User login
Anti-NMDAR encephalitis or primary psychiatric disorder?
New insights and ‘red flags’ provide clues to diagnosis
It remains difficult to distinguish anti-NMDA receptor encephalitis from a primary psychiatric disorder, but recent studies have identified clinical features and proposed screening criteria that could make it easier to identify these patients who would benefit from immunotherapy, according to an expert in the neurologic disease.
Most patients with confirmed anti-NMDA receptor encephalitis will experience substantial improvement after treatment with immunotherapy and other modalities, said Josep Dalmau, MD, PhD, professor at the Catalan Institute for Research and Advanced Studies at the University of Barcelona and adjunct professor of neurology at the University of Pennsylvania, Philadelphia.
“In our experience, being aggressive with immune therapy ... the patients do quite well, which means that basically 85%-90% of the patients substantially improved over the next few months,” Dr. Dalmau said at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
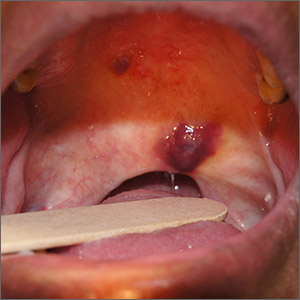
Identified for the first time a little more than a decade ago, anti-NMDA receptor encephalitis is a rare, immune-mediated disease that is usually found in children and young adults and is more common among women. It is frequently associated with ovarian tumors and teratomas, said Dr. Dalmau, and in about 90% of cases, patients will have prominent psychiatric and behavioral symptoms.
Patients develop IgG antibodies against the GluN1 subunit of the NMDA receptor. These autoantibodies represent not only a diagnostic marker of the disease, but are also pathogenic, altering NMDA receptor–related synaptic transmission, Dr. Dalmau said.
In several recent studies, investigators have attempted to cobble together a distinct phenotype on anti-NMDA receptor encephalitis to aid psychiatrists who might encounter patients with the disease, he said.
In one of the most recent studies, researchers combed the medical literature and found that, among 544 individuals with the disease, the most common psychiatric symptoms were agitation, seen in 59%, and psychotic symptoms (particularly visual-auditory hallucinations and disorganized behavior) in 54%; catatonia was seen in 42% of adults and 35% of children.
according to a report from researchers in Berlin, Dr. Dalmau added. By picking up on those clinical signs, which included seizures, catatonia, autonomic instability, or hyperkinesia, the time from symptom onset to diagnosis could be cut in half, the researchers found.
There’s also a handy acronym that could serve as a mnemonic to pick up on “diagnostic clues” of anti-NMDA receptor encephalitis among patients with new-onset psychiatric symptoms, Dr. Dalmau said.
That acronym, published in a review article by Dr. Dalmau and colleagues, is SEARCH For NMDAR-A, covering, in order: sleep dysfunction, excitement, agitation, rapid onset, child and young adult predominance, history of psychiatric disease (absent), fluctuating catatonia, negative and positive symptoms, memory deficit, decreased verbal output, antipsychotic intolerance, rule out neuroleptic malignant syndrome, and of course, antibodies (though the final “A” also stands for additional testing, including magnetic resonance imaging, cerebrospinal fluid studies, and electroencephalogram).
While the disease can be lethal, Dr. Dalmau said most patients respond to immunotherapy, and if applicable, treatment of the underlying tumor can help. The most common first-line treatments include steroids, intravenous immunoglobulin, and plasma exchange, he said, while second-line treatments include the monoclonal anti-CD20 antibody rituximab and cyclophosphamide.
Beyond immunotherapy, patients may benefit from supportive care and psychiatric treatment. Benzodiazepines are well tolerated, but Dr. Dalmau said antipsychotic intolerance is frequent, and electroconvulsive therapy has “mixed results” in these patients.
The recovery process can take months and may be complicated by hypersomnia, hyperphagia, and hypersexuality, he added.
“Some patients improve dramatically in 1 month, but this is uncommon, really,” he said, adding that an early recovery may be a “red flag” that the underlying condition is something other than anti-NMDA receptor encephalitis.
Dr. Dalmau provided disclosures related to Cellex Foundation, Safra Foundation, Caixa Health Project Foundation, and Sage Therapeutics.
SOURCE: Dalmau J. APA 2020, Abstract.
New insights and ‘red flags’ provide clues to diagnosis
New insights and ‘red flags’ provide clues to diagnosis
It remains difficult to distinguish anti-NMDA receptor encephalitis from a primary psychiatric disorder, but recent studies have identified clinical features and proposed screening criteria that could make it easier to identify these patients who would benefit from immunotherapy, according to an expert in the neurologic disease.
Most patients with confirmed anti-NMDA receptor encephalitis will experience substantial improvement after treatment with immunotherapy and other modalities, said Josep Dalmau, MD, PhD, professor at the Catalan Institute for Research and Advanced Studies at the University of Barcelona and adjunct professor of neurology at the University of Pennsylvania, Philadelphia.
“In our experience, being aggressive with immune therapy ... the patients do quite well, which means that basically 85%-90% of the patients substantially improved over the next few months,” Dr. Dalmau said at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
Identified for the first time a little more than a decade ago, anti-NMDA receptor encephalitis is a rare, immune-mediated disease that is usually found in children and young adults and is more common among women. It is frequently associated with ovarian tumors and teratomas, said Dr. Dalmau, and in about 90% of cases, patients will have prominent psychiatric and behavioral symptoms.
Patients develop IgG antibodies against the GluN1 subunit of the NMDA receptor. These autoantibodies represent not only a diagnostic marker of the disease, but are also pathogenic, altering NMDA receptor–related synaptic transmission, Dr. Dalmau said.
In several recent studies, investigators have attempted to cobble together a distinct phenotype on anti-NMDA receptor encephalitis to aid psychiatrists who might encounter patients with the disease, he said.
In one of the most recent studies, researchers combed the medical literature and found that, among 544 individuals with the disease, the most common psychiatric symptoms were agitation, seen in 59%, and psychotic symptoms (particularly visual-auditory hallucinations and disorganized behavior) in 54%; catatonia was seen in 42% of adults and 35% of children.
according to a report from researchers in Berlin, Dr. Dalmau added. By picking up on those clinical signs, which included seizures, catatonia, autonomic instability, or hyperkinesia, the time from symptom onset to diagnosis could be cut in half, the researchers found.
There’s also a handy acronym that could serve as a mnemonic to pick up on “diagnostic clues” of anti-NMDA receptor encephalitis among patients with new-onset psychiatric symptoms, Dr. Dalmau said.
That acronym, published in a review article by Dr. Dalmau and colleagues, is SEARCH For NMDAR-A, covering, in order: sleep dysfunction, excitement, agitation, rapid onset, child and young adult predominance, history of psychiatric disease (absent), fluctuating catatonia, negative and positive symptoms, memory deficit, decreased verbal output, antipsychotic intolerance, rule out neuroleptic malignant syndrome, and of course, antibodies (though the final “A” also stands for additional testing, including magnetic resonance imaging, cerebrospinal fluid studies, and electroencephalogram).
While the disease can be lethal, Dr. Dalmau said most patients respond to immunotherapy, and if applicable, treatment of the underlying tumor can help. The most common first-line treatments include steroids, intravenous immunoglobulin, and plasma exchange, he said, while second-line treatments include the monoclonal anti-CD20 antibody rituximab and cyclophosphamide.
Beyond immunotherapy, patients may benefit from supportive care and psychiatric treatment. Benzodiazepines are well tolerated, but Dr. Dalmau said antipsychotic intolerance is frequent, and electroconvulsive therapy has “mixed results” in these patients.
The recovery process can take months and may be complicated by hypersomnia, hyperphagia, and hypersexuality, he added.
“Some patients improve dramatically in 1 month, but this is uncommon, really,” he said, adding that an early recovery may be a “red flag” that the underlying condition is something other than anti-NMDA receptor encephalitis.
Dr. Dalmau provided disclosures related to Cellex Foundation, Safra Foundation, Caixa Health Project Foundation, and Sage Therapeutics.
SOURCE: Dalmau J. APA 2020, Abstract.
It remains difficult to distinguish anti-NMDA receptor encephalitis from a primary psychiatric disorder, but recent studies have identified clinical features and proposed screening criteria that could make it easier to identify these patients who would benefit from immunotherapy, according to an expert in the neurologic disease.
Most patients with confirmed anti-NMDA receptor encephalitis will experience substantial improvement after treatment with immunotherapy and other modalities, said Josep Dalmau, MD, PhD, professor at the Catalan Institute for Research and Advanced Studies at the University of Barcelona and adjunct professor of neurology at the University of Pennsylvania, Philadelphia.
“In our experience, being aggressive with immune therapy ... the patients do quite well, which means that basically 85%-90% of the patients substantially improved over the next few months,” Dr. Dalmau said at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
Identified for the first time a little more than a decade ago, anti-NMDA receptor encephalitis is a rare, immune-mediated disease that is usually found in children and young adults and is more common among women. It is frequently associated with ovarian tumors and teratomas, said Dr. Dalmau, and in about 90% of cases, patients will have prominent psychiatric and behavioral symptoms.
Patients develop IgG antibodies against the GluN1 subunit of the NMDA receptor. These autoantibodies represent not only a diagnostic marker of the disease, but are also pathogenic, altering NMDA receptor–related synaptic transmission, Dr. Dalmau said.
In several recent studies, investigators have attempted to cobble together a distinct phenotype on anti-NMDA receptor encephalitis to aid psychiatrists who might encounter patients with the disease, he said.
In one of the most recent studies, researchers combed the medical literature and found that, among 544 individuals with the disease, the most common psychiatric symptoms were agitation, seen in 59%, and psychotic symptoms (particularly visual-auditory hallucinations and disorganized behavior) in 54%; catatonia was seen in 42% of adults and 35% of children.
according to a report from researchers in Berlin, Dr. Dalmau added. By picking up on those clinical signs, which included seizures, catatonia, autonomic instability, or hyperkinesia, the time from symptom onset to diagnosis could be cut in half, the researchers found.
There’s also a handy acronym that could serve as a mnemonic to pick up on “diagnostic clues” of anti-NMDA receptor encephalitis among patients with new-onset psychiatric symptoms, Dr. Dalmau said.
That acronym, published in a review article by Dr. Dalmau and colleagues, is SEARCH For NMDAR-A, covering, in order: sleep dysfunction, excitement, agitation, rapid onset, child and young adult predominance, history of psychiatric disease (absent), fluctuating catatonia, negative and positive symptoms, memory deficit, decreased verbal output, antipsychotic intolerance, rule out neuroleptic malignant syndrome, and of course, antibodies (though the final “A” also stands for additional testing, including magnetic resonance imaging, cerebrospinal fluid studies, and electroencephalogram).
While the disease can be lethal, Dr. Dalmau said most patients respond to immunotherapy, and if applicable, treatment of the underlying tumor can help. The most common first-line treatments include steroids, intravenous immunoglobulin, and plasma exchange, he said, while second-line treatments include the monoclonal anti-CD20 antibody rituximab and cyclophosphamide.
Beyond immunotherapy, patients may benefit from supportive care and psychiatric treatment. Benzodiazepines are well tolerated, but Dr. Dalmau said antipsychotic intolerance is frequent, and electroconvulsive therapy has “mixed results” in these patients.
The recovery process can take months and may be complicated by hypersomnia, hyperphagia, and hypersexuality, he added.
“Some patients improve dramatically in 1 month, but this is uncommon, really,” he said, adding that an early recovery may be a “red flag” that the underlying condition is something other than anti-NMDA receptor encephalitis.
Dr. Dalmau provided disclosures related to Cellex Foundation, Safra Foundation, Caixa Health Project Foundation, and Sage Therapeutics.
SOURCE: Dalmau J. APA 2020, Abstract.
FROM APA 2020
Ob.gyns., peds, other PCPs seeking COVID-19 financial relief from feds
A handful of specialties – including family medicine, obstetrics/gynecology, pediatrics, and other primary care specialties – are calling for targeted and urgent relief payments from the federal government, saying that they have been left out of distributions aimed at alleviating the financial fallout associated with the novel coronavirus.
The federal government has already distributed about $150 billion – through direct payments and advances on reimbursement – to clinicians, but, to date, the money has only been given to providers who bill Medicare, and not even all of those individuals have received payments.
“It is critical that frontline physicians who may not participate in Medicare fee-for-service, in whole or in part, including obstetrician/gynecologists, pediatricians, and family physicians, have the resources they need to continue providing essential health care to patients amid the pandemic and in the months to come,” said the American Academy of Family Physicians, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists in a letter to Health & Human Services (Secretary Alex Azar.
In particular, the organizations are concerned that no money has been distributed or earmarked for clinicians who serve Medicaid recipients.
“The organizations that signed that letter are the primary providers of care to the Medicaid population,” Shawn Martin, senior VP for the AAFP, said in an interview. That’s true even for family physicians.
“Typically, in an average family medicine practice, their Medicaid panel size is equal to if not greater than the Medicare panel size,” he said.
On April 23, Mr. Azar said HHS was working on a distribution plan for providers who only take Medicaid, as well as for dentists and skilled nursing facilities. An HHS spokesperson confirmed that the agency still intends to provide money to those groups of providers and that the agency is committed to distributing funds quickly and with transparency.
Mr. Azar had also announced that the government would soon start distributing $20 billion in payments to Medicare providers, on top of the $30 billion that had already been handed out to clinicians on April 10 and 17.
That $50 billion came from the COVID-19–related $100 billion Provider Relief Fund, which was part of the Coronavirus Aid, Relief, and Economic Security Act, signed into law on March 27.
Additionally, the Centers for Medicare & Medicaid Services had distributed some $100 billion to providers who participated in Medicare Part A or B through the Medicare Advance Payment program, which is a deferred loan. The agency brought that program to a halt on April 27.
An additional $75 billion will now be available through the Public Health and Social Services Emergency Fund (PHSSEF) as part of the third congressional COVID relief package, signed into law on April 24.
Mr. Martin said that the AAFP and other physician organizations have been talking with HHS about how to distribute money from that new pool of funds. “There’s been a lot of progress, but there hasn’t been any action,” he said, adding that the purpose of the joint letter to HHS “is to say it’s time for action.”
COVID-19 damage
AAFP, AAP, and ACOG noted in the letter the damage that’s being inflicted by COVID-19. They cited data that show a 50% decline in measles, mumps, and rubella shots, a 42% drop in diphtheria and whooping cough vaccinations, and a 73% decline in human papillomavirus shots. The groups also noted a rise in child abuse injuries that are being seen in EDs and the potential for a worsening of the maternal mortality crisis in the United States.
Primary care physicians are also the go-to doctors for upper respiratory infections, noted the groups in the letter.
“Put simply, our physician members need to be able to keep their doors open and continue treating patients,” said the groups.
A study by Harvard University and Phreesia, a health care technology company, found that ambulatory practice visits had declined by at least half since early February, with a 71% drop in visits by 7- to 17-year-olds and a 59% decline in visits by neonates, infants, and toddlers (up to age 6). Overall, pediatric practices experienced a 62% drop-off in visits.
Research conducted by the Physicians Foundation and Merritt Hawkins shows that 21% of 842 physicians who responded to an early April survey said they’d been furloughed or been given a pay cut. That number rose to 30% among doctors who are not treating COVID-19 patients.
Although the majority in the survey (66%) said they planned to keep practicing in the same manner during the pandemic, 32% said they planned to change practices, opt out of patient care roles, close their practices temporarily, or retire. The survey has a margin of error of ±3.5%.
Internists seek consideration, too
The American College of Physicians also has urged HHS to give special consideration to its members. The group wrote to Mr. Azar on April 28, recommending that payments from the new $75 billion PHSSEF be prioritized for primary care, as well as for smaller practices, those that provide care in underserved areas, and internal medicine subspecialty practices.
“Internal medicine specialists and other primary care physicians have an essential role in delivering primary, preventive, and comprehensive care not only to patients with symptoms or diagnoses of COVID-19, but also to patients with other underlying medical conditions, including conditions like heart disease and diabetes that put them at greater risk of mortality from COVID-19,” wrote ACP President Jacqueline Fincher, MD, MACP.
ACP said the government could pay physicians on the basis of the amount of additional expenses incurred that were related to COVID-19, such as extra staffing or temporary relocation of their place of residence to prevent exposing family members to the virus. Pay should also be based on the percentage of revenue losses from all payers, including Medicare, Medicaid, and commercial insurers, Dr. Fincher said in the letter.
AAFP, AAP, and ACOG also had a suggestion for distributing payments to non-Medicare providers. “Given that most women’s health, pediatric, and family practices have received less financial relief to date, we recommend that HHS provide these practices with a larger proportion of funds relative to their reported revenue than is provided on average across specialties,” they wrote.
A version of this article originally appeared on Medscape.com.
A handful of specialties – including family medicine, obstetrics/gynecology, pediatrics, and other primary care specialties – are calling for targeted and urgent relief payments from the federal government, saying that they have been left out of distributions aimed at alleviating the financial fallout associated with the novel coronavirus.
The federal government has already distributed about $150 billion – through direct payments and advances on reimbursement – to clinicians, but, to date, the money has only been given to providers who bill Medicare, and not even all of those individuals have received payments.
“It is critical that frontline physicians who may not participate in Medicare fee-for-service, in whole or in part, including obstetrician/gynecologists, pediatricians, and family physicians, have the resources they need to continue providing essential health care to patients amid the pandemic and in the months to come,” said the American Academy of Family Physicians, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists in a letter to Health & Human Services (Secretary Alex Azar.
In particular, the organizations are concerned that no money has been distributed or earmarked for clinicians who serve Medicaid recipients.
“The organizations that signed that letter are the primary providers of care to the Medicaid population,” Shawn Martin, senior VP for the AAFP, said in an interview. That’s true even for family physicians.
“Typically, in an average family medicine practice, their Medicaid panel size is equal to if not greater than the Medicare panel size,” he said.
On April 23, Mr. Azar said HHS was working on a distribution plan for providers who only take Medicaid, as well as for dentists and skilled nursing facilities. An HHS spokesperson confirmed that the agency still intends to provide money to those groups of providers and that the agency is committed to distributing funds quickly and with transparency.
Mr. Azar had also announced that the government would soon start distributing $20 billion in payments to Medicare providers, on top of the $30 billion that had already been handed out to clinicians on April 10 and 17.
That $50 billion came from the COVID-19–related $100 billion Provider Relief Fund, which was part of the Coronavirus Aid, Relief, and Economic Security Act, signed into law on March 27.
Additionally, the Centers for Medicare & Medicaid Services had distributed some $100 billion to providers who participated in Medicare Part A or B through the Medicare Advance Payment program, which is a deferred loan. The agency brought that program to a halt on April 27.
An additional $75 billion will now be available through the Public Health and Social Services Emergency Fund (PHSSEF) as part of the third congressional COVID relief package, signed into law on April 24.
Mr. Martin said that the AAFP and other physician organizations have been talking with HHS about how to distribute money from that new pool of funds. “There’s been a lot of progress, but there hasn’t been any action,” he said, adding that the purpose of the joint letter to HHS “is to say it’s time for action.”
COVID-19 damage
AAFP, AAP, and ACOG noted in the letter the damage that’s being inflicted by COVID-19. They cited data that show a 50% decline in measles, mumps, and rubella shots, a 42% drop in diphtheria and whooping cough vaccinations, and a 73% decline in human papillomavirus shots. The groups also noted a rise in child abuse injuries that are being seen in EDs and the potential for a worsening of the maternal mortality crisis in the United States.
Primary care physicians are also the go-to doctors for upper respiratory infections, noted the groups in the letter.
“Put simply, our physician members need to be able to keep their doors open and continue treating patients,” said the groups.
A study by Harvard University and Phreesia, a health care technology company, found that ambulatory practice visits had declined by at least half since early February, with a 71% drop in visits by 7- to 17-year-olds and a 59% decline in visits by neonates, infants, and toddlers (up to age 6). Overall, pediatric practices experienced a 62% drop-off in visits.
Research conducted by the Physicians Foundation and Merritt Hawkins shows that 21% of 842 physicians who responded to an early April survey said they’d been furloughed or been given a pay cut. That number rose to 30% among doctors who are not treating COVID-19 patients.
Although the majority in the survey (66%) said they planned to keep practicing in the same manner during the pandemic, 32% said they planned to change practices, opt out of patient care roles, close their practices temporarily, or retire. The survey has a margin of error of ±3.5%.
Internists seek consideration, too
The American College of Physicians also has urged HHS to give special consideration to its members. The group wrote to Mr. Azar on April 28, recommending that payments from the new $75 billion PHSSEF be prioritized for primary care, as well as for smaller practices, those that provide care in underserved areas, and internal medicine subspecialty practices.
“Internal medicine specialists and other primary care physicians have an essential role in delivering primary, preventive, and comprehensive care not only to patients with symptoms or diagnoses of COVID-19, but also to patients with other underlying medical conditions, including conditions like heart disease and diabetes that put them at greater risk of mortality from COVID-19,” wrote ACP President Jacqueline Fincher, MD, MACP.
ACP said the government could pay physicians on the basis of the amount of additional expenses incurred that were related to COVID-19, such as extra staffing or temporary relocation of their place of residence to prevent exposing family members to the virus. Pay should also be based on the percentage of revenue losses from all payers, including Medicare, Medicaid, and commercial insurers, Dr. Fincher said in the letter.
AAFP, AAP, and ACOG also had a suggestion for distributing payments to non-Medicare providers. “Given that most women’s health, pediatric, and family practices have received less financial relief to date, we recommend that HHS provide these practices with a larger proportion of funds relative to their reported revenue than is provided on average across specialties,” they wrote.
A version of this article originally appeared on Medscape.com.
A handful of specialties – including family medicine, obstetrics/gynecology, pediatrics, and other primary care specialties – are calling for targeted and urgent relief payments from the federal government, saying that they have been left out of distributions aimed at alleviating the financial fallout associated with the novel coronavirus.
The federal government has already distributed about $150 billion – through direct payments and advances on reimbursement – to clinicians, but, to date, the money has only been given to providers who bill Medicare, and not even all of those individuals have received payments.
“It is critical that frontline physicians who may not participate in Medicare fee-for-service, in whole or in part, including obstetrician/gynecologists, pediatricians, and family physicians, have the resources they need to continue providing essential health care to patients amid the pandemic and in the months to come,” said the American Academy of Family Physicians, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists in a letter to Health & Human Services (Secretary Alex Azar.
In particular, the organizations are concerned that no money has been distributed or earmarked for clinicians who serve Medicaid recipients.
“The organizations that signed that letter are the primary providers of care to the Medicaid population,” Shawn Martin, senior VP for the AAFP, said in an interview. That’s true even for family physicians.
“Typically, in an average family medicine practice, their Medicaid panel size is equal to if not greater than the Medicare panel size,” he said.
On April 23, Mr. Azar said HHS was working on a distribution plan for providers who only take Medicaid, as well as for dentists and skilled nursing facilities. An HHS spokesperson confirmed that the agency still intends to provide money to those groups of providers and that the agency is committed to distributing funds quickly and with transparency.
Mr. Azar had also announced that the government would soon start distributing $20 billion in payments to Medicare providers, on top of the $30 billion that had already been handed out to clinicians on April 10 and 17.
That $50 billion came from the COVID-19–related $100 billion Provider Relief Fund, which was part of the Coronavirus Aid, Relief, and Economic Security Act, signed into law on March 27.
Additionally, the Centers for Medicare & Medicaid Services had distributed some $100 billion to providers who participated in Medicare Part A or B through the Medicare Advance Payment program, which is a deferred loan. The agency brought that program to a halt on April 27.
An additional $75 billion will now be available through the Public Health and Social Services Emergency Fund (PHSSEF) as part of the third congressional COVID relief package, signed into law on April 24.
Mr. Martin said that the AAFP and other physician organizations have been talking with HHS about how to distribute money from that new pool of funds. “There’s been a lot of progress, but there hasn’t been any action,” he said, adding that the purpose of the joint letter to HHS “is to say it’s time for action.”
COVID-19 damage
AAFP, AAP, and ACOG noted in the letter the damage that’s being inflicted by COVID-19. They cited data that show a 50% decline in measles, mumps, and rubella shots, a 42% drop in diphtheria and whooping cough vaccinations, and a 73% decline in human papillomavirus shots. The groups also noted a rise in child abuse injuries that are being seen in EDs and the potential for a worsening of the maternal mortality crisis in the United States.
Primary care physicians are also the go-to doctors for upper respiratory infections, noted the groups in the letter.
“Put simply, our physician members need to be able to keep their doors open and continue treating patients,” said the groups.
A study by Harvard University and Phreesia, a health care technology company, found that ambulatory practice visits had declined by at least half since early February, with a 71% drop in visits by 7- to 17-year-olds and a 59% decline in visits by neonates, infants, and toddlers (up to age 6). Overall, pediatric practices experienced a 62% drop-off in visits.
Research conducted by the Physicians Foundation and Merritt Hawkins shows that 21% of 842 physicians who responded to an early April survey said they’d been furloughed or been given a pay cut. That number rose to 30% among doctors who are not treating COVID-19 patients.
Although the majority in the survey (66%) said they planned to keep practicing in the same manner during the pandemic, 32% said they planned to change practices, opt out of patient care roles, close their practices temporarily, or retire. The survey has a margin of error of ±3.5%.
Internists seek consideration, too
The American College of Physicians also has urged HHS to give special consideration to its members. The group wrote to Mr. Azar on April 28, recommending that payments from the new $75 billion PHSSEF be prioritized for primary care, as well as for smaller practices, those that provide care in underserved areas, and internal medicine subspecialty practices.
“Internal medicine specialists and other primary care physicians have an essential role in delivering primary, preventive, and comprehensive care not only to patients with symptoms or diagnoses of COVID-19, but also to patients with other underlying medical conditions, including conditions like heart disease and diabetes that put them at greater risk of mortality from COVID-19,” wrote ACP President Jacqueline Fincher, MD, MACP.
ACP said the government could pay physicians on the basis of the amount of additional expenses incurred that were related to COVID-19, such as extra staffing or temporary relocation of their place of residence to prevent exposing family members to the virus. Pay should also be based on the percentage of revenue losses from all payers, including Medicare, Medicaid, and commercial insurers, Dr. Fincher said in the letter.
AAFP, AAP, and ACOG also had a suggestion for distributing payments to non-Medicare providers. “Given that most women’s health, pediatric, and family practices have received less financial relief to date, we recommend that HHS provide these practices with a larger proportion of funds relative to their reported revenue than is provided on average across specialties,” they wrote.
A version of this article originally appeared on Medscape.com.
Report details first case of PML with ocrelizumab alone
The case report was presented online as part of the 2020 American Academy of Neurology Science Highlights.
PML, an opportunistic infection of the brain caused by reactivation of the John Cunningham (JC) virus, has occurred with rituximab, another anti-CD20 therapy, in rare cases. Eight other cases of PML diagnosed after ocrelizumab initiation are considered carry-over cases related to prior treatment with natalizumab or fingolimod, according to Genentech, the manufacturer of ocrelizumab. No other PML cases have been associated with ocrelizumab alone.
The case without prior MS treatment was in a 78-year-old man. He presented with 2 weeks of progressive visual disturbance and confusion, said Marc L. Gordon, MD, chief of neurology at the Zucker Hillside Hospital in Glen Oaks, N.Y., and professor of neurology and psychiatry at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, N.Y.
The patient had a right homonymous hemianopia. “MRI revealed an enlarging non-enhancing left parietal lesion without mass effect,” reported Dr. Gordon and colleagues. “CSF PCR revealed 1,000 copies/mL of JCV, confirming the diagnosis of PML. Blood work upon diagnosis revealed grade-2 lymphopenia ... and negative HIV serology.”
“The patient’s symptoms progressed over weeks to involve bilateral visual loss, right facial droop, and dysphasia,” they said. “Ocrelizumab was discontinued and off-label pembrolizumab treatment was initiated.” The patient did not respond to therapy and became bedbound, Dr. Gordon said in an interview. The patient received palliative care and died. An autopsy is pending.
“PML occurrence may have been multifactorial, due to a combination of the immunomodulatory function of ocrelizumab, possible immune senescence, and preceding mild lymphopenia,” Dr. Gordon and coauthors said. “This case emphasizes the importance of a thorough discussion of the risks and benefits of ocrelizumab, especially in patients at higher risk for infections, such as the elderly.”
The patient, who was Dr. Gordon’s patient for 20 years, had monitored updates in drug development and had looked forward to starting ocrelizumab, the first therapy approved for primary progressive MS, when it became available after its approval in 2017. The patient was concerned about progressively worsening gait impairment and related falls caused by MS.
Antibodies indicated that the patient had prior exposure to JCV, but Dr. Gordon considered the risk of PML to be relatively small. Prior to treatment, the patient’s absolute lymphocyte count was normal or indicated mild lymphocytopenia, which Dr. Gordon did not consider clinically significant. The patient received ocrelizumab for 2 years without incident.
In an information sheet for health care professionals about ocrelizumab and PML prepared in February 2020, Genentech says the patient’s age and low absolute lymphocyte count are confounding factors, which distort “the assessment of association between exposure to a drug and an adverse event.
“As of January 31, 2020, no unconfounded PML cases associated with ocrelizumab therapy have been reported,” according to the document. “Out of more than 150,000 patients treated globally (clinical trials and post-marketing experience), there have been nine confirmed, confounded cases of PML in patients treated with ocrelizumab, of which eight were carry-over cases from a prior DMT.”
The prescribing information for the drug notes that no cases of PML were identified in ocrelizumab clinical trials, but that PML has been observed in patients treated with other anti-CD20 antibodies and other MS therapies. In addition, PML “has been associated with some risk factors (eg, immunocompromised patients, polytherapy with immunosuppressants).
“At the first sign or symptom suggestive of PML, withhold ocrelizumab and perform an appropriate diagnostic evaluation,” the prescribing information says. “MRI findings may be apparent before clinical signs or symptoms. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes.”
“It is important for people to recognize that this is at least a possibility,” Dr. Gordon said. Any change in clinical symptomatology may warrant imaging, and CSF testing may be warranted if an MRI raises concerns about PML, he said.
Dr. Gordon has received research support from MSD (Merck), Eisai, AbbVie, and Janssen.
SOURCE: Sul J et al. AAN 2020. Abstract S29.001.
The case report was presented online as part of the 2020 American Academy of Neurology Science Highlights.
PML, an opportunistic infection of the brain caused by reactivation of the John Cunningham (JC) virus, has occurred with rituximab, another anti-CD20 therapy, in rare cases. Eight other cases of PML diagnosed after ocrelizumab initiation are considered carry-over cases related to prior treatment with natalizumab or fingolimod, according to Genentech, the manufacturer of ocrelizumab. No other PML cases have been associated with ocrelizumab alone.
The case without prior MS treatment was in a 78-year-old man. He presented with 2 weeks of progressive visual disturbance and confusion, said Marc L. Gordon, MD, chief of neurology at the Zucker Hillside Hospital in Glen Oaks, N.Y., and professor of neurology and psychiatry at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, N.Y.
The patient had a right homonymous hemianopia. “MRI revealed an enlarging non-enhancing left parietal lesion without mass effect,” reported Dr. Gordon and colleagues. “CSF PCR revealed 1,000 copies/mL of JCV, confirming the diagnosis of PML. Blood work upon diagnosis revealed grade-2 lymphopenia ... and negative HIV serology.”
“The patient’s symptoms progressed over weeks to involve bilateral visual loss, right facial droop, and dysphasia,” they said. “Ocrelizumab was discontinued and off-label pembrolizumab treatment was initiated.” The patient did not respond to therapy and became bedbound, Dr. Gordon said in an interview. The patient received palliative care and died. An autopsy is pending.
“PML occurrence may have been multifactorial, due to a combination of the immunomodulatory function of ocrelizumab, possible immune senescence, and preceding mild lymphopenia,” Dr. Gordon and coauthors said. “This case emphasizes the importance of a thorough discussion of the risks and benefits of ocrelizumab, especially in patients at higher risk for infections, such as the elderly.”
The patient, who was Dr. Gordon’s patient for 20 years, had monitored updates in drug development and had looked forward to starting ocrelizumab, the first therapy approved for primary progressive MS, when it became available after its approval in 2017. The patient was concerned about progressively worsening gait impairment and related falls caused by MS.
Antibodies indicated that the patient had prior exposure to JCV, but Dr. Gordon considered the risk of PML to be relatively small. Prior to treatment, the patient’s absolute lymphocyte count was normal or indicated mild lymphocytopenia, which Dr. Gordon did not consider clinically significant. The patient received ocrelizumab for 2 years without incident.
In an information sheet for health care professionals about ocrelizumab and PML prepared in February 2020, Genentech says the patient’s age and low absolute lymphocyte count are confounding factors, which distort “the assessment of association between exposure to a drug and an adverse event.
“As of January 31, 2020, no unconfounded PML cases associated with ocrelizumab therapy have been reported,” according to the document. “Out of more than 150,000 patients treated globally (clinical trials and post-marketing experience), there have been nine confirmed, confounded cases of PML in patients treated with ocrelizumab, of which eight were carry-over cases from a prior DMT.”
The prescribing information for the drug notes that no cases of PML were identified in ocrelizumab clinical trials, but that PML has been observed in patients treated with other anti-CD20 antibodies and other MS therapies. In addition, PML “has been associated with some risk factors (eg, immunocompromised patients, polytherapy with immunosuppressants).
“At the first sign or symptom suggestive of PML, withhold ocrelizumab and perform an appropriate diagnostic evaluation,” the prescribing information says. “MRI findings may be apparent before clinical signs or symptoms. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes.”
“It is important for people to recognize that this is at least a possibility,” Dr. Gordon said. Any change in clinical symptomatology may warrant imaging, and CSF testing may be warranted if an MRI raises concerns about PML, he said.
Dr. Gordon has received research support from MSD (Merck), Eisai, AbbVie, and Janssen.
SOURCE: Sul J et al. AAN 2020. Abstract S29.001.
The case report was presented online as part of the 2020 American Academy of Neurology Science Highlights.
PML, an opportunistic infection of the brain caused by reactivation of the John Cunningham (JC) virus, has occurred with rituximab, another anti-CD20 therapy, in rare cases. Eight other cases of PML diagnosed after ocrelizumab initiation are considered carry-over cases related to prior treatment with natalizumab or fingolimod, according to Genentech, the manufacturer of ocrelizumab. No other PML cases have been associated with ocrelizumab alone.
The case without prior MS treatment was in a 78-year-old man. He presented with 2 weeks of progressive visual disturbance and confusion, said Marc L. Gordon, MD, chief of neurology at the Zucker Hillside Hospital in Glen Oaks, N.Y., and professor of neurology and psychiatry at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, N.Y.
The patient had a right homonymous hemianopia. “MRI revealed an enlarging non-enhancing left parietal lesion without mass effect,” reported Dr. Gordon and colleagues. “CSF PCR revealed 1,000 copies/mL of JCV, confirming the diagnosis of PML. Blood work upon diagnosis revealed grade-2 lymphopenia ... and negative HIV serology.”
“The patient’s symptoms progressed over weeks to involve bilateral visual loss, right facial droop, and dysphasia,” they said. “Ocrelizumab was discontinued and off-label pembrolizumab treatment was initiated.” The patient did not respond to therapy and became bedbound, Dr. Gordon said in an interview. The patient received palliative care and died. An autopsy is pending.
“PML occurrence may have been multifactorial, due to a combination of the immunomodulatory function of ocrelizumab, possible immune senescence, and preceding mild lymphopenia,” Dr. Gordon and coauthors said. “This case emphasizes the importance of a thorough discussion of the risks and benefits of ocrelizumab, especially in patients at higher risk for infections, such as the elderly.”
The patient, who was Dr. Gordon’s patient for 20 years, had monitored updates in drug development and had looked forward to starting ocrelizumab, the first therapy approved for primary progressive MS, when it became available after its approval in 2017. The patient was concerned about progressively worsening gait impairment and related falls caused by MS.
Antibodies indicated that the patient had prior exposure to JCV, but Dr. Gordon considered the risk of PML to be relatively small. Prior to treatment, the patient’s absolute lymphocyte count was normal or indicated mild lymphocytopenia, which Dr. Gordon did not consider clinically significant. The patient received ocrelizumab for 2 years without incident.
In an information sheet for health care professionals about ocrelizumab and PML prepared in February 2020, Genentech says the patient’s age and low absolute lymphocyte count are confounding factors, which distort “the assessment of association between exposure to a drug and an adverse event.
“As of January 31, 2020, no unconfounded PML cases associated with ocrelizumab therapy have been reported,” according to the document. “Out of more than 150,000 patients treated globally (clinical trials and post-marketing experience), there have been nine confirmed, confounded cases of PML in patients treated with ocrelizumab, of which eight were carry-over cases from a prior DMT.”
The prescribing information for the drug notes that no cases of PML were identified in ocrelizumab clinical trials, but that PML has been observed in patients treated with other anti-CD20 antibodies and other MS therapies. In addition, PML “has been associated with some risk factors (eg, immunocompromised patients, polytherapy with immunosuppressants).
“At the first sign or symptom suggestive of PML, withhold ocrelizumab and perform an appropriate diagnostic evaluation,” the prescribing information says. “MRI findings may be apparent before clinical signs or symptoms. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes.”
“It is important for people to recognize that this is at least a possibility,” Dr. Gordon said. Any change in clinical symptomatology may warrant imaging, and CSF testing may be warranted if an MRI raises concerns about PML, he said.
Dr. Gordon has received research support from MSD (Merck), Eisai, AbbVie, and Janssen.
SOURCE: Sul J et al. AAN 2020. Abstract S29.001.
FROM AAN 2020
TERAVOLT data suggest high death rate in lung cancer patients with COVID-19
Registry data suggest an “unexpectedly high” mortality rate among patients with thoracic cancers who develop COVID-19, according to a presenter at the AACR virtual meeting I.
Data from the TERAVOLT registry showed a 34.6% mortality rate among 200 patients with COVID-19 and thoracic cancer, according to Marina Chiara Garassino, MD, of Fondazione IRCCS Instituto Nazionale dei Tumor in Milan, Italy, who presented the data at the meeting in a session on cancer and COVID-19.
Cancer patients infected with COVID-19 have been reported to be at increased risk of death, but the magnitude of increase is uncertain (Lancet Oncol. 2020 Mar;21[3]:335-7; JAMA. 2020 Mar 23. doi: 10.1001/jama.2020.4683).
Patients with thoracic cancer may be particularly vulnerable because of older age, tobacco use, preexisting cardiopulmonary comorbidities, and the immunosuppressive effects of treatment.
The global TERAVOLT registry was begun in late March 2020 to provide outcome data for coronavirus infections in thoracic cancer patients specifically. It is hoped that the data collected will guide patient management and define factors influencing morbidity and mortality.
Dr. Garassino said institutions from 21 countries have joined the TERAVOLT registry thus far. Currently, about 17 new patients with thoracic cancer and laboratory confirmed or clinically suspected COVID-19 are added to the registry each week.
As of April 12, 2020, there were 200 patients included in the registry. Their median age was 68 years, and 70.5% were men. Non–small cell lung cancer was the histology in 75.5% and small-cell lung cancer in 14.5% of patients. Most patients (73.5%) had stage IV disease. Approximately 27% of patients had at least three comorbid conditions.
About 74% of patients were on current cancer treatment, with 19% on tyrosine kinase inhibitors alone, 32.7% on chemotherapy alone, 23.1% on immunotherapy alone, and 13.6% on chemotherapy plus immunotherapy.
In all, 152 patients (76.0%) were hospitalized. However, 91.2% of patients were not admitted to the ICU, either because of a shortage of equipment or institutional policy.
The most common complications were pneumonia/pneumonitis (79.6%), acute respiratory distress syndrome (26.8%), multiorgan failure (7.6%), and sepsis (5.1%).
A total of 66 patients (34.6%) died. Most deaths were attributed to COVID-19 and not the underlying cancer, Dr. Garassino said.
A univariate analysis showed no association between cancer treatment and an increased risk of hospitalization or death. However, Dr. Garassino and colleagues are collecting more data to confirm these results.
In a multivariate analysis, no factors were associated with the risk of death, although data from a larger number of patients may shed more light on that issue.
TERAVOLT will continue to collect and provide data to identify characteristics associated with severe COVID-19–related illness, to guide physicians with information applicable to patients with thoracic malignancies, tailored to individual risk.
Like the COVID-19 and Cancer Consortium and the ESMO CoCare registry, TERAVOLT represents a way for the patient care and translational science communities to share lessons from the COVID-19 pandemic.
AACR plans to help share those lessons as well, in another session on COVID-19 and cancer at the AACR virtual meeting II in June and at a conference on COVID-19 and cancer in July, according to session moderator Antoni Ribas, MD, PhD, of the University of California, Los Angeles.
Dr. Garassino disclosed relationships with AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, and other companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Registry data suggest an “unexpectedly high” mortality rate among patients with thoracic cancers who develop COVID-19, according to a presenter at the AACR virtual meeting I.
Data from the TERAVOLT registry showed a 34.6% mortality rate among 200 patients with COVID-19 and thoracic cancer, according to Marina Chiara Garassino, MD, of Fondazione IRCCS Instituto Nazionale dei Tumor in Milan, Italy, who presented the data at the meeting in a session on cancer and COVID-19.
Cancer patients infected with COVID-19 have been reported to be at increased risk of death, but the magnitude of increase is uncertain (Lancet Oncol. 2020 Mar;21[3]:335-7; JAMA. 2020 Mar 23. doi: 10.1001/jama.2020.4683).
Patients with thoracic cancer may be particularly vulnerable because of older age, tobacco use, preexisting cardiopulmonary comorbidities, and the immunosuppressive effects of treatment.
The global TERAVOLT registry was begun in late March 2020 to provide outcome data for coronavirus infections in thoracic cancer patients specifically. It is hoped that the data collected will guide patient management and define factors influencing morbidity and mortality.
Dr. Garassino said institutions from 21 countries have joined the TERAVOLT registry thus far. Currently, about 17 new patients with thoracic cancer and laboratory confirmed or clinically suspected COVID-19 are added to the registry each week.
As of April 12, 2020, there were 200 patients included in the registry. Their median age was 68 years, and 70.5% were men. Non–small cell lung cancer was the histology in 75.5% and small-cell lung cancer in 14.5% of patients. Most patients (73.5%) had stage IV disease. Approximately 27% of patients had at least three comorbid conditions.
About 74% of patients were on current cancer treatment, with 19% on tyrosine kinase inhibitors alone, 32.7% on chemotherapy alone, 23.1% on immunotherapy alone, and 13.6% on chemotherapy plus immunotherapy.
In all, 152 patients (76.0%) were hospitalized. However, 91.2% of patients were not admitted to the ICU, either because of a shortage of equipment or institutional policy.
The most common complications were pneumonia/pneumonitis (79.6%), acute respiratory distress syndrome (26.8%), multiorgan failure (7.6%), and sepsis (5.1%).
A total of 66 patients (34.6%) died. Most deaths were attributed to COVID-19 and not the underlying cancer, Dr. Garassino said.
A univariate analysis showed no association between cancer treatment and an increased risk of hospitalization or death. However, Dr. Garassino and colleagues are collecting more data to confirm these results.
In a multivariate analysis, no factors were associated with the risk of death, although data from a larger number of patients may shed more light on that issue.
TERAVOLT will continue to collect and provide data to identify characteristics associated with severe COVID-19–related illness, to guide physicians with information applicable to patients with thoracic malignancies, tailored to individual risk.
Like the COVID-19 and Cancer Consortium and the ESMO CoCare registry, TERAVOLT represents a way for the patient care and translational science communities to share lessons from the COVID-19 pandemic.
AACR plans to help share those lessons as well, in another session on COVID-19 and cancer at the AACR virtual meeting II in June and at a conference on COVID-19 and cancer in July, according to session moderator Antoni Ribas, MD, PhD, of the University of California, Los Angeles.
Dr. Garassino disclosed relationships with AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, and other companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Registry data suggest an “unexpectedly high” mortality rate among patients with thoracic cancers who develop COVID-19, according to a presenter at the AACR virtual meeting I.
Data from the TERAVOLT registry showed a 34.6% mortality rate among 200 patients with COVID-19 and thoracic cancer, according to Marina Chiara Garassino, MD, of Fondazione IRCCS Instituto Nazionale dei Tumor in Milan, Italy, who presented the data at the meeting in a session on cancer and COVID-19.
Cancer patients infected with COVID-19 have been reported to be at increased risk of death, but the magnitude of increase is uncertain (Lancet Oncol. 2020 Mar;21[3]:335-7; JAMA. 2020 Mar 23. doi: 10.1001/jama.2020.4683).
Patients with thoracic cancer may be particularly vulnerable because of older age, tobacco use, preexisting cardiopulmonary comorbidities, and the immunosuppressive effects of treatment.
The global TERAVOLT registry was begun in late March 2020 to provide outcome data for coronavirus infections in thoracic cancer patients specifically. It is hoped that the data collected will guide patient management and define factors influencing morbidity and mortality.
Dr. Garassino said institutions from 21 countries have joined the TERAVOLT registry thus far. Currently, about 17 new patients with thoracic cancer and laboratory confirmed or clinically suspected COVID-19 are added to the registry each week.
As of April 12, 2020, there were 200 patients included in the registry. Their median age was 68 years, and 70.5% were men. Non–small cell lung cancer was the histology in 75.5% and small-cell lung cancer in 14.5% of patients. Most patients (73.5%) had stage IV disease. Approximately 27% of patients had at least three comorbid conditions.
About 74% of patients were on current cancer treatment, with 19% on tyrosine kinase inhibitors alone, 32.7% on chemotherapy alone, 23.1% on immunotherapy alone, and 13.6% on chemotherapy plus immunotherapy.
In all, 152 patients (76.0%) were hospitalized. However, 91.2% of patients were not admitted to the ICU, either because of a shortage of equipment or institutional policy.
The most common complications were pneumonia/pneumonitis (79.6%), acute respiratory distress syndrome (26.8%), multiorgan failure (7.6%), and sepsis (5.1%).
A total of 66 patients (34.6%) died. Most deaths were attributed to COVID-19 and not the underlying cancer, Dr. Garassino said.
A univariate analysis showed no association between cancer treatment and an increased risk of hospitalization or death. However, Dr. Garassino and colleagues are collecting more data to confirm these results.
In a multivariate analysis, no factors were associated with the risk of death, although data from a larger number of patients may shed more light on that issue.
TERAVOLT will continue to collect and provide data to identify characteristics associated with severe COVID-19–related illness, to guide physicians with information applicable to patients with thoracic malignancies, tailored to individual risk.
Like the COVID-19 and Cancer Consortium and the ESMO CoCare registry, TERAVOLT represents a way for the patient care and translational science communities to share lessons from the COVID-19 pandemic.
AACR plans to help share those lessons as well, in another session on COVID-19 and cancer at the AACR virtual meeting II in June and at a conference on COVID-19 and cancer in July, according to session moderator Antoni Ribas, MD, PhD, of the University of California, Los Angeles.
Dr. Garassino disclosed relationships with AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, and other companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM AACR 2020
Substantial very late MACE risk after PCI for SIHD
Patients with stable ischemic heart disease remain at substantial risk for major adverse cardiovascular events 1-5 years after percutaneous coronary intervention, even with contemporary second-generation drug-eluting stents, according to a pooled analysis of long-term follow-up data on 10,987 patients in 19 prospective, randomized, head-to-head metallic stent trials.
The analysis showed that, although most major adverse cardiovascular events (MACE) occurred during the first year after stenting, no plateau in MACE was reached between years 1 and 5, Mahesh V. Madhavan, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
“Further studies are required to understand the mechanisms of late events and whether improvements in stent technology, revascularization technique, and adjunctive therapies may improve outcomes in patients with SIHD [stable ischemic heart disease],” said Dr. Madhavan, a cardiology fellow at Columbia University Irving Medical Center and New York–Presbyterian Hospital.
This post hoc analysis of pooled individual patient-level data from 19 randomized trials included 10,987 metallic stent recipients with SIHD. Sixty-one percent got second-generation drug-eluting stents (DES), 25% received first-generation DES, and 15% got bare metal stents (BMS). The largest prospective head-to-head RCT was SPIRIT IV, with 2,130 patients. All five TAXUS trials were also included.
The 5-year rate of the primary composite MACE endpoint composed of cardiac death, MI, or ischemia-driven target lesion revascularization was 24.1% in patients with BMS stents, 17.9% with first-gen DES, and 13.4% with second-gen DES, reflecting the advances in stent technology over time. Most of these MACE events occurred during the first year after PCI, with rates of 18%, 8.6%, and 5.3%, respectively, in the three groups. However, the MACE rate beyond the first year out through year 5 remained substantial: 10.2% with first-gen DES, 8.5% with second-gen DES, and 7.4% in the BMS group.
The cardiac death rate from PCI through year 5 was 3.8% with second-gen DES, 3.6% with first-gen DES, and 3.3% with BMS. The MI rate was 7.7% with first-gen DES, 6.1% with BMS, and 5% with second-gen DES.
Stent thrombosis occurred during the first year in 0.9% of first-gen DES and BMS recipients and in 0.7% of patients with second-gen DES. During years 1-5, the rates were 1.6% with first-gen DES, 0.9% with second-gen devices, and 0.2% with BMS.
Second-gen DES provided a big advantage in terms of lessened need for ischemia-driven target lesion revascularization through the first 5 years, with a rate of 7.3%, compared to 18.7% in patients with first-gen DES and 10.5% with BMS.
In a multivariate regression analysis, independent predictors of MACE in the first 5 years post PCI included indicators of greater lesion and/or procedural complexity, such as left main or left anterior descending disease, greater lesion length, and more than one treated lesion, as well as standard cardiovascular risk factors, including recent smoking, hypertension, and diabetes.
In contrast, hyperlipidemia was associated with a significant 15% reduction in MACE risk, which in an interview Dr. Madhavan said may have been due to aggressive lipid-lowering therapy, although he added that this is conjecture because he and his coinvestigators didn’t have access to data on the use of guideline-directed medical therapy or antiplatelet regimens.
Asked about future prospects for reducing the substantial very late risk of MACE highlighted in his study, Dr. Madhavan cited the use of adjunctive imaging during PCI as promising.
“The currently enrolling ILUMEN IV trial, among other studies, will help determine whether imaging-guided intervention can help improve intermediate and long-term rates of MACE,” he observed.
Promising medical therapies that could potentially confer benefit in terms of reducing long-term MACE in patients who’ve undergone PCI for SIHD include novel lipid-lowering drugs, tailored antithrombotic strategies, new anti-inflammatory agents, and the SGLT2 inhibitors, Dr. Madhavan continued.
In terms of advances in stent design, he cited recent evidence that ultrathin-strut stents featuring bioresorbable polymer, such as the Orsiro stent, may reduce late stent-related MACE through 3 years.
“We’ll have to see if these benefits extend to longer-term follow-up up to 5 years,” he said.
He deemed his study results “fairly consistent” with those of the ISCHEMIA trial, where ischemic events in the patients with SIHD assigned to an initial invasive strategy continued to occur in the latter years of follow-up without any clear plateau effect (N Engl J Med. 2020 Apr 9;382[15]:1395-407).
Dr. Madhavan reported no financial conflicts regarding his study, funded by an institutional research grant from the National Heart, Lung, and Blood Institute.
Shortly following Dr. Madhavan’s presentation at ACC 2020, the study results were published online (Circ Cardiovasc Interv. 2020 Apr;13[4[:e008565. doi: 10.1161/CIRCINTERVENTIONS.119.008565).
SOURCE: Madhavan MV. ACC 2020, Abstract 909-10.
Patients with stable ischemic heart disease remain at substantial risk for major adverse cardiovascular events 1-5 years after percutaneous coronary intervention, even with contemporary second-generation drug-eluting stents, according to a pooled analysis of long-term follow-up data on 10,987 patients in 19 prospective, randomized, head-to-head metallic stent trials.
The analysis showed that, although most major adverse cardiovascular events (MACE) occurred during the first year after stenting, no plateau in MACE was reached between years 1 and 5, Mahesh V. Madhavan, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
“Further studies are required to understand the mechanisms of late events and whether improvements in stent technology, revascularization technique, and adjunctive therapies may improve outcomes in patients with SIHD [stable ischemic heart disease],” said Dr. Madhavan, a cardiology fellow at Columbia University Irving Medical Center and New York–Presbyterian Hospital.
This post hoc analysis of pooled individual patient-level data from 19 randomized trials included 10,987 metallic stent recipients with SIHD. Sixty-one percent got second-generation drug-eluting stents (DES), 25% received first-generation DES, and 15% got bare metal stents (BMS). The largest prospective head-to-head RCT was SPIRIT IV, with 2,130 patients. All five TAXUS trials were also included.
The 5-year rate of the primary composite MACE endpoint composed of cardiac death, MI, or ischemia-driven target lesion revascularization was 24.1% in patients with BMS stents, 17.9% with first-gen DES, and 13.4% with second-gen DES, reflecting the advances in stent technology over time. Most of these MACE events occurred during the first year after PCI, with rates of 18%, 8.6%, and 5.3%, respectively, in the three groups. However, the MACE rate beyond the first year out through year 5 remained substantial: 10.2% with first-gen DES, 8.5% with second-gen DES, and 7.4% in the BMS group.
The cardiac death rate from PCI through year 5 was 3.8% with second-gen DES, 3.6% with first-gen DES, and 3.3% with BMS. The MI rate was 7.7% with first-gen DES, 6.1% with BMS, and 5% with second-gen DES.
Stent thrombosis occurred during the first year in 0.9% of first-gen DES and BMS recipients and in 0.7% of patients with second-gen DES. During years 1-5, the rates were 1.6% with first-gen DES, 0.9% with second-gen devices, and 0.2% with BMS.
Second-gen DES provided a big advantage in terms of lessened need for ischemia-driven target lesion revascularization through the first 5 years, with a rate of 7.3%, compared to 18.7% in patients with first-gen DES and 10.5% with BMS.
In a multivariate regression analysis, independent predictors of MACE in the first 5 years post PCI included indicators of greater lesion and/or procedural complexity, such as left main or left anterior descending disease, greater lesion length, and more than one treated lesion, as well as standard cardiovascular risk factors, including recent smoking, hypertension, and diabetes.
In contrast, hyperlipidemia was associated with a significant 15% reduction in MACE risk, which in an interview Dr. Madhavan said may have been due to aggressive lipid-lowering therapy, although he added that this is conjecture because he and his coinvestigators didn’t have access to data on the use of guideline-directed medical therapy or antiplatelet regimens.
Asked about future prospects for reducing the substantial very late risk of MACE highlighted in his study, Dr. Madhavan cited the use of adjunctive imaging during PCI as promising.
“The currently enrolling ILUMEN IV trial, among other studies, will help determine whether imaging-guided intervention can help improve intermediate and long-term rates of MACE,” he observed.
Promising medical therapies that could potentially confer benefit in terms of reducing long-term MACE in patients who’ve undergone PCI for SIHD include novel lipid-lowering drugs, tailored antithrombotic strategies, new anti-inflammatory agents, and the SGLT2 inhibitors, Dr. Madhavan continued.
In terms of advances in stent design, he cited recent evidence that ultrathin-strut stents featuring bioresorbable polymer, such as the Orsiro stent, may reduce late stent-related MACE through 3 years.
“We’ll have to see if these benefits extend to longer-term follow-up up to 5 years,” he said.
He deemed his study results “fairly consistent” with those of the ISCHEMIA trial, where ischemic events in the patients with SIHD assigned to an initial invasive strategy continued to occur in the latter years of follow-up without any clear plateau effect (N Engl J Med. 2020 Apr 9;382[15]:1395-407).
Dr. Madhavan reported no financial conflicts regarding his study, funded by an institutional research grant from the National Heart, Lung, and Blood Institute.
Shortly following Dr. Madhavan’s presentation at ACC 2020, the study results were published online (Circ Cardiovasc Interv. 2020 Apr;13[4[:e008565. doi: 10.1161/CIRCINTERVENTIONS.119.008565).
SOURCE: Madhavan MV. ACC 2020, Abstract 909-10.
Patients with stable ischemic heart disease remain at substantial risk for major adverse cardiovascular events 1-5 years after percutaneous coronary intervention, even with contemporary second-generation drug-eluting stents, according to a pooled analysis of long-term follow-up data on 10,987 patients in 19 prospective, randomized, head-to-head metallic stent trials.
The analysis showed that, although most major adverse cardiovascular events (MACE) occurred during the first year after stenting, no plateau in MACE was reached between years 1 and 5, Mahesh V. Madhavan, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
“Further studies are required to understand the mechanisms of late events and whether improvements in stent technology, revascularization technique, and adjunctive therapies may improve outcomes in patients with SIHD [stable ischemic heart disease],” said Dr. Madhavan, a cardiology fellow at Columbia University Irving Medical Center and New York–Presbyterian Hospital.
This post hoc analysis of pooled individual patient-level data from 19 randomized trials included 10,987 metallic stent recipients with SIHD. Sixty-one percent got second-generation drug-eluting stents (DES), 25% received first-generation DES, and 15% got bare metal stents (BMS). The largest prospective head-to-head RCT was SPIRIT IV, with 2,130 patients. All five TAXUS trials were also included.
The 5-year rate of the primary composite MACE endpoint composed of cardiac death, MI, or ischemia-driven target lesion revascularization was 24.1% in patients with BMS stents, 17.9% with first-gen DES, and 13.4% with second-gen DES, reflecting the advances in stent technology over time. Most of these MACE events occurred during the first year after PCI, with rates of 18%, 8.6%, and 5.3%, respectively, in the three groups. However, the MACE rate beyond the first year out through year 5 remained substantial: 10.2% with first-gen DES, 8.5% with second-gen DES, and 7.4% in the BMS group.
The cardiac death rate from PCI through year 5 was 3.8% with second-gen DES, 3.6% with first-gen DES, and 3.3% with BMS. The MI rate was 7.7% with first-gen DES, 6.1% with BMS, and 5% with second-gen DES.
Stent thrombosis occurred during the first year in 0.9% of first-gen DES and BMS recipients and in 0.7% of patients with second-gen DES. During years 1-5, the rates were 1.6% with first-gen DES, 0.9% with second-gen devices, and 0.2% with BMS.
Second-gen DES provided a big advantage in terms of lessened need for ischemia-driven target lesion revascularization through the first 5 years, with a rate of 7.3%, compared to 18.7% in patients with first-gen DES and 10.5% with BMS.
In a multivariate regression analysis, independent predictors of MACE in the first 5 years post PCI included indicators of greater lesion and/or procedural complexity, such as left main or left anterior descending disease, greater lesion length, and more than one treated lesion, as well as standard cardiovascular risk factors, including recent smoking, hypertension, and diabetes.
In contrast, hyperlipidemia was associated with a significant 15% reduction in MACE risk, which in an interview Dr. Madhavan said may have been due to aggressive lipid-lowering therapy, although he added that this is conjecture because he and his coinvestigators didn’t have access to data on the use of guideline-directed medical therapy or antiplatelet regimens.
Asked about future prospects for reducing the substantial very late risk of MACE highlighted in his study, Dr. Madhavan cited the use of adjunctive imaging during PCI as promising.
“The currently enrolling ILUMEN IV trial, among other studies, will help determine whether imaging-guided intervention can help improve intermediate and long-term rates of MACE,” he observed.
Promising medical therapies that could potentially confer benefit in terms of reducing long-term MACE in patients who’ve undergone PCI for SIHD include novel lipid-lowering drugs, tailored antithrombotic strategies, new anti-inflammatory agents, and the SGLT2 inhibitors, Dr. Madhavan continued.
In terms of advances in stent design, he cited recent evidence that ultrathin-strut stents featuring bioresorbable polymer, such as the Orsiro stent, may reduce late stent-related MACE through 3 years.
“We’ll have to see if these benefits extend to longer-term follow-up up to 5 years,” he said.
He deemed his study results “fairly consistent” with those of the ISCHEMIA trial, where ischemic events in the patients with SIHD assigned to an initial invasive strategy continued to occur in the latter years of follow-up without any clear plateau effect (N Engl J Med. 2020 Apr 9;382[15]:1395-407).
Dr. Madhavan reported no financial conflicts regarding his study, funded by an institutional research grant from the National Heart, Lung, and Blood Institute.
Shortly following Dr. Madhavan’s presentation at ACC 2020, the study results were published online (Circ Cardiovasc Interv. 2020 Apr;13[4[:e008565. doi: 10.1161/CIRCINTERVENTIONS.119.008565).
SOURCE: Madhavan MV. ACC 2020, Abstract 909-10.
FROM ACC 20
COVID-19: An opportunity, challenge for addiction treatment, NIDA boss says
The COVID-19 pandemic is posing significant challenges while also providing unique opportunities for patients with substance use disorders (SUD), a leading expert says.
Nora Volkow, MD, director of the National Institute on Drug Abuse, said that the pandemic has accelerated the use of telemedicine, making it easier for patients with SUD to access treatment. It has also led to the proliferation of more mental health hotlines, which is critical since the vast majority of these patients have comorbid mental illness.
In addition, COVID-19 has resulted in increased availability of “alternative” peer support mechanisms via cellphones or computers to aid individuals’ sobriety.
Dr. Volkow spoke at the virtual American Psychiatric Association Spring Highlights Meeting 2020, which is replacing the organization’s canceled annual meeting.
While methadone clinics have had to close during the pandemic, making it challenging for those on medically assisted treatment to receive methadone or buprenorphine, some of the rules and regulations have been relaxed in order to make these medications accessible without the need for in-person attendance at a clinic. In addition, the Substance Abuse and Mental Health Services Administration has relaxed some of its own regulations regarding telehealth and opioid treatment programs.
Social isolation, stigma intensified
A pandemic increases anxiety in the general population, but for patients with SUD who may be also be struggling with homelessness and comorbid mental illness, the situation can further exacerbate social stigma and isolation – leading to relapse, more overdoses, and overdose deaths, Dr. Volkow said. Social interaction is “extraordinarily important” for patients and “one of the most powerful tools we have” to build resilience.
Right now, said Dr. Volkow, “we are in the dark as to how COVID infections have affected the number of overdose deaths.”
However, she noted that
“So even through this devastation, we can actually extract something that may help others in future,” she said.
Dr. Volkow noted that during the pandemic it is critical to reinforce the importance of engaging in – and remaining in – treatment to SUD patients. It’s also crucial to make patients aware of social support systems and behavioral interventions to help them cope with stress and to mitigate relapse risk.
COVID-19 and relapse
Elie G. Aoun, MD, assistant professor of psychiatry at New York University and vice chair of the APA’s Council on Addiction Psychiatry, said in an interview that Dr. Volkow’s presentation provided “exactly the kind of accessible information” clinicians need.
Dr. Aoun said he sees the impact of the COVID-19 crisis in his practice every day. Patients with SUD “are getting the short end of the stick.”
Social distancing measures prompted by the pandemic can be “very triggering” for SUD patients, he said. One of his patients told him the current isolation, loneliness, movement restrictions, and boredom remind her of the way she felt when she used drugs.
Dr. Aoun said four of his patients have relapsed since the pandemic began. Two of them had just started treatment after years of using drugs, so this was a “major setback” for them.
He and his colleagues were “not really prepared” to provide care via video link, which he believes is not as effective as in-person sessions.
In addition to disrupting patient care, said Dr. Aoun, the pandemic is forcing the medical community to face social determinants of health, such as poverty and homelessness, as they relate to addiction disorders and whether or not someone receives care.
This article originally appeared on Medscape.com.
The COVID-19 pandemic is posing significant challenges while also providing unique opportunities for patients with substance use disorders (SUD), a leading expert says.
Nora Volkow, MD, director of the National Institute on Drug Abuse, said that the pandemic has accelerated the use of telemedicine, making it easier for patients with SUD to access treatment. It has also led to the proliferation of more mental health hotlines, which is critical since the vast majority of these patients have comorbid mental illness.
In addition, COVID-19 has resulted in increased availability of “alternative” peer support mechanisms via cellphones or computers to aid individuals’ sobriety.
Dr. Volkow spoke at the virtual American Psychiatric Association Spring Highlights Meeting 2020, which is replacing the organization’s canceled annual meeting.
While methadone clinics have had to close during the pandemic, making it challenging for those on medically assisted treatment to receive methadone or buprenorphine, some of the rules and regulations have been relaxed in order to make these medications accessible without the need for in-person attendance at a clinic. In addition, the Substance Abuse and Mental Health Services Administration has relaxed some of its own regulations regarding telehealth and opioid treatment programs.
Social isolation, stigma intensified
A pandemic increases anxiety in the general population, but for patients with SUD who may be also be struggling with homelessness and comorbid mental illness, the situation can further exacerbate social stigma and isolation – leading to relapse, more overdoses, and overdose deaths, Dr. Volkow said. Social interaction is “extraordinarily important” for patients and “one of the most powerful tools we have” to build resilience.
Right now, said Dr. Volkow, “we are in the dark as to how COVID infections have affected the number of overdose deaths.”
However, she noted that
“So even through this devastation, we can actually extract something that may help others in future,” she said.
Dr. Volkow noted that during the pandemic it is critical to reinforce the importance of engaging in – and remaining in – treatment to SUD patients. It’s also crucial to make patients aware of social support systems and behavioral interventions to help them cope with stress and to mitigate relapse risk.
COVID-19 and relapse
Elie G. Aoun, MD, assistant professor of psychiatry at New York University and vice chair of the APA’s Council on Addiction Psychiatry, said in an interview that Dr. Volkow’s presentation provided “exactly the kind of accessible information” clinicians need.
Dr. Aoun said he sees the impact of the COVID-19 crisis in his practice every day. Patients with SUD “are getting the short end of the stick.”
Social distancing measures prompted by the pandemic can be “very triggering” for SUD patients, he said. One of his patients told him the current isolation, loneliness, movement restrictions, and boredom remind her of the way she felt when she used drugs.
Dr. Aoun said four of his patients have relapsed since the pandemic began. Two of them had just started treatment after years of using drugs, so this was a “major setback” for them.
He and his colleagues were “not really prepared” to provide care via video link, which he believes is not as effective as in-person sessions.
In addition to disrupting patient care, said Dr. Aoun, the pandemic is forcing the medical community to face social determinants of health, such as poverty and homelessness, as they relate to addiction disorders and whether or not someone receives care.
This article originally appeared on Medscape.com.
The COVID-19 pandemic is posing significant challenges while also providing unique opportunities for patients with substance use disorders (SUD), a leading expert says.
Nora Volkow, MD, director of the National Institute on Drug Abuse, said that the pandemic has accelerated the use of telemedicine, making it easier for patients with SUD to access treatment. It has also led to the proliferation of more mental health hotlines, which is critical since the vast majority of these patients have comorbid mental illness.
In addition, COVID-19 has resulted in increased availability of “alternative” peer support mechanisms via cellphones or computers to aid individuals’ sobriety.
Dr. Volkow spoke at the virtual American Psychiatric Association Spring Highlights Meeting 2020, which is replacing the organization’s canceled annual meeting.
While methadone clinics have had to close during the pandemic, making it challenging for those on medically assisted treatment to receive methadone or buprenorphine, some of the rules and regulations have been relaxed in order to make these medications accessible without the need for in-person attendance at a clinic. In addition, the Substance Abuse and Mental Health Services Administration has relaxed some of its own regulations regarding telehealth and opioid treatment programs.
Social isolation, stigma intensified
A pandemic increases anxiety in the general population, but for patients with SUD who may be also be struggling with homelessness and comorbid mental illness, the situation can further exacerbate social stigma and isolation – leading to relapse, more overdoses, and overdose deaths, Dr. Volkow said. Social interaction is “extraordinarily important” for patients and “one of the most powerful tools we have” to build resilience.
Right now, said Dr. Volkow, “we are in the dark as to how COVID infections have affected the number of overdose deaths.”
However, she noted that
“So even through this devastation, we can actually extract something that may help others in future,” she said.
Dr. Volkow noted that during the pandemic it is critical to reinforce the importance of engaging in – and remaining in – treatment to SUD patients. It’s also crucial to make patients aware of social support systems and behavioral interventions to help them cope with stress and to mitigate relapse risk.
COVID-19 and relapse
Elie G. Aoun, MD, assistant professor of psychiatry at New York University and vice chair of the APA’s Council on Addiction Psychiatry, said in an interview that Dr. Volkow’s presentation provided “exactly the kind of accessible information” clinicians need.
Dr. Aoun said he sees the impact of the COVID-19 crisis in his practice every day. Patients with SUD “are getting the short end of the stick.”
Social distancing measures prompted by the pandemic can be “very triggering” for SUD patients, he said. One of his patients told him the current isolation, loneliness, movement restrictions, and boredom remind her of the way she felt when she used drugs.
Dr. Aoun said four of his patients have relapsed since the pandemic began. Two of them had just started treatment after years of using drugs, so this was a “major setback” for them.
He and his colleagues were “not really prepared” to provide care via video link, which he believes is not as effective as in-person sessions.
In addition to disrupting patient care, said Dr. Aoun, the pandemic is forcing the medical community to face social determinants of health, such as poverty and homelessness, as they relate to addiction disorders and whether or not someone receives care.
This article originally appeared on Medscape.com.
Trials and tribulations: Neurology research during COVID-19
However, researchers remain determined to forge ahead – with many redesigning their studies, at least in part to optimize the safety of their participants and research staff.
Keeping people engaged while protocols are on hold; expanding normal safety considerations; and re-enlisting statisticians to keep their findings as significant as possible are just some of study survival strategies underway.
Alzheimer’s disease research on hold
The pandemic is having a significant impact on Alzheimer’s disease research, and medical research in general, says Heather Snyder, PhD, vice president, Medical & Scientific Relations at the Alzheimer’s Association.
“Many clinical trials worldwide are pausing, changing, or halting the testing of the drug or the intervention,” she told Medscape Medical News. “How the teams have adapted depends on the study,” she added. “As you can imagine, things are changing on a daily basis.”
The US Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER) trial, for example, is on hold until at least May 31. The Alzheimer’s Association is helping to implement and fund the study along with Wake Forest University Medical Center.
“We’re not randomizing participants at this point in time and the intervention — which is based on a team meeting, and there is a social aspect to that — has been paused,” Dr. Snyder said.
Another pivotal study underway is the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s study (the A4 Study). Investigators are evaluating if an anti-amyloid antibody, solanezumab (Eli Lilly), can slow memory loss among people with amyloid on imaging but no symptoms of cognitive decline at baseline.
“The A4 Study is definitely continuing. However, in an effort to minimize risk to participants, site staff and study integrity, we have implemented an optional study hiatus for both the double-blind and open-label extension phases,” lead investigator Reisa Anne Sperling, MD, told Medscape Medical News.
“We wanted to prioritize the safety of our participants as well as the ability of participants to remain in the study … despite disruptions from the COVID-19 pandemic,” said Dr. Sperling, who is professor of neurology at Harvard Medical School and director of the Center for Alzheimer Research and Treatment at Brigham and Women’s Hospital and Massachusetts General Hospital in Boston.
The ultimate goal is for A4 participants to receive the full number of planned infusions and assessments, even if it takes longer, she added.
Many Alzheimer’s disease researchers outside the United States face similar challenges. “As you probably are well aware, Spain is now in a complete lockdown. This has affected research centers like ours, the Barcelona Brain Research Center, and the way we work,” José Luís Molinuevo Guix, MD, PhD, told Medscape Medical News.
All participants in observational studies like the ALFA+ study and initiatives, as well as those in trials including PENSA and AB1601, “are not allowed, by law, to come in, hence from a safety perspective we are on good grounds,” added Dr. Molinuevo Guix, who directs the Alzheimer’s disease and other cognitive disorders unit at the Hospital Clinic de Barcelona.
The investigators are creating protocols for communicating with participants during the pandemic and for restarting visits safely after the lockdown has ended.
Stroke studies amended or suspended
A similar situation is occurring in stroke trials. Stroke is “obviously an acute disease, as well as a disease that requires secondary prevention,” Mitchell Elkind, MD, president-elect of the American Heart Association, told Medscape Medical News.
“One could argue that patients with stroke are going to be in the hospital anyway – why not enroll them in a study? They’re not incurring any additional risk,” he said. “But the staff have to come in to see them, and we’re really trying to avoid exposure.”
One ongoing trial, the Atrial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke (ARCADIA), stopped randomly assigning new participants to secondary prevention with apixaban or aspirin because of COVID-19. However, Dr. Elkind and colleagues plan to provide medication to the 440 people already in the trial.
“Wherever possible, the study coordinators are shipping the drug to people and doing follow-up visits by phone or video,” said Dr. Elkind, chief of the Division of Neurology Clinical Outcomes Research and Population Sciences at Columbia University in New York City.
Protecting patients, staff, and ultimately society is a “major driving force in stopping the randomizations,” he stressed.
ARCADIA is part of the StrokeNet prevention trials network, run by the NIH’s National Institute of Neurologic Disorders and Stroke (NINDS). Additional pivotal trials include the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST) and the Multi-arm Optimization of Stroke Thrombolysis (MOST) studies, he said.
Joseph Broderick, MD, director of the national NIH StrokeNet, agreed that safety comes first. “It was the decision of the StrokeNet leadership and the principal investigators of the trials that we needed to hold recruitment of new patients while we worked on adapting processes of enrollment to ensure the safety of both patients and researchers interacting with study patients,” he told Medscape Medical News.
Potential risks vary based on the study intervention and the need for in-person interactions. Trials that include stimulation devices or physical therapy, for example, might be most affected, added Dr. Broderick, professor and director of the UC Gardner Neuroscience Institute at the University of Cincinnati in Ohio.
Nevertheless, “there are potential ways … to move as much as possible toward telemedicine and digital interactions during this time.”
Multiple challenges in multiple sclerosis
At the national level, the COVID-19 pandemic has had an “unprecedented impact on almost all the clinical trials funded by NINDS,” said Clinton Wright, MD, director of the Division of Clinical Research at NINDS. “Investigators have had to adapt quickly.”
Supplementing existing grants with money to conduct research on COVID-19 and pursuing research opportunities from different institutes are “some of the creative approaches [that] have come from the NIH [National Institutes of Health] itself,” Dr. Wright said. “Other creative approaches have come from investigators trying to keep their studies and trials going during the pandemic.”
In clinical trials, “everything from electronic consent to in-home research drug delivery is being brought to bear.”
“A few ongoing trials have been able to modify their protocols to obtain consent and carry out evaluations remotely by telephone or videoconferencing,” Dr. Wright said. “This is especially critical for trials that involve medical management of specific risk factors or conditions, where suspension of the trial could itself have adverse consequences due to reduced engagement with research participants.”
For participants already in MS studies, “each upcoming visit is assessed for whether it’s critical or could be done virtually or just skipped. If a person needs a treatment that cannot be postponed or skipped, they come in,” Jeffrey Cohen, MD, director of the Experimental Therapeutics Program at the Mellen Center for Multiple Sclerosis Treatment and Research at the Cleveland Clinic, Ohio, told Medscape Medical News.
New study enrollment is largely on hold and study visits for existing participants are limited, said Dr. Cohen, who is also president of the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS).
Some of the major ongoing trials in MS are “looking at very fundamental questions in the field,” Dr. Cohen said. The Determining the Effectiveness of earLy Intensive Versus Escalation Approaches for RRMS (DELIVER-MS) and Traditional Versus Early Aggressive Therapy for Multiple Sclerosis (TREAT-MS) trials, for example, evaluate whether treatment should be initiated with one of the less efficacious agents with escalation as needed, or whether treatment should begin with a high-efficacy agent.
Both trials are currently on hold because of the pandemic, as is the Best Available Therapy Versus Autologous Hematopoietic Stem Cell Transplant for Multiple Sclerosis (BEAT-MS) study.
“There has been a lot of interest in hematopoietic stem cell transplants and where they fit into our overall treatment strategy, and this is intended to provide a more definitive answer,” Dr. Cohen said.
Making the most of down time
“The pandemic has been challenging” in terms of ongoing MS research, said Benjamin M. Segal, MD, chair of the Department of Neurology and director of the Neuroscience Research Institute at the Ohio State University Wexner Medical Center, Columbus.
“With regard to the lab, our animal model experiments have been placed on hold. We have stopped collecting samples from clinical subjects for biomarker studies.
“However, my research team has been taking advantage of the time that has been freed up from bench work by analyzing data sets that had been placed aside, delving more deeply into the literature, and writing new grant proposals and articles,” he added.
Two of Dr. Segal’s trainees are writing review articles on the immunopathogenesis of MS and its treatment. Another postdoctoral candidate is writing a grant proposal to investigate how coinfection with a coronavirus modulates CNS pathology and the clinical course of an animal model of MS.
“I am asking my trainees to plan out experiments further in advance than they ever have before, so they are as prepared as possible to resume their research agendas once we are up and running again,” Dr. Segal said.
Confronting current challenges while planning for a future less disrupted by the pandemic is a common theme that emerges.
“The duration of this [pandemic] will dictate how we analyze the data at the end [for the US POINTER study]. There is a large group of statisticians working on this,” Dr. Snyder said.
Dr. Sperling of Harvard Medical School also remains undeterred. “This is definitely a challenging time, as we must not allow the COVID-19 to interfere with our essential mission to find a successful treatment to prevent cognitive decline in AD. We do need, however, to be as flexible as possible to protect our participants and minimize the impact to our overall study integrity,” she said.
NIH guidance
Dr. Molinuevo Guix, of the Barcelona Brain Research Center, is also determined to continue his Alzheimer’s disease research. “I am aware that after the crisis, there will be less [risk] but still a COVID-19 infection risk, so apart from trying to generate part of our visits virtually, we want to make sure we have all necessary safety measures in place. We remain very active to preserve the work we have done to keep up the fight against Alzheimer’s and dementia,” he said.
Such forward thinking also applies to major stroke trials, said Dr. Broderick of the University of Cincinnati. “As soon as we shut down enrollment in stroke trials, we immediately began to make plans about how and when we can restart our stroke trials,” he explained. “One of our trials can do every step of the trial process remotely without direct in-person interactions and will be able to restart soon.”
An individualized approach is needed, Dr. Broderick added. “For trials involving necessary in-person and hands-on assessments, we will need to consider how best to use protective equipment and expanded testing that will likely match the ongoing clinical care and requirements at a given institution.
“Even if a trial officially reopens enrollment, the decision to enroll locally will need to follow local institutional environment and guidelines. Thus, restart of trial enrollment will not likely be uniform, similar to how trials often start in the first place,” Dr. Broderick said.
The NIH published uniform standards for researchers across its institutes to help guide them during the pandemic. Future contingency plans also are underway at the NINDS.
“As the pandemic wanes and in-person research activities restart, it will be important to have in place safety measures that prevent a resurgence of the virus, such as proper personal protective equipment for staff and research participants, said Dr. Wright, the clinical research director at NINDS.
For clinical trials, NINDS is prepared to provide supplemental funds to trial investigators to help support additional activities undertaken as a result of the pandemic.
“This has been an instructive experience. The pandemic will end, and we will resume much of our old patterns of behavior,” said Ohio State’s Dr. Segal. “But some of the strategies that we have employed to get through this time will continue to influence the way we communicate information, plan experiments, and prioritize research activities in the future, to good effect.”
Drs. Snyder, Sperling, Molinuevo Guix, Elkind, Broderick, Wright, Cohen, and Segal have disclosed no relevant disclosures.
This story first appeared on Medscape.com.
However, researchers remain determined to forge ahead – with many redesigning their studies, at least in part to optimize the safety of their participants and research staff.
Keeping people engaged while protocols are on hold; expanding normal safety considerations; and re-enlisting statisticians to keep their findings as significant as possible are just some of study survival strategies underway.
Alzheimer’s disease research on hold
The pandemic is having a significant impact on Alzheimer’s disease research, and medical research in general, says Heather Snyder, PhD, vice president, Medical & Scientific Relations at the Alzheimer’s Association.
“Many clinical trials worldwide are pausing, changing, or halting the testing of the drug or the intervention,” she told Medscape Medical News. “How the teams have adapted depends on the study,” she added. “As you can imagine, things are changing on a daily basis.”
The US Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER) trial, for example, is on hold until at least May 31. The Alzheimer’s Association is helping to implement and fund the study along with Wake Forest University Medical Center.
“We’re not randomizing participants at this point in time and the intervention — which is based on a team meeting, and there is a social aspect to that — has been paused,” Dr. Snyder said.
Another pivotal study underway is the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s study (the A4 Study). Investigators are evaluating if an anti-amyloid antibody, solanezumab (Eli Lilly), can slow memory loss among people with amyloid on imaging but no symptoms of cognitive decline at baseline.
“The A4 Study is definitely continuing. However, in an effort to minimize risk to participants, site staff and study integrity, we have implemented an optional study hiatus for both the double-blind and open-label extension phases,” lead investigator Reisa Anne Sperling, MD, told Medscape Medical News.
“We wanted to prioritize the safety of our participants as well as the ability of participants to remain in the study … despite disruptions from the COVID-19 pandemic,” said Dr. Sperling, who is professor of neurology at Harvard Medical School and director of the Center for Alzheimer Research and Treatment at Brigham and Women’s Hospital and Massachusetts General Hospital in Boston.
The ultimate goal is for A4 participants to receive the full number of planned infusions and assessments, even if it takes longer, she added.
Many Alzheimer’s disease researchers outside the United States face similar challenges. “As you probably are well aware, Spain is now in a complete lockdown. This has affected research centers like ours, the Barcelona Brain Research Center, and the way we work,” José Luís Molinuevo Guix, MD, PhD, told Medscape Medical News.
All participants in observational studies like the ALFA+ study and initiatives, as well as those in trials including PENSA and AB1601, “are not allowed, by law, to come in, hence from a safety perspective we are on good grounds,” added Dr. Molinuevo Guix, who directs the Alzheimer’s disease and other cognitive disorders unit at the Hospital Clinic de Barcelona.
The investigators are creating protocols for communicating with participants during the pandemic and for restarting visits safely after the lockdown has ended.
Stroke studies amended or suspended
A similar situation is occurring in stroke trials. Stroke is “obviously an acute disease, as well as a disease that requires secondary prevention,” Mitchell Elkind, MD, president-elect of the American Heart Association, told Medscape Medical News.
“One could argue that patients with stroke are going to be in the hospital anyway – why not enroll them in a study? They’re not incurring any additional risk,” he said. “But the staff have to come in to see them, and we’re really trying to avoid exposure.”
One ongoing trial, the Atrial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke (ARCADIA), stopped randomly assigning new participants to secondary prevention with apixaban or aspirin because of COVID-19. However, Dr. Elkind and colleagues plan to provide medication to the 440 people already in the trial.
“Wherever possible, the study coordinators are shipping the drug to people and doing follow-up visits by phone or video,” said Dr. Elkind, chief of the Division of Neurology Clinical Outcomes Research and Population Sciences at Columbia University in New York City.
Protecting patients, staff, and ultimately society is a “major driving force in stopping the randomizations,” he stressed.
ARCADIA is part of the StrokeNet prevention trials network, run by the NIH’s National Institute of Neurologic Disorders and Stroke (NINDS). Additional pivotal trials include the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST) and the Multi-arm Optimization of Stroke Thrombolysis (MOST) studies, he said.
Joseph Broderick, MD, director of the national NIH StrokeNet, agreed that safety comes first. “It was the decision of the StrokeNet leadership and the principal investigators of the trials that we needed to hold recruitment of new patients while we worked on adapting processes of enrollment to ensure the safety of both patients and researchers interacting with study patients,” he told Medscape Medical News.
Potential risks vary based on the study intervention and the need for in-person interactions. Trials that include stimulation devices or physical therapy, for example, might be most affected, added Dr. Broderick, professor and director of the UC Gardner Neuroscience Institute at the University of Cincinnati in Ohio.
Nevertheless, “there are potential ways … to move as much as possible toward telemedicine and digital interactions during this time.”
Multiple challenges in multiple sclerosis
At the national level, the COVID-19 pandemic has had an “unprecedented impact on almost all the clinical trials funded by NINDS,” said Clinton Wright, MD, director of the Division of Clinical Research at NINDS. “Investigators have had to adapt quickly.”
Supplementing existing grants with money to conduct research on COVID-19 and pursuing research opportunities from different institutes are “some of the creative approaches [that] have come from the NIH [National Institutes of Health] itself,” Dr. Wright said. “Other creative approaches have come from investigators trying to keep their studies and trials going during the pandemic.”
In clinical trials, “everything from electronic consent to in-home research drug delivery is being brought to bear.”
“A few ongoing trials have been able to modify their protocols to obtain consent and carry out evaluations remotely by telephone or videoconferencing,” Dr. Wright said. “This is especially critical for trials that involve medical management of specific risk factors or conditions, where suspension of the trial could itself have adverse consequences due to reduced engagement with research participants.”
For participants already in MS studies, “each upcoming visit is assessed for whether it’s critical or could be done virtually or just skipped. If a person needs a treatment that cannot be postponed or skipped, they come in,” Jeffrey Cohen, MD, director of the Experimental Therapeutics Program at the Mellen Center for Multiple Sclerosis Treatment and Research at the Cleveland Clinic, Ohio, told Medscape Medical News.
New study enrollment is largely on hold and study visits for existing participants are limited, said Dr. Cohen, who is also president of the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS).
Some of the major ongoing trials in MS are “looking at very fundamental questions in the field,” Dr. Cohen said. The Determining the Effectiveness of earLy Intensive Versus Escalation Approaches for RRMS (DELIVER-MS) and Traditional Versus Early Aggressive Therapy for Multiple Sclerosis (TREAT-MS) trials, for example, evaluate whether treatment should be initiated with one of the less efficacious agents with escalation as needed, or whether treatment should begin with a high-efficacy agent.
Both trials are currently on hold because of the pandemic, as is the Best Available Therapy Versus Autologous Hematopoietic Stem Cell Transplant for Multiple Sclerosis (BEAT-MS) study.
“There has been a lot of interest in hematopoietic stem cell transplants and where they fit into our overall treatment strategy, and this is intended to provide a more definitive answer,” Dr. Cohen said.
Making the most of down time
“The pandemic has been challenging” in terms of ongoing MS research, said Benjamin M. Segal, MD, chair of the Department of Neurology and director of the Neuroscience Research Institute at the Ohio State University Wexner Medical Center, Columbus.
“With regard to the lab, our animal model experiments have been placed on hold. We have stopped collecting samples from clinical subjects for biomarker studies.
“However, my research team has been taking advantage of the time that has been freed up from bench work by analyzing data sets that had been placed aside, delving more deeply into the literature, and writing new grant proposals and articles,” he added.
Two of Dr. Segal’s trainees are writing review articles on the immunopathogenesis of MS and its treatment. Another postdoctoral candidate is writing a grant proposal to investigate how coinfection with a coronavirus modulates CNS pathology and the clinical course of an animal model of MS.
“I am asking my trainees to plan out experiments further in advance than they ever have before, so they are as prepared as possible to resume their research agendas once we are up and running again,” Dr. Segal said.
Confronting current challenges while planning for a future less disrupted by the pandemic is a common theme that emerges.
“The duration of this [pandemic] will dictate how we analyze the data at the end [for the US POINTER study]. There is a large group of statisticians working on this,” Dr. Snyder said.
Dr. Sperling of Harvard Medical School also remains undeterred. “This is definitely a challenging time, as we must not allow the COVID-19 to interfere with our essential mission to find a successful treatment to prevent cognitive decline in AD. We do need, however, to be as flexible as possible to protect our participants and minimize the impact to our overall study integrity,” she said.
NIH guidance
Dr. Molinuevo Guix, of the Barcelona Brain Research Center, is also determined to continue his Alzheimer’s disease research. “I am aware that after the crisis, there will be less [risk] but still a COVID-19 infection risk, so apart from trying to generate part of our visits virtually, we want to make sure we have all necessary safety measures in place. We remain very active to preserve the work we have done to keep up the fight against Alzheimer’s and dementia,” he said.
Such forward thinking also applies to major stroke trials, said Dr. Broderick of the University of Cincinnati. “As soon as we shut down enrollment in stroke trials, we immediately began to make plans about how and when we can restart our stroke trials,” he explained. “One of our trials can do every step of the trial process remotely without direct in-person interactions and will be able to restart soon.”
An individualized approach is needed, Dr. Broderick added. “For trials involving necessary in-person and hands-on assessments, we will need to consider how best to use protective equipment and expanded testing that will likely match the ongoing clinical care and requirements at a given institution.
“Even if a trial officially reopens enrollment, the decision to enroll locally will need to follow local institutional environment and guidelines. Thus, restart of trial enrollment will not likely be uniform, similar to how trials often start in the first place,” Dr. Broderick said.
The NIH published uniform standards for researchers across its institutes to help guide them during the pandemic. Future contingency plans also are underway at the NINDS.
“As the pandemic wanes and in-person research activities restart, it will be important to have in place safety measures that prevent a resurgence of the virus, such as proper personal protective equipment for staff and research participants, said Dr. Wright, the clinical research director at NINDS.
For clinical trials, NINDS is prepared to provide supplemental funds to trial investigators to help support additional activities undertaken as a result of the pandemic.
“This has been an instructive experience. The pandemic will end, and we will resume much of our old patterns of behavior,” said Ohio State’s Dr. Segal. “But some of the strategies that we have employed to get through this time will continue to influence the way we communicate information, plan experiments, and prioritize research activities in the future, to good effect.”
Drs. Snyder, Sperling, Molinuevo Guix, Elkind, Broderick, Wright, Cohen, and Segal have disclosed no relevant disclosures.
This story first appeared on Medscape.com.
However, researchers remain determined to forge ahead – with many redesigning their studies, at least in part to optimize the safety of their participants and research staff.
Keeping people engaged while protocols are on hold; expanding normal safety considerations; and re-enlisting statisticians to keep their findings as significant as possible are just some of study survival strategies underway.
Alzheimer’s disease research on hold
The pandemic is having a significant impact on Alzheimer’s disease research, and medical research in general, says Heather Snyder, PhD, vice president, Medical & Scientific Relations at the Alzheimer’s Association.
“Many clinical trials worldwide are pausing, changing, or halting the testing of the drug or the intervention,” she told Medscape Medical News. “How the teams have adapted depends on the study,” she added. “As you can imagine, things are changing on a daily basis.”
The US Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER) trial, for example, is on hold until at least May 31. The Alzheimer’s Association is helping to implement and fund the study along with Wake Forest University Medical Center.
“We’re not randomizing participants at this point in time and the intervention — which is based on a team meeting, and there is a social aspect to that — has been paused,” Dr. Snyder said.
Another pivotal study underway is the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s study (the A4 Study). Investigators are evaluating if an anti-amyloid antibody, solanezumab (Eli Lilly), can slow memory loss among people with amyloid on imaging but no symptoms of cognitive decline at baseline.
“The A4 Study is definitely continuing. However, in an effort to minimize risk to participants, site staff and study integrity, we have implemented an optional study hiatus for both the double-blind and open-label extension phases,” lead investigator Reisa Anne Sperling, MD, told Medscape Medical News.
“We wanted to prioritize the safety of our participants as well as the ability of participants to remain in the study … despite disruptions from the COVID-19 pandemic,” said Dr. Sperling, who is professor of neurology at Harvard Medical School and director of the Center for Alzheimer Research and Treatment at Brigham and Women’s Hospital and Massachusetts General Hospital in Boston.
The ultimate goal is for A4 participants to receive the full number of planned infusions and assessments, even if it takes longer, she added.
Many Alzheimer’s disease researchers outside the United States face similar challenges. “As you probably are well aware, Spain is now in a complete lockdown. This has affected research centers like ours, the Barcelona Brain Research Center, and the way we work,” José Luís Molinuevo Guix, MD, PhD, told Medscape Medical News.
All participants in observational studies like the ALFA+ study and initiatives, as well as those in trials including PENSA and AB1601, “are not allowed, by law, to come in, hence from a safety perspective we are on good grounds,” added Dr. Molinuevo Guix, who directs the Alzheimer’s disease and other cognitive disorders unit at the Hospital Clinic de Barcelona.
The investigators are creating protocols for communicating with participants during the pandemic and for restarting visits safely after the lockdown has ended.
Stroke studies amended or suspended
A similar situation is occurring in stroke trials. Stroke is “obviously an acute disease, as well as a disease that requires secondary prevention,” Mitchell Elkind, MD, president-elect of the American Heart Association, told Medscape Medical News.
“One could argue that patients with stroke are going to be in the hospital anyway – why not enroll them in a study? They’re not incurring any additional risk,” he said. “But the staff have to come in to see them, and we’re really trying to avoid exposure.”
One ongoing trial, the Atrial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke (ARCADIA), stopped randomly assigning new participants to secondary prevention with apixaban or aspirin because of COVID-19. However, Dr. Elkind and colleagues plan to provide medication to the 440 people already in the trial.
“Wherever possible, the study coordinators are shipping the drug to people and doing follow-up visits by phone or video,” said Dr. Elkind, chief of the Division of Neurology Clinical Outcomes Research and Population Sciences at Columbia University in New York City.
Protecting patients, staff, and ultimately society is a “major driving force in stopping the randomizations,” he stressed.
ARCADIA is part of the StrokeNet prevention trials network, run by the NIH’s National Institute of Neurologic Disorders and Stroke (NINDS). Additional pivotal trials include the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST) and the Multi-arm Optimization of Stroke Thrombolysis (MOST) studies, he said.
Joseph Broderick, MD, director of the national NIH StrokeNet, agreed that safety comes first. “It was the decision of the StrokeNet leadership and the principal investigators of the trials that we needed to hold recruitment of new patients while we worked on adapting processes of enrollment to ensure the safety of both patients and researchers interacting with study patients,” he told Medscape Medical News.
Potential risks vary based on the study intervention and the need for in-person interactions. Trials that include stimulation devices or physical therapy, for example, might be most affected, added Dr. Broderick, professor and director of the UC Gardner Neuroscience Institute at the University of Cincinnati in Ohio.
Nevertheless, “there are potential ways … to move as much as possible toward telemedicine and digital interactions during this time.”
Multiple challenges in multiple sclerosis
At the national level, the COVID-19 pandemic has had an “unprecedented impact on almost all the clinical trials funded by NINDS,” said Clinton Wright, MD, director of the Division of Clinical Research at NINDS. “Investigators have had to adapt quickly.”
Supplementing existing grants with money to conduct research on COVID-19 and pursuing research opportunities from different institutes are “some of the creative approaches [that] have come from the NIH [National Institutes of Health] itself,” Dr. Wright said. “Other creative approaches have come from investigators trying to keep their studies and trials going during the pandemic.”
In clinical trials, “everything from electronic consent to in-home research drug delivery is being brought to bear.”
“A few ongoing trials have been able to modify their protocols to obtain consent and carry out evaluations remotely by telephone or videoconferencing,” Dr. Wright said. “This is especially critical for trials that involve medical management of specific risk factors or conditions, where suspension of the trial could itself have adverse consequences due to reduced engagement with research participants.”
For participants already in MS studies, “each upcoming visit is assessed for whether it’s critical or could be done virtually or just skipped. If a person needs a treatment that cannot be postponed or skipped, they come in,” Jeffrey Cohen, MD, director of the Experimental Therapeutics Program at the Mellen Center for Multiple Sclerosis Treatment and Research at the Cleveland Clinic, Ohio, told Medscape Medical News.
New study enrollment is largely on hold and study visits for existing participants are limited, said Dr. Cohen, who is also president of the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS).
Some of the major ongoing trials in MS are “looking at very fundamental questions in the field,” Dr. Cohen said. The Determining the Effectiveness of earLy Intensive Versus Escalation Approaches for RRMS (DELIVER-MS) and Traditional Versus Early Aggressive Therapy for Multiple Sclerosis (TREAT-MS) trials, for example, evaluate whether treatment should be initiated with one of the less efficacious agents with escalation as needed, or whether treatment should begin with a high-efficacy agent.
Both trials are currently on hold because of the pandemic, as is the Best Available Therapy Versus Autologous Hematopoietic Stem Cell Transplant for Multiple Sclerosis (BEAT-MS) study.
“There has been a lot of interest in hematopoietic stem cell transplants and where they fit into our overall treatment strategy, and this is intended to provide a more definitive answer,” Dr. Cohen said.
Making the most of down time
“The pandemic has been challenging” in terms of ongoing MS research, said Benjamin M. Segal, MD, chair of the Department of Neurology and director of the Neuroscience Research Institute at the Ohio State University Wexner Medical Center, Columbus.
“With regard to the lab, our animal model experiments have been placed on hold. We have stopped collecting samples from clinical subjects for biomarker studies.
“However, my research team has been taking advantage of the time that has been freed up from bench work by analyzing data sets that had been placed aside, delving more deeply into the literature, and writing new grant proposals and articles,” he added.
Two of Dr. Segal’s trainees are writing review articles on the immunopathogenesis of MS and its treatment. Another postdoctoral candidate is writing a grant proposal to investigate how coinfection with a coronavirus modulates CNS pathology and the clinical course of an animal model of MS.
“I am asking my trainees to plan out experiments further in advance than they ever have before, so they are as prepared as possible to resume their research agendas once we are up and running again,” Dr. Segal said.
Confronting current challenges while planning for a future less disrupted by the pandemic is a common theme that emerges.
“The duration of this [pandemic] will dictate how we analyze the data at the end [for the US POINTER study]. There is a large group of statisticians working on this,” Dr. Snyder said.
Dr. Sperling of Harvard Medical School also remains undeterred. “This is definitely a challenging time, as we must not allow the COVID-19 to interfere with our essential mission to find a successful treatment to prevent cognitive decline in AD. We do need, however, to be as flexible as possible to protect our participants and minimize the impact to our overall study integrity,” she said.
NIH guidance
Dr. Molinuevo Guix, of the Barcelona Brain Research Center, is also determined to continue his Alzheimer’s disease research. “I am aware that after the crisis, there will be less [risk] but still a COVID-19 infection risk, so apart from trying to generate part of our visits virtually, we want to make sure we have all necessary safety measures in place. We remain very active to preserve the work we have done to keep up the fight against Alzheimer’s and dementia,” he said.
Such forward thinking also applies to major stroke trials, said Dr. Broderick of the University of Cincinnati. “As soon as we shut down enrollment in stroke trials, we immediately began to make plans about how and when we can restart our stroke trials,” he explained. “One of our trials can do every step of the trial process remotely without direct in-person interactions and will be able to restart soon.”
An individualized approach is needed, Dr. Broderick added. “For trials involving necessary in-person and hands-on assessments, we will need to consider how best to use protective equipment and expanded testing that will likely match the ongoing clinical care and requirements at a given institution.
“Even if a trial officially reopens enrollment, the decision to enroll locally will need to follow local institutional environment and guidelines. Thus, restart of trial enrollment will not likely be uniform, similar to how trials often start in the first place,” Dr. Broderick said.
The NIH published uniform standards for researchers across its institutes to help guide them during the pandemic. Future contingency plans also are underway at the NINDS.
“As the pandemic wanes and in-person research activities restart, it will be important to have in place safety measures that prevent a resurgence of the virus, such as proper personal protective equipment for staff and research participants, said Dr. Wright, the clinical research director at NINDS.
For clinical trials, NINDS is prepared to provide supplemental funds to trial investigators to help support additional activities undertaken as a result of the pandemic.
“This has been an instructive experience. The pandemic will end, and we will resume much of our old patterns of behavior,” said Ohio State’s Dr. Segal. “But some of the strategies that we have employed to get through this time will continue to influence the way we communicate information, plan experiments, and prioritize research activities in the future, to good effect.”
Drs. Snyder, Sperling, Molinuevo Guix, Elkind, Broderick, Wright, Cohen, and Segal have disclosed no relevant disclosures.
This story first appeared on Medscape.com.
Phytophotodermatitis in a Butterfly Enthusiast Induced by Common Rue
To the Editor:
Phytophotodermatitis is common in dermatology during the summer months, especially in individuals who spend time outdoors; however, identification of the offending plant can be challenging. We report a case of phytophotodermatitis in which the causative plant, common rue, was not identified until it was revealed that the patient was a butterfly enthusiast.
A 60-year-old woman presented to the outpatient dermatology clinic in late summer for a routine skin examination. An eruption was noted over the right thigh and knee that had first appeared approximately 2 weeks prior. The rash started as pruritic blisters but gradually progressed to erythema and then eventually to brown markings, which were observed at the current presentation. Physical examination revealed hyperpigmented, brown, streaky, linear patches and plaques over the right thigh, knee, and lower leg (Figure). When asked about her hobbies, the patient reported an affinity for butterflies and noted that she attracts them with specific species of plants in her garden. She recalled recently planting the herb of grace, or common rue, to attract the giant swallowtail butterfly (Papilio cresphontes). Upon further inquiry, she remembered working in the garden on her knees and digging up roots near the common rue plant while wearing shorts approximately 2 weeks prior to the current presentation. Given the streaky linear pattern of the eruption along with recent sun exposure and exposure to the common rue plant, a diagnosis of phytophotodermatitis was made. No further treatment was sought, as the eruption was not bothersome to her. She was intrigued that the common rue plant had caused the dermatitis and planned on taking proper precautions when working near the plant in the future.
In this case, the observed phototoxic skin findings resulted from exposure to common rue (Ruta graveolens),a pungently scented evergreen shrub native to the Mediterranean region and a member of the Rutaceae family. Extracts have been used in homeopathic practices for bruises, sprains, headache, neck stiffness, rheumatologic pain, neuralgia, stomach problems, and phlebitis, as well as in seasonings, soaps, creams, and perfumes.1 The most commonly encountered plants known to cause phytophotodermatitis belong to the Apiaceae and Rutaceae families.2 Members of Apiaceae include angelica, celery, dill, fennel, hogweed, parsley, and parsnip. Aside from the common rue plant, the Rutaceae family also includes bergamot orange, bitter orange, burning bush (or gas plant), grapefruit, lemon, and lime. Other potential offending agents are fig, mustard, buttercup, St. John’s wort, and scurfpea. The phototoxic properties are due to furocoumarins, which include psoralens and angelicins. They are inert until activated by UVA radiation, which inflicts direct cellular damage, causing vacuolization and apoptosis of keratinocytes, similar to a sunburn.3 Clinical findings typically present 24 hours after sun exposure with erythema, edema, pain, and occasionally vesicles or bullae in severe cases. Unlike sunburn, lesions often present in linear, streaky, or bizarre patterns, reflective of the direct contact with the plant. The lesions eventually transition to hyperpigmentation, which may take months to years to resolve.
Other considerations in cases of suspected phytophotodermatitis include polymorphic light eruption, actinic prurigo, hydroa vacciniforme, chronic actinic dermatitis, solar urticaria, drug reactions, porphyria, Smith-Lemli-Opitz syndrome, lupus erythematosus, and dermatomyositis.4 Clinicians should suspect phytophotodermatitis with phototoxic findings in bartenders, citrus farm workers, gardeners, chefs, and kitchen workers, especially those handling limes and celery. As in our case, phytophotodermatitis also should be considered in butterfly enthusiasts trying to attract the giant swallowtail butterfly. The caterpillars feed on the leaves of the common rue plant, one of a select few plants that giant swallowtail butterflies use as a host due to its bitter leaves that aid in avoiding predators.5
This case illustrates a unique perspective of phytophotodermatitis, as butterfly enthusiasm is not commonly reported in association with the common rue plant with respect to phytophotodermatitis. This case underscores the importance of inquiring about patients’ professions and hobbies, both in dermatology and other specialties.
- Atta AH, Alkofahi A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J Ethnopharmacol. 1998;60:117-124.
- McGovern TW. Dermatoses due to plants. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. Edinburgh, Scotland: Mosby; 2007:265-283.
- Hawk JLM, Calonje E. The photosensitivity disorders. In: Elder DE, ed. Lever’s Histopathology of the Skin. 9th ed. Philadelphia, Pennsylvania: Lippincott Williams and Wilkins; 2005:345-353.
- Lim HW. Abnormal responses to ultraviolet radiation: photosensitivity induced by exogenous agents. In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012:1066-1074.
- McAuslane H. Giant swallowtail. University of Florida Department of Entomology and Nematology Featured Creatures website. http://entnemdept.ufl.edu/creatures/citrus/giantswallowtail.htm. Revised January 2018. Accessed April 10, 2020.
To the Editor:
Phytophotodermatitis is common in dermatology during the summer months, especially in individuals who spend time outdoors; however, identification of the offending plant can be challenging. We report a case of phytophotodermatitis in which the causative plant, common rue, was not identified until it was revealed that the patient was a butterfly enthusiast.
A 60-year-old woman presented to the outpatient dermatology clinic in late summer for a routine skin examination. An eruption was noted over the right thigh and knee that had first appeared approximately 2 weeks prior. The rash started as pruritic blisters but gradually progressed to erythema and then eventually to brown markings, which were observed at the current presentation. Physical examination revealed hyperpigmented, brown, streaky, linear patches and plaques over the right thigh, knee, and lower leg (Figure). When asked about her hobbies, the patient reported an affinity for butterflies and noted that she attracts them with specific species of plants in her garden. She recalled recently planting the herb of grace, or common rue, to attract the giant swallowtail butterfly (Papilio cresphontes). Upon further inquiry, she remembered working in the garden on her knees and digging up roots near the common rue plant while wearing shorts approximately 2 weeks prior to the current presentation. Given the streaky linear pattern of the eruption along with recent sun exposure and exposure to the common rue plant, a diagnosis of phytophotodermatitis was made. No further treatment was sought, as the eruption was not bothersome to her. She was intrigued that the common rue plant had caused the dermatitis and planned on taking proper precautions when working near the plant in the future.

In this case, the observed phototoxic skin findings resulted from exposure to common rue (Ruta graveolens),a pungently scented evergreen shrub native to the Mediterranean region and a member of the Rutaceae family. Extracts have been used in homeopathic practices for bruises, sprains, headache, neck stiffness, rheumatologic pain, neuralgia, stomach problems, and phlebitis, as well as in seasonings, soaps, creams, and perfumes.1 The most commonly encountered plants known to cause phytophotodermatitis belong to the Apiaceae and Rutaceae families.2 Members of Apiaceae include angelica, celery, dill, fennel, hogweed, parsley, and parsnip. Aside from the common rue plant, the Rutaceae family also includes bergamot orange, bitter orange, burning bush (or gas plant), grapefruit, lemon, and lime. Other potential offending agents are fig, mustard, buttercup, St. John’s wort, and scurfpea. The phototoxic properties are due to furocoumarins, which include psoralens and angelicins. They are inert until activated by UVA radiation, which inflicts direct cellular damage, causing vacuolization and apoptosis of keratinocytes, similar to a sunburn.3 Clinical findings typically present 24 hours after sun exposure with erythema, edema, pain, and occasionally vesicles or bullae in severe cases. Unlike sunburn, lesions often present in linear, streaky, or bizarre patterns, reflective of the direct contact with the plant. The lesions eventually transition to hyperpigmentation, which may take months to years to resolve.
Other considerations in cases of suspected phytophotodermatitis include polymorphic light eruption, actinic prurigo, hydroa vacciniforme, chronic actinic dermatitis, solar urticaria, drug reactions, porphyria, Smith-Lemli-Opitz syndrome, lupus erythematosus, and dermatomyositis.4 Clinicians should suspect phytophotodermatitis with phototoxic findings in bartenders, citrus farm workers, gardeners, chefs, and kitchen workers, especially those handling limes and celery. As in our case, phytophotodermatitis also should be considered in butterfly enthusiasts trying to attract the giant swallowtail butterfly. The caterpillars feed on the leaves of the common rue plant, one of a select few plants that giant swallowtail butterflies use as a host due to its bitter leaves that aid in avoiding predators.5
This case illustrates a unique perspective of phytophotodermatitis, as butterfly enthusiasm is not commonly reported in association with the common rue plant with respect to phytophotodermatitis. This case underscores the importance of inquiring about patients’ professions and hobbies, both in dermatology and other specialties.
To the Editor:
Phytophotodermatitis is common in dermatology during the summer months, especially in individuals who spend time outdoors; however, identification of the offending plant can be challenging. We report a case of phytophotodermatitis in which the causative plant, common rue, was not identified until it was revealed that the patient was a butterfly enthusiast.
A 60-year-old woman presented to the outpatient dermatology clinic in late summer for a routine skin examination. An eruption was noted over the right thigh and knee that had first appeared approximately 2 weeks prior. The rash started as pruritic blisters but gradually progressed to erythema and then eventually to brown markings, which were observed at the current presentation. Physical examination revealed hyperpigmented, brown, streaky, linear patches and plaques over the right thigh, knee, and lower leg (Figure). When asked about her hobbies, the patient reported an affinity for butterflies and noted that she attracts them with specific species of plants in her garden. She recalled recently planting the herb of grace, or common rue, to attract the giant swallowtail butterfly (Papilio cresphontes). Upon further inquiry, she remembered working in the garden on her knees and digging up roots near the common rue plant while wearing shorts approximately 2 weeks prior to the current presentation. Given the streaky linear pattern of the eruption along with recent sun exposure and exposure to the common rue plant, a diagnosis of phytophotodermatitis was made. No further treatment was sought, as the eruption was not bothersome to her. She was intrigued that the common rue plant had caused the dermatitis and planned on taking proper precautions when working near the plant in the future.

In this case, the observed phototoxic skin findings resulted from exposure to common rue (Ruta graveolens),a pungently scented evergreen shrub native to the Mediterranean region and a member of the Rutaceae family. Extracts have been used in homeopathic practices for bruises, sprains, headache, neck stiffness, rheumatologic pain, neuralgia, stomach problems, and phlebitis, as well as in seasonings, soaps, creams, and perfumes.1 The most commonly encountered plants known to cause phytophotodermatitis belong to the Apiaceae and Rutaceae families.2 Members of Apiaceae include angelica, celery, dill, fennel, hogweed, parsley, and parsnip. Aside from the common rue plant, the Rutaceae family also includes bergamot orange, bitter orange, burning bush (or gas plant), grapefruit, lemon, and lime. Other potential offending agents are fig, mustard, buttercup, St. John’s wort, and scurfpea. The phototoxic properties are due to furocoumarins, which include psoralens and angelicins. They are inert until activated by UVA radiation, which inflicts direct cellular damage, causing vacuolization and apoptosis of keratinocytes, similar to a sunburn.3 Clinical findings typically present 24 hours after sun exposure with erythema, edema, pain, and occasionally vesicles or bullae in severe cases. Unlike sunburn, lesions often present in linear, streaky, or bizarre patterns, reflective of the direct contact with the plant. The lesions eventually transition to hyperpigmentation, which may take months to years to resolve.
Other considerations in cases of suspected phytophotodermatitis include polymorphic light eruption, actinic prurigo, hydroa vacciniforme, chronic actinic dermatitis, solar urticaria, drug reactions, porphyria, Smith-Lemli-Opitz syndrome, lupus erythematosus, and dermatomyositis.4 Clinicians should suspect phytophotodermatitis with phototoxic findings in bartenders, citrus farm workers, gardeners, chefs, and kitchen workers, especially those handling limes and celery. As in our case, phytophotodermatitis also should be considered in butterfly enthusiasts trying to attract the giant swallowtail butterfly. The caterpillars feed on the leaves of the common rue plant, one of a select few plants that giant swallowtail butterflies use as a host due to its bitter leaves that aid in avoiding predators.5
This case illustrates a unique perspective of phytophotodermatitis, as butterfly enthusiasm is not commonly reported in association with the common rue plant with respect to phytophotodermatitis. This case underscores the importance of inquiring about patients’ professions and hobbies, both in dermatology and other specialties.
- Atta AH, Alkofahi A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J Ethnopharmacol. 1998;60:117-124.
- McGovern TW. Dermatoses due to plants. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. Edinburgh, Scotland: Mosby; 2007:265-283.
- Hawk JLM, Calonje E. The photosensitivity disorders. In: Elder DE, ed. Lever’s Histopathology of the Skin. 9th ed. Philadelphia, Pennsylvania: Lippincott Williams and Wilkins; 2005:345-353.
- Lim HW. Abnormal responses to ultraviolet radiation: photosensitivity induced by exogenous agents. In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012:1066-1074.
- McAuslane H. Giant swallowtail. University of Florida Department of Entomology and Nematology Featured Creatures website. http://entnemdept.ufl.edu/creatures/citrus/giantswallowtail.htm. Revised January 2018. Accessed April 10, 2020.
- Atta AH, Alkofahi A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J Ethnopharmacol. 1998;60:117-124.
- McGovern TW. Dermatoses due to plants. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. Edinburgh, Scotland: Mosby; 2007:265-283.
- Hawk JLM, Calonje E. The photosensitivity disorders. In: Elder DE, ed. Lever’s Histopathology of the Skin. 9th ed. Philadelphia, Pennsylvania: Lippincott Williams and Wilkins; 2005:345-353.
- Lim HW. Abnormal responses to ultraviolet radiation: photosensitivity induced by exogenous agents. In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012:1066-1074.
- McAuslane H. Giant swallowtail. University of Florida Department of Entomology and Nematology Featured Creatures website. http://entnemdept.ufl.edu/creatures/citrus/giantswallowtail.htm. Revised January 2018. Accessed April 10, 2020.
Practice Points
- It is important to inquire about patients’ professions and hobbies, which may lead to the diagnosis, as in this case of a butterfly enthusiast trying to attract the giant swallowtail butterfly with the common rue plant.
- One should suspect phytophotodermatitis with phototoxic findings in bartenders, citrus farm workers, gardeners, chefs, and kitchen workers, especially those handling limes and celery
Remdesivir now ‘standard of care’ for COVID-19, Fauci says
, according to a preliminary data analysis from a U.S.-led randomized, controlled trial.
On the basis of as yet unpublished data, remdesivir “will be the standard of care” for patients with COVID-19, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases (NIAID), said during a press conference at the White House April 29.
The randomized, placebo-controlled international trial was sponsored by NIAID, which is part of the National Institutes of Health, and enrolled 1,063 patients. It began on Feb. 21.
The interim results, discussed in the press conference and in a NIAID press release, show that time to recovery (i.e., being well enough for hospital discharge or to return to normal activity level) was 31% faster for patients who received remdesivir than for those who received placebo (P < .001).
The median time to recovery was 11 days for patients treated with remdesivir, compared with 15 days for those who received placebo. Results also suggested a survival benefit, with a mortality rate of 8.0% for the group receiving remdesivir and 11.6% for the patients who received placebo (P = .059).
The study, known as the Adaptive COVID-19 Treatment Trial (ACTT), is the first clinical trial launched in the United States to evaluate an experimental treatment for COVID-19. It is being conducted at 68 sites – 47 in the United States and 21 in countries in Europe and Asia.
The data are being released after an interim review by the independent data safety monitoring board found significant benefit with the drug, Dr. Fauci said.
“The reason we are making the announcement now is something that people don’t fully appreciate: Whenever you have clear-cut evidence that a drug works, you have an ethical obligation to let the people in the placebo group know so they could have access,” he explained.
“When I was looking at the data with our team the other night, it was reminiscent of 34 years ago in 1986 when we were struggling for drugs for HIV,” said Dr. Fauci, who was a key figure in HIV/AIDS research. “We did the first randomized, placebo-controlled trial with AZT. It turned out to have an effect that was modest but that was not the endgame because, building on that, every year after, we did better and better.”
Similarly, new trials of drugs for COVID-19 will build on remdesivir, with other agents being added to block other pathways or viral enzymes, Dr. Fauci said.
The study will be submitted to a journal for peer review, he noted, but the New York Times is reporting that the Food and Drug Administration will approve remdesivir for emergency use soon.
In contrast to the positive results Dr. Fauci described from the NIAID-sponsored trial, a randomized, placebo-controlled clinical trial of remdesivir among hospitalized patients with severe COVID-19 in China was inconclusive.
The study, published online in The Lancet, showed some nonsignificant trends toward benefit but did not meet its primary endpoint.
The study was stopped early after 237 of the intended 453 patients were enrolled, owing to a lack of additional patients who met the eligibility criteria. The trial was thus underpowered.
Results showed that treatment with remdesivir did not significantly speed recovery or reduce deaths from COVID-19, but with regard to prespecified secondary outcomes, time to clinical improvement and duration of invasive mechanical ventilation were shorter among a subgroup of patients who began undergoing treatment with remdesivir within 10 days of showing symptoms, in comparison with patients who received standard care.
“To me, the studies reported here in The Lancet appear to be less promising than some statements released today from the NIH, so the situation is a bit puzzling to me,” said Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, who was not involved in the study. “I would need to look more closely at the data which NIH is looking at to understand the differences.”
Trial details
The published trial was conducted at 10 hospitals in Hubei, China. Enrollment criteria included being admitted to hospital with laboratory-confirmed SARS-CoV-2 infection within 12 days of symptom onset, having oxygen saturation of 94% or less, and having radiologically confirmed pneumonia.
Patients were randomly assigned in a 2:1 ratio to receive intravenous remdesivir (200 mg on day 1 followed by 100 mg on days 2–10 in single daily infusions) or placebo infusions for 10 days. Patients were permitted concomitant use of lopinavir-ritonavir, interferons, and corticosteroids.
The primary endpoint was time to clinical improvement to day 28, defined as the time (in days) from randomization to the point of a decline of two levels on a six-point ordinal scale of clinical status (on that scale, 1 indicated that the patient was discharged, and 6 indicated death) or to the patient’s being discharged alive from hospital, whichever came first.
The trial was stopped early because stringent public health measures used in Wuhan led to marked reductions in new patient presentations and because lack of available hospital beds resulted in most patients being enrolled later in the course of disease.
Between Feb. 6, 2020, and March 12, 2020, 237 patients were enrolled and were randomly assigned to receive either remdesivir (n = 158) or placebo (n = 79).
Results showed that use of remdesivir was not associated with a difference in time to clinical improvement (hazard ratio [HR], 1.23; 95% confidence interval [CI], 0.87-1.75).
Although not statistically significant, time to clinical improvement was numerically faster for patients who received remdesivir than for those who received placebo among patients with symptom duration of 10 days or less (median, 18 days vs. 23 days; HR, 1.52; 95% CI, 0.95-2.43).
The mortality rates were similar for the two groups (14% of patients who received remdesivir died vs. 13% of those who received placebo). There was no signal that viral load decreased differentially over time between the two groups.
Adverse events were reported in 66% of remdesivir recipients, vs. 64% of those who received placebo. Remdesivir was stopped early because of adverse events in 12% of patients; it was stopped early for 5% of those who received placebo.
The authors, led by Yeming Wang, MD, China-Japan Friendship Hospital, Beijing, noted that compared with a previous study of compassionate use of remdesivir, the population in the current study was less ill and was treated somewhat earlier in the disease course (median, 10 days vs. 12 days).
Because the study was terminated early, the researchers said they could not adequately assess whether earlier treatment with remdesivir might have provided clinical benefit.
“However, among patients who were treated within 10 days of symptom onset, remdesivir was not a significant factor but was associated with a numerical reduction of 5 days in median time to clinical improvement,” they stated.
They added that remdesivir was adequately tolerated and that no new safety concerns were identified.
In an accompanying comment in The Lancet, John David Norrie, MD, Edinburgh Clinical Trials Unit, United Kingdom, pointed out that this study “has not shown a statistically significant finding that confirms a remdesivir treatment benefit of at least the minimally clinically important difference, nor has it ruled such a benefit out.”
Dr. Norrie was cautious about the fact that the subgroup analysis suggested possible benefit for those treated within 10 days.
Although he said it seems biologically plausible that treating patients earlier could be more effective, he added that “as well as being vigilant against overinterpretation, we need to ensure that hypotheses generated in efficacy-based trials, even in subgroups, are confirmed or refuted in subsequent adequately powered trials or meta-analyses.”
Noting that several other trials of remdesivir are underway, he concluded: “With each individual study at heightened risk of being incomplete, pooling data across possibly several underpowered but high-quality studies looks like our best way to obtain robust insights into what works, safely, and on whom.”
A version of this article originally appeared on Medscape.com.
, according to a preliminary data analysis from a U.S.-led randomized, controlled trial.
On the basis of as yet unpublished data, remdesivir “will be the standard of care” for patients with COVID-19, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases (NIAID), said during a press conference at the White House April 29.
The randomized, placebo-controlled international trial was sponsored by NIAID, which is part of the National Institutes of Health, and enrolled 1,063 patients. It began on Feb. 21.
The interim results, discussed in the press conference and in a NIAID press release, show that time to recovery (i.e., being well enough for hospital discharge or to return to normal activity level) was 31% faster for patients who received remdesivir than for those who received placebo (P < .001).
The median time to recovery was 11 days for patients treated with remdesivir, compared with 15 days for those who received placebo. Results also suggested a survival benefit, with a mortality rate of 8.0% for the group receiving remdesivir and 11.6% for the patients who received placebo (P = .059).
The study, known as the Adaptive COVID-19 Treatment Trial (ACTT), is the first clinical trial launched in the United States to evaluate an experimental treatment for COVID-19. It is being conducted at 68 sites – 47 in the United States and 21 in countries in Europe and Asia.
The data are being released after an interim review by the independent data safety monitoring board found significant benefit with the drug, Dr. Fauci said.
“The reason we are making the announcement now is something that people don’t fully appreciate: Whenever you have clear-cut evidence that a drug works, you have an ethical obligation to let the people in the placebo group know so they could have access,” he explained.
“When I was looking at the data with our team the other night, it was reminiscent of 34 years ago in 1986 when we were struggling for drugs for HIV,” said Dr. Fauci, who was a key figure in HIV/AIDS research. “We did the first randomized, placebo-controlled trial with AZT. It turned out to have an effect that was modest but that was not the endgame because, building on that, every year after, we did better and better.”
Similarly, new trials of drugs for COVID-19 will build on remdesivir, with other agents being added to block other pathways or viral enzymes, Dr. Fauci said.
The study will be submitted to a journal for peer review, he noted, but the New York Times is reporting that the Food and Drug Administration will approve remdesivir for emergency use soon.
In contrast to the positive results Dr. Fauci described from the NIAID-sponsored trial, a randomized, placebo-controlled clinical trial of remdesivir among hospitalized patients with severe COVID-19 in China was inconclusive.
The study, published online in The Lancet, showed some nonsignificant trends toward benefit but did not meet its primary endpoint.
The study was stopped early after 237 of the intended 453 patients were enrolled, owing to a lack of additional patients who met the eligibility criteria. The trial was thus underpowered.
Results showed that treatment with remdesivir did not significantly speed recovery or reduce deaths from COVID-19, but with regard to prespecified secondary outcomes, time to clinical improvement and duration of invasive mechanical ventilation were shorter among a subgroup of patients who began undergoing treatment with remdesivir within 10 days of showing symptoms, in comparison with patients who received standard care.
“To me, the studies reported here in The Lancet appear to be less promising than some statements released today from the NIH, so the situation is a bit puzzling to me,” said Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, who was not involved in the study. “I would need to look more closely at the data which NIH is looking at to understand the differences.”
Trial details
The published trial was conducted at 10 hospitals in Hubei, China. Enrollment criteria included being admitted to hospital with laboratory-confirmed SARS-CoV-2 infection within 12 days of symptom onset, having oxygen saturation of 94% or less, and having radiologically confirmed pneumonia.
Patients were randomly assigned in a 2:1 ratio to receive intravenous remdesivir (200 mg on day 1 followed by 100 mg on days 2–10 in single daily infusions) or placebo infusions for 10 days. Patients were permitted concomitant use of lopinavir-ritonavir, interferons, and corticosteroids.
The primary endpoint was time to clinical improvement to day 28, defined as the time (in days) from randomization to the point of a decline of two levels on a six-point ordinal scale of clinical status (on that scale, 1 indicated that the patient was discharged, and 6 indicated death) or to the patient’s being discharged alive from hospital, whichever came first.
The trial was stopped early because stringent public health measures used in Wuhan led to marked reductions in new patient presentations and because lack of available hospital beds resulted in most patients being enrolled later in the course of disease.
Between Feb. 6, 2020, and March 12, 2020, 237 patients were enrolled and were randomly assigned to receive either remdesivir (n = 158) or placebo (n = 79).
Results showed that use of remdesivir was not associated with a difference in time to clinical improvement (hazard ratio [HR], 1.23; 95% confidence interval [CI], 0.87-1.75).
Although not statistically significant, time to clinical improvement was numerically faster for patients who received remdesivir than for those who received placebo among patients with symptom duration of 10 days or less (median, 18 days vs. 23 days; HR, 1.52; 95% CI, 0.95-2.43).
The mortality rates were similar for the two groups (14% of patients who received remdesivir died vs. 13% of those who received placebo). There was no signal that viral load decreased differentially over time between the two groups.
Adverse events were reported in 66% of remdesivir recipients, vs. 64% of those who received placebo. Remdesivir was stopped early because of adverse events in 12% of patients; it was stopped early for 5% of those who received placebo.
The authors, led by Yeming Wang, MD, China-Japan Friendship Hospital, Beijing, noted that compared with a previous study of compassionate use of remdesivir, the population in the current study was less ill and was treated somewhat earlier in the disease course (median, 10 days vs. 12 days).
Because the study was terminated early, the researchers said they could not adequately assess whether earlier treatment with remdesivir might have provided clinical benefit.
“However, among patients who were treated within 10 days of symptom onset, remdesivir was not a significant factor but was associated with a numerical reduction of 5 days in median time to clinical improvement,” they stated.
They added that remdesivir was adequately tolerated and that no new safety concerns were identified.
In an accompanying comment in The Lancet, John David Norrie, MD, Edinburgh Clinical Trials Unit, United Kingdom, pointed out that this study “has not shown a statistically significant finding that confirms a remdesivir treatment benefit of at least the minimally clinically important difference, nor has it ruled such a benefit out.”
Dr. Norrie was cautious about the fact that the subgroup analysis suggested possible benefit for those treated within 10 days.
Although he said it seems biologically plausible that treating patients earlier could be more effective, he added that “as well as being vigilant against overinterpretation, we need to ensure that hypotheses generated in efficacy-based trials, even in subgroups, are confirmed or refuted in subsequent adequately powered trials or meta-analyses.”
Noting that several other trials of remdesivir are underway, he concluded: “With each individual study at heightened risk of being incomplete, pooling data across possibly several underpowered but high-quality studies looks like our best way to obtain robust insights into what works, safely, and on whom.”
A version of this article originally appeared on Medscape.com.
, according to a preliminary data analysis from a U.S.-led randomized, controlled trial.
On the basis of as yet unpublished data, remdesivir “will be the standard of care” for patients with COVID-19, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases (NIAID), said during a press conference at the White House April 29.
The randomized, placebo-controlled international trial was sponsored by NIAID, which is part of the National Institutes of Health, and enrolled 1,063 patients. It began on Feb. 21.
The interim results, discussed in the press conference and in a NIAID press release, show that time to recovery (i.e., being well enough for hospital discharge or to return to normal activity level) was 31% faster for patients who received remdesivir than for those who received placebo (P < .001).
The median time to recovery was 11 days for patients treated with remdesivir, compared with 15 days for those who received placebo. Results also suggested a survival benefit, with a mortality rate of 8.0% for the group receiving remdesivir and 11.6% for the patients who received placebo (P = .059).
The study, known as the Adaptive COVID-19 Treatment Trial (ACTT), is the first clinical trial launched in the United States to evaluate an experimental treatment for COVID-19. It is being conducted at 68 sites – 47 in the United States and 21 in countries in Europe and Asia.
The data are being released after an interim review by the independent data safety monitoring board found significant benefit with the drug, Dr. Fauci said.
“The reason we are making the announcement now is something that people don’t fully appreciate: Whenever you have clear-cut evidence that a drug works, you have an ethical obligation to let the people in the placebo group know so they could have access,” he explained.
“When I was looking at the data with our team the other night, it was reminiscent of 34 years ago in 1986 when we were struggling for drugs for HIV,” said Dr. Fauci, who was a key figure in HIV/AIDS research. “We did the first randomized, placebo-controlled trial with AZT. It turned out to have an effect that was modest but that was not the endgame because, building on that, every year after, we did better and better.”
Similarly, new trials of drugs for COVID-19 will build on remdesivir, with other agents being added to block other pathways or viral enzymes, Dr. Fauci said.
The study will be submitted to a journal for peer review, he noted, but the New York Times is reporting that the Food and Drug Administration will approve remdesivir for emergency use soon.
In contrast to the positive results Dr. Fauci described from the NIAID-sponsored trial, a randomized, placebo-controlled clinical trial of remdesivir among hospitalized patients with severe COVID-19 in China was inconclusive.
The study, published online in The Lancet, showed some nonsignificant trends toward benefit but did not meet its primary endpoint.
The study was stopped early after 237 of the intended 453 patients were enrolled, owing to a lack of additional patients who met the eligibility criteria. The trial was thus underpowered.
Results showed that treatment with remdesivir did not significantly speed recovery or reduce deaths from COVID-19, but with regard to prespecified secondary outcomes, time to clinical improvement and duration of invasive mechanical ventilation were shorter among a subgroup of patients who began undergoing treatment with remdesivir within 10 days of showing symptoms, in comparison with patients who received standard care.
“To me, the studies reported here in The Lancet appear to be less promising than some statements released today from the NIH, so the situation is a bit puzzling to me,” said Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, who was not involved in the study. “I would need to look more closely at the data which NIH is looking at to understand the differences.”
Trial details
The published trial was conducted at 10 hospitals in Hubei, China. Enrollment criteria included being admitted to hospital with laboratory-confirmed SARS-CoV-2 infection within 12 days of symptom onset, having oxygen saturation of 94% or less, and having radiologically confirmed pneumonia.
Patients were randomly assigned in a 2:1 ratio to receive intravenous remdesivir (200 mg on day 1 followed by 100 mg on days 2–10 in single daily infusions) or placebo infusions for 10 days. Patients were permitted concomitant use of lopinavir-ritonavir, interferons, and corticosteroids.
The primary endpoint was time to clinical improvement to day 28, defined as the time (in days) from randomization to the point of a decline of two levels on a six-point ordinal scale of clinical status (on that scale, 1 indicated that the patient was discharged, and 6 indicated death) or to the patient’s being discharged alive from hospital, whichever came first.
The trial was stopped early because stringent public health measures used in Wuhan led to marked reductions in new patient presentations and because lack of available hospital beds resulted in most patients being enrolled later in the course of disease.
Between Feb. 6, 2020, and March 12, 2020, 237 patients were enrolled and were randomly assigned to receive either remdesivir (n = 158) or placebo (n = 79).
Results showed that use of remdesivir was not associated with a difference in time to clinical improvement (hazard ratio [HR], 1.23; 95% confidence interval [CI], 0.87-1.75).
Although not statistically significant, time to clinical improvement was numerically faster for patients who received remdesivir than for those who received placebo among patients with symptom duration of 10 days or less (median, 18 days vs. 23 days; HR, 1.52; 95% CI, 0.95-2.43).
The mortality rates were similar for the two groups (14% of patients who received remdesivir died vs. 13% of those who received placebo). There was no signal that viral load decreased differentially over time between the two groups.
Adverse events were reported in 66% of remdesivir recipients, vs. 64% of those who received placebo. Remdesivir was stopped early because of adverse events in 12% of patients; it was stopped early for 5% of those who received placebo.
The authors, led by Yeming Wang, MD, China-Japan Friendship Hospital, Beijing, noted that compared with a previous study of compassionate use of remdesivir, the population in the current study was less ill and was treated somewhat earlier in the disease course (median, 10 days vs. 12 days).
Because the study was terminated early, the researchers said they could not adequately assess whether earlier treatment with remdesivir might have provided clinical benefit.
“However, among patients who were treated within 10 days of symptom onset, remdesivir was not a significant factor but was associated with a numerical reduction of 5 days in median time to clinical improvement,” they stated.
They added that remdesivir was adequately tolerated and that no new safety concerns were identified.
In an accompanying comment in The Lancet, John David Norrie, MD, Edinburgh Clinical Trials Unit, United Kingdom, pointed out that this study “has not shown a statistically significant finding that confirms a remdesivir treatment benefit of at least the minimally clinically important difference, nor has it ruled such a benefit out.”
Dr. Norrie was cautious about the fact that the subgroup analysis suggested possible benefit for those treated within 10 days.
Although he said it seems biologically plausible that treating patients earlier could be more effective, he added that “as well as being vigilant against overinterpretation, we need to ensure that hypotheses generated in efficacy-based trials, even in subgroups, are confirmed or refuted in subsequent adequately powered trials or meta-analyses.”
Noting that several other trials of remdesivir are underway, he concluded: “With each individual study at heightened risk of being incomplete, pooling data across possibly several underpowered but high-quality studies looks like our best way to obtain robust insights into what works, safely, and on whom.”
A version of this article originally appeared on Medscape.com.
Purple oral plaques
The papules were clinically consistent with Kaposi sarcoma (KS) and confirmed with a biopsy from the buccal mucosa. The patient had not been given a diagnosis of human immunodeficiency virus (HIV) prior to this presentation. However, the physician confirmed the diagnosis with RNA titers. A CD4 count < 40 cells/mm3 (reference range, 500-1200 cells/mm3) pointed to her progression to AIDS. A chest X-ray revealed multiple nodules; a subsequent biopsy indicated that they were consistent with KS.
KS is a low-grade tumor of vascular origin associated with human herpesvirus-8 (HHV-8). It most often presents on the skin as flat to raised pink to deep purple lesions. It can manifest in the oral mucosa, viscera, and other organs, which can portend a worse prognosis because of the risks associated with bleeding and organ perforation.
There are 4 types of KS.
- Classic KS occurs most often in elderly men of Mediterranean descent.
- HIV-associated KS can occur at any time during HIV infection but is more common as CD4 counts fall. HIV-associated KS increased in frequency dramatically in the United States during the early years of the HIV pandemic prior to effective antiretroviral therapy (ART).
- Endemic KS occurs in equatorial Africa, where there is a natural increased transmission rate of HHV-8.
- Iatrogenic KS can occur following treatment with immunosuppressive therapies.
Our patient was admitted to the Infectious Disease Service and given ART. Chemotherapy was discussed (and sometimes is warranted in extensive visceral disease) but the patient and her specialists opted for ART alone. In addition to ART, she was started on daily trimethoprim-sulfamethoxazole for pneumocystis prophylaxis.
At 6 months’ follow-up with Infectious Disease, the patient’s oral lesions resolved, CD4 count increased above 200 cells/mm3, and HIV RNA titers fell.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Thariat J, Kirova Y, Sio T, et al. Mucosal Kaposi sarcoma, a Rare Cancer Network study. Rare Tumors. 2012;4:E49.
The papules were clinically consistent with Kaposi sarcoma (KS) and confirmed with a biopsy from the buccal mucosa. The patient had not been given a diagnosis of human immunodeficiency virus (HIV) prior to this presentation. However, the physician confirmed the diagnosis with RNA titers. A CD4 count < 40 cells/mm3 (reference range, 500-1200 cells/mm3) pointed to her progression to AIDS. A chest X-ray revealed multiple nodules; a subsequent biopsy indicated that they were consistent with KS.
KS is a low-grade tumor of vascular origin associated with human herpesvirus-8 (HHV-8). It most often presents on the skin as flat to raised pink to deep purple lesions. It can manifest in the oral mucosa, viscera, and other organs, which can portend a worse prognosis because of the risks associated with bleeding and organ perforation.
There are 4 types of KS.
- Classic KS occurs most often in elderly men of Mediterranean descent.
- HIV-associated KS can occur at any time during HIV infection but is more common as CD4 counts fall. HIV-associated KS increased in frequency dramatically in the United States during the early years of the HIV pandemic prior to effective antiretroviral therapy (ART).
- Endemic KS occurs in equatorial Africa, where there is a natural increased transmission rate of HHV-8.
- Iatrogenic KS can occur following treatment with immunosuppressive therapies.
Our patient was admitted to the Infectious Disease Service and given ART. Chemotherapy was discussed (and sometimes is warranted in extensive visceral disease) but the patient and her specialists opted for ART alone. In addition to ART, she was started on daily trimethoprim-sulfamethoxazole for pneumocystis prophylaxis.
At 6 months’ follow-up with Infectious Disease, the patient’s oral lesions resolved, CD4 count increased above 200 cells/mm3, and HIV RNA titers fell.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
The papules were clinically consistent with Kaposi sarcoma (KS) and confirmed with a biopsy from the buccal mucosa. The patient had not been given a diagnosis of human immunodeficiency virus (HIV) prior to this presentation. However, the physician confirmed the diagnosis with RNA titers. A CD4 count < 40 cells/mm3 (reference range, 500-1200 cells/mm3) pointed to her progression to AIDS. A chest X-ray revealed multiple nodules; a subsequent biopsy indicated that they were consistent with KS.
KS is a low-grade tumor of vascular origin associated with human herpesvirus-8 (HHV-8). It most often presents on the skin as flat to raised pink to deep purple lesions. It can manifest in the oral mucosa, viscera, and other organs, which can portend a worse prognosis because of the risks associated with bleeding and organ perforation.
There are 4 types of KS.
- Classic KS occurs most often in elderly men of Mediterranean descent.
- HIV-associated KS can occur at any time during HIV infection but is more common as CD4 counts fall. HIV-associated KS increased in frequency dramatically in the United States during the early years of the HIV pandemic prior to effective antiretroviral therapy (ART).
- Endemic KS occurs in equatorial Africa, where there is a natural increased transmission rate of HHV-8.
- Iatrogenic KS can occur following treatment with immunosuppressive therapies.
Our patient was admitted to the Infectious Disease Service and given ART. Chemotherapy was discussed (and sometimes is warranted in extensive visceral disease) but the patient and her specialists opted for ART alone. In addition to ART, she was started on daily trimethoprim-sulfamethoxazole for pneumocystis prophylaxis.
At 6 months’ follow-up with Infectious Disease, the patient’s oral lesions resolved, CD4 count increased above 200 cells/mm3, and HIV RNA titers fell.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Thariat J, Kirova Y, Sio T, et al. Mucosal Kaposi sarcoma, a Rare Cancer Network study. Rare Tumors. 2012;4:E49.
Thariat J, Kirova Y, Sio T, et al. Mucosal Kaposi sarcoma, a Rare Cancer Network study. Rare Tumors. 2012;4:E49.