User login
Winners and losers under bold Trump plan to slash drug rebate deals
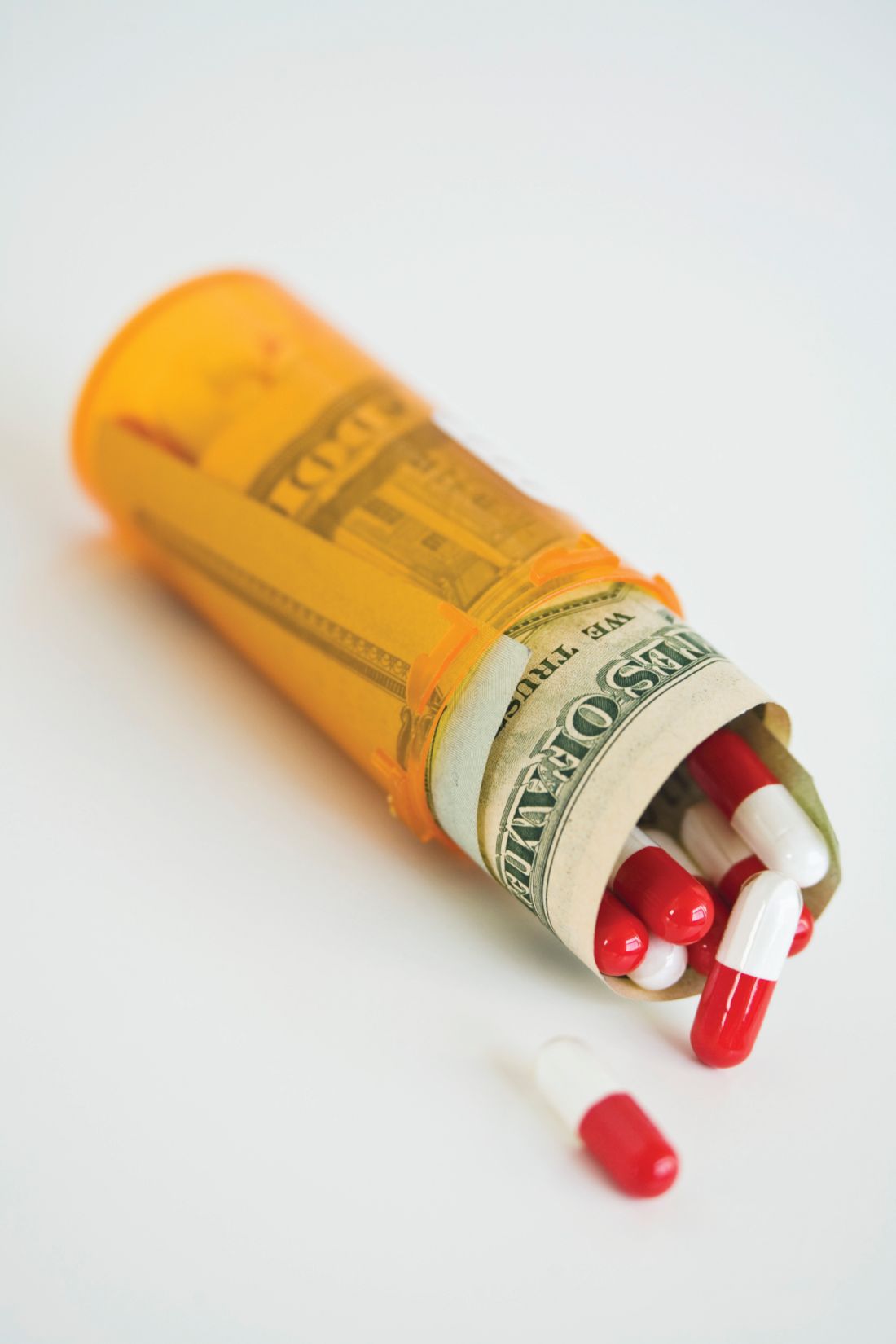
Few consumers have heard of the secret, business-to-business payments that the Trump administration wants to ban in an attempt to control drug costs.
But the administration’s plan for drug rebates, announced Jan. 31, would end the pharmaceutical business as usual, shift billions in revenue and cause far-reaching, unforeseen change, say health policy authorities.
In pointed language sure to anger middlemen who benefit from the deals, administration officials proposed banning rebates paid by drug companies to ensure coverage for their products under Medicare and Medicaid plans.
“A shadowy system of kickbacks,” was how Health and Human Services Secretary Alex Azar described the current system in a speech on Feb. 1.
The proposal is a regulatory change applying only to Medicare plans for seniors and managed Medicaid plans for low-income people. But private insurers, who often take cues from government programs, might make a similar shift, administration officials said.
Drug rebates are essentially discounts off the list price. Outlawing them would divert $29 billion in rebates now paid to insurers and pharmacy benefit managers into “seniors’ pocketbooks at the pharmacy counter,” Azar said.
The measure already faces fierce opposition from some in the industry and is unlikely to be implemented as presented or by the proposed 2020 effective date, health policy analysts said.
In any event, it’s hardly a pure win for seniors or patients in general. Consumers are unlikely to collect the full benefit of eliminated rebates.
At the same time, the change would produce uncertain ricochets, including higher drug-plan premiums for consumers, that would produce new winners and losers across the economy.
“It is the most significant proposal that the administration has introduced so far” to try to control drug prices, said Rachel Sachs, a law professor at Washington University in St. Louis. “But I’m struck by the uncertainty that the administration has in what the effects would be.”
Chronically ill patients who take lots of expensive medicine
The list price for many brand-name medicines has doubled or tripled in recent years. But virtually the only ones affected by the full increases are the many patients who pay cash or whose out-of-pocket payments are based on the posted price.
By banning rebates, the administration says its intention is to ensure discounts are passed all the way to the patient instead of the middlemen, the so-called pharmacy benefit managers or PBMs. That means consumers using expensive drugs might see their out-of-pocket costs go down.
If rebates were eliminated for commercial insurance, where deductibles and out-of-pocket costs are generally much higher, chronically ill patients could benefit much more.
Drug companies
Ending rebates would give the administration a drug-policy “win” that doesn’t directly threaten pharmaceutical company profits.
“We applaud the administration for taking steps to reform the rebate system” Stephen Ubl, CEO of PhRMA, the main lobby for branded drugs, said after the proposal came out.
The change might also slow the soaring list-price increases that have become a publicity nightmare for the industry. When list prices pop by 5% or 10% each year, drugmakers pay part of the proceeds to insurers and PBMs in the form of rebates to guarantee health-plan coverage.
No one is claiming that eliminating rebates would stop escalating list prices, even if all insurers adopted the practice. But some believe it would remove an important factor.
Pharmacy benefit managers
PBMs reap billions of dollars in rebate revenue in return for putting particular products on lists of covered drugs. The administration is essentially proposing to make those payments illegal, at least for Medicare and Medicaid plans.
PBMs, which claim they control costs by negotiating with drugmakers, might have to go back to their roots – processing pharmacy claims for a fee. After recent industry consolidation into a few enormous companies, on the other hand, they might have the market power to charge very high fees, replacing much of the lost rebate revenue.
PBMs “are concerned” that the move “would increase drug costs and force Medicare beneficiaries to pay higher premiums and out-of-pocket expenses,” said JC Scott, CEO of the Pharmaceutical Care Management Association, the PBM lobby.
Insurance companies
Insurers, who often receive rebates directly, could also be hurt financially.
“From the start, the focus on rebates has been a distraction from the real issue – the problem is the price” of the drugs, said Matt Eyles, CEO of America’s Health Insurance Plans, a trade group. “We are not middlemen – we are your bargaining power, working hard to negotiate lower prices.”
Patients without chronic conditions and high drug costs
Lower out-of-pocket costs at the pharmacy counter would be financed, at least in part, by higher premiums for Medicare and Medicaid plans paid by consumers and the government. Premiums for Medicare Part D plans could rise from $3.20 to $5.64 per month, according to consultants hired by the Department of Health and Human Services.
“There is likely to be a wide variation in how much savings people see based on the drugs they take and the point-of-sale discounts that are negotiated,” said Elizabeth Carpenter, policy practice director at Avalere, a consultancy.
Consumers who don’t need expensive drugs every month could see insurance costs go up slightly without getting the benefits of lower out-of-pocket expense for purchased drugs.
Other policy changes giving health plans more negotiating power against drugmakers would keep a lid on premium increases, administration officials argue.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Few consumers have heard of the secret, business-to-business payments that the Trump administration wants to ban in an attempt to control drug costs.
But the administration’s plan for drug rebates, announced Jan. 31, would end the pharmaceutical business as usual, shift billions in revenue and cause far-reaching, unforeseen change, say health policy authorities.
In pointed language sure to anger middlemen who benefit from the deals, administration officials proposed banning rebates paid by drug companies to ensure coverage for their products under Medicare and Medicaid plans.
“A shadowy system of kickbacks,” was how Health and Human Services Secretary Alex Azar described the current system in a speech on Feb. 1.
The proposal is a regulatory change applying only to Medicare plans for seniors and managed Medicaid plans for low-income people. But private insurers, who often take cues from government programs, might make a similar shift, administration officials said.
Drug rebates are essentially discounts off the list price. Outlawing them would divert $29 billion in rebates now paid to insurers and pharmacy benefit managers into “seniors’ pocketbooks at the pharmacy counter,” Azar said.
The measure already faces fierce opposition from some in the industry and is unlikely to be implemented as presented or by the proposed 2020 effective date, health policy analysts said.
In any event, it’s hardly a pure win for seniors or patients in general. Consumers are unlikely to collect the full benefit of eliminated rebates.
At the same time, the change would produce uncertain ricochets, including higher drug-plan premiums for consumers, that would produce new winners and losers across the economy.
“It is the most significant proposal that the administration has introduced so far” to try to control drug prices, said Rachel Sachs, a law professor at Washington University in St. Louis. “But I’m struck by the uncertainty that the administration has in what the effects would be.”
Chronically ill patients who take lots of expensive medicine
The list price for many brand-name medicines has doubled or tripled in recent years. But virtually the only ones affected by the full increases are the many patients who pay cash or whose out-of-pocket payments are based on the posted price.
By banning rebates, the administration says its intention is to ensure discounts are passed all the way to the patient instead of the middlemen, the so-called pharmacy benefit managers or PBMs. That means consumers using expensive drugs might see their out-of-pocket costs go down.
If rebates were eliminated for commercial insurance, where deductibles and out-of-pocket costs are generally much higher, chronically ill patients could benefit much more.
Drug companies
Ending rebates would give the administration a drug-policy “win” that doesn’t directly threaten pharmaceutical company profits.
“We applaud the administration for taking steps to reform the rebate system” Stephen Ubl, CEO of PhRMA, the main lobby for branded drugs, said after the proposal came out.
The change might also slow the soaring list-price increases that have become a publicity nightmare for the industry. When list prices pop by 5% or 10% each year, drugmakers pay part of the proceeds to insurers and PBMs in the form of rebates to guarantee health-plan coverage.
No one is claiming that eliminating rebates would stop escalating list prices, even if all insurers adopted the practice. But some believe it would remove an important factor.
Pharmacy benefit managers
PBMs reap billions of dollars in rebate revenue in return for putting particular products on lists of covered drugs. The administration is essentially proposing to make those payments illegal, at least for Medicare and Medicaid plans.
PBMs, which claim they control costs by negotiating with drugmakers, might have to go back to their roots – processing pharmacy claims for a fee. After recent industry consolidation into a few enormous companies, on the other hand, they might have the market power to charge very high fees, replacing much of the lost rebate revenue.
PBMs “are concerned” that the move “would increase drug costs and force Medicare beneficiaries to pay higher premiums and out-of-pocket expenses,” said JC Scott, CEO of the Pharmaceutical Care Management Association, the PBM lobby.
Insurance companies
Insurers, who often receive rebates directly, could also be hurt financially.
“From the start, the focus on rebates has been a distraction from the real issue – the problem is the price” of the drugs, said Matt Eyles, CEO of America’s Health Insurance Plans, a trade group. “We are not middlemen – we are your bargaining power, working hard to negotiate lower prices.”
Patients without chronic conditions and high drug costs
Lower out-of-pocket costs at the pharmacy counter would be financed, at least in part, by higher premiums for Medicare and Medicaid plans paid by consumers and the government. Premiums for Medicare Part D plans could rise from $3.20 to $5.64 per month, according to consultants hired by the Department of Health and Human Services.
“There is likely to be a wide variation in how much savings people see based on the drugs they take and the point-of-sale discounts that are negotiated,” said Elizabeth Carpenter, policy practice director at Avalere, a consultancy.
Consumers who don’t need expensive drugs every month could see insurance costs go up slightly without getting the benefits of lower out-of-pocket expense for purchased drugs.
Other policy changes giving health plans more negotiating power against drugmakers would keep a lid on premium increases, administration officials argue.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Few consumers have heard of the secret, business-to-business payments that the Trump administration wants to ban in an attempt to control drug costs.
But the administration’s plan for drug rebates, announced Jan. 31, would end the pharmaceutical business as usual, shift billions in revenue and cause far-reaching, unforeseen change, say health policy authorities.
In pointed language sure to anger middlemen who benefit from the deals, administration officials proposed banning rebates paid by drug companies to ensure coverage for their products under Medicare and Medicaid plans.
“A shadowy system of kickbacks,” was how Health and Human Services Secretary Alex Azar described the current system in a speech on Feb. 1.
The proposal is a regulatory change applying only to Medicare plans for seniors and managed Medicaid plans for low-income people. But private insurers, who often take cues from government programs, might make a similar shift, administration officials said.
Drug rebates are essentially discounts off the list price. Outlawing them would divert $29 billion in rebates now paid to insurers and pharmacy benefit managers into “seniors’ pocketbooks at the pharmacy counter,” Azar said.
The measure already faces fierce opposition from some in the industry and is unlikely to be implemented as presented or by the proposed 2020 effective date, health policy analysts said.
In any event, it’s hardly a pure win for seniors or patients in general. Consumers are unlikely to collect the full benefit of eliminated rebates.
At the same time, the change would produce uncertain ricochets, including higher drug-plan premiums for consumers, that would produce new winners and losers across the economy.
“It is the most significant proposal that the administration has introduced so far” to try to control drug prices, said Rachel Sachs, a law professor at Washington University in St. Louis. “But I’m struck by the uncertainty that the administration has in what the effects would be.”
Chronically ill patients who take lots of expensive medicine
The list price for many brand-name medicines has doubled or tripled in recent years. But virtually the only ones affected by the full increases are the many patients who pay cash or whose out-of-pocket payments are based on the posted price.
By banning rebates, the administration says its intention is to ensure discounts are passed all the way to the patient instead of the middlemen, the so-called pharmacy benefit managers or PBMs. That means consumers using expensive drugs might see their out-of-pocket costs go down.
If rebates were eliminated for commercial insurance, where deductibles and out-of-pocket costs are generally much higher, chronically ill patients could benefit much more.
Drug companies
Ending rebates would give the administration a drug-policy “win” that doesn’t directly threaten pharmaceutical company profits.
“We applaud the administration for taking steps to reform the rebate system” Stephen Ubl, CEO of PhRMA, the main lobby for branded drugs, said after the proposal came out.
The change might also slow the soaring list-price increases that have become a publicity nightmare for the industry. When list prices pop by 5% or 10% each year, drugmakers pay part of the proceeds to insurers and PBMs in the form of rebates to guarantee health-plan coverage.
No one is claiming that eliminating rebates would stop escalating list prices, even if all insurers adopted the practice. But some believe it would remove an important factor.
Pharmacy benefit managers
PBMs reap billions of dollars in rebate revenue in return for putting particular products on lists of covered drugs. The administration is essentially proposing to make those payments illegal, at least for Medicare and Medicaid plans.
PBMs, which claim they control costs by negotiating with drugmakers, might have to go back to their roots – processing pharmacy claims for a fee. After recent industry consolidation into a few enormous companies, on the other hand, they might have the market power to charge very high fees, replacing much of the lost rebate revenue.
PBMs “are concerned” that the move “would increase drug costs and force Medicare beneficiaries to pay higher premiums and out-of-pocket expenses,” said JC Scott, CEO of the Pharmaceutical Care Management Association, the PBM lobby.
Insurance companies
Insurers, who often receive rebates directly, could also be hurt financially.
“From the start, the focus on rebates has been a distraction from the real issue – the problem is the price” of the drugs, said Matt Eyles, CEO of America’s Health Insurance Plans, a trade group. “We are not middlemen – we are your bargaining power, working hard to negotiate lower prices.”
Patients without chronic conditions and high drug costs
Lower out-of-pocket costs at the pharmacy counter would be financed, at least in part, by higher premiums for Medicare and Medicaid plans paid by consumers and the government. Premiums for Medicare Part D plans could rise from $3.20 to $5.64 per month, according to consultants hired by the Department of Health and Human Services.
“There is likely to be a wide variation in how much savings people see based on the drugs they take and the point-of-sale discounts that are negotiated,” said Elizabeth Carpenter, policy practice director at Avalere, a consultancy.
Consumers who don’t need expensive drugs every month could see insurance costs go up slightly without getting the benefits of lower out-of-pocket expense for purchased drugs.
Other policy changes giving health plans more negotiating power against drugmakers would keep a lid on premium increases, administration officials argue.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Clinical relevance of tamoxifen pharmacogenetics in question
The clinical relevance of tamoxifen pharmacogenetics and therapeutic drug monitoring has been challenged, according to findings from an observational study.
“The objective of the prospective CYPTAM study was to associate endoxifen concentrations and CYP2D6 genotypes with clinical outcome[s] in patients with early-stage breast cancer receiving tamoxifen,” wrote Anabel Sanchez-Spitman, PharmD, of the Leiden University Medical Center, the Netherlands, and her colleagues in the Journal of Clinical Oncology.
“Although, in a retrospective study, an endoxifen threshold of 5.9 ng/mL for efficacy [has been] described, confirmation based on prospective studies is lacking,” they wrote.
The researchers followed 667 patients with early-stage breast malignancy who were treated with 20 mg of oral tamoxifen daily.
Dr. Sanchez-Spitman and her colleagues performed CYP2D6 genotype testing and measured blood levels of endoxifen, an active metabolite of tamoxifen, in study participants. Both of these measures were linked with relapse-free survival via statistical analysis.
After analysis, no significant association was found between endoxifen concentrations (P = .691) or CYP2D6 genotyping (P = .799) and relapse-free survival. These data were the same in both univariable and multivariable regression analysis.
“Neither categorizing endoxifen concentrations into quartiles nor using 5.9 ng/mL as threshold altered these results,” they wrote.
The authors acknowledged that a key limitation of the study was the absence of tamoxifen adherence testing, but the availability of persistence data was well collected, lessening this drawback.
“Our data do not justify therapeutic drug monitoring based on endoxifen concentrations in patients with breast cancer receiving tamoxifen,” they concluded.
The study was supported by grant funding provided by ZOLEON. The authors reported no conflicts of interest.
SOURCE: Sanchez-Spitman A et al. J Clin Oncol. 2019 Jan 24. doi: 10.1200/JCO.18.00307.
The clinical relevance of tamoxifen pharmacogenetics and therapeutic drug monitoring has been challenged, according to findings from an observational study.
“The objective of the prospective CYPTAM study was to associate endoxifen concentrations and CYP2D6 genotypes with clinical outcome[s] in patients with early-stage breast cancer receiving tamoxifen,” wrote Anabel Sanchez-Spitman, PharmD, of the Leiden University Medical Center, the Netherlands, and her colleagues in the Journal of Clinical Oncology.
“Although, in a retrospective study, an endoxifen threshold of 5.9 ng/mL for efficacy [has been] described, confirmation based on prospective studies is lacking,” they wrote.
The researchers followed 667 patients with early-stage breast malignancy who were treated with 20 mg of oral tamoxifen daily.
Dr. Sanchez-Spitman and her colleagues performed CYP2D6 genotype testing and measured blood levels of endoxifen, an active metabolite of tamoxifen, in study participants. Both of these measures were linked with relapse-free survival via statistical analysis.
After analysis, no significant association was found between endoxifen concentrations (P = .691) or CYP2D6 genotyping (P = .799) and relapse-free survival. These data were the same in both univariable and multivariable regression analysis.
“Neither categorizing endoxifen concentrations into quartiles nor using 5.9 ng/mL as threshold altered these results,” they wrote.
The authors acknowledged that a key limitation of the study was the absence of tamoxifen adherence testing, but the availability of persistence data was well collected, lessening this drawback.
“Our data do not justify therapeutic drug monitoring based on endoxifen concentrations in patients with breast cancer receiving tamoxifen,” they concluded.
The study was supported by grant funding provided by ZOLEON. The authors reported no conflicts of interest.
SOURCE: Sanchez-Spitman A et al. J Clin Oncol. 2019 Jan 24. doi: 10.1200/JCO.18.00307.
The clinical relevance of tamoxifen pharmacogenetics and therapeutic drug monitoring has been challenged, according to findings from an observational study.
“The objective of the prospective CYPTAM study was to associate endoxifen concentrations and CYP2D6 genotypes with clinical outcome[s] in patients with early-stage breast cancer receiving tamoxifen,” wrote Anabel Sanchez-Spitman, PharmD, of the Leiden University Medical Center, the Netherlands, and her colleagues in the Journal of Clinical Oncology.
“Although, in a retrospective study, an endoxifen threshold of 5.9 ng/mL for efficacy [has been] described, confirmation based on prospective studies is lacking,” they wrote.
The researchers followed 667 patients with early-stage breast malignancy who were treated with 20 mg of oral tamoxifen daily.
Dr. Sanchez-Spitman and her colleagues performed CYP2D6 genotype testing and measured blood levels of endoxifen, an active metabolite of tamoxifen, in study participants. Both of these measures were linked with relapse-free survival via statistical analysis.
After analysis, no significant association was found between endoxifen concentrations (P = .691) or CYP2D6 genotyping (P = .799) and relapse-free survival. These data were the same in both univariable and multivariable regression analysis.
“Neither categorizing endoxifen concentrations into quartiles nor using 5.9 ng/mL as threshold altered these results,” they wrote.
The authors acknowledged that a key limitation of the study was the absence of tamoxifen adherence testing, but the availability of persistence data was well collected, lessening this drawback.
“Our data do not justify therapeutic drug monitoring based on endoxifen concentrations in patients with breast cancer receiving tamoxifen,” they concluded.
The study was supported by grant funding provided by ZOLEON. The authors reported no conflicts of interest.
SOURCE: Sanchez-Spitman A et al. J Clin Oncol. 2019 Jan 24. doi: 10.1200/JCO.18.00307.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: An endoxifen concentration of greater than 5.9 ng/mL was not linked with clinical efficacy in patients with breast malignancy.
Study details: A prospective study of 667 patients with early-stage breast malignancy treated with adjuvant tamoxifen.
Disclosures: The study was supported by grant funding provided by ZOLEON. The authors reported no conflicts of interest.
Source: Sanchez-Spitman A et al. J Clin Oncol. 2019 Jan 24. doi: 10.1200/JCO.18.00307.
Aerobic exercise and cognitive decline
Also today, findings in seropositive arthralgia patients may help to predict rheumatoid arthritis, elevated coronary artery calcification is not linked to increased death risk in active men, and clinical benefits persist 5 years after theymectomy for myasthenia gravis.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, findings in seropositive arthralgia patients may help to predict rheumatoid arthritis, elevated coronary artery calcification is not linked to increased death risk in active men, and clinical benefits persist 5 years after theymectomy for myasthenia gravis.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, findings in seropositive arthralgia patients may help to predict rheumatoid arthritis, elevated coronary artery calcification is not linked to increased death risk in active men, and clinical benefits persist 5 years after theymectomy for myasthenia gravis.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Mapping the Pathway of Pain
What makes us pull a hand away from a hot stove or flinch at a pinprick? Researchers from the National Center for Complementary and Integrative Health say they have identified activity in the brain that governs these reactions.
Alexander Chesler, PhD, senior author of the study, says we already know a lot about local spinal cord circuits for simple reflexive responses, but “the mechanisms underlying more complex behaviors remain poorly understood.”
Using heat as the source of discomfort in their experiments, the researchers found a predictable sequence of behaviors—likened to the sequence of responding to walking cautiously on a hot beach, then hopping as the heat intensifies, then running to a water source. “This kind of ‘feed-forward’ circuitry is unique because it is an upward spiral,” says Arnab Barik, PhD, one of the study authors. “The more this pathway is activated by harmful activity, the more it reacts, leading to dramatic behavioral responses.”
The experiments showed that the parts of the brainstem involved in this circuit are the parabrachial nucleus (PBNI) and the dorsal reticular formation in the medulla (MdD). Standing on a hot surface activated a group of nerve cells in the PBNI, triggering escape responses through connections to the MdD. Interestingly, the PBNI cells express a gene that codes for substances that also contribute to multiple disease processes.
“Our data provide evidence that the PBNI produces streams of information with distinct functional significance,” says Arnab Barik, PhD, one of the study authors. “The brainstem-spinal cord pathway identified in this study selectively controls pain response and elicits appropriate behaviors based on sensory input.”
Further investigation, the researchers say, can help us understand how pain is encoded in the brain. The study findings may also offer opportunities to understand how the body becomes dysregulated during chronic pain.
What makes us pull a hand away from a hot stove or flinch at a pinprick? Researchers from the National Center for Complementary and Integrative Health say they have identified activity in the brain that governs these reactions.
Alexander Chesler, PhD, senior author of the study, says we already know a lot about local spinal cord circuits for simple reflexive responses, but “the mechanisms underlying more complex behaviors remain poorly understood.”
Using heat as the source of discomfort in their experiments, the researchers found a predictable sequence of behaviors—likened to the sequence of responding to walking cautiously on a hot beach, then hopping as the heat intensifies, then running to a water source. “This kind of ‘feed-forward’ circuitry is unique because it is an upward spiral,” says Arnab Barik, PhD, one of the study authors. “The more this pathway is activated by harmful activity, the more it reacts, leading to dramatic behavioral responses.”
The experiments showed that the parts of the brainstem involved in this circuit are the parabrachial nucleus (PBNI) and the dorsal reticular formation in the medulla (MdD). Standing on a hot surface activated a group of nerve cells in the PBNI, triggering escape responses through connections to the MdD. Interestingly, the PBNI cells express a gene that codes for substances that also contribute to multiple disease processes.
“Our data provide evidence that the PBNI produces streams of information with distinct functional significance,” says Arnab Barik, PhD, one of the study authors. “The brainstem-spinal cord pathway identified in this study selectively controls pain response and elicits appropriate behaviors based on sensory input.”
Further investigation, the researchers say, can help us understand how pain is encoded in the brain. The study findings may also offer opportunities to understand how the body becomes dysregulated during chronic pain.
What makes us pull a hand away from a hot stove or flinch at a pinprick? Researchers from the National Center for Complementary and Integrative Health say they have identified activity in the brain that governs these reactions.
Alexander Chesler, PhD, senior author of the study, says we already know a lot about local spinal cord circuits for simple reflexive responses, but “the mechanisms underlying more complex behaviors remain poorly understood.”
Using heat as the source of discomfort in their experiments, the researchers found a predictable sequence of behaviors—likened to the sequence of responding to walking cautiously on a hot beach, then hopping as the heat intensifies, then running to a water source. “This kind of ‘feed-forward’ circuitry is unique because it is an upward spiral,” says Arnab Barik, PhD, one of the study authors. “The more this pathway is activated by harmful activity, the more it reacts, leading to dramatic behavioral responses.”
The experiments showed that the parts of the brainstem involved in this circuit are the parabrachial nucleus (PBNI) and the dorsal reticular formation in the medulla (MdD). Standing on a hot surface activated a group of nerve cells in the PBNI, triggering escape responses through connections to the MdD. Interestingly, the PBNI cells express a gene that codes for substances that also contribute to multiple disease processes.
“Our data provide evidence that the PBNI produces streams of information with distinct functional significance,” says Arnab Barik, PhD, one of the study authors. “The brainstem-spinal cord pathway identified in this study selectively controls pain response and elicits appropriate behaviors based on sensory input.”
Further investigation, the researchers say, can help us understand how pain is encoded in the brain. The study findings may also offer opportunities to understand how the body becomes dysregulated during chronic pain.
Obesity-related cancers increasing in younger adults
The incidence of obesity-related cancers such as kidney and gallbladder cancer has increased significantly in young adults over the past two decades in the United States, according to an analysis of data from 25 population-based state registries in the United States.
The incidence of 6 of the 12 obesity-related cancers increased among individuals aged 25-49 years, Hyuna Sung, PhD, of the American Cancer Society, Atlanta, and her colleagues reported Feb. 4 in the Lancet Public Health.
Among more than 14.6 million incident cases of cancer diagnosed in adults aged 25-84 years between 1995 and 2014, the greatest increase in incidence, 6.23% annually, was seen with kidney cancer among the 25- to 29-year age group. Incidence, however, also increased by at least 6.17% in those aged 30-34 years, by 5.23% in those aged 35-39 years, and by 3.88% in those aged 40-44 years.
The incidence rate for kidney cancer among individuals born around 1985 was nearly fivefold higher than in individuals born in 1950, the investigators said (Lancet Public Health. 2019 Feb 4. doi: 10.1016/S2468-2667(18)30267-6).
The analysis also showed significant increases from 1995 to 2014 in the incidence of cancer of the gallbladder among younger adults: 3.71% per year among those aged 25-29 years and 2.58% per year in those aged 30-34 years.
Similarly, the incidence of uterine corpus cancer increased in the 25- to 29-year age group by 3.34% per year and by 3.22% in the 30- to 34-year age group. The incidence of colorectal cancer increased by 2.41% among those aged 25-29 years and by 2.38% in those aged 30-34 years, Dr. Sung and her associates said.
The greatest annual increase in the incidence of multiple myeloma was seen in individuals aged 30-34 years (2.21%), but significant annual increases in incidence were seen in individuals aged 30-44 years.
For pancreatic cancer, significant annual increases in incidence were seen among individuals aged 25-29 years (4.34%) and 30-34 years (2.47%).
The study also showed increases in the same obesity-related cancers – except for colorectal cancer – among adults aged 50 years and older. The incidence of colorectal cancer actually decreased annually in older adults, while the incidence of uterine corpus cancer increased among women aged 50-69 years but decreased in those over 75 years.
Dr. Sung and her coauthors suggested that these trends may be related to the rise of obesity and overweight in the United States, noting that excess body weight could be responsible for up to 60% of all endometrial cancers, 36% of gallbladder cancers, and 33% of kidney cancers in adults aged over 30 years.
“Because most epidemiological studies have primarily focused on older populations, the effect of excess bodyweight in early life or of weight change from young adulthood on cancer risk in different stages of the life course is not well characterized,” they wrote. “In concert with excess bodyweight, obesity-related health conditions and lifestyle factors can contribute to the increasing burden of obesity-related cancers in young adults, which include diabetes, gallstones, inflammatory bowel disease, and poor diet.”
The incidences of breast cancer and gastric cardia cancer were relatively stable in all age groups over the study period, and the incidence of ovarian cancer decreased in all age groups.
Researchers looked at the incidence of 30 cancers in total, including 18 cancers not related to obesity. Here they saw increases among younger adults only in the incidence of gastric noncardiac cancer – which showed a 2.16% annual increase in incidence among those aged 30-34 years – and leukemia, where there was a 1.33% annual increase in incidence in the same age group.
But the incidence of eight cancers, including those related to smoking and infection, decreased each year among younger adults.
“Our findings expose a recent change that could serve as a warning of an increased burden of obesity-related cancers to come in older adults,” study senior author Ahmedin Jemal, PhD, of the American Cancer Society, said in a statement. “Most cancers occur in older adults, which means that as the young people in our study age, the burden of obesity-related cancer cases and deaths are likely to increase even more. On the eve of World Cancer Day, it’s timely to consider what can be done to avert the impending rise.”
The future burden of these cancers could halt or even reverse the reductions in cancer mortality achieved over the past several decades, the investigators warned.
The study was funded by the American Cancer Society and the National Cancer Institute. No conflicts of interest were declared.
SOURCE: Sung H et al. Lancet Public Health. 2019 Feb 4 doi: 10.1016/ S2468-2667(18)30267-6.
Cancer was long thought of as a disease of aging, but the increase in incidence of some cancers in younger age groups has driven a recent reexamination of risk factors. This study’s most striking finding is the disproportionate increase in obesity-related cancer incidence among successively younger cohorts. Coupled with the rising incidence of obesity over the same period, it provides compelling evidence of a possible causal role for obesity in the increased incidence of these cancers.
Not all obesity-related cancers, however, show this pattern of age-specific increase in incidence, which could reflect the influence of other risk factors.
The hypothesis suggested by the study’s authors is plausible but needs to be tested more directly in experimental and population-based studies.
Catherine R. Marinac, PhD, is with the department of medical oncology at the Dana-Farber Cancer Institute, Boston, and Brenda M. Birmann, ScD, is with the department of medicine at Brigham and Women’s Hospital, Boston. These comments are taken from an accompanying editorial (Lancet Public Health. 2019 Feb 4. doi: 10.1016/S2468-2667(19)30017-9). No conflicts of interest were declared.
Cancer was long thought of as a disease of aging, but the increase in incidence of some cancers in younger age groups has driven a recent reexamination of risk factors. This study’s most striking finding is the disproportionate increase in obesity-related cancer incidence among successively younger cohorts. Coupled with the rising incidence of obesity over the same period, it provides compelling evidence of a possible causal role for obesity in the increased incidence of these cancers.
Not all obesity-related cancers, however, show this pattern of age-specific increase in incidence, which could reflect the influence of other risk factors.
The hypothesis suggested by the study’s authors is plausible but needs to be tested more directly in experimental and population-based studies.
Catherine R. Marinac, PhD, is with the department of medical oncology at the Dana-Farber Cancer Institute, Boston, and Brenda M. Birmann, ScD, is with the department of medicine at Brigham and Women’s Hospital, Boston. These comments are taken from an accompanying editorial (Lancet Public Health. 2019 Feb 4. doi: 10.1016/S2468-2667(19)30017-9). No conflicts of interest were declared.
Cancer was long thought of as a disease of aging, but the increase in incidence of some cancers in younger age groups has driven a recent reexamination of risk factors. This study’s most striking finding is the disproportionate increase in obesity-related cancer incidence among successively younger cohorts. Coupled with the rising incidence of obesity over the same period, it provides compelling evidence of a possible causal role for obesity in the increased incidence of these cancers.
Not all obesity-related cancers, however, show this pattern of age-specific increase in incidence, which could reflect the influence of other risk factors.
The hypothesis suggested by the study’s authors is plausible but needs to be tested more directly in experimental and population-based studies.
Catherine R. Marinac, PhD, is with the department of medical oncology at the Dana-Farber Cancer Institute, Boston, and Brenda M. Birmann, ScD, is with the department of medicine at Brigham and Women’s Hospital, Boston. These comments are taken from an accompanying editorial (Lancet Public Health. 2019 Feb 4. doi: 10.1016/S2468-2667(19)30017-9). No conflicts of interest were declared.
The incidence of obesity-related cancers such as kidney and gallbladder cancer has increased significantly in young adults over the past two decades in the United States, according to an analysis of data from 25 population-based state registries in the United States.
The incidence of 6 of the 12 obesity-related cancers increased among individuals aged 25-49 years, Hyuna Sung, PhD, of the American Cancer Society, Atlanta, and her colleagues reported Feb. 4 in the Lancet Public Health.
Among more than 14.6 million incident cases of cancer diagnosed in adults aged 25-84 years between 1995 and 2014, the greatest increase in incidence, 6.23% annually, was seen with kidney cancer among the 25- to 29-year age group. Incidence, however, also increased by at least 6.17% in those aged 30-34 years, by 5.23% in those aged 35-39 years, and by 3.88% in those aged 40-44 years.
The incidence rate for kidney cancer among individuals born around 1985 was nearly fivefold higher than in individuals born in 1950, the investigators said (Lancet Public Health. 2019 Feb 4. doi: 10.1016/S2468-2667(18)30267-6).
The analysis also showed significant increases from 1995 to 2014 in the incidence of cancer of the gallbladder among younger adults: 3.71% per year among those aged 25-29 years and 2.58% per year in those aged 30-34 years.
Similarly, the incidence of uterine corpus cancer increased in the 25- to 29-year age group by 3.34% per year and by 3.22% in the 30- to 34-year age group. The incidence of colorectal cancer increased by 2.41% among those aged 25-29 years and by 2.38% in those aged 30-34 years, Dr. Sung and her associates said.
The greatest annual increase in the incidence of multiple myeloma was seen in individuals aged 30-34 years (2.21%), but significant annual increases in incidence were seen in individuals aged 30-44 years.
For pancreatic cancer, significant annual increases in incidence were seen among individuals aged 25-29 years (4.34%) and 30-34 years (2.47%).
The study also showed increases in the same obesity-related cancers – except for colorectal cancer – among adults aged 50 years and older. The incidence of colorectal cancer actually decreased annually in older adults, while the incidence of uterine corpus cancer increased among women aged 50-69 years but decreased in those over 75 years.
Dr. Sung and her coauthors suggested that these trends may be related to the rise of obesity and overweight in the United States, noting that excess body weight could be responsible for up to 60% of all endometrial cancers, 36% of gallbladder cancers, and 33% of kidney cancers in adults aged over 30 years.
“Because most epidemiological studies have primarily focused on older populations, the effect of excess bodyweight in early life or of weight change from young adulthood on cancer risk in different stages of the life course is not well characterized,” they wrote. “In concert with excess bodyweight, obesity-related health conditions and lifestyle factors can contribute to the increasing burden of obesity-related cancers in young adults, which include diabetes, gallstones, inflammatory bowel disease, and poor diet.”
The incidences of breast cancer and gastric cardia cancer were relatively stable in all age groups over the study period, and the incidence of ovarian cancer decreased in all age groups.
Researchers looked at the incidence of 30 cancers in total, including 18 cancers not related to obesity. Here they saw increases among younger adults only in the incidence of gastric noncardiac cancer – which showed a 2.16% annual increase in incidence among those aged 30-34 years – and leukemia, where there was a 1.33% annual increase in incidence in the same age group.
But the incidence of eight cancers, including those related to smoking and infection, decreased each year among younger adults.
“Our findings expose a recent change that could serve as a warning of an increased burden of obesity-related cancers to come in older adults,” study senior author Ahmedin Jemal, PhD, of the American Cancer Society, said in a statement. “Most cancers occur in older adults, which means that as the young people in our study age, the burden of obesity-related cancer cases and deaths are likely to increase even more. On the eve of World Cancer Day, it’s timely to consider what can be done to avert the impending rise.”
The future burden of these cancers could halt or even reverse the reductions in cancer mortality achieved over the past several decades, the investigators warned.
The study was funded by the American Cancer Society and the National Cancer Institute. No conflicts of interest were declared.
SOURCE: Sung H et al. Lancet Public Health. 2019 Feb 4 doi: 10.1016/ S2468-2667(18)30267-6.
The incidence of obesity-related cancers such as kidney and gallbladder cancer has increased significantly in young adults over the past two decades in the United States, according to an analysis of data from 25 population-based state registries in the United States.
The incidence of 6 of the 12 obesity-related cancers increased among individuals aged 25-49 years, Hyuna Sung, PhD, of the American Cancer Society, Atlanta, and her colleagues reported Feb. 4 in the Lancet Public Health.
Among more than 14.6 million incident cases of cancer diagnosed in adults aged 25-84 years between 1995 and 2014, the greatest increase in incidence, 6.23% annually, was seen with kidney cancer among the 25- to 29-year age group. Incidence, however, also increased by at least 6.17% in those aged 30-34 years, by 5.23% in those aged 35-39 years, and by 3.88% in those aged 40-44 years.
The incidence rate for kidney cancer among individuals born around 1985 was nearly fivefold higher than in individuals born in 1950, the investigators said (Lancet Public Health. 2019 Feb 4. doi: 10.1016/S2468-2667(18)30267-6).
The analysis also showed significant increases from 1995 to 2014 in the incidence of cancer of the gallbladder among younger adults: 3.71% per year among those aged 25-29 years and 2.58% per year in those aged 30-34 years.
Similarly, the incidence of uterine corpus cancer increased in the 25- to 29-year age group by 3.34% per year and by 3.22% in the 30- to 34-year age group. The incidence of colorectal cancer increased by 2.41% among those aged 25-29 years and by 2.38% in those aged 30-34 years, Dr. Sung and her associates said.
The greatest annual increase in the incidence of multiple myeloma was seen in individuals aged 30-34 years (2.21%), but significant annual increases in incidence were seen in individuals aged 30-44 years.
For pancreatic cancer, significant annual increases in incidence were seen among individuals aged 25-29 years (4.34%) and 30-34 years (2.47%).
The study also showed increases in the same obesity-related cancers – except for colorectal cancer – among adults aged 50 years and older. The incidence of colorectal cancer actually decreased annually in older adults, while the incidence of uterine corpus cancer increased among women aged 50-69 years but decreased in those over 75 years.
Dr. Sung and her coauthors suggested that these trends may be related to the rise of obesity and overweight in the United States, noting that excess body weight could be responsible for up to 60% of all endometrial cancers, 36% of gallbladder cancers, and 33% of kidney cancers in adults aged over 30 years.
“Because most epidemiological studies have primarily focused on older populations, the effect of excess bodyweight in early life or of weight change from young adulthood on cancer risk in different stages of the life course is not well characterized,” they wrote. “In concert with excess bodyweight, obesity-related health conditions and lifestyle factors can contribute to the increasing burden of obesity-related cancers in young adults, which include diabetes, gallstones, inflammatory bowel disease, and poor diet.”
The incidences of breast cancer and gastric cardia cancer were relatively stable in all age groups over the study period, and the incidence of ovarian cancer decreased in all age groups.
Researchers looked at the incidence of 30 cancers in total, including 18 cancers not related to obesity. Here they saw increases among younger adults only in the incidence of gastric noncardiac cancer – which showed a 2.16% annual increase in incidence among those aged 30-34 years – and leukemia, where there was a 1.33% annual increase in incidence in the same age group.
But the incidence of eight cancers, including those related to smoking and infection, decreased each year among younger adults.
“Our findings expose a recent change that could serve as a warning of an increased burden of obesity-related cancers to come in older adults,” study senior author Ahmedin Jemal, PhD, of the American Cancer Society, said in a statement. “Most cancers occur in older adults, which means that as the young people in our study age, the burden of obesity-related cancer cases and deaths are likely to increase even more. On the eve of World Cancer Day, it’s timely to consider what can be done to avert the impending rise.”
The future burden of these cancers could halt or even reverse the reductions in cancer mortality achieved over the past several decades, the investigators warned.
The study was funded by the American Cancer Society and the National Cancer Institute. No conflicts of interest were declared.
SOURCE: Sung H et al. Lancet Public Health. 2019 Feb 4 doi: 10.1016/ S2468-2667(18)30267-6.
FROM THE LANCET PUBLIC HEALTH
Key clinical point: The incidence of obesity-related cancers has increased in younger adults.
Major finding: The incidence of kidney cancer has increased by more than 6% per year in younger adults since 1995.
Study details: Analysis of data from 14,672,409 cases of cancer diagnosed between 1995 and 2014.
Disclosures: The study was funded by the American Cancer Society and the National Cancer Institute. No conflicts of interest were declared.
Source: Sung H et al. Lancet Public Health. 2019 Feb 4.
New twists in medical management of PAD
SNOWMASS, COLO. – Be leery of lowering high blood pressure too much in patients with lower extremity peripheral artery disease, Robert A. Vogel, MD, cautioned the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology
“We used to worry about lowering blood pressure too much in [coronary artery disease]. I need to rekindle that thought, because you want to be very careful about lowering blood pressure too much in PAD,” said Dr. Vogel, a preventive cardiology specialist at the University of Colorado, Denver.
He cited a recent reanalysis of data from the landmark ALLHAT (Antihypertensive and Lipid-lowering Treatment to Prevent Heart Attack Trial) conducted by investigators at Stanford (Calif.) University. During a median 4.3 years of prospective follow-up of 33,357 participants with a median baseline blood pressure of 146/84 mm Hg, the risk of the composite endpoint of lower extremity PAD events – defined as PAD-related hospitalization, revascularization procedures, medical treatment, or PAD-related death – was increased by 26% in patients with a systolic blood pressure below 120 mm Hg, compared with an SBP of 120-129 mm Hg.
In a similar Cox regression analysis, the risk of PAD events was increased by 72% in patients with a diastolic blood pressure below 60 mm Hg, compared with that of patients with a DBP of 70-79 mm Hg, and to a lesser, albeit still statistically significant and clinically meaningful, extent in those with a DBP of 60-69 mm Hg (Circulation. 2018;138:1805-14).
Dr. Vogel’s cautionary note about overzealous blood pressure–lowering was one of several developments he highlighted since publication of the 2016 American Heart Association/American College of Cardiology guidelines on the medical management of lower extremity PAD (J Am Coll Cardiol. 2017;69:1465-508). Others include new data demonstrating that proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor therapy shows particularly strong benefit in the patient subgroup with PAD, as did rivaroxaban (Xarelto) at 2.5 mg b.i.d. plus aspirin 100 mg/day in the COMPASS trial.
Also, the final week of 2018 saw publication of the ACC Expert Consensus Decision Pathway on Tobacco Cessation (J Am Coll Cardiol. 2018 Dec 25;72[25]:3332-65), which Dr. Vogel considers an exemplary document every physician who cares for patients with PAD ought to read.
The Class I recommendations in the ACC/AHA guidelines for medical management of PAD include introducing a supervised exercise program before resorting to a revascularization procedure, providing advice on smoking cessation at every visit, antiplatelet therapy, a high-intensity statin, cilostazol for claudication, and coordination of the patient’s diabetes care with an endocrinologist or primary care physician.
Numerous studies have documented that physicians by and large aren’t doing so well in bringing these evidence-based therapies to bear. For example, a recent study of 155,647 Veterans Affairs patients with new-onset PAD found that 28% weren’t on a statin. Only 18.4% with PAD and comorbid coronary or carotid disease were on a high-intensity statin, as were just 6.4% with PAD only. In a multivariate adjusted analysis, high-intensity statin users had a 33% lower risk of amputation and a 26% lower risk of mortality, compared with statin nonusers (Circulation. 2018;137:1435-46).
It’s as if there’s a widespread failure to appreciate the substantial morbidity and mortality conferred by PAD, so Dr. Vogel put it into perspective: “In broad strokes, atherosclerosis starts in the aorta, moves to the coronaries, goes to the carotids, and ends up in the legs. By the time you have lower-extremity atherosclerosis, you are a vasculopath,” he explained.
Smoking cessation
“Lower-extremity PAD is a disease of smoking. Cholesterol goes to the heart, blood pressure goes to the head, and smoking goes to the legs, in broad strokes. Current smokers are 12 times more likely to have PAD than never smokers. And if you stop smoking, you reduce death by more than 50%,” the cardiologist said.
“You’ve got to get these folks to stop smoking, a difficult task. I do my clinical work at a VA hospital, and I can tell you this is a challenge,” he continued.
The new ACC Expert Consensus report is a boon in this regard.
“It’s not that long, and it’s very, very good. Very helpful. It’s not theoretical, it’s very practical,” according to Dr. Vogel. But he didn’t sugar coat what’s involved in getting PAD patients to quit smoking.
“At best, per round of smoking cessation, with pharmacology as well as multiple-session counseling, you can get 15%-20% abstinence per cycle. And it often takes many cycles of counseling to get people to stop smoking,” he added.
Low-dose rivaroxaban plus aspirin
Dr. Vogel believes the combination of low-dose rivaroxaban plus aspirin is worthy of serious consideration in patients with PAD on the strength of the COMPASS trial, a randomized, double-blind study of more than 27,000 patients with stable CAD, 27% of whom also had PAD. The PAD group on rivaroxaban 2.5 mg b.i.d. plus aspirin had a 28% relative risk reduction in the composite endpoint of cardiovascular death, stroke, or MI, compared with those on aspirin plus placebo. This benefit came at a cost of a 51% increase in the risk of major bleeding, but not fatal bleeding or bleeding causing critical damage to the brain or other organs.
Taking into account both the primary efficacy and severe bleeding rates, the net clinical benefit of low-dose rivaroxaban was 20%. The absolute risk reduction was larger in the PAD subgroup than in those with CAD-only because of their greater baseline risk (N Engl J Med. 2017;377:1319-30).
At present a 2.5-mg dose of rivaroxaban isn’t commercially available, so patients have to cut higher-dose tablets, but on the strength of the COMPASS results, a 2.5-mg tablet is in the works, Dr. Vogel said.
PCSK9 inhibitors
In the FOURIER trial of evolocumab (Praluent) versus placebo on top of maximally tolerated statin therapy in more than 27,000 patients with atherosclerotic disease, including 3,642 with PAD, the rate of MALE (major adverse limb events) was reduced by 42% in the evolocumab group, with a number-needed-to-treat in order to prevent one additional MALE event of only 16 (Circulation. 2018;137:338-50).
“This is a subgroup that really benefits from PCSK9 inhibition. It’s something to think about,” Dr. Vogel said.
Dr. Vogel reported serving as a paid consultant to the National Football League and the Pritikin Longevity Center, receiving research grants from Sanofi, and serving on speakers bureaus for Regeneron and Sanofi.
SNOWMASS, COLO. – Be leery of lowering high blood pressure too much in patients with lower extremity peripheral artery disease, Robert A. Vogel, MD, cautioned the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology
“We used to worry about lowering blood pressure too much in [coronary artery disease]. I need to rekindle that thought, because you want to be very careful about lowering blood pressure too much in PAD,” said Dr. Vogel, a preventive cardiology specialist at the University of Colorado, Denver.
He cited a recent reanalysis of data from the landmark ALLHAT (Antihypertensive and Lipid-lowering Treatment to Prevent Heart Attack Trial) conducted by investigators at Stanford (Calif.) University. During a median 4.3 years of prospective follow-up of 33,357 participants with a median baseline blood pressure of 146/84 mm Hg, the risk of the composite endpoint of lower extremity PAD events – defined as PAD-related hospitalization, revascularization procedures, medical treatment, or PAD-related death – was increased by 26% in patients with a systolic blood pressure below 120 mm Hg, compared with an SBP of 120-129 mm Hg.
In a similar Cox regression analysis, the risk of PAD events was increased by 72% in patients with a diastolic blood pressure below 60 mm Hg, compared with that of patients with a DBP of 70-79 mm Hg, and to a lesser, albeit still statistically significant and clinically meaningful, extent in those with a DBP of 60-69 mm Hg (Circulation. 2018;138:1805-14).
Dr. Vogel’s cautionary note about overzealous blood pressure–lowering was one of several developments he highlighted since publication of the 2016 American Heart Association/American College of Cardiology guidelines on the medical management of lower extremity PAD (J Am Coll Cardiol. 2017;69:1465-508). Others include new data demonstrating that proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor therapy shows particularly strong benefit in the patient subgroup with PAD, as did rivaroxaban (Xarelto) at 2.5 mg b.i.d. plus aspirin 100 mg/day in the COMPASS trial.
Also, the final week of 2018 saw publication of the ACC Expert Consensus Decision Pathway on Tobacco Cessation (J Am Coll Cardiol. 2018 Dec 25;72[25]:3332-65), which Dr. Vogel considers an exemplary document every physician who cares for patients with PAD ought to read.
The Class I recommendations in the ACC/AHA guidelines for medical management of PAD include introducing a supervised exercise program before resorting to a revascularization procedure, providing advice on smoking cessation at every visit, antiplatelet therapy, a high-intensity statin, cilostazol for claudication, and coordination of the patient’s diabetes care with an endocrinologist or primary care physician.
Numerous studies have documented that physicians by and large aren’t doing so well in bringing these evidence-based therapies to bear. For example, a recent study of 155,647 Veterans Affairs patients with new-onset PAD found that 28% weren’t on a statin. Only 18.4% with PAD and comorbid coronary or carotid disease were on a high-intensity statin, as were just 6.4% with PAD only. In a multivariate adjusted analysis, high-intensity statin users had a 33% lower risk of amputation and a 26% lower risk of mortality, compared with statin nonusers (Circulation. 2018;137:1435-46).
It’s as if there’s a widespread failure to appreciate the substantial morbidity and mortality conferred by PAD, so Dr. Vogel put it into perspective: “In broad strokes, atherosclerosis starts in the aorta, moves to the coronaries, goes to the carotids, and ends up in the legs. By the time you have lower-extremity atherosclerosis, you are a vasculopath,” he explained.
Smoking cessation
“Lower-extremity PAD is a disease of smoking. Cholesterol goes to the heart, blood pressure goes to the head, and smoking goes to the legs, in broad strokes. Current smokers are 12 times more likely to have PAD than never smokers. And if you stop smoking, you reduce death by more than 50%,” the cardiologist said.
“You’ve got to get these folks to stop smoking, a difficult task. I do my clinical work at a VA hospital, and I can tell you this is a challenge,” he continued.
The new ACC Expert Consensus report is a boon in this regard.
“It’s not that long, and it’s very, very good. Very helpful. It’s not theoretical, it’s very practical,” according to Dr. Vogel. But he didn’t sugar coat what’s involved in getting PAD patients to quit smoking.
“At best, per round of smoking cessation, with pharmacology as well as multiple-session counseling, you can get 15%-20% abstinence per cycle. And it often takes many cycles of counseling to get people to stop smoking,” he added.
Low-dose rivaroxaban plus aspirin
Dr. Vogel believes the combination of low-dose rivaroxaban plus aspirin is worthy of serious consideration in patients with PAD on the strength of the COMPASS trial, a randomized, double-blind study of more than 27,000 patients with stable CAD, 27% of whom also had PAD. The PAD group on rivaroxaban 2.5 mg b.i.d. plus aspirin had a 28% relative risk reduction in the composite endpoint of cardiovascular death, stroke, or MI, compared with those on aspirin plus placebo. This benefit came at a cost of a 51% increase in the risk of major bleeding, but not fatal bleeding or bleeding causing critical damage to the brain or other organs.
Taking into account both the primary efficacy and severe bleeding rates, the net clinical benefit of low-dose rivaroxaban was 20%. The absolute risk reduction was larger in the PAD subgroup than in those with CAD-only because of their greater baseline risk (N Engl J Med. 2017;377:1319-30).
At present a 2.5-mg dose of rivaroxaban isn’t commercially available, so patients have to cut higher-dose tablets, but on the strength of the COMPASS results, a 2.5-mg tablet is in the works, Dr. Vogel said.
PCSK9 inhibitors
In the FOURIER trial of evolocumab (Praluent) versus placebo on top of maximally tolerated statin therapy in more than 27,000 patients with atherosclerotic disease, including 3,642 with PAD, the rate of MALE (major adverse limb events) was reduced by 42% in the evolocumab group, with a number-needed-to-treat in order to prevent one additional MALE event of only 16 (Circulation. 2018;137:338-50).
“This is a subgroup that really benefits from PCSK9 inhibition. It’s something to think about,” Dr. Vogel said.
Dr. Vogel reported serving as a paid consultant to the National Football League and the Pritikin Longevity Center, receiving research grants from Sanofi, and serving on speakers bureaus for Regeneron and Sanofi.
SNOWMASS, COLO. – Be leery of lowering high blood pressure too much in patients with lower extremity peripheral artery disease, Robert A. Vogel, MD, cautioned the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology
“We used to worry about lowering blood pressure too much in [coronary artery disease]. I need to rekindle that thought, because you want to be very careful about lowering blood pressure too much in PAD,” said Dr. Vogel, a preventive cardiology specialist at the University of Colorado, Denver.
He cited a recent reanalysis of data from the landmark ALLHAT (Antihypertensive and Lipid-lowering Treatment to Prevent Heart Attack Trial) conducted by investigators at Stanford (Calif.) University. During a median 4.3 years of prospective follow-up of 33,357 participants with a median baseline blood pressure of 146/84 mm Hg, the risk of the composite endpoint of lower extremity PAD events – defined as PAD-related hospitalization, revascularization procedures, medical treatment, or PAD-related death – was increased by 26% in patients with a systolic blood pressure below 120 mm Hg, compared with an SBP of 120-129 mm Hg.
In a similar Cox regression analysis, the risk of PAD events was increased by 72% in patients with a diastolic blood pressure below 60 mm Hg, compared with that of patients with a DBP of 70-79 mm Hg, and to a lesser, albeit still statistically significant and clinically meaningful, extent in those with a DBP of 60-69 mm Hg (Circulation. 2018;138:1805-14).
Dr. Vogel’s cautionary note about overzealous blood pressure–lowering was one of several developments he highlighted since publication of the 2016 American Heart Association/American College of Cardiology guidelines on the medical management of lower extremity PAD (J Am Coll Cardiol. 2017;69:1465-508). Others include new data demonstrating that proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor therapy shows particularly strong benefit in the patient subgroup with PAD, as did rivaroxaban (Xarelto) at 2.5 mg b.i.d. plus aspirin 100 mg/day in the COMPASS trial.
Also, the final week of 2018 saw publication of the ACC Expert Consensus Decision Pathway on Tobacco Cessation (J Am Coll Cardiol. 2018 Dec 25;72[25]:3332-65), which Dr. Vogel considers an exemplary document every physician who cares for patients with PAD ought to read.
The Class I recommendations in the ACC/AHA guidelines for medical management of PAD include introducing a supervised exercise program before resorting to a revascularization procedure, providing advice on smoking cessation at every visit, antiplatelet therapy, a high-intensity statin, cilostazol for claudication, and coordination of the patient’s diabetes care with an endocrinologist or primary care physician.
Numerous studies have documented that physicians by and large aren’t doing so well in bringing these evidence-based therapies to bear. For example, a recent study of 155,647 Veterans Affairs patients with new-onset PAD found that 28% weren’t on a statin. Only 18.4% with PAD and comorbid coronary or carotid disease were on a high-intensity statin, as were just 6.4% with PAD only. In a multivariate adjusted analysis, high-intensity statin users had a 33% lower risk of amputation and a 26% lower risk of mortality, compared with statin nonusers (Circulation. 2018;137:1435-46).
It’s as if there’s a widespread failure to appreciate the substantial morbidity and mortality conferred by PAD, so Dr. Vogel put it into perspective: “In broad strokes, atherosclerosis starts in the aorta, moves to the coronaries, goes to the carotids, and ends up in the legs. By the time you have lower-extremity atherosclerosis, you are a vasculopath,” he explained.
Smoking cessation
“Lower-extremity PAD is a disease of smoking. Cholesterol goes to the heart, blood pressure goes to the head, and smoking goes to the legs, in broad strokes. Current smokers are 12 times more likely to have PAD than never smokers. And if you stop smoking, you reduce death by more than 50%,” the cardiologist said.
“You’ve got to get these folks to stop smoking, a difficult task. I do my clinical work at a VA hospital, and I can tell you this is a challenge,” he continued.
The new ACC Expert Consensus report is a boon in this regard.
“It’s not that long, and it’s very, very good. Very helpful. It’s not theoretical, it’s very practical,” according to Dr. Vogel. But he didn’t sugar coat what’s involved in getting PAD patients to quit smoking.
“At best, per round of smoking cessation, with pharmacology as well as multiple-session counseling, you can get 15%-20% abstinence per cycle. And it often takes many cycles of counseling to get people to stop smoking,” he added.
Low-dose rivaroxaban plus aspirin
Dr. Vogel believes the combination of low-dose rivaroxaban plus aspirin is worthy of serious consideration in patients with PAD on the strength of the COMPASS trial, a randomized, double-blind study of more than 27,000 patients with stable CAD, 27% of whom also had PAD. The PAD group on rivaroxaban 2.5 mg b.i.d. plus aspirin had a 28% relative risk reduction in the composite endpoint of cardiovascular death, stroke, or MI, compared with those on aspirin plus placebo. This benefit came at a cost of a 51% increase in the risk of major bleeding, but not fatal bleeding or bleeding causing critical damage to the brain or other organs.
Taking into account both the primary efficacy and severe bleeding rates, the net clinical benefit of low-dose rivaroxaban was 20%. The absolute risk reduction was larger in the PAD subgroup than in those with CAD-only because of their greater baseline risk (N Engl J Med. 2017;377:1319-30).
At present a 2.5-mg dose of rivaroxaban isn’t commercially available, so patients have to cut higher-dose tablets, but on the strength of the COMPASS results, a 2.5-mg tablet is in the works, Dr. Vogel said.
PCSK9 inhibitors
In the FOURIER trial of evolocumab (Praluent) versus placebo on top of maximally tolerated statin therapy in more than 27,000 patients with atherosclerotic disease, including 3,642 with PAD, the rate of MALE (major adverse limb events) was reduced by 42% in the evolocumab group, with a number-needed-to-treat in order to prevent one additional MALE event of only 16 (Circulation. 2018;137:338-50).
“This is a subgroup that really benefits from PCSK9 inhibition. It’s something to think about,” Dr. Vogel said.
Dr. Vogel reported serving as a paid consultant to the National Football League and the Pritikin Longevity Center, receiving research grants from Sanofi, and serving on speakers bureaus for Regeneron and Sanofi.
EXPERT ANALYSIS FROM ACC SNOWMASS 2019
CMS proposing more flexibility in Medicare Advantage, Part D
More flexibility in benefits design could be coming to Medicare Advantage and the Part D prescription drug benefit if proposals offered by the Centers for Medicare & Medicaid Services are finalized.
The agency issued its proposed update for both programs for the 2020 plan year, which would allow Medicare Advantage plan sponsors to offer more specialized supplemental benefits for beneficiaries with chronic illnesses.
“For the 2020 plan year and beyond, Medicare Advantage plans will have greater flexibility to offer chronically ill patients any benefit that improves or maintains their health,” Demetrios Kouzoukas, CMS principal deputy administrator for Medicare and director of the Center for Medicare, said during a Jan. 30 press teleconference. “For example, plans could provide home-delivered or special meals in a far broader set of circumstances than what is allowed today.”
He noted that it would be up to the plans to determine what kinds of supplemental benefits would be offered and added that the offering of these benefits would not require a waiver, but would be evaluated as part of the plan’s overall bid submitted to the agency.
“We recognize that Medicare beneficiaries frequently have multiple chronic conditions,” Mr. Kouzoukas said. “We are excited that these changes will allow these beneficiaries to have new options for improving their health as a result of innovative health plan benefits.”
For Medicare Part D, the agency is specifically encouraging plan sponsors “to provide lower cost sharing for opioid reversal agents such as naloxone,” he added. The proposal also offers additional flexibility for plans to offer targeted benefits and cost sharing reductions to patients with chronic pain or those undergoing addiction treatment, according to a fact sheet highlighting key proposals.
Comments on the proposals are due by March 1. CMS expects to finalize the changes by the beginning of April.
More flexibility in benefits design could be coming to Medicare Advantage and the Part D prescription drug benefit if proposals offered by the Centers for Medicare & Medicaid Services are finalized.
The agency issued its proposed update for both programs for the 2020 plan year, which would allow Medicare Advantage plan sponsors to offer more specialized supplemental benefits for beneficiaries with chronic illnesses.
“For the 2020 plan year and beyond, Medicare Advantage plans will have greater flexibility to offer chronically ill patients any benefit that improves or maintains their health,” Demetrios Kouzoukas, CMS principal deputy administrator for Medicare and director of the Center for Medicare, said during a Jan. 30 press teleconference. “For example, plans could provide home-delivered or special meals in a far broader set of circumstances than what is allowed today.”
He noted that it would be up to the plans to determine what kinds of supplemental benefits would be offered and added that the offering of these benefits would not require a waiver, but would be evaluated as part of the plan’s overall bid submitted to the agency.
“We recognize that Medicare beneficiaries frequently have multiple chronic conditions,” Mr. Kouzoukas said. “We are excited that these changes will allow these beneficiaries to have new options for improving their health as a result of innovative health plan benefits.”
For Medicare Part D, the agency is specifically encouraging plan sponsors “to provide lower cost sharing for opioid reversal agents such as naloxone,” he added. The proposal also offers additional flexibility for plans to offer targeted benefits and cost sharing reductions to patients with chronic pain or those undergoing addiction treatment, according to a fact sheet highlighting key proposals.
Comments on the proposals are due by March 1. CMS expects to finalize the changes by the beginning of April.
More flexibility in benefits design could be coming to Medicare Advantage and the Part D prescription drug benefit if proposals offered by the Centers for Medicare & Medicaid Services are finalized.
The agency issued its proposed update for both programs for the 2020 plan year, which would allow Medicare Advantage plan sponsors to offer more specialized supplemental benefits for beneficiaries with chronic illnesses.
“For the 2020 plan year and beyond, Medicare Advantage plans will have greater flexibility to offer chronically ill patients any benefit that improves or maintains their health,” Demetrios Kouzoukas, CMS principal deputy administrator for Medicare and director of the Center for Medicare, said during a Jan. 30 press teleconference. “For example, plans could provide home-delivered or special meals in a far broader set of circumstances than what is allowed today.”
He noted that it would be up to the plans to determine what kinds of supplemental benefits would be offered and added that the offering of these benefits would not require a waiver, but would be evaluated as part of the plan’s overall bid submitted to the agency.
“We recognize that Medicare beneficiaries frequently have multiple chronic conditions,” Mr. Kouzoukas said. “We are excited that these changes will allow these beneficiaries to have new options for improving their health as a result of innovative health plan benefits.”
For Medicare Part D, the agency is specifically encouraging plan sponsors “to provide lower cost sharing for opioid reversal agents such as naloxone,” he added. The proposal also offers additional flexibility for plans to offer targeted benefits and cost sharing reductions to patients with chronic pain or those undergoing addiction treatment, according to a fact sheet highlighting key proposals.
Comments on the proposals are due by March 1. CMS expects to finalize the changes by the beginning of April.
Expert: There’s no single treatment for fibromyalgia
SAN DIEGO – There are many potential treatments for fibromyalgia, but a large number of them – NSAIDs, opioids, cannabis and more – come with caveats and nothing beats an old stand-by: physical rehabilitation.
With exercise, “we’re getting the muscles moving, and we’re getting [patients] used to stimulation that will hopefully deaden that pain response over time,” David E.J. Bazzo, MD, said at Pain Care for Primary Care. Still, “it’s going to take multiple things to best treat your patients.”
Fibromyalgia is unique, said Dr. Bazzo, professor of family medicine and public health at the University of California, San Diego. Diagnosis is based on self-reported symptoms since no laboratory tests are available. For diagnostic criteria, he recommends those released by the American College of Rheumatology in 2010 and 2011 and updated in 2016. The criteria, he said, recognize the importance of cognitive symptoms, unrefreshing sleep, fatigue, and certain somatic symptoms (Semin Arthritis Rheum. 2016;46[3]:319-29).
Poor sleep is an especially important problem in fibromyalgia, Dr. Bazzo said, although it’s “a bit of a chicken-and-egg discussion.” It’s not clear which comes first, but “we know that both happen hand-in-hand. We need to work on people’s sleep as one of the primary targets.”
When it comes to treatment, “you have to validate this person’s symptoms and say, ‘Yes, I believe you. I know that you are suffering, and that you’re having pain,’ ” Dr. Bazzo said at the meeting held by the American Pain Society and Global Academy for Medical Education. He advised clinicians to keep in mind conditions that can accompany fibromyalgia, such as depression, that may require other treatment options.
Dr. Bazzo offered advice about these approaches to treatment:
- Exercise. Research supports treadmill and cycle ergometry (BMJ 2002;325:185).
- Opioids. “There’s no convincing evidence that opioids have a role in treating fibromyalgia initially. If you’ve tried everything and patients have had problems, are just not responsive or had side effects, you could consider opioids. But that should be at the tail end of everything because the data is not there,” he said.
- Tramadol. “It’s like an opioid with potential for addiction,” he said. “Don’t just use it willy-nilly. Make sure you have a reason and a good plan. Would it be my first thing? No. Is it something that I keep in my back pocket when other things aren’t working? Perhaps. Would I use it before an opioid? For sure.”
- Second-line therapies. According to Dr. Bazzo, these include antiepileptics such as gabapentin and pregabalin, low-dose cyclobenzaprine, and dual reuptake inhibitors such as duloxetine. There are many other second-line options, he said, from behavioral approaches to yoga to guided physical therapy.
- NSAIDs. Not helpful.
- Cannabis. May interact with other medications.
- Pain clinics. Make sure you refer patients to a pain clinic that embraces a multidisciplinary approach, he said, not one that only offers “pain pills or shots.”
Dr. Bazzo reported no relevant conflicts of interest. The Global Academy for Medical Education and this news organization are owned by the same parent company.
SAN DIEGO – There are many potential treatments for fibromyalgia, but a large number of them – NSAIDs, opioids, cannabis and more – come with caveats and nothing beats an old stand-by: physical rehabilitation.
With exercise, “we’re getting the muscles moving, and we’re getting [patients] used to stimulation that will hopefully deaden that pain response over time,” David E.J. Bazzo, MD, said at Pain Care for Primary Care. Still, “it’s going to take multiple things to best treat your patients.”
Fibromyalgia is unique, said Dr. Bazzo, professor of family medicine and public health at the University of California, San Diego. Diagnosis is based on self-reported symptoms since no laboratory tests are available. For diagnostic criteria, he recommends those released by the American College of Rheumatology in 2010 and 2011 and updated in 2016. The criteria, he said, recognize the importance of cognitive symptoms, unrefreshing sleep, fatigue, and certain somatic symptoms (Semin Arthritis Rheum. 2016;46[3]:319-29).
Poor sleep is an especially important problem in fibromyalgia, Dr. Bazzo said, although it’s “a bit of a chicken-and-egg discussion.” It’s not clear which comes first, but “we know that both happen hand-in-hand. We need to work on people’s sleep as one of the primary targets.”
When it comes to treatment, “you have to validate this person’s symptoms and say, ‘Yes, I believe you. I know that you are suffering, and that you’re having pain,’ ” Dr. Bazzo said at the meeting held by the American Pain Society and Global Academy for Medical Education. He advised clinicians to keep in mind conditions that can accompany fibromyalgia, such as depression, that may require other treatment options.
Dr. Bazzo offered advice about these approaches to treatment:
- Exercise. Research supports treadmill and cycle ergometry (BMJ 2002;325:185).
- Opioids. “There’s no convincing evidence that opioids have a role in treating fibromyalgia initially. If you’ve tried everything and patients have had problems, are just not responsive or had side effects, you could consider opioids. But that should be at the tail end of everything because the data is not there,” he said.
- Tramadol. “It’s like an opioid with potential for addiction,” he said. “Don’t just use it willy-nilly. Make sure you have a reason and a good plan. Would it be my first thing? No. Is it something that I keep in my back pocket when other things aren’t working? Perhaps. Would I use it before an opioid? For sure.”
- Second-line therapies. According to Dr. Bazzo, these include antiepileptics such as gabapentin and pregabalin, low-dose cyclobenzaprine, and dual reuptake inhibitors such as duloxetine. There are many other second-line options, he said, from behavioral approaches to yoga to guided physical therapy.
- NSAIDs. Not helpful.
- Cannabis. May interact with other medications.
- Pain clinics. Make sure you refer patients to a pain clinic that embraces a multidisciplinary approach, he said, not one that only offers “pain pills or shots.”
Dr. Bazzo reported no relevant conflicts of interest. The Global Academy for Medical Education and this news organization are owned by the same parent company.
SAN DIEGO – There are many potential treatments for fibromyalgia, but a large number of them – NSAIDs, opioids, cannabis and more – come with caveats and nothing beats an old stand-by: physical rehabilitation.
With exercise, “we’re getting the muscles moving, and we’re getting [patients] used to stimulation that will hopefully deaden that pain response over time,” David E.J. Bazzo, MD, said at Pain Care for Primary Care. Still, “it’s going to take multiple things to best treat your patients.”
Fibromyalgia is unique, said Dr. Bazzo, professor of family medicine and public health at the University of California, San Diego. Diagnosis is based on self-reported symptoms since no laboratory tests are available. For diagnostic criteria, he recommends those released by the American College of Rheumatology in 2010 and 2011 and updated in 2016. The criteria, he said, recognize the importance of cognitive symptoms, unrefreshing sleep, fatigue, and certain somatic symptoms (Semin Arthritis Rheum. 2016;46[3]:319-29).
Poor sleep is an especially important problem in fibromyalgia, Dr. Bazzo said, although it’s “a bit of a chicken-and-egg discussion.” It’s not clear which comes first, but “we know that both happen hand-in-hand. We need to work on people’s sleep as one of the primary targets.”
When it comes to treatment, “you have to validate this person’s symptoms and say, ‘Yes, I believe you. I know that you are suffering, and that you’re having pain,’ ” Dr. Bazzo said at the meeting held by the American Pain Society and Global Academy for Medical Education. He advised clinicians to keep in mind conditions that can accompany fibromyalgia, such as depression, that may require other treatment options.
Dr. Bazzo offered advice about these approaches to treatment:
- Exercise. Research supports treadmill and cycle ergometry (BMJ 2002;325:185).
- Opioids. “There’s no convincing evidence that opioids have a role in treating fibromyalgia initially. If you’ve tried everything and patients have had problems, are just not responsive or had side effects, you could consider opioids. But that should be at the tail end of everything because the data is not there,” he said.
- Tramadol. “It’s like an opioid with potential for addiction,” he said. “Don’t just use it willy-nilly. Make sure you have a reason and a good plan. Would it be my first thing? No. Is it something that I keep in my back pocket when other things aren’t working? Perhaps. Would I use it before an opioid? For sure.”
- Second-line therapies. According to Dr. Bazzo, these include antiepileptics such as gabapentin and pregabalin, low-dose cyclobenzaprine, and dual reuptake inhibitors such as duloxetine. There are many other second-line options, he said, from behavioral approaches to yoga to guided physical therapy.
- NSAIDs. Not helpful.
- Cannabis. May interact with other medications.
- Pain clinics. Make sure you refer patients to a pain clinic that embraces a multidisciplinary approach, he said, not one that only offers “pain pills or shots.”
Dr. Bazzo reported no relevant conflicts of interest. The Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM PAIN CARE FOR PRIMARY CARE
Shifting drugs from Part B to Part D could be costly to patients
A shift in Medicare drug coverage from Part B to Part D might save the government some money but could end up costing some patients in the long run.
Analysis of the 75 brand-name drugs with the highest Part B expenditures ($21.6 billion annually at 2018 prices) indicated that the government could save between $17.6 billion and $20.1 billion after rebates by switching coverage to Part D, Thomas J. Hwang of Harvard Medical School, Boston, and his associates said.
The potential for greater overall savings, however, “was constrained by the fact that 33 (44%) of the studied brand-name drugs were in protected classes, which HHS has reported precludes meaningful price negotiation by Part D plans,” they wrote.
The proposal also could have a “material impact” on patient out-of-pocket costs, although the impact would vary based on the drug as well as patients’ insurance coverage in addition to Medicare (JAMA Int Med. 2019. doi: 10.1001/jamainternmed.2018.6417).
For example, moving drug coverage to Part D would lower out-of-pocket costs for the majority of the 75 drugs for patients with Medigap supplemental insurance, but out-of-pocket costs could go up for almost 40% of products. Patients who would benefit most from the shift would be those who qualify for the low-income subsidy, which can eliminate coinsurance requirements.
“By contrast, for patients with Medigap insurance, out-of-pocket costs in Part D were estimated to exceed the annual premium costs for supplemental insurance [approximately 47-56 of the 75 drugs],” Mr. Hwang and his colleagues added. “Out-of-pocket costs would be increased under the proposed policy for beneficiaries with Medigap but without Part D coverage.”
The analysis was limited by the inability to predict the proposed transition’s impact on insurance premiums or drug utilization. Patients who were dually eligible for Medicare and Medicaid were excluded.
SOURCE: Hwang TJ et al. JAMA Int Med. 2019. doi: 10.1001/jamainternmed.2018.6417.
Policy analysts need to be careful and do their due diligence to ensure all consequences of the policy options are fully understood, especially as pharmaceuticals account for greater costs in the Medicare program. Future policy analyses must, like Mr. Hwang and his associates did, account for changes to Medicare costs as well as beneficiary costs to understand the overall effects of policy changes.
Francis Crosson, MD, chairman of the Medicare Payment Advisory Commission, and Jon Christianson, PhD, vice chairman of MedPAC, made these comments in an accompanying editorial (JAMA Int Med. doi: 10.1001/jamainternmed.2018.6146 ).
Policy analysts need to be careful and do their due diligence to ensure all consequences of the policy options are fully understood, especially as pharmaceuticals account for greater costs in the Medicare program. Future policy analyses must, like Mr. Hwang and his associates did, account for changes to Medicare costs as well as beneficiary costs to understand the overall effects of policy changes.
Francis Crosson, MD, chairman of the Medicare Payment Advisory Commission, and Jon Christianson, PhD, vice chairman of MedPAC, made these comments in an accompanying editorial (JAMA Int Med. doi: 10.1001/jamainternmed.2018.6146 ).
Policy analysts need to be careful and do their due diligence to ensure all consequences of the policy options are fully understood, especially as pharmaceuticals account for greater costs in the Medicare program. Future policy analyses must, like Mr. Hwang and his associates did, account for changes to Medicare costs as well as beneficiary costs to understand the overall effects of policy changes.
Francis Crosson, MD, chairman of the Medicare Payment Advisory Commission, and Jon Christianson, PhD, vice chairman of MedPAC, made these comments in an accompanying editorial (JAMA Int Med. doi: 10.1001/jamainternmed.2018.6146 ).
A shift in Medicare drug coverage from Part B to Part D might save the government some money but could end up costing some patients in the long run.
Analysis of the 75 brand-name drugs with the highest Part B expenditures ($21.6 billion annually at 2018 prices) indicated that the government could save between $17.6 billion and $20.1 billion after rebates by switching coverage to Part D, Thomas J. Hwang of Harvard Medical School, Boston, and his associates said.
The potential for greater overall savings, however, “was constrained by the fact that 33 (44%) of the studied brand-name drugs were in protected classes, which HHS has reported precludes meaningful price negotiation by Part D plans,” they wrote.
The proposal also could have a “material impact” on patient out-of-pocket costs, although the impact would vary based on the drug as well as patients’ insurance coverage in addition to Medicare (JAMA Int Med. 2019. doi: 10.1001/jamainternmed.2018.6417).
For example, moving drug coverage to Part D would lower out-of-pocket costs for the majority of the 75 drugs for patients with Medigap supplemental insurance, but out-of-pocket costs could go up for almost 40% of products. Patients who would benefit most from the shift would be those who qualify for the low-income subsidy, which can eliminate coinsurance requirements.
“By contrast, for patients with Medigap insurance, out-of-pocket costs in Part D were estimated to exceed the annual premium costs for supplemental insurance [approximately 47-56 of the 75 drugs],” Mr. Hwang and his colleagues added. “Out-of-pocket costs would be increased under the proposed policy for beneficiaries with Medigap but without Part D coverage.”
The analysis was limited by the inability to predict the proposed transition’s impact on insurance premiums or drug utilization. Patients who were dually eligible for Medicare and Medicaid were excluded.
SOURCE: Hwang TJ et al. JAMA Int Med. 2019. doi: 10.1001/jamainternmed.2018.6417.
A shift in Medicare drug coverage from Part B to Part D might save the government some money but could end up costing some patients in the long run.
Analysis of the 75 brand-name drugs with the highest Part B expenditures ($21.6 billion annually at 2018 prices) indicated that the government could save between $17.6 billion and $20.1 billion after rebates by switching coverage to Part D, Thomas J. Hwang of Harvard Medical School, Boston, and his associates said.
The potential for greater overall savings, however, “was constrained by the fact that 33 (44%) of the studied brand-name drugs were in protected classes, which HHS has reported precludes meaningful price negotiation by Part D plans,” they wrote.
The proposal also could have a “material impact” on patient out-of-pocket costs, although the impact would vary based on the drug as well as patients’ insurance coverage in addition to Medicare (JAMA Int Med. 2019. doi: 10.1001/jamainternmed.2018.6417).
For example, moving drug coverage to Part D would lower out-of-pocket costs for the majority of the 75 drugs for patients with Medigap supplemental insurance, but out-of-pocket costs could go up for almost 40% of products. Patients who would benefit most from the shift would be those who qualify for the low-income subsidy, which can eliminate coinsurance requirements.
“By contrast, for patients with Medigap insurance, out-of-pocket costs in Part D were estimated to exceed the annual premium costs for supplemental insurance [approximately 47-56 of the 75 drugs],” Mr. Hwang and his colleagues added. “Out-of-pocket costs would be increased under the proposed policy for beneficiaries with Medigap but without Part D coverage.”
The analysis was limited by the inability to predict the proposed transition’s impact on insurance premiums or drug utilization. Patients who were dually eligible for Medicare and Medicaid were excluded.
SOURCE: Hwang TJ et al. JAMA Int Med. 2019. doi: 10.1001/jamainternmed.2018.6417.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Shifting drug coverage to Part D would save the government money.
Major finding: For patients with Medigap plans, costs could increase on as many as 40% of the drugs studied.
Study details: Researchers examined the 75 drugs with the highest Part B expenditures in 2016 and, using 2018 prices, estimated the effect of moving these drugs into the Part D prescription drug program.
Disclosures: No relevant conflicts of interest were reported.
Source: Hwang TJ et al. JAMA Int Med. 2019. doi: 10.1001/jamainternmed.2018.6417.
Pregnancy problems predict cardiovascular future
SNOWMASS, COLO. – Think of pregnancy as a cardiovascular stress test, Carole A. Warnes, MD, urged at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Pregnancy complications may unmask a predisposition to premature cardiovascular disease. Yet a woman’s reproductive history is often overlooked in this regard, despite the fact that cardiovascular disease is the number-one cause of death in women, observed Dr. Warnes, the Snowmass conference director and professor of medicine at the Mayo Clinic in Rochester, Minn.
“I think reproductive history is often overlooked as a predictor of cardiovascular and even peripheral vascular events. I suspect many of us don’t routinely ask our patients about miscarriages and stillbirths. We might think about preeclampsia, but these are also hallmarks of trouble to come,” the cardiologist said.
Indeed, this point was underscored in a retrospective Danish national population-based cohort registry study of more than 1 million women followed for nearly 16 million person-years after one or more miscarriages, stillbirths, or live singleton births. Women with stillbirths were 2.69-fold more likely to have an MI, 2.42-fold more likely to develop renovascular hypertension, and 1.74-fold more likely to have a stroke during follow-up than those with no stillbirths.
Moreover, women with miscarriages were 1.13-, 1.2-, and 1.16-fold more likely to have an MI, renovascular hypertension, and stroke, respectively, than women with no miscarriages. And the risks were additive: For each additional miscarriage, the risks of MI, renovascular hypertension, and stroke increased by 9%, 19%, and 13%, respectively (Circulation. 2013;127[17]:1775-82).
The concept of maternal placental syndromes encompasses four events believed to originate from diseased placental blood vessels: preeclampsia, gestational hypertension, placental abruption, and placental infarction. In a population-based retrospective study known as CHAMPS (Cardiovascular Health After Maternal Placental Syndromes), conducted in more than 1 million Ontario women who were free from cardiovascular disease prior to their first delivery, 7% were diagnosed with a maternal placental syndrome. Their incidence of a composite endpoint comprised of hospitalization or revascularization for CAD, peripheral artery disease, or cerebrovascular disease at least 90 days after delivery discharge was double that of women without a maternal placental syndrome.
“These women manifested their first cardiovascular event at an average age of 38, not 50 or 60,” Dr. Warnes said.
The risk of premature cardiovascular disease was magnified 4.4-fold in women with a maternal placental syndrome plus an intrauterine fetal death, compared with those with neither, after adjustment for sociodemographic factors and other potential confounders, and by 3.1-fold in women with a maternal placental syndrome and poor fetal growth (Lancet. 2005;366[9499]:1797-803).
These findings were independently confirmed recently in a population-based retrospective study of nearly 303,000 Florida women free of prepregnancy hypertension, diabetes, heart disease, or renal disease who were followed for a median of 4.9 years after their first delivery. During that relative brief follow-up period, the adjusted risk of cardiovascular disease was increased by 19% in those with a maternal placental syndrome, compared with those without. And the risk was additive: women with more than one maternal placental syndrome had a 43% greater short-term risk of developing cardiovascular disease, compared with those with none. And when women with a maternal placental syndrome also had a preterm birth or a small-for-gestational age baby, their risk increased 45% (Am J Obstet Gynecol. 2016;215[4]:484.e1-484.e14).
It’s not just preeclampsia, which affects 3%-5% of all pregnancies, and gestational hypertension – defined as high blood pressure arising only after 20 weeks’ gestation and without proteinuria – that have been linked to future premature cardiovascular disease. In the Northern Finland Birth Cohort 1966, in which investigators have followed 10,314 women born in that year for 39 years, any form of high blood pressure during pregnancy was a harbinger of subsequent cardiovascular disease, diabetes, and chronic kidney disease. That included chronic isolated systolic and isolated diastolic hypertension (Circulation. 2013;127[6]:681-90).
The pathophysiologic processes involved in complicated pregnancies echo those of CAD and stroke: inflammation, altered angiogenesis, vasculopathy, thrombosis, and insulin resistance. Still unsettled, however, is the chicken-versus-egg question of whether preeclampsia and other pregnancy complications represent the initial expression of an adverse phenotype associated with early development of cardiovascular disease or the complications injure the vascular endothelium and thereby trigger accelerated atherosclerosis. In any case, markers of endothelial activation have been documented up to 15 years after an episode of preeclampsia, Dr. Warnes said.
All of these data underscore the importance of identifying at-risk women based upon reproductive history, scheduling additional medical checkups so they don’t drop off the radar for the next 20 years, encouraging lifestyle modification, and giving consideration to early initiation of antihypertensive and lipid-lowering therapies.
“Pregnancy complications give us a glimpse of this awful disease trajectory at a time when women are completely asymptomatic and we could intervene and perhaps change outcomes with targeted therapy when it might be expected to work better and patients might be more receptive to such interventions,” she said.
Dr. Warnes reported having no financial conflicts of interest.
SNOWMASS, COLO. – Think of pregnancy as a cardiovascular stress test, Carole A. Warnes, MD, urged at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Pregnancy complications may unmask a predisposition to premature cardiovascular disease. Yet a woman’s reproductive history is often overlooked in this regard, despite the fact that cardiovascular disease is the number-one cause of death in women, observed Dr. Warnes, the Snowmass conference director and professor of medicine at the Mayo Clinic in Rochester, Minn.
“I think reproductive history is often overlooked as a predictor of cardiovascular and even peripheral vascular events. I suspect many of us don’t routinely ask our patients about miscarriages and stillbirths. We might think about preeclampsia, but these are also hallmarks of trouble to come,” the cardiologist said.
Indeed, this point was underscored in a retrospective Danish national population-based cohort registry study of more than 1 million women followed for nearly 16 million person-years after one or more miscarriages, stillbirths, or live singleton births. Women with stillbirths were 2.69-fold more likely to have an MI, 2.42-fold more likely to develop renovascular hypertension, and 1.74-fold more likely to have a stroke during follow-up than those with no stillbirths.
Moreover, women with miscarriages were 1.13-, 1.2-, and 1.16-fold more likely to have an MI, renovascular hypertension, and stroke, respectively, than women with no miscarriages. And the risks were additive: For each additional miscarriage, the risks of MI, renovascular hypertension, and stroke increased by 9%, 19%, and 13%, respectively (Circulation. 2013;127[17]:1775-82).
The concept of maternal placental syndromes encompasses four events believed to originate from diseased placental blood vessels: preeclampsia, gestational hypertension, placental abruption, and placental infarction. In a population-based retrospective study known as CHAMPS (Cardiovascular Health After Maternal Placental Syndromes), conducted in more than 1 million Ontario women who were free from cardiovascular disease prior to their first delivery, 7% were diagnosed with a maternal placental syndrome. Their incidence of a composite endpoint comprised of hospitalization or revascularization for CAD, peripheral artery disease, or cerebrovascular disease at least 90 days after delivery discharge was double that of women without a maternal placental syndrome.
“These women manifested their first cardiovascular event at an average age of 38, not 50 or 60,” Dr. Warnes said.
The risk of premature cardiovascular disease was magnified 4.4-fold in women with a maternal placental syndrome plus an intrauterine fetal death, compared with those with neither, after adjustment for sociodemographic factors and other potential confounders, and by 3.1-fold in women with a maternal placental syndrome and poor fetal growth (Lancet. 2005;366[9499]:1797-803).
These findings were independently confirmed recently in a population-based retrospective study of nearly 303,000 Florida women free of prepregnancy hypertension, diabetes, heart disease, or renal disease who were followed for a median of 4.9 years after their first delivery. During that relative brief follow-up period, the adjusted risk of cardiovascular disease was increased by 19% in those with a maternal placental syndrome, compared with those without. And the risk was additive: women with more than one maternal placental syndrome had a 43% greater short-term risk of developing cardiovascular disease, compared with those with none. And when women with a maternal placental syndrome also had a preterm birth or a small-for-gestational age baby, their risk increased 45% (Am J Obstet Gynecol. 2016;215[4]:484.e1-484.e14).
It’s not just preeclampsia, which affects 3%-5% of all pregnancies, and gestational hypertension – defined as high blood pressure arising only after 20 weeks’ gestation and without proteinuria – that have been linked to future premature cardiovascular disease. In the Northern Finland Birth Cohort 1966, in which investigators have followed 10,314 women born in that year for 39 years, any form of high blood pressure during pregnancy was a harbinger of subsequent cardiovascular disease, diabetes, and chronic kidney disease. That included chronic isolated systolic and isolated diastolic hypertension (Circulation. 2013;127[6]:681-90).
The pathophysiologic processes involved in complicated pregnancies echo those of CAD and stroke: inflammation, altered angiogenesis, vasculopathy, thrombosis, and insulin resistance. Still unsettled, however, is the chicken-versus-egg question of whether preeclampsia and other pregnancy complications represent the initial expression of an adverse phenotype associated with early development of cardiovascular disease or the complications injure the vascular endothelium and thereby trigger accelerated atherosclerosis. In any case, markers of endothelial activation have been documented up to 15 years after an episode of preeclampsia, Dr. Warnes said.
All of these data underscore the importance of identifying at-risk women based upon reproductive history, scheduling additional medical checkups so they don’t drop off the radar for the next 20 years, encouraging lifestyle modification, and giving consideration to early initiation of antihypertensive and lipid-lowering therapies.
“Pregnancy complications give us a glimpse of this awful disease trajectory at a time when women are completely asymptomatic and we could intervene and perhaps change outcomes with targeted therapy when it might be expected to work better and patients might be more receptive to such interventions,” she said.
Dr. Warnes reported having no financial conflicts of interest.
SNOWMASS, COLO. – Think of pregnancy as a cardiovascular stress test, Carole A. Warnes, MD, urged at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Pregnancy complications may unmask a predisposition to premature cardiovascular disease. Yet a woman’s reproductive history is often overlooked in this regard, despite the fact that cardiovascular disease is the number-one cause of death in women, observed Dr. Warnes, the Snowmass conference director and professor of medicine at the Mayo Clinic in Rochester, Minn.
“I think reproductive history is often overlooked as a predictor of cardiovascular and even peripheral vascular events. I suspect many of us don’t routinely ask our patients about miscarriages and stillbirths. We might think about preeclampsia, but these are also hallmarks of trouble to come,” the cardiologist said.
Indeed, this point was underscored in a retrospective Danish national population-based cohort registry study of more than 1 million women followed for nearly 16 million person-years after one or more miscarriages, stillbirths, or live singleton births. Women with stillbirths were 2.69-fold more likely to have an MI, 2.42-fold more likely to develop renovascular hypertension, and 1.74-fold more likely to have a stroke during follow-up than those with no stillbirths.
Moreover, women with miscarriages were 1.13-, 1.2-, and 1.16-fold more likely to have an MI, renovascular hypertension, and stroke, respectively, than women with no miscarriages. And the risks were additive: For each additional miscarriage, the risks of MI, renovascular hypertension, and stroke increased by 9%, 19%, and 13%, respectively (Circulation. 2013;127[17]:1775-82).
The concept of maternal placental syndromes encompasses four events believed to originate from diseased placental blood vessels: preeclampsia, gestational hypertension, placental abruption, and placental infarction. In a population-based retrospective study known as CHAMPS (Cardiovascular Health After Maternal Placental Syndromes), conducted in more than 1 million Ontario women who were free from cardiovascular disease prior to their first delivery, 7% were diagnosed with a maternal placental syndrome. Their incidence of a composite endpoint comprised of hospitalization or revascularization for CAD, peripheral artery disease, or cerebrovascular disease at least 90 days after delivery discharge was double that of women without a maternal placental syndrome.
“These women manifested their first cardiovascular event at an average age of 38, not 50 or 60,” Dr. Warnes said.
The risk of premature cardiovascular disease was magnified 4.4-fold in women with a maternal placental syndrome plus an intrauterine fetal death, compared with those with neither, after adjustment for sociodemographic factors and other potential confounders, and by 3.1-fold in women with a maternal placental syndrome and poor fetal growth (Lancet. 2005;366[9499]:1797-803).
These findings were independently confirmed recently in a population-based retrospective study of nearly 303,000 Florida women free of prepregnancy hypertension, diabetes, heart disease, or renal disease who were followed for a median of 4.9 years after their first delivery. During that relative brief follow-up period, the adjusted risk of cardiovascular disease was increased by 19% in those with a maternal placental syndrome, compared with those without. And the risk was additive: women with more than one maternal placental syndrome had a 43% greater short-term risk of developing cardiovascular disease, compared with those with none. And when women with a maternal placental syndrome also had a preterm birth or a small-for-gestational age baby, their risk increased 45% (Am J Obstet Gynecol. 2016;215[4]:484.e1-484.e14).
It’s not just preeclampsia, which affects 3%-5% of all pregnancies, and gestational hypertension – defined as high blood pressure arising only after 20 weeks’ gestation and without proteinuria – that have been linked to future premature cardiovascular disease. In the Northern Finland Birth Cohort 1966, in which investigators have followed 10,314 women born in that year for 39 years, any form of high blood pressure during pregnancy was a harbinger of subsequent cardiovascular disease, diabetes, and chronic kidney disease. That included chronic isolated systolic and isolated diastolic hypertension (Circulation. 2013;127[6]:681-90).
The pathophysiologic processes involved in complicated pregnancies echo those of CAD and stroke: inflammation, altered angiogenesis, vasculopathy, thrombosis, and insulin resistance. Still unsettled, however, is the chicken-versus-egg question of whether preeclampsia and other pregnancy complications represent the initial expression of an adverse phenotype associated with early development of cardiovascular disease or the complications injure the vascular endothelium and thereby trigger accelerated atherosclerosis. In any case, markers of endothelial activation have been documented up to 15 years after an episode of preeclampsia, Dr. Warnes said.
All of these data underscore the importance of identifying at-risk women based upon reproductive history, scheduling additional medical checkups so they don’t drop off the radar for the next 20 years, encouraging lifestyle modification, and giving consideration to early initiation of antihypertensive and lipid-lowering therapies.
“Pregnancy complications give us a glimpse of this awful disease trajectory at a time when women are completely asymptomatic and we could intervene and perhaps change outcomes with targeted therapy when it might be expected to work better and patients might be more receptive to such interventions,” she said.
Dr. Warnes reported having no financial conflicts of interest.
EXPERT ANALYSIS FROM ACC SNOWMASS 2019