User login
ACE inhibitor and ARB therapy: Practical recommendations
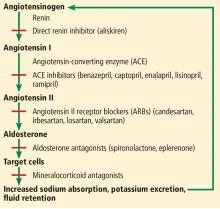
Inhibition of the renin-angiotensin-aldosterone system with angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) is widely used in the treatment of heart failure, hypertension, chronic kidney disease, and coronary artery disease with left ventricular dysfunction.
In this issue, Momoniat et al1 review the benefits of ACE inhibitors and ARBs and how to manage adverse effects. I would like to add some of my own observations.
ARE ACE INHIBITORS REALLY BETTER THAN ARBs?
ACE inhibitors have been the cornerstone of treatment for patients with heart failure with reduced ejection fraction (HFrEF), in whom their use is associated with reduced rates of morbidity and death.2,3 The use of ARBs in these patients is also associated with decreased rates of morbidity and death4,5; however, in early comparisons, ACE inhibitors were deemed more effective in decreasing the incidence of myocardial infarction, cardiovascular death, and all-cause mortality in patients with hypertension, diabetes, and increased cardiovascular risk,6 and all-cause mortality in patients with HFrEF.7
This presumed superiority of ACE inhibitors over ARBs was thought to be a result of a greater vasodilatory effect caused by inhibiting the degradation of bradykinin and leading to increased levels of nitric oxide and vasoactive prostaglandins.8 Another proposed explanation was that because ARBs block angiotensin II AT1 receptors but not AT2 receptors, the increased stimulation of markedly upregulated AT2 receptors in atheromatous plaques in response to elevated serum levels of angiotensin II was deleterious.6 Therefore, ACE inhibitors have been recommended as first-line therapy by most guidelines, whereas ARBs are recommended as second-line therapy, when patients are unable to tolerate ACE inhibitors.
Nevertheless, the much debated differences in outcomes between ACE inhibitors and ARBs do not seem to be real and may have originated from a generational gap in the trials.
The ACE inhibitor trials were performed a decade earlier than the ARB trials. Indirect comparisons of their respective placebo-controlled trials assumed that the placebo groups used for comparison in the 2 sets of trials were similar.9,10 Actually, the rate of cardiovascular disease decreased nearly 50% between the decades of 1990 to 2000 and 2000 to 2010, the likely result of aggressive primary and secondary prevention strategies in clinical practice, including revascularization and lipid-lowering therapy.10
In fact, a meta-regression analysis showed that the differences between ACE inhibitors and ARBs compared with placebo were due to higher event rates in the placebo groups in the ACE inhibitor trials than in the ARB trials for the outcomes of death, cardiovascular death, and myocardial infarction.11 Sensitivity analyses restricted to trials published after 2000 to control for this generational gap showed similar efficacy with ACE inhibitors vs placebo and with ARBs vs placebo for all clinical outcomes.11 Moreover, recent studies have shown that ARBs produce a greater decrease in cardiovascular events than ACE inhibitors, especially in patients with established cardiovascular disease.12,13
An advantage of ARBs over ACE inhibitors is fewer adverse effects: in general, ARBs are better tolerated than ACE inhibitors.14 There are also ethnic differences in the risks of adverse reactions to these medications. African Americans have a higher risk of developing angioedema with ACE inhibitors compared with the rest of the US population, and Chinese Americans have a higher risk than whites of developing cough with ACE inhibitors.9,15
HOW I MANAGE THESE MEDICATIONS
In my medical practice, I try to make sure patients with HFrEF, hypertension, chronic kidney disease, and coronary artery disease with left ventricular dysfunction receive an inhibitor of the renin-angiotensin-aldosterone system.
Which agent?
I prefer ARBs because patients tolerate them better. I continue ACE inhibitors in patients who are already taking them without adverse effects, and I change to ARBs in patients who later become unable to tolerate ACE inhibitors.
Most antihypertensive agents increase the risk of incident gout, except for calcium channel blockers and losartan.16 Losartan is the only ARB with a uricosuric effect, although a mild one,17,18 due to inhibition of the urate transporter 1,19 and therefore I prefer to use it instead of other ARBs or ACE inhibitors in patients who have a concomitant diagnosis of gout.
Which combinations of agents?
The addition of beta-blockers and mineralocorticoid receptor blockers to ACE inhibitors or ARBs is associated with a further decrease in the mortality risk for patients with HFrEF,20–22 but some patients cannot tolerate these combinations or optimized doses of these medications because of worsening hypotension or increased risk of developing acute kidney injury or hyperkalemia.
In most cases, I try not to combine ACE inhibitors with ARBs. This combination may be useful in nondiabetic patients with proteinuria refractory to maximum treatment with 1 class of these agents, but it is associated with an increased risk of hyperkalemia or acute kidney injury in patients with diabetic nephropathy without improving rates of the clinical outcomes of death or cardiovascular events.23 I prefer adding a daily low dose of a mineralocorticoid receptor blocker to an ACE inhibitor or an ARB, which is more effective in controlling refractory proteinuria.24 This regimen is associated with decreased rates of mortality, cardiovascular mortality, and hospitalization for heart failure in patients with HFrEF,22 although it can lead to a higher frequency of hyperkalemia,25 and patients on it require frequent dietary education and monitoring of serum potassium.
I avoid combining direct renin inhibitors with ACE inhibitors or ARBs, since this combination has been contraindicated by the US Food and Drug Administration due to lack of reduction in target-organ damage and an associated increased risk of hypotension, hyperkalemia, and kidney failure, and a slight increase in the risk of stroke or death in patients with diabetic nephropathy.26
Valsartan-sacubitril
Neprilysin is a membrane-bound endopeptidase that degrades vasoactive peptides, including B-type natriuretic peptide and atrial natriuretic peptide.27 The combination of the ARB valsartan and the neprilysin inhibitor sacubitril is associated with a 20% further decrease in rates of cardiovascular mortality and hospitalization and a 16% decrease in total mortality for patients with HFrEF compared with an ACE inhibitor, although there can also be more hypotension and angioedema with the combination.27,28
Very importantly, an ACE inhibitor cannot be used together with valsartan-sacubitril due to increased risk of angioedema and cough. I change ACE inhibitors or ARBs to valsartan-sacubitril in patients with HFrEF who still have symptoms of heart failure. Interestingly, a network meta-analysis showed that the combination of valsartan-sacubitril plus a mineralocorticoid receptor blocker and a beta-blocker resulted in the greatest mortality reduction in patients with HFrEF.7 A word of caution, though: one can also expect an increased risk of hypotension, hyperkalemia, and kidney failure.
Monitoring
It is crucial to monitor blood pressure, serum potassium, and renal function in patients receiving ACE inhibitors, ARBs, mineralocorticoid receptor blockers, valsartan-sacubitril, or combinations of these medications, particularly in elderly patients, who are more susceptible to complications. I use a multidisciplinary approach in my clinic: a patient educator, dietitian, pharmacist, and advanced practice nurse play key roles in educating and monitoring patients for the development of possible complications from this therapy or interactions with other medications.
A recent population-based cohort study found an association of ACE inhibitor use with a 14% relative increase in lung cancer incidence after 10 years of use, compared with ARBs,29 but this may not represent a large absolute risk (calculated number needed to harm of 2,970 after 10 years of ACE inhibitor use) and should be balanced against the improvement in morbidity and mortality gained with use of an ACE inhibitor. Additional studies with long-term follow-up are needed to investigate this possible association.
TAKE-HOME POINTS
- Blockade of the renin-angiotensin-aldosterone system is a cornerstone in the therapy of cardiovascular disease.
- ARBs are as effective as ACE inhibitors and have a better tolerability profile.
- ACE inhibitors cause more angioedema in African Americans and more cough in Chinese Americans than in the rest of the population.
- ACE inhibitors and most ARBs (except for losartan) increase the risk of gout.
- The combination of beta-blockers and mineralocorticoid receptor blockers with ACE inhibitors or ARBs and, lately, the use of the valsartan-sacubitril combination have been increasingly beneficial for patients with HFrEF.
- Momoniat T, Ilyas D, Bhandari S. ACE inhibitors and ARBs: managing potassium and renal function. Cleve Clin J Med 2019; 86(9):601–607. doi:10.3949/ccjm.86a.18024
- CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316(23):1429–1435. doi:10.1056/NEJM198706043162301
- SOLVD Investigators; Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325(5):293–302. doi:10.1056/NEJM199108013250501
- Young JB, Dunlap ME, Pfeffer MA, et al; Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) Investigators and Committees. Mortality and morbidity reduction with candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection fraction trials. Circulation 2004; 110(17):2618–2626. doi:10.1161/01.CIR.0000146819.43235.A9
- Cohn JN, Tognoni G; Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345(23):1667–1675. doi:10.1056/NEJMoa010713
- Straus MH, Hall AS. Angiotensin receptor blockers do not reduce risk of myocardial infarction, cardiovascular death, or total mortality: further evidence for the ARB-MI paradox. Circulation 2017; 135(22):2088–2090. doi:10.1161/CIRCULATIONAHA.117.026112
- Burnett H, Earley A, Voors AA, et al. Thirty years of evidence on the efficacy of drug treatments for chronic heart failure with reduced ejection fraction. A network meta-analysis. Circ Heart Fail 2017; 10(1). pii:e003529. doi:10.1161/CIRCHEARTFAILURE.116.003529
- Chobanian AV. Editorial: angiotensin inhibition. N Engl J Med 1974; 291(16):844–845. doi:10.1056/NEJM197410172911611
- Messerli FH, Bangalore S, Bavishi C, Rimoldi SF. Angiotensin-converting enzyme inhibitors in hypertension: to use or not to use? J Am Coll Cardiol 2018; 71(13):1474–1482. doi:10.1016/j.jacc.2018.01.058
- Messerli FH, Bangalore S. Angiotensin receptor blockers reduce cardiovascular events, including the risk of myocardial infarction. Circulation 2017; 135(22):2085–2087. doi:10.1161/CIRCULATIONAHA.116.025950
- Bangalore S, Fakheri R, Toklu B, Ogedegbe G, Weintraub H, Messerli FH. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers in patients without heart failure? Insights from 254,301 patients from randomized trials. Mayo Clin Proc 2016; 91(1):51–60. doi:10.1016/j.mayocp.2015.10.019
- Potier L, Roussel R, Elbez Y, et al; REACH Registry Investigators. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in high vascular risk. Heart 2017; 103(17):1339–1346. doi:10.1136/heartjnl-2016-310705
- Bangalore S, Kumar S, Wetterslev J, Messerli FH. Angiotensin receptor blockers and risk of myocardial infarction: meta-analyses and trial sequential analyses of 147,020 patients from randomized trials. BMJ 2011; 342:d2234. doi:10.1136/bmj.d2234
- Saglimbene V, Palmer SC, Ruospo M, et al; Long-Term Impact of RAS Inhibition on Cardiorenal Outcomes (LIRICO) Investigators. The long-term impact of renin-angiotensin system (RAS) inhibition on cardiorenal outcomes (LIRICO): a randomized, controlled trial. J Am Soc Nephrol 2018; 29(12):2890–2899. doi:10.1681/ASN.2018040443
- McDowell SE, Coleman JJ, Ferner RE. Systematic review and meta-analysis of ethnic differences in risks of adverse reactions to drugs used in cardiovascular medicine. BMJ 2006; 332(7551):1177–1181. doi:10.1136/bmj.38803.528113.55
- Choi HK, Soriano LC, Zhang Y, Rodríguez LA. Antihypertensive drugs and risk of incident gout among patients with hypertension: population based case-control study. BMJ 2012; 344:d8190. doi:10.1136/bmj.d8190
- Wolff ML, Cruz JL, Vanderman AJ, Brown JN. The effect of angiotensin II receptor blockers on hyperuricemia. Ther Adv Chronic Dis 2015; 6(6):339–346. doi:10.1177/2040622315596119
- Schmidt A, Gruber U, Böhmig G, Köller E, Mayer G. The effect of ACE inhibitor and angiotensin II receptor antagonist therapy on serum uric acid levels and potassium homeostasis in hypertensive renal transplant recipients treated with CsA. Nephrol Dial Transplant 2001; 16(5):1034–1037. pmid:11328912
- Hamada T, Ichida K, Hosoyamada M, et al. Uricosuric action of losartan via the inhibition of urate transporter 1 (URAT1) in hypertensive patients. Am J Hypertens 2008; 21(10):1157–1162. doi:10.1038/ajh.2008.245
- Packer M, Coats AJ, Fowler MB, et al; Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344(22):1651–1658. doi:10.1056/NEJM200105313442201
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341(10):709–717. doi:10.1056/NEJM199909023411001
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364(1):11-21. doi:10.1056/NEJMoa1009492
- Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369(20):1892–1903. doi:10.1056/NEJMoa1303154
- Chrysostomou A, Pedagogos E, MacGregor L, Becker GJ. Double-blind, placebo-controlled study on the effect of the aldosterone receptor antagonist spironolactone in patients who have persistent proteinuria and are on long-term angiotensin-converting enzyme inhibitor therapy, with or without an angiotensin II receptor blocker. Clin J Am Soc Nephrol 2006; 1(2):256–262. doi:10.2215/CJN.01040905
- Abbas S, Ihle P, Harder S, Schubert I. Risk of hyperkalemia and combined use of spironolactone and long-term ACE inhibitor/angiotensin receptor blocker therapy in heart failure using real-life data: a population- and insurance-based cohort. Pharmacoepidemiol Drug Saf 2015; 24(4):406–413. doi:10.1002/pds.3748
- US Food and Drug Administration. FDA drug safety communication: new warning and contraindication for blood pressure medicines containing aliskiren (Tekturna). www.fda.gov/Drugs/DrugSafety/ucm300889.htm. Accessed March 8, 2019.
- Jhund PS, McMurray JJ. The neprilysin pathway in heart failure: a review and guide on the use of sacubitril/valsartan. Heart 2016; 102(17):1342–1347. doi:10.1136/heartjnl-2014-306775
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371(11):993–1004. doi:10.1056/NEJMoa1409077
- Hicks BM, Filion KB, Yin H, Sakr L, Udell JA, Azoulay L. Angiotensin converting enzyme inhibitors and risk of lung cancer: population based cohort study. BMJ 2018; 363:k4209. doi:10.1136/bmj.k4209
Inhibition of the renin-angiotensin-aldosterone system with angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) is widely used in the treatment of heart failure, hypertension, chronic kidney disease, and coronary artery disease with left ventricular dysfunction.
In this issue, Momoniat et al1 review the benefits of ACE inhibitors and ARBs and how to manage adverse effects. I would like to add some of my own observations.
ARE ACE INHIBITORS REALLY BETTER THAN ARBs?
ACE inhibitors have been the cornerstone of treatment for patients with heart failure with reduced ejection fraction (HFrEF), in whom their use is associated with reduced rates of morbidity and death.2,3 The use of ARBs in these patients is also associated with decreased rates of morbidity and death4,5; however, in early comparisons, ACE inhibitors were deemed more effective in decreasing the incidence of myocardial infarction, cardiovascular death, and all-cause mortality in patients with hypertension, diabetes, and increased cardiovascular risk,6 and all-cause mortality in patients with HFrEF.7
This presumed superiority of ACE inhibitors over ARBs was thought to be a result of a greater vasodilatory effect caused by inhibiting the degradation of bradykinin and leading to increased levels of nitric oxide and vasoactive prostaglandins.8 Another proposed explanation was that because ARBs block angiotensin II AT1 receptors but not AT2 receptors, the increased stimulation of markedly upregulated AT2 receptors in atheromatous plaques in response to elevated serum levels of angiotensin II was deleterious.6 Therefore, ACE inhibitors have been recommended as first-line therapy by most guidelines, whereas ARBs are recommended as second-line therapy, when patients are unable to tolerate ACE inhibitors.
Nevertheless, the much debated differences in outcomes between ACE inhibitors and ARBs do not seem to be real and may have originated from a generational gap in the trials.
The ACE inhibitor trials were performed a decade earlier than the ARB trials. Indirect comparisons of their respective placebo-controlled trials assumed that the placebo groups used for comparison in the 2 sets of trials were similar.9,10 Actually, the rate of cardiovascular disease decreased nearly 50% between the decades of 1990 to 2000 and 2000 to 2010, the likely result of aggressive primary and secondary prevention strategies in clinical practice, including revascularization and lipid-lowering therapy.10
In fact, a meta-regression analysis showed that the differences between ACE inhibitors and ARBs compared with placebo were due to higher event rates in the placebo groups in the ACE inhibitor trials than in the ARB trials for the outcomes of death, cardiovascular death, and myocardial infarction.11 Sensitivity analyses restricted to trials published after 2000 to control for this generational gap showed similar efficacy with ACE inhibitors vs placebo and with ARBs vs placebo for all clinical outcomes.11 Moreover, recent studies have shown that ARBs produce a greater decrease in cardiovascular events than ACE inhibitors, especially in patients with established cardiovascular disease.12,13
An advantage of ARBs over ACE inhibitors is fewer adverse effects: in general, ARBs are better tolerated than ACE inhibitors.14 There are also ethnic differences in the risks of adverse reactions to these medications. African Americans have a higher risk of developing angioedema with ACE inhibitors compared with the rest of the US population, and Chinese Americans have a higher risk than whites of developing cough with ACE inhibitors.9,15
HOW I MANAGE THESE MEDICATIONS
In my medical practice, I try to make sure patients with HFrEF, hypertension, chronic kidney disease, and coronary artery disease with left ventricular dysfunction receive an inhibitor of the renin-angiotensin-aldosterone system.
Which agent?
I prefer ARBs because patients tolerate them better. I continue ACE inhibitors in patients who are already taking them without adverse effects, and I change to ARBs in patients who later become unable to tolerate ACE inhibitors.
Most antihypertensive agents increase the risk of incident gout, except for calcium channel blockers and losartan.16 Losartan is the only ARB with a uricosuric effect, although a mild one,17,18 due to inhibition of the urate transporter 1,19 and therefore I prefer to use it instead of other ARBs or ACE inhibitors in patients who have a concomitant diagnosis of gout.
Which combinations of agents?
The addition of beta-blockers and mineralocorticoid receptor blockers to ACE inhibitors or ARBs is associated with a further decrease in the mortality risk for patients with HFrEF,20–22 but some patients cannot tolerate these combinations or optimized doses of these medications because of worsening hypotension or increased risk of developing acute kidney injury or hyperkalemia.
In most cases, I try not to combine ACE inhibitors with ARBs. This combination may be useful in nondiabetic patients with proteinuria refractory to maximum treatment with 1 class of these agents, but it is associated with an increased risk of hyperkalemia or acute kidney injury in patients with diabetic nephropathy without improving rates of the clinical outcomes of death or cardiovascular events.23 I prefer adding a daily low dose of a mineralocorticoid receptor blocker to an ACE inhibitor or an ARB, which is more effective in controlling refractory proteinuria.24 This regimen is associated with decreased rates of mortality, cardiovascular mortality, and hospitalization for heart failure in patients with HFrEF,22 although it can lead to a higher frequency of hyperkalemia,25 and patients on it require frequent dietary education and monitoring of serum potassium.
I avoid combining direct renin inhibitors with ACE inhibitors or ARBs, since this combination has been contraindicated by the US Food and Drug Administration due to lack of reduction in target-organ damage and an associated increased risk of hypotension, hyperkalemia, and kidney failure, and a slight increase in the risk of stroke or death in patients with diabetic nephropathy.26
Valsartan-sacubitril
Neprilysin is a membrane-bound endopeptidase that degrades vasoactive peptides, including B-type natriuretic peptide and atrial natriuretic peptide.27 The combination of the ARB valsartan and the neprilysin inhibitor sacubitril is associated with a 20% further decrease in rates of cardiovascular mortality and hospitalization and a 16% decrease in total mortality for patients with HFrEF compared with an ACE inhibitor, although there can also be more hypotension and angioedema with the combination.27,28
Very importantly, an ACE inhibitor cannot be used together with valsartan-sacubitril due to increased risk of angioedema and cough. I change ACE inhibitors or ARBs to valsartan-sacubitril in patients with HFrEF who still have symptoms of heart failure. Interestingly, a network meta-analysis showed that the combination of valsartan-sacubitril plus a mineralocorticoid receptor blocker and a beta-blocker resulted in the greatest mortality reduction in patients with HFrEF.7 A word of caution, though: one can also expect an increased risk of hypotension, hyperkalemia, and kidney failure.
Monitoring
It is crucial to monitor blood pressure, serum potassium, and renal function in patients receiving ACE inhibitors, ARBs, mineralocorticoid receptor blockers, valsartan-sacubitril, or combinations of these medications, particularly in elderly patients, who are more susceptible to complications. I use a multidisciplinary approach in my clinic: a patient educator, dietitian, pharmacist, and advanced practice nurse play key roles in educating and monitoring patients for the development of possible complications from this therapy or interactions with other medications.
A recent population-based cohort study found an association of ACE inhibitor use with a 14% relative increase in lung cancer incidence after 10 years of use, compared with ARBs,29 but this may not represent a large absolute risk (calculated number needed to harm of 2,970 after 10 years of ACE inhibitor use) and should be balanced against the improvement in morbidity and mortality gained with use of an ACE inhibitor. Additional studies with long-term follow-up are needed to investigate this possible association.
TAKE-HOME POINTS
- Blockade of the renin-angiotensin-aldosterone system is a cornerstone in the therapy of cardiovascular disease.
- ARBs are as effective as ACE inhibitors and have a better tolerability profile.
- ACE inhibitors cause more angioedema in African Americans and more cough in Chinese Americans than in the rest of the population.
- ACE inhibitors and most ARBs (except for losartan) increase the risk of gout.
- The combination of beta-blockers and mineralocorticoid receptor blockers with ACE inhibitors or ARBs and, lately, the use of the valsartan-sacubitril combination have been increasingly beneficial for patients with HFrEF.
Inhibition of the renin-angiotensin-aldosterone system with angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) is widely used in the treatment of heart failure, hypertension, chronic kidney disease, and coronary artery disease with left ventricular dysfunction.
In this issue, Momoniat et al1 review the benefits of ACE inhibitors and ARBs and how to manage adverse effects. I would like to add some of my own observations.
ARE ACE INHIBITORS REALLY BETTER THAN ARBs?
ACE inhibitors have been the cornerstone of treatment for patients with heart failure with reduced ejection fraction (HFrEF), in whom their use is associated with reduced rates of morbidity and death.2,3 The use of ARBs in these patients is also associated with decreased rates of morbidity and death4,5; however, in early comparisons, ACE inhibitors were deemed more effective in decreasing the incidence of myocardial infarction, cardiovascular death, and all-cause mortality in patients with hypertension, diabetes, and increased cardiovascular risk,6 and all-cause mortality in patients with HFrEF.7
This presumed superiority of ACE inhibitors over ARBs was thought to be a result of a greater vasodilatory effect caused by inhibiting the degradation of bradykinin and leading to increased levels of nitric oxide and vasoactive prostaglandins.8 Another proposed explanation was that because ARBs block angiotensin II AT1 receptors but not AT2 receptors, the increased stimulation of markedly upregulated AT2 receptors in atheromatous plaques in response to elevated serum levels of angiotensin II was deleterious.6 Therefore, ACE inhibitors have been recommended as first-line therapy by most guidelines, whereas ARBs are recommended as second-line therapy, when patients are unable to tolerate ACE inhibitors.
Nevertheless, the much debated differences in outcomes between ACE inhibitors and ARBs do not seem to be real and may have originated from a generational gap in the trials.
The ACE inhibitor trials were performed a decade earlier than the ARB trials. Indirect comparisons of their respective placebo-controlled trials assumed that the placebo groups used for comparison in the 2 sets of trials were similar.9,10 Actually, the rate of cardiovascular disease decreased nearly 50% between the decades of 1990 to 2000 and 2000 to 2010, the likely result of aggressive primary and secondary prevention strategies in clinical practice, including revascularization and lipid-lowering therapy.10
In fact, a meta-regression analysis showed that the differences between ACE inhibitors and ARBs compared with placebo were due to higher event rates in the placebo groups in the ACE inhibitor trials than in the ARB trials for the outcomes of death, cardiovascular death, and myocardial infarction.11 Sensitivity analyses restricted to trials published after 2000 to control for this generational gap showed similar efficacy with ACE inhibitors vs placebo and with ARBs vs placebo for all clinical outcomes.11 Moreover, recent studies have shown that ARBs produce a greater decrease in cardiovascular events than ACE inhibitors, especially in patients with established cardiovascular disease.12,13
An advantage of ARBs over ACE inhibitors is fewer adverse effects: in general, ARBs are better tolerated than ACE inhibitors.14 There are also ethnic differences in the risks of adverse reactions to these medications. African Americans have a higher risk of developing angioedema with ACE inhibitors compared with the rest of the US population, and Chinese Americans have a higher risk than whites of developing cough with ACE inhibitors.9,15
HOW I MANAGE THESE MEDICATIONS
In my medical practice, I try to make sure patients with HFrEF, hypertension, chronic kidney disease, and coronary artery disease with left ventricular dysfunction receive an inhibitor of the renin-angiotensin-aldosterone system.
Which agent?
I prefer ARBs because patients tolerate them better. I continue ACE inhibitors in patients who are already taking them without adverse effects, and I change to ARBs in patients who later become unable to tolerate ACE inhibitors.
Most antihypertensive agents increase the risk of incident gout, except for calcium channel blockers and losartan.16 Losartan is the only ARB with a uricosuric effect, although a mild one,17,18 due to inhibition of the urate transporter 1,19 and therefore I prefer to use it instead of other ARBs or ACE inhibitors in patients who have a concomitant diagnosis of gout.
Which combinations of agents?
The addition of beta-blockers and mineralocorticoid receptor blockers to ACE inhibitors or ARBs is associated with a further decrease in the mortality risk for patients with HFrEF,20–22 but some patients cannot tolerate these combinations or optimized doses of these medications because of worsening hypotension or increased risk of developing acute kidney injury or hyperkalemia.
In most cases, I try not to combine ACE inhibitors with ARBs. This combination may be useful in nondiabetic patients with proteinuria refractory to maximum treatment with 1 class of these agents, but it is associated with an increased risk of hyperkalemia or acute kidney injury in patients with diabetic nephropathy without improving rates of the clinical outcomes of death or cardiovascular events.23 I prefer adding a daily low dose of a mineralocorticoid receptor blocker to an ACE inhibitor or an ARB, which is more effective in controlling refractory proteinuria.24 This regimen is associated with decreased rates of mortality, cardiovascular mortality, and hospitalization for heart failure in patients with HFrEF,22 although it can lead to a higher frequency of hyperkalemia,25 and patients on it require frequent dietary education and monitoring of serum potassium.
I avoid combining direct renin inhibitors with ACE inhibitors or ARBs, since this combination has been contraindicated by the US Food and Drug Administration due to lack of reduction in target-organ damage and an associated increased risk of hypotension, hyperkalemia, and kidney failure, and a slight increase in the risk of stroke or death in patients with diabetic nephropathy.26
Valsartan-sacubitril
Neprilysin is a membrane-bound endopeptidase that degrades vasoactive peptides, including B-type natriuretic peptide and atrial natriuretic peptide.27 The combination of the ARB valsartan and the neprilysin inhibitor sacubitril is associated with a 20% further decrease in rates of cardiovascular mortality and hospitalization and a 16% decrease in total mortality for patients with HFrEF compared with an ACE inhibitor, although there can also be more hypotension and angioedema with the combination.27,28
Very importantly, an ACE inhibitor cannot be used together with valsartan-sacubitril due to increased risk of angioedema and cough. I change ACE inhibitors or ARBs to valsartan-sacubitril in patients with HFrEF who still have symptoms of heart failure. Interestingly, a network meta-analysis showed that the combination of valsartan-sacubitril plus a mineralocorticoid receptor blocker and a beta-blocker resulted in the greatest mortality reduction in patients with HFrEF.7 A word of caution, though: one can also expect an increased risk of hypotension, hyperkalemia, and kidney failure.
Monitoring
It is crucial to monitor blood pressure, serum potassium, and renal function in patients receiving ACE inhibitors, ARBs, mineralocorticoid receptor blockers, valsartan-sacubitril, or combinations of these medications, particularly in elderly patients, who are more susceptible to complications. I use a multidisciplinary approach in my clinic: a patient educator, dietitian, pharmacist, and advanced practice nurse play key roles in educating and monitoring patients for the development of possible complications from this therapy or interactions with other medications.
A recent population-based cohort study found an association of ACE inhibitor use with a 14% relative increase in lung cancer incidence after 10 years of use, compared with ARBs,29 but this may not represent a large absolute risk (calculated number needed to harm of 2,970 after 10 years of ACE inhibitor use) and should be balanced against the improvement in morbidity and mortality gained with use of an ACE inhibitor. Additional studies with long-term follow-up are needed to investigate this possible association.
TAKE-HOME POINTS
- Blockade of the renin-angiotensin-aldosterone system is a cornerstone in the therapy of cardiovascular disease.
- ARBs are as effective as ACE inhibitors and have a better tolerability profile.
- ACE inhibitors cause more angioedema in African Americans and more cough in Chinese Americans than in the rest of the population.
- ACE inhibitors and most ARBs (except for losartan) increase the risk of gout.
- The combination of beta-blockers and mineralocorticoid receptor blockers with ACE inhibitors or ARBs and, lately, the use of the valsartan-sacubitril combination have been increasingly beneficial for patients with HFrEF.
- Momoniat T, Ilyas D, Bhandari S. ACE inhibitors and ARBs: managing potassium and renal function. Cleve Clin J Med 2019; 86(9):601–607. doi:10.3949/ccjm.86a.18024
- CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316(23):1429–1435. doi:10.1056/NEJM198706043162301
- SOLVD Investigators; Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325(5):293–302. doi:10.1056/NEJM199108013250501
- Young JB, Dunlap ME, Pfeffer MA, et al; Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) Investigators and Committees. Mortality and morbidity reduction with candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection fraction trials. Circulation 2004; 110(17):2618–2626. doi:10.1161/01.CIR.0000146819.43235.A9
- Cohn JN, Tognoni G; Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345(23):1667–1675. doi:10.1056/NEJMoa010713
- Straus MH, Hall AS. Angiotensin receptor blockers do not reduce risk of myocardial infarction, cardiovascular death, or total mortality: further evidence for the ARB-MI paradox. Circulation 2017; 135(22):2088–2090. doi:10.1161/CIRCULATIONAHA.117.026112
- Burnett H, Earley A, Voors AA, et al. Thirty years of evidence on the efficacy of drug treatments for chronic heart failure with reduced ejection fraction. A network meta-analysis. Circ Heart Fail 2017; 10(1). pii:e003529. doi:10.1161/CIRCHEARTFAILURE.116.003529
- Chobanian AV. Editorial: angiotensin inhibition. N Engl J Med 1974; 291(16):844–845. doi:10.1056/NEJM197410172911611
- Messerli FH, Bangalore S, Bavishi C, Rimoldi SF. Angiotensin-converting enzyme inhibitors in hypertension: to use or not to use? J Am Coll Cardiol 2018; 71(13):1474–1482. doi:10.1016/j.jacc.2018.01.058
- Messerli FH, Bangalore S. Angiotensin receptor blockers reduce cardiovascular events, including the risk of myocardial infarction. Circulation 2017; 135(22):2085–2087. doi:10.1161/CIRCULATIONAHA.116.025950
- Bangalore S, Fakheri R, Toklu B, Ogedegbe G, Weintraub H, Messerli FH. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers in patients without heart failure? Insights from 254,301 patients from randomized trials. Mayo Clin Proc 2016; 91(1):51–60. doi:10.1016/j.mayocp.2015.10.019
- Potier L, Roussel R, Elbez Y, et al; REACH Registry Investigators. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in high vascular risk. Heart 2017; 103(17):1339–1346. doi:10.1136/heartjnl-2016-310705
- Bangalore S, Kumar S, Wetterslev J, Messerli FH. Angiotensin receptor blockers and risk of myocardial infarction: meta-analyses and trial sequential analyses of 147,020 patients from randomized trials. BMJ 2011; 342:d2234. doi:10.1136/bmj.d2234
- Saglimbene V, Palmer SC, Ruospo M, et al; Long-Term Impact of RAS Inhibition on Cardiorenal Outcomes (LIRICO) Investigators. The long-term impact of renin-angiotensin system (RAS) inhibition on cardiorenal outcomes (LIRICO): a randomized, controlled trial. J Am Soc Nephrol 2018; 29(12):2890–2899. doi:10.1681/ASN.2018040443
- McDowell SE, Coleman JJ, Ferner RE. Systematic review and meta-analysis of ethnic differences in risks of adverse reactions to drugs used in cardiovascular medicine. BMJ 2006; 332(7551):1177–1181. doi:10.1136/bmj.38803.528113.55
- Choi HK, Soriano LC, Zhang Y, Rodríguez LA. Antihypertensive drugs and risk of incident gout among patients with hypertension: population based case-control study. BMJ 2012; 344:d8190. doi:10.1136/bmj.d8190
- Wolff ML, Cruz JL, Vanderman AJ, Brown JN. The effect of angiotensin II receptor blockers on hyperuricemia. Ther Adv Chronic Dis 2015; 6(6):339–346. doi:10.1177/2040622315596119
- Schmidt A, Gruber U, Böhmig G, Köller E, Mayer G. The effect of ACE inhibitor and angiotensin II receptor antagonist therapy on serum uric acid levels and potassium homeostasis in hypertensive renal transplant recipients treated with CsA. Nephrol Dial Transplant 2001; 16(5):1034–1037. pmid:11328912
- Hamada T, Ichida K, Hosoyamada M, et al. Uricosuric action of losartan via the inhibition of urate transporter 1 (URAT1) in hypertensive patients. Am J Hypertens 2008; 21(10):1157–1162. doi:10.1038/ajh.2008.245
- Packer M, Coats AJ, Fowler MB, et al; Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344(22):1651–1658. doi:10.1056/NEJM200105313442201
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341(10):709–717. doi:10.1056/NEJM199909023411001
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364(1):11-21. doi:10.1056/NEJMoa1009492
- Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369(20):1892–1903. doi:10.1056/NEJMoa1303154
- Chrysostomou A, Pedagogos E, MacGregor L, Becker GJ. Double-blind, placebo-controlled study on the effect of the aldosterone receptor antagonist spironolactone in patients who have persistent proteinuria and are on long-term angiotensin-converting enzyme inhibitor therapy, with or without an angiotensin II receptor blocker. Clin J Am Soc Nephrol 2006; 1(2):256–262. doi:10.2215/CJN.01040905
- Abbas S, Ihle P, Harder S, Schubert I. Risk of hyperkalemia and combined use of spironolactone and long-term ACE inhibitor/angiotensin receptor blocker therapy in heart failure using real-life data: a population- and insurance-based cohort. Pharmacoepidemiol Drug Saf 2015; 24(4):406–413. doi:10.1002/pds.3748
- US Food and Drug Administration. FDA drug safety communication: new warning and contraindication for blood pressure medicines containing aliskiren (Tekturna). www.fda.gov/Drugs/DrugSafety/ucm300889.htm. Accessed March 8, 2019.
- Jhund PS, McMurray JJ. The neprilysin pathway in heart failure: a review and guide on the use of sacubitril/valsartan. Heart 2016; 102(17):1342–1347. doi:10.1136/heartjnl-2014-306775
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371(11):993–1004. doi:10.1056/NEJMoa1409077
- Hicks BM, Filion KB, Yin H, Sakr L, Udell JA, Azoulay L. Angiotensin converting enzyme inhibitors and risk of lung cancer: population based cohort study. BMJ 2018; 363:k4209. doi:10.1136/bmj.k4209
- Momoniat T, Ilyas D, Bhandari S. ACE inhibitors and ARBs: managing potassium and renal function. Cleve Clin J Med 2019; 86(9):601–607. doi:10.3949/ccjm.86a.18024
- CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316(23):1429–1435. doi:10.1056/NEJM198706043162301
- SOLVD Investigators; Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325(5):293–302. doi:10.1056/NEJM199108013250501
- Young JB, Dunlap ME, Pfeffer MA, et al; Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) Investigators and Committees. Mortality and morbidity reduction with candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection fraction trials. Circulation 2004; 110(17):2618–2626. doi:10.1161/01.CIR.0000146819.43235.A9
- Cohn JN, Tognoni G; Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345(23):1667–1675. doi:10.1056/NEJMoa010713
- Straus MH, Hall AS. Angiotensin receptor blockers do not reduce risk of myocardial infarction, cardiovascular death, or total mortality: further evidence for the ARB-MI paradox. Circulation 2017; 135(22):2088–2090. doi:10.1161/CIRCULATIONAHA.117.026112
- Burnett H, Earley A, Voors AA, et al. Thirty years of evidence on the efficacy of drug treatments for chronic heart failure with reduced ejection fraction. A network meta-analysis. Circ Heart Fail 2017; 10(1). pii:e003529. doi:10.1161/CIRCHEARTFAILURE.116.003529
- Chobanian AV. Editorial: angiotensin inhibition. N Engl J Med 1974; 291(16):844–845. doi:10.1056/NEJM197410172911611
- Messerli FH, Bangalore S, Bavishi C, Rimoldi SF. Angiotensin-converting enzyme inhibitors in hypertension: to use or not to use? J Am Coll Cardiol 2018; 71(13):1474–1482. doi:10.1016/j.jacc.2018.01.058
- Messerli FH, Bangalore S. Angiotensin receptor blockers reduce cardiovascular events, including the risk of myocardial infarction. Circulation 2017; 135(22):2085–2087. doi:10.1161/CIRCULATIONAHA.116.025950
- Bangalore S, Fakheri R, Toklu B, Ogedegbe G, Weintraub H, Messerli FH. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers in patients without heart failure? Insights from 254,301 patients from randomized trials. Mayo Clin Proc 2016; 91(1):51–60. doi:10.1016/j.mayocp.2015.10.019
- Potier L, Roussel R, Elbez Y, et al; REACH Registry Investigators. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in high vascular risk. Heart 2017; 103(17):1339–1346. doi:10.1136/heartjnl-2016-310705
- Bangalore S, Kumar S, Wetterslev J, Messerli FH. Angiotensin receptor blockers and risk of myocardial infarction: meta-analyses and trial sequential analyses of 147,020 patients from randomized trials. BMJ 2011; 342:d2234. doi:10.1136/bmj.d2234
- Saglimbene V, Palmer SC, Ruospo M, et al; Long-Term Impact of RAS Inhibition on Cardiorenal Outcomes (LIRICO) Investigators. The long-term impact of renin-angiotensin system (RAS) inhibition on cardiorenal outcomes (LIRICO): a randomized, controlled trial. J Am Soc Nephrol 2018; 29(12):2890–2899. doi:10.1681/ASN.2018040443
- McDowell SE, Coleman JJ, Ferner RE. Systematic review and meta-analysis of ethnic differences in risks of adverse reactions to drugs used in cardiovascular medicine. BMJ 2006; 332(7551):1177–1181. doi:10.1136/bmj.38803.528113.55
- Choi HK, Soriano LC, Zhang Y, Rodríguez LA. Antihypertensive drugs and risk of incident gout among patients with hypertension: population based case-control study. BMJ 2012; 344:d8190. doi:10.1136/bmj.d8190
- Wolff ML, Cruz JL, Vanderman AJ, Brown JN. The effect of angiotensin II receptor blockers on hyperuricemia. Ther Adv Chronic Dis 2015; 6(6):339–346. doi:10.1177/2040622315596119
- Schmidt A, Gruber U, Böhmig G, Köller E, Mayer G. The effect of ACE inhibitor and angiotensin II receptor antagonist therapy on serum uric acid levels and potassium homeostasis in hypertensive renal transplant recipients treated with CsA. Nephrol Dial Transplant 2001; 16(5):1034–1037. pmid:11328912
- Hamada T, Ichida K, Hosoyamada M, et al. Uricosuric action of losartan via the inhibition of urate transporter 1 (URAT1) in hypertensive patients. Am J Hypertens 2008; 21(10):1157–1162. doi:10.1038/ajh.2008.245
- Packer M, Coats AJ, Fowler MB, et al; Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344(22):1651–1658. doi:10.1056/NEJM200105313442201
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341(10):709–717. doi:10.1056/NEJM199909023411001
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364(1):11-21. doi:10.1056/NEJMoa1009492
- Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369(20):1892–1903. doi:10.1056/NEJMoa1303154
- Chrysostomou A, Pedagogos E, MacGregor L, Becker GJ. Double-blind, placebo-controlled study on the effect of the aldosterone receptor antagonist spironolactone in patients who have persistent proteinuria and are on long-term angiotensin-converting enzyme inhibitor therapy, with or without an angiotensin II receptor blocker. Clin J Am Soc Nephrol 2006; 1(2):256–262. doi:10.2215/CJN.01040905
- Abbas S, Ihle P, Harder S, Schubert I. Risk of hyperkalemia and combined use of spironolactone and long-term ACE inhibitor/angiotensin receptor blocker therapy in heart failure using real-life data: a population- and insurance-based cohort. Pharmacoepidemiol Drug Saf 2015; 24(4):406–413. doi:10.1002/pds.3748
- US Food and Drug Administration. FDA drug safety communication: new warning and contraindication for blood pressure medicines containing aliskiren (Tekturna). www.fda.gov/Drugs/DrugSafety/ucm300889.htm. Accessed March 8, 2019.
- Jhund PS, McMurray JJ. The neprilysin pathway in heart failure: a review and guide on the use of sacubitril/valsartan. Heart 2016; 102(17):1342–1347. doi:10.1136/heartjnl-2014-306775
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371(11):993–1004. doi:10.1056/NEJMoa1409077
- Hicks BM, Filion KB, Yin H, Sakr L, Udell JA, Azoulay L. Angiotensin converting enzyme inhibitors and risk of lung cancer: population based cohort study. BMJ 2018; 363:k4209. doi:10.1136/bmj.k4209
Diabetes management: Beyond hemoglobin A1c
When scientists discovered the band of hemoglobin A1c during electrophoresis in the 1950s and 1960s and discerned it was elevated in patients with diabetes, little did they know the important role it would play in the diagnosis and treatment of diabetes in the decades to come.1–3 Despite some caveats, a hemoglobin A1c level of 6.5% or higher is diagnostic of diabetes across most populations, and hemoglobin A1c goals ranging from 6.5% to 7.5% have been set for different subsets of patients depending on comorbidities, complications, risk of hypoglycemia, life expectancy, disease duration, patient preferences, and available resources.4
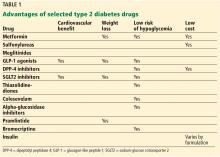
With a growing number of medications for diabetes—insulin in its various formulations and 11 other classes—hemoglobin A1c targets can now be tailored to fit individual patient profiles. Although helping patients attain their glycemic goals is paramount, other factors should be considered when prescribing or changing a drug treatment regimen, such as cardiovascular risk reduction, weight control, avoidance of hypoglycemia, and minimizing out-of-pocket drug costs (Table 1).
CARDIOVASCULAR BENEFIT
Patients with type 2 diabetes have a 2 to 3 times higher risk of clinical atherosclerotic disease, according to 20 years of surveillance data from the Framingham cohort.5
Mixed results with intensive treatment
Reducing cardiovascular risk remains an important goal in diabetes management, but unfortunately, data from the long-term clinical trials aimed at reducing macrovascular risk with intensive glycemic management have been conflicting.
The United Kingdom Prospective Diabetes Study (UKPDS),6 which enrolled more than 4,000 patients with newly diagnosed type 2 diabetes, did not initially show a statistically significant difference in the incidence of myocardial infarction with intensive control vs conventional control, although intensive treatment did reduce the incidence of microvascular disease. However, 10 years after the trial ended, the incidence was 15% lower in the intensive-treatment group than in the conventional-treatment group, and the difference was statistically significant.7
A 10-year follow-up analysis of the Veterans Affairs Diabetes Trial (VADT)8 showed that patients who had been randomly assigned to intensive glucose control for 5.6 years had 8.6 fewer major cardiovascular events per 1,000 person-years than those assigned to standard therapy, but no improvement in median overall survival. The hemoglobin A1c levels achieved during the trial were 6.9% and 8.4%, respectively.
In 2008, the US Food and Drug Administration (FDA)9 mandated that all new applications for diabetes drugs must include cardiovascular outcome studies. Therefore, we now have data on the cardiovascular benefits of two antihyperglycemic drug classes—incretins and sodium-glucose cotransporter 2 (SGLT2) inhibitors, making them attractive medications to target both cardiac and glucose concerns.
Incretins
The incretin drugs comprise 2 classes, glucagon-like peptide 1 (GLP-1) receptor agonists and dipeptidyl peptidase 4 (DPP-4) inhibitors.
Liraglutide. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial10 compared liraglutide (a GLP-1 receptor agonist) and placebo in 9,000 patients with diabetes who either had or were at high risk of cardiovascular disease. Patients in the liraglutide group had a lower risk of the primary composite end point of death from cardiovascular causes or the first episode of nonfatal (including silent) myocardial infarction or nonfatal stroke, and a lower risk of cardiovascular death, all-cause mortality, and microvascular events than those in the placebo group. The number of patients who would need to be treated to prevent 1 event in 3 years was 66 in the analysis of the primary outcome and 98 in the analysis of death from any cause.9
Lixisenatide. The Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial11 studied the effect of the once-daily GLP-1 receptor agonist lixisenatide on cardiovascular outcomes in 6,000 patients with type 2 diabetes with a recent coronary event. In contrast to LEADER, ELIXA did not show a cardiovascular benefit over placebo.
Exenatide. The Exenatide Study of Cardiovascular Event Lowering (EXSCEL)12 assessed another GLP-1 extended-release drug, exenatide, in 14,000 patients, 73% of whom had established cardiovascular disease. In those patients, the drug had a modest benefit in terms of first occurrence of any component of the composite outcome of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke (3-component major adverse cardiac event [MACE] outcome) in a time-to-event analysis, but the results were not statistically significant. However, the drug did significantly reduce all-cause mortality.
Semaglutide, another GLP-1 receptor agonist recently approved by the FDA, also showed benefit in patients who had cardiovascular disease or were at high risk, with significant reduction in the primary composite end point of death from cardiovascular causes or the first occurrence of nonfatal myocardial infarction (including silent) or nonfatal stroke.13
Dulaglutide, a newer GLP-1 drug, was associated with significantly reduced major adverse cardiovascular events (a composite end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) in about 9,900 patients with diabetes, with a median follow-up of more than 5 years. Only 31% of the patients in the trial had established cardiovascular disease.14
Comment. GLP-1 drugs as a class are a good option for patients with diabetes who require weight loss, and liraglutide is now FDA-approved for reduction of cardiovascular events in patients with type 2 diabetes with established cardiovascular disease. However, other factors should be considered when prescribing these drugs: they have adverse gastrointestinal effects, the cardiovascular benefit was not a class effect, they are relatively expensive, and they must be injected. Also, they should not be prescribed concurrently with a DPP-4 inhibitor because they target the same pathway.
SGLT2 inhibitors
The other class of diabetes drugs that have shown cardiovascular benefit are the SGLT2 inhibitors.
Empagliflozin. The Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG)15 compared the efficacy of empagliflozin vs placebo in 7,000 patients with diabetes and cardiovascular disease and showed relative risk reductions of 38% in death from cardiovascular death, 31% in sudden death, and 35% in heart failure hospitalizations. Empagliflozin also showed benefit in terms of progression of kidney disease and occurrence of clinically relevant renal events in this population.16
Canagliflozin also has cardiovascular outcome data and showed significant benefit when compared with placebo in the primary outcome of the composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, but no significant effects on cardiovascular death or all-cause mortality.17 Data from this trial also suggested a nonsignificant benefit of canagliflozin in decreasing progression of albuminuria and in the composite outcome of a sustained 40% reduction in the estimated glomerular filtration rate (eGFR), the need for renal replacement therapy, or death from renal causes.
The above data led to an additional indication from the FDA for empagliflozin—and recently, canagliflozin—to prevent cardiovascular death in patients with diabetes with established disease, but other factors should be considered when prescribing them. Patients taking canagliflozin showed a significantly increased risk of amputation. SGLT2 inhibitors as a class also increase the risk of genital infections in men and women; this is an important consideration since patients with diabetes complain of vaginal fungal and urinary tract infections even without the use of these drugs. A higher incidence of fractures with canagliflozin should also be considered when using these medications in elderly and osteoporosis-prone patients at high risk of falling.
Dapagliflozin, the third drug in this class, was associated with a lower rate of hospitalization for heart failure in about 17,160 patients—including 10,186 without atherosclerotic cardiovascular disease—who were followed for a median of 4.2 years.18 It did not show benefit for the primary safety outcome, a composite of major adverse cardiovascular events defined as cardiovascular death, myocardial infarction, or ischemic stroke.
WEIGHT MANAGEMENT
Weight loss can help overweight patients reach their hemoglobin A1c target.
Metformin should be continued as other drugs are added because it does not induce weight gain and may help with weight loss of up to 2 kg as shown in the Diabetes Prevention Program Outcomes Study.19
GLP-1 receptor agonists and SGLT2 inhibitors help with weight loss and are good additions to a basal insulin regimen to minimize weight gain.
Liraglutide was associated with a mean weight loss of 2.3 kg over 36 months of treatment compared with placebo in the LEADER trial.10
In the Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6),20 the mean body weight in the semaglutide group, compared with the placebo group, was 2.9 kg lower in the group receiving a lower dose and 4.3 kg lower in the group receiving a higher dose of the drug.
In a 24-week trial in 182 patients with type 2 diabetes inadequately controlled on metformin, dapagliflozin produced a statistically significant weight reduction of 2.08 kg (95% confidence interval 2.84–1.31; P < .0001) compared with placebo.21
Lifestyle changes aimed at weight management should be emphasized and discussed at every visit.
HYPOGLYCEMIA RISK
Hypoglycemia is a major consideration when tailoring hemoglobin A1c targets. In the Action to Control Cardiovascular Risk (ACCORD) trial,22 severe, symptomatic hypoglycemia increased the risk of death in both the intensive and conventional treatment groups. In VADT, the occurrence of a recent severe hypoglycemic event was the strongest independent predictor of death within 90 days. Further analysis showed that even though serious hypoglycemia occurred more often in the intensive therapy group, it was associated with progression of coronary artery calcification in the standard therapy group.23 Hence, it is imperative that tight glycemic control not be achieved at the cost of severe or recurrent hypoglycemia.
In terms of hypoglycemia, metformin is an excellent medication. The American Diabetes Association24 recommends metformin as the first-line therapy for newly diagnosed diabetes. Long-term follow-up data from UKPDS showed that metformin decreased mortality and the incidence of myocardial infarction and lowered treatment costs as well as the overall risk of hypoglycemia.25 When prescribed, it should be titrated to the highest dose.
The FDA26 has changed the prescribing information for metformin in patients with renal impairment. Metformin should not be started if the eGFR is less than 45 mL/min/1.73 m2, but it can be continued if the patient is already receiving it and the eGFR is between 30 and 45. Previously, creatinine levels were used to define renal impairment and suitability for metformin. This change has increased the number of patients who can benefit from this medication.
In patients who have a contraindication to metformin, DPP-4 inhibitors can be considered, as they carry a low risk of hypoglycemia as well. Sulfonylureas should be used with caution in these patients, especially if their oral intake is variable. When sulfonylureas were compared to the DPP-4 inhibitor sitagliptin as an add-on to metformin, the rate of hypoglycemia was 32% in the sulfonylurea group vs 5% in the sitagliptin group.27
Of the sulfonylureas, glipizide and glimepiride are better than glyburide because of a comparatively lower risk of hypoglycemia and a higher selectivity for binding the KATP channel on the pancreatic beta cell.28
Meglitinides can be a good option for patients who skip meals, but they are more expensive than other generic oral hypoglycemic agents and require multiple daily dosing.
GLP-1 analogues also have a low risk of hypoglycemia but are only available in injectable formulations. Patients must be willing and able to perform the injections themselves.29
LOOSER TARGETS FOR OLDER PATIENTS
In 2010, among US residents age 65 and older, 10.9 million (about 27%) had diabetes,30 and this number is projected to increase to 26.7 million by 2050.31 This population is prone to hypoglycemia when treated with insulin and sulfonylureas. An injury sustained by a fall induced by hypoglycemia can be life-altering. In addition, no randomized clinical trials show the effect of tight glycemic control on complications in older patients with diabetes because patients older than 80 are often excluded.
A reasonable goal suggested by the European Diabetes Working Party for Older People 201132 and reiterated by the American Geriatrics Society in 201333 is a hemoglobin A1c between 7% and 7.5% for relatively healthy older patients and 7.5% to 8% or 8.5% in frail elderly patients with diabetes.
Consider prescribing medications that carry a low risk of hypoglycemia, can be dose-adjusted for kidney function, and do not rely on manual dexterity for administration (ie, do not require patients to give themselves injections). These include metformin and DPP-4 inhibitors.
DRUG COMBINATIONS
Polypharmacy is a concern for all patients with diabetes, especially since it increases the risk of drug interactions and adverse effects, increases out-of-pocket costs, and decreases the likelihood that patients will remain adherent to their treatment regimen. The use of combination medications can reduce the number of pills or injections required, as well as copayments.
Due to concern for multiple drug-drug interactions (and also due to the progressive nature of diabetes), many people with type 2 diabetes are given insulin in lieu of pills to lower their blood glucose. In addition to premixed insulin combinations (such as combinations of neutral protamine Hagedorn and regular insulin or combinations of insulin analogues), long-acting basal insulins can now be prescribed with a GLP-1 drug in fixed-dose combinations such as insulin glargine plus lixisenatide and insulin degludec plus liraglutide.
COST CONSIDERATIONS
It is important to discuss medication cost with patients, because many newer diabetic drugs are expensive and add to the financial burden of patients already paying for multiple medications, such as antihypertensives and statins.
Metformin and sulfonylureas are less expensive alternatives for patients who cannot afford GLP-1 analogues or SGLT2 inhibitors. Even within the same drug class, the formulary-preferred drug may be cheaper than the nonformulary alternative. Thus, it is helpful to research formulary alternatives before discussing treatment regimens with patients.
- Allen DW, Schroeder WA, Balog J. Observations on the chromatographic heterogeneity of normal adult and fetal human hemoglobin: a study of the effects of crystallization and chromatography on the heterogeneity and isoleucine content. J Amer Chem Soc 1958; 80(7):1628–1634. doi:10.1021/ja01540a030
- Huisman TH, Dozy AM. Studies on the heterogeneity of hemoglobin. V. Binding of hemoglobin with oxidized glutathione. J Lab Clin Med 1962; 60:302–319. pmid:14449875
- Rahbar S, Blumenfeld O, Ranney HM. Studies of an unusual hemoglobin in patients with diabetes mellitus. Biochem Biophys Res Commun 1969; 36(5):838–843. pmid:5808299
- American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S55–S64. doi:10.2337/dc18-S006
- Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979; 241(19):2035–2038. pmid:430798
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352(9131):837–853. [Erratum in Lancet 1999; 354:602.] pmid:9742976
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359(15):1577–1589. doi:10.1056/NEJMoa0806470
- Hayward RA, Reaven PD, Wiitala WL, et al; VADT Investigators. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 372(23):2197–2206. doi:10.1056/NEJMoa1414266
- US Food and Drug Administration. Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. https://www.govinfo.gov/content/pkg/FR-2008-12-19/pdf/E8-30086.pdf. Accessed August 6, 2019.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375(4):311–322. doi:10.1056/NEJMoa1603827
- Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373(23):2247–2257. doi:10.1056/NEJMoa1509225
- Holman RR, Bethel MA, Mentz RJ, et al; EXSCEL Study Group. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017; 377(13):1228–1239. doi:10.1056/NEJMoa1612917
- Cosmi F, Laini R, Nicolucci A. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2017; 376(9):890. doi:10.1056/NEJMc1615712
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019; 394(10193):121–130. doi:10.1016/S0140-6736(19)31149-3
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373(22):2117–2128. doi:10.1056/NEJMoa1504720
- Wanner C, Inzucchi SE, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375(4):323–334. doi:10.1056/NEJMoa1515920
- Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377(7):644–657. doi:10.1056/NEJMoa1611925
- Wiviott SD, Raz I, Bonaca MP, et al; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2018. [Epub ahead of print] doi:10.1056/NEJMoa1812389
- Diabetes Prevention Program Research Group; Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009; 374(9702):1677–1686. doi:10.1016/S0140-6736(09)61457-4
- Marso SP, Bain SC, Consoli A, et al, for the SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375:1834–1844. doi:10.1056/NEJMoa1607141
- Bolinder J, Ljunggren Ö, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012; 97(3):1020–1031. doi:10.1210/jc.2011-2260
- Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010; 340:b4909. doi:10.1136/bmj.b4909
- Saremi A, Bahn GD, Reaven PD; Veterans Affairs Diabetes Trial (VADT). A link between hypoglycemia and progression of atherosclerosis in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care 2016; 39(3):448–454. doi:10.2337/dc15-2107
- American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S73–S85. doi:10.2337/dc18-S008
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359(15):1577–1589. doi:10.1056/NEJMoa0806470
- US Food and Drug Administration. FDA drug safety communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. www.fda.gov/Drugs/DrugSafety/ucm493244.htm. Accessed August 5, 2019.
- Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP; Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9(2):194–205. doi:10.1111/j.1463-1326.2006.00704.x
- Gangji AS, Cukierman T, Gerstein HC, Goldsmith CH, Clase CM. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care 2007; 30(2):389–394. doi:10.2337/dc06-1789
- Nauck M, Frid A, Hermansen K, et al; LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009; 32(1):84–90. doi:10.2337/dc08-1355
- Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed August 5, 2019.
- Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 2010; 8:29. doi:10.1186/1478-7954-8-29
- Sinclair AJ, Paolisso G, Castro M, Bourdel-Marchasson I, Gadsby R, Rodriguez Mañas L; European Diabetes Working Party for Older People. European Diabetes Working Party for Older People 2011 clinical guidelines for type 2 diabetes mellitus. Executive summary. Diabetes Metab 2011; 37(suppl 3):S27–S38. doi:10.1016/S1262-3636(11)70962-4
- American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus; Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc 2013; 61(11):2020–2026. doi:10.1111/jgs.12514
When scientists discovered the band of hemoglobin A1c during electrophoresis in the 1950s and 1960s and discerned it was elevated in patients with diabetes, little did they know the important role it would play in the diagnosis and treatment of diabetes in the decades to come.1–3 Despite some caveats, a hemoglobin A1c level of 6.5% or higher is diagnostic of diabetes across most populations, and hemoglobin A1c goals ranging from 6.5% to 7.5% have been set for different subsets of patients depending on comorbidities, complications, risk of hypoglycemia, life expectancy, disease duration, patient preferences, and available resources.4
With a growing number of medications for diabetes—insulin in its various formulations and 11 other classes—hemoglobin A1c targets can now be tailored to fit individual patient profiles. Although helping patients attain their glycemic goals is paramount, other factors should be considered when prescribing or changing a drug treatment regimen, such as cardiovascular risk reduction, weight control, avoidance of hypoglycemia, and minimizing out-of-pocket drug costs (Table 1).
CARDIOVASCULAR BENEFIT
Patients with type 2 diabetes have a 2 to 3 times higher risk of clinical atherosclerotic disease, according to 20 years of surveillance data from the Framingham cohort.5
Mixed results with intensive treatment
Reducing cardiovascular risk remains an important goal in diabetes management, but unfortunately, data from the long-term clinical trials aimed at reducing macrovascular risk with intensive glycemic management have been conflicting.
The United Kingdom Prospective Diabetes Study (UKPDS),6 which enrolled more than 4,000 patients with newly diagnosed type 2 diabetes, did not initially show a statistically significant difference in the incidence of myocardial infarction with intensive control vs conventional control, although intensive treatment did reduce the incidence of microvascular disease. However, 10 years after the trial ended, the incidence was 15% lower in the intensive-treatment group than in the conventional-treatment group, and the difference was statistically significant.7
A 10-year follow-up analysis of the Veterans Affairs Diabetes Trial (VADT)8 showed that patients who had been randomly assigned to intensive glucose control for 5.6 years had 8.6 fewer major cardiovascular events per 1,000 person-years than those assigned to standard therapy, but no improvement in median overall survival. The hemoglobin A1c levels achieved during the trial were 6.9% and 8.4%, respectively.
In 2008, the US Food and Drug Administration (FDA)9 mandated that all new applications for diabetes drugs must include cardiovascular outcome studies. Therefore, we now have data on the cardiovascular benefits of two antihyperglycemic drug classes—incretins and sodium-glucose cotransporter 2 (SGLT2) inhibitors, making them attractive medications to target both cardiac and glucose concerns.
Incretins
The incretin drugs comprise 2 classes, glucagon-like peptide 1 (GLP-1) receptor agonists and dipeptidyl peptidase 4 (DPP-4) inhibitors.
Liraglutide. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial10 compared liraglutide (a GLP-1 receptor agonist) and placebo in 9,000 patients with diabetes who either had or were at high risk of cardiovascular disease. Patients in the liraglutide group had a lower risk of the primary composite end point of death from cardiovascular causes or the first episode of nonfatal (including silent) myocardial infarction or nonfatal stroke, and a lower risk of cardiovascular death, all-cause mortality, and microvascular events than those in the placebo group. The number of patients who would need to be treated to prevent 1 event in 3 years was 66 in the analysis of the primary outcome and 98 in the analysis of death from any cause.9
Lixisenatide. The Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial11 studied the effect of the once-daily GLP-1 receptor agonist lixisenatide on cardiovascular outcomes in 6,000 patients with type 2 diabetes with a recent coronary event. In contrast to LEADER, ELIXA did not show a cardiovascular benefit over placebo.
Exenatide. The Exenatide Study of Cardiovascular Event Lowering (EXSCEL)12 assessed another GLP-1 extended-release drug, exenatide, in 14,000 patients, 73% of whom had established cardiovascular disease. In those patients, the drug had a modest benefit in terms of first occurrence of any component of the composite outcome of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke (3-component major adverse cardiac event [MACE] outcome) in a time-to-event analysis, but the results were not statistically significant. However, the drug did significantly reduce all-cause mortality.
Semaglutide, another GLP-1 receptor agonist recently approved by the FDA, also showed benefit in patients who had cardiovascular disease or were at high risk, with significant reduction in the primary composite end point of death from cardiovascular causes or the first occurrence of nonfatal myocardial infarction (including silent) or nonfatal stroke.13
Dulaglutide, a newer GLP-1 drug, was associated with significantly reduced major adverse cardiovascular events (a composite end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) in about 9,900 patients with diabetes, with a median follow-up of more than 5 years. Only 31% of the patients in the trial had established cardiovascular disease.14
Comment. GLP-1 drugs as a class are a good option for patients with diabetes who require weight loss, and liraglutide is now FDA-approved for reduction of cardiovascular events in patients with type 2 diabetes with established cardiovascular disease. However, other factors should be considered when prescribing these drugs: they have adverse gastrointestinal effects, the cardiovascular benefit was not a class effect, they are relatively expensive, and they must be injected. Also, they should not be prescribed concurrently with a DPP-4 inhibitor because they target the same pathway.
SGLT2 inhibitors
The other class of diabetes drugs that have shown cardiovascular benefit are the SGLT2 inhibitors.
Empagliflozin. The Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG)15 compared the efficacy of empagliflozin vs placebo in 7,000 patients with diabetes and cardiovascular disease and showed relative risk reductions of 38% in death from cardiovascular death, 31% in sudden death, and 35% in heart failure hospitalizations. Empagliflozin also showed benefit in terms of progression of kidney disease and occurrence of clinically relevant renal events in this population.16
Canagliflozin also has cardiovascular outcome data and showed significant benefit when compared with placebo in the primary outcome of the composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, but no significant effects on cardiovascular death or all-cause mortality.17 Data from this trial also suggested a nonsignificant benefit of canagliflozin in decreasing progression of albuminuria and in the composite outcome of a sustained 40% reduction in the estimated glomerular filtration rate (eGFR), the need for renal replacement therapy, or death from renal causes.
The above data led to an additional indication from the FDA for empagliflozin—and recently, canagliflozin—to prevent cardiovascular death in patients with diabetes with established disease, but other factors should be considered when prescribing them. Patients taking canagliflozin showed a significantly increased risk of amputation. SGLT2 inhibitors as a class also increase the risk of genital infections in men and women; this is an important consideration since patients with diabetes complain of vaginal fungal and urinary tract infections even without the use of these drugs. A higher incidence of fractures with canagliflozin should also be considered when using these medications in elderly and osteoporosis-prone patients at high risk of falling.
Dapagliflozin, the third drug in this class, was associated with a lower rate of hospitalization for heart failure in about 17,160 patients—including 10,186 without atherosclerotic cardiovascular disease—who were followed for a median of 4.2 years.18 It did not show benefit for the primary safety outcome, a composite of major adverse cardiovascular events defined as cardiovascular death, myocardial infarction, or ischemic stroke.
WEIGHT MANAGEMENT
Weight loss can help overweight patients reach their hemoglobin A1c target.
Metformin should be continued as other drugs are added because it does not induce weight gain and may help with weight loss of up to 2 kg as shown in the Diabetes Prevention Program Outcomes Study.19
GLP-1 receptor agonists and SGLT2 inhibitors help with weight loss and are good additions to a basal insulin regimen to minimize weight gain.
Liraglutide was associated with a mean weight loss of 2.3 kg over 36 months of treatment compared with placebo in the LEADER trial.10
In the Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6),20 the mean body weight in the semaglutide group, compared with the placebo group, was 2.9 kg lower in the group receiving a lower dose and 4.3 kg lower in the group receiving a higher dose of the drug.
In a 24-week trial in 182 patients with type 2 diabetes inadequately controlled on metformin, dapagliflozin produced a statistically significant weight reduction of 2.08 kg (95% confidence interval 2.84–1.31; P < .0001) compared with placebo.21
Lifestyle changes aimed at weight management should be emphasized and discussed at every visit.
HYPOGLYCEMIA RISK
Hypoglycemia is a major consideration when tailoring hemoglobin A1c targets. In the Action to Control Cardiovascular Risk (ACCORD) trial,22 severe, symptomatic hypoglycemia increased the risk of death in both the intensive and conventional treatment groups. In VADT, the occurrence of a recent severe hypoglycemic event was the strongest independent predictor of death within 90 days. Further analysis showed that even though serious hypoglycemia occurred more often in the intensive therapy group, it was associated with progression of coronary artery calcification in the standard therapy group.23 Hence, it is imperative that tight glycemic control not be achieved at the cost of severe or recurrent hypoglycemia.
In terms of hypoglycemia, metformin is an excellent medication. The American Diabetes Association24 recommends metformin as the first-line therapy for newly diagnosed diabetes. Long-term follow-up data from UKPDS showed that metformin decreased mortality and the incidence of myocardial infarction and lowered treatment costs as well as the overall risk of hypoglycemia.25 When prescribed, it should be titrated to the highest dose.
The FDA26 has changed the prescribing information for metformin in patients with renal impairment. Metformin should not be started if the eGFR is less than 45 mL/min/1.73 m2, but it can be continued if the patient is already receiving it and the eGFR is between 30 and 45. Previously, creatinine levels were used to define renal impairment and suitability for metformin. This change has increased the number of patients who can benefit from this medication.
In patients who have a contraindication to metformin, DPP-4 inhibitors can be considered, as they carry a low risk of hypoglycemia as well. Sulfonylureas should be used with caution in these patients, especially if their oral intake is variable. When sulfonylureas were compared to the DPP-4 inhibitor sitagliptin as an add-on to metformin, the rate of hypoglycemia was 32% in the sulfonylurea group vs 5% in the sitagliptin group.27
Of the sulfonylureas, glipizide and glimepiride are better than glyburide because of a comparatively lower risk of hypoglycemia and a higher selectivity for binding the KATP channel on the pancreatic beta cell.28
Meglitinides can be a good option for patients who skip meals, but they are more expensive than other generic oral hypoglycemic agents and require multiple daily dosing.
GLP-1 analogues also have a low risk of hypoglycemia but are only available in injectable formulations. Patients must be willing and able to perform the injections themselves.29
LOOSER TARGETS FOR OLDER PATIENTS
In 2010, among US residents age 65 and older, 10.9 million (about 27%) had diabetes,30 and this number is projected to increase to 26.7 million by 2050.31 This population is prone to hypoglycemia when treated with insulin and sulfonylureas. An injury sustained by a fall induced by hypoglycemia can be life-altering. In addition, no randomized clinical trials show the effect of tight glycemic control on complications in older patients with diabetes because patients older than 80 are often excluded.
A reasonable goal suggested by the European Diabetes Working Party for Older People 201132 and reiterated by the American Geriatrics Society in 201333 is a hemoglobin A1c between 7% and 7.5% for relatively healthy older patients and 7.5% to 8% or 8.5% in frail elderly patients with diabetes.
Consider prescribing medications that carry a low risk of hypoglycemia, can be dose-adjusted for kidney function, and do not rely on manual dexterity for administration (ie, do not require patients to give themselves injections). These include metformin and DPP-4 inhibitors.
DRUG COMBINATIONS
Polypharmacy is a concern for all patients with diabetes, especially since it increases the risk of drug interactions and adverse effects, increases out-of-pocket costs, and decreases the likelihood that patients will remain adherent to their treatment regimen. The use of combination medications can reduce the number of pills or injections required, as well as copayments.
Due to concern for multiple drug-drug interactions (and also due to the progressive nature of diabetes), many people with type 2 diabetes are given insulin in lieu of pills to lower their blood glucose. In addition to premixed insulin combinations (such as combinations of neutral protamine Hagedorn and regular insulin or combinations of insulin analogues), long-acting basal insulins can now be prescribed with a GLP-1 drug in fixed-dose combinations such as insulin glargine plus lixisenatide and insulin degludec plus liraglutide.
COST CONSIDERATIONS
It is important to discuss medication cost with patients, because many newer diabetic drugs are expensive and add to the financial burden of patients already paying for multiple medications, such as antihypertensives and statins.
Metformin and sulfonylureas are less expensive alternatives for patients who cannot afford GLP-1 analogues or SGLT2 inhibitors. Even within the same drug class, the formulary-preferred drug may be cheaper than the nonformulary alternative. Thus, it is helpful to research formulary alternatives before discussing treatment regimens with patients.
When scientists discovered the band of hemoglobin A1c during electrophoresis in the 1950s and 1960s and discerned it was elevated in patients with diabetes, little did they know the important role it would play in the diagnosis and treatment of diabetes in the decades to come.1–3 Despite some caveats, a hemoglobin A1c level of 6.5% or higher is diagnostic of diabetes across most populations, and hemoglobin A1c goals ranging from 6.5% to 7.5% have been set for different subsets of patients depending on comorbidities, complications, risk of hypoglycemia, life expectancy, disease duration, patient preferences, and available resources.4
With a growing number of medications for diabetes—insulin in its various formulations and 11 other classes—hemoglobin A1c targets can now be tailored to fit individual patient profiles. Although helping patients attain their glycemic goals is paramount, other factors should be considered when prescribing or changing a drug treatment regimen, such as cardiovascular risk reduction, weight control, avoidance of hypoglycemia, and minimizing out-of-pocket drug costs (Table 1).
CARDIOVASCULAR BENEFIT
Patients with type 2 diabetes have a 2 to 3 times higher risk of clinical atherosclerotic disease, according to 20 years of surveillance data from the Framingham cohort.5
Mixed results with intensive treatment
Reducing cardiovascular risk remains an important goal in diabetes management, but unfortunately, data from the long-term clinical trials aimed at reducing macrovascular risk with intensive glycemic management have been conflicting.
The United Kingdom Prospective Diabetes Study (UKPDS),6 which enrolled more than 4,000 patients with newly diagnosed type 2 diabetes, did not initially show a statistically significant difference in the incidence of myocardial infarction with intensive control vs conventional control, although intensive treatment did reduce the incidence of microvascular disease. However, 10 years after the trial ended, the incidence was 15% lower in the intensive-treatment group than in the conventional-treatment group, and the difference was statistically significant.7
A 10-year follow-up analysis of the Veterans Affairs Diabetes Trial (VADT)8 showed that patients who had been randomly assigned to intensive glucose control for 5.6 years had 8.6 fewer major cardiovascular events per 1,000 person-years than those assigned to standard therapy, but no improvement in median overall survival. The hemoglobin A1c levels achieved during the trial were 6.9% and 8.4%, respectively.
In 2008, the US Food and Drug Administration (FDA)9 mandated that all new applications for diabetes drugs must include cardiovascular outcome studies. Therefore, we now have data on the cardiovascular benefits of two antihyperglycemic drug classes—incretins and sodium-glucose cotransporter 2 (SGLT2) inhibitors, making them attractive medications to target both cardiac and glucose concerns.
Incretins
The incretin drugs comprise 2 classes, glucagon-like peptide 1 (GLP-1) receptor agonists and dipeptidyl peptidase 4 (DPP-4) inhibitors.
Liraglutide. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial10 compared liraglutide (a GLP-1 receptor agonist) and placebo in 9,000 patients with diabetes who either had or were at high risk of cardiovascular disease. Patients in the liraglutide group had a lower risk of the primary composite end point of death from cardiovascular causes or the first episode of nonfatal (including silent) myocardial infarction or nonfatal stroke, and a lower risk of cardiovascular death, all-cause mortality, and microvascular events than those in the placebo group. The number of patients who would need to be treated to prevent 1 event in 3 years was 66 in the analysis of the primary outcome and 98 in the analysis of death from any cause.9
Lixisenatide. The Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial11 studied the effect of the once-daily GLP-1 receptor agonist lixisenatide on cardiovascular outcomes in 6,000 patients with type 2 diabetes with a recent coronary event. In contrast to LEADER, ELIXA did not show a cardiovascular benefit over placebo.
Exenatide. The Exenatide Study of Cardiovascular Event Lowering (EXSCEL)12 assessed another GLP-1 extended-release drug, exenatide, in 14,000 patients, 73% of whom had established cardiovascular disease. In those patients, the drug had a modest benefit in terms of first occurrence of any component of the composite outcome of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke (3-component major adverse cardiac event [MACE] outcome) in a time-to-event analysis, but the results were not statistically significant. However, the drug did significantly reduce all-cause mortality.
Semaglutide, another GLP-1 receptor agonist recently approved by the FDA, also showed benefit in patients who had cardiovascular disease or were at high risk, with significant reduction in the primary composite end point of death from cardiovascular causes or the first occurrence of nonfatal myocardial infarction (including silent) or nonfatal stroke.13
Dulaglutide, a newer GLP-1 drug, was associated with significantly reduced major adverse cardiovascular events (a composite end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) in about 9,900 patients with diabetes, with a median follow-up of more than 5 years. Only 31% of the patients in the trial had established cardiovascular disease.14
Comment. GLP-1 drugs as a class are a good option for patients with diabetes who require weight loss, and liraglutide is now FDA-approved for reduction of cardiovascular events in patients with type 2 diabetes with established cardiovascular disease. However, other factors should be considered when prescribing these drugs: they have adverse gastrointestinal effects, the cardiovascular benefit was not a class effect, they are relatively expensive, and they must be injected. Also, they should not be prescribed concurrently with a DPP-4 inhibitor because they target the same pathway.
SGLT2 inhibitors
The other class of diabetes drugs that have shown cardiovascular benefit are the SGLT2 inhibitors.
Empagliflozin. The Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG)15 compared the efficacy of empagliflozin vs placebo in 7,000 patients with diabetes and cardiovascular disease and showed relative risk reductions of 38% in death from cardiovascular death, 31% in sudden death, and 35% in heart failure hospitalizations. Empagliflozin also showed benefit in terms of progression of kidney disease and occurrence of clinically relevant renal events in this population.16
Canagliflozin also has cardiovascular outcome data and showed significant benefit when compared with placebo in the primary outcome of the composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, but no significant effects on cardiovascular death or all-cause mortality.17 Data from this trial also suggested a nonsignificant benefit of canagliflozin in decreasing progression of albuminuria and in the composite outcome of a sustained 40% reduction in the estimated glomerular filtration rate (eGFR), the need for renal replacement therapy, or death from renal causes.
The above data led to an additional indication from the FDA for empagliflozin—and recently, canagliflozin—to prevent cardiovascular death in patients with diabetes with established disease, but other factors should be considered when prescribing them. Patients taking canagliflozin showed a significantly increased risk of amputation. SGLT2 inhibitors as a class also increase the risk of genital infections in men and women; this is an important consideration since patients with diabetes complain of vaginal fungal and urinary tract infections even without the use of these drugs. A higher incidence of fractures with canagliflozin should also be considered when using these medications in elderly and osteoporosis-prone patients at high risk of falling.
Dapagliflozin, the third drug in this class, was associated with a lower rate of hospitalization for heart failure in about 17,160 patients—including 10,186 without atherosclerotic cardiovascular disease—who were followed for a median of 4.2 years.18 It did not show benefit for the primary safety outcome, a composite of major adverse cardiovascular events defined as cardiovascular death, myocardial infarction, or ischemic stroke.
WEIGHT MANAGEMENT
Weight loss can help overweight patients reach their hemoglobin A1c target.
Metformin should be continued as other drugs are added because it does not induce weight gain and may help with weight loss of up to 2 kg as shown in the Diabetes Prevention Program Outcomes Study.19
GLP-1 receptor agonists and SGLT2 inhibitors help with weight loss and are good additions to a basal insulin regimen to minimize weight gain.
Liraglutide was associated with a mean weight loss of 2.3 kg over 36 months of treatment compared with placebo in the LEADER trial.10
In the Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6),20 the mean body weight in the semaglutide group, compared with the placebo group, was 2.9 kg lower in the group receiving a lower dose and 4.3 kg lower in the group receiving a higher dose of the drug.
In a 24-week trial in 182 patients with type 2 diabetes inadequately controlled on metformin, dapagliflozin produced a statistically significant weight reduction of 2.08 kg (95% confidence interval 2.84–1.31; P < .0001) compared with placebo.21
Lifestyle changes aimed at weight management should be emphasized and discussed at every visit.
HYPOGLYCEMIA RISK
Hypoglycemia is a major consideration when tailoring hemoglobin A1c targets. In the Action to Control Cardiovascular Risk (ACCORD) trial,22 severe, symptomatic hypoglycemia increased the risk of death in both the intensive and conventional treatment groups. In VADT, the occurrence of a recent severe hypoglycemic event was the strongest independent predictor of death within 90 days. Further analysis showed that even though serious hypoglycemia occurred more often in the intensive therapy group, it was associated with progression of coronary artery calcification in the standard therapy group.23 Hence, it is imperative that tight glycemic control not be achieved at the cost of severe or recurrent hypoglycemia.
In terms of hypoglycemia, metformin is an excellent medication. The American Diabetes Association24 recommends metformin as the first-line therapy for newly diagnosed diabetes. Long-term follow-up data from UKPDS showed that metformin decreased mortality and the incidence of myocardial infarction and lowered treatment costs as well as the overall risk of hypoglycemia.25 When prescribed, it should be titrated to the highest dose.
The FDA26 has changed the prescribing information for metformin in patients with renal impairment. Metformin should not be started if the eGFR is less than 45 mL/min/1.73 m2, but it can be continued if the patient is already receiving it and the eGFR is between 30 and 45. Previously, creatinine levels were used to define renal impairment and suitability for metformin. This change has increased the number of patients who can benefit from this medication.
In patients who have a contraindication to metformin, DPP-4 inhibitors can be considered, as they carry a low risk of hypoglycemia as well. Sulfonylureas should be used with caution in these patients, especially if their oral intake is variable. When sulfonylureas were compared to the DPP-4 inhibitor sitagliptin as an add-on to metformin, the rate of hypoglycemia was 32% in the sulfonylurea group vs 5% in the sitagliptin group.27
Of the sulfonylureas, glipizide and glimepiride are better than glyburide because of a comparatively lower risk of hypoglycemia and a higher selectivity for binding the KATP channel on the pancreatic beta cell.28
Meglitinides can be a good option for patients who skip meals, but they are more expensive than other generic oral hypoglycemic agents and require multiple daily dosing.
GLP-1 analogues also have a low risk of hypoglycemia but are only available in injectable formulations. Patients must be willing and able to perform the injections themselves.29
LOOSER TARGETS FOR OLDER PATIENTS
In 2010, among US residents age 65 and older, 10.9 million (about 27%) had diabetes,30 and this number is projected to increase to 26.7 million by 2050.31 This population is prone to hypoglycemia when treated with insulin and sulfonylureas. An injury sustained by a fall induced by hypoglycemia can be life-altering. In addition, no randomized clinical trials show the effect of tight glycemic control on complications in older patients with diabetes because patients older than 80 are often excluded.
A reasonable goal suggested by the European Diabetes Working Party for Older People 201132 and reiterated by the American Geriatrics Society in 201333 is a hemoglobin A1c between 7% and 7.5% for relatively healthy older patients and 7.5% to 8% or 8.5% in frail elderly patients with diabetes.
Consider prescribing medications that carry a low risk of hypoglycemia, can be dose-adjusted for kidney function, and do not rely on manual dexterity for administration (ie, do not require patients to give themselves injections). These include metformin and DPP-4 inhibitors.
DRUG COMBINATIONS
Polypharmacy is a concern for all patients with diabetes, especially since it increases the risk of drug interactions and adverse effects, increases out-of-pocket costs, and decreases the likelihood that patients will remain adherent to their treatment regimen. The use of combination medications can reduce the number of pills or injections required, as well as copayments.
Due to concern for multiple drug-drug interactions (and also due to the progressive nature of diabetes), many people with type 2 diabetes are given insulin in lieu of pills to lower their blood glucose. In addition to premixed insulin combinations (such as combinations of neutral protamine Hagedorn and regular insulin or combinations of insulin analogues), long-acting basal insulins can now be prescribed with a GLP-1 drug in fixed-dose combinations such as insulin glargine plus lixisenatide and insulin degludec plus liraglutide.
COST CONSIDERATIONS
It is important to discuss medication cost with patients, because many newer diabetic drugs are expensive and add to the financial burden of patients already paying for multiple medications, such as antihypertensives and statins.
Metformin and sulfonylureas are less expensive alternatives for patients who cannot afford GLP-1 analogues or SGLT2 inhibitors. Even within the same drug class, the formulary-preferred drug may be cheaper than the nonformulary alternative. Thus, it is helpful to research formulary alternatives before discussing treatment regimens with patients.
- Allen DW, Schroeder WA, Balog J. Observations on the chromatographic heterogeneity of normal adult and fetal human hemoglobin: a study of the effects of crystallization and chromatography on the heterogeneity and isoleucine content. J Amer Chem Soc 1958; 80(7):1628–1634. doi:10.1021/ja01540a030
- Huisman TH, Dozy AM. Studies on the heterogeneity of hemoglobin. V. Binding of hemoglobin with oxidized glutathione. J Lab Clin Med 1962; 60:302–319. pmid:14449875
- Rahbar S, Blumenfeld O, Ranney HM. Studies of an unusual hemoglobin in patients with diabetes mellitus. Biochem Biophys Res Commun 1969; 36(5):838–843. pmid:5808299
- American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S55–S64. doi:10.2337/dc18-S006
- Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979; 241(19):2035–2038. pmid:430798
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352(9131):837–853. [Erratum in Lancet 1999; 354:602.] pmid:9742976
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359(15):1577–1589. doi:10.1056/NEJMoa0806470
- Hayward RA, Reaven PD, Wiitala WL, et al; VADT Investigators. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 372(23):2197–2206. doi:10.1056/NEJMoa1414266
- US Food and Drug Administration. Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. https://www.govinfo.gov/content/pkg/FR-2008-12-19/pdf/E8-30086.pdf. Accessed August 6, 2019.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375(4):311–322. doi:10.1056/NEJMoa1603827
- Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373(23):2247–2257. doi:10.1056/NEJMoa1509225
- Holman RR, Bethel MA, Mentz RJ, et al; EXSCEL Study Group. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017; 377(13):1228–1239. doi:10.1056/NEJMoa1612917
- Cosmi F, Laini R, Nicolucci A. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2017; 376(9):890. doi:10.1056/NEJMc1615712
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019; 394(10193):121–130. doi:10.1016/S0140-6736(19)31149-3
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373(22):2117–2128. doi:10.1056/NEJMoa1504720
- Wanner C, Inzucchi SE, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375(4):323–334. doi:10.1056/NEJMoa1515920
- Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377(7):644–657. doi:10.1056/NEJMoa1611925
- Wiviott SD, Raz I, Bonaca MP, et al; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2018. [Epub ahead of print] doi:10.1056/NEJMoa1812389
- Diabetes Prevention Program Research Group; Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009; 374(9702):1677–1686. doi:10.1016/S0140-6736(09)61457-4
- Marso SP, Bain SC, Consoli A, et al, for the SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375:1834–1844. doi:10.1056/NEJMoa1607141
- Bolinder J, Ljunggren Ö, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012; 97(3):1020–1031. doi:10.1210/jc.2011-2260
- Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010; 340:b4909. doi:10.1136/bmj.b4909
- Saremi A, Bahn GD, Reaven PD; Veterans Affairs Diabetes Trial (VADT). A link between hypoglycemia and progression of atherosclerosis in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care 2016; 39(3):448–454. doi:10.2337/dc15-2107
- American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S73–S85. doi:10.2337/dc18-S008
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359(15):1577–1589. doi:10.1056/NEJMoa0806470
- US Food and Drug Administration. FDA drug safety communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. www.fda.gov/Drugs/DrugSafety/ucm493244.htm. Accessed August 5, 2019.
- Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP; Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9(2):194–205. doi:10.1111/j.1463-1326.2006.00704.x
- Gangji AS, Cukierman T, Gerstein HC, Goldsmith CH, Clase CM. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care 2007; 30(2):389–394. doi:10.2337/dc06-1789
- Nauck M, Frid A, Hermansen K, et al; LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009; 32(1):84–90. doi:10.2337/dc08-1355
- Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed August 5, 2019.
- Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 2010; 8:29. doi:10.1186/1478-7954-8-29
- Sinclair AJ, Paolisso G, Castro M, Bourdel-Marchasson I, Gadsby R, Rodriguez Mañas L; European Diabetes Working Party for Older People. European Diabetes Working Party for Older People 2011 clinical guidelines for type 2 diabetes mellitus. Executive summary. Diabetes Metab 2011; 37(suppl 3):S27–S38. doi:10.1016/S1262-3636(11)70962-4
- American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus; Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc 2013; 61(11):2020–2026. doi:10.1111/jgs.12514
- Allen DW, Schroeder WA, Balog J. Observations on the chromatographic heterogeneity of normal adult and fetal human hemoglobin: a study of the effects of crystallization and chromatography on the heterogeneity and isoleucine content. J Amer Chem Soc 1958; 80(7):1628–1634. doi:10.1021/ja01540a030
- Huisman TH, Dozy AM. Studies on the heterogeneity of hemoglobin. V. Binding of hemoglobin with oxidized glutathione. J Lab Clin Med 1962; 60:302–319. pmid:14449875
- Rahbar S, Blumenfeld O, Ranney HM. Studies of an unusual hemoglobin in patients with diabetes mellitus. Biochem Biophys Res Commun 1969; 36(5):838–843. pmid:5808299
- American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S55–S64. doi:10.2337/dc18-S006
- Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979; 241(19):2035–2038. pmid:430798
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352(9131):837–853. [Erratum in Lancet 1999; 354:602.] pmid:9742976
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359(15):1577–1589. doi:10.1056/NEJMoa0806470
- Hayward RA, Reaven PD, Wiitala WL, et al; VADT Investigators. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 372(23):2197–2206. doi:10.1056/NEJMoa1414266
- US Food and Drug Administration. Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. https://www.govinfo.gov/content/pkg/FR-2008-12-19/pdf/E8-30086.pdf. Accessed August 6, 2019.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375(4):311–322. doi:10.1056/NEJMoa1603827
- Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373(23):2247–2257. doi:10.1056/NEJMoa1509225
- Holman RR, Bethel MA, Mentz RJ, et al; EXSCEL Study Group. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017; 377(13):1228–1239. doi:10.1056/NEJMoa1612917
- Cosmi F, Laini R, Nicolucci A. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2017; 376(9):890. doi:10.1056/NEJMc1615712
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019; 394(10193):121–130. doi:10.1016/S0140-6736(19)31149-3
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373(22):2117–2128. doi:10.1056/NEJMoa1504720
- Wanner C, Inzucchi SE, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375(4):323–334. doi:10.1056/NEJMoa1515920
- Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377(7):644–657. doi:10.1056/NEJMoa1611925
- Wiviott SD, Raz I, Bonaca MP, et al; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2018. [Epub ahead of print] doi:10.1056/NEJMoa1812389
- Diabetes Prevention Program Research Group; Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009; 374(9702):1677–1686. doi:10.1016/S0140-6736(09)61457-4
- Marso SP, Bain SC, Consoli A, et al, for the SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375:1834–1844. doi:10.1056/NEJMoa1607141
- Bolinder J, Ljunggren Ö, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012; 97(3):1020–1031. doi:10.1210/jc.2011-2260
- Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010; 340:b4909. doi:10.1136/bmj.b4909
- Saremi A, Bahn GD, Reaven PD; Veterans Affairs Diabetes Trial (VADT). A link between hypoglycemia and progression of atherosclerosis in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care 2016; 39(3):448–454. doi:10.2337/dc15-2107
- American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S73–S85. doi:10.2337/dc18-S008
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359(15):1577–1589. doi:10.1056/NEJMoa0806470
- US Food and Drug Administration. FDA drug safety communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. www.fda.gov/Drugs/DrugSafety/ucm493244.htm. Accessed August 5, 2019.
- Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP; Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9(2):194–205. doi:10.1111/j.1463-1326.2006.00704.x
- Gangji AS, Cukierman T, Gerstein HC, Goldsmith CH, Clase CM. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care 2007; 30(2):389–394. doi:10.2337/dc06-1789
- Nauck M, Frid A, Hermansen K, et al; LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009; 32(1):84–90. doi:10.2337/dc08-1355
- Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed August 5, 2019.
- Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 2010; 8:29. doi:10.1186/1478-7954-8-29
- Sinclair AJ, Paolisso G, Castro M, Bourdel-Marchasson I, Gadsby R, Rodriguez Mañas L; European Diabetes Working Party for Older People. European Diabetes Working Party for Older People 2011 clinical guidelines for type 2 diabetes mellitus. Executive summary. Diabetes Metab 2011; 37(suppl 3):S27–S38. doi:10.1016/S1262-3636(11)70962-4
- American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus; Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc 2013; 61(11):2020–2026. doi:10.1111/jgs.12514
KEY POINTS
- Some glucagon-like peptide 1 (GLP-1) receptor agonists have been shown to reduce cardiovascular risk, and liraglutide carries an indication for this use.
- The sodium-glucose cotransporter 2 inhibitors empaglifozin and canaglifozin carry indications to prevent cardiovascular death in patients with diabetes with established cardiovascular disease.
- Metformin, GLP-1 receptor agonists, and dipeptidyl peptidase 4 inhibitors are beneficial in terms of promoting weight loss—or at least not causing weight gain.
- Disadvantages and adverse effects of various drugs must also be considered.
ACE inhibitors and ARBs: Managing potassium and renal function
A highly active, water- and alcohol-soluble, basic pressor substance is formed when renin and renin-activator interact, for which we suggest the name “angiotonin.”
—Irvine H. Page and O.M. Helmer, 1940.1
The renin-angiotensin-aldosterone system regulates salt and, in part, water homeostasis, and therefore blood pressure and fluid balance through its actions on the heart, kidneys, and blood vessels.2 Drugs that target this system—angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)—are used primarily to treat hypertension and also to treat chronic kidney disease and heart failure with reduced ejection fraction.
Controlling blood pressure is important, as hypertension increases the risk of myocardial infarction, cerebrovascular events, and progression of chronic kidney disease, which itself is a risk factor for cardiovascular disease. However, the benefit of these drugs is only partly due to their effect on blood pressure. They also reduce proteinuria, which is a graded risk factor for progression of kidney disease as well as morbidity and death from vascular events.3
Despite the benefits of ACE inhibitors and ARBs, concern about their adverse effects—especially hyperkalemia and a decline in renal function—has led to their underuse in patients likely to derive the greatest benefit.3
ACE INHIBITORS AND ARBs
ACE inhibitors, as their name indicates, inhibit conversion of angiotensin I to angiotensin II by ACE, resulting in vasodilation of the efferent arteriole and a drop in blood pressure. Inhibition of ACE, a kininase, also results in a rise in kinins. One of these, bradykinin, is associated with some of the side effects of this class of drugs such as cough, which affects 5% to 20% of patients.4 Elevation of bradykinin is also believed to account for ACE inhibitor-induced angioedema, an uncommon but potentially serious side effect. Kinins are also associated with desirable effects such as lowering blood pressure, increasing insulin sensitivity, and dilating blood vessels.
ARBs were developed as an alternative for patients unable to tolerate the adverse effects of ACE inhibitors. While ACE inhibitors reduce the activity of angiotensin II at both the AT1 and AT2 receptors, ARBs block only the AT1 receptors, thereby inhibiting their vasoconstricting activity on smooth muscle. ARBs also raise the levels of renin, angiotensin I, and angiotensin II as a result of feedback inhibition. Angiotensin II is associated with release of inflammatory mediators such as tumor necrosis factor alpha, cytokines, and chemokines, the consequences of which are also inhibited by ARBs, further preventing renal fibrosis and scarring from chronic inflammation.3
What is the evidence supporting the use of ACE inhibitors and ARBs?
ACE inhibitors and ARBs, used singly, reduce blood pressure and proteinuria, slow progression of kidney disease, and improve outcomes in patients who have heart failure, diabetes mellitus, or a history of myocardial infarction.5–11
While dual blockade with the combination of an ACE inhibitor and an ARB lowers blood pressure and proteinuria to a greater degree than monotherapy, dual blockade has been associated with higher rates of complications, including hyperkalemia.12–17
RISK FACTORS FOR HYPERKALEMIA
ACE inhibitors and ARBs raise potassium, especially when used in combination. Other risk factors for hyperkalemia include the following—and note that some of them are also indications for ACE inhibitors and ARBs:
Renal insufficiency. The kidneys are responsible for over 90% of potassium removal in healthy individuals,18,19 and the lower the GFR, the higher the risk of hyperkalemia.3,20,21
Heart failure
Diabetes mellitus6,21–23
Endogenous potassium load due to hemolysis, rhabdomyolysis, insulin deficiency, lactic acidosis, or gastrointestinal bleeding
Exogenous potassium load due to dietary consumption or blood products
Other medications, eg, sacubitril-valsartan, aldosterone antagonists, mineralocorticoid receptor antagonists, potassium-sparing diuretics, beta-adrenergic antagonists, nonsteroidal anti-inflammatory drugs, heparin, cyclosporine, trimethoprim, digoxin
Hypertension
Hypoaldosteronism (including type 4 renal tubular acidosis)
Addison disease
Advanced age
Lower body mass index.
Both hypokalemia and hyperkalemia are associated with a higher risk of death,20,21,24 but in patients with heart failure, the survival benefit from ACE inhibitors, ARBs, and mineralocorticoid receptor antagonists outweighs the risk of hyperkalemia.25–27 Weir and Rolfe28 concluded that patients with heart failure and chronic kidney disease are at greatest risk of hyperkalemia from renin-angiotensin-aldosterone system inhibition, but the increases in potassium levels are small (about 0.1 to 0.3 mmol/L) and unlikely to be clinically significant.
Hyperkalemia tends to recur. Einhorn et al20 found that nearly half of patients with chronic kidney disease who had an episode of hyperkalemia had 1 or more recurrent episodes within a year.
ACE INHIBITORS, ARBs, ABD RENAL FUNCTION
Another concern about using ACE inhibitors and ARBs, especially in patients with chronic kidney disease, is that the serum creatinine level tends to rise when starting these drugs,29 although several studies have shown that an acute rise in creatinine may demonstrate that the drug is actually protecting the kidney.30,31 Hirsch32 described this phenomenon as “prerenal success,” proposing that the decline in GFR is hemodynamic, secondary to a fall in intraglomerular pressure as a result of efferent vasodilation, and therefore should not be reversed.
Schmidt et al,33,34 in a study in 122,363 patients who began ACE inhibitor or ARB therapy, found that cardiorenal outcomes were worse, with higher rates of end-stage renal disease, myocardial infarction, heart failure, and death, in those in whom creatinine rose by 30% or more since starting treatment. This trend was also seen, to a lesser degree, in those with a smaller increase in creatinine, suggesting that even this group of patients should receive close monitoring.
Whether renin-angiotensin-aldosterone system inhibitors provide a benefit in advanced progressive chronic kidney disease remains unclear.35–37 The Angiotensin Converting Enzyme Inhibitor (ACEi)/Angiotensin Receptor Blocker (ARB) Withdrawal in Advanced Renal Disease trial (STOP-ACEi),38 currently under way, will provide valuable data to help close this gap in our knowledge. This open-label randomized controlled trial is testing the hypothesis that stopping ACE inhibitor or ARB treatment, or a combination of both, compared with continuing these treatments, will improve or stabilize renal function in patients with progressive stage 4 or 5 chronic kidney disease.
NEED FOR MONITORING
Taken together, the above data suggest close and regular monitoring is required in patients receiving these drugs. However, monitoring tends to be lax.34,37,39 A 2017 study of adherence to the guidelines for monitoring serum creatinine and potassium after starting an ACE inhibitor or ARB and subsequent discontinuation found that fewer than 10% of patients had follow-up within the recommended 2 weeks after starting these drugs.34 Most patients with a creatinine rise of 30% or more or a potassium level higher than 6.0 mmol/L continued treatment. There was also no evidence of increased monitoring in those deemed at higher risk of these complications.
WHAT DO THE GUIDELINES SUGGEST?
ACE inhibitors and ARBs in chronic kidney disease and hypertension
Target blood pressures vary in guidelines from different organizations.4,40–45 The 2017 joint guidelines of the American College of Cardiology and American Heart Association (ACC/AHA)40 recommend a target blood pressure of 130/80 mm Hg or less in all patients irrespective of the level of proteinuria and whether they have diabetes mellitus, based on several studies.46–48 In the elderly, other factors such as the risk of hypotension and falls must be taken into consideration in establishing the most appropriate blood pressure target.
In general, a renin-angiotensin-aldosterone system inhibitor is recommended if the patient has diabetes, stage 1, 2, or 3 chronic kidney disease, or proteinuria. For example, the guidelines recommend a renin-angiotensin-aldosterone system inhibitor in diabetic patients with albuminuria.
None of the guidelines recommend routine use of combination therapy.
ACE inhibitors and ARBs in heart failure
The 2017 ACC/AHA and Heart Failure Society of America (HFSA) guidelines for heart failure49 recommend an ACE inhibitor or ARB for patients with stage C (symptomatic) heart failure with reduced ejection fraction, in view of the known cardiovascular morbidity and mortality benefits.
The European Society of Cardiology50 recommends ACE inhibitors for patients with symptomatic heart failure with reduced ejection fraction, as well as those with asymptomatic left ventricular systolic dysfunction. In patients with stable coronary artery disease, an ACE inhibitor should be considered even with normal left ventricular function.
ARBs should be used as alternatives in those unable to tolerate ACE inhibitors.
Combination therapy should be avoided due to the increased risk of renal impairment and hyperkalemia but may be considered in patients with heart failure and reduced ejection fraction in whom other treatments are unsuitable. These include patients on beta-blockers who cannot tolerate mineralocorticoid receptor antagonists such as spironolactone. Combination therapy should be done only under strict supervision.50
Starting ACE or ARB therapy
Close monitoring of serum potassium is recommended during ACE inhibitor or ARB use. Those at greatest risk of hyperkalemia include elderly patients, those taking other medications associated with hyperkalemia, and diabetic patients, because of their higher risk of renovascular disease.
Caution is advised when starting ACE inhibitor or ARB therapy in these high-risk groups as well as in patients with potassium levels higher than 5.0 mmol/L at baseline, at high risk of prerenal acute kidney injury, with known renal insufficiency, and with previous deterioration in renal function on these medications.3,41,51
Before starting therapy, ensure that patients are volume-replete and measure baseline serum electrolytes and creatinine.41,51
The ACC/AHA and HFSA recommend starting at a low dose and titrating upward slowly. If maximal doses are not tolerated, then a lower dose should be maintained.49 The European Society of Cardiology guidelines52 suggest increasing the dose at no less than every 2 weeks unless in an inpatient setting. Blood testing should be done 7 to 14 days after starting therapy, after any titration in dosage, and every 4 months thereafter.53
The guidelines generally agree that a rise in creatinine of up to 30% and a fall in eGFR of up to 25% is acceptable, with the need for regular monitoring, particularly in high-risk groups.40–42,51,52
What if serum potassium or creatinine rises during treatment?
If hyperkalemia arises or renal function declines by a significant amount, one should first address contributing factors. If no improvement is seen, then the dose of the ACE inhibitor or ARB should be reduced by 50% and blood work repeated in 1 to 2 weeks. If the laboratory values do not return to an acceptable level, reducing the dose further or stopping the drug is advised.
Give dietary advice to all patients with chronic kidney disease being considered for a renin-angiotensin-aldosterone system inhibitor or for an increase in dose with a potassium level higher than 4.5 mmol/L. A low-potassium diet should aim for potassium intake of less than 50 or 75 mmol/day and sodium intake of less than 60 mmol/day for hypertensive patients with chronic kidney disease.
Review the patient’s medications if the baseline potassium level is higher than 5.0 mmol/L. Consider stopping potassium-sparing agents, digoxin, trimethoprim, and nonsteroidal anti-inflammatory drugs. Also think about starting a non–potassium-sparing diuretic as well as sodium bicarbonate to reduce potassium levels. Blood work should be repeated within 2 weeks after these changes.
Do not start a renin-angiotensin-aldosterone system inhibitor, or do not increase the dose, if the potassium level is elevated until measures have been taken to reduce the degree of hyperkalemia.51
In renal transplant recipients, renin-angiotensin-aldosterone system inhibitors are often preferred to manage hypertension in those who have proteinuria or cardiovascular disease. However, the risk of hyperkalemia is also greater with concomitant use of immunosuppressive drugs such as tacrolimus and cyclosporine. Management of complications should be approached according to guidelines discussed above.51
Monitor renal function, potassium. The National Institute for Health and Care Excellence guideline54 advocates that baseline renal function testing should be followed by repeat blood testing 1 to 2 weeks after starting renin-angiotensin-aldosterone system inhibitors in patients with ischemic heart disease. The advice is similar when starting therapy in patients with chronic heart failure, emphasizing the need to monitor after each dose increment and to use clinical judgment when deciding to start treatment. The AHA advises caution in patients with renal insufficiency or a potassium level above 5.0 mmol/L.49
Sick day rules. The National Institute for Health and Care Excellence encourages discussing “sick day rules” with patients starting renin-angiotensin-aldosterone system inhibitors. This means patients should be advised to temporarily stop taking nephrotoxic medications, including over-the-counter nonsteroidal anti-inflammatory drugs, in any potential state of illness or dehydration, such as diarrhea and vomiting. There is, however, little evidence that this advice can actually reduce the incidence of acute kidney injury.55,56
OUR RECOMMENDATIONS
Our advice for managing patients receiving ACE inhibitors or ARBs is summarized in Table 1.
- Page IH, Helmer OM. A crystalline pressor substance (angiotonin) resulting from the reaction between renin and renin-activator. Exp Med 1940; 71(1):29–42. doi:10.1084/jem.71.1.29
- Steddon S, Ashman N, Chesser A, Cunningham J. Oxford Handbook of Nephrology and Hypertension. 2nd ed. Oxford: Oxford University Press; 2016:203–206, 508–509.
- Barratt J, Topham P, Harris K. Oxford Desk Reference. 1st ed. Oxford: Oxford University Press; 2008.
- International Kidney Foundation. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO_BP_GL.pdf. Accessed April 3, 2019.
- Heart Outcomes Prevention Evaluation Study Investigators; Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000; 342(3):145–153. doi:10.1056/NEJM200001203420301
- Swedberg K, Kjekshus J. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). Am J Cardiol 1988; 62(2):60A–66A. pmid:2839019
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345(12):861–869. doi:10.1056/NEJMoa011161
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349(20):1893–1906. doi:10.1056/NEJMoa032292
- Epstein M. Reduction of cardiovascular risk in chronic kidney disease by mineralocorticoid receptor antagonism. Lancet Diabetes Endocrinol 2015; 3(12):993–1003. doi:10.1016/S2213-8587(15)00289-2
- SOLVD Investigators; Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325(5):293–302. doi:10.1056/NEJM199108013250501
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group; Angiotensin-Converting Enzymne Inhibition and Progression of Renal Disease. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 2001; 60(3):1131–1140. doi:10.1046/j.1523-1755.2001.0600031131.x
- Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet 2015; 385(9982):2047–2056. doi:10.1016/S0140-6736(14)62459-4
- Ruggenenti P, Perticucci E, Cravedi P, et al. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 2008; 19(6):1213–1224. doi:10.1681/ASN.2007090970
- Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH. Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomised trials. BMJ 2013; 346:f360. doi:10.1136/bmj.f360
- ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358(15):1547–1559. doi:10.1056/NEJMoa0801317
- Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369(20):1892–1903.
doi:10.1056/NEJMoa1303154 - Catalá-López F, Macías Saint-Gerons D, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med 2016; 13(3):e1001971. doi:10.1371/journal.pmed.1001971
- Agarwal R, Afzalpurkar R, Fordtran JS. Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology 1994; 107(2):548–571. pmid:8039632
- Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol 2015; 10(6):1050–1060. doi:10.2215/CJN.08580813
- Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med 2009; 169(12):1156–1162. doi:10.1001/archinternmed.2009.132
- Nakhoul GN, Huang H, Arrigain S, et al. Serum potassium, end-stage renal disease and mortality in chronic kidney disease. Am J Nephrol 2015; 41(6):456–463. doi:10.1159/000437151
- Acker CG, Johnson JP, Palevsky PM, Greenberg A. Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med 1998; 158(8):917–924. pmid:9570179
- Desai AS, Swedberg K, McMurray JJ, et al; CHARM Program Investigators. Incidence and predictors of hyperkalemia in patients with heart failure: an analysis of the CHARM Program. J Am Coll Cardiol 2007; 50(20):1959–1966. doi:10.1016/j.jacc.2007.07.067
- Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Sakhuja A, Mao MA, Erickson SB. Impact of admission serum potassium on mortality in patients with chronic kidney disease and cardiovascular disease. QJM 2017; 110(11):713–719. doi:10.1093/qjmed/hcx118
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364(1):11–21. doi:10.1056/NEJMoa1009492
- Rossignol P, Dobre D, McMurray JJ, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail 2014; 7(1):51–58. doi:10.1161/CIRCHEARTFAILURE.113.000792
- Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail 2011; 4(6):685–691. doi:10.1161/CIRCHEARTFAILURE.111.963256
- Weir M, Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol 2010; 5(3):531–548. doi:10.2215/CJN.07821109
- Valente M, Bhandari S. Renal function after new treatment with renin-angiotensin system blockers. BMJ 2017; 356:j1122. doi:10.1136/bmj.j1122
- Bakris G, Weir M. Angiotensin-converting enzyme inhibitor–associated elevations in serum creatinine. Arch Intern Med 2000; 160(5):685–693. pmid:10724055
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345(12):861–869. doi:10.1056/NEJMoa011161
- Hirsch S. Pre-renal success. Kidney Int 2012; 81(6):596. doi:10.1038/ki.2011.418
- Schmidt M, Mansfield KE, Bhaskaran K, et al. Serum creatinine elevation after renin-angiotensin system blockade and long term cardiorenal risks: cohort study. BMJ 2017; 356:j791. doi:10.1136/bmj.j791
- Schmidt M, Mansfield KE, Bhaskaran K, et al. Adherence to guidelines for creatinine and potassium monitoring and discontinuation following renin–angiotensin system blockade: a UK general practice-based cohort study. BMJ Open 2017; 7(1):e012818. doi:10.1136/bmjopen-2016-012818
- Lund LH, Carrero JJ, Farahmand B, et al. Association between enrollment in a heart failure quality registry and subsequent mortality—a nationwide cohort study. Eur J Heart Fail 2017; 19(9):1107–1116. doi:10.1002/ejhf.762
- Edner M, Benson L, Dahlstrom U, Lund LH. Association between renin-angiotensin system antagonist use and mortality in heart failure with severe renal insuffuciency: a prospective propensity score-matched cohort study. Eur Heart J 2015; 36(34):2318–2326. doi:10.1093/eurheartj/ehv268
- Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care 2015; 21(suppl 11):S212–S220. pmid:26619183
- Bhandari S, Ives N, Brettell EA, et al. Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi trial. Nephrol Dial Transplant 2016; 31(2):255–261. doi:10.1093/ndt/gfv346
- Raebel MA, Ross C, Xu S, et al. Diabetes and drug-associated hyperkalemia: effect of potassium monitoring. J Gen Intern Med 2010; 25(4):326–333. doi:10.1007/s11606-009-1228-x
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71(6):e13–e115. doi:10.1161/HYP.0000000000000065
- The Renal Association. The UK eCKD Guide. https://renal.org/information-resources/the-uk-eckd-guide. Accessed August 12, 2019.
- National Institute for Health and Care Excellence (NICE). Chronic kidney disease in adults: assessment and management. https://www.nice.org.uk/guidance/cg182. Accessed August 12, 2019.
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. https://www.nice.org.uk/Guidance/CG127. Accessed August 12, 2019.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34(28):2159–2219. doi:10.1093/eurheartj/eht151
- International Kidney Foundation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. https://www.sciencedirect.com/journal/kidney-international-supplements/vol/3/issue/1. Accessed August 12, 2019.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Wright J, Bakris G, Greene T. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease. Results from the AASK trial. ACC Current Journal Review 2003; 12(2):37–38. doi:10.1016/s1062-1458(03)00035-7
- Ku E, Bakris G, Johansen K, et al. Acute declines in renal function during intensive BP lowering: implications for future ESRD risk. J Am Soc Nephrol 2017; 28(9):2794–2801. doi:10.1681/ASN.2017010040
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136(6):e137–e161. doi:10.1161/CIR.0000000000000509
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37(27):2129–2200. doi:10.1093/eurheartj/ehw128
- Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 51):S1–S290. pmid:15114537
- Asenjo RM, Bueno H, Mcintosh M. Angiotensin converting enzyme inhibitors (ACE inhibitors) and angiotensin II receptor blockers (ARBs). ACE inhibitors and ARBs, a cornerstone in the prevention and treatment of cardiovascular disease. www.escardio.org/Education/ESC-Prevention-of-CVD-Programme/Treatment-goals/Cardio-Protective-drugs/angiotensin-converting-enzyme-inhibitors-ace-inhibitors-and-angiotensin-ii-rec. Accessed August 12, 2019.
- López-Sendón J, Swedberg K, McMurray J, et al; Task Force on ACE-inhibitors of the European Society of Cardiology. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur Heart J 2004; 25(16):1454–1470. doi:10.1016/j.ehj.2004.06.003
- National Institute for Health and Care Excellence (NICE). Myocardial infarction: cardiac rehabilitation and prevention of further cardiovascular disease. https://www.nice.org.uk/Guidance/CG172. Accessed April 3, 2019.
- National Institute for Health and Care Excellence (NICE). Acute kidney injury: prevention, detection and management. https://www.nice.org.uk/Guidance/CG169. Accessed August 12, 2019.
- Think Kidneys. “Sick day” guidance in patients at risk of acute kidney injury: a position statement from the Think Kidneys Board. https://www.thinkkidneys.nhs.uk/aki/wp-content/uploads/sites/2/2018/01/Think-Kidneys-Sick-Day-Guidance-2018.pdf. Accessed August 12, 2019.
- Meaney CJ, Beccari MV, Yang Y, Zhao J. Systematic review and meta-analysis of patiromer and sodium zirconium cyclosilicate: a new armamentarium for the treatment of hyperkalemia. Pharmacotherapy 2017; 37(4):401–411. doi:10.1002/phar.1906
A highly active, water- and alcohol-soluble, basic pressor substance is formed when renin and renin-activator interact, for which we suggest the name “angiotonin.”
—Irvine H. Page and O.M. Helmer, 1940.1
The renin-angiotensin-aldosterone system regulates salt and, in part, water homeostasis, and therefore blood pressure and fluid balance through its actions on the heart, kidneys, and blood vessels.2 Drugs that target this system—angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)—are used primarily to treat hypertension and also to treat chronic kidney disease and heart failure with reduced ejection fraction.
Controlling blood pressure is important, as hypertension increases the risk of myocardial infarction, cerebrovascular events, and progression of chronic kidney disease, which itself is a risk factor for cardiovascular disease. However, the benefit of these drugs is only partly due to their effect on blood pressure. They also reduce proteinuria, which is a graded risk factor for progression of kidney disease as well as morbidity and death from vascular events.3
Despite the benefits of ACE inhibitors and ARBs, concern about their adverse effects—especially hyperkalemia and a decline in renal function—has led to their underuse in patients likely to derive the greatest benefit.3
ACE INHIBITORS AND ARBs
ACE inhibitors, as their name indicates, inhibit conversion of angiotensin I to angiotensin II by ACE, resulting in vasodilation of the efferent arteriole and a drop in blood pressure. Inhibition of ACE, a kininase, also results in a rise in kinins. One of these, bradykinin, is associated with some of the side effects of this class of drugs such as cough, which affects 5% to 20% of patients.4 Elevation of bradykinin is also believed to account for ACE inhibitor-induced angioedema, an uncommon but potentially serious side effect. Kinins are also associated with desirable effects such as lowering blood pressure, increasing insulin sensitivity, and dilating blood vessels.
ARBs were developed as an alternative for patients unable to tolerate the adverse effects of ACE inhibitors. While ACE inhibitors reduce the activity of angiotensin II at both the AT1 and AT2 receptors, ARBs block only the AT1 receptors, thereby inhibiting their vasoconstricting activity on smooth muscle. ARBs also raise the levels of renin, angiotensin I, and angiotensin II as a result of feedback inhibition. Angiotensin II is associated with release of inflammatory mediators such as tumor necrosis factor alpha, cytokines, and chemokines, the consequences of which are also inhibited by ARBs, further preventing renal fibrosis and scarring from chronic inflammation.3
What is the evidence supporting the use of ACE inhibitors and ARBs?
ACE inhibitors and ARBs, used singly, reduce blood pressure and proteinuria, slow progression of kidney disease, and improve outcomes in patients who have heart failure, diabetes mellitus, or a history of myocardial infarction.5–11
While dual blockade with the combination of an ACE inhibitor and an ARB lowers blood pressure and proteinuria to a greater degree than monotherapy, dual blockade has been associated with higher rates of complications, including hyperkalemia.12–17
RISK FACTORS FOR HYPERKALEMIA
ACE inhibitors and ARBs raise potassium, especially when used in combination. Other risk factors for hyperkalemia include the following—and note that some of them are also indications for ACE inhibitors and ARBs:
Renal insufficiency. The kidneys are responsible for over 90% of potassium removal in healthy individuals,18,19 and the lower the GFR, the higher the risk of hyperkalemia.3,20,21
Heart failure
Diabetes mellitus6,21–23
Endogenous potassium load due to hemolysis, rhabdomyolysis, insulin deficiency, lactic acidosis, or gastrointestinal bleeding
Exogenous potassium load due to dietary consumption or blood products
Other medications, eg, sacubitril-valsartan, aldosterone antagonists, mineralocorticoid receptor antagonists, potassium-sparing diuretics, beta-adrenergic antagonists, nonsteroidal anti-inflammatory drugs, heparin, cyclosporine, trimethoprim, digoxin
Hypertension
Hypoaldosteronism (including type 4 renal tubular acidosis)
Addison disease
Advanced age
Lower body mass index.
Both hypokalemia and hyperkalemia are associated with a higher risk of death,20,21,24 but in patients with heart failure, the survival benefit from ACE inhibitors, ARBs, and mineralocorticoid receptor antagonists outweighs the risk of hyperkalemia.25–27 Weir and Rolfe28 concluded that patients with heart failure and chronic kidney disease are at greatest risk of hyperkalemia from renin-angiotensin-aldosterone system inhibition, but the increases in potassium levels are small (about 0.1 to 0.3 mmol/L) and unlikely to be clinically significant.
Hyperkalemia tends to recur. Einhorn et al20 found that nearly half of patients with chronic kidney disease who had an episode of hyperkalemia had 1 or more recurrent episodes within a year.
ACE INHIBITORS, ARBs, ABD RENAL FUNCTION
Another concern about using ACE inhibitors and ARBs, especially in patients with chronic kidney disease, is that the serum creatinine level tends to rise when starting these drugs,29 although several studies have shown that an acute rise in creatinine may demonstrate that the drug is actually protecting the kidney.30,31 Hirsch32 described this phenomenon as “prerenal success,” proposing that the decline in GFR is hemodynamic, secondary to a fall in intraglomerular pressure as a result of efferent vasodilation, and therefore should not be reversed.
Schmidt et al,33,34 in a study in 122,363 patients who began ACE inhibitor or ARB therapy, found that cardiorenal outcomes were worse, with higher rates of end-stage renal disease, myocardial infarction, heart failure, and death, in those in whom creatinine rose by 30% or more since starting treatment. This trend was also seen, to a lesser degree, in those with a smaller increase in creatinine, suggesting that even this group of patients should receive close monitoring.
Whether renin-angiotensin-aldosterone system inhibitors provide a benefit in advanced progressive chronic kidney disease remains unclear.35–37 The Angiotensin Converting Enzyme Inhibitor (ACEi)/Angiotensin Receptor Blocker (ARB) Withdrawal in Advanced Renal Disease trial (STOP-ACEi),38 currently under way, will provide valuable data to help close this gap in our knowledge. This open-label randomized controlled trial is testing the hypothesis that stopping ACE inhibitor or ARB treatment, or a combination of both, compared with continuing these treatments, will improve or stabilize renal function in patients with progressive stage 4 or 5 chronic kidney disease.
NEED FOR MONITORING
Taken together, the above data suggest close and regular monitoring is required in patients receiving these drugs. However, monitoring tends to be lax.34,37,39 A 2017 study of adherence to the guidelines for monitoring serum creatinine and potassium after starting an ACE inhibitor or ARB and subsequent discontinuation found that fewer than 10% of patients had follow-up within the recommended 2 weeks after starting these drugs.34 Most patients with a creatinine rise of 30% or more or a potassium level higher than 6.0 mmol/L continued treatment. There was also no evidence of increased monitoring in those deemed at higher risk of these complications.
WHAT DO THE GUIDELINES SUGGEST?
ACE inhibitors and ARBs in chronic kidney disease and hypertension
Target blood pressures vary in guidelines from different organizations.4,40–45 The 2017 joint guidelines of the American College of Cardiology and American Heart Association (ACC/AHA)40 recommend a target blood pressure of 130/80 mm Hg or less in all patients irrespective of the level of proteinuria and whether they have diabetes mellitus, based on several studies.46–48 In the elderly, other factors such as the risk of hypotension and falls must be taken into consideration in establishing the most appropriate blood pressure target.
In general, a renin-angiotensin-aldosterone system inhibitor is recommended if the patient has diabetes, stage 1, 2, or 3 chronic kidney disease, or proteinuria. For example, the guidelines recommend a renin-angiotensin-aldosterone system inhibitor in diabetic patients with albuminuria.
None of the guidelines recommend routine use of combination therapy.
ACE inhibitors and ARBs in heart failure
The 2017 ACC/AHA and Heart Failure Society of America (HFSA) guidelines for heart failure49 recommend an ACE inhibitor or ARB for patients with stage C (symptomatic) heart failure with reduced ejection fraction, in view of the known cardiovascular morbidity and mortality benefits.
The European Society of Cardiology50 recommends ACE inhibitors for patients with symptomatic heart failure with reduced ejection fraction, as well as those with asymptomatic left ventricular systolic dysfunction. In patients with stable coronary artery disease, an ACE inhibitor should be considered even with normal left ventricular function.
ARBs should be used as alternatives in those unable to tolerate ACE inhibitors.
Combination therapy should be avoided due to the increased risk of renal impairment and hyperkalemia but may be considered in patients with heart failure and reduced ejection fraction in whom other treatments are unsuitable. These include patients on beta-blockers who cannot tolerate mineralocorticoid receptor antagonists such as spironolactone. Combination therapy should be done only under strict supervision.50
Starting ACE or ARB therapy
Close monitoring of serum potassium is recommended during ACE inhibitor or ARB use. Those at greatest risk of hyperkalemia include elderly patients, those taking other medications associated with hyperkalemia, and diabetic patients, because of their higher risk of renovascular disease.
Caution is advised when starting ACE inhibitor or ARB therapy in these high-risk groups as well as in patients with potassium levels higher than 5.0 mmol/L at baseline, at high risk of prerenal acute kidney injury, with known renal insufficiency, and with previous deterioration in renal function on these medications.3,41,51
Before starting therapy, ensure that patients are volume-replete and measure baseline serum electrolytes and creatinine.41,51
The ACC/AHA and HFSA recommend starting at a low dose and titrating upward slowly. If maximal doses are not tolerated, then a lower dose should be maintained.49 The European Society of Cardiology guidelines52 suggest increasing the dose at no less than every 2 weeks unless in an inpatient setting. Blood testing should be done 7 to 14 days after starting therapy, after any titration in dosage, and every 4 months thereafter.53
The guidelines generally agree that a rise in creatinine of up to 30% and a fall in eGFR of up to 25% is acceptable, with the need for regular monitoring, particularly in high-risk groups.40–42,51,52
What if serum potassium or creatinine rises during treatment?
If hyperkalemia arises or renal function declines by a significant amount, one should first address contributing factors. If no improvement is seen, then the dose of the ACE inhibitor or ARB should be reduced by 50% and blood work repeated in 1 to 2 weeks. If the laboratory values do not return to an acceptable level, reducing the dose further or stopping the drug is advised.
Give dietary advice to all patients with chronic kidney disease being considered for a renin-angiotensin-aldosterone system inhibitor or for an increase in dose with a potassium level higher than 4.5 mmol/L. A low-potassium diet should aim for potassium intake of less than 50 or 75 mmol/day and sodium intake of less than 60 mmol/day for hypertensive patients with chronic kidney disease.
Review the patient’s medications if the baseline potassium level is higher than 5.0 mmol/L. Consider stopping potassium-sparing agents, digoxin, trimethoprim, and nonsteroidal anti-inflammatory drugs. Also think about starting a non–potassium-sparing diuretic as well as sodium bicarbonate to reduce potassium levels. Blood work should be repeated within 2 weeks after these changes.
Do not start a renin-angiotensin-aldosterone system inhibitor, or do not increase the dose, if the potassium level is elevated until measures have been taken to reduce the degree of hyperkalemia.51
In renal transplant recipients, renin-angiotensin-aldosterone system inhibitors are often preferred to manage hypertension in those who have proteinuria or cardiovascular disease. However, the risk of hyperkalemia is also greater with concomitant use of immunosuppressive drugs such as tacrolimus and cyclosporine. Management of complications should be approached according to guidelines discussed above.51
Monitor renal function, potassium. The National Institute for Health and Care Excellence guideline54 advocates that baseline renal function testing should be followed by repeat blood testing 1 to 2 weeks after starting renin-angiotensin-aldosterone system inhibitors in patients with ischemic heart disease. The advice is similar when starting therapy in patients with chronic heart failure, emphasizing the need to monitor after each dose increment and to use clinical judgment when deciding to start treatment. The AHA advises caution in patients with renal insufficiency or a potassium level above 5.0 mmol/L.49
Sick day rules. The National Institute for Health and Care Excellence encourages discussing “sick day rules” with patients starting renin-angiotensin-aldosterone system inhibitors. This means patients should be advised to temporarily stop taking nephrotoxic medications, including over-the-counter nonsteroidal anti-inflammatory drugs, in any potential state of illness or dehydration, such as diarrhea and vomiting. There is, however, little evidence that this advice can actually reduce the incidence of acute kidney injury.55,56
OUR RECOMMENDATIONS
Our advice for managing patients receiving ACE inhibitors or ARBs is summarized in Table 1.
A highly active, water- and alcohol-soluble, basic pressor substance is formed when renin and renin-activator interact, for which we suggest the name “angiotonin.”
—Irvine H. Page and O.M. Helmer, 1940.1
The renin-angiotensin-aldosterone system regulates salt and, in part, water homeostasis, and therefore blood pressure and fluid balance through its actions on the heart, kidneys, and blood vessels.2 Drugs that target this system—angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)—are used primarily to treat hypertension and also to treat chronic kidney disease and heart failure with reduced ejection fraction.
Controlling blood pressure is important, as hypertension increases the risk of myocardial infarction, cerebrovascular events, and progression of chronic kidney disease, which itself is a risk factor for cardiovascular disease. However, the benefit of these drugs is only partly due to their effect on blood pressure. They also reduce proteinuria, which is a graded risk factor for progression of kidney disease as well as morbidity and death from vascular events.3
Despite the benefits of ACE inhibitors and ARBs, concern about their adverse effects—especially hyperkalemia and a decline in renal function—has led to their underuse in patients likely to derive the greatest benefit.3
ACE INHIBITORS AND ARBs
ACE inhibitors, as their name indicates, inhibit conversion of angiotensin I to angiotensin II by ACE, resulting in vasodilation of the efferent arteriole and a drop in blood pressure. Inhibition of ACE, a kininase, also results in a rise in kinins. One of these, bradykinin, is associated with some of the side effects of this class of drugs such as cough, which affects 5% to 20% of patients.4 Elevation of bradykinin is also believed to account for ACE inhibitor-induced angioedema, an uncommon but potentially serious side effect. Kinins are also associated with desirable effects such as lowering blood pressure, increasing insulin sensitivity, and dilating blood vessels.
ARBs were developed as an alternative for patients unable to tolerate the adverse effects of ACE inhibitors. While ACE inhibitors reduce the activity of angiotensin II at both the AT1 and AT2 receptors, ARBs block only the AT1 receptors, thereby inhibiting their vasoconstricting activity on smooth muscle. ARBs also raise the levels of renin, angiotensin I, and angiotensin II as a result of feedback inhibition. Angiotensin II is associated with release of inflammatory mediators such as tumor necrosis factor alpha, cytokines, and chemokines, the consequences of which are also inhibited by ARBs, further preventing renal fibrosis and scarring from chronic inflammation.3
What is the evidence supporting the use of ACE inhibitors and ARBs?
ACE inhibitors and ARBs, used singly, reduce blood pressure and proteinuria, slow progression of kidney disease, and improve outcomes in patients who have heart failure, diabetes mellitus, or a history of myocardial infarction.5–11
While dual blockade with the combination of an ACE inhibitor and an ARB lowers blood pressure and proteinuria to a greater degree than monotherapy, dual blockade has been associated with higher rates of complications, including hyperkalemia.12–17
RISK FACTORS FOR HYPERKALEMIA
ACE inhibitors and ARBs raise potassium, especially when used in combination. Other risk factors for hyperkalemia include the following—and note that some of them are also indications for ACE inhibitors and ARBs:
Renal insufficiency. The kidneys are responsible for over 90% of potassium removal in healthy individuals,18,19 and the lower the GFR, the higher the risk of hyperkalemia.3,20,21
Heart failure
Diabetes mellitus6,21–23
Endogenous potassium load due to hemolysis, rhabdomyolysis, insulin deficiency, lactic acidosis, or gastrointestinal bleeding
Exogenous potassium load due to dietary consumption or blood products
Other medications, eg, sacubitril-valsartan, aldosterone antagonists, mineralocorticoid receptor antagonists, potassium-sparing diuretics, beta-adrenergic antagonists, nonsteroidal anti-inflammatory drugs, heparin, cyclosporine, trimethoprim, digoxin
Hypertension
Hypoaldosteronism (including type 4 renal tubular acidosis)
Addison disease
Advanced age
Lower body mass index.
Both hypokalemia and hyperkalemia are associated with a higher risk of death,20,21,24 but in patients with heart failure, the survival benefit from ACE inhibitors, ARBs, and mineralocorticoid receptor antagonists outweighs the risk of hyperkalemia.25–27 Weir and Rolfe28 concluded that patients with heart failure and chronic kidney disease are at greatest risk of hyperkalemia from renin-angiotensin-aldosterone system inhibition, but the increases in potassium levels are small (about 0.1 to 0.3 mmol/L) and unlikely to be clinically significant.
Hyperkalemia tends to recur. Einhorn et al20 found that nearly half of patients with chronic kidney disease who had an episode of hyperkalemia had 1 or more recurrent episodes within a year.
ACE INHIBITORS, ARBs, ABD RENAL FUNCTION
Another concern about using ACE inhibitors and ARBs, especially in patients with chronic kidney disease, is that the serum creatinine level tends to rise when starting these drugs,29 although several studies have shown that an acute rise in creatinine may demonstrate that the drug is actually protecting the kidney.30,31 Hirsch32 described this phenomenon as “prerenal success,” proposing that the decline in GFR is hemodynamic, secondary to a fall in intraglomerular pressure as a result of efferent vasodilation, and therefore should not be reversed.
Schmidt et al,33,34 in a study in 122,363 patients who began ACE inhibitor or ARB therapy, found that cardiorenal outcomes were worse, with higher rates of end-stage renal disease, myocardial infarction, heart failure, and death, in those in whom creatinine rose by 30% or more since starting treatment. This trend was also seen, to a lesser degree, in those with a smaller increase in creatinine, suggesting that even this group of patients should receive close monitoring.
Whether renin-angiotensin-aldosterone system inhibitors provide a benefit in advanced progressive chronic kidney disease remains unclear.35–37 The Angiotensin Converting Enzyme Inhibitor (ACEi)/Angiotensin Receptor Blocker (ARB) Withdrawal in Advanced Renal Disease trial (STOP-ACEi),38 currently under way, will provide valuable data to help close this gap in our knowledge. This open-label randomized controlled trial is testing the hypothesis that stopping ACE inhibitor or ARB treatment, or a combination of both, compared with continuing these treatments, will improve or stabilize renal function in patients with progressive stage 4 or 5 chronic kidney disease.
NEED FOR MONITORING
Taken together, the above data suggest close and regular monitoring is required in patients receiving these drugs. However, monitoring tends to be lax.34,37,39 A 2017 study of adherence to the guidelines for monitoring serum creatinine and potassium after starting an ACE inhibitor or ARB and subsequent discontinuation found that fewer than 10% of patients had follow-up within the recommended 2 weeks after starting these drugs.34 Most patients with a creatinine rise of 30% or more or a potassium level higher than 6.0 mmol/L continued treatment. There was also no evidence of increased monitoring in those deemed at higher risk of these complications.
WHAT DO THE GUIDELINES SUGGEST?
ACE inhibitors and ARBs in chronic kidney disease and hypertension
Target blood pressures vary in guidelines from different organizations.4,40–45 The 2017 joint guidelines of the American College of Cardiology and American Heart Association (ACC/AHA)40 recommend a target blood pressure of 130/80 mm Hg or less in all patients irrespective of the level of proteinuria and whether they have diabetes mellitus, based on several studies.46–48 In the elderly, other factors such as the risk of hypotension and falls must be taken into consideration in establishing the most appropriate blood pressure target.
In general, a renin-angiotensin-aldosterone system inhibitor is recommended if the patient has diabetes, stage 1, 2, or 3 chronic kidney disease, or proteinuria. For example, the guidelines recommend a renin-angiotensin-aldosterone system inhibitor in diabetic patients with albuminuria.
None of the guidelines recommend routine use of combination therapy.
ACE inhibitors and ARBs in heart failure
The 2017 ACC/AHA and Heart Failure Society of America (HFSA) guidelines for heart failure49 recommend an ACE inhibitor or ARB for patients with stage C (symptomatic) heart failure with reduced ejection fraction, in view of the known cardiovascular morbidity and mortality benefits.
The European Society of Cardiology50 recommends ACE inhibitors for patients with symptomatic heart failure with reduced ejection fraction, as well as those with asymptomatic left ventricular systolic dysfunction. In patients with stable coronary artery disease, an ACE inhibitor should be considered even with normal left ventricular function.
ARBs should be used as alternatives in those unable to tolerate ACE inhibitors.
Combination therapy should be avoided due to the increased risk of renal impairment and hyperkalemia but may be considered in patients with heart failure and reduced ejection fraction in whom other treatments are unsuitable. These include patients on beta-blockers who cannot tolerate mineralocorticoid receptor antagonists such as spironolactone. Combination therapy should be done only under strict supervision.50
Starting ACE or ARB therapy
Close monitoring of serum potassium is recommended during ACE inhibitor or ARB use. Those at greatest risk of hyperkalemia include elderly patients, those taking other medications associated with hyperkalemia, and diabetic patients, because of their higher risk of renovascular disease.
Caution is advised when starting ACE inhibitor or ARB therapy in these high-risk groups as well as in patients with potassium levels higher than 5.0 mmol/L at baseline, at high risk of prerenal acute kidney injury, with known renal insufficiency, and with previous deterioration in renal function on these medications.3,41,51
Before starting therapy, ensure that patients are volume-replete and measure baseline serum electrolytes and creatinine.41,51
The ACC/AHA and HFSA recommend starting at a low dose and titrating upward slowly. If maximal doses are not tolerated, then a lower dose should be maintained.49 The European Society of Cardiology guidelines52 suggest increasing the dose at no less than every 2 weeks unless in an inpatient setting. Blood testing should be done 7 to 14 days after starting therapy, after any titration in dosage, and every 4 months thereafter.53
The guidelines generally agree that a rise in creatinine of up to 30% and a fall in eGFR of up to 25% is acceptable, with the need for regular monitoring, particularly in high-risk groups.40–42,51,52
What if serum potassium or creatinine rises during treatment?
If hyperkalemia arises or renal function declines by a significant amount, one should first address contributing factors. If no improvement is seen, then the dose of the ACE inhibitor or ARB should be reduced by 50% and blood work repeated in 1 to 2 weeks. If the laboratory values do not return to an acceptable level, reducing the dose further or stopping the drug is advised.
Give dietary advice to all patients with chronic kidney disease being considered for a renin-angiotensin-aldosterone system inhibitor or for an increase in dose with a potassium level higher than 4.5 mmol/L. A low-potassium diet should aim for potassium intake of less than 50 or 75 mmol/day and sodium intake of less than 60 mmol/day for hypertensive patients with chronic kidney disease.
Review the patient’s medications if the baseline potassium level is higher than 5.0 mmol/L. Consider stopping potassium-sparing agents, digoxin, trimethoprim, and nonsteroidal anti-inflammatory drugs. Also think about starting a non–potassium-sparing diuretic as well as sodium bicarbonate to reduce potassium levels. Blood work should be repeated within 2 weeks after these changes.
Do not start a renin-angiotensin-aldosterone system inhibitor, or do not increase the dose, if the potassium level is elevated until measures have been taken to reduce the degree of hyperkalemia.51
In renal transplant recipients, renin-angiotensin-aldosterone system inhibitors are often preferred to manage hypertension in those who have proteinuria or cardiovascular disease. However, the risk of hyperkalemia is also greater with concomitant use of immunosuppressive drugs such as tacrolimus and cyclosporine. Management of complications should be approached according to guidelines discussed above.51
Monitor renal function, potassium. The National Institute for Health and Care Excellence guideline54 advocates that baseline renal function testing should be followed by repeat blood testing 1 to 2 weeks after starting renin-angiotensin-aldosterone system inhibitors in patients with ischemic heart disease. The advice is similar when starting therapy in patients with chronic heart failure, emphasizing the need to monitor after each dose increment and to use clinical judgment when deciding to start treatment. The AHA advises caution in patients with renal insufficiency or a potassium level above 5.0 mmol/L.49
Sick day rules. The National Institute for Health and Care Excellence encourages discussing “sick day rules” with patients starting renin-angiotensin-aldosterone system inhibitors. This means patients should be advised to temporarily stop taking nephrotoxic medications, including over-the-counter nonsteroidal anti-inflammatory drugs, in any potential state of illness or dehydration, such as diarrhea and vomiting. There is, however, little evidence that this advice can actually reduce the incidence of acute kidney injury.55,56
OUR RECOMMENDATIONS
Our advice for managing patients receiving ACE inhibitors or ARBs is summarized in Table 1.
- Page IH, Helmer OM. A crystalline pressor substance (angiotonin) resulting from the reaction between renin and renin-activator. Exp Med 1940; 71(1):29–42. doi:10.1084/jem.71.1.29
- Steddon S, Ashman N, Chesser A, Cunningham J. Oxford Handbook of Nephrology and Hypertension. 2nd ed. Oxford: Oxford University Press; 2016:203–206, 508–509.
- Barratt J, Topham P, Harris K. Oxford Desk Reference. 1st ed. Oxford: Oxford University Press; 2008.
- International Kidney Foundation. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO_BP_GL.pdf. Accessed April 3, 2019.
- Heart Outcomes Prevention Evaluation Study Investigators; Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000; 342(3):145–153. doi:10.1056/NEJM200001203420301
- Swedberg K, Kjekshus J. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). Am J Cardiol 1988; 62(2):60A–66A. pmid:2839019
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345(12):861–869. doi:10.1056/NEJMoa011161
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349(20):1893–1906. doi:10.1056/NEJMoa032292
- Epstein M. Reduction of cardiovascular risk in chronic kidney disease by mineralocorticoid receptor antagonism. Lancet Diabetes Endocrinol 2015; 3(12):993–1003. doi:10.1016/S2213-8587(15)00289-2
- SOLVD Investigators; Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325(5):293–302. doi:10.1056/NEJM199108013250501
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group; Angiotensin-Converting Enzymne Inhibition and Progression of Renal Disease. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 2001; 60(3):1131–1140. doi:10.1046/j.1523-1755.2001.0600031131.x
- Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet 2015; 385(9982):2047–2056. doi:10.1016/S0140-6736(14)62459-4
- Ruggenenti P, Perticucci E, Cravedi P, et al. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 2008; 19(6):1213–1224. doi:10.1681/ASN.2007090970
- Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH. Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomised trials. BMJ 2013; 346:f360. doi:10.1136/bmj.f360
- ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358(15):1547–1559. doi:10.1056/NEJMoa0801317
- Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369(20):1892–1903.
doi:10.1056/NEJMoa1303154 - Catalá-López F, Macías Saint-Gerons D, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med 2016; 13(3):e1001971. doi:10.1371/journal.pmed.1001971
- Agarwal R, Afzalpurkar R, Fordtran JS. Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology 1994; 107(2):548–571. pmid:8039632
- Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol 2015; 10(6):1050–1060. doi:10.2215/CJN.08580813
- Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med 2009; 169(12):1156–1162. doi:10.1001/archinternmed.2009.132
- Nakhoul GN, Huang H, Arrigain S, et al. Serum potassium, end-stage renal disease and mortality in chronic kidney disease. Am J Nephrol 2015; 41(6):456–463. doi:10.1159/000437151
- Acker CG, Johnson JP, Palevsky PM, Greenberg A. Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med 1998; 158(8):917–924. pmid:9570179
- Desai AS, Swedberg K, McMurray JJ, et al; CHARM Program Investigators. Incidence and predictors of hyperkalemia in patients with heart failure: an analysis of the CHARM Program. J Am Coll Cardiol 2007; 50(20):1959–1966. doi:10.1016/j.jacc.2007.07.067
- Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Sakhuja A, Mao MA, Erickson SB. Impact of admission serum potassium on mortality in patients with chronic kidney disease and cardiovascular disease. QJM 2017; 110(11):713–719. doi:10.1093/qjmed/hcx118
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364(1):11–21. doi:10.1056/NEJMoa1009492
- Rossignol P, Dobre D, McMurray JJ, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail 2014; 7(1):51–58. doi:10.1161/CIRCHEARTFAILURE.113.000792
- Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail 2011; 4(6):685–691. doi:10.1161/CIRCHEARTFAILURE.111.963256
- Weir M, Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol 2010; 5(3):531–548. doi:10.2215/CJN.07821109
- Valente M, Bhandari S. Renal function after new treatment with renin-angiotensin system blockers. BMJ 2017; 356:j1122. doi:10.1136/bmj.j1122
- Bakris G, Weir M. Angiotensin-converting enzyme inhibitor–associated elevations in serum creatinine. Arch Intern Med 2000; 160(5):685–693. pmid:10724055
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345(12):861–869. doi:10.1056/NEJMoa011161
- Hirsch S. Pre-renal success. Kidney Int 2012; 81(6):596. doi:10.1038/ki.2011.418
- Schmidt M, Mansfield KE, Bhaskaran K, et al. Serum creatinine elevation after renin-angiotensin system blockade and long term cardiorenal risks: cohort study. BMJ 2017; 356:j791. doi:10.1136/bmj.j791
- Schmidt M, Mansfield KE, Bhaskaran K, et al. Adherence to guidelines for creatinine and potassium monitoring and discontinuation following renin–angiotensin system blockade: a UK general practice-based cohort study. BMJ Open 2017; 7(1):e012818. doi:10.1136/bmjopen-2016-012818
- Lund LH, Carrero JJ, Farahmand B, et al. Association between enrollment in a heart failure quality registry and subsequent mortality—a nationwide cohort study. Eur J Heart Fail 2017; 19(9):1107–1116. doi:10.1002/ejhf.762
- Edner M, Benson L, Dahlstrom U, Lund LH. Association between renin-angiotensin system antagonist use and mortality in heart failure with severe renal insuffuciency: a prospective propensity score-matched cohort study. Eur Heart J 2015; 36(34):2318–2326. doi:10.1093/eurheartj/ehv268
- Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care 2015; 21(suppl 11):S212–S220. pmid:26619183
- Bhandari S, Ives N, Brettell EA, et al. Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi trial. Nephrol Dial Transplant 2016; 31(2):255–261. doi:10.1093/ndt/gfv346
- Raebel MA, Ross C, Xu S, et al. Diabetes and drug-associated hyperkalemia: effect of potassium monitoring. J Gen Intern Med 2010; 25(4):326–333. doi:10.1007/s11606-009-1228-x
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71(6):e13–e115. doi:10.1161/HYP.0000000000000065
- The Renal Association. The UK eCKD Guide. https://renal.org/information-resources/the-uk-eckd-guide. Accessed August 12, 2019.
- National Institute for Health and Care Excellence (NICE). Chronic kidney disease in adults: assessment and management. https://www.nice.org.uk/guidance/cg182. Accessed August 12, 2019.
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. https://www.nice.org.uk/Guidance/CG127. Accessed August 12, 2019.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34(28):2159–2219. doi:10.1093/eurheartj/eht151
- International Kidney Foundation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. https://www.sciencedirect.com/journal/kidney-international-supplements/vol/3/issue/1. Accessed August 12, 2019.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Wright J, Bakris G, Greene T. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease. Results from the AASK trial. ACC Current Journal Review 2003; 12(2):37–38. doi:10.1016/s1062-1458(03)00035-7
- Ku E, Bakris G, Johansen K, et al. Acute declines in renal function during intensive BP lowering: implications for future ESRD risk. J Am Soc Nephrol 2017; 28(9):2794–2801. doi:10.1681/ASN.2017010040
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136(6):e137–e161. doi:10.1161/CIR.0000000000000509
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37(27):2129–2200. doi:10.1093/eurheartj/ehw128
- Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 51):S1–S290. pmid:15114537
- Asenjo RM, Bueno H, Mcintosh M. Angiotensin converting enzyme inhibitors (ACE inhibitors) and angiotensin II receptor blockers (ARBs). ACE inhibitors and ARBs, a cornerstone in the prevention and treatment of cardiovascular disease. www.escardio.org/Education/ESC-Prevention-of-CVD-Programme/Treatment-goals/Cardio-Protective-drugs/angiotensin-converting-enzyme-inhibitors-ace-inhibitors-and-angiotensin-ii-rec. Accessed August 12, 2019.
- López-Sendón J, Swedberg K, McMurray J, et al; Task Force on ACE-inhibitors of the European Society of Cardiology. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur Heart J 2004; 25(16):1454–1470. doi:10.1016/j.ehj.2004.06.003
- National Institute for Health and Care Excellence (NICE). Myocardial infarction: cardiac rehabilitation and prevention of further cardiovascular disease. https://www.nice.org.uk/Guidance/CG172. Accessed April 3, 2019.
- National Institute for Health and Care Excellence (NICE). Acute kidney injury: prevention, detection and management. https://www.nice.org.uk/Guidance/CG169. Accessed August 12, 2019.
- Think Kidneys. “Sick day” guidance in patients at risk of acute kidney injury: a position statement from the Think Kidneys Board. https://www.thinkkidneys.nhs.uk/aki/wp-content/uploads/sites/2/2018/01/Think-Kidneys-Sick-Day-Guidance-2018.pdf. Accessed August 12, 2019.
- Meaney CJ, Beccari MV, Yang Y, Zhao J. Systematic review and meta-analysis of patiromer and sodium zirconium cyclosilicate: a new armamentarium for the treatment of hyperkalemia. Pharmacotherapy 2017; 37(4):401–411. doi:10.1002/phar.1906
- Page IH, Helmer OM. A crystalline pressor substance (angiotonin) resulting from the reaction between renin and renin-activator. Exp Med 1940; 71(1):29–42. doi:10.1084/jem.71.1.29
- Steddon S, Ashman N, Chesser A, Cunningham J. Oxford Handbook of Nephrology and Hypertension. 2nd ed. Oxford: Oxford University Press; 2016:203–206, 508–509.
- Barratt J, Topham P, Harris K. Oxford Desk Reference. 1st ed. Oxford: Oxford University Press; 2008.
- International Kidney Foundation. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO_BP_GL.pdf. Accessed April 3, 2019.
- Heart Outcomes Prevention Evaluation Study Investigators; Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000; 342(3):145–153. doi:10.1056/NEJM200001203420301
- Swedberg K, Kjekshus J. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). Am J Cardiol 1988; 62(2):60A–66A. pmid:2839019
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345(12):861–869. doi:10.1056/NEJMoa011161
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349(20):1893–1906. doi:10.1056/NEJMoa032292
- Epstein M. Reduction of cardiovascular risk in chronic kidney disease by mineralocorticoid receptor antagonism. Lancet Diabetes Endocrinol 2015; 3(12):993–1003. doi:10.1016/S2213-8587(15)00289-2
- SOLVD Investigators; Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325(5):293–302. doi:10.1056/NEJM199108013250501
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group; Angiotensin-Converting Enzymne Inhibition and Progression of Renal Disease. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 2001; 60(3):1131–1140. doi:10.1046/j.1523-1755.2001.0600031131.x
- Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet 2015; 385(9982):2047–2056. doi:10.1016/S0140-6736(14)62459-4
- Ruggenenti P, Perticucci E, Cravedi P, et al. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 2008; 19(6):1213–1224. doi:10.1681/ASN.2007090970
- Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH. Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomised trials. BMJ 2013; 346:f360. doi:10.1136/bmj.f360
- ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358(15):1547–1559. doi:10.1056/NEJMoa0801317
- Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369(20):1892–1903.
doi:10.1056/NEJMoa1303154 - Catalá-López F, Macías Saint-Gerons D, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med 2016; 13(3):e1001971. doi:10.1371/journal.pmed.1001971
- Agarwal R, Afzalpurkar R, Fordtran JS. Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology 1994; 107(2):548–571. pmid:8039632
- Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol 2015; 10(6):1050–1060. doi:10.2215/CJN.08580813
- Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med 2009; 169(12):1156–1162. doi:10.1001/archinternmed.2009.132
- Nakhoul GN, Huang H, Arrigain S, et al. Serum potassium, end-stage renal disease and mortality in chronic kidney disease. Am J Nephrol 2015; 41(6):456–463. doi:10.1159/000437151
- Acker CG, Johnson JP, Palevsky PM, Greenberg A. Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med 1998; 158(8):917–924. pmid:9570179
- Desai AS, Swedberg K, McMurray JJ, et al; CHARM Program Investigators. Incidence and predictors of hyperkalemia in patients with heart failure: an analysis of the CHARM Program. J Am Coll Cardiol 2007; 50(20):1959–1966. doi:10.1016/j.jacc.2007.07.067
- Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Sakhuja A, Mao MA, Erickson SB. Impact of admission serum potassium on mortality in patients with chronic kidney disease and cardiovascular disease. QJM 2017; 110(11):713–719. doi:10.1093/qjmed/hcx118
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364(1):11–21. doi:10.1056/NEJMoa1009492
- Rossignol P, Dobre D, McMurray JJ, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail 2014; 7(1):51–58. doi:10.1161/CIRCHEARTFAILURE.113.000792
- Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail 2011; 4(6):685–691. doi:10.1161/CIRCHEARTFAILURE.111.963256
- Weir M, Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol 2010; 5(3):531–548. doi:10.2215/CJN.07821109
- Valente M, Bhandari S. Renal function after new treatment with renin-angiotensin system blockers. BMJ 2017; 356:j1122. doi:10.1136/bmj.j1122
- Bakris G, Weir M. Angiotensin-converting enzyme inhibitor–associated elevations in serum creatinine. Arch Intern Med 2000; 160(5):685–693. pmid:10724055
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345(12):861–869. doi:10.1056/NEJMoa011161
- Hirsch S. Pre-renal success. Kidney Int 2012; 81(6):596. doi:10.1038/ki.2011.418
- Schmidt M, Mansfield KE, Bhaskaran K, et al. Serum creatinine elevation after renin-angiotensin system blockade and long term cardiorenal risks: cohort study. BMJ 2017; 356:j791. doi:10.1136/bmj.j791
- Schmidt M, Mansfield KE, Bhaskaran K, et al. Adherence to guidelines for creatinine and potassium monitoring and discontinuation following renin–angiotensin system blockade: a UK general practice-based cohort study. BMJ Open 2017; 7(1):e012818. doi:10.1136/bmjopen-2016-012818
- Lund LH, Carrero JJ, Farahmand B, et al. Association between enrollment in a heart failure quality registry and subsequent mortality—a nationwide cohort study. Eur J Heart Fail 2017; 19(9):1107–1116. doi:10.1002/ejhf.762
- Edner M, Benson L, Dahlstrom U, Lund LH. Association between renin-angiotensin system antagonist use and mortality in heart failure with severe renal insuffuciency: a prospective propensity score-matched cohort study. Eur Heart J 2015; 36(34):2318–2326. doi:10.1093/eurheartj/ehv268
- Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care 2015; 21(suppl 11):S212–S220. pmid:26619183
- Bhandari S, Ives N, Brettell EA, et al. Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi trial. Nephrol Dial Transplant 2016; 31(2):255–261. doi:10.1093/ndt/gfv346
- Raebel MA, Ross C, Xu S, et al. Diabetes and drug-associated hyperkalemia: effect of potassium monitoring. J Gen Intern Med 2010; 25(4):326–333. doi:10.1007/s11606-009-1228-x
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71(6):e13–e115. doi:10.1161/HYP.0000000000000065
- The Renal Association. The UK eCKD Guide. https://renal.org/information-resources/the-uk-eckd-guide. Accessed August 12, 2019.
- National Institute for Health and Care Excellence (NICE). Chronic kidney disease in adults: assessment and management. https://www.nice.org.uk/guidance/cg182. Accessed August 12, 2019.
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. https://www.nice.org.uk/Guidance/CG127. Accessed August 12, 2019.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34(28):2159–2219. doi:10.1093/eurheartj/eht151
- International Kidney Foundation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. https://www.sciencedirect.com/journal/kidney-international-supplements/vol/3/issue/1. Accessed August 12, 2019.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Wright J, Bakris G, Greene T. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease. Results from the AASK trial. ACC Current Journal Review 2003; 12(2):37–38. doi:10.1016/s1062-1458(03)00035-7
- Ku E, Bakris G, Johansen K, et al. Acute declines in renal function during intensive BP lowering: implications for future ESRD risk. J Am Soc Nephrol 2017; 28(9):2794–2801. doi:10.1681/ASN.2017010040
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136(6):e137–e161. doi:10.1161/CIR.0000000000000509
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37(27):2129–2200. doi:10.1093/eurheartj/ehw128
- Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 51):S1–S290. pmid:15114537
- Asenjo RM, Bueno H, Mcintosh M. Angiotensin converting enzyme inhibitors (ACE inhibitors) and angiotensin II receptor blockers (ARBs). ACE inhibitors and ARBs, a cornerstone in the prevention and treatment of cardiovascular disease. www.escardio.org/Education/ESC-Prevention-of-CVD-Programme/Treatment-goals/Cardio-Protective-drugs/angiotensin-converting-enzyme-inhibitors-ace-inhibitors-and-angiotensin-ii-rec. Accessed August 12, 2019.
- López-Sendón J, Swedberg K, McMurray J, et al; Task Force on ACE-inhibitors of the European Society of Cardiology. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur Heart J 2004; 25(16):1454–1470. doi:10.1016/j.ehj.2004.06.003
- National Institute for Health and Care Excellence (NICE). Myocardial infarction: cardiac rehabilitation and prevention of further cardiovascular disease. https://www.nice.org.uk/Guidance/CG172. Accessed April 3, 2019.
- National Institute for Health and Care Excellence (NICE). Acute kidney injury: prevention, detection and management. https://www.nice.org.uk/Guidance/CG169. Accessed August 12, 2019.
- Think Kidneys. “Sick day” guidance in patients at risk of acute kidney injury: a position statement from the Think Kidneys Board. https://www.thinkkidneys.nhs.uk/aki/wp-content/uploads/sites/2/2018/01/Think-Kidneys-Sick-Day-Guidance-2018.pdf. Accessed August 12, 2019.
- Meaney CJ, Beccari MV, Yang Y, Zhao J. Systematic review and meta-analysis of patiromer and sodium zirconium cyclosilicate: a new armamentarium for the treatment of hyperkalemia. Pharmacotherapy 2017; 37(4):401–411. doi:10.1002/phar.1906
KEY POINTS
- ACE inhibitors and ARBs reduce proteinuria by lowering the intraglomerular pressure, reducing hyperfiltration.
- These drugs tend to raise the serum potassium level and reduce the glomerular filtration rate (GFR). Monitoring the serum potassium and creatinine levels and the GFR is therefore imperative.
- Despite the benefits, concern for adverse effects including hyperkalemia and a rise in serum creatinine has led to reluctance to prescribe these drugs, and they are underused in the patients who may derive the greatest benefit.
In PAD, dropping statins ups death risk 43%
PARIS – , according to new research presented at the annual congress of the European Society of Cardiology.
Patients with peripheral manifestations of cardiovascular disease “are a population with an extremely high risk to suffer a heart attack or a stroke,” said Joern Dopheide, MD, during a press conference at the meeting. Despite the known benefits of statins, including the reduction of all-cause and cardiovascular death and the reduction of morbidity, adherence to guideline-directed statin therapy is far from optimal, said Dr. Dopheide of Bern (Switzerland) University Hospital.
Patients with peripheral artery disease (PAD) not taking statins had a mortality rate of 34%, more than three times that of patients adherent to an intensified statin regimen. More surprisingly, patients who had been on a statin and then stopped the medication also had a mortality rate of 33%, indistinguishable from those who had never been treated with a statin.
Although statin adherence is low in general, it’s especially low in patients with PAD, said Dr. Dopheide. Still, he said, “few systematic data exist on the prognostic value of statin adherence and the correlation between adherence and cardiovascular outcome in PAD patients.”
Accordingly, Dr. Dopheide and his coinvestigators sought to determine the association between statin adherence and survival in PAD patients. The researchers obtained baseline and follow-up data for a cohort of 691 symptomatic PAD patients seen at a single site, looking at statin dosage, LDL cholesterol levels, and survival.
The patients were followed for a period of 50 months. Dr. Dopheide said that “Over the time course, we were able to increase the statin adherence from about 73% to about 81%, and parallel to that, we were able to reduce the LDL cholesterol levels from about 97 to 83 mg/dL, and we were able to increase the intensity of patients on statin therapy.”
Dr. Dopheide said that he and his colleagues saw a dose-response effect, so that the biggest drop in cholesterol was seen in patients on high statin doses, on more potent statins, or both.
Intensity was increased in some cases by upping statin dose – the mean statin dose climbed from 50 to 58 mg daily during the study period. An alternative strategy was to switch to a more potent statin such as atorvastatin or rosuvastatin; sometimes both intensity and dose were boosted.
“We were able to see that patients who were always on their statin therapy had a pretty low mortality rate of about 20%,” a figure that was halved for patients on more intensive statin therapy, who had a mortality rate of 10% across the study period, said Dr. Dopheide. “Patients in whom we started a statin therapy still profited from it, and had only a 15% mortality,” he added.
Some of the most surprising – and disturbing – study findings involved those who reduced their statin dose: “When patients discontinued their usual dose and decreased it, they suffered an even higher mortality rate, of nearly 43%. So that was kind of surprising and shocking to us.”
Identifying these high-risk patients and keeping them adherent is a substantial clinical challenge, but an important goal, said Dr. Dopheide. “We know that patients with peripheral arterial disease are a little more underrepresented in daily practice; it’s hard to identify them, especially when they are asymptomatic,” he acknowledged. However, once a PAD patient is identified, “One should at least keep the patient on the statin dosage they have,” or initiate statins if needed.
Further, warned Dr. Dopheide, “One should never discontinue statin or decrease the dosage,” adding that PAD patients should be informed that they are at “very high risk for myocardial infarction or stroke.” These patients “should regard their statin therapy as one of the most important and life-saving medications they can take,” he said.
Dr. Dopheide reported no outside sources of funding and no conflicts of interest.
SOURCE: Dopheide, J., et al. ESC Congress 2019, Abstract P5363.
PARIS – , according to new research presented at the annual congress of the European Society of Cardiology.
Patients with peripheral manifestations of cardiovascular disease “are a population with an extremely high risk to suffer a heart attack or a stroke,” said Joern Dopheide, MD, during a press conference at the meeting. Despite the known benefits of statins, including the reduction of all-cause and cardiovascular death and the reduction of morbidity, adherence to guideline-directed statin therapy is far from optimal, said Dr. Dopheide of Bern (Switzerland) University Hospital.
Patients with peripheral artery disease (PAD) not taking statins had a mortality rate of 34%, more than three times that of patients adherent to an intensified statin regimen. More surprisingly, patients who had been on a statin and then stopped the medication also had a mortality rate of 33%, indistinguishable from those who had never been treated with a statin.
Although statin adherence is low in general, it’s especially low in patients with PAD, said Dr. Dopheide. Still, he said, “few systematic data exist on the prognostic value of statin adherence and the correlation between adherence and cardiovascular outcome in PAD patients.”
Accordingly, Dr. Dopheide and his coinvestigators sought to determine the association between statin adherence and survival in PAD patients. The researchers obtained baseline and follow-up data for a cohort of 691 symptomatic PAD patients seen at a single site, looking at statin dosage, LDL cholesterol levels, and survival.
The patients were followed for a period of 50 months. Dr. Dopheide said that “Over the time course, we were able to increase the statin adherence from about 73% to about 81%, and parallel to that, we were able to reduce the LDL cholesterol levels from about 97 to 83 mg/dL, and we were able to increase the intensity of patients on statin therapy.”
Dr. Dopheide said that he and his colleagues saw a dose-response effect, so that the biggest drop in cholesterol was seen in patients on high statin doses, on more potent statins, or both.
Intensity was increased in some cases by upping statin dose – the mean statin dose climbed from 50 to 58 mg daily during the study period. An alternative strategy was to switch to a more potent statin such as atorvastatin or rosuvastatin; sometimes both intensity and dose were boosted.
“We were able to see that patients who were always on their statin therapy had a pretty low mortality rate of about 20%,” a figure that was halved for patients on more intensive statin therapy, who had a mortality rate of 10% across the study period, said Dr. Dopheide. “Patients in whom we started a statin therapy still profited from it, and had only a 15% mortality,” he added.
Some of the most surprising – and disturbing – study findings involved those who reduced their statin dose: “When patients discontinued their usual dose and decreased it, they suffered an even higher mortality rate, of nearly 43%. So that was kind of surprising and shocking to us.”
Identifying these high-risk patients and keeping them adherent is a substantial clinical challenge, but an important goal, said Dr. Dopheide. “We know that patients with peripheral arterial disease are a little more underrepresented in daily practice; it’s hard to identify them, especially when they are asymptomatic,” he acknowledged. However, once a PAD patient is identified, “One should at least keep the patient on the statin dosage they have,” or initiate statins if needed.
Further, warned Dr. Dopheide, “One should never discontinue statin or decrease the dosage,” adding that PAD patients should be informed that they are at “very high risk for myocardial infarction or stroke.” These patients “should regard their statin therapy as one of the most important and life-saving medications they can take,” he said.
Dr. Dopheide reported no outside sources of funding and no conflicts of interest.
SOURCE: Dopheide, J., et al. ESC Congress 2019, Abstract P5363.
PARIS – , according to new research presented at the annual congress of the European Society of Cardiology.
Patients with peripheral manifestations of cardiovascular disease “are a population with an extremely high risk to suffer a heart attack or a stroke,” said Joern Dopheide, MD, during a press conference at the meeting. Despite the known benefits of statins, including the reduction of all-cause and cardiovascular death and the reduction of morbidity, adherence to guideline-directed statin therapy is far from optimal, said Dr. Dopheide of Bern (Switzerland) University Hospital.
Patients with peripheral artery disease (PAD) not taking statins had a mortality rate of 34%, more than three times that of patients adherent to an intensified statin regimen. More surprisingly, patients who had been on a statin and then stopped the medication also had a mortality rate of 33%, indistinguishable from those who had never been treated with a statin.
Although statin adherence is low in general, it’s especially low in patients with PAD, said Dr. Dopheide. Still, he said, “few systematic data exist on the prognostic value of statin adherence and the correlation between adherence and cardiovascular outcome in PAD patients.”
Accordingly, Dr. Dopheide and his coinvestigators sought to determine the association between statin adherence and survival in PAD patients. The researchers obtained baseline and follow-up data for a cohort of 691 symptomatic PAD patients seen at a single site, looking at statin dosage, LDL cholesterol levels, and survival.
The patients were followed for a period of 50 months. Dr. Dopheide said that “Over the time course, we were able to increase the statin adherence from about 73% to about 81%, and parallel to that, we were able to reduce the LDL cholesterol levels from about 97 to 83 mg/dL, and we were able to increase the intensity of patients on statin therapy.”
Dr. Dopheide said that he and his colleagues saw a dose-response effect, so that the biggest drop in cholesterol was seen in patients on high statin doses, on more potent statins, or both.
Intensity was increased in some cases by upping statin dose – the mean statin dose climbed from 50 to 58 mg daily during the study period. An alternative strategy was to switch to a more potent statin such as atorvastatin or rosuvastatin; sometimes both intensity and dose were boosted.
“We were able to see that patients who were always on their statin therapy had a pretty low mortality rate of about 20%,” a figure that was halved for patients on more intensive statin therapy, who had a mortality rate of 10% across the study period, said Dr. Dopheide. “Patients in whom we started a statin therapy still profited from it, and had only a 15% mortality,” he added.
Some of the most surprising – and disturbing – study findings involved those who reduced their statin dose: “When patients discontinued their usual dose and decreased it, they suffered an even higher mortality rate, of nearly 43%. So that was kind of surprising and shocking to us.”
Identifying these high-risk patients and keeping them adherent is a substantial clinical challenge, but an important goal, said Dr. Dopheide. “We know that patients with peripheral arterial disease are a little more underrepresented in daily practice; it’s hard to identify them, especially when they are asymptomatic,” he acknowledged. However, once a PAD patient is identified, “One should at least keep the patient on the statin dosage they have,” or initiate statins if needed.
Further, warned Dr. Dopheide, “One should never discontinue statin or decrease the dosage,” adding that PAD patients should be informed that they are at “very high risk for myocardial infarction or stroke.” These patients “should regard their statin therapy as one of the most important and life-saving medications they can take,” he said.
Dr. Dopheide reported no outside sources of funding and no conflicts of interest.
SOURCE: Dopheide, J., et al. ESC Congress 2019, Abstract P5363.
AT ESC CONGRESS 2019
Body sculpting, microneedling show strong growth
, according to a survey by the American Society for Dermatologic Surgery.
The society’s members performed an estimated 3.5 million laser/light/energy-based procedures and 2.1 million injectable neuromodulator procedures last year as the total volume of cosmetic treatments rose by more than 7% over 2017, the society reported. The total number of procedures in 2017 was 8.3 million, which represented an increase of 19% over 2016.
The largest percent increase in 2018 by type of procedure came in the body-sculpting sector, which jumped 43% from 2017 to 2018. In terms of the total number, however, body sculpting was well behind the other major categories of cosmetic treatments at 624,000 procedures performed. The most popular form of body sculpting last year was cryolipolysis (287,000 procedures), followed by radiofrequency (163,000), and deoxycholic acid (66,000), the ASDS reported.
“The coupling of scientific research and technology [is] driving innovative options for consumers seeking noninvasive cosmetic treatments,” said ASDS President Murad Alam, MD.
Among those newer options is microneedling, which was up by 45% over its 2017 total with almost 263,000 procedures in 2018. Another innovative treatment, thread lifts, in which temporary sutures visibly lift the skin around the face, appears to be gaining awareness as nearly 33,000 procedures were performed last year, according to the ASDS.
Year-over-year increases were smaller among the more established procedures: laser/light/energy-based procedures were up by 6.6%, injectable neuromodulators rose just 0.9%, injectable soft-tissue fillers were down 0.8%, and chemical peels increased by 2.4%, the society’s data show.
The survey was conducted among ASDS members from Jan. 15 to May 21, 2019, and the 596 responses were generalized to the entire ASDS membership of over 6,400 physicians.
, according to a survey by the American Society for Dermatologic Surgery.

The society’s members performed an estimated 3.5 million laser/light/energy-based procedures and 2.1 million injectable neuromodulator procedures last year as the total volume of cosmetic treatments rose by more than 7% over 2017, the society reported. The total number of procedures in 2017 was 8.3 million, which represented an increase of 19% over 2016.
The largest percent increase in 2018 by type of procedure came in the body-sculpting sector, which jumped 43% from 2017 to 2018. In terms of the total number, however, body sculpting was well behind the other major categories of cosmetic treatments at 624,000 procedures performed. The most popular form of body sculpting last year was cryolipolysis (287,000 procedures), followed by radiofrequency (163,000), and deoxycholic acid (66,000), the ASDS reported.
“The coupling of scientific research and technology [is] driving innovative options for consumers seeking noninvasive cosmetic treatments,” said ASDS President Murad Alam, MD.
Among those newer options is microneedling, which was up by 45% over its 2017 total with almost 263,000 procedures in 2018. Another innovative treatment, thread lifts, in which temporary sutures visibly lift the skin around the face, appears to be gaining awareness as nearly 33,000 procedures were performed last year, according to the ASDS.
Year-over-year increases were smaller among the more established procedures: laser/light/energy-based procedures were up by 6.6%, injectable neuromodulators rose just 0.9%, injectable soft-tissue fillers were down 0.8%, and chemical peels increased by 2.4%, the society’s data show.
The survey was conducted among ASDS members from Jan. 15 to May 21, 2019, and the 596 responses were generalized to the entire ASDS membership of over 6,400 physicians.
, according to a survey by the American Society for Dermatologic Surgery.

The society’s members performed an estimated 3.5 million laser/light/energy-based procedures and 2.1 million injectable neuromodulator procedures last year as the total volume of cosmetic treatments rose by more than 7% over 2017, the society reported. The total number of procedures in 2017 was 8.3 million, which represented an increase of 19% over 2016.
The largest percent increase in 2018 by type of procedure came in the body-sculpting sector, which jumped 43% from 2017 to 2018. In terms of the total number, however, body sculpting was well behind the other major categories of cosmetic treatments at 624,000 procedures performed. The most popular form of body sculpting last year was cryolipolysis (287,000 procedures), followed by radiofrequency (163,000), and deoxycholic acid (66,000), the ASDS reported.
“The coupling of scientific research and technology [is] driving innovative options for consumers seeking noninvasive cosmetic treatments,” said ASDS President Murad Alam, MD.
Among those newer options is microneedling, which was up by 45% over its 2017 total with almost 263,000 procedures in 2018. Another innovative treatment, thread lifts, in which temporary sutures visibly lift the skin around the face, appears to be gaining awareness as nearly 33,000 procedures were performed last year, according to the ASDS.
Year-over-year increases were smaller among the more established procedures: laser/light/energy-based procedures were up by 6.6%, injectable neuromodulators rose just 0.9%, injectable soft-tissue fillers were down 0.8%, and chemical peels increased by 2.4%, the society’s data show.
The survey was conducted among ASDS members from Jan. 15 to May 21, 2019, and the 596 responses were generalized to the entire ASDS membership of over 6,400 physicians.
HFNC 12 L/min on floor cuts down on bronchiolitis ICU transfers
SEATTLE – ICU transfers for acute bronchiolitis dropped 63% at Johns Hopkins All Children’s Hospital in St. Petersburg, Fla., after the high-flow nasal cannula limit on the floor was raised from 6 L/min to 12 L/min, and treatment was started in the emergency department, according to a presentation at Pediatric Hospital Medicine.
A year before the change was made in April 2018, there were 17 transfers among 249 bronchiolitis patients treated on the floor, a transfer rate of 6.8%. In the year after the change, there were eight among 319 patients, a transfer rate of 2.5%. Raising the limit to 12 L/min prevented an estimated 14 transfers, for a total savings of almost $250,000, said pediatric hospitalist and assistant professor Shaila Siraj, MD.
The change was made after Dr. Siraj and her colleagues noticed that when children topped out at 6 L, they sometimes only needed a slightly higher flow rate in the ICU, maybe 8 L or 10 L, for a short while before they came back to the floor. Given the safety of high-flow nasal cannula (HFNC), the ICU transfer often seemed like a waste of time and resources.
“As hospitalists, we felt we could safely take care of these patients,” Dr. Siraj said.
So she and her colleague pediatric critical care specialist Anthony Sochet, MD, also an assistant professor of pediatrics, reviewed over a year’s worth of data at All Children’s. They found that 12 L/min – roughly 1.5 L/kg/min – was the cutoff that best discriminated between patients who needed intubation and those who did not, “so that’s what we chose,” Dr. Sochet said.
For simplicity, they broke limits down by age: A maximum flow rate of 8 L/min for children up to 6 months old; 10 L for children aged 6-12 months; and up to 12 L/min for children age 12-24 months. The fraction of inspired oxygen remained the same at 50%. Children were started at maximum flows, then weaned down as they improved. Respiratory assessments were made at least every 4 hours.
The changes were part of a larger revision of the hospital’s pathway for uncomplicated bronchiolitis in children up to 2 years old; it was a joint effort involving nurses, respiratory therapists, and pediatric hospitalists, and ED and ICU teams.
Early initiation in the ED was “probably one of the most important” changes; it kept children from wearing out as they struggled to breath. Kids often start to improve right away, but when then don’t after 30-60 minutes, it’s an indication that they should probably be triaged to the ICU for possible intubation, Dr. Siraj said.
Dr. Sochet was careful to note that institutions have to assess their own situations before taking similar steps. “Not everyone has a tertiary care ICU staffed 24 and 7,” he said.
“You have to ask what floor resources you have, what’s your ability to escalate when you need to. Use data from your own institution to guide where you pick your cutoffs. Adequate staffing is really about respiratory [therapist]/nursing ratios, not the physicians,” he said.
In addition, “in an otherwise healthy child that just has [HFNC] for bronchiolitis, there is absolutely no reason why you should be withholding feeds.” Fed children will feel better and do better, he said.
The presenters had no disclosures.
SEATTLE – ICU transfers for acute bronchiolitis dropped 63% at Johns Hopkins All Children’s Hospital in St. Petersburg, Fla., after the high-flow nasal cannula limit on the floor was raised from 6 L/min to 12 L/min, and treatment was started in the emergency department, according to a presentation at Pediatric Hospital Medicine.
A year before the change was made in April 2018, there were 17 transfers among 249 bronchiolitis patients treated on the floor, a transfer rate of 6.8%. In the year after the change, there were eight among 319 patients, a transfer rate of 2.5%. Raising the limit to 12 L/min prevented an estimated 14 transfers, for a total savings of almost $250,000, said pediatric hospitalist and assistant professor Shaila Siraj, MD.
The change was made after Dr. Siraj and her colleagues noticed that when children topped out at 6 L, they sometimes only needed a slightly higher flow rate in the ICU, maybe 8 L or 10 L, for a short while before they came back to the floor. Given the safety of high-flow nasal cannula (HFNC), the ICU transfer often seemed like a waste of time and resources.
“As hospitalists, we felt we could safely take care of these patients,” Dr. Siraj said.
So she and her colleague pediatric critical care specialist Anthony Sochet, MD, also an assistant professor of pediatrics, reviewed over a year’s worth of data at All Children’s. They found that 12 L/min – roughly 1.5 L/kg/min – was the cutoff that best discriminated between patients who needed intubation and those who did not, “so that’s what we chose,” Dr. Sochet said.
For simplicity, they broke limits down by age: A maximum flow rate of 8 L/min for children up to 6 months old; 10 L for children aged 6-12 months; and up to 12 L/min for children age 12-24 months. The fraction of inspired oxygen remained the same at 50%. Children were started at maximum flows, then weaned down as they improved. Respiratory assessments were made at least every 4 hours.
The changes were part of a larger revision of the hospital’s pathway for uncomplicated bronchiolitis in children up to 2 years old; it was a joint effort involving nurses, respiratory therapists, and pediatric hospitalists, and ED and ICU teams.
Early initiation in the ED was “probably one of the most important” changes; it kept children from wearing out as they struggled to breath. Kids often start to improve right away, but when then don’t after 30-60 minutes, it’s an indication that they should probably be triaged to the ICU for possible intubation, Dr. Siraj said.
Dr. Sochet was careful to note that institutions have to assess their own situations before taking similar steps. “Not everyone has a tertiary care ICU staffed 24 and 7,” he said.
“You have to ask what floor resources you have, what’s your ability to escalate when you need to. Use data from your own institution to guide where you pick your cutoffs. Adequate staffing is really about respiratory [therapist]/nursing ratios, not the physicians,” he said.
In addition, “in an otherwise healthy child that just has [HFNC] for bronchiolitis, there is absolutely no reason why you should be withholding feeds.” Fed children will feel better and do better, he said.
The presenters had no disclosures.
SEATTLE – ICU transfers for acute bronchiolitis dropped 63% at Johns Hopkins All Children’s Hospital in St. Petersburg, Fla., after the high-flow nasal cannula limit on the floor was raised from 6 L/min to 12 L/min, and treatment was started in the emergency department, according to a presentation at Pediatric Hospital Medicine.
A year before the change was made in April 2018, there were 17 transfers among 249 bronchiolitis patients treated on the floor, a transfer rate of 6.8%. In the year after the change, there were eight among 319 patients, a transfer rate of 2.5%. Raising the limit to 12 L/min prevented an estimated 14 transfers, for a total savings of almost $250,000, said pediatric hospitalist and assistant professor Shaila Siraj, MD.
The change was made after Dr. Siraj and her colleagues noticed that when children topped out at 6 L, they sometimes only needed a slightly higher flow rate in the ICU, maybe 8 L or 10 L, for a short while before they came back to the floor. Given the safety of high-flow nasal cannula (HFNC), the ICU transfer often seemed like a waste of time and resources.
“As hospitalists, we felt we could safely take care of these patients,” Dr. Siraj said.
So she and her colleague pediatric critical care specialist Anthony Sochet, MD, also an assistant professor of pediatrics, reviewed over a year’s worth of data at All Children’s. They found that 12 L/min – roughly 1.5 L/kg/min – was the cutoff that best discriminated between patients who needed intubation and those who did not, “so that’s what we chose,” Dr. Sochet said.
For simplicity, they broke limits down by age: A maximum flow rate of 8 L/min for children up to 6 months old; 10 L for children aged 6-12 months; and up to 12 L/min for children age 12-24 months. The fraction of inspired oxygen remained the same at 50%. Children were started at maximum flows, then weaned down as they improved. Respiratory assessments were made at least every 4 hours.
The changes were part of a larger revision of the hospital’s pathway for uncomplicated bronchiolitis in children up to 2 years old; it was a joint effort involving nurses, respiratory therapists, and pediatric hospitalists, and ED and ICU teams.
Early initiation in the ED was “probably one of the most important” changes; it kept children from wearing out as they struggled to breath. Kids often start to improve right away, but when then don’t after 30-60 minutes, it’s an indication that they should probably be triaged to the ICU for possible intubation, Dr. Siraj said.
Dr. Sochet was careful to note that institutions have to assess their own situations before taking similar steps. “Not everyone has a tertiary care ICU staffed 24 and 7,” he said.
“You have to ask what floor resources you have, what’s your ability to escalate when you need to. Use data from your own institution to guide where you pick your cutoffs. Adequate staffing is really about respiratory [therapist]/nursing ratios, not the physicians,” he said.
In addition, “in an otherwise healthy child that just has [HFNC] for bronchiolitis, there is absolutely no reason why you should be withholding feeds.” Fed children will feel better and do better, he said.
The presenters had no disclosures.
REPORTING FROM PHM 2019
Key clinical point:
Major finding: ICU transfers dropped 63% after the floor limit was raised from 6 L/min to 12 L/min.
Study details: Before/after quality improvement project
Disclosures: There was no external funding, and the presenters had no disclosures.
Tips for adding cosmeceuticals to your aesthetic practice
SAN DIEGO – In the opinion of Kimberly J. Butterwick, MD, there are at least three reasons why dermatologists should consider incorporating cosmeceuticals into their aesthetic practice
First, if you don’t, patients will buy products elsewhere. “There’s good data showing that 80% of patients will purchase a product within 24 hours of an office visit,” Dr. Butterwick said at the annual Masters of Aesthetics Symposium.
“You should be the one giving them unbiased advice, because patients waste a lot of money on products which aren’t that effective. Female patients spend an average of $2,000 per year on cosmetics. The average woman uses 15 different cosmetics per day,” according to Dr. Butterwick.
A second reason to consider selling cosmeceuticals is that patients visit dermatologists in order to have healthy, beautiful skin. “Patients want and need your expertise,” said Dr. Butterwick, one of five board-certified dermatologists who practices at the San Diego-based Cosmetic Laser Dermatology. “Patients who are educated and are given advice have better compliance and outcomes. You also want to care for patients for life, to show that you have an interest in treating them beyond what they come to see you for. That will make them come back to you. They’ll get refills and visits and more advice.”
A third reason to consider selling moisturizers, bleaching agents, and other cosmeceuticals is that it’s good for business. “It can be profitable, not just to you, but it’s an opportunity for employees to be creative and earn more with a product sales incentive,” Dr. Butterwick said. “Some of them are great sellers.” She and her colleagues at Cosmetic Laser Dermatology hit more than $1 million in gross revenue from cosmeceutical sales in 2016, 2017, and 2018. In 2018 alone, they sold 167 different products across 27 skin care lines. Six product lines brought in 84% of total sales: SkinMedica, Calecim, SkinCeuticals, Neocutis, Colorescience, and Topix. “Antiaging products are always going to be the number one seller,” she said, including antioxidants, peptides, growth factors, retinoids, hydroxyacids, botanicals, nutriceuticals, teeth-whitening agents, and supplements. New serums with solid science behind them, she continued, include Multi-Action Cream, a product from Calecim that contains a cytokine and growth factor blend from umbilical cord stem cells of red deer to stimulate collagen production and healing after procedures. In 2020, Dr. Butterwick said that SkinMedica’s TNS Essential Serum will contain human fibroblasts grown at low oxygen levels. These are designed to behave as embryonic fibroblasts with more effective growth factors, resulting in better collagen production.
“You want to take the high road when selling cosmeceuticals,” said Dr. Butterwick, who also was a co-founder of SkinMedica. “Provide guidance and education to steer your patients toward products that have proven efficacy, safety, are well tolerated, and are tested and approved by office staff and patients.”
Her tips for effective dispensing include selecting products that target your patient base and the climate in your area, and starting with a specific product line such as SkinMedica, Obagi, SkinCeuticals, Colorescience, Alastin, or Skin Better. “When you choose a company, make sure they have good return policies,” she said. “Get that in writing. Make sure they’ll educate your staff, and make sure they have some system in place to monitor unauthorized sales online. A lot of companies have this now. At trade shows, I’ve learned that some companies will dump expired products, which people buy at a discount and sell online. You don’t want to be competing with that kind of situation.”
She recommends setting aside a dedicated area in your office to display products, “whether it’s the checkout counter in your waiting room or a separate room that resembles a store,” she said. “For effective dispensing, physician-directed products are best. Explain the science: why you are recommending a product and why it is effective. Staff can review the regimen and try products with the patient. A written regimen assures compliance. You also want to offer patients discounts for multiple products or a featured brand of the month. Offer free shipping for refills, and consider linking products with procedures for a discount.”
Citing independent research conducted for a major cosmetics company, Dr. Butterwick said that patients are initially excited to purchase a cosmeceutical product, but once they get home compliance wanes. Only 30% buy the product a second time, and only 12% buy it a third time. “Reasons why so many drop off include that they find it inconvenient to buy, they forgot how to use the product, they become demotivated or distracted, or they shop around for a lower price,” she explained. “Remind your patients not to buy products online. Many of these products are expired or counterfeit. There’s so much information available online, but why not be a source of truth and tell them what’s really going to help? That’s going to assure your patient of the best outcome. It will also keep your patient loyal to you and your practice.”
In addition to co-founding SkinMedica, Dr. Butterwick disclosed that she has received grants/research support from Allergan, Galderma, and Histogen, and consulting fees from Allergan, Colorescience, Evolus, Galderma, Merz, and Sinclair. She is also a member of the speakers’ bureau for Allergan and Merz.
SAN DIEGO – In the opinion of Kimberly J. Butterwick, MD, there are at least three reasons why dermatologists should consider incorporating cosmeceuticals into their aesthetic practice
First, if you don’t, patients will buy products elsewhere. “There’s good data showing that 80% of patients will purchase a product within 24 hours of an office visit,” Dr. Butterwick said at the annual Masters of Aesthetics Symposium.
“You should be the one giving them unbiased advice, because patients waste a lot of money on products which aren’t that effective. Female patients spend an average of $2,000 per year on cosmetics. The average woman uses 15 different cosmetics per day,” according to Dr. Butterwick.
A second reason to consider selling cosmeceuticals is that patients visit dermatologists in order to have healthy, beautiful skin. “Patients want and need your expertise,” said Dr. Butterwick, one of five board-certified dermatologists who practices at the San Diego-based Cosmetic Laser Dermatology. “Patients who are educated and are given advice have better compliance and outcomes. You also want to care for patients for life, to show that you have an interest in treating them beyond what they come to see you for. That will make them come back to you. They’ll get refills and visits and more advice.”
A third reason to consider selling moisturizers, bleaching agents, and other cosmeceuticals is that it’s good for business. “It can be profitable, not just to you, but it’s an opportunity for employees to be creative and earn more with a product sales incentive,” Dr. Butterwick said. “Some of them are great sellers.” She and her colleagues at Cosmetic Laser Dermatology hit more than $1 million in gross revenue from cosmeceutical sales in 2016, 2017, and 2018. In 2018 alone, they sold 167 different products across 27 skin care lines. Six product lines brought in 84% of total sales: SkinMedica, Calecim, SkinCeuticals, Neocutis, Colorescience, and Topix. “Antiaging products are always going to be the number one seller,” she said, including antioxidants, peptides, growth factors, retinoids, hydroxyacids, botanicals, nutriceuticals, teeth-whitening agents, and supplements. New serums with solid science behind them, she continued, include Multi-Action Cream, a product from Calecim that contains a cytokine and growth factor blend from umbilical cord stem cells of red deer to stimulate collagen production and healing after procedures. In 2020, Dr. Butterwick said that SkinMedica’s TNS Essential Serum will contain human fibroblasts grown at low oxygen levels. These are designed to behave as embryonic fibroblasts with more effective growth factors, resulting in better collagen production.
“You want to take the high road when selling cosmeceuticals,” said Dr. Butterwick, who also was a co-founder of SkinMedica. “Provide guidance and education to steer your patients toward products that have proven efficacy, safety, are well tolerated, and are tested and approved by office staff and patients.”
Her tips for effective dispensing include selecting products that target your patient base and the climate in your area, and starting with a specific product line such as SkinMedica, Obagi, SkinCeuticals, Colorescience, Alastin, or Skin Better. “When you choose a company, make sure they have good return policies,” she said. “Get that in writing. Make sure they’ll educate your staff, and make sure they have some system in place to monitor unauthorized sales online. A lot of companies have this now. At trade shows, I’ve learned that some companies will dump expired products, which people buy at a discount and sell online. You don’t want to be competing with that kind of situation.”
She recommends setting aside a dedicated area in your office to display products, “whether it’s the checkout counter in your waiting room or a separate room that resembles a store,” she said. “For effective dispensing, physician-directed products are best. Explain the science: why you are recommending a product and why it is effective. Staff can review the regimen and try products with the patient. A written regimen assures compliance. You also want to offer patients discounts for multiple products or a featured brand of the month. Offer free shipping for refills, and consider linking products with procedures for a discount.”
Citing independent research conducted for a major cosmetics company, Dr. Butterwick said that patients are initially excited to purchase a cosmeceutical product, but once they get home compliance wanes. Only 30% buy the product a second time, and only 12% buy it a third time. “Reasons why so many drop off include that they find it inconvenient to buy, they forgot how to use the product, they become demotivated or distracted, or they shop around for a lower price,” she explained. “Remind your patients not to buy products online. Many of these products are expired or counterfeit. There’s so much information available online, but why not be a source of truth and tell them what’s really going to help? That’s going to assure your patient of the best outcome. It will also keep your patient loyal to you and your practice.”
In addition to co-founding SkinMedica, Dr. Butterwick disclosed that she has received grants/research support from Allergan, Galderma, and Histogen, and consulting fees from Allergan, Colorescience, Evolus, Galderma, Merz, and Sinclair. She is also a member of the speakers’ bureau for Allergan and Merz.
SAN DIEGO – In the opinion of Kimberly J. Butterwick, MD, there are at least three reasons why dermatologists should consider incorporating cosmeceuticals into their aesthetic practice
First, if you don’t, patients will buy products elsewhere. “There’s good data showing that 80% of patients will purchase a product within 24 hours of an office visit,” Dr. Butterwick said at the annual Masters of Aesthetics Symposium.
“You should be the one giving them unbiased advice, because patients waste a lot of money on products which aren’t that effective. Female patients spend an average of $2,000 per year on cosmetics. The average woman uses 15 different cosmetics per day,” according to Dr. Butterwick.
A second reason to consider selling cosmeceuticals is that patients visit dermatologists in order to have healthy, beautiful skin. “Patients want and need your expertise,” said Dr. Butterwick, one of five board-certified dermatologists who practices at the San Diego-based Cosmetic Laser Dermatology. “Patients who are educated and are given advice have better compliance and outcomes. You also want to care for patients for life, to show that you have an interest in treating them beyond what they come to see you for. That will make them come back to you. They’ll get refills and visits and more advice.”
A third reason to consider selling moisturizers, bleaching agents, and other cosmeceuticals is that it’s good for business. “It can be profitable, not just to you, but it’s an opportunity for employees to be creative and earn more with a product sales incentive,” Dr. Butterwick said. “Some of them are great sellers.” She and her colleagues at Cosmetic Laser Dermatology hit more than $1 million in gross revenue from cosmeceutical sales in 2016, 2017, and 2018. In 2018 alone, they sold 167 different products across 27 skin care lines. Six product lines brought in 84% of total sales: SkinMedica, Calecim, SkinCeuticals, Neocutis, Colorescience, and Topix. “Antiaging products are always going to be the number one seller,” she said, including antioxidants, peptides, growth factors, retinoids, hydroxyacids, botanicals, nutriceuticals, teeth-whitening agents, and supplements. New serums with solid science behind them, she continued, include Multi-Action Cream, a product from Calecim that contains a cytokine and growth factor blend from umbilical cord stem cells of red deer to stimulate collagen production and healing after procedures. In 2020, Dr. Butterwick said that SkinMedica’s TNS Essential Serum will contain human fibroblasts grown at low oxygen levels. These are designed to behave as embryonic fibroblasts with more effective growth factors, resulting in better collagen production.
“You want to take the high road when selling cosmeceuticals,” said Dr. Butterwick, who also was a co-founder of SkinMedica. “Provide guidance and education to steer your patients toward products that have proven efficacy, safety, are well tolerated, and are tested and approved by office staff and patients.”
Her tips for effective dispensing include selecting products that target your patient base and the climate in your area, and starting with a specific product line such as SkinMedica, Obagi, SkinCeuticals, Colorescience, Alastin, or Skin Better. “When you choose a company, make sure they have good return policies,” she said. “Get that in writing. Make sure they’ll educate your staff, and make sure they have some system in place to monitor unauthorized sales online. A lot of companies have this now. At trade shows, I’ve learned that some companies will dump expired products, which people buy at a discount and sell online. You don’t want to be competing with that kind of situation.”
She recommends setting aside a dedicated area in your office to display products, “whether it’s the checkout counter in your waiting room or a separate room that resembles a store,” she said. “For effective dispensing, physician-directed products are best. Explain the science: why you are recommending a product and why it is effective. Staff can review the regimen and try products with the patient. A written regimen assures compliance. You also want to offer patients discounts for multiple products or a featured brand of the month. Offer free shipping for refills, and consider linking products with procedures for a discount.”
Citing independent research conducted for a major cosmetics company, Dr. Butterwick said that patients are initially excited to purchase a cosmeceutical product, but once they get home compliance wanes. Only 30% buy the product a second time, and only 12% buy it a third time. “Reasons why so many drop off include that they find it inconvenient to buy, they forgot how to use the product, they become demotivated or distracted, or they shop around for a lower price,” she explained. “Remind your patients not to buy products online. Many of these products are expired or counterfeit. There’s so much information available online, but why not be a source of truth and tell them what’s really going to help? That’s going to assure your patient of the best outcome. It will also keep your patient loyal to you and your practice.”
In addition to co-founding SkinMedica, Dr. Butterwick disclosed that she has received grants/research support from Allergan, Galderma, and Histogen, and consulting fees from Allergan, Colorescience, Evolus, Galderma, Merz, and Sinclair. She is also a member of the speakers’ bureau for Allergan and Merz.
EXPERT ANALYSIS FROM MOAS 2019
Should you market your aesthetic services to the ‘Me Me Me Generation’?
SAN DIEGO – If the idea of marketing your aesthetic dermatology services to Millennials is an afterthought, Brian Biesman, MD, recommends that you reconsider that outlook. At the annual Masters of Aesthetics Symposium, Dr. Biesman told attendees that the age group dubbed as the “Me Me Me Generation” by Joel Stein of Time Magazine is slowly overtaking Baby Boomers as the largest shopping generation in history.
A large consumer survey conducted by Accenture found that by 2020, spending by Millennials will account for $1.4 trillion in U.S. retail sales. This segment of the population, which the Pew Research Center defines as those born from 1981 to 1996, also spends more online than any other generation. According to data from the consulting firm Bain & Company, 25% of luxury goods will be purchased online by 2025, up from 8% in 2016. “Millennials are going to be a huge economic driving force,” Dr. Biesman said.
Dr. Biesman, an oculofacial plastic surgeon who practices in Nashville, Tenn., said Millennials were born into a digital age. “They are very socially connected, sometimes to their detriment,” said Dr. Biesman, who is a past president of the American Society for Laser Medicine and Surgery. “They’re definitely in debt ... but they’re comfortable with that and don’t mind spending. Their priorities are different. They tend to put off marriage and having kids, and they’re driven by social media.
They also hold a strong interest in appearance, said Dr, Biesman, who noted that the average Millennial woman is more likely to be aware of beauty issues by a factor of 10 years younger than her mother’s generation. “At age 25, Millennial women are getting interested in aesthetics, whereas the older generation didn’t start until about 35,” he said. Millennials “are educated, and they use the Internet to read up on procedures.” In his clinical experience, The most popular procedures include neuromodulators, fillers (especially in the lips and in the infraorbital hollow), minimally invasive laser hair removal, superficial laser resurfacing, and prescription skin care and cosmeceuticals.
According to a 2018 survey of 500 Millennials conducted by the aesthetics site Zalea, 32% were considering a cosmetic procedure and 6.6% had undergone one. Of the 149 Millennials who completed all of the survey questions, 65% indicated that they relied on Google search for information about cosmetic treatments, which was a higher proportion than for physicians (63%), friends and family (60%), and social networks such as Instagram, Facebook, and Twitter (25%). Dr. Biesman said that a paradigm shift is under way in aesthetic dermatology, in which the traditional means of achieving a strong reputation amongst patients by excellent training, publications, and research can be replaced by building a visible social media presence/personality.
“The social media influencer factor is a real phenomenon, and can carry tremendous weight due to their perceived relationship with their audience/followers,” Dr. Biesman said. “Some physicians are influencers, while others collaborate with influencers.” He emphasized that the decision to work with social media influencers depends on your preference, your comfort level/trust, the professionalism of the influencer, and your overall social media strategy. “The more you share about yourself, the more successful your social media account will be,” he said. “You need to determine your comfort zone, such as how much of your life you want to share.”
He advises aesthetic dermatologists to develop a strategy for reaching out to and incorporating Millennials into their practice. “Be deliberate in assessing the profile of your practice demographics, and determine which patient groups you want to serve, and to what extent,” he said. “If your practice is focused on minimally invasive aesthetics, it’s important to understand the Millennial mindset, because this is the largest group of consumers.”
Dr. Biesman reported having no relevant disclosures related to his presentation.
SAN DIEGO – If the idea of marketing your aesthetic dermatology services to Millennials is an afterthought, Brian Biesman, MD, recommends that you reconsider that outlook. At the annual Masters of Aesthetics Symposium, Dr. Biesman told attendees that the age group dubbed as the “Me Me Me Generation” by Joel Stein of Time Magazine is slowly overtaking Baby Boomers as the largest shopping generation in history.
A large consumer survey conducted by Accenture found that by 2020, spending by Millennials will account for $1.4 trillion in U.S. retail sales. This segment of the population, which the Pew Research Center defines as those born from 1981 to 1996, also spends more online than any other generation. According to data from the consulting firm Bain & Company, 25% of luxury goods will be purchased online by 2025, up from 8% in 2016. “Millennials are going to be a huge economic driving force,” Dr. Biesman said.
Dr. Biesman, an oculofacial plastic surgeon who practices in Nashville, Tenn., said Millennials were born into a digital age. “They are very socially connected, sometimes to their detriment,” said Dr. Biesman, who is a past president of the American Society for Laser Medicine and Surgery. “They’re definitely in debt ... but they’re comfortable with that and don’t mind spending. Their priorities are different. They tend to put off marriage and having kids, and they’re driven by social media.
They also hold a strong interest in appearance, said Dr, Biesman, who noted that the average Millennial woman is more likely to be aware of beauty issues by a factor of 10 years younger than her mother’s generation. “At age 25, Millennial women are getting interested in aesthetics, whereas the older generation didn’t start until about 35,” he said. Millennials “are educated, and they use the Internet to read up on procedures.” In his clinical experience, The most popular procedures include neuromodulators, fillers (especially in the lips and in the infraorbital hollow), minimally invasive laser hair removal, superficial laser resurfacing, and prescription skin care and cosmeceuticals.
According to a 2018 survey of 500 Millennials conducted by the aesthetics site Zalea, 32% were considering a cosmetic procedure and 6.6% had undergone one. Of the 149 Millennials who completed all of the survey questions, 65% indicated that they relied on Google search for information about cosmetic treatments, which was a higher proportion than for physicians (63%), friends and family (60%), and social networks such as Instagram, Facebook, and Twitter (25%). Dr. Biesman said that a paradigm shift is under way in aesthetic dermatology, in which the traditional means of achieving a strong reputation amongst patients by excellent training, publications, and research can be replaced by building a visible social media presence/personality.
“The social media influencer factor is a real phenomenon, and can carry tremendous weight due to their perceived relationship with their audience/followers,” Dr. Biesman said. “Some physicians are influencers, while others collaborate with influencers.” He emphasized that the decision to work with social media influencers depends on your preference, your comfort level/trust, the professionalism of the influencer, and your overall social media strategy. “The more you share about yourself, the more successful your social media account will be,” he said. “You need to determine your comfort zone, such as how much of your life you want to share.”
He advises aesthetic dermatologists to develop a strategy for reaching out to and incorporating Millennials into their practice. “Be deliberate in assessing the profile of your practice demographics, and determine which patient groups you want to serve, and to what extent,” he said. “If your practice is focused on minimally invasive aesthetics, it’s important to understand the Millennial mindset, because this is the largest group of consumers.”
Dr. Biesman reported having no relevant disclosures related to his presentation.
SAN DIEGO – If the idea of marketing your aesthetic dermatology services to Millennials is an afterthought, Brian Biesman, MD, recommends that you reconsider that outlook. At the annual Masters of Aesthetics Symposium, Dr. Biesman told attendees that the age group dubbed as the “Me Me Me Generation” by Joel Stein of Time Magazine is slowly overtaking Baby Boomers as the largest shopping generation in history.
A large consumer survey conducted by Accenture found that by 2020, spending by Millennials will account for $1.4 trillion in U.S. retail sales. This segment of the population, which the Pew Research Center defines as those born from 1981 to 1996, also spends more online than any other generation. According to data from the consulting firm Bain & Company, 25% of luxury goods will be purchased online by 2025, up from 8% in 2016. “Millennials are going to be a huge economic driving force,” Dr. Biesman said.
Dr. Biesman, an oculofacial plastic surgeon who practices in Nashville, Tenn., said Millennials were born into a digital age. “They are very socially connected, sometimes to their detriment,” said Dr. Biesman, who is a past president of the American Society for Laser Medicine and Surgery. “They’re definitely in debt ... but they’re comfortable with that and don’t mind spending. Their priorities are different. They tend to put off marriage and having kids, and they’re driven by social media.
They also hold a strong interest in appearance, said Dr, Biesman, who noted that the average Millennial woman is more likely to be aware of beauty issues by a factor of 10 years younger than her mother’s generation. “At age 25, Millennial women are getting interested in aesthetics, whereas the older generation didn’t start until about 35,” he said. Millennials “are educated, and they use the Internet to read up on procedures.” In his clinical experience, The most popular procedures include neuromodulators, fillers (especially in the lips and in the infraorbital hollow), minimally invasive laser hair removal, superficial laser resurfacing, and prescription skin care and cosmeceuticals.
According to a 2018 survey of 500 Millennials conducted by the aesthetics site Zalea, 32% were considering a cosmetic procedure and 6.6% had undergone one. Of the 149 Millennials who completed all of the survey questions, 65% indicated that they relied on Google search for information about cosmetic treatments, which was a higher proportion than for physicians (63%), friends and family (60%), and social networks such as Instagram, Facebook, and Twitter (25%). Dr. Biesman said that a paradigm shift is under way in aesthetic dermatology, in which the traditional means of achieving a strong reputation amongst patients by excellent training, publications, and research can be replaced by building a visible social media presence/personality.
“The social media influencer factor is a real phenomenon, and can carry tremendous weight due to their perceived relationship with their audience/followers,” Dr. Biesman said. “Some physicians are influencers, while others collaborate with influencers.” He emphasized that the decision to work with social media influencers depends on your preference, your comfort level/trust, the professionalism of the influencer, and your overall social media strategy. “The more you share about yourself, the more successful your social media account will be,” he said. “You need to determine your comfort zone, such as how much of your life you want to share.”
He advises aesthetic dermatologists to develop a strategy for reaching out to and incorporating Millennials into their practice. “Be deliberate in assessing the profile of your practice demographics, and determine which patient groups you want to serve, and to what extent,” he said. “If your practice is focused on minimally invasive aesthetics, it’s important to understand the Millennial mindset, because this is the largest group of consumers.”
Dr. Biesman reported having no relevant disclosures related to his presentation.
EXPERT ANALYSIS FROM MOAS 2019
Standardized communication may prevent anticoagulant adverse drug events
Background: With increased use of anticoagulants, the amount of related ADEs has also increased. ADEs may be preventable through improved communication during transitions of care. The key communication elements are not standardized.
Study design: Delphi method.
Setting: Consensus panel in New York state.
Synopsis: The New York State Anticoagulation Coalition (NYSACC) tasked an expert multidisciplinary panel of physicians, pharmacists, nurse practitioners, and physician assistants to develop a list of minimum required data elements (RDEs) for transitions of care using the Delphi method.
The following items are the 15 RDEs that require documentation: (1) current anticoagulants; (2) indications; (3) new or previous user; (4) if new, start date, (5) short-term or long-term use; (6) if short term, intended duration; (7) last two doses given; (8) next dose due; (9) latest renal function; (10) provision of patient education materials; (11) assessment of patient/caregiver understanding; (12) future anticoagulation provider; and if warfarin, (13) the target range, (14) at least 2-3 consecutive international normalized ratio results, and (15) next INR level.
Bottom line: Standardized communication during transitions of care regarding anticoagulation may reduce anticoagulant ADEs. Objective evidence showing reduction of ADEs after implementation of the list is needed.
Citation: Triller D et al. Defining minimum necessary anticoagulation-related communication at discharge: Consensus of the Care Transitions Task Force of the New York State Anticoagulation Coalition. Jt Comm J Qual Patient Saf. 2018;44(11):630-40.
Dr. Vuong is an associate physician in the division of hospital medicine at the University of California, San Diego.
Background: With increased use of anticoagulants, the amount of related ADEs has also increased. ADEs may be preventable through improved communication during transitions of care. The key communication elements are not standardized.
Study design: Delphi method.
Setting: Consensus panel in New York state.
Synopsis: The New York State Anticoagulation Coalition (NYSACC) tasked an expert multidisciplinary panel of physicians, pharmacists, nurse practitioners, and physician assistants to develop a list of minimum required data elements (RDEs) for transitions of care using the Delphi method.
The following items are the 15 RDEs that require documentation: (1) current anticoagulants; (2) indications; (3) new or previous user; (4) if new, start date, (5) short-term or long-term use; (6) if short term, intended duration; (7) last two doses given; (8) next dose due; (9) latest renal function; (10) provision of patient education materials; (11) assessment of patient/caregiver understanding; (12) future anticoagulation provider; and if warfarin, (13) the target range, (14) at least 2-3 consecutive international normalized ratio results, and (15) next INR level.
Bottom line: Standardized communication during transitions of care regarding anticoagulation may reduce anticoagulant ADEs. Objective evidence showing reduction of ADEs after implementation of the list is needed.
Citation: Triller D et al. Defining minimum necessary anticoagulation-related communication at discharge: Consensus of the Care Transitions Task Force of the New York State Anticoagulation Coalition. Jt Comm J Qual Patient Saf. 2018;44(11):630-40.
Dr. Vuong is an associate physician in the division of hospital medicine at the University of California, San Diego.
Background: With increased use of anticoagulants, the amount of related ADEs has also increased. ADEs may be preventable through improved communication during transitions of care. The key communication elements are not standardized.
Study design: Delphi method.
Setting: Consensus panel in New York state.
Synopsis: The New York State Anticoagulation Coalition (NYSACC) tasked an expert multidisciplinary panel of physicians, pharmacists, nurse practitioners, and physician assistants to develop a list of minimum required data elements (RDEs) for transitions of care using the Delphi method.
The following items are the 15 RDEs that require documentation: (1) current anticoagulants; (2) indications; (3) new or previous user; (4) if new, start date, (5) short-term or long-term use; (6) if short term, intended duration; (7) last two doses given; (8) next dose due; (9) latest renal function; (10) provision of patient education materials; (11) assessment of patient/caregiver understanding; (12) future anticoagulation provider; and if warfarin, (13) the target range, (14) at least 2-3 consecutive international normalized ratio results, and (15) next INR level.
Bottom line: Standardized communication during transitions of care regarding anticoagulation may reduce anticoagulant ADEs. Objective evidence showing reduction of ADEs after implementation of the list is needed.
Citation: Triller D et al. Defining minimum necessary anticoagulation-related communication at discharge: Consensus of the Care Transitions Task Force of the New York State Anticoagulation Coalition. Jt Comm J Qual Patient Saf. 2018;44(11):630-40.
Dr. Vuong is an associate physician in the division of hospital medicine at the University of California, San Diego.
Cephalosporins remain empiric therapy for skin infections in pediatric AD
“Clindamycin, tetracyclines, or TMP‐SMX can be considered in patients suspected to have, or with a history of, MRSA [methicillin‐resistant S. aureus] infection,” wrote Cristopher C. Briscoe, MD, of the Washington University School of Medicine in St. Louis, Missouri, and his coauthors. The study was published in Pediatric Dermatology.
To determine the optimal empiric antibiotic for pediatric AD patients with skin infections, the researchers analyzed skin cultures from 106 patients seen at Saint Louis Children’s Hospital (SLCH). The results were also compared to cultures from pediatric patients who presented at the SLCH emergency department (ED) with S. aureus skin abscesses.
Of the 170 cultures that grew S. aureus, 130 (77.8%) grew MSSA, and 37 (22.2%) grew MRSA. Three of the cultures grew both. The prevalence of MRSA in the cohort differed from the prevalence in the ED patients (44%). The prevalence of either infection did not differ significantly by age, sex or race, though the average number of cultures in African American patients topped the average for Caucasian patients (1.8 vs. 1.2, P less than .003).
All patients with MSSA – in both the cohort and the ED – proved 100% susceptible to cefazolin. Cohort patients with MSSA saw lower susceptibility to doxycycline compared to the ED patients (89.4% vs. 97%), as did MRSA cohort patients to trimethoprim‐sulfamethoxazole (92% vs. 98%).
“When a patient with AD walks into your office and looks like they have an infection of their eczema, your go-to antibiotic is going to be one that targets MSSA,” said coauthor Carrie Coughlin, MD, of the Washington University School of Medicine in an interview. “You’ll still do a culture to prove or disprove that assumption, but it gives you a guide to help make that patient better in the short term while you work things up.”
“Also, remember that MSSA is not ‘better’ to have than MRSA,” she added. “You can now see some of the virulence factors from MRSA strains in MSSA strains, so treating both of them is important.”
The authors acknowledged their study’s limitations, including the limited generalizability of a single-center design and a lack of information as to the body sites from which the cultures were obtained. They were also unable to reliably determine prior antibiotic exposure, noting that “future work could examine whether prior exposure differed significantly in the MRSA and MSSA groups.”
The study was funded by grants from the Agency for Healthcare Research and Quality. The authors reported no conflicts of interest.
SOURCE: Briscoe CC et al. Pediatr Dermatol. 2019 May 24. doi: 10.1111/pde.13867.
“Clindamycin, tetracyclines, or TMP‐SMX can be considered in patients suspected to have, or with a history of, MRSA [methicillin‐resistant S. aureus] infection,” wrote Cristopher C. Briscoe, MD, of the Washington University School of Medicine in St. Louis, Missouri, and his coauthors. The study was published in Pediatric Dermatology.
To determine the optimal empiric antibiotic for pediatric AD patients with skin infections, the researchers analyzed skin cultures from 106 patients seen at Saint Louis Children’s Hospital (SLCH). The results were also compared to cultures from pediatric patients who presented at the SLCH emergency department (ED) with S. aureus skin abscesses.
Of the 170 cultures that grew S. aureus, 130 (77.8%) grew MSSA, and 37 (22.2%) grew MRSA. Three of the cultures grew both. The prevalence of MRSA in the cohort differed from the prevalence in the ED patients (44%). The prevalence of either infection did not differ significantly by age, sex or race, though the average number of cultures in African American patients topped the average for Caucasian patients (1.8 vs. 1.2, P less than .003).
All patients with MSSA – in both the cohort and the ED – proved 100% susceptible to cefazolin. Cohort patients with MSSA saw lower susceptibility to doxycycline compared to the ED patients (89.4% vs. 97%), as did MRSA cohort patients to trimethoprim‐sulfamethoxazole (92% vs. 98%).
“When a patient with AD walks into your office and looks like they have an infection of their eczema, your go-to antibiotic is going to be one that targets MSSA,” said coauthor Carrie Coughlin, MD, of the Washington University School of Medicine in an interview. “You’ll still do a culture to prove or disprove that assumption, but it gives you a guide to help make that patient better in the short term while you work things up.”
“Also, remember that MSSA is not ‘better’ to have than MRSA,” she added. “You can now see some of the virulence factors from MRSA strains in MSSA strains, so treating both of them is important.”
The authors acknowledged their study’s limitations, including the limited generalizability of a single-center design and a lack of information as to the body sites from which the cultures were obtained. They were also unable to reliably determine prior antibiotic exposure, noting that “future work could examine whether prior exposure differed significantly in the MRSA and MSSA groups.”
The study was funded by grants from the Agency for Healthcare Research and Quality. The authors reported no conflicts of interest.
SOURCE: Briscoe CC et al. Pediatr Dermatol. 2019 May 24. doi: 10.1111/pde.13867.
“Clindamycin, tetracyclines, or TMP‐SMX can be considered in patients suspected to have, or with a history of, MRSA [methicillin‐resistant S. aureus] infection,” wrote Cristopher C. Briscoe, MD, of the Washington University School of Medicine in St. Louis, Missouri, and his coauthors. The study was published in Pediatric Dermatology.
To determine the optimal empiric antibiotic for pediatric AD patients with skin infections, the researchers analyzed skin cultures from 106 patients seen at Saint Louis Children’s Hospital (SLCH). The results were also compared to cultures from pediatric patients who presented at the SLCH emergency department (ED) with S. aureus skin abscesses.
Of the 170 cultures that grew S. aureus, 130 (77.8%) grew MSSA, and 37 (22.2%) grew MRSA. Three of the cultures grew both. The prevalence of MRSA in the cohort differed from the prevalence in the ED patients (44%). The prevalence of either infection did not differ significantly by age, sex or race, though the average number of cultures in African American patients topped the average for Caucasian patients (1.8 vs. 1.2, P less than .003).
All patients with MSSA – in both the cohort and the ED – proved 100% susceptible to cefazolin. Cohort patients with MSSA saw lower susceptibility to doxycycline compared to the ED patients (89.4% vs. 97%), as did MRSA cohort patients to trimethoprim‐sulfamethoxazole (92% vs. 98%).
“When a patient with AD walks into your office and looks like they have an infection of their eczema, your go-to antibiotic is going to be one that targets MSSA,” said coauthor Carrie Coughlin, MD, of the Washington University School of Medicine in an interview. “You’ll still do a culture to prove or disprove that assumption, but it gives you a guide to help make that patient better in the short term while you work things up.”
“Also, remember that MSSA is not ‘better’ to have than MRSA,” she added. “You can now see some of the virulence factors from MRSA strains in MSSA strains, so treating both of them is important.”
The authors acknowledged their study’s limitations, including the limited generalizability of a single-center design and a lack of information as to the body sites from which the cultures were obtained. They were also unable to reliably determine prior antibiotic exposure, noting that “future work could examine whether prior exposure differed significantly in the MRSA and MSSA groups.”
The study was funded by grants from the Agency for Healthcare Research and Quality. The authors reported no conflicts of interest.
SOURCE: Briscoe CC et al. Pediatr Dermatol. 2019 May 24. doi: 10.1111/pde.13867.
FROM PEDIATRIC DERMATOLOGY