User login
Part 2: The Leader as Pioneer
Leadership is both a science and an art, requiring a process, influence, and vision.1 And—coincidentally?—we could also define nursing in those terms. After all, health care leaders must possess the vision and ability to translate their passion and establish an environment of trust with their teams to achieve the desired outcome.
When I reflect on my experiences as an NP, I think about those who influenced me to pursue this career. My mentors were both scientists and artists who translated their passion to me. Without their combination of caring and competence, I wouldn’t be where I am today. There’d be one less NP—and for me, part of being an NP is showing others the essential value of our profession.
Throughout my career, my mission was to bring NPs into more health care settings—such as urgent care and emergency departments in rural areas—where greater access to care was needed and where NPs were woefully underutilized. Advocating for NPs also led me into nonclinical settings, such as my time as a Health Policy Coordinator. As mentioned last week, this position required me to communicate the needs of both patients and health care providers to multiple groups, including industry associations, federal agencies, and professional licensure boards. No matter the setting, I had to understand its specific function and needs in order to envision and then explain how it would benefit from a stronger NP presence. This always included how NPs would complement other health care providers and professionals.
My mission to bring the NP role to new settings led me to surprising places. For example, I had the opportunity to be the first NP to serve as a Medical Officer on an expedition for JASON Learning—a pioneering nonprofit organization established to engage students in scientific research and expeditions led by leading scientists.2 How did I do that, you ask? Well, as a graduate student, I worked on a project with the JASON team. When I had the chance to speak with the expedition coordinator, I mentioned my interest in marine biology. I then asked about the composition of the expedition support team and discovered that they didn’t have an NP. Here was the chance to utilize my communication skills to sell the idea of having a pediatric NP (PNP) on an expedition! I explained my vision of the role a PNP could play, emphasizing how an expedition would benefit from an NP with experience in caring for teenagers.
I must have been persuasive, because that conversation landed me with a primary role as the health care provider on an expedition to the Everglades National Park in Florida. In addition to reviewing the medical history of each “Argonaut” (participant), I treated minor illnesses and injuries and prevented sunburn during the expedition. I also had the opportunity to become certified in Snorkel and SCUBA—and I still use the former today. As a bonus, I met Bob Ballard (known for finding the wreckage of the RMS Titanic) and learned how to tag an alligator.
The excursion to the Everglades was an accomplishment that needed a process, influence, and vision. Later, when championing the creation of AANP, I was able to put these skills into action again. In 1984, when there was no group dedicated to representing NPs, I challenged an audience of colleagues to start a new organization and offered up seed money. A small group of us soon banded together and eventually rallied other NPs to embrace the concept of an NP-specific organization. We developed bylaws, and a year later, our organization was incorporated as a 501(c)(3). The legal address was my home in Lowell, Massachusetts.
Each of these situations required a relationship with the people on the team and a mission to expand the NP profession. In every situation, I translated my vision to others, and we worked together to achieve goals—just as I had been taught through the example of my own mentors.
Continue to: Keep the concepts of...
Keep the concepts of relationship and vison in mind as we continue discussing leadership next Thursday in Part 3. In the meantime, please share your thoughts, approaches, and accomplishments as a leader.
1. McArthur DB. The nurse practitioner as leader. J Am Acad Nurse Pract. 2006;18(1):8-10.
2. JASON Learning website. https://www.jason.org. Accessed September 10, 2019.
Leadership is both a science and an art, requiring a process, influence, and vision.1 And—coincidentally?—we could also define nursing in those terms. After all, health care leaders must possess the vision and ability to translate their passion and establish an environment of trust with their teams to achieve the desired outcome.
When I reflect on my experiences as an NP, I think about those who influenced me to pursue this career. My mentors were both scientists and artists who translated their passion to me. Without their combination of caring and competence, I wouldn’t be where I am today. There’d be one less NP—and for me, part of being an NP is showing others the essential value of our profession.
Throughout my career, my mission was to bring NPs into more health care settings—such as urgent care and emergency departments in rural areas—where greater access to care was needed and where NPs were woefully underutilized. Advocating for NPs also led me into nonclinical settings, such as my time as a Health Policy Coordinator. As mentioned last week, this position required me to communicate the needs of both patients and health care providers to multiple groups, including industry associations, federal agencies, and professional licensure boards. No matter the setting, I had to understand its specific function and needs in order to envision and then explain how it would benefit from a stronger NP presence. This always included how NPs would complement other health care providers and professionals.
My mission to bring the NP role to new settings led me to surprising places. For example, I had the opportunity to be the first NP to serve as a Medical Officer on an expedition for JASON Learning—a pioneering nonprofit organization established to engage students in scientific research and expeditions led by leading scientists.2 How did I do that, you ask? Well, as a graduate student, I worked on a project with the JASON team. When I had the chance to speak with the expedition coordinator, I mentioned my interest in marine biology. I then asked about the composition of the expedition support team and discovered that they didn’t have an NP. Here was the chance to utilize my communication skills to sell the idea of having a pediatric NP (PNP) on an expedition! I explained my vision of the role a PNP could play, emphasizing how an expedition would benefit from an NP with experience in caring for teenagers.
I must have been persuasive, because that conversation landed me with a primary role as the health care provider on an expedition to the Everglades National Park in Florida. In addition to reviewing the medical history of each “Argonaut” (participant), I treated minor illnesses and injuries and prevented sunburn during the expedition. I also had the opportunity to become certified in Snorkel and SCUBA—and I still use the former today. As a bonus, I met Bob Ballard (known for finding the wreckage of the RMS Titanic) and learned how to tag an alligator.
The excursion to the Everglades was an accomplishment that needed a process, influence, and vision. Later, when championing the creation of AANP, I was able to put these skills into action again. In 1984, when there was no group dedicated to representing NPs, I challenged an audience of colleagues to start a new organization and offered up seed money. A small group of us soon banded together and eventually rallied other NPs to embrace the concept of an NP-specific organization. We developed bylaws, and a year later, our organization was incorporated as a 501(c)(3). The legal address was my home in Lowell, Massachusetts.
Each of these situations required a relationship with the people on the team and a mission to expand the NP profession. In every situation, I translated my vision to others, and we worked together to achieve goals—just as I had been taught through the example of my own mentors.
Continue to: Keep the concepts of...
Keep the concepts of relationship and vison in mind as we continue discussing leadership next Thursday in Part 3. In the meantime, please share your thoughts, approaches, and accomplishments as a leader.
Leadership is both a science and an art, requiring a process, influence, and vision.1 And—coincidentally?—we could also define nursing in those terms. After all, health care leaders must possess the vision and ability to translate their passion and establish an environment of trust with their teams to achieve the desired outcome.
When I reflect on my experiences as an NP, I think about those who influenced me to pursue this career. My mentors were both scientists and artists who translated their passion to me. Without their combination of caring and competence, I wouldn’t be where I am today. There’d be one less NP—and for me, part of being an NP is showing others the essential value of our profession.
Throughout my career, my mission was to bring NPs into more health care settings—such as urgent care and emergency departments in rural areas—where greater access to care was needed and where NPs were woefully underutilized. Advocating for NPs also led me into nonclinical settings, such as my time as a Health Policy Coordinator. As mentioned last week, this position required me to communicate the needs of both patients and health care providers to multiple groups, including industry associations, federal agencies, and professional licensure boards. No matter the setting, I had to understand its specific function and needs in order to envision and then explain how it would benefit from a stronger NP presence. This always included how NPs would complement other health care providers and professionals.
My mission to bring the NP role to new settings led me to surprising places. For example, I had the opportunity to be the first NP to serve as a Medical Officer on an expedition for JASON Learning—a pioneering nonprofit organization established to engage students in scientific research and expeditions led by leading scientists.2 How did I do that, you ask? Well, as a graduate student, I worked on a project with the JASON team. When I had the chance to speak with the expedition coordinator, I mentioned my interest in marine biology. I then asked about the composition of the expedition support team and discovered that they didn’t have an NP. Here was the chance to utilize my communication skills to sell the idea of having a pediatric NP (PNP) on an expedition! I explained my vision of the role a PNP could play, emphasizing how an expedition would benefit from an NP with experience in caring for teenagers.
I must have been persuasive, because that conversation landed me with a primary role as the health care provider on an expedition to the Everglades National Park in Florida. In addition to reviewing the medical history of each “Argonaut” (participant), I treated minor illnesses and injuries and prevented sunburn during the expedition. I also had the opportunity to become certified in Snorkel and SCUBA—and I still use the former today. As a bonus, I met Bob Ballard (known for finding the wreckage of the RMS Titanic) and learned how to tag an alligator.
The excursion to the Everglades was an accomplishment that needed a process, influence, and vision. Later, when championing the creation of AANP, I was able to put these skills into action again. In 1984, when there was no group dedicated to representing NPs, I challenged an audience of colleagues to start a new organization and offered up seed money. A small group of us soon banded together and eventually rallied other NPs to embrace the concept of an NP-specific organization. We developed bylaws, and a year later, our organization was incorporated as a 501(c)(3). The legal address was my home in Lowell, Massachusetts.
Each of these situations required a relationship with the people on the team and a mission to expand the NP profession. In every situation, I translated my vision to others, and we worked together to achieve goals—just as I had been taught through the example of my own mentors.
Continue to: Keep the concepts of...
Keep the concepts of relationship and vison in mind as we continue discussing leadership next Thursday in Part 3. In the meantime, please share your thoughts, approaches, and accomplishments as a leader.
1. McArthur DB. The nurse practitioner as leader. J Am Acad Nurse Pract. 2006;18(1):8-10.
2. JASON Learning website. https://www.jason.org. Accessed September 10, 2019.
1. McArthur DB. The nurse practitioner as leader. J Am Acad Nurse Pract. 2006;18(1):8-10.
2. JASON Learning website. https://www.jason.org. Accessed September 10, 2019.
New genotype of S. pyrogenes found in rise of scarlet fever in U.K.
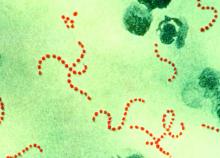
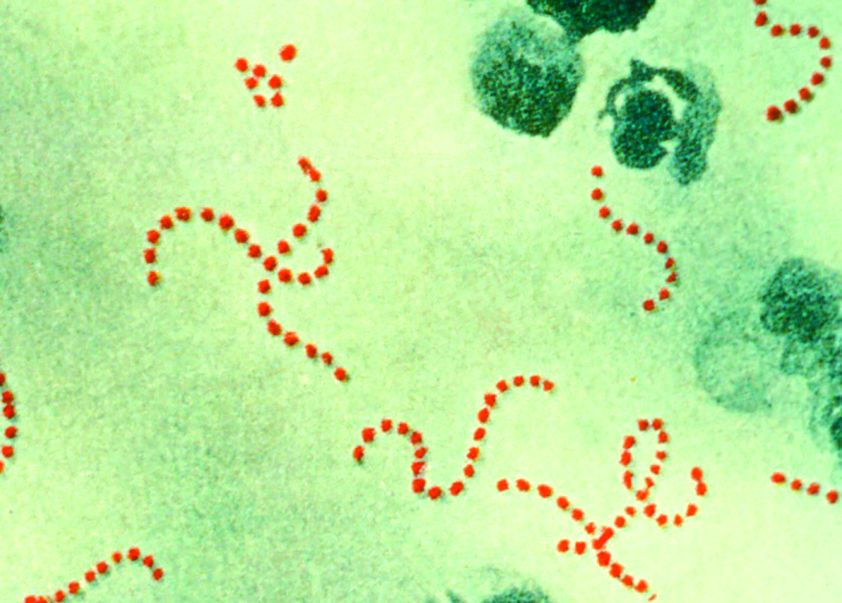
A new Streptococcus pyogenes genotype (designated M1UK) emerged in 2014 in England causing an increase in scarlet fever “unprecedented in modern times.” Researchers discovered that this new genotype became dominant during this increased period of scarlet fever. This new genotype was characterized by an increased production of streptococcal pyrogenic exotoxin A (SpeA, also known as scarlet fever or erythrogenic toxin A) compared to previous isolates, according to a report in The Lancet Infectious Diseases.
The researchers analyzed changes in S. pyogenes emm1 genotypes sampled from scarlet fever and invasive disease cases in 2014-2016. The emm1 gene encodes the cell surface M virulence protein and is used for serotyping S. pyogenes isolates. Using regional (northwest London) and national (England and Wales) data, they compared genomes of 135 noninvasive and 552 invasive emm1 isolates from 2009-2016 with 2,800 global emm1 sequences.
During the increase in scarlet fever and invasive disease, emm1 S. pyogenes upper respiratory tract isolates increased significantly in northwest London during the March to May periods over 3 years from 5% of isolates in 2014 to 19% isolates in 2015 to 33% isolates in 2016. Similarly, invasive emm1 isolates collected nationally in the same period increased from 31% of isolates in 2015 to 42% in 2016 (P less than .0001). Sequences of emm1 isolates from 2009-2016 showed emergence of a new emm1 lineage (designated M1UK), which could be genotypically distinguished from pandemic emm1 isolates (M1global) by 27 single-nucleotide polymorphisms. In addition, the median SpeA protein concentration was 9 times greater among M1UK isolates than among M1global isolates. By 2016, M1UK expanded nationally to comprise 84% of all emm1 genomes tested. Dataset analysis also identified single M1UK isolates present in Denmark and the United States.
“The expansion of such a lineage within the community reservoir of S. pyogenes might be sufficient to explain England’s recent increase in invasive infection. Further research to assess the likely effects of M1UK on infection transmissibility, treatment response, disease burden, and severity is required, coupled with consideration of public health interventions to limit transmission where appropriate,” Dr. Lynskey and colleagues concluded.
The authors reported that they had no disclosures.
SOURCE: Linskey NN et al. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30446-3.
A new Streptococcus pyogenes genotype (designated M1UK) emerged in 2014 in England causing an increase in scarlet fever “unprecedented in modern times.” Researchers discovered that this new genotype became dominant during this increased period of scarlet fever. This new genotype was characterized by an increased production of streptococcal pyrogenic exotoxin A (SpeA, also known as scarlet fever or erythrogenic toxin A) compared to previous isolates, according to a report in The Lancet Infectious Diseases.
The researchers analyzed changes in S. pyogenes emm1 genotypes sampled from scarlet fever and invasive disease cases in 2014-2016. The emm1 gene encodes the cell surface M virulence protein and is used for serotyping S. pyogenes isolates. Using regional (northwest London) and national (England and Wales) data, they compared genomes of 135 noninvasive and 552 invasive emm1 isolates from 2009-2016 with 2,800 global emm1 sequences.
During the increase in scarlet fever and invasive disease, emm1 S. pyogenes upper respiratory tract isolates increased significantly in northwest London during the March to May periods over 3 years from 5% of isolates in 2014 to 19% isolates in 2015 to 33% isolates in 2016. Similarly, invasive emm1 isolates collected nationally in the same period increased from 31% of isolates in 2015 to 42% in 2016 (P less than .0001). Sequences of emm1 isolates from 2009-2016 showed emergence of a new emm1 lineage (designated M1UK), which could be genotypically distinguished from pandemic emm1 isolates (M1global) by 27 single-nucleotide polymorphisms. In addition, the median SpeA protein concentration was 9 times greater among M1UK isolates than among M1global isolates. By 2016, M1UK expanded nationally to comprise 84% of all emm1 genomes tested. Dataset analysis also identified single M1UK isolates present in Denmark and the United States.
“The expansion of such a lineage within the community reservoir of S. pyogenes might be sufficient to explain England’s recent increase in invasive infection. Further research to assess the likely effects of M1UK on infection transmissibility, treatment response, disease burden, and severity is required, coupled with consideration of public health interventions to limit transmission where appropriate,” Dr. Lynskey and colleagues concluded.
The authors reported that they had no disclosures.
SOURCE: Linskey NN et al. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30446-3.
A new Streptococcus pyogenes genotype (designated M1UK) emerged in 2014 in England causing an increase in scarlet fever “unprecedented in modern times.” Researchers discovered that this new genotype became dominant during this increased period of scarlet fever. This new genotype was characterized by an increased production of streptococcal pyrogenic exotoxin A (SpeA, also known as scarlet fever or erythrogenic toxin A) compared to previous isolates, according to a report in The Lancet Infectious Diseases.
The researchers analyzed changes in S. pyogenes emm1 genotypes sampled from scarlet fever and invasive disease cases in 2014-2016. The emm1 gene encodes the cell surface M virulence protein and is used for serotyping S. pyogenes isolates. Using regional (northwest London) and national (England and Wales) data, they compared genomes of 135 noninvasive and 552 invasive emm1 isolates from 2009-2016 with 2,800 global emm1 sequences.
During the increase in scarlet fever and invasive disease, emm1 S. pyogenes upper respiratory tract isolates increased significantly in northwest London during the March to May periods over 3 years from 5% of isolates in 2014 to 19% isolates in 2015 to 33% isolates in 2016. Similarly, invasive emm1 isolates collected nationally in the same period increased from 31% of isolates in 2015 to 42% in 2016 (P less than .0001). Sequences of emm1 isolates from 2009-2016 showed emergence of a new emm1 lineage (designated M1UK), which could be genotypically distinguished from pandemic emm1 isolates (M1global) by 27 single-nucleotide polymorphisms. In addition, the median SpeA protein concentration was 9 times greater among M1UK isolates than among M1global isolates. By 2016, M1UK expanded nationally to comprise 84% of all emm1 genomes tested. Dataset analysis also identified single M1UK isolates present in Denmark and the United States.
“The expansion of such a lineage within the community reservoir of S. pyogenes might be sufficient to explain England’s recent increase in invasive infection. Further research to assess the likely effects of M1UK on infection transmissibility, treatment response, disease burden, and severity is required, coupled with consideration of public health interventions to limit transmission where appropriate,” Dr. Lynskey and colleagues concluded.
The authors reported that they had no disclosures.
SOURCE: Linskey NN et al. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30446-3.
FROM THE LANCET INFECTIOUS DISEASES
Key clinical point: An Streptococcus pyrogenes isolate with increased scarlet fever toxin production has become dominant.
Major finding: By 2016, M1UK expanded nationally to constitute 84% of all emm1 genomes tested.
Study details: Genomic comparison of 135 noninvasive and 552 invasive emm1 isolates from 2009-2016 with 2,800 global emm1 sequences.
Disclosures: The authors reported that they had no disclosures.
Source: Linskey NN et al. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30446-3.
Judge blocks North Dakota abortion law
A district judge has temporarily blocked a North Dakota law that forces physicians to tell patients that medication abortions may be reversed, calling the measure devoid of scientific support.
In a Sept. 10 ruling, Judge Daniel Hovland of the U.S. District Court for the District of North Dakota temporarily halted enforcement of North Dakota House Bill 1336, a law that required doctors to tell women that reversing the effects of abortion-inducing drugs is possible if patients changed their minds, but that “time is of the essence.” In his 24-page decision, Judge Hovland said the North Dakota law violates a physician’s right not to speak and goes far beyond any informed consent laws addressed by the U.S. Supreme Court or other courts to date.
“Legislation which forces physicians to tell their patients, as part of informed consent, that ‘it may be possible’ to reverse or cure an ailment, disease, illness, surgical procedure, or the effects of any medication – in the absence of any medical or scientific evidence to support such a message – is unsound, misplaced, and would not survive a constitutional challenge under any level of scrutiny,” Judge Hovland wrote in his ruling. “State legislatures should not be mandating unproven medical treatments, or requiring physicians to provide patients with misleading and inaccurate information.”
North Dakota Governor Doug Burgum (R), signed HB 1336 into law in March. The measure requires that physicians inform patients at least 24 hours before a medication abortion that it may be possible to reverse the effects if they change their mind, and also mandates that doctors provide printed materials to patients with information about reversing the effects of an abortion-inducing drug.
The American Medical Association and the Red River Women’s Clinic based in Fargo, issued a joint legal challenge against North Dakota Attorney General Wayne Stenehjem in June over the law. The plaintiffs contend the North Dakota law violates physicians’ First Amendment rights that protect doctors from being compelled to speak against their will. The plaintiffs also argued that so-called abortion reversals are based on controversial, unproven theories that are rejected by major medical organizations.
The lawsuit also challenges an existing North Dakota law called the Abortion Control Act, that requires physicians to tell patients that abortion terminates “the life of a whole, separate, unique, living human being,” which the plaintiffs contend is a controversial, ideological, and nonmedical message that forces physicians to act as the mouthpiece of the state. That part of the lawsuit was not addressed in Judge Hovland’s ruling.
AMA President Patrice A. Harris, MD, said the association was pleased that the judge blocked enforcement of HB 1336 while the case advances in the court system.
“The AMA filed this lawsuit in North Dakota because we strongly believe the government should not dictate what physicians say to their patients,” Dr. Harris said in a statement. “With this ruling, physicians in North Dakota will not be forced by law to provide patients with false, misleading, non-medical information about reproductive health that contradicts reality and science.”
A spokeswoman for Mr. Stenehjem’s office said the attorney general is reviewing the order and declined to comment further.
At least seven other states have passed similar laws requiring physicians to tell patients about the possibility of medication abortion reversals, including Arkansas, Idaho, Kentucky, Nebraska, Oklahoma, South Dakota, and Utah.
A district judge has temporarily blocked a North Dakota law that forces physicians to tell patients that medication abortions may be reversed, calling the measure devoid of scientific support.
In a Sept. 10 ruling, Judge Daniel Hovland of the U.S. District Court for the District of North Dakota temporarily halted enforcement of North Dakota House Bill 1336, a law that required doctors to tell women that reversing the effects of abortion-inducing drugs is possible if patients changed their minds, but that “time is of the essence.” In his 24-page decision, Judge Hovland said the North Dakota law violates a physician’s right not to speak and goes far beyond any informed consent laws addressed by the U.S. Supreme Court or other courts to date.
“Legislation which forces physicians to tell their patients, as part of informed consent, that ‘it may be possible’ to reverse or cure an ailment, disease, illness, surgical procedure, or the effects of any medication – in the absence of any medical or scientific evidence to support such a message – is unsound, misplaced, and would not survive a constitutional challenge under any level of scrutiny,” Judge Hovland wrote in his ruling. “State legislatures should not be mandating unproven medical treatments, or requiring physicians to provide patients with misleading and inaccurate information.”
North Dakota Governor Doug Burgum (R), signed HB 1336 into law in March. The measure requires that physicians inform patients at least 24 hours before a medication abortion that it may be possible to reverse the effects if they change their mind, and also mandates that doctors provide printed materials to patients with information about reversing the effects of an abortion-inducing drug.
The American Medical Association and the Red River Women’s Clinic based in Fargo, issued a joint legal challenge against North Dakota Attorney General Wayne Stenehjem in June over the law. The plaintiffs contend the North Dakota law violates physicians’ First Amendment rights that protect doctors from being compelled to speak against their will. The plaintiffs also argued that so-called abortion reversals are based on controversial, unproven theories that are rejected by major medical organizations.
The lawsuit also challenges an existing North Dakota law called the Abortion Control Act, that requires physicians to tell patients that abortion terminates “the life of a whole, separate, unique, living human being,” which the plaintiffs contend is a controversial, ideological, and nonmedical message that forces physicians to act as the mouthpiece of the state. That part of the lawsuit was not addressed in Judge Hovland’s ruling.
AMA President Patrice A. Harris, MD, said the association was pleased that the judge blocked enforcement of HB 1336 while the case advances in the court system.
“The AMA filed this lawsuit in North Dakota because we strongly believe the government should not dictate what physicians say to their patients,” Dr. Harris said in a statement. “With this ruling, physicians in North Dakota will not be forced by law to provide patients with false, misleading, non-medical information about reproductive health that contradicts reality and science.”
A spokeswoman for Mr. Stenehjem’s office said the attorney general is reviewing the order and declined to comment further.
At least seven other states have passed similar laws requiring physicians to tell patients about the possibility of medication abortion reversals, including Arkansas, Idaho, Kentucky, Nebraska, Oklahoma, South Dakota, and Utah.
A district judge has temporarily blocked a North Dakota law that forces physicians to tell patients that medication abortions may be reversed, calling the measure devoid of scientific support.
In a Sept. 10 ruling, Judge Daniel Hovland of the U.S. District Court for the District of North Dakota temporarily halted enforcement of North Dakota House Bill 1336, a law that required doctors to tell women that reversing the effects of abortion-inducing drugs is possible if patients changed their minds, but that “time is of the essence.” In his 24-page decision, Judge Hovland said the North Dakota law violates a physician’s right not to speak and goes far beyond any informed consent laws addressed by the U.S. Supreme Court or other courts to date.
“Legislation which forces physicians to tell their patients, as part of informed consent, that ‘it may be possible’ to reverse or cure an ailment, disease, illness, surgical procedure, or the effects of any medication – in the absence of any medical or scientific evidence to support such a message – is unsound, misplaced, and would not survive a constitutional challenge under any level of scrutiny,” Judge Hovland wrote in his ruling. “State legislatures should not be mandating unproven medical treatments, or requiring physicians to provide patients with misleading and inaccurate information.”
North Dakota Governor Doug Burgum (R), signed HB 1336 into law in March. The measure requires that physicians inform patients at least 24 hours before a medication abortion that it may be possible to reverse the effects if they change their mind, and also mandates that doctors provide printed materials to patients with information about reversing the effects of an abortion-inducing drug.
The American Medical Association and the Red River Women’s Clinic based in Fargo, issued a joint legal challenge against North Dakota Attorney General Wayne Stenehjem in June over the law. The plaintiffs contend the North Dakota law violates physicians’ First Amendment rights that protect doctors from being compelled to speak against their will. The plaintiffs also argued that so-called abortion reversals are based on controversial, unproven theories that are rejected by major medical organizations.
The lawsuit also challenges an existing North Dakota law called the Abortion Control Act, that requires physicians to tell patients that abortion terminates “the life of a whole, separate, unique, living human being,” which the plaintiffs contend is a controversial, ideological, and nonmedical message that forces physicians to act as the mouthpiece of the state. That part of the lawsuit was not addressed in Judge Hovland’s ruling.
AMA President Patrice A. Harris, MD, said the association was pleased that the judge blocked enforcement of HB 1336 while the case advances in the court system.
“The AMA filed this lawsuit in North Dakota because we strongly believe the government should not dictate what physicians say to their patients,” Dr. Harris said in a statement. “With this ruling, physicians in North Dakota will not be forced by law to provide patients with false, misleading, non-medical information about reproductive health that contradicts reality and science.”
A spokeswoman for Mr. Stenehjem’s office said the attorney general is reviewing the order and declined to comment further.
At least seven other states have passed similar laws requiring physicians to tell patients about the possibility of medication abortion reversals, including Arkansas, Idaho, Kentucky, Nebraska, Oklahoma, South Dakota, and Utah.
Lack of High-Quality Clinical Evidence Hampers Cannabis Treatment for PTSD
Researchers from University College London in the United Kingdom and University of Amsterdam in the Netherlands who conducted a systematic review of available research found a “striking” lack of evidence, considering the vast interest in the potential of the treatment and the “overwhelming demand by veterans.”
The researchers wanted to conduct a “fine-grained evaluation” of cannabinoid effectiveness in posttraumatic stress disorder (PTSD). They identified 10 studies investigating medicinal cannabinoids for patients with PTSD who were experiencing symptoms that were measured by a clinical psychometric, such as the Clinician-Administered PTSD Scale. Only 1 was a randomized, double-blind, placebo-controlled crossover clinical trial. Three studies used nabilone, a synthetic delta9-tetrahydrocannabinol (THC) analog; 1 used oral THC; 2 used cannabidiol (CBD) oil, and 4 used smoked herbal preparations of cannabis.
In line with previous reviews, the researchers found insufficient evidence to support the use of cannabinoids as a psychopharmacologic treatment for PTSD. In fact, they suggest that the lack of evidence poses a public health risk. However, the researchers say, this is mainly because the available support so far has been limited to small, “low-quality” studies, anecdotal reports, and some experimental evidence. There are reasons to keep investigating the possibilities, they conclude.
For instance, there is concurrence among studies that medicinal cannabinoids can help with sleep disturbances, and thus may be more effective, with less risk of addiction than benzodiazepines or opiate-based medications. Self-reports, anecdotal accounts, and case reports suggest that medical cannabis can dramatically reduce not only sleep symptoms, such as insomnia and nightmares, but may help with traumatic intrusions, hyperarousal, stress, anxiety, and depression.
The researchers also cite a study that found veterans who use cannabis believe it to be more effective and less complicated by adverse effects (AEs) than are alcohol and other psychopharmaceuticals. The AEs are generally mild to moderate, such as dry mouth and feeling “stoned,” but compared with the AEs of currently prescribed drugs are considered less burdensome.
Safety concerns are particularly critical in this population, though. Some research has shown that rates of cannabis use disorder are greater among patients who have PTSD compared with those who do not. A study of veterans admitted to US Department of Veterans Affairs treatment programs found recreational cannabis users with PTSD had poorer outcomes on severity of symptoms, violent behavior, and other drug use. Cannabinoids have also been associated with severe AEs in people with a history of psychosis—a consideration in combat veterans who have hallucinations or delusions.
Although they used strict inclusion criteria, the researchers say the studies they used still had “significant” limitations. Future well-controlled, randomized, double-blind clinical trials are highly warranted, they add, to address the “large unmet need” for effective PTSD treatments.
Researchers from University College London in the United Kingdom and University of Amsterdam in the Netherlands who conducted a systematic review of available research found a “striking” lack of evidence, considering the vast interest in the potential of the treatment and the “overwhelming demand by veterans.”
The researchers wanted to conduct a “fine-grained evaluation” of cannabinoid effectiveness in posttraumatic stress disorder (PTSD). They identified 10 studies investigating medicinal cannabinoids for patients with PTSD who were experiencing symptoms that were measured by a clinical psychometric, such as the Clinician-Administered PTSD Scale. Only 1 was a randomized, double-blind, placebo-controlled crossover clinical trial. Three studies used nabilone, a synthetic delta9-tetrahydrocannabinol (THC) analog; 1 used oral THC; 2 used cannabidiol (CBD) oil, and 4 used smoked herbal preparations of cannabis.
In line with previous reviews, the researchers found insufficient evidence to support the use of cannabinoids as a psychopharmacologic treatment for PTSD. In fact, they suggest that the lack of evidence poses a public health risk. However, the researchers say, this is mainly because the available support so far has been limited to small, “low-quality” studies, anecdotal reports, and some experimental evidence. There are reasons to keep investigating the possibilities, they conclude.
For instance, there is concurrence among studies that medicinal cannabinoids can help with sleep disturbances, and thus may be more effective, with less risk of addiction than benzodiazepines or opiate-based medications. Self-reports, anecdotal accounts, and case reports suggest that medical cannabis can dramatically reduce not only sleep symptoms, such as insomnia and nightmares, but may help with traumatic intrusions, hyperarousal, stress, anxiety, and depression.
The researchers also cite a study that found veterans who use cannabis believe it to be more effective and less complicated by adverse effects (AEs) than are alcohol and other psychopharmaceuticals. The AEs are generally mild to moderate, such as dry mouth and feeling “stoned,” but compared with the AEs of currently prescribed drugs are considered less burdensome.
Safety concerns are particularly critical in this population, though. Some research has shown that rates of cannabis use disorder are greater among patients who have PTSD compared with those who do not. A study of veterans admitted to US Department of Veterans Affairs treatment programs found recreational cannabis users with PTSD had poorer outcomes on severity of symptoms, violent behavior, and other drug use. Cannabinoids have also been associated with severe AEs in people with a history of psychosis—a consideration in combat veterans who have hallucinations or delusions.
Although they used strict inclusion criteria, the researchers say the studies they used still had “significant” limitations. Future well-controlled, randomized, double-blind clinical trials are highly warranted, they add, to address the “large unmet need” for effective PTSD treatments.
Researchers from University College London in the United Kingdom and University of Amsterdam in the Netherlands who conducted a systematic review of available research found a “striking” lack of evidence, considering the vast interest in the potential of the treatment and the “overwhelming demand by veterans.”
The researchers wanted to conduct a “fine-grained evaluation” of cannabinoid effectiveness in posttraumatic stress disorder (PTSD). They identified 10 studies investigating medicinal cannabinoids for patients with PTSD who were experiencing symptoms that were measured by a clinical psychometric, such as the Clinician-Administered PTSD Scale. Only 1 was a randomized, double-blind, placebo-controlled crossover clinical trial. Three studies used nabilone, a synthetic delta9-tetrahydrocannabinol (THC) analog; 1 used oral THC; 2 used cannabidiol (CBD) oil, and 4 used smoked herbal preparations of cannabis.
In line with previous reviews, the researchers found insufficient evidence to support the use of cannabinoids as a psychopharmacologic treatment for PTSD. In fact, they suggest that the lack of evidence poses a public health risk. However, the researchers say, this is mainly because the available support so far has been limited to small, “low-quality” studies, anecdotal reports, and some experimental evidence. There are reasons to keep investigating the possibilities, they conclude.
For instance, there is concurrence among studies that medicinal cannabinoids can help with sleep disturbances, and thus may be more effective, with less risk of addiction than benzodiazepines or opiate-based medications. Self-reports, anecdotal accounts, and case reports suggest that medical cannabis can dramatically reduce not only sleep symptoms, such as insomnia and nightmares, but may help with traumatic intrusions, hyperarousal, stress, anxiety, and depression.
The researchers also cite a study that found veterans who use cannabis believe it to be more effective and less complicated by adverse effects (AEs) than are alcohol and other psychopharmaceuticals. The AEs are generally mild to moderate, such as dry mouth and feeling “stoned,” but compared with the AEs of currently prescribed drugs are considered less burdensome.
Safety concerns are particularly critical in this population, though. Some research has shown that rates of cannabis use disorder are greater among patients who have PTSD compared with those who do not. A study of veterans admitted to US Department of Veterans Affairs treatment programs found recreational cannabis users with PTSD had poorer outcomes on severity of symptoms, violent behavior, and other drug use. Cannabinoids have also been associated with severe AEs in people with a history of psychosis—a consideration in combat veterans who have hallucinations or delusions.
Although they used strict inclusion criteria, the researchers say the studies they used still had “significant” limitations. Future well-controlled, randomized, double-blind clinical trials are highly warranted, they add, to address the “large unmet need” for effective PTSD treatments.
Antimalarial exposure above recommended doses in lupus contributes most retinopathy risk
Antimalarial retinal complications occurred in about 5% of patients with systemic lupus erythematosus (SLE) exposed to hydroxychloroquine or chloroquine during an average of nearly 13 years of follow-up, without any cases of retinal toxicity occurring within the first 5 years of use, according to findings from a case-control study.
The rate of retinal complications observed in the study is slightly lower or within the range reported in other studies of antimalarial use by patients with SLE, first author Elvis-Raymond Mukwikwi of the University of Montreal and coauthors from McGill University, Montreal, reported in the Journal of Rheumatology.
Hydroxychloroquine (HCQ) and chloroquine (CQ) are commonly used to treat SLE and rheumatoid arthritis, and they are being investigated for use in diabetes, cancer, and cardiovascular disease. However, in rare cases, the drugs can lead to irreversible retinopathy.
To better understand the factors associated with retinopathy, the researchers analyzed data from 326 records obtained from the McGill University Health Center Lupus Clinic Registry. A total of 18 patients (5.5%) had confirmed retinal toxicity, and the investigators matched each of these patients to 3 control patients with SLE and exposure to HCQ/CQ who did not develop retinopathy and had the same duration of SLE and age at SLE diagnosis.
The minimum number of years of HCQ/CQ exposure before retinopathy developed was 8 years, and the maximum number was 33 years. Overall, 17 retinopathy cases had exposure to HCQ, and 12 of those cases (71%) received average doses higher than current recommendations. Eight patients were exposed to average daily doses of HCQ higher than 5 mg/kg and four to average daily doses of CQ above 2.3 mg/kg. Exposure to an average dose higher than currently recommended occurred in 49% of controls. The exposure to doses higher than current recommendations was “not surprising given that these were issued in 2016 and most of our patients had been taking HCQ for longer than this,” the authors wrote.
High-dose exposure was common, with 83% of controls and all retinopathy cases having been exposed to HCQ/CQ doses above recommendations, as determined during at least one annual assessment.
Among patients with retinopathy, exposure to CQ was three times more frequent than it was for those without the condition (39% vs. 13%; 95% confidence interval, 1.8%-52%), although all of the patients exposed to CQ also were exposed to long periods of HCQ, making it impossible to determine retinopathy risk to CQ exposure alone.
The researchers also found gaps in monitoring. In the 5 years before discontinuation of medication, 53% of cases had missed one or more annual ophthalmologic assessments to screen for retinal damage, compared with 75% of controls.
Concomitant renal damage, believed to be a risk factor for retinal toxicity, occurred more often in the retinopathy group, though the difference failed to meet statistical significance (23% versus 15%). Patients with retinopathy were less likely to be Caucasian (61% versus 74%), but this also did not reach statistical significance.
The authors did not disclose information on funding or conflicts of interest.
SOURCE: Mukwikwi ER et al. J Rheumatol. 2019 Sep 1. doi: 10.3899/jrheum.181102
Antimalarial retinal complications occurred in about 5% of patients with systemic lupus erythematosus (SLE) exposed to hydroxychloroquine or chloroquine during an average of nearly 13 years of follow-up, without any cases of retinal toxicity occurring within the first 5 years of use, according to findings from a case-control study.
The rate of retinal complications observed in the study is slightly lower or within the range reported in other studies of antimalarial use by patients with SLE, first author Elvis-Raymond Mukwikwi of the University of Montreal and coauthors from McGill University, Montreal, reported in the Journal of Rheumatology.
Hydroxychloroquine (HCQ) and chloroquine (CQ) are commonly used to treat SLE and rheumatoid arthritis, and they are being investigated for use in diabetes, cancer, and cardiovascular disease. However, in rare cases, the drugs can lead to irreversible retinopathy.
To better understand the factors associated with retinopathy, the researchers analyzed data from 326 records obtained from the McGill University Health Center Lupus Clinic Registry. A total of 18 patients (5.5%) had confirmed retinal toxicity, and the investigators matched each of these patients to 3 control patients with SLE and exposure to HCQ/CQ who did not develop retinopathy and had the same duration of SLE and age at SLE diagnosis.
The minimum number of years of HCQ/CQ exposure before retinopathy developed was 8 years, and the maximum number was 33 years. Overall, 17 retinopathy cases had exposure to HCQ, and 12 of those cases (71%) received average doses higher than current recommendations. Eight patients were exposed to average daily doses of HCQ higher than 5 mg/kg and four to average daily doses of CQ above 2.3 mg/kg. Exposure to an average dose higher than currently recommended occurred in 49% of controls. The exposure to doses higher than current recommendations was “not surprising given that these were issued in 2016 and most of our patients had been taking HCQ for longer than this,” the authors wrote.
High-dose exposure was common, with 83% of controls and all retinopathy cases having been exposed to HCQ/CQ doses above recommendations, as determined during at least one annual assessment.
Among patients with retinopathy, exposure to CQ was three times more frequent than it was for those without the condition (39% vs. 13%; 95% confidence interval, 1.8%-52%), although all of the patients exposed to CQ also were exposed to long periods of HCQ, making it impossible to determine retinopathy risk to CQ exposure alone.
The researchers also found gaps in monitoring. In the 5 years before discontinuation of medication, 53% of cases had missed one or more annual ophthalmologic assessments to screen for retinal damage, compared with 75% of controls.
Concomitant renal damage, believed to be a risk factor for retinal toxicity, occurred more often in the retinopathy group, though the difference failed to meet statistical significance (23% versus 15%). Patients with retinopathy were less likely to be Caucasian (61% versus 74%), but this also did not reach statistical significance.
The authors did not disclose information on funding or conflicts of interest.
SOURCE: Mukwikwi ER et al. J Rheumatol. 2019 Sep 1. doi: 10.3899/jrheum.181102
Antimalarial retinal complications occurred in about 5% of patients with systemic lupus erythematosus (SLE) exposed to hydroxychloroquine or chloroquine during an average of nearly 13 years of follow-up, without any cases of retinal toxicity occurring within the first 5 years of use, according to findings from a case-control study.
The rate of retinal complications observed in the study is slightly lower or within the range reported in other studies of antimalarial use by patients with SLE, first author Elvis-Raymond Mukwikwi of the University of Montreal and coauthors from McGill University, Montreal, reported in the Journal of Rheumatology.
Hydroxychloroquine (HCQ) and chloroquine (CQ) are commonly used to treat SLE and rheumatoid arthritis, and they are being investigated for use in diabetes, cancer, and cardiovascular disease. However, in rare cases, the drugs can lead to irreversible retinopathy.
To better understand the factors associated with retinopathy, the researchers analyzed data from 326 records obtained from the McGill University Health Center Lupus Clinic Registry. A total of 18 patients (5.5%) had confirmed retinal toxicity, and the investigators matched each of these patients to 3 control patients with SLE and exposure to HCQ/CQ who did not develop retinopathy and had the same duration of SLE and age at SLE diagnosis.
The minimum number of years of HCQ/CQ exposure before retinopathy developed was 8 years, and the maximum number was 33 years. Overall, 17 retinopathy cases had exposure to HCQ, and 12 of those cases (71%) received average doses higher than current recommendations. Eight patients were exposed to average daily doses of HCQ higher than 5 mg/kg and four to average daily doses of CQ above 2.3 mg/kg. Exposure to an average dose higher than currently recommended occurred in 49% of controls. The exposure to doses higher than current recommendations was “not surprising given that these were issued in 2016 and most of our patients had been taking HCQ for longer than this,” the authors wrote.
High-dose exposure was common, with 83% of controls and all retinopathy cases having been exposed to HCQ/CQ doses above recommendations, as determined during at least one annual assessment.
Among patients with retinopathy, exposure to CQ was three times more frequent than it was for those without the condition (39% vs. 13%; 95% confidence interval, 1.8%-52%), although all of the patients exposed to CQ also were exposed to long periods of HCQ, making it impossible to determine retinopathy risk to CQ exposure alone.
The researchers also found gaps in monitoring. In the 5 years before discontinuation of medication, 53% of cases had missed one or more annual ophthalmologic assessments to screen for retinal damage, compared with 75% of controls.
Concomitant renal damage, believed to be a risk factor for retinal toxicity, occurred more often in the retinopathy group, though the difference failed to meet statistical significance (23% versus 15%). Patients with retinopathy were less likely to be Caucasian (61% versus 74%), but this also did not reach statistical significance.
The authors did not disclose information on funding or conflicts of interest.
SOURCE: Mukwikwi ER et al. J Rheumatol. 2019 Sep 1. doi: 10.3899/jrheum.181102
FROM THE JOURNAL OF RHEUMATOLOGY
Severe Pretibial Myxedema Refractory to Systemic Immunosuppressants
To the Editor:
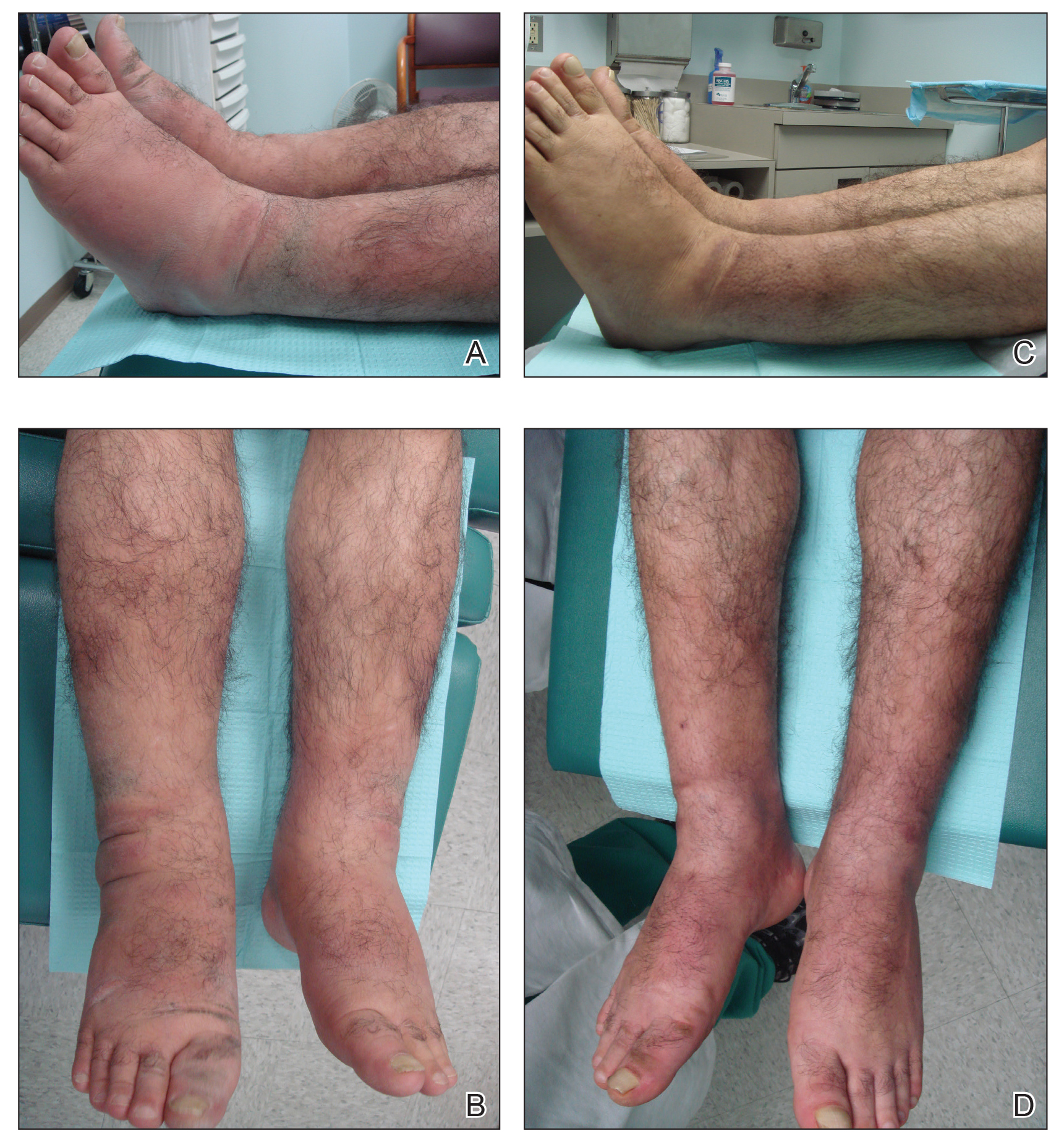
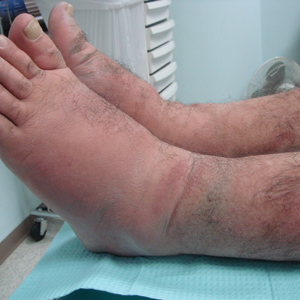
A 55-year-old man with a history of Graves disease treated with radioactive iodine and Graves ophthalmopathy was referred to our dermatology clinic by his endocrinologist with a 2-year history of severe pretibial myxedema (PM) that had failed treatment with systemic immunosuppressants after diagnosis by an outside dermatologist in the United Kingdom approximately 2 years prior. In addition to burning pain and difficulty walking associated with progressive “enlarging” of the lower legs and feet (Figure, A and B), the patient reported that he consistently had to find larger shoes (size 13 at the current presentation). His medications included gabapentin for foot pain and levothyroxine for hypothyroidism.
Physical examination revealed diffuse, waxy, indurated, flesh-colored and erythematous plaques and nodules with a peau d’orange appearance on the dorsal feet, ankles, and lower legs. Laboratory evaluation revealed a thyroid stimulating immunoglobulin level of 617% (reference range, <140%) and mild anemia. His thyroid stimulating hormone and free T4 levels, a comprehensive metabolic panel, and lipid panel were all within reference range.
Treatment with oral, intravenous, and intralesional steroids; cyclosporine; and azathioprine were tried prior to presentation to our clinic with no improvement. The patient was started on pentoxifylline (400 mg 3 times daily), intralesional triamcinolone acetonide (5 mg/mL every 3–4 weeks), clobetasol propionate ointment 0.05% under occlusion twice daily, short-stretch bandages, and compression stockings (20–30 mm Hg). The baseline circumference of the extremities also were measured (right ankle, 12 in; left ankle, 11.5 in; right and left mid-plantar feet, 12 in).
At 3-week follow-up, the lesions had flattened with softening of the skin. The patient reported his legs were smaller and he had bought a new pair of shoes at size 8.5 (Figure, C). He noted less pain and difficulty with walking. The circumference of the extremities was measured again (right ankle, 10.2 in; left ankle, 10 in; right and left mid-plantar feet, 10.5 in). The patient continued treatment and was followed for 3 months. At each visit, clinical improvement was noted as well as report of decreased pain while walking (Figure, D).
Pretibial myxedema is a known manifestation of Graves disease that almost always occurs in the presence of Graves ophthalmopathy. Pretibial myxedema occurs in 0.5% to 4.3% of patients with Graves disease and variably manifests as diffuse nonpitting edema or localized, waxy, indurated plaques or nodules.1,2
The proposed pathogenesis of PM is that autoantibodies directed against the thyroid receptors cross-react with the fibroblasts of the skin,2,3 which stimulates the fibroblasts to produce high amounts of glycosaminoglycans, especially hyaluronic acid, in the dermis and subcutis of the pretibial area. It is not known why there is a predilection for the anterior shins, but mechanical factors and dependent position (ie, leg position is lower than the level of the heart) may be involved.4
The mainstay of treatment for PM is topical and intralesional corticosteroids, which may have a benefit in mild to moderate disease; however, in cases of severe disease that is refractory to intralesional and topical corticosteroids under occlusion, more aggressive treatment is required. Systemic immunosuppressants such as cyclosporine, azathioprine, and corticosteroids have proven useful in some but not all cases.5,6
Our patient did not respond to treatment with systemic and intralesional corticosteroids, cyclosporine, or azathioprine before he presented to our clinic; however, the lesions were dramatically improved after 3 weeks of treatment with pentoxifylline, intralesional and topical corticosteroids under occlusion, short-stretch bandages, and compression stockings.
Pentoxifylline inhibits the proliferation and glycosaminoglycan synthesis of cultured fibroblasts derived from patients with Graves ophthalmology and PM.7 It has been shown to reduce thickness of skin lesions when used in combination with topical or intralesional steroids.3,8 Corticosteroids are thought to block fibroblast-mediated glycosaminoglycan production.3,9 The deposition of mucin, which is comprised of glycosaminoglycans, expands the dermal tissue and causes fluid to accumulate; it also causes compression of dermal lymphatics, worsening the dermal edema. Because fluid accumulates, the use of short-stretch bandages and compression stockings may provide additional benefit, as was seen in our patient, whose shoe size decreased from a 13 to an 8.5 within 3 weeks of treatment.
In conclusion, the combination of pentoxifylline, intralesional and topical corticosteroids under occlusion, short-stretch bandages, and compression garments can cause substantial improvement in severe PM refractory to systemic immunosuppressants.
- Susser WS, Heermans AG, Chapman MS, et al. Elephantiasic pretibial myxedema: a novel treatment for an uncommon disorder. J Am Acad Dermatol. 2002;46:723-726.
- Kriss J. Pathogenesis and treatment of pretibial myxedema. Endocrinol Metab Clin North Am. 1987;16:409-415.
- Pineda AM, Tianco EA, Tan JB, et al. Oral pentoxifylline and topical clobetasol propionate ointment in the treatment of pretibial myxoedema, with concomitant improvement of Graves’ ophthalmopathy. J Eur Acad Dermatol Venereol. 2007; 21:1441-1443.
- Fatourechi V. Pretibial myxedema. Am J Clin Dermatol. 2005;6:295-309.
- Benoit FL, Greenspan FS. Corticoid therapy for pretibial myxedema: observations on the long-acting thyroid stimulator. Ann Intern Med. 1967;66:711-720.
- Hanke CW, Bergfeld WF, Guirguis MN, et al. Pretibial myxedema (elephantiasis form): treatment with cytotoxic therapy. Cleve Clin Q. 1983;50:183-188.
- Chang CC, Chang TC, Kao SC, et al. Pentoxifylline inhibits the proliferation and glycosaminoglycan synthesis of cultured fibroblasts derived from patients with Graves’ ophthalmopathy and pretibial myxoedema. Acta Endocrinol (Copenh). 1993;129:322-327.
- Engin B, Gümüs¸el M, Ozdemir M, et al. Successful combined pentoxifylline and intralesional triamcinolone acetonide treatment of severe pretibial myxedema. Dermatol Online J. 2007;13:16.
- Lang PG, Sisson JC, Lynch PJ. Intralesional triamcinolone therapy for pretibial myxedema. Arch Dermatol. 1975;111:197-202.
To the Editor:
A 55-year-old man with a history of Graves disease treated with radioactive iodine and Graves ophthalmopathy was referred to our dermatology clinic by his endocrinologist with a 2-year history of severe pretibial myxedema (PM) that had failed treatment with systemic immunosuppressants after diagnosis by an outside dermatologist in the United Kingdom approximately 2 years prior. In addition to burning pain and difficulty walking associated with progressive “enlarging” of the lower legs and feet (Figure, A and B), the patient reported that he consistently had to find larger shoes (size 13 at the current presentation). His medications included gabapentin for foot pain and levothyroxine for hypothyroidism.
Physical examination revealed diffuse, waxy, indurated, flesh-colored and erythematous plaques and nodules with a peau d’orange appearance on the dorsal feet, ankles, and lower legs. Laboratory evaluation revealed a thyroid stimulating immunoglobulin level of 617% (reference range, <140%) and mild anemia. His thyroid stimulating hormone and free T4 levels, a comprehensive metabolic panel, and lipid panel were all within reference range.
Treatment with oral, intravenous, and intralesional steroids; cyclosporine; and azathioprine were tried prior to presentation to our clinic with no improvement. The patient was started on pentoxifylline (400 mg 3 times daily), intralesional triamcinolone acetonide (5 mg/mL every 3–4 weeks), clobetasol propionate ointment 0.05% under occlusion twice daily, short-stretch bandages, and compression stockings (20–30 mm Hg). The baseline circumference of the extremities also were measured (right ankle, 12 in; left ankle, 11.5 in; right and left mid-plantar feet, 12 in).
At 3-week follow-up, the lesions had flattened with softening of the skin. The patient reported his legs were smaller and he had bought a new pair of shoes at size 8.5 (Figure, C). He noted less pain and difficulty with walking. The circumference of the extremities was measured again (right ankle, 10.2 in; left ankle, 10 in; right and left mid-plantar feet, 10.5 in). The patient continued treatment and was followed for 3 months. At each visit, clinical improvement was noted as well as report of decreased pain while walking (Figure, D).
Pretibial myxedema is a known manifestation of Graves disease that almost always occurs in the presence of Graves ophthalmopathy. Pretibial myxedema occurs in 0.5% to 4.3% of patients with Graves disease and variably manifests as diffuse nonpitting edema or localized, waxy, indurated plaques or nodules.1,2
The proposed pathogenesis of PM is that autoantibodies directed against the thyroid receptors cross-react with the fibroblasts of the skin,2,3 which stimulates the fibroblasts to produce high amounts of glycosaminoglycans, especially hyaluronic acid, in the dermis and subcutis of the pretibial area. It is not known why there is a predilection for the anterior shins, but mechanical factors and dependent position (ie, leg position is lower than the level of the heart) may be involved.4
The mainstay of treatment for PM is topical and intralesional corticosteroids, which may have a benefit in mild to moderate disease; however, in cases of severe disease that is refractory to intralesional and topical corticosteroids under occlusion, more aggressive treatment is required. Systemic immunosuppressants such as cyclosporine, azathioprine, and corticosteroids have proven useful in some but not all cases.5,6
Our patient did not respond to treatment with systemic and intralesional corticosteroids, cyclosporine, or azathioprine before he presented to our clinic; however, the lesions were dramatically improved after 3 weeks of treatment with pentoxifylline, intralesional and topical corticosteroids under occlusion, short-stretch bandages, and compression stockings.
Pentoxifylline inhibits the proliferation and glycosaminoglycan synthesis of cultured fibroblasts derived from patients with Graves ophthalmology and PM.7 It has been shown to reduce thickness of skin lesions when used in combination with topical or intralesional steroids.3,8 Corticosteroids are thought to block fibroblast-mediated glycosaminoglycan production.3,9 The deposition of mucin, which is comprised of glycosaminoglycans, expands the dermal tissue and causes fluid to accumulate; it also causes compression of dermal lymphatics, worsening the dermal edema. Because fluid accumulates, the use of short-stretch bandages and compression stockings may provide additional benefit, as was seen in our patient, whose shoe size decreased from a 13 to an 8.5 within 3 weeks of treatment.
In conclusion, the combination of pentoxifylline, intralesional and topical corticosteroids under occlusion, short-stretch bandages, and compression garments can cause substantial improvement in severe PM refractory to systemic immunosuppressants.
To the Editor:
A 55-year-old man with a history of Graves disease treated with radioactive iodine and Graves ophthalmopathy was referred to our dermatology clinic by his endocrinologist with a 2-year history of severe pretibial myxedema (PM) that had failed treatment with systemic immunosuppressants after diagnosis by an outside dermatologist in the United Kingdom approximately 2 years prior. In addition to burning pain and difficulty walking associated with progressive “enlarging” of the lower legs and feet (Figure, A and B), the patient reported that he consistently had to find larger shoes (size 13 at the current presentation). His medications included gabapentin for foot pain and levothyroxine for hypothyroidism.
Physical examination revealed diffuse, waxy, indurated, flesh-colored and erythematous plaques and nodules with a peau d’orange appearance on the dorsal feet, ankles, and lower legs. Laboratory evaluation revealed a thyroid stimulating immunoglobulin level of 617% (reference range, <140%) and mild anemia. His thyroid stimulating hormone and free T4 levels, a comprehensive metabolic panel, and lipid panel were all within reference range.
Treatment with oral, intravenous, and intralesional steroids; cyclosporine; and azathioprine were tried prior to presentation to our clinic with no improvement. The patient was started on pentoxifylline (400 mg 3 times daily), intralesional triamcinolone acetonide (5 mg/mL every 3–4 weeks), clobetasol propionate ointment 0.05% under occlusion twice daily, short-stretch bandages, and compression stockings (20–30 mm Hg). The baseline circumference of the extremities also were measured (right ankle, 12 in; left ankle, 11.5 in; right and left mid-plantar feet, 12 in).
At 3-week follow-up, the lesions had flattened with softening of the skin. The patient reported his legs were smaller and he had bought a new pair of shoes at size 8.5 (Figure, C). He noted less pain and difficulty with walking. The circumference of the extremities was measured again (right ankle, 10.2 in; left ankle, 10 in; right and left mid-plantar feet, 10.5 in). The patient continued treatment and was followed for 3 months. At each visit, clinical improvement was noted as well as report of decreased pain while walking (Figure, D).
Pretibial myxedema is a known manifestation of Graves disease that almost always occurs in the presence of Graves ophthalmopathy. Pretibial myxedema occurs in 0.5% to 4.3% of patients with Graves disease and variably manifests as diffuse nonpitting edema or localized, waxy, indurated plaques or nodules.1,2
The proposed pathogenesis of PM is that autoantibodies directed against the thyroid receptors cross-react with the fibroblasts of the skin,2,3 which stimulates the fibroblasts to produce high amounts of glycosaminoglycans, especially hyaluronic acid, in the dermis and subcutis of the pretibial area. It is not known why there is a predilection for the anterior shins, but mechanical factors and dependent position (ie, leg position is lower than the level of the heart) may be involved.4
The mainstay of treatment for PM is topical and intralesional corticosteroids, which may have a benefit in mild to moderate disease; however, in cases of severe disease that is refractory to intralesional and topical corticosteroids under occlusion, more aggressive treatment is required. Systemic immunosuppressants such as cyclosporine, azathioprine, and corticosteroids have proven useful in some but not all cases.5,6
Our patient did not respond to treatment with systemic and intralesional corticosteroids, cyclosporine, or azathioprine before he presented to our clinic; however, the lesions were dramatically improved after 3 weeks of treatment with pentoxifylline, intralesional and topical corticosteroids under occlusion, short-stretch bandages, and compression stockings.
Pentoxifylline inhibits the proliferation and glycosaminoglycan synthesis of cultured fibroblasts derived from patients with Graves ophthalmology and PM.7 It has been shown to reduce thickness of skin lesions when used in combination with topical or intralesional steroids.3,8 Corticosteroids are thought to block fibroblast-mediated glycosaminoglycan production.3,9 The deposition of mucin, which is comprised of glycosaminoglycans, expands the dermal tissue and causes fluid to accumulate; it also causes compression of dermal lymphatics, worsening the dermal edema. Because fluid accumulates, the use of short-stretch bandages and compression stockings may provide additional benefit, as was seen in our patient, whose shoe size decreased from a 13 to an 8.5 within 3 weeks of treatment.
In conclusion, the combination of pentoxifylline, intralesional and topical corticosteroids under occlusion, short-stretch bandages, and compression garments can cause substantial improvement in severe PM refractory to systemic immunosuppressants.
- Susser WS, Heermans AG, Chapman MS, et al. Elephantiasic pretibial myxedema: a novel treatment for an uncommon disorder. J Am Acad Dermatol. 2002;46:723-726.
- Kriss J. Pathogenesis and treatment of pretibial myxedema. Endocrinol Metab Clin North Am. 1987;16:409-415.
- Pineda AM, Tianco EA, Tan JB, et al. Oral pentoxifylline and topical clobetasol propionate ointment in the treatment of pretibial myxoedema, with concomitant improvement of Graves’ ophthalmopathy. J Eur Acad Dermatol Venereol. 2007; 21:1441-1443.
- Fatourechi V. Pretibial myxedema. Am J Clin Dermatol. 2005;6:295-309.
- Benoit FL, Greenspan FS. Corticoid therapy for pretibial myxedema: observations on the long-acting thyroid stimulator. Ann Intern Med. 1967;66:711-720.
- Hanke CW, Bergfeld WF, Guirguis MN, et al. Pretibial myxedema (elephantiasis form): treatment with cytotoxic therapy. Cleve Clin Q. 1983;50:183-188.
- Chang CC, Chang TC, Kao SC, et al. Pentoxifylline inhibits the proliferation and glycosaminoglycan synthesis of cultured fibroblasts derived from patients with Graves’ ophthalmopathy and pretibial myxoedema. Acta Endocrinol (Copenh). 1993;129:322-327.
- Engin B, Gümüs¸el M, Ozdemir M, et al. Successful combined pentoxifylline and intralesional triamcinolone acetonide treatment of severe pretibial myxedema. Dermatol Online J. 2007;13:16.
- Lang PG, Sisson JC, Lynch PJ. Intralesional triamcinolone therapy for pretibial myxedema. Arch Dermatol. 1975;111:197-202.
- Susser WS, Heermans AG, Chapman MS, et al. Elephantiasic pretibial myxedema: a novel treatment for an uncommon disorder. J Am Acad Dermatol. 2002;46:723-726.
- Kriss J. Pathogenesis and treatment of pretibial myxedema. Endocrinol Metab Clin North Am. 1987;16:409-415.
- Pineda AM, Tianco EA, Tan JB, et al. Oral pentoxifylline and topical clobetasol propionate ointment in the treatment of pretibial myxoedema, with concomitant improvement of Graves’ ophthalmopathy. J Eur Acad Dermatol Venereol. 2007; 21:1441-1443.
- Fatourechi V. Pretibial myxedema. Am J Clin Dermatol. 2005;6:295-309.
- Benoit FL, Greenspan FS. Corticoid therapy for pretibial myxedema: observations on the long-acting thyroid stimulator. Ann Intern Med. 1967;66:711-720.
- Hanke CW, Bergfeld WF, Guirguis MN, et al. Pretibial myxedema (elephantiasis form): treatment with cytotoxic therapy. Cleve Clin Q. 1983;50:183-188.
- Chang CC, Chang TC, Kao SC, et al. Pentoxifylline inhibits the proliferation and glycosaminoglycan synthesis of cultured fibroblasts derived from patients with Graves’ ophthalmopathy and pretibial myxoedema. Acta Endocrinol (Copenh). 1993;129:322-327.
- Engin B, Gümüs¸el M, Ozdemir M, et al. Successful combined pentoxifylline and intralesional triamcinolone acetonide treatment of severe pretibial myxedema. Dermatol Online J. 2007;13:16.
- Lang PG, Sisson JC, Lynch PJ. Intralesional triamcinolone therapy for pretibial myxedema. Arch Dermatol. 1975;111:197-202.
Practice Points
- Pretibial myxedema (PM) is a known manifestation of Graves disease that almost always occurs in the presence of Graves ophthalmopathy.
- The proposed pathogenesis of PM is cross-reaction of autoantibodies directed against the thyroid receptors with the fibroblasts of the skin. It is not known why there is a predilection for the anterior shins, but mechanical factors and dependent position may be involved.
- The mainstay of treatment for PM is topical and intralesional corticosteroids, which may have a benefit in mild to moderate disease; however, in cases of severe disease that is refractory to intralesional and topical corticosteroids under occlusion, more aggressive treatment is required.
Expert spotlights telltale clinical signs of xeroderma pigmentosum
AUSTIN, TEX. – If a child presents with acute (XP), a rare autosomal recessive disorder.
Other telltale symptoms of XP include the presence of skin cancer at an early age and a large number of skin cancers.
At the annual meeting of the Society for Pediatric Dermatology, John J. DiGiovanna, MD, described XP as a disorder of genomic instability, which has no cure. It’s caused by a mutation in genes XPA through XPG and the XP variant (XPV) gene. “The genome controls our genes, and UV rays damage DNA,” said Dr. DiGiovanna, who is a senior research physician at the National Cancer Institute’s Laboratory of Cancer and Biology and Genetics, Bethesda, Md. “This damage from UV radiation is similar to damage from chemical agents that form DNA adducts, such as cigarette smoke and certain chemotherapy agents such as cisplatinum.”
XP patients present with or without acute burning after minimal sun exposure, while children with both subtypes develop “freckling” by the time they reach 2 years of age. Dr. DiGiovanna pointed out that lentigo maligna lesions associated with XP resemble freckles at first glance, yet they vary in size, intensity, and border. Meanwhile, freckles in healthy patients are similar in size, are light tan in color, and have a regular border.
“The burning with minimal sun exposure that occurs during childhood leads to pigmentary changes, atrophy, xerosis, and telangiectasias,” he said. A follow-up analysis of 106 XP patients admitted to the National Institutes of Health between 1971 and 2009 found that patients were diagnosed with their first nonmelanoma skin cancer at a median age of 9 years, compared with age 67 among those in the general population (J Med Genet 2011 Mar;48[3]:168-76). “This is a 58-year decrease in age at risk, which is a 10,000-fold increase in skin cancer,” said Dr. DiGiovanna, who was one of the study authors.
Melanoma also occurs at an earlier age among XP patients – a median age of 22 years, compared with a median of 55 years in the general population. “In the general population, melanoma occurs at a younger age than nonmelanoma skin cancer, while in the XP population, melanoma occurs at an older age,” he said. “This is giving us a good biologic lesson that the melanoma induction mechanism must be different from nonmelanoma skin cancer.”
He recalled one XP patient who was followed by NIH researchers for 4 decades. She worked in a doctor’s office and drove a car, but developed progressive neurologic degeneration and died at the age of 40. “This was not due to unrepaired UV damage, but there are other agents which damage other neurons,” Dr. DiGiovanna explained. “Over time, what you get is a decrease in brain volume, an increase in the brain ventricles, and a loss of brain tissue. At postmortem examination, her brain was of infantile size, compared with that of an equivalent 40-year-old. This is a disease of neuronal loss, and it’s progressive. Only about 20%-25% of XP patients experience neural degeneration.”
Management of XP involves strict sun avoidance, including use of a portable UV meter and many layers of UV protection, including application of sunscreen, wearing protective clothing, sunglasses, hats, and face shields, and the use of UV-blocking window film, LED lights, and a vitamin D diet or oral supplementation. Affected individuals also require frequent skin monitoring by the patients and their family members, frequent dermatologic exams by clinicians, biopsy of suspicious lesions, removal of any skin cancers found, field treatments with agents such as 5-fluorouracil and imiquimod, and chemoprevention with oral retinoids for patients who are actively developing large numbers of new lesions (N Engl J Med. 1988 Jun23;318[25]:1633-7).
“Probably the most important thing you can do is refer them to patient support groups,” Dr. DiGiovanna said. “They are present in many countries and can help them manage the day-to-day issues of their condition.” Support groups based in North America include the XP Family Support Group, XP Society, and XP Grupo Luz De Esperanza.
Dr. DiGiovanna reported having no financial disclosures.
AUSTIN, TEX. – If a child presents with acute (XP), a rare autosomal recessive disorder.
Other telltale symptoms of XP include the presence of skin cancer at an early age and a large number of skin cancers.
At the annual meeting of the Society for Pediatric Dermatology, John J. DiGiovanna, MD, described XP as a disorder of genomic instability, which has no cure. It’s caused by a mutation in genes XPA through XPG and the XP variant (XPV) gene. “The genome controls our genes, and UV rays damage DNA,” said Dr. DiGiovanna, who is a senior research physician at the National Cancer Institute’s Laboratory of Cancer and Biology and Genetics, Bethesda, Md. “This damage from UV radiation is similar to damage from chemical agents that form DNA adducts, such as cigarette smoke and certain chemotherapy agents such as cisplatinum.”
XP patients present with or without acute burning after minimal sun exposure, while children with both subtypes develop “freckling” by the time they reach 2 years of age. Dr. DiGiovanna pointed out that lentigo maligna lesions associated with XP resemble freckles at first glance, yet they vary in size, intensity, and border. Meanwhile, freckles in healthy patients are similar in size, are light tan in color, and have a regular border.
“The burning with minimal sun exposure that occurs during childhood leads to pigmentary changes, atrophy, xerosis, and telangiectasias,” he said. A follow-up analysis of 106 XP patients admitted to the National Institutes of Health between 1971 and 2009 found that patients were diagnosed with their first nonmelanoma skin cancer at a median age of 9 years, compared with age 67 among those in the general population (J Med Genet 2011 Mar;48[3]:168-76). “This is a 58-year decrease in age at risk, which is a 10,000-fold increase in skin cancer,” said Dr. DiGiovanna, who was one of the study authors.
Melanoma also occurs at an earlier age among XP patients – a median age of 22 years, compared with a median of 55 years in the general population. “In the general population, melanoma occurs at a younger age than nonmelanoma skin cancer, while in the XP population, melanoma occurs at an older age,” he said. “This is giving us a good biologic lesson that the melanoma induction mechanism must be different from nonmelanoma skin cancer.”
He recalled one XP patient who was followed by NIH researchers for 4 decades. She worked in a doctor’s office and drove a car, but developed progressive neurologic degeneration and died at the age of 40. “This was not due to unrepaired UV damage, but there are other agents which damage other neurons,” Dr. DiGiovanna explained. “Over time, what you get is a decrease in brain volume, an increase in the brain ventricles, and a loss of brain tissue. At postmortem examination, her brain was of infantile size, compared with that of an equivalent 40-year-old. This is a disease of neuronal loss, and it’s progressive. Only about 20%-25% of XP patients experience neural degeneration.”
Management of XP involves strict sun avoidance, including use of a portable UV meter and many layers of UV protection, including application of sunscreen, wearing protective clothing, sunglasses, hats, and face shields, and the use of UV-blocking window film, LED lights, and a vitamin D diet or oral supplementation. Affected individuals also require frequent skin monitoring by the patients and their family members, frequent dermatologic exams by clinicians, biopsy of suspicious lesions, removal of any skin cancers found, field treatments with agents such as 5-fluorouracil and imiquimod, and chemoprevention with oral retinoids for patients who are actively developing large numbers of new lesions (N Engl J Med. 1988 Jun23;318[25]:1633-7).
“Probably the most important thing you can do is refer them to patient support groups,” Dr. DiGiovanna said. “They are present in many countries and can help them manage the day-to-day issues of their condition.” Support groups based in North America include the XP Family Support Group, XP Society, and XP Grupo Luz De Esperanza.
Dr. DiGiovanna reported having no financial disclosures.
AUSTIN, TEX. – If a child presents with acute (XP), a rare autosomal recessive disorder.
Other telltale symptoms of XP include the presence of skin cancer at an early age and a large number of skin cancers.
At the annual meeting of the Society for Pediatric Dermatology, John J. DiGiovanna, MD, described XP as a disorder of genomic instability, which has no cure. It’s caused by a mutation in genes XPA through XPG and the XP variant (XPV) gene. “The genome controls our genes, and UV rays damage DNA,” said Dr. DiGiovanna, who is a senior research physician at the National Cancer Institute’s Laboratory of Cancer and Biology and Genetics, Bethesda, Md. “This damage from UV radiation is similar to damage from chemical agents that form DNA adducts, such as cigarette smoke and certain chemotherapy agents such as cisplatinum.”
XP patients present with or without acute burning after minimal sun exposure, while children with both subtypes develop “freckling” by the time they reach 2 years of age. Dr. DiGiovanna pointed out that lentigo maligna lesions associated with XP resemble freckles at first glance, yet they vary in size, intensity, and border. Meanwhile, freckles in healthy patients are similar in size, are light tan in color, and have a regular border.
“The burning with minimal sun exposure that occurs during childhood leads to pigmentary changes, atrophy, xerosis, and telangiectasias,” he said. A follow-up analysis of 106 XP patients admitted to the National Institutes of Health between 1971 and 2009 found that patients were diagnosed with their first nonmelanoma skin cancer at a median age of 9 years, compared with age 67 among those in the general population (J Med Genet 2011 Mar;48[3]:168-76). “This is a 58-year decrease in age at risk, which is a 10,000-fold increase in skin cancer,” said Dr. DiGiovanna, who was one of the study authors.
Melanoma also occurs at an earlier age among XP patients – a median age of 22 years, compared with a median of 55 years in the general population. “In the general population, melanoma occurs at a younger age than nonmelanoma skin cancer, while in the XP population, melanoma occurs at an older age,” he said. “This is giving us a good biologic lesson that the melanoma induction mechanism must be different from nonmelanoma skin cancer.”
He recalled one XP patient who was followed by NIH researchers for 4 decades. She worked in a doctor’s office and drove a car, but developed progressive neurologic degeneration and died at the age of 40. “This was not due to unrepaired UV damage, but there are other agents which damage other neurons,” Dr. DiGiovanna explained. “Over time, what you get is a decrease in brain volume, an increase in the brain ventricles, and a loss of brain tissue. At postmortem examination, her brain was of infantile size, compared with that of an equivalent 40-year-old. This is a disease of neuronal loss, and it’s progressive. Only about 20%-25% of XP patients experience neural degeneration.”
Management of XP involves strict sun avoidance, including use of a portable UV meter and many layers of UV protection, including application of sunscreen, wearing protective clothing, sunglasses, hats, and face shields, and the use of UV-blocking window film, LED lights, and a vitamin D diet or oral supplementation. Affected individuals also require frequent skin monitoring by the patients and their family members, frequent dermatologic exams by clinicians, biopsy of suspicious lesions, removal of any skin cancers found, field treatments with agents such as 5-fluorouracil and imiquimod, and chemoprevention with oral retinoids for patients who are actively developing large numbers of new lesions (N Engl J Med. 1988 Jun23;318[25]:1633-7).
“Probably the most important thing you can do is refer them to patient support groups,” Dr. DiGiovanna said. “They are present in many countries and can help them manage the day-to-day issues of their condition.” Support groups based in North America include the XP Family Support Group, XP Society, and XP Grupo Luz De Esperanza.
Dr. DiGiovanna reported having no financial disclosures.
REPORTING FROM SPD 2019
Metformin-TKI combo improves PFS in EGFR-mutated lung cancer
Combination metformin and epidermal growth factor receptor–tyrosine kinase inhibitor (EGFR-TKI) therapy improved progression-free survival in patients with EGFR-mutated lung adenocarcinoma, according to results from a phase 2 trial.
“This is the first study to prospectively show that the addition of metformin to standard EGFR-TKIs therapy in patients with advanced lung adenocarcinoma significantly improves PFS [progression-free survival],” wrote Oscar Arrieta, MD, of the Instituto Nacional de Cancerología, Mexico, and colleagues in JAMA Oncology.
The open-label, randomized study included 139 patients, 69 of whom were randomly assigned to receive metformin plus EGFR-TKI therapy and 70 of whom received EGFR-TKI monotherapy.
EGFR-TKI therapy was selected based on physician choice and consisted of either afatinib dimaleate, gefitinib, or erlotinib hydrochloride at regular doses. Study patients in the combination arm received metformin 500 mg twice daily.
The primary endpoint was PFS (intent-to-treat population). Secondary endpoints included overall survival (OS), objective response rate, and safety.
After analysis, the researchers found that the median PFS was 13.1 months with metformin-EGFR-TKI combination therapy and 9.9 months with EGFR-TKI monotherapy (hazard ratio, 0.60; P = .03).
In addition, the median OS was also significantly prolonged for patients receiving combined treatment (31.7 months vs. 17.5 months; P = .02).
“Multivariable analysis showed that treatment with metformin is independently associated with longer PFS and OS,” the researchers wrote.
With respect to safety, no significant rise in adverse events was observed, and toxicities were comparable across both treatment groups.
The researchers acknowledged that a key limitation of the study was the absence of a double-blinded design. As a result, various biases could have influenced the results.
“The results from this phase 2 study warrant the design of a larger, phase 3, placebo-controlled study to draw more robust conclusions,” they said.
The study was funded by the National Council for Science and Technology in Mexico. The authors reported financial affiliations with AbbVie, AstraZeneca, Boehringer Ingelheim, Lilly, Bristol-Myers Squibb, Merck, Pfizer, Roche, and several others.
SOURCE: Arrieta O et al. JAMA Oncol. 2019 Sep 5. doi: 10.1001/jamaoncol.2019.2553.
Combination metformin and epidermal growth factor receptor–tyrosine kinase inhibitor (EGFR-TKI) therapy improved progression-free survival in patients with EGFR-mutated lung adenocarcinoma, according to results from a phase 2 trial.
“This is the first study to prospectively show that the addition of metformin to standard EGFR-TKIs therapy in patients with advanced lung adenocarcinoma significantly improves PFS [progression-free survival],” wrote Oscar Arrieta, MD, of the Instituto Nacional de Cancerología, Mexico, and colleagues in JAMA Oncology.
The open-label, randomized study included 139 patients, 69 of whom were randomly assigned to receive metformin plus EGFR-TKI therapy and 70 of whom received EGFR-TKI monotherapy.
EGFR-TKI therapy was selected based on physician choice and consisted of either afatinib dimaleate, gefitinib, or erlotinib hydrochloride at regular doses. Study patients in the combination arm received metformin 500 mg twice daily.
The primary endpoint was PFS (intent-to-treat population). Secondary endpoints included overall survival (OS), objective response rate, and safety.
After analysis, the researchers found that the median PFS was 13.1 months with metformin-EGFR-TKI combination therapy and 9.9 months with EGFR-TKI monotherapy (hazard ratio, 0.60; P = .03).
In addition, the median OS was also significantly prolonged for patients receiving combined treatment (31.7 months vs. 17.5 months; P = .02).
“Multivariable analysis showed that treatment with metformin is independently associated with longer PFS and OS,” the researchers wrote.
With respect to safety, no significant rise in adverse events was observed, and toxicities were comparable across both treatment groups.
The researchers acknowledged that a key limitation of the study was the absence of a double-blinded design. As a result, various biases could have influenced the results.
“The results from this phase 2 study warrant the design of a larger, phase 3, placebo-controlled study to draw more robust conclusions,” they said.
The study was funded by the National Council for Science and Technology in Mexico. The authors reported financial affiliations with AbbVie, AstraZeneca, Boehringer Ingelheim, Lilly, Bristol-Myers Squibb, Merck, Pfizer, Roche, and several others.
SOURCE: Arrieta O et al. JAMA Oncol. 2019 Sep 5. doi: 10.1001/jamaoncol.2019.2553.
Combination metformin and epidermal growth factor receptor–tyrosine kinase inhibitor (EGFR-TKI) therapy improved progression-free survival in patients with EGFR-mutated lung adenocarcinoma, according to results from a phase 2 trial.
“This is the first study to prospectively show that the addition of metformin to standard EGFR-TKIs therapy in patients with advanced lung adenocarcinoma significantly improves PFS [progression-free survival],” wrote Oscar Arrieta, MD, of the Instituto Nacional de Cancerología, Mexico, and colleagues in JAMA Oncology.
The open-label, randomized study included 139 patients, 69 of whom were randomly assigned to receive metformin plus EGFR-TKI therapy and 70 of whom received EGFR-TKI monotherapy.
EGFR-TKI therapy was selected based on physician choice and consisted of either afatinib dimaleate, gefitinib, or erlotinib hydrochloride at regular doses. Study patients in the combination arm received metformin 500 mg twice daily.
The primary endpoint was PFS (intent-to-treat population). Secondary endpoints included overall survival (OS), objective response rate, and safety.
After analysis, the researchers found that the median PFS was 13.1 months with metformin-EGFR-TKI combination therapy and 9.9 months with EGFR-TKI monotherapy (hazard ratio, 0.60; P = .03).
In addition, the median OS was also significantly prolonged for patients receiving combined treatment (31.7 months vs. 17.5 months; P = .02).
“Multivariable analysis showed that treatment with metformin is independently associated with longer PFS and OS,” the researchers wrote.
With respect to safety, no significant rise in adverse events was observed, and toxicities were comparable across both treatment groups.
The researchers acknowledged that a key limitation of the study was the absence of a double-blinded design. As a result, various biases could have influenced the results.
“The results from this phase 2 study warrant the design of a larger, phase 3, placebo-controlled study to draw more robust conclusions,” they said.
The study was funded by the National Council for Science and Technology in Mexico. The authors reported financial affiliations with AbbVie, AstraZeneca, Boehringer Ingelheim, Lilly, Bristol-Myers Squibb, Merck, Pfizer, Roche, and several others.
SOURCE: Arrieta O et al. JAMA Oncol. 2019 Sep 5. doi: 10.1001/jamaoncol.2019.2553.
FROM JAMA ONCOLOGY
Older IBD patients are most at risk of postdischarge VTE
Hospitalized patients with inflammatory bowel diseases (IBD) are most likely to be readmitted for venous thromboembolism (VTE) within 60 days of discharge, according to a new study that analyzed 5 years of U.S. readmissions data.
“Given increased thrombotic risk postdischarge, as well as overall safety of VTE prophylaxis, extending prophylaxis for those at highest risk may have significant benefits,” wrote Adam S. Faye, MD, of Columbia University, and coauthors. The study was published in Clinical Gastroenterology and Hepatology.
To determine which IBD patients would be most in need of postdischarge VTE prophylaxis, as well as when to administer it, the researchers analyzed 2010-2014 data from the Nationwide Readmissions Database (NRD). They found a total of 872,122 index admissions for IBD patients; 4% of those patients had a prior VTE. Of the index admissions, 1,160 led to a VTE readmission within 90 days. Readmitted patients had a relatively equal proportion of ulcerative colitis (n = 522) and Crohn’s disease (n = 638).
More than 90% of VTE readmissions occurred within 60 days of discharge; the risk was highest over the first 10 days and then decreased in each ensuing 10-day period until a slight increase at the 81- to 90-day period. All patients over age 30 had higher rates of readmission than those of patients under age 18, with the highest risk in patients between the ages of 66 and 80 years (risk ratio 4.04; 95% confidence interval, 2.54-6.44, P less than .01). Women were at lower risk (RR 0.82; 95% CI, 0.73-0.92, P less than .01). Higher risks of readmission were also associated with being on Medicare (RR 1.39; 95% CI, 1.23-1.58, P less than .01) compared with being on private insurance and being cared for at a large hospital (RR 1.26; 95% CI, 1.04-1.52, P = .02) compared with a small hospital.
The highest risk of VTE readmission was associated with a prior history of VTE (RR 2.89; 95% CI, 2.40-3.48, P less than .01), having two or more comorbidities (RR 2.57; 95% CI, 2.11-3.12, P less than .01) and having a Clostridioides difficile infection as of index admission (RR 1.90; 95% CI, 1.51-2.38, P less than .01). In addition, increased risk was associated with being discharged to a nursing or care facility (RR 1.85; 95% CI, 1.56-2.20, P less than .01) or home with health services (RR 2.05; 95% CI, 1.78-2.38, P less than .01) compared with a routine discharge.
In their multivariable analysis, similar factors such as a history of VTE (adjusted RR 2.41; 95% CI, 1.99-2.90, P less than .01), two or more comorbidities (aRR 1.78; 95% CI, 1.44-2.20, P less than .01) and C. difficile infection (aRR 1.47; 95% CI, 1.17-1.85, P less than.01) continued to be associated with higher risk of VTE readmission.
Though they emphasized that the use of NRD data offered the impressive ability to “review over 15 million discharges across the U.S. annually,” Dr. Faye and coauthors acknowledged that their study did have limitations. These included the inability to verify via chart review the study’s outcomes and covariates. In addition, they were unable to assess potential contributing risk factors such as medication use, use of VTE prophylaxis during hospitalization, disease severity, and family history. Finally, though unlikely, they admitted the possibility that patients could be counted more than once if they were readmitted with a VTE each year of the study.
The authors reported being supported by grants from the National Institutes of Health and various pharmaceutical companies, as well as receiving honoraria and serving as consultants.
SOURCE: Faye AS et al. Clin Gastroenterol Hepatol. 2019 July 20. doi: 10.1016/j.cgh.2019.07.028.
Hospitalized patients with inflammatory bowel diseases (IBD) are most likely to be readmitted for venous thromboembolism (VTE) within 60 days of discharge, according to a new study that analyzed 5 years of U.S. readmissions data.
“Given increased thrombotic risk postdischarge, as well as overall safety of VTE prophylaxis, extending prophylaxis for those at highest risk may have significant benefits,” wrote Adam S. Faye, MD, of Columbia University, and coauthors. The study was published in Clinical Gastroenterology and Hepatology.
To determine which IBD patients would be most in need of postdischarge VTE prophylaxis, as well as when to administer it, the researchers analyzed 2010-2014 data from the Nationwide Readmissions Database (NRD). They found a total of 872,122 index admissions for IBD patients; 4% of those patients had a prior VTE. Of the index admissions, 1,160 led to a VTE readmission within 90 days. Readmitted patients had a relatively equal proportion of ulcerative colitis (n = 522) and Crohn’s disease (n = 638).
More than 90% of VTE readmissions occurred within 60 days of discharge; the risk was highest over the first 10 days and then decreased in each ensuing 10-day period until a slight increase at the 81- to 90-day period. All patients over age 30 had higher rates of readmission than those of patients under age 18, with the highest risk in patients between the ages of 66 and 80 years (risk ratio 4.04; 95% confidence interval, 2.54-6.44, P less than .01). Women were at lower risk (RR 0.82; 95% CI, 0.73-0.92, P less than .01). Higher risks of readmission were also associated with being on Medicare (RR 1.39; 95% CI, 1.23-1.58, P less than .01) compared with being on private insurance and being cared for at a large hospital (RR 1.26; 95% CI, 1.04-1.52, P = .02) compared with a small hospital.
The highest risk of VTE readmission was associated with a prior history of VTE (RR 2.89; 95% CI, 2.40-3.48, P less than .01), having two or more comorbidities (RR 2.57; 95% CI, 2.11-3.12, P less than .01) and having a Clostridioides difficile infection as of index admission (RR 1.90; 95% CI, 1.51-2.38, P less than .01). In addition, increased risk was associated with being discharged to a nursing or care facility (RR 1.85; 95% CI, 1.56-2.20, P less than .01) or home with health services (RR 2.05; 95% CI, 1.78-2.38, P less than .01) compared with a routine discharge.
In their multivariable analysis, similar factors such as a history of VTE (adjusted RR 2.41; 95% CI, 1.99-2.90, P less than .01), two or more comorbidities (aRR 1.78; 95% CI, 1.44-2.20, P less than .01) and C. difficile infection (aRR 1.47; 95% CI, 1.17-1.85, P less than.01) continued to be associated with higher risk of VTE readmission.
Though they emphasized that the use of NRD data offered the impressive ability to “review over 15 million discharges across the U.S. annually,” Dr. Faye and coauthors acknowledged that their study did have limitations. These included the inability to verify via chart review the study’s outcomes and covariates. In addition, they were unable to assess potential contributing risk factors such as medication use, use of VTE prophylaxis during hospitalization, disease severity, and family history. Finally, though unlikely, they admitted the possibility that patients could be counted more than once if they were readmitted with a VTE each year of the study.
The authors reported being supported by grants from the National Institutes of Health and various pharmaceutical companies, as well as receiving honoraria and serving as consultants.
SOURCE: Faye AS et al. Clin Gastroenterol Hepatol. 2019 July 20. doi: 10.1016/j.cgh.2019.07.028.
Hospitalized patients with inflammatory bowel diseases (IBD) are most likely to be readmitted for venous thromboembolism (VTE) within 60 days of discharge, according to a new study that analyzed 5 years of U.S. readmissions data.
“Given increased thrombotic risk postdischarge, as well as overall safety of VTE prophylaxis, extending prophylaxis for those at highest risk may have significant benefits,” wrote Adam S. Faye, MD, of Columbia University, and coauthors. The study was published in Clinical Gastroenterology and Hepatology.
To determine which IBD patients would be most in need of postdischarge VTE prophylaxis, as well as when to administer it, the researchers analyzed 2010-2014 data from the Nationwide Readmissions Database (NRD). They found a total of 872,122 index admissions for IBD patients; 4% of those patients had a prior VTE. Of the index admissions, 1,160 led to a VTE readmission within 90 days. Readmitted patients had a relatively equal proportion of ulcerative colitis (n = 522) and Crohn’s disease (n = 638).
More than 90% of VTE readmissions occurred within 60 days of discharge; the risk was highest over the first 10 days and then decreased in each ensuing 10-day period until a slight increase at the 81- to 90-day period. All patients over age 30 had higher rates of readmission than those of patients under age 18, with the highest risk in patients between the ages of 66 and 80 years (risk ratio 4.04; 95% confidence interval, 2.54-6.44, P less than .01). Women were at lower risk (RR 0.82; 95% CI, 0.73-0.92, P less than .01). Higher risks of readmission were also associated with being on Medicare (RR 1.39; 95% CI, 1.23-1.58, P less than .01) compared with being on private insurance and being cared for at a large hospital (RR 1.26; 95% CI, 1.04-1.52, P = .02) compared with a small hospital.
The highest risk of VTE readmission was associated with a prior history of VTE (RR 2.89; 95% CI, 2.40-3.48, P less than .01), having two or more comorbidities (RR 2.57; 95% CI, 2.11-3.12, P less than .01) and having a Clostridioides difficile infection as of index admission (RR 1.90; 95% CI, 1.51-2.38, P less than .01). In addition, increased risk was associated with being discharged to a nursing or care facility (RR 1.85; 95% CI, 1.56-2.20, P less than .01) or home with health services (RR 2.05; 95% CI, 1.78-2.38, P less than .01) compared with a routine discharge.
In their multivariable analysis, similar factors such as a history of VTE (adjusted RR 2.41; 95% CI, 1.99-2.90, P less than .01), two or more comorbidities (aRR 1.78; 95% CI, 1.44-2.20, P less than .01) and C. difficile infection (aRR 1.47; 95% CI, 1.17-1.85, P less than.01) continued to be associated with higher risk of VTE readmission.
Though they emphasized that the use of NRD data offered the impressive ability to “review over 15 million discharges across the U.S. annually,” Dr. Faye and coauthors acknowledged that their study did have limitations. These included the inability to verify via chart review the study’s outcomes and covariates. In addition, they were unable to assess potential contributing risk factors such as medication use, use of VTE prophylaxis during hospitalization, disease severity, and family history. Finally, though unlikely, they admitted the possibility that patients could be counted more than once if they were readmitted with a VTE each year of the study.
The authors reported being supported by grants from the National Institutes of Health and various pharmaceutical companies, as well as receiving honoraria and serving as consultants.
SOURCE: Faye AS et al. Clin Gastroenterol Hepatol. 2019 July 20. doi: 10.1016/j.cgh.2019.07.028.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Readmission for VTE in patients with inflammatory bowel diseases most often occurs within 60 days of discharge.
Major finding: The highest readmission risk was in patients between the ages of 66 and 80 (risk ratio 4.04; 95% confidence interval, 2.54-6.44, P less than .01).
Study details: A retrospective cohort study of 1,160 IBD patients who had VTE readmissions via 2010-2014 data from the Nationwide Readmissions Database.
Disclosures: The authors reported being supported by grants from the National Institutes of Health and various pharmaceutical companies, as well as receiving honoraria and serving as consultants.
Source: Faye AS et al. Clin Gastroenterol Hepatol. 2019 July 20. doi: 10.1016/j.cgh.2019.07.028.
How to distinguish Stevens-Johnson syndrome from its mimickers
NEW YORK – said Lucia Seminario-Vidal, MD, PhD, speaking at a hospital dermatology–focused session of the summer meeting of the American Academy of Dermatology.
Even before histopathologic results are available, dermatologists can help take some of the panic out of the room when Stevens-Johnson syndrome or toxic epidermal necrolysis (SJS/TEN) is first on the differential diagnosis for the referring physician, she said. Physical findings, a careful review of systems, and past history – especially the introduction of any new medications – can go a long way toward ruling out SJS/TEN among many patients.
In a recent study coauthored by Dr. Seminario-Vidal, of the University of South Florida, Tampa, 208 inpatients with suspected SJS/TEN, 72% were given another diagnosis after a dermatologist consultation (J Am Acad Dermatol. 2019 Sep;81[3]:749-57). The study’s authors, led by Allison Weinkle, MD, a dermatology resident at the University of South Florida, performed a retrospective chart review of 208 patients suspected of having SJS/TEN and received inpatient dermatology consultations.
One surprising finding from the review is that “known risk factors for SJS/TEN such as drug exposure, malignancy, and HIV/AIDS are not helpful to differentiate it from its mimickers,” Dr. Seminario-Vidal said.
However, close attention to the clinical presentation is helpful in differentiating the mimickers from true SJS/TEN. Fever, painful skin, Nikolsky sign, and mucosal involvement were all more common in patients with SJS/TEN. “This was something that was really helpful to discuss with the primary teams at our institutions,” Dr. Seminario-Vidal said.
The most common mimickers in the cases she and her colleagues reviewed were severe cutaneous adverse drug reactions (SCARs) and erythema multiforme; in general, the mimickers were conditions that have severe mucocutaneous involvement.
Patients with SJS/TEN will have fever and pain, typically, and the primary lesions are atypical targets with a positive Nikolsky sign. Hepatic enzyme elevation is seen in 15% of patients, and lymphopenia may be present. Common drugs provoking SJS/TEN include antibiotics, anticonvulsants, NSAIDs, allopurinol, and – importantly – programmed cell death protein 1 (PD-1) inhibitors. Because PD-1s are being used for an ever-expanding range of oncology indications, the rate of SJS/TEN may be expected to rise, Dr. Seminario-Vidal noted.
Other common mimics can include a florid generalized fixed drug eruption, which can also be painful and have atypical targets and a positive Nikolsky sign. Here, large, dusky patches may be present, with perioral, genital, and hand/foot involvement common, but fever is absent. Lesions are sharply localized, round or oval, and they recur at the same site with reexposure to the provoking agent. “Histopathologic studies are diagnostic in these patients, but many times, if you have a good clinical history, you don’t need histopathology,” Dr. Seminario-Vidal said.
As with SJS/TEN, mucosal involvement can be present with fixed drug eruptions. However, hepatic enzymes and the complete blood count will not show abnormalities related to the fixed drug eruption.
Sulfonamides have been associated with up to 75% of fixed drug eruptions reported in some case series, she said, noting that common over-the-counter medications such as nonprescription analgesics, loratadine, and pseudoephedrine can also provoke fixed drug eruptions.
Bullous conditions that can mimic SJS/TEN include linear Immunoglobulin A bullous dermatosis. “We know that the classic presentation is the beautiful ‘string of pearls’ sign, but most patients won’t have this,” Dr. Seminario-Vidal said. The condition often appears in intertriginous locations, and Koebnerization can be a strong clue to this diagnosis, she added.
This painful condition rarely has an associated fever, and will occasionally involve the mucosa, but it doesn’t cause alterations in laboratory values. About 70% of cases of drug-induced linear IgA bullous dermatosis are caused by vancomycin, a consideration when patients may be on several antibiotics. “When I first started, I used to stop everything,” said Dr. Seminario-Vidal. Now, she first discontinues vancomycin and rechecks the patient the next day. In the absence of progression, vancomycin can be presumed to be the culprit, she said.
In bullous pemphigoid, mucosal involvement has been reported in up to 30% of patients, which can initially confuse the diagnosis. “A clue is the presence of tense bullae that initially are very pruritic, but they can become painful over time,” Dr. Seminario-Vidal said. “With tense bullae, with no specific primary lesion, but the patient is very itchy, think bullous pemphigoid.” Pain is sometimes present, but fever is rare. Eosinophilia occurs about 50% of the time, offering an additional diagnostic clue.
Furosemide is a common culprit in drug-induced bullous pemphigoid, as is its cousin bumetanide. Certain analgesics, amoxicillin, ciprofloxacin, and angiotensin converting enzyme inhibitors have also been implicated, said Dr. Seminario-Vidal, though most cases are not drug induced. “Go over the drug history of the patients. It’s something that I cannot emphasize enough … because if you find the drug that causes this disease, you can avoid lengthy systemic treatment.”
Pemphigus foliaceus has a characteristic crusting scale that forms with the rupture of flaccid bullae. Nikolsky’s sign is positive. Mucosal involvement is rare, but is sometimes seen on the occasions when the condition is drug-induced, and laboratory values are usually normal, she said. “The clues to diagnosis of these patients are involvement in the seborrheic areas, including the face; there’s a lot of crust in pemphigus foliaceus.”
Drug-induced pemphigus foliaceus is associated with drugs that have a thiol group, or with sulfur groups that are converted to thiol (–SH) groups. These include both angiotensin converting enzyme inhibitors and angiotensin receptor blockers, penicillins, cephalosporins, and piroxicam. However, said Dr. Seminario-Vidal, penicillamine alone accounts for 50% of drug-induced pemphigus foliaceus.
Dr. Seminario-Vidal reported financial relationships with Eli Lilly, Soligenix, Helsinn, Eisai, Boehringer Ingelheim, Corrona, Akros, Novartis, AbbVie, BMS, Celgene, Glenmark, and Kyowa Kirin.
SOURCE: Seminario-Vidal L. Summer AAD 2019, Session F-025.
NEW YORK – said Lucia Seminario-Vidal, MD, PhD, speaking at a hospital dermatology–focused session of the summer meeting of the American Academy of Dermatology.
Even before histopathologic results are available, dermatologists can help take some of the panic out of the room when Stevens-Johnson syndrome or toxic epidermal necrolysis (SJS/TEN) is first on the differential diagnosis for the referring physician, she said. Physical findings, a careful review of systems, and past history – especially the introduction of any new medications – can go a long way toward ruling out SJS/TEN among many patients.
In a recent study coauthored by Dr. Seminario-Vidal, of the University of South Florida, Tampa, 208 inpatients with suspected SJS/TEN, 72% were given another diagnosis after a dermatologist consultation (J Am Acad Dermatol. 2019 Sep;81[3]:749-57). The study’s authors, led by Allison Weinkle, MD, a dermatology resident at the University of South Florida, performed a retrospective chart review of 208 patients suspected of having SJS/TEN and received inpatient dermatology consultations.
One surprising finding from the review is that “known risk factors for SJS/TEN such as drug exposure, malignancy, and HIV/AIDS are not helpful to differentiate it from its mimickers,” Dr. Seminario-Vidal said.
However, close attention to the clinical presentation is helpful in differentiating the mimickers from true SJS/TEN. Fever, painful skin, Nikolsky sign, and mucosal involvement were all more common in patients with SJS/TEN. “This was something that was really helpful to discuss with the primary teams at our institutions,” Dr. Seminario-Vidal said.
The most common mimickers in the cases she and her colleagues reviewed were severe cutaneous adverse drug reactions (SCARs) and erythema multiforme; in general, the mimickers were conditions that have severe mucocutaneous involvement.
Patients with SJS/TEN will have fever and pain, typically, and the primary lesions are atypical targets with a positive Nikolsky sign. Hepatic enzyme elevation is seen in 15% of patients, and lymphopenia may be present. Common drugs provoking SJS/TEN include antibiotics, anticonvulsants, NSAIDs, allopurinol, and – importantly – programmed cell death protein 1 (PD-1) inhibitors. Because PD-1s are being used for an ever-expanding range of oncology indications, the rate of SJS/TEN may be expected to rise, Dr. Seminario-Vidal noted.
Other common mimics can include a florid generalized fixed drug eruption, which can also be painful and have atypical targets and a positive Nikolsky sign. Here, large, dusky patches may be present, with perioral, genital, and hand/foot involvement common, but fever is absent. Lesions are sharply localized, round or oval, and they recur at the same site with reexposure to the provoking agent. “Histopathologic studies are diagnostic in these patients, but many times, if you have a good clinical history, you don’t need histopathology,” Dr. Seminario-Vidal said.
As with SJS/TEN, mucosal involvement can be present with fixed drug eruptions. However, hepatic enzymes and the complete blood count will not show abnormalities related to the fixed drug eruption.
Sulfonamides have been associated with up to 75% of fixed drug eruptions reported in some case series, she said, noting that common over-the-counter medications such as nonprescription analgesics, loratadine, and pseudoephedrine can also provoke fixed drug eruptions.
Bullous conditions that can mimic SJS/TEN include linear Immunoglobulin A bullous dermatosis. “We know that the classic presentation is the beautiful ‘string of pearls’ sign, but most patients won’t have this,” Dr. Seminario-Vidal said. The condition often appears in intertriginous locations, and Koebnerization can be a strong clue to this diagnosis, she added.
This painful condition rarely has an associated fever, and will occasionally involve the mucosa, but it doesn’t cause alterations in laboratory values. About 70% of cases of drug-induced linear IgA bullous dermatosis are caused by vancomycin, a consideration when patients may be on several antibiotics. “When I first started, I used to stop everything,” said Dr. Seminario-Vidal. Now, she first discontinues vancomycin and rechecks the patient the next day. In the absence of progression, vancomycin can be presumed to be the culprit, she said.
In bullous pemphigoid, mucosal involvement has been reported in up to 30% of patients, which can initially confuse the diagnosis. “A clue is the presence of tense bullae that initially are very pruritic, but they can become painful over time,” Dr. Seminario-Vidal said. “With tense bullae, with no specific primary lesion, but the patient is very itchy, think bullous pemphigoid.” Pain is sometimes present, but fever is rare. Eosinophilia occurs about 50% of the time, offering an additional diagnostic clue.
Furosemide is a common culprit in drug-induced bullous pemphigoid, as is its cousin bumetanide. Certain analgesics, amoxicillin, ciprofloxacin, and angiotensin converting enzyme inhibitors have also been implicated, said Dr. Seminario-Vidal, though most cases are not drug induced. “Go over the drug history of the patients. It’s something that I cannot emphasize enough … because if you find the drug that causes this disease, you can avoid lengthy systemic treatment.”
Pemphigus foliaceus has a characteristic crusting scale that forms with the rupture of flaccid bullae. Nikolsky’s sign is positive. Mucosal involvement is rare, but is sometimes seen on the occasions when the condition is drug-induced, and laboratory values are usually normal, she said. “The clues to diagnosis of these patients are involvement in the seborrheic areas, including the face; there’s a lot of crust in pemphigus foliaceus.”
Drug-induced pemphigus foliaceus is associated with drugs that have a thiol group, or with sulfur groups that are converted to thiol (–SH) groups. These include both angiotensin converting enzyme inhibitors and angiotensin receptor blockers, penicillins, cephalosporins, and piroxicam. However, said Dr. Seminario-Vidal, penicillamine alone accounts for 50% of drug-induced pemphigus foliaceus.
Dr. Seminario-Vidal reported financial relationships with Eli Lilly, Soligenix, Helsinn, Eisai, Boehringer Ingelheim, Corrona, Akros, Novartis, AbbVie, BMS, Celgene, Glenmark, and Kyowa Kirin.
SOURCE: Seminario-Vidal L. Summer AAD 2019, Session F-025.
NEW YORK – said Lucia Seminario-Vidal, MD, PhD, speaking at a hospital dermatology–focused session of the summer meeting of the American Academy of Dermatology.
Even before histopathologic results are available, dermatologists can help take some of the panic out of the room when Stevens-Johnson syndrome or toxic epidermal necrolysis (SJS/TEN) is first on the differential diagnosis for the referring physician, she said. Physical findings, a careful review of systems, and past history – especially the introduction of any new medications – can go a long way toward ruling out SJS/TEN among many patients.
In a recent study coauthored by Dr. Seminario-Vidal, of the University of South Florida, Tampa, 208 inpatients with suspected SJS/TEN, 72% were given another diagnosis after a dermatologist consultation (J Am Acad Dermatol. 2019 Sep;81[3]:749-57). The study’s authors, led by Allison Weinkle, MD, a dermatology resident at the University of South Florida, performed a retrospective chart review of 208 patients suspected of having SJS/TEN and received inpatient dermatology consultations.
One surprising finding from the review is that “known risk factors for SJS/TEN such as drug exposure, malignancy, and HIV/AIDS are not helpful to differentiate it from its mimickers,” Dr. Seminario-Vidal said.
However, close attention to the clinical presentation is helpful in differentiating the mimickers from true SJS/TEN. Fever, painful skin, Nikolsky sign, and mucosal involvement were all more common in patients with SJS/TEN. “This was something that was really helpful to discuss with the primary teams at our institutions,” Dr. Seminario-Vidal said.
The most common mimickers in the cases she and her colleagues reviewed were severe cutaneous adverse drug reactions (SCARs) and erythema multiforme; in general, the mimickers were conditions that have severe mucocutaneous involvement.
Patients with SJS/TEN will have fever and pain, typically, and the primary lesions are atypical targets with a positive Nikolsky sign. Hepatic enzyme elevation is seen in 15% of patients, and lymphopenia may be present. Common drugs provoking SJS/TEN include antibiotics, anticonvulsants, NSAIDs, allopurinol, and – importantly – programmed cell death protein 1 (PD-1) inhibitors. Because PD-1s are being used for an ever-expanding range of oncology indications, the rate of SJS/TEN may be expected to rise, Dr. Seminario-Vidal noted.
Other common mimics can include a florid generalized fixed drug eruption, which can also be painful and have atypical targets and a positive Nikolsky sign. Here, large, dusky patches may be present, with perioral, genital, and hand/foot involvement common, but fever is absent. Lesions are sharply localized, round or oval, and they recur at the same site with reexposure to the provoking agent. “Histopathologic studies are diagnostic in these patients, but many times, if you have a good clinical history, you don’t need histopathology,” Dr. Seminario-Vidal said.
As with SJS/TEN, mucosal involvement can be present with fixed drug eruptions. However, hepatic enzymes and the complete blood count will not show abnormalities related to the fixed drug eruption.
Sulfonamides have been associated with up to 75% of fixed drug eruptions reported in some case series, she said, noting that common over-the-counter medications such as nonprescription analgesics, loratadine, and pseudoephedrine can also provoke fixed drug eruptions.
Bullous conditions that can mimic SJS/TEN include linear Immunoglobulin A bullous dermatosis. “We know that the classic presentation is the beautiful ‘string of pearls’ sign, but most patients won’t have this,” Dr. Seminario-Vidal said. The condition often appears in intertriginous locations, and Koebnerization can be a strong clue to this diagnosis, she added.
This painful condition rarely has an associated fever, and will occasionally involve the mucosa, but it doesn’t cause alterations in laboratory values. About 70% of cases of drug-induced linear IgA bullous dermatosis are caused by vancomycin, a consideration when patients may be on several antibiotics. “When I first started, I used to stop everything,” said Dr. Seminario-Vidal. Now, she first discontinues vancomycin and rechecks the patient the next day. In the absence of progression, vancomycin can be presumed to be the culprit, she said.
In bullous pemphigoid, mucosal involvement has been reported in up to 30% of patients, which can initially confuse the diagnosis. “A clue is the presence of tense bullae that initially are very pruritic, but they can become painful over time,” Dr. Seminario-Vidal said. “With tense bullae, with no specific primary lesion, but the patient is very itchy, think bullous pemphigoid.” Pain is sometimes present, but fever is rare. Eosinophilia occurs about 50% of the time, offering an additional diagnostic clue.
Furosemide is a common culprit in drug-induced bullous pemphigoid, as is its cousin bumetanide. Certain analgesics, amoxicillin, ciprofloxacin, and angiotensin converting enzyme inhibitors have also been implicated, said Dr. Seminario-Vidal, though most cases are not drug induced. “Go over the drug history of the patients. It’s something that I cannot emphasize enough … because if you find the drug that causes this disease, you can avoid lengthy systemic treatment.”
Pemphigus foliaceus has a characteristic crusting scale that forms with the rupture of flaccid bullae. Nikolsky’s sign is positive. Mucosal involvement is rare, but is sometimes seen on the occasions when the condition is drug-induced, and laboratory values are usually normal, she said. “The clues to diagnosis of these patients are involvement in the seborrheic areas, including the face; there’s a lot of crust in pemphigus foliaceus.”
Drug-induced pemphigus foliaceus is associated with drugs that have a thiol group, or with sulfur groups that are converted to thiol (–SH) groups. These include both angiotensin converting enzyme inhibitors and angiotensin receptor blockers, penicillins, cephalosporins, and piroxicam. However, said Dr. Seminario-Vidal, penicillamine alone accounts for 50% of drug-induced pemphigus foliaceus.
Dr. Seminario-Vidal reported financial relationships with Eli Lilly, Soligenix, Helsinn, Eisai, Boehringer Ingelheim, Corrona, Akros, Novartis, AbbVie, BMS, Celgene, Glenmark, and Kyowa Kirin.
SOURCE: Seminario-Vidal L. Summer AAD 2019, Session F-025.
EXPERT ANALYSIS FROM SUMMER AAD 2019