User login
List of medications linked to drug-induced lupus expands
leaving the overall number now standing at 118.
Among the 118 suspected drugs found in VigiBase, the WHO’s global deduplicated individual case safety reports (ICSR) database, 42 had not been previously reported in association with drug-induced lupus (DIL) and 76 had been previously reported in association with DIL in Medline. DIL was reported as a serious adverse event in 55.4% of cases, according to French researchers led by Laurent Arnaud, MD, PhD, of the department of rheumatology at Hôpitaux Universitaires de Strasbourg and Centre National de Références des Maladies Systémiques Rares, Strasbourg, France.
Dr. Arnaud and his colleagues conducted a case-noncase analysis for each drug associated with DIL in order to compare the proportion of specific adverse drug reactions (ADRs) reported for a single drug with the proportion of the same ADR for all other treatments in VigiBase, which receives reports from more than 130 country members of the WHO Programme for International Drug Monitoring and contains over 16 million deduplicated ICSRs recorded by pharmacovigilance centers since 1967. They searched for cases classified as systemic lupus erythematosus (SLE) and identified 12,166 ICSRs of DIL; from these they found 118 suspected drugs with significant pharmacovigilance signal from 8,163 ICSRs that mostly originated from the Americas (65%) and Europe (23%).
In line with what the study authors expected, the drugs associated with the highest number of DIL cases were the antitumor necrosis factor agents infliximab, adalimumab, and etanercept, and the drugs associated with the highest disproportional reporting of DIL were procainamide and hydralazine.
“This is an important finding because these are the two drugs associated with the highest risk of DIL in the literature, therefore confirming the reliability of our approach using a large pharmacovigilance database,” the researchers wrote in Annals of the Rheumatic Diseases.
Overall, DIL was considered definite for 9 drugs (procainamide, hydralazine, minocycline, quinidine, isoniazid, terbinafine, methyldopa, dihydralazine, and chlorpromazine), probable for 19 drugs, and possible for 45 drugs.
The median age of DIL onset was 49 years, which the authors noted was about 2 decades older than that of spontaneous SLE.
They also observed a marked predominance in females (female to male sex ratio, 4.3), a finding that contrasted with previous studies reporting a female to male sex ratio closer to 1:1.
Dr. Arnaud and his colleagues stated that their finding of a median delay between the reported start of suspected treatment and DIL occurrence of 172 days (interquartile range, 35-610 days) suggested that DIL mostly appears after a few months and usually within the first 2 years of treatment with the suspected drug.
“The analysis of the median reporting years for each suspected drug shows a clear evolution of suspected drugs during the past decades. This further underlines that the constantly changing spectrum of DIL should be monitored continuously, and further validates the interest of our approach using the WHO international pharmacovigilance database, the biggest database of this kind with over 16 million deduplicated ICSRs,” they wrote.
The researchers added that distinguishing DIL from SLE is important because its prognosis is usually good when the drug is withdrawn, but the spectrum of DIL is constantly evolving, with drugs once described as strongly linked to DIL now prescribed less frequently.
“The first case of DIL was reported in 1945 with sulfadiazine, while hydralazine DIL was first reported in 1953. Since then, pharmacopoeia has strongly evolved, and one could hypothesize that so has the spectrum of drugs that can induce DIL,” they wrote.
“The detailed list of suspected drugs may prove useful to physicians when confronted with potential DIL cases. Altogether, these findings may help in improving the identification of this constantly evolving disease,” they concluded.
The current study was limited by the lack of a uniform set of criteria for the diagnosis of DIL and by the level of reported details available in VigiBase.
The authors had no outside funding for the study and reported having no conflicts of interest.
SOURCE: Arnaud L et al. Ann Rheum Dis. 2019 Feb 4. doi: 10.1136/annrheumdis-2018-214598.
This new and updated list of possible lupus-inducing drugs includes a growing range of treatment categories, chemical structures, and pharmacologic actions. Yet it is still unclear what the common denominator is that links them.
Drug-induced lupus (DIL) is a peculiar adverse drug reaction that appears to be unrelated to any known property of the inducing agent, although cytokine modulating biologics are a possible exception. Nevertheless, the in vivo metabolism of dissimilar drugs to products with a common, reactive property may go some way to explaining how compounds with different pharmacologic and chemical structures could induce similar adverse reactions.
The findings by Arnaud et al. need better documentation than just positive pharmacovigilance signals. For example, a drug with a relatively high signal does not necessarily translate to a high propensity for causing lupus-like symptoms. It may be a reflection of high drug usage or an awareness of the report contributors for detecting new-onset systemic lupus erythematosus.
Regardless, this research serves to help and inform the medical community to increase the vigilance of previously unreported DIL and perhaps motivate the publication of novel, convincing case reports.
Robert L. Rubin, PhD, is with the University of New Mexico, Albuquerque. His comments are adapted from an editorial accompanying the report by Arnaud et al. (Ann Rheum Dis. 2019 Feb 13. doi: annrheumdis-2018-214785). He reported having no relevant disclosures.
This new and updated list of possible lupus-inducing drugs includes a growing range of treatment categories, chemical structures, and pharmacologic actions. Yet it is still unclear what the common denominator is that links them.
Drug-induced lupus (DIL) is a peculiar adverse drug reaction that appears to be unrelated to any known property of the inducing agent, although cytokine modulating biologics are a possible exception. Nevertheless, the in vivo metabolism of dissimilar drugs to products with a common, reactive property may go some way to explaining how compounds with different pharmacologic and chemical structures could induce similar adverse reactions.
The findings by Arnaud et al. need better documentation than just positive pharmacovigilance signals. For example, a drug with a relatively high signal does not necessarily translate to a high propensity for causing lupus-like symptoms. It may be a reflection of high drug usage or an awareness of the report contributors for detecting new-onset systemic lupus erythematosus.
Regardless, this research serves to help and inform the medical community to increase the vigilance of previously unreported DIL and perhaps motivate the publication of novel, convincing case reports.
Robert L. Rubin, PhD, is with the University of New Mexico, Albuquerque. His comments are adapted from an editorial accompanying the report by Arnaud et al. (Ann Rheum Dis. 2019 Feb 13. doi: annrheumdis-2018-214785). He reported having no relevant disclosures.
This new and updated list of possible lupus-inducing drugs includes a growing range of treatment categories, chemical structures, and pharmacologic actions. Yet it is still unclear what the common denominator is that links them.
Drug-induced lupus (DIL) is a peculiar adverse drug reaction that appears to be unrelated to any known property of the inducing agent, although cytokine modulating biologics are a possible exception. Nevertheless, the in vivo metabolism of dissimilar drugs to products with a common, reactive property may go some way to explaining how compounds with different pharmacologic and chemical structures could induce similar adverse reactions.
The findings by Arnaud et al. need better documentation than just positive pharmacovigilance signals. For example, a drug with a relatively high signal does not necessarily translate to a high propensity for causing lupus-like symptoms. It may be a reflection of high drug usage or an awareness of the report contributors for detecting new-onset systemic lupus erythematosus.
Regardless, this research serves to help and inform the medical community to increase the vigilance of previously unreported DIL and perhaps motivate the publication of novel, convincing case reports.
Robert L. Rubin, PhD, is with the University of New Mexico, Albuquerque. His comments are adapted from an editorial accompanying the report by Arnaud et al. (Ann Rheum Dis. 2019 Feb 13. doi: annrheumdis-2018-214785). He reported having no relevant disclosures.
leaving the overall number now standing at 118.
Among the 118 suspected drugs found in VigiBase, the WHO’s global deduplicated individual case safety reports (ICSR) database, 42 had not been previously reported in association with drug-induced lupus (DIL) and 76 had been previously reported in association with DIL in Medline. DIL was reported as a serious adverse event in 55.4% of cases, according to French researchers led by Laurent Arnaud, MD, PhD, of the department of rheumatology at Hôpitaux Universitaires de Strasbourg and Centre National de Références des Maladies Systémiques Rares, Strasbourg, France.
Dr. Arnaud and his colleagues conducted a case-noncase analysis for each drug associated with DIL in order to compare the proportion of specific adverse drug reactions (ADRs) reported for a single drug with the proportion of the same ADR for all other treatments in VigiBase, which receives reports from more than 130 country members of the WHO Programme for International Drug Monitoring and contains over 16 million deduplicated ICSRs recorded by pharmacovigilance centers since 1967. They searched for cases classified as systemic lupus erythematosus (SLE) and identified 12,166 ICSRs of DIL; from these they found 118 suspected drugs with significant pharmacovigilance signal from 8,163 ICSRs that mostly originated from the Americas (65%) and Europe (23%).
In line with what the study authors expected, the drugs associated with the highest number of DIL cases were the antitumor necrosis factor agents infliximab, adalimumab, and etanercept, and the drugs associated with the highest disproportional reporting of DIL were procainamide and hydralazine.
“This is an important finding because these are the two drugs associated with the highest risk of DIL in the literature, therefore confirming the reliability of our approach using a large pharmacovigilance database,” the researchers wrote in Annals of the Rheumatic Diseases.
Overall, DIL was considered definite for 9 drugs (procainamide, hydralazine, minocycline, quinidine, isoniazid, terbinafine, methyldopa, dihydralazine, and chlorpromazine), probable for 19 drugs, and possible for 45 drugs.
The median age of DIL onset was 49 years, which the authors noted was about 2 decades older than that of spontaneous SLE.
They also observed a marked predominance in females (female to male sex ratio, 4.3), a finding that contrasted with previous studies reporting a female to male sex ratio closer to 1:1.
Dr. Arnaud and his colleagues stated that their finding of a median delay between the reported start of suspected treatment and DIL occurrence of 172 days (interquartile range, 35-610 days) suggested that DIL mostly appears after a few months and usually within the first 2 years of treatment with the suspected drug.
“The analysis of the median reporting years for each suspected drug shows a clear evolution of suspected drugs during the past decades. This further underlines that the constantly changing spectrum of DIL should be monitored continuously, and further validates the interest of our approach using the WHO international pharmacovigilance database, the biggest database of this kind with over 16 million deduplicated ICSRs,” they wrote.
The researchers added that distinguishing DIL from SLE is important because its prognosis is usually good when the drug is withdrawn, but the spectrum of DIL is constantly evolving, with drugs once described as strongly linked to DIL now prescribed less frequently.
“The first case of DIL was reported in 1945 with sulfadiazine, while hydralazine DIL was first reported in 1953. Since then, pharmacopoeia has strongly evolved, and one could hypothesize that so has the spectrum of drugs that can induce DIL,” they wrote.
“The detailed list of suspected drugs may prove useful to physicians when confronted with potential DIL cases. Altogether, these findings may help in improving the identification of this constantly evolving disease,” they concluded.
The current study was limited by the lack of a uniform set of criteria for the diagnosis of DIL and by the level of reported details available in VigiBase.
The authors had no outside funding for the study and reported having no conflicts of interest.
SOURCE: Arnaud L et al. Ann Rheum Dis. 2019 Feb 4. doi: 10.1136/annrheumdis-2018-214598.
leaving the overall number now standing at 118.
Among the 118 suspected drugs found in VigiBase, the WHO’s global deduplicated individual case safety reports (ICSR) database, 42 had not been previously reported in association with drug-induced lupus (DIL) and 76 had been previously reported in association with DIL in Medline. DIL was reported as a serious adverse event in 55.4% of cases, according to French researchers led by Laurent Arnaud, MD, PhD, of the department of rheumatology at Hôpitaux Universitaires de Strasbourg and Centre National de Références des Maladies Systémiques Rares, Strasbourg, France.
Dr. Arnaud and his colleagues conducted a case-noncase analysis for each drug associated with DIL in order to compare the proportion of specific adverse drug reactions (ADRs) reported for a single drug with the proportion of the same ADR for all other treatments in VigiBase, which receives reports from more than 130 country members of the WHO Programme for International Drug Monitoring and contains over 16 million deduplicated ICSRs recorded by pharmacovigilance centers since 1967. They searched for cases classified as systemic lupus erythematosus (SLE) and identified 12,166 ICSRs of DIL; from these they found 118 suspected drugs with significant pharmacovigilance signal from 8,163 ICSRs that mostly originated from the Americas (65%) and Europe (23%).
In line with what the study authors expected, the drugs associated with the highest number of DIL cases were the antitumor necrosis factor agents infliximab, adalimumab, and etanercept, and the drugs associated with the highest disproportional reporting of DIL were procainamide and hydralazine.
“This is an important finding because these are the two drugs associated with the highest risk of DIL in the literature, therefore confirming the reliability of our approach using a large pharmacovigilance database,” the researchers wrote in Annals of the Rheumatic Diseases.
Overall, DIL was considered definite for 9 drugs (procainamide, hydralazine, minocycline, quinidine, isoniazid, terbinafine, methyldopa, dihydralazine, and chlorpromazine), probable for 19 drugs, and possible for 45 drugs.
The median age of DIL onset was 49 years, which the authors noted was about 2 decades older than that of spontaneous SLE.
They also observed a marked predominance in females (female to male sex ratio, 4.3), a finding that contrasted with previous studies reporting a female to male sex ratio closer to 1:1.
Dr. Arnaud and his colleagues stated that their finding of a median delay between the reported start of suspected treatment and DIL occurrence of 172 days (interquartile range, 35-610 days) suggested that DIL mostly appears after a few months and usually within the first 2 years of treatment with the suspected drug.
“The analysis of the median reporting years for each suspected drug shows a clear evolution of suspected drugs during the past decades. This further underlines that the constantly changing spectrum of DIL should be monitored continuously, and further validates the interest of our approach using the WHO international pharmacovigilance database, the biggest database of this kind with over 16 million deduplicated ICSRs,” they wrote.
The researchers added that distinguishing DIL from SLE is important because its prognosis is usually good when the drug is withdrawn, but the spectrum of DIL is constantly evolving, with drugs once described as strongly linked to DIL now prescribed less frequently.
“The first case of DIL was reported in 1945 with sulfadiazine, while hydralazine DIL was first reported in 1953. Since then, pharmacopoeia has strongly evolved, and one could hypothesize that so has the spectrum of drugs that can induce DIL,” they wrote.
“The detailed list of suspected drugs may prove useful to physicians when confronted with potential DIL cases. Altogether, these findings may help in improving the identification of this constantly evolving disease,” they concluded.
The current study was limited by the lack of a uniform set of criteria for the diagnosis of DIL and by the level of reported details available in VigiBase.
The authors had no outside funding for the study and reported having no conflicts of interest.
SOURCE: Arnaud L et al. Ann Rheum Dis. 2019 Feb 4. doi: 10.1136/annrheumdis-2018-214598.
FROM ANNALS OF THE RHEUMATIC DISEASES
Meta-analysis: Combo therapies best for neuroendocrine tumors
Multiple treatment options, which have shown to be safe and effective for patients with neuroendocrine tumors (NET), are presently available, according to a systematic review and meta-analysis of 30 randomized clinical trials.
“We aimed to identify all [randomized clinical trials] comparing therapeutic interventions in [neuroendocrine tumors],” Reto M. Kaderli, MD, of the University of Bern in Switzerland, and his colleagues wrote in JAMA Oncology.
The researchers searched major databases for studies that compared treatment options for patients with neuroendocrine tumors. After applying the search criteria, the team found 38 studies that included a total of 30 randomized trials.
Various outcome measures were used, including progression-free survival, overall survival, disease control, quality of life, and adverse events. The majority of studies compared an intervention to a comparator of unclear efficacy or placebo. In addition, Dr. Kaderli and his colleagues completed a network meta-analysis to analyze the efficacy of each treatment option.
“The results suggest a superiority of combination therapies, especially of those including somatostatin analogues. In pNETs [pancreatic NETs], somatostatin analogues plus interferon, everolimus, or everolimus plus bevacizumab were highly efficacious. The certainty of evidence for these therapies was variable and was the highest for somatostatin analogues plus everolimus,” Dr. Kaderli and his associates wrote.
Furthermore, the results “suggest a range of monotherapies that are superior to placebo, including everolimus, interferon, and sunitinib in pNETs, and somatostatin analogues in pNETs and GI [gastrointestinal]-NETs,” they said.
The researchers acknowledged that a key limitation of the study was the inability to acquire unpublished data. Consequently, Dr. Kaderli and his colleagues reported there may be a risk of publication bias.
The study was supported by funding from the Insula Stiftung zur Förderung der viszeralchirurgischen Forschung. The authors reported no conflicts of interest.
SOURCE: Kaderli RM et al. JAMA Oncol. 2019 Feb 14. doi: 10.1001/jamaoncol.2018.6720.
One question that remains from the study by Reto M. Kaderli, MD, and his colleagues is the comparative advantages and disadvantages of the various therapies used to treat patients with neuroendocrine tumors.
Recently, several novel therapies have been approved for the treatment of pancreatic and gastrointestinal neuroendocrine tumors, which include agents used for both tumor and symptom control. The majority of studies that evaluated these agents compared them with a comparator of unclear efficacy or placebo. As a result, it is challenging to compare and contrast these competing therapies with respect to efficacy and safety.
Other challenges also exist, such as the biological and clinical diversity seen with neuroendocrine tumors. The current body of literature contains studies that examined different tumor types, including pancreatic, midgut, or gastroenteropancreatic neuroendocrine tumors, which caused significant variation in outcomes. Similar difficulties were seen with the response criteria across these studies.
These results support the active recruitment of patients into randomized comparison studies, which compare these therapies directly. While these types of studies will require larger sample sizes versus placebo-controlled trials, they are necessary to better understand which agents are best in different subtypes of neuroendocrine tumors.
Jonathan R. Strosberg, MD, and Taymeyah Al-Toubah, MPH, are affiliated with the department of gastrointestinal oncology at H. Lee Moffitt Cancer Center and Research Institute in Tampa. Mauro Cives, MD, is affiliated with the department of biomedical sciences and human oncology at the University of Bari in Italy. Dr. Strosberg reported having financial affiliations with Ipsen, Lexicon, and Novartis. These comments are adapted from their accompanying editorial (JAMA Oncol. 2019 Feb 14. doi: 10.1001/jamaoncol.2018.6694 .
One question that remains from the study by Reto M. Kaderli, MD, and his colleagues is the comparative advantages and disadvantages of the various therapies used to treat patients with neuroendocrine tumors.
Recently, several novel therapies have been approved for the treatment of pancreatic and gastrointestinal neuroendocrine tumors, which include agents used for both tumor and symptom control. The majority of studies that evaluated these agents compared them with a comparator of unclear efficacy or placebo. As a result, it is challenging to compare and contrast these competing therapies with respect to efficacy and safety.
Other challenges also exist, such as the biological and clinical diversity seen with neuroendocrine tumors. The current body of literature contains studies that examined different tumor types, including pancreatic, midgut, or gastroenteropancreatic neuroendocrine tumors, which caused significant variation in outcomes. Similar difficulties were seen with the response criteria across these studies.
These results support the active recruitment of patients into randomized comparison studies, which compare these therapies directly. While these types of studies will require larger sample sizes versus placebo-controlled trials, they are necessary to better understand which agents are best in different subtypes of neuroendocrine tumors.
Jonathan R. Strosberg, MD, and Taymeyah Al-Toubah, MPH, are affiliated with the department of gastrointestinal oncology at H. Lee Moffitt Cancer Center and Research Institute in Tampa. Mauro Cives, MD, is affiliated with the department of biomedical sciences and human oncology at the University of Bari in Italy. Dr. Strosberg reported having financial affiliations with Ipsen, Lexicon, and Novartis. These comments are adapted from their accompanying editorial (JAMA Oncol. 2019 Feb 14. doi: 10.1001/jamaoncol.2018.6694 .
One question that remains from the study by Reto M. Kaderli, MD, and his colleagues is the comparative advantages and disadvantages of the various therapies used to treat patients with neuroendocrine tumors.
Recently, several novel therapies have been approved for the treatment of pancreatic and gastrointestinal neuroendocrine tumors, which include agents used for both tumor and symptom control. The majority of studies that evaluated these agents compared them with a comparator of unclear efficacy or placebo. As a result, it is challenging to compare and contrast these competing therapies with respect to efficacy and safety.
Other challenges also exist, such as the biological and clinical diversity seen with neuroendocrine tumors. The current body of literature contains studies that examined different tumor types, including pancreatic, midgut, or gastroenteropancreatic neuroendocrine tumors, which caused significant variation in outcomes. Similar difficulties were seen with the response criteria across these studies.
These results support the active recruitment of patients into randomized comparison studies, which compare these therapies directly. While these types of studies will require larger sample sizes versus placebo-controlled trials, they are necessary to better understand which agents are best in different subtypes of neuroendocrine tumors.
Jonathan R. Strosberg, MD, and Taymeyah Al-Toubah, MPH, are affiliated with the department of gastrointestinal oncology at H. Lee Moffitt Cancer Center and Research Institute in Tampa. Mauro Cives, MD, is affiliated with the department of biomedical sciences and human oncology at the University of Bari in Italy. Dr. Strosberg reported having financial affiliations with Ipsen, Lexicon, and Novartis. These comments are adapted from their accompanying editorial (JAMA Oncol. 2019 Feb 14. doi: 10.1001/jamaoncol.2018.6694 .
Multiple treatment options, which have shown to be safe and effective for patients with neuroendocrine tumors (NET), are presently available, according to a systematic review and meta-analysis of 30 randomized clinical trials.
“We aimed to identify all [randomized clinical trials] comparing therapeutic interventions in [neuroendocrine tumors],” Reto M. Kaderli, MD, of the University of Bern in Switzerland, and his colleagues wrote in JAMA Oncology.
The researchers searched major databases for studies that compared treatment options for patients with neuroendocrine tumors. After applying the search criteria, the team found 38 studies that included a total of 30 randomized trials.
Various outcome measures were used, including progression-free survival, overall survival, disease control, quality of life, and adverse events. The majority of studies compared an intervention to a comparator of unclear efficacy or placebo. In addition, Dr. Kaderli and his colleagues completed a network meta-analysis to analyze the efficacy of each treatment option.
“The results suggest a superiority of combination therapies, especially of those including somatostatin analogues. In pNETs [pancreatic NETs], somatostatin analogues plus interferon, everolimus, or everolimus plus bevacizumab were highly efficacious. The certainty of evidence for these therapies was variable and was the highest for somatostatin analogues plus everolimus,” Dr. Kaderli and his associates wrote.
Furthermore, the results “suggest a range of monotherapies that are superior to placebo, including everolimus, interferon, and sunitinib in pNETs, and somatostatin analogues in pNETs and GI [gastrointestinal]-NETs,” they said.
The researchers acknowledged that a key limitation of the study was the inability to acquire unpublished data. Consequently, Dr. Kaderli and his colleagues reported there may be a risk of publication bias.
The study was supported by funding from the Insula Stiftung zur Förderung der viszeralchirurgischen Forschung. The authors reported no conflicts of interest.
SOURCE: Kaderli RM et al. JAMA Oncol. 2019 Feb 14. doi: 10.1001/jamaoncol.2018.6720.
Multiple treatment options, which have shown to be safe and effective for patients with neuroendocrine tumors (NET), are presently available, according to a systematic review and meta-analysis of 30 randomized clinical trials.
“We aimed to identify all [randomized clinical trials] comparing therapeutic interventions in [neuroendocrine tumors],” Reto M. Kaderli, MD, of the University of Bern in Switzerland, and his colleagues wrote in JAMA Oncology.
The researchers searched major databases for studies that compared treatment options for patients with neuroendocrine tumors. After applying the search criteria, the team found 38 studies that included a total of 30 randomized trials.
Various outcome measures were used, including progression-free survival, overall survival, disease control, quality of life, and adverse events. The majority of studies compared an intervention to a comparator of unclear efficacy or placebo. In addition, Dr. Kaderli and his colleagues completed a network meta-analysis to analyze the efficacy of each treatment option.
“The results suggest a superiority of combination therapies, especially of those including somatostatin analogues. In pNETs [pancreatic NETs], somatostatin analogues plus interferon, everolimus, or everolimus plus bevacizumab were highly efficacious. The certainty of evidence for these therapies was variable and was the highest for somatostatin analogues plus everolimus,” Dr. Kaderli and his associates wrote.
Furthermore, the results “suggest a range of monotherapies that are superior to placebo, including everolimus, interferon, and sunitinib in pNETs, and somatostatin analogues in pNETs and GI [gastrointestinal]-NETs,” they said.
The researchers acknowledged that a key limitation of the study was the inability to acquire unpublished data. Consequently, Dr. Kaderli and his colleagues reported there may be a risk of publication bias.
The study was supported by funding from the Insula Stiftung zur Förderung der viszeralchirurgischen Forschung. The authors reported no conflicts of interest.
SOURCE: Kaderli RM et al. JAMA Oncol. 2019 Feb 14. doi: 10.1001/jamaoncol.2018.6720.
FROM JAMA ONCOLOGY
Shaping the future of hospital medicine
Dr. Therese Franco leads SHM’s Pacific Northwest chapter
Therese Franco, MD, SFHM, a hospitalist at the Virginia Mason Medical Center in Seattle, is the current president of SHM’s Pacific Northwest chapter.
The Hospitalist recently sat down with her to learn about her background and discuss some of the initiatives that the Pacific Northwest chapter has been working on.
Can you tell us about your education and training on the way to becoming a hospitalist?
My undergraduate degree is in engineering from Michigan State University. I then went to the University of Michigan in Ann Arbor and did one degree at the School of Public Health in environmental and industrial health, and another degree in the College of Engineering in industrial and operations engineering. In my work with the safety department at an automotive company, I found I was spending a lot of time looking at data, and not talking to people. I got into a conversation with one of the occupational medicine physicians there, and he said, “You ought to try this.” I spoke with a good friend, who was a medical student, and she agreed.
So then I went to medical school thinking that I would practice occupational medicine. I went to medical school at Wayne State University in Detroit and did a couple of rotations in occupational medicine. I wasn’t sure that was the right fit, so I then went off to residency in internal medicine at the University of Connecticut and really enjoyed my wards experience. I liked the pace, I liked the variety, and just really liked all of hospital medicine. So that’s what I decided to do.
What are your areas of research interest?
This year I’m doing a research fellowship through the Center for Healthcare Improvement Science, at Virginia Mason. Through SHM’s mentored implementation program, I have done a lot of work on diabetes and glycemic control but never really published much of it. I think it is so important to share what you learn, so I’m working on publishing some of our results from the diabetes work.
Another area of interest is advanced-practice providers in hospital medicine, which I think is very important, given all the issues that health care is facing. I think that medicine has gotten more complex and that we’re going to have to look at working in a collaborative, inter-professional, multidisciplinary way. I think that advanced practice can really improve the care of hospitalized patients, if we practice appropriate skill-task alignment, develop a culture of mutual respect, and find the best ways to deploy our advanced-practice providers and our physicians.
That can be challenging. Some people, I think, are worried about losing their jobs, and some people feel like they want to “own” all of the patient, because it’s such a part of the culture of medicine. So it’s a really complicated issue, and I think that doctors are going to have to get used to delegating tasks that they used to perform.
So a collaborative practice requires both a professional and a cultural shift?
I think so. I was our inaugural program director for an advanced-practice fellowship in hospital medicine, and in that role, I attended conferences and learning events for program development. I think that many institutions are facing some of the same challenges. For the most part, I’m optimistic about things. I think we’re on the right track, and help is on the way – we just have to figure out how to use it.
Has your institution made any changes along these lines?
We’re primarily using the fellowship as a tool to recruit and retain some of the brightest and best. We’ve got three fellows that matriculated from our program and are currently working in the section of hospital medicine. Everyone’s been really flexible and open to the idea that the job description is emerging. I think my colleagues are very appreciative of our advanced-practice providers. We’ve got two nurse practitioners and one physician assistant who is also a PhD-trained pharmacist. They’ve been great additions to our team.
What are some of the other issues that the Pacific Northwest chapter members are concerned about?
One of our most successful meetings was around telemedicine. There’s a lot of interest in that, and it’s very financially and technically complex. Some hospitals in the area are really doing novel things. One of the most interesting things is an addiction medicine teleconsult.
That’s out of Swedish Medical Center, Seattle. Of course there’s telestroke, which I think is picking up in popularity. We had speakers from Virginia Mason who presented on telestroke. Some institutions are even doing admissions this way. The University of Washington is doing some good antimicrobial stewardship work. They present cases and they teleconference and have an infectious disease consultant. It’s not a program directed at revenue generation, but is focused instead on sharing and spreading expertise.
Our chapter also hosted a presentation on burnout that was pretty well attended. And then, unfortunately, we did lose a hospitalist to suicide over the summer. That was the inspiration for offering the screening of the movie, “Do No Harm: Exposing the Hippocratic Hoax.”
What was the program that you put together around the screening?
We had the filmmaker come for the screening, and we organized a panel discussion with a wellness officer from a local clinic and a psychiatrist who used to be on the board of the Physician Health Program. John Nelson, MD, MHM, one of SHM’s cofounders and a local hospitalist here, also participated as a panelist.
Overall, the event was well received. There were some things that I didn’t really expect. I’m not sure that the film resonated with too many people in the room. It is very much directed at the educational process – med students and residents – and at times the dialogue is a little inflammatory.
I learned a few important things from the film. I did not realize that the tragedy of physician suicide is not unique to the United States – it’s an international issue. And we sometimes use the term “pimping” to talk about questioning interns or residents on rounds. Apparently, that stands for “put in my place,” which is very condescending and unacceptable. I will not use the term again.
I think future conversations need to come from thoughtful, rational, respectful leaders who are willing to work with regulatory agencies, hospitals, and administrators. If we want to move forward, physicians, administrators, and the public need to come together in the best interest of the patient and of public health. And I don’t know who leads that conversation.
Will your chapter have another event around that subject?
We will do what our membership wants and needs. We meet quarterly, and once a year we hold a people’s choice meeting and I solicit topics. If members want to keep the conversation moving, I’m going to do what I can to support them.
What are some other issues that stand out as important to your chapter?
One key topic is the financial side of hospitalist practice, and dealing with issues that seem to create inefficiencies – regulatory issues, documentation issues, things that are important because we want to tell the story of what we’re doing. We certainly want to be reimbursed for the value-added work that we’re doing, but a lot of value-added work creates inefficiencies of practice, and I hear a lot of dissatisfaction around documentation, coding, billing, and other issues related to reimbursement. While people are concerned about these problems, nobody wants to talk about them. They just want somebody to fix it. So I’m not sure what to do with that, because I think if I had a meeting about coding and billing, I would have three attendees.
But our annual poster meeting is always well attended. We always do it at the end of the year, to kick off the holiday season. It’s a nice opportunity to connect socially with colleagues because you mix and you mingle and look at the posters. We had some really great posters, and our top three prize winners were medical students, which is inspirational. They make you feel good about the future.
Our chapter is trying to diversify geographically and clinically. We were fortunate to receive a development funds grant to use technology to do streaming meetings. Our hope is that we can host streaming meetings and eventually transition hosting to rotate around the state. Once there’s large enough attendance, the different delegates can develop their own leadership teams and, eventually, their own chapter. We’re hoping to grow the organization that way.
What else is on the horizon for hospitalists in the Pacific Northwest?
I’d like to see more frequent meetings and a greater variety of meetings. I think there’s interest in adding some kind of service element to the chapter. Maybe we can do a blood pressure screening at a sporting event.
I think we’ll also be focusing on students and residents and trying to create support for them. We held a student event around financial planning, and that was very well attended. I think we would like to do something around mentorship. Of course it’s hard to find mentors, because everybody is so busy.
Our chapter really needs to leverage our technology if we want to have the reach that I’m talking about. I’m looking forward to piloting the streaming meeting concept, and I hope to do some live polling of our meeting attendees to get them engaged. I hope we continue to grow and keep the dialogue going about what matters in hospital medicine, and do our part to shape the future in the way we want it.
Dr. Therese Franco leads SHM’s Pacific Northwest chapter
Dr. Therese Franco leads SHM’s Pacific Northwest chapter
Therese Franco, MD, SFHM, a hospitalist at the Virginia Mason Medical Center in Seattle, is the current president of SHM’s Pacific Northwest chapter.
The Hospitalist recently sat down with her to learn about her background and discuss some of the initiatives that the Pacific Northwest chapter has been working on.
Can you tell us about your education and training on the way to becoming a hospitalist?
My undergraduate degree is in engineering from Michigan State University. I then went to the University of Michigan in Ann Arbor and did one degree at the School of Public Health in environmental and industrial health, and another degree in the College of Engineering in industrial and operations engineering. In my work with the safety department at an automotive company, I found I was spending a lot of time looking at data, and not talking to people. I got into a conversation with one of the occupational medicine physicians there, and he said, “You ought to try this.” I spoke with a good friend, who was a medical student, and she agreed.
So then I went to medical school thinking that I would practice occupational medicine. I went to medical school at Wayne State University in Detroit and did a couple of rotations in occupational medicine. I wasn’t sure that was the right fit, so I then went off to residency in internal medicine at the University of Connecticut and really enjoyed my wards experience. I liked the pace, I liked the variety, and just really liked all of hospital medicine. So that’s what I decided to do.
What are your areas of research interest?
This year I’m doing a research fellowship through the Center for Healthcare Improvement Science, at Virginia Mason. Through SHM’s mentored implementation program, I have done a lot of work on diabetes and glycemic control but never really published much of it. I think it is so important to share what you learn, so I’m working on publishing some of our results from the diabetes work.
Another area of interest is advanced-practice providers in hospital medicine, which I think is very important, given all the issues that health care is facing. I think that medicine has gotten more complex and that we’re going to have to look at working in a collaborative, inter-professional, multidisciplinary way. I think that advanced practice can really improve the care of hospitalized patients, if we practice appropriate skill-task alignment, develop a culture of mutual respect, and find the best ways to deploy our advanced-practice providers and our physicians.
That can be challenging. Some people, I think, are worried about losing their jobs, and some people feel like they want to “own” all of the patient, because it’s such a part of the culture of medicine. So it’s a really complicated issue, and I think that doctors are going to have to get used to delegating tasks that they used to perform.
So a collaborative practice requires both a professional and a cultural shift?
I think so. I was our inaugural program director for an advanced-practice fellowship in hospital medicine, and in that role, I attended conferences and learning events for program development. I think that many institutions are facing some of the same challenges. For the most part, I’m optimistic about things. I think we’re on the right track, and help is on the way – we just have to figure out how to use it.
Has your institution made any changes along these lines?
We’re primarily using the fellowship as a tool to recruit and retain some of the brightest and best. We’ve got three fellows that matriculated from our program and are currently working in the section of hospital medicine. Everyone’s been really flexible and open to the idea that the job description is emerging. I think my colleagues are very appreciative of our advanced-practice providers. We’ve got two nurse practitioners and one physician assistant who is also a PhD-trained pharmacist. They’ve been great additions to our team.
What are some of the other issues that the Pacific Northwest chapter members are concerned about?
One of our most successful meetings was around telemedicine. There’s a lot of interest in that, and it’s very financially and technically complex. Some hospitals in the area are really doing novel things. One of the most interesting things is an addiction medicine teleconsult.
That’s out of Swedish Medical Center, Seattle. Of course there’s telestroke, which I think is picking up in popularity. We had speakers from Virginia Mason who presented on telestroke. Some institutions are even doing admissions this way. The University of Washington is doing some good antimicrobial stewardship work. They present cases and they teleconference and have an infectious disease consultant. It’s not a program directed at revenue generation, but is focused instead on sharing and spreading expertise.
Our chapter also hosted a presentation on burnout that was pretty well attended. And then, unfortunately, we did lose a hospitalist to suicide over the summer. That was the inspiration for offering the screening of the movie, “Do No Harm: Exposing the Hippocratic Hoax.”
What was the program that you put together around the screening?
We had the filmmaker come for the screening, and we organized a panel discussion with a wellness officer from a local clinic and a psychiatrist who used to be on the board of the Physician Health Program. John Nelson, MD, MHM, one of SHM’s cofounders and a local hospitalist here, also participated as a panelist.
Overall, the event was well received. There were some things that I didn’t really expect. I’m not sure that the film resonated with too many people in the room. It is very much directed at the educational process – med students and residents – and at times the dialogue is a little inflammatory.
I learned a few important things from the film. I did not realize that the tragedy of physician suicide is not unique to the United States – it’s an international issue. And we sometimes use the term “pimping” to talk about questioning interns or residents on rounds. Apparently, that stands for “put in my place,” which is very condescending and unacceptable. I will not use the term again.
I think future conversations need to come from thoughtful, rational, respectful leaders who are willing to work with regulatory agencies, hospitals, and administrators. If we want to move forward, physicians, administrators, and the public need to come together in the best interest of the patient and of public health. And I don’t know who leads that conversation.
Will your chapter have another event around that subject?
We will do what our membership wants and needs. We meet quarterly, and once a year we hold a people’s choice meeting and I solicit topics. If members want to keep the conversation moving, I’m going to do what I can to support them.
What are some other issues that stand out as important to your chapter?
One key topic is the financial side of hospitalist practice, and dealing with issues that seem to create inefficiencies – regulatory issues, documentation issues, things that are important because we want to tell the story of what we’re doing. We certainly want to be reimbursed for the value-added work that we’re doing, but a lot of value-added work creates inefficiencies of practice, and I hear a lot of dissatisfaction around documentation, coding, billing, and other issues related to reimbursement. While people are concerned about these problems, nobody wants to talk about them. They just want somebody to fix it. So I’m not sure what to do with that, because I think if I had a meeting about coding and billing, I would have three attendees.
But our annual poster meeting is always well attended. We always do it at the end of the year, to kick off the holiday season. It’s a nice opportunity to connect socially with colleagues because you mix and you mingle and look at the posters. We had some really great posters, and our top three prize winners were medical students, which is inspirational. They make you feel good about the future.
Our chapter is trying to diversify geographically and clinically. We were fortunate to receive a development funds grant to use technology to do streaming meetings. Our hope is that we can host streaming meetings and eventually transition hosting to rotate around the state. Once there’s large enough attendance, the different delegates can develop their own leadership teams and, eventually, their own chapter. We’re hoping to grow the organization that way.
What else is on the horizon for hospitalists in the Pacific Northwest?
I’d like to see more frequent meetings and a greater variety of meetings. I think there’s interest in adding some kind of service element to the chapter. Maybe we can do a blood pressure screening at a sporting event.
I think we’ll also be focusing on students and residents and trying to create support for them. We held a student event around financial planning, and that was very well attended. I think we would like to do something around mentorship. Of course it’s hard to find mentors, because everybody is so busy.
Our chapter really needs to leverage our technology if we want to have the reach that I’m talking about. I’m looking forward to piloting the streaming meeting concept, and I hope to do some live polling of our meeting attendees to get them engaged. I hope we continue to grow and keep the dialogue going about what matters in hospital medicine, and do our part to shape the future in the way we want it.
Therese Franco, MD, SFHM, a hospitalist at the Virginia Mason Medical Center in Seattle, is the current president of SHM’s Pacific Northwest chapter.
The Hospitalist recently sat down with her to learn about her background and discuss some of the initiatives that the Pacific Northwest chapter has been working on.
Can you tell us about your education and training on the way to becoming a hospitalist?
My undergraduate degree is in engineering from Michigan State University. I then went to the University of Michigan in Ann Arbor and did one degree at the School of Public Health in environmental and industrial health, and another degree in the College of Engineering in industrial and operations engineering. In my work with the safety department at an automotive company, I found I was spending a lot of time looking at data, and not talking to people. I got into a conversation with one of the occupational medicine physicians there, and he said, “You ought to try this.” I spoke with a good friend, who was a medical student, and she agreed.
So then I went to medical school thinking that I would practice occupational medicine. I went to medical school at Wayne State University in Detroit and did a couple of rotations in occupational medicine. I wasn’t sure that was the right fit, so I then went off to residency in internal medicine at the University of Connecticut and really enjoyed my wards experience. I liked the pace, I liked the variety, and just really liked all of hospital medicine. So that’s what I decided to do.
What are your areas of research interest?
This year I’m doing a research fellowship through the Center for Healthcare Improvement Science, at Virginia Mason. Through SHM’s mentored implementation program, I have done a lot of work on diabetes and glycemic control but never really published much of it. I think it is so important to share what you learn, so I’m working on publishing some of our results from the diabetes work.
Another area of interest is advanced-practice providers in hospital medicine, which I think is very important, given all the issues that health care is facing. I think that medicine has gotten more complex and that we’re going to have to look at working in a collaborative, inter-professional, multidisciplinary way. I think that advanced practice can really improve the care of hospitalized patients, if we practice appropriate skill-task alignment, develop a culture of mutual respect, and find the best ways to deploy our advanced-practice providers and our physicians.
That can be challenging. Some people, I think, are worried about losing their jobs, and some people feel like they want to “own” all of the patient, because it’s such a part of the culture of medicine. So it’s a really complicated issue, and I think that doctors are going to have to get used to delegating tasks that they used to perform.
So a collaborative practice requires both a professional and a cultural shift?
I think so. I was our inaugural program director for an advanced-practice fellowship in hospital medicine, and in that role, I attended conferences and learning events for program development. I think that many institutions are facing some of the same challenges. For the most part, I’m optimistic about things. I think we’re on the right track, and help is on the way – we just have to figure out how to use it.
Has your institution made any changes along these lines?
We’re primarily using the fellowship as a tool to recruit and retain some of the brightest and best. We’ve got three fellows that matriculated from our program and are currently working in the section of hospital medicine. Everyone’s been really flexible and open to the idea that the job description is emerging. I think my colleagues are very appreciative of our advanced-practice providers. We’ve got two nurse practitioners and one physician assistant who is also a PhD-trained pharmacist. They’ve been great additions to our team.
What are some of the other issues that the Pacific Northwest chapter members are concerned about?
One of our most successful meetings was around telemedicine. There’s a lot of interest in that, and it’s very financially and technically complex. Some hospitals in the area are really doing novel things. One of the most interesting things is an addiction medicine teleconsult.
That’s out of Swedish Medical Center, Seattle. Of course there’s telestroke, which I think is picking up in popularity. We had speakers from Virginia Mason who presented on telestroke. Some institutions are even doing admissions this way. The University of Washington is doing some good antimicrobial stewardship work. They present cases and they teleconference and have an infectious disease consultant. It’s not a program directed at revenue generation, but is focused instead on sharing and spreading expertise.
Our chapter also hosted a presentation on burnout that was pretty well attended. And then, unfortunately, we did lose a hospitalist to suicide over the summer. That was the inspiration for offering the screening of the movie, “Do No Harm: Exposing the Hippocratic Hoax.”
What was the program that you put together around the screening?
We had the filmmaker come for the screening, and we organized a panel discussion with a wellness officer from a local clinic and a psychiatrist who used to be on the board of the Physician Health Program. John Nelson, MD, MHM, one of SHM’s cofounders and a local hospitalist here, also participated as a panelist.
Overall, the event was well received. There were some things that I didn’t really expect. I’m not sure that the film resonated with too many people in the room. It is very much directed at the educational process – med students and residents – and at times the dialogue is a little inflammatory.
I learned a few important things from the film. I did not realize that the tragedy of physician suicide is not unique to the United States – it’s an international issue. And we sometimes use the term “pimping” to talk about questioning interns or residents on rounds. Apparently, that stands for “put in my place,” which is very condescending and unacceptable. I will not use the term again.
I think future conversations need to come from thoughtful, rational, respectful leaders who are willing to work with regulatory agencies, hospitals, and administrators. If we want to move forward, physicians, administrators, and the public need to come together in the best interest of the patient and of public health. And I don’t know who leads that conversation.
Will your chapter have another event around that subject?
We will do what our membership wants and needs. We meet quarterly, and once a year we hold a people’s choice meeting and I solicit topics. If members want to keep the conversation moving, I’m going to do what I can to support them.
What are some other issues that stand out as important to your chapter?
One key topic is the financial side of hospitalist practice, and dealing with issues that seem to create inefficiencies – regulatory issues, documentation issues, things that are important because we want to tell the story of what we’re doing. We certainly want to be reimbursed for the value-added work that we’re doing, but a lot of value-added work creates inefficiencies of practice, and I hear a lot of dissatisfaction around documentation, coding, billing, and other issues related to reimbursement. While people are concerned about these problems, nobody wants to talk about them. They just want somebody to fix it. So I’m not sure what to do with that, because I think if I had a meeting about coding and billing, I would have three attendees.
But our annual poster meeting is always well attended. We always do it at the end of the year, to kick off the holiday season. It’s a nice opportunity to connect socially with colleagues because you mix and you mingle and look at the posters. We had some really great posters, and our top three prize winners were medical students, which is inspirational. They make you feel good about the future.
Our chapter is trying to diversify geographically and clinically. We were fortunate to receive a development funds grant to use technology to do streaming meetings. Our hope is that we can host streaming meetings and eventually transition hosting to rotate around the state. Once there’s large enough attendance, the different delegates can develop their own leadership teams and, eventually, their own chapter. We’re hoping to grow the organization that way.
What else is on the horizon for hospitalists in the Pacific Northwest?
I’d like to see more frequent meetings and a greater variety of meetings. I think there’s interest in adding some kind of service element to the chapter. Maybe we can do a blood pressure screening at a sporting event.
I think we’ll also be focusing on students and residents and trying to create support for them. We held a student event around financial planning, and that was very well attended. I think we would like to do something around mentorship. Of course it’s hard to find mentors, because everybody is so busy.
Our chapter really needs to leverage our technology if we want to have the reach that I’m talking about. I’m looking forward to piloting the streaming meeting concept, and I hope to do some live polling of our meeting attendees to get them engaged. I hope we continue to grow and keep the dialogue going about what matters in hospital medicine, and do our part to shape the future in the way we want it.
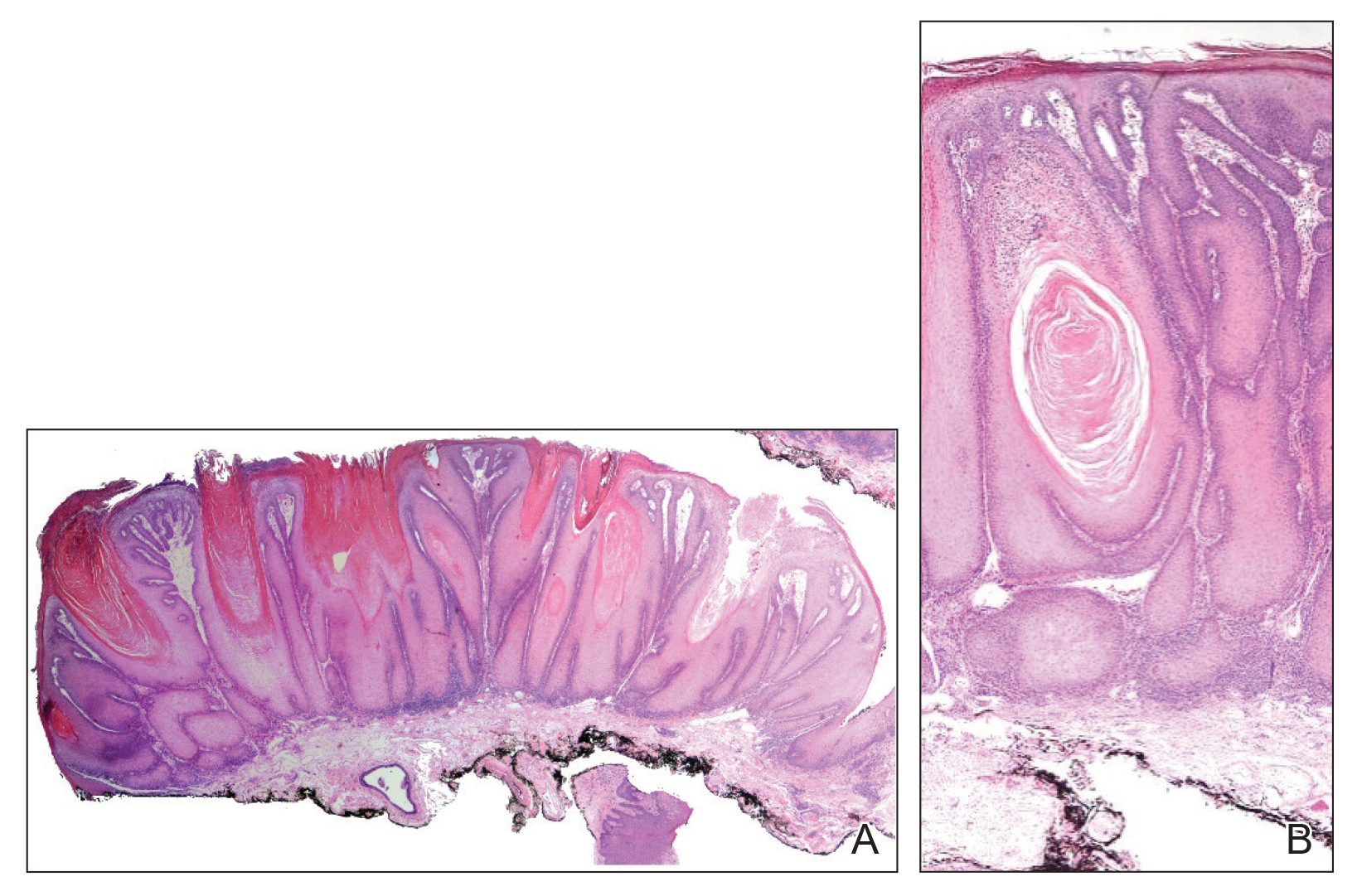
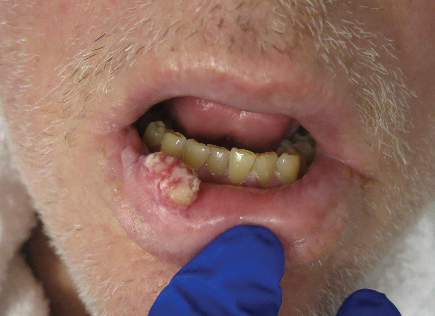
Outcomes similar for skin graft, synthetic mesh in incisional hernia repair
Giant incisional , according to findings published in Hernia.
In a prospective, randomized study of 52 patients (24 who received FTSG and 28 who received synthetic mesh), two recurrences were found at 1-year follow up in each group. Results for pain, patient satisfaction, and aesthetic outcome did not differ significantly between the groups, reported Viktor Holmdahl of the department of surgery and perioperative sciences at Umeå (Sweden) University and his coauthors.
Patients were aged 18 years or older, with symptomatic giant (greater than 10 cm) ventral incisional hernia. All patients had computerized tomography (CT) imaging of the abdominal wall, and were assessed for abdominal wall muscle strength via the Biodex Multi-Joint System-4.
The synthetic mesh used was a polypropylene mesh overlapping the repaired hernia defect by at least 5 cm. The FTSG was always placed in an onlay position, and was taken from excess skin adjacent to the midline incision made for the hernia repair. Postoperative care was identical for both groups, Dr. Holmdahl and his colleagues reported.
At 1 year, all patients received a follow-up Biodex assessment and were evaluated for recurrence. Aesthetic outcomes such as scar appearance and wound healing were assessed. Patients self-reported pain levels using a visual analogue scale.
Two patients in both the FTSG group (8.3%) and the synthetic mesh group (7.1%) had recurrence (P = 1.000). Differences between groups for the other measures were not significant, including for well-healed scars (91.3% for FTSG vs. 96.4% for synthetic mesh) and excess skin (60.9% vs. 57.1%). Biodex results also did not differ significantly between the groups.
The similarities in results between the two techniques “indicate that FTSG possibly has a future role in hernia repair, but more research is needed,” the authors concluded.
The study was supported by the Swedish Research Council and by a regional agreement between Umeå University and Västerbotten County Council. The investigators declared no conflicts of interest.
SOURCE: Holmdahl V et al. Hernia 2019 Feb 8. doi: 10.1007/s10029-019-01900-4.
Giant incisional , according to findings published in Hernia.
In a prospective, randomized study of 52 patients (24 who received FTSG and 28 who received synthetic mesh), two recurrences were found at 1-year follow up in each group. Results for pain, patient satisfaction, and aesthetic outcome did not differ significantly between the groups, reported Viktor Holmdahl of the department of surgery and perioperative sciences at Umeå (Sweden) University and his coauthors.
Patients were aged 18 years or older, with symptomatic giant (greater than 10 cm) ventral incisional hernia. All patients had computerized tomography (CT) imaging of the abdominal wall, and were assessed for abdominal wall muscle strength via the Biodex Multi-Joint System-4.
The synthetic mesh used was a polypropylene mesh overlapping the repaired hernia defect by at least 5 cm. The FTSG was always placed in an onlay position, and was taken from excess skin adjacent to the midline incision made for the hernia repair. Postoperative care was identical for both groups, Dr. Holmdahl and his colleagues reported.
At 1 year, all patients received a follow-up Biodex assessment and were evaluated for recurrence. Aesthetic outcomes such as scar appearance and wound healing were assessed. Patients self-reported pain levels using a visual analogue scale.
Two patients in both the FTSG group (8.3%) and the synthetic mesh group (7.1%) had recurrence (P = 1.000). Differences between groups for the other measures were not significant, including for well-healed scars (91.3% for FTSG vs. 96.4% for synthetic mesh) and excess skin (60.9% vs. 57.1%). Biodex results also did not differ significantly between the groups.
The similarities in results between the two techniques “indicate that FTSG possibly has a future role in hernia repair, but more research is needed,” the authors concluded.
The study was supported by the Swedish Research Council and by a regional agreement between Umeå University and Västerbotten County Council. The investigators declared no conflicts of interest.
SOURCE: Holmdahl V et al. Hernia 2019 Feb 8. doi: 10.1007/s10029-019-01900-4.
Giant incisional , according to findings published in Hernia.
In a prospective, randomized study of 52 patients (24 who received FTSG and 28 who received synthetic mesh), two recurrences were found at 1-year follow up in each group. Results for pain, patient satisfaction, and aesthetic outcome did not differ significantly between the groups, reported Viktor Holmdahl of the department of surgery and perioperative sciences at Umeå (Sweden) University and his coauthors.
Patients were aged 18 years or older, with symptomatic giant (greater than 10 cm) ventral incisional hernia. All patients had computerized tomography (CT) imaging of the abdominal wall, and were assessed for abdominal wall muscle strength via the Biodex Multi-Joint System-4.
The synthetic mesh used was a polypropylene mesh overlapping the repaired hernia defect by at least 5 cm. The FTSG was always placed in an onlay position, and was taken from excess skin adjacent to the midline incision made for the hernia repair. Postoperative care was identical for both groups, Dr. Holmdahl and his colleagues reported.
At 1 year, all patients received a follow-up Biodex assessment and were evaluated for recurrence. Aesthetic outcomes such as scar appearance and wound healing were assessed. Patients self-reported pain levels using a visual analogue scale.
Two patients in both the FTSG group (8.3%) and the synthetic mesh group (7.1%) had recurrence (P = 1.000). Differences between groups for the other measures were not significant, including for well-healed scars (91.3% for FTSG vs. 96.4% for synthetic mesh) and excess skin (60.9% vs. 57.1%). Biodex results also did not differ significantly between the groups.
The similarities in results between the two techniques “indicate that FTSG possibly has a future role in hernia repair, but more research is needed,” the authors concluded.
The study was supported by the Swedish Research Council and by a regional agreement between Umeå University and Västerbotten County Council. The investigators declared no conflicts of interest.
SOURCE: Holmdahl V et al. Hernia 2019 Feb 8. doi: 10.1007/s10029-019-01900-4.
FROM HERNIA
Possible mechanism for fluoroquinolone-induced aortopathy uncovered
A new study finds that patients taking fluoroquinolone antibiotics may be at higher risk of aortopathy in part because of human aortic myofibroblast–mediated extracellular matrix (ECM) dysregulation.
“Emerging evidence supports pharmacologic-associated aortopathy in patients receiving fluoroquinolone [FQ] antibiotics,” said first author David G. Guzzardi, PhD, and his colleagues, citing previous research showing that, “compared with patients receiving amoxicillin antibiotics, those receiving FQ have a 66% higher risk of aneurysm or dissection within a 2-month period after commencing FQ use.”
Based upon such data, the Food and Drug Administration issued a December 2018 warning about the increased risk of ruptures or tears in the aorta with fluoroquinolone antibiotics in certain patients, updating their May 2017 warning regarding “disabling and potentially permanent side effects of the tendons, muscles, joints, nerves, and central nervous system that can occur together in the same patient,” upon exposure to this class of antibiotics. Earlier in 2018, the FDA had reinforced their safety information about serious and potentially fatal low blood sugar levels and mental health side effects with fluoroquinolone antibiotics.
Dr. Guzzardi and his colleagues at the University of Calgary (Alta.) performed a study to attempt to determine the possible cellular mechanisms for the observed aortopathy. In their study published in the Journal of Thoracic and Cardiovascular Surgery, Dr. Guzzardi and his colleagues isolated human aortic myofibroblasts from nine patients with aortopathy who were undergoing elective ascending aortic resection.
Following exposure of cells to FQ, the researchers assessed secreted matrix metalloproteinases relative to tissue inhibitors of matrix metalloproteinases (TIMPs). In addition, they examined ECM degradation by using a three-dimensional gelatin-fluorescein isothiocyanate fluorescence microgel assay. Aortic cellular collagen type I expression following FQ exposure was determined by immunoblotting and immunofluorescent staining. Dr. Guzzardi and his colleagues also looked at cell apoptosis, necrosis, and metabolic viability using two versions of vital staining.
They found that FQ exposure significantly decreased aortic cell TIMP-1 (P less than .004) and TIMP-2 (P less than .0004) protein expression, compared with controls, and the ratio of matrix metalloproteinase 9/TIMP-2 was increased (P less than .01). This suggests an increased capacity for ECM degradation after FQ exposure, according to the researchers.
In addition, FQ exposure attenuated collagen type I expression as assessed by immunoblotting (P less than .002) and immunofluorescence (P less than .02).
“FQ induces human aortic myofibroblast–mediated ECM dysregulation by decreasing TIMP expression and preventing compensatory collagen deposition. These data provide novel insights into the mechanisms that may underlie the clinical association of FQ exposure and increased risk of acute aortic events in the community. Our data suggest cautious use of FQ in selected patient populations with preexistent aortopathy and connective tissue disorders,” the researchers concluded.
In an accompanying editorial, while warning that these are preliminary observations based upon a small number of patients with aortopathy, Ari A. Mennander, MD, PhD, of Tampere (Finland) University, wrote that, “for the first time, the wild theory of fluoroquinolone-associated aortopathy has a molecular hint that is based on collagen degeneration and progression of aortic disease. ... This theory is in line with previous observations revealing antifibrotic activity and decreased collagen-1 protein expression with fluoroquinolones. The enigmatic puzzle of the progression of some aortic events may alarmingly be iatrogenic, and the clinician may wisely consider a prudent use of fluoroquinolones in patients with aortic dilatation.”
The authors and commentators reported that they had no commercial conflicts to disclose.
SOURCE: Guzzardi DG et al. J Thorac Cardiovasc Surg. 2019;157:109-19.
The issue of fluoroquinolones is certainly of concern. I wonder how many of my patients who have suffered a ruptured aneurysm were on one of these drugs? In the last few years, Cipro (ciprofloxacin) and Levaquin (levofloxacin) were commonly used by our family practice and internal medicine colleagues for almost all outpatient infections. It was so common that even my wife, who is not a physician, would request Cipro whenever she had a sneeze. We would also bring large bottles of Cipro every time we went traveling to some exotic destination, reassuring ourselves that the only “runs” we would have would be in the airport trying to catch a flight. A cousin of mine, prescribed Levaquin by a well-meaning physician while he was cruising the Nile River, ruptured his Achilles tendon.
Russell H. Samson, MD, FACS, DFSVS , is a clinical professor of surgery at Florida State University, Sarasota, a senior surgeon at Sarasota Vascular Specialists, and President of the Mote Vascular Foundation.
The issue of fluoroquinolones is certainly of concern. I wonder how many of my patients who have suffered a ruptured aneurysm were on one of these drugs? In the last few years, Cipro (ciprofloxacin) and Levaquin (levofloxacin) were commonly used by our family practice and internal medicine colleagues for almost all outpatient infections. It was so common that even my wife, who is not a physician, would request Cipro whenever she had a sneeze. We would also bring large bottles of Cipro every time we went traveling to some exotic destination, reassuring ourselves that the only “runs” we would have would be in the airport trying to catch a flight. A cousin of mine, prescribed Levaquin by a well-meaning physician while he was cruising the Nile River, ruptured his Achilles tendon.
Russell H. Samson, MD, FACS, DFSVS , is a clinical professor of surgery at Florida State University, Sarasota, a senior surgeon at Sarasota Vascular Specialists, and President of the Mote Vascular Foundation.
The issue of fluoroquinolones is certainly of concern. I wonder how many of my patients who have suffered a ruptured aneurysm were on one of these drugs? In the last few years, Cipro (ciprofloxacin) and Levaquin (levofloxacin) were commonly used by our family practice and internal medicine colleagues for almost all outpatient infections. It was so common that even my wife, who is not a physician, would request Cipro whenever she had a sneeze. We would also bring large bottles of Cipro every time we went traveling to some exotic destination, reassuring ourselves that the only “runs” we would have would be in the airport trying to catch a flight. A cousin of mine, prescribed Levaquin by a well-meaning physician while he was cruising the Nile River, ruptured his Achilles tendon.
Russell H. Samson, MD, FACS, DFSVS , is a clinical professor of surgery at Florida State University, Sarasota, a senior surgeon at Sarasota Vascular Specialists, and President of the Mote Vascular Foundation.
A new study finds that patients taking fluoroquinolone antibiotics may be at higher risk of aortopathy in part because of human aortic myofibroblast–mediated extracellular matrix (ECM) dysregulation.
“Emerging evidence supports pharmacologic-associated aortopathy in patients receiving fluoroquinolone [FQ] antibiotics,” said first author David G. Guzzardi, PhD, and his colleagues, citing previous research showing that, “compared with patients receiving amoxicillin antibiotics, those receiving FQ have a 66% higher risk of aneurysm or dissection within a 2-month period after commencing FQ use.”
Based upon such data, the Food and Drug Administration issued a December 2018 warning about the increased risk of ruptures or tears in the aorta with fluoroquinolone antibiotics in certain patients, updating their May 2017 warning regarding “disabling and potentially permanent side effects of the tendons, muscles, joints, nerves, and central nervous system that can occur together in the same patient,” upon exposure to this class of antibiotics. Earlier in 2018, the FDA had reinforced their safety information about serious and potentially fatal low blood sugar levels and mental health side effects with fluoroquinolone antibiotics.
Dr. Guzzardi and his colleagues at the University of Calgary (Alta.) performed a study to attempt to determine the possible cellular mechanisms for the observed aortopathy. In their study published in the Journal of Thoracic and Cardiovascular Surgery, Dr. Guzzardi and his colleagues isolated human aortic myofibroblasts from nine patients with aortopathy who were undergoing elective ascending aortic resection.
Following exposure of cells to FQ, the researchers assessed secreted matrix metalloproteinases relative to tissue inhibitors of matrix metalloproteinases (TIMPs). In addition, they examined ECM degradation by using a three-dimensional gelatin-fluorescein isothiocyanate fluorescence microgel assay. Aortic cellular collagen type I expression following FQ exposure was determined by immunoblotting and immunofluorescent staining. Dr. Guzzardi and his colleagues also looked at cell apoptosis, necrosis, and metabolic viability using two versions of vital staining.
They found that FQ exposure significantly decreased aortic cell TIMP-1 (P less than .004) and TIMP-2 (P less than .0004) protein expression, compared with controls, and the ratio of matrix metalloproteinase 9/TIMP-2 was increased (P less than .01). This suggests an increased capacity for ECM degradation after FQ exposure, according to the researchers.
In addition, FQ exposure attenuated collagen type I expression as assessed by immunoblotting (P less than .002) and immunofluorescence (P less than .02).
“FQ induces human aortic myofibroblast–mediated ECM dysregulation by decreasing TIMP expression and preventing compensatory collagen deposition. These data provide novel insights into the mechanisms that may underlie the clinical association of FQ exposure and increased risk of acute aortic events in the community. Our data suggest cautious use of FQ in selected patient populations with preexistent aortopathy and connective tissue disorders,” the researchers concluded.
In an accompanying editorial, while warning that these are preliminary observations based upon a small number of patients with aortopathy, Ari A. Mennander, MD, PhD, of Tampere (Finland) University, wrote that, “for the first time, the wild theory of fluoroquinolone-associated aortopathy has a molecular hint that is based on collagen degeneration and progression of aortic disease. ... This theory is in line with previous observations revealing antifibrotic activity and decreased collagen-1 protein expression with fluoroquinolones. The enigmatic puzzle of the progression of some aortic events may alarmingly be iatrogenic, and the clinician may wisely consider a prudent use of fluoroquinolones in patients with aortic dilatation.”
The authors and commentators reported that they had no commercial conflicts to disclose.
SOURCE: Guzzardi DG et al. J Thorac Cardiovasc Surg. 2019;157:109-19.
A new study finds that patients taking fluoroquinolone antibiotics may be at higher risk of aortopathy in part because of human aortic myofibroblast–mediated extracellular matrix (ECM) dysregulation.
“Emerging evidence supports pharmacologic-associated aortopathy in patients receiving fluoroquinolone [FQ] antibiotics,” said first author David G. Guzzardi, PhD, and his colleagues, citing previous research showing that, “compared with patients receiving amoxicillin antibiotics, those receiving FQ have a 66% higher risk of aneurysm or dissection within a 2-month period after commencing FQ use.”
Based upon such data, the Food and Drug Administration issued a December 2018 warning about the increased risk of ruptures or tears in the aorta with fluoroquinolone antibiotics in certain patients, updating their May 2017 warning regarding “disabling and potentially permanent side effects of the tendons, muscles, joints, nerves, and central nervous system that can occur together in the same patient,” upon exposure to this class of antibiotics. Earlier in 2018, the FDA had reinforced their safety information about serious and potentially fatal low blood sugar levels and mental health side effects with fluoroquinolone antibiotics.
Dr. Guzzardi and his colleagues at the University of Calgary (Alta.) performed a study to attempt to determine the possible cellular mechanisms for the observed aortopathy. In their study published in the Journal of Thoracic and Cardiovascular Surgery, Dr. Guzzardi and his colleagues isolated human aortic myofibroblasts from nine patients with aortopathy who were undergoing elective ascending aortic resection.
Following exposure of cells to FQ, the researchers assessed secreted matrix metalloproteinases relative to tissue inhibitors of matrix metalloproteinases (TIMPs). In addition, they examined ECM degradation by using a three-dimensional gelatin-fluorescein isothiocyanate fluorescence microgel assay. Aortic cellular collagen type I expression following FQ exposure was determined by immunoblotting and immunofluorescent staining. Dr. Guzzardi and his colleagues also looked at cell apoptosis, necrosis, and metabolic viability using two versions of vital staining.
They found that FQ exposure significantly decreased aortic cell TIMP-1 (P less than .004) and TIMP-2 (P less than .0004) protein expression, compared with controls, and the ratio of matrix metalloproteinase 9/TIMP-2 was increased (P less than .01). This suggests an increased capacity for ECM degradation after FQ exposure, according to the researchers.
In addition, FQ exposure attenuated collagen type I expression as assessed by immunoblotting (P less than .002) and immunofluorescence (P less than .02).
“FQ induces human aortic myofibroblast–mediated ECM dysregulation by decreasing TIMP expression and preventing compensatory collagen deposition. These data provide novel insights into the mechanisms that may underlie the clinical association of FQ exposure and increased risk of acute aortic events in the community. Our data suggest cautious use of FQ in selected patient populations with preexistent aortopathy and connective tissue disorders,” the researchers concluded.
In an accompanying editorial, while warning that these are preliminary observations based upon a small number of patients with aortopathy, Ari A. Mennander, MD, PhD, of Tampere (Finland) University, wrote that, “for the first time, the wild theory of fluoroquinolone-associated aortopathy has a molecular hint that is based on collagen degeneration and progression of aortic disease. ... This theory is in line with previous observations revealing antifibrotic activity and decreased collagen-1 protein expression with fluoroquinolones. The enigmatic puzzle of the progression of some aortic events may alarmingly be iatrogenic, and the clinician may wisely consider a prudent use of fluoroquinolones in patients with aortic dilatation.”
The authors and commentators reported that they had no commercial conflicts to disclose.
SOURCE: Guzzardi DG et al. J Thorac Cardiovasc Surg. 2019;157:109-19.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Growing Painful Nodule on the Lower Lip
The Diagnosis: Verrucous Carcinoma
An excisional biopsy revealed an endophytic and exophytic squamous proliferation with a papillomatous growth pattern, bulbous pushing border, and confluent parakeratosis (Figure). No fungal organisms were seen. Due to clinical and histological findings, a diagnosis of verrucous carcinoma (VC) was made.
Verrucous carcinoma is a rare variant of squamous cell carcinoma (SCC) with specific clinical and histological features.1 These tumors have a slow and localized growth pattern but can be locally aggressive. Metastasis of VC is rare, giving VC an overall good prognosis, with a 5-year survival rate greater than 75%.2 Verrucous carcinoma typically occurs in 1 of 3 locations: the oropharynx, genitals, or soles of the feet. Depending on the site of involvement, various names have been used in the literature to describe this entity, including Ackerman tumor (solitary oral mucosal lesion), Buschke-Lowenstein tumor (genital involvement), florid oral papillomatosis (multiple oral lesions), and carcinoma cuniculatum (sole of the foot).3 The most common sites for VC in the oral cavity are the buccal mucosa and gingiva.4
Verrucous carcinoma occurs more often among men in the sixth decade of life.3 The etiology of oral VC remains unclear; however, use of chewing tobacco, chemical carcinogens, chronic irritation, human papillomavirus (HPV), and poor oral hygiene have been reported as predisposing risk factors.4,5 The role of HPV in the pathogenesis of VC remains controversial, but both low-risk types HPV-6 and HPV-11 and high-risk types HPV-16 and HPV-18 have been found in association with VC.5,6
Clinically, oral VC lesions most often present as pink-white erythematous papules or plaques with exophytic cauliflowerlike surface alterations. Although the tumors are slow growing with little risk for metastasis, they may be locally invasive with deep involvement of the surrounding
structures.1 Histopathologically, VC displays proliferation of the epithelium with downward growth into the connective tissue but usually without a pattern of true invasion. The epithelium is well differentiated and displays little pleomorphism or mitoses.5,7 Obtaining a generous biopsy specimen is essential to view the diagnostic architecture of VC and rule out other entities, such as viral verruca, blastomycosis, SCC, and verruciform xanthoma. Squamous cell carcinoma characteristically has a more infiltrative border as opposed to the bulbous border of VC. In addition, the distribution of p53 and Ki-67 staining differs between SCC and VC. Squamous cell carcinoma shows positive p53 and Ki-67 staining for the full thickness of the epidermis, while VC has positive staining only in the lower third of the epidermis.5
Surgical resection is considered the first-line treatment of VC through excision or Mohs micrographic surgery. Radiation therapy is controversial due to the risk for anaplastic transformation. When surgery is not ideal due to the tumor size or location or the patient’s preference, other treatment modalities with reported success include intralesional interferon alfa; cryosurgery; topical imiquimod; and topical or systemic cytostatic agents such as bleomycin, 5-fluorouracil, cisplatin, or methotrexate.1,2
- Pattee SF, Bordeaux J, Mahalingam M, et al. Verrucous carcinoma of the scalp. J Am Acad Dermatol. 2006;56:506-508.
- Nikkels AF, Thirion L, Quatresooz P, et al. Photodynamic therapy for cutaneous verrucous carcinoma. J Am Acad Dermatol. 2007;57:516-519.
- Ho J, Diven DG, Butler PJ, et al. An ulcerating verrucous plaque on the foot. Arch Dermatol. 2000;136:547-552.
- Sonalika WG, Anand T. Oral verrucous carcinoma: a retrospective analysis for clinicopathologic features. J Cancer Res Ther. 2016;12:142-145.
- Dubina M, Goldenberg G. Viral-associated nonmelanoma skin cancers: a review. Am J Dermatopathol. 2009;31:561-573.
- Geusau A, Heinz-Peer G, Volc-Platzer B, et al. Regression of deeply infiltrating giant condyloma (Buschke-Lowenstein tumor) following long-term intralesional interferon alpha therapy. Arch Dermatol. 2000;136:707-710.
- Ansai S, Kimura T, Hayashi M. Fatal genital verrucous carcinoma. Am J Dermatopathol. 2007;29:68-71.
The Diagnosis: Verrucous Carcinoma
An excisional biopsy revealed an endophytic and exophytic squamous proliferation with a papillomatous growth pattern, bulbous pushing border, and confluent parakeratosis (Figure). No fungal organisms were seen. Due to clinical and histological findings, a diagnosis of verrucous carcinoma (VC) was made.
Verrucous carcinoma is a rare variant of squamous cell carcinoma (SCC) with specific clinical and histological features.1 These tumors have a slow and localized growth pattern but can be locally aggressive. Metastasis of VC is rare, giving VC an overall good prognosis, with a 5-year survival rate greater than 75%.2 Verrucous carcinoma typically occurs in 1 of 3 locations: the oropharynx, genitals, or soles of the feet. Depending on the site of involvement, various names have been used in the literature to describe this entity, including Ackerman tumor (solitary oral mucosal lesion), Buschke-Lowenstein tumor (genital involvement), florid oral papillomatosis (multiple oral lesions), and carcinoma cuniculatum (sole of the foot).3 The most common sites for VC in the oral cavity are the buccal mucosa and gingiva.4
Verrucous carcinoma occurs more often among men in the sixth decade of life.3 The etiology of oral VC remains unclear; however, use of chewing tobacco, chemical carcinogens, chronic irritation, human papillomavirus (HPV), and poor oral hygiene have been reported as predisposing risk factors.4,5 The role of HPV in the pathogenesis of VC remains controversial, but both low-risk types HPV-6 and HPV-11 and high-risk types HPV-16 and HPV-18 have been found in association with VC.5,6
Clinically, oral VC lesions most often present as pink-white erythematous papules or plaques with exophytic cauliflowerlike surface alterations. Although the tumors are slow growing with little risk for metastasis, they may be locally invasive with deep involvement of the surrounding
structures.1 Histopathologically, VC displays proliferation of the epithelium with downward growth into the connective tissue but usually without a pattern of true invasion. The epithelium is well differentiated and displays little pleomorphism or mitoses.5,7 Obtaining a generous biopsy specimen is essential to view the diagnostic architecture of VC and rule out other entities, such as viral verruca, blastomycosis, SCC, and verruciform xanthoma. Squamous cell carcinoma characteristically has a more infiltrative border as opposed to the bulbous border of VC. In addition, the distribution of p53 and Ki-67 staining differs between SCC and VC. Squamous cell carcinoma shows positive p53 and Ki-67 staining for the full thickness of the epidermis, while VC has positive staining only in the lower third of the epidermis.5
Surgical resection is considered the first-line treatment of VC through excision or Mohs micrographic surgery. Radiation therapy is controversial due to the risk for anaplastic transformation. When surgery is not ideal due to the tumor size or location or the patient’s preference, other treatment modalities with reported success include intralesional interferon alfa; cryosurgery; topical imiquimod; and topical or systemic cytostatic agents such as bleomycin, 5-fluorouracil, cisplatin, or methotrexate.1,2
The Diagnosis: Verrucous Carcinoma
An excisional biopsy revealed an endophytic and exophytic squamous proliferation with a papillomatous growth pattern, bulbous pushing border, and confluent parakeratosis (Figure). No fungal organisms were seen. Due to clinical and histological findings, a diagnosis of verrucous carcinoma (VC) was made.
Verrucous carcinoma is a rare variant of squamous cell carcinoma (SCC) with specific clinical and histological features.1 These tumors have a slow and localized growth pattern but can be locally aggressive. Metastasis of VC is rare, giving VC an overall good prognosis, with a 5-year survival rate greater than 75%.2 Verrucous carcinoma typically occurs in 1 of 3 locations: the oropharynx, genitals, or soles of the feet. Depending on the site of involvement, various names have been used in the literature to describe this entity, including Ackerman tumor (solitary oral mucosal lesion), Buschke-Lowenstein tumor (genital involvement), florid oral papillomatosis (multiple oral lesions), and carcinoma cuniculatum (sole of the foot).3 The most common sites for VC in the oral cavity are the buccal mucosa and gingiva.4
Verrucous carcinoma occurs more often among men in the sixth decade of life.3 The etiology of oral VC remains unclear; however, use of chewing tobacco, chemical carcinogens, chronic irritation, human papillomavirus (HPV), and poor oral hygiene have been reported as predisposing risk factors.4,5 The role of HPV in the pathogenesis of VC remains controversial, but both low-risk types HPV-6 and HPV-11 and high-risk types HPV-16 and HPV-18 have been found in association with VC.5,6
Clinically, oral VC lesions most often present as pink-white erythematous papules or plaques with exophytic cauliflowerlike surface alterations. Although the tumors are slow growing with little risk for metastasis, they may be locally invasive with deep involvement of the surrounding
structures.1 Histopathologically, VC displays proliferation of the epithelium with downward growth into the connective tissue but usually without a pattern of true invasion. The epithelium is well differentiated and displays little pleomorphism or mitoses.5,7 Obtaining a generous biopsy specimen is essential to view the diagnostic architecture of VC and rule out other entities, such as viral verruca, blastomycosis, SCC, and verruciform xanthoma. Squamous cell carcinoma characteristically has a more infiltrative border as opposed to the bulbous border of VC. In addition, the distribution of p53 and Ki-67 staining differs between SCC and VC. Squamous cell carcinoma shows positive p53 and Ki-67 staining for the full thickness of the epidermis, while VC has positive staining only in the lower third of the epidermis.5
Surgical resection is considered the first-line treatment of VC through excision or Mohs micrographic surgery. Radiation therapy is controversial due to the risk for anaplastic transformation. When surgery is not ideal due to the tumor size or location or the patient’s preference, other treatment modalities with reported success include intralesional interferon alfa; cryosurgery; topical imiquimod; and topical or systemic cytostatic agents such as bleomycin, 5-fluorouracil, cisplatin, or methotrexate.1,2
- Pattee SF, Bordeaux J, Mahalingam M, et al. Verrucous carcinoma of the scalp. J Am Acad Dermatol. 2006;56:506-508.
- Nikkels AF, Thirion L, Quatresooz P, et al. Photodynamic therapy for cutaneous verrucous carcinoma. J Am Acad Dermatol. 2007;57:516-519.
- Ho J, Diven DG, Butler PJ, et al. An ulcerating verrucous plaque on the foot. Arch Dermatol. 2000;136:547-552.
- Sonalika WG, Anand T. Oral verrucous carcinoma: a retrospective analysis for clinicopathologic features. J Cancer Res Ther. 2016;12:142-145.
- Dubina M, Goldenberg G. Viral-associated nonmelanoma skin cancers: a review. Am J Dermatopathol. 2009;31:561-573.
- Geusau A, Heinz-Peer G, Volc-Platzer B, et al. Regression of deeply infiltrating giant condyloma (Buschke-Lowenstein tumor) following long-term intralesional interferon alpha therapy. Arch Dermatol. 2000;136:707-710.
- Ansai S, Kimura T, Hayashi M. Fatal genital verrucous carcinoma. Am J Dermatopathol. 2007;29:68-71.
- Pattee SF, Bordeaux J, Mahalingam M, et al. Verrucous carcinoma of the scalp. J Am Acad Dermatol. 2006;56:506-508.
- Nikkels AF, Thirion L, Quatresooz P, et al. Photodynamic therapy for cutaneous verrucous carcinoma. J Am Acad Dermatol. 2007;57:516-519.
- Ho J, Diven DG, Butler PJ, et al. An ulcerating verrucous plaque on the foot. Arch Dermatol. 2000;136:547-552.
- Sonalika WG, Anand T. Oral verrucous carcinoma: a retrospective analysis for clinicopathologic features. J Cancer Res Ther. 2016;12:142-145.
- Dubina M, Goldenberg G. Viral-associated nonmelanoma skin cancers: a review. Am J Dermatopathol. 2009;31:561-573.
- Geusau A, Heinz-Peer G, Volc-Platzer B, et al. Regression of deeply infiltrating giant condyloma (Buschke-Lowenstein tumor) following long-term intralesional interferon alpha therapy. Arch Dermatol. 2000;136:707-710.
- Ansai S, Kimura T, Hayashi M. Fatal genital verrucous carcinoma. Am J Dermatopathol. 2007;29:68-71.
Enterovirus in at-risk children associated with later celiac disease
“We found a significant association between exposure to enterovirus and subsequent risk of celiac disease,” wrote lead author Christian R. Kahrs of the University of Oslo and his coauthors, adding that “adenovirus was not associated with celiac disease.” The study was published in the BMJ.
From 2001 to 2007, 46,939 newborns in Norway were screened for the HLA-DQ2/DQ8 genotype, which is associated with an increased risk of celiac disease. The genotype was identified in 912 children, and blood and stool sample collection began at 3 months. Children who were still contributing blood samples by 2014-2016 were invited to be screened for celiac disease.
Of the 220 children screened, 25 were diagnosed with celiac disease. Enterovirus was detected in 370 (17%) of the 2,135 stool samples and was more frequent in children who developed celiac disease antibodies than in matched controls (adjusted odds ratio, 1.49; 95% confidence interval, 1.07-2.06; P = .02). There was a significant association between later development of celiac disease and the commonly identified enterovirus A (aOR, 1.62; 95% CI, 1.04-2.53; P = .03) and enterovirus B (aOR, 2.27; 95% CI, 1.33-3.88; P = .003). No adenovirus types were associated with development of celiac disease.
The authors acknowledged their study’s limitations, including the possibility that children might be diagnosed with celiac disease later than the study’s roughly 10-year follow-up and the limited number of children with the disease despite a large number of analyzed samples. They noted that, “given the limited number of cases, we call for corroboration in similar studies and preferably interventional studies to reach conclusions about causality.”
The study was funded by the Research Council of Norway, the Project for the Conceptual Development of Research Organization, and the Norwegian Coeliac Society. Two authors reported grant support from trusts and foundations in Norway and Switzerland; no conflicts of interest were reported.
SOURCE: Kahrs CR et al. BMJ. 2019 Feb 13. doi: 10.1136/bmj.l231.
“We found a significant association between exposure to enterovirus and subsequent risk of celiac disease,” wrote lead author Christian R. Kahrs of the University of Oslo and his coauthors, adding that “adenovirus was not associated with celiac disease.” The study was published in the BMJ.
From 2001 to 2007, 46,939 newborns in Norway were screened for the HLA-DQ2/DQ8 genotype, which is associated with an increased risk of celiac disease. The genotype was identified in 912 children, and blood and stool sample collection began at 3 months. Children who were still contributing blood samples by 2014-2016 were invited to be screened for celiac disease.
Of the 220 children screened, 25 were diagnosed with celiac disease. Enterovirus was detected in 370 (17%) of the 2,135 stool samples and was more frequent in children who developed celiac disease antibodies than in matched controls (adjusted odds ratio, 1.49; 95% confidence interval, 1.07-2.06; P = .02). There was a significant association between later development of celiac disease and the commonly identified enterovirus A (aOR, 1.62; 95% CI, 1.04-2.53; P = .03) and enterovirus B (aOR, 2.27; 95% CI, 1.33-3.88; P = .003). No adenovirus types were associated with development of celiac disease.
The authors acknowledged their study’s limitations, including the possibility that children might be diagnosed with celiac disease later than the study’s roughly 10-year follow-up and the limited number of children with the disease despite a large number of analyzed samples. They noted that, “given the limited number of cases, we call for corroboration in similar studies and preferably interventional studies to reach conclusions about causality.”
The study was funded by the Research Council of Norway, the Project for the Conceptual Development of Research Organization, and the Norwegian Coeliac Society. Two authors reported grant support from trusts and foundations in Norway and Switzerland; no conflicts of interest were reported.
SOURCE: Kahrs CR et al. BMJ. 2019 Feb 13. doi: 10.1136/bmj.l231.
“We found a significant association between exposure to enterovirus and subsequent risk of celiac disease,” wrote lead author Christian R. Kahrs of the University of Oslo and his coauthors, adding that “adenovirus was not associated with celiac disease.” The study was published in the BMJ.
From 2001 to 2007, 46,939 newborns in Norway were screened for the HLA-DQ2/DQ8 genotype, which is associated with an increased risk of celiac disease. The genotype was identified in 912 children, and blood and stool sample collection began at 3 months. Children who were still contributing blood samples by 2014-2016 were invited to be screened for celiac disease.
Of the 220 children screened, 25 were diagnosed with celiac disease. Enterovirus was detected in 370 (17%) of the 2,135 stool samples and was more frequent in children who developed celiac disease antibodies than in matched controls (adjusted odds ratio, 1.49; 95% confidence interval, 1.07-2.06; P = .02). There was a significant association between later development of celiac disease and the commonly identified enterovirus A (aOR, 1.62; 95% CI, 1.04-2.53; P = .03) and enterovirus B (aOR, 2.27; 95% CI, 1.33-3.88; P = .003). No adenovirus types were associated with development of celiac disease.
The authors acknowledged their study’s limitations, including the possibility that children might be diagnosed with celiac disease later than the study’s roughly 10-year follow-up and the limited number of children with the disease despite a large number of analyzed samples. They noted that, “given the limited number of cases, we call for corroboration in similar studies and preferably interventional studies to reach conclusions about causality.”
The study was funded by the Research Council of Norway, the Project for the Conceptual Development of Research Organization, and the Norwegian Coeliac Society. Two authors reported grant support from trusts and foundations in Norway and Switzerland; no conflicts of interest were reported.
SOURCE: Kahrs CR et al. BMJ. 2019 Feb 13. doi: 10.1136/bmj.l231.
FROM THE BMJ
Delays of 1-2+ years in IBD diagnosis are common, patients say
LAS VEGAS – Delays in diagnosis of inflammatory bowel disease (IBD) appear to be very common and often extensive, a new survey of U.S. patients suggests. Nearly two-thirds said their diagnosis was delayed past symptom onset for more than a year, and almost half reported a delay of more than 2 years.
On average, patients who experienced diagnosis delays said they’d seen an average of 3.5 physicians. “Most patients reported that they received an uncertain or wrong diagnosis by their primary care physician or gastroenterologist,” said study coauthor Ryan C. Ungaro, MD, of Icahn School of Medicine at Mount Sinai, New York, in an interview prior to the presentation of the study findings at the Crohn’s & Colitis Congress - a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
“Working at a tertiary care IBD center, we noticed that many patients tell us it took them a long time to get diagnosed with Crohn’s disease [CD] or ulcerative colitis [UC],” said Dr. Ungaro. “There are some studies on delay in diagnosis in Europe but none in the U.S. We hypothesized that diagnostic delay is a major issue for IBD patients in the U.S.”
The study authors offered a survey to 2,341 patients with IBD; 1,121 responded to the questions. Of those, 68% reported their diagnosis was delayed, with 64% reporting a delay of over 1 year and 48% reporting a delay over 2 years.
Compared with those with UC, patients with CD were more likely to report more than 1-year delays (70% vs. 48%; P less than .0001) and more than 2-year delays (52% vs. 37%; P = .0008).
Patients who reported delays said they saw an average of 3.5 physicians before getting an IBD diagnosis. The patients most commonly blamed their incorrect diagnosis on primary care providers (58%) and gastroenterologists (28%).
“Most likely, CD may be misdiagnosed because the common presenting symptoms – abdominal pain, diarrhea – are also seen in other common gastrointestinal conditions such as irritable bowel syndrome,” Dr. Ungaro said. “In contrast, most patients with UC present with rectal bleeding which is a ‘red flag’ symptom that is more likely to get worked up.”
In some cases, patients blamed themselves, reporting “that they personally did not feel their symptoms warranted work-up or were too embarrassed by their symptoms to tell anyone,” Dr. Ungaro said. “The other theme that was noted was access – delay or difficulty seeing a gastroenterologist.”
Going forward, “diagnostic delay may be improved through patient education regarding awareness of alarm symptoms for IBD,” said gastroenterologist and study lead author Zane Gallinger, MD, FRCPC, of the University of Toronto at Mount Sinai Hospital, in an interview. According to him, these symptoms include diarrhea, abdominal pain, weight loss, family history of CD, perianal abscess, and fistula and fever.
At the primary care level, Dr. Gallinger said that noninvasive tests such as fecal calprotectin can help identify patients with inflammatory conditions and that “more rapid access to gastroenterologists for earlier diagnosis of IBD can improve patient outcomes.”
The Crohn’s and Colitis Foundation funded the study. Dr. Gallinger reported relationships with Takeda and AbbVie.
SOURCE: Gallinger Z et al. Crohn’s & Colitis Congress, Abstract P030.
LAS VEGAS – Delays in diagnosis of inflammatory bowel disease (IBD) appear to be very common and often extensive, a new survey of U.S. patients suggests. Nearly two-thirds said their diagnosis was delayed past symptom onset for more than a year, and almost half reported a delay of more than 2 years.
On average, patients who experienced diagnosis delays said they’d seen an average of 3.5 physicians. “Most patients reported that they received an uncertain or wrong diagnosis by their primary care physician or gastroenterologist,” said study coauthor Ryan C. Ungaro, MD, of Icahn School of Medicine at Mount Sinai, New York, in an interview prior to the presentation of the study findings at the Crohn’s & Colitis Congress - a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
“Working at a tertiary care IBD center, we noticed that many patients tell us it took them a long time to get diagnosed with Crohn’s disease [CD] or ulcerative colitis [UC],” said Dr. Ungaro. “There are some studies on delay in diagnosis in Europe but none in the U.S. We hypothesized that diagnostic delay is a major issue for IBD patients in the U.S.”
The study authors offered a survey to 2,341 patients with IBD; 1,121 responded to the questions. Of those, 68% reported their diagnosis was delayed, with 64% reporting a delay of over 1 year and 48% reporting a delay over 2 years.
Compared with those with UC, patients with CD were more likely to report more than 1-year delays (70% vs. 48%; P less than .0001) and more than 2-year delays (52% vs. 37%; P = .0008).
Patients who reported delays said they saw an average of 3.5 physicians before getting an IBD diagnosis. The patients most commonly blamed their incorrect diagnosis on primary care providers (58%) and gastroenterologists (28%).
“Most likely, CD may be misdiagnosed because the common presenting symptoms – abdominal pain, diarrhea – are also seen in other common gastrointestinal conditions such as irritable bowel syndrome,” Dr. Ungaro said. “In contrast, most patients with UC present with rectal bleeding which is a ‘red flag’ symptom that is more likely to get worked up.”
In some cases, patients blamed themselves, reporting “that they personally did not feel their symptoms warranted work-up or were too embarrassed by their symptoms to tell anyone,” Dr. Ungaro said. “The other theme that was noted was access – delay or difficulty seeing a gastroenterologist.”
Going forward, “diagnostic delay may be improved through patient education regarding awareness of alarm symptoms for IBD,” said gastroenterologist and study lead author Zane Gallinger, MD, FRCPC, of the University of Toronto at Mount Sinai Hospital, in an interview. According to him, these symptoms include diarrhea, abdominal pain, weight loss, family history of CD, perianal abscess, and fistula and fever.
At the primary care level, Dr. Gallinger said that noninvasive tests such as fecal calprotectin can help identify patients with inflammatory conditions and that “more rapid access to gastroenterologists for earlier diagnosis of IBD can improve patient outcomes.”
The Crohn’s and Colitis Foundation funded the study. Dr. Gallinger reported relationships with Takeda and AbbVie.
SOURCE: Gallinger Z et al. Crohn’s & Colitis Congress, Abstract P030.
LAS VEGAS – Delays in diagnosis of inflammatory bowel disease (IBD) appear to be very common and often extensive, a new survey of U.S. patients suggests. Nearly two-thirds said their diagnosis was delayed past symptom onset for more than a year, and almost half reported a delay of more than 2 years.
On average, patients who experienced diagnosis delays said they’d seen an average of 3.5 physicians. “Most patients reported that they received an uncertain or wrong diagnosis by their primary care physician or gastroenterologist,” said study coauthor Ryan C. Ungaro, MD, of Icahn School of Medicine at Mount Sinai, New York, in an interview prior to the presentation of the study findings at the Crohn’s & Colitis Congress - a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
“Working at a tertiary care IBD center, we noticed that many patients tell us it took them a long time to get diagnosed with Crohn’s disease [CD] or ulcerative colitis [UC],” said Dr. Ungaro. “There are some studies on delay in diagnosis in Europe but none in the U.S. We hypothesized that diagnostic delay is a major issue for IBD patients in the U.S.”
The study authors offered a survey to 2,341 patients with IBD; 1,121 responded to the questions. Of those, 68% reported their diagnosis was delayed, with 64% reporting a delay of over 1 year and 48% reporting a delay over 2 years.
Compared with those with UC, patients with CD were more likely to report more than 1-year delays (70% vs. 48%; P less than .0001) and more than 2-year delays (52% vs. 37%; P = .0008).
Patients who reported delays said they saw an average of 3.5 physicians before getting an IBD diagnosis. The patients most commonly blamed their incorrect diagnosis on primary care providers (58%) and gastroenterologists (28%).
“Most likely, CD may be misdiagnosed because the common presenting symptoms – abdominal pain, diarrhea – are also seen in other common gastrointestinal conditions such as irritable bowel syndrome,” Dr. Ungaro said. “In contrast, most patients with UC present with rectal bleeding which is a ‘red flag’ symptom that is more likely to get worked up.”
In some cases, patients blamed themselves, reporting “that they personally did not feel their symptoms warranted work-up or were too embarrassed by their symptoms to tell anyone,” Dr. Ungaro said. “The other theme that was noted was access – delay or difficulty seeing a gastroenterologist.”
Going forward, “diagnostic delay may be improved through patient education regarding awareness of alarm symptoms for IBD,” said gastroenterologist and study lead author Zane Gallinger, MD, FRCPC, of the University of Toronto at Mount Sinai Hospital, in an interview. According to him, these symptoms include diarrhea, abdominal pain, weight loss, family history of CD, perianal abscess, and fistula and fever.
At the primary care level, Dr. Gallinger said that noninvasive tests such as fecal calprotectin can help identify patients with inflammatory conditions and that “more rapid access to gastroenterologists for earlier diagnosis of IBD can improve patient outcomes.”
The Crohn’s and Colitis Foundation funded the study. Dr. Gallinger reported relationships with Takeda and AbbVie.
SOURCE: Gallinger Z et al. Crohn’s & Colitis Congress, Abstract P030.
REPORTING FROM THE CROHN’S & COLITIS CONGRESS
One postdelivery antibiotic dose nearly halves infection in operative delivery
LAS VEGAS – A randomized controlled trial comparing a single postdelivery intravenous dose of antibiotic after operative delivery found that antibiotics nearly halved the risk for maternal infection.
For women who received a single dose of amoxicillin-clavulanic acid, the risk ratio was 0.58 for suspected or confirmed infection, compared with those who received an intravenous dose of saline solution (95% confidence interval, 0.49-0.69, P less than .001). Culture-confirmed systemic infections were similarly reduced by a risk ratio (RR) of 0.44 (95% CI, 0.22-0.89; P =.018).
Superficial and deep incisional infections were also significantly less likely in the women who had received antibiotics (RRs 0.53 and 0.46, respectively; P less than .001 for both). Although sepsis occurred in numerically fewer women who received antibiotics, the numbers were, overall, small and not statistically significant.
By 6 weeks after delivery, patients receiving antibiotics were less likely to have outpatient or home visits for perineal problems or concerns as well (P less than .001).
“This trial shows clear benefit of a single dose of prophylactic antibiotic after operative vaginal birth, and this should be introduced into routine practice,” said Marian Knight, MBChB, DPhil.
Dr. Knight presented findings of the randomized trial, dubbed ANODE, at a late-breaking abstract session of the meeting, which was sponsored by the Society for Maternal-Fetal Medicine. Dr. Knight, professor of maternal and child population health at the University of Oxford (England), explained that ANODE aimed to determine whether a single dose of prophylactic amoxicillin-clavulanic acid was clinically effective in preventing confirmed or suspected maternal infection after operative vaginal birth.
The study tips the scales in favor of the anti-infective properties of the single antibiotic dose after operative delivery, and comes at a time when unacceptable levels of maternal morbidity and mortality coexist with pressing worries about antibiotic resistance, Dr. Knight said.
The multicenter randomized, blinded, placebo-controlled trial was conducted at 27 sites in the United Kingdom between March 2016 and June 2018.
Women at the study sites who underwent operative delivery, whether by forceps or vacuum extraction, received either a single dose of intravenous amoxicillin-clavulanic acid (1 gm/200 mg), or a placebo dose of saline solution. Antibiotics were given in the window of 0 to 6 hours post-delivery.
The primary outcome measure was confirmed or suspected maternal infection within 6 weeks of delivery. Women were positive for infection if they were prescribed antibiotics for perineal wound infections, if they experienced endometrial or uterine infections, if they had urinary tract infections with “systemic features, or if they had other systemic infections. Other criteria for infection were culture-confirmed systemic infection, or endometritis by criteria established by the Centers for Disease Control and Prevention.
Dr. Knight and her colleagues used an intention-to-treat analysis that looked at the primary outcome as a risk ratio, with a 95% CI. Secondary outcomes, also presented as risk ratios, were considered with a 99% CI.
A total of 3,427 women were randomized. In all, 1,715 women in the active arm and 1,705 in the placebo arm were included in the outcomes analyses. Women were interviewed by telephone, they completed questionnaires, and they received a questionnaire by mail or completed one online. Slightly more than 1,500 women in each arm completed the phone interview, and nearly 1,300 in each arm completed the initial questionnaire.
The mean age was 30 years, and most of the participants (84%-87%) were white. Most were of normal weight, with a median body mass index at the initial prenatal visit of 25 kg/m2.
Though women with multiple pregnancies were included in the study, just 11 in the active arm and 9 in the placebo arm delivered twins. There were no triplets. Most women (76%-78%) were primiparous, and just 7%-8% of women had prior cesarean delivery.
The mode of operative delivery for most of the participants (63%-67%) was forceps, with all but 10 of the remaining women receiving vacuum extraction (the remaining 10 had spontaneous vaginal deliveries).
The reasons for instrumental delivery were approximately evenly divided between failure of labor to progress and fetal compromise.
Nearly 90% of the women – more than 1,500 in each study arm – received episiotomies, a figure that Dr. Knight said she found surprising. She noted that mediolateral incisions are the standard of care in the United Kingdom. Still, 29%-33% of the women experienced a tear, with most being second-degree tears. Third- and fourth-degree tears occurred in two women overall. Almost all of the women (99%) had their wounds sutured.
Three serious adverse events were reported. One woman in the placebo arm required intensive care unit admission for severe sepsis, and another placebo participant required a transfusion after postpartum hemorrhage. One patient who received antibiotic had immediate diffuse itching and a swollen throat. However, antibiotic side effects were reported in only 2 of the 1,715 active arm participants, Dr. Knight said.
The competing concerns of maternal safety and antibiotic stewardship are weighed against a global backdrop of high maternal infection rates, Dr. Knight said. Sepsis causes 11% of global maternal deaths, a rate that drops to about 5% in higher-income nations. However, she pointed out, that figure rises to about 13% in the United States.
“For every woman that dies from pregnancy-related infection, a further 70 have severe infection and survive,” she said.
Known risk factors for infection include operative vaginal delivery and cesarean deliveries. For cesareans performed after the onset of labor, the adjusted odds ratio reaches 6.7 for severe infection, Dr. Knight said (PLoS Med. 2014;11:e1001672). A systematic review estimated that the rate for any infection following cesarean delivery approaches one in four women, she said (Cochrane Database Syst Rev. 2014 Oct 28;[10]:CD007482).
The same systematic review found that prophylactic antibiotics reduced incidence of wound infection, endometritis, and serious maternal wound infection after cesarean delivery (RR 0.40, 0.38, and 0.31, respectively).
For operative vaginal deliveries, however, a Cochrane review found one study of 393 women. Although no women given antibiotics developed endometritis compared with seven cases of endometritis in the no-antibiotics group for a RR of .07, the 95% confidence interval in the Cochrane analysis included zero, so the findings weren’t statistically significant. Hospital length of stay didn’t differ between the two groups (Cochrane Database Syst Rev. 2014 Oct 13;[10]:CD004455).
Citing this review, the United Kingdom’s Royal College of Gynecologists had concluded that evidence was insufficient to support routine antibiotic prophylaxis in operative deliveries. The American College of Obstetricians and Gynecologists make no mention of antibiotic prophylaxis or postdelivery infection in its guidelines for operative delivery, Dr. Knight said.
Since confirmed or suspected infection was still seen in 11% of women who received antibiotics, further analysis “is needed to investigate whether early administration, prenatal administration, or repeated administration is more likely to be effective,” she said. Women in the ANODE trial received their dose at a median of 3 hours after delivery.
“Until these analyses are completed, there is no indication for administration of more than a single dose of prophylactic antibiotic, or for predelivery administration,” she said.
Dr. Knight reported that ANODE was funded by the U.K.’s National Institute for Health Research. She reported that she had no conflicts of interest.
SOURCE: Knight M et al. Am J Obstet Gynecol. 2019 Jan;220;1:S685. Abstract LB 3.
LAS VEGAS – A randomized controlled trial comparing a single postdelivery intravenous dose of antibiotic after operative delivery found that antibiotics nearly halved the risk for maternal infection.
For women who received a single dose of amoxicillin-clavulanic acid, the risk ratio was 0.58 for suspected or confirmed infection, compared with those who received an intravenous dose of saline solution (95% confidence interval, 0.49-0.69, P less than .001). Culture-confirmed systemic infections were similarly reduced by a risk ratio (RR) of 0.44 (95% CI, 0.22-0.89; P =.018).
Superficial and deep incisional infections were also significantly less likely in the women who had received antibiotics (RRs 0.53 and 0.46, respectively; P less than .001 for both). Although sepsis occurred in numerically fewer women who received antibiotics, the numbers were, overall, small and not statistically significant.
By 6 weeks after delivery, patients receiving antibiotics were less likely to have outpatient or home visits for perineal problems or concerns as well (P less than .001).
“This trial shows clear benefit of a single dose of prophylactic antibiotic after operative vaginal birth, and this should be introduced into routine practice,” said Marian Knight, MBChB, DPhil.
Dr. Knight presented findings of the randomized trial, dubbed ANODE, at a late-breaking abstract session of the meeting, which was sponsored by the Society for Maternal-Fetal Medicine. Dr. Knight, professor of maternal and child population health at the University of Oxford (England), explained that ANODE aimed to determine whether a single dose of prophylactic amoxicillin-clavulanic acid was clinically effective in preventing confirmed or suspected maternal infection after operative vaginal birth.
The study tips the scales in favor of the anti-infective properties of the single antibiotic dose after operative delivery, and comes at a time when unacceptable levels of maternal morbidity and mortality coexist with pressing worries about antibiotic resistance, Dr. Knight said.
The multicenter randomized, blinded, placebo-controlled trial was conducted at 27 sites in the United Kingdom between March 2016 and June 2018.
Women at the study sites who underwent operative delivery, whether by forceps or vacuum extraction, received either a single dose of intravenous amoxicillin-clavulanic acid (1 gm/200 mg), or a placebo dose of saline solution. Antibiotics were given in the window of 0 to 6 hours post-delivery.
The primary outcome measure was confirmed or suspected maternal infection within 6 weeks of delivery. Women were positive for infection if they were prescribed antibiotics for perineal wound infections, if they experienced endometrial or uterine infections, if they had urinary tract infections with “systemic features, or if they had other systemic infections. Other criteria for infection were culture-confirmed systemic infection, or endometritis by criteria established by the Centers for Disease Control and Prevention.
Dr. Knight and her colleagues used an intention-to-treat analysis that looked at the primary outcome as a risk ratio, with a 95% CI. Secondary outcomes, also presented as risk ratios, were considered with a 99% CI.
A total of 3,427 women were randomized. In all, 1,715 women in the active arm and 1,705 in the placebo arm were included in the outcomes analyses. Women were interviewed by telephone, they completed questionnaires, and they received a questionnaire by mail or completed one online. Slightly more than 1,500 women in each arm completed the phone interview, and nearly 1,300 in each arm completed the initial questionnaire.
The mean age was 30 years, and most of the participants (84%-87%) were white. Most were of normal weight, with a median body mass index at the initial prenatal visit of 25 kg/m2.
Though women with multiple pregnancies were included in the study, just 11 in the active arm and 9 in the placebo arm delivered twins. There were no triplets. Most women (76%-78%) were primiparous, and just 7%-8% of women had prior cesarean delivery.
The mode of operative delivery for most of the participants (63%-67%) was forceps, with all but 10 of the remaining women receiving vacuum extraction (the remaining 10 had spontaneous vaginal deliveries).
The reasons for instrumental delivery were approximately evenly divided between failure of labor to progress and fetal compromise.
Nearly 90% of the women – more than 1,500 in each study arm – received episiotomies, a figure that Dr. Knight said she found surprising. She noted that mediolateral incisions are the standard of care in the United Kingdom. Still, 29%-33% of the women experienced a tear, with most being second-degree tears. Third- and fourth-degree tears occurred in two women overall. Almost all of the women (99%) had their wounds sutured.
Three serious adverse events were reported. One woman in the placebo arm required intensive care unit admission for severe sepsis, and another placebo participant required a transfusion after postpartum hemorrhage. One patient who received antibiotic had immediate diffuse itching and a swollen throat. However, antibiotic side effects were reported in only 2 of the 1,715 active arm participants, Dr. Knight said.
The competing concerns of maternal safety and antibiotic stewardship are weighed against a global backdrop of high maternal infection rates, Dr. Knight said. Sepsis causes 11% of global maternal deaths, a rate that drops to about 5% in higher-income nations. However, she pointed out, that figure rises to about 13% in the United States.
“For every woman that dies from pregnancy-related infection, a further 70 have severe infection and survive,” she said.
Known risk factors for infection include operative vaginal delivery and cesarean deliveries. For cesareans performed after the onset of labor, the adjusted odds ratio reaches 6.7 for severe infection, Dr. Knight said (PLoS Med. 2014;11:e1001672). A systematic review estimated that the rate for any infection following cesarean delivery approaches one in four women, she said (Cochrane Database Syst Rev. 2014 Oct 28;[10]:CD007482).
The same systematic review found that prophylactic antibiotics reduced incidence of wound infection, endometritis, and serious maternal wound infection after cesarean delivery (RR 0.40, 0.38, and 0.31, respectively).
For operative vaginal deliveries, however, a Cochrane review found one study of 393 women. Although no women given antibiotics developed endometritis compared with seven cases of endometritis in the no-antibiotics group for a RR of .07, the 95% confidence interval in the Cochrane analysis included zero, so the findings weren’t statistically significant. Hospital length of stay didn’t differ between the two groups (Cochrane Database Syst Rev. 2014 Oct 13;[10]:CD004455).
Citing this review, the United Kingdom’s Royal College of Gynecologists had concluded that evidence was insufficient to support routine antibiotic prophylaxis in operative deliveries. The American College of Obstetricians and Gynecologists make no mention of antibiotic prophylaxis or postdelivery infection in its guidelines for operative delivery, Dr. Knight said.
Since confirmed or suspected infection was still seen in 11% of women who received antibiotics, further analysis “is needed to investigate whether early administration, prenatal administration, or repeated administration is more likely to be effective,” she said. Women in the ANODE trial received their dose at a median of 3 hours after delivery.
“Until these analyses are completed, there is no indication for administration of more than a single dose of prophylactic antibiotic, or for predelivery administration,” she said.
Dr. Knight reported that ANODE was funded by the U.K.’s National Institute for Health Research. She reported that she had no conflicts of interest.
SOURCE: Knight M et al. Am J Obstet Gynecol. 2019 Jan;220;1:S685. Abstract LB 3.
LAS VEGAS – A randomized controlled trial comparing a single postdelivery intravenous dose of antibiotic after operative delivery found that antibiotics nearly halved the risk for maternal infection.
For women who received a single dose of amoxicillin-clavulanic acid, the risk ratio was 0.58 for suspected or confirmed infection, compared with those who received an intravenous dose of saline solution (95% confidence interval, 0.49-0.69, P less than .001). Culture-confirmed systemic infections were similarly reduced by a risk ratio (RR) of 0.44 (95% CI, 0.22-0.89; P =.018).
Superficial and deep incisional infections were also significantly less likely in the women who had received antibiotics (RRs 0.53 and 0.46, respectively; P less than .001 for both). Although sepsis occurred in numerically fewer women who received antibiotics, the numbers were, overall, small and not statistically significant.
By 6 weeks after delivery, patients receiving antibiotics were less likely to have outpatient or home visits for perineal problems or concerns as well (P less than .001).
“This trial shows clear benefit of a single dose of prophylactic antibiotic after operative vaginal birth, and this should be introduced into routine practice,” said Marian Knight, MBChB, DPhil.
Dr. Knight presented findings of the randomized trial, dubbed ANODE, at a late-breaking abstract session of the meeting, which was sponsored by the Society for Maternal-Fetal Medicine. Dr. Knight, professor of maternal and child population health at the University of Oxford (England), explained that ANODE aimed to determine whether a single dose of prophylactic amoxicillin-clavulanic acid was clinically effective in preventing confirmed or suspected maternal infection after operative vaginal birth.
The study tips the scales in favor of the anti-infective properties of the single antibiotic dose after operative delivery, and comes at a time when unacceptable levels of maternal morbidity and mortality coexist with pressing worries about antibiotic resistance, Dr. Knight said.
The multicenter randomized, blinded, placebo-controlled trial was conducted at 27 sites in the United Kingdom between March 2016 and June 2018.
Women at the study sites who underwent operative delivery, whether by forceps or vacuum extraction, received either a single dose of intravenous amoxicillin-clavulanic acid (1 gm/200 mg), or a placebo dose of saline solution. Antibiotics were given in the window of 0 to 6 hours post-delivery.
The primary outcome measure was confirmed or suspected maternal infection within 6 weeks of delivery. Women were positive for infection if they were prescribed antibiotics for perineal wound infections, if they experienced endometrial or uterine infections, if they had urinary tract infections with “systemic features, or if they had other systemic infections. Other criteria for infection were culture-confirmed systemic infection, or endometritis by criteria established by the Centers for Disease Control and Prevention.
Dr. Knight and her colleagues used an intention-to-treat analysis that looked at the primary outcome as a risk ratio, with a 95% CI. Secondary outcomes, also presented as risk ratios, were considered with a 99% CI.
A total of 3,427 women were randomized. In all, 1,715 women in the active arm and 1,705 in the placebo arm were included in the outcomes analyses. Women were interviewed by telephone, they completed questionnaires, and they received a questionnaire by mail or completed one online. Slightly more than 1,500 women in each arm completed the phone interview, and nearly 1,300 in each arm completed the initial questionnaire.
The mean age was 30 years, and most of the participants (84%-87%) were white. Most were of normal weight, with a median body mass index at the initial prenatal visit of 25 kg/m2.
Though women with multiple pregnancies were included in the study, just 11 in the active arm and 9 in the placebo arm delivered twins. There were no triplets. Most women (76%-78%) were primiparous, and just 7%-8% of women had prior cesarean delivery.
The mode of operative delivery for most of the participants (63%-67%) was forceps, with all but 10 of the remaining women receiving vacuum extraction (the remaining 10 had spontaneous vaginal deliveries).
The reasons for instrumental delivery were approximately evenly divided between failure of labor to progress and fetal compromise.
Nearly 90% of the women – more than 1,500 in each study arm – received episiotomies, a figure that Dr. Knight said she found surprising. She noted that mediolateral incisions are the standard of care in the United Kingdom. Still, 29%-33% of the women experienced a tear, with most being second-degree tears. Third- and fourth-degree tears occurred in two women overall. Almost all of the women (99%) had their wounds sutured.
Three serious adverse events were reported. One woman in the placebo arm required intensive care unit admission for severe sepsis, and another placebo participant required a transfusion after postpartum hemorrhage. One patient who received antibiotic had immediate diffuse itching and a swollen throat. However, antibiotic side effects were reported in only 2 of the 1,715 active arm participants, Dr. Knight said.
The competing concerns of maternal safety and antibiotic stewardship are weighed against a global backdrop of high maternal infection rates, Dr. Knight said. Sepsis causes 11% of global maternal deaths, a rate that drops to about 5% in higher-income nations. However, she pointed out, that figure rises to about 13% in the United States.
“For every woman that dies from pregnancy-related infection, a further 70 have severe infection and survive,” she said.
Known risk factors for infection include operative vaginal delivery and cesarean deliveries. For cesareans performed after the onset of labor, the adjusted odds ratio reaches 6.7 for severe infection, Dr. Knight said (PLoS Med. 2014;11:e1001672). A systematic review estimated that the rate for any infection following cesarean delivery approaches one in four women, she said (Cochrane Database Syst Rev. 2014 Oct 28;[10]:CD007482).
The same systematic review found that prophylactic antibiotics reduced incidence of wound infection, endometritis, and serious maternal wound infection after cesarean delivery (RR 0.40, 0.38, and 0.31, respectively).
For operative vaginal deliveries, however, a Cochrane review found one study of 393 women. Although no women given antibiotics developed endometritis compared with seven cases of endometritis in the no-antibiotics group for a RR of .07, the 95% confidence interval in the Cochrane analysis included zero, so the findings weren’t statistically significant. Hospital length of stay didn’t differ between the two groups (Cochrane Database Syst Rev. 2014 Oct 13;[10]:CD004455).
Citing this review, the United Kingdom’s Royal College of Gynecologists had concluded that evidence was insufficient to support routine antibiotic prophylaxis in operative deliveries. The American College of Obstetricians and Gynecologists make no mention of antibiotic prophylaxis or postdelivery infection in its guidelines for operative delivery, Dr. Knight said.
Since confirmed or suspected infection was still seen in 11% of women who received antibiotics, further analysis “is needed to investigate whether early administration, prenatal administration, or repeated administration is more likely to be effective,” she said. Women in the ANODE trial received their dose at a median of 3 hours after delivery.
“Until these analyses are completed, there is no indication for administration of more than a single dose of prophylactic antibiotic, or for predelivery administration,” she said.
Dr. Knight reported that ANODE was funded by the U.K.’s National Institute for Health Research. She reported that she had no conflicts of interest.
SOURCE: Knight M et al. Am J Obstet Gynecol. 2019 Jan;220;1:S685. Abstract LB 3.
REPORTING FROM THE PREGNANCY MEETING
Severe skin reaction to AEDs
Also today, adherence to statins is lower among younger patients, women, and minorities, the flu season rages on, and how medication-assisted treatment impacts pregnant women with opioid use disorder.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, adherence to statins is lower among younger patients, women, and minorities, the flu season rages on, and how medication-assisted treatment impacts pregnant women with opioid use disorder.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, adherence to statins is lower among younger patients, women, and minorities, the flu season rages on, and how medication-assisted treatment impacts pregnant women with opioid use disorder.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify