User login
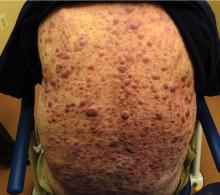
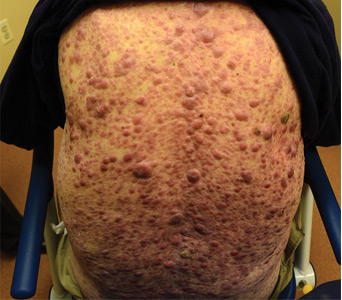
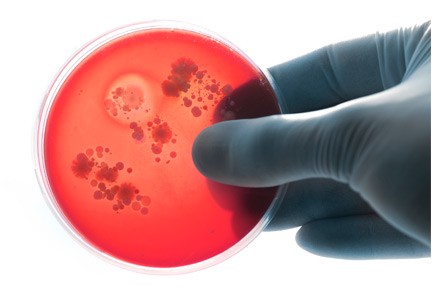
Aleukemic leukemia cutis
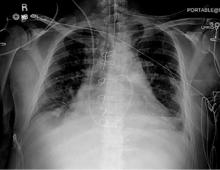
On examination, the numerous firm, indurated nodules ranged in size from 1 to 4 cm. There was no palpable lymphadenopathy.
Results of a peripheral blood cell count showed the following:
- Hemoglobin 12.5 g/dL (reference range 13.0–17.0)
- Platelet count 154 × 109/L (130–400)
- White blood cell count 5.0 × 109/L (4.0–11.0)
- Neutrophils 1.7 × 109/L (1.5–8.0)
- Lymphocytes 2.2 × 109/L (1.0–4.0)
- Monocytes 1.0 × 109/L (0.2–1.0)
- Eosinophils 0 (0–0.4)
- Basophils 0 (0–0.2)
- Blasts 0.
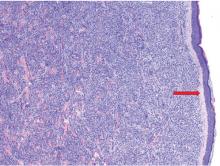
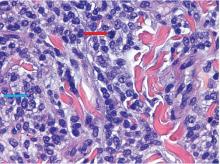
The findings were consistent with leukemic cells with monocytic differentiation. The infiltrate was unusual because leukemic infiltrates typically demonstrate a high nuclear-to-cytoplasmic ratio, but in this case the malignant cells had moderate amounts of cytoplasm due to the monocytic differentiation. Also, a grenz zone is more typically seen in B-cell lymphomas, and T cells more typically demonstrate epidermotropism.
Bone marrow aspiration was performed and revealed a hypercellular bone marrow with trilineage maturation with only 2% blasts. The fluorescence in situ hybridization testing for myelodysplastic syndrome and acute myeloid leukemia was normal. A diagnosis of aleukemic leukemia cutis was made.
After 2 months of chemotherapy with azacitidine, the nodules were less indurated. Treatment was briefly withdrawn due to the development of acute pneumonia, leading to a rapid progression of cutaneous involvement. Despite restarting chemotherapy, the patient died.
ALEUKEMIC LEUKEMIA CUTIS
The differential diagnosis of leukemia cutis is diverse and extensive. Patients often present with painless, firm, indurated nodules, papules, and plaques.1 The lesions can be small, involving a small amount of body surface area, but can also be very large and diffuse.
In our patient’s case, there were no new drugs or exposures to suggest a drug-related eruption, or pruritus or pain to suggest an inflammatory process. The rapid progression of the lesions suggested either an infectious or malignant process. The top 3 conditions in the differential diagnosis, based on his clinical presentation, were cutaneous T-cell lymphoma, cutaneous CD30+ anaplastic large-cell lymphoma, and a drug-induced cutaneous pseudolymphoma.
Skin biopsy is required to differentiate leukemia cutis from the other conditions. On skin biopsy study, leukemia cutis is characterized by infiltration of the skin by leukemic cells and is seen in 10% to 15% of patients with acute myeloid leukemia.2 In 5% of cases, leukemia cutis can present without bone marrow or peripheral signs of leukemia, hence the term aleukemic leukemia cutis.3 Cutaneous signs can occur before, after, or simultaneously with systemic leukemia.4
In the absence of systemic symptoms, the diagnosis is made when progressive cutaneous symptoms are present. The prognosis for aleukemic leukemia cutis is poor. Prompt diagnosis with skin biopsy is paramount to improve outcomes.
Acknowledgment: We would like to recognize Maanasa Devabhaktuni for her assistance in reporting this case.
- Yonal I, Hindilerden F, Coskun R, Dogan OI, Nalcaci M. Aleukemic leukemia cutis manifesting with disseminated nodular eruptions and a plaque preceding acute monocytic leukemia: a case report. Case Rep Oncol 2011; 4(3):547–554. doi:10.1159/000334745
- Cho-Vega JH, Medeiros LJ, Prieto VG, Vega F. Leukemia cutis. Am J Clin Pathol 2008; 129(1):130–142. doi:10.1309/WYACYWF6NGM3WBRT
- Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci 2013; 28(4):614–619. doi:10.3346/jkms.2013.28.4.614
- Obiozor C, Ganguly S, Fraga GR. Leukemia cutis with lymphoglandular bodies: a clue to acute lymphoblastic leukemia cutis. Dermatol Online J 2015; 21(8)pii:13030/qt6m18g35f. pmid:26437164
On examination, the numerous firm, indurated nodules ranged in size from 1 to 4 cm. There was no palpable lymphadenopathy.
Results of a peripheral blood cell count showed the following:
- Hemoglobin 12.5 g/dL (reference range 13.0–17.0)
- Platelet count 154 × 109/L (130–400)
- White blood cell count 5.0 × 109/L (4.0–11.0)
- Neutrophils 1.7 × 109/L (1.5–8.0)
- Lymphocytes 2.2 × 109/L (1.0–4.0)
- Monocytes 1.0 × 109/L (0.2–1.0)
- Eosinophils 0 (0–0.4)
- Basophils 0 (0–0.2)
- Blasts 0.
The findings were consistent with leukemic cells with monocytic differentiation. The infiltrate was unusual because leukemic infiltrates typically demonstrate a high nuclear-to-cytoplasmic ratio, but in this case the malignant cells had moderate amounts of cytoplasm due to the monocytic differentiation. Also, a grenz zone is more typically seen in B-cell lymphomas, and T cells more typically demonstrate epidermotropism.
Bone marrow aspiration was performed and revealed a hypercellular bone marrow with trilineage maturation with only 2% blasts. The fluorescence in situ hybridization testing for myelodysplastic syndrome and acute myeloid leukemia was normal. A diagnosis of aleukemic leukemia cutis was made.
After 2 months of chemotherapy with azacitidine, the nodules were less indurated. Treatment was briefly withdrawn due to the development of acute pneumonia, leading to a rapid progression of cutaneous involvement. Despite restarting chemotherapy, the patient died.
ALEUKEMIC LEUKEMIA CUTIS
The differential diagnosis of leukemia cutis is diverse and extensive. Patients often present with painless, firm, indurated nodules, papules, and plaques.1 The lesions can be small, involving a small amount of body surface area, but can also be very large and diffuse.
In our patient’s case, there were no new drugs or exposures to suggest a drug-related eruption, or pruritus or pain to suggest an inflammatory process. The rapid progression of the lesions suggested either an infectious or malignant process. The top 3 conditions in the differential diagnosis, based on his clinical presentation, were cutaneous T-cell lymphoma, cutaneous CD30+ anaplastic large-cell lymphoma, and a drug-induced cutaneous pseudolymphoma.
Skin biopsy is required to differentiate leukemia cutis from the other conditions. On skin biopsy study, leukemia cutis is characterized by infiltration of the skin by leukemic cells and is seen in 10% to 15% of patients with acute myeloid leukemia.2 In 5% of cases, leukemia cutis can present without bone marrow or peripheral signs of leukemia, hence the term aleukemic leukemia cutis.3 Cutaneous signs can occur before, after, or simultaneously with systemic leukemia.4
In the absence of systemic symptoms, the diagnosis is made when progressive cutaneous symptoms are present. The prognosis for aleukemic leukemia cutis is poor. Prompt diagnosis with skin biopsy is paramount to improve outcomes.
Acknowledgment: We would like to recognize Maanasa Devabhaktuni for her assistance in reporting this case.
On examination, the numerous firm, indurated nodules ranged in size from 1 to 4 cm. There was no palpable lymphadenopathy.
Results of a peripheral blood cell count showed the following:
- Hemoglobin 12.5 g/dL (reference range 13.0–17.0)
- Platelet count 154 × 109/L (130–400)
- White blood cell count 5.0 × 109/L (4.0–11.0)
- Neutrophils 1.7 × 109/L (1.5–8.0)
- Lymphocytes 2.2 × 109/L (1.0–4.0)
- Monocytes 1.0 × 109/L (0.2–1.0)
- Eosinophils 0 (0–0.4)
- Basophils 0 (0–0.2)
- Blasts 0.
The findings were consistent with leukemic cells with monocytic differentiation. The infiltrate was unusual because leukemic infiltrates typically demonstrate a high nuclear-to-cytoplasmic ratio, but in this case the malignant cells had moderate amounts of cytoplasm due to the monocytic differentiation. Also, a grenz zone is more typically seen in B-cell lymphomas, and T cells more typically demonstrate epidermotropism.
Bone marrow aspiration was performed and revealed a hypercellular bone marrow with trilineage maturation with only 2% blasts. The fluorescence in situ hybridization testing for myelodysplastic syndrome and acute myeloid leukemia was normal. A diagnosis of aleukemic leukemia cutis was made.
After 2 months of chemotherapy with azacitidine, the nodules were less indurated. Treatment was briefly withdrawn due to the development of acute pneumonia, leading to a rapid progression of cutaneous involvement. Despite restarting chemotherapy, the patient died.
ALEUKEMIC LEUKEMIA CUTIS
The differential diagnosis of leukemia cutis is diverse and extensive. Patients often present with painless, firm, indurated nodules, papules, and plaques.1 The lesions can be small, involving a small amount of body surface area, but can also be very large and diffuse.
In our patient’s case, there were no new drugs or exposures to suggest a drug-related eruption, or pruritus or pain to suggest an inflammatory process. The rapid progression of the lesions suggested either an infectious or malignant process. The top 3 conditions in the differential diagnosis, based on his clinical presentation, were cutaneous T-cell lymphoma, cutaneous CD30+ anaplastic large-cell lymphoma, and a drug-induced cutaneous pseudolymphoma.
Skin biopsy is required to differentiate leukemia cutis from the other conditions. On skin biopsy study, leukemia cutis is characterized by infiltration of the skin by leukemic cells and is seen in 10% to 15% of patients with acute myeloid leukemia.2 In 5% of cases, leukemia cutis can present without bone marrow or peripheral signs of leukemia, hence the term aleukemic leukemia cutis.3 Cutaneous signs can occur before, after, or simultaneously with systemic leukemia.4
In the absence of systemic symptoms, the diagnosis is made when progressive cutaneous symptoms are present. The prognosis for aleukemic leukemia cutis is poor. Prompt diagnosis with skin biopsy is paramount to improve outcomes.
Acknowledgment: We would like to recognize Maanasa Devabhaktuni for her assistance in reporting this case.
- Yonal I, Hindilerden F, Coskun R, Dogan OI, Nalcaci M. Aleukemic leukemia cutis manifesting with disseminated nodular eruptions and a plaque preceding acute monocytic leukemia: a case report. Case Rep Oncol 2011; 4(3):547–554. doi:10.1159/000334745
- Cho-Vega JH, Medeiros LJ, Prieto VG, Vega F. Leukemia cutis. Am J Clin Pathol 2008; 129(1):130–142. doi:10.1309/WYACYWF6NGM3WBRT
- Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci 2013; 28(4):614–619. doi:10.3346/jkms.2013.28.4.614
- Obiozor C, Ganguly S, Fraga GR. Leukemia cutis with lymphoglandular bodies: a clue to acute lymphoblastic leukemia cutis. Dermatol Online J 2015; 21(8)pii:13030/qt6m18g35f. pmid:26437164
- Yonal I, Hindilerden F, Coskun R, Dogan OI, Nalcaci M. Aleukemic leukemia cutis manifesting with disseminated nodular eruptions and a plaque preceding acute monocytic leukemia: a case report. Case Rep Oncol 2011; 4(3):547–554. doi:10.1159/000334745
- Cho-Vega JH, Medeiros LJ, Prieto VG, Vega F. Leukemia cutis. Am J Clin Pathol 2008; 129(1):130–142. doi:10.1309/WYACYWF6NGM3WBRT
- Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci 2013; 28(4):614–619. doi:10.3346/jkms.2013.28.4.614
- Obiozor C, Ganguly S, Fraga GR. Leukemia cutis with lymphoglandular bodies: a clue to acute lymphoblastic leukemia cutis. Dermatol Online J 2015; 21(8)pii:13030/qt6m18g35f. pmid:26437164
Dancing sternal wires: A radiologic sign of sternal dehiscence
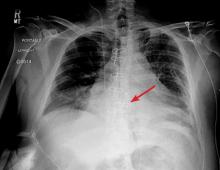
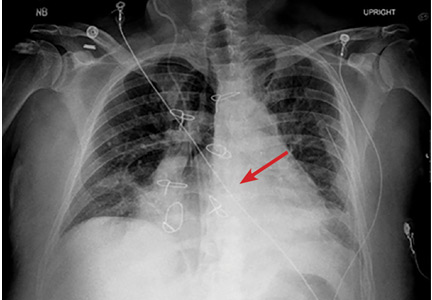
The next day, routine radiography showed widely separated sternal wires (Figure 3), indicating significant progression of sternal dehiscence. The patient subsequently underwent open reduction and internal fixation of the sternum.
STERNAL DEHISCENCE
Physical examination may reveal tenderness to palpation, but findings that are more characteristic are an audible click and rocking of the sternum with coughing or forced chest movements.3
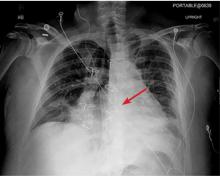
Plain chest radiography can clearly show early signs of sternal dehiscence; however, physicians rarely scrutinize the films for wire placement. Subtle signs include loss of sternal alignment with shifting of the segments and central sternal lucency. Gross signs start to appear when 2 or more wires are displaced; these signs are dramatic and rarely missed.
Loss of alignment and central sternal lucency are the earliest radiographic signs of dehiscence. Awareness of early subtle signs can lead to prompt diagnosis and treatment to prevent progression to gross sternal dehiscence.
- Olbrecht VA, Barreiro CJ, Bonde PN, et al. Clinical outcomes of noninfectious sternal dehiscence after median sternotomy. Ann Thorac Surg 2006; 82(3):902–907. doi:10.1016/j.athoracsur.2006.04.058
- Efthymiou CA, Kay PH, Nair UR. Repair of spontaneous right ventricular rupture following sternal dehiscence. A novel technique. Interact Cardiovasc Thorac Surg 2010; 10(1):12–13. doi:10.1510/icvts.2009.217810
- Santarpino G, Pfeiffer S, Concistré G, Fischlein T. Sternal wound dehiscence from intense coughing in a cardiac surgery patient: could it be prevented? G Chir 2013; 34(4):112-113. pmid:23660161
The next day, routine radiography showed widely separated sternal wires (Figure 3), indicating significant progression of sternal dehiscence. The patient subsequently underwent open reduction and internal fixation of the sternum.
STERNAL DEHISCENCE
Physical examination may reveal tenderness to palpation, but findings that are more characteristic are an audible click and rocking of the sternum with coughing or forced chest movements.3
Plain chest radiography can clearly show early signs of sternal dehiscence; however, physicians rarely scrutinize the films for wire placement. Subtle signs include loss of sternal alignment with shifting of the segments and central sternal lucency. Gross signs start to appear when 2 or more wires are displaced; these signs are dramatic and rarely missed.
Loss of alignment and central sternal lucency are the earliest radiographic signs of dehiscence. Awareness of early subtle signs can lead to prompt diagnosis and treatment to prevent progression to gross sternal dehiscence.
The next day, routine radiography showed widely separated sternal wires (Figure 3), indicating significant progression of sternal dehiscence. The patient subsequently underwent open reduction and internal fixation of the sternum.
STERNAL DEHISCENCE
Physical examination may reveal tenderness to palpation, but findings that are more characteristic are an audible click and rocking of the sternum with coughing or forced chest movements.3
Plain chest radiography can clearly show early signs of sternal dehiscence; however, physicians rarely scrutinize the films for wire placement. Subtle signs include loss of sternal alignment with shifting of the segments and central sternal lucency. Gross signs start to appear when 2 or more wires are displaced; these signs are dramatic and rarely missed.
Loss of alignment and central sternal lucency are the earliest radiographic signs of dehiscence. Awareness of early subtle signs can lead to prompt diagnosis and treatment to prevent progression to gross sternal dehiscence.
- Olbrecht VA, Barreiro CJ, Bonde PN, et al. Clinical outcomes of noninfectious sternal dehiscence after median sternotomy. Ann Thorac Surg 2006; 82(3):902–907. doi:10.1016/j.athoracsur.2006.04.058
- Efthymiou CA, Kay PH, Nair UR. Repair of spontaneous right ventricular rupture following sternal dehiscence. A novel technique. Interact Cardiovasc Thorac Surg 2010; 10(1):12–13. doi:10.1510/icvts.2009.217810
- Santarpino G, Pfeiffer S, Concistré G, Fischlein T. Sternal wound dehiscence from intense coughing in a cardiac surgery patient: could it be prevented? G Chir 2013; 34(4):112-113. pmid:23660161
- Olbrecht VA, Barreiro CJ, Bonde PN, et al. Clinical outcomes of noninfectious sternal dehiscence after median sternotomy. Ann Thorac Surg 2006; 82(3):902–907. doi:10.1016/j.athoracsur.2006.04.058
- Efthymiou CA, Kay PH, Nair UR. Repair of spontaneous right ventricular rupture following sternal dehiscence. A novel technique. Interact Cardiovasc Thorac Surg 2010; 10(1):12–13. doi:10.1510/icvts.2009.217810
- Santarpino G, Pfeiffer S, Concistré G, Fischlein T. Sternal wound dehiscence from intense coughing in a cardiac surgery patient: could it be prevented? G Chir 2013; 34(4):112-113. pmid:23660161
Repeating blood cultures after initial bacteremia: When and how often?
Repeat cultures are indicated in specific scenarios, but for most patients, frequent and indiscriminate repetition after an initial positive culture is unnecessary and may be associated with excessive use of resources. Prospective studies and practice guidelines are needed to help further define the indications.
THE TENDENCY TO REPEAT CULTURES
Current literature lacks strong evidence for repeating previously positive blood cultures collected appropriately—ie, 10 mL of blood for aerobic culture and 10 mL for anaerobic culture from 2 different sites, and a positive result from both sets. However, because of the risk of serious complications of bacteremia, particularly in critically ill patients, many clinicians order multiple, repeated sets of blood cultures.
Tabriz et al1 found that one-third of hospitalized patients got repeat cultures after an initial set, regardless of the result of the first set. Most (83.4%) of those cultures yielded no growth, 9.1% grew the same pathogen, and 5.0% were contaminated. Finding a new pathogen was rare, occurring in only 2.5% of repeated cultures.
Wiggers et al2 reported an even higher number of repeat cultures ordered for patients who had an initially positive culture: 38.9%.2 And in another study,3 half of the patients received more than 2 consecutive cultures.
Drawbacks
Unrestrained ordering of repeat blood cultures can increase the risk of a false-positive result, leading to more cultures, echocardiography, other imaging tests, and unnecessary antimicrobial therapy, all of which puts patients at risk of adverse effects of treatment and missed alternative diagnoses and increases the length and cost of hospitalization.4
Advantages
On the other hand, repeat blood cultures may increase the diagnostic yield for conditions such as infective endocarditis and may have implications for the duration of antibiotic therapy.1 The duration of therapy for bacteremia is usually determined from the last negative culture; hence, documenting clearance of bacteremia can determine a precise end-date for antibiotic therapy.
Bacteremia due to Staphylococcus aureus and to endovascular and epidural sources has been found to be independently associated with persistent bacteremia, detected in 6.6% of 1,801 index cases of bacteremia in a retrospective cohort study.2 An endovascular source (adjusted odds ratio [OR] 7.66, 95% confidence interval [CI] 2.30–25.48), an epidural source (adjusted OR 26.99, 95% CI, 1.91–391.08), and S aureus bacteremia (adjusted OR 4.49, 95% CI 1.88–10.73) were independently associated with persistent bacteremia. Escherichia coli (5.1%, P = .006), viridans group streptococci (1.7%, P = .035), and beta-hemolytic streptococci (0%, P = .028) were associated with a lower likelihood of persistent bacteremia. Patients with persistent bacteremia were less likely to have achieved source control within 48 hours of the index event (29.7% vs 52.5%, P < .001).2
WHEN REPEATING CULTURES IS APPROPRIATE
Repeating blood cultures after an initial positive result is superfluous, except in certain situations.
Suspected endovascular infection
Patients with endocarditis, thrombophlebitis, an indwelling device for epidural access, or a cardiovascular implantable electronic device should have repeat cultures after an initial positive culture. Implantable electronic device infection is suspected in the following cases: sustained positive blood culture (> 24 hours); relapsing bacteremia despite a course of appropriate antibiotic therapy; presence of an implantable cardioverter defibrillator; presence of a prosthetic cardiac valve; and an episode of bacteremia within 3 months of device placement.5
S aureus bacteremia
Repeat blood culture is warranted for S aureus bacteremia regardless of methicillin susceptibility.1 But persistent methicillin-resistant S aureus (MRSA) bacteremia changes the management of these patients.6 For example, the source of infection should be identified, followed by debridement or drainage, and then either high-dose or combination antimicrobial therapy.6 Infective endocarditis from persistent MRSA bacteremia is an indication for surgery.6
Persistent S aureus bacteremia may change the duration of therapy, as the common practice is to continue treating uncomplicated gram-positive bacteremia for 14 days from the date of the first negative culture. Infection leading to infective endocarditis increases the duration of antibiotic therapy to at least 4 weeks.
Candidemia
Candidemia is an absolute indication for repeat blood culture.7 Patients with persistent candidemia should undergo imaging of the genitourinary tract, liver, and spleen as part of the evaluation for a deep-tissue source of infection.7 Also, if the patient is initially treated with an echinocandin, therapy can be transitioned to fluconazole if the isolate is azole-susceptible, the patient’s condition is clinically stable, and repeat cultures are negative.7 Therefore, repeating cultures has therapeutic implications.
Confirming response to therapy
In patients with infective endocarditis or other endovascular infection caused by S aureus, Enterococcus species, or gram-negative bacilli,1 repeat blood culture should be done to confirm therapeutic response. Patients with infective endocarditis whose condition is stable can be discharged to receive outpatient parenteral antibiotic therapy. However, patients with uncontrolled heart failure, systemic emboli, abscess, persistent fever, or persistently positive cultures are not candidates for outpatient therapy and require repeat cultures.8
Multidrug-resistant gram-negative bacilli
Bacteremia due to multidrug-resistant gram-negative bacilli requires repeat blood cultures to document clearance of bacteremia and to ensure the efficacy of antibiotics, as these organisms pose a higher risk of treatment failure, and combination synergistic regimens may be needed if bacteremia does not clear.
Febrile neutropenia
Blood cultures are important in the management of febrile neutropenia. In a study by Rosenblum et al,9 repeat cultures were positive in 10.9% of patients with febrile neutropenia after an initial negative culture, but many of those organisms were of low pathogenicity, and a significant proportion were coagulase-negative staphylococci.10 Another study showed that the frequency of detecting new pathogens by repeat culture in recurrent febrile neutropenia was higher than that in persistent febrile neutropenia (8% vs 2%) (P = .0491); a history of recent bacteremia was identified as a significant predictor of positive culture in recurrent febrile neutropenia.11
Persistent or new infection
Persistence of fever, leukocytosis, or other signs of infection 72 hours after appropriate antibiotic therapy is started requires follow-up blood cultures.
New episode of sepsis. A new episode of sepsis should be confirmed12 using the systemic inflammatory response syndrome criteria, the newer definition of Sepsis-related Organ Failure Assessment (SOFA) in the intensive-care unit, or the quick SOFA in general units. If the patient develops new signs of sepsis after response to treatment for initial bacteremia, repeat blood cultures should be considered.
Central line-associated bloodstream infection requires repeat cultures.13 Persistence of bacteremia in this type of infection extends the duration of therapy, as most clinicians determine treatment duration from the last negative culture. Persistent bacteremia also influences the decision to salvage or remove the catheter. Microbiologic clearance of bacteremia on blood culture can also guide the time of reinsertion if the catheter was removed.
Concern for an unresolved focus of infection such as abscess, joint infection, or retained catheter is an indication for repeat blood cultures.
Bacteremia of unknown source. In clinical practice, we encounter scenarios in which blood cultures are positive but no source can be identified. In those situations, it is important to repeat blood cultures to document clearance. If bacteremia persists, we need to continue searching for the source.
WHEN ROUTINELY REPEATING CULTURES IS NOT INDICATED
Repeat blood cultures are not routinely indicated in patients with streptococcal bacteremia, uncomplicated gram-negative bacteremia, and bacteremia associated with localized infection such as cellulitis, community-acquired pneumonia, or pyelonephritis.2,4 A study of patients with gram-negative bacteremia found that 17 repeated cultures needed to be drawn to yield 1 positive culture.14
Isolated fever or leukocytosis does not accurately predict bacteremia.4 A study that excluded neutropenic and intensive-care patients reported none of the initially negative cultures to be positive when repeated.15
Ordering repeat cultures in response to persistent fever is a common practice, even though fever is typical in the first 72 hours of antibiotic therapy. Such cultures rarely if ever reveal new pathogens, and results can be predicted based on cultures before the start of antibiotics.15 For patients on antibiotics, physicians should therefore wait for results of the preantibiotic cultures rather than order new cultures in response to persistent fever.15
WOULD WE MISS PERSISTENT BACTEREMIA?
In theory, not repeating blood cultures could miss persistent bacteremia, but this is unlikely if the concerns discussed above are considered. Further, persistent bacteremia would result in clinical signs and symptoms that should prompt repeat cultures.
FREQUENCY OF REPEAT BLOOD CULTURES
There are no evidence-based guidelines for the frequency of repeating cultures. The Infectious Diseases Society of America recommends repeating blood cultures 2 to 4 days after the index positive culture in the case of multidrug-resistant S aureus bacteremia, and every day or every other day for candidemia.6,7,9
A study evaluating the practice patterns of repeating cultures after an initial bacteremia showed that 34.7% were done within 24 hours and 44.7% were done in 2 to 4 days.1 There is no evidence that repeating blood cultures daily is necessary in these patients. As a general rule, it should be done 48 to 72 hours after a positive culture.
- Tabriz MS, Riederer K, Baran J Jr, Khatib R. Repeating blood cultures during hospital stay: practice pattern at a teaching hospital and a proposal for guidelines. Clin Microbiol Infect 2004; 10(7):624–627. doi:10.1111/j.1469-0691.2004.00893.x
- Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis 2016; 16:286. doi:10.1186/s12879-016-1622-z
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case–control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Coburn B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood cultures? JAMA 2012; 308(5):502–511. doi:10.1001/jama.2012.8262
- Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; Council on Cardiovascular Disease in Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Interdisciplinary Council on Quality of Care; American Heart Association. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121(3):458–477. doi:10.1161/CIRCULATIONAHA.109.192665
- Liu C, Bayer A, Cosgrove SE, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52(3):e18–e55. doi:10.1093/cid/ciq146
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62(4):e1–e50. doi:10.1093/cid/civ933
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Rosenblum J, Lin J, Kim M, Levy AS. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2013; 60(6):923–927. doi:10.1002/pbc.24358
- Thomas MW, Chauvenet AR, O'Suoji C. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2014; 61(2):194. doi:10.1002/pbc.24834
- Kimura SI, Gomyo A, Hayakawa J, et al. Clinical significance of repeat blood cultures during febrile neutropenia in adult acute myeloid leukaemia patients undergoing intensive chemotherapy. Infect Dis (Lond) 2017; 49(10):748–757. doi:10.1080/23744235.2017.1340665
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315(8):801–810. doi:10.1001/jama.2016.0287
- Shah H, Bosch W, Thompson KM, Hellinger WC. Intravascular catheter-related bloodstream infection. Neurohospitalist 2013; 3(3):144–151. doi:10.1177/1941874413476043
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Grace CJ, Lieberman J, Pierce K, Littenberg B. Usefulness of blood culture for hospitalized patients who are receiving antibiotic therapy. Clin Infect Dis 2001; 32(11):1651–1655. doi:10.1086/320527
Repeat cultures are indicated in specific scenarios, but for most patients, frequent and indiscriminate repetition after an initial positive culture is unnecessary and may be associated with excessive use of resources. Prospective studies and practice guidelines are needed to help further define the indications.
THE TENDENCY TO REPEAT CULTURES
Current literature lacks strong evidence for repeating previously positive blood cultures collected appropriately—ie, 10 mL of blood for aerobic culture and 10 mL for anaerobic culture from 2 different sites, and a positive result from both sets. However, because of the risk of serious complications of bacteremia, particularly in critically ill patients, many clinicians order multiple, repeated sets of blood cultures.
Tabriz et al1 found that one-third of hospitalized patients got repeat cultures after an initial set, regardless of the result of the first set. Most (83.4%) of those cultures yielded no growth, 9.1% grew the same pathogen, and 5.0% were contaminated. Finding a new pathogen was rare, occurring in only 2.5% of repeated cultures.
Wiggers et al2 reported an even higher number of repeat cultures ordered for patients who had an initially positive culture: 38.9%.2 And in another study,3 half of the patients received more than 2 consecutive cultures.
Drawbacks
Unrestrained ordering of repeat blood cultures can increase the risk of a false-positive result, leading to more cultures, echocardiography, other imaging tests, and unnecessary antimicrobial therapy, all of which puts patients at risk of adverse effects of treatment and missed alternative diagnoses and increases the length and cost of hospitalization.4
Advantages
On the other hand, repeat blood cultures may increase the diagnostic yield for conditions such as infective endocarditis and may have implications for the duration of antibiotic therapy.1 The duration of therapy for bacteremia is usually determined from the last negative culture; hence, documenting clearance of bacteremia can determine a precise end-date for antibiotic therapy.
Bacteremia due to Staphylococcus aureus and to endovascular and epidural sources has been found to be independently associated with persistent bacteremia, detected in 6.6% of 1,801 index cases of bacteremia in a retrospective cohort study.2 An endovascular source (adjusted odds ratio [OR] 7.66, 95% confidence interval [CI] 2.30–25.48), an epidural source (adjusted OR 26.99, 95% CI, 1.91–391.08), and S aureus bacteremia (adjusted OR 4.49, 95% CI 1.88–10.73) were independently associated with persistent bacteremia. Escherichia coli (5.1%, P = .006), viridans group streptococci (1.7%, P = .035), and beta-hemolytic streptococci (0%, P = .028) were associated with a lower likelihood of persistent bacteremia. Patients with persistent bacteremia were less likely to have achieved source control within 48 hours of the index event (29.7% vs 52.5%, P < .001).2
WHEN REPEATING CULTURES IS APPROPRIATE
Repeating blood cultures after an initial positive result is superfluous, except in certain situations.
Suspected endovascular infection
Patients with endocarditis, thrombophlebitis, an indwelling device for epidural access, or a cardiovascular implantable electronic device should have repeat cultures after an initial positive culture. Implantable electronic device infection is suspected in the following cases: sustained positive blood culture (> 24 hours); relapsing bacteremia despite a course of appropriate antibiotic therapy; presence of an implantable cardioverter defibrillator; presence of a prosthetic cardiac valve; and an episode of bacteremia within 3 months of device placement.5
S aureus bacteremia
Repeat blood culture is warranted for S aureus bacteremia regardless of methicillin susceptibility.1 But persistent methicillin-resistant S aureus (MRSA) bacteremia changes the management of these patients.6 For example, the source of infection should be identified, followed by debridement or drainage, and then either high-dose or combination antimicrobial therapy.6 Infective endocarditis from persistent MRSA bacteremia is an indication for surgery.6
Persistent S aureus bacteremia may change the duration of therapy, as the common practice is to continue treating uncomplicated gram-positive bacteremia for 14 days from the date of the first negative culture. Infection leading to infective endocarditis increases the duration of antibiotic therapy to at least 4 weeks.
Candidemia
Candidemia is an absolute indication for repeat blood culture.7 Patients with persistent candidemia should undergo imaging of the genitourinary tract, liver, and spleen as part of the evaluation for a deep-tissue source of infection.7 Also, if the patient is initially treated with an echinocandin, therapy can be transitioned to fluconazole if the isolate is azole-susceptible, the patient’s condition is clinically stable, and repeat cultures are negative.7 Therefore, repeating cultures has therapeutic implications.
Confirming response to therapy
In patients with infective endocarditis or other endovascular infection caused by S aureus, Enterococcus species, or gram-negative bacilli,1 repeat blood culture should be done to confirm therapeutic response. Patients with infective endocarditis whose condition is stable can be discharged to receive outpatient parenteral antibiotic therapy. However, patients with uncontrolled heart failure, systemic emboli, abscess, persistent fever, or persistently positive cultures are not candidates for outpatient therapy and require repeat cultures.8
Multidrug-resistant gram-negative bacilli
Bacteremia due to multidrug-resistant gram-negative bacilli requires repeat blood cultures to document clearance of bacteremia and to ensure the efficacy of antibiotics, as these organisms pose a higher risk of treatment failure, and combination synergistic regimens may be needed if bacteremia does not clear.
Febrile neutropenia
Blood cultures are important in the management of febrile neutropenia. In a study by Rosenblum et al,9 repeat cultures were positive in 10.9% of patients with febrile neutropenia after an initial negative culture, but many of those organisms were of low pathogenicity, and a significant proportion were coagulase-negative staphylococci.10 Another study showed that the frequency of detecting new pathogens by repeat culture in recurrent febrile neutropenia was higher than that in persistent febrile neutropenia (8% vs 2%) (P = .0491); a history of recent bacteremia was identified as a significant predictor of positive culture in recurrent febrile neutropenia.11
Persistent or new infection
Persistence of fever, leukocytosis, or other signs of infection 72 hours after appropriate antibiotic therapy is started requires follow-up blood cultures.
New episode of sepsis. A new episode of sepsis should be confirmed12 using the systemic inflammatory response syndrome criteria, the newer definition of Sepsis-related Organ Failure Assessment (SOFA) in the intensive-care unit, or the quick SOFA in general units. If the patient develops new signs of sepsis after response to treatment for initial bacteremia, repeat blood cultures should be considered.
Central line-associated bloodstream infection requires repeat cultures.13 Persistence of bacteremia in this type of infection extends the duration of therapy, as most clinicians determine treatment duration from the last negative culture. Persistent bacteremia also influences the decision to salvage or remove the catheter. Microbiologic clearance of bacteremia on blood culture can also guide the time of reinsertion if the catheter was removed.
Concern for an unresolved focus of infection such as abscess, joint infection, or retained catheter is an indication for repeat blood cultures.
Bacteremia of unknown source. In clinical practice, we encounter scenarios in which blood cultures are positive but no source can be identified. In those situations, it is important to repeat blood cultures to document clearance. If bacteremia persists, we need to continue searching for the source.
WHEN ROUTINELY REPEATING CULTURES IS NOT INDICATED
Repeat blood cultures are not routinely indicated in patients with streptococcal bacteremia, uncomplicated gram-negative bacteremia, and bacteremia associated with localized infection such as cellulitis, community-acquired pneumonia, or pyelonephritis.2,4 A study of patients with gram-negative bacteremia found that 17 repeated cultures needed to be drawn to yield 1 positive culture.14
Isolated fever or leukocytosis does not accurately predict bacteremia.4 A study that excluded neutropenic and intensive-care patients reported none of the initially negative cultures to be positive when repeated.15
Ordering repeat cultures in response to persistent fever is a common practice, even though fever is typical in the first 72 hours of antibiotic therapy. Such cultures rarely if ever reveal new pathogens, and results can be predicted based on cultures before the start of antibiotics.15 For patients on antibiotics, physicians should therefore wait for results of the preantibiotic cultures rather than order new cultures in response to persistent fever.15
WOULD WE MISS PERSISTENT BACTEREMIA?
In theory, not repeating blood cultures could miss persistent bacteremia, but this is unlikely if the concerns discussed above are considered. Further, persistent bacteremia would result in clinical signs and symptoms that should prompt repeat cultures.
FREQUENCY OF REPEAT BLOOD CULTURES
There are no evidence-based guidelines for the frequency of repeating cultures. The Infectious Diseases Society of America recommends repeating blood cultures 2 to 4 days after the index positive culture in the case of multidrug-resistant S aureus bacteremia, and every day or every other day for candidemia.6,7,9
A study evaluating the practice patterns of repeating cultures after an initial bacteremia showed that 34.7% were done within 24 hours and 44.7% were done in 2 to 4 days.1 There is no evidence that repeating blood cultures daily is necessary in these patients. As a general rule, it should be done 48 to 72 hours after a positive culture.
Repeat cultures are indicated in specific scenarios, but for most patients, frequent and indiscriminate repetition after an initial positive culture is unnecessary and may be associated with excessive use of resources. Prospective studies and practice guidelines are needed to help further define the indications.
THE TENDENCY TO REPEAT CULTURES
Current literature lacks strong evidence for repeating previously positive blood cultures collected appropriately—ie, 10 mL of blood for aerobic culture and 10 mL for anaerobic culture from 2 different sites, and a positive result from both sets. However, because of the risk of serious complications of bacteremia, particularly in critically ill patients, many clinicians order multiple, repeated sets of blood cultures.
Tabriz et al1 found that one-third of hospitalized patients got repeat cultures after an initial set, regardless of the result of the first set. Most (83.4%) of those cultures yielded no growth, 9.1% grew the same pathogen, and 5.0% were contaminated. Finding a new pathogen was rare, occurring in only 2.5% of repeated cultures.
Wiggers et al2 reported an even higher number of repeat cultures ordered for patients who had an initially positive culture: 38.9%.2 And in another study,3 half of the patients received more than 2 consecutive cultures.
Drawbacks
Unrestrained ordering of repeat blood cultures can increase the risk of a false-positive result, leading to more cultures, echocardiography, other imaging tests, and unnecessary antimicrobial therapy, all of which puts patients at risk of adverse effects of treatment and missed alternative diagnoses and increases the length and cost of hospitalization.4
Advantages
On the other hand, repeat blood cultures may increase the diagnostic yield for conditions such as infective endocarditis and may have implications for the duration of antibiotic therapy.1 The duration of therapy for bacteremia is usually determined from the last negative culture; hence, documenting clearance of bacteremia can determine a precise end-date for antibiotic therapy.
Bacteremia due to Staphylococcus aureus and to endovascular and epidural sources has been found to be independently associated with persistent bacteremia, detected in 6.6% of 1,801 index cases of bacteremia in a retrospective cohort study.2 An endovascular source (adjusted odds ratio [OR] 7.66, 95% confidence interval [CI] 2.30–25.48), an epidural source (adjusted OR 26.99, 95% CI, 1.91–391.08), and S aureus bacteremia (adjusted OR 4.49, 95% CI 1.88–10.73) were independently associated with persistent bacteremia. Escherichia coli (5.1%, P = .006), viridans group streptococci (1.7%, P = .035), and beta-hemolytic streptococci (0%, P = .028) were associated with a lower likelihood of persistent bacteremia. Patients with persistent bacteremia were less likely to have achieved source control within 48 hours of the index event (29.7% vs 52.5%, P < .001).2
WHEN REPEATING CULTURES IS APPROPRIATE
Repeating blood cultures after an initial positive result is superfluous, except in certain situations.
Suspected endovascular infection
Patients with endocarditis, thrombophlebitis, an indwelling device for epidural access, or a cardiovascular implantable electronic device should have repeat cultures after an initial positive culture. Implantable electronic device infection is suspected in the following cases: sustained positive blood culture (> 24 hours); relapsing bacteremia despite a course of appropriate antibiotic therapy; presence of an implantable cardioverter defibrillator; presence of a prosthetic cardiac valve; and an episode of bacteremia within 3 months of device placement.5
S aureus bacteremia
Repeat blood culture is warranted for S aureus bacteremia regardless of methicillin susceptibility.1 But persistent methicillin-resistant S aureus (MRSA) bacteremia changes the management of these patients.6 For example, the source of infection should be identified, followed by debridement or drainage, and then either high-dose or combination antimicrobial therapy.6 Infective endocarditis from persistent MRSA bacteremia is an indication for surgery.6
Persistent S aureus bacteremia may change the duration of therapy, as the common practice is to continue treating uncomplicated gram-positive bacteremia for 14 days from the date of the first negative culture. Infection leading to infective endocarditis increases the duration of antibiotic therapy to at least 4 weeks.
Candidemia
Candidemia is an absolute indication for repeat blood culture.7 Patients with persistent candidemia should undergo imaging of the genitourinary tract, liver, and spleen as part of the evaluation for a deep-tissue source of infection.7 Also, if the patient is initially treated with an echinocandin, therapy can be transitioned to fluconazole if the isolate is azole-susceptible, the patient’s condition is clinically stable, and repeat cultures are negative.7 Therefore, repeating cultures has therapeutic implications.
Confirming response to therapy
In patients with infective endocarditis or other endovascular infection caused by S aureus, Enterococcus species, or gram-negative bacilli,1 repeat blood culture should be done to confirm therapeutic response. Patients with infective endocarditis whose condition is stable can be discharged to receive outpatient parenteral antibiotic therapy. However, patients with uncontrolled heart failure, systemic emboli, abscess, persistent fever, or persistently positive cultures are not candidates for outpatient therapy and require repeat cultures.8
Multidrug-resistant gram-negative bacilli
Bacteremia due to multidrug-resistant gram-negative bacilli requires repeat blood cultures to document clearance of bacteremia and to ensure the efficacy of antibiotics, as these organisms pose a higher risk of treatment failure, and combination synergistic regimens may be needed if bacteremia does not clear.
Febrile neutropenia
Blood cultures are important in the management of febrile neutropenia. In a study by Rosenblum et al,9 repeat cultures were positive in 10.9% of patients with febrile neutropenia after an initial negative culture, but many of those organisms were of low pathogenicity, and a significant proportion were coagulase-negative staphylococci.10 Another study showed that the frequency of detecting new pathogens by repeat culture in recurrent febrile neutropenia was higher than that in persistent febrile neutropenia (8% vs 2%) (P = .0491); a history of recent bacteremia was identified as a significant predictor of positive culture in recurrent febrile neutropenia.11
Persistent or new infection
Persistence of fever, leukocytosis, or other signs of infection 72 hours after appropriate antibiotic therapy is started requires follow-up blood cultures.
New episode of sepsis. A new episode of sepsis should be confirmed12 using the systemic inflammatory response syndrome criteria, the newer definition of Sepsis-related Organ Failure Assessment (SOFA) in the intensive-care unit, or the quick SOFA in general units. If the patient develops new signs of sepsis after response to treatment for initial bacteremia, repeat blood cultures should be considered.
Central line-associated bloodstream infection requires repeat cultures.13 Persistence of bacteremia in this type of infection extends the duration of therapy, as most clinicians determine treatment duration from the last negative culture. Persistent bacteremia also influences the decision to salvage or remove the catheter. Microbiologic clearance of bacteremia on blood culture can also guide the time of reinsertion if the catheter was removed.
Concern for an unresolved focus of infection such as abscess, joint infection, or retained catheter is an indication for repeat blood cultures.
Bacteremia of unknown source. In clinical practice, we encounter scenarios in which blood cultures are positive but no source can be identified. In those situations, it is important to repeat blood cultures to document clearance. If bacteremia persists, we need to continue searching for the source.
WHEN ROUTINELY REPEATING CULTURES IS NOT INDICATED
Repeat blood cultures are not routinely indicated in patients with streptococcal bacteremia, uncomplicated gram-negative bacteremia, and bacteremia associated with localized infection such as cellulitis, community-acquired pneumonia, or pyelonephritis.2,4 A study of patients with gram-negative bacteremia found that 17 repeated cultures needed to be drawn to yield 1 positive culture.14
Isolated fever or leukocytosis does not accurately predict bacteremia.4 A study that excluded neutropenic and intensive-care patients reported none of the initially negative cultures to be positive when repeated.15
Ordering repeat cultures in response to persistent fever is a common practice, even though fever is typical in the first 72 hours of antibiotic therapy. Such cultures rarely if ever reveal new pathogens, and results can be predicted based on cultures before the start of antibiotics.15 For patients on antibiotics, physicians should therefore wait for results of the preantibiotic cultures rather than order new cultures in response to persistent fever.15
WOULD WE MISS PERSISTENT BACTEREMIA?
In theory, not repeating blood cultures could miss persistent bacteremia, but this is unlikely if the concerns discussed above are considered. Further, persistent bacteremia would result in clinical signs and symptoms that should prompt repeat cultures.
FREQUENCY OF REPEAT BLOOD CULTURES
There are no evidence-based guidelines for the frequency of repeating cultures. The Infectious Diseases Society of America recommends repeating blood cultures 2 to 4 days after the index positive culture in the case of multidrug-resistant S aureus bacteremia, and every day or every other day for candidemia.6,7,9
A study evaluating the practice patterns of repeating cultures after an initial bacteremia showed that 34.7% were done within 24 hours and 44.7% were done in 2 to 4 days.1 There is no evidence that repeating blood cultures daily is necessary in these patients. As a general rule, it should be done 48 to 72 hours after a positive culture.
- Tabriz MS, Riederer K, Baran J Jr, Khatib R. Repeating blood cultures during hospital stay: practice pattern at a teaching hospital and a proposal for guidelines. Clin Microbiol Infect 2004; 10(7):624–627. doi:10.1111/j.1469-0691.2004.00893.x
- Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis 2016; 16:286. doi:10.1186/s12879-016-1622-z
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case–control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Coburn B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood cultures? JAMA 2012; 308(5):502–511. doi:10.1001/jama.2012.8262
- Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; Council on Cardiovascular Disease in Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Interdisciplinary Council on Quality of Care; American Heart Association. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121(3):458–477. doi:10.1161/CIRCULATIONAHA.109.192665
- Liu C, Bayer A, Cosgrove SE, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52(3):e18–e55. doi:10.1093/cid/ciq146
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62(4):e1–e50. doi:10.1093/cid/civ933
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Rosenblum J, Lin J, Kim M, Levy AS. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2013; 60(6):923–927. doi:10.1002/pbc.24358
- Thomas MW, Chauvenet AR, O'Suoji C. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2014; 61(2):194. doi:10.1002/pbc.24834
- Kimura SI, Gomyo A, Hayakawa J, et al. Clinical significance of repeat blood cultures during febrile neutropenia in adult acute myeloid leukaemia patients undergoing intensive chemotherapy. Infect Dis (Lond) 2017; 49(10):748–757. doi:10.1080/23744235.2017.1340665
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315(8):801–810. doi:10.1001/jama.2016.0287
- Shah H, Bosch W, Thompson KM, Hellinger WC. Intravascular catheter-related bloodstream infection. Neurohospitalist 2013; 3(3):144–151. doi:10.1177/1941874413476043
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Grace CJ, Lieberman J, Pierce K, Littenberg B. Usefulness of blood culture for hospitalized patients who are receiving antibiotic therapy. Clin Infect Dis 2001; 32(11):1651–1655. doi:10.1086/320527
- Tabriz MS, Riederer K, Baran J Jr, Khatib R. Repeating blood cultures during hospital stay: practice pattern at a teaching hospital and a proposal for guidelines. Clin Microbiol Infect 2004; 10(7):624–627. doi:10.1111/j.1469-0691.2004.00893.x
- Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis 2016; 16:286. doi:10.1186/s12879-016-1622-z
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case–control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Coburn B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood cultures? JAMA 2012; 308(5):502–511. doi:10.1001/jama.2012.8262
- Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; Council on Cardiovascular Disease in Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Interdisciplinary Council on Quality of Care; American Heart Association. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121(3):458–477. doi:10.1161/CIRCULATIONAHA.109.192665
- Liu C, Bayer A, Cosgrove SE, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52(3):e18–e55. doi:10.1093/cid/ciq146
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62(4):e1–e50. doi:10.1093/cid/civ933
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Rosenblum J, Lin J, Kim M, Levy AS. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2013; 60(6):923–927. doi:10.1002/pbc.24358
- Thomas MW, Chauvenet AR, O'Suoji C. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2014; 61(2):194. doi:10.1002/pbc.24834
- Kimura SI, Gomyo A, Hayakawa J, et al. Clinical significance of repeat blood cultures during febrile neutropenia in adult acute myeloid leukaemia patients undergoing intensive chemotherapy. Infect Dis (Lond) 2017; 49(10):748–757. doi:10.1080/23744235.2017.1340665
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315(8):801–810. doi:10.1001/jama.2016.0287
- Shah H, Bosch W, Thompson KM, Hellinger WC. Intravascular catheter-related bloodstream infection. Neurohospitalist 2013; 3(3):144–151. doi:10.1177/1941874413476043
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Grace CJ, Lieberman J, Pierce K, Littenberg B. Usefulness of blood culture for hospitalized patients who are receiving antibiotic therapy. Clin Infect Dis 2001; 32(11):1651–1655. doi:10.1086/320527
Follow-up blood cultures are often needed after bacteremia
Bacteremia is common and associated with significant morbidity and mortality. Bloodstream infections rank among the leading causes of death in North America and Europe.1
In this issue, Mushtaq et al2 contend that follow-up blood cultures after initial bacteremia are not needed for most hospitalized patients. Not repeating blood cultures after initial bacteremia has been proposed to decrease hospitalization length, consultations, and healthcare costs in some clinical settings. However, without follow-up cultures, it can be difficult to assess the adequacy of treatment of bacteremia and associated underlying infections.
GRAM-NEGATIVE ORGANISMS
Results of retrospective studies indicate that follow-up cultures may not be routinely needed for gram-negative bacteremia. In a review by Canzoneri et al of 383 cases with subsequent follow-up cultures,3 55 (14%) were positive. The mean duration of bacteremia was 2.8 days (range 1 to 15 days). Of the 55 persistently positive blood cultures, only 8 (15%) were caused by gram-negative organisms. Limitations to this study included the lack of patient outcome data, a low event rate, and the retrospective design.4
In a retrospective case-control study of follow-up cultures for 862 episodes of Klebsiella pneumoniae bacteremia,5 independent risk factors for persistent bacteremia were intra-abdominal infection, higher Charlson comorbidity index score, solid-organ transplant, and unfavorable treatment response.
These studies confirm that persistent bacteremia is uncommon with gram-negative organisms. They also support using comorbidities and treatment response to guide the ordering of follow-up blood cultures.
WHEN IS FOLLOW-UP CULTURE USEFUL?
Although follow-up blood cultures may not be needed routinely in patients with gram- negative bacteremia, it would be difficult to extrapolate this to gram-positive organisms, especially Staphylococcus aureus.
In Canzoneri et al,3 43 (78%) of the 55 positive follow-up cultures were due to gram-positive organisms. Factors associated with positive follow-up cultures were concurrent fever, presence of a central intravenous line, end-stage renal disease on hemodialysis, and diabetes mellitus. In addition, infectious disease consultation to decide the need for follow-up cultures for S aureus bacteremia has been associated with fewer deaths, fewer relapses, and lower readmission rates.6,7
In certain clinical scenarios, follow-up blood cultures can provide useful information, such as when the source of bacteremia is endocarditis or cardiac device infection, a vascular graft, or an intravascular line. In the Infectious Diseases Society of America guidelines for diagnosis and management of catheter-related bloodstream infections, persistent or relapsing bacteremia for some organisms is a criterion for removal of a long-term central venous catheter.8
Follow-up cultures are especially useful when the focus of infection is protected from antibiotic penetration, such as in the central nervous system, joints, and abdominal or other abscess. These foci may require drainage for cure. In these cases or in the setting of unfavorable clinical treatment response, follow-up blood cultures showing persistent bacteremia can prompt a search for unaddressed or incompletely addressed foci of infection and allow for source control.
The timing of follow-up cultures is generally 1 to 2 days after the initial culture. Although Mushtaq et al propose a different approach, traditional teaching has been that the last blood culture should not be positive, and this leads to ordering follow-up blood cultures until clearance of bacteremia is documented.
- Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 2013; 19(6):501–509. doi:10.1111/1469-0691.12195
- Mushtaq A, Bredell B, Soubani A. Repeating blood cultures after an initial bacteremia: when and how often? Cleve Clin J Med 2019; 86(2):89–92. doi:10.3949/ccjm.86a.18001
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Jones RB, Paruchuri A, Shah SS. Prospective trials are required to alter practice for follow-up blood cultures for gram-negative bacilli bacteremia. Clin Infect Dis 2018; 67(2):315–316. doi:10.1093/cid/ciy070
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case-control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Honda H, Krauss MJ, Jones JC, Olsen MA, Warren DK. The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med 2010; 123(7):631–637. doi:10.1016/j.amjmed.2010.01.015
- Fowler VG Jr, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 1998; 27(3):478–486. pmid:9770144
- Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49(1):1–45. doi:10.1086/599376
Bacteremia is common and associated with significant morbidity and mortality. Bloodstream infections rank among the leading causes of death in North America and Europe.1
In this issue, Mushtaq et al2 contend that follow-up blood cultures after initial bacteremia are not needed for most hospitalized patients. Not repeating blood cultures after initial bacteremia has been proposed to decrease hospitalization length, consultations, and healthcare costs in some clinical settings. However, without follow-up cultures, it can be difficult to assess the adequacy of treatment of bacteremia and associated underlying infections.
GRAM-NEGATIVE ORGANISMS
Results of retrospective studies indicate that follow-up cultures may not be routinely needed for gram-negative bacteremia. In a review by Canzoneri et al of 383 cases with subsequent follow-up cultures,3 55 (14%) were positive. The mean duration of bacteremia was 2.8 days (range 1 to 15 days). Of the 55 persistently positive blood cultures, only 8 (15%) were caused by gram-negative organisms. Limitations to this study included the lack of patient outcome data, a low event rate, and the retrospective design.4
In a retrospective case-control study of follow-up cultures for 862 episodes of Klebsiella pneumoniae bacteremia,5 independent risk factors for persistent bacteremia were intra-abdominal infection, higher Charlson comorbidity index score, solid-organ transplant, and unfavorable treatment response.
These studies confirm that persistent bacteremia is uncommon with gram-negative organisms. They also support using comorbidities and treatment response to guide the ordering of follow-up blood cultures.
WHEN IS FOLLOW-UP CULTURE USEFUL?
Although follow-up blood cultures may not be needed routinely in patients with gram- negative bacteremia, it would be difficult to extrapolate this to gram-positive organisms, especially Staphylococcus aureus.
In Canzoneri et al,3 43 (78%) of the 55 positive follow-up cultures were due to gram-positive organisms. Factors associated with positive follow-up cultures were concurrent fever, presence of a central intravenous line, end-stage renal disease on hemodialysis, and diabetes mellitus. In addition, infectious disease consultation to decide the need for follow-up cultures for S aureus bacteremia has been associated with fewer deaths, fewer relapses, and lower readmission rates.6,7
In certain clinical scenarios, follow-up blood cultures can provide useful information, such as when the source of bacteremia is endocarditis or cardiac device infection, a vascular graft, or an intravascular line. In the Infectious Diseases Society of America guidelines for diagnosis and management of catheter-related bloodstream infections, persistent or relapsing bacteremia for some organisms is a criterion for removal of a long-term central venous catheter.8
Follow-up cultures are especially useful when the focus of infection is protected from antibiotic penetration, such as in the central nervous system, joints, and abdominal or other abscess. These foci may require drainage for cure. In these cases or in the setting of unfavorable clinical treatment response, follow-up blood cultures showing persistent bacteremia can prompt a search for unaddressed or incompletely addressed foci of infection and allow for source control.
The timing of follow-up cultures is generally 1 to 2 days after the initial culture. Although Mushtaq et al propose a different approach, traditional teaching has been that the last blood culture should not be positive, and this leads to ordering follow-up blood cultures until clearance of bacteremia is documented.
Bacteremia is common and associated with significant morbidity and mortality. Bloodstream infections rank among the leading causes of death in North America and Europe.1
In this issue, Mushtaq et al2 contend that follow-up blood cultures after initial bacteremia are not needed for most hospitalized patients. Not repeating blood cultures after initial bacteremia has been proposed to decrease hospitalization length, consultations, and healthcare costs in some clinical settings. However, without follow-up cultures, it can be difficult to assess the adequacy of treatment of bacteremia and associated underlying infections.
GRAM-NEGATIVE ORGANISMS
Results of retrospective studies indicate that follow-up cultures may not be routinely needed for gram-negative bacteremia. In a review by Canzoneri et al of 383 cases with subsequent follow-up cultures,3 55 (14%) were positive. The mean duration of bacteremia was 2.8 days (range 1 to 15 days). Of the 55 persistently positive blood cultures, only 8 (15%) were caused by gram-negative organisms. Limitations to this study included the lack of patient outcome data, a low event rate, and the retrospective design.4
In a retrospective case-control study of follow-up cultures for 862 episodes of Klebsiella pneumoniae bacteremia,5 independent risk factors for persistent bacteremia were intra-abdominal infection, higher Charlson comorbidity index score, solid-organ transplant, and unfavorable treatment response.
These studies confirm that persistent bacteremia is uncommon with gram-negative organisms. They also support using comorbidities and treatment response to guide the ordering of follow-up blood cultures.
WHEN IS FOLLOW-UP CULTURE USEFUL?
Although follow-up blood cultures may not be needed routinely in patients with gram- negative bacteremia, it would be difficult to extrapolate this to gram-positive organisms, especially Staphylococcus aureus.
In Canzoneri et al,3 43 (78%) of the 55 positive follow-up cultures were due to gram-positive organisms. Factors associated with positive follow-up cultures were concurrent fever, presence of a central intravenous line, end-stage renal disease on hemodialysis, and diabetes mellitus. In addition, infectious disease consultation to decide the need for follow-up cultures for S aureus bacteremia has been associated with fewer deaths, fewer relapses, and lower readmission rates.6,7
In certain clinical scenarios, follow-up blood cultures can provide useful information, such as when the source of bacteremia is endocarditis or cardiac device infection, a vascular graft, or an intravascular line. In the Infectious Diseases Society of America guidelines for diagnosis and management of catheter-related bloodstream infections, persistent or relapsing bacteremia for some organisms is a criterion for removal of a long-term central venous catheter.8
Follow-up cultures are especially useful when the focus of infection is protected from antibiotic penetration, such as in the central nervous system, joints, and abdominal or other abscess. These foci may require drainage for cure. In these cases or in the setting of unfavorable clinical treatment response, follow-up blood cultures showing persistent bacteremia can prompt a search for unaddressed or incompletely addressed foci of infection and allow for source control.
The timing of follow-up cultures is generally 1 to 2 days after the initial culture. Although Mushtaq et al propose a different approach, traditional teaching has been that the last blood culture should not be positive, and this leads to ordering follow-up blood cultures until clearance of bacteremia is documented.
- Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 2013; 19(6):501–509. doi:10.1111/1469-0691.12195
- Mushtaq A, Bredell B, Soubani A. Repeating blood cultures after an initial bacteremia: when and how often? Cleve Clin J Med 2019; 86(2):89–92. doi:10.3949/ccjm.86a.18001
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Jones RB, Paruchuri A, Shah SS. Prospective trials are required to alter practice for follow-up blood cultures for gram-negative bacilli bacteremia. Clin Infect Dis 2018; 67(2):315–316. doi:10.1093/cid/ciy070
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case-control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Honda H, Krauss MJ, Jones JC, Olsen MA, Warren DK. The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med 2010; 123(7):631–637. doi:10.1016/j.amjmed.2010.01.015
- Fowler VG Jr, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 1998; 27(3):478–486. pmid:9770144
- Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49(1):1–45. doi:10.1086/599376
- Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 2013; 19(6):501–509. doi:10.1111/1469-0691.12195
- Mushtaq A, Bredell B, Soubani A. Repeating blood cultures after an initial bacteremia: when and how often? Cleve Clin J Med 2019; 86(2):89–92. doi:10.3949/ccjm.86a.18001
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Jones RB, Paruchuri A, Shah SS. Prospective trials are required to alter practice for follow-up blood cultures for gram-negative bacilli bacteremia. Clin Infect Dis 2018; 67(2):315–316. doi:10.1093/cid/ciy070
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case-control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Honda H, Krauss MJ, Jones JC, Olsen MA, Warren DK. The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med 2010; 123(7):631–637. doi:10.1016/j.amjmed.2010.01.015
- Fowler VG Jr, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 1998; 27(3):478–486. pmid:9770144
- Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49(1):1–45. doi:10.1086/599376
Peer-reviewers for 2018
We thank those who reviewed manuscripts submitted to the Cleveland Clinic Journal of Medicine in 2018. Reviewing papers for the Journal—both for specialty content and for relevance to our readership—is an arduous task that involves considerable time and effort. Our publication decisions depend in no small part on the timely efforts of reviewers, and we are indebted to them for contributing their expertise this past year.
—Brian F. Mandell, MD, PhD, Editor in Chief
We thank those who reviewed manuscripts submitted to the Cleveland Clinic Journal of Medicine in 2018. Reviewing papers for the Journal—both for specialty content and for relevance to our readership—is an arduous task that involves considerable time and effort. Our publication decisions depend in no small part on the timely efforts of reviewers, and we are indebted to them for contributing their expertise this past year.
—Brian F. Mandell, MD, PhD, Editor in Chief
We thank those who reviewed manuscripts submitted to the Cleveland Clinic Journal of Medicine in 2018. Reviewing papers for the Journal—both for specialty content and for relevance to our readership—is an arduous task that involves considerable time and effort. Our publication decisions depend in no small part on the timely efforts of reviewers, and we are indebted to them for contributing their expertise this past year.
—Brian F. Mandell, MD, PhD, Editor in Chief
Patient, Caregiver, and Clinician Perspectives on Expectations for Home Healthcare after Discharge: A Qualitative Case Study
Patients who are discharged from the hospital with home healthcare (HHC) are older, sicker, and more likely to be readmitted to the hospital than patients discharged home without HHC.1-3 Communication between clinicians in different settings is a key factor in successful transitions. In prior work, we focused on communication between primary care providers, hospitalists, and HHC nurses to inform efforts to improve care transitions.4,5 In one study, HHC nurses described that patients frequently have expectations beyond the scope of what skilled HHC provides,5 which prompted us to also question experiences of patients and caregivers after discharge with skilled HHC (eg, nursing and physical therapy).
In a prior qualitative study by Foust and colleagues, HHC patients and caregivers described disparate experiences around preparation for hospital discharge—patients expressed knowing about the timing and plans for discharge, and the caregivers frequently felt left out of this discussion.6 In other studies, caregivers of recently discharged patients have described feeling excluded from interactions with clinicians both before and after discharge.7,8 In another recent qualitative study, caregivers described uncertainty about their role compared with the HHC role in caring for the patient.9
As of 2016, a majority of states had passed the Caregiver Advise, Record, and Enable (CARE) Act, which requires hospitals to (1) record a family caregiver in the medical record, (2) inform this caregiver about discharge, and (3) deliver instructions with education about medical tasks that they will need to complete after discharge.10
METHODS
Study Design
In this qualitative descriptive case study, we interviewed HHC patients, an involved caregiver, and the HHC clinician completing the first HHC visit within 7-14 days following hospital discharge. We chose this timeframe to allow patients to receive one or more HHC visits following hospital discharge.
Population
- >65 years old,12 eligibility was initially limited to patients
- >65 years old. Due to recruitment challenges, the age range was broadened to
- >50 years old in October 2017.
Because our goal was to better understand the experience of general medicine patients with multiple comorbidities, we recruited patients from one general medicine unit at an academic hospital in Colorado. Patients on this unit were screened for eligibility Monday-Friday (excluding weekends and holidays) based on research assistant availability.
Criteria included are as follows: HHC referral, three or more comorbidities, resides in the community prior to admission (ie, not in a facility), cognitively intact, English speaking, and able to identify a caregiver participating in their care. Eligible patients were approached for written consent prior to discharge to allow us to contact them 7-14 days after discharge for an interview by phone or in their home, per their preference. At the time of consent, patients provided contact information for their informal caregiver. Caregiver eligibility criteria included the following: age ≥18 years and provides caregiving at least one hour a week before hospital discharge. HHC clinicians approached for interviews had completed the first HHC visit for the patient following discharge. Both caregivers and HHC clinicians provided verbal consent for interviews. All participants received a $25 gift card for participation in the study.
Framework and Data Collection
Our interview guides were organized by the Agency for Healthcare Research and Quality Care Coordination Framework, an approach we have taken in prior work.4,5,13 We added questions about patient preparation and self-management support to build on findings from a prior study with HHC nurses and on prior work by Coleman and colleagues.5,14 Sample questions from the interview guides for patients, caregivers, and HHC clinicians within key analysis domains are included in Appendix 1. The patient and caregiver interviews were completed by an individual with prior experience in social work and healthcare (SS). The HHC clinician interviews were completed by either this individual (SS) or a physician-researcher with experience in qualitative methods (CJ). Patients and caregivers could choose to be interviewed individually or together. All interviews were digitally recorded and transcribed verbatim.
Analysis
Patient interviews lasted an average of 43 minutes, caregiver interviews an average of 41 minutes, and HHC clinician interviews an average of 25 minutes
We observed the two main themes of clear and unclear expectations for HHC after discharge. Clear expectations occur when the patient and/or caregiver have expectations for HHC that align with the services they receive. Unclear expectations occur when the patient and/or caregiver expectations are either uncertain or misaligned with the services they receive. Although not all interviews yielded codes about clear or unclear expectations, patients described clear expectations in five cases and unclear expectations in another five cases.
In nine cases with more than one perspective available, expectations were compared within cases and found to be clear (three cases), unclear (three cases), or discordant (three cases) across perspectives. For the discordant cases, the description of clear and unclear expectations differed between patients and either their caregiver or their HHC clinician. Patients and caregivers with clear expectations for HHC frequently described prior experiences with skilled HHC or work experience within the healthcare field. In most cases with unclear expectations, the patient and caregiver did not have prior experience with HHC. In addition, the desire for assistance with personal care for patients such as showering and housekeeping was described by caregivers with unclear expectations. The results are organized into clear, unclear, and discordant expectations from the perspectives of patients, caregivers, and HHC clinicians within cases.
Clear Expectations within Cases
Clear expectations for HHC were identified across perspectives in three cases, with sample quotes provided in Table 2. In the case of patient 1, the patient and HHC nurse had known each other for over two years because the patient had a wound requiring long-term HHC services. A caregiver did not complete an interview in this case. With patient 2, the patient, caregiver, and HHC physical therapist (PT) all describe that the patient had clear expectations for HHC. In this case, the patient and caregiver describe feeling prepared because of previously receiving HHC, prior work experience in the healthcare field, and a caregiver with experience working in HHC. In the case of patient 3, the patient had previously received HHC from the same HHC nurse.
Unclear Expectations across Cases
For the three cases in which unclear expectations were described across perspectives, two of the patients described being new to HHC, with representative quotes in Table 2. Patient 4 and her caregiver are new to HHC and describe unclear expectations for both the HHC referral and the HHC role, which was also noted by the HHC clinician. Of note, the caregiver for patient 4 further described that she was unable to be present for the first HHC visit. In the case of patient 5, although the patient had previously received HHC, the patient describes not knowing why the HHC PT needs to see her after discharge, which is also noted by the HHC PT. Finally, both patient 6 and her HHC PT describe that the patient was not sure about their expectations for HHC and that HHC was a new experience for them.
Discordant Expectation Clarity across Cases
In three of the cases, the description of clear and unclear expectations was discrepant across roles. In case 7, the caregiver and patient are new to HHC and express different perspectives about expectations for HHC. The HHC clinician, in this case, did not complete an interview. The caregiver describes not being present for the first HHC visit and no awareness that the patient was being discharged with HHC:
Caregiver: Well, we didn’t even know she had home health until she got home.
The same caregiver also expresses unclear expectations for HHC:
Caregiver: It’s pretty cloudy. They (the HHC clinicians) don’t help her with her laundry, they don’t help with the housekeeping, they don’t help… with her showers so somebody is there when she showers. They don’t do anything. The only two things like I said is the…home healthcare comes in on Wednesdays to see what she needs and then the therapy comes in one day a week.
However, the patient expresses more clear expectations that are being met by HHC.
Patient: They (HHC) have met my expectations. They come in twice a week. They do vitals, take vitals and discuss with me, you know, what my feelings are, how I’m doing and I know they have met my expectations.
In case 8, although the patient describes knowing about the HHC PT involvement in her care, she expresses some unclear expectations about an HHC nurse after discharge.
Patient: As far as home health, I didn’t have a real …plan there at the hospital… They knew about (the HHC PT) coming once a week but as far as, you know, a nurse coming by to check on me, no.
However, the HHC PT describes feeling that the patient had clear expectations for HHC after discharge:
Interviewer: Can you reflect on whether she was prepared to receive home healthcare?
HHC PT: Yeah, she was ready.
Interviewer: …do you feel like she was prepared to know what to expect from you?
HHC PT: Yeah, but I think that comes from being a previous patient also.
Finally, in case 9, the patient describes clear expectations for HHC even though they were new to HHC:
Patient: …I knew what the PT was going to do and …I still need her because I’ve lost so much weight so she’s been really good, instrumental, at giving me exercises… Occupational therapist…she’s going to teach me how to shave, she’s going to teach me how to get ready for the day.
The HHC PT describes that although the patient knew the PT role, they reflect that the patient may have been somewhat unclear about expectations for the first HHC visit:
HHC PT: He knew all that it entailed with the exception of he didn’t really know what the first day was going to be like and the first day I don’t usually do treatment because it does take a long time to get all the paperwork signed, to do the evaluation and the fact that it takes two hours to do that note.
DISCUSSION
In this qualitative case study with HHC patients, caregivers, and clinicians, the participants described varying levels of expectation clarity for HHC after discharge. We triangulated across and within cases and found three cases with clear expectations and three cases with unclear expectations for HHC across perspectives. In three additional cases, we found discordant expectations across perspectives: patients and HHC clinician expectations differed in two of the cases and a patient and caregiver differed in one case. Of interest, in all three cases of clear expectations across perspectives, the patients and/or caregivers had prior HHC or healthcare work experience. In contrast,
Prior studies in this area have included a qualitative study HHC patients, caregivers, and clinicians by Foust and colleagues in which multiple caregivers described finding out about the discharge from the patient or other caregivers, rather than being actively engaged by clinicians.6 In another recent qualitative study by Arbaje and colleagues, a majority of caregivers described “mismatched expectations” about HHC services, in which caregivers
When caregivers have unclear expectations for HHC, they could be expressing the need for more support after hospital discharge, which suggests an active role for hospital teams to assess and address additional support needs with the patients and caregivers. For example, if the patient or caregiver request additional personal care services, a home health aide could help to reduce caregiver burden and improve the support network for the patient. In a prior study in which patients were asked what would help them to make informed decisions about postacute care options, the patients described wanting to receive practical information that could describe how it would apply to their specific situation and perceived needs.18 To provide this for patients and caregivers, it would follow that hospitals could provide information about skilled HHC nursing and therapies and information about services that could meet additional needs, such as home health aides.
Limitations of this study include that it was a small qualitative case study of patients, caregivers, and HHC clinicians from one medical unit at one academic medical center. Most patients in this study had Medicare insurance, were 65 years and older, white, and female.
In conclusion, to improve care transitions for HHC patients and their caregivers, emphasizing engagement of caregivers is key to ensure that they are educated about HHC, provided with additional support as needed, and included in initial HHC visits once the patients are at home. Even though patients and caregivers with prior HHC experience often had clear expectations for HHC, a strategy to uniformly engage caregivers across a range of experience can ensure caregivers have all the information and support needed to optimize care transitions to HHC.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Christine Jones is supported by grant number K08HS024569 from the Agency for Healthcare Research and Quality. Jason Falvey was supported by grant F31AG056069 from the National Institute on Aging, National Institutes of Health and is currently supported by T32AG019134. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality or the National Institutes of Health.
1. Jones CD, Wald HL, Boxer RS, et al. Characteristics associated with home health care referrals at hospital discharge: results from the 2012 National Inpatient Sample. Health Serv Res. 2017;52(2):879-894. doi: 10.1111/1475-6773. PubMed
2. Avalere Health. Home Health Chartbook 2015: Prepared for the Alliance for Home Health Quality and Innovation. 2016.
3. Hospital Compare. https://www.medicare.gov/hospitalcompare/search.html. Accessed May 1, 2017.
4. Jones CD, Vu MB, O’Donnell CM, et al. A failure to communicate: a qualitative exploration of care coordination between hospitalists and primary care providers around patient hospitalizations. J Gen Intern Med. 2015;30(4):417-424. doi: 10.1007/s11606-014-3056-x. PubMed
5. Jones CD, Jones J, Richard A, et al. “Connecting the dots”: a qualitative study of home health nurse perspectives on coordinating care for recently discharged patients. J Gen Intern Med. 2017;32(10):1114-1121. doi: 10.1007/s11606-017-4104-0. PubMed
6. Foust JB, Vuckovic N, Henriquez E. Hospital to home health care transition: patient, caregiver, and clinician perspectives. West J Nurs Res. 2012;34(2):194-212. doi: 10.1177/0193945911400448. PubMed
7. Blair J, Volpe M, Aggarwal B. Challenges, needs, and experiences of recently hospitalized cardiac patients and their informal caregivers. J Cardiovasc Nurs. 2014;29(1):29-37. doi: 10.1097/JCN.0b013e3182784123. PubMed
8. Coleman EA, Roman SP. Family caregivers’ experiences during transitions out of hospital. J Healthc Qual. 2015;37(1):12-21. doi: 10.1097/01.JHQ.0000460117.83437.b3. PubMed
9. Arbaje AI, Hughes A, Werner N, et al. Information management goals and process failures during home visits for middle-aged and older adults receiving skilled home healthcare services after hospital discharge: a multisite, qualitative study. BMJ Qual Saf. 2018. doi: 10.1136/bmjqs-2018-008163. PubMed
10. Coleman EA. Family caregivers as partners in care transitions: the caregiver advise record and enable act. J Hosp Med. 2016;11(12):883-885. doi: 10.1002/jhm.2637. PubMed
11. Jones AL, Harris-Kojetin L, Valverde R. Characteristics and use of home health care by men and women aged 65 and over. Natl Health Stat Report. 2012(52):1-7. PubMed
12. Tian W. An all-payer view of hospital discharge to postacute care, 2013. HCUP Statistical Brief #205. Rockville, Maryland; 2016. PubMed
13. McDonald KM, Sundaram V, Bravata DM, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol. 7: Care Coordination). Rockville, Maryland; 2007. PubMed
14. Coleman EA, Smith JD, Frank JC, Eilertsen TB, Thiare JN, Kramer AM. Development and testing of a measure designed to assess the quality of care transitions. Int J Integr Care. 2002;2:e02. doi: 10.5334/ijic.60. PubMed
15. Jones J, Nowels CT, Sudore R, Ahluwalia S, Bekelman DB. The future as a series of transitions: qualitative study of heart failure patients and their informal caregivers. J Gen Intern Med. 2015;30(2):176-182. doi: 10.1007/s11606-014-3085-5. PubMed
16. Lum HD, Jones J, Lahoff D, et al. Unique challenges of hospice for patients with heart failure: a qualitative study of hospice clinicians. Am Heart J. 2015;170(3):524-530 e523. doi: 10.1016/j.ahj.2015.06.019. PubMed
17. Kerr C, Nixon A, Wild D. Assessing and demonstrating data saturation in qualitative inquiry supporting patient-reported outcomes research. Expert Rev Pharmacoecon Outcomes Res. 2010;10(3):269-281. doi: 10.1586/erp.10.30. PubMed
18. Sefcik JS, Nock RH, Flores EJ, et al. Patient preferences for information on post-acute care services. Res Gerontol Nurs. 2016;9(4):175-182. doi: 10.3928/19404921-20160120-01. PubMed
19. Coleman EA, Roman SP, Hall KA, Min SJ. Enhancing the care transitions intervention protocol to better address the needs of family caregivers. J Healthc Qual. 2015;37(1):2-11. doi: 10.1097/01.JHQ.0000460118.60567.fe. PubMed
Patients who are discharged from the hospital with home healthcare (HHC) are older, sicker, and more likely to be readmitted to the hospital than patients discharged home without HHC.1-3 Communication between clinicians in different settings is a key factor in successful transitions. In prior work, we focused on communication between primary care providers, hospitalists, and HHC nurses to inform efforts to improve care transitions.4,5 In one study, HHC nurses described that patients frequently have expectations beyond the scope of what skilled HHC provides,5 which prompted us to also question experiences of patients and caregivers after discharge with skilled HHC (eg, nursing and physical therapy).
In a prior qualitative study by Foust and colleagues, HHC patients and caregivers described disparate experiences around preparation for hospital discharge—patients expressed knowing about the timing and plans for discharge, and the caregivers frequently felt left out of this discussion.6 In other studies, caregivers of recently discharged patients have described feeling excluded from interactions with clinicians both before and after discharge.7,8 In another recent qualitative study, caregivers described uncertainty about their role compared with the HHC role in caring for the patient.9
As of 2016, a majority of states had passed the Caregiver Advise, Record, and Enable (CARE) Act, which requires hospitals to (1) record a family caregiver in the medical record, (2) inform this caregiver about discharge, and (3) deliver instructions with education about medical tasks that they will need to complete after discharge.10
METHODS
Study Design
In this qualitative descriptive case study, we interviewed HHC patients, an involved caregiver, and the HHC clinician completing the first HHC visit within 7-14 days following hospital discharge. We chose this timeframe to allow patients to receive one or more HHC visits following hospital discharge.
Population
- >65 years old,12 eligibility was initially limited to patients
- >65 years old. Due to recruitment challenges, the age range was broadened to
- >50 years old in October 2017.
Because our goal was to better understand the experience of general medicine patients with multiple comorbidities, we recruited patients from one general medicine unit at an academic hospital in Colorado. Patients on this unit were screened for eligibility Monday-Friday (excluding weekends and holidays) based on research assistant availability.
Criteria included are as follows: HHC referral, three or more comorbidities, resides in the community prior to admission (ie, not in a facility), cognitively intact, English speaking, and able to identify a caregiver participating in their care. Eligible patients were approached for written consent prior to discharge to allow us to contact them 7-14 days after discharge for an interview by phone or in their home, per their preference. At the time of consent, patients provided contact information for their informal caregiver. Caregiver eligibility criteria included the following: age ≥18 years and provides caregiving at least one hour a week before hospital discharge. HHC clinicians approached for interviews had completed the first HHC visit for the patient following discharge. Both caregivers and HHC clinicians provided verbal consent for interviews. All participants received a $25 gift card for participation in the study.
Framework and Data Collection
Our interview guides were organized by the Agency for Healthcare Research and Quality Care Coordination Framework, an approach we have taken in prior work.4,5,13 We added questions about patient preparation and self-management support to build on findings from a prior study with HHC nurses and on prior work by Coleman and colleagues.5,14 Sample questions from the interview guides for patients, caregivers, and HHC clinicians within key analysis domains are included in Appendix 1. The patient and caregiver interviews were completed by an individual with prior experience in social work and healthcare (SS). The HHC clinician interviews were completed by either this individual (SS) or a physician-researcher with experience in qualitative methods (CJ). Patients and caregivers could choose to be interviewed individually or together. All interviews were digitally recorded and transcribed verbatim.
Analysis
Patient interviews lasted an average of 43 minutes, caregiver interviews an average of 41 minutes, and HHC clinician interviews an average of 25 minutes
We observed the two main themes of clear and unclear expectations for HHC after discharge. Clear expectations occur when the patient and/or caregiver have expectations for HHC that align with the services they receive. Unclear expectations occur when the patient and/or caregiver expectations are either uncertain or misaligned with the services they receive. Although not all interviews yielded codes about clear or unclear expectations, patients described clear expectations in five cases and unclear expectations in another five cases.
In nine cases with more than one perspective available, expectations were compared within cases and found to be clear (three cases), unclear (three cases), or discordant (three cases) across perspectives. For the discordant cases, the description of clear and unclear expectations differed between patients and either their caregiver or their HHC clinician. Patients and caregivers with clear expectations for HHC frequently described prior experiences with skilled HHC or work experience within the healthcare field. In most cases with unclear expectations, the patient and caregiver did not have prior experience with HHC. In addition, the desire for assistance with personal care for patients such as showering and housekeeping was described by caregivers with unclear expectations. The results are organized into clear, unclear, and discordant expectations from the perspectives of patients, caregivers, and HHC clinicians within cases.
Clear Expectations within Cases
Clear expectations for HHC were identified across perspectives in three cases, with sample quotes provided in Table 2. In the case of patient 1, the patient and HHC nurse had known each other for over two years because the patient had a wound requiring long-term HHC services. A caregiver did not complete an interview in this case. With patient 2, the patient, caregiver, and HHC physical therapist (PT) all describe that the patient had clear expectations for HHC. In this case, the patient and caregiver describe feeling prepared because of previously receiving HHC, prior work experience in the healthcare field, and a caregiver with experience working in HHC. In the case of patient 3, the patient had previously received HHC from the same HHC nurse.
Unclear Expectations across Cases
For the three cases in which unclear expectations were described across perspectives, two of the patients described being new to HHC, with representative quotes in Table 2. Patient 4 and her caregiver are new to HHC and describe unclear expectations for both the HHC referral and the HHC role, which was also noted by the HHC clinician. Of note, the caregiver for patient 4 further described that she was unable to be present for the first HHC visit. In the case of patient 5, although the patient had previously received HHC, the patient describes not knowing why the HHC PT needs to see her after discharge, which is also noted by the HHC PT. Finally, both patient 6 and her HHC PT describe that the patient was not sure about their expectations for HHC and that HHC was a new experience for them.
Discordant Expectation Clarity across Cases
In three of the cases, the description of clear and unclear expectations was discrepant across roles. In case 7, the caregiver and patient are new to HHC and express different perspectives about expectations for HHC. The HHC clinician, in this case, did not complete an interview. The caregiver describes not being present for the first HHC visit and no awareness that the patient was being discharged with HHC:
Caregiver: Well, we didn’t even know she had home health until she got home.
The same caregiver also expresses unclear expectations for HHC:
Caregiver: It’s pretty cloudy. They (the HHC clinicians) don’t help her with her laundry, they don’t help with the housekeeping, they don’t help… with her showers so somebody is there when she showers. They don’t do anything. The only two things like I said is the…home healthcare comes in on Wednesdays to see what she needs and then the therapy comes in one day a week.
However, the patient expresses more clear expectations that are being met by HHC.
Patient: They (HHC) have met my expectations. They come in twice a week. They do vitals, take vitals and discuss with me, you know, what my feelings are, how I’m doing and I know they have met my expectations.
In case 8, although the patient describes knowing about the HHC PT involvement in her care, she expresses some unclear expectations about an HHC nurse after discharge.
Patient: As far as home health, I didn’t have a real …plan there at the hospital… They knew about (the HHC PT) coming once a week but as far as, you know, a nurse coming by to check on me, no.
However, the HHC PT describes feeling that the patient had clear expectations for HHC after discharge:
Interviewer: Can you reflect on whether she was prepared to receive home healthcare?
HHC PT: Yeah, she was ready.
Interviewer: …do you feel like she was prepared to know what to expect from you?
HHC PT: Yeah, but I think that comes from being a previous patient also.
Finally, in case 9, the patient describes clear expectations for HHC even though they were new to HHC:
Patient: …I knew what the PT was going to do and …I still need her because I’ve lost so much weight so she’s been really good, instrumental, at giving me exercises… Occupational therapist…she’s going to teach me how to shave, she’s going to teach me how to get ready for the day.
The HHC PT describes that although the patient knew the PT role, they reflect that the patient may have been somewhat unclear about expectations for the first HHC visit:
HHC PT: He knew all that it entailed with the exception of he didn’t really know what the first day was going to be like and the first day I don’t usually do treatment because it does take a long time to get all the paperwork signed, to do the evaluation and the fact that it takes two hours to do that note.
DISCUSSION
In this qualitative case study with HHC patients, caregivers, and clinicians, the participants described varying levels of expectation clarity for HHC after discharge. We triangulated across and within cases and found three cases with clear expectations and three cases with unclear expectations for HHC across perspectives. In three additional cases, we found discordant expectations across perspectives: patients and HHC clinician expectations differed in two of the cases and a patient and caregiver differed in one case. Of interest, in all three cases of clear expectations across perspectives, the patients and/or caregivers had prior HHC or healthcare work experience. In contrast,
Prior studies in this area have included a qualitative study HHC patients, caregivers, and clinicians by Foust and colleagues in which multiple caregivers described finding out about the discharge from the patient or other caregivers, rather than being actively engaged by clinicians.6 In another recent qualitative study by Arbaje and colleagues, a majority of caregivers described “mismatched expectations” about HHC services, in which caregivers
When caregivers have unclear expectations for HHC, they could be expressing the need for more support after hospital discharge, which suggests an active role for hospital teams to assess and address additional support needs with the patients and caregivers. For example, if the patient or caregiver request additional personal care services, a home health aide could help to reduce caregiver burden and improve the support network for the patient. In a prior study in which patients were asked what would help them to make informed decisions about postacute care options, the patients described wanting to receive practical information that could describe how it would apply to their specific situation and perceived needs.18 To provide this for patients and caregivers, it would follow that hospitals could provide information about skilled HHC nursing and therapies and information about services that could meet additional needs, such as home health aides.
Limitations of this study include that it was a small qualitative case study of patients, caregivers, and HHC clinicians from one medical unit at one academic medical center. Most patients in this study had Medicare insurance, were 65 years and older, white, and female.
In conclusion, to improve care transitions for HHC patients and their caregivers, emphasizing engagement of caregivers is key to ensure that they are educated about HHC, provided with additional support as needed, and included in initial HHC visits once the patients are at home. Even though patients and caregivers with prior HHC experience often had clear expectations for HHC, a strategy to uniformly engage caregivers across a range of experience can ensure caregivers have all the information and support needed to optimize care transitions to HHC.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Christine Jones is supported by grant number K08HS024569 from the Agency for Healthcare Research and Quality. Jason Falvey was supported by grant F31AG056069 from the National Institute on Aging, National Institutes of Health and is currently supported by T32AG019134. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality or the National Institutes of Health.
Patients who are discharged from the hospital with home healthcare (HHC) are older, sicker, and more likely to be readmitted to the hospital than patients discharged home without HHC.1-3 Communication between clinicians in different settings is a key factor in successful transitions. In prior work, we focused on communication between primary care providers, hospitalists, and HHC nurses to inform efforts to improve care transitions.4,5 In one study, HHC nurses described that patients frequently have expectations beyond the scope of what skilled HHC provides,5 which prompted us to also question experiences of patients and caregivers after discharge with skilled HHC (eg, nursing and physical therapy).
In a prior qualitative study by Foust and colleagues, HHC patients and caregivers described disparate experiences around preparation for hospital discharge—patients expressed knowing about the timing and plans for discharge, and the caregivers frequently felt left out of this discussion.6 In other studies, caregivers of recently discharged patients have described feeling excluded from interactions with clinicians both before and after discharge.7,8 In another recent qualitative study, caregivers described uncertainty about their role compared with the HHC role in caring for the patient.9
As of 2016, a majority of states had passed the Caregiver Advise, Record, and Enable (CARE) Act, which requires hospitals to (1) record a family caregiver in the medical record, (2) inform this caregiver about discharge, and (3) deliver instructions with education about medical tasks that they will need to complete after discharge.10
METHODS
Study Design
In this qualitative descriptive case study, we interviewed HHC patients, an involved caregiver, and the HHC clinician completing the first HHC visit within 7-14 days following hospital discharge. We chose this timeframe to allow patients to receive one or more HHC visits following hospital discharge.
Population
- >65 years old,12 eligibility was initially limited to patients
- >65 years old. Due to recruitment challenges, the age range was broadened to
- >50 years old in October 2017.
Because our goal was to better understand the experience of general medicine patients with multiple comorbidities, we recruited patients from one general medicine unit at an academic hospital in Colorado. Patients on this unit were screened for eligibility Monday-Friday (excluding weekends and holidays) based on research assistant availability.
Criteria included are as follows: HHC referral, three or more comorbidities, resides in the community prior to admission (ie, not in a facility), cognitively intact, English speaking, and able to identify a caregiver participating in their care. Eligible patients were approached for written consent prior to discharge to allow us to contact them 7-14 days after discharge for an interview by phone or in their home, per their preference. At the time of consent, patients provided contact information for their informal caregiver. Caregiver eligibility criteria included the following: age ≥18 years and provides caregiving at least one hour a week before hospital discharge. HHC clinicians approached for interviews had completed the first HHC visit for the patient following discharge. Both caregivers and HHC clinicians provided verbal consent for interviews. All participants received a $25 gift card for participation in the study.
Framework and Data Collection
Our interview guides were organized by the Agency for Healthcare Research and Quality Care Coordination Framework, an approach we have taken in prior work.4,5,13 We added questions about patient preparation and self-management support to build on findings from a prior study with HHC nurses and on prior work by Coleman and colleagues.5,14 Sample questions from the interview guides for patients, caregivers, and HHC clinicians within key analysis domains are included in Appendix 1. The patient and caregiver interviews were completed by an individual with prior experience in social work and healthcare (SS). The HHC clinician interviews were completed by either this individual (SS) or a physician-researcher with experience in qualitative methods (CJ). Patients and caregivers could choose to be interviewed individually or together. All interviews were digitally recorded and transcribed verbatim.
Analysis
Patient interviews lasted an average of 43 minutes, caregiver interviews an average of 41 minutes, and HHC clinician interviews an average of 25 minutes
We observed the two main themes of clear and unclear expectations for HHC after discharge. Clear expectations occur when the patient and/or caregiver have expectations for HHC that align with the services they receive. Unclear expectations occur when the patient and/or caregiver expectations are either uncertain or misaligned with the services they receive. Although not all interviews yielded codes about clear or unclear expectations, patients described clear expectations in five cases and unclear expectations in another five cases.
In nine cases with more than one perspective available, expectations were compared within cases and found to be clear (three cases), unclear (three cases), or discordant (three cases) across perspectives. For the discordant cases, the description of clear and unclear expectations differed between patients and either their caregiver or their HHC clinician. Patients and caregivers with clear expectations for HHC frequently described prior experiences with skilled HHC or work experience within the healthcare field. In most cases with unclear expectations, the patient and caregiver did not have prior experience with HHC. In addition, the desire for assistance with personal care for patients such as showering and housekeeping was described by caregivers with unclear expectations. The results are organized into clear, unclear, and discordant expectations from the perspectives of patients, caregivers, and HHC clinicians within cases.
Clear Expectations within Cases
Clear expectations for HHC were identified across perspectives in three cases, with sample quotes provided in Table 2. In the case of patient 1, the patient and HHC nurse had known each other for over two years because the patient had a wound requiring long-term HHC services. A caregiver did not complete an interview in this case. With patient 2, the patient, caregiver, and HHC physical therapist (PT) all describe that the patient had clear expectations for HHC. In this case, the patient and caregiver describe feeling prepared because of previously receiving HHC, prior work experience in the healthcare field, and a caregiver with experience working in HHC. In the case of patient 3, the patient had previously received HHC from the same HHC nurse.
Unclear Expectations across Cases
For the three cases in which unclear expectations were described across perspectives, two of the patients described being new to HHC, with representative quotes in Table 2. Patient 4 and her caregiver are new to HHC and describe unclear expectations for both the HHC referral and the HHC role, which was also noted by the HHC clinician. Of note, the caregiver for patient 4 further described that she was unable to be present for the first HHC visit. In the case of patient 5, although the patient had previously received HHC, the patient describes not knowing why the HHC PT needs to see her after discharge, which is also noted by the HHC PT. Finally, both patient 6 and her HHC PT describe that the patient was not sure about their expectations for HHC and that HHC was a new experience for them.
Discordant Expectation Clarity across Cases
In three of the cases, the description of clear and unclear expectations was discrepant across roles. In case 7, the caregiver and patient are new to HHC and express different perspectives about expectations for HHC. The HHC clinician, in this case, did not complete an interview. The caregiver describes not being present for the first HHC visit and no awareness that the patient was being discharged with HHC:
Caregiver: Well, we didn’t even know she had home health until she got home.
The same caregiver also expresses unclear expectations for HHC:
Caregiver: It’s pretty cloudy. They (the HHC clinicians) don’t help her with her laundry, they don’t help with the housekeeping, they don’t help… with her showers so somebody is there when she showers. They don’t do anything. The only two things like I said is the…home healthcare comes in on Wednesdays to see what she needs and then the therapy comes in one day a week.
However, the patient expresses more clear expectations that are being met by HHC.
Patient: They (HHC) have met my expectations. They come in twice a week. They do vitals, take vitals and discuss with me, you know, what my feelings are, how I’m doing and I know they have met my expectations.
In case 8, although the patient describes knowing about the HHC PT involvement in her care, she expresses some unclear expectations about an HHC nurse after discharge.
Patient: As far as home health, I didn’t have a real …plan there at the hospital… They knew about (the HHC PT) coming once a week but as far as, you know, a nurse coming by to check on me, no.
However, the HHC PT describes feeling that the patient had clear expectations for HHC after discharge:
Interviewer: Can you reflect on whether she was prepared to receive home healthcare?
HHC PT: Yeah, she was ready.
Interviewer: …do you feel like she was prepared to know what to expect from you?
HHC PT: Yeah, but I think that comes from being a previous patient also.
Finally, in case 9, the patient describes clear expectations for HHC even though they were new to HHC:
Patient: …I knew what the PT was going to do and …I still need her because I’ve lost so much weight so she’s been really good, instrumental, at giving me exercises… Occupational therapist…she’s going to teach me how to shave, she’s going to teach me how to get ready for the day.
The HHC PT describes that although the patient knew the PT role, they reflect that the patient may have been somewhat unclear about expectations for the first HHC visit:
HHC PT: He knew all that it entailed with the exception of he didn’t really know what the first day was going to be like and the first day I don’t usually do treatment because it does take a long time to get all the paperwork signed, to do the evaluation and the fact that it takes two hours to do that note.
DISCUSSION
In this qualitative case study with HHC patients, caregivers, and clinicians, the participants described varying levels of expectation clarity for HHC after discharge. We triangulated across and within cases and found three cases with clear expectations and three cases with unclear expectations for HHC across perspectives. In three additional cases, we found discordant expectations across perspectives: patients and HHC clinician expectations differed in two of the cases and a patient and caregiver differed in one case. Of interest, in all three cases of clear expectations across perspectives, the patients and/or caregivers had prior HHC or healthcare work experience. In contrast,
Prior studies in this area have included a qualitative study HHC patients, caregivers, and clinicians by Foust and colleagues in which multiple caregivers described finding out about the discharge from the patient or other caregivers, rather than being actively engaged by clinicians.6 In another recent qualitative study by Arbaje and colleagues, a majority of caregivers described “mismatched expectations” about HHC services, in which caregivers
When caregivers have unclear expectations for HHC, they could be expressing the need for more support after hospital discharge, which suggests an active role for hospital teams to assess and address additional support needs with the patients and caregivers. For example, if the patient or caregiver request additional personal care services, a home health aide could help to reduce caregiver burden and improve the support network for the patient. In a prior study in which patients were asked what would help them to make informed decisions about postacute care options, the patients described wanting to receive practical information that could describe how it would apply to their specific situation and perceived needs.18 To provide this for patients and caregivers, it would follow that hospitals could provide information about skilled HHC nursing and therapies and information about services that could meet additional needs, such as home health aides.
Limitations of this study include that it was a small qualitative case study of patients, caregivers, and HHC clinicians from one medical unit at one academic medical center. Most patients in this study had Medicare insurance, were 65 years and older, white, and female.
In conclusion, to improve care transitions for HHC patients and their caregivers, emphasizing engagement of caregivers is key to ensure that they are educated about HHC, provided with additional support as needed, and included in initial HHC visits once the patients are at home. Even though patients and caregivers with prior HHC experience often had clear expectations for HHC, a strategy to uniformly engage caregivers across a range of experience can ensure caregivers have all the information and support needed to optimize care transitions to HHC.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Christine Jones is supported by grant number K08HS024569 from the Agency for Healthcare Research and Quality. Jason Falvey was supported by grant F31AG056069 from the National Institute on Aging, National Institutes of Health and is currently supported by T32AG019134. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality or the National Institutes of Health.
1. Jones CD, Wald HL, Boxer RS, et al. Characteristics associated with home health care referrals at hospital discharge: results from the 2012 National Inpatient Sample. Health Serv Res. 2017;52(2):879-894. doi: 10.1111/1475-6773. PubMed
2. Avalere Health. Home Health Chartbook 2015: Prepared for the Alliance for Home Health Quality and Innovation. 2016.
3. Hospital Compare. https://www.medicare.gov/hospitalcompare/search.html. Accessed May 1, 2017.
4. Jones CD, Vu MB, O’Donnell CM, et al. A failure to communicate: a qualitative exploration of care coordination between hospitalists and primary care providers around patient hospitalizations. J Gen Intern Med. 2015;30(4):417-424. doi: 10.1007/s11606-014-3056-x. PubMed
5. Jones CD, Jones J, Richard A, et al. “Connecting the dots”: a qualitative study of home health nurse perspectives on coordinating care for recently discharged patients. J Gen Intern Med. 2017;32(10):1114-1121. doi: 10.1007/s11606-017-4104-0. PubMed
6. Foust JB, Vuckovic N, Henriquez E. Hospital to home health care transition: patient, caregiver, and clinician perspectives. West J Nurs Res. 2012;34(2):194-212. doi: 10.1177/0193945911400448. PubMed
7. Blair J, Volpe M, Aggarwal B. Challenges, needs, and experiences of recently hospitalized cardiac patients and their informal caregivers. J Cardiovasc Nurs. 2014;29(1):29-37. doi: 10.1097/JCN.0b013e3182784123. PubMed
8. Coleman EA, Roman SP. Family caregivers’ experiences during transitions out of hospital. J Healthc Qual. 2015;37(1):12-21. doi: 10.1097/01.JHQ.0000460117.83437.b3. PubMed
9. Arbaje AI, Hughes A, Werner N, et al. Information management goals and process failures during home visits for middle-aged and older adults receiving skilled home healthcare services after hospital discharge: a multisite, qualitative study. BMJ Qual Saf. 2018. doi: 10.1136/bmjqs-2018-008163. PubMed
10. Coleman EA. Family caregivers as partners in care transitions: the caregiver advise record and enable act. J Hosp Med. 2016;11(12):883-885. doi: 10.1002/jhm.2637. PubMed
11. Jones AL, Harris-Kojetin L, Valverde R. Characteristics and use of home health care by men and women aged 65 and over. Natl Health Stat Report. 2012(52):1-7. PubMed
12. Tian W. An all-payer view of hospital discharge to postacute care, 2013. HCUP Statistical Brief #205. Rockville, Maryland; 2016. PubMed
13. McDonald KM, Sundaram V, Bravata DM, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol. 7: Care Coordination). Rockville, Maryland; 2007. PubMed
14. Coleman EA, Smith JD, Frank JC, Eilertsen TB, Thiare JN, Kramer AM. Development and testing of a measure designed to assess the quality of care transitions. Int J Integr Care. 2002;2:e02. doi: 10.5334/ijic.60. PubMed
15. Jones J, Nowels CT, Sudore R, Ahluwalia S, Bekelman DB. The future as a series of transitions: qualitative study of heart failure patients and their informal caregivers. J Gen Intern Med. 2015;30(2):176-182. doi: 10.1007/s11606-014-3085-5. PubMed
16. Lum HD, Jones J, Lahoff D, et al. Unique challenges of hospice for patients with heart failure: a qualitative study of hospice clinicians. Am Heart J. 2015;170(3):524-530 e523. doi: 10.1016/j.ahj.2015.06.019. PubMed
17. Kerr C, Nixon A, Wild D. Assessing and demonstrating data saturation in qualitative inquiry supporting patient-reported outcomes research. Expert Rev Pharmacoecon Outcomes Res. 2010;10(3):269-281. doi: 10.1586/erp.10.30. PubMed
18. Sefcik JS, Nock RH, Flores EJ, et al. Patient preferences for information on post-acute care services. Res Gerontol Nurs. 2016;9(4):175-182. doi: 10.3928/19404921-20160120-01. PubMed
19. Coleman EA, Roman SP, Hall KA, Min SJ. Enhancing the care transitions intervention protocol to better address the needs of family caregivers. J Healthc Qual. 2015;37(1):2-11. doi: 10.1097/01.JHQ.0000460118.60567.fe. PubMed
1. Jones CD, Wald HL, Boxer RS, et al. Characteristics associated with home health care referrals at hospital discharge: results from the 2012 National Inpatient Sample. Health Serv Res. 2017;52(2):879-894. doi: 10.1111/1475-6773. PubMed
2. Avalere Health. Home Health Chartbook 2015: Prepared for the Alliance for Home Health Quality and Innovation. 2016.
3. Hospital Compare. https://www.medicare.gov/hospitalcompare/search.html. Accessed May 1, 2017.
4. Jones CD, Vu MB, O’Donnell CM, et al. A failure to communicate: a qualitative exploration of care coordination between hospitalists and primary care providers around patient hospitalizations. J Gen Intern Med. 2015;30(4):417-424. doi: 10.1007/s11606-014-3056-x. PubMed
5. Jones CD, Jones J, Richard A, et al. “Connecting the dots”: a qualitative study of home health nurse perspectives on coordinating care for recently discharged patients. J Gen Intern Med. 2017;32(10):1114-1121. doi: 10.1007/s11606-017-4104-0. PubMed
6. Foust JB, Vuckovic N, Henriquez E. Hospital to home health care transition: patient, caregiver, and clinician perspectives. West J Nurs Res. 2012;34(2):194-212. doi: 10.1177/0193945911400448. PubMed
7. Blair J, Volpe M, Aggarwal B. Challenges, needs, and experiences of recently hospitalized cardiac patients and their informal caregivers. J Cardiovasc Nurs. 2014;29(1):29-37. doi: 10.1097/JCN.0b013e3182784123. PubMed
8. Coleman EA, Roman SP. Family caregivers’ experiences during transitions out of hospital. J Healthc Qual. 2015;37(1):12-21. doi: 10.1097/01.JHQ.0000460117.83437.b3. PubMed
9. Arbaje AI, Hughes A, Werner N, et al. Information management goals and process failures during home visits for middle-aged and older adults receiving skilled home healthcare services after hospital discharge: a multisite, qualitative study. BMJ Qual Saf. 2018. doi: 10.1136/bmjqs-2018-008163. PubMed
10. Coleman EA. Family caregivers as partners in care transitions: the caregiver advise record and enable act. J Hosp Med. 2016;11(12):883-885. doi: 10.1002/jhm.2637. PubMed
11. Jones AL, Harris-Kojetin L, Valverde R. Characteristics and use of home health care by men and women aged 65 and over. Natl Health Stat Report. 2012(52):1-7. PubMed
12. Tian W. An all-payer view of hospital discharge to postacute care, 2013. HCUP Statistical Brief #205. Rockville, Maryland; 2016. PubMed
13. McDonald KM, Sundaram V, Bravata DM, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol. 7: Care Coordination). Rockville, Maryland; 2007. PubMed
14. Coleman EA, Smith JD, Frank JC, Eilertsen TB, Thiare JN, Kramer AM. Development and testing of a measure designed to assess the quality of care transitions. Int J Integr Care. 2002;2:e02. doi: 10.5334/ijic.60. PubMed
15. Jones J, Nowels CT, Sudore R, Ahluwalia S, Bekelman DB. The future as a series of transitions: qualitative study of heart failure patients and their informal caregivers. J Gen Intern Med. 2015;30(2):176-182. doi: 10.1007/s11606-014-3085-5. PubMed
16. Lum HD, Jones J, Lahoff D, et al. Unique challenges of hospice for patients with heart failure: a qualitative study of hospice clinicians. Am Heart J. 2015;170(3):524-530 e523. doi: 10.1016/j.ahj.2015.06.019. PubMed
17. Kerr C, Nixon A, Wild D. Assessing and demonstrating data saturation in qualitative inquiry supporting patient-reported outcomes research. Expert Rev Pharmacoecon Outcomes Res. 2010;10(3):269-281. doi: 10.1586/erp.10.30. PubMed
18. Sefcik JS, Nock RH, Flores EJ, et al. Patient preferences for information on post-acute care services. Res Gerontol Nurs. 2016;9(4):175-182. doi: 10.3928/19404921-20160120-01. PubMed
19. Coleman EA, Roman SP, Hall KA, Min SJ. Enhancing the care transitions intervention protocol to better address the needs of family caregivers. J Healthc Qual. 2015;37(1):2-11. doi: 10.1097/01.JHQ.0000460118.60567.fe. PubMed
© 2019 Society of Hospital Medicine
Outpatient Parenteral Antimicrobial Therapy in Vulnerable Populations—People Who Inject Drugs and the Homeless
Outpatient parenteral antimicrobial therapy (OPAT) programs allow patients to receive antibiotic therapy at home or in other settings.1-3 Bacterial infections among people who inject drugs (PWID) and the homeless are common, leading to complicated treatment strategies. Those with opioid dependence have frequent hospitalizations.4 Bacteremia and endocarditis frequently require intravenous (IV) antibiotics5-7 and may be difficult to treat. Creating outpatient treatment plans for PWID and the homeless is challenging, and there is a paucity of data on OPAT effectiveness in these groups as they are often excluded from OPAT services.1,2,8
We evaluated treatment outcomes in PWID and the homeless in our OPAT program.
METHODS
We conducted a retrospective cohort study of hospitalized adults discharged from Harborview Medical Center (HMC) with OPAT from January 1, 2015 to April 30, 2016. HMC is a county hospital in Seattle, Washington, affiliated with the University of Washington (UW). Infectious disease specialists supervise our OPAT program and provide follow-up care. We partner with a medical respite facility, a discharge option for homeless patients.9 Respite is staffed by HMC nurses, mental health specialists, and case managers.
Patients aged >18 years were enrolled in OPAT if they were discharged with >2 weeks of IV therapy or required laboratory monitoring while on oral antibiotics. Patients with multiple hospitalizations were included for their initial OPAT encounter only. PWID discharged to respite were instructed not to use their vascular access to inject drugs, but drug abstinence was not required. A tamper-evident sticker was placed over lines that nurses evaluated daily. Patients violating line-tampering restrictions were discharged from respite, and OPAT providers developed alternative antibiotic plans.
The two primary exposures evaluated were patient-reported injection drug use and housing status, and our primary exposure measure was the four-category combination: (1) housed non-PWID, (2) housed PWID, (3) homeless non-PWID, and (4) homeless PWID. Current drug use was defined as use within three months of hospitalization. Homelessness was defined as lack of stable housing. Patients receiving chemotherapy, prolonged steroids, biologic agents, or those with organ transplant were considered immunocompromised.
The primary outcome was clinical cure, defined as completion of antibiotic therapy and resolution of infection, determined by OPAT providers. Patients who were placed on oral suppressive antibiotics or died before treatment completion were considered not cured. Unknown status, including care transfer and lost to follow-up, were noted separately. Lost to follow-up was assumed if patients did not return for care, their care was not formally transferred, and no other medical information was available.
Secondary outcomes included hospital length of stay (LOS), secondary bacteremia, line-tampering, and 30-day readmissions. Secondary bacteremia was defined as bacteremia with a different pathogen from the index illness, which occurred during the initial treatment course. Readmission included readmissions related to OPAT (ie, recurrent or worsening infection, treatment-related toxicities, line-tampering, secondary bacteremia, and line-associated complications).
Data collection was performed using REDCap, a data-capturing software program linked to the electronic medical record (EMR).10 Hospitalization dates and demographics were electronically populated from the EMR. Details regarding drug use, homelessness, comorbidities, diagnosis, discharge complications, clinical cure, and lost to follow-up were manually entered.
Statistical Analysis
Statistical calculations were performed using SAS (v. 9.4). Chi-square testing and analysis of variance were conducted to assess group differences in demographics, infection types, and clinical outcomes.
Primary and secondary outcomes were further evaluated by univariable logistic regression and presented as odds ratios, with the non-PWID housed group serving as the reference. Given the large number of PWID and homeless patients lost to follow-up, sensitivity analyses were conducted using the assumption that patients with unknown clinical outcomes did not achieve cure (ie, chronic infection or death). Multivariable regression was performed on the outcomes of cure and 30-day readmission to OPAT using backward elimination to select a final model, initially including potential confounders of age, sex, and relevant comorbidities (DM and HIV). We assumed that those lost to follow-up were not cured (or readmitted). Other secondary outcomes were either rare events or those of uncertain relevance (eg, hospital LOS) to be evaluated in the multivariable analysis.
Our study did not meet the definition of research by the UW’s institutional review board. It was a quality improvement project funded by a UW Medicine Patient Safety Innovations Program Grant.
RESULTS
Overall, 596 patients received OPAT over 16 months. OPAT patients were categorized into groups as follows: homeless PWID (9%, n = 53), housed PWID (8%, n = 48), homeless non-PWID (8%, n = 45), and housed non-PWID (75%, n = 450).
PWID were younger than non-PWID, and the majority of patients in all groups were men (Table 1). PWID were more likely to have hepatitis C. Non-PWID appeared more likely to have diabetes and be immunosuppressed.
Patients had a total of 960 types of infection (Table 1). Bacteremia was the most common infection among PWID. Osteomyelitis was the most frequent infection in non-PWID.
Discharge location varied widely (P < .001; Table 1). The majority of patients with housing (housed PWID 60.4%, housed non-PWID 59.1%) were discharged to home, although 36.7% of housed non-PWID went to nursing facilities. Among homeless patients, 58.5% of PWID and 42.2% of non-PWID were discharged to respite; 10 patients were discharged to a shelter or street. Data specific to transition from IV to oral therapy were not recorded.
Cure rates among participants with known outcomes did not differ by group (Table 1; P = .85). In a sensitivity analysis of clinical cure, assuming those with unknown outcomes were not cured, housing status and drug use were significantly associated with cure (Table 1; P < .001, in the overall test), with rates lower among housed and homeless PWID groups (50.0% and 47.2%, respectively) compared with housed and homeless non-PWID groups (73.1% and 82.2%, respectively). In the multivariable analysis after backward elimination of noninfluential measures, only PWID and housing status were associated with cure; PWID, whether housed (OR = 0.37) or not (OR = 0.33), had lower odds of cure relative to housed non-PWID (Table 2).
Secondary outcomes, evaluated on all patients regardless of cure, differed by group (Table 1). Mean LOS appeared to be shortest for homeless PWID (15.5 days versus ≥18.0 for other groups; P < .001 for overall test). Homeless PWID patients appeared more likely to have secondary bacteremia (13.2% versus <4.2% in other groups; P < .001 for overall test), line tampering (35.9% versus <2.2% in other groups; P < .001), and 30-day readmission related to OPAT (26.4% versus <16.7% in other groups; P = .004). Compared with housed non-PWID using logistic regression, homeless PWID had a higher risk of secondary bacteremia (OR = 12.9; 95% CI 3.8-37.8; P < .001), line tampering (OR 88.4; 95% CI 24.5-318.3; P < .001), and readmission for OPAT (OR 2.4; 95% CI 1.2-4.6; P = .007). After adjusting for age, sex, and comorbidities, readmission for OPAT remained elevated in homeless PWID (OR = 2.4; 95% CI 1.2-4.6). No significant differences in secondary outcomes were found between housed non-PWID and also between housed PWID and homeless non-PWID.
Among homeless persons, discharge to respite care was not associated with improved outcomes, assuming those lost to follow-up did not achieve cure. Among non-PWID discharged to respite, the cure rate was 74% (14/19) compared with 88% (23/26) discharged elsewhere (P = .20). Among PWID, 48% (15/31) discharged to respite were cured compared with 45% (10/22) discharged elsewhere (P = .83).
DISCUSSION
Our study compares the outcomes of 596 OPAT patients, including PWID and the homeless. Among those retained in care, PWID achieved similar rates of cure compared with non-PWID groups. When assuming that all lost to follow-up had poor outcomes, the cure rates were markedly lower for PWID, with no difference noted by housing status.
Data on PWID and homeless enrolled in OPAT programs are limited.5,11,12 Few studies have reported the outcomes of infections in PWID and the homeless, as these populations often experience significant loss to follow-up due to transiency, lack of care continuity, and effective means of communication.
Cure was achieved in less than half of PWID, when lack of cure was assumed for unknown outcomes. This rate was substantially less than that for non-PWID groups. The assumption that those lost to follow-up did not achieve cure dramatically alters the inference; the truth may lie somewhere between the primary and sensitivity analyses. Homeless PWID remained at the highest risk for lost to follow-up, secondary bacteremia, line-tampering, and 30-day readmission related to OPAT.
PWID have traditionally been considered as a high-risk group for OPAT,1,2,8 but to completely restrict PWID from OPAT may not be appropriate. Ho et al. studied 29 PWID who were selectively enrolled to receive OPAT, and 28 completed IV therapy without any instances of line-tampering, death, or unknown clinical status.6 Recent literature suggests that some candidates can succeed with OPAT, despite drug use.13,14
Homelessness is also considered a barrier to OPAT.1,8 Medical respite is a harm-reduction model implemented for patients who require subacute care.9 In our study, among homeless patients, PWID status was the primary determinant of whether therapy was successful, rather than respite care.
Our study may have limited generalizability to other populations. We are a single-center facility in a large, urban city. PWID and housing status were self-reported but were verified before discharge. Most of our patients were men and white; thus, outcomes may differ for others. Due to the nature of the data, cost effectiveness could not be directly calculated. LOS and readmissions serve as proxy measures.
When patients remain engaged in care, PWID and the homeless achieved comparable clinical cure rates to those of housed non-PWID. Moving forward, OPAT can be more effective in PWID and the homeless with careful patient selection and close clinical support. Access to medication-assisted therapy, such as methadone or buprenorphine,15 may improve follow-up rates and linkage to outpatient care. Additional treatment strategies to improve retention in and adherence to care may promote successful outcomes in these vulnerable populations.
Disclosures
Presented at the Poster Abstract Session: Clinical Practice Issues at ID Week, October 26–30, 2016, New Orleans, LA. No conflicts of interested related to this work for all authors.
Funding
AW and AM received NIH NIAID grant K24 AI 071113-06 and UW Medicine Patient Safety Innovations Program Grant.
1. Tice, AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004; 38(12):1651-1672. doi: 10.1086/420939. PubMed
2. Williams DN, Baker CA, Kind AC, Sannes MR. The history and evolution of outpatient parenteral antibiotic therapy (OPAT). Int J Antimicrob Agents. 2015;46(3):307-312. doi: 10.1016/j.ijantimicag.2015.07.001. PubMed
3. Gilchrist M, Seaton RA. Outpatient parenteral antimicrobial therapy and antimicrobial stewardship: challenges and checklists. J Antimicrob Chemother. 2015;70(4);965-970. doi: 10.1093/jac/dku517. PubMed
4. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections from 2002-2012. Health Aff (Milwood). 2016;35(5):832-837. doi: 10.1377/hlthaff.2015.1424. PubMed
5. Beieler AM, Dellit TH, Chan JD, et al. Successful implementation of outpatient parenteral antibiotic therapy at a medical respite facility for homeless patients. J Hosp Med. 2016;11(8):531-535. doi: 10.1002/jhm.2597. PubMed
6. Ho J, Archuleta S, Sulaiman Z, Fisher D. Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65:2641-2644. doi: 10.1093/jac/dkq355. PubMed
7. Suleyman G, Kenney R, Zervos MJ, Weinmann A. Safety and efficacy of outpatient parenteral antibiotic therapy in an academic infectious disease clinic. J Clin Pharm Ther. 2017;42(1):39-43. doi: 10.1111/jcpt.12465. PubMed
8. Bhavan KP, Brown LS, Haley RW. Self-administered outpatient antimicrobial infusion by uninsured patients discharged from a safety-net hospital: a propensity-score-balanced retrospective cohort study. PLoS Med. 2015;12(12):e1001922. doi: 10.1371/journal.pmed. PubMed
9. Seattle-King County Medical Respite. https://www.kingcounty.gov/depts/health/locations/homeless-health/healthcare-for-the-homeless/services/medical-respite.aspx. Accessed October 2, 2018.
10. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-3781. doi: 10.1016/j.jbi.2008.08.010. PubMed
11. Buerhle DJ, Shields RK, Shah N, Shoff C, Sheridan K. Risk factors associated with outpatient parenteral antibiotic therapy program failure among intravenous drug users. Open Forum Infect Dis.2017;4(3):ofx102. doi: 10.1093/ofid/ofx102. PubMed
12. Hernandez W, Price C, Knepper B, McLees M, Young H. Outpatient parenteral antimicrobial therapy administration in a homeless population. J Infus Nurs. 2016;39(2):81-85. doi: 10.1097/NAN.0000000000000165. PubMed
13. Sukuki J, Johnson J, Montgomery M, Hayden M, Price C. Outpatient parenteral antimicrobial therapy among people who inject drugs: a review of the literature. Open Forum Infect Dis. 2018;5(9):ofy194. doi: 10.1093/ofid/ofy194. PubMed
14. D’Couto HT, Robbins GK, Ard KL, Wakeman SE, Alves J, Nelson SB. Outcomes according to discharge location for persons who inject drugs receiving outpatient parenteral antimicrobial therapy. Open Forum Infect Dis. 2018;5(5):ofy056. doi: 10.1093/ofid/ofy056. PubMed
15. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. doi: 10.1016/j.amjmed.2015.09.024. PubMed
Outpatient parenteral antimicrobial therapy (OPAT) programs allow patients to receive antibiotic therapy at home or in other settings.1-3 Bacterial infections among people who inject drugs (PWID) and the homeless are common, leading to complicated treatment strategies. Those with opioid dependence have frequent hospitalizations.4 Bacteremia and endocarditis frequently require intravenous (IV) antibiotics5-7 and may be difficult to treat. Creating outpatient treatment plans for PWID and the homeless is challenging, and there is a paucity of data on OPAT effectiveness in these groups as they are often excluded from OPAT services.1,2,8
We evaluated treatment outcomes in PWID and the homeless in our OPAT program.
METHODS
We conducted a retrospective cohort study of hospitalized adults discharged from Harborview Medical Center (HMC) with OPAT from January 1, 2015 to April 30, 2016. HMC is a county hospital in Seattle, Washington, affiliated with the University of Washington (UW). Infectious disease specialists supervise our OPAT program and provide follow-up care. We partner with a medical respite facility, a discharge option for homeless patients.9 Respite is staffed by HMC nurses, mental health specialists, and case managers.
Patients aged >18 years were enrolled in OPAT if they were discharged with >2 weeks of IV therapy or required laboratory monitoring while on oral antibiotics. Patients with multiple hospitalizations were included for their initial OPAT encounter only. PWID discharged to respite were instructed not to use their vascular access to inject drugs, but drug abstinence was not required. A tamper-evident sticker was placed over lines that nurses evaluated daily. Patients violating line-tampering restrictions were discharged from respite, and OPAT providers developed alternative antibiotic plans.
The two primary exposures evaluated were patient-reported injection drug use and housing status, and our primary exposure measure was the four-category combination: (1) housed non-PWID, (2) housed PWID, (3) homeless non-PWID, and (4) homeless PWID. Current drug use was defined as use within three months of hospitalization. Homelessness was defined as lack of stable housing. Patients receiving chemotherapy, prolonged steroids, biologic agents, or those with organ transplant were considered immunocompromised.
The primary outcome was clinical cure, defined as completion of antibiotic therapy and resolution of infection, determined by OPAT providers. Patients who were placed on oral suppressive antibiotics or died before treatment completion were considered not cured. Unknown status, including care transfer and lost to follow-up, were noted separately. Lost to follow-up was assumed if patients did not return for care, their care was not formally transferred, and no other medical information was available.
Secondary outcomes included hospital length of stay (LOS), secondary bacteremia, line-tampering, and 30-day readmissions. Secondary bacteremia was defined as bacteremia with a different pathogen from the index illness, which occurred during the initial treatment course. Readmission included readmissions related to OPAT (ie, recurrent or worsening infection, treatment-related toxicities, line-tampering, secondary bacteremia, and line-associated complications).
Data collection was performed using REDCap, a data-capturing software program linked to the electronic medical record (EMR).10 Hospitalization dates and demographics were electronically populated from the EMR. Details regarding drug use, homelessness, comorbidities, diagnosis, discharge complications, clinical cure, and lost to follow-up were manually entered.
Statistical Analysis
Statistical calculations were performed using SAS (v. 9.4). Chi-square testing and analysis of variance were conducted to assess group differences in demographics, infection types, and clinical outcomes.
Primary and secondary outcomes were further evaluated by univariable logistic regression and presented as odds ratios, with the non-PWID housed group serving as the reference. Given the large number of PWID and homeless patients lost to follow-up, sensitivity analyses were conducted using the assumption that patients with unknown clinical outcomes did not achieve cure (ie, chronic infection or death). Multivariable regression was performed on the outcomes of cure and 30-day readmission to OPAT using backward elimination to select a final model, initially including potential confounders of age, sex, and relevant comorbidities (DM and HIV). We assumed that those lost to follow-up were not cured (or readmitted). Other secondary outcomes were either rare events or those of uncertain relevance (eg, hospital LOS) to be evaluated in the multivariable analysis.
Our study did not meet the definition of research by the UW’s institutional review board. It was a quality improvement project funded by a UW Medicine Patient Safety Innovations Program Grant.
RESULTS
Overall, 596 patients received OPAT over 16 months. OPAT patients were categorized into groups as follows: homeless PWID (9%, n = 53), housed PWID (8%, n = 48), homeless non-PWID (8%, n = 45), and housed non-PWID (75%, n = 450).
PWID were younger than non-PWID, and the majority of patients in all groups were men (Table 1). PWID were more likely to have hepatitis C. Non-PWID appeared more likely to have diabetes and be immunosuppressed.
Patients had a total of 960 types of infection (Table 1). Bacteremia was the most common infection among PWID. Osteomyelitis was the most frequent infection in non-PWID.
Discharge location varied widely (P < .001; Table 1). The majority of patients with housing (housed PWID 60.4%, housed non-PWID 59.1%) were discharged to home, although 36.7% of housed non-PWID went to nursing facilities. Among homeless patients, 58.5% of PWID and 42.2% of non-PWID were discharged to respite; 10 patients were discharged to a shelter or street. Data specific to transition from IV to oral therapy were not recorded.
Cure rates among participants with known outcomes did not differ by group (Table 1; P = .85). In a sensitivity analysis of clinical cure, assuming those with unknown outcomes were not cured, housing status and drug use were significantly associated with cure (Table 1; P < .001, in the overall test), with rates lower among housed and homeless PWID groups (50.0% and 47.2%, respectively) compared with housed and homeless non-PWID groups (73.1% and 82.2%, respectively). In the multivariable analysis after backward elimination of noninfluential measures, only PWID and housing status were associated with cure; PWID, whether housed (OR = 0.37) or not (OR = 0.33), had lower odds of cure relative to housed non-PWID (Table 2).
Secondary outcomes, evaluated on all patients regardless of cure, differed by group (Table 1). Mean LOS appeared to be shortest for homeless PWID (15.5 days versus ≥18.0 for other groups; P < .001 for overall test). Homeless PWID patients appeared more likely to have secondary bacteremia (13.2% versus <4.2% in other groups; P < .001 for overall test), line tampering (35.9% versus <2.2% in other groups; P < .001), and 30-day readmission related to OPAT (26.4% versus <16.7% in other groups; P = .004). Compared with housed non-PWID using logistic regression, homeless PWID had a higher risk of secondary bacteremia (OR = 12.9; 95% CI 3.8-37.8; P < .001), line tampering (OR 88.4; 95% CI 24.5-318.3; P < .001), and readmission for OPAT (OR 2.4; 95% CI 1.2-4.6; P = .007). After adjusting for age, sex, and comorbidities, readmission for OPAT remained elevated in homeless PWID (OR = 2.4; 95% CI 1.2-4.6). No significant differences in secondary outcomes were found between housed non-PWID and also between housed PWID and homeless non-PWID.
Among homeless persons, discharge to respite care was not associated with improved outcomes, assuming those lost to follow-up did not achieve cure. Among non-PWID discharged to respite, the cure rate was 74% (14/19) compared with 88% (23/26) discharged elsewhere (P = .20). Among PWID, 48% (15/31) discharged to respite were cured compared with 45% (10/22) discharged elsewhere (P = .83).
DISCUSSION
Our study compares the outcomes of 596 OPAT patients, including PWID and the homeless. Among those retained in care, PWID achieved similar rates of cure compared with non-PWID groups. When assuming that all lost to follow-up had poor outcomes, the cure rates were markedly lower for PWID, with no difference noted by housing status.
Data on PWID and homeless enrolled in OPAT programs are limited.5,11,12 Few studies have reported the outcomes of infections in PWID and the homeless, as these populations often experience significant loss to follow-up due to transiency, lack of care continuity, and effective means of communication.
Cure was achieved in less than half of PWID, when lack of cure was assumed for unknown outcomes. This rate was substantially less than that for non-PWID groups. The assumption that those lost to follow-up did not achieve cure dramatically alters the inference; the truth may lie somewhere between the primary and sensitivity analyses. Homeless PWID remained at the highest risk for lost to follow-up, secondary bacteremia, line-tampering, and 30-day readmission related to OPAT.
PWID have traditionally been considered as a high-risk group for OPAT,1,2,8 but to completely restrict PWID from OPAT may not be appropriate. Ho et al. studied 29 PWID who were selectively enrolled to receive OPAT, and 28 completed IV therapy without any instances of line-tampering, death, or unknown clinical status.6 Recent literature suggests that some candidates can succeed with OPAT, despite drug use.13,14
Homelessness is also considered a barrier to OPAT.1,8 Medical respite is a harm-reduction model implemented for patients who require subacute care.9 In our study, among homeless patients, PWID status was the primary determinant of whether therapy was successful, rather than respite care.
Our study may have limited generalizability to other populations. We are a single-center facility in a large, urban city. PWID and housing status were self-reported but were verified before discharge. Most of our patients were men and white; thus, outcomes may differ for others. Due to the nature of the data, cost effectiveness could not be directly calculated. LOS and readmissions serve as proxy measures.
When patients remain engaged in care, PWID and the homeless achieved comparable clinical cure rates to those of housed non-PWID. Moving forward, OPAT can be more effective in PWID and the homeless with careful patient selection and close clinical support. Access to medication-assisted therapy, such as methadone or buprenorphine,15 may improve follow-up rates and linkage to outpatient care. Additional treatment strategies to improve retention in and adherence to care may promote successful outcomes in these vulnerable populations.
Disclosures
Presented at the Poster Abstract Session: Clinical Practice Issues at ID Week, October 26–30, 2016, New Orleans, LA. No conflicts of interested related to this work for all authors.
Funding
AW and AM received NIH NIAID grant K24 AI 071113-06 and UW Medicine Patient Safety Innovations Program Grant.
Outpatient parenteral antimicrobial therapy (OPAT) programs allow patients to receive antibiotic therapy at home or in other settings.1-3 Bacterial infections among people who inject drugs (PWID) and the homeless are common, leading to complicated treatment strategies. Those with opioid dependence have frequent hospitalizations.4 Bacteremia and endocarditis frequently require intravenous (IV) antibiotics5-7 and may be difficult to treat. Creating outpatient treatment plans for PWID and the homeless is challenging, and there is a paucity of data on OPAT effectiveness in these groups as they are often excluded from OPAT services.1,2,8
We evaluated treatment outcomes in PWID and the homeless in our OPAT program.
METHODS
We conducted a retrospective cohort study of hospitalized adults discharged from Harborview Medical Center (HMC) with OPAT from January 1, 2015 to April 30, 2016. HMC is a county hospital in Seattle, Washington, affiliated with the University of Washington (UW). Infectious disease specialists supervise our OPAT program and provide follow-up care. We partner with a medical respite facility, a discharge option for homeless patients.9 Respite is staffed by HMC nurses, mental health specialists, and case managers.
Patients aged >18 years were enrolled in OPAT if they were discharged with >2 weeks of IV therapy or required laboratory monitoring while on oral antibiotics. Patients with multiple hospitalizations were included for their initial OPAT encounter only. PWID discharged to respite were instructed not to use their vascular access to inject drugs, but drug abstinence was not required. A tamper-evident sticker was placed over lines that nurses evaluated daily. Patients violating line-tampering restrictions were discharged from respite, and OPAT providers developed alternative antibiotic plans.
The two primary exposures evaluated were patient-reported injection drug use and housing status, and our primary exposure measure was the four-category combination: (1) housed non-PWID, (2) housed PWID, (3) homeless non-PWID, and (4) homeless PWID. Current drug use was defined as use within three months of hospitalization. Homelessness was defined as lack of stable housing. Patients receiving chemotherapy, prolonged steroids, biologic agents, or those with organ transplant were considered immunocompromised.
The primary outcome was clinical cure, defined as completion of antibiotic therapy and resolution of infection, determined by OPAT providers. Patients who were placed on oral suppressive antibiotics or died before treatment completion were considered not cured. Unknown status, including care transfer and lost to follow-up, were noted separately. Lost to follow-up was assumed if patients did not return for care, their care was not formally transferred, and no other medical information was available.
Secondary outcomes included hospital length of stay (LOS), secondary bacteremia, line-tampering, and 30-day readmissions. Secondary bacteremia was defined as bacteremia with a different pathogen from the index illness, which occurred during the initial treatment course. Readmission included readmissions related to OPAT (ie, recurrent or worsening infection, treatment-related toxicities, line-tampering, secondary bacteremia, and line-associated complications).
Data collection was performed using REDCap, a data-capturing software program linked to the electronic medical record (EMR).10 Hospitalization dates and demographics were electronically populated from the EMR. Details regarding drug use, homelessness, comorbidities, diagnosis, discharge complications, clinical cure, and lost to follow-up were manually entered.
Statistical Analysis
Statistical calculations were performed using SAS (v. 9.4). Chi-square testing and analysis of variance were conducted to assess group differences in demographics, infection types, and clinical outcomes.
Primary and secondary outcomes were further evaluated by univariable logistic regression and presented as odds ratios, with the non-PWID housed group serving as the reference. Given the large number of PWID and homeless patients lost to follow-up, sensitivity analyses were conducted using the assumption that patients with unknown clinical outcomes did not achieve cure (ie, chronic infection or death). Multivariable regression was performed on the outcomes of cure and 30-day readmission to OPAT using backward elimination to select a final model, initially including potential confounders of age, sex, and relevant comorbidities (DM and HIV). We assumed that those lost to follow-up were not cured (or readmitted). Other secondary outcomes were either rare events or those of uncertain relevance (eg, hospital LOS) to be evaluated in the multivariable analysis.
Our study did not meet the definition of research by the UW’s institutional review board. It was a quality improvement project funded by a UW Medicine Patient Safety Innovations Program Grant.
RESULTS
Overall, 596 patients received OPAT over 16 months. OPAT patients were categorized into groups as follows: homeless PWID (9%, n = 53), housed PWID (8%, n = 48), homeless non-PWID (8%, n = 45), and housed non-PWID (75%, n = 450).
PWID were younger than non-PWID, and the majority of patients in all groups were men (Table 1). PWID were more likely to have hepatitis C. Non-PWID appeared more likely to have diabetes and be immunosuppressed.
Patients had a total of 960 types of infection (Table 1). Bacteremia was the most common infection among PWID. Osteomyelitis was the most frequent infection in non-PWID.
Discharge location varied widely (P < .001; Table 1). The majority of patients with housing (housed PWID 60.4%, housed non-PWID 59.1%) were discharged to home, although 36.7% of housed non-PWID went to nursing facilities. Among homeless patients, 58.5% of PWID and 42.2% of non-PWID were discharged to respite; 10 patients were discharged to a shelter or street. Data specific to transition from IV to oral therapy were not recorded.
Cure rates among participants with known outcomes did not differ by group (Table 1; P = .85). In a sensitivity analysis of clinical cure, assuming those with unknown outcomes were not cured, housing status and drug use were significantly associated with cure (Table 1; P < .001, in the overall test), with rates lower among housed and homeless PWID groups (50.0% and 47.2%, respectively) compared with housed and homeless non-PWID groups (73.1% and 82.2%, respectively). In the multivariable analysis after backward elimination of noninfluential measures, only PWID and housing status were associated with cure; PWID, whether housed (OR = 0.37) or not (OR = 0.33), had lower odds of cure relative to housed non-PWID (Table 2).
Secondary outcomes, evaluated on all patients regardless of cure, differed by group (Table 1). Mean LOS appeared to be shortest for homeless PWID (15.5 days versus ≥18.0 for other groups; P < .001 for overall test). Homeless PWID patients appeared more likely to have secondary bacteremia (13.2% versus <4.2% in other groups; P < .001 for overall test), line tampering (35.9% versus <2.2% in other groups; P < .001), and 30-day readmission related to OPAT (26.4% versus <16.7% in other groups; P = .004). Compared with housed non-PWID using logistic regression, homeless PWID had a higher risk of secondary bacteremia (OR = 12.9; 95% CI 3.8-37.8; P < .001), line tampering (OR 88.4; 95% CI 24.5-318.3; P < .001), and readmission for OPAT (OR 2.4; 95% CI 1.2-4.6; P = .007). After adjusting for age, sex, and comorbidities, readmission for OPAT remained elevated in homeless PWID (OR = 2.4; 95% CI 1.2-4.6). No significant differences in secondary outcomes were found between housed non-PWID and also between housed PWID and homeless non-PWID.
Among homeless persons, discharge to respite care was not associated with improved outcomes, assuming those lost to follow-up did not achieve cure. Among non-PWID discharged to respite, the cure rate was 74% (14/19) compared with 88% (23/26) discharged elsewhere (P = .20). Among PWID, 48% (15/31) discharged to respite were cured compared with 45% (10/22) discharged elsewhere (P = .83).
DISCUSSION
Our study compares the outcomes of 596 OPAT patients, including PWID and the homeless. Among those retained in care, PWID achieved similar rates of cure compared with non-PWID groups. When assuming that all lost to follow-up had poor outcomes, the cure rates were markedly lower for PWID, with no difference noted by housing status.
Data on PWID and homeless enrolled in OPAT programs are limited.5,11,12 Few studies have reported the outcomes of infections in PWID and the homeless, as these populations often experience significant loss to follow-up due to transiency, lack of care continuity, and effective means of communication.
Cure was achieved in less than half of PWID, when lack of cure was assumed for unknown outcomes. This rate was substantially less than that for non-PWID groups. The assumption that those lost to follow-up did not achieve cure dramatically alters the inference; the truth may lie somewhere between the primary and sensitivity analyses. Homeless PWID remained at the highest risk for lost to follow-up, secondary bacteremia, line-tampering, and 30-day readmission related to OPAT.
PWID have traditionally been considered as a high-risk group for OPAT,1,2,8 but to completely restrict PWID from OPAT may not be appropriate. Ho et al. studied 29 PWID who were selectively enrolled to receive OPAT, and 28 completed IV therapy without any instances of line-tampering, death, or unknown clinical status.6 Recent literature suggests that some candidates can succeed with OPAT, despite drug use.13,14
Homelessness is also considered a barrier to OPAT.1,8 Medical respite is a harm-reduction model implemented for patients who require subacute care.9 In our study, among homeless patients, PWID status was the primary determinant of whether therapy was successful, rather than respite care.
Our study may have limited generalizability to other populations. We are a single-center facility in a large, urban city. PWID and housing status were self-reported but were verified before discharge. Most of our patients were men and white; thus, outcomes may differ for others. Due to the nature of the data, cost effectiveness could not be directly calculated. LOS and readmissions serve as proxy measures.
When patients remain engaged in care, PWID and the homeless achieved comparable clinical cure rates to those of housed non-PWID. Moving forward, OPAT can be more effective in PWID and the homeless with careful patient selection and close clinical support. Access to medication-assisted therapy, such as methadone or buprenorphine,15 may improve follow-up rates and linkage to outpatient care. Additional treatment strategies to improve retention in and adherence to care may promote successful outcomes in these vulnerable populations.
Disclosures
Presented at the Poster Abstract Session: Clinical Practice Issues at ID Week, October 26–30, 2016, New Orleans, LA. No conflicts of interested related to this work for all authors.
Funding
AW and AM received NIH NIAID grant K24 AI 071113-06 and UW Medicine Patient Safety Innovations Program Grant.
1. Tice, AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004; 38(12):1651-1672. doi: 10.1086/420939. PubMed
2. Williams DN, Baker CA, Kind AC, Sannes MR. The history and evolution of outpatient parenteral antibiotic therapy (OPAT). Int J Antimicrob Agents. 2015;46(3):307-312. doi: 10.1016/j.ijantimicag.2015.07.001. PubMed
3. Gilchrist M, Seaton RA. Outpatient parenteral antimicrobial therapy and antimicrobial stewardship: challenges and checklists. J Antimicrob Chemother. 2015;70(4);965-970. doi: 10.1093/jac/dku517. PubMed
4. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections from 2002-2012. Health Aff (Milwood). 2016;35(5):832-837. doi: 10.1377/hlthaff.2015.1424. PubMed
5. Beieler AM, Dellit TH, Chan JD, et al. Successful implementation of outpatient parenteral antibiotic therapy at a medical respite facility for homeless patients. J Hosp Med. 2016;11(8):531-535. doi: 10.1002/jhm.2597. PubMed
6. Ho J, Archuleta S, Sulaiman Z, Fisher D. Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65:2641-2644. doi: 10.1093/jac/dkq355. PubMed
7. Suleyman G, Kenney R, Zervos MJ, Weinmann A. Safety and efficacy of outpatient parenteral antibiotic therapy in an academic infectious disease clinic. J Clin Pharm Ther. 2017;42(1):39-43. doi: 10.1111/jcpt.12465. PubMed
8. Bhavan KP, Brown LS, Haley RW. Self-administered outpatient antimicrobial infusion by uninsured patients discharged from a safety-net hospital: a propensity-score-balanced retrospective cohort study. PLoS Med. 2015;12(12):e1001922. doi: 10.1371/journal.pmed. PubMed
9. Seattle-King County Medical Respite. https://www.kingcounty.gov/depts/health/locations/homeless-health/healthcare-for-the-homeless/services/medical-respite.aspx. Accessed October 2, 2018.
10. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-3781. doi: 10.1016/j.jbi.2008.08.010. PubMed
11. Buerhle DJ, Shields RK, Shah N, Shoff C, Sheridan K. Risk factors associated with outpatient parenteral antibiotic therapy program failure among intravenous drug users. Open Forum Infect Dis.2017;4(3):ofx102. doi: 10.1093/ofid/ofx102. PubMed
12. Hernandez W, Price C, Knepper B, McLees M, Young H. Outpatient parenteral antimicrobial therapy administration in a homeless population. J Infus Nurs. 2016;39(2):81-85. doi: 10.1097/NAN.0000000000000165. PubMed
13. Sukuki J, Johnson J, Montgomery M, Hayden M, Price C. Outpatient parenteral antimicrobial therapy among people who inject drugs: a review of the literature. Open Forum Infect Dis. 2018;5(9):ofy194. doi: 10.1093/ofid/ofy194. PubMed
14. D’Couto HT, Robbins GK, Ard KL, Wakeman SE, Alves J, Nelson SB. Outcomes according to discharge location for persons who inject drugs receiving outpatient parenteral antimicrobial therapy. Open Forum Infect Dis. 2018;5(5):ofy056. doi: 10.1093/ofid/ofy056. PubMed
15. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. doi: 10.1016/j.amjmed.2015.09.024. PubMed
1. Tice, AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004; 38(12):1651-1672. doi: 10.1086/420939. PubMed
2. Williams DN, Baker CA, Kind AC, Sannes MR. The history and evolution of outpatient parenteral antibiotic therapy (OPAT). Int J Antimicrob Agents. 2015;46(3):307-312. doi: 10.1016/j.ijantimicag.2015.07.001. PubMed
3. Gilchrist M, Seaton RA. Outpatient parenteral antimicrobial therapy and antimicrobial stewardship: challenges and checklists. J Antimicrob Chemother. 2015;70(4);965-970. doi: 10.1093/jac/dku517. PubMed
4. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections from 2002-2012. Health Aff (Milwood). 2016;35(5):832-837. doi: 10.1377/hlthaff.2015.1424. PubMed
5. Beieler AM, Dellit TH, Chan JD, et al. Successful implementation of outpatient parenteral antibiotic therapy at a medical respite facility for homeless patients. J Hosp Med. 2016;11(8):531-535. doi: 10.1002/jhm.2597. PubMed
6. Ho J, Archuleta S, Sulaiman Z, Fisher D. Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65:2641-2644. doi: 10.1093/jac/dkq355. PubMed
7. Suleyman G, Kenney R, Zervos MJ, Weinmann A. Safety and efficacy of outpatient parenteral antibiotic therapy in an academic infectious disease clinic. J Clin Pharm Ther. 2017;42(1):39-43. doi: 10.1111/jcpt.12465. PubMed
8. Bhavan KP, Brown LS, Haley RW. Self-administered outpatient antimicrobial infusion by uninsured patients discharged from a safety-net hospital: a propensity-score-balanced retrospective cohort study. PLoS Med. 2015;12(12):e1001922. doi: 10.1371/journal.pmed. PubMed
9. Seattle-King County Medical Respite. https://www.kingcounty.gov/depts/health/locations/homeless-health/healthcare-for-the-homeless/services/medical-respite.aspx. Accessed October 2, 2018.
10. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-3781. doi: 10.1016/j.jbi.2008.08.010. PubMed
11. Buerhle DJ, Shields RK, Shah N, Shoff C, Sheridan K. Risk factors associated with outpatient parenteral antibiotic therapy program failure among intravenous drug users. Open Forum Infect Dis.2017;4(3):ofx102. doi: 10.1093/ofid/ofx102. PubMed
12. Hernandez W, Price C, Knepper B, McLees M, Young H. Outpatient parenteral antimicrobial therapy administration in a homeless population. J Infus Nurs. 2016;39(2):81-85. doi: 10.1097/NAN.0000000000000165. PubMed
13. Sukuki J, Johnson J, Montgomery M, Hayden M, Price C. Outpatient parenteral antimicrobial therapy among people who inject drugs: a review of the literature. Open Forum Infect Dis. 2018;5(9):ofy194. doi: 10.1093/ofid/ofy194. PubMed
14. D’Couto HT, Robbins GK, Ard KL, Wakeman SE, Alves J, Nelson SB. Outcomes according to discharge location for persons who inject drugs receiving outpatient parenteral antimicrobial therapy. Open Forum Infect Dis. 2018;5(5):ofy056. doi: 10.1093/ofid/ofy056. PubMed
15. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. doi: 10.1016/j.amjmed.2015.09.024. PubMed
© 2019 Society of Hospital Medicine
Things We Do For No Reason: Sliding-Scale Insulin as Monotherapy for Glycemic Control in Hospitalized Patients
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A CLINICAL SCENARIO
A 60-year-old man with a past medical history of obesity and type 2 diabetes presented to the emergency department with one week of myalgias and fever up to 103.5°F (39.7°C). Other vital signs were normal. He had no localizing symptoms, and physical examination was unrevealing, except for a small scab from a tick bite sustained two weeks prior to symptom onset. Before admission, he had been managing his diabetes with metformin 1,000 mg twice a day, and on arrival, his blood sugar level was 275 mg/dL. The admitting provider decided to hold the patient’s metformin and replace it with insulin per a sliding scale. Is monotherapy with sliding-scale insulin the best inpatient management option for this patient’s type 2 diabetes?
WHY YOU MIGHT THINK SLIDING-SCALE INSULIN AS MONOTHERAPY IS HELPFUL
The basic premise of sliding-scale insulin (SSI) is to correct hyperglycemia through the frequent administration of short-acting insulin dosed according to a patient’s blood glucose level with the help of a prespecified rubric. When blood glucose levels are low, patients receive little or no insulin, and when blood glucose levels are high, higher doses are given. This approach to inpatient blood glucose management was first popularized by Joslin in 1934,1 and it remains a common strategy today. For example, a 2007 survey of 44 hospitals in the United States showed that approximately 43% of all noncritically ill patients with hyperglycemia were treated with SSI alone.2 More recently, a single-center study showed that 30% of clinicians continued to use SSI as monotherapy even after the implementation of order sets designed to limit this practice.3
The rationale for SSI as monotherapy appears to have two components. First, guidelines suggest that certain patients should be screened periodically in the hospital for hyperglycemia (blood glucose persistently greater than 180 mg/dL) and that, if identified, hyperglycemia should be treated.4 By pairing finger-stick glucose monitoring with SSI, the diagnosis and treatment—although not the prevention—of hyperglycemia can be accomplished simultaneously. Second, inpatient providers do not want to cause harm in the form of hypoglycemia. SSI as monotherapy is sometimes viewed as a cautious approach in this regard as insulin is administered only if the blood sugar level is high.
Convenience is probably another key contributor to the enduring use of SSI as monotherapy. Several hospitals have ready-made order sets for SSI that are easier to prescribe than a patient-specific regimen including both short- and long-acting insulin. In at least one single-center survey, physicians and staff were found to favor convenience over perceived efficacy when asked about their attitudes toward inpatient glycemic control.5 Although efforts at individual hospitals to change practice patterns among residents have shown promise,6 reform on a broader scale remains elusive.
WHY SSI AS MONOTHERAPY IS NOT HELPFUL
SSI administration does not attempt to replicate normal pancreatic physiology, which involves basal insulin secretion to impair hepatic gluconeogenesis and meal-associated insulin spikes to promote uptake into glucose-avid tissues. SSI is a reactive strategy, not a proactive one, and perhaps unsurprisingly, to our knowledge, it has never been shown to prevent hyperglycemia in hospitalized patients, an impression corroborated by a systematic review of the topic between 1964 and 2003.7 More recently, one multicenter trial analyzed the effect of adding SSI to oral antihyperglycemic medications in hospitalized diabetics and found no differences in rates of hyperglycemia.8 Another study found that 84% of administered SSI doses failed to correct hyperglycemia.9
However, does adding basal insulin to SSI raise a patient’s risk of hypoglycemia? When basal insulin is dosed carefully, the answer appears to be no. In a trial in which diabetic long-term care residents who were receiving SSI at baseline were randomized to either continued SSI or basal-bolus insulin, the investigators found that the basal-bolus group experienced significantly lower average blood glucose levels without an increase in adverse glycemic events.10 Perhaps the most significant milestone to date, however, was the RABBIT 2 multicenter trial, published in 2007, that randomized hospitalized, insulin-naïve diabetics to either a weight-based regimen of basal and prandial insulin or SSI only.11 Rates of hypoglycemia and length of stay did not differ between the groups, and 66% of patients receiving basal-prandial insulin achieved their glycemic control target as opposed to just 38% of patients in the SSI-only group. The SSI group also required more total insulin. A weight-based, basal-bolus strategy was later proven to be similarly effective, without causing severe hypoglycemia, for patients undergoing surgery who could not maintain consistent oral alimentation.12 Basal-bolus insulin was associated with fewer surgical complications, and it produced a cost savings of $751 per day as determined by a post hoc comparative effectiveness study.13
Prolonged use of SSI as monotherapy may be not only ineffective but also harmful. Clearly, the absence of basal insulin will harm type 1 diabetics, who need basal insulin to prevent diabetic ketoacidosis. However, even for type 2 diabetics and nondiabetics, hyperglycemia has been established as a marker for adverse outcomes among hospitalized patients,14 and SSI monotherapy has been associated with a three-fold higher risk of hyperglycemia compared with the use of a sliding scale plus other forms of insulin.15 At least one other study has also linked this practice with a significantly increased length of stay compared with patients who were receiving insulin proactively.16 We believe that the potential for harm is difficult to disregard, especially because safer alternatives are available. Ultimately, it can be stated that in hospitalized patients with persistent hyperglycemia who require insulin, SSI alone should not be the preferred treatment choice regardless of whether the patient carries a known diagnosis of diabetes mellitus or has used insulin previously.
WHEN YOU MIGHT CONSIDER USING SSI AS MONOTHERAPY
As discussed above, there is no known clinical scenario in which SSI as monotherapy has been proven to be effective; however, the use of SSI as monotherapy as a short-term approach has not been well studied. Hospitalized patients who are at risk for adverse glycemic events should be monitored with periodic finger-stick blood glucose draws per guidelines, and in the first 24 hours, it may be reasonable to withhold basal insulin for insulin-naive patients, particularly if the medication reconciliation or other key components of the history are in doubt, or if there are risk factors for hypoglycemia such as a history of bariatric surgery. The amount of insulin received in the first 24 hours of such monitoring may inform subsequent insulin dosing, but this method of “dose finding” has not been validated in the literature.
Uncertain or interrupted alimentation status or stress hyperglycemia may complicate the assessment of a patient’s insulin needs. One of the insights from the RABBIT 2 surgery trial is that even with interrupted alimentation, patients on a weight-based, long-acting insulin regimen did not experience severe hypoglycemia. Nevertheless, if a patient without type 1 diabetes is felt to be at high risk for a severe hypoglycemic event, it may be prudent to withhold long-acting insulin. However, in that situation, adding SSI to finger-stick monitoring is unlikely to be beneficial. Cases of stress hyperglycemia in nondiabetics can also be challenging, as the persistence of hyperglycemia can be difficult to predict. Guidelines state that if hyperglycemia is persistent, then insulin therapy should be initiated and that this therapy is best accomplished in the form of a basal-prandial regimen.17
WHAT YOU SHOULD DO INSTEAD
Current guidelines from the American Diabetes Association17 and the American Association of Clinical Endocrinologists18 for hospitalized patients with hyperglycemia who require insulin recommend against the prolonged use of SSI as monotherapy (category A recommendation) and support the use of basal plus correctional insulin with the addition of nutritional insulin for patients with consistent oral intake (category A recommendation). Although a complete discourse on the determination of the appropriate starting dose of insulin is outside of the scope of this cas presentation, the basic approach begins with calculating a weight-based total daily dose of insulin, approximately half of which can be given as basal insulin with the remainder given with meals along with correctional insulin as needed to account for premeal hyperglycemia.4 For example, the protocol used in the RABBIT 2 trial, which involved known type 2 diabetics, started insulin based on a total daily dose of 0.4 units/kg for patients presenting with blood sugar levels ≤200 mg/dL and 0.5 units/kg for those with higher initial glucose levels.7 Half of the total daily dose was given as basal insulin, and the other half was divided among meals. Caution with insulin dosing may be required in patients aged >70 years, in those with impaired renal function, and in situations in which steroid doses are fluctuating. The Society of Hospital Medicine has formulated an online subcutaneous insulin order implementation guideline, eQUIPS, that can be a helpful resource to centers that are interested in changing their practice patterns.19
RECOMMENDATIONS
- Instead of using SSI monotherapy for hospitalized patients who require insulin, add basal and prandial insulin, using a weight-based approach if necessary for insulin-naive patients.
- Engage with leadership at your center to learn how inpatient hyperglycemia protocols and blood sugar management teams can help provide evidence-based and individualized treatment plans for your patients.
- If no infrastructure exists at your center, the Society of Hospital Medicine offers training and guidance through its eQUIPS inpatient hyperglycemia management program.
CONCLUSION
In the case presentation, the hyperglycemic patient whose metformin was on hold should have been started on a combination of basal and prandial insulin as determined by his weight and current renal function as opposed to monotherapy with SSI. Using SSI as monotherapy for hyperglycemia is a common practice, and although well-intentioned, it is an ineffective and possibly dangerous approach. Continued efforts must be made to address the gap between guidelines and suboptimal practice patterns locally and nationally.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailingTWDFNR@hospitalmedicine.org.
Acknowledgments
The authors would like to thank Dr. Asem Ali of the Division of Endocrinology at UMass Memorial Medical Center for his review of the manuscript.
Disclosures
The authors have nothing to disclose.
1. Joslin EP. A Diabetic Manual for the Mutual Use of Doctor and Patient. Philadelphia, PA: Lea & Febiger; 1934:108.
2. Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care. 2007;30(2):367-369. doi: 10.2337/dc06-1715. PubMed
3. Valgardson JD, Merino M, Redgrave J, Hudson JI, Hudson MS. Effectiveness of inpatient insulin order sets using human insulins in noncritically ill patients in a rural hospital. Endocr Pract. 2015;21(7):794-806. doi: 10.4158/EP14153. PubMed
4. Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553-591. doi: 10.2337/diacare.27.2.553. PubMed
5. Beliard R, Muzykovsky K, Vincent W, 3rd, Shah B, Davanos E. Perceptions, barriers, and knowledge of inpatient glycemic control: a survey of health care workers. J Pharm Pract. 2016;29(4):348-354. doi: 10.1177/0897190014566309. PubMed
6. Baldwin D, Villanueva G, McNutt R, Bhatnagar S. Eliminating inpatient sliding-scale insulin: a reeducation project with medical house staff. Diabetes Care. 2005;28(5):1008-1011. doi: 10.2337/diacare.28.12.2987-a. PubMed
7. Browning LA, Dumo P. Sliding-scale insulin: an antiquated approach to glycemic control in hospitalized patients. Am J Health Syst Pharm. 2004;61(15):1611-1614. PubMed
8. Dickerson LM, Ye X, Sack JL, Hueston WJ. Glycemic control in medical inpatients with type 2 diabetes mellitus receiving sliding scale insulin regimens versus routine diabetes medications: a multicenter randomized controlled trial. Ann Fam Med. 2003;1(1):29-35. PubMed
9. Golightly LK, Jones MA, Hamamura DH, Stolpman NM, McDermott MT. Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding-scale insulin therapy. Pharmacotherapy. 2006;26(10):1421-1432. doi: 10.1592/phco.26.10.1421. PubMed
10. Dharmarajan TS, Mahajan D, Zambrano A, et al. Sliding scale insulin vs basal-bolus insulin therapy in long-term care: a 21-day randomized controlled trial comparing efficacy, safety and feasibility. J Am Med Dir Assoc. 2016;17(3):206-213. doi: 10.1016/j.jamda.2015.08.015. PubMed
11. Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30(9):2181-2186. doi: 10.2337/dc07-0295. PubMed
12. Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256-261. doi: 10.2337/dc10-1407. PubMed
13. Phillips VL, Byrd AL, Adeel S, Peng L, Smiley DD, Umpierrez GE. A comparison of inpatient cost per day in general surgery patients with type 2 diabetes treated with basal-bolus versus sliding scale insulin regimens. Pharmacoecon Open. 2017;1(2):109-115. doi: 10.1007/s41669-017-0020-9.. PubMed
14. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982. doi: 10.1210/jcem.87.3.8341. PubMed
15. Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157(5):545-552. PubMed
16. Gearhart JG, Duncan JL, 3rd, Replogle WH, Forbes RC, Walley EJ. Efficacy of sliding-scale insulin therapy: a comparison with prospective regimens. Fam Pract Res J. 1994;14(4):313-322. PubMed
17. American Diabetes A. 14. Diabetes care in the hospital: Standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S144-S151. doi: 10.2337/dc18-S014. PubMed
18. Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353-369. doi: 10.2337/dc09-9029. PubMed
19. Maynard G, Wesorick DH, O’Malley C, Inzucchi SE, Society of Hospital Medicine Glycemic Control Task F. Subcutaneous insulin order sets and protocols: effective design and implementation strategies. J Hosp Med. 2008;3(5 Suppl):29-41. doi: 10.1002/jhm.354. PubMed
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A CLINICAL SCENARIO
A 60-year-old man with a past medical history of obesity and type 2 diabetes presented to the emergency department with one week of myalgias and fever up to 103.5°F (39.7°C). Other vital signs were normal. He had no localizing symptoms, and physical examination was unrevealing, except for a small scab from a tick bite sustained two weeks prior to symptom onset. Before admission, he had been managing his diabetes with metformin 1,000 mg twice a day, and on arrival, his blood sugar level was 275 mg/dL. The admitting provider decided to hold the patient’s metformin and replace it with insulin per a sliding scale. Is monotherapy with sliding-scale insulin the best inpatient management option for this patient’s type 2 diabetes?
WHY YOU MIGHT THINK SLIDING-SCALE INSULIN AS MONOTHERAPY IS HELPFUL
The basic premise of sliding-scale insulin (SSI) is to correct hyperglycemia through the frequent administration of short-acting insulin dosed according to a patient’s blood glucose level with the help of a prespecified rubric. When blood glucose levels are low, patients receive little or no insulin, and when blood glucose levels are high, higher doses are given. This approach to inpatient blood glucose management was first popularized by Joslin in 1934,1 and it remains a common strategy today. For example, a 2007 survey of 44 hospitals in the United States showed that approximately 43% of all noncritically ill patients with hyperglycemia were treated with SSI alone.2 More recently, a single-center study showed that 30% of clinicians continued to use SSI as monotherapy even after the implementation of order sets designed to limit this practice.3
The rationale for SSI as monotherapy appears to have two components. First, guidelines suggest that certain patients should be screened periodically in the hospital for hyperglycemia (blood glucose persistently greater than 180 mg/dL) and that, if identified, hyperglycemia should be treated.4 By pairing finger-stick glucose monitoring with SSI, the diagnosis and treatment—although not the prevention—of hyperglycemia can be accomplished simultaneously. Second, inpatient providers do not want to cause harm in the form of hypoglycemia. SSI as monotherapy is sometimes viewed as a cautious approach in this regard as insulin is administered only if the blood sugar level is high.
Convenience is probably another key contributor to the enduring use of SSI as monotherapy. Several hospitals have ready-made order sets for SSI that are easier to prescribe than a patient-specific regimen including both short- and long-acting insulin. In at least one single-center survey, physicians and staff were found to favor convenience over perceived efficacy when asked about their attitudes toward inpatient glycemic control.5 Although efforts at individual hospitals to change practice patterns among residents have shown promise,6 reform on a broader scale remains elusive.
WHY SSI AS MONOTHERAPY IS NOT HELPFUL
SSI administration does not attempt to replicate normal pancreatic physiology, which involves basal insulin secretion to impair hepatic gluconeogenesis and meal-associated insulin spikes to promote uptake into glucose-avid tissues. SSI is a reactive strategy, not a proactive one, and perhaps unsurprisingly, to our knowledge, it has never been shown to prevent hyperglycemia in hospitalized patients, an impression corroborated by a systematic review of the topic between 1964 and 2003.7 More recently, one multicenter trial analyzed the effect of adding SSI to oral antihyperglycemic medications in hospitalized diabetics and found no differences in rates of hyperglycemia.8 Another study found that 84% of administered SSI doses failed to correct hyperglycemia.9
However, does adding basal insulin to SSI raise a patient’s risk of hypoglycemia? When basal insulin is dosed carefully, the answer appears to be no. In a trial in which diabetic long-term care residents who were receiving SSI at baseline were randomized to either continued SSI or basal-bolus insulin, the investigators found that the basal-bolus group experienced significantly lower average blood glucose levels without an increase in adverse glycemic events.10 Perhaps the most significant milestone to date, however, was the RABBIT 2 multicenter trial, published in 2007, that randomized hospitalized, insulin-naïve diabetics to either a weight-based regimen of basal and prandial insulin or SSI only.11 Rates of hypoglycemia and length of stay did not differ between the groups, and 66% of patients receiving basal-prandial insulin achieved their glycemic control target as opposed to just 38% of patients in the SSI-only group. The SSI group also required more total insulin. A weight-based, basal-bolus strategy was later proven to be similarly effective, without causing severe hypoglycemia, for patients undergoing surgery who could not maintain consistent oral alimentation.12 Basal-bolus insulin was associated with fewer surgical complications, and it produced a cost savings of $751 per day as determined by a post hoc comparative effectiveness study.13
Prolonged use of SSI as monotherapy may be not only ineffective but also harmful. Clearly, the absence of basal insulin will harm type 1 diabetics, who need basal insulin to prevent diabetic ketoacidosis. However, even for type 2 diabetics and nondiabetics, hyperglycemia has been established as a marker for adverse outcomes among hospitalized patients,14 and SSI monotherapy has been associated with a three-fold higher risk of hyperglycemia compared with the use of a sliding scale plus other forms of insulin.15 At least one other study has also linked this practice with a significantly increased length of stay compared with patients who were receiving insulin proactively.16 We believe that the potential for harm is difficult to disregard, especially because safer alternatives are available. Ultimately, it can be stated that in hospitalized patients with persistent hyperglycemia who require insulin, SSI alone should not be the preferred treatment choice regardless of whether the patient carries a known diagnosis of diabetes mellitus or has used insulin previously.
WHEN YOU MIGHT CONSIDER USING SSI AS MONOTHERAPY
As discussed above, there is no known clinical scenario in which SSI as monotherapy has been proven to be effective; however, the use of SSI as monotherapy as a short-term approach has not been well studied. Hospitalized patients who are at risk for adverse glycemic events should be monitored with periodic finger-stick blood glucose draws per guidelines, and in the first 24 hours, it may be reasonable to withhold basal insulin for insulin-naive patients, particularly if the medication reconciliation or other key components of the history are in doubt, or if there are risk factors for hypoglycemia such as a history of bariatric surgery. The amount of insulin received in the first 24 hours of such monitoring may inform subsequent insulin dosing, but this method of “dose finding” has not been validated in the literature.
Uncertain or interrupted alimentation status or stress hyperglycemia may complicate the assessment of a patient’s insulin needs. One of the insights from the RABBIT 2 surgery trial is that even with interrupted alimentation, patients on a weight-based, long-acting insulin regimen did not experience severe hypoglycemia. Nevertheless, if a patient without type 1 diabetes is felt to be at high risk for a severe hypoglycemic event, it may be prudent to withhold long-acting insulin. However, in that situation, adding SSI to finger-stick monitoring is unlikely to be beneficial. Cases of stress hyperglycemia in nondiabetics can also be challenging, as the persistence of hyperglycemia can be difficult to predict. Guidelines state that if hyperglycemia is persistent, then insulin therapy should be initiated and that this therapy is best accomplished in the form of a basal-prandial regimen.17
WHAT YOU SHOULD DO INSTEAD
Current guidelines from the American Diabetes Association17 and the American Association of Clinical Endocrinologists18 for hospitalized patients with hyperglycemia who require insulin recommend against the prolonged use of SSI as monotherapy (category A recommendation) and support the use of basal plus correctional insulin with the addition of nutritional insulin for patients with consistent oral intake (category A recommendation). Although a complete discourse on the determination of the appropriate starting dose of insulin is outside of the scope of this cas presentation, the basic approach begins with calculating a weight-based total daily dose of insulin, approximately half of which can be given as basal insulin with the remainder given with meals along with correctional insulin as needed to account for premeal hyperglycemia.4 For example, the protocol used in the RABBIT 2 trial, which involved known type 2 diabetics, started insulin based on a total daily dose of 0.4 units/kg for patients presenting with blood sugar levels ≤200 mg/dL and 0.5 units/kg for those with higher initial glucose levels.7 Half of the total daily dose was given as basal insulin, and the other half was divided among meals. Caution with insulin dosing may be required in patients aged >70 years, in those with impaired renal function, and in situations in which steroid doses are fluctuating. The Society of Hospital Medicine has formulated an online subcutaneous insulin order implementation guideline, eQUIPS, that can be a helpful resource to centers that are interested in changing their practice patterns.19
RECOMMENDATIONS
- Instead of using SSI monotherapy for hospitalized patients who require insulin, add basal and prandial insulin, using a weight-based approach if necessary for insulin-naive patients.
- Engage with leadership at your center to learn how inpatient hyperglycemia protocols and blood sugar management teams can help provide evidence-based and individualized treatment plans for your patients.
- If no infrastructure exists at your center, the Society of Hospital Medicine offers training and guidance through its eQUIPS inpatient hyperglycemia management program.
CONCLUSION
In the case presentation, the hyperglycemic patient whose metformin was on hold should have been started on a combination of basal and prandial insulin as determined by his weight and current renal function as opposed to monotherapy with SSI. Using SSI as monotherapy for hyperglycemia is a common practice, and although well-intentioned, it is an ineffective and possibly dangerous approach. Continued efforts must be made to address the gap between guidelines and suboptimal practice patterns locally and nationally.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailingTWDFNR@hospitalmedicine.org.
Acknowledgments
The authors would like to thank Dr. Asem Ali of the Division of Endocrinology at UMass Memorial Medical Center for his review of the manuscript.
Disclosures
The authors have nothing to disclose.
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A CLINICAL SCENARIO
A 60-year-old man with a past medical history of obesity and type 2 diabetes presented to the emergency department with one week of myalgias and fever up to 103.5°F (39.7°C). Other vital signs were normal. He had no localizing symptoms, and physical examination was unrevealing, except for a small scab from a tick bite sustained two weeks prior to symptom onset. Before admission, he had been managing his diabetes with metformin 1,000 mg twice a day, and on arrival, his blood sugar level was 275 mg/dL. The admitting provider decided to hold the patient’s metformin and replace it with insulin per a sliding scale. Is monotherapy with sliding-scale insulin the best inpatient management option for this patient’s type 2 diabetes?
WHY YOU MIGHT THINK SLIDING-SCALE INSULIN AS MONOTHERAPY IS HELPFUL
The basic premise of sliding-scale insulin (SSI) is to correct hyperglycemia through the frequent administration of short-acting insulin dosed according to a patient’s blood glucose level with the help of a prespecified rubric. When blood glucose levels are low, patients receive little or no insulin, and when blood glucose levels are high, higher doses are given. This approach to inpatient blood glucose management was first popularized by Joslin in 1934,1 and it remains a common strategy today. For example, a 2007 survey of 44 hospitals in the United States showed that approximately 43% of all noncritically ill patients with hyperglycemia were treated with SSI alone.2 More recently, a single-center study showed that 30% of clinicians continued to use SSI as monotherapy even after the implementation of order sets designed to limit this practice.3
The rationale for SSI as monotherapy appears to have two components. First, guidelines suggest that certain patients should be screened periodically in the hospital for hyperglycemia (blood glucose persistently greater than 180 mg/dL) and that, if identified, hyperglycemia should be treated.4 By pairing finger-stick glucose monitoring with SSI, the diagnosis and treatment—although not the prevention—of hyperglycemia can be accomplished simultaneously. Second, inpatient providers do not want to cause harm in the form of hypoglycemia. SSI as monotherapy is sometimes viewed as a cautious approach in this regard as insulin is administered only if the blood sugar level is high.
Convenience is probably another key contributor to the enduring use of SSI as monotherapy. Several hospitals have ready-made order sets for SSI that are easier to prescribe than a patient-specific regimen including both short- and long-acting insulin. In at least one single-center survey, physicians and staff were found to favor convenience over perceived efficacy when asked about their attitudes toward inpatient glycemic control.5 Although efforts at individual hospitals to change practice patterns among residents have shown promise,6 reform on a broader scale remains elusive.
WHY SSI AS MONOTHERAPY IS NOT HELPFUL
SSI administration does not attempt to replicate normal pancreatic physiology, which involves basal insulin secretion to impair hepatic gluconeogenesis and meal-associated insulin spikes to promote uptake into glucose-avid tissues. SSI is a reactive strategy, not a proactive one, and perhaps unsurprisingly, to our knowledge, it has never been shown to prevent hyperglycemia in hospitalized patients, an impression corroborated by a systematic review of the topic between 1964 and 2003.7 More recently, one multicenter trial analyzed the effect of adding SSI to oral antihyperglycemic medications in hospitalized diabetics and found no differences in rates of hyperglycemia.8 Another study found that 84% of administered SSI doses failed to correct hyperglycemia.9
However, does adding basal insulin to SSI raise a patient’s risk of hypoglycemia? When basal insulin is dosed carefully, the answer appears to be no. In a trial in which diabetic long-term care residents who were receiving SSI at baseline were randomized to either continued SSI or basal-bolus insulin, the investigators found that the basal-bolus group experienced significantly lower average blood glucose levels without an increase in adverse glycemic events.10 Perhaps the most significant milestone to date, however, was the RABBIT 2 multicenter trial, published in 2007, that randomized hospitalized, insulin-naïve diabetics to either a weight-based regimen of basal and prandial insulin or SSI only.11 Rates of hypoglycemia and length of stay did not differ between the groups, and 66% of patients receiving basal-prandial insulin achieved their glycemic control target as opposed to just 38% of patients in the SSI-only group. The SSI group also required more total insulin. A weight-based, basal-bolus strategy was later proven to be similarly effective, without causing severe hypoglycemia, for patients undergoing surgery who could not maintain consistent oral alimentation.12 Basal-bolus insulin was associated with fewer surgical complications, and it produced a cost savings of $751 per day as determined by a post hoc comparative effectiveness study.13
Prolonged use of SSI as monotherapy may be not only ineffective but also harmful. Clearly, the absence of basal insulin will harm type 1 diabetics, who need basal insulin to prevent diabetic ketoacidosis. However, even for type 2 diabetics and nondiabetics, hyperglycemia has been established as a marker for adverse outcomes among hospitalized patients,14 and SSI monotherapy has been associated with a three-fold higher risk of hyperglycemia compared with the use of a sliding scale plus other forms of insulin.15 At least one other study has also linked this practice with a significantly increased length of stay compared with patients who were receiving insulin proactively.16 We believe that the potential for harm is difficult to disregard, especially because safer alternatives are available. Ultimately, it can be stated that in hospitalized patients with persistent hyperglycemia who require insulin, SSI alone should not be the preferred treatment choice regardless of whether the patient carries a known diagnosis of diabetes mellitus or has used insulin previously.
WHEN YOU MIGHT CONSIDER USING SSI AS MONOTHERAPY
As discussed above, there is no known clinical scenario in which SSI as monotherapy has been proven to be effective; however, the use of SSI as monotherapy as a short-term approach has not been well studied. Hospitalized patients who are at risk for adverse glycemic events should be monitored with periodic finger-stick blood glucose draws per guidelines, and in the first 24 hours, it may be reasonable to withhold basal insulin for insulin-naive patients, particularly if the medication reconciliation or other key components of the history are in doubt, or if there are risk factors for hypoglycemia such as a history of bariatric surgery. The amount of insulin received in the first 24 hours of such monitoring may inform subsequent insulin dosing, but this method of “dose finding” has not been validated in the literature.
Uncertain or interrupted alimentation status or stress hyperglycemia may complicate the assessment of a patient’s insulin needs. One of the insights from the RABBIT 2 surgery trial is that even with interrupted alimentation, patients on a weight-based, long-acting insulin regimen did not experience severe hypoglycemia. Nevertheless, if a patient without type 1 diabetes is felt to be at high risk for a severe hypoglycemic event, it may be prudent to withhold long-acting insulin. However, in that situation, adding SSI to finger-stick monitoring is unlikely to be beneficial. Cases of stress hyperglycemia in nondiabetics can also be challenging, as the persistence of hyperglycemia can be difficult to predict. Guidelines state that if hyperglycemia is persistent, then insulin therapy should be initiated and that this therapy is best accomplished in the form of a basal-prandial regimen.17
WHAT YOU SHOULD DO INSTEAD
Current guidelines from the American Diabetes Association17 and the American Association of Clinical Endocrinologists18 for hospitalized patients with hyperglycemia who require insulin recommend against the prolonged use of SSI as monotherapy (category A recommendation) and support the use of basal plus correctional insulin with the addition of nutritional insulin for patients with consistent oral intake (category A recommendation). Although a complete discourse on the determination of the appropriate starting dose of insulin is outside of the scope of this cas presentation, the basic approach begins with calculating a weight-based total daily dose of insulin, approximately half of which can be given as basal insulin with the remainder given with meals along with correctional insulin as needed to account for premeal hyperglycemia.4 For example, the protocol used in the RABBIT 2 trial, which involved known type 2 diabetics, started insulin based on a total daily dose of 0.4 units/kg for patients presenting with blood sugar levels ≤200 mg/dL and 0.5 units/kg for those with higher initial glucose levels.7 Half of the total daily dose was given as basal insulin, and the other half was divided among meals. Caution with insulin dosing may be required in patients aged >70 years, in those with impaired renal function, and in situations in which steroid doses are fluctuating. The Society of Hospital Medicine has formulated an online subcutaneous insulin order implementation guideline, eQUIPS, that can be a helpful resource to centers that are interested in changing their practice patterns.19
RECOMMENDATIONS
- Instead of using SSI monotherapy for hospitalized patients who require insulin, add basal and prandial insulin, using a weight-based approach if necessary for insulin-naive patients.
- Engage with leadership at your center to learn how inpatient hyperglycemia protocols and blood sugar management teams can help provide evidence-based and individualized treatment plans for your patients.
- If no infrastructure exists at your center, the Society of Hospital Medicine offers training and guidance through its eQUIPS inpatient hyperglycemia management program.
CONCLUSION
In the case presentation, the hyperglycemic patient whose metformin was on hold should have been started on a combination of basal and prandial insulin as determined by his weight and current renal function as opposed to monotherapy with SSI. Using SSI as monotherapy for hyperglycemia is a common practice, and although well-intentioned, it is an ineffective and possibly dangerous approach. Continued efforts must be made to address the gap between guidelines and suboptimal practice patterns locally and nationally.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailingTWDFNR@hospitalmedicine.org.
Acknowledgments
The authors would like to thank Dr. Asem Ali of the Division of Endocrinology at UMass Memorial Medical Center for his review of the manuscript.
Disclosures
The authors have nothing to disclose.
1. Joslin EP. A Diabetic Manual for the Mutual Use of Doctor and Patient. Philadelphia, PA: Lea & Febiger; 1934:108.
2. Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care. 2007;30(2):367-369. doi: 10.2337/dc06-1715. PubMed
3. Valgardson JD, Merino M, Redgrave J, Hudson JI, Hudson MS. Effectiveness of inpatient insulin order sets using human insulins in noncritically ill patients in a rural hospital. Endocr Pract. 2015;21(7):794-806. doi: 10.4158/EP14153. PubMed
4. Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553-591. doi: 10.2337/diacare.27.2.553. PubMed
5. Beliard R, Muzykovsky K, Vincent W, 3rd, Shah B, Davanos E. Perceptions, barriers, and knowledge of inpatient glycemic control: a survey of health care workers. J Pharm Pract. 2016;29(4):348-354. doi: 10.1177/0897190014566309. PubMed
6. Baldwin D, Villanueva G, McNutt R, Bhatnagar S. Eliminating inpatient sliding-scale insulin: a reeducation project with medical house staff. Diabetes Care. 2005;28(5):1008-1011. doi: 10.2337/diacare.28.12.2987-a. PubMed
7. Browning LA, Dumo P. Sliding-scale insulin: an antiquated approach to glycemic control in hospitalized patients. Am J Health Syst Pharm. 2004;61(15):1611-1614. PubMed
8. Dickerson LM, Ye X, Sack JL, Hueston WJ. Glycemic control in medical inpatients with type 2 diabetes mellitus receiving sliding scale insulin regimens versus routine diabetes medications: a multicenter randomized controlled trial. Ann Fam Med. 2003;1(1):29-35. PubMed
9. Golightly LK, Jones MA, Hamamura DH, Stolpman NM, McDermott MT. Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding-scale insulin therapy. Pharmacotherapy. 2006;26(10):1421-1432. doi: 10.1592/phco.26.10.1421. PubMed
10. Dharmarajan TS, Mahajan D, Zambrano A, et al. Sliding scale insulin vs basal-bolus insulin therapy in long-term care: a 21-day randomized controlled trial comparing efficacy, safety and feasibility. J Am Med Dir Assoc. 2016;17(3):206-213. doi: 10.1016/j.jamda.2015.08.015. PubMed
11. Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30(9):2181-2186. doi: 10.2337/dc07-0295. PubMed
12. Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256-261. doi: 10.2337/dc10-1407. PubMed
13. Phillips VL, Byrd AL, Adeel S, Peng L, Smiley DD, Umpierrez GE. A comparison of inpatient cost per day in general surgery patients with type 2 diabetes treated with basal-bolus versus sliding scale insulin regimens. Pharmacoecon Open. 2017;1(2):109-115. doi: 10.1007/s41669-017-0020-9.. PubMed
14. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982. doi: 10.1210/jcem.87.3.8341. PubMed
15. Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157(5):545-552. PubMed
16. Gearhart JG, Duncan JL, 3rd, Replogle WH, Forbes RC, Walley EJ. Efficacy of sliding-scale insulin therapy: a comparison with prospective regimens. Fam Pract Res J. 1994;14(4):313-322. PubMed
17. American Diabetes A. 14. Diabetes care in the hospital: Standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S144-S151. doi: 10.2337/dc18-S014. PubMed
18. Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353-369. doi: 10.2337/dc09-9029. PubMed
19. Maynard G, Wesorick DH, O’Malley C, Inzucchi SE, Society of Hospital Medicine Glycemic Control Task F. Subcutaneous insulin order sets and protocols: effective design and implementation strategies. J Hosp Med. 2008;3(5 Suppl):29-41. doi: 10.1002/jhm.354. PubMed
1. Joslin EP. A Diabetic Manual for the Mutual Use of Doctor and Patient. Philadelphia, PA: Lea & Febiger; 1934:108.
2. Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care. 2007;30(2):367-369. doi: 10.2337/dc06-1715. PubMed
3. Valgardson JD, Merino M, Redgrave J, Hudson JI, Hudson MS. Effectiveness of inpatient insulin order sets using human insulins in noncritically ill patients in a rural hospital. Endocr Pract. 2015;21(7):794-806. doi: 10.4158/EP14153. PubMed
4. Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553-591. doi: 10.2337/diacare.27.2.553. PubMed
5. Beliard R, Muzykovsky K, Vincent W, 3rd, Shah B, Davanos E. Perceptions, barriers, and knowledge of inpatient glycemic control: a survey of health care workers. J Pharm Pract. 2016;29(4):348-354. doi: 10.1177/0897190014566309. PubMed
6. Baldwin D, Villanueva G, McNutt R, Bhatnagar S. Eliminating inpatient sliding-scale insulin: a reeducation project with medical house staff. Diabetes Care. 2005;28(5):1008-1011. doi: 10.2337/diacare.28.12.2987-a. PubMed
7. Browning LA, Dumo P. Sliding-scale insulin: an antiquated approach to glycemic control in hospitalized patients. Am J Health Syst Pharm. 2004;61(15):1611-1614. PubMed
8. Dickerson LM, Ye X, Sack JL, Hueston WJ. Glycemic control in medical inpatients with type 2 diabetes mellitus receiving sliding scale insulin regimens versus routine diabetes medications: a multicenter randomized controlled trial. Ann Fam Med. 2003;1(1):29-35. PubMed
9. Golightly LK, Jones MA, Hamamura DH, Stolpman NM, McDermott MT. Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding-scale insulin therapy. Pharmacotherapy. 2006;26(10):1421-1432. doi: 10.1592/phco.26.10.1421. PubMed
10. Dharmarajan TS, Mahajan D, Zambrano A, et al. Sliding scale insulin vs basal-bolus insulin therapy in long-term care: a 21-day randomized controlled trial comparing efficacy, safety and feasibility. J Am Med Dir Assoc. 2016;17(3):206-213. doi: 10.1016/j.jamda.2015.08.015. PubMed
11. Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30(9):2181-2186. doi: 10.2337/dc07-0295. PubMed
12. Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256-261. doi: 10.2337/dc10-1407. PubMed
13. Phillips VL, Byrd AL, Adeel S, Peng L, Smiley DD, Umpierrez GE. A comparison of inpatient cost per day in general surgery patients with type 2 diabetes treated with basal-bolus versus sliding scale insulin regimens. Pharmacoecon Open. 2017;1(2):109-115. doi: 10.1007/s41669-017-0020-9.. PubMed
14. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982. doi: 10.1210/jcem.87.3.8341. PubMed
15. Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157(5):545-552. PubMed
16. Gearhart JG, Duncan JL, 3rd, Replogle WH, Forbes RC, Walley EJ. Efficacy of sliding-scale insulin therapy: a comparison with prospective regimens. Fam Pract Res J. 1994;14(4):313-322. PubMed
17. American Diabetes A. 14. Diabetes care in the hospital: Standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S144-S151. doi: 10.2337/dc18-S014. PubMed
18. Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353-369. doi: 10.2337/dc09-9029. PubMed
19. Maynard G, Wesorick DH, O’Malley C, Inzucchi SE, Society of Hospital Medicine Glycemic Control Task F. Subcutaneous insulin order sets and protocols: effective design and implementation strategies. J Hosp Med. 2008;3(5 Suppl):29-41. doi: 10.1002/jhm.354. PubMed
© 2018 Society of Hospital Medicine
Should the Diagnosis of UTI in Young Febrile Infants Require a Positive Urinalysis?
Reduction of antibiotic overuse is an important goal for improving the quality of care for children and is highlighted in many of the Choosing Wisely® recommendations across disciplines.1-3 However, the evidence supporting these recommendations vary widely and many are derived from expert opinion and clinical practice guidelines rather than from original research studies.2 In this issue of the Journal of Hospital Medicine, Schroeder and colleagues identify a potential area of antibiotic overuse among young febrile infants with possible urinary tract infection (UTI).4 A wide variation in antibiotic treatment rates (0%-35%) was observed across 124 hospitals in the United States for febrile infants 7-60 days of age with uropathogen detection by urine culture but a negative urinalysis (UA). Treated infants with a negative UA were more likely to be younger (7-30 days), have respiratory symptoms, and were less likely to have abnormal inflammatory markers than infants with a positive UA.
Clinicians faced with the decision of whether or not to treat a febrile infant with uropathogen detection in the setting of a negative UA must weigh the potential benefits and harms of antibiotic use in this population. Withholding antibiotics for a young infant with UTI may increase the risk of recurrent UTI and renal scarring,5,6 while antibiotic treatment in young infants can lead to the disruption of the gut microbiome, resulting in long-term consequences that are only beginning to be understood.7-10
The American Academy of Pediatrics (AAP) UTI practice parameter requires a positive UA to establish the diagnosis of UTI in children 2-24 months of age.11 This recommendation is based primarily on studies demonstrating that uropathogen detection in the setting of a negative UA commonly represents asymptomatic bacteriuria or contamination rather than true infection.12-14 This is supported by research showing that the UA demonstrates near perfect (>99%) sensitivity for UTI in children with bacteremic UTI,12,15 and studies demonstrating lower rates of subsequent urinary infections and renal injury among infants with uropathogen detection and a negative UA compared with those with uropathogen detection and a positive UA.13,14,16
An important question is whether febrile infants within the first two months of life with uropathogen detection should be treated with antimicrobials regardless of UA findings or specifically in the setting of a negative UA. The AAP practice guideline11 deliberately omits these young infants, recognizing that evidence derived from studies of older infants and children may not be applicable to this young age group, as they may not mount as robust an inflammatory response and thus may not demonstrate pyuria in the setting of a bacterial urinary infection. Schroeder et al. demonstrate lower rates of abnormal inflammatory markers in UA negative compared with UA positive infants, a finding the authors argue supports the possibility of asymptomatic bacteriuria or contamination rather than true infection.4 The counterargument is that young infants may not mount a significant inflammatory response to true infections.
The authors appropriately highlight the paucity of literature to help differentiate true infection from asymptomatic bacteriuria or contamination in infants less than two months of age. As infants in this age group are usually treated with antibiotics for a positive urine culture regardless of UA result, robust data on short- and long-term outcomes of untreated infants are lacking. Much of the existing literature evaluates the test performance of the UA for UTI using the urine culture as the reference standard, which presents inherent limitations with incorporating the results of the UA into the definition of UTI using these data. Additionally, reported test performance of the UA for UTI varies by uropathogen type,17 fever duration,18 associated bacteremia,19 and urine concentration,20 which are important considerations when applying a strict definition of UTI that includes the UA in this age group. Conversely, more recent studies have demonstrated improved sensitivity of the dipstick and microscopic UA for the detection of UTI.15,20,21 The improved test performance may not only enhance the use of the UA as a screen for UTI in this high-risk population but also allow its potential inclusion into the definition of UTI as the authors suggest, as previous false-negative UTIs would be less frequent with improved UA testing modalities.
Ultimately, what’s missing from the equation is whether treatment of young febrile infants with uropathogen detection in the setting of a negative UA affects either short-term or long-term complications of UTI. Unfortunately, limited information exists to help inform the decision to initiate antibiotic treatment for these infants. Ideally, this question can only be answered by either an observational study evaluating outcomes of untreated infants or a randomized trial of antibiotics for infants less than two months of age with uropathogen detection in the setting of a negative UA. Until then, we may continue to observe a wide variation in antibiotic treatment rates for febrile young infants with uropathogen detection in the setting of a negative UA.
Disclosures
The authors have nothing to disclose.
1. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479-485. doi: 10.1002/jhm.2064. PubMed
2. Admon AJ, Gupta A, Williams M, et al. Appraising the evidence supporting Choosing Wisely® recommendations. J Hosp Med. 2018;13(10):688-691. doi: 10.12788/jhm.2964. PubMed
3. Reyes M, Paulus E, Hronek C, et al. Choosing Wisely Campaign: Report card and achievable benchmarks of care for children’s hospitals. Hosp Pediatr. 2017;7(11):633-641. doi: 10.1542/hpeds.2017-0029. PubMed
4. Schroeder AR, Lucas BP, Garber MD, McCulloh RJ, Joshi-Patel AA BE. Negative urinalyses in febrile infants 7-60 days of age treated for urinary tract infection. J Hosp Med. 2019;14(2):101-104. doi: 10.12788/jhm.3120..
5. Shaikh N, Mattoo TK, Keren R, et al. Early antibiotic treatment for pediatric febrile urinary tract infection and renal scarring. JAMA Pediatr. 2016;170(9):848-854. doi: 10.1001/jamapediatrics.2016.1181. PubMed
6. Keren R, Shaikh N, Pohl H, et al. Risk factors for recurrent urinary tract infection and renal scarring. Pediatrics. 2015;136(1):e13-e21. doi: 10.1542/peds.2015-0409. PubMed
7. Stiemsma LT, Michels KB. The role of the microbiome in the developmental origins of health and disease. Pediatrics. 2018;141(4):e20172437. doi: 10.1542/peds.2017-2437. PubMed
8. Gibson MK, Crofts TS, Dantas G. Antibiotics and the developing infant gut microbiota and resistome. Curr Opin Microbiol. 2015;27:51-56. doi: 10.1016/j.mib.2015.07.007. PubMed
9. Arboleya S, Sánchez B, Milani C, et al. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J Pediatr. 2015;166(3):538-544. doi: 10.1016/j.jpeds.2014.09.041. PubMed
10. Dardas M, Gill SR, Grier A, et al. The impact of postnatal antibiotics on the preterm intestinal microbiome. Pediatr Res. 2014;76(2):150-158. doi: 10.1038/pr.2014.69. PubMed
11. Roberts KB, Downs SM, Finnell SM, et al. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128(3):595-610. doi: 10.1542/peds.2011-1330. PubMed
12. Schroeder AR, Chang PW, Shen MW, Biondi EA, Greenhow TL. Diagnostic accuracy of the urinalysis for urinary tract infection in infants < 3 months of age. Pediatrics. 2015;135(6):965-971. doi: 10.1542/peds.2015-0012. PubMed
13. Wettergren B, Hellström M, Stokland E, Jodal U. Six year follow up of infants with bacteriuria on screening. BMJ. 1990;301(6756):845-848. doi: 10.1136/bmj.301.6756.845. PubMed
14. Wettergren B, Jodal U. Spontaneous clearance of asymptomatic bacteriuria in infants. Acta Paediatr Scand. 1990;79(3):300-304. doi: 10.1111/j.1651-2227.1990.tb11460.x. PubMed
15. Tzimenatos L, Mahajan P, Dayan PS, et al. Accuracy of the urinalysis for urinary tract infections in febrile infants 60 days and younger. Pediatrics. 2018;141(2):e20173068. doi: 10.1542/peds.2017-3068. PubMed
16. Wettergren B, Jodal U, Jonasson G. Epidemiology of bacteriuria during the first year of life. Acta Paediatr Scand. 1985;74(6):925-933. doi: 10.1111/j.1651-2227.1985.tb10059.x. PubMed
17. Shaikh N, Shope TR, Hoberman A, Vigliotti A, Kurs-Lasky M, Martin JM. Association between uropathogen and pyuria. Pediatrics. 2016;138(1):e20160087. doi: 10.1542/peds.2016-0087. PubMed
18. Hoberman A, Wald ER, Reynolds EA, Penchansky L, Charron M. Is urine culture necessary to rule out urinary tract infection in young febrile children? Pediatr Infect Dis J. 1996;15(4):304-309. PubMed
19. Roman HK, Chang PW, Schroeder AR. Diagnosis and management of bacteremic urinary tract infection in infants. Hosp Pediatr. 2015;5(1):1-8. doi: 10.1542/hpeds.2014-0051. PubMed
20. Chaudhari PP, Monuteaux MC, Bachur RG. Urine concentration and pyuria for identifying UTI in infants. Pediatrics. 2016;138(5):e20162370. PubMed
21. Glissmeyer EW, Korgenski EK, Wilkes J, et al. Dipstick screening for urinary tract infection in febrile infants. Pediatrics. 2014;133(5):e1121-e1127. doi: 10.1542/peds.2013-3291. PubMed
Reduction of antibiotic overuse is an important goal for improving the quality of care for children and is highlighted in many of the Choosing Wisely® recommendations across disciplines.1-3 However, the evidence supporting these recommendations vary widely and many are derived from expert opinion and clinical practice guidelines rather than from original research studies.2 In this issue of the Journal of Hospital Medicine, Schroeder and colleagues identify a potential area of antibiotic overuse among young febrile infants with possible urinary tract infection (UTI).4 A wide variation in antibiotic treatment rates (0%-35%) was observed across 124 hospitals in the United States for febrile infants 7-60 days of age with uropathogen detection by urine culture but a negative urinalysis (UA). Treated infants with a negative UA were more likely to be younger (7-30 days), have respiratory symptoms, and were less likely to have abnormal inflammatory markers than infants with a positive UA.
Clinicians faced with the decision of whether or not to treat a febrile infant with uropathogen detection in the setting of a negative UA must weigh the potential benefits and harms of antibiotic use in this population. Withholding antibiotics for a young infant with UTI may increase the risk of recurrent UTI and renal scarring,5,6 while antibiotic treatment in young infants can lead to the disruption of the gut microbiome, resulting in long-term consequences that are only beginning to be understood.7-10
The American Academy of Pediatrics (AAP) UTI practice parameter requires a positive UA to establish the diagnosis of UTI in children 2-24 months of age.11 This recommendation is based primarily on studies demonstrating that uropathogen detection in the setting of a negative UA commonly represents asymptomatic bacteriuria or contamination rather than true infection.12-14 This is supported by research showing that the UA demonstrates near perfect (>99%) sensitivity for UTI in children with bacteremic UTI,12,15 and studies demonstrating lower rates of subsequent urinary infections and renal injury among infants with uropathogen detection and a negative UA compared with those with uropathogen detection and a positive UA.13,14,16
An important question is whether febrile infants within the first two months of life with uropathogen detection should be treated with antimicrobials regardless of UA findings or specifically in the setting of a negative UA. The AAP practice guideline11 deliberately omits these young infants, recognizing that evidence derived from studies of older infants and children may not be applicable to this young age group, as they may not mount as robust an inflammatory response and thus may not demonstrate pyuria in the setting of a bacterial urinary infection. Schroeder et al. demonstrate lower rates of abnormal inflammatory markers in UA negative compared with UA positive infants, a finding the authors argue supports the possibility of asymptomatic bacteriuria or contamination rather than true infection.4 The counterargument is that young infants may not mount a significant inflammatory response to true infections.
The authors appropriately highlight the paucity of literature to help differentiate true infection from asymptomatic bacteriuria or contamination in infants less than two months of age. As infants in this age group are usually treated with antibiotics for a positive urine culture regardless of UA result, robust data on short- and long-term outcomes of untreated infants are lacking. Much of the existing literature evaluates the test performance of the UA for UTI using the urine culture as the reference standard, which presents inherent limitations with incorporating the results of the UA into the definition of UTI using these data. Additionally, reported test performance of the UA for UTI varies by uropathogen type,17 fever duration,18 associated bacteremia,19 and urine concentration,20 which are important considerations when applying a strict definition of UTI that includes the UA in this age group. Conversely, more recent studies have demonstrated improved sensitivity of the dipstick and microscopic UA for the detection of UTI.15,20,21 The improved test performance may not only enhance the use of the UA as a screen for UTI in this high-risk population but also allow its potential inclusion into the definition of UTI as the authors suggest, as previous false-negative UTIs would be less frequent with improved UA testing modalities.
Ultimately, what’s missing from the equation is whether treatment of young febrile infants with uropathogen detection in the setting of a negative UA affects either short-term or long-term complications of UTI. Unfortunately, limited information exists to help inform the decision to initiate antibiotic treatment for these infants. Ideally, this question can only be answered by either an observational study evaluating outcomes of untreated infants or a randomized trial of antibiotics for infants less than two months of age with uropathogen detection in the setting of a negative UA. Until then, we may continue to observe a wide variation in antibiotic treatment rates for febrile young infants with uropathogen detection in the setting of a negative UA.
Disclosures
The authors have nothing to disclose.
Reduction of antibiotic overuse is an important goal for improving the quality of care for children and is highlighted in many of the Choosing Wisely® recommendations across disciplines.1-3 However, the evidence supporting these recommendations vary widely and many are derived from expert opinion and clinical practice guidelines rather than from original research studies.2 In this issue of the Journal of Hospital Medicine, Schroeder and colleagues identify a potential area of antibiotic overuse among young febrile infants with possible urinary tract infection (UTI).4 A wide variation in antibiotic treatment rates (0%-35%) was observed across 124 hospitals in the United States for febrile infants 7-60 days of age with uropathogen detection by urine culture but a negative urinalysis (UA). Treated infants with a negative UA were more likely to be younger (7-30 days), have respiratory symptoms, and were less likely to have abnormal inflammatory markers than infants with a positive UA.
Clinicians faced with the decision of whether or not to treat a febrile infant with uropathogen detection in the setting of a negative UA must weigh the potential benefits and harms of antibiotic use in this population. Withholding antibiotics for a young infant with UTI may increase the risk of recurrent UTI and renal scarring,5,6 while antibiotic treatment in young infants can lead to the disruption of the gut microbiome, resulting in long-term consequences that are only beginning to be understood.7-10
The American Academy of Pediatrics (AAP) UTI practice parameter requires a positive UA to establish the diagnosis of UTI in children 2-24 months of age.11 This recommendation is based primarily on studies demonstrating that uropathogen detection in the setting of a negative UA commonly represents asymptomatic bacteriuria or contamination rather than true infection.12-14 This is supported by research showing that the UA demonstrates near perfect (>99%) sensitivity for UTI in children with bacteremic UTI,12,15 and studies demonstrating lower rates of subsequent urinary infections and renal injury among infants with uropathogen detection and a negative UA compared with those with uropathogen detection and a positive UA.13,14,16
An important question is whether febrile infants within the first two months of life with uropathogen detection should be treated with antimicrobials regardless of UA findings or specifically in the setting of a negative UA. The AAP practice guideline11 deliberately omits these young infants, recognizing that evidence derived from studies of older infants and children may not be applicable to this young age group, as they may not mount as robust an inflammatory response and thus may not demonstrate pyuria in the setting of a bacterial urinary infection. Schroeder et al. demonstrate lower rates of abnormal inflammatory markers in UA negative compared with UA positive infants, a finding the authors argue supports the possibility of asymptomatic bacteriuria or contamination rather than true infection.4 The counterargument is that young infants may not mount a significant inflammatory response to true infections.
The authors appropriately highlight the paucity of literature to help differentiate true infection from asymptomatic bacteriuria or contamination in infants less than two months of age. As infants in this age group are usually treated with antibiotics for a positive urine culture regardless of UA result, robust data on short- and long-term outcomes of untreated infants are lacking. Much of the existing literature evaluates the test performance of the UA for UTI using the urine culture as the reference standard, which presents inherent limitations with incorporating the results of the UA into the definition of UTI using these data. Additionally, reported test performance of the UA for UTI varies by uropathogen type,17 fever duration,18 associated bacteremia,19 and urine concentration,20 which are important considerations when applying a strict definition of UTI that includes the UA in this age group. Conversely, more recent studies have demonstrated improved sensitivity of the dipstick and microscopic UA for the detection of UTI.15,20,21 The improved test performance may not only enhance the use of the UA as a screen for UTI in this high-risk population but also allow its potential inclusion into the definition of UTI as the authors suggest, as previous false-negative UTIs would be less frequent with improved UA testing modalities.
Ultimately, what’s missing from the equation is whether treatment of young febrile infants with uropathogen detection in the setting of a negative UA affects either short-term or long-term complications of UTI. Unfortunately, limited information exists to help inform the decision to initiate antibiotic treatment for these infants. Ideally, this question can only be answered by either an observational study evaluating outcomes of untreated infants or a randomized trial of antibiotics for infants less than two months of age with uropathogen detection in the setting of a negative UA. Until then, we may continue to observe a wide variation in antibiotic treatment rates for febrile young infants with uropathogen detection in the setting of a negative UA.
Disclosures
The authors have nothing to disclose.
1. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479-485. doi: 10.1002/jhm.2064. PubMed
2. Admon AJ, Gupta A, Williams M, et al. Appraising the evidence supporting Choosing Wisely® recommendations. J Hosp Med. 2018;13(10):688-691. doi: 10.12788/jhm.2964. PubMed
3. Reyes M, Paulus E, Hronek C, et al. Choosing Wisely Campaign: Report card and achievable benchmarks of care for children’s hospitals. Hosp Pediatr. 2017;7(11):633-641. doi: 10.1542/hpeds.2017-0029. PubMed
4. Schroeder AR, Lucas BP, Garber MD, McCulloh RJ, Joshi-Patel AA BE. Negative urinalyses in febrile infants 7-60 days of age treated for urinary tract infection. J Hosp Med. 2019;14(2):101-104. doi: 10.12788/jhm.3120..
5. Shaikh N, Mattoo TK, Keren R, et al. Early antibiotic treatment for pediatric febrile urinary tract infection and renal scarring. JAMA Pediatr. 2016;170(9):848-854. doi: 10.1001/jamapediatrics.2016.1181. PubMed
6. Keren R, Shaikh N, Pohl H, et al. Risk factors for recurrent urinary tract infection and renal scarring. Pediatrics. 2015;136(1):e13-e21. doi: 10.1542/peds.2015-0409. PubMed
7. Stiemsma LT, Michels KB. The role of the microbiome in the developmental origins of health and disease. Pediatrics. 2018;141(4):e20172437. doi: 10.1542/peds.2017-2437. PubMed
8. Gibson MK, Crofts TS, Dantas G. Antibiotics and the developing infant gut microbiota and resistome. Curr Opin Microbiol. 2015;27:51-56. doi: 10.1016/j.mib.2015.07.007. PubMed
9. Arboleya S, Sánchez B, Milani C, et al. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J Pediatr. 2015;166(3):538-544. doi: 10.1016/j.jpeds.2014.09.041. PubMed
10. Dardas M, Gill SR, Grier A, et al. The impact of postnatal antibiotics on the preterm intestinal microbiome. Pediatr Res. 2014;76(2):150-158. doi: 10.1038/pr.2014.69. PubMed
11. Roberts KB, Downs SM, Finnell SM, et al. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128(3):595-610. doi: 10.1542/peds.2011-1330. PubMed
12. Schroeder AR, Chang PW, Shen MW, Biondi EA, Greenhow TL. Diagnostic accuracy of the urinalysis for urinary tract infection in infants < 3 months of age. Pediatrics. 2015;135(6):965-971. doi: 10.1542/peds.2015-0012. PubMed
13. Wettergren B, Hellström M, Stokland E, Jodal U. Six year follow up of infants with bacteriuria on screening. BMJ. 1990;301(6756):845-848. doi: 10.1136/bmj.301.6756.845. PubMed
14. Wettergren B, Jodal U. Spontaneous clearance of asymptomatic bacteriuria in infants. Acta Paediatr Scand. 1990;79(3):300-304. doi: 10.1111/j.1651-2227.1990.tb11460.x. PubMed
15. Tzimenatos L, Mahajan P, Dayan PS, et al. Accuracy of the urinalysis for urinary tract infections in febrile infants 60 days and younger. Pediatrics. 2018;141(2):e20173068. doi: 10.1542/peds.2017-3068. PubMed
16. Wettergren B, Jodal U, Jonasson G. Epidemiology of bacteriuria during the first year of life. Acta Paediatr Scand. 1985;74(6):925-933. doi: 10.1111/j.1651-2227.1985.tb10059.x. PubMed
17. Shaikh N, Shope TR, Hoberman A, Vigliotti A, Kurs-Lasky M, Martin JM. Association between uropathogen and pyuria. Pediatrics. 2016;138(1):e20160087. doi: 10.1542/peds.2016-0087. PubMed
18. Hoberman A, Wald ER, Reynolds EA, Penchansky L, Charron M. Is urine culture necessary to rule out urinary tract infection in young febrile children? Pediatr Infect Dis J. 1996;15(4):304-309. PubMed
19. Roman HK, Chang PW, Schroeder AR. Diagnosis and management of bacteremic urinary tract infection in infants. Hosp Pediatr. 2015;5(1):1-8. doi: 10.1542/hpeds.2014-0051. PubMed
20. Chaudhari PP, Monuteaux MC, Bachur RG. Urine concentration and pyuria for identifying UTI in infants. Pediatrics. 2016;138(5):e20162370. PubMed
21. Glissmeyer EW, Korgenski EK, Wilkes J, et al. Dipstick screening for urinary tract infection in febrile infants. Pediatrics. 2014;133(5):e1121-e1127. doi: 10.1542/peds.2013-3291. PubMed
1. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479-485. doi: 10.1002/jhm.2064. PubMed
2. Admon AJ, Gupta A, Williams M, et al. Appraising the evidence supporting Choosing Wisely® recommendations. J Hosp Med. 2018;13(10):688-691. doi: 10.12788/jhm.2964. PubMed
3. Reyes M, Paulus E, Hronek C, et al. Choosing Wisely Campaign: Report card and achievable benchmarks of care for children’s hospitals. Hosp Pediatr. 2017;7(11):633-641. doi: 10.1542/hpeds.2017-0029. PubMed
4. Schroeder AR, Lucas BP, Garber MD, McCulloh RJ, Joshi-Patel AA BE. Negative urinalyses in febrile infants 7-60 days of age treated for urinary tract infection. J Hosp Med. 2019;14(2):101-104. doi: 10.12788/jhm.3120..
5. Shaikh N, Mattoo TK, Keren R, et al. Early antibiotic treatment for pediatric febrile urinary tract infection and renal scarring. JAMA Pediatr. 2016;170(9):848-854. doi: 10.1001/jamapediatrics.2016.1181. PubMed
6. Keren R, Shaikh N, Pohl H, et al. Risk factors for recurrent urinary tract infection and renal scarring. Pediatrics. 2015;136(1):e13-e21. doi: 10.1542/peds.2015-0409. PubMed
7. Stiemsma LT, Michels KB. The role of the microbiome in the developmental origins of health and disease. Pediatrics. 2018;141(4):e20172437. doi: 10.1542/peds.2017-2437. PubMed
8. Gibson MK, Crofts TS, Dantas G. Antibiotics and the developing infant gut microbiota and resistome. Curr Opin Microbiol. 2015;27:51-56. doi: 10.1016/j.mib.2015.07.007. PubMed
9. Arboleya S, Sánchez B, Milani C, et al. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J Pediatr. 2015;166(3):538-544. doi: 10.1016/j.jpeds.2014.09.041. PubMed
10. Dardas M, Gill SR, Grier A, et al. The impact of postnatal antibiotics on the preterm intestinal microbiome. Pediatr Res. 2014;76(2):150-158. doi: 10.1038/pr.2014.69. PubMed
11. Roberts KB, Downs SM, Finnell SM, et al. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128(3):595-610. doi: 10.1542/peds.2011-1330. PubMed
12. Schroeder AR, Chang PW, Shen MW, Biondi EA, Greenhow TL. Diagnostic accuracy of the urinalysis for urinary tract infection in infants < 3 months of age. Pediatrics. 2015;135(6):965-971. doi: 10.1542/peds.2015-0012. PubMed
13. Wettergren B, Hellström M, Stokland E, Jodal U. Six year follow up of infants with bacteriuria on screening. BMJ. 1990;301(6756):845-848. doi: 10.1136/bmj.301.6756.845. PubMed
14. Wettergren B, Jodal U. Spontaneous clearance of asymptomatic bacteriuria in infants. Acta Paediatr Scand. 1990;79(3):300-304. doi: 10.1111/j.1651-2227.1990.tb11460.x. PubMed
15. Tzimenatos L, Mahajan P, Dayan PS, et al. Accuracy of the urinalysis for urinary tract infections in febrile infants 60 days and younger. Pediatrics. 2018;141(2):e20173068. doi: 10.1542/peds.2017-3068. PubMed
16. Wettergren B, Jodal U, Jonasson G. Epidemiology of bacteriuria during the first year of life. Acta Paediatr Scand. 1985;74(6):925-933. doi: 10.1111/j.1651-2227.1985.tb10059.x. PubMed
17. Shaikh N, Shope TR, Hoberman A, Vigliotti A, Kurs-Lasky M, Martin JM. Association between uropathogen and pyuria. Pediatrics. 2016;138(1):e20160087. doi: 10.1542/peds.2016-0087. PubMed
18. Hoberman A, Wald ER, Reynolds EA, Penchansky L, Charron M. Is urine culture necessary to rule out urinary tract infection in young febrile children? Pediatr Infect Dis J. 1996;15(4):304-309. PubMed
19. Roman HK, Chang PW, Schroeder AR. Diagnosis and management of bacteremic urinary tract infection in infants. Hosp Pediatr. 2015;5(1):1-8. doi: 10.1542/hpeds.2014-0051. PubMed
20. Chaudhari PP, Monuteaux MC, Bachur RG. Urine concentration and pyuria for identifying UTI in infants. Pediatrics. 2016;138(5):e20162370. PubMed
21. Glissmeyer EW, Korgenski EK, Wilkes J, et al. Dipstick screening for urinary tract infection in febrile infants. Pediatrics. 2014;133(5):e1121-e1127. doi: 10.1542/peds.2013-3291. PubMed
© 2019 Society of Hospital Medicine
Admittedly Simple? The Quest for Clarity in Medicare Claims Data
Every reader of a certain age will recognize this acronym: ADCVANDIML. In simpler times, we “admitted” to a location: medical intensive care unit, bone marrow transplant unit. At some point, admission orders changed from a synonym for “hospitalize” to chart evidence necessary for inpatient payment to the hospital. In the billing and payment world, “inpatient” and “outpatient” hospitalizations are paid at different rates. Observation stays are one type of “outpatient hospitalization,” a confusing and contradictory term to physicians and patients alike. In their article published in this month’s Journal of Hospital Medicine, Sheehy and colleagues attempt the herculean task of defining a reproducible methodology to identify observation hospital stays using Medicare claims data.1 They highlight the complexity of claims data, the variability of revenue codes used, and the probable high frequency of status changes from outpatient observation to inpatient, and vice-versa, during a single hospitalization. They also argue for reform to simplify payment policy for hospitalized patients.
In October 2013, the Center for Medicare and Medicaid Services (CMS) changed the definition of “inpatient” in the Hospital Inpatient Prospective Payment System rule.2 This change is known colloquially as the “two-midnight rule” and occurred on the heels of several years of Recovery Audit contractor (RAC) retroactive denials of short-stay inpatient payments to hospitals around the country. These denials appear to have been based solely on the visit status under which a claim was billed, rather than a dispute over the actual medical care delivered.3 The RAC audits alleged billions of dollars of improper payment to hospitals and resulted in a log-jam of hundreds of thousands of cases in the federal appeal system.4 The two-midnight rule altered the subjective characterization of an inpatient from patient-based (severity of illness) and physician-based (intensity of service) to an objective, time-based payment definition. For the hospital to submit a claim to Medicare Part A, a medical provider with admitting privileges should expect that the patient will need, for medically necessary reasons, a hospitalization that will span at least two midnights of hospital care. Notable exceptions to the rule include patients undergoing a procedure on the Medicare Inpatient Only list and hospitalizations that include an unplanned mechanical intubation. To receive payment for observation (an outpatient service billed under Part B) the physician must place an observation order in addition to other requirements. At its core, the two-midnight rule is a payment rule, not a patient care rule.
This change in the criteria for an inpatient hospitalization from a subjective to a more objective and measurable time-based criterion might lead us to believe that the process for determining the correct visit status would now be simple. Unfortunately, we are dealing with a messy real-world scenario, where doctors can make different judgments and patients can have an unpredictable hospital course. Physicians are familiar with the issues surrounding the choice of the “correct” admission order. In many hospitals, the Medicare patients in “observation” and those with an “inpatient” order can be on the same floor and even share the same room. From a hospital resource, nurse’s, and physician’s standpoint, the patients are often indistinguishable. While some facilities have observation units often associated with their emergency departments, the elderly and those patients with certain comorbidities can be excluded from these units based on protocols designed to improve outcomes and patient safety.
Additionally, most patients who spend at least one night in the hospital for medical treatment would not think that they could be an “outpatient.” To address this, CMS has produced specific beneficiary information5 and now requires hospitals to provide patients with the Medicare outpatient observation notice (MOON) if patients spend more than 24 hours in observation status.6 Beneficiaries must sign this notice, but unlike those admitted as inpatients, Medicare observation patients have no appeal rights. Recent articles in the lay press highlight the interplay between observation status, out-of-pocket expenses, and impact on postacute care.7,8
Following the implementation of the two-midnight rule, CMS directed the regional Medicare Administrative Contractors to perform audits in every hospital in the country. This has led to system-based processes at most facilities directing the “proper” visit class orders for our patients: direct education to providers, electronic medical record fixes and hard-stops, and real-time communications from the utilization review nurses and staff. These processes, based on a payment rule are burdensome to patients, physicians, and hospital support staff.
It’s not surprising to see that the billing of hospital-based observation care is also a quagmire. The methods and results sections of Sheehy et al.’s article reads like a calculus textbook written in a foreign language on first pass, even to an expert. Adding to an already complex issue, since October 2013, a hospital’s Utilization Review physicians can also “self-deny” Medicare inpatient stays that do not meet the two-midnight rule payment criteria and still bill for most of Part B charges. These cases are sometimes referred to as “Part A to B rebills” and may or may not have been captured in the claims data reported by CMS and reviewed by Sheehy et al. These cases represent another important status change that should be tracked.
There is a multitude of opinions on the pros and cons of observation care as a payment policy, and the data presented by Sheehy et al. is further evidence that the line between inpatient and observation hospitalizations remains blurred and mutable. The authors demonstrate the need for a consistent methodology to define observation stays and ultimately to study them using claims-based data. Simplicity may be the answer, but first, we must know what we are doing, then we can have a debate on whether or not it needs to change.
Disclosures
The authors have nothing to disclose.
1. Sheehy AM, Shi F, Kind AJH. Identifying observation stays in Medicare data. J Hosp Med. 2019;14(2):96-100. doi: 10.2788/jhm.3038. PubMed
2. Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the Long- Term Care; Hospital Prospective Payment System and Fiscal Year 2014 Rates; Quality Reporting Requirements for Specific Providers; Hospital Conditions of Participation; Payment Policies Related to Patient Status; Final Rule. https://www.gpo.gov/fdsys/pkg/FR-2013-08-19/pdf/2013-18956.pdf. Accessed November 1, 2018.
3. Sheehy AM, Locke C, Engel JZ, et al. Recovery audit contractor audits and appeals at three academic medical centers. J Hosp Med. 2015;10(4):212-219. doi: 10.1002/jhm.2332. PubMed
4. Office of Medicare Hearings and Appeals. Memorandum to OMHA MedicareAppellants. http://www.modernhealthcare.com/assets/pdf/CH92573110.pdf. Accessed November 4, 2018.
5. Center for Medicare and Medicaid Services. Are You a Hospital Inpatient or Outpatient? https://www.medicare.gov/sites/default/files/2018-09/11435-Are-You-an-Inpatient-or-Outpatient.pdf. Accessed November 4, 2018.
6. Center for Medicare and Medicaid Services. Medicare Outpatient Observation Notice website. https://www.cms.gov/Medicare/Medicare-General-Information/BNI/MOON.html. Accessed November 1, 2018.
7. Kodjak, A. How Medicare’s Conflicting Hospitalization Rules MostMe Thousands of Dollars. https://www.npr.org/sections/health-shots/2018/04/20/583338114/how-medicares-conflicting-hospitalization-rules-cost-me-thousands-of-dollars. Accessed November 1, 2018.
8. Schroeder, MO. Have You Really Been Admitted as an Inpatient to the Hospital? https://health.usnews.com/health-care/patient-advice/articles/2018-10-18/have-you-really-been-admitted-as-an-inpatient-to-the-hospital. Accessed November 1, 2018.
Every reader of a certain age will recognize this acronym: ADCVANDIML. In simpler times, we “admitted” to a location: medical intensive care unit, bone marrow transplant unit. At some point, admission orders changed from a synonym for “hospitalize” to chart evidence necessary for inpatient payment to the hospital. In the billing and payment world, “inpatient” and “outpatient” hospitalizations are paid at different rates. Observation stays are one type of “outpatient hospitalization,” a confusing and contradictory term to physicians and patients alike. In their article published in this month’s Journal of Hospital Medicine, Sheehy and colleagues attempt the herculean task of defining a reproducible methodology to identify observation hospital stays using Medicare claims data.1 They highlight the complexity of claims data, the variability of revenue codes used, and the probable high frequency of status changes from outpatient observation to inpatient, and vice-versa, during a single hospitalization. They also argue for reform to simplify payment policy for hospitalized patients.
In October 2013, the Center for Medicare and Medicaid Services (CMS) changed the definition of “inpatient” in the Hospital Inpatient Prospective Payment System rule.2 This change is known colloquially as the “two-midnight rule” and occurred on the heels of several years of Recovery Audit contractor (RAC) retroactive denials of short-stay inpatient payments to hospitals around the country. These denials appear to have been based solely on the visit status under which a claim was billed, rather than a dispute over the actual medical care delivered.3 The RAC audits alleged billions of dollars of improper payment to hospitals and resulted in a log-jam of hundreds of thousands of cases in the federal appeal system.4 The two-midnight rule altered the subjective characterization of an inpatient from patient-based (severity of illness) and physician-based (intensity of service) to an objective, time-based payment definition. For the hospital to submit a claim to Medicare Part A, a medical provider with admitting privileges should expect that the patient will need, for medically necessary reasons, a hospitalization that will span at least two midnights of hospital care. Notable exceptions to the rule include patients undergoing a procedure on the Medicare Inpatient Only list and hospitalizations that include an unplanned mechanical intubation. To receive payment for observation (an outpatient service billed under Part B) the physician must place an observation order in addition to other requirements. At its core, the two-midnight rule is a payment rule, not a patient care rule.
This change in the criteria for an inpatient hospitalization from a subjective to a more objective and measurable time-based criterion might lead us to believe that the process for determining the correct visit status would now be simple. Unfortunately, we are dealing with a messy real-world scenario, where doctors can make different judgments and patients can have an unpredictable hospital course. Physicians are familiar with the issues surrounding the choice of the “correct” admission order. In many hospitals, the Medicare patients in “observation” and those with an “inpatient” order can be on the same floor and even share the same room. From a hospital resource, nurse’s, and physician’s standpoint, the patients are often indistinguishable. While some facilities have observation units often associated with their emergency departments, the elderly and those patients with certain comorbidities can be excluded from these units based on protocols designed to improve outcomes and patient safety.
Additionally, most patients who spend at least one night in the hospital for medical treatment would not think that they could be an “outpatient.” To address this, CMS has produced specific beneficiary information5 and now requires hospitals to provide patients with the Medicare outpatient observation notice (MOON) if patients spend more than 24 hours in observation status.6 Beneficiaries must sign this notice, but unlike those admitted as inpatients, Medicare observation patients have no appeal rights. Recent articles in the lay press highlight the interplay between observation status, out-of-pocket expenses, and impact on postacute care.7,8
Following the implementation of the two-midnight rule, CMS directed the regional Medicare Administrative Contractors to perform audits in every hospital in the country. This has led to system-based processes at most facilities directing the “proper” visit class orders for our patients: direct education to providers, electronic medical record fixes and hard-stops, and real-time communications from the utilization review nurses and staff. These processes, based on a payment rule are burdensome to patients, physicians, and hospital support staff.
It’s not surprising to see that the billing of hospital-based observation care is also a quagmire. The methods and results sections of Sheehy et al.’s article reads like a calculus textbook written in a foreign language on first pass, even to an expert. Adding to an already complex issue, since October 2013, a hospital’s Utilization Review physicians can also “self-deny” Medicare inpatient stays that do not meet the two-midnight rule payment criteria and still bill for most of Part B charges. These cases are sometimes referred to as “Part A to B rebills” and may or may not have been captured in the claims data reported by CMS and reviewed by Sheehy et al. These cases represent another important status change that should be tracked.
There is a multitude of opinions on the pros and cons of observation care as a payment policy, and the data presented by Sheehy et al. is further evidence that the line between inpatient and observation hospitalizations remains blurred and mutable. The authors demonstrate the need for a consistent methodology to define observation stays and ultimately to study them using claims-based data. Simplicity may be the answer, but first, we must know what we are doing, then we can have a debate on whether or not it needs to change.
Disclosures
The authors have nothing to disclose.
Every reader of a certain age will recognize this acronym: ADCVANDIML. In simpler times, we “admitted” to a location: medical intensive care unit, bone marrow transplant unit. At some point, admission orders changed from a synonym for “hospitalize” to chart evidence necessary for inpatient payment to the hospital. In the billing and payment world, “inpatient” and “outpatient” hospitalizations are paid at different rates. Observation stays are one type of “outpatient hospitalization,” a confusing and contradictory term to physicians and patients alike. In their article published in this month’s Journal of Hospital Medicine, Sheehy and colleagues attempt the herculean task of defining a reproducible methodology to identify observation hospital stays using Medicare claims data.1 They highlight the complexity of claims data, the variability of revenue codes used, and the probable high frequency of status changes from outpatient observation to inpatient, and vice-versa, during a single hospitalization. They also argue for reform to simplify payment policy for hospitalized patients.
In October 2013, the Center for Medicare and Medicaid Services (CMS) changed the definition of “inpatient” in the Hospital Inpatient Prospective Payment System rule.2 This change is known colloquially as the “two-midnight rule” and occurred on the heels of several years of Recovery Audit contractor (RAC) retroactive denials of short-stay inpatient payments to hospitals around the country. These denials appear to have been based solely on the visit status under which a claim was billed, rather than a dispute over the actual medical care delivered.3 The RAC audits alleged billions of dollars of improper payment to hospitals and resulted in a log-jam of hundreds of thousands of cases in the federal appeal system.4 The two-midnight rule altered the subjective characterization of an inpatient from patient-based (severity of illness) and physician-based (intensity of service) to an objective, time-based payment definition. For the hospital to submit a claim to Medicare Part A, a medical provider with admitting privileges should expect that the patient will need, for medically necessary reasons, a hospitalization that will span at least two midnights of hospital care. Notable exceptions to the rule include patients undergoing a procedure on the Medicare Inpatient Only list and hospitalizations that include an unplanned mechanical intubation. To receive payment for observation (an outpatient service billed under Part B) the physician must place an observation order in addition to other requirements. At its core, the two-midnight rule is a payment rule, not a patient care rule.
This change in the criteria for an inpatient hospitalization from a subjective to a more objective and measurable time-based criterion might lead us to believe that the process for determining the correct visit status would now be simple. Unfortunately, we are dealing with a messy real-world scenario, where doctors can make different judgments and patients can have an unpredictable hospital course. Physicians are familiar with the issues surrounding the choice of the “correct” admission order. In many hospitals, the Medicare patients in “observation” and those with an “inpatient” order can be on the same floor and even share the same room. From a hospital resource, nurse’s, and physician’s standpoint, the patients are often indistinguishable. While some facilities have observation units often associated with their emergency departments, the elderly and those patients with certain comorbidities can be excluded from these units based on protocols designed to improve outcomes and patient safety.
Additionally, most patients who spend at least one night in the hospital for medical treatment would not think that they could be an “outpatient.” To address this, CMS has produced specific beneficiary information5 and now requires hospitals to provide patients with the Medicare outpatient observation notice (MOON) if patients spend more than 24 hours in observation status.6 Beneficiaries must sign this notice, but unlike those admitted as inpatients, Medicare observation patients have no appeal rights. Recent articles in the lay press highlight the interplay between observation status, out-of-pocket expenses, and impact on postacute care.7,8
Following the implementation of the two-midnight rule, CMS directed the regional Medicare Administrative Contractors to perform audits in every hospital in the country. This has led to system-based processes at most facilities directing the “proper” visit class orders for our patients: direct education to providers, electronic medical record fixes and hard-stops, and real-time communications from the utilization review nurses and staff. These processes, based on a payment rule are burdensome to patients, physicians, and hospital support staff.
It’s not surprising to see that the billing of hospital-based observation care is also a quagmire. The methods and results sections of Sheehy et al.’s article reads like a calculus textbook written in a foreign language on first pass, even to an expert. Adding to an already complex issue, since October 2013, a hospital’s Utilization Review physicians can also “self-deny” Medicare inpatient stays that do not meet the two-midnight rule payment criteria and still bill for most of Part B charges. These cases are sometimes referred to as “Part A to B rebills” and may or may not have been captured in the claims data reported by CMS and reviewed by Sheehy et al. These cases represent another important status change that should be tracked.
There is a multitude of opinions on the pros and cons of observation care as a payment policy, and the data presented by Sheehy et al. is further evidence that the line between inpatient and observation hospitalizations remains blurred and mutable. The authors demonstrate the need for a consistent methodology to define observation stays and ultimately to study them using claims-based data. Simplicity may be the answer, but first, we must know what we are doing, then we can have a debate on whether or not it needs to change.
Disclosures
The authors have nothing to disclose.
1. Sheehy AM, Shi F, Kind AJH. Identifying observation stays in Medicare data. J Hosp Med. 2019;14(2):96-100. doi: 10.2788/jhm.3038. PubMed
2. Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the Long- Term Care; Hospital Prospective Payment System and Fiscal Year 2014 Rates; Quality Reporting Requirements for Specific Providers; Hospital Conditions of Participation; Payment Policies Related to Patient Status; Final Rule. https://www.gpo.gov/fdsys/pkg/FR-2013-08-19/pdf/2013-18956.pdf. Accessed November 1, 2018.
3. Sheehy AM, Locke C, Engel JZ, et al. Recovery audit contractor audits and appeals at three academic medical centers. J Hosp Med. 2015;10(4):212-219. doi: 10.1002/jhm.2332. PubMed
4. Office of Medicare Hearings and Appeals. Memorandum to OMHA MedicareAppellants. http://www.modernhealthcare.com/assets/pdf/CH92573110.pdf. Accessed November 4, 2018.
5. Center for Medicare and Medicaid Services. Are You a Hospital Inpatient or Outpatient? https://www.medicare.gov/sites/default/files/2018-09/11435-Are-You-an-Inpatient-or-Outpatient.pdf. Accessed November 4, 2018.
6. Center for Medicare and Medicaid Services. Medicare Outpatient Observation Notice website. https://www.cms.gov/Medicare/Medicare-General-Information/BNI/MOON.html. Accessed November 1, 2018.
7. Kodjak, A. How Medicare’s Conflicting Hospitalization Rules MostMe Thousands of Dollars. https://www.npr.org/sections/health-shots/2018/04/20/583338114/how-medicares-conflicting-hospitalization-rules-cost-me-thousands-of-dollars. Accessed November 1, 2018.
8. Schroeder, MO. Have You Really Been Admitted as an Inpatient to the Hospital? https://health.usnews.com/health-care/patient-advice/articles/2018-10-18/have-you-really-been-admitted-as-an-inpatient-to-the-hospital. Accessed November 1, 2018.
1. Sheehy AM, Shi F, Kind AJH. Identifying observation stays in Medicare data. J Hosp Med. 2019;14(2):96-100. doi: 10.2788/jhm.3038. PubMed
2. Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the Long- Term Care; Hospital Prospective Payment System and Fiscal Year 2014 Rates; Quality Reporting Requirements for Specific Providers; Hospital Conditions of Participation; Payment Policies Related to Patient Status; Final Rule. https://www.gpo.gov/fdsys/pkg/FR-2013-08-19/pdf/2013-18956.pdf. Accessed November 1, 2018.
3. Sheehy AM, Locke C, Engel JZ, et al. Recovery audit contractor audits and appeals at three academic medical centers. J Hosp Med. 2015;10(4):212-219. doi: 10.1002/jhm.2332. PubMed
4. Office of Medicare Hearings and Appeals. Memorandum to OMHA MedicareAppellants. http://www.modernhealthcare.com/assets/pdf/CH92573110.pdf. Accessed November 4, 2018.
5. Center for Medicare and Medicaid Services. Are You a Hospital Inpatient or Outpatient? https://www.medicare.gov/sites/default/files/2018-09/11435-Are-You-an-Inpatient-or-Outpatient.pdf. Accessed November 4, 2018.
6. Center for Medicare and Medicaid Services. Medicare Outpatient Observation Notice website. https://www.cms.gov/Medicare/Medicare-General-Information/BNI/MOON.html. Accessed November 1, 2018.
7. Kodjak, A. How Medicare’s Conflicting Hospitalization Rules MostMe Thousands of Dollars. https://www.npr.org/sections/health-shots/2018/04/20/583338114/how-medicares-conflicting-hospitalization-rules-cost-me-thousands-of-dollars. Accessed November 1, 2018.
8. Schroeder, MO. Have You Really Been Admitted as an Inpatient to the Hospital? https://health.usnews.com/health-care/patient-advice/articles/2018-10-18/have-you-really-been-admitted-as-an-inpatient-to-the-hospital. Accessed November 1, 2018.
© 2019 Society of Hospital Medicine