User login
Limits of care: What events can you prevent?
Psychotic patient declines hospital admission, drives into an office building
Cook County (IL) Circuit Court
The patient, age 43, had been treated for mental illness for many years. He was voluntarily admitted to a hospital under the care of his psychiatrist, and was discharged at his own request a few days later. He had improved and was not considered a candidate for involuntary admission because he was not a danger to himself or others.
The patient then informed the psychiatrist that he did not want to continue treatment and said he had an appointment with a new psychiatrist within 2 weeks.
Five days later, the patient went to another hospital for voluntary admission. He was seen by an emergency room physician, who determined the patient was a candidate for voluntary admission. The patient, however, decided to leave the hospital while a bed was being arranged.
Two days later, the patient began having auditory and visual hallucinations. He then drove his car through the glass doors of an office building. No one was injured, but the patient was arrested and convicted of felony damage to property.
In his suit, the patient alleged his longtime psychiatrist was negligent and failed to properly treat him to avoid development of hallucinations. The psychiatrist argued that involuntary admission was not indicated and that the care given was appropriate.
- A defense verdict was returned
Patient commits suicide after discharge
Cook County (IL) Circuit Court
A patient, age 45, committed suicide by taking lethal doses of medication prescribed by her psychiatrist. The patient had suffered from severe depression, personality disorder, and substance abuse. The day before her death, she went to a hospital emergency room, where she was assessed for suicide and released without the psychiatrist having been notified.
The patient’s family claimed that the psychiatrist was negligent because he did not adequately assess or monitor the patient’s clinical condition at sufficient intervals over the 3 months preceding her suicide. The family also alleged that the psychiatrist prescribed oxycodone inappropriately.
The psychiatrist argued that proper care was given and that the patient failed to provide a complete, accurate medical history at the emergency room visit and did not to consent to admission.
- A defense verdict was returned
Could admission have prevented patient’s suicide?
Douglas County (NE) District Court
A patient in his mid-60s with a history of depression committed suicide with a gunshot wound to the head. Before his suicide, the patient was seeing a psychiatrist and psychologist for depression and emotional problems.
The patient’s family alleged the psychiatrist failed to diagnose the severity of the patient’s problems and admit him to a hospital for treatment and observation. The psychiatrist and psychologist denied negligence.
- A defense verdict was returned
Medical malpractice law is constantly evolving to determine what constitutes “negligent care.” The legal standard requires a patient who brings a negligence claim against a psychiatrist to prove:
- a relationship between patient and psychiatrist such that a duty of care exists
- the duty was breached—meaning the standard of care was not met
- the breach of duty caused the injury.
Relationship rules
The first case highlights issues surrounding the patient-psychiatrist relationship. In general, once you have agreed to treat a patient, a doctor-patient relationship and duty of care exists.
In the first case, the patient informed his longtime psychiatrist that he no longer wanted to continue care after discharge. A psychiatrist who terminates a doctor-patient relationship should provide written notice, an explanation of termination, and referrals and continue to care for the patient for a reasonable period.1 No such duty exists, however, when the patient ends treatment. Courts have found that the patient has not been abandoned when he or she voluntarily and unilaterally terminates the relationship.2,3
The relationship ends the moment the patient terminates care, unless the patient is not competent to make that unilateral decision. In that situation, your duty of care to the patient continues.2 When a competent patient terminates care, document the date and time of termination and the patient’s competence.
When relationships begin
The patient in the first case had an appointment with a new psychiatrist within 2 weeks. Is the new psychiatrist liable for what happens in the intervening period or does the relationship begin when the patient has been examined or treated? The legal question of when a physician-patient relationship is created remains problematic. Standards vary from state to state, but general principles offer some guidance.
The physician-patient relationship is a contract. The court would examine parties’ actions to ascertain their intent to determine if the patient reasonably believed that the physician—by actions or words—agreed to provide necessary medical care. Additionally, whether a relationship exists depends on the specific facts and circumstances of each situation.
There is some authority, across many jurisdictions, that a physician-patient relationship is established only when a physician conducts the initial history and physical examination. In some cases, however, the relationship has been found to exist at an earlier point, such as when a physician gave a referred patient an appointment for a consultation. When in doubt, assume the relationship exists.4
Duty of care
These cases raise areas where possible duty of care was breached:
- negligent prescription of medication
- failure to assess suicidal thinking.
Assessing suicide risk. Negligence in the second and third cases is based upon failure to assess suicidal thoughts. The legal system recognizes that psychiatrists cannot predict suicide,6 and mistakes in clinical judgment are not the same as negligence. Psychiatrists, however, are required to assess suicide risk and intervene appropriately.
When defending a negligence claim, the profession’s custom—reflected by the standard of care common to others with the practitioner’s training—is the benchmark against which the courts measure negligence. Therefore, take steps determined appropriate by the profession and document this risk assessment.7 For example, ask the patient about:
- suicidal thoughts and intent
- stressors
- history of suicidal behavior/attempts
- substance use
- signs and symptoms of depression
- bipolar disorder
- psychosis.8
Prescriptions. No clear line defines negligence when potentially dangerous medications are prescribed to a suicidal patient. Some psychiatrists dispense limited quantities of medications and see the patient weekly to monitor mood and medication. But even then a psychiatrist cannot prevent suicide—for example, the patient may have multiple prescribers or hoard medications. The concept of “sufficient intervals” to see a patient is determined case-by-case.
Documentation. Make suicide assessments an ongoing process. Document all aspects of the patient’s care, stability, and suicide risk, and reasons for the visit intervals. Indicate in the records your risk-benefit assessment in making treatment decisions.
Cases are selected byfrom Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Drug brand name
- Oxycodone • Percocet
1. American Medical Association Code of Medical Ethics, Opinion 8.115.
2. Knapp v. Eppright, 783 SW2d 293 (Tex 1989).
3. Saunders v. Tisher (Maine Sup. Jud. Ct. 2006).
4. Physicians Risk Management Update. The physician-patient relationship: when does it begin? Available at: http://www.phyins.com/pi/risk/updates/mayjun04.html. Accessed December 28, 2006.
5. American Psychiatric Association. Principles of medical ethics with annotations especially applicable to psychiatry. Washington, DC; 2006. Available at: http://www.psych.org/psych_pract/ethics/ppaethics.cfm. Accessed December 28, 2006.
6. Pokorny A. Prediction of suicide in psychiatric patients. Report of a prospective study. Arch Gen Psychiatry 1983;40(3):249-57.
7. Packman WL, Pennuto TO, Bongar B, Orthwein J. Legal issues of professional negligence in suicide cases. Behav Sci Law 2004;22:697-713.
8. Simon RI. The suicidal patient. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law: a comprehensive handbook. Cambridge, MA: Harvard University Press; 1998:166-86.
9. Maunz v. Perales, 276 Kan. 313, 76 P.3d 1027 (Kan 2003).
Psychotic patient declines hospital admission, drives into an office building
Cook County (IL) Circuit Court
The patient, age 43, had been treated for mental illness for many years. He was voluntarily admitted to a hospital under the care of his psychiatrist, and was discharged at his own request a few days later. He had improved and was not considered a candidate for involuntary admission because he was not a danger to himself or others.
The patient then informed the psychiatrist that he did not want to continue treatment and said he had an appointment with a new psychiatrist within 2 weeks.
Five days later, the patient went to another hospital for voluntary admission. He was seen by an emergency room physician, who determined the patient was a candidate for voluntary admission. The patient, however, decided to leave the hospital while a bed was being arranged.
Two days later, the patient began having auditory and visual hallucinations. He then drove his car through the glass doors of an office building. No one was injured, but the patient was arrested and convicted of felony damage to property.
In his suit, the patient alleged his longtime psychiatrist was negligent and failed to properly treat him to avoid development of hallucinations. The psychiatrist argued that involuntary admission was not indicated and that the care given was appropriate.
- A defense verdict was returned
Patient commits suicide after discharge
Cook County (IL) Circuit Court
A patient, age 45, committed suicide by taking lethal doses of medication prescribed by her psychiatrist. The patient had suffered from severe depression, personality disorder, and substance abuse. The day before her death, she went to a hospital emergency room, where she was assessed for suicide and released without the psychiatrist having been notified.
The patient’s family claimed that the psychiatrist was negligent because he did not adequately assess or monitor the patient’s clinical condition at sufficient intervals over the 3 months preceding her suicide. The family also alleged that the psychiatrist prescribed oxycodone inappropriately.
The psychiatrist argued that proper care was given and that the patient failed to provide a complete, accurate medical history at the emergency room visit and did not to consent to admission.
- A defense verdict was returned
Could admission have prevented patient’s suicide?
Douglas County (NE) District Court
A patient in his mid-60s with a history of depression committed suicide with a gunshot wound to the head. Before his suicide, the patient was seeing a psychiatrist and psychologist for depression and emotional problems.
The patient’s family alleged the psychiatrist failed to diagnose the severity of the patient’s problems and admit him to a hospital for treatment and observation. The psychiatrist and psychologist denied negligence.
- A defense verdict was returned
Medical malpractice law is constantly evolving to determine what constitutes “negligent care.” The legal standard requires a patient who brings a negligence claim against a psychiatrist to prove:
- a relationship between patient and psychiatrist such that a duty of care exists
- the duty was breached—meaning the standard of care was not met
- the breach of duty caused the injury.
Relationship rules
The first case highlights issues surrounding the patient-psychiatrist relationship. In general, once you have agreed to treat a patient, a doctor-patient relationship and duty of care exists.
In the first case, the patient informed his longtime psychiatrist that he no longer wanted to continue care after discharge. A psychiatrist who terminates a doctor-patient relationship should provide written notice, an explanation of termination, and referrals and continue to care for the patient for a reasonable period.1 No such duty exists, however, when the patient ends treatment. Courts have found that the patient has not been abandoned when he or she voluntarily and unilaterally terminates the relationship.2,3
The relationship ends the moment the patient terminates care, unless the patient is not competent to make that unilateral decision. In that situation, your duty of care to the patient continues.2 When a competent patient terminates care, document the date and time of termination and the patient’s competence.
When relationships begin
The patient in the first case had an appointment with a new psychiatrist within 2 weeks. Is the new psychiatrist liable for what happens in the intervening period or does the relationship begin when the patient has been examined or treated? The legal question of when a physician-patient relationship is created remains problematic. Standards vary from state to state, but general principles offer some guidance.
The physician-patient relationship is a contract. The court would examine parties’ actions to ascertain their intent to determine if the patient reasonably believed that the physician—by actions or words—agreed to provide necessary medical care. Additionally, whether a relationship exists depends on the specific facts and circumstances of each situation.
There is some authority, across many jurisdictions, that a physician-patient relationship is established only when a physician conducts the initial history and physical examination. In some cases, however, the relationship has been found to exist at an earlier point, such as when a physician gave a referred patient an appointment for a consultation. When in doubt, assume the relationship exists.4
Duty of care
These cases raise areas where possible duty of care was breached:
- negligent prescription of medication
- failure to assess suicidal thinking.
Assessing suicide risk. Negligence in the second and third cases is based upon failure to assess suicidal thoughts. The legal system recognizes that psychiatrists cannot predict suicide,6 and mistakes in clinical judgment are not the same as negligence. Psychiatrists, however, are required to assess suicide risk and intervene appropriately.
When defending a negligence claim, the profession’s custom—reflected by the standard of care common to others with the practitioner’s training—is the benchmark against which the courts measure negligence. Therefore, take steps determined appropriate by the profession and document this risk assessment.7 For example, ask the patient about:
- suicidal thoughts and intent
- stressors
- history of suicidal behavior/attempts
- substance use
- signs and symptoms of depression
- bipolar disorder
- psychosis.8
Prescriptions. No clear line defines negligence when potentially dangerous medications are prescribed to a suicidal patient. Some psychiatrists dispense limited quantities of medications and see the patient weekly to monitor mood and medication. But even then a psychiatrist cannot prevent suicide—for example, the patient may have multiple prescribers or hoard medications. The concept of “sufficient intervals” to see a patient is determined case-by-case.
Documentation. Make suicide assessments an ongoing process. Document all aspects of the patient’s care, stability, and suicide risk, and reasons for the visit intervals. Indicate in the records your risk-benefit assessment in making treatment decisions.
Cases are selected byfrom Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Drug brand name
- Oxycodone • Percocet
Psychotic patient declines hospital admission, drives into an office building
Cook County (IL) Circuit Court
The patient, age 43, had been treated for mental illness for many years. He was voluntarily admitted to a hospital under the care of his psychiatrist, and was discharged at his own request a few days later. He had improved and was not considered a candidate for involuntary admission because he was not a danger to himself or others.
The patient then informed the psychiatrist that he did not want to continue treatment and said he had an appointment with a new psychiatrist within 2 weeks.
Five days later, the patient went to another hospital for voluntary admission. He was seen by an emergency room physician, who determined the patient was a candidate for voluntary admission. The patient, however, decided to leave the hospital while a bed was being arranged.
Two days later, the patient began having auditory and visual hallucinations. He then drove his car through the glass doors of an office building. No one was injured, but the patient was arrested and convicted of felony damage to property.
In his suit, the patient alleged his longtime psychiatrist was negligent and failed to properly treat him to avoid development of hallucinations. The psychiatrist argued that involuntary admission was not indicated and that the care given was appropriate.
- A defense verdict was returned
Patient commits suicide after discharge
Cook County (IL) Circuit Court
A patient, age 45, committed suicide by taking lethal doses of medication prescribed by her psychiatrist. The patient had suffered from severe depression, personality disorder, and substance abuse. The day before her death, she went to a hospital emergency room, where she was assessed for suicide and released without the psychiatrist having been notified.
The patient’s family claimed that the psychiatrist was negligent because he did not adequately assess or monitor the patient’s clinical condition at sufficient intervals over the 3 months preceding her suicide. The family also alleged that the psychiatrist prescribed oxycodone inappropriately.
The psychiatrist argued that proper care was given and that the patient failed to provide a complete, accurate medical history at the emergency room visit and did not to consent to admission.
- A defense verdict was returned
Could admission have prevented patient’s suicide?
Douglas County (NE) District Court
A patient in his mid-60s with a history of depression committed suicide with a gunshot wound to the head. Before his suicide, the patient was seeing a psychiatrist and psychologist for depression and emotional problems.
The patient’s family alleged the psychiatrist failed to diagnose the severity of the patient’s problems and admit him to a hospital for treatment and observation. The psychiatrist and psychologist denied negligence.
- A defense verdict was returned
Medical malpractice law is constantly evolving to determine what constitutes “negligent care.” The legal standard requires a patient who brings a negligence claim against a psychiatrist to prove:
- a relationship between patient and psychiatrist such that a duty of care exists
- the duty was breached—meaning the standard of care was not met
- the breach of duty caused the injury.
Relationship rules
The first case highlights issues surrounding the patient-psychiatrist relationship. In general, once you have agreed to treat a patient, a doctor-patient relationship and duty of care exists.
In the first case, the patient informed his longtime psychiatrist that he no longer wanted to continue care after discharge. A psychiatrist who terminates a doctor-patient relationship should provide written notice, an explanation of termination, and referrals and continue to care for the patient for a reasonable period.1 No such duty exists, however, when the patient ends treatment. Courts have found that the patient has not been abandoned when he or she voluntarily and unilaterally terminates the relationship.2,3
The relationship ends the moment the patient terminates care, unless the patient is not competent to make that unilateral decision. In that situation, your duty of care to the patient continues.2 When a competent patient terminates care, document the date and time of termination and the patient’s competence.
When relationships begin
The patient in the first case had an appointment with a new psychiatrist within 2 weeks. Is the new psychiatrist liable for what happens in the intervening period or does the relationship begin when the patient has been examined or treated? The legal question of when a physician-patient relationship is created remains problematic. Standards vary from state to state, but general principles offer some guidance.
The physician-patient relationship is a contract. The court would examine parties’ actions to ascertain their intent to determine if the patient reasonably believed that the physician—by actions or words—agreed to provide necessary medical care. Additionally, whether a relationship exists depends on the specific facts and circumstances of each situation.
There is some authority, across many jurisdictions, that a physician-patient relationship is established only when a physician conducts the initial history and physical examination. In some cases, however, the relationship has been found to exist at an earlier point, such as when a physician gave a referred patient an appointment for a consultation. When in doubt, assume the relationship exists.4
Duty of care
These cases raise areas where possible duty of care was breached:
- negligent prescription of medication
- failure to assess suicidal thinking.
Assessing suicide risk. Negligence in the second and third cases is based upon failure to assess suicidal thoughts. The legal system recognizes that psychiatrists cannot predict suicide,6 and mistakes in clinical judgment are not the same as negligence. Psychiatrists, however, are required to assess suicide risk and intervene appropriately.
When defending a negligence claim, the profession’s custom—reflected by the standard of care common to others with the practitioner’s training—is the benchmark against which the courts measure negligence. Therefore, take steps determined appropriate by the profession and document this risk assessment.7 For example, ask the patient about:
- suicidal thoughts and intent
- stressors
- history of suicidal behavior/attempts
- substance use
- signs and symptoms of depression
- bipolar disorder
- psychosis.8
Prescriptions. No clear line defines negligence when potentially dangerous medications are prescribed to a suicidal patient. Some psychiatrists dispense limited quantities of medications and see the patient weekly to monitor mood and medication. But even then a psychiatrist cannot prevent suicide—for example, the patient may have multiple prescribers or hoard medications. The concept of “sufficient intervals” to see a patient is determined case-by-case.
Documentation. Make suicide assessments an ongoing process. Document all aspects of the patient’s care, stability, and suicide risk, and reasons for the visit intervals. Indicate in the records your risk-benefit assessment in making treatment decisions.
Cases are selected byfrom Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Drug brand name
- Oxycodone • Percocet
1. American Medical Association Code of Medical Ethics, Opinion 8.115.
2. Knapp v. Eppright, 783 SW2d 293 (Tex 1989).
3. Saunders v. Tisher (Maine Sup. Jud. Ct. 2006).
4. Physicians Risk Management Update. The physician-patient relationship: when does it begin? Available at: http://www.phyins.com/pi/risk/updates/mayjun04.html. Accessed December 28, 2006.
5. American Psychiatric Association. Principles of medical ethics with annotations especially applicable to psychiatry. Washington, DC; 2006. Available at: http://www.psych.org/psych_pract/ethics/ppaethics.cfm. Accessed December 28, 2006.
6. Pokorny A. Prediction of suicide in psychiatric patients. Report of a prospective study. Arch Gen Psychiatry 1983;40(3):249-57.
7. Packman WL, Pennuto TO, Bongar B, Orthwein J. Legal issues of professional negligence in suicide cases. Behav Sci Law 2004;22:697-713.
8. Simon RI. The suicidal patient. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law: a comprehensive handbook. Cambridge, MA: Harvard University Press; 1998:166-86.
9. Maunz v. Perales, 276 Kan. 313, 76 P.3d 1027 (Kan 2003).
1. American Medical Association Code of Medical Ethics, Opinion 8.115.
2. Knapp v. Eppright, 783 SW2d 293 (Tex 1989).
3. Saunders v. Tisher (Maine Sup. Jud. Ct. 2006).
4. Physicians Risk Management Update. The physician-patient relationship: when does it begin? Available at: http://www.phyins.com/pi/risk/updates/mayjun04.html. Accessed December 28, 2006.
5. American Psychiatric Association. Principles of medical ethics with annotations especially applicable to psychiatry. Washington, DC; 2006. Available at: http://www.psych.org/psych_pract/ethics/ppaethics.cfm. Accessed December 28, 2006.
6. Pokorny A. Prediction of suicide in psychiatric patients. Report of a prospective study. Arch Gen Psychiatry 1983;40(3):249-57.
7. Packman WL, Pennuto TO, Bongar B, Orthwein J. Legal issues of professional negligence in suicide cases. Behav Sci Law 2004;22:697-713.
8. Simon RI. The suicidal patient. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law: a comprehensive handbook. Cambridge, MA: Harvard University Press; 1998:166-86.
9. Maunz v. Perales, 276 Kan. 313, 76 P.3d 1027 (Kan 2003).
Clarification
The method for skin grafting surgical defects of the nasal alar region known as the drumhead graft was invented and developed by Dr. J. Michael Wentzell of Billings, Mont. ('Drumhead' Technique May Spare Alar Graft Depressions, SKIN & ALLERGY NEWS, January 2007, p. 32). Dr. Bradley K. Draper of Billings presented Dr. Wentzell's technique at the annual meeting of the American Society for Dermatologic Surgery.
The method for skin grafting surgical defects of the nasal alar region known as the drumhead graft was invented and developed by Dr. J. Michael Wentzell of Billings, Mont. ('Drumhead' Technique May Spare Alar Graft Depressions, SKIN & ALLERGY NEWS, January 2007, p. 32). Dr. Bradley K. Draper of Billings presented Dr. Wentzell's technique at the annual meeting of the American Society for Dermatologic Surgery.
The method for skin grafting surgical defects of the nasal alar region known as the drumhead graft was invented and developed by Dr. J. Michael Wentzell of Billings, Mont. ('Drumhead' Technique May Spare Alar Graft Depressions, SKIN & ALLERGY NEWS, January 2007, p. 32). Dr. Bradley K. Draper of Billings presented Dr. Wentzell's technique at the annual meeting of the American Society for Dermatologic Surgery.
Brief Report / Krinsley
The last 15 years have brought reports in the medical literature of exciting advances in describing the relationship between hyperglycemia and adverse outcomes in a variety of clinical contexts involving acutely ill patients.19 Hyperglycemia in hospitalized patients was long thought to be an adaptive mechanism and, at least in the intensive care setting, was rarely treated below threshold values of 225‐250 mg/dL. The pioneering work of Furnary et al. and the Portland Diabetic Project was the first to demonstrate that close monitoring and treatment of hyperglycemia in diabetic patients undergoing cardiovascular surgery decreased the occurrence of deep sternal wound infections, a dreaded postoperative complication.10 A second publication documented the steady decrease in mortality among these patients over the years as the group's glycemic target was steadily lowered.11 In the last several years the mortality rate of diabetic patients undergoing cardiovascular surgery has decreased so that it now approximates that of nondiabetics, eliminating the diabetic disadvantage. This work set the stage for the landmark Leuven study, performed at Catholic University in Belgium and published by Van den Berghe's group in 2001.12 This prospective, randomized, controlled study involving 1548 mechanically ventilated patients in a surgical intensive care unit, 63% of whom had undergone cardiovascular surgery, compared the outcomes of patients treated with continuous intravenous insulin to achieve euglycemia (80‐110 mg/dL) to those of a control group that received treatment only when glucose level exceeded 210 mg/dL. The outcomes including a 37% reduction in hospital mortality in the treated group and a 40%‐50% reduction in numerous morbid conditions, including the need for renal replacement therapy, prolonged mechanical ventilation, prolonged antibiotic use, and critical illness polyneuropathy, that spawned a paradigm shift in ICU medicine. A large before‐and‐after study performed in a mixed medical‐surgical ICU of a university‐affiliated community hospital confirmed the mortality benefits of glycemic management, using a more modest target of 80‐140 mg/dL.13 Finally, a prospective, randomized, controlled trial in a medical ICU population by the Leuven investigators reported improvement in several morbidities and a mortality advantage from intensive glycemic control, targeting 80‐100 mg/dL, among patients with ICU stays longer than 3 days.14 Consequently, intensive glycemic management of critically ill patients is rapidly becoming a worldwide standard of care, presenting an array of challenges to clinicians involved in the care of these patients. This article presents an overview of the issues surrounding promulgation of protocols implementing tight glycemic control (TGC).
Building Blocks for Implementation of a Successful TGC Protocol
Data management tools
According to Curtis et al., A successful quality project requires transparent and informative data reporting. In the absence of timely and informative data reporting, interest wanes and projects lose momentum. On the other hand, actionable and interpretable data empower the ICU team, affirm that quality improvement efforts are making a difference, and increase the chances for sustainability.15
It is impossible to build a successful TGC program without proper data management tools. Conceptually, there are 2 levels of data reporting. At a minimum, an ICU must develop methods to demonstrate the effect of the protocol on glycemic levels. Optimally, there should also be a mechanism to report clinical and even financial outcomes resulting from the work. Quite simply, without ready access to these types of data it is unlikely that ICU cliniciansnurses, dieticians, and physicianswill continue to do the hard work necessary to allow a TGC program to achieve sustained success.
Examples of glycemic reports
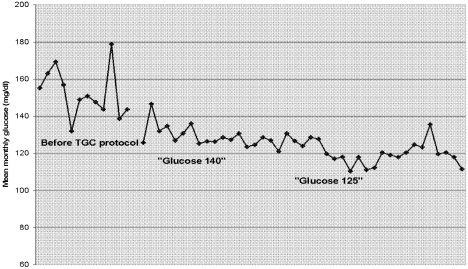
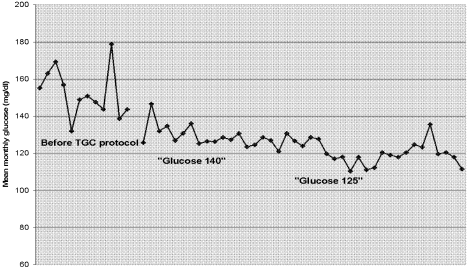
Figure 1 shows a simple and powerful graphic used in the Stamford Hospital ICUthe mean monthly glucose value. This simple calculation does not account for severity of illness or prevalence of underlying diabetes, but it is readily understood and easy to create. The run chart below demonstrates the ICU's success in first implementing a treatment threshold of 140 mg/dL and, later, a treatment threshold of 125 mg/dL.
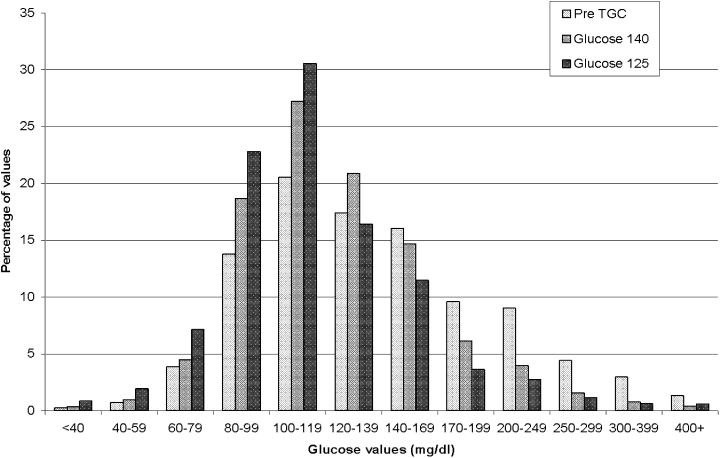
Another tool used in the Stamford Hospital ICU is a histogram that shows the percentage of glucose values that fall within discrete increments. Figure 2 details the outcomes in 3 periods: pre‐TGC, glucose 140, and glucose 125. This type of display powerfully demonstrates how the TGC protocols resulted in a marked increase in euglycemic values and dramatically reduced marked hyperglycemia.
The ability to capture useful sorts of data like these requires the assistance of the hospital's information technology department to create a link from the laboratory database to a data repository that the ICU's glycemic champion can regularly access and that displays the data in graphic form. Purchasing a point‐of‐care data management application provides an alternative solution. These applications can provide detailed reports on a unit's glycemic control, such as those displayed in Figures 1 and 2; some also have the capacity to delineate data by unit, individual practitioner, and patient.
Outcome data
The facility of an ICU to report data on glycemic control in a timely manner fulfills the minimum data requirement for successful implementation of a TGC protocol. However, sustained success depends on the unit's capacity to report information on relevant outcomes. It is not enough for an ICU director to be able to tell the hospital administration that the mean glucose level has decreased, from 160 to 135 mg/dL, for example, 6 months after institution of such a labor‐intensive program. The more relevant information is whether this intervention has had an effect on severity‐adjusted mortality, length of stay, and important comorbid conditions such as ICU‐acquired infections.
With innumerable measures that an ICU nursing or medical director might want to track, how should the measures to use be chosen?
A data set for a beginner might include the following parameters: demographics, including age, sex, and, possibly, ethnicity; admission and discharge dates and times; length of stay (LOS), ideally measured in exact time rather than number of calendar days; diagnosis; and ICU and hospital survival. The ICU data manager must develop a system to validate each patient's final discharge status from the hospital; some patients survive the ICU stay but die before hospital discharge, which therefore affects the ICU's hospital mortality rate.
The intermediate level of outcome reporting might include 2 additional elements: severity scoring and detailed information about episodes of mechanical ventilation. The most widely used models for scoring the severity of illness of ICU patients include the Acute Physiology and Chronic Health Evaluation (APACHE), the Simplified Acute Physiology Score (SAPS), and the Mortality Prediction Model (MPM).1620 The APACHE II system is the most widely quoted in the medical literature but is based on a validation cohort more than 25 years old.16 The scoring algorithms for APACHE III and APACHE IV have been released on the Web; the most recent iteration, APACHE IV, was developed using data from more than 100,000 admissions to a variety of types of ICUs between January 1, 2002, and December 31, 2003, and also includes predictions for ICU LOS.18 Use of these tools allows the ICU clinician to benchmark the unit's performance against this large heterogeneous group of ICU patients treated using contemporary ICU practice patterns. Important features of mechanical ventilation episodes worth tracking include: time of start and finish of each episode (to calculate ventilator LOS); whether the patient had an unplanned extubation; the percentage of patients who required reintubation after planned extubation; tracheostomy rate; and the use of continuous intravenous sedatives or paralytics.
An advanced data outcome system would be linked to various hospital data silos, allowing capture of all laboratory, pharmacy, and radiology charges into the ICU database, allowing financial analysis of ICU performance. Another link would funnel all important laboratory results into the database. Additional types of useful data include: ultimate discharge status of the patient (eg, home, skilled nursing facility, rehabilitation facility, another acute care hospital); procedures done in the ICU; infections acquired in the ICU; and comorbidities based on ICD‐9 codes. Several examples of the output possible with the use of the advanced data outcome system developed for use in the Stamford Hospital ICU are reported later in this article.
Protocol‐driven collaborative culture
Successful implementation of TGC is most likely in an environment that embraces standardized care using evidence‐based best practices. All routine aspects of care in the Stamford Hospital ICU are protocol driven. Some examples include deep‐vein thrombosis prophylaxis, stress ulcer prophylaxis, ventilator weaning, ventilator sedation, enteral nutrition, and potassium, phosphate, and magnesium repletion. These protocols were all in place when discussions began in the ICU about how to create a TGC protocol. The nurses were comfortable using protocols, and there were no longer any counterproductive arguments about physician autonomy of treatment decisions centered on these basic care issues. These factors facilitated adoption of the TGC protocol. Finally, the strength of the relationship binding the nursing and medical leadership of the ICU was fundamental to the program's success. A complex initiative such as TGC mandates that these parties share the same vision for the ICU.
Overcoming resistance
Adoption of TGC by an ICU will undoubtedly encounter resistance from the staff. The factors responsible for this are very real. An understanding and patient attitude by the unit's leadership will greatly facilitate implementation. Factors that are the basis for this resistance in part include:
-
TGC represents a fundamental paradigm shift in ICU care. Until recently, hyperglycemia, even at levels as high as 200‐250 mg/dL, has until recently been tolerated and ignored, as it has been considered a normal adaptive response to acute and severe illness.
-
Doing TGC correctly is hard work. This work includes the logistics of monitoring, explaining to families and patients the reasons for frequent finger sticks or blood testing (But Grandma isn't even a diabetic), being aware of the potential for significant discomfort to the patient, and having to make treatment decisions in response to all the newly acquired data.
-
Fear of hypoglycemia. Nurses want to protect, and not hurt, their patients. Insulin therapy, especially when targeting euglycemia or near‐euglycemia, is potentially dangerous.
An effective educational program directed to the staff, including nurses, staff physicians, and pharmacists, will help surmount this resistance. The components of this educational program should include: the basis in the medical literature for instituting intensive programs to monitor and treat patient glycemic levels; a review of the insulin formulations (subcutaneous, intravenous, long acting, and short acting) with emphasis on the different pharmacokinetic implications underlying their use; and a detailed analysis of factors associated with hypoglycemia.21, 22
Specific Issues Regarding TGC Implementation
Setting the glycemic target
What is the correct glycemic target? Van den Berghe et al. used a treatment threshold of 110 mg/dL for both her surgical ICU and medical ICU studies. The Stamford Hospital ICU trial, with a mixed population of medical, surgical, and cardiac patients, targeted 140 mg/dL.13
A detailed review of a very large cohort of patients treated in the Stamford Hospital ICU suggests that patients who achieve low euglycemia have the best survival (see Fig. 3). This analysis used APACHE methodology to analyze expected and actual mortality in relation to each patient's mean glucose during the ICU stay. The APACHE III and IV mortality prediction models use age, presence or absence of a group of important comorbidities, admitting diagnosis to the ICU, length of time in the ICU before ICU admission, location of the patient prior to ICU admission, and the most abnormal values of a large group of physiological parameters during the first 24 hours of ICU admission to derive a discrete prediction of hospital mortality for that patient. A standardized mortality ratio (SMR) can be calculated by dividing the patients' actual hospital mortality rate by the mean of all the individual predictions of mortality (SMR = actual/predicted mortality). A value less than 1 suggests that the patients in the observed cohort had a lower mortality rate than that predicted by the model.
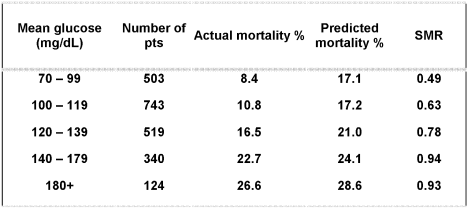
Patients who achieved euglycemia (<110 mg/dL) in the surgical ICU study of Van den Berghe et al. also had the lowest mortality rates as well as the lowest incidence of the various comorbidities measured compared to those with intermediate blood glucose levels (110‐150 mg/dL). Those with the worst glycemic control (blood glucose > 150 mg/dL) had the highest mortality rate and the highest incidence of various serious comorbid conditions.23
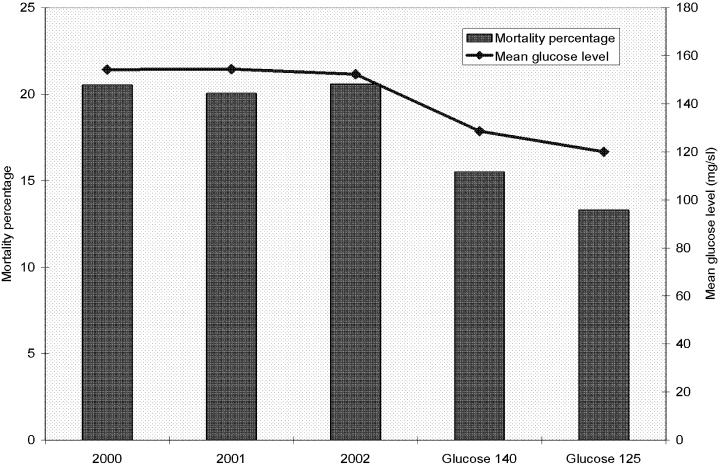
Although available data support a euglycemic target, is this unequivocally the correct target for an ICU beginning TGC implementation? Not necessarily. Targeting 110 mg/dL requires an intensity of treatment that may be intimidating to an ICU staff, especially one without experience managing protocols. Moreover, the lower the glycemic target, the greater the risk for iatrogenic hypoglycemia. An ICU considering implementation of a TGC protocol might consider staged adoption. The initial target might be as high as 175 mg/dL. As the clinicians gain experience using the protocol, including acquiring and reporting data, the treatment threshold could be lowered. The Stamford Hospital ICU staff, with more than 5 years of experience developing a model of standardized care using evidence‐based best‐practice patient care protocols, spent several months arguing about the glycemic target when TGC was first discussed following publication of the initial Van den Berghe study.12 The director of Critical Care wanted to replicate Van den Berghe's work and urged a target of 110 mg/dL. The nurses refused. A compromise was reached: a 140 mg/dL treatment threshold. This confirms an important lesson: the ICU team must choose an achievable goal. It is noteworthy that after 2 years of successful use of the glucose 140 protocol, the Stamford Hospital ICU nurses initiated a revision of the protocol, deciding they wanted to target 125 mg/dL. Figure 4 illustrates the glycemic and mortality results comparing the last 3 years before TGC with the glucose 140 and glucose 125 periods.
Choosing a protocol
After choosing a glycemic target, the ICU leadership must agree on a protocol to achieve the objective. TGC protocols can be broadly characterized as directive or nondirective.
The Stamford Hospital ICU TGC protocol is an example of a nondirective protocol.13 The nursing staff considers the document a starting point for therapy decisions. Many patients receive insulin dosing at variance with the guidelines established by the document. A nurse is empowered to make these treatment decisions. This is not dissimilar to the process ICU nurses use when titrating a vasopressor to achieve a targeted goal for mean arterial pressure. Nondirective protocols are most suitable for ICU staffs that have had considerable prior experience using nurse‐driven protocols in an environment that supports and accepts standardized care.
A number of directive protocols have been published in the literature.24 Their unifying feature is the goal of prescribing a specific insulin dose for each set of circumstances a nurse may encounter. The patient's previous glucose level and the rate of change in glucose level are considered, and the document typically details the choices for insulin dosing in several columns based on the patient's previously documented sensitivity to insulin. Although this sort of protocol can be helpful in providing explicit guidance with insulin dosing, its complexity may impede adoption.
Another option is the use of tools that have been developed to assist an ICU in initiating and promulgating TGC protocols, including software applications that automatically calculate insulin dosing. Finally, work has been initiated on the development of monitors that provide near‐continuous monitoring of glucose levels at bedside.25, 26 Adoption of such monitoring will facilitate the implementation of TGC protocols because of its impact on eliminating the workflow burdens of intensive glycemic monitoring as well as markedly diminishing the risk of hypoglycemia.
Hypoglycemia
In the Van den Berghe et al. surgical ICU study, severe hypoglycemia, defined as a glucose level less than 40 mg/dL, occurred at least once among 5.1% of the patients in the intensively treated group versus in 0.8% of the patients in the conventionally treated group.12 The hypoglycemia was described as transient, a result of the frequency of monitoring during the study, and was not associated with overt adverse consequences. The incidence of severe hypoglycemia (<40 mg/dL) was described differently in the Stamford Hospital trial: 0.35% of all the values obtained during the baseline period, compared to 0.34% of those obtained during the treatment period, again without any overt adverse consequences.13 Nevertheless, it is not known with certainty whether having even a single episode of severe hypoglycemia independently contributes to the risk of mortality.
Vreisendorp recently identified a group of predisposing factors for the development of severe hypoglycemia among ICU patients undergoing TGC.21 The most important include: a decrease in the administration of nutrition without a concomitant change in insulin dosing; diabetes mellitus; insulin treatment; sepsis; inotropic support; and renal failure. The Stamford Hospital ICU TGC protocol document now includes a black box warning highlighting renal failure (associated with decreased clearance of administered insulin), hepatic failure, and sepsis (associated with decreased hepatic gluconeogenesis) as major risk factors for severe hypoglycemia. Ongoing reinforcement is necessary to encourage the ICU staff recognize these risk factors for severe hypoglycemia and respond by adopting more conservative insulin dosing and instituting more frequent glucose monitoring.
Economic Benefits of TGC
Recently published data support the economic benefits of intensive glycemic management. Van den Berghe et al. quantified costs attributable to ICU days, mechanical ventilation, and use of antibiotics, vasopressors, intotropic agents, and transfusions in the 2 treatment groups in their surgical ICU study. The savings per patient in the intensively treated group totaled $2638; mean LOS was 6.6 days.27, 28 Data from the Stamford Hospital ICU trial was analyzed differently, with quantification of all laboratory, pharmacy, and diagnostic imaging costs, as well as costs associated with ICU days, mechanical ventilation and days in the hospital after ICU discharge.29 The savings per patient in the intensively treated group totaled $1560. Notably, this occurred in the context of a much shorter LOS than that seen in the Belgian trial; mean and median LOS were only 3.4 and 1.7 days, respectively.
CONCLUSIONS
Intensive glycemic management of critically ill patients is emerging as a standard of care, based on data demonstrating its effectiveness in reducing mortality, morbidity, and costs. Intensive care unit staffs need to make important choices about the type of protocol most suitable for use, the glycemic target, and the mechanisms for avoiding hypoglycemia. The implementation of appropriate data management tools in a protocol‐driven environment that supports standardization of care will facilitate adoption of TGC.
- .Hyperglycemia during critical illness.J Parenter Enteral Nutr.2006;30:254–258.
- ,,, et al.Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.Lancet.2000;355:773–778.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,, et al.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,, et al.Admission glucose level and clinical outcomes in the NINDS rt‐PA Stroke Trial.Neurology.2002;59:669–674.
- ,,, et al.Outcomes and perioperative hyperglycemia in patients with or without diabetes mellitus undergoing coronary artery bypass grafting.Ann Thorac Surg.2003;75:1392–1399.
- ,,.Admission hyperglycemia as a prognostic indicator in trauma.J Trauma.2003;55:33–38.
- ,,.Perioperative diabetic and hyperglycemic management issues.Crit Care Med.2004;32:S116–S125.
- .Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients.Mayo Clinic Proc.2003;78:1471–1478.
- ,,, et al.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thorac Surg.1999;67:352–360.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125:1007–1021.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive care unit quality improvement: A “how‐to” guide for the interdisciplinary team.Crit Care Med.2006;34:211–218.
- ,,, et al.APACHE II. A severity of disease classification system.Crit Care Med.1985;13:818–829.
- ,,, et al.The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults.Chest.1991;100:1619–1636.
- http://www.cerner.com/public/Cerner_3.asp?id=3562. Accessed December 12,2006.
- ,,, et al.SAPS II revisited.Int Care Med.2005;31:416–423.
- ,,, et al.Mortality probability models (MPM II) based on an international cohort of intensive care unit patients.JAMA.1993;270:2478–86.
- ,,, et al.Predisposing factors for hypoglycemia in the intensive care unit.Crit Care Med.2006;34:96–101.
- ,,, et al.Evaluation of short‐term outcomes of hypoglycemia in the intensive care unit.Crit Care Med.2006;34:2714–1218.
- ,,, et al.Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control.Crit Care Med.2003;31:359–366.
- http://www.glycemiccontrol.net/Published_Protocols.htm. Accessed December 12,2006.
- ,,, et al.Validation of the OptiScanner, a new continuous glucose monitor.Crit Care Med.2005;33:S265.
- ,,, et al.ICU validation of the OptiScanner, a continuous glucose monitoring device.Crit Care Med.2006;34:A67.
- ,,, et al.Analysis of healthcare resource utilization with intensive insulin therapy in critically ill patients.Crit Care Med.2006;34:612–616.
- .A simple intervention that saves lives and money.Crit Care Med.2006;34:896.
- ,.Cost analysis of intensive glycemic control in critically ill adult patients.Chest.2006;129:644–650.
The last 15 years have brought reports in the medical literature of exciting advances in describing the relationship between hyperglycemia and adverse outcomes in a variety of clinical contexts involving acutely ill patients.19 Hyperglycemia in hospitalized patients was long thought to be an adaptive mechanism and, at least in the intensive care setting, was rarely treated below threshold values of 225‐250 mg/dL. The pioneering work of Furnary et al. and the Portland Diabetic Project was the first to demonstrate that close monitoring and treatment of hyperglycemia in diabetic patients undergoing cardiovascular surgery decreased the occurrence of deep sternal wound infections, a dreaded postoperative complication.10 A second publication documented the steady decrease in mortality among these patients over the years as the group's glycemic target was steadily lowered.11 In the last several years the mortality rate of diabetic patients undergoing cardiovascular surgery has decreased so that it now approximates that of nondiabetics, eliminating the diabetic disadvantage. This work set the stage for the landmark Leuven study, performed at Catholic University in Belgium and published by Van den Berghe's group in 2001.12 This prospective, randomized, controlled study involving 1548 mechanically ventilated patients in a surgical intensive care unit, 63% of whom had undergone cardiovascular surgery, compared the outcomes of patients treated with continuous intravenous insulin to achieve euglycemia (80‐110 mg/dL) to those of a control group that received treatment only when glucose level exceeded 210 mg/dL. The outcomes including a 37% reduction in hospital mortality in the treated group and a 40%‐50% reduction in numerous morbid conditions, including the need for renal replacement therapy, prolonged mechanical ventilation, prolonged antibiotic use, and critical illness polyneuropathy, that spawned a paradigm shift in ICU medicine. A large before‐and‐after study performed in a mixed medical‐surgical ICU of a university‐affiliated community hospital confirmed the mortality benefits of glycemic management, using a more modest target of 80‐140 mg/dL.13 Finally, a prospective, randomized, controlled trial in a medical ICU population by the Leuven investigators reported improvement in several morbidities and a mortality advantage from intensive glycemic control, targeting 80‐100 mg/dL, among patients with ICU stays longer than 3 days.14 Consequently, intensive glycemic management of critically ill patients is rapidly becoming a worldwide standard of care, presenting an array of challenges to clinicians involved in the care of these patients. This article presents an overview of the issues surrounding promulgation of protocols implementing tight glycemic control (TGC).
Building Blocks for Implementation of a Successful TGC Protocol
Data management tools
According to Curtis et al., A successful quality project requires transparent and informative data reporting. In the absence of timely and informative data reporting, interest wanes and projects lose momentum. On the other hand, actionable and interpretable data empower the ICU team, affirm that quality improvement efforts are making a difference, and increase the chances for sustainability.15
It is impossible to build a successful TGC program without proper data management tools. Conceptually, there are 2 levels of data reporting. At a minimum, an ICU must develop methods to demonstrate the effect of the protocol on glycemic levels. Optimally, there should also be a mechanism to report clinical and even financial outcomes resulting from the work. Quite simply, without ready access to these types of data it is unlikely that ICU cliniciansnurses, dieticians, and physicianswill continue to do the hard work necessary to allow a TGC program to achieve sustained success.
Examples of glycemic reports
Figure 1 shows a simple and powerful graphic used in the Stamford Hospital ICUthe mean monthly glucose value. This simple calculation does not account for severity of illness or prevalence of underlying diabetes, but it is readily understood and easy to create. The run chart below demonstrates the ICU's success in first implementing a treatment threshold of 140 mg/dL and, later, a treatment threshold of 125 mg/dL.

Another tool used in the Stamford Hospital ICU is a histogram that shows the percentage of glucose values that fall within discrete increments. Figure 2 details the outcomes in 3 periods: pre‐TGC, glucose 140, and glucose 125. This type of display powerfully demonstrates how the TGC protocols resulted in a marked increase in euglycemic values and dramatically reduced marked hyperglycemia.

The ability to capture useful sorts of data like these requires the assistance of the hospital's information technology department to create a link from the laboratory database to a data repository that the ICU's glycemic champion can regularly access and that displays the data in graphic form. Purchasing a point‐of‐care data management application provides an alternative solution. These applications can provide detailed reports on a unit's glycemic control, such as those displayed in Figures 1 and 2; some also have the capacity to delineate data by unit, individual practitioner, and patient.
Outcome data
The facility of an ICU to report data on glycemic control in a timely manner fulfills the minimum data requirement for successful implementation of a TGC protocol. However, sustained success depends on the unit's capacity to report information on relevant outcomes. It is not enough for an ICU director to be able to tell the hospital administration that the mean glucose level has decreased, from 160 to 135 mg/dL, for example, 6 months after institution of such a labor‐intensive program. The more relevant information is whether this intervention has had an effect on severity‐adjusted mortality, length of stay, and important comorbid conditions such as ICU‐acquired infections.
With innumerable measures that an ICU nursing or medical director might want to track, how should the measures to use be chosen?
A data set for a beginner might include the following parameters: demographics, including age, sex, and, possibly, ethnicity; admission and discharge dates and times; length of stay (LOS), ideally measured in exact time rather than number of calendar days; diagnosis; and ICU and hospital survival. The ICU data manager must develop a system to validate each patient's final discharge status from the hospital; some patients survive the ICU stay but die before hospital discharge, which therefore affects the ICU's hospital mortality rate.
The intermediate level of outcome reporting might include 2 additional elements: severity scoring and detailed information about episodes of mechanical ventilation. The most widely used models for scoring the severity of illness of ICU patients include the Acute Physiology and Chronic Health Evaluation (APACHE), the Simplified Acute Physiology Score (SAPS), and the Mortality Prediction Model (MPM).1620 The APACHE II system is the most widely quoted in the medical literature but is based on a validation cohort more than 25 years old.16 The scoring algorithms for APACHE III and APACHE IV have been released on the Web; the most recent iteration, APACHE IV, was developed using data from more than 100,000 admissions to a variety of types of ICUs between January 1, 2002, and December 31, 2003, and also includes predictions for ICU LOS.18 Use of these tools allows the ICU clinician to benchmark the unit's performance against this large heterogeneous group of ICU patients treated using contemporary ICU practice patterns. Important features of mechanical ventilation episodes worth tracking include: time of start and finish of each episode (to calculate ventilator LOS); whether the patient had an unplanned extubation; the percentage of patients who required reintubation after planned extubation; tracheostomy rate; and the use of continuous intravenous sedatives or paralytics.
An advanced data outcome system would be linked to various hospital data silos, allowing capture of all laboratory, pharmacy, and radiology charges into the ICU database, allowing financial analysis of ICU performance. Another link would funnel all important laboratory results into the database. Additional types of useful data include: ultimate discharge status of the patient (eg, home, skilled nursing facility, rehabilitation facility, another acute care hospital); procedures done in the ICU; infections acquired in the ICU; and comorbidities based on ICD‐9 codes. Several examples of the output possible with the use of the advanced data outcome system developed for use in the Stamford Hospital ICU are reported later in this article.
Protocol‐driven collaborative culture
Successful implementation of TGC is most likely in an environment that embraces standardized care using evidence‐based best practices. All routine aspects of care in the Stamford Hospital ICU are protocol driven. Some examples include deep‐vein thrombosis prophylaxis, stress ulcer prophylaxis, ventilator weaning, ventilator sedation, enteral nutrition, and potassium, phosphate, and magnesium repletion. These protocols were all in place when discussions began in the ICU about how to create a TGC protocol. The nurses were comfortable using protocols, and there were no longer any counterproductive arguments about physician autonomy of treatment decisions centered on these basic care issues. These factors facilitated adoption of the TGC protocol. Finally, the strength of the relationship binding the nursing and medical leadership of the ICU was fundamental to the program's success. A complex initiative such as TGC mandates that these parties share the same vision for the ICU.
Overcoming resistance
Adoption of TGC by an ICU will undoubtedly encounter resistance from the staff. The factors responsible for this are very real. An understanding and patient attitude by the unit's leadership will greatly facilitate implementation. Factors that are the basis for this resistance in part include:
-
TGC represents a fundamental paradigm shift in ICU care. Until recently, hyperglycemia, even at levels as high as 200‐250 mg/dL, has until recently been tolerated and ignored, as it has been considered a normal adaptive response to acute and severe illness.
-
Doing TGC correctly is hard work. This work includes the logistics of monitoring, explaining to families and patients the reasons for frequent finger sticks or blood testing (But Grandma isn't even a diabetic), being aware of the potential for significant discomfort to the patient, and having to make treatment decisions in response to all the newly acquired data.
-
Fear of hypoglycemia. Nurses want to protect, and not hurt, their patients. Insulin therapy, especially when targeting euglycemia or near‐euglycemia, is potentially dangerous.
An effective educational program directed to the staff, including nurses, staff physicians, and pharmacists, will help surmount this resistance. The components of this educational program should include: the basis in the medical literature for instituting intensive programs to monitor and treat patient glycemic levels; a review of the insulin formulations (subcutaneous, intravenous, long acting, and short acting) with emphasis on the different pharmacokinetic implications underlying their use; and a detailed analysis of factors associated with hypoglycemia.21, 22
Specific Issues Regarding TGC Implementation
Setting the glycemic target
What is the correct glycemic target? Van den Berghe et al. used a treatment threshold of 110 mg/dL for both her surgical ICU and medical ICU studies. The Stamford Hospital ICU trial, with a mixed population of medical, surgical, and cardiac patients, targeted 140 mg/dL.13
A detailed review of a very large cohort of patients treated in the Stamford Hospital ICU suggests that patients who achieve low euglycemia have the best survival (see Fig. 3). This analysis used APACHE methodology to analyze expected and actual mortality in relation to each patient's mean glucose during the ICU stay. The APACHE III and IV mortality prediction models use age, presence or absence of a group of important comorbidities, admitting diagnosis to the ICU, length of time in the ICU before ICU admission, location of the patient prior to ICU admission, and the most abnormal values of a large group of physiological parameters during the first 24 hours of ICU admission to derive a discrete prediction of hospital mortality for that patient. A standardized mortality ratio (SMR) can be calculated by dividing the patients' actual hospital mortality rate by the mean of all the individual predictions of mortality (SMR = actual/predicted mortality). A value less than 1 suggests that the patients in the observed cohort had a lower mortality rate than that predicted by the model.

Patients who achieved euglycemia (<110 mg/dL) in the surgical ICU study of Van den Berghe et al. also had the lowest mortality rates as well as the lowest incidence of the various comorbidities measured compared to those with intermediate blood glucose levels (110‐150 mg/dL). Those with the worst glycemic control (blood glucose > 150 mg/dL) had the highest mortality rate and the highest incidence of various serious comorbid conditions.23
Although available data support a euglycemic target, is this unequivocally the correct target for an ICU beginning TGC implementation? Not necessarily. Targeting 110 mg/dL requires an intensity of treatment that may be intimidating to an ICU staff, especially one without experience managing protocols. Moreover, the lower the glycemic target, the greater the risk for iatrogenic hypoglycemia. An ICU considering implementation of a TGC protocol might consider staged adoption. The initial target might be as high as 175 mg/dL. As the clinicians gain experience using the protocol, including acquiring and reporting data, the treatment threshold could be lowered. The Stamford Hospital ICU staff, with more than 5 years of experience developing a model of standardized care using evidence‐based best‐practice patient care protocols, spent several months arguing about the glycemic target when TGC was first discussed following publication of the initial Van den Berghe study.12 The director of Critical Care wanted to replicate Van den Berghe's work and urged a target of 110 mg/dL. The nurses refused. A compromise was reached: a 140 mg/dL treatment threshold. This confirms an important lesson: the ICU team must choose an achievable goal. It is noteworthy that after 2 years of successful use of the glucose 140 protocol, the Stamford Hospital ICU nurses initiated a revision of the protocol, deciding they wanted to target 125 mg/dL. Figure 4 illustrates the glycemic and mortality results comparing the last 3 years before TGC with the glucose 140 and glucose 125 periods.

Choosing a protocol
After choosing a glycemic target, the ICU leadership must agree on a protocol to achieve the objective. TGC protocols can be broadly characterized as directive or nondirective.
The Stamford Hospital ICU TGC protocol is an example of a nondirective protocol.13 The nursing staff considers the document a starting point for therapy decisions. Many patients receive insulin dosing at variance with the guidelines established by the document. A nurse is empowered to make these treatment decisions. This is not dissimilar to the process ICU nurses use when titrating a vasopressor to achieve a targeted goal for mean arterial pressure. Nondirective protocols are most suitable for ICU staffs that have had considerable prior experience using nurse‐driven protocols in an environment that supports and accepts standardized care.
A number of directive protocols have been published in the literature.24 Their unifying feature is the goal of prescribing a specific insulin dose for each set of circumstances a nurse may encounter. The patient's previous glucose level and the rate of change in glucose level are considered, and the document typically details the choices for insulin dosing in several columns based on the patient's previously documented sensitivity to insulin. Although this sort of protocol can be helpful in providing explicit guidance with insulin dosing, its complexity may impede adoption.
Another option is the use of tools that have been developed to assist an ICU in initiating and promulgating TGC protocols, including software applications that automatically calculate insulin dosing. Finally, work has been initiated on the development of monitors that provide near‐continuous monitoring of glucose levels at bedside.25, 26 Adoption of such monitoring will facilitate the implementation of TGC protocols because of its impact on eliminating the workflow burdens of intensive glycemic monitoring as well as markedly diminishing the risk of hypoglycemia.
Hypoglycemia
In the Van den Berghe et al. surgical ICU study, severe hypoglycemia, defined as a glucose level less than 40 mg/dL, occurred at least once among 5.1% of the patients in the intensively treated group versus in 0.8% of the patients in the conventionally treated group.12 The hypoglycemia was described as transient, a result of the frequency of monitoring during the study, and was not associated with overt adverse consequences. The incidence of severe hypoglycemia (<40 mg/dL) was described differently in the Stamford Hospital trial: 0.35% of all the values obtained during the baseline period, compared to 0.34% of those obtained during the treatment period, again without any overt adverse consequences.13 Nevertheless, it is not known with certainty whether having even a single episode of severe hypoglycemia independently contributes to the risk of mortality.
Vreisendorp recently identified a group of predisposing factors for the development of severe hypoglycemia among ICU patients undergoing TGC.21 The most important include: a decrease in the administration of nutrition without a concomitant change in insulin dosing; diabetes mellitus; insulin treatment; sepsis; inotropic support; and renal failure. The Stamford Hospital ICU TGC protocol document now includes a black box warning highlighting renal failure (associated with decreased clearance of administered insulin), hepatic failure, and sepsis (associated with decreased hepatic gluconeogenesis) as major risk factors for severe hypoglycemia. Ongoing reinforcement is necessary to encourage the ICU staff recognize these risk factors for severe hypoglycemia and respond by adopting more conservative insulin dosing and instituting more frequent glucose monitoring.
Economic Benefits of TGC
Recently published data support the economic benefits of intensive glycemic management. Van den Berghe et al. quantified costs attributable to ICU days, mechanical ventilation, and use of antibiotics, vasopressors, intotropic agents, and transfusions in the 2 treatment groups in their surgical ICU study. The savings per patient in the intensively treated group totaled $2638; mean LOS was 6.6 days.27, 28 Data from the Stamford Hospital ICU trial was analyzed differently, with quantification of all laboratory, pharmacy, and diagnostic imaging costs, as well as costs associated with ICU days, mechanical ventilation and days in the hospital after ICU discharge.29 The savings per patient in the intensively treated group totaled $1560. Notably, this occurred in the context of a much shorter LOS than that seen in the Belgian trial; mean and median LOS were only 3.4 and 1.7 days, respectively.
CONCLUSIONS
Intensive glycemic management of critically ill patients is emerging as a standard of care, based on data demonstrating its effectiveness in reducing mortality, morbidity, and costs. Intensive care unit staffs need to make important choices about the type of protocol most suitable for use, the glycemic target, and the mechanisms for avoiding hypoglycemia. The implementation of appropriate data management tools in a protocol‐driven environment that supports standardization of care will facilitate adoption of TGC.
The last 15 years have brought reports in the medical literature of exciting advances in describing the relationship between hyperglycemia and adverse outcomes in a variety of clinical contexts involving acutely ill patients.19 Hyperglycemia in hospitalized patients was long thought to be an adaptive mechanism and, at least in the intensive care setting, was rarely treated below threshold values of 225‐250 mg/dL. The pioneering work of Furnary et al. and the Portland Diabetic Project was the first to demonstrate that close monitoring and treatment of hyperglycemia in diabetic patients undergoing cardiovascular surgery decreased the occurrence of deep sternal wound infections, a dreaded postoperative complication.10 A second publication documented the steady decrease in mortality among these patients over the years as the group's glycemic target was steadily lowered.11 In the last several years the mortality rate of diabetic patients undergoing cardiovascular surgery has decreased so that it now approximates that of nondiabetics, eliminating the diabetic disadvantage. This work set the stage for the landmark Leuven study, performed at Catholic University in Belgium and published by Van den Berghe's group in 2001.12 This prospective, randomized, controlled study involving 1548 mechanically ventilated patients in a surgical intensive care unit, 63% of whom had undergone cardiovascular surgery, compared the outcomes of patients treated with continuous intravenous insulin to achieve euglycemia (80‐110 mg/dL) to those of a control group that received treatment only when glucose level exceeded 210 mg/dL. The outcomes including a 37% reduction in hospital mortality in the treated group and a 40%‐50% reduction in numerous morbid conditions, including the need for renal replacement therapy, prolonged mechanical ventilation, prolonged antibiotic use, and critical illness polyneuropathy, that spawned a paradigm shift in ICU medicine. A large before‐and‐after study performed in a mixed medical‐surgical ICU of a university‐affiliated community hospital confirmed the mortality benefits of glycemic management, using a more modest target of 80‐140 mg/dL.13 Finally, a prospective, randomized, controlled trial in a medical ICU population by the Leuven investigators reported improvement in several morbidities and a mortality advantage from intensive glycemic control, targeting 80‐100 mg/dL, among patients with ICU stays longer than 3 days.14 Consequently, intensive glycemic management of critically ill patients is rapidly becoming a worldwide standard of care, presenting an array of challenges to clinicians involved in the care of these patients. This article presents an overview of the issues surrounding promulgation of protocols implementing tight glycemic control (TGC).
Building Blocks for Implementation of a Successful TGC Protocol
Data management tools
According to Curtis et al., A successful quality project requires transparent and informative data reporting. In the absence of timely and informative data reporting, interest wanes and projects lose momentum. On the other hand, actionable and interpretable data empower the ICU team, affirm that quality improvement efforts are making a difference, and increase the chances for sustainability.15
It is impossible to build a successful TGC program without proper data management tools. Conceptually, there are 2 levels of data reporting. At a minimum, an ICU must develop methods to demonstrate the effect of the protocol on glycemic levels. Optimally, there should also be a mechanism to report clinical and even financial outcomes resulting from the work. Quite simply, without ready access to these types of data it is unlikely that ICU cliniciansnurses, dieticians, and physicianswill continue to do the hard work necessary to allow a TGC program to achieve sustained success.
Examples of glycemic reports
Figure 1 shows a simple and powerful graphic used in the Stamford Hospital ICUthe mean monthly glucose value. This simple calculation does not account for severity of illness or prevalence of underlying diabetes, but it is readily understood and easy to create. The run chart below demonstrates the ICU's success in first implementing a treatment threshold of 140 mg/dL and, later, a treatment threshold of 125 mg/dL.

Another tool used in the Stamford Hospital ICU is a histogram that shows the percentage of glucose values that fall within discrete increments. Figure 2 details the outcomes in 3 periods: pre‐TGC, glucose 140, and glucose 125. This type of display powerfully demonstrates how the TGC protocols resulted in a marked increase in euglycemic values and dramatically reduced marked hyperglycemia.

The ability to capture useful sorts of data like these requires the assistance of the hospital's information technology department to create a link from the laboratory database to a data repository that the ICU's glycemic champion can regularly access and that displays the data in graphic form. Purchasing a point‐of‐care data management application provides an alternative solution. These applications can provide detailed reports on a unit's glycemic control, such as those displayed in Figures 1 and 2; some also have the capacity to delineate data by unit, individual practitioner, and patient.
Outcome data
The facility of an ICU to report data on glycemic control in a timely manner fulfills the minimum data requirement for successful implementation of a TGC protocol. However, sustained success depends on the unit's capacity to report information on relevant outcomes. It is not enough for an ICU director to be able to tell the hospital administration that the mean glucose level has decreased, from 160 to 135 mg/dL, for example, 6 months after institution of such a labor‐intensive program. The more relevant information is whether this intervention has had an effect on severity‐adjusted mortality, length of stay, and important comorbid conditions such as ICU‐acquired infections.
With innumerable measures that an ICU nursing or medical director might want to track, how should the measures to use be chosen?
A data set for a beginner might include the following parameters: demographics, including age, sex, and, possibly, ethnicity; admission and discharge dates and times; length of stay (LOS), ideally measured in exact time rather than number of calendar days; diagnosis; and ICU and hospital survival. The ICU data manager must develop a system to validate each patient's final discharge status from the hospital; some patients survive the ICU stay but die before hospital discharge, which therefore affects the ICU's hospital mortality rate.
The intermediate level of outcome reporting might include 2 additional elements: severity scoring and detailed information about episodes of mechanical ventilation. The most widely used models for scoring the severity of illness of ICU patients include the Acute Physiology and Chronic Health Evaluation (APACHE), the Simplified Acute Physiology Score (SAPS), and the Mortality Prediction Model (MPM).1620 The APACHE II system is the most widely quoted in the medical literature but is based on a validation cohort more than 25 years old.16 The scoring algorithms for APACHE III and APACHE IV have been released on the Web; the most recent iteration, APACHE IV, was developed using data from more than 100,000 admissions to a variety of types of ICUs between January 1, 2002, and December 31, 2003, and also includes predictions for ICU LOS.18 Use of these tools allows the ICU clinician to benchmark the unit's performance against this large heterogeneous group of ICU patients treated using contemporary ICU practice patterns. Important features of mechanical ventilation episodes worth tracking include: time of start and finish of each episode (to calculate ventilator LOS); whether the patient had an unplanned extubation; the percentage of patients who required reintubation after planned extubation; tracheostomy rate; and the use of continuous intravenous sedatives or paralytics.
An advanced data outcome system would be linked to various hospital data silos, allowing capture of all laboratory, pharmacy, and radiology charges into the ICU database, allowing financial analysis of ICU performance. Another link would funnel all important laboratory results into the database. Additional types of useful data include: ultimate discharge status of the patient (eg, home, skilled nursing facility, rehabilitation facility, another acute care hospital); procedures done in the ICU; infections acquired in the ICU; and comorbidities based on ICD‐9 codes. Several examples of the output possible with the use of the advanced data outcome system developed for use in the Stamford Hospital ICU are reported later in this article.
Protocol‐driven collaborative culture
Successful implementation of TGC is most likely in an environment that embraces standardized care using evidence‐based best practices. All routine aspects of care in the Stamford Hospital ICU are protocol driven. Some examples include deep‐vein thrombosis prophylaxis, stress ulcer prophylaxis, ventilator weaning, ventilator sedation, enteral nutrition, and potassium, phosphate, and magnesium repletion. These protocols were all in place when discussions began in the ICU about how to create a TGC protocol. The nurses were comfortable using protocols, and there were no longer any counterproductive arguments about physician autonomy of treatment decisions centered on these basic care issues. These factors facilitated adoption of the TGC protocol. Finally, the strength of the relationship binding the nursing and medical leadership of the ICU was fundamental to the program's success. A complex initiative such as TGC mandates that these parties share the same vision for the ICU.
Overcoming resistance
Adoption of TGC by an ICU will undoubtedly encounter resistance from the staff. The factors responsible for this are very real. An understanding and patient attitude by the unit's leadership will greatly facilitate implementation. Factors that are the basis for this resistance in part include:
-
TGC represents a fundamental paradigm shift in ICU care. Until recently, hyperglycemia, even at levels as high as 200‐250 mg/dL, has until recently been tolerated and ignored, as it has been considered a normal adaptive response to acute and severe illness.
-
Doing TGC correctly is hard work. This work includes the logistics of monitoring, explaining to families and patients the reasons for frequent finger sticks or blood testing (But Grandma isn't even a diabetic), being aware of the potential for significant discomfort to the patient, and having to make treatment decisions in response to all the newly acquired data.
-
Fear of hypoglycemia. Nurses want to protect, and not hurt, their patients. Insulin therapy, especially when targeting euglycemia or near‐euglycemia, is potentially dangerous.
An effective educational program directed to the staff, including nurses, staff physicians, and pharmacists, will help surmount this resistance. The components of this educational program should include: the basis in the medical literature for instituting intensive programs to monitor and treat patient glycemic levels; a review of the insulin formulations (subcutaneous, intravenous, long acting, and short acting) with emphasis on the different pharmacokinetic implications underlying their use; and a detailed analysis of factors associated with hypoglycemia.21, 22
Specific Issues Regarding TGC Implementation
Setting the glycemic target
What is the correct glycemic target? Van den Berghe et al. used a treatment threshold of 110 mg/dL for both her surgical ICU and medical ICU studies. The Stamford Hospital ICU trial, with a mixed population of medical, surgical, and cardiac patients, targeted 140 mg/dL.13
A detailed review of a very large cohort of patients treated in the Stamford Hospital ICU suggests that patients who achieve low euglycemia have the best survival (see Fig. 3). This analysis used APACHE methodology to analyze expected and actual mortality in relation to each patient's mean glucose during the ICU stay. The APACHE III and IV mortality prediction models use age, presence or absence of a group of important comorbidities, admitting diagnosis to the ICU, length of time in the ICU before ICU admission, location of the patient prior to ICU admission, and the most abnormal values of a large group of physiological parameters during the first 24 hours of ICU admission to derive a discrete prediction of hospital mortality for that patient. A standardized mortality ratio (SMR) can be calculated by dividing the patients' actual hospital mortality rate by the mean of all the individual predictions of mortality (SMR = actual/predicted mortality). A value less than 1 suggests that the patients in the observed cohort had a lower mortality rate than that predicted by the model.

Patients who achieved euglycemia (<110 mg/dL) in the surgical ICU study of Van den Berghe et al. also had the lowest mortality rates as well as the lowest incidence of the various comorbidities measured compared to those with intermediate blood glucose levels (110‐150 mg/dL). Those with the worst glycemic control (blood glucose > 150 mg/dL) had the highest mortality rate and the highest incidence of various serious comorbid conditions.23
Although available data support a euglycemic target, is this unequivocally the correct target for an ICU beginning TGC implementation? Not necessarily. Targeting 110 mg/dL requires an intensity of treatment that may be intimidating to an ICU staff, especially one without experience managing protocols. Moreover, the lower the glycemic target, the greater the risk for iatrogenic hypoglycemia. An ICU considering implementation of a TGC protocol might consider staged adoption. The initial target might be as high as 175 mg/dL. As the clinicians gain experience using the protocol, including acquiring and reporting data, the treatment threshold could be lowered. The Stamford Hospital ICU staff, with more than 5 years of experience developing a model of standardized care using evidence‐based best‐practice patient care protocols, spent several months arguing about the glycemic target when TGC was first discussed following publication of the initial Van den Berghe study.12 The director of Critical Care wanted to replicate Van den Berghe's work and urged a target of 110 mg/dL. The nurses refused. A compromise was reached: a 140 mg/dL treatment threshold. This confirms an important lesson: the ICU team must choose an achievable goal. It is noteworthy that after 2 years of successful use of the glucose 140 protocol, the Stamford Hospital ICU nurses initiated a revision of the protocol, deciding they wanted to target 125 mg/dL. Figure 4 illustrates the glycemic and mortality results comparing the last 3 years before TGC with the glucose 140 and glucose 125 periods.

Choosing a protocol
After choosing a glycemic target, the ICU leadership must agree on a protocol to achieve the objective. TGC protocols can be broadly characterized as directive or nondirective.
The Stamford Hospital ICU TGC protocol is an example of a nondirective protocol.13 The nursing staff considers the document a starting point for therapy decisions. Many patients receive insulin dosing at variance with the guidelines established by the document. A nurse is empowered to make these treatment decisions. This is not dissimilar to the process ICU nurses use when titrating a vasopressor to achieve a targeted goal for mean arterial pressure. Nondirective protocols are most suitable for ICU staffs that have had considerable prior experience using nurse‐driven protocols in an environment that supports and accepts standardized care.
A number of directive protocols have been published in the literature.24 Their unifying feature is the goal of prescribing a specific insulin dose for each set of circumstances a nurse may encounter. The patient's previous glucose level and the rate of change in glucose level are considered, and the document typically details the choices for insulin dosing in several columns based on the patient's previously documented sensitivity to insulin. Although this sort of protocol can be helpful in providing explicit guidance with insulin dosing, its complexity may impede adoption.
Another option is the use of tools that have been developed to assist an ICU in initiating and promulgating TGC protocols, including software applications that automatically calculate insulin dosing. Finally, work has been initiated on the development of monitors that provide near‐continuous monitoring of glucose levels at bedside.25, 26 Adoption of such monitoring will facilitate the implementation of TGC protocols because of its impact on eliminating the workflow burdens of intensive glycemic monitoring as well as markedly diminishing the risk of hypoglycemia.
Hypoglycemia
In the Van den Berghe et al. surgical ICU study, severe hypoglycemia, defined as a glucose level less than 40 mg/dL, occurred at least once among 5.1% of the patients in the intensively treated group versus in 0.8% of the patients in the conventionally treated group.12 The hypoglycemia was described as transient, a result of the frequency of monitoring during the study, and was not associated with overt adverse consequences. The incidence of severe hypoglycemia (<40 mg/dL) was described differently in the Stamford Hospital trial: 0.35% of all the values obtained during the baseline period, compared to 0.34% of those obtained during the treatment period, again without any overt adverse consequences.13 Nevertheless, it is not known with certainty whether having even a single episode of severe hypoglycemia independently contributes to the risk of mortality.
Vreisendorp recently identified a group of predisposing factors for the development of severe hypoglycemia among ICU patients undergoing TGC.21 The most important include: a decrease in the administration of nutrition without a concomitant change in insulin dosing; diabetes mellitus; insulin treatment; sepsis; inotropic support; and renal failure. The Stamford Hospital ICU TGC protocol document now includes a black box warning highlighting renal failure (associated with decreased clearance of administered insulin), hepatic failure, and sepsis (associated with decreased hepatic gluconeogenesis) as major risk factors for severe hypoglycemia. Ongoing reinforcement is necessary to encourage the ICU staff recognize these risk factors for severe hypoglycemia and respond by adopting more conservative insulin dosing and instituting more frequent glucose monitoring.
Economic Benefits of TGC
Recently published data support the economic benefits of intensive glycemic management. Van den Berghe et al. quantified costs attributable to ICU days, mechanical ventilation, and use of antibiotics, vasopressors, intotropic agents, and transfusions in the 2 treatment groups in their surgical ICU study. The savings per patient in the intensively treated group totaled $2638; mean LOS was 6.6 days.27, 28 Data from the Stamford Hospital ICU trial was analyzed differently, with quantification of all laboratory, pharmacy, and diagnostic imaging costs, as well as costs associated with ICU days, mechanical ventilation and days in the hospital after ICU discharge.29 The savings per patient in the intensively treated group totaled $1560. Notably, this occurred in the context of a much shorter LOS than that seen in the Belgian trial; mean and median LOS were only 3.4 and 1.7 days, respectively.
CONCLUSIONS
Intensive glycemic management of critically ill patients is emerging as a standard of care, based on data demonstrating its effectiveness in reducing mortality, morbidity, and costs. Intensive care unit staffs need to make important choices about the type of protocol most suitable for use, the glycemic target, and the mechanisms for avoiding hypoglycemia. The implementation of appropriate data management tools in a protocol‐driven environment that supports standardization of care will facilitate adoption of TGC.
- .Hyperglycemia during critical illness.J Parenter Enteral Nutr.2006;30:254–258.
- ,,, et al.Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.Lancet.2000;355:773–778.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,, et al.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,, et al.Admission glucose level and clinical outcomes in the NINDS rt‐PA Stroke Trial.Neurology.2002;59:669–674.
- ,,, et al.Outcomes and perioperative hyperglycemia in patients with or without diabetes mellitus undergoing coronary artery bypass grafting.Ann Thorac Surg.2003;75:1392–1399.
- ,,.Admission hyperglycemia as a prognostic indicator in trauma.J Trauma.2003;55:33–38.
- ,,.Perioperative diabetic and hyperglycemic management issues.Crit Care Med.2004;32:S116–S125.
- .Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients.Mayo Clinic Proc.2003;78:1471–1478.
- ,,, et al.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thorac Surg.1999;67:352–360.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125:1007–1021.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive care unit quality improvement: A “how‐to” guide for the interdisciplinary team.Crit Care Med.2006;34:211–218.
- ,,, et al.APACHE II. A severity of disease classification system.Crit Care Med.1985;13:818–829.
- ,,, et al.The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults.Chest.1991;100:1619–1636.
- http://www.cerner.com/public/Cerner_3.asp?id=3562. Accessed December 12,2006.
- ,,, et al.SAPS II revisited.Int Care Med.2005;31:416–423.
- ,,, et al.Mortality probability models (MPM II) based on an international cohort of intensive care unit patients.JAMA.1993;270:2478–86.
- ,,, et al.Predisposing factors for hypoglycemia in the intensive care unit.Crit Care Med.2006;34:96–101.
- ,,, et al.Evaluation of short‐term outcomes of hypoglycemia in the intensive care unit.Crit Care Med.2006;34:2714–1218.
- ,,, et al.Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control.Crit Care Med.2003;31:359–366.
- http://www.glycemiccontrol.net/Published_Protocols.htm. Accessed December 12,2006.
- ,,, et al.Validation of the OptiScanner, a new continuous glucose monitor.Crit Care Med.2005;33:S265.
- ,,, et al.ICU validation of the OptiScanner, a continuous glucose monitoring device.Crit Care Med.2006;34:A67.
- ,,, et al.Analysis of healthcare resource utilization with intensive insulin therapy in critically ill patients.Crit Care Med.2006;34:612–616.
- .A simple intervention that saves lives and money.Crit Care Med.2006;34:896.
- ,.Cost analysis of intensive glycemic control in critically ill adult patients.Chest.2006;129:644–650.
- .Hyperglycemia during critical illness.J Parenter Enteral Nutr.2006;30:254–258.
- ,,, et al.Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.Lancet.2000;355:773–778.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,, et al.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,, et al.Admission glucose level and clinical outcomes in the NINDS rt‐PA Stroke Trial.Neurology.2002;59:669–674.
- ,,, et al.Outcomes and perioperative hyperglycemia in patients with or without diabetes mellitus undergoing coronary artery bypass grafting.Ann Thorac Surg.2003;75:1392–1399.
- ,,.Admission hyperglycemia as a prognostic indicator in trauma.J Trauma.2003;55:33–38.
- ,,.Perioperative diabetic and hyperglycemic management issues.Crit Care Med.2004;32:S116–S125.
- .Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients.Mayo Clinic Proc.2003;78:1471–1478.
- ,,, et al.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thorac Surg.1999;67:352–360.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125:1007–1021.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive care unit quality improvement: A “how‐to” guide for the interdisciplinary team.Crit Care Med.2006;34:211–218.
- ,,, et al.APACHE II. A severity of disease classification system.Crit Care Med.1985;13:818–829.
- ,,, et al.The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults.Chest.1991;100:1619–1636.
- http://www.cerner.com/public/Cerner_3.asp?id=3562. Accessed December 12,2006.
- ,,, et al.SAPS II revisited.Int Care Med.2005;31:416–423.
- ,,, et al.Mortality probability models (MPM II) based on an international cohort of intensive care unit patients.JAMA.1993;270:2478–86.
- ,,, et al.Predisposing factors for hypoglycemia in the intensive care unit.Crit Care Med.2006;34:96–101.
- ,,, et al.Evaluation of short‐term outcomes of hypoglycemia in the intensive care unit.Crit Care Med.2006;34:2714–1218.
- ,,, et al.Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control.Crit Care Med.2003;31:359–366.
- http://www.glycemiccontrol.net/Published_Protocols.htm. Accessed December 12,2006.
- ,,, et al.Validation of the OptiScanner, a new continuous glucose monitor.Crit Care Med.2005;33:S265.
- ,,, et al.ICU validation of the OptiScanner, a continuous glucose monitoring device.Crit Care Med.2006;34:A67.
- ,,, et al.Analysis of healthcare resource utilization with intensive insulin therapy in critically ill patients.Crit Care Med.2006;34:612–616.
- .A simple intervention that saves lives and money.Crit Care Med.2006;34:896.
- ,.Cost analysis of intensive glycemic control in critically ill adult patients.Chest.2006;129:644–650.
Tight Glycemic Control / Michota and Braithwaite
Hyperglycemia is common in the hospital among patients with diabetes and those without. The exact overall prevalence of diabetes in the hospital is unknown; however, in 2000, 12.4% of U.S. hospital discharges listed diabetes as a diagnosis. Among cardiac surgery patients, the prevalence of diabetes is as high as 29%.2 Another study reported a 26% prevalence of diabetes in a community teaching hospital, with an additional 12% of patients having unrecognized diabetes or hospital‐related hyperglycemia.3 Levetan et al. found laboratory‐documented hyperglycemia in 13% of 1034 consecutively hospitalized patients.4 A subsequent chart review found that more than one‐third of patients with hyperglycemia identified by laboratory testing remained unrecognized as having diabetes documented in the discharge summary, although diabetes or hyperglycemia was noted in the progress notes. In a retrospective chart review study, Umpierrez et al. similarly found 38% of 1886 consecutively hospitalized patients who had glucose measurements on admission were hyperglycemic.3 One‐third of these patients were not previously known to have diabetes, and compared to patients with diagnosed diabetes, they were more likely to require admission to the intensive care unit, had longer hospital stays, and were less likely to be discharged straight home.
Until recently, most clinicians viewed tight glucose control in the hospitalized patient as an intervention with little immediate benefit and significant potential for harm. The goal was simply to prevent excessive hyperglycemia and avoid ketoacidosis or significant fluid derangements while minimizing the risk for hypoglycemia. Today, a growing body of evidence suggests a close correlation between tight glucose control and improved clinical outcomes. Among those who have had a myocardial infarction and those in the surgical intensive care unit, it is known that intensive glycemic control reduces mortality.5, 6 Maintaining normoglycemia in patients in the surgical intensive care unit through intravenous insulin infusion also reduces the incidence of comorbidities such as transfusion requirements, renal failure, sepsis, and neuropathy and reduces the duration of ventilator dependence.6 Although trials using glucose‐insulin‐potassium infusions (GIK), when conducted such that lowering of blood glucose occurred, have shown benefit in the settings of myocardial infarction5, 7 and cardiac surgery,8 not all studies of GIK therapy have yielded positive results. The negative results of the CREATE‐ECLA study suggest that GIK therapy per se is not beneficial unless it reduces blood glucose.9 An abundance of additional observational data and comparisons with historical control data suggest that favorable outcomes might be causally dependent on euglycemia. The outcomes studied include hospital or critical care unit mortality and nosocomial infection,1014 specifically outcomes of strokes,1522 trauma,2325 renal transplantation,2628 myocardial infarction,2936 endocarditis,37 acute lymphocytic leukemia,38 community‐acquired pneumonia,39 infectious complications in the hospital,4046 and cardiac surgery,9, 44, 45, 4751 as well as length of stay and costs.11, 25, 5156
It is important for each hospital to consider the methodology used for blood glucose measurement, realizing that measurements in the Leuven Belgium studies were performed on arterial whole blood using a blood gas analyzer. With recognition that the normal range for blood glucose is method dependent, the data presented above form the basis for the recommended glycemic targets for hospitalized patients:
Target range blood glucose (AACE et al., 2004)
-
Preprandial: < 110 mg/dL
-
Peak postprandial: < 180 mg/dL
-
Critically ill surgical patients: 80‐110 mg/dL Target range blood glucose (ADA, 2006)
-
Critically ill: Blood glucose as close to 110 mg/dL as possible and generally < 180 mg/dL. These patients generally will require IV insulin.
-
Noncritically ill: Premeal blood glucose as close to 90‐130 mg/dL as possible (midpoint 110 mg/dL). Postprandial blood glucose < 180 mg/dL.
This supplement, Avoiding Complications in the Hospitalized Patient: The Case for Tight Glycemic Control, reviews several aspects of hyperglycemia in the hospital setting. Evidence that supports more intensive glucose control is reviewed, along with a real‐world success story that demonstrates how to apply the new glycemic targets in a multidisciplinary performance improvement project. In addition, the standard insulin sliding scale is examined in terms of efficacy, safety, and potential for meeting the new recommended glycemic targets.
- Tierney E: Data from the national hospital discharge survey database 2000.Centers for Disease Control and Prevention, Division of Diabetes translation,Atlanta, GA,2003.
- Moghissi E: Hospital management of diabetes: beyond the sliding scale.Clev Clin J Med.2004;71:801–808.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,,Ratner RE: Unrecognized diabetes among hospitalized patients.Diabetes Care.1998;21:246–249.
- , for theDIGAMI study group.Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.BMJ.1997;314:1512–1515.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.J Am Coll Cardiol.1995;26:57–65.
- ,,,,,.Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events.Circulation.2004;109:1497–1502.
- CREATE‐ECLA Trial Group Investigators.Effect of glucose‐insulin‐potassium infusion on mortality in patients with acute st‐segment elevation myocardial infarction: the CREATE‐ECLA randomized controlled trial.JAMA.2005;293:437–446.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,.Reduction of nosocomial infections in the surgical intensive‐care unit by strict glycemic control.Endocr Pract.2004;10(suppl 2):46–52.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125:1007–1021.
- ,,,,,.Mortalilty in hospitalized patients with hypoglycemia and severe hyperglycemia.Mt Sinai J Med.1995;62:422–426.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,.Glucose control and mortality in critically ill patients.JAMA.2003;290:2041–2047.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004:79:992–1000.
- ,,.Insulin therapy for critically ill hospitalized patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2004;164:2005–2011.
- ,,, et al.Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome.Stroke.2003;34:2208–2214.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nodiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,, et al.Admission glucose level and clinical outcomes in the NINDS rt‐PA Stroke Trial.Neurology.2002;59:669–674.
- .Effect of hyperglycemia on stroke outcomes.Endocr Pract.2004;10(suppl 2):34–39.
- ,,, et al.Predictors of hyperacute clinical worsening in ischemic stroke patients receiving thrombolytic therapy.Stroke.2004;35:1903–1907.
- ,.Hyperglycemia in acute stroke.Stroke.2004;35:363–364.
- ,,,,.Decreased mortality by normalizing blood glucose after acute ischemic stroke.Acad Emerg Med.2006;13:174–180.
- ,,.Blood glucose control after acute stroke: a retrospective study.Acad Emerg Med.2003;10:432.
- ,,,,.Relationship of early hyperglycemia to mortality in trauma patients.J Trauma.2004;56:1058–1062.
- ,,,,,.Admission hyperglycemia is predictive of outcome in critically ill trauma patients.J Trauma.2005;59:80–83.
- ,,, et al.Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke.Neurology.2002;59(1):67–71.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,,,.Early peri‐operative hyperglycaemia and renal allograft rejection in patients without diabetes.BMC Nephrol.2000;1:1.
- ,,,,.Protective effect of insulin on ischemic renal injury in diabetes mellitus.Kidney Int.2002;61:1383–1392.
- ,,,,,.Impaired glucose metabolism predicts mortality after a myocardial infarction.Int J Cardiol.2001;79 (2–3):207–214.
- ,,,.Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.Lancet.2000;355:773–778.
- ,,, et al.A single serum glucose measurement predicts adverse outcomes across the whole range of acute coronary syndromes.Heart.2003;89:512–516.
- ,,,,,.Intensification of therapeutic approaches reduces mortality in diabetic patients with acute myocardial infarction: the Munich registry.Diabetes Care.2004;27:455–460.
- ,,, et al.Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus.Arch Intern Med.2004;164:982–988.
- ,,,.Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the diabetes and insulin‐glucose infusion in acute myocardial infarction (DIGAMI) study.Circulation.1999;99:2626–2632.
- ,,,,,.Plasma glucose at hospital admission and previous metabolic control determine myocardial infarct size and survival in patients with and without type 2 diabetes: the Langendreer Myocardial Infarction and Blood Glucose in Diabetic Patients Assessment (LAMBDA).Diabetes Care.2005;28:2551–2553.
- ,,, et al.Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes.Circulation.2005;111:3078–3086.
- ,,, et al.Early predictors of in‐hospital death in infective endocarditis.Circulation.2004;109:1745–1749.
- .Relation between the duration of remission and hyperglycemia in induction chemotherapy for acute lymphocytic leukemia.Cancer.2004;100:1179–1185.
- ,,,,.Etiology and outcome of community‐acquired pneumonia in patients with diabetes mellitus.Chest.2005;128:3233–3239.
- ,,,.Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes.Diabetes Care.1999;22:1408–1414.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enteral Nutr.1998;22(2):77–81.
- ,,,,.The association of diabetes and glucose control with surgical‐site infections among cardiothoracic surgery patients.Infect Control Hosp Epidemiol.2001;22:607–612.
- ,,,,.Early post‐operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study.Eur J Vasc Endovasc Surg.2004;5:520–525.
- ,,.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356–61.
- ,,,.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thorac Surg.1999;67:352–362.
- ,,, et al.Improving outcomes for diabetic patients undergoing vascular surgery.Diabetes Spectr.2005;18(1):53–60.
- ,,.Early postoperative outcome and medium‐term survival in 540 diabetic and 2239 nondiabetic patients undergoing coronary artery bypass grafting.Ann Thorac Surg.2002;74:712–719.
- ,,,,.Diabetes and coronary artery bypass surgery: an examination of perioperative glycemic control and outcomes.Diabetes Care.2003;26:1518–1524.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland diabetic project.Endocr Pract.2004;10(suppl 2):21–33.
- ,,,,.Glucose‐insulin‐potassium solutions improve outcomes in diabetics who have coronary artery operations.Ann Thorac Surg.2000;70:145–150.
- ,,, et al.Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients.Mayo Clin Proc.2005;80:862–866.
- ,,,.Postoperative hyperglycemia prolongs length of stay in diabetic CABG patients.Circulation. II2000;102(II):556 (abstract).
- .Reduction of hospital costs and length of stay by good control of blood glucose levels.Endocr Pract.2004;10(suppl 2):53–56.
Hyperglycemia is common in the hospital among patients with diabetes and those without. The exact overall prevalence of diabetes in the hospital is unknown; however, in 2000, 12.4% of U.S. hospital discharges listed diabetes as a diagnosis. Among cardiac surgery patients, the prevalence of diabetes is as high as 29%.2 Another study reported a 26% prevalence of diabetes in a community teaching hospital, with an additional 12% of patients having unrecognized diabetes or hospital‐related hyperglycemia.3 Levetan et al. found laboratory‐documented hyperglycemia in 13% of 1034 consecutively hospitalized patients.4 A subsequent chart review found that more than one‐third of patients with hyperglycemia identified by laboratory testing remained unrecognized as having diabetes documented in the discharge summary, although diabetes or hyperglycemia was noted in the progress notes. In a retrospective chart review study, Umpierrez et al. similarly found 38% of 1886 consecutively hospitalized patients who had glucose measurements on admission were hyperglycemic.3 One‐third of these patients were not previously known to have diabetes, and compared to patients with diagnosed diabetes, they were more likely to require admission to the intensive care unit, had longer hospital stays, and were less likely to be discharged straight home.
Until recently, most clinicians viewed tight glucose control in the hospitalized patient as an intervention with little immediate benefit and significant potential for harm. The goal was simply to prevent excessive hyperglycemia and avoid ketoacidosis or significant fluid derangements while minimizing the risk for hypoglycemia. Today, a growing body of evidence suggests a close correlation between tight glucose control and improved clinical outcomes. Among those who have had a myocardial infarction and those in the surgical intensive care unit, it is known that intensive glycemic control reduces mortality.5, 6 Maintaining normoglycemia in patients in the surgical intensive care unit through intravenous insulin infusion also reduces the incidence of comorbidities such as transfusion requirements, renal failure, sepsis, and neuropathy and reduces the duration of ventilator dependence.6 Although trials using glucose‐insulin‐potassium infusions (GIK), when conducted such that lowering of blood glucose occurred, have shown benefit in the settings of myocardial infarction5, 7 and cardiac surgery,8 not all studies of GIK therapy have yielded positive results. The negative results of the CREATE‐ECLA study suggest that GIK therapy per se is not beneficial unless it reduces blood glucose.9 An abundance of additional observational data and comparisons with historical control data suggest that favorable outcomes might be causally dependent on euglycemia. The outcomes studied include hospital or critical care unit mortality and nosocomial infection,1014 specifically outcomes of strokes,1522 trauma,2325 renal transplantation,2628 myocardial infarction,2936 endocarditis,37 acute lymphocytic leukemia,38 community‐acquired pneumonia,39 infectious complications in the hospital,4046 and cardiac surgery,9, 44, 45, 4751 as well as length of stay and costs.11, 25, 5156
It is important for each hospital to consider the methodology used for blood glucose measurement, realizing that measurements in the Leuven Belgium studies were performed on arterial whole blood using a blood gas analyzer. With recognition that the normal range for blood glucose is method dependent, the data presented above form the basis for the recommended glycemic targets for hospitalized patients:
Target range blood glucose (AACE et al., 2004)
-
Preprandial: < 110 mg/dL
-
Peak postprandial: < 180 mg/dL
-
Critically ill surgical patients: 80‐110 mg/dL Target range blood glucose (ADA, 2006)
-
Critically ill: Blood glucose as close to 110 mg/dL as possible and generally < 180 mg/dL. These patients generally will require IV insulin.
-
Noncritically ill: Premeal blood glucose as close to 90‐130 mg/dL as possible (midpoint 110 mg/dL). Postprandial blood glucose < 180 mg/dL.
This supplement, Avoiding Complications in the Hospitalized Patient: The Case for Tight Glycemic Control, reviews several aspects of hyperglycemia in the hospital setting. Evidence that supports more intensive glucose control is reviewed, along with a real‐world success story that demonstrates how to apply the new glycemic targets in a multidisciplinary performance improvement project. In addition, the standard insulin sliding scale is examined in terms of efficacy, safety, and potential for meeting the new recommended glycemic targets.
Hyperglycemia is common in the hospital among patients with diabetes and those without. The exact overall prevalence of diabetes in the hospital is unknown; however, in 2000, 12.4% of U.S. hospital discharges listed diabetes as a diagnosis. Among cardiac surgery patients, the prevalence of diabetes is as high as 29%.2 Another study reported a 26% prevalence of diabetes in a community teaching hospital, with an additional 12% of patients having unrecognized diabetes or hospital‐related hyperglycemia.3 Levetan et al. found laboratory‐documented hyperglycemia in 13% of 1034 consecutively hospitalized patients.4 A subsequent chart review found that more than one‐third of patients with hyperglycemia identified by laboratory testing remained unrecognized as having diabetes documented in the discharge summary, although diabetes or hyperglycemia was noted in the progress notes. In a retrospective chart review study, Umpierrez et al. similarly found 38% of 1886 consecutively hospitalized patients who had glucose measurements on admission were hyperglycemic.3 One‐third of these patients were not previously known to have diabetes, and compared to patients with diagnosed diabetes, they were more likely to require admission to the intensive care unit, had longer hospital stays, and were less likely to be discharged straight home.
Until recently, most clinicians viewed tight glucose control in the hospitalized patient as an intervention with little immediate benefit and significant potential for harm. The goal was simply to prevent excessive hyperglycemia and avoid ketoacidosis or significant fluid derangements while minimizing the risk for hypoglycemia. Today, a growing body of evidence suggests a close correlation between tight glucose control and improved clinical outcomes. Among those who have had a myocardial infarction and those in the surgical intensive care unit, it is known that intensive glycemic control reduces mortality.5, 6 Maintaining normoglycemia in patients in the surgical intensive care unit through intravenous insulin infusion also reduces the incidence of comorbidities such as transfusion requirements, renal failure, sepsis, and neuropathy and reduces the duration of ventilator dependence.6 Although trials using glucose‐insulin‐potassium infusions (GIK), when conducted such that lowering of blood glucose occurred, have shown benefit in the settings of myocardial infarction5, 7 and cardiac surgery,8 not all studies of GIK therapy have yielded positive results. The negative results of the CREATE‐ECLA study suggest that GIK therapy per se is not beneficial unless it reduces blood glucose.9 An abundance of additional observational data and comparisons with historical control data suggest that favorable outcomes might be causally dependent on euglycemia. The outcomes studied include hospital or critical care unit mortality and nosocomial infection,1014 specifically outcomes of strokes,1522 trauma,2325 renal transplantation,2628 myocardial infarction,2936 endocarditis,37 acute lymphocytic leukemia,38 community‐acquired pneumonia,39 infectious complications in the hospital,4046 and cardiac surgery,9, 44, 45, 4751 as well as length of stay and costs.11, 25, 5156
It is important for each hospital to consider the methodology used for blood glucose measurement, realizing that measurements in the Leuven Belgium studies were performed on arterial whole blood using a blood gas analyzer. With recognition that the normal range for blood glucose is method dependent, the data presented above form the basis for the recommended glycemic targets for hospitalized patients:
Target range blood glucose (AACE et al., 2004)
-
Preprandial: < 110 mg/dL
-
Peak postprandial: < 180 mg/dL
-
Critically ill surgical patients: 80‐110 mg/dL Target range blood glucose (ADA, 2006)
-
Critically ill: Blood glucose as close to 110 mg/dL as possible and generally < 180 mg/dL. These patients generally will require IV insulin.
-
Noncritically ill: Premeal blood glucose as close to 90‐130 mg/dL as possible (midpoint 110 mg/dL). Postprandial blood glucose < 180 mg/dL.
This supplement, Avoiding Complications in the Hospitalized Patient: The Case for Tight Glycemic Control, reviews several aspects of hyperglycemia in the hospital setting. Evidence that supports more intensive glucose control is reviewed, along with a real‐world success story that demonstrates how to apply the new glycemic targets in a multidisciplinary performance improvement project. In addition, the standard insulin sliding scale is examined in terms of efficacy, safety, and potential for meeting the new recommended glycemic targets.
- Tierney E: Data from the national hospital discharge survey database 2000.Centers for Disease Control and Prevention, Division of Diabetes translation,Atlanta, GA,2003.
- Moghissi E: Hospital management of diabetes: beyond the sliding scale.Clev Clin J Med.2004;71:801–808.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,,Ratner RE: Unrecognized diabetes among hospitalized patients.Diabetes Care.1998;21:246–249.
- , for theDIGAMI study group.Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.BMJ.1997;314:1512–1515.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.J Am Coll Cardiol.1995;26:57–65.
- ,,,,,.Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events.Circulation.2004;109:1497–1502.
- CREATE‐ECLA Trial Group Investigators.Effect of glucose‐insulin‐potassium infusion on mortality in patients with acute st‐segment elevation myocardial infarction: the CREATE‐ECLA randomized controlled trial.JAMA.2005;293:437–446.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,.Reduction of nosocomial infections in the surgical intensive‐care unit by strict glycemic control.Endocr Pract.2004;10(suppl 2):46–52.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125:1007–1021.
- ,,,,,.Mortalilty in hospitalized patients with hypoglycemia and severe hyperglycemia.Mt Sinai J Med.1995;62:422–426.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,.Glucose control and mortality in critically ill patients.JAMA.2003;290:2041–2047.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004:79:992–1000.
- ,,.Insulin therapy for critically ill hospitalized patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2004;164:2005–2011.
- ,,, et al.Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome.Stroke.2003;34:2208–2214.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nodiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,, et al.Admission glucose level and clinical outcomes in the NINDS rt‐PA Stroke Trial.Neurology.2002;59:669–674.
- .Effect of hyperglycemia on stroke outcomes.Endocr Pract.2004;10(suppl 2):34–39.
- ,,, et al.Predictors of hyperacute clinical worsening in ischemic stroke patients receiving thrombolytic therapy.Stroke.2004;35:1903–1907.
- ,.Hyperglycemia in acute stroke.Stroke.2004;35:363–364.
- ,,,,.Decreased mortality by normalizing blood glucose after acute ischemic stroke.Acad Emerg Med.2006;13:174–180.
- ,,.Blood glucose control after acute stroke: a retrospective study.Acad Emerg Med.2003;10:432.
- ,,,,.Relationship of early hyperglycemia to mortality in trauma patients.J Trauma.2004;56:1058–1062.
- ,,,,,.Admission hyperglycemia is predictive of outcome in critically ill trauma patients.J Trauma.2005;59:80–83.
- ,,, et al.Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke.Neurology.2002;59(1):67–71.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,,,.Early peri‐operative hyperglycaemia and renal allograft rejection in patients without diabetes.BMC Nephrol.2000;1:1.
- ,,,,.Protective effect of insulin on ischemic renal injury in diabetes mellitus.Kidney Int.2002;61:1383–1392.
- ,,,,,.Impaired glucose metabolism predicts mortality after a myocardial infarction.Int J Cardiol.2001;79 (2–3):207–214.
- ,,,.Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.Lancet.2000;355:773–778.
- ,,, et al.A single serum glucose measurement predicts adverse outcomes across the whole range of acute coronary syndromes.Heart.2003;89:512–516.
- ,,,,,.Intensification of therapeutic approaches reduces mortality in diabetic patients with acute myocardial infarction: the Munich registry.Diabetes Care.2004;27:455–460.
- ,,, et al.Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus.Arch Intern Med.2004;164:982–988.
- ,,,.Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the diabetes and insulin‐glucose infusion in acute myocardial infarction (DIGAMI) study.Circulation.1999;99:2626–2632.
- ,,,,,.Plasma glucose at hospital admission and previous metabolic control determine myocardial infarct size and survival in patients with and without type 2 diabetes: the Langendreer Myocardial Infarction and Blood Glucose in Diabetic Patients Assessment (LAMBDA).Diabetes Care.2005;28:2551–2553.
- ,,, et al.Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes.Circulation.2005;111:3078–3086.
- ,,, et al.Early predictors of in‐hospital death in infective endocarditis.Circulation.2004;109:1745–1749.
- .Relation between the duration of remission and hyperglycemia in induction chemotherapy for acute lymphocytic leukemia.Cancer.2004;100:1179–1185.
- ,,,,.Etiology and outcome of community‐acquired pneumonia in patients with diabetes mellitus.Chest.2005;128:3233–3239.
- ,,,.Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes.Diabetes Care.1999;22:1408–1414.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enteral Nutr.1998;22(2):77–81.
- ,,,,.The association of diabetes and glucose control with surgical‐site infections among cardiothoracic surgery patients.Infect Control Hosp Epidemiol.2001;22:607–612.
- ,,,,.Early post‐operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study.Eur J Vasc Endovasc Surg.2004;5:520–525.
- ,,.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356–61.
- ,,,.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thorac Surg.1999;67:352–362.
- ,,, et al.Improving outcomes for diabetic patients undergoing vascular surgery.Diabetes Spectr.2005;18(1):53–60.
- ,,.Early postoperative outcome and medium‐term survival in 540 diabetic and 2239 nondiabetic patients undergoing coronary artery bypass grafting.Ann Thorac Surg.2002;74:712–719.
- ,,,,.Diabetes and coronary artery bypass surgery: an examination of perioperative glycemic control and outcomes.Diabetes Care.2003;26:1518–1524.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland diabetic project.Endocr Pract.2004;10(suppl 2):21–33.
- ,,,,.Glucose‐insulin‐potassium solutions improve outcomes in diabetics who have coronary artery operations.Ann Thorac Surg.2000;70:145–150.
- ,,, et al.Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients.Mayo Clin Proc.2005;80:862–866.
- ,,,.Postoperative hyperglycemia prolongs length of stay in diabetic CABG patients.Circulation. II2000;102(II):556 (abstract).
- .Reduction of hospital costs and length of stay by good control of blood glucose levels.Endocr Pract.2004;10(suppl 2):53–56.
- Tierney E: Data from the national hospital discharge survey database 2000.Centers for Disease Control and Prevention, Division of Diabetes translation,Atlanta, GA,2003.
- Moghissi E: Hospital management of diabetes: beyond the sliding scale.Clev Clin J Med.2004;71:801–808.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,,Ratner RE: Unrecognized diabetes among hospitalized patients.Diabetes Care.1998;21:246–249.
- , for theDIGAMI study group.Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.BMJ.1997;314:1512–1515.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.J Am Coll Cardiol.1995;26:57–65.
- ,,,,,.Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events.Circulation.2004;109:1497–1502.
- CREATE‐ECLA Trial Group Investigators.Effect of glucose‐insulin‐potassium infusion on mortality in patients with acute st‐segment elevation myocardial infarction: the CREATE‐ECLA randomized controlled trial.JAMA.2005;293:437–446.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,.Reduction of nosocomial infections in the surgical intensive‐care unit by strict glycemic control.Endocr Pract.2004;10(suppl 2):46–52.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125:1007–1021.
- ,,,,,.Mortalilty in hospitalized patients with hypoglycemia and severe hyperglycemia.Mt Sinai J Med.1995;62:422–426.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,.Glucose control and mortality in critically ill patients.JAMA.2003;290:2041–2047.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004:79:992–1000.
- ,,.Insulin therapy for critically ill hospitalized patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2004;164:2005–2011.
- ,,, et al.Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome.Stroke.2003;34:2208–2214.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nodiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,, et al.Admission glucose level and clinical outcomes in the NINDS rt‐PA Stroke Trial.Neurology.2002;59:669–674.
- .Effect of hyperglycemia on stroke outcomes.Endocr Pract.2004;10(suppl 2):34–39.
- ,,, et al.Predictors of hyperacute clinical worsening in ischemic stroke patients receiving thrombolytic therapy.Stroke.2004;35:1903–1907.
- ,.Hyperglycemia in acute stroke.Stroke.2004;35:363–364.
- ,,,,.Decreased mortality by normalizing blood glucose after acute ischemic stroke.Acad Emerg Med.2006;13:174–180.
- ,,.Blood glucose control after acute stroke: a retrospective study.Acad Emerg Med.2003;10:432.
- ,,,,.Relationship of early hyperglycemia to mortality in trauma patients.J Trauma.2004;56:1058–1062.
- ,,,,,.Admission hyperglycemia is predictive of outcome in critically ill trauma patients.J Trauma.2005;59:80–83.
- ,,, et al.Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke.Neurology.2002;59(1):67–71.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,,,.Early peri‐operative hyperglycaemia and renal allograft rejection in patients without diabetes.BMC Nephrol.2000;1:1.
- ,,,,.Protective effect of insulin on ischemic renal injury in diabetes mellitus.Kidney Int.2002;61:1383–1392.
- ,,,,,.Impaired glucose metabolism predicts mortality after a myocardial infarction.Int J Cardiol.2001;79 (2–3):207–214.
- ,,,.Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.Lancet.2000;355:773–778.
- ,,, et al.A single serum glucose measurement predicts adverse outcomes across the whole range of acute coronary syndromes.Heart.2003;89:512–516.
- ,,,,,.Intensification of therapeutic approaches reduces mortality in diabetic patients with acute myocardial infarction: the Munich registry.Diabetes Care.2004;27:455–460.
- ,,, et al.Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus.Arch Intern Med.2004;164:982–988.
- ,,,.Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the diabetes and insulin‐glucose infusion in acute myocardial infarction (DIGAMI) study.Circulation.1999;99:2626–2632.
- ,,,,,.Plasma glucose at hospital admission and previous metabolic control determine myocardial infarct size and survival in patients with and without type 2 diabetes: the Langendreer Myocardial Infarction and Blood Glucose in Diabetic Patients Assessment (LAMBDA).Diabetes Care.2005;28:2551–2553.
- ,,, et al.Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes.Circulation.2005;111:3078–3086.
- ,,, et al.Early predictors of in‐hospital death in infective endocarditis.Circulation.2004;109:1745–1749.
- .Relation between the duration of remission and hyperglycemia in induction chemotherapy for acute lymphocytic leukemia.Cancer.2004;100:1179–1185.
- ,,,,.Etiology and outcome of community‐acquired pneumonia in patients with diabetes mellitus.Chest.2005;128:3233–3239.
- ,,,.Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes.Diabetes Care.1999;22:1408–1414.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enteral Nutr.1998;22(2):77–81.
- ,,,,.The association of diabetes and glucose control with surgical‐site infections among cardiothoracic surgery patients.Infect Control Hosp Epidemiol.2001;22:607–612.
- ,,,,.Early post‐operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study.Eur J Vasc Endovasc Surg.2004;5:520–525.
- ,,.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356–61.
- ,,,.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thorac Surg.1999;67:352–362.
- ,,, et al.Improving outcomes for diabetic patients undergoing vascular surgery.Diabetes Spectr.2005;18(1):53–60.
- ,,.Early postoperative outcome and medium‐term survival in 540 diabetic and 2239 nondiabetic patients undergoing coronary artery bypass grafting.Ann Thorac Surg.2002;74:712–719.
- ,,,,.Diabetes and coronary artery bypass surgery: an examination of perioperative glycemic control and outcomes.Diabetes Care.2003;26:1518–1524.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland diabetic project.Endocr Pract.2004;10(suppl 2):21–33.
- ,,,,.Glucose‐insulin‐potassium solutions improve outcomes in diabetics who have coronary artery operations.Ann Thorac Surg.2000;70:145–150.
- ,,, et al.Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients.Mayo Clin Proc.2005;80:862–866.
- ,,,.Postoperative hyperglycemia prolongs length of stay in diabetic CABG patients.Circulation. II2000;102(II):556 (abstract).
- .Reduction of hospital costs and length of stay by good control of blood glucose levels.Endocr Pract.2004;10(suppl 2):53–56.
Benefits of Euglycemia
Until recently it had been argued that hospitalization was not the time in the life of a patient to insist on tight glycemic control. Hyperglycemia was understood to be a consequence of medical stress.1 It was well known that infection, sepsis, or other medical stress might exacerbate hyperglycemia or promote a diabetic crisis.25 Admittedly, the severity of hyperglycemia among patients who have diabetes was thought to predict the risk for hospitalization with infection as well as the outcome of the infectious condition.68 However, until recently strict glycemic control in the hospital was not strongly advocated because hypoglycemia might occur and might be directly and uniquely traceable to actions taken in the hospital.9, 10 Furthermore, the complications of diabetes were thought to be divided into acute metabolic emergencies and chronic tissue complications such as polyneuropathy, retinopathy, nephropathy, and macrovascular disease that would have evolved over a period longer than the duration of hospitalization, and the possibility that short‐term hyperglycemia might affect outcomes was considered unproven.
The purpose of this article is to define the specific populations, disorders, and hospital settings for which there now is strong evidence that short‐term glycemic control will affect the outcome of a course of hospital treatment.
PHYSIOLOGIC LINK BETWEEN HYPERGLYCEMIA AND ADVERSE OUTCOMES
Five years ago a caregiver would not have been likely to think of glycemic control as a contributing factor when considering specific complex problems such as pump failure or arrhythmia after cardiac surgery, long‐term mortality after myocardial infarction, acute renal failure, the need for transfusion during treatment of a complicated surgical illness, or prolonged dependence on a ventilator in the surgical ICU. Although it now known that these and other complications are linked to hospital hyperglycemia, the mechanisms of harm are several steps removed from the hyperglycemia itself. The causes of these adverse outcomes are multifactorial. The causal dependence of the injury on hyperglycemia is not easy to see. In fact, it required randomized prospectively designed trials to convincingly demonstrate the contributory role of hyperglycemia to these and other adverse outcomes.
Now that this link has been convincingly demonstrated, there is intense interest in discovering the probable mechanisms by which control of hyperglycemia and specifically the use of insulin might improve outcomes.1114 Mortality, predominantly sepsis related, was the primary outcome for which the Leuven, Belgium, report of 2001 on the surgical ICU showed improvement.15 Simplistically, in seeking a mechanism of benefit with respect to sepsis, it might be argued that if gross hyperglycemia were prevented in patients with surgical wounds, improvement of host defenses against infective organisms might be expected.1622 However, additional mechanisms of protection probably should be invoked, including those by which glycemic control and specifically insulin therapy affect endothelial function and the coagulation pathway, thus improving the ability of a patient to withstand and recover from sepsis. Insulin promotes beneficial nitric oxide synthase activation (e‐NOS) in capillary endothelium.2326 In patients with prolonged critical illness, intensive insulin therapy lowers ICAM‐1 levels, reflecting reduced endothelial activation. Whereas e‐NOS exerts a beneficial endothelial effect, hepatic iNOS activation is harmful. One proposed mechanism of benefit from adequate insulin therapy is suppression of excessive hepatic iNOS‐induced release of circulating NO, which might contribute to endothelial dysfunction, organ failure, and death.27
Additional proposed mechanisms of hyperglycemia‐induced harm to hospitalized patients, some potentially specifically reduced by insulin therapy, resemble those discussed in relation to macrovascular disease and include activation of inflammatory cytokines, matrix metalloproteinases, and adhesion molecules, and the adverse arrhythmogenic effects of elevated circulating nonesterified fatty acids (Figure 1).2834
CRITICAL WINDOW OF TIME
The results of several retrospectively or prospectively conducted single‐institution observational studies suggest there is a critical window of time within which clinicians must get it right, when attempting glycemic control or else jeopardize therapeutic goals, such as duration of remission in treatment of acute lymphocytic leukemia35 or avoidance of acute rejection in renal allograft surgery.36 Additionally, delayed risk of infection appears to be linked to previous glycemic control during specific early time frames surrounding surgery, renal transplantation, admission for trauma, and induction chemotherapy for leukemia (Table 1).3541 For patients who have diabetes, the potential to reduce the consequences of infections through intensified glycemic control probably begins prior to admission and in the hospital has still not been fully realized.4244
| Patients | Ascertainment of hyperglycemia | Delayed events among patients with early hyperglycemia |
|---|---|---|
| 100 postoperative uninfected diabetic patients undergoing elective surgery, monitored prospectively37 | First postoperative day | Postoperative nosocomial infection rate within 14 days was 2.7 times higher for patients having at least one BG > 220 mg/dL (33.3% vs. 11.5%). |
| 990 historical controls and 595 patients in the interventional group of postoperative cardiac diabetic patients40 | First 2 postoperative days | Incidence of deep sternal wound infection was reduced from 2.4% to 1.5% (P < 0.02) after introduction of protocol to maintain mean BG < 200 mg/dL. |
| 423 renal allograft recipients receiving their first cadaveric transplant36 | First 100 hours; first day. | A mean of 10.8 2.3 days after transplantation, 70% of patients developed postoperative infection, and after a mean of 7.7 2.6 days, 40% developed acute rejection. Every patient with mean BG over 200 mg/dL during the first 100 hours developed postoperative infection. On the first postoperative day the mean BG had been 248.4 mg/dL among those developing infection and 167.4 mg/dL among those without infection (P < .001), and the mean BG had been 270 mmol/L among those developing rejection and 194 mmol/L among those without rejection. |
| 516 trauma patients admitted to the ICU38 | Either of first 2 hospital days | Hyperglycemia 200 mg/dL was associated with a higher infection rate (32% vs. 22%, P = .04) and with greater mortality (34% vs. 13%, P < .0001) |
| 275 patients having lower‐extremity peripheral vascular surgery39 | First 48 hours | Postoperative infections within 30 days were 5.1 times more frequent in the top quartile for BG versus the lowest quartile (confidence interval 1.6‐17.1, P = .007). |
| 278 adult patients receiving induction chemotherapy for acute lymphocytic leukemia35 | First 30 days | Hyperglycemia defined as 2 BG 200 mg/dL was associated with a greater likelihood of sepsis (16.5% vs. 8.0%, P = .03) or any complicated infection (38.8% vs. 25.1%, P = .016), shorter duration of complete remission (24 vs. 52 months), and with shorter median survival (29 vs. 88 months, P = .001). |
KEY STUDIES SHOWING CLINICAL BENEFIT OF TIGHT GLYCEMIC CONTROL
A summary of several key studies that demonstrated the clinical benefit of tight glycemic control is shown in Table 2. These studies successfully separated the intensively and conventionally managed study groups according blood glucose. Although trials using glucose‐insulin‐potassium infusions (GIK) such that blood glucose was lowered have shown benefit for patients who have had myocardial infarctions4547 or cardiac surgery,48 not all GIK studies have yielded positive results. The negative results of the CREATE‐ECLA study suggest that GIK therapy per se is not beneficial unless it reduces blood glucose.49 In the setting of acute myocardial infarction, the DIGAMI 2 trial and the HI‐5 trials failed to achieve the intended separation between treatment groups.50, 51 It has been speculated that if insulin is delivered so as to not achieve normoglycemia, then hyperinsulinemia in the presence of hyperglycemia actually may be proinflammatory.52
| Population and/or setting and patients | Study design and/or intervention | Principal findings |
|---|---|---|
| Patients with diabetes mellitus and with hyperglycemia > 11 mmol/L or similar hyperglycemia without known diabetes who had had acute myocardial within the preceding 24 hours.4547 There were 620 patients, of whom 15 of the controls and 10 patients in the intensive group had no prior diagnosis of diabetes. | Prospective, randomized, controlled clinical trial. Controls received standard treatment. Treatment group received infusion of glucose‐insulin for at least 24 hours, followed by multiple injections of subcutaneous insulin for at least 3 months. | Overall mortality after 1 year was l9% in the insulin group compared to 26% among controls (P < .05). The most frequent cause of death of all patients was congestive heart failure. The benefit continued for at least 3.5 years, with an absolute reduction in mortality of 11%. |
| Diabetic cardiac surgery population.40, 5557 To date, 5510 cardiac surgery patients who were either admitted or discharged with the diagnosis of diabetes have been studied. | Prospective nonrandomized study of the effects of hyperglycemia and its pharmacologic reduction with intensive intravenous insulin regimens on outcomes. | The 3‐day average of perioperative BG (3‐BG) correlated with mortality (P < .0001, odds ratio 2.0 per 50 mg/dL increase in 3‐BG). Continuous intravenous insulin infusion independently reduced risk of death 60% (RR = 0.4, P < .001). Length of stay in the CABG population increased by 1 day for every increase of 50 mg/dL in 3‐BG. |
| Critically ill surgical patients receiving mechanical ventilation. Overall, 13% of 1548 participants had previously diagnosed diabetes. | Prospective, randomized, controlled clinical trial. Intravenous insulin infusion targeting BG 80‐110 mg/dL was initiated in the intensive group for BG > 110 mg/dL. Intravenous insulin infusion targeting BG 180‐200 was initiated in the conventional group for BG > 215 mg/dL. A whole‐blood glucose method using a gas analyzer was employed to determine arterial blood glucose. | In the group assigned to intensive insulin therapy mortality was reduced during intensive care from 8.0% with conventional treatment to 4.6% (P < .04), or a risk reduction of 42%. In the group remaining in the ICU for more than 5 days, mortality was reduced from 20.2% with conventional treatment to 10.6% with intensive insulin therapy; P = .005. The intensive group experienced reductions in overall in‐hospital mortality of 34%, in bloodstream infections of 46%, and in acute renal failure requiring dialysis or hemofiltration of 41%. Also reduced were the need for red‐cell transfusions, critical‐illness neuropathy, duration of mechanical ventilation, and length of stay in the ICU. |
| Critically ill medical patients. The intention to treat group included 1200 participants, of whom 16.9% had a history of diabetes.54 | Randomized controlled clinical trial, as above. | In the intensive group, in‐hospital mortality was not significantly reduced, and among the 433 who stayed in the ICU for less than 3 days, mortality was actually higher. However, in the intention‐to‐treat group there was reduction in newly acquired kidney injury, prolonged mechanical ventilation, and length of stay in the ICU and in the hospital. Among the 767 patients who stayed in the ICU for 3 or more days, intensive insulin therapy reduced in‐hospital mortality from 52.5% to 43.0%. |
| Patients within 24 hours of having an acute stroke and who had poststroke hyperglycemia. The results of the first 452 patients recruited to the GIST‐UK study showed that 15.3% had previously recognized diabetes.62 | Randomized controlled clinical trial. The intensive group received glucose‐potassium‐insulin (GKI) infusion, and the conventional group received saline infusion. | Although mean glucose declined in both groups, the GKI infusion safely achieved separation of groups by blood glucose. Outcomes are pending. |
The findings of the negative intensive insulin studies do not offset the evidence favoring glycemic control, derived from studies that actually achieved the lowering of blood glucose in the intensive groups, such as the successful prospectively designed trials in myocardial infarction or surgical or medical intensive care units15, 45, 47, 53, 54 and the long‐running, large, prospectively monitored Portland series utilizing insulin infusion for cardiac surgery,40, 5557 which have demonstrated the beneficial effects of euglycemia on mortality and morbidity, or the findings of Krinsley, reported elsewhere in this issue.58, 59 The well‐recognized correlation between outcomes of acute stroke and poststroke hyperglycemia has led to the design of a multicenter trial of glucose‐insulin‐potassium infusion for stroke, in which separation of groups by blood glucose has been achieved.6062 The success of GIK therapy in controlling hyperglycemia depends in part on the particular formulation of the infusion as it matches patient needs, and it is probable that insulin infusion following stroke is capable of safely achieving even tighter glycemic control than GIK.63 Intravenous insulin infusion therapy is more difficult to conduct than GIK therapy. However, because of concern about the proinflammatory and prothrombotic effects of hyperglycemia and recognition of the occasional failure of GIK infusions to control hyperglycemia and of the anti‐inflammatory, antithrombotic, and vasodilatory actions of insulin, there have been calls for additional trials of insulin infusions (as opposed to glucose‐insulin infusions) for both acute myocardial infarction and stroke.52, 64
HYPOGLYCEMIA
Serious or fatal sequelae of hypoglycemia are the principal safety risks in intensive insulin management.9, 65 Case ascertainment cannot be assured by glucose averaging methods but instead requires a method of searching for isolated episodes of hypoglycemia.10 One of the most dreaded consequences of nonfatal hypoglycemia is permanent impairment of intellectual function. Because many euglycemic medically ill patients experience alteration of sensorium while acutely ill, there is a risk that the consequences of an actual episode of severe hypoglycemia will be ascribed to other comorbidities, overlooking or misattributing the altered cognitive function that may persist at discharge to causes other than the obvious iatrogenic one. Because there is an increased risk of hypoglycemia during intensive insulin therapy, controversy has arisen over glycemic targets, especially among critically ill nonsurgical patients.54, 6669
On the other hand, the consequences of hypoglycemia during intensive intravenous insulin therapy in a surgical ICU were said to be negligible.15 In hospitalized elderly patients, hypoglycemia in association with increased mortality risk may not be an independent predictor.70 In the critical care unit, predictors of hypoglycemia are identifiable after the introduction of strict glycemic control that include not only insulin therapy but also CVVH treatment with bicarbonate substitution fluid, discontinuation of nutrition without insulin adjustment, prior diagnosis of diabetes mellitus, sepsis, and the need for inotropic or vasopressor drugs.71 A prudent policy for the future would be not only to treat hypoglycemia promptly when it does occur, with attention to subsequent monitoring to avoid relapse or recurrence, but also to actively introduce strategies for predicting the risk of hypoglycemia and preventing it, especially when high risk is identified.10 The fear of hypoglycemia should not paralyze efforts to achieve better glycemic control in hospitalized patients.
UNANSWERED QUESTIONS AND VISION FOR THE FUTURE
On general wards, 38% of admitted patients may have hyperglycemia.72 As shown in the Leuven, Belgium, study, to achieve the target BG of less than 110 mg/dL in the intensive group in the surgical critical care unit, it was necessary to administer intravenous insulin infusion to essentially all patients. In a study that utilized continuous glucose monitoring, normoglycemia in patients in the intensive care unit was achieved as little as 22% of the time.73
An actively debated subject is how best to assess hospital performance in glycemic control (glucometrics). Hospitals need to show satisfactory control of variability between patients and within the treatment course of individual patients. That is, it is not sufficient to be satisfied with reasonable results of average blood glucose, using blood glucose as the unit of observation (where n is the number of blood glucose determinations). Alternatives are to use patient day or individual patient as a unit of observation (where n is the number of patient days and the number of patients, respectively). Time‐weighting methods are cumbersome but increase the validity of the averaging method used. When the patient is the unit of observation, possible measures include a per‐patient blood glucose average, percentage of blood glucose measurements with certain ranges, time spent within certain blood glucose ranges, or area under the curve of blood glucose versus time. Caveats about glucometrics pertain to both intravenous insulin infusion and also subcutaneous insulin management.74
Although awaiting additional evidence, diabetes experts have widely accepted the proposition that hospitals should focus on prevention of hyperglycemia as an important patient safety factor.75 Although the target range for glycemic control remains controversial, many students of the subject have endorsed the recommendations mentioned in the lead article in this issue, with the understanding that these criteria were developed from the results of the Leuven, Belgium, study, in which a whole blood glucose analyzer was used for measurement, and that the method of measurement of blood glucose must be considered in interpreting applicability of the target range at individual hospitals, many of which use plasma‐correlated methods yielding higher results. However, in new settings and for medical conditions that have not yet been rigorously evaluated by clinical trials, it is an unanswered question whether intensification of glycemic control can be achieved safely outside the critical care unit and, if so, what type of insulin therapy should be used and for what conditions the benefits would outweigh the risks and justify the costs.
Most inpatient management probably will continue to be conducted using subcutaneous injection therapy,7682 designed to match carbohydrate exposure through the appropriate use of analogs or conventional insulin products. One argument for the use of insulin analogs in the hospital, using basal‐prandial‐correction therapy, is the probability of reducing hypoglycemia and getting closer to target range control among patients who are eating but who are at risk for abrupt suspension of meals. Aggressive subcutaneous management strategies are likely to be most effective when standardized protocols, order sets, and informative computerized order entry systems gain widespread hospital acceptance.
If the importance of gaining glycemic control is highly time dependent and if hyperglycemia is uncontrolled, then a strong argument can be made for routine use of intravenous insulin infusion. For appropriately selected patients, intravenous insulin infusion is cost effective,83, 84 and its use can be extended to appropriate patients outside the critical care unit.8587 Many hospitals have protocols for intravenous insulin but use them only sporadically. For patients who already are in the intensive care setting, it is imperative to develop policies that require introduction of intravenous insulin infusion at a given glycemic threshold. On general wards that lack sufficient staffing to conduct intravenous insulin therapy, it is appropriate either to transfer candidate patients to a ward that has adequate staffing when medical condition requires improved control or to develop policies and procedures that will extend the use of intravenous insulin infusion to general wards. In the future, new technologies can be envisioned that will unburden nursing staff, making intravenous insulin infusion realizable as the treatment of choice for hemodynamically stable patients in most hospital settings. These technologies will include continuous monitoring of blood glucose, dose‐defining algorithms, and the eventual development of a fully automated closed‐loop system of monitoring and delivery that might automatically match insulin delivery to carbohydrate exposure and patient insulin sensitivity.8893
- ,,.Stress‐induced hyperglycemia.Crit Care Clin.2001;17(1):107–124.
- ,,,,.Hyperosmolarity and acidosis in diabetes mellitus: a three‐year experience in Rhode Island.J Gen Intern Med.1991:495–502.
- ,,.Prognostic factors in the diabetic hyperosmolar state.J Am Geriatr Soc.1987;35:737–741.
- ,,.Predisposing factors for the diabetic hyperosmolar state.Arch Intern Med.1987;147:499–501.
- .The diabetic hyperosmolar state.Clin Geriatr Med.1990;6:797–806.
- ,,,,,.Bacteremia in adult diabetic patients.Diabetes Care.1991;14(2):89–94.
- ,,,,,.Influence of diabetes mellitus and glycaemic control on the characteristics and outcome of common infections.Diabet Med.1996:457–463.
- ,,.Diabetes and the risk of infection‐related mortality in the U.S.Diabetes Care.2001;24:1044–1049.
- ,,.Hypoglycemia in hospitalized patients.N Engl J Med.1986;315:1245–1250.
- ,,, et al.Hospital hypoglycemia: not only treatment but also prevention.Endocr Pract.2004;10(suppl 2):71–80.
- .Effect of insulin therapy on nonglycemic variables during acute illness.Endocr Pract.2004;10(suppl 2):63–70.
- .Molecular mechanisms by which metabolic control may improve outcomes.Endocr Pract.2004;10(suppl 2):57–62.
- ,,,,.Insulin infusion in acute illness.J Clin Invest.2005;115:2069–2072.
- ,,.Intensive insulin therapy in the intensive care unit: update on clinical impact and mechanisms of action.Endocr Pract.2006;12(suppl 3):14–21.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,.Impaired granulocyte adherence. A reversible defect in host defense in patients with poorly controlled diabetes.Diabetes.1978;27:677–681.
- ,,.Impaired leukocyte function in patients with poorly controlled diabetes.Diabetes.1974;23(1):9–15.
- ,,, et al.The effect of diabetes mellitus on chemotactic and bactericidal activity of human polymorphonuclear leukocytes.Diabetes Res Clin Pract.1987;4:27–35.
- ,,,,.Polymorphonuclear leukocytes in non‐insulin‐dependent diabetes mellitus: abnormalities in metabolism and function.Ann Intern Med.1995;123:919–924.
- ,,,,.Agonist‐dependent failure of neutrophil function in diabetes correlates with extent of hyperglycemia.J Leukoc Biol.2001;70:395–404.
- ,,,,,.Insulin increases neutrophil count and phagocytic capacity after cardiac surgery.Anesth Analg.2002;94:1113–1119.
- ,,,.In vivo evidences that insulin regulates human polymorphonuclear neutrophil functions.J Leukoc Biol.2004;76:1104–1110.
- ,.Insulin‐stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells.J Clin Invest.1996;98:894–898.
- ,,,,.Insulin‐mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release.J Clin Invest.1994;94:1172–1179.
- ,.Effect of insulin on human aortic endothelial nitric oxide synthase.Metabolism.2000;49(2):147–50.
- ,.Nitric oxide, platelet function, myocardial infarction and reperfusion therapies.Heart Fail Rev.2003;8(1):47–54.
- ,,, et al.Intensive insulin therapy protects the endothelium of critically ill patients.J Clin Invest.2005;115:2277–2286.
- ,,, et al.Elevated circulating free fatty acid levels impair endothelium‐dependent vasodilation.J Clin Invest.1997;100:1230–1239.
- ,,, et al.Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti‐inflammatory effect?J Clin Endocrinol Metab.2001;86:3257–3265.
- ,,.The anti‐inflammatory and potential anti‐atherogenic effect of insulin: a new paradigm.Diabetologia.2002;45:924–930.
- ,,,.The potential influence of inflammation and insulin resistance on the pathogenesis and treatment of atherosclerosis‐related complications in type 2 diabetes.J Clin Endocrinol Metab.2003;88:2422–2429.
- ,,.The potential therapeutic role of insulin in acute myocardial infarction in patients admitted to intensive care and in those with unspecified hyperglycemia.Diabetes Care.2003;26:516–519.
- ,,.Insulin treatment improves the systemic inflammatory reaction to severe trauma.Ann Surg.2004;239:553–560.
- ,,, et al.Anti‐inflammatory and profibrinolytic effect of insulin in acute ST‐segment‐elevation myocardial infarction.Circulation.2004;109:849–854.
- .Relation between the duration of remission and hyperglycemia in induction chemotherapy for acute lymphocytic leukemia.Cancer.2004;100:1179–1185.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enteral Nutr.1998;22(2):77–81.
- ,,,,.Relationship of early hyperglycemia to mortality in trauma patients.J Trauma.2004;56:1058–1062.
- ,,,,.Early post‐operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study.Eur J Vasc Endovasc Surg.2004;5:520–525.
- ,,,.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thorac Surg.1999;67:352–362.
- ,.Effects on outcome of in‐hospital transition from intravenous insulin infusion to subcutaneous therapy.Am J Cardiol.2006;98:557–564.
- ,,,.The impact of diabetes in patients with necrotizing soft tissue infections.Surg Infect.2005;6:427–438.
- ,,,.Infections in patients with diabetes mellitus.N Engl J Med.1999;341:1906–1912.
- ,,.Quantifying the risk of infectious diseases for people with diabetes.Diabetes Care.2003;26:510–513.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.J Am Coll Cardiol.1995;26:57–65.
- ,,, et al.Effects of insulin treatment on cause‐specific one‐year mortality and morbidity in diabetic patients with acute myocardial infarction.Eur Heart J.1996;17:1337–1344.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,,,,.Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events.Circulation.2004;109:1497–1502.
- .Effect of Glucose‐Insulin‐Potassium Infusion on Mortality in Patients With Acute ST‐Segment Elevation Myocardial Infarction: The CREATE‐ECLA Randomized Controlled Trial.JAMA.2005;293:437–446.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity.Eur Heart J.2005;26:650–661.
- ,,.The Hyperglycemia: Intensive Insulin Infusion In Infarction (HI‐5) Study: A randomized controlled trial of insulin infusion therapy for myocardial infarction.Diabetes Care.2006;29:765–770.
- .Inpatient diabetes: review of data from the cardiac care unit.Endocr Pract.2006;12(suppl 3):27–34.
- ,.Reduction of nosocomial infections in the surgical intensive‐care unit by strict glycemic control.Endocr Pract.2004;10(suppl 2):46–52.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356–361.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125:1007–1021.
- ,.Clinical effects of hyperglycemia in the cardiac surgery population: the Portland diabetic project.Endocr Pract.2006;12(suppl 3):22–26.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000.
- .Decreased mortality of critically ill patients with the use of an intensive glycemic management protocol.Mayo Clin Proc.2003;78:1471–1478.
- ,,, et al.Admission glucose level and clinical outcomes in the NINDS rt‐PA Stroke. Trial.Neurology.2002;59:669–74.
- ,,,,,.Glucose potassium insulin infusions in the treatment of acute stroke patients with mild to moderate hyperglycemia: the Glucose Insulin in Stroke. Trial (GIST).Stroke.1999;30:793–799.
- ,,,.Poststroke hyperglycemia: natural history and immediate management.Stroke.2004;35(1):122–126.
- ,,,.IV insulin during acute cerebral infarction in diabetic patients.Neurology.2004;62:1441–1442.
- ,,,.Hyperglycemia, insulin, and acute ischemic stroke: a mechanistic justification for a trial of insulin infusion therapy.Stroke.2006;37(1):267–273.
- ,,.Hypoglycemia and cardiac arrest in a critically ill patient on strict glycemic control.Anesth Analg.2006;102:549–551.
- ,,.Strict glucose control in the critically ill.Br Med J.2006;332:865–866.
- .Intensive Insulin in Intensive Care.N Engl J Med.2006;354:516–518.
- ,.Counterpoint: Inpatient glucose management: a premature call to arms?Diabetes Care.2005;28:976–979.
- ,,,.Glucose control and mortality in critically ill patients.JAMA.2003;290:2041–2047.
- ,,, et al.Hypoglycemia as a predictor of mortality in hospitalized elderly patients.Arch Intern Med.2003;163:1825–1829.
- ,,, et al.Predisposing factors for hypoglycemia in the intensive care unit.Crit Care Med.2006;34:96–101.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,.Intensive insulin therapy in the intensive care unit: assessment by continuous glucose monitoring.Diabetes Care.2006;29:1750–1756.
- ,,,.No patient left behind: evaluation and design of intravenous insulin infusion algorithms.Endocr Pract.2006;12(suppl 3):72–78.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,,.Eliminating Inpatient Sliding‐Scale Insulin: A reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,.70/30 Insulin algorithm versus sliding scale insulin.Ann Pharmacother.2005;39:1606–1609.
- ,.Hospital management of hyperglycemia.Clin Diabetes.2004;22(2):81–88.
- ,.Subcutaneous insulin therapy in the hospital setting: issues, concerns, and implementation.Endocr Pract.2004;10(suppl 2):81–88.
- ,,,,.Hyperglycemia in the hospital.Diabetes Spectr.2005;18(1):20–27.
- .Insulin Analogues.N Engl J Med.2005;352:174–183.
- .Insulin management of diabetic patients on general medical and surgical floors.Endocr Pract.2006;12(suppl 3):86–90.
- ,,, et al.Improved perioperative glycemic control by continuous insulin infusion under supervision of an endocrinologist does not increase costs in patients with diabetes.Endocr Pract.2004;10(2):112–118.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project.Endocr Pract.2004;10(suppl 2):21–33.
- ,,,.New insulin infusion protocol improves blood glucose control in hospitalized patients without increasing hypoglycemia.J Qual Patient Saf.2005;31:141–147.
- ,,,,.Optimizing hospital use of intravenous insulin therapy: improved management of hyperglycemia and error reduction with a new nomogram.Endocr Pract.2005;11:240–253.
- ,,,,.Implementation of a new intravenous insulin method on intermediate‐care units in hospitalized patients.Diabetes Educ.2005;31:818–823.
- ,,.Clinical results of an updated insulin infusion protocol in critically ill patients.Diabetes Spectr.2005;18(3):188–191.
- ,,.Glucommander: A computer‐directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation.Diabetes Care.2005;28:2418–2423.
- ,,, et al.Improving hyperglycemia management in the intensive care unit: preliminary report of a nurse‐driven quality improvement project using a redesigned insulin infusion algorithm.Diabetes Educ.2006;32:394–403.
- ,,, et al.Performance of a dose‐defining insulin infusion protocol among trauma service ICU admissions.Diabetes Technol Ther.2006;8:476–488.
- ,,, et al.A simple insulin‐nutrition protocol for tight glycemic control in critical illness: development and protocol comparison.Diabetes Technol Ther.2006;8:191–206.
- ,,, et al.Evaluation of the impact of chiropodist care in the secondary prevention of foot ulcerations in diabetic subjects.Diabetes Care.2003;26:1691–1695.
Until recently it had been argued that hospitalization was not the time in the life of a patient to insist on tight glycemic control. Hyperglycemia was understood to be a consequence of medical stress.1 It was well known that infection, sepsis, or other medical stress might exacerbate hyperglycemia or promote a diabetic crisis.25 Admittedly, the severity of hyperglycemia among patients who have diabetes was thought to predict the risk for hospitalization with infection as well as the outcome of the infectious condition.68 However, until recently strict glycemic control in the hospital was not strongly advocated because hypoglycemia might occur and might be directly and uniquely traceable to actions taken in the hospital.9, 10 Furthermore, the complications of diabetes were thought to be divided into acute metabolic emergencies and chronic tissue complications such as polyneuropathy, retinopathy, nephropathy, and macrovascular disease that would have evolved over a period longer than the duration of hospitalization, and the possibility that short‐term hyperglycemia might affect outcomes was considered unproven.
The purpose of this article is to define the specific populations, disorders, and hospital settings for which there now is strong evidence that short‐term glycemic control will affect the outcome of a course of hospital treatment.
PHYSIOLOGIC LINK BETWEEN HYPERGLYCEMIA AND ADVERSE OUTCOMES
Five years ago a caregiver would not have been likely to think of glycemic control as a contributing factor when considering specific complex problems such as pump failure or arrhythmia after cardiac surgery, long‐term mortality after myocardial infarction, acute renal failure, the need for transfusion during treatment of a complicated surgical illness, or prolonged dependence on a ventilator in the surgical ICU. Although it now known that these and other complications are linked to hospital hyperglycemia, the mechanisms of harm are several steps removed from the hyperglycemia itself. The causes of these adverse outcomes are multifactorial. The causal dependence of the injury on hyperglycemia is not easy to see. In fact, it required randomized prospectively designed trials to convincingly demonstrate the contributory role of hyperglycemia to these and other adverse outcomes.
Now that this link has been convincingly demonstrated, there is intense interest in discovering the probable mechanisms by which control of hyperglycemia and specifically the use of insulin might improve outcomes.1114 Mortality, predominantly sepsis related, was the primary outcome for which the Leuven, Belgium, report of 2001 on the surgical ICU showed improvement.15 Simplistically, in seeking a mechanism of benefit with respect to sepsis, it might be argued that if gross hyperglycemia were prevented in patients with surgical wounds, improvement of host defenses against infective organisms might be expected.1622 However, additional mechanisms of protection probably should be invoked, including those by which glycemic control and specifically insulin therapy affect endothelial function and the coagulation pathway, thus improving the ability of a patient to withstand and recover from sepsis. Insulin promotes beneficial nitric oxide synthase activation (e‐NOS) in capillary endothelium.2326 In patients with prolonged critical illness, intensive insulin therapy lowers ICAM‐1 levels, reflecting reduced endothelial activation. Whereas e‐NOS exerts a beneficial endothelial effect, hepatic iNOS activation is harmful. One proposed mechanism of benefit from adequate insulin therapy is suppression of excessive hepatic iNOS‐induced release of circulating NO, which might contribute to endothelial dysfunction, organ failure, and death.27
Additional proposed mechanisms of hyperglycemia‐induced harm to hospitalized patients, some potentially specifically reduced by insulin therapy, resemble those discussed in relation to macrovascular disease and include activation of inflammatory cytokines, matrix metalloproteinases, and adhesion molecules, and the adverse arrhythmogenic effects of elevated circulating nonesterified fatty acids (Figure 1).2834

CRITICAL WINDOW OF TIME
The results of several retrospectively or prospectively conducted single‐institution observational studies suggest there is a critical window of time within which clinicians must get it right, when attempting glycemic control or else jeopardize therapeutic goals, such as duration of remission in treatment of acute lymphocytic leukemia35 or avoidance of acute rejection in renal allograft surgery.36 Additionally, delayed risk of infection appears to be linked to previous glycemic control during specific early time frames surrounding surgery, renal transplantation, admission for trauma, and induction chemotherapy for leukemia (Table 1).3541 For patients who have diabetes, the potential to reduce the consequences of infections through intensified glycemic control probably begins prior to admission and in the hospital has still not been fully realized.4244
| Patients | Ascertainment of hyperglycemia | Delayed events among patients with early hyperglycemia |
|---|---|---|
| 100 postoperative uninfected diabetic patients undergoing elective surgery, monitored prospectively37 | First postoperative day | Postoperative nosocomial infection rate within 14 days was 2.7 times higher for patients having at least one BG > 220 mg/dL (33.3% vs. 11.5%). |
| 990 historical controls and 595 patients in the interventional group of postoperative cardiac diabetic patients40 | First 2 postoperative days | Incidence of deep sternal wound infection was reduced from 2.4% to 1.5% (P < 0.02) after introduction of protocol to maintain mean BG < 200 mg/dL. |
| 423 renal allograft recipients receiving their first cadaveric transplant36 | First 100 hours; first day. | A mean of 10.8 2.3 days after transplantation, 70% of patients developed postoperative infection, and after a mean of 7.7 2.6 days, 40% developed acute rejection. Every patient with mean BG over 200 mg/dL during the first 100 hours developed postoperative infection. On the first postoperative day the mean BG had been 248.4 mg/dL among those developing infection and 167.4 mg/dL among those without infection (P < .001), and the mean BG had been 270 mmol/L among those developing rejection and 194 mmol/L among those without rejection. |
| 516 trauma patients admitted to the ICU38 | Either of first 2 hospital days | Hyperglycemia 200 mg/dL was associated with a higher infection rate (32% vs. 22%, P = .04) and with greater mortality (34% vs. 13%, P < .0001) |
| 275 patients having lower‐extremity peripheral vascular surgery39 | First 48 hours | Postoperative infections within 30 days were 5.1 times more frequent in the top quartile for BG versus the lowest quartile (confidence interval 1.6‐17.1, P = .007). |
| 278 adult patients receiving induction chemotherapy for acute lymphocytic leukemia35 | First 30 days | Hyperglycemia defined as 2 BG 200 mg/dL was associated with a greater likelihood of sepsis (16.5% vs. 8.0%, P = .03) or any complicated infection (38.8% vs. 25.1%, P = .016), shorter duration of complete remission (24 vs. 52 months), and with shorter median survival (29 vs. 88 months, P = .001). |
KEY STUDIES SHOWING CLINICAL BENEFIT OF TIGHT GLYCEMIC CONTROL
A summary of several key studies that demonstrated the clinical benefit of tight glycemic control is shown in Table 2. These studies successfully separated the intensively and conventionally managed study groups according blood glucose. Although trials using glucose‐insulin‐potassium infusions (GIK) such that blood glucose was lowered have shown benefit for patients who have had myocardial infarctions4547 or cardiac surgery,48 not all GIK studies have yielded positive results. The negative results of the CREATE‐ECLA study suggest that GIK therapy per se is not beneficial unless it reduces blood glucose.49 In the setting of acute myocardial infarction, the DIGAMI 2 trial and the HI‐5 trials failed to achieve the intended separation between treatment groups.50, 51 It has been speculated that if insulin is delivered so as to not achieve normoglycemia, then hyperinsulinemia in the presence of hyperglycemia actually may be proinflammatory.52
| Population and/or setting and patients | Study design and/or intervention | Principal findings |
|---|---|---|
| Patients with diabetes mellitus and with hyperglycemia > 11 mmol/L or similar hyperglycemia without known diabetes who had had acute myocardial within the preceding 24 hours.4547 There were 620 patients, of whom 15 of the controls and 10 patients in the intensive group had no prior diagnosis of diabetes. | Prospective, randomized, controlled clinical trial. Controls received standard treatment. Treatment group received infusion of glucose‐insulin for at least 24 hours, followed by multiple injections of subcutaneous insulin for at least 3 months. | Overall mortality after 1 year was l9% in the insulin group compared to 26% among controls (P < .05). The most frequent cause of death of all patients was congestive heart failure. The benefit continued for at least 3.5 years, with an absolute reduction in mortality of 11%. |
| Diabetic cardiac surgery population.40, 5557 To date, 5510 cardiac surgery patients who were either admitted or discharged with the diagnosis of diabetes have been studied. | Prospective nonrandomized study of the effects of hyperglycemia and its pharmacologic reduction with intensive intravenous insulin regimens on outcomes. | The 3‐day average of perioperative BG (3‐BG) correlated with mortality (P < .0001, odds ratio 2.0 per 50 mg/dL increase in 3‐BG). Continuous intravenous insulin infusion independently reduced risk of death 60% (RR = 0.4, P < .001). Length of stay in the CABG population increased by 1 day for every increase of 50 mg/dL in 3‐BG. |
| Critically ill surgical patients receiving mechanical ventilation. Overall, 13% of 1548 participants had previously diagnosed diabetes. | Prospective, randomized, controlled clinical trial. Intravenous insulin infusion targeting BG 80‐110 mg/dL was initiated in the intensive group for BG > 110 mg/dL. Intravenous insulin infusion targeting BG 180‐200 was initiated in the conventional group for BG > 215 mg/dL. A whole‐blood glucose method using a gas analyzer was employed to determine arterial blood glucose. | In the group assigned to intensive insulin therapy mortality was reduced during intensive care from 8.0% with conventional treatment to 4.6% (P < .04), or a risk reduction of 42%. In the group remaining in the ICU for more than 5 days, mortality was reduced from 20.2% with conventional treatment to 10.6% with intensive insulin therapy; P = .005. The intensive group experienced reductions in overall in‐hospital mortality of 34%, in bloodstream infections of 46%, and in acute renal failure requiring dialysis or hemofiltration of 41%. Also reduced were the need for red‐cell transfusions, critical‐illness neuropathy, duration of mechanical ventilation, and length of stay in the ICU. |
| Critically ill medical patients. The intention to treat group included 1200 participants, of whom 16.9% had a history of diabetes.54 | Randomized controlled clinical trial, as above. | In the intensive group, in‐hospital mortality was not significantly reduced, and among the 433 who stayed in the ICU for less than 3 days, mortality was actually higher. However, in the intention‐to‐treat group there was reduction in newly acquired kidney injury, prolonged mechanical ventilation, and length of stay in the ICU and in the hospital. Among the 767 patients who stayed in the ICU for 3 or more days, intensive insulin therapy reduced in‐hospital mortality from 52.5% to 43.0%. |
| Patients within 24 hours of having an acute stroke and who had poststroke hyperglycemia. The results of the first 452 patients recruited to the GIST‐UK study showed that 15.3% had previously recognized diabetes.62 | Randomized controlled clinical trial. The intensive group received glucose‐potassium‐insulin (GKI) infusion, and the conventional group received saline infusion. | Although mean glucose declined in both groups, the GKI infusion safely achieved separation of groups by blood glucose. Outcomes are pending. |
The findings of the negative intensive insulin studies do not offset the evidence favoring glycemic control, derived from studies that actually achieved the lowering of blood glucose in the intensive groups, such as the successful prospectively designed trials in myocardial infarction or surgical or medical intensive care units15, 45, 47, 53, 54 and the long‐running, large, prospectively monitored Portland series utilizing insulin infusion for cardiac surgery,40, 5557 which have demonstrated the beneficial effects of euglycemia on mortality and morbidity, or the findings of Krinsley, reported elsewhere in this issue.58, 59 The well‐recognized correlation between outcomes of acute stroke and poststroke hyperglycemia has led to the design of a multicenter trial of glucose‐insulin‐potassium infusion for stroke, in which separation of groups by blood glucose has been achieved.6062 The success of GIK therapy in controlling hyperglycemia depends in part on the particular formulation of the infusion as it matches patient needs, and it is probable that insulin infusion following stroke is capable of safely achieving even tighter glycemic control than GIK.63 Intravenous insulin infusion therapy is more difficult to conduct than GIK therapy. However, because of concern about the proinflammatory and prothrombotic effects of hyperglycemia and recognition of the occasional failure of GIK infusions to control hyperglycemia and of the anti‐inflammatory, antithrombotic, and vasodilatory actions of insulin, there have been calls for additional trials of insulin infusions (as opposed to glucose‐insulin infusions) for both acute myocardial infarction and stroke.52, 64
HYPOGLYCEMIA
Serious or fatal sequelae of hypoglycemia are the principal safety risks in intensive insulin management.9, 65 Case ascertainment cannot be assured by glucose averaging methods but instead requires a method of searching for isolated episodes of hypoglycemia.10 One of the most dreaded consequences of nonfatal hypoglycemia is permanent impairment of intellectual function. Because many euglycemic medically ill patients experience alteration of sensorium while acutely ill, there is a risk that the consequences of an actual episode of severe hypoglycemia will be ascribed to other comorbidities, overlooking or misattributing the altered cognitive function that may persist at discharge to causes other than the obvious iatrogenic one. Because there is an increased risk of hypoglycemia during intensive insulin therapy, controversy has arisen over glycemic targets, especially among critically ill nonsurgical patients.54, 6669
On the other hand, the consequences of hypoglycemia during intensive intravenous insulin therapy in a surgical ICU were said to be negligible.15 In hospitalized elderly patients, hypoglycemia in association with increased mortality risk may not be an independent predictor.70 In the critical care unit, predictors of hypoglycemia are identifiable after the introduction of strict glycemic control that include not only insulin therapy but also CVVH treatment with bicarbonate substitution fluid, discontinuation of nutrition without insulin adjustment, prior diagnosis of diabetes mellitus, sepsis, and the need for inotropic or vasopressor drugs.71 A prudent policy for the future would be not only to treat hypoglycemia promptly when it does occur, with attention to subsequent monitoring to avoid relapse or recurrence, but also to actively introduce strategies for predicting the risk of hypoglycemia and preventing it, especially when high risk is identified.10 The fear of hypoglycemia should not paralyze efforts to achieve better glycemic control in hospitalized patients.
UNANSWERED QUESTIONS AND VISION FOR THE FUTURE
On general wards, 38% of admitted patients may have hyperglycemia.72 As shown in the Leuven, Belgium, study, to achieve the target BG of less than 110 mg/dL in the intensive group in the surgical critical care unit, it was necessary to administer intravenous insulin infusion to essentially all patients. In a study that utilized continuous glucose monitoring, normoglycemia in patients in the intensive care unit was achieved as little as 22% of the time.73
An actively debated subject is how best to assess hospital performance in glycemic control (glucometrics). Hospitals need to show satisfactory control of variability between patients and within the treatment course of individual patients. That is, it is not sufficient to be satisfied with reasonable results of average blood glucose, using blood glucose as the unit of observation (where n is the number of blood glucose determinations). Alternatives are to use patient day or individual patient as a unit of observation (where n is the number of patient days and the number of patients, respectively). Time‐weighting methods are cumbersome but increase the validity of the averaging method used. When the patient is the unit of observation, possible measures include a per‐patient blood glucose average, percentage of blood glucose measurements with certain ranges, time spent within certain blood glucose ranges, or area under the curve of blood glucose versus time. Caveats about glucometrics pertain to both intravenous insulin infusion and also subcutaneous insulin management.74
Although awaiting additional evidence, diabetes experts have widely accepted the proposition that hospitals should focus on prevention of hyperglycemia as an important patient safety factor.75 Although the target range for glycemic control remains controversial, many students of the subject have endorsed the recommendations mentioned in the lead article in this issue, with the understanding that these criteria were developed from the results of the Leuven, Belgium, study, in which a whole blood glucose analyzer was used for measurement, and that the method of measurement of blood glucose must be considered in interpreting applicability of the target range at individual hospitals, many of which use plasma‐correlated methods yielding higher results. However, in new settings and for medical conditions that have not yet been rigorously evaluated by clinical trials, it is an unanswered question whether intensification of glycemic control can be achieved safely outside the critical care unit and, if so, what type of insulin therapy should be used and for what conditions the benefits would outweigh the risks and justify the costs.
Most inpatient management probably will continue to be conducted using subcutaneous injection therapy,7682 designed to match carbohydrate exposure through the appropriate use of analogs or conventional insulin products. One argument for the use of insulin analogs in the hospital, using basal‐prandial‐correction therapy, is the probability of reducing hypoglycemia and getting closer to target range control among patients who are eating but who are at risk for abrupt suspension of meals. Aggressive subcutaneous management strategies are likely to be most effective when standardized protocols, order sets, and informative computerized order entry systems gain widespread hospital acceptance.
If the importance of gaining glycemic control is highly time dependent and if hyperglycemia is uncontrolled, then a strong argument can be made for routine use of intravenous insulin infusion. For appropriately selected patients, intravenous insulin infusion is cost effective,83, 84 and its use can be extended to appropriate patients outside the critical care unit.8587 Many hospitals have protocols for intravenous insulin but use them only sporadically. For patients who already are in the intensive care setting, it is imperative to develop policies that require introduction of intravenous insulin infusion at a given glycemic threshold. On general wards that lack sufficient staffing to conduct intravenous insulin therapy, it is appropriate either to transfer candidate patients to a ward that has adequate staffing when medical condition requires improved control or to develop policies and procedures that will extend the use of intravenous insulin infusion to general wards. In the future, new technologies can be envisioned that will unburden nursing staff, making intravenous insulin infusion realizable as the treatment of choice for hemodynamically stable patients in most hospital settings. These technologies will include continuous monitoring of blood glucose, dose‐defining algorithms, and the eventual development of a fully automated closed‐loop system of monitoring and delivery that might automatically match insulin delivery to carbohydrate exposure and patient insulin sensitivity.8893
Until recently it had been argued that hospitalization was not the time in the life of a patient to insist on tight glycemic control. Hyperglycemia was understood to be a consequence of medical stress.1 It was well known that infection, sepsis, or other medical stress might exacerbate hyperglycemia or promote a diabetic crisis.25 Admittedly, the severity of hyperglycemia among patients who have diabetes was thought to predict the risk for hospitalization with infection as well as the outcome of the infectious condition.68 However, until recently strict glycemic control in the hospital was not strongly advocated because hypoglycemia might occur and might be directly and uniquely traceable to actions taken in the hospital.9, 10 Furthermore, the complications of diabetes were thought to be divided into acute metabolic emergencies and chronic tissue complications such as polyneuropathy, retinopathy, nephropathy, and macrovascular disease that would have evolved over a period longer than the duration of hospitalization, and the possibility that short‐term hyperglycemia might affect outcomes was considered unproven.
The purpose of this article is to define the specific populations, disorders, and hospital settings for which there now is strong evidence that short‐term glycemic control will affect the outcome of a course of hospital treatment.
PHYSIOLOGIC LINK BETWEEN HYPERGLYCEMIA AND ADVERSE OUTCOMES
Five years ago a caregiver would not have been likely to think of glycemic control as a contributing factor when considering specific complex problems such as pump failure or arrhythmia after cardiac surgery, long‐term mortality after myocardial infarction, acute renal failure, the need for transfusion during treatment of a complicated surgical illness, or prolonged dependence on a ventilator in the surgical ICU. Although it now known that these and other complications are linked to hospital hyperglycemia, the mechanisms of harm are several steps removed from the hyperglycemia itself. The causes of these adverse outcomes are multifactorial. The causal dependence of the injury on hyperglycemia is not easy to see. In fact, it required randomized prospectively designed trials to convincingly demonstrate the contributory role of hyperglycemia to these and other adverse outcomes.
Now that this link has been convincingly demonstrated, there is intense interest in discovering the probable mechanisms by which control of hyperglycemia and specifically the use of insulin might improve outcomes.1114 Mortality, predominantly sepsis related, was the primary outcome for which the Leuven, Belgium, report of 2001 on the surgical ICU showed improvement.15 Simplistically, in seeking a mechanism of benefit with respect to sepsis, it might be argued that if gross hyperglycemia were prevented in patients with surgical wounds, improvement of host defenses against infective organisms might be expected.1622 However, additional mechanisms of protection probably should be invoked, including those by which glycemic control and specifically insulin therapy affect endothelial function and the coagulation pathway, thus improving the ability of a patient to withstand and recover from sepsis. Insulin promotes beneficial nitric oxide synthase activation (e‐NOS) in capillary endothelium.2326 In patients with prolonged critical illness, intensive insulin therapy lowers ICAM‐1 levels, reflecting reduced endothelial activation. Whereas e‐NOS exerts a beneficial endothelial effect, hepatic iNOS activation is harmful. One proposed mechanism of benefit from adequate insulin therapy is suppression of excessive hepatic iNOS‐induced release of circulating NO, which might contribute to endothelial dysfunction, organ failure, and death.27
Additional proposed mechanisms of hyperglycemia‐induced harm to hospitalized patients, some potentially specifically reduced by insulin therapy, resemble those discussed in relation to macrovascular disease and include activation of inflammatory cytokines, matrix metalloproteinases, and adhesion molecules, and the adverse arrhythmogenic effects of elevated circulating nonesterified fatty acids (Figure 1).2834

CRITICAL WINDOW OF TIME
The results of several retrospectively or prospectively conducted single‐institution observational studies suggest there is a critical window of time within which clinicians must get it right, when attempting glycemic control or else jeopardize therapeutic goals, such as duration of remission in treatment of acute lymphocytic leukemia35 or avoidance of acute rejection in renal allograft surgery.36 Additionally, delayed risk of infection appears to be linked to previous glycemic control during specific early time frames surrounding surgery, renal transplantation, admission for trauma, and induction chemotherapy for leukemia (Table 1).3541 For patients who have diabetes, the potential to reduce the consequences of infections through intensified glycemic control probably begins prior to admission and in the hospital has still not been fully realized.4244
| Patients | Ascertainment of hyperglycemia | Delayed events among patients with early hyperglycemia |
|---|---|---|
| 100 postoperative uninfected diabetic patients undergoing elective surgery, monitored prospectively37 | First postoperative day | Postoperative nosocomial infection rate within 14 days was 2.7 times higher for patients having at least one BG > 220 mg/dL (33.3% vs. 11.5%). |
| 990 historical controls and 595 patients in the interventional group of postoperative cardiac diabetic patients40 | First 2 postoperative days | Incidence of deep sternal wound infection was reduced from 2.4% to 1.5% (P < 0.02) after introduction of protocol to maintain mean BG < 200 mg/dL. |
| 423 renal allograft recipients receiving their first cadaveric transplant36 | First 100 hours; first day. | A mean of 10.8 2.3 days after transplantation, 70% of patients developed postoperative infection, and after a mean of 7.7 2.6 days, 40% developed acute rejection. Every patient with mean BG over 200 mg/dL during the first 100 hours developed postoperative infection. On the first postoperative day the mean BG had been 248.4 mg/dL among those developing infection and 167.4 mg/dL among those without infection (P < .001), and the mean BG had been 270 mmol/L among those developing rejection and 194 mmol/L among those without rejection. |
| 516 trauma patients admitted to the ICU38 | Either of first 2 hospital days | Hyperglycemia 200 mg/dL was associated with a higher infection rate (32% vs. 22%, P = .04) and with greater mortality (34% vs. 13%, P < .0001) |
| 275 patients having lower‐extremity peripheral vascular surgery39 | First 48 hours | Postoperative infections within 30 days were 5.1 times more frequent in the top quartile for BG versus the lowest quartile (confidence interval 1.6‐17.1, P = .007). |
| 278 adult patients receiving induction chemotherapy for acute lymphocytic leukemia35 | First 30 days | Hyperglycemia defined as 2 BG 200 mg/dL was associated with a greater likelihood of sepsis (16.5% vs. 8.0%, P = .03) or any complicated infection (38.8% vs. 25.1%, P = .016), shorter duration of complete remission (24 vs. 52 months), and with shorter median survival (29 vs. 88 months, P = .001). |
KEY STUDIES SHOWING CLINICAL BENEFIT OF TIGHT GLYCEMIC CONTROL
A summary of several key studies that demonstrated the clinical benefit of tight glycemic control is shown in Table 2. These studies successfully separated the intensively and conventionally managed study groups according blood glucose. Although trials using glucose‐insulin‐potassium infusions (GIK) such that blood glucose was lowered have shown benefit for patients who have had myocardial infarctions4547 or cardiac surgery,48 not all GIK studies have yielded positive results. The negative results of the CREATE‐ECLA study suggest that GIK therapy per se is not beneficial unless it reduces blood glucose.49 In the setting of acute myocardial infarction, the DIGAMI 2 trial and the HI‐5 trials failed to achieve the intended separation between treatment groups.50, 51 It has been speculated that if insulin is delivered so as to not achieve normoglycemia, then hyperinsulinemia in the presence of hyperglycemia actually may be proinflammatory.52
| Population and/or setting and patients | Study design and/or intervention | Principal findings |
|---|---|---|
| Patients with diabetes mellitus and with hyperglycemia > 11 mmol/L or similar hyperglycemia without known diabetes who had had acute myocardial within the preceding 24 hours.4547 There were 620 patients, of whom 15 of the controls and 10 patients in the intensive group had no prior diagnosis of diabetes. | Prospective, randomized, controlled clinical trial. Controls received standard treatment. Treatment group received infusion of glucose‐insulin for at least 24 hours, followed by multiple injections of subcutaneous insulin for at least 3 months. | Overall mortality after 1 year was l9% in the insulin group compared to 26% among controls (P < .05). The most frequent cause of death of all patients was congestive heart failure. The benefit continued for at least 3.5 years, with an absolute reduction in mortality of 11%. |
| Diabetic cardiac surgery population.40, 5557 To date, 5510 cardiac surgery patients who were either admitted or discharged with the diagnosis of diabetes have been studied. | Prospective nonrandomized study of the effects of hyperglycemia and its pharmacologic reduction with intensive intravenous insulin regimens on outcomes. | The 3‐day average of perioperative BG (3‐BG) correlated with mortality (P < .0001, odds ratio 2.0 per 50 mg/dL increase in 3‐BG). Continuous intravenous insulin infusion independently reduced risk of death 60% (RR = 0.4, P < .001). Length of stay in the CABG population increased by 1 day for every increase of 50 mg/dL in 3‐BG. |
| Critically ill surgical patients receiving mechanical ventilation. Overall, 13% of 1548 participants had previously diagnosed diabetes. | Prospective, randomized, controlled clinical trial. Intravenous insulin infusion targeting BG 80‐110 mg/dL was initiated in the intensive group for BG > 110 mg/dL. Intravenous insulin infusion targeting BG 180‐200 was initiated in the conventional group for BG > 215 mg/dL. A whole‐blood glucose method using a gas analyzer was employed to determine arterial blood glucose. | In the group assigned to intensive insulin therapy mortality was reduced during intensive care from 8.0% with conventional treatment to 4.6% (P < .04), or a risk reduction of 42%. In the group remaining in the ICU for more than 5 days, mortality was reduced from 20.2% with conventional treatment to 10.6% with intensive insulin therapy; P = .005. The intensive group experienced reductions in overall in‐hospital mortality of 34%, in bloodstream infections of 46%, and in acute renal failure requiring dialysis or hemofiltration of 41%. Also reduced were the need for red‐cell transfusions, critical‐illness neuropathy, duration of mechanical ventilation, and length of stay in the ICU. |
| Critically ill medical patients. The intention to treat group included 1200 participants, of whom 16.9% had a history of diabetes.54 | Randomized controlled clinical trial, as above. | In the intensive group, in‐hospital mortality was not significantly reduced, and among the 433 who stayed in the ICU for less than 3 days, mortality was actually higher. However, in the intention‐to‐treat group there was reduction in newly acquired kidney injury, prolonged mechanical ventilation, and length of stay in the ICU and in the hospital. Among the 767 patients who stayed in the ICU for 3 or more days, intensive insulin therapy reduced in‐hospital mortality from 52.5% to 43.0%. |
| Patients within 24 hours of having an acute stroke and who had poststroke hyperglycemia. The results of the first 452 patients recruited to the GIST‐UK study showed that 15.3% had previously recognized diabetes.62 | Randomized controlled clinical trial. The intensive group received glucose‐potassium‐insulin (GKI) infusion, and the conventional group received saline infusion. | Although mean glucose declined in both groups, the GKI infusion safely achieved separation of groups by blood glucose. Outcomes are pending. |
The findings of the negative intensive insulin studies do not offset the evidence favoring glycemic control, derived from studies that actually achieved the lowering of blood glucose in the intensive groups, such as the successful prospectively designed trials in myocardial infarction or surgical or medical intensive care units15, 45, 47, 53, 54 and the long‐running, large, prospectively monitored Portland series utilizing insulin infusion for cardiac surgery,40, 5557 which have demonstrated the beneficial effects of euglycemia on mortality and morbidity, or the findings of Krinsley, reported elsewhere in this issue.58, 59 The well‐recognized correlation between outcomes of acute stroke and poststroke hyperglycemia has led to the design of a multicenter trial of glucose‐insulin‐potassium infusion for stroke, in which separation of groups by blood glucose has been achieved.6062 The success of GIK therapy in controlling hyperglycemia depends in part on the particular formulation of the infusion as it matches patient needs, and it is probable that insulin infusion following stroke is capable of safely achieving even tighter glycemic control than GIK.63 Intravenous insulin infusion therapy is more difficult to conduct than GIK therapy. However, because of concern about the proinflammatory and prothrombotic effects of hyperglycemia and recognition of the occasional failure of GIK infusions to control hyperglycemia and of the anti‐inflammatory, antithrombotic, and vasodilatory actions of insulin, there have been calls for additional trials of insulin infusions (as opposed to glucose‐insulin infusions) for both acute myocardial infarction and stroke.52, 64
HYPOGLYCEMIA
Serious or fatal sequelae of hypoglycemia are the principal safety risks in intensive insulin management.9, 65 Case ascertainment cannot be assured by glucose averaging methods but instead requires a method of searching for isolated episodes of hypoglycemia.10 One of the most dreaded consequences of nonfatal hypoglycemia is permanent impairment of intellectual function. Because many euglycemic medically ill patients experience alteration of sensorium while acutely ill, there is a risk that the consequences of an actual episode of severe hypoglycemia will be ascribed to other comorbidities, overlooking or misattributing the altered cognitive function that may persist at discharge to causes other than the obvious iatrogenic one. Because there is an increased risk of hypoglycemia during intensive insulin therapy, controversy has arisen over glycemic targets, especially among critically ill nonsurgical patients.54, 6669
On the other hand, the consequences of hypoglycemia during intensive intravenous insulin therapy in a surgical ICU were said to be negligible.15 In hospitalized elderly patients, hypoglycemia in association with increased mortality risk may not be an independent predictor.70 In the critical care unit, predictors of hypoglycemia are identifiable after the introduction of strict glycemic control that include not only insulin therapy but also CVVH treatment with bicarbonate substitution fluid, discontinuation of nutrition without insulin adjustment, prior diagnosis of diabetes mellitus, sepsis, and the need for inotropic or vasopressor drugs.71 A prudent policy for the future would be not only to treat hypoglycemia promptly when it does occur, with attention to subsequent monitoring to avoid relapse or recurrence, but also to actively introduce strategies for predicting the risk of hypoglycemia and preventing it, especially when high risk is identified.10 The fear of hypoglycemia should not paralyze efforts to achieve better glycemic control in hospitalized patients.
UNANSWERED QUESTIONS AND VISION FOR THE FUTURE
On general wards, 38% of admitted patients may have hyperglycemia.72 As shown in the Leuven, Belgium, study, to achieve the target BG of less than 110 mg/dL in the intensive group in the surgical critical care unit, it was necessary to administer intravenous insulin infusion to essentially all patients. In a study that utilized continuous glucose monitoring, normoglycemia in patients in the intensive care unit was achieved as little as 22% of the time.73
An actively debated subject is how best to assess hospital performance in glycemic control (glucometrics). Hospitals need to show satisfactory control of variability between patients and within the treatment course of individual patients. That is, it is not sufficient to be satisfied with reasonable results of average blood glucose, using blood glucose as the unit of observation (where n is the number of blood glucose determinations). Alternatives are to use patient day or individual patient as a unit of observation (where n is the number of patient days and the number of patients, respectively). Time‐weighting methods are cumbersome but increase the validity of the averaging method used. When the patient is the unit of observation, possible measures include a per‐patient blood glucose average, percentage of blood glucose measurements with certain ranges, time spent within certain blood glucose ranges, or area under the curve of blood glucose versus time. Caveats about glucometrics pertain to both intravenous insulin infusion and also subcutaneous insulin management.74
Although awaiting additional evidence, diabetes experts have widely accepted the proposition that hospitals should focus on prevention of hyperglycemia as an important patient safety factor.75 Although the target range for glycemic control remains controversial, many students of the subject have endorsed the recommendations mentioned in the lead article in this issue, with the understanding that these criteria were developed from the results of the Leuven, Belgium, study, in which a whole blood glucose analyzer was used for measurement, and that the method of measurement of blood glucose must be considered in interpreting applicability of the target range at individual hospitals, many of which use plasma‐correlated methods yielding higher results. However, in new settings and for medical conditions that have not yet been rigorously evaluated by clinical trials, it is an unanswered question whether intensification of glycemic control can be achieved safely outside the critical care unit and, if so, what type of insulin therapy should be used and for what conditions the benefits would outweigh the risks and justify the costs.
Most inpatient management probably will continue to be conducted using subcutaneous injection therapy,7682 designed to match carbohydrate exposure through the appropriate use of analogs or conventional insulin products. One argument for the use of insulin analogs in the hospital, using basal‐prandial‐correction therapy, is the probability of reducing hypoglycemia and getting closer to target range control among patients who are eating but who are at risk for abrupt suspension of meals. Aggressive subcutaneous management strategies are likely to be most effective when standardized protocols, order sets, and informative computerized order entry systems gain widespread hospital acceptance.
If the importance of gaining glycemic control is highly time dependent and if hyperglycemia is uncontrolled, then a strong argument can be made for routine use of intravenous insulin infusion. For appropriately selected patients, intravenous insulin infusion is cost effective,83, 84 and its use can be extended to appropriate patients outside the critical care unit.8587 Many hospitals have protocols for intravenous insulin but use them only sporadically. For patients who already are in the intensive care setting, it is imperative to develop policies that require introduction of intravenous insulin infusion at a given glycemic threshold. On general wards that lack sufficient staffing to conduct intravenous insulin therapy, it is appropriate either to transfer candidate patients to a ward that has adequate staffing when medical condition requires improved control or to develop policies and procedures that will extend the use of intravenous insulin infusion to general wards. In the future, new technologies can be envisioned that will unburden nursing staff, making intravenous insulin infusion realizable as the treatment of choice for hemodynamically stable patients in most hospital settings. These technologies will include continuous monitoring of blood glucose, dose‐defining algorithms, and the eventual development of a fully automated closed‐loop system of monitoring and delivery that might automatically match insulin delivery to carbohydrate exposure and patient insulin sensitivity.8893
- ,,.Stress‐induced hyperglycemia.Crit Care Clin.2001;17(1):107–124.
- ,,,,.Hyperosmolarity and acidosis in diabetes mellitus: a three‐year experience in Rhode Island.J Gen Intern Med.1991:495–502.
- ,,.Prognostic factors in the diabetic hyperosmolar state.J Am Geriatr Soc.1987;35:737–741.
- ,,.Predisposing factors for the diabetic hyperosmolar state.Arch Intern Med.1987;147:499–501.
- .The diabetic hyperosmolar state.Clin Geriatr Med.1990;6:797–806.
- ,,,,,.Bacteremia in adult diabetic patients.Diabetes Care.1991;14(2):89–94.
- ,,,,,.Influence of diabetes mellitus and glycaemic control on the characteristics and outcome of common infections.Diabet Med.1996:457–463.
- ,,.Diabetes and the risk of infection‐related mortality in the U.S.Diabetes Care.2001;24:1044–1049.
- ,,.Hypoglycemia in hospitalized patients.N Engl J Med.1986;315:1245–1250.
- ,,, et al.Hospital hypoglycemia: not only treatment but also prevention.Endocr Pract.2004;10(suppl 2):71–80.
- .Effect of insulin therapy on nonglycemic variables during acute illness.Endocr Pract.2004;10(suppl 2):63–70.
- .Molecular mechanisms by which metabolic control may improve outcomes.Endocr Pract.2004;10(suppl 2):57–62.
- ,,,,.Insulin infusion in acute illness.J Clin Invest.2005;115:2069–2072.
- ,,.Intensive insulin therapy in the intensive care unit: update on clinical impact and mechanisms of action.Endocr Pract.2006;12(suppl 3):14–21.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,.Impaired granulocyte adherence. A reversible defect in host defense in patients with poorly controlled diabetes.Diabetes.1978;27:677–681.
- ,,.Impaired leukocyte function in patients with poorly controlled diabetes.Diabetes.1974;23(1):9–15.
- ,,, et al.The effect of diabetes mellitus on chemotactic and bactericidal activity of human polymorphonuclear leukocytes.Diabetes Res Clin Pract.1987;4:27–35.
- ,,,,.Polymorphonuclear leukocytes in non‐insulin‐dependent diabetes mellitus: abnormalities in metabolism and function.Ann Intern Med.1995;123:919–924.
- ,,,,.Agonist‐dependent failure of neutrophil function in diabetes correlates with extent of hyperglycemia.J Leukoc Biol.2001;70:395–404.
- ,,,,,.Insulin increases neutrophil count and phagocytic capacity after cardiac surgery.Anesth Analg.2002;94:1113–1119.
- ,,,.In vivo evidences that insulin regulates human polymorphonuclear neutrophil functions.J Leukoc Biol.2004;76:1104–1110.
- ,.Insulin‐stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells.J Clin Invest.1996;98:894–898.
- ,,,,.Insulin‐mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release.J Clin Invest.1994;94:1172–1179.
- ,.Effect of insulin on human aortic endothelial nitric oxide synthase.Metabolism.2000;49(2):147–50.
- ,.Nitric oxide, platelet function, myocardial infarction and reperfusion therapies.Heart Fail Rev.2003;8(1):47–54.
- ,,, et al.Intensive insulin therapy protects the endothelium of critically ill patients.J Clin Invest.2005;115:2277–2286.
- ,,, et al.Elevated circulating free fatty acid levels impair endothelium‐dependent vasodilation.J Clin Invest.1997;100:1230–1239.
- ,,, et al.Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti‐inflammatory effect?J Clin Endocrinol Metab.2001;86:3257–3265.
- ,,.The anti‐inflammatory and potential anti‐atherogenic effect of insulin: a new paradigm.Diabetologia.2002;45:924–930.
- ,,,.The potential influence of inflammation and insulin resistance on the pathogenesis and treatment of atherosclerosis‐related complications in type 2 diabetes.J Clin Endocrinol Metab.2003;88:2422–2429.
- ,,.The potential therapeutic role of insulin in acute myocardial infarction in patients admitted to intensive care and in those with unspecified hyperglycemia.Diabetes Care.2003;26:516–519.
- ,,.Insulin treatment improves the systemic inflammatory reaction to severe trauma.Ann Surg.2004;239:553–560.
- ,,, et al.Anti‐inflammatory and profibrinolytic effect of insulin in acute ST‐segment‐elevation myocardial infarction.Circulation.2004;109:849–854.
- .Relation between the duration of remission and hyperglycemia in induction chemotherapy for acute lymphocytic leukemia.Cancer.2004;100:1179–1185.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enteral Nutr.1998;22(2):77–81.
- ,,,,.Relationship of early hyperglycemia to mortality in trauma patients.J Trauma.2004;56:1058–1062.
- ,,,,.Early post‐operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study.Eur J Vasc Endovasc Surg.2004;5:520–525.
- ,,,.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thorac Surg.1999;67:352–362.
- ,.Effects on outcome of in‐hospital transition from intravenous insulin infusion to subcutaneous therapy.Am J Cardiol.2006;98:557–564.
- ,,,.The impact of diabetes in patients with necrotizing soft tissue infections.Surg Infect.2005;6:427–438.
- ,,,.Infections in patients with diabetes mellitus.N Engl J Med.1999;341:1906–1912.
- ,,.Quantifying the risk of infectious diseases for people with diabetes.Diabetes Care.2003;26:510–513.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.J Am Coll Cardiol.1995;26:57–65.
- ,,, et al.Effects of insulin treatment on cause‐specific one‐year mortality and morbidity in diabetic patients with acute myocardial infarction.Eur Heart J.1996;17:1337–1344.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,,,,.Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events.Circulation.2004;109:1497–1502.
- .Effect of Glucose‐Insulin‐Potassium Infusion on Mortality in Patients With Acute ST‐Segment Elevation Myocardial Infarction: The CREATE‐ECLA Randomized Controlled Trial.JAMA.2005;293:437–446.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity.Eur Heart J.2005;26:650–661.
- ,,.The Hyperglycemia: Intensive Insulin Infusion In Infarction (HI‐5) Study: A randomized controlled trial of insulin infusion therapy for myocardial infarction.Diabetes Care.2006;29:765–770.
- .Inpatient diabetes: review of data from the cardiac care unit.Endocr Pract.2006;12(suppl 3):27–34.
- ,.Reduction of nosocomial infections in the surgical intensive‐care unit by strict glycemic control.Endocr Pract.2004;10(suppl 2):46–52.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356–361.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125:1007–1021.
- ,.Clinical effects of hyperglycemia in the cardiac surgery population: the Portland diabetic project.Endocr Pract.2006;12(suppl 3):22–26.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000.
- .Decreased mortality of critically ill patients with the use of an intensive glycemic management protocol.Mayo Clin Proc.2003;78:1471–1478.
- ,,, et al.Admission glucose level and clinical outcomes in the NINDS rt‐PA Stroke. Trial.Neurology.2002;59:669–74.
- ,,,,,.Glucose potassium insulin infusions in the treatment of acute stroke patients with mild to moderate hyperglycemia: the Glucose Insulin in Stroke. Trial (GIST).Stroke.1999;30:793–799.
- ,,,.Poststroke hyperglycemia: natural history and immediate management.Stroke.2004;35(1):122–126.
- ,,,.IV insulin during acute cerebral infarction in diabetic patients.Neurology.2004;62:1441–1442.
- ,,,.Hyperglycemia, insulin, and acute ischemic stroke: a mechanistic justification for a trial of insulin infusion therapy.Stroke.2006;37(1):267–273.
- ,,.Hypoglycemia and cardiac arrest in a critically ill patient on strict glycemic control.Anesth Analg.2006;102:549–551.
- ,,.Strict glucose control in the critically ill.Br Med J.2006;332:865–866.
- .Intensive Insulin in Intensive Care.N Engl J Med.2006;354:516–518.
- ,.Counterpoint: Inpatient glucose management: a premature call to arms?Diabetes Care.2005;28:976–979.
- ,,,.Glucose control and mortality in critically ill patients.JAMA.2003;290:2041–2047.
- ,,, et al.Hypoglycemia as a predictor of mortality in hospitalized elderly patients.Arch Intern Med.2003;163:1825–1829.
- ,,, et al.Predisposing factors for hypoglycemia in the intensive care unit.Crit Care Med.2006;34:96–101.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,.Intensive insulin therapy in the intensive care unit: assessment by continuous glucose monitoring.Diabetes Care.2006;29:1750–1756.
- ,,,.No patient left behind: evaluation and design of intravenous insulin infusion algorithms.Endocr Pract.2006;12(suppl 3):72–78.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,,.Eliminating Inpatient Sliding‐Scale Insulin: A reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,.70/30 Insulin algorithm versus sliding scale insulin.Ann Pharmacother.2005;39:1606–1609.
- ,.Hospital management of hyperglycemia.Clin Diabetes.2004;22(2):81–88.
- ,.Subcutaneous insulin therapy in the hospital setting: issues, concerns, and implementation.Endocr Pract.2004;10(suppl 2):81–88.
- ,,,,.Hyperglycemia in the hospital.Diabetes Spectr.2005;18(1):20–27.
- .Insulin Analogues.N Engl J Med.2005;352:174–183.
- .Insulin management of diabetic patients on general medical and surgical floors.Endocr Pract.2006;12(suppl 3):86–90.
- ,,, et al.Improved perioperative glycemic control by continuous insulin infusion under supervision of an endocrinologist does not increase costs in patients with diabetes.Endocr Pract.2004;10(2):112–118.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project.Endocr Pract.2004;10(suppl 2):21–33.
- ,,,.New insulin infusion protocol improves blood glucose control in hospitalized patients without increasing hypoglycemia.J Qual Patient Saf.2005;31:141–147.
- ,,,,.Optimizing hospital use of intravenous insulin therapy: improved management of hyperglycemia and error reduction with a new nomogram.Endocr Pract.2005;11:240–253.
- ,,,,.Implementation of a new intravenous insulin method on intermediate‐care units in hospitalized patients.Diabetes Educ.2005;31:818–823.
- ,,.Clinical results of an updated insulin infusion protocol in critically ill patients.Diabetes Spectr.2005;18(3):188–191.
- ,,.Glucommander: A computer‐directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation.Diabetes Care.2005;28:2418–2423.
- ,,, et al.Improving hyperglycemia management in the intensive care unit: preliminary report of a nurse‐driven quality improvement project using a redesigned insulin infusion algorithm.Diabetes Educ.2006;32:394–403.
- ,,, et al.Performance of a dose‐defining insulin infusion protocol among trauma service ICU admissions.Diabetes Technol Ther.2006;8:476–488.
- ,,, et al.A simple insulin‐nutrition protocol for tight glycemic control in critical illness: development and protocol comparison.Diabetes Technol Ther.2006;8:191–206.
- ,,, et al.Evaluation of the impact of chiropodist care in the secondary prevention of foot ulcerations in diabetic subjects.Diabetes Care.2003;26:1691–1695.
- ,,.Stress‐induced hyperglycemia.Crit Care Clin.2001;17(1):107–124.
- ,,,,.Hyperosmolarity and acidosis in diabetes mellitus: a three‐year experience in Rhode Island.J Gen Intern Med.1991:495–502.
- ,,.Prognostic factors in the diabetic hyperosmolar state.J Am Geriatr Soc.1987;35:737–741.
- ,,.Predisposing factors for the diabetic hyperosmolar state.Arch Intern Med.1987;147:499–501.
- .The diabetic hyperosmolar state.Clin Geriatr Med.1990;6:797–806.
- ,,,,,.Bacteremia in adult diabetic patients.Diabetes Care.1991;14(2):89–94.
- ,,,,,.Influence of diabetes mellitus and glycaemic control on the characteristics and outcome of common infections.Diabet Med.1996:457–463.
- ,,.Diabetes and the risk of infection‐related mortality in the U.S.Diabetes Care.2001;24:1044–1049.
- ,,.Hypoglycemia in hospitalized patients.N Engl J Med.1986;315:1245–1250.
- ,,, et al.Hospital hypoglycemia: not only treatment but also prevention.Endocr Pract.2004;10(suppl 2):71–80.
- .Effect of insulin therapy on nonglycemic variables during acute illness.Endocr Pract.2004;10(suppl 2):63–70.
- .Molecular mechanisms by which metabolic control may improve outcomes.Endocr Pract.2004;10(suppl 2):57–62.
- ,,,,.Insulin infusion in acute illness.J Clin Invest.2005;115:2069–2072.
- ,,.Intensive insulin therapy in the intensive care unit: update on clinical impact and mechanisms of action.Endocr Pract.2006;12(suppl 3):14–21.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,.Impaired granulocyte adherence. A reversible defect in host defense in patients with poorly controlled diabetes.Diabetes.1978;27:677–681.
- ,,.Impaired leukocyte function in patients with poorly controlled diabetes.Diabetes.1974;23(1):9–15.
- ,,, et al.The effect of diabetes mellitus on chemotactic and bactericidal activity of human polymorphonuclear leukocytes.Diabetes Res Clin Pract.1987;4:27–35.
- ,,,,.Polymorphonuclear leukocytes in non‐insulin‐dependent diabetes mellitus: abnormalities in metabolism and function.Ann Intern Med.1995;123:919–924.
- ,,,,.Agonist‐dependent failure of neutrophil function in diabetes correlates with extent of hyperglycemia.J Leukoc Biol.2001;70:395–404.
- ,,,,,.Insulin increases neutrophil count and phagocytic capacity after cardiac surgery.Anesth Analg.2002;94:1113–1119.
- ,,,.In vivo evidences that insulin regulates human polymorphonuclear neutrophil functions.J Leukoc Biol.2004;76:1104–1110.
- ,.Insulin‐stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells.J Clin Invest.1996;98:894–898.
- ,,,,.Insulin‐mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release.J Clin Invest.1994;94:1172–1179.
- ,.Effect of insulin on human aortic endothelial nitric oxide synthase.Metabolism.2000;49(2):147–50.
- ,.Nitric oxide, platelet function, myocardial infarction and reperfusion therapies.Heart Fail Rev.2003;8(1):47–54.
- ,,, et al.Intensive insulin therapy protects the endothelium of critically ill patients.J Clin Invest.2005;115:2277–2286.
- ,,, et al.Elevated circulating free fatty acid levels impair endothelium‐dependent vasodilation.J Clin Invest.1997;100:1230–1239.
- ,,, et al.Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti‐inflammatory effect?J Clin Endocrinol Metab.2001;86:3257–3265.
- ,,.The anti‐inflammatory and potential anti‐atherogenic effect of insulin: a new paradigm.Diabetologia.2002;45:924–930.
- ,,,.The potential influence of inflammation and insulin resistance on the pathogenesis and treatment of atherosclerosis‐related complications in type 2 diabetes.J Clin Endocrinol Metab.2003;88:2422–2429.
- ,,.The potential therapeutic role of insulin in acute myocardial infarction in patients admitted to intensive care and in those with unspecified hyperglycemia.Diabetes Care.2003;26:516–519.
- ,,.Insulin treatment improves the systemic inflammatory reaction to severe trauma.Ann Surg.2004;239:553–560.
- ,,, et al.Anti‐inflammatory and profibrinolytic effect of insulin in acute ST‐segment‐elevation myocardial infarction.Circulation.2004;109:849–854.
- .Relation between the duration of remission and hyperglycemia in induction chemotherapy for acute lymphocytic leukemia.Cancer.2004;100:1179–1185.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enteral Nutr.1998;22(2):77–81.
- ,,,,.Relationship of early hyperglycemia to mortality in trauma patients.J Trauma.2004;56:1058–1062.
- ,,,,.Early post‐operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study.Eur J Vasc Endovasc Surg.2004;5:520–525.
- ,,,.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thorac Surg.1999;67:352–362.
- ,.Effects on outcome of in‐hospital transition from intravenous insulin infusion to subcutaneous therapy.Am J Cardiol.2006;98:557–564.
- ,,,.The impact of diabetes in patients with necrotizing soft tissue infections.Surg Infect.2005;6:427–438.
- ,,,.Infections in patients with diabetes mellitus.N Engl J Med.1999;341:1906–1912.
- ,,.Quantifying the risk of infectious diseases for people with diabetes.Diabetes Care.2003;26:510–513.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.J Am Coll Cardiol.1995;26:57–65.
- ,,, et al.Effects of insulin treatment on cause‐specific one‐year mortality and morbidity in diabetic patients with acute myocardial infarction.Eur Heart J.1996;17:1337–1344.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,,,,.Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events.Circulation.2004;109:1497–1502.
- .Effect of Glucose‐Insulin‐Potassium Infusion on Mortality in Patients With Acute ST‐Segment Elevation Myocardial Infarction: The CREATE‐ECLA Randomized Controlled Trial.JAMA.2005;293:437–446.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity.Eur Heart J.2005;26:650–661.
- ,,.The Hyperglycemia: Intensive Insulin Infusion In Infarction (HI‐5) Study: A randomized controlled trial of insulin infusion therapy for myocardial infarction.Diabetes Care.2006;29:765–770.
- .Inpatient diabetes: review of data from the cardiac care unit.Endocr Pract.2006;12(suppl 3):27–34.
- ,.Reduction of nosocomial infections in the surgical intensive‐care unit by strict glycemic control.Endocr Pract.2004;10(suppl 2):46–52.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356–361.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125:1007–1021.
- ,.Clinical effects of hyperglycemia in the cardiac surgery population: the Portland diabetic project.Endocr Pract.2006;12(suppl 3):22–26.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000.
- .Decreased mortality of critically ill patients with the use of an intensive glycemic management protocol.Mayo Clin Proc.2003;78:1471–1478.
- ,,, et al.Admission glucose level and clinical outcomes in the NINDS rt‐PA Stroke. Trial.Neurology.2002;59:669–74.
- ,,,,,.Glucose potassium insulin infusions in the treatment of acute stroke patients with mild to moderate hyperglycemia: the Glucose Insulin in Stroke. Trial (GIST).Stroke.1999;30:793–799.
- ,,,.Poststroke hyperglycemia: natural history and immediate management.Stroke.2004;35(1):122–126.
- ,,,.IV insulin during acute cerebral infarction in diabetic patients.Neurology.2004;62:1441–1442.
- ,,,.Hyperglycemia, insulin, and acute ischemic stroke: a mechanistic justification for a trial of insulin infusion therapy.Stroke.2006;37(1):267–273.
- ,,.Hypoglycemia and cardiac arrest in a critically ill patient on strict glycemic control.Anesth Analg.2006;102:549–551.
- ,,.Strict glucose control in the critically ill.Br Med J.2006;332:865–866.
- .Intensive Insulin in Intensive Care.N Engl J Med.2006;354:516–518.
- ,.Counterpoint: Inpatient glucose management: a premature call to arms?Diabetes Care.2005;28:976–979.
- ,,,.Glucose control and mortality in critically ill patients.JAMA.2003;290:2041–2047.
- ,,, et al.Hypoglycemia as a predictor of mortality in hospitalized elderly patients.Arch Intern Med.2003;163:1825–1829.
- ,,, et al.Predisposing factors for hypoglycemia in the intensive care unit.Crit Care Med.2006;34:96–101.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,.Intensive insulin therapy in the intensive care unit: assessment by continuous glucose monitoring.Diabetes Care.2006;29:1750–1756.
- ,,,.No patient left behind: evaluation and design of intravenous insulin infusion algorithms.Endocr Pract.2006;12(suppl 3):72–78.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,,.Eliminating Inpatient Sliding‐Scale Insulin: A reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,.70/30 Insulin algorithm versus sliding scale insulin.Ann Pharmacother.2005;39:1606–1609.
- ,.Hospital management of hyperglycemia.Clin Diabetes.2004;22(2):81–88.
- ,.Subcutaneous insulin therapy in the hospital setting: issues, concerns, and implementation.Endocr Pract.2004;10(suppl 2):81–88.
- ,,,,.Hyperglycemia in the hospital.Diabetes Spectr.2005;18(1):20–27.
- .Insulin Analogues.N Engl J Med.2005;352:174–183.
- .Insulin management of diabetic patients on general medical and surgical floors.Endocr Pract.2006;12(suppl 3):86–90.
- ,,, et al.Improved perioperative glycemic control by continuous insulin infusion under supervision of an endocrinologist does not increase costs in patients with diabetes.Endocr Pract.2004;10(2):112–118.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project.Endocr Pract.2004;10(suppl 2):21–33.
- ,,,.New insulin infusion protocol improves blood glucose control in hospitalized patients without increasing hypoglycemia.J Qual Patient Saf.2005;31:141–147.
- ,,,,.Optimizing hospital use of intravenous insulin therapy: improved management of hyperglycemia and error reduction with a new nomogram.Endocr Pract.2005;11:240–253.
- ,,,,.Implementation of a new intravenous insulin method on intermediate‐care units in hospitalized patients.Diabetes Educ.2005;31:818–823.
- ,,.Clinical results of an updated insulin infusion protocol in critically ill patients.Diabetes Spectr.2005;18(3):188–191.
- ,,.Glucommander: A computer‐directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation.Diabetes Care.2005;28:2418–2423.
- ,,, et al.Improving hyperglycemia management in the intensive care unit: preliminary report of a nurse‐driven quality improvement project using a redesigned insulin infusion algorithm.Diabetes Educ.2006;32:394–403.
- ,,, et al.Performance of a dose‐defining insulin infusion protocol among trauma service ICU admissions.Diabetes Technol Ther.2006;8:476–488.
- ,,, et al.A simple insulin‐nutrition protocol for tight glycemic control in critical illness: development and protocol comparison.Diabetes Technol Ther.2006;8:191–206.
- ,,, et al.Evaluation of the impact of chiropodist care in the secondary prevention of foot ulcerations in diabetic subjects.Diabetes Care.2003;26:1691–1695.
Charts to Screens
Federal policy makers have set 2014 as the target year for all Americans to have an electronic health record. While researchers claim that health information technology (IT) holds great promise to improve the quality and efficiency of healthcare delivery, the path to effecting the transition to computer-based documentation systems is fraught with obstacles. In addition to large initial capital investments for upgraded hardware and software, hospitals face other barriers to IT adoption. The challenges experienced by hospitals making this change include steep learning curves, workflow disruptions, and time delays.

—Richard Todd, MD
Advancements and Glitches
A 2005 American Hospital Association (AHA) survey of 900 community hospitals found a wide range of IT usage. Some hospitals have completed installation of bar coding for medication management, while a small minority are using advanced computerized physician order entry (CPOE) systems.1 Typical of many hospitals in the AHA survey, Abbott Northwestern Hospital in Minneapolis chose an incremental IT implementation approach.
Academic hospitalist Debra L. Burgy, MD, is the lead physician in Abbott Northwestern General Medicine Associates Group, affiliated with the internal medicine program at the University of Minnesota (Minneapolis), where she is also adjunct assistant professor of medicine. Hers was the first group of physicians to go live with the hospital’s electronic documentation system 16 months ago, in July of 2005.
“We went up on July 1 because we thought it might be an advantage to have a long weekend with a lower census,” she recalls. As it turned out, her group of academic hospitalists was caught short-staffed on the holiday weekend, having to adjust to their new IT roles, take care of patients, and orient the brand-new interns.
“It was kind of a sad weekend for me,” she remarks wryly.
Of the launch in July 2005, Dr. Burgy observes that the learning curve “was longer than I expected, but once you achieve it and you’re adept at most of the functions I do find [electronic documentation] better overall in many ways.”
One advantage: As an academic hospitalist, she consults with her residents and emergency department admitting physicians in real time by pulling up patients’ charts from any location.
Dr. Burgy and her colleagues still find the time required to enter the narrative part of the patient’s history of present illness difficult, as well as the discharge notes. Another bug: The system is designed to prompt the physician to complete medication reconciliation (Medication Administration Record, or MAR) at admission, transfer, and discharge. Because the medications are not organized in alphabetical order or side by side, however, the logistics of reconciling more than a few medications can be frustrating.
“Most of us end up printing out the current MAR, which seems to defeat the purpose of the computerized record,” says Dr. Burgy.
A Staged Approach
According to Mary A. Dallas, MD, chief medical information officer for Presbyterian Healthcare Services (PHS), an integrated healthcare delivery network in Albuquerque, N.M., PHS launched CPOE in the main hospital’s inpatient services area as the final step in the pharmacy automation process designed to improve patient safety and prevent medication errors.
Five years ago, the main hospital began the process of developing a closed-loop pharmacy order system. Now, with this system in place, medication orders go directly from the physician’s fingertips to a pharmacy work queue. The verified drug order is then messaged to the pharmacy robot for packaging. On the floor, nurses’ hand-held devices flash a message that the drug order is ready. Upon delivery to the floor, a nurse scans the bar code on the packaged medication, matches it to the patient’s bracelet bar code, and scans his or her badge before administering the medication. This verifies the 5 “Rs” of medication safety: right medication, right dose, right route, right patient, and right time, as well as concurrently creating the electronic MAR.
As the former medical director of the hospital’s Adult Hospitalist Service, Dr. Dallas understands the physician’s point of view. When launching the hospital’s CPOE, she was aware that, “especially in the hospitalist arena, we were adding some extra learning curve to their day.”
She also admits, “It does take longer to log onto a computer system and wait for the program to boot than it does to just scribble a medication order on paper. There’s no way to avoid that.”
As she has worked to build order sets tailored for various specialties, however, Dr. Dallas has been sensitive to challenges that can be softened. Automatic prompts at the point of order entry are carefully monitored, she points out because “surplus of medication” alert pop-ups can sometimes produce physician “alert fatigue,” and doctors may begin to ignore—rather than address—the alerts. “You have to start light and then work to get more stringent as people tolerate and get used to that system,” she says.
Getting Physician Buy-In
The launch of the CPOE system at Presbyterian Hospital in Albuquerque was the fourth such experience for Richard Todd, MD, medical director of the hospital’s Adult Hospitalist Group. He sees speed—or the lack thereof—as a major barrier to physician adoption of computerized documentation systems. He has observed that some hospitals don’t invest in the appropriate hardware required to handle such technically demanding software. As a result, a user may have to wait 25 or 30 seconds for an order entry system to boot up.
“That is an eternity in computing time,” he says, and a physician who experiences this difficulty more than twice may no longer have the patience to work with the system.

—Richard H. Bailey, MD
Physicians should be part of the IT design and selection process, Dr. Todd believes. “To get a successful adoption by physicians, the engineers need to come to the physicians’ table and not the other way around,” he says, pointing to the success of Wiz Order, Vanderbilt University School of Medicine’s order-entry system, which is part of an electronic medical record custom-built with input from doctors.
“The biggest mistake you can make is to have physicians feel that you’re forcing something down their throats that slows them down,” says Dr. Todd. “Every physician is under tremendous time pressure to get the primary job done, so if you do anything that even makes them perceive that it’s going to make them less efficient, you’re not going to get buy-in.”
S. Trent Rosenbloom, MD, MPH, trained as an internist and pediatrician and spent some time as a hospitalist early in his career. Currently, he is assistant professor in the departments of Biomedical Informatics, Internal Medicine, and Pediatrics, and in the School of Nursing at Vanderbilt University Medical Center. He and his research colleagues have investigated the factors which influence providers’ perceptions of clinical documentation tools.2
“The key issue is not so much time, but the perception of time and work flow,” he explains. “It [a computerized documentation system] could be twice as fast, but if I have to go out of my way to do it, then I might perceive it as taking more effort and more time.”
For instance, writing a drug order on paper can appear to be a faster process than finding a computer, sitting down, logging on to the system, finding the patient in the menu, opening the patient file, and then entering a drug order. Dr. Rosenbloom points out that when physicians think about these two processes, however, they may not factor in the other time factors for the paper order, such as walking to the chart, finding the chart, turning to the right page, and entering the drug order. And although computer systems are not error-free, CPOE tends to reduce transcription and other errors that in themselves can be time-consuming, if not life threatening, for the patient.
Keys to Success
Sources agreed that IT adoption by physicians increases in direct proportion to their participation in the process. “[Hospitalists and other physicians] need to make sure that their hospital includes physicians in every step of the due diligence process: looking through systems, going to the sales, actually banging on the product, and making sure that they perceive it as meeting their needs,” advises Dr. Rosenbloom.
Vendors differ in their methods for bringing client hospitals online. “A staged approach is probably best, based on what we know currently,” he suggests.
Finally, flexibility is key—for vendors and users. Dr. Rosenbloom advises teams to “expect to fail, and learn from that.” It’s important to recognize, he says, “that even if you’re putting in a computer system that has been implemented in 50% of hospitals—which hasn’t yet happened—there are idiosyncrasies and differences in your own center that will cause the implementation process to be different.”
Given hospitalists’ interest in hospital processes, leading the IT adoption effort is a natural role for hospitalist leaders, believes Richard H. Bailey, MD, medical director of Inpatient Care and Hospitalist Services at Saint Clare’s Hospital in Weston, Wis. “I was one of the most computer illiterate people I knew,” he relates. “But somehow, I got thrown into the role. We took a potential lemon, embraced it early on, and made lemonade.” TH
Gretchen Henkel also writes about benchmarking hospital medicine programs in this issue.
References
- American Hospital Association. Forward momentum: hospital use of information technology. October 2005. Available at: www.aha.org/aha/research-and-trends/AHA-policy-research/2005.html. Last accessed November 29, 2006.
- Rosenbloom ST, Crow AN, Blackford JU, et al. Cognitive factors influencing perceptions of clinical documentation tools. J Biomed Inform. 2006 Jul 8; [Epub ahead of print].
Federal policy makers have set 2014 as the target year for all Americans to have an electronic health record. While researchers claim that health information technology (IT) holds great promise to improve the quality and efficiency of healthcare delivery, the path to effecting the transition to computer-based documentation systems is fraught with obstacles. In addition to large initial capital investments for upgraded hardware and software, hospitals face other barriers to IT adoption. The challenges experienced by hospitals making this change include steep learning curves, workflow disruptions, and time delays.

—Richard Todd, MD
Advancements and Glitches
A 2005 American Hospital Association (AHA) survey of 900 community hospitals found a wide range of IT usage. Some hospitals have completed installation of bar coding for medication management, while a small minority are using advanced computerized physician order entry (CPOE) systems.1 Typical of many hospitals in the AHA survey, Abbott Northwestern Hospital in Minneapolis chose an incremental IT implementation approach.
Academic hospitalist Debra L. Burgy, MD, is the lead physician in Abbott Northwestern General Medicine Associates Group, affiliated with the internal medicine program at the University of Minnesota (Minneapolis), where she is also adjunct assistant professor of medicine. Hers was the first group of physicians to go live with the hospital’s electronic documentation system 16 months ago, in July of 2005.
“We went up on July 1 because we thought it might be an advantage to have a long weekend with a lower census,” she recalls. As it turned out, her group of academic hospitalists was caught short-staffed on the holiday weekend, having to adjust to their new IT roles, take care of patients, and orient the brand-new interns.
“It was kind of a sad weekend for me,” she remarks wryly.
Of the launch in July 2005, Dr. Burgy observes that the learning curve “was longer than I expected, but once you achieve it and you’re adept at most of the functions I do find [electronic documentation] better overall in many ways.”
One advantage: As an academic hospitalist, she consults with her residents and emergency department admitting physicians in real time by pulling up patients’ charts from any location.
Dr. Burgy and her colleagues still find the time required to enter the narrative part of the patient’s history of present illness difficult, as well as the discharge notes. Another bug: The system is designed to prompt the physician to complete medication reconciliation (Medication Administration Record, or MAR) at admission, transfer, and discharge. Because the medications are not organized in alphabetical order or side by side, however, the logistics of reconciling more than a few medications can be frustrating.
“Most of us end up printing out the current MAR, which seems to defeat the purpose of the computerized record,” says Dr. Burgy.
A Staged Approach
According to Mary A. Dallas, MD, chief medical information officer for Presbyterian Healthcare Services (PHS), an integrated healthcare delivery network in Albuquerque, N.M., PHS launched CPOE in the main hospital’s inpatient services area as the final step in the pharmacy automation process designed to improve patient safety and prevent medication errors.
Five years ago, the main hospital began the process of developing a closed-loop pharmacy order system. Now, with this system in place, medication orders go directly from the physician’s fingertips to a pharmacy work queue. The verified drug order is then messaged to the pharmacy robot for packaging. On the floor, nurses’ hand-held devices flash a message that the drug order is ready. Upon delivery to the floor, a nurse scans the bar code on the packaged medication, matches it to the patient’s bracelet bar code, and scans his or her badge before administering the medication. This verifies the 5 “Rs” of medication safety: right medication, right dose, right route, right patient, and right time, as well as concurrently creating the electronic MAR.
As the former medical director of the hospital’s Adult Hospitalist Service, Dr. Dallas understands the physician’s point of view. When launching the hospital’s CPOE, she was aware that, “especially in the hospitalist arena, we were adding some extra learning curve to their day.”
She also admits, “It does take longer to log onto a computer system and wait for the program to boot than it does to just scribble a medication order on paper. There’s no way to avoid that.”
As she has worked to build order sets tailored for various specialties, however, Dr. Dallas has been sensitive to challenges that can be softened. Automatic prompts at the point of order entry are carefully monitored, she points out because “surplus of medication” alert pop-ups can sometimes produce physician “alert fatigue,” and doctors may begin to ignore—rather than address—the alerts. “You have to start light and then work to get more stringent as people tolerate and get used to that system,” she says.
Getting Physician Buy-In
The launch of the CPOE system at Presbyterian Hospital in Albuquerque was the fourth such experience for Richard Todd, MD, medical director of the hospital’s Adult Hospitalist Group. He sees speed—or the lack thereof—as a major barrier to physician adoption of computerized documentation systems. He has observed that some hospitals don’t invest in the appropriate hardware required to handle such technically demanding software. As a result, a user may have to wait 25 or 30 seconds for an order entry system to boot up.
“That is an eternity in computing time,” he says, and a physician who experiences this difficulty more than twice may no longer have the patience to work with the system.

—Richard H. Bailey, MD
Physicians should be part of the IT design and selection process, Dr. Todd believes. “To get a successful adoption by physicians, the engineers need to come to the physicians’ table and not the other way around,” he says, pointing to the success of Wiz Order, Vanderbilt University School of Medicine’s order-entry system, which is part of an electronic medical record custom-built with input from doctors.
“The biggest mistake you can make is to have physicians feel that you’re forcing something down their throats that slows them down,” says Dr. Todd. “Every physician is under tremendous time pressure to get the primary job done, so if you do anything that even makes them perceive that it’s going to make them less efficient, you’re not going to get buy-in.”
S. Trent Rosenbloom, MD, MPH, trained as an internist and pediatrician and spent some time as a hospitalist early in his career. Currently, he is assistant professor in the departments of Biomedical Informatics, Internal Medicine, and Pediatrics, and in the School of Nursing at Vanderbilt University Medical Center. He and his research colleagues have investigated the factors which influence providers’ perceptions of clinical documentation tools.2
“The key issue is not so much time, but the perception of time and work flow,” he explains. “It [a computerized documentation system] could be twice as fast, but if I have to go out of my way to do it, then I might perceive it as taking more effort and more time.”
For instance, writing a drug order on paper can appear to be a faster process than finding a computer, sitting down, logging on to the system, finding the patient in the menu, opening the patient file, and then entering a drug order. Dr. Rosenbloom points out that when physicians think about these two processes, however, they may not factor in the other time factors for the paper order, such as walking to the chart, finding the chart, turning to the right page, and entering the drug order. And although computer systems are not error-free, CPOE tends to reduce transcription and other errors that in themselves can be time-consuming, if not life threatening, for the patient.
Keys to Success
Sources agreed that IT adoption by physicians increases in direct proportion to their participation in the process. “[Hospitalists and other physicians] need to make sure that their hospital includes physicians in every step of the due diligence process: looking through systems, going to the sales, actually banging on the product, and making sure that they perceive it as meeting their needs,” advises Dr. Rosenbloom.
Vendors differ in their methods for bringing client hospitals online. “A staged approach is probably best, based on what we know currently,” he suggests.
Finally, flexibility is key—for vendors and users. Dr. Rosenbloom advises teams to “expect to fail, and learn from that.” It’s important to recognize, he says, “that even if you’re putting in a computer system that has been implemented in 50% of hospitals—which hasn’t yet happened—there are idiosyncrasies and differences in your own center that will cause the implementation process to be different.”
Given hospitalists’ interest in hospital processes, leading the IT adoption effort is a natural role for hospitalist leaders, believes Richard H. Bailey, MD, medical director of Inpatient Care and Hospitalist Services at Saint Clare’s Hospital in Weston, Wis. “I was one of the most computer illiterate people I knew,” he relates. “But somehow, I got thrown into the role. We took a potential lemon, embraced it early on, and made lemonade.” TH
Gretchen Henkel also writes about benchmarking hospital medicine programs in this issue.
References
- American Hospital Association. Forward momentum: hospital use of information technology. October 2005. Available at: www.aha.org/aha/research-and-trends/AHA-policy-research/2005.html. Last accessed November 29, 2006.
- Rosenbloom ST, Crow AN, Blackford JU, et al. Cognitive factors influencing perceptions of clinical documentation tools. J Biomed Inform. 2006 Jul 8; [Epub ahead of print].
Federal policy makers have set 2014 as the target year for all Americans to have an electronic health record. While researchers claim that health information technology (IT) holds great promise to improve the quality and efficiency of healthcare delivery, the path to effecting the transition to computer-based documentation systems is fraught with obstacles. In addition to large initial capital investments for upgraded hardware and software, hospitals face other barriers to IT adoption. The challenges experienced by hospitals making this change include steep learning curves, workflow disruptions, and time delays.

—Richard Todd, MD
Advancements and Glitches
A 2005 American Hospital Association (AHA) survey of 900 community hospitals found a wide range of IT usage. Some hospitals have completed installation of bar coding for medication management, while a small minority are using advanced computerized physician order entry (CPOE) systems.1 Typical of many hospitals in the AHA survey, Abbott Northwestern Hospital in Minneapolis chose an incremental IT implementation approach.
Academic hospitalist Debra L. Burgy, MD, is the lead physician in Abbott Northwestern General Medicine Associates Group, affiliated with the internal medicine program at the University of Minnesota (Minneapolis), where she is also adjunct assistant professor of medicine. Hers was the first group of physicians to go live with the hospital’s electronic documentation system 16 months ago, in July of 2005.
“We went up on July 1 because we thought it might be an advantage to have a long weekend with a lower census,” she recalls. As it turned out, her group of academic hospitalists was caught short-staffed on the holiday weekend, having to adjust to their new IT roles, take care of patients, and orient the brand-new interns.
“It was kind of a sad weekend for me,” she remarks wryly.
Of the launch in July 2005, Dr. Burgy observes that the learning curve “was longer than I expected, but once you achieve it and you’re adept at most of the functions I do find [electronic documentation] better overall in many ways.”
One advantage: As an academic hospitalist, she consults with her residents and emergency department admitting physicians in real time by pulling up patients’ charts from any location.
Dr. Burgy and her colleagues still find the time required to enter the narrative part of the patient’s history of present illness difficult, as well as the discharge notes. Another bug: The system is designed to prompt the physician to complete medication reconciliation (Medication Administration Record, or MAR) at admission, transfer, and discharge. Because the medications are not organized in alphabetical order or side by side, however, the logistics of reconciling more than a few medications can be frustrating.
“Most of us end up printing out the current MAR, which seems to defeat the purpose of the computerized record,” says Dr. Burgy.
A Staged Approach
According to Mary A. Dallas, MD, chief medical information officer for Presbyterian Healthcare Services (PHS), an integrated healthcare delivery network in Albuquerque, N.M., PHS launched CPOE in the main hospital’s inpatient services area as the final step in the pharmacy automation process designed to improve patient safety and prevent medication errors.
Five years ago, the main hospital began the process of developing a closed-loop pharmacy order system. Now, with this system in place, medication orders go directly from the physician’s fingertips to a pharmacy work queue. The verified drug order is then messaged to the pharmacy robot for packaging. On the floor, nurses’ hand-held devices flash a message that the drug order is ready. Upon delivery to the floor, a nurse scans the bar code on the packaged medication, matches it to the patient’s bracelet bar code, and scans his or her badge before administering the medication. This verifies the 5 “Rs” of medication safety: right medication, right dose, right route, right patient, and right time, as well as concurrently creating the electronic MAR.
As the former medical director of the hospital’s Adult Hospitalist Service, Dr. Dallas understands the physician’s point of view. When launching the hospital’s CPOE, she was aware that, “especially in the hospitalist arena, we were adding some extra learning curve to their day.”
She also admits, “It does take longer to log onto a computer system and wait for the program to boot than it does to just scribble a medication order on paper. There’s no way to avoid that.”
As she has worked to build order sets tailored for various specialties, however, Dr. Dallas has been sensitive to challenges that can be softened. Automatic prompts at the point of order entry are carefully monitored, she points out because “surplus of medication” alert pop-ups can sometimes produce physician “alert fatigue,” and doctors may begin to ignore—rather than address—the alerts. “You have to start light and then work to get more stringent as people tolerate and get used to that system,” she says.
Getting Physician Buy-In
The launch of the CPOE system at Presbyterian Hospital in Albuquerque was the fourth such experience for Richard Todd, MD, medical director of the hospital’s Adult Hospitalist Group. He sees speed—or the lack thereof—as a major barrier to physician adoption of computerized documentation systems. He has observed that some hospitals don’t invest in the appropriate hardware required to handle such technically demanding software. As a result, a user may have to wait 25 or 30 seconds for an order entry system to boot up.
“That is an eternity in computing time,” he says, and a physician who experiences this difficulty more than twice may no longer have the patience to work with the system.

—Richard H. Bailey, MD
Physicians should be part of the IT design and selection process, Dr. Todd believes. “To get a successful adoption by physicians, the engineers need to come to the physicians’ table and not the other way around,” he says, pointing to the success of Wiz Order, Vanderbilt University School of Medicine’s order-entry system, which is part of an electronic medical record custom-built with input from doctors.
“The biggest mistake you can make is to have physicians feel that you’re forcing something down their throats that slows them down,” says Dr. Todd. “Every physician is under tremendous time pressure to get the primary job done, so if you do anything that even makes them perceive that it’s going to make them less efficient, you’re not going to get buy-in.”
S. Trent Rosenbloom, MD, MPH, trained as an internist and pediatrician and spent some time as a hospitalist early in his career. Currently, he is assistant professor in the departments of Biomedical Informatics, Internal Medicine, and Pediatrics, and in the School of Nursing at Vanderbilt University Medical Center. He and his research colleagues have investigated the factors which influence providers’ perceptions of clinical documentation tools.2
“The key issue is not so much time, but the perception of time and work flow,” he explains. “It [a computerized documentation system] could be twice as fast, but if I have to go out of my way to do it, then I might perceive it as taking more effort and more time.”
For instance, writing a drug order on paper can appear to be a faster process than finding a computer, sitting down, logging on to the system, finding the patient in the menu, opening the patient file, and then entering a drug order. Dr. Rosenbloom points out that when physicians think about these two processes, however, they may not factor in the other time factors for the paper order, such as walking to the chart, finding the chart, turning to the right page, and entering the drug order. And although computer systems are not error-free, CPOE tends to reduce transcription and other errors that in themselves can be time-consuming, if not life threatening, for the patient.
Keys to Success
Sources agreed that IT adoption by physicians increases in direct proportion to their participation in the process. “[Hospitalists and other physicians] need to make sure that their hospital includes physicians in every step of the due diligence process: looking through systems, going to the sales, actually banging on the product, and making sure that they perceive it as meeting their needs,” advises Dr. Rosenbloom.
Vendors differ in their methods for bringing client hospitals online. “A staged approach is probably best, based on what we know currently,” he suggests.
Finally, flexibility is key—for vendors and users. Dr. Rosenbloom advises teams to “expect to fail, and learn from that.” It’s important to recognize, he says, “that even if you’re putting in a computer system that has been implemented in 50% of hospitals—which hasn’t yet happened—there are idiosyncrasies and differences in your own center that will cause the implementation process to be different.”
Given hospitalists’ interest in hospital processes, leading the IT adoption effort is a natural role for hospitalist leaders, believes Richard H. Bailey, MD, medical director of Inpatient Care and Hospitalist Services at Saint Clare’s Hospital in Weston, Wis. “I was one of the most computer illiterate people I knew,” he relates. “But somehow, I got thrown into the role. We took a potential lemon, embraced it early on, and made lemonade.” TH
Gretchen Henkel also writes about benchmarking hospital medicine programs in this issue.
References
- American Hospital Association. Forward momentum: hospital use of information technology. October 2005. Available at: www.aha.org/aha/research-and-trends/AHA-policy-research/2005.html. Last accessed November 29, 2006.
- Rosenbloom ST, Crow AN, Blackford JU, et al. Cognitive factors influencing perceptions of clinical documentation tools. J Biomed Inform. 2006 Jul 8; [Epub ahead of print].
Skilled Labor
Emalie Gibbons Baker, CNM, arrives at St Mary’s Hospital at 7 a.m. Half an hour later she is scrubbed in, first assisting a community OB/GYN who performs a repeat cesarean delivery. By 9 a.m., the baby is safely delivered and resting with his mom, and the physician is seeing patients in her private practice a few miles from the hospital.
Now Baker cares for a nervous first-time mother in labor, sitting close to her bed and softly encouraging her through each contraction, praising her efforts when each pain subsides. She steps out to monitor an outpatient who has arrived for a labor check, performs a sterile speculum exam, and confirms the well-being of the fetus. Then reviews the signs of labor with the expectant mother, gives her a pep talk and a hug, and discharges her.
This is a typical start to a busy day for Baker, a certified nurse-midwife (CNM) laborist at St. Mary’s Hospital in Leonardtown, Md. CNM laborists like Baker work cooperatively with their collaborating physicians and midwife colleagues in the ambulatory setting and in the hospital, leaving their colleagues with time to care for high-risk women in the hospital and to hold office hours in their private practices.
Like most CNM laborists, Baker provides care during labor and the post-partum period for pregnant women and new mothers in the hospital setting. She oversees labor induction, augmentation, and pain management, including epidurals, for patients on the ward, works with the nursing staff, provides hands-on care for patients, provides first assists in cesarean deliveries, and evaluates pregnant patients who present to the emergency department (ED). When a new need arises, Baker can often provide the necessary service, and the list continues to grow as she adds circumcisions and interpretation of fetal fibronectin results to her responsibilities.
Skilled Laborers
Certified nurse-midwives are a valuable addition to the field of hospital-based healthcare. The approximately 7,000 practicing certified nurse-midwives in the United States delivered more than 310,000 babies in 2003, representing more than 10% of the vaginal deliveries in this country.
Many people are unaware that 98% of CNM-attended deliveries in the United States occur in a hospital. Certified nurse-midwives are qualified professionals who have graduated from an accredited university-based program and passed a national certification exam. Baker, like all CNMs, is a registered nurse. She earned her master’s degree in midwifery in 1994 at State University of New York Downstate University, one of 40 midwifery education programs in the United States.
Certified nurse-midwives practice in a variety of settings, including hospital and office-based practices, community health centers, and public health facilities. CNMs are licensed in all 50 states. They are reimbursed by Medicare, are Medicaid-mandated service providers, and are widely included in managed care provider listings. CNMs are experts in the management of normal birth. Studies have demonstrated that the outcomes of nurse-midwifery care are at least equivalent to those of patients managed by physicians for normal maternity care, and patients repeatedly indicate high levels of satisfaction with the care provided by nurse-midwives.
“Having [Baker] at the hospital has been a big selling point for patients. She helps by massaging the patients who are anxious or need things explained to them. I think this provides the patients with a sense of security, and they also appreciate that this is a unique and different service we provide here,” says Valinda Nwadike, MD, an OB/GYN at St. Mary’s County who previously worked with nurse-midwives in a large urban hospital in Washington, D.C. “All in all, having [Baker] on board as a CNM laborist means better patient interaction and increased quality and continuity of care. It is a very useful tool, one that improves both patient care and our quality of life as community physicians. It’s a win-win situation.”
Filling the Gap
The Southern Maryland community served by St. Mary’s Hospital is quickly growing. The hospital serves as the birth site for the nearby Patuxent Naval Air Station. The number of births at the 100-bed facility recently jumped from 600-800 births a year to more than 1,000. The four OB/GYNs serving this county of 90,000 are all in private practice. With growing businesses, these community providers found that juggling busy outpatient schedules with inpatient demands for labor support or hospital-based procedures was resulting in disruption in their clinics, lost revenue, and frustration for them and their patients alike.
Collectively, the community OB/GYNs got together and decided to hire a CNM laborist to help cover the bases. Lawrence Tilley, MD, chief of obstetrics and gynecology at the hospital, had watched the success of the hospitalist model at St. Mary’s and has a certified nurse-midwife on staff at his private office. He finds that offering nurse-midwifery services in his practice acts as a draw for patients. At the hospital, he would like to add more midwives to the staff, for 24/7 coverage.
Hundreds of miles away, in a busy urban healthcare delivery system with different needs and rhythms than those in rural Maryland, the CNM laborist model also fits the bill. At Mt. Sinai Hospital in Chicago Laborist Darryn Dunbar, CNM, attends the births of nurse-midwifery patients served by the Access Community Health Network, a large healthcare organization that manages 44 Federally Qualified Health Centers in the Chicago area. The hospital sees 4,000 births a year, of which close to 10% are attended by midwives. Dunbar is one of two CNM laborists at Mt. Sinai who care for Access midwifery patients, most of whom are on Medicaid. He works solely in the hospital, providing inpatient coverage after hours and on weekends to the clients of a seven-midwife team that, with the addition of his laborist services, is able to offer almost continuous midwifery coverage.
“The goals were to extend midwifery coverage, to increase patient satisfaction and safety by having continuity of on-site care for this group of patients, … to improve staffing ratios in labor and delivery, and [to] provide relief for the residents and house officers,” says Dunbar.
His many years of experience as a full-scope CNM in busy, urban settings with high volume and increased social and medical risk factors make him well suited to providing care in this setting. In addition, in Illinois (as of this year) Dunbar can bill directly for his services under Medicaid and receives 100% of the physician reimbursement rate. He can also serve as the billing provider when he supervises the deliveries of residents in the hospital training program.
Dunbar is a valuable member of the OB team. He receives patients who come in through the ED and helps with OB triage, first assists with cesarean deliveries on occasion, and “runs the board” when the residents are off the floor for educational obligations, are in surgery, or are busy with other patients. The nursing staff, house attendings, and residents have all come to rely on his watchful eyes and helping hands.
Nurse-midwives, according to their professional philosophy, believe the best model of healthcare for a woman and her family is one that promotes a continuous and compassionate partnership, including individualized methods of care guided by the best evidence available, therapeutic use of human presence, and skillful communication. They believe in watchful waiting and non-intervention in normal processes, the appropriate use of interventions and technology for current or potential health problems, and consultation, collaboration, and referral with other members of the healthcare team, as needed, to provide optimal healthcare.
The ability to provide this kind of care is one of the greatest strengths certified nurse-midwives bring to the communities they serve, especially in busy hospitals where the healthcare needs of women and their newborns are great and the demands on providers’ time are high.
Struggling to increase patient safety, decrease costs, and optimize productivity while maintaining good health outcomes, hospitals are increasingly turning to nurse-midwives. Meanwhile, many community OB/GYN providers are reducing their OB call due to burnout and quality of life issues, increased liability insurance premiums, and fear of litigation. Resident work-hours have decreased due to safety concerns and mandated work limits. There is a need for providers who can care for laboring women in the hospital setting, providing continuity and quality of service during their hospital admission. On-site CNM laborists fill in the gap.
Increase Safety and Quality
Having a laborist on board in the OB/GYN department of the hospital helps Yaacov Zamel, MD, a pediatric hospitalist at St. Mary’s, by allowing him to establish a working relationship with someone whose availability and practice patterns he can rely on. He also notes that this improves care for the women and babies. “The better the support for OB, the better it is for newborns. Ultimately, more patients will want to come here,” says Dr. Zamel.
Dr. Nwadike agrees that having a nurse-midwife on staff increases patient safety and the quality of care. “In our community, we needed help specifically with coverage for hospital patients and procedures, and Baker’s skills are the perfect match,” says Dr. Nwadike. “Now she is an invaluable resource and can do all of those things, as well as provide ED triage, care for unassigned patients, or manage precipitous deliveries. As a continuous presence on labor and delivery, she is a great resource for patients and can provide them with more depth, more education. There are really limitless possibilities for her role to expand.”
As experts in caring for healthy women and their newborns, with a history of achieving excellent perinatal outcomes while caring for underserved populations, certified nurse-midwives are ideal healthcare providers for women who arrive at hospitals seeking quality care. “Working as a laborist, I enjoy being able to use all of my skills. That has been very exciting,” says Baker.
At 4:30 p.m., Baker wraps up for the day. The community OB/GYN on call arrives from his office to assume care for a laboring patient on his panel. Baker updates him on the woman’s status, than wraps the patient in a warm parting hug. TH
Rima Jolivet is the senior technical advisor at the American College of Nurse-Midwives.
Emalie Gibbons Baker, CNM, arrives at St Mary’s Hospital at 7 a.m. Half an hour later she is scrubbed in, first assisting a community OB/GYN who performs a repeat cesarean delivery. By 9 a.m., the baby is safely delivered and resting with his mom, and the physician is seeing patients in her private practice a few miles from the hospital.
Now Baker cares for a nervous first-time mother in labor, sitting close to her bed and softly encouraging her through each contraction, praising her efforts when each pain subsides. She steps out to monitor an outpatient who has arrived for a labor check, performs a sterile speculum exam, and confirms the well-being of the fetus. Then reviews the signs of labor with the expectant mother, gives her a pep talk and a hug, and discharges her.
This is a typical start to a busy day for Baker, a certified nurse-midwife (CNM) laborist at St. Mary’s Hospital in Leonardtown, Md. CNM laborists like Baker work cooperatively with their collaborating physicians and midwife colleagues in the ambulatory setting and in the hospital, leaving their colleagues with time to care for high-risk women in the hospital and to hold office hours in their private practices.
Like most CNM laborists, Baker provides care during labor and the post-partum period for pregnant women and new mothers in the hospital setting. She oversees labor induction, augmentation, and pain management, including epidurals, for patients on the ward, works with the nursing staff, provides hands-on care for patients, provides first assists in cesarean deliveries, and evaluates pregnant patients who present to the emergency department (ED). When a new need arises, Baker can often provide the necessary service, and the list continues to grow as she adds circumcisions and interpretation of fetal fibronectin results to her responsibilities.
Skilled Laborers
Certified nurse-midwives are a valuable addition to the field of hospital-based healthcare. The approximately 7,000 practicing certified nurse-midwives in the United States delivered more than 310,000 babies in 2003, representing more than 10% of the vaginal deliveries in this country.
Many people are unaware that 98% of CNM-attended deliveries in the United States occur in a hospital. Certified nurse-midwives are qualified professionals who have graduated from an accredited university-based program and passed a national certification exam. Baker, like all CNMs, is a registered nurse. She earned her master’s degree in midwifery in 1994 at State University of New York Downstate University, one of 40 midwifery education programs in the United States.
Certified nurse-midwives practice in a variety of settings, including hospital and office-based practices, community health centers, and public health facilities. CNMs are licensed in all 50 states. They are reimbursed by Medicare, are Medicaid-mandated service providers, and are widely included in managed care provider listings. CNMs are experts in the management of normal birth. Studies have demonstrated that the outcomes of nurse-midwifery care are at least equivalent to those of patients managed by physicians for normal maternity care, and patients repeatedly indicate high levels of satisfaction with the care provided by nurse-midwives.
“Having [Baker] at the hospital has been a big selling point for patients. She helps by massaging the patients who are anxious or need things explained to them. I think this provides the patients with a sense of security, and they also appreciate that this is a unique and different service we provide here,” says Valinda Nwadike, MD, an OB/GYN at St. Mary’s County who previously worked with nurse-midwives in a large urban hospital in Washington, D.C. “All in all, having [Baker] on board as a CNM laborist means better patient interaction and increased quality and continuity of care. It is a very useful tool, one that improves both patient care and our quality of life as community physicians. It’s a win-win situation.”
Filling the Gap
The Southern Maryland community served by St. Mary’s Hospital is quickly growing. The hospital serves as the birth site for the nearby Patuxent Naval Air Station. The number of births at the 100-bed facility recently jumped from 600-800 births a year to more than 1,000. The four OB/GYNs serving this county of 90,000 are all in private practice. With growing businesses, these community providers found that juggling busy outpatient schedules with inpatient demands for labor support or hospital-based procedures was resulting in disruption in their clinics, lost revenue, and frustration for them and their patients alike.
Collectively, the community OB/GYNs got together and decided to hire a CNM laborist to help cover the bases. Lawrence Tilley, MD, chief of obstetrics and gynecology at the hospital, had watched the success of the hospitalist model at St. Mary’s and has a certified nurse-midwife on staff at his private office. He finds that offering nurse-midwifery services in his practice acts as a draw for patients. At the hospital, he would like to add more midwives to the staff, for 24/7 coverage.
Hundreds of miles away, in a busy urban healthcare delivery system with different needs and rhythms than those in rural Maryland, the CNM laborist model also fits the bill. At Mt. Sinai Hospital in Chicago Laborist Darryn Dunbar, CNM, attends the births of nurse-midwifery patients served by the Access Community Health Network, a large healthcare organization that manages 44 Federally Qualified Health Centers in the Chicago area. The hospital sees 4,000 births a year, of which close to 10% are attended by midwives. Dunbar is one of two CNM laborists at Mt. Sinai who care for Access midwifery patients, most of whom are on Medicaid. He works solely in the hospital, providing inpatient coverage after hours and on weekends to the clients of a seven-midwife team that, with the addition of his laborist services, is able to offer almost continuous midwifery coverage.
“The goals were to extend midwifery coverage, to increase patient satisfaction and safety by having continuity of on-site care for this group of patients, … to improve staffing ratios in labor and delivery, and [to] provide relief for the residents and house officers,” says Dunbar.
His many years of experience as a full-scope CNM in busy, urban settings with high volume and increased social and medical risk factors make him well suited to providing care in this setting. In addition, in Illinois (as of this year) Dunbar can bill directly for his services under Medicaid and receives 100% of the physician reimbursement rate. He can also serve as the billing provider when he supervises the deliveries of residents in the hospital training program.
Dunbar is a valuable member of the OB team. He receives patients who come in through the ED and helps with OB triage, first assists with cesarean deliveries on occasion, and “runs the board” when the residents are off the floor for educational obligations, are in surgery, or are busy with other patients. The nursing staff, house attendings, and residents have all come to rely on his watchful eyes and helping hands.
Nurse-midwives, according to their professional philosophy, believe the best model of healthcare for a woman and her family is one that promotes a continuous and compassionate partnership, including individualized methods of care guided by the best evidence available, therapeutic use of human presence, and skillful communication. They believe in watchful waiting and non-intervention in normal processes, the appropriate use of interventions and technology for current or potential health problems, and consultation, collaboration, and referral with other members of the healthcare team, as needed, to provide optimal healthcare.
The ability to provide this kind of care is one of the greatest strengths certified nurse-midwives bring to the communities they serve, especially in busy hospitals where the healthcare needs of women and their newborns are great and the demands on providers’ time are high.
Struggling to increase patient safety, decrease costs, and optimize productivity while maintaining good health outcomes, hospitals are increasingly turning to nurse-midwives. Meanwhile, many community OB/GYN providers are reducing their OB call due to burnout and quality of life issues, increased liability insurance premiums, and fear of litigation. Resident work-hours have decreased due to safety concerns and mandated work limits. There is a need for providers who can care for laboring women in the hospital setting, providing continuity and quality of service during their hospital admission. On-site CNM laborists fill in the gap.
Increase Safety and Quality
Having a laborist on board in the OB/GYN department of the hospital helps Yaacov Zamel, MD, a pediatric hospitalist at St. Mary’s, by allowing him to establish a working relationship with someone whose availability and practice patterns he can rely on. He also notes that this improves care for the women and babies. “The better the support for OB, the better it is for newborns. Ultimately, more patients will want to come here,” says Dr. Zamel.
Dr. Nwadike agrees that having a nurse-midwife on staff increases patient safety and the quality of care. “In our community, we needed help specifically with coverage for hospital patients and procedures, and Baker’s skills are the perfect match,” says Dr. Nwadike. “Now she is an invaluable resource and can do all of those things, as well as provide ED triage, care for unassigned patients, or manage precipitous deliveries. As a continuous presence on labor and delivery, she is a great resource for patients and can provide them with more depth, more education. There are really limitless possibilities for her role to expand.”
As experts in caring for healthy women and their newborns, with a history of achieving excellent perinatal outcomes while caring for underserved populations, certified nurse-midwives are ideal healthcare providers for women who arrive at hospitals seeking quality care. “Working as a laborist, I enjoy being able to use all of my skills. That has been very exciting,” says Baker.
At 4:30 p.m., Baker wraps up for the day. The community OB/GYN on call arrives from his office to assume care for a laboring patient on his panel. Baker updates him on the woman’s status, than wraps the patient in a warm parting hug. TH
Rima Jolivet is the senior technical advisor at the American College of Nurse-Midwives.
Emalie Gibbons Baker, CNM, arrives at St Mary’s Hospital at 7 a.m. Half an hour later she is scrubbed in, first assisting a community OB/GYN who performs a repeat cesarean delivery. By 9 a.m., the baby is safely delivered and resting with his mom, and the physician is seeing patients in her private practice a few miles from the hospital.
Now Baker cares for a nervous first-time mother in labor, sitting close to her bed and softly encouraging her through each contraction, praising her efforts when each pain subsides. She steps out to monitor an outpatient who has arrived for a labor check, performs a sterile speculum exam, and confirms the well-being of the fetus. Then reviews the signs of labor with the expectant mother, gives her a pep talk and a hug, and discharges her.
This is a typical start to a busy day for Baker, a certified nurse-midwife (CNM) laborist at St. Mary’s Hospital in Leonardtown, Md. CNM laborists like Baker work cooperatively with their collaborating physicians and midwife colleagues in the ambulatory setting and in the hospital, leaving their colleagues with time to care for high-risk women in the hospital and to hold office hours in their private practices.
Like most CNM laborists, Baker provides care during labor and the post-partum period for pregnant women and new mothers in the hospital setting. She oversees labor induction, augmentation, and pain management, including epidurals, for patients on the ward, works with the nursing staff, provides hands-on care for patients, provides first assists in cesarean deliveries, and evaluates pregnant patients who present to the emergency department (ED). When a new need arises, Baker can often provide the necessary service, and the list continues to grow as she adds circumcisions and interpretation of fetal fibronectin results to her responsibilities.
Skilled Laborers
Certified nurse-midwives are a valuable addition to the field of hospital-based healthcare. The approximately 7,000 practicing certified nurse-midwives in the United States delivered more than 310,000 babies in 2003, representing more than 10% of the vaginal deliveries in this country.
Many people are unaware that 98% of CNM-attended deliveries in the United States occur in a hospital. Certified nurse-midwives are qualified professionals who have graduated from an accredited university-based program and passed a national certification exam. Baker, like all CNMs, is a registered nurse. She earned her master’s degree in midwifery in 1994 at State University of New York Downstate University, one of 40 midwifery education programs in the United States.
Certified nurse-midwives practice in a variety of settings, including hospital and office-based practices, community health centers, and public health facilities. CNMs are licensed in all 50 states. They are reimbursed by Medicare, are Medicaid-mandated service providers, and are widely included in managed care provider listings. CNMs are experts in the management of normal birth. Studies have demonstrated that the outcomes of nurse-midwifery care are at least equivalent to those of patients managed by physicians for normal maternity care, and patients repeatedly indicate high levels of satisfaction with the care provided by nurse-midwives.
“Having [Baker] at the hospital has been a big selling point for patients. She helps by massaging the patients who are anxious or need things explained to them. I think this provides the patients with a sense of security, and they also appreciate that this is a unique and different service we provide here,” says Valinda Nwadike, MD, an OB/GYN at St. Mary’s County who previously worked with nurse-midwives in a large urban hospital in Washington, D.C. “All in all, having [Baker] on board as a CNM laborist means better patient interaction and increased quality and continuity of care. It is a very useful tool, one that improves both patient care and our quality of life as community physicians. It’s a win-win situation.”
Filling the Gap
The Southern Maryland community served by St. Mary’s Hospital is quickly growing. The hospital serves as the birth site for the nearby Patuxent Naval Air Station. The number of births at the 100-bed facility recently jumped from 600-800 births a year to more than 1,000. The four OB/GYNs serving this county of 90,000 are all in private practice. With growing businesses, these community providers found that juggling busy outpatient schedules with inpatient demands for labor support or hospital-based procedures was resulting in disruption in their clinics, lost revenue, and frustration for them and their patients alike.
Collectively, the community OB/GYNs got together and decided to hire a CNM laborist to help cover the bases. Lawrence Tilley, MD, chief of obstetrics and gynecology at the hospital, had watched the success of the hospitalist model at St. Mary’s and has a certified nurse-midwife on staff at his private office. He finds that offering nurse-midwifery services in his practice acts as a draw for patients. At the hospital, he would like to add more midwives to the staff, for 24/7 coverage.
Hundreds of miles away, in a busy urban healthcare delivery system with different needs and rhythms than those in rural Maryland, the CNM laborist model also fits the bill. At Mt. Sinai Hospital in Chicago Laborist Darryn Dunbar, CNM, attends the births of nurse-midwifery patients served by the Access Community Health Network, a large healthcare organization that manages 44 Federally Qualified Health Centers in the Chicago area. The hospital sees 4,000 births a year, of which close to 10% are attended by midwives. Dunbar is one of two CNM laborists at Mt. Sinai who care for Access midwifery patients, most of whom are on Medicaid. He works solely in the hospital, providing inpatient coverage after hours and on weekends to the clients of a seven-midwife team that, with the addition of his laborist services, is able to offer almost continuous midwifery coverage.
“The goals were to extend midwifery coverage, to increase patient satisfaction and safety by having continuity of on-site care for this group of patients, … to improve staffing ratios in labor and delivery, and [to] provide relief for the residents and house officers,” says Dunbar.
His many years of experience as a full-scope CNM in busy, urban settings with high volume and increased social and medical risk factors make him well suited to providing care in this setting. In addition, in Illinois (as of this year) Dunbar can bill directly for his services under Medicaid and receives 100% of the physician reimbursement rate. He can also serve as the billing provider when he supervises the deliveries of residents in the hospital training program.
Dunbar is a valuable member of the OB team. He receives patients who come in through the ED and helps with OB triage, first assists with cesarean deliveries on occasion, and “runs the board” when the residents are off the floor for educational obligations, are in surgery, or are busy with other patients. The nursing staff, house attendings, and residents have all come to rely on his watchful eyes and helping hands.
Nurse-midwives, according to their professional philosophy, believe the best model of healthcare for a woman and her family is one that promotes a continuous and compassionate partnership, including individualized methods of care guided by the best evidence available, therapeutic use of human presence, and skillful communication. They believe in watchful waiting and non-intervention in normal processes, the appropriate use of interventions and technology for current or potential health problems, and consultation, collaboration, and referral with other members of the healthcare team, as needed, to provide optimal healthcare.
The ability to provide this kind of care is one of the greatest strengths certified nurse-midwives bring to the communities they serve, especially in busy hospitals where the healthcare needs of women and their newborns are great and the demands on providers’ time are high.
Struggling to increase patient safety, decrease costs, and optimize productivity while maintaining good health outcomes, hospitals are increasingly turning to nurse-midwives. Meanwhile, many community OB/GYN providers are reducing their OB call due to burnout and quality of life issues, increased liability insurance premiums, and fear of litigation. Resident work-hours have decreased due to safety concerns and mandated work limits. There is a need for providers who can care for laboring women in the hospital setting, providing continuity and quality of service during their hospital admission. On-site CNM laborists fill in the gap.
Increase Safety and Quality
Having a laborist on board in the OB/GYN department of the hospital helps Yaacov Zamel, MD, a pediatric hospitalist at St. Mary’s, by allowing him to establish a working relationship with someone whose availability and practice patterns he can rely on. He also notes that this improves care for the women and babies. “The better the support for OB, the better it is for newborns. Ultimately, more patients will want to come here,” says Dr. Zamel.
Dr. Nwadike agrees that having a nurse-midwife on staff increases patient safety and the quality of care. “In our community, we needed help specifically with coverage for hospital patients and procedures, and Baker’s skills are the perfect match,” says Dr. Nwadike. “Now she is an invaluable resource and can do all of those things, as well as provide ED triage, care for unassigned patients, or manage precipitous deliveries. As a continuous presence on labor and delivery, she is a great resource for patients and can provide them with more depth, more education. There are really limitless possibilities for her role to expand.”
As experts in caring for healthy women and their newborns, with a history of achieving excellent perinatal outcomes while caring for underserved populations, certified nurse-midwives are ideal healthcare providers for women who arrive at hospitals seeking quality care. “Working as a laborist, I enjoy being able to use all of my skills. That has been very exciting,” says Baker.
At 4:30 p.m., Baker wraps up for the day. The community OB/GYN on call arrives from his office to assume care for a laboring patient on his panel. Baker updates him on the woman’s status, than wraps the patient in a warm parting hug. TH
Rima Jolivet is the senior technical advisor at the American College of Nurse-Midwives.
Hospital Advertising
By now Americans are accustomed to seeing advertisements for medical goods and services. The steady supply of direct-to-consumer TV advertisements by the pharmaceutical industry is probably the most high-profile example. But while much has been written about the negative effects of these advertisements, the impact of healthcare service advertising—by hospitals as well as by individual physicians—receives comparatively little attention and almost no debate.
While advertising by doctors and hospitals has been legal for 30 years, until recently, professional taboos discouraged the practice. Increasing economic pressures and changing cultural norms have led, however, to the demise of these informal proscriptions, and advertisements produced by hospitals and individual providers are now common.
Yet arguments against healthcare-service advertising can be made on both ethical and economic grounds. While advocates of healthcare service advertising argue that the practice is harmless, often educational, and economically essential, several recent studies of healthcare service advertising reveal that medical centers and individual physicians often create advertisements that:
- Manipulate patients’ ignorance and vulnerability; and
- Stimulate demand for unproven or ineffective therapies.1-3
These advertising practices may lead patients not only to make poor decisions about disease treatment or health maintenance, they may also encourage unnecessary risks or foster unrealistic expectations. Further, the relatively unrestrained manner in which advertising for medical services is now practiced may increase the overall cost of healthcare.
Hospital Advertising and the Ethics of Patient Decision-Making
Those who support healthcare service advertising argue that on the whole decisions regarding the purchase of medical services are not significantly different than those related to any other kind of purchase. In their opinion, buying a car and buying a cholecystectomy are—in economic terms at least—not significantly different. They argue that while consumers of healthcare—like their car-purchasing brethren—should be protected from false advertising they don’t warrant protection from more subtle or manipulative appeals.
But if the “purchasing” of medical services is unique among commercial transactions, then one could argue that consumers of healthcare are ethically entitled to special treatment. Is medicine fundamentally different? It is in both the milieu in which purchase decisions are made and the special nature of the patient-as-consumer situation.
In the majority of circumstances, the consumer of healthcare services can’t truly be informed about what he or she is buying. Assessing the efficacy and safety of medical treatments requires time, reflection, and often expertise that most patients don’t have. Even if their sponsors’ intentions are honorable it is extremely difficult for medical service advertisements to convey the complex risks-and-benefits ratios that underlie intelligent medical decision-making. Complicating matters further, indicators of quality in medicine are extremely difficult to assess for the healthcare professional—let alone the layperson. As one author has put it, “the sheer complexity of medicine, and the quality measures it has available, virtually guarantees that any statement about quality that can fit comfortably in a popular advertising format will be deceptive … .”4
Admittedly, medicine isn’t the only area in which purchasers of goods or services have limited knowledge about the items they are buying. Few people—including this author—actually understand how computers or cars work. Medicine, however, is unique in that purchasers of medical services are not only relatively uninformed, but they are also uniquely vulnerable and dependent. More often than not, patients making decisions about medical services are under severe emotional and/or physical duress. They also depend on the skills, goodwill, and conscientiousness of healthcare providers.
Yet while the vulnerable and dependent position of patients should encourage scrupulous avoidance of manipulative or emotional messages in medical service advertising, frequently just the opposite is true.1,5 In a study of advertisements produced for academic medical centers, Larson and colleagues found that more than 60% of the advertisements directly appealed to patients’ emotions. Further, the same study found that medical centers consistently promoted procedures or therapies with unproven benefits.
Hospital Advertising and Its Effect on the Cost of Healthcare Services
Hospitals, medical centers, and individual physicians currently spend millions of dollars annually advertising themselves to the public.6 The question is, “What is the return on all this money,” or (put another way) “Is all this spending worth it?” Certainly, the pervasive and increasing use of advertising by healthcare institutions indicates that the advertisers, at least, believe it is. But beyond the salutary effect advertising may have on a single institution, what is the cost of healthcare advertising for the healthcare system as a whole?
When hospital advertising first became widespread, one of the most pervasive justifications for its use was that it was not advertising at all; it was simply education.7,8 Advertising, it was argued, was a way for hospitals to educate the public on the need or availability of vital healthcare services. Defenders reasoned that it was not a matter of stimulating demand but rather of increasing utilization. While admittedly there are instances in which healthcare service advertising has increased the demand for necessary and efficacious services, it is just as likely to promote expensive, unnecessary, or inefficacious ones; for example, the aggressive advertising of whole-body computed tomographic and magnetic resonance imaging screening tests (a procedure whose benefit has never been proven and that may expose patients to invasive and costly follow-up tests).1,3,9
Admittedly, the costs of these screening tests are borne by the individual consumer, but the expensive and often unnecessary follow-up testing they may provoke are covered by all of us. Evidence also indicates that hospital advertising may (in part) be responsible for the public’s demand for costly and ineffectual treatments around the end of life, given the perception that higher technology and more advanced procedures are always better.1,9
We shouldn’t be surprised that the expansion of healthcare advertising has led to this situation. In essence, healthcare institutions that advertise without regard to the actual need for their products or services are simply behaving the same way more obviously commercial enterprises do. General Motors Corp. doesn’t need to consider the actual transportation needs of the public when it introduces a new car—only whether or not the company can sell it. By the same token, without standards for healthcare advertising that explicitly address the effect these advertisements may have on demand for unnecessary services, promotion of these often-profitable services will only continue and grow.
The Costs of Competition
Supporters of healthcare advertising also suggest that advertising is good for the healthcare consumer. They cite marketing theorists’ contentions that by providing the public with free and useful information, advertising lowers search costs—the costs associated with finding a good or service—and makes consumers more sensitive to product characteristics. The consequence, they contend, is that advertising not only ultimately lowers consumers’ cost, but it can also drive an increase in quality.10 These observations may have some merit with other sectors of the economy; they have little relevance in healthcare.
First, aggressive and well-funded advertising can easily overwhelm the disincentive of purchasing low-quality goods or services—particularly in a field like healthcare, in which quality is so difficult to measure objectively. Further, in an area like healthcare, in which there are legal restrictions on price competition and consumers typically pay through a third-party intermediary, there is little if any room for advertising to promote lower costs. The fact is that healthcare advertising is more likely to be inflationary.
When a hospital spends money to promote its new open-heart surgery program, it is most likely competing with other institutions for the same pool of patients. Because the supply of potential consumers of this service is limited, other institutions will be forced to spend more money promoting their own programs simply to maintain the market share they already have.11 As a result, advertising by one institution only increases pressure on advertising budgets across the board—a situation that inevitably leads to higher costs universally.
Advocates of healthcare advertising also argue that it can be good for the community. They argue that advertising may increase revenue for a healthcare institution, thus enabling the institution to more vigorously pursue its mission. Because the demand for legitimate healthcare services remains relatively fixed, however, the only growth healthcare advertising typically creates comes at the expense of a competitor.12 The consequence of this “zero sum game” becomes starkly apparent when one considers that hospitals and medical centers tend to compete only for the most well-insured or affluent patients. There is little healthcare advertising directed at conditions that disproportionately affect the poor or uninsured. Hospitals or medical centers with the best or most aggressive advertising campaigns tend to “cherry-pick” the highest-paying patients, leaving those patients who are less likely to pay concentrated at centers that are unable to compete. This concentration of poorly reimbursed or free care at institutions struggling to maintain financial viability can, over time, lead to lower quality and, if the institutions fail, decreased access for the most vulnerable.
Conclusion
With economic pressures and competition for healthcare expenditures growing, hospitals and individual physicians will continue to look to advertising healthcare services as a means to increase revenue. Yet patients are fundamentally different than other types of consumers. Given the typical patient’s combination of vulnerability and inequity of knowledge, it is clear that healthcare consumers deserve special protection from advertisements that play to emotions or ignorance.
Additionally, because we as a society collectively foot the bill for healthcare costs, we must think about whether we can count on individual hospitals and healthcare providers—with their own narrow financial agendas—to abstain from advertising that unnecessarily promotes increased expenditures and costs.
More studies on the direct costs of healthcare service advertising need to be done, and more light needs to be shed on the effects of the millions of dollars advertisers spend annually. Some advertising of medical services may indeed be necessary, but it cannot be allowed to threaten informed patient decision-making or the economic viability of our healthcare system. TH
Dr. Oxman is a hospitalist, a critical care and infectious diseases fellow in Boston, and a former fellow in medical ethics at Harvard.
References
- Larson RJ, Schwartz LM, Woloshin S, et al. Advertising by academic medical centers. Arch Intern Med. 2005 Mar 28;165(6):645-651.
- Finn R. Hospital marketing practices: when is it appropriate to advertise new technology? J Natl Cancer Inst. 2001 Jan;93(1):6-7.
- Illes J, Kann D, Karetsky K, et al. Advertising, patient decision making and self-referral for computed tomographic and magnetic resonance imaging. Arch Intern Med. 2004 Dec 13-27;164(22):2415-2419.
- Latham SR. Ethics in the marketing of medical services. Mt Sinai J Med. 2004 Sep;71(4):243-250.
- Greer S, Greenbaum P. Fear-based advertising and the increase in psychiatric hospitalization of adolescents. Hosp Community Psychiatry. 1992 Oct;43(10):1038-1039.
- McKneally MF. Controversies in cardiothoracic surgery: is it ethical to advertise surgical results to increase referrals? J Thorac Cardiovasc Surg. 2002 May;123(5):839-841.
- Berger JD. The ethical side of advertising. Hosp Forum. 1981 Nov-Dec;24(6):35, 38-39.
- Bonner JW III. Hospital advertising. Hosp Community Psychiatry. 1993 Apr;44(4):391-392.
- Manning S, Schneiderman LJ. Miracles or limits: what message from the medical marketplace? HEC Forum. 1996 Mar;8(2):103-108.
- Hammond KL, Jurkus AF. Healthcare professionals and the ethics of healthcare marketing. Health Mark Q. 1993;11(1-2):9-17.
- MacStravic RE. Should hospitals market? Hosp Prog. 1977 Aug;58(8):56-59, 82.
- Parrington M. The ethics and etiquette of marketing. Healthc Forum J. 1989 Jan-Feb;32(1):42.
By now Americans are accustomed to seeing advertisements for medical goods and services. The steady supply of direct-to-consumer TV advertisements by the pharmaceutical industry is probably the most high-profile example. But while much has been written about the negative effects of these advertisements, the impact of healthcare service advertising—by hospitals as well as by individual physicians—receives comparatively little attention and almost no debate.
While advertising by doctors and hospitals has been legal for 30 years, until recently, professional taboos discouraged the practice. Increasing economic pressures and changing cultural norms have led, however, to the demise of these informal proscriptions, and advertisements produced by hospitals and individual providers are now common.
Yet arguments against healthcare-service advertising can be made on both ethical and economic grounds. While advocates of healthcare service advertising argue that the practice is harmless, often educational, and economically essential, several recent studies of healthcare service advertising reveal that medical centers and individual physicians often create advertisements that:
- Manipulate patients’ ignorance and vulnerability; and
- Stimulate demand for unproven or ineffective therapies.1-3
These advertising practices may lead patients not only to make poor decisions about disease treatment or health maintenance, they may also encourage unnecessary risks or foster unrealistic expectations. Further, the relatively unrestrained manner in which advertising for medical services is now practiced may increase the overall cost of healthcare.
Hospital Advertising and the Ethics of Patient Decision-Making
Those who support healthcare service advertising argue that on the whole decisions regarding the purchase of medical services are not significantly different than those related to any other kind of purchase. In their opinion, buying a car and buying a cholecystectomy are—in economic terms at least—not significantly different. They argue that while consumers of healthcare—like their car-purchasing brethren—should be protected from false advertising they don’t warrant protection from more subtle or manipulative appeals.
But if the “purchasing” of medical services is unique among commercial transactions, then one could argue that consumers of healthcare are ethically entitled to special treatment. Is medicine fundamentally different? It is in both the milieu in which purchase decisions are made and the special nature of the patient-as-consumer situation.
In the majority of circumstances, the consumer of healthcare services can’t truly be informed about what he or she is buying. Assessing the efficacy and safety of medical treatments requires time, reflection, and often expertise that most patients don’t have. Even if their sponsors’ intentions are honorable it is extremely difficult for medical service advertisements to convey the complex risks-and-benefits ratios that underlie intelligent medical decision-making. Complicating matters further, indicators of quality in medicine are extremely difficult to assess for the healthcare professional—let alone the layperson. As one author has put it, “the sheer complexity of medicine, and the quality measures it has available, virtually guarantees that any statement about quality that can fit comfortably in a popular advertising format will be deceptive … .”4
Admittedly, medicine isn’t the only area in which purchasers of goods or services have limited knowledge about the items they are buying. Few people—including this author—actually understand how computers or cars work. Medicine, however, is unique in that purchasers of medical services are not only relatively uninformed, but they are also uniquely vulnerable and dependent. More often than not, patients making decisions about medical services are under severe emotional and/or physical duress. They also depend on the skills, goodwill, and conscientiousness of healthcare providers.
Yet while the vulnerable and dependent position of patients should encourage scrupulous avoidance of manipulative or emotional messages in medical service advertising, frequently just the opposite is true.1,5 In a study of advertisements produced for academic medical centers, Larson and colleagues found that more than 60% of the advertisements directly appealed to patients’ emotions. Further, the same study found that medical centers consistently promoted procedures or therapies with unproven benefits.
Hospital Advertising and Its Effect on the Cost of Healthcare Services
Hospitals, medical centers, and individual physicians currently spend millions of dollars annually advertising themselves to the public.6 The question is, “What is the return on all this money,” or (put another way) “Is all this spending worth it?” Certainly, the pervasive and increasing use of advertising by healthcare institutions indicates that the advertisers, at least, believe it is. But beyond the salutary effect advertising may have on a single institution, what is the cost of healthcare advertising for the healthcare system as a whole?
When hospital advertising first became widespread, one of the most pervasive justifications for its use was that it was not advertising at all; it was simply education.7,8 Advertising, it was argued, was a way for hospitals to educate the public on the need or availability of vital healthcare services. Defenders reasoned that it was not a matter of stimulating demand but rather of increasing utilization. While admittedly there are instances in which healthcare service advertising has increased the demand for necessary and efficacious services, it is just as likely to promote expensive, unnecessary, or inefficacious ones; for example, the aggressive advertising of whole-body computed tomographic and magnetic resonance imaging screening tests (a procedure whose benefit has never been proven and that may expose patients to invasive and costly follow-up tests).1,3,9
Admittedly, the costs of these screening tests are borne by the individual consumer, but the expensive and often unnecessary follow-up testing they may provoke are covered by all of us. Evidence also indicates that hospital advertising may (in part) be responsible for the public’s demand for costly and ineffectual treatments around the end of life, given the perception that higher technology and more advanced procedures are always better.1,9
We shouldn’t be surprised that the expansion of healthcare advertising has led to this situation. In essence, healthcare institutions that advertise without regard to the actual need for their products or services are simply behaving the same way more obviously commercial enterprises do. General Motors Corp. doesn’t need to consider the actual transportation needs of the public when it introduces a new car—only whether or not the company can sell it. By the same token, without standards for healthcare advertising that explicitly address the effect these advertisements may have on demand for unnecessary services, promotion of these often-profitable services will only continue and grow.
The Costs of Competition
Supporters of healthcare advertising also suggest that advertising is good for the healthcare consumer. They cite marketing theorists’ contentions that by providing the public with free and useful information, advertising lowers search costs—the costs associated with finding a good or service—and makes consumers more sensitive to product characteristics. The consequence, they contend, is that advertising not only ultimately lowers consumers’ cost, but it can also drive an increase in quality.10 These observations may have some merit with other sectors of the economy; they have little relevance in healthcare.
First, aggressive and well-funded advertising can easily overwhelm the disincentive of purchasing low-quality goods or services—particularly in a field like healthcare, in which quality is so difficult to measure objectively. Further, in an area like healthcare, in which there are legal restrictions on price competition and consumers typically pay through a third-party intermediary, there is little if any room for advertising to promote lower costs. The fact is that healthcare advertising is more likely to be inflationary.
When a hospital spends money to promote its new open-heart surgery program, it is most likely competing with other institutions for the same pool of patients. Because the supply of potential consumers of this service is limited, other institutions will be forced to spend more money promoting their own programs simply to maintain the market share they already have.11 As a result, advertising by one institution only increases pressure on advertising budgets across the board—a situation that inevitably leads to higher costs universally.
Advocates of healthcare advertising also argue that it can be good for the community. They argue that advertising may increase revenue for a healthcare institution, thus enabling the institution to more vigorously pursue its mission. Because the demand for legitimate healthcare services remains relatively fixed, however, the only growth healthcare advertising typically creates comes at the expense of a competitor.12 The consequence of this “zero sum game” becomes starkly apparent when one considers that hospitals and medical centers tend to compete only for the most well-insured or affluent patients. There is little healthcare advertising directed at conditions that disproportionately affect the poor or uninsured. Hospitals or medical centers with the best or most aggressive advertising campaigns tend to “cherry-pick” the highest-paying patients, leaving those patients who are less likely to pay concentrated at centers that are unable to compete. This concentration of poorly reimbursed or free care at institutions struggling to maintain financial viability can, over time, lead to lower quality and, if the institutions fail, decreased access for the most vulnerable.
Conclusion
With economic pressures and competition for healthcare expenditures growing, hospitals and individual physicians will continue to look to advertising healthcare services as a means to increase revenue. Yet patients are fundamentally different than other types of consumers. Given the typical patient’s combination of vulnerability and inequity of knowledge, it is clear that healthcare consumers deserve special protection from advertisements that play to emotions or ignorance.
Additionally, because we as a society collectively foot the bill for healthcare costs, we must think about whether we can count on individual hospitals and healthcare providers—with their own narrow financial agendas—to abstain from advertising that unnecessarily promotes increased expenditures and costs.
More studies on the direct costs of healthcare service advertising need to be done, and more light needs to be shed on the effects of the millions of dollars advertisers spend annually. Some advertising of medical services may indeed be necessary, but it cannot be allowed to threaten informed patient decision-making or the economic viability of our healthcare system. TH
Dr. Oxman is a hospitalist, a critical care and infectious diseases fellow in Boston, and a former fellow in medical ethics at Harvard.
References
- Larson RJ, Schwartz LM, Woloshin S, et al. Advertising by academic medical centers. Arch Intern Med. 2005 Mar 28;165(6):645-651.
- Finn R. Hospital marketing practices: when is it appropriate to advertise new technology? J Natl Cancer Inst. 2001 Jan;93(1):6-7.
- Illes J, Kann D, Karetsky K, et al. Advertising, patient decision making and self-referral for computed tomographic and magnetic resonance imaging. Arch Intern Med. 2004 Dec 13-27;164(22):2415-2419.
- Latham SR. Ethics in the marketing of medical services. Mt Sinai J Med. 2004 Sep;71(4):243-250.
- Greer S, Greenbaum P. Fear-based advertising and the increase in psychiatric hospitalization of adolescents. Hosp Community Psychiatry. 1992 Oct;43(10):1038-1039.
- McKneally MF. Controversies in cardiothoracic surgery: is it ethical to advertise surgical results to increase referrals? J Thorac Cardiovasc Surg. 2002 May;123(5):839-841.
- Berger JD. The ethical side of advertising. Hosp Forum. 1981 Nov-Dec;24(6):35, 38-39.
- Bonner JW III. Hospital advertising. Hosp Community Psychiatry. 1993 Apr;44(4):391-392.
- Manning S, Schneiderman LJ. Miracles or limits: what message from the medical marketplace? HEC Forum. 1996 Mar;8(2):103-108.
- Hammond KL, Jurkus AF. Healthcare professionals and the ethics of healthcare marketing. Health Mark Q. 1993;11(1-2):9-17.
- MacStravic RE. Should hospitals market? Hosp Prog. 1977 Aug;58(8):56-59, 82.
- Parrington M. The ethics and etiquette of marketing. Healthc Forum J. 1989 Jan-Feb;32(1):42.
By now Americans are accustomed to seeing advertisements for medical goods and services. The steady supply of direct-to-consumer TV advertisements by the pharmaceutical industry is probably the most high-profile example. But while much has been written about the negative effects of these advertisements, the impact of healthcare service advertising—by hospitals as well as by individual physicians—receives comparatively little attention and almost no debate.
While advertising by doctors and hospitals has been legal for 30 years, until recently, professional taboos discouraged the practice. Increasing economic pressures and changing cultural norms have led, however, to the demise of these informal proscriptions, and advertisements produced by hospitals and individual providers are now common.
Yet arguments against healthcare-service advertising can be made on both ethical and economic grounds. While advocates of healthcare service advertising argue that the practice is harmless, often educational, and economically essential, several recent studies of healthcare service advertising reveal that medical centers and individual physicians often create advertisements that:
- Manipulate patients’ ignorance and vulnerability; and
- Stimulate demand for unproven or ineffective therapies.1-3
These advertising practices may lead patients not only to make poor decisions about disease treatment or health maintenance, they may also encourage unnecessary risks or foster unrealistic expectations. Further, the relatively unrestrained manner in which advertising for medical services is now practiced may increase the overall cost of healthcare.
Hospital Advertising and the Ethics of Patient Decision-Making
Those who support healthcare service advertising argue that on the whole decisions regarding the purchase of medical services are not significantly different than those related to any other kind of purchase. In their opinion, buying a car and buying a cholecystectomy are—in economic terms at least—not significantly different. They argue that while consumers of healthcare—like their car-purchasing brethren—should be protected from false advertising they don’t warrant protection from more subtle or manipulative appeals.
But if the “purchasing” of medical services is unique among commercial transactions, then one could argue that consumers of healthcare are ethically entitled to special treatment. Is medicine fundamentally different? It is in both the milieu in which purchase decisions are made and the special nature of the patient-as-consumer situation.
In the majority of circumstances, the consumer of healthcare services can’t truly be informed about what he or she is buying. Assessing the efficacy and safety of medical treatments requires time, reflection, and often expertise that most patients don’t have. Even if their sponsors’ intentions are honorable it is extremely difficult for medical service advertisements to convey the complex risks-and-benefits ratios that underlie intelligent medical decision-making. Complicating matters further, indicators of quality in medicine are extremely difficult to assess for the healthcare professional—let alone the layperson. As one author has put it, “the sheer complexity of medicine, and the quality measures it has available, virtually guarantees that any statement about quality that can fit comfortably in a popular advertising format will be deceptive … .”4
Admittedly, medicine isn’t the only area in which purchasers of goods or services have limited knowledge about the items they are buying. Few people—including this author—actually understand how computers or cars work. Medicine, however, is unique in that purchasers of medical services are not only relatively uninformed, but they are also uniquely vulnerable and dependent. More often than not, patients making decisions about medical services are under severe emotional and/or physical duress. They also depend on the skills, goodwill, and conscientiousness of healthcare providers.
Yet while the vulnerable and dependent position of patients should encourage scrupulous avoidance of manipulative or emotional messages in medical service advertising, frequently just the opposite is true.1,5 In a study of advertisements produced for academic medical centers, Larson and colleagues found that more than 60% of the advertisements directly appealed to patients’ emotions. Further, the same study found that medical centers consistently promoted procedures or therapies with unproven benefits.
Hospital Advertising and Its Effect on the Cost of Healthcare Services
Hospitals, medical centers, and individual physicians currently spend millions of dollars annually advertising themselves to the public.6 The question is, “What is the return on all this money,” or (put another way) “Is all this spending worth it?” Certainly, the pervasive and increasing use of advertising by healthcare institutions indicates that the advertisers, at least, believe it is. But beyond the salutary effect advertising may have on a single institution, what is the cost of healthcare advertising for the healthcare system as a whole?
When hospital advertising first became widespread, one of the most pervasive justifications for its use was that it was not advertising at all; it was simply education.7,8 Advertising, it was argued, was a way for hospitals to educate the public on the need or availability of vital healthcare services. Defenders reasoned that it was not a matter of stimulating demand but rather of increasing utilization. While admittedly there are instances in which healthcare service advertising has increased the demand for necessary and efficacious services, it is just as likely to promote expensive, unnecessary, or inefficacious ones; for example, the aggressive advertising of whole-body computed tomographic and magnetic resonance imaging screening tests (a procedure whose benefit has never been proven and that may expose patients to invasive and costly follow-up tests).1,3,9
Admittedly, the costs of these screening tests are borne by the individual consumer, but the expensive and often unnecessary follow-up testing they may provoke are covered by all of us. Evidence also indicates that hospital advertising may (in part) be responsible for the public’s demand for costly and ineffectual treatments around the end of life, given the perception that higher technology and more advanced procedures are always better.1,9
We shouldn’t be surprised that the expansion of healthcare advertising has led to this situation. In essence, healthcare institutions that advertise without regard to the actual need for their products or services are simply behaving the same way more obviously commercial enterprises do. General Motors Corp. doesn’t need to consider the actual transportation needs of the public when it introduces a new car—only whether or not the company can sell it. By the same token, without standards for healthcare advertising that explicitly address the effect these advertisements may have on demand for unnecessary services, promotion of these often-profitable services will only continue and grow.
The Costs of Competition
Supporters of healthcare advertising also suggest that advertising is good for the healthcare consumer. They cite marketing theorists’ contentions that by providing the public with free and useful information, advertising lowers search costs—the costs associated with finding a good or service—and makes consumers more sensitive to product characteristics. The consequence, they contend, is that advertising not only ultimately lowers consumers’ cost, but it can also drive an increase in quality.10 These observations may have some merit with other sectors of the economy; they have little relevance in healthcare.
First, aggressive and well-funded advertising can easily overwhelm the disincentive of purchasing low-quality goods or services—particularly in a field like healthcare, in which quality is so difficult to measure objectively. Further, in an area like healthcare, in which there are legal restrictions on price competition and consumers typically pay through a third-party intermediary, there is little if any room for advertising to promote lower costs. The fact is that healthcare advertising is more likely to be inflationary.
When a hospital spends money to promote its new open-heart surgery program, it is most likely competing with other institutions for the same pool of patients. Because the supply of potential consumers of this service is limited, other institutions will be forced to spend more money promoting their own programs simply to maintain the market share they already have.11 As a result, advertising by one institution only increases pressure on advertising budgets across the board—a situation that inevitably leads to higher costs universally.
Advocates of healthcare advertising also argue that it can be good for the community. They argue that advertising may increase revenue for a healthcare institution, thus enabling the institution to more vigorously pursue its mission. Because the demand for legitimate healthcare services remains relatively fixed, however, the only growth healthcare advertising typically creates comes at the expense of a competitor.12 The consequence of this “zero sum game” becomes starkly apparent when one considers that hospitals and medical centers tend to compete only for the most well-insured or affluent patients. There is little healthcare advertising directed at conditions that disproportionately affect the poor or uninsured. Hospitals or medical centers with the best or most aggressive advertising campaigns tend to “cherry-pick” the highest-paying patients, leaving those patients who are less likely to pay concentrated at centers that are unable to compete. This concentration of poorly reimbursed or free care at institutions struggling to maintain financial viability can, over time, lead to lower quality and, if the institutions fail, decreased access for the most vulnerable.
Conclusion
With economic pressures and competition for healthcare expenditures growing, hospitals and individual physicians will continue to look to advertising healthcare services as a means to increase revenue. Yet patients are fundamentally different than other types of consumers. Given the typical patient’s combination of vulnerability and inequity of knowledge, it is clear that healthcare consumers deserve special protection from advertisements that play to emotions or ignorance.
Additionally, because we as a society collectively foot the bill for healthcare costs, we must think about whether we can count on individual hospitals and healthcare providers—with their own narrow financial agendas—to abstain from advertising that unnecessarily promotes increased expenditures and costs.
More studies on the direct costs of healthcare service advertising need to be done, and more light needs to be shed on the effects of the millions of dollars advertisers spend annually. Some advertising of medical services may indeed be necessary, but it cannot be allowed to threaten informed patient decision-making or the economic viability of our healthcare system. TH
Dr. Oxman is a hospitalist, a critical care and infectious diseases fellow in Boston, and a former fellow in medical ethics at Harvard.
References
- Larson RJ, Schwartz LM, Woloshin S, et al. Advertising by academic medical centers. Arch Intern Med. 2005 Mar 28;165(6):645-651.
- Finn R. Hospital marketing practices: when is it appropriate to advertise new technology? J Natl Cancer Inst. 2001 Jan;93(1):6-7.
- Illes J, Kann D, Karetsky K, et al. Advertising, patient decision making and self-referral for computed tomographic and magnetic resonance imaging. Arch Intern Med. 2004 Dec 13-27;164(22):2415-2419.
- Latham SR. Ethics in the marketing of medical services. Mt Sinai J Med. 2004 Sep;71(4):243-250.
- Greer S, Greenbaum P. Fear-based advertising and the increase in psychiatric hospitalization of adolescents. Hosp Community Psychiatry. 1992 Oct;43(10):1038-1039.
- McKneally MF. Controversies in cardiothoracic surgery: is it ethical to advertise surgical results to increase referrals? J Thorac Cardiovasc Surg. 2002 May;123(5):839-841.
- Berger JD. The ethical side of advertising. Hosp Forum. 1981 Nov-Dec;24(6):35, 38-39.
- Bonner JW III. Hospital advertising. Hosp Community Psychiatry. 1993 Apr;44(4):391-392.
- Manning S, Schneiderman LJ. Miracles or limits: what message from the medical marketplace? HEC Forum. 1996 Mar;8(2):103-108.
- Hammond KL, Jurkus AF. Healthcare professionals and the ethics of healthcare marketing. Health Mark Q. 1993;11(1-2):9-17.
- MacStravic RE. Should hospitals market? Hosp Prog. 1977 Aug;58(8):56-59, 82.
- Parrington M. The ethics and etiquette of marketing. Healthc Forum J. 1989 Jan-Feb;32(1):42.
Unforgettable
Recently The Hospitalist asked readers to share their reminiscences of patients who had left their mark in some special way on the docs’ lives. Here are those stories.
Patient al Dente
Sandi Verbin, MD, pediatric hospitalist at Temple University Children's Hospital, Philadelphia, and part-time pediatric hospitalist at Holy Redeemer Hospital, Meadowbrook, Pa.
It was midnight in the ER. My shift was ending, and I had to be up early in the morning to drive to my niece’s third birthday party about two hours away. The ER showed no signs of slowing, however, and one of the nurses approached me, asking if I could see the “quick” patient in Room 4: a child with a piece of macaroni stuck up his nose.
In over 10 years of pediatric practice, the allure of placing foreign objects in body orifices has eluded me. Why is this fun? Nevertheless, a parade of toddlers have presented themselves to me with such varied objects as crayons, tissue paper, beads, coffee beans, Play-Doh, M&Ms, rocks, and magnets in their ears and nostrils. (This doesn’t count the unfortunate innocent bystanders who presented with insects having claimed “squatter’s rights” to the patients’ auditory canals.)
Invariably, when asked, the children deny knowing how the object came to be there—at best, a lame “it just fell in” is offered. When questioned as to how the offending object came so close to the involved area, I am met with silence or a shrug. One memorable child told me he did not believe I was the doctor because I was a girl. I let it pass, considering he had put a rabbit food pellet in his ear after being dared to do so by his older brother. I felt his overall judgment was somewhat questionable.
I entered Room 4, prepared to see the usual anxious—or, as in some cases, oblivious—toddler. Instead, to my surprise, a sheepish-looking eight-year-old boy sat on the table, accompanied by his exasperated mother. The pair had been waiting for several hours for the anticipated pasta-ectomy.
Unable to stop myself, I blurted out, “What is a big boy like you doing putting macaroni up his nose? I expect to see this in little kids, but not eight-year-old boys!” The child answered that he had put the macaroni up his nose “when I was in preschool.”
“You mean five or six years ago?” I asked incredulously. When he reluctantly said yes, I explained that I would look up his nose, but that that piece of macaroni was long gone—dissolved or swallowed lo these many years ago. Sure enough, an exam revealed turbinates and mucus but no complex carbohydrates.
I explained to the mother again that there was no way the food item could have survived in the child’s nose for five years, that he would have presented with sinusitis years ago had the macaroni not been swallowed, dissolved, or sneezed out, and that there was no place else to which it could have migrated.
My best diagnosis was that the child had an unusually dry, sharp-feeling piece of mucus in his nose. The discomfort of this had caused him to admit his transgression, committed in the reckless days of toddlerhood, one which had clearly been a source of guilt for him ever since. After some saline nose drops, and no doubt due in no small part to the soul-cleansing effects of confession, the boy felt better. He and mom went home.
I left the ER to contemplate what antics the next day’s group of three-year-olds would cook up. I vowed to keep a close eye on the Jelly Beans.
Great Foster Mom
Alison Holmes, MD, MPH, pediatric hospitalist, Concord Hospital, Concord, N.H., and assistant professor of Community and Family Medicine, Dartmouth Medical School, contributed two stories.
When I was a resident, there was one chronically ill baby who was born at 34 weeks and had significant cyanotic heart disease. He would need a number of high-risk cardiac surgeries, and he also had a portion of his small intestine removed for necrotizing enterocolitis [caused by] his prematurity. After that it can be hard to absorb [nutrients] and grow. The baby had a lot of trouble with diarrhea and dehydration. We put him on the GI service, and the fluid overload from rehydration caused him to go into heart failure, and nobody could ever get the balance right.
He’d go back and forth between the GI service and the cardiology service. All the residents knew him, and he was in a horrible social situation. His mother was a drug user, and after his birth she never visited; we didn’t know anything about the father. The baby was this high-risk infant who basically had laid in the hospital with the TV on for the first five months of his life. Nobody paid attention to him, and I remember thinking, “This is horrible. He’s not going to get any love or nurturing. He’s not going to be normal, because nobody picks him up and holds him and talks to him.”
He was discharged into foster care, and I became his primary doctor. He just had the greatest foster mother in the world. She didn’t care that he had these medical problems; she was so glad to have a baby. She had been a foster parent for a while and had cared for troubled older children and had had enough of that, and she had her own 11-year-old. She was so thrilled to have this baby, and she just loved him and loved him and loved him.
I watched over the next year as he regained normal development despite all his early setbacks—both medical and social. Eventually his father did get involved; he went back into the father’s care, and the father rallied his whole family. The foster mother stays in touch with the family and is the child’s godmother.
By the time I finished residency, he was about three and a half years old, had been through three major cardiac surgeries, and was completely developmentally normal. I’ll never forget that no matter what we do medically, it’s people like that foster mother who make a difference for children.
Doing Wonders
I cared for a growth-retarded baby whose mother was in her late 30s. She was a drug user, and she had lost custody of her three prior children. Here she was with a fourth child, without any supports. She had used cocaine until about the fifth month of her pregnancy, when she decided to get some help.
I thought, “What are this baby’s chances?” But [the mom] did it. She did not go back to using drugs. She stayed clean and reconciled with the father. Life wasn’t always so kind to her. She couldn’t always hold down the same job, but at least she always had a job. She did wonders for this little girl, and she was able to turn her own life around.
Yet One More Challenge
Sandeep Sachdeva, MD, hospitalist at Swedish Medical Center; lead hospitalist, Swedish Medical Center’s Stroke Program; and clinical instructor, University of Washington, Seattle.
The patient I was most impressed by was a lady who came into the hospital about a year ago. I think she was in her mid 60s and had been blind and deaf from birth. She had obviously faced huge challenges and was living alone.
She had a very good support system: a non-governmental organization (NGO) here that provides close support for people in this type of situation. Even though she didn’t have any family support, these volunteers from the NGO would come out to make sure she was doing OK.
As I recall, she had tripped over something and had fallen and broken her hip, and she was admitted to the hospital for hip surgery. Just looking after her was a tremendous learning experience for me: She couldn't see me; she couldn’t hear me; and the only way we could communicate was through a sign language interpreter, where she had to feel the hands of the person doing sign language. She was very involved in her own care; she would ask questions, and there was no dearth of communication.
It was fascinating to deal with this lady who is—in essence—in a different universe with no sound and no sight, and who was facing the challenge of being in the hospital and recovering from her surgery. It felt very satisfying to look after her and to be inspired by her—a patient who was able to overcome yet another challenge when she is already disadvantaged. She did very well, and I believe she went home. I could still feel that she was trying to be independent and be the take-charge person that she had always been.
Patient in a Pickle
Eric Kupersmith, MD, assistant professor of medicine and director of the Hospitalist Program at Cooper Hospital, Camden, N.J. Dr. Kupersmith has plenty of patients he’ll never forget. Here are the stories of few.
There was a patient who would get admitted every month with congestive heart failure. He would develop pulmonary edema as an acute event like clockwork, and no one could figure out what was causing this. The seventh consecutive time, he was placed on my service. We eventually discovered that each month he would buy a jar of pickles and eat the whole jar. Then—at the end of the month when he finished the pickles—he would drink the entire jar full of juice.
The salt in the pickle juice caused the acute pulmonary edema, but until this piece of history was taken no one could pin down the problem. It really was a medical mystery. Figuring it out prevented his readmissions and is just the kind of thing that represents how hospitalists sometimes have to serve as diagnostic detectives.
You and Who Else?
An old woman was brought in by someone and was admitted to my service. When I entered the room, the daughter who lives [with] and cares for her said, “I know she has cancer, I know she’s demented, I think it is time to let her go. I live with her. I’m her power of attorney. There’s no document, but I’m her only daughter.”
What do I do? I figure hospice care; she’s dying in the hospital. I don’t cure her pneumonia. I let her go, and—three days later [after she had died]—I get a phone call from her two sons.
They say, “Hi Doctor, we brought our mother in. What’s the plan of care?” It turns out there was no power of attorney. The daughter did live with the patient, but apparently the daughter was mad at her two brothers, so she didn’t tell me the whole story. The sons threatened me—not with malpractice—but with going to tell the district attorney that I committed murder. And one of the sons mentioned murdering me.
Both threats resolved with tears and empathy as I met with them and acknowledged the error; after full explanations, they agreed with the final decision.
Put Bar Codes on Families, Too!
A mildly demented older man was admitted for something small. In comes another man and says, “This is my brother, and he’s ready to go.”
“Really?” I asked. “That’s your brother?”
“Yes,” he answered. “That’s my brother Jim.”
“OK, great,” I replied. And Jim takes him home.
Two hours later, the family shows up and tells me he doesn’t have a brother. It turns out that at the church the patient attends, they call each other “brother.’”
When They Know, They Know
I got called to see a patient. “What is the matter?” I asked him.
“I’m dying,” he said.
“What do you mean?” I asked. “Do you have chest pain?”
“No,” he replied.
“Are you short of breath?” I asked.
“No,” he said.
“Are you feeling a fever?” I asked.
“No! I’m dying!” he exclaimed.
I found nothing from the interview. I did a physical exam and found nothing. I called other physicians in who were seeing the patient. Everyone said, “I don’t know what he means.” The patient died within the hour.
I’ve had this happen to me four times. In three of those cases, they said, “I’m going to die today.”
In the other case, the patient said, “I don’t feel right.” When I asked him what he meant, he said, “I don’t know. I just feel weird.” And then he died that day.
Ask the Patient Why
I have a number of patients with sickle cell disease who have chronic pain syndrome. I had a female patient—about 26 years old—who basically stayed immobile for two weeks. The staff was upset with her about that.
To each other, they referred to her as noncompliant, and we wondered, “Why won’t she get up? She won’t even try to get out of bed.”
When I was assigned her case, I said to her, “Everyone says you won’t get out of bed. Will you tell me why?”
“You’re the first person to ask me that,” she said.
“Well, then,” I asked, “why?”
“I have an artificial hip,” she said, “and it is dislocated.”
When the physicians and other staff had urged her to get up, she had simply said, “My hip hurts.” It turns out that she was clinically depressed and was angry because she felt frustrated that she was always being judged.
Ultimately, she died two years later from the same problem in another hospital where she had developed DVT. In that other hospital, she’d done the same thing: She had refused to move, and the staff had told her she had to move—but no one had asked her why she hadn’t. TH
Andrea Sattinger writes frequently for The Hospitalist.
Recently The Hospitalist asked readers to share their reminiscences of patients who had left their mark in some special way on the docs’ lives. Here are those stories.
Patient al Dente
Sandi Verbin, MD, pediatric hospitalist at Temple University Children's Hospital, Philadelphia, and part-time pediatric hospitalist at Holy Redeemer Hospital, Meadowbrook, Pa.
It was midnight in the ER. My shift was ending, and I had to be up early in the morning to drive to my niece’s third birthday party about two hours away. The ER showed no signs of slowing, however, and one of the nurses approached me, asking if I could see the “quick” patient in Room 4: a child with a piece of macaroni stuck up his nose.
In over 10 years of pediatric practice, the allure of placing foreign objects in body orifices has eluded me. Why is this fun? Nevertheless, a parade of toddlers have presented themselves to me with such varied objects as crayons, tissue paper, beads, coffee beans, Play-Doh, M&Ms, rocks, and magnets in their ears and nostrils. (This doesn’t count the unfortunate innocent bystanders who presented with insects having claimed “squatter’s rights” to the patients’ auditory canals.)
Invariably, when asked, the children deny knowing how the object came to be there—at best, a lame “it just fell in” is offered. When questioned as to how the offending object came so close to the involved area, I am met with silence or a shrug. One memorable child told me he did not believe I was the doctor because I was a girl. I let it pass, considering he had put a rabbit food pellet in his ear after being dared to do so by his older brother. I felt his overall judgment was somewhat questionable.
I entered Room 4, prepared to see the usual anxious—or, as in some cases, oblivious—toddler. Instead, to my surprise, a sheepish-looking eight-year-old boy sat on the table, accompanied by his exasperated mother. The pair had been waiting for several hours for the anticipated pasta-ectomy.
Unable to stop myself, I blurted out, “What is a big boy like you doing putting macaroni up his nose? I expect to see this in little kids, but not eight-year-old boys!” The child answered that he had put the macaroni up his nose “when I was in preschool.”
“You mean five or six years ago?” I asked incredulously. When he reluctantly said yes, I explained that I would look up his nose, but that that piece of macaroni was long gone—dissolved or swallowed lo these many years ago. Sure enough, an exam revealed turbinates and mucus but no complex carbohydrates.
I explained to the mother again that there was no way the food item could have survived in the child’s nose for five years, that he would have presented with sinusitis years ago had the macaroni not been swallowed, dissolved, or sneezed out, and that there was no place else to which it could have migrated.
My best diagnosis was that the child had an unusually dry, sharp-feeling piece of mucus in his nose. The discomfort of this had caused him to admit his transgression, committed in the reckless days of toddlerhood, one which had clearly been a source of guilt for him ever since. After some saline nose drops, and no doubt due in no small part to the soul-cleansing effects of confession, the boy felt better. He and mom went home.
I left the ER to contemplate what antics the next day’s group of three-year-olds would cook up. I vowed to keep a close eye on the Jelly Beans.
Great Foster Mom
Alison Holmes, MD, MPH, pediatric hospitalist, Concord Hospital, Concord, N.H., and assistant professor of Community and Family Medicine, Dartmouth Medical School, contributed two stories.
When I was a resident, there was one chronically ill baby who was born at 34 weeks and had significant cyanotic heart disease. He would need a number of high-risk cardiac surgeries, and he also had a portion of his small intestine removed for necrotizing enterocolitis [caused by] his prematurity. After that it can be hard to absorb [nutrients] and grow. The baby had a lot of trouble with diarrhea and dehydration. We put him on the GI service, and the fluid overload from rehydration caused him to go into heart failure, and nobody could ever get the balance right.
He’d go back and forth between the GI service and the cardiology service. All the residents knew him, and he was in a horrible social situation. His mother was a drug user, and after his birth she never visited; we didn’t know anything about the father. The baby was this high-risk infant who basically had laid in the hospital with the TV on for the first five months of his life. Nobody paid attention to him, and I remember thinking, “This is horrible. He’s not going to get any love or nurturing. He’s not going to be normal, because nobody picks him up and holds him and talks to him.”
He was discharged into foster care, and I became his primary doctor. He just had the greatest foster mother in the world. She didn’t care that he had these medical problems; she was so glad to have a baby. She had been a foster parent for a while and had cared for troubled older children and had had enough of that, and she had her own 11-year-old. She was so thrilled to have this baby, and she just loved him and loved him and loved him.
I watched over the next year as he regained normal development despite all his early setbacks—both medical and social. Eventually his father did get involved; he went back into the father’s care, and the father rallied his whole family. The foster mother stays in touch with the family and is the child’s godmother.
By the time I finished residency, he was about three and a half years old, had been through three major cardiac surgeries, and was completely developmentally normal. I’ll never forget that no matter what we do medically, it’s people like that foster mother who make a difference for children.
Doing Wonders
I cared for a growth-retarded baby whose mother was in her late 30s. She was a drug user, and she had lost custody of her three prior children. Here she was with a fourth child, without any supports. She had used cocaine until about the fifth month of her pregnancy, when she decided to get some help.
I thought, “What are this baby’s chances?” But [the mom] did it. She did not go back to using drugs. She stayed clean and reconciled with the father. Life wasn’t always so kind to her. She couldn’t always hold down the same job, but at least she always had a job. She did wonders for this little girl, and she was able to turn her own life around.
Yet One More Challenge
Sandeep Sachdeva, MD, hospitalist at Swedish Medical Center; lead hospitalist, Swedish Medical Center’s Stroke Program; and clinical instructor, University of Washington, Seattle.
The patient I was most impressed by was a lady who came into the hospital about a year ago. I think she was in her mid 60s and had been blind and deaf from birth. She had obviously faced huge challenges and was living alone.
She had a very good support system: a non-governmental organization (NGO) here that provides close support for people in this type of situation. Even though she didn’t have any family support, these volunteers from the NGO would come out to make sure she was doing OK.
As I recall, she had tripped over something and had fallen and broken her hip, and she was admitted to the hospital for hip surgery. Just looking after her was a tremendous learning experience for me: She couldn't see me; she couldn’t hear me; and the only way we could communicate was through a sign language interpreter, where she had to feel the hands of the person doing sign language. She was very involved in her own care; she would ask questions, and there was no dearth of communication.
It was fascinating to deal with this lady who is—in essence—in a different universe with no sound and no sight, and who was facing the challenge of being in the hospital and recovering from her surgery. It felt very satisfying to look after her and to be inspired by her—a patient who was able to overcome yet another challenge when she is already disadvantaged. She did very well, and I believe she went home. I could still feel that she was trying to be independent and be the take-charge person that she had always been.
Patient in a Pickle
Eric Kupersmith, MD, assistant professor of medicine and director of the Hospitalist Program at Cooper Hospital, Camden, N.J. Dr. Kupersmith has plenty of patients he’ll never forget. Here are the stories of few.
There was a patient who would get admitted every month with congestive heart failure. He would develop pulmonary edema as an acute event like clockwork, and no one could figure out what was causing this. The seventh consecutive time, he was placed on my service. We eventually discovered that each month he would buy a jar of pickles and eat the whole jar. Then—at the end of the month when he finished the pickles—he would drink the entire jar full of juice.
The salt in the pickle juice caused the acute pulmonary edema, but until this piece of history was taken no one could pin down the problem. It really was a medical mystery. Figuring it out prevented his readmissions and is just the kind of thing that represents how hospitalists sometimes have to serve as diagnostic detectives.
You and Who Else?
An old woman was brought in by someone and was admitted to my service. When I entered the room, the daughter who lives [with] and cares for her said, “I know she has cancer, I know she’s demented, I think it is time to let her go. I live with her. I’m her power of attorney. There’s no document, but I’m her only daughter.”
What do I do? I figure hospice care; she’s dying in the hospital. I don’t cure her pneumonia. I let her go, and—three days later [after she had died]—I get a phone call from her two sons.
They say, “Hi Doctor, we brought our mother in. What’s the plan of care?” It turns out there was no power of attorney. The daughter did live with the patient, but apparently the daughter was mad at her two brothers, so she didn’t tell me the whole story. The sons threatened me—not with malpractice—but with going to tell the district attorney that I committed murder. And one of the sons mentioned murdering me.
Both threats resolved with tears and empathy as I met with them and acknowledged the error; after full explanations, they agreed with the final decision.
Put Bar Codes on Families, Too!
A mildly demented older man was admitted for something small. In comes another man and says, “This is my brother, and he’s ready to go.”
“Really?” I asked. “That’s your brother?”
“Yes,” he answered. “That’s my brother Jim.”
“OK, great,” I replied. And Jim takes him home.
Two hours later, the family shows up and tells me he doesn’t have a brother. It turns out that at the church the patient attends, they call each other “brother.’”
When They Know, They Know
I got called to see a patient. “What is the matter?” I asked him.
“I’m dying,” he said.
“What do you mean?” I asked. “Do you have chest pain?”
“No,” he replied.
“Are you short of breath?” I asked.
“No,” he said.
“Are you feeling a fever?” I asked.
“No! I’m dying!” he exclaimed.
I found nothing from the interview. I did a physical exam and found nothing. I called other physicians in who were seeing the patient. Everyone said, “I don’t know what he means.” The patient died within the hour.
I’ve had this happen to me four times. In three of those cases, they said, “I’m going to die today.”
In the other case, the patient said, “I don’t feel right.” When I asked him what he meant, he said, “I don’t know. I just feel weird.” And then he died that day.
Ask the Patient Why
I have a number of patients with sickle cell disease who have chronic pain syndrome. I had a female patient—about 26 years old—who basically stayed immobile for two weeks. The staff was upset with her about that.
To each other, they referred to her as noncompliant, and we wondered, “Why won’t she get up? She won’t even try to get out of bed.”
When I was assigned her case, I said to her, “Everyone says you won’t get out of bed. Will you tell me why?”
“You’re the first person to ask me that,” she said.
“Well, then,” I asked, “why?”
“I have an artificial hip,” she said, “and it is dislocated.”
When the physicians and other staff had urged her to get up, she had simply said, “My hip hurts.” It turns out that she was clinically depressed and was angry because she felt frustrated that she was always being judged.
Ultimately, she died two years later from the same problem in another hospital where she had developed DVT. In that other hospital, she’d done the same thing: She had refused to move, and the staff had told her she had to move—but no one had asked her why she hadn’t. TH
Andrea Sattinger writes frequently for The Hospitalist.
Recently The Hospitalist asked readers to share their reminiscences of patients who had left their mark in some special way on the docs’ lives. Here are those stories.
Patient al Dente
Sandi Verbin, MD, pediatric hospitalist at Temple University Children's Hospital, Philadelphia, and part-time pediatric hospitalist at Holy Redeemer Hospital, Meadowbrook, Pa.
It was midnight in the ER. My shift was ending, and I had to be up early in the morning to drive to my niece’s third birthday party about two hours away. The ER showed no signs of slowing, however, and one of the nurses approached me, asking if I could see the “quick” patient in Room 4: a child with a piece of macaroni stuck up his nose.
In over 10 years of pediatric practice, the allure of placing foreign objects in body orifices has eluded me. Why is this fun? Nevertheless, a parade of toddlers have presented themselves to me with such varied objects as crayons, tissue paper, beads, coffee beans, Play-Doh, M&Ms, rocks, and magnets in their ears and nostrils. (This doesn’t count the unfortunate innocent bystanders who presented with insects having claimed “squatter’s rights” to the patients’ auditory canals.)
Invariably, when asked, the children deny knowing how the object came to be there—at best, a lame “it just fell in” is offered. When questioned as to how the offending object came so close to the involved area, I am met with silence or a shrug. One memorable child told me he did not believe I was the doctor because I was a girl. I let it pass, considering he had put a rabbit food pellet in his ear after being dared to do so by his older brother. I felt his overall judgment was somewhat questionable.
I entered Room 4, prepared to see the usual anxious—or, as in some cases, oblivious—toddler. Instead, to my surprise, a sheepish-looking eight-year-old boy sat on the table, accompanied by his exasperated mother. The pair had been waiting for several hours for the anticipated pasta-ectomy.
Unable to stop myself, I blurted out, “What is a big boy like you doing putting macaroni up his nose? I expect to see this in little kids, but not eight-year-old boys!” The child answered that he had put the macaroni up his nose “when I was in preschool.”
“You mean five or six years ago?” I asked incredulously. When he reluctantly said yes, I explained that I would look up his nose, but that that piece of macaroni was long gone—dissolved or swallowed lo these many years ago. Sure enough, an exam revealed turbinates and mucus but no complex carbohydrates.
I explained to the mother again that there was no way the food item could have survived in the child’s nose for five years, that he would have presented with sinusitis years ago had the macaroni not been swallowed, dissolved, or sneezed out, and that there was no place else to which it could have migrated.
My best diagnosis was that the child had an unusually dry, sharp-feeling piece of mucus in his nose. The discomfort of this had caused him to admit his transgression, committed in the reckless days of toddlerhood, one which had clearly been a source of guilt for him ever since. After some saline nose drops, and no doubt due in no small part to the soul-cleansing effects of confession, the boy felt better. He and mom went home.
I left the ER to contemplate what antics the next day’s group of three-year-olds would cook up. I vowed to keep a close eye on the Jelly Beans.
Great Foster Mom
Alison Holmes, MD, MPH, pediatric hospitalist, Concord Hospital, Concord, N.H., and assistant professor of Community and Family Medicine, Dartmouth Medical School, contributed two stories.
When I was a resident, there was one chronically ill baby who was born at 34 weeks and had significant cyanotic heart disease. He would need a number of high-risk cardiac surgeries, and he also had a portion of his small intestine removed for necrotizing enterocolitis [caused by] his prematurity. After that it can be hard to absorb [nutrients] and grow. The baby had a lot of trouble with diarrhea and dehydration. We put him on the GI service, and the fluid overload from rehydration caused him to go into heart failure, and nobody could ever get the balance right.
He’d go back and forth between the GI service and the cardiology service. All the residents knew him, and he was in a horrible social situation. His mother was a drug user, and after his birth she never visited; we didn’t know anything about the father. The baby was this high-risk infant who basically had laid in the hospital with the TV on for the first five months of his life. Nobody paid attention to him, and I remember thinking, “This is horrible. He’s not going to get any love or nurturing. He’s not going to be normal, because nobody picks him up and holds him and talks to him.”
He was discharged into foster care, and I became his primary doctor. He just had the greatest foster mother in the world. She didn’t care that he had these medical problems; she was so glad to have a baby. She had been a foster parent for a while and had cared for troubled older children and had had enough of that, and she had her own 11-year-old. She was so thrilled to have this baby, and she just loved him and loved him and loved him.
I watched over the next year as he regained normal development despite all his early setbacks—both medical and social. Eventually his father did get involved; he went back into the father’s care, and the father rallied his whole family. The foster mother stays in touch with the family and is the child’s godmother.
By the time I finished residency, he was about three and a half years old, had been through three major cardiac surgeries, and was completely developmentally normal. I’ll never forget that no matter what we do medically, it’s people like that foster mother who make a difference for children.
Doing Wonders
I cared for a growth-retarded baby whose mother was in her late 30s. She was a drug user, and she had lost custody of her three prior children. Here she was with a fourth child, without any supports. She had used cocaine until about the fifth month of her pregnancy, when she decided to get some help.
I thought, “What are this baby’s chances?” But [the mom] did it. She did not go back to using drugs. She stayed clean and reconciled with the father. Life wasn’t always so kind to her. She couldn’t always hold down the same job, but at least she always had a job. She did wonders for this little girl, and she was able to turn her own life around.
Yet One More Challenge
Sandeep Sachdeva, MD, hospitalist at Swedish Medical Center; lead hospitalist, Swedish Medical Center’s Stroke Program; and clinical instructor, University of Washington, Seattle.
The patient I was most impressed by was a lady who came into the hospital about a year ago. I think she was in her mid 60s and had been blind and deaf from birth. She had obviously faced huge challenges and was living alone.
She had a very good support system: a non-governmental organization (NGO) here that provides close support for people in this type of situation. Even though she didn’t have any family support, these volunteers from the NGO would come out to make sure she was doing OK.
As I recall, she had tripped over something and had fallen and broken her hip, and she was admitted to the hospital for hip surgery. Just looking after her was a tremendous learning experience for me: She couldn't see me; she couldn’t hear me; and the only way we could communicate was through a sign language interpreter, where she had to feel the hands of the person doing sign language. She was very involved in her own care; she would ask questions, and there was no dearth of communication.
It was fascinating to deal with this lady who is—in essence—in a different universe with no sound and no sight, and who was facing the challenge of being in the hospital and recovering from her surgery. It felt very satisfying to look after her and to be inspired by her—a patient who was able to overcome yet another challenge when she is already disadvantaged. She did very well, and I believe she went home. I could still feel that she was trying to be independent and be the take-charge person that she had always been.
Patient in a Pickle
Eric Kupersmith, MD, assistant professor of medicine and director of the Hospitalist Program at Cooper Hospital, Camden, N.J. Dr. Kupersmith has plenty of patients he’ll never forget. Here are the stories of few.
There was a patient who would get admitted every month with congestive heart failure. He would develop pulmonary edema as an acute event like clockwork, and no one could figure out what was causing this. The seventh consecutive time, he was placed on my service. We eventually discovered that each month he would buy a jar of pickles and eat the whole jar. Then—at the end of the month when he finished the pickles—he would drink the entire jar full of juice.
The salt in the pickle juice caused the acute pulmonary edema, but until this piece of history was taken no one could pin down the problem. It really was a medical mystery. Figuring it out prevented his readmissions and is just the kind of thing that represents how hospitalists sometimes have to serve as diagnostic detectives.
You and Who Else?
An old woman was brought in by someone and was admitted to my service. When I entered the room, the daughter who lives [with] and cares for her said, “I know she has cancer, I know she’s demented, I think it is time to let her go. I live with her. I’m her power of attorney. There’s no document, but I’m her only daughter.”
What do I do? I figure hospice care; she’s dying in the hospital. I don’t cure her pneumonia. I let her go, and—three days later [after she had died]—I get a phone call from her two sons.
They say, “Hi Doctor, we brought our mother in. What’s the plan of care?” It turns out there was no power of attorney. The daughter did live with the patient, but apparently the daughter was mad at her two brothers, so she didn’t tell me the whole story. The sons threatened me—not with malpractice—but with going to tell the district attorney that I committed murder. And one of the sons mentioned murdering me.
Both threats resolved with tears and empathy as I met with them and acknowledged the error; after full explanations, they agreed with the final decision.
Put Bar Codes on Families, Too!
A mildly demented older man was admitted for something small. In comes another man and says, “This is my brother, and he’s ready to go.”
“Really?” I asked. “That’s your brother?”
“Yes,” he answered. “That’s my brother Jim.”
“OK, great,” I replied. And Jim takes him home.
Two hours later, the family shows up and tells me he doesn’t have a brother. It turns out that at the church the patient attends, they call each other “brother.’”
When They Know, They Know
I got called to see a patient. “What is the matter?” I asked him.
“I’m dying,” he said.
“What do you mean?” I asked. “Do you have chest pain?”
“No,” he replied.
“Are you short of breath?” I asked.
“No,” he said.
“Are you feeling a fever?” I asked.
“No! I’m dying!” he exclaimed.
I found nothing from the interview. I did a physical exam and found nothing. I called other physicians in who were seeing the patient. Everyone said, “I don’t know what he means.” The patient died within the hour.
I’ve had this happen to me four times. In three of those cases, they said, “I’m going to die today.”
In the other case, the patient said, “I don’t feel right.” When I asked him what he meant, he said, “I don’t know. I just feel weird.” And then he died that day.
Ask the Patient Why
I have a number of patients with sickle cell disease who have chronic pain syndrome. I had a female patient—about 26 years old—who basically stayed immobile for two weeks. The staff was upset with her about that.
To each other, they referred to her as noncompliant, and we wondered, “Why won’t she get up? She won’t even try to get out of bed.”
When I was assigned her case, I said to her, “Everyone says you won’t get out of bed. Will you tell me why?”
“You’re the first person to ask me that,” she said.
“Well, then,” I asked, “why?”
“I have an artificial hip,” she said, “and it is dislocated.”
When the physicians and other staff had urged her to get up, she had simply said, “My hip hurts.” It turns out that she was clinically depressed and was angry because she felt frustrated that she was always being judged.
Ultimately, she died two years later from the same problem in another hospital where she had developed DVT. In that other hospital, she’d done the same thing: She had refused to move, and the staff had told her she had to move—but no one had asked her why she hadn’t. TH
Andrea Sattinger writes frequently for The Hospitalist.
Meeting Reviews
The Mayo 3rd Annual Update in Hospital Medicine
The Third Annual Update in Hospital Medicine presented by Mayo School of Continuing Medical Education was Nov. 8-11 in Tucson, Ariz. The course directors, Ellen Willis, MD, and Adriane Budavari, MD, designed a course to cover not only general hospital medicine, but also to apply research to clinical practice, ethics, provider burnout, and medical history. The course format provided the attendees with five hours of early morning learning for four days, leaving afternoons and evenings free to enjoy Tucson.
After years of continuing medical education (CME) experience, including a stint as associate director of CME at Mayo Arizona from 1995 to 2001, Dr. Willis held the first Mayo Hospital Medicine course in Rochester, Minn., in 2004, with an attendance of 52. In 2005, the venue moved to Tucson, where attendance reached 146, climbing to 161 in 2006. The course continues to improve; we know because of attendees’ reviews.
The 2006 course began with a review of medical history followed by a discussion of disaster management and the role of the hospitalist. The course continued with review of therapeutic hypothermia in the ICU. The next topics were advance directives, ischemic stroke treatments, management of intracerebral hemorrhage, and the proposed change in definition of transient ischemic attack. The first day concluded with common ethical considerations during acute care.
Day two started with evidence-based medicine and a research-based literature review of critical care management strategies. The course continued with presentations on dementia, seizure disorders in the elderly, blistering skin disorders, and wound management principles. Palliative care was reviewed in an interactive format. The day concluded with an array of “toxic syndromes” seen in acutely ill patients, and an evidence-based review supporting more aggressive diabetes mellitus management.
The third day the course started with an exploration of alternative medicines. Then it covered avian influenza, evidence-based recommendations for radiological work-ups in commonly seen inpatient problems, delirium, and psychiatry. After that, there was an update on retrievable IVC filters and one on hospital safety. The day concluded with a panel discussion reviewing delirium case studies with attendees’ participation.
The fourth day covered diagnostic approaches to primary aldosteronism, pheochromocytoma, and also abnormal liver tests. Pulmonary literature was reviewed including the relationship between acetaminophen and respiratory disease. Discussions of common cardiac conditions and upper GI bleeding concluded the conference.
For those of you who need to store some Vitamin D before the winter, or who would like a great review of hospital medicine, the next Update in Hospital Medicine will be back in sunny Tucson Nov. 14-17, 2007.
The 7th Annual Southern Hospital Medicine Update
Ochsner Health System and the Emory University of School of Medicine (Atlanta) hosted the 7th Annual Southern Hospital Medicine Update Nov. 2-4 in New Orleans. Both institutions combined their two successful hospital medicine conferences into one regional symposium after Hurricane Katrina last year.
Steve Deitelzweig, MD, FACP, and David Lee, MD, MBA, FACP, from Ochsner partnered with Mark Williams, MD, FACP, and Dan Dressler, MD, MsC, from Emory. More than 200 healthcare professionals participated in 20 hours of continuing medical education (CME) and the rebirth of New Orleans following the devastation of Katrina last year. The update took place in the heart of the revived French Quarter at the Royal Sonesta Hotel.
The 2006 update started with a New Orleans breakfast of beignets and café au lait. Russell Holman, MD, SHM president-elect, gave a snapshot of the status of hospital medicine, followed by an inspirational talk on leadership by Ochsner CEO Patrick Quinlan.
Cardiology topics were followed by an acute coronary syndrome update, discussion of optimizing management of renal artery stenosis, and updates on congestive heart failure management. Pulmonary and critical care and perioperative management were the topics of the afternoon. Robert Centor of University of Alabama, Birmingham (UAB) worked through various cases of sodium and acid-base problems. Mike Heisler discussed the indications and various modes of mechanical ventilation. Amir Jaffer, MD, of the Cleveland Clinic concluded the day with preoperative assessment and postoperative complications. After a long day of CME guests enjoyed a wine and cheese reception in the hotel’s central courtyard, complete with live jazz music. Then it was “laissez le bon temps rouler” on Bourbon Street.
Day two commenced with vascular medicine and use of clinical case-based teaching pertaining to stroke and critical care. Steve Deitelzweig, MD, reviewed the current advances in venous thromboembolism and urged us to improve prophylaxis in the hospital. Dan Dressler, MD, presented a systematic approach to workup of syncope. The afternoon broke into two concurrent sessions.
The first session included endoscopic approaches to the management of pancreas and biliary diseases, acute gastrointestinal bleeding, and complex endocrine and rheumatology cases. The concurrent session was highlighted by the presentation by Mark Williams, MD, editor of the Journal of Hospital Medicine, on how to optimize the discharge process and Dr. Renee Meadows’ review of strategies to improve safety in the hospital. Then it was on to the French Quarter for the night.
The last half-day began with emerging infectious diseases and hospital-acquired pneumonias. The Review of Medical Literature was followed by a discussion by Jeff Wiese, MD, (Tulane, New Orleans) concerning a hypothetical case that involved the review of the most current literature in 2006. The conference concluded with the wrap-up and review of pearls by David Lee, MD, and left participants with wonderful memories of the atmosphere, foods, and music of New Orleans.
The response from the conference participants has been so positive that the conference directors decided to host the 2007 8th Annual Southern Hospital Medicine Update in New Orleans again and then move to Atlanta in 2008. The conference will expand to include pre-session procedure training, administrative courses and abstract competition next year. TH
The Mayo 3rd Annual Update in Hospital Medicine
The Third Annual Update in Hospital Medicine presented by Mayo School of Continuing Medical Education was Nov. 8-11 in Tucson, Ariz. The course directors, Ellen Willis, MD, and Adriane Budavari, MD, designed a course to cover not only general hospital medicine, but also to apply research to clinical practice, ethics, provider burnout, and medical history. The course format provided the attendees with five hours of early morning learning for four days, leaving afternoons and evenings free to enjoy Tucson.
After years of continuing medical education (CME) experience, including a stint as associate director of CME at Mayo Arizona from 1995 to 2001, Dr. Willis held the first Mayo Hospital Medicine course in Rochester, Minn., in 2004, with an attendance of 52. In 2005, the venue moved to Tucson, where attendance reached 146, climbing to 161 in 2006. The course continues to improve; we know because of attendees’ reviews.
The 2006 course began with a review of medical history followed by a discussion of disaster management and the role of the hospitalist. The course continued with review of therapeutic hypothermia in the ICU. The next topics were advance directives, ischemic stroke treatments, management of intracerebral hemorrhage, and the proposed change in definition of transient ischemic attack. The first day concluded with common ethical considerations during acute care.
Day two started with evidence-based medicine and a research-based literature review of critical care management strategies. The course continued with presentations on dementia, seizure disorders in the elderly, blistering skin disorders, and wound management principles. Palliative care was reviewed in an interactive format. The day concluded with an array of “toxic syndromes” seen in acutely ill patients, and an evidence-based review supporting more aggressive diabetes mellitus management.
The third day the course started with an exploration of alternative medicines. Then it covered avian influenza, evidence-based recommendations for radiological work-ups in commonly seen inpatient problems, delirium, and psychiatry. After that, there was an update on retrievable IVC filters and one on hospital safety. The day concluded with a panel discussion reviewing delirium case studies with attendees’ participation.
The fourth day covered diagnostic approaches to primary aldosteronism, pheochromocytoma, and also abnormal liver tests. Pulmonary literature was reviewed including the relationship between acetaminophen and respiratory disease. Discussions of common cardiac conditions and upper GI bleeding concluded the conference.
For those of you who need to store some Vitamin D before the winter, or who would like a great review of hospital medicine, the next Update in Hospital Medicine will be back in sunny Tucson Nov. 14-17, 2007.
The 7th Annual Southern Hospital Medicine Update
Ochsner Health System and the Emory University of School of Medicine (Atlanta) hosted the 7th Annual Southern Hospital Medicine Update Nov. 2-4 in New Orleans. Both institutions combined their two successful hospital medicine conferences into one regional symposium after Hurricane Katrina last year.
Steve Deitelzweig, MD, FACP, and David Lee, MD, MBA, FACP, from Ochsner partnered with Mark Williams, MD, FACP, and Dan Dressler, MD, MsC, from Emory. More than 200 healthcare professionals participated in 20 hours of continuing medical education (CME) and the rebirth of New Orleans following the devastation of Katrina last year. The update took place in the heart of the revived French Quarter at the Royal Sonesta Hotel.
The 2006 update started with a New Orleans breakfast of beignets and café au lait. Russell Holman, MD, SHM president-elect, gave a snapshot of the status of hospital medicine, followed by an inspirational talk on leadership by Ochsner CEO Patrick Quinlan.
Cardiology topics were followed by an acute coronary syndrome update, discussion of optimizing management of renal artery stenosis, and updates on congestive heart failure management. Pulmonary and critical care and perioperative management were the topics of the afternoon. Robert Centor of University of Alabama, Birmingham (UAB) worked through various cases of sodium and acid-base problems. Mike Heisler discussed the indications and various modes of mechanical ventilation. Amir Jaffer, MD, of the Cleveland Clinic concluded the day with preoperative assessment and postoperative complications. After a long day of CME guests enjoyed a wine and cheese reception in the hotel’s central courtyard, complete with live jazz music. Then it was “laissez le bon temps rouler” on Bourbon Street.
Day two commenced with vascular medicine and use of clinical case-based teaching pertaining to stroke and critical care. Steve Deitelzweig, MD, reviewed the current advances in venous thromboembolism and urged us to improve prophylaxis in the hospital. Dan Dressler, MD, presented a systematic approach to workup of syncope. The afternoon broke into two concurrent sessions.
The first session included endoscopic approaches to the management of pancreas and biliary diseases, acute gastrointestinal bleeding, and complex endocrine and rheumatology cases. The concurrent session was highlighted by the presentation by Mark Williams, MD, editor of the Journal of Hospital Medicine, on how to optimize the discharge process and Dr. Renee Meadows’ review of strategies to improve safety in the hospital. Then it was on to the French Quarter for the night.
The last half-day began with emerging infectious diseases and hospital-acquired pneumonias. The Review of Medical Literature was followed by a discussion by Jeff Wiese, MD, (Tulane, New Orleans) concerning a hypothetical case that involved the review of the most current literature in 2006. The conference concluded with the wrap-up and review of pearls by David Lee, MD, and left participants with wonderful memories of the atmosphere, foods, and music of New Orleans.
The response from the conference participants has been so positive that the conference directors decided to host the 2007 8th Annual Southern Hospital Medicine Update in New Orleans again and then move to Atlanta in 2008. The conference will expand to include pre-session procedure training, administrative courses and abstract competition next year. TH
The Mayo 3rd Annual Update in Hospital Medicine
The Third Annual Update in Hospital Medicine presented by Mayo School of Continuing Medical Education was Nov. 8-11 in Tucson, Ariz. The course directors, Ellen Willis, MD, and Adriane Budavari, MD, designed a course to cover not only general hospital medicine, but also to apply research to clinical practice, ethics, provider burnout, and medical history. The course format provided the attendees with five hours of early morning learning for four days, leaving afternoons and evenings free to enjoy Tucson.
After years of continuing medical education (CME) experience, including a stint as associate director of CME at Mayo Arizona from 1995 to 2001, Dr. Willis held the first Mayo Hospital Medicine course in Rochester, Minn., in 2004, with an attendance of 52. In 2005, the venue moved to Tucson, where attendance reached 146, climbing to 161 in 2006. The course continues to improve; we know because of attendees’ reviews.
The 2006 course began with a review of medical history followed by a discussion of disaster management and the role of the hospitalist. The course continued with review of therapeutic hypothermia in the ICU. The next topics were advance directives, ischemic stroke treatments, management of intracerebral hemorrhage, and the proposed change in definition of transient ischemic attack. The first day concluded with common ethical considerations during acute care.
Day two started with evidence-based medicine and a research-based literature review of critical care management strategies. The course continued with presentations on dementia, seizure disorders in the elderly, blistering skin disorders, and wound management principles. Palliative care was reviewed in an interactive format. The day concluded with an array of “toxic syndromes” seen in acutely ill patients, and an evidence-based review supporting more aggressive diabetes mellitus management.
The third day the course started with an exploration of alternative medicines. Then it covered avian influenza, evidence-based recommendations for radiological work-ups in commonly seen inpatient problems, delirium, and psychiatry. After that, there was an update on retrievable IVC filters and one on hospital safety. The day concluded with a panel discussion reviewing delirium case studies with attendees’ participation.
The fourth day covered diagnostic approaches to primary aldosteronism, pheochromocytoma, and also abnormal liver tests. Pulmonary literature was reviewed including the relationship between acetaminophen and respiratory disease. Discussions of common cardiac conditions and upper GI bleeding concluded the conference.
For those of you who need to store some Vitamin D before the winter, or who would like a great review of hospital medicine, the next Update in Hospital Medicine will be back in sunny Tucson Nov. 14-17, 2007.
The 7th Annual Southern Hospital Medicine Update
Ochsner Health System and the Emory University of School of Medicine (Atlanta) hosted the 7th Annual Southern Hospital Medicine Update Nov. 2-4 in New Orleans. Both institutions combined their two successful hospital medicine conferences into one regional symposium after Hurricane Katrina last year.
Steve Deitelzweig, MD, FACP, and David Lee, MD, MBA, FACP, from Ochsner partnered with Mark Williams, MD, FACP, and Dan Dressler, MD, MsC, from Emory. More than 200 healthcare professionals participated in 20 hours of continuing medical education (CME) and the rebirth of New Orleans following the devastation of Katrina last year. The update took place in the heart of the revived French Quarter at the Royal Sonesta Hotel.
The 2006 update started with a New Orleans breakfast of beignets and café au lait. Russell Holman, MD, SHM president-elect, gave a snapshot of the status of hospital medicine, followed by an inspirational talk on leadership by Ochsner CEO Patrick Quinlan.
Cardiology topics were followed by an acute coronary syndrome update, discussion of optimizing management of renal artery stenosis, and updates on congestive heart failure management. Pulmonary and critical care and perioperative management were the topics of the afternoon. Robert Centor of University of Alabama, Birmingham (UAB) worked through various cases of sodium and acid-base problems. Mike Heisler discussed the indications and various modes of mechanical ventilation. Amir Jaffer, MD, of the Cleveland Clinic concluded the day with preoperative assessment and postoperative complications. After a long day of CME guests enjoyed a wine and cheese reception in the hotel’s central courtyard, complete with live jazz music. Then it was “laissez le bon temps rouler” on Bourbon Street.
Day two commenced with vascular medicine and use of clinical case-based teaching pertaining to stroke and critical care. Steve Deitelzweig, MD, reviewed the current advances in venous thromboembolism and urged us to improve prophylaxis in the hospital. Dan Dressler, MD, presented a systematic approach to workup of syncope. The afternoon broke into two concurrent sessions.
The first session included endoscopic approaches to the management of pancreas and biliary diseases, acute gastrointestinal bleeding, and complex endocrine and rheumatology cases. The concurrent session was highlighted by the presentation by Mark Williams, MD, editor of the Journal of Hospital Medicine, on how to optimize the discharge process and Dr. Renee Meadows’ review of strategies to improve safety in the hospital. Then it was on to the French Quarter for the night.
The last half-day began with emerging infectious diseases and hospital-acquired pneumonias. The Review of Medical Literature was followed by a discussion by Jeff Wiese, MD, (Tulane, New Orleans) concerning a hypothetical case that involved the review of the most current literature in 2006. The conference concluded with the wrap-up and review of pearls by David Lee, MD, and left participants with wonderful memories of the atmosphere, foods, and music of New Orleans.
The response from the conference participants has been so positive that the conference directors decided to host the 2007 8th Annual Southern Hospital Medicine Update in New Orleans again and then move to Atlanta in 2008. The conference will expand to include pre-session procedure training, administrative courses and abstract competition next year. TH