User login
When is electroconvulsive therapy (ECT) indicated?
Know the general work-up and contraindications
Case
A 56-year-old female comes to the hospitalist service for presumed sepsis with acute renal insufficiency. She has a history of steadily progressive Parkinson’s disease. Vital signs show a temperature of 104° F; heart rate,135; BP, 100/70; respiratory rate, 20; oxygen saturation, 100% on room air. She is rigid on exam with creatine kinase, 2450 IU/L, and serum creatinine, 2.2. History reveals the patient’s levodopa was increased to 1,200 mg/day recently, then stopped by the family after she became paranoid. A diagnosis of neuroleptic malignant syndrome (NMS) is made.
Background
Electroconvulsive therapy (ECT) has been the gold standard for treatment of refractory psychiatric disease for decades. While it has proven beneficial for both medical and psychiatric disorders, it remains surrounded in controversy. Additionally, there is a significant degree of discomfort among nonpsychiatric providers on when to consider ECT, as well as how to evaluate the patient and manage their comorbidities before and during the procedure1.
Hospitalists should be familiar with the relative contraindications and general work-up for ECT, which can expedite both psychiatric and anesthesia evaluations and minimize adverse outcomes.
While the mechanism of action still is not known, ECT exerts a variety of effects in the brain and periphery. The dominant theory is that ECT increases neurotransmitter activity throughout the brain. Studies have shown increased GABA transmission, normalized glutamate transmission, and resetting of the hypothalamic-pituitary axis, as well as activation of downstream signal transduction pathways leading to increased synaptic connectivity in the brain. Many of ECT’s results may be caused by combinations of the above mechanisms2.
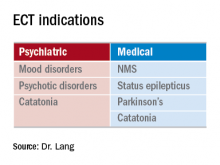
ECT principally is indicated for refractory mood and psychotic disorders. These include schizophrenia, bipolar disorder, and major depression. ECT-responsive patients typically have failed multiple appropriate medication trials and often have prolonged hospitalizations. What is less known are the medical indications for this procedure. Examples include Parkinson’s disease (especially with on/off phenomenon), status epilepticus, and neuroleptic malignant syndrome. Additionally, ECT has been shown to be beneficial for slow-to-resolve delirium and catatonia (regardless of etiology).
A psychiatrist also may take into consideration factors such as past response to ECT or the level of urgency to the patient’s presentation. A general work-up includes basic comprehensive metabolic panel, complete blood count, chest x-ray, EKG, and other testing based on history, physical, and past medical history.
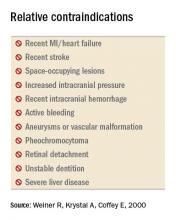
While there are no absolute contraindications to ECT, several relative contraindications exist. These include recent MI or stroke (generally within the last 30 days), increased intracranial pressure, active bleeding (especially from the central nervous system), retinal detachment, and unstable dentition. Apart from making sure the technique is medically indicated, an ECT consultant also evaluates the medical comorbidities. The patient may require treatment, such as removal of unstable dentition prior to the procedure, if clinical urgency does not preclude a delay.
Select patients require more detailed consultation prior to the onset of anesthesia. Examples would include patients with pseudocholinesterase deficiency, myasthenia gravis, or pregnancy. Pregnancy often is considered a contraindication, but ECT has no notable effect on labor & delivery, fetal injury, or development. It would be a preferred modality over medications, especially in unstable mothers during the first trimester. ECT exerts little effect on the fetus, as the amount of current that actually gets to the fetus is negligible6.
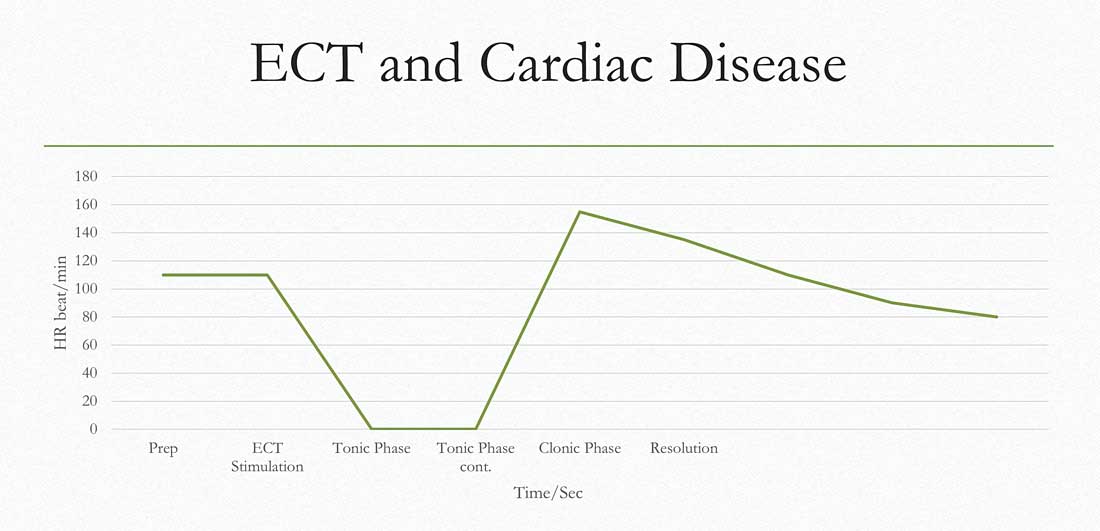
Outside the central nervous system, ECT exerts the most influence over the cardiovascular system. During the tonic phase of a seizure, increased vagal tone can depress the heart rate to asystole in some patients (see chart below). This may last for 3-4 seconds until the clonic phase occurs (with a noradrenergic surge), whereupon the heart rate can accelerate to the 140s. Unless unstable cardiac disease is present, patients typically tolerate this extremely well without any adverse sequela7. Studies involving patients who have severe aortic stenosis and pacemakers/defibrillators show overall excellent tolerability8,9.
Medications can have an impact on the onset, quality, and duration of seizures. Thus, a careful medication review is needed. A consultant will look first for medications such as benzodiazepines or anticonvulsants that would raise the seizure threshold. Ideally, the medications would be stopped, but if not feasible, they can be held the night before (or the day before in the case of such long half-life agents as diazepam) to minimize their impact.
As for anticonvulsants, the doses can be reduced, along with modest increases in energy settings to facilitate seizure. If used for mood stabilization only, one could consider stopping them completely, but this is usually not required (it is not recommended to stop them if used for epilepsy). Lithium can lead to prolonged neuromuscular blockade, prolonged seizures, or postictal delirium. However, discontinuation of lithium also has a risk-benefit consideration, so usually, doses are reduced and/or decreased doses of neuromuscular blockade are employed. Theophylline can induce extended seizures or status epilepticus so it is usually held prior to ECT.
Back to the case
Given the patient’s severe Parkinson’s disease and concurrent NMS, ECT was initiated. By the second treatment, fever and tachycardia resolved. By the sixth treatment, all NMS symptoms and associated paranoia had completely resolved and her Parkinson’s disease rating scale score went from 142 to 42. Her levodopa dose was reduced from 1,200 to 300 mg/day. She remained stable for years afterward.
Bottom line
ECT is both effective and well tolerated in patients who have received appropriate medical evaluation.
Dr. Lang is clinical associate professor in the departments of psychiatry and internal medicine and director of the electroconvulsive therapy and transcranial magnetic stimulation programs at East Carolina University, Greenville, N.C.
Key points
- ECT is indicated for psychotic and depressive disorders, with high efficacy and rapid response.
- ECT also has proven benefits for NMS, catatonia, delirium, status epilepticus, and Parkinson’s disease.
- Evaluation and focused treatment of relative contraindications maximizes both safety and tolerability of ECT.
References
1. Weiner R et al. “Electroconvulsive therapy in the medical & neurologic patient” in A Stoudemire, BS Fogel & D Greenberg (eds) Psychiatric Care of the Medical Patient, 2nd ed., New York, Oxford Univ Press. 2000:419-28. (Second edition is out of print.)
2. Baghai T et al. Electroconvulsive therapy and its different indications. Dialogues Clin Neurosci. Mar 2008;10(1):105-17.
3. Ozer F et al. Electroconvulsive therapy in drug-induced psychiatric states and neuroleptic malignant syndrome. J ECT. 2005 Jun;21(2):125-7.
4. Taylor S. Electroconvulsive therapy: A review of history, patient selection, technique, and medication management. South Med J. 2007 May;100(5):494-8.
5. The Practice of Electroconvulsive Therapy, 2nd edition. A Task Force Report of the American Psychiatric Association. 2001. pp. 84-85.
6. Miller LJ. Use of electroconvulsive therapy during pregnancy. Hosp Community Psychiatry. 1994 May;45(5):444-50.
7. Miller R et al. ECT: Physiologic Effects. Miller’s Anesthesia. 7th Edition. 2009.
8. Mueller PS et al. The Safety of electroconvulsive therapy in patients with severe aortic stenosis. Mayo Clin Proc. 2007 Nov;82(11):1360-3.
9. Dolenc TJ et al. Electroconvulsive therapy in patients with cardiac pacemakers & implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 2004 Sep;27(9):1257-63.
Suggested readings
The practice of electroconvulsive therapy: Recommendations for treatment, training, and privileging (A Task Force Report of the American Psychiatric Association), 2nd Edition. APA Publishing. 2001.
Weiner R et al. “Electroconvulsive therapy in the medical & neurologic patient” in A Stoudemire, BS Fogel & D Greenberg (eds) Psychiatric Care of the Medical Patient, 2nd ed., New York, Oxford Univ Press. 2000:419-28. (Second edition is out of print.)
Rosenquist P et al. Charting the course of electroconvulsive therapy: Where have we been and where are we headed? J Psychosoc Nurs Ment Health Serv. 2016 Dec 1;54(12):39-43.
QUIZ
1. All of the following are indications for ECT except?
A. Schizophrenia.
B. Panic attacks.
C. Bipolar mania.
D. Catatonia.
Answer: B. Panic attacks. ECT is not effective for anxiety disorders including panic, generalized anxiety, PTSD, or OCD.
2. The most commonly accepted mechanism of action for ECT is?
A. Reduction in glutamate levels.
B. Altering signal transduction pathways.
C. Increased neurotransmitter activity.
D. Increased cerebral blood flow.
Answer: C. Increased neurotransmitter activity. There are data to support all, but neurotransmitter flow is most accepted thus far.
3. Which of the following is a common side effect of ECT?
A. Bronchospasm.
B. Diarrhea.
C. Delirium.
D. Visual changes.
Answer: C. Delirium. The rest are rare or not noted.
4. Which of the following is a relative contraindication for ECT?
A. Pregnancy.
B. Epilepsy.
C. Advanced age.
D. Increased intracranial pressure.
Answer: D. Increased intracranial pressure.
Know the general work-up and contraindications
Know the general work-up and contraindications
Case
A 56-year-old female comes to the hospitalist service for presumed sepsis with acute renal insufficiency. She has a history of steadily progressive Parkinson’s disease. Vital signs show a temperature of 104° F; heart rate,135; BP, 100/70; respiratory rate, 20; oxygen saturation, 100% on room air. She is rigid on exam with creatine kinase, 2450 IU/L, and serum creatinine, 2.2. History reveals the patient’s levodopa was increased to 1,200 mg/day recently, then stopped by the family after she became paranoid. A diagnosis of neuroleptic malignant syndrome (NMS) is made.
Background
Electroconvulsive therapy (ECT) has been the gold standard for treatment of refractory psychiatric disease for decades. While it has proven beneficial for both medical and psychiatric disorders, it remains surrounded in controversy. Additionally, there is a significant degree of discomfort among nonpsychiatric providers on when to consider ECT, as well as how to evaluate the patient and manage their comorbidities before and during the procedure1.
Hospitalists should be familiar with the relative contraindications and general work-up for ECT, which can expedite both psychiatric and anesthesia evaluations and minimize adverse outcomes.
While the mechanism of action still is not known, ECT exerts a variety of effects in the brain and periphery. The dominant theory is that ECT increases neurotransmitter activity throughout the brain. Studies have shown increased GABA transmission, normalized glutamate transmission, and resetting of the hypothalamic-pituitary axis, as well as activation of downstream signal transduction pathways leading to increased synaptic connectivity in the brain. Many of ECT’s results may be caused by combinations of the above mechanisms2.
ECT principally is indicated for refractory mood and psychotic disorders. These include schizophrenia, bipolar disorder, and major depression. ECT-responsive patients typically have failed multiple appropriate medication trials and often have prolonged hospitalizations. What is less known are the medical indications for this procedure. Examples include Parkinson’s disease (especially with on/off phenomenon), status epilepticus, and neuroleptic malignant syndrome. Additionally, ECT has been shown to be beneficial for slow-to-resolve delirium and catatonia (regardless of etiology).
A psychiatrist also may take into consideration factors such as past response to ECT or the level of urgency to the patient’s presentation. A general work-up includes basic comprehensive metabolic panel, complete blood count, chest x-ray, EKG, and other testing based on history, physical, and past medical history.
While there are no absolute contraindications to ECT, several relative contraindications exist. These include recent MI or stroke (generally within the last 30 days), increased intracranial pressure, active bleeding (especially from the central nervous system), retinal detachment, and unstable dentition. Apart from making sure the technique is medically indicated, an ECT consultant also evaluates the medical comorbidities. The patient may require treatment, such as removal of unstable dentition prior to the procedure, if clinical urgency does not preclude a delay.
Select patients require more detailed consultation prior to the onset of anesthesia. Examples would include patients with pseudocholinesterase deficiency, myasthenia gravis, or pregnancy. Pregnancy often is considered a contraindication, but ECT has no notable effect on labor & delivery, fetal injury, or development. It would be a preferred modality over medications, especially in unstable mothers during the first trimester. ECT exerts little effect on the fetus, as the amount of current that actually gets to the fetus is negligible6.
Outside the central nervous system, ECT exerts the most influence over the cardiovascular system. During the tonic phase of a seizure, increased vagal tone can depress the heart rate to asystole in some patients (see chart below). This may last for 3-4 seconds until the clonic phase occurs (with a noradrenergic surge), whereupon the heart rate can accelerate to the 140s. Unless unstable cardiac disease is present, patients typically tolerate this extremely well without any adverse sequela7. Studies involving patients who have severe aortic stenosis and pacemakers/defibrillators show overall excellent tolerability8,9.
Medications can have an impact on the onset, quality, and duration of seizures. Thus, a careful medication review is needed. A consultant will look first for medications such as benzodiazepines or anticonvulsants that would raise the seizure threshold. Ideally, the medications would be stopped, but if not feasible, they can be held the night before (or the day before in the case of such long half-life agents as diazepam) to minimize their impact.
As for anticonvulsants, the doses can be reduced, along with modest increases in energy settings to facilitate seizure. If used for mood stabilization only, one could consider stopping them completely, but this is usually not required (it is not recommended to stop them if used for epilepsy). Lithium can lead to prolonged neuromuscular blockade, prolonged seizures, or postictal delirium. However, discontinuation of lithium also has a risk-benefit consideration, so usually, doses are reduced and/or decreased doses of neuromuscular blockade are employed. Theophylline can induce extended seizures or status epilepticus so it is usually held prior to ECT.
Back to the case
Given the patient’s severe Parkinson’s disease and concurrent NMS, ECT was initiated. By the second treatment, fever and tachycardia resolved. By the sixth treatment, all NMS symptoms and associated paranoia had completely resolved and her Parkinson’s disease rating scale score went from 142 to 42. Her levodopa dose was reduced from 1,200 to 300 mg/day. She remained stable for years afterward.
Bottom line
ECT is both effective and well tolerated in patients who have received appropriate medical evaluation.
Dr. Lang is clinical associate professor in the departments of psychiatry and internal medicine and director of the electroconvulsive therapy and transcranial magnetic stimulation programs at East Carolina University, Greenville, N.C.
Key points
- ECT is indicated for psychotic and depressive disorders, with high efficacy and rapid response.
- ECT also has proven benefits for NMS, catatonia, delirium, status epilepticus, and Parkinson’s disease.
- Evaluation and focused treatment of relative contraindications maximizes both safety and tolerability of ECT.
References
1. Weiner R et al. “Electroconvulsive therapy in the medical & neurologic patient” in A Stoudemire, BS Fogel & D Greenberg (eds) Psychiatric Care of the Medical Patient, 2nd ed., New York, Oxford Univ Press. 2000:419-28. (Second edition is out of print.)
2. Baghai T et al. Electroconvulsive therapy and its different indications. Dialogues Clin Neurosci. Mar 2008;10(1):105-17.
3. Ozer F et al. Electroconvulsive therapy in drug-induced psychiatric states and neuroleptic malignant syndrome. J ECT. 2005 Jun;21(2):125-7.
4. Taylor S. Electroconvulsive therapy: A review of history, patient selection, technique, and medication management. South Med J. 2007 May;100(5):494-8.
5. The Practice of Electroconvulsive Therapy, 2nd edition. A Task Force Report of the American Psychiatric Association. 2001. pp. 84-85.
6. Miller LJ. Use of electroconvulsive therapy during pregnancy. Hosp Community Psychiatry. 1994 May;45(5):444-50.
7. Miller R et al. ECT: Physiologic Effects. Miller’s Anesthesia. 7th Edition. 2009.
8. Mueller PS et al. The Safety of electroconvulsive therapy in patients with severe aortic stenosis. Mayo Clin Proc. 2007 Nov;82(11):1360-3.
9. Dolenc TJ et al. Electroconvulsive therapy in patients with cardiac pacemakers & implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 2004 Sep;27(9):1257-63.
Suggested readings
The practice of electroconvulsive therapy: Recommendations for treatment, training, and privileging (A Task Force Report of the American Psychiatric Association), 2nd Edition. APA Publishing. 2001.
Weiner R et al. “Electroconvulsive therapy in the medical & neurologic patient” in A Stoudemire, BS Fogel & D Greenberg (eds) Psychiatric Care of the Medical Patient, 2nd ed., New York, Oxford Univ Press. 2000:419-28. (Second edition is out of print.)
Rosenquist P et al. Charting the course of electroconvulsive therapy: Where have we been and where are we headed? J Psychosoc Nurs Ment Health Serv. 2016 Dec 1;54(12):39-43.
QUIZ
1. All of the following are indications for ECT except?
A. Schizophrenia.
B. Panic attacks.
C. Bipolar mania.
D. Catatonia.
Answer: B. Panic attacks. ECT is not effective for anxiety disorders including panic, generalized anxiety, PTSD, or OCD.
2. The most commonly accepted mechanism of action for ECT is?
A. Reduction in glutamate levels.
B. Altering signal transduction pathways.
C. Increased neurotransmitter activity.
D. Increased cerebral blood flow.
Answer: C. Increased neurotransmitter activity. There are data to support all, but neurotransmitter flow is most accepted thus far.
3. Which of the following is a common side effect of ECT?
A. Bronchospasm.
B. Diarrhea.
C. Delirium.
D. Visual changes.
Answer: C. Delirium. The rest are rare or not noted.
4. Which of the following is a relative contraindication for ECT?
A. Pregnancy.
B. Epilepsy.
C. Advanced age.
D. Increased intracranial pressure.
Answer: D. Increased intracranial pressure.
Case
A 56-year-old female comes to the hospitalist service for presumed sepsis with acute renal insufficiency. She has a history of steadily progressive Parkinson’s disease. Vital signs show a temperature of 104° F; heart rate,135; BP, 100/70; respiratory rate, 20; oxygen saturation, 100% on room air. She is rigid on exam with creatine kinase, 2450 IU/L, and serum creatinine, 2.2. History reveals the patient’s levodopa was increased to 1,200 mg/day recently, then stopped by the family after she became paranoid. A diagnosis of neuroleptic malignant syndrome (NMS) is made.
Background
Electroconvulsive therapy (ECT) has been the gold standard for treatment of refractory psychiatric disease for decades. While it has proven beneficial for both medical and psychiatric disorders, it remains surrounded in controversy. Additionally, there is a significant degree of discomfort among nonpsychiatric providers on when to consider ECT, as well as how to evaluate the patient and manage their comorbidities before and during the procedure1.
Hospitalists should be familiar with the relative contraindications and general work-up for ECT, which can expedite both psychiatric and anesthesia evaluations and minimize adverse outcomes.
While the mechanism of action still is not known, ECT exerts a variety of effects in the brain and periphery. The dominant theory is that ECT increases neurotransmitter activity throughout the brain. Studies have shown increased GABA transmission, normalized glutamate transmission, and resetting of the hypothalamic-pituitary axis, as well as activation of downstream signal transduction pathways leading to increased synaptic connectivity in the brain. Many of ECT’s results may be caused by combinations of the above mechanisms2.
ECT principally is indicated for refractory mood and psychotic disorders. These include schizophrenia, bipolar disorder, and major depression. ECT-responsive patients typically have failed multiple appropriate medication trials and often have prolonged hospitalizations. What is less known are the medical indications for this procedure. Examples include Parkinson’s disease (especially with on/off phenomenon), status epilepticus, and neuroleptic malignant syndrome. Additionally, ECT has been shown to be beneficial for slow-to-resolve delirium and catatonia (regardless of etiology).
A psychiatrist also may take into consideration factors such as past response to ECT or the level of urgency to the patient’s presentation. A general work-up includes basic comprehensive metabolic panel, complete blood count, chest x-ray, EKG, and other testing based on history, physical, and past medical history.
While there are no absolute contraindications to ECT, several relative contraindications exist. These include recent MI or stroke (generally within the last 30 days), increased intracranial pressure, active bleeding (especially from the central nervous system), retinal detachment, and unstable dentition. Apart from making sure the technique is medically indicated, an ECT consultant also evaluates the medical comorbidities. The patient may require treatment, such as removal of unstable dentition prior to the procedure, if clinical urgency does not preclude a delay.
Select patients require more detailed consultation prior to the onset of anesthesia. Examples would include patients with pseudocholinesterase deficiency, myasthenia gravis, or pregnancy. Pregnancy often is considered a contraindication, but ECT has no notable effect on labor & delivery, fetal injury, or development. It would be a preferred modality over medications, especially in unstable mothers during the first trimester. ECT exerts little effect on the fetus, as the amount of current that actually gets to the fetus is negligible6.
Outside the central nervous system, ECT exerts the most influence over the cardiovascular system. During the tonic phase of a seizure, increased vagal tone can depress the heart rate to asystole in some patients (see chart below). This may last for 3-4 seconds until the clonic phase occurs (with a noradrenergic surge), whereupon the heart rate can accelerate to the 140s. Unless unstable cardiac disease is present, patients typically tolerate this extremely well without any adverse sequela7. Studies involving patients who have severe aortic stenosis and pacemakers/defibrillators show overall excellent tolerability8,9.
Medications can have an impact on the onset, quality, and duration of seizures. Thus, a careful medication review is needed. A consultant will look first for medications such as benzodiazepines or anticonvulsants that would raise the seizure threshold. Ideally, the medications would be stopped, but if not feasible, they can be held the night before (or the day before in the case of such long half-life agents as diazepam) to minimize their impact.
As for anticonvulsants, the doses can be reduced, along with modest increases in energy settings to facilitate seizure. If used for mood stabilization only, one could consider stopping them completely, but this is usually not required (it is not recommended to stop them if used for epilepsy). Lithium can lead to prolonged neuromuscular blockade, prolonged seizures, or postictal delirium. However, discontinuation of lithium also has a risk-benefit consideration, so usually, doses are reduced and/or decreased doses of neuromuscular blockade are employed. Theophylline can induce extended seizures or status epilepticus so it is usually held prior to ECT.
Back to the case
Given the patient’s severe Parkinson’s disease and concurrent NMS, ECT was initiated. By the second treatment, fever and tachycardia resolved. By the sixth treatment, all NMS symptoms and associated paranoia had completely resolved and her Parkinson’s disease rating scale score went from 142 to 42. Her levodopa dose was reduced from 1,200 to 300 mg/day. She remained stable for years afterward.
Bottom line
ECT is both effective and well tolerated in patients who have received appropriate medical evaluation.
Dr. Lang is clinical associate professor in the departments of psychiatry and internal medicine and director of the electroconvulsive therapy and transcranial magnetic stimulation programs at East Carolina University, Greenville, N.C.
Key points
- ECT is indicated for psychotic and depressive disorders, with high efficacy and rapid response.
- ECT also has proven benefits for NMS, catatonia, delirium, status epilepticus, and Parkinson’s disease.
- Evaluation and focused treatment of relative contraindications maximizes both safety and tolerability of ECT.
References
1. Weiner R et al. “Electroconvulsive therapy in the medical & neurologic patient” in A Stoudemire, BS Fogel & D Greenberg (eds) Psychiatric Care of the Medical Patient, 2nd ed., New York, Oxford Univ Press. 2000:419-28. (Second edition is out of print.)
2. Baghai T et al. Electroconvulsive therapy and its different indications. Dialogues Clin Neurosci. Mar 2008;10(1):105-17.
3. Ozer F et al. Electroconvulsive therapy in drug-induced psychiatric states and neuroleptic malignant syndrome. J ECT. 2005 Jun;21(2):125-7.
4. Taylor S. Electroconvulsive therapy: A review of history, patient selection, technique, and medication management. South Med J. 2007 May;100(5):494-8.
5. The Practice of Electroconvulsive Therapy, 2nd edition. A Task Force Report of the American Psychiatric Association. 2001. pp. 84-85.
6. Miller LJ. Use of electroconvulsive therapy during pregnancy. Hosp Community Psychiatry. 1994 May;45(5):444-50.
7. Miller R et al. ECT: Physiologic Effects. Miller’s Anesthesia. 7th Edition. 2009.
8. Mueller PS et al. The Safety of electroconvulsive therapy in patients with severe aortic stenosis. Mayo Clin Proc. 2007 Nov;82(11):1360-3.
9. Dolenc TJ et al. Electroconvulsive therapy in patients with cardiac pacemakers & implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 2004 Sep;27(9):1257-63.
Suggested readings
The practice of electroconvulsive therapy: Recommendations for treatment, training, and privileging (A Task Force Report of the American Psychiatric Association), 2nd Edition. APA Publishing. 2001.
Weiner R et al. “Electroconvulsive therapy in the medical & neurologic patient” in A Stoudemire, BS Fogel & D Greenberg (eds) Psychiatric Care of the Medical Patient, 2nd ed., New York, Oxford Univ Press. 2000:419-28. (Second edition is out of print.)
Rosenquist P et al. Charting the course of electroconvulsive therapy: Where have we been and where are we headed? J Psychosoc Nurs Ment Health Serv. 2016 Dec 1;54(12):39-43.
QUIZ
1. All of the following are indications for ECT except?
A. Schizophrenia.
B. Panic attacks.
C. Bipolar mania.
D. Catatonia.
Answer: B. Panic attacks. ECT is not effective for anxiety disorders including panic, generalized anxiety, PTSD, or OCD.
2. The most commonly accepted mechanism of action for ECT is?
A. Reduction in glutamate levels.
B. Altering signal transduction pathways.
C. Increased neurotransmitter activity.
D. Increased cerebral blood flow.
Answer: C. Increased neurotransmitter activity. There are data to support all, but neurotransmitter flow is most accepted thus far.
3. Which of the following is a common side effect of ECT?
A. Bronchospasm.
B. Diarrhea.
C. Delirium.
D. Visual changes.
Answer: C. Delirium. The rest are rare or not noted.
4. Which of the following is a relative contraindication for ECT?
A. Pregnancy.
B. Epilepsy.
C. Advanced age.
D. Increased intracranial pressure.
Answer: D. Increased intracranial pressure.
FDA: Faulty hematology analyzers face class I recall
The Food and Drug Administration is alerting laboratories and providers to a class I recall on Beckman Coulter hematology analyzers because of the potential for inaccurate platelet count results.
A class I recall indicates reasonable probability of serious adverse health consequences or death associated with use, according to the FDA.
The recall is related to the devices’ platelet analyzing function; among other uses, these devices help assess patients fitness for surgery, so a faulty reading on platelet counts could result in increased risk for life-threatening bleeding during a procedure in patients who have unidentified severe thrombocytopenia, according to a statement from the agency.
“Because this may cause serious injury, or even death, to a patient, we are urging health care professionals to be aware of the potential for inaccurate diagnostic results with these analyzers and to take appropriate actions including the use of alternative diagnostic testing or confirming analyzer results with manual scanning or estimate of platelets,” Tim Stenzel, MD, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health, said in the statement.
The recall applies to the UniCel DxH 800 Coulter Cellular Analysis System, UniCel DxH 600 Coulter Cellular Analysis System, and UniCel DxH 900 Coulter Cellular Analysis System. The faulty devices were first identified in 2018, and the manufacturer released an urgent medical device correction letter at that time. The company has more recently released a software patch for the devices, but the FDA has not yet assessed whether it resolves the problem. The agency has released detailed actions and recommendations related to these devices.
At this time, the FDA is unaware of any serious adverse events that have been directly linked to these devices, but the agency recommends that any events be reported through its MedWatch reporting system.
The Food and Drug Administration is alerting laboratories and providers to a class I recall on Beckman Coulter hematology analyzers because of the potential for inaccurate platelet count results.
A class I recall indicates reasonable probability of serious adverse health consequences or death associated with use, according to the FDA.
The recall is related to the devices’ platelet analyzing function; among other uses, these devices help assess patients fitness for surgery, so a faulty reading on platelet counts could result in increased risk for life-threatening bleeding during a procedure in patients who have unidentified severe thrombocytopenia, according to a statement from the agency.
“Because this may cause serious injury, or even death, to a patient, we are urging health care professionals to be aware of the potential for inaccurate diagnostic results with these analyzers and to take appropriate actions including the use of alternative diagnostic testing or confirming analyzer results with manual scanning or estimate of platelets,” Tim Stenzel, MD, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health, said in the statement.
The recall applies to the UniCel DxH 800 Coulter Cellular Analysis System, UniCel DxH 600 Coulter Cellular Analysis System, and UniCel DxH 900 Coulter Cellular Analysis System. The faulty devices were first identified in 2018, and the manufacturer released an urgent medical device correction letter at that time. The company has more recently released a software patch for the devices, but the FDA has not yet assessed whether it resolves the problem. The agency has released detailed actions and recommendations related to these devices.
At this time, the FDA is unaware of any serious adverse events that have been directly linked to these devices, but the agency recommends that any events be reported through its MedWatch reporting system.
The Food and Drug Administration is alerting laboratories and providers to a class I recall on Beckman Coulter hematology analyzers because of the potential for inaccurate platelet count results.
A class I recall indicates reasonable probability of serious adverse health consequences or death associated with use, according to the FDA.
The recall is related to the devices’ platelet analyzing function; among other uses, these devices help assess patients fitness for surgery, so a faulty reading on platelet counts could result in increased risk for life-threatening bleeding during a procedure in patients who have unidentified severe thrombocytopenia, according to a statement from the agency.
“Because this may cause serious injury, or even death, to a patient, we are urging health care professionals to be aware of the potential for inaccurate diagnostic results with these analyzers and to take appropriate actions including the use of alternative diagnostic testing or confirming analyzer results with manual scanning or estimate of platelets,” Tim Stenzel, MD, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health, said in the statement.
The recall applies to the UniCel DxH 800 Coulter Cellular Analysis System, UniCel DxH 600 Coulter Cellular Analysis System, and UniCel DxH 900 Coulter Cellular Analysis System. The faulty devices were first identified in 2018, and the manufacturer released an urgent medical device correction letter at that time. The company has more recently released a software patch for the devices, but the FDA has not yet assessed whether it resolves the problem. The agency has released detailed actions and recommendations related to these devices.
At this time, the FDA is unaware of any serious adverse events that have been directly linked to these devices, but the agency recommends that any events be reported through its MedWatch reporting system.
Sometimes You Should Order Another Test
On August 7, 2012, a 44-year-old electrical engineer sustained a knee injury. He initially sought treatment at an emergency department (ED) in Indiana, where he lived, and was released with a splint on his leg.
On August 9, the patient presented to an Illinois medical clinic. He was seen by a physician who referred the patient to another physician at the clinic for evaluation for surgery. The procedure, to repair ruptured ligaments in the patient’s left knee, was scheduled for August 16.
After returning home, the patient called the first physician with complaints that his splinted left knee, calf, and leg felt hot. The physician sent approval for the patient to undergo Doppler imaging of his left leg at a hospital in Indiana. The Doppler was performed on August 10, and the results were sent to the referring physician. The imaging was negative for any abnormalities.
On the morning of August 13, the patient presented to the Illinois medical clinic for presurgical clearance. The examination was performed by an NP, and then the patient had a presurgical consultation with the surgeon. The patient allegedly reported to the NP and the surgeon that he had continuing pain, swelling, and heat sensation in his left leg. Relying on the Doppler performed a few days earlier, the surgeon told the patient that these symptoms were related to the knee trauma he had sustained. No additional Doppler imaging was ordered.
On August 16, the patient was anesthetized in preparation for surgery and soon thereafter suffered a pulmonary embolism (PE) when a deep vein thrombosis (DVT) in his left leg detached and traveled to his lung. He went into pulmonary arrest, coded, and was declared brain dead within hours of arriving for surgery.
The decedent left behind a wife and 2 daughters, ages 12 and 14. His wife, as the administrator of her husband’s estate, sued the NP and her employer. The 2 orthopedic physicians, a treating cardiologist, and their employer were named as respondents in discovery. The physicians’ employer was ultimately added as a defendant, along with the NP’s employer. Prior to trial, the 3 physicians and the NP were dismissed from the case. The matter proceeded against the 2 employing organizations.
The estate alleged that the NP and operating surgeon/physician, each as agents of their respective employers, failed to order a second Doppler image of the decedent’s left leg during presurgical clearance procedures and in the 4 days leading up to the surgery. The estate alleged that a second Doppler was needed because the decedent had complaints that were consistent with DVT—such as continuing pain, swelling, and heat sensation—in his left leg at the August 13 visit. The estate argued that the failure to order a second Doppler led to a failure to diagnose the DVT from which the decedent was suffering symptoms. The estate alleged that the earlier Doppler was performed too soon after the decedent’s injury to show a DVT, as DVTs do not develop immediately after trauma but grow and spread over time.
Continue to: While pain, swelling, and warmth...
While pain, swelling, and warmth/heat sensation are symptoms that accompany trauma, the estate asserted that these are also symptoms of a DVT and that the surgeon, as a reasonable orthopedic physician, should have tested the decedent for a DVT on August 13, on the morning of the surgery, or any day in between and that he also should have ordered a hematologic consult. The estate’s orthopedic surgery expert testified that the surgeon violated the standard of care by failing to appreciate the symptoms and risk factors the decedent was experiencing. The expert testified that the decedent had 4 of 5 high-risk factors for a DVT: Although there was no family history of DVT, the decedent had sustained leg trauma, he was older than 40, his leg was immobilized, and he was considered obese (BMI > 30).
The same orthopedist further opined that a Doppler performed on August 13 more likely than not would have shown the DVT, and the August 16 knee surgery would have been delayed until it was treated. He added that the failure to perform a second Doppler before surgery constituted negligence that caused the decedent’s death. The estate’s hematology expert testified that the decedent was a candidate for prophylactic anticoagulation on August 13 and, if a Doppler had been performed, the need for such medication would have been discovered.
The defense argued that the decedent’s signs and symptoms did not change following the Doppler on August 10 and, therefore, there was no reason for the surgeon to order a second Doppler prior to performing surgery. The defense further argued that the NP, in the scope of her practice, was not allowed to order a Doppler, knew that the surgeon would be seeing the decedent during the same visit, and could rely on the surgeon to order the necessary presurgical tests. The defense’s orthopedics expert testified that, unless there was an increase in signs and/or symptoms, the standard of care did not require the surgeon to order another Doppler. The expert further testified that the surgeon did not place the decedent on a presurgical anticoagulant because this would have increased his risk for bleeding. The defense’s hematology expert testified that there was no guarantee an anticoagulant would have prevented the PE because of the large size of the clot. He further stated that the decedent was not a candidate for prophylactic anticoagulants prior to surgery because the Doppler was negative for clotting, and there was no increase in his symptoms after the Doppler was performed.
The estate’s NP expert testified that, while performing the presurgical clearance, the defendant NP failed to obtain the Doppler history or a full description of the patient’s symptoms (which resulted from a DVT) and failed to order a Doppler. The defense’s NP expert testified that, based on the defendant NP’s testimony, she was not allowed to order a Doppler, and that, as an NP, she would have had a document in her credentials setting forth what she can and cannot recommend. Since such a document was not produced, this could not be determined, she opined.
VERDICT
After an 11-day trial and 2.5 hours of deliberation, the jury found in favor of the plaintiff estate. The jury found the NP’s employer not liable but the physicians’ employer responsible. Damages totaling $5,511,567 were awarded to the estate.
Continue to: COMMENTARY
COMMENTARY
If the Grim Reaper had an Employee of the Month plaque, DVT would proudly see its name etched thereon about 8 months of the year. Missed bleeding takes second place (muttering under its breath, promising to “up its game” next year). You may think the pathophysiology is boring. DVTs don’t care. They just kill.
While these comments may seem flip, the intention is not to minimize the threat posed by DVTs but rather to underscore it. DVT/PE is one of the most missed clinical entities giving rise to litigation; it is legally problematic because its development is often foreseeable. There is a clear setup (eg, surgery or immobilization) and a disease process that is easily understood by even lay people. Jurors understand the concept of a “clot”—if you aren’t moving much, you are apt to get a “clog” and if the clog is discovered and “dissolved” the threat goes away, but if it “breaks loose” it could kill. Jurors need not understand highbrow concepts such as the renin-angiotensin-aldosterone system or the hypothalamic pituitary adrenal axis; it is a clog. This is common sense; during deliberations, jurors will reason that if they “get it,” why couldn’t you?
To add insult to (endothelial) injury, DVT and PE are generally curable; most patients recover fully with proper treatment. Plaintiff’s counsel can trot out the tried-and-true argument: “If a simple, painless ultrasound test had been done, [the patient] would be having dinner with his family tonight.” Furthermore, affected patients are apt to be on the younger side, with a lengthy employment life ahead of them—potentially giving rise to substantial loss-of-earnings damages.
In the case presented here, we are told that the decedent complained of “continuing pain, swelling, and heat sensation” when he saw both an NP and a surgeon for presurgical clearance. We do not know if his leg was examined, but if it had been, the positive and negative findings likely would have been discussed in the case synopsis. It appears the NP and the surgeon saw the leg in the immobilizer and decided to rely on the previous negative Doppler.
First, let’s address the diagnosis of DVT: We should all realize that Homan’s sign sucks. You have permission to elicit Homan’s sign if you are in a museum for antiquated medicine (where other artifacts include AZT monotherapy, bite-and-swallow nifedipine for hypertensive urgency, and meperidine for sphincter of Oddi spasm). Everywhere else on the planet, Homan’s sign has always been bad and is certainly not the standard of care. If you are still attempting to elicit Homan’s sign—cut it out. It is the 1970s leisure suit in your closet: never was any good, never is going to be. Declutter your clinical test arsenal and KonMari Homan’s sign straight to the junk pile.
Continue to: A diagnostic tool...
A diagnostic tool that works better is the Wells’ Criteria, which operates on a points system (with 3-8 points indicating high probability of DVT, 1-2 points indicating moderate probability, and less than 1 point indicating low probability).1 Patients are assessed according to the following criteria:
- Paralysis, paresis, or recent orthopedic casting of lower extremity (1 pt)
- Recently bedridden (> 3 d) or major surgery within past 4 weeks (1 pt)
- Localized tenderness in deep vein system (1 pt)
- Swelling of entire leg (1 pt)
- Calf swelling 3 cm greater than other leg (measured 10 cm below the tibial tuberosity) (1 pt)
- Pitting edema greater in the symptomatic leg (1 pt)
- Collateral nonvaricose superficial veins (1 pt)
- Active cancer or cancer treated within 6 months (1 pt)
- Alternative diagnosis more likely than DVT (Baker cyst, cellulitis, muscle damage, superficial venous thrombosis, postphlebitic syndrome, inguinal lymphadenopathy, external venous compression) (–2 pts).1
Moving through the Wells’ score system, we don’t have enough clinical data input for this patient. We do know he had leg pain and possibly swelling (1 pt). His knee was immobilized (albeit without plaster) (1 pt). We don’t know how “bedridden” he was. I’ll argue we should not deduct for “an alternative diagnosis more likely” because the decedent injured his knee and we can expect knee pain and knee swelling, not symptoms and findings involving the entire leg. So this patient would score at least 1 point, possibly 2, and thus be stratified as “moderate probability.”
There was an initial suspicion of DVT in this patient, and a Doppler was ordered on August 10. The patient’s symptoms persisted. However, in light of the negative Doppler results, the continued symptoms were attributed to the knee derangement and not a DVT.
Which brings us to the first malpractice trap: reliance on a prior negative study to rule out a dynamic condition. For any condition that can evolve, do not hesitate to order a repeat test when needed. DVT is a dynamic process; given the right clinical setup (in this case, immobility and ongoing/increasing symptoms), a clinician should not be bashful about ordering a follow-up study. As providers, we recognize the static nature of certain studies and have no reservations about ordering serial complete blood counts or a repeat chest film. Yet we are more reluctant to order repeat studies for equally dynamic disease processes—even when they are required by the standard of care. Here, reliance on a 3-day-old Doppler was problematic. Don’t rely on an old study if the disease under suspicion evolves rapidly.
The second trap: Do not allow yourself to be scolded (or engage in self-scolding) if a correctly ordered test is negative. A clinical decision is correct if it is based on science and in the interest of safeguarding the patient. Don’t buy into the trap that your decision needs to be validated by a positive result. Here, the persisting or worsening leg pain with entire leg swelling warranted a new study—even if the result was expected to be negative.
Continue to: When your decision to order...
When your decision to order such a test is challenged, my favorite rhetorical defense is “Those are some pretty big dice to roll.” That is what you are doing if you skip a test that should be ordered. The bounceback kid with a prior negative lumbar puncture, who now appears toxic, needs a repeat tap. Why? Because you cannot afford to miss meningitis—that would be a risky roll of the dice.
One final point about this case: The NP argued “she was not allowed to order a Doppler” and her NP expert made the argument that “she would have had a document in her credentials setting forth what she can and cannot recommend.” The expert testified she could not find such a document and “could not determine” whether the defendant NP could “recommend” that test. I don’t fault the tactical decision to use this argument, in this case, when the surgeon also saw the patient on the same day. However, we should all recognize this will not normally work. A clinician cannot credibly argue she is not “credentialed” to recommend a course of action she can’t presently deliver.
Consider a clinician employed in an urgent care center without direct access to order CT. She evaluates an 80-year-old woman on warfarin who slipped and struck her head on a marble table. The standard of care requires a CT scan (likely several) to rule out an intracranial bleed. The urgent care clinician cannot send the patient away and later claim she was “not credentialed” to recommend CT imaging to rule out an intracranial bleed. As a matter of the standard of care, our hypothetical clinician would be duty bound to advise the patient of the risk for bleeding and then take steps to arrange for that care—even though she is not in a position to personally deliver it.
IN SUMMARY
Protect your patients from evolving cases by ordering updated tests. Do not be afraid of a negative result. Instead, fear the Reaper; keep him away from your patients. Let him get his “more cowbell” somewhere else.
1. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350(9094):1795-1798.
On August 7, 2012, a 44-year-old electrical engineer sustained a knee injury. He initially sought treatment at an emergency department (ED) in Indiana, where he lived, and was released with a splint on his leg.
On August 9, the patient presented to an Illinois medical clinic. He was seen by a physician who referred the patient to another physician at the clinic for evaluation for surgery. The procedure, to repair ruptured ligaments in the patient’s left knee, was scheduled for August 16.
After returning home, the patient called the first physician with complaints that his splinted left knee, calf, and leg felt hot. The physician sent approval for the patient to undergo Doppler imaging of his left leg at a hospital in Indiana. The Doppler was performed on August 10, and the results were sent to the referring physician. The imaging was negative for any abnormalities.
On the morning of August 13, the patient presented to the Illinois medical clinic for presurgical clearance. The examination was performed by an NP, and then the patient had a presurgical consultation with the surgeon. The patient allegedly reported to the NP and the surgeon that he had continuing pain, swelling, and heat sensation in his left leg. Relying on the Doppler performed a few days earlier, the surgeon told the patient that these symptoms were related to the knee trauma he had sustained. No additional Doppler imaging was ordered.
On August 16, the patient was anesthetized in preparation for surgery and soon thereafter suffered a pulmonary embolism (PE) when a deep vein thrombosis (DVT) in his left leg detached and traveled to his lung. He went into pulmonary arrest, coded, and was declared brain dead within hours of arriving for surgery.
The decedent left behind a wife and 2 daughters, ages 12 and 14. His wife, as the administrator of her husband’s estate, sued the NP and her employer. The 2 orthopedic physicians, a treating cardiologist, and their employer were named as respondents in discovery. The physicians’ employer was ultimately added as a defendant, along with the NP’s employer. Prior to trial, the 3 physicians and the NP were dismissed from the case. The matter proceeded against the 2 employing organizations.
The estate alleged that the NP and operating surgeon/physician, each as agents of their respective employers, failed to order a second Doppler image of the decedent’s left leg during presurgical clearance procedures and in the 4 days leading up to the surgery. The estate alleged that a second Doppler was needed because the decedent had complaints that were consistent with DVT—such as continuing pain, swelling, and heat sensation—in his left leg at the August 13 visit. The estate argued that the failure to order a second Doppler led to a failure to diagnose the DVT from which the decedent was suffering symptoms. The estate alleged that the earlier Doppler was performed too soon after the decedent’s injury to show a DVT, as DVTs do not develop immediately after trauma but grow and spread over time.
Continue to: While pain, swelling, and warmth...
While pain, swelling, and warmth/heat sensation are symptoms that accompany trauma, the estate asserted that these are also symptoms of a DVT and that the surgeon, as a reasonable orthopedic physician, should have tested the decedent for a DVT on August 13, on the morning of the surgery, or any day in between and that he also should have ordered a hematologic consult. The estate’s orthopedic surgery expert testified that the surgeon violated the standard of care by failing to appreciate the symptoms and risk factors the decedent was experiencing. The expert testified that the decedent had 4 of 5 high-risk factors for a DVT: Although there was no family history of DVT, the decedent had sustained leg trauma, he was older than 40, his leg was immobilized, and he was considered obese (BMI > 30).
The same orthopedist further opined that a Doppler performed on August 13 more likely than not would have shown the DVT, and the August 16 knee surgery would have been delayed until it was treated. He added that the failure to perform a second Doppler before surgery constituted negligence that caused the decedent’s death. The estate’s hematology expert testified that the decedent was a candidate for prophylactic anticoagulation on August 13 and, if a Doppler had been performed, the need for such medication would have been discovered.
The defense argued that the decedent’s signs and symptoms did not change following the Doppler on August 10 and, therefore, there was no reason for the surgeon to order a second Doppler prior to performing surgery. The defense further argued that the NP, in the scope of her practice, was not allowed to order a Doppler, knew that the surgeon would be seeing the decedent during the same visit, and could rely on the surgeon to order the necessary presurgical tests. The defense’s orthopedics expert testified that, unless there was an increase in signs and/or symptoms, the standard of care did not require the surgeon to order another Doppler. The expert further testified that the surgeon did not place the decedent on a presurgical anticoagulant because this would have increased his risk for bleeding. The defense’s hematology expert testified that there was no guarantee an anticoagulant would have prevented the PE because of the large size of the clot. He further stated that the decedent was not a candidate for prophylactic anticoagulants prior to surgery because the Doppler was negative for clotting, and there was no increase in his symptoms after the Doppler was performed.
The estate’s NP expert testified that, while performing the presurgical clearance, the defendant NP failed to obtain the Doppler history or a full description of the patient’s symptoms (which resulted from a DVT) and failed to order a Doppler. The defense’s NP expert testified that, based on the defendant NP’s testimony, she was not allowed to order a Doppler, and that, as an NP, she would have had a document in her credentials setting forth what she can and cannot recommend. Since such a document was not produced, this could not be determined, she opined.
VERDICT
After an 11-day trial and 2.5 hours of deliberation, the jury found in favor of the plaintiff estate. The jury found the NP’s employer not liable but the physicians’ employer responsible. Damages totaling $5,511,567 were awarded to the estate.
Continue to: COMMENTARY
COMMENTARY
If the Grim Reaper had an Employee of the Month plaque, DVT would proudly see its name etched thereon about 8 months of the year. Missed bleeding takes second place (muttering under its breath, promising to “up its game” next year). You may think the pathophysiology is boring. DVTs don’t care. They just kill.
While these comments may seem flip, the intention is not to minimize the threat posed by DVTs but rather to underscore it. DVT/PE is one of the most missed clinical entities giving rise to litigation; it is legally problematic because its development is often foreseeable. There is a clear setup (eg, surgery or immobilization) and a disease process that is easily understood by even lay people. Jurors understand the concept of a “clot”—if you aren’t moving much, you are apt to get a “clog” and if the clog is discovered and “dissolved” the threat goes away, but if it “breaks loose” it could kill. Jurors need not understand highbrow concepts such as the renin-angiotensin-aldosterone system or the hypothalamic pituitary adrenal axis; it is a clog. This is common sense; during deliberations, jurors will reason that if they “get it,” why couldn’t you?
To add insult to (endothelial) injury, DVT and PE are generally curable; most patients recover fully with proper treatment. Plaintiff’s counsel can trot out the tried-and-true argument: “If a simple, painless ultrasound test had been done, [the patient] would be having dinner with his family tonight.” Furthermore, affected patients are apt to be on the younger side, with a lengthy employment life ahead of them—potentially giving rise to substantial loss-of-earnings damages.
In the case presented here, we are told that the decedent complained of “continuing pain, swelling, and heat sensation” when he saw both an NP and a surgeon for presurgical clearance. We do not know if his leg was examined, but if it had been, the positive and negative findings likely would have been discussed in the case synopsis. It appears the NP and the surgeon saw the leg in the immobilizer and decided to rely on the previous negative Doppler.
First, let’s address the diagnosis of DVT: We should all realize that Homan’s sign sucks. You have permission to elicit Homan’s sign if you are in a museum for antiquated medicine (where other artifacts include AZT monotherapy, bite-and-swallow nifedipine for hypertensive urgency, and meperidine for sphincter of Oddi spasm). Everywhere else on the planet, Homan’s sign has always been bad and is certainly not the standard of care. If you are still attempting to elicit Homan’s sign—cut it out. It is the 1970s leisure suit in your closet: never was any good, never is going to be. Declutter your clinical test arsenal and KonMari Homan’s sign straight to the junk pile.
Continue to: A diagnostic tool...
A diagnostic tool that works better is the Wells’ Criteria, which operates on a points system (with 3-8 points indicating high probability of DVT, 1-2 points indicating moderate probability, and less than 1 point indicating low probability).1 Patients are assessed according to the following criteria:
- Paralysis, paresis, or recent orthopedic casting of lower extremity (1 pt)
- Recently bedridden (> 3 d) or major surgery within past 4 weeks (1 pt)
- Localized tenderness in deep vein system (1 pt)
- Swelling of entire leg (1 pt)
- Calf swelling 3 cm greater than other leg (measured 10 cm below the tibial tuberosity) (1 pt)
- Pitting edema greater in the symptomatic leg (1 pt)
- Collateral nonvaricose superficial veins (1 pt)
- Active cancer or cancer treated within 6 months (1 pt)
- Alternative diagnosis more likely than DVT (Baker cyst, cellulitis, muscle damage, superficial venous thrombosis, postphlebitic syndrome, inguinal lymphadenopathy, external venous compression) (–2 pts).1
Moving through the Wells’ score system, we don’t have enough clinical data input for this patient. We do know he had leg pain and possibly swelling (1 pt). His knee was immobilized (albeit without plaster) (1 pt). We don’t know how “bedridden” he was. I’ll argue we should not deduct for “an alternative diagnosis more likely” because the decedent injured his knee and we can expect knee pain and knee swelling, not symptoms and findings involving the entire leg. So this patient would score at least 1 point, possibly 2, and thus be stratified as “moderate probability.”
There was an initial suspicion of DVT in this patient, and a Doppler was ordered on August 10. The patient’s symptoms persisted. However, in light of the negative Doppler results, the continued symptoms were attributed to the knee derangement and not a DVT.
Which brings us to the first malpractice trap: reliance on a prior negative study to rule out a dynamic condition. For any condition that can evolve, do not hesitate to order a repeat test when needed. DVT is a dynamic process; given the right clinical setup (in this case, immobility and ongoing/increasing symptoms), a clinician should not be bashful about ordering a follow-up study. As providers, we recognize the static nature of certain studies and have no reservations about ordering serial complete blood counts or a repeat chest film. Yet we are more reluctant to order repeat studies for equally dynamic disease processes—even when they are required by the standard of care. Here, reliance on a 3-day-old Doppler was problematic. Don’t rely on an old study if the disease under suspicion evolves rapidly.
The second trap: Do not allow yourself to be scolded (or engage in self-scolding) if a correctly ordered test is negative. A clinical decision is correct if it is based on science and in the interest of safeguarding the patient. Don’t buy into the trap that your decision needs to be validated by a positive result. Here, the persisting or worsening leg pain with entire leg swelling warranted a new study—even if the result was expected to be negative.
Continue to: When your decision to order...
When your decision to order such a test is challenged, my favorite rhetorical defense is “Those are some pretty big dice to roll.” That is what you are doing if you skip a test that should be ordered. The bounceback kid with a prior negative lumbar puncture, who now appears toxic, needs a repeat tap. Why? Because you cannot afford to miss meningitis—that would be a risky roll of the dice.
One final point about this case: The NP argued “she was not allowed to order a Doppler” and her NP expert made the argument that “she would have had a document in her credentials setting forth what she can and cannot recommend.” The expert testified she could not find such a document and “could not determine” whether the defendant NP could “recommend” that test. I don’t fault the tactical decision to use this argument, in this case, when the surgeon also saw the patient on the same day. However, we should all recognize this will not normally work. A clinician cannot credibly argue she is not “credentialed” to recommend a course of action she can’t presently deliver.
Consider a clinician employed in an urgent care center without direct access to order CT. She evaluates an 80-year-old woman on warfarin who slipped and struck her head on a marble table. The standard of care requires a CT scan (likely several) to rule out an intracranial bleed. The urgent care clinician cannot send the patient away and later claim she was “not credentialed” to recommend CT imaging to rule out an intracranial bleed. As a matter of the standard of care, our hypothetical clinician would be duty bound to advise the patient of the risk for bleeding and then take steps to arrange for that care—even though she is not in a position to personally deliver it.
IN SUMMARY
Protect your patients from evolving cases by ordering updated tests. Do not be afraid of a negative result. Instead, fear the Reaper; keep him away from your patients. Let him get his “more cowbell” somewhere else.
On August 7, 2012, a 44-year-old electrical engineer sustained a knee injury. He initially sought treatment at an emergency department (ED) in Indiana, where he lived, and was released with a splint on his leg.
On August 9, the patient presented to an Illinois medical clinic. He was seen by a physician who referred the patient to another physician at the clinic for evaluation for surgery. The procedure, to repair ruptured ligaments in the patient’s left knee, was scheduled for August 16.
After returning home, the patient called the first physician with complaints that his splinted left knee, calf, and leg felt hot. The physician sent approval for the patient to undergo Doppler imaging of his left leg at a hospital in Indiana. The Doppler was performed on August 10, and the results were sent to the referring physician. The imaging was negative for any abnormalities.
On the morning of August 13, the patient presented to the Illinois medical clinic for presurgical clearance. The examination was performed by an NP, and then the patient had a presurgical consultation with the surgeon. The patient allegedly reported to the NP and the surgeon that he had continuing pain, swelling, and heat sensation in his left leg. Relying on the Doppler performed a few days earlier, the surgeon told the patient that these symptoms were related to the knee trauma he had sustained. No additional Doppler imaging was ordered.
On August 16, the patient was anesthetized in preparation for surgery and soon thereafter suffered a pulmonary embolism (PE) when a deep vein thrombosis (DVT) in his left leg detached and traveled to his lung. He went into pulmonary arrest, coded, and was declared brain dead within hours of arriving for surgery.
The decedent left behind a wife and 2 daughters, ages 12 and 14. His wife, as the administrator of her husband’s estate, sued the NP and her employer. The 2 orthopedic physicians, a treating cardiologist, and their employer were named as respondents in discovery. The physicians’ employer was ultimately added as a defendant, along with the NP’s employer. Prior to trial, the 3 physicians and the NP were dismissed from the case. The matter proceeded against the 2 employing organizations.
The estate alleged that the NP and operating surgeon/physician, each as agents of their respective employers, failed to order a second Doppler image of the decedent’s left leg during presurgical clearance procedures and in the 4 days leading up to the surgery. The estate alleged that a second Doppler was needed because the decedent had complaints that were consistent with DVT—such as continuing pain, swelling, and heat sensation—in his left leg at the August 13 visit. The estate argued that the failure to order a second Doppler led to a failure to diagnose the DVT from which the decedent was suffering symptoms. The estate alleged that the earlier Doppler was performed too soon after the decedent’s injury to show a DVT, as DVTs do not develop immediately after trauma but grow and spread over time.
Continue to: While pain, swelling, and warmth...
While pain, swelling, and warmth/heat sensation are symptoms that accompany trauma, the estate asserted that these are also symptoms of a DVT and that the surgeon, as a reasonable orthopedic physician, should have tested the decedent for a DVT on August 13, on the morning of the surgery, or any day in between and that he also should have ordered a hematologic consult. The estate’s orthopedic surgery expert testified that the surgeon violated the standard of care by failing to appreciate the symptoms and risk factors the decedent was experiencing. The expert testified that the decedent had 4 of 5 high-risk factors for a DVT: Although there was no family history of DVT, the decedent had sustained leg trauma, he was older than 40, his leg was immobilized, and he was considered obese (BMI > 30).
The same orthopedist further opined that a Doppler performed on August 13 more likely than not would have shown the DVT, and the August 16 knee surgery would have been delayed until it was treated. He added that the failure to perform a second Doppler before surgery constituted negligence that caused the decedent’s death. The estate’s hematology expert testified that the decedent was a candidate for prophylactic anticoagulation on August 13 and, if a Doppler had been performed, the need for such medication would have been discovered.
The defense argued that the decedent’s signs and symptoms did not change following the Doppler on August 10 and, therefore, there was no reason for the surgeon to order a second Doppler prior to performing surgery. The defense further argued that the NP, in the scope of her practice, was not allowed to order a Doppler, knew that the surgeon would be seeing the decedent during the same visit, and could rely on the surgeon to order the necessary presurgical tests. The defense’s orthopedics expert testified that, unless there was an increase in signs and/or symptoms, the standard of care did not require the surgeon to order another Doppler. The expert further testified that the surgeon did not place the decedent on a presurgical anticoagulant because this would have increased his risk for bleeding. The defense’s hematology expert testified that there was no guarantee an anticoagulant would have prevented the PE because of the large size of the clot. He further stated that the decedent was not a candidate for prophylactic anticoagulants prior to surgery because the Doppler was negative for clotting, and there was no increase in his symptoms after the Doppler was performed.
The estate’s NP expert testified that, while performing the presurgical clearance, the defendant NP failed to obtain the Doppler history or a full description of the patient’s symptoms (which resulted from a DVT) and failed to order a Doppler. The defense’s NP expert testified that, based on the defendant NP’s testimony, she was not allowed to order a Doppler, and that, as an NP, she would have had a document in her credentials setting forth what she can and cannot recommend. Since such a document was not produced, this could not be determined, she opined.
VERDICT
After an 11-day trial and 2.5 hours of deliberation, the jury found in favor of the plaintiff estate. The jury found the NP’s employer not liable but the physicians’ employer responsible. Damages totaling $5,511,567 were awarded to the estate.
Continue to: COMMENTARY
COMMENTARY
If the Grim Reaper had an Employee of the Month plaque, DVT would proudly see its name etched thereon about 8 months of the year. Missed bleeding takes second place (muttering under its breath, promising to “up its game” next year). You may think the pathophysiology is boring. DVTs don’t care. They just kill.
While these comments may seem flip, the intention is not to minimize the threat posed by DVTs but rather to underscore it. DVT/PE is one of the most missed clinical entities giving rise to litigation; it is legally problematic because its development is often foreseeable. There is a clear setup (eg, surgery or immobilization) and a disease process that is easily understood by even lay people. Jurors understand the concept of a “clot”—if you aren’t moving much, you are apt to get a “clog” and if the clog is discovered and “dissolved” the threat goes away, but if it “breaks loose” it could kill. Jurors need not understand highbrow concepts such as the renin-angiotensin-aldosterone system or the hypothalamic pituitary adrenal axis; it is a clog. This is common sense; during deliberations, jurors will reason that if they “get it,” why couldn’t you?
To add insult to (endothelial) injury, DVT and PE are generally curable; most patients recover fully with proper treatment. Plaintiff’s counsel can trot out the tried-and-true argument: “If a simple, painless ultrasound test had been done, [the patient] would be having dinner with his family tonight.” Furthermore, affected patients are apt to be on the younger side, with a lengthy employment life ahead of them—potentially giving rise to substantial loss-of-earnings damages.
In the case presented here, we are told that the decedent complained of “continuing pain, swelling, and heat sensation” when he saw both an NP and a surgeon for presurgical clearance. We do not know if his leg was examined, but if it had been, the positive and negative findings likely would have been discussed in the case synopsis. It appears the NP and the surgeon saw the leg in the immobilizer and decided to rely on the previous negative Doppler.
First, let’s address the diagnosis of DVT: We should all realize that Homan’s sign sucks. You have permission to elicit Homan’s sign if you are in a museum for antiquated medicine (where other artifacts include AZT monotherapy, bite-and-swallow nifedipine for hypertensive urgency, and meperidine for sphincter of Oddi spasm). Everywhere else on the planet, Homan’s sign has always been bad and is certainly not the standard of care. If you are still attempting to elicit Homan’s sign—cut it out. It is the 1970s leisure suit in your closet: never was any good, never is going to be. Declutter your clinical test arsenal and KonMari Homan’s sign straight to the junk pile.
Continue to: A diagnostic tool...
A diagnostic tool that works better is the Wells’ Criteria, which operates on a points system (with 3-8 points indicating high probability of DVT, 1-2 points indicating moderate probability, and less than 1 point indicating low probability).1 Patients are assessed according to the following criteria:
- Paralysis, paresis, or recent orthopedic casting of lower extremity (1 pt)
- Recently bedridden (> 3 d) or major surgery within past 4 weeks (1 pt)
- Localized tenderness in deep vein system (1 pt)
- Swelling of entire leg (1 pt)
- Calf swelling 3 cm greater than other leg (measured 10 cm below the tibial tuberosity) (1 pt)
- Pitting edema greater in the symptomatic leg (1 pt)
- Collateral nonvaricose superficial veins (1 pt)
- Active cancer or cancer treated within 6 months (1 pt)
- Alternative diagnosis more likely than DVT (Baker cyst, cellulitis, muscle damage, superficial venous thrombosis, postphlebitic syndrome, inguinal lymphadenopathy, external venous compression) (–2 pts).1
Moving through the Wells’ score system, we don’t have enough clinical data input for this patient. We do know he had leg pain and possibly swelling (1 pt). His knee was immobilized (albeit without plaster) (1 pt). We don’t know how “bedridden” he was. I’ll argue we should not deduct for “an alternative diagnosis more likely” because the decedent injured his knee and we can expect knee pain and knee swelling, not symptoms and findings involving the entire leg. So this patient would score at least 1 point, possibly 2, and thus be stratified as “moderate probability.”
There was an initial suspicion of DVT in this patient, and a Doppler was ordered on August 10. The patient’s symptoms persisted. However, in light of the negative Doppler results, the continued symptoms were attributed to the knee derangement and not a DVT.
Which brings us to the first malpractice trap: reliance on a prior negative study to rule out a dynamic condition. For any condition that can evolve, do not hesitate to order a repeat test when needed. DVT is a dynamic process; given the right clinical setup (in this case, immobility and ongoing/increasing symptoms), a clinician should not be bashful about ordering a follow-up study. As providers, we recognize the static nature of certain studies and have no reservations about ordering serial complete blood counts or a repeat chest film. Yet we are more reluctant to order repeat studies for equally dynamic disease processes—even when they are required by the standard of care. Here, reliance on a 3-day-old Doppler was problematic. Don’t rely on an old study if the disease under suspicion evolves rapidly.
The second trap: Do not allow yourself to be scolded (or engage in self-scolding) if a correctly ordered test is negative. A clinical decision is correct if it is based on science and in the interest of safeguarding the patient. Don’t buy into the trap that your decision needs to be validated by a positive result. Here, the persisting or worsening leg pain with entire leg swelling warranted a new study—even if the result was expected to be negative.
Continue to: When your decision to order...
When your decision to order such a test is challenged, my favorite rhetorical defense is “Those are some pretty big dice to roll.” That is what you are doing if you skip a test that should be ordered. The bounceback kid with a prior negative lumbar puncture, who now appears toxic, needs a repeat tap. Why? Because you cannot afford to miss meningitis—that would be a risky roll of the dice.
One final point about this case: The NP argued “she was not allowed to order a Doppler” and her NP expert made the argument that “she would have had a document in her credentials setting forth what she can and cannot recommend.” The expert testified she could not find such a document and “could not determine” whether the defendant NP could “recommend” that test. I don’t fault the tactical decision to use this argument, in this case, when the surgeon also saw the patient on the same day. However, we should all recognize this will not normally work. A clinician cannot credibly argue she is not “credentialed” to recommend a course of action she can’t presently deliver.
Consider a clinician employed in an urgent care center without direct access to order CT. She evaluates an 80-year-old woman on warfarin who slipped and struck her head on a marble table. The standard of care requires a CT scan (likely several) to rule out an intracranial bleed. The urgent care clinician cannot send the patient away and later claim she was “not credentialed” to recommend CT imaging to rule out an intracranial bleed. As a matter of the standard of care, our hypothetical clinician would be duty bound to advise the patient of the risk for bleeding and then take steps to arrange for that care—even though she is not in a position to personally deliver it.
IN SUMMARY
Protect your patients from evolving cases by ordering updated tests. Do not be afraid of a negative result. Instead, fear the Reaper; keep him away from your patients. Let him get his “more cowbell” somewhere else.
1. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350(9094):1795-1798.
1. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350(9094):1795-1798.
Practical advice for colorectal cancer screening
Douglas K. Rex, MD, AGAF, MACP, MACG, FASGE; offers advice and insight for colorectal cancer screening, highlighting:
- Clinically relevant facts about the spectrum of precancerous lesions that can be targeted during screening;
- Key practical aspects of the 3 screening tests that receive significant use in the United States.
- Why some tests are used more than others
Click HERE to read the supplement
Douglas K. Rex, MD, AGAF, MACP, MACG, FASGE; offers advice and insight for colorectal cancer screening, highlighting:
- Clinically relevant facts about the spectrum of precancerous lesions that can be targeted during screening;
- Key practical aspects of the 3 screening tests that receive significant use in the United States.
- Why some tests are used more than others
Click HERE to read the supplement
Douglas K. Rex, MD, AGAF, MACP, MACG, FASGE; offers advice and insight for colorectal cancer screening, highlighting:
- Clinically relevant facts about the spectrum of precancerous lesions that can be targeted during screening;
- Key practical aspects of the 3 screening tests that receive significant use in the United States.
- Why some tests are used more than others
Click HERE to read the supplement
By the numbers: Readmissions for skin conditions
Almost 10% of patients
Data from the Nationwide Readmissions Database also showed that the same-cause readmission rate was 3.3% after 30 days and 7.8% within the calendar year (CY) over the 5-year study period of 2010-2014, Myron Zhang, MD, of the department of dermatology at Weill Cornell Medicine, New York, and his associates reported in the Journal of the American Academy of Dermatology.
The total cost of the CY readmissions was $2.54 billion, which works out to $508 million per year or $8,995 per visit. The most common dermatologic diagnosis – cellulitis made up 83.6% of all hospitalizations – was also the most expensive in terms of readmissions, resulting in $1.9 billion in CY costs, Dr. Zhang and associates wrote.
Overall readmission rates for cellulitis were not provided, but annual rates ranged from 9.1% to 9.3% (30-day all cause), from 7.7% to 8.1% (CY same cause), and from 3.1% to 3.3% (30-day same cause), they wrote.
The dermatologic diagnosis with the highest 30-day same-cause readmission rate was vascular hamartomas at 21.1%, followed by dermatomyositis (18.3%) and thrombotic microangiopathy (13.7%). Dermatomyositis had the highest CY same-cause readmission rate (30.8%) and mycosis fungoides had the highest 30-day all-cause rate (32.3%), according to the investigators.
“Diseases, characteristics, and comorbidities associated with high readmission rates should trigger hospitals to consider dermatology consultation, coordinate outpatient follow-up, and support underinsured outpatient access. These measures have been shown to reduce readmissions or hospital visits in general dermatologic settings, but outcomes in individual diseases are not well studied,” Dr. Zhang and associates wrote. They noted that there have been “very few prior studies of readmissions for skin diseases.”
rfranki@mdedge.com
SOURCE: Zhang M et al. J Am Acad. Dermatol. 2019. doi: 10.1016/j.jaad.2019.05.023. .
Almost 10% of patients

Data from the Nationwide Readmissions Database also showed that the same-cause readmission rate was 3.3% after 30 days and 7.8% within the calendar year (CY) over the 5-year study period of 2010-2014, Myron Zhang, MD, of the department of dermatology at Weill Cornell Medicine, New York, and his associates reported in the Journal of the American Academy of Dermatology.
The total cost of the CY readmissions was $2.54 billion, which works out to $508 million per year or $8,995 per visit. The most common dermatologic diagnosis – cellulitis made up 83.6% of all hospitalizations – was also the most expensive in terms of readmissions, resulting in $1.9 billion in CY costs, Dr. Zhang and associates wrote.
Overall readmission rates for cellulitis were not provided, but annual rates ranged from 9.1% to 9.3% (30-day all cause), from 7.7% to 8.1% (CY same cause), and from 3.1% to 3.3% (30-day same cause), they wrote.
The dermatologic diagnosis with the highest 30-day same-cause readmission rate was vascular hamartomas at 21.1%, followed by dermatomyositis (18.3%) and thrombotic microangiopathy (13.7%). Dermatomyositis had the highest CY same-cause readmission rate (30.8%) and mycosis fungoides had the highest 30-day all-cause rate (32.3%), according to the investigators.
“Diseases, characteristics, and comorbidities associated with high readmission rates should trigger hospitals to consider dermatology consultation, coordinate outpatient follow-up, and support underinsured outpatient access. These measures have been shown to reduce readmissions or hospital visits in general dermatologic settings, but outcomes in individual diseases are not well studied,” Dr. Zhang and associates wrote. They noted that there have been “very few prior studies of readmissions for skin diseases.”
rfranki@mdedge.com
SOURCE: Zhang M et al. J Am Acad. Dermatol. 2019. doi: 10.1016/j.jaad.2019.05.023. .
Almost 10% of patients

Data from the Nationwide Readmissions Database also showed that the same-cause readmission rate was 3.3% after 30 days and 7.8% within the calendar year (CY) over the 5-year study period of 2010-2014, Myron Zhang, MD, of the department of dermatology at Weill Cornell Medicine, New York, and his associates reported in the Journal of the American Academy of Dermatology.
The total cost of the CY readmissions was $2.54 billion, which works out to $508 million per year or $8,995 per visit. The most common dermatologic diagnosis – cellulitis made up 83.6% of all hospitalizations – was also the most expensive in terms of readmissions, resulting in $1.9 billion in CY costs, Dr. Zhang and associates wrote.
Overall readmission rates for cellulitis were not provided, but annual rates ranged from 9.1% to 9.3% (30-day all cause), from 7.7% to 8.1% (CY same cause), and from 3.1% to 3.3% (30-day same cause), they wrote.
The dermatologic diagnosis with the highest 30-day same-cause readmission rate was vascular hamartomas at 21.1%, followed by dermatomyositis (18.3%) and thrombotic microangiopathy (13.7%). Dermatomyositis had the highest CY same-cause readmission rate (30.8%) and mycosis fungoides had the highest 30-day all-cause rate (32.3%), according to the investigators.
“Diseases, characteristics, and comorbidities associated with high readmission rates should trigger hospitals to consider dermatology consultation, coordinate outpatient follow-up, and support underinsured outpatient access. These measures have been shown to reduce readmissions or hospital visits in general dermatologic settings, but outcomes in individual diseases are not well studied,” Dr. Zhang and associates wrote. They noted that there have been “very few prior studies of readmissions for skin diseases.”
rfranki@mdedge.com
SOURCE: Zhang M et al. J Am Acad. Dermatol. 2019. doi: 10.1016/j.jaad.2019.05.023. .
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
SEER data show that pancreatic cancer mortality rates differ by ethnicity
SAN DIEGO – .
However, between 2013 and 2015, mortality rates began to fall among all ethnicities, for reasons that remain unclear. “There is still a significant disparity between the [white] and African American populations,” lead study author Mohamed M. Gad, MD, said at the annual Digestive Disease Week. “Trends for the Asian/American Indian populations were similar to that of the [white] population.”
While most patients diagnosed with pancreatic cancer are white men, the disparities among racial and ethnic groups have not been addressed on a large scale, said Dr. Gad, an internal medicine resident at the Cleveland Clinic. In an effort to investigate the racial disparities in mortality rates in patients with pancreatic cancer, he and colleagues collected data from the National Cancer Institute’s Surveillance, Epidemiology and End-Results Program (SEER) 9 database during 1973 to 2015. The researchers calculated mortality rates for all pancreatic cancer cases by race. Next, they calculated the Observed/Expected (O/E) ratio and the excess risk per 10,000 person-years to estimate the change of risk following the diagnosis in different ethnicities, when compared to the general population, using Joinpoint Regression software.
Dr. Gad reported results from 65,985 patients who died from pancreatic cancer between 1973 and 2015. The overall mortality rate was highest for African Americans (9.4%), followed by whites (6.4%), and Asians/American Indians (5.4%). The researchers observed that mortality rates of all racial groups continued to increase from 1973 until 2013. However, between 2013 and 2015, the mortality rates decreased by 20.5% for African Americans, by 27.1% for whites, and by 25.1% for Asians/American Indians (P less than .001 for all decreases). Socioeconomic status and access to health care have been hypothesized to play a role in these observed outcomes, Dr. Gad said, but more research is needed to understand the observed disparities and assess the need for intervention. For example, he said, one line of research would be to evaluate the difference between access to health care and the quality of health care delivery for African American patients compared with their white counterparts. “Once we identify that, how can we improve outcomes for the African American population?” Dr. Gad asked.
He reported having no financial disclosures.
SAN DIEGO – .
However, between 2013 and 2015, mortality rates began to fall among all ethnicities, for reasons that remain unclear. “There is still a significant disparity between the [white] and African American populations,” lead study author Mohamed M. Gad, MD, said at the annual Digestive Disease Week. “Trends for the Asian/American Indian populations were similar to that of the [white] population.”
While most patients diagnosed with pancreatic cancer are white men, the disparities among racial and ethnic groups have not been addressed on a large scale, said Dr. Gad, an internal medicine resident at the Cleveland Clinic. In an effort to investigate the racial disparities in mortality rates in patients with pancreatic cancer, he and colleagues collected data from the National Cancer Institute’s Surveillance, Epidemiology and End-Results Program (SEER) 9 database during 1973 to 2015. The researchers calculated mortality rates for all pancreatic cancer cases by race. Next, they calculated the Observed/Expected (O/E) ratio and the excess risk per 10,000 person-years to estimate the change of risk following the diagnosis in different ethnicities, when compared to the general population, using Joinpoint Regression software.
Dr. Gad reported results from 65,985 patients who died from pancreatic cancer between 1973 and 2015. The overall mortality rate was highest for African Americans (9.4%), followed by whites (6.4%), and Asians/American Indians (5.4%). The researchers observed that mortality rates of all racial groups continued to increase from 1973 until 2013. However, between 2013 and 2015, the mortality rates decreased by 20.5% for African Americans, by 27.1% for whites, and by 25.1% for Asians/American Indians (P less than .001 for all decreases). Socioeconomic status and access to health care have been hypothesized to play a role in these observed outcomes, Dr. Gad said, but more research is needed to understand the observed disparities and assess the need for intervention. For example, he said, one line of research would be to evaluate the difference between access to health care and the quality of health care delivery for African American patients compared with their white counterparts. “Once we identify that, how can we improve outcomes for the African American population?” Dr. Gad asked.
He reported having no financial disclosures.
SAN DIEGO – .
However, between 2013 and 2015, mortality rates began to fall among all ethnicities, for reasons that remain unclear. “There is still a significant disparity between the [white] and African American populations,” lead study author Mohamed M. Gad, MD, said at the annual Digestive Disease Week. “Trends for the Asian/American Indian populations were similar to that of the [white] population.”
While most patients diagnosed with pancreatic cancer are white men, the disparities among racial and ethnic groups have not been addressed on a large scale, said Dr. Gad, an internal medicine resident at the Cleveland Clinic. In an effort to investigate the racial disparities in mortality rates in patients with pancreatic cancer, he and colleagues collected data from the National Cancer Institute’s Surveillance, Epidemiology and End-Results Program (SEER) 9 database during 1973 to 2015. The researchers calculated mortality rates for all pancreatic cancer cases by race. Next, they calculated the Observed/Expected (O/E) ratio and the excess risk per 10,000 person-years to estimate the change of risk following the diagnosis in different ethnicities, when compared to the general population, using Joinpoint Regression software.
Dr. Gad reported results from 65,985 patients who died from pancreatic cancer between 1973 and 2015. The overall mortality rate was highest for African Americans (9.4%), followed by whites (6.4%), and Asians/American Indians (5.4%). The researchers observed that mortality rates of all racial groups continued to increase from 1973 until 2013. However, between 2013 and 2015, the mortality rates decreased by 20.5% for African Americans, by 27.1% for whites, and by 25.1% for Asians/American Indians (P less than .001 for all decreases). Socioeconomic status and access to health care have been hypothesized to play a role in these observed outcomes, Dr. Gad said, but more research is needed to understand the observed disparities and assess the need for intervention. For example, he said, one line of research would be to evaluate the difference between access to health care and the quality of health care delivery for African American patients compared with their white counterparts. “Once we identify that, how can we improve outcomes for the African American population?” Dr. Gad asked.
He reported having no financial disclosures.
REPORTING FROM DDW 2019
Revision total joint replacements linked to higher infection risk
, according to the findings of a large cohort study.
In absolute terms, these rates were 15.6% after revision total knee replacement and 8.6% after revision total hip replacement, vs. 2.1% and 2.1% after the respective primary surgeries, wrote Charles E. Edmiston Jr., PhD, and associates. “This result is consistent with results from several other studies that have reported an increased risk after revision procedures,” they added. Such information is essential for developing care bundles based on presenting risk factors, they wrote in a report published in the American Journal of Infection Control.
Population aging and comorbidities are fueling the need for primary and revision joint replacement surgery. “Such trends highlight the need for solutions to cost-effectively prevent and treat surgical site infections, one of the common complications after total joint replacement procedures,” the researchers wrote. Using IBM MarketScan and Medicare data, they studied 335,134 total knee replacements and 163,547 total hip replacements performed in the United States between 2009 and 2015.
After adjustment for potential confounders, the comorbidities that were most strongly linked to surgical site infections during the 90 days after primary or revision joint replacement were AIDS, paralysis, coagulopathy, metastatic cancer, heart failure, alcohol use disorder, obesity, fluid electrolyte disorders, and chronic pulmonary disorders. The estimated odds ratios for these correlates ranged from 1.33 to 1.58, and all confidence intervals reached statistical significance.
In general, these findings reflect those of smaller studies, the researchers noted. “The challenge for the future will be the development of evidence-based surgical care bundles that focus on the peri-, intra-, and postoperative components of orthopedic patient care, especially in patients undergoing periprosthetic revision.”
Johnson & Johnson Medical Device Companies provided funding. Three coinvestigators reported employment and stockholder ties to the company. In addition, Dr. Edmiston and one coinvestigator reported speaker fees for Ethicon, a Johnson & Johnson company.
SOURCE: Edmiston CE et al. Am J Infect Control. 2019 May 6. doi: 10.1016/j.ajic.2019.03.030.
, according to the findings of a large cohort study.
In absolute terms, these rates were 15.6% after revision total knee replacement and 8.6% after revision total hip replacement, vs. 2.1% and 2.1% after the respective primary surgeries, wrote Charles E. Edmiston Jr., PhD, and associates. “This result is consistent with results from several other studies that have reported an increased risk after revision procedures,” they added. Such information is essential for developing care bundles based on presenting risk factors, they wrote in a report published in the American Journal of Infection Control.
Population aging and comorbidities are fueling the need for primary and revision joint replacement surgery. “Such trends highlight the need for solutions to cost-effectively prevent and treat surgical site infections, one of the common complications after total joint replacement procedures,” the researchers wrote. Using IBM MarketScan and Medicare data, they studied 335,134 total knee replacements and 163,547 total hip replacements performed in the United States between 2009 and 2015.
After adjustment for potential confounders, the comorbidities that were most strongly linked to surgical site infections during the 90 days after primary or revision joint replacement were AIDS, paralysis, coagulopathy, metastatic cancer, heart failure, alcohol use disorder, obesity, fluid electrolyte disorders, and chronic pulmonary disorders. The estimated odds ratios for these correlates ranged from 1.33 to 1.58, and all confidence intervals reached statistical significance.
In general, these findings reflect those of smaller studies, the researchers noted. “The challenge for the future will be the development of evidence-based surgical care bundles that focus on the peri-, intra-, and postoperative components of orthopedic patient care, especially in patients undergoing periprosthetic revision.”
Johnson & Johnson Medical Device Companies provided funding. Three coinvestigators reported employment and stockholder ties to the company. In addition, Dr. Edmiston and one coinvestigator reported speaker fees for Ethicon, a Johnson & Johnson company.
SOURCE: Edmiston CE et al. Am J Infect Control. 2019 May 6. doi: 10.1016/j.ajic.2019.03.030.
, according to the findings of a large cohort study.
In absolute terms, these rates were 15.6% after revision total knee replacement and 8.6% after revision total hip replacement, vs. 2.1% and 2.1% after the respective primary surgeries, wrote Charles E. Edmiston Jr., PhD, and associates. “This result is consistent with results from several other studies that have reported an increased risk after revision procedures,” they added. Such information is essential for developing care bundles based on presenting risk factors, they wrote in a report published in the American Journal of Infection Control.
Population aging and comorbidities are fueling the need for primary and revision joint replacement surgery. “Such trends highlight the need for solutions to cost-effectively prevent and treat surgical site infections, one of the common complications after total joint replacement procedures,” the researchers wrote. Using IBM MarketScan and Medicare data, they studied 335,134 total knee replacements and 163,547 total hip replacements performed in the United States between 2009 and 2015.
After adjustment for potential confounders, the comorbidities that were most strongly linked to surgical site infections during the 90 days after primary or revision joint replacement were AIDS, paralysis, coagulopathy, metastatic cancer, heart failure, alcohol use disorder, obesity, fluid electrolyte disorders, and chronic pulmonary disorders. The estimated odds ratios for these correlates ranged from 1.33 to 1.58, and all confidence intervals reached statistical significance.
In general, these findings reflect those of smaller studies, the researchers noted. “The challenge for the future will be the development of evidence-based surgical care bundles that focus on the peri-, intra-, and postoperative components of orthopedic patient care, especially in patients undergoing periprosthetic revision.”
Johnson & Johnson Medical Device Companies provided funding. Three coinvestigators reported employment and stockholder ties to the company. In addition, Dr. Edmiston and one coinvestigator reported speaker fees for Ethicon, a Johnson & Johnson company.
SOURCE: Edmiston CE et al. Am J Infect Control. 2019 May 6. doi: 10.1016/j.ajic.2019.03.030.
FROM THE AMERICAN JOURNAL OF INFECTION CONTROL
mTORC1 inhibitor protects elderly asthmatics from viral respiratory tract infections
DALLAS – A molecule that boosts innate viral immunity may protect elderly people with asthma from the root cause of most exacerbations – viral respiratory tract infections.
Dubbed RTB101, the oral medication is a selective, potent inhibitor of target of rapamycin complex 1 (TORC1). In phase 2b data presented at the American Thoracic Society’s international conference, RTB101 decreased by 52% the number of elderly subjects with severe, lab-confirmed respiratory tract infections (RTI) symptoms.
But the molecule was even more effective in patients with asthma aged 65 years and older, Joan Mannick, MD, said in an interview during the meeting. In this group, it reduced by 69% the percentage of subjects who developed RTIs and reduced the rate of infection by about 79%, compared with placebo.
“The core cause of asthma exacerbations in these patients is viral respiratory tract infection,” said Dr. Mannick, chief medical officer of resTORbio, the Boston company developing RTB101. “About 80% of the viruses detected in these infections are rhinoviruses, and there are 170 rhinovirus serotypes. We have never been able to develop a vaccine against rhinovirus, and we have no treatment other than to treat the inflammation caused by the infection.”
Centers for Disease Control and Prevention mortality records confirm the impact of viral respiratory infections on older people who experience asthma exacerbations: 6 of 10,000 will die, compared with less than 2 per 10,000 for all other age groups. Decreasing the number of these infections in older people with asthma would prevent morbidity and mortality and save considerable health care dollars.
“One of the reasons that asthmatics have such difficulty when they get respiratory infections is that they seem to have deficient antiviral immunity in the airways,” Dr. Mannick said. She pointed to a 2008 study of bronchial epithelial cells from both patients with asthma and healthy controls. When inoculated with rhinovirus, the cells from asthmatic airways were unable to mount a healthy immune response and were particularly deficient in producing interferon-beta.
By inhibiting mammalian TORC1 (mTORC1), RBT101 also inhibits sterol regulatory element binding transcription factor 2, a pathway that influences cholesterol synthesis. Cells perceive cholesterol synthesis attenuation as a threat, Dr. Mannick said, and react by up-regulating a number of immune response genes – including some specifically antiviral genes that up-regulate interferon-alpha and -beta production and immune cytokine signaling pathways.
RTB101 is not a particularly new molecule; Novartis originally investigated it as an anticancer agent. “It failed, because it was too selective for mTORC1,” Dr. Mannick said. After Novartis dropped the molecule, resTORbio, a Novartis spin-off, began to investigate it as an immunotherapy for RTIs, particularly in patients with asthma.
reSTORbio’s phase 2 studies on RTB101 comprised 264 healthy subjects aged 65 years and older, who received placebo or 10 mg RTB101 daily for 6 weeks, during cold and flu season. They were followed for a year, confirming the antiviral gene up-regulation. Treatment was also associated with a 42% reduction in the rate of respiratory tract infections.
Conversations with the Food and Drug Administration and payers collected, Dr. Mannick said. “They said that where this drug could really make a difference was if it could decrease these infections in high-risk elderly, who are expensive to treat. So, we targeted people 65 years and older with asthma, chronic obstructive pulmonary disease, and smokers, and people who are 85 years or older.”
The phase 2b trial comprised 652 of these elderly high-risk subjects randomized to the following treatment arms: RTB101 5 mg once daily (n = 61), RTB101 10 mg once daily (n = 176), RTB101 10 mg b.i.d. (n = 120), RTB101 10 mg plus everolimus 0.1 mg daily (n = 115), or matching placebo (n = 180) over 16 weeks, during the entire cold and flu season. The primary endpoint was laboratory-confirmed RTIs in all groups.
The RTB101 10-mg, once-daily group had the best results with a 30.6% reduction in the percentage of patients with lab-confirmed RTIs, compared with placebo, and a 52% reduction in the percentage with severe symptoms.
A subgroup analysis found even more benefit to those with asthma. Among these patients, RTB101 effected a 58.2% decrease in patients with RTIs, and a 66.4% decrease in the rate of infections, compared with placebo.
RTB101 was most effective against rhinoviruses, but it also prevented RTIs associated with influenza A and coronavirus OC43. It also decreased the incidence of RTIs caused by respiratory syncytial virus, parainfluenza 4, influenza B, metapneumovirus, or other coronavirus serotypes.
There were no safety signals, Dr. Mannick noted. Adverse events were similar in both placebo and active groups, and none were deemed related to the study drug. About 5% of each group discontinued the drug because an adverse event.
Plans for a phase 3 trial are underway. A phase 3, placebo-controlled study in the Southern Hemisphere is now ongoing, during the winter cold and flu season. The Northern Hemisphere phase 3 will commence fall and winter of 2019.
Whether RBT101 can help younger people with asthma is an open question. Elderly patients not only have the asthma-related immune deficiency, but also the general age-related immune issues. Younger patients, however, still express the same asthma-related impairment of bronchial immunity.
“We would like to investigate this in younger people and in children, but that will have to wait until our other phase 3 studies are complete,” Dr. Mannick said.
The trial was sponsored by resTORbio.
SOURCE: Mannick J et al. ATS 2019, Abstract A2623.
CORRECTION 5/24/2019 The article was corrected to state a decreased the incidence of RTIs caused by respiratory syncytial virus, parainfluenza 4, influenza B, metapneumovirus, or other coronavirus serotypes.
DALLAS – A molecule that boosts innate viral immunity may protect elderly people with asthma from the root cause of most exacerbations – viral respiratory tract infections.
Dubbed RTB101, the oral medication is a selective, potent inhibitor of target of rapamycin complex 1 (TORC1). In phase 2b data presented at the American Thoracic Society’s international conference, RTB101 decreased by 52% the number of elderly subjects with severe, lab-confirmed respiratory tract infections (RTI) symptoms.
But the molecule was even more effective in patients with asthma aged 65 years and older, Joan Mannick, MD, said in an interview during the meeting. In this group, it reduced by 69% the percentage of subjects who developed RTIs and reduced the rate of infection by about 79%, compared with placebo.
“The core cause of asthma exacerbations in these patients is viral respiratory tract infection,” said Dr. Mannick, chief medical officer of resTORbio, the Boston company developing RTB101. “About 80% of the viruses detected in these infections are rhinoviruses, and there are 170 rhinovirus serotypes. We have never been able to develop a vaccine against rhinovirus, and we have no treatment other than to treat the inflammation caused by the infection.”
Centers for Disease Control and Prevention mortality records confirm the impact of viral respiratory infections on older people who experience asthma exacerbations: 6 of 10,000 will die, compared with less than 2 per 10,000 for all other age groups. Decreasing the number of these infections in older people with asthma would prevent morbidity and mortality and save considerable health care dollars.
“One of the reasons that asthmatics have such difficulty when they get respiratory infections is that they seem to have deficient antiviral immunity in the airways,” Dr. Mannick said. She pointed to a 2008 study of bronchial epithelial cells from both patients with asthma and healthy controls. When inoculated with rhinovirus, the cells from asthmatic airways were unable to mount a healthy immune response and were particularly deficient in producing interferon-beta.
By inhibiting mammalian TORC1 (mTORC1), RBT101 also inhibits sterol regulatory element binding transcription factor 2, a pathway that influences cholesterol synthesis. Cells perceive cholesterol synthesis attenuation as a threat, Dr. Mannick said, and react by up-regulating a number of immune response genes – including some specifically antiviral genes that up-regulate interferon-alpha and -beta production and immune cytokine signaling pathways.
RTB101 is not a particularly new molecule; Novartis originally investigated it as an anticancer agent. “It failed, because it was too selective for mTORC1,” Dr. Mannick said. After Novartis dropped the molecule, resTORbio, a Novartis spin-off, began to investigate it as an immunotherapy for RTIs, particularly in patients with asthma.
reSTORbio’s phase 2 studies on RTB101 comprised 264 healthy subjects aged 65 years and older, who received placebo or 10 mg RTB101 daily for 6 weeks, during cold and flu season. They were followed for a year, confirming the antiviral gene up-regulation. Treatment was also associated with a 42% reduction in the rate of respiratory tract infections.
Conversations with the Food and Drug Administration and payers collected, Dr. Mannick said. “They said that where this drug could really make a difference was if it could decrease these infections in high-risk elderly, who are expensive to treat. So, we targeted people 65 years and older with asthma, chronic obstructive pulmonary disease, and smokers, and people who are 85 years or older.”
The phase 2b trial comprised 652 of these elderly high-risk subjects randomized to the following treatment arms: RTB101 5 mg once daily (n = 61), RTB101 10 mg once daily (n = 176), RTB101 10 mg b.i.d. (n = 120), RTB101 10 mg plus everolimus 0.1 mg daily (n = 115), or matching placebo (n = 180) over 16 weeks, during the entire cold and flu season. The primary endpoint was laboratory-confirmed RTIs in all groups.
The RTB101 10-mg, once-daily group had the best results with a 30.6% reduction in the percentage of patients with lab-confirmed RTIs, compared with placebo, and a 52% reduction in the percentage with severe symptoms.
A subgroup analysis found even more benefit to those with asthma. Among these patients, RTB101 effected a 58.2% decrease in patients with RTIs, and a 66.4% decrease in the rate of infections, compared with placebo.
RTB101 was most effective against rhinoviruses, but it also prevented RTIs associated with influenza A and coronavirus OC43. It also decreased the incidence of RTIs caused by respiratory syncytial virus, parainfluenza 4, influenza B, metapneumovirus, or other coronavirus serotypes.
There were no safety signals, Dr. Mannick noted. Adverse events were similar in both placebo and active groups, and none were deemed related to the study drug. About 5% of each group discontinued the drug because an adverse event.
Plans for a phase 3 trial are underway. A phase 3, placebo-controlled study in the Southern Hemisphere is now ongoing, during the winter cold and flu season. The Northern Hemisphere phase 3 will commence fall and winter of 2019.
Whether RBT101 can help younger people with asthma is an open question. Elderly patients not only have the asthma-related immune deficiency, but also the general age-related immune issues. Younger patients, however, still express the same asthma-related impairment of bronchial immunity.
“We would like to investigate this in younger people and in children, but that will have to wait until our other phase 3 studies are complete,” Dr. Mannick said.
The trial was sponsored by resTORbio.
SOURCE: Mannick J et al. ATS 2019, Abstract A2623.
CORRECTION 5/24/2019 The article was corrected to state a decreased the incidence of RTIs caused by respiratory syncytial virus, parainfluenza 4, influenza B, metapneumovirus, or other coronavirus serotypes.
DALLAS – A molecule that boosts innate viral immunity may protect elderly people with asthma from the root cause of most exacerbations – viral respiratory tract infections.
Dubbed RTB101, the oral medication is a selective, potent inhibitor of target of rapamycin complex 1 (TORC1). In phase 2b data presented at the American Thoracic Society’s international conference, RTB101 decreased by 52% the number of elderly subjects with severe, lab-confirmed respiratory tract infections (RTI) symptoms.
But the molecule was even more effective in patients with asthma aged 65 years and older, Joan Mannick, MD, said in an interview during the meeting. In this group, it reduced by 69% the percentage of subjects who developed RTIs and reduced the rate of infection by about 79%, compared with placebo.
“The core cause of asthma exacerbations in these patients is viral respiratory tract infection,” said Dr. Mannick, chief medical officer of resTORbio, the Boston company developing RTB101. “About 80% of the viruses detected in these infections are rhinoviruses, and there are 170 rhinovirus serotypes. We have never been able to develop a vaccine against rhinovirus, and we have no treatment other than to treat the inflammation caused by the infection.”
Centers for Disease Control and Prevention mortality records confirm the impact of viral respiratory infections on older people who experience asthma exacerbations: 6 of 10,000 will die, compared with less than 2 per 10,000 for all other age groups. Decreasing the number of these infections in older people with asthma would prevent morbidity and mortality and save considerable health care dollars.
“One of the reasons that asthmatics have such difficulty when they get respiratory infections is that they seem to have deficient antiviral immunity in the airways,” Dr. Mannick said. She pointed to a 2008 study of bronchial epithelial cells from both patients with asthma and healthy controls. When inoculated with rhinovirus, the cells from asthmatic airways were unable to mount a healthy immune response and were particularly deficient in producing interferon-beta.
By inhibiting mammalian TORC1 (mTORC1), RBT101 also inhibits sterol regulatory element binding transcription factor 2, a pathway that influences cholesterol synthesis. Cells perceive cholesterol synthesis attenuation as a threat, Dr. Mannick said, and react by up-regulating a number of immune response genes – including some specifically antiviral genes that up-regulate interferon-alpha and -beta production and immune cytokine signaling pathways.
RTB101 is not a particularly new molecule; Novartis originally investigated it as an anticancer agent. “It failed, because it was too selective for mTORC1,” Dr. Mannick said. After Novartis dropped the molecule, resTORbio, a Novartis spin-off, began to investigate it as an immunotherapy for RTIs, particularly in patients with asthma.
reSTORbio’s phase 2 studies on RTB101 comprised 264 healthy subjects aged 65 years and older, who received placebo or 10 mg RTB101 daily for 6 weeks, during cold and flu season. They were followed for a year, confirming the antiviral gene up-regulation. Treatment was also associated with a 42% reduction in the rate of respiratory tract infections.
Conversations with the Food and Drug Administration and payers collected, Dr. Mannick said. “They said that where this drug could really make a difference was if it could decrease these infections in high-risk elderly, who are expensive to treat. So, we targeted people 65 years and older with asthma, chronic obstructive pulmonary disease, and smokers, and people who are 85 years or older.”
The phase 2b trial comprised 652 of these elderly high-risk subjects randomized to the following treatment arms: RTB101 5 mg once daily (n = 61), RTB101 10 mg once daily (n = 176), RTB101 10 mg b.i.d. (n = 120), RTB101 10 mg plus everolimus 0.1 mg daily (n = 115), or matching placebo (n = 180) over 16 weeks, during the entire cold and flu season. The primary endpoint was laboratory-confirmed RTIs in all groups.
The RTB101 10-mg, once-daily group had the best results with a 30.6% reduction in the percentage of patients with lab-confirmed RTIs, compared with placebo, and a 52% reduction in the percentage with severe symptoms.
A subgroup analysis found even more benefit to those with asthma. Among these patients, RTB101 effected a 58.2% decrease in patients with RTIs, and a 66.4% decrease in the rate of infections, compared with placebo.
RTB101 was most effective against rhinoviruses, but it also prevented RTIs associated with influenza A and coronavirus OC43. It also decreased the incidence of RTIs caused by respiratory syncytial virus, parainfluenza 4, influenza B, metapneumovirus, or other coronavirus serotypes.
There were no safety signals, Dr. Mannick noted. Adverse events were similar in both placebo and active groups, and none were deemed related to the study drug. About 5% of each group discontinued the drug because an adverse event.
Plans for a phase 3 trial are underway. A phase 3, placebo-controlled study in the Southern Hemisphere is now ongoing, during the winter cold and flu season. The Northern Hemisphere phase 3 will commence fall and winter of 2019.
Whether RBT101 can help younger people with asthma is an open question. Elderly patients not only have the asthma-related immune deficiency, but also the general age-related immune issues. Younger patients, however, still express the same asthma-related impairment of bronchial immunity.
“We would like to investigate this in younger people and in children, but that will have to wait until our other phase 3 studies are complete,” Dr. Mannick said.
The trial was sponsored by resTORbio.
SOURCE: Mannick J et al. ATS 2019, Abstract A2623.
CORRECTION 5/24/2019 The article was corrected to state a decreased the incidence of RTIs caused by respiratory syncytial virus, parainfluenza 4, influenza B, metapneumovirus, or other coronavirus serotypes.
REPORTING FROM ATS 2019
Coffee, tea, and soda all up GERD risk
SAN DIEGO – .
In an interview following the oral presentation, Raaj S. Mehta, MD, said that patients in his primary care panel at Massachusetts General Hospital, Boston, where he’s a senior resident, frequently came to him with GERD. In addition to questions about diet, patients frequently wanted to know which beverages might provoke or exacerbate their GERD.
In trying to help his patients, Dr. Mehta said he realized that there wasn’t a prospective evidence base to answer their questions about beverages and GERD, so he and his colleagues used data from the Nurses’ Health Study II (NHS II), a prospective cohort study, to look at the association between various beverages and the incidence of GERD.
“What’s exciting is that we were able to find that coffee, tea, and soda – all three – increase your risk for gastroesophageal reflux disease,” Dr. Mehta said in a video interview. “At the highest quintile level, so looking at people who consume six or more cups per day, you’re looking at maybe a 25%-35% increase in risk of reflux disease.”
There was a dose-response relationship as well: “You do see a slight increase as you go from one cup, to two, to three, and so on, all the way up to six cups” of the offending beverages, said Dr. Mehta.
Overall, the risk for GERD rose from 1.17 to 1.34 with coffee consumption as servings per day increased from less than one to six or more (P for trend less than .0001). Tea consumption was associated with increased GERD risk ranging from 1.08 to 1.26 as consumption rose (P for trend .001). For soda, the increased risk went from 1.12 at less than one serving daily, to 1.41 at four to five servings daily, and then fell to 1.29 at six or more daily servings (P for trend less than .0001).
Whether the beverages were caffeinated or not, said Dr. Mehta, only made a “minimal difference” in GERD risk.
“In contrast, we didn’t see an association for beverages like water, juice, and milk,” he said – reassuring findings in light of fruit juice’s anecdotal status as a GERD culprit.
The NHS II collected data every 2 years from 48,308 female nurses aged 42-62 years at the beginning of the study. Every 4 years dietary information was collected, and on the opposite 4-year cycle, participants answered questions about GERD. Medication use, including the incident use of proton pump inhibitors, was collected every 2 years.
Patients with baseline GERD or use of PPIs or H2 receptor antagonists were excluded from participation.
The quantity and type of beverages were assessed by food frequency questionnaires; other demographic, dietary, and medication variables were also gathered and used to adjust the statistical analysis.
A substitution analysis answered the “what-if” question of the effect of substituting two glasses of plain water daily for either coffee, tea, or soda. Dr. Mehta and colleagues saw a modest reduction in risk for GERD with this strategy.
In addition to the prospective nature of the study (abstract 514, doi: 10.1016/S0016-5085(19)37044-1), the large sample size, high follow-up rates, and well validated dietary data were all strengths, said Dr. Mehta. However, the study’s population is relatively homogeneous, and residual confounding couldn’t be excluded. Also, GERD was defined by self-report, though participants were asked to respond to clear, validated criteria.
For Dr. Mehta, he’s glad to have a clear answer to a common clinic question. “I think that this is one additional thing that I can recommend as a primary care provider to my patients when they come into my office,” he said.
Dr. Mehta reported no conflicts of interest.
SAN DIEGO – .
In an interview following the oral presentation, Raaj S. Mehta, MD, said that patients in his primary care panel at Massachusetts General Hospital, Boston, where he’s a senior resident, frequently came to him with GERD. In addition to questions about diet, patients frequently wanted to know which beverages might provoke or exacerbate their GERD.
In trying to help his patients, Dr. Mehta said he realized that there wasn’t a prospective evidence base to answer their questions about beverages and GERD, so he and his colleagues used data from the Nurses’ Health Study II (NHS II), a prospective cohort study, to look at the association between various beverages and the incidence of GERD.
“What’s exciting is that we were able to find that coffee, tea, and soda – all three – increase your risk for gastroesophageal reflux disease,” Dr. Mehta said in a video interview. “At the highest quintile level, so looking at people who consume six or more cups per day, you’re looking at maybe a 25%-35% increase in risk of reflux disease.”
There was a dose-response relationship as well: “You do see a slight increase as you go from one cup, to two, to three, and so on, all the way up to six cups” of the offending beverages, said Dr. Mehta.
Overall, the risk for GERD rose from 1.17 to 1.34 with coffee consumption as servings per day increased from less than one to six or more (P for trend less than .0001). Tea consumption was associated with increased GERD risk ranging from 1.08 to 1.26 as consumption rose (P for trend .001). For soda, the increased risk went from 1.12 at less than one serving daily, to 1.41 at four to five servings daily, and then fell to 1.29 at six or more daily servings (P for trend less than .0001).
Whether the beverages were caffeinated or not, said Dr. Mehta, only made a “minimal difference” in GERD risk.
“In contrast, we didn’t see an association for beverages like water, juice, and milk,” he said – reassuring findings in light of fruit juice’s anecdotal status as a GERD culprit.
The NHS II collected data every 2 years from 48,308 female nurses aged 42-62 years at the beginning of the study. Every 4 years dietary information was collected, and on the opposite 4-year cycle, participants answered questions about GERD. Medication use, including the incident use of proton pump inhibitors, was collected every 2 years.
Patients with baseline GERD or use of PPIs or H2 receptor antagonists were excluded from participation.
The quantity and type of beverages were assessed by food frequency questionnaires; other demographic, dietary, and medication variables were also gathered and used to adjust the statistical analysis.
A substitution analysis answered the “what-if” question of the effect of substituting two glasses of plain water daily for either coffee, tea, or soda. Dr. Mehta and colleagues saw a modest reduction in risk for GERD with this strategy.
In addition to the prospective nature of the study (abstract 514, doi: 10.1016/S0016-5085(19)37044-1), the large sample size, high follow-up rates, and well validated dietary data were all strengths, said Dr. Mehta. However, the study’s population is relatively homogeneous, and residual confounding couldn’t be excluded. Also, GERD was defined by self-report, though participants were asked to respond to clear, validated criteria.
For Dr. Mehta, he’s glad to have a clear answer to a common clinic question. “I think that this is one additional thing that I can recommend as a primary care provider to my patients when they come into my office,” he said.
Dr. Mehta reported no conflicts of interest.
SAN DIEGO – .
In an interview following the oral presentation, Raaj S. Mehta, MD, said that patients in his primary care panel at Massachusetts General Hospital, Boston, where he’s a senior resident, frequently came to him with GERD. In addition to questions about diet, patients frequently wanted to know which beverages might provoke or exacerbate their GERD.
In trying to help his patients, Dr. Mehta said he realized that there wasn’t a prospective evidence base to answer their questions about beverages and GERD, so he and his colleagues used data from the Nurses’ Health Study II (NHS II), a prospective cohort study, to look at the association between various beverages and the incidence of GERD.
“What’s exciting is that we were able to find that coffee, tea, and soda – all three – increase your risk for gastroesophageal reflux disease,” Dr. Mehta said in a video interview. “At the highest quintile level, so looking at people who consume six or more cups per day, you’re looking at maybe a 25%-35% increase in risk of reflux disease.”
There was a dose-response relationship as well: “You do see a slight increase as you go from one cup, to two, to three, and so on, all the way up to six cups” of the offending beverages, said Dr. Mehta.
Overall, the risk for GERD rose from 1.17 to 1.34 with coffee consumption as servings per day increased from less than one to six or more (P for trend less than .0001). Tea consumption was associated with increased GERD risk ranging from 1.08 to 1.26 as consumption rose (P for trend .001). For soda, the increased risk went from 1.12 at less than one serving daily, to 1.41 at four to five servings daily, and then fell to 1.29 at six or more daily servings (P for trend less than .0001).
Whether the beverages were caffeinated or not, said Dr. Mehta, only made a “minimal difference” in GERD risk.
“In contrast, we didn’t see an association for beverages like water, juice, and milk,” he said – reassuring findings in light of fruit juice’s anecdotal status as a GERD culprit.
The NHS II collected data every 2 years from 48,308 female nurses aged 42-62 years at the beginning of the study. Every 4 years dietary information was collected, and on the opposite 4-year cycle, participants answered questions about GERD. Medication use, including the incident use of proton pump inhibitors, was collected every 2 years.
Patients with baseline GERD or use of PPIs or H2 receptor antagonists were excluded from participation.
The quantity and type of beverages were assessed by food frequency questionnaires; other demographic, dietary, and medication variables were also gathered and used to adjust the statistical analysis.
A substitution analysis answered the “what-if” question of the effect of substituting two glasses of plain water daily for either coffee, tea, or soda. Dr. Mehta and colleagues saw a modest reduction in risk for GERD with this strategy.
In addition to the prospective nature of the study (abstract 514, doi: 10.1016/S0016-5085(19)37044-1), the large sample size, high follow-up rates, and well validated dietary data were all strengths, said Dr. Mehta. However, the study’s population is relatively homogeneous, and residual confounding couldn’t be excluded. Also, GERD was defined by self-report, though participants were asked to respond to clear, validated criteria.
For Dr. Mehta, he’s glad to have a clear answer to a common clinic question. “I think that this is one additional thing that I can recommend as a primary care provider to my patients when they come into my office,” he said.
Dr. Mehta reported no conflicts of interest.
REPORTING FROM DDW 2019
Lambert-Eaton Myasthenic Syndrome and Merkel Cell Carcinoma
Merkel cell carcinoma (MCC) is an aggressive neuroendocrine malignancy of the skin that is thought to arise from neural crest cells. It has an estimated annual incidence of 0.6 per 100,000 individuals, typically occurs in the elderly population, and is most common in white males.1 The tumor presents as a rapidly growing, violaceous nodule in sun-exposed areas of the skin; early in the course, it can be mistaken for a benign entity such as an epidermal cyst.2 Merkel cell carcinoma has a propensity to spread to regional lymph nodes, and in some cases, it occurs in the absence of skin findings.3 Histologically, MCC is nearly indistinguishable from small cell lung carcinoma (SCLC).4 The overall prognosis for patients with MCC is poor and largely dependent on the stage at diagnosis. Patients with regional and distant metastases have a 5-year survival rate of 26% to 42% and 18%, respectively.3
Lambert-Eaton myasthenic syndrome (LEMS) is a paraneoplastic or autoimmune disorder of the neuromuscular junction that is found in 3% of cases of SCLC.4 Reported cases of LEMS in patients with MCC are exceedingly rare.5-8 We provide a full report and longitudinal clinical follow-up of a case that was briefly discussed by Simmons et al,8 and we review the literature regarding paraneoplastic syndromes associated with MCC and other extrapulmonary small cell carcinomas (EPSCCs).
Case Report
A 63-year-old man was evaluated in the neurology clinic due to difficulty walking, climbing stairs, and performing push-ups over the last month. Prior to the onset of symptoms, he was otherwise healthy, walking 3 miles daily; however, at presentation he required use of a cane. Leg weakness worsened as the day progressed. In addition, he reported constipation, urinary urgency, dry mouth, mild dysphagia, reduced sensation below the knees, and a nasal quality in his speech. He had no ptosis, diplopia, dysarthria, muscle cramps, myalgia, or facial weakness. He denied fevers, chills, and night sweats but did admit to an unintentional 10- to 15-lb weight loss over the preceding few months.
The neurologic examination revealed mild proximal upper extremity weakness in the bilateral shoulder abductors, infraspinatus, hip extensors, and hip flexors (Medical Research Council muscle scale grade 4). All deep tendon reflexes, except the Achilles reflex, were present. Despite subjective sensory concerns, objective examination of all sensory modalities was normal. Cranial nerve examination was normal, except for a slight nasal quality to his voice.
A qualitative assay was positive for the presence of P/Q-type voltage-gated calcium channel (VGCC) antibodies. Other laboratory studies were within reference range, including acetylcholine-receptor antibodies (blocking, binding, and modulating) and muscle-specific kinase antibodies.
Lumbar and cervical spine magnetic resonance imaging revealed multilevel neuroforaminal stenosis without spinal canal stenosis or myelopathy. Computed tomography (CT) of the chest was notable for 2 pathologically enlarged lymph nodes in the left axilla and no evidence of primary pulmonary malignancy. Nerve-conduction studies (NCSs) in conjunction with other clinical findings were consistent with the diagnosis of LEMS.
Ultrasound-guided biopsy of the enlarged axillary lymph nodes demonstrated sheets and nests of small round blue tumor cells with minimal cytoplasm, high mitotic rate, and foci of necrosis (Figure 1). The tumor cells were positive for pancytokeratin (Lu-5) and cytokeratin (CK) 20 in a perinuclear dotlike pattern (Figure 2), as well as for the neuroendocrine markers synaptophysin (Figure 3), chromogranin A, and CD56. The tumor cells showed no immunoreactivity for CK7, thyroid transcription factor 1, CD3, CD5, or CD20. Flow cytometry demonstrated low cellularity, low specimen viability, and no evidence of an abnormal B-cell population. These findings were consistent with the diagnosis of MCC.
The patient underwent surgical excision of the involved lymph nodes. Four weeks after surgery, he reported dramatic improvement in strength, with complete resolution of the nasal speech, dysphagia, dry mouth, urinary retention, and constipation. Two months after surgery, his strength had normalized, except for slight persistent weakness in the bilateral shoulder abductors, trace weakness in the hip flexors, and a slight Trendelenburg gait. He was able to rise from a chair without using his arms and no longer required a cane for ambulation.
The patient underwent adjuvant radiation therapy after 2-month surgical follow-up with 5000-cGy radiation treatment to the left axillary region. Six months following primary definitive surgery and 4 months following adjuvant radiation therapy, he reported a 95% subjective return of physical strength. The patient was able to return to near-baseline physical activity. He continued to deny symptoms of dry mouth, incontinence, or constipation. Objectively, he had no focal neurologic deficits or weakness; no evidence of new skin lesions or lymphadenopathy was noted.
Comment
MCC vs SCLC
Merkel cell carcinoma is classified as a type of EPSCC. The histologic appearance of MCC is indistinguishable from SCLC. Both tumors are composed of uniform sheets of small round cells with a high nucleus to cytoplasm ratio, and both can express neuroendocrine markers, such as neuron-specific enolase, chromogranin A, and synaptophysin.9 Immunohistochemical positivity for CK20 and neurofilaments in combination with negative staining for thyroid transcription factor 1 and CK7 effectively differentiate MCC from SCLC.9 In addition, MCC often displays CK20 positivity in a perinuclear dotlike or punctate pattern, which is characteristic of this tumor.3,9,10 Negative immunohistochemical markers for B cells (CD20) and T cells (CD3) are important in excluding lymphoma.
LEMS Diagnosis
Lambert-Eaton myasthenic syndrome is a paraneoplastic or autoimmune disorder involving the neuromuscular junction. Autoantibodies to VGCC impair calcium influx into the presynaptic terminal, resulting in marked reduction of acetylcholine release into the synaptic cleft. The reduction in acetylcholine activity impairs production of muscle fiber action potentials, resulting in clinical weakness. The diagnosis of LEMS rests on clinical presentation, positive serology, and confirmatory neurophysiologic testing by NCS. Clinically, patients present with proximal weakness, hyporeflexia or areflexia, and autonomic dysfunction. Antibodies to P/Q-type VGCCs are found in 85% to 90% of cases of LEMS and are thought to play a direct causative role in the development of weakness.11 The finding of postexercise facilitation on motor NCS is the neurophysiologic hallmark and is highly specific for the diagnosis.
Approximately 50% to 60% of patients who present with LEMS have an underlying tumor, the vast majority of which are SCLC.11 There are a few reports of LEMS associated with other malignancies, including lymphoma; thymoma; neuroblastoma; and carcinoma of the breast, stomach, prostate, bladder, kidney, and gallbladder.12 Patients with nontumor or autoimmune LEMS tend to be younger, and there is no male predominance, as there is in paraneoplastic LEMS.13 Given the risk of underlying malignancy in LEMS, Titulaer et al14 proposed a screening protocol for patients presenting with LEMS, recommending initial primary screening using CT of the chest. If the CT scan is negative, total-body fludeoxyglucose positron emission tomography should be performed to assess for fludeoxyglucose avid lesions. If both initial studies are negative, routine follow-up with CT of the chest at 6-month intervals for a minimum of 2 to 4 years after the initial diagnosis of LEMS was recommended. An exception to this protocol was suggested to allow consideration to stop screening after the first 6-month follow-up chest CT for patients younger than 45 years who have never smoked and who have an HLA 8.1 haplotype for which nontumor LEMS would be a more probable diagnosis.14
In addition to a screening protocol, a validated prediction tool, the Dutch-English LEMS Tumor Association prediction score, was developed. It uses common signs and symptoms of LEMS and risk factors for SCLC to help guide the need for further screening.15
Paraneoplastic Syndromes Associated With MCC
Other paraneoplastic syndromes have been reported in association with MCC. A patient with brainstem encephalitis associated with MCC was reported in a trial of a novel immunotherapy for paraneoplastic neurologic syndromes.16,17 A syndrome of inappropriate antidiuretic hormone (SIADH) secretion was reported in a patient with N-type calcium channel antibodies.18 Two cases of paraneoplastic cerebellar degeneration have been reported; the first was associated with a novel 70-kD antibody,19 and the second was associated with the P/Q-type VGCC antibody.20 Anti-Hu antibodies have been found in a handful of reports of neurologic deterioration in patients with MCC. Hocar et al21 reported a severe necrotizing myopathy; Greenlee et al22 described a syndrome of progressive sensorimotor and autonomic neuropathy with encephalopathy; and Lopez et al23 described a constellation of vision changes, gait imbalance, and proximal weakness. Support for a pathophysiologic connection among these 3 cases is suggested by the finding of Hu antigen expression by MCC in 2 studies.24,25 Because MCC can present with occult lymph node involvement in the absence of primary cutaneous findings,3 there are more cases of paraneoplastic neurologic syndromes that were not recognized.
Extrapulmonary small cell carcinomas such as MCC are morphologically indistinguishable from their pulmonary counterparts and have been reported in most anatomic regions of the body, including gynecologic organs (eg, ovaries, cervix), genitourinary organs (eg, bladder, prostate), the gastrointestinal tract (eg, esophagus), skin (eg, MCC), and the head and neck region. Extrapulmonary small cell carcinoma is a rare entity, with the most common form found in the gynecologic tract, representing only 2% of gynecologic malignancies.26
Paraneoplastic syndromes of EPSCC are rare given the paucity of the malignancy. Several case reports discuss findings of SIADH in EPSCC of the cervix, as well as hypercalcemia, polyneuropathy, Cushing syndrome, limbic encephalitis, and peripheral neuropathy in EPSCC of the prostate.27,28 In contrast, SCLC has long been associated with paraneoplastic syndromes. Numerous case reports have been published describing SCLC-associated paraneoplastic syndromes to include hypercalcemia, Cushing syndrome, SIADH, vasoactive peptide production, cerebellar degeneration, limbic encephalitis, visceral plexopathy, autonomic dysfunction, and LEMS.29 As more cases of EPSCC with paraneoplastic syndromes are identified and reported, we might gain a better understanding of this interesting phenomenon.
Conclusion
Merkel cell carcinoma is an aggressive neuroendocrine malignancy associated with paraneoplastic neurologic syndromes, including LEMS. A thorough search for an underlying malignancy is highly recommended in patients with diagnosed LEMS without clear cause. Early identification and treatment of the primary tumor can lead to improvement of neurologic symptoms.
We present a case of LEMS with no clearly identifiable cause on presentation with later diagnosis of metastatic MCC of unknown primary origin. After surgical excision of affected lymph nodes and adjuvant radiation therapy, the patient had near-complete resolution of LEMS symptoms at 6-month follow-up, without additional findings of lymphadenopathy or skin lesions. Although this patient is not undergoing routine surveillance imaging to monitor for recurrence of MCC, a chest CT or positron emission tomography–CT for secondary screening would be considered if the patient experienced clinical symptoms consistent with LEMS.
In cases of LEMS without pulmonary malignancy, we recommend considering MCC in the differential diagnosis during the workup of an underlying malignancy
- Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2010;37:20-27.
- Senchenkov A, Moran SL. Merkel cell carcinoma: diagnosis, management, and outcomes. Plast Reconstr Surg. 2013;131:E771-E778.
- Han SY, North JP, Canavan T, et al. Merkel cell carcinoma. Hematol Oncol Clin N Am. 2012;26:1351-1374.
- Vernino S. Paraneoplastic disorders affecting the neuromuscular junction or anterior horn cell. CONTINUUM Lifelong Learning in Neurology. 2009;15:132-146.
- Eggers SD, Salomao DR, Dinapoli RP, et al. Paraneoplastic and metastatic neurologic complications of Merkel cell carcinoma. Mayo Clin Proc. 2001;76:327-330.
- Siau RT, Morris A, Karoo RO. Surgery results in complete cure of Lambert-Eaton myasthenic syndrome in a patient with metastatic Merkel cell carcinoma. J Plast Reconstr Aesthet Surg. 2014;67:e162-e164.
- Bombelli F, Lispi L, Calabrò F, et al. Lambert-Eaton myasthenic syndrome associated to Merkel cell carcinoma: report of a case. Neurol Sci. 2015;36:1491-1492.
- Simmons DB, Duginski TM, McClean JC, et al. Lambert-eaton myasthenic syndrome and merkel cell carcinoma. Muscle Nerve. 2015;53:325-326.
- Bobos M, Hytiroglou P, Kostopoulos I, et al. Immunohistochemical distinction between Merkel cell carcinoma and small cell carcinoma of the lung. Am J Dermatopathol. 2006;28:99-104.
- Jensen K, Kohler S, Rouse RV. Cytokeratin staining in Merkel cell carcinoma: an immunohistochemical study of cytokeratins 5/6, 7, 17, and 20. Appl Immunohistochem Mol Morphol. 2000;8:310-315.
- Titulaer MJ, Lang B, Verschuuren JJ. Lambert-Eaton myasthenic syndrome: from clinical characteristics to therapeutic strategies. Lancet Neurol. 2011;10:1098-1107.
- Sanders DB. Lambert-Eaton myasthenic syndrome. In: Daroff R, Aminoff MJ, eds. Encyclopedia of the Neurological Sciences. Vol 2. New York, NY: Elsevier; 2009:819-822.
- Wirtz PW, Smallegange TM, Wintzen AR, et al. Differences in clinical features between the Lambert-Eaton myasthenic syndrome with and without cancer: an analysis of 227 published cases. Clin Neurol Neurosurg. 2002;104:359-363.
- Titulaer MJ, Wirtz PW, Willems LN, et al. Screening for small-cell lung cancer: a follow-up study of patients with Lambert-Eaton myasthenic syndrome. J Clin Oncol. 2008;26:4276-4281.
- Titulaer MJ, Maddison P, Sont JK, et al. Clinical Dutch-English Lambert-Eaton Myasthenic Syndrome (LEMS) Tumor Association prediction score accurately predicts small-cell lung cancer in the LEMS. J Clin Oncol. 2011;7:902-908.
- Cher LM, Hochberg FH, Teruya J, et al. Therapy for paraneoplastic neurologic syndromes in six patients with protein A column immunoadsorption. Cancer. 1995;75:1678-1683.
- Batchelor TT, Platten M, Hochberg FH. Immunoadsorption therapy for paraneoplastic syndromes. J Neurooncol. 1998;40:131-136.
- Blondin NA, Vortmeyer AO, Harel NY. Paraneoplastic syndrome of inappropriate antidiuretic hormone mimicking limbic encephalitis. Arch Neurol. 2011;68:1591-1594.
- Balegno S, Ceroni M, Corato M, et al. Antibodies to cerebellar nerve fibres in two patients with paraneoplastic cerebellar ataxia. Anticancer Res. 2005;25:3211-3214.
- Zhang C, Emery L, Lancaster E. Paraneoplastic cerebellar degeneration associated with noncutaneous Merkel cell carcinoma. Neurol Neuroimmunol Neuroinflamm. 2014;1:e17.
- Hocar O, Poszepczynska-Guigné E, Faye O, et al. Severe necrotizing myopathy subsequent to Merkel cell carcinoma. Ann Dermatol Venereol. 2011;138:130-134.
- Greenlee JE, Steffens JD, Clawson SA, et al. Anti-Hu antibodies in Merkel cell carcinoma. Ann Neurol. 2002;52:111-115.
- Lopez MC, Pericay C, Agustí M, et al. Merkel cell carcinoma associated with a paraneoplastic neurologic syndrome. Histopathology. 2004;44:628-629.
- Dalmau J, Furneaux HM, Cordon-Cardo C, et al. The expression of the Hu (paraneoplastic encephalomyelitis/sensory neuronopathy) antigen in human normal and tumor tissues. Am J Pathol. 1992;141:881-886.
- Gultekin SH, Rosai J, Demopoulos A, et al. Hu immunolabeling as a marker of neural and neuroendocrine differentiation in normal and neoplastic human tissues: assessment using a recombinant anti-Hu Fab fragment. Int J Surg Pathol. 2000;8:109-117.
- Zheng X, Liu D, Fallon JT, et al. Distinct genetic alterations in small cell carcinoma from different anatomic sites. Exp Hematol Oncol. 2015;4:2.
- Kim D, Yun H, Lee Y, et al. Small cell neuroendocrine carcinoma of the uterine cervix presenting with syndrome of inappropriate antidiuretic hormone secretion. Obstet Gynecol Sci. 2013;56:420-425.
- Venkatesh PK, Motwani B, Sherman N, et al. Metastatic pure small-cell carcinoma of prostate. Am J Med Sci. 2004;328:286-289.
- Kaltsas G, Androulakis II, de Herder WW, et al. Paraneoplastic syndromes secondary to neuroendocrine tumours. Endocr Relat Cancer. 2010;17:R173-R193.
Merkel cell carcinoma (MCC) is an aggressive neuroendocrine malignancy of the skin that is thought to arise from neural crest cells. It has an estimated annual incidence of 0.6 per 100,000 individuals, typically occurs in the elderly population, and is most common in white males.1 The tumor presents as a rapidly growing, violaceous nodule in sun-exposed areas of the skin; early in the course, it can be mistaken for a benign entity such as an epidermal cyst.2 Merkel cell carcinoma has a propensity to spread to regional lymph nodes, and in some cases, it occurs in the absence of skin findings.3 Histologically, MCC is nearly indistinguishable from small cell lung carcinoma (SCLC).4 The overall prognosis for patients with MCC is poor and largely dependent on the stage at diagnosis. Patients with regional and distant metastases have a 5-year survival rate of 26% to 42% and 18%, respectively.3
Lambert-Eaton myasthenic syndrome (LEMS) is a paraneoplastic or autoimmune disorder of the neuromuscular junction that is found in 3% of cases of SCLC.4 Reported cases of LEMS in patients with MCC are exceedingly rare.5-8 We provide a full report and longitudinal clinical follow-up of a case that was briefly discussed by Simmons et al,8 and we review the literature regarding paraneoplastic syndromes associated with MCC and other extrapulmonary small cell carcinomas (EPSCCs).
Case Report
A 63-year-old man was evaluated in the neurology clinic due to difficulty walking, climbing stairs, and performing push-ups over the last month. Prior to the onset of symptoms, he was otherwise healthy, walking 3 miles daily; however, at presentation he required use of a cane. Leg weakness worsened as the day progressed. In addition, he reported constipation, urinary urgency, dry mouth, mild dysphagia, reduced sensation below the knees, and a nasal quality in his speech. He had no ptosis, diplopia, dysarthria, muscle cramps, myalgia, or facial weakness. He denied fevers, chills, and night sweats but did admit to an unintentional 10- to 15-lb weight loss over the preceding few months.
The neurologic examination revealed mild proximal upper extremity weakness in the bilateral shoulder abductors, infraspinatus, hip extensors, and hip flexors (Medical Research Council muscle scale grade 4). All deep tendon reflexes, except the Achilles reflex, were present. Despite subjective sensory concerns, objective examination of all sensory modalities was normal. Cranial nerve examination was normal, except for a slight nasal quality to his voice.
A qualitative assay was positive for the presence of P/Q-type voltage-gated calcium channel (VGCC) antibodies. Other laboratory studies were within reference range, including acetylcholine-receptor antibodies (blocking, binding, and modulating) and muscle-specific kinase antibodies.
Lumbar and cervical spine magnetic resonance imaging revealed multilevel neuroforaminal stenosis without spinal canal stenosis or myelopathy. Computed tomography (CT) of the chest was notable for 2 pathologically enlarged lymph nodes in the left axilla and no evidence of primary pulmonary malignancy. Nerve-conduction studies (NCSs) in conjunction with other clinical findings were consistent with the diagnosis of LEMS.
Ultrasound-guided biopsy of the enlarged axillary lymph nodes demonstrated sheets and nests of small round blue tumor cells with minimal cytoplasm, high mitotic rate, and foci of necrosis (Figure 1). The tumor cells were positive for pancytokeratin (Lu-5) and cytokeratin (CK) 20 in a perinuclear dotlike pattern (Figure 2), as well as for the neuroendocrine markers synaptophysin (Figure 3), chromogranin A, and CD56. The tumor cells showed no immunoreactivity for CK7, thyroid transcription factor 1, CD3, CD5, or CD20. Flow cytometry demonstrated low cellularity, low specimen viability, and no evidence of an abnormal B-cell population. These findings were consistent with the diagnosis of MCC.
The patient underwent surgical excision of the involved lymph nodes. Four weeks after surgery, he reported dramatic improvement in strength, with complete resolution of the nasal speech, dysphagia, dry mouth, urinary retention, and constipation. Two months after surgery, his strength had normalized, except for slight persistent weakness in the bilateral shoulder abductors, trace weakness in the hip flexors, and a slight Trendelenburg gait. He was able to rise from a chair without using his arms and no longer required a cane for ambulation.
The patient underwent adjuvant radiation therapy after 2-month surgical follow-up with 5000-cGy radiation treatment to the left axillary region. Six months following primary definitive surgery and 4 months following adjuvant radiation therapy, he reported a 95% subjective return of physical strength. The patient was able to return to near-baseline physical activity. He continued to deny symptoms of dry mouth, incontinence, or constipation. Objectively, he had no focal neurologic deficits or weakness; no evidence of new skin lesions or lymphadenopathy was noted.
Comment
MCC vs SCLC
Merkel cell carcinoma is classified as a type of EPSCC. The histologic appearance of MCC is indistinguishable from SCLC. Both tumors are composed of uniform sheets of small round cells with a high nucleus to cytoplasm ratio, and both can express neuroendocrine markers, such as neuron-specific enolase, chromogranin A, and synaptophysin.9 Immunohistochemical positivity for CK20 and neurofilaments in combination with negative staining for thyroid transcription factor 1 and CK7 effectively differentiate MCC from SCLC.9 In addition, MCC often displays CK20 positivity in a perinuclear dotlike or punctate pattern, which is characteristic of this tumor.3,9,10 Negative immunohistochemical markers for B cells (CD20) and T cells (CD3) are important in excluding lymphoma.
LEMS Diagnosis
Lambert-Eaton myasthenic syndrome is a paraneoplastic or autoimmune disorder involving the neuromuscular junction. Autoantibodies to VGCC impair calcium influx into the presynaptic terminal, resulting in marked reduction of acetylcholine release into the synaptic cleft. The reduction in acetylcholine activity impairs production of muscle fiber action potentials, resulting in clinical weakness. The diagnosis of LEMS rests on clinical presentation, positive serology, and confirmatory neurophysiologic testing by NCS. Clinically, patients present with proximal weakness, hyporeflexia or areflexia, and autonomic dysfunction. Antibodies to P/Q-type VGCCs are found in 85% to 90% of cases of LEMS and are thought to play a direct causative role in the development of weakness.11 The finding of postexercise facilitation on motor NCS is the neurophysiologic hallmark and is highly specific for the diagnosis.
Approximately 50% to 60% of patients who present with LEMS have an underlying tumor, the vast majority of which are SCLC.11 There are a few reports of LEMS associated with other malignancies, including lymphoma; thymoma; neuroblastoma; and carcinoma of the breast, stomach, prostate, bladder, kidney, and gallbladder.12 Patients with nontumor or autoimmune LEMS tend to be younger, and there is no male predominance, as there is in paraneoplastic LEMS.13 Given the risk of underlying malignancy in LEMS, Titulaer et al14 proposed a screening protocol for patients presenting with LEMS, recommending initial primary screening using CT of the chest. If the CT scan is negative, total-body fludeoxyglucose positron emission tomography should be performed to assess for fludeoxyglucose avid lesions. If both initial studies are negative, routine follow-up with CT of the chest at 6-month intervals for a minimum of 2 to 4 years after the initial diagnosis of LEMS was recommended. An exception to this protocol was suggested to allow consideration to stop screening after the first 6-month follow-up chest CT for patients younger than 45 years who have never smoked and who have an HLA 8.1 haplotype for which nontumor LEMS would be a more probable diagnosis.14
In addition to a screening protocol, a validated prediction tool, the Dutch-English LEMS Tumor Association prediction score, was developed. It uses common signs and symptoms of LEMS and risk factors for SCLC to help guide the need for further screening.15
Paraneoplastic Syndromes Associated With MCC
Other paraneoplastic syndromes have been reported in association with MCC. A patient with brainstem encephalitis associated with MCC was reported in a trial of a novel immunotherapy for paraneoplastic neurologic syndromes.16,17 A syndrome of inappropriate antidiuretic hormone (SIADH) secretion was reported in a patient with N-type calcium channel antibodies.18 Two cases of paraneoplastic cerebellar degeneration have been reported; the first was associated with a novel 70-kD antibody,19 and the second was associated with the P/Q-type VGCC antibody.20 Anti-Hu antibodies have been found in a handful of reports of neurologic deterioration in patients with MCC. Hocar et al21 reported a severe necrotizing myopathy; Greenlee et al22 described a syndrome of progressive sensorimotor and autonomic neuropathy with encephalopathy; and Lopez et al23 described a constellation of vision changes, gait imbalance, and proximal weakness. Support for a pathophysiologic connection among these 3 cases is suggested by the finding of Hu antigen expression by MCC in 2 studies.24,25 Because MCC can present with occult lymph node involvement in the absence of primary cutaneous findings,3 there are more cases of paraneoplastic neurologic syndromes that were not recognized.
Extrapulmonary small cell carcinomas such as MCC are morphologically indistinguishable from their pulmonary counterparts and have been reported in most anatomic regions of the body, including gynecologic organs (eg, ovaries, cervix), genitourinary organs (eg, bladder, prostate), the gastrointestinal tract (eg, esophagus), skin (eg, MCC), and the head and neck region. Extrapulmonary small cell carcinoma is a rare entity, with the most common form found in the gynecologic tract, representing only 2% of gynecologic malignancies.26
Paraneoplastic syndromes of EPSCC are rare given the paucity of the malignancy. Several case reports discuss findings of SIADH in EPSCC of the cervix, as well as hypercalcemia, polyneuropathy, Cushing syndrome, limbic encephalitis, and peripheral neuropathy in EPSCC of the prostate.27,28 In contrast, SCLC has long been associated with paraneoplastic syndromes. Numerous case reports have been published describing SCLC-associated paraneoplastic syndromes to include hypercalcemia, Cushing syndrome, SIADH, vasoactive peptide production, cerebellar degeneration, limbic encephalitis, visceral plexopathy, autonomic dysfunction, and LEMS.29 As more cases of EPSCC with paraneoplastic syndromes are identified and reported, we might gain a better understanding of this interesting phenomenon.
Conclusion
Merkel cell carcinoma is an aggressive neuroendocrine malignancy associated with paraneoplastic neurologic syndromes, including LEMS. A thorough search for an underlying malignancy is highly recommended in patients with diagnosed LEMS without clear cause. Early identification and treatment of the primary tumor can lead to improvement of neurologic symptoms.
We present a case of LEMS with no clearly identifiable cause on presentation with later diagnosis of metastatic MCC of unknown primary origin. After surgical excision of affected lymph nodes and adjuvant radiation therapy, the patient had near-complete resolution of LEMS symptoms at 6-month follow-up, without additional findings of lymphadenopathy or skin lesions. Although this patient is not undergoing routine surveillance imaging to monitor for recurrence of MCC, a chest CT or positron emission tomography–CT for secondary screening would be considered if the patient experienced clinical symptoms consistent with LEMS.
In cases of LEMS without pulmonary malignancy, we recommend considering MCC in the differential diagnosis during the workup of an underlying malignancy
Merkel cell carcinoma (MCC) is an aggressive neuroendocrine malignancy of the skin that is thought to arise from neural crest cells. It has an estimated annual incidence of 0.6 per 100,000 individuals, typically occurs in the elderly population, and is most common in white males.1 The tumor presents as a rapidly growing, violaceous nodule in sun-exposed areas of the skin; early in the course, it can be mistaken for a benign entity such as an epidermal cyst.2 Merkel cell carcinoma has a propensity to spread to regional lymph nodes, and in some cases, it occurs in the absence of skin findings.3 Histologically, MCC is nearly indistinguishable from small cell lung carcinoma (SCLC).4 The overall prognosis for patients with MCC is poor and largely dependent on the stage at diagnosis. Patients with regional and distant metastases have a 5-year survival rate of 26% to 42% and 18%, respectively.3
Lambert-Eaton myasthenic syndrome (LEMS) is a paraneoplastic or autoimmune disorder of the neuromuscular junction that is found in 3% of cases of SCLC.4 Reported cases of LEMS in patients with MCC are exceedingly rare.5-8 We provide a full report and longitudinal clinical follow-up of a case that was briefly discussed by Simmons et al,8 and we review the literature regarding paraneoplastic syndromes associated with MCC and other extrapulmonary small cell carcinomas (EPSCCs).
Case Report
A 63-year-old man was evaluated in the neurology clinic due to difficulty walking, climbing stairs, and performing push-ups over the last month. Prior to the onset of symptoms, he was otherwise healthy, walking 3 miles daily; however, at presentation he required use of a cane. Leg weakness worsened as the day progressed. In addition, he reported constipation, urinary urgency, dry mouth, mild dysphagia, reduced sensation below the knees, and a nasal quality in his speech. He had no ptosis, diplopia, dysarthria, muscle cramps, myalgia, or facial weakness. He denied fevers, chills, and night sweats but did admit to an unintentional 10- to 15-lb weight loss over the preceding few months.
The neurologic examination revealed mild proximal upper extremity weakness in the bilateral shoulder abductors, infraspinatus, hip extensors, and hip flexors (Medical Research Council muscle scale grade 4). All deep tendon reflexes, except the Achilles reflex, were present. Despite subjective sensory concerns, objective examination of all sensory modalities was normal. Cranial nerve examination was normal, except for a slight nasal quality to his voice.
A qualitative assay was positive for the presence of P/Q-type voltage-gated calcium channel (VGCC) antibodies. Other laboratory studies were within reference range, including acetylcholine-receptor antibodies (blocking, binding, and modulating) and muscle-specific kinase antibodies.
Lumbar and cervical spine magnetic resonance imaging revealed multilevel neuroforaminal stenosis without spinal canal stenosis or myelopathy. Computed tomography (CT) of the chest was notable for 2 pathologically enlarged lymph nodes in the left axilla and no evidence of primary pulmonary malignancy. Nerve-conduction studies (NCSs) in conjunction with other clinical findings were consistent with the diagnosis of LEMS.
Ultrasound-guided biopsy of the enlarged axillary lymph nodes demonstrated sheets and nests of small round blue tumor cells with minimal cytoplasm, high mitotic rate, and foci of necrosis (Figure 1). The tumor cells were positive for pancytokeratin (Lu-5) and cytokeratin (CK) 20 in a perinuclear dotlike pattern (Figure 2), as well as for the neuroendocrine markers synaptophysin (Figure 3), chromogranin A, and CD56. The tumor cells showed no immunoreactivity for CK7, thyroid transcription factor 1, CD3, CD5, or CD20. Flow cytometry demonstrated low cellularity, low specimen viability, and no evidence of an abnormal B-cell population. These findings were consistent with the diagnosis of MCC.
The patient underwent surgical excision of the involved lymph nodes. Four weeks after surgery, he reported dramatic improvement in strength, with complete resolution of the nasal speech, dysphagia, dry mouth, urinary retention, and constipation. Two months after surgery, his strength had normalized, except for slight persistent weakness in the bilateral shoulder abductors, trace weakness in the hip flexors, and a slight Trendelenburg gait. He was able to rise from a chair without using his arms and no longer required a cane for ambulation.
The patient underwent adjuvant radiation therapy after 2-month surgical follow-up with 5000-cGy radiation treatment to the left axillary region. Six months following primary definitive surgery and 4 months following adjuvant radiation therapy, he reported a 95% subjective return of physical strength. The patient was able to return to near-baseline physical activity. He continued to deny symptoms of dry mouth, incontinence, or constipation. Objectively, he had no focal neurologic deficits or weakness; no evidence of new skin lesions or lymphadenopathy was noted.
Comment
MCC vs SCLC
Merkel cell carcinoma is classified as a type of EPSCC. The histologic appearance of MCC is indistinguishable from SCLC. Both tumors are composed of uniform sheets of small round cells with a high nucleus to cytoplasm ratio, and both can express neuroendocrine markers, such as neuron-specific enolase, chromogranin A, and synaptophysin.9 Immunohistochemical positivity for CK20 and neurofilaments in combination with negative staining for thyroid transcription factor 1 and CK7 effectively differentiate MCC from SCLC.9 In addition, MCC often displays CK20 positivity in a perinuclear dotlike or punctate pattern, which is characteristic of this tumor.3,9,10 Negative immunohistochemical markers for B cells (CD20) and T cells (CD3) are important in excluding lymphoma.
LEMS Diagnosis
Lambert-Eaton myasthenic syndrome is a paraneoplastic or autoimmune disorder involving the neuromuscular junction. Autoantibodies to VGCC impair calcium influx into the presynaptic terminal, resulting in marked reduction of acetylcholine release into the synaptic cleft. The reduction in acetylcholine activity impairs production of muscle fiber action potentials, resulting in clinical weakness. The diagnosis of LEMS rests on clinical presentation, positive serology, and confirmatory neurophysiologic testing by NCS. Clinically, patients present with proximal weakness, hyporeflexia or areflexia, and autonomic dysfunction. Antibodies to P/Q-type VGCCs are found in 85% to 90% of cases of LEMS and are thought to play a direct causative role in the development of weakness.11 The finding of postexercise facilitation on motor NCS is the neurophysiologic hallmark and is highly specific for the diagnosis.
Approximately 50% to 60% of patients who present with LEMS have an underlying tumor, the vast majority of which are SCLC.11 There are a few reports of LEMS associated with other malignancies, including lymphoma; thymoma; neuroblastoma; and carcinoma of the breast, stomach, prostate, bladder, kidney, and gallbladder.12 Patients with nontumor or autoimmune LEMS tend to be younger, and there is no male predominance, as there is in paraneoplastic LEMS.13 Given the risk of underlying malignancy in LEMS, Titulaer et al14 proposed a screening protocol for patients presenting with LEMS, recommending initial primary screening using CT of the chest. If the CT scan is negative, total-body fludeoxyglucose positron emission tomography should be performed to assess for fludeoxyglucose avid lesions. If both initial studies are negative, routine follow-up with CT of the chest at 6-month intervals for a minimum of 2 to 4 years after the initial diagnosis of LEMS was recommended. An exception to this protocol was suggested to allow consideration to stop screening after the first 6-month follow-up chest CT for patients younger than 45 years who have never smoked and who have an HLA 8.1 haplotype for which nontumor LEMS would be a more probable diagnosis.14
In addition to a screening protocol, a validated prediction tool, the Dutch-English LEMS Tumor Association prediction score, was developed. It uses common signs and symptoms of LEMS and risk factors for SCLC to help guide the need for further screening.15
Paraneoplastic Syndromes Associated With MCC
Other paraneoplastic syndromes have been reported in association with MCC. A patient with brainstem encephalitis associated with MCC was reported in a trial of a novel immunotherapy for paraneoplastic neurologic syndromes.16,17 A syndrome of inappropriate antidiuretic hormone (SIADH) secretion was reported in a patient with N-type calcium channel antibodies.18 Two cases of paraneoplastic cerebellar degeneration have been reported; the first was associated with a novel 70-kD antibody,19 and the second was associated with the P/Q-type VGCC antibody.20 Anti-Hu antibodies have been found in a handful of reports of neurologic deterioration in patients with MCC. Hocar et al21 reported a severe necrotizing myopathy; Greenlee et al22 described a syndrome of progressive sensorimotor and autonomic neuropathy with encephalopathy; and Lopez et al23 described a constellation of vision changes, gait imbalance, and proximal weakness. Support for a pathophysiologic connection among these 3 cases is suggested by the finding of Hu antigen expression by MCC in 2 studies.24,25 Because MCC can present with occult lymph node involvement in the absence of primary cutaneous findings,3 there are more cases of paraneoplastic neurologic syndromes that were not recognized.
Extrapulmonary small cell carcinomas such as MCC are morphologically indistinguishable from their pulmonary counterparts and have been reported in most anatomic regions of the body, including gynecologic organs (eg, ovaries, cervix), genitourinary organs (eg, bladder, prostate), the gastrointestinal tract (eg, esophagus), skin (eg, MCC), and the head and neck region. Extrapulmonary small cell carcinoma is a rare entity, with the most common form found in the gynecologic tract, representing only 2% of gynecologic malignancies.26
Paraneoplastic syndromes of EPSCC are rare given the paucity of the malignancy. Several case reports discuss findings of SIADH in EPSCC of the cervix, as well as hypercalcemia, polyneuropathy, Cushing syndrome, limbic encephalitis, and peripheral neuropathy in EPSCC of the prostate.27,28 In contrast, SCLC has long been associated with paraneoplastic syndromes. Numerous case reports have been published describing SCLC-associated paraneoplastic syndromes to include hypercalcemia, Cushing syndrome, SIADH, vasoactive peptide production, cerebellar degeneration, limbic encephalitis, visceral plexopathy, autonomic dysfunction, and LEMS.29 As more cases of EPSCC with paraneoplastic syndromes are identified and reported, we might gain a better understanding of this interesting phenomenon.
Conclusion
Merkel cell carcinoma is an aggressive neuroendocrine malignancy associated with paraneoplastic neurologic syndromes, including LEMS. A thorough search for an underlying malignancy is highly recommended in patients with diagnosed LEMS without clear cause. Early identification and treatment of the primary tumor can lead to improvement of neurologic symptoms.
We present a case of LEMS with no clearly identifiable cause on presentation with later diagnosis of metastatic MCC of unknown primary origin. After surgical excision of affected lymph nodes and adjuvant radiation therapy, the patient had near-complete resolution of LEMS symptoms at 6-month follow-up, without additional findings of lymphadenopathy or skin lesions. Although this patient is not undergoing routine surveillance imaging to monitor for recurrence of MCC, a chest CT or positron emission tomography–CT for secondary screening would be considered if the patient experienced clinical symptoms consistent with LEMS.
In cases of LEMS without pulmonary malignancy, we recommend considering MCC in the differential diagnosis during the workup of an underlying malignancy
- Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2010;37:20-27.
- Senchenkov A, Moran SL. Merkel cell carcinoma: diagnosis, management, and outcomes. Plast Reconstr Surg. 2013;131:E771-E778.
- Han SY, North JP, Canavan T, et al. Merkel cell carcinoma. Hematol Oncol Clin N Am. 2012;26:1351-1374.
- Vernino S. Paraneoplastic disorders affecting the neuromuscular junction or anterior horn cell. CONTINUUM Lifelong Learning in Neurology. 2009;15:132-146.
- Eggers SD, Salomao DR, Dinapoli RP, et al. Paraneoplastic and metastatic neurologic complications of Merkel cell carcinoma. Mayo Clin Proc. 2001;76:327-330.
- Siau RT, Morris A, Karoo RO. Surgery results in complete cure of Lambert-Eaton myasthenic syndrome in a patient with metastatic Merkel cell carcinoma. J Plast Reconstr Aesthet Surg. 2014;67:e162-e164.
- Bombelli F, Lispi L, Calabrò F, et al. Lambert-Eaton myasthenic syndrome associated to Merkel cell carcinoma: report of a case. Neurol Sci. 2015;36:1491-1492.
- Simmons DB, Duginski TM, McClean JC, et al. Lambert-eaton myasthenic syndrome and merkel cell carcinoma. Muscle Nerve. 2015;53:325-326.
- Bobos M, Hytiroglou P, Kostopoulos I, et al. Immunohistochemical distinction between Merkel cell carcinoma and small cell carcinoma of the lung. Am J Dermatopathol. 2006;28:99-104.
- Jensen K, Kohler S, Rouse RV. Cytokeratin staining in Merkel cell carcinoma: an immunohistochemical study of cytokeratins 5/6, 7, 17, and 20. Appl Immunohistochem Mol Morphol. 2000;8:310-315.
- Titulaer MJ, Lang B, Verschuuren JJ. Lambert-Eaton myasthenic syndrome: from clinical characteristics to therapeutic strategies. Lancet Neurol. 2011;10:1098-1107.
- Sanders DB. Lambert-Eaton myasthenic syndrome. In: Daroff R, Aminoff MJ, eds. Encyclopedia of the Neurological Sciences. Vol 2. New York, NY: Elsevier; 2009:819-822.
- Wirtz PW, Smallegange TM, Wintzen AR, et al. Differences in clinical features between the Lambert-Eaton myasthenic syndrome with and without cancer: an analysis of 227 published cases. Clin Neurol Neurosurg. 2002;104:359-363.
- Titulaer MJ, Wirtz PW, Willems LN, et al. Screening for small-cell lung cancer: a follow-up study of patients with Lambert-Eaton myasthenic syndrome. J Clin Oncol. 2008;26:4276-4281.
- Titulaer MJ, Maddison P, Sont JK, et al. Clinical Dutch-English Lambert-Eaton Myasthenic Syndrome (LEMS) Tumor Association prediction score accurately predicts small-cell lung cancer in the LEMS. J Clin Oncol. 2011;7:902-908.
- Cher LM, Hochberg FH, Teruya J, et al. Therapy for paraneoplastic neurologic syndromes in six patients with protein A column immunoadsorption. Cancer. 1995;75:1678-1683.
- Batchelor TT, Platten M, Hochberg FH. Immunoadsorption therapy for paraneoplastic syndromes. J Neurooncol. 1998;40:131-136.
- Blondin NA, Vortmeyer AO, Harel NY. Paraneoplastic syndrome of inappropriate antidiuretic hormone mimicking limbic encephalitis. Arch Neurol. 2011;68:1591-1594.
- Balegno S, Ceroni M, Corato M, et al. Antibodies to cerebellar nerve fibres in two patients with paraneoplastic cerebellar ataxia. Anticancer Res. 2005;25:3211-3214.
- Zhang C, Emery L, Lancaster E. Paraneoplastic cerebellar degeneration associated with noncutaneous Merkel cell carcinoma. Neurol Neuroimmunol Neuroinflamm. 2014;1:e17.
- Hocar O, Poszepczynska-Guigné E, Faye O, et al. Severe necrotizing myopathy subsequent to Merkel cell carcinoma. Ann Dermatol Venereol. 2011;138:130-134.
- Greenlee JE, Steffens JD, Clawson SA, et al. Anti-Hu antibodies in Merkel cell carcinoma. Ann Neurol. 2002;52:111-115.
- Lopez MC, Pericay C, Agustí M, et al. Merkel cell carcinoma associated with a paraneoplastic neurologic syndrome. Histopathology. 2004;44:628-629.
- Dalmau J, Furneaux HM, Cordon-Cardo C, et al. The expression of the Hu (paraneoplastic encephalomyelitis/sensory neuronopathy) antigen in human normal and tumor tissues. Am J Pathol. 1992;141:881-886.
- Gultekin SH, Rosai J, Demopoulos A, et al. Hu immunolabeling as a marker of neural and neuroendocrine differentiation in normal and neoplastic human tissues: assessment using a recombinant anti-Hu Fab fragment. Int J Surg Pathol. 2000;8:109-117.
- Zheng X, Liu D, Fallon JT, et al. Distinct genetic alterations in small cell carcinoma from different anatomic sites. Exp Hematol Oncol. 2015;4:2.
- Kim D, Yun H, Lee Y, et al. Small cell neuroendocrine carcinoma of the uterine cervix presenting with syndrome of inappropriate antidiuretic hormone secretion. Obstet Gynecol Sci. 2013;56:420-425.
- Venkatesh PK, Motwani B, Sherman N, et al. Metastatic pure small-cell carcinoma of prostate. Am J Med Sci. 2004;328:286-289.
- Kaltsas G, Androulakis II, de Herder WW, et al. Paraneoplastic syndromes secondary to neuroendocrine tumours. Endocr Relat Cancer. 2010;17:R173-R193.
- Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2010;37:20-27.
- Senchenkov A, Moran SL. Merkel cell carcinoma: diagnosis, management, and outcomes. Plast Reconstr Surg. 2013;131:E771-E778.
- Han SY, North JP, Canavan T, et al. Merkel cell carcinoma. Hematol Oncol Clin N Am. 2012;26:1351-1374.
- Vernino S. Paraneoplastic disorders affecting the neuromuscular junction or anterior horn cell. CONTINUUM Lifelong Learning in Neurology. 2009;15:132-146.
- Eggers SD, Salomao DR, Dinapoli RP, et al. Paraneoplastic and metastatic neurologic complications of Merkel cell carcinoma. Mayo Clin Proc. 2001;76:327-330.
- Siau RT, Morris A, Karoo RO. Surgery results in complete cure of Lambert-Eaton myasthenic syndrome in a patient with metastatic Merkel cell carcinoma. J Plast Reconstr Aesthet Surg. 2014;67:e162-e164.
- Bombelli F, Lispi L, Calabrò F, et al. Lambert-Eaton myasthenic syndrome associated to Merkel cell carcinoma: report of a case. Neurol Sci. 2015;36:1491-1492.
- Simmons DB, Duginski TM, McClean JC, et al. Lambert-eaton myasthenic syndrome and merkel cell carcinoma. Muscle Nerve. 2015;53:325-326.
- Bobos M, Hytiroglou P, Kostopoulos I, et al. Immunohistochemical distinction between Merkel cell carcinoma and small cell carcinoma of the lung. Am J Dermatopathol. 2006;28:99-104.
- Jensen K, Kohler S, Rouse RV. Cytokeratin staining in Merkel cell carcinoma: an immunohistochemical study of cytokeratins 5/6, 7, 17, and 20. Appl Immunohistochem Mol Morphol. 2000;8:310-315.
- Titulaer MJ, Lang B, Verschuuren JJ. Lambert-Eaton myasthenic syndrome: from clinical characteristics to therapeutic strategies. Lancet Neurol. 2011;10:1098-1107.
- Sanders DB. Lambert-Eaton myasthenic syndrome. In: Daroff R, Aminoff MJ, eds. Encyclopedia of the Neurological Sciences. Vol 2. New York, NY: Elsevier; 2009:819-822.
- Wirtz PW, Smallegange TM, Wintzen AR, et al. Differences in clinical features between the Lambert-Eaton myasthenic syndrome with and without cancer: an analysis of 227 published cases. Clin Neurol Neurosurg. 2002;104:359-363.
- Titulaer MJ, Wirtz PW, Willems LN, et al. Screening for small-cell lung cancer: a follow-up study of patients with Lambert-Eaton myasthenic syndrome. J Clin Oncol. 2008;26:4276-4281.
- Titulaer MJ, Maddison P, Sont JK, et al. Clinical Dutch-English Lambert-Eaton Myasthenic Syndrome (LEMS) Tumor Association prediction score accurately predicts small-cell lung cancer in the LEMS. J Clin Oncol. 2011;7:902-908.
- Cher LM, Hochberg FH, Teruya J, et al. Therapy for paraneoplastic neurologic syndromes in six patients with protein A column immunoadsorption. Cancer. 1995;75:1678-1683.
- Batchelor TT, Platten M, Hochberg FH. Immunoadsorption therapy for paraneoplastic syndromes. J Neurooncol. 1998;40:131-136.
- Blondin NA, Vortmeyer AO, Harel NY. Paraneoplastic syndrome of inappropriate antidiuretic hormone mimicking limbic encephalitis. Arch Neurol. 2011;68:1591-1594.
- Balegno S, Ceroni M, Corato M, et al. Antibodies to cerebellar nerve fibres in two patients with paraneoplastic cerebellar ataxia. Anticancer Res. 2005;25:3211-3214.
- Zhang C, Emery L, Lancaster E. Paraneoplastic cerebellar degeneration associated with noncutaneous Merkel cell carcinoma. Neurol Neuroimmunol Neuroinflamm. 2014;1:e17.
- Hocar O, Poszepczynska-Guigné E, Faye O, et al. Severe necrotizing myopathy subsequent to Merkel cell carcinoma. Ann Dermatol Venereol. 2011;138:130-134.
- Greenlee JE, Steffens JD, Clawson SA, et al. Anti-Hu antibodies in Merkel cell carcinoma. Ann Neurol. 2002;52:111-115.
- Lopez MC, Pericay C, Agustí M, et al. Merkel cell carcinoma associated with a paraneoplastic neurologic syndrome. Histopathology. 2004;44:628-629.
- Dalmau J, Furneaux HM, Cordon-Cardo C, et al. The expression of the Hu (paraneoplastic encephalomyelitis/sensory neuronopathy) antigen in human normal and tumor tissues. Am J Pathol. 1992;141:881-886.
- Gultekin SH, Rosai J, Demopoulos A, et al. Hu immunolabeling as a marker of neural and neuroendocrine differentiation in normal and neoplastic human tissues: assessment using a recombinant anti-Hu Fab fragment. Int J Surg Pathol. 2000;8:109-117.
- Zheng X, Liu D, Fallon JT, et al. Distinct genetic alterations in small cell carcinoma from different anatomic sites. Exp Hematol Oncol. 2015;4:2.
- Kim D, Yun H, Lee Y, et al. Small cell neuroendocrine carcinoma of the uterine cervix presenting with syndrome of inappropriate antidiuretic hormone secretion. Obstet Gynecol Sci. 2013;56:420-425.
- Venkatesh PK, Motwani B, Sherman N, et al. Metastatic pure small-cell carcinoma of prostate. Am J Med Sci. 2004;328:286-289.
- Kaltsas G, Androulakis II, de Herder WW, et al. Paraneoplastic syndromes secondary to neuroendocrine tumours. Endocr Relat Cancer. 2010;17:R173-R193.
Practice Points
- Approximately 50% to 60% of patients with Lambert-Eaton myasthenic syndrome (LEMS) have an underlying tumor, most commonly small cell lung carcinoma.
- A thorough search for an underlying malignancy is highly recommended in patients with diagnosed LEMS without clear cause; to this end, a screening protocol comprising computed tomography and total-body fludeoxyglucose positron emission tomography has been established.
- Because Merkel cell carcinoma (MCC) can present as occult lymph node involvement with primary cutaneous findings absent, it is recommended that MCC be considered in the differential diagnosis of an underlying malignancy in a LEMS patient.
- Early identification and treatment of the primary tumor can lead to improvement of neurologic symptoms.