invasive Neurosurg 2010 ;53(5–6):218–222. doi:10.1055/s-0030-1267973
- Perneczky A, Fries G. Endoscope-assisted brain surgery: part 1—evolution, basic concept, and current technique. Neurosurgery 1998; 42(2):219–224. doi:10.1097/00006123-199802000-00001
- Ebner FH, Marquardt JS, Hirt B, Feigl GC, Tatagiba M, Schuhmann MU. Broadening horizons of neuroendoscopy with a variable-view rigid endoscope: an anatomical study. Eur J Surg Oncol 2010; 36(2):195–200. doi:10.1016/j.ejso.2009.07.185
meningioma, peripheral nerve, spinal canal, minimally invasive, carpal tunnel, ventricular neuroendoscopy, craniosynostosis, degenerative spine disease, Luigi Rigante, Hamid Borghei-Razavi, Pablo Recinos, Florian Roser
meningioma, peripheral nerve, spinal canal, minimally invasive, carpal tunnel, ventricular neuroendoscopy, craniosynostosis, degenerative spine disease, Luigi Rigante, Hamid Borghei-Razavi, Pablo Recinos, Florian Roser
KEY POINTS
- An increasing number of neurosurgical patients are undergoing endoscopic surgeries of the brain, spine, and peripheral nerves. Familiarization with these techniques provides medical specialists with important knowledge regarding appropriate patient care.
- The combination of classic microscopic and endoscopic procedures improves surgical outcomes by increasing surgical maneuverability and reducing manipulation of eloquent structures.
- Further innovations in optical physics, electronics, and robotics will dramatically improve the potential of endoscopic neurosurgery in the next decades.
A few pearls can help prepare the mind
We need to recognize the diverse problems that patients with potential multisystem disease can develop, lobby when necessary for them to be seen promptly by the relevant specialists, and initiate appropriate diagnostic testing and management in less-urgent scenarios. Most of us need frequent refreshers on the clinical manifestations of these disorders so that we can recognize them when they appear unannounced in our exam rooms.
The caregiver with a prepared mind is more likely to experience the diagnostic epiphany, and then use point-of-care references to hone in on the details. With many patients and clinical conundrums, the basics matter.
Dr. Chester Oddis, in this issue of the Journal, reviews the basics of several primary muscle disorders. He discusses, in a case-based format extracted from his recent Medicine Grand Rounds presentation at Cleveland Clinic, nuances of specific diagnoses and the clinical progression of diseases that are critical to be aware of in order to recognize and manage them, and expeditiously refer the patient to our appropriate subspecialty colleagues.
Major challenges exist in recognizing the inflammatory myopathies and their mimics early in their course. These are serious but uncommon entities, and in part because patients and physicians often attribute their early symptoms to more-common causes, diagnosis can be elusive—until the possibility is considered. We hope that Dr. Oddis’s article will make it easier to rapidly recognize these muscle disorders.
Patients often struggle to explain their symptoms of early muscle dysfunction. Since patients often verbalize their fatigue as “feeling weak,” we often misconstrue complaints of true muscle weakness (like difficulty walking up steps) as being due to fatigue. Add in some anemia from chronic inflammation and some “liver test” abnormalities, and it is easy to see how the recognition of true muscle weakness can be delayed.
We can tease muscle weakness from fatigue or dyspnea by asking the patient to specifically and functionally describe their “weakness,” and then by asking pointed questions: “Do you have difficulty getting up from the toilet without using your arms? Do you have trouble brushing your hair or teeth?” Physical examination can clearly help here, but without routine examination of muscle strength in normal fragile elderly patients, the degree of muscle weakness can be difficult to assess. Likewise challenging is detecting the early onset of weakness by examination in a 280-lb power-lifter.
Obtaining an accurate functional and behavioral history is often critical to the early recognition of muscle disease. Muscle pain, as Dr. Oddis notes, is not a characteristic feature of many myopathies, whereas, paradoxically, the coexistence of new-onset symmetrical small-joint pain (especially with arthritis) along with muscle weakness can be a powerful clue to the diagnosis of an inflammatory myopathy.
An elevated creatine kinase (CK) level generally points directly to a muscle disease, although some neurologic disorders are associated with elevations in CK, and the entity of benign “hyperCKemia” must be recognized and not overmanaged. The latter becomes a problem when laboratory tests are allowed to drive the diagnostic evaluation in a vacuum of clinical details.
A more common scenario is the misinterpretation of common laboratory test abnormalities in the setting of a patient with “fatigue” or generalized weakness who has elevations in aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Although AST and ALT are often called “liver function tests,” these enzymes are also abundant in skeletal muscle, and since they are included on routine biochemical panels, their elevation often leads to liver imaging and sometimes even biopsy before anyone recognizes muscle disease as the cause of the patient’s symptoms and laboratory test abnormalities. Hence, a muscle source (or hemolysis) should at least be considered when AST and ALT are elevated in the absence of elevated alkaline phosphatase or gamma-glutamyl transferase.
When evaluating innumerable clinical scenarios, experienced clinicians can most certainly generate similar principles of diagnostic reasoning, based on having a few fundamental facts at their fingertips. Increasing the chances of having a prepared mind when confronted with a patient with a less-than-straightforward set of symptoms is one of my major arguments in support of continuing to read and generate internal medicine teaching literature and to attend and participate in clinical teaching conferences such as Medicine Grand Rounds. It is also why we will continue to appreciate and publish presentations like this one in the Journal.
I don’t expect to retain all the details from these and similar papers, and I know we all carry virtually infinite databases in our pockets. But keeping a few clinical pearls outside of my specialty in my head comes in handy. Having a prepared mind makes it much easier to converse with patients, to promptly initiate appropriate testing, plans, and consultations, and to then decide what to search for on my smartphone between patients.
We need to recognize the diverse problems that patients with potential multisystem disease can develop, lobby when necessary for them to be seen promptly by the relevant specialists, and initiate appropriate diagnostic testing and management in less-urgent scenarios. Most of us need frequent refreshers on the clinical manifestations of these disorders so that we can recognize them when they appear unannounced in our exam rooms.
The caregiver with a prepared mind is more likely to experience the diagnostic epiphany, and then use point-of-care references to hone in on the details. With many patients and clinical conundrums, the basics matter.
Dr. Chester Oddis, in this issue of the Journal, reviews the basics of several primary muscle disorders. He discusses, in a case-based format extracted from his recent Medicine Grand Rounds presentation at Cleveland Clinic, nuances of specific diagnoses and the clinical progression of diseases that are critical to be aware of in order to recognize and manage them, and expeditiously refer the patient to our appropriate subspecialty colleagues.
Major challenges exist in recognizing the inflammatory myopathies and their mimics early in their course. These are serious but uncommon entities, and in part because patients and physicians often attribute their early symptoms to more-common causes, diagnosis can be elusive—until the possibility is considered. We hope that Dr. Oddis’s article will make it easier to rapidly recognize these muscle disorders.
Patients often struggle to explain their symptoms of early muscle dysfunction. Since patients often verbalize their fatigue as “feeling weak,” we often misconstrue complaints of true muscle weakness (like difficulty walking up steps) as being due to fatigue. Add in some anemia from chronic inflammation and some “liver test” abnormalities, and it is easy to see how the recognition of true muscle weakness can be delayed.
We can tease muscle weakness from fatigue or dyspnea by asking the patient to specifically and functionally describe their “weakness,” and then by asking pointed questions: “Do you have difficulty getting up from the toilet without using your arms? Do you have trouble brushing your hair or teeth?” Physical examination can clearly help here, but without routine examination of muscle strength in normal fragile elderly patients, the degree of muscle weakness can be difficult to assess. Likewise challenging is detecting the early onset of weakness by examination in a 280-lb power-lifter.
Obtaining an accurate functional and behavioral history is often critical to the early recognition of muscle disease. Muscle pain, as Dr. Oddis notes, is not a characteristic feature of many myopathies, whereas, paradoxically, the coexistence of new-onset symmetrical small-joint pain (especially with arthritis) along with muscle weakness can be a powerful clue to the diagnosis of an inflammatory myopathy.
An elevated creatine kinase (CK) level generally points directly to a muscle disease, although some neurologic disorders are associated with elevations in CK, and the entity of benign “hyperCKemia” must be recognized and not overmanaged. The latter becomes a problem when laboratory tests are allowed to drive the diagnostic evaluation in a vacuum of clinical details.
A more common scenario is the misinterpretation of common laboratory test abnormalities in the setting of a patient with “fatigue” or generalized weakness who has elevations in aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Although AST and ALT are often called “liver function tests,” these enzymes are also abundant in skeletal muscle, and since they are included on routine biochemical panels, their elevation often leads to liver imaging and sometimes even biopsy before anyone recognizes muscle disease as the cause of the patient’s symptoms and laboratory test abnormalities. Hence, a muscle source (or hemolysis) should at least be considered when AST and ALT are elevated in the absence of elevated alkaline phosphatase or gamma-glutamyl transferase.
When evaluating innumerable clinical scenarios, experienced clinicians can most certainly generate similar principles of diagnostic reasoning, based on having a few fundamental facts at their fingertips. Increasing the chances of having a prepared mind when confronted with a patient with a less-than-straightforward set of symptoms is one of my major arguments in support of continuing to read and generate internal medicine teaching literature and to attend and participate in clinical teaching conferences such as Medicine Grand Rounds. It is also why we will continue to appreciate and publish presentations like this one in the Journal.
I don’t expect to retain all the details from these and similar papers, and I know we all carry virtually infinite databases in our pockets. But keeping a few clinical pearls outside of my specialty in my head comes in handy. Having a prepared mind makes it much easier to converse with patients, to promptly initiate appropriate testing, plans, and consultations, and to then decide what to search for on my smartphone between patients.
We need to recognize the diverse problems that patients with potential multisystem disease can develop, lobby when necessary for them to be seen promptly by the relevant specialists, and initiate appropriate diagnostic testing and management in less-urgent scenarios. Most of us need frequent refreshers on the clinical manifestations of these disorders so that we can recognize them when they appear unannounced in our exam rooms.
The caregiver with a prepared mind is more likely to experience the diagnostic epiphany, and then use point-of-care references to hone in on the details. With many patients and clinical conundrums, the basics matter.
Dr. Chester Oddis, in this issue of the Journal, reviews the basics of several primary muscle disorders. He discusses, in a case-based format extracted from his recent Medicine Grand Rounds presentation at Cleveland Clinic, nuances of specific diagnoses and the clinical progression of diseases that are critical to be aware of in order to recognize and manage them, and expeditiously refer the patient to our appropriate subspecialty colleagues.
Major challenges exist in recognizing the inflammatory myopathies and their mimics early in their course. These are serious but uncommon entities, and in part because patients and physicians often attribute their early symptoms to more-common causes, diagnosis can be elusive—until the possibility is considered. We hope that Dr. Oddis’s article will make it easier to rapidly recognize these muscle disorders.
Patients often struggle to explain their symptoms of early muscle dysfunction. Since patients often verbalize their fatigue as “feeling weak,” we often misconstrue complaints of true muscle weakness (like difficulty walking up steps) as being due to fatigue. Add in some anemia from chronic inflammation and some “liver test” abnormalities, and it is easy to see how the recognition of true muscle weakness can be delayed.
We can tease muscle weakness from fatigue or dyspnea by asking the patient to specifically and functionally describe their “weakness,” and then by asking pointed questions: “Do you have difficulty getting up from the toilet without using your arms? Do you have trouble brushing your hair or teeth?” Physical examination can clearly help here, but without routine examination of muscle strength in normal fragile elderly patients, the degree of muscle weakness can be difficult to assess. Likewise challenging is detecting the early onset of weakness by examination in a 280-lb power-lifter.
Obtaining an accurate functional and behavioral history is often critical to the early recognition of muscle disease. Muscle pain, as Dr. Oddis notes, is not a characteristic feature of many myopathies, whereas, paradoxically, the coexistence of new-onset symmetrical small-joint pain (especially with arthritis) along with muscle weakness can be a powerful clue to the diagnosis of an inflammatory myopathy.
An elevated creatine kinase (CK) level generally points directly to a muscle disease, although some neurologic disorders are associated with elevations in CK, and the entity of benign “hyperCKemia” must be recognized and not overmanaged. The latter becomes a problem when laboratory tests are allowed to drive the diagnostic evaluation in a vacuum of clinical details.
A more common scenario is the misinterpretation of common laboratory test abnormalities in the setting of a patient with “fatigue” or generalized weakness who has elevations in aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Although AST and ALT are often called “liver function tests,” these enzymes are also abundant in skeletal muscle, and since they are included on routine biochemical panels, their elevation often leads to liver imaging and sometimes even biopsy before anyone recognizes muscle disease as the cause of the patient’s symptoms and laboratory test abnormalities. Hence, a muscle source (or hemolysis) should at least be considered when AST and ALT are elevated in the absence of elevated alkaline phosphatase or gamma-glutamyl transferase.
When evaluating innumerable clinical scenarios, experienced clinicians can most certainly generate similar principles of diagnostic reasoning, based on having a few fundamental facts at their fingertips. Increasing the chances of having a prepared mind when confronted with a patient with a less-than-straightforward set of symptoms is one of my major arguments in support of continuing to read and generate internal medicine teaching literature and to attend and participate in clinical teaching conferences such as Medicine Grand Rounds. It is also why we will continue to appreciate and publish presentations like this one in the Journal.
I don’t expect to retain all the details from these and similar papers, and I know we all carry virtually infinite databases in our pockets. But keeping a few clinical pearls outside of my specialty in my head comes in handy. Having a prepared mind makes it much easier to converse with patients, to promptly initiate appropriate testing, plans, and consultations, and to then decide what to search for on my smartphone between patients.
Vulvar and gluteal manifestations of Crohn disease
A 37-year-old woman presented with recurring painful swelling and erythema of the vulva over the last year. Despite a series of negative vaginal cultures, she was prescribed multiple courses of antifungal and antibacterial treatments, while her symptoms continued to worsen. She had no other relevant medical history except for occasional diarrhea and abdominal cramping, which were attributed to irritable bowel syndrome.
CROHN DISEASE OUTSIDE THE GASTROINTESTINAL TRACT
Crohn disease primarily affects the gastrointestinal tract but is associated with extraintestinal manifestations (in the oral cavity, eyes, skin, and joints) in up to 45% of patients.1
The most common mucocutaneous manifestations are granulomatous lesions that extend directly from the gastrointestinal tract, including perianal and peristomal skin tags, fistulas, and perineal ulcerations. In most cases, the onset of cutaneous manifestations follows intestinal disease, but vulvar Crohn disease may precede gastrointestinal symptoms in approximately 25% of patients, with the average age at onset in the mid-30s.1
The pathogenesis of vulvar Crohn disease remains unclear. One theory involves production of immune complexes from the gastrointestinal tract and a possible T-lymphocyte-mediated type IV hypersensitivity reaction.2
The diagnosis of vulvar Crohn disease should be considered in a patient who has vulvar pain, edema, and ulcerations not otherwise explained, whether or not gastrointestinal Crohn disease is present. The diagnosis is established with clinical history and characteristic histopathology on biopsy. Multiple biopsies may be needed, and early endoscopy is recommended to establish the diagnosis. The histologic features include noncaseating and nonnecrotizing granulomatous dermatitis or vulvitis with occasional reports of eosinophilic infiltrates and necrobiosis.5,6 An imaging study such as ultrasonography is sometimes used to differentiate between a specific cutaneous manifestation of Crohn disease and its complications such as perianal fistula or abscess.
Clinical vulvar lesions are nonspecific, and those of Crohn disease are frequently mistaken for infectious, inflammatory, or traumatic vulvitis. Diagnostic biopsy for histologic analysis is warranted.
- Andreani SM, Ratnasingham K, Dang HH, Gravante G, Giordano P. Crohn’s disease of the vulva. Int J Surg 2010; 8(1):2–5. doi:10.1016/j.ijsu.2009.09.012
- Siroy A, Wasman J. Metastatic Crohn disease: a rare cutaneous entity. Arch Pathol Lab Med 2012; 136(3):329–332. doi:10.5858/arpa.2010-0666-RS
- Foo WC, Papalas JA, Robboy SJ, Selim MA. Vulvar manifestations of Crohn’s disease. Am J Dermatopathol 2011; 33(6):588–593. doi:10.1097/DAD.0b013e31820a2635
- Amankwah Y, Haefner H. Vulvar edema. Dermatol Clin 2010; 28(4):765–777. doi:10.1016/j.det.2010.08.001
- Emanuel PO, Phelps RG. Metastatic Crohn’s disease: a histopathologic study of 12 cases. J Cutan Pathol 2008; 35(5):457–461. doi:10.1111/j.1600-0560.2007.00849.x
- Hackzell-Bradley M, Hedblad MA, Stephansson EA. Metastatic Crohn’s disease: report of 3 cases with special reference to histopathologic findings. Arch Dermatol 1996; 132(8):928–932.
A 37-year-old woman presented with recurring painful swelling and erythema of the vulva over the last year. Despite a series of negative vaginal cultures, she was prescribed multiple courses of antifungal and antibacterial treatments, while her symptoms continued to worsen. She had no other relevant medical history except for occasional diarrhea and abdominal cramping, which were attributed to irritable bowel syndrome.
CROHN DISEASE OUTSIDE THE GASTROINTESTINAL TRACT
Crohn disease primarily affects the gastrointestinal tract but is associated with extraintestinal manifestations (in the oral cavity, eyes, skin, and joints) in up to 45% of patients.1
The most common mucocutaneous manifestations are granulomatous lesions that extend directly from the gastrointestinal tract, including perianal and peristomal skin tags, fistulas, and perineal ulcerations. In most cases, the onset of cutaneous manifestations follows intestinal disease, but vulvar Crohn disease may precede gastrointestinal symptoms in approximately 25% of patients, with the average age at onset in the mid-30s.1
The pathogenesis of vulvar Crohn disease remains unclear. One theory involves production of immune complexes from the gastrointestinal tract and a possible T-lymphocyte-mediated type IV hypersensitivity reaction.2
The diagnosis of vulvar Crohn disease should be considered in a patient who has vulvar pain, edema, and ulcerations not otherwise explained, whether or not gastrointestinal Crohn disease is present. The diagnosis is established with clinical history and characteristic histopathology on biopsy. Multiple biopsies may be needed, and early endoscopy is recommended to establish the diagnosis. The histologic features include noncaseating and nonnecrotizing granulomatous dermatitis or vulvitis with occasional reports of eosinophilic infiltrates and necrobiosis.5,6 An imaging study such as ultrasonography is sometimes used to differentiate between a specific cutaneous manifestation of Crohn disease and its complications such as perianal fistula or abscess.
Clinical vulvar lesions are nonspecific, and those of Crohn disease are frequently mistaken for infectious, inflammatory, or traumatic vulvitis. Diagnostic biopsy for histologic analysis is warranted.
A 37-year-old woman presented with recurring painful swelling and erythema of the vulva over the last year. Despite a series of negative vaginal cultures, she was prescribed multiple courses of antifungal and antibacterial treatments, while her symptoms continued to worsen. She had no other relevant medical history except for occasional diarrhea and abdominal cramping, which were attributed to irritable bowel syndrome.
CROHN DISEASE OUTSIDE THE GASTROINTESTINAL TRACT
Crohn disease primarily affects the gastrointestinal tract but is associated with extraintestinal manifestations (in the oral cavity, eyes, skin, and joints) in up to 45% of patients.1
The most common mucocutaneous manifestations are granulomatous lesions that extend directly from the gastrointestinal tract, including perianal and peristomal skin tags, fistulas, and perineal ulcerations. In most cases, the onset of cutaneous manifestations follows intestinal disease, but vulvar Crohn disease may precede gastrointestinal symptoms in approximately 25% of patients, with the average age at onset in the mid-30s.1
The pathogenesis of vulvar Crohn disease remains unclear. One theory involves production of immune complexes from the gastrointestinal tract and a possible T-lymphocyte-mediated type IV hypersensitivity reaction.2
The diagnosis of vulvar Crohn disease should be considered in a patient who has vulvar pain, edema, and ulcerations not otherwise explained, whether or not gastrointestinal Crohn disease is present. The diagnosis is established with clinical history and characteristic histopathology on biopsy. Multiple biopsies may be needed, and early endoscopy is recommended to establish the diagnosis. The histologic features include noncaseating and nonnecrotizing granulomatous dermatitis or vulvitis with occasional reports of eosinophilic infiltrates and necrobiosis.5,6 An imaging study such as ultrasonography is sometimes used to differentiate between a specific cutaneous manifestation of Crohn disease and its complications such as perianal fistula or abscess.
Clinical vulvar lesions are nonspecific, and those of Crohn disease are frequently mistaken for infectious, inflammatory, or traumatic vulvitis. Diagnostic biopsy for histologic analysis is warranted.
- Andreani SM, Ratnasingham K, Dang HH, Gravante G, Giordano P. Crohn’s disease of the vulva. Int J Surg 2010; 8(1):2–5. doi:10.1016/j.ijsu.2009.09.012
- Siroy A, Wasman J. Metastatic Crohn disease: a rare cutaneous entity. Arch Pathol Lab Med 2012; 136(3):329–332. doi:10.5858/arpa.2010-0666-RS
- Foo WC, Papalas JA, Robboy SJ, Selim MA. Vulvar manifestations of Crohn’s disease. Am J Dermatopathol 2011; 33(6):588–593. doi:10.1097/DAD.0b013e31820a2635
- Amankwah Y, Haefner H. Vulvar edema. Dermatol Clin 2010; 28(4):765–777. doi:10.1016/j.det.2010.08.001
- Emanuel PO, Phelps RG. Metastatic Crohn’s disease: a histopathologic study of 12 cases. J Cutan Pathol 2008; 35(5):457–461. doi:10.1111/j.1600-0560.2007.00849.x
- Hackzell-Bradley M, Hedblad MA, Stephansson EA. Metastatic Crohn’s disease: report of 3 cases with special reference to histopathologic findings. Arch Dermatol 1996; 132(8):928–932.
- Andreani SM, Ratnasingham K, Dang HH, Gravante G, Giordano P. Crohn’s disease of the vulva. Int J Surg 2010; 8(1):2–5. doi:10.1016/j.ijsu.2009.09.012
- Siroy A, Wasman J. Metastatic Crohn disease: a rare cutaneous entity. Arch Pathol Lab Med 2012; 136(3):329–332. doi:10.5858/arpa.2010-0666-RS
- Foo WC, Papalas JA, Robboy SJ, Selim MA. Vulvar manifestations of Crohn’s disease. Am J Dermatopathol 2011; 33(6):588–593. doi:10.1097/DAD.0b013e31820a2635
- Amankwah Y, Haefner H. Vulvar edema. Dermatol Clin 2010; 28(4):765–777. doi:10.1016/j.det.2010.08.001
- Emanuel PO, Phelps RG. Metastatic Crohn’s disease: a histopathologic study of 12 cases. J Cutan Pathol 2008; 35(5):457–461. doi:10.1111/j.1600-0560.2007.00849.x
- Hackzell-Bradley M, Hedblad MA, Stephansson EA. Metastatic Crohn’s disease: report of 3 cases with special reference to histopathologic findings. Arch Dermatol 1996; 132(8):928–932.
A complication of enoxaparin injection
A 78-year-old woman presented to the emergency department with shortness of breath and palpitations and was found to have atrial fibrillation with rapid ventricular response. Medical therapy with drug therapy and cardioversion proved ineffective. She then underwent atrioventricular node ablation and placement of a pacemaker.
At the time of admission, anticoagulation was started with full-dose enoxaparin, injected subcutaneously on the left side of the abdominal wall, as her CHA2DS2-VASc score (http://chadvasc.org) was 5, due to age, female sex, and history of heart failure and hypertension.
Four days after admission, she reported lower abdominal pain, and her urine output was minimal. A bladder scan showed more than 500 mL of residual urine. She was hemodynamically stable, but physical examination revealed mild abdominal distention and tenderness in the suprapubic region. Laboratory testing showed a sharp rise in serum creatinine and a drop in hematocrit.
The patient was initially managed conservatively with serial physical examinations, monitoring of the hematocrit, serial imaging studies, and discontinuation of anticoagulation, but the pain and anuria persisted. Repeat computed tomography 15 days after admission showed that the hematoma had expanded, and she now had hydronephrosis on the right side as well, requiring urologic intervention with bilateral nephrostomy tube placement.
The size of the hematoma was evaluated with serial abdominal and pelvic examinations. After several days, her urine output had improved, the nephrostomy tubes were removed, and she was discharged.
RECTUS SHEATH HEMATOMA
Our patient had a giant pelvic hematoma, probably arising from the rectus sheath. This uncommon problem can arise from trauma, anticoagulation, or increased intra-abdominal pressure, but it can also occur spontaneously.1
In rectus sheath hematoma, a branch of the inferior epigastric artery is injured at its insertion into the rectus abdominis muscle. Symptoms arise if bleeding does not stop spontaneously from a tamponade effect.2
We speculate that in our patient, deep injection of enoxaparin into the abdominal wall injured the inferior epigastric artery, which started the hematoma, and the bleeding was exacerbated by the anticoagulation effect of the enoxaparin.
Another form of pelvic hematoma is retroperitoneal. It is most commonly caused by trauma but can occur due to rupture of the aorta, compression from tumors, or, infrequently, anticoagulation therapy.3
The role of anticoagulation
Spontaneous pelvic hematoma is usually missed as a cause of abdominal pain in patients on anticoagulation therapy and is mistaken for common acute conditions such as ulcer, diverticulitis, appendicitis, ovarian cyst torsion, and tumor.4 It usually develops within 5 days of starting anticoagulation therapy. Symptoms vary depending on the location of the hematoma and are best diagnosed with abdominal computed tomography, with sensitivity as high as 100%.
MANAGEMENT
Conservative management, reserved for patients in stable condition, includes temporarily stopping and reevaluating the risks and benefits of anticoagulation and antiplatelet agents, giving blood transfusions, and controlling pain. If conservative measures fail, options are arterial embolization, stent grafting, and blood vessel ligation.5 If these measures fail, patients should undergo surgical evacuation of the hematoma and ligation of bleeding vessels.6
TAKE-HOME MESSAGE
Subcutaneous injections, especially of anticoagulants, into the abdominal wall can increase the risk of hematoma. Other risk factors are older age, female sex, and thin body habitus with less abdominal fat.7 Healthcare professionals should avoid deep injections into the abdomen and should counsel patients and their caregivers about this, as well. The deltoid region could be a safer alternative.
- Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore) 2006; 85(2):105–110. doi:10.1097/01.md.0000216818.13067.5a
- Hatjipetrou A, Anyfantakis D, Kastanakis M. Rectus sheath hematoma: a review of the literature. Int J Surg 2015; 13:267–271. doi:10.1016/j.ijsu.2014.12.015
- Haq MM, Taimur SDM, Khan SR, Rahman MA. Retroperitoneal hematoma following enoxaparin treatment in an elderly woman—a case report. Cardiovasc J 2010; 3(1):94–97. doi:10.3329/cardio.v3i1.6434
- Luhmann A, Williams EV. Rectus sheath hematoma: a series of unfortunate events. World J Surg 2006; 30(11):2050–2055. doi:10.1007/s00268-005-0702-9
- Pace F, Colombo GM, Del Vecchio LR, et al. Low molecular weight heparin and fatal spontaneous extraperitoneal hematoma in the elderly. Geriatr Gerontol Int 2012; 12(1):172–174. doi:10.1111/j.1447-0594.2011.00742.x
- Velicki L, Cemerlic-Adic N, Bogdanovic D, Mrdanin T. Rectus sheath haematoma: enoxaparin-related complication. Acta Clin Belg 2013; 68(2):147–149. doi:10.2143/ACB.68.2.3213
- Sheth HS, Kumar R, DiNella J, Janov C, Kaldas H, Smith RE. Evaluation of risk factors for rectus sheath hematoma. Clin Appl Thromb Hemost 2016; 22(3):292–296. doi:10.1177/1076029614553024
A 78-year-old woman presented to the emergency department with shortness of breath and palpitations and was found to have atrial fibrillation with rapid ventricular response. Medical therapy with drug therapy and cardioversion proved ineffective. She then underwent atrioventricular node ablation and placement of a pacemaker.
At the time of admission, anticoagulation was started with full-dose enoxaparin, injected subcutaneously on the left side of the abdominal wall, as her CHA2DS2-VASc score (http://chadvasc.org) was 5, due to age, female sex, and history of heart failure and hypertension.
Four days after admission, she reported lower abdominal pain, and her urine output was minimal. A bladder scan showed more than 500 mL of residual urine. She was hemodynamically stable, but physical examination revealed mild abdominal distention and tenderness in the suprapubic region. Laboratory testing showed a sharp rise in serum creatinine and a drop in hematocrit.
The patient was initially managed conservatively with serial physical examinations, monitoring of the hematocrit, serial imaging studies, and discontinuation of anticoagulation, but the pain and anuria persisted. Repeat computed tomography 15 days after admission showed that the hematoma had expanded, and she now had hydronephrosis on the right side as well, requiring urologic intervention with bilateral nephrostomy tube placement.
The size of the hematoma was evaluated with serial abdominal and pelvic examinations. After several days, her urine output had improved, the nephrostomy tubes were removed, and she was discharged.
RECTUS SHEATH HEMATOMA
Our patient had a giant pelvic hematoma, probably arising from the rectus sheath. This uncommon problem can arise from trauma, anticoagulation, or increased intra-abdominal pressure, but it can also occur spontaneously.1
In rectus sheath hematoma, a branch of the inferior epigastric artery is injured at its insertion into the rectus abdominis muscle. Symptoms arise if bleeding does not stop spontaneously from a tamponade effect.2
We speculate that in our patient, deep injection of enoxaparin into the abdominal wall injured the inferior epigastric artery, which started the hematoma, and the bleeding was exacerbated by the anticoagulation effect of the enoxaparin.
Another form of pelvic hematoma is retroperitoneal. It is most commonly caused by trauma but can occur due to rupture of the aorta, compression from tumors, or, infrequently, anticoagulation therapy.3
The role of anticoagulation
Spontaneous pelvic hematoma is usually missed as a cause of abdominal pain in patients on anticoagulation therapy and is mistaken for common acute conditions such as ulcer, diverticulitis, appendicitis, ovarian cyst torsion, and tumor.4 It usually develops within 5 days of starting anticoagulation therapy. Symptoms vary depending on the location of the hematoma and are best diagnosed with abdominal computed tomography, with sensitivity as high as 100%.
MANAGEMENT
Conservative management, reserved for patients in stable condition, includes temporarily stopping and reevaluating the risks and benefits of anticoagulation and antiplatelet agents, giving blood transfusions, and controlling pain. If conservative measures fail, options are arterial embolization, stent grafting, and blood vessel ligation.5 If these measures fail, patients should undergo surgical evacuation of the hematoma and ligation of bleeding vessels.6
TAKE-HOME MESSAGE
Subcutaneous injections, especially of anticoagulants, into the abdominal wall can increase the risk of hematoma. Other risk factors are older age, female sex, and thin body habitus with less abdominal fat.7 Healthcare professionals should avoid deep injections into the abdomen and should counsel patients and their caregivers about this, as well. The deltoid region could be a safer alternative.
A 78-year-old woman presented to the emergency department with shortness of breath and palpitations and was found to have atrial fibrillation with rapid ventricular response. Medical therapy with drug therapy and cardioversion proved ineffective. She then underwent atrioventricular node ablation and placement of a pacemaker.
At the time of admission, anticoagulation was started with full-dose enoxaparin, injected subcutaneously on the left side of the abdominal wall, as her CHA2DS2-VASc score (http://chadvasc.org) was 5, due to age, female sex, and history of heart failure and hypertension.
Four days after admission, she reported lower abdominal pain, and her urine output was minimal. A bladder scan showed more than 500 mL of residual urine. She was hemodynamically stable, but physical examination revealed mild abdominal distention and tenderness in the suprapubic region. Laboratory testing showed a sharp rise in serum creatinine and a drop in hematocrit.
The patient was initially managed conservatively with serial physical examinations, monitoring of the hematocrit, serial imaging studies, and discontinuation of anticoagulation, but the pain and anuria persisted. Repeat computed tomography 15 days after admission showed that the hematoma had expanded, and she now had hydronephrosis on the right side as well, requiring urologic intervention with bilateral nephrostomy tube placement.
The size of the hematoma was evaluated with serial abdominal and pelvic examinations. After several days, her urine output had improved, the nephrostomy tubes were removed, and she was discharged.
RECTUS SHEATH HEMATOMA
Our patient had a giant pelvic hematoma, probably arising from the rectus sheath. This uncommon problem can arise from trauma, anticoagulation, or increased intra-abdominal pressure, but it can also occur spontaneously.1
In rectus sheath hematoma, a branch of the inferior epigastric artery is injured at its insertion into the rectus abdominis muscle. Symptoms arise if bleeding does not stop spontaneously from a tamponade effect.2
We speculate that in our patient, deep injection of enoxaparin into the abdominal wall injured the inferior epigastric artery, which started the hematoma, and the bleeding was exacerbated by the anticoagulation effect of the enoxaparin.
Another form of pelvic hematoma is retroperitoneal. It is most commonly caused by trauma but can occur due to rupture of the aorta, compression from tumors, or, infrequently, anticoagulation therapy.3
The role of anticoagulation
Spontaneous pelvic hematoma is usually missed as a cause of abdominal pain in patients on anticoagulation therapy and is mistaken for common acute conditions such as ulcer, diverticulitis, appendicitis, ovarian cyst torsion, and tumor.4 It usually develops within 5 days of starting anticoagulation therapy. Symptoms vary depending on the location of the hematoma and are best diagnosed with abdominal computed tomography, with sensitivity as high as 100%.
MANAGEMENT
Conservative management, reserved for patients in stable condition, includes temporarily stopping and reevaluating the risks and benefits of anticoagulation and antiplatelet agents, giving blood transfusions, and controlling pain. If conservative measures fail, options are arterial embolization, stent grafting, and blood vessel ligation.5 If these measures fail, patients should undergo surgical evacuation of the hematoma and ligation of bleeding vessels.6
TAKE-HOME MESSAGE
Subcutaneous injections, especially of anticoagulants, into the abdominal wall can increase the risk of hematoma. Other risk factors are older age, female sex, and thin body habitus with less abdominal fat.7 Healthcare professionals should avoid deep injections into the abdomen and should counsel patients and their caregivers about this, as well. The deltoid region could be a safer alternative.
- Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore) 2006; 85(2):105–110. doi:10.1097/01.md.0000216818.13067.5a
- Hatjipetrou A, Anyfantakis D, Kastanakis M. Rectus sheath hematoma: a review of the literature. Int J Surg 2015; 13:267–271. doi:10.1016/j.ijsu.2014.12.015
- Haq MM, Taimur SDM, Khan SR, Rahman MA. Retroperitoneal hematoma following enoxaparin treatment in an elderly woman—a case report. Cardiovasc J 2010; 3(1):94–97. doi:10.3329/cardio.v3i1.6434
- Luhmann A, Williams EV. Rectus sheath hematoma: a series of unfortunate events. World J Surg 2006; 30(11):2050–2055. doi:10.1007/s00268-005-0702-9
- Pace F, Colombo GM, Del Vecchio LR, et al. Low molecular weight heparin and fatal spontaneous extraperitoneal hematoma in the elderly. Geriatr Gerontol Int 2012; 12(1):172–174. doi:10.1111/j.1447-0594.2011.00742.x
- Velicki L, Cemerlic-Adic N, Bogdanovic D, Mrdanin T. Rectus sheath haematoma: enoxaparin-related complication. Acta Clin Belg 2013; 68(2):147–149. doi:10.2143/ACB.68.2.3213
- Sheth HS, Kumar R, DiNella J, Janov C, Kaldas H, Smith RE. Evaluation of risk factors for rectus sheath hematoma. Clin Appl Thromb Hemost 2016; 22(3):292–296. doi:10.1177/1076029614553024
- Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore) 2006; 85(2):105–110. doi:10.1097/01.md.0000216818.13067.5a
- Hatjipetrou A, Anyfantakis D, Kastanakis M. Rectus sheath hematoma: a review of the literature. Int J Surg 2015; 13:267–271. doi:10.1016/j.ijsu.2014.12.015
- Haq MM, Taimur SDM, Khan SR, Rahman MA. Retroperitoneal hematoma following enoxaparin treatment in an elderly woman—a case report. Cardiovasc J 2010; 3(1):94–97. doi:10.3329/cardio.v3i1.6434
- Luhmann A, Williams EV. Rectus sheath hematoma: a series of unfortunate events. World J Surg 2006; 30(11):2050–2055. doi:10.1007/s00268-005-0702-9
- Pace F, Colombo GM, Del Vecchio LR, et al. Low molecular weight heparin and fatal spontaneous extraperitoneal hematoma in the elderly. Geriatr Gerontol Int 2012; 12(1):172–174. doi:10.1111/j.1447-0594.2011.00742.x
- Velicki L, Cemerlic-Adic N, Bogdanovic D, Mrdanin T. Rectus sheath haematoma: enoxaparin-related complication. Acta Clin Belg 2013; 68(2):147–149. doi:10.2143/ACB.68.2.3213
- Sheth HS, Kumar R, DiNella J, Janov C, Kaldas H, Smith RE. Evaluation of risk factors for rectus sheath hematoma. Clin Appl Thromb Hemost 2016; 22(3):292–296. doi:10.1177/1076029614553024
What are the risks to inpatients during hospital construction or renovation?
Hospital-acquired infections related to construction and renovation activities account for more than 5,000 deaths per year across the United States.1
Hospital construction, renovation, and demolition projects ultimately serve the interests of patients, but they also can put inpatients at risk of mold infection, Legionnaires disease, sleep deprivation, exacerbation of lung disease, and in rare cases, physical injury.
Hospitals are in a continuous state of transformation to meet the needs of medical and technologic advances and an increasing patient population,1 and in the last 10 years, more than $200 billion has been spent on construction projects at US healthcare facilities. Therefore, constant attention is needed to reduce the risks to the health of hospitalized patients during these projects.
HOSPITAL-ACQUIRED INFECTIONS
Mold infections
Construction can cause substantial dust contamination and scatter large amounts of fungal spores. An analysis conducted during a period of excavation at a hospital campus showed a significant association between excavation activities and hospital-acquired mold infections (hazard ratio [HR] 2.8, P = .01) but not yeast infections (HR 0.75, P = .78).2
Aspergillus species have been the organisms most commonly involved in hospital-acquired mold infection. In a review of 53 studies including 458 patients,3A fumigatus was identified in 154 patients, and A flavus was identified in 101 patients. A niger, A terreus, A nidulans, Zygomycetes, and other fungi were also identified, but to a much lesser extent. Hematologic malignancies were the predominant underlying morbidity in 299 patients. Half of the sources of healthcare-associated Aspergillus outbreaks were estimated to result from construction and renovation activities within or surrounding the hospital.3
Heavy demolition and transportation of wreckage have been found to cause the greatest concentrations of Aspergillus species,1 but even small concentrations may be sufficient to cause infection in high-risk hospitalized patients.3 Invasive pulmonary aspergillosis is the mold infection most commonly associated with these activities, particularly in immunocompromised and critically ill patients. It is characterized by invasion of lung tissue by Aspergillus hyphae. Hematogenous dissemination occurs in about 25% of patients, and the death rate often exceeds 50%.4
A review of cases of fungal infection during hospital construction, renovation, and demolition projects from 1976 to 2014 identified 372 infected patients, of whom 180 died.5 The majority of infections were due to Aspergillus. Other fungi included Rhizopus, Candida, and Fusarium. Infections occurred mainly in patients with hematologic malignancies and patients who had undergone stem cell transplant (76%), followed by patients with other malignancies or transplant (19%). Rarely affected were patients in the intensive care unit or patients with rheumatologic diseases or on hemodialysis.5
Legionnaires disease
Legionnaires disease is a form of atypical pneumonia caused by the bacterium Legionella, often associated with differing degrees of gastrointestinal symptoms. Legionella species are the bacteria most often associated with construction in hospitals, as construction and demolition often result in collections of stagnant water.
The primary mode of transmission is inhalation of contaminated mist or aerosols. Legionella species can also colonize newly constructed hospital buildings within weeks of installation of water fixtures.
In a large university-affiliated hospital, 2 cases of nosocomial legionellosis were identified during a period of major construction.6 An epidemiologic investigation traced the source to a widespread contamination of potable water within the hospital. One patient’s isolate was similar to that of a water sample from the faucet in his room, and an association between Legionnaires disease and construction was postulated.
Another institution’s newly constructed hematology-oncology unit identified 10 cases of Legionnaires disease over a 12-week period in patients and visitors with exposure to the unit during and within the incubation period.7 A clinical and environmental assessment found 3 clinical isolates of Legionella identical to environmental isolates found from the unit, strongly implicating the potable water system as the likely source.7
In Ohio, 11 cases of hospital-acquired Legionnaires disease were identified in patients moved to a newly constructed 12-story addition to a hospital, and 1 of those died.8
Legionella infections appear to be less common than mold infections when reviewing the available literature on patients exposed to hospital construction, renovation, or demolition activities. Yet unlike mold infections, which occur mostly in immunocompromised patients, Legionella also affects people with normal immunity.1
NONCOMMUNICABLE ILLNESSES
Sleep deprivation
Noise in hospitals has been linked to sleep disturbances in inpatients. A study using noise dosimeters in a university hospital found a mean continuous noise level of 63.5 dBA (A-weighting of decibels indicates risk of hearing loss) over a 24-hour period, a level more than 2 times higher than the recommended 30 dBA.9 The same study also found a significant correlation between sleep disturbance in inpatients and increasing noise levels, in a dose-response manner.
Common sources of noise during construction may include power generators, welding and cutting equipment, and transport of materials. While construction activities themselves have yet to be directly linked to sleep deprivation in patients, construction is inevitably accompanied by noise.
Noise is the most common factor interfering with sleep reported by hospitalized patients. Other effects of noise on patients include a rise in heart rate and blood pressure, increased cholesterol and triglyceride levels, increased use of sedatives, and longer length of stay.9,10 Although construction is rarely done at night, patients generally take naps during the day, so the noise is disruptive.
Physical injuries
Hospitalized patients rarely suffer injuries related to hospital construction. However, these incidents may be underreported. Few cases of physical injury in patients exposed to construction or renovation in healthcare facilities can be found through a Web search.11,12
Exacerbation of lung disease
Inhalation of indoor air pollutants exposed during renovation can directly trigger an inflammatory response and cause exacerbation in patients with chronic lung diseases such as asthma and chronic obstructive pulmonary disease. No study has specifically examined the effect of hospital construction or renovation on exacerbation of chronic lung diseases in hospitalized patients. Nevertheless, dust and indoor air pollutants from building renovation have often been reported as agents associated with work-related asthma.13
THE MESSAGE
Although the risks to inpatients during hospital construction projects appear minimal, their effect can at times be detrimental, especially to the immunocompromised. Hospitals should adhere to infection control risk assessment protocols during construction events. The small number of outbreaks of construction-related infections can make the diagnosis of nosocomial origin of these infections challenging; a high index of suspicion is needed.
Currently in the United States, there is no standard regarding acceptable levels of airborne mold concentrations, and data to support routine hospital air sampling or validation of available air samplers are inadequate. This remains an area for future research.14,15
Certain measures have been shown to significantly decrease the risk of mold infections and other nosocomial infections during construction projects, including16:
- Effective dust control through containment units and barriers
- Consistent use of high-efficiency particulate air filters in hospital units that care for immunocompromised and critically ill patients
- Routine surveillance.
Noise and vibration can be reduced by temporary walls and careful tool selection and scheduling. Similarly, temporary walls and other barriers help protect healthcare employees and patients from the risk of direct physical injury.
Preconstruction risk assessments that address infection control, safety, noise, and air quality are crucial, and the Joint Commission generally requires such assessments. Further, education of hospital staff and members of the construction team about the potential detrimental effects of hospital construction and renovation is essential to secure a safe environment.
- Clair JD, Colatrella S. Opening Pandora’s (tool) box: health care construction and associated risk for nosocomial infection. Infect Disord Drug Targets 2013; 13(3):177–183. pmid:23961740
- Pokala HR, Leonard D, Cox J, et al. Association of hospital construction with the development of healthcare associated environmental mold infections (HAEMI) in pediatric patients with leukemia. Pediatr Blood Cancer 2014; 61(2):276–280. doi:10.1002/pbc.24685
- Vonberg RP, Gastmeier P. Nosocomial aspergillosis in outbreak settings. J Hosp Infect 2006; 63(3):246–254. doi:10.1016/j.jhin.2006.02.014
- Kanj A, Abdallah N, Soubani AO. The spectrum of pulmonary aspergillosis. Respir Med 2018; 141:121–131. doi:10.1016/j.rmed.2018.06.029
- Kanamori H, Rutala WA, Sickbert-Bennett EE, Weber DJ. Review of fungal outbreaks and infection prevention in healthcare settings during construction and renovation. Clin Infect Dis 2015; 61(3):433–444. doi:10.1093/cid/civ297
- Perola O, Kauppinen J, Kusnetsov J, Heikkinen J, Jokinen C, Katila ML. Nosocomial Legionella pneumophila serogroup 5 outbreak associated with persistent colonization of a hospital water system. APMIS 2002; 110(12):863–868. pmid:12645664
- Francois Watkins LK, Toews KE, Harris AM, et al. Lessons from an outbreak of Legionnaires disease on a hematology-oncology unit. Infect Control Hosp Epidemiol 2017; 38(3):306–313. doi:10.1017/ice.2016.281
- Lin YE, Stout JE, Yu VL. Prevention of hospital-acquired legionellosis. Curr Opin Infect Dis 2011; 24(4):350–356. doi:10.1097/QCO.0b013e3283486c6e
- Park MJ, Yoo JH, Cho BW, Kim KT, Jeong WC, Ha M. Noise in hospital rooms and sleep disturbance in hospitalized medical patients. Environ Health Toxicol 2014; 29:e2014006. doi:10.5620/eht.2014.29.e2014006
- Buxton OM, Ellenbogen JM, Wang W, et al. Sleep disruption due to hospital noises: a prospective evaluation. Ann Intern Med 2012; 157(3):170–179. doi:10.7326/0003-4819-157-3-201208070-00472
- Heldt D; The Gazette. Accident will delay University of Iowa Hospitals construction work for several days. www.thegazette.com/2013/03/08/university-of-iowa-hospitals-patient-injured-by-falling-construction-debris. Accessed July 22, 2019.
- Darrah N; Fox News. Texas hospital explosion kills 1, leaves 12 injured. www.foxnews.com/us/texas-hospital-explosion-kills-1-leaves-12-injured. Accessed July 22, 2019.
- Centers for Disease Control and Prevention (CDC). Work-related asthma: most frequently reported agents associated with work-related asthma cases by state, 2009–2012. wwwn.cdc.gov/eworld/Data/926. Accessed July 22, 2019.
- Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of Aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63(4):e1–e60. doi:10.1093/cid/ciw326
- Chang CC, Athan E, Morrissey CO, Slavin MA. Preventing invasive fungal infection during hospital building works. Intern Med J 2008; 38(6b):538–541. doi:10.1111/j.1445-5994.2008.01727.x
- Oren I, Haddad N, Finkelstein R, Rowe JM. Invasive pulmonary aspergillosis in neutropenic patients during hospital construction: before and after chemoprophylaxis and institution of HEPA filters. Am J Hematol 2001; 66(4):257–262. doi:10.1002/ajh.1054
Hospital-acquired infections related to construction and renovation activities account for more than 5,000 deaths per year across the United States.1
Hospital construction, renovation, and demolition projects ultimately serve the interests of patients, but they also can put inpatients at risk of mold infection, Legionnaires disease, sleep deprivation, exacerbation of lung disease, and in rare cases, physical injury.
Hospitals are in a continuous state of transformation to meet the needs of medical and technologic advances and an increasing patient population,1 and in the last 10 years, more than $200 billion has been spent on construction projects at US healthcare facilities. Therefore, constant attention is needed to reduce the risks to the health of hospitalized patients during these projects.
HOSPITAL-ACQUIRED INFECTIONS
Mold infections
Construction can cause substantial dust contamination and scatter large amounts of fungal spores. An analysis conducted during a period of excavation at a hospital campus showed a significant association between excavation activities and hospital-acquired mold infections (hazard ratio [HR] 2.8, P = .01) but not yeast infections (HR 0.75, P = .78).2
Aspergillus species have been the organisms most commonly involved in hospital-acquired mold infection. In a review of 53 studies including 458 patients,3A fumigatus was identified in 154 patients, and A flavus was identified in 101 patients. A niger, A terreus, A nidulans, Zygomycetes, and other fungi were also identified, but to a much lesser extent. Hematologic malignancies were the predominant underlying morbidity in 299 patients. Half of the sources of healthcare-associated Aspergillus outbreaks were estimated to result from construction and renovation activities within or surrounding the hospital.3
Heavy demolition and transportation of wreckage have been found to cause the greatest concentrations of Aspergillus species,1 but even small concentrations may be sufficient to cause infection in high-risk hospitalized patients.3 Invasive pulmonary aspergillosis is the mold infection most commonly associated with these activities, particularly in immunocompromised and critically ill patients. It is characterized by invasion of lung tissue by Aspergillus hyphae. Hematogenous dissemination occurs in about 25% of patients, and the death rate often exceeds 50%.4
A review of cases of fungal infection during hospital construction, renovation, and demolition projects from 1976 to 2014 identified 372 infected patients, of whom 180 died.5 The majority of infections were due to Aspergillus. Other fungi included Rhizopus, Candida, and Fusarium. Infections occurred mainly in patients with hematologic malignancies and patients who had undergone stem cell transplant (76%), followed by patients with other malignancies or transplant (19%). Rarely affected were patients in the intensive care unit or patients with rheumatologic diseases or on hemodialysis.5
Legionnaires disease
Legionnaires disease is a form of atypical pneumonia caused by the bacterium Legionella, often associated with differing degrees of gastrointestinal symptoms. Legionella species are the bacteria most often associated with construction in hospitals, as construction and demolition often result in collections of stagnant water.
The primary mode of transmission is inhalation of contaminated mist or aerosols. Legionella species can also colonize newly constructed hospital buildings within weeks of installation of water fixtures.
In a large university-affiliated hospital, 2 cases of nosocomial legionellosis were identified during a period of major construction.6 An epidemiologic investigation traced the source to a widespread contamination of potable water within the hospital. One patient’s isolate was similar to that of a water sample from the faucet in his room, and an association between Legionnaires disease and construction was postulated.
Another institution’s newly constructed hematology-oncology unit identified 10 cases of Legionnaires disease over a 12-week period in patients and visitors with exposure to the unit during and within the incubation period.7 A clinical and environmental assessment found 3 clinical isolates of Legionella identical to environmental isolates found from the unit, strongly implicating the potable water system as the likely source.7
In Ohio, 11 cases of hospital-acquired Legionnaires disease were identified in patients moved to a newly constructed 12-story addition to a hospital, and 1 of those died.8
Legionella infections appear to be less common than mold infections when reviewing the available literature on patients exposed to hospital construction, renovation, or demolition activities. Yet unlike mold infections, which occur mostly in immunocompromised patients, Legionella also affects people with normal immunity.1
NONCOMMUNICABLE ILLNESSES
Sleep deprivation
Noise in hospitals has been linked to sleep disturbances in inpatients. A study using noise dosimeters in a university hospital found a mean continuous noise level of 63.5 dBA (A-weighting of decibels indicates risk of hearing loss) over a 24-hour period, a level more than 2 times higher than the recommended 30 dBA.9 The same study also found a significant correlation between sleep disturbance in inpatients and increasing noise levels, in a dose-response manner.
Common sources of noise during construction may include power generators, welding and cutting equipment, and transport of materials. While construction activities themselves have yet to be directly linked to sleep deprivation in patients, construction is inevitably accompanied by noise.
Noise is the most common factor interfering with sleep reported by hospitalized patients. Other effects of noise on patients include a rise in heart rate and blood pressure, increased cholesterol and triglyceride levels, increased use of sedatives, and longer length of stay.9,10 Although construction is rarely done at night, patients generally take naps during the day, so the noise is disruptive.
Physical injuries
Hospitalized patients rarely suffer injuries related to hospital construction. However, these incidents may be underreported. Few cases of physical injury in patients exposed to construction or renovation in healthcare facilities can be found through a Web search.11,12
Exacerbation of lung disease
Inhalation of indoor air pollutants exposed during renovation can directly trigger an inflammatory response and cause exacerbation in patients with chronic lung diseases such as asthma and chronic obstructive pulmonary disease. No study has specifically examined the effect of hospital construction or renovation on exacerbation of chronic lung diseases in hospitalized patients. Nevertheless, dust and indoor air pollutants from building renovation have often been reported as agents associated with work-related asthma.13
THE MESSAGE
Although the risks to inpatients during hospital construction projects appear minimal, their effect can at times be detrimental, especially to the immunocompromised. Hospitals should adhere to infection control risk assessment protocols during construction events. The small number of outbreaks of construction-related infections can make the diagnosis of nosocomial origin of these infections challenging; a high index of suspicion is needed.
Currently in the United States, there is no standard regarding acceptable levels of airborne mold concentrations, and data to support routine hospital air sampling or validation of available air samplers are inadequate. This remains an area for future research.14,15
Certain measures have been shown to significantly decrease the risk of mold infections and other nosocomial infections during construction projects, including16:
- Effective dust control through containment units and barriers
- Consistent use of high-efficiency particulate air filters in hospital units that care for immunocompromised and critically ill patients
- Routine surveillance.
Noise and vibration can be reduced by temporary walls and careful tool selection and scheduling. Similarly, temporary walls and other barriers help protect healthcare employees and patients from the risk of direct physical injury.
Preconstruction risk assessments that address infection control, safety, noise, and air quality are crucial, and the Joint Commission generally requires such assessments. Further, education of hospital staff and members of the construction team about the potential detrimental effects of hospital construction and renovation is essential to secure a safe environment.
Hospital-acquired infections related to construction and renovation activities account for more than 5,000 deaths per year across the United States.1
Hospital construction, renovation, and demolition projects ultimately serve the interests of patients, but they also can put inpatients at risk of mold infection, Legionnaires disease, sleep deprivation, exacerbation of lung disease, and in rare cases, physical injury.
Hospitals are in a continuous state of transformation to meet the needs of medical and technologic advances and an increasing patient population,1 and in the last 10 years, more than $200 billion has been spent on construction projects at US healthcare facilities. Therefore, constant attention is needed to reduce the risks to the health of hospitalized patients during these projects.
HOSPITAL-ACQUIRED INFECTIONS
Mold infections
Construction can cause substantial dust contamination and scatter large amounts of fungal spores. An analysis conducted during a period of excavation at a hospital campus showed a significant association between excavation activities and hospital-acquired mold infections (hazard ratio [HR] 2.8, P = .01) but not yeast infections (HR 0.75, P = .78).2
Aspergillus species have been the organisms most commonly involved in hospital-acquired mold infection. In a review of 53 studies including 458 patients,3A fumigatus was identified in 154 patients, and A flavus was identified in 101 patients. A niger, A terreus, A nidulans, Zygomycetes, and other fungi were also identified, but to a much lesser extent. Hematologic malignancies were the predominant underlying morbidity in 299 patients. Half of the sources of healthcare-associated Aspergillus outbreaks were estimated to result from construction and renovation activities within or surrounding the hospital.3
Heavy demolition and transportation of wreckage have been found to cause the greatest concentrations of Aspergillus species,1 but even small concentrations may be sufficient to cause infection in high-risk hospitalized patients.3 Invasive pulmonary aspergillosis is the mold infection most commonly associated with these activities, particularly in immunocompromised and critically ill patients. It is characterized by invasion of lung tissue by Aspergillus hyphae. Hematogenous dissemination occurs in about 25% of patients, and the death rate often exceeds 50%.4
A review of cases of fungal infection during hospital construction, renovation, and demolition projects from 1976 to 2014 identified 372 infected patients, of whom 180 died.5 The majority of infections were due to Aspergillus. Other fungi included Rhizopus, Candida, and Fusarium. Infections occurred mainly in patients with hematologic malignancies and patients who had undergone stem cell transplant (76%), followed by patients with other malignancies or transplant (19%). Rarely affected were patients in the intensive care unit or patients with rheumatologic diseases or on hemodialysis.5
Legionnaires disease
Legionnaires disease is a form of atypical pneumonia caused by the bacterium Legionella, often associated with differing degrees of gastrointestinal symptoms. Legionella species are the bacteria most often associated with construction in hospitals, as construction and demolition often result in collections of stagnant water.
The primary mode of transmission is inhalation of contaminated mist or aerosols. Legionella species can also colonize newly constructed hospital buildings within weeks of installation of water fixtures.
In a large university-affiliated hospital, 2 cases of nosocomial legionellosis were identified during a period of major construction.6 An epidemiologic investigation traced the source to a widespread contamination of potable water within the hospital. One patient’s isolate was similar to that of a water sample from the faucet in his room, and an association between Legionnaires disease and construction was postulated.
Another institution’s newly constructed hematology-oncology unit identified 10 cases of Legionnaires disease over a 12-week period in patients and visitors with exposure to the unit during and within the incubation period.7 A clinical and environmental assessment found 3 clinical isolates of Legionella identical to environmental isolates found from the unit, strongly implicating the potable water system as the likely source.7
In Ohio, 11 cases of hospital-acquired Legionnaires disease were identified in patients moved to a newly constructed 12-story addition to a hospital, and 1 of those died.8
Legionella infections appear to be less common than mold infections when reviewing the available literature on patients exposed to hospital construction, renovation, or demolition activities. Yet unlike mold infections, which occur mostly in immunocompromised patients, Legionella also affects people with normal immunity.1
NONCOMMUNICABLE ILLNESSES
Sleep deprivation
Noise in hospitals has been linked to sleep disturbances in inpatients. A study using noise dosimeters in a university hospital found a mean continuous noise level of 63.5 dBA (A-weighting of decibels indicates risk of hearing loss) over a 24-hour period, a level more than 2 times higher than the recommended 30 dBA.9 The same study also found a significant correlation between sleep disturbance in inpatients and increasing noise levels, in a dose-response manner.
Common sources of noise during construction may include power generators, welding and cutting equipment, and transport of materials. While construction activities themselves have yet to be directly linked to sleep deprivation in patients, construction is inevitably accompanied by noise.
Noise is the most common factor interfering with sleep reported by hospitalized patients. Other effects of noise on patients include a rise in heart rate and blood pressure, increased cholesterol and triglyceride levels, increased use of sedatives, and longer length of stay.9,10 Although construction is rarely done at night, patients generally take naps during the day, so the noise is disruptive.
Physical injuries
Hospitalized patients rarely suffer injuries related to hospital construction. However, these incidents may be underreported. Few cases of physical injury in patients exposed to construction or renovation in healthcare facilities can be found through a Web search.11,12
Exacerbation of lung disease
Inhalation of indoor air pollutants exposed during renovation can directly trigger an inflammatory response and cause exacerbation in patients with chronic lung diseases such as asthma and chronic obstructive pulmonary disease. No study has specifically examined the effect of hospital construction or renovation on exacerbation of chronic lung diseases in hospitalized patients. Nevertheless, dust and indoor air pollutants from building renovation have often been reported as agents associated with work-related asthma.13
THE MESSAGE
Although the risks to inpatients during hospital construction projects appear minimal, their effect can at times be detrimental, especially to the immunocompromised. Hospitals should adhere to infection control risk assessment protocols during construction events. The small number of outbreaks of construction-related infections can make the diagnosis of nosocomial origin of these infections challenging; a high index of suspicion is needed.
Currently in the United States, there is no standard regarding acceptable levels of airborne mold concentrations, and data to support routine hospital air sampling or validation of available air samplers are inadequate. This remains an area for future research.14,15
Certain measures have been shown to significantly decrease the risk of mold infections and other nosocomial infections during construction projects, including16:
- Effective dust control through containment units and barriers
- Consistent use of high-efficiency particulate air filters in hospital units that care for immunocompromised and critically ill patients
- Routine surveillance.
Noise and vibration can be reduced by temporary walls and careful tool selection and scheduling. Similarly, temporary walls and other barriers help protect healthcare employees and patients from the risk of direct physical injury.
Preconstruction risk assessments that address infection control, safety, noise, and air quality are crucial, and the Joint Commission generally requires such assessments. Further, education of hospital staff and members of the construction team about the potential detrimental effects of hospital construction and renovation is essential to secure a safe environment.
- Clair JD, Colatrella S. Opening Pandora’s (tool) box: health care construction and associated risk for nosocomial infection. Infect Disord Drug Targets 2013; 13(3):177–183. pmid:23961740
- Pokala HR, Leonard D, Cox J, et al. Association of hospital construction with the development of healthcare associated environmental mold infections (HAEMI) in pediatric patients with leukemia. Pediatr Blood Cancer 2014; 61(2):276–280. doi:10.1002/pbc.24685
- Vonberg RP, Gastmeier P. Nosocomial aspergillosis in outbreak settings. J Hosp Infect 2006; 63(3):246–254. doi:10.1016/j.jhin.2006.02.014
- Kanj A, Abdallah N, Soubani AO. The spectrum of pulmonary aspergillosis. Respir Med 2018; 141:121–131. doi:10.1016/j.rmed.2018.06.029
- Kanamori H, Rutala WA, Sickbert-Bennett EE, Weber DJ. Review of fungal outbreaks and infection prevention in healthcare settings during construction and renovation. Clin Infect Dis 2015; 61(3):433–444. doi:10.1093/cid/civ297
- Perola O, Kauppinen J, Kusnetsov J, Heikkinen J, Jokinen C, Katila ML. Nosocomial Legionella pneumophila serogroup 5 outbreak associated with persistent colonization of a hospital water system. APMIS 2002; 110(12):863–868. pmid:12645664
- Francois Watkins LK, Toews KE, Harris AM, et al. Lessons from an outbreak of Legionnaires disease on a hematology-oncology unit. Infect Control Hosp Epidemiol 2017; 38(3):306–313. doi:10.1017/ice.2016.281
- Lin YE, Stout JE, Yu VL. Prevention of hospital-acquired legionellosis. Curr Opin Infect Dis 2011; 24(4):350–356. doi:10.1097/QCO.0b013e3283486c6e
- Park MJ, Yoo JH, Cho BW, Kim KT, Jeong WC, Ha M. Noise in hospital rooms and sleep disturbance in hospitalized medical patients. Environ Health Toxicol 2014; 29:e2014006. doi:10.5620/eht.2014.29.e2014006
- Buxton OM, Ellenbogen JM, Wang W, et al. Sleep disruption due to hospital noises: a prospective evaluation. Ann Intern Med 2012; 157(3):170–179. doi:10.7326/0003-4819-157-3-201208070-00472
- Heldt D; The Gazette. Accident will delay University of Iowa Hospitals construction work for several days. www.thegazette.com/2013/03/08/university-of-iowa-hospitals-patient-injured-by-falling-construction-debris. Accessed July 22, 2019.
- Darrah N; Fox News. Texas hospital explosion kills 1, leaves 12 injured. www.foxnews.com/us/texas-hospital-explosion-kills-1-leaves-12-injured. Accessed July 22, 2019.
- Centers for Disease Control and Prevention (CDC). Work-related asthma: most frequently reported agents associated with work-related asthma cases by state, 2009–2012. wwwn.cdc.gov/eworld/Data/926. Accessed July 22, 2019.
- Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of Aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63(4):e1–e60. doi:10.1093/cid/ciw326
- Chang CC, Athan E, Morrissey CO, Slavin MA. Preventing invasive fungal infection during hospital building works. Intern Med J 2008; 38(6b):538–541. doi:10.1111/j.1445-5994.2008.01727.x
- Oren I, Haddad N, Finkelstein R, Rowe JM. Invasive pulmonary aspergillosis in neutropenic patients during hospital construction: before and after chemoprophylaxis and institution of HEPA filters. Am J Hematol 2001; 66(4):257–262. doi:10.1002/ajh.1054
- Clair JD, Colatrella S. Opening Pandora’s (tool) box: health care construction and associated risk for nosocomial infection. Infect Disord Drug Targets 2013; 13(3):177–183. pmid:23961740
- Pokala HR, Leonard D, Cox J, et al. Association of hospital construction with the development of healthcare associated environmental mold infections (HAEMI) in pediatric patients with leukemia. Pediatr Blood Cancer 2014; 61(2):276–280. doi:10.1002/pbc.24685
- Vonberg RP, Gastmeier P. Nosocomial aspergillosis in outbreak settings. J Hosp Infect 2006; 63(3):246–254. doi:10.1016/j.jhin.2006.02.014
- Kanj A, Abdallah N, Soubani AO. The spectrum of pulmonary aspergillosis. Respir Med 2018; 141:121–131. doi:10.1016/j.rmed.2018.06.029
- Kanamori H, Rutala WA, Sickbert-Bennett EE, Weber DJ. Review of fungal outbreaks and infection prevention in healthcare settings during construction and renovation. Clin Infect Dis 2015; 61(3):433–444. doi:10.1093/cid/civ297
- Perola O, Kauppinen J, Kusnetsov J, Heikkinen J, Jokinen C, Katila ML. Nosocomial Legionella pneumophila serogroup 5 outbreak associated with persistent colonization of a hospital water system. APMIS 2002; 110(12):863–868. pmid:12645664
- Francois Watkins LK, Toews KE, Harris AM, et al. Lessons from an outbreak of Legionnaires disease on a hematology-oncology unit. Infect Control Hosp Epidemiol 2017; 38(3):306–313. doi:10.1017/ice.2016.281
- Lin YE, Stout JE, Yu VL. Prevention of hospital-acquired legionellosis. Curr Opin Infect Dis 2011; 24(4):350–356. doi:10.1097/QCO.0b013e3283486c6e
- Park MJ, Yoo JH, Cho BW, Kim KT, Jeong WC, Ha M. Noise in hospital rooms and sleep disturbance in hospitalized medical patients. Environ Health Toxicol 2014; 29:e2014006. doi:10.5620/eht.2014.29.e2014006
- Buxton OM, Ellenbogen JM, Wang W, et al. Sleep disruption due to hospital noises: a prospective evaluation. Ann Intern Med 2012; 157(3):170–179. doi:10.7326/0003-4819-157-3-201208070-00472
- Heldt D; The Gazette. Accident will delay University of Iowa Hospitals construction work for several days. www.thegazette.com/2013/03/08/university-of-iowa-hospitals-patient-injured-by-falling-construction-debris. Accessed July 22, 2019.
- Darrah N; Fox News. Texas hospital explosion kills 1, leaves 12 injured. www.foxnews.com/us/texas-hospital-explosion-kills-1-leaves-12-injured. Accessed July 22, 2019.
- Centers for Disease Control and Prevention (CDC). Work-related asthma: most frequently reported agents associated with work-related asthma cases by state, 2009–2012. wwwn.cdc.gov/eworld/Data/926. Accessed July 22, 2019.
- Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of Aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63(4):e1–e60. doi:10.1093/cid/ciw326
- Chang CC, Athan E, Morrissey CO, Slavin MA. Preventing invasive fungal infection during hospital building works. Intern Med J 2008; 38(6b):538–541. doi:10.1111/j.1445-5994.2008.01727.x
- Oren I, Haddad N, Finkelstein R, Rowe JM. Invasive pulmonary aspergillosis in neutropenic patients during hospital construction: before and after chemoprophylaxis and institution of HEPA filters. Am J Hematol 2001; 66(4):257–262. doi:10.1002/ajh.1054