User login
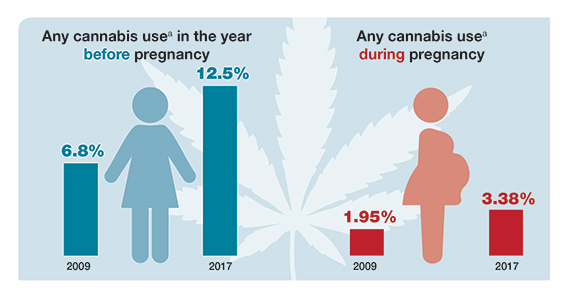
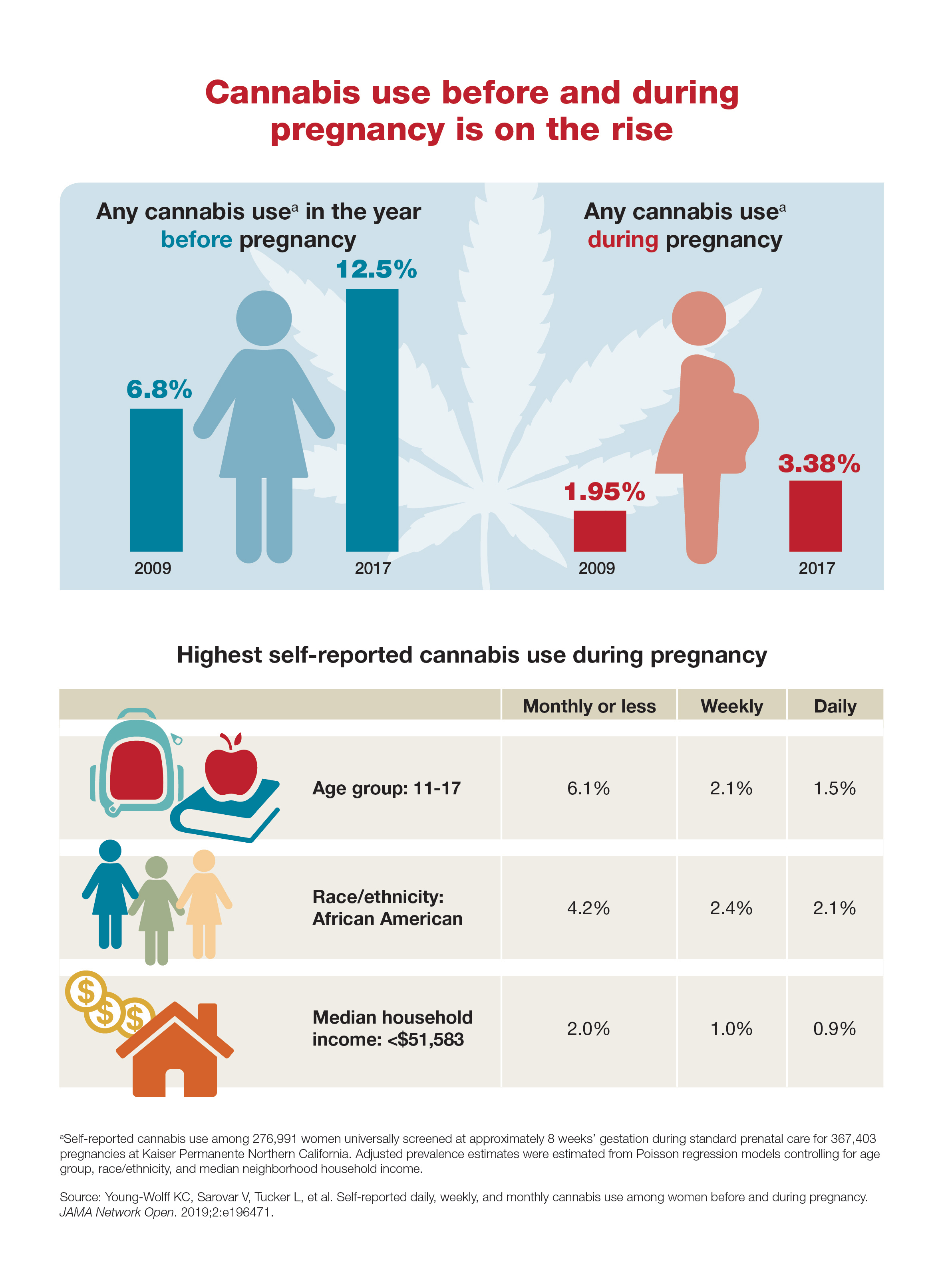
Cannabis use before and during pregnancy is on the rise


Measles cases continue to decline
according to the Centers for Disease Control and Prevention.

A total of 24 measles cases were confirmed in August, and the total for the year is now 1,241 cases in 31 states. Only seven of those cases were added during the most recent reporting week, which ended Sept. 5, but five were older cases that had just been reported, the CDC said Sept. 9.
With the ending of the measles outbreak in New York, announced Sept. 3, the largest of the three remaining active outbreaks in the country is in Rockland County, N.Y., just north of the city, which has reported 312 cases since it began in 2018.
The two other outbreaks are located in El Paso, Tex., where six cases have been reported so far, and Wyoming County in western New York State, where five cases have occurred.
according to the Centers for Disease Control and Prevention.

A total of 24 measles cases were confirmed in August, and the total for the year is now 1,241 cases in 31 states. Only seven of those cases were added during the most recent reporting week, which ended Sept. 5, but five were older cases that had just been reported, the CDC said Sept. 9.
With the ending of the measles outbreak in New York, announced Sept. 3, the largest of the three remaining active outbreaks in the country is in Rockland County, N.Y., just north of the city, which has reported 312 cases since it began in 2018.
The two other outbreaks are located in El Paso, Tex., where six cases have been reported so far, and Wyoming County in western New York State, where five cases have occurred.
according to the Centers for Disease Control and Prevention.

A total of 24 measles cases were confirmed in August, and the total for the year is now 1,241 cases in 31 states. Only seven of those cases were added during the most recent reporting week, which ended Sept. 5, but five were older cases that had just been reported, the CDC said Sept. 9.
With the ending of the measles outbreak in New York, announced Sept. 3, the largest of the three remaining active outbreaks in the country is in Rockland County, N.Y., just north of the city, which has reported 312 cases since it began in 2018.
The two other outbreaks are located in El Paso, Tex., where six cases have been reported so far, and Wyoming County in western New York State, where five cases have occurred.
Coming acne drugs might particularly benefit skin of color patients
NEW YORK – The most recently approved therapy for acne, sarecycline, as well as several agents in late stages of clinical testing, might represent a particular advance for treating black patients or others with darker skin tones due to a reduced risk of irritation, according to a review presented at the Skin of Color Update 2019.
a complication many consider worse than the acne itself, according to Andrew Alexis, MD, director of the Skin of Color Center and chair of the department of dermatology at Mount Sinai St. Luke’s, New York.
“The importance of PIH is that it alters our endpoint in patients of color. Not only are we treating the pustules, comedones, and other classic features of acne, but we have to treat all the way through to the resolution of the PIH if we want a satisfied patient,” he said.
There are data to back this up. In one of the surveys cited by Dr. Alexis, 42% of nonwhite patients identified resolution of PIH as the most important goal in the treatment of their acne.
As in those with light skin, acute acne lesions in darker skin can resolve relatively rapidly after initiating an effective regimen that includes established therapies such as retinoids or antibiotics. However, PIH, once it develops, might take 6-12 months to resolve, according to Dr. Alexis, who is a professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
“You have to keep in mind the subclinical inflammation, which can be a slow burning process beneath the surface of the skin,” he said. He cited a biopsy study that demonstrated inflammation even in nonlesional skin of black patients with acne.
Because of the slow reversal of PIH, it is imperative in skin of color patients to employ therapies with the least risk of exacerbating PIH. While this includes judicious use of currently available agents, Dr. Alexis believes that newer agents might have a larger therapeutic window, reducing the potential for inflammation at effective doses.
This advantage has yet to be confirmed in head-to-head studies, but Dr. Alexis is optimistic. In the case of sarecycline, which became the first antibiotic approved specifically for acne when it was approved by the Food and Drug Administration in 2018, about 20% of those included in the phase 3 registration trial were nonwhite, he said.
The results were “impressive” regardless of skin color in the phase 3 study, according to Dr. Alexis. He conceded that this is not the only antibiotic with anti-inflammatory activity, but he suggested that a high degree of efficacy might be relevant for early acne control and a reduced risk of PIH.
The same can be said for trifarotene, a novel topical retinoid that was associated with highly significant reductions in both inflammatory and noninflammatory lesion counts in a recently published phase 3 trial (J Am Acad Dermatol. 2019 Jun;80[6]:1691-9). According to Dr. Alexis, the impact of this therapy on PIH has not been specifically tested, but he expects those data to be forthcoming.
A new 0.045% lotion formulation of tazarotene might also widen the therapeutic window relative to current tazarotene formulations based on clinical trials he cited. Despite a concentration that is about half that of the currently available tazarotene cream, the efficacy of this product appeared to be at least as good “without the baggage of a greater potential for irritation,” he said.
After “a few years of drought” regarding new options for treatment of acne, these are not the only promising agents in clinical trials, according to Dr. Alexis. If these agents prove to offer greater efficacy with less irritation, their increased clinical value might prove most meaningful to patients with darker skin.
“There is a delicate balance between maximizing efficacy without causing irritation that leads to PIH in patients with skin of color,” he cautioned. He is hopeful that the newer agents will make this balance easier to achieve.
Dr. Alexis has financial relationships with many pharmaceutical companies, including many that market drugs for acne.
NEW YORK – The most recently approved therapy for acne, sarecycline, as well as several agents in late stages of clinical testing, might represent a particular advance for treating black patients or others with darker skin tones due to a reduced risk of irritation, according to a review presented at the Skin of Color Update 2019.
a complication many consider worse than the acne itself, according to Andrew Alexis, MD, director of the Skin of Color Center and chair of the department of dermatology at Mount Sinai St. Luke’s, New York.
“The importance of PIH is that it alters our endpoint in patients of color. Not only are we treating the pustules, comedones, and other classic features of acne, but we have to treat all the way through to the resolution of the PIH if we want a satisfied patient,” he said.
There are data to back this up. In one of the surveys cited by Dr. Alexis, 42% of nonwhite patients identified resolution of PIH as the most important goal in the treatment of their acne.
As in those with light skin, acute acne lesions in darker skin can resolve relatively rapidly after initiating an effective regimen that includes established therapies such as retinoids or antibiotics. However, PIH, once it develops, might take 6-12 months to resolve, according to Dr. Alexis, who is a professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
“You have to keep in mind the subclinical inflammation, which can be a slow burning process beneath the surface of the skin,” he said. He cited a biopsy study that demonstrated inflammation even in nonlesional skin of black patients with acne.
Because of the slow reversal of PIH, it is imperative in skin of color patients to employ therapies with the least risk of exacerbating PIH. While this includes judicious use of currently available agents, Dr. Alexis believes that newer agents might have a larger therapeutic window, reducing the potential for inflammation at effective doses.
This advantage has yet to be confirmed in head-to-head studies, but Dr. Alexis is optimistic. In the case of sarecycline, which became the first antibiotic approved specifically for acne when it was approved by the Food and Drug Administration in 2018, about 20% of those included in the phase 3 registration trial were nonwhite, he said.
The results were “impressive” regardless of skin color in the phase 3 study, according to Dr. Alexis. He conceded that this is not the only antibiotic with anti-inflammatory activity, but he suggested that a high degree of efficacy might be relevant for early acne control and a reduced risk of PIH.
The same can be said for trifarotene, a novel topical retinoid that was associated with highly significant reductions in both inflammatory and noninflammatory lesion counts in a recently published phase 3 trial (J Am Acad Dermatol. 2019 Jun;80[6]:1691-9). According to Dr. Alexis, the impact of this therapy on PIH has not been specifically tested, but he expects those data to be forthcoming.
A new 0.045% lotion formulation of tazarotene might also widen the therapeutic window relative to current tazarotene formulations based on clinical trials he cited. Despite a concentration that is about half that of the currently available tazarotene cream, the efficacy of this product appeared to be at least as good “without the baggage of a greater potential for irritation,” he said.
After “a few years of drought” regarding new options for treatment of acne, these are not the only promising agents in clinical trials, according to Dr. Alexis. If these agents prove to offer greater efficacy with less irritation, their increased clinical value might prove most meaningful to patients with darker skin.
“There is a delicate balance between maximizing efficacy without causing irritation that leads to PIH in patients with skin of color,” he cautioned. He is hopeful that the newer agents will make this balance easier to achieve.
Dr. Alexis has financial relationships with many pharmaceutical companies, including many that market drugs for acne.
NEW YORK – The most recently approved therapy for acne, sarecycline, as well as several agents in late stages of clinical testing, might represent a particular advance for treating black patients or others with darker skin tones due to a reduced risk of irritation, according to a review presented at the Skin of Color Update 2019.
a complication many consider worse than the acne itself, according to Andrew Alexis, MD, director of the Skin of Color Center and chair of the department of dermatology at Mount Sinai St. Luke’s, New York.
“The importance of PIH is that it alters our endpoint in patients of color. Not only are we treating the pustules, comedones, and other classic features of acne, but we have to treat all the way through to the resolution of the PIH if we want a satisfied patient,” he said.
There are data to back this up. In one of the surveys cited by Dr. Alexis, 42% of nonwhite patients identified resolution of PIH as the most important goal in the treatment of their acne.
As in those with light skin, acute acne lesions in darker skin can resolve relatively rapidly after initiating an effective regimen that includes established therapies such as retinoids or antibiotics. However, PIH, once it develops, might take 6-12 months to resolve, according to Dr. Alexis, who is a professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
“You have to keep in mind the subclinical inflammation, which can be a slow burning process beneath the surface of the skin,” he said. He cited a biopsy study that demonstrated inflammation even in nonlesional skin of black patients with acne.
Because of the slow reversal of PIH, it is imperative in skin of color patients to employ therapies with the least risk of exacerbating PIH. While this includes judicious use of currently available agents, Dr. Alexis believes that newer agents might have a larger therapeutic window, reducing the potential for inflammation at effective doses.
This advantage has yet to be confirmed in head-to-head studies, but Dr. Alexis is optimistic. In the case of sarecycline, which became the first antibiotic approved specifically for acne when it was approved by the Food and Drug Administration in 2018, about 20% of those included in the phase 3 registration trial were nonwhite, he said.
The results were “impressive” regardless of skin color in the phase 3 study, according to Dr. Alexis. He conceded that this is not the only antibiotic with anti-inflammatory activity, but he suggested that a high degree of efficacy might be relevant for early acne control and a reduced risk of PIH.
The same can be said for trifarotene, a novel topical retinoid that was associated with highly significant reductions in both inflammatory and noninflammatory lesion counts in a recently published phase 3 trial (J Am Acad Dermatol. 2019 Jun;80[6]:1691-9). According to Dr. Alexis, the impact of this therapy on PIH has not been specifically tested, but he expects those data to be forthcoming.
A new 0.045% lotion formulation of tazarotene might also widen the therapeutic window relative to current tazarotene formulations based on clinical trials he cited. Despite a concentration that is about half that of the currently available tazarotene cream, the efficacy of this product appeared to be at least as good “without the baggage of a greater potential for irritation,” he said.
After “a few years of drought” regarding new options for treatment of acne, these are not the only promising agents in clinical trials, according to Dr. Alexis. If these agents prove to offer greater efficacy with less irritation, their increased clinical value might prove most meaningful to patients with darker skin.
“There is a delicate balance between maximizing efficacy without causing irritation that leads to PIH in patients with skin of color,” he cautioned. He is hopeful that the newer agents will make this balance easier to achieve.
Dr. Alexis has financial relationships with many pharmaceutical companies, including many that market drugs for acne.
EXPERT ANALYSIS FROM SOC 2019
FDA issues warning to JUUL on illegal marketing of e-cigarettes
, citing violation of the Federal Food, Drug, and Cosmetic Act.
According to the letter, JUUL has marketed its e-cigarettes and e-liquids as modified-risk tobacco products without receiving FDA authorization to do so. JUUL’s labeling, advertising, and other consumer-oriented activities to this effect could reasonably lead consumers to believe that JUUL products represent a lower risk of tobacco-related disease, compared with other tobacco products; that they contain a reduced level of a substance; and that they are free of a particular substance or substances.
As evidence, the letter cited testimony given at a July 2019 hearing held by the Subcommittee on Economic and Consumer Policy of the Committee on Oversight and Reform of the House of Representatives, in which a representative from JUUL, speaking to students at a school presentation, said that JUUL products were “much safer than cigarettes” and that the “FDA would approve it any day,” that JUUL products were “totally safe,” that a student “should mention JUUL to his [nicotine-addicted] friend ... because that’s a safer alternative than smoking cigarettes, and it would be better for the kid to use,” and that the FDA “was about to come out and say it [JUUL] was 99% safer than cigarettes ... and that ... would happen very soon.”
In addition, a “Letter from the CEO” that appeared on the JUUL website and was emailed to a parent in response to her complaint that the company sold JUUL products to her child stated that “[JUUL’s] simple and convenient system incorporates temperature regulation to heat nicotine liquid and deliver smokers the satisfaction that they want without the combustion and the harm associated with it.”
In a related press release, acting FDA Commissioner Ned Sharpless, MD, said that “regardless of where products like e-cigarettes fall on the continuum of tobacco product risk, the law is clear that, before marketing tobacco products for reduced risk, companies must demonstrate with scientific evidence that their specific product does in fact pose less risk or is less harmful. JUUL has ignored the law, and very concerningly, has made some of these statements in school to our nation’s youth.”
The FDA has requested a response from JUUL within 15 working days of the letter’s issue. Failure to comply with the Federal Food, Drug, and Cosmetic Act could result in the FDA’s initiating further actions such as civil money penalties, seizure, and/or injunction.
, citing violation of the Federal Food, Drug, and Cosmetic Act.
According to the letter, JUUL has marketed its e-cigarettes and e-liquids as modified-risk tobacco products without receiving FDA authorization to do so. JUUL’s labeling, advertising, and other consumer-oriented activities to this effect could reasonably lead consumers to believe that JUUL products represent a lower risk of tobacco-related disease, compared with other tobacco products; that they contain a reduced level of a substance; and that they are free of a particular substance or substances.
As evidence, the letter cited testimony given at a July 2019 hearing held by the Subcommittee on Economic and Consumer Policy of the Committee on Oversight and Reform of the House of Representatives, in which a representative from JUUL, speaking to students at a school presentation, said that JUUL products were “much safer than cigarettes” and that the “FDA would approve it any day,” that JUUL products were “totally safe,” that a student “should mention JUUL to his [nicotine-addicted] friend ... because that’s a safer alternative than smoking cigarettes, and it would be better for the kid to use,” and that the FDA “was about to come out and say it [JUUL] was 99% safer than cigarettes ... and that ... would happen very soon.”
In addition, a “Letter from the CEO” that appeared on the JUUL website and was emailed to a parent in response to her complaint that the company sold JUUL products to her child stated that “[JUUL’s] simple and convenient system incorporates temperature regulation to heat nicotine liquid and deliver smokers the satisfaction that they want without the combustion and the harm associated with it.”
In a related press release, acting FDA Commissioner Ned Sharpless, MD, said that “regardless of where products like e-cigarettes fall on the continuum of tobacco product risk, the law is clear that, before marketing tobacco products for reduced risk, companies must demonstrate with scientific evidence that their specific product does in fact pose less risk or is less harmful. JUUL has ignored the law, and very concerningly, has made some of these statements in school to our nation’s youth.”
The FDA has requested a response from JUUL within 15 working days of the letter’s issue. Failure to comply with the Federal Food, Drug, and Cosmetic Act could result in the FDA’s initiating further actions such as civil money penalties, seizure, and/or injunction.
, citing violation of the Federal Food, Drug, and Cosmetic Act.
According to the letter, JUUL has marketed its e-cigarettes and e-liquids as modified-risk tobacco products without receiving FDA authorization to do so. JUUL’s labeling, advertising, and other consumer-oriented activities to this effect could reasonably lead consumers to believe that JUUL products represent a lower risk of tobacco-related disease, compared with other tobacco products; that they contain a reduced level of a substance; and that they are free of a particular substance or substances.
As evidence, the letter cited testimony given at a July 2019 hearing held by the Subcommittee on Economic and Consumer Policy of the Committee on Oversight and Reform of the House of Representatives, in which a representative from JUUL, speaking to students at a school presentation, said that JUUL products were “much safer than cigarettes” and that the “FDA would approve it any day,” that JUUL products were “totally safe,” that a student “should mention JUUL to his [nicotine-addicted] friend ... because that’s a safer alternative than smoking cigarettes, and it would be better for the kid to use,” and that the FDA “was about to come out and say it [JUUL] was 99% safer than cigarettes ... and that ... would happen very soon.”
In addition, a “Letter from the CEO” that appeared on the JUUL website and was emailed to a parent in response to her complaint that the company sold JUUL products to her child stated that “[JUUL’s] simple and convenient system incorporates temperature regulation to heat nicotine liquid and deliver smokers the satisfaction that they want without the combustion and the harm associated with it.”
In a related press release, acting FDA Commissioner Ned Sharpless, MD, said that “regardless of where products like e-cigarettes fall on the continuum of tobacco product risk, the law is clear that, before marketing tobacco products for reduced risk, companies must demonstrate with scientific evidence that their specific product does in fact pose less risk or is less harmful. JUUL has ignored the law, and very concerningly, has made some of these statements in school to our nation’s youth.”
The FDA has requested a response from JUUL within 15 working days of the letter’s issue. Failure to comply with the Federal Food, Drug, and Cosmetic Act could result in the FDA’s initiating further actions such as civil money penalties, seizure, and/or injunction.
Native tissue repair of POP: Apical suspension, anterior repair, and posterior repair



Videos courtesy of Mayo Clinic
Read the related article: Native tissue repair of POP: Surgical techniques to improve outcomes



Videos courtesy of Mayo Clinic
Read the related article: Native tissue repair of POP: Surgical techniques to improve outcomes



Videos courtesy of Mayo Clinic
Read the related article: Native tissue repair of POP: Surgical techniques to improve outcomes
Colorectal screening cost effective in cystic fibrosis patients
Screening for colorectal cancer in patients with cystic fibrosis is cost effective, and should be started at a younger age and performed more often, new research suggests.
While colorectal cancer (CRC) screening traditionally begins at age 50 years in people at average risk for the disease, those at high risk usually begin undergoing colonoscopies at an earlier age. Patients with cystic fibrosis fall under the latter category, wrote Andrea Gini, of the department of public health at Erasmus Medical Center in Rotterdam, the Netherlands, and colleagues, with an incidence of CRC up to 30 times higher than the general population, but their shorter lifespan has led to a “different trade-off between the benefits and harms of CRC screening.”
Between 2000 and 2015, the median predicted survival age for patients with cystic fibrosis increased from 33.3 years to 41.7 years; this increased survival has brought increased risk for other diseases, particularly in the GI tract, Mr. Gini and colleagues wrote in Gastroenterology. By using the Microsimulation Screening Analysis–Colon model – a joint project between Erasmus Medical Center and Memorial Sloan Kettering Cancer Center in New York – the investigators assessed the cost-effectiveness of CRC screening in patients with cystic fibrosis.
Three cohorts of 10 million patients each were simulated, with one cohort having undergone transplant, one cohort not having transplant, and one cohort of individuals without cystic fibrosis. The simulated patient age was 30 years in 2017. A total of 76 different colonoscopy-screening strategies were assessed, with each differing in screening interval (3, 5, or 10 years for colonoscopy), age to start screening (30, 35, 40, 45, or 50 years), and age to end screening (55, 60, 65, 70, or 75 years). The optimal screening strategy was determined based on a willingness-to-pay threshold of $100,000 per life-year gained, the investigators wrote.
In the absence of screening, the mortality rate for nontransplant cystic fibrosis patients was 19.1 per 1,000 people, and the rate for cystic fibrosis patients who had undergone transplant was 22.3 per 1,000 people. The standard screening strategy prevented more than 73% of CRC deaths in the general population, 66% of deaths in nontransplant cystic fibrosis patients, and 36% of deaths in cystic fibrosis patients with transplant; however, the model predicted that only 22% of individuals who received a transplant and 36% of those who did not would reach the age of 50 years.
According to the model, the optimal colonoscopy-screening strategy for nontransplant patients was one screen every 5 years, starting at 40 and screening until the age of 75. The incremental cost-effectiveness ratio (ICER) was $84,000 per life-year gained; CRC incidence was reduced by 52% and CRC mortality was reduced by 79%. For transplant patients, the best strategy was one screen every 3 years between the ages of 35 and 55, which reduced CRC mortality by 82% at an ICER of $71,000 per life-year gained.
In a separate analysis of fecal immunochemical testing, a less-demanding alternative to colonoscopy, the optimal screening strategy was an annual test between the age of 35 and 75 years for nontransplant cystic fibrosis patients, for an ICER of $47,000 per life-year gained and a CRC mortality reduction of 78%. The best strategy for transplant patients was once a year between the ages of 30 and 60, which reduced CRC mortality by 77% at an ICER of $86,000 per life-year gained. While fecal immunochemical testing may be more cost effective than colonoscopy, “specific evidence of its performance in the cystic fibrosis population is required before considering this screening modality,” the investigators noted.
“This study indicates that there is benefit to earlier CRC screening in the cystic fibrosis population and [that it] can be done at acceptable costs,” the investigators wrote. “The findings of this analysis support clinicians, researchers, and policy makers who aim to define a tailored CRC screening for individuals with cystic fibrosis in the United States.”
The study was funded by the Cystic Fibrosis Foundation, the Cancer Intervention and Surveillance Modeling Network consortium, and Memorial Sloan Kettering Cancer Center. The investigators reported no conflicts of interest.
lfranki@mdedge.com
SOURCE: Gini A et al. Gastroenterology. 2017 Dec 27. doi: 10.1053/j.gastro.2017.12.011.
Screening for colorectal cancer in patients with cystic fibrosis is cost effective, and should be started at a younger age and performed more often, new research suggests.
While colorectal cancer (CRC) screening traditionally begins at age 50 years in people at average risk for the disease, those at high risk usually begin undergoing colonoscopies at an earlier age. Patients with cystic fibrosis fall under the latter category, wrote Andrea Gini, of the department of public health at Erasmus Medical Center in Rotterdam, the Netherlands, and colleagues, with an incidence of CRC up to 30 times higher than the general population, but their shorter lifespan has led to a “different trade-off between the benefits and harms of CRC screening.”
Between 2000 and 2015, the median predicted survival age for patients with cystic fibrosis increased from 33.3 years to 41.7 years; this increased survival has brought increased risk for other diseases, particularly in the GI tract, Mr. Gini and colleagues wrote in Gastroenterology. By using the Microsimulation Screening Analysis–Colon model – a joint project between Erasmus Medical Center and Memorial Sloan Kettering Cancer Center in New York – the investigators assessed the cost-effectiveness of CRC screening in patients with cystic fibrosis.
Three cohorts of 10 million patients each were simulated, with one cohort having undergone transplant, one cohort not having transplant, and one cohort of individuals without cystic fibrosis. The simulated patient age was 30 years in 2017. A total of 76 different colonoscopy-screening strategies were assessed, with each differing in screening interval (3, 5, or 10 years for colonoscopy), age to start screening (30, 35, 40, 45, or 50 years), and age to end screening (55, 60, 65, 70, or 75 years). The optimal screening strategy was determined based on a willingness-to-pay threshold of $100,000 per life-year gained, the investigators wrote.
In the absence of screening, the mortality rate for nontransplant cystic fibrosis patients was 19.1 per 1,000 people, and the rate for cystic fibrosis patients who had undergone transplant was 22.3 per 1,000 people. The standard screening strategy prevented more than 73% of CRC deaths in the general population, 66% of deaths in nontransplant cystic fibrosis patients, and 36% of deaths in cystic fibrosis patients with transplant; however, the model predicted that only 22% of individuals who received a transplant and 36% of those who did not would reach the age of 50 years.
According to the model, the optimal colonoscopy-screening strategy for nontransplant patients was one screen every 5 years, starting at 40 and screening until the age of 75. The incremental cost-effectiveness ratio (ICER) was $84,000 per life-year gained; CRC incidence was reduced by 52% and CRC mortality was reduced by 79%. For transplant patients, the best strategy was one screen every 3 years between the ages of 35 and 55, which reduced CRC mortality by 82% at an ICER of $71,000 per life-year gained.
In a separate analysis of fecal immunochemical testing, a less-demanding alternative to colonoscopy, the optimal screening strategy was an annual test between the age of 35 and 75 years for nontransplant cystic fibrosis patients, for an ICER of $47,000 per life-year gained and a CRC mortality reduction of 78%. The best strategy for transplant patients was once a year between the ages of 30 and 60, which reduced CRC mortality by 77% at an ICER of $86,000 per life-year gained. While fecal immunochemical testing may be more cost effective than colonoscopy, “specific evidence of its performance in the cystic fibrosis population is required before considering this screening modality,” the investigators noted.
“This study indicates that there is benefit to earlier CRC screening in the cystic fibrosis population and [that it] can be done at acceptable costs,” the investigators wrote. “The findings of this analysis support clinicians, researchers, and policy makers who aim to define a tailored CRC screening for individuals with cystic fibrosis in the United States.”
The study was funded by the Cystic Fibrosis Foundation, the Cancer Intervention and Surveillance Modeling Network consortium, and Memorial Sloan Kettering Cancer Center. The investigators reported no conflicts of interest.
lfranki@mdedge.com
SOURCE: Gini A et al. Gastroenterology. 2017 Dec 27. doi: 10.1053/j.gastro.2017.12.011.
Screening for colorectal cancer in patients with cystic fibrosis is cost effective, and should be started at a younger age and performed more often, new research suggests.
While colorectal cancer (CRC) screening traditionally begins at age 50 years in people at average risk for the disease, those at high risk usually begin undergoing colonoscopies at an earlier age. Patients with cystic fibrosis fall under the latter category, wrote Andrea Gini, of the department of public health at Erasmus Medical Center in Rotterdam, the Netherlands, and colleagues, with an incidence of CRC up to 30 times higher than the general population, but their shorter lifespan has led to a “different trade-off between the benefits and harms of CRC screening.”
Between 2000 and 2015, the median predicted survival age for patients with cystic fibrosis increased from 33.3 years to 41.7 years; this increased survival has brought increased risk for other diseases, particularly in the GI tract, Mr. Gini and colleagues wrote in Gastroenterology. By using the Microsimulation Screening Analysis–Colon model – a joint project between Erasmus Medical Center and Memorial Sloan Kettering Cancer Center in New York – the investigators assessed the cost-effectiveness of CRC screening in patients with cystic fibrosis.
Three cohorts of 10 million patients each were simulated, with one cohort having undergone transplant, one cohort not having transplant, and one cohort of individuals without cystic fibrosis. The simulated patient age was 30 years in 2017. A total of 76 different colonoscopy-screening strategies were assessed, with each differing in screening interval (3, 5, or 10 years for colonoscopy), age to start screening (30, 35, 40, 45, or 50 years), and age to end screening (55, 60, 65, 70, or 75 years). The optimal screening strategy was determined based on a willingness-to-pay threshold of $100,000 per life-year gained, the investigators wrote.
In the absence of screening, the mortality rate for nontransplant cystic fibrosis patients was 19.1 per 1,000 people, and the rate for cystic fibrosis patients who had undergone transplant was 22.3 per 1,000 people. The standard screening strategy prevented more than 73% of CRC deaths in the general population, 66% of deaths in nontransplant cystic fibrosis patients, and 36% of deaths in cystic fibrosis patients with transplant; however, the model predicted that only 22% of individuals who received a transplant and 36% of those who did not would reach the age of 50 years.
According to the model, the optimal colonoscopy-screening strategy for nontransplant patients was one screen every 5 years, starting at 40 and screening until the age of 75. The incremental cost-effectiveness ratio (ICER) was $84,000 per life-year gained; CRC incidence was reduced by 52% and CRC mortality was reduced by 79%. For transplant patients, the best strategy was one screen every 3 years between the ages of 35 and 55, which reduced CRC mortality by 82% at an ICER of $71,000 per life-year gained.
In a separate analysis of fecal immunochemical testing, a less-demanding alternative to colonoscopy, the optimal screening strategy was an annual test between the age of 35 and 75 years for nontransplant cystic fibrosis patients, for an ICER of $47,000 per life-year gained and a CRC mortality reduction of 78%. The best strategy for transplant patients was once a year between the ages of 30 and 60, which reduced CRC mortality by 77% at an ICER of $86,000 per life-year gained. While fecal immunochemical testing may be more cost effective than colonoscopy, “specific evidence of its performance in the cystic fibrosis population is required before considering this screening modality,” the investigators noted.
“This study indicates that there is benefit to earlier CRC screening in the cystic fibrosis population and [that it] can be done at acceptable costs,” the investigators wrote. “The findings of this analysis support clinicians, researchers, and policy makers who aim to define a tailored CRC screening for individuals with cystic fibrosis in the United States.”
The study was funded by the Cystic Fibrosis Foundation, the Cancer Intervention and Surveillance Modeling Network consortium, and Memorial Sloan Kettering Cancer Center. The investigators reported no conflicts of interest.
lfranki@mdedge.com
SOURCE: Gini A et al. Gastroenterology. 2017 Dec 27. doi: 10.1053/j.gastro.2017.12.011.
FROM GASTROENTEROLOGY
Key clinical point: Colorectal cancer screening in patients with cystic fibrosis is cost effective and should be performed more often and at a younger age.
Major finding:
Study details: The Microsimulation Screening Analysis–Colon, involving three simulated cohorts of 10 million people.
Disclosures: The study was funded by the Cystic Fibrosis Foundation, the Cancer Intervention and Surveillance Modeling Network consortium, and Memorial Sloan Kettering Cancer Center. The investigators reported no conflicts of interest.
Source: Gini A et al. Gastroenterology. 2017 Dec 27. doi: 10.1053/j.gastro.2017.12.011.
Supporting our gender-diverse patients
CASE Patient has adverse effects from halted estrogen pills
JR twists her hands nervously as you step into the room. “They stopped my hormones,” she sighs as you pull up her lab results.
JR recently had been admitted to an inpatient cardiology unit for several days for a heart failure exacerbation. Her ankles are still swollen beneath her floral print skirt, but she is breathing much easier now. She is back at your primary care office, hoping to get clearance to restart her estrogen pills.
JR reports having mood swings and terrible nightmares while not taking her hormones, which she has been taking for more than 3 years. She hesitates before sharing, “One of the doctors kept asking me questions about my sex life that had nothing to do with my heart condition. I don’t want to go back there.”
Providing compassionate and comprehensive care to gender-nonconforming individuals is challenging for a multitude of reasons, from clinician ignorance to systemic discrimination. About 33% of transgender patients reported being harassed, denied care, or even being assaulted when seeking health care, while 23% reported avoiding going to the doctor altogether when sick or injured out of fear of discrimination.1
Unfortunately, now, further increases to barriers to care may be put in place. In late May of this year, the Department of Health and Human Services (HHS) proposed new regulations that would reverse previous regulations granted through section 1557 of the Affordable Care Act (ACA)—the Health Care Rights Law—which affirmed the rights of gender nonbinary persons to medical care. Among the proposed changes is the elimination of protections against discrimination in health care based on gender identity.2 The proposed regulation changes come on the heels of a federal court case, which seeks to declare that hospital systems may turn away patients based on gender identity.3
Unraveling rights afforded under the ACA
The Health Care Rights Law was passed under the ACA; it prohibits discrimination based on race, color, national origin, sex, age, and disability in health programs and activities receiving federal financial assistance. Multiple lower courts have supported that the rights of transgender individuals is included within these protections against discrimination on the basis of sex.4 These court rulings not only have ensured the ability of gender-diverse individuals to access care but also have enforced insurance coverage of therapies for gender dysphoria. It was only in 2014 that Medicaid began providing coverage for gender-affirming surgeries and eliminating language that such procedures were “experimental” or “cosmetic.” The 2016 passage of the ACA mandated that private insurance companies follow suit. Unfortunately, the recent proposed regulation changes to the Health Care Rights Law may spark a reversal from insurance companies as well. Such a setback would affect gender-diverse individuals’ hormone treatments as well as their ability to access a full spectrum of care within the health care system.
Continue to: ACOG urges nondiscriminatory practices...
ACOG urges nondiscriminatory practices
The proposed regulation changes to the Health Care Rights Law are from the Conscience and Religious Freedom Division of the HHS Office for Civil Rights, which was established in 2018 and has been advocating for the rights of health care providers to refuse to treat patients based on their own religious beliefs.5 We argue, however, that providing care to persons of varying backgrounds is not an assault on our individual liberties but rather a privilege as providers. As obstetrician-gynecologists, it may be easy to only consider cis-gendered women our responsibility. But our field also emphasizes individual empowerment above all else—we fight every day for our patients’ rights to contraception, fertility, pregnancy, parenthood, and sexual freedoms. Let us continue speaking up for the rights of all those who need gynecologic care, regardless of the pronouns they use.
“The American College of Obstetricians and Gynecologists urges health care providers to foster nondiscriminatory practices and policies to increase identification and to facilitate quality health care for transgender individuals, both in assisting with the transition if desired as well as providing long-term preventive health care.”6
We urge you to take action
- Reach out to your local representatives about protecting transgender health access
- Educate yourself on the unique needs of transgender individuals
- Read personal accounts
- Share your personal story
- Find referring providers near your practice
- 2015 US Transgender Survey. December 2016. https://www.transequality.org/sites/default/files/docs/USTS-Full-Report-FINAL.PDF. Accessed August 30, 2019.
- Musumeci M, Kates J, Dawson J, et al. HHS’ proposed changes to non-discrimination regulations under ACA section 1557. July 1, 2019. https://www.kff.org/disparities-policy/issue-brief/hhss-proposed-changes-to-non-discrimination-regulations-under-aca-section-1557/. Accessed August 30, 2019.
- Franciscan Alliance v. Burwell. ACLU website. https://www.aclu.org/cases/franciscan-alliance-v-burwell. Accessed August 30, 2019.
- Pear R. Trump plan would cut back health care protections for transgender people. April 21, 2018. https://www.nytimes.com/2018/04/21/us/politics/trump-transgender-health-care.html. Accessed August 30, 2019.
- U.S. Department of Health and Human Services. HHS announces new conscience and religious freedom division. January 18, 2018. https://www.hhs.gov/about/news/2018/01/18/hhs-ocr-announces-new-conscience-and-religious-freedom-division.html. Accessed August 30, 2019.
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee Opinion no. 512: health care for transgender individuals. Obstet Gynecol. 2011;118:1454–1458.
CASE Patient has adverse effects from halted estrogen pills
JR twists her hands nervously as you step into the room. “They stopped my hormones,” she sighs as you pull up her lab results.
JR recently had been admitted to an inpatient cardiology unit for several days for a heart failure exacerbation. Her ankles are still swollen beneath her floral print skirt, but she is breathing much easier now. She is back at your primary care office, hoping to get clearance to restart her estrogen pills.
JR reports having mood swings and terrible nightmares while not taking her hormones, which she has been taking for more than 3 years. She hesitates before sharing, “One of the doctors kept asking me questions about my sex life that had nothing to do with my heart condition. I don’t want to go back there.”
Providing compassionate and comprehensive care to gender-nonconforming individuals is challenging for a multitude of reasons, from clinician ignorance to systemic discrimination. About 33% of transgender patients reported being harassed, denied care, or even being assaulted when seeking health care, while 23% reported avoiding going to the doctor altogether when sick or injured out of fear of discrimination.1
Unfortunately, now, further increases to barriers to care may be put in place. In late May of this year, the Department of Health and Human Services (HHS) proposed new regulations that would reverse previous regulations granted through section 1557 of the Affordable Care Act (ACA)—the Health Care Rights Law—which affirmed the rights of gender nonbinary persons to medical care. Among the proposed changes is the elimination of protections against discrimination in health care based on gender identity.2 The proposed regulation changes come on the heels of a federal court case, which seeks to declare that hospital systems may turn away patients based on gender identity.3
Unraveling rights afforded under the ACA
The Health Care Rights Law was passed under the ACA; it prohibits discrimination based on race, color, national origin, sex, age, and disability in health programs and activities receiving federal financial assistance. Multiple lower courts have supported that the rights of transgender individuals is included within these protections against discrimination on the basis of sex.4 These court rulings not only have ensured the ability of gender-diverse individuals to access care but also have enforced insurance coverage of therapies for gender dysphoria. It was only in 2014 that Medicaid began providing coverage for gender-affirming surgeries and eliminating language that such procedures were “experimental” or “cosmetic.” The 2016 passage of the ACA mandated that private insurance companies follow suit. Unfortunately, the recent proposed regulation changes to the Health Care Rights Law may spark a reversal from insurance companies as well. Such a setback would affect gender-diverse individuals’ hormone treatments as well as their ability to access a full spectrum of care within the health care system.
Continue to: ACOG urges nondiscriminatory practices...
ACOG urges nondiscriminatory practices
The proposed regulation changes to the Health Care Rights Law are from the Conscience and Religious Freedom Division of the HHS Office for Civil Rights, which was established in 2018 and has been advocating for the rights of health care providers to refuse to treat patients based on their own religious beliefs.5 We argue, however, that providing care to persons of varying backgrounds is not an assault on our individual liberties but rather a privilege as providers. As obstetrician-gynecologists, it may be easy to only consider cis-gendered women our responsibility. But our field also emphasizes individual empowerment above all else—we fight every day for our patients’ rights to contraception, fertility, pregnancy, parenthood, and sexual freedoms. Let us continue speaking up for the rights of all those who need gynecologic care, regardless of the pronouns they use.
“The American College of Obstetricians and Gynecologists urges health care providers to foster nondiscriminatory practices and policies to increase identification and to facilitate quality health care for transgender individuals, both in assisting with the transition if desired as well as providing long-term preventive health care.”6
We urge you to take action
- Reach out to your local representatives about protecting transgender health access
- Educate yourself on the unique needs of transgender individuals
- Read personal accounts
- Share your personal story
- Find referring providers near your practice
CASE Patient has adverse effects from halted estrogen pills
JR twists her hands nervously as you step into the room. “They stopped my hormones,” she sighs as you pull up her lab results.
JR recently had been admitted to an inpatient cardiology unit for several days for a heart failure exacerbation. Her ankles are still swollen beneath her floral print skirt, but she is breathing much easier now. She is back at your primary care office, hoping to get clearance to restart her estrogen pills.
JR reports having mood swings and terrible nightmares while not taking her hormones, which she has been taking for more than 3 years. She hesitates before sharing, “One of the doctors kept asking me questions about my sex life that had nothing to do with my heart condition. I don’t want to go back there.”
Providing compassionate and comprehensive care to gender-nonconforming individuals is challenging for a multitude of reasons, from clinician ignorance to systemic discrimination. About 33% of transgender patients reported being harassed, denied care, or even being assaulted when seeking health care, while 23% reported avoiding going to the doctor altogether when sick or injured out of fear of discrimination.1
Unfortunately, now, further increases to barriers to care may be put in place. In late May of this year, the Department of Health and Human Services (HHS) proposed new regulations that would reverse previous regulations granted through section 1557 of the Affordable Care Act (ACA)—the Health Care Rights Law—which affirmed the rights of gender nonbinary persons to medical care. Among the proposed changes is the elimination of protections against discrimination in health care based on gender identity.2 The proposed regulation changes come on the heels of a federal court case, which seeks to declare that hospital systems may turn away patients based on gender identity.3
Unraveling rights afforded under the ACA
The Health Care Rights Law was passed under the ACA; it prohibits discrimination based on race, color, national origin, sex, age, and disability in health programs and activities receiving federal financial assistance. Multiple lower courts have supported that the rights of transgender individuals is included within these protections against discrimination on the basis of sex.4 These court rulings not only have ensured the ability of gender-diverse individuals to access care but also have enforced insurance coverage of therapies for gender dysphoria. It was only in 2014 that Medicaid began providing coverage for gender-affirming surgeries and eliminating language that such procedures were “experimental” or “cosmetic.” The 2016 passage of the ACA mandated that private insurance companies follow suit. Unfortunately, the recent proposed regulation changes to the Health Care Rights Law may spark a reversal from insurance companies as well. Such a setback would affect gender-diverse individuals’ hormone treatments as well as their ability to access a full spectrum of care within the health care system.
Continue to: ACOG urges nondiscriminatory practices...
ACOG urges nondiscriminatory practices
The proposed regulation changes to the Health Care Rights Law are from the Conscience and Religious Freedom Division of the HHS Office for Civil Rights, which was established in 2018 and has been advocating for the rights of health care providers to refuse to treat patients based on their own religious beliefs.5 We argue, however, that providing care to persons of varying backgrounds is not an assault on our individual liberties but rather a privilege as providers. As obstetrician-gynecologists, it may be easy to only consider cis-gendered women our responsibility. But our field also emphasizes individual empowerment above all else—we fight every day for our patients’ rights to contraception, fertility, pregnancy, parenthood, and sexual freedoms. Let us continue speaking up for the rights of all those who need gynecologic care, regardless of the pronouns they use.
“The American College of Obstetricians and Gynecologists urges health care providers to foster nondiscriminatory practices and policies to increase identification and to facilitate quality health care for transgender individuals, both in assisting with the transition if desired as well as providing long-term preventive health care.”6
We urge you to take action
- Reach out to your local representatives about protecting transgender health access
- Educate yourself on the unique needs of transgender individuals
- Read personal accounts
- Share your personal story
- Find referring providers near your practice
- 2015 US Transgender Survey. December 2016. https://www.transequality.org/sites/default/files/docs/USTS-Full-Report-FINAL.PDF. Accessed August 30, 2019.
- Musumeci M, Kates J, Dawson J, et al. HHS’ proposed changes to non-discrimination regulations under ACA section 1557. July 1, 2019. https://www.kff.org/disparities-policy/issue-brief/hhss-proposed-changes-to-non-discrimination-regulations-under-aca-section-1557/. Accessed August 30, 2019.
- Franciscan Alliance v. Burwell. ACLU website. https://www.aclu.org/cases/franciscan-alliance-v-burwell. Accessed August 30, 2019.
- Pear R. Trump plan would cut back health care protections for transgender people. April 21, 2018. https://www.nytimes.com/2018/04/21/us/politics/trump-transgender-health-care.html. Accessed August 30, 2019.
- U.S. Department of Health and Human Services. HHS announces new conscience and religious freedom division. January 18, 2018. https://www.hhs.gov/about/news/2018/01/18/hhs-ocr-announces-new-conscience-and-religious-freedom-division.html. Accessed August 30, 2019.
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee Opinion no. 512: health care for transgender individuals. Obstet Gynecol. 2011;118:1454–1458.
- 2015 US Transgender Survey. December 2016. https://www.transequality.org/sites/default/files/docs/USTS-Full-Report-FINAL.PDF. Accessed August 30, 2019.
- Musumeci M, Kates J, Dawson J, et al. HHS’ proposed changes to non-discrimination regulations under ACA section 1557. July 1, 2019. https://www.kff.org/disparities-policy/issue-brief/hhss-proposed-changes-to-non-discrimination-regulations-under-aca-section-1557/. Accessed August 30, 2019.
- Franciscan Alliance v. Burwell. ACLU website. https://www.aclu.org/cases/franciscan-alliance-v-burwell. Accessed August 30, 2019.
- Pear R. Trump plan would cut back health care protections for transgender people. April 21, 2018. https://www.nytimes.com/2018/04/21/us/politics/trump-transgender-health-care.html. Accessed August 30, 2019.
- U.S. Department of Health and Human Services. HHS announces new conscience and religious freedom division. January 18, 2018. https://www.hhs.gov/about/news/2018/01/18/hhs-ocr-announces-new-conscience-and-religious-freedom-division.html. Accessed August 30, 2019.
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee Opinion no. 512: health care for transgender individuals. Obstet Gynecol. 2011;118:1454–1458.
Apply to become the next SVS PSO Associate Medical Director
The SVS PSO is looking for a part-time Associate Medical Director. This person will be responsible for assisting the SVS PSO Medical Director and the SVS PSO staff, with guidance and over site in its clinical operations. The new associate director will be nominated by the SVS PSO Executive Committee and approved the SVS and the SVS Executive Board. He or she will serve a one-year term, with the opportunity to serve two additional one-year terms. There is a modest honorary associated with this position and the potential to advance into the role of SVS PSO Medical Director. Submit your application before Oct. 11 to be considered. Read the full job description here. Please email your completed resume to HRResumes@vascularsociety.org.
The SVS PSO is looking for a part-time Associate Medical Director. This person will be responsible for assisting the SVS PSO Medical Director and the SVS PSO staff, with guidance and over site in its clinical operations. The new associate director will be nominated by the SVS PSO Executive Committee and approved the SVS and the SVS Executive Board. He or she will serve a one-year term, with the opportunity to serve two additional one-year terms. There is a modest honorary associated with this position and the potential to advance into the role of SVS PSO Medical Director. Submit your application before Oct. 11 to be considered. Read the full job description here. Please email your completed resume to HRResumes@vascularsociety.org.
The SVS PSO is looking for a part-time Associate Medical Director. This person will be responsible for assisting the SVS PSO Medical Director and the SVS PSO staff, with guidance and over site in its clinical operations. The new associate director will be nominated by the SVS PSO Executive Committee and approved the SVS and the SVS Executive Board. He or she will serve a one-year term, with the opportunity to serve two additional one-year terms. There is a modest honorary associated with this position and the potential to advance into the role of SVS PSO Medical Director. Submit your application before Oct. 11 to be considered. Read the full job description here. Please email your completed resume to HRResumes@vascularsociety.org.
ARNIs effective for acute decompensated heart failure
Background: The PARADIGM-HF trial demonstrated that patients with chronic HFrEF treated with an ARNI (sacubitril/valsartan) had significantly reduced cardiovascular mortality and hospitalizations when compared with enalapril. Patients with acute decompensated heart failure were excluded from this trial. The PIONEER-HF trial was designed to determine whether initiation of an ARNI in patients with acute decompensated heart failure is effective.
Study design: Multicenter, randomized, double-blind, active-controlled trial.
Setting: A total of 129 centers in the United States.
Synopsis: Of 881 patients with acute HFrEF, 440 were randomized to receive sacubitril/valsartan and 441 were randomized to receive enalapril. The majority of patients were men; mean age was 61 years. The primary outcome was the mean reduction in NT-proBNP concentration at weeks 4 and 8 as compared with baseline. In the sacubitril/valsartan group, there was a 46.7% reduction from baseline, and in the enalapril group, there was a 25.3% reduction from baseline. With regard to drug safety, there was no difference between groups in worsening renal function, symptomatic hypotension, or hyperkalemia.
A limitation of this study is that 20% of patients in each group discontinued treatment by 8 weeks secondary to an adverse event. Additionally, a clinical measure such as cardiovascular mortality, all-cause mortality, or rehospitalization for heart failure was not included in the primary outcome.
Bottom line: In patients with acute decompensated HFrEF, ARNIs are more effective at reducing NT-proBNP levels than enalapril, while maintaining a similar safety profile. Further investigation to evaluate clinical outcomes needs to be completed.
Citation: Velazquez EJ et al. Angiotensin–neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2018 Nov 11. doi: 10.1056/NEJMoa1812851.
Dr. McIntyre is an associate physician in the division of hospital medicine at the University of California, San Diego.
Background: The PARADIGM-HF trial demonstrated that patients with chronic HFrEF treated with an ARNI (sacubitril/valsartan) had significantly reduced cardiovascular mortality and hospitalizations when compared with enalapril. Patients with acute decompensated heart failure were excluded from this trial. The PIONEER-HF trial was designed to determine whether initiation of an ARNI in patients with acute decompensated heart failure is effective.
Study design: Multicenter, randomized, double-blind, active-controlled trial.
Setting: A total of 129 centers in the United States.
Synopsis: Of 881 patients with acute HFrEF, 440 were randomized to receive sacubitril/valsartan and 441 were randomized to receive enalapril. The majority of patients were men; mean age was 61 years. The primary outcome was the mean reduction in NT-proBNP concentration at weeks 4 and 8 as compared with baseline. In the sacubitril/valsartan group, there was a 46.7% reduction from baseline, and in the enalapril group, there was a 25.3% reduction from baseline. With regard to drug safety, there was no difference between groups in worsening renal function, symptomatic hypotension, or hyperkalemia.
A limitation of this study is that 20% of patients in each group discontinued treatment by 8 weeks secondary to an adverse event. Additionally, a clinical measure such as cardiovascular mortality, all-cause mortality, or rehospitalization for heart failure was not included in the primary outcome.
Bottom line: In patients with acute decompensated HFrEF, ARNIs are more effective at reducing NT-proBNP levels than enalapril, while maintaining a similar safety profile. Further investigation to evaluate clinical outcomes needs to be completed.
Citation: Velazquez EJ et al. Angiotensin–neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2018 Nov 11. doi: 10.1056/NEJMoa1812851.
Dr. McIntyre is an associate physician in the division of hospital medicine at the University of California, San Diego.
Background: The PARADIGM-HF trial demonstrated that patients with chronic HFrEF treated with an ARNI (sacubitril/valsartan) had significantly reduced cardiovascular mortality and hospitalizations when compared with enalapril. Patients with acute decompensated heart failure were excluded from this trial. The PIONEER-HF trial was designed to determine whether initiation of an ARNI in patients with acute decompensated heart failure is effective.
Study design: Multicenter, randomized, double-blind, active-controlled trial.
Setting: A total of 129 centers in the United States.
Synopsis: Of 881 patients with acute HFrEF, 440 were randomized to receive sacubitril/valsartan and 441 were randomized to receive enalapril. The majority of patients were men; mean age was 61 years. The primary outcome was the mean reduction in NT-proBNP concentration at weeks 4 and 8 as compared with baseline. In the sacubitril/valsartan group, there was a 46.7% reduction from baseline, and in the enalapril group, there was a 25.3% reduction from baseline. With regard to drug safety, there was no difference between groups in worsening renal function, symptomatic hypotension, or hyperkalemia.
A limitation of this study is that 20% of patients in each group discontinued treatment by 8 weeks secondary to an adverse event. Additionally, a clinical measure such as cardiovascular mortality, all-cause mortality, or rehospitalization for heart failure was not included in the primary outcome.
Bottom line: In patients with acute decompensated HFrEF, ARNIs are more effective at reducing NT-proBNP levels than enalapril, while maintaining a similar safety profile. Further investigation to evaluate clinical outcomes needs to be completed.
Citation: Velazquez EJ et al. Angiotensin–neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2018 Nov 11. doi: 10.1056/NEJMoa1812851.
Dr. McIntyre is an associate physician in the division of hospital medicine at the University of California, San Diego.
SVS Now Accepting International Scholar Applications
If you are a young vascular surgeon from outside North America, consider applying for the International Scholars Program. Recipients of the award will receive a $5,000 stipend, spend two weeks in the U.S, visiting universities and clinics, and attend the 2020 VAM in Toronto. Scholars will work with a mentor to schedule various vascular program visits, including clinical, teaching and research programs. Apply before Sept. 16 to be considered. Learn more.
If you are a young vascular surgeon from outside North America, consider applying for the International Scholars Program. Recipients of the award will receive a $5,000 stipend, spend two weeks in the U.S, visiting universities and clinics, and attend the 2020 VAM in Toronto. Scholars will work with a mentor to schedule various vascular program visits, including clinical, teaching and research programs. Apply before Sept. 16 to be considered. Learn more.
If you are a young vascular surgeon from outside North America, consider applying for the International Scholars Program. Recipients of the award will receive a $5,000 stipend, spend two weeks in the U.S, visiting universities and clinics, and attend the 2020 VAM in Toronto. Scholars will work with a mentor to schedule various vascular program visits, including clinical, teaching and research programs. Apply before Sept. 16 to be considered. Learn more.