User login
No difference between ipilimumab/nivolumab combo and immunotherapy plus VEGF for metastatic RCC
There is no significant difference in response or survival rates between the combination of ipilimumab/nivolumab (ipi-nivo) and immuno-oncology plus vascular endothelial growth factor inhibition (IOVE) for patients with metastatic renal cell carcinoma.
Therefore, the treatment should probably be directed by patient preferences, among other things, Shaan Dudani, MD, and colleagues wrote in European Oncology.
“Given the current lack of evidence to suggest a difference in efficacy between treatment strategies, patients, clinicians and policy makers are likely to take into account other considerations, such as toxicity, cost, logistics, prognostic categories, and patient preferences in deciding between the various front-line [immuno-oncology] combination regimens,” wrote Dr. Dudani, of the University of Calgary, and coauthors.
The team examined response rates among 263 patients with metastatic renal cell carcinoma from the International Metastatic Renal-Cell Carcinoma Database Consortium (IMDC) dataset. Patients treated with any first-line IOVE combination (n = 113) were compared with those treated with ipi-nivo (n = 75). Patients were about 62 years old. The most common sites of metastasis were liver (about 20%) and bone (about 33%), and about 20% had sarcomatoid features (about 20%). Most (about 75%) had multiple metastatic sites.
Thirty percent of those in the IOVE group and 40% in the ipi-nivo group had received second-line treatments. These included axitinib, levantinib plus severolimus, nivolumab alone, pazopanib, and sunitinib, as well as other treatments.
At a mean follow-up of 11.7 months, the response rates were 33% for IOVE and 40% for ipi-nivo. This difference was not statistically significant (between group difference, 7%; 95% confidence interval, –8% to 22%; P = .4). Complete response occurred in 2% in IOVE and 5% of the ipi-nivo group.
The time to treatment failure was 14.3 months for IOVE and 10.2 months for ipi-nivo – again not a significant difference (P = .2). Time to next treatment also was not significantly different (19.7 vs. 17.9 months; P = .4). Neither group met the study’s overall survival goal.
After adjustment for IMDC risk score, hazard ratios for death were 0.71 for IOVE and 1.74 for ipi-nivo. There were no significant between-group differences when comparing intermediate- and poor-risk patients or when the analysis was restricted only to favorable-risk patients. Among 55 who received second-line therapy, there was also no significant difference in time to treatment failure.
“It was interesting, though not surprising, to observe that the majority [88%] of second-line therapies in this cohort were VEGF-based following ipi-nivo vs. IOVE combinations,” the authors noted. “The higher response rates observed in patients receiving second-line VEGF combinations is noteworthy and thought provoking. Biologically, it is plausible that VEGF-based second-line therapy would be more likely to be effective in the VEGF-naive ipi-nivo cohort. It remains to be seen whether the numerical difference in time to treatment failure becomes significant with increased sample size and further follow-up, and whether this contributes to differences in overall survival, which ultimately impacts treatment selections in the first-line setting.”
Dr. Dudani had no financial disclosures.
SOURCE: Dudani S et al. Euro Onc. 2019 Aug 22. doi: 10.1016/j.eururo.2019.07.048.
There is no significant difference in response or survival rates between the combination of ipilimumab/nivolumab (ipi-nivo) and immuno-oncology plus vascular endothelial growth factor inhibition (IOVE) for patients with metastatic renal cell carcinoma.
Therefore, the treatment should probably be directed by patient preferences, among other things, Shaan Dudani, MD, and colleagues wrote in European Oncology.
“Given the current lack of evidence to suggest a difference in efficacy between treatment strategies, patients, clinicians and policy makers are likely to take into account other considerations, such as toxicity, cost, logistics, prognostic categories, and patient preferences in deciding between the various front-line [immuno-oncology] combination regimens,” wrote Dr. Dudani, of the University of Calgary, and coauthors.
The team examined response rates among 263 patients with metastatic renal cell carcinoma from the International Metastatic Renal-Cell Carcinoma Database Consortium (IMDC) dataset. Patients treated with any first-line IOVE combination (n = 113) were compared with those treated with ipi-nivo (n = 75). Patients were about 62 years old. The most common sites of metastasis were liver (about 20%) and bone (about 33%), and about 20% had sarcomatoid features (about 20%). Most (about 75%) had multiple metastatic sites.
Thirty percent of those in the IOVE group and 40% in the ipi-nivo group had received second-line treatments. These included axitinib, levantinib plus severolimus, nivolumab alone, pazopanib, and sunitinib, as well as other treatments.
At a mean follow-up of 11.7 months, the response rates were 33% for IOVE and 40% for ipi-nivo. This difference was not statistically significant (between group difference, 7%; 95% confidence interval, –8% to 22%; P = .4). Complete response occurred in 2% in IOVE and 5% of the ipi-nivo group.
The time to treatment failure was 14.3 months for IOVE and 10.2 months for ipi-nivo – again not a significant difference (P = .2). Time to next treatment also was not significantly different (19.7 vs. 17.9 months; P = .4). Neither group met the study’s overall survival goal.
After adjustment for IMDC risk score, hazard ratios for death were 0.71 for IOVE and 1.74 for ipi-nivo. There were no significant between-group differences when comparing intermediate- and poor-risk patients or when the analysis was restricted only to favorable-risk patients. Among 55 who received second-line therapy, there was also no significant difference in time to treatment failure.
“It was interesting, though not surprising, to observe that the majority [88%] of second-line therapies in this cohort were VEGF-based following ipi-nivo vs. IOVE combinations,” the authors noted. “The higher response rates observed in patients receiving second-line VEGF combinations is noteworthy and thought provoking. Biologically, it is plausible that VEGF-based second-line therapy would be more likely to be effective in the VEGF-naive ipi-nivo cohort. It remains to be seen whether the numerical difference in time to treatment failure becomes significant with increased sample size and further follow-up, and whether this contributes to differences in overall survival, which ultimately impacts treatment selections in the first-line setting.”
Dr. Dudani had no financial disclosures.
SOURCE: Dudani S et al. Euro Onc. 2019 Aug 22. doi: 10.1016/j.eururo.2019.07.048.
There is no significant difference in response or survival rates between the combination of ipilimumab/nivolumab (ipi-nivo) and immuno-oncology plus vascular endothelial growth factor inhibition (IOVE) for patients with metastatic renal cell carcinoma.
Therefore, the treatment should probably be directed by patient preferences, among other things, Shaan Dudani, MD, and colleagues wrote in European Oncology.
“Given the current lack of evidence to suggest a difference in efficacy between treatment strategies, patients, clinicians and policy makers are likely to take into account other considerations, such as toxicity, cost, logistics, prognostic categories, and patient preferences in deciding between the various front-line [immuno-oncology] combination regimens,” wrote Dr. Dudani, of the University of Calgary, and coauthors.
The team examined response rates among 263 patients with metastatic renal cell carcinoma from the International Metastatic Renal-Cell Carcinoma Database Consortium (IMDC) dataset. Patients treated with any first-line IOVE combination (n = 113) were compared with those treated with ipi-nivo (n = 75). Patients were about 62 years old. The most common sites of metastasis were liver (about 20%) and bone (about 33%), and about 20% had sarcomatoid features (about 20%). Most (about 75%) had multiple metastatic sites.
Thirty percent of those in the IOVE group and 40% in the ipi-nivo group had received second-line treatments. These included axitinib, levantinib plus severolimus, nivolumab alone, pazopanib, and sunitinib, as well as other treatments.
At a mean follow-up of 11.7 months, the response rates were 33% for IOVE and 40% for ipi-nivo. This difference was not statistically significant (between group difference, 7%; 95% confidence interval, –8% to 22%; P = .4). Complete response occurred in 2% in IOVE and 5% of the ipi-nivo group.
The time to treatment failure was 14.3 months for IOVE and 10.2 months for ipi-nivo – again not a significant difference (P = .2). Time to next treatment also was not significantly different (19.7 vs. 17.9 months; P = .4). Neither group met the study’s overall survival goal.
After adjustment for IMDC risk score, hazard ratios for death were 0.71 for IOVE and 1.74 for ipi-nivo. There were no significant between-group differences when comparing intermediate- and poor-risk patients or when the analysis was restricted only to favorable-risk patients. Among 55 who received second-line therapy, there was also no significant difference in time to treatment failure.
“It was interesting, though not surprising, to observe that the majority [88%] of second-line therapies in this cohort were VEGF-based following ipi-nivo vs. IOVE combinations,” the authors noted. “The higher response rates observed in patients receiving second-line VEGF combinations is noteworthy and thought provoking. Biologically, it is plausible that VEGF-based second-line therapy would be more likely to be effective in the VEGF-naive ipi-nivo cohort. It remains to be seen whether the numerical difference in time to treatment failure becomes significant with increased sample size and further follow-up, and whether this contributes to differences in overall survival, which ultimately impacts treatment selections in the first-line setting.”
Dr. Dudani had no financial disclosures.
SOURCE: Dudani S et al. Euro Onc. 2019 Aug 22. doi: 10.1016/j.eururo.2019.07.048.
FROM EUROPEAN ONCOLOGY
Snapshots of an oncologist
It’s 6:30 on a Friday night, and I am triaging three admissions to the leukemia service at once. The call from the ED about you makes me pause. I recognize your name – you were my patient a few years before. At the time, you were undergoing chemotherapy for acute myeloid leukemia, and I cared for you during the aftermath. I now pull up your chart and fill in the gaps of the last 2 years. You got into remission and received a bone marrow transplant. For 2 years, you were cured. But today, you are back. The ED has picked up an abundance of blasts – cancer cells – in your blood. I walk to your ED gurney slowly, thinking of how to tell you this. You recognize me, too. And I can see in your eyes that you already know. “I am so sorry this is happening,” I say.
You are here for your third cycle of chemotherapy. It’s a standard check-in. The first cycle was tolerable, the second cycle was rough, and now you are exhausted. You wonder if it’s normal to be so beat up from this. You ask how much nausea is too much nausea. But your hair didn’t fall out – isn’t that strange? Is it a sure thing that it will? And, by the way, is there anything to prevent the neuropathy? You wiggle your fingers as if to emphasize the point. We go through each of your symptoms and strategize ways to make this cycle better than the last. “OK,” you conclude triumphantly. “I got this!”
It’s your 1-month follow-up and it’s time to pivot. After you were diagnosed with an aggressive triple-negative breast cancer, you met with a medical oncologist and a surgeon. Chemotherapy first, they agreed. The chemo would shrink the tumor, they said, so that it could all be scooped out with surgery. The medications were rough, but you knew it was for the best. But now it’s been two cycles and the lump in your breast is getting bigger not smaller. I ask if I may draw on your skin, promising I’ll wash it off. I gently trace the mass in pen and pull out a tape measure. Yes. It is bigger. I listen to your heart and hear it racing. “What now?” you ask.
When you saw your doctor for bloating and were told it’s not gas, actually, but stage 4 cancer, you didn’t cry. You didn’t deny it. You prepared. You called your lawyer and made a will. You contacted your job and planned for retirement. You organized your things so your children wouldn’t have to. Your oncologist recommended palliative chemotherapy as it could give you some more good days. The best case scenario would be 1 year. That was 2½ years ago. You still like to be prepared, you tell me, but that’s on the back burner now. You are busy, after all – your feet still ache from dancing all night in heels at your niece’s wedding last weekend. I pull up your latest PET scan and we look together: Again, wonderfully, everything appears stable. “See you in 3 months,” I say.
You called three times to move up this appointment because you didn’t know if you’d be alive this long. You want a second opinion. When your kidney cancer grew after surgery, two immunotherapy drugs, and a chemotherapy pill, the latest setback has been fevers up to 104 ° with drenching night sweats. They found a deep infection gnawing around the edges of your tumor, and antibiotics aren’t touching it. The only chance to stop the cancer is more chemotherapy, but that could make the infection worse and lead to a rapid demise. You can’t decide. Today, in the exam room, you are sweating. Your temperature is 101 °. Your partner is trying to keep it together, but the crumpled tissues in her hand give it away. She looks at me earnestly: “What would you do if this were your family member?”
You teach about this disease in your classes and never thought it would happen to you. It started simply enough – you were bruising. Your joints ached. Small things; odd things. The ER doctor cleverly noticed that some numbers were off in your blood counts and sent you to a hematology-oncology doctor, who then cleverly ordered a molecular blood test. It was a long shot. He didn’t really expect it to come back with chronic myeloid leukemia. But there it is, and here we are. You return to talk about treatment options. You understand in detail the biology of how they work. What you don’t know is which is best for you. I go through the four choices and unpleasant effects of each. Muscle aches; diarrhea; risk of bleeding; twice a day dosing tied to mealtimes. “Is there an Option 5?” you wonder.
You have been in the hospital for 34 days, but who’s counting? You are. Because it has been Thirty. Four. Days. You knew the chemotherapy would suppress your blood counts. Now you know what “impaired immune system” really means. You had the bloodstream bacterial infection, requiring 2 days in the ICU. You had the invasive fungus growing in your lungs. The nurses post a calendar on your wall and kindly fill it in every day with your white blood cell count so you don’t have to ask. For days, it’s the same. Your bag stays packed – “just in case,” you explain. Your spouse diligently keeps your children – 2 and 4 years old – away, as kids are notorious germ factories. Then one Sunday morning and – finally! “Put me on speakerphone,” you tell your spouse. “Daddy is coming home!”
One of the most precious parts of hematology and oncology is the relationships. You are there not just for one difficult moment, but for the journey. I await getting to help you over the years to come. For now, I will settle for snapshots.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz and listen to her each week on the Blood & Cancer podcast.
It’s 6:30 on a Friday night, and I am triaging three admissions to the leukemia service at once. The call from the ED about you makes me pause. I recognize your name – you were my patient a few years before. At the time, you were undergoing chemotherapy for acute myeloid leukemia, and I cared for you during the aftermath. I now pull up your chart and fill in the gaps of the last 2 years. You got into remission and received a bone marrow transplant. For 2 years, you were cured. But today, you are back. The ED has picked up an abundance of blasts – cancer cells – in your blood. I walk to your ED gurney slowly, thinking of how to tell you this. You recognize me, too. And I can see in your eyes that you already know. “I am so sorry this is happening,” I say.
You are here for your third cycle of chemotherapy. It’s a standard check-in. The first cycle was tolerable, the second cycle was rough, and now you are exhausted. You wonder if it’s normal to be so beat up from this. You ask how much nausea is too much nausea. But your hair didn’t fall out – isn’t that strange? Is it a sure thing that it will? And, by the way, is there anything to prevent the neuropathy? You wiggle your fingers as if to emphasize the point. We go through each of your symptoms and strategize ways to make this cycle better than the last. “OK,” you conclude triumphantly. “I got this!”
It’s your 1-month follow-up and it’s time to pivot. After you were diagnosed with an aggressive triple-negative breast cancer, you met with a medical oncologist and a surgeon. Chemotherapy first, they agreed. The chemo would shrink the tumor, they said, so that it could all be scooped out with surgery. The medications were rough, but you knew it was for the best. But now it’s been two cycles and the lump in your breast is getting bigger not smaller. I ask if I may draw on your skin, promising I’ll wash it off. I gently trace the mass in pen and pull out a tape measure. Yes. It is bigger. I listen to your heart and hear it racing. “What now?” you ask.
When you saw your doctor for bloating and were told it’s not gas, actually, but stage 4 cancer, you didn’t cry. You didn’t deny it. You prepared. You called your lawyer and made a will. You contacted your job and planned for retirement. You organized your things so your children wouldn’t have to. Your oncologist recommended palliative chemotherapy as it could give you some more good days. The best case scenario would be 1 year. That was 2½ years ago. You still like to be prepared, you tell me, but that’s on the back burner now. You are busy, after all – your feet still ache from dancing all night in heels at your niece’s wedding last weekend. I pull up your latest PET scan and we look together: Again, wonderfully, everything appears stable. “See you in 3 months,” I say.
You called three times to move up this appointment because you didn’t know if you’d be alive this long. You want a second opinion. When your kidney cancer grew after surgery, two immunotherapy drugs, and a chemotherapy pill, the latest setback has been fevers up to 104 ° with drenching night sweats. They found a deep infection gnawing around the edges of your tumor, and antibiotics aren’t touching it. The only chance to stop the cancer is more chemotherapy, but that could make the infection worse and lead to a rapid demise. You can’t decide. Today, in the exam room, you are sweating. Your temperature is 101 °. Your partner is trying to keep it together, but the crumpled tissues in her hand give it away. She looks at me earnestly: “What would you do if this were your family member?”
You teach about this disease in your classes and never thought it would happen to you. It started simply enough – you were bruising. Your joints ached. Small things; odd things. The ER doctor cleverly noticed that some numbers were off in your blood counts and sent you to a hematology-oncology doctor, who then cleverly ordered a molecular blood test. It was a long shot. He didn’t really expect it to come back with chronic myeloid leukemia. But there it is, and here we are. You return to talk about treatment options. You understand in detail the biology of how they work. What you don’t know is which is best for you. I go through the four choices and unpleasant effects of each. Muscle aches; diarrhea; risk of bleeding; twice a day dosing tied to mealtimes. “Is there an Option 5?” you wonder.
You have been in the hospital for 34 days, but who’s counting? You are. Because it has been Thirty. Four. Days. You knew the chemotherapy would suppress your blood counts. Now you know what “impaired immune system” really means. You had the bloodstream bacterial infection, requiring 2 days in the ICU. You had the invasive fungus growing in your lungs. The nurses post a calendar on your wall and kindly fill it in every day with your white blood cell count so you don’t have to ask. For days, it’s the same. Your bag stays packed – “just in case,” you explain. Your spouse diligently keeps your children – 2 and 4 years old – away, as kids are notorious germ factories. Then one Sunday morning and – finally! “Put me on speakerphone,” you tell your spouse. “Daddy is coming home!”
One of the most precious parts of hematology and oncology is the relationships. You are there not just for one difficult moment, but for the journey. I await getting to help you over the years to come. For now, I will settle for snapshots.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz and listen to her each week on the Blood & Cancer podcast.
It’s 6:30 on a Friday night, and I am triaging three admissions to the leukemia service at once. The call from the ED about you makes me pause. I recognize your name – you were my patient a few years before. At the time, you were undergoing chemotherapy for acute myeloid leukemia, and I cared for you during the aftermath. I now pull up your chart and fill in the gaps of the last 2 years. You got into remission and received a bone marrow transplant. For 2 years, you were cured. But today, you are back. The ED has picked up an abundance of blasts – cancer cells – in your blood. I walk to your ED gurney slowly, thinking of how to tell you this. You recognize me, too. And I can see in your eyes that you already know. “I am so sorry this is happening,” I say.
You are here for your third cycle of chemotherapy. It’s a standard check-in. The first cycle was tolerable, the second cycle was rough, and now you are exhausted. You wonder if it’s normal to be so beat up from this. You ask how much nausea is too much nausea. But your hair didn’t fall out – isn’t that strange? Is it a sure thing that it will? And, by the way, is there anything to prevent the neuropathy? You wiggle your fingers as if to emphasize the point. We go through each of your symptoms and strategize ways to make this cycle better than the last. “OK,” you conclude triumphantly. “I got this!”
It’s your 1-month follow-up and it’s time to pivot. After you were diagnosed with an aggressive triple-negative breast cancer, you met with a medical oncologist and a surgeon. Chemotherapy first, they agreed. The chemo would shrink the tumor, they said, so that it could all be scooped out with surgery. The medications were rough, but you knew it was for the best. But now it’s been two cycles and the lump in your breast is getting bigger not smaller. I ask if I may draw on your skin, promising I’ll wash it off. I gently trace the mass in pen and pull out a tape measure. Yes. It is bigger. I listen to your heart and hear it racing. “What now?” you ask.
When you saw your doctor for bloating and were told it’s not gas, actually, but stage 4 cancer, you didn’t cry. You didn’t deny it. You prepared. You called your lawyer and made a will. You contacted your job and planned for retirement. You organized your things so your children wouldn’t have to. Your oncologist recommended palliative chemotherapy as it could give you some more good days. The best case scenario would be 1 year. That was 2½ years ago. You still like to be prepared, you tell me, but that’s on the back burner now. You are busy, after all – your feet still ache from dancing all night in heels at your niece’s wedding last weekend. I pull up your latest PET scan and we look together: Again, wonderfully, everything appears stable. “See you in 3 months,” I say.
You called three times to move up this appointment because you didn’t know if you’d be alive this long. You want a second opinion. When your kidney cancer grew after surgery, two immunotherapy drugs, and a chemotherapy pill, the latest setback has been fevers up to 104 ° with drenching night sweats. They found a deep infection gnawing around the edges of your tumor, and antibiotics aren’t touching it. The only chance to stop the cancer is more chemotherapy, but that could make the infection worse and lead to a rapid demise. You can’t decide. Today, in the exam room, you are sweating. Your temperature is 101 °. Your partner is trying to keep it together, but the crumpled tissues in her hand give it away. She looks at me earnestly: “What would you do if this were your family member?”
You teach about this disease in your classes and never thought it would happen to you. It started simply enough – you were bruising. Your joints ached. Small things; odd things. The ER doctor cleverly noticed that some numbers were off in your blood counts and sent you to a hematology-oncology doctor, who then cleverly ordered a molecular blood test. It was a long shot. He didn’t really expect it to come back with chronic myeloid leukemia. But there it is, and here we are. You return to talk about treatment options. You understand in detail the biology of how they work. What you don’t know is which is best for you. I go through the four choices and unpleasant effects of each. Muscle aches; diarrhea; risk of bleeding; twice a day dosing tied to mealtimes. “Is there an Option 5?” you wonder.
You have been in the hospital for 34 days, but who’s counting? You are. Because it has been Thirty. Four. Days. You knew the chemotherapy would suppress your blood counts. Now you know what “impaired immune system” really means. You had the bloodstream bacterial infection, requiring 2 days in the ICU. You had the invasive fungus growing in your lungs. The nurses post a calendar on your wall and kindly fill it in every day with your white blood cell count so you don’t have to ask. For days, it’s the same. Your bag stays packed – “just in case,” you explain. Your spouse diligently keeps your children – 2 and 4 years old – away, as kids are notorious germ factories. Then one Sunday morning and – finally! “Put me on speakerphone,” you tell your spouse. “Daddy is coming home!”
One of the most precious parts of hematology and oncology is the relationships. You are there not just for one difficult moment, but for the journey. I await getting to help you over the years to come. For now, I will settle for snapshots.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz and listen to her each week on the Blood & Cancer podcast.
October 2019 Advances in Federal Mental Health Care
Click here to access October 2019 Advances in Federal Mental Health Care
Table of Contents
- Research News
- Open Clinical Trials for Veterans With Suicidal Ideation
- Validation of the Timberlawn Couple and Family Evaluation Scales-Self-Report in Veterans with PTSD
- Perceived Barriers and Facilitators of Clozapine Use: A National Survey of Veterans Affairs Prescribers
- Standardizing the Use of Mental Health Screening Instruments in Patients With Pain
- Assessing Refill Data Among Different Classes of Antidepressants

Click here to access October 2019 Advances in Federal Mental Health Care
Table of Contents
- Research News
- Open Clinical Trials for Veterans With Suicidal Ideation
- Validation of the Timberlawn Couple and Family Evaluation Scales-Self-Report in Veterans with PTSD
- Perceived Barriers and Facilitators of Clozapine Use: A National Survey of Veterans Affairs Prescribers
- Standardizing the Use of Mental Health Screening Instruments in Patients With Pain
- Assessing Refill Data Among Different Classes of Antidepressants

Click here to access October 2019 Advances in Federal Mental Health Care
Table of Contents
- Research News
- Open Clinical Trials for Veterans With Suicidal Ideation
- Validation of the Timberlawn Couple and Family Evaluation Scales-Self-Report in Veterans with PTSD
- Perceived Barriers and Facilitators of Clozapine Use: A National Survey of Veterans Affairs Prescribers
- Standardizing the Use of Mental Health Screening Instruments in Patients With Pain
- Assessing Refill Data Among Different Classes of Antidepressants

VA Health Care Facilities Enter a New Smoke-Free Era
The updated smoking policy goes into effect for employees, patients, visitors, volunteers, contractors, and vendors, whether they smoke cigarettes, cigars, pipes, or even electronic and vaping devices, and whenever they are on the grounds of VA health care facilities, including parking areas.
The new policy comes after the VA reviewed research on second- and thirdhand smoke and best practices in the health care industry. “There is no risk-free level of exposure to tobacco smoke,” the VA’s Smokefree website says. Overwhelming evidence shows exposure to secondhand smoke has significant medical risks. Moreover, a growing body of evidence shows exposure to thirdhand smoke (residual nicotine and other chemicals left on indoor surfaces) also is a health hazard. The residue is thought to react with indoor pollutants to create a toxic mix that clings long after smoking has stopped and cannot be eliminated by opening windows, or using fans, or other means of clearing rooms.
“We are not alone in recognizing the importance of creating a smoke-free campus,” said VA Secretary Robert Wilkie. He notes that as of 2014, 4000 health care facilities and 4 national health care systems in the US have implemented smoke-free grounds.
National Association of Government employees will begin implementing the policy as of October 1, and have until January 1, 2020, to fully comply. Smoking shelters will be closed, although each facility will independently determine the disposition of smoking areas and shelters.
The new policy does not mean anyone has to quit smoking but to encourage quitting, the VA offers resources, including www.publichealth.va.gov/smoking/quit/index.asp. More tips and tools are available at the Smokefree Veteran website: https://veterans.smokefree.gov. SmokefreeVET is a text-messaging program (https://veterans.smokefree.gov/tools-tips-vet/smokefreevet) that provides 24/7 support to help veterans quit for good. Employees can contact their facility for resources.
The policies are available at https://www.va.gov/health/smokefree.
The updated smoking policy goes into effect for employees, patients, visitors, volunteers, contractors, and vendors, whether they smoke cigarettes, cigars, pipes, or even electronic and vaping devices, and whenever they are on the grounds of VA health care facilities, including parking areas.
The new policy comes after the VA reviewed research on second- and thirdhand smoke and best practices in the health care industry. “There is no risk-free level of exposure to tobacco smoke,” the VA’s Smokefree website says. Overwhelming evidence shows exposure to secondhand smoke has significant medical risks. Moreover, a growing body of evidence shows exposure to thirdhand smoke (residual nicotine and other chemicals left on indoor surfaces) also is a health hazard. The residue is thought to react with indoor pollutants to create a toxic mix that clings long after smoking has stopped and cannot be eliminated by opening windows, or using fans, or other means of clearing rooms.
“We are not alone in recognizing the importance of creating a smoke-free campus,” said VA Secretary Robert Wilkie. He notes that as of 2014, 4000 health care facilities and 4 national health care systems in the US have implemented smoke-free grounds.
National Association of Government employees will begin implementing the policy as of October 1, and have until January 1, 2020, to fully comply. Smoking shelters will be closed, although each facility will independently determine the disposition of smoking areas and shelters.
The new policy does not mean anyone has to quit smoking but to encourage quitting, the VA offers resources, including www.publichealth.va.gov/smoking/quit/index.asp. More tips and tools are available at the Smokefree Veteran website: https://veterans.smokefree.gov. SmokefreeVET is a text-messaging program (https://veterans.smokefree.gov/tools-tips-vet/smokefreevet) that provides 24/7 support to help veterans quit for good. Employees can contact their facility for resources.
The policies are available at https://www.va.gov/health/smokefree.
The updated smoking policy goes into effect for employees, patients, visitors, volunteers, contractors, and vendors, whether they smoke cigarettes, cigars, pipes, or even electronic and vaping devices, and whenever they are on the grounds of VA health care facilities, including parking areas.
The new policy comes after the VA reviewed research on second- and thirdhand smoke and best practices in the health care industry. “There is no risk-free level of exposure to tobacco smoke,” the VA’s Smokefree website says. Overwhelming evidence shows exposure to secondhand smoke has significant medical risks. Moreover, a growing body of evidence shows exposure to thirdhand smoke (residual nicotine and other chemicals left on indoor surfaces) also is a health hazard. The residue is thought to react with indoor pollutants to create a toxic mix that clings long after smoking has stopped and cannot be eliminated by opening windows, or using fans, or other means of clearing rooms.
“We are not alone in recognizing the importance of creating a smoke-free campus,” said VA Secretary Robert Wilkie. He notes that as of 2014, 4000 health care facilities and 4 national health care systems in the US have implemented smoke-free grounds.
National Association of Government employees will begin implementing the policy as of October 1, and have until January 1, 2020, to fully comply. Smoking shelters will be closed, although each facility will independently determine the disposition of smoking areas and shelters.
The new policy does not mean anyone has to quit smoking but to encourage quitting, the VA offers resources, including www.publichealth.va.gov/smoking/quit/index.asp. More tips and tools are available at the Smokefree Veteran website: https://veterans.smokefree.gov. SmokefreeVET is a text-messaging program (https://veterans.smokefree.gov/tools-tips-vet/smokefreevet) that provides 24/7 support to help veterans quit for good. Employees can contact their facility for resources.
The policies are available at https://www.va.gov/health/smokefree.
Streaked Discoloration on the Upper Body
The Diagnosis: Bleomycin-Induced Flagellate Hyperpigmentation
Histopathology of the affected skin demonstrated a slight increase in collagen bundle thickness, a chronic dermal perivascular inflammation, and associated pigment incontinence with dermal melanophages compared to unaffected skin (Figure). CD34 was faintly decreased, and dermal mucin increased in affected skin. This postinflammatory pigmentary alteration with subtle dermal sclerosis had persisted unchanged for more than 5 years after cessation of bleomycin therapy. Topical hydroquinone, physical blocker photoprotection, and laser modalities such as the Q-switched alexandrite (755-nm)/Nd:YAG (1064-nm) and ablative CO2 resurfacing lasers were attempted with minimal overall impact on cosmesis.
Bleomycin is a chemotherapeutic antibiotic that has been commonly used to treat Hodgkin lymphoma, germ cell tumors, and recurrent malignant pleural effusions.1 The drug is inactivated in most tissues by the enzyme bleomycin hydrolase. This enzyme is not present in skin and lung tissue; as a result, these organs are the most common sites of bleomycin toxicity.1 There are a variety of cutaneous effects associated with bleomycin including alopecia, hyperpigmentation, acral erythema, Raynaud phenomenon, and nail dystrophy.2 Flagellate hyperpigmentation is a less common cutaneous toxicity. It is an unusual eruption that appears as whiplike linear streaks on the upper chest and back, limbs, and flanks.3 This cutaneous manifestation was once thought to be specific to bleomycin use; however, it also has been described in dermatomyositis, adult-onset Still disease, and after the ingestion of uncooked or undercooked shiitake mushrooms.4 Flagellate hyperpigmentation also was once thought to be dose dependent; however, it has been described in even very small doses.5 The eruption has been described as independent of the route of drug administration, appearing with intravenous, subcutaneous, and intramuscular bleomycin.2 The association of bleomycin and flagellate hyperpigmentation has been reported since 1970; however, it is less commonly seen in clinical practice with the declining use of bleomycin.1
The exact mechanism for the hyperpigmentation is unknown. It has been proposed that the linear lesions are related to areas of pruritus and subsequent excoriations.1 Dermatographism may be present to a limited extent, but it is unlikely to be a chief cause of flagellate hyperpigmentation, as linear streaks have been reported in the absence of trauma. It also has been proposed that bleomycin has a direct toxic effect on the melanocytes, which stimulates increased melanin secretion.2 The hyperpigmentation also may be due to pigmentary incontinence secondary to inflammation.5 Histopathologic findings usually are varied and nonspecific.2 There may be a deep perivascular lymphocytic infiltrate, which is nonspecific but can be associated with drug-induced pathology.4 Bleomycin also is used to induce localized scleroderma in mouse-model research6 and has been reported to cause localized scleroderma at an infusion site or after an intralesional injection,7,8 which is not typically reported in flagellate erythema, but bleomycin's sclerosing effects may have played a role in the visible and sclerosing atrophy noted in our patient. Yamamoto et al9 reported a similar case of dermal sclerosis induced by bleomycin.
Flagellate hyperpigmentation typically lasts for up to 6 months.3 Patients with cutaneous manifestations from bleomycin therapy usually respond to steroid therapy and discontinuation of the drug. Bleomycin re-exposure should be avoided, as it may cause extension or widespread recurrence of flagellate hyperpigmentation.3 Postinflammatory pigment alteration may persist in patients with darker skin types and in patients with dramatic inciting inflammation.
Atrophoderma of Pasini and Pierini is a form of dermal atrophy that presents with 1 or more sharply demarcated depressed patches. There is some debate whether it is a distinct entity or a primary atrophic morphea.10 Linear atrophoderma of Moulin has a similar morphology with hyperpigmented depressions and "cliff-drop" borders, but these lesions follow the lines of Blaschko.11 Linear morphea initially can present as a linear erythematous streak but more commonly appears as a plaque-type morphea lesion that forms a scarlike band.12 Erythema dyschromicum perstans is an ashy dermatosis characterized by gray or blue-brown macules seen in Fitzpatrick skin types III through V and typically is chronic and progressive.13
- Lee HY, Lim KH, Ryu Y, et al. Bleomycininduced flagellate erythema: a case report and review of the literature. Oncol Lett. 2014;8:933-935.
- Simpson RC, Da Forno P, Nagarajan C, et al. A pruritic rash in a patient with Hodgkin lymphoma. Clin Exp Dermatol. 2011;36:680-682.
- Fyfe AJ, McKay P. Toxicities associated with bleomycin. J R Coll Physicians Edinb. 2010;40:213-215.
- Lu CC, Lu YY, Wang QR, et al. Bleomycin-induced flagellate erythema. Balkan Med J. 2014;31:189-190.
- Abess A, Keel DM, Graham BS. Flagellate hyperpigmentation following intralesional bleomycin treatment of verruca plantaris. Arch Dermatol. 2003;139:337-339.
- Yamamoto T. The bleomycin-induced scleroderma model: what have we learned for scleroderma pathogenesis? Arch Dermatol Res. 2006;297:333-344.
- Kim KH, Yoon TJ, Oh CW, et al. A case of bleomycin-induced scleroderma. J Korean Med Sci. 1996;11:454-456.
- Kerr LD, Spiera H. Scleroderma in association with the use of bleomycin: a report of 3 cases. J Rheumatol. 1992;19:294-296.
- Yamamoto T, Yokozeki H, Nishioka K. Dermal sclerosis in the lesional skin of 'flagellate' erythema (scratch dermatitis) induced by bleomycin. Dermatology. 1998;197:399-400.
- Kencka D, Blaszczyk M, Jablońska S. Atrophoderma Pasini-Pierini is a primary atrophic abortive morphea. Dermatology. 1995;190:203-206.
- Moulin G, Hill MP, Guillaud V, et al. Acquired atrophic pigmented band-like lesions following Blaschko's lines. Ann Dermatol Venereol. 1992;119:729-736.
- Fett N, Werth VP. Update on morphea: part I. epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64:217-228.
- Zaynoun S, Rubeiz N, Kibbi AG. Ashy dermatosis--a critical review of literature and a proposed simplified clinical classification. Int J Dermatol. 2008;47:542-544.
The Diagnosis: Bleomycin-Induced Flagellate Hyperpigmentation
Histopathology of the affected skin demonstrated a slight increase in collagen bundle thickness, a chronic dermal perivascular inflammation, and associated pigment incontinence with dermal melanophages compared to unaffected skin (Figure). CD34 was faintly decreased, and dermal mucin increased in affected skin. This postinflammatory pigmentary alteration with subtle dermal sclerosis had persisted unchanged for more than 5 years after cessation of bleomycin therapy. Topical hydroquinone, physical blocker photoprotection, and laser modalities such as the Q-switched alexandrite (755-nm)/Nd:YAG (1064-nm) and ablative CO2 resurfacing lasers were attempted with minimal overall impact on cosmesis.
Bleomycin is a chemotherapeutic antibiotic that has been commonly used to treat Hodgkin lymphoma, germ cell tumors, and recurrent malignant pleural effusions.1 The drug is inactivated in most tissues by the enzyme bleomycin hydrolase. This enzyme is not present in skin and lung tissue; as a result, these organs are the most common sites of bleomycin toxicity.1 There are a variety of cutaneous effects associated with bleomycin including alopecia, hyperpigmentation, acral erythema, Raynaud phenomenon, and nail dystrophy.2 Flagellate hyperpigmentation is a less common cutaneous toxicity. It is an unusual eruption that appears as whiplike linear streaks on the upper chest and back, limbs, and flanks.3 This cutaneous manifestation was once thought to be specific to bleomycin use; however, it also has been described in dermatomyositis, adult-onset Still disease, and after the ingestion of uncooked or undercooked shiitake mushrooms.4 Flagellate hyperpigmentation also was once thought to be dose dependent; however, it has been described in even very small doses.5 The eruption has been described as independent of the route of drug administration, appearing with intravenous, subcutaneous, and intramuscular bleomycin.2 The association of bleomycin and flagellate hyperpigmentation has been reported since 1970; however, it is less commonly seen in clinical practice with the declining use of bleomycin.1
The exact mechanism for the hyperpigmentation is unknown. It has been proposed that the linear lesions are related to areas of pruritus and subsequent excoriations.1 Dermatographism may be present to a limited extent, but it is unlikely to be a chief cause of flagellate hyperpigmentation, as linear streaks have been reported in the absence of trauma. It also has been proposed that bleomycin has a direct toxic effect on the melanocytes, which stimulates increased melanin secretion.2 The hyperpigmentation also may be due to pigmentary incontinence secondary to inflammation.5 Histopathologic findings usually are varied and nonspecific.2 There may be a deep perivascular lymphocytic infiltrate, which is nonspecific but can be associated with drug-induced pathology.4 Bleomycin also is used to induce localized scleroderma in mouse-model research6 and has been reported to cause localized scleroderma at an infusion site or after an intralesional injection,7,8 which is not typically reported in flagellate erythema, but bleomycin's sclerosing effects may have played a role in the visible and sclerosing atrophy noted in our patient. Yamamoto et al9 reported a similar case of dermal sclerosis induced by bleomycin.
Flagellate hyperpigmentation typically lasts for up to 6 months.3 Patients with cutaneous manifestations from bleomycin therapy usually respond to steroid therapy and discontinuation of the drug. Bleomycin re-exposure should be avoided, as it may cause extension or widespread recurrence of flagellate hyperpigmentation.3 Postinflammatory pigment alteration may persist in patients with darker skin types and in patients with dramatic inciting inflammation.
Atrophoderma of Pasini and Pierini is a form of dermal atrophy that presents with 1 or more sharply demarcated depressed patches. There is some debate whether it is a distinct entity or a primary atrophic morphea.10 Linear atrophoderma of Moulin has a similar morphology with hyperpigmented depressions and "cliff-drop" borders, but these lesions follow the lines of Blaschko.11 Linear morphea initially can present as a linear erythematous streak but more commonly appears as a plaque-type morphea lesion that forms a scarlike band.12 Erythema dyschromicum perstans is an ashy dermatosis characterized by gray or blue-brown macules seen in Fitzpatrick skin types III through V and typically is chronic and progressive.13
The Diagnosis: Bleomycin-Induced Flagellate Hyperpigmentation
Histopathology of the affected skin demonstrated a slight increase in collagen bundle thickness, a chronic dermal perivascular inflammation, and associated pigment incontinence with dermal melanophages compared to unaffected skin (Figure). CD34 was faintly decreased, and dermal mucin increased in affected skin. This postinflammatory pigmentary alteration with subtle dermal sclerosis had persisted unchanged for more than 5 years after cessation of bleomycin therapy. Topical hydroquinone, physical blocker photoprotection, and laser modalities such as the Q-switched alexandrite (755-nm)/Nd:YAG (1064-nm) and ablative CO2 resurfacing lasers were attempted with minimal overall impact on cosmesis.
Bleomycin is a chemotherapeutic antibiotic that has been commonly used to treat Hodgkin lymphoma, germ cell tumors, and recurrent malignant pleural effusions.1 The drug is inactivated in most tissues by the enzyme bleomycin hydrolase. This enzyme is not present in skin and lung tissue; as a result, these organs are the most common sites of bleomycin toxicity.1 There are a variety of cutaneous effects associated with bleomycin including alopecia, hyperpigmentation, acral erythema, Raynaud phenomenon, and nail dystrophy.2 Flagellate hyperpigmentation is a less common cutaneous toxicity. It is an unusual eruption that appears as whiplike linear streaks on the upper chest and back, limbs, and flanks.3 This cutaneous manifestation was once thought to be specific to bleomycin use; however, it also has been described in dermatomyositis, adult-onset Still disease, and after the ingestion of uncooked or undercooked shiitake mushrooms.4 Flagellate hyperpigmentation also was once thought to be dose dependent; however, it has been described in even very small doses.5 The eruption has been described as independent of the route of drug administration, appearing with intravenous, subcutaneous, and intramuscular bleomycin.2 The association of bleomycin and flagellate hyperpigmentation has been reported since 1970; however, it is less commonly seen in clinical practice with the declining use of bleomycin.1
The exact mechanism for the hyperpigmentation is unknown. It has been proposed that the linear lesions are related to areas of pruritus and subsequent excoriations.1 Dermatographism may be present to a limited extent, but it is unlikely to be a chief cause of flagellate hyperpigmentation, as linear streaks have been reported in the absence of trauma. It also has been proposed that bleomycin has a direct toxic effect on the melanocytes, which stimulates increased melanin secretion.2 The hyperpigmentation also may be due to pigmentary incontinence secondary to inflammation.5 Histopathologic findings usually are varied and nonspecific.2 There may be a deep perivascular lymphocytic infiltrate, which is nonspecific but can be associated with drug-induced pathology.4 Bleomycin also is used to induce localized scleroderma in mouse-model research6 and has been reported to cause localized scleroderma at an infusion site or after an intralesional injection,7,8 which is not typically reported in flagellate erythema, but bleomycin's sclerosing effects may have played a role in the visible and sclerosing atrophy noted in our patient. Yamamoto et al9 reported a similar case of dermal sclerosis induced by bleomycin.
Flagellate hyperpigmentation typically lasts for up to 6 months.3 Patients with cutaneous manifestations from bleomycin therapy usually respond to steroid therapy and discontinuation of the drug. Bleomycin re-exposure should be avoided, as it may cause extension or widespread recurrence of flagellate hyperpigmentation.3 Postinflammatory pigment alteration may persist in patients with darker skin types and in patients with dramatic inciting inflammation.
Atrophoderma of Pasini and Pierini is a form of dermal atrophy that presents with 1 or more sharply demarcated depressed patches. There is some debate whether it is a distinct entity or a primary atrophic morphea.10 Linear atrophoderma of Moulin has a similar morphology with hyperpigmented depressions and "cliff-drop" borders, but these lesions follow the lines of Blaschko.11 Linear morphea initially can present as a linear erythematous streak but more commonly appears as a plaque-type morphea lesion that forms a scarlike band.12 Erythema dyschromicum perstans is an ashy dermatosis characterized by gray or blue-brown macules seen in Fitzpatrick skin types III through V and typically is chronic and progressive.13
- Lee HY, Lim KH, Ryu Y, et al. Bleomycininduced flagellate erythema: a case report and review of the literature. Oncol Lett. 2014;8:933-935.
- Simpson RC, Da Forno P, Nagarajan C, et al. A pruritic rash in a patient with Hodgkin lymphoma. Clin Exp Dermatol. 2011;36:680-682.
- Fyfe AJ, McKay P. Toxicities associated with bleomycin. J R Coll Physicians Edinb. 2010;40:213-215.
- Lu CC, Lu YY, Wang QR, et al. Bleomycin-induced flagellate erythema. Balkan Med J. 2014;31:189-190.
- Abess A, Keel DM, Graham BS. Flagellate hyperpigmentation following intralesional bleomycin treatment of verruca plantaris. Arch Dermatol. 2003;139:337-339.
- Yamamoto T. The bleomycin-induced scleroderma model: what have we learned for scleroderma pathogenesis? Arch Dermatol Res. 2006;297:333-344.
- Kim KH, Yoon TJ, Oh CW, et al. A case of bleomycin-induced scleroderma. J Korean Med Sci. 1996;11:454-456.
- Kerr LD, Spiera H. Scleroderma in association with the use of bleomycin: a report of 3 cases. J Rheumatol. 1992;19:294-296.
- Yamamoto T, Yokozeki H, Nishioka K. Dermal sclerosis in the lesional skin of 'flagellate' erythema (scratch dermatitis) induced by bleomycin. Dermatology. 1998;197:399-400.
- Kencka D, Blaszczyk M, Jablońska S. Atrophoderma Pasini-Pierini is a primary atrophic abortive morphea. Dermatology. 1995;190:203-206.
- Moulin G, Hill MP, Guillaud V, et al. Acquired atrophic pigmented band-like lesions following Blaschko's lines. Ann Dermatol Venereol. 1992;119:729-736.
- Fett N, Werth VP. Update on morphea: part I. epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64:217-228.
- Zaynoun S, Rubeiz N, Kibbi AG. Ashy dermatosis--a critical review of literature and a proposed simplified clinical classification. Int J Dermatol. 2008;47:542-544.
- Lee HY, Lim KH, Ryu Y, et al. Bleomycininduced flagellate erythema: a case report and review of the literature. Oncol Lett. 2014;8:933-935.
- Simpson RC, Da Forno P, Nagarajan C, et al. A pruritic rash in a patient with Hodgkin lymphoma. Clin Exp Dermatol. 2011;36:680-682.
- Fyfe AJ, McKay P. Toxicities associated with bleomycin. J R Coll Physicians Edinb. 2010;40:213-215.
- Lu CC, Lu YY, Wang QR, et al. Bleomycin-induced flagellate erythema. Balkan Med J. 2014;31:189-190.
- Abess A, Keel DM, Graham BS. Flagellate hyperpigmentation following intralesional bleomycin treatment of verruca plantaris. Arch Dermatol. 2003;139:337-339.
- Yamamoto T. The bleomycin-induced scleroderma model: what have we learned for scleroderma pathogenesis? Arch Dermatol Res. 2006;297:333-344.
- Kim KH, Yoon TJ, Oh CW, et al. A case of bleomycin-induced scleroderma. J Korean Med Sci. 1996;11:454-456.
- Kerr LD, Spiera H. Scleroderma in association with the use of bleomycin: a report of 3 cases. J Rheumatol. 1992;19:294-296.
- Yamamoto T, Yokozeki H, Nishioka K. Dermal sclerosis in the lesional skin of 'flagellate' erythema (scratch dermatitis) induced by bleomycin. Dermatology. 1998;197:399-400.
- Kencka D, Blaszczyk M, Jablońska S. Atrophoderma Pasini-Pierini is a primary atrophic abortive morphea. Dermatology. 1995;190:203-206.
- Moulin G, Hill MP, Guillaud V, et al. Acquired atrophic pigmented band-like lesions following Blaschko's lines. Ann Dermatol Venereol. 1992;119:729-736.
- Fett N, Werth VP. Update on morphea: part I. epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64:217-228.
- Zaynoun S, Rubeiz N, Kibbi AG. Ashy dermatosis--a critical review of literature and a proposed simplified clinical classification. Int J Dermatol. 2008;47:542-544.
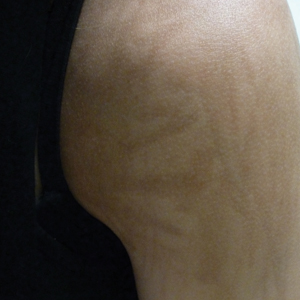
An 18-year-old woman presented to our dermatology clinic with persistent diffuse discoloration on the upper body of more than 5 years’ duration. Her medical history was notable for primary mediastinal classical Hodgkin lymphoma treated with ABVE-PC (doxorubicin, bleomycin, vincristine, etoposide, prednisone, cyclophosphamide) chemotherapy and 22 Gy radiation therapy to the chest 5 years prior. She reported the initial onset of diffuse pruritus with associated scratching and persistent skin discoloration while receiving a course of chemotherapy. Physical examination revealed numerous thin, flagellate, faintly hyperpigmented streaks with subtle atrophy in a parallel configuration on the bilateral shoulders (top), upper back (bottom), and abdomen. Punch biopsies (5 mm) of both affected and unaffected skin on the left side of the lateral upper back were performed.
When providing contraceptive counseling to women with migraine headaches, how do you identify migraine with aura?
Most physicians know that migraine with aura is a risk factor for ischemic stroke and that the use of an estrogen-containing contraceptive further increases this risk.1-3 Additional important and prevalent risk factors for ischemic stroke include cigarette smoking, hypertension, diabetes, and ischemic heart disease.1 The American College of Obstetricians and Gynecologists (ACOG)2 and the Centers for Disease Control and Prevention (CDC)3 recommend against the use of estrogen-containing contraceptives for women with migraine with aura because of the increased risk of ischemic stroke (Medical Eligibility Criteria [MEC] category 4—unacceptable health risk, method not to be used).
However, those who have migraine with aura can use nonhormonal and progestin-only forms of contraception, including copper- and levonorgestrel-intrauterine devices, the etonogestrel subdermal implant, depot medroxyprogesterone acetate, and progestin-only pills (MEC category 1—no restriction).2,3 ACOG and the CDC advise that estrogen-containing contraceptives can be used for those with migraine without aura who have no other risk factors for stroke (MEC category 2—advantages generally outweigh theoretical or proven risks).2,3 Given the high prevalence of migraine in reproductive-age women, accurate diagnosis of aura is of paramount importance in order to provide appropriate contraceptive counseling.
When is migraine with aura the right diagnosis?
In clinical practice, there is a high level of confusion about the migraine symptoms that warrant a diagnosis of migraine with aura. One approach to improving the accuracy of such a diagnosis is to refer every woman seeking contraceptive counseling who has migraine headaches to a neurologist for expert adjudication of the presence or absence of aura. But in the clinical context of contraceptive counseling, neurology consultation is not always readily available, and requiring consultation increases barriers to care. However, there are tools—such as the Visual Aura Rating Scale (VARS), which is discussed below—that may help non-neurologists identify migraine with aura.4 First, let us review the data that links migraine with aura with increased risk of ischemic stroke.

Migraine with aura is a risk factor for stroke
Multiple case-control studies report that migraine with aura is a risk factor for ischemic stroke.1,5,6 Studies also report that women with migraine with aura who use estrogen-containing contraceptives have an even greater risk of ischemic stroke. For example, one recent case-control study used a commercial claims database of 1,884 cases of ischemic stroke among individuals who identify as women 15 to 49 years of age matched to 7,536 controls without ischemic stroke.1 In this study, the risk of ischemic stroke was increased more than 2.5-fold by cigarette smoking (adjusted odds ratio [aOR], 2.59), hypertension (aOR, 2.73), diabetes (aOR, 2.78), migraine with aura (aOR, 2.89), and ischemic heart disease (aOR, 5.49). For those with migraine with aura who also used an estrogen-containing contraceptive, the aOR for ischemic stroke was 6.08. By contrast, the risk for stroke among those with migraine with aura who were not using an estrogen-containing contraceptive was 2.65. Furthermore, among those with migraine without aura, the risk of ischemic stroke was only 1.77 with the use of an estrogen-containing contraceptive.
Continue to: Although women with migraine...
Although women with migraine with and without aura are at increased risk for stroke, the absolute risk is still very low. For example, one review reported that the incidence of ischemic stroke per 100,000 person-years among women 20 to 44 years of age was 2.5 for those without migraine not taking estrogen-containing contraceptives, 5.9 for those with migraine with aura not taking estrogen-containing contraceptives, and 14.5 among those with migraine with aura and taking estrogen-containing contraceptives.6 Another important observation is that the incidence of thrombotic stroke dramatically increases from adolescence (3.4 per 100,000 person-years) to 45-49 years of age (64.4 per 100,000 person-years).7 Therefore, older women with migraine are at greater risk for stroke than adolescents.
Diagnostic criteria for migraine with and without aura
In contraceptive counseling, if an estrogen-containing contraceptive is being considered, it is important to identify women with migraine headache, determine migraine subtype, assess the frequency of migraines and identify other cardiovascular risk factors, such as hypertension and cigarette smoking. The International Headache Society has evolved the diagnostic criteria for migraine with and without aura, and now endorses the criteria published in the 3rd edition of the International Classification of Headache Disorders (ICHD-3; TABLES 1 and 2).8 For non-neurologists, these criteria may be difficult to remember and impractical to utilize in daily contraceptive counseling. Two simplified tools, the ID Migraine Questionnaire9 and the Visual Aura Rating Scale (TABLE 3)4 may help identify women who have migraine headaches and assess for the presence of aura.


The ID Migraine Questionnaire
In a study of 563 people seeking primary care who had headaches in the past 3 months, 3 questions were identified as being helpful in identifying women with migraine. This 3-question screening tool had reasonable sensitivity (81%), specificity (75%), and positive predictive value (93%) compared with expert diagnosis using the ICHD-3.9 The 3 questions in this screening tool, which are answered “Yes” or “No,” are:
During the last 3 months did you have the following symptoms with your headaches:
- Feel nauseated or sick to your stomach?
- Light bothered you?
- Your headaches limited your ability to work, study or do what you needed to do for at least 1 day?
If two questions are answered “Yes” the patient may have migraine headaches.
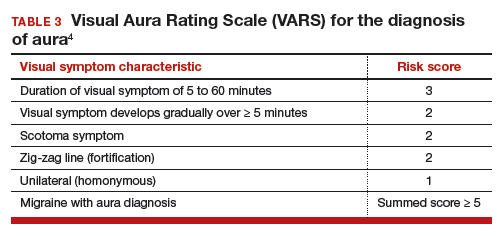
Visual Aura Rating Scale for the diagnosis of migraine with aura
More than 90% of women with migraine with aura have visual auras, leaving only a minority with non–visual aura, such as tingling or numbness in a limb, speech or language problems, or muscle weakness. Hence for non-neurologists, it is reasonable to focus on the accurate diagnosis of visual aura to identify those with migraine with aura.
In the clinical context of contraceptive counseling, the Visual Aura Rating Scale (VARS) is especially useful because it has good sensitivity and specificity, and it is easy to use in practice (TABLE 3).4 VARS assesses for 5 characteristics of a visual aura, and each characteristic is associated with a weighted risk score. The 5 symptoms assessed include:
- duration of visual symptom between 5 and 60 minutes (3 points)
- visual symptom develops gradually over 5 minutes (2 points)
- scotoma (2 points)
- zig-zag line (2 points)
- unilateral (1 point).
Continue to: Of note, visual aura is usually...
Of note, visual aura is usually slow-spreading and persists for more than 5 minutes but less than 60 minutes. If a visual symptom has a sudden onset and persists for much longer than 60 minutes, concern is heightened for a more serious neurologic diagnosis such as transient ischemic attack or stroke. A summed score of 5 or more points supports the diagnosis of migraine with aura. In one study, VARS had a sensitivity of 91% and specificity of 96% for identifying women with migraine with aura diagnosed by the ICHD-3 criteria.4
Consider using VARS to identify migraine with aura
Epidemiologic studies report that about 17% of adults have migraine, and about 5% have migraine with aura.10,11 Consequently, migraine with aura is one of the most common medical conditions encountered during contraceptive counseling. The CDC MEC recommend against the use of estrogen-containing contraceptives in women with migraine with aura (Category 4 rating). The VARS may help clinicians identify those who have migraine with aura who should not be offered estrogen-containing contraceptives. Equally important, the use of VARS could help reduce the number of women who are inappropriately diagnosed as having migraine with aura based on fleeting visual symptoms lasting far less than 5 minutes during a migraine headache.
- Champaloux SW, Tepper NK, Monsour M, et al. Use of combined hormonal contraceptives among women with migraine and risk of ischemic stroke. Am J Obstet Gynecol. 2017;216:489.e1-e7.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Eriksen MK, Thomsen LL, Olesen J. The Visual Aura Rating Scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914.
- Sacco S, Merki-Feld G, Aegidius KL, et al. Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain. 2017;18:108.
- Lidegaard Ø, Lokkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
- Headache Classification Committee of the International Headache Society. International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
- Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: the ID Migraine validation study. Neurology. 2003;12;61:375-382.
- Lipton RB, Scher AI, Kolodner K, et al. Migraine in the United States: epidemiology and patterns of health care use. Neurology. 2002;58:885-894.
- Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
Most physicians know that migraine with aura is a risk factor for ischemic stroke and that the use of an estrogen-containing contraceptive further increases this risk.1-3 Additional important and prevalent risk factors for ischemic stroke include cigarette smoking, hypertension, diabetes, and ischemic heart disease.1 The American College of Obstetricians and Gynecologists (ACOG)2 and the Centers for Disease Control and Prevention (CDC)3 recommend against the use of estrogen-containing contraceptives for women with migraine with aura because of the increased risk of ischemic stroke (Medical Eligibility Criteria [MEC] category 4—unacceptable health risk, method not to be used).
However, those who have migraine with aura can use nonhormonal and progestin-only forms of contraception, including copper- and levonorgestrel-intrauterine devices, the etonogestrel subdermal implant, depot medroxyprogesterone acetate, and progestin-only pills (MEC category 1—no restriction).2,3 ACOG and the CDC advise that estrogen-containing contraceptives can be used for those with migraine without aura who have no other risk factors for stroke (MEC category 2—advantages generally outweigh theoretical or proven risks).2,3 Given the high prevalence of migraine in reproductive-age women, accurate diagnosis of aura is of paramount importance in order to provide appropriate contraceptive counseling.
When is migraine with aura the right diagnosis?
In clinical practice, there is a high level of confusion about the migraine symptoms that warrant a diagnosis of migraine with aura. One approach to improving the accuracy of such a diagnosis is to refer every woman seeking contraceptive counseling who has migraine headaches to a neurologist for expert adjudication of the presence or absence of aura. But in the clinical context of contraceptive counseling, neurology consultation is not always readily available, and requiring consultation increases barriers to care. However, there are tools—such as the Visual Aura Rating Scale (VARS), which is discussed below—that may help non-neurologists identify migraine with aura.4 First, let us review the data that links migraine with aura with increased risk of ischemic stroke.

Migraine with aura is a risk factor for stroke
Multiple case-control studies report that migraine with aura is a risk factor for ischemic stroke.1,5,6 Studies also report that women with migraine with aura who use estrogen-containing contraceptives have an even greater risk of ischemic stroke. For example, one recent case-control study used a commercial claims database of 1,884 cases of ischemic stroke among individuals who identify as women 15 to 49 years of age matched to 7,536 controls without ischemic stroke.1 In this study, the risk of ischemic stroke was increased more than 2.5-fold by cigarette smoking (adjusted odds ratio [aOR], 2.59), hypertension (aOR, 2.73), diabetes (aOR, 2.78), migraine with aura (aOR, 2.89), and ischemic heart disease (aOR, 5.49). For those with migraine with aura who also used an estrogen-containing contraceptive, the aOR for ischemic stroke was 6.08. By contrast, the risk for stroke among those with migraine with aura who were not using an estrogen-containing contraceptive was 2.65. Furthermore, among those with migraine without aura, the risk of ischemic stroke was only 1.77 with the use of an estrogen-containing contraceptive.
Continue to: Although women with migraine...
Although women with migraine with and without aura are at increased risk for stroke, the absolute risk is still very low. For example, one review reported that the incidence of ischemic stroke per 100,000 person-years among women 20 to 44 years of age was 2.5 for those without migraine not taking estrogen-containing contraceptives, 5.9 for those with migraine with aura not taking estrogen-containing contraceptives, and 14.5 among those with migraine with aura and taking estrogen-containing contraceptives.6 Another important observation is that the incidence of thrombotic stroke dramatically increases from adolescence (3.4 per 100,000 person-years) to 45-49 years of age (64.4 per 100,000 person-years).7 Therefore, older women with migraine are at greater risk for stroke than adolescents.
Diagnostic criteria for migraine with and without aura
In contraceptive counseling, if an estrogen-containing contraceptive is being considered, it is important to identify women with migraine headache, determine migraine subtype, assess the frequency of migraines and identify other cardiovascular risk factors, such as hypertension and cigarette smoking. The International Headache Society has evolved the diagnostic criteria for migraine with and without aura, and now endorses the criteria published in the 3rd edition of the International Classification of Headache Disorders (ICHD-3; TABLES 1 and 2).8 For non-neurologists, these criteria may be difficult to remember and impractical to utilize in daily contraceptive counseling. Two simplified tools, the ID Migraine Questionnaire9 and the Visual Aura Rating Scale (TABLE 3)4 may help identify women who have migraine headaches and assess for the presence of aura.


The ID Migraine Questionnaire
In a study of 563 people seeking primary care who had headaches in the past 3 months, 3 questions were identified as being helpful in identifying women with migraine. This 3-question screening tool had reasonable sensitivity (81%), specificity (75%), and positive predictive value (93%) compared with expert diagnosis using the ICHD-3.9 The 3 questions in this screening tool, which are answered “Yes” or “No,” are:
During the last 3 months did you have the following symptoms with your headaches:
- Feel nauseated or sick to your stomach?
- Light bothered you?
- Your headaches limited your ability to work, study or do what you needed to do for at least 1 day?
If two questions are answered “Yes” the patient may have migraine headaches.
Visual Aura Rating Scale for the diagnosis of migraine with aura
More than 90% of women with migraine with aura have visual auras, leaving only a minority with non–visual aura, such as tingling or numbness in a limb, speech or language problems, or muscle weakness. Hence for non-neurologists, it is reasonable to focus on the accurate diagnosis of visual aura to identify those with migraine with aura.
In the clinical context of contraceptive counseling, the Visual Aura Rating Scale (VARS) is especially useful because it has good sensitivity and specificity, and it is easy to use in practice (TABLE 3).4 VARS assesses for 5 characteristics of a visual aura, and each characteristic is associated with a weighted risk score. The 5 symptoms assessed include:
- duration of visual symptom between 5 and 60 minutes (3 points)
- visual symptom develops gradually over 5 minutes (2 points)
- scotoma (2 points)
- zig-zag line (2 points)
- unilateral (1 point).
Continue to: Of note, visual aura is usually...
Of note, visual aura is usually slow-spreading and persists for more than 5 minutes but less than 60 minutes. If a visual symptom has a sudden onset and persists for much longer than 60 minutes, concern is heightened for a more serious neurologic diagnosis such as transient ischemic attack or stroke. A summed score of 5 or more points supports the diagnosis of migraine with aura. In one study, VARS had a sensitivity of 91% and specificity of 96% for identifying women with migraine with aura diagnosed by the ICHD-3 criteria.4

Consider using VARS to identify migraine with aura
Epidemiologic studies report that about 17% of adults have migraine, and about 5% have migraine with aura.10,11 Consequently, migraine with aura is one of the most common medical conditions encountered during contraceptive counseling. The CDC MEC recommend against the use of estrogen-containing contraceptives in women with migraine with aura (Category 4 rating). The VARS may help clinicians identify those who have migraine with aura who should not be offered estrogen-containing contraceptives. Equally important, the use of VARS could help reduce the number of women who are inappropriately diagnosed as having migraine with aura based on fleeting visual symptoms lasting far less than 5 minutes during a migraine headache.
Most physicians know that migraine with aura is a risk factor for ischemic stroke and that the use of an estrogen-containing contraceptive further increases this risk.1-3 Additional important and prevalent risk factors for ischemic stroke include cigarette smoking, hypertension, diabetes, and ischemic heart disease.1 The American College of Obstetricians and Gynecologists (ACOG)2 and the Centers for Disease Control and Prevention (CDC)3 recommend against the use of estrogen-containing contraceptives for women with migraine with aura because of the increased risk of ischemic stroke (Medical Eligibility Criteria [MEC] category 4—unacceptable health risk, method not to be used).
However, those who have migraine with aura can use nonhormonal and progestin-only forms of contraception, including copper- and levonorgestrel-intrauterine devices, the etonogestrel subdermal implant, depot medroxyprogesterone acetate, and progestin-only pills (MEC category 1—no restriction).2,3 ACOG and the CDC advise that estrogen-containing contraceptives can be used for those with migraine without aura who have no other risk factors for stroke (MEC category 2—advantages generally outweigh theoretical or proven risks).2,3 Given the high prevalence of migraine in reproductive-age women, accurate diagnosis of aura is of paramount importance in order to provide appropriate contraceptive counseling.
When is migraine with aura the right diagnosis?
In clinical practice, there is a high level of confusion about the migraine symptoms that warrant a diagnosis of migraine with aura. One approach to improving the accuracy of such a diagnosis is to refer every woman seeking contraceptive counseling who has migraine headaches to a neurologist for expert adjudication of the presence or absence of aura. But in the clinical context of contraceptive counseling, neurology consultation is not always readily available, and requiring consultation increases barriers to care. However, there are tools—such as the Visual Aura Rating Scale (VARS), which is discussed below—that may help non-neurologists identify migraine with aura.4 First, let us review the data that links migraine with aura with increased risk of ischemic stroke.

Migraine with aura is a risk factor for stroke
Multiple case-control studies report that migraine with aura is a risk factor for ischemic stroke.1,5,6 Studies also report that women with migraine with aura who use estrogen-containing contraceptives have an even greater risk of ischemic stroke. For example, one recent case-control study used a commercial claims database of 1,884 cases of ischemic stroke among individuals who identify as women 15 to 49 years of age matched to 7,536 controls without ischemic stroke.1 In this study, the risk of ischemic stroke was increased more than 2.5-fold by cigarette smoking (adjusted odds ratio [aOR], 2.59), hypertension (aOR, 2.73), diabetes (aOR, 2.78), migraine with aura (aOR, 2.89), and ischemic heart disease (aOR, 5.49). For those with migraine with aura who also used an estrogen-containing contraceptive, the aOR for ischemic stroke was 6.08. By contrast, the risk for stroke among those with migraine with aura who were not using an estrogen-containing contraceptive was 2.65. Furthermore, among those with migraine without aura, the risk of ischemic stroke was only 1.77 with the use of an estrogen-containing contraceptive.
Continue to: Although women with migraine...
Although women with migraine with and without aura are at increased risk for stroke, the absolute risk is still very low. For example, one review reported that the incidence of ischemic stroke per 100,000 person-years among women 20 to 44 years of age was 2.5 for those without migraine not taking estrogen-containing contraceptives, 5.9 for those with migraine with aura not taking estrogen-containing contraceptives, and 14.5 among those with migraine with aura and taking estrogen-containing contraceptives.6 Another important observation is that the incidence of thrombotic stroke dramatically increases from adolescence (3.4 per 100,000 person-years) to 45-49 years of age (64.4 per 100,000 person-years).7 Therefore, older women with migraine are at greater risk for stroke than adolescents.
Diagnostic criteria for migraine with and without aura
In contraceptive counseling, if an estrogen-containing contraceptive is being considered, it is important to identify women with migraine headache, determine migraine subtype, assess the frequency of migraines and identify other cardiovascular risk factors, such as hypertension and cigarette smoking. The International Headache Society has evolved the diagnostic criteria for migraine with and without aura, and now endorses the criteria published in the 3rd edition of the International Classification of Headache Disorders (ICHD-3; TABLES 1 and 2).8 For non-neurologists, these criteria may be difficult to remember and impractical to utilize in daily contraceptive counseling. Two simplified tools, the ID Migraine Questionnaire9 and the Visual Aura Rating Scale (TABLE 3)4 may help identify women who have migraine headaches and assess for the presence of aura.


The ID Migraine Questionnaire
In a study of 563 people seeking primary care who had headaches in the past 3 months, 3 questions were identified as being helpful in identifying women with migraine. This 3-question screening tool had reasonable sensitivity (81%), specificity (75%), and positive predictive value (93%) compared with expert diagnosis using the ICHD-3.9 The 3 questions in this screening tool, which are answered “Yes” or “No,” are:
During the last 3 months did you have the following symptoms with your headaches:
- Feel nauseated or sick to your stomach?
- Light bothered you?
- Your headaches limited your ability to work, study or do what you needed to do for at least 1 day?
If two questions are answered “Yes” the patient may have migraine headaches.
Visual Aura Rating Scale for the diagnosis of migraine with aura
More than 90% of women with migraine with aura have visual auras, leaving only a minority with non–visual aura, such as tingling or numbness in a limb, speech or language problems, or muscle weakness. Hence for non-neurologists, it is reasonable to focus on the accurate diagnosis of visual aura to identify those with migraine with aura.
In the clinical context of contraceptive counseling, the Visual Aura Rating Scale (VARS) is especially useful because it has good sensitivity and specificity, and it is easy to use in practice (TABLE 3).4 VARS assesses for 5 characteristics of a visual aura, and each characteristic is associated with a weighted risk score. The 5 symptoms assessed include:
- duration of visual symptom between 5 and 60 minutes (3 points)
- visual symptom develops gradually over 5 minutes (2 points)
- scotoma (2 points)
- zig-zag line (2 points)
- unilateral (1 point).
Continue to: Of note, visual aura is usually...
Of note, visual aura is usually slow-spreading and persists for more than 5 minutes but less than 60 minutes. If a visual symptom has a sudden onset and persists for much longer than 60 minutes, concern is heightened for a more serious neurologic diagnosis such as transient ischemic attack or stroke. A summed score of 5 or more points supports the diagnosis of migraine with aura. In one study, VARS had a sensitivity of 91% and specificity of 96% for identifying women with migraine with aura diagnosed by the ICHD-3 criteria.4

Consider using VARS to identify migraine with aura
Epidemiologic studies report that about 17% of adults have migraine, and about 5% have migraine with aura.10,11 Consequently, migraine with aura is one of the most common medical conditions encountered during contraceptive counseling. The CDC MEC recommend against the use of estrogen-containing contraceptives in women with migraine with aura (Category 4 rating). The VARS may help clinicians identify those who have migraine with aura who should not be offered estrogen-containing contraceptives. Equally important, the use of VARS could help reduce the number of women who are inappropriately diagnosed as having migraine with aura based on fleeting visual symptoms lasting far less than 5 minutes during a migraine headache.
- Champaloux SW, Tepper NK, Monsour M, et al. Use of combined hormonal contraceptives among women with migraine and risk of ischemic stroke. Am J Obstet Gynecol. 2017;216:489.e1-e7.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Eriksen MK, Thomsen LL, Olesen J. The Visual Aura Rating Scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914.
- Sacco S, Merki-Feld G, Aegidius KL, et al. Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain. 2017;18:108.
- Lidegaard Ø, Lokkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
- Headache Classification Committee of the International Headache Society. International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
- Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: the ID Migraine validation study. Neurology. 2003;12;61:375-382.
- Lipton RB, Scher AI, Kolodner K, et al. Migraine in the United States: epidemiology and patterns of health care use. Neurology. 2002;58:885-894.
- Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
- Champaloux SW, Tepper NK, Monsour M, et al. Use of combined hormonal contraceptives among women with migraine and risk of ischemic stroke. Am J Obstet Gynecol. 2017;216:489.e1-e7.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Eriksen MK, Thomsen LL, Olesen J. The Visual Aura Rating Scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914.
- Sacco S, Merki-Feld G, Aegidius KL, et al. Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain. 2017;18:108.
- Lidegaard Ø, Lokkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
- Headache Classification Committee of the International Headache Society. International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
- Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: the ID Migraine validation study. Neurology. 2003;12;61:375-382.
- Lipton RB, Scher AI, Kolodner K, et al. Migraine in the United States: epidemiology and patterns of health care use. Neurology. 2002;58:885-894.
- Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
Can we discern optimal long-term osteoporosis treatment for women?
In a recent systematic review, Fink and colleagues attempted to summarize the published evidence of the efficacy and safety of long-term (> 3 years) therapy for osteoporosis.1 Unfortunately, they arrived at very limited and tentative conclusions because, as they point out, of the paucity of such evidence.
Why long-term studies stop short
Only 3 of the several tens of placebo-controlled fracture end-point studies (about 58 trials and observational studies) that Fink and colleagues reviewed evaluated treatment for more than 3 years. The nonavailability of longer-term studies is the direct consequence of a requirement by regulatory agencies for a 3-year fracture end-point study in order to register a new drug for osteoporosis. Hence, longer, placebo-controlled studies do not benefit the industry sponsor, and enrolling patients with osteoporosis or who are at high risk for fracture in any, much less long, placebo-controlled trials is now considered to be unethical.
What the authors did observe
From this limited set of information with which to evaluate, Fink and colleagues observed that long-term therapy with raloxifene reduces the risk of vertebral fractures but is associated with thromboembolic complications. In addition, treatment for more than 3 years with bisphosphonates reduces the risk of vertebral and nonvertebral fractures but may increase risk of rare adverse events (including femoral shaft fractures with atypical radiographic features).
The bisphosphonate holiday. The authors refer to the even more limited evidence about the effects of discontinuing bisphosphonate therapy. Unlike the rapid loss of bone mass density (BMD) and fracture protection upon stopping estrogen or denosumab, the offset of these treatment benefits is slower when bisphosphonates are discontinued. This, coupled with concern about increasing risk with long-term bisphosphonate therapy, led to the confusing concept of a “bisphosphonate holiday.” While recommendations to consider temporary discontinuation of bisphosphonates in patients at low risk for fracture have been made by expert panels,2 very little information exists about the benefits/risks of this strategy, how long the treatment interruption should be, or how to decide when and with what to restart therapy. Unfortunately, overall, Fink and colleagues’ observations provide little practical guidance for clinicians.
Continue to: What we can learn from longer term and recent studies of ideal treatment...
What we can learn from longer term and recent studies of ideal treatment
Since we have no “cure” for osteoporosis, and since the benefits of therapy, including protection from fractures, abate upon stopping treatment (as they do when we stop treating hypertension or diabetes), very long term if not lifelong management is required for patients with osteoporosis. Persistent or even greater reduction of fracture risk with treatment up to 10 years, compared with the rate of fracture in the placebo or treated group during the first 3 years of the study, has been observed with zoledronate and denosumab.3-5 Denosumab was not included in the systematic review by Fink and colleagues since the pivotal fracture trial with that agent was placebo-controlled for only 3 years.6
Sequential drug treatment may be best. Fink and colleagues also did not consider new evidence, which suggests that the use of osteoporosis drugs in sequence—rather than a single agent for a long time—may be the most effective management strategy.7,8
More consideration should be given to the use of estrogen and raloxifene in younger postmenopausal women at risk for vertebral but not hip fracture.
Only treat high-risk patients. Using osteoporosis therapies to only treat patients at high risk for fracture will optimize the benefit:risk ratio and cost-effectiveness of therapy.
Bisphosphonate holidays may not be as important as once thought. BMD and fracture risk reduction does not improve after 5 years of bisphosphonate therapy, and longer treatment may increase the risk of atypic
Hip BMD may serve as indicator for treatment decisions. Recent evidence indicating that the change in hip BMD with treatment or the level of hip BMD achieved on treatment correlates with fracture risk reduction may provide a useful clinical target to guide treatment decisions.9,10
Because we have a lack of pristine evidence does not mean that we shouldn’t treat osteoporosis; we have to do the best we can with the limited evidence we have. Therapy must be individualized, for we are not just treating osteoporosis, we are treating patients with osteoporosis.
- Fink HA, MacDonald R, Forte ML, et al. Long-term drug therapy and drug discontinuations and holidays for osteoporosis fracture prevention: a systematic review. Ann Intern Med. 2019;171:37-50.
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:16-35.
- Black DM, Reid IR, Cauley JA, et al. The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2015;30:934-944.
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513-523.
- Ferrari S, Butler PW, Kendler DL, et al. Further nonvertebral fracture reduction beyond 3 years for up to 10 years of denosumab treatment. J Clin Endocrinol Metab. 2019;104:3450-3461.
- Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Cosman F, Nieves JW, Dempster DW. Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res. 2017;32:198-202.
- Hanley DA, McClung MR, Davison KS, et al; Writing Group for the Western Osteoporosis Alliance. Western Osteoporosis Alliance Clinical Practice Series: evaluating the balance of benefits and risks of long-term osteoporosis therapies. Am J Med. 2017;130:862.e1-862.e7.
- Bouxsein ML, Eastell R, Lui LY, et al; FNIH Bone Quality Project. Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res. 2019;34:632-642.
- Ferrari S, Libanati C, Lin CJF, et al. Relationship between bone mineral density T-score and nonvertebral fracture risk over 10 years of denosumab treatment. J Bone Miner Res. 2019;34:1033-1040.
In a recent systematic review, Fink and colleagues attempted to summarize the published evidence of the efficacy and safety of long-term (> 3 years) therapy for osteoporosis.1 Unfortunately, they arrived at very limited and tentative conclusions because, as they point out, of the paucity of such evidence.
Why long-term studies stop short
Only 3 of the several tens of placebo-controlled fracture end-point studies (about 58 trials and observational studies) that Fink and colleagues reviewed evaluated treatment for more than 3 years. The nonavailability of longer-term studies is the direct consequence of a requirement by regulatory agencies for a 3-year fracture end-point study in order to register a new drug for osteoporosis. Hence, longer, placebo-controlled studies do not benefit the industry sponsor, and enrolling patients with osteoporosis or who are at high risk for fracture in any, much less long, placebo-controlled trials is now considered to be unethical.
What the authors did observe
From this limited set of information with which to evaluate, Fink and colleagues observed that long-term therapy with raloxifene reduces the risk of vertebral fractures but is associated with thromboembolic complications. In addition, treatment for more than 3 years with bisphosphonates reduces the risk of vertebral and nonvertebral fractures but may increase risk of rare adverse events (including femoral shaft fractures with atypical radiographic features).
The bisphosphonate holiday. The authors refer to the even more limited evidence about the effects of discontinuing bisphosphonate therapy. Unlike the rapid loss of bone mass density (BMD) and fracture protection upon stopping estrogen or denosumab, the offset of these treatment benefits is slower when bisphosphonates are discontinued. This, coupled with concern about increasing risk with long-term bisphosphonate therapy, led to the confusing concept of a “bisphosphonate holiday.” While recommendations to consider temporary discontinuation of bisphosphonates in patients at low risk for fracture have been made by expert panels,2 very little information exists about the benefits/risks of this strategy, how long the treatment interruption should be, or how to decide when and with what to restart therapy. Unfortunately, overall, Fink and colleagues’ observations provide little practical guidance for clinicians.
Continue to: What we can learn from longer term and recent studies of ideal treatment...
What we can learn from longer term and recent studies of ideal treatment
Since we have no “cure” for osteoporosis, and since the benefits of therapy, including protection from fractures, abate upon stopping treatment (as they do when we stop treating hypertension or diabetes), very long term if not lifelong management is required for patients with osteoporosis. Persistent or even greater reduction of fracture risk with treatment up to 10 years, compared with the rate of fracture in the placebo or treated group during the first 3 years of the study, has been observed with zoledronate and denosumab.3-5 Denosumab was not included in the systematic review by Fink and colleagues since the pivotal fracture trial with that agent was placebo-controlled for only 3 years.6
Sequential drug treatment may be best. Fink and colleagues also did not consider new evidence, which suggests that the use of osteoporosis drugs in sequence—rather than a single agent for a long time—may be the most effective management strategy.7,8
More consideration should be given to the use of estrogen and raloxifene in younger postmenopausal women at risk for vertebral but not hip fracture.
Only treat high-risk patients. Using osteoporosis therapies to only treat patients at high risk for fracture will optimize the benefit:risk ratio and cost-effectiveness of therapy.
Bisphosphonate holidays may not be as important as once thought. BMD and fracture risk reduction does not improve after 5 years of bisphosphonate therapy, and longer treatment may increase the risk of atypic
Hip BMD may serve as indicator for treatment decisions. Recent evidence indicating that the change in hip BMD with treatment or the level of hip BMD achieved on treatment correlates with fracture risk reduction may provide a useful clinical target to guide treatment decisions.9,10
Because we have a lack of pristine evidence does not mean that we shouldn’t treat osteoporosis; we have to do the best we can with the limited evidence we have. Therapy must be individualized, for we are not just treating osteoporosis, we are treating patients with osteoporosis.
In a recent systematic review, Fink and colleagues attempted to summarize the published evidence of the efficacy and safety of long-term (> 3 years) therapy for osteoporosis.1 Unfortunately, they arrived at very limited and tentative conclusions because, as they point out, of the paucity of such evidence.
Why long-term studies stop short
Only 3 of the several tens of placebo-controlled fracture end-point studies (about 58 trials and observational studies) that Fink and colleagues reviewed evaluated treatment for more than 3 years. The nonavailability of longer-term studies is the direct consequence of a requirement by regulatory agencies for a 3-year fracture end-point study in order to register a new drug for osteoporosis. Hence, longer, placebo-controlled studies do not benefit the industry sponsor, and enrolling patients with osteoporosis or who are at high risk for fracture in any, much less long, placebo-controlled trials is now considered to be unethical.
What the authors did observe
From this limited set of information with which to evaluate, Fink and colleagues observed that long-term therapy with raloxifene reduces the risk of vertebral fractures but is associated with thromboembolic complications. In addition, treatment for more than 3 years with bisphosphonates reduces the risk of vertebral and nonvertebral fractures but may increase risk of rare adverse events (including femoral shaft fractures with atypical radiographic features).
The bisphosphonate holiday. The authors refer to the even more limited evidence about the effects of discontinuing bisphosphonate therapy. Unlike the rapid loss of bone mass density (BMD) and fracture protection upon stopping estrogen or denosumab, the offset of these treatment benefits is slower when bisphosphonates are discontinued. This, coupled with concern about increasing risk with long-term bisphosphonate therapy, led to the confusing concept of a “bisphosphonate holiday.” While recommendations to consider temporary discontinuation of bisphosphonates in patients at low risk for fracture have been made by expert panels,2 very little information exists about the benefits/risks of this strategy, how long the treatment interruption should be, or how to decide when and with what to restart therapy. Unfortunately, overall, Fink and colleagues’ observations provide little practical guidance for clinicians.
Continue to: What we can learn from longer term and recent studies of ideal treatment...
What we can learn from longer term and recent studies of ideal treatment
Since we have no “cure” for osteoporosis, and since the benefits of therapy, including protection from fractures, abate upon stopping treatment (as they do when we stop treating hypertension or diabetes), very long term if not lifelong management is required for patients with osteoporosis. Persistent or even greater reduction of fracture risk with treatment up to 10 years, compared with the rate of fracture in the placebo or treated group during the first 3 years of the study, has been observed with zoledronate and denosumab.3-5 Denosumab was not included in the systematic review by Fink and colleagues since the pivotal fracture trial with that agent was placebo-controlled for only 3 years.6
Sequential drug treatment may be best. Fink and colleagues also did not consider new evidence, which suggests that the use of osteoporosis drugs in sequence—rather than a single agent for a long time—may be the most effective management strategy.7,8
More consideration should be given to the use of estrogen and raloxifene in younger postmenopausal women at risk for vertebral but not hip fracture.
Only treat high-risk patients. Using osteoporosis therapies to only treat patients at high risk for fracture will optimize the benefit:risk ratio and cost-effectiveness of therapy.
Bisphosphonate holidays may not be as important as once thought. BMD and fracture risk reduction does not improve after 5 years of bisphosphonate therapy, and longer treatment may increase the risk of atypic
Hip BMD may serve as indicator for treatment decisions. Recent evidence indicating that the change in hip BMD with treatment or the level of hip BMD achieved on treatment correlates with fracture risk reduction may provide a useful clinical target to guide treatment decisions.9,10
Because we have a lack of pristine evidence does not mean that we shouldn’t treat osteoporosis; we have to do the best we can with the limited evidence we have. Therapy must be individualized, for we are not just treating osteoporosis, we are treating patients with osteoporosis.
- Fink HA, MacDonald R, Forte ML, et al. Long-term drug therapy and drug discontinuations and holidays for osteoporosis fracture prevention: a systematic review. Ann Intern Med. 2019;171:37-50.
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:16-35.
- Black DM, Reid IR, Cauley JA, et al. The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2015;30:934-944.
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513-523.
- Ferrari S, Butler PW, Kendler DL, et al. Further nonvertebral fracture reduction beyond 3 years for up to 10 years of denosumab treatment. J Clin Endocrinol Metab. 2019;104:3450-3461.
- Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Cosman F, Nieves JW, Dempster DW. Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res. 2017;32:198-202.
- Hanley DA, McClung MR, Davison KS, et al; Writing Group for the Western Osteoporosis Alliance. Western Osteoporosis Alliance Clinical Practice Series: evaluating the balance of benefits and risks of long-term osteoporosis therapies. Am J Med. 2017;130:862.e1-862.e7.
- Bouxsein ML, Eastell R, Lui LY, et al; FNIH Bone Quality Project. Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res. 2019;34:632-642.
- Ferrari S, Libanati C, Lin CJF, et al. Relationship between bone mineral density T-score and nonvertebral fracture risk over 10 years of denosumab treatment. J Bone Miner Res. 2019;34:1033-1040.
- Fink HA, MacDonald R, Forte ML, et al. Long-term drug therapy and drug discontinuations and holidays for osteoporosis fracture prevention: a systematic review. Ann Intern Med. 2019;171:37-50.
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:16-35.
- Black DM, Reid IR, Cauley JA, et al. The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2015;30:934-944.
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513-523.
- Ferrari S, Butler PW, Kendler DL, et al. Further nonvertebral fracture reduction beyond 3 years for up to 10 years of denosumab treatment. J Clin Endocrinol Metab. 2019;104:3450-3461.
- Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Cosman F, Nieves JW, Dempster DW. Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res. 2017;32:198-202.
- Hanley DA, McClung MR, Davison KS, et al; Writing Group for the Western Osteoporosis Alliance. Western Osteoporosis Alliance Clinical Practice Series: evaluating the balance of benefits and risks of long-term osteoporosis therapies. Am J Med. 2017;130:862.e1-862.e7.
- Bouxsein ML, Eastell R, Lui LY, et al; FNIH Bone Quality Project. Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res. 2019;34:632-642.
- Ferrari S, Libanati C, Lin CJF, et al. Relationship between bone mineral density T-score and nonvertebral fracture risk over 10 years of denosumab treatment. J Bone Miner Res. 2019;34:1033-1040.
Ovarian tumor markers: What to draw and when
Tumor markers are serum measures that are valuable in the discrimination of an adnexal mass. However, given the long list from which to choose, it can be confusing to know exactly which might best serve your diagnostic needs. I am commonly asked by obstetrician/gynecologists and primary care doctors for guidance on this subject. In this column I will explore some of the decision making that I use when determining which markers might be most helpful for individual patients.
So which tumor markers should you order when you have diagnosed an adnexal mass? Because tumor marker profiles can differ dramatically based on the cell type of the neoplasm, perhaps the first question to ask is what is the most likely category of neoplasm based on other clinical data? Ovarian neoplasms fit into the following subgroups: epithelial (including the most common cell type, serous ovarian cancer, but also the less common mucinous and low malignant potential tumors), sex cord-stromal tumors, germ cell tumors, and metastatic tumors. Table 1 summarizes which tumor markers should be considered based on the clinical setting.
You should suspect an epithelial tumor if there is an adnexal mass with significant cystic components in older, postmenopausal patients, or the presence of peritoneal carcinomatosis on imaging. The tumor markers most commonly elevated in this clinical setting are cancer antigen 125 (CA 125), carcinoembryonic antigen (CEA), and possibly CA 19-9. The CA 125 antigen is a glycoprotein derived from the epithelium of peritoneum, pleura, pericardium, and Müllerian tissues. The multiple sites of origin of this glycoprotein speaks to the poor specificity associated with its elevation, as it is well known to be elevated in both benign conditions such as endometriosis, fibroids, pregnancy, ovulation, cirrhosis, and pericarditis as well as in nongynecologic malignancies, particularly those metastatic to the peritoneal cavity. Multiple different assays are available to measure CA 125, and each is associated with a slightly different reference range. Therefore, if measuring serial values, it is best to have these assessed by the same laboratory. Similarly, as it can be physiologically elevated during the menstrual cycle, premenopausal women should have serial assessments at the same point in their menstrual cycle or ideally within the first 2 weeks of their cycle.
The sensitivity of CA 125 in detecting ovarian cancer is only 78%, which is limited by the fact that not all epithelial ovarian cancer cell types (including some clear cell, carcinosarcoma, and mucinous) express elevations in this tumor marker, and because CA 125 is elevated in less than half of stage I ovarian cancers.1 Therefore, given the lack of sensitivity and specificity for this tumor marker, you should integrate other clinical data, such as imaging findings, age of the patient, and associated benign medical conditions, when evaluating the likelihood of cancer. The American College of Obstetricians and Gynecologists (ACOG) recommends that in the setting of an adnexal mass, referral to gynecologic oncology is recommended when the CA 125 value is greater than 200 U/mL in premenopausal women, or greater than 35U/mL in postmenopausal women.2
CEA is a protein that can be expressed in the colon but not in other normal tissues after birth, and therefore its elevation is commonly associated with metastatic GI tumors to the ovary and peritoneum, or mucinous ovarian tumors, including borderline tumors. Metastatic GI tumors typically are suspected when there are bilateral ovarian solid masses. Right-sided ovarian cysts also can be associated with appendiceal pathology and checking a CEA level can be considered in these cases. I will commonly draw both CA 125 and CEA tumor markers in the setting of cystic +/– solid ovarian masses. This allows the recognition of CA 125-negative/CEA-positive ovarian cancers, such as mucinous tumors, which aids in later surveillance or increases my suspicion for an occult GI tumor (particularly if there is a disproportionately higher elevation in CEA than CA 125).3 If tumor marker profiles are suggestive of an occult GI tumor, I often will consider a preoperative colonoscopy and upper GI endoscopic assessment.
CA 19-9 is a much less specific tumor marker which can be elevated in a variety of solid organ tumors including pancreatic, hepatobiliary, gastric and ovarian tumors. I typically reserve adding this marker for atypical clinical presentations of ovarian cancer, such as carcinomatosis in the absence of pelvic masses.
Ovarian sex cord-stromal neoplasms most commonly present as solid tumors in the ovary. The ovarian stroma includes the bland fibroblasts and the hormone-producing sex-cord granulosa, Sertoli and Leydig cells. Therefore the sex cord-stromal tumors commonly are associated with elevations in serum inhibin, anti-Müllerian hormone, and potentially androstenedione and dehydroepiandrosterone.4 These tumors rarely have advanced disease at diagnosis. Granulosa cell tumors should be suspected in women with a solid ovarian mass and abnormal uterine bleeding (including postmenopausal bleeding), and the appropriate tumor markers (inhibin and anti-Müllerian hormone) can guide this diagnosis preoperatively.4 Androgen-secreting stromal tumors such as Sertoli-Leydig tumors often present with virilization or menstrual irregularities. Interestingly, these patients may have dramatic clinical symptoms with corresponding nonvisible or very small solid adnexal lesions seen on imaging. In the case of fibromas, these solid tumors have normal hormonal tumor markers but may present with ascites and pleural effusions as part of Meigs syndrome, which can confuse the clinician who may suspect advanced-stage epithelial cancer especially as this condition may be associated with elevated CA 125.
Germ cell tumors make up the other main group of primary ovarian tumors, and typically strongly express tumor markers. These tumors typically are solid and highly vascularized on imaging, can be bilateral, and may be very large at the time of diagnosis.5 They most commonly are unilateral and arise among younger women (including usually in the second and third decades of life). Table 1 demonstrates the different tumor markers associated with different germ cell tumors. It is my practice to order a panel of all of these germ cell markers in young women with solid adnexal masses in whom germ cell tumors are suspected, but I will not routinely draw this expansive panel for older women with cystic lesions.
Tumor marker panels (such as OVA 1, Overa, Risk of Malignancy Algorithm or ROMA) have become popular in recent years. These panels include multiple serum markers (such as CA 125, beta-2 microglobulin, human epididymis secretory protein 4, transferrin, etc.) evaluated in concert with the goal being a more nuanced assessment of likelihood for malignancy.6,7 These assays typically are stratified by age or menopausal status given the physiologic differences in normal reference ranges that occur between these groups. While these studies do improve upon the sensitivity and specificity for identifying malignancy, compared with single-assay tests, they are not definitively diagnostic for this purpose. Therefore, I typically recommend these assays if a referring doctor needs additional risk stratification to guide whether or not to refer to an oncologist for surgery.
Not all tumor markers are of equal value in all patients with an adnexal mass. I recommend careful consideration of other clinical factors such as age, menopausal status, ultrasonographic features, and associated findings such as GI symptoms or manifestations of hormonal alterations when considering which markers to assess.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Hum Reprod. 1989 Jan;4(1):1-12.
2. Obstet Gynecol. 2016 Nov;128(5):e210-e26.
3. Dan Med Bull. 2011 Nov;58(11):A4331.
4. Int J Cancer. 2015 Oct 1;137(7):1661-71.
5. Obstet Gynecol. 2000 Jan;95(1):128-33.
6. Obstet Gynecol. 2011 Jun;117(6):1289-97.
7. Obstet Gynecol. 2011 Aug;118(2 Pt 1):280-8.
Tumor markers are serum measures that are valuable in the discrimination of an adnexal mass. However, given the long list from which to choose, it can be confusing to know exactly which might best serve your diagnostic needs. I am commonly asked by obstetrician/gynecologists and primary care doctors for guidance on this subject. In this column I will explore some of the decision making that I use when determining which markers might be most helpful for individual patients.

So which tumor markers should you order when you have diagnosed an adnexal mass? Because tumor marker profiles can differ dramatically based on the cell type of the neoplasm, perhaps the first question to ask is what is the most likely category of neoplasm based on other clinical data? Ovarian neoplasms fit into the following subgroups: epithelial (including the most common cell type, serous ovarian cancer, but also the less common mucinous and low malignant potential tumors), sex cord-stromal tumors, germ cell tumors, and metastatic tumors. Table 1 summarizes which tumor markers should be considered based on the clinical setting.
You should suspect an epithelial tumor if there is an adnexal mass with significant cystic components in older, postmenopausal patients, or the presence of peritoneal carcinomatosis on imaging. The tumor markers most commonly elevated in this clinical setting are cancer antigen 125 (CA 125), carcinoembryonic antigen (CEA), and possibly CA 19-9. The CA 125 antigen is a glycoprotein derived from the epithelium of peritoneum, pleura, pericardium, and Müllerian tissues. The multiple sites of origin of this glycoprotein speaks to the poor specificity associated with its elevation, as it is well known to be elevated in both benign conditions such as endometriosis, fibroids, pregnancy, ovulation, cirrhosis, and pericarditis as well as in nongynecologic malignancies, particularly those metastatic to the peritoneal cavity. Multiple different assays are available to measure CA 125, and each is associated with a slightly different reference range. Therefore, if measuring serial values, it is best to have these assessed by the same laboratory. Similarly, as it can be physiologically elevated during the menstrual cycle, premenopausal women should have serial assessments at the same point in their menstrual cycle or ideally within the first 2 weeks of their cycle.
The sensitivity of CA 125 in detecting ovarian cancer is only 78%, which is limited by the fact that not all epithelial ovarian cancer cell types (including some clear cell, carcinosarcoma, and mucinous) express elevations in this tumor marker, and because CA 125 is elevated in less than half of stage I ovarian cancers.1 Therefore, given the lack of sensitivity and specificity for this tumor marker, you should integrate other clinical data, such as imaging findings, age of the patient, and associated benign medical conditions, when evaluating the likelihood of cancer. The American College of Obstetricians and Gynecologists (ACOG) recommends that in the setting of an adnexal mass, referral to gynecologic oncology is recommended when the CA 125 value is greater than 200 U/mL in premenopausal women, or greater than 35U/mL in postmenopausal women.2
CEA is a protein that can be expressed in the colon but not in other normal tissues after birth, and therefore its elevation is commonly associated with metastatic GI tumors to the ovary and peritoneum, or mucinous ovarian tumors, including borderline tumors. Metastatic GI tumors typically are suspected when there are bilateral ovarian solid masses. Right-sided ovarian cysts also can be associated with appendiceal pathology and checking a CEA level can be considered in these cases. I will commonly draw both CA 125 and CEA tumor markers in the setting of cystic +/– solid ovarian masses. This allows the recognition of CA 125-negative/CEA-positive ovarian cancers, such as mucinous tumors, which aids in later surveillance or increases my suspicion for an occult GI tumor (particularly if there is a disproportionately higher elevation in CEA than CA 125).3 If tumor marker profiles are suggestive of an occult GI tumor, I often will consider a preoperative colonoscopy and upper GI endoscopic assessment.
CA 19-9 is a much less specific tumor marker which can be elevated in a variety of solid organ tumors including pancreatic, hepatobiliary, gastric and ovarian tumors. I typically reserve adding this marker for atypical clinical presentations of ovarian cancer, such as carcinomatosis in the absence of pelvic masses.
Ovarian sex cord-stromal neoplasms most commonly present as solid tumors in the ovary. The ovarian stroma includes the bland fibroblasts and the hormone-producing sex-cord granulosa, Sertoli and Leydig cells. Therefore the sex cord-stromal tumors commonly are associated with elevations in serum inhibin, anti-Müllerian hormone, and potentially androstenedione and dehydroepiandrosterone.4 These tumors rarely have advanced disease at diagnosis. Granulosa cell tumors should be suspected in women with a solid ovarian mass and abnormal uterine bleeding (including postmenopausal bleeding), and the appropriate tumor markers (inhibin and anti-Müllerian hormone) can guide this diagnosis preoperatively.4 Androgen-secreting stromal tumors such as Sertoli-Leydig tumors often present with virilization or menstrual irregularities. Interestingly, these patients may have dramatic clinical symptoms with corresponding nonvisible or very small solid adnexal lesions seen on imaging. In the case of fibromas, these solid tumors have normal hormonal tumor markers but may present with ascites and pleural effusions as part of Meigs syndrome, which can confuse the clinician who may suspect advanced-stage epithelial cancer especially as this condition may be associated with elevated CA 125.
Germ cell tumors make up the other main group of primary ovarian tumors, and typically strongly express tumor markers. These tumors typically are solid and highly vascularized on imaging, can be bilateral, and may be very large at the time of diagnosis.5 They most commonly are unilateral and arise among younger women (including usually in the second and third decades of life). Table 1 demonstrates the different tumor markers associated with different germ cell tumors. It is my practice to order a panel of all of these germ cell markers in young women with solid adnexal masses in whom germ cell tumors are suspected, but I will not routinely draw this expansive panel for older women with cystic lesions.
Tumor marker panels (such as OVA 1, Overa, Risk of Malignancy Algorithm or ROMA) have become popular in recent years. These panels include multiple serum markers (such as CA 125, beta-2 microglobulin, human epididymis secretory protein 4, transferrin, etc.) evaluated in concert with the goal being a more nuanced assessment of likelihood for malignancy.6,7 These assays typically are stratified by age or menopausal status given the physiologic differences in normal reference ranges that occur between these groups. While these studies do improve upon the sensitivity and specificity for identifying malignancy, compared with single-assay tests, they are not definitively diagnostic for this purpose. Therefore, I typically recommend these assays if a referring doctor needs additional risk stratification to guide whether or not to refer to an oncologist for surgery.
Not all tumor markers are of equal value in all patients with an adnexal mass. I recommend careful consideration of other clinical factors such as age, menopausal status, ultrasonographic features, and associated findings such as GI symptoms or manifestations of hormonal alterations when considering which markers to assess.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Hum Reprod. 1989 Jan;4(1):1-12.
2. Obstet Gynecol. 2016 Nov;128(5):e210-e26.
3. Dan Med Bull. 2011 Nov;58(11):A4331.
4. Int J Cancer. 2015 Oct 1;137(7):1661-71.
5. Obstet Gynecol. 2000 Jan;95(1):128-33.
6. Obstet Gynecol. 2011 Jun;117(6):1289-97.
7. Obstet Gynecol. 2011 Aug;118(2 Pt 1):280-8.
Tumor markers are serum measures that are valuable in the discrimination of an adnexal mass. However, given the long list from which to choose, it can be confusing to know exactly which might best serve your diagnostic needs. I am commonly asked by obstetrician/gynecologists and primary care doctors for guidance on this subject. In this column I will explore some of the decision making that I use when determining which markers might be most helpful for individual patients.

So which tumor markers should you order when you have diagnosed an adnexal mass? Because tumor marker profiles can differ dramatically based on the cell type of the neoplasm, perhaps the first question to ask is what is the most likely category of neoplasm based on other clinical data? Ovarian neoplasms fit into the following subgroups: epithelial (including the most common cell type, serous ovarian cancer, but also the less common mucinous and low malignant potential tumors), sex cord-stromal tumors, germ cell tumors, and metastatic tumors. Table 1 summarizes which tumor markers should be considered based on the clinical setting.
You should suspect an epithelial tumor if there is an adnexal mass with significant cystic components in older, postmenopausal patients, or the presence of peritoneal carcinomatosis on imaging. The tumor markers most commonly elevated in this clinical setting are cancer antigen 125 (CA 125), carcinoembryonic antigen (CEA), and possibly CA 19-9. The CA 125 antigen is a glycoprotein derived from the epithelium of peritoneum, pleura, pericardium, and Müllerian tissues. The multiple sites of origin of this glycoprotein speaks to the poor specificity associated with its elevation, as it is well known to be elevated in both benign conditions such as endometriosis, fibroids, pregnancy, ovulation, cirrhosis, and pericarditis as well as in nongynecologic malignancies, particularly those metastatic to the peritoneal cavity. Multiple different assays are available to measure CA 125, and each is associated with a slightly different reference range. Therefore, if measuring serial values, it is best to have these assessed by the same laboratory. Similarly, as it can be physiologically elevated during the menstrual cycle, premenopausal women should have serial assessments at the same point in their menstrual cycle or ideally within the first 2 weeks of their cycle.
The sensitivity of CA 125 in detecting ovarian cancer is only 78%, which is limited by the fact that not all epithelial ovarian cancer cell types (including some clear cell, carcinosarcoma, and mucinous) express elevations in this tumor marker, and because CA 125 is elevated in less than half of stage I ovarian cancers.1 Therefore, given the lack of sensitivity and specificity for this tumor marker, you should integrate other clinical data, such as imaging findings, age of the patient, and associated benign medical conditions, when evaluating the likelihood of cancer. The American College of Obstetricians and Gynecologists (ACOG) recommends that in the setting of an adnexal mass, referral to gynecologic oncology is recommended when the CA 125 value is greater than 200 U/mL in premenopausal women, or greater than 35U/mL in postmenopausal women.2
CEA is a protein that can be expressed in the colon but not in other normal tissues after birth, and therefore its elevation is commonly associated with metastatic GI tumors to the ovary and peritoneum, or mucinous ovarian tumors, including borderline tumors. Metastatic GI tumors typically are suspected when there are bilateral ovarian solid masses. Right-sided ovarian cysts also can be associated with appendiceal pathology and checking a CEA level can be considered in these cases. I will commonly draw both CA 125 and CEA tumor markers in the setting of cystic +/– solid ovarian masses. This allows the recognition of CA 125-negative/CEA-positive ovarian cancers, such as mucinous tumors, which aids in later surveillance or increases my suspicion for an occult GI tumor (particularly if there is a disproportionately higher elevation in CEA than CA 125).3 If tumor marker profiles are suggestive of an occult GI tumor, I often will consider a preoperative colonoscopy and upper GI endoscopic assessment.
CA 19-9 is a much less specific tumor marker which can be elevated in a variety of solid organ tumors including pancreatic, hepatobiliary, gastric and ovarian tumors. I typically reserve adding this marker for atypical clinical presentations of ovarian cancer, such as carcinomatosis in the absence of pelvic masses.
Ovarian sex cord-stromal neoplasms most commonly present as solid tumors in the ovary. The ovarian stroma includes the bland fibroblasts and the hormone-producing sex-cord granulosa, Sertoli and Leydig cells. Therefore the sex cord-stromal tumors commonly are associated with elevations in serum inhibin, anti-Müllerian hormone, and potentially androstenedione and dehydroepiandrosterone.4 These tumors rarely have advanced disease at diagnosis. Granulosa cell tumors should be suspected in women with a solid ovarian mass and abnormal uterine bleeding (including postmenopausal bleeding), and the appropriate tumor markers (inhibin and anti-Müllerian hormone) can guide this diagnosis preoperatively.4 Androgen-secreting stromal tumors such as Sertoli-Leydig tumors often present with virilization or menstrual irregularities. Interestingly, these patients may have dramatic clinical symptoms with corresponding nonvisible or very small solid adnexal lesions seen on imaging. In the case of fibromas, these solid tumors have normal hormonal tumor markers but may present with ascites and pleural effusions as part of Meigs syndrome, which can confuse the clinician who may suspect advanced-stage epithelial cancer especially as this condition may be associated with elevated CA 125.
Germ cell tumors make up the other main group of primary ovarian tumors, and typically strongly express tumor markers. These tumors typically are solid and highly vascularized on imaging, can be bilateral, and may be very large at the time of diagnosis.5 They most commonly are unilateral and arise among younger women (including usually in the second and third decades of life). Table 1 demonstrates the different tumor markers associated with different germ cell tumors. It is my practice to order a panel of all of these germ cell markers in young women with solid adnexal masses in whom germ cell tumors are suspected, but I will not routinely draw this expansive panel for older women with cystic lesions.
Tumor marker panels (such as OVA 1, Overa, Risk of Malignancy Algorithm or ROMA) have become popular in recent years. These panels include multiple serum markers (such as CA 125, beta-2 microglobulin, human epididymis secretory protein 4, transferrin, etc.) evaluated in concert with the goal being a more nuanced assessment of likelihood for malignancy.6,7 These assays typically are stratified by age or menopausal status given the physiologic differences in normal reference ranges that occur between these groups. While these studies do improve upon the sensitivity and specificity for identifying malignancy, compared with single-assay tests, they are not definitively diagnostic for this purpose. Therefore, I typically recommend these assays if a referring doctor needs additional risk stratification to guide whether or not to refer to an oncologist for surgery.
Not all tumor markers are of equal value in all patients with an adnexal mass. I recommend careful consideration of other clinical factors such as age, menopausal status, ultrasonographic features, and associated findings such as GI symptoms or manifestations of hormonal alterations when considering which markers to assess.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Hum Reprod. 1989 Jan;4(1):1-12.
2. Obstet Gynecol. 2016 Nov;128(5):e210-e26.
3. Dan Med Bull. 2011 Nov;58(11):A4331.
4. Int J Cancer. 2015 Oct 1;137(7):1661-71.
5. Obstet Gynecol. 2000 Jan;95(1):128-33.
6. Obstet Gynecol. 2011 Jun;117(6):1289-97.
7. Obstet Gynecol. 2011 Aug;118(2 Pt 1):280-8.
To AROM or not to AROM: Does early amniotomy during induction of labor increase the risk of cesarean delivery?
De Vivo V, Carbone L, Saccone G, et al. Early amniotomy after cervical ripening for induction of labor: a systematic review and meta-analysis of randomized controlled trials. Am J Obstet Gynecol. 2019. doi: 10.1016/j.ajog.2019.07.049.
EXPERT COMMENTARY
Induction of labor has doubled over the past 2 decades, with almost 25% of parturients currently undergoing induction in the United States.1 Labor induction at term is associated with perinatal outcomes similar to those with spontaneous labor, without an increase in the CD rate.1-3 Although numerous methods for cervical ripening have been evaluated, the safest and most effective method has yet to be determined.2
Amniotomy—or artificial rupture of membranes (AROM)—has long been used as a technique for labor induction and for augmentation in women in spontaneous labor. Purported benefits include an increased responsiveness to exogenous oxytocin, decreased interval to delivery, and an increased likelihood of spontaneous vaginal delivery. Risks of amniotomy include injury to the fetus or surrounding tissues, bleeding, nonreassuring fetal testing, cord prolapse, and prolonged rupture of membranes (defined as longer than 18 hours), which is a risk factor for intra-amniotic infection.
The optimal timing of amniotomy is not known. The recent study by De Vivo and colleagues was designed to better understand the risk/benefit ratio of early amniotomy after cervical ripening in women undergoing induction of labor.
Details of the study
The authors conducted a systematic review and meta-analysis that included 1,273 women in 4 randomized controlled trials to determine the effectiveness of routine early amniotomy versus late amniotomy/spontaneous rupture of membranes after cervical ripening (with either a Foley catheter or prostaglandins) in women with a singleton vertex fetus undergoing induction of labor in the term or late preterm period.
Early amniotomy was defined as AROM “soon after cervical ripening” (cases); late amniotomy was defined as AROM after the active phase of labor or spontaneous rupture of membranes (controls).
The primary outcome was the incidence of CD. Secondary outcomes included the overall length of labor, latency from induction to delivery, and neonatal morbidity (a composite of birth weight, Apgar scores, meconium-stained amniotic fluid, neonatal sepsis, need for resuscitation, and admission to the neonatal intensive care unit).
Continue to: Findings...
Findings. Women randomly assigned to early amniotomy had a similar risk of CD compared with controls (31.1% vs 30.9% [relative risk (RR), 1.05; 95% confidence interval (CI), 0.71–1.56]) and a shorter interval from induction to delivery of about 5 hours (mean difference, -4.95 hours [95% CI, -8.12 to -1.78]).
There was no difference in any of the secondary outcome measures, although the number of events was small. Specifically, there was no significant difference in rates of chorioamnionitis between the early and late amniotomy cohorts (7.3% vs 4.8% [RR, 1.47; 95% CI, 0.95–2.28]).
Study strengths and limitations
This is the first systematic review to evaluate early versus late amniotomy after cervical ripening for induction of labor. “Systematic review and meta-analysis” is not synonymous with a review of the literature. It has its own methodology and is regarded as original research. A strength of this study is that it was performed by a highly credible team who followed established Cochrane and PRISMA methodological and reporting guidelines.
Study weaknesses include the fact that the meta-analysis contained a relatively small number of trials and study participants. It was significantly underpowered to address issues related to neonatal outcome. The 4 trials included were highly variable in terms of maternal parity and indications for labor induction and CD. The definition of “early amniotomy” was inconsistent, and the overall rate of CD varied greatly among the studies (7.9%–41.1%). Multiple pregnancies were excluded. Taken together, these findings may have limited generalizability.
This is the first systematic review to evaluate early versus late amniotomy/spontaneous rupture of membranes after cervical ripening for induction of labor. The study results suggest that amniotomy soon after cervical ripening does not change the likelihood of CD, but it does shorten the induction-to-delivery interval by around 5 hours. Prior studies have shown that early amniotomy in women in spontaneous labor decreases time to delivery by an average of 3 hours.4 Now we know that this is true also of early amniotomy following cervical ripening for induction of labor.
A number of questions still remain before early amniotomy is introduced into routine practice: Does group B streptococcus colonization status matter? Does this practice increase the risk of chorioamnionitis? At this time, it seems most prudent to individualize amniotomy timing based on a woman's obstetric history, indication for induction, and response to cervical ripening.
ERROL R. NORWITZ, MD, PHD, MBA, AND DIANA KOLETTIS, MD
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. ACOG practice bulletin no. 107: Induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386-397.
- Saccone G, Berghella V. Induction of labor at full term in uncomplicated singleton gestations: a systematic review and metaanalysis of randomized controlled trials. Am J Obstet Gynecol. 2015;213:629-636.
- Grobman WA, Rice MM, Reddy UM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- Frigoletto FD Jr, Lieberman E, Lang JM, et al. A clinical trial of active management of labor. N Engl J Med. 1995;333:745-750.
De Vivo V, Carbone L, Saccone G, et al. Early amniotomy after cervical ripening for induction of labor: a systematic review and meta-analysis of randomized controlled trials. Am J Obstet Gynecol. 2019. doi: 10.1016/j.ajog.2019.07.049.
EXPERT COMMENTARY
Induction of labor has doubled over the past 2 decades, with almost 25% of parturients currently undergoing induction in the United States.1 Labor induction at term is associated with perinatal outcomes similar to those with spontaneous labor, without an increase in the CD rate.1-3 Although numerous methods for cervical ripening have been evaluated, the safest and most effective method has yet to be determined.2
Amniotomy—or artificial rupture of membranes (AROM)—has long been used as a technique for labor induction and for augmentation in women in spontaneous labor. Purported benefits include an increased responsiveness to exogenous oxytocin, decreased interval to delivery, and an increased likelihood of spontaneous vaginal delivery. Risks of amniotomy include injury to the fetus or surrounding tissues, bleeding, nonreassuring fetal testing, cord prolapse, and prolonged rupture of membranes (defined as longer than 18 hours), which is a risk factor for intra-amniotic infection.
The optimal timing of amniotomy is not known. The recent study by De Vivo and colleagues was designed to better understand the risk/benefit ratio of early amniotomy after cervical ripening in women undergoing induction of labor.
Details of the study
The authors conducted a systematic review and meta-analysis that included 1,273 women in 4 randomized controlled trials to determine the effectiveness of routine early amniotomy versus late amniotomy/spontaneous rupture of membranes after cervical ripening (with either a Foley catheter or prostaglandins) in women with a singleton vertex fetus undergoing induction of labor in the term or late preterm period.
Early amniotomy was defined as AROM “soon after cervical ripening” (cases); late amniotomy was defined as AROM after the active phase of labor or spontaneous rupture of membranes (controls).
The primary outcome was the incidence of CD. Secondary outcomes included the overall length of labor, latency from induction to delivery, and neonatal morbidity (a composite of birth weight, Apgar scores, meconium-stained amniotic fluid, neonatal sepsis, need for resuscitation, and admission to the neonatal intensive care unit).
Continue to: Findings...
Findings. Women randomly assigned to early amniotomy had a similar risk of CD compared with controls (31.1% vs 30.9% [relative risk (RR), 1.05; 95% confidence interval (CI), 0.71–1.56]) and a shorter interval from induction to delivery of about 5 hours (mean difference, -4.95 hours [95% CI, -8.12 to -1.78]).
There was no difference in any of the secondary outcome measures, although the number of events was small. Specifically, there was no significant difference in rates of chorioamnionitis between the early and late amniotomy cohorts (7.3% vs 4.8% [RR, 1.47; 95% CI, 0.95–2.28]).
Study strengths and limitations
This is the first systematic review to evaluate early versus late amniotomy after cervical ripening for induction of labor. “Systematic review and meta-analysis” is not synonymous with a review of the literature. It has its own methodology and is regarded as original research. A strength of this study is that it was performed by a highly credible team who followed established Cochrane and PRISMA methodological and reporting guidelines.
Study weaknesses include the fact that the meta-analysis contained a relatively small number of trials and study participants. It was significantly underpowered to address issues related to neonatal outcome. The 4 trials included were highly variable in terms of maternal parity and indications for labor induction and CD. The definition of “early amniotomy” was inconsistent, and the overall rate of CD varied greatly among the studies (7.9%–41.1%). Multiple pregnancies were excluded. Taken together, these findings may have limited generalizability.
This is the first systematic review to evaluate early versus late amniotomy/spontaneous rupture of membranes after cervical ripening for induction of labor. The study results suggest that amniotomy soon after cervical ripening does not change the likelihood of CD, but it does shorten the induction-to-delivery interval by around 5 hours. Prior studies have shown that early amniotomy in women in spontaneous labor decreases time to delivery by an average of 3 hours.4 Now we know that this is true also of early amniotomy following cervical ripening for induction of labor.
A number of questions still remain before early amniotomy is introduced into routine practice: Does group B streptococcus colonization status matter? Does this practice increase the risk of chorioamnionitis? At this time, it seems most prudent to individualize amniotomy timing based on a woman's obstetric history, indication for induction, and response to cervical ripening.
ERROL R. NORWITZ, MD, PHD, MBA, AND DIANA KOLETTIS, MD
De Vivo V, Carbone L, Saccone G, et al. Early amniotomy after cervical ripening for induction of labor: a systematic review and meta-analysis of randomized controlled trials. Am J Obstet Gynecol. 2019. doi: 10.1016/j.ajog.2019.07.049.
EXPERT COMMENTARY
Induction of labor has doubled over the past 2 decades, with almost 25% of parturients currently undergoing induction in the United States.1 Labor induction at term is associated with perinatal outcomes similar to those with spontaneous labor, without an increase in the CD rate.1-3 Although numerous methods for cervical ripening have been evaluated, the safest and most effective method has yet to be determined.2
Amniotomy—or artificial rupture of membranes (AROM)—has long been used as a technique for labor induction and for augmentation in women in spontaneous labor. Purported benefits include an increased responsiveness to exogenous oxytocin, decreased interval to delivery, and an increased likelihood of spontaneous vaginal delivery. Risks of amniotomy include injury to the fetus or surrounding tissues, bleeding, nonreassuring fetal testing, cord prolapse, and prolonged rupture of membranes (defined as longer than 18 hours), which is a risk factor for intra-amniotic infection.
The optimal timing of amniotomy is not known. The recent study by De Vivo and colleagues was designed to better understand the risk/benefit ratio of early amniotomy after cervical ripening in women undergoing induction of labor.
Details of the study
The authors conducted a systematic review and meta-analysis that included 1,273 women in 4 randomized controlled trials to determine the effectiveness of routine early amniotomy versus late amniotomy/spontaneous rupture of membranes after cervical ripening (with either a Foley catheter or prostaglandins) in women with a singleton vertex fetus undergoing induction of labor in the term or late preterm period.
Early amniotomy was defined as AROM “soon after cervical ripening” (cases); late amniotomy was defined as AROM after the active phase of labor or spontaneous rupture of membranes (controls).
The primary outcome was the incidence of CD. Secondary outcomes included the overall length of labor, latency from induction to delivery, and neonatal morbidity (a composite of birth weight, Apgar scores, meconium-stained amniotic fluid, neonatal sepsis, need for resuscitation, and admission to the neonatal intensive care unit).
Continue to: Findings...
Findings. Women randomly assigned to early amniotomy had a similar risk of CD compared with controls (31.1% vs 30.9% [relative risk (RR), 1.05; 95% confidence interval (CI), 0.71–1.56]) and a shorter interval from induction to delivery of about 5 hours (mean difference, -4.95 hours [95% CI, -8.12 to -1.78]).
There was no difference in any of the secondary outcome measures, although the number of events was small. Specifically, there was no significant difference in rates of chorioamnionitis between the early and late amniotomy cohorts (7.3% vs 4.8% [RR, 1.47; 95% CI, 0.95–2.28]).
Study strengths and limitations
This is the first systematic review to evaluate early versus late amniotomy after cervical ripening for induction of labor. “Systematic review and meta-analysis” is not synonymous with a review of the literature. It has its own methodology and is regarded as original research. A strength of this study is that it was performed by a highly credible team who followed established Cochrane and PRISMA methodological and reporting guidelines.
Study weaknesses include the fact that the meta-analysis contained a relatively small number of trials and study participants. It was significantly underpowered to address issues related to neonatal outcome. The 4 trials included were highly variable in terms of maternal parity and indications for labor induction and CD. The definition of “early amniotomy” was inconsistent, and the overall rate of CD varied greatly among the studies (7.9%–41.1%). Multiple pregnancies were excluded. Taken together, these findings may have limited generalizability.
This is the first systematic review to evaluate early versus late amniotomy/spontaneous rupture of membranes after cervical ripening for induction of labor. The study results suggest that amniotomy soon after cervical ripening does not change the likelihood of CD, but it does shorten the induction-to-delivery interval by around 5 hours. Prior studies have shown that early amniotomy in women in spontaneous labor decreases time to delivery by an average of 3 hours.4 Now we know that this is true also of early amniotomy following cervical ripening for induction of labor.
A number of questions still remain before early amniotomy is introduced into routine practice: Does group B streptococcus colonization status matter? Does this practice increase the risk of chorioamnionitis? At this time, it seems most prudent to individualize amniotomy timing based on a woman's obstetric history, indication for induction, and response to cervical ripening.
ERROL R. NORWITZ, MD, PHD, MBA, AND DIANA KOLETTIS, MD
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. ACOG practice bulletin no. 107: Induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386-397.
- Saccone G, Berghella V. Induction of labor at full term in uncomplicated singleton gestations: a systematic review and metaanalysis of randomized controlled trials. Am J Obstet Gynecol. 2015;213:629-636.
- Grobman WA, Rice MM, Reddy UM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- Frigoletto FD Jr, Lieberman E, Lang JM, et al. A clinical trial of active management of labor. N Engl J Med. 1995;333:745-750.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. ACOG practice bulletin no. 107: Induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386-397.
- Saccone G, Berghella V. Induction of labor at full term in uncomplicated singleton gestations: a systematic review and metaanalysis of randomized controlled trials. Am J Obstet Gynecol. 2015;213:629-636.
- Grobman WA, Rice MM, Reddy UM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- Frigoletto FD Jr, Lieberman E, Lang JM, et al. A clinical trial of active management of labor. N Engl J Med. 1995;333:745-750.
Flying toward equity and inclusion
Diversity is a ‘team sport’
These are challenging, and sometimes tragic, times in the history of the United States. The image of a father and child face down in the Rio Grande River, drowning as they tried to cross from Mexico into Texas, is heart breaking. Irrespective of your political affiliation, we can agree that the immigration process is far from ideal and that no one should die in pursuit of a better life.
The United States has a complicated history with equity and inclusion, for all persons, and we are now living in times when the scab is being ripped off and these wounds are raw. What role can the Society of Hospital Medicine play to help heal these wounds?
I am a first-generation immigrant to the United States. I remember walking down the streets of my neighborhood in Uganda when my attention was drawn to a plane flying overhead. I thought to myself, “Some lucky duck is going to the U.S.” The United States was the land of opportunity and I was determined to come here. Through hard work and some luck, I arrived in the United States on June 15, 1991, with a single suitcase packed full of hope, dreams, and $3,000.
Fast-forward 28 years. I am now a hospitalist and faculty at the Johns Hopkins University, Baltimore, the associate director of the division of hospital medicine, and the vice chair for clinical operations at Johns Hopkins Bayview Medical Center. I learned about hospital medicine during my third year of medical school at the University of Minnesota, Minneapolis. While I loved general medicine, I could not see myself practicing anywhere outside of the hospital.
Following residency at Johns Hopkins Bayview, I still felt that a hospital-based practice was tailor-made for me. As I matured professionally, I worked to improve the provision of care within my hospital, and then started developing educational and practice programs in hospital medicine, both locally and internationally. My passion for hospital medicine led me to serve on committees for SHM, and this year, I was honored to join the SHM Board of Directors.
It is hard to answer the question of why, or how, one person immigrates to the United States and finds success while another loses their life. A quote attributed to Edmund Burke says, “the only thing necessary for the triumph of evil is for good [wo]men to do nothing.” One of SHM’s core values is to promote diversity and inclusion. A major step taken by the society to promote work in this area was to establish the diversity and inclusion Special Interest Group in 2018. I am the board liaison for the diversity and inclusion SIG and will work alongside this group, which aims to:
- Foster diversity, equity, and inclusion in SHM.
- Increase visibility of diversity, equity, and inclusion to the broader hospital medicine community.
- Support hospital medicine groups in matching their work forces to their diverse patient populations.
- Develop tool kits to improve the provision of care for our diverse patient population.
- Engender diversity among hospitalists.
- Develop opportunities for expanding the fund of knowledge on diversity in hospital medicine through research and discovery.
- Participate in SHM’s advocacy efforts related to diversity and inclusion.
- Develop partnerships with other key organizations to advance diversity, equity, and inclusion platforms so as to increase the scalability of SHM’s efforts.
We have been successful at Hopkins with diversity and inclusion, but that did not occur by chance. I believe that diversity and inclusion is a team sport and that everyone can be an important part of that team. In my hospitalist group, we actively engage women, men, doctors, NPs, PAs, administrators, minorities, and nonminorities. We recruit to – and cherish members of – our group irrespective of religious beliefs or sexual orientation. We believe that a heterogeneous group of people leads to an engaged and high-performing culture.
I have traveled a convoluted path since my arrival in 1991. Along the way, I was blessed with a husband and son who anchor me. Every day they remind me that the hard work I do is to build on the past to improve the future. My husband, an immigrant from Uganda like me, reminds me that we are lucky to have made it to the United States and that the ability and freedom to work hard and be rewarded for that hard work is a great privilege. My son reminds me of the many other children who look at me and know that they too can dare to dream. Occasionally, I still look up and see a plane, and I am reminded of that day many years ago. Hospital medicine is my suitcase packed with hopes and dreams for me, for this specialty, and for this country.
Dr. Kisuule is associate director of the division of hospital medicine at Johns Hopkins Bayview and assistant professor at Johns Hopkins University, both in Baltimore, and a member of the SHM Board of Directors.
Diversity is a ‘team sport’
Diversity is a ‘team sport’
These are challenging, and sometimes tragic, times in the history of the United States. The image of a father and child face down in the Rio Grande River, drowning as they tried to cross from Mexico into Texas, is heart breaking. Irrespective of your political affiliation, we can agree that the immigration process is far from ideal and that no one should die in pursuit of a better life.
The United States has a complicated history with equity and inclusion, for all persons, and we are now living in times when the scab is being ripped off and these wounds are raw. What role can the Society of Hospital Medicine play to help heal these wounds?
I am a first-generation immigrant to the United States. I remember walking down the streets of my neighborhood in Uganda when my attention was drawn to a plane flying overhead. I thought to myself, “Some lucky duck is going to the U.S.” The United States was the land of opportunity and I was determined to come here. Through hard work and some luck, I arrived in the United States on June 15, 1991, with a single suitcase packed full of hope, dreams, and $3,000.
Fast-forward 28 years. I am now a hospitalist and faculty at the Johns Hopkins University, Baltimore, the associate director of the division of hospital medicine, and the vice chair for clinical operations at Johns Hopkins Bayview Medical Center. I learned about hospital medicine during my third year of medical school at the University of Minnesota, Minneapolis. While I loved general medicine, I could not see myself practicing anywhere outside of the hospital.
Following residency at Johns Hopkins Bayview, I still felt that a hospital-based practice was tailor-made for me. As I matured professionally, I worked to improve the provision of care within my hospital, and then started developing educational and practice programs in hospital medicine, both locally and internationally. My passion for hospital medicine led me to serve on committees for SHM, and this year, I was honored to join the SHM Board of Directors.
It is hard to answer the question of why, or how, one person immigrates to the United States and finds success while another loses their life. A quote attributed to Edmund Burke says, “the only thing necessary for the triumph of evil is for good [wo]men to do nothing.” One of SHM’s core values is to promote diversity and inclusion. A major step taken by the society to promote work in this area was to establish the diversity and inclusion Special Interest Group in 2018. I am the board liaison for the diversity and inclusion SIG and will work alongside this group, which aims to:
- Foster diversity, equity, and inclusion in SHM.
- Increase visibility of diversity, equity, and inclusion to the broader hospital medicine community.
- Support hospital medicine groups in matching their work forces to their diverse patient populations.
- Develop tool kits to improve the provision of care for our diverse patient population.
- Engender diversity among hospitalists.
- Develop opportunities for expanding the fund of knowledge on diversity in hospital medicine through research and discovery.
- Participate in SHM’s advocacy efforts related to diversity and inclusion.
- Develop partnerships with other key organizations to advance diversity, equity, and inclusion platforms so as to increase the scalability of SHM’s efforts.
We have been successful at Hopkins with diversity and inclusion, but that did not occur by chance. I believe that diversity and inclusion is a team sport and that everyone can be an important part of that team. In my hospitalist group, we actively engage women, men, doctors, NPs, PAs, administrators, minorities, and nonminorities. We recruit to – and cherish members of – our group irrespective of religious beliefs or sexual orientation. We believe that a heterogeneous group of people leads to an engaged and high-performing culture.
I have traveled a convoluted path since my arrival in 1991. Along the way, I was blessed with a husband and son who anchor me. Every day they remind me that the hard work I do is to build on the past to improve the future. My husband, an immigrant from Uganda like me, reminds me that we are lucky to have made it to the United States and that the ability and freedom to work hard and be rewarded for that hard work is a great privilege. My son reminds me of the many other children who look at me and know that they too can dare to dream. Occasionally, I still look up and see a plane, and I am reminded of that day many years ago. Hospital medicine is my suitcase packed with hopes and dreams for me, for this specialty, and for this country.
Dr. Kisuule is associate director of the division of hospital medicine at Johns Hopkins Bayview and assistant professor at Johns Hopkins University, both in Baltimore, and a member of the SHM Board of Directors.
These are challenging, and sometimes tragic, times in the history of the United States. The image of a father and child face down in the Rio Grande River, drowning as they tried to cross from Mexico into Texas, is heart breaking. Irrespective of your political affiliation, we can agree that the immigration process is far from ideal and that no one should die in pursuit of a better life.
The United States has a complicated history with equity and inclusion, for all persons, and we are now living in times when the scab is being ripped off and these wounds are raw. What role can the Society of Hospital Medicine play to help heal these wounds?
I am a first-generation immigrant to the United States. I remember walking down the streets of my neighborhood in Uganda when my attention was drawn to a plane flying overhead. I thought to myself, “Some lucky duck is going to the U.S.” The United States was the land of opportunity and I was determined to come here. Through hard work and some luck, I arrived in the United States on June 15, 1991, with a single suitcase packed full of hope, dreams, and $3,000.
Fast-forward 28 years. I am now a hospitalist and faculty at the Johns Hopkins University, Baltimore, the associate director of the division of hospital medicine, and the vice chair for clinical operations at Johns Hopkins Bayview Medical Center. I learned about hospital medicine during my third year of medical school at the University of Minnesota, Minneapolis. While I loved general medicine, I could not see myself practicing anywhere outside of the hospital.
Following residency at Johns Hopkins Bayview, I still felt that a hospital-based practice was tailor-made for me. As I matured professionally, I worked to improve the provision of care within my hospital, and then started developing educational and practice programs in hospital medicine, both locally and internationally. My passion for hospital medicine led me to serve on committees for SHM, and this year, I was honored to join the SHM Board of Directors.
It is hard to answer the question of why, or how, one person immigrates to the United States and finds success while another loses their life. A quote attributed to Edmund Burke says, “the only thing necessary for the triumph of evil is for good [wo]men to do nothing.” One of SHM’s core values is to promote diversity and inclusion. A major step taken by the society to promote work in this area was to establish the diversity and inclusion Special Interest Group in 2018. I am the board liaison for the diversity and inclusion SIG and will work alongside this group, which aims to:
- Foster diversity, equity, and inclusion in SHM.
- Increase visibility of diversity, equity, and inclusion to the broader hospital medicine community.
- Support hospital medicine groups in matching their work forces to their diverse patient populations.
- Develop tool kits to improve the provision of care for our diverse patient population.
- Engender diversity among hospitalists.
- Develop opportunities for expanding the fund of knowledge on diversity in hospital medicine through research and discovery.
- Participate in SHM’s advocacy efforts related to diversity and inclusion.
- Develop partnerships with other key organizations to advance diversity, equity, and inclusion platforms so as to increase the scalability of SHM’s efforts.
We have been successful at Hopkins with diversity and inclusion, but that did not occur by chance. I believe that diversity and inclusion is a team sport and that everyone can be an important part of that team. In my hospitalist group, we actively engage women, men, doctors, NPs, PAs, administrators, minorities, and nonminorities. We recruit to – and cherish members of – our group irrespective of religious beliefs or sexual orientation. We believe that a heterogeneous group of people leads to an engaged and high-performing culture.
I have traveled a convoluted path since my arrival in 1991. Along the way, I was blessed with a husband and son who anchor me. Every day they remind me that the hard work I do is to build on the past to improve the future. My husband, an immigrant from Uganda like me, reminds me that we are lucky to have made it to the United States and that the ability and freedom to work hard and be rewarded for that hard work is a great privilege. My son reminds me of the many other children who look at me and know that they too can dare to dream. Occasionally, I still look up and see a plane, and I am reminded of that day many years ago. Hospital medicine is my suitcase packed with hopes and dreams for me, for this specialty, and for this country.
Dr. Kisuule is associate director of the division of hospital medicine at Johns Hopkins Bayview and assistant professor at Johns Hopkins University, both in Baltimore, and a member of the SHM Board of Directors.