User login
What's your diagnosis?
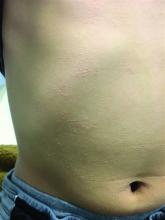
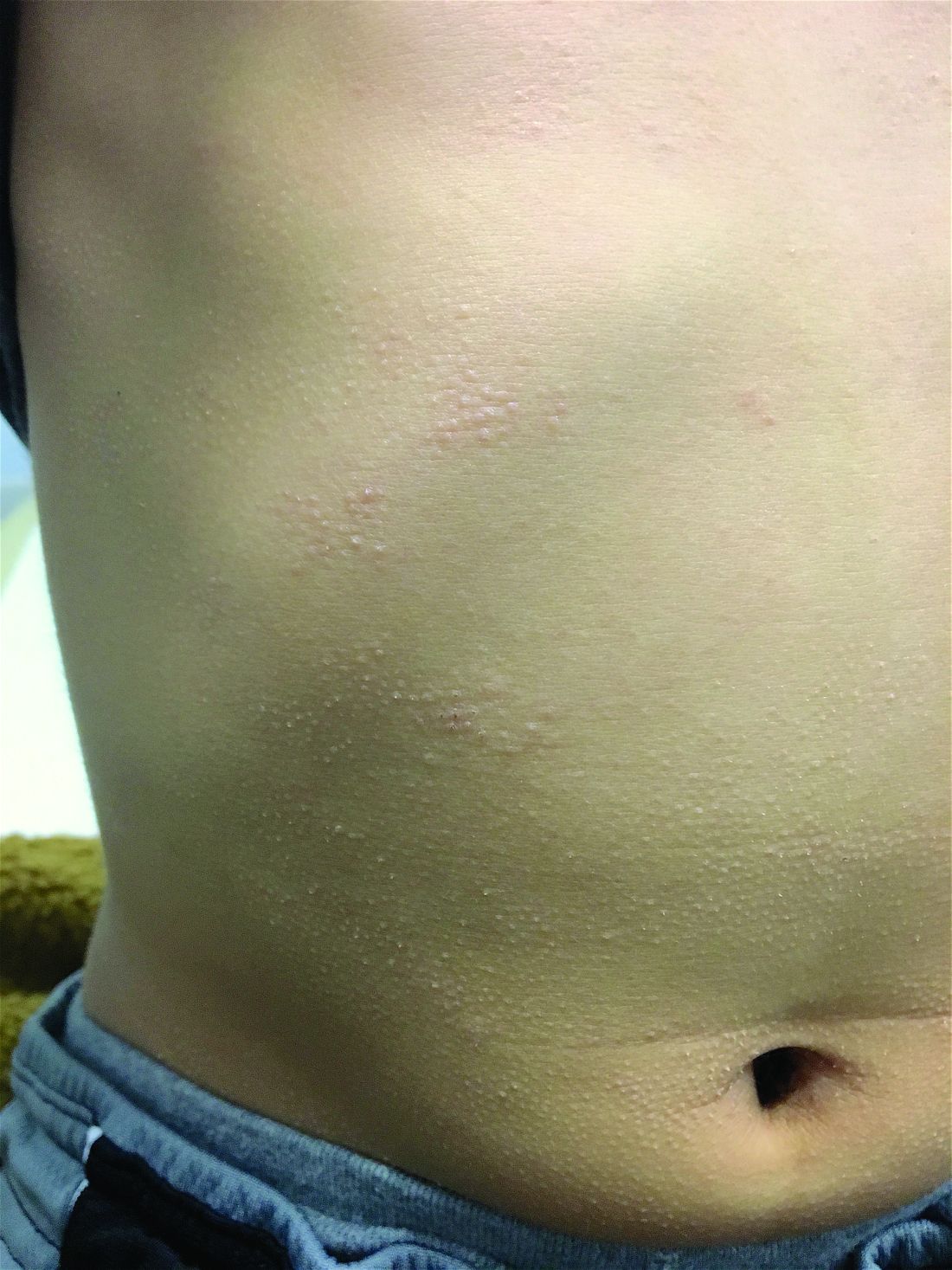
The patient was diagnosed with lichen spinulosus (LS) based on the physical appearance of the lesions (hyperkeratotic spiny papules forming plaques), the lack of pruritus, and negative personal history of atopic dermatitis.
Lichen spinulosus is an underreported entity, first described in 1908 by Adamson as superficial circumscribed chronic dermatitis in children and adolescents. The median age of presentation is age 16 years. There are several reports of possible associations with systemic infections such as HIV, fungi, and syphilis, as well as chronic diseases such as Crohn’s disease, Hodgkin disease, seborrhea, and secondary to certain medications such as omeprazole. There are no prior reports of infliximab being associated with LS, but it has been reported to cause other lichenoid reactions such as lichen planus and lichen planopilaris.
Clinically the lesions are characterized by asymptomatic, small (1 cm), skin color, hyperkeratotic, follicular papules that coalesce into plaques. The lesions usually occur on the extensor surfaces of the arms, neck, torso, and buttocks. Mild pruritus can occur in some patients.
The lesions in keratosis pilaris can be very similar to lichen spinulosus, but usually they don’t coalesce into plaques and are commonly present on the extensor surfaces of the arms, thighs, and cheeks. Histopathology of both conditions is very similar.
Another condition to consider includes papular eczema. The lesions in papular eczema tend to be pruritic and are not as circumscribed as LS lesions. Papular eczema responds well to the use of topical corticosteroids, while LS lesions usually do not. Lichen nitidus (LN) is characterized by monomorphic, skin color, 1-mm, flat-topped papules. Lesions tend to occur in crops rather than circumscribed papules forming plaques. LN most commonly presents on the extensor surface of the arms, trunk, dorsal hands, and genitalia. Koebner phenomenon is usually seen. Although uncommon in children, a more generalized type of follicular mucinosis can present very similar to lichen spinulosus. A recent study found LS-like lesions with associated hypopigmentation and hair loss should be suspicious for folliculotropic mycosis fungoides.
Keratolytics such as lactic acid, urea, and salicylic acid can help improve LS, although they do not cure it. Other reported treatments include the use of topical adapalene, tacalcitol cream, and tretinoin gel with hydroactive adhesive.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at pdnews@mdedge.com.
The patient was diagnosed with lichen spinulosus (LS) based on the physical appearance of the lesions (hyperkeratotic spiny papules forming plaques), the lack of pruritus, and negative personal history of atopic dermatitis.
Lichen spinulosus is an underreported entity, first described in 1908 by Adamson as superficial circumscribed chronic dermatitis in children and adolescents. The median age of presentation is age 16 years. There are several reports of possible associations with systemic infections such as HIV, fungi, and syphilis, as well as chronic diseases such as Crohn’s disease, Hodgkin disease, seborrhea, and secondary to certain medications such as omeprazole. There are no prior reports of infliximab being associated with LS, but it has been reported to cause other lichenoid reactions such as lichen planus and lichen planopilaris.
Clinically the lesions are characterized by asymptomatic, small (1 cm), skin color, hyperkeratotic, follicular papules that coalesce into plaques. The lesions usually occur on the extensor surfaces of the arms, neck, torso, and buttocks. Mild pruritus can occur in some patients.
The lesions in keratosis pilaris can be very similar to lichen spinulosus, but usually they don’t coalesce into plaques and are commonly present on the extensor surfaces of the arms, thighs, and cheeks. Histopathology of both conditions is very similar.
Another condition to consider includes papular eczema. The lesions in papular eczema tend to be pruritic and are not as circumscribed as LS lesions. Papular eczema responds well to the use of topical corticosteroids, while LS lesions usually do not. Lichen nitidus (LN) is characterized by monomorphic, skin color, 1-mm, flat-topped papules. Lesions tend to occur in crops rather than circumscribed papules forming plaques. LN most commonly presents on the extensor surface of the arms, trunk, dorsal hands, and genitalia. Koebner phenomenon is usually seen. Although uncommon in children, a more generalized type of follicular mucinosis can present very similar to lichen spinulosus. A recent study found LS-like lesions with associated hypopigmentation and hair loss should be suspicious for folliculotropic mycosis fungoides.
Keratolytics such as lactic acid, urea, and salicylic acid can help improve LS, although they do not cure it. Other reported treatments include the use of topical adapalene, tacalcitol cream, and tretinoin gel with hydroactive adhesive.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at pdnews@mdedge.com.
The patient was diagnosed with lichen spinulosus (LS) based on the physical appearance of the lesions (hyperkeratotic spiny papules forming plaques), the lack of pruritus, and negative personal history of atopic dermatitis.
Lichen spinulosus is an underreported entity, first described in 1908 by Adamson as superficial circumscribed chronic dermatitis in children and adolescents. The median age of presentation is age 16 years. There are several reports of possible associations with systemic infections such as HIV, fungi, and syphilis, as well as chronic diseases such as Crohn’s disease, Hodgkin disease, seborrhea, and secondary to certain medications such as omeprazole. There are no prior reports of infliximab being associated with LS, but it has been reported to cause other lichenoid reactions such as lichen planus and lichen planopilaris.
Clinically the lesions are characterized by asymptomatic, small (1 cm), skin color, hyperkeratotic, follicular papules that coalesce into plaques. The lesions usually occur on the extensor surfaces of the arms, neck, torso, and buttocks. Mild pruritus can occur in some patients.
The lesions in keratosis pilaris can be very similar to lichen spinulosus, but usually they don’t coalesce into plaques and are commonly present on the extensor surfaces of the arms, thighs, and cheeks. Histopathology of both conditions is very similar.
Another condition to consider includes papular eczema. The lesions in papular eczema tend to be pruritic and are not as circumscribed as LS lesions. Papular eczema responds well to the use of topical corticosteroids, while LS lesions usually do not. Lichen nitidus (LN) is characterized by monomorphic, skin color, 1-mm, flat-topped papules. Lesions tend to occur in crops rather than circumscribed papules forming plaques. LN most commonly presents on the extensor surface of the arms, trunk, dorsal hands, and genitalia. Koebner phenomenon is usually seen. Although uncommon in children, a more generalized type of follicular mucinosis can present very similar to lichen spinulosus. A recent study found LS-like lesions with associated hypopigmentation and hair loss should be suspicious for folliculotropic mycosis fungoides.
Keratolytics such as lactic acid, urea, and salicylic acid can help improve LS, although they do not cure it. Other reported treatments include the use of topical adapalene, tacalcitol cream, and tretinoin gel with hydroactive adhesive.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at pdnews@mdedge.com.
A 7-year-old male with a history of Crohn's disease presents with 6 months of asymptomatic, bumpy lesions on the torso and extremities. He has been using over-the-counter hydrocortisone and moisturizers without it helping. His Crohn's disease has been controlled with infliximab infusions for 2 years. The mother is concerned the rash could be a side effect of the medication.
He denies any prior history of atopic dermatitis or psoriasis. The mother had eczema as a child. He has two brothers who have been diagnosed and treated for allergic rhinitis.
On physical examination, he is a thin, pleasant young boy in no distress.
His skin is somewhat dry, and there are several hyperkeratotic follicular papules forming plaques on the torso and extremities. There is no associated hair loss on the affected areas or inflammation noted.
FDA approves Adacel for repeat Tdap vaccinations
The Food and Drug Administration has approved the expanded use of Adacel (Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis (Tdap) Vaccine Adsorbed) to include repeat vaccinations 8 years or more after the first vaccination in people aged 10-64 years.
The expanded indication was based on results of a randomized, controlled trial, published in the Journal of the Pediatric Infectious Diseases Society, in which more than 1,300 adults aged 18-64 years received either Adacel or a Td (tetanus-diphtheria) vaccine 8-12 years after receiving a previous dose of Adacel.
Over the course of the study, no significant difference in adverse event incidence was observed between groups. Injection-site reaction was the most common adverse event during the study, occurring in 87.7% of those who received Adacel and 88.0% of those who received the Td vaccine. Other common adverse events associated with Adacel include headache, body ache or muscle weakness, tiredness, muscle aches, and general discomfort.
“While strong vaccination programs are in place for young adolescents, a single Tdap immunization does not offer lifetime protection against pertussis due to waning immunity. The licensure of Adacel as the first Tdap vaccine in the U.S. for repeat vaccination is an important step for eligible patients and offers flexibility for health care providers to help manage their immunization schedules,” said David P. Greenberg, MD, regional medical head North America at Sanofi Pasteur, in the press release.
Find the full press release on the Sanofi website.
The Food and Drug Administration has approved the expanded use of Adacel (Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis (Tdap) Vaccine Adsorbed) to include repeat vaccinations 8 years or more after the first vaccination in people aged 10-64 years.
The expanded indication was based on results of a randomized, controlled trial, published in the Journal of the Pediatric Infectious Diseases Society, in which more than 1,300 adults aged 18-64 years received either Adacel or a Td (tetanus-diphtheria) vaccine 8-12 years after receiving a previous dose of Adacel.
Over the course of the study, no significant difference in adverse event incidence was observed between groups. Injection-site reaction was the most common adverse event during the study, occurring in 87.7% of those who received Adacel and 88.0% of those who received the Td vaccine. Other common adverse events associated with Adacel include headache, body ache or muscle weakness, tiredness, muscle aches, and general discomfort.
“While strong vaccination programs are in place for young adolescents, a single Tdap immunization does not offer lifetime protection against pertussis due to waning immunity. The licensure of Adacel as the first Tdap vaccine in the U.S. for repeat vaccination is an important step for eligible patients and offers flexibility for health care providers to help manage their immunization schedules,” said David P. Greenberg, MD, regional medical head North America at Sanofi Pasteur, in the press release.
Find the full press release on the Sanofi website.
The Food and Drug Administration has approved the expanded use of Adacel (Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis (Tdap) Vaccine Adsorbed) to include repeat vaccinations 8 years or more after the first vaccination in people aged 10-64 years.
The expanded indication was based on results of a randomized, controlled trial, published in the Journal of the Pediatric Infectious Diseases Society, in which more than 1,300 adults aged 18-64 years received either Adacel or a Td (tetanus-diphtheria) vaccine 8-12 years after receiving a previous dose of Adacel.
Over the course of the study, no significant difference in adverse event incidence was observed between groups. Injection-site reaction was the most common adverse event during the study, occurring in 87.7% of those who received Adacel and 88.0% of those who received the Td vaccine. Other common adverse events associated with Adacel include headache, body ache or muscle weakness, tiredness, muscle aches, and general discomfort.
“While strong vaccination programs are in place for young adolescents, a single Tdap immunization does not offer lifetime protection against pertussis due to waning immunity. The licensure of Adacel as the first Tdap vaccine in the U.S. for repeat vaccination is an important step for eligible patients and offers flexibility for health care providers to help manage their immunization schedules,” said David P. Greenberg, MD, regional medical head North America at Sanofi Pasteur, in the press release.
Find the full press release on the Sanofi website.
Lower grip strength associated with worse health outcomes
Background: Previous studies have shown that lower muscle function is associated with increased mortality; however, studies have not been able to fully examine associations with age and disease-specific mortality.
Study design: Prospective, population-based study.
Setting: Large population cohort in the United Kingdom (UK Biobank).
Synopsis: The UK Biobank population included 502,293 individuals, aged 40-69 years, recruited during April 2007–December 2010, with grip strength data available. Mean follow-up was 7.1 years for all-cause and disease-specific mortality. Cox proportional hazard models were used to report hazard ratios (HR) per 5-kg decrease in grip strength and were controlled for multiple sociodemographic and lifestyle factors. A lower grip strength was found to correlate with all-cause mortality (HR, 1.16 in women; HR, 1.20 in men) as well as incidence of and mortality from cardiovascular disease, respiratory disease, and cancer. Hazard ratios were higher among younger age groups with similar lower grip strength. The use of grip strength also improved the prediction of an office-based mortality risk score from cardiovascular disease.
Bottom line: Grip strength is a useful and easy-to-obtain measurement that is associated with all-cause and disease-specific morbidity and can be used to improve the prediction of an office-based risk score.
Citation: Celis-Morales CA et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all-cause mortality: Prospective cohort study of half a million UK Biobank participants. BMJ. 2018;361:k1651.
Dr. Marr is assistant professor of medicine and an academic hospitalist, University of Utah, Salt Lake City.
Background: Previous studies have shown that lower muscle function is associated with increased mortality; however, studies have not been able to fully examine associations with age and disease-specific mortality.
Study design: Prospective, population-based study.
Setting: Large population cohort in the United Kingdom (UK Biobank).
Synopsis: The UK Biobank population included 502,293 individuals, aged 40-69 years, recruited during April 2007–December 2010, with grip strength data available. Mean follow-up was 7.1 years for all-cause and disease-specific mortality. Cox proportional hazard models were used to report hazard ratios (HR) per 5-kg decrease in grip strength and were controlled for multiple sociodemographic and lifestyle factors. A lower grip strength was found to correlate with all-cause mortality (HR, 1.16 in women; HR, 1.20 in men) as well as incidence of and mortality from cardiovascular disease, respiratory disease, and cancer. Hazard ratios were higher among younger age groups with similar lower grip strength. The use of grip strength also improved the prediction of an office-based mortality risk score from cardiovascular disease.
Bottom line: Grip strength is a useful and easy-to-obtain measurement that is associated with all-cause and disease-specific morbidity and can be used to improve the prediction of an office-based risk score.
Citation: Celis-Morales CA et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all-cause mortality: Prospective cohort study of half a million UK Biobank participants. BMJ. 2018;361:k1651.
Dr. Marr is assistant professor of medicine and an academic hospitalist, University of Utah, Salt Lake City.
Background: Previous studies have shown that lower muscle function is associated with increased mortality; however, studies have not been able to fully examine associations with age and disease-specific mortality.
Study design: Prospective, population-based study.
Setting: Large population cohort in the United Kingdom (UK Biobank).
Synopsis: The UK Biobank population included 502,293 individuals, aged 40-69 years, recruited during April 2007–December 2010, with grip strength data available. Mean follow-up was 7.1 years for all-cause and disease-specific mortality. Cox proportional hazard models were used to report hazard ratios (HR) per 5-kg decrease in grip strength and were controlled for multiple sociodemographic and lifestyle factors. A lower grip strength was found to correlate with all-cause mortality (HR, 1.16 in women; HR, 1.20 in men) as well as incidence of and mortality from cardiovascular disease, respiratory disease, and cancer. Hazard ratios were higher among younger age groups with similar lower grip strength. The use of grip strength also improved the prediction of an office-based mortality risk score from cardiovascular disease.
Bottom line: Grip strength is a useful and easy-to-obtain measurement that is associated with all-cause and disease-specific morbidity and can be used to improve the prediction of an office-based risk score.
Citation: Celis-Morales CA et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all-cause mortality: Prospective cohort study of half a million UK Biobank participants. BMJ. 2018;361:k1651.
Dr. Marr is assistant professor of medicine and an academic hospitalist, University of Utah, Salt Lake City.
Checkpoint inhibitors linked to rare, but serious immune-related side effects
Checkpoint inhibitors can cause rare, but serious, hematological immune-related adverse events (hem-irAEs), which require early detection and intervention, according to a recent French study.
Immune thrombocytopenia, hemolytic anemia, and neutropenia were the most common hem-irAEs in the population, reported lead author, Nicolas Delanoy, MD, of Gustave Roussy, Université Paris-Saclay, Villejuif, France, and his colleagues.
“About 71% of patients treated have any-grade irAEs and 10% have grade 3-4 irAEs after anti-PD-1 immunotherapy,” the investigators wrote. The report is in The Lancet Haematology. “In most cases, they involve the skin, gastrointestinal tract, thyroid or endocrine glands, liver, lungs, or joints. However, all organs can potentially be affected, including the hemopoietic system.”
Despite this possibility, few reports detail the frequency or character of hematological toxicities from immunotherapy.
The present study involved 948 patients who entered into three French registries between 2014 and 2018. The first registry, consisting of 745 patients, was observed prospectively during checkpoint inhibitor therapy. The other two registries provided retrospective data on confirmed irAEs or hem-irAEs.
Among 745 patients followed during checkpoint inhibitor therapy, four developed hem-irAEs, providing an incidence rate of 0.5%. The other two databases added 31 patients with confirmed hem-irAEs, allowing for characterization of 35 total cases.
The group of 35 patients had a median age of 65 years, with more men (n = 21) than women (n = 14). Melanoma was the most common type of malignancy (43%), followed by non–small-cell lung cancer (34%), lymphoma (11%), and others. The majority of patients received nivolumab (57%), slightly fewer received pembrolizumab (40%), and a small minority received atezolizumab (3%).
Immune thrombocytopenia, hemolytic anemia, and neutropenia were the most common hem-irAEs, each occurring in nine patients (26%). Five patients (14%) had aplastic anemia or pancytopenia, two patients had bicytopenia (6%; neutropenia and anemia or thrombocytopenia and anemia), and one patient had pure red cell aplasia (3%).
Hem-irAEs resolved in 60% of patients, but two patients (6%) died due to febrile neutropenia. Overall, 71% of hem-irAEs were grade 4.
These findings suggest that hem-irAEs are rare, but they are often serious, and potentially life-threatening, the researchers noted.
In 7 of 35 patients (20%) who were rechallenged with checkpoint inhibitor therapy, 3 (43%) had recurrence of hem-irAEs. This finding should elicit caution and close monitoring if rechallenge is elected.
“This observational study encourages further, in-depth investigations of hematological immune toxicities, to search for biomarkers that can be helpful for earlier detection,” the investigators concluded.
This study was funded by Gustave Roussy and the Gustave Roussy Immunotherapy Program. Dr. Delanoy reported nonfinancial support from Sanofi and other authors reported financial relationships with pharmaceutical companies.
SOURCE: Delanoy N et al. Lancet Haematol. 2018 Dec 4. doi: 10.1016/S2352-3026(18)30175-3.
Checkpoint inhibitors can cause rare, but serious, hematological immune-related adverse events (hem-irAEs), which require early detection and intervention, according to a recent French study.
Immune thrombocytopenia, hemolytic anemia, and neutropenia were the most common hem-irAEs in the population, reported lead author, Nicolas Delanoy, MD, of Gustave Roussy, Université Paris-Saclay, Villejuif, France, and his colleagues.
“About 71% of patients treated have any-grade irAEs and 10% have grade 3-4 irAEs after anti-PD-1 immunotherapy,” the investigators wrote. The report is in The Lancet Haematology. “In most cases, they involve the skin, gastrointestinal tract, thyroid or endocrine glands, liver, lungs, or joints. However, all organs can potentially be affected, including the hemopoietic system.”
Despite this possibility, few reports detail the frequency or character of hematological toxicities from immunotherapy.
The present study involved 948 patients who entered into three French registries between 2014 and 2018. The first registry, consisting of 745 patients, was observed prospectively during checkpoint inhibitor therapy. The other two registries provided retrospective data on confirmed irAEs or hem-irAEs.
Among 745 patients followed during checkpoint inhibitor therapy, four developed hem-irAEs, providing an incidence rate of 0.5%. The other two databases added 31 patients with confirmed hem-irAEs, allowing for characterization of 35 total cases.
The group of 35 patients had a median age of 65 years, with more men (n = 21) than women (n = 14). Melanoma was the most common type of malignancy (43%), followed by non–small-cell lung cancer (34%), lymphoma (11%), and others. The majority of patients received nivolumab (57%), slightly fewer received pembrolizumab (40%), and a small minority received atezolizumab (3%).
Immune thrombocytopenia, hemolytic anemia, and neutropenia were the most common hem-irAEs, each occurring in nine patients (26%). Five patients (14%) had aplastic anemia or pancytopenia, two patients had bicytopenia (6%; neutropenia and anemia or thrombocytopenia and anemia), and one patient had pure red cell aplasia (3%).
Hem-irAEs resolved in 60% of patients, but two patients (6%) died due to febrile neutropenia. Overall, 71% of hem-irAEs were grade 4.
These findings suggest that hem-irAEs are rare, but they are often serious, and potentially life-threatening, the researchers noted.
In 7 of 35 patients (20%) who were rechallenged with checkpoint inhibitor therapy, 3 (43%) had recurrence of hem-irAEs. This finding should elicit caution and close monitoring if rechallenge is elected.
“This observational study encourages further, in-depth investigations of hematological immune toxicities, to search for biomarkers that can be helpful for earlier detection,” the investigators concluded.
This study was funded by Gustave Roussy and the Gustave Roussy Immunotherapy Program. Dr. Delanoy reported nonfinancial support from Sanofi and other authors reported financial relationships with pharmaceutical companies.
SOURCE: Delanoy N et al. Lancet Haematol. 2018 Dec 4. doi: 10.1016/S2352-3026(18)30175-3.
Checkpoint inhibitors can cause rare, but serious, hematological immune-related adverse events (hem-irAEs), which require early detection and intervention, according to a recent French study.
Immune thrombocytopenia, hemolytic anemia, and neutropenia were the most common hem-irAEs in the population, reported lead author, Nicolas Delanoy, MD, of Gustave Roussy, Université Paris-Saclay, Villejuif, France, and his colleagues.
“About 71% of patients treated have any-grade irAEs and 10% have grade 3-4 irAEs after anti-PD-1 immunotherapy,” the investigators wrote. The report is in The Lancet Haematology. “In most cases, they involve the skin, gastrointestinal tract, thyroid or endocrine glands, liver, lungs, or joints. However, all organs can potentially be affected, including the hemopoietic system.”
Despite this possibility, few reports detail the frequency or character of hematological toxicities from immunotherapy.
The present study involved 948 patients who entered into three French registries between 2014 and 2018. The first registry, consisting of 745 patients, was observed prospectively during checkpoint inhibitor therapy. The other two registries provided retrospective data on confirmed irAEs or hem-irAEs.
Among 745 patients followed during checkpoint inhibitor therapy, four developed hem-irAEs, providing an incidence rate of 0.5%. The other two databases added 31 patients with confirmed hem-irAEs, allowing for characterization of 35 total cases.
The group of 35 patients had a median age of 65 years, with more men (n = 21) than women (n = 14). Melanoma was the most common type of malignancy (43%), followed by non–small-cell lung cancer (34%), lymphoma (11%), and others. The majority of patients received nivolumab (57%), slightly fewer received pembrolizumab (40%), and a small minority received atezolizumab (3%).
Immune thrombocytopenia, hemolytic anemia, and neutropenia were the most common hem-irAEs, each occurring in nine patients (26%). Five patients (14%) had aplastic anemia or pancytopenia, two patients had bicytopenia (6%; neutropenia and anemia or thrombocytopenia and anemia), and one patient had pure red cell aplasia (3%).
Hem-irAEs resolved in 60% of patients, but two patients (6%) died due to febrile neutropenia. Overall, 71% of hem-irAEs were grade 4.
These findings suggest that hem-irAEs are rare, but they are often serious, and potentially life-threatening, the researchers noted.
In 7 of 35 patients (20%) who were rechallenged with checkpoint inhibitor therapy, 3 (43%) had recurrence of hem-irAEs. This finding should elicit caution and close monitoring if rechallenge is elected.
“This observational study encourages further, in-depth investigations of hematological immune toxicities, to search for biomarkers that can be helpful for earlier detection,” the investigators concluded.
This study was funded by Gustave Roussy and the Gustave Roussy Immunotherapy Program. Dr. Delanoy reported nonfinancial support from Sanofi and other authors reported financial relationships with pharmaceutical companies.
SOURCE: Delanoy N et al. Lancet Haematol. 2018 Dec 4. doi: 10.1016/S2352-3026(18)30175-3.
FROM THE LANCET HAEMATOLOGY
Key clinical point:
Major finding: Checkpoint inhibitor therapy led to hematological toxicity in 0.5% of patients.
Study details: A study of 948 patients in French registries who were observed prospectively or retrospectively, including a case series of 35 patients treated with checkpoint inhibitor therapy who developed hematologic, immune-related adverse events.
Disclosures: This study was funded by Gustave Roussy and the Gustave Roussy Immunotherapy Program. Dr. Delanoy reported nonfinancial support from Sanofi and other authors reported financial relationships with pharmaceutical companies.
Source: Delanoy N et al. Lancet Haematol. 2018 Dec 4. doi: 10.1016/S2352-3026(18)30175-3.
Know the red flags for synaptic autoimmune psychosis
BARCELONA – Consider the possibility of an autoantibody-related etiology in all cases of first-onset psychosis, Josep Dalmau, MD, PhD, urged at the annual congress of the European College of Neuropsychopharmacology.
“There are patients in our clinics all of us – neurologists and psychiatrists – are missing. These patients are believed to have psychiatric presentations, but they do not. They are autoimmune,” said Dr. Dalmau, professor of neurology at the University of Barcelona.
Dr. Dalmau urged psychiatrists to become familiar with the red flags suggestive of synaptic autoimmunity as the underlying cause of first-episode, out-of-the-blue psychosis.
“If you have a patient with a classical presentation of schizophrenia or bipolar disorder, you probably won’t find antibodies,” according to the neurologist.
It’s important to have a high index of suspicion, because anti–NMDA receptor encephalitis is treatable with immunotherapy. And firm evidence shows that earlier recognition and treatment lead to improved outcomes. Also, the disorder is refractory to antipsychotics; indeed, 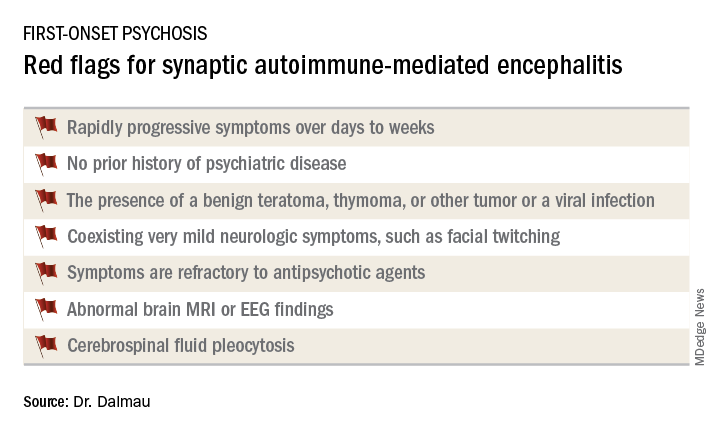
Manifestations of anti–NMDA receptor encephalitis follow a characteristic pattern, beginning with a prodromal flulike phase lasting several days to a week. This is followed by acute-onset bizarre behavioral changes, irritability, and psychosis with delusions and/or hallucinations, often progressing to catatonia. After 1-4 weeks of this, florid neurologic symptoms usually appear, including seizures, abnormal movements, autonomic dysregulation, and hypoventilation requiring prolonged ICU support for weeks to months. This is followed by a prolonged recovery phase lasting 5-24 months, and a period marked by deficits in executive function and working memory, impulsivity, and disinhibition. Impressively, the patient has no memory of the illness.
In one large series of patients with confirmed anti–NMDA receptor encephalitis reported by Dr. Dalmau and coinvestigators, psychiatric symptoms occurred in isolation without subsequent neurologic involvement in just 4% of cases (JAMA Neurol. 2013 Sep 1;70[9]:1133-9).
Dr. Dalmau was senior author of an international cohort study including 577 patients with anti-NMDA receptor encephalitis with serial follow-up for 24 months. The study provided an unprecedented picture of the epidemiology and clinical features of the disorder.
“It’s a disease predominantly of women and young people,” he observed.
Indeed, the median age of the study population was 21 years, and 37% of subjects were less than 18 years of age. Roughly 80% of patients were female and most of them had a benign ovarian teratoma, which played a key role in their neuropsychiatric disease (Lancet Neurol. 2013 Feb;12[2]:157-65). These benign tumors express the NMDA receptor in ectopic nerve tissue, triggering a systemic immune response.
One or more relapses – again treatable via immunotherapy – occurred in 12% of patients during 24 months of follow-up.
When a red flag suggestive of synaptic autoimmunity is present, it’s important to obtain a cerebrospinal fluid (CSF) sample for analysis, along with an EEG and/or brain MRI.
“I don’t know if you as psychiatrists are set up to do spinal taps in all persons with first presentation of psychosis, but this would be my suggestion. It’s extremely useful in this situation,” Dr. Dalmau said.
The vast majority of patients with anti–NMDA receptor encephalitis have CSF pleocytosis with a mild lymphocytic predominance. The MRI is abnormal in about 35% of cases. EEG abnormalities are common but nonspecific. The diagnosis is confirmed by identification of anti–NMDA receptor antibodies in the CSF.
First-line therapy is corticosteroids, intravenous immunoglobulin, and/or plasma exchange to remove the pathogenic antibodies, along with resection of the tumor if present. These treatments are effective in almost half of affected patients. When they’re not, the second-line options are rituximab (Rituxan) and cyclophosphamide, alone or combined.
Antibodies to the NMDA receptor are far and away the most common cause of synaptic autoimmunity-induced psychosis, but other targets of autoimmunity have been documented as well, including the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, contactin-associated protein-like 2 (CASPR2), and neurexin-3-alpha.
Dr. Dalmau and various collaborators continue to advance the understanding of this novel category of neuropsychiatric disease. They have developed a simple 5-point score, known as the NEOS score, that predicts 1-year functional status in patients with anti–NMDA receptor encephalitis (Neurology. 2018 Dec 21. doi: 10.1212/WNL.0000000000006783). He and his colleagues have also recently shown in a prospective study that herpes simplex encephalitis can result in an autoimmune encephalitis, with NMDA receptor antibodies present in most cases (Lancet Neurol. 2018 Sep;17[9]:760-72).
Dr. Dalmau’s research is supported by the U.S. National Institute of Neurological Disorders and Stroke, the Spanish Ministry of Health, and Spanish research foundations. He reported receiving royalties from the use of several neuronal antibody tests.
BARCELONA – Consider the possibility of an autoantibody-related etiology in all cases of first-onset psychosis, Josep Dalmau, MD, PhD, urged at the annual congress of the European College of Neuropsychopharmacology.
“There are patients in our clinics all of us – neurologists and psychiatrists – are missing. These patients are believed to have psychiatric presentations, but they do not. They are autoimmune,” said Dr. Dalmau, professor of neurology at the University of Barcelona.
Dr. Dalmau urged psychiatrists to become familiar with the red flags suggestive of synaptic autoimmunity as the underlying cause of first-episode, out-of-the-blue psychosis.
“If you have a patient with a classical presentation of schizophrenia or bipolar disorder, you probably won’t find antibodies,” according to the neurologist.
It’s important to have a high index of suspicion, because anti–NMDA receptor encephalitis is treatable with immunotherapy. And firm evidence shows that earlier recognition and treatment lead to improved outcomes. Also, the disorder is refractory to antipsychotics; indeed, 
Manifestations of anti–NMDA receptor encephalitis follow a characteristic pattern, beginning with a prodromal flulike phase lasting several days to a week. This is followed by acute-onset bizarre behavioral changes, irritability, and psychosis with delusions and/or hallucinations, often progressing to catatonia. After 1-4 weeks of this, florid neurologic symptoms usually appear, including seizures, abnormal movements, autonomic dysregulation, and hypoventilation requiring prolonged ICU support for weeks to months. This is followed by a prolonged recovery phase lasting 5-24 months, and a period marked by deficits in executive function and working memory, impulsivity, and disinhibition. Impressively, the patient has no memory of the illness.
In one large series of patients with confirmed anti–NMDA receptor encephalitis reported by Dr. Dalmau and coinvestigators, psychiatric symptoms occurred in isolation without subsequent neurologic involvement in just 4% of cases (JAMA Neurol. 2013 Sep 1;70[9]:1133-9).
Dr. Dalmau was senior author of an international cohort study including 577 patients with anti-NMDA receptor encephalitis with serial follow-up for 24 months. The study provided an unprecedented picture of the epidemiology and clinical features of the disorder.
“It’s a disease predominantly of women and young people,” he observed.
Indeed, the median age of the study population was 21 years, and 37% of subjects were less than 18 years of age. Roughly 80% of patients were female and most of them had a benign ovarian teratoma, which played a key role in their neuropsychiatric disease (Lancet Neurol. 2013 Feb;12[2]:157-65). These benign tumors express the NMDA receptor in ectopic nerve tissue, triggering a systemic immune response.
One or more relapses – again treatable via immunotherapy – occurred in 12% of patients during 24 months of follow-up.
When a red flag suggestive of synaptic autoimmunity is present, it’s important to obtain a cerebrospinal fluid (CSF) sample for analysis, along with an EEG and/or brain MRI.
“I don’t know if you as psychiatrists are set up to do spinal taps in all persons with first presentation of psychosis, but this would be my suggestion. It’s extremely useful in this situation,” Dr. Dalmau said.
The vast majority of patients with anti–NMDA receptor encephalitis have CSF pleocytosis with a mild lymphocytic predominance. The MRI is abnormal in about 35% of cases. EEG abnormalities are common but nonspecific. The diagnosis is confirmed by identification of anti–NMDA receptor antibodies in the CSF.
First-line therapy is corticosteroids, intravenous immunoglobulin, and/or plasma exchange to remove the pathogenic antibodies, along with resection of the tumor if present. These treatments are effective in almost half of affected patients. When they’re not, the second-line options are rituximab (Rituxan) and cyclophosphamide, alone or combined.
Antibodies to the NMDA receptor are far and away the most common cause of synaptic autoimmunity-induced psychosis, but other targets of autoimmunity have been documented as well, including the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, contactin-associated protein-like 2 (CASPR2), and neurexin-3-alpha.
Dr. Dalmau and various collaborators continue to advance the understanding of this novel category of neuropsychiatric disease. They have developed a simple 5-point score, known as the NEOS score, that predicts 1-year functional status in patients with anti–NMDA receptor encephalitis (Neurology. 2018 Dec 21. doi: 10.1212/WNL.0000000000006783). He and his colleagues have also recently shown in a prospective study that herpes simplex encephalitis can result in an autoimmune encephalitis, with NMDA receptor antibodies present in most cases (Lancet Neurol. 2018 Sep;17[9]:760-72).
Dr. Dalmau’s research is supported by the U.S. National Institute of Neurological Disorders and Stroke, the Spanish Ministry of Health, and Spanish research foundations. He reported receiving royalties from the use of several neuronal antibody tests.
BARCELONA – Consider the possibility of an autoantibody-related etiology in all cases of first-onset psychosis, Josep Dalmau, MD, PhD, urged at the annual congress of the European College of Neuropsychopharmacology.
“There are patients in our clinics all of us – neurologists and psychiatrists – are missing. These patients are believed to have psychiatric presentations, but they do not. They are autoimmune,” said Dr. Dalmau, professor of neurology at the University of Barcelona.
Dr. Dalmau urged psychiatrists to become familiar with the red flags suggestive of synaptic autoimmunity as the underlying cause of first-episode, out-of-the-blue psychosis.
“If you have a patient with a classical presentation of schizophrenia or bipolar disorder, you probably won’t find antibodies,” according to the neurologist.
It’s important to have a high index of suspicion, because anti–NMDA receptor encephalitis is treatable with immunotherapy. And firm evidence shows that earlier recognition and treatment lead to improved outcomes. Also, the disorder is refractory to antipsychotics; indeed, 
Manifestations of anti–NMDA receptor encephalitis follow a characteristic pattern, beginning with a prodromal flulike phase lasting several days to a week. This is followed by acute-onset bizarre behavioral changes, irritability, and psychosis with delusions and/or hallucinations, often progressing to catatonia. After 1-4 weeks of this, florid neurologic symptoms usually appear, including seizures, abnormal movements, autonomic dysregulation, and hypoventilation requiring prolonged ICU support for weeks to months. This is followed by a prolonged recovery phase lasting 5-24 months, and a period marked by deficits in executive function and working memory, impulsivity, and disinhibition. Impressively, the patient has no memory of the illness.
In one large series of patients with confirmed anti–NMDA receptor encephalitis reported by Dr. Dalmau and coinvestigators, psychiatric symptoms occurred in isolation without subsequent neurologic involvement in just 4% of cases (JAMA Neurol. 2013 Sep 1;70[9]:1133-9).
Dr. Dalmau was senior author of an international cohort study including 577 patients with anti-NMDA receptor encephalitis with serial follow-up for 24 months. The study provided an unprecedented picture of the epidemiology and clinical features of the disorder.
“It’s a disease predominantly of women and young people,” he observed.
Indeed, the median age of the study population was 21 years, and 37% of subjects were less than 18 years of age. Roughly 80% of patients were female and most of them had a benign ovarian teratoma, which played a key role in their neuropsychiatric disease (Lancet Neurol. 2013 Feb;12[2]:157-65). These benign tumors express the NMDA receptor in ectopic nerve tissue, triggering a systemic immune response.
One or more relapses – again treatable via immunotherapy – occurred in 12% of patients during 24 months of follow-up.
When a red flag suggestive of synaptic autoimmunity is present, it’s important to obtain a cerebrospinal fluid (CSF) sample for analysis, along with an EEG and/or brain MRI.
“I don’t know if you as psychiatrists are set up to do spinal taps in all persons with first presentation of psychosis, but this would be my suggestion. It’s extremely useful in this situation,” Dr. Dalmau said.
The vast majority of patients with anti–NMDA receptor encephalitis have CSF pleocytosis with a mild lymphocytic predominance. The MRI is abnormal in about 35% of cases. EEG abnormalities are common but nonspecific. The diagnosis is confirmed by identification of anti–NMDA receptor antibodies in the CSF.
First-line therapy is corticosteroids, intravenous immunoglobulin, and/or plasma exchange to remove the pathogenic antibodies, along with resection of the tumor if present. These treatments are effective in almost half of affected patients. When they’re not, the second-line options are rituximab (Rituxan) and cyclophosphamide, alone or combined.
Antibodies to the NMDA receptor are far and away the most common cause of synaptic autoimmunity-induced psychosis, but other targets of autoimmunity have been documented as well, including the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, contactin-associated protein-like 2 (CASPR2), and neurexin-3-alpha.
Dr. Dalmau and various collaborators continue to advance the understanding of this novel category of neuropsychiatric disease. They have developed a simple 5-point score, known as the NEOS score, that predicts 1-year functional status in patients with anti–NMDA receptor encephalitis (Neurology. 2018 Dec 21. doi: 10.1212/WNL.0000000000006783). He and his colleagues have also recently shown in a prospective study that herpes simplex encephalitis can result in an autoimmune encephalitis, with NMDA receptor antibodies present in most cases (Lancet Neurol. 2018 Sep;17[9]:760-72).
Dr. Dalmau’s research is supported by the U.S. National Institute of Neurological Disorders and Stroke, the Spanish Ministry of Health, and Spanish research foundations. He reported receiving royalties from the use of several neuronal antibody tests.
REPORTING FROM THE ECNP CONGRESS
Progress in cancer research slowed by broken system
NEW YORK – Conflicts of interest, whether reported or unreported, are just one component of a broken system that has misaligned incentives with meaningful advances in breast cancer research, agreed a panel of experts who participated in an evening program sponsored by SHARE, a breast cancer patient advocacy group.
“The issue of disclosures, while it is important, is not really the key issue. The key issue for me is about the amazing amount of money that is changing hands,” said Fran Visco, president of the National Breast Cancer Coalition, Washington. She contended not enough money in breast cancer research is being applied to save women’s lives rather than driving profits for institutions, physicians, and the pharmaceutical industry.
The other two participants in this panel, called Conflicts of Interest in Research and Treatment: What Breast Cancer Patients Should Know, largely agreed. In turn, they each explained that major sums of money in play for physicians and institutions collaborating with industry are earmarked for generating income much more than improving outcomes.
“Most of where this money is going is for these little tiny improvements and there is no association between the outcome increment that you get and what you pay for that outcome increment,” said Robert Cook-Deegan, MD, a bioethicist and a professor at the School for Innovation in Society, Arizona State University, Tempe.
Leaving aside whether physician-scientists working at nonprofit research institutions can avoid conflicts of interest when money is funneled to them from start-up investment opportunities, pharmaceutical board memberships, and direct compensation deals for consultancies and other services, Dr. Cook-Deegan was referring to the bigger problem of misaligned incentives. Large financial rewards are available for any drug with market potential instead of being limited to those that save lives, he said.
Tracing the current system to the Bayh-Dole Act in 1980, which permitted researchers to claim patents for intellectual property developed with federal funding, Dr. Cook-Deegan described a system of minnows, fish, and sharks. Researchers, who are the minnows, seek potential new therapeutics to be developed by for-profit startups or other commercial entities, which are the fish. If the drug continues to show promise, it brings investors, who are the sharks.
Once the new therapy reaches the sharks, the excitement about a new and more effective treatment has been overshadowed by the excitement about profits, which are essential to pay for the large investment required for the clinical development that brings new drugs to market. If the drug is marketable, it is considered a success even when the clinical benefits barely register.
“There have been about 20 new [cancer] drugs approved by the [Food and Drug Administration] in the last 2 years. The increased median disease-free survival is usually about 3 months,” said Otis W. Brawley, MD, who recently joined the Kimmel Cancer Center at Johns Hopkins University after resigning his position as chief medical officer at the American Cancer Society (ACS).
Dr. Brawley joined the others in declaring: “The system is broken.” The flow of money is not the only incentive for researchers to participate in clinical development programs focused on minor advances rather than blockbuster discoveries. Unrestricted research grants are now much harder to obtain, according to Dr. Brawley, making collaboration with industry essential not just for research funding but career advancement. Research at major cancer centers is still being conducted at the highest level, but there are unavoidable interdependent relationships for nonprofit institutions and for-profit enterprises.
“The doc who prescribes the drug to the average patient probably does not have this conflict of interest,” he said. “However, they are influenced by people who have these conflicts.”
The SHARE panel on conflicts of interest was convened after a series of articles co-reported by ProPublica and the New York Times described the failure of the chief medical officer at Memorial Sloan Kettering Cancer Center, José Baselga, MD, to disclose his financial conflicts of interest in publications, such as the New England Journal of Medicine, where they are required. Dr. Baselga resigned his position.
However, the failure to disclose financial relationships with industry was a minor topic during the SHARE panel. Robert Bazell, PhD, an adjunct professor in the division of molecular, cellular, and developmental biology at Yale University, New Haven, Conn., moderated the panel. He said in his opening remarks that the issues “go much deeper.”
While the articles did not reveal “anything illegal, it opened a lot of peoples’ eyes as to how much money there was in the system,” said Dr. Bazell, who was for many years the chief science and health correspondent for NBC News.
Specifically, it has heightened concern about whether profit motives are subverting the goals of science, according to Dr. Bazell. “This money bomb has fallen on a lot of science but it has particularly fallen on oncology,” he said.
Not everyone, including the bioethicist, agreed that profits by themselves are the problem. Big rewards may be a reasonable price for discoveries that save lives, but all agreed that the system does not necessarily reserve big rewards for life-saving advances.
“The focus isn’t really on drugs that will save lives, its just on more drugs,” said Ms. Visco, echoing the incentive system described by Dr. Cook-Deegan. She believes the current system handsomely rewards scientists and physicians for developing drugs with little or no significant clinical benefit. When a drug shows a progression-free survival benefit relative to a previous standard, even of modest statistical or clinical significance, it will be prescribed and profits will be generated.
Early in her tenure at the National Breast Cancer Coalition, Ms. Visco, who was a practicing lawyer prior to taking the helm of the coalition, considered fund raising for research the primary goal. She hoped that the coalition could help the breast cancer research community identify and fund priorities, but her perspective has changed.
“I used to think that we should be collaborators but now I am coming to the conclusion that we should be in charge. Educated, trained patient advocates with a constituency should be in charge, because our only agenda is to save lives and end breast cancer,” Ms. Visco said. She no longer believes in simply increasing funding even at research centers such as the National Cancer Institute without a fundamental reorganization of priorities.
Many leaders in medicine are aware of the problem, according to Dr. Brawley, who cited a recent statement by the American Society of Clinical Oncology that expressed concern about the plethora of cancer trials showing very small gains. He did agree with the others, however, that incentives are now misaligned.
“We are designing clinical trials not to look for big gains,” Dr. Brawley agreed. But he also cautioned that the system is complex. One reason that drugs offering modest or little gain over a previous standard are highly marketable is that patients themselves demand them. Direct-to-consumer marketing supports drugs with little or even nothing to offer.
“I see patients who want drugs that they should not get,” said Dr. Brawley, who indicated that clinicians are under pressure to offer something to desperate patients even when options are expensive and not shown to provide any change in outcome.
Overall, in the shake-up at Memorial Sloan Kettering, it was the failure to disclose significant financial relationships rather than the financial relationships themselves that represented an important breach in ethics, according to Dr. Brawley, who indicated that researcher relationships with industry are not inherently wrong. In an article published in November 2018, the New York Times reported that Dr. Brawley left his post at the ACS because of “his dismay” over some partnerships the ACS had formed with industry, but Dr. Brawley would not confirm or deny this report.
Relative to conflicts of interest at major research institutions, Dr. Cook-Deegan was more circumspect about whether disclosure is enough. Although he agreed with the premise that close collaboration with industry might be clinically valuable, which one investigator at Memorial Sloan Kettering claimed when speaking with the New York Times, he questioned whether there is a line over which the relationship is too intertwined.
“Do you really need to serve on the board? Do you really need the types of financial ties that have the potential to influence clinical decisions?” he asked.
Although Dr. Cook-Deegan agreed with the others that he does not know exactly how best to fix the system, he believes a fix may be coming.
“I think we are at an inflection point,” he said. “The symptoms of a system that has been running off the rails for a while are getting severe enough that we are starting to pay attention,” he said. One sign of movement is that both political parties have “introduced bills to address pricing, which is directly related to the problems we are talking about.”
NEW YORK – Conflicts of interest, whether reported or unreported, are just one component of a broken system that has misaligned incentives with meaningful advances in breast cancer research, agreed a panel of experts who participated in an evening program sponsored by SHARE, a breast cancer patient advocacy group.
“The issue of disclosures, while it is important, is not really the key issue. The key issue for me is about the amazing amount of money that is changing hands,” said Fran Visco, president of the National Breast Cancer Coalition, Washington. She contended not enough money in breast cancer research is being applied to save women’s lives rather than driving profits for institutions, physicians, and the pharmaceutical industry.
The other two participants in this panel, called Conflicts of Interest in Research and Treatment: What Breast Cancer Patients Should Know, largely agreed. In turn, they each explained that major sums of money in play for physicians and institutions collaborating with industry are earmarked for generating income much more than improving outcomes.
“Most of where this money is going is for these little tiny improvements and there is no association between the outcome increment that you get and what you pay for that outcome increment,” said Robert Cook-Deegan, MD, a bioethicist and a professor at the School for Innovation in Society, Arizona State University, Tempe.
Leaving aside whether physician-scientists working at nonprofit research institutions can avoid conflicts of interest when money is funneled to them from start-up investment opportunities, pharmaceutical board memberships, and direct compensation deals for consultancies and other services, Dr. Cook-Deegan was referring to the bigger problem of misaligned incentives. Large financial rewards are available for any drug with market potential instead of being limited to those that save lives, he said.
Tracing the current system to the Bayh-Dole Act in 1980, which permitted researchers to claim patents for intellectual property developed with federal funding, Dr. Cook-Deegan described a system of minnows, fish, and sharks. Researchers, who are the minnows, seek potential new therapeutics to be developed by for-profit startups or other commercial entities, which are the fish. If the drug continues to show promise, it brings investors, who are the sharks.
Once the new therapy reaches the sharks, the excitement about a new and more effective treatment has been overshadowed by the excitement about profits, which are essential to pay for the large investment required for the clinical development that brings new drugs to market. If the drug is marketable, it is considered a success even when the clinical benefits barely register.
“There have been about 20 new [cancer] drugs approved by the [Food and Drug Administration] in the last 2 years. The increased median disease-free survival is usually about 3 months,” said Otis W. Brawley, MD, who recently joined the Kimmel Cancer Center at Johns Hopkins University after resigning his position as chief medical officer at the American Cancer Society (ACS).
Dr. Brawley joined the others in declaring: “The system is broken.” The flow of money is not the only incentive for researchers to participate in clinical development programs focused on minor advances rather than blockbuster discoveries. Unrestricted research grants are now much harder to obtain, according to Dr. Brawley, making collaboration with industry essential not just for research funding but career advancement. Research at major cancer centers is still being conducted at the highest level, but there are unavoidable interdependent relationships for nonprofit institutions and for-profit enterprises.
“The doc who prescribes the drug to the average patient probably does not have this conflict of interest,” he said. “However, they are influenced by people who have these conflicts.”
The SHARE panel on conflicts of interest was convened after a series of articles co-reported by ProPublica and the New York Times described the failure of the chief medical officer at Memorial Sloan Kettering Cancer Center, José Baselga, MD, to disclose his financial conflicts of interest in publications, such as the New England Journal of Medicine, where they are required. Dr. Baselga resigned his position.
However, the failure to disclose financial relationships with industry was a minor topic during the SHARE panel. Robert Bazell, PhD, an adjunct professor in the division of molecular, cellular, and developmental biology at Yale University, New Haven, Conn., moderated the panel. He said in his opening remarks that the issues “go much deeper.”
While the articles did not reveal “anything illegal, it opened a lot of peoples’ eyes as to how much money there was in the system,” said Dr. Bazell, who was for many years the chief science and health correspondent for NBC News.
Specifically, it has heightened concern about whether profit motives are subverting the goals of science, according to Dr. Bazell. “This money bomb has fallen on a lot of science but it has particularly fallen on oncology,” he said.
Not everyone, including the bioethicist, agreed that profits by themselves are the problem. Big rewards may be a reasonable price for discoveries that save lives, but all agreed that the system does not necessarily reserve big rewards for life-saving advances.
“The focus isn’t really on drugs that will save lives, its just on more drugs,” said Ms. Visco, echoing the incentive system described by Dr. Cook-Deegan. She believes the current system handsomely rewards scientists and physicians for developing drugs with little or no significant clinical benefit. When a drug shows a progression-free survival benefit relative to a previous standard, even of modest statistical or clinical significance, it will be prescribed and profits will be generated.
Early in her tenure at the National Breast Cancer Coalition, Ms. Visco, who was a practicing lawyer prior to taking the helm of the coalition, considered fund raising for research the primary goal. She hoped that the coalition could help the breast cancer research community identify and fund priorities, but her perspective has changed.
“I used to think that we should be collaborators but now I am coming to the conclusion that we should be in charge. Educated, trained patient advocates with a constituency should be in charge, because our only agenda is to save lives and end breast cancer,” Ms. Visco said. She no longer believes in simply increasing funding even at research centers such as the National Cancer Institute without a fundamental reorganization of priorities.
Many leaders in medicine are aware of the problem, according to Dr. Brawley, who cited a recent statement by the American Society of Clinical Oncology that expressed concern about the plethora of cancer trials showing very small gains. He did agree with the others, however, that incentives are now misaligned.
“We are designing clinical trials not to look for big gains,” Dr. Brawley agreed. But he also cautioned that the system is complex. One reason that drugs offering modest or little gain over a previous standard are highly marketable is that patients themselves demand them. Direct-to-consumer marketing supports drugs with little or even nothing to offer.
“I see patients who want drugs that they should not get,” said Dr. Brawley, who indicated that clinicians are under pressure to offer something to desperate patients even when options are expensive and not shown to provide any change in outcome.
Overall, in the shake-up at Memorial Sloan Kettering, it was the failure to disclose significant financial relationships rather than the financial relationships themselves that represented an important breach in ethics, according to Dr. Brawley, who indicated that researcher relationships with industry are not inherently wrong. In an article published in November 2018, the New York Times reported that Dr. Brawley left his post at the ACS because of “his dismay” over some partnerships the ACS had formed with industry, but Dr. Brawley would not confirm or deny this report.
Relative to conflicts of interest at major research institutions, Dr. Cook-Deegan was more circumspect about whether disclosure is enough. Although he agreed with the premise that close collaboration with industry might be clinically valuable, which one investigator at Memorial Sloan Kettering claimed when speaking with the New York Times, he questioned whether there is a line over which the relationship is too intertwined.
“Do you really need to serve on the board? Do you really need the types of financial ties that have the potential to influence clinical decisions?” he asked.
Although Dr. Cook-Deegan agreed with the others that he does not know exactly how best to fix the system, he believes a fix may be coming.
“I think we are at an inflection point,” he said. “The symptoms of a system that has been running off the rails for a while are getting severe enough that we are starting to pay attention,” he said. One sign of movement is that both political parties have “introduced bills to address pricing, which is directly related to the problems we are talking about.”
NEW YORK – Conflicts of interest, whether reported or unreported, are just one component of a broken system that has misaligned incentives with meaningful advances in breast cancer research, agreed a panel of experts who participated in an evening program sponsored by SHARE, a breast cancer patient advocacy group.
“The issue of disclosures, while it is important, is not really the key issue. The key issue for me is about the amazing amount of money that is changing hands,” said Fran Visco, president of the National Breast Cancer Coalition, Washington. She contended not enough money in breast cancer research is being applied to save women’s lives rather than driving profits for institutions, physicians, and the pharmaceutical industry.
The other two participants in this panel, called Conflicts of Interest in Research and Treatment: What Breast Cancer Patients Should Know, largely agreed. In turn, they each explained that major sums of money in play for physicians and institutions collaborating with industry are earmarked for generating income much more than improving outcomes.
“Most of where this money is going is for these little tiny improvements and there is no association between the outcome increment that you get and what you pay for that outcome increment,” said Robert Cook-Deegan, MD, a bioethicist and a professor at the School for Innovation in Society, Arizona State University, Tempe.
Leaving aside whether physician-scientists working at nonprofit research institutions can avoid conflicts of interest when money is funneled to them from start-up investment opportunities, pharmaceutical board memberships, and direct compensation deals for consultancies and other services, Dr. Cook-Deegan was referring to the bigger problem of misaligned incentives. Large financial rewards are available for any drug with market potential instead of being limited to those that save lives, he said.
Tracing the current system to the Bayh-Dole Act in 1980, which permitted researchers to claim patents for intellectual property developed with federal funding, Dr. Cook-Deegan described a system of minnows, fish, and sharks. Researchers, who are the minnows, seek potential new therapeutics to be developed by for-profit startups or other commercial entities, which are the fish. If the drug continues to show promise, it brings investors, who are the sharks.
Once the new therapy reaches the sharks, the excitement about a new and more effective treatment has been overshadowed by the excitement about profits, which are essential to pay for the large investment required for the clinical development that brings new drugs to market. If the drug is marketable, it is considered a success even when the clinical benefits barely register.
“There have been about 20 new [cancer] drugs approved by the [Food and Drug Administration] in the last 2 years. The increased median disease-free survival is usually about 3 months,” said Otis W. Brawley, MD, who recently joined the Kimmel Cancer Center at Johns Hopkins University after resigning his position as chief medical officer at the American Cancer Society (ACS).
Dr. Brawley joined the others in declaring: “The system is broken.” The flow of money is not the only incentive for researchers to participate in clinical development programs focused on minor advances rather than blockbuster discoveries. Unrestricted research grants are now much harder to obtain, according to Dr. Brawley, making collaboration with industry essential not just for research funding but career advancement. Research at major cancer centers is still being conducted at the highest level, but there are unavoidable interdependent relationships for nonprofit institutions and for-profit enterprises.
“The doc who prescribes the drug to the average patient probably does not have this conflict of interest,” he said. “However, they are influenced by people who have these conflicts.”
The SHARE panel on conflicts of interest was convened after a series of articles co-reported by ProPublica and the New York Times described the failure of the chief medical officer at Memorial Sloan Kettering Cancer Center, José Baselga, MD, to disclose his financial conflicts of interest in publications, such as the New England Journal of Medicine, where they are required. Dr. Baselga resigned his position.
However, the failure to disclose financial relationships with industry was a minor topic during the SHARE panel. Robert Bazell, PhD, an adjunct professor in the division of molecular, cellular, and developmental biology at Yale University, New Haven, Conn., moderated the panel. He said in his opening remarks that the issues “go much deeper.”
While the articles did not reveal “anything illegal, it opened a lot of peoples’ eyes as to how much money there was in the system,” said Dr. Bazell, who was for many years the chief science and health correspondent for NBC News.
Specifically, it has heightened concern about whether profit motives are subverting the goals of science, according to Dr. Bazell. “This money bomb has fallen on a lot of science but it has particularly fallen on oncology,” he said.
Not everyone, including the bioethicist, agreed that profits by themselves are the problem. Big rewards may be a reasonable price for discoveries that save lives, but all agreed that the system does not necessarily reserve big rewards for life-saving advances.
“The focus isn’t really on drugs that will save lives, its just on more drugs,” said Ms. Visco, echoing the incentive system described by Dr. Cook-Deegan. She believes the current system handsomely rewards scientists and physicians for developing drugs with little or no significant clinical benefit. When a drug shows a progression-free survival benefit relative to a previous standard, even of modest statistical or clinical significance, it will be prescribed and profits will be generated.
Early in her tenure at the National Breast Cancer Coalition, Ms. Visco, who was a practicing lawyer prior to taking the helm of the coalition, considered fund raising for research the primary goal. She hoped that the coalition could help the breast cancer research community identify and fund priorities, but her perspective has changed.
“I used to think that we should be collaborators but now I am coming to the conclusion that we should be in charge. Educated, trained patient advocates with a constituency should be in charge, because our only agenda is to save lives and end breast cancer,” Ms. Visco said. She no longer believes in simply increasing funding even at research centers such as the National Cancer Institute without a fundamental reorganization of priorities.
Many leaders in medicine are aware of the problem, according to Dr. Brawley, who cited a recent statement by the American Society of Clinical Oncology that expressed concern about the plethora of cancer trials showing very small gains. He did agree with the others, however, that incentives are now misaligned.
“We are designing clinical trials not to look for big gains,” Dr. Brawley agreed. But he also cautioned that the system is complex. One reason that drugs offering modest or little gain over a previous standard are highly marketable is that patients themselves demand them. Direct-to-consumer marketing supports drugs with little or even nothing to offer.
“I see patients who want drugs that they should not get,” said Dr. Brawley, who indicated that clinicians are under pressure to offer something to desperate patients even when options are expensive and not shown to provide any change in outcome.
Overall, in the shake-up at Memorial Sloan Kettering, it was the failure to disclose significant financial relationships rather than the financial relationships themselves that represented an important breach in ethics, according to Dr. Brawley, who indicated that researcher relationships with industry are not inherently wrong. In an article published in November 2018, the New York Times reported that Dr. Brawley left his post at the ACS because of “his dismay” over some partnerships the ACS had formed with industry, but Dr. Brawley would not confirm or deny this report.
Relative to conflicts of interest at major research institutions, Dr. Cook-Deegan was more circumspect about whether disclosure is enough. Although he agreed with the premise that close collaboration with industry might be clinically valuable, which one investigator at Memorial Sloan Kettering claimed when speaking with the New York Times, he questioned whether there is a line over which the relationship is too intertwined.
“Do you really need to serve on the board? Do you really need the types of financial ties that have the potential to influence clinical decisions?” he asked.
Although Dr. Cook-Deegan agreed with the others that he does not know exactly how best to fix the system, he believes a fix may be coming.
“I think we are at an inflection point,” he said. “The symptoms of a system that has been running off the rails for a while are getting severe enough that we are starting to pay attention,” he said. One sign of movement is that both political parties have “introduced bills to address pricing, which is directly related to the problems we are talking about.”
NIH announces new clinical trial assessing FMT for recurrent CDAD
A clinical trial has begun which will examine whether fecal microbiota transplantation (FMT) by enema is safe and can prevent recurrent Clostridium difficile–associated disease (CDAD), according to a press release from the National Institutes of Health.
CDAD is normally treated with antibiotics such as vancomycin or fidaxomicin; however, it recurs in about 20% of people who receive this treatment. FMT is effective at curing patients with recurring C. diff infections, but long-term safety and a standardized process have yet to be established.
An estimated 162 people aged 18 years or older who have had two or more episodes of CDAD within the previous 6 months will be included in the clinical trial. These patients will be split into two groups: The first will receive an antidiarrheal medication and an FMT delivered by retention enema; the second will receive an antidiarrheal and a placebo colored to look like an active stool sample.
All patients will provide stool and blood samples at designated time points for 1 year after they undergo treatment for CDAD. Stool samples will be examined for gut microbial diversity changes and infectious pathogens; blood samples will be examined for metabolic syndrome markers.
“Clostridium difficile–associated disease, a significant problem in health care facilities, causes an estimated 15,000 deaths in the United States each year. This randomized, controlled trial aims to provide critical data on the efficacy and long-term safety of using fecal microbiota transplants by enema to cure C. diff infections,” NIAID director Anthony S. Fauci, MD, said in the press release.
The full trial page can be found at Clinicaltrials.gov.
A clinical trial has begun which will examine whether fecal microbiota transplantation (FMT) by enema is safe and can prevent recurrent Clostridium difficile–associated disease (CDAD), according to a press release from the National Institutes of Health.
CDAD is normally treated with antibiotics such as vancomycin or fidaxomicin; however, it recurs in about 20% of people who receive this treatment. FMT is effective at curing patients with recurring C. diff infections, but long-term safety and a standardized process have yet to be established.
An estimated 162 people aged 18 years or older who have had two or more episodes of CDAD within the previous 6 months will be included in the clinical trial. These patients will be split into two groups: The first will receive an antidiarrheal medication and an FMT delivered by retention enema; the second will receive an antidiarrheal and a placebo colored to look like an active stool sample.
All patients will provide stool and blood samples at designated time points for 1 year after they undergo treatment for CDAD. Stool samples will be examined for gut microbial diversity changes and infectious pathogens; blood samples will be examined for metabolic syndrome markers.
“Clostridium difficile–associated disease, a significant problem in health care facilities, causes an estimated 15,000 deaths in the United States each year. This randomized, controlled trial aims to provide critical data on the efficacy and long-term safety of using fecal microbiota transplants by enema to cure C. diff infections,” NIAID director Anthony S. Fauci, MD, said in the press release.
The full trial page can be found at Clinicaltrials.gov.
A clinical trial has begun which will examine whether fecal microbiota transplantation (FMT) by enema is safe and can prevent recurrent Clostridium difficile–associated disease (CDAD), according to a press release from the National Institutes of Health.
CDAD is normally treated with antibiotics such as vancomycin or fidaxomicin; however, it recurs in about 20% of people who receive this treatment. FMT is effective at curing patients with recurring C. diff infections, but long-term safety and a standardized process have yet to be established.
An estimated 162 people aged 18 years or older who have had two or more episodes of CDAD within the previous 6 months will be included in the clinical trial. These patients will be split into two groups: The first will receive an antidiarrheal medication and an FMT delivered by retention enema; the second will receive an antidiarrheal and a placebo colored to look like an active stool sample.
All patients will provide stool and blood samples at designated time points for 1 year after they undergo treatment for CDAD. Stool samples will be examined for gut microbial diversity changes and infectious pathogens; blood samples will be examined for metabolic syndrome markers.
“Clostridium difficile–associated disease, a significant problem in health care facilities, causes an estimated 15,000 deaths in the United States each year. This randomized, controlled trial aims to provide critical data on the efficacy and long-term safety of using fecal microbiota transplants by enema to cure C. diff infections,” NIAID director Anthony S. Fauci, MD, said in the press release.
The full trial page can be found at Clinicaltrials.gov.
FDA approves cabozantinib for previously treated HCC
The Food and Drug Administration has approved cabozantinib tablets (Cabometyx) for patients with hepatocellular carcinoma (HCC) who have been previously treated with sorafenib.
Approval was based on an improvement in overall survival over placebo seen in the phase 3 CELESTIAL trial for patients with advanced HCC who received prior sorafenib.
Median overall survival was 10.2 months with cabozantinib versus 8.0 months with placebo (hazard ratio, 0.76; 95% confidence interval, 0.63-0.92; P = .0049). Median progression-free survival was 5.2 months with cabozantinib and 1.9 months with placebo (HR, 0.44; 95% CI, 0.36-0.52; P less than .0001). Objective response rates were 4% with cabozantinib and 0.4% with placebo (P = .0086), Exelixis, makers of the drug, said in a press release.
The most common grade 3 or 4 adverse events in the patients who received cabozantinib, compared with those who received placebo, were palmar-plantar erythrodysesthesia (17% vs. 0%), hypertension (16% vs. 2%), increased aspartate aminotransferase (12% vs. 7%), fatigue (10% vs. 4%), and diarrhea (10% vs. 2%). Treatment-related grade 5 adverse events occurred in six patients in the cabozantinib group (hepatic failure, esophagobronchial fistula, portal vein thrombosis, upper gastrointestinal hemorrhage, pulmonary embolism, and hepatorenal syndrome) and in one patient in the placebo group (hepatic failure).
Cabozantinib is also approved to treat renal cell carcinoma and medullary thyroid cancer.
Checkpoint inhibitor pembrolizumab was granted accelerated approval for the same HCC indication – to treat patients who have been previously treated with sorafenib – in late 2018.
Exelixis and its partner Ipsen have launched a phase 3 trial of cabozantinib in combination with the checkpoint inhibitor atezolizumab versus sorafenib in previously untreated advanced HCC. The trial will also explore single-agent activity of cabozantinib in the first-line setting, the company said in the press release.
The Food and Drug Administration has approved cabozantinib tablets (Cabometyx) for patients with hepatocellular carcinoma (HCC) who have been previously treated with sorafenib.
Approval was based on an improvement in overall survival over placebo seen in the phase 3 CELESTIAL trial for patients with advanced HCC who received prior sorafenib.
Median overall survival was 10.2 months with cabozantinib versus 8.0 months with placebo (hazard ratio, 0.76; 95% confidence interval, 0.63-0.92; P = .0049). Median progression-free survival was 5.2 months with cabozantinib and 1.9 months with placebo (HR, 0.44; 95% CI, 0.36-0.52; P less than .0001). Objective response rates were 4% with cabozantinib and 0.4% with placebo (P = .0086), Exelixis, makers of the drug, said in a press release.
The most common grade 3 or 4 adverse events in the patients who received cabozantinib, compared with those who received placebo, were palmar-plantar erythrodysesthesia (17% vs. 0%), hypertension (16% vs. 2%), increased aspartate aminotransferase (12% vs. 7%), fatigue (10% vs. 4%), and diarrhea (10% vs. 2%). Treatment-related grade 5 adverse events occurred in six patients in the cabozantinib group (hepatic failure, esophagobronchial fistula, portal vein thrombosis, upper gastrointestinal hemorrhage, pulmonary embolism, and hepatorenal syndrome) and in one patient in the placebo group (hepatic failure).
Cabozantinib is also approved to treat renal cell carcinoma and medullary thyroid cancer.
Checkpoint inhibitor pembrolizumab was granted accelerated approval for the same HCC indication – to treat patients who have been previously treated with sorafenib – in late 2018.
Exelixis and its partner Ipsen have launched a phase 3 trial of cabozantinib in combination with the checkpoint inhibitor atezolizumab versus sorafenib in previously untreated advanced HCC. The trial will also explore single-agent activity of cabozantinib in the first-line setting, the company said in the press release.
The Food and Drug Administration has approved cabozantinib tablets (Cabometyx) for patients with hepatocellular carcinoma (HCC) who have been previously treated with sorafenib.
Approval was based on an improvement in overall survival over placebo seen in the phase 3 CELESTIAL trial for patients with advanced HCC who received prior sorafenib.
Median overall survival was 10.2 months with cabozantinib versus 8.0 months with placebo (hazard ratio, 0.76; 95% confidence interval, 0.63-0.92; P = .0049). Median progression-free survival was 5.2 months with cabozantinib and 1.9 months with placebo (HR, 0.44; 95% CI, 0.36-0.52; P less than .0001). Objective response rates were 4% with cabozantinib and 0.4% with placebo (P = .0086), Exelixis, makers of the drug, said in a press release.
The most common grade 3 or 4 adverse events in the patients who received cabozantinib, compared with those who received placebo, were palmar-plantar erythrodysesthesia (17% vs. 0%), hypertension (16% vs. 2%), increased aspartate aminotransferase (12% vs. 7%), fatigue (10% vs. 4%), and diarrhea (10% vs. 2%). Treatment-related grade 5 adverse events occurred in six patients in the cabozantinib group (hepatic failure, esophagobronchial fistula, portal vein thrombosis, upper gastrointestinal hemorrhage, pulmonary embolism, and hepatorenal syndrome) and in one patient in the placebo group (hepatic failure).
Cabozantinib is also approved to treat renal cell carcinoma and medullary thyroid cancer.
Checkpoint inhibitor pembrolizumab was granted accelerated approval for the same HCC indication – to treat patients who have been previously treated with sorafenib – in late 2018.
Exelixis and its partner Ipsen have launched a phase 3 trial of cabozantinib in combination with the checkpoint inhibitor atezolizumab versus sorafenib in previously untreated advanced HCC. The trial will also explore single-agent activity of cabozantinib in the first-line setting, the company said in the press release.
CRS/HIPEC safety concerns may be outdated
, according to a retrospective study involving more than 34,000 cases.
Compared with four other surgical oncology procedures considered high risk, CRS/HIPEC had the lowest 30-day mortality rate, reported lead author Jason M. Foster, MD, of the University of Nebraska Medical Center in Omaha, and his colleagues.
“The perception of high morbidity, high mortality, and poor surgical outcomes remains a barrier to CRS/HIPEC patient referral as well as clinical trial development in the United States, despite the published noncomparative data establishing contemporary safety,” the investigators wrote in JAMA Network Open.
The study involved 34,114 patients from the American College of Surgeons National Surgical Quality Improvement Project (NSQIP) database who underwent CRS/HIPEC (n = 1,822), trisegmental hepatectomy (n = 2,449), right lobe hepatectomy (n = 5,109), pancreaticoduodenectomy (Whipple; n = 16,793), or esophagectomy (n = 7,941) during 2005-2015. The investigators rates of overall 30-day postoperative mortality, superficial incisional infection, deep incisional infection, organ space infection, return to operating room, and length of hospital stay.
Analysis revealed that CRS/HIPEC had a 30-day mortality rate of 1.1%, which was lower than rates of 2.5%-3.9% for the comparative procedures. Similarly, organ space infection rate was lowest for CRS/HIPEC (7.2%). Superficial and deep incisional infection rates were 5.4% and 1.7%, respectively, for CRS/HIPEC, lower than all procedures except right lobe hepatectomy, with rates of 4.6% and 1.5%. Return to OR was necessary for 6.8% of CRS/HIPEC patients, a rate similar to the other procedures except esophagectomy, in which return to OR was necessary 14.4% of the time. Finally, CRS/HIPEC had a median length of stay of 8 days, which was slightly longer than right lobe or trisegmental hepatectomy (7 days), but shorter than Whipple procedure or esophagectomy (10 days.)
“This study found that CRS/HIPEC had the lowest mortality risk, almost 50%-75% lower than other advanced oncology surgical procedures,” the investigators noted. “These findings provide objective data to dispel the misperception of morbidity and mortality concerns surrounding CRS/HIPEC, and surgical risk should no longer remain a deterrent to patient referral or development of clinical trials for CRS/HIPEC.”
The study was funded by the Platon Foundation and the Hill Foundation. The authors reported no conflicts of interest.
SOURCE: Foster JM et al. JAMA Netw Open. 2019 Jan 11. doi: 10.1001/jamanetworkopen.2018.6847.
The recent study by Foster et al. provides insight into the national safety of reductive surgery combined with hyperthermic intraperitoneal chemotherapy (CRS/HIPEC); however, more detailed safety and efficacy data are needed to influence current practices, according to Margaret Smith, MD, and Hari Nathan, MD, PhD.
A closer look at the Foster et al. study reveals three key limitations: First, “cytoreductive surgery encompasses a wide range of procedures, from resection of one peritoneal nodule to multivisceral resection with peritoneal stripping, and, thus, reflects a wide range of possible morbidity,” the authors wrote in an editorial for JAMA Network Open. Therefore, the findings may not represent certain patient populations.
Second, “comparison with other procedures for different indications constructs a straw man.” In contrast with some candidates for CRS/HIPEC, “a patient with pancreatic cancer has no other curative option besides a Whipple procedure.” This imperfect comparison should be considered as such.
Third, the safety of CRS/HIPEC may not be the procedure’s primary limitation. “A more salient concern may be its oncologic effectiveness,” the authors wrote.
Although a clinical randomized trial from 2003 involving patients with colorectal peritoneal carcinomatosis showed a near doubling of overall survival with CRS/HIPEC, compared with systemic chemotherapy alone (22 vs. 12.5 months), a comprehensive understanding of safety and efficacy is lacking, particularly regarding the inclusion of HIPEC. For example, the recent phase 3 Prodige 7 trial showed that addition of HIPEC to CRS added morbidity without survival advantage in patients with colorectal peritoneal carcinomatosis; in contrast, a separate phase 3 trial in epithelial ovarian cancer showed that adding HIPEC to CRS did extend survival.
“…Others have cautioned against changing practice based on these results given concerns over small sample size, imbalances in effects seen across centers, and overall survival with CRS/HIPEC that was similar to other studies’ reported survival following interval debulking alone. Legitimate concerns regarding the efficacy of CRS/HIPEC exist, and appropriate patient selection for this aggressive treatment remains a challenge. Foster et al. demonstrates acceptable morbidity and mortality rates for CRS/HIPEC in this highly selected patient cohort. However, until the benefit for individual patients is more thoroughly understood, clinician referral and treatment practices will remain difficult to transform,” the authors wrote.
Dr. Smith and Dr. Nathan are affiliated with Michigan Medicine at the University of Michigan in Ann Arbor. These comments are adapted from the accompanying editorial (JAMA Netw Open 2019 Jan 11. doi:10.1001/jamanetworkopen.2018.6839).
The recent study by Foster et al. provides insight into the national safety of reductive surgery combined with hyperthermic intraperitoneal chemotherapy (CRS/HIPEC); however, more detailed safety and efficacy data are needed to influence current practices, according to Margaret Smith, MD, and Hari Nathan, MD, PhD.
A closer look at the Foster et al. study reveals three key limitations: First, “cytoreductive surgery encompasses a wide range of procedures, from resection of one peritoneal nodule to multivisceral resection with peritoneal stripping, and, thus, reflects a wide range of possible morbidity,” the authors wrote in an editorial for JAMA Network Open. Therefore, the findings may not represent certain patient populations.
Second, “comparison with other procedures for different indications constructs a straw man.” In contrast with some candidates for CRS/HIPEC, “a patient with pancreatic cancer has no other curative option besides a Whipple procedure.” This imperfect comparison should be considered as such.
Third, the safety of CRS/HIPEC may not be the procedure’s primary limitation. “A more salient concern may be its oncologic effectiveness,” the authors wrote.
Although a clinical randomized trial from 2003 involving patients with colorectal peritoneal carcinomatosis showed a near doubling of overall survival with CRS/HIPEC, compared with systemic chemotherapy alone (22 vs. 12.5 months), a comprehensive understanding of safety and efficacy is lacking, particularly regarding the inclusion of HIPEC. For example, the recent phase 3 Prodige 7 trial showed that addition of HIPEC to CRS added morbidity without survival advantage in patients with colorectal peritoneal carcinomatosis; in contrast, a separate phase 3 trial in epithelial ovarian cancer showed that adding HIPEC to CRS did extend survival.
“…Others have cautioned against changing practice based on these results given concerns over small sample size, imbalances in effects seen across centers, and overall survival with CRS/HIPEC that was similar to other studies’ reported survival following interval debulking alone. Legitimate concerns regarding the efficacy of CRS/HIPEC exist, and appropriate patient selection for this aggressive treatment remains a challenge. Foster et al. demonstrates acceptable morbidity and mortality rates for CRS/HIPEC in this highly selected patient cohort. However, until the benefit for individual patients is more thoroughly understood, clinician referral and treatment practices will remain difficult to transform,” the authors wrote.
Dr. Smith and Dr. Nathan are affiliated with Michigan Medicine at the University of Michigan in Ann Arbor. These comments are adapted from the accompanying editorial (JAMA Netw Open 2019 Jan 11. doi:10.1001/jamanetworkopen.2018.6839).
The recent study by Foster et al. provides insight into the national safety of reductive surgery combined with hyperthermic intraperitoneal chemotherapy (CRS/HIPEC); however, more detailed safety and efficacy data are needed to influence current practices, according to Margaret Smith, MD, and Hari Nathan, MD, PhD.
A closer look at the Foster et al. study reveals three key limitations: First, “cytoreductive surgery encompasses a wide range of procedures, from resection of one peritoneal nodule to multivisceral resection with peritoneal stripping, and, thus, reflects a wide range of possible morbidity,” the authors wrote in an editorial for JAMA Network Open. Therefore, the findings may not represent certain patient populations.
Second, “comparison with other procedures for different indications constructs a straw man.” In contrast with some candidates for CRS/HIPEC, “a patient with pancreatic cancer has no other curative option besides a Whipple procedure.” This imperfect comparison should be considered as such.
Third, the safety of CRS/HIPEC may not be the procedure’s primary limitation. “A more salient concern may be its oncologic effectiveness,” the authors wrote.
Although a clinical randomized trial from 2003 involving patients with colorectal peritoneal carcinomatosis showed a near doubling of overall survival with CRS/HIPEC, compared with systemic chemotherapy alone (22 vs. 12.5 months), a comprehensive understanding of safety and efficacy is lacking, particularly regarding the inclusion of HIPEC. For example, the recent phase 3 Prodige 7 trial showed that addition of HIPEC to CRS added morbidity without survival advantage in patients with colorectal peritoneal carcinomatosis; in contrast, a separate phase 3 trial in epithelial ovarian cancer showed that adding HIPEC to CRS did extend survival.
“…Others have cautioned against changing practice based on these results given concerns over small sample size, imbalances in effects seen across centers, and overall survival with CRS/HIPEC that was similar to other studies’ reported survival following interval debulking alone. Legitimate concerns regarding the efficacy of CRS/HIPEC exist, and appropriate patient selection for this aggressive treatment remains a challenge. Foster et al. demonstrates acceptable morbidity and mortality rates for CRS/HIPEC in this highly selected patient cohort. However, until the benefit for individual patients is more thoroughly understood, clinician referral and treatment practices will remain difficult to transform,” the authors wrote.
Dr. Smith and Dr. Nathan are affiliated with Michigan Medicine at the University of Michigan in Ann Arbor. These comments are adapted from the accompanying editorial (JAMA Netw Open 2019 Jan 11. doi:10.1001/jamanetworkopen.2018.6839).
, according to a retrospective study involving more than 34,000 cases.
Compared with four other surgical oncology procedures considered high risk, CRS/HIPEC had the lowest 30-day mortality rate, reported lead author Jason M. Foster, MD, of the University of Nebraska Medical Center in Omaha, and his colleagues.
“The perception of high morbidity, high mortality, and poor surgical outcomes remains a barrier to CRS/HIPEC patient referral as well as clinical trial development in the United States, despite the published noncomparative data establishing contemporary safety,” the investigators wrote in JAMA Network Open.
The study involved 34,114 patients from the American College of Surgeons National Surgical Quality Improvement Project (NSQIP) database who underwent CRS/HIPEC (n = 1,822), trisegmental hepatectomy (n = 2,449), right lobe hepatectomy (n = 5,109), pancreaticoduodenectomy (Whipple; n = 16,793), or esophagectomy (n = 7,941) during 2005-2015. The investigators rates of overall 30-day postoperative mortality, superficial incisional infection, deep incisional infection, organ space infection, return to operating room, and length of hospital stay.
Analysis revealed that CRS/HIPEC had a 30-day mortality rate of 1.1%, which was lower than rates of 2.5%-3.9% for the comparative procedures. Similarly, organ space infection rate was lowest for CRS/HIPEC (7.2%). Superficial and deep incisional infection rates were 5.4% and 1.7%, respectively, for CRS/HIPEC, lower than all procedures except right lobe hepatectomy, with rates of 4.6% and 1.5%. Return to OR was necessary for 6.8% of CRS/HIPEC patients, a rate similar to the other procedures except esophagectomy, in which return to OR was necessary 14.4% of the time. Finally, CRS/HIPEC had a median length of stay of 8 days, which was slightly longer than right lobe or trisegmental hepatectomy (7 days), but shorter than Whipple procedure or esophagectomy (10 days.)
“This study found that CRS/HIPEC had the lowest mortality risk, almost 50%-75% lower than other advanced oncology surgical procedures,” the investigators noted. “These findings provide objective data to dispel the misperception of morbidity and mortality concerns surrounding CRS/HIPEC, and surgical risk should no longer remain a deterrent to patient referral or development of clinical trials for CRS/HIPEC.”
The study was funded by the Platon Foundation and the Hill Foundation. The authors reported no conflicts of interest.
SOURCE: Foster JM et al. JAMA Netw Open. 2019 Jan 11. doi: 10.1001/jamanetworkopen.2018.6847.
, according to a retrospective study involving more than 34,000 cases.
Compared with four other surgical oncology procedures considered high risk, CRS/HIPEC had the lowest 30-day mortality rate, reported lead author Jason M. Foster, MD, of the University of Nebraska Medical Center in Omaha, and his colleagues.
“The perception of high morbidity, high mortality, and poor surgical outcomes remains a barrier to CRS/HIPEC patient referral as well as clinical trial development in the United States, despite the published noncomparative data establishing contemporary safety,” the investigators wrote in JAMA Network Open.
The study involved 34,114 patients from the American College of Surgeons National Surgical Quality Improvement Project (NSQIP) database who underwent CRS/HIPEC (n = 1,822), trisegmental hepatectomy (n = 2,449), right lobe hepatectomy (n = 5,109), pancreaticoduodenectomy (Whipple; n = 16,793), or esophagectomy (n = 7,941) during 2005-2015. The investigators rates of overall 30-day postoperative mortality, superficial incisional infection, deep incisional infection, organ space infection, return to operating room, and length of hospital stay.
Analysis revealed that CRS/HIPEC had a 30-day mortality rate of 1.1%, which was lower than rates of 2.5%-3.9% for the comparative procedures. Similarly, organ space infection rate was lowest for CRS/HIPEC (7.2%). Superficial and deep incisional infection rates were 5.4% and 1.7%, respectively, for CRS/HIPEC, lower than all procedures except right lobe hepatectomy, with rates of 4.6% and 1.5%. Return to OR was necessary for 6.8% of CRS/HIPEC patients, a rate similar to the other procedures except esophagectomy, in which return to OR was necessary 14.4% of the time. Finally, CRS/HIPEC had a median length of stay of 8 days, which was slightly longer than right lobe or trisegmental hepatectomy (7 days), but shorter than Whipple procedure or esophagectomy (10 days.)
“This study found that CRS/HIPEC had the lowest mortality risk, almost 50%-75% lower than other advanced oncology surgical procedures,” the investigators noted. “These findings provide objective data to dispel the misperception of morbidity and mortality concerns surrounding CRS/HIPEC, and surgical risk should no longer remain a deterrent to patient referral or development of clinical trials for CRS/HIPEC.”
The study was funded by the Platon Foundation and the Hill Foundation. The authors reported no conflicts of interest.
SOURCE: Foster JM et al. JAMA Netw Open. 2019 Jan 11. doi: 10.1001/jamanetworkopen.2018.6847.
FROM JAMA NETWORK OPEN
Key clinical point: Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) appears safe, and concerns about high complication rates may be outdated.
Major finding: CRS/HIPEC had a 30-day mortality rate of 1.1%, which was lower than rates of 2.5%-3.9% for comparative high-risk surgical oncology procedures.
Study details: A retrospective study of 34,114 patients from the American College of Surgeons National Surgical Quality Improvement Project (NSQIP) database who underwent CRS/HIPEC (n = 1,822), trisegmental hepatectomy (n = 2,449), right lobe hepatectomy (n = 5,109), pancreaticoduodenectomy (Whipple; n = 16,793), or esophagectomy (n = 7,941) during 2005-2015.
Disclosures: The study was funded by the Platon Foundation and the Hill Foundation. The authors reported no conflicts of interest.
Source: Foster JM et al. JAMA Netw Open. 2019 Jan 11. doi: 10.1001/jamanetworkopen.2018.6847.
Oncology research does crowdsourcing
Crowdsourcing is now happening at multiple steps in cancer research, from linking patients with studies to data collection, to an end game that offers the data to anyone interested in taking a crack at analyzing it, drawing conclusions, or generating hypotheses for further study. Generally defined today as electronic-based communication that links otherwise disparate individuals or groups, crowdsourcing has quietly over the past decade or so begun to transform a significant segment of all types of biomedical research, with effects on oncology that seemingly are among the strongest.
Crowdsourcing data analysis “is an opportunity to take on complex problems in cancer research and attract researchers who would not usually work on it. With the complexity of cancer we need to tap into every resource; this is a way to do it with low overhead,” explained Daniel Gallahan, PhD, deputy director of the Division of Cancer Biology of the National Cancer Institute. “It’s been a workable model that we’ve had success with, and there is not a lot of upfront cost,” Dr. Gallahan said in an interview.
“A lot of data is produced in oncology, and the ability of an individual to analyze the data is fairly limited, but if we can focus a community of people around a specific question, we can answer it much more quickly,” explained James C. Costello, PhD, a bioinformatics researcher at the University of Colorado in Aurora.
Traced to first usage in 2006, the term crowdsourcing as it applies to data analysis means combining “the bottom-up creative intelligence of a community that volunteers solutions with the top-down management of an organization that poses the problem,” according to a 2016 review (Nat Rev Genet. 2016 July 15;17[8]:470-86). The bioinformatics experts who wrote this review, as well as most anyone else who saw the way crowdsourcing has taken hold over the last decade, link this increased role to the huge amounts of data generated by and needing analysis in biomedical research in general – and in oncology in particular. Companies like Sage Bionetworks have came on the scene built to expedite crowdsourced data analysis.
Dr. Gallahan cited as examples of crowdsourced cancer studies a pair of DREAM (Dialogue for Reverse Engineering Assessments and Methods) challenges, one for developing an improved algorithm for predicting drug sensitivity in breast cancer cells (Nat Biotechnol. 2014 Dec;32[12]:1202-12) and a second that developed a better prognostic model for patients with metastatic, castration-resistant prostate cancer (Lancet Oncol. 2017 Jan;18[1]:132-42). The National Cancer Institute “has been actively involved in DREAM challenges,” he noted.
In addition to competing research teams working individually to find the best solution to these problems, the prostate cancer challenge, for example, then “asked the best performing teams to work together to further improve the methodology,” said Dr. Costello, who helped organize and run both the breast cancer and prostate cancer challenges cited by Dr. Gallahan. “The challenge phase was separate, but then [the teams] worked together to come up with even better solutions. We put together the best of seven models, and none of the teams had ever worked together before,” explained Dr. Costello, (JCO Clin Cancer Inform. 2017 Aug 4. doi: 10.1200/CCI.17.00018). He called this opportunity another attraction of a crowdsourced challenge for problem solving.
Crowdsourced data analysis is also vulnerable to several possible problems. It potentially can “lose specificity and the hypothesis-driven aspect,” cautioned Dr. Costello. “There are also issues of who owns the data and who receives credit” for a solution based on the data, and there are inherent limitations in finding associations that are independent of a study’s original primary endpoint. “It’s a different approach that does not replace the traditional, standard research approach of hypothesis-driven studies,” he said.
Other caveats on the output of DREAM challenges and other crowdsourced data analysis are the need for validation of the solution and a way to follow up on a solution to take it from an academic exercise to a useful application, said Dr. Gallahan.
Despite their limitations crowdsourced analyses began appearing a little more than a decade ago and have steadily grown in number “as more and more data get generated,” more than can be easily analyzed by the people collecting the data, Dr. Gallahan noted.
In fact, some studies now accumulate data with the express intent of posting the findings online and making them available for crowdsourced analyses. The Count Me In study is a collaboration of researchers at Dana-Farber Cancer Institute in Boston and three other organizations to engage and collect data from patients with a wide range of cancer types. The study began enrolling patients with metastatic breast cancer, with about 5,100 of these patients included by early 2019 in the Metastatic Breast Cancer Project. Count Me In has expanded to also include patients with prostate cancer, angiosarcoma, and gastroesophageal cancer. The program’s overall goal is to enroll more than 100,000 patients diagnosed with any type of cancer.
“The concept is using online recruitment to involve patients who wouldn’t otherwise engage” with this sort of study, said Eliezer van Allen, MD, a Dana-Farber oncologist who runs the prostate cancer arm of Count Me In. “Patients are the ones who spread the word” about Count Me In to other patients, largely through social media, an approach representing another way that crowdsourcing has seeped into cancer research.
“There was an inflection point in about 2013-2015” when a critical mass of patients with cancers interacted with other cancer patients in various social media and other online groups sufficient to make Count Me In viable, Dr. van Allen said in an interview.
Once data come in – from donated specimens that underwent genetic analysis, blood work, and clinical records – the researchers formated the information and then put it out with open access at the cBioPortal. Among the research questions that Dr. van Allen looks forward to getting addressed with these data are what factors define exceptional treatment responders, what are the genetic profiles of metastatic tumors, and what are characteristics of patients not treated at academic medical centers.
But the coolest thing about crowdsourcing these data is that some people “will use it to answer questions that I haven’t thought of,” Dr. van Allen said.
He conceded that the clinical-record data are limited by their uncertain reliability and that the model for patient recruitment introduces bias, although the organizers of Count Me In are developing paper-based tools for patient enrollment to complement electronic enrollment. “Our goal is to cast a wide net for patients,” with eventual expansion to all cancer types. The prostate cancer arm of Count Me In tallied 623 participating patients as of the start of 2019 and after the first year of patient recruitment into the prostate cancer section. Dr. van Allen planned to release the first batch of data from the prostate cancer study by the end of January 2019.
The first publication of findings from the breast cancer project of Count Me In appeared in late 2018, an analysis of acquired HER2 mutations found in tumor biopsies from eight patients with estrogen-receptor-positive metastatic breast cancer and the linkage of these mutations to drug resistance (Nature Gen. 2018 Dec 10. doi: 10.1038/s41588-018-0287-5).
Crowdsourcing to get cancer patients into studies is also an initiative of patients themselves, like the patient-trial matching service provided by the Myeloma Crowd, which works in partnership with the SparkCures website. Myeloma Crowd began in 2013 as a series of interviews with researchers running cancer trials who explained their studies and the types of patients they were seeking. By 2015, this effort added a partnership with SparkCures that runs an online tool that matches myeloma patients with an individualized short list of available trials.
In late 2018, Myeloma Crowd launched a new website, HealthTree, that combines at one site disease information, trial matching (still using SparkCures), and several platforms for patient interaction. Myeloma Crowd also seeks HealthTree to be a vehicle for patient-data collection, such as a recent survey on vaccination experiences following myeloma treatment, said Jenny Ahlstrom, a myeloma patient and founder of Myeloma Crowd. “We use tech to build a patient constituency, and now we’re using it to collect data and do research,” she said in an interview. Patients “invite each other to contribute data,” an approach that makes Myeloma Crowd unique, she maintained. One goal is to “speed up the clinical trial process,” as well as link patients with the more conventional trials they qualify for, Ms. Ahlstrom said.
Any effective effort to link patients with appropriate trials is a big plus, commented Dr. Gallahan. It both “empowers patients,” while also offering a novel path for informing patients about trials. “Any time you can get more patients into trials, it’s a success,” although of course patients must still meet enrollment criteria for a trial, he said. Privacy-protected online patient networks also provide an easier way for patients to submit their data into a trial.
But often, these issues aren’t so easily resolved. “It’s difficult to guarantee whether participants meet eligibility criteria” when they enter through a crowdsourced portal because “there is no way to validate,” said Young Ji Lee, PhD, a biomedical informatics researcher at the University of Pittsburgh. An analysis based on patient data collected by crowdsourcing is also subject to a selection bias, and confidentiality is not easily ensured. A crowdsourced study still needs institutional review board oversight, she said in an interview,
Dr. Lee cited a 2018 review that identified 202 studies published through March 2016 that involved health-related crowdsourcing, with 36% involving research. Oncology-related studies made up 7% of the total, fourth highest after public health, psychiatry, and surgery (J Med Internet Res. 2018 May;20[5]:e187). Dr. Lee ran her own study of published reports of crowdsourcing research in cancer through June 2016 and identified 12 studies (Cancer Med. 2017 Nov;6[11]:2595-605). “Crowdsourcing will continue to expand in biomedical research, including oncology, with the growing trends of patient-centered, participatory medicine,” she concluded.
Dr. Gallahan, Dr. Costello, and Dr. Lee had no disclosures. Dr. van Allen has been a consultant or advisor to Dynamo, Foresite Capital, Genome Medical, Illumina, Invitae, and Tango Therapeutics, he has received research funding from Bristol-Myers Squibb and Novartis, and he has an equity interest in Genome Medical, Microsoft, Synapse, and Tango Therapeutics. Ms. Ahlstrom said that Myeloma Crowd has received funding from Bristol-Myers Squibb, Celgene, Janssen, Sanofi, and Takeda.
Updated January 16, 2019.
Crowdsourcing is now happening at multiple steps in cancer research, from linking patients with studies to data collection, to an end game that offers the data to anyone interested in taking a crack at analyzing it, drawing conclusions, or generating hypotheses for further study. Generally defined today as electronic-based communication that links otherwise disparate individuals or groups, crowdsourcing has quietly over the past decade or so begun to transform a significant segment of all types of biomedical research, with effects on oncology that seemingly are among the strongest.
Crowdsourcing data analysis “is an opportunity to take on complex problems in cancer research and attract researchers who would not usually work on it. With the complexity of cancer we need to tap into every resource; this is a way to do it with low overhead,” explained Daniel Gallahan, PhD, deputy director of the Division of Cancer Biology of the National Cancer Institute. “It’s been a workable model that we’ve had success with, and there is not a lot of upfront cost,” Dr. Gallahan said in an interview.
“A lot of data is produced in oncology, and the ability of an individual to analyze the data is fairly limited, but if we can focus a community of people around a specific question, we can answer it much more quickly,” explained James C. Costello, PhD, a bioinformatics researcher at the University of Colorado in Aurora.
Traced to first usage in 2006, the term crowdsourcing as it applies to data analysis means combining “the bottom-up creative intelligence of a community that volunteers solutions with the top-down management of an organization that poses the problem,” according to a 2016 review (Nat Rev Genet. 2016 July 15;17[8]:470-86). The bioinformatics experts who wrote this review, as well as most anyone else who saw the way crowdsourcing has taken hold over the last decade, link this increased role to the huge amounts of data generated by and needing analysis in biomedical research in general – and in oncology in particular. Companies like Sage Bionetworks have came on the scene built to expedite crowdsourced data analysis.
Dr. Gallahan cited as examples of crowdsourced cancer studies a pair of DREAM (Dialogue for Reverse Engineering Assessments and Methods) challenges, one for developing an improved algorithm for predicting drug sensitivity in breast cancer cells (Nat Biotechnol. 2014 Dec;32[12]:1202-12) and a second that developed a better prognostic model for patients with metastatic, castration-resistant prostate cancer (Lancet Oncol. 2017 Jan;18[1]:132-42). The National Cancer Institute “has been actively involved in DREAM challenges,” he noted.
In addition to competing research teams working individually to find the best solution to these problems, the prostate cancer challenge, for example, then “asked the best performing teams to work together to further improve the methodology,” said Dr. Costello, who helped organize and run both the breast cancer and prostate cancer challenges cited by Dr. Gallahan. “The challenge phase was separate, but then [the teams] worked together to come up with even better solutions. We put together the best of seven models, and none of the teams had ever worked together before,” explained Dr. Costello, (JCO Clin Cancer Inform. 2017 Aug 4. doi: 10.1200/CCI.17.00018). He called this opportunity another attraction of a crowdsourced challenge for problem solving.
Crowdsourced data analysis is also vulnerable to several possible problems. It potentially can “lose specificity and the hypothesis-driven aspect,” cautioned Dr. Costello. “There are also issues of who owns the data and who receives credit” for a solution based on the data, and there are inherent limitations in finding associations that are independent of a study’s original primary endpoint. “It’s a different approach that does not replace the traditional, standard research approach of hypothesis-driven studies,” he said.
Other caveats on the output of DREAM challenges and other crowdsourced data analysis are the need for validation of the solution and a way to follow up on a solution to take it from an academic exercise to a useful application, said Dr. Gallahan.
Despite their limitations crowdsourced analyses began appearing a little more than a decade ago and have steadily grown in number “as more and more data get generated,” more than can be easily analyzed by the people collecting the data, Dr. Gallahan noted.
In fact, some studies now accumulate data with the express intent of posting the findings online and making them available for crowdsourced analyses. The Count Me In study is a collaboration of researchers at Dana-Farber Cancer Institute in Boston and three other organizations to engage and collect data from patients with a wide range of cancer types. The study began enrolling patients with metastatic breast cancer, with about 5,100 of these patients included by early 2019 in the Metastatic Breast Cancer Project. Count Me In has expanded to also include patients with prostate cancer, angiosarcoma, and gastroesophageal cancer. The program’s overall goal is to enroll more than 100,000 patients diagnosed with any type of cancer.
“The concept is using online recruitment to involve patients who wouldn’t otherwise engage” with this sort of study, said Eliezer van Allen, MD, a Dana-Farber oncologist who runs the prostate cancer arm of Count Me In. “Patients are the ones who spread the word” about Count Me In to other patients, largely through social media, an approach representing another way that crowdsourcing has seeped into cancer research.
“There was an inflection point in about 2013-2015” when a critical mass of patients with cancers interacted with other cancer patients in various social media and other online groups sufficient to make Count Me In viable, Dr. van Allen said in an interview.
Once data come in – from donated specimens that underwent genetic analysis, blood work, and clinical records – the researchers formated the information and then put it out with open access at the cBioPortal. Among the research questions that Dr. van Allen looks forward to getting addressed with these data are what factors define exceptional treatment responders, what are the genetic profiles of metastatic tumors, and what are characteristics of patients not treated at academic medical centers.
But the coolest thing about crowdsourcing these data is that some people “will use it to answer questions that I haven’t thought of,” Dr. van Allen said.
He conceded that the clinical-record data are limited by their uncertain reliability and that the model for patient recruitment introduces bias, although the organizers of Count Me In are developing paper-based tools for patient enrollment to complement electronic enrollment. “Our goal is to cast a wide net for patients,” with eventual expansion to all cancer types. The prostate cancer arm of Count Me In tallied 623 participating patients as of the start of 2019 and after the first year of patient recruitment into the prostate cancer section. Dr. van Allen planned to release the first batch of data from the prostate cancer study by the end of January 2019.
The first publication of findings from the breast cancer project of Count Me In appeared in late 2018, an analysis of acquired HER2 mutations found in tumor biopsies from eight patients with estrogen-receptor-positive metastatic breast cancer and the linkage of these mutations to drug resistance (Nature Gen. 2018 Dec 10. doi: 10.1038/s41588-018-0287-5).
Crowdsourcing to get cancer patients into studies is also an initiative of patients themselves, like the patient-trial matching service provided by the Myeloma Crowd, which works in partnership with the SparkCures website. Myeloma Crowd began in 2013 as a series of interviews with researchers running cancer trials who explained their studies and the types of patients they were seeking. By 2015, this effort added a partnership with SparkCures that runs an online tool that matches myeloma patients with an individualized short list of available trials.
In late 2018, Myeloma Crowd launched a new website, HealthTree, that combines at one site disease information, trial matching (still using SparkCures), and several platforms for patient interaction. Myeloma Crowd also seeks HealthTree to be a vehicle for patient-data collection, such as a recent survey on vaccination experiences following myeloma treatment, said Jenny Ahlstrom, a myeloma patient and founder of Myeloma Crowd. “We use tech to build a patient constituency, and now we’re using it to collect data and do research,” she said in an interview. Patients “invite each other to contribute data,” an approach that makes Myeloma Crowd unique, she maintained. One goal is to “speed up the clinical trial process,” as well as link patients with the more conventional trials they qualify for, Ms. Ahlstrom said.
Any effective effort to link patients with appropriate trials is a big plus, commented Dr. Gallahan. It both “empowers patients,” while also offering a novel path for informing patients about trials. “Any time you can get more patients into trials, it’s a success,” although of course patients must still meet enrollment criteria for a trial, he said. Privacy-protected online patient networks also provide an easier way for patients to submit their data into a trial.
But often, these issues aren’t so easily resolved. “It’s difficult to guarantee whether participants meet eligibility criteria” when they enter through a crowdsourced portal because “there is no way to validate,” said Young Ji Lee, PhD, a biomedical informatics researcher at the University of Pittsburgh. An analysis based on patient data collected by crowdsourcing is also subject to a selection bias, and confidentiality is not easily ensured. A crowdsourced study still needs institutional review board oversight, she said in an interview,
Dr. Lee cited a 2018 review that identified 202 studies published through March 2016 that involved health-related crowdsourcing, with 36% involving research. Oncology-related studies made up 7% of the total, fourth highest after public health, psychiatry, and surgery (J Med Internet Res. 2018 May;20[5]:e187). Dr. Lee ran her own study of published reports of crowdsourcing research in cancer through June 2016 and identified 12 studies (Cancer Med. 2017 Nov;6[11]:2595-605). “Crowdsourcing will continue to expand in biomedical research, including oncology, with the growing trends of patient-centered, participatory medicine,” she concluded.
Dr. Gallahan, Dr. Costello, and Dr. Lee had no disclosures. Dr. van Allen has been a consultant or advisor to Dynamo, Foresite Capital, Genome Medical, Illumina, Invitae, and Tango Therapeutics, he has received research funding from Bristol-Myers Squibb and Novartis, and he has an equity interest in Genome Medical, Microsoft, Synapse, and Tango Therapeutics. Ms. Ahlstrom said that Myeloma Crowd has received funding from Bristol-Myers Squibb, Celgene, Janssen, Sanofi, and Takeda.
Updated January 16, 2019.
Crowdsourcing is now happening at multiple steps in cancer research, from linking patients with studies to data collection, to an end game that offers the data to anyone interested in taking a crack at analyzing it, drawing conclusions, or generating hypotheses for further study. Generally defined today as electronic-based communication that links otherwise disparate individuals or groups, crowdsourcing has quietly over the past decade or so begun to transform a significant segment of all types of biomedical research, with effects on oncology that seemingly are among the strongest.
Crowdsourcing data analysis “is an opportunity to take on complex problems in cancer research and attract researchers who would not usually work on it. With the complexity of cancer we need to tap into every resource; this is a way to do it with low overhead,” explained Daniel Gallahan, PhD, deputy director of the Division of Cancer Biology of the National Cancer Institute. “It’s been a workable model that we’ve had success with, and there is not a lot of upfront cost,” Dr. Gallahan said in an interview.
“A lot of data is produced in oncology, and the ability of an individual to analyze the data is fairly limited, but if we can focus a community of people around a specific question, we can answer it much more quickly,” explained James C. Costello, PhD, a bioinformatics researcher at the University of Colorado in Aurora.
Traced to first usage in 2006, the term crowdsourcing as it applies to data analysis means combining “the bottom-up creative intelligence of a community that volunteers solutions with the top-down management of an organization that poses the problem,” according to a 2016 review (Nat Rev Genet. 2016 July 15;17[8]:470-86). The bioinformatics experts who wrote this review, as well as most anyone else who saw the way crowdsourcing has taken hold over the last decade, link this increased role to the huge amounts of data generated by and needing analysis in biomedical research in general – and in oncology in particular. Companies like Sage Bionetworks have came on the scene built to expedite crowdsourced data analysis.
Dr. Gallahan cited as examples of crowdsourced cancer studies a pair of DREAM (Dialogue for Reverse Engineering Assessments and Methods) challenges, one for developing an improved algorithm for predicting drug sensitivity in breast cancer cells (Nat Biotechnol. 2014 Dec;32[12]:1202-12) and a second that developed a better prognostic model for patients with metastatic, castration-resistant prostate cancer (Lancet Oncol. 2017 Jan;18[1]:132-42). The National Cancer Institute “has been actively involved in DREAM challenges,” he noted.
In addition to competing research teams working individually to find the best solution to these problems, the prostate cancer challenge, for example, then “asked the best performing teams to work together to further improve the methodology,” said Dr. Costello, who helped organize and run both the breast cancer and prostate cancer challenges cited by Dr. Gallahan. “The challenge phase was separate, but then [the teams] worked together to come up with even better solutions. We put together the best of seven models, and none of the teams had ever worked together before,” explained Dr. Costello, (JCO Clin Cancer Inform. 2017 Aug 4. doi: 10.1200/CCI.17.00018). He called this opportunity another attraction of a crowdsourced challenge for problem solving.
Crowdsourced data analysis is also vulnerable to several possible problems. It potentially can “lose specificity and the hypothesis-driven aspect,” cautioned Dr. Costello. “There are also issues of who owns the data and who receives credit” for a solution based on the data, and there are inherent limitations in finding associations that are independent of a study’s original primary endpoint. “It’s a different approach that does not replace the traditional, standard research approach of hypothesis-driven studies,” he said.
Other caveats on the output of DREAM challenges and other crowdsourced data analysis are the need for validation of the solution and a way to follow up on a solution to take it from an academic exercise to a useful application, said Dr. Gallahan.
Despite their limitations crowdsourced analyses began appearing a little more than a decade ago and have steadily grown in number “as more and more data get generated,” more than can be easily analyzed by the people collecting the data, Dr. Gallahan noted.
In fact, some studies now accumulate data with the express intent of posting the findings online and making them available for crowdsourced analyses. The Count Me In study is a collaboration of researchers at Dana-Farber Cancer Institute in Boston and three other organizations to engage and collect data from patients with a wide range of cancer types. The study began enrolling patients with metastatic breast cancer, with about 5,100 of these patients included by early 2019 in the Metastatic Breast Cancer Project. Count Me In has expanded to also include patients with prostate cancer, angiosarcoma, and gastroesophageal cancer. The program’s overall goal is to enroll more than 100,000 patients diagnosed with any type of cancer.
“The concept is using online recruitment to involve patients who wouldn’t otherwise engage” with this sort of study, said Eliezer van Allen, MD, a Dana-Farber oncologist who runs the prostate cancer arm of Count Me In. “Patients are the ones who spread the word” about Count Me In to other patients, largely through social media, an approach representing another way that crowdsourcing has seeped into cancer research.
“There was an inflection point in about 2013-2015” when a critical mass of patients with cancers interacted with other cancer patients in various social media and other online groups sufficient to make Count Me In viable, Dr. van Allen said in an interview.
Once data come in – from donated specimens that underwent genetic analysis, blood work, and clinical records – the researchers formated the information and then put it out with open access at the cBioPortal. Among the research questions that Dr. van Allen looks forward to getting addressed with these data are what factors define exceptional treatment responders, what are the genetic profiles of metastatic tumors, and what are characteristics of patients not treated at academic medical centers.
But the coolest thing about crowdsourcing these data is that some people “will use it to answer questions that I haven’t thought of,” Dr. van Allen said.
He conceded that the clinical-record data are limited by their uncertain reliability and that the model for patient recruitment introduces bias, although the organizers of Count Me In are developing paper-based tools for patient enrollment to complement electronic enrollment. “Our goal is to cast a wide net for patients,” with eventual expansion to all cancer types. The prostate cancer arm of Count Me In tallied 623 participating patients as of the start of 2019 and after the first year of patient recruitment into the prostate cancer section. Dr. van Allen planned to release the first batch of data from the prostate cancer study by the end of January 2019.
The first publication of findings from the breast cancer project of Count Me In appeared in late 2018, an analysis of acquired HER2 mutations found in tumor biopsies from eight patients with estrogen-receptor-positive metastatic breast cancer and the linkage of these mutations to drug resistance (Nature Gen. 2018 Dec 10. doi: 10.1038/s41588-018-0287-5).
Crowdsourcing to get cancer patients into studies is also an initiative of patients themselves, like the patient-trial matching service provided by the Myeloma Crowd, which works in partnership with the SparkCures website. Myeloma Crowd began in 2013 as a series of interviews with researchers running cancer trials who explained their studies and the types of patients they were seeking. By 2015, this effort added a partnership with SparkCures that runs an online tool that matches myeloma patients with an individualized short list of available trials.
In late 2018, Myeloma Crowd launched a new website, HealthTree, that combines at one site disease information, trial matching (still using SparkCures), and several platforms for patient interaction. Myeloma Crowd also seeks HealthTree to be a vehicle for patient-data collection, such as a recent survey on vaccination experiences following myeloma treatment, said Jenny Ahlstrom, a myeloma patient and founder of Myeloma Crowd. “We use tech to build a patient constituency, and now we’re using it to collect data and do research,” she said in an interview. Patients “invite each other to contribute data,” an approach that makes Myeloma Crowd unique, she maintained. One goal is to “speed up the clinical trial process,” as well as link patients with the more conventional trials they qualify for, Ms. Ahlstrom said.
Any effective effort to link patients with appropriate trials is a big plus, commented Dr. Gallahan. It both “empowers patients,” while also offering a novel path for informing patients about trials. “Any time you can get more patients into trials, it’s a success,” although of course patients must still meet enrollment criteria for a trial, he said. Privacy-protected online patient networks also provide an easier way for patients to submit their data into a trial.
But often, these issues aren’t so easily resolved. “It’s difficult to guarantee whether participants meet eligibility criteria” when they enter through a crowdsourced portal because “there is no way to validate,” said Young Ji Lee, PhD, a biomedical informatics researcher at the University of Pittsburgh. An analysis based on patient data collected by crowdsourcing is also subject to a selection bias, and confidentiality is not easily ensured. A crowdsourced study still needs institutional review board oversight, she said in an interview,
Dr. Lee cited a 2018 review that identified 202 studies published through March 2016 that involved health-related crowdsourcing, with 36% involving research. Oncology-related studies made up 7% of the total, fourth highest after public health, psychiatry, and surgery (J Med Internet Res. 2018 May;20[5]:e187). Dr. Lee ran her own study of published reports of crowdsourcing research in cancer through June 2016 and identified 12 studies (Cancer Med. 2017 Nov;6[11]:2595-605). “Crowdsourcing will continue to expand in biomedical research, including oncology, with the growing trends of patient-centered, participatory medicine,” she concluded.
Dr. Gallahan, Dr. Costello, and Dr. Lee had no disclosures. Dr. van Allen has been a consultant or advisor to Dynamo, Foresite Capital, Genome Medical, Illumina, Invitae, and Tango Therapeutics, he has received research funding from Bristol-Myers Squibb and Novartis, and he has an equity interest in Genome Medical, Microsoft, Synapse, and Tango Therapeutics. Ms. Ahlstrom said that Myeloma Crowd has received funding from Bristol-Myers Squibb, Celgene, Janssen, Sanofi, and Takeda.
Updated January 16, 2019.