User login
Progress report: Elimination of neonatal tetanus
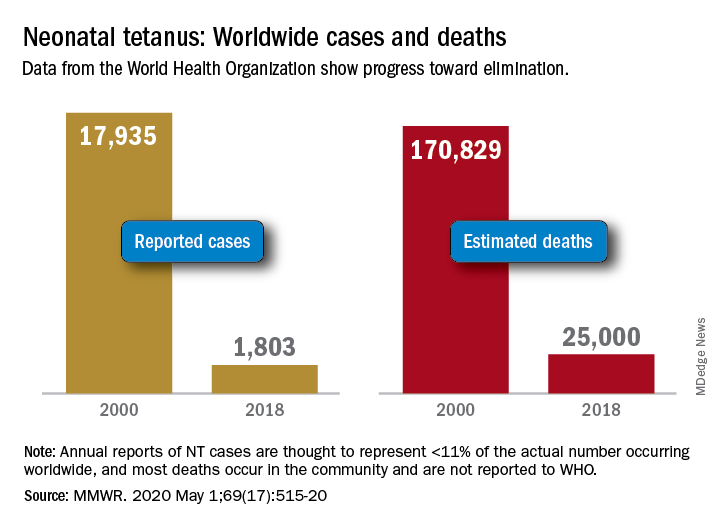
Worldwide cases of neonatal tetanus fell by 90% from 2000 to 2018, deaths dropped by 85%, and 45 countries achieved elimination of maternal and neonatal tetanus (MNT), according to the Centers for Disease Control and Prevention.
“Despite this progress, some countries that achieved elimination are still struggling to sustain performance indicators; war and insecurity pose challenges in countries that have not achieved MNT elimination,” Henry N. Njuguna, MD, of the CDC’s global immunization division, and associates wrote in the Morbidity and Mortality Weekly Report.
Other worldwide measures also improved from 2000 to 2018: and the percentage of deliveries attended by a skilled birth attendant increased from 62% during 2000-2005 to 81% in 2013-2018, they reported.
The MNT elimination initiative, which began in 1999 and targeted 59 priority countries, immunized approximately 154 million women of reproductive age with at least two doses of tetanus toxoid–containing vaccine from 2000 to 2018, the investigators wrote, based on data from the World Health Organization and the United Nations Children’s Fund.
With 14 of the priority countries – including Nigeria, Pakistan, and Yemen – still dealing with MNT, however, numerous challenges remain, they noted. About 47 million women and their babies are still unprotected, and 49 million women have not received tetanus toxoid–containing vaccine.
This lack of coverage “can be attributed to weak health systems, including conflict and security issues that limit access to vaccination services, competing priorities that limit the implementation of planned MNT elimination activities, and withdrawal of donor funding,” Dr. Njuguna and associates wrote.
SOURCE: Njuguna HN et al. MMWR. 2020 May 1;69(17):515-20.
Worldwide cases of neonatal tetanus fell by 90% from 2000 to 2018, deaths dropped by 85%, and 45 countries achieved elimination of maternal and neonatal tetanus (MNT), according to the Centers for Disease Control and Prevention.
“Despite this progress, some countries that achieved elimination are still struggling to sustain performance indicators; war and insecurity pose challenges in countries that have not achieved MNT elimination,” Henry N. Njuguna, MD, of the CDC’s global immunization division, and associates wrote in the Morbidity and Mortality Weekly Report.
Other worldwide measures also improved from 2000 to 2018: and the percentage of deliveries attended by a skilled birth attendant increased from 62% during 2000-2005 to 81% in 2013-2018, they reported.
The MNT elimination initiative, which began in 1999 and targeted 59 priority countries, immunized approximately 154 million women of reproductive age with at least two doses of tetanus toxoid–containing vaccine from 2000 to 2018, the investigators wrote, based on data from the World Health Organization and the United Nations Children’s Fund.
With 14 of the priority countries – including Nigeria, Pakistan, and Yemen – still dealing with MNT, however, numerous challenges remain, they noted. About 47 million women and their babies are still unprotected, and 49 million women have not received tetanus toxoid–containing vaccine.
This lack of coverage “can be attributed to weak health systems, including conflict and security issues that limit access to vaccination services, competing priorities that limit the implementation of planned MNT elimination activities, and withdrawal of donor funding,” Dr. Njuguna and associates wrote.
SOURCE: Njuguna HN et al. MMWR. 2020 May 1;69(17):515-20.
Worldwide cases of neonatal tetanus fell by 90% from 2000 to 2018, deaths dropped by 85%, and 45 countries achieved elimination of maternal and neonatal tetanus (MNT), according to the Centers for Disease Control and Prevention.
“Despite this progress, some countries that achieved elimination are still struggling to sustain performance indicators; war and insecurity pose challenges in countries that have not achieved MNT elimination,” Henry N. Njuguna, MD, of the CDC’s global immunization division, and associates wrote in the Morbidity and Mortality Weekly Report.
Other worldwide measures also improved from 2000 to 2018: and the percentage of deliveries attended by a skilled birth attendant increased from 62% during 2000-2005 to 81% in 2013-2018, they reported.
The MNT elimination initiative, which began in 1999 and targeted 59 priority countries, immunized approximately 154 million women of reproductive age with at least two doses of tetanus toxoid–containing vaccine from 2000 to 2018, the investigators wrote, based on data from the World Health Organization and the United Nations Children’s Fund.
With 14 of the priority countries – including Nigeria, Pakistan, and Yemen – still dealing with MNT, however, numerous challenges remain, they noted. About 47 million women and their babies are still unprotected, and 49 million women have not received tetanus toxoid–containing vaccine.
This lack of coverage “can be attributed to weak health systems, including conflict and security issues that limit access to vaccination services, competing priorities that limit the implementation of planned MNT elimination activities, and withdrawal of donor funding,” Dr. Njuguna and associates wrote.
SOURCE: Njuguna HN et al. MMWR. 2020 May 1;69(17):515-20.
FROM MMWR
New study of diabetes drug for COVID-19 raises eyebrows
A just-launched study of the type 2 diabetes agent dapagliflozin (Farxiga, AstraZeneca) in patients with mild to moderate COVID-19 is raising eyebrows, given that several expert groups have advised that drugs in this class – the sodium-glucose cotransporter 2 (SGLT2) inhibitors – be stopped in all patients hospitalized with COVID-19 because of the increased risk for diabetic ketoacidosis (DKA).
The randomized, double-blind, placebo-controlled, phase 3 Dapagliflozin in Respiratory Failure in Patients With COVID-19 (DARE-19) study is sponsored by AstraZeneca and Saint Luke’s Mid America Heart Institute.
The trial will assess whether dapagliflozin reduces the risks of disease progression, clinical complications, and death because of COVID-19 in patients with type 2 diabetes, cardiovascular disease, and/or mild to moderate chronic kidney disease (CKD).
“Dapagliflozin has demonstrated cardio- and renal-protective benefits and improved outcomes in high-risk patients with type 2 diabetes, heart failure with reduced ejection fraction, and CKD,” said the principal investigator of DARE-19, Mikhail N. Kosiborod, MD, a cardiologist at Saint Luke’s Mid America Heart Institute, Kansas City, Mo.
And “patients with COVID-19 and underlying cardiometabolic disease appear to be at the highest risk of morbid complications,” he explained in an AstraZeneca statement.
“Through DARE-19, we hope to decrease the severity of illness, and prevent cardiovascular, respiratory, and kidney decompensation, which are common in patients with COVID-19,” Dr. Kosiborod continued.
However, advice to stop SGLT2 inhibitors in patients hospitalized with COVID-19 because of its associated DKA risk has come from several channels.
These include initial guidance from Diabetes UK; experts who spoke during an American Diabetes Association webinar; and most recently, an international panel of diabetes experts.
Some clinicians went so far as to say that they view the trial as potentially dangerous, while others said they could see some logic to it, as long as it is carefully managed.
“A dangerous proposition – a DARE I would not take”
Partha Kar, MD, of Portsmouth Hospitals NHS Trust and national clinical director of diabetes at NHS England, said in an interview: “It’s interesting to see [AstraZeneca] embark on a study with a particular class of drug whereby ... [in] the UK we have said that if you get sent to hospital with COVID-19 you should stop [SGLT2 inhibitors] immediately.”
It “sounds like a risky proposition to go ahead with, [and it] definitely made me raise an eyebrow,” he added.
Nephrologist Bruce R. Leslie, MD, of Seventh Doctor Consulting in Princeton, N.J., agreed with Dr. Kar.
“Giving SGLT2 inhibitors to patients in the DARE-19 study is a dangerous proposition because these drugs can induce ketoacidosis during the stress of acute illness such as COVID-19. ... Moreover, ketoacidosis is associated with hypercoagulability which could be especially dangerous in COVID-19, given that it has been causing thrombophilia with large-vessel occlusive strokes in young patients,” he said in an interview.
“One wonders how these risks were assessed by the authorities that approved the DARE-19 study,” said Dr. Leslie, who formerly worked for Bristol-Myers Squibb.
“How does the sponsor intend to secure informed consent given the risks? This is a DARE I would not take,” he said.
Asked to address these concerns, Dr. Kosiborod said in an interview that “the DARE-19 trial will assess both the efficacy and the safety of dapagliflozin in this patient population in a closely monitored environment of a rigorously designed randomized clinical trial. The trial protocol excludes patients with type 1 diabetes or at high risk for DKA.
“Furthermore, the protocol includes detailed specific instructions to ensure careful monitoring for DKA, including frequent assessments of acid-base status in the hospital setting. The safety data will be closely monitored by an independent data-monitoring committee,” he continued.
Dr. Kosiborod also pointed out that there is “no systematically collected information on the use of dapagliflozin or any other SGLT2 inhibitor in patients being treated for COVID-19, including the associated potential benefits, possible risks such as DKA, and the balance of these potential benefits and risks.”
DARE-19 design: Several outcomes will be examined
The DARE-19 trial is designed to enroll 900 adults with confirmed SARS-CoV-2 infection and oxygen saturation of 94% or greater.
Inclusion criteria include a medical history of hypertension, type 2 diabetes, atherosclerotic cardiovascular disease, heart failure, and/or stage 3-4 CKD. Exclusion criteria include current SGLT2 inhibitor treatment, type 1 diabetes, severe CKD, and severe COVID-19.
Dapagliflozin is approved in the EU for use in some patients with type 1 diabetes; this is not the case in the United States, although SGLT2 inhibitors in general are sometimes used off label in these patients.
Patients in DARE-19 will be randomized to 10 mg/day dapagliflozin or placebo for 30 days, in addition to standard care, in participating hospital. Primary outcomes are time to first occurrence of either death or new or worsened organ dysfunction, including respiratory decompensation, new or worsening heart failure, requirement for vasopressor therapy, ventricular tachycardia, and renal failure.
Secondary outcomes include a composite of time to death from any cause, time to new/worsened organ dysfunction, clinical status at day 30, and time to hospital discharge.
Rationale for the study
Irl B. Hirsch, MD, professor and diabetes treatment and teaching chair at the University of Washington, Seattle, said in an interview that he does see some logic to the trial.
Admitting that he doesn’t know much about “COVID-19 cardiomyopathy” – which would be one of the targets of dapagliflozin – other than it is quite common, he said that this, along with the potential renal benefits of dapagliflozin in the setting of COVID-19, make the study “intriguing.”
“Perhaps there is some rationale to it,” he said. However, “my concern is these sick COVID-19 patients are often acidemic, and besides the very complex acid-base challenges we see with intubated patients, these patients likely have combination lactic and ketoacidemia, the latter at least some from starvation.
“Still, if enough dextrose and insulin are provided to prevent ketoacid accumulation, my guess is it would do at least as well as hydroxychloroquine,” he said.
And Simon Heller, MD, professor of clinical diabetes at the University of Sheffield (England), said in an interview: “I think it is quite a brave study, mainly because of the increased risk of DKA.
“However, on the basis that these patients will be carefully monitored, the risk of DKA shouldn’t be great. I think it is important that patients with type 2 diabetes can participate whenever possible in such trials,” he said.
The estimated completion date for DARE-19 is December 2020.
Dr. Kosiborod has reported receiving grant support, honoraria, and/or research support from AstraZeneca, Boehringer Ingelheim, Sanofi, Amgen, Novo Nordisk, Merck, Eisai, Janssen, Bayer, GlaxoSmithKline, Glytec, Intarcia Therapeutics, Novartis, Applied Therapeutics, Amarin, and Eli Lilly. Dr. Leslie has reported owning stock in Bristol-Myers Squibb, Pfizer, and Lilly. Dr. Hirsch has reported consulting for Abbott Diabetes Care, Roche, and Bigfoot Biomedical, conducting research for Medtronic, and is a diabetes editor for UpToDate. Dr. Heller has received advisory or consultation fees from Lilly, Novo Nordisk, Takeda, MSD, and Becton Dickinson; has served as a speaker for AstraZeneca, Lilly, Novo Nordisk, Boehringer Ingelheim, and Takeda; and has received research support from Medtronic UK. He is on the advisory board for Medscape. Dr. Kar has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A just-launched study of the type 2 diabetes agent dapagliflozin (Farxiga, AstraZeneca) in patients with mild to moderate COVID-19 is raising eyebrows, given that several expert groups have advised that drugs in this class – the sodium-glucose cotransporter 2 (SGLT2) inhibitors – be stopped in all patients hospitalized with COVID-19 because of the increased risk for diabetic ketoacidosis (DKA).
The randomized, double-blind, placebo-controlled, phase 3 Dapagliflozin in Respiratory Failure in Patients With COVID-19 (DARE-19) study is sponsored by AstraZeneca and Saint Luke’s Mid America Heart Institute.
The trial will assess whether dapagliflozin reduces the risks of disease progression, clinical complications, and death because of COVID-19 in patients with type 2 diabetes, cardiovascular disease, and/or mild to moderate chronic kidney disease (CKD).
“Dapagliflozin has demonstrated cardio- and renal-protective benefits and improved outcomes in high-risk patients with type 2 diabetes, heart failure with reduced ejection fraction, and CKD,” said the principal investigator of DARE-19, Mikhail N. Kosiborod, MD, a cardiologist at Saint Luke’s Mid America Heart Institute, Kansas City, Mo.
And “patients with COVID-19 and underlying cardiometabolic disease appear to be at the highest risk of morbid complications,” he explained in an AstraZeneca statement.
“Through DARE-19, we hope to decrease the severity of illness, and prevent cardiovascular, respiratory, and kidney decompensation, which are common in patients with COVID-19,” Dr. Kosiborod continued.
However, advice to stop SGLT2 inhibitors in patients hospitalized with COVID-19 because of its associated DKA risk has come from several channels.
These include initial guidance from Diabetes UK; experts who spoke during an American Diabetes Association webinar; and most recently, an international panel of diabetes experts.
Some clinicians went so far as to say that they view the trial as potentially dangerous, while others said they could see some logic to it, as long as it is carefully managed.
“A dangerous proposition – a DARE I would not take”
Partha Kar, MD, of Portsmouth Hospitals NHS Trust and national clinical director of diabetes at NHS England, said in an interview: “It’s interesting to see [AstraZeneca] embark on a study with a particular class of drug whereby ... [in] the UK we have said that if you get sent to hospital with COVID-19 you should stop [SGLT2 inhibitors] immediately.”
It “sounds like a risky proposition to go ahead with, [and it] definitely made me raise an eyebrow,” he added.
Nephrologist Bruce R. Leslie, MD, of Seventh Doctor Consulting in Princeton, N.J., agreed with Dr. Kar.
“Giving SGLT2 inhibitors to patients in the DARE-19 study is a dangerous proposition because these drugs can induce ketoacidosis during the stress of acute illness such as COVID-19. ... Moreover, ketoacidosis is associated with hypercoagulability which could be especially dangerous in COVID-19, given that it has been causing thrombophilia with large-vessel occlusive strokes in young patients,” he said in an interview.
“One wonders how these risks were assessed by the authorities that approved the DARE-19 study,” said Dr. Leslie, who formerly worked for Bristol-Myers Squibb.
“How does the sponsor intend to secure informed consent given the risks? This is a DARE I would not take,” he said.
Asked to address these concerns, Dr. Kosiborod said in an interview that “the DARE-19 trial will assess both the efficacy and the safety of dapagliflozin in this patient population in a closely monitored environment of a rigorously designed randomized clinical trial. The trial protocol excludes patients with type 1 diabetes or at high risk for DKA.
“Furthermore, the protocol includes detailed specific instructions to ensure careful monitoring for DKA, including frequent assessments of acid-base status in the hospital setting. The safety data will be closely monitored by an independent data-monitoring committee,” he continued.
Dr. Kosiborod also pointed out that there is “no systematically collected information on the use of dapagliflozin or any other SGLT2 inhibitor in patients being treated for COVID-19, including the associated potential benefits, possible risks such as DKA, and the balance of these potential benefits and risks.”
DARE-19 design: Several outcomes will be examined
The DARE-19 trial is designed to enroll 900 adults with confirmed SARS-CoV-2 infection and oxygen saturation of 94% or greater.
Inclusion criteria include a medical history of hypertension, type 2 diabetes, atherosclerotic cardiovascular disease, heart failure, and/or stage 3-4 CKD. Exclusion criteria include current SGLT2 inhibitor treatment, type 1 diabetes, severe CKD, and severe COVID-19.
Dapagliflozin is approved in the EU for use in some patients with type 1 diabetes; this is not the case in the United States, although SGLT2 inhibitors in general are sometimes used off label in these patients.
Patients in DARE-19 will be randomized to 10 mg/day dapagliflozin or placebo for 30 days, in addition to standard care, in participating hospital. Primary outcomes are time to first occurrence of either death or new or worsened organ dysfunction, including respiratory decompensation, new or worsening heart failure, requirement for vasopressor therapy, ventricular tachycardia, and renal failure.
Secondary outcomes include a composite of time to death from any cause, time to new/worsened organ dysfunction, clinical status at day 30, and time to hospital discharge.
Rationale for the study
Irl B. Hirsch, MD, professor and diabetes treatment and teaching chair at the University of Washington, Seattle, said in an interview that he does see some logic to the trial.
Admitting that he doesn’t know much about “COVID-19 cardiomyopathy” – which would be one of the targets of dapagliflozin – other than it is quite common, he said that this, along with the potential renal benefits of dapagliflozin in the setting of COVID-19, make the study “intriguing.”
“Perhaps there is some rationale to it,” he said. However, “my concern is these sick COVID-19 patients are often acidemic, and besides the very complex acid-base challenges we see with intubated patients, these patients likely have combination lactic and ketoacidemia, the latter at least some from starvation.
“Still, if enough dextrose and insulin are provided to prevent ketoacid accumulation, my guess is it would do at least as well as hydroxychloroquine,” he said.
And Simon Heller, MD, professor of clinical diabetes at the University of Sheffield (England), said in an interview: “I think it is quite a brave study, mainly because of the increased risk of DKA.
“However, on the basis that these patients will be carefully monitored, the risk of DKA shouldn’t be great. I think it is important that patients with type 2 diabetes can participate whenever possible in such trials,” he said.
The estimated completion date for DARE-19 is December 2020.
Dr. Kosiborod has reported receiving grant support, honoraria, and/or research support from AstraZeneca, Boehringer Ingelheim, Sanofi, Amgen, Novo Nordisk, Merck, Eisai, Janssen, Bayer, GlaxoSmithKline, Glytec, Intarcia Therapeutics, Novartis, Applied Therapeutics, Amarin, and Eli Lilly. Dr. Leslie has reported owning stock in Bristol-Myers Squibb, Pfizer, and Lilly. Dr. Hirsch has reported consulting for Abbott Diabetes Care, Roche, and Bigfoot Biomedical, conducting research for Medtronic, and is a diabetes editor for UpToDate. Dr. Heller has received advisory or consultation fees from Lilly, Novo Nordisk, Takeda, MSD, and Becton Dickinson; has served as a speaker for AstraZeneca, Lilly, Novo Nordisk, Boehringer Ingelheim, and Takeda; and has received research support from Medtronic UK. He is on the advisory board for Medscape. Dr. Kar has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A just-launched study of the type 2 diabetes agent dapagliflozin (Farxiga, AstraZeneca) in patients with mild to moderate COVID-19 is raising eyebrows, given that several expert groups have advised that drugs in this class – the sodium-glucose cotransporter 2 (SGLT2) inhibitors – be stopped in all patients hospitalized with COVID-19 because of the increased risk for diabetic ketoacidosis (DKA).
The randomized, double-blind, placebo-controlled, phase 3 Dapagliflozin in Respiratory Failure in Patients With COVID-19 (DARE-19) study is sponsored by AstraZeneca and Saint Luke’s Mid America Heart Institute.
The trial will assess whether dapagliflozin reduces the risks of disease progression, clinical complications, and death because of COVID-19 in patients with type 2 diabetes, cardiovascular disease, and/or mild to moderate chronic kidney disease (CKD).
“Dapagliflozin has demonstrated cardio- and renal-protective benefits and improved outcomes in high-risk patients with type 2 diabetes, heart failure with reduced ejection fraction, and CKD,” said the principal investigator of DARE-19, Mikhail N. Kosiborod, MD, a cardiologist at Saint Luke’s Mid America Heart Institute, Kansas City, Mo.
And “patients with COVID-19 and underlying cardiometabolic disease appear to be at the highest risk of morbid complications,” he explained in an AstraZeneca statement.
“Through DARE-19, we hope to decrease the severity of illness, and prevent cardiovascular, respiratory, and kidney decompensation, which are common in patients with COVID-19,” Dr. Kosiborod continued.
However, advice to stop SGLT2 inhibitors in patients hospitalized with COVID-19 because of its associated DKA risk has come from several channels.
These include initial guidance from Diabetes UK; experts who spoke during an American Diabetes Association webinar; and most recently, an international panel of diabetes experts.
Some clinicians went so far as to say that they view the trial as potentially dangerous, while others said they could see some logic to it, as long as it is carefully managed.
“A dangerous proposition – a DARE I would not take”
Partha Kar, MD, of Portsmouth Hospitals NHS Trust and national clinical director of diabetes at NHS England, said in an interview: “It’s interesting to see [AstraZeneca] embark on a study with a particular class of drug whereby ... [in] the UK we have said that if you get sent to hospital with COVID-19 you should stop [SGLT2 inhibitors] immediately.”
It “sounds like a risky proposition to go ahead with, [and it] definitely made me raise an eyebrow,” he added.
Nephrologist Bruce R. Leslie, MD, of Seventh Doctor Consulting in Princeton, N.J., agreed with Dr. Kar.
“Giving SGLT2 inhibitors to patients in the DARE-19 study is a dangerous proposition because these drugs can induce ketoacidosis during the stress of acute illness such as COVID-19. ... Moreover, ketoacidosis is associated with hypercoagulability which could be especially dangerous in COVID-19, given that it has been causing thrombophilia with large-vessel occlusive strokes in young patients,” he said in an interview.
“One wonders how these risks were assessed by the authorities that approved the DARE-19 study,” said Dr. Leslie, who formerly worked for Bristol-Myers Squibb.
“How does the sponsor intend to secure informed consent given the risks? This is a DARE I would not take,” he said.
Asked to address these concerns, Dr. Kosiborod said in an interview that “the DARE-19 trial will assess both the efficacy and the safety of dapagliflozin in this patient population in a closely monitored environment of a rigorously designed randomized clinical trial. The trial protocol excludes patients with type 1 diabetes or at high risk for DKA.
“Furthermore, the protocol includes detailed specific instructions to ensure careful monitoring for DKA, including frequent assessments of acid-base status in the hospital setting. The safety data will be closely monitored by an independent data-monitoring committee,” he continued.
Dr. Kosiborod also pointed out that there is “no systematically collected information on the use of dapagliflozin or any other SGLT2 inhibitor in patients being treated for COVID-19, including the associated potential benefits, possible risks such as DKA, and the balance of these potential benefits and risks.”
DARE-19 design: Several outcomes will be examined
The DARE-19 trial is designed to enroll 900 adults with confirmed SARS-CoV-2 infection and oxygen saturation of 94% or greater.
Inclusion criteria include a medical history of hypertension, type 2 diabetes, atherosclerotic cardiovascular disease, heart failure, and/or stage 3-4 CKD. Exclusion criteria include current SGLT2 inhibitor treatment, type 1 diabetes, severe CKD, and severe COVID-19.
Dapagliflozin is approved in the EU for use in some patients with type 1 diabetes; this is not the case in the United States, although SGLT2 inhibitors in general are sometimes used off label in these patients.
Patients in DARE-19 will be randomized to 10 mg/day dapagliflozin or placebo for 30 days, in addition to standard care, in participating hospital. Primary outcomes are time to first occurrence of either death or new or worsened organ dysfunction, including respiratory decompensation, new or worsening heart failure, requirement for vasopressor therapy, ventricular tachycardia, and renal failure.
Secondary outcomes include a composite of time to death from any cause, time to new/worsened organ dysfunction, clinical status at day 30, and time to hospital discharge.
Rationale for the study
Irl B. Hirsch, MD, professor and diabetes treatment and teaching chair at the University of Washington, Seattle, said in an interview that he does see some logic to the trial.
Admitting that he doesn’t know much about “COVID-19 cardiomyopathy” – which would be one of the targets of dapagliflozin – other than it is quite common, he said that this, along with the potential renal benefits of dapagliflozin in the setting of COVID-19, make the study “intriguing.”
“Perhaps there is some rationale to it,” he said. However, “my concern is these sick COVID-19 patients are often acidemic, and besides the very complex acid-base challenges we see with intubated patients, these patients likely have combination lactic and ketoacidemia, the latter at least some from starvation.
“Still, if enough dextrose and insulin are provided to prevent ketoacid accumulation, my guess is it would do at least as well as hydroxychloroquine,” he said.
And Simon Heller, MD, professor of clinical diabetes at the University of Sheffield (England), said in an interview: “I think it is quite a brave study, mainly because of the increased risk of DKA.
“However, on the basis that these patients will be carefully monitored, the risk of DKA shouldn’t be great. I think it is important that patients with type 2 diabetes can participate whenever possible in such trials,” he said.
The estimated completion date for DARE-19 is December 2020.
Dr. Kosiborod has reported receiving grant support, honoraria, and/or research support from AstraZeneca, Boehringer Ingelheim, Sanofi, Amgen, Novo Nordisk, Merck, Eisai, Janssen, Bayer, GlaxoSmithKline, Glytec, Intarcia Therapeutics, Novartis, Applied Therapeutics, Amarin, and Eli Lilly. Dr. Leslie has reported owning stock in Bristol-Myers Squibb, Pfizer, and Lilly. Dr. Hirsch has reported consulting for Abbott Diabetes Care, Roche, and Bigfoot Biomedical, conducting research for Medtronic, and is a diabetes editor for UpToDate. Dr. Heller has received advisory or consultation fees from Lilly, Novo Nordisk, Takeda, MSD, and Becton Dickinson; has served as a speaker for AstraZeneca, Lilly, Novo Nordisk, Boehringer Ingelheim, and Takeda; and has received research support from Medtronic UK. He is on the advisory board for Medscape. Dr. Kar has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Economic burden of migraine increases with the number of treatment failures
, researchers wrote. Utilization of health care resources and associated costs are greater among patients with three or more treatment failures, compared with patients with fewer treatment failures. This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Migraine entails a significant economic burden, including direct costs (e.g., medical costs) and indirect costs (e.g., lost productivity). Information about the burden associated with failed preventive treatments among migraineurs is limited, however. Lawrence C. Newman, MD, director of the division of headache at NYU Langone Health in New York, and colleagues conducted a study to characterize health care resource utilization (HCRU) and its associated costs among migraineurs, stratified by the number of preventive treatment failures.
About one quarter of patients had two treatment failures
Using data from the IBM MarketScan Commercial and Medicare Supplemental database, Dr. Newman and colleagues identified patients who received a new diagnosis of migraine between Jan. 1, 2011, and June 30, 2015. Next, they identified the number of treatment failures during the 2 years following the initial migraine diagnosis. They assessed HCRU and associated costs during the 12 months following an index event. The index was the date of initiation of the second preventive treatment for patients with one treatment failure, the date of initiation of the third treatment for patients with two treatment failures, and the date of initiation of the fourth treatment for patients with three or more treatment failures.
Dr. Newman’s group identified 44,181 patients with incident migraine who had failed preventive treatments. Of this population, 27,112 patients (61.4%) had one treatment failure, 10,583 (24%) had two treatment failures, and 6,486 (14.7%) had three or more treatment failures.
The total medical cost per patient, including emergency room (ER), inpatient (IP), and outpatient (OP) care, increased with increasing number of treatment failures ($10,329 for one, $13,774 for two, and $35,392 for three or more). When the investigators added prescription drug costs, the total health care costs also increased with number of treatment failures ($13,946 for one, $18,685 for two, and $41,864 for three or more).
Similarly, the per-patient annual health care provider visits increased with increasing numbers of treatment failures. The number of ER visits per year was 0.54, 0.69, and 1.02 for patients with one, two, and three or more treatment failures, respectively. The annual number of IP visits was 0.46, 0.59, and 0.97, for patients with one, two, and three or more treatment failures, respectively. OP visits showed a similar trend. The annual number of office visits was 9.47 for patients with one, 11.24 for patients with two, and 14.26 for patients with three or more treatment failures. The annual number of other visits was 13.15 for patients with one, 15.73 for patients with two, and 19.96 for patients with three or more treatment failures.
Guidelines could enable appropriate treatment
Reasons for treatment failure include misdiagnosis of the headache disorder, failure to identify and account for comorbidities, overlooking concurrent acute medication overuse, and inappropriate dose or formulation, said Dr. Newman. “Common pitfalls in prevention that lead to treatment failure include not using evidence-based treatments, starting at too low of a dose and not increasing, starting too high or increasing the dose too quickly, discontinuing the medication before an effect can be seen (before 8 weeks), patient nonadherence, and not establishing realistic expectations.”
Available resources could help clinicians treat migraine effectively. “The American Headache Society (AHS)/AAN preventive guidelines have been retired, yet they offered several levels of effectiveness of pharmacologic treatments that were evidence-based,” said Dr. Newman. “Furthermore, in 2019, the AHS published a consensus statement on integrating new migraine treatments into clinical practice. This statement offered advice about the new anti-CGRP agents, onabotulinum toxin, and neuromodulation devices. I think this is a good starting point for neurologists to be familiar with to choose appropriate therapeutic options for people living with migraine.”
Earlier treatment may reduce patients’ economic burden
The study’s weaknesses included its observational design and its reliance on diagnostic codes, which raised the possibility that comorbidities were inadequately recognized, said Dr. Newman. The reasons that patients changed medications are unknown, and the results are not generalizable to patients aged 65 years or older, he added.
Major strengths of Dr. Newman’s study are its large sample size and wealth of available data, said Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles. “The multiple subcategories suggest that this was a carefully organized and implemented study,” he added. If any diagnoses of migraine were provided by general practitioners with little knowledge of migraine, this would weaken the study, said Dr. Rapoport, editor-in-chief of Neurology Reviews.
“We can ease the economic burden of migraineurs by improving acute care therapy with better selection and earlier starting of effective preventive therapy,” he continued. “Going for migraine-specific acute care therapy is better than pain medications or other nonspecific therapies. If you do not stop a migraine attack with effective therapy, you increase the odds that the patient will go on to chronic migraine. It is always important to effectively teach doctors and nurses to improve their diagnostic skills and use the optimal acute and preventive therapy.” For their next trial, maximizing an accurate diagnosis and performing a prospective study measuring treatment outcomes will be particularly valuable, Dr. Rapoport concluded.
Dr. Newman’s study was supported by Novartis Pharma in Basel, Switzerland. Together with Amgen, Novartis developed erenumab. Dr. Newman has received compensation from Allergan, Alder, Amgen, Biohaven, Novartis, Teva, Supernus, and Theranica for consulting, serving on a scientific advisory board, speaking, or other activities. He has received compensation from Springer Scientific for editorial services.
SOURCE: Newman L et al. AAN 2020, Abstract S47.009.
, researchers wrote. Utilization of health care resources and associated costs are greater among patients with three or more treatment failures, compared with patients with fewer treatment failures. This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Migraine entails a significant economic burden, including direct costs (e.g., medical costs) and indirect costs (e.g., lost productivity). Information about the burden associated with failed preventive treatments among migraineurs is limited, however. Lawrence C. Newman, MD, director of the division of headache at NYU Langone Health in New York, and colleagues conducted a study to characterize health care resource utilization (HCRU) and its associated costs among migraineurs, stratified by the number of preventive treatment failures.
About one quarter of patients had two treatment failures
Using data from the IBM MarketScan Commercial and Medicare Supplemental database, Dr. Newman and colleagues identified patients who received a new diagnosis of migraine between Jan. 1, 2011, and June 30, 2015. Next, they identified the number of treatment failures during the 2 years following the initial migraine diagnosis. They assessed HCRU and associated costs during the 12 months following an index event. The index was the date of initiation of the second preventive treatment for patients with one treatment failure, the date of initiation of the third treatment for patients with two treatment failures, and the date of initiation of the fourth treatment for patients with three or more treatment failures.
Dr. Newman’s group identified 44,181 patients with incident migraine who had failed preventive treatments. Of this population, 27,112 patients (61.4%) had one treatment failure, 10,583 (24%) had two treatment failures, and 6,486 (14.7%) had three or more treatment failures.
The total medical cost per patient, including emergency room (ER), inpatient (IP), and outpatient (OP) care, increased with increasing number of treatment failures ($10,329 for one, $13,774 for two, and $35,392 for three or more). When the investigators added prescription drug costs, the total health care costs also increased with number of treatment failures ($13,946 for one, $18,685 for two, and $41,864 for three or more).
Similarly, the per-patient annual health care provider visits increased with increasing numbers of treatment failures. The number of ER visits per year was 0.54, 0.69, and 1.02 for patients with one, two, and three or more treatment failures, respectively. The annual number of IP visits was 0.46, 0.59, and 0.97, for patients with one, two, and three or more treatment failures, respectively. OP visits showed a similar trend. The annual number of office visits was 9.47 for patients with one, 11.24 for patients with two, and 14.26 for patients with three or more treatment failures. The annual number of other visits was 13.15 for patients with one, 15.73 for patients with two, and 19.96 for patients with three or more treatment failures.
Guidelines could enable appropriate treatment
Reasons for treatment failure include misdiagnosis of the headache disorder, failure to identify and account for comorbidities, overlooking concurrent acute medication overuse, and inappropriate dose or formulation, said Dr. Newman. “Common pitfalls in prevention that lead to treatment failure include not using evidence-based treatments, starting at too low of a dose and not increasing, starting too high or increasing the dose too quickly, discontinuing the medication before an effect can be seen (before 8 weeks), patient nonadherence, and not establishing realistic expectations.”
Available resources could help clinicians treat migraine effectively. “The American Headache Society (AHS)/AAN preventive guidelines have been retired, yet they offered several levels of effectiveness of pharmacologic treatments that were evidence-based,” said Dr. Newman. “Furthermore, in 2019, the AHS published a consensus statement on integrating new migraine treatments into clinical practice. This statement offered advice about the new anti-CGRP agents, onabotulinum toxin, and neuromodulation devices. I think this is a good starting point for neurologists to be familiar with to choose appropriate therapeutic options for people living with migraine.”
Earlier treatment may reduce patients’ economic burden
The study’s weaknesses included its observational design and its reliance on diagnostic codes, which raised the possibility that comorbidities were inadequately recognized, said Dr. Newman. The reasons that patients changed medications are unknown, and the results are not generalizable to patients aged 65 years or older, he added.
Major strengths of Dr. Newman’s study are its large sample size and wealth of available data, said Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles. “The multiple subcategories suggest that this was a carefully organized and implemented study,” he added. If any diagnoses of migraine were provided by general practitioners with little knowledge of migraine, this would weaken the study, said Dr. Rapoport, editor-in-chief of Neurology Reviews.
“We can ease the economic burden of migraineurs by improving acute care therapy with better selection and earlier starting of effective preventive therapy,” he continued. “Going for migraine-specific acute care therapy is better than pain medications or other nonspecific therapies. If you do not stop a migraine attack with effective therapy, you increase the odds that the patient will go on to chronic migraine. It is always important to effectively teach doctors and nurses to improve their diagnostic skills and use the optimal acute and preventive therapy.” For their next trial, maximizing an accurate diagnosis and performing a prospective study measuring treatment outcomes will be particularly valuable, Dr. Rapoport concluded.
Dr. Newman’s study was supported by Novartis Pharma in Basel, Switzerland. Together with Amgen, Novartis developed erenumab. Dr. Newman has received compensation from Allergan, Alder, Amgen, Biohaven, Novartis, Teva, Supernus, and Theranica for consulting, serving on a scientific advisory board, speaking, or other activities. He has received compensation from Springer Scientific for editorial services.
SOURCE: Newman L et al. AAN 2020, Abstract S47.009.
, researchers wrote. Utilization of health care resources and associated costs are greater among patients with three or more treatment failures, compared with patients with fewer treatment failures. This research was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Migraine entails a significant economic burden, including direct costs (e.g., medical costs) and indirect costs (e.g., lost productivity). Information about the burden associated with failed preventive treatments among migraineurs is limited, however. Lawrence C. Newman, MD, director of the division of headache at NYU Langone Health in New York, and colleagues conducted a study to characterize health care resource utilization (HCRU) and its associated costs among migraineurs, stratified by the number of preventive treatment failures.
About one quarter of patients had two treatment failures
Using data from the IBM MarketScan Commercial and Medicare Supplemental database, Dr. Newman and colleagues identified patients who received a new diagnosis of migraine between Jan. 1, 2011, and June 30, 2015. Next, they identified the number of treatment failures during the 2 years following the initial migraine diagnosis. They assessed HCRU and associated costs during the 12 months following an index event. The index was the date of initiation of the second preventive treatment for patients with one treatment failure, the date of initiation of the third treatment for patients with two treatment failures, and the date of initiation of the fourth treatment for patients with three or more treatment failures.
Dr. Newman’s group identified 44,181 patients with incident migraine who had failed preventive treatments. Of this population, 27,112 patients (61.4%) had one treatment failure, 10,583 (24%) had two treatment failures, and 6,486 (14.7%) had three or more treatment failures.
The total medical cost per patient, including emergency room (ER), inpatient (IP), and outpatient (OP) care, increased with increasing number of treatment failures ($10,329 for one, $13,774 for two, and $35,392 for three or more). When the investigators added prescription drug costs, the total health care costs also increased with number of treatment failures ($13,946 for one, $18,685 for two, and $41,864 for three or more).
Similarly, the per-patient annual health care provider visits increased with increasing numbers of treatment failures. The number of ER visits per year was 0.54, 0.69, and 1.02 for patients with one, two, and three or more treatment failures, respectively. The annual number of IP visits was 0.46, 0.59, and 0.97, for patients with one, two, and three or more treatment failures, respectively. OP visits showed a similar trend. The annual number of office visits was 9.47 for patients with one, 11.24 for patients with two, and 14.26 for patients with three or more treatment failures. The annual number of other visits was 13.15 for patients with one, 15.73 for patients with two, and 19.96 for patients with three or more treatment failures.
Guidelines could enable appropriate treatment
Reasons for treatment failure include misdiagnosis of the headache disorder, failure to identify and account for comorbidities, overlooking concurrent acute medication overuse, and inappropriate dose or formulation, said Dr. Newman. “Common pitfalls in prevention that lead to treatment failure include not using evidence-based treatments, starting at too low of a dose and not increasing, starting too high or increasing the dose too quickly, discontinuing the medication before an effect can be seen (before 8 weeks), patient nonadherence, and not establishing realistic expectations.”
Available resources could help clinicians treat migraine effectively. “The American Headache Society (AHS)/AAN preventive guidelines have been retired, yet they offered several levels of effectiveness of pharmacologic treatments that were evidence-based,” said Dr. Newman. “Furthermore, in 2019, the AHS published a consensus statement on integrating new migraine treatments into clinical practice. This statement offered advice about the new anti-CGRP agents, onabotulinum toxin, and neuromodulation devices. I think this is a good starting point for neurologists to be familiar with to choose appropriate therapeutic options for people living with migraine.”
Earlier treatment may reduce patients’ economic burden
The study’s weaknesses included its observational design and its reliance on diagnostic codes, which raised the possibility that comorbidities were inadequately recognized, said Dr. Newman. The reasons that patients changed medications are unknown, and the results are not generalizable to patients aged 65 years or older, he added.
Major strengths of Dr. Newman’s study are its large sample size and wealth of available data, said Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles. “The multiple subcategories suggest that this was a carefully organized and implemented study,” he added. If any diagnoses of migraine were provided by general practitioners with little knowledge of migraine, this would weaken the study, said Dr. Rapoport, editor-in-chief of Neurology Reviews.
“We can ease the economic burden of migraineurs by improving acute care therapy with better selection and earlier starting of effective preventive therapy,” he continued. “Going for migraine-specific acute care therapy is better than pain medications or other nonspecific therapies. If you do not stop a migraine attack with effective therapy, you increase the odds that the patient will go on to chronic migraine. It is always important to effectively teach doctors and nurses to improve their diagnostic skills and use the optimal acute and preventive therapy.” For their next trial, maximizing an accurate diagnosis and performing a prospective study measuring treatment outcomes will be particularly valuable, Dr. Rapoport concluded.
Dr. Newman’s study was supported by Novartis Pharma in Basel, Switzerland. Together with Amgen, Novartis developed erenumab. Dr. Newman has received compensation from Allergan, Alder, Amgen, Biohaven, Novartis, Teva, Supernus, and Theranica for consulting, serving on a scientific advisory board, speaking, or other activities. He has received compensation from Springer Scientific for editorial services.
SOURCE: Newman L et al. AAN 2020, Abstract S47.009.
From AAN 2020
Case series suggests biologics, JAK inhibitors safe during pandemic
Use of biologics and Janus kinase (JAK) inhibitors was not associated with worse outcomes in 86 people with inflammatory diseases who contracted COVID-19, according to a case series from New York University Langone Health.
“We are not seeing worse outcomes with overall use of either. It’s reassuring” that the data support continued use during the pandemic, said rheumatologist and senior investigator Jose Scher, MD, an associate professor at New York University.
There have been concerns among rheumatologists, gastroenterologists, and dermatologists that underlying inflammatory diseases and the agents used to treat them would impact outcomes in COVID-19.
Dr. Scher and colleagues, including lead author and rheumatologist Rebecca Haberman, MD, wanted to address the issue, so they reviewed the experience in their own health system of patients with inflammatory diseases – most commonly psoriatic arthritis, RA, and Crohn’s disease – who were assessed for COVID-19 from March 3 to April 3.
Fever, cough, and shortness of breath were the most common symptoms. The infection was confirmed by polymerase chain reaction in 59 (69%) and highly suspected in 27.
A total of 62 patients (72%) were on JAK inhibitors or biologics at baseline, including 38 (44%) on tumor necrosis factor inhibitors.
Overall, 14 patients (16%) were hospitalized with COVID-19, which is consistent the 26% hospitalization rate among the general population in New York City.
Baseline biologic and JAK inhibitor use was actually lower among hospitalized patients than among those who weren’t hospitalized (50% vs. 76%), and the hospitalization rate was only 11% among 62 subjects who had been on the agents long term, more than a year among most.
Hospitalized patients tended to be slightly older (mean, 50 vs. 46 years) with a higher prevalence of hypertension, diabetes, and chronic obstructive pulmonary disease. They also had a higher prevalence of RA (43% vs. 19%), methotrexate use (43% vs. 15%), and use of hydroxychloroquine (21% vs. 7%) and oral glucocorticoids (29% vs. 6%).
It’s unknown what to make of those findings for now, Dr. Scher said. The study didn’t address differences in the severity of the underlying inflammatory illness, but a new and significantly larger case series is in the works that will analyze that and other potential confounders.
Dr. Scher noted that he’s particularly interested in drilling down further on the higher prevalence of RA and methotrexate in hospitalized patients. “We want to understand those signals better. All of this needs further validation,” he said.
Of the 14 hospitalized patients, 11 (79%) were discharged after a mean of 5.6 days. One died in the ED, and two remained hospitalized as of April 3, including one in the ICU.
The investigators are contributing to COVID-19 registries for inflammatory disease patients. The registries are tending to report higher hospitalization rates, but Dr. Scher noted they might be biased towards more severe cases, among other issues.
As for the current situation in New York City, he said that the “last week in March and first 3 in April were indescribable in terms of admissions, intubations, and deaths. Over the last week or so, it has calmed down significantly.”
There was no external funding. Dr. Haberman reported ties to Janssen, and Dr. Scher reported ties to Janssen, Novartis, Pfizer, and other companies.
aotto@mdedge.com
SOURCE: Haberman R et al. N Engl J Med. 2020 Apr 29. doi: 10.1056/NEJMc2009567.
Use of biologics and Janus kinase (JAK) inhibitors was not associated with worse outcomes in 86 people with inflammatory diseases who contracted COVID-19, according to a case series from New York University Langone Health.
“We are not seeing worse outcomes with overall use of either. It’s reassuring” that the data support continued use during the pandemic, said rheumatologist and senior investigator Jose Scher, MD, an associate professor at New York University.
There have been concerns among rheumatologists, gastroenterologists, and dermatologists that underlying inflammatory diseases and the agents used to treat them would impact outcomes in COVID-19.
Dr. Scher and colleagues, including lead author and rheumatologist Rebecca Haberman, MD, wanted to address the issue, so they reviewed the experience in their own health system of patients with inflammatory diseases – most commonly psoriatic arthritis, RA, and Crohn’s disease – who were assessed for COVID-19 from March 3 to April 3.
Fever, cough, and shortness of breath were the most common symptoms. The infection was confirmed by polymerase chain reaction in 59 (69%) and highly suspected in 27.
A total of 62 patients (72%) were on JAK inhibitors or biologics at baseline, including 38 (44%) on tumor necrosis factor inhibitors.
Overall, 14 patients (16%) were hospitalized with COVID-19, which is consistent the 26% hospitalization rate among the general population in New York City.
Baseline biologic and JAK inhibitor use was actually lower among hospitalized patients than among those who weren’t hospitalized (50% vs. 76%), and the hospitalization rate was only 11% among 62 subjects who had been on the agents long term, more than a year among most.
Hospitalized patients tended to be slightly older (mean, 50 vs. 46 years) with a higher prevalence of hypertension, diabetes, and chronic obstructive pulmonary disease. They also had a higher prevalence of RA (43% vs. 19%), methotrexate use (43% vs. 15%), and use of hydroxychloroquine (21% vs. 7%) and oral glucocorticoids (29% vs. 6%).
It’s unknown what to make of those findings for now, Dr. Scher said. The study didn’t address differences in the severity of the underlying inflammatory illness, but a new and significantly larger case series is in the works that will analyze that and other potential confounders.
Dr. Scher noted that he’s particularly interested in drilling down further on the higher prevalence of RA and methotrexate in hospitalized patients. “We want to understand those signals better. All of this needs further validation,” he said.
Of the 14 hospitalized patients, 11 (79%) were discharged after a mean of 5.6 days. One died in the ED, and two remained hospitalized as of April 3, including one in the ICU.
The investigators are contributing to COVID-19 registries for inflammatory disease patients. The registries are tending to report higher hospitalization rates, but Dr. Scher noted they might be biased towards more severe cases, among other issues.
As for the current situation in New York City, he said that the “last week in March and first 3 in April were indescribable in terms of admissions, intubations, and deaths. Over the last week or so, it has calmed down significantly.”
There was no external funding. Dr. Haberman reported ties to Janssen, and Dr. Scher reported ties to Janssen, Novartis, Pfizer, and other companies.
aotto@mdedge.com
SOURCE: Haberman R et al. N Engl J Med. 2020 Apr 29. doi: 10.1056/NEJMc2009567.
Use of biologics and Janus kinase (JAK) inhibitors was not associated with worse outcomes in 86 people with inflammatory diseases who contracted COVID-19, according to a case series from New York University Langone Health.
“We are not seeing worse outcomes with overall use of either. It’s reassuring” that the data support continued use during the pandemic, said rheumatologist and senior investigator Jose Scher, MD, an associate professor at New York University.
There have been concerns among rheumatologists, gastroenterologists, and dermatologists that underlying inflammatory diseases and the agents used to treat them would impact outcomes in COVID-19.
Dr. Scher and colleagues, including lead author and rheumatologist Rebecca Haberman, MD, wanted to address the issue, so they reviewed the experience in their own health system of patients with inflammatory diseases – most commonly psoriatic arthritis, RA, and Crohn’s disease – who were assessed for COVID-19 from March 3 to April 3.
Fever, cough, and shortness of breath were the most common symptoms. The infection was confirmed by polymerase chain reaction in 59 (69%) and highly suspected in 27.
A total of 62 patients (72%) were on JAK inhibitors or biologics at baseline, including 38 (44%) on tumor necrosis factor inhibitors.
Overall, 14 patients (16%) were hospitalized with COVID-19, which is consistent the 26% hospitalization rate among the general population in New York City.
Baseline biologic and JAK inhibitor use was actually lower among hospitalized patients than among those who weren’t hospitalized (50% vs. 76%), and the hospitalization rate was only 11% among 62 subjects who had been on the agents long term, more than a year among most.
Hospitalized patients tended to be slightly older (mean, 50 vs. 46 years) with a higher prevalence of hypertension, diabetes, and chronic obstructive pulmonary disease. They also had a higher prevalence of RA (43% vs. 19%), methotrexate use (43% vs. 15%), and use of hydroxychloroquine (21% vs. 7%) and oral glucocorticoids (29% vs. 6%).
It’s unknown what to make of those findings for now, Dr. Scher said. The study didn’t address differences in the severity of the underlying inflammatory illness, but a new and significantly larger case series is in the works that will analyze that and other potential confounders.
Dr. Scher noted that he’s particularly interested in drilling down further on the higher prevalence of RA and methotrexate in hospitalized patients. “We want to understand those signals better. All of this needs further validation,” he said.
Of the 14 hospitalized patients, 11 (79%) were discharged after a mean of 5.6 days. One died in the ED, and two remained hospitalized as of April 3, including one in the ICU.
The investigators are contributing to COVID-19 registries for inflammatory disease patients. The registries are tending to report higher hospitalization rates, but Dr. Scher noted they might be biased towards more severe cases, among other issues.
As for the current situation in New York City, he said that the “last week in March and first 3 in April were indescribable in terms of admissions, intubations, and deaths. Over the last week or so, it has calmed down significantly.”
There was no external funding. Dr. Haberman reported ties to Janssen, and Dr. Scher reported ties to Janssen, Novartis, Pfizer, and other companies.
aotto@mdedge.com
SOURCE: Haberman R et al. N Engl J Med. 2020 Apr 29. doi: 10.1056/NEJMc2009567.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
COVID-19 and pregnancy: Is miscarriage a risk?

- Are you treating pregnant patients with COVID-19? Take this brief survey: https://www.surveymonkey.com/r/CDZ7VFK
- Enroll your patients in PRIORITY: Pregnancy Coronavirus Outcomes Registry
- Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection JAMA. April 30, 2020

- Are you treating pregnant patients with COVID-19? Take this brief survey: https://www.surveymonkey.com/r/CDZ7VFK
- Enroll your patients in PRIORITY: Pregnancy Coronavirus Outcomes Registry
- Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection JAMA. April 30, 2020

- Are you treating pregnant patients with COVID-19? Take this brief survey: https://www.surveymonkey.com/r/CDZ7VFK
- Enroll your patients in PRIORITY: Pregnancy Coronavirus Outcomes Registry
- Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection JAMA. April 30, 2020
Noninvasive tests boost risk stratification in obese compensated ACLD
Readily available and inexpensive noninvasive tests, when used in combination with liver markers obtained with the extra-large probe, can improve the ability to predict risk for decompensation and other adverse outcomes in obese and overweight patients with compensated advanced chronic liver disease (cACLD), according to study results reported in the upcoming issue of the journal Clinical Gastroenterology and Hepatology.
The retrospective study of 272 obese and overweight patients in Bern, Switzerland, and Montreal with cACLD is the first to fully assess the noninvasive marker of portal hypertension along with using the extra-large probe for controlled attenuation parameter (CAP) to determine risk, wrote Yuly Mendoza, MD, of the University of Bern and colleagues. Decompensation in cACLD carries a higher risk of death. The study noted that portal hypertension is a key driver of progression to decompensation, “and as such, it should be identified as soon as possible and treated as needed.”
“Prediction of prognosis in cACLD is challenging, and noninvasive tests are important tools for clinicians to avoid as much as possible the use of more invasive tests,” wrote Dr. Mendoza and colleagues. Based on the extra-large probe, 76% (n = 206) of study patients had metabolic syndrome, sometimes with other etiologies of liver disease, and 57% (n = 154) had cACLD because of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH).
Twelve patients had decompensation and five developed severe bacterial infections.
“Readily available noninvasive tests can be used to identify obese or overweight patients with cACLD who are at increased risk for decompensation and severe bacterial infections,” wrote the researchers.
The study noted that obesity is a challenge for noninvasive tests and is a major limitation to liver stiffness measurement on transient elastography using the standard M probe. The XL probe has been specifically designed to overcome this challenge in obese patients, but it hasn’t been evaluated for the prediction of clinical decompensation in obese patients with cACLD.
This study claimed to provide further evidence that liver stiffness measurement in combination with noninvasive tests for liver stiffness measurement, spleen size/platelet count (LSPS), portal hypertension and portal hypertension risk score can help identify patients at risk for clinical decompensation and severe bacterial infections.
The study used average area under the receiving operator curve (AUC) to calculate the ability of the markers to distinguish risk, all with 95% confidence interval: 0.803 for liver stiffness measurement, 0.829 for portal hypertension risk score, and 0.845 for LSPS (P < .001). The markers showed an even better ability to differentiate between patients at risk for developing classical clinical decompensation in follow-up from those not at risk (all 95% CI): 0.848 for liver stiffness measurement, 0.881 for portal hypertension risk score, and 0.890 for LSPS (P < .001).
“The results of the present study validate the use of [extra-large] probe for liver stiffness measurement and CAP to stratify the risk of clinical decompensation and clinically relevant events in overweight/obese patients with cACLD, particularly in case of NAFLD/NASH etiology,” wrote Dr. Mendoza and colleagues.
All study participants were followed for at least 6 months, with a median of 17 months. Patients who developed decompensation or severe bacterial infections had slightly worse liver function (higher international normalized ratio and lower albumin), lower mean platelet count (117 vs. 179 x 109/L; P < .001) and lower mean CAP (297 vs. 318 dBm; P = .030) than did patients who stayed compensated.
CAP above 220 dB/m was marginally associated with a lower risk of decompensation or severe bacterial infections on univariate analysis, as were elevated Model for End-Stage Liver Disease score, elevated Child Pugh score, low platelet count, low serum albumin, elevated serum bilirubin and increased liver stiffness measurement, LSPS, and portal hypertension risk scores.
Dr. Mendoza and colleagues have no relevant financial disclosures. The study received funding from the Swiss government.
SOURCE: Mendoza Y et al. Clin Gastroenterol Hepatol. 2020. doi: 10.1016/j.cgh.2020.04.018.
Readily available and inexpensive noninvasive tests, when used in combination with liver markers obtained with the extra-large probe, can improve the ability to predict risk for decompensation and other adverse outcomes in obese and overweight patients with compensated advanced chronic liver disease (cACLD), according to study results reported in the upcoming issue of the journal Clinical Gastroenterology and Hepatology.
The retrospective study of 272 obese and overweight patients in Bern, Switzerland, and Montreal with cACLD is the first to fully assess the noninvasive marker of portal hypertension along with using the extra-large probe for controlled attenuation parameter (CAP) to determine risk, wrote Yuly Mendoza, MD, of the University of Bern and colleagues. Decompensation in cACLD carries a higher risk of death. The study noted that portal hypertension is a key driver of progression to decompensation, “and as such, it should be identified as soon as possible and treated as needed.”
“Prediction of prognosis in cACLD is challenging, and noninvasive tests are important tools for clinicians to avoid as much as possible the use of more invasive tests,” wrote Dr. Mendoza and colleagues. Based on the extra-large probe, 76% (n = 206) of study patients had metabolic syndrome, sometimes with other etiologies of liver disease, and 57% (n = 154) had cACLD because of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH).
Twelve patients had decompensation and five developed severe bacterial infections.
“Readily available noninvasive tests can be used to identify obese or overweight patients with cACLD who are at increased risk for decompensation and severe bacterial infections,” wrote the researchers.
The study noted that obesity is a challenge for noninvasive tests and is a major limitation to liver stiffness measurement on transient elastography using the standard M probe. The XL probe has been specifically designed to overcome this challenge in obese patients, but it hasn’t been evaluated for the prediction of clinical decompensation in obese patients with cACLD.
This study claimed to provide further evidence that liver stiffness measurement in combination with noninvasive tests for liver stiffness measurement, spleen size/platelet count (LSPS), portal hypertension and portal hypertension risk score can help identify patients at risk for clinical decompensation and severe bacterial infections.
The study used average area under the receiving operator curve (AUC) to calculate the ability of the markers to distinguish risk, all with 95% confidence interval: 0.803 for liver stiffness measurement, 0.829 for portal hypertension risk score, and 0.845 for LSPS (P < .001). The markers showed an even better ability to differentiate between patients at risk for developing classical clinical decompensation in follow-up from those not at risk (all 95% CI): 0.848 for liver stiffness measurement, 0.881 for portal hypertension risk score, and 0.890 for LSPS (P < .001).
“The results of the present study validate the use of [extra-large] probe for liver stiffness measurement and CAP to stratify the risk of clinical decompensation and clinically relevant events in overweight/obese patients with cACLD, particularly in case of NAFLD/NASH etiology,” wrote Dr. Mendoza and colleagues.
All study participants were followed for at least 6 months, with a median of 17 months. Patients who developed decompensation or severe bacterial infections had slightly worse liver function (higher international normalized ratio and lower albumin), lower mean platelet count (117 vs. 179 x 109/L; P < .001) and lower mean CAP (297 vs. 318 dBm; P = .030) than did patients who stayed compensated.
CAP above 220 dB/m was marginally associated with a lower risk of decompensation or severe bacterial infections on univariate analysis, as were elevated Model for End-Stage Liver Disease score, elevated Child Pugh score, low platelet count, low serum albumin, elevated serum bilirubin and increased liver stiffness measurement, LSPS, and portal hypertension risk scores.
Dr. Mendoza and colleagues have no relevant financial disclosures. The study received funding from the Swiss government.
SOURCE: Mendoza Y et al. Clin Gastroenterol Hepatol. 2020. doi: 10.1016/j.cgh.2020.04.018.
Readily available and inexpensive noninvasive tests, when used in combination with liver markers obtained with the extra-large probe, can improve the ability to predict risk for decompensation and other adverse outcomes in obese and overweight patients with compensated advanced chronic liver disease (cACLD), according to study results reported in the upcoming issue of the journal Clinical Gastroenterology and Hepatology.
The retrospective study of 272 obese and overweight patients in Bern, Switzerland, and Montreal with cACLD is the first to fully assess the noninvasive marker of portal hypertension along with using the extra-large probe for controlled attenuation parameter (CAP) to determine risk, wrote Yuly Mendoza, MD, of the University of Bern and colleagues. Decompensation in cACLD carries a higher risk of death. The study noted that portal hypertension is a key driver of progression to decompensation, “and as such, it should be identified as soon as possible and treated as needed.”
“Prediction of prognosis in cACLD is challenging, and noninvasive tests are important tools for clinicians to avoid as much as possible the use of more invasive tests,” wrote Dr. Mendoza and colleagues. Based on the extra-large probe, 76% (n = 206) of study patients had metabolic syndrome, sometimes with other etiologies of liver disease, and 57% (n = 154) had cACLD because of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH).
Twelve patients had decompensation and five developed severe bacterial infections.
“Readily available noninvasive tests can be used to identify obese or overweight patients with cACLD who are at increased risk for decompensation and severe bacterial infections,” wrote the researchers.
The study noted that obesity is a challenge for noninvasive tests and is a major limitation to liver stiffness measurement on transient elastography using the standard M probe. The XL probe has been specifically designed to overcome this challenge in obese patients, but it hasn’t been evaluated for the prediction of clinical decompensation in obese patients with cACLD.
This study claimed to provide further evidence that liver stiffness measurement in combination with noninvasive tests for liver stiffness measurement, spleen size/platelet count (LSPS), portal hypertension and portal hypertension risk score can help identify patients at risk for clinical decompensation and severe bacterial infections.
The study used average area under the receiving operator curve (AUC) to calculate the ability of the markers to distinguish risk, all with 95% confidence interval: 0.803 for liver stiffness measurement, 0.829 for portal hypertension risk score, and 0.845 for LSPS (P < .001). The markers showed an even better ability to differentiate between patients at risk for developing classical clinical decompensation in follow-up from those not at risk (all 95% CI): 0.848 for liver stiffness measurement, 0.881 for portal hypertension risk score, and 0.890 for LSPS (P < .001).
“The results of the present study validate the use of [extra-large] probe for liver stiffness measurement and CAP to stratify the risk of clinical decompensation and clinically relevant events in overweight/obese patients with cACLD, particularly in case of NAFLD/NASH etiology,” wrote Dr. Mendoza and colleagues.
All study participants were followed for at least 6 months, with a median of 17 months. Patients who developed decompensation or severe bacterial infections had slightly worse liver function (higher international normalized ratio and lower albumin), lower mean platelet count (117 vs. 179 x 109/L; P < .001) and lower mean CAP (297 vs. 318 dBm; P = .030) than did patients who stayed compensated.
CAP above 220 dB/m was marginally associated with a lower risk of decompensation or severe bacterial infections on univariate analysis, as were elevated Model for End-Stage Liver Disease score, elevated Child Pugh score, low platelet count, low serum albumin, elevated serum bilirubin and increased liver stiffness measurement, LSPS, and portal hypertension risk scores.
Dr. Mendoza and colleagues have no relevant financial disclosures. The study received funding from the Swiss government.
SOURCE: Mendoza Y et al. Clin Gastroenterol Hepatol. 2020. doi: 10.1016/j.cgh.2020.04.018.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Use of cannabinoids in dermatology here to stay
In the clinical opinion of .
“There’s no question in my mind about that. Don’t play catch-up; be at the forefront, because at a minimum your patients are going to ask you about this,” he said in a video presentation during a virtual meeting held by the George Washington University department of dermatology.
In 2018, officials at Health Canada reviewed literature and international reviews concerning potential therapeutic uses and harmful effects of cannabis and cannabinoids and published a free downloadable guide for health care professionals. “In the book, dermatology doesn’t have its own section,” said Dr. Friedman, professor and interim chair of dermatology at George Washington University, Washington. “It falls under inflammation and makes up four paragraphs of the entire book, which is weird, given that if you survey the dispensaries in Canada, the majority of them led in with dermatologic indications, many of which are completely unsubstantiated.”
In the United States, a recent survey of 531 dermatologists led by Elizabeth S. Robinson, MD, of George Washington University, found that 55% reported at least one patient-initiated discussion about cannabinoids in the last year (J Drugs Dermatol. 2018;17[2]:1273-8). However, 48% were concerned about a negative stigma when proposing cannabinoid therapies to patients. While most respondents (86%) were willing to prescribe an FDA-approved cannabinoid as a topical treatment, fewer (71%) were willing to prescribe an oral form. In an unpublished study conducted 2 years later, 155 dermatologists were asked if they had ever recommended medical cannabis products for the treatment/management of a dermatologic condition. More than 80% said they had not.
“It’s important to recognize that if we have a strong fund of knowledge, we can guide these patients to use the right cannabinoids for the right indications, so long as we have some evidence supporting it,” said Dr. Friedman, residency program director and director of translational research in George Washington University’s department of dermatology.
According to existing medical literature, cannabinoids may ultimately play a role in the treatment of eczema (J Am Acad Dermatol. 2020 May. doi: 10.1016/j.jaad.2020.01.036 and ClinicalTrials.gov NCT03824405), psoriasis, acne, and certain collagen vascular diseases, including scleroderma, dermatomyositis, and cutaneous lupus erythematosus (CLE). Most of the evidence for its use in collagen vascular diseases comes from the investigation of a synthetic cannabinoid known as anabasum, which is derived from TCH, but it has no affinity for the CB1 receptor. “Rather, it goes after the CB2 receptor, which is heavily prevalent in the immune system,” he noted.
In the summer of 2018, the FDA granted Orphan Drug Designation to Corbus Pharmaceuticals for lenabasum, a derivative of anabasum, for the treatment of dermatomyositis. “Hopefully, we’ll see this in the next year,” said Dr. Friedman, who consults for Corbus. A more recent study showed that lenabasum could reduce the production of interleukin-31 (Br. J Dermatol 2018;179[3]:669-78), which “I think will have broader implications in dermatology beyond dermatomyositis,” he said.
Dr. Friedman also reviewed data on a topical endocannabinoid nanoparticle-based formulation his team developed and is studying for the treatment of CLE. “There is a huge unmet need as there are no topical therapies approved for CLE,” he said. “Our animal data are very promising and we plan to move forward to human studies shortly.”
Resources for clinicians to improve their understanding about the potential use of cannabinoids in dermatology include an online certificate program in cannabis medicine offered by Thomas Jefferson University, as well as their state departments of health. Other resources include the International Cannabinoid Research Society, the International Association for Cannabinoid Medicines, the University of California’s Center for Medicinal Cannabis Research, and the Canadian Consortium for the Investigation of Cannabinoids.
Dr. Friedman noted that marijuana may exacerbate appetite, sleepiness, dizziness, low blood pressure, dry mouth/eyes, decreased urination, hallucinations, paranoia, anxiety, poor balance and posture in patients with dyskinetic disorders, and impaired attention, memory, and psychomotor performance. High concentrations can cause hyperemesis syndrome and exacerbate existing psychoses. With respect to cannabidiol (CBD), “unless you go with super high concentrations, over 50 mg/kg per day, you’re probably not going to run into so much trouble,” Dr. Friedman said. “Above that, you do get some liver function test abnormalities. The problem is, a lot of CBD-based products have impurities in them.”
Different state-based requirements exist for recommending cannabinoid products to your patients “so it’s important to know those requirements,” Dr. Friedman said. “I have patients sign a cannabis contrast. There are examples of these online. My mantra is start low and go slow, and stay low as much as possible.”
The virtual meeting at George Washington University included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic. Dr. Friedman reported that he serves as a consultant and/or adviser to numerous pharmaceutical companies, including some that produce cannabinoids. He is a speaker for Regeneron/Sanofi, Abbvie, and Dermira, and has received grants from Pfizer and the Dermatology Foundation.
In the clinical opinion of .
“There’s no question in my mind about that. Don’t play catch-up; be at the forefront, because at a minimum your patients are going to ask you about this,” he said in a video presentation during a virtual meeting held by the George Washington University department of dermatology.
In 2018, officials at Health Canada reviewed literature and international reviews concerning potential therapeutic uses and harmful effects of cannabis and cannabinoids and published a free downloadable guide for health care professionals. “In the book, dermatology doesn’t have its own section,” said Dr. Friedman, professor and interim chair of dermatology at George Washington University, Washington. “It falls under inflammation and makes up four paragraphs of the entire book, which is weird, given that if you survey the dispensaries in Canada, the majority of them led in with dermatologic indications, many of which are completely unsubstantiated.”
In the United States, a recent survey of 531 dermatologists led by Elizabeth S. Robinson, MD, of George Washington University, found that 55% reported at least one patient-initiated discussion about cannabinoids in the last year (J Drugs Dermatol. 2018;17[2]:1273-8). However, 48% were concerned about a negative stigma when proposing cannabinoid therapies to patients. While most respondents (86%) were willing to prescribe an FDA-approved cannabinoid as a topical treatment, fewer (71%) were willing to prescribe an oral form. In an unpublished study conducted 2 years later, 155 dermatologists were asked if they had ever recommended medical cannabis products for the treatment/management of a dermatologic condition. More than 80% said they had not.
“It’s important to recognize that if we have a strong fund of knowledge, we can guide these patients to use the right cannabinoids for the right indications, so long as we have some evidence supporting it,” said Dr. Friedman, residency program director and director of translational research in George Washington University’s department of dermatology.
According to existing medical literature, cannabinoids may ultimately play a role in the treatment of eczema (J Am Acad Dermatol. 2020 May. doi: 10.1016/j.jaad.2020.01.036 and ClinicalTrials.gov NCT03824405), psoriasis, acne, and certain collagen vascular diseases, including scleroderma, dermatomyositis, and cutaneous lupus erythematosus (CLE). Most of the evidence for its use in collagen vascular diseases comes from the investigation of a synthetic cannabinoid known as anabasum, which is derived from TCH, but it has no affinity for the CB1 receptor. “Rather, it goes after the CB2 receptor, which is heavily prevalent in the immune system,” he noted.
In the summer of 2018, the FDA granted Orphan Drug Designation to Corbus Pharmaceuticals for lenabasum, a derivative of anabasum, for the treatment of dermatomyositis. “Hopefully, we’ll see this in the next year,” said Dr. Friedman, who consults for Corbus. A more recent study showed that lenabasum could reduce the production of interleukin-31 (Br. J Dermatol 2018;179[3]:669-78), which “I think will have broader implications in dermatology beyond dermatomyositis,” he said.
Dr. Friedman also reviewed data on a topical endocannabinoid nanoparticle-based formulation his team developed and is studying for the treatment of CLE. “There is a huge unmet need as there are no topical therapies approved for CLE,” he said. “Our animal data are very promising and we plan to move forward to human studies shortly.”
Resources for clinicians to improve their understanding about the potential use of cannabinoids in dermatology include an online certificate program in cannabis medicine offered by Thomas Jefferson University, as well as their state departments of health. Other resources include the International Cannabinoid Research Society, the International Association for Cannabinoid Medicines, the University of California’s Center for Medicinal Cannabis Research, and the Canadian Consortium for the Investigation of Cannabinoids.
Dr. Friedman noted that marijuana may exacerbate appetite, sleepiness, dizziness, low blood pressure, dry mouth/eyes, decreased urination, hallucinations, paranoia, anxiety, poor balance and posture in patients with dyskinetic disorders, and impaired attention, memory, and psychomotor performance. High concentrations can cause hyperemesis syndrome and exacerbate existing psychoses. With respect to cannabidiol (CBD), “unless you go with super high concentrations, over 50 mg/kg per day, you’re probably not going to run into so much trouble,” Dr. Friedman said. “Above that, you do get some liver function test abnormalities. The problem is, a lot of CBD-based products have impurities in them.”
Different state-based requirements exist for recommending cannabinoid products to your patients “so it’s important to know those requirements,” Dr. Friedman said. “I have patients sign a cannabis contrast. There are examples of these online. My mantra is start low and go slow, and stay low as much as possible.”
The virtual meeting at George Washington University included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic. Dr. Friedman reported that he serves as a consultant and/or adviser to numerous pharmaceutical companies, including some that produce cannabinoids. He is a speaker for Regeneron/Sanofi, Abbvie, and Dermira, and has received grants from Pfizer and the Dermatology Foundation.
In the clinical opinion of .
“There’s no question in my mind about that. Don’t play catch-up; be at the forefront, because at a minimum your patients are going to ask you about this,” he said in a video presentation during a virtual meeting held by the George Washington University department of dermatology.
In 2018, officials at Health Canada reviewed literature and international reviews concerning potential therapeutic uses and harmful effects of cannabis and cannabinoids and published a free downloadable guide for health care professionals. “In the book, dermatology doesn’t have its own section,” said Dr. Friedman, professor and interim chair of dermatology at George Washington University, Washington. “It falls under inflammation and makes up four paragraphs of the entire book, which is weird, given that if you survey the dispensaries in Canada, the majority of them led in with dermatologic indications, many of which are completely unsubstantiated.”
In the United States, a recent survey of 531 dermatologists led by Elizabeth S. Robinson, MD, of George Washington University, found that 55% reported at least one patient-initiated discussion about cannabinoids in the last year (J Drugs Dermatol. 2018;17[2]:1273-8). However, 48% were concerned about a negative stigma when proposing cannabinoid therapies to patients. While most respondents (86%) were willing to prescribe an FDA-approved cannabinoid as a topical treatment, fewer (71%) were willing to prescribe an oral form. In an unpublished study conducted 2 years later, 155 dermatologists were asked if they had ever recommended medical cannabis products for the treatment/management of a dermatologic condition. More than 80% said they had not.
“It’s important to recognize that if we have a strong fund of knowledge, we can guide these patients to use the right cannabinoids for the right indications, so long as we have some evidence supporting it,” said Dr. Friedman, residency program director and director of translational research in George Washington University’s department of dermatology.
According to existing medical literature, cannabinoids may ultimately play a role in the treatment of eczema (J Am Acad Dermatol. 2020 May. doi: 10.1016/j.jaad.2020.01.036 and ClinicalTrials.gov NCT03824405), psoriasis, acne, and certain collagen vascular diseases, including scleroderma, dermatomyositis, and cutaneous lupus erythematosus (CLE). Most of the evidence for its use in collagen vascular diseases comes from the investigation of a synthetic cannabinoid known as anabasum, which is derived from TCH, but it has no affinity for the CB1 receptor. “Rather, it goes after the CB2 receptor, which is heavily prevalent in the immune system,” he noted.
In the summer of 2018, the FDA granted Orphan Drug Designation to Corbus Pharmaceuticals for lenabasum, a derivative of anabasum, for the treatment of dermatomyositis. “Hopefully, we’ll see this in the next year,” said Dr. Friedman, who consults for Corbus. A more recent study showed that lenabasum could reduce the production of interleukin-31 (Br. J Dermatol 2018;179[3]:669-78), which “I think will have broader implications in dermatology beyond dermatomyositis,” he said.
Dr. Friedman also reviewed data on a topical endocannabinoid nanoparticle-based formulation his team developed and is studying for the treatment of CLE. “There is a huge unmet need as there are no topical therapies approved for CLE,” he said. “Our animal data are very promising and we plan to move forward to human studies shortly.”
Resources for clinicians to improve their understanding about the potential use of cannabinoids in dermatology include an online certificate program in cannabis medicine offered by Thomas Jefferson University, as well as their state departments of health. Other resources include the International Cannabinoid Research Society, the International Association for Cannabinoid Medicines, the University of California’s Center for Medicinal Cannabis Research, and the Canadian Consortium for the Investigation of Cannabinoids.
Dr. Friedman noted that marijuana may exacerbate appetite, sleepiness, dizziness, low blood pressure, dry mouth/eyes, decreased urination, hallucinations, paranoia, anxiety, poor balance and posture in patients with dyskinetic disorders, and impaired attention, memory, and psychomotor performance. High concentrations can cause hyperemesis syndrome and exacerbate existing psychoses. With respect to cannabidiol (CBD), “unless you go with super high concentrations, over 50 mg/kg per day, you’re probably not going to run into so much trouble,” Dr. Friedman said. “Above that, you do get some liver function test abnormalities. The problem is, a lot of CBD-based products have impurities in them.”
Different state-based requirements exist for recommending cannabinoid products to your patients “so it’s important to know those requirements,” Dr. Friedman said. “I have patients sign a cannabis contrast. There are examples of these online. My mantra is start low and go slow, and stay low as much as possible.”
The virtual meeting at George Washington University included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic. Dr. Friedman reported that he serves as a consultant and/or adviser to numerous pharmaceutical companies, including some that produce cannabinoids. He is a speaker for Regeneron/Sanofi, Abbvie, and Dermira, and has received grants from Pfizer and the Dermatology Foundation.
Antitumor treatment may increase risk of severe events in COVID-19 patients
Cancer patients who received antitumor treatment within 14 days of COVID-19 diagnosis had an increased risk of severe events, according to data from three hospitals in Wuhan.
Patients with patchy consolidation at hospital admission also had an increased risk of severe events, defined as ICU admission, mechanical ventilation, or death.
However, these findings are limited by the small number of patients studied and the retrospective nature of the analysis, according to researchers.
Li Zhang, MD, PhD, of Tongji Hospital in Wuhan, China, presented this research at the AACR virtual meeting I. Some of the data were previously published in Annals of Oncology.
The researchers studied 28 patients with cancer among 1,276 patients with COVID-19 treated at three hospitals in Wuhan. The most common cancer types were lung (n = 7), esophageal (n = 4), and breast (n = 3). Patients had other gastrointestinal, gynecologic, genitourinary, and head and neck cancers as well.
The patients’ median age was 65 years (range, 56-70 years), 60.9% were men, 35.7% had stage IV cancer, and 28.6% had hospital-acquired COVID-19. Antitumor treatments included chemotherapy (n = 22), surgery (n = 21), radiotherapy (n = 21), targeted therapy (n = 5), and immune checkpoint inhibitors (n = 2).
COVID-19 treatment
Most patients (n = 22) received oxygen as their only respiratory intervention, although 10 received mechanical ventilation.
For systemic therapy, patients received antibiotic treatment (n = 23), corticosteroids (n = 15), intravenous immunoglobulin (n = 10), and tocilizumab (n = 1).
Antiviral treatments included umifenovir (n = 14), lopinavir/ritonavir (n = 10), ganciclovir (n = 9), ribavirin (n = 1), or a combination of antiviral drugs (n = 9).
“No cancer patients were enrolled in clinical trials, so no one received hydroxychloroquine or remdesivir,” Dr. Zhang noted.
Outcomes
In all, 15 patients (53.6%) had severe events. The median time from COVID-19 diagnosis to severe events was 7 days (range, 5-15 days).
A total of eight patients (28.6%) died – three with lung cancer, two with prostate cancer, one with liver cancer, one with rectal cancer, and one with testicular cancer.
Causes of death were acute respiratory distress syndrome (n = 5), septic shock (n = 1), suspected pulmonary embolism (n = 1), and acute myocardial infarction (n = 1).
By April 4, 14 patients had been discharged from the hospital, and 6 were still hospitalized. The median duration of hospitalization was 18.4 days for discharged patients and 29.4 days for patients still in hospital.
Follow-up CT scans showed improvement in 13 patients, no changes in 5 patients, and deterioration in 6 patients.
Factors associated with severe events
In a multivariable analysis, receiving antitumor treatment within 14 days of COVID-19 diagnosis was associated with severe events (hazard ratio, 4.079; P = .037).
However, only seven patients received antitumor treatments within 14 days of COVID-19 diagnosis – three chemotherapy, two targeted therapy, one radiotherapy, and one immune checkpoint inhibitor. Five of these seven patients had severe events.
Another factor associated with severe events in multivariable analysis was patchy consolidation on CT scan at admission (HR, 5.438; P = .01). Age and gender were not significantly associated with severe events.
Immune checkpoint inhibitors
Dr. Zhang and colleagues also analyzed a second group of cancer patients and their family members to determine if patients on immune checkpoint inhibitors have an increased risk of COVID-19.
This group included 124 cancer patients treated with immune checkpoint inhibitors for at least 2 months. The patients had a median age of 59 years (range, 54-65 years), and 61.8% were men. Most patients (95.2%) had stage IV cancer, and the most common cancers were lung (54.0%), esophageal (18.6%), and head and neck (10.7%).
In this group, only one cancer patient developed COVID-19 (via nosocomial infection). In another case, a patient’s spouse developed COVID-19, but the patient did not.
Dr. Zhang said this “limited information did not suggest cancer patients treated with immune checkpoint inhibitors were more vulnerable to COVID infection.”
Dr. Zhang and colleagues reported no conflicts of interest. This research was funded by the National Natural Science Foundation of China and Huazhong University of Science and Technology COVID-19 Rapid Response Call China.
SOURCE: Zhang L et al. Ann Oncol. 2020 Mar 26. doi: 10.1016/j.annonc.2020.03.296.
Cancer patients who received antitumor treatment within 14 days of COVID-19 diagnosis had an increased risk of severe events, according to data from three hospitals in Wuhan.
Patients with patchy consolidation at hospital admission also had an increased risk of severe events, defined as ICU admission, mechanical ventilation, or death.
However, these findings are limited by the small number of patients studied and the retrospective nature of the analysis, according to researchers.
Li Zhang, MD, PhD, of Tongji Hospital in Wuhan, China, presented this research at the AACR virtual meeting I. Some of the data were previously published in Annals of Oncology.
The researchers studied 28 patients with cancer among 1,276 patients with COVID-19 treated at three hospitals in Wuhan. The most common cancer types were lung (n = 7), esophageal (n = 4), and breast (n = 3). Patients had other gastrointestinal, gynecologic, genitourinary, and head and neck cancers as well.
The patients’ median age was 65 years (range, 56-70 years), 60.9% were men, 35.7% had stage IV cancer, and 28.6% had hospital-acquired COVID-19. Antitumor treatments included chemotherapy (n = 22), surgery (n = 21), radiotherapy (n = 21), targeted therapy (n = 5), and immune checkpoint inhibitors (n = 2).
COVID-19 treatment
Most patients (n = 22) received oxygen as their only respiratory intervention, although 10 received mechanical ventilation.
For systemic therapy, patients received antibiotic treatment (n = 23), corticosteroids (n = 15), intravenous immunoglobulin (n = 10), and tocilizumab (n = 1).
Antiviral treatments included umifenovir (n = 14), lopinavir/ritonavir (n = 10), ganciclovir (n = 9), ribavirin (n = 1), or a combination of antiviral drugs (n = 9).
“No cancer patients were enrolled in clinical trials, so no one received hydroxychloroquine or remdesivir,” Dr. Zhang noted.
Outcomes
In all, 15 patients (53.6%) had severe events. The median time from COVID-19 diagnosis to severe events was 7 days (range, 5-15 days).
A total of eight patients (28.6%) died – three with lung cancer, two with prostate cancer, one with liver cancer, one with rectal cancer, and one with testicular cancer.
Causes of death were acute respiratory distress syndrome (n = 5), septic shock (n = 1), suspected pulmonary embolism (n = 1), and acute myocardial infarction (n = 1).
By April 4, 14 patients had been discharged from the hospital, and 6 were still hospitalized. The median duration of hospitalization was 18.4 days for discharged patients and 29.4 days for patients still in hospital.
Follow-up CT scans showed improvement in 13 patients, no changes in 5 patients, and deterioration in 6 patients.
Factors associated with severe events
In a multivariable analysis, receiving antitumor treatment within 14 days of COVID-19 diagnosis was associated with severe events (hazard ratio, 4.079; P = .037).
However, only seven patients received antitumor treatments within 14 days of COVID-19 diagnosis – three chemotherapy, two targeted therapy, one radiotherapy, and one immune checkpoint inhibitor. Five of these seven patients had severe events.
Another factor associated with severe events in multivariable analysis was patchy consolidation on CT scan at admission (HR, 5.438; P = .01). Age and gender were not significantly associated with severe events.
Immune checkpoint inhibitors
Dr. Zhang and colleagues also analyzed a second group of cancer patients and their family members to determine if patients on immune checkpoint inhibitors have an increased risk of COVID-19.
This group included 124 cancer patients treated with immune checkpoint inhibitors for at least 2 months. The patients had a median age of 59 years (range, 54-65 years), and 61.8% were men. Most patients (95.2%) had stage IV cancer, and the most common cancers were lung (54.0%), esophageal (18.6%), and head and neck (10.7%).
In this group, only one cancer patient developed COVID-19 (via nosocomial infection). In another case, a patient’s spouse developed COVID-19, but the patient did not.
Dr. Zhang said this “limited information did not suggest cancer patients treated with immune checkpoint inhibitors were more vulnerable to COVID infection.”
Dr. Zhang and colleagues reported no conflicts of interest. This research was funded by the National Natural Science Foundation of China and Huazhong University of Science and Technology COVID-19 Rapid Response Call China.
SOURCE: Zhang L et al. Ann Oncol. 2020 Mar 26. doi: 10.1016/j.annonc.2020.03.296.
Cancer patients who received antitumor treatment within 14 days of COVID-19 diagnosis had an increased risk of severe events, according to data from three hospitals in Wuhan.
Patients with patchy consolidation at hospital admission also had an increased risk of severe events, defined as ICU admission, mechanical ventilation, or death.
However, these findings are limited by the small number of patients studied and the retrospective nature of the analysis, according to researchers.
Li Zhang, MD, PhD, of Tongji Hospital in Wuhan, China, presented this research at the AACR virtual meeting I. Some of the data were previously published in Annals of Oncology.
The researchers studied 28 patients with cancer among 1,276 patients with COVID-19 treated at three hospitals in Wuhan. The most common cancer types were lung (n = 7), esophageal (n = 4), and breast (n = 3). Patients had other gastrointestinal, gynecologic, genitourinary, and head and neck cancers as well.
The patients’ median age was 65 years (range, 56-70 years), 60.9% were men, 35.7% had stage IV cancer, and 28.6% had hospital-acquired COVID-19. Antitumor treatments included chemotherapy (n = 22), surgery (n = 21), radiotherapy (n = 21), targeted therapy (n = 5), and immune checkpoint inhibitors (n = 2).
COVID-19 treatment
Most patients (n = 22) received oxygen as their only respiratory intervention, although 10 received mechanical ventilation.
For systemic therapy, patients received antibiotic treatment (n = 23), corticosteroids (n = 15), intravenous immunoglobulin (n = 10), and tocilizumab (n = 1).
Antiviral treatments included umifenovir (n = 14), lopinavir/ritonavir (n = 10), ganciclovir (n = 9), ribavirin (n = 1), or a combination of antiviral drugs (n = 9).
“No cancer patients were enrolled in clinical trials, so no one received hydroxychloroquine or remdesivir,” Dr. Zhang noted.
Outcomes
In all, 15 patients (53.6%) had severe events. The median time from COVID-19 diagnosis to severe events was 7 days (range, 5-15 days).
A total of eight patients (28.6%) died – three with lung cancer, two with prostate cancer, one with liver cancer, one with rectal cancer, and one with testicular cancer.
Causes of death were acute respiratory distress syndrome (n = 5), septic shock (n = 1), suspected pulmonary embolism (n = 1), and acute myocardial infarction (n = 1).
By April 4, 14 patients had been discharged from the hospital, and 6 were still hospitalized. The median duration of hospitalization was 18.4 days for discharged patients and 29.4 days for patients still in hospital.
Follow-up CT scans showed improvement in 13 patients, no changes in 5 patients, and deterioration in 6 patients.
Factors associated with severe events
In a multivariable analysis, receiving antitumor treatment within 14 days of COVID-19 diagnosis was associated with severe events (hazard ratio, 4.079; P = .037).
However, only seven patients received antitumor treatments within 14 days of COVID-19 diagnosis – three chemotherapy, two targeted therapy, one radiotherapy, and one immune checkpoint inhibitor. Five of these seven patients had severe events.
Another factor associated with severe events in multivariable analysis was patchy consolidation on CT scan at admission (HR, 5.438; P = .01). Age and gender were not significantly associated with severe events.
Immune checkpoint inhibitors
Dr. Zhang and colleagues also analyzed a second group of cancer patients and their family members to determine if patients on immune checkpoint inhibitors have an increased risk of COVID-19.
This group included 124 cancer patients treated with immune checkpoint inhibitors for at least 2 months. The patients had a median age of 59 years (range, 54-65 years), and 61.8% were men. Most patients (95.2%) had stage IV cancer, and the most common cancers were lung (54.0%), esophageal (18.6%), and head and neck (10.7%).
In this group, only one cancer patient developed COVID-19 (via nosocomial infection). In another case, a patient’s spouse developed COVID-19, but the patient did not.
Dr. Zhang said this “limited information did not suggest cancer patients treated with immune checkpoint inhibitors were more vulnerable to COVID infection.”
Dr. Zhang and colleagues reported no conflicts of interest. This research was funded by the National Natural Science Foundation of China and Huazhong University of Science and Technology COVID-19 Rapid Response Call China.
SOURCE: Zhang L et al. Ann Oncol. 2020 Mar 26. doi: 10.1016/j.annonc.2020.03.296.
FROM AACR 2020
Repeat TAVR outcomes ‘reassuring’
Redo transcatheter aortic valve replacement (TAVR) is a reasonably safe and effective option for selected patients with valve dysfunction after TAVR, new registry data suggest.
“Redo TAVR is about to become a much more common procedure and it’s reassuring to see that the outcomes that can be achieved by these procedures are quite good,” said Uri Landes, MD, Vancouver General Hospital, British Columbia, Canada.
Landes and colleagues reported results from the Redo-TAVR Registry in the April 28 issue of the Journal of the American College of Cardiology.
The Redo-TAVR Registry is an investigator-initiated effort designed to collect information on patients who undergo a second TAVR within a dysfunctional transcatheter heart valve (THV).
From 63,876 TAVR procedures done at 37 participating centers, 212 (0.33%) were redo-TAVR procedures. Seventy-four of the redo procedures were done within 1 year of the initial TAVR and the remaining 138 were beyond 1 year. Median time from TAVR-to-redo-TAVR for these two groups was 68 (38 to 154) days and 5 (3 to 6) years, respectively.
“It’s important to understand that this is probably a highly selected group of patients and these numbers do no reliably reflect the ratio of patients who will need a redo TAVR,” said Landes in an interview with theheart.org | Medscape Cardiology.
“We don’t know how many patients were excluded from redo TAVR because of prohibitive anatomical factors, such as an anticipated high risk for coronary occlusion, or a patient prosthesis mismatch. Also, some of these individuals received their THVs more recently, so if they will suffer THV valve dysfunction, it may not have happened yet,” he added.
In the early redo group, the indication for redo-TAVR was most often combined aortic THV stenosis and regurgitation (83.8%). Pure THV stenosis was seen in only 16.2% of patients.
For those with redo procedures after 1 year, THV stenosis was seen in 51 (37.0%) patients and regurgitation or combined stenosis-regurgitation in 86 (62.3%).
Device success using VARC-2 criteria was achieved in 85.1%, with no difference seen between those presenting within or beyond 1 year. Most failures were attributable to high residual gradients (14.1%) or regurgitation (8.9%).
No significant difference was seen in 30-day (94.6% and 98.5%) and 1-year survival (83.6% and 88.3%) in patients who presented within 1 year or later.
At 30-day and 1-year follow-up, residual gradients were 12.6 ± 7.5 mm Hg and 12.9 ± 9.0 mm Hg, respectively. High residual gradients (320 mm Hg) were seen in about 14% of patients.
Aortic valve areas were 1.63 ± 0.61 cm2 at 30 days and 1.51 ± 0.57 cm2 at 1 year. Regurgitation was mild or less in 91% of patients at both time points.
Periprocedural complication rates were relatively low. There were three strokes (1.4%), one valve malposition (3.3%), two coronary obstructions (0.9%), and 20 new permanent pacemaker implants (9.6%). Importantly, no procedure-related mortality was seen, only one patient converted to open heart surgery, and symptomatic improvements were substantial.
“We are currently working on an analysis that compares TAVI-in-TAVI versus TAVI in surgical valves, and we are happy to see that it appears as if TAVI-in-TAVI outcomes don’t fall short,” said Landes. More analysis is also needed to see if perhaps some THVs work better or worse for redo procedures.
“We also want to understand which of the many combinations of heart valves available are better than others, thinking that supra-annular leaflets inside intra-annular leaflet devices may function differently than vice versa,” said Landes.
Vinod Thourani, MD, chief of cardiovascular surgery at Piedmont Heart Institute, Atlanta, considers these new observational data “reassuring” and “robust,” albeit with some limitations. He was first author on an editorial comment on this paper and spoke to theheart.org | Medscape Cardiology.
“This is unadjudicated registry data but you can’t lie about death and I feel good seeing that if you need a second TAVR inside of a TAVR, your mortality risk is pretty good,” said Thourani.
That said, he questions whether these data can really be extrapolated to lower-risk patients. “I think this is an early snapshot and it’s a relatively big sample, but it’s a selected sample and we don’t know how many patients needed redo TAVR and didn’t get it or didn’t want it,” he added.
On the comforting side, there has been ongoing concern that a redo procedure that involves “propping open” a degenerated TAVR prosthesis’s leaflets with a new TAVR valve may occlude the coronary ostium by closing the flow within the open cells.
“Luckily, the investigators show an extremely low risk of coronary obstruction of only 0.9% in an anatomically high-risk patient population,” he said. This incidence, however, may increase as the use of TAVR rises in younger and less risky patients, he added.
Thourani would also like to see a longer follow-up on these patients. Median follow-up post redo TAVR was 15 months in this analysis.
“What I think we need to concentrate on as we do these studies is the life-long management of aortic stenosis wherein we try to minimize the overall number of invasive procedures as much as we can,” said Thourani.
Landes reported no conflict of interest. Thourani reported he is an advisor and/or researcher for Abbott Vascular, Boston Scientific, and Edwards Lifesciences.
This article first appeared on Medscape.com.
Redo transcatheter aortic valve replacement (TAVR) is a reasonably safe and effective option for selected patients with valve dysfunction after TAVR, new registry data suggest.
“Redo TAVR is about to become a much more common procedure and it’s reassuring to see that the outcomes that can be achieved by these procedures are quite good,” said Uri Landes, MD, Vancouver General Hospital, British Columbia, Canada.
Landes and colleagues reported results from the Redo-TAVR Registry in the April 28 issue of the Journal of the American College of Cardiology.
The Redo-TAVR Registry is an investigator-initiated effort designed to collect information on patients who undergo a second TAVR within a dysfunctional transcatheter heart valve (THV).
From 63,876 TAVR procedures done at 37 participating centers, 212 (0.33%) were redo-TAVR procedures. Seventy-four of the redo procedures were done within 1 year of the initial TAVR and the remaining 138 were beyond 1 year. Median time from TAVR-to-redo-TAVR for these two groups was 68 (38 to 154) days and 5 (3 to 6) years, respectively.
“It’s important to understand that this is probably a highly selected group of patients and these numbers do no reliably reflect the ratio of patients who will need a redo TAVR,” said Landes in an interview with theheart.org | Medscape Cardiology.
“We don’t know how many patients were excluded from redo TAVR because of prohibitive anatomical factors, such as an anticipated high risk for coronary occlusion, or a patient prosthesis mismatch. Also, some of these individuals received their THVs more recently, so if they will suffer THV valve dysfunction, it may not have happened yet,” he added.
In the early redo group, the indication for redo-TAVR was most often combined aortic THV stenosis and regurgitation (83.8%). Pure THV stenosis was seen in only 16.2% of patients.
For those with redo procedures after 1 year, THV stenosis was seen in 51 (37.0%) patients and regurgitation or combined stenosis-regurgitation in 86 (62.3%).
Device success using VARC-2 criteria was achieved in 85.1%, with no difference seen between those presenting within or beyond 1 year. Most failures were attributable to high residual gradients (14.1%) or regurgitation (8.9%).
No significant difference was seen in 30-day (94.6% and 98.5%) and 1-year survival (83.6% and 88.3%) in patients who presented within 1 year or later.
At 30-day and 1-year follow-up, residual gradients were 12.6 ± 7.5 mm Hg and 12.9 ± 9.0 mm Hg, respectively. High residual gradients (320 mm Hg) were seen in about 14% of patients.
Aortic valve areas were 1.63 ± 0.61 cm2 at 30 days and 1.51 ± 0.57 cm2 at 1 year. Regurgitation was mild or less in 91% of patients at both time points.
Periprocedural complication rates were relatively low. There were three strokes (1.4%), one valve malposition (3.3%), two coronary obstructions (0.9%), and 20 new permanent pacemaker implants (9.6%). Importantly, no procedure-related mortality was seen, only one patient converted to open heart surgery, and symptomatic improvements were substantial.
“We are currently working on an analysis that compares TAVI-in-TAVI versus TAVI in surgical valves, and we are happy to see that it appears as if TAVI-in-TAVI outcomes don’t fall short,” said Landes. More analysis is also needed to see if perhaps some THVs work better or worse for redo procedures.
“We also want to understand which of the many combinations of heart valves available are better than others, thinking that supra-annular leaflets inside intra-annular leaflet devices may function differently than vice versa,” said Landes.
Vinod Thourani, MD, chief of cardiovascular surgery at Piedmont Heart Institute, Atlanta, considers these new observational data “reassuring” and “robust,” albeit with some limitations. He was first author on an editorial comment on this paper and spoke to theheart.org | Medscape Cardiology.
“This is unadjudicated registry data but you can’t lie about death and I feel good seeing that if you need a second TAVR inside of a TAVR, your mortality risk is pretty good,” said Thourani.
That said, he questions whether these data can really be extrapolated to lower-risk patients. “I think this is an early snapshot and it’s a relatively big sample, but it’s a selected sample and we don’t know how many patients needed redo TAVR and didn’t get it or didn’t want it,” he added.
On the comforting side, there has been ongoing concern that a redo procedure that involves “propping open” a degenerated TAVR prosthesis’s leaflets with a new TAVR valve may occlude the coronary ostium by closing the flow within the open cells.
“Luckily, the investigators show an extremely low risk of coronary obstruction of only 0.9% in an anatomically high-risk patient population,” he said. This incidence, however, may increase as the use of TAVR rises in younger and less risky patients, he added.
Thourani would also like to see a longer follow-up on these patients. Median follow-up post redo TAVR was 15 months in this analysis.
“What I think we need to concentrate on as we do these studies is the life-long management of aortic stenosis wherein we try to minimize the overall number of invasive procedures as much as we can,” said Thourani.
Landes reported no conflict of interest. Thourani reported he is an advisor and/or researcher for Abbott Vascular, Boston Scientific, and Edwards Lifesciences.
This article first appeared on Medscape.com.
Redo transcatheter aortic valve replacement (TAVR) is a reasonably safe and effective option for selected patients with valve dysfunction after TAVR, new registry data suggest.
“Redo TAVR is about to become a much more common procedure and it’s reassuring to see that the outcomes that can be achieved by these procedures are quite good,” said Uri Landes, MD, Vancouver General Hospital, British Columbia, Canada.
Landes and colleagues reported results from the Redo-TAVR Registry in the April 28 issue of the Journal of the American College of Cardiology.
The Redo-TAVR Registry is an investigator-initiated effort designed to collect information on patients who undergo a second TAVR within a dysfunctional transcatheter heart valve (THV).
From 63,876 TAVR procedures done at 37 participating centers, 212 (0.33%) were redo-TAVR procedures. Seventy-four of the redo procedures were done within 1 year of the initial TAVR and the remaining 138 were beyond 1 year. Median time from TAVR-to-redo-TAVR for these two groups was 68 (38 to 154) days and 5 (3 to 6) years, respectively.
“It’s important to understand that this is probably a highly selected group of patients and these numbers do no reliably reflect the ratio of patients who will need a redo TAVR,” said Landes in an interview with theheart.org | Medscape Cardiology.
“We don’t know how many patients were excluded from redo TAVR because of prohibitive anatomical factors, such as an anticipated high risk for coronary occlusion, or a patient prosthesis mismatch. Also, some of these individuals received their THVs more recently, so if they will suffer THV valve dysfunction, it may not have happened yet,” he added.
In the early redo group, the indication for redo-TAVR was most often combined aortic THV stenosis and regurgitation (83.8%). Pure THV stenosis was seen in only 16.2% of patients.
For those with redo procedures after 1 year, THV stenosis was seen in 51 (37.0%) patients and regurgitation or combined stenosis-regurgitation in 86 (62.3%).
Device success using VARC-2 criteria was achieved in 85.1%, with no difference seen between those presenting within or beyond 1 year. Most failures were attributable to high residual gradients (14.1%) or regurgitation (8.9%).
No significant difference was seen in 30-day (94.6% and 98.5%) and 1-year survival (83.6% and 88.3%) in patients who presented within 1 year or later.
At 30-day and 1-year follow-up, residual gradients were 12.6 ± 7.5 mm Hg and 12.9 ± 9.0 mm Hg, respectively. High residual gradients (320 mm Hg) were seen in about 14% of patients.
Aortic valve areas were 1.63 ± 0.61 cm2 at 30 days and 1.51 ± 0.57 cm2 at 1 year. Regurgitation was mild or less in 91% of patients at both time points.
Periprocedural complication rates were relatively low. There were three strokes (1.4%), one valve malposition (3.3%), two coronary obstructions (0.9%), and 20 new permanent pacemaker implants (9.6%). Importantly, no procedure-related mortality was seen, only one patient converted to open heart surgery, and symptomatic improvements were substantial.
“We are currently working on an analysis that compares TAVI-in-TAVI versus TAVI in surgical valves, and we are happy to see that it appears as if TAVI-in-TAVI outcomes don’t fall short,” said Landes. More analysis is also needed to see if perhaps some THVs work better or worse for redo procedures.
“We also want to understand which of the many combinations of heart valves available are better than others, thinking that supra-annular leaflets inside intra-annular leaflet devices may function differently than vice versa,” said Landes.
Vinod Thourani, MD, chief of cardiovascular surgery at Piedmont Heart Institute, Atlanta, considers these new observational data “reassuring” and “robust,” albeit with some limitations. He was first author on an editorial comment on this paper and spoke to theheart.org | Medscape Cardiology.
“This is unadjudicated registry data but you can’t lie about death and I feel good seeing that if you need a second TAVR inside of a TAVR, your mortality risk is pretty good,” said Thourani.
That said, he questions whether these data can really be extrapolated to lower-risk patients. “I think this is an early snapshot and it’s a relatively big sample, but it’s a selected sample and we don’t know how many patients needed redo TAVR and didn’t get it or didn’t want it,” he added.
On the comforting side, there has been ongoing concern that a redo procedure that involves “propping open” a degenerated TAVR prosthesis’s leaflets with a new TAVR valve may occlude the coronary ostium by closing the flow within the open cells.
“Luckily, the investigators show an extremely low risk of coronary obstruction of only 0.9% in an anatomically high-risk patient population,” he said. This incidence, however, may increase as the use of TAVR rises in younger and less risky patients, he added.
Thourani would also like to see a longer follow-up on these patients. Median follow-up post redo TAVR was 15 months in this analysis.
“What I think we need to concentrate on as we do these studies is the life-long management of aortic stenosis wherein we try to minimize the overall number of invasive procedures as much as we can,” said Thourani.
Landes reported no conflict of interest. Thourani reported he is an advisor and/or researcher for Abbott Vascular, Boston Scientific, and Edwards Lifesciences.
This article first appeared on Medscape.com.
Circulating biomarker, genetic data improve pancreatic cancer risk modeling in general population
Risk models that incorporate genetic and circulating biomarker data in addition to established risk factors may improve risk modeling for pancreatic cancer in the general population, according to investigators.
Identifying high-risk individuals could facilitate earlier disease detection, which is essential for curing pancreatic cancer, reported lead author Jihye Kim, PhD, of Harvard School of Public Health, Boston, and colleagues.
“Given the late stage at presentation for most patients with pancreatic cancer, earlier detection approaches are worthy of significant investment as a critical means to reduce mortality from pancreatic cancer, soon to be the second-leading cause of cancer death in the United States,” the investigators wrote in Cancer Epidemiology, Biomarkers & Prevention.
According to the investigators, a variety of risk factors for pancreatic cancer are well established and include clinical, demographic, and lifestyle factors, while recent studies have reported associations with genetic and circulating biomarkers.
“Although risk factors have been investigated individually, their joint contribution to risk discrimination remains largely unknown,” the investigators wrote.
To learn more, Dr. Kim and colleagues performed a nested case-control study in which 500 patients with primary pancreatic adenocarcinoma were matched with 1,091 healthy controls. Data were drawn from four prospective studies: the Nurses’ Health Study, the Health Professionals Follow-up Study, the Women’s Health Initiative Observational Study, and the Physicians’ Health Study I. Via these studies, cases provided blood samples prior to diagnosis with pancreatic cancer.
“Importantly, because all our subjects were enrolled in prospective cohorts, all risk factor data and circulating markers were measured before the cases’ diagnosis of pancreatic cancer,” the investigators wrote. “This design faithfully recapitulates the situation faced by primary care physicians, where decisions related to disease screening are made in the prediagnostic setting using data collected in the several years prior to cancer diagnosis.”
In the present study, the investigators collected patient data for a variety of risk factors, including clinical and lifestyle characteristics, circulating biomarkers such as interleukin-6 and proinsulin, and 22 single-nucleotide polymorphisms. Frequencies and distributions of these factors were used to develop three multivariate risk models: a clinical model, a clinical/genetic model, and a clinical/genetic/biomarker model. To determine absolute risk of pancreatic cancer, these three models were combined with U.S. epidemiologic data, including incidence and mortality rates.
Cross-validation showed that the risk models became increasingly accurate with each added dataset; the area under the curve increased from 0.55 for the clinical model to 0.61 for the clinical/genetic model and ultimately to 0.62 for the clinical/genetic/biomarker model. Consequently, each model identified a greater number of individuals with at least a threefold risk of pancreatic cancer over a 10-year period. For example, the clinical model identified 1.5% of women and 0.2% of men with at least threefold risk, whereas the model that also included genetic and biomarker data identified 2.6% of women and 3.7% of men.
Absolute risk modeling allowed for generation of risk stratification percentiles by age. Women in the 99th risk percentile had a 1.7% risk of developing pancreatic cancer by age 70 years, and a 3.6% risk by age 80. For men, the highest-risk group had a 2.0% risk of pancreatic cancer by age 70 years and a 3.8% risk by age 80. Conversely, both men and women in the 10th risk percentile had a 0.2% risk by age 70 years and a 0.4% risk by age 80.
“[T]he addition of genetic variants and circulating markers added discriminatory ability beyond clinical factors that could be solicited in a physician’s office,” the investigators wrote.
“Further refinement and validation in independent samples will be necessary to make these models clinically actionable and impact survival of patients with pancreatic cancer,” they concluded.
The study was supported by the National Institutes of Health. The investigators reported additional relationships with Bayer, Celgene, Eli Lilly, and others.
SOURCE: Kim J et al. Cancer Epidemiol Biomarkers Prev. 2020 Apr 22. doi: 10.1158/1055-9965.EPI-19-1389.
Risk models that incorporate genetic and circulating biomarker data in addition to established risk factors may improve risk modeling for pancreatic cancer in the general population, according to investigators.
Identifying high-risk individuals could facilitate earlier disease detection, which is essential for curing pancreatic cancer, reported lead author Jihye Kim, PhD, of Harvard School of Public Health, Boston, and colleagues.
“Given the late stage at presentation for most patients with pancreatic cancer, earlier detection approaches are worthy of significant investment as a critical means to reduce mortality from pancreatic cancer, soon to be the second-leading cause of cancer death in the United States,” the investigators wrote in Cancer Epidemiology, Biomarkers & Prevention.
According to the investigators, a variety of risk factors for pancreatic cancer are well established and include clinical, demographic, and lifestyle factors, while recent studies have reported associations with genetic and circulating biomarkers.
“Although risk factors have been investigated individually, their joint contribution to risk discrimination remains largely unknown,” the investigators wrote.
To learn more, Dr. Kim and colleagues performed a nested case-control study in which 500 patients with primary pancreatic adenocarcinoma were matched with 1,091 healthy controls. Data were drawn from four prospective studies: the Nurses’ Health Study, the Health Professionals Follow-up Study, the Women’s Health Initiative Observational Study, and the Physicians’ Health Study I. Via these studies, cases provided blood samples prior to diagnosis with pancreatic cancer.
“Importantly, because all our subjects were enrolled in prospective cohorts, all risk factor data and circulating markers were measured before the cases’ diagnosis of pancreatic cancer,” the investigators wrote. “This design faithfully recapitulates the situation faced by primary care physicians, where decisions related to disease screening are made in the prediagnostic setting using data collected in the several years prior to cancer diagnosis.”
In the present study, the investigators collected patient data for a variety of risk factors, including clinical and lifestyle characteristics, circulating biomarkers such as interleukin-6 and proinsulin, and 22 single-nucleotide polymorphisms. Frequencies and distributions of these factors were used to develop three multivariate risk models: a clinical model, a clinical/genetic model, and a clinical/genetic/biomarker model. To determine absolute risk of pancreatic cancer, these three models were combined with U.S. epidemiologic data, including incidence and mortality rates.
Cross-validation showed that the risk models became increasingly accurate with each added dataset; the area under the curve increased from 0.55 for the clinical model to 0.61 for the clinical/genetic model and ultimately to 0.62 for the clinical/genetic/biomarker model. Consequently, each model identified a greater number of individuals with at least a threefold risk of pancreatic cancer over a 10-year period. For example, the clinical model identified 1.5% of women and 0.2% of men with at least threefold risk, whereas the model that also included genetic and biomarker data identified 2.6% of women and 3.7% of men.
Absolute risk modeling allowed for generation of risk stratification percentiles by age. Women in the 99th risk percentile had a 1.7% risk of developing pancreatic cancer by age 70 years, and a 3.6% risk by age 80. For men, the highest-risk group had a 2.0% risk of pancreatic cancer by age 70 years and a 3.8% risk by age 80. Conversely, both men and women in the 10th risk percentile had a 0.2% risk by age 70 years and a 0.4% risk by age 80.
“[T]he addition of genetic variants and circulating markers added discriminatory ability beyond clinical factors that could be solicited in a physician’s office,” the investigators wrote.
“Further refinement and validation in independent samples will be necessary to make these models clinically actionable and impact survival of patients with pancreatic cancer,” they concluded.
The study was supported by the National Institutes of Health. The investigators reported additional relationships with Bayer, Celgene, Eli Lilly, and others.
SOURCE: Kim J et al. Cancer Epidemiol Biomarkers Prev. 2020 Apr 22. doi: 10.1158/1055-9965.EPI-19-1389.
Risk models that incorporate genetic and circulating biomarker data in addition to established risk factors may improve risk modeling for pancreatic cancer in the general population, according to investigators.
Identifying high-risk individuals could facilitate earlier disease detection, which is essential for curing pancreatic cancer, reported lead author Jihye Kim, PhD, of Harvard School of Public Health, Boston, and colleagues.
“Given the late stage at presentation for most patients with pancreatic cancer, earlier detection approaches are worthy of significant investment as a critical means to reduce mortality from pancreatic cancer, soon to be the second-leading cause of cancer death in the United States,” the investigators wrote in Cancer Epidemiology, Biomarkers & Prevention.
According to the investigators, a variety of risk factors for pancreatic cancer are well established and include clinical, demographic, and lifestyle factors, while recent studies have reported associations with genetic and circulating biomarkers.
“Although risk factors have been investigated individually, their joint contribution to risk discrimination remains largely unknown,” the investigators wrote.
To learn more, Dr. Kim and colleagues performed a nested case-control study in which 500 patients with primary pancreatic adenocarcinoma were matched with 1,091 healthy controls. Data were drawn from four prospective studies: the Nurses’ Health Study, the Health Professionals Follow-up Study, the Women’s Health Initiative Observational Study, and the Physicians’ Health Study I. Via these studies, cases provided blood samples prior to diagnosis with pancreatic cancer.
“Importantly, because all our subjects were enrolled in prospective cohorts, all risk factor data and circulating markers were measured before the cases’ diagnosis of pancreatic cancer,” the investigators wrote. “This design faithfully recapitulates the situation faced by primary care physicians, where decisions related to disease screening are made in the prediagnostic setting using data collected in the several years prior to cancer diagnosis.”
In the present study, the investigators collected patient data for a variety of risk factors, including clinical and lifestyle characteristics, circulating biomarkers such as interleukin-6 and proinsulin, and 22 single-nucleotide polymorphisms. Frequencies and distributions of these factors were used to develop three multivariate risk models: a clinical model, a clinical/genetic model, and a clinical/genetic/biomarker model. To determine absolute risk of pancreatic cancer, these three models were combined with U.S. epidemiologic data, including incidence and mortality rates.
Cross-validation showed that the risk models became increasingly accurate with each added dataset; the area under the curve increased from 0.55 for the clinical model to 0.61 for the clinical/genetic model and ultimately to 0.62 for the clinical/genetic/biomarker model. Consequently, each model identified a greater number of individuals with at least a threefold risk of pancreatic cancer over a 10-year period. For example, the clinical model identified 1.5% of women and 0.2% of men with at least threefold risk, whereas the model that also included genetic and biomarker data identified 2.6% of women and 3.7% of men.
Absolute risk modeling allowed for generation of risk stratification percentiles by age. Women in the 99th risk percentile had a 1.7% risk of developing pancreatic cancer by age 70 years, and a 3.6% risk by age 80. For men, the highest-risk group had a 2.0% risk of pancreatic cancer by age 70 years and a 3.8% risk by age 80. Conversely, both men and women in the 10th risk percentile had a 0.2% risk by age 70 years and a 0.4% risk by age 80.
“[T]he addition of genetic variants and circulating markers added discriminatory ability beyond clinical factors that could be solicited in a physician’s office,” the investigators wrote.
“Further refinement and validation in independent samples will be necessary to make these models clinically actionable and impact survival of patients with pancreatic cancer,” they concluded.
The study was supported by the National Institutes of Health. The investigators reported additional relationships with Bayer, Celgene, Eli Lilly, and others.
SOURCE: Kim J et al. Cancer Epidemiol Biomarkers Prev. 2020 Apr 22. doi: 10.1158/1055-9965.EPI-19-1389.
FROM CANCER EPIDEMIOLOGY, BIOMARKERS & PREVENTION